WO2001066078A1 - Cosmetic composition having improved absorption and adhesion characteristics - Google Patents
Cosmetic composition having improved absorption and adhesion characteristics Download PDFInfo
- Publication number
- WO2001066078A1 WO2001066078A1 PCT/GB2000/003948 GB0003948W WO0166078A1 WO 2001066078 A1 WO2001066078 A1 WO 2001066078A1 GB 0003948 W GB0003948 W GB 0003948W WO 0166078 A1 WO0166078 A1 WO 0166078A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- waxy
- starch
- superabsorbent polymer
- powder
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/91—Graft copolymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/732—Starch; Amylose; Amylopectin; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/81—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- A61K8/8141—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- A61K8/8147—Homopolymers or copolymers of acids; Metal or ammonium salts thereof, e.g. crotonic acid, (meth)acrylic acid; Compositions of derivatives of such polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/54—Polymers characterized by specific structures/properties
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/12—Face or body powders for grooming, adorning or absorbing
Definitions
- the present invention relates generally to personal care and cosmetic compositions in powder form providing excellent moisture absorbency and adhesive properties. More particularly, the present invention relates to personal care and cosmetic compositions in powder form comprising superabsorbent polymers.
- talc which is a natural hydrous magnesium silicate
- various other ingredients have also been proposed and utilized for body powders including starches, cellulose derivatives, polymeric substances and the like.
- talc and non-talc compositions are available through commercial channels, numerous attempts to develop improved body powder compositions have been on-going. See for example, U.S. Patent No. 4,185,086 to Zeitx which teaches and claims a body powder composition comprising talc, fragrance and from about 0.1 to 1 wt. % polyethylene glycol. Since one of the primary purposes of a body powder is to absorb moisture, the effectiveness of the body powder is diminished when the powder has reached its capacity for absorbency.
- U.S. Patent No. 5,417,963 to Murphy et al. discloses body and body powder compositions with enhanced deodorant and moist absorbency characteristics consisting of from about 70 wt. % talc and the remainder comprising micro-crystallite particles of a bicarbonate salt coated with a hydrophobic polymer such as xanthan gum, carrageenan, alginate salt, guar gum, gum arabic, casein, dextran, agar, methyl cellulose, carboxymethyl cellulose, and the like.
- a hydrophobic polymer such as xanthan gum, carrageenan, alginate salt, guar gum, gum arabic, casein, dextran, agar, methyl cellulose, carboxymethyl cellulose, and the like.
- Superabsorbent polymers are known and are mainly used in the production of diapers and incontinence articles as well as wound dressings.
- U.S. Patent No. 5,064,653 to Sessions et al. discloses the use of superabsorbent polymers in a foam composition for applications in sanitary napkins, diapers, and the like.
- Superabsorbent polymers are also added in some cosmetic compacted powders to add in the absorbency.
- U.S. Patent No. 5,211 ,892 discloses the addition of superabsorbent polymers to pharmaceutical or cosmetic compacted powder comprising
- thermoplastic product such as polyethylene wax to provide an absorbent or partially friable compacted product.
- U.S. Patent No. 4,661,476 discloses the addition of superabsorbent polymers to skin lotion comprising about 75 to 99 wt. % water, to cool the surface of skin that has been overheated by excessive exposure to sun and/or wind.
- U.S. Patent No. 6,013,271 discloses the addition of superabsorbent materials to an oil-in-water dispersion which comprises from about 30 to 98.89 wt. % of water.
- a method to improve the resistance to wash-off characteristic of a skin care, cosmetic or pharmaceutical composition comprising from 25 wt. % to 99 wt. % (preferably from 25 wt. % to 95 wt. %) a powder carrier, by including in said composition from 0.01 wt. % to 20 wt. % of a superabsorbent polymer.
- a skin care, cosmetic or pharmaceutical composition having superior resistance to wash-off characteristics, the composition comprising from 25 wt. % to 99 wt. % (preferably from 25 wt.% to 95 wt. % ) a powder carrier and from 0.01 wt. % to 20 wt. % of a superabsorbent polymer, wherein the adherence to skin of said composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
- the skin care, cosmetic or pharmaceutical composition is preferably in loose powder form.
- the composition is preferably in loose powder form when it is a cosmetic composition.
- compositions of the present invention comprise one of a number of superabsorbent polymers combined with several other ingredients well-known in the cosmetic, skin care, or pharmaceutical art. It has surprisingly been found that superabsorbent polymers, when added to the composition of the present invention, surprisingly and unexpectedly provide and promote resistance to wash-off of the compositions from the skin.
- Binder superabsorbent polymers are water-insoluble, crosslinked, high molecular weight polymers capable of absorbing and retaining large quantities of aqueous fluids. These polymers are well known in the art by a few other names such as hydrogels, hydrocoUoids, water absorbent hydrophilic polymers, etc.
- these super-absorbent polymers are wide-mesh crosslinked, water-insoluble polymers based on alkali metal salts of polyacrylic acid or copolymers of alkali metal salts of acrylic acid and acrylamide which are obtained by radical-initiated copolymerization of acrylic acid and polyfunctional monomers, such as divinylbenzene, ethylene glycol dimethacrylate, ethylene glycol diallylether, butanediol acrylate, hexanediol methacrylate, polyglycol diacrylate, trimethylol propane diacrylate, allyl acrylate, diallyl acrylamide, triallylamine, diallylether, methylene bis-acrylamide and N- methylol acrylamide.
- polymers of this type are capable of absorbing large quantities of liquids, swelling and forming hydrogels in the process, and of retaining them, even under pressure.
- Superabsorbent polymers include but are not limited to cross-linked polyacrylamide copolymer, sodium polyacrylate, starch-graft copolymer, hydrolyzed starch acrylonitrile copolymer, cross-linked polyacrylamide copolymer, and other water- swellable, and/or water absorbing agents.
- Superabsorbent polymers which have been found suitable for the compositions of the present invention are hydrolyzed starch-polyacrylonitrile graft copolymer salts, commercially available from Grain Processing Corporation under the trademark Waterlock®. These polymers have the chemical name starch-g-poly(2-propenamide-co- 2-propenoic acid, mixed sodium and aluminum salt). Another suitable example is poly- 2-propenoic acid, sodium salt, commercially available from Sanyo Corp.
- the superabsorbent polymer may be used alone, or in combination to achieve the desired absorptivity and adhesion characteristics in the compositions.
- the superabsorbent polymers are added to the composition in an amount of from 0.01 wt. % to 20 wt. %. Preferably these are added in an amount of from 5 to 15 wt. % and more preferably in an amount of about 10 wt. %.
- Powder Carrier The superabsorbent polymer is then combined with a typical powder carrier which comprises the bulk of the composition of the present invention.
- Powder carriers include but are not limited to starch or flour.
- Typical sources for the starches and flours are cereals, tubers, roots, legumes and fruits.
- the native source can be corn, white corn, pea, potato, sweet potato, banana, barley, wheat, rice, sago, amaranth, tapioca, sorghum, waxy maize, waxy tapioca, waxy pea, waxy wheat, waxy rice, waxy barley, waxy potato, waxy sorghum, high amylose starches containing greater than 40% amylose, and the like.
- Preferred starches are potato, corn, rice, oat, and waxy starches such as waxy maize, waxy tapioca, waxy rice, and waxy barley, legume starches, soy starch, turnip starch, microcrystalline cellulose, aluminum starch octenyl succinate, kaolin, and mixtures thereof.
- a most preferred powder carrier is cornstarch, which consists of a carbohydrate polymer derived chiefly from waxy maize corn. In a form of a white powder, it rapidly swells in water.
- Powder carriers typically comprise from 25%o to 99%o, preferably from 30% to 80%, more preferably from 35% to 75%, and most preferably from 40% to 70%, by weight of the composition.
- Optional Anti-caking and Anti-clustering agents are selected from the group comprising calcium phosphates (e.g., calcium triphosphate), aluminum calcium sulfate, silicas (or silicon dioxide), silicates, carbonates and mixtures thereof.
- the anti-clustering / anti-caking agent also acts as a buffer and is incorporated in the body powder in amounts of from about 0.1 to 2 wt. % and preferably in an amount of from about 1 to 1.5 wt. %.
- hydrophobic silicon dioxide can be also added in an amount of preferably 0.1 to 2% by weight to further impart free-flowing properties to the composition.
- compositions of the present invention may also contain compounds which function as anti -bacterial agents to combat bacteria which cause diaper rash, perspiration odor and/or athlete's foot in personal care applications.
- the anti-bacterial compounds may be present in an anti-bacterially effective amount of from 0.025 to 2%o by weight, preferably 0.05 to 1% by weight. Examples of suitable anti-bacterial compounds are 8-hydroxyquinoline, methylbenzethonium chloride, benzethonium chloride, benzalkonium chloride, and the like.
- the compositions of the present invention also optionally include skin aids.
- skin aids refers to skin protectants, emollients, moisturizers, astringents, and antioxidants.
- Preferred skin protectants are corn starch, kaolin, mineral oil, sodium bicarbonate, dimethicone, zinc oxide, colloidal oatmeal, and mixtures thereof.
- the skin protectants comprise from about 0.1% to about 80%), preferably from about 0.1%o to about 30%, most preferably from about 0.1% to about 10%), by weight of the composition.
- Typical emollients and moisturizers useful in the present invention are tocopherol, tocopheryl acetate, aloe, mineral oil, jojoba oil, and mixtures thereof.
- the emollients/moisturizers comprise from about 0.1% to about 50%, preferably from about 0.1% to about 25%o, most preferably from about 0.1%) to about 10%), by weight of the composition.
- Antioxidants useful in the present invention include retinol, retinyl acetate, and retinyl palmitate, and more preferred, encapsulated antioxidants.
- Optional antioxidants comprise up to about 25%, preferably from about 0.1%o to about 10%, by weight of the composition.
- compositions may further contain zinc oxide in baby powder applications for the relief of skin rash and chafing in addition to acting as a whitening agent and also imparting astringent properties.
- the zinc oxide may be present in an amount of 1 to 30%) by weight, preferably 5 to 20% by weight of the composition.
- the perfumes which are useful in the present invention are any commercial perfumes which result in the fragrance desired by the formula.
- Commercial perfumes are mixtures of many components and these components all contribute to the particular fragrance which is characteristic of the mixture.
- Examples of typical perfume which can be formulated to make up a particular pleasant aroma include lemon oil, musk ketone, ionone, diphenyl oxide, cedarwood-terpeneless; geranyl acetate; ylang ylang oil; cedryl acetate, isoeugenol; cinnamic alcohol, aurantheol, methyl anthranilate; vanillin, oil bergamot, eugenol; oil of cananga; citral; tetrahydro linalool; oil patchouly, methyl isoeugenol; hexylcinnamic aldehyde; resil oilbanum, resin balsam fir; musk aurbrette, resin balsam Peru;
- the perfume is typically utilized in an amount of from 0.005 to 1 wt. % of the total composition, preferably from about 0.1 to 0.3 wt. %.
- composition of the present invention may optionally include odor control agents such as uncomplexed cyclodextrin, zeolites, carbon odor-controlling agents, sodium bicarbonates, and/or antimicrobial agents for added body odor control.
- odor control agents such as uncomplexed cyclodextrin, zeolites, carbon odor-controlling agents, sodium bicarbonates, and/or antimicrobial agents for added body odor control.
- the composition of the present invention may be specially formulated to provide good skin feel characteristics, by further comprising skin feel components in an amount of up to 50%) by weight of the composition.
- skin feel components commonly refer to three groups of ingredients which are 1) stearates and/or fatty acid derivatives, 2) spherical particles, and 3) platelet-shaped particles.
- Stearates and/or fatty acid derivatives useful herein include metallic stearates such as magnesium stearate or zinc stearate, and similar fatty acid derivatives such as fatty acid esters which possess unctuous, oily characteristics.
- Spherical particles useful herein include nylon, polyethylene, and polytetrafluoroethylene. Platelet-shaped particles which are useful herein include mica, talc, lauroyl lysine, boron nitride, and barium sulfate.
- compositions comprising superabsorbent polymers of the present invention may also, optionally, be utilized as topical active carrier systems.
- suitable topical active ingredients include anti-microbials; anti-fungals; topical analgesics and anesthetics; anti-inflammatories; protectants; astringents; anti-septics such as benzenthonium chloride; skin moisturizer compounds such as lauryl lysine, waxes, petroleum jelly and oils; skin treatment compounds such as alpha hydroxy acids; antioxidants; medicinals such as camphor, zinc oxide and sulfur; among others, including vitamins such as vitamin A, D and E, in amounts ranging from about 0.1% to about 50%) by weight of the final compositions.
- inert and active agents can be added if desired, such as dyes, colorants, and/or preservatives, for visual appeal and performance impression.
- compositions of the present invention can be conveniently prepared in the form of dusting powders, liquid body powders, stick forms or cream forms.
- a most preferred application form is a dusting powder for the application to the skin.
- compositions of the present invention can be prepared by well-known mixing or blending procedures using a commercial blender.
- the superabsorbent polymer and optional additives can be pre-mixed and then blended directly into the powder carriers and other ingredients or through a liquid addition bar.
- Example 1 Powder compositions were prepared as follows:
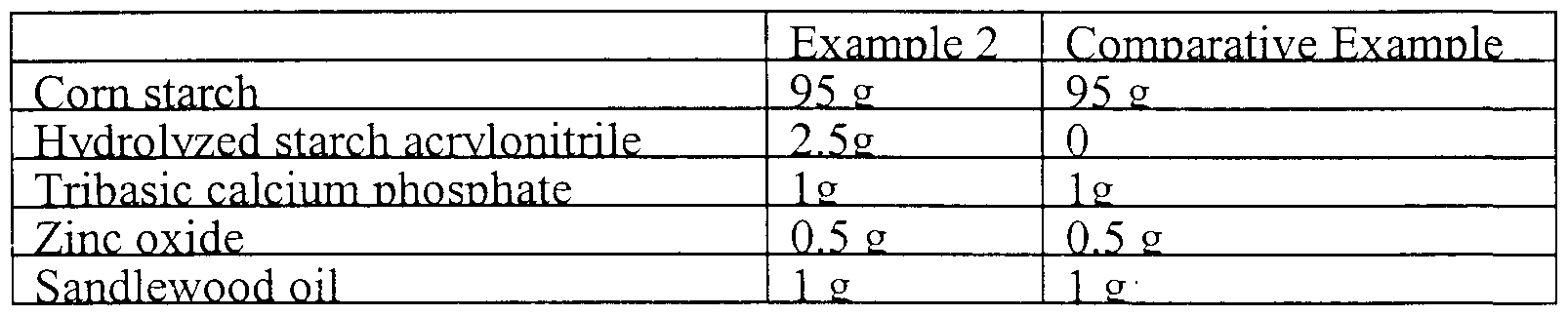
- Example 2 Compositions were prepared as follows:
- the formulated powder was liberally applied to buttocks and thighs of an infant suffering from diaper rash.
- the composition of Example 2 visibly adhered to the skin better and for a much longer period of time as compared to a comparative example without any superabsorbent polymers added.
- the rash completely cleared up. There was not evidence in any instance of the caking of the powder on the skin, nor there was any sign of irritation of the portions of the infant's skin treated with the composition of the present invention.
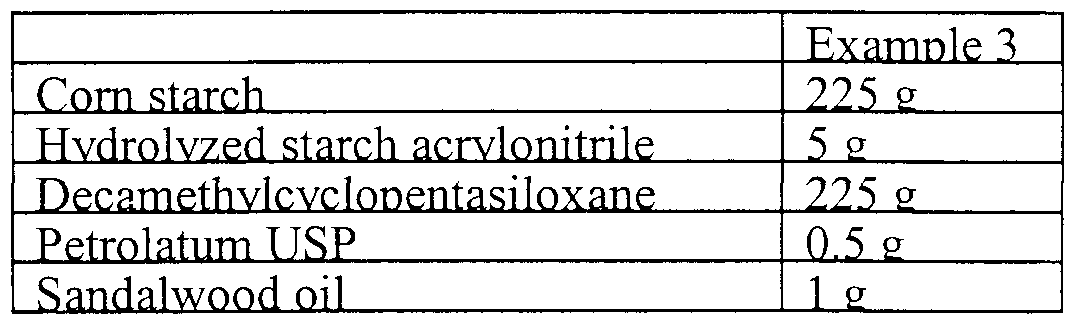
- Example 3 A formulation for a liquid powder of the present invention with excellent adhesion properties can be prepared by mixing melted petroleum jelly with decamethylcyclopentasiloxane, and then adding the resulting colloidal mixture to corn starch to form a liquid body powder mixture according to the formula:
- Example 4 A face powder with superior adhesion properties compared to a comparative product which does not contain any superabsorbent polymers can be prepared using the following formulation:
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Cosmetics (AREA)
- Medicinal Preparation (AREA)
Abstract
A skin care, cosmetic or pharmaceutical composition having superior resistance to wash-off, comprises from 25 wt.% to 99 wt.% a powder carrier and 0.01 wt.% to 20 wt.% of a superabsorbent polymer, wherein the adherence to skin of said composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
Description
COSMETIC COMPOSITION HAVING IMPROVED ABSORPTION AND ADHESION CHARACTERISTICS
Field of the Invention The present invention relates generally to personal care and cosmetic compositions in powder form providing excellent moisture absorbency and adhesive properties. More particularly, the present invention relates to personal care and cosmetic compositions in powder form comprising superabsorbent polymers.
Background of the invention
Human skin secretes substances such as eccrine and apocrine sweat, and lipid- soluble sebum. Skin secretions provide food and a moist environment for microbes to proliferate, which may result in embarrassing body odor and even fungal or bacterial skin infections. Additionally, moisture from skin secretions can result in skin rashes and other uncomfortable skin related disorders. Cosmetic skin and personal care powders have long been available to absorb moisture. Body powders have also been used extensively on babies to prevent diaper rash and to otherwise help promote dryness.
High-grade talc, which is a natural hydrous magnesium silicate, had for many years been used in the form of powder for application to the skin. Aside from talc, various other ingredients have also been proposed and utilized for body powders including starches, cellulose derivatives, polymeric substances and the like. Although many satisfactory talc and non-talc compositions are available through commercial channels, numerous attempts to develop improved body powder compositions have been on-going. See for example, U.S. Patent No. 4,185,086 to Zeitx which teaches and claims a body powder composition comprising talc, fragrance and from about 0.1 to 1 wt. % polyethylene glycol. Since one of the primary purposes of a body powder is to absorb moisture, the effectiveness of the body powder is diminished when the powder has reached its capacity for absorbency.
U.S. Patent No. 5,417,963 to Murphy et al. discloses body and body powder compositions with enhanced deodorant and moist absorbency characteristics consisting
of from about 70 wt. % talc and the remainder comprising micro-crystallite particles of a bicarbonate salt coated with a hydrophobic polymer such as xanthan gum, carrageenan, alginate salt, guar gum, gum arabic, casein, dextran, agar, methyl cellulose, carboxymethyl cellulose, and the like.
Superabsorbent polymers are known and are mainly used in the production of diapers and incontinence articles as well as wound dressings. U.S. Patent No. 5,064,653 to Sessions et al. discloses the use of superabsorbent polymers in a foam composition for applications in sanitary napkins, diapers, and the like.
Superabsorbent polymers are also added in some cosmetic compacted powders to add in the absorbency. U.S. Patent No. 5,211 ,892 discloses the addition of superabsorbent polymers to pharmaceutical or cosmetic compacted powder comprising
5 to 80% of a thermoplastic product such as polyethylene wax to provide an absorbent or partially friable compacted product.
U.S. Patent No. 4,661,476 discloses the addition of superabsorbent polymers to skin lotion comprising about 75 to 99 wt. % water, to cool the surface of skin that has been overheated by excessive exposure to sun and/or wind. U.S. Patent No. 6,013,271 discloses the addition of superabsorbent materials to an oil-in-water dispersion which comprises from about 30 to 98.89 wt. % of water.
In finding a pharmaceutical, cosmetic skin, or personal care composition with greater absorbent properties, there has, according to the present invention, been discovered an unexpected and surprising benefit to superabsorbent polymers besides its inherent absorbent characteristics. It has been found that the addition of superabsorbent polymer compounds to pharmaceutical, cosmetic skin, or personal care compositions in loose powder form, besides providing the expected greater absorbent properties, surprisingly and unexpectedly promotes superior resistance to wash-off for better topical agent delivery.
SUMMARY OF THE INVENTION
According to the invention, there is provided a method to improve the resistance to wash-off characteristic of a skin care, cosmetic or pharmaceutical composition comprising from 25 wt. % to 99 wt. % (preferably from 25 wt. % to 95 wt. %) a powder carrier, by including in said composition from 0.01 wt. % to 20 wt. % of a superabsorbent polymer.
According to the invention there is further provided a skin care, cosmetic or pharmaceutical composition having superior resistance to wash-off characteristics, the composition comprising from 25 wt. % to 99 wt. % (preferably from 25 wt.% to 95 wt. % ) a powder carrier and from 0.01 wt. % to 20 wt. % of a superabsorbent polymer, wherein the adherence to skin of said composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
The skin care, cosmetic or pharmaceutical composition is preferably in loose powder form. In particular, the composition is preferably in loose powder form when it is a cosmetic composition.
DETAILED DESCRIPTION OF THE INVENTION The compositions of the present invention comprise one of a number of superabsorbent polymers combined with several other ingredients well-known in the cosmetic, skin care, or pharmaceutical art. It has surprisingly been found that superabsorbent polymers, when added to the composition of the present invention, surprisingly and unexpectedly provide and promote resistance to wash-off of the compositions from the skin.
Binder superabsorbent polymers. Superabsorbent polymers are water-insoluble, crosslinked, high molecular weight polymers capable of absorbing and retaining large quantities of aqueous fluids. These polymers are well known in the art by a few other names such as hydrogels, hydrocoUoids, water absorbent hydrophilic polymers, etc. In general, these super-absorbent polymers are wide-mesh crosslinked, water-insoluble polymers based on alkali metal salts of polyacrylic acid or copolymers of alkali metal
salts of acrylic acid and acrylamide which are obtained by radical-initiated copolymerization of acrylic acid and polyfunctional monomers, such as divinylbenzene, ethylene glycol dimethacrylate, ethylene glycol diallylether, butanediol acrylate, hexanediol methacrylate, polyglycol diacrylate, trimethylol propane diacrylate, allyl acrylate, diallyl acrylamide, triallylamine, diallylether, methylene bis-acrylamide and N- methylol acrylamide. By virtue of their structure, polymers of this type are capable of absorbing large quantities of liquids, swelling and forming hydrogels in the process, and of retaining them, even under pressure.
Superabsorbent polymers include but are not limited to cross-linked polyacrylamide copolymer, sodium polyacrylate, starch-graft copolymer, hydrolyzed starch acrylonitrile copolymer, cross-linked polyacrylamide copolymer, and other water- swellable, and/or water absorbing agents.
Superabsorbent polymers which have been found suitable for the compositions of the present invention are hydrolyzed starch-polyacrylonitrile graft copolymer salts, commercially available from Grain Processing Corporation under the trademark Waterlock®. These polymers have the chemical name starch-g-poly(2-propenamide-co- 2-propenoic acid, mixed sodium and aluminum salt). Another suitable example is poly- 2-propenoic acid, sodium salt, commercially available from Sanyo Corp. The superabsorbent polymer may be used alone, or in combination to achieve the desired absorptivity and adhesion characteristics in the compositions.
The superabsorbent polymers are added to the composition in an amount of from 0.01 wt. % to 20 wt. %. Preferably these are added in an amount of from 5 to 15 wt. % and more preferably in an amount of about 10 wt. %.
Powder Carrier. The superabsorbent polymer is then combined with a typical powder carrier which comprises the bulk of the composition of the present invention. Powder carriers include but are not limited to starch or flour. Typical sources for the starches and flours are cereals, tubers, roots, legumes and fruits. The native source can be corn, white corn, pea, potato, sweet potato, banana, barley, wheat, rice, sago,
amaranth, tapioca, sorghum, waxy maize, waxy tapioca, waxy pea, waxy wheat, waxy rice, waxy barley, waxy potato, waxy sorghum, high amylose starches containing greater than 40% amylose, and the like. Preferred starches are potato, corn, rice, oat, and waxy starches such as waxy maize, waxy tapioca, waxy rice, and waxy barley, legume starches, soy starch, turnip starch, microcrystalline cellulose, aluminum starch octenyl succinate, kaolin, and mixtures thereof.
A most preferred powder carrier is cornstarch, which consists of a carbohydrate polymer derived chiefly from waxy maize corn. In a form of a white powder, it rapidly swells in water.
Powder carriers typically comprise from 25%o to 99%o, preferably from 30% to 80%, more preferably from 35% to 75%, and most preferably from 40% to 70%, by weight of the composition.
Optional Anti-caking and Anti-clustering agents. Optional anti-clustering / anti-caking agents are selected from the group comprising calcium phosphates (e.g., calcium triphosphate), aluminum calcium sulfate, silicas (or silicon dioxide), silicates, carbonates and mixtures thereof. The anti-clustering / anti-caking agent also acts as a buffer and is incorporated in the body powder in amounts of from about 0.1 to 2 wt. % and preferably in an amount of from about 1 to 1.5 wt. %. In addition to the typical anti- caking and anti-clustering agents, hydrophobic silicon dioxide can be also added in an amount of preferably 0.1 to 2% by weight to further impart free-flowing properties to the composition.
Other Optional Ingredients. The compositions of the present invention may also contain compounds which function as anti -bacterial agents to combat bacteria which cause diaper rash, perspiration odor and/or athlete's foot in personal care applications. The anti-bacterial compounds may be present in an anti-bacterially effective amount of from 0.025 to 2%o by weight, preferably 0.05 to 1% by weight. Examples of suitable anti-bacterial compounds are 8-hydroxyquinoline, methylbenzethonium chloride, benzethonium chloride, benzalkonium chloride, and the like.
The compositions of the present invention also optionally include skin aids. The term "skin aids," as used herein, refers to skin protectants, emollients, moisturizers, astringents, and antioxidants. Preferred skin protectants are corn starch, kaolin, mineral oil, sodium bicarbonate, dimethicone, zinc oxide, colloidal oatmeal, and mixtures thereof. When present, the skin protectants comprise from about 0.1% to about 80%), preferably from about 0.1%o to about 30%, most preferably from about 0.1% to about 10%), by weight of the composition.
Typical emollients and moisturizers useful in the present invention are tocopherol, tocopheryl acetate, aloe, mineral oil, jojoba oil, and mixtures thereof. When present, the emollients/moisturizers comprise from about 0.1% to about 50%, preferably from about 0.1% to about 25%o, most preferably from about 0.1%) to about 10%), by weight of the composition.
Antioxidants useful in the present invention include retinol, retinyl acetate, and retinyl palmitate, and more preferred, encapsulated antioxidants. Optional antioxidants comprise up to about 25%, preferably from about 0.1%o to about 10%, by weight of the composition.
The compositions may further contain zinc oxide in baby powder applications for the relief of skin rash and chafing in addition to acting as a whitening agent and also imparting astringent properties. The zinc oxide may be present in an amount of 1 to 30%) by weight, preferably 5 to 20% by weight of the composition.
The perfumes which are useful in the present invention are any commercial perfumes which result in the fragrance desired by the formula. Commercial perfumes are mixtures of many components and these components all contribute to the particular fragrance which is characteristic of the mixture. Examples of typical perfume which can be formulated to make up a particular pleasant aroma include lemon oil, musk ketone, ionone, diphenyl oxide, cedarwood-terpeneless; geranyl acetate; ylang ylang oil; cedryl acetate, isoeugenol; cinnamic alcohol, aurantheol, methyl anthranilate; vanillin,
oil bergamot, eugenol; oil of cananga; citral; tetrahydro linalool; oil patchouly, methyl isoeugenol; hexylcinnamic aldehyde; resil oilbanum, resin balsam fir; musk aurbrette, resin balsam Peru; oil sandalwood, geraniol; terpenyl acetate, benzyl isoeugenol; oil copaiba; oil nutmeg, rhodinol; diphenyl methane; hydroxycitronellal; methyl benzoate; benzyl propionate; oil palmarose; oil orange, oil geranium; methyl gamma ionone; oil of lavender; and mixtures thereof.
The perfume is typically utilized in an amount of from 0.005 to 1 wt. % of the total composition, preferably from about 0.1 to 0.3 wt. %.
In addition to perfumes and when used as a body / baby powder, the composition of the present invention may optionally include odor control agents such as uncomplexed cyclodextrin, zeolites, carbon odor-controlling agents, sodium bicarbonates, and/or antimicrobial agents for added body odor control.
When used as in body / baby powder applications, the composition of the present invention may be specially formulated to provide good skin feel characteristics, by further comprising skin feel components in an amount of up to 50%) by weight of the composition. "Skin feel components," commonly refer to three groups of ingredients which are 1) stearates and/or fatty acid derivatives, 2) spherical particles, and 3) platelet-shaped particles. Stearates and/or fatty acid derivatives useful herein include metallic stearates such as magnesium stearate or zinc stearate, and similar fatty acid derivatives such as fatty acid esters which possess unctuous, oily characteristics. Spherical particles useful herein include nylon, polyethylene, and polytetrafluoroethylene. Platelet-shaped particles which are useful herein include mica, talc, lauroyl lysine, boron nitride, and barium sulfate.
The compositions comprising superabsorbent polymers of the present invention may also, optionally, be utilized as topical active carrier systems. Suitable topical active ingredients include anti-microbials; anti-fungals; topical analgesics and anesthetics; anti-inflammatories; protectants; astringents; anti-septics such as benzenthonium chloride; skin moisturizer compounds such as lauryl lysine, waxes,
petroleum jelly and oils; skin treatment compounds such as alpha hydroxy acids; antioxidants; medicinals such as camphor, zinc oxide and sulfur; among others, including vitamins such as vitamin A, D and E, in amounts ranging from about 0.1% to about 50%) by weight of the final compositions.
Other components typically found in pharmaceutical, cosmetic and personal care applications including both inert and active agents can be added if desired, such as dyes, colorants, and/or preservatives, for visual appeal and performance impression.
Preparation. The compositions of the present invention can be conveniently prepared in the form of dusting powders, liquid body powders, stick forms or cream forms. A most preferred application form is a dusting powder for the application to the skin.
The compositions of the present invention can be prepared by well-known mixing or blending procedures using a commercial blender. The superabsorbent polymer and optional additives can be pre-mixed and then blended directly into the powder carriers and other ingredients or through a liquid addition bar.
EXAMPLES The following examples are provided to more fully set forth some preferred formulations of the compositions of the present invention. They are provided in order to further illustrate, and in no way to limit the scope of, the invention.
Example 1. Powder compositions were prepared as follows:
About 0.3 g of the formulated powder with sodium polyacrylate was placed on a human forearm and spread by hand in about one inch by six-inch strip. Another 0.3 g sample of powder without the super absorbent polymer was applied to the other forearm in the same manner. Water was added to powder on the forearm by means of a spray
water bottle. Most of the powder without the superabsorbent polymer was washed off the skin by the fine spray of water. This experiment was repeated for the formula with superabsorbent polymer added with excellent adhesion of the powder. The addition of the superabsorbent polymer resulted in gel-like matrix that could not be removed except by mechanical means.
Example 2. Compositions were prepared as follows:
The formulated powder was liberally applied to buttocks and thighs of an infant suffering from diaper rash. The composition of Example 2 visibly adhered to the skin better and for a much longer period of time as compared to a comparative example without any superabsorbent polymers added. Within a period of three days, the rash completely cleared up. There was not evidence in any instance of the caking of the powder on the skin, nor there was any sign of irritation of the portions of the infant's skin treated with the composition of the present invention.
Example 3. A formulation for a liquid powder of the present invention with excellent adhesion properties can be prepared by mixing melted petroleum jelly with decamethylcyclopentasiloxane, and then adding the resulting colloidal mixture to corn starch to form a liquid body powder mixture according to the formula:
Claims
1. A method of improving resistance to wash-off of a composition selected from the group consisting of a skin care composition, a cosmetic composition, and a pharmaceutical composition, said composition comprising from 25 wt. % to 99 wt. % of a powder carrier, said method comprising including in said composition from 0.01 wt. % to 20 wt. % of a superabsorbent polymer, wherein the resistance to wash-off from skin of said composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
2. A method according to claim 1, wherein said superabsorbent polymer is included in an amount of from 5 wt. % to 15 wt. %.
3. A method according to claim 1 or claim 2, wherein said superabsorbent polymer is selected from the group consisting of: cross-linked polyacrylamide copolymer, sodium polyacrylate, starch graft co-polymer, hydrolyzed starch acrylonitrile copolymer, cross-linked polyacrylamide copolymer, natural gums, synthetic gums, hydrocoUoids, cellulose and its derivatives and mixtures thereof.
4. A method according to claim 3, wherein said superabsorbent polymer is a hydrolyzed starch-polyacrylonitrile graft copolymer salt.
5. A method according to any of claims 1 to 4, wherein said composition is a skin care composition comprising from 25 wt. % to 95 wt. % of a starch powder carrier.
6. A method according to any of claims 1 to 4, wherein said composition is a cosmetic face powder composition comprising from 25 wt. % to 95 wt. % of a starch powder carrier.
7. A method according to claim 5 or claim 6, further comprising up to 50 wt. % of talc.
8. A method according to any of claims 1 to 4, wherein said composition is a pharmaceutical powder composition further comprising at least a topically active ingredient selected from the group consisting of: anti-microbials; anti-fungals; topical analgesics and anaesthetics; anti-inflammatories; and mixtures thereof.
9. A method according to any of claims 5 to 7, wherein said starch is corn, white corn, pea, potato, sweet potato, banana, barley, wheat, rice, sago, amaranth, tapioca, sorghum, waxy maize, waxy tapioca, waxy pea, waxy wheat, waxy rice, waxy barley, waxy potato, waxy sorghum, or a starch having an amylose content of 40%) or greater.
10. A composition selected from the group consisting of a skin care composition and a pharmaceutical composition comprising:
(a) from 25 wt. % to 95 wt. % of a starch powder carrier; and (b) from 0.01 wt. % to 20 wt. % of a superabsorbent polymer; wherein the resistance to wash-off from skin of said personal care composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
11. A composition according to claim 10. further comprising up to 50 wt. % of talc.
12. A composition according to claim 10 or claim 11, wherein said superabsorbent polymer is selected from the group consisting of: cross-linked polyacrylamide copolymer, sodium polyacrylate, starch graft co-polymer, hydrolyzed starch acrylonitrile copolymer, cross-linked polyacrylamide copolymer, natural gums, synthetic gums, hydrocoUoids, cellulose and its derivatives, and mixtures thereof.
13. A composition according to any of claims 10 to 12 in the form of a body powder.
14. A composition according to any of claims 10 to 12 in the form of a body powder.
15. A composition according to any of claims 10 to 14, wherein said starch is corn, white corn, pea, potato, sweet potato, banana, barley, wheat, rice, sago, amaranth, tapioca, sorghum, waxy maize, waxy tapioca, waxy pea, waxy wheat, waxy rice, waxy barley, waxy potato, waxy sorghum, or a starch having an amylose content of 40% or greater.
16. A composition according to any of claims 10 to 15 in the form of a semi-solid.
17. A composition according to any of claims 10 to 16, further comprising at least an additive selected from the group consisting of preservatives, stabilizers, anti-caking agents, topically active ingredient, dyes, colorants, and mixtures thereof.
18. A composition according to claim 17, wherein said topically active ingredient is selected from the group consisting of anti-microbials, anti-fungals, anti-inflammatories, topical analgesic, topical anesthetics, skin moisturizer compounds, protectants, astringents and mixtures thereof.
19. Use of a superabsorbent polymer in a method of improving the resistance to wash-off of a composition selected from the group consisting of a skin care composition, a cosmetic composition, and a pharmaceutical composition, said composition comprising from 25 wt. % to 99 wt. % of a powder carrier, said method comprising including in said composition from 0.01 wt.%o to 20 wt.%o of said superabsorbent polymer, whereby the resistance to wash-off from skin of said composition is improved as compared to an analogous composition not containing said superabsorbent polymer.
20. Use according to claim 19, wherein said superabsorbent polymer is included in an amount of from 5 wt % to 15 wt. %.
21. Use according to claim 19 or claim 20, wherein said superabsorbent polymer is selected from the group consisting of: cross-linked polyacrylamide copolymer, sodium polyacrylate, starch graft co-polymer, hydrolyzed starch acrylonitrile copolymer, cross- linked polyacrylamide copolymer, natural gums, synthetic gums, hydrocoUoids, cellulose and its derivatives and mixtures thereof.
22. Use according to claim 21, wherein said superabsorbent polymer is a hydrolyzed starch-polyacrylonitrile graft copolymer salt.
23. Use according to any of claims 19 to 22, wherein said composition is a skin care composition comprising from 25 wt. % to 95 wt. % of a starch powder carrier.
24. Use according to any of claims 19 to 22, wherein said composition is a cosmetic face powder composition comprising from 25 wt. % to 95 wt. % of a starch powder carrier.
25. Use according to claim 23 or claim 24, further comprising up to 50 wt. % of talc.
26. Use according to any of claims 19 to 22, wherein said composition is a pharmaceutical powder composition further comprising at least a topically active ingredient selected from the group consisting of: anti-microbials; anti-fungals; topical analgesics and anaesthetics; anti-inflammatories; and mixtures thereof.
27. Use according to any of claims 23 to 25, wherein said starch is corn, white corn, pea, potato, sweet potato, banana, barley, wheat, rice, sago, amaranth, tapioca, sorghum, waxy maize, waxy tapioca, waxy pea, waxy wheat, waxy rice, waxy barley, waxy potato, waxy sorghum, or a starch having an amylose content of 40% or greater.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2000278058A AU2000278058A1 (en) | 2000-03-08 | 2000-10-13 | Cosmetic composition having improved absorption and adhesion characteristics |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US52116500A | 2000-03-08 | 2000-03-08 | |
| US09/521,165 | 2000-03-08 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2001066078A1 true WO2001066078A1 (en) | 2001-09-13 |
Family
ID=24075633
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2000/003948 WO2001066078A1 (en) | 2000-03-08 | 2000-10-13 | Cosmetic composition having improved absorption and adhesion characteristics |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU2000278058A1 (en) |
| WO (1) | WO2001066078A1 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003030854A1 (en) * | 2001-10-05 | 2003-04-17 | Colgate-Palmolive Company | Underarm gel products with superabsorbent polymer component |
| WO2003030853A1 (en) * | 2001-10-05 | 2003-04-17 | Colgate-Palmolive Company | Underarm products with water lock component |
| WO2004000254A1 (en) * | 2002-06-24 | 2003-12-31 | Colgate-Palmolive Company | Soft solid antiperspirant with cooling and drying effect |
| WO2004000255A1 (en) * | 2002-06-24 | 2003-12-31 | Colgate-Palmolive Company | Antiperspirant stick with cooling and drying effect |
| WO2005087181A2 (en) * | 2004-02-27 | 2005-09-22 | Colgate-Palmolive Company | Dry deodorant containing a sesquiterpene alcohol and zinc oxide |
| WO2005107695A1 (en) * | 2004-05-10 | 2005-11-17 | Unilever Plc | Cosmetic compositons with tapioca starch |
| FR2874174A1 (en) * | 2004-08-11 | 2006-02-17 | Bourjois Soc Par Actions Simpl | COSMETIC COMPOSITION COMPRISING A SUPERABSORBENT POLYMER |
| FR2902004A1 (en) * | 2006-04-14 | 2007-12-14 | Lvmh Rech | COSMETIC COMPOSITION OF MASK TYPE FOR SKIN CARE |
| WO2012059343A1 (en) * | 2010-11-05 | 2012-05-10 | L'oreal | Composition in the powder form comprising at least one essential oil and one modified tapioca starch |
| US9549891B2 (en) | 2012-03-19 | 2017-01-24 | The Procter & Gamble Company | Superabsorbent polymers and sunscreen actives for use in skin care compositions |
| US10285926B2 (en) | 2015-06-29 | 2019-05-14 | The Procter & Gamble Company | Superabsorbent polymers and starch powders for use in skin care compositions |
| EP3708146A1 (en) * | 2019-03-12 | 2020-09-16 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| EP3708147A1 (en) * | 2019-03-12 | 2020-09-16 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| US11376199B2 (en) | 2019-03-12 | 2022-07-05 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2384493A1 (en) * | 1977-03-23 | 1978-10-20 | Stiefel Laboratories | COMPOSITION OF ABSORBENT POWDER FOR BODY TOILET |
| US4272514A (en) * | 1979-11-06 | 1981-06-09 | Spenco Medical Corporation | High absorption body powder |
| JPS6081120A (en) * | 1983-10-13 | 1985-05-09 | Shiseido Co Ltd | Cosmetic |
| JPH04356415A (en) * | 1990-08-02 | 1992-12-10 | Kao Corp | Cosmetic |
-
2000
- 2000-10-13 WO PCT/GB2000/003948 patent/WO2001066078A1/en active Application Filing
- 2000-10-13 AU AU2000278058A patent/AU2000278058A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2384493A1 (en) * | 1977-03-23 | 1978-10-20 | Stiefel Laboratories | COMPOSITION OF ABSORBENT POWDER FOR BODY TOILET |
| US4272514A (en) * | 1979-11-06 | 1981-06-09 | Spenco Medical Corporation | High absorption body powder |
| JPS6081120A (en) * | 1983-10-13 | 1985-05-09 | Shiseido Co Ltd | Cosmetic |
| JPH04356415A (en) * | 1990-08-02 | 1992-12-10 | Kao Corp | Cosmetic |
Non-Patent Citations (2)
| Title |
|---|
| DATABASE WPI Week 198525, Derwent World Patents Index; AN 1985-149123, XP002157853 * |
| DATABASE WPI Week 199304, Derwent World Patents Index; AN 1993-030479, XP002157854 * |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003030854A1 (en) * | 2001-10-05 | 2003-04-17 | Colgate-Palmolive Company | Underarm gel products with superabsorbent polymer component |
| WO2003030853A1 (en) * | 2001-10-05 | 2003-04-17 | Colgate-Palmolive Company | Underarm products with water lock component |
| WO2004000254A1 (en) * | 2002-06-24 | 2003-12-31 | Colgate-Palmolive Company | Soft solid antiperspirant with cooling and drying effect |
| WO2004000255A1 (en) * | 2002-06-24 | 2003-12-31 | Colgate-Palmolive Company | Antiperspirant stick with cooling and drying effect |
| US6793915B1 (en) | 2002-06-24 | 2004-09-21 | Colgate-Palmolive Company | Cool and dry soft solid antiperspirant |
| US6805855B2 (en) | 2002-06-24 | 2004-10-19 | Colgate-Palmolive Company | Cool and dry antiperspirant stick |
| WO2005087181A2 (en) * | 2004-02-27 | 2005-09-22 | Colgate-Palmolive Company | Dry deodorant containing a sesquiterpene alcohol and zinc oxide |
| WO2005087181A3 (en) * | 2004-02-27 | 2006-01-05 | Colgate Palmolive Co | Dry deodorant containing a sesquiterpene alcohol and zinc oxide |
| WO2005107695A1 (en) * | 2004-05-10 | 2005-11-17 | Unilever Plc | Cosmetic compositons with tapioca starch |
| AU2005239805B2 (en) * | 2004-05-10 | 2008-01-24 | Unilever Plc | Cosmetic compositons with tapioca starch |
| FR2874174A1 (en) * | 2004-08-11 | 2006-02-17 | Bourjois Soc Par Actions Simpl | COSMETIC COMPOSITION COMPRISING A SUPERABSORBENT POLYMER |
| WO2006024768A2 (en) * | 2004-08-11 | 2006-03-09 | Bourjois | Cosmetic composition comprising a superabsorbent polymer |
| WO2006024768A3 (en) * | 2004-08-11 | 2006-12-28 | Bourjois | Cosmetic composition comprising a superabsorbent polymer |
| FR2902004A1 (en) * | 2006-04-14 | 2007-12-14 | Lvmh Rech | COSMETIC COMPOSITION OF MASK TYPE FOR SKIN CARE |
| WO2012059343A1 (en) * | 2010-11-05 | 2012-05-10 | L'oreal | Composition in the powder form comprising at least one essential oil and one modified tapioca starch |
| FR2967060A1 (en) * | 2010-11-05 | 2012-05-11 | Oreal | POWDER COMPOSITION COMPRISING AT LEAST ONE ESSENTIAL OIL, MODIFIED TAPIOCA STARCH |
| US9549891B2 (en) | 2012-03-19 | 2017-01-24 | The Procter & Gamble Company | Superabsorbent polymers and sunscreen actives for use in skin care compositions |
| US9839598B2 (en) | 2012-03-19 | 2017-12-12 | The Procter & Gamble Company | Superabsorbent polymers and sunscreen actives for use in skin care compositions |
| US10285926B2 (en) | 2015-06-29 | 2019-05-14 | The Procter & Gamble Company | Superabsorbent polymers and starch powders for use in skin care compositions |
| EP3708146A1 (en) * | 2019-03-12 | 2020-09-16 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| EP3708147A1 (en) * | 2019-03-12 | 2020-09-16 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| WO2020185457A1 (en) * | 2019-03-12 | 2020-09-17 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| WO2020185458A1 (en) * | 2019-03-12 | 2020-09-17 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
| US11376199B2 (en) | 2019-03-12 | 2022-07-05 | The Procter & Gamble Company | Anhydrous cosmetic compositions and uses |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2000278058A1 (en) | 2001-09-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6004584A (en) | Highly absorbent body powders | |
| US6720006B2 (en) | Anti-microbial body care product | |
| AU735255B2 (en) | Hydroalcoholic compositions thickened using polymers | |
| WO2001066078A1 (en) | Cosmetic composition having improved absorption and adhesion characteristics | |
| US4913896A (en) | Multi-purpose body powder composition | |
| JP5089165B2 (en) | Antibacterial substance | |
| MXPA06002166A (en) | Skin care topical ointment. | |
| CZ147499A3 (en) | Bad smell absorbing preparation bad smell and moisture absorbing preparation and their use | |
| JP2002507559A (en) | Polyalkoxy copolymers as lipase inhibitors and their compositions | |
| EP1322343A1 (en) | Multi component controlled release system for sanitary paper products | |
| JP2001139451A (en) | Gelatinous cosmetic | |
| CA2327729A1 (en) | Cream cosmetic base with powdery feel | |
| US8603054B2 (en) | Delivery product for topical compositions | |
| US20160175214A1 (en) | Anhydrous cosmetic composition for topical application | |
| JPH11511752A (en) | Cosmetic composition | |
| NZ552309A (en) | Antimicrobial compositions comprising acetic acid, vinegar, or citric acid, and EDTA, and methods of use thereof | |
| JP2000327516A (en) | Gel-like composition for external application to skin | |
| EP3233044B1 (en) | Anhydrous cosmetic composition for topical application in intimate areas | |
| WO2002039950A2 (en) | Anhydrous cosmetic composition and gel vehicle | |
| US20170165395A1 (en) | Systems for managing intimate skin | |
| JP2001206816A (en) | Milky lotion having skin-softening property and antimicrobial activity, cosmetic preparation and skin- cleansing article | |
| JP3686756B2 (en) | Antiperspirant | |
| WO2024209264A1 (en) | Antiperspirant and deodorant composition | |
| JP2023153449A (en) | Wrinkle inhibitor and skin cosmetics including wrinkle inhibitor | |
| KR20000052842A (en) | Compositions for reducing body odors and excess moisture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: JP |