CN110903374A - Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold - Google Patents
Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold Download PDFInfo
- Publication number
- CN110903374A CN110903374A CN201911322819.9A CN201911322819A CN110903374A CN 110903374 A CN110903374 A CN 110903374A CN 201911322819 A CN201911322819 A CN 201911322819A CN 110903374 A CN110903374 A CN 110903374A
- Authority
- CN
- China
- Prior art keywords
- silk fibroin
- solution
- usnic acid
- composite skin
- aqueous solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/43504—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates
- C07K14/43563—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates from insects
- C07K14/43586—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from invertebrates from insects from silkworms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/22—Polypeptides or derivatives thereof, e.g. degradation products
- A61L27/227—Other specific proteins or polypeptides not covered by A61L27/222, A61L27/225 or A61L27/24
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/58—Materials at least partially resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/60—Materials for use in artificial skin
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01F—CHEMICAL FEATURES IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS; APPARATUS SPECIALLY ADAPTED FOR THE MANUFACTURE OF CARBON FILAMENTS
- D01F1/00—General methods for the manufacture of artificial filaments or the like
- D01F1/02—Addition of substances to the spinning solution or to the melt
- D01F1/10—Other agents for modifying properties
- D01F1/103—Agents inhibiting growth of microorganisms
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01F—CHEMICAL FEATURES IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS; APPARATUS SPECIALLY ADAPTED FOR THE MANUFACTURE OF CARBON FILAMENTS
- D01F4/00—Monocomponent artificial filaments or the like of proteins; Manufacture thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Dermatology (AREA)
- Transplantation (AREA)
- Epidemiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Textile Engineering (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Tropical Medicine & Parasitology (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Insects & Arthropods (AREA)
- Manufacturing & Machinery (AREA)
- Biomedical Technology (AREA)
- Peptides Or Proteins (AREA)
- Materials For Medical Uses (AREA)
Abstract
The invention discloses an extraction method of a silk fibroin aqueous solution, which comprises the following steps: 1) cutting and cleaning silkworm cocoon, degumming by sodium carbonate, washing and drying to obtain silk fibroin; 2) putting silk fibroin into a lithium bromide aqueous solution to obtain a silk fibroin solution; 3) shearing the dialysis bag, placing the dialysis bag in a mixed solution of sodium bicarbonate and EDTA for boiling treatment, and then placing the dialysis bag in an EDTA solution for boiling treatment; 4) and pouring the dissolved solution into a dialysis bag for dialysis, and freezing and centrifuging to obtain the product. Also discloses a composite skin scaffold based on silk fibroin/usnic acid and a preparation method thereof: 1) extracting silk fibroin by the method; 2) drying silk fibroin aqueous solution to form silk fibroin membrane, grinding, adding formic acid, stirring, freezing and centrifuging to obtain silk fibroin/formic acid completely dissolved solution; 3) mixing usnic acid solid powder with the silk fibroin/formic acid complete solution to obtain silk fibroin/usnic acid mixed solution; 4) and (3) carrying out electrostatic spinning on the silk fibroin/usnic acid mixed solution to obtain the silk fibroin/usnic acid composite material.
Description
Technical Field
The invention belongs to the technical field of biological materials, and particularly relates to a silk fibroin extraction method, a composite skin scaffold based on silk fibroin/usnic acid and a preparation method of the composite skin scaffold.
Background
In recent years, more and more tissue engineering fields have introduced composite wound dressings based on natural flexible polymers, of which silk fibroin is the most studied. Silk fibroin (silk fibroin, abbreviated as SF) is a natural, biocompatible, biodegradable, low-cost polymer fibrin extracted from silk. SF in silkworm cocoon is 5263 amino acid residues consisting of glycine, alanine, serine, tyrosine and valine, and the other 15 amino acids account for only 4.7%. The silk fibroin protein-containing composite material has the advantages of high mechanical strength, good elasticity, excellent biocompatibility, biodegradability, high water and oxygen uptake, low immunogenicity, easy film formation and good form maintenance, and is very suitable for preparing artificial skin, so that the silk fibroin protein is more and more concerned in the field of tissue engineering as a natural biomaterial with excellent performance.
The two main proteins contained in silkworm cocoon are silk fibroin and sericin, which exist in a complex form and are wrapped by the sericin. It has been shown that the protein in this complex form produces an adaptive immune response in vivo, and that the remaining silk fibroin does not produce this immunogenicity when the sericin is removed. The traditional extraction method of silk fibroin comprises using hydrochloric acid/formic acid, formic acid/calcium chloride, formic acid/lithium bromide and HFIP (hexafluoroisopropanol) as solvent to obtain silk fibroin solution. Although only a small amount of hydrogen bonds are destroyed in the method, the fiber structure and the performance of the natural silk fibroin can be greatly protected, but a large number of defects exist in the method, the aqueous solution state cannot be kept in a formic acid/calcium chloride system, and protein can be separated out by adding water; in a formic acid/lithium bromide system, the obtained aqueous solution must be stored in an environment at 4 ℃ to avoid premature gelling; while HFIP is a toxic agent. Another common extraction method is to use inorganic salts (lithium bromide, calcium chloride/ethanol/water), high concentration acid and high ion concentration solution to obtain silk fibroin aqueous solution after dissolution, dialysis and centrifugation. By using the method for dissolving, most inorganic salt ions can enter the interior of the silk fibroin, most original hydrogen bonds are cut off, and the silk fibroin is sheared at the same time, so that the silk fibroin is fully dissolved, and a series of defects of instability of silk fibroin aqueous solution and the like caused by dissolution in a mild environment are avoided. However, the traditional dialysis method is difficult to completely remove inorganic salt ions and other impurity ions dissolved in the solution, which brings great inconvenience to the storage, processing and other aspects of the silk fibroin aqueous solution. In summary, the aqueous fibroin solution extracted by the conventional dialysis method has different limitations in different aspects, and thus a novel extraction method is required.
The skin, as the largest organ in the human body, plays an important role in the processes of sensing external stimuli, regulating body temperature, regulating and controlling water evaporation loss, protecting various tissues and organs in the body from physical or chemical damage, resisting the invasion of pathogenic microorganisms and the like. The skin participates in the metabolism process of the human body, and has important significance for keeping the stability of the internal environment of the human body and maintaining the normal physiological function of the organism. However, skin damage and ulceration caused by skin lesions or external injuries such as burns, scalds, etc. often results in slow skin repair due to lack of implantable autologous skin and severely affects various physiological functions of the skin.
Bacterial infection is one of the main causes affecting the healing of skin wounds, and a large amount of inflammatory factors, proteases and free radicals contained in wound exudate can slow down the healing speed of the wounds. In this case, the novel skin dressing needs excellent antibacterial performance, and can protect the wound surface, prevent the loss of body fluid/protein, and prevent bacteria from invading to cause inflammation, and can also inhibit the bacterial infection of the wound surface by adding bioactive components such as antibiotics or growth factors into the skin dressing to promote the wound healing.
At present, people mostly prevent and treat infection by locally applying broad-spectrum antibiotics to wound surfaces or adding antibiotics into dressings, so that bacterial resistance is generated, even some antibacterial agents have certain biological toxicity, for example, vancomycin can cause anaphylactic reaction, renal toxicity and the like. Clinically, the antibacterial property of the dressing is enhanced by adding functional components such as nano metal, antibiotics and the like, but cytotoxicity and drug resistance are caused, and the safety of organisms is threatened. In addition, the antibacterial material and the cotton fabric are often used in a composite way, the wound dressing has poor biocompatibility, is not suitable for being directly contacted with skin, can possibly cause rejection and inflammatory reaction of a body in a long-term use process, and is not beneficial to healing and repairing of a wound surface. Therefore, the preparation of the skin scaffold which can simultaneously overcome all the defects has important significance for wound healing.
The existing research shows that the silk fibroin can be used as a carrier for wound functional dressing, drug sustained release agent and the like; the silk fibroin functional dressing loaded with specific substances (such as antibiotics and the like) has a certain promotion effect on the antibiosis and repair of wounds. To date, in addition to dressings made of pure silk fibroin, dressings made of many bioactive substances such as natural and synthetic polymers, metal ions, antimicrobial agents, etc. mixed with silk fibroin scaffolds have been developed and widely used for skin tissue engineering, such as chitosan, polyvinyl alcohol, silver ions, etc. However, the existing dressing has more or less problems, so that a novel preparation mode is needed.
The research on the active action mechanism of the usnic acid in the aspects of antibiosis, antivirus, drug-resistant bacteria biofilm, tumor resistance and the like is continuously broken through, and the usnic acid is used for resisting candida albicans and the formed biofilm, so that the usnic acid has ultrahigh antagonism to the candida albicans and the formed biofilm. In addition, the results of in vitro antibacterial studies of the antibacterial material prepared from usnic acid, acrylic resin and polyvinylpyrrolidone show that the fibrous scaffold has high inhibitory effect on staphylococcus aureus. However, since usnic acid has extremely low solubility and is insoluble in most solvents, it has a great limitation in the use of usnic acid. Therefore, it is very important to select a suitable way to mix the silk fibroin and the mixture uniformly.
Disclosure of Invention
The invention aims to solve the problems and provides a silk fibroin extraction method, a silk fibroin/usnic acid composite skin scaffold and a preparation method thereof.
The technical scheme adopted by the invention for realizing the purpose is as follows:
a method for extracting silk fibroin aqueous solution comprises the following steps:
1) shearing silkworm cocoons, ultrasonically cleaning, boiling and degumming in a sodium carbonate solution, washing with deionized water, and drying to obtain degummed silk fibroin;
2) preparing a lithium bromide aqueous solution, and placing the degummed silk fibroin obtained in the step 1) into the lithium bromide aqueous solution to obtain a silk fibroin solution;
3) respectively preparing an EDTA solution and a sodium bicarbonate solution, mixing the sodium bicarbonate solution with part of the EDTA solution to obtain a mixed solution of sodium bicarbonate and EDTA, shearing a dialysis bag, placing the dialysis bag in the mixed solution of sodium bicarbonate and EDTA for boiling treatment for 8-12 min, taking out the dialysis bag, placing the dialysis bag in the EDTA solution for boiling treatment for 6-10 min, and sealing the solution for later use;
4) pouring the dissolved solution obtained in the step 2) into the dialysis bag treated in the step 3), dialyzing by using pure water under the condition of magnetic stirring, taking out the dialyzate after the dialysis is finished, freezing and centrifuging, and taking the supernatant for low-temperature storage to obtain the silk fibroin aqueous solution.
The concentration of the EDTA solution prepared in the step 3) is 1mM, the concentration of the sodium bicarbonate solution is 0.02g/ml, and the EDTA solution and the sodium bicarbonate solution are mixed according to the volume ratio of 1: 0.8-1.2.
The EDTA solution in the step 3) needs to be firstly adjusted to pH 7.8-8.0 by using a sodium hydroxide solution.
In the step 4), the solution obtained in the step 2) is poured into the dialysis bag processed in the step 3) and dialyzed by pure water for 48-60 hours, and the time of freezing and centrifuging is 15-20 min.
A preparation method of a silk fibroin/usnic acid-based composite skin scaffold comprises the following steps:
1) extracting the silk fibroin aqueous solution by adopting the method;
2) drying the silk fibroin aqueous solution obtained in the step 1) into a silk fibroin membrane, grinding, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 18-20%, stirring until the silk fibroin membrane is completely dissolved, and then freezing and centrifuging the dissolved solution for 10-15 min to obtain a silk fibroin/formic acid completely dissolved solution;
3) mixing usnic acid solid powder with the silk fibroin/formic acid complete solution obtained in the step 2), and stirring until usnic acid and the silk fibroin/formic acid complete solution are fully mixed, wherein the final concentration of usnic acid is 0.5-5 mg/ml, so as to obtain a silk fibroin/usnic acid mixed solution;
4) and (3) carrying out electrostatic spinning on the silk fibroin/usnic acid mixed solution to obtain the silk fibroin/usnic acid-based composite skin scaffold.
The condition for dissolving the fibroin film in the step 2) is that the fibroin film is dissolved by magnetic stirring at 180-200 rpm at room temperature, and the stirring time is 48-72 hours.
The stirring condition in the step 3) is that the mixture is stirred and mixed by magnetic force at 140-160 rpm at room temperature, and the stirring time is 24-36 hours.
The step 4) of adjusting electrostatic spinning parameters specifically refers to: the external applied voltage is 18-22 kV, the distance between the needle head and the receiving plate is 11-15 cm, the propelling speed of the injection pump is 0.006-0.01 ml/min, the temperature is 22-24 ℃, the relative humidity is 40-45%, and the duration time of electrostatic spinning is 3-4.5 h.
A composite skin scaffold based on silk fibroin/usnic acid is prepared by the method.
The invention has the beneficial effects that: the extracted silk fibroin aqueous solution has extremely high purity, and the novel dialysis mode avoids the influence of a small amount of residual impurity ions in the solution after the traditional dialysis on the stability of the silk fibroin aqueous solution in the long-term storage process and brings great convenience to operators in the aspects of subsequent processing and the like. The silk fibroin aqueous solution has excellent biocompatibility and biodegradability inherent to silk fibroin, and is extracted by a long-time boiling degumming and novel dialysis mode, so that the silk fibroin aqueous solution is guaranteed to be thoroughly separated from sericin, and the silk fibroin aqueous solution is used as a natural high polymer flexible substrate, so that a series of rejection reactions such as inflammatory reaction, immunoreaction and the like generated by the conventional commercial dressing substrate to wounds are overcome.
The preparation method of the composite skin scaffold firstly solves a series of problems caused by uneven dispersion of usnic acid due to insolubility of usnic acid in most solvents, and the silk fibroin/usnic acid flexible skin scaffold prepared by the method has good mechanical stretching, high specific surface area and porosity, so that the silk fibroin substrate has ultrahigh usnic acid loading capacity and usnic acid is uniformly distributed in the scaffold. The silk fibroin/usnic acid composite skin scaffold has excellent biodegradable performance of silk fibroin, overcomes a series of problems of secondary damage and the like to patients when dressing such as the existing commercial gauze and the like are removed, the usnic acid powder loaded in the dressing has excellent antibacterial performance, is slow and durable in release, overcomes explosive release of medicine in common dressing, has a durable antibacterial effect, and is more beneficial to wound healing; the antibacterial agent has obvious inhibition effect on the growth of gram-positive bacteria and gram-negative bacteria, and overcomes the problem of single antibacterial; and has excellent performances of good permeability and soft texture, so that the stent and the wound have interaction: the wound exudate is absorbed, the wound exudate is seamlessly attached to the wound, and the wound exudate has activity, can promote active substances to play a role, and accelerates wound healing; the composite skin stent has important application prospects in the aspects of biosensors, drug delivery/release, tissue engineering and biomedicine.
Drawings
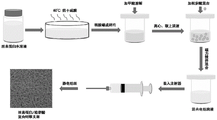
Fig. 1 is a preparation schematic diagram of the silk fibroin/usnic acid-based composite skin scaffold of the present invention.
Fig. 2 is an electron microscope scan of composite skin scaffolds prepared from different concentrations of silk fibroin according to the present invention, wherein a is composite skin scaffold sample 5, and b is composite skin scaffold sample 1.
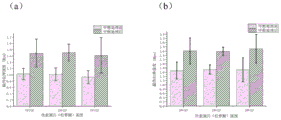
Fig. 3 is a graph showing the secondary structure change of the composite skin scaffold prepared by the method of the present invention before and after methanol treatment, wherein a is the secondary structure of the composite skin scaffold before methanol treatment, and b is the secondary structure change of the composite skin scaffold after methanol treatment.
FIG. 4 is a mechanical property test chart of the composite skin stent prepared by the method of the invention
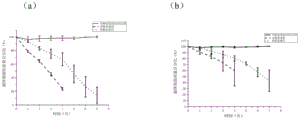
Fig. 5 is a graph of the degradation performance of composite skin scaffolds prepared by the method of the present invention.
Fig. 6 is an antibacterial performance test using the composite skin scaffold of the present invention, wherein a, b, c, d are inhibitory effects of the composite skin scaffold on the growth of staphylococcus aureus, streptococcus pyogenes, escherichia coli, and pseudomonas aeruginosa, respectively.
Fig. 7 is the result of a cell compatibility test using the composite skin scaffold of the present invention.
Detailed Description
The invention is further illustrated by the following examples, which are not intended to be limiting.
The experimental procedures in the following examples are conventional unless otherwise specified.
Example 1 extraction of aqueous Silk fibroin solution
First, prepare silk fibroin aqueous solution sample 1
Extracting a silk fibroin aqueous solution according to the following steps:
1) taking 15 silkworm cocoons with uniform size, shearing each silkworm cocoon into 4 parts, ultrasonically cleaning for 5min, repeating for 2 times, wringing, and drying in an oven at 60 ℃; boiling and degumming the dried silkworm cocoons in 0.02M sodium carbonate solution for 30min, taking out the degumming silk, firstly carrying out ultrasonic cleaning, repeating for 2 times, wherein each time is at least 10min, then thoroughly washing with ultrapure water, and drying to obtain the silk fibroin.
2) Preparing 9.3M lithium bromide solution for later use, taking out 3g of the silk fibroin obtained in the step 1), tightly pressing the silk fibroin at the bottom of a 50ml beaker, adding 12ml of 9.3M lithium bromide solution, sealing the preservative film, and placing the silk fibroin in a water bath kettle at 60 ℃ for continuous dissolution for 4.5 hours.
3) Preparing 200ml of 1mM EDTA solution, adjusting the pH value to 7.8 by using 0.1M sodium hydroxide, mixing 100ml of EDTA solution with 80ml of sodium bicarbonate solution with the concentration of 0.02g/ml, shearing a dialysis bag (the cut-off molecular weight of the dialysis bag is 7000KDA) of 13cm in the mixed solution, boiling for 12min, placing in the remaining 100ml of EDTA solution, boiling for 10min, and sealing for later use.
4) Pouring the solution obtained in the step 2) into the dialysis bag treated in the step 3), fixing the dialysis bag by using a dialysis clamp, placing the dialysis bag into ultrapure water, magnetically stirring and dialyzing for 60 hours, taking out the dialysate after the completion, and carrying out refrigerated centrifugation at 4 ℃ and 10000rpm for 20 minutes to obtain the silk fibroin aqueous solution with the concentration of about 0.065 g/ml.
Secondly, preparing a silk fibroin aqueous solution sample 2
Extracting a silk fibroin aqueous solution according to the following steps:
1) taking 15 silkworm cocoons with uniform size, shearing each silkworm cocoon into 4 parts, ultrasonically cleaning for 5min, repeating for 2 times, wringing, and drying in an oven at 60 ℃; boiling and degumming the dried silkworm cocoons in 0.02M sodium carbonate solution for 45min, taking out the degumming silk, firstly carrying out ultrasonic cleaning, repeating for 2 times, wherein each time is at least 10min, then thoroughly washing with ultrapure water, and drying to obtain the silk fibroin.
2) Preparing 9.3M lithium bromide solution for later use, taking out 3g of the silk fibroin obtained in the step 1), tightly pressing the silk fibroin at the bottom of a 50ml beaker, adding 11ml of 9.3M lithium bromide solution, sealing the preservative film, and placing the silk fibroin in a water bath kettle at 60 ℃ for continuous dissolution for 4 hours.
3) Preparing 200ml of 1mM EDTA solution, adjusting the pH value to 7.9 by using 0.1M sodium hydroxide, mixing 100ml of EDTA solution with 100ml of sodium bicarbonate solution with the concentration of 0.02g/ml, shearing a dialysis bag (the cut-off molecular weight of the dialysis bag is 7000KDA) of 13cm into the mixed solution, boiling for 10min, placing the dialysis bag in the remaining 100ml of EDTA solution, boiling for 8min, and sealing for later use.
4) Pouring the solution obtained in the step 2) into the dialysis bag treated in the step 3), fixing the dialysis bag by using a dialysis clamp, placing the dialysis bag into ultrapure water, magnetically stirring and dialyzing for 54 hours, taking out the dialysate after the completion, and carrying out refrigerated centrifugation at 8500rpm and 4 ℃ for 18 minutes to obtain the silk fibroin aqueous solution with the concentration of about 0.063 g/ml.
Thirdly, preparing a silk fibroin aqueous solution sample 3
Extracting a silk fibroin aqueous solution according to the following steps:
1) taking 15 silkworm cocoons with uniform size, shearing each silkworm cocoon into 4 parts, ultrasonically cleaning for 5min, repeating for 2 times, wringing, and drying in an oven at 60 ℃; boiling and degumming the dried silkworm cocoons in 0.02M sodium carbonate solution for 60min, taking out the degumming silk, firstly carrying out ultrasonic cleaning, repeating for 2 times, wherein each time is at least 10min, then thoroughly washing with ultrapure water, and drying to obtain the silk fibroin.
2) Preparing 9.3M lithium bromide solution for later use, taking out 3g of the silk fibroin obtained in the step 1), tightly pressing the silk fibroin at the bottom of a 50ml beaker, adding 10.5ml of 9.3M lithium bromide solution, sealing the opening of a preservative film, and placing the silk fibroin in a 60 ℃ water bath kettle for continuously dissolving for 3.5 hours.
3) Preparing 200ml of 1mM EDTA solution, adjusting the pH value to 8.0 by using 0.1M sodium hydroxide, mixing 100ml of EDTA solution with 120ml of sodium bicarbonate solution with the concentration of 0.02g/ml, shearing a dialysis bag (the cut-off molecular weight of the dialysis bag is 7000KDA) of 13cm in the mixed solution, boiling for 8min, placing in the remaining 100ml of EDTA solution, boiling for 6min, and sealing for later use.
4) Pouring the solution obtained in the step 2) into the dialysis bag treated in the step 3), fixing the dialysis bag by using a dialysis clamp, placing the dialysis bag into ultrapure water, magnetically stirring and dialyzing for 48 hours, taking out the dialysate after the completion, and carrying out refrigerated centrifugation at 7000rpm and 4 ℃ for 15 minutes to obtain the silk fibroin aqueous solution with the concentration of about 0.062 g/ml.
The silk fibroin aqueous solution prepared by the dissolution method and the novel dialysis method in the embodiment 1 has the following two advantages: firstly, the concentration is stable. Because the degummed silk fibroin is dissolved in a high-concentration lithium bromide aqueous solution in a severe environment, most hydrogen bonds in the silk fibroin can be broken, so that the silk fibroin almost reaches the degree of complete dissolution, the problem of partial insolubility in other dissolution systems is avoided, insoluble substances are prevented from existing in a precipitation form in the centrifugation process, and the concentration stability of the silk fibroin aqueous solution is ensured to a great extent. Secondly, the purity is high. The new dialysis method can remove almost all impurity ions in the silk fibroin dissolving solution to a great extent, so that the purity of the silk fibroin aqueous solution is quite high, various reactions are difficult to occur even in the solution in the long-term storage process, and great convenience is brought to subsequent operations.
Example 2 preparation of composite skin scaffolds based on silk fibroin/usnic acid
A preparation method of a composite skin scaffold based on silk fibroin/usnic acid comprises the following steps (figure 1 is a schematic diagram of the method):
first, a silk fibroin/usnic acid composite skin scaffold sample 1 is prepared
1) An aqueous silk fibroin solution sample 1 was prepared as in example 1;
2) placing 40ml of silk fibroin aqueous solution sample 1 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 20% (namely when the silk fibroin is 20g, the formic acid is 100ml), sealing the beaker, dissolving for 72 hours under the condition of magnetic stirring at 200rpm, freezing and centrifuging the dissolved solution at 4 ℃ for 15min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete dissolved solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid of 0.5mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 36 hours at 160rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 4ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 22kV, the distance between the needle head and the receiving plate is 15cm, the propelling speed of the injection pump is 0.01ml/min, the temperature is 22 ℃, the relative humidity is 45 percent, and the duration time of electrostatic spinning is 3 h;
5) and after the electrostatic spinning is finished, taking down the receiving device, and continuously drying the receiving device in a vacuum drying oven at the temperature of 23 ℃ for 26 hours. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Secondly, preparing a silk fibroin/usnic acid composite skin scaffold sample 2
1) An aqueous silk fibroin solution sample 1 was prepared as in example 1;
2) placing 40ml of silk fibroin aqueous solution sample 1 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 20% (namely when the silk fibroin is 20g, the formic acid is 100ml), sealing the beaker, dissolving for 72 hours under the condition of magnetic stirring at 200rpm, freezing and centrifuging the dissolved solution at 4 ℃ for 15min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete dissolved solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid being 1mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 36 hours at 160rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 4ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 22kV, the distance between the needle head and the receiving plate is 15cm, the propelling speed of the injection pump is 0.01ml/min, the temperature is 22 ℃, the relative humidity is 45 percent, and the duration time of electrostatic spinning is 3 h;
5) and after the electrostatic spinning is finished, taking down the receiving device, and continuously drying the receiving device in a vacuum drying oven at the temperature of 23 ℃ for 26 hours. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Thirdly, preparing the silk fibroin/usnic acid composite skin stent 3
1) An aqueous silk fibroin solution sample 2 was prepared as in example 1;
2) placing 40ml of silk fibroin aqueous solution sample 2 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 19% (namely when the silk fibroin is 19g, the formic acid is 100ml), sealing the beaker, dissolving for 60 hours under the magnetic stirring condition of 190rpm, freezing and centrifuging the solution at 4 ℃ for 13min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid of 2mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 30 hours at 150rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 5ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 20kV, the distance between the needle head and the receiving plate is 13cm, the propelling speed of the injection pump is 0.008ml/min, the temperature is 23 ℃, the relative humidity is 43 percent, and the duration time of electrostatic spinning is 3.8 h;
5) and taking the receiving device after electrostatic spinning is finished, and placing the receiving device in a vacuum drying oven at the temperature of 24 ℃ for continuous drying for 24 hours. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Fourthly, preparing the silk fibroin/usnic acid composite skin stent 4
1) An aqueous silk fibroin solution sample 2 was prepared as in example 1.
2) Placing 40ml of silk fibroin aqueous solution sample 2 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 19% (namely when the silk fibroin is 19g, the formic acid is 100ml), sealing the beaker, dissolving for 60 hours under the magnetic stirring condition of 190rpm, freezing and centrifuging the solution at 4 ℃ for 13min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid being 3mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 30 hours at 150rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 5ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 20kV, the distance between the needle head and the receiving plate is 13cm, the propelling speed of the injection pump is 0.008ml/min, the temperature is 23 ℃, the relative humidity is 43 percent, and the duration time of electrostatic spinning is 3.8 h;
5) and taking the receiving device after electrostatic spinning is finished, and placing the receiving device in a vacuum drying oven at the temperature of 24 ℃ for continuous drying for 24 hours. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Fifthly, preparing the silk fibroin/usnic acid composite skin stent 5
1) An aqueous silk fibroin solution sample 3 was prepared as in example 1.
2) Placing 40ml of silk fibroin aqueous solution sample 3 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 18% (namely when the silk fibroin is 18g, the formic acid is 100ml), sealing the beaker, dissolving for 48 hours under the condition of magnetic stirring at 180rpm, freezing and centrifuging the dissolved solution at 4 ℃ for 10min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete dissolved solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid of 4mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 24 hours at 140rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 6ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 18kV, the distance between the needle head and the receiving plate is 11cm, the propelling speed of the injection pump is 0.006ml/min, the temperature is 24 ℃, the relative humidity is 40%, and the electrostatic spinning duration is 4.5 h;
5) and taking the receiving device after electrostatic spinning is finished, and placing the receiving device in a vacuum drying oven at the temperature of 25 ℃ for continuous drying for 23 h. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Sixthly, preparing the silk fibroin/usnic acid composite skin stent 6
1) An aqueous silk fibroin solution sample 3 was prepared as in example 1.
2) Placing 40ml of silk fibroin aqueous solution sample 3 in a glass culture dish with the diameter of 10cm, drying at 45 ℃ to form a silk fibroin film, grinding and placing in a beaker, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 18% (namely when the silk fibroin is 18g, the formic acid is 100ml), sealing the beaker, dissolving for 48 hours under the condition of magnetic stirring at 180rpm, freezing and centrifuging the dissolved solution at 4 ℃ for 10min after the completion, and taking the supernatant to obtain a silk fibroin/formic acid complete dissolved solution;
3) weighing certain mass of usnic acid solid powder according to the final concentration of usnic acid of 5mg/ml, and magnetically stirring and mixing the silk fibroin/formic acid complete dissolved solution obtained in the step 2) for 24 hours at 140rpm to obtain a silk fibroin/usnic acid mixed solution;
4) taking 6ml of silk fibroin/usnic acid mixed solution in a 10ml disposable syringe, and adjusting various parameters of electrostatic spinning as follows: the external applied voltage is 18kV, the distance between the needle head and the receiving plate is 11cm, the propelling speed of the injection pump is 0.006ml/min, the temperature is 24 ℃, the relative humidity is 40%, and the electrostatic spinning duration is 4.5 h;
5) and taking the receiving device after electrostatic spinning is finished, and placing the receiving device in a vacuum drying oven at the temperature of 25 ℃ for continuous drying for 23 h. And then, taking down the composite fiber stent from the surface of the receiving plate, and then cutting to obtain small round block composite skin stents with uniform size and thickness.
Observing the obtained microscopic surface structure of the scaffold, wherein fig. 2 is an electron microscope scanning image of a composite scaffold obtained by electrostatic spinning of silk fibroin with different concentrations, wherein fig. 2a is a composite skin scaffold sample 5, fig. 2b is a composite skin scaffold sample 1, and it can be seen from the image that the microscopic structure of the composite skin scaffold surface is a porous structure with uniform fiber size and nano-fiber layer-by-layer uniform accumulation, which shows that the scaffold has good permeability, and it can be seen that the fiber diameter is related to the concentration of the silk fibroin, and the fiber diameter is increased along with the increase of the concentration of the silk fibroin, and the three-dimensional structure is more obvious, which shows that the prepared composite scaffold has a three-dimensional porous cross-linked structure.
And (2) carrying out methanol treatment on the obtained composite skin stent sample, namely soaking the composite skin stent sample for 15min by using anhydrous methanol (with the relative molecular mass of 32.04), then thoroughly soaking and rinsing the composite skin stent by using pure water, and taking out the composite skin stent for natural drying at room temperature, wherein a secondary structure change diagram of the composite skin stent sample 1 before and after the methanol treatment is shown in fig. 3, wherein a diagram of β -sheet content inside the stent before the methanol treatment is shown in fig. 3a, and a diagram of β -sheet content inside the stent after the methanol treatment is shown in fig. 3b, which shows that the internal structure of the prepared composite skin stent is changed after the methanol treatment, the β -sheet content is increased, and the problem of instant dissolution when meeting water is solved.
FIG. 4 is a test chart of mechanical properties of each scaffold prepared by the method of the present invention, which shows that the mechanical properties of the scaffold are not changed by adding usnic acid, and the mechanical properties are only related to the concentration of silk fibroin and are enhanced as the concentration of silk fibroin increases.
Fig. 5 is a graph of the degradation performance of each of the scaffolds prepared by the method of the present invention, wherein fig. 5a is composite skin scaffold sample 5 and fig. 5b is composite skin scaffold sample 1. Fig. 4 and fig. 5 show that the scaffold substrate prepared by the method of the present invention has excellent mechanical properties and biodegradability.
Example 3 application example
A silk fibroin/usnic acid composite skin scaffold is used for inhibiting the growth of different types of bacteria:
composite skin scaffolds containing different concentrations of usnic acid were prepared according to the preparation method of composite skin scaffold sample 1 in example 2, and controls were set, wherein the mass-to-volume ratio of silk fibroin to formic acid used in the scaffold was 20%, and the concentrations of usnic acid were 0mg/ml (control group), 0.5mg/ml, 1mg/ml, 2mg/ml, 3mg/ml, 4mg/ml, and 5mg/ml, respectively.
FIGS. 6a and 6b show the inhibitory effect of the composite skin scaffold on the growth of gram-positive bacteria (Staphylococcus aureus and Streptococcus pyogenes, respectively), and FIGS. 6c and 6d show the inhibitory effect of the composite skin scaffold on the growth of gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa, respectively), wherein the positions of the scaffolds corresponding to the use amounts of usnic acid of 0, 0.5mg/ml, 1mg/ml, 2mg/ml, and 3mg/ml in the respective figures are respectively the middle, upper right, upper left, lower right, and lower left, and the positions of the scaffolds corresponding to the use amounts of usnic acid of 0, 2mg/ml, 3mg/ml, 4mg/ml, and 5mg/ml in the respective figures are the middle, upper right, upper left, lower right, and lower left. The sizes of the antibacterial apertures around the bracket on the surface of the flat plate can be seen visually, the usnic acid with different concentrations has obvious inhibition effect on the growth of four different types of bacteria, the sizes of the antibacterial apertures are different according to the concentration of the usnic acid contained in the bracket, and the sizes of the antibacterial apertures are positively correlated with the concentration of the usnic acid.
Secondly, detecting the cell compatibility of the silk fibroin/usnic acid composite skin scaffold:
respectively placing the previous composite skin scaffold samples 1, 2 and 6 in a complete cell culture medium, after soaking in a cell incubator for a period of time, taking out soaking solutions with usnic acid concentrations of 0.5mg/ml, 1mg/ml and 5mg/ml in the scaffold, respectively serving as low-concentration, medium-concentration and high-concentration experimental group culture solutions to normally culture NIH3T3 fibroblasts, and observing the cell growth morphology after a period of time, as shown in FIG. 7a, counting and measuring the cells by using CCK-8, so that the number of the cells cultured in the soaking solutions with the concentrations is not obviously different, and the dressing does not influence the normal growth of the cells, and as shown in FIGS. 7b, 7c, 7d, 7e and 7f, respectively, the normal culture of the fibroblasts by using the common cell culture medium and the composite skin scaffold soaking solution with usnic acid concentrations of 0, 0.5mg/ml, 1mg/ml and 5mg/ml is performed on the NIH3T3 fibroblasts And the growth form of the cells is observed after a period of time, which shows that no significant difference exists in the cell form, and the antibacterial dressing has good cell compatibility.
Claims (9)
1. The extraction method of the silk fibroin aqueous solution is characterized by comprising the following steps:
1) shearing silkworm cocoons, ultrasonically cleaning, boiling and degumming in a sodium carbonate solution, washing with deionized water, and drying to obtain degummed silk fibroin;
2) preparing a lithium bromide aqueous solution, and dissolving the degummed silk fibroin obtained in the step 1) in the lithium bromide aqueous solution to obtain a silk fibroin solution;
3) respectively preparing an EDTA solution and a sodium bicarbonate solution, mixing the sodium bicarbonate solution with part of the EDTA solution to obtain a mixed solution of sodium bicarbonate and EDTA, shearing a dialysis bag, placing the dialysis bag in the mixed solution of sodium bicarbonate and EDTA for boiling treatment for 8-12 min, taking out the dialysis bag, placing the dialysis bag in the EDTA solution for boiling treatment for 6-10 min, and sealing the solution for later use;
4) pouring the dissolved solution obtained in the step 2) into the dialysis bag treated in the step 3), dialyzing by using pure water under the condition of magnetic stirring, taking out the dialyzate after the dialysis is finished, freezing and centrifuging, and taking the supernatant for low-temperature storage to obtain the silk fibroin aqueous solution.
2. The extraction method of the silk fibroin aqueous solution of claim 1, wherein the concentration of the EDTA solution prepared in the step 3) is 1mM, the concentration of the sodium bicarbonate solution is 0.02g/ml, and the EDTA solution and the sodium bicarbonate solution are mixed according to a volume ratio of 1: 0.8-1.2.
3. The method for extracting aqueous solution of silk fibroin according to claim 1, wherein the EDTA solution in step 3) is first adjusted to pH 7.8-8.0 with sodium hydroxide solution.
4. The method for extracting the silk fibroin aqueous solution as claimed in claim 1, wherein the time for pouring the solution obtained in step 2) into the dialysis bag treated in step 3) for dialysis with pure water in step 4) is 48-60 h, and the time for freezing and centrifuging is 15-20 min.
5. A preparation method of a silk fibroin/usnic acid-based composite skin scaffold is characterized by comprising the following steps:
1) extracting an aqueous solution of silk fibroin by the method of claim 1;
2) drying the silk fibroin aqueous solution obtained in the step 1) into a silk fibroin membrane, grinding, adding formic acid according to the mass volume ratio of the silk fibroin to the formic acid of 18-20%, stirring until the silk fibroin membrane is completely dissolved, and then freezing and centrifuging the dissolved solution for 10-15 min to obtain a silk fibroin/formic acid completely dissolved solution;
3) mixing usnic acid solid powder with the silk fibroin/formic acid complete solution obtained in the step 2), and stirring until usnic acid and the silk fibroin/formic acid complete solution are fully mixed, wherein the final concentration of usnic acid is 0.5-5 mg/ml, so as to obtain a silk fibroin/usnic acid mixed solution;
4) and (3) carrying out electrostatic spinning on the silk fibroin/usnic acid mixed solution to obtain the silk fibroin/usnic acid-based composite skin scaffold.
6. The method for preparing the silk fibroin/usnic acid-based composite skin scaffold according to claim 5, wherein the silk fibroin film in the step 2) is dissolved under the condition of magnetic stirring at 180-200 rpm at room temperature for 48-72 h.
7. The preparation method of the silk fibroin/usnic acid-based composite skin scaffold according to claim 5, wherein the stirring condition in the step 3) is that the magnetic stirring is carried out at room temperature at 140-160 rpm for 24-36 h.
8. The method for preparing the silk fibroin/usnic acid-based composite skin scaffold according to claim 5, wherein the step 4) of adjusting the electrospinning parameters specifically means: the external applied voltage is 18-22 kV, the distance between the needle head and the receiving plate is 11-15 cm, the propelling speed of the injection pump is 0.006-0.01 ml/min, the temperature is 22-24 ℃, the relative humidity is 40-45%, and the duration time of electrostatic spinning is 3-4.5 h.
9. A composite skin scaffold based on silk fibroin/usnic acid, characterized in that it is prepared by any one of the methods of claims 5 to 8.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911322819.9A CN110903374A (en) | 2019-12-20 | 2019-12-20 | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911322819.9A CN110903374A (en) | 2019-12-20 | 2019-12-20 | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN110903374A true CN110903374A (en) | 2020-03-24 |
Family
ID=69826764
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201911322819.9A Pending CN110903374A (en) | 2019-12-20 | 2019-12-20 | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110903374A (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112326743A (en) * | 2020-11-05 | 2021-02-05 | 重庆医科大学 | C-SF-FA flexible conductive film based on silk fibroin, wearable wound monitoring sensor and preparation method of wearable wound monitoring sensor |
| CN112931611A (en) * | 2021-02-03 | 2021-06-11 | 中国科学院上海微系统与信息技术研究所 | Silk fibroin solution, preparation method thereof and food preservation method |
| CN113249876A (en) * | 2021-06-10 | 2021-08-13 | 上海科技大学 | Ion conductor material and preparation method and application thereof |
| CN114699556A (en) * | 2022-04-08 | 2022-07-05 | 深圳高性能医疗器械国家研究院有限公司 | Preparation method of silk fibroin repair patch and silk fibroin repair patch |
| CN114748683A (en) * | 2022-04-26 | 2022-07-15 | 深圳湾实验室 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
| CN114748682A (en) * | 2022-04-26 | 2022-07-15 | 深圳湾实验室 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
| CN114832144A (en) * | 2022-04-26 | 2022-08-02 | 深圳湾实验室 | Broad-spectrum antibacterial antioxidant silk fibroin band-aid and preparation and application thereof |
| CN114831967A (en) * | 2022-04-26 | 2022-08-02 | 深圳湾实验室 | Broad-spectrum antioxidant silk fibroin adhesive bandage and preparation and application thereof |
| CN115778936A (en) * | 2022-12-16 | 2023-03-14 | 中国农业大学 | Application of usnic acid synergistic polymyxin in resisting gram-negative bacterial infection |
| WO2023206055A1 (en) * | 2022-04-26 | 2023-11-02 | 深圳湾实验室 | Modification and use of silk fibroin |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109706531A (en) * | 2019-01-16 | 2019-05-03 | 南通纺织丝绸产业技术研究院 | Regenerated silk fibroin/hydroxypropyl methyl cellulose nanofiber preparation method |
| CN110453378A (en) * | 2019-07-03 | 2019-11-15 | 上海大学 | A kind of sulfonic acid based quantum dot/fibroin albumen composite nano-fiber membrane and its preparation method and application |
-
2019
- 2019-12-20 CN CN201911322819.9A patent/CN110903374A/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109706531A (en) * | 2019-01-16 | 2019-05-03 | 南通纺织丝绸产业技术研究院 | Regenerated silk fibroin/hydroxypropyl methyl cellulose nanofiber preparation method |
| CN110453378A (en) * | 2019-07-03 | 2019-11-15 | 上海大学 | A kind of sulfonic acid based quantum dot/fibroin albumen composite nano-fiber membrane and its preparation method and application |
Non-Patent Citations (4)
| Title |
|---|
| SEMIH ÇALAMAK,ET AL: "Silk fibroin based antibacterial bionanotextiles as wound", 《MATERIALS SCIENCE AND ENGINEERING C》 * |
| 张锴: "静电纺超细纤维的形态结构控制及其在水净化领域的应用研究", 《中国优秀博硕士学位论文全文数据库(博士) 工程科技I辑》 * |
| 李冰冰等: "《生化与分子生物学实验指导》", 30 August 2014 * |
| 罗姮等: "松萝酸-丝素蛋白膜新型抗菌生物敷料的制备及性能研究", 《第七届全国地衣生物学研讨会》 * |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112326743A (en) * | 2020-11-05 | 2021-02-05 | 重庆医科大学 | C-SF-FA flexible conductive film based on silk fibroin, wearable wound monitoring sensor and preparation method of wearable wound monitoring sensor |
| CN112326743B (en) * | 2020-11-05 | 2024-01-16 | 重庆医科大学 | C-SF-FA flexible conductive film based on silk fibroin, wearable wound monitoring sensor and preparation method thereof |
| CN112931611A (en) * | 2021-02-03 | 2021-06-11 | 中国科学院上海微系统与信息技术研究所 | Silk fibroin solution, preparation method thereof and food preservation method |
| CN113249876A (en) * | 2021-06-10 | 2021-08-13 | 上海科技大学 | Ion conductor material and preparation method and application thereof |
| CN114699556A (en) * | 2022-04-08 | 2022-07-05 | 深圳高性能医疗器械国家研究院有限公司 | Preparation method of silk fibroin repair patch and silk fibroin repair patch |
| CN114748683A (en) * | 2022-04-26 | 2022-07-15 | 深圳湾实验室 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
| CN114748682A (en) * | 2022-04-26 | 2022-07-15 | 深圳湾实验室 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
| CN114832144A (en) * | 2022-04-26 | 2022-08-02 | 深圳湾实验室 | Broad-spectrum antibacterial antioxidant silk fibroin band-aid and preparation and application thereof |
| CN114831967A (en) * | 2022-04-26 | 2022-08-02 | 深圳湾实验室 | Broad-spectrum antioxidant silk fibroin adhesive bandage and preparation and application thereof |
| WO2023206055A1 (en) * | 2022-04-26 | 2023-11-02 | 深圳湾实验室 | Modification and use of silk fibroin |
| CN115778936A (en) * | 2022-12-16 | 2023-03-14 | 中国农业大学 | Application of usnic acid synergistic polymyxin in resisting gram-negative bacterial infection |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110903374A (en) | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold | |
| Gholipourmalekabadi et al. | Silk fibroin for skin injury repair: where do things stand? | |
| Yao et al. | Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair | |
| Divyashri et al. | Applications of hydrogel‐based delivery systems in wound care and treatment: an up‐to‐date review | |
| Liu et al. | Magnesium oxide-incorporated electrospun membranes inhibit bacterial infections and promote the healing process of infected wounds | |
| Zhang et al. | Advances in wound dressing based on electrospinning nanofibers | |
| US20090162439A1 (en) | Silk fibroin coating | |
| CN110639050A (en) | Silk fibroin nanofiber and preparation method of silver-loaded antibacterial dressing based on silk fibroin nanofiber | |
| RU2422133C1 (en) | Hydrophylic gel, method of its obtaining (versions), wound covering and based on it bandage means | |
| CN112480434B (en) | Copper ion antibacterial hydrogel and preparation method and application thereof | |
| Alam et al. | Surface modified thin film from silk and gelatin for sustained drug release to heal wound | |
| CN115124738B (en) | Double-layer bionic drug-loaded hydrogel and preparation and application thereof | |
| Ajmal et al. | PLGA/Gelatin-based electrospun nanofiber scaffold encapsulating antibacterial and antioxidant molecules for accelerated tissue regeneration | |
| Chandika et al. | Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly (ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold | |
| Wang et al. | Sustained release of EGF/bFGF growth factors achieved by mussel-inspired core–shell nanofibers with hemostatic and anti-inflammatory effects for promoting wound healing | |
| Li et al. | Chitosan-based injectable hydrogel with multifunction for wound healing: A critical review | |
| Seifi et al. | A novel multifunctional chitosan-gelatin/carboxymethyl cellulose-alginate bilayer hydrogel containing human placenta extract for accelerating full-thickness wound healing | |
| CN107469137B (en) | Injectable hemostatic hydrogel material and preparation method and application thereof | |
| TWI814462B (en) | Manufacturing method of hemostatic material and hemostatic material prepared thereby | |
| CN114288464B (en) | Antibacterial healing-promoting hydrogel dressing and preparation method and application thereof | |
| Davis et al. | Passive and interactive dressing materials | |
| CN115850733B (en) | Nanoclay hydrogel for injection and preparation method and application thereof | |
| KR100608192B1 (en) | A Method for Producing a Neutralized Chitosan Sponge for Wound Dressing and Scaffold, and the Chitosan Sponge produced by the same | |
| CN115584034B (en) | Injectable hydrogel material for wound repair and preparation method thereof | |
| KR100546793B1 (en) | Foam dressing using chitosan and Method of preparing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |