WO2020040298A1 - 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 - Google Patents
有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 Download PDFInfo
- Publication number
- WO2020040298A1 WO2020040298A1 PCT/JP2019/033069 JP2019033069W WO2020040298A1 WO 2020040298 A1 WO2020040298 A1 WO 2020040298A1 JP 2019033069 W JP2019033069 W JP 2019033069W WO 2020040298 A1 WO2020040298 A1 WO 2020040298A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- component
- ring
- aryl
- alkyl
- compound
- Prior art date
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 450
- 239000011254 layer-forming composition Substances 0.000 title claims description 18
- 238000005286 illumination Methods 0.000 title description 3
- 230000003111 delayed effect Effects 0.000 claims abstract description 83
- 229910052796 boron Inorganic materials 0.000 claims abstract description 82
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical group [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims abstract description 77
- 238000002189 fluorescence spectrum Methods 0.000 claims abstract description 57
- 239000000126 substance Substances 0.000 claims abstract description 45
- -1 arylheteroarylamino Chemical group 0.000 claims description 337
- 239000010410 layer Substances 0.000 claims description 287
- 125000003118 aryl group Chemical group 0.000 claims description 248
- 125000000217 alkyl group Chemical group 0.000 claims description 221
- 125000001072 heteroaryl group Chemical group 0.000 claims description 201
- 239000000203 mixture Substances 0.000 claims description 109
- 125000004432 carbon atom Chemical group C* 0.000 claims description 102
- 229910052739 hydrogen Inorganic materials 0.000 claims description 98
- 239000001257 hydrogen Substances 0.000 claims description 98
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 90
- 125000003545 alkoxy group Chemical group 0.000 claims description 65
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 64
- 125000004986 diarylamino group Chemical group 0.000 claims description 64
- 229920000642 polymer Polymers 0.000 claims description 64
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 56
- 230000005284 excitation Effects 0.000 claims description 52
- 125000004104 aryloxy group Chemical group 0.000 claims description 49
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 43
- 239000002904 solvent Substances 0.000 claims description 41
- 229910052717 sulfur Inorganic materials 0.000 claims description 41
- 238000001296 phosphorescence spectrum Methods 0.000 claims description 35
- 230000036961 partial effect Effects 0.000 claims description 32
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical group [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 30
- 229910052805 deuterium Chemical group 0.000 claims description 30
- 229910052736 halogen Inorganic materials 0.000 claims description 30
- 150000002367 halogens Chemical group 0.000 claims description 30
- 150000002431 hydrogen Chemical class 0.000 claims description 30
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 28
- 229910052757 nitrogen Inorganic materials 0.000 claims description 27
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 26
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 26
- 229910052782 aluminium Inorganic materials 0.000 claims description 25
- 125000000732 arylene group Chemical group 0.000 claims description 23
- 238000000576 coating method Methods 0.000 claims description 22
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 19
- 239000003960 organic solvent Substances 0.000 claims description 19
- 229910052799 carbon Inorganic materials 0.000 claims description 18
- 239000011248 coating agent Substances 0.000 claims description 18
- 125000005647 linker group Chemical group 0.000 claims description 16
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 15
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzonitrile Chemical compound N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 claims description 15
- 238000009835 boiling Methods 0.000 claims description 15
- 238000005401 electroluminescence Methods 0.000 claims description 15
- 125000005549 heteroarylene group Chemical group 0.000 claims description 15
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 15
- 229910052760 oxygen Inorganic materials 0.000 claims description 15
- 125000004429 atom Chemical group 0.000 claims description 14
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 claims description 13
- 229910052698 phosphorus Inorganic materials 0.000 claims description 13
- 125000003107 substituted aryl group Chemical group 0.000 claims description 13
- 125000005110 aryl thio group Chemical group 0.000 claims description 12
- 125000005553 heteroaryloxy group Chemical group 0.000 claims description 12
- 125000005368 heteroarylthio group Chemical group 0.000 claims description 12
- 125000001181 organosilyl group Chemical class [SiH3]* 0.000 claims description 12
- 101150112582 dad2 gene Proteins 0.000 claims description 11
- 125000005240 diheteroarylamino group Chemical group 0.000 claims description 11
- 125000005346 substituted cycloalkyl group Chemical group 0.000 claims description 11
- 238000000862 absorption spectrum Methods 0.000 claims description 10
- 230000014509 gene expression Effects 0.000 claims description 9
- KZTYYGOKRVBIMI-UHFFFAOYSA-N diphenyl sulfone Chemical compound C=1C=CC=CC=1S(=O)(=O)C1=CC=CC=C1 KZTYYGOKRVBIMI-UHFFFAOYSA-N 0.000 claims description 7
- 229910052733 gallium Inorganic materials 0.000 claims description 7
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 6
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 claims description 6
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 claims description 6
- 229910052785 arsenic Inorganic materials 0.000 claims description 6
- 150000002894 organic compounds Chemical class 0.000 claims description 6
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 claims description 6
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 claims description 6
- 150000003852 triazoles Chemical class 0.000 claims description 6
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 claims description 5
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 claims description 5
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 5
- 229910052801 chlorine Inorganic materials 0.000 claims description 5
- 229910052740 iodine Inorganic materials 0.000 claims description 5
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 claims description 5
- TZMSYXZUNZXBOL-UHFFFAOYSA-N 10H-phenoxazine Chemical compound C1=CC=C2NC3=CC=CC=C3OC2=C1 TZMSYXZUNZXBOL-UHFFFAOYSA-N 0.000 claims description 4
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 claims description 4
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 claims description 4
- 239000012965 benzophenone Substances 0.000 claims description 4
- LAQPNDIUHRHNCV-UHFFFAOYSA-N isophthalonitrile Chemical compound N#CC1=CC=CC(C#N)=C1 LAQPNDIUHRHNCV-UHFFFAOYSA-N 0.000 claims description 4
- XQZYPMVTSDWCCE-UHFFFAOYSA-N phthalonitrile Chemical compound N#CC1=CC=CC=C1C#N XQZYPMVTSDWCCE-UHFFFAOYSA-N 0.000 claims description 4
- 229920006391 phthalonitrile polymer Polymers 0.000 claims description 4
- 229910052711 selenium Inorganic materials 0.000 claims description 4
- 125000000547 substituted alkyl group Chemical group 0.000 claims description 4
- PQJUJGAVDBINPI-UHFFFAOYSA-N 9H-thioxanthene Chemical compound C1=CC=C2CC3=CC=CC=C3SC2=C1 PQJUJGAVDBINPI-UHFFFAOYSA-N 0.000 claims description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 3
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 claims description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 3
- 229910052794 bromium Inorganic materials 0.000 claims description 3
- 239000000460 chlorine Substances 0.000 claims description 3
- 239000011630 iodine Substances 0.000 claims description 3
- 229940126062 Compound A Drugs 0.000 claims description 2
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 claims description 2
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 claims description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 2
- 102100039104 Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit DAD1 Human genes 0.000 claims 3
- 101000884921 Homo sapiens Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit DAD1 Proteins 0.000 claims 3
- 125000001424 substituent group Chemical group 0.000 description 106
- 238000000034 method Methods 0.000 description 101
- 239000010408 film Substances 0.000 description 94
- 239000002019 doping agent Substances 0.000 description 91
- 239000000463 material Substances 0.000 description 90
- 230000015572 biosynthetic process Effects 0.000 description 46
- 238000002347 injection Methods 0.000 description 43
- 239000007924 injection Substances 0.000 description 43
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 41
- 230000032258 transport Effects 0.000 description 40
- 230000000052 comparative effect Effects 0.000 description 36
- 239000000758 substrate Substances 0.000 description 33
- 238000003786 synthesis reaction Methods 0.000 description 28
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 26
- 230000005525 hole transport Effects 0.000 description 23
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 22
- 239000000370 acceptor Substances 0.000 description 22
- 125000000623 heterocyclic group Chemical group 0.000 description 21
- 230000002829 reductive effect Effects 0.000 description 21
- 239000000243 solution Substances 0.000 description 21
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 18
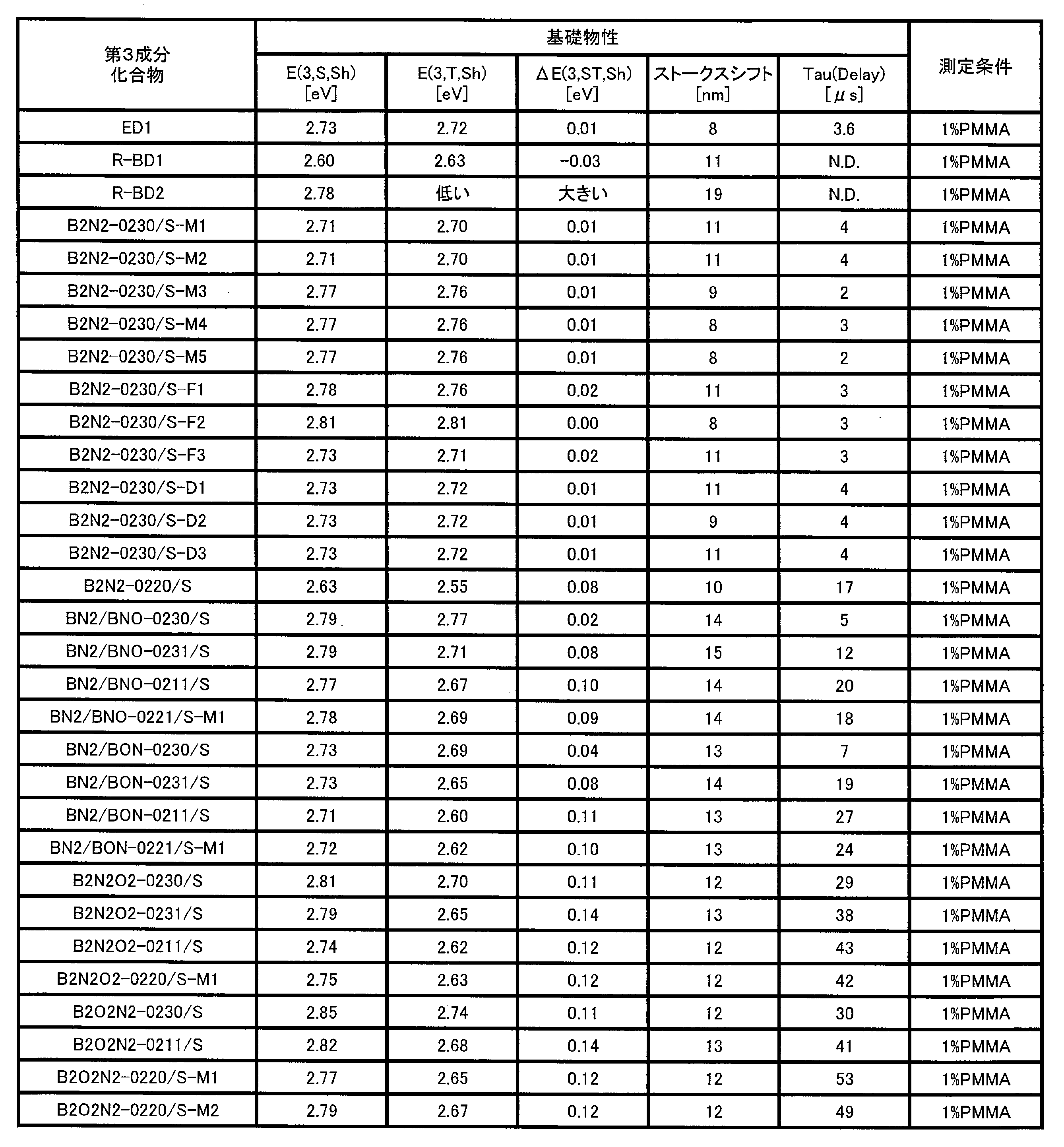
- 238000005259 measurement Methods 0.000 description 18
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 17
- 239000011521 glass Substances 0.000 description 17
- 238000004519 manufacturing process Methods 0.000 description 17
- 0 IC1C(*C2C(*C3C(*4)CCCCCCCCCC3)C4CCCCCCCC2)CCCCCCCCC1 Chemical compound IC1C(*C2C(*C3C(*4)CCCCCCCCCC3)C4CCCCCCCC2)CCCCCCCCC1 0.000 description 16
- 150000004945 aromatic hydrocarbons Chemical group 0.000 description 15
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 15
- 238000007740 vapor deposition Methods 0.000 description 15
- 238000000151 deposition Methods 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 238000000295 emission spectrum Methods 0.000 description 14
- 229910052751 metal Inorganic materials 0.000 description 14
- 239000002184 metal Substances 0.000 description 14
- 238000010438 heat treatment Methods 0.000 description 13
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 12
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 12
- PQXKHYXIUOZZFA-UHFFFAOYSA-M lithium fluoride Chemical compound [Li+].[F-] PQXKHYXIUOZZFA-UHFFFAOYSA-M 0.000 description 12
- 239000011159 matrix material Substances 0.000 description 12
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 12
- 238000004528 spin coating Methods 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 11
- 230000008021 deposition Effects 0.000 description 11
- 238000010586 diagram Methods 0.000 description 11
- 238000001035 drying Methods 0.000 description 11
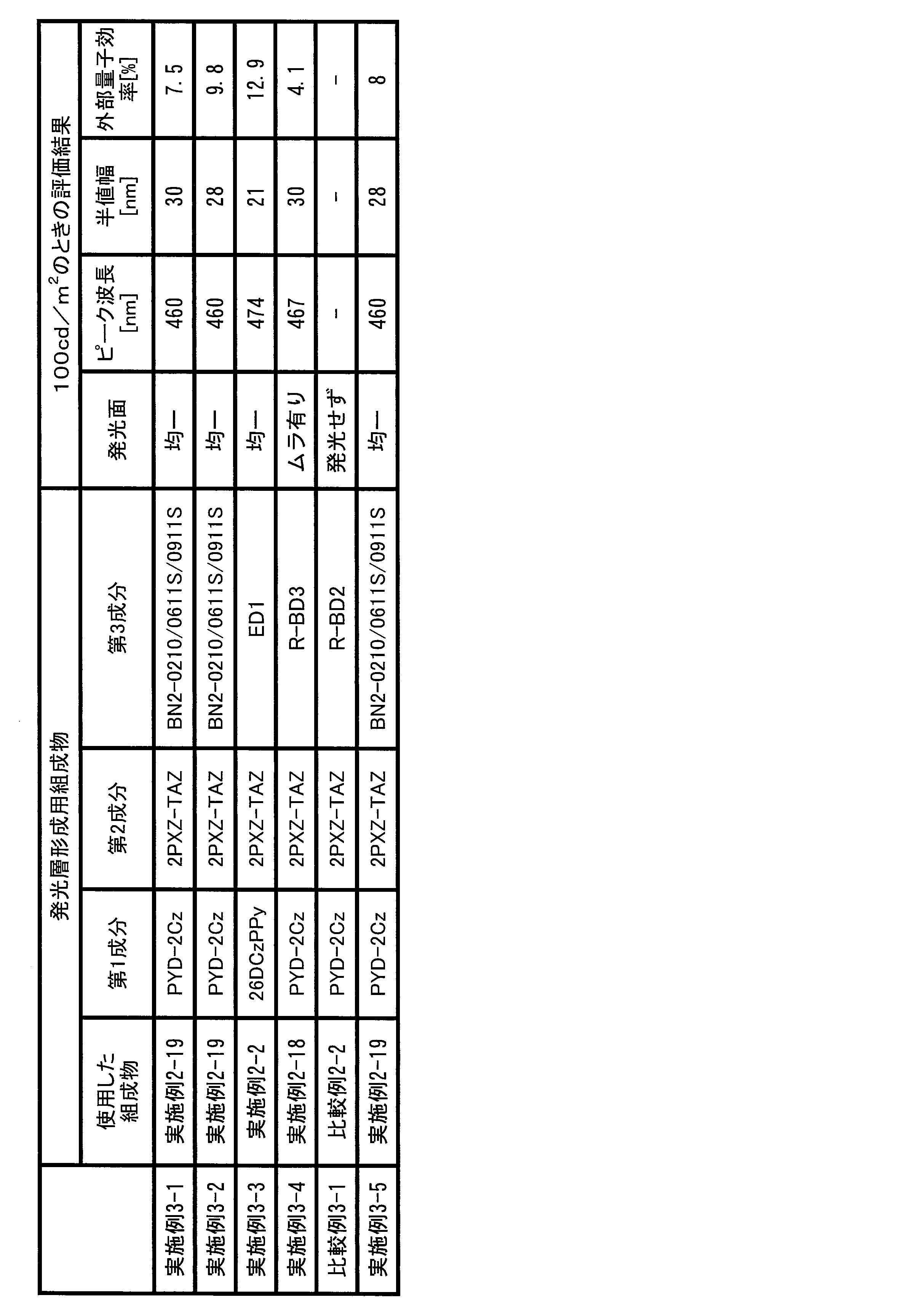
- 238000011156 evaluation Methods 0.000 description 11
- 125000001624 naphthyl group Chemical group 0.000 description 11
- 239000010409 thin film Substances 0.000 description 11
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 10
- 238000010521 absorption reaction Methods 0.000 description 10
- 229910052792 caesium Inorganic materials 0.000 description 10
- 230000005281 excited state Effects 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 238000004770 highest occupied molecular orbital Methods 0.000 description 10
- 230000007246 mechanism Effects 0.000 description 10
- 239000004094 surface-active agent Substances 0.000 description 10
- 238000001771 vacuum deposition Methods 0.000 description 10
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 9
- 239000012043 crude product Substances 0.000 description 9
- 230000005283 ground state Effects 0.000 description 9
- 238000004768 lowest unoccupied molecular orbital Methods 0.000 description 9
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 9
- 239000012044 organic layer Substances 0.000 description 9
- 239000011368 organic material Substances 0.000 description 9
- 239000001301 oxygen Substances 0.000 description 9
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 8
- 238000001704 evaporation Methods 0.000 description 8
- 125000001828 phenalenyl group Chemical group C1(C=CC2=CC=CC3=CC=CC1=C23)* 0.000 description 8
- 238000001228 spectrum Methods 0.000 description 8
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 7
- 229910052783 alkali metal Inorganic materials 0.000 description 7
- 150000001340 alkali metals Chemical class 0.000 description 7
- 239000003086 colorant Substances 0.000 description 7
- 150000002739 metals Chemical class 0.000 description 7
- 229920003227 poly(N-vinyl carbazole) Polymers 0.000 description 7
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 7
- 239000002994 raw material Substances 0.000 description 7
- 230000002441 reversible effect Effects 0.000 description 7
- 238000001308 synthesis method Methods 0.000 description 7
- HMUNWXXNJPVALC-UHFFFAOYSA-N 1-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)C(CN1CC2=C(CC1)NN=N2)=O HMUNWXXNJPVALC-UHFFFAOYSA-N 0.000 description 6
- VZSRBBMJRBPUNF-UHFFFAOYSA-N 2-(2,3-dihydro-1H-inden-2-ylamino)-N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]pyrimidine-5-carboxamide Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C(=O)NCCC(N1CC2=C(CC1)NN=N2)=O VZSRBBMJRBPUNF-UHFFFAOYSA-N 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 229920000144 PEDOT:PSS Polymers 0.000 description 6
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 6
- SLGBZMMZGDRARJ-UHFFFAOYSA-N Triphenylene Chemical group C1=CC=C2C3=CC=CC=C3C3=CC=CC=C3C2=C1 SLGBZMMZGDRARJ-UHFFFAOYSA-N 0.000 description 6
- 125000001931 aliphatic group Chemical group 0.000 description 6
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 6
- 239000011230 binding agent Substances 0.000 description 6
- 238000005859 coupling reaction Methods 0.000 description 6
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 6
- NNBZCPXTIHJBJL-UHFFFAOYSA-N decalin Chemical compound C1CCCC2CCCCC21 NNBZCPXTIHJBJL-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- MPQXHAGKBWFSNV-UHFFFAOYSA-N oxidophosphanium Chemical class [PH3]=O MPQXHAGKBWFSNV-UHFFFAOYSA-N 0.000 description 6
- 125000005561 phenanthryl group Chemical group 0.000 description 6
- 230000000704 physical effect Effects 0.000 description 6
- 229910052700 potassium Inorganic materials 0.000 description 6
- 125000001725 pyrenyl group Chemical group 0.000 description 6
- 150000003222 pyridines Chemical class 0.000 description 6
- 125000004076 pyridyl group Chemical group 0.000 description 6
- 238000006862 quantum yield reaction Methods 0.000 description 6
- 229920005989 resin Polymers 0.000 description 6
- 239000011347 resin Substances 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- 125000001544 thienyl group Chemical group 0.000 description 6
- 150000003918 triazines Chemical class 0.000 description 6
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 5
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 5
- NSMJMUQZRGZMQC-UHFFFAOYSA-N 2-naphthalen-1-yl-1H-imidazo[4,5-f][1,10]phenanthroline Chemical compound C12=CC=CN=C2C2=NC=CC=C2C2=C1NC(C=1C3=CC=CC=C3C=CC=1)=N2 NSMJMUQZRGZMQC-UHFFFAOYSA-N 0.000 description 5
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 5
- 125000005577 anthracene group Chemical group 0.000 description 5
- 230000004888 barrier function Effects 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- 150000004696 coordination complex Chemical class 0.000 description 5
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 5
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 5
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 5
- 125000002541 furyl group Chemical group 0.000 description 5
- 125000002883 imidazolyl group Chemical group 0.000 description 5
- 229910052744 lithium Inorganic materials 0.000 description 5
- 125000002971 oxazolyl group Chemical group 0.000 description 5
- 125000003933 pentacenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC5=CC=CC=C5C=C4C=C3C=C12)* 0.000 description 5
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 5
- 239000004926 polymethyl methacrylate Substances 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 150000003230 pyrimidines Chemical class 0.000 description 5
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 5
- 230000001603 reducing effect Effects 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 5
- 239000011593 sulfur Chemical group 0.000 description 5
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 5
- 238000012546 transfer Methods 0.000 description 5
- UDONPJKEOAWFGI-UHFFFAOYSA-N 1-methyl-3-phenoxybenzene Chemical compound CC1=CC=CC(OC=2C=CC=CC=2)=C1 UDONPJKEOAWFGI-UHFFFAOYSA-N 0.000 description 4
- QPUYECUOLPXSFR-UHFFFAOYSA-N 1-methylnaphthalene Chemical compound C1=CC=C2C(C)=CC=CC2=C1 QPUYECUOLPXSFR-UHFFFAOYSA-N 0.000 description 4
- 238000005160 1H NMR spectroscopy Methods 0.000 description 4
- HKMTVMBEALTRRR-UHFFFAOYSA-N Benzo[a]fluorene Chemical class C1=CC=CC2=C3CC4=CC=CC=C4C3=CC=C21 HKMTVMBEALTRRR-UHFFFAOYSA-N 0.000 description 4
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical group CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 4
- 229920001214 Polysorbate 60 Polymers 0.000 description 4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 4
- JAWMENYCRQKKJY-UHFFFAOYSA-N [3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-ylmethyl)-1-oxa-2,8-diazaspiro[4.5]dec-2-en-8-yl]-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]methanone Chemical compound N1N=NC=2CN(CCC=21)CC1=NOC2(C1)CCN(CC2)C(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F JAWMENYCRQKKJY-UHFFFAOYSA-N 0.000 description 4
- 125000004054 acenaphthylenyl group Chemical group C1(=CC2=CC=CC3=CC=CC1=C23)* 0.000 description 4
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 4
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 4
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 4
- 150000004996 alkyl benzenes Chemical class 0.000 description 4
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 4
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 description 4
- 125000005013 aryl ether group Chemical group 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 4
- 125000002619 bicyclic group Chemical group 0.000 description 4
- XJHCXCQVJFPJIK-UHFFFAOYSA-M caesium fluoride Chemical compound [F-].[Cs+] XJHCXCQVJFPJIK-UHFFFAOYSA-M 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 230000007547 defect Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 239000003480 eluent Substances 0.000 description 4
- 230000008020 evaporation Effects 0.000 description 4
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N fluorene Chemical compound C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 description 4
- 229910052731 fluorine Inorganic materials 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 125000005842 heteroatom Chemical group 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 4
- 125000001041 indolyl group Chemical group 0.000 description 4
- 229910010272 inorganic material Inorganic materials 0.000 description 4
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 4
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 4
- 125000000842 isoxazolyl group Chemical group 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 229910052749 magnesium Inorganic materials 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000012299 nitrogen atmosphere Substances 0.000 description 4
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 4
- 125000001715 oxadiazolyl group Chemical group 0.000 description 4
- 125000001644 phenoxazinyl group Chemical group C1(=CC=CC=2OC3=CC=CC=C3NC12)* 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 238000001556 precipitation Methods 0.000 description 4
- 238000007639 printing Methods 0.000 description 4
- 125000003373 pyrazinyl group Chemical group 0.000 description 4
- 125000003226 pyrazolyl group Chemical group 0.000 description 4
- 125000000714 pyrimidinyl group Chemical group 0.000 description 4
- 125000000168 pyrrolyl group Chemical group 0.000 description 4
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 4
- 229910052701 rubidium Inorganic materials 0.000 description 4
- 238000007789 sealing Methods 0.000 description 4
- 229910052710 silicon Inorganic materials 0.000 description 4
- 239000010703 silicon Substances 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000004544 sputter deposition Methods 0.000 description 4
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 3
- 125000005955 1H-indazolyl group Chemical group 0.000 description 3
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 3
- 125000006176 2-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(C([H])([H])*)C([H])([H])C([H])([H])[H] 0.000 description 3
- YLZOPXRUQYQQID-UHFFFAOYSA-N 3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-1-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]propan-1-one Chemical compound N1N=NC=2CN(CCC=21)CCC(=O)N1CCN(CC1)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F YLZOPXRUQYQQID-UHFFFAOYSA-N 0.000 description 3
- 125000004920 4-methyl-2-pentyl group Chemical group CC(CC(C)*)C 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 3
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 3
- XECAHXYUAAWDEL-UHFFFAOYSA-N acrylonitrile butadiene styrene Chemical compound C=CC=C.C=CC#N.C=CC1=CC=CC=C1 XECAHXYUAAWDEL-UHFFFAOYSA-N 0.000 description 3
- 239000004676 acrylonitrile butadiene styrene Substances 0.000 description 3
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 3
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 3
- 150000001342 alkaline earth metals Chemical class 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 125000000304 alkynyl group Chemical group 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 125000005605 benzo group Chemical group 0.000 description 3
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 3
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 3
- 235000010290 biphenyl Nutrition 0.000 description 3
- 239000004305 biphenyl Chemical group 0.000 description 3
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 239000003638 chemical reducing agent Substances 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- HHNHBFLGXIUXCM-GFCCVEGCSA-N cyclohexylbenzene Chemical compound [CH]1CCCC[C@@H]1C1=CC=CC=C1 HHNHBFLGXIUXCM-GFCCVEGCSA-N 0.000 description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 3
- SQIFACVGCPWBQZ-UHFFFAOYSA-N delta-Terpineol Chemical compound CC(C)(O)C1CCC(=C)CC1 SQIFACVGCPWBQZ-UHFFFAOYSA-N 0.000 description 3
- TXCDCPKCNAJMEE-UHFFFAOYSA-N dibenzofuran Chemical group C1=CC=C2C3=CC=CC=C3OC2=C1 TXCDCPKCNAJMEE-UHFFFAOYSA-N 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- 125000004222 fluoren-1-yl group Chemical group [H]C1=C([H])C2=C(C([H])=C1[H])C([H])([H])C1=C(*)C([H])=C([H])C([H])=C21 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 238000007641 inkjet printing Methods 0.000 description 3
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 3
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000001786 isothiazolyl group Chemical group 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000004973 liquid crystal related substance Substances 0.000 description 3
- 125000002950 monocyclic group Chemical group 0.000 description 3
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 3
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 3
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 125000003261 o-tolyl group Chemical group [H]C1=C([H])C(*)=C(C([H])=C1[H])C([H])([H])[H] 0.000 description 3
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 3
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 3
- 125000004225 phenalen-1-yl group Chemical group [H]C1=C([H])C2=C3C(C([H])=C([H])C([H])(*)C3=C([H])C([H])=C2[H])=C1[H] 0.000 description 3
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical group C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 3
- 125000001484 phenothiazinyl group Chemical group C1(=CC=CC=2SC3=CC=CC=C3NC12)* 0.000 description 3
- 238000000206 photolithography Methods 0.000 description 3
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 3
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical class N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 3
- 125000001742 pyren-1-yl group Chemical group [H]C1=C([H])C2=C([H])C([H])=C3C(*)=C([H])C([H])=C4C([H])=C([H])C(=C1[H])C2=C34 0.000 description 3
- 125000001732 pyren-2-yl group Chemical group [H]C1=C([H])C2=C([H])C([H])=C3C([H])=C(*)C([H])=C4C([H])=C([H])C(=C1[H])C2=C34 0.000 description 3
- 125000001486 pyren-4-yl group Chemical group [H]C1=C(C2=C34)C([H])=C([H])C([H])=C2C([*])=C([H])C3=C([H])C([H])=C([H])C4=C1[H] 0.000 description 3
- 125000002098 pyridazinyl group Chemical group 0.000 description 3
- 239000010453 quartz Substances 0.000 description 3
- 238000010791 quenching Methods 0.000 description 3
- 230000000171 quenching effect Effects 0.000 description 3
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 3
- 229910052761 rare earth metal Inorganic materials 0.000 description 3
- 150000002910 rare earth metals Chemical class 0.000 description 3
- 239000013557 residual solvent Substances 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- 239000004065 semiconductor Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 3
- 125000006850 spacer group Chemical group 0.000 description 3
- 125000004434 sulfur atom Chemical group 0.000 description 3
- 230000003746 surface roughness Effects 0.000 description 3
- 125000001973 tert-pentyl group Chemical group [H]C([H])([H])C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 125000003831 tetrazolyl group Chemical group 0.000 description 3
- 125000001113 thiadiazolyl group Chemical group 0.000 description 3
- 125000004627 thianthrenyl group Chemical group C1(=CC=CC=2SC3=CC=CC=C3SC12)* 0.000 description 3
- 125000000335 thiazolyl group Chemical group 0.000 description 3
- 229910052718 tin Inorganic materials 0.000 description 3
- 239000011135 tin Substances 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 125000004306 triazinyl group Chemical group 0.000 description 3
- 125000001425 triazolyl group Chemical group 0.000 description 3
- 125000002875 triphenylen-1-yl group Chemical group [H]C1=C([H])C2=C(C([H])=C1[H])C1=C(*)C([H])=C([H])C([H])=C1C1=C([H])C([H])=C([H])C([H])=C21 0.000 description 3
- 125000005580 triphenylene group Chemical group 0.000 description 3
- 125000003960 triphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C3=CC=CC=C3C12)* 0.000 description 3
- 229910052725 zinc Inorganic materials 0.000 description 3
- 239000011701 zinc Substances 0.000 description 3
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 2
- TXBFHHYSJNVGBX-UHFFFAOYSA-N (4-diphenylphosphorylphenyl)-triphenylsilane Chemical compound C=1C=CC=CC=1P(C=1C=CC(=CC=1)[Si](C=1C=CC=CC=1)(C=1C=CC=CC=1)C=1C=CC=CC=1)(=O)C1=CC=CC=C1 TXBFHHYSJNVGBX-UHFFFAOYSA-N 0.000 description 2
- CRUILBNAQILVHZ-UHFFFAOYSA-N 1,2,3-trimethoxybenzene Chemical compound COC1=CC=CC(OC)=C1OC CRUILBNAQILVHZ-UHFFFAOYSA-N 0.000 description 2
- FYGHSUNMUKGBRK-UHFFFAOYSA-N 1,2,3-trimethylbenzene Chemical compound CC1=CC=CC(C)=C1C FYGHSUNMUKGBRK-UHFFFAOYSA-N 0.000 description 2
- GWHJZXXIDMPWGX-UHFFFAOYSA-N 1,2,4-trimethylbenzene Chemical compound CC1=CC=C(C)C(C)=C1 GWHJZXXIDMPWGX-UHFFFAOYSA-N 0.000 description 2
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 2
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical compound C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 2
- FNKCOUREFBNNHG-UHFFFAOYSA-N 1,3-dibromo-5-chlorobenzene Chemical compound ClC1=CC(Br)=CC(Br)=C1 FNKCOUREFBNNHG-UHFFFAOYSA-N 0.000 description 2
- CHLICZRVGGXEOD-UHFFFAOYSA-N 1-Methoxy-4-methylbenzene Chemical compound COC1=CC=C(C)C=C1 CHLICZRVGGXEOD-UHFFFAOYSA-N 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- IANQTJSKSUMEQM-UHFFFAOYSA-N 1-benzofuran Chemical group C1=CC=C2OC=CC2=C1 IANQTJSKSUMEQM-UHFFFAOYSA-N 0.000 description 2
- VIPWUFMFHBIKQI-UHFFFAOYSA-N 1-fluoro-4-methoxybenzene Chemical compound COC1=CC=C(F)C=C1 VIPWUFMFHBIKQI-UHFFFAOYSA-N 0.000 description 2
- BBMCTIGTTCKYKF-UHFFFAOYSA-N 1-heptanol Chemical compound CCCCCCCO BBMCTIGTTCKYKF-UHFFFAOYSA-N 0.000 description 2
- WULZIURTBVLJRF-UHFFFAOYSA-N 1-n,3-n-diphenylbenzene-1,3-diamine Chemical compound C=1C=CC(NC=2C=CC=CC=2)=CC=1NC1=CC=CC=C1 WULZIURTBVLJRF-UHFFFAOYSA-N 0.000 description 2
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Chemical compound C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 2
- OXQOBQJCDNLAPO-UHFFFAOYSA-N 2,3-Dimethylpyrazine Chemical compound CC1=NC=CN=C1C OXQOBQJCDNLAPO-UHFFFAOYSA-N 0.000 description 2
- HBEDSQVIWPRPAY-UHFFFAOYSA-N 2,3-dihydrobenzofuran Chemical compound C1=CC=C2OCCC2=C1 HBEDSQVIWPRPAY-UHFFFAOYSA-N 0.000 description 2
- JFJNVIPVOCESGZ-UHFFFAOYSA-N 2,3-dipyridin-2-ylpyridine Chemical compound N1=CC=CC=C1C1=CC=CN=C1C1=CC=CC=N1 JFJNVIPVOCESGZ-UHFFFAOYSA-N 0.000 description 2
- STTGYIUESPWXOW-UHFFFAOYSA-N 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline Chemical compound C=12C=CC3=C(C=4C=CC=CC=4)C=C(C)N=C3C2=NC(C)=CC=1C1=CC=CC=C1 STTGYIUESPWXOW-UHFFFAOYSA-N 0.000 description 2
- LDXJRKWFNNFDSA-UHFFFAOYSA-N 2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-1-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]ethanone Chemical compound C1CN(CC2=NNN=C21)CC(=O)N3CCN(CC3)C4=CN=C(N=C4)NCC5=CC(=CC=C5)OC(F)(F)F LDXJRKWFNNFDSA-UHFFFAOYSA-N 0.000 description 2
- XLLIQLLCWZCATF-UHFFFAOYSA-N 2-methoxyethyl acetate Chemical compound COCCOC(C)=O XLLIQLLCWZCATF-UHFFFAOYSA-N 0.000 description 2
- NWPNXBQSRGKSJB-UHFFFAOYSA-N 2-methylbenzonitrile Chemical compound CC1=CC=CC=C1C#N NWPNXBQSRGKSJB-UHFFFAOYSA-N 0.000 description 2
- QENGPZGAWFQWCZ-UHFFFAOYSA-N 3-Methylthiophene Chemical compound CC=1C=CSC=1 QENGPZGAWFQWCZ-UHFFFAOYSA-N 0.000 description 2
- DDTHMESPCBONDT-UHFFFAOYSA-N 4-(4-oxocyclohexa-2,5-dien-1-ylidene)cyclohexa-2,5-dien-1-one Chemical compound C1=CC(=O)C=CC1=C1C=CC(=O)C=C1 DDTHMESPCBONDT-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 2
- 241000284156 Clerodendrum quadriloculare Species 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 2
- LTEQMZWBSYACLV-UHFFFAOYSA-N Hexylbenzene Chemical compound CCCCCCC1=CC=CC=C1 LTEQMZWBSYACLV-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 229920000877 Melamine resin Polymers 0.000 description 2
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 2
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 2
- URLKBWYHVLBVBO-UHFFFAOYSA-N Para-Xylene Chemical group CC1=CC=C(C)C=C1 URLKBWYHVLBVBO-UHFFFAOYSA-N 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- NWGKJDSIEKMTRX-AAZCQSIUSA-N Sorbitan monooleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O NWGKJDSIEKMTRX-AAZCQSIUSA-N 0.000 description 2
- IYFATESGLOUGBX-YVNJGZBMSA-N Sorbitan monopalmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O IYFATESGLOUGBX-YVNJGZBMSA-N 0.000 description 2
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 2
- 101150088517 TCTA gene Proteins 0.000 description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- LWZFANDGMFTDAV-BURFUSLBSA-N [(2r)-2-[(2r,3r,4s)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O LWZFANDGMFTDAV-BURFUSLBSA-N 0.000 description 2
- 125000003172 aldehyde group Chemical group 0.000 description 2
- 125000002723 alicyclic group Chemical group 0.000 description 2
- 150000005215 alkyl ethers Chemical class 0.000 description 2
- 125000004414 alkyl thio group Chemical group 0.000 description 2
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical compound [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- YCSBALJAGZKWFF-UHFFFAOYSA-N anthracen-2-amine Chemical compound C1=CC=CC2=CC3=CC(N)=CC=C3C=C21 YCSBALJAGZKWFF-UHFFFAOYSA-N 0.000 description 2
- 150000001454 anthracenes Chemical class 0.000 description 2
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 2
- 150000004056 anthraquinones Chemical class 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 150000004832 aryl thioethers Chemical group 0.000 description 2
- XJHABGPPCLHLLV-UHFFFAOYSA-N benzo[de]isoquinoline-1,3-dione Chemical class C1=CC(C(=O)NC2=O)=C3C2=CC=CC3=C1 XJHABGPPCLHLLV-UHFFFAOYSA-N 0.000 description 2
- WZJYKHNJTSNBHV-UHFFFAOYSA-N benzoquinoline Natural products C1=CN=C2C3=CC=CC=C3C=CC2=C1 WZJYKHNJTSNBHV-UHFFFAOYSA-N 0.000 description 2
- 229910052790 beryllium Inorganic materials 0.000 description 2
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical group C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 2
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 2
- UORVGPXVDQYIDP-BJUDXGSMSA-N borane Chemical class [10BH3] UORVGPXVDQYIDP-BJUDXGSMSA-N 0.000 description 2
- ILAHWRKJUDSMFH-UHFFFAOYSA-N boron tribromide Chemical compound BrB(Br)Br ILAHWRKJUDSMFH-UHFFFAOYSA-N 0.000 description 2
- QARVLSVVCXYDNA-UHFFFAOYSA-N bromobenzene Chemical compound BrC1=CC=CC=C1 QARVLSVVCXYDNA-UHFFFAOYSA-N 0.000 description 2
- XSIFPSYPOVKYCO-UHFFFAOYSA-N butyl benzoate Chemical compound CCCCOC(=O)C1=CC=CC=C1 XSIFPSYPOVKYCO-UHFFFAOYSA-N 0.000 description 2
- OCKPCBLVNKHBMX-UHFFFAOYSA-N butylbenzene Chemical compound CCCCC1=CC=CC=C1 OCKPCBLVNKHBMX-UHFFFAOYSA-N 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011651 chromium Substances 0.000 description 2
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 2
- 125000005390 cinnolyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 150000001893 coumarin derivatives Chemical class 0.000 description 2
- RWGFKTVRMDUZSP-UHFFFAOYSA-N cumene Chemical compound CC(C)C1=CC=CC=C1 RWGFKTVRMDUZSP-UHFFFAOYSA-N 0.000 description 2
- 150000003997 cyclic ketones Chemical class 0.000 description 2
- 125000000392 cycloalkenyl group Chemical group 0.000 description 2
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- ZSWFCLXCOIISFI-UHFFFAOYSA-N cyclopentadiene Chemical compound C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 2
- LPIQUOYDBNQMRZ-UHFFFAOYSA-N cyclopentene Chemical compound C1CC=CC1 LPIQUOYDBNQMRZ-UHFFFAOYSA-N 0.000 description 2
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- 230000009849 deactivation Effects 0.000 description 2
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 2
- 238000007872 degassing Methods 0.000 description 2
- IYYZUPMFVPLQIF-UHFFFAOYSA-N dibenzothiophene Chemical compound C1=CC=C2C3=CC=CC=C3SC2=C1 IYYZUPMFVPLQIF-UHFFFAOYSA-N 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- LQZZUXJYWNFBMV-UHFFFAOYSA-N dodecan-1-ol Chemical compound CCCCCCCCCCCCO LQZZUXJYWNFBMV-UHFFFAOYSA-N 0.000 description 2
- MTZQAGJQAFMTAQ-UHFFFAOYSA-N ethyl benzoate Chemical compound CCOC(=O)C1=CC=CC=C1 MTZQAGJQAFMTAQ-UHFFFAOYSA-N 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 229920002313 fluoropolymer Polymers 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 125000003838 furazanyl group Chemical group 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- NNRLDGQZIVUQTE-UHFFFAOYSA-N gamma-Terpineol Chemical compound CC(C)=C1CCC(C)(O)CC1 NNRLDGQZIVUQTE-UHFFFAOYSA-N 0.000 description 2
- 230000009477 glass transition Effects 0.000 description 2
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 2
- 150000002484 inorganic compounds Chemical class 0.000 description 2
- 239000011147 inorganic material Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 2
- 125000001977 isobenzofuranyl group Chemical group C=1(OC=C2C=CC=CC12)* 0.000 description 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 2
- 125000005956 isoquinolyl group Chemical group 0.000 description 2
- 238000010030 laminating Methods 0.000 description 2
- 239000002346 layers by function Substances 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- IVSZLXZYQVIEFR-UHFFFAOYSA-N m-xylene Chemical group CC1=CC=CC(C)=C1 IVSZLXZYQVIEFR-UHFFFAOYSA-N 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 2
- CETWDUZRCINIHU-UHFFFAOYSA-N methyl-n-amyl-carbinol Natural products CCCCCC(C)O CETWDUZRCINIHU-UHFFFAOYSA-N 0.000 description 2
- 239000012046 mixed solvent Substances 0.000 description 2
- 238000004776 molecular orbital Methods 0.000 description 2
- 229910052750 molybdenum Inorganic materials 0.000 description 2
- 239000011733 molybdenum Substances 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- SFMJNHNUOVADRW-UHFFFAOYSA-N n-[5-[9-[4-(methanesulfonamido)phenyl]-2-oxobenzo[h][1,6]naphthyridin-1-yl]-2-methylphenyl]prop-2-enamide Chemical compound C1=C(NC(=O)C=C)C(C)=CC=C1N1C(=O)C=CC2=C1C1=CC(C=3C=CC(NS(C)(=O)=O)=CC=3)=CC=C1N=C2 SFMJNHNUOVADRW-UHFFFAOYSA-N 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- LQNUZADURLCDLV-UHFFFAOYSA-N nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC=C1 LQNUZADURLCDLV-UHFFFAOYSA-N 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- ZWRUINPWMLAQRD-UHFFFAOYSA-N nonan-1-ol Chemical compound CCCCCCCCCO ZWRUINPWMLAQRD-UHFFFAOYSA-N 0.000 description 2
- SJWFXCIHNDVPSH-UHFFFAOYSA-N octane-2-ol Natural products CCCCCCC(C)O SJWFXCIHNDVPSH-UHFFFAOYSA-N 0.000 description 2
- 238000007645 offset printing Methods 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 235000012736 patent blue V Nutrition 0.000 description 2
- DGBWPZSGHAXYGK-UHFFFAOYSA-N perinone Chemical class C12=NC3=CC=CC=C3N2C(=O)C2=CC=C3C4=C2C1=CC=C4C(=O)N1C2=CC=CC=C2N=C13 DGBWPZSGHAXYGK-UHFFFAOYSA-N 0.000 description 2
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 description 2
- 150000005041 phenanthrolines Chemical class 0.000 description 2
- 125000005954 phenoxathiinyl group Chemical group 0.000 description 2
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 2
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical class [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 2
- 238000005424 photoluminescence Methods 0.000 description 2
- 239000002985 plastic film Substances 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920000548 poly(silane) polymer Polymers 0.000 description 2
- 229920002492 poly(sulfone) Polymers 0.000 description 2
- 229920000767 polyaniline Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 239000004800 polyvinyl chloride Substances 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 2
- 239000011698 potassium fluoride Substances 0.000 description 2
- UOHMMEJUHBCKEE-UHFFFAOYSA-N prehnitene Chemical compound CC1=CC=C(C)C(C)=C1C UOHMMEJUHBCKEE-UHFFFAOYSA-N 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 2
- 125000005493 quinolyl group Chemical group 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000001226 reprecipitation Methods 0.000 description 2
- 230000027756 respiratory electron transport chain Effects 0.000 description 2
- 229910052814 silicon oxide Inorganic materials 0.000 description 2
- 239000005361 soda-lime glass Substances 0.000 description 2
- 229950006451 sorbitan laurate Drugs 0.000 description 2
- 235000011067 sorbitan monolaureate Nutrition 0.000 description 2
- 229950004959 sorbitan oleate Drugs 0.000 description 2
- 229950003429 sorbitan palmitate Drugs 0.000 description 2
- 229950011392 sorbitan stearate Drugs 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229920003002 synthetic resin Polymers 0.000 description 2
- 239000000057 synthetic resin Substances 0.000 description 2
- YTZKOQUCBOVLHL-UHFFFAOYSA-N tert-butylbenzene Chemical compound CC(C)(C)C1=CC=CC=C1 YTZKOQUCBOVLHL-UHFFFAOYSA-N 0.000 description 2
- NHGXDBSUJJNIRV-UHFFFAOYSA-M tetrabutylammonium chloride Chemical compound [Cl-].CCCC[N+](CCCC)(CCCC)CCCC NHGXDBSUJJNIRV-UHFFFAOYSA-M 0.000 description 2
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 2
- VDZOOKBUILJEDG-UHFFFAOYSA-M tetrabutylammonium hydroxide Chemical compound [OH-].CCCC[N+](CCCC)(CCCC)CCCC VDZOOKBUILJEDG-UHFFFAOYSA-M 0.000 description 2
- HLZKNKRTKFSKGZ-UHFFFAOYSA-N tetradecan-1-ol Chemical compound CCCCCCCCCCCCCCO HLZKNKRTKFSKGZ-UHFFFAOYSA-N 0.000 description 2
- ZUHZGEOKBKGPSW-UHFFFAOYSA-N tetraglyme Chemical compound COCCOCCOCCOCCOC ZUHZGEOKBKGPSW-UHFFFAOYSA-N 0.000 description 2
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 2
- GVIJJXMXTUZIOD-UHFFFAOYSA-N thianthrene Chemical group C1=CC=C2SC3=CC=CC=C3SC2=C1 GVIJJXMXTUZIOD-UHFFFAOYSA-N 0.000 description 2
- 150000003577 thiophenes Chemical class 0.000 description 2
- 125000003944 tolyl group Chemical group 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 239000008096 xylene Substances 0.000 description 2
- JWZZKOKVBUJMES-UHFFFAOYSA-N (+-)-Isoprenaline Chemical compound CC(C)NCC(O)C1=CC=C(O)C(O)=C1 JWZZKOKVBUJMES-UHFFFAOYSA-N 0.000 description 1
- GDTMIGSCWLYRHS-UHFFFAOYSA-K (2,6-diphenylphenoxy)-bis[(2-methylquinolin-8-yl)oxy]alumane Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.[O-]C1=C(C=2C=CC=CC=2)C=CC=C1C1=CC=CC=C1 GDTMIGSCWLYRHS-UHFFFAOYSA-K 0.000 description 1
- WUOACPNHFRMFPN-SECBINFHSA-N (S)-(-)-alpha-terpineol Chemical compound CC1=CC[C@@H](C(C)(C)O)CC1 WUOACPNHFRMFPN-SECBINFHSA-N 0.000 description 1
- RUJPNZNXGCHGID-UHFFFAOYSA-N (Z)-beta-Terpineol Natural products CC(=C)C1CCC(C)(O)CC1 RUJPNZNXGCHGID-UHFFFAOYSA-N 0.000 description 1
- QYGBYAQGBVHMDD-XQRVVYSFSA-N (z)-2-cyano-3-thiophen-2-ylprop-2-enoic acid Chemical compound OC(=O)C(\C#N)=C/C1=CC=CS1 QYGBYAQGBVHMDD-XQRVVYSFSA-N 0.000 description 1
- FQDMMQLZHXGRLP-UHFFFAOYSA-N 1,1,2,2-tetramethyl-3H-indeno[2,1-a]acridine Chemical compound CC1(CC=C2C(=CC3=C4C=C5C=CC=CC5=C4C=CC3=N2)C1(C)C)C FQDMMQLZHXGRLP-UHFFFAOYSA-N 0.000 description 1
- XOVCXMKIBNFMAL-UHFFFAOYSA-N 1,1-dimethyl-2h-acridine Chemical compound C1=CC=C2C=C3C(C)(C)CC=CC3=NC2=C1 XOVCXMKIBNFMAL-UHFFFAOYSA-N 0.000 description 1
- PYVPMCZSTWELEJ-UHFFFAOYSA-N 1,2,3,4-tetramethyl-9h-carbazole Chemical compound C1=CC=C2C3=C(C)C(C)=C(C)C(C)=C3NC2=C1 PYVPMCZSTWELEJ-UHFFFAOYSA-N 0.000 description 1
- UGUHFDPGDQDVGX-UHFFFAOYSA-N 1,2,3-thiadiazole Chemical group C1=CSN=N1 UGUHFDPGDQDVGX-UHFFFAOYSA-N 0.000 description 1
- GSMMBRRXAPIYSR-UHFFFAOYSA-N 1,2-bis(benzenesulfonyl)benzene Chemical compound C=1C=CC=C(S(=O)(=O)C=2C=CC=CC=2)C=1S(=O)(=O)C1=CC=CC=C1 GSMMBRRXAPIYSR-UHFFFAOYSA-N 0.000 description 1
- ZIZMDHZLHJBNSQ-UHFFFAOYSA-N 1,2-dihydrophenazine Chemical compound C1=CC=C2N=C(C=CCC3)C3=NC2=C1 ZIZMDHZLHJBNSQ-UHFFFAOYSA-N 0.000 description 1
- MCHLMNHHIVZROI-UHFFFAOYSA-N 1,2-dimethoxy-9h-carbazole Chemical compound C1=CC=C2NC3=C(OC)C(OC)=CC=C3C2=C1 MCHLMNHHIVZROI-UHFFFAOYSA-N 0.000 description 1
- FVHAWXWFPBPFOS-UHFFFAOYSA-N 1,2-dimethyl-3-nitrobenzene Chemical compound CC1=CC=CC([N+]([O-])=O)=C1C FVHAWXWFPBPFOS-UHFFFAOYSA-N 0.000 description 1
- BOCODUXWXDKEJP-UHFFFAOYSA-N 1,2-dimethyl-9h-carbazole Chemical compound C1=CC=C2C3=CC=C(C)C(C)=C3NC2=C1 BOCODUXWXDKEJP-UHFFFAOYSA-N 0.000 description 1
- FQYVVSNFPLKMNU-UHFFFAOYSA-N 1,2-dipentylbenzene Chemical compound CCCCCC1=CC=CC=C1CCCCC FQYVVSNFPLKMNU-UHFFFAOYSA-N 0.000 description 1
- ACXNSKYDNPWWAG-UHFFFAOYSA-N 1,2-ditert-butyl-9h-carbazole Chemical compound C1=CC=C2C3=CC=C(C(C)(C)C)C(C(C)(C)C)=C3NC2=C1 ACXNSKYDNPWWAG-UHFFFAOYSA-N 0.000 description 1
- FTNJQNQLEGKTGD-UHFFFAOYSA-N 1,3-benzodioxole Chemical compound C1=CC=C2OCOC2=C1 FTNJQNQLEGKTGD-UHFFFAOYSA-N 0.000 description 1
- 125000000355 1,3-benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- JSRLURSZEMLAFO-UHFFFAOYSA-N 1,3-dibromobenzene Chemical compound BrC1=CC=CC(Br)=C1 JSRLURSZEMLAFO-UHFFFAOYSA-N 0.000 description 1
- OCJBOOLMMGQPQU-UHFFFAOYSA-N 1,4-dichlorobenzene Chemical compound ClC1=CC=C(Cl)C=C1 OCJBOOLMMGQPQU-UHFFFAOYSA-N 0.000 description 1
- FLBAYUMRQUHISI-UHFFFAOYSA-N 1,8-naphthyridine Chemical group N1=CC=CC2=CC=CN=C21 FLBAYUMRQUHISI-UHFFFAOYSA-N 0.000 description 1
- UHXOHPVVEHBKKT-UHFFFAOYSA-N 1-(2,2-diphenylethenyl)-4-[4-(2,2-diphenylethenyl)phenyl]benzene Chemical group C=1C=C(C=2C=CC(C=C(C=3C=CC=CC=3)C=3C=CC=CC=3)=CC=2)C=CC=1C=C(C=1C=CC=CC=1)C1=CC=CC=C1 UHXOHPVVEHBKKT-UHFFFAOYSA-N 0.000 description 1
- OHVLMTFVQDZYHP-UHFFFAOYSA-N 1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-2-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]ethanone Chemical compound N1N=NC=2CN(CCC=21)C(CN1CCN(CC1)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)=O OHVLMTFVQDZYHP-UHFFFAOYSA-N 0.000 description 1
- LZRCTLNAHMQEKM-UHFFFAOYSA-N 1-(butoxymethyl)-4-methylbenzene Chemical compound CCCCOCC1=CC=C(C)C=C1 LZRCTLNAHMQEKM-UHFFFAOYSA-N 0.000 description 1
- OSIGJGFTADMDOB-UHFFFAOYSA-N 1-Methoxy-3-methylbenzene Chemical compound COC1=CC=CC(C)=C1 OSIGJGFTADMDOB-UHFFFAOYSA-N 0.000 description 1
- NGFHEKCFOXQCQD-UHFFFAOYSA-N 1-N,1-N,3-N,3-N,5-N-pentakis-phenylbenzene-1,3,5-triamine Chemical compound C1(=CC=CC=C1)NC1=CC(=CC(=C1)N(C1=CC=CC=C1)C1=CC=CC=C1)N(C1=CC=CC=C1)C1=CC=CC=C1 NGFHEKCFOXQCQD-UHFFFAOYSA-N 0.000 description 1
- HYLLZXPMJRMUHH-UHFFFAOYSA-N 1-[2-(2-methoxyethoxy)ethoxy]butane Chemical compound CCCCOCCOCCOC HYLLZXPMJRMUHH-UHFFFAOYSA-N 0.000 description 1
- SNAQINZKMQFYFV-UHFFFAOYSA-N 1-[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]butane Chemical compound CCCCOCCOCCOCCOC SNAQINZKMQFYFV-UHFFFAOYSA-N 0.000 description 1
- PHBJYIUTTPNUBD-UHFFFAOYSA-N 1-[4-(10-naphthalen-2-ylanthracen-9-yl)phenyl]-2-phenylbenzimidazole Chemical compound C1=CC=CC=C1C1=NC2=CC=CC=C2N1C1=CC=C(C=2C3=CC=CC=C3C(C=3C=C4C=CC=CC4=CC=3)=C3C=CC=CC3=2)C=C1 PHBJYIUTTPNUBD-UHFFFAOYSA-N 0.000 description 1
- KPLZANXKTMWBMS-UHFFFAOYSA-N 1-benzo[a]anthracen-1-ylcyclohexa-3,5-diene-1,3-diamine Chemical compound C1(=CC=CC2=CC=C3C=C4C=CC=CC4=CC3=C12)C1(CC(=CC=C1)N)N KPLZANXKTMWBMS-UHFFFAOYSA-N 0.000 description 1
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical group C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 1
- FNPVYRJTBXHIPB-UHFFFAOYSA-N 1-chloro-3-fluoro-2-methylbenzene Chemical compound CC1=C(F)C=CC=C1Cl FNPVYRJTBXHIPB-UHFFFAOYSA-N 0.000 description 1
- RRQYJINTUHWNHW-UHFFFAOYSA-N 1-ethoxy-2-(2-ethoxyethoxy)ethane Chemical compound CCOCCOCCOCC RRQYJINTUHWNHW-UHFFFAOYSA-N 0.000 description 1
- AWLDSXJCQWTJPC-UHFFFAOYSA-N 1-fluoro-2,3-dimethylbenzene Chemical group CC1=CC=CC(F)=C1C AWLDSXJCQWTJPC-UHFFFAOYSA-N 0.000 description 1
- JIXDOBAQOWOUPA-UHFFFAOYSA-N 1-fluoro-2-methoxybenzene Chemical compound COC1=CC=CC=C1F JIXDOBAQOWOUPA-UHFFFAOYSA-N 0.000 description 1
- IWFKMNAEFPEIOY-UHFFFAOYSA-N 1-fluoro-3,5-dimethoxybenzene Chemical compound COC1=CC(F)=CC(OC)=C1 IWFKMNAEFPEIOY-UHFFFAOYSA-N 0.000 description 1
- MFJNOXOAIFNSBX-UHFFFAOYSA-N 1-fluoro-3-methoxybenzene Chemical compound COC1=CC=CC(F)=C1 MFJNOXOAIFNSBX-UHFFFAOYSA-N 0.000 description 1
- RERATEUBWLKDFE-UHFFFAOYSA-N 1-methoxy-2-[2-(2-methoxypropoxy)propoxy]propane Chemical compound COCC(C)OCC(C)OCC(C)OC RERATEUBWLKDFE-UHFFFAOYSA-N 0.000 description 1
- JCHJBEZBHANKGA-UHFFFAOYSA-N 1-methoxy-3,5-dimethylbenzene Chemical compound COC1=CC(C)=CC(C)=C1 JCHJBEZBHANKGA-UHFFFAOYSA-N 0.000 description 1
- XHONYVFDZSPELQ-UHFFFAOYSA-N 1-methoxy-3-(trifluoromethyl)benzene Chemical compound COC1=CC=CC(C(F)(F)F)=C1 XHONYVFDZSPELQ-UHFFFAOYSA-N 0.000 description 1
- ALLIZEAXNXSFGD-UHFFFAOYSA-N 1-methyl-2-phenylbenzene Chemical group CC1=CC=CC=C1C1=CC=CC=C1 ALLIZEAXNXSFGD-UHFFFAOYSA-N 0.000 description 1
- VYWRZFKFPKKOLU-UHFFFAOYSA-N 1-methyl-4-(pentoxymethyl)benzene Chemical compound CCCCCOCC1=CC=C(C)C=C1 VYWRZFKFPKKOLU-UHFFFAOYSA-N 0.000 description 1
- HGHKXIOBIUJSCS-UHFFFAOYSA-N 1-methyl-4-(propoxymethyl)benzene Chemical compound CCCOCC1=CC=C(C)C=C1 HGHKXIOBIUJSCS-UHFFFAOYSA-N 0.000 description 1
- IYZMXHQDXZKNCY-UHFFFAOYSA-N 1-n,1-n-diphenyl-4-n,4-n-bis[4-(n-phenylanilino)phenyl]benzene-1,4-diamine Chemical compound C1=CC=CC=C1N(C=1C=CC(=CC=1)N(C=1C=CC(=CC=1)N(C=1C=CC=CC=1)C=1C=CC=CC=1)C=1C=CC(=CC=1)N(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 IYZMXHQDXZKNCY-UHFFFAOYSA-N 0.000 description 1
- TVQCZIFSQMWQSO-UHFFFAOYSA-N 1-phenyl-1,2-dihydroindolo[3,2-c]carbazole Chemical compound C1(=CC=CC=C1)C1C=2C(C=CC1)=NC1=CC=C3C=4C=CC=CC=4N=C3C1=2 TVQCZIFSQMWQSO-UHFFFAOYSA-N 0.000 description 1
- IKHFIMAZKKDZEF-UHFFFAOYSA-N 1-phenyl-2-[4-(10-phenylanthracen-9-yl)phenyl]benzimidazole Chemical compound C1=CC=CC=C1C(C1=CC=CC=C11)=C(C=CC=C2)C2=C1C1=CC=C(C=2N(C3=CC=CC=C3N=2)C=2C=CC=CC=2)C=C1 IKHFIMAZKKDZEF-UHFFFAOYSA-N 0.000 description 1
- IVXBGKPGZOETEW-UHFFFAOYSA-N 10-[4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl]-9,9-dimethylacridine Chemical compound CC1(C)C2=C(C=CC=C2)N(C2=CC=C(C=C2)C2=NC(=NC(=N2)C2=CC=CC=C2)C2=CC=CC=C2)C2=C1C=CC=C2 IVXBGKPGZOETEW-UHFFFAOYSA-N 0.000 description 1
- ZABORCXHTNWZRV-UHFFFAOYSA-N 10-[4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl]phenoxazine Chemical compound O1C2=CC=CC=C2N(C2=CC=C(C=C2)C2=NC(=NC(=N2)C2=CC=CC=C2)C2=CC=CC=C2)C2=C1C=CC=C2 ZABORCXHTNWZRV-UHFFFAOYSA-N 0.000 description 1
- WJFKNYWRSNBZNX-UHFFFAOYSA-N 10H-phenothiazine Chemical compound C1=CC=C2NC3=CC=CC=C3SC2=C1 WJFKNYWRSNBZNX-UHFFFAOYSA-N 0.000 description 1
- GLYYLMBVQZMMMS-UHFFFAOYSA-N 12h-[1]benzothiolo[3,2-a]carbazole Chemical compound S1C2=CC=CC=C2C2=C1C=CC1=C2NC2=CC=CC=C12 GLYYLMBVQZMMMS-UHFFFAOYSA-N 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical group C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 1
- ZVFJWYZMQAEBMO-UHFFFAOYSA-N 1h-benzo[h]quinolin-10-one Chemical compound C1=CNC2=C3C(=O)C=CC=C3C=CC2=C1 ZVFJWYZMQAEBMO-UHFFFAOYSA-N 0.000 description 1
- HCLXLZGAYCTHQJ-UHFFFAOYSA-N 1h-cyclohepta[b]pyridine Chemical compound C1=CC=CC=C2NC=CC=C21 HCLXLZGAYCTHQJ-UHFFFAOYSA-N 0.000 description 1
- WLODWTPNUWYZKN-UHFFFAOYSA-N 1h-pyrrol-2-ol Chemical compound OC1=CC=CN1 WLODWTPNUWYZKN-UHFFFAOYSA-N 0.000 description 1
- CJGXJKVMUHXVHL-UHFFFAOYSA-N 2,2-dimethylpropylbenzene Chemical compound CC(C)(C)CC1=CC=CC=C1 CJGXJKVMUHXVHL-UHFFFAOYSA-N 0.000 description 1
- HBKCANVCZLBZGI-UHFFFAOYSA-N 2,3-dimethyl-2H-anthracen-1-one Chemical compound CC=1C(C(C2=CC3=CC=CC=C3C=C2C=1)=O)C HBKCANVCZLBZGI-UHFFFAOYSA-N 0.000 description 1
- MVWPVABZQQJTPL-UHFFFAOYSA-N 2,3-diphenylcyclohexa-2,5-diene-1,4-dione Chemical class O=C1C=CC(=O)C(C=2C=CC=CC=2)=C1C1=CC=CC=C1 MVWPVABZQQJTPL-UHFFFAOYSA-N 0.000 description 1
- PRWATGACIORDEL-UHFFFAOYSA-N 2,4,5,6-tetra(carbazol-9-yl)benzene-1,3-dicarbonitrile Chemical compound C12=CC=CC=C2C2=CC=CC=C2N1C1=C(C#N)C(N2C3=CC=CC=C3C3=CC=CC=C32)=C(N2C3=CC=CC=C3C3=CC=CC=C32)C(N2C3=CC=CC=C3C3=CC=CC=C32)=C1C#N PRWATGACIORDEL-UHFFFAOYSA-N 0.000 description 1
- VTSWSQGDJQFXHB-UHFFFAOYSA-N 2,4,6-trichloro-5-methylpyrimidine Chemical compound CC1=C(Cl)N=C(Cl)N=C1Cl VTSWSQGDJQFXHB-UHFFFAOYSA-N 0.000 description 1
- UJCFZCTTZWHRNL-UHFFFAOYSA-N 2,4-Dimethylanisole Chemical compound COC1=CC=C(C)C=C1C UJCFZCTTZWHRNL-UHFFFAOYSA-N 0.000 description 1
- UXFZNPGAWHMSRK-UHFFFAOYSA-N 2,4-dimethylquinolin-8-ol Chemical compound C1=CC=C(O)C2=NC(C)=CC(C)=C21 UXFZNPGAWHMSRK-UHFFFAOYSA-N 0.000 description 1
- 125000005810 2,5-xylyl group Chemical group [H]C1=C([H])C(=C(*)C([H])=C1C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- GBHMUSBVGVHVLQ-UHFFFAOYSA-N 2-(2'-benzo[h]quinolin-2-yl-9,9'-spirobi[fluorene]-2-yl)benzo[h]quinoline Chemical compound C1=CC=C2C3=NC(C4=CC=C5C=6C(C7(C8=CC(=CC=C8C8=CC=CC=C87)C=7N=C8C9=CC=CC=C9C=CC8=CC=7)C5=C4)=CC=CC=6)=CC=C3C=CC2=C1 GBHMUSBVGVHVLQ-UHFFFAOYSA-N 0.000 description 1
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 1
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 description 1
- MTQADTZTLQGIRT-UHFFFAOYSA-N 2-(4-dinaphthalen-1-ylphosphorylphenyl)-1,8-naphthyridine Chemical compound C1=CC=C2C(P(C=3C=CC(=CC=3)C=3N=C4N=CC=CC4=CC=3)(C=3C4=CC=CC=C4C=CC=3)=O)=CC=CC2=C1 MTQADTZTLQGIRT-UHFFFAOYSA-N 0.000 description 1
- NBYLBWHHTUWMER-UHFFFAOYSA-N 2-Methylquinolin-8-ol Chemical compound C1=CC=C(O)C2=NC(C)=CC=C21 NBYLBWHHTUWMER-UHFFFAOYSA-N 0.000 description 1
- LVXKJHDSZNWVEE-UHFFFAOYSA-N 2-[10-(1,10-phenanthrolin-2-yl)anthracen-9-yl]-1,10-phenanthroline Chemical compound C1=CN=C2C3=NC(C4=C5C=CC=CC5=C(C=5N=C6C7=NC=CC=C7C=CC6=CC=5)C5=CC=CC=C54)=CC=C3C=CC2=C1 LVXKJHDSZNWVEE-UHFFFAOYSA-N 0.000 description 1
- QWKWMRMSNXMFOE-UHFFFAOYSA-N 2-[2,3-bis(1-phenylbenzimidazol-2-yl)phenyl]-1-phenylbenzimidazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2N=C1C1=CC=CC(C=2N(C3=CC=CC=C3N=2)C=2C=CC=CC=2)=C1C1=NC2=CC=CC=C2N1C1=CC=CC=C1 QWKWMRMSNXMFOE-UHFFFAOYSA-N 0.000 description 1
- RJBIZCOYFBKBIM-UHFFFAOYSA-N 2-[2-(2-methoxyethoxy)ethoxy]propane Chemical compound COCCOCCOC(C)C RJBIZCOYFBKBIM-UHFFFAOYSA-N 0.000 description 1
- HFBUZPXNXFVACY-UHFFFAOYSA-N 2-[3-(10-naphthalen-2-ylanthracen-9-yl)phenyl]-1-phenylbenzimidazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2N=C1C1=CC=CC(C=2C3=CC=CC=C3C(C=3C=C4C=CC=CC4=CC=3)=C3C=CC=CC3=2)=C1 HFBUZPXNXFVACY-UHFFFAOYSA-N 0.000 description 1
- MCNJVPRHIXCHLM-UHFFFAOYSA-N 2-[4-(10-naphthalen-2-ylanthracen-9-yl)phenyl]-1-phenylbenzimidazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2N=C1C1=CC=C(C=2C3=CC=CC=C3C(C=3C=C4C=CC=CC4=CC=3)=C3C=CC=CC3=2)C=C1 MCNJVPRHIXCHLM-UHFFFAOYSA-N 0.000 description 1
- VOZBMWWMIQGZGM-UHFFFAOYSA-N 2-[4-(9,10-dinaphthalen-2-ylanthracen-2-yl)phenyl]-1-phenylbenzimidazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2N=C1C1=CC=C(C=2C=C3C(C=4C=C5C=CC=CC5=CC=4)=C4C=CC=CC4=C(C=4C=C5C=CC=CC5=CC=4)C3=CC=2)C=C1 VOZBMWWMIQGZGM-UHFFFAOYSA-N 0.000 description 1
- IXHWGNYCZPISET-UHFFFAOYSA-N 2-[4-(dicyanomethylidene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene]propanedinitrile Chemical compound FC1=C(F)C(=C(C#N)C#N)C(F)=C(F)C1=C(C#N)C#N IXHWGNYCZPISET-UHFFFAOYSA-N 0.000 description 1
- WZFUQSJFWNHZHM-UHFFFAOYSA-N 2-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)CC(=O)N1CC2=C(CC1)NN=N2 WZFUQSJFWNHZHM-UHFFFAOYSA-N 0.000 description 1
- IHCCLXNEEPMSIO-UHFFFAOYSA-N 2-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperidin-1-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C1CCN(CC1)CC(=O)N1CC2=C(CC1)NN=N2 IHCCLXNEEPMSIO-UHFFFAOYSA-N 0.000 description 1
- JTAUTNBVFDTYTI-UHFFFAOYSA-N 2-fluoro-1,3-dimethylbenzene Chemical group CC1=CC=CC(C)=C1F JTAUTNBVFDTYTI-UHFFFAOYSA-N 0.000 description 1
- GDHXJNRAJRCGMX-UHFFFAOYSA-N 2-fluorobenzonitrile Chemical compound FC1=CC=CC=C1C#N GDHXJNRAJRCGMX-UHFFFAOYSA-N 0.000 description 1
- GFNZJAUVJCGWLW-UHFFFAOYSA-N 2-methoxy-1,3-dimethylbenzene Chemical compound COC1=C(C)C=CC=C1C GFNZJAUVJCGWLW-UHFFFAOYSA-N 0.000 description 1
- SJZAUIVYZWPNAS-UHFFFAOYSA-N 2-methoxy-1,4-dimethylbenzene Chemical compound COC1=CC(C)=CC=C1C SJZAUIVYZWPNAS-UHFFFAOYSA-N 0.000 description 1
- CRWNQZTZTZWPOF-UHFFFAOYSA-N 2-methyl-4-phenylpyridine Chemical compound C1=NC(C)=CC(C=2C=CC=CC=2)=C1 CRWNQZTZTZWPOF-UHFFFAOYSA-N 0.000 description 1
- XWKFPIODWVPXLX-UHFFFAOYSA-N 2-methyl-5-methylpyridine Natural products CC1=CC=C(C)N=C1 XWKFPIODWVPXLX-UHFFFAOYSA-N 0.000 description 1
- DTFKRVXLBCAIOZ-UHFFFAOYSA-N 2-methylanisole Chemical compound COC1=CC=CC=C1C DTFKRVXLBCAIOZ-UHFFFAOYSA-N 0.000 description 1
- HONWGFNQCPRRFM-UHFFFAOYSA-N 2-n-(3-methylphenyl)-1-n,1-n,2-n-triphenylbenzene-1,2-diamine Chemical compound CC1=CC=CC(N(C=2C=CC=CC=2)C=2C(=CC=CC=2)N(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 HONWGFNQCPRRFM-UHFFFAOYSA-N 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- FZTBAQBBLSYHJZ-UHFFFAOYSA-N 2-phenyl-1,3-oxazol-4-ol Chemical compound OC1=COC(C=2C=CC=CC=2)=N1 FZTBAQBBLSYHJZ-UHFFFAOYSA-N 0.000 description 1
- DWYHDSLIWMUSOO-UHFFFAOYSA-N 2-phenyl-1h-benzimidazole Chemical compound C1=CC=CC=C1C1=NC2=CC=CC=C2N1 DWYHDSLIWMUSOO-UHFFFAOYSA-N 0.000 description 1
- GYUPAYHPAZQUMB-UHFFFAOYSA-N 2-phenyl-9-[3-(9-phenyl-1,10-phenanthrolin-2-yl)phenyl]-1,10-phenanthroline Chemical compound C1=CC=CC=C1C1=CC=C(C=CC=2C3=NC(=CC=2)C=2C=C(C=CC=2)C=2N=C4C5=NC(=CC=C5C=CC4=CC=2)C=2C=CC=CC=2)C3=N1 GYUPAYHPAZQUMB-UHFFFAOYSA-N 0.000 description 1
- OXPDQFOKSZYEMJ-UHFFFAOYSA-N 2-phenylpyrimidine Chemical compound C1=CC=CC=C1C1=NC=CC=N1 OXPDQFOKSZYEMJ-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- DEQUFFZCXSTYJC-UHFFFAOYSA-N 3,4-diphenylbenzene-1,2-diamine Chemical compound C=1C=CC=CC=1C1=C(N)C(N)=CC=C1C1=CC=CC=C1 DEQUFFZCXSTYJC-UHFFFAOYSA-N 0.000 description 1
- QCAHUFWKIQLBNB-UHFFFAOYSA-N 3-(3-methoxypropoxy)propan-1-ol Chemical compound COCCCOCCCO QCAHUFWKIQLBNB-UHFFFAOYSA-N 0.000 description 1
- JZTPKAROPNTQQV-UHFFFAOYSA-N 3-fluorobenzonitrile Chemical compound FC1=CC=CC(C#N)=C1 JZTPKAROPNTQQV-UHFFFAOYSA-N 0.000 description 1
- OGGKVJMNFFSDEV-UHFFFAOYSA-N 3-methyl-n-[4-[4-(n-(3-methylphenyl)anilino)phenyl]phenyl]-n-phenylaniline Chemical group CC1=CC=CC(N(C=2C=CC=CC=2)C=2C=CC(=CC=2)C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C=C(C)C=CC=2)=C1 OGGKVJMNFFSDEV-UHFFFAOYSA-N 0.000 description 1
- XNXIYYFOYIUJIW-UHFFFAOYSA-N 3-methylbutylbenzene Chemical compound CC(C)CCC1=CC=CC=C1 XNXIYYFOYIUJIW-UHFFFAOYSA-N 0.000 description 1
- VATRWWPJWVCZTA-UHFFFAOYSA-N 3-oxo-n-[2-(trifluoromethyl)phenyl]butanamide Chemical compound CC(=O)CC(=O)NC1=CC=CC=C1C(F)(F)F VATRWWPJWVCZTA-UHFFFAOYSA-N 0.000 description 1
- DHDHJYNTEFLIHY-UHFFFAOYSA-N 4,7-diphenyl-1,10-phenanthroline Chemical compound C1=CC=CC=C1C1=CC=NC2=C1C=CC1=C(C=3C=CC=CC=3)C=CN=C21 DHDHJYNTEFLIHY-UHFFFAOYSA-N 0.000 description 1
- DAGKHJDZYJFWSO-UHFFFAOYSA-N 4-fluoro-1,2-dimethoxybenzene Chemical compound COC1=CC=C(F)C=C1OC DAGKHJDZYJFWSO-UHFFFAOYSA-N 0.000 description 1
- LVUBSVWMOWKPDJ-UHFFFAOYSA-N 4-methoxy-1,2-dimethylbenzene Chemical compound COC1=CC=C(C)C(C)=C1 LVUBSVWMOWKPDJ-UHFFFAOYSA-N 0.000 description 1
- 229940077398 4-methyl anisole Drugs 0.000 description 1
- UQRONKZLYKUEMO-UHFFFAOYSA-N 4-methyl-1-(2,4,6-trimethylphenyl)pent-4-en-2-one Chemical group CC(=C)CC(=O)Cc1c(C)cc(C)cc1C UQRONKZLYKUEMO-UHFFFAOYSA-N 0.000 description 1
- ZOKIJILZFXPFTO-UHFFFAOYSA-N 4-methyl-n-[4-[1-[4-(4-methyl-n-(4-methylphenyl)anilino)phenyl]cyclohexyl]phenyl]-n-(4-methylphenyl)aniline Chemical compound C1=CC(C)=CC=C1N(C=1C=CC(=CC=1)C1(CCCCC1)C=1C=CC(=CC=1)N(C=1C=CC(C)=CC=1)C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 ZOKIJILZFXPFTO-UHFFFAOYSA-N 0.000 description 1
- AOQKGYRILLEVJV-UHFFFAOYSA-N 4-naphthalen-1-yl-3,5-diphenyl-1,2,4-triazole Chemical compound C1=CC=CC=C1C(N1C=2C3=CC=CC=C3C=CC=2)=NN=C1C1=CC=CC=C1 AOQKGYRILLEVJV-UHFFFAOYSA-N 0.000 description 1
- LZASVYBZAXHNCX-UHFFFAOYSA-N 5,6-dimethyl-1,2,3,4-tetraphenylacridine-1,2-diamine Chemical compound CC=1C(=C2N=C3C(=C(C(C(C3=CC2=CC1)(N)C1=CC=CC=C1)(N)C1=CC=CC=C1)C1=CC=CC=C1)C1=CC=CC=C1)C LZASVYBZAXHNCX-UHFFFAOYSA-N 0.000 description 1
- FNERQQDCQZWYOC-UHFFFAOYSA-N 5-(10-naphthalen-2-ylanthracen-9-yl)-1,2-diphenylbenzimidazole Chemical compound C1=CC=CC=C1C1=NC2=CC(C=3C4=CC=CC=C4C(C=4C=C5C=CC=CC5=CC=4)=C4C=CC=CC4=3)=CC=C2N1C1=CC=CC=C1 FNERQQDCQZWYOC-UHFFFAOYSA-N 0.000 description 1
- DEYHZYIPSXJFEO-UHFFFAOYSA-N 5-[3,5-bis(1,10-phenanthrolin-5-yl)phenyl]-1,10-phenanthroline Chemical compound C12=CC=CN=C2C2=NC=CC=C2C=C1C1=CC(C=2C3=CC=CN=C3C3=NC=CC=C3C=2)=CC(C=2C3=CC=CN=C3C3=NC=CC=C3C=2)=C1 DEYHZYIPSXJFEO-UHFFFAOYSA-N 0.000 description 1
- CKOCSOGJXPVDMI-UHFFFAOYSA-N 5-[6-(1,10-phenanthrolin-5-yl)pyridin-2-yl]-1,10-phenanthroline Chemical compound C1=CC=C2C(C=3C=CC=C(N=3)C=3C4=CC=CN=C4C4=NC=CC=C4C=3)=CC3=CC=CN=C3C2=N1 CKOCSOGJXPVDMI-UHFFFAOYSA-N 0.000 description 1
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical group N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 1
- NNPSETUREXYULF-UHFFFAOYSA-N 9-[2,3-bis(3,6-dimethylcarbazol-9-yl)-5-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl]-3,6-dimethylcarbazole Chemical compound C1(=CC=CC=C1)C1=NC(=NC(=N1)C1=CC=CC=C1)C=1C=C(C(=C(C=1)N1C2=CC=C(C=C2C=2C=C(C=CC1=2)C)C)N1C2=CC=C(C=C2C=2C=C(C=CC1=2)C)C)N1C2=CC=C(C=C2C=2C=C(C=CC1=2)C)C NNPSETUREXYULF-UHFFFAOYSA-N 0.000 description 1
- BCGGKXAVNZIPAL-UHFFFAOYSA-N 9-phenyl-N-[1-phenyl-4-[4-(N-(9-phenylcarbazol-3-yl)anilino)phenyl]cyclohexa-2,4-dien-1-yl]carbazol-3-amine Chemical compound C1(=CC=CC=C1)C1(CC=C(C=C1)C1=CC=C(C=C1)N(C=1C=CC=2N(C3=CC=CC=C3C2C1)C1=CC=CC=C1)C1=CC=CC=C1)NC=1C=CC=2N(C3=CC=CC=C3C2C1)C1=CC=CC=C1 BCGGKXAVNZIPAL-UHFFFAOYSA-N 0.000 description 1
- VIJYEGDOKCKUOL-UHFFFAOYSA-N 9-phenylcarbazole Chemical compound C1=CC=CC=C1N1C2=CC=CC=C2C2=CC=CC=C21 VIJYEGDOKCKUOL-UHFFFAOYSA-N 0.000 description 1
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229920000178 Acrylic resin Polymers 0.000 description 1
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- 229910001148 Al-Li alloy Inorganic materials 0.000 description 1
- 241000156724 Antirhea Species 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- SLJNLVQLNZVMSG-UHFFFAOYSA-N C(C(CC(C1)C2)C3)C1CC23c1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 Chemical compound C(C(CC(C1)C2)C3)C1CC23c1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 SLJNLVQLNZVMSG-UHFFFAOYSA-N 0.000 description 1
- DFWQIDUMUFQCGK-UHFFFAOYSA-N C(C1C23)=CC=C2N(c2ccccc2)c2ccccc2B3c2ccccc2N1c1ccccc1 Chemical compound C(C1C23)=CC=C2N(c2ccccc2)c2ccccc2B3c2ccccc2N1c1ccccc1 DFWQIDUMUFQCGK-UHFFFAOYSA-N 0.000 description 1
- HWBYKUUZWINGTP-UHFFFAOYSA-N C(CCC)OCC1=CC=CC=C1C1(CC=C(C=C1)COCCCCCCC)C Chemical compound C(CCC)OCC1=CC=CC=C1C1(CC=C(C=C1)COCCCCCCC)C HWBYKUUZWINGTP-UHFFFAOYSA-N 0.000 description 1
- JUQCGSDZVFVHQU-UHFFFAOYSA-N C1(=CC=CC=C1)C1(CC(=CC(=C1)N(C1=CC=CC=C1)C1=CC=CC=C1)N(C1=CC=CC=C1)C1=CC=CC=C1)N Chemical compound C1(=CC=CC=C1)C1(CC(=CC(=C1)N(C1=CC=CC=C1)C1=CC=CC=C1)N(C1=CC=CC=C1)C1=CC=CC=C1)N JUQCGSDZVFVHQU-UHFFFAOYSA-N 0.000 description 1
- VONZRMHLQXCFCC-UHFFFAOYSA-N C1=CC=CC=2CC=3C4=C(C=5C(=CC=CC5C=C4C=CC3)N)C12 Chemical compound C1=CC=CC=2CC=3C4=C(C=5C(=CC=CC5C=C4C=CC3)N)C12 VONZRMHLQXCFCC-UHFFFAOYSA-N 0.000 description 1
- ZAJWPXVXVHDPFX-UHFFFAOYSA-N C1c2ccccc2N(c2cc(N(c3ccccc3)c3cccc-4c3B3c5c6[n]-4c4ccccc4c6ccc5)c3cc2)c2c1cccc2 Chemical compound C1c2ccccc2N(c2cc(N(c3ccccc3)c3cccc-4c3B3c5c6[n]-4c4ccccc4c6ccc5)c3cc2)c2c1cccc2 ZAJWPXVXVHDPFX-UHFFFAOYSA-N 0.000 description 1
- SVMXCGOMPPMHCP-UHFFFAOYSA-N CC=1C(=C(C=CC=1)B(C1=C(C(=CC=C1)C)C)C1=C(C(=CC=C1)C)C)C Chemical compound CC=1C(=C(C=CC=1)B(C1=C(C(=CC=C1)C)C)C1=C(C(=CC=C1)C)C)C SVMXCGOMPPMHCP-UHFFFAOYSA-N 0.000 description 1
- XZDSIYYRBYJKMS-UHFFFAOYSA-N CCCCc(cc1)cc(B2c(c(N3c4ccccc4)c4)ccc4N(c4ccccc4)c4ccccc4)c1N(c1ccc(CCCC)c(C)c1)c1c2c3ccc1 Chemical compound CCCCc(cc1)cc(B2c(c(N3c4ccccc4)c4)ccc4N(c4ccccc4)c4ccccc4)c1N(c1ccc(CCCC)c(C)c1)c1c2c3ccc1 XZDSIYYRBYJKMS-UHFFFAOYSA-N 0.000 description 1
- NXOVRLKWTMUOLM-UHFFFAOYSA-N CCCCc1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 Chemical compound CCCCc1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 NXOVRLKWTMUOLM-UHFFFAOYSA-N 0.000 description 1
- SDFLTYHTFPTIGX-UHFFFAOYSA-N C[n]1c(cccc2)c2c2c1cccc2 Chemical compound C[n]1c(cccc2)c2c2c1cccc2 SDFLTYHTFPTIGX-UHFFFAOYSA-N 0.000 description 1
- XCZSSFBOHUMROA-UHFFFAOYSA-N Cc(cc1)c2Oc3ccccc3B(c3ccc4)c2c1-[n]1c3c4c2ccccc12 Chemical compound Cc(cc1)c2Oc3ccccc3B(c3ccc4)c2c1-[n]1c3c4c2ccccc12 XCZSSFBOHUMROA-UHFFFAOYSA-N 0.000 description 1
- QVYVUDHKZKBNGD-UHFFFAOYSA-N Cc1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 Chemical compound Cc1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 QVYVUDHKZKBNGD-UHFFFAOYSA-N 0.000 description 1
- 239000004709 Chlorinated polyethylene Substances 0.000 description 1
- 229920000089 Cyclic olefin copolymer Polymers 0.000 description 1
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 239000004641 Diallyl-phthalate Substances 0.000 description 1
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 1
- QMMFVYPAHWMCMS-UHFFFAOYSA-N Dimethyl sulfide Chemical group CSC QMMFVYPAHWMCMS-UHFFFAOYSA-N 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 229920000219 Ethylene vinyl alcohol Polymers 0.000 description 1
- VVCWPJCGHSLBNV-UHFFFAOYSA-N Fc(cc1F)ccc1N(c1cc(N(c(ccc(F)c2)c2F)c2c(B3c4ccccc4N4c5ccccc5)c4cc(N(c4ccccc4)c4ccccc4)c2)c3cc1B1c2ccccc2N2c3ccccc3)c3c1c2cc(N(c1ccccc1)c1ccccc1)c3 Chemical compound Fc(cc1F)ccc1N(c1cc(N(c(ccc(F)c2)c2F)c2c(B3c4ccccc4N4c5ccccc5)c4cc(N(c4ccccc4)c4ccccc4)c2)c3cc1B1c2ccccc2N2c3ccccc3)c3c1c2cc(N(c1ccccc1)c1ccccc1)c3 VVCWPJCGHSLBNV-UHFFFAOYSA-N 0.000 description 1
- HCQNQJFCFNIIIT-UHFFFAOYSA-N Fc1cc(N(c2cc(N(c(cc3F)cc(F)c3F)c3c(B4c(cccc5)c5N5c6ccccc6)c5cc(N(c5ccccc5)c5ccccc5)c3)c4cc2B2c3ccccc3N3c4ccccc4)c4c2c3cc(N(c2ccccc2)c2ccccc2)c4)cc(F)c1F Chemical compound Fc1cc(N(c2cc(N(c(cc3F)cc(F)c3F)c3c(B4c(cccc5)c5N5c6ccccc6)c5cc(N(c5ccccc5)c5ccccc5)c3)c4cc2B2c3ccccc3N3c4ccccc4)c4c2c3cc(N(c2ccccc2)c2ccccc2)c4)cc(F)c1F HCQNQJFCFNIIIT-UHFFFAOYSA-N 0.000 description 1
- QKLZGVRZOUOUGX-UHFFFAOYSA-N Fc1cccc(F)c1N(c1ccccc1)c1cc(N(c2ccccc2)c2cc(N(c3ccccc3)c3cc(N(c4ccccc4)c(c(F)ccc4)c4F)cc4c3B3c(cccc5)c5N4c(c(F)ccc4)c4F)c3cc2B2c3ccccc3N3c(c(F)ccc4)c4F)c2c3c1 Chemical compound Fc1cccc(F)c1N(c1ccccc1)c1cc(N(c2ccccc2)c2cc(N(c3ccccc3)c3cc(N(c4ccccc4)c(c(F)ccc4)c4F)cc4c3B3c(cccc5)c5N4c(c(F)ccc4)c4F)c3cc2B2c3ccccc3N3c(c(F)ccc4)c4F)c2c3c1 QKLZGVRZOUOUGX-UHFFFAOYSA-N 0.000 description 1
- HIHNCDHNUUTCBX-UHFFFAOYSA-N Fc1cccc(F)c1N1c2cc(N(c3ccccc3)c3ccccc3)cc(N(c3ccccc3)c3c4cccc3)c2B4c(cc2B(c3ccccc3N(c3ccccc3)c3c4)c3c3cc4N(c4ccccc4)c4ccccc4)c1cc2N3c(c(F)ccc1)c1F Chemical compound Fc1cccc(F)c1N1c2cc(N(c3ccccc3)c3ccccc3)cc(N(c3ccccc3)c3c4cccc3)c2B4c(cc2B(c3ccccc3N(c3ccccc3)c3c4)c3c3cc4N(c4ccccc4)c4ccccc4)c1cc2N3c(c(F)ccc1)c1F HIHNCDHNUUTCBX-UHFFFAOYSA-N 0.000 description 1
- 229910000846 In alloy Inorganic materials 0.000 description 1
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical class C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 1
- 229910021578 Iron(III) chloride Inorganic materials 0.000 description 1
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-diisopropylethylamine Substances CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 1
- HUWZNFLLGUSKBD-UHFFFAOYSA-N N,N-diphenyl-9H-carbazol-1-amine Chemical compound C1=CC=CC=C1N(C=1C=2NC3=CC=CC=C3C=2C=CC=1)C1=CC=CC=C1 HUWZNFLLGUSKBD-UHFFFAOYSA-N 0.000 description 1
- MKYBYDHXWVHEJW-UHFFFAOYSA-N N-[1-oxo-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propan-2-yl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(C(C)NC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 MKYBYDHXWVHEJW-UHFFFAOYSA-N 0.000 description 1
- AFCARXCZXQIEQB-UHFFFAOYSA-N N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(CCNC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 AFCARXCZXQIEQB-UHFFFAOYSA-N 0.000 description 1
- JSGBQPDSAPOUAB-UHFFFAOYSA-N N-phenylaniline 2-phenylaniline Chemical compound C1(=C(C=CC=C1)N)C1=CC=CC=C1.C1(=CC=CC=C1)NC1=CC=CC=C1 JSGBQPDSAPOUAB-UHFFFAOYSA-N 0.000 description 1
- GPCVKMHOIXIFSL-UHFFFAOYSA-N N1=NC2=NC=CC(NN=N3)=C2C3=N1 Chemical compound N1=NC2=NC=CC(NN=N3)=C2C3=N1 GPCVKMHOIXIFSL-UHFFFAOYSA-N 0.000 description 1
- HGVNXEVNBBVJGZ-UHFFFAOYSA-N O1C2=C(N(C3=CC=CC=C13)C1=CC=C(C3=CC(C#N)=C(C#N)C=C3C3=CC=C(N4C5=CC=CC=C5OC5=C4C=CC=C5)C=C3)C=C1)C=CC=C2 Chemical compound O1C2=C(N(C3=CC=CC=C13)C1=CC=C(C3=CC(C#N)=C(C#N)C=C3C3=CC=C(N4C5=CC=CC=C5OC5=C4C=CC=C5)C=C3)C=C1)C=CC=C2 HGVNXEVNBBVJGZ-UHFFFAOYSA-N 0.000 description 1
- MIQQGEVEPAFKQY-UHFFFAOYSA-N O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.Cl.Cl Chemical compound O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.O=C(C=CC1=CC=CC=C1)C=CC1=CC=CC=C1.Cl.Cl MIQQGEVEPAFKQY-UHFFFAOYSA-N 0.000 description 1
- 229920006197 POE laurate Polymers 0.000 description 1
- 241000282376 Panthera tigris Species 0.000 description 1
- PWATWSYOIIXYMA-UHFFFAOYSA-N Pentylbenzene Chemical compound CCCCCC1=CC=CC=C1 PWATWSYOIIXYMA-UHFFFAOYSA-N 0.000 description 1
- 240000004760 Pimpinella anisum Species 0.000 description 1
- 239000004962 Polyamide-imide Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 229920000265 Polyparaphenylene Polymers 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 229920001328 Polyvinylidene chloride Polymers 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- 229910004205 SiNX Inorganic materials 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- MDYOLVRUBBJPFM-UHFFFAOYSA-N Tropolone Natural products OC1=CC=CC=CC1=O MDYOLVRUBBJPFM-UHFFFAOYSA-N 0.000 description 1
- 229920001807 Urea-formaldehyde Polymers 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- NYXIPJBBAPFGIR-UHFFFAOYSA-K [Al+3].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-] Chemical compound [Al+3].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-].C1(=CC=CC=C1)C=1C=C(C=CC1)[O-] NYXIPJBBAPFGIR-UHFFFAOYSA-K 0.000 description 1
- OADHDOBGGVWIRP-UHFFFAOYSA-K [Al+3].CC1=C(C(=C(C(=C1)C)C)C)[O-].CC1=NC2=C(C=CC=C2C=C1)[O-].CC1=NC2=C(C=CC=C2C=C1)[O-] Chemical compound [Al+3].CC1=C(C(=C(C(=C1)C)C)C)[O-].CC1=NC2=C(C=CC=C2C=C1)[O-].CC1=NC2=C(C=CC=C2C=C1)[O-] OADHDOBGGVWIRP-UHFFFAOYSA-K 0.000 description 1
- LZGPSPLQOINXDX-UHFFFAOYSA-K [Al+3].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-] Chemical compound [Al+3].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-].CC1=NC2=C(C=CC(=C2C=C1)C(F)(F)F)[O-] LZGPSPLQOINXDX-UHFFFAOYSA-K 0.000 description 1
- FIXWTUSAJYMHPC-UHFFFAOYSA-K [Al+3].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-] Chemical compound [Al+3].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-].CC1=NC2=C(C=CC=C2C(=C1)OC)[O-] FIXWTUSAJYMHPC-UHFFFAOYSA-K 0.000 description 1
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 1
- 125000004062 acenaphthenyl group Chemical group C1(CC2=CC=CC3=CC=CC1=C23)* 0.000 description 1
- 229920001893 acrylonitrile styrene Polymers 0.000 description 1
- 229920001923 acrylonitrile-ethylene-styrene Polymers 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000007605 air drying Methods 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910001508 alkali metal halide Inorganic materials 0.000 description 1
- 150000008045 alkali metal halides Chemical class 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 229910001615 alkaline earth metal halide Inorganic materials 0.000 description 1
- 229920000180 alkyd Polymers 0.000 description 1
- OVKDFILSBMEKLT-UHFFFAOYSA-N alpha-Terpineol Natural products CC(=C)C1(O)CCC(C)=CC1 OVKDFILSBMEKLT-UHFFFAOYSA-N 0.000 description 1
- WUOACPNHFRMFPN-UHFFFAOYSA-N alpha-terpineol Chemical compound CC1=CCC(C(C)(C)O)CC1 WUOACPNHFRMFPN-UHFFFAOYSA-N 0.000 description 1
- 229940088601 alpha-terpineol Drugs 0.000 description 1
- AZIDZQPJVYZBCI-UHFFFAOYSA-K aluminum;2,3-dimethylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC=CC([O-])=C1C.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 AZIDZQPJVYZBCI-UHFFFAOYSA-K 0.000 description 1
- LYPCCIQMOSCMGG-UHFFFAOYSA-K aluminum;2,4-dimethylquinolin-8-olate Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC=C([O-])C2=NC(C)=CC(C)=C21 LYPCCIQMOSCMGG-UHFFFAOYSA-K 0.000 description 1
- MIRXSQSUOBXMJX-UHFFFAOYSA-K aluminum;2,4-dimethylquinolin-8-olate;2-phenylphenolate Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC=C([O-])C2=NC(C)=CC(C)=C21.[O-]C1=CC=CC=C1C1=CC=CC=C1 MIRXSQSUOBXMJX-UHFFFAOYSA-K 0.000 description 1
- RLAHWXMEUXXBSY-UHFFFAOYSA-K aluminum;2,4-dimethylquinolin-8-olate;4-phenylphenolate Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC([O-])=CC=C1C1=CC=CC=C1 RLAHWXMEUXXBSY-UHFFFAOYSA-K 0.000 description 1
- HJIPNTADLUEEED-UHFFFAOYSA-K aluminum;2,6-dimethylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC=CC(C)=C1[O-].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 HJIPNTADLUEEED-UHFFFAOYSA-K 0.000 description 1
- WEEMWGRPEJDDTR-UHFFFAOYSA-K aluminum;2-methylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC=CC=C1[O-].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 WEEMWGRPEJDDTR-UHFFFAOYSA-K 0.000 description 1
- DLIRCHDELWASPG-UHFFFAOYSA-K aluminum;3,4-dimethylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC=C([O-])C=C1C.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 DLIRCHDELWASPG-UHFFFAOYSA-K 0.000 description 1
- YTNLZZMHIQJAHM-UHFFFAOYSA-K aluminum;3,4-dimethylquinolin-8-olate Chemical compound [Al+3].[O-]C1=CC=CC2=C(C)C(C)=CN=C21.[O-]C1=CC=CC2=C(C)C(C)=CN=C21.[O-]C1=CC=CC2=C(C)C(C)=CN=C21 YTNLZZMHIQJAHM-UHFFFAOYSA-K 0.000 description 1
- AGYQALRMKURINO-UHFFFAOYSA-K aluminum;3,5-dimethylphenolate;2,4-dimethylquinolin-8-olate Chemical compound [Al+3].CC1=CC(C)=CC([O-])=C1.C1=CC=C([O-])C2=NC(C)=CC(C)=C21.C1=CC=C([O-])C2=NC(C)=CC(C)=C21 AGYQALRMKURINO-UHFFFAOYSA-K 0.000 description 1
- BZHLLHPAFRKZAV-UHFFFAOYSA-K aluminum;3,5-dimethylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC(C)=CC([O-])=C1.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 BZHLLHPAFRKZAV-UHFFFAOYSA-K 0.000 description 1
- NXJQXEAVOUKGEY-UHFFFAOYSA-K aluminum;3-methylphenolate;2-methylquinolin-8-olate Chemical compound [Al+3].CC1=CC=CC([O-])=C1.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 NXJQXEAVOUKGEY-UHFFFAOYSA-K 0.000 description 1
- KBQCKHMZXMVXSK-UHFFFAOYSA-K aluminum;4,5-dimethylquinolin-8-olate Chemical compound [Al+3].C1=CC(C)=C2C(C)=CC=NC2=C1[O-].C1=CC(C)=C2C(C)=CC=NC2=C1[O-].C1=CC(C)=C2C(C)=CC=NC2=C1[O-] KBQCKHMZXMVXSK-UHFFFAOYSA-K 0.000 description 1
- NKLUNHCWDRAGIH-UHFFFAOYSA-K aluminum;4,6-dimethylquinolin-8-olate Chemical compound [Al+3].N1=CC=C(C)C2=CC(C)=CC([O-])=C21.N1=CC=C(C)C2=CC(C)=CC([O-])=C21.N1=CC=C(C)C2=CC(C)=CC([O-])=C21 NKLUNHCWDRAGIH-UHFFFAOYSA-K 0.000 description 1
- 229940107816 ammonium iodide Drugs 0.000 description 1
- 239000010405 anode material Substances 0.000 description 1
- RGHILYZRVFRRNK-UHFFFAOYSA-N anthracene-1,2-dione Chemical compound C1=CC=C2C=C(C(C(=O)C=C3)=O)C3=CC2=C1 RGHILYZRVFRRNK-UHFFFAOYSA-N 0.000 description 1
- 229940058303 antinematodal benzimidazole derivative Drugs 0.000 description 1
- 229940054051 antipsychotic indole derivative Drugs 0.000 description 1
- 229940027991 antiseptic and disinfectant quinoline derivative Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- WUICPPBYLKNKNS-UHFFFAOYSA-N benzene-1,2,3-tricarbonitrile Chemical compound N#CC1=CC=CC(C#N)=C1C#N WUICPPBYLKNKNS-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- HFACYLZERDEVSX-UHFFFAOYSA-N benzidine Chemical class C1=CC(N)=CC=C1C1=CC=C(N)C=C1 HFACYLZERDEVSX-UHFFFAOYSA-N 0.000 description 1
- UDEWPOVQBGFNGE-UHFFFAOYSA-N benzoic acid n-propyl ester Natural products CCCOC(=O)C1=CC=CC=C1 UDEWPOVQBGFNGE-UHFFFAOYSA-N 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical group C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- ATBAMAFKBVZNFJ-UHFFFAOYSA-N beryllium atom Chemical compound [Be] ATBAMAFKBVZNFJ-UHFFFAOYSA-N 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229920001222 biopolymer Chemical class 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- QUDWYFHPNIMBFC-UHFFFAOYSA-N bis(prop-2-enyl) benzene-1,2-dicarboxylate Chemical compound C=CCOC(=O)C1=CC=CC=C1C(=O)OCC=C QUDWYFHPNIMBFC-UHFFFAOYSA-N 0.000 description 1
- MPCSRDRXSVSWPI-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-(2-phenylphenoxy)alumane Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.[O-]C1=CC=CC=C1C1=CC=CC=C1 MPCSRDRXSVSWPI-UHFFFAOYSA-K 0.000 description 1
- WDIXNURAWJTREU-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-(3-phenylphenoxy)alumane Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.[O-]C1=CC=CC(C=2C=CC=CC=2)=C1 WDIXNURAWJTREU-UHFFFAOYSA-K 0.000 description 1
- UFVXQDWNSAGPHN-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-(4-phenylphenoxy)alumane Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC([O-])=CC=C1C1=CC=CC=C1 UFVXQDWNSAGPHN-UHFFFAOYSA-K 0.000 description 1
- YNIPMNHYVHUEBM-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-naphthalen-1-yloxyalumane Chemical compound [Al+3].C1=CC=C2C([O-])=CC=CC2=C1.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 YNIPMNHYVHUEBM-UHFFFAOYSA-K 0.000 description 1
- YUXXRZQLFGGXRJ-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-naphthalen-2-yloxyalumane Chemical compound [Al+3].C1=CC=CC2=CC([O-])=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21 YUXXRZQLFGGXRJ-UHFFFAOYSA-K 0.000 description 1
- CCAADOWYZMBENE-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-phenoxyalumane Chemical compound C12=NC(C)=CC=C2C=CC=C1O[Al](OC=1C2=NC(C)=CC=C2C=CC=1)OC1=CC=CC=C1 CCAADOWYZMBENE-UHFFFAOYSA-K 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 125000000707 boryl group Chemical group B* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- HQABUPZFAYXKJW-UHFFFAOYSA-O butylazanium Chemical compound CCCC[NH3+] HQABUPZFAYXKJW-UHFFFAOYSA-O 0.000 description 1
- NAVKJPVRJRHSGH-UHFFFAOYSA-N c(cc1)cc(B2c(cc3S(c(cccc4)c4Oc4ccc5)c4c5Oc3c3)c3O3)c1Oc1c2c3ccc1 Chemical compound c(cc1)cc(B2c(cc3S(c(cccc4)c4Oc4ccc5)c4c5Oc3c3)c3O3)c1Oc1c2c3ccc1 NAVKJPVRJRHSGH-UHFFFAOYSA-N 0.000 description 1
- NQPPZVBLFAVOBP-UHFFFAOYSA-N c(cc1)cc2c1Sc1cccc(Sc3c4)c1B2c3cc1c4Sc2cccc3c2B1c(cccc1)c1S3 Chemical compound c(cc1)cc2c1Sc1cccc(Sc3c4)c1B2c3cc1c4Sc2cccc3c2B1c(cccc1)c1S3 NQPPZVBLFAVOBP-UHFFFAOYSA-N 0.000 description 1
- VOOMCEYFGNFUQU-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)cc2c1c(-c1ccc(-c3ccccn3)nc1)c(cccc1)c1c2-c(cc1)cnc1-c1ncccc1 Chemical compound c(cc1)ccc1-c(cc1)cc2c1c(-c1ccc(-c3ccccn3)nc1)c(cccc1)c1c2-c(cc1)cnc1-c1ncccc1 VOOMCEYFGNFUQU-UHFFFAOYSA-N 0.000 description 1
- LYDUQPPTTRDAKD-UHFFFAOYSA-N c(cc1)ccc1N(c1cccc-2c1B1c3c4[n]-2c2ccccc2c4ccc3)c2c1ccc(-[n]1c3ccccc3c3c1cccc3)c2 Chemical compound c(cc1)ccc1N(c1cccc-2c1B1c3c4[n]-2c2ccccc2c4ccc3)c2c1ccc(-[n]1c3ccccc3c3c1cccc3)c2 LYDUQPPTTRDAKD-UHFFFAOYSA-N 0.000 description 1
- SZGHWAZZVXJRCX-UHFFFAOYSA-N c(cc1)ccc1N(c1cccc-2c1B1c3c4[n]-2c2ccccc2c4ccc3)c2c1ccc(N1c(cccc3)c3Oc3c1cccc3)c2 Chemical compound c(cc1)ccc1N(c1cccc-2c1B1c3c4[n]-2c2ccccc2c4ccc3)c2c1ccc(N1c(cccc3)c3Oc3c1cccc3)c2 SZGHWAZZVXJRCX-UHFFFAOYSA-N 0.000 description 1
- JVPUIBHYTGRXEE-UHFFFAOYSA-N c(cc1)ccc1N(c1ccccc1)c1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 Chemical compound c(cc1)ccc1N(c1ccccc1)c1cc(N(c2ccccc2)c2cccc-3c2B2c4c5[n]-3c3ccccc3c5ccc4)c2cc1 JVPUIBHYTGRXEE-UHFFFAOYSA-N 0.000 description 1
- MWOLQTPIBFMGAZ-UHFFFAOYSA-N c(cc1)ccc1N(c1ccccc11)c2cccc(N(c3ccccc3)c3c4)c2B1c3cc(B1c(cccc2)c2N2c3ccccc3)c4N(c3ccccc3)c3c1c2ccc3 Chemical compound c(cc1)ccc1N(c1ccccc11)c2cccc(N(c3ccccc3)c3c4)c2B1c3cc(B1c(cccc2)c2N2c3ccccc3)c4N(c3ccccc3)c3c1c2ccc3 MWOLQTPIBFMGAZ-UHFFFAOYSA-N 0.000 description 1
- FMJQRMRQZRRPEH-UHFFFAOYSA-N c(cc1)ccc1N1c2cccc(-[n](c3c4)c5c6cccc5c3ccc4-c3ccc4Oc5cccc(-[n]7c(c8ccc9)c9c9ccccc79)c5B8c4c3)c2B6c2c1cccc2 Chemical compound c(cc1)ccc1N1c2cccc(-[n](c3c4)c5c6cccc5c3ccc4-c3ccc4Oc5cccc(-[n]7c(c8ccc9)c9c9ccccc79)c5B8c4c3)c2B6c2c1cccc2 FMJQRMRQZRRPEH-UHFFFAOYSA-N 0.000 description 1
- QEKBTPXKLDGHFO-UHFFFAOYSA-N c(cc12)ccc1-c1ccccc1C21c(cc(cc2)-c3ccc(-c4ccccn4)nc3)c2-c(cc2)c1cc2-c1ccc(-c2ccccn2)nc1 Chemical compound c(cc12)ccc1-c1ccccc1C21c(cc(cc2)-c3ccc(-c4ccccn4)nc3)c2-c(cc2)c1cc2-c1ccc(-c2ccccn2)nc1 QEKBTPXKLDGHFO-UHFFFAOYSA-N 0.000 description 1
- IITQULMOOBIEDJ-UHFFFAOYSA-N c(cc1c2c3c4ccc2)ccc1[n]3-c1cccc-2c1B4c1c3[n]-2c(cccc2)c2c3ccc1 Chemical compound c(cc1c2c3c4ccc2)ccc1[n]3-c1cccc-2c1B4c1c3[n]-2c(cccc2)c2c3ccc1 IITQULMOOBIEDJ-UHFFFAOYSA-N 0.000 description 1
- YAELXYZSNJSOCR-UHFFFAOYSA-N c1ccc2c(cc(cc3)-c4ccc(-c5ncccc5)nc4)c3c(ccc(-c(cc3)cnc3-c3ccccn3)c3)c3c2c1 Chemical compound c1ccc2c(cc(cc3)-c4ccc(-c5ncccc5)nc4)c3c(ccc(-c(cc3)cnc3-c3ccccn3)c3)c3c2c1 YAELXYZSNJSOCR-UHFFFAOYSA-N 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- KOPBYBDAPCDYFK-UHFFFAOYSA-N caesium oxide Chemical compound [O-2].[Cs+].[Cs+] KOPBYBDAPCDYFK-UHFFFAOYSA-N 0.000 description 1
- 229910001942 caesium oxide Inorganic materials 0.000 description 1
- KVUAALJSMIVURS-ZEDZUCNESA-L calcium folinate Chemical compound [Ca+2].C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C=C1 KVUAALJSMIVURS-ZEDZUCNESA-L 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 125000002676 chrysenyl group Chemical group C1(=CC=CC=2C3=CC=C4C=CC=CC4=C3C=CC12)* 0.000 description 1
- 238000010549 co-Evaporation Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 1
- OMZSGWSJDCOLKM-UHFFFAOYSA-N copper(II) sulfide Chemical compound [S-2].[Cu+2] OMZSGWSJDCOLKM-UHFFFAOYSA-N 0.000 description 1
- GBRBMTNGQBKBQE-UHFFFAOYSA-L copper;diiodide Chemical compound I[Cu]I GBRBMTNGQBKBQE-UHFFFAOYSA-L 0.000 description 1
- 150000001907 coumarones Chemical class 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- KGVUXGUFPMJPCQ-UHFFFAOYSA-N cycloocta-1,4-diene nickel Chemical compound [Ni].C1CC=CCC=CC1.C1CC=CCC=CC1 KGVUXGUFPMJPCQ-UHFFFAOYSA-N 0.000 description 1
- NLUNLVTVUDIHFE-UHFFFAOYSA-N cyclooctylcyclooctane Chemical group C1CCCCCCC1C1CCCCCCC1 NLUNLVTVUDIHFE-UHFFFAOYSA-N 0.000 description 1
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 229930007927 cymene Natural products 0.000 description 1
- ACUZDYFTRHEKOS-UHFFFAOYSA-N decan-2-ol Chemical compound CCCCCCCCC(C)O ACUZDYFTRHEKOS-UHFFFAOYSA-N 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- IYYZUPMFVPLQIF-ALWQSETLSA-N dibenzothiophene Chemical group C1=CC=CC=2[34S]C3=C(C=21)C=CC=C3 IYYZUPMFVPLQIF-ALWQSETLSA-N 0.000 description 1
- 125000005509 dibenzothiophenyl group Chemical group 0.000 description 1
- 229940117389 dichlorobenzene Drugs 0.000 description 1
- YMWUJEATGCHHMB-UHFFFAOYSA-N dichloromethane Substances ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 1
- ZBQUMMFUJLOTQC-UHFFFAOYSA-L dichloronickel;3-diphenylphosphanylpropyl(diphenyl)phosphane Chemical compound Cl[Ni]Cl.C=1C=CC=CC=1P(C=1C=CC=CC=1)CCCP(C=1C=CC=CC=1)C1=CC=CC=C1 ZBQUMMFUJLOTQC-UHFFFAOYSA-L 0.000 description 1
- 150000005690 diesters Chemical group 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 229940019778 diethylene glycol diethyl ether Drugs 0.000 description 1
- 229940028356 diethylene glycol monobutyl ether Drugs 0.000 description 1
- 229940105990 diglycerin Drugs 0.000 description 1
- GPLRAVKSCUXZTP-UHFFFAOYSA-N diglycerol Chemical compound OCC(O)COCC(O)CO GPLRAVKSCUXZTP-UHFFFAOYSA-N 0.000 description 1
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 1
- RXKJFZQQPQGTFL-UHFFFAOYSA-N dihydroxyacetone Chemical compound OCC(=O)CO RXKJFZQQPQGTFL-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- XSWSEQPWKOWORN-UHFFFAOYSA-N dodecan-2-ol Chemical compound CCCCCCCCCCC(C)O XSWSEQPWKOWORN-UHFFFAOYSA-N 0.000 description 1
- KWKXNDCHNDYVRT-UHFFFAOYSA-N dodecylbenzene Chemical compound CCCCCCCCCCCCC1=CC=CC=C1 KWKXNDCHNDYVRT-UHFFFAOYSA-N 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000005566 electron beam evaporation Methods 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000010304 firing Methods 0.000 description 1
- HVQAJTFOCKOKIN-UHFFFAOYSA-N flavonol Natural products O1C2=CC=CC=C2C(=O)C(O)=C1C1=CC=CC=C1 HVQAJTFOCKOKIN-UHFFFAOYSA-N 0.000 description 1
- 235000011957 flavonols Nutrition 0.000 description 1
- 125000003914 fluoranthenyl group Chemical class C1(=CC=C2C=CC=C3C4=CC=CC=C4C1=C23)* 0.000 description 1
- 238000001506 fluorescence spectroscopy Methods 0.000 description 1
- 125000003709 fluoroalkyl group Chemical group 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 150000002258 gallium Chemical class 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 238000012835 hanging drop method Methods 0.000 description 1
- RBTKNAXYKSUFRK-UHFFFAOYSA-N heliogen blue Chemical compound [Cu].[N-]1C2=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=NC([N-]1)=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=N2 RBTKNAXYKSUFRK-UHFFFAOYSA-N 0.000 description 1
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 1
- URRIMVUDCOMQIT-UHFFFAOYSA-N heptoxymethylbenzene Chemical compound CCCCCCCOCC1=CC=CC=C1 URRIMVUDCOMQIT-UHFFFAOYSA-N 0.000 description 1
- 125000004404 heteroalkyl group Chemical group 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 1
- CBXXPNJELNWJCH-UHFFFAOYSA-N hexoxymethylbenzene Chemical compound CCCCCCOCC1=CC=CC=C1 CBXXPNJELNWJCH-UHFFFAOYSA-N 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 125000002887 hydroxy group Chemical class [H]O* 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- 125000004857 imidazopyridinyl group Chemical class N1C(=NC2=C1C=CC=N2)* 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 229910003437 indium oxide Inorganic materials 0.000 description 1
- PJXISJQVUVHSOJ-UHFFFAOYSA-N indium(iii) oxide Chemical compound [O-2].[O-2].[O-2].[In+3].[In+3] PJXISJQVUVHSOJ-UHFFFAOYSA-N 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 150000002475 indoles Chemical class 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 229910017053 inorganic salt Inorganic materials 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229940079865 intestinal antiinfectives imidazole derivative Drugs 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000007733 ion plating Methods 0.000 description 1
- 229920000554 ionomer Polymers 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical group C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- QDLAGTHXVHQKRE-UHFFFAOYSA-N lichenxanthone Natural products COC1=CC(O)=C2C(=O)C3=C(C)C=C(OC)C=C3OC2=C1 QDLAGTHXVHQKRE-UHFFFAOYSA-N 0.000 description 1
- 239000001989 lithium alloy Substances 0.000 description 1
- FUJCRWPEOMXPAD-UHFFFAOYSA-N lithium oxide Chemical compound [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 1
- 229910001947 lithium oxide Inorganic materials 0.000 description 1
- SKEDXQSRJSUMRP-UHFFFAOYSA-N lithium;quinolin-8-ol Chemical compound [Li].C1=CN=C2C(O)=CC=CC2=C1 SKEDXQSRJSUMRP-UHFFFAOYSA-N 0.000 description 1
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 1
- SJCKRGFTWFGHGZ-UHFFFAOYSA-N magnesium silver Chemical compound [Mg].[Ag] SJCKRGFTWFGHGZ-UHFFFAOYSA-N 0.000 description 1
- JDSHMPZPIAZGSV-UHFFFAOYSA-N melamine Chemical compound NC1=NC(N)=NC(N)=N1 JDSHMPZPIAZGSV-UHFFFAOYSA-N 0.000 description 1
- 230000005499 meniscus Effects 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- AUHZEENZYGFFBQ-UHFFFAOYSA-N mesitylene Substances CC1=CC(C)=CC(C)=C1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 1
- 125000001827 mesitylenyl group Chemical group [H]C1=C(C(*)=C(C([H])=C1C([H])([H])[H])C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- OLXYLDUSSBULGU-UHFFFAOYSA-N methyl pyridine-4-carboxylate Chemical compound COC(=O)C1=CC=NC=C1 OLXYLDUSSBULGU-UHFFFAOYSA-N 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- IBHBKWKFFTZAHE-UHFFFAOYSA-N n-[4-[4-(n-naphthalen-1-ylanilino)phenyl]phenyl]-n-phenylnaphthalen-1-amine Chemical group C1=CC=CC=C1N(C=1C2=CC=CC=C2C=CC=1)C1=CC=C(C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C3=CC=CC=C3C=CC=2)C=C1 IBHBKWKFFTZAHE-UHFFFAOYSA-N 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 125000004957 naphthylene group Chemical group 0.000 description 1
- 150000005054 naphthyridines Chemical class 0.000 description 1
- KFBKRCXOTTUAFS-UHFFFAOYSA-N nickel;triphenylphosphane Chemical compound [Ni].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 KFBKRCXOTTUAFS-UHFFFAOYSA-N 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000002868 norbornyl group Chemical group C12(CCC(CC1)C2)* 0.000 description 1
- 229940078552 o-xylene Drugs 0.000 description 1
- NXZJVIKVJAKQOU-UHFFFAOYSA-N octoxymethylbenzene Chemical compound CCCCCCCCOCC1=CC=CC=C1 NXZJVIKVJAKQOU-UHFFFAOYSA-N 0.000 description 1
- 229920002114 octoxynol-9 Polymers 0.000 description 1
- CDKDZKXSXLNROY-UHFFFAOYSA-N octylbenzene Chemical group CCCCCCCCC1=CC=CC=C1 CDKDZKXSXLNROY-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-M oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC([O-])=O ZQPPMHVWECSIRJ-KTKRTIGZSA-M 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- 230000010355 oscillation Effects 0.000 description 1
- 150000004866 oxadiazoles Chemical class 0.000 description 1
- 150000004880 oxines Chemical class 0.000 description 1
- JCGNDDUYTRNOFT-UHFFFAOYSA-N oxolane-2,4-dione Chemical compound O=C1COC(=O)C1 JCGNDDUYTRNOFT-UHFFFAOYSA-N 0.000 description 1
- HFPZCAJZSCWRBC-UHFFFAOYSA-N p-cymene Chemical compound CC(C)C1=CC=C(C)C=C1 HFPZCAJZSCWRBC-UHFFFAOYSA-N 0.000 description 1
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 238000000059 patterning Methods 0.000 description 1
- RJROSTOBLTZWNX-UHFFFAOYSA-N pentacene-5,13-diamine Chemical compound C1=CC=CC2=CC3=CC4=C(C5=CC=CC=C5C=C4C(=C3C=C12)N)N RJROSTOBLTZWNX-UHFFFAOYSA-N 0.000 description 1
- XDFPTBAEJOMVDG-UHFFFAOYSA-N pentacyclo[11.7.1.02,7.09,21.015,20]henicosa-1(21),2,4,6,8,10,12,15,17,19-decaene Chemical compound C1=CC(CC=2C3=CC=CC=2)=C2C3=C(C=CC=C3)C3=CC2=C1 XDFPTBAEJOMVDG-UHFFFAOYSA-N 0.000 description 1
- RSDLTJVQMXAXCA-UHFFFAOYSA-N pentoxymethylbenzene Chemical compound CCCCCOCC1=CC=CC=C1 RSDLTJVQMXAXCA-UHFFFAOYSA-N 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 125000001791 phenazinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3N=C12)* 0.000 description 1
- DLRJIFUOBPOJNS-UHFFFAOYSA-N phenetole Chemical compound CCOC1=CC=CC=C1 DLRJIFUOBPOJNS-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-M phenolate Chemical compound [O-]C1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-M 0.000 description 1
- 229940031826 phenolate Drugs 0.000 description 1
- 229920001568 phenolic resin Polymers 0.000 description 1
- 239000005011 phenolic resin Substances 0.000 description 1
- 229950000688 phenothiazine Drugs 0.000 description 1
- GJSGGHOYGKMUPT-UHFFFAOYSA-N phenoxathiine Chemical group C1=CC=C2OC3=CC=CC=C3SC2=C1 GJSGGHOYGKMUPT-UHFFFAOYSA-N 0.000 description 1
- 125000004932 phenoxathinyl group Chemical group 0.000 description 1
- 229960005323 phenoxyethanol Drugs 0.000 description 1
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- LEVJVKGPFAQPOI-UHFFFAOYSA-N phenylmethanone Chemical compound O=[C]C1=CC=CC=C1 LEVJVKGPFAQPOI-UHFFFAOYSA-N 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical group C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920006255 plastic film Polymers 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920000636 poly(norbornene) polymer Polymers 0.000 description 1
- 229920002312 polyamide-imide Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920005596 polymer binder Polymers 0.000 description 1
- 239000002491 polymer binding agent Substances 0.000 description 1
- 239000003505 polymerization initiator Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920000056 polyoxyethylene ether Polymers 0.000 description 1
- 229940051841 polyoxyethylene ether Drugs 0.000 description 1
- 229920013636 polyphenyl ether polymer Polymers 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001021 polysulfide Polymers 0.000 description 1
- 239000005077 polysulfide Substances 0.000 description 1
- 150000008117 polysulfides Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920000123 polythiophene Polymers 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000005033 polyvinylidene chloride Substances 0.000 description 1
- 150000004033 porphyrin derivatives Chemical class 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 235000003270 potassium fluoride Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- SCUZVMOVTVSBLE-UHFFFAOYSA-N prop-2-enenitrile;styrene Chemical compound C=CC#N.C=CC1=CC=CC=C1 SCUZVMOVTVSBLE-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- LLHKCFNBLRBOGN-UHFFFAOYSA-N propylene glycol methyl ether acetate Chemical compound COCC(C)OC(C)=O LLHKCFNBLRBOGN-UHFFFAOYSA-N 0.000 description 1
- CPNGPNLZQNNVQM-UHFFFAOYSA-N pteridine Chemical group N1=CN=CC2=NC=CN=C21 CPNGPNLZQNNVQM-UHFFFAOYSA-N 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- OTVZGAXESBAAQQ-UHFFFAOYSA-N pyrazine-2,3-dicarbonitrile Chemical compound N#CC1=NC=CN=C1C#N OTVZGAXESBAAQQ-UHFFFAOYSA-N 0.000 description 1
- 150000003216 pyrazines Chemical class 0.000 description 1
- 150000003217 pyrazoles Chemical class 0.000 description 1
- 150000003219 pyrazolines Chemical class 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical group C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- GPHQHTOMRSGBNZ-UHFFFAOYSA-N pyridine-4-carbonitrile Chemical compound N#CC1=CC=NC=C1 GPHQHTOMRSGBNZ-UHFFFAOYSA-N 0.000 description 1
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 description 1
- 150000003233 pyrroles Chemical class 0.000 description 1
- 150000004322 quinolinols Chemical class 0.000 description 1
- 150000004059 quinone derivatives Chemical class 0.000 description 1
- IVYZWJPQNNZOHV-UHFFFAOYSA-N quinoxaline-2-carbonitrile Chemical compound C1=CC=CC2=NC(C#N)=CN=C21 IVYZWJPQNNZOHV-UHFFFAOYSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 229910001404 rare earth metal oxide Inorganic materials 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 238000007650 screen-printing Methods 0.000 description 1
- ZJMWRROPUADPEA-UHFFFAOYSA-N sec-butylbenzene Chemical compound CCC(C)C1=CC=CC=C1 ZJMWRROPUADPEA-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000010898 silica gel chromatography Methods 0.000 description 1
- 150000003377 silicon compounds Chemical group 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 229940100515 sorbitan Drugs 0.000 description 1
- VNFWTIYUKDMAOP-UHFFFAOYSA-N sphos Chemical compound COC1=CC=CC(OC)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 VNFWTIYUKDMAOP-UHFFFAOYSA-N 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical class C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 150000003440 styrenes Chemical class 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 238000000859 sublimation Methods 0.000 description 1
- 230000008022 sublimation Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- 239000005061 synthetic rubber Substances 0.000 description 1
- QJVXKWHHAMZTBY-GCPOEHJPSA-N syringin Chemical compound COC1=CC(\C=C\CO)=CC(OC)=C1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 QJVXKWHHAMZTBY-GCPOEHJPSA-N 0.000 description 1
- 229940042055 systemic antimycotics triazole derivative Drugs 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 229940116411 terpineol Drugs 0.000 description 1
- 229940073455 tetraethylammonium hydroxide Drugs 0.000 description 1
- LRGJRHZIDJQFCL-UHFFFAOYSA-M tetraethylazanium;hydroxide Chemical compound [OH-].CC[N+](CC)(CC)CC LRGJRHZIDJQFCL-UHFFFAOYSA-M 0.000 description 1
- 150000003536 tetrazoles Chemical group 0.000 description 1
- 238000007725 thermal activation Methods 0.000 description 1
- 150000004867 thiadiazoles Chemical class 0.000 description 1
- NZFNXWQNBYZDAQ-UHFFFAOYSA-N thioridazine hydrochloride Chemical class Cl.C12=CC(SC)=CC=C2SC2=CC=CC=C2N1CCC1CCCCN1C NZFNXWQNBYZDAQ-UHFFFAOYSA-N 0.000 description 1
- YRHRIQCWCFGUEQ-UHFFFAOYSA-N thioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3SC2=C1 YRHRIQCWCFGUEQ-UHFFFAOYSA-N 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 1
- 125000005259 triarylamine group Chemical group 0.000 description 1
- JLGLQAWTXXGVEM-UHFFFAOYSA-N triethylene glycol monomethyl ether Chemical compound COCCOCCOCCO JLGLQAWTXXGVEM-UHFFFAOYSA-N 0.000 description 1
- YFNKIDBQEZZDLK-UHFFFAOYSA-N triglyme Chemical compound COCCOCCOCCOC YFNKIDBQEZZDLK-UHFFFAOYSA-N 0.000 description 1
- 229940030010 trimethoxybenzene Drugs 0.000 description 1
- IECKAVQTURBPON-UHFFFAOYSA-N trimethoxymethylbenzene Chemical compound COC(OC)(OC)C1=CC=CC=C1 IECKAVQTURBPON-UHFFFAOYSA-N 0.000 description 1
- TZPKFPYZCMHDHL-UHFFFAOYSA-N trimethoxytoluene Natural products COC1=CC(OC)=C(C)C(OC)=C1 TZPKFPYZCMHDHL-UHFFFAOYSA-N 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000001651 triphenylamine derivatives Chemical class 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 235000019798 tripotassium phosphate Nutrition 0.000 description 1
- XRXDCKUSXVGNCW-UHFFFAOYSA-K tris[(2-methylquinolin-8-yl)oxy]alumane Chemical compound C1=C(C)N=C2C(O[Al](OC=3C4=NC(C)=CC=C4C=CC=3)OC3=CC=CC4=CC=C(N=C43)C)=CC=CC2=C1 XRXDCKUSXVGNCW-UHFFFAOYSA-K 0.000 description 1
- SXXNJJQVBPWGTP-UHFFFAOYSA-K tris[(4-methylquinolin-8-yl)oxy]alumane Chemical compound [Al+3].C1=CC=C2C(C)=CC=NC2=C1[O-].C1=CC=C2C(C)=CC=NC2=C1[O-].C1=CC=C2C(C)=CC=NC2=C1[O-] SXXNJJQVBPWGTP-UHFFFAOYSA-K 0.000 description 1
- HSRBHVUVCOUJAC-UHFFFAOYSA-K tris[(5-methylquinolin-8-yl)oxy]alumane Chemical compound [Al+3].C1=CC=C2C(C)=CC=C([O-])C2=N1.C1=CC=C2C(C)=CC=C([O-])C2=N1.C1=CC=C2C(C)=CC=C([O-])C2=N1 HSRBHVUVCOUJAC-UHFFFAOYSA-K 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- KJIOQYGWTQBHNH-UHFFFAOYSA-N undecanol Chemical compound CCCCCCCCCCCO KJIOQYGWTQBHNH-UHFFFAOYSA-N 0.000 description 1
- 229920006337 unsaturated polyester resin Polymers 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- ABDKAPXRBAPSQN-UHFFFAOYSA-N veratrole Chemical compound COC1=CC=CC=C1OC ABDKAPXRBAPSQN-UHFFFAOYSA-N 0.000 description 1
- PXXNTAGJWPJAGM-UHFFFAOYSA-N vertaline Natural products C1C2C=3C=C(OC)C(OC)=CC=3OC(C=C3)=CC=C3CCC(=O)OC1CC1N2CCCC1 PXXNTAGJWPJAGM-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 125000005023 xylyl group Chemical group 0.000 description 1
- YVTHLONGBIQYBO-UHFFFAOYSA-N zinc indium(3+) oxygen(2-) Chemical compound [O--].[Zn++].[In+3] YVTHLONGBIQYBO-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/658—Organoboranes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09F—DISPLAYING; ADVERTISING; SIGNS; LABELS OR NAME-PLATES; SEALS
- G09F9/00—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements
- G09F9/30—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements in which the desired character or characters are formed by combining individual elements
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/361—Polynuclear complexes, i.e. complexes comprising two or more metal centers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/18—Metal complexes
- C09K2211/188—Metal complexes of other metals not provided for in one of the previous groups
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
Definitions
- the present invention relates to an organic electroluminescent device including a host compound, a thermally activated delayed phosphor and a compound having a boron atom, and a display device and a lighting device including the organic electroluminescent device.
- the present invention also relates to a composition and a compound for forming a light emitting layer of an organic electroluminescent device.
- the organic electroluminescent element has a structure including a pair of electrodes including an anode and a cathode, and one or more layers including an organic compound disposed between the pair of electrodes.
- the layer containing an organic compound include a light-emitting layer and a charge transport / injection layer that transports or injects charges such as holes and electrons.
- Various organic materials suitable for these layers have been developed.
- ⁇ ⁇ There are mainly two light emission mechanisms of the organic electroluminescent element: fluorescence emission using light emission from an excited singlet state and phosphorescence emission using light emission from an excited triplet state.
- Common fluorescent light emitting materials have low exciton utilization efficiency, about 25%, and have triplet-triplet fusion (TTF: Triplet-Triplet @ Fusion or triplet-triplet annihilation, TTA: Triplet-Triplet @ Annihilation). ), The exciton utilization efficiency is 62.5%.
- phosphorescent materials may have an exciton utilization efficiency of 100% in some cases, but have difficulty in achieving deep blue light emission, and have a problem that the color purity is low due to the wide emission spectrum.
- Non-Patent Document 1 a thermally activated delayed fluorescent (TADF) mechanism (see Non-Patent Document 1), and the use of a thermally activated delayed fluorescent substance to emit light.
- the exciton utilization efficiency has reached 100%.
- the heat-activated delayed fluorescent substance gives a broad emission spectrum with low color purity due to its structure, the speed of inverse intersystem crossing is extremely high.
- Adachi and colleagues take advantage of this advantage, using thermally activated delayed phosphors as assisting dopants (Assisting Dopant: AD), and using narrow half bandwidth dopants as emitting dopants (Emitting Dopant: ED).
- Hyper Fluorescence TM TADF Assisting Fluorescence: also called TAF
- TAF Trigger Fluorescence TM
- a high-efficiency, high-color-purity, and long-life element has been developed as a red- and green-emitting organic electroluminescent element (see Non-Patent Document 2).
- deep blue emission has problems in any of efficiency, color purity, and lifetime because both the emitting dopant and the assisting dopant require high energy.
- Patent Document 3 a new molecular design that dramatically improves the color purity of TADF materials has been proposed by Professor Kwansei Gakuin University Hatakeyama (see Non-Patent Document 3).
- the compound (1-401) disclosed has a robust planar structure utilizing the multiple resonance effect of boron (electron donating) and nitrogen (electron withdrawing), resulting in absorption and emission. Successfully obtained an emission spectrum having a small Stokes shift and a high color purity.
- the dimer compound represented by the formula (1-422) two borons and two nitrogens are bonded to the central benzene ring, so that the multiple resonance effect is further enhanced in the central benzene ring. As a result, it is possible to emit light having an extremely narrow emission peak width.
- organic electroluminescent properties such as luminescent properties are further enhanced, and options of organic electroluminescent materials such as a material for a light emitting layer are increased.
- combinations of compounds that have not been specifically known hitherto are desired.
- the present inventors have conducted intensive studies to solve the above problems, and as a result, using a host compound, a thermally activated delayed fluorescent substance, and a light emitting layer containing a compound having a boron atom in a molecule, their excited singlet energy levels It has been found that by defining the positional relationship, an excellent organic electroluminescent device can be obtained, and the present invention has been completed. Specifically, the present invention has the following configuration.
- An organic electroluminescent device having a light emitting layer, wherein the light emitting layer is As a first component, at least one host compound; As a second component, at least one heat-activated delayed phosphor; A compound having at least one type of boron atom as the third component,
- the excitation singlet energy level obtained from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the first component is E (1, S, Sh), and the excitation singlet energy level is obtained from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the second component.
- the excited singlet energy level is E (2, S, Sh)
- the excited singlet energy level determined from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the third component is E (3, S, Sh).
- the first component may be included as a polymer compound having a structure in which two hydrogen atoms of the host compound are eliminated as a repeating unit
- the second component may be included as a polymer compound having a structure in which two hydrogen atoms of the thermally activated delayed fluorescent substance are eliminated as a repeating unit
- the third component may be included as a polymer compound having a structure in which two hydrogen atoms of the compound having a boron atom are eliminated as a repeating unit, Organic electroluminescent device.
- a ring, B ring and C ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- a ring, B ring, C ring and D ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- Y is B (boron), X 1 , X 2 , X 3 And X 4 Are independently>O,>NR,> CR 2 ,> S or> Se, and the R of> NR and> CR 2 R is an optionally substituted aryl, an optionally substituted heteroaryl, an optionally substituted cycloalkyl or an optionally substituted alkyl; May be bonded to at least one selected from the A ring, B ring, C ring and D ring by a group or a single bond, R 1 And R 2 Is independently hydrogen, alky
- a ring, B ring and C ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- X 1 , X 2 And X 3 Is independently O, NR,> CR 2 , S or Se, R of the NR and> CR 2 R is an optionally substituted aryl, an optionally substituted heteroaryl, an optionally substituted cycloalkyl or alkyl, and R of the above NR is a linking group or a single bond.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 And R 11 Is each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy, which may be further substituted with aryl, heteroaryl or alkyl; , R 1 ⁇ R 3 , R 4 ⁇ R 7 And R 8 ⁇ R 11 May be bonded to each other to form an aryl ring or a heteroaryl ring together with the a ring, b ring or c ring, and the formed ring is aryl, heteroaryl, diarylamino, alkyl, cyclo May be substituted with at least one selected from alkyl, alkoxy and aryloxy, which may be further substituted with at least one selected from aryl, heteroaryl and alkyl; X 1 And X
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 And R 14 Is each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino, arylheteroarylamino, alkyl, cycloalkyl, alkoxy, aryloxy, heteroaryloxy, arylthio, heteroarylthio or An alkyl-substituted silyl wherein at least one hydrogen is optionally substituted with aryl, heteroaryl or alkyl; 5 ⁇ R 7 And R 10 ⁇ R 12 And adjacent groups may form an aryl ring or a heteroaryl ring together with the b-ring or the d-ring, and at least one hydrogen in the formed ring is aryl, heteroaryl, diarylamino, Diheteroary
- CR 2 R is -O-, -S-, -C (-R) 2 -Or a single bond may be bonded to at least one selected from the a ring, the b ring, the c ring and the d ring; 2 R in-is hydrogen or alkyl having 1 to 6 carbons, Where X 1 , X 2 , X 3 , And X 4 Is simultaneously> CR 2 Will not be And At least one hydrogen in the compound represented by the formula (2) may be substituted with cyano, halogen, or deuterium.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 9 , R 10 And R 11 Is each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy, which are further substituted with at least one selected from aryl, heteroaryl and alkyl And R 1 ⁇ R 3 , R 4 ⁇ R 6 And R 9 ⁇ R 11 May be bonded to each other to form an aryl ring or a heteroaryl ring together with the a ring, b ring or c ring, and the formed ring is aryl, heteroaryl, diarylamino, alkyl, cyclo May be substituted with at least one selected from alkyl, alkoxy and aryloxy, which may be further substituted with at least one selected from aryl, heteroaryl, cycloalkyl and alkyl;
- X 1 is aryl, hetero
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 And R 14 Is each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy, which are further substituted with at least one selected from aryl, heteroaryl and alkyl And R 1 ⁇ R 3 , R 4 ⁇ R 7 , R 8 ⁇ R 10 And R 11 ⁇ R 14 Among adjacent groups may be bonded to each other to form an aryl ring or a heteroaryl ring together with the a ring, the b ring, the c ring or the d ring, and the formed ring is aryl, heteroaryl, diarylamino, Optionally substituted with at least one selected from alkyl, cycloalkyl, alk
- X and L are simultaneously> CR 2 Will not be And At least one hydrogen in the compounds and structures represented by formula (4) may be substituted with cyano, halogen or deuterium.
- the third component at least one compound represented by any of the formulas (1), (2) and (4) is included, In the above formula (1), X 1 And X 2 Are each independently> O or> NR, In the above formula (2), X 1 , X 2 , X 3 And X 4 Are each independently> O or> NR, In the above formula (4), X is> O and> NR, and L is a single bond.
- the organic electroluminescent device according to [3].
- At least one compound represented by any of the formulas (1), (2), (3), and (4) is included, and an atom constituting a ring present in the compound includes: At least one selected from aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino, arylheteroarylamino, alkyl, cycloalkyl, alkoxy, aryloxy, heteroaryloxy, arylthio, heteroarylthio and alkyl-substituted silyl
- At least one compound represented by the formula (2) is included, and the atoms constituting the ring present in the compound are aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino , Arylheteroarylamino, alkyl, cycloalkyl, alkoxy, aryloxy, heteroaryloxy, arylthio, heteroarylthio and alkyl-substituted silyl, which are further substituted with aryl, heteroaryl, cycloalkyl
- the organic electroluminescent device according to [5] which may be substituted with at least one selected from alkyl and alkyl.
- each hydrogen in the partial structure may be independently substituted with aryl, heteroaryl, diarylamino, alkyl, cycloalkyl, alkoxy or aryloxy, and these are further substituted with aryl, heteroaryl, cycloalkyl and alkyl. And may be substituted with at least one selected from [8]
- the compound represented by any of the above formulas (1) to (4) is 3 Sp bonded to m or p position with respect to carbon or boron atom 2
- the organic electroluminescent device according to [3], wherein the compound represented by the formula (2) is the following compound.
- the organic electroluminescent device according to [4], wherein the compound represented by the formula (2) is the following compound.
- L 1 Is arylene, heteroarylene, heteroarylenearylene or aryleneheteroarylenearylene having 6 to 24 carbon atoms; At least one hydrogen in the compounds represented by each of the above formulas may be substituted with alkyl having 1 to 6 carbon atoms, cyano, halogen or deuterium.
- the second component is selected from carbazole, phenoxazine, acridine, triazine, pyrimidine, pyrazine, thioxanthene, benzonitrile, phthalonitrile, isophthalonitrile, diphenylsulfone, triazole, oxadiazole, thiadiazole and benzophenone as a partial structure.
- the organic electroluminescent device according to any one of [1] to [13], comprising at least one of the following: [15] The organic compound according to any one of [1] to [14], wherein the second component contains at least one compound represented by any of the following formulas (AD1), (AD2) and (AD3). Electroluminescent device.
- M is each independently a single bond, —O—,> N—Ar or> CAr 2
- J is each independently an arylene having 6 to 18 carbon atoms, and the arylene is substituted with at least one selected from phenyl, alkyl having 1 to 6 carbons, and cycloalkyl having 3 to 12 carbons.
- Ar is each independently hydrogen, aryl having 6 to 18 carbons, heteroaryl having 6 to 18 carbons, alkyl having 1 to 6 carbons or cycloalkyl having 3 to 12 carbons, At least one hydrogen in the arylene may be substituted with phenyl, alkyl having 1 to 6 carbons or cycloalkyl having 3 to 12 carbons, m is 1 or 2, n is an integer of 2 to (6-m); At least one hydrogen in the compounds represented by the above formulas may be substituted with halogen or deuterium.
- D 2 -L 2 -A 2 -L 3 -D 3 (DAD2) (In the above formula (DAD2), D 2 And D 3 Are each independently a donor group, and L 2 And L 3 Are each independently a single bond or a conjugated linking group; 2 Is an acceptor group.
- the organic electroluminescent device according to [17].
- the excitation singlet energy level obtained from the shoulder on the peak short wavelength side of the fluorescence spectrum of the second component is E (2, S, Sh)
- the excitation singlet energy level is obtained from the shoulder on the peak short wavelength side of the phosphorescence spectrum of the second component.
- the excited triplet energy level is E (2, T, Sh)
- the excited singlet energy level determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the third component is E (3, S, Sh).
- the singlet triplet energy difference ( ⁇ E (2, The organic electroluminescent device according to any one of [1] to [23], wherein ST, Sh) and ⁇ E (3, ST, Sh)) have the following relationship.
- the excitation singlet energy level obtained from the shoulder on the peak short wavelength side of the fluorescence spectrum of the second component is E (2, S, Sh)
- the excitation singlet energy level is obtained from the shoulder on the peak short wavelength side of the phosphorescence spectrum of the second component.
- the excited triplet energy level is E (2, T, Sh)
- the excited singlet energy level determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the third component is E (3, S, Sh).
- the singlet triplet energy difference ( ⁇ E (2, The organic electroluminescent device according to any one of [1] to [24], wherein ST, Sh) and ⁇ E (3, ST, Sh)) have the following relationship.
- ⁇ E (2, ST, Sh) E (2, S, Sh) ⁇ E (2, T, Sh) ⁇ 0.30 eV [27]
- the organic electroluminescent device according to any one of [1] to [26], wherein a singlet / triplet energy difference ( ⁇ E (2, ST, Sh)) of the second component has the following relationship.
- ⁇ E (2, ST, Sh) E (2, S, Sh) ⁇ E (2, T, Sh) ⁇ 0.15 eV
- the excitation singlet energy level obtained from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the second component is E (2, S, Sh), and the excitation singlet energy level is obtained from the shoulder on the short wavelength side of the peak of the phosphorescence spectrum of the second component.
- the excited triplet energy level is E (2, T, Sh)
- the excited singlet energy level determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the third component is E (3, S, Sh).
- E (3, T, Sh) the excited triplet energy level obtained from the shoulder on the short wavelength side of the peak of the phosphorescence spectrum of the third component
- a display device comprising the organic electroluminescent device according to any one of [1] to [32].
- a lighting device comprising the organic electroluminescent element according to any one of [1] to [32].
- a composition for forming a light-emitting layer for coating and forming a light-emitting layer of an organic electroluminescent device comprising at least one organic solvent as a fourth component in addition to the first component, the second component, and the third component according to any one of [1] to [32].
- the fourth component includes a good solvent (GS) and a poor solvent (PS) for at least one of the first component, the second component, and the compound that is the third component, and the good solvent (GS) Boiling point (BP GS ) Is the boiling point (BP) of the poor solvent (PS) PS
- the first component is 0.0998% by mass to 4.0% by mass with respect to the total mass of the composition for forming a light emitting layer; 0.0001% by mass to 2.0% by mass of the second component based on the total mass of the composition for forming a light emitting layer;
- the third component is 0.0001% by mass to 2.0% by mass based on the total mass of the composition for forming a light emitting layer; 90.0% by mass to 99.9% by mass of the fourth component, based on the total mass of the composition for forming a light emitting layer;
- the composition for forming a light emitting layer according to any one of [35] to [37].
- [39] An organic electroluminescent device having a light emitting layer formed using the composition for forming a light emitting layer according to any one of [38] to [38].
- R 40 And R 41 Is an alkyl having 2 to 10 carbon atoms which may be bonded, and the wavy line is a bonding site to another structure.
- a repeating unit containing a structure in which two hydrogen atoms are eliminated from a compound having a boron atom, a repeating unit containing a structure in which two hydrogen atoms are eliminated from a thermally activated delayed fluorescent substance, and two hydrogen atoms from a host compound A polymer compound comprising at least two kinds of repeating units selected from repeating units having a structure from which is eliminated.
- At least one kind of repeating unit containing a structure in which two hydrogen atoms are eliminated from a compound having a boron atom at least one kind of repeating unit containing a structure in which two hydrogen atoms are eliminated from a thermally activated delayed fluorescent substance, and a polymer compound containing at least one repeating unit having a structure in which two hydrogen atoms are eliminated from a host compound.
- the organic electroluminescent device of the present invention includes, in the light-emitting layer, a host compound, a heat-activated delayed fluorescent substance, and a compound having a boron atom in a molecule, and their excited singlet energy levels satisfy a predetermined condition. Thereby, the organic electroluminescence characteristics can be further enhanced.
- FIG. 2 is an energy level diagram showing an example of an energy relationship between a host, an assisting dopant, and an emitting dopant of the organic electroluminescent device to which the present invention is applied.
- FIG. 4 is an energy level diagram showing another example of the energy relationship between the host, the assisting dopant, and the emitting dopant of the organic electroluminescent device to which the present invention is applied.
- FIG. 9 is an energy level diagram showing still another example of the energy relationship between the host, the assisting dopant, and the emitting dopant of the organic electroluminescent device to which the present invention is applied.
- FIG. 1 is a schematic cross-sectional view illustrating an organic electroluminescent device according to an embodiment.
- FIG. 1 is a schematic cross-sectional view illustrating an organic electroluminescent device according to an embodiment.
- FIG. 4 is an energy level diagram showing an energy relationship among a host, an assisting dopant, and an emitting dopant of a TAF element using a general fluorescent dopant.
- FIG. 3 is a diagram illustrating a method for manufacturing an organic electroluminescent element on a substrate having a bank by using an inkjet method.
- FIG. 2 is a view showing the relationship between the basic skeleton of a compound and Tau (Delay) and Stokes shift.
- FIG. 3 is a diagram showing the relationship between the basic skeleton of a compound and the half-value width, external quantum efficiency, and device lifetime.
- FIG. 3 is a view showing the relationship between substituents of a compound and Tau (Delay) and Stokes shift.
- FIG. 3 is a diagram showing the relationship among substituents of a compound and half-value width, external quantum efficiency, and device lifetime.
- FIG. 3 is a diagram showing the relationship between the substituents of a compound and Stokes shift and half width.
- a numerical range represented by using “to” means a range including numerical values described before and after “to” as a lower limit and an upper limit.
- Room temperature means 20 ° C.
- the organic electroluminescent device of the present invention utilizes a host compound, a thermally activated delayed fluorescent substance, and a compound having a boron atom in a molecule.
- the “host compound” in the present invention means that the excited singlet energy level determined from the shoulder on the short wavelength side of the peak of the fluorescence spectrum is a thermally activated delayed phosphor as the second component, and It means a compound higher than a compound having a boron atom.
- thermally activated delayed phosphor refers to the absorption of thermal energy, which causes an inverse intersystem crossing from an excited triplet state to an excited singlet state, and radiation inactivation from the excited singlet state to cause delayed fluorescence.
- thermally activated delayed fluorescence includes those that undergo higher-order triplets in the process of excitation from the excited triplet state to the excited singlet state.
- a paper by Durk University Monkman et al. NATURE COMMUNICATIONS, 7: 13680, DOI: 10.1038 / ncomms13680
- a paper by Hosogai et al. Sato et al. Scientific Reports, 7: 4820, DOI: 10.1038 / s41598-017-05007-7)
- Sato et al At Kyoto University (The 98th Annual Meeting of the Chemical Society of Japan, publication number: 2I4- 15.
- the fluorescence lifetime of a sample containing a target compound is measured at 300 K, it is determined that the target compound is a “heat-activated delayed fluorescent substance” when a slow fluorescent component is observed.
- the slow fluorescent component refers to a component having a fluorescence lifetime of 0.1 ⁇ sec or more.
- the measurement of the fluorescence lifetime can be performed using, for example, a fluorescence lifetime measuring device (C11367-01, manufactured by Hamamatsu Photonics KK).
- the ⁇ compound having a boron atom in the molecule '' can function as an emitting dopant, and the ⁇ thermally-activated delayed fluorescent substance '' can be used as an assisting dopant to assist the emission of a compound having a boron atom in the molecule.
- a thermally activated delayed phosphor as an assisting dopant
- TAF device TADF Assisting Fluorescence device
- FIG. 5 shows an energy level diagram of a light emitting layer of a TAF element using a general fluorescent dopant as an emitting dopant (ED).
- the energy level of the ground state of the host is E (1, G)
- the excited singlet energy level determined from the shoulder on the short wavelength side of the fluorescence spectrum of the host is E (1, S, Sh)
- the excited triplet energy level determined from the shoulder on the short wavelength side of the phosphorescence spectrum is E (1, T, Sh)
- the ground state energy level of the assisting dopant as the second component is E (2, G).
- the excitation singlet energy level determined from the short wavelength shoulder of the fluorescence spectrum of the assisting dopant as the second component is E (2, S, Sh)
- the phosphorescent spectrum of the assisting dopant as the second component is E (2, S, Sh).
- the excited triplet energy level determined from the shoulder on the short wavelength side is E (2, T, Sh)
- the ground state energy level of the emitting dopant as the third component is E (3, G)
- the excitation singlet energy level determined from the shoulder on the short wavelength side of the fluorescence spectrum of the three-component emitting dopant is E (3, S, Sh)
- the short-wavelength of the phosphorescent spectrum of the third component is the dopant.
- the excited triplet energy level determined from the shoulder on the side is E (3, T, Sh).
- E (3, T, Sh) When a general fluorescent dopant is used as an emitting dopant (ED), the energy up-converted by the assisting dopant is changed to the excited singlet energy level E (3, S, Sh) of the emitting dopant. The light is shifted. However, some of the excited triplet energy E (2, T, Sh) on the assisting dopant moves to the excited triplet energy level E (3, T, Sh) of the emitting dopant, , An intersystem crossing from the excited singlet energy level E (3, S, Sh) to the excited triplet energy level E (3, T, Sh) occurs, and then heat is transferred to the ground state E (3, G). Deactivate. Some energy is not used for light emission by this route, and energy is wasted.
- the energy transferred from the assisting dopant to the emitting dopant can be efficiently used for light emission, thereby realizing high luminous efficiency. This is presumed to be due to the following light emission mechanism. That is, a preferable energy relationship in the organic electroluminescent device of the present invention is shown in FIG.
- the compound having a boron atom as the emitting dopant has a high excited triplet energy level E (3, T, Sh).
- the excited singlet energy up-converted by the assisting dopant intersects with the excited triplet energy level E (3, T, Sh) by the emitting dopant, the excited singlet energy remains on the emitting dopant. It is up-converted or recovered to the excited triplet energy level E (2, T, Sh) on the assisting dopant (thermally activated delayed phosphor). Therefore, the generated excitation energy can be used for light emission without waste.
- the functions of up-conversion and luminescence into two types of molecules, each of which is excellent, it is expected that the residence time of high energy is reduced and the burden on the compound is reduced.
- the excited triplet energy involved in the forward and reverse intersystem crossing from the excited triplet state to the excited singlet state is calculated by molecular orbital calculation.
- it may not be the excited triplet energy observed by the phosphorescence spectrum but a higher-order excited triplet energy (The 98th Annual Meeting of the Chemical Society of Japan, Presentation No .: 2I4-15, Mechanism of high-efficiency light emission in organic electroluminescence using DABNA as a light-emitting molecule, presented by Professor Toru Sato of the graduate School of Engineering, Kyoto University).
- the inverse intersystem crossing in DABNA2 having a boron atom in the molecule is an FvHT (Fluorescence via Higher Triplet) mechanism using higher-order triplet orbitals, and the transition from higher-order triplet orbitals to the ground state is performed. It is suggested that the transition from higher-order triplet orbit to excited singlet orbit occurs because of the suppression.
- FvHT Fluorescence via Higher Triplet
- the energy relation in the light-emitting layer of the organic electroluminescent device of the present invention is such that the higher-order excited triplet energy level E (3, Tn) is the excited singlet energy level. If it is slightly lower (in the case of the TADF mechanism), it can be represented by the energy level diagram of FIG. 2, and if the higher-order excited triplet energy level is slightly higher than the excited singlet energy level (FvHT function). 3) can be represented by the energy level diagram of FIG. In any case, since deactivation from the lowest triplet orbit is suppressed, it is expected that good device characteristics will be provided similarly to the energy relationship in FIG.
- the excited triplet energy level is determined from the phosphorescence spectrum because it can be measured spectroscopically.
- the organic electroluminescent device of the present invention is not limited to those shown in FIGS. It does not exclude having the energy relationship shown by.
- a DA-type thermally activated delayed phosphor (D represents an electron-donating atomic group and A represents an electron-accepting atomic group) has a high up-conversion rate. , The half-width of light emission is wide, and the color purity is low.
- a thermally activated delayed phosphor of the multiple resonance effect (MRE) type has a slow up-conversion speed, a narrow emission half width, a high color purity, a high fluorescence quantum yield (PLQY), and It is characterized by a high light emission speed.
- the organic electroluminescent device of the present invention is designed to take advantage of these molecules. As a result, it is possible to realize a spectrum with a good halftone width and a good color, high external quantum efficiency, improved roll-off, and long life.
- the host compound first component
- the thermally activated delayed phosphor assisting dopant, second component
- the boron atom in the organic electroluminescent device of the present invention were used.
- the relationship between the energies of the compounds (emission dopant, third component) is summarized below.
- the excitation singlet energy level determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the first component is E (1, S, Sh)
- the excitation singlet determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the second component is E (2, S, Sh)
- the excited singlet energy level obtained from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the third component is E (3, S, Sh)
- E (1, S, Sh) -E (2, S, Sh) is preferably from 0 to 1.0 eV
- E (2, S, Sh) -E (3, S, Sh) is It is preferably 0 to 0.20 eV.
- the excitation singlet energy level obtained from the short wavelength side peak top of the fluorescence spectrum of the first component is E (1, S, PT)
- the excitation singlet energy obtained from the short wavelength side peak top of the second component fluorescence spectrum is obtained.
- energy level is E (2, S, PT)
- the excited singlet energy level obtained from the peak top on the short wavelength side of the fluorescence spectrum of the third component is E (3, S, PT)
- the present invention can be applied to E (2, S, PT) and E (3, S, PT) whichever is larger, but E (3, S, PT)> E (2, S, PT) It is preferable that
- the excitation singlet energy level determined from the shoulder on the peak short wavelength side of the fluorescence spectrum of the second component is E (2, S, Sh)
- the excitation triplet determined from the shoulder on the peak short wavelength side of the phosphorescence spectrum of the second component is E (2, T, Sh)
- the term energy level is E (2, T, Sh)
- the excited singlet energy level obtained from the shoulder on the short wavelength side of the peak of the fluorescence spectrum of the third component is E (3, S, Sh)
- the energy of the third component is Assuming that the excited triplet energy level obtained from the shoulder on the short wavelength side of the peak of the phosphorescence spectrum is E (3, T, Sh), the singlet triplet energy difference ( ⁇ E (2, ST, Sh)) obtained from these is determined.
- ⁇ E (3, ST, Sh)) preferably have the following relationship.
- ⁇ E (2, ST, Sh) E (2, S, Sh) ⁇ E (2, T, Sh) ⁇ 0.50 eV
- ⁇ E (3, ST, Sh) E (3, S, Sh) ⁇ E (3, T, Sh) ⁇ 0.20 eV
- ⁇ E (2, ST, Sh) is more preferably 0.30 eV or less, further preferably 0.15 eV or less, and even more preferably 0.10 eV or less.
- ⁇ E (3, ST, Sh) is more preferably 0.15 eV or less, and even more preferably 0.10 eV or less.
- ⁇ E (2, ST, Sh) and ⁇ E (3, ST, Sh) preferably have the following relationship. ⁇ E (2, ST, Sh) ⁇ ⁇ E (3, ST, Sh)
- E (2, S, Sh) -E (3, S, Sh) is preferably 0 to 0.20 eV
- E (3, T, Sh) -E (2, T, Sh) is It is preferably 0 to 0.20 eV.
- the inverse intersecting speed of the second component is k (2, RISC)
- the inverse intersecting speed of the third component is k (3, RISC)
- the emission speed of the second component is k (2, Prompt)
- the excitation singlet energy level determined from the shoulder on the short wavelength side of the fluorescence spectrum is E (1, S, Sh)
- the peak top on the short wavelength side of the fluorescence spectrum is defined as E (1, S, Sh).
- the excitation singlet energy level determined from the above is defined as E (1, S, PT)
- the excitation triplet energy level determined from the shoulder on the short wavelength side of the phosphorescence spectrum is defined as E (1, T, Sh).
- the excited triplet energy level determined from the peak top on the short wavelength side of the spectrum is E (1, T, PT).
- PT the excited triplet energy level E (T, Sh) determined from the shoulder on the short wavelength side of the phosphorescence spectrum, and the excited triplet energy level E (T) determined from the peak top on the short wavelength side of the phosphorescence spectrum.
- PT the inverse intersystem crossing speed, and the light emission speed are calculated as follows.
- the shoulder on the peak short wavelength side means an inflection point on the short wavelength side of the emission peak
- the peak top on the short wavelength side is the emission maximum value of the emission peak, It means the position on the peak corresponding to the emission maximum value on the shortest wavelength side.
- the thickness of the polymethyl methacrylate film in which the target compound is dispersed may be any thickness that can provide sufficient intensity for the measurement of the absorption spectrum, the fluorescence spectrum, and the phosphorescence spectrum. If it is strong, it may be thick.
- the wavelength of the absorption peak obtained in the absorption spectrum is used.
- blue emission is in the range of 400 to 500 nm
- green emission is Is determined in the range of 480 to 600 nm
- the respective energy levels are obtained using data obtained from the emission peaks appearing in the range of 580 to 700 nm.
- Excited singlet energy level E (S, Sh) obtained from the shoulder on the peak short wavelength side of the fluorescence spectrum
- the measurement sample containing the target compound is irradiated with excitation light at 77 K, and the fluorescence spectrum is observed.
- a tangent line passing through the inflection point (shoulder) on the shorter wavelength side is drawn to the emission peak appearing in the fluorescence spectrum, and the following equation is obtained from the wavelength (B Sh ) [nm] at the intersection of the tangent line and the baseline. Is used to calculate the excited singlet energy level E (S, Sh).
- E (S, Sh) [eV] 1240 / B Sh [2]
- Excited singlet energy level E (S, PT) obtained from the peak top on the short wavelength side of the fluorescence spectrum
- the measurement sample containing the target compound is irradiated with excitation light at 77 K, and the fluorescence spectrum is observed.
- the wavelength (emission maximum wavelength, B PT ) [nm] corresponding to the peak top on the shortest wavelength side of the emission peak appearing in the fluorescence spectrum the excited singlet energy level E (S, PT) is obtained using the following equation. Is calculated.
- E (S, PT) [eV] 1240 / B PT [3]
- Excited triplet energy level E (T, Sh) obtained from the shoulder on the peak short wavelength side of the phosphorescence spectrum
- the measurement sample containing the target compound is irradiated with excitation light at 77 K, and the phosphorescence spectrum is observed.
- a tangent line passing through the inflection point (shoulder) on the shorter wavelength side is drawn with respect to the emission peak appearing in the phosphorescence spectrum, and from the wavelength (C Sh ) [nm] at the intersection of the tangent line and the base line, the following equation is obtained. Is used to calculate the excited triplet energy level E (T, Sh).
- E (T, Sh) [eV] 1240 / C Sh [4]
- Excited triplet energy level E (T, PT) obtained from the peak top on the short wavelength side of the phosphorescence spectrum
- the measurement sample containing the target compound is irradiated with excitation light at 77 K, and the phosphorescence spectrum is observed.
- the excited triplet energy level E (T, PT) is calculated using the following equation. Is calculated.
- E (T, PT) [eV] 1240 / C PT
- the DA (donor-acceptor) type TADF material and the MRE (Multi Resonance Effect, multiple resonance) type compound have different emission widths of the fluorescence and phosphorescence spectra due to the robustness of the molecule. Even in the same case, it is considered that the DA type thermally activated delayed fluorescent substance has a wider range of energy than the MRE type compound molecule.
- the TAF element it is necessary to accurately estimate the energy transfer between the components and design the configuration. Therefore, the excited singlet energy level and the excited triplet energy level are estimated from the shoulder on the short wavelength side of the spectrum. In general, the intersection of the tangent and the baseline passing through the inflection point on the short wavelength side of the spectrum is the energy determined from the shoulder on the short wavelength side.
- the excited singlet energy level E (S, Sh) and the excited triplet energy level E (T, Sh) obtained from the shoulder on the short wavelength side are used for calculation and discussion of ⁇ E (ST), and the first component It is also used to discuss the confinement and transfer of energy between the host compound and the assisting dopant, and the confinement and transfer of energy between the assisting dopant and the emitting dopant.
- the inverse intersystem crossing speed indicates the speed of the inverse intersystem crossing from the excited triplet to the excited singlet.
- the inverse intersystem crossing rate of the assisting dopant and the emitting dopant is calculated by transient fluorescence spectrometry using the method described in Nat. Commun. 2015, 6, 8476. or Organic Electronics 2013, 14, 2721-2726.
- the assisting dopant has an inverse intersystem crossing speed of 10 5 s ⁇ 1 , and more preferably 10 6 s ⁇ 1 .
- the light emission rate indicates a rate at which a transition from an excited singlet to a ground state occurs via fluorescence emission without going through a TADF process.
- the emission velocities of the assisting dopant and the emitting dopant are calculated using the method described in Nat. Commun. 2015, 6, 8476. Specifically, the inverse intersystem crossing speed of the emitting dopant is 10 7 s ⁇ 1 , and more preferably 10 8 s ⁇ 1 .
- the light emitting layer in the organic electroluminescent device includes at least a host compound as a first component, a thermally activated delayed phosphor as a second component, and a compound having a boron atom as a third component.
- the thermally activated delayed fluorescent substance as the second component is referred to as “assisting dopant” (compound)
- the compound having a boron atom as the third component is referred to as “emitting dopant” (compound).
- the light emitting layer may be a single layer or a plurality of layers.
- the host compound, the thermally activated delayed fluorescent substance, and the compound having a boron atom may be contained in the same layer, or at least one component may be contained in a plurality of layers.
- the host compound, the thermally activated delayed fluorescent substance, and the compound having a boron atom contained in the light emitting layer may be of one type or a combination of a plurality of types.
- the assisting dopant and the emitting dopant may be entirely or partially contained in the host compound as the matrix.
- the emitting layer doped with the assisting dopant and the emitting dopant is formed by depositing the host compound, the assisting dopant, and the emitting dopant by a ternary co-evaporation method, and the host compound, the assisting dopant, and the emitting dopant are mixed in advance. And then simultaneously vapor deposition, applying a composition (paint) for forming a light-emitting layer prepared by dissolving a host compound, an assisting dopant and an emitting dopant in an organic solvent, or a wet film-forming method. it can.
- the amount of the host compound used depends on the type of the host compound, and may be determined according to the characteristics of the host compound.
- the standard of the amount of the host compound used is preferably 40 to 99.999% by mass, more preferably 50 to 99.99% by mass, and still more preferably 60 to 99.9% by mass of the whole material for the light emitting layer. It is. The above range is preferable, for example, in terms of efficient charge transport and efficient energy transfer to the dopant.
- the amount of the assisting dopant (thermally activated delayed fluorescent material) used varies depending on the kind of the assisting dopant, and may be determined according to the characteristics of the assisting dopant.
- the standard of the amount of the assisting dopant to be used is preferably 1 to 60% by mass, more preferably 2 to 50% by mass, further preferably 5 to 30% by mass of the whole material for the light emitting layer. The above range is preferable, for example, in that energy can be efficiently transferred to the emitting dopant.
- the amount of the emitting dopant (compound having a boron atom) used depends on the type of the emitting dopant, and may be determined according to the characteristics of the emitting dopant.
- the standard of the usage amount of the emitting dopant is preferably 0.001 to 30% by mass, more preferably 0.01 to 20% by mass, and still more preferably 0.1 to 10% by mass of the whole material for the light emitting layer. %.
- the above range is preferable, for example, in that the density quenching phenomenon can be prevented.
- the amount of the emitting dopant used is low, since the concentration quenching phenomenon can be prevented. It is preferable that the amount of the assisting dopant used is high from the viewpoint of the efficiency of the thermally activated delayed fluorescence mechanism. Further, from the viewpoint of the efficiency of the thermally activated delayed fluorescence mechanism of the assisting dopant, it is preferable that the amount of the emitting dopant used is lower than that of the assisting dopant.
- the host compound known compounds can be used, and examples thereof include a compound having at least one of a carbazole ring and a furan ring. Among them, at least one of a furanyl group and a carbazolyl group, It is preferable to use a compound in which at least one of the above is bonded. Specific examples include mCP and mCBP.
- the excited triplet energy level E (1, T, Sh) obtained from the shoulder on the short wavelength side of the peak of the phosphorescence spectrum of the host compound is determined from the viewpoint of promoting the generation of TADF in the light emitting layer without inhibiting the light emitting layer. It is preferable that the emission dopant or the emission dopant having the highest excitation triplet energy level among the excitation triplet energy levels E (2, T, Sh) and E (3, T, Sh) be higher than the above.
- the excited triplet energy level E (1, T, Sh) of the host compound is 0.01 eV or more as compared with E (2, T, Sh) and E (3, T, Sh). Preferably, it is 0.03 eV or more, more preferably, 0.1 eV or more.
- a TADF-active compound may be used as the host compound.
- L 1 is arylene having 6 to 24 carbon atoms, heteroarylene having 2 to 24 carbon atoms, heteroarylene arylene having 6 to 24 carbon atoms or 6 to 24 carbon atoms.
- Arylene heteroarylene arylene preferably an arylene having 6 to 16 carbon atoms, more preferably an arylene having 6 to 12 carbon atoms, particularly preferably an arylene having 6 to 10 carbon atoms, specifically, a benzene ring and a biphenyl ring , A terphenyl ring and a fluorene ring.
- a heteroarylene having 2 to 24 carbon atoms is preferable, a heteroarylene having 2 to 20 carbon atoms is more preferable, a heteroarylene having 2 to 15 carbon atoms is further preferable, and a heteroarylene having 2 to 10 carbon atoms is particularly preferable.
- the host compound is preferably a compound represented by any of the structural formulas listed below.
- at least one hydrogen may be substituted with halogen, cyano, alkyl having 1 to 4 carbons (eg, methyl or t-butyl), phenyl or naphthyl.
- the heat-activated delayed fluorescent substance (TADF compound) used in the present invention is capable of forming a HOMO (Highest Occupied Molecular Orbital) in a molecule by using an electron-donating substituent called a donor and an electron-accepting substituent called an acceptor.
- TADF compound heat-activated delayed fluorescent substance
- DA type heat-activated delayed phosphor designed to localize LUMO (Lowest Unoccupied Molecular Orbital) and to cause efficient reverse intersystem crossing (TADF compound).
- the term “electron-donating substituent” refers to a substituent and a partial structure in which a LUMO orbital is localized in a thermally activated delayed fluorescent molecule.
- the term “electron-accepting substituent” means a substituent and a partial structure in which a HOMO orbital is localized in a thermally activated delayed fluorescent molecule.
- a thermally activated delayed phosphor using a donor or an acceptor has a large spin orbit coupling (SOC) due to its structure, and has a small exchange interaction between HOMO and LUMO and ⁇ E ( Since ST) is small, a very fast inverse intersystem crossing speed is obtained.
- thermally activated delayed phosphors using donors and acceptors have a large degree of structural relaxation in the excited state.
- Some molecules have different stable structures between the ground state and the excited state. When the conversion to the excited state occurs, the structure changes to a stable structure in the excited state), so that a broad emission spectrum is provided. Therefore, when used as a light-emitting material, color purity may be reduced.
- heat-activated delayed fluorescent substance of the present invention for example, a compound in which a donor and an acceptor are bound directly or via a spacer can be used.
- structure of the donor and acceptor used in the heat-activated delayed fluorescent substance of the present invention for example, the structure described in Chemistry of Materials, 2017, 29, 1946-1963 can be used.
- Examples of the donor structure include carbazole, dimethylcarbazole, di-tert-butylcarbazole, dimethoxycarbazole, tetramethylcarbazole, benzofluorocarbazole, benzothienocarbazole, phenyldihydroindolocarbazole, phenylbicarbazole, bicarbazole, and tercarbazole.
- Acceptable structures include sulfonyldibenzene, benzophenone, phenylenebis (phenylmethanone), benzonitrile, isonicotinonitrile, phthalonitrile, isophthalonitrile, paraphthalonitrile, benzenetricarbonitrile, triazole, oxazole, thiadiazole , Benzothiazole, benzobis (thiazole), benzoxazole, benzobis (oxazole), quinoline, benzimidazole, dibenzoquinoxaline, heptaazaphenalene, thioxanthone dioxide, dimethylanthracenone, anthracenedione, cycloheptapyridine, fluorangecarbonitrile, Triephenyltriazine, pyrazinedicarbonitrile, pyrimidine, phenylpyrimidine, methylpyrimidine, pyridi Dicarbonitrile, dibenzo quinoxaline-carbon
- the compound having heat-activated delayed fluorescence of the present invention as a partial structure, carbazole, phenoxazine, acridine, triazine, pyrimidine, pyrazine, thioxanthene, benzonitrile, phthalonitrile, isophthalonitrile, diphenylsulfone, triazole And a compound having at least one selected from oxadiazole, thiadiazole and benzophenone.
- the compound used as the second component of the light emitting layer of the present invention is a heat-activated delayed fluorescent substance, and is preferably a compound whose emission spectrum at least partially overlaps the absorption peak of the emitting dopant.
- compounds that can be used as the second component (heat-activated delayed fluorescent substance) of the light emitting layer of the present invention will be exemplified.
- the compounds that can be used as the heat-activated delayed fluorescent substance in the present invention are not limited to the following exemplified compounds.
- Me represents methyl
- t-Bu represents t-butyl
- Ph represents phenyl
- wavy lines represent bonding positions.
- a compound represented by any of the following formulas (AD1), (AD2) and (AD3) can also be used as the heat-activated delayed phosphor.
- M is each independently a single bond, —O—,> N—Ar or> CAr 2 , and represents the HOMO depth and the excited singlet energy level and the excited triplet energy level of the partial structure to be formed. From the viewpoint of height, a single bond, —O— or> N—Ar is preferable.
- J is a spacer structure that separates a donor partial structure and an acceptor partial structure, each independently being an arylene having 6 to 18 carbon atoms, and a conjugate leaching from the donor partial structure and the acceptor partial structure. From the viewpoint of the size, arylene having 6 to 12 carbon atoms is preferable.
- Ar is each independently hydrogen, aryl having 6 to 24 carbons, heteroaryl having 2 to 24 carbons, alkyl having 1 to 12 carbons or cycloalkyl having 3 to 18 carbons, and the partial structure to be formed From the viewpoint of the depth of the HOMO and the height of the excited singlet energy level and the excited triplet energy level of the HOMO, preferably hydrogen, aryl having 6 to 12 carbons, heteroaryl having 2 to 14 carbons, carbon number Alkyl of 1-4 or cycloalkyl of 6-10, more preferably hydrogen, phenyl, tolyl, xylyl, mesityl, biphenyl, pyridyl, bipyridyl, triazyl, carbazolyl, dimethylcarbazolyl, di-tert-butyl Carbazolyl, benzimidazole or phenylbenzimidazole, more preferably hydrogen Phenyl or carbazolyl.
- n is 1 or 2.
- n is an integer of-(6-m), and preferably an integer of 4- (6-m) from the viewpoint of steric hindrance.
- at least one hydrogen in the compound represented by each of the above formulas may be substituted with halogen or deuterium.
- the compound used as the second component of the light emitting layer of the present invention is 4CzBN, 4CzBN-Ph, 5CzBN, 3Cz2DPhCzBN, 4CzIPN, 2PXZ-TAZ, Cz-TRZ3, BDPCC-TPTA, MA-TA, PA -TA, FA-TA, PXZ-TRZ, DMAC-TRZ, BCzT, DCzTrz, DDCzTRz, spiroAC-TRZ, Ac-HPM, Ac-PPM, Ac-MPM, TCzTrz, TmCzTrz and DCzmCzTrz are preferred.
- the compound used as the second component of the light emitting layer of the present invention may be a donor-acceptor type TADF compound represented by DA in which one donor D and one acceptor A are bonded directly or via a linking group.
- the organic electroluminescent device has a structure represented by the following formula (DAD1) in which a plurality of donors D are bonded to one acceptor A through a direct bond or a linking group. It is preferable because it becomes more excellent.
- D 1 -L 1 nA 1 (DAD1)
- DAD1 includes a compound represented by the following formula (DAD2).
- D 1 , D 2, and D 3 each independently represent a donor group.
- the donor group the above donor structure can be employed.
- a 1 and A 2 each independently represent an acceptor group.
- the acceptor group the above-described acceptor structure can be employed.
- L 1 , L 2 and L 3 each independently represent a single bond or a conjugated linking group.
- the conjugated linking group has a spacer structure for separating the donor group and the acceptor group, and is preferably an arylene having 6 to 18 carbon atoms, more preferably an arylene having 6 to 12 carbon atoms.
- L 1 , L 2 and L 3 are more preferably each independently phenylene, methylphenylene or dimethylphenylene.
- n is a 2 or more in the formula (DAD1), represents an integer less than or equal to the maximum number of A 1 may be substituted. n may be selected, for example, in the range of 2 to 10, or in the range of 2 to 6. When n is 2, the compound represented by the formula (DAD2) is obtained. n number of D 1 may be different even in the same, the n L 1 may be different even in the same.
- Preferred specific examples of the compounds represented by the formulas (DAD1) and (DAD2) include 2PXZ-TAZ and the following compounds. Not limited.
- the light emitting layer of the organic electroluminescent device of the present invention contains a compound having a boron atom as the third component.
- the light emitting layer includes, as a compound having a boron atom, a compound represented by any of the following formulas (i), (ii), and (iii), and a multimer having a plurality of structures represented by the following formula (i) Preferably it comprises at least one of the compounds.
- the light emitting layer of the organic electroluminescent device of the present invention comprises a compound represented by any of the following formulas (1), (2), (3) and (4) as a third component (compound having a boron atom). More preferably, at least one is included.
- a light emitting material for an organic electroluminescent display three kinds of materials, a fluorescent material, a phosphorescent material, and a thermally activated delayed fluorescent (TADF) material, are used. About 62.5%.
- the phosphorescent material and the TADF material sometimes have luminous efficiencies as high as 100%, but both have a problem that the color purity is low (the emission spectrum is wide).
- the display expresses various colors by mixing the three primary colors of light, red, green and blue, but if the color purity is low, colors that cannot be reproduced will be created, and the image quality of the display will be reduced. It greatly decreases.
- the half width of the blue emission spectrum of a commercially available smartphone is about 20 to 25 nm, but the half width of a general fluorescent material is about 40 to 60 nm, the phosphorescent material is about 60 to 90 nm, and the TADF material is And about 70 to 100 nm.
- the half width of a fluorescent material it is only necessary to remove some unnecessary colors because the half width is relatively narrow.
- a phosphorescent material or a TADF material it is necessary to remove half or more. From such a background, development of a luminescent material having both luminous efficiency and color purity has been desired.
- a TADF material uses an electron-donating substituent called a donor and an electron-accepting substituent called an acceptor to localize HOMO and LUMO in a molecule, so that an efficient reverse intersystem (reverse intersystem) is used.
- reverse intersystem reverse intersystem
- crossing is designed to occur, the use of donors and acceptors increases the structural relaxation in the excited state (for some molecules, the stable state is different between the ground state and the excited state, so the ground state is stimulated by external stimuli)
- the structure changes to a stable structure in the excited state after that), thereby giving a broad emission spectrum with low color purity.
- WO 2015/102118 proposes a new molecular design that dramatically improves the color purity of a TADF material.
- a TADF material For example, in the compound (1-401) disclosed in the literature, three carbons on a benzene ring composed of six carbons are obtained by utilizing the multiple resonance effect of boron (electron donating) and nitrogen (electron withdrawing). (Black circles) successfully localized HOMO and the remaining three carbons (open circles) localized LUMO. Due to this efficient inverse intersystem crossing, the luminous efficiency of the compound reaches 100% at the maximum.
- boron and nitrogen of the compound (1-401) not only localize HOMO and LUMO, but also maintain a robust planar structure by condensing three benzene rings, and reduce structural relaxation in an excited state. It also plays a role of suppressing, and as a result, has succeeded in obtaining an emission spectrum with a small Stokes shift of absorption and emission peaks and high color purity.
- the half width of the emission spectrum is 28 nm, which indicates a level of color purity that surpasses even that of a high-purity fluorescent material that is in practical use.
- the dimer compound represented by the formula (1-422) two borons and two nitrogens are bonded to the central benzene ring, thereby further enhancing the multiple resonance effect in the central benzene ring. As a result, it is possible to emit light having an extremely narrow emission peak width.
- the present compound in the device of the present invention, by utilizing the present compound as an emitting dopant, a high energy transfer efficiency from the assisting dopant to the emitting dopant, an appropriate emission wavelength and a half width of the emission spectrum, a high color purity, and a high device Efficiency and small roll-off, and long life are realized.
- Compounds represented by any of the above formulas (i), (ii) and (iii), multimeric compounds having a plurality of structures represented by the formula (i), formulas (1), (2), ( The compound represented by any one of 3) and (4) is a compound obtained by further studying these specific compound examples.
- the third component may be a normal phosphor or a thermally activated delayed phosphor.
- a ring, B ring and C ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- X 1 and X 2 are each independently O, NR,> CR 2 , S or Se, wherein R of the NR and R of> CR 2 are an optionally substituted aryl, Is a heteroaryl, an optionally substituted cycloalkyl or an alkyl, and R of the NR is at least one selected from the above-mentioned ring A, ring B and ring C by a linking group or a single bond. May be combined, and At least one hydrogen in the compound or structure represented by formula (i) may
- R 1 to R 11 each independently represent hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy (hereinafter, the first) Substituents), which may be further substituted with at least one (or more, a second substituent) selected from aryl, heteroaryl, and alkyl, and R 1 to R 3 , R 4 to R 7 and adjacent groups among R 8 to R 11 may be bonded to each other to form an aryl ring or a heteroaryl ring together with the a ring, b ring or c ring, and the formed ring may be an aryl or heteroaryl , Diarylamino, alkyl, cycloalkyl, alkoxy and aryloxy (at least one of
- the “aryl” (first substituent) such as R 1 may be a single ring or a condensed ring obtained by condensing two or more aromatic hydrocarbon rings, and may have two or more aromatic hydrocarbon rings linked to each other. It may be a connected ring. When two or more aromatic hydrocarbon rings are linked, they may be linked linearly or may be linked branched.
- “Aryl” is, for example, an aryl having 6 to 30 carbon atoms, preferably an aryl having 6 to 20 carbon atoms, more preferably an aryl having 6 to 16 carbon atoms, further preferably an aryl having 6 to 12 carbon atoms. Aryl of the number 6 to 10 is particularly preferred.
- aryl examples include phenyl which is a monocyclic system, biphenylyl which is a bicyclic system, naphthyl which is a condensed bicyclic system, terphenylyl which is a tricyclic system (m-terphenylyl, o-terphenylyl, p-terphenylyl), condensed Examples include tricyclic, acenaphthenyl, fluorenyl, phenalenyl, phenanthrenyl, fused tetracyclic, triphenylenyl, pyrenyl, naphthacenyl, fused pentacyclic perylenyl, pentacenyl, and the like.
- Heteroaryl (first substituent) such as R 1 is a condensed ring in which one or more heterocycles and one or more heterocycles or one or more aromatic hydrocarbon rings are condensed even if they are monocyclic. Alternatively, a connecting ring in which two or more heterocycles are connected may be used. When two or more heterocycles are linked, they may be linked linearly or may be linked branched. “Heteroaryl” is, for example, a heteroaryl having 2 to 30 carbon atoms, preferably a heteroaryl having 2 to 25 carbon atoms, more preferably a heteroaryl having 2 to 20 carbon atoms, and a heteroaryl having 2 to 15 carbon atoms.
- heteroaryl having 2 to 10 carbon atoms is particularly preferable.
- the heteroaryl is, for example, a heterocyclic ring containing 1 to 5 heteroatoms selected from oxygen, sulfur and nitrogen in addition to carbon as ring-constituting atoms.
- heteroaryl examples include, for example, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl, oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl, triazinyl, indolyl, isoindolyl, 1H-indazolyl, Benzimidazolyl, benzoxazolyl, benzothiazolyl, 1H-benzotriazolyl, quinolinyl, isoquinolinyl, cinnolinyl, quinazolinyl, quinoxalinyl, phthalazinyl, naphthyridinyl, prinyl, pteridinyl, carbazolyl, acridinyl, phen
- aryl in “diarylamino” (first substituent) such as R 1 and the “aryl” in “aryloxy” (first substituent), the description of aryl described above can be cited.
- aryl in “diarylboryl” (first substituent) such as R 1 , the above description of aryl can be cited.
- Alkyl (first substituent) such as R 1 may be linear or branched, and is, for example, linear alkyl having 1 to 24 carbons or branched alkyl having 3 to 24 carbons.
- Alkyl having 1 to 18 carbons (branched alkyl having 3 to 18 carbons) is preferable, alkyl having 1 to 12 carbons (branched alkyl having 3 to 12 carbons) is more preferable, and alkyl having 1 to 6 carbons is preferable.
- Branched alkyl having 3 to 6 carbons is more preferable, and alkyl having 1 to 4 carbons (branched alkyl having 3 to 4 carbons) is particularly preferable.
- alkyl examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, n-pentyl, isopentyl, neopentyl, t-pentyl, n-hexyl, 1-methyl Pentyl, 4-methyl-2-pentyl, 3,3-dimethylbutyl, 2-ethylbutyl, n-heptyl, 1-methylhexyl, n-octyl, t-octyl, 1-methylheptyl, 2-ethylhexyl, 2-propyl Pentyl, n-nonyl, 2,2-dimethylheptyl, 2,6-dimethyl-4-heptyl, 3,5,5-trimethylhexyl, n-decyl, n-undecyl, 1-methyldecyl, methyl
- Cycloalkyl (first substituent) such as R 1 includes cycloalkyl consisting of one ring, cycloalkyl consisting of a plurality of rings, cycloalkyl containing a double bond not conjugated in a ring, and branching outside the ring. Any of the included cycloalkyls may be used, for example, cycloalkyl having 3 to 12 carbon atoms. Cycloalkyl having 5 to 10 carbon atoms is preferable, and cycloalkyl having 6 to 10 carbon atoms is more preferable.
- cycloalkyl examples include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, bicyclo [2,2,1] heptyl, bicyclo [2.2.2] octyl, decahydronaphthyl, adamantyl and the like. No.
- Alkoxy (first substituent) such as R 1 may be linear or branched. For example, it is a straight-chain alkoxy having 1 to 24 carbon atoms or a branched alkoxy having 3 to 24 carbon atoms. Alkoxy having 1 to 18 carbon atoms (alkoxy having a branched chain having 3 to 18 carbon atoms) is preferable, alkoxy having 1 to 12 carbons (alkoxy having a branched chain having 3 to 12 carbon atoms) is more preferable, and alkoxy having 1 to 6 carbon atoms is preferable. (Alkoxy having a branched chain having 3 to 6 carbon atoms) is more preferred, and alkoxy having 1 to 4 carbons (an alkoxy having a branched chain having 3 to 4 carbon atoms) is particularly preferred.
- alkoxy includes methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, s-butoxy, t-butoxy, pentyloxy, hexyloxy, heptyloxy, octyloxy and the like.
- the emission wavelength can be adjusted by the steric hindrance, electron-donating property and electron-withdrawing property of the structure of R 1 or the like (first substituent), and is preferably a group represented by the following formula. And more preferably methyl, t-butyl, bicyclooctyl, cyclohexyl, adamantyl, phenyl, o-tolyl, p-tolyl, 2,4-xylyl, 2,5-xylyl, 2,6-xylyl, 2,4, 6-mesityl, diphenylamino, di-p-tolylamino, bis (p- (t-butyl) phenyl) amino, diphenylboryl, dimesitylboryl, dibenzooxaborinyl, phenyldibenzodiborinyl, carbazolyl, 3,6-dimethylcarba Zolyl, 3,6-di-t-butylcarbazolyl and
- steric hindrance is large for selective synthesis.
- t-butyl, o-tolyl, 2,6-xylyl, 2,4,6-mesityl , 3,6-dimethylcarbazolyl and 3,6-di-t-butylcarbazolyl are preferred.
- Me represents methyl
- tBu represents t-butyl
- the wavy line represents the bonding position
- Adjacent groups among R 1 to R 3 , R 4 to R 7 and R 8 to R 11 in the formula (1) are bonded to each other to form an aryl ring or a heteroaryl ring together with the a ring, b ring or c ring.
- the ring structure of the polycyclic aromatic compound represented by the formula (1) changes depending on the mutual bonding form of the substituents on the a-ring, b-ring and c-ring.
- R 3 of a ring and R 4 of b ring, R 7 of b ring and R 8 of c ring, R 11 of c ring and R 1 of a ring do not correspond to “adjacent groups”. , They do not combine. That is, “adjacent groups” means groups that are adjacent on the same ring.
- the “aryl ring” or “heteroaryl ring” formed is an unvalent ring of the aryl or heteroaryl as the first substituent described above.
- the carbon number of the formed ring includes the carbon number of the ring before condensation.
- Aryl, heteroaryl, diarylamino, alkyl, alkoxy, or aryloxy (the above, the first substituent) which substitutes on the formed aryl ring or heteroaryl ring, and aryl, hetero, which further substitutes the first substituent
- the aryl or alkyl (the above is the second substituent)
- the description of aryl, heteroaryl, diarylamino, alkyl, alkoxy or aryloxy as R 1 and the like can be cited.
- X in the formula (1) is>O,>NR,> CR 2 ,> S or> Se, and preferably> O and> NR.
- aryl is R of CR 2, heteroaryl or alkyl (more first substituent), also aryl further substituted to the first substituent, heteroaryl or alkyl (more, the the 2 substituents), aryl as above R 1 etc. (first substituent), a description of the heteroaryl or alkyl can be cited.
- the compound represented by the formula (1) is preferably a compound having the following partial structure.
- Me represents methyl
- tBu and t-Bu represent t-butyl
- Ph represents phenyl
- a ring, B ring, C ring and D ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- Y is B (boron),
- X 1 , X 2 , X 3 and X 4 are each independently>O,>NR,> CR 2 ,> S or> Se, and R of> NR and> CR 2 R is an optionally substituted aryl, an optionally substituted heteroaryl, an optionally substituted cycloalkyl or an optionally substituted alkyl, and R of the formula> NR represents a linking group.
- R 1 and R 2 each independently represent hydrogen, alkyl having 1 to 6 carbons, cycloalkyl having 3 to 12 carbons, aryl having 6 to 12 carbons, heteroaryl or diarylamino having 2 to 15 carbons (Wherein aryl is aryl having 6 to 12 carbon atoms)
- At least one hydrogen in the compound represented by the formula (ii) may be substituted with cyano, halogen, or deuterium.
- R 1 to R 14 each independently represent hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino, arylheteroarylamino, alkyl, cycloalkyl , alkoxy, aryloxy, heteroaryloxy, arylthio, a heteroarylthio or alkyl-substituted silyl, at least one hydrogen in these, aryl may be substituted with a heteroaryl or alkyl, also, R 5 ⁇ R 7 and the adjacent groups among R 10 to R 12 may be bonded to each other to form an aryl ring or a heteroaryl ring together with the b-ring or the d-ring, and at least one hydrogen in the formed ring is aryl , Heteroaryl, diarylamin
- aryls having up to 12 carbon atoms, heteroaryl having 2 to 15 carbon atoms, cycloalkyl having 3 to 12 carbon atoms or alkyl having 1 to 6 carbon atoms, and R of> NR and R of> CR 2 are —O—, —S—, —C (—R) 2 — or a single bond may be bonded to at least one selected from the a ring, b ring, c ring and d ring; R of —R) 2 — is hydrogen or alkyl having 1 to 6 carbons; However, X 1 , X 2 , X 3 , and X 4 are not simultaneously> CR 2 ; And At least one hydrogen in the compounds and structures represented by formula (2) may be substituted with cyano, halogen, or deuterium. )
- aryl, heteroaryl, diarylamino, alkyl, alkoxy or aryloxy or more, the first substituent
- aryl, heteroaryl or alkyl or more, further substituting the first substituent) the second substituent
- aryl as above R 1 etc. first substituent
- heteroaryl, diarylamino, alkyl a description of the alkoxy or aryloxy
- the compound represented by the above formula (2) is preferably a compound containing the following partial structure.
- R in the above structure is any of the following groups.
- a ring, B ring and C ring are each independently an aryl ring or a heteroaryl ring, and at least one hydrogen in these rings may be substituted;
- X 1 , X 2 and X 3 are each independently O, NR,> CR 2 , S or Se, and R of NR and R of> CR 2 may be substituted Aryl, optionally substituted heteroaryl, optionally substituted cycloalkyl or alkyl, and R of the NR is selected from the A ring, B ring and C ring by a linking group or a single bond.
- And may be associated with at least one of At least one hydrogen in the compound or structure represented by formula (iii)
- R 1 to R 11 are each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy, Further, it may be substituted with at least one selected from aryl, heteroaryl and alkyl, and adjacent groups among R 1 to R 3 , R 4 to R 6 and R 9 to R 11 are bonded to each other To form an aryl ring or a heteroaryl ring together with the a ring, b ring or c ring, and the formed ring is selected from aryl, heteroaryl, diarylamino, alkyl, cycloalkyl, alkoxy and aryloxy.
- X 1 , X 2 and X 3 are each independently>O,> NR, or> CR 2 , wherein R of> NR and R of> CR 2 are aryl, heteroaryl, cycloaryl Alkyl or alkyl, which may be substituted with at least one selected from aryl, heteroaryl and alkyl; However, X 1 , X 2 , and X 3 are not simultaneously> CR 2 .
- At least one hydrogen in the compounds and structures represented by formula (3) may be substituted with cyano, halogen or deuterium.
- aryl, heteroaryl, diarylamino, alkyl, alkoxy or aryloxy (or more, a first substituent), or aryl, heteroaryl or alkyl (or more, which further substitutes the first substituent) the second substituent), aryl as above R 1 etc. (first substituent), heteroaryl, diarylamino, alkyl, a description of the alkoxy or aryloxy can be cited.
- R 1 to R 14 are each independently hydrogen, aryl, heteroaryl, diarylamino, diarylboryl, alkyl, cycloalkyl, alkoxy or aryloxy; Further, it may be substituted with at least one selected from aryl, heteroaryl and alkyl, and further, among R 1 to R 3 , R 4 to R 7 , R 8 to R 10 and R 11 to R 14 Adjacent groups may combine with each other to form an aryl ring or a heteroaryl ring together with the a ring, b ring, c ring or d ring, and the formed ring is aryl, heteroaryl, diarylamino, alkyl, cyclo May be substituted with at least one selected from alkyl
- L is a single bond,> CR 2 ,>O,> S or> NR
- R in the above> CR 2 and> NR is each independently hydrogen, aryl, heteroaryl, diarylamino , Alkyl, alkoxy or aryloxy, which may be further substituted with at least one selected from aryl, heteroaryl and alkyl;
- X and L are simultaneously> not be a CR 2
- At least one hydrogen in the compounds and structures represented by formula (4) may be substituted with cyano, halogen or deuterium.
- aryl, heteroaryl, diarylamino, alkyl, alkoxy or aryloxy or more, the first substituent
- aryl, heteroaryl or alkyl or more, further substituting the first substituent) the second substituent
- aryl as above R 1 etc. first substituent
- heteroaryl, diarylamino, alkyl a description of the alkoxy or aryloxy
- L in the formula (4) is a single bond,> CR 2 ,>O,> S or> NR, preferably a single bond,> O or> NR, and more preferably a single bond.
- Aryl, heteroaryl, diarylamino, alkyl, alkoxy or aryloxy (hereinafter, the first substituent) which is R of> CR 2 and> NR, and aryl and heteroaryl further substituting the first substituent or alkyl (more second substituent) as may cite aryl as above R 1 etc. (first substituent), heteroaryl, diarylamino, alkyl, a description of the alkoxy or aryloxy.
- the compound represented by the formula (4) is preferably a compound having the following partial structure.
- the third component of the present invention is at least one of the compounds represented by formulas (i) to (iii), and more specifically, at least one of the compounds represented by formulas (1) to (4). It is preferably one. From the viewpoint of high PLQY, the compounds represented by the formulas (i) and (ii) are preferable, and the compound represented by the formula (ii) is more preferable. More specifically, it is preferable that the planarity of the conjugated structure containing a boron atom is higher, the formulas (1), (2) and (4) are preferable, and the formulas (2) and (4) are more preferable.
- the compounds represented by the formulas (i) and (ii) are preferable, and the compound represented by the formula (ii) is more preferable. More specifically, it is preferable that X, X 1 , X 2 , X 3 and X 4 are nitrogen, and formulas (1), (2) and (4) are preferable, and formulas (1) and (4) More preferred. From the viewpoint of a large SOC, the formulas (i) and (ii) are preferable. More specifically, it is better that the conjugated structure containing a boron atom is not a perfect plane but is distorted. Formulas (1), (2) and (4) are preferable, and formulas (1) and (4) are more preferable. , Formula (1) is more preferable.
- the substituent is bonded to the ring present in the compound. It may be an unsubstituted compound which is not substituted, but it is preferable to use a compound substituted with an appropriate substituent.
- a compound substituted with an appropriate substituent By using a compound substituted with an appropriate substituent as the third component, the characteristics of the organic electroluminescent device are more excellent than when using an unsubstituted compound.
- Preferred substituents include aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino, arylheteroarylamino, alkyl, cycloalkyl, alkoxy, aryloxy, heteroaryloxy, arylthio, heteroarylthio and alkyl-substituted silyl. More preferred substituents include aryl, heteroaryl, diarylamino, alkyl, cycloalkyl, alkoxy and aryloxy; more preferred substituents include aryl, diarylamino, alkyl Even more preferred substituents are diarylamino and alkyl; particularly preferred substituents are diarylamino.
- aryl, heteroaryl, diarylamino, diarylboryl, diheteroarylamino, arylheteroarylamino, alkyl, cycloalkyl, alkoxy, aryloxy, heteroaryloxy, arylthio, heteroarylthio and alkyl-substituted silyl are May be substituted with at least one selected from aryl, heteroaryl, cycloalkyl and alkyl.
- the two aryls in the diarylamino may be the same or different, but are preferably the same.
- the aryl include phenyl which is a monocyclic system, biphenylyl which is a bicyclic system, naphthyl which is a condensed bicyclic system, terphenylyl which is a tricyclic system (m-terphenylyl, o-terphenylyl, p-terphenylyl), condensed tricyclic system Acenaphthylenyl, fluorenyl, phenalenyl, phenanthrenyl, condensed tetracyclic triphenylenyl, pyrenyl, naphthacenyl, condensed pentacyclic perylenyl, pentacenyl and the like.
- the aryl of the diarylamino is substituted, it is preferably substituted with at least one selected from aryl and alkyl. Further, a group in which two aryl groups constituting diarylamino are not bonded to each other may be particularly selected and used. For example, diphenylamino, di (4-methylphenyl) amino, di (4-t-butylphenyl) amino and the like can be mentioned.
- alkyl examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, n-pentyl, isopentyl, neopentyl, t-pentyl, n-hexyl, 1-methyl Pentyl, 4-methyl-2-pentyl, 3,3-dimethylbutyl, 2-ethylbutyl, n-heptyl, 1-methylhexyl, n-octyl, t-octyl, 1-methylheptyl, 2-ethylhexyl, 2-propyl Pentyl, n-nonyl, 2,2-dimethylheptyl, 2,6-dimethyl-4-heptyl, 3,5,5-trimethylhexyl, n-decyl, n-undecyl, 1-methyldecyl, methyl
- the third component By using a compound having a preferred substituent as the third component, it is possible to improve the external quantum yield of the organic electroluminescent device, to reduce the half width, and to increase the lifetime of the device. Can be Further, by substituting with an appropriate substituent, the lifetime Tau (Delay) of the delayed fluorescence can be shortened, and the Stokes shift can be reduced, thereby improving the performance of the organic electroluminescent device. .
- the following particularly preferred energy structures can be constructed and adopted for the second component and the third component by employing a technique such as appropriately selecting a substituent. E (3, T, Sh) ⁇ E (2, T, Sh) ⁇ E (2, ST, Sh) ⁇ ⁇ E (3, ST, Sh)
- the Stokes shift of the third component is 10 nm or less.
- the Stokes shift of the third component is 15 nm or less. Further, the following energy structure is also preferable. E (3, T, Sh) ⁇ E (2, T, Sh) ⁇ E (2, ST, Sh) ⁇ ⁇ E (3, ST, Sh)
- the Stokes shift of the third component is larger than 15 nm. In any of the above energy structures, it is preferable that E (3, S, PT) ⁇ E (2, S, PT).
- the first component contained in the light emitting layer of the organic electroluminescent device of the present invention may be a polymer compound containing, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from a host compound.
- the second component contained in the light emitting layer of the organic electroluminescent device of the present invention may be a polymer compound containing, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance.
- the third component contained in the light emitting layer of the organic electroluminescent device of the present invention may be a polymer compound containing, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from a compound having a boron atom.
- the two hydrogen atoms to be eliminated can be any two atoms in the compound. It may or may not be two hydrogen atoms bonded to the same ring structure.
- the polymer compound contained in the light-emitting layer contains, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from the host compound, and also contains, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance. It may be a polymer compound.
- the polymer compound contained in the light-emitting layer contains, as a repeating unit, a structure in which two hydrogen atoms are eliminated from a host compound and a structure in which two hydrogen atoms are eliminated from a compound containing a boron atom as a repeating unit. It may be a molecular compound.
- the polymer compound contained in the light emitting layer contains, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance, and a structure in which two hydrogen atoms have been eliminated from a compound containing a boron atom. It may be a polymer compound containing as a unit. Further, the polymer compound contained in the light emitting layer contains, as a repeating unit, a structure in which two hydrogen atoms have been eliminated from the host compound, and a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance. And a polymer compound containing, as a repeating unit, a structure in which two hydrogen atoms are eliminated from a compound containing a boron atom.
- the polymer compound contained in the light emitting layer may contain two or more kinds of repeating units having a structure in which two hydrogen atoms are eliminated from the host compound.
- the polymer compound contained in the light emitting layer may contain two or more kinds of repeating units having a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance. Two or more kinds of repeating units having a structure in which two hydrogen atoms are eliminated from a compound containing a boron atom, which is contained in the polymer compound contained in the light emitting layer, may be used.
- the polymer compound contained in the light emitting layer includes a repeating unit having a structure in which two hydrogen atoms have been eliminated from the host compound, a repeating unit having a structure in which two hydrogen atoms have been eliminated from the thermally activated delayed fluorescent substance, and boron.
- the compound may contain one or more kinds of repeating units different from these.
- a repeating unit including a structure exhibiting a hole transporting property a repeating unit including a structure exhibiting an electron transporting property, a repeating unit not exhibiting a hole transporting property or an electron transporting property, and the like are appropriately selected and employed. can do.
- the arylene and heteroarylene mentioned herein may be substituted, and examples of the substituent include alkyl having 1 to 30 carbons, aryl having 6 to 22 carbons, and heteroaryl having 5 to 22 ring skeleton constituting atoms. be able to.
- Examples of RA include alkyl having 1 to 30 carbons, aryl having 6 to 22 carbons, and heteroaryl having 5 to 22 ring skeleton-constituting atoms.
- Specific examples of the repeating unit include the following structures.
- the hydrogen atoms present in the structures below may be substituted with alkyl having 1 to 30 carbons, aryl having 6 to 22 carbons, heteroaryl having 5 to 22 ring skeleton-constituting atoms, or the like.
- each repeating unit constituting the polymer compound contained in the light emitting layer is not particularly limited.
- a repeating unit having a structure in which two hydrogen atoms are eliminated from a host compound a repeating unit having a structure in which two hydrogen atoms are eliminated from a thermally activated delayed fluorescent substance, and two hydrogen atoms are eliminated from a compound containing a boron atom
- each repeating unit can be selected within the range of 0.01 to 100 mol%.
- the molar ratio of the repeating unit can be selected from the range of 0.01 to 99.99 mol%.
- R B is alkyl optionally substituted, an optionally substituted cycloalkyl, or also aryl such as optionally substituted.
- R C and R G are a hydrogen atom, an optionally substituted alkyl, an optionally substituted cycloalkyl, or the like, and two or more R C or R G are connected to each other to form a cyclic structure.
- RD is Li, Na, K, Rb, Cs or the like.
- RE and RF are a chlorine atom, a bromine atom or an iodine atom.
- the coupling reaction is preferably performed in the presence of a catalyst.
- a catalyst bis (triphenylphosphine) palladium (II) dichloride, bis (tris-o-methoxyphenylphosphine) palladium (II) dichloride, tetrakis (triphenylphosphine) palladium (0), tris (dibenzylideneacetone) dichloride Palladium (0), palladium acetate, tetrakis (triphenylphosphine) nickel (0), [1,3-bis (diphenylphosphino) propane) nickel (II) dichloride, bis (1,4-cyclooctadiene) nickel ( 0), sodium carbonate, potassium carbonate, cesium carbonate, potassium fluoride, cesium fluoride, tripotassium phosphate, tetrabutylammonium fluoride, tetraethylammonium hydroxide, tetra
- the organic electroluminescent device of the present invention may have one or more organic layers in addition to the light emitting layer.
- the organic layer include an electron transport layer, a hole transport layer, an electron injection layer, a hole injection layer, and the like, and may further include another organic layer.
- FIG. 4 shows an example of a layer configuration of an organic electroluminescent device including these organic layers.
- reference numeral 101 denotes a substrate
- 102 denotes an anode
- 103 denotes a hole injection layer
- 104 denotes a hole transport layer
- 105 denotes a light emitting layer
- 106 denotes an electron transport layer
- 107 denotes an electron injection layer
- 108 denotes a cathode.
- an organic layer, a cathode and an anode, and a substrate provided in addition to the light emitting layer in the organic electroluminescent device will be described.
- the electron injection layer and the electron transport layer 107 in the organic electroluminescent element play a role of efficiently injecting electrons moving from the cathode 108 into the light emitting layer 105 or the electron transport layer 106.
- the electron transport layer 106 plays a role in efficiently transporting electrons injected from the cathode 108 or electrons injected from the cathode 108 through the electron injection layer 107 to the light emitting layer 105.
- Each of the electron transport layer 106 and the electron injection layer 107 is formed by laminating and mixing one or more of the electron transport / injection materials.
- the electron injection / transport layer is a layer that injects electrons from the cathode and transports the electrons. It is desirable that the electron injection efficiency is high and the injected electrons are transported efficiently.
- the substance be a substance having a high electron affinity, a high electron mobility, excellent stability, and hardly generating impurities serving as traps during production and use.
- the electron transport capability is not so high. Even if it is not high, the effect of improving the luminous efficiency is equivalent to a material having a high electron transporting ability. Therefore, the electron injecting / transporting layer in the present embodiment may include a function of a layer that can efficiently block the movement of holes.
- a material (electron transporting material) for forming the electron transporting layer 106 or the electron injecting layer 107 a compound conventionally used as an electron transporting compound in a photoconductive material, an electron injecting layer and an electron transporting layer of an organic electroluminescent element can be used. Any of the known compounds used can be arbitrarily selected and used.
- condensed ring type aromatic ring derivatives such as naphthalene and anthracene
- styryl type aromatic ring derivatives typified by 4,4′-bis (diphenylethenyl) biphenyl
- perinone derivatives such as naphthalene and anthracene
- coumarin derivatives such as naphthalimide derivatives
- quinone derivatives such as anthraquinone and diphenoquinone, phosphorus oxide derivatives, carbazole derivatives and indole derivatives.
- the metal complex having an electron accepting nitrogen include a hydroxyazole complex such as a hydroxyphenyloxazole complex, an azomethine complex, a tropolone metal complex, a flavonol metal complex, and a benzoquinoline metal complex. These materials may be used alone or in combination with different materials.
- electron transfer compounds include pyridine derivatives, naphthalene derivatives, anthracene derivatives, phenanthroline derivatives, perinone derivatives, coumarin derivatives, naphthalimide derivatives, anthraquinone derivatives, diphenoquinone derivatives, diphenylquinone derivatives, perylene derivatives, and oxadiazole.
- Derivatives such as 1,3-bis [(4-t-butylphenyl) 1,3,4-oxadiazolyl] phenylene), thiophene derivatives, and triazole derivatives (N-naphthyl-2,5-diphenyl-1,3,4- Triazole), metal complexes of thiadiazole derivatives, oxine derivatives, quinolinol-based metal complexes, quinoxaline derivatives, polymers of quinoxaline derivatives, benzazoles, gallium complexes, pyrazole derivatives, perfluorinated Nylene derivatives, triazine derivatives, pyrazine derivatives, benzoquinoline derivatives (such as 2,2'-bis (benzo [h] quinolin-2-yl) -9,9'-spirobifluorene), imidazopyridine derivatives, borane derivatives, benzones Imidazole derivatives (such as tris (N-phenylbenzimidazol
- the above-mentioned materials may be used alone, but may be used in combination with different materials.
- borane derivatives pyridine derivatives, fluoranthene derivatives, BO derivatives, anthracene derivatives, benzofluorene derivatives, phosphine oxide derivatives, pyrimidine derivatives, carbazole derivatives, triazine derivatives, benzimidazole derivatives, phenanthroline derivatives, and quinolinol-based metals Complexes are preferred.
- the pyridine derivative is, for example, a compound represented by the following formula (ETM-2), and preferably a compound represented by the formula (ETM-2-1) or (ETM-2-2).
- ⁇ is an n-valent aryl ring (preferably an n-valent benzene ring, naphthalene ring, anthracene ring, fluorene ring, benzofluorene ring, phenalene ring, phenanthrene ring or triphenylene ring), and n is an integer of 1 to 4. is there.
- R 11 to R 18 are each independently hydrogen, alkyl (preferably alkyl having 1 to 24 carbon atoms), cycloalkyl (preferably cycloalkyl having 3 to 12 carbon atoms) Alkyl) or aryl (preferably aryl having 6 to 30 carbon atoms).
- R 11 and R 12 are each independently hydrogen, alkyl (preferably alkyl having 1 to 24 carbon atoms), cycloalkyl (preferably cycloalkyl having 3 to 12 carbon atoms) Alkyl) or aryl (preferably aryl having 6 to 30 carbon atoms), and R 11 and R 12 may combine to form a ring.
- the “pyridine-based substituent” is any of the following formulas (Py-1) to (Py-15), and the pyridine-based substituents are each independently substituted with alkyl having 1 to 4 carbon atoms. It may be. Further, the pyridine-based substituent may be bonded to ⁇ , an anthracene ring or a fluorene ring in each formula via a phenylene group or a naphthylene group.
- the pyridine-based substituent is any of the above formulas (Py-1) to (Py-15), and among them, any of the following formulas (Py-21) to (Py-44) Is preferred.
- At least one hydrogen in each pyridine derivative may be substituted with deuterium, and among the two “pyridine-based substituents” in the above formula (ETM-2-1) and formula (ETM-2-2) May be replaced by an aryl.
- the “alkyl” for R 11 to R 18 may be linear or branched, and includes, for example, linear alkyl having 1 to 24 carbons or branched alkyl having 3 to 24 carbons.
- Preferred “alkyl” is alkyl having 1 to 18 carbons (branched alkyl having 3 to 18 carbons). More preferred “alkyl” is alkyl having 1 to 12 carbons (branched alkyl having 3 to 12 carbons). More preferred “alkyl” is alkyl having 1 to 6 carbons (branched alkyl having 3 to 6 carbons). Particularly preferred “alkyl” is alkyl having 1 to 4 carbons (branched alkyl having 3 to 4 carbons).
- alkyl includes methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, n-pentyl, isopentyl, neopentyl, t-pentyl, n-hexyl, -Methylpentyl, 4-methyl-2-pentyl, 3,3-dimethylbutyl, 2-ethylbutyl, n-heptyl, 1-methylhexyl, n-octyl, t-octyl, 1-methylheptyl, 2-ethylhexyl, -Propylpentyl, n-nonyl, 2,2-dimethylheptyl, 2,6-dimethyl-4-heptyl, 3,5,5-trimethylhexyl, n-decyl, n-undecyl, 1-methyl,
- alkyl having 1 to 4 carbon atoms to be substituted with the pyridine-based substituent the description of the above alkyl can be cited.
- Cycloalkyl for R 11 to R 18 includes, for example, cycloalkyl having 3 to 12 carbon atoms.
- Preferred “cycloalkyl” is cycloalkyl having 3 to 10 carbon atoms. More preferred “cycloalkyl” is cycloalkyl having 3 to 8 carbon atoms. More preferred “cycloalkyl” is cycloalkyl having 3 to 6 carbon atoms.
- cycloalkyl includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, methylcyclopentyl, cycloheptyl, methylcyclohexyl, cyclooctyl, dimethylcyclohexyl and the like.
- the “aryl” in R 11 to R 18 may be a single ring, a condensed ring in which two or more aromatic hydrocarbon rings are fused, or a connecting ring in which two or more aromatic hydrocarbon rings are linked. There may be. When two or more aromatic hydrocarbon rings are linked, they may be linked linearly or may be linked branched. Preferred aryl is aryl having 6 to 30 carbons, more preferred aryl is aryl having 6 to 18 carbons, still more preferred is aryl having 6 to 14 carbons, and particularly preferred is aryl having 6 to 12 carbons. It is.
- aryl examples include, for example, phenyl which is a monocyclic aryl, biphenylyl which is a bicyclic aryl (2-biphenylyl, 3-biphenylyl, 4-biphenylyl), and naphthyl (1-naphthyl) which is a fused bicyclic aryl , 2-naphthyl), terphenylyl which is a tricyclic aryl (m-terphenyl-2'-yl, m-terphenyl-4'-yl, m-terphenyl-5'-yl, o-terphenyl-3 '-Yl, o-terphenyl-4'-yl, p-terphenyl-2'-yl, m-terphenyl-2-yl, m-terphenyl-3-yl, m-terphenyl-4-yl , O-terphenyl-2-yl, o-terphenyl,
- Preferred “aryl having 6 to 30 carbon atoms” include phenyl, naphthyl, phenanthryl, chrysenyl or triphenylenyl, more preferably phenyl, 1-naphthyl, 2-naphthyl or phenanthryl, and particularly preferably phenyl, -Naphthyl or 2-naphthyl.
- R 11 and R 12 in the above formula (ETM-2-2) may combine to form a ring, and as a result, the 5-membered ring of the fluorene skeleton has cyclobutane, cyclopentane, cyclopentene, cyclopentadiene, Cyclohexane, fluorene or indene may be spiro-bonded.
- pyridine derivative examples include, for example, the following compounds.
- This pyridine derivative can be produced using a known raw material and a known synthesis method.
- the phosphine oxide derivative is, for example, a compound represented by the following formula (ETM-7-1). The details are also described in WO2013 / 079217.
- R 5 is a substituted or unsubstituted alkyl having 1 to 20 carbons, an aryl having 6 to 20 carbons or a heteroaryl having 5 to 20 carbons
- R 6 is CN, substituted or unsubstituted alkyl having 1 to 20 carbons, heteroalkyl having 1 to 20 carbons, aryl having 6 to 20 carbons, heteroaryl having 5 to 20 carbons, 1 to carbons 20 alkoxy or aryloxy having 6 to 20 carbon atoms
- R 7 and R 8 are each independently a substituted or unsubstituted aryl having 6 to 20 carbons or a heteroaryl having 5 to 20 carbons
- R 9 is oxygen or sulfur
- j is 0 or 1
- k is 0 or 1
- r is an integer of 0 to 4
- q is
- the phosphine oxide derivative may be, for example, a compound represented by the following formula (ETM-7-2).
- R 1 to R 3 may be the same or different, and include hydrogen, an alkyl group, a cycloalkyl group, an aralkyl group, an alkenyl group, a cycloalkenyl group, an alkynyl group, an alkoxy group, an alkylthio group, an arylether group, and an arylthioether group.
- Ar 1 may be the same or different and is an arylene group or a heteroarylene group
- Ar 2 may be the same or different and is an aryl group or a heteroaryl group.
- at least one of Ar 1 and Ar 2 has a substituent or forms a condensed ring with an adjacent substituent.
- n is an integer of 0 to 3. When n is 0, there is no unsaturated structure part, and when n is 3, R 1 does not exist.
- the alkyl group means a saturated aliphatic hydrocarbon group such as a methyl group, an ethyl group, a propyl group, and a butyl group, which may be unsubstituted or substituted.
- the substituent is not particularly limited, and examples thereof include an alkyl group, an aryl group, and a heterocyclic group. This point is also common to the following description.
- the number of carbon atoms in the alkyl group is not particularly limited, but is usually in the range of 1 to 20 from the viewpoint of availability and cost.
- cycloalkyl group refers to, for example, a saturated alicyclic hydrocarbon group such as cyclopropyl, cyclohexyl, norbornyl, and adamantyl, which may be unsubstituted or substituted.
- the number of carbon atoms in the alkyl group is not particularly limited, but is usually in the range of 3 to 20.
- the aralkyl group refers to, for example, an aromatic hydrocarbon group via an aliphatic hydrocarbon such as a benzyl group and a phenylethyl group, and both the aliphatic hydrocarbon and the aromatic hydrocarbon are unsubstituted or substituted. It doesn't matter.
- the carbon number of the aliphatic moiety is not particularly limited, but is usually in the range of 1 to 20.
- Alkenyl group refers to an unsaturated aliphatic hydrocarbon group containing a double bond such as a vinyl group, an allyl group and a butadienyl group, which may be unsubstituted or substituted.
- the number of carbon atoms of the alkenyl group is not particularly limited, but is usually in the range of 2 to 20.
- the cycloalkenyl group refers to, for example, an unsaturated alicyclic hydrocarbon group containing a double bond such as a cyclopentenyl group, a cyclopentadienyl group, and a cyclohexene group, which may be unsubstituted or substituted. I don't care.
- Alkynyl group means, for example, an unsaturated aliphatic hydrocarbon group containing a triple bond such as an acetylenyl group, which may be unsubstituted or substituted.
- the number of carbon atoms in the alkynyl group is not particularly limited, but is usually in the range of 2 to 20.
- Alkoxy group means, for example, an aliphatic hydrocarbon group via an ether bond such as a methoxy group, and the aliphatic hydrocarbon group may be unsubstituted or substituted.
- the carbon number of the alkoxy group is not particularly limited, it is usually in the range of 1 to 20.
- Alkylthio group is a group in which an oxygen atom of an ether bond of an alkoxy group is substituted with a sulfur atom.
- the aryl ether group refers to, for example, an aromatic hydrocarbon group via an ether bond such as a phenoxy group, and the aromatic hydrocarbon group may be unsubstituted or substituted.
- the number of carbon atoms in the aryl ether group is not particularly limited, but is usually in the range of 6 to 40.
- the arylthioether group is a group in which the oxygen atom of the ether bond of the arylether group is substituted with a sulfur atom.
- the aryl group refers to, for example, an aromatic hydrocarbon group such as a phenyl group, a naphthyl group, a biphenylyl group, a phenanthryl group, a terphenyl group, and a pyrenyl group.
- the aryl group may be unsubstituted or substituted.
- the carbon number of the aryl group is not particularly limited, but is usually in the range of 6 to 40.
- heterocyclic group refers to, for example, a cyclic structure group having an atom other than carbon, such as a furanyl group, a thiophenyl group, an oxazolyl group, a pyridyl group, a quinolinyl group, and a carbazolyl group. It doesn't matter.
- the carbon number of the heterocyclic group is not particularly limited, but is usually in the range of 2 to 30.
- Halogen refers to fluorine, chlorine, bromine and iodine.
- the aldehyde group, carbonyl group, and amino group may also include groups substituted with an aliphatic hydrocarbon, an alicyclic hydrocarbon, an aromatic hydrocarbon, a heterocyclic ring, and the like.
- aliphatic hydrocarbons aliphatic hydrocarbons, alicyclic hydrocarbons, aromatic hydrocarbons, and heterocycles may be unsubstituted or substituted.
- silyl group means a silicon compound group such as a trimethylsilyl group, which may be unsubstituted or substituted.
- carbon number of the silyl group is not particularly limited, it is usually in the range of 3 to 20. Further, the number of silicon is usually 1 to 6.
- the condensed ring formed between adjacent substituents is, for example, Ar 1 and R 2 , Ar 1 and R 3 , Ar 2 and R 2 , Ar 2 and R 3 , R 2 and R 3 , Ar 1 and It is a conjugated or non-conjugated fused ring formed between Ar 2 and the like.
- n when n is 1, may be formed conjugated or non-conjugated fused ring with two of R 1 each other.
- These condensed rings may contain nitrogen, oxygen and sulfur atoms in the ring structure, or may be condensed with another ring.
- phosphine oxide derivative examples include the following compounds.
- This phosphine oxide derivative can be produced using a known raw material and a known synthesis method.
- the pyrimidine derivative is, for example, a compound represented by the following formula (ETM-8), and preferably a compound represented by the following formula (ETM-8-1). The details are also described in WO 2011/021689.
- Ar is each independently an optionally substituted aryl or an optionally substituted heteroaryl.
- n is an integer of 1 to 4, preferably an integer of 1 to 3, and more preferably 2 or 3.
- the “aryl” of the “optionally substituted aryl” may be a single ring, a condensed ring obtained by condensing two or more aromatic hydrocarbon rings, or two or more aromatic hydrocarbon rings linked to each other. It may be a connected ring. When two or more aromatic hydrocarbon rings are linked, they may be linked linearly or may be linked branched.
- “Aryl” includes, for example, aryl having 6 to 30 carbon atoms, preferably aryl having 6 to 24 carbon atoms, more preferably aryl having 6 to 20 carbon atoms, and still more preferably aryl having 6 to 12 carbon atoms. It is.
- aryl examples include, for example, phenyl which is a monocyclic aryl, biphenylyl which is a bicyclic aryl (2-biphenylyl, 3-biphenylyl, 4-biphenylyl), and naphthyl (1-naphthyl) which is a fused bicyclic aryl , 2-naphthyl), terphenylyl which is a tricyclic aryl (m-terphenyl-2'-yl, m-terphenyl-4'-yl, m-terphenyl-5'-yl, o-terphenyl-3 '-Yl, o-terphenyl-4'-yl, p-terphenyl-2'-yl, m-terphenyl-2-yl, m-terphenyl-3-yl, m-terphenyl-4-yl , O-terphenyl-2-yl, o-terphenyl,
- heteroaryl of the “optionally substituted heteroaryl” may be a single ring or a condensed ring in which one or more heterocycles and one or more heterocycles or one or more aromatic hydrocarbon rings are fused. Alternatively, it may be a connecting ring in which two or more heterocycles are connected. When two or more heterocycles are linked, they may be linked linearly or may be linked branched. “Heteroaryl” includes, for example, heteroaryl having 2 to 30 carbon atoms, preferably heteroaryl having 2 to 25 carbon atoms, more preferably heteroaryl having 2 to 20 carbon atoms, and 2 to 15 carbon atoms.
- Heteroaryl is more preferred, and heteroaryl having 2 to 10 carbon atoms is particularly preferred.
- the heteroaryl includes, for example, a heterocyclic ring containing 1 to 5 hetero atoms selected from oxygen, sulfur and nitrogen in addition to carbon as ring-constituting atoms.
- heteroaryl examples include, for example, furyl, thienyl, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl, pyrazolyl, oxadiazolyl, furazanil, thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl, triazinyl, benzofuranyl, benzofuranyl, Isobenzofuranyl, benzo [b] thienyl, indolyl, isoindolyl, 1H-indazolyl, benzimidazolyl, benzoxazolyl, benzothiazolyl, 1H-benzotriazolyl, quinolyl, isoquinolyl, cinnolyl, quinazolyl, quinoxalinyl,
- the above aryl and heteroaryl may be substituted, for example, each of the above aryl and heteroaryl may be substituted.
- pyrimidine derivative examples include, for example, the following compounds.
- This pyrimidine derivative can be produced using a known raw material and a known synthesis method.
- Triazine derivative is, for example, a compound represented by the following formula (ETM-10), and preferably a compound represented by the following formula (ETM-10-1). Details are described in U.S. Publication No. 2011/0156013.
- Ar is each independently an optionally substituted aryl or an optionally substituted heteroaryl.
- n is an integer of 1 to 4, preferably an integer of 1 to 3, and more preferably 2 or 3.
- aryl of the “optionally substituted aryl” includes, for example, aryl having 6 to 30 carbon atoms, preferably aryl having 6 to 24 carbon atoms, more preferably aryl having 6 to 20 carbon atoms, More preferably, it is an aryl having 6 to 12 carbon atoms.
- aryl examples include, for example, phenyl which is a monocyclic aryl, biphenylyl which is a bicyclic aryl (2-biphenylyl, 3-biphenylyl, 4-biphenylyl), and naphthyl (1-naphthyl) which is a fused bicyclic aryl , 2-naphthyl), terphenylyl which is a tricyclic aryl (m-terphenyl-2'-yl, m-terphenyl-4'-yl, m-terphenyl-5'-yl, o-terphenyl-3 '-Yl, o-terphenyl-4'-yl, p-terphenyl-2'-yl, m-terphenyl-2-yl, m-terphenyl-3-yl, m-terphenyl-4-yl , O-terphenyl-2-yl, o-terphenyl,
- heteroaryl of the “optionally substituted heteroaryl” may be a single ring or a condensed ring in which one or more heterocycles and one or more heterocycles or one or more aromatic hydrocarbon rings are fused. Alternatively, it may be a connecting ring in which two or more heterocycles are connected. When two or more heterocycles are linked, they may be linked linearly or may be linked branched. “Heteroaryl” includes, for example, heteroaryl having 2 to 30 carbon atoms, preferably heteroaryl having 2 to 25 carbon atoms, more preferably heteroaryl having 2 to 20 carbon atoms, and 2 to 15 carbon atoms.
- Heteroaryl is more preferred, and heteroaryl having 2 to 10 carbon atoms is particularly preferred.
- the heteroaryl includes, for example, a heterocyclic ring containing 1 to 5 hetero atoms selected from oxygen, sulfur and nitrogen in addition to carbon as ring-constituting atoms.
- heteroaryl examples include, for example, furyl, thienyl, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl, pyrazolyl, oxadiazolyl, furazanil, thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl, triazinyl, benzofuranyl, benzofuranyl, Isobenzofuranyl, benzo [b] thienyl, indolyl, isoindolyl, 1H-indazolyl, benzimidazolyl, benzoxazolyl, benzothiazolyl, 1H-benzotriazolyl, quinolyl, isoquinolyl, cinnolyl, quinazolyl, quinoxalinyl,
- the above aryl and heteroaryl may be substituted, for example, each of the above aryl and heteroaryl may be substituted.
- triazine derivative examples include, for example, the following compounds.
- This triazine derivative can be produced using a known raw material and a known synthesis method.
- Benzimidazole derivative is, for example, a compound represented by the following formula (ETM-11).
- ⁇ is an n-valent aryl ring (preferably an n-valent benzene ring, naphthalene ring, anthracene ring, fluorene ring, benzofluorene ring, phenalene ring, phenanthrene ring or triphenylene ring), and n is an integer of 1 to 4.
- the “benzimidazole-based substituent” means that the pyridyl group in the “pyridine-based substituent” in the above formulas (ETM-2), (ETM-2-1) and (ETM-2-2) is benzo. It is a substituent replacing the imidazole group, and at least one hydrogen in the benzimidazole derivative may be substituted with deuterium.
- R 11 in the benzimidazole group is hydrogen, alkyl having 1 to 24 carbons, cycloalkyl having 3 to 12 carbons or aryl having 6 to 30 carbons, and is represented by the above formula (ETM-2-1) or ( It may be cited to the description of R 11 in ETM-2-2).
- ⁇ is further preferably an anthracene ring or a fluorene ring, and in this case, the structure described in the above formula (ETM-2-1) or (ETM-2-2) can be referred to.
- R 11 to R 18 can refer to the description in the above formula (ETM-2-1) or formula (ETM-2-2).
- two pyridine-based substituents are described as being bonded. However, when these are replaced with benzimidazole-based substituents, both are substituted.
- benzimidazole derivative examples include, for example, 1-phenyl-2- (4- (10-phenylanthracen-9-yl) phenyl) -1H-benzo [d] imidazole, 2- (4- (10- ( Naphthalen-2-yl) anthracen-9-yl) phenyl) -1-phenyl-1H-benzo [d] imidazole, 2- (3- (10- (naphthalen-2-yl) anthracen-9-yl) phenyl) -1-phenyl-1H-benzo [d] imidazole, 5- (10- (naphthalen-2-yl) anthracen-9-yl) -1,2-diphenyl-1H-benzo [d] imidazole, 1- (4 -(10- (naphthalen-2-yl) anthracen-9-yl) phenyl) -2-phenyl-1H-benzo [d] imidazole, 2- (4- (9,10 Di (naphthalen-2
- This benzimidazole derivative can be produced using a known raw material and a known synthesis method.
- Phenanthroline derivative is, for example, a compound represented by the following formula (ETM-12) or (ETM-12-1). Details are described in WO 2006/021982.
- ⁇ is an n-valent aryl ring (preferably an n-valent benzene ring, naphthalene ring, anthracene ring, fluorene ring, benzofluorene ring, phenalene ring, phenanthrene ring or triphenylene ring), and n is an integer of 1 to 4. is there.
- R 11 to R 18 in each formula are each independently hydrogen, alkyl (preferably alkyl having 1 to 24 carbons), cycloalkyl (preferably cycloalkyl having 3 to 12 carbons) or aryl (preferably carbon Aryl of formulas 6 to 30).
- alkyl preferably alkyl having 1 to 24 carbons
- cycloalkyl preferably cycloalkyl having 3 to 12 carbons
- aryl preferably carbon Aryl of formulas 6 to 30.
- each phenanthroline derivative may be replaced with deuterium.
- R 11 ⁇ R 18, cycloalkyl and aryl may be cited to the description of R 11 ⁇ R 18 in the formula (ETM-2).
- ⁇ is, for example, the following structural formula in addition to the above examples.
- R in the following structural formulas is each independently hydrogen, methyl, ethyl, isopropyl, cyclohexyl, phenyl, 1-naphthyl, 2-naphthyl, biphenylyl or terphenylyl.
- phenanthroline derivative examples include, for example, 4,7-diphenyl-1,10-phenanthroline, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline, 9,10-di (1,10- Phenanthroline-2-yl) anthracene, 2,6-di (1,10-phenanthroline-5-yl) pyridine, 1,3,5-tri (1,10-phenanthroline-5-yl) benzene, 9,9 ′ -Difluoro-bis (1,10-phenanthroline-5-yl), bathocuproine, 1,3-bis (2-phenyl-1,10-phenanthroline-9-yl) benzene and the like.
- This phenanthroline derivative can be produced using a known raw material and a known synthesis method.
- the quinolinol-based metal complex is, for example, a compound represented by the following formula (ETM-13).
- R 1 to R 6 are hydrogen or a substituent
- M is Li, Al, Ga, Be or Zn
- n is an integer of 1 to 3.
- quinolinol-based metal complexes include 8-quinolinol lithium, tris (8-quinolinolate) aluminum, tris (4-methyl-8-quinolinolate) aluminum, tris (5-methyl-8-quinolinolate) aluminum, tris (3 , 4-Dimethyl-8-quinolinolate) aluminum, tris (4,5-dimethyl-8-quinolinolate) aluminum, tris (4,6-dimethyl-8-quinolinolate) aluminum, bis (2-methyl-8-quinolinolate) ( Phenolate) aluminum, bis (2-methyl-8-quinolinolate) (2-methylphenolate) aluminum, bis (2-methyl-8-quinolinolate) (3-methylphenolate) aluminum, bis (2-methyl-8- Quinolinolate) (4- Butylphenolate) aluminum, bis (2-methyl-8-quinolinolate) (2-phenylphenolate) aluminum, bis (2-methyl-8-quinolinolate) (3-phenylphenolate) aluminum, bis (2-methyl- 8-quinol lithium
- This quinolinol-based metal complex can be produced using a known raw material and a known synthesis method.
- the electron transport layer or the electron injection layer may further contain a substance capable of reducing a material forming the electron transport layer or the electron injection layer.
- a substance capable of reducing a material forming the electron transport layer or the electron injection layer As the reducing substance, various substances having a certain reducing property are used, for example, alkali metals, alkaline earth metals, rare earth metals, alkali metal oxides, alkali metal halides, alkali metals, and the like. From the group consisting of earth metal oxides, alkaline earth metal halides, rare earth metal oxides, rare earth metal halides, alkali metal organic complexes, alkaline earth metal organic complexes, and rare earth metal organic complexes At least one selected can be suitably used.
- Preferred reducing substances include alkali metals such as Na (2.36 eV), K (2.28 eV), Rb (2.16 eV) or Cs (1.95 eV), and Ca (2. eV).
- Alkaline earth metals such as 9 eV), Sr (2.0 to 2.5 eV) and Ba (2.52 eV), and those having a work function of 2.9 eV or less are particularly preferable.
- a more preferable reducing substance is an alkali metal of K, Rb or Cs, further preferably Rb or Cs, and most preferably Cs.
- alkali metals have particularly high reducing ability, and the addition of a relatively small amount to the material forming the electron transporting layer or the electron injecting layer can improve the emission luminance and extend the life of the organic electroluminescent device.
- a reducing substance having a work function of 2.9 eV or less a combination of two or more of these alkali metals is also preferable.
- a combination containing Cs for example, Cs and Na, Cs and K, Cs and Rb, or A combination of Cs, Na and K is preferred.
- the cathode in the organic electroluminescent device plays a role of injecting electrons into the light emitting layer 105 via the electron injection layer 107 and the electron transport layer.
- the material for forming the cathode 108 is not particularly limited as long as the material can efficiently inject electrons into the organic layer, and the same material as the material for forming the anode 102 can be used.
- metals such as tin, indium, calcium, aluminum, silver, copper, nickel, chromium, gold, platinum, iron, zinc, lithium, sodium, potassium, cesium and magnesium or alloys thereof (magnesium-silver alloy, magnesium) -An indium alloy, an aluminum-lithium alloy such as lithium fluoride / aluminum, etc.).
- lithium, sodium, potassium, cesium, calcium, magnesium or an alloy containing these low work function metals is effective.
- metals such as platinum, gold, silver, copper, iron, tin, aluminum and indium, or alloys using these metals, and inorganic substances such as silica, titania and silicon nitride, polyvinyl alcohol, and vinyl chloride It is preferable to laminate a hydrocarbon polymer compound and the like.
- the method for producing these electrodes is not particularly limited as long as conduction can be achieved, such as resistance heating, electron beam evaporation, sputtering, ion plating, and coating.
- the hole injection layer and the hole transport layer 103 in the organic electroluminescent element serve to inject holes moving from the anode 102 into the light emitting layer 105 or the hole transport layer 104 efficiently.
- the hole transport layer 104 plays a role in efficiently transporting holes injected from the anode 102 or holes injected from the anode 102 through the hole injection layer 103 to the light-emitting layer 105.
- the hole injection layer 103 and the hole transport layer 104 are each formed by laminating and mixing one or more of the hole injection / transport materials or a mixture of the hole injection / transport material and the polymer binder. Is done. Further, a layer may be formed by adding an inorganic salt such as iron (III) chloride to the hole injecting / transporting material.
- a hole injection / transport substance As a hole injection / transport substance, it is necessary to efficiently inject and transport holes from the positive electrode between the electrodes to which an electric field is applied, and the hole injection efficiency is high, and the injected holes are efficiently transported. It is desirable to do. For that purpose, it is preferable that the ionization potential is small, the hole mobility is large, the stability is further improved, and impurities serving as traps are less likely to be generated during production and use.
- a compound conventionally used as a hole charge transport material in a photoconductive material, a p-type semiconductor, and a hole injection layer of an organic electroluminescent element are used. Any of the known materials used for the layer and the hole transport layer can be selected and used. Specific examples thereof include a carbazole derivative (N-phenylcarbazole, polyvinylcarbazole, etc.), a biscarbazole derivative such as bis (N-arylcarbazole) or bis (N-alkylcarbazole), and a triarylamine derivative (aromatic tertiary).
- polycarbonates having the above monomers in the side chain polycarbonates having the above monomers in the side chain, styrene derivatives, polyvinyl carbazole, polysilanes and the like are preferable, but light emission is preferred.
- the compound is not particularly limited as long as it is a compound capable of forming a thin film required for manufacturing an element, injecting holes from the anode, and transporting holes.
- an organic semiconductor matrix material is composed of a compound having a good electron donating property or a compound having a good electron accepting property.
- Strong electron acceptors such as tetracyanoquinonedimethane (TCNQ) or 2,3,5,6-tetrafluorotetracyano-1,4-benzoquinonedimethane (F4TCNQ) are known for doping of electron donors.
- TCNQ tetracyanoquinonedimethane
- F4TCNQ 2,3,5,6-tetrafluorotetracyano-1,4-benzoquinonedimethane
- a material for forming the hole injection layer 103 and the hole transport layer 104 by using a wet film formation method a material for forming the hole injection layer 103 and the hole transport layer 104 used for the above-described vapor deposition is used.
- a hole-injecting and hole-transporting polymer, a hole-injecting and hole-transporting crosslinkable polymer, a hole-injecting and hole-transporting polymer precursor, and a polymer An initiator or the like can be used.
- PEDOT PSS
- polyaniline compounds described in JP-A-2005-108828, WO 2010/058776, WO 2013/042623, etc.
- fluorene polymers JP-A-2011-251984, 2011-501449, JP 2012-533661, etc.
- the anode 102 in the organic electroluminescent device plays a role of injecting holes into the light emitting layer 105. Note that when the hole injection layer 103 and / or the hole transport layer 104 are provided between the anode 102 and the light-emitting layer 105, holes are injected into the light-emitting layer 105 through these layers. .
- an inorganic compound and an organic compound can be given.
- the inorganic compound include metals (aluminum, gold, silver, nickel, palladium, chromium, etc.), metal oxides (indium oxide, tin oxide, indium-tin oxide (ITO), indium-zinc oxide) (IZO), metal halides (eg, copper iodide), copper sulfide, carbon black, ITO glass, Nesa glass, and the like.
- the organic compound include conductive polymers such as polythiophene such as poly (3-methylthiophene), polypyrrole, and polyaniline. In addition, it can be appropriately selected from the substances used as the anode of the organic electroluminescent element.
- the resistance of the transparent electrode is not limited as long as a current sufficient for light emission of the light emitting element can be supplied, but is preferably low from the viewpoint of power consumption of the light emitting element.
- an ITO substrate having a resistance of 300 ⁇ / ⁇ or less functions as an element electrode.
- a substrate of about 10 ⁇ / ⁇ can be supplied at present, for example, 100 to 5 ⁇ / ⁇ , preferably 50 to 5 ⁇ . It is particularly desirable to use a low-resistance product of /.
- the thickness of ITO can be arbitrarily selected according to the resistance value, but is usually used in a range of 50 to 300 nm.
- the anode in the organic electroluminescent element may have a bank (partition material).
- a bank partition material
- an arbitrary layer can be obtained by dropping a composition for forming each layer or a composition for forming a light emitting layer in a bank and drying the composition.
- Photolithography technology can be used for manufacturing the bank.
- a bank material that can be used for photolithography an inorganic material and an organic material can be used.
- the inorganic material for example, SiNx, SiOx and a mixture thereof
- the organic material for example, a positive resist Materials and negative resist materials can be used.
- a patterning printing method such as a sputtering method, an inkjet method, gravure offset printing, reverse offset printing, and screen printing can also be used. In that case, a permanent resist material can be used.
- the bank may have a multilayer structure, and different types of materials may be used.
- organic material used for the bank examples include polysaccharides and derivatives thereof, homopolymers and copolymers of ethylenic monomers having hydroxyls, biopolymer compounds, polyacryloyl compounds, polyesters, polystyrene, polyimide, polyamideimide, and poly (imide).
- Ether imide polysulfide, polysulfone, polyphenylene, polyphenyl ether, polyurethane, epoxy (meth) acrylate, melamine (meth) acrylate, polyolefin, cyclic polyolefin, acrylonitrile-butadiene-styrene copolymer (ABS), silicone resin, polyvinyl chloride , Chlorinated polyethylene, chlorinated polypropylene, polyacetate, polynorbornene, synthetic rubber, polyfluorovinylidene, polytetrafluoroethylene, poly Fluorinated polymers hexafluoropropylene etc., fluoroolefins - hydrocarbonoxy olefin copolymer, fluorocarbon polymers, and the like, but is not so limited.
- a resin layer is formed by applying a material having liquid repellency to the functional layer forming composition such as the light emitting layer forming composition on the element substrate on which the electrodes are formed, and drying the applied material.
- a material having liquid repellency to the functional layer forming composition such as the light emitting layer forming composition on the element substrate on which the electrodes are formed, and drying the applied material.
- a bank can be formed on the element substrate on which the electrodes are formed.
- a process such as a washing / drying process with a solvent or an ultraviolet treatment may be performed to remove impurities on the surface of the bank in order to spread the composition for forming a functional layer evenly.
- the substrate 101 serves as a support for the organic electroluminescent device 100, and is usually made of quartz, glass, metal, plastic, or the like.
- the substrate 101 is formed in a plate shape, a film shape, or a sheet shape depending on the purpose.
- a glass plate, a metal plate, a metal foil, a plastic film, a plastic sheet, or the like is used.
- a glass plate and a plate made of a transparent synthetic resin such as polyester, polymethacrylate, polycarbonate, and polysulfone are preferable.
- the thickness only needs to be 0.2 mm or more, as long as it has a thickness sufficient to maintain mechanical strength.
- the upper limit of the thickness is, for example, 2 mm or less, preferably 1 mm or less.
- alkali-free glass is preferable because it is preferable that the amount of ions eluted from the glass is small, but soda lime glass with a barrier coat such as SiO 2 is also commercially available. it can.
- the substrate 101 may be provided with a gas barrier film such as a dense silicon oxide film on at least one side in order to enhance gas barrier properties.
- a plate, film, or sheet made of a synthetic resin having low gas barrier properties is used as the substrate 101. When used, it is preferable to provide a gas barrier film.
- Each layer constituting the organic electroluminescent device is formed by vapor deposition, resistance heating vapor deposition, electron beam vapor deposition, sputtering, molecular lamination, printing, spin coating or casting, in which the materials constituting each layer are formed. It can be formed by forming a thin film by a method such as a coating method.
- the thickness of each layer thus formed is not particularly limited and can be appropriately set according to the properties of the material, but is usually in the range of 2 nm to 5000 nm. The film thickness can usually be measured with a quartz oscillation type film thickness measuring device or the like.
- the evaporation conditions vary depending on the type of material, the target crystal structure, association structure, and the like of the film.
- the deposition conditions are as follows: heating temperature of the crucible for deposition +50 to + 400 ° C., vacuum degree of 10 ⁇ 6 to 10 ⁇ 3 Pa, deposition rate of 0.01 to 50 nm / sec, substrate temperature of ⁇ 150 to + 300 ° C., and film thickness of 2 nm. It is preferable to set appropriately within a range of 5 ⁇ m.
- an anode / a hole injection layer / a hole transport layer / a host compound, a light-emitting layer containing a thermally activated delayed phosphor and a compound having a boron atom / electron transport A method for producing an organic electroluminescent device comprising a layer / electron injection layer / cathode will be described.
- Evaporation Method A thin film of an anode material is formed on a suitable substrate by an evaporation method or the like to produce an anode, and then a thin film of a hole injection layer and a hole transport layer is formed on the anode.
- a host compound, a thermally activated delayed phosphor and a compound having a boron atom are co-evaporated to form a thin film to form a light emitting layer, and an electron transport layer and an electron injection layer are formed on the light emitting layer,
- a target organic electroluminescent element is obtained by forming a thin film made of a material for a cathode by a vapor deposition method or the like to form a cathode.
- the production order may be reversed, and the cathode, the electron injection layer, the electron transport layer, the light emitting layer, the hole transport layer, the hole injection layer, and the anode may be produced in this order. It is possible.
- a compound represented by the formula (ii) or a compound represented by the formula (2) as the third component it is preferable to select and use a compound in which at least one of R 1 to R 14 in the formula (2) is a substituent.
- a preferable substituent in the above-mentioned third component can be employed.
- alkyl having 1 to 24 carbon atoms and optionally substituted diarylamino can be particularly preferably employed.
- the external quantum efficiency of the organic electroluminescent device is higher and the characteristics are better than when formed by a vapor deposition method using the compound represented by the formula (4).
- the light-emitting layer is formed by a vapor deposition method using the compound represented by the formula (i) or (iii) as the third component
- a preferable substituent in the third component It is particularly preferable to use a compound having an alkyl having 1 to 24 carbon atoms and an optionally substituted diarylamino.
- the external quantum efficiency of the organic electroluminescent device is higher than when the light-emitting layer is formed by a vapor deposition method using a corresponding compound having no substituent. Long life and excellent characteristics.
- the film is formed by using a wet film forming method.
- the wet film forming method generally forms a coating film through a coating step of applying a composition for forming a light emitting layer to a substrate and a drying step of removing a solvent from the applied composition for forming a light emitting layer.
- the method using a spin coater can be changed to a spin coating method, a slit coating method using a slit coater, a gravure using a plate, offset, reverse offset, flexographic printing method, a method using an ink jet printer to an ink jet method, a mist form
- the spraying method is called a spray method.
- the drying step includes methods such as air drying, heating, and vacuum drying. The drying step may be performed only once, or may be performed a plurality of times using different methods and conditions. Further, for example, different methods such as firing under reduced pressure may be used in combination.
- the wet film forming method is a film forming method using a solution, and for example, a partial printing method (ink jet method), a spin coating method or a casting method, a coating method, and the like.
- the wet film formation method does not require an expensive vacuum deposition apparatus unlike the vacuum deposition method, and can form a film under atmospheric pressure.
- the wet film forming method enables a large area and continuous production, which leads to a reduction in manufacturing cost.
- the wet film formation method is difficult to laminate.
- a multilayer film is formed by a wet film formation method, it is necessary to prevent the dissolution of the lower layer by the composition of the upper layer, a composition having controlled solubility, crosslinking of the lower layer, and an orthogonal solvent (orthogonal solvent, which dissolve each other). No solvent).
- the organic electroluminescent device thus manufactured is preferably covered with a sealing layer (not shown) to protect it from moisture and oxygen.
- a sealing layer for example, an inorganic insulating material such as silicon oxynitride (SiON) having low permeability to moisture or oxygen can be used.
- the organic electroluminescent element may be sealed by attaching a sealing substrate such as a transparent glass or an opaque ceramic to an element substrate on which the organic electroluminescent element is formed via an adhesive.
- the present invention can also be applied to a display device including the organic electroluminescent element, a lighting device including the organic electroluminescent element, and the like.
- a display device or a lighting device equipped with the organic electroluminescent element can be manufactured by a known method such as connecting the organic electroluminescent element according to the present embodiment to a known driving device, and can be driven by direct current, pulse, or alternating current. Driving can be performed by appropriately using a known driving method such as driving.
- Examples of the display device include a panel display such as a color flat panel display and a flexible display such as a flexible color organic electroluminescence (EL) display (for example, JP-A-10-335066, JP-A-2003-321546). Gazette, JP-A-2004-281086).
- Examples of the display method of the display include a matrix and / or segment method. Note that the matrix display and the segment display may coexist in the same panel.
- pixels for display are two-dimensionally arranged such as in a lattice or mosaic, and a set of pixels displays a character or an image.
- the shape and size of the pixel depend on the application. For example, a square pixel having a side of 300 ⁇ m or less is normally used for displaying images and characters on a personal computer, a monitor, and a television. In the case of a large display such as a display panel, a pixel having a side of mm order is used. become.
- pixels of the same color may be arranged, but in the case of color display, red, green and blue pixels are displayed side by side. In this case, there are typically a delta type and a stripe type.
- the matrix may be driven by either a line-sequential driving method or an active matrix.
- the line-sequential driving has the advantage that the structure is simpler, but the active matrix is sometimes superior when the operating characteristics are taken into consideration.
- a pattern is formed so as to display predetermined information, and a predetermined area emits light.
- a time display and a temperature display on a digital clock or a thermometer an operation state display of an audio device or an electromagnetic cooker, and a panel display of a car.
- Illumination devices include, for example, illumination devices such as interior lighting, backlights of liquid crystal display devices (for example, JP-A-2003-257621, JP-A-2003-277741, and JP-A-2004-119211). Etc.).
- a backlight is mainly used for the purpose of improving the visibility of a display device that does not emit light, and is used for a liquid crystal display device, a clock, an audio device, an automobile panel, a display panel, a sign, and the like.
- the present embodiment is considered to be difficult to make thin because the conventional method is made up of a fluorescent lamp and a light guide plate.
- the backlight using the light emitting element according to the above is characterized by being thin and lightweight.
- the light emitting layer forming composition of the present invention is a composition for forming a light emitting layer of an organic electroluminescent device by a wet method.
- the composition for forming a light-emitting layer comprises a compound having at least one host compound as a first component, at least one heat-activated delayed phosphor as a second component, and at least one boron atom as a third component. And a composition containing at least one organic solvent as the fourth component.
- the host compound the thermally activated delayed fluorescent substance, and the compound having a boron atom, the compounds described in the description of the light emitting layer in the organic electroluminescent device can be used.
- the composition for forming a light emitting layer of the present invention preferably contains at least one organic solvent.
- the evaporation rate of the organic solvent at the time of film formation, it is possible to control and improve the film formability and the presence / absence of defects in the coating film, surface roughness, and smoothness.
- the meniscus stability at the pinhole of the inkjet head can be controlled, and the ejection property can be controlled and improved.
- the drying rate of the film and the orientation of the derivative molecules the electric characteristics, light-emitting characteristics, efficiency, and lifetime of the organic electroluminescent device having the light-emitting layer obtained from the light-emitting layer forming composition are improved. be able to.
- the boiling point of at least one organic solvent contained as the fourth component in the composition for forming a light emitting layer is from 130 ° C. to 350 ° C., preferably from 140 ° C. to 300 ° C., more preferably from 150 ° C. to 250 ° C. More preferred.
- the boiling point is higher than 130 ° C., it is preferable from the viewpoint of ink jet discharge properties.
- the boiling point is lower than 350 ° C., it is preferable from the viewpoints of coating film defects, surface roughness, residual solvent and smoothness. From the viewpoints of good ink jetting properties, film forming properties, smoothness, and low residual solvent, a configuration containing two or more organic solvents is more preferable.
- the composition may be in a solid state by removing a solvent from the composition for forming a light emitting layer in consideration of transportability and the like.
- the composition for forming a light-emitting layer of the present invention contains, as a fourth component, a good solvent (GS) and a poor solvent (PS) for at least one of the compounds as the first component, the second component, and the third component. It is particularly preferred that the boiling point (BP GS ) of the solvent ( GS ) is lower than the boiling point (BP PS ) of the poor solvent (PS).
- a poor solvent having a high boiling point a good solvent having a low boiling point volatilizes first during the film formation, and the concentration of the components contained in the composition and the concentration of the poor solvent are increased, thereby promoting a rapid film formation. Thereby, a coating film with few defects, small surface roughness, and high smoothness can be obtained.
- the difference in solubility is preferably at least 1%, more preferably at least 3%, even more preferably at least 5%.
- the difference in boiling points is preferably at least 10 ° C., more preferably at least 30 ° C., even more preferably at least 50 ° C.
- the organic solvent is removed from the coating film by a drying process such as vacuum, reduced pressure, and heating.
- a drying process such as vacuum, reduced pressure, and heating.
- organic solvent examples include a hydrocarbon solvent, an alkylbenzene solvent, a phenyl ether solvent, an alkyl ether solvent, a cyclic ketone solvent, an aliphatic ketone solvent, and a simple solvent. Examples thereof include a cyclic ketone solvent, a solvent having a diester skeleton, and a fluorinated solvent.
- an alkylbenzene-based solvent, a phenylether-based solvent, or a mixed solvent thereof is preferable.
- the alkylbenzene-based solvent cyclohexylbenzene is preferable, and as the phenylether-based solvent, 3-phenoxytoluene is preferable.
- a mixed solvent of cyclohexylbenzene and 3-phenoxytoluene is also preferable.
- the mass ratio of the two is not particularly limited, but may be, for example, 2: 8 to 8: 2, and is preferably 5: 5 to 8: 2.
- composition for forming an optional component light emitting layer may contain optional components as long as the properties are not impaired.
- Optional components include a binder and a surfactant.
- the composition for forming a binder light emitting layer may contain a binder.
- the binder forms a film during film formation and bonds the obtained film to the substrate. In addition, it plays a role of dissolving, dispersing, and binding other components in the light emitting layer forming composition.
- binder used in the composition for forming a light emitting layer examples include acrylic resin, polyethylene terephthalate, ethylene-vinyl acetate copolymer, ethylene-vinyl alcohol copolymer, acrylonitrile-ethylene-styrene copolymer (AES) resin, Ionomer, chlorinated polyether, diallyl phthalate resin, unsaturated polyester resin, polyethylene, polypropylene, polyvinyl chloride, polyvinylidene chloride, polystyrene, polyvinyl acetate, Teflon (registered trademark), acrylonitrile-butadiene-styrene copolymer (ABS) ) Resins, acrylonitrile-styrene copolymer (AS) resins, phenolic resins, epoxy resins, melamine resins, urea resins, alkyd resins, polyurethanes, and copolymers of the above resins and polymers. Is but
- the binder used in the composition for forming a light emitting layer may be only one kind or a mixture of plural kinds.
- the composition for forming a surfactant light emitting layer may contain, for example, a surfactant for controlling the film surface uniformity, the solvent affinity and the liquid repellency of the film surface of the light emitting layer forming composition.
- Surfactants are classified into ionic and nonionic according to the structure of the hydrophilic group, and further classified into alkyl, silicon and fluorine based on the structure of the hydrophobic group. Further, according to the molecular structure, they are classified into a monomolecular system having a relatively small molecular weight and a simple structure and a high molecular system having a large molecular weight and having side chains or branches.
- the composition is classified into a single system and a mixed system in which two or more surfactants and a base material are mixed from the composition.
- surfactants that can be used in the composition for forming a light emitting layer all kinds of surfactants can be used.
- surfactant examples include Polyflow No. 45, Polyflow KL-245, Polyflow No. 75, polyflow no. 90, polyflow no. 95 (trade name, manufactured by Kyoeisha Chemical Industry Co., Ltd.), Disperbyk 161, Disperbake 162, Disperbake 163, Disperbake 164, Disperbake 166, Disperbake 170, Disperbake 180, Disperbake 181, Disperbake Bake 182, BYK300, BYK306, BYK310, BYK320, BYK330, BYK342, BYK344, BYK346 (trade name, manufactured by BYK Japan KK), KP-341, KP-358, KP-368, KF-96-50CS, KF -50-100CS (trade name, manufactured by Shin-Etsu Chemical Co., Ltd.), Surflon SC-101, Surflon KH-40 (trade name, manufactured by Seimi Chemical Co., Ltd.), Futergent 222F, Futerge 251;
- the first component, the second component, and the third component have excellent solubility, film formability, wet coatability, and thermal stability.
- a compound that satisfies at least one of properties and in-plane orientation is selected.
- alkyl having 1 to 24 carbon atoms, diarylamino, cycloalkyl having 5 to 24 carbon atoms, and cycloalkyl having 6 to 24 carbon atoms are preferable. It is preferable to select a compound substituted with aryl and heteroaryl having 5 to 24 carbon atoms.
- the first component it is preferable to select a compound having phenylene, triazine, pyridine, carbazole, dibenzofuran or dibenzothiophene having a substituent at the m-position in the molecule.
- a compound having alkyl or cycloalkyl in the molecule is preferable from the viewpoint of solubility and film formability, and a rod-like molecule having a high oversight is preferable from the viewpoint of efficiency.
- Compounds having azole, thiadiazole and triazole in the molecule are preferred, and Formula 2PXZ-TAZ is preferred.
- a compound having alkyl or cycloalkyl in the molecule is preferable from the viewpoint of solubility and film-forming properties, and a rod-like molecule having a high overhead is preferable from the viewpoint of efficiency.
- the compound represented by 2) is preferable, and B2N4-0230 / S-M1, B2N4-0220 / S-M1, B2N4-0211 / S-M1, BN2BNO-0230 / S-M1, and B2O2N2-0220 / S-M1 It is preferred to select a compound that is
- each component in the composition for forming a light emitting layer of the present invention is not particularly limited, but the content of the first component is preferably based on the total mass of the first component, the second component, and the third component. It is from 40% by mass to 98.999% by mass, more preferably from 50% by mass to 99.99% by mass, still more preferably from 60% by mass to 94.9% by mass.
- the content of the second component is 1% by mass to 60% by mass, more preferably 2% by mass to 50% by mass, based on the total mass of the first component, the second component and the third component. Preferably it is 5% by mass to 30% by mass.
- the content of the third component is preferably 0.001% by mass to 30% by mass, more preferably 0.01% to 20% by mass, based on the total mass of the first component, the second component and the third component. And more preferably 0.1 to 10% by mass.
- the above range is preferable, for example, in that the density quenching phenomenon can be prevented.
- the content of each of the first component, the second component and the third component is determined by the good dissolution of each component in the composition for forming a light emitting layer. , Storage stability and film-forming properties, and good film quality of a coating film obtained from the composition for forming a light-emitting layer, and also good dischargeability when using an inkjet method, produced using the composition. It may be determined from the viewpoints of good electric characteristics, light-emitting characteristics, efficiency, and life of the organic electroluminescent element having the light-emitting layer.
- the first component is 40 to 98.999% by mass
- the second component is 1% by mass based on the total mass of the first component, the second component, and the third component of the composition for forming a light emitting layer.
- % To 60% by mass and the third component is preferably 0.001% to 30% by mass. More preferably, the first component is 50% to 97.9% by mass, and the second component is 2% by mass, based on the total mass of the first, second and third components of the composition for forming a light emitting layer.
- the third component is 0.01 to 20% by mass.
- the first component is 60% by mass to 94.9% by mass
- the second component is 5% by mass, based on the total mass of the first component, the second component, and the third component of the composition for forming a light emitting layer.
- the third component is 0.1-10% by mass.
- each component in the composition for forming a light-emitting layer of the present invention is good solubility, storage stability and film formability of each component in the composition for forming a light-emitting layer, and the composition for forming a light-emitting layer.
- Good film quality of the coating film obtained from the above, and also good ejection property when using the ink jet method, good electric characteristics and light emitting characteristics of the organic electroluminescent element having the light emitting layer manufactured using the composition It may be determined from the viewpoints of efficiency, life, and life.
- the first component is included in an amount of 0.0998% by mass to 4.0% by mass
- the second component is included in the total mass of the light emitting layer forming composition.
- the third component is 0.0001% by mass to 2.0% by mass
- the fourth component is the light emitting layer with respect to the total mass of the composition for forming a light emitting layer. It is preferably from 90.0% to 99.9% by mass relative to the total mass of the forming composition.
- the first component is 0.17% by mass to 4.0% by mass with respect to the total mass of the light emitting layer forming composition
- the second component is with respect to the total mass of the light emitting layer forming composition.
- the third component being 0.03% by mass to 1.0% by mass
- the fourth component being the composition for forming the light emitting layer, based on the total mass of the composition for forming the light emitting layer. 93.0% by mass to 99.77% by mass with respect to the total mass of the product.
- the first component is 0.25% by mass to 2.5% by mass with respect to the total mass of the light emitting layer forming composition
- the second component is the total mass of the light emitting layer forming composition.
- the third component being 0.05% to 0.5% by mass
- the fourth component being the composition for forming the light emitting layer, based on the total mass of the composition for forming the light emitting layer. 96.5% by mass to 99.7% by mass relative to the total mass of the product.
- the first component is 0.095% by mass to 4.0% by mass, based on the total mass of the light emitting layer forming composition
- the second component is the total mass of the light emitting layer forming composition.
- the third component is 0.002% by mass to 1.0% by mass
- the fourth component is the light emitting layer with respect to the total mass of the composition for forming a light emitting layer. It is 92.0% by mass to 99.9% by mass relative to the total mass of the forming composition.
- the composition for forming a light-emitting layer can be produced by appropriately selecting the above-mentioned components by stirring, mixing, heating, cooling, dissolving, dispersing and the like by a known method. After the preparation, filtration, degassing (also referred to as degassing), ion exchange treatment, inert gas replacement / sealing treatment, and the like may be appropriately selected and performed.
- the viscosity of the composition for forming a light emitting layer is preferably from 0.3 mPa ⁇ s to 3 mPa ⁇ s at 25 ° C., more preferably from 1 mPa ⁇ s to 3 mPa ⁇ s.
- the viscosity is a value measured using a conical plate type rotary viscometer (cone plate type).
- the viscosity of the composition for forming a light emitting layer preferably has a surface tension at 25 ° C. of 20 mN / m to 40 mN / m, and more preferably 20 mN / m to 30 mN / m.
- the surface tension is a value measured using the hanging drop method.
- reaction solution was cooled to room temperature, filtered using silica gel (eluent: toluene), and the solvent was distilled off under reduced pressure to obtain a crude product. After dissolving the obtained crude product in toluene, an appropriate amount was distilled off under reduced pressure, and hexane was added for reprecipitation, whereby N 1 , N 3 -diphenylbenzene-1,3-diamine (16.5 g, (60% yield) as a white solid.
- reaction solution was cooled to room temperature, filtered using silica gel (eluent: toluene), and the solvent was distilled off under reduced pressure to obtain a crude product. After dissolving the obtained crude product in toluene, the solution is distilled off under reduced pressure to prepare a saturated solution, and hexane is added thereto for reprecipitation to give 5-chloro-N 1 , N 1 , N 3 , N 3. -Tetraphenylbenzene-1,3-diamine (5.66 g, 43% yield) was obtained as a white solid.
- Compound (1-49) was synthesized according to the method described in “Comparative Synthesis Example (1)” of JP-A-2016-88927.
- the compound When thinning only the compound to be evaluated for evaluation, the compound was vacuum-deposited on a glass substrate with a thickness of 30 to 100 nm to obtain a sample.
- a commercially available PMMA (polymethyl methacrylate) was used as a matrix material when the compound to be evaluated was dispersed in an appropriate matrix material.
- a sample was prepared by dissolving PMMA and a compound to be evaluated in toluene and then forming a thin film having a thickness of 10 nm on a transparent support substrate (10 mm ⁇ 10 mm) made of quartz by spin coating. .
- the concentration of the sample was 1% by mass.
- the measurement of the absorption spectrum of the evaluation sample for the absorption characteristics and the emission characteristics was performed using an ultraviolet-visible-near-infrared spectrophotometer (UV-2600, Shimadzu Corporation).
- UV-2600 ultraviolet-visible-near-infrared spectrophotometer
- the fluorescence spectrum and phosphorescence spectrum of the sample were measured using a spectrofluorometer (F-7000, manufactured by Hitachi High-Tech Co., Ltd.).
- the photoluminescence was measured by exciting at an appropriate excitation wavelength of about 340 nm at room temperature.
- the sample was immersed in liquid nitrogen (temperature 77 K) using an attached cooling unit.
- the delay time from the irradiation of the excitation light to the start of the measurement was adjusted using an optical chopper. The sample was excited at the appropriate excitation wavelength and photoluminescence was measured.
- the Stokes shift was determined from the difference between the peak top of the absorption spectrum and the peak top of the emission spectrum at room temperature.
- the fluorescence quantum yield (PLQY) was measured using an absolute PL quantum yield measurement device (C9920-02G, manufactured by Hamamatsu Photonics KK). Furthermore, the time until the light emission intensity became 50% of the initial value was measured by continuously applying a direct current, and the device life (LT50) was evaluated.
- Fluorescence Lifetime (Delayed Fluorescence) The fluorescence lifetime was measured at 300K using a fluorescence lifetime measuring device (C11367-01, manufactured by Hamamatsu Photonics KK). Specifically, at the maximum emission wavelength measured at an appropriate excitation wavelength, a light emission component having a short fluorescence lifetime and a light emission component having a long fluorescence lifetime were observed. In the measurement of the fluorescence lifetime of a general organic electroluminescent material that emits fluorescence at room temperature, a slow emission component involving a triplet component derived from phosphorescence due to deactivation of the triplet component due to heat is not observed. rare.
- the fluorescence lifetime of the delayed fluorescence was measured as Tau (Delay).
- the singlet excitation energy level of the first component is E (1, S, Sh)
- the singlet excitation energy level of the second component is E (2, S, Sh)
- the singlet excitation energy level is the single component.
- Term is E (3, S, Sh)
- the triplet excitation energy level of the first component is E (1, T, Sh)
- the triplet excitation energy level of the second component is E (2, T, Sh)
- the triplet excitation energy level of the third component is denoted as E (3, T, Sh).
- ⁇ E (ST) is the energy difference between E (S, Sh) and E (T, Sh).
- ⁇ E (ST) E (S, Sh) ⁇ E (T, Sh).
- ⁇ E (ST) is, for example, “Purely organic electroluminescent material realizing 100% conversion from electricity to light”, H. Kaji, H. Suzuki, T. Fukushima, K. Shizu, K. Katsuaki, S. Kubo, T Komino, H. Oiwa, F. Suzuki, A. Wakamiya, Y. Murata, C. Adachi, Nat. Commun. 2015, 6, 8476.
- ⁇ E (ST) of the first component is ⁇ E (1, ST, Sh)
- ⁇ E (ST) of the second component is ⁇ E (2, ST, Sh)
- ⁇ E (ST) of the third component is ⁇ E (ST).
- ⁇ E (3, ST, Sh) is displayed.
- the following table shows the results of measuring the maximum peak emission wavelength of the fluorescence spectrum of the main compound.
- Configuration A is a configuration suitable for a heat-activated delayed fluorescence material.
- Configuration A is an element configuration that can be expected to have high efficiency as shown in the literature (Adv. Mater. 2016, 28, 2777-2781).
- the application of the compound of the present invention is not limited to these structures, and the film thickness of each layer and constituent materials can be appropriately changed depending on the basic physical properties of the compound of the present invention.
- Examples 1-1 to 1-3 and Comparative Examples 1-1 to 1-4> A 26 mm ⁇ 28 mm ⁇ 0.7 mm glass substrate (manufactured by OptoScience Corp.), which was formed by polishing ITO formed to a thickness of 200 nm by sputtering to 50 nm, was used as a transparent support substrate.
- This transparent support substrate was fixed to a substrate holder of a commercially available vapor deposition device (manufactured by Choshu Sangyo Co., Ltd.), and NPD, TcTa, mCP, the first component (mCBP), the second component (the compounds described in the table below), A tantalum evaporation boat containing the third component (ED1) and TSPO1, respectively, and an aluminum nitride evaporation boat containing LiF and aluminum, respectively, were mounted.
- the following layers were sequentially formed on the ITO film of the transparent support substrate.
- the pressure in the vacuum chamber was reduced to 5 ⁇ 10 ⁇ 4 Pa.
- NPD was heated to deposit a film to a thickness of 40 nm
- TcTa was heated to deposit a film to a thickness of 15 nm to form two layers.
- the mCP was heated to be deposited to a thickness of 15 nm to form an electron blocking layer.
- the first component, the second component, and the third component described in the table below were simultaneously heated and co-evaporated to a thickness of 20 nm to form a light emitting layer.
- the deposition rate was adjusted so that the mass ratio of the first component, the second component, and the third component was as shown in the table below.
- TSPO1 was heated and evaporated to a thickness of 30 nm to form an electron transport layer.
- the deposition rate of each of the above layers was 0.01 to 1 nm / sec.
- the cathode is formed by heating LiF so as to have a film thickness of 1 nm at a deposition rate of 0.01 to 0.1 nm / sec, and then heating aluminum so as to have a film thickness of 100 nm.
- the deposition rate of aluminum was adjusted to be 1 nm to 10 nm / sec.
- Examples 1-1 to 1-3, Comparative Examples 1-3 and Comparative Examples 1-4 were deep blue (deep blue), and Comparative Examples 1-1 were sky blue (sky blue) to greenish. Blue (Greenish blue), and Comparative Example 1-2 was blue (blue).
- Examples 1-4 to 1-11 and Comparative Example 1-5 The organic electroluminescent devices of Examples 1-4 to 1-11 and Comparative Example 1-5 were manufactured in the same procedure as Example 1-1, except that the materials described in the table below were used. did. In Example 1-4, Comparative Example 1-5, and Example 1-5, the compound of the third component was changed. In Examples 1-6 to 1-11, the compound of the first component was changed.
- Example 1-5 For each of the fabricated organic electroluminescent devices of Examples 1-4 to 1-11 and Comparative Example 1-5, a DC voltage was applied using the ITO electrode as the anode and the aluminum electrode as the cathode in the same manner as in Example 1-1, and the fluorescent light was emitted. The peak wavelength, half width and external quantum efficiency were measured. The results are shown in the table below.
- Examples 1-4 to 1-11 and Comparative Example 1-5 all satisfy the relational expression of E (1, S, Sh) ⁇ E (2, S, Sh) ⁇ E (3, S, Sh).
- the first component, the second component, and the third component are used in combination in the light-emitting layer, compared to Comparative Example 1-5 using R-BD2 which is a compound having no boron atom as the third component,
- the half-value width was smaller and the external quantum efficiency was higher.
- Example 1-5 using RD-3 which is the compound of formula (i) as the third component RD-1 which is the compound of formula (ii) corresponding to the dimer of formula (i)
- the half width was smaller and more preferable characteristics were exhibited.
- E (3, T, Sh) the external quantum efficiency is equal to or more than that of Example 1-5.
- the ED1 when ED1 having a structure substituted with a diarylamino group is used as the third component, the ED1 has a particularly small half-width, a high external quantum efficiency, and further excellent characteristics as an organic electroluminescent device. It was confirmed that.
- Examples 1-12 to 1-15 and Comparative Example 1-6 The organic electroluminescent devices of Examples 1-4 to 1-11 and Comparative Example 1-5 were manufactured in the same procedure as Example 1-1, except that the materials described in the table below were used. did. In these specific examples, the compound of the second component is changed.
- Example 1-1 For each of the fabricated organic electroluminescent devices of Examples 1-12 to 1-15 and Comparative Example 1-6, a DC voltage was applied using the ITO electrode as the anode and the aluminum electrode as the cathode in the same manner as in Example 1-1, and the fluorescent light was emitted. The peak wavelength, half width and external quantum efficiency were measured. The results are shown in the table below.
- Examples 1-12 to 1-15 have a half-width compared to Comparative Example 1-6 in which the relationship of E (1, S, Sh) ⁇ E (2, S, Sh) ⁇ E (3, S, Sh) is not satisfied. And the external quantum yield was high. The results of Examples 1-12 to 1-15 show that good characteristics are maintained even when the type of the second component is changed.
- Examples 1-16 to 1-26 Using the materials described in the table below, the electron transport layer was changed to a configuration B by forming two layers of an electron transport layer 1 having a thickness of 10 nm and an electron transport layer 2 having a thickness of 20 nm. According to the same procedure as in 1-1, the organic electroluminescent devices of Examples 1-16 to 1-26 were manufactured.
- the electron transporting layer 2 of Example 1-23 was formed by heating BPy-TP2 and Liq and vapor-depositing them to a thickness of 20 nm so that the respective ratios became 7: 3 by mass.
- the electron transport layer 2 of Example 1-24 was formed by heating SF3-TRZ and Liq and vapor-depositing the film to a thickness of 20 nm so that the respective ratios became 7: 3 by mass.
- Examples 1-27 to 1-55 and Comparative Example 1-7 The organic electroluminescence of Configuration B of Examples 1-27 to 1-55 and Comparative Example 1-7 was conducted in the same manner as in Example 1-14, except that the materials described in the table below were used. The device was manufactured. In Examples 1-27 to 1-55, various compounds represented by the formula (ii) were evaluated as the third component.
- Example 1-7 For each of the prepared organic electroluminescent devices of Examples 1-27 to 1-55 and Comparative Example 1-7, a DC voltage was applied using the ITO electrode as the anode and the aluminum electrode as the cathode in the same manner as in Example 1-16, and the fluorescent light was emitted. The peak wavelength, half width, external quantum efficiency, and device lifetime (LT50) were measured. The results are shown in the table below.
- Examples 1-27 to 1-55 and Comparative Example 1-7 the first component that satisfies E (1, S, Sh) ⁇ E (2, S, Sh) ⁇ E (3, S, Sh) ,
- the second component and the third component are used in combination in the light-emitting layer.
- R-BD2 which is a compound having no boron atom as the third component
- the half width was smaller, the external quantum efficiency was higher, and the device life was longer.
- the relational expressions of E (1, T, Sh)> E (3, T, Sh) ⁇ E (2, T, Sh) are used.
- Example 1-39 shows ⁇ E (3, ST, Sh). ) was sufficiently low at 0.08 eV, so that the half width was small and the external quantum efficiency was high.
- Comparative Example 1-7 no phosphorescence was emitted from the third component, R-BD2, and ⁇ E (3, ST, Sh) was large, so that the half width was large and the external quantum efficiency was low.
- Examples 1-27 to 1-55 all have a structure represented by the formula (ii). Since the skeleton of the formula (i) has a dimerized multimeric skeleton and the skeleton has a symmetrical shape, it is considered to show good characteristics.
- Examples 1-56 to 1-72 and Comparative Example 1-8 The organic electroluminescence of Configuration B of Examples 1-56 to 1-72 and Comparative Example 1-8 was performed in the same manner as in Example 1-14, except that the materials described in the following table were used. The device was manufactured. In Examples 1-56 to 1-72, various compounds represented by the formula (i) were evaluated as the third component.
- Example 1-8 For each of the fabricated organic electroluminescent devices of Examples 1-56 to 1-72 and Comparative Example 1-8, a DC voltage was applied using the ITO electrode as the anode and the aluminum electrode as the cathode in the same manner as in Example 1-16, and the fluorescent The peak wavelength, half width, external quantum efficiency, and device lifetime (LT50) were measured.
- Examples 1-56 to 1-66 and Comparative Example 1 where E (3, T, Sh) is less than 0.3 eV and ⁇ E (2, ST, Sh) is greater than ⁇ E (3, ST, Sh).
- the measurement results of -8 are shown in the following table. Further, the measurements of Examples 1-67 to 1-72 in which E (3, T, Sh) is 0.3 eV or more and ⁇ E (3, ST, Sh) is larger than ⁇ E (2, ST, Sh). The results are shown in the table below.
- the first component, the second component, and the third component satisfying the relationship of E (1, S, Sh) ⁇ E (2, S, Sh) ⁇ E (3, S, Sh) are combined to form a light emitting layer.
- the device lifetimes (LT50) of Examples 1-56 to 1-72 used are long, the relationship of E (1, S, Sh) ⁇ E (2, S, Sh) ⁇ E (3, S, Sh) It was confirmed that the element life (LT50) of Comparative Example 1-8 which was not satisfied was short.
- Examples 1-56 to 1-72 have a half width in the range of 22 to 30 nm, an external quantum efficiency in the range of 12.0 to 16.8%, and an element lifetime (LT50) of 82 to 116 hours. Was within the range.
- FIG. 7 also shows the results of averaging the Tau (Delay) and Stokes shift of the compounds having each of these basic skeletons and forming a graph. 7 and 8, it can be seen that a device having a small Tau (Delay) has a longer device life (LT50), a smaller half width, and a higher external quantum efficiency.
- LT50 device life
- the compound represented by the formula (ii) has a smaller Tau (Delay) than the compound represented by the formula (i), and the characteristics of the organic electroluminescent device using the compound represented by the formula (ii) as the third component Is better.
- a compound having a basic skeleton of B2N4 is particularly preferable.
- FIG. 10 is a graph showing the relationship between the external quantum efficiency and the device lifetime (LT50).
- FIG. 9 shows a graph of the average value of Tau (Delay) and Stokes shift for each substituent. 9 and 10, the graphs are divided into carbazolyl (Cz), diphenylamino (DPA), compounds substituted with both diphenylamino and fluorine atoms (DPA & F), phenyl (Ph), and tert-butyl (tBu). ing.
- diphenylamino (DPA) and phenyl (Ph) include those substituted with alkyl. 9 and 10, the compound substituted with diphenylamino (DPA) has a small Tau (Delay) and Stokes shift, a long device lifetime (LT50), a small half width, and a low external quantum efficiency. Is also expensive. However, DPA & F substituted by a fluorine atom together with diphenylamino has a device life (LT50) that is not as long as when substituted by diphenylamino alone.
- LT50 device life
- diphenylamino shows a particularly excellent effect in improving the device characteristics
- carbazolyl (Cz), phenyl (Ph), and tert-butyl (tBu) also show a good effect.
- FIG. 11 shows the types of substituents substituted on the basic skeleton and the average of Stokes shift based on the measurement results of Examples 1-56 to 1-66 using the compound represented by formula (i) as the third component.
- 6 is a graph showing the relationship between the value and the average value of the half width. It was confirmed that the Stokes shift of the compound substituted with phenyl (Ph) was small and the half width was narrow. In particular, the effect of the phenyl group in which the ortho position was alkyl-substituted was excellent.
- FIG. 7 shows that the compounds represented by the formula (i), BONf and BOnN, have a small Stokes shift.
- ⁇ E (3, ST, Sh) is slightly larger than 0.3 eV, and therefore, Examples 1-67 to 1-72 using these compounds as the third component show E (1, T, Sh).
- the evaluation results of the Stokes shift of the third component and the device characteristics of Examples 1-67 to 1-72 are shown in the following table. The results in the table below show that the smaller the Stokes shift, the longer the device lifetime (LT50). From this, even when E (1, T, Sh)> E (2, T, Sh)> E (3, T, Sh), a compound having a small Stokes shift as the third component is used. It was shown that the element characteristics were improved when used.
- composition for forming light-emitting layer Next, in order to describe the present invention in more detail, specific examples of the composition for forming a light-emitting layer of the present invention and evaluation results thereof will be described. Not limited.
- Examples 2-1 to 2-32> The first component, the second component, the third component, and the fourth component shown in the table below were mixed at a ratio shown in the table and stirred to prepare a composition for forming a light emitting layer.
- Tol used as the fourth component is toluene
- DHNp is decahydronaphthalene
- 3PxT is 3-phenoxytoluene
- c6B is cyclohexylbenzene
- Anis is anisole
- Xyl is xylene (mixture)
- 1MNp 1-methylnaphthalene
- 8B n-octylbenzene
- DPE diphenyl ether
- 4FAnis is 4-fluoroanisole.
- Inkjet Using an inkjet, the ink was discharged into a pixel of 100 ppi and dried at 100 ° C. The discharge stability of the inkjet was also evaluated immediately after the start of the inkjet discharge and after the continuous operation for 24 hours. A sample with poor ejection stability was rated "x”, a sample with good ejection stability was rated “O”, and a sample with extremely good ejection stability was rated " ⁇ ".
- Comparative Example 2-2 using compound R-BD2 having no boron atom as the third component was poor in solubility and could not be evaluated thereafter.
- Comparative Example 2-1 using unsubstituted R-BD1 having a large molecular weight had poor solubility.
- Examples 2-1 to 2-33 using unsubstituted R-BD3 having a small molecular weight and a compound having a substituent even if having a large molecular weight are compounds having a boron atom, Both the film properties and the ink jet ejection stability were good.
- XLP-101 as a polymer hole transport compound was synthesized by the following reaction.
- a copolymer in which M5 or M6 was bonded next to M4 was obtained, and the ratio of each unit was estimated to be 40:10:50 (molar ratio) based on the charging ratio.
- Bpin is pinacolate boryl.
- PEDOT PSS Solution A commercially available PEDOT: PSS solution (Clevios TM PVP AI4083, aqueous dispersion of PEDOT: PSS, Heraeus Holdings) was used.
- OTPD OTPD
- LT-N159 manufactured by Luminescence Technology Corp.
- IK-2 photocationic polymerization initiator, manufactured by San Apro
- XLP-101 was dissolved in xylene at a concentration of 0.6% by mass to prepare a 0.6% by mass XLP-101 solution.
- PCz polyvinyl carbazole
- Example 3-1 A PEDOT: PSS solution was spin-coated on a glass substrate on which ITO was deposited to a thickness of 150 nm, and baked on a hot plate at 200 ° C. for 1 hour to form a PEDOT: PSS film having a thickness of 40 nm. (Hole injection layer). Next, the OTPD solution is spin-coated, dried on a hot plate at 80 ° C. for 10 minutes, exposed to light at an exposure intensity of 100 mJ / cm 2 with an exposure machine, and baked on a hot plate at 100 ° C. for 1 hour to obtain a solution. A OTPD film having a thickness of 30 nm, which was insoluble in the above, was formed (hole transport layer). Next, the composition for forming a light emitting layer prepared in Example 2-19 was spin-coated and baked on a hot plate at 120 ° C. for 1 hour to form a light emitting layer having a thickness of 20 nm.
- the produced multilayer film was fixed to a substrate holder of a commercially available vapor deposition apparatus (manufactured by Showa Vacuum Co., Ltd.), and a molybdenum vapor deposition boat containing 2CzBN and BPy-TP2, a molybdenum vapor deposition boat containing LiF, and aluminum
- the inserted tungsten deposition boat was mounted.
- 2CzBN was heated and vapor-deposited to a thickness of 10 nm to form the electron transport layer 1.
- BPy-TP2 was heated and vapor-deposited to a thickness of 20 nm to form an electron transport layer 2.
- the deposition rate at the time of forming the electron transport layer was 1 nm / sec. Thereafter, LiF was heated to be deposited at a deposition rate of 0.01 to 0.1 nm / sec so as to have a film thickness of 1 nm. Next, aluminum was heated and vapor-deposited to a thickness of 100 nm to form a cathode. Thus, an organic electroluminescent device was obtained.
- Example 3-2 An organic electroluminescent device was obtained in the same manner as in Example 3-1. Note that the hole transport layer was formed by spin-coating an XLP-101 solution and baking it on a hot plate at 200 ° C. for 1 hour to form a film having a thickness of 30 nm.
- Example 3-3 An organic electroluminescent device was manufactured in the same procedure as in Example 3-1 except that the composition for forming a light emitting layer prepared in Example 2-2 was used instead of the composition for forming a light emitting layer prepared in Example 2-19.
- the hole transport layer was formed by spin-coating an XLP-101 solution and baking it on a hot plate at 200 ° C. for 1 hour to form a film having a thickness of 30 nm.
- Example 3-4 An organic electroluminescent device was prepared in the same procedure as in Example 3-1 except that the composition for forming a light emitting layer prepared in Example 2-18 was used instead of the composition for forming a light emitting layer prepared in Example 2-19.
- the hole transport layer was formed by spin-coating an XLP-101 solution and baking it on a hot plate at 200 ° C. for 1 hour to form a film having a thickness of 30 nm.
- Example 3-5 An organic electroluminescent device was obtained in the same manner as in Example 3-1.
- the hole transport layer was formed by spin-coating a PCz solution and baking it on a hot plate at 120 ° C. for 1 hour to form a film having a thickness of 30 nm.
- an organic electroluminescent device could be manufactured using the composition for forming a light emitting layer of the present invention.
- R-BD3 which is a compound containing boron, has excellent solubility, emits light in an organic electroluminescent device manufactured using a wet film formation method, has a blue peak wavelength, a small half-value width, The taste was excellent. Further, comparing Example 3-2 and Example 3-4, the device using the emitting dopant having a substituent was excellent in external quantum efficiency.
- composition containing polymer compound The composition for forming a light emitting layer of the present invention may contain a polymer compound or a crosslinkable compound. Further, the organic electroluminescent device of the present invention may contain a polymer compound or a crosslinkable compound.
- Example 4-1 According to the method described in International Patent Publication No. WO2019 / 004248, a polymer containing a structure having a host as the first component and a boron atom as the third component can be synthesized.
- the above polymer is a polymer containing the first component and the third component in the molecule.
- Addition of the heat-activated delayed phosphor as the second component is the composition for forming a light-emitting layer of the present invention.
- Example 4-2> According to the method described in International Patent Publication No. WO2019 / 004248, it is possible to synthesize a polymer having the following structure of the host as the first component and the thermally activated delayed phosphor as the second component.
- the above polymer is a polymer containing the first component and the second component in the molecule.
- a compound having a boron atom as the third component is added, the composition for forming a light emitting layer of the present invention is obtained.
- a polymer containing a structure having a host having the first component, a thermally activated delayed fluorescent material having the second component, and a boron atom being the third component is synthesized as follows. can do.
- Example 4-4 According to the method described in International Patent Publication No. WO2019 / 004248, a polymer containing a structure having a host as the first component and a boron atom as the third component can be synthesized. Addition of the heat-activated delayed phosphor as the second component is the composition for forming a light-emitting layer of the present invention.
- a polymer containing a structure having a host as the first component and a boron atom as the third component can be synthesized.
- Addition of the heat-activated delayed phosphor as the second component is the composition for forming a light-emitting layer of the present invention.
- the first component, the second component, or the third component added to the polymer a single molecule that can be used as the first component, the second component, or the third component in the present invention can be used.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Organic Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Theoretical Computer Science (AREA)
- High Energy & Nuclear Physics (AREA)
- Inorganic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
- Devices For Indicating Variable Information By Combining Individual Elements (AREA)
Abstract
Description
発光層を有する有機電界発光素子であって、前記発光層が、
第1成分として、少なくとも1種のホスト化合物と、
第2成分として、少なくとも1種の熱活性化型遅延蛍光体と、
第3成分として、少なくとも1種のホウ素原子を有する化合物とを含み、
前記第1成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(1,S,Sh)、前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)としたとき、以下の関係式(1)を満たし、
前記第1成分は、前記ホスト化合物の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよく、
前記第2成分は、前記熱活性化型遅延蛍光体の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよく、
前記第3成分は、前記ホウ素原子を有する化合物の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよい、
有機電界発光素子。
関係式(1): E(1,S,Sh)≧E(2,S,Sh)≧E(3,S,Sh)
[2]
前記第3成分として、下記式(i)、(ii)および(iii)のいずれかで表される化合物、および下記式(i)で表される構造を複数有する多量体化合物の少なくとも1つを含む、[1]に記載の有機電界発光素子。
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1およびX2は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(i)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。)
A環、B環、C環およびD環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-R、>CR2、>Sまたは>Seであり、前記>N-RのRおよび>CR2のRは、置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたは置換されていてもよいアルキルであり、また、前記>N-RのRは連結基または単結合により前記A環、B環、C環およびD環から選択される少なくとも1つと結合していてもよく、
R1およびR2は、それぞれ独立して、水素、炭素数1~6のアルキル、炭素数3~12のシクロアルキル、炭素数6~12のアリール、炭素数2~15のヘテロアリールまたはジアリールアミノ(ただしアリールは炭素数6~12のアリール)であり、
式(ii)で表される化合物における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1、X2およびX3は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(iii)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。)
[3]
前記第3成分として、下記式(1)、(2)、(3)および(4)のいずれかで表される化合物を少なくとも1つ含む、[1]または[2]に記載の有機電界発光素子。
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10およびR11は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、また、R1~R3、R4~R7およびR8~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
X1およびX2は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1およびX2は、同時に>CR2になることはなく、
そして、
式(1)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13およびR14は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルであり、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、また、R5~R7およびR10~R12のうちの隣接する基同士が結合してb環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環における少なくとも1つの水素は、アリール、ヘテロアリール、ジアリールアミノ、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルで置換されていてもよく、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRは、炭素数6~12のアリール、炭素数2~15のヘテロアリール、炭素数3~12のシクロアルキルまたは炭素数1~6のアルキルであり、また、前記>N-RのRおよび>CR2のRは、-O-、-S-、-C(-R)2-または単結合により前記a環、b環、c環およびd環から選択される少なくとも1つと結合していてもよく、前記-C(-R)2-のRは水素または炭素数1~6のアルキルであり、
ただし、X1、X2、X3、およびX4は、同時に>CR2になることはなく、
そして、
式(2)で表される化合物における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R9、R10およびR11は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R6およびR9~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
X1、X2およびX3は、それぞれ独立して、>O、>N-R、または>CR2であり、前記>N-RのRおよび>CR2 のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1、X2、およびX3は、同時に>CR2になることはなく、
そして、
式(3)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13およびR14は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R7、R8~R10およびR11~R14のうちの隣接する基同士が結合してa環、b環、c環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Xは、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRはアリール、ヘテロアリールまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Lは、単結合、>CR2、>O、>Sまたは>N-Rであり、前記>CR2および>N-RにおけるRは、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、アルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、XおよびLは、同時に>CR2 になることはなく、
そして、
式(4)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
[4]
前記第3成分として、前記式(1)、(2)および(4)のいずれかで表される化合物を少なくとも1つを含み、
前記式(1)において、X1およびX2が、それぞれ独立して、>Oまたは>N-Rであり、
前記式(2)において、X1、X2、X3およびX4が、それぞれ独立して、>Oまたは>N-Rであり、
前記式(4)において、Xが、>Oおよび>N-Rであり、Lが、単結合である、
[3]に記載の有機電界発光素子。
[5]
前記第3成分として、前記式(1)、(2)、(3)および(4)のいずれかで表される化合物を少なくとも1つを含み、その化合物に存在する環を構成する原子が、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルから選択される少なくとも1つで置換されている、[3]または[4]に記載の有機電界発光素子。
[6]
前記第3成分として、前記式(2)で表される化合物を少なくとも1つを含み、その化合物に存在する環を構成する原子が、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルから選択される少なくとも1つで置換され、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよい、[5]に記載の有機電界発光素子。
[7]
前記第3成分が、下記式で表される部分構造を含む、[1]~[6]のいずれか一項に記載の有機電界発光素子。ただし、前記部分構造における水素は、それぞれ独立して、アリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシまたはアリールオキシで置換されてもよく、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよい。
前記式(1)~(4)のいずれかで表される化合物が、以下に記載のいずれかの部分構造を含む、[3]~[6]のいずれか一項に記載の有機電界発光素子。
Meはメチルを表し、tBuはt-ブチルを表し、波線は結合位置を表す。
ただし、上記部分構造式における水素は、
それぞれ独立して、アリール、ヘテロアリール、ジアリールアミノ、アルキル、アルコキシまたはアリールオキシで置換されていてもよく、前記アリールにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、前記ヘテロアリールにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、前記ジアリールアミノにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよい。)
[9]
前記式(1)~(4)のいずれかで表される化合物が、sp3炭素、ホウ素原子に対してm位またはp位に結合するsp2炭素原子、または、ホウ素に対してp位に置換する窒素原子、をいずれか1つ有する、[3]~[8]のいずれか一項に記載の有機電界発光素子。
[10]
前記式(2)で表される化合物が、以下の化合物である、[3]に記載の有機電界発光素子。
前記式(2)で表される化合物が、以下の化合物である、[4]に記載の有機電界発光素子。
前記第1成分が、部分構造として、カルバゾールおよびフランから選択される少なくとも一つを有する化合物である、[1]~[11]のいずれか一項に記載の有機電界発光素子。
[13]
前記第1成分が、下記式(H1)、(H2)および(H3)のいずれかで表される化合物を少なくとも一つ含有する、[1]~[12]のいずれか一項に記載の有機電界発光素子。
上記各式で表される化合物における少なくとも1つの水素は、炭素数1~6のアルキル、シアノ、ハロゲンまたは重水素で置換されていてもよい。)
[14]
前記第2成分が、部分構造として、カルバゾール、フェノキサジン、アクリジン、トリアジン、ピリミジン、ピラジン、チオキサンテン、ベンゾニトリル、フタロニトリル、イソフタロニトリル、ジフェニルスルホン、トリアゾール、オキサジアゾール、チアジアゾールおよびベンゾフェノンから選択される少なくとも一つを有する、[1]~[13]のいずれか一項に記載の有機電界発光素子。
[15]
前記第2成分が、下記式(AD1)、(AD2)および(AD3)のいずれかで表される化合物を少なくとも一つ含有する、[1]~[14]のいずれか一項に記載の有機電界発光素子。
Mは、それぞれ独立して、単結合、-O-、>N-Arまたは>CAr2であり、
Jは、それぞれ独立して、炭素数6~18のアリーレンであり、前記アリーレンは、フェニル、炭素数1~6のアルキルおよび炭素数3~12のシクロアルキルから選択される少なくとも1つで置換されてもよく、
Qは、それぞれ独立して、=C(-H)-または=N-であり、
Arは、それぞれ独立して、水素、炭素数6~18のアリール、炭素数6~18のヘテロアリール、炭素数1~6のアルキルまたは炭素数3~12のシクロアルキルであり、前記アリールおよびヘテロアリーレンにおける少なくとも1つの水素は、フェニル、炭素数1~6のアルキルまたは炭素数3~12のシクロアルキルで置換されてもよく、
mは、1または2であり、
nは、2~(6-m)の整数であり、
上記各式で表される化合物における少なくとも1つの水素は、ハロゲンまたは重水素で置換されていてもよい。)
[16]
前記第2成分が、下記式(DAD1)で表される化合物を少なくとも一つ含有する、[1]~[14]のいずれか一項に記載の有機電界発光素子。
(D1-L1)n-A1 (DAD1)
(上記式(DAD1)中、D1はドナー性基であり、L1は単結合または共役連結基であり、A1はアクセプター性基であり、nは2以上であってA1が置換しうる最大数以下である整数である。)
[17]
前記第2成分が、下記式(DAD2)で表される化合物を少なくとも一つ含有する、[16]に記載の有機電界発光素子。
D2-L2-A2-L3-D3 (DAD2)
(上記式(DAD2)中、D2およびD3はそれぞれ独立してドナー性基であり、L2およびL3はそれぞれ独立しては単結合または共役連結基であり、A2はアクセプター性基である。)
[18]
前記式(AD1)、(AD2)および(AD3)において、
Mは、それぞれ独立して、単結合、-O-または>N-Arであり、
Jは、それぞれ独立して、フェニレンであり、前記フェニレンは、炭素数1~4のアルキルで置換されてもよく、
Qは、それぞれ独立して、=Nーであり、
Arは、それぞれ独立して、水素またはフェニルであり、前記フェニルは、フェニル、炭素数1~4のアルキルで置換されてもよく、
mは、1または2であり、
nは、4~(6-m)の整数である、
[17]に記載の有機電界発光素子。
[19]
前記第2成分の逆項間交差速度が、105s-1以上である、[1]~[18]のいずれか一項に記載の有機電界発光素子。
[20]
前記第3成分の遅延蛍光寿命が、0.05μsec~40μsecである、[1]~[19]のいずれか一項に記載の有機電界発光素子。
[21]
前記第3成分の遅延蛍光寿命が、0.05μsec~20μsecである、[1]~[19]のいずれか一項に記載の有機電界発光素子。
[22]
前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、14nm以下である、[1]~[21]のいずれか一項に記載の有機電界発光素子。
[23]
前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、10nm以下である、[1]~[21]のいずれか一項に記載の有機電界発光素子。
[24]
前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらから求められる一重項三重項エネルギー差(ΔE(2,ST,Sh)およびΔE(3,ST,Sh))が以下の関係にある、[1]~[23]のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.50 eV
ΔE(3,ST,Sh)=E(3,S,Sh)ーE(3,T,Sh)≦ 0.20 eV
[25]
前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらから求められる一重項三重項エネルギー差(ΔE(2,ST,Sh)およびΔE(3,ST,Sh))が以下の関係にある、[1]~[24]のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)
ΔE(3,ST,Sh)=E(3,S,Sh)ーE(3,T,Sh)
ΔE(2,ST,Sh)≧ ΔE(3,ST,Sh)
[26]
前記第2成分の一重項三重項エネルギー差(ΔE(2,ST,Sh))が以下の関係にある、[1]~[25]のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.30 eV
[27]
前記第2成分の一重項三重項エネルギー差(ΔE(2,ST,Sh))が以下の関係にある、[1]~[26]のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.15 eV
[28]
前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらが以下の関係にある、[1]~[27]のいずれか一項に記載の有機電界発光素子。
E(2,S,Sh)≧E(3,S,Sh)
E(2,T,Sh)≦E(3,T,Sh)
[29]
前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、10nm以下である、[1]~[28]のいずれか一項に記載の有機電界発光素子。
[30]
前記第3成分が、前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物として含まれている、[1]~[29]のいずれか一項に記載の有機電界発光素子。
[31]
前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物が、前記ホスト化合物の水素原子2個が脱離した基も繰り返し単位として有する、[30]に記載の有機電界発光素子。
[32]
前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物が、前記遅延蛍光体の水素原子2個が脱離した基も繰り返し単位として有する、[30]または[31]に記載の有機電界発光素子。
[33]
[1]~[32]のいずれか一項に記載の有機電界発光素子を備えた表示装置。
[34]
[1]~[32]のいずれか1項に記載の有機電界発光素子を備えた照明装置。
[35]
有機電界発光素子の発光層を塗布形成するための発光層形成用組成物であって、
[1]~[32]のいずれか一項に記載の第1成分、第2成分および第3成分に加えて、第4成分として、少なくとも1種の有機溶媒を含む、発光層形成用組成物(ただし、前記第3成分は下記化合物ではない。)。
前記第4成分における少なくとも1種の有機溶媒の沸点が130℃~350℃である、[35]に記載の発光層形成用組成物。
[37]
前記第4成分が、前記第1成分、前記第2成分、および前記第3成分である化合物の少なくとも1種に対する良溶媒(GS)と貧溶媒(PS)とを含み、前記良溶媒(GS)の沸点(BPGS)が前記貧溶媒(PS)の沸点(BPPS)よりも低い、[35]または[36]に記載の発光層形成用組成物。
[38]
前記第1成分が発光層形成用組成物の全質量に対して0.0998質量%~4.0質量%であり、
前記第2成分が発光層形成用組成物の全質量に対して0.0001質量%~2.0質量%であり、
前記第3成分が発光層形成用組成物の全質量に対して0.0001質量%~2.0質量%であり、
前記第4成分が発光層形成用組成物の全質量に対して90.0質量%~99.9質量%である、
[35]~[37]のいずれか一項に記載の発光層形成用組成物。
[39]
[35]~[38]のいずれか一項に記載の発光層形成用組成物を用いて形成される発光層を有する有機電界発光素子。
[40]
[2]に記載の式(ii)で表される化合物の少なくとも1つの水素が、下記部分構造(B)、塩素、臭素、またはヨウ素により置換された化合物。
[41]
ホウ素原子を有する化合物から水素原子2個を脱離した構造を含む繰り返し単位、熱活性化型遅延蛍光体から水素原子2個を脱離した構造を含む繰り返し単位、およびホスト化合物から水素原子2個を脱離した構造を含む繰り返し単位から選択される少なくとも2種の繰り返し単位を含む高分子化合物。
[42]
ホウ素原子を有する化合物から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種、熱活性化型遅延蛍光体から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種、およびホスト化合物から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種を含む高分子化合物。
本発明の有機電界発光素子は、ホスト化合物、熱活性化型遅延蛍光体、分子中にホウ素原子を有する化合物を利用したものである。
本発明における「ホスト化合物」とは、蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位が、第2成分としての熱活性化型遅延蛍光体、および、第3成分としてのホウ素原子を有する化合物よりも高い化合物のことを意味する。
「熱活性化型遅延蛍光体」とは、熱エネルギーを吸収して励起三重項状態から励起一重項状態への逆項間交差を起こし、その励起一重項状態から放射失活して遅延蛍光を放射しうる化合物のことを意味する。ただし、「熱活性化型遅延蛍光」とは、励起三重項状態から励起一重項状態への励起過程で高次三重項を経るものも含む。例えば、Durham大学 Monkmanらによる論文(NATURE COMMUNICATIONS,7:13680,DOI: 10.1038/ncomms13680)、産業技術総合研究所 細貝らによる論文(Hosokai et al., Sci. Adv. 2017;3: e1603282)、京都大学 佐藤らによる論文(Scientific Reports,7:4820, DOI:10.1038/s41598-017-05007-7)および、同じく京都大学 佐藤らによる学会発表(日本化学会第98春季年会、発表番号:2I4-15、DABNAを発光分子として用いた有機電界発光における高効率発光の機構、京都大学大学院工学研究科)などが挙げられる。本発明では、対象化合物を含むサンプルについて、300Kで蛍光寿命を測定したとき、遅い蛍光成分が観測されたことをもって該対象化合物が「熱活性化型遅延蛍光体」であると判定することとする。ここで、遅い蛍光成分とは、蛍光寿命が0.1μsec以上であるもののことを言う。蛍光寿命の測定は、例えば蛍光寿命測定装置(浜松ホトニクス社製、C11367-01)を用いて行うことができる。
「分子中にホウ素原子を有する化合物」は、エミッティングドーパントとして機能させることができ、「熱活性化型遅延蛍光体」は、分子中にホウ素原子を有する化合物の発光をアシストするアシスティングドーパントとして機能させることができる。
以下の説明では、熱活性化型遅延蛍光体をアシスティングドーパントとして用いる有機電界発光素子を、「TAF素子」(TADF Assisting Fluorescence素子)ということがある。
すなわち、本発明の有機電界発光素子における好ましいエネルギー関係を図1に示す。本発明の有機電界発光素子においては、エミッティングドーパントとしての、ホウ素原子を有する化合物が高い励起三重項エネルギー準位E(3,T,Sh)を有する。そのため、アシスティングドーパントでアップコンバージョンされた励起一重項エネルギーが、例え、エミッティングドーパントで励起三重項エネルギー準位E(3,T,Sh)へ項間交差した場合にも、エミッティングドーパント上でアップコンバージョンされるか、アシスティングドーパント(熱活性化型遅延蛍光体)上の励起三重項エネルギー準位E(2,T,Sh)へ回収される。したがって、生成した励起エネルギーを無駄なく発光に使用することができる。また、アップコンバージョンおよび発光の機能をそれぞれが得意な2種の分子に分けることで、高いエネルギーの滞留時間が減少し、化合物への負担が減少すると予想される。
関係式(1): E(1,S,Sh)≧E(2,S,Sh)≧E(3,S,Sh)
つまり、エネルギーの閉じ込めおよび/または伝達は第1成分であるホスト化合物が、発光は第3成分であるエミッティングドーパントがそれぞれ担う。
ここで、E(1,S,Sh)ーE(2,S,Sh)は0~1.0eVであることが好ましく、E(2,S,Sh)ーE(3,S,Sh)は0~0.20eVであることが好ましい。
E(1,S,PT)>E(2,S,PT)
E(1,S,PT)>E(3,S,PT)
E(2,S,PT)とE(3,S,PT)はいずれが大きくても本発明を適用することができるが、E(3,S,PT)>E(2,S,PT)であることが好ましい。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.50 eV
ΔE(3,ST,Sh)=E(3,S,Sh)ーE(3,T,Sh)≦ 0.20 eV
つまり、第2成分においては、TADF活性の指標としてΔE(ST)の大きさを用いる。ΔE(ST)が小さければ小さいほどTADF活性を示すには有利になる。
ここで、ΔE(2,ST,Sh)は0.30eV以下であることがより好ましく、0.15eV以下であることがさらに好ましく、0.10eV以下であることがさらにより好ましい。ΔE(3,ST,Sh)は、0.15eV以下であることがより好ましく、0.10eV以下であることがさらに好ましい。
ΔE(2,ST,Sh)とΔE(3,ST,Sh)は以下の関係にあることが好ましい。
ΔE(2,ST,Sh)≧ΔE(3,ST,Sh)
E(2,S,Sh)≧E(3,S,Sh)
E(2,T,Sh)≦E(3,T,Sh)
ここで、E(2,S,Sh)ーE(3,S,Sh)は0~0.20eVであることが好ましく、E(3,T,Sh)ーE(2,T,Sh)は0~0.20eVであることが好ましい。
これらは図1で示した本発明のTAF素子の設計を示す。
k(2,RISC)>k(3,RISC)
k(2,Prompt)<k(3,Prompt)
ここで、「ピーク短波長側の肩」とは、発光ピークの短波長側の変曲点のことを意味し、「短波長側のピークトップ」とは、発光ピークの発光極大値のうち、最も短波長側の発光極大値に対応するピーク上の位置のことを意味する。
また、各エネルギー準位を測定するための測定サンプルとして、対象化合物がホスト化合物またはアシスティングドーパントである場合には、ガラス基板上に形成した対象化合物の単独膜(Neat膜、厚さ:50nm)を使用し、対象化合物がエミッティングドーパントである場合には、ガラス基板上に形成した、対象化合物を分散させたポリメチルメタクリレート膜(厚さ:
10μm、対象化合物の濃度:1質量%)を使用する。対象化合物を分散させたポリメチルメタクリレート膜の膜厚については、吸収スペクトル、蛍光スペクトルおよび燐光スペクトルの測定に十分な強度が得られる膜厚であればよく、強度が弱い場合には厚く、強度が強い場合には厚くすればよい。励起光には、吸収スペクトルにおいて得られた吸収ピークの波長を使用し、蛍光スペクトルまたは燐光スペクトルに出現した発光ピークのうち、青色の発光の場合は400~500nmの範囲に、緑色の発光の場合は480~600nmの範囲に、赤色の場合は580~700nmの範囲にそれぞれ出現した発光ピークから得たデータを用いて各エネルギー準位を求めることとする。また、吸収ピークと発光ピークが近く、発光ピーク中に励起光が混合する場合には、より短波長側の吸収ピークや吸収肩を用いてもよい。
[1]蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位E(S,Sh)
対象化合物を含む測定サンプルに、77Kで励起光を照射して蛍光スペクトルを観測する。その蛍光スペクトルに現れた発光ピークに対して、その短波長側の変曲点(肩)を通る接線をひき、その接線とベースラインとの交点の波長(BSh)[nm]から、下記式を用いて励起一重項エネルギー準位E(S,Sh)を算出する。
E(S,Sh) [eV]=1240/BSh
[2]蛍光スペクトルの短波長側のピークトップより求められる励起一重項エネルギー準位E(S,PT)
対象化合物を含む測定サンプルに、77Kで励起光を照射して蛍光スペクトルを観測する。その蛍光スペクトルに現れた発光ピークの最も短波長側のピークトップに対応する波長(発光極大波長、BPT)[nm]から、下記式を用いて励起一重項エネルギー準位E(S,PT)を算出する。
E(S,PT) [eV]=1240/BPT
[3]燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位E(T,Sh)
対象化合物を含む測定サンプルに、77Kで励起光を照射して燐光スペクトルを観測する。その燐光スペクトルに現れた発光ピークに対して、その短波長側の変曲点(肩)を通る接線をひき、その接線とベースラインとの交点の波長(CSh)[nm]から、下記式を用いて励起三重項エネルギー準位E(T,Sh)を算出する。
E(T,Sh) [eV]=1240/CSh
[4]燐光スペクトルの短波長側のピークトップより求められる励起三重項エネルギー準位E(T,PT)
対象化合物を含む測定サンプルに、77Kで励起光を照射して燐光スペクトルを観測する。その燐光スペクトルに現れた発光ピークの最も短波長側のピークトップに対応する波長(発光極大波長、CPT)[nm]から、下記式を用いて励起三重項エネルギー準位E(T,PT)を算出する。
E(T,PT) [eV]=1240/CPT
短波長側の肩から求められる励起一重項エネルギー準位E(S,Sh)および励起三重項エネルギー準位E(T,Sh)は、ΔE(ST)の算出と議論に用いるとともに、第1成分であるホスト化合物とアシスティングドーパントとのエネルギーの閉じ込めおよび授受、アシスティングドーパントとエミッティングドーパントとのエネルギーの閉じ込めおよび授受の議論にも用いる。
逆項間交差速度は、励起三重項から励起一重項への逆項間交差の速度を示す。アシスティングドーパントおよびエミッティングドーパントの逆項間交差速度は、過渡蛍光分光測定により、Nat. Commun. 2015, 6, 8476.またはOrganic Electronics 2013, 14, 2721-2726に記載の方法を用いて算出することができ、具体的には、アシスティングドーパントの逆項間交差速度は105s-1であり、さらに好ましくは、106s-1である。
発光速度は、TADF過程を経ないで励起一重項から基底状態へ蛍光発光を経て遷移する速度を示す。アシスティングドーパントおよびエミッティングドーパントの発光速度は、逆項間交差速度と同様にNat. Commun. 2015, 6, 8476.またはOrganic Electronics 2013, 14, 2721-2726に記載の方法を用いて算出することができ、具体的には、エミッティングドーパントの逆項間交差速度は107s-1であり、さらに好ましくは、108s-1である。
以下において、本発明の有機電界発光素子を構成する各層について説明する。
2-1.有機電界発光素子における発光層
発光層は、第1成分としてのホスト化合物、第2成分としての熱活性化型遅延蛍光体、および第3成分としてのホウ素原子を有する化合物を少なくとも含む。
本明細書中では、第2成分としての熱活性化型遅延蛍光体を「アシスティングドーパント」(化合物)といい、第3成分としての、ホウ素原子を有する化合物を「エミッティングドーパント」(化合物)ということがある。
発光層は単一層でも複数層からなってもどちらでもよい。また、ホスト化合物、熱活性化型遅延蛍光体およびホウ素原子を有する化合物は、同一の層内に含まれていてもよく、複数層に少なくとも1成分ずつ含まれていてもよい。発光層が含むホスト化合物、熱活性化型遅延蛍光体およびホウ素原子を有する化合物は、それぞれ一種類であっても、複数の組み合わせであっても、いずれでもよい。アシスティングドーパントおよびエミッティングドーパントは、マトリックスとしてのホスト化合物中に、全体的に含まれていてもよいし、部分的に含まれていてもよい。アシスティングドーパントおよびエミッティングドーパントがドープされた発光層は、ホスト化合物とアシスティングドーパントとエミッティングドーパントを三元共蒸着法によって成膜する方法、ホスト化合物とアシスティングドーパントとエミッティングドーパントを予め混合してから同時に蒸着する方法、ホスト化合物とアシスティングドーパントとエミッティングドーパントを有機溶媒に溶解して調製した発光層形成用組成物(塗料)を塗布する、湿式成膜法等により形成することができる。
ホスト化合物としては、公知のものを用いることができ、例えばカルバゾール環およびフラン環の少なくとも一方を有する化合物を挙げることができ、中でも、フラニル基およびカルバゾリル基の少なくとも一方と、アリーレンおよびヘテロアリーレンの少なくとも一方とが結合した化合物を用いることが好ましい。具体例として、mCPやmCBPなどが挙げられる。
上記各式で表される化合物における少なくとも1つの水素は、炭素数1~6のアルキル、シアノ、ハロゲンまたは重水素で置換されていてもよい。
本発明で用いる熱活性化型遅延蛍光体(TADF化合物)は、ドナーと呼ばれる電子供与性の置換基とアクセプターと呼ばれる電子受容性の置換基を用いて分子内のHOMO(Highest Occupied Molecular Orbital)とLUMO(Lowest Unoccupied Molecular Orbital)を局在化させて、効率的な逆項間交差(reverse intersystem crossing)が起きるようにデザインされた、ドナー-アクセプター型熱活性化型遅延蛍光体(D-A型TADF化合物)であることが好ましい。
ここで、本明細書中において「電子供与性の置換基」(ドナー)とは、熱活性化型遅延蛍光体分子中でLUMO軌道が局在する置換基および部分構造のことを意味し、「電子受容性の置換基」(アクセプター)とは、熱活性化型遅延蛍光体分子中でHOMO軌道が局在する置換基および部分構造のことを意味することとする。
一般的に、ドナーやアクセプターを用いた熱活性化型遅延蛍光体は、構造に起因してスピン軌道結合(SOC: Spin Orbit Coupling)が大きく、かつ、HOMOとLUMOの交換相互作用が小さくΔE(ST)が小さいために、非常に速い逆項間交差速度が得られる。一方、ドナーやアクセプターを用いた熱活性化型遅延蛍光体は、励起状態での構造緩和が大きくなり(ある分子においては、基底状態と励起状態では安定構造が異なるため、外部刺激により基底状態から励起状態への変換が起きると、その後、励起状態における安定構造へと構造が変化する)、幅広な発光スペクトルを与えるため、発光材料として使うと色純度を低下させる可能性がある。
Mは、それぞれ独立して、単結合、-O-、>N-Arまたは>CAr2であり、形成する部分構造のHOMOの深さおよび励起一重項エネルギー準位および励起三重項エネルギー準位の高さの観点から、好ましくは、単結合、-O-または>N-Arである。Jはドナー性の部分構造とアクセプター性の部分構造を分けるスペーサー構造であり、それぞれ独立して、炭素数6~18のアリーレンであり、ドナー性の部分構造とアクセプター性の部分構造から染み出す共役の大きさの観点から、炭素数6~12のアリーレンが好ましい。より具体的には、フェニレン、メチルフェニレンおよびジメチルフェニレンが挙げられる。Qは、それぞれ独立して、=C(-H)-または=N-であり、形成する部分構造のLUMOの浅さおよび励起一重項エネルギー準位および励起三重項エネルギー準位の高さの観点から、好ましくは、=N-である。Arは、それぞれ独立して、水素、炭素数6~24のアリール、炭素数2~24のヘテロアリール、炭素数1~12のアルキルまたは炭素数3~18のシクロアルキルであり、形成する部分構造のHOMOの深さおよび励起一重項エネルギー準位および励起三重項エネルギー準位の高さの観点から、好ましくは、水素、炭素数6~12のアリール、炭素数2~14のヘテロアリール、炭素数1~4のアルキルまたは炭素数6~10のシクロアルキルであり、より好ましくは、水素、フェニル、トリル、キシリル、メシチル、ビフェニル、ピリジル、ビピリジル、トリアジル、カルバゾリル、ジメチルカルバゾリル、ジーtert-ブチルカルバゾリル、ベンゾイミダゾールまたはフェニルベンゾイミダゾールであり、さらに好ましくは、水素、フェニルまたはカルバゾリルである。mは、1または2である。nは、~(6-m)の整数であり、立体障害の観点から、好ましくは、4~(6-m)の整数である。さらに、上記各式で表される化合物における少なくとも1つの水素は、ハロゲンまたは重水素で置換されていてもよい。
(D1-L1)n-A1 (DAD1)
式(DAD1)には、下記式(DAD2)で表される化合物が含まれる。
D2-L2-A2-L3-D3 (DAD2)
式(DAD1)および式(DAD2)において、D1、D2およびD3はそれぞれ独立してドナー性基を表す。ドナー性基としては、上記のドナー性の構造を採用することができる。A1およびA2はそれぞれ独立してアクセプター性基を表す、アクセプター性基としては、上記のアクセプター性の構造を採用することができる。L1、L2およびL3はそれぞれ独立して単結合または共役連結基を表す。共役連結基はドナー性基とアクセプター性基を分けるスペーサー構造であり、炭素数6~18のアリーレンであることが好ましく、炭素数6~12のアリーレンがより好ましい。L1、L2およびL3は、それぞれ独立してフェニレン、メチルフェニレンまたはジメチルフェニレンであることがさらに好ましい。式(DAD1)におけるnは2以上であって、A1が置換しうる最大数以下の整数を表す。nは例えば2~10の範囲内で選択したり、2~6の範囲内で選択したりしてもよい。nが2であるとき、式(DAD2)で表される化合物になる。n個のD1は同一であっても異なっていてもよく、n個のL1は同一であっても異なっていてもよい。式(DAD1)および式(DAD2)で表される化合物の好ましい具体例として、2PXZ-TAZや下記の化合物をあげることができるが、本発明で採用することができる第2成分はこれらの化合物に限定されない。
本発明の有機電界発光素子の発光層は、第3成分としてホウ素原子を有する化合物を含む。発光層は、ホウ素原子を有する化合物として、下記式(i)、(ii)、および(iii)のいずれかで表される化合物、および下記式(i)で表される構造を複数有する多量体化合物の少なくとも1つを含むことが好ましい。
上記の式(i)、(ii)、および(iii)のいずれかで表される化合物、式(i)で表される構造を複数有する多量体化合物、式(1)、(2)、(3)および(4)のいずれかで表される化合物は、これらの具体的な化合物例について、さらに検討を行って一般化したものである。
第3成分は通常の蛍光体であっても、熱活性化型遅延蛍光体であってもよい。
以下において、各式およびその具体例について説明する。
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1およびX2は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(i)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。
R1~R11(以降、「R1等」ともいう)は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシ(以上、第1置換基)であり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つ(以上、第2置換基)で置換されていてもよく、また、R1~R3、R4~R7およびR8~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つ(以上、第1置換基)で置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つ(以上、第2置換基)で置換されていてもよく、
X1およびX2は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1およびX2は、同時に>CR2になることはなく、
そして、
式(1)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。
A環、B環、C環およびD環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-R、>CR2、>Sまたは>Seであり、前記>N-RのRおよび>CR2のRは、置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたは置換されていてもよいアルキルであり、また、前記>N-RのRは連結基または単結合により前記A環、B環、C環およびD環から選択される少なくとも1つと結合していてもよく、
R1およびR2は、それぞれ独立して、水素、炭素数1~6のアルキル、炭素数3~12のシクロアルキル、炭素数6~12のアリール、炭素数2~15のヘテロアリールまたはジアリールアミノ(ただしアリールは炭素数6~12のアリール)であり、
式(ii)で表される化合物における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。
R1~R14(以降、「R1等」ともいう)は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルであり、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、また、R5~R7およびR10~R12のうちの隣接する基同士が結合してb環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環における少なくとも1つの水素は、アリール、ヘテロアリール、ジアリールアミノ、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルで置換されていてもよく、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRは、炭素数6~12のアリール、炭素数2~15のヘテロアリール、炭素数3~12のシクロアルキルまたは炭素数1~6のアルキルであり、また、前記>N-RのRおよび>CR2のRは、-O-、-S-、-C(-R)2-または単結合により前記a環、b環、c環およびd環から選択される少なくとも1つと結合していてもよく、前記-C(-R)2-のRは水素または炭素数1~6のアルキルであり、
ただし、X1、X2、X3、およびX4は、同時に>CR2になることはなく、
そして、
式(2)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1、X2およびX3は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(iii)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。
R1~R11(以降、「R1等」ともいう)は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R6およびR9~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
X1、X2およびX3は、それぞれ独立して、>O、>N-R、または>CR2であり、前記>N-RのRおよび>CR2 のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1、X2、およびX3は、同時に>CR2になることはなく、
そして、
式(3)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。
上記の式(i)で表される化合物は、下記式(4)で表される化合物であることも好ましい。
R1~R14(以降、「R1等」ともいう)は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R7、R8~R10およびR11~R14のうちの隣接する基同士が結合してa環、b環、c環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Xは、>O、>N-Rまたは>CR2であり、前記>N-RのRはアリール、ヘテロアリールまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Lは、単結合、>CR2、>O、>Sまたは>N-Rであり、前記>CR2および>N-RにおけるRは、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、アルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、XおよびLは、同時に>CR2になることはなく、
そして、
式(4)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。
第3成分として用いることができる式(i)~(iii)および式(1)~(4)で表される化合物は、化合物内に存在する環に置換基が結合していない無置換体であってもよいが、適切な置換基で置換されている化合物を用いることが好ましい。適切な置換基で置換されている化合物を第3成分として用いることにより、無置換体を用いた場合よりも、有機電界発光素子の特性がより優れたものになる。好ましい置換基として、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルをあげることができ;より好ましい置換基として、アリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシをあげることができ;さらに好ましい置換基としてアリール、ジアリールアミノ、アルキルをあげることができ;さらにより好ましい置換基としてジアリールアミノ、アルキルをあげることができ;特に好ましい置換基としてジアリールアミノをあげることができる。ここでいうアリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルは、これらにおける少なくとも1つの水素が、アリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよい。
適切に置換基を選択する等の手法をとることにより、第2成分と第3成分については下記の特に好ましいエネルギー構造を構築し、採用することができる。
E(3,T,Sh)≧E(2,T,Sh)
ΔE(2,ST,Sh)≧ΔE(3,ST,Sh)
第3成分のストークスシフトが10nm以下
また、下記のエネルギー構造も好ましい。
E(3,T,Sh)≦E(2,T,Sh)
ΔE(2,ST,Sh)≦ΔE(3,ST,Sh)
第3成分のストークスシフトが15nm以下
さらに、下記のエネルギー構造も好ましい。
E(3,T,Sh)≦E(2,T,Sh)
ΔE(2,ST,Sh)≧ΔE(3,ST,Sh)
第3成分のストークスシフトが15nmより大きい
また、上記のいずれのエネルギー構造においても、E(3,S,PT)≧E(2,S,PT)であることが好ましい。
本発明の有機電界発光素子の発光層に含まれる第1成分は、ホスト化合物から水素原子2個が脱離した構造を繰り返し単位として含む高分子化合物であってもよい。本発明の有機電界発光素子の発光層に含まれる第2成分は、熱活性型遅延蛍光体から水素原子2個が脱離した構造を繰り返し単位として含む高分子化合物であってもよい。本発明の有機電界発光素子の発光層に含まれる第3成分は、ホウ素原子を有する化合物から水素原子2個が脱離した構造を繰り返し単位として含む高分子化合物であってもよい。脱離する2個の水素原子は、化合物中の任意の2原子とすることができる。同一の環構造に結合している2個の水素原子であってもよいし、そうでなくてもよい。
本発明の有機電界発光素子は、発光層の他に、1以上の有機層を有していてもよい。有機層としては、例えば、電子輸送層、正孔輸送層、電子注入層および正孔注入層等を挙げることができ、さらに、その他の有機層を有していてもよい。
図4に、これらの有機層を備えた有機電界発光素子の層構成の一例を示す。図4において、101は基板、102は陽極、103は正孔注入層、104は正孔輸送層、105は発光層、106は電子輸送層、107は電子注入層、108は陰極をそれぞれ示す。
以下において、有機電界発光素子において、発光層の他に設けられる有機層、陰極および陽極、基板について説明する。
電子注入層107は、陰極108から移動してくる電子を、効率よく発光層105内または電子輸送層106内に注入する役割を果たす。電子輸送層106は、陰極108から注入された電子または陰極108から電子注入層107を介して注入された電子を、効率よく発光層105に輸送する役割を果たす。電子輸送層106および電子注入層107は、それぞれ、電子輸送・注入材料の一種または二種以上を積層、混合により形成される。
具体的な「シクロアルキル」としては、シクロプロピル、シクロブチル、シクロペンチル、シクロヘキシル、メチルシクロペンチル、シクロヘプチル、メチルシクロヘキシル、シクロオクチルまたはジメチルシクロヘキシルなどが挙げられる。
ホスフィンオキサイド誘導体は、例えば下記式(ETM-7-1)で表される化合物である。詳細は国際公開第2013/079217号公報にも記載されている。
R6は、CN、置換または無置換の、炭素数1~20のアルキル、炭素数1~20のヘテロアルキル、炭素数6~20のアリール、炭素数5~20のヘテロアリール、炭素数1~20のアルコキシまたは炭素数6~20のアリールオキシであり、
R7およびR8は、それぞれ独立して、置換または無置換の、炭素数6~20のアリールまたは炭素数5~20のヘテロアリールであり、
R9は酸素または硫黄であり、
jは0または1であり、kは0または1であり、rは0~4の整数であり、qは1~3の整数である。
ピリミジン誘導体は、例えば下記式(ETM-8)で表される化合物であり、好ましくは下記式(ETM-8-1)で表される化合物である。詳細は国際公開第2011/021689号公報にも記載されている。
トリアジン誘導体は、例えば下記式(ETM-10)で表される化合物であり、好ましくは下記式(ETM-10-1)で表される化合物である。詳細は米国公開公報2011/0156013号公報に記載されている。
フェナントロリン誘導体は、例えば下記式(ETM-12)または式(ETM-12-1)で表される化合物である。詳細は国際公開2006/021982号公報に記載されている。
キノリノール系金属錯体は、例えば下記式(ETM-13)で表される化合物である。
陰極108は、電子注入層107および電子輸送層106を介して、発光層105に電子を注入する役割を果たす。
正孔注入層103は、陽極102から移動してくる正孔を、効率よく発光層105内または正孔輸送層104内に注入する役割を果たすものである。正孔輸送層104は、陽極102から注入された正孔または陽極102から正孔注入層103を介して注入された正孔を、効率よく発光層105に輸送する役割を果たすものである。正孔注入層103および正孔輸送層104は、それぞれ、正孔注入・輸送材料の一種または二種以上を積層、混合するか、正孔注入・輸送材料と高分子結着剤の混合物により形成される。また、正孔注入・輸送材料に塩化鉄(III)のような無機塩を添加して層を形成してもよい。
陽極102は、発光層105へ正孔を注入する役割を果たすものである。なお、陽極102と発光層105との間に正孔注入層103および/または正孔輸送層104が設けられている場合には、これらを介して発光層105へ正孔を注入することになる。
基板101は、有機電界発光素子100の支持体となるものであり、通常、石英、ガラス、金属、プラスチックなどが用いられる。基板101は、目的に応じて板状、フィルム状、またはシート状に形成され、例えば、ガラス板、金属板、金属箔、プラスチックフィルム、プラスチックシートなどが用いられる。なかでも、ガラス板、および、ポリエステル、ポリメタクリレート、ポリカーボネート、ポリスルホンなどの透明な合成樹脂製の板が好ましい。ガラス基板であれば、ソーダライムガラスや無アルカリガラスなどが用いられ、また、厚みも機械的強度を保つのに十分な厚みがあればよいので、例えば、0.2mm以上あればよい。厚さの上限値としては、例えば、2mm以下、好ましくは1mm以下である。ガラスの材質については、ガラスからの溶出イオンが少ない方がよいので無アルカリガラスの方が好ましいが、SiO2などのバリアコートを施したソーダライムガラスも市販されているのでこれを使用することができる。また、基板101には、ガスバリア性を高めるために、少なくとも片面に緻密なシリコン酸化膜などのガスバリア膜を設けてもよく、特にガスバリア性が低い合成樹脂製の板、フィルムまたはシートを基板101として用いる場合にはガスバリア膜を設けるのが好ましい。
有機電界発光素子を構成する各層は、各層を構成すべき材料を蒸着法、抵抗加熱蒸着、電子ビーム蒸着、スパッタリング、分子積層法、印刷法、スピンコート法またはキャスト法、コーティング法などの方法で薄膜とすることにより、形成することができる。このようにして形成された各層の膜厚については特に限定はなく、材料の性質に応じて適宜設定することができるが、通常2nm~5000nmの範囲である。膜厚は通常、水晶発振式膜厚測定装置などで測定できる。蒸着法を用いて薄膜化する場合、その蒸着条件は、材料の種類、膜の目的とする結晶構造および会合構造などにより異なる。蒸着条件は一般的に、蒸着用ルツボの加熱温度+50~+400℃、真空度10-6~10-3Pa、蒸着速度0.01~50nm/秒、基板温度-150~+300℃、膜厚2nm~5μmの範囲で適宜設定することが好ましい。
適当な基板上に、陽極材料の薄膜を蒸着法などにより形成させて陽極を作製した後、この陽極上に正孔注入層および正孔輸送層の薄膜を形成させる。この上に、ホスト化合物、熱活性化型遅延蛍光体およびホウ素原子を有する化合物を共蒸着し薄膜を形成させて発光層とし、この発光層の上に電子輸送層、電子注入層を形成させ、さらに陰極用物質からなる薄膜を蒸着法などにより形成させて陰極とすることにより、目的の有機電界発光素子が得られる。なお、上述の有機電界発光素子の作製においては、作製順序を逆にして、陰極、電子注入層、電子輸送層、発光層、正孔輸送層、正孔注入層、陽極の順に作製することも可能である。
蒸着法で発光層を形成する際には、第3成分として式(ii)で表される化合物や式(2)で表される化合物を選択して用いることが好ましい。特に式(2)のR1~R14の少なくとも1つが置換基である化合物を選択して用いることが好ましい。ここでいう置換基としては、上記の第3成分における好ましい置換基を採用することができる。中でも、炭素数1~24のアルキル、置換されていてもよいジアリールアミノを特に好ましく採用することができる。これらの置換基を有する化合物を用いて発光層を蒸着法で形成した場合は、置換基を有しない対応化合物、式(i)で表される化合物、式(iii)で表される化合物、あるいは式(4)で表される化合物を用いて蒸着法で形成した場合よりも、有機電界発光素子の外部量子効率が高くて特性が優れている。
また、第3成分として式(i)または式(iii)で表される化合物を用いて蒸着法で発光層を形成する場合は、上記の第3成分における好ましい置換基を採用することが好ましく、炭素数1~24のアルキル、置換されていてもよいジアリールアミノを有する化合物を用いることが特に好ましい。これらの置換基を有する化合物を用いて発光層を蒸着法で形成した場合は、置換基を有しない対応化合物を用いて蒸着法で形成した場合よりも、有機電界発光素子の外部量子効率が高くて長寿命であり特性が優れている。
発光層形成用組成物を使用する場合は、湿式成膜法を用いることによって成膜される。
そこで、一般的には、幾つかの層だけを湿式成膜法を用い、残りを真空蒸着法で有機電界発光素子を作製するという方法が採用される。
(手順1)陽極の真空蒸着法による成膜
(手順2)正孔注入層の湿式成膜法による成膜
(手順3)正孔輸送層の湿式成膜法による成膜
(手順4)ホスト化合物、熱活性化型遅延蛍光体およびホウ素原子を有する化合物を含む発光層形成用組成物の湿式成膜法による成膜
(手順5)電子輸送層の真空蒸着法による成膜
(手順6)電子注入層の真空蒸着法による成膜
(手順7)陰極の真空蒸着法による成膜
この手順を経ることで、陽極/正孔注入層/正孔輸送層/ホスト材料、熱活性化型遅延蛍光体およびホウ素原子を有する化合物を含む発光層/電子輸送層/電子注入層/陰極からなる有機電界発光素子が得られる。
図6を参考にして、バンクを有する基板にインクジェット法を用いて有機電界発光素子を作製する方法を説明する。まず、バンク(200)は基板(110)上の電極(120)の上に設けられている。この場合、インクジェットヘッド(300)より、バンク(200)間にインクの液滴(310)を滴下し、乾燥させることで塗膜(130)を作製することができる。これを繰り返し、次の塗膜(140)、さらに発光層(150)まで作製し、真空蒸着法を用い電子輸送層、電子注入層および電極を成膜すれば、バンク材で発光部位が区切られた有機電界発光素子を作製することができる。
また、本発明は、有機電界発光素子を備えた表示装置または有機電界発光素子を備えた照明装置などにも応用することができる。
有機電界発光素子を備えた表示装置または照明装置は、本実施形態にかかる有機電界発光素子と公知の駆動装置とを接続するなど公知の方法によって製造することができ、直流駆動、パルス駆動、交流駆動など公知の駆動方法を適宜用いて駆動することができる。
本発明の発光層形成用組成物は、有機電界発光素子の発光層を湿式法により形成するための組成物である。発光層形成用組成物は、第1成分として少なくとも1種のホスト化合物と、第2成分として少なくとも1種の熱活性化型遅延蛍光体と、第3成分として少なくとも1種のホウ素原子を有する化合物と、第4成分として少なくとも1種の有機溶媒を含む組成物である。ホスト化合物、熱活性化型遅延蛍光体およびホウ素原子を有する化合物については、上記の有機電界発光素子における発光層の説明にて記載した化合物を用いることができる。
本発明の発光層形成用組成物は、少なくとも1種の有機溶媒を含むことが好ましい。成膜時に有機溶媒の蒸発速度を制御することで、成膜性および塗膜の欠陥の有無、表面粗さ、平滑性を制御および改善することができる。また、インクジェット法を用いた成膜時は、インクジェットヘッドのピンホールでのメニスカス安定性を制御し、吐出性を制御・改善することができる。加えて、膜の乾燥速度および誘導体分子の配向を制御することで、該発光層形成用組成物より得られる発光層を有する有機電界発光素子の電気特性、発光特性、効率、および寿命を改善することができる。
発光層形成用組成物に第4成分として含まれる少なくとも1種の有機溶媒の沸点は、130℃~350℃であり、140℃~300℃がより好ましく、150℃~250℃がさらに好ましい。沸点が130℃より高い場合、インクジェットの吐出性の観点から好ましい。また、沸点が350℃より低い場合、塗膜の欠陥、表面粗さ、残留溶媒および平滑性の観点から好ましい。良好なインクジェットの吐出性、製膜性、平滑性および低い残留溶媒の観点から、2種以上の有機溶媒を含む構成がより好ましい。一方で、場合によっては、運搬性などを考慮し、発光層形成用組成物中から溶媒を除去することで固形状態とした組成物であってもよい。
高沸点の貧溶媒を加えることで成膜時に低沸点の良溶媒が先に揮発し、組成物中の含有物の濃度と貧溶媒の濃度が増加し速やかな成膜が促される。これにより、欠陥が少なく、表面粗さが小さい、平滑性の高い塗膜が得られる。
発光層形成用組成物に用いられる有機溶媒としては、炭化水素系溶媒、アルキルベンゼン系溶媒、フェニルエーテル系溶媒、アルキルエーテル系溶媒、環状ケトン系溶媒、脂肪族ケトン系溶媒、単環性ケトン系溶媒、ジエステル骨格を有する溶媒および含フッ素系溶媒などが挙げられ、具体例として、ペンタノール、ヘキサノール、ヘプタノール、オクタノール、ノナノール、デカノール、ウンデカノール、ドデカノール、テトラデカノール、ヘキサン-2-オール、ヘプタン-2-オール、オクタン-2-オール、デカン-2-オール、ドデカン-2-オール、シクロヘキサノール、α-テルピネオール、β-テルピネオール、γ-テルピネオール、δ-テルピネオール、テルピネオール(混合物)、エチレングリコールモノメチルエーテルアセテート、プロピレングリコールモノメチルエーテルアセテート、ジエチレングリコールジメチルエーテル、ジプロピレングリコールジメチルエーテル、ジエチレングリコールエチルメチルエーテル、ジエチレングリコールイソプロピルメチルエーテル、ジプロピレングリコールモノメチルエーテル、ジエチレングリコールジエチルエーテル、ジエチレングリコールモノメチルエーテル、ジエチレングリコールブチルメチルエーテル、トリプロピレングリコールジメチルエーテル、トリエチレングリコールジメチルエーテル、ジエチレングリコールモノブチルエーテル、エチレングリコールモノフェニルエーテル、トリエチレングリコールモノメチルエーテル、ジエチレングリコールジブチルエーテル、トリエチレングリコールブチルメチルエーテル、ポリエチレングリコールジメチルエーテル、テトラエチレングリコールジメチルエーテル、p-キシレン、m-キシレン、o-キシレン、2,6-ルチジン、2-フルオロ-m-キシレン、3-フルオロ-o-キシレン、2-クロロベンゾ三フッ化物、クメン、トルエン、2-クロロ-6-フルオロトルエン、2-フルオロアニソール、アニソール、2,3-ジメチルピラジン、ブロモベンゼン、4-フルオロアニソール、3-フルオロアニソール、3-トリフルオロメチルアニソール、メシチレン、1,2,4-トリメチルベンゼン、t-ブチルベンゼン、2-メチルアニソール、フェネトール、ベンゾジオキソール、4-メチルアニソール、s-ブチルベンゼン、3-メチルアニソール、4-フルオロ-3-メチルアニソール、シメン、1,2,3-トリメチルベンゼン、1,2-ジクロロベンゼン、2-フルオロベンゾニトリル、4-フルオロベラトロール、2,6-ジメチルアニソール、n-ブチルベンゼン、3-フルオロベンゾニトリル、デカリン(デカヒドロナフタレン)、ネオペンチルベンゼン、2,5-ジメチルアニソール、2,4-ジメチルアニソール、ベンゾニトリル、3,5-ジメチルアニソール、ジフェニルエーテル、1-フルオロ-3,5-ジメトキシベンゼン、安息香酸メチル、イソペンチルベンゼン、3,4-ジメチルアニソール、o-トルニトリル、n-アミルベンゼン、ベラトロール、1,2,3,4-テトラヒドロナフタレン、安息香酸エチル、n-ヘキシルベンゼン、安息香酸プロピル、シクロヘキシルベンゼン、1-メチルナフタレン、安息香酸ブチル、2-メチルビフェニル、3-フェノキシトルエン、2,2’-ビトリル、ドデシルベンゼン、ジペンチルベンゼン、テトラメチルベンゼン、トリメトキシベンゼン、トリメトキシトルエン、2,3-ジヒドロベンゾフラン、1-メチル-4-(プロポキシメチル)ベンゼン、1-メチル-4-(ブチルオキシメチル)ベンゼン、1-メチル-4-(ペンチルオキシメチル)ベンゼン、1-メチル-4-(ヘキシルオキシメチル)ベンゼン、1-メチル-4-(ヘプチルオキシメチル)ベンゼンベンジルブチルエーテル、ベンジルペンチルエーテル、ベンジルヘキシルエーテル、ベンジルヘプチルエーテル、ベンジルオクチルエーテル、ニトロベンゼン、ジメチルニトロベンゼン、アミノビフェニル、ジフェニルアミンなどが挙げられるが、それだけに限定されない。また、溶媒は単一で用いてもよく、混合してもよい。
発光層形成用組成物は、その性質を損なわない範囲で、任意成分を含んでいてもよい。任意成分としては、バインダーおよび界面活性剤等が挙げられる。
発光層形成用組成物は、バインダーを含有していてもよい。バインダーは、成膜時には膜を形成するとともに、得られた膜を基板と接合する。また、該発光層形成用組成物中で他の成分を溶解および分散および結着させる役割を果たす。
発光層形成用組成物は、例えば、発光層形成用組成物の膜面均一性、膜表面の親溶媒性および撥液性の制御のために界面活性剤を含有してもよい。界面活性剤は、親水性基の構造からイオン性および非イオン性に分類され、さらに、疎水性基の構造からアルキル系およびシリコン系およびフッ素系に分類される。また、分子の構造から、分子量が比較的小さく単純な構造を有する単分子系および分子量が大きく側鎖や枝分かれを有する高分子系に分類される。また、組成から、単一系、二種以上の界面活性剤および基材を混合した混合系に分類される。該発光層形成用組成物に用いることのできる界面活性剤としては、全ての種類の界面活性剤を用いることができる。
界面活性剤は1種で用いてもよく、2種以上を併用してもよい。
本発明の発光層形成用組成物では、第1成分、第2成分および第3成分として、優れた溶解性、成膜性、湿式塗布性、熱的安定性、および面内配向性の少なくとも1つを満たす化合物を選択する。また、優れた溶解性、製膜性、湿式塗布性、および面内配向性の観点から、炭素数1~24のアルキル、ジアリールアミノ、炭素数5~24のシクロアルキル、炭素数6~24のアリールおよび炭素数5~24のヘテロアリールで置換されている化合物を選択することが好ましい。
以下に、実施例で使用した化合物の合成例を示す。
合成例(1)
化合物(ED1):N7,N7,N13,N13,5,9,11,15-オクタフェニル-5,9,11,15-テトラヒドロ-5,9,11,15-テトラアザ-19b,20b-ジボラジナフト[3,2,1-de:1’,2’,3’-jk]ペンタセン-7,13-ジアミンの合成
窒素雰囲気下、1,3-ジブロモベンゼン(25.0g、106mmol)、アニリン(20.3ml、223mmol)、トリス(ジベンジリデンアセトン)二パラジウム(0)(Pd2(dba)3)(971mg、1.06mmol)、2,2’-ビス(ジフェニルホスフィノ)-1,1’-ビナフチル(BINAP:1.98g、3.18mmol)、NaOtBu(25.5g、265mmol)およびトルエン(400ml)の入ったフラスコを110℃に加熱し、18時間撹拌した。反応液を室温まで冷却し、シリカゲルを用いて濾過し(溶離液:トルエン)、溶媒を減圧留去して粗生成物を得た。得られた粗生成物をトルエンに溶解させた後、適当量を減圧留去し、ヘキサンを加え再沈殿させることで、N1,N3-ジフェニルベンゼン-1,3-ジアミン(16.5g、収率60%)を白色固体として得た。
1H-NMR(400MHz,CDCl3):δ=5.63(s,2H)、6.60(dd,2H)、6.74(t,1H)、6.90(t,2H)、7.06(d,4H)、7.12(t,1H)、7.24(dt,4H).
窒素雰囲気下、1,3-ジブロモ-5-クロロベンゼン(8.11g、30mmol)、ジフェニルアミン(10.1g、60mmol)、Pd2(dba)3(550mg、0.6mmol)、2-ジシクロヘキシルフェニルホスフィノ-2’,6’-ジメトキシジフェニル(SPhos:0.493g、1.2mmol)、ナトリウム tert-ブトキシド(NaOtBu)(8.60g、90mmol)およびトルエン(300ml)の入ったフラスコを80℃に加熱し、15時間撹拌した。反応液を室温まで冷却し、シリカゲルを用いて濾過し(溶離液:トルエン)、溶媒を減圧留去して粗生成物を得た。得られた粗生成物をトルエンに溶解させた後、減圧留去することで飽和溶液を調製し、ヘキサンを加え再沈殿させることで、5-クロロ-N1,N1,N3,N3-テトラフェニルベンゼン-1,3-ジアミン(5.66g、収率43%)を白色固体として得た。
1H-NMR(400MHz,CDCl3):δ=6.56(d,2H)、6.64(t,1H)、7.00(t,4H)、7.05(d,8H)、7.21(dd,8H).
窒素雰囲気下、第1段で合成したN1,N3-ジフェニルベンゼン-1,3-ジアミン(1.34g、5.1mmol)、第2段で合成した5-クロロ-N1,N1,N3,N3-テトラフェニルベンゼン-1,3-ジアミン(4.80g、11mmol)、Pd2(dba)3(0.140g、0.15mmol)、トリ-tert-ブチルホスフィン(60.7mg、0.30mmol)、NaOtBu(1.47g、15mmol)およびトルエン(200ml)の入ったフラスコを110℃に加熱し、8時間撹拌した。反応液を室温まで冷却し、シリカゲルを用いて濾過し(溶離液:トルエン)、溶媒を減圧留去して粗生成物を得た。得られた粗生成物をヘキサン、メタノールの順に洗浄することで、N1,N1’-(1,3-フェニレン)ビス(N1,N3,N3,N5,N5-ペンタフェニルベンゼン-1,3,5-トリアミン(4.80g、収率87%)を白色固体として得た。
1H-NMR(400MHz,CDCl3):δ=6.38(d,4H)、6.41(t,2H)、6.58(dd,2H)、6.70(t,1H)、6.88-6.90(m,14H)、6.85(t,1H)、6.99(d,16H)、7.08-7.15(m,20H).
N1,N1’-(1,3-フェニレン)ビス(N1,N3,N3,N5,N5-ペンタフェニルベンゼン-1,3,5-トリアミン(3.24g、3.0mmol)およびオルトジクロロベンゼン(400ml)の入ったフラスコに、窒素雰囲気下、室温で、三臭化ホウ素(1.13ml、12mmol)を加えた。滴下終了後、180℃まで昇温して20時間撹拌した。その後、再び室温まで冷却して、N-ジイソプロピルエチルアミン(7.70ml、45mmol)を加え、発熱が収まるまで撹拌した。その後、60℃で減圧下、反応溶液を留去して粗生成物を得た。得られた粗生成物をアセトニトリル、メタノール、トルエンの順に洗浄し、シリカゲルカラムクロマトグラフィー(溶離液:トルエン)で精製後粗体をo-ジクロロベンゼンで2回再結晶を行い、その後1×10-4mmHgの減圧下、440℃にて昇華精製を行うことで、化合物(ED1)を1.17g得た。
1H-NMR(400MHz,CDCl3):δ=5.72(s,2H)、5.74(s,2H)、5.86(s,1H)、6.83(d,2H)、6.88-6.93(m,12H)、7.05(t,8H)、7.12-7.19(m,6H)、7.24-7.26(m,4H)、7.05(d,4H)、7.12(dd,8H)、7.12-7.19(m,6H)、7.32(d,4H)、7.38(dd,2H)、7.42(t,2H)、7.46(dd,2H)、7.47(dd,4H)、9.30(d,2H)、10.5(s,1H).
化合物(1-41):2,12-ジ-t-ブチル-5,9-ビス(4-(t-ブチル)フェニル)-7-メチル-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセンの合成
化合物(1-53):2,12-ジ-t-ブチル-5,9-ビス(4-(t-ブチル)フェニル)-7-(9H-カルバゾール-9-イル)-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセンの合成
化合物(1-37):3,12-ジ-t-ブチル-9-(4-(t-ブチル)フェニル)-5-(3,5-ジ-t-ブチルフェニル)-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセンの合成
化合物(1-46):3,12-ジ-t-ブチル-9-(4-(t-ブチル)フェニル)-5-(3,5-ジ-t-ブチルフェニル)-7-メチル-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセンの合成
化合物(1-50):2,12-ジ-t-ブチル-N,N,5,9-テトラキス(4-(t-ブチル)フェニル)-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセン-7-アミンの合成
化合物(1-49):2,12-ジ-t-ブチル-5,9-ビス(4-(t-ブチル)フェニル)-N,N-ジフェニル-5,9-ジヒドロ-5,9-ジアザ-13b-ボラナフト[3,2,1-de]アントラセン-7-アミンの合成
式(1-340)の化合物:15,15-ジメチル-N,N-ジフェニル-15H-5,9-ジオキサ-16b-ボラインデノ[1,2-b]ナフト[1,2,3-fg]アントラセン-13-アミンの合成
式(1-351)の化合物:5-([1,1’-ビフェニル]-4-イル)-15,15-ジメチル-N,N,2-トリフェニル-5H,15H-9-オキサ-5-アザ-16b-ボラインデノ[1,2-b]ナフト[1,2,3-fg]アントラセン-13-アミンの合成
サンプルの準備
評価対象の化合物の吸収特性と発光特性(蛍光と燐光)を評価する際には、評価対象の化合物のみを薄膜化し評価するか、あるいは、評価対象の化合物を適切なマトリックス材料中に分散して薄膜化して評価した。
評価対象の化合物を適切なマトリックス材料中に分散する際のマトリックス材料としては、市販のPMMA(ポリメチルメタクリレート)を用いた。本実施例では、PMMAと評価対象の化合物をトルエン中で溶解させた後、スピンコーティング法により石英製の透明支持基板(10mm×10mm)上に厚さ10nmの薄膜を形成してサンプルを作製した。サンプルの濃度は1質量%とした。
サンプルの吸収スペクトルの測定は、紫外可視近赤外分光光度計((株)島津製作所、UV-2600)を用いて行った。また、サンプルの蛍光スペクトルおよび燐光スペクトルの測定は、分光蛍光光度計(日立ハイテク(株)製、F-7000)を用いて行った。
さらに、直流電流を連続的に印加して発光強度が初期の50%になるまでの時間を測定し、素子寿命(LT50)を評価した。
蛍光寿命測定装置(浜松ホトニクス(株)製、C11367-01)を用いて300Kで蛍光寿命を測定した。具体的には、適切な励起波長で測定される極大発光波長において蛍光寿命の早い発光成分と遅い発光成分を観測した。蛍光を発光する一般的な有機電界発光材料の室温における蛍光寿命測定では、熱による3重項成分の失活により、燐光に由来する3重項成分が関与する遅い発光成分が観測されることはほとんどない。評価対象の化合物において遅い発光成分が観測された場合は、励起寿命の長い3重項エネルギーが熱活性化により1重項エネルギーに移動して遅延蛍光として観測されたことを示すことになる。ここでは、遅延蛍光の蛍光寿命をTau(Delay)として測定した。
一重項励起エネルギー準位E(S,Sh)は、蛍光スペクトルのピーク短波長側の肩を通る接線とベースラインとの交点における波長BSh(nm)から、E(S,Sh)=1240/BShで算出した。また、三重項励起エネルギー準位E(T,Sh)は、燐光スペクトルのピーク短波長側の肩を通る接線とベースラインとの交点における波長CSh(nm)から、E(T,Sh)=1240/CShで算出した。さらに蛍光スペクトルの極大ピーク発光波長も測定した。
本明細書では、第1成分の一重項励起エネルギー準位をE(1,S,Sh)、第2成分の一重項励起エネルギー準位をE(2,S,Sh)、第3成分の一重項励起エネルギー準位をE(3,S,Sh)、第1成分の三重項励起エネルギー準位をE(1,T,Sh)、第2成分の三重項励起エネルギー準位をE(2,T,Sh)、第3成分の三重項励起エネルギー準位をE(3,T,Sh)と表示する。
本明細書では、第1成分のΔE(ST)をΔE(1,ST,Sh)、第2成分のΔE(ST)をΔE(2,ST,Sh)、第3成分のΔE(ST)をΔE(3,ST,Sh)と表示する。
有機電界発光素子を作製し、電圧を印加して電流密度、輝度、色度および外部量子効率を測定した。作製した有機電界発光素子の構成として、まず以下の構成A(表1)を選定して評価した。構成Aは熱活性化型遅延蛍光用材料に適合した構成である。構成Aは文献(Adv. Mater. 2016, 28, 2777-2781)で示された高い効率を期待できる素子構成である。ただし、本発明の化合物の適用はこれらの構成に限定されず、各層の膜厚や構成材料は本発明の化合物の基礎物性によって適宜変更することができる。
スパッタリングにより200nmの厚さに製膜したITOを50nmまで研磨した、26mm×28mm×0.7mmのガラス基板((株)オプトサイエンス製)を透明支持基板とした。この透明支持基板を市販の蒸着装置(長州産業(株)製)の基板ホルダーに固定し、NPD、TcTa、mCP、第1成分(mCBP)、第2成分(下の表に記載の化合物)、第3成分(ED1)およびTSPO1をそれぞれ入れたタンタル製蒸着用ボート、LiFおよびアルミニウムをそれぞれ入れた窒化アルミニウム製蒸着用ボートを装着した。
下の表に記載される材料を用いた点を変更し、その他は実施例1-1と同じ手順により、実施例1-4から1-11および比較例1-5の有機電界発光素子を製造した。実施例1-4、比較例1-5、実施例1-5では第3成分の化合物を替え、実施例1-6から1-11では第1成分の化合物を替えている。
また、式(i)の化合物であるRD-3を第3成分として用いた実施例1-5よりも、式(i)の二量体に相当する式(ii)の化合物であるRD-1やED1を第3成分として用いた実施例1-4および1-6から1-11の方が半値幅が小さくて、より好ましい特性を示した。また、実施例の中では、E(1,T,Sh)>E(3,T,Sh)>E(2,T,Sh)である実施例1-4および1-6から1-11の方が、E(1,T,Sh)>E(2,T,Sh)>E(3,T,Sh)である実施例1-5よりも、半値幅が小さくて外部量子効率も同等以上であった。特に、ジアリールアミノ基で置換された構造を有するED1を第3成分として用いた場合には、特に半値幅が小さくて外部量子効率が高く、有機電界発光素子としての特性が一段と優れたものであることが確認された。
下の表に記載される材料を用いた点を変更し、その他は実施例1-1と同じ手順により、実施例1-4から1-11および比較例1-5の有機電界発光素子を製造した。これらの具体例では、第2成分の化合物を替えている。
下の表に記載される材料を用いて、電子輸送層を厚み10nmの電子輸送層1と厚み20nmの電子輸送層2の2層形成して構成Bとした点を変更し、その他は実施例1-1と同じ手順により、実施例1-16から1-26の有機電界発光素子を製造した。実施例1-23の電子輸送層2は、BPy-TP2およびLiqを加熱してそれぞれの比が質量比で7:3になるように膜厚20nmに蒸着することにより形成した。また、実施例1-24の電子輸送層2は、SF3-TRZおよびLiqを加熱してそれぞれの比が質量比で7:3になるように膜厚20nmに蒸着することにより形成した。
下の表に記載される材料を用いた点を変更し、その他は実施例1-14と同じ手順により、実施例1-27から1-55および比較例1-7の構成Bの有機電界発光素子を製造した。実施例1-27から1-55では、式(ii)で表される多様な化合物を第3成分として用いて評価した。
実施例1-27から1-38および1-40から1-55は、いずれもE(1,T,Sh)>E(3,T,Sh)≧E(2,T,Sh)の関係式を満たすため、素子寿命が長く外部量子効率も高かった。一方、E(1,T,Sh)>E(2,T,Sh)>E(3,T,Sh)である有機電界発光素子のうち、実施例1-39はΔE(3,ST,Sh)が0.08eVで十分に低いことから、半値幅が小さく外部量子効率が高かった。一方、比較例1-7は、第3成分であるR-BD2からの燐光発光が認められず、ΔE(3,ST,Sh)が大きいことから、半値幅が大きくて外部量子効率が低かった。
実施例1-27から1-55は、いずれも式(ii)で表される構造を有する。式(i)の骨格が二量化した多量体骨格を有するとともに、骨格が対称形を有していることから、良好な特性を示すものと考えられる。
下の表に記載される材料を用いた点を変更し、その他は実施例1-14と同じ手順により、実施例1-56から1-72および比較例1-8の構成Bの有機電界発光素子を製造した。実施例1-56から1-72では、式(i)で表される多様な化合物を第3成分として用いて評価した。
実施例1-56から1-72の半値幅は22~30nmの範囲内であり、外部量子効率は12.0~16.8%の範囲内であり、素子寿命(LT50)は82~116時間の範囲内であった。いずれも良好な特性ではあるが、上記の実施例1-27から1-55の方がさらに良好な特性を示した。このことは、式(i)で表される構造を有する化合物を第3成分として用いる場合よりも、式(ii)のように式(i)が多量化した構造を有する化合物を第3化合物として用いる方が、特性が一段と良好になることを示している。
次に、本発明をさらに詳細に説明するために、本発明の発光層形成用組成物の具体例とその評価結果を示すが、本発明はこれらの具体例に限定されない。
下の表に示す第1成分、第2成分、第3成分および第4成分を表に記載される比率で混合して攪拌することにより、発光層形成用組成物を調製した。表中、第4成分として用いているTolはトルエン、DHNpはデカヒドロナフタレン、3PxTは3-フェノキシトルエン、c6Bはシクロヘキシルベンゼン、Anisはアニソール、Xylはキシレン(混合物)、1MNpは1-メチルナフタレン、8Bはn-オクチルベンゼン、DPEはジフェニルエーテル、4FAnisは4-フルオロアニソールである。
インクジェット塗布用インク組成物としての有用性を評価するために、各発光層形成用組成物の濁りおよび沈殿を確認して溶解性を評価した。濁りおよび沈殿のないものを「OK」、濁りまたは沈殿が起きたものを「NG」とした。
溶解性の評価において「OK」であった発光層形成用組成物に関して、下記の手順でスピンコート成膜またはインクジェット印刷後に得られた膜の製膜性を評価した。製膜後に、膜に、ピンホールまたは析出またはムラのあるものを「×」、ピンホール、化合物の析出およびムラのないものを「○」、ピンホール、化合物の析出およびムラがなく、平滑性が高いもの(Ra<5nm)を「◎」で示した。
厚み0.5mm、サイズ28×26mmの清浄なガラス基板に、照射エネルギー1000mJ/cm2(低圧水銀灯(254ナノメートル))を照射することでUV-O3処理を行った。次いで、0.3~0.6mLのインク組成物をガラス上に滴下し、スピンコート(スロープ、5秒間→500~5000rpm、10秒間→スロープ、5秒間)を行った。さらに、120℃のホットプレート上で10分間乾燥させた。
インクジェットを用いて、100ppiのピクセル内に吐出し、100℃で乾燥させた。
インクジェットの吐出安定性についても、インクジェット吐出開始直後と24時間連続運転後にそれぞれ評価を行った。吐出安定性が不良であるものを「×」、良好であるものを「○」、極めて良好であるものを「◎」とした。
次に、有機層を塗布形成して得られる有機電界発光素子について説明する。発光層の形成する際には、発光層形成用組成物を使用した。
まず、特開2018-61028号公報に記載の方法に従い、下記の反応により高分子正孔輸送化合物であるXLP-101を合成した。M4の隣にM5またはM6が結合した共重合体が得られ、仕込み比より各ユニットは40:10:50(モル比)であると推測される。なお、下記式において、Bpinはピナコラートボリルである。
市販のPEDOT:PSS溶液(Clevios(TM) P VP AI4083、PEDOT:PSSの水分散液、Heraeus Holdings社製)を用いた。
OTPD(LT-N159、Luminescence Technology Corp社製)およびIK-2(光カチオン重合開始剤、サンアプロ社製)をトルエンに溶解させ、OTPD濃度0.7質量%、IK-2濃度0.007質量%のOTPD溶液を調製した。
キシレンにXLP-101を0.6質量%の濃度で溶解させ、0.6質量%XLP-101溶液を調製した。
ITOが150nmの厚さに蒸着されたガラス基板上に、PEDOT:PSS溶液をスピンコートし、200℃のホットプレート上で1時間焼成することで、膜厚40nmのPEDOT:PSS膜を成膜した(正孔注入層)。次いで、OTPD溶液をスピンコートし、80℃のホットプレート上で10分間乾燥した後、露光機で露光強度100mJ/cm2で露光し、100℃のホットプレート上で1時間焼成することで、溶液に不溶な膜厚30nmのOTPD膜を成膜した(正孔輸送層)。次いで、実施例2-19で調製した発光層形成用組成物をスピンコートし、120℃のホットプレート上で1時間焼成することで、膜厚20nmの発光層を成膜した。
実施例3-1と同様の方法で有機電界発光素子を得た。なお、正孔輸送層は、XLP-101溶液をスピンコートし、200℃のホットプレート上で1時間焼成することで、膜厚30nmの膜を成膜した。
実施例2-19で調製した発光層形成用組成物の替わりに実施例2-2で調製した発光層形成用組成物を用いた以外、実施例3-1と同様の手順で有機電界発光素子を作製した。なお、正孔輸送層は、XLP-101溶液をスピンコートし、200℃のホットプレート上で1時間焼成することで、膜厚30nmの膜を成膜した。
実施例2-19で調製した発光層形成用組成物の替わりに実施例2-18で調製した発光層形成用組成物を用いた以外、実施例3-1と同様の手順で有機電界発光素子を作製した。なお、正孔輸送層は、XLP-101溶液をスピンコートし、200℃のホットプレート上で1時間焼成することで、膜厚30nmの膜を成膜した。
実施例2-18で調製した発光層形成用組成物の替わりに比較例2-2で調製した発光層形成用組成物を用いた以外、実施例3-4と同様の手順で有機電界発光素子を作製した。
実施例3-1と同様の方法で有機電界発光素子を得た。なお、正孔輸送層は、PCz溶液をスピンコートし、120℃のホットプレート上で1時間焼成することで、膜厚30nmの膜を成膜した。
本発明の発光層形成用組成物は高分子化合物や架橋性の化合物を含んでもよい。また、本発明の有機電界発光素子は高分子化合物や架橋性の化合物を含んでもよい。
国際特許公開番号WO2019/004248に記載の方法で以下の第1成分であるホストと第2成分である熱活性化型遅延蛍光体と第3成分であるホウ素原子を有する構造を含む高分子を合成することができる。
国際特許公開番号WO2019/004248に記載の方法で以下の第1成分であるホストと第3成分であるホウ素原子を有する構造を含む高分子を合成することができる。第2成分である熱活性化型遅延蛍光体を加えると本発明の発光層形成用組成物である。
101 基板
102 陽極
103 正孔注入層
104 正孔輸送層
105 発光層
106 電子輸送層
107 電子注入層
108 陰極
Claims (42)
- 発光層を有する有機電界発光素子であって、前記発光層が、
第1成分として、少なくとも1種のホスト化合物と、
第2成分として、少なくとも1種の熱活性化型遅延蛍光体と、
第3成分として、少なくとも1種のホウ素原子を有する化合物とを含み、
前記第1成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(1,S,Sh)、前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)としたとき、以下の関係式(1)を満たし、
前記第1成分は、前記ホスト化合物の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよく、
前記第2成分は、前記熱活性化型遅延蛍光体の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよく、
前記第3成分は、前記ホウ素原子を有する化合物の水素原子2個が脱離した構造を繰り返し単位とする高分子化合物として含まれていてもよい、
有機電界発光素子。
関係式(1): E(1,S,Sh)≧E(2,S,Sh)≧E(3,S,Sh) - 前記第3成分として、下記式(i)、(ii)および(iii)のいずれかで表される化合物、および下記式(i)で表される構造を複数有する多量体化合物の少なくとも1つを含む、請求項1に記載の有機電界発光素子。
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1およびX2は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(i)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。)
A環、B環、C環およびD環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-R、>CR2、>Sまたは>Seであり、前記>N-RのRおよび>CR2のRは、置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたは置換されていてもよいアルキルであり、また、前記>N-RのRは連結基または単結合により前記A環、B環、C環およびD環から選択される少なくとも1つと結合していてもよく、
R1およびR2は、それぞれ独立して、水素、炭素数1~6のアルキル、炭素数3~12のシクロアルキル、炭素数6~12のアリール、炭素数2~15のヘテロアリールまたはジアリールアミノ(ただしアリールは炭素数6~12のアリール)であり、
式(ii)で表される化合物における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
A環、B環およびC環は、それぞれ独立して、アリール環またはヘテロアリール環であり、これらの環における少なくとも1つの水素は置換されていてもよく、
Y1は、B、P、P=O、P=S、Al、Ga、As、Si-RまたはGe-Rであり、前記Si-RおよびGe-RのRはアリールまたはアルキルであり、
X1、X2およびX3は、それぞれ独立して、O、N-R、>CR2、SまたはSeであり、前記N-RのRおよび>CR2のRは置換されていてもよいアリール、置換されていてもよいヘテロアリール、置換されてもよいシクロアルキルまたはアルキルであり、また、前記N-RのRは連結基または単結合により前記A環、B環およびC環から選択される少なくとも1つと結合していてもよく、そして、
式(iii)で表される化合物または構造における少なくとも1つの水素がシアノ、ハロゲンまたは重水素で置換されていてもよい。) - 前記第3成分として、下記式(1)、(2)、(3)および(4)のいずれかで表される化合物を少なくとも1つ含む、請求項1または2に記載の有機電界発光素子。
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10およびR11は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、また、R1~R3、R4~R7およびR8~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
X1およびX2は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1およびX2は、同時に>CR2になることはなく、
そして、
式(1)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13およびR14は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルであり、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、また、R5~R7およびR10~R12のうちの隣接する基同士が結合してb環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環における少なくとも1つの水素は、アリール、ヘテロアリール、ジアリールアミノ、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオまたはアルキル置換シリルで置換されていてもよく、これらにおける少なくとも1つの水素は、アリール、ヘテロアリールまたはアルキルで置換されていてもよく、
YはB(ホウ素)であり、
X1、X2、X3およびX4は、それぞれ独立して、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2のRは、炭素数6~12のアリール、炭素数2~15のヘテロアリール、炭素数3~12のシクロアルキルまたは炭素数1~6のアルキルであり、また、前記>N-RのRおよび>CR2のRは、-O-、-S-、-C(-R)2-または単結合により前記a環、b環、c環およびd環から選択される少なくとも1つと結合していてもよく、前記-C(-R)2-のRは水素または炭素数1~6のアルキルであり、
ただし、X1、X2、X3、およびX4は、同時に>CR2になることはなく、
そして、
式(2)で表される化合物における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R9、R10およびR11は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R6およびR9~R11のうちの隣接する基同士が結合してa環、b環またはc環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよく、
X1、X2およびX3は、それぞれ独立して、>O、>N-R、または>CR2であり、前記>N-RのRおよび>CR2 のRはアリール、ヘテロアリール、シクロアルキルまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、X1、X2、およびX3は、同時に>CR2になることはなく、
そして、
式(3)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。)
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13およびR14は、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、アルキル、シクロアルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、また、R1~R3、R4~R7、R8~R10およびR11~R14のうちの隣接する基同士が結合してa環、b環、c環またはd環と共にアリール環またはヘテロアリール環を形成していてもよく、形成された環はアリール、ヘテロアリール、ジアリールアミノ、アルキル、シクロアルキル、アルコキシおよびアリールオキシから選択される少なくとも1つで置換されていてもよく、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Xは、>O、>N-Rまたは>CR2であり、前記>N-RのRおよび>CR2 のRはアリール、ヘテロアリールまたはアルキルであり、これらはアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
Lは、単結合、>CR2、>O、>Sまたは>N-Rであり、前記>CR2および>N-RにおけるRは、それぞれ独立して、水素、アリール、ヘテロアリール、ジアリールアミノ、アルキル、アルコキシまたはアリールオキシであり、これらはさらにアリール、ヘテロアリールおよびアルキルから選択される少なくとも1つで置換されていてもよく、
ただし、XおよびLは、同時に>CR2になることはなく、
そして、
式(4)で表される化合物および構造における少なくとも1つの水素はシアノ、ハロゲンまたは重水素で置換されていてもよい。) - 前記第3成分として、前記式(1)、(2)および(4)のいずれかで表される化合物を少なくとも1つを含み、
前記式(1)において、X1およびX2が、それぞれ独立して、>Oまたは>N-Rであり、
前記式(2)において、X1、X2、X3およびX4が、それぞれ独立して、>Oまたは>N-Rであり、
前記式(4)において、Xが、>Oおよび>N-Rであり、Lが、単結合である、
請求項3に記載の有機電界発光素子。 - 前記第3成分として、前記式(1)、(2)、(3)および(4)のいずれかで表される化合物を少なくとも1つを含み、その化合物に存在する環を構成する原子が、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルから選択される少なくとも1つで置換されている、請求項3または4に記載の有機電界発光素子。
- 前記第3成分として、前記式(2)で表される化合物を少なくとも1つを含み、その化合物に存在する環を構成する原子が、アリール、ヘテロアリール、ジアリールアミノ、ジアリールボリル、ジヘテロアリールアミノ、アリールヘテロアリールアミノ、アルキル、シクロアルキル、アルコキシ、アリールオキシ、ヘテロアリールオキシ、アリールチオ、ヘテロアリールチオおよびアルキル置換シリルから選択される少なくとも1つで置換され、これらはさらにアリール、ヘテロアリール、シクロアルキルおよびアルキルから選択される少なくとも1つで置換されていてもよい、請求項5に記載の有機電界発光素子。
- 前記式(1)~(4)のいずれかで表される化合物が、以下に記載のいずれかの部分構造を含む、請求項3~6のいずれか一項に記載の有機電界発光素子。
Meはメチルを表し、tBuはt-ブチルを表し、波線は結合位置を表す。
ただし、上記部分構造式における水素は、
それぞれ独立して、アリール、ヘテロアリール、ジアリールアミノ、アルキル、アルコキシまたはアリールオキシで置換されていてもよく、前記アリールにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、前記ヘテロアリールにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよく、前記ジアリールアミノにおける水素はさらにアリール、ヘテロアリールまたはアルキルで置換されていてもよい。) - 前記式(1)~(4)のいずれかで表される化合物が、sp3炭素、ホウ素原子に対してm位またはp位に結合するsp2炭素原子、または、ホウ素に対してp位に置換する窒素原子、をいずれか1つ有する、請求項3~8のいずれか一項に記載の有機電界発光素子。
- 前記第1成分が、部分構造として、カルバゾールおよびフランから選択される少なくとも一つを有する化合物である、請求項1~11のいずれか一項に記載の有機電界発光素子。
- 前記第2成分が、部分構造として、カルバゾール、フェノキサジン、アクリジン、トリアジン、ピリミジン、ピラジン、チオキサンテン、ベンゾニトリル、フタロニトリル、イソフタロニトリル、ジフェニルスルホン、トリアゾール、オキサジアゾール、チアジアゾールおよびベンゾフェノンから選択される少なくとも一つを有する、請求項1~13のいずれか一項に記載の有機電界発光素子。
- 前記第2成分が、下記式(AD1)、(AD2)および(AD3)のいずれかで表される化合物を少なくとも一つ含有する、請求項1~14のいずれか一項に記載の有機電界発光素子。
Mは、それぞれ独立して、単結合、-O-、>N-Arまたは>CAr2であり、
Jは、それぞれ独立して、炭素数6~18のアリーレンであり、前記アリーレンは、フェニル、炭素数1~6のアルキルおよび炭素数3~12のシクロアルキルから選択される少なくとも1つで置換されてもよく、
Qは、それぞれ独立して、=C(-H)-または=N-であり、
Arは、それぞれ独立して、水素、炭素数6~18のアリール、炭素数6~18のヘテロアリール、炭素数1~6のアルキルまたは炭素数3~12のシクロアルキルであり、前記アリールおよびヘテロアリーレンにおける少なくとも1つの水素は、フェニル、炭素数1~6のアルキルまたは炭素数3~12のシクロアルキルで置換されてもよく、
mは、1または2であり、
nは、2~(6-m)の整数であり、
上記各式で表される化合物における少なくとも1つの水素は、ハロゲンまたは重水素で置換されていてもよい。) - 前記第2成分が、下記式(DAD1)で表される化合物を少なくとも一つ含有する、請求項1~14のいずれか一項に記載の有機電界発光素子。
(D1-L1)n-A1 (DAD1)
(上記式(DAD1)中、D1はドナー性基であり、L1は単結合または共役連結基であり、A1はアクセプター性基であり、nは2以上であってA1が置換しうる最大数以下である整数である。) - 前記第2成分が、下記式(DAD2)で表される化合物を少なくとも一つ含有する、請求項16に記載の有機電界発光素子。
D2-L2-A2-L3-D3 (DAD2)
(上記式(DAD2)中、D2およびD3はそれぞれ独立してドナー性基であり、L2およびL3はそれぞれ独立しては単結合または共役連結基であり、A2はアクセプター性基である。) - 前記式(AD1)、(AD2)および(AD3)において、
Mは、それぞれ独立して、単結合、-O-または>N-Arであり、
Jは、それぞれ独立して、フェニレンであり、前記フェニレンは、炭素数1~4のアルキルで置換されてもよく、
Qは、それぞれ独立して、=Nーであり、
Arは、それぞれ独立して、水素またはフェニルであり、前記フェニルは、フェニル、炭素数1~4のアルキルで置換されてもよく、
mは、1または2であり、
nは、4~(6-m)の整数である、
請求項17に記載の有機電界発光素子。 - 前記第2成分の逆項間交差速度が、105s-1以上である、請求項1~18のいずれか一項に記載の有機電界発光素子。
- 前記第3成分の遅延蛍光寿命が、0.05μsec~40μsecである、請求項1~19のいずれか一項に記載の有機電界発光素子。
- 前記第3成分の遅延蛍光寿命が、0.05μsec~20μsecである、請求項1~19のいずれか一項に記載の有機電界発光素子。
- 前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、14nm以下である、請求項1~21のいずれか一項に記載の有機電界発光素子。
- 前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、10nm以下である、請求項1~21のいずれか一項に記載の有機電界発光素子。
- 前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらから求められる一重項三重項エネルギー差(ΔE(2,ST,Sh)およびΔE(3,ST,Sh))が以下の関係にある、請求項1~23のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.50 eV
ΔE(3,ST,Sh)=E(3,S,Sh)ーE(3,T,Sh)≦ 0.20 eV - 前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらから求められる一重項三重項エネルギー差(ΔE(2,ST,Sh)およびΔE(3,ST,Sh))が以下の関係にある、請求項1~24のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)
ΔE(3,ST,Sh)=E(3,S,Sh)ーE(3,T,Sh)
ΔE(2,ST,Sh)≧ ΔE(3,ST,Sh) - 前記第2成分の一重項三重項エネルギー差(ΔE(2,ST,Sh))が以下の関係にある、請求項1~25のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.30 eV - 前記第2成分の一重項三重項エネルギー差(ΔE(2,ST,Sh))が以下の関係にある、請求項1~26のいずれか一項に記載の有機電界発光素子。
ΔE(2,ST,Sh)=E(2,S,Sh)ーE(2,T,Sh)≦ 0.15 eV - 前記第2成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(2,S,Sh)、前記第2成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(2,T,Sh)、前記第3成分の蛍光スペクトルのピーク短波長側の肩より求められる励起一重項エネルギー準位をE(3,S,Sh)、前記第3成分の燐光スペクトルのピーク短波長側の肩より求められる励起三重項エネルギー準位をE(3,T,Sh)としたとき、これらが以下の関係にある、請求項1~27のいずれか一項に記載の有機電界発光素子。
E(2,S,Sh)≧E(3,S,Sh)
E(2,T,Sh)≦E(3,T,Sh) - 前記第3成分の蛍光スペクトルのピークトップおよび吸収スペクトルのピークトップの差より求められるストークスシフトが、10nm以下である、請求項1~28のいずれか一項に記載の有機電界発光素子。
- 前記第3成分が、前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物として含まれている、請求項1~29のいずれか一項に記載の有機電界発光素子。
- 前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物が、前記ホスト化合物の水素原子2個が脱離した基も繰り返し単位として有する、請求項30に記載の有機電界発光素子。
- 前記ホウ素原子を有する化合物の水素原子2個が脱離した基を繰り返し単位とする高分子化合物が、前記遅延蛍光体の水素原子2個が脱離した基も繰り返し単位として有する、請求項30または31に記載の有機電界発光素子。
- 請求項1~32のいずれか一項に記載の有機電界発光素子を備えた表示装置。
- 請求項1~32のいずれか1項に記載の有機電界発光素子を備えた照明装置。
- 前記第4成分における少なくとも1種の有機溶媒の沸点が130℃~350℃である、請求項35に記載の発光層形成用組成物。
- 前記第4成分が、前記第1成分、前記第2成分、および前記第3成分である化合物の少なくとも1種に対する良溶媒(GS)と貧溶媒(PS)とを含み、前記良溶媒(GS)の沸点(BPGS)が前記貧溶媒(PS)の沸点(BPPS)よりも低い、請求項35または36に記載の発光層形成用組成物。
- 前記第1成分が発光層形成用組成物の全質量に対して0.0998質量%~4.0質量%であり、
前記第2成分が発光層形成用組成物の全質量に対して0.0001質量%~2.0質量%であり、
前記第3成分が発光層形成用組成物の全質量に対して0.0001質量%~2.0質量%であり、
前記第4成分が発光層形成用組成物の全質量に対して90.0質量%~99.9質量%である、
請求項35~37のいずれか一項に記載の発光層形成用組成物。 - 請求項35~38のいずれか一項に記載の発光層形成用組成物を用いて形成される発光層を有する有機電界発光素子。
- ホウ素原子を有する化合物から水素原子2個を脱離した構造を含む繰り返し単位、熱活性化型遅延蛍光体から水素原子2個を脱離した構造を含む繰り返し単位、およびホスト化合物から水素原子2個を脱離した構造を含む繰り返し単位から選択される少なくとも2種の繰り返し単位を含む高分子化合物。
- ホウ素原子を有する化合物から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種、熱活性化型遅延蛍光体から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種、およびホスト化合物から水素原子2個を脱離した構造を含む繰り返し単位の少なくとも1種を含む高分子化合物。
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020538493A JP7388658B2 (ja) | 2018-08-23 | 2019-08-23 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
| KR1020217008660A KR20210050537A (ko) | 2018-08-23 | 2019-08-23 | 유기전계 발광소자, 표시장치, 조명장치, 발광층형성용 조성물, 및 화합물 |
| CN201980066048.3A CN113169285A (zh) | 2018-08-23 | 2019-08-23 | 有机电致发光元件、显示装置、照明装置、发光层形成用组合物和化合物 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018156720 | 2018-08-23 | ||
| JP2018-156720 | 2018-08-23 | ||
| JP2018215107 | 2018-11-15 | ||
| JP2018-215107 | 2018-11-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020040298A1 true WO2020040298A1 (ja) | 2020-02-27 |
Family
ID=69593198
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/033069 WO2020040298A1 (ja) | 2018-08-23 | 2019-08-23 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP7388658B2 (ja) |
| KR (1) | KR20210050537A (ja) |
| CN (1) | CN113169285A (ja) |
| WO (1) | WO2020040298A1 (ja) |
Cited By (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020120096A (ja) * | 2019-01-22 | 2020-08-06 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機発光素子、及びそれを含む表示装置 |
| JP2020167391A (ja) * | 2019-03-29 | 2020-10-08 | 住友化学株式会社 | 発光素子及び発光素子用組成物 |
| WO2020218558A1 (ja) * | 2019-04-26 | 2020-10-29 | 学校法人関西学院 | 化合物、有機デバイス用材料、発光層形成用組成物、有機電界効果トランジスタ、有機薄膜太陽電池、有機電界発光素子、表示装置、および照明装置 |
| US20210273175A1 (en) * | 2020-02-19 | 2021-09-02 | Samsung Display Co., Ltd. | Organic electroluminescence device and polycyclic compound for organic electroluminescence device |
| WO2021200252A1 (ja) | 2020-03-31 | 2021-10-07 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子 |
| WO2021230658A1 (ko) * | 2020-05-12 | 2021-11-18 | 머티어리얼사이언스 주식회사 | 유기 전계 발광 소자 |
| KR20210138512A (ko) * | 2020-05-12 | 2021-11-19 | 머티어리얼사이언스 주식회사 | 유기 전계 발광 소자 |
| WO2022024664A1 (ja) * | 2020-07-28 | 2022-02-03 | 住友化学株式会社 | 組成物及び発光素子 |
| WO2022045272A1 (ja) | 2020-08-28 | 2022-03-03 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子 |
| CN114256429A (zh) * | 2020-09-25 | 2022-03-29 | 江苏三月科技股份有限公司 | 一种敏化荧光有机电致发光器件及其应用 |
| KR20220046465A (ko) * | 2020-10-06 | 2022-04-14 | 삼성디스플레이 주식회사 | 발광 소자 |
| WO2022084505A1 (en) * | 2020-10-23 | 2022-04-28 | Cynora Gmbh | Organic molecules for optoelectronic devices |
| CN114464747A (zh) * | 2020-11-10 | 2022-05-10 | 乐金显示有限公司 | 有机发光二极管和包括其的有机发光装置 |
| WO2022138790A1 (ja) * | 2020-12-24 | 2022-06-30 | 三菱ケミカル株式会社 | 有機電界発光素子の発光層形成用組成物、有機電界発光素子、有機el表示装置及び有機el照明 |
| WO2022138822A1 (ja) * | 2020-12-23 | 2022-06-30 | 三菱ケミカル株式会社 | 有機電界発光素子、有機el表示装置、有機el照明及び有機電界発光素子の製造方法 |
| CN114685359A (zh) * | 2020-12-30 | 2022-07-01 | 北京鼎材科技有限公司 | 一种化合物及其应用、包含其的有机电致发光器件 |
| JP2022104798A (ja) * | 2020-12-29 | 2022-07-11 | エルジー ディスプレイ カンパニー リミテッド | 有機発光ダイオードおよび有機発光装置 |
| US11472820B2 (en) | 2019-05-08 | 2022-10-18 | Samsung Display Co., Ltd. | Organic molecules for optoelectronic devices |
| WO2022249750A1 (ja) * | 2021-05-28 | 2022-12-01 | 株式会社Kyulux | トップエミッション方式の有機エレクトロルミネッセンス素子およびその設計方法 |
| WO2022270113A1 (ja) * | 2021-06-23 | 2022-12-29 | 株式会社Kyulux | 有機エレクトロルミネッセンス素子 |
| JP2023003372A (ja) * | 2021-06-23 | 2023-01-11 | 株式会社Kyulux | 化合物、発光材料および有機発光素子 |
| WO2023282224A1 (ja) * | 2021-07-06 | 2023-01-12 | 株式会社Kyulux | 有機発光素子およびその設計方法 |
| KR20230065958A (ko) * | 2020-10-06 | 2023-05-12 | 삼성디스플레이 주식회사 | 발광 소자 |
| WO2023094936A1 (ja) * | 2021-11-26 | 2023-06-01 | 株式会社半導体エネルギー研究所 | 発光デバイス、発光装置、有機化合物、電子機器、および照明装置 |
| CN116218283A (zh) * | 2023-04-13 | 2023-06-06 | 义乌清越光电技术研究院有限公司 | 一种用于tfb空穴传输层的量子点墨水及其应用 |
| KR20230093426A (ko) | 2020-10-26 | 2023-06-27 | 미쯔비시 케미컬 주식회사 | 유기 전계 발광 소자, 유기 el 표시 장치 및 유기 el 조명 |
| KR20240035434A (ko) | 2021-07-16 | 2024-03-15 | 고쿠리쓰다이가쿠호진 규슈다이가쿠 | 붕소 함유 화합물, 발광 재료 및 그것을 사용한 발광 소자 |
| WO2024101071A1 (ja) * | 2022-11-11 | 2024-05-16 | 住友化学株式会社 | 組成物及びそれを含有する発光素子、並びに化合物 |
| WO2024143995A1 (ko) * | 2022-12-28 | 2024-07-04 | 덕산네오룩스 주식회사 | 유기전기 소자용 화합물을 이용한 유기전기소자 및 그 전자 장치 |
| US12146087B2 (en) | 2017-12-28 | 2024-11-19 | Idemitsu Kosan Co., Ltd. | Compound and organic electroluminescence device |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020203875A (ja) * | 2019-06-13 | 2020-12-24 | 学校法人関西学院 | 多環芳香族化合物 |
| WO2023224400A1 (ko) * | 2022-05-20 | 2023-11-23 | 경상국립대학교산학협력단 | 신규한 헤테로고리 화합물 및 이를 이용한 유기 발광 소자 |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016006033A (ja) * | 2014-04-23 | 2016-01-14 | シャープ株式会社 | 化合物、標識剤、太陽電池モジュール、太陽光発電装置、有機薄膜太陽電池、表示装置及び有機el素子 |
| JP2016122672A (ja) * | 2013-03-18 | 2016-07-07 | 出光興産株式会社 | 発光装置 |
| WO2017146192A1 (ja) * | 2016-02-24 | 2017-08-31 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子、及び電子機器 |
| US20180123049A1 (en) * | 2015-03-27 | 2018-05-03 | Industry-Academic Cooperation Foundation, Dankook University | Ortho-substituted thermally activated delayed fluorescence material and organic light-emitting device comprising same |
| WO2018181188A1 (ja) * | 2017-03-31 | 2018-10-04 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子および電子機器 |
| WO2018203666A1 (ko) * | 2017-05-02 | 2018-11-08 | 주식회사 엘지화학 | 신규한 화합물 및 이를 이용한 유기발광 소자 |
| WO2019004247A1 (ja) * | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | 発光素子及びその製造に有用な高分子化合物 |
| WO2019004248A1 (ja) * | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | 高分子化合物及びそれを用いた発光素子 |
| WO2019062685A1 (zh) * | 2017-09-29 | 2019-04-04 | 江苏三月光电科技有限公司 | 一种含硼有机电致发光器件及其制备方法 |
| KR20190078541A (ko) * | 2017-12-26 | 2019-07-04 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| WO2019132506A1 (ko) * | 2017-12-26 | 2019-07-04 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| WO2019151204A1 (ja) * | 2018-02-05 | 2019-08-08 | 学校法人関西学院 | 多環芳香族化合物の発光材料を用いた有機電界発光素子 |
| WO2019163808A1 (ja) * | 2018-02-20 | 2019-08-29 | 国立大学法人九州大学 | 発光性粒子の製造方法、発光性粒子およびバイオイメージング材料 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI636056B (zh) * | 2014-02-18 | 2018-09-21 | 學校法人關西學院 | 多環芳香族化合物及其製造方法、有機元件用材料及其應用 |
| WO2015136880A1 (ja) * | 2014-03-11 | 2015-09-17 | 保土谷化学工業株式会社 | アザフルオレン環構造を有するスピロ化合物、発光材料および有機エレクトロルミネッセンス素子 |
| US10680186B2 (en) * | 2015-03-09 | 2020-06-09 | Kwansei Gakuin Educational Foundation | Polycyclic aromatic compound and light emitting layer-forming composition |
| US20200190115A1 (en) * | 2017-05-16 | 2020-06-18 | Kwansei Gakuin Educational Foundation | Polycyclic aromatic compound |
-
2019
- 2019-08-23 CN CN201980066048.3A patent/CN113169285A/zh active Pending
- 2019-08-23 KR KR1020217008660A patent/KR20210050537A/ko not_active Application Discontinuation
- 2019-08-23 WO PCT/JP2019/033069 patent/WO2020040298A1/ja active Application Filing
- 2019-08-23 JP JP2020538493A patent/JP7388658B2/ja active Active
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016122672A (ja) * | 2013-03-18 | 2016-07-07 | 出光興産株式会社 | 発光装置 |
| JP2016006033A (ja) * | 2014-04-23 | 2016-01-14 | シャープ株式会社 | 化合物、標識剤、太陽電池モジュール、太陽光発電装置、有機薄膜太陽電池、表示装置及び有機el素子 |
| US20180123049A1 (en) * | 2015-03-27 | 2018-05-03 | Industry-Academic Cooperation Foundation, Dankook University | Ortho-substituted thermally activated delayed fluorescence material and organic light-emitting device comprising same |
| WO2017146192A1 (ja) * | 2016-02-24 | 2017-08-31 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子、及び電子機器 |
| WO2018181188A1 (ja) * | 2017-03-31 | 2018-10-04 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子および電子機器 |
| WO2018203666A1 (ko) * | 2017-05-02 | 2018-11-08 | 주식회사 엘지화학 | 신규한 화합물 및 이를 이용한 유기발광 소자 |
| WO2019004247A1 (ja) * | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | 発光素子及びその製造に有用な高分子化合物 |
| WO2019004248A1 (ja) * | 2017-06-30 | 2019-01-03 | 住友化学株式会社 | 高分子化合物及びそれを用いた発光素子 |
| WO2019062685A1 (zh) * | 2017-09-29 | 2019-04-04 | 江苏三月光电科技有限公司 | 一种含硼有机电致发光器件及其制备方法 |
| KR20190078541A (ko) * | 2017-12-26 | 2019-07-04 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| WO2019132506A1 (ko) * | 2017-12-26 | 2019-07-04 | 주식회사 엘지화학 | 화합물 및 이를 포함하는 유기 발광 소자 |
| WO2019151204A1 (ja) * | 2018-02-05 | 2019-08-08 | 学校法人関西学院 | 多環芳香族化合物の発光材料を用いた有機電界発光素子 |
| WO2019163808A1 (ja) * | 2018-02-20 | 2019-08-29 | 国立大学法人九州大学 | 発光性粒子の製造方法、発光性粒子およびバイオイメージング材料 |
Non-Patent Citations (2)
| Title |
|---|
| HAN, SI-HYUN ET AL.: "Ideal blue thermally activated delayed fluorescence emission assisted by a thermally activated delayed fluorescence assistant dopant through a fast reverse intersystem crossing mediated cascade energy transfer process", JOURNAL OF MATERIALS CHEMISTRY C, vol. 7, 14 March 2019 (2019-03-14), pages 3082 - 3089, XP055687954 * |
| HOSOKAI, TAKUYA ET AL.: "Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states", SCIENCE ADVANCES, vol. 3, 10 May 2017 (2017-05-10), pages e1603282, XP055499754, DOI: 10.1126/sciadv.1603282 * |
Cited By (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12146087B2 (en) | 2017-12-28 | 2024-11-19 | Idemitsu Kosan Co., Ltd. | Compound and organic electroluminescence device |
| JP7250589B2 (ja) | 2019-01-22 | 2023-04-03 | 三星ディスプレイ株式會社 | 有機発光素子、及びそれを含む表示装置 |
| JP2020120096A (ja) * | 2019-01-22 | 2020-08-06 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | 有機発光素子、及びそれを含む表示装置 |
| JP2020167391A (ja) * | 2019-03-29 | 2020-10-08 | 住友化学株式会社 | 発光素子及び発光素子用組成物 |
| WO2020203211A1 (ja) * | 2019-03-29 | 2020-10-08 | 住友化学株式会社 | 発光素子及び発光素子用組成物 |
| WO2020218558A1 (ja) * | 2019-04-26 | 2020-10-29 | 学校法人関西学院 | 化合物、有機デバイス用材料、発光層形成用組成物、有機電界効果トランジスタ、有機薄膜太陽電池、有機電界発光素子、表示装置、および照明装置 |
| CN113784972A (zh) * | 2019-04-26 | 2021-12-10 | 学校法人关西学院 | 化合物、有机设备用材料、发光层形成用组合物、有机场效应晶体管、有机薄膜太阳能电池、有机电致发光元件、显示装置和照明装置 |
| US11472820B2 (en) | 2019-05-08 | 2022-10-18 | Samsung Display Co., Ltd. | Organic molecules for optoelectronic devices |
| US20210273175A1 (en) * | 2020-02-19 | 2021-09-02 | Samsung Display Co., Ltd. | Organic electroluminescence device and polycyclic compound for organic electroluminescence device |
| US11800793B2 (en) * | 2020-02-19 | 2023-10-24 | Samsung Display Co., Ltd. | Organic electroluminescence device and polycyclic compound for organic electroluminescence device |
| WO2021200252A1 (ja) | 2020-03-31 | 2021-10-07 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子 |
| KR20220160573A (ko) | 2020-03-31 | 2022-12-06 | 닛테츠 케미컬 앤드 머티리얼 가부시키가이샤 | 유기 전계 발광 소자 |
| WO2021230658A1 (ko) * | 2020-05-12 | 2021-11-18 | 머티어리얼사이언스 주식회사 | 유기 전계 발광 소자 |
| KR20210138512A (ko) * | 2020-05-12 | 2021-11-19 | 머티어리얼사이언스 주식회사 | 유기 전계 발광 소자 |
| KR102550442B1 (ko) * | 2020-05-12 | 2023-07-03 | 머티어리얼사이언스 주식회사 | 유기 전계 발광 소자 |
| WO2022024664A1 (ja) * | 2020-07-28 | 2022-02-03 | 住友化学株式会社 | 組成物及び発光素子 |
| EP4190879A4 (en) * | 2020-07-28 | 2024-08-21 | Sumitomo Chemical Co | COMPOSITION AND ELECTROLUMINESCENT ELEMENT |
| KR20230057337A (ko) | 2020-08-28 | 2023-04-28 | 닛테츠 케미컬 앤드 머티리얼 가부시키가이샤 | 유기 전계 발광 소자 |
| WO2022045272A1 (ja) | 2020-08-28 | 2022-03-03 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子 |
| CN114256429B (zh) * | 2020-09-25 | 2024-06-07 | 江苏三月科技股份有限公司 | 一种敏化荧光有机电致发光器件及其应用 |
| CN114256429A (zh) * | 2020-09-25 | 2022-03-29 | 江苏三月科技股份有限公司 | 一种敏化荧光有机电致发光器件及其应用 |
| KR102689550B1 (ko) * | 2020-10-06 | 2024-07-31 | 삼성디스플레이 주식회사 | 발광 소자 |
| KR20230065958A (ko) * | 2020-10-06 | 2023-05-12 | 삼성디스플레이 주식회사 | 발광 소자 |
| KR102529651B1 (ko) * | 2020-10-06 | 2023-05-10 | 삼성디스플레이 주식회사 | 발광 소자 |
| KR20220046465A (ko) * | 2020-10-06 | 2022-04-14 | 삼성디스플레이 주식회사 | 발광 소자 |
| WO2022084505A1 (en) * | 2020-10-23 | 2022-04-28 | Cynora Gmbh | Organic molecules for optoelectronic devices |
| KR20230093426A (ko) | 2020-10-26 | 2023-06-27 | 미쯔비시 케미컬 주식회사 | 유기 전계 발광 소자, 유기 el 표시 장치 및 유기 el 조명 |
| JP7246453B2 (ja) | 2020-11-10 | 2023-03-27 | エルジー ディスプレイ カンパニー リミテッド | 有機発光ダイオードおよび有機発光装置 |
| CN114464747A (zh) * | 2020-11-10 | 2022-05-10 | 乐金显示有限公司 | 有机发光二极管和包括其的有机发光装置 |
| EP3995500A1 (en) * | 2020-11-10 | 2022-05-11 | LG Display Co., Ltd. | Organic light emitting diode and organic light emitting device including the same |
| JP2022077011A (ja) * | 2020-11-10 | 2022-05-20 | エルジー ディスプレイ カンパニー リミテッド | 有機発光ダイオードおよび有機発光装置 |
| CN114464747B (zh) * | 2020-11-10 | 2024-04-09 | 乐金显示有限公司 | 有机发光二极管和包括其的有机发光装置 |
| WO2022138822A1 (ja) * | 2020-12-23 | 2022-06-30 | 三菱ケミカル株式会社 | 有機電界発光素子、有機el表示装置、有機el照明及び有機電界発光素子の製造方法 |
| WO2022138790A1 (ja) * | 2020-12-24 | 2022-06-30 | 三菱ケミカル株式会社 | 有機電界発光素子の発光層形成用組成物、有機電界発光素子、有機el表示装置及び有機el照明 |
| JP7288033B2 (ja) | 2020-12-29 | 2023-06-06 | エルジー ディスプレイ カンパニー リミテッド | 有機発光ダイオードおよび有機発光装置 |
| JP2022104798A (ja) * | 2020-12-29 | 2022-07-11 | エルジー ディスプレイ カンパニー リミテッド | 有機発光ダイオードおよび有機発光装置 |
| CN114685359A (zh) * | 2020-12-30 | 2022-07-01 | 北京鼎材科技有限公司 | 一种化合物及其应用、包含其的有机电致发光器件 |
| WO2022249750A1 (ja) * | 2021-05-28 | 2022-12-01 | 株式会社Kyulux | トップエミッション方式の有機エレクトロルミネッセンス素子およびその設計方法 |
| JP7222159B2 (ja) | 2021-06-23 | 2023-02-15 | 株式会社Kyulux | 化合物、発光材料および有機発光素子 |
| WO2022270113A1 (ja) * | 2021-06-23 | 2022-12-29 | 株式会社Kyulux | 有機エレクトロルミネッセンス素子 |
| JP2023003372A (ja) * | 2021-06-23 | 2023-01-11 | 株式会社Kyulux | 化合物、発光材料および有機発光素子 |
| WO2023282224A1 (ja) * | 2021-07-06 | 2023-01-12 | 株式会社Kyulux | 有機発光素子およびその設計方法 |
| KR20240035434A (ko) | 2021-07-16 | 2024-03-15 | 고쿠리쓰다이가쿠호진 규슈다이가쿠 | 붕소 함유 화합물, 발광 재료 및 그것을 사용한 발광 소자 |
| WO2023094936A1 (ja) * | 2021-11-26 | 2023-06-01 | 株式会社半導体エネルギー研究所 | 発光デバイス、発光装置、有機化合物、電子機器、および照明装置 |
| WO2024101071A1 (ja) * | 2022-11-11 | 2024-05-16 | 住友化学株式会社 | 組成物及びそれを含有する発光素子、並びに化合物 |
| WO2024143995A1 (ko) * | 2022-12-28 | 2024-07-04 | 덕산네오룩스 주식회사 | 유기전기 소자용 화합물을 이용한 유기전기소자 및 그 전자 장치 |
| CN116218283A (zh) * | 2023-04-13 | 2023-06-06 | 义乌清越光电技术研究院有限公司 | 一种用于tfb空穴传输层的量子点墨水及其应用 |
| CN116218283B (zh) * | 2023-04-13 | 2024-04-05 | 义乌清越光电技术研究院有限公司 | 一种用于tfb空穴传输层的量子点墨水及其应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2020040298A1 (ja) | 2021-09-24 |
| KR20210050537A (ko) | 2021-05-07 |
| JP7388658B2 (ja) | 2023-11-29 |
| CN113169285A (zh) | 2021-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7388658B2 (ja) | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 | |
| US20240360158A1 (en) | Polycyclic aromatic compound | |
| KR102714692B1 (ko) | 다환 방향족 화합물 및 그의 다량체 | |
| JP7531159B2 (ja) | 多環芳香族化合物 | |
| KR102595325B1 (ko) | 다환 방향족 화합물 및 발광층 형성용 조성물 | |
| WO2020045681A1 (ja) | 多環芳香族化合物の発光材料を用いた有機電界発光素子 | |
| JPWO2019235452A1 (ja) | ターシャリーアルキル置換多環芳香族化合物 | |
| KR20210092256A (ko) | 유기 전계 발광 소자, 표시 장치, 및 조명 장치 | |
| WO2020080528A1 (ja) | 多環芳香族化合物 | |
| KR20200143653A (ko) | 다환 방향족 화합물 | |
| KR20220004116A (ko) | 화합물, 유기 디바이스용 재료, 발광층 형성용 조성물, 유기 전계효과 트랜지스터, 유기 박막 태양전지, 유기 전계 발광 소자, 표시 장치, 및 조명 장치 | |
| JP2022074041A (ja) | 多環芳香族化合物 | |
| JP2021077890A (ja) | 有機電界発光素子、表示装置、および照明装置、ならびに発光層形成用組成物 | |
| KR20210043466A (ko) | 다환 방향족 화합물, 유기 디바이스용 재료, 유기전계 발광소자, 표시 장치 및 조명 장치 | |
| JP2021077889A (ja) | 有機電界発光素子、表示装置、および照明装置、ならびに発光層形成用組成物 | |
| WO2022185896A1 (ja) | 多環芳香族化合物および有機電界発光素子 | |
| WO2022185897A1 (ja) | 多環芳香族化合物 | |
| KR20230141577A (ko) | 유기 전계 발광 소자 | |
| KR20240047307A (ko) | 다환방향족 화합물 | |
| KR20240100291A (ko) | 다환 방향족 화합물 | |
| JP2022179317A (ja) | 多環芳香族化合物 | |
| CN116997557A (zh) | 多环芳香族化合物及有机电致发光元件 | |
| KR20210007909A (ko) | 발광층 형성용 조성물 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19851825 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020538493 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20217008660 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19851825 Country of ref document: EP Kind code of ref document: A1 |