CN114748682A - Composition for preparing burn wound dressing, preparation and preparation method thereof - Google Patents
Composition for preparing burn wound dressing, preparation and preparation method thereof Download PDFInfo
- Publication number
- CN114748682A CN114748682A CN202210442226.1A CN202210442226A CN114748682A CN 114748682 A CN114748682 A CN 114748682A CN 202210442226 A CN202210442226 A CN 202210442226A CN 114748682 A CN114748682 A CN 114748682A
- Authority
- CN
- China
- Prior art keywords
- wound dressing
- burn wound
- iodine
- silk fibroin
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 27
- 238000002360 preparation method Methods 0.000 title claims abstract description 22
- 108010022355 Fibroins Proteins 0.000 claims abstract description 74
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 62
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 claims abstract description 48
- 239000011630 iodine Substances 0.000 claims abstract description 47
- 229910052740 iodine Inorganic materials 0.000 claims abstract description 47
- 229910001868 water Inorganic materials 0.000 claims abstract description 40
- 238000010521 absorption reaction Methods 0.000 claims abstract description 32
- 239000000463 material Substances 0.000 claims abstract description 29
- 229920000642 polymer Polymers 0.000 claims abstract description 19
- 229920001661 Chitosan Polymers 0.000 claims abstract description 17
- 229920000858 Cyclodextrin Polymers 0.000 claims abstract description 11
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 claims abstract description 9
- 239000001116 FEMA 4028 Substances 0.000 claims abstract description 8
- 235000011175 beta-cyclodextrine Nutrition 0.000 claims abstract description 8
- 229960004853 betadex Drugs 0.000 claims abstract description 8
- 230000004888 barrier function Effects 0.000 claims abstract description 3
- 239000000243 solution Substances 0.000 claims description 63
- 239000008367 deionised water Substances 0.000 claims description 22
- 229910021641 deionized water Inorganic materials 0.000 claims description 22
- 238000000034 method Methods 0.000 claims description 19
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 17
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 17
- 239000011575 calcium Substances 0.000 claims description 16
- 229910052791 calcium Inorganic materials 0.000 claims description 16
- 239000000499 gel Substances 0.000 claims description 15
- 239000007788 liquid Substances 0.000 claims description 13
- 239000011148 porous material Substances 0.000 claims description 13
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 11
- 235000021355 Stearic acid Nutrition 0.000 claims description 8
- 238000010438 heat treatment Methods 0.000 claims description 8
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 claims description 8
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 claims description 8
- 239000008117 stearic acid Substances 0.000 claims description 8
- 239000002202 Polyethylene glycol Substances 0.000 claims description 6
- 239000001913 cellulose Substances 0.000 claims description 6
- 229920002678 cellulose Polymers 0.000 claims description 6
- 229920001223 polyethylene glycol Polymers 0.000 claims description 6
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical group OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 claims description 5
- 108010010803 Gelatin Proteins 0.000 claims description 5
- 239000008273 gelatin Substances 0.000 claims description 5
- 229920000159 gelatin Polymers 0.000 claims description 5
- 235000019322 gelatine Nutrition 0.000 claims description 5
- 235000011852 gelatine desserts Nutrition 0.000 claims description 5
- 238000002156 mixing Methods 0.000 claims description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 4
- 235000001014 amino acid Nutrition 0.000 claims description 4
- 229940024606 amino acid Drugs 0.000 claims description 4
- 150000001413 amino acids Chemical class 0.000 claims description 4
- 239000002738 chelating agent Substances 0.000 claims description 4
- 238000005213 imbibition Methods 0.000 claims description 4
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 4
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 4
- 210000002966 serum Anatomy 0.000 claims description 4
- 150000003384 small molecules Chemical class 0.000 claims description 4
- 239000011780 sodium chloride Substances 0.000 claims description 4
- 102000008186 Collagen Human genes 0.000 claims description 3
- 108010035532 Collagen Proteins 0.000 claims description 3
- 238000004113 cell culture Methods 0.000 claims description 3
- 229920001436 collagen Polymers 0.000 claims description 3
- 239000003995 emulsifying agent Substances 0.000 claims description 3
- 239000008055 phosphate buffer solution Substances 0.000 claims description 3
- 239000004014 plasticizer Substances 0.000 claims description 3
- 229920001495 poly(sodium acrylate) polymer Polymers 0.000 claims description 3
- 229920002401 polyacrylamide Polymers 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Chemical compound [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 claims description 3
- 239000011800 void material Substances 0.000 claims description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 2
- 229920001450 Alpha-Cyclodextrin Polymers 0.000 claims description 2
- 239000004475 Arginine Substances 0.000 claims description 2
- FTEDXVNDVHYDQW-UHFFFAOYSA-N BAPTA Chemical compound OC(=O)CN(CC(O)=O)C1=CC=CC=C1OCCOC1=CC=CC=C1N(CC(O)=O)CC(O)=O FTEDXVNDVHYDQW-UHFFFAOYSA-N 0.000 claims description 2
- CKLJMWTZIZZHCS-UHFFFAOYSA-N D-OH-Asp Natural products OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 claims description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 2
- 239000004471 Glycine Substances 0.000 claims description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 claims description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 claims description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 2
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 2
- 229920002472 Starch Polymers 0.000 claims description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 claims description 2
- 239000004473 Threonine Substances 0.000 claims description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 2
- GNJXVFXIDHCCKR-UHFFFAOYSA-N acetyloxymethyl 2-[[2-(acetyloxymethoxy)-2-oxoethyl]-[2-[2-[2-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]ethoxy]ethoxy]ethyl]amino]acetate Chemical compound CC(=O)OCOC(=O)CN(CC(=O)OCOC(C)=O)CCOCCOCCN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O GNJXVFXIDHCCKR-UHFFFAOYSA-N 0.000 claims description 2
- 239000002671 adjuvant Substances 0.000 claims description 2
- 235000004279 alanine Nutrition 0.000 claims description 2
- HFHDHCJBZVLPGP-RWMJIURBSA-N alpha-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO HFHDHCJBZVLPGP-RWMJIURBSA-N 0.000 claims description 2
- 229940043377 alpha-cyclodextrin Drugs 0.000 claims description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 2
- 235000003704 aspartic acid Nutrition 0.000 claims description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims description 2
- 235000018417 cysteine Nutrition 0.000 claims description 2
- 239000012530 fluid Substances 0.000 claims description 2
- GDSRMADSINPKSL-HSEONFRVSA-N gamma-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO GDSRMADSINPKSL-HSEONFRVSA-N 0.000 claims description 2
- 229940080345 gamma-cyclodextrin Drugs 0.000 claims description 2
- 235000013922 glutamic acid Nutrition 0.000 claims description 2
- 239000004220 glutamic acid Substances 0.000 claims description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 2
- 229960000310 isoleucine Drugs 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 239000003960 organic solvent Substances 0.000 claims description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 2
- 235000008729 phenylalanine Nutrition 0.000 claims description 2
- 239000008107 starch Substances 0.000 claims description 2
- 235000019698 starch Nutrition 0.000 claims description 2
- 230000008719 thickening Effects 0.000 claims description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 2
- 239000004474 valine Substances 0.000 claims description 2
- 239000013022 formulation composition Substances 0.000 claims 1
- 230000000813 microbial effect Effects 0.000 claims 1
- 230000000844 anti-bacterial effect Effects 0.000 abstract description 21
- 230000003078 antioxidant effect Effects 0.000 abstract description 21
- 239000003963 antioxidant agent Substances 0.000 abstract description 13
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 abstract description 7
- 230000003020 moisturizing effect Effects 0.000 abstract description 4
- 230000003647 oxidation Effects 0.000 abstract description 4
- 238000007254 oxidation reaction Methods 0.000 abstract description 4
- 230000035699 permeability Effects 0.000 abstract description 4
- 230000003064 anti-oxidating effect Effects 0.000 abstract description 3
- 229940079593 drug Drugs 0.000 abstract description 3
- 239000003814 drug Substances 0.000 abstract description 3
- 230000014759 maintenance of location Effects 0.000 abstract description 3
- 230000003115 biocidal effect Effects 0.000 abstract description 2
- 239000002131 composite material Substances 0.000 abstract 1
- 208000027418 Wounds and injury Diseases 0.000 description 93
- 206010052428 Wound Diseases 0.000 description 92
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 33
- 238000003756 stirring Methods 0.000 description 17
- PEDCQBHIVMGVHV-UHFFFAOYSA-N glycerol group Chemical group OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 15
- 101710143098 Paralytic peptide 1 Proteins 0.000 description 12
- 239000003642 reactive oxygen metabolite Substances 0.000 description 11
- 235000019441 ethanol Nutrition 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 230000000694 effects Effects 0.000 description 9
- 239000000839 emulsion Substances 0.000 description 8
- 238000004108 freeze drying Methods 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 238000001035 drying Methods 0.000 description 6
- 239000000835 fiber Substances 0.000 description 6
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 6
- 230000035876 healing Effects 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 230000029663 wound healing Effects 0.000 description 6
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 5
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- TUJKJAMUKRIRHC-UHFFFAOYSA-N hydroxyl Chemical compound [OH] TUJKJAMUKRIRHC-UHFFFAOYSA-N 0.000 description 5
- 244000005700 microbiome Species 0.000 description 5
- 230000011506 response to oxidative stress Effects 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- GUTLYIVDDKVIGB-OUBTZVSYSA-N Cobalt-60 Chemical compound [60Co] GUTLYIVDDKVIGB-OUBTZVSYSA-N 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- 241000191967 Staphylococcus aureus Species 0.000 description 4
- -1 anhydride compounds Chemical class 0.000 description 4
- 239000003242 anti bacterial agent Substances 0.000 description 4
- 238000005520 cutting process Methods 0.000 description 4
- 239000012024 dehydrating agents Substances 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 238000001704 evaporation Methods 0.000 description 4
- 230000003203 everyday effect Effects 0.000 description 4
- 238000007710 freezing Methods 0.000 description 4
- 230000008014 freezing Effects 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- AMXOYNBUYSYVKV-UHFFFAOYSA-M lithium bromide Chemical compound [Li+].[Br-] AMXOYNBUYSYVKV-UHFFFAOYSA-M 0.000 description 4
- 238000004806 packaging method and process Methods 0.000 description 4
- 238000011056 performance test Methods 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 230000001954 sterilising effect Effects 0.000 description 4
- 238000004659 sterilization and disinfection Methods 0.000 description 4
- 238000009210 therapy by ultrasound Methods 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 3
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 3
- 108010024636 Glutathione Proteins 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 210000001124 body fluid Anatomy 0.000 description 3
- 239000010839 body fluid Substances 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 229960003180 glutathione Drugs 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 230000002458 infectious effect Effects 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 238000011031 large-scale manufacturing process Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 230000036542 oxidative stress Effects 0.000 description 3
- 239000002504 physiological saline solution Substances 0.000 description 3
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 3
- 238000011725 BALB/c mouse Methods 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 241000222122 Candida albicans Species 0.000 description 2
- 102000016938 Catalase Human genes 0.000 description 2
- 108010053835 Catalase Proteins 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 208000028990 Skin injury Diseases 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 230000006851 antioxidant defense Effects 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000003385 bacteriostatic effect Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- 229940095731 candida albicans Drugs 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 208000006111 contracture Diseases 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 210000000416 exudates and transudate Anatomy 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 230000003859 lipid peroxidation Effects 0.000 description 2
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical compound O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 description 2
- 230000001338 necrotic effect Effects 0.000 description 2
- 229910052755 nonmetal Inorganic materials 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000003908 quality control method Methods 0.000 description 2
- 238000004445 quantitative analysis Methods 0.000 description 2
- 230000008439 repair process Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 208000035404 Autolysis Diseases 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 206010051814 Eschar Diseases 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 102000006587 Glutathione peroxidase Human genes 0.000 description 1
- 108700016172 Glutathione peroxidases Proteins 0.000 description 1
- 101000588302 Homo sapiens Nuclear factor erythroid 2-related factor 2 Proteins 0.000 description 1
- 101001023770 Homo sapiens Transcription factor NF-E2 45 kDa subunit Proteins 0.000 description 1
- 239000006142 Luria-Bertani Agar Substances 0.000 description 1
- 208000034486 Multi-organ failure Diseases 0.000 description 1
- 208000010718 Multiple Organ Failure Diseases 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 206010062575 Muscle contracture Diseases 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 102100031701 Nuclear factor erythroid 2-related factor 2 Human genes 0.000 description 1
- 239000008118 PEG 6000 Substances 0.000 description 1
- QGMRQYFBGABWDR-UHFFFAOYSA-M Pentobarbital sodium Chemical compound [Na+].CCCC(C)C1(CC)C(=O)NC(=O)[N-]C1=O QGMRQYFBGABWDR-UHFFFAOYSA-M 0.000 description 1
- 229920002535 Polyethylene Glycol 1500 Polymers 0.000 description 1
- 229920002538 Polyethylene Glycol 20000 Polymers 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- 229920001030 Polyethylene Glycol 4000 Polymers 0.000 description 1
- 229920002582 Polyethylene Glycol 600 Polymers 0.000 description 1
- 229920002584 Polyethylene Glycol 6000 Polymers 0.000 description 1
- 206010039509 Scab Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 206010072170 Skin wound Diseases 0.000 description 1
- BCKXLBQYZLBQEK-KVVVOXFISA-M Sodium oleate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC([O-])=O BCKXLBQYZLBQEK-KVVVOXFISA-M 0.000 description 1
- 102000019197 Superoxide Dismutase Human genes 0.000 description 1
- 108010012715 Superoxide dismutase Proteins 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 102100035412 Transcription factor NF-E2 45 kDa subunit Human genes 0.000 description 1
- 206010053716 Wound necrosis Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000003592 biomimetic effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229910000420 cerium oxide Inorganic materials 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000006196 deacetylation Effects 0.000 description 1
- 238000003381 deacetylation reaction Methods 0.000 description 1
- 238000001804 debridement Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000008260 defense mechanism Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000000635 electron micrograph Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 231100000333 eschar Toxicity 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- SZVJSHCCFOBDDC-UHFFFAOYSA-N ferrosoferric oxide Chemical compound O=[Fe]O[Fe]O[Fe]=O SZVJSHCCFOBDDC-UHFFFAOYSA-N 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 150000002357 guanidines Chemical class 0.000 description 1
- 150000003278 haem Chemical class 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 230000003166 hypermetabolic effect Effects 0.000 description 1
- 238000009616 inductively coupled plasma Methods 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- OCVXZQOKBHXGRU-UHFFFAOYSA-N iodine(1+) Chemical compound [I+] OCVXZQOKBHXGRU-UHFFFAOYSA-N 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 208000029744 multiple organ dysfunction syndrome Diseases 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000004792 oxidative damage Effects 0.000 description 1
- BMMGVYCKOGBVEV-UHFFFAOYSA-N oxo(oxoceriooxy)cerium Chemical compound [Ce]=O.O=[Ce]=O BMMGVYCKOGBVEV-UHFFFAOYSA-N 0.000 description 1
- SOQBVABWOPYFQZ-UHFFFAOYSA-N oxygen(2-);titanium(4+) Chemical compound [O-2].[O-2].[Ti+4] SOQBVABWOPYFQZ-UHFFFAOYSA-N 0.000 description 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000001991 pathophysiological effect Effects 0.000 description 1
- 229960002275 pentobarbital sodium Drugs 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229940114930 potassium stearate Drugs 0.000 description 1
- ANBFRLKBEIFNQU-UHFFFAOYSA-M potassium;octadecanoate Chemical compound [K+].CCCCCCCCCCCCCCCCCC([O-])=O ANBFRLKBEIFNQU-UHFFFAOYSA-M 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 230000002000 scavenging effect Effects 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 230000028043 self proteolysis Effects 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 239000002352 surface water Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- VUYXVWGKCKTUMF-UHFFFAOYSA-N tetratriacontaethylene glycol monomethyl ether Chemical compound COCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO VUYXVWGKCKTUMF-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000009772 tissue formation Effects 0.000 description 1
- 230000007838 tissue remodeling Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0061—Use of materials characterised by their function or physical properties
- A61L26/0085—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0004—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form containing inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0009—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form containing macromolecular materials
- A61L26/0023—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0009—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form containing macromolecular materials
- A61L26/0028—Polypeptides; Proteins; Degradation products thereof
- A61L26/0047—Specific proteins or polypeptides not covered by groups A61L26/0033 - A61L26/0042
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0061—Use of materials characterised by their function or physical properties
- A61L26/0066—Medicaments; Biocides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L26/00—Chemical aspects of, or use of materials for, wound dressings or bandages in liquid, gel or powder form
- A61L26/0061—Use of materials characterised by their function or physical properties
- A61L26/008—Hydrogels or hydrocolloids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/10—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing inorganic materials
- A61L2300/106—Halogens or compounds thereof, e.g. iodine, chlorite
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/252—Polypeptides, proteins, e.g. glycoproteins, lipoproteins, cytokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Materials Engineering (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Materials For Medical Uses (AREA)
Abstract
The invention belongs to the technical field of medical medicines, and particularly relates to a composition for preparing a burn wound dressing, a preparation and a preparation method thereof. The biological dressing of the invention takes silk fibroin as an antioxidant; filling elemental iodine into beta-cyclodextrin by using ultrasonic waves to serve as active iodine with a broad-spectrum antibacterial function; the silk fibroin with oxidation resistance and the active iodine with broad-spectrum antibacterial function are loaded on a chitosan-water absorption molecule skeleton to prepare the porous asymmetric burn wound dressing with oxidation resistance, antibacterial property and moisturizing function. The burn wound dressing consists of fibroin, active iodine, a framework and auxiliary materials according to a mass ratio of 1: 0.5-2: 0.1-10; the skeleton consists of chitosan and a water-absorbing polymer according to a mass ratio of 1: 0.1-5; the active iodine consists of elemental iodine and a slow release material of iodine. The burn wound dressing is a novel composite dressing with the advantages of antioxidation, antibiosis, high moisture retention, high strength, air permeability, barrier property, easy uncovering and the like.
Description
Technical Field
The invention belongs to the field of biomedicine, and particularly relates to a composition for preparing a burn wound dressing, a preparation and a preparation method thereof.
Background
Burns are one of the major catastrophic events for mankind and are also the fourth most common trauma worldwide. After burn, serious hypermetabolic reaction and damage of oxidation resistance and cell defense mechanisms, local or whole body oxidative stress reaction have important influence on wound recovery of patients, and can cause serious consequences such as long-term inflammatory infiltration of burn wounds, no healing and scarring of the wound, even multiple organ failure and the like.
It is well known that oxidative stress is a severe damage of DNA, lipids, proteins and carbohydrates at the wound site by excess Reactive Oxygen Species (ROS). Thus, unbalanced ROS alter cellular function, leading to abnormal signaling pathways, inducing inflammation and scar contractures. At present, antioxidant therapy has been shown to minimize the pathophysiological damage of burns, such as tissue lipid peroxidation, tissue necrosis, and decreased mortality, compared to other burn wound treatment methods. However, dermal contracture/fibrosis caused by burns remains a major clinical challenge.
Under pathological conditions, ROS are produced mainly by mitochondria, resulting in excessive reactive oxygen species being produced in the cell, thereby inducing unregulated cellular redox, leading to fibrosis. However, existing antioxidant strategies focus on the quenching of extracellular reactive oxygen species, and few burn dressings are used to modulate reactive oxygen species in an intracellular/extracellular synergistic manner.
It is well recognized that antioxidant defenses in biological systems can precisely coordinate the regulation of reactive oxygen species produced from different cells, such as: 1) antioxidase (superoxide dismutase (SODs), Catalase (CAT), glutathione peroxidase (GSH-Px), heme catalytic enzyme and various metal coordination proteins can decompose high-activity active oxygen into inert molecule H2O and O2(ii) a 2) The natural extracellular matrix (hyaluronic acid, HA) eliminates intracellular reactive oxygen species by mediating the major redox transcription factor, the nuclear factor NF-E2-related factor (Nrf 2); 3) small molecule antioxidants, such as vitamin e (ve), can precisely penetrate into membranes, terminating chain reactions that prevent lipid peroxidation. Thus, the antioxidant system in the body can precisely counter unbalanced ROS from different cells to maintain the balance of oxidation and antioxidant in the body. However, to date, few studies have explored biomimetic antioxidant defense dressings for skin wound care.
The artificial nano enzyme materials are developed vigorously in the aspect of resisting oxidative damage, such as gold nano materials, cerium oxide nano particles, nano manganese dioxide and the like, and the artificial nano enzyme materials show strong active oxygen scavenging activity. However, these used materials not only cause cytotoxicity and inflammation due to their nano-size, but also lack activity for wound care. Under physiological conditions, wound healing involves many complex processes such as hemostasis, inflammation, new tissue formation and remodeling of skin appendages. An ideal wound dressing would promote wound healing, retain water, maintain electrolyte balance and stop bleeding, provide not only a similar extracellular matrix (ECM), but would also rapidly cover the wound and prevent bacteria and other pathogens from invading the wound. Most importantly, the burn dressing has good biological safety, can maintain the tissue microenvironment required by the burn wound by reducing the oxidative stress reaction of the wound, thereby promoting the autolysis debridement of necrotic eschar tissue, preventing wound necrosis, promoting cell proliferation and migration, has rich raw material sources, and is convenient for large-scale production. Therefore, the industrial production of the nanometer materials with the anti-oxidation effect is still a challenge when the nanometer materials are applied to the clinical treatment of burn induced oxidative stress skin injury.
The invention patent publication US20140044758a1 discloses a wound dressing that is bacteriostatic and hygroscopic. The chitosan fiber utilized by the invention has antibacterial property, and the chemically modified cellulose has fluid absorption capacity, but the oxidative stress reaction of the burn wound surface caused in the ROS environment cannot be effectively solved.
Disclosure of Invention
In order to solve the problems, the invention selects silk fibroin with good repair function as an antioxidant for reducing the oxidative stress reaction of the wound surface; mixing iodine (I)2) The solution is mixed with beta-Cyclodextrin (CD) solution, and iodine is filled into the cyclodextrin by using ultrasonic waves, so that the active iodine with the broad-spectrum antibacterial function is prepared. The invention loads Silk Fibroin (SF) with antioxidant effect and active iodine (CD-I) with broad-spectrum antibacterial function on a Chitosan (CTS) -water-absorbing polymer skeleton, and then modifies the smooth surface of sponge with stearic acid to prepare the burn wound dressing with antioxidant, antibacterial and moisturizing functions, which is named as CTS-GEL/SF/CD-I/SA.
One of the objects of the present invention is to provide a composition for preparing a burn wound dressing.
In order to achieve the purpose, the invention adopts the following technical scheme:
the composition comprises silk fibroin, a framework and active iodine according to a mass ratio of 1: 0.5-2: 0.1-10; the skeleton consists of chitosan and a water-absorbing polymer according to a mass ratio of 1: 0.1-5; the active iodine consists of elementary iodine and a slow release material of iodine.
Further, the silk fibroin is natural silk fibroin and/or modified silk fibroin; the modified silk fibroin is selected from one or more of silk fibroin with calcium partially or completely removed, silk fibroin subjected to heating treatment and derivatives thereof, silk fibroin subjected to ultraviolet irradiation and derivatives thereof, and silk fibroin subjected to organic solvent treatment and derivatives thereof.
Further, the water-absorbing polymer is selected from natural water-absorbing polymers and/or synthetic water-absorbing polymers, the natural water-absorbing polymers are selected from collagen, gelatin, cellulose and derivatives thereof, and the synthetic water-absorbing polymers are selected from polyethylene glycol, polyacrylamide, sodium polyacrylate or polyvinyl alcohol.
Further, the chitosan is selected from acid-soluble chitosan, and/or water-soluble chitosan, and/or anhydride modified chitosan derived wound, and/or high deacetylation degree chitosan, and/or chitosan modified by anhydride compounds.
Further, the water-absorbing polymer is selected from natural water-absorbing polymers and/or synthetic water-absorbing polymers; the natural water-absorbing polymer is collagen, gelatin, cellulose and derivatives thereof, and the artificially synthesized water-absorbing polymer is polyethylene glycol, polyacrylamide, sodium polyacrylate or polyvinyl alcohol.
Furthermore, the polyethylene glycol is preferably one or more of PEG-400, PEG-600, PEG-1500, PEG-4000, PEG-6000 and PEG-20000.
Further, the antibacterial agent is selected from a nano nonmetal antibacterial material, and/or a nano metal antibacterial material, and/or a quaternary ammonium salt antibacterial material, and/or an oxidizing material; the nano nonmetal antibacterial material comprises nano ferroferric oxide, nano zinc oxide and nano titanium dioxide, the nano metal antibacterial material comprises nano gold, nano silver and nano zinc, the quaternary ammonium salt antibacterial material comprises chitosan quaternary ammonium salt and guanidine salt, and the oxidizing material comprises elementary iodine.
Further, the slow release material of the elemental iodine is selected from a polymer material and/or a small molecule material, the polymer material comprises starch, cellulose and derivatives thereof, polyvinylpyrrolidone and polyethylene glycol, and the small molecule material comprises alpha-cyclodextrin, beta-cyclodextrin and gamma-cyclodextrin.
The invention also aims to provide a preparation composition for preparing the burn wound dressing.
In order to achieve the purpose, the invention adopts the following technical scheme:
the preparation composition is prepared from the silk fibroin, the skeleton, the active iodine and auxiliary materials according to the weight ratio of 1: 0.5-2: 0.1-10 by mass; the auxiliary materials are plasticizers and/or emulsifiers.
Further, the plasticizer is selected from glycerol, propylene glycol or sorbitol.
Further, the emulsifier is selected from any one or more of sodium stearate, potassium stearate, sodium oleate, sorbitan fatty acid and hexadecyl sulfated castor oil.
The invention also aims to provide a method for preparing the burn wound dressing by using the composition or the dressing preparation composition, and the method for preparing the biological dressing does not need a separation and purification process, thereby saving the cost, facilitating the quality control and being beneficial to large-scale production.
In order to achieve the purpose, the invention adopts the following technical scheme:
the method for preparing the burn wound dressing specifically comprises the following steps:
s1: preparing silk fibroin solution and active iodine;
s2: mixing the obtained product of S1 with skeleton and adjuvants.
Further, modifying the product obtained from S2 with stearic acid after S2 in S3.
Further, the S2 is prepared by mixing the product obtained from S1 with a skeleton and auxiliary materials, stirring and emulsifying, standing at 4 ℃ for 1h, standing at-20 ℃ for 4h, and standing at-70 ℃ for 6h, and freeze-drying.
Further, the S3 is prepared by completely swelling the product obtained in S2 in water, dripping stearic acid solution on the surface of the product, washing the product with absolute ethyl alcohol, standing the product at-20 ℃ for 2 hours, standing the product at-70 ℃ for 6 hours, then freeze-drying the product and drying the product.
Further, a chelating agent of calcium or an amino acid capable of chelating with calcium is added in the preparation process of S1; the chelating agent of calcium is EDTA and its derivatives, EGTA AM and its derivatives, BAPTA and its derivatives; the amino acid capable of chelating calcium comprises any one or more of glutamic acid, alanine, aspartic acid, phenylalanine, asparaginic acid, arginine, threonine, tyrosine, tryptophan, glycine, serine, valine, histidine, isoleucine, cysteine and derivatives thereof.
The fourth purpose of the invention is to provide a burn wound dressing which has broad-spectrum antioxidant effect, can promote the healing of chronic difficult-to-heal wound surfaces in oxidative stress microenvironment, and has the characteristics of moisture retention, high strength, air permeability, barrier property, easy uncovering property and the like.
In order to achieve the purpose, the invention adopts the following technical scheme:
a burn wound dressing obtained by the above-described method for producing a burn wound dressing.
Furthermore, the burn wound dressing is of a porous structure, the porosity of the burn wound dressing is 60% -80%, and the pore size of the burn wound dressing is 0.5-2 mm.
Further, the water absorption rate of the burn wound dressing is 1-20 times; the water absorption multiplying power can be calculated by the following formula: q = (M2-M1)/M1; q is the water absorption multiplying power, and the unit is g/g; m1 is the sample mass before imbibition, in g; m2 is the mass of the sample after pipetting in g.
Further, the water absorption rates of the burn wound dressing in different media are as follows: the water absorption rate in deionized water is 14-16; the water absorption rate in saline water is 12-14; the water absorption rate in the phosphate buffer solution is 9-11; the water absorption rate in the cell culture solution is 7-10; the water absorption rate in serum is 5-7.
Further, the burn wound dressing is subjected to freeze-drying treatment, and/or low-temperature treatment, and/or high-temperature treatment, and/or alcohol modification and/or ray irradiation.
The fifth purpose of the invention is to provide a method for adsorbing liquid, which provides a new idea for effectively adsorbing liquid.
In order to achieve the purpose, the invention adopts the following technical scheme:
the method is characterized in that the burn wound dressing adsorbs liquid, and the liquid enters the pore structure of the burn wound dressing, so that the pore wall of the burn wound dressing is thickened and gelatinized to ensure that a tube cavity is eliminated.
The sixth purpose of the invention is to provide a method for blocking microorganisms, which provides a new idea for effectively blocking microorganisms.
In order to realize the purpose, the invention adopts the following technical scheme:
isolating microorganisms by a method comprising adsorbing the liquid, adsorbing the liquid by the method, thickening and gelling the wall of the burn wound dressing pore space to make the lumen void, and isolating microorganisms.
The invention has the beneficial effects that:
1) the burn and wound dressing provided by the invention can reduce the oxidative stress reaction of the wound surface, recover the repair function of repairing related cells and accelerate the healing of the wound surface.
2) The water-absorbing macromolecules or polymers with a proper proportion in the burn wound dressing provided by the invention have certain water-absorbing and water-locking effects. When the dressing is contacted with body fluid, the material swells to form gel, so that the wound surface can be effectively isolated from the outside, and the dressing has good air permeability. The moisture-locking function of the burn wound dressing enables the contact surface to keep certain humidity, thereby being beneficial to accelerating the formation of epithelial tissues, relieving pain, decomposing necrotic tissues and slowly releasing antibacterial agents.
3) The antibacterial agent slow-release carrier in the burn wound dressing provided by the invention can play a role of slow-release antibacterial agent for a long time, and reduce or prevent the growth of microorganisms on the wound surface.
4) The burn wound dressing provided by the invention can be tightly attached to a wound surface, seals the wound surface, prevents harmful particles from contacting the wound surface, has good air permeability, and cannot be adhered to the wound surface tissue.
5) The burn wound dressing provided by the invention is prepared by adopting a freeze-drying method, and a separation and purification process is not needed in the middle, so that the cost is saved, the quality control is convenient, and the large-scale production is facilitated.
Drawings
Fig. 1 is an electron micrograph of a sample of the burn wound dressing prepared in example 5.
Detailed Description
The technical solution of the present invention will be further clearly and completely described with reference to the following specific examples. It is to be understood that the described embodiments are merely a few embodiments of the invention and are not to be taken as the full scope of the invention. Therefore, all other embodiments obtained by those skilled in the art without inventive efforts shall fall within the scope of the present invention.
Unless otherwise specified, the percentages in the examples all represent the mass fraction of the solvent.
Example 1 preparation of modified Silk fibroin solution sample 1
1) Taking 10g of silk fibroin fiber, adding 100mL of calcium chloride ternary solution (calcium chloride: water: ethanol =1: 1-5: 1-20), dissolving at 80 ℃, dialyzing for 3 days, and changing deionized water for 3 times a day to obtain silk fibroin solution;
2) taking 20mL of the silk fibroin solution obtained in the step 1), adding 2mL of EDTA aqueous solution with the concentration of 100mmol/L for reaction for 1h, dialyzing for 3 days, and changing deionized water for 3 times every day to obtain a low-calcium or calcium-free silk fibroin solution sample 1.
Example 2 preparation of modified Silk fibroin solution sample 2
1) Taking 10g of silk fibroin fiber, adding 100mL of 10mol/L lithium bromide solution, dissolving at 80 ℃, dialyzing for 3 days, and changing deionized water for 3 times a day to obtain silk fibroin solution;
2) taking 20mL of the silk fibroin solution obtained in the step 1), adding 2mL of EDTA aqueous solution with the concentration of 100mmol/L for reaction for 1h, dialyzing for 3 days, and changing deionized water for 3 times every day to obtain a low-calcium or calcium-free silk fibroin solution sample 2.
Example 3 preparation of modified Silk fibroin solution sample 3
1) Taking 1g of silk fibroin fiber, adding 20ml of EDTA aqueous solution with the concentration of 100mmol/L for reaction for 24h, dialyzing for 3 days, changing deionized water for 3 times every day, and drying at 50 ℃ to obtain the low-calcium or calcium-free silk fibroin fiber;
2) taking 10g of the low-calcium or calcium-free silk fibroin fibers obtained in the step 1), adding 100mL of a lithium bromide solution with the concentration of 10mol/L, reacting at 80 ℃ for 24h, dialyzing for 3 days, and changing deionized water for 3 times every day to obtain a low-calcium or calcium-free silk fibroin solution sample 3.
Example 4 in vitro broad-spectrum antioxidant Performance test of modified Silk fibroin solution samples
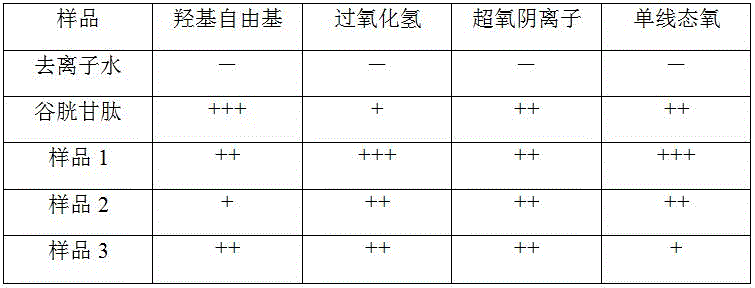
The low calcium or calcium-free silk fibroin solution samples prepared in examples 1-3 were subjected to a broad-spectrum antioxidant performance test. Each of the samples prepared in examples 1-3, glutathione and deionized water were reacted with superoxide anion, hydroxyl radical and H, respectively2O2Reacting, and then using a superoxide anion test kit, a hydroxyl radical test kit and a hydrogen peroxide quantitative analysis kit to test that 5 groups of samples respectively eliminate superoxide anions, hydroxyl radicals and H2O2Of the cell.
The antioxidant effect evaluation of each sample is shown in table 1. As can be seen from Table 1, the silk fibroin solutions prepared by different preparation processes can scavenge superoxide anion, hydroxyl radical and H2O2But 3 groups of samples prepared by the three processes of the invention all have good antioxidation.
TABLE 1 evaluation of antioxidant action
Note: "-" indicates no antioxidant effect; "+" indicates a clearance of 10% to 50%; "+ +" indicates a clearance of 50% -90%; "+ + + + +" indicates a clearance > 90%.
EXAMPLE 5 preparation of burn wound dressing sample 1
1) Dissolving the elementary iodine in 75% ethanol solution to prepare iodine with mass fraction of 5%. Heating to dissolve 2% beta-cyclodextrin, adding 5% iodine tincture into beta-cyclodextrin solution, and performing ultrasonic treatment for 30 min. Evaporating the liquid by a rotary evaporator, and collecting solid powder for later use;
2) heating 20mL of 4% silk fibroin solution at 95 ℃ for 2h, adding 1mL of pure glycerol, mechanically stirring for 10min, adding 10mL of 5% gelatin solution, mechanically stirring for 10min to form white emulsion, adding 10mL of 2% chitosan solution into the white emulsion, and mechanically stirring for 30min for later use;
3) dissolving 1g of the product obtained in the step 1) in 4mL of deionized water, adding the deionized water into the product obtained in the step 2), mechanically stirring for 10min, pouring the mixture into a container with the size of 100 multiplied by 150mm, standing the mixture at the temperature of 4 ℃ for 1h, standing the mixture at the temperature of-20 ℃ for 4h, standing the mixture at the temperature of-70 ℃ for 6h, and freeze-drying the mixture by a freeze-dryer to obtain CTS-GEL/SF/CD-I sponge for later use;
4) allowing the CTS-GEL/SF/CD-I sponge to fully absorb deionized water, standing at-20 ℃ for 4h, uniformly pouring 8mL of stearic acid solution (40 mmol/L ethanol, DCC as a dehydrating agent) on the smooth surface of the CTS-GEL/SF/CD-I sponge, freezing for 2h, washing the smooth surface of the CTS-GEL/SF/CD-I dressing with absolute ethanol at 20 ℃ for 3 times, drying to obtain the CTS-GEL/SF/CD-I/SA dressing, cutting, packaging, and performing cobalt 60 irradiation sterilization to obtain the CTS-GEL/SF/CD-I/SA dressing.
Example 6 preparation of burn wound dressing sample 2
1) Dissolving elementary iodine in 75% ethanol solution to obtain 5% iodine tincture. Adding 5% iodine tincture into 2% polyvinylpyrrolidone solution, and performing ultrasonic treatment for 30 min. Evaporating the liquid by a rotary evaporator, and collecting solid powder for later use;
2) heating 20mL of 4% silk fibroin solution at 95 ℃ for 2h, adding 1mL of glycerol, mechanically stirring for 10min, adding 10mL of 5% gelatin solution, mechanically stirring for 10min to form white emulsion, adding 10mL of 2% chitosan solution into the white emulsion, and mechanically stirring for 30min for later use;
3) dissolving 1g of the product obtained in the step 1) in 5mL of deionized water, adding the deionized water into the product obtained in the step 2), mechanically stirring for 10min, pouring the mixture into a container with the size of 100 multiplied by 150mm, standing the mixture at the temperature of 4 ℃ for 1h, standing the mixture at the temperature of-20 ℃ for 4h, standing the mixture at the temperature of-70 ℃ for 6h, and freeze-drying the mixture by a freeze dryer to obtain CTS-GEL/SF/PP-I sponge for later use;
4) allowing the CTS-GEL/SF/PP-I sponge to fully absorb deionized water, standing at-20 ℃ for 4h, uniformly pouring 8mL of stearic acid solution (40 mmol/L ethanol, DCC as a dehydrating agent) on the smooth surface of the CTS-GEL/SF/PP-I sponge, freezing for 2h, washing the smooth surface of the CTS-GEL/SF/PP-I dressing with absolute ethanol at 20 ℃ for 3 times, drying to obtain the CTS-GEL/SF/PP-I/SA dressing, cutting, packaging, and performing cobalt 60 irradiation sterilization to obtain the CTS-GEL/SF/PP-I/SA dressing.
EXAMPLE 7 preparation of burn wound dressing sample 3
1) Dissolving the elementary iodine in 75% ethanol solution to prepare iodine with mass fraction of 5%. Heating to dissolve 2% beta-cyclodextrin, adding 5% iodine tincture into beta-cyclodextrin solution, and performing ultrasonic treatment for 30 min. Evaporating the liquid by a rotary evaporator, and collecting solid powder for later use;
2) heating 20mL of 4% silk fibroin solution at 95 ℃ for 2h, adding 1mL of glycerol, mechanically stirring for 10min, adding 10mL of 5% polyvinyl alcohol solution, mechanically stirring for 10min to form white emulsion, adding 10mL of 2% chitosan solution into the white emulsion, and mechanically stirring for 30min for later use;
3) dissolving 1g of the product obtained in the step 1) in 5mL of deionized water, adding the solution into the product obtained in the step 2), mechanically stirring for 10min, pouring the solution into a container with the size of 100 multiplied by 150mm, standing the solution at the temperature of 4 ℃ for 1h, standing the solution at the temperature of-20 ℃ for 4h, standing the solution at the temperature of-70 ℃ for 6h, and freeze-drying the solution by a freeze dryer to obtain CTS-PVA/SF/CD-I sponge for later use;
4) allowing the CTS-PVA/SF/CD-I sponge to fully absorb deionized water, standing at-20 ℃ for 4h, uniformly pouring 8mL of stearic acid solution (40 mmol/L ethanol, DCC as a dehydrating agent) on the smooth surface of the CTS-PVA/SF/CD-I sponge, freezing for 2h, washing the smooth surface of the CTS-PVA/SF/CD-I dressing with absolute ethanol at 20 ℃ for 3 times, drying to obtain the CTS-PVA/SF/CD-I/SA dressing, cutting, packaging, and performing cobalt 60 irradiation sterilization to obtain the CTS-PVA/SF/CD-I/SA dressing.
EXAMPLE 8 preparation of burn wound dressing sample 4
1) Dissolving the elementary iodine in 75% ethanol solution to prepare iodine with mass fraction of 5%. Adding 5% iodine tincture into 2% polyvinylpyrrolidone solution, and performing ultrasonic treatment for 30 min. Evaporating the liquid by a rotary evaporator, and collecting solid powder for later use;
2) heating 20mL of 4% silk fibroin solution at 95 ℃ for 2h, adding 1mL of glycerol, mechanically stirring for 10min, adding 10mL of 5% polyvinyl alcohol solution, mechanically stirring for 10min to form white emulsion, adding 10mL of 2% chitosan solution into the white emulsion, and mechanically stirring for 30min for later use;
3) dissolving 1g of the product obtained in the step 1) in 5mL of deionized water, adding the solution into the product obtained in the step 2), mechanically stirring for 10min, pouring the solution into a container with the size of 100 multiplied by 150mm, standing the solution at the temperature of 4 ℃ for 1h, standing the solution at the temperature of-20 ℃ for 4h, standing the solution at the temperature of-70 ℃ for 6h, and freeze-drying the solution by a freeze dryer to obtain CTS-PVA/SF/PP-I sponge for later use;
4) allowing CTS-PVA/SF/PP-I sponge to fully absorb deionized water, standing at-20 ℃ for 4h, uniformly pouring 8mL of stearic acid solution (40 mmol/L ethanol, DCC as a dehydrating agent) on the smooth surface of the CTS-PVA/SF/PP-I sponge, freezing for 2h, washing the smooth surface of the CTS-PVA/SF/PP-I dressing with absolute ethanol at 20 ℃ for 3 times, drying to obtain the CTS-PVA/SF/PP-I/SA dressing, cutting, packaging, and performing cobalt 60 irradiation sterilization to obtain the CTS-PVA/SF/PP-I/SA dressing.
Example 9 physical and structural characterization
The samples of the burned wound dressings prepared in examples 5-8 were elastic, curlable, and free of off-flavor. General structural observation was performed and microstructure observation was performed using a scanning electron microscope, as represented by the burned wound dressing sample 1 prepared in example 5. As shown in FIG. 1, the samples are all interconnected porous structures, and the porosity is between 60% and 80%. After the mutually penetrated pore structure is contacted with body fluid, the pore structure of the pore structure quickly sucks the body fluid into the pores, and the water-absorbing polymer quickly gelatinizes, so that on one hand, the pore wall thickens and gelatinizes after absorbing water, the tube cavity narrows, and the viscoelasticity of the tube wall increases; on the other hand, the rapidly gelled dressing begins to slowly release active iodine, and has the functions of broad-spectrum antibiosis and healing promotion.
Example 10 physical Property characterization
The burn wound dressings prepared in examples 5 to 8 were subjected to a moisture retention test, and the control group was a general burn wound dressing.
The results show that the burn wound dressings obtained in examples 5 to 8 have a longer moisturizing time than the control group, and the moisturizing time is 15 hours or more.
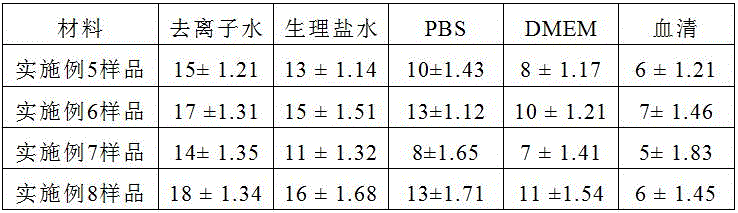
The burn wound dressing samples prepared in examples 5 to 8 were tested for water absorption rate, 0.1g of the samples prepared in examples 5 to 8 were accurately weighed, respectively, immersed in deionized water (pH = 7.0), physiological saline, phosphate buffer, DMEM medium, and blood serum, completely swollen by absorbing water at 37 ℃, surface water was removed, the samples were weighed for mass after imbibing, and the water absorption rate of the samples was calculated. The water absorption multiplying power Q calculation formula is as follows: q = (M2-M1)/M1, wherein Q is the water (saline) absorption multiplying power and the unit is g/g; m1 is the mass of the sample before imbibition, and the unit is g; m2 is the mass of the sample after imbibition in g.
As a result, as shown in Table 2, samples 1 to 4 of the burn wound dressing exhibited similar water absorption properties. Taking the example of the burn wound dressing sample 1 prepared in example 5, the water absorption rates in different media were: the water absorption rate in deionized water is 14-16; the water absorption rate in saline water is 12-14; the water absorption rate in the phosphate buffer solution is 9-11; the water absorption rate in the cell culture solution is 7-10; the water absorption rate in serum is 5-7.
TABLE 2 Water absorption Capacity of different media
The burnt wound dressing samples prepared in the examples 5 and 6 are subjected to iodine slow release function test, 0.1g of the sample samples prepared in the examples 5 and 6 are accurately weighed respectively, the sample samples are immersed in physiological saline at 37 ℃, and the content of iodine released by the physiological saline is detected by an inductively coupled plasma spectrometer for 2h, 4h, 8h, 12h, 24h, 36h, 48h, 60h and 72h respectively.
The results are shown in Table 3 below, where the iodine release for both groups of samples increased slowly from 2h until 72h reached a higher level. The burn wound dressing prepared by the invention has a good iodine slow release function, and the burn wound dressing prepared in example 6 has a better iodine slow release effect.
TABLE 3 evaluation of iodine Release Functions
Example 11 in vitro broad-spectrum antioxidant Performance test
The samples of the burn wound dressings prepared in examples 5 to 8 were tested for their broad spectrum antioxidant ability by mixing each of the samples prepared in examples 5 to 8, glutathione solution and deionized water with a mixture containing superoxide anion, hydroxyl radical and H2O2The solution is reacted, and 6 groups of samples are tested to respectively remove superoxide anions, hydroxyl radicals and H by using a superoxide anion test kit, a hydroxyl radical test kit and a hydrogen peroxide quantitative analysis kit2O2The ability of the cell to perform.
As shown in Table 4 below, the samples of the burn wound dressings prepared in examples 5 to 8 and glutathione had good broad-spectrum antioxidant properties, with the sample of the burn wound dressing prepared in example 5 having the best antioxidant effect.
TABLE 4 broad-spectrum antioxidant Properties
Note: "-" indicates no antioxidant effect; "+" indicates a clearance of 10% to 50%; "+ +" indicates a clearance of 50% -90%; "+ + + + +" indicates a clearance > 90%.
EXAMPLE 12 in vitro broad-spectrum antibacterial Performance test
The antibacterial activity of the burn wound dressings prepared in examples 5 to 8 was tested by the bacteriostatic ring method, and the antibacterial activity of the burn wound dressings was evaluated using staphylococcus aureus, drug-resistant staphylococcus aureus, escherichia coli, pseudomonas aeruginosa, drug-resistant pseudomonas aeruginosa, and candida albicans.
After 70. mu.L of the bacterial suspension (1X 108 CFU/mL) was spread on an LB agar plate, sterile gauze, iodine-containing gauze, and the burn wound dressing prepared in examples 5 to 8 were placed on the surface of the agar, and incubated at 37 ℃ for 12 hours, the diameter of the zone of inhibition was measured.
Table 5 below shows the killing effect of the burn wound dressing samples prepared in examples 5-8 and two control groups on 6 strains (Candida albicans, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus aureus resistant strains and Pseudomonas aeruginosa resistant strains). The results of analysis of the size of the zone of inhibition show that the burn wound dressings prepared in examples 5 to 8 have good antibacterial effect; the inhibition zone is smaller than that of iodine-containing gauze group, which shows that the iodine-containing gauze group has good slow release effect.
TABLE 5 evaluation of antibacterial Effect
Note: non-antibacterial "-"; the antibacterial activity is indicated by "+", wherein the diameter of the zone of inhibition is greater than 3mm is indicated by "+".
Example 13 in vivo assessment of infectious wound healing Effect
Shenzhen bay laboratory animal ethics committee approved in vivo animal experiments. 120 BALB/c mice, males, weighing approximately 18g + -2 g each, were randomized into 6 groups (20 mice per group). Pentobarbital sodium (20 mg/kg) is injected into the abdominal cavity to anaesthetize the mice, the skin is removed, the back of each mouse is made into phi 1cm full-layer skin injury, pseudomonas aeruginosa bacterial liquid with the concentration of 1 multiplied by 108 CFU is dripped on the wound surface, and 80 mul of each wound surface forms an infected wound surface. The wound was tightly covered with the samples of examples 5-8 of the present invention, iodine-containing gauze and sterile gauze, respectively. Changes were made 2 times per week. The wound healing effect of the antioxidant antibacterial healing-promoting burn wound dressing is evaluated according to the repairing condition of the infected wound of a BALB/c mouse.
During the experiment, the wounds treated by 6 groups of samples all have scabbing in different degrees, and the wounds shrink. The treatment results are shown in Table 6, and the wound healing of the samples of examples 5-8 of the present invention reached 50.42 + -2.23, 43.32 + -1.21, 48.56 + -2.13 and 40.52 + -1.34 respectively at day 3 after wounding, and the iodine-containing gauze and gauze group reached only 19.34 + -1.32 and 28.23 + -1.08 respectively. Examples 5-8 samples of the treatment groups showed no redness at the wound margins, redness at the wound margins with iodine-containing gauze groups, and some infectious exudate remained in the gauze groups. The healing rate of the treatment groups of the samples of the examples 5-8 is 67.43 +/-2.12-79.73 +/-1.24 through the observation of the wound surface of the mice on the 7 th day after the wound. The iodine-containing gauze has serious adhesion with the wound surface and obvious red and swollen edges of the wound surface. The granulation of the gauze group was evident and a partial inflammatory exudate remained. On day 12 after surgery, the best of the sample treatment groups of example 5 could reach 99.01 ± 1.31, the sample treatment groups of examples 6-8 were 93.24 ± 1.12, 95.41 ± 1.36 and 90.47 ± 1.67, respectively, while the iodine gauze group was only 67.02 ± 1.41, which may be wound injury caused by excess iodine after infection is eliminated.
The 6 groups of samples had significant statistical differences in their healing rates, with the best treatment groups and the second order gauze group of examples 5-8, inferior to iodine-containing gauze. The wound surface repaired by the burn wound dressing prepared in the example 5 is more obvious in shrinkage and optimal in treatment effect.
TABLE 6 wound healing Rate of infectious wound
Claims (14)
1. The composition for preparing the burn wound dressing is characterized by comprising silk fibroin, a framework and active iodine according to a mass ratio of 1: 0.5-2: 0.1-10; the skeleton consists of chitosan and a water-absorbing polymer according to a mass ratio of 1: 0.1-5; the active iodine consists of elementary iodine and a slow release material of iodine.
2. The composition of claim 1, wherein the silk fibroin is native and/or modified silk fibroin; the modified silk fibroin is selected from one or more of silk fibroin with calcium partially or completely removed, silk fibroin subjected to heating treatment and derivatives thereof, silk fibroin subjected to ultraviolet irradiation and derivatives thereof, and silk fibroin subjected to organic solvent treatment and derivatives thereof.
3. The composition according to claim 1, wherein the water-absorbing polymer is selected from natural water-absorbing polymers selected from collagen, gelatin, cellulose and derivatives thereof and/or synthetic water-absorbing polymers selected from polyethylene glycol, polyacrylamide, sodium polyacrylate or polyvinyl alcohol.
4. The composition of claim 1, wherein the slow release material of elemental iodine is selected from the group consisting of polymeric materials comprising starch, cellulose and its derivatives, polyvinylpyrrolidone, polyethylene glycol, and/or small molecule materials comprising alpha-cyclodextrin, beta-cyclodextrin, and gamma-cyclodextrin.
5. The preparation composition for preparing the burn wound dressing is characterized by comprising the silk fibroin, the skeleton, the active iodine and the auxiliary materials according to the mass ratio of 1: 0.5-2: 0.1-10 in the claim 1; the auxiliary materials are plasticizers and/or emulsifiers.
6. A method of making a burn wound dressing, characterized by using the composition of claim 1 or the formulation composition of claim 5, comprising the steps of:
s1: preparing silk fibroin solution and active iodine;
s2: mixing the obtained product of S1 with skeleton and adjuvants.
7. The method of claim 6, further comprising, after S2, modifying the result of S2 with stearic acid S3.
8. The method according to claim 6, wherein S1 is prepared by adding a chelating agent for calcium or an amino acid capable of chelating calcium; the chelating agent of calcium is EDTA and its derivatives, EGTA AM and its derivatives, BAPTA and its derivatives; the amino acid capable of chelating calcium comprises any one or more of glutamic acid, alanine, aspartic acid, phenylalanine, asparaginic acid, arginine, threonine, tyrosine, tryptophan, glycine, serine, valine, histidine, isoleucine, cysteine and derivatives thereof.
9. Burn wound dressing obtainable by the process for the preparation of a burn wound dressing according to claims 6-8.
10. The burn wound dressing of claim 9, wherein the burn wound dressing is a porous structure having a porosity of 55% to 80% and a pore size of 0.5 mm to 2 mm.
11. The burn wound dressing of claim 9, wherein the burn wound dressing has a water absorption rate of 1-20 times; the water absorption multiplying power can be calculated by the following formula: q = (M2-M1)/M1; q is the water absorption multiplying power, and the unit is g/g; m1 is the sample mass before imbibition, in g; m2 is the mass of the sample after pipetting in g.
12. The burn wound dressing of claim 11, wherein said burn wound dressing has a water absorption rate in different media of: the water absorption rate in deionized water is 14-16; the water absorption rate in saline water is 12-14; the water absorption rate in the phosphate buffer solution is 9-11; the water absorption rate in the cell culture solution is 7-10; the water absorption rate in serum is 5-7.
13. The method of adsorbing a liquid with the burn wound dressing of claims 9-12, wherein the burn wound dressing adsorbs a liquid that enters the pore structure of the burn wound dressing, thickening and gelling the walls of the pores of the burn wound dressing to render the lumen void.
14. A method for the microbial barrier comprising the method of claim 13, wherein the fluid is adsorbed by the burn wound dressing, and wherein the microbes are sequestered after the walls of the pores of the burn wound dressing thicken and gel to render the lumen void.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210442226.1A CN114748682A (en) | 2022-04-26 | 2022-04-26 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210442226.1A CN114748682A (en) | 2022-04-26 | 2022-04-26 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN114748682A true CN114748682A (en) | 2022-07-15 |
Family
ID=82333960
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210442226.1A Pending CN114748682A (en) | 2022-04-26 | 2022-04-26 | Composition for preparing burn wound dressing, preparation and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114748682A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118141979A (en) * | 2024-05-13 | 2024-06-07 | 中山大学 | Antibacterial spray dressing composition, preparation method thereof and antibacterial dressing containing antibacterial spray dressing composition |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012112757A2 (en) * | 2011-02-17 | 2012-08-23 | Allergan, Inc. | Compositions and improved soft tissue replacement methods |
| WO2015189586A1 (en) * | 2014-06-12 | 2015-12-17 | Cell Therapy Limited | Hybrid composition |
| US20170027168A1 (en) * | 2015-07-27 | 2017-02-02 | Stephan HEATH | Methods, products, and systems relating to making, providing, and using nanocrystalline (nc) products comprising nanocrystalline cellulose (ncc), nanocrystalline (nc) polymers and/or nanocrystalline (nc) plastics or other nanocrystals of cellulose composites or structures, in combination with other materials |
| CN110903374A (en) * | 2019-12-20 | 2020-03-24 | 重庆医科大学 | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold |
| CN112546289A (en) * | 2020-12-11 | 2021-03-26 | 北京中卫医正科技有限公司 | Composite biological hydrogel dressing and preparation method thereof |
| CN113174074A (en) * | 2021-02-08 | 2021-07-27 | 四川大学华西医院 | Conductive silk fibroin film and preparation method and application thereof |
-
2022
- 2022-04-26 CN CN202210442226.1A patent/CN114748682A/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012112757A2 (en) * | 2011-02-17 | 2012-08-23 | Allergan, Inc. | Compositions and improved soft tissue replacement methods |
| WO2015189586A1 (en) * | 2014-06-12 | 2015-12-17 | Cell Therapy Limited | Hybrid composition |
| US20170027168A1 (en) * | 2015-07-27 | 2017-02-02 | Stephan HEATH | Methods, products, and systems relating to making, providing, and using nanocrystalline (nc) products comprising nanocrystalline cellulose (ncc), nanocrystalline (nc) polymers and/or nanocrystalline (nc) plastics or other nanocrystals of cellulose composites or structures, in combination with other materials |
| CN110903374A (en) * | 2019-12-20 | 2020-03-24 | 重庆医科大学 | Silk fibroin extraction method, composite skin scaffold based on silk fibroin/usnic acid and preparation method of composite skin scaffold |
| CN112546289A (en) * | 2020-12-11 | 2021-03-26 | 北京中卫医正科技有限公司 | Composite biological hydrogel dressing and preparation method thereof |
| CN113174074A (en) * | 2021-02-08 | 2021-07-27 | 四川大学华西医院 | Conductive silk fibroin film and preparation method and application thereof |
Non-Patent Citations (2)
| Title |
|---|
| 张文元等: "壳聚糖-丝素支架与真皮间充质干细胞的体外复合培养", 《中国组织工程研究与临床康复》 * |
| 杨欣等: "壳聚糖在伤口敷料中的应用研究进展", 《精细与专用化学品》 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118141979A (en) * | 2024-05-13 | 2024-06-07 | 中山大学 | Antibacterial spray dressing composition, preparation method thereof and antibacterial dressing containing antibacterial spray dressing composition |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zhang et al. | A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing | |
| Cao et al. | Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy | |
| He et al. | Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing | |
| CN110354295B (en) | Photo-thermal conversion material and preparation method thereof | |
| CN112370567B (en) | Hydrogel active dressing with antibacterial and anti-inflammatory functions | |
| CN112300420B (en) | Injectable antibacterial interpenetrating double-network hydrogel and preparation method and application thereof | |
| CN110152051A (en) | Wound antiseptic dressing and its preparation method and application is burnt in a kind of water suction | |
| Singh et al. | Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties | |
| RU2240830C1 (en) | Wound coating and method for its preparing | |
| CN112250887B (en) | Copper metal organic framework nanoparticle functionalized hydrogel and preparation method and application thereof | |
| Wei et al. | Quaternized chitosan/cellulose composites as enhanced hemostatic and antibacterial sponges for wound healing | |
| CN110975000A (en) | Preparation and application of antibacterial modified exosome burn wound healing promotion biological dressing | |
| CN108853570B (en) | Hemostatic sponge and preparation method thereof | |
| Lin et al. | The role and mechanism of polydopamine and cuttlefish ink melanin carrying copper ion nanoparticles in antibacterial properties and promoting wound healing | |
| Wei et al. | Facile preparation of polyphenol-crosslinked chitosan-based hydrogels for cutaneous wound repair | |
| Cheng et al. | An agar–polyvinyl alcohol hydrogel loaded with tannic acid with efficient hemostatic and antibacterial capacity for wound dressing | |
| CN105228658A (en) | A kind of medical dressing hydrogel compound fabric and its preparation method and application | |
| Cui et al. | A chitosan-based self-healing hydrogel for accelerating infected wound healing | |
| CN103755965A (en) | Polylysine hydrogel and preparation method and application thereof | |
| Han et al. | Hydrogen sulfide-releasing polyurethane/gelatin/keratin–TA conjugate mats for wound healing | |
| Jiang et al. | Carboxymethyl chitosan-based multifunctional hydrogels incorporated with photothermal therapy against drug-resistant bacterial wound infection | |
| Parwani et al. | Gum acacia-PVA hydrogel blends for wound healing | |
| CN114524950A (en) | Magnetic targeting hydrophobic drug carrier hydrogel and preparation method and application thereof | |
| CN116650710A (en) | Mussel inspired multifunctional double-network crosslinked hydrogel wound dressing | |
| Mirjalili et al. | Controlled release of protein from gelatin/chitosan hydrogel containing platelet-rich fibrin encapsulated in chitosan nanoparticles for accelerated wound healing in an animal model |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20220715 |