WO2019004248A1 - 高分子化合物及びそれを用いた発光素子 - Google Patents
高分子化合物及びそれを用いた発光素子 Download PDFInfo
- Publication number
- WO2019004248A1 WO2019004248A1 PCT/JP2018/024289 JP2018024289W WO2019004248A1 WO 2019004248 A1 WO2019004248 A1 WO 2019004248A1 JP 2018024289 W JP2018024289 W JP 2018024289W WO 2019004248 A1 WO2019004248 A1 WO 2019004248A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- formula
- substituent
- represented
- groups
- Prior art date
Links
- 229920002521 macromolecule Polymers 0.000 title abstract description 4
- 150000001875 compounds Chemical class 0.000 claims abstract description 266
- 125000000623 heterocyclic group Chemical group 0.000 claims abstract description 130
- 125000003118 aryl group Chemical group 0.000 claims abstract description 97
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 95
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 64
- 239000000470 constituent Substances 0.000 claims abstract description 50
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 claims abstract description 15
- 229910052717 sulfur Inorganic materials 0.000 claims abstract description 14
- 125000004434 sulfur atom Chemical group 0.000 claims abstract description 14
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical group [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims abstract description 9
- 229910052796 boron Inorganic materials 0.000 claims abstract description 9
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical group [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 claims abstract description 5
- 229910052711 selenium Inorganic materials 0.000 claims abstract description 5
- 125000001424 substituent group Chemical group 0.000 claims description 212
- 229920000642 polymer Polymers 0.000 claims description 130
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 86
- 239000000463 material Substances 0.000 claims description 67
- 125000000732 arylene group Chemical group 0.000 claims description 64
- 239000000203 mixture Substances 0.000 claims description 60
- 125000003545 alkoxy group Chemical group 0.000 claims description 41
- 125000000000 cycloalkoxy group Chemical group 0.000 claims description 39
- 239000002904 solvent Substances 0.000 claims description 22
- 125000004432 carbon atom Chemical group C* 0.000 claims description 21
- 238000002347 injection Methods 0.000 claims description 21
- 239000007924 injection Substances 0.000 claims description 21
- 125000002947 alkylene group Chemical group 0.000 claims description 19
- 125000004104 aryloxy group Chemical group 0.000 claims description 17
- 125000003277 amino group Chemical group 0.000 claims description 16
- 125000002993 cycloalkylene group Chemical group 0.000 claims description 15
- 239000012044 organic layer Substances 0.000 claims description 15
- 230000005525 hole transport Effects 0.000 claims description 14
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 12
- 239000003963 antioxidant agent Substances 0.000 claims description 9
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 8
- 230000003078 antioxidant effect Effects 0.000 claims description 7
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical group [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 6
- 125000005647 linker group Chemical group 0.000 claims description 5
- 229910052757 nitrogen Inorganic materials 0.000 claims description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 4
- 229910052698 phosphorus Inorganic materials 0.000 claims description 4
- 125000004437 phosphorous atom Chemical group 0.000 claims description 3
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical group [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 claims description 2
- 229910052785 arsenic Inorganic materials 0.000 claims description 2
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical group [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 claims description 2
- 229910052733 gallium Inorganic materials 0.000 claims description 2
- 125000001072 heteroaryl group Chemical group 0.000 claims description 2
- 229910052760 oxygen Inorganic materials 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 abstract description 15
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 124
- -1 2-ethylhexyl Chemical group 0.000 description 57
- 239000010410 layer Substances 0.000 description 49
- 229910052799 carbon Inorganic materials 0.000 description 44
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 44
- 150000001721 carbon Chemical group 0.000 description 43
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 36
- 230000015572 biosynthetic process Effects 0.000 description 33
- 238000000034 method Methods 0.000 description 33
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 32
- 238000006243 chemical reaction Methods 0.000 description 31
- 238000003786 synthesis reaction Methods 0.000 description 31
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 30
- 239000000243 solution Substances 0.000 description 28
- 239000003921 oil Substances 0.000 description 23
- 238000004128 high performance liquid chromatography Methods 0.000 description 22
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- 0 CC(C)(N(*)*)[Al] Chemical compound CC(C)(N(*)*)[Al] 0.000 description 21
- 239000000741 silica gel Substances 0.000 description 19
- 229910002027 silica gel Inorganic materials 0.000 description 19
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 18
- 150000004696 coordination complex Chemical class 0.000 description 17
- 229940125782 compound 2 Drugs 0.000 description 16
- 239000000706 filtrate Substances 0.000 description 16
- 239000012046 mixed solvent Substances 0.000 description 16
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 15
- 229910001873 dinitrogen Inorganic materials 0.000 description 15
- 239000007787 solid Substances 0.000 description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 14
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 14
- 239000000843 powder Substances 0.000 description 14
- 239000002994 raw material Substances 0.000 description 14
- 238000010898 silica gel chromatography Methods 0.000 description 14
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 13
- 239000012043 crude product Substances 0.000 description 13
- 125000005843 halogen group Chemical group 0.000 description 13
- 239000000758 substrate Substances 0.000 description 13
- 239000000178 monomer Substances 0.000 description 11
- 238000012643 polycondensation polymerization Methods 0.000 description 11
- 239000002244 precipitate Substances 0.000 description 11
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 10
- 229940125904 compound 1 Drugs 0.000 description 10
- 229940126214 compound 3 Drugs 0.000 description 10
- 125000004093 cyano group Chemical group *C#N 0.000 description 10
- 229910052763 palladium Inorganic materials 0.000 description 10
- 150000003384 small molecules Chemical class 0.000 description 10
- PUZPDOWCWNUUKD-UHFFFAOYSA-M sodium fluoride Chemical compound [F-].[Na+] PUZPDOWCWNUUKD-UHFFFAOYSA-M 0.000 description 10
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 10
- 239000008096 xylene Substances 0.000 description 10
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 9
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 9
- 229910052786 argon Inorganic materials 0.000 description 9
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 8
- 150000004945 aromatic hydrocarbons Chemical group 0.000 description 8
- 239000003054 catalyst Substances 0.000 description 8
- 229940125898 compound 5 Drugs 0.000 description 8
- 229910052751 metal Inorganic materials 0.000 description 8
- 239000002184 metal Substances 0.000 description 8
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 8
- 238000005160 1H NMR spectroscopy Methods 0.000 description 7
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 7
- 238000001914 filtration Methods 0.000 description 7
- 239000007789 gas Substances 0.000 description 7
- 150000002500 ions Chemical class 0.000 description 7
- 150000002605 large molecules Chemical class 0.000 description 7
- 238000004528 spin coating Methods 0.000 description 7
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- 125000004429 atom Chemical group 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- 238000000295 emission spectrum Methods 0.000 description 6
- 238000011156 evaluation Methods 0.000 description 6
- 238000010438 heat treatment Methods 0.000 description 6
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 6
- 239000011572 manganese Substances 0.000 description 6
- 239000003444 phase transfer catalyst Substances 0.000 description 6
- 238000006862 quantum yield reaction Methods 0.000 description 6
- 229940073455 tetraethylammonium hydroxide Drugs 0.000 description 6
- LRGJRHZIDJQFCL-UHFFFAOYSA-M tetraethylazanium;hydroxide Chemical compound [OH-].CC[N+](CC)(CC)CC LRGJRHZIDJQFCL-UHFFFAOYSA-M 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- 125000003342 alkenyl group Chemical group 0.000 description 5
- 125000000304 alkynyl group Chemical group 0.000 description 5
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 5
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 5
- 238000000576 coating method Methods 0.000 description 5
- 238000013329 compounding Methods 0.000 description 5
- 229920001940 conductive polymer Polymers 0.000 description 5
- 238000001816 cooling Methods 0.000 description 5
- 229910052731 fluorine Inorganic materials 0.000 description 5
- 125000001153 fluoro group Chemical group F* 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- 125000005842 heteroatom Chemical group 0.000 description 5
- 239000011261 inert gas Substances 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 238000007639 printing Methods 0.000 description 5
- 239000011775 sodium fluoride Substances 0.000 description 5
- 235000013024 sodium fluoride Nutrition 0.000 description 5
- HBENZIXOGRCSQN-VQWWACLZSA-N (1S,2S,6R,14R,15R,16R)-5-(cyclopropylmethyl)-16-[(2S)-2-hydroxy-3,3-dimethylpentan-2-yl]-15-methoxy-13-oxa-5-azahexacyclo[13.2.2.12,8.01,6.02,14.012,20]icosa-8(20),9,11-trien-11-ol Chemical compound N1([C@@H]2CC=3C4=C(C(=CC=3)O)O[C@H]3[C@@]5(OC)CC[C@@]2([C@@]43CC1)C[C@@H]5[C@](C)(O)C(C)(C)CC)CC1CC1 HBENZIXOGRCSQN-VQWWACLZSA-N 0.000 description 4
- PHDIJLFSKNMCMI-ITGJKDDRSA-N (3R,4S,5R,6R)-6-(hydroxymethyl)-4-(8-quinolin-6-yloxyoctoxy)oxane-2,3,5-triol Chemical compound OC[C@@H]1[C@H]([C@@H]([C@H](C(O1)O)O)OCCCCCCCCOC=1C=C2C=CC=NC2=CC=1)O PHDIJLFSKNMCMI-ITGJKDDRSA-N 0.000 description 4
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 4
- WJFKNYWRSNBZNX-UHFFFAOYSA-N 10H-phenothiazine Chemical compound C1=CC=C2NC3=CC=CC=C3SC2=C1 WJFKNYWRSNBZNX-UHFFFAOYSA-N 0.000 description 4
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 4
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 4
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 4
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 4
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- 235000011114 ammonium hydroxide Nutrition 0.000 description 4
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 4
- XJHCXCQVJFPJIK-UHFFFAOYSA-M caesium fluoride Chemical compound [F-].[Cs+] XJHCXCQVJFPJIK-UHFFFAOYSA-M 0.000 description 4
- 229910052801 chlorine Inorganic materials 0.000 description 4
- 125000001309 chloro group Chemical group Cl* 0.000 description 4
- 239000012230 colorless oil Substances 0.000 description 4
- 229940125773 compound 10 Drugs 0.000 description 4
- TXCDCPKCNAJMEE-UHFFFAOYSA-N dibenzofuran Chemical compound C1=CC=C2C3=CC=CC=C3OC2=C1 TXCDCPKCNAJMEE-UHFFFAOYSA-N 0.000 description 4
- IYYZUPMFVPLQIF-UHFFFAOYSA-N dibenzothiophene Chemical compound C1=CC=C2C3=CC=CC=C3SC2=C1 IYYZUPMFVPLQIF-UHFFFAOYSA-N 0.000 description 4
- 229910052740 iodine Inorganic materials 0.000 description 4
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 4
- GVOISEJVFFIGQE-YCZSINBZSA-N n-[(1r,2s,5r)-5-[methyl(propan-2-yl)amino]-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](N(C)C(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 GVOISEJVFFIGQE-YCZSINBZSA-N 0.000 description 4
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 4
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 4
- 229950000688 phenothiazine Drugs 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- 238000010992 reflux Methods 0.000 description 4
- 238000001542 size-exclusion chromatography Methods 0.000 description 4
- YTZKOQUCBOVLHL-UHFFFAOYSA-N tert-butylbenzene Chemical compound CC(C)(C)C1=CC=CC=C1 YTZKOQUCBOVLHL-UHFFFAOYSA-N 0.000 description 4
- 238000007740 vapor deposition Methods 0.000 description 4
- CXNIUSPIQKWYAI-UHFFFAOYSA-N xantphos Chemical compound C=12OC3=C(P(C=4C=CC=CC=4)C=4C=CC=CC=4)C=CC=C3C(C)(C)C2=CC=CC=1P(C=1C=CC=CC=1)C1=CC=CC=C1 CXNIUSPIQKWYAI-UHFFFAOYSA-N 0.000 description 4
- TZMSYXZUNZXBOL-UHFFFAOYSA-N 10H-phenoxazine Chemical compound C1=CC=C2NC3=CC=CC=C3OC2=C1 TZMSYXZUNZXBOL-UHFFFAOYSA-N 0.000 description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- OPEKHRGERHDLRK-UHFFFAOYSA-N 4-tert-butyl-n-(4-tert-butylphenyl)aniline Chemical compound C1=CC(C(C)(C)C)=CC=C1NC1=CC=C(C(C)(C)C)C=C1 OPEKHRGERHDLRK-UHFFFAOYSA-N 0.000 description 3
- 229910000838 Al alloy Inorganic materials 0.000 description 3
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 3
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- IPWKHHSGDUIRAH-UHFFFAOYSA-N bis(pinacolato)diboron Chemical compound O1C(C)(C)C(C)(C)OB1B1OC(C)(C)C(C)(C)O1 IPWKHHSGDUIRAH-UHFFFAOYSA-N 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052802 copper Inorganic materials 0.000 description 3
- 239000010949 copper Substances 0.000 description 3
- 125000000392 cycloalkenyl group Chemical group 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 238000007641 inkjet printing Methods 0.000 description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 3
- 238000006116 polymerization reaction Methods 0.000 description 3
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 229930192474 thiophene Natural products 0.000 description 3
- 229910052723 transition metal Inorganic materials 0.000 description 3
- 150000003624 transition metals Chemical class 0.000 description 3
- MNKCGUKVRJZKEQ-MIXQCLKLSA-N (1z,5z)-cycloocta-1,5-diene;iridium;methanol Chemical compound [Ir].[Ir].OC.OC.C\1C\C=C/CC\C=C/1.C\1C\C=C/CC\C=C/1 MNKCGUKVRJZKEQ-MIXQCLKLSA-N 0.000 description 2
- UVNPEUJXKZFWSJ-LMTQTHQJSA-N (R)-N-[(4S)-8-[6-amino-5-[(3,3-difluoro-2-oxo-1H-pyrrolo[2,3-b]pyridin-4-yl)sulfanyl]pyrazin-2-yl]-2-oxa-8-azaspiro[4.5]decan-4-yl]-2-methylpropane-2-sulfinamide Chemical compound CC(C)(C)[S@@](=O)N[C@@H]1COCC11CCN(CC1)c1cnc(Sc2ccnc3NC(=O)C(F)(F)c23)c(N)n1 UVNPEUJXKZFWSJ-LMTQTHQJSA-N 0.000 description 2
- UBOXGVDOUJQMTN-UHFFFAOYSA-N 1,1,2-trichloroethane Chemical compound ClCC(Cl)Cl UBOXGVDOUJQMTN-UHFFFAOYSA-N 0.000 description 2
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- LVEYOSJUKRVCCF-UHFFFAOYSA-N 1,3-bis(diphenylphosphino)propane Chemical compound C=1C=CC=CC=1P(C=1C=CC=CC=1)CCCP(C=1C=CC=CC=1)C1=CC=CC=C1 LVEYOSJUKRVCCF-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- 125000001140 1,4-phenylene group Chemical group [H]C1=C([H])C([*:2])=C([H])C([H])=C1[*:1] 0.000 description 2
- CHLICZRVGGXEOD-UHFFFAOYSA-N 1-Methoxy-4-methylbenzene Chemical compound COC1=CC=C(C)C=C1 CHLICZRVGGXEOD-UHFFFAOYSA-N 0.000 description 2
- RMSGQZDGSZOJMU-UHFFFAOYSA-N 1-butyl-2-phenylbenzene Chemical group CCCCC1=CC=CC=C1C1=CC=CC=C1 RMSGQZDGSZOJMU-UHFFFAOYSA-N 0.000 description 2
- KWKAKUADMBZCLK-UHFFFAOYSA-N 1-octene Chemical group CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 description 2
- FGRBYDKOBBBPOI-UHFFFAOYSA-N 10,10-dioxo-2-[4-(N-phenylanilino)phenyl]thioxanthen-9-one Chemical compound O=C1c2ccccc2S(=O)(=O)c2ccc(cc12)-c1ccc(cc1)N(c1ccccc1)c1ccccc1 FGRBYDKOBBBPOI-UHFFFAOYSA-N 0.000 description 2
- OVEMTTZEBOCJDV-UHFFFAOYSA-N 4-hexylaniline Chemical compound CCCCCCC1=CC=C(N)C=C1 OVEMTTZEBOCJDV-UHFFFAOYSA-N 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- 229910001316 Ag alloy Inorganic materials 0.000 description 2
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 2
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical compound C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 2
- QBXVXKRWOVBUDB-GRKNLSHJSA-N ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C Chemical compound ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C QBXVXKRWOVBUDB-GRKNLSHJSA-N 0.000 description 2
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 2
- YNQLUTRBYVCPMQ-UHFFFAOYSA-N Ethylbenzene Chemical compound CCC1=CC=CC=C1 YNQLUTRBYVCPMQ-UHFFFAOYSA-N 0.000 description 2
- LTEQMZWBSYACLV-UHFFFAOYSA-N Hexylbenzene Chemical compound CCCCCCC1=CC=CC=C1 LTEQMZWBSYACLV-UHFFFAOYSA-N 0.000 description 2
- 229910000846 In alloy Inorganic materials 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- NUGPIZCTELGDOS-QHCPKHFHSA-N N-[(1S)-3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-pyridin-3-ylpropyl]cyclopentanecarboxamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CC[C@@H](C=1C=NC=CC=1)NC(=O)C1CCCC1)C NUGPIZCTELGDOS-QHCPKHFHSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical group [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 239000005456 alcohol based solvent Substances 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 150000001450 anions Chemical class 0.000 description 2
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 2
- 150000004982 aromatic amines Chemical group 0.000 description 2
- 238000007611 bar coating method Methods 0.000 description 2
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229910052792 caesium Inorganic materials 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- WCZVZNOTHYJIEI-UHFFFAOYSA-N cinnoline Chemical compound N1=NC=CC2=CC=CC=C21 WCZVZNOTHYJIEI-UHFFFAOYSA-N 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 125000004956 cyclohexylene group Chemical group 0.000 description 2
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 2
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 229910003472 fullerene Inorganic materials 0.000 description 2
- 229910002804 graphite Inorganic materials 0.000 description 2
- 239000010439 graphite Substances 0.000 description 2
- 125000004836 hexamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 2
- 229910052741 iridium Inorganic materials 0.000 description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- PQXKHYXIUOZZFA-UHFFFAOYSA-M lithium fluoride Chemical compound [Li+].[F-] PQXKHYXIUOZZFA-UHFFFAOYSA-M 0.000 description 2
- UBJFKNSINUCEAL-UHFFFAOYSA-N lithium;2-methylpropane Chemical compound [Li+].C[C-](C)C UBJFKNSINUCEAL-UHFFFAOYSA-N 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- AUHZEENZYGFFBQ-UHFFFAOYSA-N mesitylene Substances CC1=CC(C)=CC(C)=C1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 2
- 125000001827 mesitylenyl group Chemical group [H]C1=C(C(*)=C(C([H])=C1C([H])([H])[H])C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 2
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 2
- 239000011259 mixed solution Substances 0.000 description 2
- QAPTWHXHEYAIKG-RCOXNQKVSA-N n-[(1r,2s,5r)-5-(tert-butylamino)-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](NC(C)(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 QAPTWHXHEYAIKG-RCOXNQKVSA-N 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- BKIMMITUMNQMOS-UHFFFAOYSA-N nonane Chemical compound CCCCCCCCC BKIMMITUMNQMOS-UHFFFAOYSA-N 0.000 description 2
- 229940078552 o-xylene Drugs 0.000 description 2
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 2
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical compound C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000011698 potassium fluoride Substances 0.000 description 2
- 235000003270 potassium fluoride Nutrition 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 229910052701 rubidium Inorganic materials 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- 238000004544 sputter deposition Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- NHGXDBSUJJNIRV-UHFFFAOYSA-M tetrabutylammonium chloride Chemical compound [Cl-].CCCC[N+](CCCC)(CCCC)CCCC NHGXDBSUJJNIRV-UHFFFAOYSA-M 0.000 description 2
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 2
- VDZOOKBUILJEDG-UHFFFAOYSA-M tetrabutylammonium hydroxide Chemical compound [OH-].CCCC[N+](CCCC)(CCCC)CCCC VDZOOKBUILJEDG-UHFFFAOYSA-M 0.000 description 2
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 2
- 239000011135 tin Substances 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- COIOYMYWGDAQPM-UHFFFAOYSA-N tris(2-methylphenyl)phosphane Chemical compound CC1=CC=CC=C1P(C=1C(=CC=CC=1)C)C1=CC=CC=C1C COIOYMYWGDAQPM-UHFFFAOYSA-N 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- RRKODOZNUZCUBN-CCAGOZQPSA-N (1z,3z)-cycloocta-1,3-diene Chemical compound C1CC\C=C/C=C\C1 RRKODOZNUZCUBN-CCAGOZQPSA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 1
- UXJHQQLYKUVLIE-UHFFFAOYSA-N 1,2-dihydroacridine Chemical compound C1=CC=C2N=C(C=CCC3)C3=CC2=C1 UXJHQQLYKUVLIE-UHFFFAOYSA-N 0.000 description 1
- 229940015975 1,2-hexanediol Drugs 0.000 description 1
- 125000002030 1,2-phenylene group Chemical group [H]C1=C([H])C([*:1])=C([*:2])C([H])=C1[H] 0.000 description 1
- YWDUZLFWHVQCHY-UHFFFAOYSA-N 1,3,5-tribromobenzene Chemical compound BrC1=CC(Br)=CC(Br)=C1 YWDUZLFWHVQCHY-UHFFFAOYSA-N 0.000 description 1
- KARUMYWDGQTPFL-UHFFFAOYSA-N 1,3-dibromo-5-iodobenzene Chemical compound BrC1=CC(Br)=CC(I)=C1 KARUMYWDGQTPFL-UHFFFAOYSA-N 0.000 description 1
- 125000001989 1,3-phenylene group Chemical group [H]C1=C([H])C([*:1])=C([H])C([*:2])=C1[H] 0.000 description 1
- AZQWKYJCGOJGHM-UHFFFAOYSA-N 1,4-benzoquinone Chemical compound O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 description 1
- CEOHKQQUAYAZNB-UHFFFAOYSA-N 1-bromo-2,3-dichloro-5-methylbenzene Chemical compound CC1=CC(Cl)=C(Cl)C(Br)=C1 CEOHKQQUAYAZNB-UHFFFAOYSA-N 0.000 description 1
- HVKCZUVMQPUWSX-UHFFFAOYSA-N 1-bromo-2,3-dichlorobenzene Chemical compound ClC1=CC=CC(Br)=C1Cl HVKCZUVMQPUWSX-UHFFFAOYSA-N 0.000 description 1
- BUOWTUULDKULFI-UHFFFAOYSA-N 1-bromo-3,5-ditert-butylbenzene Chemical compound CC(C)(C)C1=CC(Br)=CC(C(C)(C)C)=C1 BUOWTUULDKULFI-UHFFFAOYSA-N 0.000 description 1
- CTPUUDQIXKUAMO-UHFFFAOYSA-N 1-bromo-3-iodobenzene Chemical compound BrC1=CC=CC(I)=C1 CTPUUDQIXKUAMO-UHFFFAOYSA-N 0.000 description 1
- 125000006039 1-hexenyl group Chemical group 0.000 description 1
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 1
- 125000005978 1-naphthyloxy group Chemical group 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- DJMUYABFXCIYSC-UHFFFAOYSA-N 1H-phosphole Chemical compound C=1C=CPC=1 DJMUYABFXCIYSC-UHFFFAOYSA-N 0.000 description 1
- FKNIDKXOANSRCS-UHFFFAOYSA-N 2,3,4-trinitrofluoren-1-one Chemical compound C1=CC=C2C3=C([N+](=O)[O-])C([N+]([O-])=O)=C([N+]([O-])=O)C(=O)C3=CC2=C1 FKNIDKXOANSRCS-UHFFFAOYSA-N 0.000 description 1
- VFBJMPNFKOMEEW-UHFFFAOYSA-N 2,3-diphenylbut-2-enedinitrile Chemical group C=1C=CC=CC=1C(C#N)=C(C#N)C1=CC=CC=C1 VFBJMPNFKOMEEW-UHFFFAOYSA-N 0.000 description 1
- IXHWGNYCZPISET-UHFFFAOYSA-N 2-[4-(dicyanomethylidene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene]propanedinitrile Chemical compound FC1=C(F)C(=C(C#N)C#N)C(F)=C(F)C1=C(C#N)C#N IXHWGNYCZPISET-UHFFFAOYSA-N 0.000 description 1
- CFMPIQSLDJXNPD-UHFFFAOYSA-N 2-bromo-5-chloro-1,3-dimethylbenzene Chemical group CC1=CC(Cl)=CC(C)=C1Br CFMPIQSLDJXNPD-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- 125000005979 2-naphthyloxy group Chemical group 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- MJKNHXCPGXUEDO-UHFFFAOYSA-N 3,5-ditert-butylaniline Chemical compound CC(C)(C)C1=CC(N)=CC(C(C)(C)C)=C1 MJKNHXCPGXUEDO-UHFFFAOYSA-N 0.000 description 1
- YDXLVFKTOSKBKT-UHFFFAOYSA-N 3-bromo-n,n-diphenylaniline Chemical compound BrC1=CC=CC(N(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 YDXLVFKTOSKBKT-UHFFFAOYSA-N 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- 125000006201 3-phenylpropyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- CMSGUKVDXXTJDQ-UHFFFAOYSA-N 4-(2-naphthalen-1-ylethylamino)-4-oxobutanoic acid Chemical compound C1=CC=C2C(CCNC(=O)CCC(=O)O)=CC=CC2=C1 CMSGUKVDXXTJDQ-UHFFFAOYSA-N 0.000 description 1
- DDTHMESPCBONDT-UHFFFAOYSA-N 4-(4-oxocyclohexa-2,5-dien-1-ylidene)cyclohexa-2,5-dien-1-one Chemical compound C1=CC(=O)C=CC1=C1C=CC(=O)C=C1 DDTHMESPCBONDT-UHFFFAOYSA-N 0.000 description 1
- 229940077398 4-methyl anisole Drugs 0.000 description 1
- TXNLQUKVUJITMX-UHFFFAOYSA-N 4-tert-butyl-2-(4-tert-butylpyridin-2-yl)pyridine Chemical group CC(C)(C)C1=CC=NC(C=2N=CC=C(C=2)C(C)(C)C)=C1 TXNLQUKVUJITMX-UHFFFAOYSA-N 0.000 description 1
- 125000006043 5-hexenyl group Chemical group 0.000 description 1
- IGDNJMOBPOHHRN-UHFFFAOYSA-N 5h-benzo[b]phosphindole Chemical compound C1=CC=C2C3=CC=CC=C3PC2=C1 IGDNJMOBPOHHRN-UHFFFAOYSA-N 0.000 description 1
- 239000005725 8-Hydroxyquinoline Substances 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- DKGXZFRGARPPLX-UHFFFAOYSA-N Bc(cc1)ccc1N(c1ccc(C)cc1)c(cc1)cc(C2(CCCCCCCC)CCCCCCCC)c1-c(cc1)c2cc1N(c1ccc(C)cc1)c(cc1)ccc1Br Chemical compound Bc(cc1)ccc1N(c1ccc(C)cc1)c(cc1)cc(C2(CCCCCCCC)CCCCCCCC)c1-c(cc1)c2cc1N(c1ccc(C)cc1)c(cc1)ccc1Br DKGXZFRGARPPLX-UHFFFAOYSA-N 0.000 description 1
- KYNSBQPICQTCGU-UHFFFAOYSA-N Benzopyrane Chemical compound C1=CC=C2C=CCOC2=C1 KYNSBQPICQTCGU-UHFFFAOYSA-N 0.000 description 1
- DKPFZGUDAPQIHT-UHFFFAOYSA-N Butyl acetate Natural products CCCCOC(C)=O DKPFZGUDAPQIHT-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 102100033040 Carbonic anhydrase 12 Human genes 0.000 description 1
- 102100033041 Carbonic anhydrase 13 Human genes 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- 101000867855 Homo sapiens Carbonic anhydrase 12 Proteins 0.000 description 1
- 101000867860 Homo sapiens Carbonic anhydrase 13 Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- CRZQGDNQQAALAY-UHFFFAOYSA-N Me ester-Phenylacetic acid Natural products COC(=O)CC1=CC=CC=C1 CRZQGDNQQAALAY-UHFFFAOYSA-N 0.000 description 1
- 229910000861 Mg alloy Inorganic materials 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- LFZAGIJXANFPFN-UHFFFAOYSA-N N-[3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-thiophen-2-ylpropyl]acetamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CCC(C=1SC=CC=1)NC(C)=O)C LFZAGIJXANFPFN-UHFFFAOYSA-N 0.000 description 1
- 229930192627 Naphthoquinone Natural products 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 229920000265 Polyparaphenylene Polymers 0.000 description 1
- 229920000292 Polyquinoline Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- NPYPAHLBTDXSSS-UHFFFAOYSA-N Potassium ion Chemical compound [K+] NPYPAHLBTDXSSS-UHFFFAOYSA-N 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical class C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102100031083 Uteroglobin Human genes 0.000 description 1
- 108090000203 Uteroglobin Proteins 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- ULGYAEQHFNJYML-UHFFFAOYSA-N [AlH3].[Ca] Chemical compound [AlH3].[Ca] ULGYAEQHFNJYML-UHFFFAOYSA-N 0.000 description 1
- GANNOFFDYMSBSZ-UHFFFAOYSA-N [AlH3].[Mg] Chemical compound [AlH3].[Mg] GANNOFFDYMSBSZ-UHFFFAOYSA-N 0.000 description 1
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 1
- JHYLKGDXMUDNEO-UHFFFAOYSA-N [Mg].[In] Chemical compound [Mg].[In] JHYLKGDXMUDNEO-UHFFFAOYSA-N 0.000 description 1
- YLEIFZAVNWDOBM-ZTNXSLBXSA-N ac1l9hc7 Chemical compound C([C@H]12)C[C@@H](C([C@@H](O)CC3)(C)C)[C@@]43C[C@@]14CC[C@@]1(C)[C@@]2(C)C[C@@H]2O[C@]3(O)[C@H](O)C(C)(C)O[C@@H]3[C@@H](C)[C@H]12 YLEIFZAVNWDOBM-ZTNXSLBXSA-N 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- SRVFFFJZQVENJC-IHRRRGAJSA-N aloxistatin Chemical compound CCOC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCCC(C)C SRVFFFJZQVENJC-IHRRRGAJSA-N 0.000 description 1
- 229920005603 alternating copolymer Polymers 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 1
- 150000004056 anthraquinones Chemical class 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- 229910052790 beryllium Inorganic materials 0.000 description 1
- ATBAMAFKBVZNFJ-UHFFFAOYSA-N beryllium atom Chemical compound [Be] ATBAMAFKBVZNFJ-UHFFFAOYSA-N 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- HQABUPZFAYXKJW-UHFFFAOYSA-O butylazanium Chemical compound CCCC[NH3+] HQABUPZFAYXKJW-UHFFFAOYSA-O 0.000 description 1
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- HHNHBFLGXIUXCM-GFCCVEGCSA-N cyclohexylbenzene Chemical compound [CH]1CCCC[C@@H]1C1=CC=CC=C1 HHNHBFLGXIUXCM-GFCCVEGCSA-N 0.000 description 1
- WVIIMZNLDWSIRH-UHFFFAOYSA-N cyclohexylcyclohexane Chemical group C1CCCCC1C1CCCCC1 WVIIMZNLDWSIRH-UHFFFAOYSA-N 0.000 description 1
- 125000004210 cyclohexylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000002933 cyclohexyloxy group Chemical group C1(CCCCC1)O* 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000006612 decyloxy group Chemical group 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 125000004431 deuterium atom Chemical group 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 125000004986 diarylamino group Chemical group 0.000 description 1
- WMKGGPCROCCUDY-PHEQNACWSA-N dibenzylideneacetone Chemical compound C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 WMKGGPCROCCUDY-PHEQNACWSA-N 0.000 description 1
- ZBQUMMFUJLOTQC-UHFFFAOYSA-L dichloronickel;3-diphenylphosphanylpropyl(diphenyl)phosphane Chemical compound Cl[Ni]Cl.C=1C=CC=CC=1P(C=1C=CC=CC=1)CCCP(C=1C=CC=CC=1)C1=CC=CC=C1 ZBQUMMFUJLOTQC-UHFFFAOYSA-L 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000005678 ethenylene group Chemical group [H]C([*:1])=C([H])[*:2] 0.000 description 1
- 239000004210 ether based solvent Substances 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- YLQWCDOCJODRMT-UHFFFAOYSA-N fluoren-9-one Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3C2=C1 YLQWCDOCJODRMT-UHFFFAOYSA-N 0.000 description 1
- RMBPEFMHABBEKP-UHFFFAOYSA-N fluorene Chemical compound C1=CC=C2C3=C[CH]C=CC3=CC2=C1 RMBPEFMHABBEKP-UHFFFAOYSA-N 0.000 description 1
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- 150000002240 furans Chemical class 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229920000578 graft copolymer Polymers 0.000 description 1
- 238000007756 gravure coating Methods 0.000 description 1
- RBTKNAXYKSUFRK-UHFFFAOYSA-N heliogen blue Chemical compound [Cu].[N-]1C2=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=NC([N-]1)=C(C=CC=C3)C3=C1N=C([N-]1)C3=CC=CC=C3C1=N2 RBTKNAXYKSUFRK-UHFFFAOYSA-N 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005446 heptyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- FHKSXSQHXQEMOK-UHFFFAOYSA-N hexane-1,2-diol Chemical compound CCCCC(O)CO FHKSXSQHXQEMOK-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003707 hexyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000005980 hexynyl group Chemical group 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- LHJOPRPDWDXEIY-UHFFFAOYSA-N indium lithium Chemical compound [Li].[In] LHJOPRPDWDXEIY-UHFFFAOYSA-N 0.000 description 1
- 229910003437 indium oxide Inorganic materials 0.000 description 1
- YZASAXHKAQYPEH-UHFFFAOYSA-N indium silver Chemical compound [Ag].[In] YZASAXHKAQYPEH-UHFFFAOYSA-N 0.000 description 1
- PJXISJQVUVHSOJ-UHFFFAOYSA-N indium(iii) oxide Chemical compound [O-2].[O-2].[O-2].[In+3].[In+3] PJXISJQVUVHSOJ-UHFFFAOYSA-N 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- VVVPGLRKXQSQSZ-UHFFFAOYSA-N indolo[3,2-c]carbazole Chemical compound C1=CC=CC2=NC3=C4C5=CC=CC=C5N=C4C=CC3=C21 VVVPGLRKXQSQSZ-UHFFFAOYSA-N 0.000 description 1
- 229960005544 indolocarbazole Drugs 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229940030980 inova Drugs 0.000 description 1
- 230000002687 intercalation Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 150000002503 iridium Chemical class 0.000 description 1
- 125000002510 isobutoxy group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])O* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 239000005453 ketone based solvent Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 229910001416 lithium ion Inorganic materials 0.000 description 1
- GCICAPWZNUIIDV-UHFFFAOYSA-N lithium magnesium Chemical compound [Li].[Mg] GCICAPWZNUIIDV-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- SJCKRGFTWFGHGZ-UHFFFAOYSA-N magnesium silver Chemical compound [Mg].[Ag] SJCKRGFTWFGHGZ-UHFFFAOYSA-N 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 229910001512 metal fluoride Inorganic materials 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- GYNNXHKOJHMOHS-UHFFFAOYSA-N methyl-cycloheptane Natural products CC1CCCCCC1 GYNNXHKOJHMOHS-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229940094933 n-dodecane Drugs 0.000 description 1
- 150000002791 naphthoquinones Chemical class 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 150000002815 nickel Chemical class 0.000 description 1
- KFBKRCXOTTUAFS-UHFFFAOYSA-N nickel;triphenylphosphane Chemical compound [Ni].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 KFBKRCXOTTUAFS-UHFFFAOYSA-N 0.000 description 1
- 125000006611 nonyloxy group Chemical group 0.000 description 1
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N o-biphenylenemethane Natural products C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 description 1
- 125000004365 octenyl group Chemical group C(=CCCCCCC)* 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005447 octyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 238000007645 offset printing Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 1
- 125000001820 oxy group Chemical group [*:1]O[*:2] 0.000 description 1
- 229960003540 oxyquinoline Drugs 0.000 description 1
- 150000002940 palladium Chemical class 0.000 description 1
- LXNAVEXFUKBNMK-UHFFFAOYSA-N palladium(II) acetate Substances [Pd].CC(O)=O.CC(O)=O LXNAVEXFUKBNMK-UHFFFAOYSA-N 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 1
- 125000004115 pentoxy group Chemical group [*]OC([H])([H])C([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000005981 pentynyl group Chemical group 0.000 description 1
- 125000005003 perfluorobutyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 125000005005 perfluorohexyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 125000005007 perfluorooctyl group Chemical group FC(C(C(C(C(C(C(C(F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)* 0.000 description 1
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 1
- CSHWQDPOILHKBI-UHFFFAOYSA-N peryrene Natural products C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 CSHWQDPOILHKBI-UHFFFAOYSA-N 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- RPGWZZNNEUHDAQ-UHFFFAOYSA-N phenylphosphine Chemical compound PC1=CC=CC=C1 RPGWZZNNEUHDAQ-UHFFFAOYSA-N 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920003227 poly(N-vinyl carbazole) Polymers 0.000 description 1
- 229920000553 poly(phenylenevinylene) Polymers 0.000 description 1
- 229920001467 poly(styrenesulfonates) Polymers 0.000 description 1
- 229920000767 polyaniline Polymers 0.000 description 1
- 229920000412 polyarylene Polymers 0.000 description 1
- 229920002098 polyfluorene Polymers 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 239000011970 polystyrene sulfonate Substances 0.000 description 1
- 229960002796 polystyrene sulfonate Drugs 0.000 description 1
- 229920000123 polythiophene Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 229910001414 potassium ion Inorganic materials 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical compound C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 238000001226 reprecipitation Methods 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- IGLNJRXAVVLDKE-UHFFFAOYSA-N rubidium atom Chemical compound [Rb] IGLNJRXAVVLDKE-UHFFFAOYSA-N 0.000 description 1
- 238000007650 screen-printing Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- DZLFLBLQUQXARW-UHFFFAOYSA-N tetrabutylammonium Chemical compound CCCC[N+](CCCC)(CCCC)CCCC DZLFLBLQUQXARW-UHFFFAOYSA-N 0.000 description 1
- JRMUNVKIHCOMHV-UHFFFAOYSA-M tetrabutylammonium bromide Chemical compound [Br-].CCCC[N+](CCCC)(CCCC)CCCC JRMUNVKIHCOMHV-UHFFFAOYSA-M 0.000 description 1
- IFLREYGFSNHWGE-UHFFFAOYSA-N tetracene Chemical compound C1=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C21 IFLREYGFSNHWGE-UHFFFAOYSA-N 0.000 description 1
- NLDYACGHTUPAQU-UHFFFAOYSA-N tetracyanoethylene Chemical group N#CC(C#N)=C(C#N)C#N NLDYACGHTUPAQU-UHFFFAOYSA-N 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 150000003577 thiophenes Chemical class 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 238000010937 topological data analysis Methods 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- WLPUWLXVBWGYMZ-UHFFFAOYSA-N tricyclohexylphosphine Chemical compound C1CCCCC1P(C1CCCCC1)C1CCCCC1 WLPUWLXVBWGYMZ-UHFFFAOYSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 235000019798 tripotassium phosphate Nutrition 0.000 description 1
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 1
- 238000007738 vacuum evaporation Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- UGOMMVLRQDMAQQ-UHFFFAOYSA-N xphos Chemical compound CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 UGOMMVLRQDMAQQ-UHFFFAOYSA-N 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- YVTHLONGBIQYBO-UHFFFAOYSA-N zinc indium(3+) oxygen(2-) Chemical compound [O--].[Zn++].[In+3] YVTHLONGBIQYBO-UHFFFAOYSA-N 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
- C08G61/122—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/02—Macromolecular compounds containing only carbon atoms in the main chain of the macromolecule, e.g. polyxylylenes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L65/00—Compositions of macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/115—Polyfluorene; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/151—Copolymers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/636—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising heteroaromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/10—Definition of the polymer structure
- C08G2261/12—Copolymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/10—Definition of the polymer structure
- C08G2261/13—Morphological aspects
- C08G2261/135—Cross-linked structures
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/31—Monomer units or repeat units incorporating structural elements in the main chain incorporating aromatic structural elements in the main chain
- C08G2261/314—Condensed aromatic systems, e.g. perylene, anthracene or pyrene
- C08G2261/3142—Condensed aromatic systems, e.g. perylene, anthracene or pyrene fluorene-based, e.g. fluorene, indenofluorene, or spirobifluorene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/31—Monomer units or repeat units incorporating structural elements in the main chain incorporating aromatic structural elements in the main chain
- C08G2261/316—Monomer units or repeat units incorporating structural elements in the main chain incorporating aromatic structural elements in the main chain bridged by heteroatoms, e.g. N, P, Si or B
- C08G2261/3162—Arylamines
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/34—Monomer units or repeat units incorporating structural elements in the main chain incorporating partially-aromatic structural elements in the main chain
- C08G2261/344—Monomer units or repeat units incorporating structural elements in the main chain incorporating partially-aromatic structural elements in the main chain containing heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/40—Polymerisation processes
- C08G2261/41—Organometallic coupling reactions
- C08G2261/411—Suzuki reactions
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/50—Physical properties
- C08G2261/51—Charge transport
- C08G2261/512—Hole transport
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/50—Physical properties
- C08G2261/52—Luminescence
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/70—Post-treatment
- C08G2261/76—Post-treatment crosslinking
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/90—Applications
- C08G2261/95—Use in organic luminescent diodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/14—Carrier transporting layers
- H10K50/15—Hole transporting layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/17—Carrier injection layers
- H10K50/171—Electron injection layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/658—Organoboranes
Definitions
- the present invention relates to a polymer compound and a light emitting device using the same.
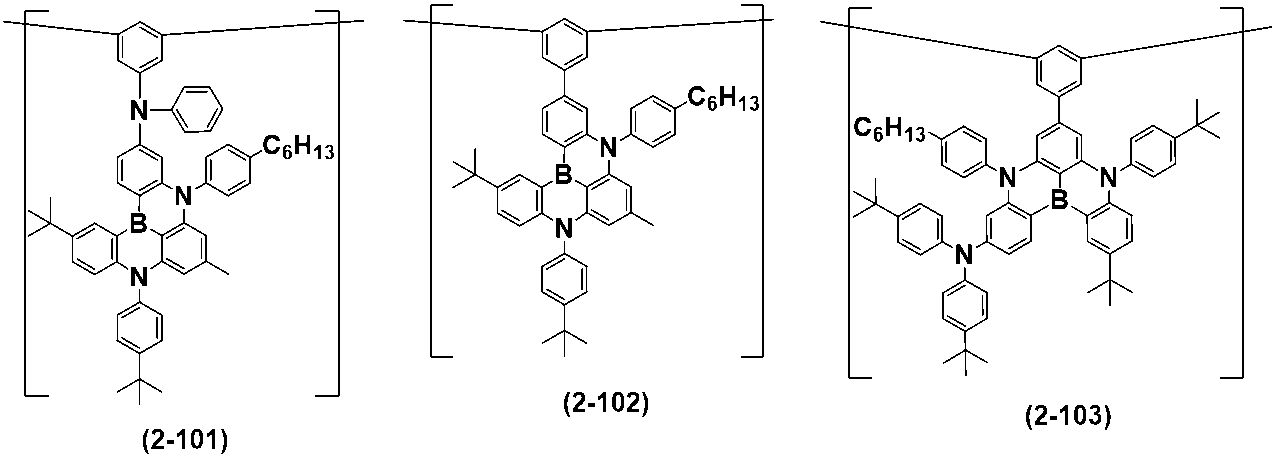
- Patent Document 1 a polymer compound represented by the following formula is studied (Patent Document 1).
- a light emitting element manufactured using the above-described polymer compound does not necessarily have a sufficient luminance life. Then, this invention aims at providing a high molecular compound useful to manufacture of the light emitting element which is excellent in the brightness
- the present invention provides the following [1] to [15].
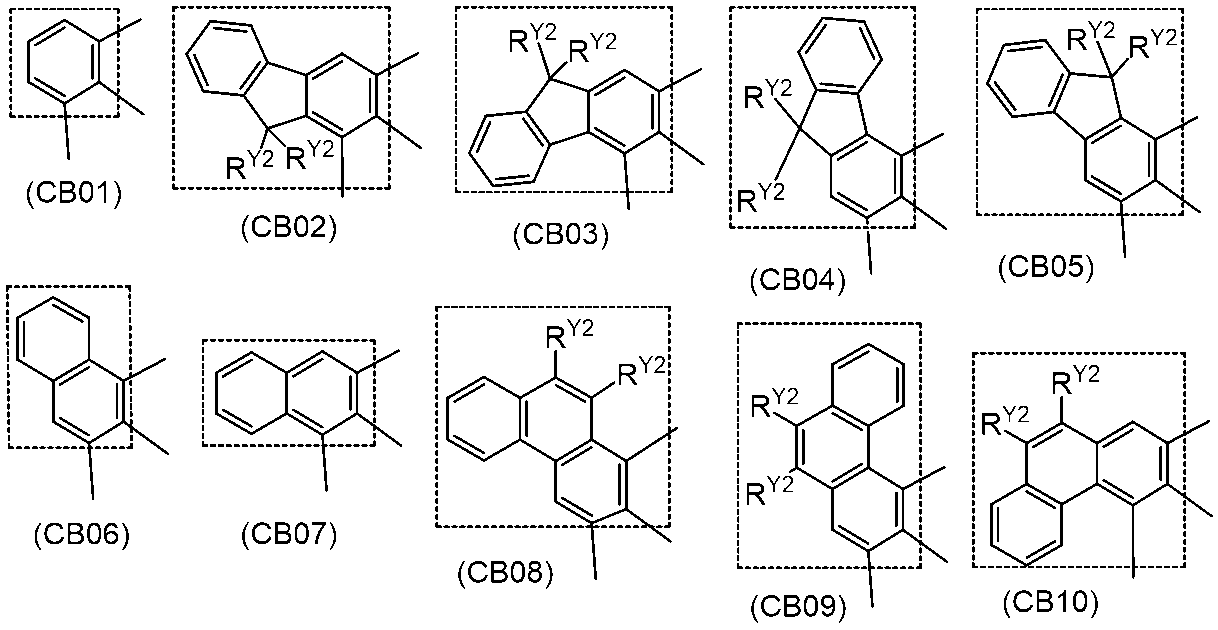
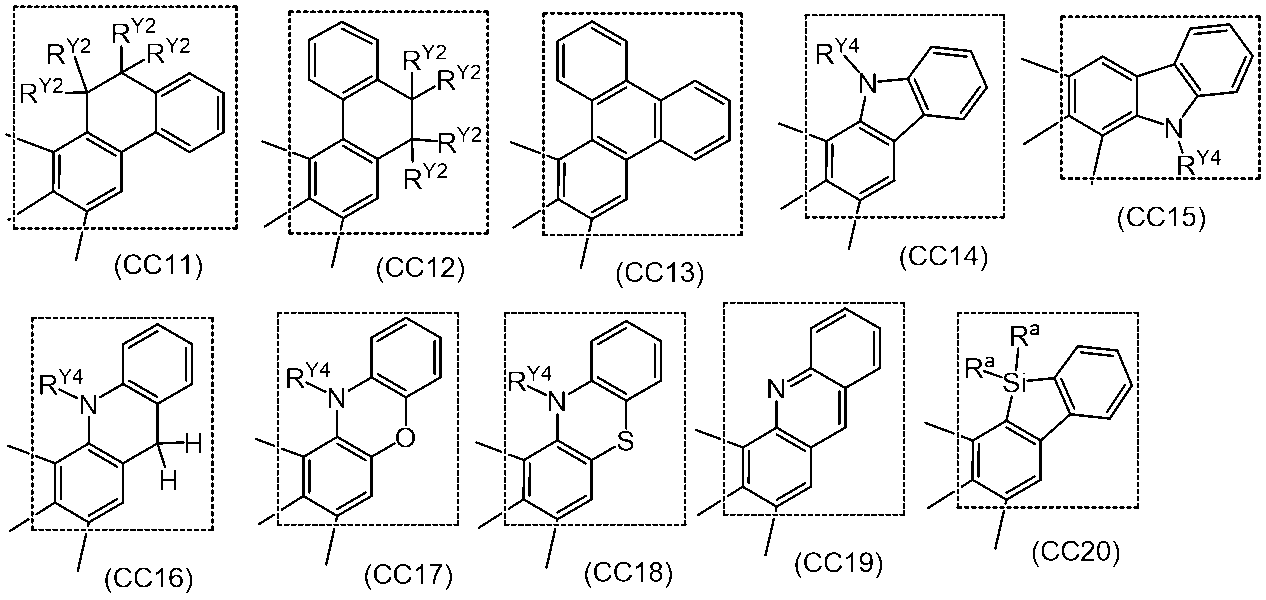
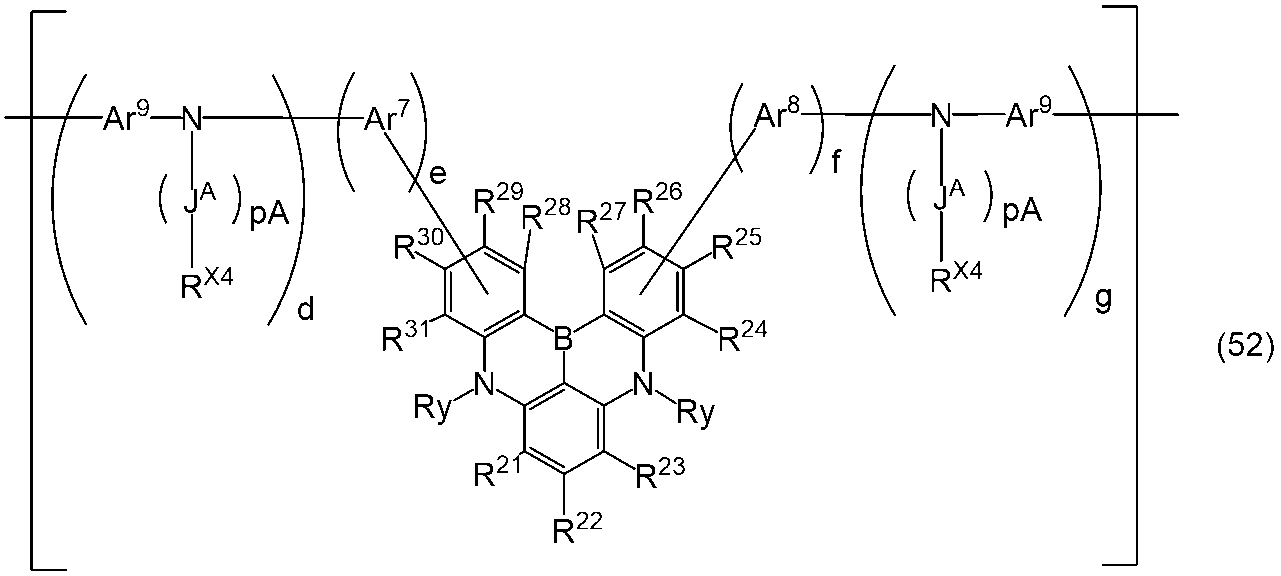
- a polymer compound comprising a constituent unit having a residue of a compound represented by the formula (1) and a constituent unit having no residue of the compound represented by the formula (1).
- Ring A, ring B and ring C each independently represent an aromatic hydrocarbon ring or a heteroaromatic ring, and these rings may have a substituent.
- Rx represents an aryl group or an alkyl group, and these groups may have a substituent.
- Y 1 represents N—Ry, a sulfur atom or a selenium atom.
- Y 2 and Y 3 each independently represent an oxygen atom, N—Ry, a sulfur atom or a selenium atom.
- Ry represents a hydrogen atom, an aryl group, a monovalent heterocyclic group, or an alkyl group, and these groups may have a substituent. When there are a plurality of Ry, they may be the same or different. Ry may be bonded to the A ring, the B ring or the C ring directly or via a linking group.
- n3 is 0 or 1; When n3 is 0, -Y 3 -is absent.
- nA is an integer of 0 to 5.
- n is 1 or 2;
- Ar 3 represents an aromatic hydrocarbon group or a heterocyclic group, and these groups may have a substituent.
- L A is an alkylene group, a cycloalkylene group, an arylene group, a divalent heterocyclic group, the group represented by -NR'-, an oxygen atom or a sulfur atom, these groups have a substituent It is also good.
- R ′ represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. If L A is plurally present, they may be the same or different.
- Q represents a residue of the compound represented by the formula (1). ] [In the formula, m is an integer of 1 to 4; mA is an integer of 0-5. When there are a plurality of mAs, they may be the same or different. c is 0 or 1; Ar 4 and Ar 6 each independently represent an arylene group or a divalent heterocyclic group, and these groups may have a substituent.
- Ar 5 represents an aromatic hydrocarbon group, a heterocyclic group, or a group in which at least one aromatic hydrocarbon ring and at least one heterocyclic ring are bonded, and these groups each have a substituent
- Ar 4 , Ar 5 and Ar 6 are each directly bonded to a group other than the group bonded to the nitrogen atom to which the group is bonded, or bonded through an oxygen atom or a sulfur atom And may form a ring.
- K A represents an alkylene group, a cycloalkylene group, an arylene group, a divalent heterocyclic group, a group represented by —NR′—, an oxygen atom or a sulfur atom, and these groups each have a substituent It is also good.
- R ′ represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. If K A there are a plurality, they may be the same or different.
- Q ′ represents a residue of a compound represented by the above formula (1), a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups each have a substituent May be When a plurality of Q 'are present, they may be the same or different. However, at least one Q ′ is a residue of the compound represented by the formula (1).
- d, e, f and g are each independently an integer of 0 to 2.
- pA is an integer of 0 to 5. When two or more pA exist, they may be the same or different.
- J A is an alkylene group, a cycloalkylene group, an arylene group, a divalent heterocyclic group, the group represented by -NR'-, an oxygen atom or a sulfur atom, these groups have a substituent It is also good.
- R ′ represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. If J A there are a plurality, they may be the same or different.
- Ar 7 , Ar 8 and Ar 9 each independently represent an arylene group or a divalent heterocyclic group, and these groups may have a substituent. When a plurality of Ar 7 , Ar 8 and Ar 9 exist, they may be the same or different.
- R X4 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- R X4 When a plurality of R X4 are present, they may be the same or different.
- Q ′ ′ represents a residue of the compound represented by the above formula (1).
- [4] The polymer compound according to any one of [1] to [3], wherein X is a boron atom.
- [5] The polymer compound according to any one of [1] to [4], wherein Y 1 , Y 2 and Y 3 are N-Ry.
- Y 1 , Y 2 and Y 3 are N-Ry.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 each independently represent a bond, a hydrogen atom, an alkyl group, a cycloalkyl group , An alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, a monovalent heterocyclic group, or a substituted amino group, and these groups may have a substituent.
- one of R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 is a bond.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 is a bond.
- Ry, Ar 4 , Ar 6 , K A and mA have the same meanings as described above.
- Ry ′ represents a direct bond, an arylene group, a divalent heterocyclic group, or an alkylene group, and these groups may have a substituent.
- Plural Ry's may be the same or different.
- Ar 7 , Ar 8 , Ar 9 , R X4 , J A , pA, d, e, f and g have the same meanings as described above.
- the constituent unit having no residue of the compound represented by the above formula (1) is selected from the group consisting of the constituent unit represented by the formula (X) and the constituent unit represented by the formula (Y)
- Each of a 1 and a 2 is independently an integer of 0 to 2.
- Ar X1 and Ar X3 each independently represent an arylene group or a divalent heterocyclic group, and these groups may have a substituent.
- Ar X2 and Ar X4 each independently represent an arylene group, a divalent heterocyclic group, or a divalent group in which at least one arylene group and at least one divalent heterocyclic group are bonded These groups may have a substituent.
- Ar X2 and Ar X4 When a plurality of Ar X2 and Ar X4 exist, they may be the same or different.
- R X1 , R X2 and R X3 independently represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- R X2 and R X3 may be the same or different.
- Ar Y 1 represents an arylene group, a divalent heterocyclic group, or a divalent group in which at least one type of arylene group and at least one type of divalent heterocyclic group are bonded, The group may have a substituent.
- the structural unit represented by the formula (Y) is a structural unit represented by the formula (Y-1) or a structural unit represented by the formula (Y-2), according to [10] Polymer compound.
- R Y1 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent
- Plural R Y1 may be the same or different, and adjacent R Y1 may be bonded to each other to form a ring together with the carbon atoms to which they are bonded.
- R Y1 represents the same meaning as described above.
- R Y2 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- a plurality of R Y2 may be the same or different, and R Y2 may be bonded to each other to form a ring together with the carbon atoms to which they are bonded.
- the total amount of the structural unit represented by the formula (2) and the structural unit represented by the formula (2 ′) is 0. 0 to the total amount of all the structural units contained in the polymer compound.
- a light emitting device comprising an organic layer containing the polymer compound according to any one of [1] to [13].
- a polymer compound useful for the production of a light emitting device excellent in luminance life it is possible to provide a polymer compound useful for the production of a light emitting device excellent in luminance life. Further, according to the present invention, a composition containing the polymer compound and a light emitting device containing the polymer compound can be provided.
- Me represents a methyl group
- Et represents an ethyl group
- Bu represents a butyl group
- i-Pr represents an isopropyl group
- t-Bu represents a tert-butyl group.
- the hydrogen atom may be a deuterium atom or a light hydrogen atom.
- the solid line representing the bond to the central metal means a covalent bond or a coordinate bond.
- the “polymer compound” means a polymer having a molecular weight distribution and having a polystyrene-equivalent number average molecular weight of 1 ⁇ 10 3 to 1 ⁇ 10 8 .
- the “low molecular weight compound” means a compound having no molecular weight distribution and having a molecular weight of 1 ⁇ 10 4 or less.
- the "constituent unit” means a unit which is present one or more in the polymer compound.
- the “alkyl group” may be linear or branched.
- the carbon atom number of the linear alkyl group is usually 1 to 50, preferably 3 to 30, and more preferably 4 to 20, not including the carbon atom number of the substituent.
- the carbon atom number of the branched alkyl group is usually 3 to 50, preferably 3 to 30, and more preferably 4 to 20, not including the carbon atom number of the substituent.
- the alkyl group may have a substituent, and examples thereof include a methyl group, an ethyl group, a propyl group, an isopropyl group, a butyl group, a 2-butyl group, an isobutyl group, a tert-butyl group, a pentyl group and an isoamyl group 2-ethylbutyl, hexyl, heptyl, octyl, 2-ethylhexyl, 3-propylheptyl, decyl, 3,7-dimethyloctyl, 2-ethyloctyl, 2-hexyldecyl, dodecyl And a group in which a hydrogen atom in these groups is substituted with a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, a fluorine atom or the like (for example, a trifluoromethyl group
- the number of carbon atoms of the "cycloalkyl group” is usually 3 to 50, preferably 3 to 30, and more preferably 4 to 20, not including the number of carbon atoms of the substituent.
- the cycloalkyl group may have a substituent, and examples thereof include a cyclohexyl group, a cyclohexylmethyl group and a cyclohexylethyl group.
- the “aryl group” means an atomic group remaining after removing one hydrogen atom directly bonded to a carbon atom constituting a ring from an aromatic hydrocarbon.
- the carbon atom number of the aryl group is usually 6 to 60, preferably 6 to 20, more preferably 6 to 10, not including the carbon atom number of the substituent.
- the aryl group may have a substituent, and examples thereof include phenyl, 1-naphthyl, 2-naphthyl, 1-anthracenyl, 2-anthracenyl, 9-anthracenyl, 1-pyrenyl, 2 -Pyrenyl group, 4-pyrenyl group, 2-fluorenyl group, 3-fluorenyl group, 4-fluorenyl group, 2-phenylphenyl group, 3-phenylphenyl group, 4-phenylphenyl group, and hydrogen atom in these groups Are groups substituted with an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, a fluorine atom or the like.
- the "alkoxy group” may be linear or branched.
- the carbon atom number of the linear alkoxy group is usually 1 to 40, preferably 4 to 10, not including the carbon atom number of the substituent.
- the carbon atom number of the branched alkoxy group is usually 3 to 40, preferably 4 to 10, not including the carbon atom number of the substituent.
- the alkoxy group may have a substituent, and examples thereof include a methoxy group, an ethoxy group, a propyloxy group, an isopropyloxy group, a butyloxy group, an isobutyloxy group, a tert-butyloxy group, a pentyloxy group, a hexyloxy group, And heptyloxy group, octyloxy group, 2-ethylhexyloxy group, nonyloxy group, decyloxy group, 3, 7-dimethyloctyloxy group, lauryloxy group, and a hydrogen atom in these groups is a cycloalkyl group, an alkoxy group, Examples thereof include groups substituted with a cycloalkoxy group, an aryl group, a fluorine atom and the like.
- the carbon atom number of the "cycloalkoxy group” is usually 3 to 40, preferably 4 to 10, not including the carbon atom number of the substituent.
- the cycloalkoxy group may have a substituent, and examples thereof include a cyclohexyloxy group.
- the number of carbon atoms of the “aryloxy group” is usually 6 to 60, preferably 6 to 48, not including the number of carbon atoms of the substituent.
- the aryloxy group may have a substituent, and examples thereof include phenoxy group, 1-naphthyloxy group, 2-naphthyloxy group, 1-anthracenyloxy group, 9-anthracenyloxy group, 1- Pyrenyloxy groups and groups in which a hydrogen atom in these groups is substituted with an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, a fluorine atom or the like can be mentioned.
- the “p-valent heterocyclic group” (p represents an integer of 1 or more) means p out of hydrogen atoms directly bonded to a carbon atom or a hetero atom constituting a ring from the heterocyclic compound. Means the remaining atomic groups excluding the hydrogen atom of Among p-valent heterocyclic groups, carbon atoms constituting the ring or the remaining atomic groups obtained by removing p hydrogen atoms from hydrogen atoms directly bonded to a hetero atom from an aromatic heterocyclic compound "P-valent aromatic heterocyclic group” is preferred.
- the “aromatic heterocyclic compound” is a complex such as oxadiazole, thiadiazole, thiazole, oxazole, thiophene, pyrrole, phosphole, furan, pyridine, pyrazine, pyrimidine, triazine, pyridazine, quinoline, isoquinoline, carbazole, dibenzophosphole etc.
- Compounds in which the ring itself exhibits aromaticity, and heterocycles such as phenoxazine, phenothiazine, dibenzoborole, dibenzosilole, benzopyran and the like themselves do not exhibit aromaticity, but an aromatic ring is fused to the heterocycle. It means a compound.
- the carbon atom number of the monovalent heterocyclic group is usually 2 to 60, preferably 4 to 20, not including the carbon atom number of the substituent.
- the monovalent heterocyclic group may have a substituent, and examples thereof include thienyl group, pyrrolyl group, furyl group, pyridyl group, piperidinyl group, quinolinyl group, isoquinolinyl group, pyrimidinyl group, triazinyl group, and the like Groups in which a hydrogen atom in any of the groups is substituted with an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group or the like.
- halogen atom represents a fluorine atom, a chlorine atom, a bromine atom or an iodine atom.
- amino group may have a substituent and is preferably a substituted amino group.
- a substituent which an amino group has an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group is preferable.
- substituted amino group examples include dialkylamino group, dicycloalkylamino group and diarylamino group.
- amino group examples include dimethylamino, diethylamino, diphenylamino, bis (4-methylphenyl) amino, bis (4-tert-butylphenyl) amino, and bis (3,5-di-tert-). And butylphenyl) amino.
- alkenyl group may be linear or branched.
- the carbon atom number of the linear alkenyl group is usually 2 to 30, preferably 3 to 20, not including the carbon atom number of the substituent.
- the carbon atom number of the branched alkenyl group is usually 3 to 30, preferably 4 to 20, not including the carbon atom number of the substituent.
- the number of carbon atoms of the "cycloalkenyl group” is usually 3 to 30, preferably 4 to 20, not including the number of carbon atoms of the substituent.
- the alkenyl group and cycloalkenyl group may have a substituent, and examples thereof include a vinyl group, 1-propenyl group, 2-propenyl group, 2-butenyl group, 3-butenyl group, 3-pentenyl group, 4- Examples include pentenyl group, 1-hexenyl group, 5-hexenyl group, 7-octenyl group, and groups in which these groups have a substituent.
- alkynyl group may be linear or branched.
- the carbon atom number of the alkynyl group is usually 2 to 20, preferably 3 to 20, not including the carbon atom of the substituent.
- the carbon atom number of the branched alkynyl group is usually 4 to 30, preferably 4 to 20, not including the carbon atom of the substituent.
- the number of carbon atoms of the “cycloalkynyl group” is usually 4 to 30, preferably 4 to 20, not including the carbon atom of the substituent.
- the alkynyl group and cycloalkynyl group may have a substituent, and examples thereof include ethynyl group, 1-propynyl group, 2-propynyl group, 2-butynyl group, 3-butynyl group, 3-pentynyl group, 4- Examples include pentynyl group, 1-hexynyl group, 5-hexynyl group, and groups in which these groups have a substituent.
- the "arylene group” means an atomic group remaining after removing two hydrogen atoms directly bonded to carbon atoms constituting a ring from an aromatic hydrocarbon.
- the carbon atom number of the arylene group is usually 6 to 60, preferably 6 to 30, and more preferably 6 to 18, not including the carbon atom number of the substituent.
- the arylene group may have a substituent, and examples thereof include phenylene group, naphthalenediyl group, anthracenediyl group, phenanthrendiyl group, dihydrophenanthrendiyl group, naphthacene diyl group, fluorenediyl group, pyrene diyl group, perylene diyl group, There may be mentioned a chrysendiyl group and a group in which these groups have a substituent, and preferably a group represented by the formula (A-1) to the formula (A-20).
- the arylene group includes a group in which a plurality of these groups are bonded.
- R and R a each independently represent a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group.
- Plural R and R a may be respectively the same or different, and R a may be bonded to each other to form a ring together with the atoms to which each is bonded.
- the carbon atom number of the divalent heterocyclic group is usually 2 to 60, preferably 3 to 20, more preferably 4 to 15, not including the carbon atom number of the substituent.
- the divalent heterocyclic group may have a substituent, and examples thereof include pyridine, diazabenzene, triazine, azanaphthalene, diazanaphthalene, carbazole, dibenzofuran, dibenzothiophene, dibenzosilole, phenoxazine, phenothiazine, acridine, and the like.
- Preferred are dihydroacridines, furans, thiophenes, azoles, diazoles and triazoles, and divalent groups in which two hydrogen atoms of hydrogen atoms directly bonded to ring carbon atoms or hetero atoms are removed, preferably Is a group represented by formula (AA-1) to formula (AA-34).
- the divalent heterocyclic group includes a group in which a plurality of these groups are bonded.
- the “substituent” is a halogen atom, cyano group, alkyl group, cycloalkyl group, aryl group, monovalent heterocyclic group, alkoxy group, cycloalkoxy group, aryloxy group, amino group, substituted amino group, alkenyl group And a cycloalkenyl group, an alkynyl group or a cycloalkynyl group.
- the polymer compound of the present invention comprises a constituent unit having a residue of the compound represented by the formula (1) and a constituent unit having no residue of the compound represented by the formula (1).
- the residue of the compound represented by the formula (1) is a group in which one or two or more hydrogen atoms have been removed from the skeleton structure of the compound represented by the formula (1) (that is, a moiety other than a substituent). is there.
- the residue of the compound represented by the formula (1) may be present in any of the main chain, side chain and terminal of the polymer compound.
- the constituent unit having a residue of the compound represented by the formula (1) and the constituent unit not having a residue of the compound represented by the formula (1) are each contained in the polymer compound alone. Or two or more may be included.
- the carbon atom number of the aromatic hydrocarbon ring represented by A ring, B ring and C ring is usually 6 to 60, preferably 6 to 18, not including the carbon atom number of the substituent. More preferably, it is 6 to 10, and particularly preferably 6.
- the aromatic hydrocarbon ring include benzene, fluorene, naphthalene, anthracene and phenanthrene, preferably benzene.
- the carbon atom number of the aromatic heterocyclic ring represented by A ring, B ring and C ring is usually 2 to 60, preferably 3 to 20, not including the carbon atom number of the substituent. Preferably it is 4-15.
- the aromatic heterocyclic ring include pyridine, diazabenzene, azanaphthalene, diazanaphthalene, carbazole, indolocarbazole, dibenzofuran, dibenzothiophene, dibenzosilole, phenoxazine, phenothiazine, acridine, dihydroacridine, furan and thiophene. .
- the luminance life of the light emitting device of the present invention is excellent, and preferably an alkyl group, an aryl group, a monovalent heterocyclic group, or It is a substituted amino group, more preferably an alkyl group, an aryl group or a substituted amino group, and these groups may have a substituent.
- CA As a detailed structure (CA) of ring A, for example, structures represented by formula (CA01) to formula (CA38) can be mentioned, and the luminance life of the light emitting element of the present invention is excellent. It is a structure represented by formula (CA19), more preferably a structure represented by formula (CA01) to formula (CA05), still more preferably a structure represented by formula (CA01).
- R a represents the same meaning as described above.
- the hydrogen atom may be replaced by a substituent which may be possessed by ring A.
- R Y2 represents an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- two or more R Y2 are present R Y2's may combine with each other to form a ring.
- Plural R Y2 may be the same or different.
- R Y4 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. When two or more R Y4 exist, they may be the same or different. ]
- R Y2 is preferably an alkyl group, a cycloalkyl group or an aryl group, and these groups may have a substituent.
- CB of ring B for example, structures represented by formula (CB01) to formula (CB24) can be mentioned, and since the device life is excellent, it is preferable to use a formula (CB01) to formula (CB13) It is a structure represented, more preferably a structure represented by Formula (CB01) to Formula (CB05), and particularly preferably a structure represented by Formula (CB01).
- R Y2 , R Y4 and R a represent the same meaning as described above.
- the hydrogen atom may be replaced by a substituent which may be possessed by the B ring.
- Examples of the detailed structure (CC) of the C ring include the structures represented by the formulas (CC01) to (CC24), and preferably the structures represented by the formulas (CC01) to (CC13) More preferably, it is a structure represented by Formula (CC01) to Formula (CC05), and more preferably a structure represented by Formula (CC01).
- R Y2 , R Y4 and R a represent the same meaning as described above.
- the hydrogen atom may be replaced by a substituent which may be possessed by the C ring.
- R Y2 is preferably both an alkyl group or a cycloalkyl group, both are an aryl group, both are a monovalent heterocyclic group, or one is an alkyl group or a cycloalkyl group and the other is an aryl group or a monovalent group. It is a heterocyclic group, more preferably one is an alkyl group or a cycloalkyl group and the other is an aryl group, and these groups may have a substituent.
- a group represented by —C (R Y2 ) C (R Y2 ) —
- the combination of two R Y2 therein is preferably both an alkyl group or a cycloalkyl group, or one is an alkyl group or a cycloalkyl group and the other is an aryl group, and these groups have a substituent May be
- R Y2 in the group are preferably an alkyl group which may have a substituent or a cycloalkyl group which may have a substituent.
- a plurality of R Y2 may be bonded to each other to form a ring together with the carbon atom to which each is bonded, and in the case where R Y2 forms a ring, —C (R Y2 ) 2 —C (R Y2 ) 2 —
- the group represented by is preferably a group represented by the formula (Y-B1) to the formula (Y-B5), more preferably a group represented by the formula (Y-B3), and these groups May have a substituent.
- R Y2 represents the same meaning as described above.
- R Y4 is preferably an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group It is a group, an aryl group or a monovalent heterocyclic group, more preferably an aryl group, and these groups may have a substituent.
- the luminance life of the light emitting device of the present invention is excellent, so preferably, the A ring is represented by Formula (CA01) to Formula (CA05).
- the ring B is a structure represented by the formula (CB01) to the formula (CB05), and the ring C is a structure represented by the formula (CC01) to the formula (CC05), more preferably Is a structure in which ring A is represented by formula (CA01), a structure in which ring B is represented by formula (CB01) to formula (CB05), and ring C is a group represented by formula (CC01) to formula (CC05) And more preferably a structure in which the ring A is a structure represented by formula (CA01), a structure in which a ring B is a structure represented by formula (CB01), and a ring C a It is a structure represented by CC01).
- N3 is preferably 0 because the luminance life of the light emitting device of the present invention is more excellent.
- Y 1 , Y 2 and Y 3 are preferably N-Ry or a sulfur atom since the stability of the polymer compound of the present invention is good, and more preferably N-Ry It is Ry.
- At least one of Y 1 , Y 2 and Y 3 is preferably N-Ry because the stability of the polymer compound of the present invention is good, and Y 1 , Y 2 and More preferably, all of Y 3 are N-Ry. However, when n3 is 0, both Y 1 and Y 2 are N-Ry.
- Ry is preferably a hydrogen atom, an aryl group which may have a substituent, or a monovalent heterocyclic ring which may have a substituent, because the luminance life of the light emitting device of the present invention is more excellent. It is a group, more preferably a hydrogen atom or an aryl group which may have a substituent, and still more preferably an aryl group which may have a substituent.
- the linking group is, for example, a divalent group such as -O-, -S- or -CH 2- , boron And trivalent groups such as atoms.
- Ry When Ry is linked to the A ring, B ring or C ring via a trivalent group, it is usually linked to the A ring and the substituent on the A ring, or linked to the B ring and the substituent on the B ring Or linked to a C ring and a substituent on the C ring.
- Examples of the structure in which Ry is directly or linked to the A ring, B ring or C ring via a linking group include structures represented by Formula (E01) to Formula (E16).
- the constituent unit having a residue of the compound represented by Formula (1) is preferably a constituent unit represented by Formula (2), Formula (2 ′) or Formula (3).
- nA is preferably 0 or 1, and more preferably 0, because the luminance life of the light emitting device of the present invention is more excellent.
- N is preferably 1, because the luminance life of the light emitting device of the present invention is more excellent.
- Ar 3 is preferably an aromatic hydrocarbon group which may have a substituent, because the luminance life of the light emitting device of the present invention is more excellent.
- the number of carbon atoms of the aromatic hydrocarbon group represented by Ar 3 is usually 6 to 60, preferably 6 to 30, and more preferably 6 to 18, not including the number of carbon atoms of the substituent. is there.
- the arylene group moiety excluding the n substituents of the aromatic hydrocarbon group represented by Ar 3 is preferably a group represented by Formula (A-1) to Formula (A-20), More preferably, a group represented by Formula (A-1), Formula (A-2), Formula (A-6) to Formula (A-10), Formula (A-19) or Formula (A-20) And more preferably a group represented by Formula (A-1), Formula (A-2), Formula (A-7), Formula (A-9) or Formula (A-19), Groups may have a substituent.
- the carbon atom number of the heterocyclic group represented by Ar 3 is usually 2 to 60, preferably 3 to 30, and more preferably 4 to 18, not including the carbon atom number of the substituent.
- the divalent heterocyclic group moiety excluding the n substituents of the heterocyclic group represented by Ar 3 is preferably a group represented by Formula (AA-1) to Formula (AA-34) is there.
- the aromatic hydrocarbon group and the heterocyclic group represented by Ar 3 may have a substituent, and as the substituent, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group can be mentioned Groups, halogen atoms, monovalent heterocyclic groups and cyano groups.
- the alkylene group represented by L A is usually 1 to 20, preferably 1 to 15, and more preferably 1 to 10, not including the number of carbon atoms of the substituent.
- the cycloalkylene group represented by L A is usually 3 to 20, not including the number of carbon atoms of the substituent.
- the alkylene group and the cycloalkylene group may have a substituent, and examples thereof include a methylene group, an ethylene group, a propylene group, a butylene group, a hexylene group, a cyclohexylene group and an octylene group.
- substituent which the alkylene group and the cycloalkylene group may have include an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, a halogen atom and a cyano group.
- Arylene group represented by L A may have a substituent.
- the arylene group includes o-phenylene, m-phenylene and p-phenylene.
- substituent which the arylene group may have include an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, a monovalent heterocyclic group, a halogen atom and a cyano group.
- aryl group represented by R ' a phenyl group, a naphthyl group, and a fluorenyl group are mentioned.
- substituent which the aryl group may have include an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, a monovalent heterocyclic group, a halogen atom and a cyano group. It can be mentioned.
- the structural unit represented by the formula (2) may be contained alone or in combination of two or more in the polymer compound.
- mA is preferably 0 or 1, and more preferably 0, because the luminance life of the light emitting device of the present invention is more excellent.
- M is preferably 2, because the luminance life of the light emitting device of the present invention is more excellent.
- C is preferably 0, because the polymer compound of the present invention can be easily produced and the luminance life of the light emitting device of the present invention is more excellent.
- Ar 5 is preferably an aromatic hydrocarbon group which may have a substituent, because the luminance life of the light emitting device of the present invention is more excellent.
- the definition and examples of the divalent heterocyclic group moiety excluding the m substituents of the heterocyclic group represented by Ar 5 are the divalent heterocyclic group represented by Ar X2 in the formula (X) described later It is the same as the definition and example of the part.
- divalent groups other than m substituents of a group in which at least one aromatic hydrocarbon ring represented by Ar 5 and at least one hetero ring are directly bonded are represented by the formula (described later) It is the same as the definition or example of the divalent group in which at least one arylene group represented by Ar X2 in X) and at least one divalent heterocyclic group are directly bonded.
- Ar 4 and Ar 6 are preferably arylene groups which may have a substituent, because the luminance lifetime of the light emitting device of the present invention is more excellent.
- the definitions and examples of the arylene group and divalent heterocyclic group represented by Ar 4 and Ar 6 are respectively the arylene group and divalent heterocyclic ring represented by Ar X1 and Ar X3 in Formula (X) described later. The same as the definition and example of the group.
- the group represented by Ar 4 , Ar 5 and Ar 6 may have a substituent, and as the substituent, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, A halogen atom, a monovalent heterocyclic group and a cyano group can be mentioned.
- alkylene group, cycloalkylene group, arylene group and divalent heterocyclic group represented by K A are respectively the alkylene group, cycloalkylene group, arylene group and divalent hetero group represented by L A The same as the definition and the example of the ring group.
- K A is preferably an alkylene group, an arylene group or a group represented by —NR′—, because K can facilitate the production of the polymer compound of the present invention, and more preferably a phenylene group, an alkylene group or — N (C 6 H 5 )-, and these groups may have a substituent.
- the constituent unit represented by the formula (2 ′) may be contained alone or in combination of two or more kinds in the polymer compound.
- the total amount of the constituent unit represented by the formula (2) and the constituent unit represented by the formula (2 ′) contained in the polymer compound of the present invention is excellent in the stability of the polymer compound, and thus the polymer compound
- the amount is preferably 0.1 to 50 mol%, more preferably 0.1 to 30 mol%, still more preferably 0.1 to 10 mol%, particularly preferably 0.5 to 50 mol%, based on the total amount of all the structural units contained in 5 mol%.
- Examples of the structural unit represented by the formula (2) include structural units represented by the formulas (2-1) to (2-17).
- Examples of the structural unit represented by the formula (2 ′) include structural units represented by the formulas (2′-1) to (2′-14).
- Structural units more preferably structural units represented by Formula (2-5) to Formula (2-14), or Formula (2′-1) to Formula (2′-6), and Preferably, they are structural units represented by Formula (2-5) to Formula (2-10), or Formula (2′-1) to Formula (2′-3).
- the structural unit represented by the formula (2) for example, the structural unit represented by the formula (31), the formula (32), the formula (35), the formula (36), the formula (38) and the formula (32) And the structural unit represented by Formula (31) or (32), and more preferably the structural unit represented by Formula (31). .
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , Ry, Ry ', Ar 3 , L A and nA are as defined above Represents the same meaning.
- R 4 ′ , R 5 ′ , R 6 ′ , R 7 ′ , R 8 ′ , R 9 ′ , R 10 ′ and R 11 ′ are each independently a bond, a hydrogen atom, an alkyl group, a cycloalkyl group, It represents an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, a monovalent heterocyclic group, or a substituted amino group, and these groups may have a substituent.
- one of R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 is a bond.
- One of ' , R 10' and R 11 ' is a bond.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 which are not a bond in the structural units represented by the formulas (31), (32) and (39)
- the structural unit represented by (49) is mentioned, Preferably it is a structural unit represented by Formula (41) or Formula (42), More preferably, it is a structural unit represented by Formula (41).
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , Ry, Ry ', Ar 4 , Ar 6 , K A , and mA are , Represents the same meaning as described above.
- R 4 ′ , R 5 ′ , R 6 ′ , R 7 ′ , R 8 ′ , R 9 ′ , R 10 ′ and R 11 ′ represent the same meaning as described above.
- one of R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 is a bond.
- One of ' , R 10' and R 11 ' is a bond.
- d, e, f and g are preferably 0 or 1, and more preferably 0, because the luminance life of the light emitting device of the present invention is more excellent.
- PA is preferably 0 or 1, and more preferably 0, because the luminance life of the light emitting device of the present invention is more excellent.
- Alkylene group represented by J A is not including the carbon atom number of substituent is usually 1 to 20, preferably 1 to 15, more preferably 1 to 10. Cycloalkylene group represented by J A is not including the carbon atom number of substituent is usually 3 to 20.
- the alkylene group and the cycloalkylene group may have a substituent, and examples thereof include a methylene group, an ethylene group, a propylene group, a butylene group, a hexylene group, a cyclohexylene group and an octylene group.
- substituent which the alkylene group and the cycloalkylene group may have include an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, a halogen atom and a cyano group.
- Arylene group represented by J A may have a substituent.
- the arylene group include o-phenylene group, m-phenylene group and p-phenylene group.
- the substituent which the arylene group may have include an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group, an aryloxy group, a monovalent heterocyclic group, a halogen atom and a cyano group.
- Ar 7 , Ar 8 and Ar 9 each are preferably an arylene group which may have a substituent, because the luminance life of the light emitting device of the present invention is more excellent.
- the number of carbon atoms of the arylene group represented by Ar 7 , Ar 8 and Ar 9 is usually 6 to 60, preferably 6 to 30, and more preferably 6 regardless of the number of carbon atoms of the substituent. It is ⁇ 18.
- the arylene group represented by Ar 7 , Ar 8 and Ar 9 is preferably a group represented by Formula (A-1) to Formula (A-20), and more preferably a group represented by Formula (A-1) A group represented by Formula (A-2), Formula (A-6) to Formula (A-10), Formula (A-19) or Formula (A-20), and more preferably A-1), a group represented by the formula (A-2), a formula (A-7), a formula (A-9) or a formula (A-19), these groups each having a substituent May be
- the carbon atom number of the divalent heterocyclic group represented by Ar 7 , Ar 8 and Ar 9 is usually 2 to 60, preferably 3 to 30, not including the carbon atom number of the substituent. More preferably, it is 4-18.
- the divalent heterocyclic group represented by Ar 7 , Ar 8 and Ar 9 is preferably a group represented by Formula (AA-1) to Formula (AA-34).
- the arylene group and divalent heterocyclic group represented by Ar 7 , Ar 8 and Ar 9 may have a substituent, and as the substituent, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group And aryl groups, aryloxy groups, halogen atoms, monovalent heterocyclic groups and cyano groups.
- R X4 is preferably an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, more preferably an aryl group, and these groups may have a substituent.
- Examples of the structural unit represented by the formula (3) include structural units represented by the formulas (3-1) to (3-12). Among these, since the luminance life of the light emitting device of the present invention is excellent, the structural units represented by formulas (3-1) to (3-6) are preferable, and more preferably A structural unit represented by the formula (3-3), more preferably a structural unit represented by the formula (3-1).
- Examples of the structural unit represented by the formula (3) include the structural unit represented by the formula (51) and the structural units represented by the formulas (52) to (55). Or the structural unit represented by Formula (51) or the structural unit represented by Formula (53), More preferably, it is a structural unit represented by Formula (51).
- R 21 , R 22 , R 23 , R 24 , R 25 , R 26 , R 27 , R 28 , R 29 , R 30 , R 31 , Ry, Ry ', Ar 7 , Ar 8 , Ar 9 , J A , R X4 , pA, d, e, f and g have the same meaning as described above.
- R X4 ′ represents a direct bond, an alkylene group, a cycloalkylene group, an arylene group or a divalent heterocyclic group, and these groups may have a substituent.
- Ar 9 ′ represents a trivalent aromatic hydrocarbon group or a trivalent heterocyclic group, and these groups may have a substituent.
- one of R 24 , R 25 , R 26 and R 27 is a bond
- one of R 28 , R 29 , R 30 and R 31 is a bond
- one of R 21 , R 22 , R 23 , R 24 , R 25 , R 26 , R 27 , R 28 , R 29 , R 30 and R 31 is a bond
- one of R 21 , R 24 , R 25 , R 26 , R 27 , R 28 , R 29 , R 30 and R 31 is a bond.
- one of R 21 , R 24 , R 25 , R 26 , R 27 , R 28 , R 29 , R 30 and R 31 is a bond.
- Examples of the structural unit represented by the formula (3) include structural units represented by the formulas (3-101) to (3-129).
- the total amount of the structural units represented by the formula (3) is preferably 0.1 to 50 moles relative to the total amount of all the structural units contained in the polymer compound because the stability of the polymer compound of the present invention is excellent. %, More preferably 0.1 to 30 mol%, still more preferably 0.1 to 10 mol%, particularly preferably 0.5 to 5 mol%.
- the structural unit a 1 represented by the formula (X) is preferably 1, because the luminance life of the light emitting device of the present invention is more excellent.
- the luminance lifetime of the light emitting device of the present invention is more excellent, it is preferably 0.
- R X1 , R X2 and R X3 are preferably an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, more preferably an aryl group, and these groups have a substituent It is also good.
- the arylene group represented by Ar X1 and Ar X3 is more preferably a group represented by Formula (A-1) or Formula (A-9), and still more preferably represented by Formula (A-1) These groups may have a substituent.
- the divalent heterocyclic group represented by Ar X1 and Ar X3 is more preferably represented by Formula (AA-1), Formula (AA-2) or Formula (AA-7) to Formula (AA-26) These groups may have a substituent.
- Ar X1 and Ar X3 are preferably arylene groups which may have a substituent.
- the arylene group represented by Ar X2 and Ar X4 is more preferably represented by Formula (A-1), Formula (A-6), Formula (A-7), Formula (A-9) to Formula (A-11) Or a group represented by formula (A-19), and these groups may have a substituent.
- the more preferable range of the divalent heterocyclic group represented by Ar X2 and Ar X4 is the same as the more preferable range of the divalent heterocyclic group represented by Ar X1 and Ar X3 .
- the more preferable ranges are the same as the more preferable ranges and the further preferable ranges of the arylene group and the divalent heterocyclic group represented by Ar X1 and Ar X3 respectively.
- Examples of the divalent group in which at least one arylene group represented by Ar X2 and Ar X4 and at least one bivalent heterocyclic group are directly bonded to each other include, for example, groups represented by the following formulas: These may have a substituent.
- R XX represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- R XX is preferably an alkyl group, a cycloalkyl group or an aryl group, and these groups may have a substituent.
- Ar X2 and Ar X4 are preferably arylene groups which may have a substituent.
- the substituent which the group represented by Ar X1 to Ar X4 and R X1 to R X3 may have is preferably an alkyl group, a cycloalkyl group or an aryl group, and these groups further have a substituent. It may be done.
- the structural unit represented by the formula (X) is preferably a structural unit represented by the formulas (X-1) to (X-7), and more preferably a formula (X-3) to a formula (X) -7), and more preferably structural units represented by Formula (X-3) to Formula (X-6).
- R X4 and R X5 are each independently a hydrogen atom, alkyl group, cycloalkyl group, alkoxy group, cycloalkoxy group, aryl group, aryloxy group, halogen atom, monovalent heterocyclic group or cyano Represents a group, and these groups may have a substituent.
- a plurality of R X4 may be the same or different.
- Plural R X5 s may be the same or different, and adjacent R X5 s may be bonded to each other to form a ring together with the carbon atoms to which they are bonded.
- the structural unit represented by the formula (X) is excellent in hole transportability, and is preferably 0.1 to 50 mol%, more preferably 0.5 based on the total amount of all structural units contained in the polymer compound. It is -30 mol%, more preferably 1 to 8 mol%, particularly preferably 1 to 5 mol%.
- structural units represented by the formula (X) for example, structural units represented by the formulas (X1-1) to (X1-7) can be mentioned, with preference given to the formula (X1-1) and the formula (X1) And 5) a structural unit represented by formula (X1-7).
- the arylene group represented by the structural unit Ar Y1 represented by the formula (Y) is more preferably represented by the formula (A-1), the formula (A-6), the formula (A-7) or the formula (A-9) A group represented by formula (A-11), formula (A-13) or formula (A-19), and more preferably a group represented by formula (A-1), formula (A-7) or formula (A) -9) or a group represented by Formula (A-19), and particularly preferably a group represented by Formula (A-1) or Formula (A-9), and these groups have a substituent It may be done.
- the divalent heterocyclic group represented by Ar Y1 is more preferably a group represented by the formula (AA-4), a formula (AA-10), a formula (AA-13), a formula (AA-15) or a formula (AA-18).
- a group represented by formula (AA-20), more preferably represented by formula (AA-4), formula (AA-10), formula (AA-18) or formula (AA-20) may have a substituent.
- the ranges are respectively the same as the more preferable range and the further preferable range of the arylene group and the divalent heterocyclic group represented by Ar Y1 described above.
- Examples of the divalent group in which at least one arylene group represented by Ar Y1 and at least one divalent heterocyclic group are directly bonded to each other include at least one represented by Ar X2 and Ar X4 in the formula (X)
- the same groups as the divalent group in which one kind of arylene group and at least one kind of divalent heterocyclic group are directly bonded to each other can be mentioned.
- the substituent which the group represented by Ar Y1 may have is preferably an alkyl group, a cycloalkyl group or an aryl group, more preferably an alkyl group or an aryl group, and these groups may be further substituted You may have.
- Examples of the structural unit represented by the formula (Y) include structural units represented by the formulas (Y-1) to (Y-7), and from the viewpoint of the luminance life of the light emitting device of the present invention, And preferably a structural unit represented by the formula (Y-1) or the formula (Y-2), and from the viewpoint of the electron transportability, it is preferably a table represented by the formula (Y-3) or the formula (Y-4) Preferably, from the viewpoint of hole transportability, they are structural units represented by formulas (Y-5) to (Y-7).
- R Y1 is preferably a hydrogen atom, an alkyl group, a cycloalkyl group or an aryl group, and these groups may have a substituent.
- the constitutional unit represented by the formula (Y-1) is preferably a constitutional unit represented by the formula (Y-1 ′).
- R Y11 represents an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- Plural R Y11 may be the same or different.
- R Y11 is preferably an alkyl group, a cycloalkyl group or an aryl group, more preferably an alkyl group or a cycloalkyl group, and these groups may have a substituent.
- R Y2 is preferably an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, more preferably an alkyl group, a cycloalkyl group or an aryl group, still more preferably Is an alkyl group or an aryl group, and these groups may have a substituent.
- the combination of two R Y2 in the group represented by —C (R Y2 ) 2 — is preferably both an alkyl group or a cycloalkyl group, both an aryl group, and both of which are monovalent A ring group, or one of which is an alkyl group or a cycloalkyl group and the other is an aryl group or a monovalent heterocyclic group, more preferably one of which is an alkyl group or a cycloalkyl group and the other of which is an aryl group; May have a substituent.
- R Y2 's may be bonded to each other to form a ring together with the atoms to which each is attached, and when R Y2 forms a ring, a group represented by —C (R Y2 ) 2 — Is preferably a group represented by formula (Y-A1) to formula (Y-A5), more preferably a group represented by formula (Y-A4), and these groups have a substituent It may be done.
- R Y2 in the group represented by —C (R Y2 ) 2 —C (R Y2 ) 2 — in X Y1 is an alkyl group or a cycloalkyl group which may have a substituent. It is. More than one R Y2 may be bonded to each other to form a ring with the atoms to which each is attached, and in the case where R Y2 forms a ring, -C (R Y2 ) 2 -C (R Y2 ) 2-
- the group represented is preferably a group represented by formulas (Y-B1) to (Y-B5), more preferably a group represented by formula (Y-B3), and these groups are substituted It may have a group.
- R Y2 represents the same meaning as described above.
- the structural unit represented by the formula (Y-2) is preferably a structural unit represented by the formula (Y-2 ′).
- R Y1 represents the same meaning as described above.
- R Y3 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- R Y3 is preferably an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, more preferably an aryl group, and these groups have a substituent May be
- R Y1 has the same meaning as described above.
- R Y4 represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- R Y4 is preferably an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, more preferably an aryl group, and these groups have a substituent May be
- Examples of the structural unit represented by the formula (Y) include structural units represented by the formulas (Y-11) to (Y-30).
- the structural unit represented by the formula (Y), in which Ar Y1 is an arylene group, is more excellent in the luminance life of the light emitting device of the present invention, and thus the total amount of all the structural units contained in the polymer compound Preferably, it is 50 to 99.5 mol%, more preferably 70 to 99.5 mol%.
- the structural unit which is a group of is preferably 1 to 30 mol%, more preferably 1 to 30 mol%, based on the total amount of all the structural units contained in the polymer compound, because the charge transportability of the light emitting device of the present invention is excellent. It is 1 to 10 mol%.
- polymer compound of the present invention examples include polymer compounds P-1 to P-16.
- the “other constituent unit” does not include the residue of the compound represented by the formula (1), and the formula (2), the formula (2 ′), the formula (3), the formula (X) and the formula It means a constitutional unit other than the constitutional unit represented by (Y).
- the end group of the polymer compound of the present invention is preferably such that the light emission characteristics and the luminance life may be reduced when the polymer compound is used for the preparation of a light emitting device if the polymerization active group remains as it is. It is a stable group.
- the terminal group is preferably a group conjugated with the main chain, and includes a group bonded to an aryl group or a monovalent heterocyclic group via a carbon-carbon bond.
- the polymer compound of the present invention may be any of a block copolymer, a random copolymer, an alternating copolymer, and a graft copolymer, and may be other embodiments, but plural kinds of raw materials It is preferable that it is a copolymer formed by copolymerizing a monomer.
- a raw material monomer used for condensation polymerization By reacting the compound represented by the formula (1) with a compound having a group selected from the group consisting of a substituent group A and a substituent group B described later, a raw material monomer used for condensation polymerization can be obtained (described below See equation). By subjecting this raw material monomer to condensation polymerization, a structural unit having a residue of the compound represented by Formula (1) is derived.
- the compound used for manufacture of the high molecular compound of this invention may be generically called a "raw material monomer.”
- Q represents the same meaning as described above.
- d represents an integer of 1 to 4;
- Z C0 represents a group selected from the group consisting of a substituent group A and a substituent group B described later. When two or more Z C0 exist, they may be the same or different.
- a compound represented by Formula (M-1), Formula (M-4) or Formula (M-5) is obtained as a raw material monomer used for condensation polymerization.
- This raw material monomer is a constituent unit having no residue of the compound represented by Formula (1).
- the polymer compound of the present invention includes, for example, a compound represented by Formula (M-1), a compound represented by Formula (M-2), and / or a compound represented by Formula (M-3) It can be produced by subjecting another compound (for example, a compound represented by the formula (M-4) and / or a compound represented by the formula (M-5)) to condensation polymerization.
- the polymer compound of the present invention includes, for example, a compound represented by the formula (M-1), at least one compound selected from the group of compounds represented by the formula (M-6), and the like It can be produced by condensation polymerization of a compound (for example, a compound represented by the formula (M-4) and / or a compound represented by the formula (M-5).
- nA, n, Ar 3, L A, Q, mA, m, c, Ar 4 ⁇ Ar 6, K A, Q ', Ar Y1, a 1, a 2, Ar X1 ⁇ Ar X4, and R X1 ⁇ R X3 represents the same meaning as described above.
- Z C1 to Z C10 each independently represent a group selected from the group consisting of Substituent Group A and Substituent Group B.
- Z C11 and Z C12 each independently represent a group selected from the group consisting of Substituent Group A and Substituent Group B.
- Z C1 , Z C2 , Z C3 , Z C4 , Z C5 , Z C6 , Z C11 and Z C12 are groups selected from Substituent Group A, Z C7 , Z C8 , Z C9 and Z C10 are And Z C1 , Z C2 , Z C3 , Z C4 , Z C5 , Z C6 , Z C11 and Z C12 are groups selected from Substituent Group B by selecting a group selected from Substituent Group B; C7 , Zc8 , Zc9 and Zc10 select a group selected from the substituent group A.
- ⁇ Substituent group A> Chlorine atom, bromine atom, iodine atom, —O—S ( O) 2 R C1 (wherein, R C1 represents an alkyl group, a cycloalkyl group or an aryl group, and these groups have a substituent)
- R C1 represents an alkyl group, a cycloalkyl group or an aryl group, and these groups have a substituent
- a group represented by —BF 3 Q ′ (wherein, Q ′ represents Li, Na, K, Rb or Cs); A group represented by the formula -MgY '(wherein Y' represents a chlorine atom, a bromine atom or an iodine atom); A group represented by —ZnY ′ ′ (wherein Y ′ ′ represents a chlorine atom, a bromine atom or an iodine atom); -Sn (R C3) 3 (wherein, R C3 represents a hydrogen atom, an alkyl group, a cycloalkyl group or an aryl group, these groups may have a substituent. More existing R C3 is And groups which may be the same or different and may be linked to each other to form a ring structure together with the tin atoms to which they are attached.
- Examples of the group represented by —B (OR C2 ) 2 include groups represented by the following formulae.
- the compound having a group selected from the substituent group A and the compound having a group selected from the substituent group B are condensation-polymerized by a known coupling reaction to form a group selected from the substituent group A and the substituent group B And a carbon atom bonded to a group selected from Therefore, if a compound having two groups selected from the substituent group A and a compound having two groups selected from the substituent group B are subjected to a known coupling reaction, condensation polymerization results in condensation of these compounds. Polymers can be obtained.
- the condensation polymerization is usually carried out in the presence of a catalyst, a base and a solvent, but if necessary, it may be carried out in the coexistence of a phase transfer catalyst.
- a catalyst for example, bis (triphenylphosphine) palladium (II) dichloride, bis (tris-o-methoxyphenylphosphine) palladium (II) dichloride, tetrakis (triphenylphosphine) palladium (0), tris (dibenzylideneacetone) And palladium complexes such as palladium acetate, palladium acetate, etc., tetrakis (triphenylphosphine) nickel (0), [1,3-bis (diphenylphosphino) propane) nickel (II) dichloride, bis (1,4) Transition metal complexes such as nickel complexes such as cyclooctadiene) nickel (0); these transition metal complexes further include triphenylphosphine, tri (o-tolyl) phosphine, tri (tert-butyl) phosphine, tricyclohexylphosphine,
- the amount of the catalyst used is usually 0.00001 to 3 molar equivalents as the amount of transition metal based on the total number of moles of the raw material monomer.
- a base and a phase transfer catalyst for example, inorganic bases such as sodium carbonate, potassium carbonate, cesium carbonate, potassium fluoride, cesium fluoride, tripotassium phosphate and the like; tetrabutyl ammonium fluoride, tetraethyl ammonium hydroxide, tetra hydroxide hydroxide Organic bases such as butyl ammonium; phase transfer catalysts such as tetrabutyl ammonium chloride and tetrabutyl ammonium bromide.
- the base and the phase transfer catalyst may be used alone or in combination of two or more.
- the amount of the base and phase transfer catalyst used is usually 0.001 to 100 molar equivalents relative to the total number of moles of the raw material monomers.
- the solvent examples include organic solvents such as toluene, xylene, mesitylene, tetrahydrofuran, 1,4-dioxane, dimethoxyethane, N, N-dimethylacetamide, N, N-dimethylformamide, and water.
- organic solvents such as toluene, xylene, mesitylene, tetrahydrofuran, 1,4-dioxane, dimethoxyethane, N, N-dimethylacetamide, N, N-dimethylformamide, and water.
- the solvents may be used alone or in combination of two or more.
- the amount of the solvent used is usually 10 to 100,000 parts by mass with respect to 100 parts by mass in total of the raw material monomers.
- the reaction temperature of the condensation polymerization is usually ⁇ 100 to 200 ° C.
- the reaction time of the condensation polymerization is usually 1 hour or more.
- Post-treatment of the polymerization reaction is carried out by a known method, for example, a method of removing water-soluble impurities by liquid separation, adding a reaction solution after the polymerization reaction to a lower alcohol such as methanol, filtering the deposited precipitate and drying it.
- the method of making it etc. is carried out alone or in combination.
- the purity of the polymer compound is low, it can be purified by a usual method such as crystallization, reprecipitation, continuous extraction with a Soxhlet extractor, column chromatography and the like.
- composition of the present invention comprises at least one material selected from the group consisting of a hole transport material, a hole injection material, an electron transport material, an electron injection material, a light emitting material, an antioxidant and a solvent; And a molecular compound.
- composition containing the polymer compound of the present invention and a solvent is suitable for producing a light emitting device using a printing method such as an inkjet printing method or a nozzle printing method.
- the viscosity of the ink may be adjusted according to the type of printing method, but in the case where a solution such as an inkjet printing method is applied to a printing method via a discharge device, in order to prevent clogging at the discharge and flying deflection. And preferably 1 to 20 mPa ⁇ s at 25 ° C.
- the solvent is preferably a solvent that can dissolve or uniformly disperse the solid content in the ink.
- chlorinated solvents such as 1,2-dichloroethane, 1,1,2-trichloroethane, chlorobenzene, o-dichlorobenzene, etc .
- ether solvents such as tetrahydrofuran, dioxane, anisole, 4-methylanisole; toluene,
- Aromatic hydrocarbon solvents such as xylene, mesitylene, ethylbenzene, n-hexylbenzene, cyclohexylbenzene; cyclohexane, methylcyclohexane, n-pentane, n-hexane, n-heptane, n-octane, n-nonane, n- Aliphatic hydrocarbon solvents such as decane, n-dodecane and bicyclohexyl
- the compounding amount of the solvent is usually 1000 to 100000 parts by mass, preferably 2000 to 20000 parts by mass with respect to 100 parts by mass of the polymer compound of the present invention.
- the solvents may be used alone or in combination of two or more.
- Hole transport materials are classified into low molecular weight compounds and high molecular weight compounds, and high molecular weight compounds are more preferable.
- the polymer compound include polyvinylcarbazole and derivatives thereof; polyarylenes having an aromatic amine structure in the side chain or main chain and derivatives thereof.
- the macromolecular compound may be a compound having an electron accepting moiety bound thereto. Examples of the electron accepting moiety include fullerene, tetrafluorotetracyanoquinodimethane, tetracyanoethylene, trinitrofluorenone and the like, with preference given to fullerene.
- the compounding amount of the hole transport material is usually 1 to 400 parts by mass, preferably 5 to 150 parts by mass with respect to 100 parts by mass of the polymer compound of the present invention.
- the hole transport material may be used alone or in combination of two or more.
- Electron transport materials are classified into low molecular weight compounds and high molecular weight compounds.
- the low molecular weight compound for example, metal complexes having 8-hydroxyquinoline as a ligand, oxadiazole, anthraquinodimethane, benzoquinone, naphthoquinone, anthraquinone, tetracyanoanthraquinodimethane, fluorenone, diphenyldicyanoethylene, and And diphenoquinone, as well as their derivatives.
- a high molecular compound polyphenylene, polyfluorene, and these derivatives are mentioned, for example.
- the polymer compound may be doped with metal.
- the compounding amount of the electron transporting material is usually 1 to 400 parts by mass, preferably 5 to 150 parts by mass with respect to 100 parts by mass of the polymer compound of the present invention.
- the electron transporting material may be used alone or in combination of two or more.
- the hole injecting material and the electron injecting material are classified into low molecular weight compounds and high molecular weight compounds, respectively.
- metal phthalocyanines such as copper phthalocyanine; Carbon; Metal oxides, such as molybdenum and tungsten; Metal fluorides, such as lithium fluoride, sodium fluoride, cesium fluoride, potassium fluoride, etc. are mentioned, for example.
- the polymer compound for example, polyaniline, polythiophene, polypyrrole, polyphenylene vinylene, polythienylene vinylene, polyquinoline, and polyquinoxaline and derivatives thereof; polymers containing an aromatic amine structure in the main chain or side chain, etc. And conductive polymers.
- the compounding amount of the hole injecting material and the electron injecting material is usually 1 to 400 parts by mass, preferably 5 to 150 parts by weight per 100 parts by mass of the polymer compound of the present invention. It is a mass part.
- the hole injecting material and the electron injecting material may be used alone or in combination of two or more.
- the conductivity of the conductive polymer is preferably 1 ⁇ 10 ⁇ 5 S / cm to 1 ⁇ 10 3 S / cm. is there.
- the conductive polymer can be doped with an appropriate amount of ions in order to bring the conductivity of the conductive polymer into such a range.
- the type of ion to be doped is an anion if it is a hole injecting material, and a cation if it is an electron injecting material.
- the anion include polystyrene sulfonate ion, alkyl benzene sulfonate ion and camphor sulfonate ion.
- the cation include lithium ion, sodium ion, potassium ion and tetrabutyl ammonium ion. Only one ion or two or more ions may be doped.
- Light emitting materials are classified into low molecular weight compounds and high molecular weight compounds.
- the low molecular weight compounds include, for example, naphthalene and its derivatives, anthracene and its derivatives, perylene and its derivatives, and a triplet light emitting complex having iridium, platinum or europium as a central metal.
- polymer compound for example, phenylene group, naphthalenediyl group, fluorenedyl group, phenanthrendiyl group, dihydrophenanthrendiyl group, group represented by formula (X), carbazole diyl group, phenoxazine diyl group, phenothiazine diyl Examples thereof include polymer compounds containing a group, an anthracenediyl group, a pyrenediyl group and the like.
- the light emitting material may contain a low molecular weight compound and a high molecular weight compound, and preferably contains a triplet light emitting complex and a high molecular weight compound.
- a triplet light emitting complex iridium complexes such as metal complexes represented by the formulas Ir-1 to Ir-5 are preferable.
- R D1 to R D8 , R D11 to R D20 , R D21 to R D26 and R D31 to R D37 each independently represent a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or an aryl group Represents an oxy group, a monovalent heterocyclic group or a halogen atom, and these groups may have a substituent.
- R D1 to R D8 , R D11 to R D20 , R D21 to R D26 and R D31 to R D37 are present in plurality, they may be the same or different.
- a D2 represents an anionic bidentate ligand
- a D1 and A D2 each independently represent a carbon atom, an oxygen atom or a nitrogen atom bonded to an iridium atom, and these The atom of may be an atom constituting a ring.
- n D1 represents 1, 2 or 3 and n D2 represents 1 or 2.
- At least one of R D1 to R D8 is preferably a group represented by the formula (DA).
- R D11 to R D20 is a group represented by the formula (DA).
- R D1 to R D8 and R D11 to R D20 is a group represented by the formula (DA).
- R 21 to R D26 is a group represented by the formula (DA).
- RD31 to RD37 is a group represented by the formula (DA).
- m DA1 , m DA2 and m DA3 each independently represent an integer of 0 or more.
- G DA represents a nitrogen atom, an aromatic hydrocarbon group or a heterocyclic group, and these groups may have a substituent.
- Ar DA1 , Ar DA2 and Ar DA3 each independently represent an arylene group or a divalent heterocyclic group, and these groups may have a substituent.
- T DA represents an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. The plurality of TDAs may be the same or different.
- m DA1 , m DA2 and m DA3 are usually an integer of 10 or less, preferably 5 or less, more preferably 0 or 1.
- m DA1 , m DA2 and m DA3 are the same integer.
- G DA is preferably a group represented by formulas (GDA-11) to (GDA-15), and these groups may have a substituent.
- R DA represents a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group, an aryl group or a monovalent heterocyclic group, and these groups may further have a substituent.
- RDAs When there are a plurality of RDAs , they may be the same or different.
- R DA is preferably a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group or a cycloalkoxy group, more preferably a hydrogen atom, an alkyl group or a cycloalkyl group, and these groups have a substituent May be
- Ar DA1 , Ar DA2 and Ar DA3 are preferably groups represented by Formula (ArDA-1) to Formula (ArDA-3).
- R DA has the same meaning as described above.
- R DB represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent. When there are multiple R DBs , they may be the same or different. ]
- T DA is preferably a group represented by Formula (TDA-1) to Formula (TDA-3).
- R DA and R DB represent the same meaning as described above.
- the group represented by the formula (D-A) is preferably a group represented by the formula (D-A1) to the formula (D-A3).
- R p1 , R p2 and R p3 each independently represent an alkyl group, a cycloalkyl group, an alkoxy group, a cycloalkoxy group or a halogen atom.
- R p1 and R p2 may be the same or different.
- np1 represents an integer of 0 to 5
- np2 represents an integer of 0 to 3
- np3 represents 0 or 1.
- the plurality of np1 may be the same or different.
- np1 is preferably an integer of 0 to 3, more preferably an integer of 1 to 3, and still more preferably 1.
- np2 is preferably 0 or 1, more preferably 0.
- np3 is preferably 0.
- R p1 , R p2 and R p3 are preferably an alkyl group or a cycloalkyl group.
- Examples of the anionic bidentate ligand represented by -A D1 --- A D2- include a ligand represented by the following formula.
- the metal complex represented by the formula Ir-1 is preferably a metal complex represented by the formula Ir-11 to Ir-13.
- the metal complex represented by the formula Ir-2 is preferably a metal complex represented by the formula Ir-21.
- the metal complex represented by the formula Ir-3 is preferably a metal complex represented by the formulas Ir-31 to Ir-33.
- the metal complex represented by the formula Ir-4 is preferably a metal complex represented by the formulas Ir-41 to Ir-43.
- the metal complex represented by the formula Ir-5 is preferably a metal complex represented by the formula Ir-51 to Ir-53.
- n D2 represents 1 or 2; D represents a group represented by formula (DA).
- a plurality of D may be the same or different.
- R DC represents a hydrogen atom, an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- a plurality of R DC may be the same or different.
- R DD represents an alkyl group, a cycloalkyl group, an aryl group or a monovalent heterocyclic group, and these groups may have a substituent.
- Plural R DD may be the same or different.
- the metal complex shown below is mentioned, for example.
- the compounding amount of the light emitting material is usually 0.1 to 400 parts by mass with respect to 100 parts by mass of the polymer compound of the present invention.
- the light emitting materials may be used alone or in combination of two or more.
- the antioxidant may be a compound which is soluble in the same solvent as the polymer compound of the present invention and does not inhibit light emission and charge transport, and examples thereof include phenol-based antioxidants and phosphorus-based antioxidants.
- the blending amount of the antioxidant is usually 0.001 to 10 parts by mass with respect to 100 parts by mass of the polymer compound of the present invention.
- the antioxidant may be used alone or in combination of two or more.
- the light emitting device of the present invention is a light emitting device provided with an organic layer containing the polymer compound of the present invention.
- the light emitting device of the present invention has, for example, an electrode comprising an anode and a cathode, and a layer containing the polymer compound of the present invention provided between the electrodes.
- the layer containing the polymer compound of the present invention is usually at least one layer of a light emitting layer, a hole transporting layer, a hole injecting layer, an electron transporting layer and an electron injecting layer, preferably a light emitting layer .
- Each of these layers contains a light emitting material, a hole transporting material, a hole injecting material, an electron transporting material, and an electron injecting material.
- Each of these layers is prepared by dissolving a light emitting material, a hole transporting material, a hole injecting material, an electron transporting material, and an electron injecting material in the above-mentioned solvent to prepare an ink, for example, spin coating, casting Method, microgravure coating method, gravure coating method, bar coating method, roll coating method, wire bar coating method, dip coating method, spray coating method, screen printing method, flexographic printing method, offset printing method, ink jet printing method, capillary It can be formed by a coating method or a nozzle coating method.
- the light emitting element has a light emitting layer between the anode and the cathode.
- the light emitting device of the present invention preferably has at least one of a hole injecting layer and a hole transporting layer between the anode and the light emitting layer from the viewpoint of the hole injecting property and the hole transporting property, From the viewpoint of injectability and electron transportability, it is preferable to have at least one of an electron injection layer and an electron transport layer between the cathode and the light emitting layer.
- the hole transport layer As materials of the hole transport layer, the electron transport layer, the light emitting layer, the hole injection layer, and the electron injection layer, in addition to the polymer compound of the present invention, the above-mentioned hole transport material, electron transport material, light emission Materials, hole injection materials, and electron injection materials can be mentioned.
- vacuum evaporation from powder for example A method, a method by film formation from a solution or molten state may be mentioned, and when using a polymer compound, for example, a method by film formation from a solution or molten state may be mentioned.
- the order, number, and thickness of the layers to be stacked may be adjusted in consideration of the light emission efficiency and the device life.
- the substrate in the light emitting element may be any substrate that can form an electrode and does not change chemically when forming an organic layer, and is, for example, a substrate made of a material such as glass, plastic, or silicon. In the case of an opaque substrate, it is preferred that the electrode furthest from the substrate be transparent or translucent.
- Examples of the material of the anode include conductive metal oxides and semitransparent metals, preferably indium oxide, zinc oxide, tin oxide; indium tin oxide (ITO), indium zinc oxide, etc. Conductive compounds; complexes of silver, palladium and copper (APC); NESA, gold, platinum, silver, copper.
- the material of the cathode includes, for example, metals such as lithium, sodium, potassium, rubidium, cesium, beryllium, magnesium, calcium, strontium, barium, aluminum, zinc and indium; alloys of two or more of them; one of them And alloys thereof with one or more species of silver, copper, manganese, titanium, cobalt, nickel, tungsten, and tin; and graphite and graphite intercalation compounds.
- the alloy include magnesium-silver alloy, magnesium-indium alloy, magnesium-aluminium alloy, indium-silver alloy, lithium-aluminium alloy, lithium-magnesium alloy, lithium-indium alloy, calcium-aluminium alloy.
- the anode and the cathode may each have a laminated structure of two or more layers.
- a planar anode and a cathode may be arranged to overlap.
- a method of installing a mask provided with a pattern-like window on the surface of a planar light-emitting element is used.
- both the anode and the cathode may be formed in stripes and arranged orthogonal to each other.
- Partial color display and multi-color display can be performed by a method of separately coating a plurality of types of polymer compounds different in emission color and a method of using a color filter or a fluorescence conversion filter.
- the dot matrix display device can be driven passively, or can be driven active in combination with a TFT or the like.
- These display devices can be used as displays of computers, televisions, portable terminals, and the like.
- a planar light emitting element can be suitably used as a planar light source for backlight of a liquid crystal display device or a planar light source for illumination. If a flexible substrate is used, it can be used as a curved light source and a display device.
- the polystyrene equivalent number average molecular weight (Mn) of the polymer compound and the polystyrene equivalent weight average molecular weight (Mw) are any of the following size exclusion chromatography (SEC) using tetrahydrofuran as a mobile phase: Determined by The SEC measurement conditions are as follows.
- the polymer compound to be measured was dissolved in tetrahydrofuran at a concentration of about 0.05% by mass, and 10 ⁇ L was injected into SEC.
- the mobile phase flowed at a flow rate of 1.0 mL / min.
- PLgel MIXED-B manufactured by Polymer Laboratories
- a UV-VIS detector manufactured by Tosoh, trade name: UV-8320GPC was used.
- LC-MS was measured by the following method.
- the measurement sample was dissolved in chloroform or tetrahydrofuran to a concentration of about 2 mg / mL, and about 1 ⁇ L was injected into LC-MS (manufactured by Agilent, trade name: 1290 Infinity LC and 6230 TOF LC / MS).
- the mobile phase of LC-MS was used while changing the ratio of acetonitrile and tetrahydrofuran, and flowed at a flow rate of 1.0 mL / min.
- SUMIPAX ODS Z-CLUE manufactured by Sumika Analysis Center, inner diameter: 4.6 mm, length: 250 mm, particle diameter 3 ⁇ m
- NMR NMR was measured by the following method. About 5 to 10 mg of a sample to be measured was added to about 0.5 mL of heavy chloroform (CDCl 3 ), heavy tetrahydrofuran, heavy dimethyl sulfoxide, heavy acetone, heavy N, N-dimethylformamide, heavy toluene, heavy methanol, heavy ethanol, heavy 2-propanol Alternatively, they were dissolved in methylene dichloride and measured using an NMR apparatus (manufactured by Agilent, trade name: INOVA 300, or manufactured by JEOL RESONANCE, trade name: JNM-ECZ400S / L1).
- HPLC high performance liquid chromatography
- an ODS column having SUMIPAX ODS Z-CLUE (manufactured by Sumika Analysis Center, inner diameter: 4.6 mm, length: 250 mm, particle diameter 3 ⁇ m) or equivalent performance was used.
- a detector a photodiode array detector (manufactured by Shimadzu Corporation, trade name: SPD-M20A) was used.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 1a (29.6 g, colorless oil).
- the HPLC area percentage value of compound 1a was 99.2%.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 1b (14.9 g, colorless oil).
- the HPLC area percentage value of compound 1b was 99.0%.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 1c (9.2 g, colorless oil).
- the HPLC area percentage value of compound 1c was 92.2%.
- the reaction vessel was changed to a nitrogen gas atmosphere, Compound 1c (7.5 g) and tert-butylbenzene (56 mL) were added, and after cooling to 0 ° C., t-BuLi ⁇ pentane solution was maintained there while maintaining 0 ° C. (1.5 M, 9.6 mL) was added slowly. The resulting mixture was stirred at 60 ° C. for 3 hours, and then pentane was distilled off under reduced pressure. The resulting mixture was cooled to ⁇ 50 ° C., BBr 3 (4.5 g) was added, and the mixture was stirred at ⁇ 50 ° C. for 0.5 hour. The resulting mixture was heated to 120 ° C. and allowed to react at 120 ° C.
- Synthesis Example 3 Synthesis and Acquisition of Compounds 3 to 8 and Compounds 11 to 12
- Compound 3 was synthesized according to the method described in JP-A-2010-189630.
- Compound 4 was synthesized according to the method described in JP-A-2007-512249.
- Compound 5 was synthesized according to the method described in JP-A-2008-106241.
- the compound 6 used the commercial item.
- Compound 7 was synthesized according to the method described in WO 2009/131255.
- Compound 8 was synthesized according to the method described in WO 2016/031639.
- Synthesis Example 4 Synthesis of Polymer Compound 1 (Step 1) After setting the inside of the reaction vessel to an inert gas atmosphere, compound 3 (3.995 g), compound 4 (6.237 g), compound 5 (0.519 g), dichlorobis (tris-o-methoxyphenylphosphine) palladium (7.1) mg) and toluene (190 mL) were added and heated to 105.degree. (Step 2) Thereafter, a 20% by mass aqueous tetraethylammonium hydroxide solution (28 mL) was dropped thereto, and the mixture was refluxed for 4 hours.
- phenylboronic acid 97.5 mg
- 20% by mass aqueous tetraethylammonium hydroxide 28 mL
- dichlorobis tris-o-methoxyphenylphosphine
- palladium 7. mg
- the resulting reaction mixture was cooled, and then washed twice with water, twice with a 10% by mass aqueous hydrochloric acid solution, twice with a 3% by mass aqueous ammonia solution, and twice with water.
- the resulting solution was added dropwise to methanol and stirred, whereupon a precipitate formed.
- the obtained precipitate was dissolved in toluene and purified by passing through an alumina column and a silica gel column in this order. The resulting solution was added dropwise to methanol and stirred, whereupon a precipitate formed. The obtained precipitate was collected by filtration and dried to obtain 6.65 g of polymer compound 1.
- the Mn of the polymer compound 1 was 2.6 ⁇ 10 4
- the Mw was 1.4 ⁇ 10 5 .
- the polymer compound 1 has a structural unit derived from the compound 3, a structural unit derived from the compound 4, and a structural unit derived from the compound 5 in the theoretical value determined from the amount of the feed material: 50: It is a copolymer comprised by the molar ratio of 42.5: 7.5.
- Step 1 Synthesis of Polymer Compound 2
- compound 2 (0.104 g)
- compound 6 0.502 g
- compound 7 0.628 g
- dichlorobis tris-o-methoxyphenylphosphine
- toluene 27 mL
- Step 2 After that, a 10% by mass aqueous tetraethylammonium hydroxide solution (18 mL) was added dropwise thereto, and the mixture was refluxed for 4 hours.
- the resulting solution was added dropwise to methanol and stirred, whereupon a precipitate formed.
- the obtained precipitate was collected by filtration and dried to obtain 0.68 g of polymer compound 2.
- the Mn of the polymer compound 2 was 4.5 ⁇ 10 4
- the Mw was 1.1 ⁇ 10 5 .
- the polymer compound 2 has a theoretical value determined from the amount of the feed materials: the constituent unit derived from the compound 6, the constituent unit derived from the compound 7, and the constituent unit derived from the compound 2: 45 It is a copolymer comprised by the molar ratio of 50: 5.
- Step 1 Synthesis of Polymer Compound 3
- (Step 1) is “in the reaction vessel with an inert gas atmosphere, then Compound 8 (0.096 g), Compound 6 (0.916 g), Compound Polymer compound 3 in the same manner as in Example 1 except that 7 (1.119 g), dichlorobis (phenylphosphine) palladium (1.5 mg) and toluene (47 mL) were mixed and heated to 105.degree. I got 1.1 g.
- the Mn of the polymer compound 3 was 5.0 ⁇ 10 4
- the Mw was 1.1 ⁇ 10 5 .
- the polymer compound 3 is a theoretical value determined from the amount of the charged raw material, and the constituent unit derived from the compound 6, the constituent unit derived from the compound 7, and the constituent unit derived from the compound 8 are 45: It is a copolymer comprised by the molar ratio of 50: 5.
- Example D1 Production and Evaluation of Light-Emitting Element D1 An anode was formed by attaching an ITO film with a thickness of 45 nm to a glass substrate by a sputtering method. Form a hole injection material ND-3202 (manufactured by Nissan Chemical Industries) on the anode to a thickness of 35 nm by spin coating, and heat it on a hot plate at 240 ° C for 15 minutes in an air atmosphere. Thus, a hole injection layer was formed. The polymer compound 1 was dissolved in xylene at a concentration of 0.6% by mass.
- ND-3202 manufactured by Nissan Chemical Industries
- a film is formed to a thickness of 20 nm by spin coating on the hole injection layer, and positive heating is performed by heating at 200 ° C. for 30 minutes on a hot plate under a nitrogen gas atmosphere. A hole transport layer was formed.
- the polymer compound 2 was dissolved in xylene at a concentration of 1.3% by mass.
- a film is formed to a thickness of 60 nm by spin coating on the hole transport layer, and light emission is obtained by heating at 150 ° C. for 10 minutes on a hot plate under a nitrogen gas atmosphere. A layer was formed.
- sodium fluoride is about 7 nm on the light emitting layer and then on the sodium fluoride layer as a cathode.
- Aluminum was deposited to about 120 nm.
- sealing was performed using a glass substrate, to fabricate a light emitting device D1.
- the maximum external quantum yield was 4.1%, and EL emission having a maximum peak wavelength of the emission spectrum at 460 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.142, 0.071).
- driving was performed at a constant current density, and the time change of luminance was measured. The time for the luminance to reach 60% of the initial luminance was 44 hours.
- Example D2 Production and Evaluation of Light-Emitting Element D2
- the composition of Polymer Compound 2 and Polymer Compound 3 was used in place of Polymer Compound 2 in a mass ratio of 50:50.
- a light emitting device D2 was produced in the same manner as in Example D1.
- the maximum external quantum yield was 3.3%, and EL emission having the maximum peak wavelength of the emission spectrum at 460 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.145, 0.067).
- driving was performed at a constant current density, and the time change of luminance was measured. The time for the luminance to reach 60% of the initial luminance was 54 hours.
- Example CD1 Comparative Example CD1 Production and Evaluation of Light Emitting Element CD1
- a light emitting element CD1 was produced in the same manner as in Example D1, except that the polymer compound 3 was used in place of the polymer compound 1 in Example D1.
- the maximum external quantum yield was 3.1%
- EL emission having the maximum peak wavelength of the light emission spectrum at 440 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.156, 0.063).
- driving was performed with a constant current, and the time change of luminance was measured. The time for the luminance to reach 60% of the initial luminance was 38 hours.
- reaction vessel was changed to an argon gas atmosphere, and then compound 9-1 (169.5 g), 4-hexylaniline (84.8 g), Pd 2 (dba) 3 (dba) 0.75 (6.26 g), t-Bu 3 PHBF 4 (3.33 g), t-BuONa (55.13 g) and toluene (2117 mL) were added and stirred at 20 ° C. for 1.5 hours.
- the resulting mixture was filtered through a short column stacked with silica gel and celite, and the obtained filtrate was concentrated under reduced pressure to obtain a crude product.
- the obtained crude product was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 9-2 (181.3 g, oil).
- the HPLC area percentage value of compound 9-2 was 99%.
- the required amount of compound 9-2 was obtained by repeating these operations.
- the reaction vessel was changed to an argon gas atmosphere, then 1-bromo-2,3-dichlorobenzene (77.2 g), compound 9-2 (197.5 g), Pd 2 (dba) 3 (dba) 0.75 (6.11 g), t-Bu 3 PHBF 4 (3.24 g), t-BuONa (53.3 g) and toluene (1337 g) were added and stirred at 50 ° C. for 4 hours. The resulting mixture was cooled to room temperature and then filtered through a short column stacked with silica gel and celite. The obtained filtrate was concentrated under reduced pressure to obtain a crude product.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 9-4 (149 g, colorless oil).
- the HPLC area percentage value of compound 9-4 was 94.5%.
- reaction vessel was changed to a nitrogen gas atmosphere, compound 9-4 (132.4 g) and tert-butylbenzene (811 mL) were added, and after cooling to 0 ° C., t-BuLi ⁇ pentane solution (1.5 M, 151 mL) was added added. The resulting mixture was stirred at 40 ° C. for 1 hour and then at 50 ° C. for 1 hour. The resulting mixture was cooled to ⁇ 50 ° C., BBr 3 (27.7 mL) was added, and the mixture was stirred at ⁇ 50 ° C. for 0.5 hours.
- the resulting mixture was warmed to 0 ° C., and N, N-diisopropylamine (48.8 mL) was added. The temperature of the resulting mixture was raised to 60 ° C., and pentane was distilled off. The resulting mixture was heated to 120 ° C. and allowed to react for 3 hours. The resulting mixture was cooled to room temperature, aqueous sodium acetate solution and ethyl acetate were added, and the obtained organic layer was washed with ion-exchanged water. The obtained organic layer was concentrated under reduced pressure to obtain a yellow oil.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 9-5 (16.4 g, yellow solid).
- the HPLC area percentage value of compound 9-5 was 96.9%.
- the resulting mixture was filtered through a short silica gel column, and the obtained filtrate was concentrated under reduced pressure to give a yellow oil.
- the obtained oil was recrystallized with heptane to obtain a pale yellow powder.
- the obtained powder was recrystallized with a mixed solution of toluene and ethanol, and dried to give compound 9-6 (7.56 g, yellow powder).
- the HPLC area percentage value of compound 9-6 was 90.7%.
- the reaction vessel was changed to a nitrogen gas atmosphere, and then 1,3,5-tribromobenzene (10.4 g), compound 9-6 (7.5 g), toluene (112 mL) and tetrakis (triphenylphosphine) palladium (0) ( 0.228 g) was added, and then 12 mass% tetrabutylammonium hydroxide aqueous solution (43 g) was added, and the mixture was heated to reflux for 3 hours. The resulting mixture was cooled to room temperature, then the aqueous layer was separated, and the resulting organic layer was washed twice with water.
- the obtained crude product was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 10-2 (5.26 g, pale red white powder).
- the HPLC area percentage value of compound 10-2 was 89.8%.
- the obtained crude product was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 10-3 (13.16 g, pale yellow solid).
- the HPLC area percentage value of compound 10-3 was 99.3%.
- the reaction vessel was an argon gas atmosphere, compound 10-2 (5.20 g), compound 10-3 (5.25g), Pd 2 ( dba) 3 (dba) 0.75 (0.452g), t-Bu 3 PHBF 4 (0.252 g), t-BuONa (2.39 g) and toluene (104 mL) were added and stirred at 50 ° C. for 2 hours.
- the resulting mixture was cooled to room temperature and then filtered through a short column stacked with silica gel and celite. The obtained filtrate was concentrated under reduced pressure to obtain a brown solid.
- the obtained solid was purified by silica gel column chromatography (mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain compound 10-4 (5.69 g, white solid).
- the HPLC area percentage value of compound 10-4 was 98.4%.
- reaction vessel was changed to a nitrogen gas atmosphere, and then compound 10-4 (4.60 g), o-dichlorobenzene (64 mL) and BBr 3 (3.3 g) were added, and the mixture was stirred at 140 ° C. for 6 hours.
- the resulting mixture was cooled to room temperature, then toluene (64 mL) and N, N-diisopropylamine (7.9 mL) were added and stirred.
- the resulting mixture was filtered through a short column stacked with silica gel and celite, and the obtained filtrate was concentrated under reduced pressure to obtain a brown oil.
- the obtained oil was purified by silica gel column chromatography (a mixed solvent of hexane and toluene), concentrated under reduced pressure, and dried to obtain a yellow powder.
- the obtained powder was recrystallized with a mixed solvent of toluene and acetonitrile to give compound 10-5 (1.55 g, yellow solid).
- the HPLC area percentage value of compound 10-5 was 99.3%.
- the required amount of compound 10-5 was obtained by repeating these operations.
- the obtained oil was recrystallized with acetonitrile to obtain a pale yellow powder.
- the obtained powder was dissolved in a mixed solvent of toluene and heptane, and then activated carbon was added and stirred. The resulting mixture was filtered using celite, concentrated under reduced pressure, and dried to give a yellow powder.
- the obtained powder was recrystallized with a mixed solution of toluene and acetonitrile, and the operation of drying was repeated twice to obtain Compound 10 (1.48 g, yellow powder).
- the HPLC area percentage value of compound 10 was 99.8%.
- Example 2 Synthesis of polymer compound 4 After setting the inside of the reaction vessel to an inert gas atmosphere, compound 6 (0.633 g), compound 8 (0.063 g), compound 7 (0.723 g), compound 9 (0.026 g) Dichlorobis (tris-o-methoxyphenylphosphine) palladium (1.02 mg) and toluene (24.2 g) were mixed and heated to 80.degree. Then, 10 mass% tetraethylammonium hydroxide aqueous solution (20 mL) was dripped there, and it was made to reflux for 4.5 hours.
- phenylboronic acid 55.8 mg
- dichlorobis tris-o-methoxyphenylphosphine
- palladium 1.01 mg
- the reaction solution was washed once with water, twice with 10% by mass aqueous hydrochloric acid solution, twice with 3% by mass aqueous ammonia solution, and twice with water.
- the water was removed by distilling the obtained organic layer under reduced pressure.
- the resulting solution was purified by passing it through a column packed with a mixture of alumina and silica gel.
- the obtained solution was added dropwise to methanol and stirred, and then the obtained precipitate was collected by filtration and dried, to obtain 0.54 g of polymer compound 4.
- the Mn of the polymer compound 4 was 4.5 ⁇ 10 4
- the Mw was 1.1 ⁇ 10 5 .
- the polymer compound 4 is a theoretical value determined from the amount of the raw materials charged, and the structural unit derived from the compound 6, the structural unit derived from the compound 8, the structural unit derived from the compound 7, and the compound 9 It is a copolymer in which the constituent units to be derived are constituted at a molar ratio of 45: 5: 49: 1.
- phenylboronic acid 112.2 g
- dichlorobis tris-o-methoxyphenylphosphine
- palladium 2.13 mg
- the reaction solution was washed once with water, twice with 10 wt% aqueous hydrochloric acid solution, twice with 3 wt% aqueous ammonia solution, and twice with water.
- the water was removed by distilling the obtained organic layer under reduced pressure.
- the resulting solution was purified by passing it through a column packed with a mixture of alumina and silica gel.
- the obtained solution was added dropwise to methanol and stirred, and then the resulting precipitate was collected by filtration and dried to obtain 1.06 g of polymer compound Polymer compound 5.
- the Mn of the polymer compound 5 was 7.7 ⁇ 10 4 and the Mw was 1.7 ⁇ 10 5 .
- the polymer compound 5 is a theoretical value determined from the amount of the raw materials charged, and the structural unit derived from the compound 6, the structural unit derived from the compound 8, the structural unit derived from the compound 10, and the compound 7 It is a copolymer in which the constituent units to be derived are constituted at a molar ratio of 44: 5: 1: 50.
- Synthesis Example 8 Synthesis of Polymer Compound 6 Polymer compound 6 was synthesized according to the method of polymer example 1 described in WO 2014/102543.
- Example D3 Production and Evaluation of Light-Emitting Element D3 An anode was formed by attaching an ITO film with a thickness of 45 nm to a glass substrate by a sputtering method. Form a hole injection material ND-3202 (manufactured by Nissan Chemical Industries) on the anode to a thickness of 35 nm by spin coating, and heat it on a hot plate at 240 ° C for 15 minutes in an air atmosphere. Thus, a hole injection layer was formed. The polymer compound 6 was dissolved in xylene at a concentration of 0.6% by mass.
- ND-3202 manufactured by Nissan Chemical Industries
- a film is formed to a thickness of 20 nm by spin coating on the hole injection layer, and positive heating is performed by heating at 200 ° C. for 30 minutes on a hot plate under a nitrogen gas atmosphere. A hole transport layer was formed.
- the polymer compound 4 was dissolved in xylene at a concentration of 1.3% by mass.
- a film is formed to a thickness of 60 nm by spin coating on the hole transport layer, and light emission is obtained by heating at 150 ° C. for 10 minutes on a hot plate under a nitrogen gas atmosphere. A layer was formed.
- sodium fluoride is about 7 nm on the light emitting layer and then on the sodium fluoride layer as a cathode.
- Aluminum was deposited to about 120 nm.
- sealing was performed using a glass substrate, to fabricate a light emitting device D3.
- the maximum external quantum yield was 8.1%, and EL emission having the maximum peak wavelength of the emission spectrum at 465 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.131, 0.095).
- driving was performed at a constant current density, and the time change of luminance was measured. The time for the luminance to reach 50% of the initial luminance was 42 hours.
- Example D4 Production and Evaluation of Light Emitting Element D4
- a light emitting element D4 was produced in the same manner as in Example D3, except that the polymer compound 5 was used instead of the polymer compound 4 in Example D3. .
- the maximum external quantum yield was 7.8%, and EL light emission having a maximum peak wavelength of the light emission spectrum at 450 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.149, 0.045).
- driving was performed at a constant current density, and the time change of luminance was measured. The time for the luminance to reach 50% of the initial luminance was 44 hours.
- Example CD2 Comparative Example CD2 Production and Evaluation of Light Emitting Element CD2
- a light emitting element CD2 was produced in the same manner as in Example D3, except that the polymer compound 3 was used in place of the polymer compound 4 in Example D3.
- the maximum external quantum yield was 7.0%, and EL emission having a maximum peak wavelength of the emission spectrum at 440 nm was observed.
- the CIE 1931 chromaticity coordinates at this time were (0.153, 0.055).
- driving was performed at a constant current density, and the time change of luminance was measured. The time for the luminance to reach 50% of the initial luminance was 21 hours.
- a polymer compound useful for the production of a light emitting device excellent in luminance life it is possible to provide a polymer compound useful for the production of a light emitting device excellent in luminance life. Further, according to the present invention, a composition containing the polymer compound and a light emitting device containing the polymer compound can be provided.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Polymers & Plastics (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
- Polyoxymethylene Polymers And Polymers With Carbon-To-Carbon Bonds (AREA)
- Optics & Photonics (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
そこで、本発明は、輝度寿命に優れる発光素子の製造に有用な高分子化合物を提供することを目的とする。
A環、B環及びC環は、それぞれ独立に、芳香族炭化水素環又は芳香族複素環を表し、これらの環は置換基を有していてもよい。
Xは、ホウ素原子、リン原子、P=O、P=S、アルミニウム原子、ガリウム原子、ヒ素原子、Si-Rx又はGe-Rxを表す。Rxは、アリール基又はアルキル基を表し、これらの基は置換基を有していてもよい。
Y1は、N-Ry、硫黄原子又はセレン原子を表す。Y2及びY3は、それぞれ独立に、酸素原子、N-Ry、硫黄原子又はセレン原子を表す。Ryは、水素原子、アリール基、1価の複素環基、又はアルキル基を表し、これらの基は置換基を有していてもよい。Ryが複数存在する場合、同一であっても異なっていてもよい。Ryは、直接又は連結基を介して、前記A環、前記B環又は前記C環と結合していてもよい。
n3は、0又は1である。n3が0である場合、-Y3-は存在しない。]
[2]前記式(1)で表される化合物の残基を有する構成単位が、式(2)又は式(2')で表される構成単位である、[1]に記載の高分子化合物。
nAは、0~5の整数である。
nは、1又は2である。
Ar3は、芳香族炭化水素基又は複素環基を表し、これらの基は置換基を有していてもよい。
LAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。LAが複数存在する場合、それらは同一でも異なっていてもよい。
Qは、前記式(1)で表される化合物の残基を表す。]
mは1~4の整数である。
mAは0~5の整数である。mAが複数存在する場合、それらは同一でも異なっていてもよい。
cは0又は1である。
Ar4及びAr6は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。
Ar5は、芳香族炭化水素基、複素環基、又は、少なくとも1種の芳香族炭化水素環と少なくとも1種の複素環とが結合した基を表し、これらの基は置換基を有していてもよい。
Ar4、Ar5及びAr6は、それぞれ、該基が結合している窒素原子に結合している該基以外の基と、直接結合して、又は、酸素原子若しくは硫黄原子を介して結合して、環を形成していてもよい。
KAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。KAが複数存在する場合、それらは同一でも異なっていてもよい。
Q’は、前記式(1)で表される化合物の残基、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。Q’が複数存在する場合、それらは同一でも異なっていてもよい。但し、少なくとも1つのQ’は、前記式(1)で表される化合物の残基である。]
[3]前記式(1)で表される化合物の残基を有する構成単位が、式(3)で表される構成単位である、[1]に記載の高分子化合物。
d、e、f及びgは、それぞれ独立に、0~2の整数である。
pAは、0~5の整数である。pAが複数存在する場合、それらは同一でも異なっていてもよい。
JAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。JAが複数存在する場合、それらは同一でも異なっていてもよい。
Ar7、Ar8及びAr9は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。Ar7、Ar8及びAr9が複数存在する場合、それらは各々同一でも異なっていてもよい。
RX4は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RX4が複数存在する場合、それらは同一でも異なっていてもよい。
Q’’は、前記式(1)で表される化合物の残基を表す。]
[4]前記Xがホウ素原子である、[1]~[3]のいずれかに記載の高分子化合物。
[5]前記Y1、Y2及びY3がN-Ryである、[1]~[4]のいずれかに記載の高分子化合物。
[6]前記n3が0である、[1]~[5]のいずれかに記載の高分子化合物。
[7]前記式(2)で表される構成単位が式(31)で表される構成単位である、[2]に記載の高分子化合物。
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11は、それぞれ独立に、結合手、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基、又は置換アミノ基を表し、これらの基は置換基を有していてもよい。但し、R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11のうちの1つは結合手である。
Ry、Ar3、LA及びnAは、前記と同じ意味を表す。]
[8]前記式(2')で表される構成単位が式(41)で表される構成単位である、[2]に記載の高分子化合物。
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11は、それぞれ独立に、結合手、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基、又は、置換アミノ基を表し、これらの基は置換基を有していてもよい。但し、R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11のうちの1つは結合手である。
Ry、Ar4、Ar6、KA及びmAは、前記と同じ意味を表す。]
[9]前記式(3)で表される構成単位が式(51)で表される構成単位である、[3]に記載の高分子化合物
R21、R22、R23、R24、R25、R26、R27、R28、R29、R30及びR31は、それぞれ独立に、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基、又は、置換アミノ基を表し、これらの基は置換基を有していてもよい。
Ry’は、直接結合、アリーレン基、2価の複素環基、又はアルキレン基を表し、これらの基は置換基を有していてもよい。複数存在するRy’は、同一でも異なっていてもよい。
Ar7、Ar8、Ar9、RX4、JA,pA、d、e、f及びgは、前記と同じ意味を表す。]
[10]前記式(1)で表される化合物の残基を有さない構成単位が、式(X)で表される構成単位及び式(Y)で表される構成単位からなる群から選ばれる少なくとも1種の構成単位である、[1]~[9]のいずれかに記載の高分子化合物。
a1及びa2は、それぞれ独立に、0~2の整数である。
ArX1及びArX3は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。
ArX2及びArX4は、それぞれ独立に、アリーレン基、2価の複素環基、又は、少なくとも1種のアリーレン基と少なくとも1種の2価の複素環基とが結合した2価の基を表し、これらの基は置換基を有していてもよい。ArX2及びArX4が複数存在する場合、それらは同一でも異なっていてもよい。
RX1、RX2及びRX3は、それぞれ独立に、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RX2及びRX3が複数存在する場合、それらは同一でも異なっていてもよい。]
[11]前記式(Y)で表される構成単位が、式(Y-1)で表される構成単位又は式(Y-2)で表される構成単位である、[10]に記載の高分子化合物。
RY1は前記と同じ意味を表す。
XY1は、-C(RY2)2-、-C(RY2)=C(RY2)-又は-C(RY2)2-C(RY2)2-で表される基を表す。RY2は、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。複数存在するRY2は、同一でも異なっていてもよく、RY2同士は互いに結合して、それぞれが結合する炭素原子と共に環を形成していてもよい。]
[12]前記式(2)で表される構成単位、及び式(2')で表される構成単位の合計量が、高分子化合物に含まれる全構成単位の合計量に対して、0.1~50モル%である、[2]、[4]~[8]、[10]及び[11]のいずれかに記載の高分子化合物。
[13]前記式(3)で表される構成単位の合計量が、高分子化合物に含まれる全構成単位の合計量に対して、0.1~50モル%である、[3]~[6]及び[9]~[11]のいずれかに記載の高分子化合物。
[14]正孔輸送材料、正孔注入材料、電子輸送材料、電子注入材料、発光材料、酸化防止剤及び溶媒からなる群より選ばれる少なくとも1種の材料と、[1]~[13]のいずれかに記載の高分子化合物と、を含有する組成物。
[15][1]~[13]のいずれかに記載の高分子化合物を含有する有機層を備えた発光素子。
本明細書で共通して用いられる用語は、特記しない限り、以下の意味である。
本発明の高分子化合物は、前記式(1)で表される化合物の残基を有する構成単位と、前記式(1)で表される化合物の残基を有さない構成単位とを含む。式(1)で表される化合物の残基とは、式(1)で表される化合物の骨格構造(即ち、置換基以外の部分)から水素原子を1個又は2個以上除いた基である。式(1)で表される化合物の残基は、高分子化合物の主鎖、側鎖及び末端のいずれに存在していてもよい。式(1)で表される化合物の残基を有する構成単位及び式(1)で表される化合物の残基を有さない構成単位は、各々、高分子化合物中に、1種のみ含まれていても2種以上含まれていてもよい。
A環、B環及びC環で表される芳香族炭化水素環の炭素原子数は、置換基の炭素原子数を含めないで、通常、6~60であり、好ましくは6~18であり、より好ましくは6~10であり、特に好ましくは6である。芳香族炭化水素環としては、例えば、ベンゼン、フルオレン、ナフタレン、アントラセン、フェナントレンが挙げられ、好ましくはベンゼンである。
Raは、前記と同じ意味を表す。
水素原子は、A環が有していてもよい置換基に置き換わっていてもよい。
RY2は、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。-C(RY2)2-、-CRY2=CRY2-、又は-C(RY2)2-C(RY2)2-で表される基において、複数存在するRY2のうち、2個のRY2が互いに結合して環を形成していてもよい。複数存在するRY2は、同一でも異なっていてもよい。
RY4は、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RY4が複数存在する場合、それらは同一でも異なっていてもよい。]
nAは、本発明の発光素子の輝度寿命がより優れるので、好ましくは0又は1であり、より好ましくは0である。
mAは、本発明の発光素子の輝度寿命がより優れるので、好ましくは0又は1であり、より好ましくは0である。
式(2)で表される構成単位としては、例えば、式(2-1)~式(2-17)で表される構成単位が挙げられる。式(2')で表される構成単位としては、例えば、式(2'-1)~式(2'-14)で表される構成単位が挙げられる。これらの中でも、本発明の発光素子の輝度寿命が優れるので、好ましくは式(2-1)~式(2-14)、又は式(2'-1)~式(2'-9)で表される構成単位であり、より好ましくは式(2-5)~式(2-14)、又は式(2'-1)~式(2'-6)で表される構成単位であり、更に好ましくは式(2-5)~式(2-10)、又は式(2'-1)~式(2'-3)で表される構成単位である。
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、Ry、Ry'、Ar3、LA、及びnAは、前記と同じ意味を表す。
R4’、R5’、R6’、R7’、R8’、R9’、R10’及びR11’は、それぞれ独立に、結合手、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基、又は置換アミノ基を表し、これらの基は置換基を有していてもよい。
但し、式(32)において、R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11のうちの1つは結合手であり、式(38)において、R1、R4、R5、R6、R9、R10、R11、R4’、R5’、R6’、R7’、R8’、R9’、R10’及びR11’のうちの1つは結合手である。]
R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、Ry、Ry'、Ar4、Ar6、KA、及びmAは、前記と同じ意味を表す。
R4’、R5’、R6’、R7’、R8’、R9’、R10’及びR11’は、前記と同じ意味を表す。
但し、式(42)において、R1、R2、R3、R4、R5、R6、R7、R8、R9、R10及びR11のうちの1つは結合手であり、式(48)において、R1、R4、R5、R6、R9、R10、R11、R4’、R5’、R6’、R7’、R8’、R9’、R10’及びR11’のうちの1つは結合手である。]
d、e、f及びgは、本発明の発光素子の輝度寿命がより優れるので、好ましくは、0又は1であり、より好ましくは0である。
式(3)で表される構成単位としては、例えば、式(3-1)~式(3-12)で表される構成単位が挙げられる。これらの中でも、本発明の発光素子の輝度寿命が優れるので、好ましくは、式(3-1)~式(3-6)で表される構成単位であり、より好ましくは、式(3-1)~式(3-3)で表される構成単位であり、更に好ましくは、式(3-1)で表される構成単位である。
R21、R22、R23、R24、R25、R26、R27、R28、R29、R30、R31、Ry、Ry'、Ar7、Ar8、Ar9、JA、RX4、pA、d、e、f及びgは、前記と同じ意味を表す。
RX4’は、直接結合、アルキレン基、シクロアルキレン基、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。
Ar9’は、3価の芳香族炭化水素基または3価の複素環基を表し、これらの基は置換基を有していてもよい。
但し、式(52)において、R24、R25、R26及びR27のうちの1つは結合手であり、且つ、R28、R29、R30及びR31のうちの1つは結合手であり、式(53)において、R21、R22、R23、R24、R25、R26、R27、R28、R29、R30及びR31のうちの1つは結合手であり、式(55)において、R21、R24、R25、R26、R27、R28、R29、R30、及びR31のうちの1つは結合手である。]
式(1)で表される化合物の残基を有さない構成単位としては、例えば、式(X)で表される構成単位及び式(Y)で表される構成単位が挙げられる。
式(X)で表される構成単位、及び、式(Y)で表される構成単位は、各々、高分子化合物中に、1種のみ含まれていても2種以上含まれていてもよい。
a1は、本発明の発光素子の輝度寿命がより優れるので、好ましくは1である。
ArY1で表されるアリーレン基は、より好ましくは式(A-1)、式(A-6)、式(A-7)、式(A-9)~式(A-11)、式(A-13)又は式(A-19)で表される基であり、更に好ましくは式(A-1)、式(A-7)、式(A-9)又は式(A-19)で表される基であり、特に好ましくは式(A-1)又は式(A-9)で表される基であり、これらの基は置換基を有していてもよい。
RY1は前記と同じ意味を表す。
RY3は、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。]
RY1は前記を同じ意味を表す。
RY4は、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。]
本発明の高分子化合物の製造方法について説明する。式(1)で表される化合物に後述の置換基A群及び置換基B群からなる群から選ばれる基を有する化合物をとを反応させることで、縮合重合に用いる原料モノマーが得られる(下記式参照)。この原料モノマーを縮合重合に供することで、式(1)で表される化合物の残基を有する構成単位が誘導される。なお、本明細書において、本発明の高分子化合物の製造に使用される化合物を総称して、「原料モノマー」ということがある。
Qは、前記と同じ意味を表す。
dは、1~4の整数を表す。
ZC0は、後述の置換基A群及び置換基B群からなる群から選ばれる基を表す。ZC0が複数存在する場合、それらは同一であっても異なっていてもよい。]
nA、n、Ar3、LA、Q、mA、m、c、Ar4~Ar6、KA、Q’、ArY1、a1、a2、ArX1~ArX4、及びRX1~RX3は、前記と同じ意味を表す。
ZC1~ZC10は、それぞれ独立に、置換基A群及び置換基B群からなる群から選ばれる基を表す。]
塩素原子、臭素原子、ヨウ素原子、-O-S(=O)2RC1(式中、RC1は、アルキル基、シクロアルキル基又はアリール基を表し、これらの基は置換基を有していてもよい。)で表される基。
-B(ORC2)2(式中、RC2は、水素原子、アルキル基、シクロアルキル基又はアリール基を表し、これらの基は置換基を有していてもよい。複数存在するRC2は同一でも異なっていてもよく、互いに連結して、それぞれが結合する酸素原子とともに環構造を形成していてもよい。)で表される基;
-BF3Q'(式中、Q'は、Li、Na、K、Rb又はCsを表す。)で表される基;
-MgY'(式中、Y'は、塩素原子、臭素原子又はヨウ素原子を表す。)で表される基;
-ZnY''(式中、Y''は、塩素原子、臭素原子又はヨウ素原子を表す。)で表される基;及び、
-Sn(RC3)3(式中、RC3は、水素原子、アルキル基、シクロアルキル基又はアリール基を表し、これらの基は置換基を有していてもよい。複数存在するRC3は同一でも異なっていてもよく、互いに連結して、それぞれが結合するスズ原子とともに環構造を形成していてもよい。)で表される基。
本発明の組成物は、正孔輸送材料、正孔注入材料、電子輸送材料、電子注入材料、発光材料、酸化防止剤及び溶媒からなる群から選ばれる少なくとも1種の材料と、本発明の高分子化合物とを含有する。
溶媒は、1種単独で用いても2種以上を併用してもよい。
正孔輸送材料は、低分子化合物と高分子化合物とに分類され、高分子化合物がより好ましい。
高分子化合物としては、例えば、ポリビニルカルバゾール及びその誘導体;側鎖又は主鎖に芳香族アミン構造を有するポリアリーレン及びその誘導体が挙げられる。高分子化合物は、電子受容性部位が結合された化合物でもよい。電子受容性部位としては、例えば、フラーレン、テトラフルオロテトラシアノキノジメタン、テトラシアノエチレン、トリニトロフルオレノン等が挙げられ、好ましくはフラーレンである。
本発明の組成物において、正孔輸送材料の配合量は、本発明の高分子化合物100質量部に対して、通常、1~400質量部であり、好ましくは5~150質量部である。
正孔輸送材料は、1種単独で用いても2種以上を併用してもよい。
電子輸送材料は、低分子化合物と高分子化合物とに分類される。
低分子化合物としては、例えば、8-ヒドロキシキノリンを配位子とする金属錯体、オキサジアゾール、アントラキノジメタン、ベンゾキノン、ナフトキノン、アントラキノン、テトラシアノアントラキノジメタン、フルオレノン、ジフェニルジシアノエチレン、及び、ジフェノキノン、並びに、これらの誘導体が挙げられる。
高分子化合物としては、例えば、ポリフェニレン、ポリフルオレン、及び、これらの誘導体が挙げられる。高分子化合物は、金属でドープされていてもよい。
本発明の組成物において、電子輸送材料の配合量は、本発明の高分子化合物100質量部に対して、通常、1~400質量部であり、好ましくは5~150質量部である。
電子輸送材料は、1種単独で用いても2種以上を併用してもよい。
正孔注入材料及び電子注入材料は、各々、低分子化合物と高分子化合物とに分類される。
低分子化合物としては、例えば、銅フタロシアニン等の金属フタロシアニン;カーボン;モリブデン、タングステン等の金属酸化物;フッ化リチウム、フッ化ナトリウム、フッ化セシウム、フッ化カリウム等の金属フッ化物が挙げられる。
高分子化合物としては、例えば、ポリアニリン、ポリチオフェン、ポリピロール、ポリフェニレンビニレン、ポリチエニレンビニレン、ポリキノリン、及び、ポリキノキサリン、並びに、これらの誘導体;芳香族アミン構造を主鎖又は側鎖に含む重合体等の導電性高分子が挙げられる。
本発明の組成物において、正孔注入材料及び電子注入材料の配合量は、各々、本発明の高分子化合物100質量部に対して、通常、1~400質量部であり、好ましくは5~150質量部である。
正孔注入材料及び電子注入材料は、各々、1種単独で用いても2種以上を併用してもよい。
正孔注入材料又は電子注入材料が導電性高分子を含む場合、導電性高分子の電気伝導度は、好ましくは、1×10-5S/cm~1×103S/cmである。導電性高分子の電気伝導度をかかる範囲とするために、導電性高分子に適量のイオンをドープすることができる。
ドープするイオンの種類は、正孔注入材料であればアニオン、電子注入材料であればカチオンである。アニオンとしては、例えば、ポリスチレンスルホン酸イオン、アルキルベンゼンスルホン酸イオン、樟脳スルホン酸イオンが挙げられる。カチオンとしては、例えば、リチウムイオン、ナトリウムイオン、カリウムイオン、テトラブチルアンモニウムイオンが挙げられる。
ドープするイオンは、1種のみでも2種以上でもよい。
発光材料は、低分子化合物と高分子化合物とに分類される。
低分子化合物としては、例えば、ナフタレン及びその誘導体、アントラセン及びその誘導体、ペリレン及びその誘導体、並びに、イリジウム、白金又はユーロピウムを中心金属とする三重項発光錯体が挙げられる。
高分子化合物としては、例えば、フェニレン基、ナフタレンジイル基、フルオレンジイル基、フェナントレンジイル基、ジヒドロフェナントレンジイル基、式(X)で表される基、カルバゾールジイル基、フェノキサジンジイル基、フェノチアジンジイル基、アントラセンジイル基、ピレンジイル基等を含む高分子化合物が挙げられる。
RD1~RD8、RD11~RD20、RD21~RD26及びRD31~RD37は、それぞれ独立に、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基又はハロゲン原子を表し、これらの基は置換基を有していてもよい。RD1~RD8、RD11~RD20、RD21~RD26及びRD31~RD37が複数存在する場合、それらはそれぞれ同一でも異なっていてもよい。
-AD1---AD2-は、アニオン性の2座配位子を表し、AD1及びAD2は、それぞれ独立に、イリジウム原子と結合する炭素原子、酸素原子又は窒素原子を表し、これらの原子は環を構成する原子であってもよい。-AD1---AD2-が複数存在する場合、それらは同一でも異なっていてもよい。
nD1は、1、2又は3を表し、nD2は、1又は2を表す。]
mDA1、mDA2及びmDA3は、それぞれ独立に、0以上の整数を表す。
GDAは、窒素原子、芳香族炭化水素基又は複素環基を表し、これらの基は置換基を有していてもよい。
ArDA1、ArDA2及びArDA3は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。ArDA1、ArDA2及びArDA3が複数ある場合、それらはそれぞれ同一でも異なっていてもよい。
TDAは、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。複数あるTDAは、同一でも異なっていてもよい。]
*、**及び***は、各々、ArDA1、ArDA2、ArDA3との結合を表す。
RDAは、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は更に置換基を有していてもよい。RDAが複数ある場合、それらは同一でも異なっていてもよい。]
RDAは前記と同じ意味を表す。
RDBは、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RDBが複数ある場合、それらは同一でも異なっていてもよい。]
Rp1、Rp2及びRp3は、それぞれ独立に、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基又はハロゲン原子を表す。Rp1及びRp2が複数ある場合、それらはそれぞれ同一であっても異なっていてもよい。
np1は、0~5の整数を表し、np2は0~3の整数を表し、np3は0又は1を表す。複数あるnp1は、同一でも異なっていてもよい。]
np2は、好ましくは0又は1であり、より好ましくは0である。
np3は好ましくは0である。
nD2は、1又は2を表す。
Dは、式(D-A)で表される基を表す。複数存在するDは、同一でも異なっていてもよい。
RDCは、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。複数存在するRDCは、同一でも異なっていてもよい。
RDDは、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。複数存在するRDDは、同一でも異なっていてもよい。]
発光材料は、1種単独で用いても2種以上を併用してもよい。
酸化防止剤は、本発明の高分子化合物と同じ溶媒に可溶であり、発光及び電荷輸送を阻害しない化合物であればよく、例えば、フェノール系酸化防止剤、リン系酸化防止剤が挙げられる。
本発明の組成物において、酸化防止剤の配合量は、本発明の高分子化合物100質量部に対して、通常、0.001~10質量部である。
酸化防止剤は、1種単独で用いても2種以上を併用してもよい。
本発明の発光素子は、本発明の高分子化合物を含有する有機層を備えた発光素子である。本発明の発光素子の構成としては、例えば、陽極及び陰極からなる電極と、該電極間に設けられた本発明の高分子化合物を含有する層とを有する。
本発明の高分子化合物を含有する層は、通常、発光層、正孔輸送層、正孔注入層、電子輸送層、電子注入層の1種以上の層であり、好ましくは、発光層である。これらの層は、各々、発光材料、正孔輸送材料、正孔注入材料、電子輸送材料、電子注入材料を含有する。これらの層は、各々、発光材料、正孔輸送材料、正孔注入材料、電子輸送材料、電子注入材料を、上述した溶媒に溶解させ、インクを調製して用い、例えば、スピンコート法、キャスティング法、マイクログラビアコート法、グラビアコート法、バーコート法、ロールコート法、ワイヤーバーコート法、ディップコート法、スプレーコート法、スクリーン印刷法、フレキソ印刷法、オフセット印刷法、インクジェット印刷法、キャピラリ-コート法、ノズルコート法により形成することができる。
発光素子における基板は、電極を形成することができ、かつ、有機層を形成する際に化学的に変化しない基板であればよく、例えば、ガラス、プラスチック、シリコン等の材料からなる基板である。不透明な基板の場合には、基板から最も遠くにある電極が透明又は半透明であることが好ましい。
発光素子を用いて面状の発光を得るためには、面状の陽極と陰極が重なり合うように配置すればよい。パターン状の発光を得るためには、面状の発光素子の表面にパターン状の窓を設けたマスクを設置する方法、非発光部にしたい層を極端に厚く形成し実質的に非発光とする方法、陽極若しくは陰極、又は両方の電極をパターン状に形成する方法がある。これらのいずれかの方法でパターンを形成し、いくつかの電極を独立にON/OFFできるように配置することにより、数字、文字等を表示できるセグメントタイプの表示装置が得られる。ドットマトリックス表示装置とするためには、陽極と陰極を共にストライプ状に形成して直交するように配置すればよい。複数の種類の発光色の異なる高分子化合物を塗り分ける方法、カラーフィルター又は蛍光変換フィルターを用いる方法により、部分カラー表示、マルチカラー表示が可能となる。ドットマトリックス表示装置は、パッシブ駆動も可能であるし、TFT等と組み合わせてアクティブ駆動も可能である。これらの表示装置は、コンピュータ、テレビ、携帯端末等のディスプレイに用いることができる。面状の発光素子は、液晶表示装置のバックライト用の面状光源、又は、面状の照明用光源として好適に用いることができる。フレキシブルな基板を用いれば、曲面状の光源、及び、表示装置としても使用できる。
測定試料を約2mg/mLの濃度になるようにクロロホルム又はテトラヒドロフランに溶解させ、LC-MS(Agilent製、商品名:1290 Infinity LC及び6230 TOF LC/MS)に約1μL注入した。LC-MSの移動相には、アセトニトリル及びテトラヒドロフランの比率を変化させながら用い、1.0mL/分の流量で流した。カラムは、SUMIPAX ODS Z-CLUE(住化分析センター製、内径:4.6mm、長さ:250mm、粒径3μm)を用いた。
5~10mgの測定試料を約0.5mLの重クロロホルム(CDCl3)、重テトラヒドロフラン、重ジメチルスルホキシド、重アセトン、重N,N-ジメチルホルムアミド、重トルエン、重メタノール、重エタノール、重2-プロパノール又は重塩化メチレンに溶解させ、NMR装置(Agilent製、商品名:INOVA300、又は、JEOL RESONANCE製、商品名:JNM-ECZ400S/L1)を用いて測定した。
(化合物1aの合成)
1H-NMR(CD2Cl2,400MHz)δ(ppm):0.92(m,3H),1.36(m,6H),1.39(s,9H),1.44(s,9H),1.58(m,2H),2.08(s,3H),2.61(dd,2H),5.92(d,2H),6.19(d,1H),6.61(d,1H),6.87(dd,1H),7.02(t,2H),7.07(m,6H),7.2-7.3(m,8H),7.42(dd,1H),7.68(d,2H),8.67(d,1H),8.79(d,1H).
化合物3は、特開2010-189630号公報に記載の方法に従って合成した。
化合物4は、特表2007-512249号公報に記載の方法に従って合成した。
化合物5は、特開2008-106241号公報に記載の方法に従って合成した。
化合物6は、市販品を用いた。
化合物7は、国際公開第2009/131255号に記載の方法に従って合成した。
化合物8は、国際公開第2016/031639号に記載の方法に従って合成した。
(工程1)反応容器内を不活性ガス雰囲気とした後、化合物3(3.995g)、化合物4(6.237g)、化合物5(0.519g)、ジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(7.1mg)及びトルエン(190mL)を加え、105℃に加熱した。
(工程2)その後、そこへ、20質量%水酸化テトラエチルアンモニウム水溶液(28mL)を滴下し、4時間還流させた。その後、そこへ、フェニルボロン酸(97.5mg),20質量%水酸化テトラエチルアンモニウム水溶液(28mL)及びジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(7.1mg)を加え、6時間還流させた。得られた反応混合物を冷却した後、水で2回、10質量%塩酸水溶液で2回、3質量%アンモニア水で2回、水で2回洗浄した。得られた溶液をメタノールに滴下し、攪拌したところ、沈澱が生じた。得られた沈殿物をトルエンに溶解させ、アルミナカラム、シリカゲルカラムの順番に通液することにより精製した。得られた溶液をメタノールに滴下し、撹拌したところ、沈殿が生じた。得られた沈殿物をろ取し、乾燥させることにより、高分子化合物1を6.65g得た。高分子化合物1のMnは2.6×104であり、Mwは1.4×105であった。
(工程1)反応容器内を不活性ガス雰囲気とした後、化合物2(0.104g)、化合物6(0.502g)、化合物7(0.628g)、ジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(0.86mg)及びトルエン(27mL)を混合し、105℃に加熱した。
(工程2)その後、そこへ、10質量%水酸化テトラエチルアンモニウム水溶液(18mL)を滴下し、4時間還流させた。その後、そこへ、フェニルボロン酸(48.8mg)及びジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(0.9mg)を加え、6時間還流させた。得られた反応混合物を冷却した後、水で2回、10質量%塩酸水溶液で2回、3質量%アンモニア水で2回、水で2回洗浄した。得られた溶液をメタノールに滴下し、攪拌したところ、沈澱が生じた。得られた沈殿物をトルエンに溶解させ、アルミナカラム、シリカゲルカラムの順番に通液することにより精製した。得られた溶液をメタノールに滴下し、撹拌したところ、沈殿が生じた。得られた沈殿物をろ取し、乾燥させることにより、高分子化合物2を0.68g得た。高分子化合物2のMnは4.5×104であり、Mwは1.1×105であった。
実施例1において、(工程1)を、「反応容器内を不活性ガス雰囲気とした後、化合物8(0.096g)、化合物6(0.916g)、化合物7(1.119g)、ジクロロビス(フェニルホスフィン)パラジウム(1.5mg)及びトルエン(47mL)を混合し、105℃に加熱した。」とする以外は、実施例1と同様にして、高分子化合物3を1.1g得た。高分子化合物3のMnは5.0×104であり、Mwは1.1×105であった。
ガラス基板にスパッタ法により45nmの厚みでITO膜を付けることにより陽極を形成した。陽極上に、正孔注入材料であるND-3202(日産化学工業製)をスピンコート法により35nmの厚さで成膜し、大気雰囲気下において、ホットプレート上で240℃、15分間加熱することにより正孔注入層を形成した。
キシレンに、高分子化合物1を0.6質量%の濃度で溶解させた。得られたキシレン溶液を用いて、正孔注入層の上に、スピンコート法により20nmの厚さで成膜し、窒素ガス雰囲気下において、ホットプレート上で200℃、30分間加熱することにより正孔輸送層を形成した。
キシレンに、高分子化合物2を1.3質量%の濃度で溶解させた。得られたキシレン溶液を用いて、正孔輸送層の上に、スピンコート法により60nmの厚さで成膜し、窒素ガス雰囲気下において、ホットプレート上で150℃、10分加熱することにより発光層を形成した。
発光層を形成した基板を蒸着機内において、1×10-4Pa以下にまで減圧した後、陰極として、発光層の上に、フッ化ナトリウムを約7nm、次いで、フッ化ナトリウム層の上に、アルミニウムを約120nm蒸着した。蒸着後、ガラス基板を用いて封止することにより、発光素子D1を作製した。
発光素子D1に電圧を印加することにより、最大外部量子収率は4.1%であり、460nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.142,0.071)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流密度で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の60%となるまでの時間は、44時間であった。
実施例D1において、高分子化合物2に代えて、高分子化合化合物2と高分子化合物3の質量比で50:50の組成物を用いた以外は、実施例D1と同様にして、発光素子D2を作製した。
発光素子D2に電圧を印加することにより、最大外部量子収率は3.3%であり、460nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.145,0.067)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流密度で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の60%となるまでの時間は、54時間であった。
実施例D1において、高分子化合物1に代えて、高分子化合物3を用いた以外は、実施例D1と同様にして、発光素子CD1を作製した。
発光素子CD1に電圧を印加することにより、最大外部量子収率は3.1%であり、440nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.156,0.063)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の60%となるまでの時間は、38時間であった。
(化合物9-1の合成)
1H-NMR(CDCl3,400MHz)δ(ppm):7.26(d,4H),7.16(m,1H),7.03-6.97(m,3H),6.91(ddd,1H),1.28(s,18H).
1H-NMR(CDCl3,400MHz)δ(ppm):7.24-7.15(m,6H),7.08(d,1H),7.07(s,1H),7.04-6.95(m,6H),6.88(d,2H),6.58(dd,1H),6.52(dd,1H),6.45(dd,1H),2.50(dd,2H),1.57(m,2H),1.28(m,24H),0.87(t,3H).
1H-NMR(CD2Cl2,400MHz)δ(ppm):8.81(d,1H),8.66(d,1H),7.67(d,2H),7.42(dd,1H), 7.30-7.20(m,8H),7.12(t,1H),7.10(d,2H),6.99(d,4H),6.84(dd,1H),6.66(d,1H),6.33(d,1H),6.05(d,1H),5.99(d,1H),2.59(m,2H),1.61(m,2H),1.43(s,9H),1.40(s,9H),1.39-1.27(m,24H),0.89(t,3H).
LC-MS(ESI,positive):1128[M+H]+
1H-NMR(CDCl3,400MHz)δ(ppm):8.87(s,1H),8.70(d,1H),7.70(d,2H),7.46(m,2H), 7.31-7.18(m,10H),7.12(d,2H),7.03(d,4H),6.95(d,1H),6.74(d,1H),6.36(d,1H),6.09(d,2H),2.59(m,2H),1.62(m,2H),1.44(s,18H),1.32(m,24H),0.89(t,3H).
(化合物10-1の合成)
LC-MS(ESI,positive):1232.9[M+H]+
1H-NMR(CDCl3,400MHz)δ(ppm):9.12(s,2H),7.67(s,4H),7.43(d,2H),6.98(s,2H), 6.82(s,4H),6.59(d,2H),5.46(br.s,2H),1.86(s,12H),1.46(s,18H),1.36(s,24H),1.13(s,36H).
反応容器内を不活性ガス雰囲気とした後、化合物6(0.633g)、化合物8(0.063g)、化合物7(0.723g)、化合物9(0.026g)、ジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(1.02mg)及びトルエン(24.2g)を混合し、80℃に加熱した。その後、そこへ、10質量%水酸化テトラエチルアンモニウム水溶液(20mL)を滴下し、4.5時間還流させた。その後、そこへ、フェニルボロン酸(55.8mg)及びジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(1.01mg)を加え、8時間還流させた。得られた反応混合物を冷却した後、反応液を水で1回、10質量%塩酸水溶液で2回、3質量%アンモニア水溶液で2回、水で2回洗浄した。得られた有機層を減圧下で蒸留することにより水分を除いた。得られた溶液を、アルミナとシリカゲルとの混合物を充填したカラムに通すことにより精製した。得られた溶液をメタノールに滴下し、撹拌した後、得られた沈殿物をろ取し、乾燥させることにより、高分子化合物4を0.54g得た。高分子化合物4のMnは4.5×104であり、Mwは1.1×105であった。
反応容器内を不活性ガス雰囲気とした後、化合物6(1.267g)、化合物8(0.127g)、化合物10(0.057g)、化合物7(1.486g)、ジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(2.09mg)及びトルエン(30.5g)を混合し、80℃に加熱した。その後、そこへ、20質量%水酸化テトラエチルアンモニウム水溶液(25mL)を滴下し、3.5時間還流させた。その後、そこへ、フェニルボロン酸(112.2g)及びジクロロビス(トリス-o-メトキシフェニルホスフィン)パラジウム(2.13mg)を加え、5時間還流させた。得られた反応混合物を冷却した後、反応液を水で1回、10重量%塩酸水溶液で2回、3重量%アンモニア水溶液で2回、水で2回洗浄した。得られた有機層を減圧下で蒸留することにより水分を除いた。得られた溶液を、アルミナとシリカゲルとの混合物を充填したカラムに通すことにより精製した。得られた溶液をメタノールに滴下し、撹拌した後、得られた沈殿物をろ取し、乾燥させることにより、高分子化合物高分子化合物5を1.06g得た。高分子化合物5のMnは7.7×104であり、Mwは1.7×105であった。
高分子化合物6は、国際公開第2014/102543号に記載のポリマー実施例1の方法に従って合成した。
ガラス基板にスパッタ法により45nmの厚みでITO膜を付けることにより陽極を形成した。陽極上に、正孔注入材料であるND-3202(日産化学工業製)をスピンコート法により35nmの厚さで成膜し、大気雰囲気下において、ホットプレート上で240℃、15分間加熱することにより正孔注入層を形成した。
キシレンに、高分子化合物6を0.6質量%の濃度で溶解させた。得られたキシレン溶液を用いて、正孔注入層の上に、スピンコート法により20nmの厚さで成膜し、窒素ガス雰囲気下において、ホットプレート上で200℃、30分間加熱することにより正孔輸送層を形成した。
キシレンに、高分子化合物4を1.3質量%の濃度で溶解させた。得られたキシレン溶液を用いて、正孔輸送層の上に、スピンコート法により60nmの厚さで成膜し、窒素ガス雰囲気下において、ホットプレート上で150℃、10分加熱することにより発光層を形成した。
発光層を形成した基板を蒸着機内において、1×10-4Pa以下にまで減圧した後、陰極として、発光層の上に、フッ化ナトリウムを約7nm、次いで、フッ化ナトリウム層の上に、アルミニウムを約120nm蒸着した。蒸着後、ガラス基板を用いて封止することにより、発光素子D3を作製した。
発光素子D3に電圧を印加することにより、最大外部量子収率は8.1%であり、465nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.131,0.095)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流密度で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の50%となるまでの時間は、42時間であった。
実施例D3において、高分子化合物4に代えて、高分子化合化合物5を用いた以外は、実施例D3と同様にして、発光素子D4を作製した。
発光素子D4に電圧を印加することにより、最大外部量子収率は7.8%であり、450nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.149,0.045)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流密度で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の50%となるまでの時間は、44時間であった。
実施例D3において、高分子化合物4に代えて、高分子化合物3を用いた以外は、実施例D3と同様にして、発光素子CD2を作製した。
発光素子CD2に電圧を印加することにより、最大外部量子収率は7.0%であり、440nmに発光スペクトルの最大ピーク波長を有するEL発光が観測された。この時のCIE1931色度座標は、(0.153,0.055)であった。電流密度が10mA/cm2となるように電流値を設定後、定電流密度で駆動させ、輝度の時間変化を測定した。輝度が初期輝度の50%となるまでの時間は、21時間であった。
Claims (15)
- 式(1)で表される化合物の残基を有する構成単位と、前記式(1)で表される化合物の残基を有さない構成単位とを含む、高分子化合物。
A環、B環及びC環は、それぞれ独立に、芳香族炭化水素環又は芳香族複素環を表し、これらの環は置換基を有していてもよい。
Xは、ホウ素原子、リン原子、P=O、P=S、アルミニウム原子、ガリウム原子、ヒ素原子、Si-Rx又はGe-Rxを表す。Rxは、アリール基又はアルキル基を表し、これらの基は置換基を有していてもよい。
Y1は、N-Ry、硫黄原子又はセレン原子を表す。Y2及びY3は、それぞれ独立に、酸素原子、N-Ry、硫黄原子又はセレン原子を表す。Ryは、水素原子、アリール基、1価の複素環基、又はアルキル基を表し、これらの基は置換基を有していてもよい。Ryが複数存在する場合、同一であっても異なっていてもよい。Ryは、直接又は連結基を介して、前記A環、前記B環又は前記C環と結合していてもよい。
n3は、0又は1である。n3が0である場合、-Y3-は存在しない。] - 前記式(1)で表される化合物の残基を有する構成単位が、式(2)又は式(2')で表される構成単位である、請求項1に記載の高分子化合物。
nAは、0~5の整数である。
nは、1又は2である。
Ar3は、芳香族炭化水素基又は複素環基を表し、これらの基は置換基を有していてもよい。
LAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。LAが複数存在する場合、それらは同一でも異なっていてもよい。
Qは、前記式(1)で表される化合物の残基を表す。]
mは1~4の整数である。
mAは0~5の整数である。mAが複数存在する場合、それらは同一でも異なっていてもよい。
cは0又は1である。
Ar4及びAr6は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。
Ar5は、芳香族炭化水素基、複素環基、又は、少なくとも1種の芳香族炭化水素環と少なくとも1種の複素環とが結合した基を表し、これらの基は置換基を有していてもよい。
Ar4、Ar5及びAr6は、それぞれ、該基が結合している窒素原子に結合している該基以外の基と、直接結合して、又は、酸素原子若しくは硫黄原子を介して結合して、環を形成していてもよい。
KAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。KAが複数存在する場合、それらは同一でも異なっていてもよい。
Q’は、前記式(1)で表される化合物の残基、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。Q’が複数存在する場合、それらは同一でも異なっていてもよい。但し、少なくとも1つのQ’は、前記式(1)で表される化合物の残基である。] - 前記式(1)で表される化合物の残基を有する構成単位が、式(3)で表される構成単位である、請求項1に記載の高分子化合物。
d、e、f及びgは、それぞれ独立に、0~2の整数である。
pAは、0~5の整数である。pAが複数存在する場合、それらは同一でも異なっていてもよい。
JAは、アルキレン基、シクロアルキレン基、アリーレン基、2価の複素環基、-NR’-で表される基、酸素原子又は硫黄原子を表し、これらの基は置換基を有していてもよい。R’は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。JAが複数存在する場合、それらは同一でも異なっていてもよい。
Ar7、Ar8及びAr9は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。Ar7、Ar8及びAr9が複数存在する場合、それらは各々同一でも異なっていてもよい。
RX4は、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RX4が複数存在する場合、それらは同一でも異なっていてもよい。
Q’’は、前記式(1)で表される化合物の残基を表す。] - 前記Xがホウ素原子である、請求項1~3のいずれか一項に記載の高分子化合物。
- 前記Y1、Y2及びY3がN-Ryである、請求項1~4のいずれか一項に記載の高分子化合物。
- 前記n3が0である、請求項1~5のいずれか一項に記載の高分子化合物。
- 前記式(3)で表される構成単位が式(51)で表される構成単位である、請求項3に記載の高分子化合物
R21、R22、R23、R24、R25、R26、R27、R28、R29、R30及びR31は、それぞれ独立に、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基、アリールオキシ基、1価の複素環基、又は、置換アミノ基を表し、これらの基は置換基を有していてもよい。
Ry’は、直接結合、アリーレン基、2価の複素環基、又はアルキレン基を表し、これらの基は置換基を有していてもよい。複数存在するRy’は、同一でも異なっていてもよい。
Ar7、Ar8、Ar9、RX4、JA,pA、d、e、f及びgは、前記と同じ意味を表す。] - 前記式(1)で表される化合物の残基を有さない構成単位が、式(X)で表される構成単位及び式(Y)で表される構成単位からなる群から選ばれる少なくとも1種の構成単位である、請求項1~9のいずれか一項に記載の高分子化合物。
a1及びa2は、それぞれ独立に、0~2の整数である。
ArX1及びArX3は、それぞれ独立に、アリーレン基又は2価の複素環基を表し、これらの基は置換基を有していてもよい。
ArX2及びArX4は、それぞれ独立に、アリーレン基、2価の複素環基、又は、少なくとも1種のアリーレン基と少なくとも1種の2価の複素環基とが結合した2価の基を表し、これらの基は置換基を有していてもよい。ArX2及びArX4が複数存在する場合、それらは同一でも異なっていてもよい。
RX1、RX2及びRX3は、それぞれ独立に、水素原子、アルキル基、シクロアルキル基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。RX2及びRX3が複数存在する場合、それらは同一でも異なっていてもよい。]
- 前記式(Y)で表される構成単位が、式(Y-1)で表される構成単位又は式(Y-2)で表される構成単位である、請求項10に記載の高分子化合物。
RY1は前記と同じ意味を表す。
XY1は、-C(RY2)2-、-C(RY2)=C(RY2)-又は-C(RY2)2-C(RY2)2-で表される基を表す。RY2は、水素原子、アルキル基、シクロアルキル基、アルコキシ基、シクロアルコキシ基、アリール基又は1価の複素環基を表し、これらの基は置換基を有していてもよい。複数存在するRY2は、同一でも異なっていてもよく、RY2同士は互いに結合して、それぞれが結合する炭素原子と共に環を形成していてもよい。] - 前記式(2)で表される構成単位、及び式(2')で表される構成単位の合計量が、高分子化合物に含まれる全構成単位の合計量に対して、0.1~50モル%である、請求項2、4~8、10及び11のいずれか一項に記載の高分子化合物。
- 前記式(3)で表される構成単位の合計量が、高分子化合物に含まれる全構成単位の合計量に対して、0.1~50モル%である、請求項3~6及び9~11のいずれか一項に記載の高分子化合物。
- 正孔輸送材料、正孔注入材料、電子輸送材料、電子注入材料、発光材料、酸化防止剤及び溶媒からなる群より選ばれる少なくとも1種の材料と、請求項1~13のいずれか一項に記載の高分子化合物と、を含有する組成物。
- 請求項1~13のいずれか一項に記載の高分子化合物を含有する有機層を備えた発光素子。
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/620,605 US11021568B2 (en) | 2017-06-30 | 2018-06-27 | Polymer compound and light emitting device using the same |
| CN201880042746.5A CN110799571B (zh) | 2017-06-30 | 2018-06-27 | 高分子化合物及使用其的发光元件 |
| JP2019526961A JP7173006B2 (ja) | 2017-06-30 | 2018-06-27 | 高分子化合物及びそれを用いた発光素子 |
| KR1020207001947A KR102526389B1 (ko) | 2017-06-30 | 2018-06-27 | 고분자 화합물 및 그것을 사용한 발광 소자 |
| EP18824800.9A EP3647338A4 (en) | 2017-06-30 | 2018-06-27 | MACROMOLECULAR COMPOUND AND LIGHT EMITTING ELEMENT WITH USE THEREOF |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017-128629 | 2017-06-30 | ||
| JP2017128629 | 2017-06-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019004248A1 true WO2019004248A1 (ja) | 2019-01-03 |
Family
ID=64741674
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/024289 WO2019004248A1 (ja) | 2017-06-30 | 2018-06-27 | 高分子化合物及びそれを用いた発光素子 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11021568B2 (ja) |
| EP (1) | EP3647338A4 (ja) |
| JP (1) | JP7173006B2 (ja) |
| KR (1) | KR102526389B1 (ja) |
| CN (1) | CN110799571B (ja) |
| WO (1) | WO2019004248A1 (ja) |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020040298A1 (ja) * | 2018-08-23 | 2020-02-27 | 学校法人関西学院 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
| WO2020045681A1 (ja) * | 2018-08-31 | 2020-03-05 | 学校法人関西学院 | 多環芳香族化合物の発光材料を用いた有機電界発光素子 |
| WO2020203203A1 (ja) * | 2019-03-29 | 2020-10-08 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子用重合体及び有機電界発光素子 |
| CN112047967A (zh) * | 2019-06-07 | 2020-12-08 | 学校法人关西学院 | 多环芳香族化合物、反应性化合物、高分子化合物、悬挂型高分子化合物、及使用其的用途 |
| WO2021122740A1 (de) | 2019-12-19 | 2021-06-24 | Merck Patent Gmbh | Polycyclische verbindungen für organische elektrolumineszenzvorrichtungen |
| WO2021191229A1 (en) * | 2020-03-24 | 2021-09-30 | Cambridge Display Technology Limited | Light emitting marker and assay |
| WO2021199948A1 (ja) | 2020-03-31 | 2021-10-07 | 住友化学株式会社 | 組成物及びそれを含有する発光素子 |
| CN113646356A (zh) * | 2019-03-29 | 2021-11-12 | 住友化学株式会社 | 发光元件和发光元件用组合物 |
| WO2022024664A1 (ja) * | 2020-07-28 | 2022-02-03 | 住友化学株式会社 | 組成物及び発光素子 |
| EP3950766A4 (en) * | 2019-03-29 | 2022-12-07 | Sumitomo Chemical Company Limited | LUMINESCENT ELEMENT AND COMPOSITION FOR LUMINESCENT ELEMENT |
| US11600787B2 (en) | 2019-08-30 | 2023-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023052313A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052314A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052272A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052275A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023054110A1 (ja) * | 2021-09-29 | 2023-04-06 | 住友化学株式会社 | 発光素子 |
| WO2024013004A1 (de) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2024132993A1 (de) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| US12058927B2 (en) | 2019-07-30 | 2024-08-06 | Samsung Display Co., Ltd. | Organic electroluminescence device and polycyclic compound for organic electroluminescence device |
| WO2024218109A1 (de) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113698519B (zh) * | 2020-05-20 | 2023-07-04 | 广州华睿光电材料有限公司 | 一种聚合物及其在有机电子器件中的应用 |
| CN114621271B (zh) * | 2020-12-10 | 2024-03-29 | 季华实验室 | 一种硼氮化合物、有机电致发光组合物及包含其的有机电致发光器件 |
| CN114621272B (zh) * | 2020-12-10 | 2024-03-29 | 季华实验室 | 一种硼氮化合物、有机电致发光组合物及包含其的有机电致发光器件 |
| CN114621273B (zh) * | 2020-12-10 | 2024-03-22 | 季华实验室 | 一种硼氮化合物、有机电致发光组合物及包含其的有机电致发光器件 |
| CN114621274B (zh) * | 2020-12-10 | 2024-03-22 | 季华实验室 | 一种硼氮化合物、有机电致发光组合物及包含其的有机电致发光器件 |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007512249A (ja) | 2003-11-14 | 2007-05-17 | 住友化学株式会社 | ハロゲン化ビスジアリールアミノ多環式芳香族化合物及びそのポリマー |
| JP2008106241A (ja) | 2006-09-25 | 2008-05-08 | Sumitomo Chemical Co Ltd | 高分子化合物及びそれを用いた高分子発光素子 |
| WO2009131255A1 (ja) | 2008-04-25 | 2009-10-29 | 住友化学株式会社 | 含窒素複素環式化合物の残基を有する高分子化合物 |
| JP2010189630A (ja) | 2009-01-20 | 2010-09-02 | Sumitomo Chemical Co Ltd | メタフェニレン系高分子化合物及びそれを用いた発光素子 |
| WO2013013753A2 (en) | 2011-07-25 | 2013-01-31 | Merck Patent Gmbh | Polymers and oligomers with functionalized side groups |
| WO2014102543A2 (en) | 2012-12-24 | 2014-07-03 | Cambridge Display Technology Limited | Polymer and device |
| WO2016031639A1 (ja) | 2014-08-28 | 2016-03-03 | 住友化学株式会社 | 高分子化合物およびそれを用いた発光素子 |
| WO2016152544A1 (ja) * | 2015-03-24 | 2016-09-29 | 学校法人関西学院 | 有機電界発光素子 |
| JP2016196610A (ja) * | 2015-04-06 | 2016-11-24 | 住友化学株式会社 | 高分子化合物およびそれを用いた発光素子 |
| WO2017018326A1 (ja) * | 2015-07-24 | 2017-02-02 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| JP2017079267A (ja) * | 2015-10-20 | 2017-04-27 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、有機エレクトロルミネッセンス素子の製造方法、表示装置、照明装置及び有機エレクトロルミネッセンス素子材料 |
| JP2018061028A (ja) * | 2016-09-29 | 2018-04-12 | 住友化学株式会社 | 発光素子 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5551428B2 (ja) * | 2009-01-06 | 2014-07-16 | ユー・ディー・シー アイルランド リミテッド | 電荷輸送材料及び有機電界発光素子 |
| US20140217378A1 (en) * | 2011-06-24 | 2014-08-07 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| CN105473569B (zh) * | 2013-11-13 | 2021-01-01 | 出光兴产株式会社 | 化合物、有机电致发光元件用材料、有机电致发光元件和电子设备 |
| TWI636056B (zh) | 2014-02-18 | 2018-09-21 | 學校法人關西學院 | 多環芳香族化合物及其製造方法、有機元件用材料及其應用 |
| US10374166B2 (en) | 2014-02-18 | 2019-08-06 | Kwansei Gakuin Educational Foundation | Polycyclic aromatic compound |
| WO2016152418A1 (ja) * | 2015-03-25 | 2016-09-29 | 学校法人関西学院 | 多環芳香族化合物および発光層形成用組成物 |
| JP2017178919A (ja) * | 2015-12-24 | 2017-10-05 | 出光興産株式会社 | 新規な化合物 |
| CN108640940A (zh) | 2016-07-29 | 2018-10-12 | 江苏三月光电科技有限公司 | 一种稳定型含硼有机电致发光化合物及其应用 |
-
2018
- 2018-06-27 EP EP18824800.9A patent/EP3647338A4/en not_active Withdrawn
- 2018-06-27 US US16/620,605 patent/US11021568B2/en active Active
- 2018-06-27 WO PCT/JP2018/024289 patent/WO2019004248A1/ja active Application Filing
- 2018-06-27 CN CN201880042746.5A patent/CN110799571B/zh active Active
- 2018-06-27 JP JP2019526961A patent/JP7173006B2/ja active Active
- 2018-06-27 KR KR1020207001947A patent/KR102526389B1/ko active IP Right Grant
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007512249A (ja) | 2003-11-14 | 2007-05-17 | 住友化学株式会社 | ハロゲン化ビスジアリールアミノ多環式芳香族化合物及びそのポリマー |
| JP2008106241A (ja) | 2006-09-25 | 2008-05-08 | Sumitomo Chemical Co Ltd | 高分子化合物及びそれを用いた高分子発光素子 |
| WO2009131255A1 (ja) | 2008-04-25 | 2009-10-29 | 住友化学株式会社 | 含窒素複素環式化合物の残基を有する高分子化合物 |
| JP2010189630A (ja) | 2009-01-20 | 2010-09-02 | Sumitomo Chemical Co Ltd | メタフェニレン系高分子化合物及びそれを用いた発光素子 |
| WO2013013753A2 (en) | 2011-07-25 | 2013-01-31 | Merck Patent Gmbh | Polymers and oligomers with functionalized side groups |
| WO2014102543A2 (en) | 2012-12-24 | 2014-07-03 | Cambridge Display Technology Limited | Polymer and device |
| WO2016031639A1 (ja) | 2014-08-28 | 2016-03-03 | 住友化学株式会社 | 高分子化合物およびそれを用いた発光素子 |
| WO2016152544A1 (ja) * | 2015-03-24 | 2016-09-29 | 学校法人関西学院 | 有機電界発光素子 |
| JP2016196610A (ja) * | 2015-04-06 | 2016-11-24 | 住友化学株式会社 | 高分子化合物およびそれを用いた発光素子 |
| WO2017018326A1 (ja) * | 2015-07-24 | 2017-02-02 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、表示装置及び照明装置 |
| JP2017079267A (ja) * | 2015-10-20 | 2017-04-27 | コニカミノルタ株式会社 | 有機エレクトロルミネッセンス素子、有機エレクトロルミネッセンス素子の製造方法、表示装置、照明装置及び有機エレクトロルミネッセンス素子材料 |
| JP2018061028A (ja) * | 2016-09-29 | 2018-04-12 | 住友化学株式会社 | 発光素子 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3647338A4 |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2020040298A1 (ja) * | 2018-08-23 | 2021-09-24 | 学校法人関西学院 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
| JP7388658B2 (ja) | 2018-08-23 | 2023-11-29 | 学校法人関西学院 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
| WO2020040298A1 (ja) * | 2018-08-23 | 2020-02-27 | 学校法人関西学院 | 有機電界発光素子、表示装置、照明装置、発光層形成用組成物、および化合物 |
| WO2020045681A1 (ja) * | 2018-08-31 | 2020-03-05 | 学校法人関西学院 | 多環芳香族化合物の発光材料を用いた有機電界発光素子 |
| EP3950766A4 (en) * | 2019-03-29 | 2022-12-07 | Sumitomo Chemical Company Limited | LUMINESCENT ELEMENT AND COMPOSITION FOR LUMINESCENT ELEMENT |
| WO2020203203A1 (ja) * | 2019-03-29 | 2020-10-08 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子用重合体及び有機電界発光素子 |
| JP7472106B2 (ja) | 2019-03-29 | 2024-04-22 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子用重合体及び有機電界発光素子 |
| EP3950764A4 (en) * | 2019-03-29 | 2022-12-07 | Sumitomo Chemical Company Limited | ELECTROLUMINESCENT ELEMENT AND COMPOSITION FOR ELECTROLUMINESCENT ELEMENT |
| JPWO2020203203A1 (ja) * | 2019-03-29 | 2020-10-08 | ||
| CN113631626A (zh) * | 2019-03-29 | 2021-11-09 | 日铁化学材料株式会社 | 有机电场发光元件用聚合物及有机电场发光元件 |
| CN113646356A (zh) * | 2019-03-29 | 2021-11-12 | 住友化学株式会社 | 发光元件和发光元件用组合物 |
| CN112047967A (zh) * | 2019-06-07 | 2020-12-08 | 学校法人关西学院 | 多环芳香族化合物、反应性化合物、高分子化合物、悬挂型高分子化合物、及使用其的用途 |
| US12058927B2 (en) | 2019-07-30 | 2024-08-06 | Samsung Display Co., Ltd. | Organic electroluminescence device and polycyclic compound for organic electroluminescence device |
| US11600787B2 (en) | 2019-08-30 | 2023-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2021122740A1 (de) | 2019-12-19 | 2021-06-24 | Merck Patent Gmbh | Polycyclische verbindungen für organische elektrolumineszenzvorrichtungen |
| WO2021191229A1 (en) * | 2020-03-24 | 2021-09-30 | Cambridge Display Technology Limited | Light emitting marker and assay |
| WO2021199948A1 (ja) | 2020-03-31 | 2021-10-07 | 住友化学株式会社 | 組成物及びそれを含有する発光素子 |
| WO2022024664A1 (ja) * | 2020-07-28 | 2022-02-03 | 住友化学株式会社 | 組成物及び発光素子 |
| EP4190879A4 (en) * | 2020-07-28 | 2024-08-21 | Sumitomo Chemical Co | COMPOSITION AND ELECTROLUMINESCENT ELEMENT |
| WO2023052313A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052314A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052272A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023052275A1 (de) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2023054110A1 (ja) * | 2021-09-29 | 2023-04-06 | 住友化学株式会社 | 発光素子 |
| WO2024013004A1 (de) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2024132993A1 (de) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
| WO2024218109A1 (de) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materialien für elektronische vorrichtungen |
Also Published As
| Publication number | Publication date |
|---|---|
| CN110799571B (zh) | 2022-09-20 |
| KR20200023397A (ko) | 2020-03-04 |
| EP3647338A1 (en) | 2020-05-06 |
| US20210087330A1 (en) | 2021-03-25 |
| CN110799571A (zh) | 2020-02-14 |
| KR102526389B1 (ko) | 2023-04-28 |
| EP3647338A4 (en) | 2021-03-24 |
| US11021568B2 (en) | 2021-06-01 |
| JPWO2019004248A1 (ja) | 2020-04-30 |
| JP7173006B2 (ja) | 2022-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7173006B2 (ja) | 高分子化合物及びそれを用いた発光素子 | |
| JP6500891B2 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| JP2021120952A (ja) | 組成物の製造方法 | |
| CN108026254B (zh) | 高分子化合物及使用其的发光元件 | |
| JP6323093B2 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| WO2018198975A1 (ja) | 発光素子 | |
| WO2016047536A1 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| JP6826930B2 (ja) | 発光素子 | |
| JP6519108B2 (ja) | 組成物およびそれを用いた発光素子 | |
| JP6642428B2 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| JP2017125087A (ja) | 高分子化合物及びそれを用いた発光素子 | |
| WO2018198974A1 (ja) | 発光素子 | |
| JP6468928B2 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| JP2015174824A (ja) | 金属錯体およびそれを用いた発光素子 | |
| WO2017077904A1 (ja) | 発光素子の駆動方法および発光装置 | |
| JP6825494B2 (ja) | 組成物、高分子化合物及びそれらを用いた発光素子 | |
| JP6707909B2 (ja) | 高分子化合物およびそれを用いた発光素子 | |
| WO2017038613A1 (ja) | 組成物及びそれを用いた発光素子 | |
| JP6804465B2 (ja) | 組成物及びそれを用いた発光素子 | |
| JP2016064998A (ja) | 金属錯体およびそれを用いた発光素子 | |
| JP6740643B2 (ja) | 高分子化合物及びそれを用いた発光素子 | |
| JP6572682B2 (ja) | 化合物及びそれを用いた発光素子 | |
| JP7354557B2 (ja) | 高分子化合物及びそれを用いた発光素子 | |
| JP6907739B2 (ja) | 組成物及びそれを用いた発光素子 | |
| JP6327019B2 (ja) | 高分子化合物およびそれを用いた発光素子 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18824800 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2019526961 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20207001947 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018824800 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2018824800 Country of ref document: EP Effective date: 20200130 |