CN110354295B - Photo-thermal conversion material and preparation method thereof - Google Patents
Photo-thermal conversion material and preparation method thereof Download PDFInfo
- Publication number
- CN110354295B CN110354295B CN201910415080.XA CN201910415080A CN110354295B CN 110354295 B CN110354295 B CN 110354295B CN 201910415080 A CN201910415080 A CN 201910415080A CN 110354295 B CN110354295 B CN 110354295B
- Authority
- CN
- China
- Prior art keywords
- natural
- metal ion
- tannic acid
- mixed solution
- combining
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K41/00—Medicinal preparations obtained by treating materials with wave energy or particle radiation ; Therapies using these preparations
- A61K41/0052—Thermotherapy; Hyperthermia; Magnetic induction; Induction heating therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/18—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/20—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing organic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/28—Polysaccharides or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/32—Proteins, polypeptides; Degradation products or derivatives thereof, e.g. albumin, collagen, fibrin, gelatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/425—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/46—Deodorants or malodour counteractants, e.g. to inhibit the formation of ammonia or bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/62—Compostable, hydrosoluble or hydrodegradable materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0015—Medicaments; Biocides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0036—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0042—Materials resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/02—Surgical adhesives or cements; Adhesives for colostomy devices containing inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/04—Surgical adhesives or cements; Adhesives for colostomy devices containing macromolecular materials
- A61L24/08—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/04—Surgical adhesives or cements; Adhesives for colostomy devices containing macromolecular materials
- A61L24/10—Polypeptides; Proteins
- A61L24/108—Specific proteins or polypeptides not covered by groups A61L24/102 - A61L24/106
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/04—Materials for stopping bleeding
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Hematology (AREA)
- Surgery (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Medicinal Preparation (AREA)
- Materials For Medical Uses (AREA)
Abstract
The invention provides a tannin/metal ion complex-based NIR photothermal conversion material and a preparation method thereof, and mainly aims to prepare TA and metal ions (Fe) with good biocompatibility3+、Ru3+、V3+) Adding into medical material, using TA and metal ion (Fe)3+、Ru3+、V3+) The NIR photothermal conversion effect of the formed complex enables the material to generate higher thermal effect under the stimulation of NIR, thereby achieving the aim of antibiosis. The material has excellent liquid absorption capacity when being used as a photo-thermal antibacterial hemostatic skin dressing, and the TA/metal ions significantly improve the cell/tissue affinity of the material, thereby having wide application prospect in the field of medical treatment.
Description
Technical Field
The invention belongs to the technical field of biological materials, and particularly relates to a photothermal conversion and antibacterial material.
Technical Field
Natural polymer-based skin dressings (films, cryogels, hydrogels, etc.) are widely used for wound healing due to their excellent biocompatibility and biodegradability. However, most natural polymer dressings do not have antibacterial activity and it is difficult to avoid microbial infection during wound healing. Currently, the addition of antibacterial agents (antibiotics, metal ions and quaternary ammonium salts) is a common means of improving the antibacterial activity of wound dressings. However, the widespread use of antibiotics may lead to the development of bacterial resistance, and the long-term cytotoxicity of metal ions and quaternary ammonium salts will greatly reduce the rate of wound healing.
In recent years, Near Infrared (NIR) photothermal therapy of bacterial infections has received much attention from researchers because of its ability to effectively circumvent many of the problems associated with the use of traditional antibiotics, such as narrow antimicrobial spectrum, drug resistance, and high drug toxicity. The near infrared photo-thermal antibacterial technology does not have specific targeting, so that the generation of drug-resistant bacteria can not be induced while bacteria are killed. Currently, a large number of near-infrared photosensitizers are: noble metal nanoparticles, carbon nanomaterials, oxides of metals and nonmetals, some polymeric photosensitizers, etc. have been gradually applied to near-infrared photothermal antibacterial and antitumor treatments. However, these near-infrared photosensitizers still have problems such as high cost, low thermal efficiency of near-infrared light, poor cell compatibility, and the like.
Disclosure of Invention
Aiming at the defects of the prior art, the invention provides an NIR photothermal conversion material based on a tannic acid/metal ion complex and a preparation method thereof, and the medical photothermal antibacterial and tumor treatment material which has good cell compatibility, high near infrared thermal efficiency, low cost and no toxic or side effect is obtained.
The main technical idea of the invention is that Tannic Acid (TA) is a polyphenol compound of natural plant origin, which has the characteristics of oxidation resistance, free radical capture and bacteriostasis. TA and metal ions (Fe) with good biocompatibility3+、Ru3+、V3+) Adding into medical material, using TA and metal ion (Fe)3+、Ru3+、V3+) The NIR photothermal conversion effect of the formed complex enables the material to generate higher thermal effect under the stimulation of NIR, thereby achieving the aim of antibiosis. Meanwhile, because the material has a porous structure,has excellent imbibition and hemostatic effects. In addition, NIR illumination is beneficial to tissue regeneration, plays a positive role in promoting wound healing, and has wide application prospects in the aspects of protecting wounds from bacterial infection and promoting tissue repair. The tannin/metal ion complex has wide raw material source, simple preparation process and low cost, is an ideal NIR photothermal conversion material, can be widely applied to the field of medical treatment, can greatly reduce the treatment cost, and has practical clinical application value.
The invention provides a material, in particular to a NIR photothermal conversion material based on tannic acid/metal ion complex, which comprises tannic acid/metal ion complex, wherein the metal ion is Fe3+、Ru3+、V3+At least one of (1).
Further, the material has good cell, tissue affinity and compatibility, and further is a natural material, and preferably, the natural material is selected from one or two of natural polysaccharide and natural protein.
The invention provides a medical material, in particular to a NIR photothermal antibacterial material based on a tannic acid/metal ion complex, which comprises the tannic acid/metal ion complex, natural polysaccharide and natural protein.
Furthermore, the natural polysaccharide and the natural protein form a porous scaffold, and TA and metal ions are adsorbed on the surface and in the pore structure of the porous scaffold.
Further, in the tannin/metal ion complex, the metal ion is Fe3+、Ru3+、V3+At least one of (1). The complex formed by the metal ions and TA has better photothermal conversion capability, generates higher thermal effect under the stimulation of NIR, effectively kills bacteria at a wound part and promotes the healing of tissues.
Further, according to the mass ratio, the natural polysaccharide: natural protein: TA: metal ions (50-80) and (20-50) and (0.1-10): (0.05-5). Preferably, the mass ratio of the natural polysaccharide to the natural protein is 2:1, and the mechanical property of the scaffold material is better than that of other scaffold materials in the mass ratio.
Further, the natural polysaccharide is at least one of chitin, chitosan, cellulose, konjac glucan, sodium alginate, hyaluronic acid, starch and spirulina polysaccharide.
Further, the natural protein is at least one of silk fibroin, fibronectin, albumin, gelatin and collagen. The natural protein has better biocompatibility and can promote the proliferation of cells.
The medical material provided by the invention can be used for photothermal treatment and photothermal anti-tumor treatment of bacterial infection. In particular, it can be used as antibacterial skin dressing, wound dressing and hemostatic material. The wound dressing and the hemostatic material have excellent liquid absorption capacity, can absorb blood exuded from the wound and perform rapid hemostasis on the wound; in addition, the dressing can convert external near infrared stimulation into local high heat, and the local high heat can effectively inhibit or kill bacteria so as to avoid bacterial infection during wound healing; meanwhile, TA/metal ions can obviously improve the cell/tissue affinity of the dressing, and further can promote skin tissue regeneration and accelerate wound healing.
The preparation method of the NIR photothermal antibacterial material provided by the invention comprises the following steps:
(1) a tannic acid/metal ion complex, or tannic acid and metal ions;
(2) a native protein;
(3) a natural polysaccharide;
(4) mixing in a solvent; further, the solvent is selected from water;
(5) and (5) freeze drying.
In the above method, the above materials can be obtained by combining the contents of items (1) to (4) at the same time, or by combining two items (1) to (3) with item (4) first and then combining the resultant with the remaining items (1) to (3) and item (4), for example, by combining items (2), (3) and (4) first and then combining the resultant with items (1) and (4) and freeze-drying the resultant mixture; for example, the materials are obtained by combining the materials (1), (2) and (4) and then combining the obtained substances with the materials (3) and (4) and freeze-drying the obtained mixture; for example, the material can be obtained by combining the components (1), (3) and (4), combining the obtained substance with the components (2) and (4), and freeze-drying the obtained mixture.
The mixing may be carried out by a dropwise manner, for example, by adding a solution of metal ions dropwise to the solution in which tannic acid is dispersed.
Further, the natural polysaccharide and the natural protein are dissolved in water to prepare a natural polysaccharide/natural protein mixed solution with the total concentration of the natural polysaccharide and the natural protein being 10-100 mg/mL.
Further, the amount of tannic acid added is such that the concentration of tannic acid in the mixed solution is 0.1 to 10 mg/mL.
Further, the dropping amount of the metal ion solution is such that the concentration of the metal ions in the mixed solution obtained after the dropping is 0.05-5 mg/mL.
The invention provides a composition, which comprises natural polysaccharide, natural protein, TA and metal ions, wherein the natural polysaccharide comprises the following components in parts by mass: natural protein: TA: metal ions (50-80) and (20-50) and (0.1-10): (0.05-5); further, the metal ion is Fe3+、Ru3+、V3+At least one of (1).
In the composition: TA and metal ions can form a stable complex, the complex has the effect of photothermal conversion and good cell compatibility, and the complex can be combined with natural polysaccharide and natural protein to obtain a medical material with photothermal antibacterial effect, so that the medical material is applied to photothermal antibacterial treatment and photothermal antitumor treatment.
Compared with the prior art, the invention has the beneficial effects that:
1. the invention provides a novel photothermal antibacterial medical material, a wound dressing and a hemostatic material, which are prepared from natural animals and plants, have good biocompatibility and biodegradability, and meanwhile, the TA/metal ion complex has the advantages of simple preparation method, high photothermal conversion efficiency and excellent cell/affinity.
2. The photothermal antibacterial material prepared by the invention has high near-infrared photothermal conversion efficiency of TA/metal ions, is natural, has no toxic or side effect, has excellent cell/affinity, and can effectively avoid the problems of long-term cytotoxicity, drug resistance and the like of the conventional antibacterial dressing. In addition, compared with the traditional photothermal agent, TA/metal ions have excellent tissue affinity, and can accelerate cell proliferation, promote tissue regeneration and accelerate wound healing.
3. The antibacterial material prepared by the invention has excellent liquid absorption capacity when being used as a hemostatic material, and can rapidly absorb and stop blood at the wound surface.
Drawings
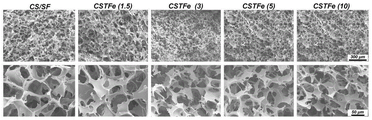
Fig. 1 is a topographical view of the antimicrobial skin wound dressing prepared in example 1.
Fig. 2 is a graph of the in vitro antimicrobial performance of the antimicrobial skin wound dressing prepared in example 1.
Fig. 3 is a graph of the hemostatic properties of the antimicrobial skin wound dressing prepared in example 1.
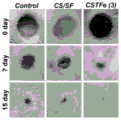
Fig. 4 is the in vivo skin repair results for the antimicrobial skin wound dressing prepared in example 1.
Detailed Description
The present invention will be described in further detail with reference to examples. It should be understood that the specific examples described herein are intended to be illustrative only and are not intended to be limiting. Any modification made without departing from the spirit and principle of the present invention and equivalent replacement or improvement made by the common knowledge and conventional means in the field shall be included in the protection scope of the present invention.
Example 1
A. The chitosan and silk fibroin were dissolved in water to prepare a 30mg/mL chitosan/silk fibroin (CS/SF) mixture (chitosan/silk fibroin mass ratio: 2: 1).
B. Uniformly dispersing TA into the chitosan/silk fibroin mixed solution prepared in the step A to ensure that the concentration of the TA is 0.3mg/mL, and then dropwise adding Fe into the mixed solution3+The solution was mixed so that the concentration of iron ions in the mixture was 0.15 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing. The prepared dressing is subjected to shape characterization and knottingAs shown in fig. 1, the resulting dressing is seen to have a rich porous structure. CSTFE (1.5) refers to a scaffold structure composed of chitosan, silk protein, tannin, and iron ion, wherein TA and Fe3+The contents of (b) were 150. mu.g/mL and 75. mu.g/mL, respectively. CSTFE (3), CSTFE (5), CSTFE (10) represent that the TA content increases sequentially, 300. mu.g/mL, 500. mu.g/mL, 1000. mu.g/mL. Therefore, as the TA content is increased, the size of the pores is shrunk, but the good porous morphology is still maintained, so that the material has good repeated compression and moisture absorption performance.
The prepared dressing was subjected to an antibacterial test
The test method comprises the following steps: the prepared antibacterial skin dressing is prepared in advance and sterilized, then the sterilized antibacterial skin dressing is flatly laid at the bottom of a pore plate, and escherichia coli or staphylococcus aureus (106CFU) is added. The antibacterial dressing was replaced with pure chitosan/fibroin (tannic acid and metal ions were not introduced) and a pure chitosan/fibroin group was set according to the same method, and a group of blank control groups was set. The three groups are respectively arranged in parallel, one group is subjected to near-infrared illumination, and the other group is not subjected to illumination. All three groups were oven-cultured at 37 ℃ for 12 hours. And (4) carrying out solid plate experiment on the incubated bacterial liquid, then culturing for 12 hours, and photographing to count bacterial colonies.
And (3) testing results: the obtained dressing has good photothermal antibacterial effect as shown in fig. 2. Compared with a control group and a pure chitosan/fibroin group, the antibacterial dressing group has a better photothermal effect, and the antibacterial rate of the antibacterial dressing group to gram-positive bacteria (staphylococcus aureus) or gram-negative bacteria (escherichia coli) can reach nearly 100%.
Hemostasis test was performed on the prepared dressing
The test method comprises the following steps: the SD rat is subjected to tail breaking model establishment, one third of the tail of the rat is cut off for setting four groups, and gauze, chitosan/silk protein and the prepared protein containing TA/Fe are respectively used3+The dressing of (a) was subjected to hemostatic treatment, and the dressing without any treatment was used as a control group. A piece of clean filter paper is placed at the bottom of the dressing, the blood loss is counted, and a picture is taken.
And (3) testing results: as shown in fig. 3, the antibacterial dressing group prepared by the invention has better hemostatic effect, and can play a role in hemostasis within 30 seconds.
Skin tissue repair experiment is carried out on the prepared auxiliary materials
The experimental method comprises the following steps: a circular defect model of 8 mm in diameter was created on the back of the mouse, and then the sterilized dressing prepared according to the present invention was spread on the wound surface while a control group was set without any treatment. The mouse defect sites were photographed on days 0,7 and 15, respectively, and the skin repair effect was compared.
The experimental results are as follows: as shown in figure 4, the dressing prepared by the invention can effectively promote skin tissue repair, has better capability of promoting tissue repair, can completely heal the wound within 15 days, and can repair the tissue.
Example 2
A. Hyaluronic acid and collagen were dissolved in water to prepare a hyaluronic acid/collagen mixture solution of 30mg/mL (hyaluronic acid/collagen mass ratio: 1).
B. Uniformly dispersing TA into the mixed solution of hyaluronic acid/collagen prepared in the step A to make the concentration of the mixed solution of hyaluronic acid/collagen be 0.2mg/mL, and then dropwise adding V into the mixed solution3+The solution was mixed so that the concentration of vanadium ions in the mixture was 0.1 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 3
A. Sodium alginate and albumin were dissolved in water to prepare a 40mg/mL sodium alginate/albumin mixture (sodium alginate/albumin mass ratio: 3: 1).
B. Uniformly dispersing TA into the mixed solution of sodium alginate/albumin prepared in the step A to ensure that the concentration of the TA is 1mg/mL, and then dropwise adding Fe into the mixed solution3+And the concentration of iron ions in the mixed solution was adjusted to 0.5 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 4
A. The chitosan and the silk fibroin were dissolved in water to prepare a 30mg/mL chitosan/silk fibroin mixed solution (the mass ratio of chitosan/silk fibroin was 2: 1).
B. Uniformly dispersing TA into the chitosan/silk fibroin mixed solution prepared in the step A to ensure that the concentration of the TA is 0.3mg/mL, and then dropwise adding Fe into the mixed solution3+And the concentration of iron ions in the mixed solution was adjusted to 0.15 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 5
A. The chitosan and gelatin were dissolved in water to prepare a chitosan/gelatin mixture (chitosan/gelatin mass ratio: 3: 1) of 40 mg/mL.
B. Uniformly dispersing TA into the chitosan/gelatin mixed solution prepared in the step A to ensure that the concentration of the TA is 1mg/mL, and then dropwise adding Fe into the mixed solution3+And the concentration of iron ions in the mixed solution was adjusted to 0.5 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 6
A. The chitosan and the silk fibroin were dissolved in water to prepare a 30mg/mL chitosan/silk fibroin mixed solution (the mass ratio of chitosan/silk fibroin was 2: 1).
B. Uniformly dispersing TA into the chitosan/silk fibroin mixed solution prepared in the step A to ensure that the concentration of the TA is 0.3mg/mL, and then dropwise adding Ru into the mixed solution3+And making Ru in the mixed solution3+The concentration of (2) was 0.15 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 7
A. Cellulose and silk fibroin fibers were dissolved in water to prepare a 40mg/mL mixed solution of cellulose and silk fibroin fibers (mass ratio of cellulose to silk fibroin fibers is 1: 1).
B. Uniformly dispersing TA into the chitosan/silk fibroin mixed solution prepared in the step A to ensure that the concentration of the TA is 0.6mg/mL, and then dropwise adding Ru into the mixed solution3+And the concentration of the metal ions in the mixed solution was adjusted to 0.3 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Example 8
A. Konjac dextran and fibronectin were dissolved in water to prepare a 30mg/mL mixed solution of Konjac dextran and fibronectin (the mass ratio of Konjac dextran to fibronectin was 2: 1).
B. Uniformly dispersing TA into the chitosan/silk fibroin mixed solution prepared in the step A to ensure that the concentration of the TA is 3mg/mL, and then dropwise adding V into the mixed solution3+And the concentration of the metal ions in the mixed solution was adjusted to 2 mg/mL.
C. And C, placing the mixed solution prepared in the step B into a mold, transferring the mixed solution into a low-temperature environment to be condensed into ice, and finally drying the mixed solution by utilizing a freeze drying technology to form the near-infrared photo-thermal antibacterial skin dressing.
Claims (8)
1. The NIR photothermal antibacterial medical material is characterized by comprising a tannin/metal ion complex, natural polysaccharide and natural protein, wherein the natural polysaccharide comprises the following components in parts by mass: natural protein: tannic acid: metal ion = (50-80): (20-50): 0.1-10): (0.05-5); the natural polysaccharide and the natural protein form a porous bracket, and the tannic acid and the metal ions are adsorbed on the surface and in the pore structure of the porous bracket;
the preparation method of the material comprises the following steps:
(1) a tannic acid/metal ion complex, or tannic acid and metal ions;
(2) a native protein;
(3) a natural polysaccharide;
(4) mixing in a solvent;
(5) freeze drying;
the material can be obtained by combining the items (1) to (4) at the same time, or by combining two of the items (1) to (3) with the item (4) first, and then combining the resultant with the remaining items (1) to (3) and the item (4), and freeze-drying the resultant mixture.
2. The material of claim 1, wherein the tannin/metal ion complex comprises a metal ion selected from the group consisting of Fe and Fe3+、Ru3+、V3+At least one of (1).
3. The material of claim 1, wherein the mass ratio of the natural polysaccharide to the natural protein is 2: 1.
4. The material of claim 3, wherein the natural polysaccharide is at least one of chitin, chitosan, cellulose, konjac glucan, sodium alginate, hyaluronic acid, starch, and spirulina polysaccharide; the natural protein is at least one of silk fibroin, silk fibroin fiber, fibronectin, albumin, gelatin and collagen.
5. A method for preparing a material according to claim 1, characterized in that it comprises the following steps:
(1) a tannic acid/metal ion complex, or tannic acid and metal ions;
(2) a native protein;
(3) a natural polysaccharide;
(4) mixing in a solvent;
(5) freeze drying;
the material can be obtained by combining the items (1) to (4) at the same time, or by combining two of the items (1) to (3) with the item (4) first, and then combining the resultant with the remaining items (1) to (3) and the item (4), and freeze-drying the resultant mixture.
6. The method of claim 5, wherein: the solvent is selected from water.
7. The method according to claim 5, wherein the natural polysaccharide and the natural protein are dissolved in water to prepare a mixed solution, the tannic acid is dispersed in the obtained solution, the obtained solution is mixed with the metal ion solution, and finally the obtained mixture is freeze-dried.
8. Use of a material according to any one of claims 1 to 4 in the manufacture of an adjuvant for promoting skin tissue repair.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910415080.XA CN110354295B (en) | 2019-05-17 | 2019-05-17 | Photo-thermal conversion material and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910415080.XA CN110354295B (en) | 2019-05-17 | 2019-05-17 | Photo-thermal conversion material and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN110354295A CN110354295A (en) | 2019-10-22 |
| CN110354295B true CN110354295B (en) | 2021-12-07 |
Family
ID=68215551
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201910415080.XA Active CN110354295B (en) | 2019-05-17 | 2019-05-17 | Photo-thermal conversion material and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110354295B (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110665051B (en) * | 2019-10-23 | 2022-05-20 | 四川大学 | Preparation method of hemostatic and antibacterial frozen gel stent |
| CN113633627B (en) * | 2020-04-24 | 2023-06-16 | 华中科技大学 | Transparent photo-thermal antibacterial hydrogel patch, preparation and application thereof |
| CN112245651A (en) * | 2020-10-09 | 2021-01-22 | 苏州康瑞健生物医疗科技有限公司 | Temperature-sensitive composite antibacterial hydrogel with good photothermal effect and application method and application thereof |
| CN112316204B (en) * | 2020-11-13 | 2021-08-27 | 四川大学 | Metal polyphenol collagen membrane material, preparation method and application thereof |
| CN114181425B (en) * | 2020-12-18 | 2023-04-07 | 青岛尼希米生物科技有限公司 | Flame-retardant, antibacterial and mildewproof cellulose-based foam material as well as preparation method and application thereof |
| KR102512038B1 (en) * | 2021-03-23 | 2023-03-21 | 충남대학교산학협력단 | Mask pack for LED and preparation method thereof |
| CN113069588B (en) * | 2021-04-17 | 2022-04-22 | 北京化工大学 | Application of polyphenol metal ion coagulation promoting coating in preparation of hemostatic material |
| CN113413468B (en) * | 2021-06-29 | 2023-05-12 | 首都医科大学附属北京儿童医院 | Photothermal-hardening combined treatment targeting nano-drug delivery system |
| CN113712044A (en) * | 2021-08-18 | 2021-11-30 | 华南理工大学 | Modified gold nanorod photothermal bacteriostatic preparation as well as preparation method and application thereof |
| CN114031807B (en) * | 2021-11-19 | 2022-12-02 | 浙江海洋大学 | Cellulose chitosan copper tannate composite gel sponge for healing and repairing tissue wounds and preparation method thereof |
| CN114682178B (en) * | 2022-04-07 | 2023-02-10 | 合肥工业大学 | Shape memory type composite aerogel for inhibiting biofouling, preparation method and application thereof |
| CN115501173B (en) * | 2022-08-31 | 2024-07-09 | 四川大学 | Multistage pore hydrogel drug slow-release system based on natural polyphenol and preparation method thereof |
| CN115429927A (en) * | 2022-09-14 | 2022-12-06 | 广东省东莞市质量监督检测中心 | Photo-thermal antibacterial dressing prepared from sodium alginate and apple polyphenol as well as preparation method and application of photo-thermal antibacterial dressing |
| CN116251233B (en) * | 2023-05-16 | 2023-08-01 | 北京大学第三医院(北京大学第三临床医学院) | High-adhesion hydrogel for promoting meniscus injury repair and preparation method thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101783448B1 (en) * | 2014-05-15 | 2017-09-29 | 포항공과대학교 산학협력단 | Bioadhesive hydrogel with surface-modified nanofiber |
| EP3177332B1 (en) * | 2014-08-08 | 2021-06-30 | Surmodics, Inc. | Article coatings including oligomerized polyphenol layer and biological methods of use |
| CN106540308A (en) * | 2016-11-29 | 2017-03-29 | 温州生物材料与工程研究所 | A kind of degradable multiporous microsphere and Preparation method and use |
| CN108341973A (en) * | 2018-03-22 | 2018-07-31 | 吉林大学 | A kind of preparation method of high strength ionic response lubricating hydrogel |
| CN108653741B (en) * | 2018-05-24 | 2021-06-25 | 华中科技大学同济医学院附属协和医院 | Metal organic coordination polymer coated natural sericin microsphere and preparation method and application thereof |
| CN109651624A (en) * | 2018-12-13 | 2019-04-19 | 福建农林大学 | A kind of high tenacity is freeze proof/heat resistanceheat resistant/antibacterial plant polyphenol nano-cellulose conductive hydrogel preparation method |
-
2019
- 2019-05-17 CN CN201910415080.XA patent/CN110354295B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN110354295A (en) | 2019-10-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110354295B (en) | Photo-thermal conversion material and preparation method thereof | |
| Chen et al. | Photothermal-promoted multi-functional dual network polysaccharide hydrogel adhesive for infected and susceptible wound healing | |
| Han et al. | Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion | |
| WO2019091150A1 (en) | Alginate wound repair dressing and preparation method thereof | |
| CN101927029B (en) | Preparation method of chitosan/polyvinyl alcohol sponge dressing containing nano-silver | |
| CN112480434B (en) | Copper ion antibacterial hydrogel and preparation method and application thereof | |
| CN1810298A (en) | Bacteriostatic porous polyelectrolyte material and its prepn process | |
| CN103356692A (en) | Composite antibacterial gel and preparation method thereof | |
| Wei et al. | EGCG-crosslinked carboxymethyl chitosan-based hydrogels with inherent desired functions for full-thickness skin wound healing | |
| CN111035801B (en) | Silver nanocluster based chitosan hydrogel dressing and preparation method and application thereof | |
| Jiang et al. | Carboxymethyl chitosan-based multifunctional hydrogels incorporated with photothermal therapy against drug-resistant bacterial wound infection | |
| CN106110383A (en) | A kind of chitosan alginate dressing and freeze-drying process thereof | |
| CN112451738B (en) | Silver ion polysaccharide polymer antibacterial dressing and preparation method and application thereof | |
| CN110152055A (en) | The functional drug that alginic acid amination derivative/bacteria cellulose nanocomposite gel is constructed is sustained medical dressing | |
| CN106344954A (en) | Bio-antimicrobial bacterial cellulose dressing and preparation method thereof | |
| Ma et al. | Collagen Scaffolds Functionalized by Cu2+‐Chelated EGCG Nanoparticles with Anti‐Inflammatory, Anti‐Oxidation, Vascularization, and Anti‐Bacterial Activities for Accelerating Wound Healing | |
| CN115487337A (en) | Dressing patch for skin repair and preparation method thereof | |
| CN104740141B (en) | A kind of antimicrobial spray and preparation method thereof | |
| Fang et al. | Highly water-absorptive and antibacterial hydrogel dressings for rapid postoperative detumescence | |
| CN110124082A (en) | Swelling type medical bio gel filler based on Polysaccharide from Portulaca oleracea and chromocor extract | |
| CN113509591A (en) | Antibacterial cationic injectable hydrogel dressing and preparation method thereof | |
| Jiang et al. | Muscle-inspired lamellar chitosan sponge with photothermal antibacterial and antioxidant properties for hemostasis and accelerated bacteria infected wound healing | |
| CN115850733B (en) | Nanoclay hydrogel for injection and preparation method and application thereof | |
| CN115624647B (en) | Biological film medical dressing compounded with wound healing medicine and film essence, and preparation method and application thereof | |
| CN111658815A (en) | Antibacterial alginate dressing and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |