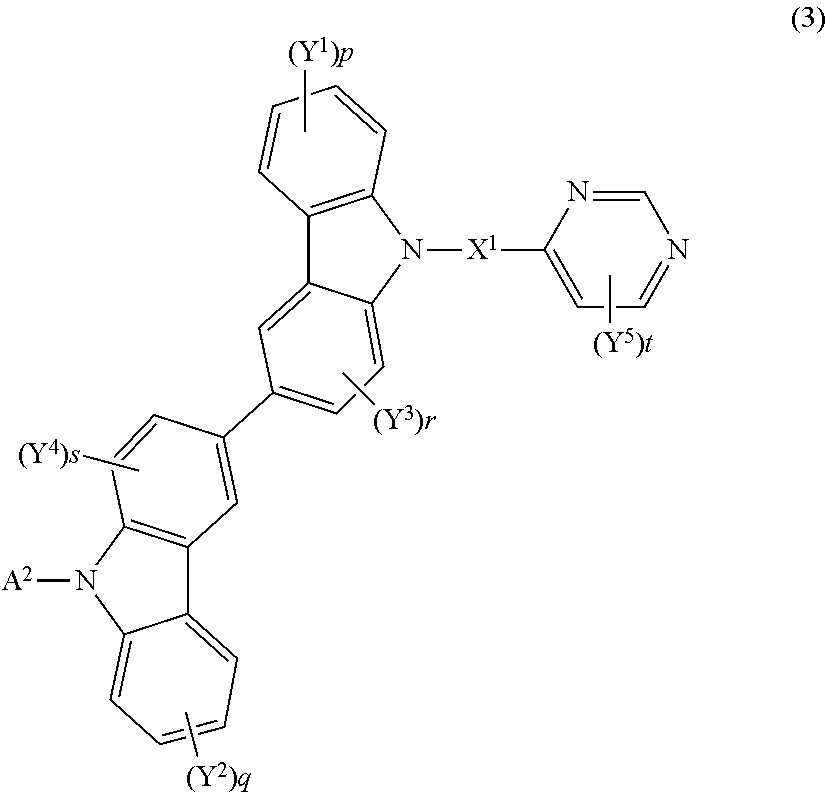
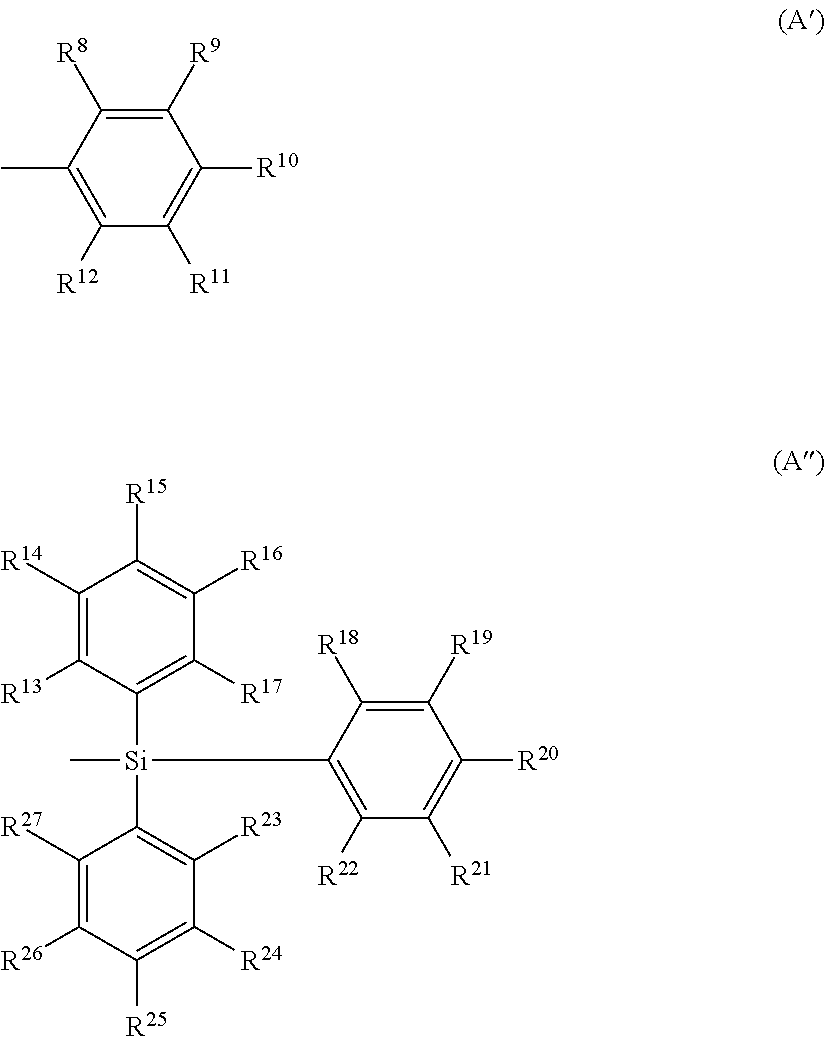
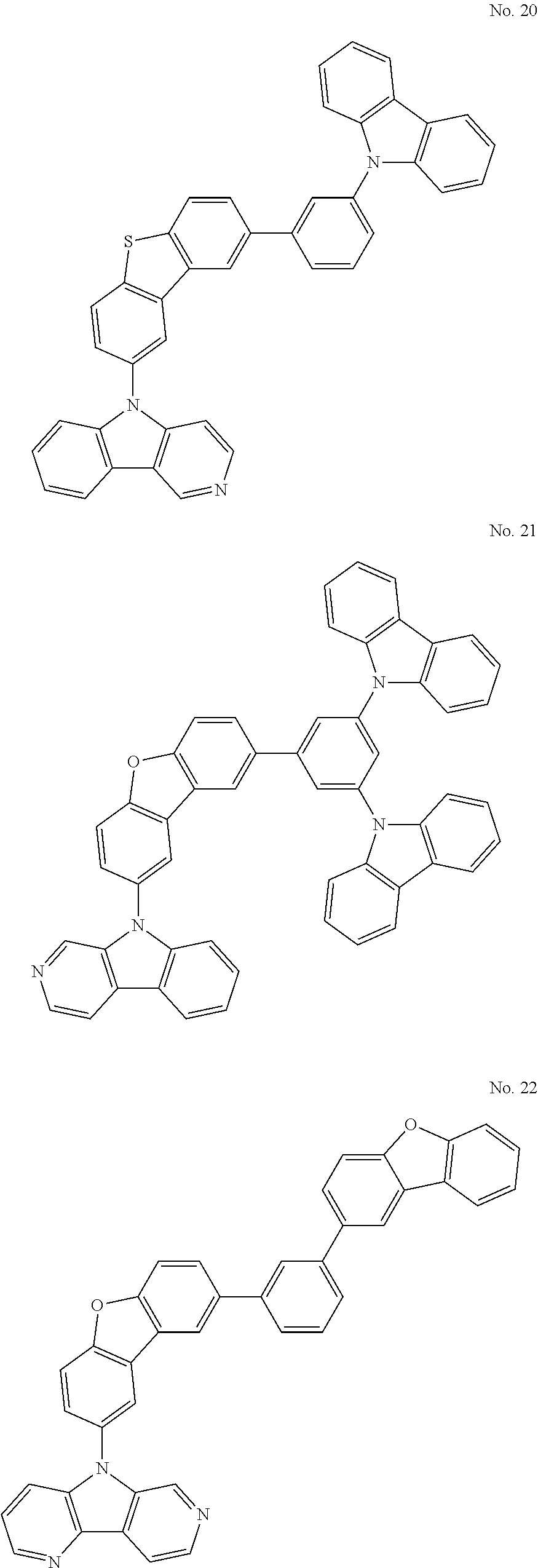
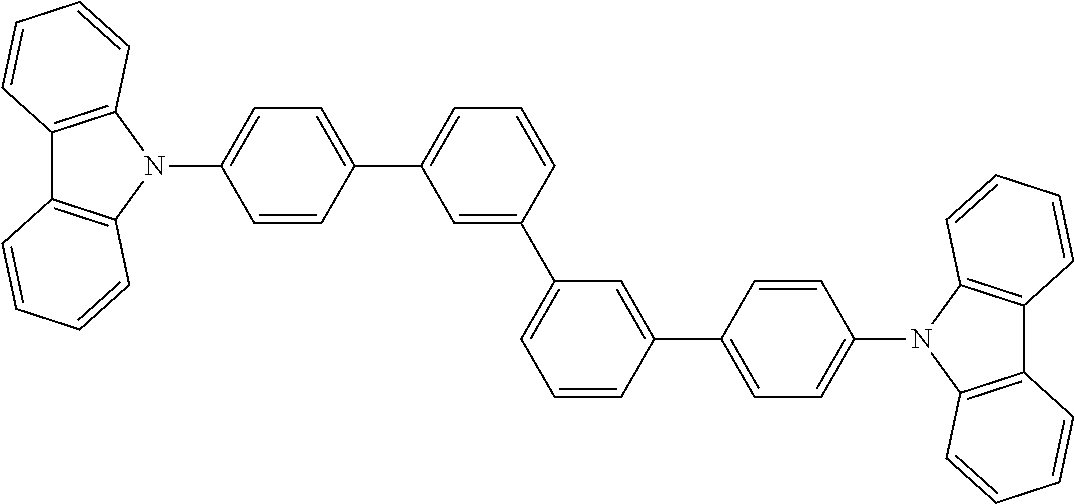
US20150228912A1 - Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same - Google Patents
Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same Download PDFInfo
- Publication number
- US20150228912A1 US20150228912A1 US14/692,010 US201514692010A US2015228912A1 US 20150228912 A1 US20150228912 A1 US 20150228912A1 US 201514692010 A US201514692010 A US 201514692010A US 2015228912 A1 US2015228912 A1 US 2015228912A1
- Authority
- US
- United States
- Prior art keywords
- group
- substituted
- unsubstituted
- carbon atoms
- ring
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 239000000463 material Substances 0.000 title claims description 163
- 238000005401 electroluminescence Methods 0.000 title claims description 14
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract description 403
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 claims abstract description 129
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims abstract description 42
- 125000000623 heterocyclic group Chemical group 0.000 claims abstract description 29
- 125000005647 linker group Chemical group 0.000 claims abstract description 7
- -1 nitrogen-containing compound Chemical class 0.000 claims description 583
- 150000001875 compounds Chemical class 0.000 claims description 122
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims description 87
- 125000000217 alkyl group Chemical group 0.000 claims description 56
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 34
- 125000003545 alkoxy group Chemical group 0.000 claims description 32
- 239000010409 thin film Substances 0.000 claims description 27
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 22
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 17
- 229910052751 metal Inorganic materials 0.000 claims description 17
- 239000002184 metal Substances 0.000 claims description 17
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 15
- 229910052731 fluorine Inorganic materials 0.000 claims description 14
- 125000001188 haloalkyl group Chemical group 0.000 claims description 14
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 claims description 11
- 125000005103 alkyl silyl group Chemical group 0.000 claims description 11
- 125000005104 aryl silyl group Chemical group 0.000 claims description 10
- 229910052741 iridium Inorganic materials 0.000 claims description 9
- 229910052697 platinum Inorganic materials 0.000 claims description 9
- 125000001153 fluoro group Chemical group F* 0.000 claims description 8
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 8
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims description 8
- 125000004429 atom Chemical group 0.000 claims description 7
- 229910052762 osmium Inorganic materials 0.000 claims description 6
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 claims description 6
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 6
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 claims description 4
- 125000001424 substituent group Chemical group 0.000 abstract description 38
- 239000010410 layer Substances 0.000 description 196
- 125000003118 aryl group Chemical group 0.000 description 73
- 0 *c(c(*)c1*)c(*)c(*)c1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 Chemical compound *c(c(*)c1*)c(*)c(*)c1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 0.000 description 44
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 30
- 230000015572 biosynthetic process Effects 0.000 description 30
- 239000002019 doping agent Substances 0.000 description 30
- 239000000243 solution Substances 0.000 description 30
- 238000003786 synthesis reaction Methods 0.000 description 28
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 27
- 239000007787 solid Substances 0.000 description 27
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 26
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 description 25
- 125000001072 heteroaryl group Chemical group 0.000 description 24
- 238000004020 luminiscence type Methods 0.000 description 23
- 238000006243 chemical reaction Methods 0.000 description 22
- 125000001624 naphthyl group Chemical group 0.000 description 22
- 239000000758 substrate Substances 0.000 description 21
- 125000005843 halogen group Chemical group 0.000 description 20
- 239000010408 film Substances 0.000 description 18
- 125000005842 heteroatom Chemical group 0.000 description 18
- 239000012044 organic layer Substances 0.000 description 18
- 125000000753 cycloalkyl group Chemical group 0.000 description 16
- 238000000034 method Methods 0.000 description 16
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 15
- 239000011521 glass Substances 0.000 description 15
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 15
- 239000000126 substance Substances 0.000 description 15
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 14
- 125000003710 aryl alkyl group Chemical group 0.000 description 14
- 229940126062 Compound A Drugs 0.000 description 13
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 13
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 13
- 238000010992 reflux Methods 0.000 description 13
- 229910052701 rubidium Inorganic materials 0.000 description 13
- 125000003277 amino group Chemical group 0.000 description 12
- 150000004696 coordination complex Chemical class 0.000 description 12
- 238000010898 silica gel chromatography Methods 0.000 description 12
- 229910052783 alkali metal Inorganic materials 0.000 description 11
- 125000003282 alkyl amino group Chemical group 0.000 description 11
- 229940125904 compound 1 Drugs 0.000 description 11
- 125000004122 cyclic group Chemical group 0.000 description 11
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 description 11
- 229910052705 radium Inorganic materials 0.000 description 11
- 125000006413 ring segment Chemical group 0.000 description 11
- 239000004065 semiconductor Substances 0.000 description 11
- 125000003960 triphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C3=CC=CC=C3C12)* 0.000 description 11
- 125000004104 aryloxy group Chemical group 0.000 description 10
- 239000012298 atmosphere Substances 0.000 description 10
- 239000003446 ligand Substances 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 10
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 10
- VQGHOUODWALEFC-UHFFFAOYSA-N 2-phenylpyridine Chemical compound C1=CC=CC=C1C1=CC=CC=N1 VQGHOUODWALEFC-UHFFFAOYSA-N 0.000 description 9
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 9
- 125000001691 aryl alkyl amino group Chemical group 0.000 description 9
- 125000000732 arylene group Chemical group 0.000 description 9
- 125000002993 cycloalkylene group Chemical group 0.000 description 9
- 238000000151 deposition Methods 0.000 description 9
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 9
- 125000004076 pyridyl group Chemical group 0.000 description 9
- 125000005493 quinolyl group Chemical group 0.000 description 9
- 229910052717 sulfur Inorganic materials 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 8
- 238000000434 field desorption mass spectrometry Methods 0.000 description 8
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 150000002430 hydrocarbons Chemical group 0.000 description 8
- 125000004857 imidazopyridinyl group Chemical group N1C(=NC2=C1C=CC=N2)* 0.000 description 8
- 239000003960 organic solvent Substances 0.000 description 8
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical group C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 8
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 8
- 150000001340 alkali metals Chemical class 0.000 description 7
- 229910052786 argon Inorganic materials 0.000 description 7
- 125000001769 aryl amino group Chemical group 0.000 description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 7
- 125000003914 fluoranthenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC=C4C1=C23)* 0.000 description 7
- 239000011737 fluorine Substances 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 7
- 125000004433 nitrogen atom Chemical group N* 0.000 description 7
- 125000001388 picenyl group Chemical group C1(=CC=CC2=CC=C3C4=CC=C5C=CC=CC5=C4C=CC3=C21)* 0.000 description 7
- GETQZCLCWQTVFV-UHFFFAOYSA-N CN(C)C Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 6
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 6
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 6
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 6
- 229910052782 aluminium Inorganic materials 0.000 description 6
- 239000007864 aqueous solution Substances 0.000 description 6
- JZOIZKBKSZMVRV-UHFFFAOYSA-N benzo(a)triphenylene Chemical group C1=CC=CC2=C3C4=CC=CC=C4C=CC3=C(C=CC=C3)C3=C21 JZOIZKBKSZMVRV-UHFFFAOYSA-N 0.000 description 6
- 125000006267 biphenyl group Chemical group 0.000 description 6
- 239000000460 chlorine Substances 0.000 description 6
- 229910052801 chlorine Inorganic materials 0.000 description 6
- 229940126214 compound 3 Drugs 0.000 description 6
- 238000001914 filtration Methods 0.000 description 6
- GVEPBJHOBDJJJI-UHFFFAOYSA-N fluoranthene Chemical compound C1=CC(C2=CC=CC=C22)=C3C2=CC=CC3=C1 GVEPBJHOBDJJJI-UHFFFAOYSA-N 0.000 description 6
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 6
- 125000004430 oxygen atom Chemical group O* 0.000 description 6
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 6
- 150000003230 pyrimidines Chemical group 0.000 description 6
- 125000004434 sulfur atom Chemical group 0.000 description 6
- 125000005580 triphenylene group Chemical group 0.000 description 6
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 5
- ATRTUAFATSADNN-UHFFFAOYSA-N CC.CC.CC.CC.CC.CCN1C2=C(C=CC=C2)C2=C/C=C/C=C\21.CCN1C2=C(C=CC=C2)C2=C\C=C/C=C\21 Chemical compound CC.CC.CC.CC.CC.CCN1C2=C(C=CC=C2)C2=C/C=C/C=C\21.CCN1C2=C(C=CC=C2)C2=C\C=C/C=C\21 ATRTUAFATSADNN-UHFFFAOYSA-N 0.000 description 5
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical group C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 5
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 5
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 5
- 150000004982 aromatic amines Chemical class 0.000 description 5
- 125000005129 aryl carbonyl group Chemical group 0.000 description 5
- 125000005110 aryl thio group Chemical group 0.000 description 5
- TUAHORSUHVUKBD-UHFFFAOYSA-N benzo[c]phenanthrene Chemical group C1=CC=CC2=C3C4=CC=CC=C4C=CC3=CC=C21 TUAHORSUHVUKBD-UHFFFAOYSA-N 0.000 description 5
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 5
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 5
- 230000002349 favourable effect Effects 0.000 description 5
- 150000002219 fluoranthenes Chemical group 0.000 description 5
- 150000002367 halogens Chemical class 0.000 description 5
- 125000005549 heteroarylene group Chemical group 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 239000001257 hydrogen Substances 0.000 description 5
- 239000005457 ice water Substances 0.000 description 5
- 239000012212 insulator Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 5
- 125000004957 naphthylene group Chemical group 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 5
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 5
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical compound C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 5
- 229910052761 rare earth metal Inorganic materials 0.000 description 5
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 5
- SLGBZMMZGDRARJ-UHFFFAOYSA-N triphenylene Chemical group C1=CC=C2C3=CC=CC=C3C3=CC=CC=C3C2=C1 SLGBZMMZGDRARJ-UHFFFAOYSA-N 0.000 description 5
- 238000001771 vacuum deposition Methods 0.000 description 5
- 229910052727 yttrium Inorganic materials 0.000 description 5
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 4
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 description 4
- JFJNVIPVOCESGZ-UHFFFAOYSA-N 2,3-dipyridin-2-ylpyridine Chemical compound N1=CC=CC=C1C1=CC=CN=C1C1=CC=CC=N1 JFJNVIPVOCESGZ-UHFFFAOYSA-N 0.000 description 4
- GAMYYCRTACQSBR-UHFFFAOYSA-N 4-azabenzimidazole Chemical compound C1=CC=C2NC=NC2=N1 GAMYYCRTACQSBR-UHFFFAOYSA-N 0.000 description 4
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 4
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 4
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 4
- 239000004215 Carbon black (E152) Substances 0.000 description 4
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 4
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 4
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 4
- 125000003342 alkenyl group Chemical group 0.000 description 4
- 150000001555 benzenes Chemical group 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 4
- 229910052794 bromium Inorganic materials 0.000 description 4
- WDECIBYCCFPHNR-UHFFFAOYSA-N chrysene Chemical compound C1=CC=CC2=CC=C3C4=CC=CC=C4C=CC3=C21 WDECIBYCCFPHNR-UHFFFAOYSA-N 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 229940125782 compound 2 Drugs 0.000 description 4
- 229940125898 compound 5 Drugs 0.000 description 4
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 4
- 230000002950 deficient Effects 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 150000002460 imidazoles Chemical class 0.000 description 4
- 150000002484 inorganic compounds Chemical class 0.000 description 4
- 229910010272 inorganic material Inorganic materials 0.000 description 4
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 4
- 229960003540 oxyquinoline Drugs 0.000 description 4
- 125000003373 pyrazinyl group Chemical group 0.000 description 4
- 125000001725 pyrenyl group Chemical group 0.000 description 4
- XSCHRSMBECNVNS-UHFFFAOYSA-N quinoxaline Chemical compound N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 4
- 150000002910 rare earth metals Chemical class 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- 238000004528 spin coating Methods 0.000 description 4
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- NAWXUBYGYWOOIX-SFHVURJKSA-N (2s)-2-[[4-[2-(2,4-diaminoquinazolin-6-yl)ethyl]benzoyl]amino]-4-methylidenepentanedioic acid Chemical compound C1=CC2=NC(N)=NC(N)=C2C=C1CCC1=CC=C(C(=O)N[C@@H](CC(=C)C(O)=O)C(O)=O)C=C1 NAWXUBYGYWOOIX-SFHVURJKSA-N 0.000 description 3
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 3
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 3
- 239000005725 8-Hydroxyquinoline Substances 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- NNPPMTNAJDCUHE-UHFFFAOYSA-N CC(C)C Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 3
- DAZXVJBJRMWXJP-UHFFFAOYSA-N CCN(C)C Chemical compound CCN(C)C DAZXVJBJRMWXJP-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical group C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- FUJCRWPEOMXPAD-UHFFFAOYSA-N Li2O Inorganic materials [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 3
- 229910000861 Mg alloy Inorganic materials 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 3
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 3
- 238000000862 absorption spectrum Methods 0.000 description 3
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 229910052788 barium Inorganic materials 0.000 description 3
- FTOVXSOBNPWTSH-UHFFFAOYSA-N benzo[b]fluoranthene Chemical group C12=CC=CC=C1C1=CC3=CC=CC=C3C3=C1C2=CC=C3 FTOVXSOBNPWTSH-UHFFFAOYSA-N 0.000 description 3
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 3
- KYYFFLQBMZRHNB-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)n1 KYYFFLQBMZRHNB-UHFFFAOYSA-N 0.000 description 3
- 150000004770 chalcogenides Chemical class 0.000 description 3
- 150000001846 chrysenes Chemical group 0.000 description 3
- 125000006165 cyclic alkyl group Chemical group 0.000 description 3
- YMHQVDAATAEZLO-UHFFFAOYSA-N cyclohexane-1,1-diamine Chemical compound NC1(N)CCCCC1 YMHQVDAATAEZLO-UHFFFAOYSA-N 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- XUCJHNOBJLKZNU-UHFFFAOYSA-M dilithium;hydroxide Chemical compound [Li+].[Li+].[OH-] XUCJHNOBJLKZNU-UHFFFAOYSA-M 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 229910052738 indium Inorganic materials 0.000 description 3
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 239000011630 iodine Substances 0.000 description 3
- QWTDNUCVQCZILF-UHFFFAOYSA-N isopentane Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- 150000002736 metal compounds Chemical class 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 3
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical group C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 3
- 150000004866 oxadiazoles Chemical class 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 125000001828 phenalenyl group Chemical group C1(C=CC2=CC=CC3=CC=CC1=C23)* 0.000 description 3
- 125000005561 phenanthryl group Chemical group 0.000 description 3
- 230000000704 physical effect Effects 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 125000005551 pyridylene group Chemical group 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 3
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 3
- 235000019798 tripotassium phosphate Nutrition 0.000 description 3
- 238000007740 vapor deposition Methods 0.000 description 3
- 229910052725 zinc Inorganic materials 0.000 description 3
- 239000011701 zinc Substances 0.000 description 3
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical group C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 2
- FLBAYUMRQUHISI-UHFFFAOYSA-N 1,8-naphthyridine Chemical compound N1=CC=CC2=CC=CN=C21 FLBAYUMRQUHISI-UHFFFAOYSA-N 0.000 description 2
- 125000004343 1-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])(*)C([H])([H])[H] 0.000 description 2
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical group C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 2
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 2
- FZKCAHQKNJXICB-UHFFFAOYSA-N 2,1-benzoxazole Chemical group C1=CC=CC2=CON=C21 FZKCAHQKNJXICB-UHFFFAOYSA-N 0.000 description 2
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 2
- VEPOHXYIFQMVHW-XOZOLZJESA-N 2,3-dihydroxybutanedioic acid (2S,3S)-3,4-dimethyl-2-phenylmorpholine Chemical compound OC(C(O)C(O)=O)C(O)=O.C[C@H]1[C@@H](OCCN1C)c1ccccc1 VEPOHXYIFQMVHW-XOZOLZJESA-N 0.000 description 2
- HLCPWBZNUKCSBN-UHFFFAOYSA-N 2-aminobenzonitrile Chemical compound NC1=CC=CC=C1C#N HLCPWBZNUKCSBN-UHFFFAOYSA-N 0.000 description 2
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 2
- 125000005916 2-methylpentyl group Chemical group 0.000 description 2
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 2
- VHMICKWLTGFITH-UHFFFAOYSA-N 2H-isoindole Chemical compound C1=CC=CC2=CNC=C21 VHMICKWLTGFITH-UHFFFAOYSA-N 0.000 description 2
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 2
- 125000005917 3-methylpentyl group Chemical group 0.000 description 2
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical class [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- NPDLYUOYAGBHFB-WDSKDSINSA-N Asn-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@H](C(O)=O)CCCN=C(N)N NPDLYUOYAGBHFB-WDSKDSINSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 2
- KHNYNFUTFKJLDD-UHFFFAOYSA-N Benzo[j]fluoranthene Chemical class C1=CC(C=2C3=CC=CC=C3C=CC=22)=C3C2=CC=CC3=C1 KHNYNFUTFKJLDD-UHFFFAOYSA-N 0.000 description 2
- BHDKGJHLCKRHAA-UHFFFAOYSA-N C1=CC(C2=C/C3=C(\C=C/2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1 Chemical compound C1=CC(C2=C/C3=C(\C=C/2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1 BHDKGJHLCKRHAA-UHFFFAOYSA-N 0.000 description 2
- BSHCIQBUBBKPOZ-UHFFFAOYSA-N CC.CC.CC.CC.CC.CN1C2=CC=CC=C2C2=C1C=CC(C1=CC=C3C(=C1)C1=C(/C=C\C=C/1)N3CC1=CC=NC=N1)=C2 Chemical compound CC.CC.CC.CC.CC.CN1C2=CC=CC=C2C2=C1C=CC(C1=CC=C3C(=C1)C1=C(/C=C\C=C/1)N3CC1=CC=NC=N1)=C2 BSHCIQBUBBKPOZ-UHFFFAOYSA-N 0.000 description 2
- OQQJIBWLRPZXCO-UHFFFAOYSA-N CC.CC.CC.CC.CCN1C2=C(C=C(C3=C/C=C4C(=C/3)\C3=C(C=CC=C3)N\4CC)C=C2)C2=C/C=C/C=C\21 Chemical compound CC.CC.CC.CC.CCN1C2=C(C=C(C3=C/C=C4C(=C/3)\C3=C(C=CC=C3)N\4CC)C=C2)C2=C/C=C/C=C\21 OQQJIBWLRPZXCO-UHFFFAOYSA-N 0.000 description 2
- VGIVLIHKENZQHQ-UHFFFAOYSA-N CN(C)CN(C)C Chemical compound CN(C)CN(C)C VGIVLIHKENZQHQ-UHFFFAOYSA-N 0.000 description 2
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 229910018096 ScF3 Inorganic materials 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 229910004299 TbF3 Inorganic materials 0.000 description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 2
- 229910009520 YbF3 Inorganic materials 0.000 description 2
- 229910052769 Ytterbium Inorganic materials 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical group [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- CWRYPZZKDGJXCA-UHFFFAOYSA-N acenaphthene Chemical compound C1=CC(CC2)=C3C2=CC=CC3=C1 CWRYPZZKDGJXCA-UHFFFAOYSA-N 0.000 description 2
- HXGDTGSAIMULJN-UHFFFAOYSA-N acetnaphthylene Natural products C1=CC(C=C2)=C3C2=CC=CC3=C1 HXGDTGSAIMULJN-UHFFFAOYSA-N 0.000 description 2
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 2
- 150000001339 alkali metal compounds Chemical class 0.000 description 2
- 125000004450 alkenylene group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- LZCZIHQBSCVGRD-UHFFFAOYSA-N benzenecarboximidamide;hydron;chloride Chemical compound [Cl-].NC(=[NH2+])C1=CC=CC=C1 LZCZIHQBSCVGRD-UHFFFAOYSA-N 0.000 description 2
- 125000002529 biphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C12)* 0.000 description 2
- 239000005388 borosilicate glass Substances 0.000 description 2
- BXULDUDPDXYLRG-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1N(c1ccccc1)c(cc1)ccc1-c(cc1)ccc1N(c(cc1)ccc1-c1ccccc1)c(cc1)ccc1-c1ccccc1 Chemical compound c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1N(c1ccccc1)c(cc1)ccc1-c(cc1)ccc1N(c(cc1)ccc1-c1ccccc1)c(cc1)ccc1-c1ccccc1 BXULDUDPDXYLRG-UHFFFAOYSA-N 0.000 description 2
- 229910052792 caesium Inorganic materials 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- 150000004697 chelate complex Chemical class 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- JNGZXGGOCLZBFB-IVCQMTBJSA-N compound E Chemical compound N([C@@H](C)C(=O)N[C@@H]1C(N(C)C2=CC=CC=C2C(C=2C=CC=CC=2)=N1)=O)C(=O)CC1=CC(F)=CC(F)=C1 JNGZXGGOCLZBFB-IVCQMTBJSA-N 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000004956 cyclohexylene group Chemical group 0.000 description 2
- ZSWFCLXCOIISFI-UHFFFAOYSA-N cyclopentadiene Chemical compound C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 229910052805 deuterium Inorganic materials 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N fluorene Chemical compound C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 description 2
- 229910052733 gallium Inorganic materials 0.000 description 2
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 2
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 2
- HOBCFUWDNJPFHB-UHFFFAOYSA-N indolizine Chemical compound C1=CC=CN2C=CC=C21 HOBCFUWDNJPFHB-UHFFFAOYSA-N 0.000 description 2
- CECAIMUJVYQLKA-UHFFFAOYSA-N iridium 1-phenylisoquinoline Chemical compound [Ir].C1=CC=CC=C1C1=NC=CC2=CC=CC=C12.C1=CC=CC=C1C1=NC=CC2=CC=CC=C12.C1=CC=CC=C1C1=NC=CC2=CC=CC=C12 CECAIMUJVYQLKA-UHFFFAOYSA-N 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 125000003564 m-cyanobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(C#N)=C1[H])C([H])([H])* 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000037230 mobility Effects 0.000 description 2
- 238000001451 molecular beam epitaxy Methods 0.000 description 2
- 150000002790 naphthalenes Chemical class 0.000 description 2
- 150000004767 nitrides Chemical class 0.000 description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 2
- 150000002829 nitrogen Chemical class 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 125000006504 o-cyanobenzyl group Chemical group [H]C1=C([H])C(C#N)=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 125000006505 p-cyanobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C#N)C([H])([H])* 0.000 description 2
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 2
- JKPCLJPYZMKPHM-UHFFFAOYSA-N pentahelicene Chemical compound C1=CC=C2C3=C4C5=CC=CC=C5C=CC4=CC=C3C=CC2=C1 JKPCLJPYZMKPHM-UHFFFAOYSA-N 0.000 description 2
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 2
- ANRQGKOBLBYXFM-UHFFFAOYSA-M phenylmagnesium bromide Chemical compound Br[Mg]C1=CC=CC=C1 ANRQGKOBLBYXFM-UHFFFAOYSA-M 0.000 description 2
- 238000001296 phosphorescence spectrum Methods 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- NOTVAPJNGZMVSD-UHFFFAOYSA-N potassium monoxide Inorganic materials [K]O[K] NOTVAPJNGZMVSD-UHFFFAOYSA-N 0.000 description 2
- CHWRSCGUEQEHOH-UHFFFAOYSA-N potassium oxide Chemical compound [O-2].[K+].[K+] CHWRSCGUEQEHOH-UHFFFAOYSA-N 0.000 description 2
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 2
- CPNGPNLZQNNVQM-UHFFFAOYSA-N pteridine Chemical compound N1=CN=CC2=NC=CN=C21 CPNGPNLZQNNVQM-UHFFFAOYSA-N 0.000 description 2
- 125000005548 pyrenylene group Chemical group 0.000 description 2
- 239000010453 quartz Substances 0.000 description 2
- 150000002909 rare earth metal compounds Chemical class 0.000 description 2
- 229910052706 scandium Inorganic materials 0.000 description 2
- OEKDNFRQVZLFBZ-UHFFFAOYSA-K scandium fluoride Chemical compound F[Sc](F)F OEKDNFRQVZLFBZ-UHFFFAOYSA-K 0.000 description 2
- 229910052711 selenium Inorganic materials 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 238000004544 sputter deposition Methods 0.000 description 2
- 229910052712 strontium Inorganic materials 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- ILMRJRBKQSSXGY-UHFFFAOYSA-N tert-butyl(dimethyl)silicon Chemical group C[Si](C)C(C)(C)C ILMRJRBKQSSXGY-UHFFFAOYSA-N 0.000 description 2
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 2
- 150000003852 triazoles Chemical group 0.000 description 2
- LKNRQYTYDPPUOX-UHFFFAOYSA-K trifluoroterbium Chemical compound F[Tb](F)F LKNRQYTYDPPUOX-UHFFFAOYSA-K 0.000 description 2
- BWHDROKFUHTORW-UHFFFAOYSA-N tritert-butylphosphane Chemical compound CC(C)(C)P(C(C)(C)C)C(C)(C)C BWHDROKFUHTORW-UHFFFAOYSA-N 0.000 description 2
- CAYQIZIAYYNFCS-UHFFFAOYSA-N (4-chlorophenyl)boronic acid Chemical compound OB(O)C1=CC=C(Cl)C=C1 CAYQIZIAYYNFCS-UHFFFAOYSA-N 0.000 description 1
- UGUHFDPGDQDVGX-UHFFFAOYSA-N 1,2,3-thiadiazole Chemical group C1=CSN=N1 UGUHFDPGDQDVGX-UHFFFAOYSA-N 0.000 description 1
- VERMWGQSKPXSPZ-BUHFOSPRSA-N 1-[(e)-2-phenylethenyl]anthracene Chemical class C=1C=CC2=CC3=CC=CC=C3C=C2C=1\C=C\C1=CC=CC=C1 VERMWGQSKPXSPZ-BUHFOSPRSA-N 0.000 description 1
- 125000006083 1-bromoethyl group Chemical group 0.000 description 1
- 125000001478 1-chloroethyl group Chemical group [H]C([H])([H])C([H])(Cl)* 0.000 description 1
- 125000004066 1-hydroxyethyl group Chemical group [H]OC([H])([*])C([H])([H])[H] 0.000 description 1
- PXSUMUYPXZEXDT-UHFFFAOYSA-M 1-phenyl-2-pyridin-1-ium-1-ylethanone;bromide Chemical compound [Br-].C=1C=CC=CC=1C(=O)C[N+]1=CC=CC=C1 PXSUMUYPXZEXDT-UHFFFAOYSA-M 0.000 description 1
- 125000001462 1-pyrrolyl group Chemical group [*]N1C([H])=C([H])C([H])=C1[H] 0.000 description 1
- UIWLITBBFICQKW-UHFFFAOYSA-N 1h-benzo[h]quinolin-2-one Chemical compound C1=CC=C2C3=NC(O)=CC=C3C=CC2=C1 UIWLITBBFICQKW-UHFFFAOYSA-N 0.000 description 1
- GIKMWFAAEIACRF-UHFFFAOYSA-N 2,4,5-trichloropyrimidine Chemical compound ClC1=NC=C(Cl)C(Cl)=N1 GIKMWFAAEIACRF-UHFFFAOYSA-N 0.000 description 1
- 125000005810 2,5-xylyl group Chemical group [H]C1=C([H])C(=C(*)C([H])=C1C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- 125000006280 2-bromobenzyl group Chemical group [H]C1=C([H])C(Br)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000005999 2-bromoethyl group Chemical group 0.000 description 1
- 125000006282 2-chlorobenzyl group Chemical group [H]C1=C([H])C(Cl)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000001340 2-chloroethyl group Chemical group [H]C([H])(Cl)C([H])([H])* 0.000 description 1
- 125000001731 2-cyanoethyl group Chemical group [H]C([H])(*)C([H])([H])C#N 0.000 description 1
- 125000006290 2-hydroxybenzyl group Chemical group [H]OC1=C(C([H])=C([H])C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- 125000006481 2-iodobenzyl group Chemical group [H]C1=C([H])C(I)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- FZTBAQBBLSYHJZ-UHFFFAOYSA-N 2-phenyl-1,3-oxazol-4-ol Chemical compound OC1=COC(C=2C=CC=CC=2)=N1 FZTBAQBBLSYHJZ-UHFFFAOYSA-N 0.000 description 1
- CCMLIFHRMDXEBM-UHFFFAOYSA-N 2-phenyl-1,3-thiazol-4-ol Chemical compound OC1=CSC(C=2C=CC=CC=2)=N1 CCMLIFHRMDXEBM-UHFFFAOYSA-N 0.000 description 1
- HJJXCBIOYBUVBH-UHFFFAOYSA-N 2-phenyl-1h-benzimidazol-4-ol Chemical compound N1C=2C(O)=CC=CC=2N=C1C1=CC=CC=C1 HJJXCBIOYBUVBH-UHFFFAOYSA-N 0.000 description 1
- VHRHRMPFHJXSNR-UHFFFAOYSA-N 2-phenylpyridin-3-ol Chemical compound OC1=CC=CN=C1C1=CC=CC=C1 VHRHRMPFHJXSNR-UHFFFAOYSA-N 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- JMTMSDXUXJISAY-UHFFFAOYSA-N 2H-benzotriazol-4-ol Chemical compound OC1=CC=CC2=C1N=NN2 JMTMSDXUXJISAY-UHFFFAOYSA-N 0.000 description 1
- DMEVMYSQZPJFOK-UHFFFAOYSA-N 3,4,5,6,9,10-hexazatetracyclo[12.4.0.02,7.08,13]octadeca-1(18),2(7),3,5,8(13),9,11,14,16-nonaene Chemical group N1=NN=C2C3=CC=CC=C3C3=CC=NN=C3C2=N1 DMEVMYSQZPJFOK-UHFFFAOYSA-N 0.000 description 1
- SUISZCALMBHJQX-UHFFFAOYSA-N 3-bromobenzaldehyde Chemical compound BrC1=CC=CC(C=O)=C1 SUISZCALMBHJQX-UHFFFAOYSA-N 0.000 description 1
- 125000006279 3-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Br)=C1[H])C([H])([H])* 0.000 description 1
- 125000003852 3-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Cl)=C1[H])C([H])([H])* 0.000 description 1
- 125000006291 3-hydroxybenzyl group Chemical group [H]OC1=C([H])C([H])=C([H])C(=C1[H])C([H])([H])* 0.000 description 1
- 125000006482 3-iodobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(I)=C1[H])C([H])([H])* 0.000 description 1
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- WDBQJSCPCGTAFG-QHCPKHFHSA-N 4,4-difluoro-N-[(1S)-3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-pyridin-3-ylpropyl]cyclohexane-1-carboxamide Chemical compound FC1(CCC(CC1)C(=O)N[C@@H](CCN1CCC(CC1)N1C(=NN=C1C)C(C)C)C=1C=NC=CC=1)F WDBQJSCPCGTAFG-QHCPKHFHSA-N 0.000 description 1
- BWGRDBSNKQABCB-UHFFFAOYSA-N 4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-thiophen-2-ylpropyl]cyclohexane-1-carboxamide Chemical compound CC(C)C1=NN=C(C)N1C1CC2CCC(C1)N2CCC(NC(=O)C1CCC(F)(F)CC1)C1=CC=CS1 BWGRDBSNKQABCB-UHFFFAOYSA-N 0.000 description 1
- CMSGUKVDXXTJDQ-UHFFFAOYSA-N 4-(2-naphthalen-1-ylethylamino)-4-oxobutanoic acid Chemical compound C1=CC=C2C(CCNC(=O)CCC(=O)O)=CC=CC2=C1 CMSGUKVDXXTJDQ-UHFFFAOYSA-N 0.000 description 1
- ZRYZBQLXDKPBDU-UHFFFAOYSA-N 4-bromobenzaldehyde Chemical compound BrC1=CC=C(C=O)C=C1 ZRYZBQLXDKPBDU-UHFFFAOYSA-N 0.000 description 1
- UBGURUDPLUZTHH-UHFFFAOYSA-N 4-bromobenzoic acid;hydrochloride Chemical compound Cl.OC(=O)C1=CC=C(Br)C=C1 UBGURUDPLUZTHH-UHFFFAOYSA-N 0.000 description 1
- 125000006281 4-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Br)C([H])([H])* 0.000 description 1
- 125000006283 4-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Cl)C([H])([H])* 0.000 description 1
- 125000003143 4-hydroxybenzyl group Chemical group [H]C([*])([H])C1=C([H])C([H])=C(O[H])C([H])=C1[H] 0.000 description 1
- 125000006483 4-iodobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1I)C([H])([H])* 0.000 description 1
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- 229910015810 BaxCa1-xO Inorganic materials 0.000 description 1
- 229910015847 BaxSr1-xO Inorganic materials 0.000 description 1
- VBDZGYLQMBHYIU-RLGPSGOWSA-M BrC1=CC(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)=CC=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.Cl.N=C(N)C1=CC=CC=C1.O=CC1=CC=CC(Br)=C1.O=COO[K].[2H]CF.[KH] Chemical compound BrC1=CC(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)=CC=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.Cl.N=C(N)C1=CC=CC=C1.O=CC1=CC=CC(Br)=C1.O=COO[K].[2H]CF.[KH] VBDZGYLQMBHYIU-RLGPSGOWSA-M 0.000 description 1
- GLQODAVNUIQIKY-JKJMDBSTSA-O BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=C(C6=C\C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.CC(=O)O.N.O=C(/C=C/C1=CC=C(Br)C=C1)C1=CC=CC=C1.O=C(C[NH+]1C=CC=CC1)C1=CC=CC=C1.[Br-] Chemical compound BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=C(C6=C\C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.CC(=O)O.N.O=C(/C=C/C1=CC=C(Br)C=C1)C1=CC=CC=C1.O=C(C[NH+]1C=CC=CC1)C1=CC=CC=C1.[Br-] GLQODAVNUIQIKY-JKJMDBSTSA-O 0.000 description 1
- IRNZHXGFZZGJTP-UHFFFAOYSA-N BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC2=C(C=C1)C1=C/C(C3=CC4=C(C=C3)C/C3=C/C=C/C=C\43)=C/C=C\1N2.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=C/C7=C(/C=C/6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC2=C(C=C1)C1=C/C(C3=CC4=C(C=C3)C/C3=C/C=C/C=C\43)=C/C=C\1N2.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=C/C7=C(/C=C/6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 IRNZHXGFZZGJTP-UHFFFAOYSA-N 0.000 description 1
- YELZFFPZMUQPBE-QDCNDKHVSA-L BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC2=C(C=C1)C1=C/C=C/C=C\1C2.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1.CC(=O)C1=CC=CC=C1.CCO.CCO.CCO.Cl.IC1=CC2=C(C=C1)C/C1=C/C=C/C=C\21.N=C(N)C1=CC=CC=C1.O=C(/C=C/C1=CC=C(Br)C=C1)C1=CC=CC=C1.O=CC1=CC=C(Br)C=C1.OB(O)C1=CC2=C(C=C1)N(C1=CC=CC=C1)/C1=C/C=C/C=C\21.O[Na].O[Na] Chemical compound BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC2=C(C=C1)C1=C/C=C/C=C\1C2.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1.CC(=O)C1=CC=CC=C1.CCO.CCO.CCO.Cl.IC1=CC2=C(C=C1)C/C1=C/C=C/C=C\21.N=C(N)C1=CC=CC=C1.O=C(/C=C/C1=CC=C(Br)C=C1)C1=CC=CC=C1.O=CC1=CC=C(Br)C=C1.OB(O)C1=CC2=C(C=C1)N(C1=CC=CC=C1)/C1=C/C=C/C=C\21.O[Na].O[Na] YELZFFPZMUQPBE-QDCNDKHVSA-L 0.000 description 1
- KURGOYHLMHQQIY-UHFFFAOYSA-N BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1 Chemical compound BrC1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1 KURGOYHLMHQQIY-UHFFFAOYSA-N 0.000 description 1
- KOYFUMVEDFDJLL-UHFFFAOYSA-M BrC1=CC=C(C2=NC3=CC=CC=C3C(C3=CC=CC=C3)=N2)C=C1.Br[Mg]C1=CC=CC=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)CC4=C5/C=C\C=C/4)=C/C=C\32)C=C1.N#CC1=CC=CC=C1N.O=CClC1=CC=C(Br)C=C1 Chemical compound BrC1=CC=C(C2=NC3=CC=CC=C3C(C3=CC=CC=C3)=N2)C=C1.Br[Mg]C1=CC=CC=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)CC4=C5/C=C\C=C/4)=C/C=C\32)C=C1.N#CC1=CC=CC=C1N.O=CClC1=CC=C(Br)C=C1 KOYFUMVEDFDJLL-UHFFFAOYSA-M 0.000 description 1
- DJIFKJNRLSREDU-UHFFFAOYSA-M BrC1=CC=CC(C2=NC3=CC=CC=C3C(C3=CC=CC=C3)=N2)=C1.Br[Mg]C1=CC=CC=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=CC(N4C5=C(C=C(C6=C\C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)CC4=C5/C=C\C=C/4)=C/C=C\32)C=C1.N#CC1=CC=CC=C1N.O=C(Cl)C1=CC=CC(Br)=C1 Chemical compound BrC1=CC=CC(C2=NC3=CC=CC=C3C(C3=CC=CC=C3)=N2)=C1.Br[Mg]C1=CC=CC=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=CC(N4C5=C(C=C(C6=C\C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)CC4=C5/C=C\C=C/4)=C/C=C\32)C=C1.N#CC1=CC=CC=C1N.O=C(Cl)C1=CC=CC(Br)=C1 DJIFKJNRLSREDU-UHFFFAOYSA-M 0.000 description 1
- FICUUZQLWPLWDZ-UHFFFAOYSA-N C.C.CCN(C)C Chemical compound C.C.CCN(C)C FICUUZQLWPLWDZ-UHFFFAOYSA-N 0.000 description 1
- CHRUYWZFMJPZFJ-UHFFFAOYSA-N C.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)OC2=C3C=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)SC2=C3C=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC2=C(C=C1)C1=C(O2)C(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)C=C3)=C2)=CC=C1.C1=CC=C(C2=CC3=C(C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C2)C2=C(C=CC(C4=CC=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C2)O3)C=C1 Chemical compound C.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)OC2=C3C=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)SC2=C3C=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC2=C(C=C1)C1=C(O2)C(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)C=C3)=C2)=CC=C1.C1=CC=C(C2=CC3=C(C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C2)C2=C(C=CC(C4=CC=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C2)O3)C=C1 CHRUYWZFMJPZFJ-UHFFFAOYSA-N 0.000 description 1
- OWVITGZMXCFRNS-UHFFFAOYSA-N C.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)OC2=C3C=CC(N3C4=C(N=CC=C4)C4=C3C=CC=N4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)SC2=C3C=CC(N3C4=C(C=CN=C4)C4=C3C=NC=C4)=C2)=C1.C1=CC=C(C2=CC3=C(C(N4C5=C(C=CC=C5)C5=C4N=CC=C5)=C2)C2=C(C=CC(C4=CC=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C2)O3)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C2/C=N\C(C2=CC4=C(C=C2)OC2=C4C=C(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC=C4)C=C2)=C/3)C=C1 Chemical compound C.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)OC2=C3C=CC(N3C4=C(N=CC=C4)C4=C3C=CC=N4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC3=C(C=C2)SC2=C3C=CC(N3C4=C(C=CN=C4)C4=C3C=NC=C4)=C2)=C1.C1=CC=C(C2=CC3=C(C(N4C5=C(C=CC=C5)C5=C4N=CC=C5)=C2)C2=C(C=CC(C4=CC=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C2)O3)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C2/C=N\C(C2=CC4=C(C=C2)OC2=C4C=C(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC=C4)C=C2)=C/3)C=C1 OWVITGZMXCFRNS-UHFFFAOYSA-N 0.000 description 1
- MWMYVXFMYYOKML-UHFFFAOYSA-N C/C1=C/C=C2/C=C\C3=CC=CC4=C3C2=C1C=C4.CC1=CC2=C(C=C1)C1=C(C=CC=C1)C2(C)C.CC1=CC2=C(C=CC=C2)C=C1.CC1=CC=C(C2=CC=CC=C2)C=C1.CC1=CC=C2C3=C(C=CC=C3)C3=CC=CC1=C32.CC1=CC=CC2=C1C=CC=C2.CC1=CC=CC=C1 Chemical compound C/C1=C/C=C2/C=C\C3=CC=CC4=C3C2=C1C=C4.CC1=CC2=C(C=C1)C1=C(C=CC=C1)C2(C)C.CC1=CC2=C(C=CC=C2)C=C1.CC1=CC=C(C2=CC=CC=C2)C=C1.CC1=CC=C2C3=C(C=CC=C3)C3=CC=CC1=C32.CC1=CC=CC2=C1C=CC=C2.CC1=CC=CC=C1 MWMYVXFMYYOKML-UHFFFAOYSA-N 0.000 description 1
- JLOYLDIJOCIZAK-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(C3=CC4=C(C=C3)OC3=C4C=CC=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CN=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1O2.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)SC4=C5/C=C(N5C6=C(N=CC=C6)C6=C5/C=C\C=N/6)/C=C\4)=C3)=CC=C1S2 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(C3=CC4=C(C=C3)OC3=C4C=CC=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CN=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1O2.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)SC4=C5/C=C(N5C6=C(N=CC=C6)C6=C5/C=C\C=N/6)/C=C\4)=C3)=CC=C1S2 JLOYLDIJOCIZAK-UHFFFAOYSA-N 0.000 description 1
- WRCZHWIKLBNLGK-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1 WRCZHWIKLBNLGK-UHFFFAOYSA-N 0.000 description 1
- DDXTZHHMGBXNRW-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 DDXTZHHMGBXNRW-UHFFFAOYSA-N 0.000 description 1
- RQPIYRRGLMOVEP-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CN=C4)C4=C3/C=C\C=N/4)/C=C\2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=NC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CN=C4)C4=C3/C=C\C=N/4)/C=C\2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=NC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 RQPIYRRGLMOVEP-UHFFFAOYSA-N 0.000 description 1
- LGPANWWASXPJPZ-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CN=C4)C4=C3/C=N\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=NC=C4)C4=C3/C=C\N=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(/C=C\C=C/5)C5=C4N=CC=C5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=CN=C4)C4=C3/C=N\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3/C=C(N3C4=C(C=NC=C4)C4=C3/C=C\N=C/4)/C=C\2)=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(/C=C\C=C/5)C5=C4N=CC=C5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1 LGPANWWASXPJPZ-UHFFFAOYSA-N 0.000 description 1
- VDBUEZSIMDPXQM-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC3=C(C=C2)SC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=NC=C4)C4=C3C=CN=C4)C=C2)=C1.C1=CC(C2=CC3=C(C=C2)SC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC3=C(C=C2)SC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1 VDBUEZSIMDPXQM-UHFFFAOYSA-N 0.000 description 1
- KKTQKDYAOWOFOU-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)SC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC=C(C2=CC=C3SC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)C3=C2)C=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)SC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC=C(C2=CC=C3SC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)C3=C2)C=C1 KKTQKDYAOWOFOU-UHFFFAOYSA-N 0.000 description 1
- FAPHNNCNUVGSOX-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(N=CC=C4)C4=C3C=CC=N4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CN=C4)C4=C3C=CC=N4)=CC(N3C4=C(C=CN=C4)C4=C3C=CC=N4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC=C(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)C=C2)=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)/C=C\2)=CC(C2=CC(N3C4=C(N=CC=C4)C4=C3C=CC=N4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CN=C4)C4=C3C=CC=N4)=CC(N3C4=C(C=CN=C4)C4=C3C=CC=N4)=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC=C(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)C=C2)=C1 FAPHNNCNUVGSOX-UHFFFAOYSA-N 0.000 description 1
- IACJZFBVDCCYDM-UHFFFAOYSA-N C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(N=CC=C4)C4=C3/C=N\C=N/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)SC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC=C(C2=CC=C3SC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=NC=C7)C7=C6/C=C\N=C/7)/C=C\5)C=C4)C3=C2)C=C1 Chemical compound C1=CC(C2=CC3=C(C=C2)SC2=C3/C=C(N3C4=C(N=CC=C4)C4=C3/C=N\C=N/4)/C=C\2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)SC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=C2)=CC(C2=CC=C3OC4=C(C=CC=C4)C3=C2)=C1.C1=CC=C(C2=CC=C3SC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=NC=C7)C7=C6/C=C\N=C/7)/C=C\5)C=C4)C3=C2)C=C1 IACJZFBVDCCYDM-UHFFFAOYSA-N 0.000 description 1
- AUSZQXITAZDJLU-UHFFFAOYSA-N C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=CC=C2)=C1 Chemical compound C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=CC=C2)=C1 AUSZQXITAZDJLU-UHFFFAOYSA-N 0.000 description 1
- LMBWNYDOONCKFC-UHFFFAOYSA-N C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=CC=C2)=C1.C1=CC2=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C3/C=C\C=C/C3=C2C=C1.C1=CC=C(C2=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4C=CC=C3)=C2)C=C1 Chemical compound C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=CC=C2)=C1.C1=CC2=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C3/C=C\C=C/C3=C2C=C1.C1=CC=C(C2=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4C=CC=C3)=C2)C=C1 LMBWNYDOONCKFC-UHFFFAOYSA-N 0.000 description 1
- XLQLSGDAJQCQKA-UHFFFAOYSA-N C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2/C=C\C=C/3)=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1 Chemical compound C1=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2/C=C\C=C/3)=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1 XLQLSGDAJQCQKA-UHFFFAOYSA-N 0.000 description 1
- IAACORKOMAVSAY-UHFFFAOYSA-N C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=NC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=NC=C4)=C2)=C1 Chemical compound C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC(N3C4=C(C=CC=C4)C4=C3C=NC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=NC=C4)=C2)=C1 IAACORKOMAVSAY-UHFFFAOYSA-N 0.000 description 1
- KVXDINRZXNIKSP-UHFFFAOYSA-N C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=C2)=CC(C2=CC=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1 Chemical compound C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)/C=C\3)=C2)=CC(C2=CC=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1.C1=CC(C2=CC=CC(C3=CC4=C(C=C3)OC3=C4/C=C(N4C5=C(C=CC=C5)C5=C4/C=N\C=C/5)/C=C\3)=C2)=CC(C2=CC=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1 KVXDINRZXNIKSP-UHFFFAOYSA-N 0.000 description 1
- KXNQYYGTWYWEST-UHFFFAOYSA-N C1=CC(C2=CC=CC(C3=C\C4=C(\C=C/3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=C3C=C(C3=CC=C4OC5=C(C=C(C6=C\C7=C(\C=C/6)OC6=C7C=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5)C4=C3)C=C1)C1=C2C=CC=C1 Chemical compound C1=CC(C2=CC=CC(C3=C\C4=C(\C=C/3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)=CC(C2=CC3=C(C=C2)OC2=C3C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=C3C=C(C3=CC=C4OC5=C(C=C(C6=C\C7=C(\C=C/6)OC6=C7C=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5)C4=C3)C=C1)C1=C2C=CC=C1 KXNQYYGTWYWEST-UHFFFAOYSA-N 0.000 description 1
- FQFIYKKLLHHYGA-UHFFFAOYSA-N C1=CC2=C(C=C1)C1=C(C=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)=C3)=C1)O2.C1=CC2=C(C=C1)N(C1=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)C=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=NC=C6)C6=C5/C=C\N=C/6)/C=C\4)C=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 Chemical compound C1=CC2=C(C=C1)C1=C(C=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)=C3)=C1)O2.C1=CC2=C(C=C1)N(C1=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)C=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC(C3=CC=C(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=NC=C6)C6=C5/C=C\N=C/6)/C=C\4)C=C3)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C1)C1=C2C=CC=C1 FQFIYKKLLHHYGA-UHFFFAOYSA-N 0.000 description 1
- OTYBODLRUNBURW-UHFFFAOYSA-N C1=CC2=C(C=C1)C1=C(C=CC=C1)C(N(C1=CC3=C(C=CC=C3)C3=C1C=CC=C3)C1=CC3=C(C=CC=C3)C3=C1C=CC=C3)=C2.C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)C=C2C=C1.C1=CC2=CC=C(N(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1 Chemical compound C1=CC2=C(C=C1)C1=C(C=CC=C1)C(N(C1=CC3=C(C=CC=C3)C3=C1C=CC=C3)C1=CC3=C(C=CC=C3)C3=C1C=CC=C3)=C2.C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)C=C2C=C1.C1=CC2=CC=C(N(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1 OTYBODLRUNBURW-UHFFFAOYSA-N 0.000 description 1
- MUOMEDOWCJKHHI-UHFFFAOYSA-N C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1O2.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)SC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1S2.C1=CC=C(C2=CC=C3OC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=N\C=C/7)/C=C\5)C=C4)C3=C2)C=C1 Chemical compound C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1O2.C1=CC2=C(C=C1)C1=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)SC4=C5/C=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)/C=C\4)=C3)=CC=C1S2.C1=CC=C(C2=CC=C3OC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=N\C=C/7)/C=C\5)C=C4)C3=C2)C=C1 MUOMEDOWCJKHHI-UHFFFAOYSA-N 0.000 description 1
- JIOGPPCOOFWNOD-UHFFFAOYSA-N C1=CC2=C(C=C1)C1=NC=CN1C=C2.C1=CC2=C(C=C1)N1C=CN=C1C=C2.C1=CC2=C(C=C1)N=CC=C2.C1=CC2=C(C=C1)N=CC=N2.C1=CC2=C(C=CN=C2)N=C1.C1=CC2=C(N=C1)N=CC=N2.C1=CC2=CC3=C(C=CC=C3)N=C2C=C1.C1=CC2=CC3=NC=CN3C=C2C=C1.C1=CC2=NC=CN2C=C1.C1=CC2=NC=CN2C=N1.C1=CC2=NC=CN2N=C1.C1=CC=NC=C1.C1=CC=NN=C1.C1=CN2C=CN=C2C=N1.C1=CN2C=CN=C2N=C1.C1=CN2C=CN=C2N=N1.C1=CN2N=CC=NC2=N1.C1=CN2N=CC=NC2=N1.C1=CN2N=CN=CC2=N1.C1=CN=CC=N1.C1=CN=CN=C1.C1=NC=NC=N1.C1=NC=NN=C1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn] Chemical compound C1=CC2=C(C=C1)C1=NC=CN1C=C2.C1=CC2=C(C=C1)N1C=CN=C1C=C2.C1=CC2=C(C=C1)N=CC=C2.C1=CC2=C(C=C1)N=CC=N2.C1=CC2=C(C=CN=C2)N=C1.C1=CC2=C(N=C1)N=CC=N2.C1=CC2=CC3=C(C=CC=C3)N=C2C=C1.C1=CC2=CC3=NC=CN3C=C2C=C1.C1=CC2=NC=CN2C=C1.C1=CC2=NC=CN2C=N1.C1=CC2=NC=CN2N=C1.C1=CC=NC=C1.C1=CC=NN=C1.C1=CN2C=CN=C2C=N1.C1=CN2C=CN=C2N=C1.C1=CN2C=CN=C2N=N1.C1=CN2N=CC=NC2=N1.C1=CN2N=CC=NC2=N1.C1=CN2N=CN=CC2=N1.C1=CN=CC=N1.C1=CN=CN=C1.C1=NC=NC=N1.C1=NC=NN=C1.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.CC.C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn].C[Rn] JIOGPPCOOFWNOD-UHFFFAOYSA-N 0.000 description 1
- RDJFJXIENYPJOH-UHFFFAOYSA-N C1=CC2=C(C=C1)C=C(N(C1=CC=C(C3=C/C4=C(C=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)/C=C\3)C=C1)C1=CC=C3/C=C\C=C/C3=C1)C=C2.C1=CC=C(N(C2=CC=C(C3=C/C4=C(C=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)/C=C\3)C=C2)C2=CC3=C(C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=C3)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C4C(=C2)C=CC2=C4C=CC(C4=C5C=CC=CC5=C5C=CC=CC5=C4)=C2)C=C3)C=C1 Chemical compound C1=CC2=C(C=C1)C=C(N(C1=CC=C(C3=C/C4=C(C=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)/C=C\3)C=C1)C1=CC=C3/C=C\C=C/C3=C1)C=C2.C1=CC=C(N(C2=CC=C(C3=C/C4=C(C=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)/C=C\3)C=C2)C2=CC3=C(C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=C3)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C4C(=C2)C=CC2=C4C=CC(C4=C5C=CC=CC5=C5C=CC=CC5=C4)=C2)C=C3)C=C1 RDJFJXIENYPJOH-UHFFFAOYSA-N 0.000 description 1
- WNIAZAGCLQHZDU-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=C3/C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)\C=C/1)C1=C2C=CC=C1.C1=CC=C(C2=CC=C3OC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)C3=C2)C=C1.C1=CC=C(N2C3=C(C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=C2C=CC(N2C4=C(C=CC=C4)C4=C2C=CC=C4)=C3)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=C3/C=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)\C=C/1)C1=C2C=CC=C1.C1=CC=C(C2=CC=C3OC4=C(C=C(C5=CC6=C(C=C5)OC5=C6/C=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)/C=C\5)C=C4)C3=C2)C=C1.C1=CC=C(N2C3=C(C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=C2C=CC(N2C4=C(C=CC=C4)C4=C2C=CC=C4)=C3)C=C1 WNIAZAGCLQHZDU-UHFFFAOYSA-N 0.000 description 1
- SQLHQLKSVANRTA-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=CC=C(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C13)C1=C2C=CC=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=CC(N(C3=CC=CC(C4=CC=CC=C4)=C3)C3=CC(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C3)=C2)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC3=C(C=C1)OC1=CC=C(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C13)C1=C2C=CC=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=CC(N(C3=CC=CC(C4=CC=CC=C4)=C3)C3=CC(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C3)=C2)C=C1 SQLHQLKSVANRTA-UHFFFAOYSA-N 0.000 description 1
- GWJGVGUBARXHOD-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=CC=C4)=C3)=C2)C=C1.C1=CC=C(C2=CC=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)C=C2)C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=CC=C4)=C3)=C2)C=C1.C1=CC=C(C2=CC=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3/C=C\C=C/4)C=C2)C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1 GWJGVGUBARXHOD-UHFFFAOYSA-N 0.000 description 1
- XHYBKFQHMAIJBV-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 XHYBKFQHMAIJBV-UHFFFAOYSA-N 0.000 description 1
- BXCIJDFDLSMLTR-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)C=C1)C1=C2C=CC=C1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C(C)C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=N1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)C=C1)C1=C2C=CC=C1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C(C)C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=N1 BXCIJDFDLSMLTR-UHFFFAOYSA-N 0.000 description 1
- HEXUYAXIYVFLGK-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC5=C4C=CC=C5)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=C(C6=CC=CC=C6)S5)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)S2)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC5=C4C=CC=C5)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=C(C6=CC=CC=C6)S5)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)S2)C=C1 HEXUYAXIYVFLGK-UHFFFAOYSA-N 0.000 description 1
- YFXUUABRSPKPTF-UHFFFAOYSA-N C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=C(C6=CC=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C3=C2C=CC=C3)C=C1 Chemical compound C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC2=CC(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)=C3C=CC=CC3=C2C=C1.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=C(C6=CC=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C3=C2C=CC=C3)C=C1 YFXUUABRSPKPTF-UHFFFAOYSA-N 0.000 description 1
- DVOBQOFVMZBBIC-UHFFFAOYSA-N C1=CC2=CC3=C(C=CC=C3)C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=CC6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2C=C1.C1=CC2=CC3=C(C=CC=C3)C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=CC6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)=C2C=C1.C1=CC2=CC=CC(C3=CC=C(N(C4=CC=C(C5=CC=CC6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)=C2C=C1.C1=CC2=CC=CC(C3=CC=C(N(C4=CC=C(C5=CC=CC6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)=C2C=C1 Chemical compound C1=CC2=CC3=C(C=CC=C3)C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=CC6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2C=C1.C1=CC2=CC3=C(C=CC=C3)C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=CC6=C5C=CC=C6)C=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)=C2C=C1.C1=CC2=CC=CC(C3=CC=C(N(C4=CC=C(C5=CC=CC6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)=C2C=C1.C1=CC2=CC=CC(C3=CC=C(N(C4=CC=C(C5=CC=CC6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=CC=C4)C=C3)=C2C=C1 DVOBQOFVMZBBIC-UHFFFAOYSA-N 0.000 description 1
- BQJDPRYESLERCU-UHFFFAOYSA-N C1=CC2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C=C5)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)C=C2C=C1.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=C(C6=CC=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=C(C5=CC=CC=C5)S4)C=C3)C3=CC=C(C4=CC=C(C5=CC=CC=C5)S4)C=C3)C=C2)C=C1 Chemical compound C1=CC2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC6=C(C=CC=C6)C=C5)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)C=C2C=C1.C1=CC=C(C2=C3C=CC=CC3=C(C3=CC=C(N(C4=CC=C(C5=C6C=CC=CC6=C(C6=CC=CC=C6)C6=C5C=CC=C6)C=C4)C4=C5C=CC=CC5=C5C=CC=CC5=C4)C=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=C(C5=CC=CC=C5)S4)C=C3)C3=CC=C(C4=CC=C(C5=CC=CC=C5)S4)C=C3)C=C2)C=C1 BQJDPRYESLERCU-UHFFFAOYSA-N 0.000 description 1
- LMBSSEKICOKIRU-UHFFFAOYSA-N C1=CC2=CC=C(N(C3=CC(C4=CC5=C(C=C4)C=C(C4=CC6=C(C=CC=C6)C6=C4C=CC=C6)C=C5)=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1.C1=CC=C(N(C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C2=CC=CN=C2)C=C1.C1=CC=C(N(C2=CC=C3C=CC=CC3=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=NC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=C2)C=C(/C2=C/C5=C(C=CC=C5)C5=C2C=CC=C5)C=C4)C=C3)C=C1 Chemical compound C1=CC2=CC=C(N(C3=CC(C4=CC5=C(C=C4)C=C(C4=CC6=C(C=CC=C6)C6=C4C=CC=C6)C=C5)=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1.C1=CC=C(N(C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C2=CC=CN=C2)C=C1.C1=CC=C(N(C2=CC=C3C=CC=CC3=C2)C2=CC(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CN=C5C(=C3)C=CC3=C5N=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=NC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=C2)C=C(/C2=C/C5=C(C=CC=C5)C5=C2C=CC=C5)C=C4)C=C3)C=C1 LMBSSEKICOKIRU-UHFFFAOYSA-N 0.000 description 1
- STMHGKLWJLACIF-UHFFFAOYSA-N C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)C=C2C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C3C(=C2)C=CC2=C3N=CC=C2)C=C1.C1=CC=C(N(C2=CN=CN=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CN=C(N(C2=CN=CN=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)N=C1 Chemical compound C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)C=C2C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C3C(=C2)C=CC2=C3N=CC=C2)C=C1.C1=CC=C(N(C2=CN=CN=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CN=C(N(C2=CN=CN=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)N=C1 STMHGKLWJLACIF-UHFFFAOYSA-N 0.000 description 1
- QBTCMAZSZLLNDU-UHFFFAOYSA-N C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)C=C2C=C1.C1=CC2=CC=C(N(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1.C1=CC2=CC=CC(N(C3=C4C=CC=CC4=CC=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)=C2C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)/C2=C/C3=CC=C4C5=CC=C6C=CC=CC6=C5C=CC4=C3C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=CC=CC=C2C2=C4C=CC5=C6C=CC=CC6=CC=C5C4=CC=C32)C=C1 Chemical compound C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)C=C2C=C1.C1=CC2=CC=C(N(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C3=CC4=C(C=CC=C4)C=C3)C=C2C=C1.C1=CC2=CC=CC(N(C3=C4C=CC=CC4=CC=C3)C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)=C2C=C1.C1=CC=C(N(C2=CC3=C(C=CC=C3)C=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)/C2=C/C3=CC=C4C5=CC=C6C=CC=CC6=C5C=CC4=C3C3=CC=CC=C32)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=CC=CC=C2C2=C4C=CC5=C6C=CC=CC6=CC=C5C4=CC=C32)C=C1 QBTCMAZSZLLNDU-UHFFFAOYSA-N 0.000 description 1
- KUSMSCWMNNYGFV-UHFFFAOYSA-N C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=CC4=C(C=CC(C5=C6C=CC=CC6=C6C=CC=CC6=C5)=C4)C=C3)C=C2C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=C4C5=C(C=CC=C5)C5=C4C3=CC=C5)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=CC=C4)C4=C2C=CC=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC(C4=C5C=CC=CC5=C5C=CC=CC5=C4)=C3)C=C2)C=C1 Chemical compound C1=CC2=CC=C(N(C3=CC4=C(C=CC=C4)C=C3)C3=CC4=C(C=CC(C5=C6C=CC=CC6=C6C=CC=CC6=C5)=C4)C=C3)C=C2C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=C4C5=C(C=CC=C5)C5=C4C3=CC=C5)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=CC=C4)C4=C2C=CC=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC(C4=C5C=CC=CC5=C5C=CC=CC5=C4)=C3)C=C2)C=C1 KUSMSCWMNNYGFV-UHFFFAOYSA-N 0.000 description 1
- XPMIQRBAAQBPAA-UHFFFAOYSA-N C1=CC2=CC=CC3=C2C(=C1)/C=C\3.C1=CC2=CC=CC3=C2C(=C1)/C=C\3.C1=CC=C2C=CC=CC2=C1.C1=CC=CC=C1 Chemical compound C1=CC2=CC=CC3=C2C(=C1)/C=C\3.C1=CC2=CC=CC3=C2C(=C1)/C=C\3.C1=CC=C2C=CC=CC2=C1.C1=CC=CC=C1 XPMIQRBAAQBPAA-UHFFFAOYSA-N 0.000 description 1
- WBCFNFAZPUPCPK-PMJWHUJKSA-I C1=CC2=CC=N3[Ir]C4=C(C=CC=C4)C3=C2C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC(C(C)(C)C)=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC=C3C=CC=CC3=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(C=CC=C2)N=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(CCCC2)N=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C=CN=C1C1=CC=CC=C1 Chemical compound C1=CC2=CC=N3[Ir]C4=C(C=CC=C4)C3=C2C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC(C(C)(C)C)=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC=C3C=CC=CC3=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(C=CC=C2)N=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(CCCC2)N=C1C1=CC=CC=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C=CN=C1C1=CC=CC=C1 WBCFNFAZPUPCPK-PMJWHUJKSA-I 0.000 description 1
- JRRXPFHLBIAGRE-UHFFFAOYSA-N C1=CC2=CC=N3[Ir]C4=C(SC=C4)C3=C2C=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir]21C2=C(C=CC=C2)C2=C3C=CC=CC3=CC=N21 Chemical compound C1=CC2=CC=N3[Ir]C4=C(SC=C4)C3=C2C=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir]21C2=C(C=CC=C2)C2=C3C=CC=CC3=CC=N21 JRRXPFHLBIAGRE-UHFFFAOYSA-N 0.000 description 1
- JEVKQQQCIAUPIF-QFLKYYPSSA-N C1=CC2=N(C=C1)[Pt]C1=C2SC=C1.C1=CC=C2C(=C1)C1=N(C=CC=C1)[Ru@]21N2=CC=CC=C2C2=N1C=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC=C1C1=N2C2=C(C=CC=C2)S1.CC1=CC2=N(C=C1)[Os]([PH](C)(C1=CC=CC=C1)C1=CC=CC=C1)([PH](C)(C1=CC=CC=C1)C1=CC=CC=C1)N1N=C(C)N=C21.CCC1=C(CC)C2=N3C1=CC1=C(CC)C(CC)=C4/C=C5/C(CC)=C(CC)C6=N5[Pt@]3(N14)N1/C(=C\2)C(CC)=C(CC)/C1=C/6 Chemical compound C1=CC2=N(C=C1)[Pt]C1=C2SC=C1.C1=CC=C2C(=C1)C1=N(C=CC=C1)[Ru@]21N2=CC=CC=C2C2=N1C=CC=C2.CC1=CC(C)=O[Ir]2(O1)C1=C(SC3=C1C=CC=C3)C1=CC=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=CC=CC=C1C1=N2C2=C(C=CC=C2)S1.CC1=CC2=N(C=C1)[Os]([PH](C)(C1=CC=CC=C1)C1=CC=CC=C1)([PH](C)(C1=CC=CC=C1)C1=CC=CC=C1)N1N=C(C)N=C21.CCC1=C(CC)C2=N3C1=CC1=C(CC)C(CC)=C4/C=C5/C(CC)=C(CC)C6=N5[Pt@]3(N14)N1/C(=C\2)C(CC)=C(CC)/C1=C/6 JEVKQQQCIAUPIF-QFLKYYPSSA-N 0.000 description 1
- RXYLAYAYTDGXIU-UHFFFAOYSA-N C1=CC2=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CN2C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CN3C=CC=CC3=N2)C(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1 Chemical compound C1=CC2=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CN2C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CN3C=CC=CC3=N2)C(C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)=C1 RXYLAYAYTDGXIU-UHFFFAOYSA-N 0.000 description 1
- NAWAQCGKYVSGGL-UHFFFAOYSA-N C1=CC2=NC(C3=CC=C(C4CC5CC4CC5C4=CC=C(N5C6=C(C=CC=C6)C6=C/C=C/C=C\65)C=C4)C=C3)=CN2C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CN=C(C2=CC(C3(C4=CC=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)C=C4)CCCCC3)=CC(C3=NC=CC=C3)=N2)C=C1 Chemical compound C1=CC2=NC(C3=CC=C(C4CC5CC4CC5C4=CC=C(N5C6=C(C=CC=C6)C6=C/C=C/C=C\65)C=C4)C=C3)=CN2C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CN=C(C2=CC(C3(C4=CC=C(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)C=C4)CCCCC3)=CC(C3=NC=CC=C3)=N2)C=C1 NAWAQCGKYVSGGL-UHFFFAOYSA-N 0.000 description 1
- GVQIXUJGHWEAFF-UHFFFAOYSA-N C1=CC2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CN2C=C1.C1=CC=C(C2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C3C=CC=CN32)C=C1.C1=CC=C(C2=C(\C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N3C=CC=C\C3=N\2)C=C1 Chemical compound C1=CC2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CN2C=C1.C1=CC=C(C2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C3C=CC=CN32)C=C1.C1=CC=C(C2=C(\C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N3C=CC=C\C3=N\2)C=C1 GVQIXUJGHWEAFF-UHFFFAOYSA-N 0.000 description 1
- WOJHEVXTGSPCTF-UHFFFAOYSA-N C1=CC2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CN2C=C1.C1=CC=C(C2=C3C=CC=CN3C=C2C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1.C1=CC=C(C2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=NC(C3=CC=CC=N3)=N2)C=C1 Chemical compound C1=CC2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CN2C=C1.C1=CC=C(C2=C3C=CC=CN3C=C2C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1.C1=CC=C(C2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=NC(C3=CC=CC=N3)=N2)C=C1 WOJHEVXTGSPCTF-UHFFFAOYSA-N 0.000 description 1
- NHALJYHLTKPEDW-UHFFFAOYSA-N C1=CC2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CN2C=C1.C1=CC=C(C2=C3C=CC=CN3C=C2C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CC=C(C2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=NC(C3=CC=CC=N3)=N2)C=C1 Chemical compound C1=CC2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CN2C=C1.C1=CC=C(C2=C3C=CC=CN3C=C2C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CC=C(C2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=NC(C3=CC=CC=N3)=N2)C=C1 NHALJYHLTKPEDW-UHFFFAOYSA-N 0.000 description 1
- LLAVJTKPGIELSM-UHFFFAOYSA-N C1=CC=C(/C2=C(\C3=CC=CC=C3)N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=NC=CC=C32)C=C1.C1=CC=C(/C2=C/C3=CC=CN=C3N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(/C2=C/N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=NC=CC=C32)C=C1 Chemical compound C1=CC=C(/C2=C(\C3=CC=CC=C3)N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=NC=CC=C32)C=C1.C1=CC=C(/C2=C/C3=CC=CN=C3N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(/C2=C/N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=NC=CC=C32)C=C1 LLAVJTKPGIELSM-UHFFFAOYSA-N 0.000 description 1
- YYHAOJUGNPSLOL-UHFFFAOYSA-N C1=CC=C(/C2=C/N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=CC=C32)C=C1.C1=CC=C(C2=CC=C(/C3=C(\C4=CC=CC=C4)C4=CC=CC=C4N3C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(/C3=C/C4=CC=CC=C4N3C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(/C2=C/N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=CC=C32)C=C1.C1=CC=C(C2=CC=C(/C3=C(\C4=CC=CC=C4)C4=CC=CC=C4N3C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(/C3=C/C4=CC=CC=C4N3C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1 YYHAOJUGNPSLOL-UHFFFAOYSA-N 0.000 description 1
- SROSJQSCFXFWDB-UHFFFAOYSA-N C1=CC=C(C2=C(C3=CC4=C(C=C3)C3=CC=C(C5=CC6=C(C=C5)C=C(C5=CC=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C=C5)C=C6)C=C3/C=C\4)C=CC3=C2C=CC=C3)C=C1.C1=CC=C(C2=CC=C3C=CC=CC3=C2C2=CC3=C(C=C2)C2=CC=C(C4=CC5=C(C=C4)C=C(C4=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C4)C=C5)C=C2/C=C\3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C(C6=CC=C7C=CC=CC7=C6)=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)/C=C\C3=C5C=CC(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(C6=CC7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=C(C3=CC4=C(C=C3)C3=CC=C(C5=CC6=C(C=C5)C=C(C5=CC=C(N(C7=CC=CC=C7)C7=CC=CC=C7)C=C5)C=C6)C=C3/C=C\4)C=CC3=C2C=CC=C3)C=C1.C1=CC=C(C2=CC=C3C=CC=CC3=C2C2=CC3=C(C=C2)C2=CC=C(C4=CC5=C(C=C4)C=C(C4=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C4)C=C5)C=C2/C=C\3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C(C6=CC=C7C=CC=CC7=C6)=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)/C=C\C3=C5C=CC(C5=CC6=C(C=CC=C6)C6=C5C=CC=C6)=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(C6=CC7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)C=C3)C=C2)C=C1 SROSJQSCFXFWDB-UHFFFAOYSA-N 0.000 description 1
- HDDRPJSQLUUZGC-UHFFFAOYSA-N C1=CC=C(C2=C(C3=CC=C(N4C5=CC=C(C6=CC=C(C7=C(C8=CC=CC=C8)N(C8=CC=CC=C8)C8=CC=CC=C87)C=C6)C=C5C5=C4C=CC(C4=CC=C(C6=C(C7=CC=CC=C7)N(C7=CC=CC=C7)C7=C6C=CC=C7)C=C4)=C5)C=C3)C3=C(C=CC=C3)C2C2=CC=CC=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=C(C5=CC=CC=C5)N(C5=CC=CC=C5)C5=C4C=CC=C5)C=C2)C2=CC=C(C4=CC=C(C5=C(C6=CC=CC=C6)N(C6=CC=CC=C6)C6=CC=CC=C65)C=C4)C=C23)C=C1 Chemical compound C1=CC=C(C2=C(C3=CC=C(N4C5=CC=C(C6=CC=C(C7=C(C8=CC=CC=C8)N(C8=CC=CC=C8)C8=CC=CC=C87)C=C6)C=C5C5=C4C=CC(C4=CC=C(C6=C(C7=CC=CC=C7)N(C7=CC=CC=C7)C7=C6C=CC=C7)C=C4)=C5)C=C3)C3=C(C=CC=C3)C2C2=CC=CC=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=C(C5=CC=CC=C5)N(C5=CC=CC=C5)C5=C4C=CC=C5)C=C2)C2=CC=C(C4=CC=C(C5=C(C6=CC=CC=C6)N(C6=CC=CC=C6)C6=CC=CC=C65)C=C4)C=C23)C=C1 HDDRPJSQLUUZGC-UHFFFAOYSA-N 0.000 description 1
- IEJRXFIKCQKEDG-UHFFFAOYSA-N C1=CC=C(C2=C(C3=CC=CC=C3)N(C3=CC=CC=C3)=C(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)C=C3)N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C(C3=CC=CC=C3)N2C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=CC=CC=C3N5C3=CC=CC=C3)=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)C=C(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)C=C4)=N2)C=C1 Chemical compound C1=CC=C(C2=C(C3=CC=CC=C3)N(C3=CC=CC=C3)=C(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)C=C3)N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C(C3=CC=CC=C3)N2C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=CC=CC=C3N5C3=CC=CC=C3)=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)C=C(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)C=C4)=N2)C=C1 IEJRXFIKCQKEDG-UHFFFAOYSA-N 0.000 description 1
- LVMBMXOBJSQTJH-UHFFFAOYSA-N C1=CC=C(C2=C(\C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)N3C=CC=C\C3=N\2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)C=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=C(\C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)N3C=CC=C\C3=N\2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC(N5C6=CC=CC=C6C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)C=C3)=C2)C=C1 LVMBMXOBJSQTJH-UHFFFAOYSA-N 0.000 description 1
- RXZQRVXMSKADQR-UHFFFAOYSA-N C1=CC=C(C2=C(\C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)N3C=CC=C\C3=N\2)C=C1.C1=CC=C(C2=NN(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C(C3=CC=CC=C3)C2)C=C1.C1=CC=C(C2=NN(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C(C3=CC=CC=C3)C2C2=CC=CC=C2)C=C1 Chemical compound C1=CC=C(C2=C(\C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)N3C=CC=C\C3=N\2)C=C1.C1=CC=C(C2=NN(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C(C3=CC=CC=C3)C2)C=C1.C1=CC=C(C2=NN(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C(C3=CC=CC=C3)C2C2=CC=CC=C2)C=C1 RXZQRVXMSKADQR-UHFFFAOYSA-N 0.000 description 1
- JNKBGFRZUHEZFA-UHFFFAOYSA-N C1=CC=C(C2=C/C3=C(\C=C/2)N(C2=CC=CC=C2)C2=C3C=C(C3=CC=C(C4=CN5C=CC=CC5=N4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=CC=C4)C4=C5/C=C\C=C/4)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=C(C4=CN5C=CC=CC5=N4)C=C3)C3=C2/C=C\C(C2=CN4C=CC=CC4=N2)=C/3)C=C1 Chemical compound C1=CC=C(C2=C/C3=C(\C=C/2)N(C2=CC=CC=C2)C2=C3C=C(C3=CC=C(C4=CN5C=CC=CC5=N4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=CC=C4)C4=C5/C=C\C=C/4)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=C(C4=CN5C=CC=CC5=N4)C=C3)C3=C2/C=C\C(C2=CN4C=CC=CC4=N2)=C/3)C=C1 JNKBGFRZUHEZFA-UHFFFAOYSA-N 0.000 description 1
- GJZHYOZYYPYCCW-UHFFFAOYSA-N C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C\C=C/C=C\54)C=C3)=N2)C=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=CC(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C\C=C/C=C\54)C=C3)=N2)C=C1.C1=CC=C(C2=C3C=CC=CC3=NC(C3=CC=CC(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)=C3)=N2)C=C1 GJZHYOZYYPYCCW-UHFFFAOYSA-N 0.000 description 1
- IKOCHGGOVFQQBC-UHFFFAOYSA-N C1=CC=C(C2=C3C=CC=CC3=NC(N3C4=C(C=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C4=C\C=C/C=C\43)=N2)C=C1.C1=CC=C(C2=NC(N3C4=C(C=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C4=C/C=C\C=C\43)=C3C=CC=CC3=N2)C=C1.C1=CC=C(C2=NC3=C(C=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)C=C3)C(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=C3C=CC=CC3=NC(N3C4=C(C=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C4=C\C=C/C=C\43)=N2)C=C1.C1=CC=C(C2=NC(N3C4=C(C=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C4=C/C=C\C=C\43)=C3C=CC=CC3=N2)C=C1.C1=CC=C(C2=NC3=C(C=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)C=C3)C(C3=CC=CC=C3)=N2)C=C1 IKOCHGGOVFQQBC-UHFFFAOYSA-N 0.000 description 1
- WJGCNGYJLYDJBR-UHFFFAOYSA-N C1=CC=C(C2=C3C=CC=CN3C=C2C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC=C(C5=CN6C=CC=CC6=N5)C=C4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 Chemical compound C1=CC=C(C2=C3C=CC=CN3C=C2C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC=C(C5=CN6C=CC=CC6=N5)C=C4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 WJGCNGYJLYDJBR-UHFFFAOYSA-N 0.000 description 1
- KZJNIBWFYUWJQR-UHFFFAOYSA-N C1=CC=C(C2=C3C=CC=CN3C=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CN5C=CC=CC5=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 Chemical compound C1=CC=C(C2=C3C=CC=CN3C=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CN5C=CC=CC5=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 KZJNIBWFYUWJQR-UHFFFAOYSA-N 0.000 description 1
- SXXOFMKUTKGINF-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=C4C=CC=CC4=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=C4C=CC=CC4=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 SXXOFMKUTKGINF-UHFFFAOYSA-N 0.000 description 1
- XIKIRQNWUPPLAT-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)=CC=C2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C/C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)=C\C=C\43)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)=CC=C2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C/C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)=C\C=C\43)C=C2)C=C1 XIKIRQNWUPPLAT-UHFFFAOYSA-N 0.000 description 1
- UCFUHHSDPAXNEP-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1 UCFUHHSDPAXNEP-UHFFFAOYSA-N 0.000 description 1
- ZAFKZSDSZOYKIM-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 ZAFKZSDSZOYKIM-UHFFFAOYSA-N 0.000 description 1
- KDDAOYULBQTOHH-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 KDDAOYULBQTOHH-UHFFFAOYSA-N 0.000 description 1
- RGHWPICUBLBBJY-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 RGHWPICUBLBBJY-UHFFFAOYSA-N 0.000 description 1
- RYYJJFPQRSYPKD-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(C4=CC=CC=C4)=NC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=NC(C8=CC=CC(C9=CC=CC=C9)=C8)=CC(C8=CC=CC=C8)=N6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=N3)=CC=C2)C=C1.C1=CC=C(C2=CC=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC(C6=CC=CC(C7=CC=CC=C7)=C6)=NC(C6=CC=CC=C6)=N4)=C5)=NC(C4=CC=CC=C4)=N3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(C4=CC=CC=C4)=NC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=NC(C8=CC=CC(C9=CC=CC=C9)=C8)=CC(C8=CC=CC=C8)=N6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=N3)=CC=C2)C=C1.C1=CC=C(C2=CC=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC(C6=CC=CC(C7=CC=CC=C7)=C6)=NC(C6=CC=CC=C6)=N4)=C5)=NC(C4=CC=CC=C4)=N3)=C2)C=C1 RYYJJFPQRSYPKD-UHFFFAOYSA-N 0.000 description 1
- HFLLKWVLWNWQRU-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=CC=CC=C3C3=C2C=CC(C2=CC=C4C(=C2)C2=C(/C=C\C=C/2)N4C2=CC=CC=C2)=C3)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(N2C3=CC=CC=C3C3=C2C=CC(C2=CC=C4C(=C2)C2=C(/C=C\C=C/2)N4C2=CC=CC=C2)=C3)C=C1 HFLLKWVLWNWQRU-UHFFFAOYSA-N 0.000 description 1
- BTZVCAYNJRFWCV-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1 BTZVCAYNJRFWCV-UHFFFAOYSA-N 0.000 description 1
- HUROMQLEJDKZHG-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 HUROMQLEJDKZHG-UHFFFAOYSA-N 0.000 description 1
- VYOUIDOGTSQWNJ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC4=C(C=C3)C=C(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)C=C4)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)C=CC(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)=C4)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC4=C(C=C3)C=C(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)C=C4)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)C=CC(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)=C4)=N2)C=C1 VYOUIDOGTSQWNJ-UHFFFAOYSA-N 0.000 description 1
- LHOACNDOINZHME-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC4=C(C=C3)C=CC(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)=C4)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC4=C(C=C3)C=CC(N3C5=CC=CC=C5C5=C3C=CC(C3=CC=C6C(=C3)C3=CC=CC=C3N6C3=CC=CC=C3)=C5)=C4)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1 LHOACNDOINZHME-UHFFFAOYSA-N 0.000 description 1
- RPPSCEFMJDMURC-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.[C-]#[N+]C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=C(C#N)C=C4)C4=CC=CC5=C4OC4=C5C=CC=C4)C=C3)C=C2)C2=C3OC4=C(C=CC=C4)C3=CC=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.[C-]#[N+]C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=C(C#N)C=C4)C4=CC=CC5=C4OC4=C5C=CC=C4)C=C3)C=C2)C2=C3OC4=C(C=CC=C4)C3=CC=C2)C=C1 RPPSCEFMJDMURC-UHFFFAOYSA-N 0.000 description 1
- PLHNJIXCWFGFCJ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=C2)N=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)=C2)N=C1 PLHNJIXCWFGFCJ-UHFFFAOYSA-N 0.000 description 1
- RDCDXJZKUNASHZ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CN(C34CC5CC(CC(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)(C5)C3)C4)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3CC4CCC3CC4C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CN(C34CC5CC(CC(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)(C5)C3)C4)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3CC4CCC3CC4C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=N2)C=C1 RDCDXJZKUNASHZ-UHFFFAOYSA-N 0.000 description 1
- VPOOTWNCLQZXSH-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)N=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)N=C1 VPOOTWNCLQZXSH-UHFFFAOYSA-N 0.000 description 1
- XNGVPCSWEIQPSI-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=CC=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=CC=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 XNGVPCSWEIQPSI-UHFFFAOYSA-N 0.000 description 1
- GETPMKHOLQSSDJ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC6=C(C=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=C4C=CC=CC4=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC6=C(C=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=C4C=CC=CC4=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=N2)C=C1 GETPMKHOLQSSDJ-UHFFFAOYSA-N 0.000 description 1
- SSWVALFBVXNJBC-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC=CC=C3)=C4)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=CC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC=CC=C3)=C4)=NC(C3=CC=CC=C3)=N2)C=C1 SSWVALFBVXNJBC-UHFFFAOYSA-N 0.000 description 1
- HGYUKECVFJMALE-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C(C6=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N6)C=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=C(C6=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N6)C=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=NC(C3=CC=CC=C3)=N2)C=C1 HGYUKECVFJMALE-UHFFFAOYSA-N 0.000 description 1
- KUXNBIPKPFYBKR-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=C(C6=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N6)C=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC=CC=C4C4=C3C=CC(C3=C/C=C5C(=C/3)/C3=C(C=CC=C3)N/5C3=CC=CC=C3)=C4)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=C(C6=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N6)C=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC=CC=C4C4=C3C=CC(C3=C/C=C5C(=C/3)/C3=C(C=CC=C3)N/5C3=CC=CC=C3)=C4)=N2)C=C1 KUXNBIPKPFYBKR-UHFFFAOYSA-N 0.000 description 1
- UNAXUPFCCDVSBH-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 UNAXUPFCCDVSBH-UHFFFAOYSA-N 0.000 description 1
- AVYWVWFKKBMELQ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C3[Ir]N4=CC=CC=C4C3=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C3[Ir]N4=CC=CC=C4C3=C2)C=C1 AVYWVWFKKBMELQ-UHFFFAOYSA-N 0.000 description 1
- BAMJTSZQYJVCTF-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC7=C4C=CC=C7)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=C4C=CC=CC4=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC4=C(C=CC=C4)C=C3)=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC7=C4C=CC=C7)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=C4C=CC=CC4=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC4=C(C=CC=C4)C=C3)=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 BAMJTSZQYJVCTF-UHFFFAOYSA-N 0.000 description 1
- ULWVQUZCPBTJPB-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)=C3)=C2)C=C1.FC1=CC2=C(C=C1)N(C1=CC(C3=CC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=CC=C1)C1=C2C=C(F)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)=C3)=C2)C=C1.FC1=CC2=C(C=C1)N(C1=CC(C3=CC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=CC=C1)C1=C2C=C(F)C=C1 ULWVQUZCPBTJPB-UHFFFAOYSA-N 0.000 description 1
- TUTWQYFBYFIWSW-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 TUTWQYFBYFIWSW-UHFFFAOYSA-N 0.000 description 1
- LUQXEUJHKDHZFA-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)=C2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=C(C8=NC=CC=C8)C=C7)=NC(C7=CC=C(C8=CC=CC=N8)C=C7)=C6)C=C4)C4=CC=CC=C45)=C\C=C\32)C=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N1)C=C2 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)=C2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=C(C8=NC=CC=C8)C=C7)=NC(C7=CC=C(C8=CC=CC=N8)C=C7)=C6)C=C4)C4=CC=CC=C45)=C\C=C\32)C=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N1)C=C2 LUQXEUJHKDHZFA-UHFFFAOYSA-N 0.000 description 1
- CYJJAMCTQUSGRY-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=C(C=CC=C4)C4=C/C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)=C\C=C\43)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C/C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)=C\C=C\54)=CC=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=C(C=CC=C4)C4=C/C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)=C\C=C\43)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C/C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=C8)C=C6)C6=CC=CC=C67)=C\C=C\54)=CC=C3)C=C2)C=C1 CYJJAMCTQUSGRY-UHFFFAOYSA-N 0.000 description 1
- CGINVQINRFGCGP-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=C/C4=C(\C=C/3)N(C3=CC=CC=C3)C3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C(C4=C6C=CC=CC6=CC=C4)C4=C5/C=C\C=C/4)=C\C=C\32)C=C1.C1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC(C6=CC(C7=CC=NC=C7)=CC=N6)=C4)N/5C4=CC=CC=C4)C=C3)C3=C2/C=C\C(C2=CC(C4=CC=NC=C4)=CC=N2)=C/3)C=C1.C1=CC=C(N2C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)C=C3C3=C2C=CC=C3)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=C/C4=C(\C=C/3)N(C3=CC=CC=C3)C3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2C3=C(C=CC=C3)C3=C/C(C4=CC5=C(C=C4)C(C4=C6C=CC=CC6=CC=C4)C4=C5/C=C\C=C/4)=C\C=C\32)C=C1.C1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC(C6=CC(C7=CC=NC=C7)=CC=N6)=C4)N/5C4=CC=CC=C4)C=C3)C3=C2/C=C\C(C2=CC(C4=CC=NC=C4)=CC=N2)=C/3)C=C1.C1=CC=C(N2C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)/C6=C(C=CC=C6)N/7C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C4/C=C\C=C/5)C=C3C3=C2C=CC=C3)C=C1 CGINVQINRFGCGP-UHFFFAOYSA-N 0.000 description 1
- IXAVORQUQAEXHT-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1 IXAVORQUQAEXHT-UHFFFAOYSA-N 0.000 description 1
- JDGVHEJIFSWOFV-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 JDGVHEJIFSWOFV-UHFFFAOYSA-N 0.000 description 1
- GNMRDJJKWRBBTI-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 GNMRDJJKWRBBTI-UHFFFAOYSA-N 0.000 description 1
- XSYNCQSEMHAHEW-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 XSYNCQSEMHAHEW-UHFFFAOYSA-N 0.000 description 1
- MYQSDQRUOAXKEQ-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC=C3)=N2)C=C1 MYQSDQRUOAXKEQ-UHFFFAOYSA-N 0.000 description 1
- QZRPJPYQYBFVAP-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 QZRPJPYQYBFVAP-UHFFFAOYSA-N 0.000 description 1
- FDLVTYLTBMMOTP-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=NC=C5)C5=C4C4=C(C=C5)C5=C(C=CC=C5)N4C4=CC=CC=C4)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC6=C(C=C5C5=C4C=CC=C5)C4=C(C=CC=C4)N6C4=CC=CC=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C4=C(C=C5)C5=C(C=CC=C5)N4C4=CC=CC=C4)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=C(C=CC=C4)C4=C3C3=C(C=C4)C4=C(C=CC=C4)N3C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=NC=C5)C5=C4C4=C(C=C5)C5=C(C=CC=C5)N4C4=CC=CC=C4)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC6=C(C=C5C5=C4C=CC=C5)C4=C(C=CC=C4)N6C4=CC=CC=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C4=C(C=C5)C5=C(C=CC=C5)N4C4=CC=CC=C4)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=C(C=CC=C4)C4=C3C3=C(C=C4)C4=C(C=CC=C4)N3C3=CC=CC=C3)=N2)C=C1 FDLVTYLTBMMOTP-UHFFFAOYSA-N 0.000 description 1
- ACXQOLBFYGOWQB-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1 ACXQOLBFYGOWQB-UHFFFAOYSA-N 0.000 description 1
- IJYKKDZYAVQEME-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=N2)C=C1 IJYKKDZYAVQEME-UHFFFAOYSA-N 0.000 description 1
- OQJUNJJTNLYBAY-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=C(C=CC=C4)C4=C3C=CC(C3=CC5=C(C=C3)N(C3=CC=CC=C3)C3=CC=CC=C35)=C4)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=C(C=CC=C4)C4=C3C=CC(C3=CC5=C(C=C3)N(C3=CC=CC=C3)C3=CC=CC=C35)=C4)=N2)C=C1 OQJUNJJTNLYBAY-UHFFFAOYSA-N 0.000 description 1
- APSPBEVTOFFTKN-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC(C3=CC=CC=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC(C3=CC=CC=C3)=N2)C=C1 APSPBEVTOFFTKN-UHFFFAOYSA-N 0.000 description 1
- NFLIBBUVYHBQKE-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N3)=C4)=NC(C3=CC=CC=C3)=N2)C=C1.CC1(C)C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC=C2C2=C1C=CC=C2 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1.C1=CC=C(C2=CC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N3)=C4)=NC(C3=CC=CC=C3)=N2)C=C1.CC1(C)C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC=C2C2=C1C=CC=C2 NFLIBBUVYHBQKE-UHFFFAOYSA-N 0.000 description 1
- OKPFTKBEIWKNDG-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC=CC=C3)=C4)=N2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NC(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C5C(=C3)C3=C(C=CC=C3)N5C3=CC=CC=C3)=C4)=N2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=CC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 OKPFTKBEIWKNDG-UHFFFAOYSA-N 0.000 description 1
- MNOUGUNLSOXPNO-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=NN2C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)=CC(N4C5=C(C=C(C6=CC=CC=C6)C=C5)C5=C4C=CC(C4=CC=CC=C4)=C5)=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C(C3=CC=CC=C3)N2C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=NN2C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=CC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)=CC(N4C5=C(C=C(C6=CC=CC=C6)C=C5)C5=C4C=CC(C4=CC=CC=C4)=C5)=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C(C3=CC=CC=C3)N2C2=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=CC(N3C4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1 MNOUGUNLSOXPNO-UHFFFAOYSA-N 0.000 description 1
- OWMFCXVASUJTDS-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=C2)N=C1 Chemical compound C1=CC=C(C2=CC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=CN=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)N=C3)=C2)N=C1 OWMFCXVASUJTDS-UHFFFAOYSA-N 0.000 description 1
- AXYHWXQWLXRFAL-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CN=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=CC=C4)=C5)N=C3)=C2)C=C1 AXYHWXQWLXRFAL-UHFFFAOYSA-N 0.000 description 1
- CMXQBWLUOQYYPL-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=C2)N=C1 Chemical compound C1=CC=C(C2=CC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=CC(C3=NC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=N3)=C2)N=C1 CMXQBWLUOQYYPL-UHFFFAOYSA-N 0.000 description 1
- HGZQTDIWNPXWGC-UHFFFAOYSA-N C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)C=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)C(C3=CC=CC=C3)=N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NCC(C3=CC=CC=C3)=N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)C=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)C(C3=CC=CC=C3)=N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NCC(C3=CC=CC=C3)=N2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1 HGZQTDIWNPXWGC-UHFFFAOYSA-N 0.000 description 1
- SJQBYXHVHJPOOY-UHFFFAOYSA-N C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)C=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)C=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=C(C6CCCCC6)C=C5)C5=C4C=CC(C4CCCCC4)=C5)C=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1 SJQBYXHVHJPOOY-UHFFFAOYSA-N 0.000 description 1
- WQTZGAILEFFIRX-UHFFFAOYSA-N C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)C=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=NC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)=CC=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=NC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)=CC=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC3=C(C=C2)N(C2=CC=C(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)C=N2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=NC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)=CC=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC3=C(C=C2)N(C2=NC(C4=CC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=N4)=CC=C2)C2=C3C=C(C3=CC=CC=C3)C=C2)C=C1 WQTZGAILEFFIRX-UHFFFAOYSA-N 0.000 description 1
- JFZOPWVUQGOVSM-DWXAKWDBSA-L C1=CC=C(C2=CC3=C(C=C2)[Ir]N2=CC=CC=C32)C=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir@@]21C2=C(C=CC=C2)C2=N1C=CC1=C2C=CC=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir@@]21C2=C(C=CC=C2)C2=N1C=CC1=C2C=CC=C1.C1=CC=N2[Pt]C3=C(C=CC=C3)C2=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=C(SC=C1)C1=N2C=CC=C1.FC(F)(F)C1=CC2=C(C=C1)C1=CC=CC=N1[Ir]2 Chemical compound C1=CC=C(C2=CC3=C(C=C2)[Ir]N2=CC=CC=C32)C=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir@@]21C2=C(C=CC=C2)C2=N1C=CC1=C2C=CC=C1.C1=CC=N2C(=C1)C1=C(C=CC=C1)[Ir@@]21C2=C(C=CC=C2)C2=N1C=CC1=C2C=CC=C1.C1=CC=N2[Pt]C3=C(C=CC=C3)C2=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=N2C2=C(C=CC=C2)O1.CC1=CC(C)=O[Ir]2(O1)C1=C(SC=C1)C1=N2C=CC=C1.FC(F)(F)C1=CC2=C(C=C1)C1=CC=CC=N1[Ir]2 JFZOPWVUQGOVSM-DWXAKWDBSA-L 0.000 description 1
- LPCZQDRAOORCQW-UHFFFAOYSA-N C1=CC=C(C2=CC3=C(C=CC=C3)C(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC9=C8C=CC=C9)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=C9C=CC=CC9=N8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC3=C2C=CC=C3)C=C1 Chemical compound C1=CC=C(C2=CC3=C(C=CC=C3)C(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC9=C8C=CC=C9)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=C9C=CC=CC9=N8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=NC3=C2C=CC=C3)C=C1 LPCZQDRAOORCQW-UHFFFAOYSA-N 0.000 description 1
- XFAZRDRTUCJDNM-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=CC(C8=CC=CC=C8)=N7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=CC(C8=CC=CC=C8)=N7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 XFAZRDRTUCJDNM-UHFFFAOYSA-N 0.000 description 1
- USZKEOQWVDPTFY-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1 USZKEOQWVDPTFY-UHFFFAOYSA-N 0.000 description 1
- KNZDIESIKWHFRR-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2/C=C\C=C/3)=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(C2=CC3=C(C=CC=C3)C=C2)=CC(N2C3=CC=CC=C3C3=C2C=CC=C3)=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)=CC(N2C3=C(C=CC=C3)C3=C2/C=C\C=C/3)=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(C2=CC3=C(C=CC=C3)C=C2)=CC(N2C3=CC=CC=C3C3=C2C=CC=C3)=C1 KNZDIESIKWHFRR-UHFFFAOYSA-N 0.000 description 1
- MXEUWHVDWQQUEX-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=CC(C4=CC=CC=C4)=N3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=CC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=C/C=C4C(=C/1)\C1=C(C=CC=C1)N\4C1=CC=CC=C1)=C3)C=C2 Chemical compound C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=CC(C4=CC=C(C5=CC=CC=C5)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=CC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=CC(C4=CC=CC=C4)=N3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=CC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=C/C=C4C(=C/1)\C1=C(C=CC=C1)N\4C1=CC=CC=C1)=C3)C=C2 MXEUWHVDWQQUEX-UHFFFAOYSA-N 0.000 description 1
- ADQLVSQJEPZLOQ-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.CC1(C)C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC=C2C2=C1C=CC=C2 Chemical compound C1=CC=C(C2=CC=C(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.CC1(C)C2=CC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=CC=C2C2=C1C=CC=C2 ADQLVSQJEPZLOQ-UHFFFAOYSA-N 0.000 description 1
- VTSAYWZCLNPTGP-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C=C3)C=C2)C=C1 VTSAYWZCLNPTGP-UHFFFAOYSA-N 0.000 description 1
- QBOBTLGXNYILEH-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C4=CC=C(C5=C6\OC7=C(C=CC=C7)\C6=C/C=C\5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=C6OC7=C(C=CC=C7)C6=CC=C5)C=C4)C4=CC=C(C5=C6\OC7=C(C=CC=C7)\C6=C/C=C\5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)C=C3)C=C2)C=C1 QBOBTLGXNYILEH-UHFFFAOYSA-N 0.000 description 1
- ROYWKGSQIYSYEY-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=CC=C5)C=C4)C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=C5C=CC=CC5=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=CC=C5)C=C4)C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=CC=C5)C=C4)C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=C5C=CC=CC5=C4)C4=CC5=C(C=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=C4C=CC=CC4=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=CC=C5)C=C4)C4=C3C=CC=C4)C=C2)C=C1 ROYWKGSQIYSYEY-UHFFFAOYSA-N 0.000 description 1
- QTHREDLLSGJKHA-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC4=C(C=C3)C3=C(C=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C3)C4(C3=CC=CC=C3)C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC4=C(C=C3)C3=C(C=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C3)C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC4=C(C=C3)C3=C(C=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C3)C4(C3=CC=CC=C3)C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC4=C(C=C3)C3=C(C=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C3)C4)C=C2)C=C1 QTHREDLLSGJKHA-UHFFFAOYSA-N 0.000 description 1
- GQFVKSUOPMXCIX-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC3=C(C=CC=C3)N2C2=CC=C(C3=C4C=CC=CC4=C(C4=CC=CC=C4)C4=CC=CC=C43)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC3=C(C=CC=C3)N2C2=CC=C(C3=C4C=CC=CC4=C(C4=CC=CC=C4)C4=CC=CC=C43)C=C2)C=C1 GQFVKSUOPMXCIX-UHFFFAOYSA-N 0.000 description 1
- DMAWPHXQHAQIAU-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=C(C7=CC=CC=C7)C=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=C5C=CC=CC5=CC=C4)C=C3)C=C2)C2=CC=CC3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1 DMAWPHXQHAQIAU-UHFFFAOYSA-N 0.000 description 1
- OPXUIRSRYGWYRB-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=C5C=CC=CC5=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC5=C4C=CC=C5)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=C(C7=CC=CC=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=C5C=CC=CC5=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC5=C4C=CC=C5)C=C3)C=C2)C=C1 OPXUIRSRYGWYRB-UHFFFAOYSA-N 0.000 description 1
- DXRGSERKQMPYBK-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=N7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(C5=CC=CC=C5)C=C4)=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=N3)C=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=NC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=N7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1 DXRGSERKQMPYBK-UHFFFAOYSA-N 0.000 description 1
- YCLCLXFKOCRWFJ-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=CC(C4=CC=CC=C4)=N3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 Chemical compound C1=CC=C(C2=CC=C(C3=NC(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=CC=C7C(=C5)C5=C(C=CC=C5)N7C5=CC=CC=C5)=C6)C=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=CC(C4=CC=CC=C4)=N3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 YCLCLXFKOCRWFJ-UHFFFAOYSA-N 0.000 description 1
- BQFIIADCVFHAKC-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5/C=C\C=C/6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C3)C=C2)C=C1 BQFIIADCVFHAKC-UHFFFAOYSA-N 0.000 description 1
- KDMQNJNHMFKOJM-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(C5=CC=C(C6=NN=C(C7=CC=C(C8=CC=CC=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(OC5=CC=C(C6=NN=C(C7=CC=C(C8=CC=CC=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(C5=CC=C(C6=NN=C(C7=CC=C(C8=CC=C(C(C)(C)C)C=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=CC=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=NN=C(C3=CC=C(C4=CC=CC=C4)C=C3)O2)C=C1.CC1=CC(C2=CC(C)=C(C3=NN=C(C4=C5C=CC=CC5=CC=C4)O3)C=C2)=CC=C1C1=NN=C(C2=CC=CC3=C2C=CC=C3)O1 Chemical compound C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(C5=CC=C(C6=NN=C(C7=CC=C(C8=CC=CC=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(OC5=CC=C(C6=NN=C(C7=CC=C(C8=CC=CC=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=C(C5=CC=C(C6=NN=C(C7=CC=C(C8=CC=C(C(C)(C)C)C=C8)C=C7)O6)C=C5)C=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=CC=C(C3=NN=C(C4=CC=CC=C4)O3)C=C2)C=C1.CC(C)(C)C1=CC=C(C2=NN=C(C3=CC=C(C4=CC=CC=C4)C=C3)O2)C=C1.CC1=CC(C2=CC(C)=C(C3=NN=C(C4=C5C=CC=CC5=CC=C4)O3)C=C2)=CC=C1C1=NN=C(C2=CC=CC3=C2C=CC=C3)O1 KDMQNJNHMFKOJM-UHFFFAOYSA-N 0.000 description 1
- YXYXCWUXSPLEBW-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC=CC(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=CC(C4=CC=C(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)=C3)C=C2)C=C1.CC1(C)C2=CC=C(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2C2=C1C=CC=C2.CC1(C)C2=CC=C(C3=CC4=C(C=C3)SC3=C4C=C(N4C5=C(C=NC=C5)C5=C4C=CN=C5)C=C3)C=C2C2=C1C=CC=C2 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC=CC(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=CC(C4=CC=C(C5=CC6=C(C=C5)OC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)=C3)C=C2)C=C1.CC1(C)C2=CC=C(C3=CC4=C(C=C3)OC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2C2=C1C=CC=C2.CC1(C)C2=CC=C(C3=CC4=C(C=C3)SC3=C4C=C(N4C5=C(C=NC=C5)C5=C4C=CN=C5)C=C3)C=C2C2=C1C=CC=C2 YXYXCWUXSPLEBW-UHFFFAOYSA-N 0.000 description 1
- MSKORAIQEZDWTE-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC=CC(C5=CC6=C(C=C5)SC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=CC(N(C3=CC=CC(C4=CC=CC=C4)=C3)C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)SC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC(C4=CC=CC(C5=CC6=C(C=C5)SC5=C6C=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=CC(N(C3=CC=CC(C4=CC=CC=C4)=C3)C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(C4=CC5=C(C=C4)OC4=C5C=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC4=C(C=C3)SC3=C4C=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 MSKORAIQEZDWTE-UHFFFAOYSA-N 0.000 description 1
- TWCSCOKAPVZRAM-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=C6C=CC=CC6=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=C6C=CC=CC6=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1 TWCSCOKAPVZRAM-UHFFFAOYSA-N 0.000 description 1
- LMFILLIQPDTJQZ-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC(C7=CC=CC=C7)=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C4C(=C3)O/C3=C/C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)=C\C=C\43)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)/C=C\21 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC(C7=CC=CC=C7)=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C4C(=C3)O/C3=C/C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)=C\C=C\43)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)/C=C\21 LMFILLIQPDTJQZ-UHFFFAOYSA-N 0.000 description 1
- ZMHAVZRLAGXFBV-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC3=C(C=CC=C3)N2C2=CC=C(C3=C4C=CC=CC4=C(C4=CC=C5C=CC=CC5=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=C(C7=CC=C(N(C8=CC=CC=C8)C8=CC=CC=C8)C=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C2C(=C1)[Ir]N1=C2C2=C(C=CC=C2)C=C1.[C-]#[N+]C1=NC2=C3N=C([N+]#[C-])C(C#N)=NC3=C3N=C(C#N)C(C#N)=NC3=C2N=C1C#N Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=NC3=C(C=CC=C3)N2C2=CC=C(C3=C4C=CC=CC4=C(C4=CC=C5C=CC=CC5=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(C5=CC=C(N(C6=CC=CC=C6)C6=CC=C(C7=CC=C(N(C8=CC=CC=C8)C8=CC=CC=C8)C=C7)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C2C(=C1)[Ir]N1=C2C2=C(C=CC=C2)C=C1.[C-]#[N+]C1=NC2=C3N=C([N+]#[C-])C(C#N)=NC3=C3N=C(C#N)C(C#N)=NC3=C2N=C1C#N ZMHAVZRLAGXFBV-UHFFFAOYSA-N 0.000 description 1
- AQXLKXAPAXDCQS-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6OC7=C(C=C(C8=CC=CC=C8)C=C7)C6=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5OC6=C(C=C(C7=CC=CC=C7)C=C6)C5=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6OC7=C(C=C(C8=CC=CC=C8)C=C7)C6=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(C5=CC=C6OC7=C(C=C(C8=CC=CC=C8)C=C7)C6=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5OC6=C(C=C(C7=CC=CC=C7)C=C6)C5=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6OC7=C(C=C(C8=CC=CC=C8)C=C7)C6=C5)C=C4)C=C3)C=C2)C=C1 AQXLKXAPAXDCQS-UHFFFAOYSA-N 0.000 description 1
- VZGISWPJRHZRJX-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C6C(=C5)CC5=C6C=CC=C5)C5=CC6=C(C=C5)C5=C(C=CC=C5)C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C6C(=C5)CC5=C6C=CC=C5)C5=CC6=C(C=C5)C5=C(C=CC=C5)C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1 VZGISWPJRHZRJX-UHFFFAOYSA-N 0.000 description 1
- YUNIHQKRRZSLON-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C6C=CC=CC6=C5)C5=CC6=C(C=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC(C6=CC=CC=C6)=C5)C5=CC(C6=CC=CC=C6)=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C6C=CC=CC6=C5)C5=CC6=C(C=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC(C6=CC=CC=C6)=C5)C5=CC(C6=CC=CC=C6)=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C=C3)C=C2)C=C1 YUNIHQKRRZSLON-UHFFFAOYSA-N 0.000 description 1
- SMUYJPXNRNQPAA-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(OC4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)C=C2)C=C1.CC(C)C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=C(C(C)C)C=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=C(OC4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)C=C2)C=C1.CC(C)C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=C(C(C)C)C=C4)C=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC=C6)C=C5)C5=CC=C(C6=CC=CC=C6)C=C5)C=C4)C=C3)C=C2)C=C1 SMUYJPXNRNQPAA-UHFFFAOYSA-N 0.000 description 1
- AZLONVAUUPEURC-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)C=C3)C=C2)C=C1 AZLONVAUUPEURC-UHFFFAOYSA-N 0.000 description 1
- AUCGZDCHOWBHDI-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC7=C6C=CC=C7)C=C5)C5=CC=C(C6=CC=CC7=C6C=CC=C7)C=C5)C=C4)C=C3)C=C2)C=C1.CC(C)C1=CC=C(N(C2=CC=C(C(C)C)C=C2)C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(N3C4=CC=CC=C4C4=C3C=CC=C4)/C=C\21 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(C5=CC=C6C(=C5)C5=C(C=CC=C5)N6C5=CC=CC=C5)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=C(C6=CC=CC7=C6C=CC=C7)C=C5)C5=CC=C(C6=CC=CC7=C6C=CC=C7)C=C5)C=C4)C=C3)C=C2)C=C1.CC(C)C1=CC=C(N(C2=CC=C(C(C)C)C=C2)C2=C3C=CC=CC3=C(N(C3=CC=CC=C3)C3=CC=CC=C3)C3=C2C=CC=C3)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=C(C4=CC=CC=C4)C=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(N3C4=CC=CC=C4C4=C3C=CC=C4)/C=C\21 AUCGZDCHOWBHDI-UHFFFAOYSA-N 0.000 description 1
- NSHZMBKQXWHOML-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N5C6=C(C=C(C7=CC=CC=C7)C=C6)C6=C5C=CC(C5=CC=CC=C5)=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=CC=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)/C=C\21 Chemical compound C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N5C6=C(C=C(C7=CC=CC=C7)C=C6)C6=C5C=CC(C5=CC=CC=C5)=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(C2=CC=C(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=CC=C3)C3=CC=C(C4=CC=CC=C4)C=C3)=C2)C2=C\C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)/C=C\21 NSHZMBKQXWHOML-UHFFFAOYSA-N 0.000 description 1
- GCLULAHXLWIWSU-UHFFFAOYSA-N C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=CC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=CC(C8=CC=CC=C8)=NC(C8=CC=CC=C8)=C7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)=C2)C=C1 GCLULAHXLWIWSU-UHFFFAOYSA-N 0.000 description 1
- KMHLLQKEXLOLEQ-UHFFFAOYSA-N C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC(C6=CC=CC=C6)=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=CC(C4=CC=CC(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC(C5=CC=CC=C5)=C4)=C3)C=C2)C=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC(C3=CC=C(N4C5=CC=C(C6=CC=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N1)C=C2 Chemical compound C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC(C6=CC=CC=C6)=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=CC(C4=CC=CC(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)=C4)=CC=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC(C5=CC=CC=C5)=C4)=C3)C=C2)C=C1.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC(C3=CC=C(N4C5=CC=C(C6=CC=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N1)C=C2 KMHLLQKEXLOLEQ-UHFFFAOYSA-N 0.000 description 1
- ADGFCSAHMLXRIV-UHFFFAOYSA-N C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC(C6=CC=CC=C6)=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC(C3=CC=CC(C4=NC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)=C3)=CC=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=CC=C(C4=NC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC(C6=CC=CC=C6)=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=C3)C=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC(C3=CC=CC(C4=NC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)=C3)=CC=C2)C=C1.C1=CC=C(C2=CC=C3C(=C2)C2=C(C=CC(C4=CC=C5C(=C4)C4=C(C=CC=C4)N5C4=CC=CC=C4)=C2)N3C2=CC=C(C3=CC=C(C4=NC(C5=CC=CC=C5)=NC(C5=CC=CC=C5)=C4)C=C3)C=C2)C=C1 ADGFCSAHMLXRIV-UHFFFAOYSA-N 0.000 description 1
- HRQWOTBADVPNTC-UHFFFAOYSA-N C1=CC=C(C2=CC=CC(C3=CC(C4=CC=CC(C5=CC=CC=C5)=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=N3)=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CN4C=CC=CC4=N3)=C2)=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC=C2)=C1 Chemical compound C1=CC=C(C2=CC=CC(C3=CC(C4=CC=CC(C5=CC=CC=C5)=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=N3)=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC(C3=CN4C=CC=CC4=N3)=C2)=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=CC=C2)=C1 HRQWOTBADVPNTC-UHFFFAOYSA-N 0.000 description 1
- KIZVAMDHPVQLHI-UHFFFAOYSA-N C1=CC=C(C2=CC=CC(C3=NC(C4=CC=CC(C5=CC=CC=C5)=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 Chemical compound C1=CC=C(C2=CC=CC(C3=NC(C4=CC=CC(C5=CC=CC=C5)=C4)=NC(C4=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=CC(N5C6=C(C=CC=C6)C6=C5C=CC=C6)=C4)=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1 KIZVAMDHPVQLHI-UHFFFAOYSA-N 0.000 description 1
- VCBQENYKEFSXTM-LEMDDYFSSA-L C1=CC=C(C2=CC=CC=C2O[Pt@@]23C4=C(C=CC=C4C4=N2C=CC=C4)C2=CC=CC=N23)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(/C=C\C(F)=C/1)C1=CC(N(C)C)=CC=N12.CC1=CC2=C(C=C1)C1=CC=CC=N1[Ir]2.CC1=CC2=C(C=C1)[Ir]N1=CC=CC=C21.CC1=CC=N2[Ir]C3=C(C2=C1)/C(F)=C\C(F)=C/3.FC1=C/C2=C(\C=C/1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC=N1[Ir]2 Chemical compound C1=CC=C(C2=CC=CC=C2O[Pt@@]23C4=C(C=CC=C4C4=N2C=CC=C4)C2=CC=CC=N23)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(/C=C\C(F)=C/1)C1=CC(N(C)C)=CC=N12.CC1=CC2=C(C=C1)C1=CC=CC=N1[Ir]2.CC1=CC2=C(C=C1)[Ir]N1=CC=CC=C21.CC1=CC=N2[Ir]C3=C(C2=C1)/C(F)=C\C(F)=C/3.FC1=C/C2=C(\C=C/1)C1=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=CC=N1[Ir]2 VCBQENYKEFSXTM-LEMDDYFSSA-L 0.000 description 1
- AQMHXVVFOMPZRE-UHFFFAOYSA-N C1=CC=C(C2=CN=C(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)N=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.N#CC1=CC(C#N)=CC(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1 Chemical compound C1=CC=C(C2=CN=C(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)N=C2)C=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.N#CC1=CC(C#N)=CC(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=C1 AQMHXVVFOMPZRE-UHFFFAOYSA-N 0.000 description 1
- ZURLFUYCTLUNLW-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC4=C(C=CC=C4)C=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC4=C(C=CC=C4)C=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC6=C4C=CC=C6)=C5)C=C3)=N2)C=C1 ZURLFUYCTLUNLW-UHFFFAOYSA-N 0.000 description 1
- AOHXRFWRQPEXGX-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=C(C5=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=N7)=C6)C=C5)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=CC=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=CC=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=C(C5=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=N7)=C6)C=C5)C=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=CC=C4)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=CC=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1 AOHXRFWRQPEXGX-UHFFFAOYSA-N 0.000 description 1
- YFENVEDFBBPDCT-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=C(C5=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=N7)=N6)C=C5)C=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=CC=C4)C4=C5/C=C(C5=CC=C(C6=CC(C7=CC=CC=N7)=NC(C7=CC=CC=C7)=N6)C=C5)\C=C/4)C=C3)=C2)C=C1.C1=CC=C(N2C3=C(C=C(C4=CC=C(C5=CN6C=CC=CC6=N5)C=C4)C=C3)C3=C2/C=C\C(C2=CC=C(C4=CN5C=CC=CC5=N4)C=C2)=C/3)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(C4=C/C5=C(\C=C/4)N(C4=CC=CC=C4)C4=C5C=C(C5=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=N7)=N6)C=C5)C=C4)C=C3)=NC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=CC=C4)C4=C5/C=C(C5=CC=C(C6=CC(C7=CC=CC=N7)=NC(C7=CC=CC=C7)=N6)C=C5)\C=C/4)C=C3)=C2)C=C1.C1=CC=C(N2C3=C(C=C(C4=CC=C(C5=CN6C=CC=CC6=N5)C=C4)C=C3)C3=C2/C=C\C(C2=CC=C(C4=CN5C=CC=CC5=N4)C=C2)=C/3)C=C1 YFENVEDFBBPDCT-UHFFFAOYSA-N 0.000 description 1
- AGXYXYVLFCVEBG-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)C=C3)=C3C=CC=CC3=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C\C=C/C=C\54)=C3)=C3C=CC=CC3=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C/C=C\C=C\54)C=C3)=C3C=CC=CC3=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC(N4C5=C(C=C(C6=CC=C7C(=C6)C6=C(C=CC=C6)N7C6=CC=CC=C6)C=C5)C5=C\C=C/C=C\54)=C3)=C3C=CC=CC3=N2)C=C1 AGXYXYVLFCVEBG-UHFFFAOYSA-N 0.000 description 1
- WIIMAWHZDINYQU-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)N=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC=C(C3=CN4C=CC=CC4=N3)C=C2)=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=CC=N3)=N2)C=C1.C1=CC=C(C2=NC(C3=NC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)N=C1.C1=CC=C2C(=C1)C1=C(C=CC=C1)N2C1=CC(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=CC(C2=CC=C(C3=CN4C=CC=CC4=N3)C=C2)=C1 WIIMAWHZDINYQU-UHFFFAOYSA-N 0.000 description 1
- MEGMHSBPFXODIE-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.N#CC1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1.N#CC1=CC=C(C2=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=N2)C=C1 MEGMHSBPFXODIE-UHFFFAOYSA-N 0.000 description 1
- OHERBWYQWOBKDK-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=C4C=CC=CC4=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC6=C(C=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC7=C4C=CC=C7)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=C4C=CC=CC4=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC6=C(C=CC=C6)C=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC7=C4C=CC=C7)N6C4=CC=CC=C4)=C5)C=C3)=N2)C=C1 OHERBWYQWOBKDK-UHFFFAOYSA-N 0.000 description 1
- IFOJYMQRUOOUSX-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1 IFOJYMQRUOOUSX-UHFFFAOYSA-N 0.000 description 1
- COJSKFCUFBJUHL-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=NC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=N2)C=C1 COJSKFCUFBJUHL-UHFFFAOYSA-N 0.000 description 1
- UTSITMZDFIMAHG-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=N3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=C(N6C7=CC=CC=C7C7=C6C=CC(C6=C/C=C8C(=C/6)/C6=C(C=CC=C6)N/8C6=CC=CC=C6)=C7)C=C5)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(C5=CC=CC(N6C7=CC=CC=C7C7=C6C=CC(C6=CC=C8C(=C6)C6=C(C=CC=C6)N8C6=CC=CC=C6)=C7)=C5)=C4)=CC=C3)=N2)C=C1.CC1(C)C2=C(C=CC(C3=NC(C4=CC=CC=C4)=NC(C4=CC=CC=C4)=N3)=C2)C2=C1C=C(N1C3=CC=CC=C3C3=C1C=CC(C1=CC=C4C(=C1)C1=C(C=CC=C1)N4C1=CC=CC=C1)=C3)C=C2 UTSITMZDFIMAHG-UHFFFAOYSA-N 0.000 description 1
- OVDZOWICJOQGPI-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(C4=CC=CC(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC(C5=C/C=C7C(=C/5)/C5=C(C=CC=C5)N/7C5=CC=CC=C5)=C6)C=C4)C=C3)=N2)C=C1 OVDZOWICJOQGPI-UHFFFAOYSA-N 0.000 description 1
- RXOAVHMDTNEBQG-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2)C=C1.C1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC=C4)N/5C4=CC=CC=C4)C=C3)C3=C2/C=C\C=C/3)C=C1.CC.CC.CC.CC.CC.CC.CC1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC=C4)N/5C4=CC=C(C)C=C4)C=C3)C3=C2/C=C\C=C/3)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2)C=C1.C1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC=C4)N/5C4=CC=CC=C4)C=C3)C3=C2/C=C\C=C/3)C=C1.CC.CC.CC.CC.CC.CC.CC1=CC=C(N2C3=C(C=C(C4=C/C=C5C(=C/4)/C4=C(C=CC=C4)N/5C4=CC=C(C)C=C4)C=C3)C3=C2/C=C\C=C/3)C=C1 RXOAVHMDTNEBQG-UHFFFAOYSA-N 0.000 description 1
- YVUOHIHLSCOPEP-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC6=C(C=C5C5=C4C=CC=C5)C4=C(C=CN=C4)N6C4=CC=CC=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC5=C(C=C4C4=C3C=CC=C4)N(C3=CC=CC=C3)C3=C5C=CC4=C3C=CC=C4)=C2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC(N4C5=CC6=C(C=C5C5=C4C=CC=C5)C4=C(C=CN=C4)N6C4=CC=CC=C4)=CC=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC5=C(C=C4C4=C3C=CC=C4)N(C3=CC=CC=C3)C3=C5C=CC4=C3C=CC=C4)=C2)C=C1 YVUOHIHLSCOPEP-UHFFFAOYSA-N 0.000 description 1
- KHRIRTDQHLRTFB-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1.ClC1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.ClC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1.ClC1=NC(Cl)=NC(Cl)=N1.OB(O)C1=CC=C(Cl)C=C1.OB(O)C1=CC=CC=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=C(C=C(C6=C/C=C7C(=C/6)\C6=C(C=CC=C6)N\7C6=CC=CC=C6)C=C5)C5=C/C=C/C=C\54)C=C3)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C\C=C\54)=C/C=C\32)C=C1.ClC1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=N2)C=C1.ClC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1.ClC1=NC(Cl)=NC(Cl)=N1.OB(O)C1=CC=C(Cl)C=C1.OB(O)C1=CC=CC=C1 KHRIRTDQHLRTFB-UHFFFAOYSA-N 0.000 description 1
- NIWGKELMWQNKNF-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=CC(C8=NC(C9=CC=CC=C9)=NC(C9=CC=CC=C9)=N8)=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)=C3)=N2)C=C1 NIWGKELMWQNKNF-UHFFFAOYSA-N 0.000 description 1
- QWRUWGVKCCZPRN-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=CC=CC=C4N6C4=CC=C6C=CC=CC6=C4)=C5)C=C3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=N5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)=N2)C=C1 QWRUWGVKCCZPRN-UHFFFAOYSA-N 0.000 description 1
- FFGPMCMGZGNPSL-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC(N4C5=CC=CC=C5C5=C4C=CC(C4=C/C=C6C(=C/4)/C4=C(C=CC=C4)N/6C4=CC=CC=C4)=C5)=C3)=N2)C=C1 FFGPMCMGZGNPSL-UHFFFAOYSA-N 0.000 description 1
- DESHUYJMPKKTRL-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N(C3=CC=CC=C3)C3=CC=C(C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4)C=C3)=N2)C=C1 DESHUYJMPKKTRL-UHFFFAOYSA-N 0.000 description 1
- JMQLEOHBTGGNQH-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=C(C=C(C5=C\C=C6C(=C/5)/C5=C(C=CC=C5)N/6C5=CC=CC=C5)C=C4)C4=C\C=C/C=C\43)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.ClC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(N3C4=C(C=C(C5=C\C=C6C(=C/5)/C5=C(C=CC=C5)N/6C5=CC=CC=C5)C=C4)C4=C\C=C/C=C\43)=N2)C=C1.C1=CC=C(N2C3=C(C=CC=C3)C3=C\C(C4=CC5=C(C=C4)C/C4=C/C=C/C=C\54)=C/C=C\32)C=C1.ClC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 JMQLEOHBTGGNQH-UHFFFAOYSA-N 0.000 description 1
- KEMBRXLJDOCHMZ-UHFFFAOYSA-N C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=NC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=NC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=N2)C=C1 Chemical compound C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=C2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=CC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=CC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=N2)C=C1.C1=CC=C(C2=NC(C3=CC=CC=N3)=NC(C3=CC4=C(C=C3)N(C3=CC=CC=C3)C3=C4/C=C(C4=NC(C5=CC=CC=N5)=NC(C5=CC=CC=C5)=N4)\C=C/3)=N2)C=C1 KEMBRXLJDOCHMZ-UHFFFAOYSA-N 0.000 description 1
- ITNSOCGFDSKPIX-MBPDYOLISA-I C1=CC=C(C2=NC3=C(C=CC=C3)N3=C2C2=C(C=CC=C2)[Ir]3)C=C1.CC(C)(C)C1=CC2=C(O[Pt@]34OC5=C(C(C)(C)C)C=C(C(C)(C)C)C=C5/C=N\3C3=C(C=CC=C3)/N4=C/2)C(C(C)(C)C)=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=C(C3=CC=CC=C3)C=C1)C1=CC=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C3C=CC=CC3=C3C=CC=CC3=N12.CC1=O[Ir@@]2(OC3=C1C(=O)CCC3)C1=C(C=CC=C1)C1=C3C=CC=CC3=CC=N12.FC1(F)C2=CC=CC3=C2C2=C4C(=CC=N2[Ir]3)C=CC=C41 Chemical compound C1=CC=C(C2=NC3=C(C=CC=C3)N3=C2C2=C(C=CC=C2)[Ir]3)C=C1.CC(C)(C)C1=CC2=C(O[Pt@]34OC5=C(C(C)(C)C)C=C(C(C)(C)C)C=C5/C=N\3C3=C(C=CC=C3)/N4=C/2)C(C(C)(C)C)=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=C(C3=CC=CC=C3)C=C1)C1=CC=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C3C=CC=CC3=C3C=CC=CC3=N12.CC1=O[Ir@@]2(OC3=C1C(=O)CCC3)C1=C(C=CC=C1)C1=C3C=CC=CC3=CC=N12.FC1(F)C2=CC=CC3=C2C2=C4C(=CC=N2[Ir]3)C=CC=C41 ITNSOCGFDSKPIX-MBPDYOLISA-I 0.000 description 1
- DNFPUHLKAMRBCT-UHFFFAOYSA-N C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1 Chemical compound C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=NC=C3C(=C2)C2=C(C=CC=C2)N3C2=CN=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)N=C2)C=C1 DNFPUHLKAMRBCT-UHFFFAOYSA-N 0.000 description 1
- WAABRBMLGKPARF-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=C4C=CC=C5C6=C(C=CC=C6)C(=C54)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=C4C=CC=C5C6=C(C=CC=C6)C(=C54)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)=CC=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=C4C=CC=C5C6=C(C=CC=C6)C(=C54)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=C4C=CC=C5C6=C(C=CC=C6)C(=C54)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)=CC=C2)C=C1 WAABRBMLGKPARF-UHFFFAOYSA-N 0.000 description 1
- UPSOTWOUNJNSRX-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C=C(C5=CC=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C=C(C5=CC=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)N=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)N=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)OC2=C3C=C(C3=CC=C4C=C(C5=CC=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C=C(C5=C6C=CC=CC6=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C(\C=C/2)SC2=C3C=C(C3=CC=C4C=C(C5=CC=C6C=CC=CC6=C5)C=CC4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)N=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)N=C2)C=C1 UPSOTWOUNJNSRX-UHFFFAOYSA-N 0.000 description 1
- RSDZQRLMSIIJAG-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C/C=C\C4=CC=CC(=C2)C43)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\CCC(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC=C4)C3=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C/C3=C/C=C\C4=CC=CC(=C2)C43)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\CCC(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC=C4)C3=C2)C=C1 RSDZQRLMSIIJAG-UHFFFAOYSA-N 0.000 description 1
- KPYMBEMHHRBLCE-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\C5=C(C=C6C=CC=CC6=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC=C2)C2=C3C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC3=C2C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC5=C4C=CC4=C5C=CC=C4)C3=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC5=C4C=CC=C5)C3=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\C5=C(C=C6C=CC=CC6=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC=C2)C2=C3C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC3=C2C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC3=C2C=CC=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC5=C4C=CC4=C5C=CC=C4)C3=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C=CC4=C(C=CC5=C4C=CC=C5)C3=C2)C=C1 KPYMBEMHHRBLCE-UHFFFAOYSA-N 0.000 description 1
- MQGZYZLLMSNXCU-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC4=C(C=CC=C4)C3=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC4=C(C=CC=C4)C3=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC4=C3C=CC=C4)C3=C2C=CC=C3)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=C3/C=C\C=C4\C5=C(C=CC=C5)C(=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC4=C(C=CC=C4)C3=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC4=C(C=CC=C4)C3=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C3C(=C2)C2=C(C=CC=C2)C2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=C3C=CC=CC3=C3C(=C2)C=CC2=C3C=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=CC4=C3C=CC=C4)C3=C2C=CC=C3)C=C1 MQGZYZLLMSNXCU-UHFFFAOYSA-N 0.000 description 1
- OZMJNAIMYTWJSD-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=CC(C4=CC(C5=CC=CC(C6=CC(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)=CC=C6)=C5)=CC=C4)=C3)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=C/C5=C(C=C(C6=CC=C(C7=CC8=C(C=CC=C8)C=C7)C=C6)C=C5)/C=C\4)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC5=C(C=C4)C=C(C4=CC=C6C=C(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)C=CC6=C4)C=C5)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC6=C(C=C5)C=C(C5=CC7=C(C=CC=C7)C7=C5C=CC=C7)C=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(C6=CC=C(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC(C3=CC=CC(C4=CC(C5=CC=CC(C6=CC(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)=CC=C6)=C5)=CC=C4)=C3)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=C/C5=C(C=C(C6=CC=C(C7=CC8=C(C=CC=C8)C=C7)C=C6)C=C5)/C=C\4)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC5=C(C=C4)C=C(C4=CC=C6C=C(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)C=CC6=C4)C=C5)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC6=C(C=C5)C=C(C5=CC7=C(C=CC=C7)C7=C5C=CC=C7)C=C6)C=C4)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(C6=CC=C(C7=CC8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5)C=C4)C=C3)C=C2)C=C1 OZMJNAIMYTWJSD-UHFFFAOYSA-N 0.000 description 1
- GFNFDVDMVGWCES-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=C2)C=C(C2=CC5=C(C=CC=C5)C5=C2C=CC=C5)C=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=CC=C6C7=CC=C8C=CC=CC8=C7C=CC6=C5C5=CC=CC=C35)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C3=CC=CC=C3C3=C6C=CC7=C8C=CC=CC8=CC=C7C6=CC=C53)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC7=C6C=CC6=C7C=CC=C6)C5=C3)C=C4)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=C2)C=C(C2=CC5=C(C=CC=C5)C5=C2C=CC=C5)C=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC=C(C4=CC5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=CC=C6C7=CC=C8C=CC=CC8=C7C=CC6=C5C5=CC=CC=C35)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C3=CC=CC=C3C3=C6C=CC7=C8C=CC=CC8=CC=C7C6=CC=C53)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC7=C6C=CC6=C7C=CC=C6)C5=C3)C=C4)C=C2)C=C1 GFNFDVDMVGWCES-UHFFFAOYSA-N 0.000 description 1
- LEVBELTWOSGJFD-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=CC=C4)C4=C2C=CC=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4C=CC=CC4=C4C(=C3)C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=CC5=C4C=CC=C5)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC(C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC(C3=CC4=C(C=CC=C4)C=C3)=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC3=C(C=C2)C=C(C2=CC4=C(C=CC=C4)C4=C2C=CC=C4)C=C3)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4C=CC=CC4=C4C(=C3)C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=CC5=C4C=CC=C5)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC(C3=CC4=C(C=CC=C4)C4=C3C=CC=C4)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C=CC2=C3C=CC(C3=CC4=C(C=CC=C4)C=C3)=C2)C=C1 LEVBELTWOSGJFD-UHFFFAOYSA-N 0.000 description 1
- GRHVWNPSNWAZBB-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4/C=C\C=C5/C4=C(C=C3)C3C=CC=CC53)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4/C=C\C=C5\C6=C(C=C7C=CC=CC7=C6)C(=C45)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4C=CC=CC4=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C=CC3=C4C=CC4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=CC5=C(C=CC6=C5C=CC=C6)C4=C3)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4/C=C\C=C5/C4=C(C=C3)C3C=CC=CC53)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4/C=C\C=C5\C6=C(C=C7C=CC=CC7=C6)C(=C45)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=C4C=CC=CC4=C4C(=C3)C3=C(C=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=C(C=CC=C3)C3=C4C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C=CC3=C4C=CC4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=CC5=C(C=CC6=C5C=CC=C6)C4=C3)C=C2)C=C1 GRHVWNPSNWAZBB-UHFFFAOYSA-N 0.000 description 1
- KNAYAJKNKVCDPP-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C/C5=C/C=C\C6=CC=CC(=C3)C65)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC6=C5C=CC=C6)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC=C6)C5=C3)C=C4)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C/C5=C/C=C\C6=CC=CC(=C3)C65)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC6=C5C=CC=C6)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC=C6)C5=C3)C=C4)C=C2)C=C1 KNAYAJKNKVCDPP-UHFFFAOYSA-N 0.000 description 1
- CFLSVNYXHMSAOW-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C5/C=C\C=C6\C7=C(C=C8C=CC=CC8=C7)C(=C56)C=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C5/C=C\C=C6\C7=C(C=CC=C7)C(=C56)C=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C3C=CC3=C6C=CC=C3)C3C=CC=CC3C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC7=C6C=CC=C7)C5=C3)C=C4)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C5/C=C\C=C6\C7=C(C=C8C=CC=CC8=C7)C(=C56)C=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=C5/C=C\C=C6\C7=C(C=CC=C7)C(=C56)C=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C3C=CC3=C6C=CC=C3)C3C=CC=CC3C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C=CC3=C5C=CC5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C=CC6=C(C=CC7=C6C=CC=C7)C5=C3)C=C4)C=C2)C=C1 CFLSVNYXHMSAOW-UHFFFAOYSA-N 0.000 description 1
- RBHXXPAODNSAAV-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C=C5)C5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C3C=CC=C6)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=C3)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C3=C(C=CC=C3)C3=C5C5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C=C5)C5=C3C=CC3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C(C=CC=C6)C=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C6=C3C=CC=C6)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC5=C(C=C3)C3=C(C=CC=C3)C3=C5C=CC=C3)C=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C(C=C3)C=C(C3=CC=C5C(=C3)C3=C(C=CC=C3)C3=C5C5=C(C=CC=C5)C5=C3C=CC=C5)C=C4)C=C2)C=C1 RBHXXPAODNSAAV-UHFFFAOYSA-N 0.000 description 1
- RHTJWDBLYCESPD-UHFFFAOYSA-N C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C/C=C5\C6=CC=C7C=CC=CC7=C6C=C\C5=C\4C4=CC=CC=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=CC=CC=C3C3=C5C=CC6=C7C=CC=CC7=CC=C6C5=CC=C43)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C=CC3=C4C=CC4=C3C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=CC5=C(C=CC6=C5C=CC5=C6C=CC=C5)C4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C(C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)N=C2)C=C1.C1=CC=C(N(C2=CC=NC=C2)C2=CN=C(C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)N=C2)C=C1 Chemical compound C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC4=C/C=C5\C6=CC=C7C=CC=CC7=C6C=C\C5=C\4C4=CC=CC=C34)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=CC=CC=C3C3=C5C=CC6=C7C=CC=CC7=CC=C6C5=CC=C43)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C=CC3=C4C=CC4=C3C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C=CC5=C(C=CC6=C5C=CC5=C6C=CC=C5)C4=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CN=C(C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)N=C2)C=C1.C1=CC=C(N(C2=CC=NC=C2)C2=CN=C(C3=C4/C=C\C=C5\C6=C(C=CC=C6)C(=C45)C=C3)N=C2)C=C1 RHTJWDBLYCESPD-UHFFFAOYSA-N 0.000 description 1
- YDSHNFIJQMWJRE-UHFFFAOYSA-N C1=CC=C2C(=C1)C1=CC=CC3=C(C4=CC(C5=CC=C6C=C(C7=C8C=CC=CC8=C8C=CC=CC8=C7)C=CC6=C5)=CC=C4)C=CC2=C13 Chemical compound C1=CC=C2C(=C1)C1=CC=CC3=C(C4=CC(C5=CC=C6C=C(C7=C8C=CC=CC8=C8C=CC=CC8=C7)C=CC6=C5)=CC=C4)C=CC2=C13 YDSHNFIJQMWJRE-UHFFFAOYSA-N 0.000 description 1
- XTOFSDXKBBDCBW-UHFFFAOYSA-H C1=CC=C2C(=C1)SC1=N2[Zn@@]2(SC3=C1C=C(C1=CC=C4C=CC=CC4=C1)C=C3)[SH]1C3=C(C=C(C4=CC=C5C=CC=CC5=C4)C=C3)C3=N(C4=CC=CC=C4S3)[Zn@@]13N1=C(SC4=CC=CC=C41)C1=CC(C4=CC=C5C=CC=CC5=C4)=CC=C1[S@@H]23.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC=C3C(=C1)C1=N(C4=CC=CC=C4S1)[Zn@]14N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC7=C(C=C6)C6=C(C=CC=C6)C7(C)C)=C5)[SH]1[Zn@@]1(SC5=C(C=C(C6=CC7=C(C=C6)C6=C(C=CC=C6)C7(C)C)C=C5)C5=N1C1=CC=CC=C1S5)[S@H]34)C=C2.FC1=CC=C(C2=CC=C3C(=C2)C2=N(C4=CC=CC=C4S2)[Zn@]24N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC=C(F)C=C6)=C5)[SH]2[Zn@@]2(SC5=C(C=C(C6=CC=C(F)C=C6)C=C5)C5=N2C2=CC=CC=C2S5)[S@H]34)C=C1.[Cl-].[Cl-].[Cl-] Chemical compound C1=CC=C2C(=C1)SC1=N2[Zn@@]2(SC3=C1C=C(C1=CC=C4C=CC=CC4=C1)C=C3)[SH]1C3=C(C=C(C4=CC=C5C=CC=CC5=C4)C=C3)C3=N(C4=CC=CC=C4S3)[Zn@@]13N1=C(SC4=CC=CC=C41)C1=CC(C4=CC=C5C=CC=CC5=C4)=CC=C1[S@@H]23.CC1(C)C2=C(C=CC=C2)C2=C1C=C(C1=CC=C3C(=C1)C1=N(C4=CC=CC=C4S1)[Zn@]14N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC7=C(C=C6)C6=C(C=CC=C6)C7(C)C)=C5)[SH]1[Zn@@]1(SC5=C(C=C(C6=CC7=C(C=C6)C6=C(C=CC=C6)C7(C)C)C=C5)C5=N1C1=CC=CC=C1S5)[S@H]34)C=C2.FC1=CC=C(C2=CC=C3C(=C2)C2=N(C4=CC=CC=C4S2)[Zn@]24N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC=C(F)C=C6)=C5)[SH]2[Zn@@]2(SC5=C(C=C(C6=CC=C(F)C=C6)C=C5)C5=N2C2=CC=CC=C2S5)[S@H]34)C=C1.[Cl-].[Cl-].[Cl-] XTOFSDXKBBDCBW-UHFFFAOYSA-H 0.000 description 1
- SUZXJNGWXCBCHZ-UHFFFAOYSA-N C1=CC=C2C=CC=CC2=C1.C1=CC=C2CCC=CC2=C1.CC.C[RaH].C[Rb] Chemical compound C1=CC=C2C=CC=CC2=C1.C1=CC=C2CCC=CC2=C1.CC.C[RaH].C[Rb] SUZXJNGWXCBCHZ-UHFFFAOYSA-N 0.000 description 1
- XMGALDXPRLXHJJ-LEMDDYFSSA-M C1=CC=N2[Ir]C3=C(C=CC=C3)C2=C1.CC1(C)C2=CC3=C(C=C2C2=C1C=CC=C2)[Ir]N1=CC=CN31.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC3=C(C=CC=C3)C=N12.CN1C2=C(C=CC=C2)N2C3=C(C=CC=C3)[Ir][C@H]12.CN1C=CN2C3=C(C=CC4=C3OC3=C4C=CC=C3)[Ir][C@H]12.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir]21N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21 Chemical compound C1=CC=N2[Ir]C3=C(C=CC=C3)C2=C1.CC1(C)C2=CC3=C(C=C2C2=C1C=CC=C2)[Ir]N1=CC=CN31.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC3=C(C=CC=C3)C=N12.CN1C2=C(C=CC=C2)N2C3=C(C=CC=C3)[Ir][C@H]12.CN1C=CN2C3=C(C=CC4=C3OC3=C4C=CC=C3)[Ir][C@H]12.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir]21N2=CC=CN2B(N2C=CC=N2)(N2C=CC=N2)N2C=CC=N21 XMGALDXPRLXHJJ-LEMDDYFSSA-M 0.000 description 1
- IXPQJOYYVZGTLN-UHFFFAOYSA-N C=NC.CN Chemical compound C=NC.CN IXPQJOYYVZGTLN-UHFFFAOYSA-N 0.000 description 1
- KNHGCCXXUNSOKW-UHFFFAOYSA-N CC(C)(C)(C)C.CC(C)C(C)C.CC(C)C(C)C.CC(C)C(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)CC.CCC(C)CC.CCC(C)CC.CCC(C)CC.CCCC(C)C.CCCC(C)C.CCCC(C)C.CCCC(C)C.CCCCCC Chemical compound CC(C)(C)(C)C.CC(C)C(C)C.CC(C)C(C)C.CC(C)C(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)(C)C.CCC(C)CC.CCC(C)CC.CCC(C)CC.CCC(C)CC.CCCC(C)C.CCCC(C)C.CCCC(C)C.CCCC(C)C.CCCCCC KNHGCCXXUNSOKW-UHFFFAOYSA-N 0.000 description 1
- YTTBXDIHPWDSEB-UHFFFAOYSA-N CC(C)(C)(C)C.CCCC(C)C Chemical compound CC(C)(C)(C)C.CCCC(C)C YTTBXDIHPWDSEB-UHFFFAOYSA-N 0.000 description 1
- MQLUPRLOCSMTMI-UHFFFAOYSA-N CC(C)(C)C.CCC(C)C Chemical compound CC(C)(C)C.CCC(C)C MQLUPRLOCSMTMI-UHFFFAOYSA-N 0.000 description 1
- UEDPFXBHDSDYQJ-UHFFFAOYSA-N CC(C)(C)C.CCCCC Chemical compound CC(C)(C)C.CCCCC UEDPFXBHDSDYQJ-UHFFFAOYSA-N 0.000 description 1
- UHLTWJXMWLMPSL-UHFFFAOYSA-N CC(C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.CCC(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.CCCCCCC1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound CC(C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.CCC(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.CCCCCCC1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 UHLTWJXMWLMPSL-UHFFFAOYSA-N 0.000 description 1
- XRRLCAVVHOQDIB-UHFFFAOYSA-L CC(C)(C)C1=CC=C(C2=CC=C3C(=C2)C2=N(C4=CC=CC=C4S2)[Zn@]24N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC=C(C(C)(C)C)C=C6)=C5)[SH]2[Zn@@]2(SC5=C(C=C(C6=CC=C(C(C)(C)C)C=C6)C=C5)C5=N2C2=CC=CC=C2S5)[S@H]34)C=C1.[Cl-] Chemical compound CC(C)(C)C1=CC=C(C2=CC=C3C(=C2)C2=N(C4=CC=CC=C4S2)[Zn@]24N5=C(SC6=CC=CC=C65)C5=C(C=CC(C6=CC=C(C(C)(C)C)C=C6)=C5)[SH]2[Zn@@]2(SC5=C(C=C(C6=CC=C(C(C)(C)C)C=C6)C=C5)C5=N2C2=CC=CC=C2S5)[S@H]34)C=C1.[Cl-] XRRLCAVVHOQDIB-UHFFFAOYSA-L 0.000 description 1
- HGEKOCCDACFQKV-UHFFFAOYSA-N CC(C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.CCC(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.CCCCCCCC1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 Chemical compound CC(C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.CCC(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.CCCCCCCC1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 HGEKOCCDACFQKV-UHFFFAOYSA-N 0.000 description 1
- SACVMZXWHDVJKY-UHFFFAOYSA-N CC(C)(C)[Si](C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C[Si](C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 Chemical compound CC(C)(C)[Si](C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1.C[Si](C)(C)C1=CC=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C=C1 SACVMZXWHDVJKY-UHFFFAOYSA-N 0.000 description 1
- UQLOWQCTJKUGGP-UHFFFAOYSA-N CC(C)(C)[Si](C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.C[Si](C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 Chemical compound CC(C)(C)[Si](C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1.C[Si](C)(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 UQLOWQCTJKUGGP-UHFFFAOYSA-N 0.000 description 1
- AIOCJLQBYNJCAI-UHFFFAOYSA-N CC(C)(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 Chemical compound CC(C)(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 AIOCJLQBYNJCAI-UHFFFAOYSA-N 0.000 description 1
- ONIBWACTBWGRGH-UHFFFAOYSA-N CC(C)(C)c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)c1 Chemical compound CC(C)(C)c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)c1 ONIBWACTBWGRGH-UHFFFAOYSA-N 0.000 description 1
- ZFFMLCVRJBZUDZ-UHFFFAOYSA-N CC(C)C(C)C Chemical compound CC(C)C(C)C ZFFMLCVRJBZUDZ-UHFFFAOYSA-N 0.000 description 1
- CWXAZUJJQLVNLF-UHFFFAOYSA-N CC(C)C.CC(C)C(C)C Chemical compound CC(C)C.CC(C)C(C)C CWXAZUJJQLVNLF-UHFFFAOYSA-N 0.000 description 1
- SUAICDWVYXQSNC-UHFFFAOYSA-N CC(C)C.CCCC Chemical compound CC(C)C.CCCC SUAICDWVYXQSNC-UHFFFAOYSA-N 0.000 description 1
- RWQBLRCVTMNUAH-UHFFFAOYSA-N CC(CC=C1)c2c1c1ccccc1[n]2-c(cc1)ccc1-c1nc(-c2ccccn2)nc(-c2ncccc2)c1 Chemical compound CC(CC=C1)c2c1c1ccccc1[n]2-c(cc1)ccc1-c1nc(-c2ccccn2)nc(-c2ncccc2)c1 RWQBLRCVTMNUAH-UHFFFAOYSA-N 0.000 description 1
- JNLUMNSZVFTIEI-UHFFFAOYSA-N CC.CC.CC.CC.CCN1C2=C(C=CC=C2)C2=C1/C=C1/C=CC=C/C1=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C1=C2C=CC=C1.CCN1C2=C(C=CC=C2)C2=C1/C=C\C1=C2C=CC=C1.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1C1=C(C=CC=C1)/C=C\2 Chemical compound CC.CC.CC.CC.CCN1C2=C(C=CC=C2)C2=C1/C=C1/C=CC=C/C1=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C1=C2C=CC=C1.CCN1C2=C(C=CC=C2)C2=C1/C=C\C1=C2C=CC=C1.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1C1=C(C=CC=C1)/C=C\2 JNLUMNSZVFTIEI-UHFFFAOYSA-N 0.000 description 1
- VNEUTHAACZVCEV-UHFFFAOYSA-N CC.CC.CCN1C2=C(C=CC=C2)C2=C1/C=C1/C=CC=C/C1=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1C1=C(C=CC=C1)/C=C\2 Chemical compound CC.CC.CCN1C2=C(C=CC=C2)C2=C1/C=C1/C=CC=C/C1=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1/C=C\C=C/2.CCN1C2=C(C=CC=C2)C2=C1C1=C(C=CC=C1)/C=C\2 VNEUTHAACZVCEV-UHFFFAOYSA-N 0.000 description 1
- CCPNCVCHXXMYST-UHFFFAOYSA-N CC1=C(C)C2=C(C(C)=C1C)C1=C3C2=C(C)C(C)=C(C)/C3=C(C)/C(C)=C\1C Chemical compound CC1=C(C)C2=C(C(C)=C1C)C1=C3C2=C(C)C(C)=C(C)/C3=C(C)/C(C)=C\1C CCPNCVCHXXMYST-UHFFFAOYSA-N 0.000 description 1
- YZQAFLLSORKTKM-UHFFFAOYSA-N CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=C1.CC1=CC2=C(C=C1)N=C(C1=CC=CC=C1)C(C1=CC=CC=C1)=N2.CC1=CC2=C(C=C1)N=C(C1=CC=CC=N1)C(C1=NC=CC=C1)=N2.CC1=CC=CC2=NC(C3=CC=CC=C3)=CN12.CC1=CC=CC2=NC(C3=CC=CC=C3)=CN12.CC1=CN2C=CC=CC2=N1.CC1=CN=C(C2=CC=CC=C2)N=N1.CC1=NC2=C(C=CC=C2)C(C2=CC=CC=C2)=C1.CC1=NC2=C(C=CC=C2)N=C1.CC1=NC2=C(C=CC=N2)N=C1.CC1=NC2=C(C=CN=C2)N=C1 Chemical compound CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=C1.CC1=CC2=C(C=C1)N=C(C1=CC=CC=C1)C(C1=CC=CC=C1)=N2.CC1=CC2=C(C=C1)N=C(C1=CC=CC=N1)C(C1=NC=CC=C1)=N2.CC1=CC=CC2=NC(C3=CC=CC=C3)=CN12.CC1=CC=CC2=NC(C3=CC=CC=C3)=CN12.CC1=CN2C=CC=CC2=N1.CC1=CN=C(C2=CC=CC=C2)N=N1.CC1=NC2=C(C=CC=C2)C(C2=CC=CC=C2)=C1.CC1=NC2=C(C=CC=C2)N=C1.CC1=NC2=C(C=CC=N2)N=C1.CC1=NC2=C(C=CN=C2)N=C1 YZQAFLLSORKTKM-UHFFFAOYSA-N 0.000 description 1
- FQHPPTJXXJEHOJ-LEMDDYFSSA-M CC1=CC(C)=O[Ir@@]2(O1)C1=C(/C=C\C(F)=C/1)C1=CC=CC=N12.FC1=C/C2=C(C3=CC=CC=N3[Ir]2)/C(F)=C\1.FC1=C/C2=C(\C=C/1)C1=CC=CC=N1[Ir]2.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir@]21C2=C(/C=C\C(F)=C/2)C2=CC=CC=N21.OCC1=CC=N2[Ir]C3=C(/C=C\C(F)=C/3)C2=C1.OCC1=CC=N2[Ir]C3=C(C2=C1)/C(F)=C\C(F)=C/3 Chemical compound CC1=CC(C)=O[Ir@@]2(O1)C1=C(/C=C\C(F)=C/1)C1=CC=CC=N12.FC1=C/C2=C(C3=CC=CC=N3[Ir]2)/C(F)=C\1.FC1=C/C2=C(\C=C/1)C1=CC=CC=N1[Ir]2.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir@]21C2=C(/C=C\C(F)=C/2)C2=CC=CC=N21.OCC1=CC=N2[Ir]C3=C(/C=C\C(F)=C/3)C2=C1.OCC1=CC=N2[Ir]C3=C(C2=C1)/C(F)=C\C(F)=C/3 FQHPPTJXXJEHOJ-LEMDDYFSSA-M 0.000 description 1
- FTBPPJXGLCMYQJ-NJJUSXBSSA-I CC1=CC(C)=O[Ir]2(O1)C1=C(C3=C(C=CC=C3)C=C1)C1=N2C2=C(C=CC=C2)S1.CC1=CC(C)=O[Ir]2(O1)C1=C(C3=CC4=C(C=CC=C4)C=N32)C2=C(C=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=C3C=CC=CC3=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CN=C1)C1=N2C=CC2=C1C=CC=C2.CCN(C)C1=CC2=C(C=C1)C1=C(C(=O)O2)C2=N(C3=CC=CC=C3S2)[Ir]12OC(C)=CC(C)=O2 Chemical compound CC1=CC(C)=O[Ir]2(O1)C1=C(C3=C(C=CC=C3)C=C1)C1=N2C2=C(C=CC=C2)S1.CC1=CC(C)=O[Ir]2(O1)C1=C(C3=CC4=C(C=CC=C4)C=N32)C2=C(C=CC=C2)C=C1.CC1=CC(C)=O[Ir]2(O1)C1=C(C=C3C=CC=CC3=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CN=C1)C1=N2C=CC2=C1C=CC=C2.CCN(C)C1=CC2=C(C=C1)C1=C(C(=O)O2)C2=N(C3=CC=CC=C3S2)[Ir]12OC(C)=CC(C)=O2 FTBPPJXGLCMYQJ-NJJUSXBSSA-I 0.000 description 1
- KLVQWZWWGYRALQ-XAQBZWGFSA-I CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C(C3=CC=CC=C3)N=C(C3=CC=CC=C3)C=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC=CC=N12.CC1=CC2=C(C(C(F)(F)F)=C1)[Ir]1(OC(=O)C3=CC=CC=N31)N1=CC=CC=C21.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir]21N2N=C(C(F)(F)F)N=C2C2=CC=CC=N21.O=C1O[Ir]2(C3=C(C(F)=CC(F)=C3)C3=CC=CC=N32)N2=CC=CC=C12 Chemical compound CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C(C3=CC=CC=C3)N=C(C3=CC=CC=C3)C=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=C3C=CC=CC3=CC=N12.CC1=CC(C)=O[Ir]2(O1)C1=C(C=CC=C1)C1=CC=CC=N12.CC1=CC2=C(C(C(F)(F)F)=C1)[Ir]1(OC(=O)C3=CC=CC=N31)N1=CC=CC=C21.FC1=CC2=C(C(F)=C1)C1=CC=CC=N1[Ir]21N2N=C(C(F)(F)F)N=C2C2=CC=CC=N21.O=C1O[Ir]2(C3=C(C(F)=CC(F)=C3)C3=CC=CC=N32)N2=CC=CC=C12 KLVQWZWWGYRALQ-XAQBZWGFSA-I 0.000 description 1
- OLAHQJPGDCDOSU-UHFFFAOYSA-N CC1=CC(C2=C3C=CC=CC3=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=C3C=CC=CC3=NC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC3=C(C=CC=C3)C=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC=CC=N2)=NC(C2=NC=CC=C2)=C1.CC1=CC(C2=CC=NC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=NC=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 Chemical compound CC1=CC(C2=C3C=CC=CC3=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=C3C=CC=CC3=NC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC3=C(C=CC=C3)C=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=CC=CC=N2)=NC(C2=NC=CC=C2)=C1.CC1=CC(C2=CC=NC=C2)=NC(C2=CC=CC=C2)=N1.CC1=CC(C2=NC=CC=C2)=NC(C2=CC=CC=C2)=N1.CC1=NC(C2=CC=CC=C2)=NC(C2=CC=CC=C2)=N1 OLAHQJPGDCDOSU-UHFFFAOYSA-N 0.000 description 1
- WDOGKXCLOAYKAH-UHFFFAOYSA-N CC1=CC2=C(C=C1)C1=C(C=CC=C1)C2.CC1=CC2=C(C=C1)C1=C(C=CC=C1)O2.CC1=CC2=C(C=C1)C1=C(C=CC=C1)S2.CC1=CC2=C(C=C1)C1=C(C=NC=N1)N2.CC1=CC2=C(C=C1)C1=C(N=CC=N1)N2.CC1=CC2=C(C=C1)CC1=C2C=CC=C1.CC1=CC2=C(C=C1)NC1=C2N=CN=C1.CC1=CC2=C(C=C1)OC1=C2C=CC=C1.CC1=CC2=C(C=C1)SC1=C2C=CC=C1 Chemical compound CC1=CC2=C(C=C1)C1=C(C=CC=C1)C2.CC1=CC2=C(C=C1)C1=C(C=CC=C1)O2.CC1=CC2=C(C=C1)C1=C(C=CC=C1)S2.CC1=CC2=C(C=C1)C1=C(C=NC=N1)N2.CC1=CC2=C(C=C1)C1=C(N=CC=N1)N2.CC1=CC2=C(C=C1)CC1=C2C=CC=C1.CC1=CC2=C(C=C1)NC1=C2N=CN=C1.CC1=CC2=C(C=C1)OC1=C2C=CC=C1.CC1=CC2=C(C=C1)SC1=C2C=CC=C1 WDOGKXCLOAYKAH-UHFFFAOYSA-N 0.000 description 1
- UWMPHJACKNPWJZ-UHFFFAOYSA-N CC1=CC2=C(C=C1)C1=C(C=CC=N1)N2.CC1=CC2=C(C=C1)C1=C(C=CN=C1)N2.CC1=CC2=C(C=C1)C1=C(C=NC=C1)N2.CC1=CC2=C(C=C1)C1=C(N=CC=C1)N2.CC1=CC2=C(C=C1)C1=C(N=CN=C1)N2.CC1=CC2=C(C=C1)NC1=C2C=CC=N1.CC1=CC2=C(C=C1)NC1=C2C=CN=C1.CC1=CC2=C(C=C1)NC1=C2C=NC=C1.CC1=CC2=C(C=C1)NC1=C2C=NC=N1.CC1=CC2=C(C=C1)NC1=C2N=CC=C1.CC1=CC2=C(C=C1)NC1=C2N=CC=N1.CN1C2=C(N=CC=N2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1C=NC=N2.CN1C2=C(N=CN=C2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1N=CN=C2 Chemical compound CC1=CC2=C(C=C1)C1=C(C=CC=N1)N2.CC1=CC2=C(C=C1)C1=C(C=CN=C1)N2.CC1=CC2=C(C=C1)C1=C(C=NC=C1)N2.CC1=CC2=C(C=C1)C1=C(N=CC=C1)N2.CC1=CC2=C(C=C1)C1=C(N=CN=C1)N2.CC1=CC2=C(C=C1)NC1=C2C=CC=N1.CC1=CC2=C(C=C1)NC1=C2C=CN=C1.CC1=CC2=C(C=C1)NC1=C2C=NC=C1.CC1=CC2=C(C=C1)NC1=C2C=NC=N1.CC1=CC2=C(C=C1)NC1=C2N=CC=C1.CC1=CC2=C(C=C1)NC1=C2N=CC=N1.CN1C2=C(N=CC=N2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1C=NC=N2.CN1C2=C(N=CN=C2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1N=CC=N2.CN1C2=C(N=CN=C2)C2=C1N=CN=C2 UWMPHJACKNPWJZ-UHFFFAOYSA-N 0.000 description 1
- XVGCIZXSPFINQP-UHFFFAOYSA-N CC1=CC=C(C)C=C1.CC1=CN=C(C)C=C1 Chemical compound CC1=CC=C(C)C=C1.CC1=CN=C(C)C=C1 XVGCIZXSPFINQP-UHFFFAOYSA-N 0.000 description 1
- YVSMRTOGMKSFCA-UHFFFAOYSA-N CC1=NN=C(C)O1.CC1=NN=C(CC2=NN=C(C)O2)O1.CC1=NN=C(COCC2=NN=C(C)O2)O1 Chemical compound CC1=NN=C(C)O1.CC1=NN=C(CC2=NN=C(C)O2)O1.CC1=NN=C(COCC2=NN=C(C)O2)O1 YVSMRTOGMKSFCA-UHFFFAOYSA-N 0.000 description 1
- KMPDAARNDVINAG-UHFFFAOYSA-N CCC(C)(C)C.CCC(C)CC Chemical compound CCC(C)(C)C.CCC(C)CC KMPDAARNDVINAG-UHFFFAOYSA-N 0.000 description 1
- QVGLDPPIMKSVBG-UHFFFAOYSA-N CCC(C)C.CCC(C)C Chemical compound CCC(C)C.CCC(C)C QVGLDPPIMKSVBG-UHFFFAOYSA-N 0.000 description 1
- NZZMNINUUFWWNR-UHFFFAOYSA-N CCC(C)C.CCCCC Chemical compound CCC(C)C.CCCCC NZZMNINUUFWWNR-UHFFFAOYSA-N 0.000 description 1
- PFEOZHBOMNWTJB-UHFFFAOYSA-N CCC(C)CC Chemical compound CCC(C)CC PFEOZHBOMNWTJB-UHFFFAOYSA-N 0.000 description 1
- RPTLOYKCFOSTCX-UHFFFAOYSA-N CCC(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1c2ccccc22)ccc1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 Chemical compound CCC(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1c2ccccc22)ccc1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 RPTLOYKCFOSTCX-UHFFFAOYSA-N 0.000 description 1
- YEFSKSIEASDBFQ-UHFFFAOYSA-N CCC(C)c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)c1 Chemical compound CCC(C)c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)c1 YEFSKSIEASDBFQ-UHFFFAOYSA-N 0.000 description 1
- CWORCDDPHMKDSY-UHFFFAOYSA-N CCCCCCCc(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1c2ccccc22)ccc1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 Chemical compound CCCCCCCc(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1c2ccccc22)ccc1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 CWORCDDPHMKDSY-UHFFFAOYSA-N 0.000 description 1
- LDPXNICTGDKODX-UHFFFAOYSA-N CCCCCCc(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 Chemical compound CCCCCCc(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 LDPXNICTGDKODX-UHFFFAOYSA-N 0.000 description 1
- PYWXXKAFOOQVLB-UHFFFAOYSA-N CN1C2=C(C=CC=C2)C2=C1C=CC=N2.CN1C2=C(C=CC=C2)C2=C1C=CN=C2.CN1C2=C(C=CC=C2)C2=C1C=NC=C2.CN1C2=C(C=CC=C2)C2=C1C=NC=N2.CN1C2=C(C=CC=C2)C2=C1N=CC=C2.CN1C2=C(C=CC=C2)C2=C1N=CC=N2.CN1C2=C(C=CC=C2)C2=C1N=CN=C2.CN1C2=C(C=CC=N2)C2=C1N=CC=C2.CN1C2=C(C=CN=C2)C2=C1C=CC=N2.CN1C2=C(C=CN=C2)C2=C1C=NC=C2.CN1C2=C(C=CN=C2)C2=C1N=CC=C2.CN1C2=C(C=CN=C2)C2=C1N=CC=C2.CN1C2=C(C=NC=C2)C2=C1C=CN=C2.CN1C2=C(C=NC=C2)C2=C1C=NC=C2.CN1C2=C(C=NC=C2)C2=C1N=CC=C2.CN1C2=C(C=NC=C2)C2=C1N=CC=C2.CN1C2=C(N=CC=C2)C2=C1C=CC=N2.CN1C2=C(N=CC=C2)C2=C1N=CC=C2 Chemical compound CN1C2=C(C=CC=C2)C2=C1C=CC=N2.CN1C2=C(C=CC=C2)C2=C1C=CN=C2.CN1C2=C(C=CC=C2)C2=C1C=NC=C2.CN1C2=C(C=CC=C2)C2=C1C=NC=N2.CN1C2=C(C=CC=C2)C2=C1N=CC=C2.CN1C2=C(C=CC=C2)C2=C1N=CC=N2.CN1C2=C(C=CC=C2)C2=C1N=CN=C2.CN1C2=C(C=CC=N2)C2=C1N=CC=C2.CN1C2=C(C=CN=C2)C2=C1C=CC=N2.CN1C2=C(C=CN=C2)C2=C1C=NC=C2.CN1C2=C(C=CN=C2)C2=C1N=CC=C2.CN1C2=C(C=CN=C2)C2=C1N=CC=C2.CN1C2=C(C=NC=C2)C2=C1C=CN=C2.CN1C2=C(C=NC=C2)C2=C1C=NC=C2.CN1C2=C(C=NC=C2)C2=C1N=CC=C2.CN1C2=C(C=NC=C2)C2=C1N=CC=C2.CN1C2=C(N=CC=C2)C2=C1C=CC=N2.CN1C2=C(N=CC=C2)C2=C1N=CC=C2 PYWXXKAFOOQVLB-UHFFFAOYSA-N 0.000 description 1
- JDGBYDWTCODHLZ-UHFFFAOYSA-N CN1C2=C(C=CC=N2)C2=C1N=CC=N2.CN1C2=C(C=CC=N2)C2=C1N=CN=C2.CN1C2=C(C=CN=C2)C2=C1C=CC=N2.CN1C2=C(C=CN=C2)C2=C1C=NC=N2.CN1C2=C(C=CN=C2)C2=C1N=CC=N2.CN1C2=C(C=CN=C2)C2=C1N=CN=C2.CN1C2=C(C=NC=C2)C2=C1C=CC=N2.CN1C2=C(C=NC=C2)C2=C1C=CC=N2.CN1C2=C(C=NC=C2)C2=C1C=NC=C2.CN1C2=C(C=NC=C2)C2=C1C=NC=N2.CN1C2=C(C=NC=C2)C2=C1N=CC=N2.CN1C2=C(C=NC=C2)C2=C1N=CN=C2.CN1C2=C(C=NC=N2)C2=C1N=CC=N2.CN1C2=C(N=CC=C2)C2=C1C=NC=N2.CN1C2=C(N=CC=C2)C2=C1N=CC=C2.CN1C2=C(N=CC=C2)C2=C1N=CC=N2.CN1C2=C(N=CC=C2)C2=C1N=CN=C2.CN1C2=C(N=CN=C2)C2=C1N=CC=C2 Chemical compound CN1C2=C(C=CC=N2)C2=C1N=CC=N2.CN1C2=C(C=CC=N2)C2=C1N=CN=C2.CN1C2=C(C=CN=C2)C2=C1C=CC=N2.CN1C2=C(C=CN=C2)C2=C1C=NC=N2.CN1C2=C(C=CN=C2)C2=C1N=CC=N2.CN1C2=C(C=CN=C2)C2=C1N=CN=C2.CN1C2=C(C=NC=C2)C2=C1C=CC=N2.CN1C2=C(C=NC=C2)C2=C1C=CC=N2.CN1C2=C(C=NC=C2)C2=C1C=NC=C2.CN1C2=C(C=NC=C2)C2=C1C=NC=N2.CN1C2=C(C=NC=C2)C2=C1N=CC=N2.CN1C2=C(C=NC=C2)C2=C1N=CN=C2.CN1C2=C(C=NC=N2)C2=C1N=CC=N2.CN1C2=C(N=CC=C2)C2=C1C=NC=N2.CN1C2=C(N=CC=C2)C2=C1N=CC=C2.CN1C2=C(N=CC=C2)C2=C1N=CC=N2.CN1C2=C(N=CC=C2)C2=C1N=CN=C2.CN1C2=C(N=CN=C2)C2=C1N=CC=C2 JDGBYDWTCODHLZ-UHFFFAOYSA-N 0.000 description 1
- DXIUZKHQPKGUPH-UHFFFAOYSA-N CN1C2=CC3=C(C=C2C2=C1C=CC=C2)C1=CC=CC=C1N3C.CN1C2=CC3=C(C=C2C2=C1C=CC=C2)N(C)C1=CC=CC=C13.CN1C2=CC=CC=C2C2=C1C1=C(C=C2)C2=C(C=CC=C2)N1C Chemical compound CN1C2=CC3=C(C=C2C2=C1C=CC=C2)C1=CC=CC=C1N3C.CN1C2=CC3=C(C=C2C2=C1C=CC=C2)N(C)C1=CC=CC=C13.CN1C2=CC=CC=C2C2=C1C1=C(C=C2)C2=C(C=CC=C2)N1C DXIUZKHQPKGUPH-UHFFFAOYSA-N 0.000 description 1
- XUXSZNUIDQAHNL-UHFFFAOYSA-N CNC1C2(CC2)C1 Chemical compound CNC1C2(CC2)C1 XUXSZNUIDQAHNL-UHFFFAOYSA-N 0.000 description 1
- DRSHXJFUUPIBHX-UHFFFAOYSA-N COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 Chemical compound COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 DRSHXJFUUPIBHX-UHFFFAOYSA-N 0.000 description 1
- GZMNAMHQWIQTHJ-UHFFFAOYSA-N C[Si](C)(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 Chemical compound C[Si](C)(C)c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c(cc1)ccc1-c1cc(-c2ccccc2)nc(-c2ccccc2)n1 GZMNAMHQWIQTHJ-UHFFFAOYSA-N 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- CVDYKLYEXFLSAR-UHFFFAOYSA-N Cl.OC(=O)C1=CC=CC(Br)=C1 Chemical compound Cl.OC(=O)C1=CC=CC(Br)=C1 CVDYKLYEXFLSAR-UHFFFAOYSA-N 0.000 description 1
- 241000284156 Clerodendrum quadriloculare Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- KOPBYBDAPCDYFK-UHFFFAOYSA-N Cs2O Inorganic materials [O-2].[Cs+].[Cs+] KOPBYBDAPCDYFK-UHFFFAOYSA-N 0.000 description 1
- BOJZRNWPFWOENA-UHFFFAOYSA-N FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=C1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=N1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=NC=CC=C2)=C1 Chemical compound FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=C1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=N1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CC(C2=CC=CC=N2)=NC(C2=NC=CC=C2)=C1 BOJZRNWPFWOENA-UHFFFAOYSA-N 0.000 description 1
- MSMQAEPZEZABCE-UHFFFAOYSA-N FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CN2C=CC=CC2=C1C1=CC=CC=C1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CN2C=CC=CC2=N1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=NC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=N1 Chemical compound FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CN2C=CC=CC2=C1C1=CC=CC=C1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=CN2C=CC=CC2=N1.FC1=C(F)C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)=C(F)C(F)=C1C1=NC(C2=CC=CC=N2)=NC(C2=CC=CC=C2)=N1 MSMQAEPZEZABCE-UHFFFAOYSA-N 0.000 description 1
- GKVNLIGAINCWPZ-UHFFFAOYSA-N FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=C3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=N3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=NC=CC=C4)=C3)C=C1)C1=C2C=C(F)C=C1 Chemical compound FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=C3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=N3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=CC=N4)=NC(C4=NC=CC=C4)=C3)C=C1)C1=C2C=C(F)C=C1 GKVNLIGAINCWPZ-UHFFFAOYSA-N 0.000 description 1
- UPGLAPRTLAOSCR-UHFFFAOYSA-N FC1=CC2=C(C=C1)N(C1=CC=C(C3=CN4C=CC=CC4=C3C3=CC=CC=C3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CN4C=CC=CC4=N3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=NC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=N3)C=C1)C1=C2C=C(F)C=C1 Chemical compound FC1=CC2=C(C=C1)N(C1=CC=C(C3=CN4C=CC=CC4=C3C3=CC=CC=C3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=CN4C=CC=CC4=N3)C=C1)C1=C2C=C(F)C=C1.FC1=CC2=C(C=C1)N(C1=CC=C(C3=NC(C4=CC=CC=N4)=NC(C4=CC=CC=C4)=N3)C=C1)C1=C2C=C(F)C=C1 UPGLAPRTLAOSCR-UHFFFAOYSA-N 0.000 description 1
- FQDQRSGIYGZNTK-FUUMYVBZSA-N FC1=CC2=C(C=C1)N(C1=CC=CC=C1)C1=CC=C(C3=CC4=C(C=C3)N(C3=CC=C(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)C=C3)C3=CC=CC=C34)C=C12.FC1=CC=C2C(=C1)C1=C(C=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=C1)N2C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1.[2H]C1=C([2H])C([2H])=C2C(=C1[2H])C1=C(C([2H])=C([2H])C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=C1[2H])N2C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1 Chemical compound FC1=CC2=C(C=C1)N(C1=CC=CC=C1)C1=CC=C(C3=CC4=C(C=C3)N(C3=CC=C(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)C=C3)C3=CC=CC=C34)C=C12.FC1=CC=C2C(=C1)C1=C(C=CC(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=C1)N2C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1.[2H]C1=C([2H])C([2H])=C2C(=C1[2H])C1=C(C([2H])=C([2H])C(C3=CC=C4C(=C3)C3=C(C=CC=C3)N4C3=CC=CC=C3)=C1[2H])N2C1=CC=C(C2=NC(C3=CC=CC=C3)=NC(C3=CC=CC=C3)=C2)C=C1 FQDQRSGIYGZNTK-FUUMYVBZSA-N 0.000 description 1
- BDFMGESOUGTUBM-UHFFFAOYSA-N Fc(cc1c2cc(-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3cc(-c4ccccc4)nc(-c4ccccc4)n3)ccc22)ccc1[n]2-c1ccccc1 Chemical compound Fc(cc1c2cc(-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3cc(-c4ccccc4)nc(-c4ccccc4)n3)ccc22)ccc1[n]2-c1ccccc1 BDFMGESOUGTUBM-UHFFFAOYSA-N 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 229910005693 GdF3 Inorganic materials 0.000 description 1
- NAFKMUWLIYQHHI-UHFFFAOYSA-N I[n]1c2ccccc2c2c1cccc2 Chemical compound I[n]1c2ccccc2c2c1cccc2 NAFKMUWLIYQHHI-UHFFFAOYSA-N 0.000 description 1
- 229910000846 In alloy Inorganic materials 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical group C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 1
- LFZAGIJXANFPFN-UHFFFAOYSA-N N-[3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-thiophen-2-ylpropyl]acetamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CCC(C=1SC=CC=1)NC(C)=O)C LFZAGIJXANFPFN-UHFFFAOYSA-N 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N N-phenyl amine Natural products NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- FIWILGQIZHDAQG-UHFFFAOYSA-N NC1=C(C(=O)NCC2=CC=C(C=C2)OCC(F)(F)F)C=C(C(=N1)N)N1N=C(N=C1)C1(CC1)C(F)(F)F Chemical compound NC1=C(C(=O)NCC2=CC=C(C=C2)OCC(F)(F)F)C=C(C(=N1)N)N1N=C(N=C1)C1(CC1)C(F)(F)F FIWILGQIZHDAQG-UHFFFAOYSA-N 0.000 description 1
- KKCBUQHMOMHUOY-UHFFFAOYSA-N Na2O Inorganic materials [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical group C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical group C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 1
- 229910018101 ScO3 Inorganic materials 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 229910052771 Terbium Inorganic materials 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- OZYVKCNEZGRBMP-VMUADKMGSA-N [2H]C1=C([2H])C([2H])=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C([2H])=C1[2H].[2H]C1=C([2H])C([2H])=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C([2H])=C1[2H].[2H]C1=C([2H])C2=C(C([2H])=C1[2H])N(C1=CC=CC=C1)C1=C([2H])C([2H])=C(C3=CC4=C(C=C3)N(C3=CC=C(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)C=C3)C3=CC=CC=C34)C([2H])=C21 Chemical compound [2H]C1=C([2H])C([2H])=C(C2=CC(C3=CC=C(N4C5=CC=CC=C5C5=C4C=CC(C4=CC=C6C(=C4)C4=C(C=CC=C4)N6C4=CC=CC=C4)=C5)C=C3)=NC(C3=CC=CC=C3)=N2)C([2H])=C1[2H].[2H]C1=C([2H])C([2H])=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(C6=NC(C7=CC=CC=C7)=NC(C7=CC=CC=C7)=C6)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C([2H])=C1[2H].[2H]C1=C([2H])C2=C(C([2H])=C1[2H])N(C1=CC=CC=C1)C1=C([2H])C([2H])=C(C3=CC4=C(C=C3)N(C3=CC=C(C5=NC(C6=CC=CC=C6)=NC(C6=CC=CC=C6)=C5)C=C3)C3=CC=CC=C34)C([2H])=C21 OZYVKCNEZGRBMP-VMUADKMGSA-N 0.000 description 1
- BMWXZUBKUCPGPK-UHFFFAOYSA-N [AlH2][n]1c2ccccc2c2c1cccc2 Chemical compound [AlH2][n]1c2ccccc2c2c1cccc2 BMWXZUBKUCPGPK-UHFFFAOYSA-N 0.000 description 1
- QOMDXTSOVDMSMM-UHFFFAOYSA-N [C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C(C)C(C)=N1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)CC(C)=N1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=N1 Chemical compound [C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C(C)C(C)=N1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)CC(C)=N1.[C-]#[N+]C1=C([N+]#[C-])N=C(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)CC(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)=N1 QOMDXTSOVDMSMM-UHFFFAOYSA-N 0.000 description 1
- KJNGJIPPQOFCSK-UHFFFAOYSA-N [H][Sr][H] Chemical compound [H][Sr][H] KJNGJIPPQOFCSK-UHFFFAOYSA-N 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 125000004062 acenaphthenyl group Chemical group C1(CC2=CC=CC3=CC=CC1=C23)* 0.000 description 1
- 125000004054 acenaphthylenyl group Chemical group C1(=CC2=CC=CC3=CC=CC1=C23)* 0.000 description 1
- XIVOUNPJCNJBPR-UHFFFAOYSA-N acridin-1-ol Chemical compound C1=CC=C2C=C3C(O)=CC=CC3=NC2=C1 XIVOUNPJCNJBPR-UHFFFAOYSA-N 0.000 description 1
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 239000005354 aluminosilicate glass Substances 0.000 description 1
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 125000005428 anthryl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C(*)=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- 229910001632 barium fluoride Inorganic materials 0.000 description 1
- YZWGEMSQAMDWEM-UHFFFAOYSA-N benzo[c]chrysene Chemical compound C1=CC2=CC=C3C=CC=CC3=C2C2=C1C1=CC=CC=C1C=C2 YZWGEMSQAMDWEM-UHFFFAOYSA-N 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- JZKFIPKXQBZXMW-UHFFFAOYSA-L beryllium difluoride Chemical compound F[Be]F JZKFIPKXQBZXMW-UHFFFAOYSA-L 0.000 description 1
- 229910001633 beryllium fluoride Inorganic materials 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 125000005997 bromomethyl group Chemical group 0.000 description 1
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- KMEIELQLHFTHAC-UHFFFAOYSA-N c([n](cccc1)c1c1-c2ccccc2)c1-c(cc1)ccc1-[n]1c(cccc2)c2c2c1cccc2 Chemical compound c([n](cccc1)c1c1-c2ccccc2)c1-c(cc1)ccc1-[n]1c(cccc2)c2c2c1cccc2 KMEIELQLHFTHAC-UHFFFAOYSA-N 0.000 description 1
- CQFDVMPOTOTZPG-UHFFFAOYSA-N c(cc1)cc(c2c3cccc2)c1[n]3-c(cc1)ccc1-c(cc1)ccc1-c1cc(-c2ccccn2)nc(-c2ncccc2)c1 Chemical compound c(cc1)cc(c2c3cccc2)c1[n]3-c(cc1)ccc1-c(cc1)ccc1-c1cc(-c2ccccn2)nc(-c2ncccc2)c1 CQFDVMPOTOTZPG-UHFFFAOYSA-N 0.000 description 1
- KTROWFUVQOCQOP-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2ccccc2c2c3)c2ccc3-c(cc2)cc(c3ccccc33)c2[n]3-c(cc2)ccc2-c2cc(-c3ccccc3)nc(-c3ccccc3)n2)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2ccccc2c2c3)c2ccc3-c(cc2)cc(c3ccccc33)c2[n]3-c(cc2)ccc2-c2cc(-c3ccccc3)nc(-c3ccccc3)n2)nc(-c2ccccc2)n1 KTROWFUVQOCQOP-UHFFFAOYSA-N 0.000 description 1
- ANUXEXKYTQZCBJ-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)cc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)cc(-c2ccccc2)n1 ANUXEXKYTQZCBJ-UHFFFAOYSA-N 0.000 description 1
- POWKBSUEBLNABG-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-c2cc(-[n]3c(cccc4)c4c4c3cccc4)cc(-[n]3c4ccccc4c4c3cccc4)c2)cc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-c2cc(-[n]3c(cccc4)c4c4c3cccc4)cc(-[n]3c4ccccc4c4c3cccc4)c2)cc(-c2ccccc2)n1 POWKBSUEBLNABG-UHFFFAOYSA-N 0.000 description 1
- ANPIEUANHDEIGO-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2c3cc(-c4nc(-c5ccccc5)nc(-c5ncccc5)c4)ccc33)ccc2[n]3-c2ccccc2)cc(-c2ncccc2)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2c3cc(-c4nc(-c5ccccc5)nc(-c5ncccc5)c4)ccc33)ccc2[n]3-c2ccccc2)cc(-c2ncccc2)n1 ANPIEUANHDEIGO-UHFFFAOYSA-N 0.000 description 1
- VBZMXRKCMGGGBG-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c2nc(-c3ccccc3)nc(-[n]3c(ccc(-c(cc4c5ccccc55)ccc4[n]5-c4nc(-c5ccccc5)nc(-c5cc(-c6ccccc6)ccc5)c4)c4)c4c4ccccc34)c2)ccc1 Chemical compound c(cc1)ccc1-c1cc(-c2nc(-c3ccccc3)nc(-[n]3c(ccc(-c(cc4c5ccccc55)ccc4[n]5-c4nc(-c5ccccc5)nc(-c5cc(-c6ccccc6)ccc5)c4)c4)c4c4ccccc34)c2)ccc1 VBZMXRKCMGGGBG-UHFFFAOYSA-N 0.000 description 1
- VOPMLFHTGOZWQP-UHFFFAOYSA-N c(cc1)ccc1-c1cccc(-c2nc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c3nc(-c4ccccc4)cc(-c4cc(-c5ccccc5)ccc4)n3)nc(-c3ccccc3)c2)c1 Chemical compound c(cc1)ccc1-c1cccc(-c2nc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c3nc(-c4ccccc4)cc(-c4cc(-c5ccccc5)ccc4)n3)nc(-c3ccccc3)c2)c1 VOPMLFHTGOZWQP-UHFFFAOYSA-N 0.000 description 1
- WBTNLIFABZXAMW-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc2c1cccc2 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc2c1cccc2 WBTNLIFABZXAMW-UHFFFAOYSA-N 0.000 description 1
- QVSJCRDHNCCXFC-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)n1 QVSJCRDHNCCXFC-UHFFFAOYSA-N 0.000 description 1
- HVBTWHQJLYMDQG-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c3cc(cccc4)c4cc3c3c2ccc(-c(cc2c4ccccc44)ccc2[n]4-c2ccccc2)c3)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c3cc(cccc4)c4cc3c3c2ccc(-c(cc2c4ccccc44)ccc2[n]4-c2ccccc2)c3)nc(-c2ccccc2)n1 HVBTWHQJLYMDQG-UHFFFAOYSA-N 0.000 description 1
- VXNSCEVRZZQUCS-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)cnc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)cnc2-[n]2c(ccc(-c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)c3)c3c3ccccc23)nc(-c2ccccc2)c1 VXNSCEVRZZQUCS-UHFFFAOYSA-N 0.000 description 1
- AVVGMGORDZDVDP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2cc(-[n]3c(ccc(-c(cc4c5c6cccc5)ccc4[n]6-c4ccccc4)c4)c4c4ccccc34)ccc2)nc2c1cccc2 Chemical compound c(cc1)ccc1-c1nc(-c2cc(-[n]3c(ccc(-c(cc4c5c6cccc5)ccc4[n]6-c4ccccc4)c4)c4c4ccccc34)ccc2)nc2c1cccc2 AVVGMGORDZDVDP-UHFFFAOYSA-N 0.000 description 1
- LPUKGHGDZUYNMP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)cc3c2ccc(-[n]2c(ccc(-c(cc4c5c6cccc5)ccc4[n]6-c4ccccc4)c4)c4c4ccccc24)c3)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)cc3c2ccc(-[n]2c(ccc(-c(cc4c5c6cccc5)ccc4[n]6-c4ccccc4)c4)c4c4ccccc24)c3)c1 LPUKGHGDZUYNMP-UHFFFAOYSA-N 0.000 description 1
- LPMMTRAUAPYMEI-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccc(cc(cc3)-[n]4c(ccc(-c(cc5c6c7cccc6)ccc5[n]7-c5ccccc5)c5)c5c5ccccc45)c3c2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccc(cc(cc3)-[n]4c(ccc(-c(cc5c6c7cccc6)ccc5[n]7-c5ccccc5)c5)c5c5ccccc45)c3c2)c1 LPMMTRAUAPYMEI-UHFFFAOYSA-N 0.000 description 1
- YCFVUWRDFHREHN-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2cnc(-[n]3c(ccc(-c(cc4)cc(c5c6cccc5)c4[n]6-c4ccccc4)c4)c4c4ccccc34)nc2)nc(-c2ccccc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2cnc(-[n]3c(ccc(-c(cc4)cc(c5c6cccc5)c4[n]6-c4ccccc4)c4)c4c4ccccc34)nc2)nc(-c2ccccc2)c1 YCFVUWRDFHREHN-UHFFFAOYSA-N 0.000 description 1
- KMHGKDHFODVOSE-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)cc(c3cc(-c4cc(-c5ncccc5)nc(-c5ccccc5)n4)ccc33)c2[n]3-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)cc(c3cc(-c4cc(-c5ncccc5)nc(-c5ccccc5)n4)ccc33)c2[n]3-c2ccccc2)n1 KMHGKDHFODVOSE-UHFFFAOYSA-N 0.000 description 1
- JRCUEURRICHGPF-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)ccc2-c(cc2)ccc2-[n]2c(cccc3)c3c3c2cccc3)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)ccc2-c(cc2)ccc2-[n]2c(cccc3)c3c3c2cccc3)c1 JRCUEURRICHGPF-UHFFFAOYSA-N 0.000 description 1
- OFMRARSHODMUBE-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)ccc2-c(cc2)ccc2-[n]2c3ccccc3c3c2cccc3)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ncccc2)cc(-c(cc2)ccc2-c(cc2)ccc2-[n]2c3ccccc3c3c2cccc3)n1 OFMRARSHODMUBE-UHFFFAOYSA-N 0.000 description 1
- ZRQBPIMBWIIAHX-UHFFFAOYSA-N c(cc1)ccc1-c1nc(cccc2)c2c(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)n1 Chemical compound c(cc1)ccc1-c1nc(cccc2)c2c(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)n1 ZRQBPIMBWIIAHX-UHFFFAOYSA-N 0.000 description 1
- FZRUKUANEWOATA-UHFFFAOYSA-N c1c(-c(cc2)ccc2-[n]2c(ccc(-c3ccccc3)c3)c3c3cc(-c4ccccc4)ccc23)nc2[n]1cccc2 Chemical compound c1c(-c(cc2)ccc2-[n]2c(ccc(-c3ccccc3)c3)c3c3cc(-c4ccccc4)ccc23)nc2[n]1cccc2 FZRUKUANEWOATA-UHFFFAOYSA-N 0.000 description 1
- BKKCBMXNYPEDIX-UHFFFAOYSA-N c1c(-c(cc2)ccc2-c2cc(-[n]3c4ccccc4c4c3cccc4)cc(-[n]3c4ccccc4c4ccccc34)c2)nc2[n]1cccc2 Chemical compound c1c(-c(cc2)ccc2-c2cc(-[n]3c4ccccc4c4c3cccc4)cc(-[n]3c4ccccc4c4ccccc34)c2)nc2[n]1cccc2 BKKCBMXNYPEDIX-UHFFFAOYSA-N 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- WUKWITHWXAAZEY-UHFFFAOYSA-L calcium difluoride Chemical compound [F-].[F-].[Ca+2] WUKWITHWXAAZEY-UHFFFAOYSA-L 0.000 description 1
- 229910001634 calcium fluoride Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 229910000421 cerium(III) oxide Inorganic materials 0.000 description 1
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 1
- 125000002676 chrysenyl group Chemical group C1(=CC=CC=2C3=CC=C4C=CC=CC4=C3C=CC12)* 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- TXCDCPKCNAJMEE-UHFFFAOYSA-N dibenzofuran Chemical group C1=CC=C2C3=CC=CC=C3OC2=C1 TXCDCPKCNAJMEE-UHFFFAOYSA-N 0.000 description 1
- 125000005509 dibenzothiophenyl group Chemical group 0.000 description 1
- AKUNKIJLSDQFLS-UHFFFAOYSA-M dicesium;hydroxide Chemical compound [OH-].[Cs+].[Cs+] AKUNKIJLSDQFLS-UHFFFAOYSA-M 0.000 description 1
- 229960004132 diethyl ether Drugs 0.000 description 1
- AFABGHUZZDYHJO-UHFFFAOYSA-N dimethyl butane Natural products CCCC(C)C AFABGHUZZDYHJO-UHFFFAOYSA-N 0.000 description 1
- 125000000532 dioxanyl group Chemical group 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- VDQVEACBQKUUSU-UHFFFAOYSA-M disodium;sulfanide Chemical compound [Na+].[Na+].[SH-] VDQVEACBQKUUSU-UHFFFAOYSA-M 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000007772 electrode material Substances 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 150000008376 fluorenones Chemical class 0.000 description 1
- 125000005567 fluorenylene group Chemical group 0.000 description 1
- 150000002222 fluorine compounds Chemical class 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- XLYOFNOQVPJJNP-ZSJDYOACSA-N heavy water Substances [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 1
- 125000004836 hexamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 125000003707 hexyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 150000007857 hydrazones Chemical class 0.000 description 1
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 125000001977 isobenzofuranyl group Chemical group C=1(OC=C2C=CC=CC12)* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 239000005355 lead glass Substances 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 1
- 229910001635 magnesium fluoride Inorganic materials 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- IBHBKWKFFTZAHE-UHFFFAOYSA-N n-[4-[4-(n-naphthalen-1-ylanilino)phenyl]phenyl]-n-phenylnaphthalen-1-amine Chemical group C1=CC=CC=C1N(C=1C2=CC=CC=C2C=CC=1)C1=CC=C(C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C3=CC=CC=C3C=CC=2)C=C1 IBHBKWKFFTZAHE-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003261 o-tolyl group Chemical group [H]C1=C([H])C(*)=C(C([H])=C1[H])C([H])([H])[H] 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 238000007122 ortho-metalation reaction Methods 0.000 description 1
- 150000007978 oxazole derivatives Chemical class 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000001820 oxy group Chemical group [*:1]O[*:2] 0.000 description 1
- 125000006503 p-nitrobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1[N+]([O-])=O)C([H])([H])* 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- 125000004817 pentamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 125000004115 pentoxy group Chemical group [*]OC([H])([H])C([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 1
- 125000005563 perylenylene group Chemical group 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 150000002987 phenanthrenes Chemical class 0.000 description 1
- 125000005560 phenanthrenylene group Chemical group 0.000 description 1
- KELCFVWDYYCEOQ-UHFFFAOYSA-N phenanthridin-1-ol Chemical compound C1=CC=CC2=C3C(O)=CC=CC3=NC=C21 KELCFVWDYYCEOQ-UHFFFAOYSA-N 0.000 description 1
- 125000004625 phenanthrolinyl group Chemical group N1=C(C=CC2=CC=C3C=CC=NC3=C12)* 0.000 description 1
- 150000004986 phenylenediamines Chemical class 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 229920003227 poly(N-vinyl carbazole) Polymers 0.000 description 1
- 229920000548 poly(silane) polymer Polymers 0.000 description 1
- 229920002492 poly(sulfone) Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 150000004032 porphyrins Chemical class 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 235000011118 potassium hydroxide Nutrition 0.000 description 1
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 1
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical class O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 description 1
- 150000003219 pyrazolines Chemical class 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical group C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- 238000006862 quantum yield reaction Methods 0.000 description 1
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- SIXSYDAISGFNSX-UHFFFAOYSA-N scandium atom Chemical compound [Sc] SIXSYDAISGFNSX-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000005361 soda-lime glass Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- VPQBLCVGUWPDHV-UHFFFAOYSA-N sodium selenide Chemical compound [Na+].[Na+].[Se-2] VPQBLCVGUWPDHV-UHFFFAOYSA-N 0.000 description 1
- 229910052979 sodium sulfide Inorganic materials 0.000 description 1
- 229910001637 strontium fluoride Inorganic materials 0.000 description 1
- FVRNDBHWWSPNOM-UHFFFAOYSA-L strontium fluoride Chemical compound [F-].[F-].[Sr+2] FVRNDBHWWSPNOM-UHFFFAOYSA-L 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- 125000002306 tributylsilyl group Chemical group C(CCC)[Si](CCCC)(CCCC)* 0.000 description 1
- ODHXBMXNKOYIBV-UHFFFAOYSA-N triphenylamine Chemical compound C1=CC=CC=C1N(C=1C=CC=CC=1)C1=CC=CC=C1 ODHXBMXNKOYIBV-UHFFFAOYSA-N 0.000 description 1
- 125000006617 triphenylamine group Chemical group 0.000 description 1
- 238000000870 ultraviolet spectroscopy Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- RUDFQVOCFDJEEF-UHFFFAOYSA-N yttrium(III) oxide Inorganic materials [O-2].[O-2].[O-2].[Y+3].[Y+3] RUDFQVOCFDJEEF-UHFFFAOYSA-N 0.000 description 1
- YVTHLONGBIQYBO-UHFFFAOYSA-N zinc indium(3+) oxygen(2-) Chemical compound [O--].[Zn++].[In+3] YVTHLONGBIQYBO-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H01L51/0072—
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B57/00—Other synthetic dyes of known constitution
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H01L51/0067—
-
- H01L51/5024—
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/20—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the material in which the electroluminescent material is embedded
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/12—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers comprising dopants
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1059—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/10—Triplet emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/90—Multiple hosts in the emissive layer
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/14—Carrier transporting layers
- H10K50/16—Electron transporting layers
- H10K50/165—Electron transporting layers comprising dopants
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/17—Carrier injection layers
- H10K50/171—Electron injection layers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- the present invention relates to a biscarbazole derivative, a material for an organic electroluminescence device, and an organic electroluminescence device using those.
- a known organic electroluminescence device includes an organic thin-film layer between an anode and a cathode, the organic thin-film layer including an emitting layer, and emits light using exciton energy generated by a recombination of holes and electrons injected into the emitting layer (see Patent Literature 1: WO2003-080760, Patent Literature 2: Japanese Patent No.: 3139321, Patent Literature 3: Japanese Patent No.: 4357781, Patent Literature 4: JP-A-2003-151774, Patent Literature 5: JP-A-2008-135498, Patent Literature 6: JP-A-2009-21336, Patent Literature 7: JP-A-2008-214307).
- Such an organic EL device which has the advantages as a self-emitting device, is expected to serve as an emitting device excellent in luminous efficiency, image quality, power consumption and thin design.
- a doping method according to which an emitting material (dopant) is doped to a host, has been known as a usable method.
- the emitting layer formed by the doping method can efficiently generate excitons from electric charges injected into the host. With the exciton energy generated by the excitons being transferred to the dopant, the dopant can emit light with high efficiency.
- an organic electroluminescence device (hereinafter, occasionally referred to as an organic EL device)
- a doping method has been further studied to find a suitable host material.
- Patent Literatures 1 to 7 disclose a compound including a carbazole skeleton and a nitrogen-containing aromatic ring in the same molecule and a compound including a plurality of carbazole skeletons in the same molecule, as shown in the following compounds I to VIII.
- the compounds I and II disclosed in Patent Literature 1 each have a structure in which a carbazole skeleton is bonded to a benzene ring and an electron-deficient nitrogen-containing hetero aromatic ring structure.
- a carbazole skeleton which is represented by polyvinyl carbazole, has been known as a main skeleton of a hole transporting material.
- the electron-deficient nitrogen-containing hetero aromatic ring structure has been known as a structure having a high electron transporting capability.
- the compounds I and II disclosed in Patent Literature 1 are materials for balancing charge transportation by combining a hole transporting skeleton and an electron transporting skeleton.
- the compound I has only a single carbazole skeleton and lacks a hole transporting capability, so that a favorable luminescence property cannot be obtained.
- the compound II has two carbazolyl groups that are branched to left and right relative to a bond axis of a pyrimidine ring and a benzene ring (two conjugated aromatic ring). Accordingly, an overlapping margin of the carbazole skeleton between molecules is impaired, so that a hole transporting capability is insufficient and a re-bonding position of charges is likely to be closer to the anode. Consequently, favorable luminescence property and lifetime property cannot be obtained.
- the compound VII disclosed in Patent Literature 6 has an electron-deficient nitrogen-containing hetero aromatic ring structure and a carbazole-linking structure.
- two carbazole skeletons are bonded to a carbon atom at 3-position by a nitrogen atom.
- the two carbazole skeletons are twisted to each other to lose flatness. Accordingly, the overlapping margin between the molecules becomes small and a hole transporting capability becomes insufficient, so that favorable luminescence property and lifetime property cannot be obtained.
- the compound VIII disclosed in Patent Literature 7 has a structure in which a bipyridyl group (a nitrogen-containing aromatic heterocyclic group) is bonded to a benzene ring of a carbazole skeleton.
- the compound is not disclosed as a phosphorescent host material although being used as a material for an electron transporting layer.
- the compound since the compound is considered to exhibit a high electron transporting capability, when used as a host material, the compound provides a poor carrier balance within the emitting layer and fails to exhibit a favorable luminescence property.
- an object of the invention is to provide a novel biscarbazole derivative having a hole transporting capability and an electron transporting capability and exhibiting an excellent carrier balance, a material for an organic EL device (hereinafter, referred to as an organic-EL-device material) and a phosphorescent and long-life organic EL device using those.
- a biscarbazole derivative according to an aspect of the invention is represented by a formula (1) below.
- hydrogen is meant to also include deuterium.
- a 1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 carbon atoms forming a ring (hereinafter, referred to as ring carbon atoms);
- a 2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- X 1 and X 2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- Y 1 to Y 4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fuse
- adjacent ones of Y 1 to Y 4 may be bonded to each other to form a ring structure
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- a plurality of Y 1 to Y 4 may be the same or different.
- the ring structure is exemplified by structures represented by the following formulae.
- the biscarbazole derivative according to the aspect of the invention is preferably represented by a formula (2) below.
- a 1 , A 2 , X 1 , X 2 , Y 1 to Y 4 , p, q, r and s represent the same as A 1 , A 2 , X 1 , X 2 , Y 1 to Y 4 , p, q, r and s in the formula (1).
- a 1 in the formula (1A) or (1B) is preferably selected from the group consisting of a substituted or unsubstituted pyridine ring, substituted or unsubstituted pyrimidine ring and substituted or unsubstituted triazine ring, more preferably selected from a substituted or unsubstituted pyrimidine ring or substituted or unsubstituted triazine ring, particularly preferably a substituted or unsubstituted pyrimidine ring.
- the biscarbazole derivative according to the aspect of the invention is preferably represented by a formula (3) below in the formula (2)
- a 2 , X 1 , Y 1 to Y 4 , p, q, r and s represent the same as A 2 , X 1 , Y 1 to Y 4 , p, q, r and s of the formula (1);
- Y 5 represent the same as Y 1 to Y 4 of the formula (1);
- t represents an integer from 1 to 3; and when t is an integer of 2 to 3, a plurality of Y 5 may be the same or different.
- a 1 is preferably a substituted or unsubstituted quinazoline ring.
- the organic-EL-device material according to another aspect of the invention contains the above biscarbazole derivative.
- the organic EL device includes: a cathode; an anode; and a plurality of organic thin-film layers provided between the cathode and the anode, and the organic thin-film layer including an emitting layer, in which at least one layer of the organic thin-film layers contains the above-described organic-EL-device material.
- the emitting layer preferably contains the organic-EL-device material according to the aspect of the invention as a host material.
- the emitting layer includes a phosphorescent material.
- the phosphorescent material is preferably an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- the organic EL device includes: a cathode; an anode; and a plurality of organic thin-film layers provided between a cathode and an anode, and the organic thin-film layer includes an emitting layer, in which at least one of the organic thin-film layers is the emitting layer containing a first host material, a second host material and a phosphorescent material providing phosphorescence, the first host material being a compound represented by a formula (4) below.
- a 1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms
- X 1 and X 2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- Y 1 to Y 4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fuse
- adjacent ones of Y 1 to Y 4 may be bonded to each other to form a ring structure
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- a plurality of Y 1 to Y 4 may be the same or different.
- the ring structure is exemplified by the same structures as ones listed when Y 1 to Y 4 are bonded to each other to form a ring structure in the formula (1).
- the second host material is preferably represented by either one of a formula (5) or (6) below.
- Cz represents a substituted or unsubstituted arylcarbazolyl group or carbazolylaryl group
- a 3 represents a group represented by a formula (7A) or (7B) below;
- a and b each represent an integer of 1 to 3.
- M 1 and M 2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M 1 and M 2 may be the same or different;
- L 5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- M 3 and M 4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 6 to 40 ring carbon atoms; M 3 and M 4 may be the same or different;
- L 6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- the second host material is preferably represented by a formula (8) below.
- R 101 to R 106 each independently represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to
- R 101 to R 106 is a substituted or unsubstituted 9-carbazolyl group, substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms, or -L-9-carbazolyl group;
- L represents a substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group
- Xa represents a sulfur atom, oxygen atom or N—R 108 ;
- R 108 represents the same as R 101 to R 106 .
- the second host material is preferably a compound selected from the group consisting of polycyclic aromatic compounds represented by formulae (9A), (9B) and (9C) below.
- Ar 101 , Ar 102 , Ar 103 , Ra and Rb represent a polycyclic aromatic skeleton having 6 to 60 ring carbon atoms selected from a substituted or unsubstituted benzene ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted chrysene ring, substituted or unsubstituted fluoranthene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzophenanthrene ring, substituted or unsubstituted dibenzophenanthrene ring, substituted or unsubstituted triphenylene ring, substituted or unsubstituted benzo[a]triphenylene ring, substituted or unsubstituted benzochrysene ring, substituted or unsubstituted benzo[b]fluoranthene ring,
- either one or both of Ra and Rb are preferably selected from the group consisting of a substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzo[c]phenanthrene ring and substituted or unsubstituted fluoranthene ring.
- the second host material is preferably a monoamine derivative represented by any one of formulae (10) to (12) below.
- Ar 111 , Ar 112 and Ar 113 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar 114 , Ar 115 and Ar 117 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar 116 is a substituted or unsubstituted arylene group or heteroarylene group.
- Ar 118 , Ar 119 and Ar 121 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar 120 is a substituted or unsubstituted arylene group or heteroarylene group.
- n is an integer of 2 to 5: when n is 2 or more, Ar 120 may be the same or different.
- the second host material is represented by a formula (13) or (14) below.
- X 3 represents a substituted or unsubstituted arylene group having 10 to 40 ring carbon atoms
- a 3 to A 6 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- a 7 to A 9 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- the second host material is more preferably represented by any one of formulae (15) to (19) below.
- a 10 to A 19 each represent a substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 40 carbon atoms, substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic amino group, or substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic heterocyclic group;
- a 10 , A 13 , A 15 and A 17 are adapted to be respectively bonded to A 11 , A 14 , A 16 and A 18 to form a ring;
- X 4 to X 9 represent a single bond or a linking group having 1 to 30 carbon atoms
- Y 6 to Y 24 represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted alkenyl group having 2 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted alkylsilyl group having 3 to 20 carbon atoms, substituted or unsubstituted arylsilyl group having 8 to 40 carbon atoms, substituted or unsubstituted aralkylsilyl group having 8 to
- X A , X B , X C , X D , X E each represent a sulfur atom, an oxygen atom or a monoaryl-substituted nitrogen atom.
- the emitting layer includes a host material and a phosphorescent material, the phosphorescent material being an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- an electron injecting layer is provided between the cathode and the emitting layer and includes a nitrogen-containing cyclic derivative.
- an electron transporting layer is provided between the cathode and the emitting layer and includes the above-described organic-EL-device material.
- a reduction-causing dopant is present at an interfacial region between the cathode and the organic thin-film layer.
- the organic EL device preferably includes a cathode, an anode, and an organic layer between the cathode and the anode, in which the organic layer include the biscarbazole derivative represented by the formulae (1) to (3).
- the organic layer preferably includes a phosphorescent material, the phosphorescent material being an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- the biscarbazole derivative is used as the organic-EL-device material, a long-life organic electroluminescence device can be provided.
- the organic-EL-device material is effective as organic-electron-device material for an organic solar cell, an organic semiconductor laser, a sensor using an organic substance and an organic TFT.
- FIG. 1 schematically shows an exemplary arrangement of an organic electroluminescence device according to an exemplary embodiment of the invention.
- FIG. 2A shows an energy diagram of an emitting layer in an organic EL device according to Examples of the invention.
- FIG. 2B shows an energy diagram of an emitting layer in an organic EL device according to Examples of the invention.
- anode/emitting layer/cathode (2) anode/hole injecting layer/emitting layer/cathode; (3) anode/emitting layer/electron injecting•transporting layer/cathode; (4) anode/hole injecting layer/emitting layer/electron injecting•transporting layer/cathode; (5) anode/organic semiconductor layer/emitting layer/cathode; (6) anode/organic semiconductor layer/electron blocking layer/emitting layer/cathode; (7) anode/organic semiconductor layer/emitting layer/adhesion improving layer/cathode; (8) anode/hole injecting•transporting layer/emitting layer/electron injecting•transporting layer/cathode; (9) anode/insulating layer/emitting layer/insulating layer/cathode; (10) anode/inorganic semiconductor layer/insulating layer/emitting layer/insulating layer/cathode;
- FIG. 1 schematically shows an exemplary arrangement of an organic EL device according to a first exemplary embodiment of the invention.
- the organic EL device 1 includes a transparent substrate 2 , an anode 3 , a cathode 4 and an organic thin-film layer 10 positioned between the anode 3 and the cathode 4 .
- the organic thin-film layer 10 includes a phosphorescent-emitting layer 5 containing a phosphorescent host (a host material) and a phosphorescent dopant (a phosphorescent material).
- a layer such as a hole injecting/transporting layer 6 may be provided between the phosphorescent-emitting layer 5 and the anode 3 while a layer such as an electron injecting/transporting layer 7 may be provided between the phosphorescent-emitting layer 5 and the cathode 4 .
- an electron blocking layer may be provided to the phosphorescent-emitting layer 5 adjacent to the anode 3 while a hole blocking layer may be provided to the phosphorescent-emitting layer 5 adjacent to the cathode 4 .
- a “fluorescent host” and a “phosphorescent host” herein respectively mean a host combined with a fluorescent dopant and a host combined with a phosphorescent dopant, and that a distinction between the fluorescent host and phosphorescent host is not unambiguously derived only from a molecular structure of the host in a limited manner.
- the fluorescent host herein means a material for forming a fluorescent-emitting layer containing a fluorescent dopant, and does not mean a host that is only usable as a host of a fluorescent material.
- the phosphorescent host herein means a material for forming a phosphorescent-emitting layer containing a phosphorescent dopant, and does not mean a host that is only usable as a host of a phosphorescent material.
- the “hole injecting/transporting layer” herein means “at least either one of a hole injecting layer and a hole transporting layer” while the “electron injecting/transporting layer” herein means “at least either one of an electron injecting layer and an electron transporting layer.”
- the organic EL device according to this exemplary embodiment is formed on a light-transmissive substrate.
- the light-transmissive plate, which supports the organic EL device is preferably a smoothly-shaped substrate that transmits 50% or more of light in a visible region of 400 nm to 700 nm.
- a glass plate, a polymer plate, and the like are preferable.
- glass plate materials such as soda-lime glass, barium/strontium-containing glass, lead glass, aluminosilicate glass, borosilicate glass, barium borosilicate glass and quartz can be used.
- polystyrene resin for the polymer plate, materials such as polycarbonate, acryl, polyethylene terephthalate, polyether sulfide and polysulfone can be used.
- the anode of the organic EL device is used for injecting holes into the hole injecting layer, the hole transporting layer or the emitting layer. It is effective that the anode has a work function of 4.5 eV or more.
- Exemplary materials for the anode are alloys of indium-tin oxide (ITO), tin oxide (NESA), indium zinc oxide, gold, silver, platinum and copper.
- ITO indium-tin oxide
- NESA tin oxide
- indium zinc oxide gold, silver, platinum and copper.
- the anode may be made by forming a thin film from these electrode materials through methods such as vapor deposition and sputtering.
- the anode When light from the emitting layer is to be emitted through the anode as in this embodiment, the anode preferably transmits more than 10% of the light in the visible region.
- Sheet resistance of the anode is preferably several hundreds ⁇ /square or lower.
- thickness of the anode is typically in a range of 10 nm to 1 ⁇ m, and preferably in a range of 10 to 200 nm.
- the cathode is preferably formed of a material with smaller work function in order to inject electrons into the electron injecting layer, the electron transporting layer and the emitting layer.
- a material for the cathode is subject to no specific limitation, examples of the material are indium, aluminum, magnesium, alloy of magnesium and indium, alloy of magnesium and aluminum, alloy of aluminum and lithium, alloy of aluminum, scandium and lithium, alloy of magnesium and silver and the like.
- the cathode may be made by forming a thin film from the above materials through a method such as vapor deposition or sputtering.
- the light may be emitted through the cathode.
- the emitting layer of the organic EL device is an organic thin-film layer having a function for providing conditions for recombination of the electrons and the holes to emit light.
- Injectability of the holes may differ from that of the electrons and transporting capabilities of the hole and the electrons (represented by mobilities of the holes and the electrons) may differ from each other.
- known methods such as vapor deposition, spin coating and an LB method may be employed.
- the emitting layer is preferably a molecular deposit film.
- the molecular deposit film means a thin film formed by depositing a material compound in gas phase or a film formed by solidifying a material compound in a solution state or in liquid phase.
- the molecular deposit film is typically distinguished from a thin film formed by the LB method (molecular accumulation film) by differences in aggregation structures, higher order structures and functional differences arising therefrom.
- the emitting layer can be formed from a thin film formed by spin coating or the like, the thin film being formed from a solution prepared by dissolving a binder (e.g. a resin) and a material compound in a solvent.
- a binder e.g. a resin
- An organic EL device includes: a cathode; an anode; and a single or a plurality of organic thin-film layers provided between the cathode and the anode, in which the organic thin-film layer(s) includes at least one emitting layer, and at least one of the organic thin-film layers includes at least one phosphorescent material and, as an organic-EL-device material, at least one biscarbazole derivative according to this exemplary embodiment (described later). It is also preferable that at least one emitting layer includes the biscarbazole derivative as the organic-EL-device material according to this exemplary embodiment and at least one phosphorescent material.
- the biscarbazole derivative according to this exemplary embodiment is represented by a formula (1) below.
- a 1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms
- a 2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- X 1 and X 2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- Y 1 to Y 4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fuse
- adjacent ones of Y 1 to Y 4 may be bonded to each other to form a ring structure
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- a plurality of Y 1 to Y 4 may be the same or different.
- the ring structure is exemplified by structures represented by the following formulae.
- the biscarbazole derivative represented by the formula (1) is more preferably represented by a formula (2) below.
- a 1 , A 2 , X 1 , X 2 , Y 1 to Y 4 , p, q, r and s are the same as those in the formula (1).
- a 1 and A 2 are preferably a nitrogen-containing heterocyclic group at the same time. More preferably, A 1 and A 2 are a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- a 1 in the formula (2) is preferably selected from the group consisting of a substituted or unsubstituted pyridine ring, substituted or unsubstituted pyrimidine ring and substituted or unsubstituted triazine ring, more preferably selected from a substituted or unsubstituted pyrimidine ring or substituted or unsubstituted triazine ring.
- a 1 in the formula (2) is further preferably a substituted or unsubstituted pyrimidine ring and is particularly preferably represented by a formula (3) below.
- a 2 , X 1 , Y 1 to Y 4 , p, q, r and s are the same as those in the formula (1); Y 5 represents the same as Y 1 to Y 4 of the formula (1); t represents an integer from 1 to 3; and when t is an integer of 2 to 3, a plurality of Y 5 may be the same or different.
- a 2 is preferably a nitrogen-containing heterocyclic group. More preferably, A 2 is a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- a 1 is preferably a substituted or unsubstituted quinazoline ring.
- X 1 is preferably a single bond or a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 30 ring carbon atoms, more preferably a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 30 ring carbon atoms, particularly preferably a benzene ring or naphthalene ring.
- a 1 and the carbazolyl group, which are bonded to X 1 are preferably in meta positions or para positions. Particularly preferably, X 1 is unsubstituted para-phenylene.
- the pyridine ring, pyrimidine ring and triazine ring are more preferably represented by the following formulae.
- Y and Y′ represent a substituent. Examples of the substituent are the same groups as those represented by Y 1 to Y 4 as described above. Y and Y′ may be the same or different. Preferred examples thereof are the substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, and the substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- * represents a bonding position to X 1 or X 2 .
- the quinazoline ring is represented by the following formula.
- Y represents a substituent.
- u represents an integer of 1 to 5.
- a plurality of Y may be the same or different.
- the substituent Y the same groups as those for the above Y 1 to Y 4 are usable, among which preferred examples thereof are the substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, and the substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- * represents a bonding position to X 1 or X 2 .
- the alkyl group, alkoxy group, haloalkyl group, haloalkoxy group and alkylsilyl group which are represented by Y 1 to Y 5 , may be linear, branched or cyclic.
- examples of the alkyl group having 1 to 20 carbon atoms are a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, n-nonyl group, n-decyl group, n-undecyl group, n-dodecyl group, n-tridecyl group, n-tetradecyl group, n-pentadecyl group, n-hexadecyl group, n-heptadecyl group, n-octadecyl group, neo-pentyl group, 1-methylpentyl group, 2-methylpentyl group, 1-p
- alkyl group having 1 to 10 carbon atoms examples include a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, cyclopentyl group, cyclohexyl group and cycloheptyl group.
- an alkoxy group having 1 to 6 carbon atoms is preferable and specific examples thereof are a methoxy group, ethoxy group, propoxy group, butoxy group, pentyloxy group, and hexyloxy group.
- the haloalkyl group having 1 to 20 carbon atoms is exemplified by an haloalkyl group provided by substituting the alkyl group having 1 to 20 carbon atoms with one or more halogen atoms. Preferred one of the halogen atoms is fluorine.
- the haloalkyl group is exemplified by a trifluoromethyl group and a 2,2,2-trifluoroethyl group.
- the haloalkoxy group having 1 to 20 carbon atoms is exemplified by a haloalkoxy group provided by substituting the alkoxy group having 1 to 20 carbon atoms with one or more halogen atoms.
- alkylsilyl group having 1 to 10 carbon atoms examples are a trimethylsilyl group, triethylsilyl group, tributylsilyl group, dimethylethylsilyl group, dimethylisopropylsilyl group, dimethylpropylsilyl group, dimethylbutylsilyl group, dimethyl-tertiary-butylsilyl group and diethylisopropylsilyl group.
- arylsilyl group having 6 to 30 carbon atoms examples are a phenyldimethylsilyl group, diphenylmethylsilyl group, diphenyl-tertiary-butylsilyl group and triphenylsilyl group.
- aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms are a pyroryl group, pyrazinyl group, pyridinyl group, indolyl group, isoindolyl group, furyl group, benzofuranyl group, isobenzofuranyl group, dibenzofuranyl group, dibenzothiophenyl group, quinolyl group, isoquinolyl group, quinoxalinyl group, carbazolyl group, phenantridinyl group, acridinyl group, phenanthrolinyl group, thienyl group and a group formed from a pyridine ring, pyrazine ring, pyrimidine ring, pyridazine ring, triazine ring, indol ring, quinoline ring, acridine ring, pirrolidine ring, dioxane ring, piperidine ring, morph
- Examples of the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms are a phenyl group, naphthyl group, phenanthryl group, biphenyl group, terphenyl group, quarterphenyl group, fluoranthenyl group, triphenylenyl group, phenanthrenyl group, pyrenyl group, chrysenyl group, fluorenyl group, and 9,9-dimethylfluorenyl group.
- the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 20 ring carbon atoms is preferable.
- the substituents are preferably a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkoxy group having 1 to 20 carbon atoms; linear, branched or cyclic haloalkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkylsilyl group having 1 to 10 carbon atoms; arylsilyl group having 6 to 30 ring carbon atoms; cyano group; halogen atom; aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms; or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- linear, branched or cyclic alkyl group having 1 to 20 carbon atoms examples include linear, branched or cyclic alkoxy group having 1 to 20 carbon atoms; linear, branched or cyclic haloalkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkylsilyl group having 1 to 10 carbon atoms; arylsilyl group having 6 to 30 ring carbon atoms; aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms; and aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms are the above-described groups.
- the halogen atom is exemplified by a fluorine atom.
- Examples of compounds for the biscarbazole derivative according to this exemplary embodiment represented by the formulae (1) to (3) are as follows.
- An organic-EL-device material contains the above biscarbazole derivative.
- the organic-EL-device material according to this exemplary embodiment includes the biscarbazole derivative represented by any one of the above formulae (1) to (3).
- the organic EL device includes a cathode, an anode, and an organic layer between the cathode and the anode, in which the organic layer include the biscarbazole derivative any one of the above formulae (1) to (3).
- the emitting layer may preferably contain the organic-EL-device material according to this exemplary embodiment.
- the organic EL device according to this exemplary embodiment may preferably contain the electron injecting/transporting layer, in which the electron injecting/transporting layer may preferably contain the organic-EL-device material according to this exemplary embodiment.
- the organic EL device according to this exemplary embodiment may preferably contain at least one of the electron injecting/transporting layer and the hole blocking layer that contains the organic-EL-device material according to this exemplary embodiment.
- the organic EL device according to this exemplary embodiment may preferably include the hole transporting layer (hole injecting layer) that contains the organic-EL-device material according to this exemplary embodiment.
- the phosphorescent material preferably contains a metal complex, and the metal complex preferably has a metal atom selected from Ir, Pt, Os, Au, Cu, Re and Ru, and a ligand.
- the ligand preferably has an ortho-metal bond.
- the phosphorescent material is preferably a compound containing a metal selected from iridium (Ir), osmium (Os) and platinum (Pt) because such a compound, which exhibits high phosphorescence quantum yield, can further enhance external quantum efficiency of the emitting device.
- the phosphorescent material is more preferably a metal complex such as an iridium complex, osmium complex or platinum complex, among which an iridium complex and platinum complex are more preferable and ortho metalation of an iridium complex is the most preferable.
- At least one of the phosphorescent material contained in the emitting layer preferably emits light with the maximum wavelength of 450 nm to 720 nm.
- the organic EL device can exhibit high efficiency.
- a reduction-causing dopant may be preferably contained in an interfacial region between the cathode and the organic thin-film layer.
- the organic EL device can emit light with enhanced luminance intensity and have a longer lifetime.
- the reduction-causing dopant may be at least one compound selected from an alkali metal, alkali metal complex, alkali metal compound, alkali earth metal, alkali earth metal complex, alkali earth metal compound, rare-earth metal, rare-earth metal complex, rare-earth metal compound and the like.
- the alkali metal examples include Na (work function: 2.36 eV), K (work function: 2.28 eV), Rb (work function: 2.16 eV), Cs (work function: 1.95 eV) and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable.
- the reduction-causing dopant is preferably K, Rb or Cs, more preferably Rb or Cs, the most preferably Cs.
- alkali earth metal examples include Ca (work function: 2.9 eV), Sr (work function: 2.0 to 2.5 eV), Ba (work function: 2.52 eV) and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable.
- Examples of the rare-earth metal are Sc, Y, Ce, Tb, Yb and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable.
- alkali metal compound examples include an alkali oxide such as Li 2 O, Cs 2 O and K 2 O, and an alkali halide such as LiF, NaF, CsF and KF. LiF, Li 2 O, and NaF are preferable.
- alkali earth metal compound examples include BaO, SrO, CaO and their mixture such as Ba x Sr 1-x O (0 ⁇ x ⁇ 1) and Ba x Ca 1-x O (0 ⁇ x ⁇ 1). BaO, SrO, and CaO are preferable.
- rare earth metal compound examples include YbF 3 , ScF 3 , ScO 3 , Y 2 O 3 , Ce 2 O 3 , GdF 3 and TbF 3 .
- YbF 3 , ScF 3 , and TbF 3 are preferable.
- the alkali metal complex, alkali earth metal complex and rare earth metal complex are not specifically limited as long as they contain at least one metal ion of an alkali metal ion, an alkali earth metal ion and a rare earth metal ion.
- a ligand for each of the complexes is preferably quinolinol, benzoquinolinol, acridinol, phenanthridinol, hydroxyphenyl oxazole, hydroxyphenyl thiazole, hydroxydiaryl oxadiazole, hydroxydiaryl thiadiazole, hydroxyphenyl pyridine, hydroxyphenyl benzoimidazole, hydroxybenzo triazole, hydroxy fluborane, bipyridyl, phenanthroline, phthalocyanine, porphyrin, cyclopentadiene, ⁇ -diketones, azomethines, or a derivative thereof, but the ligand is not limited thereto.
- the reduction-causing dopant is added to preferably form a layer or an island pattern in the interfacial region.
- the layer of the reduction-causing dopant or the island pattern of the reduction-causing dopant is preferably formed by depositing the reduction-causing dopant by resistance heating deposition while an emitting material for forming the interfacial region or an organic substance as an electron-injecting material are simultaneously deposited, so that the reduction-causing dopant is dispersed in the organic substance.
- Dispersion concentration at which the reduction-causing dopant is dispersed in the organic substance is a mole ratio (organic substance to reduction-causing dopant) of 100:1 to 1:100, preferably 5:1 to 1:5.
- the emitting material or the electron injecting material for forming the organic layer of the interfacial region is initially layered, and the reduction-causing dopant is subsequently deposited singularly thereon by resistance heating deposition to form a preferably 0.1 to 15 nm-thick layer.
- the emitting material or the electron injecting material for forming the organic layer of the interfacial region is initially formed in an island shape, and the reduction-causing dopant is subsequently deposited singularly thereon by resistance heating deposition to form a preferably 0.05 to 1 nm-thick island shape.
- a ratio of the main component to the reduction-causing dopant in the organic EL device according to this exemplary embodiment is preferably a mole ratio (main component to reduction-causing dopant) of 5:1 to 1:5, more preferably 2:1 to 1:2.
- the electron injecting layer or the electron transporting layer which aids injection of the electrons into the emitting layer, has a large electron mobility.
- the electron injecting layer is provided for adjusting energy level, by which, for instance, sudden changes of the energy level can be reduced.
- the organic EL device preferably includes the electron injecting layer between the emitting layer and the cathode, and the electron injecting layer preferably contains a nitrogen-containing cyclic derivative as the main component.
- the electron injecting layer may serve as the electron transporting layer.
- the main component means that the nitrogen-containing cyclic derivative is contained in the electron injecting layer at a content of 50 mass % or more.
- a preferable example of an electron transporting material for forming the electron injecting layer is an aromatic heterocyclic compound having in the molecule at least one heteroatom.
- a nitrogen-containing cyclic derivative is preferable.
- the nitrogen-containing cyclic derivative is preferably an aromatic ring having a nitrogen-containing six-membered or five-membered ring skeleton, or a fused aromatic cyclic compound having a nitrogen-containing six-membered or five-membered ring skeleton.
- a preferable example of the nitrogen-containing cyclic derivative is a nitrogen-containing cyclic metal chelate complex represented by the following formula (A).
- R 2 to R 7 in the formula (A) each independently represent a hydrogen atom, a halogen atom, an oxy group, an amino group, a hydrocarbon group having 1 to 40 carbon atoms, an alkoxy group, an aryloxy group, an alkoxycarbonyl group, or an aromatic heterocyclic group. These groups may be substituted or unsubstituted.
- halogen atom examples include fluorine, chlorine, bromine, and iodine.
- substituted or unsubstituted amino group include an alkylamino group, an arylamino group, and an aralkylamino group.
- the alkoxycarbonyl group is represented by —COOY′.
- Y′ are the same as the examples of the alkyl group.
- the alkylamino group and the aralkylamino group are represented by —NQ 1 Q 2 .
- Examples for each of Q 1 and Q 2 are the same as the examples described in relation to the alkyl group and the aralkyl group, and preferable examples for each of Q 1 and Q 2 are also the same as those described in relation to the alkyl group and the aralkyl group.
- Either one of Q 1 and Q 2 may be a hydrogen atom.
- the arylamino group is represented by —NAr 1 Ar 2 .
- Examples for each of Ar 1 and Ar e are the same as the examples described in relation to the non-fused aromatic hydrocarbon group and the fused aromatic hydrocarbon group.
- Either one of Ar 1 and Ar 2 may be a hydrogen atom.
- M aluminum (Al), gallium (Ga) or indium (In), among which In is preferable.
- L in the formula (A) represents a group represented by the following formula (A′) or the following formula (A′′).
- R 8 to R 12 each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 40 carbon atoms. Adjacent groups may form a cyclic structure.
- R 13 to R 27 each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 40 carbon atoms. Adjacent groups may form a cyclic structure.
- Examples of the hydrocarbon group having 1 to 40 carbon atoms represented by each of R 8 to R 12 and R 13 to R 27 in the formulae (A′) and (A′′) are the same as those of R 2 to R 7 in the formula (A).
- Examples of a divalent group formed when an adjacent set of R 8 to R 12 and R 13 to R 27 forms a cyclic structure are a tetramethylene group, a pentamethylene group, a hexamethylene group, a diphenylmethane-2,2′-diyl group, a diphenylethane-3,3′-diyl group, a diphenylpropane-4,4′-diyl group and the like.
- the electron transporting layer may contain the biscarbazole derivatives represented by the formulae (1) to (3) (or the formulae (4) to (6)).
- 8-hydroxyquinoline or a metal complex of its derivative As an electron transporting compound for the electron injecting layer or the electron transporting layer, 8-hydroxyquinoline or a metal complex of its derivative, an oxadiazole derivative and a nitrogen-containing heterocyclic derivative are preferable.
- An example of the 8-hydroxyquinoline or the metal complex of its derivative is a metal chelate oxinoid compound containing a chelate of oxine (typically 8-quinolinol or 8-hydroxyquinoline).
- oxine typically 8-quinolinol or 8-hydroxyquinoline
- tris(8-quinolinol) aluminum can be used.
- the oxadiazole derivative are as follows.
- Ar 17 , Ar 18 , Ar 19 , Ar 21 , Ar 22 and Ar 25 each represent a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms.
- Ar 17 , Ar 19 and Ar 22 may be the same as or different from Ar 18 , Ar 21 and Ar 25 respectively.
- the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms are a phenyl group, biphenyl group, anthranil group, perylenyl group and pyrenyl group.
- substituent therefor are an alkyl group having 1 to 10 carbon atoms, alkoxy group having 1 to 10 carbon atoms and cyano group.
- Ar 20 , Ar 23 and Ar 24 each represent a substituted or unsubstituted divalent aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms.
- Ar 23 and Ar 24 may be mutually the same or different.
- Examples of the divalent aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms are a phenylene group, naphthylene group, biphenylene group, anthranylene group, perylenylene group and pyrenylene group.
- Examples of the substituent therefor are an alkyl group having 1 to 10 carbon atoms, alkoxy group having 1 to 10 carbon atoms and cyano group.
- Such an electron transport compound is preferably an electron transport compound that can be favorably formed into a thin film(s).
- Examples of the electron transporting compounds are as follows.
- nitrogen-containing heterocyclic derivative as the electron transporting compound is a nitrogen-containing compound that is not a metal complex, the derivative being formed of an organic compound represented by one of the following general formulae.
- nitrogen-containing heterocyclic derivative are a five-membered ring or six-membered ring derivative having a skeleton represented by the following formula (A) and a derivative having a structure represented by the following formula (B).
- X represents a carbon atom or a nitrogen atom.
- Z 1 and Z 2 each independently represent a group of atoms capable of forming a nitrogen-containing heterocycle.
- the nitrogen-containing heterocyclic derivative is an organic compound having a nitrogen-containing aromatic polycyclic group having a five-membered ring or six-membered ring.
- the nitrogen-containing heterocyclic derivative may be a nitrogen-containing aromatic polycyclic organic compound having a skeleton formed by a combination of the skeletons respectively represented by the formulae (A) and (B), or by a combination of the skeletons respectively represented by the formulae (A) and (C).
- a nitrogen-containing group of the nitrogen-containing aromatic polycyclic organic compound is selected from nitrogen-containing heterocyclic groups respectively represented by the following general formulae.
- R represents an aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms; aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms; alkyl group having 1 to 20 carbon atoms or alkoxy group having 1 to 20 carbon atoms; and n represents an integer in a range of 0 to 5.
- n is an integer of 2 or more, plural R may be mutually the same or different.
- a preferable specific compound is a nitrogen-containing heterocyclic derivative represented by the following formula.
- HAr represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 40 ring carbon atoms
- L 1 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms
- Ar 1 represents a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 40 ring carbon atoms
- Ar 2 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms.
- HAr is exemplarily selected from the following group.
- L 1 is exemplarily selected from the following group.
- Ar 1 is exemplarily selected from the following arylanthranil groups.
- R 1 to R 14 each independently represent a hydrogen atom, halogen atom, alkyl group having 1 to 20 carbon atoms, alkoxy group having 1 to 20 carbon atoms, aryloxy group having 6 to 40 ring carbon atoms, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms; and Ar 3 represents aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms.
- All of R 1 to R 8 of a nitrogen-containing heterocyclic derivative may be hydrogen atoms.
- Ar 2 is exemplarily selected from the following group.
- the following compound (see JP-A-9-3448) can be favorably used for the nitrogen-containing aromatic polycyclic organic compound as the electron transporting compound.
- R 1 to R 4 each independently represent a hydrogen atom, substituted or unsubstituted aliphatic group, substituted or unsubstituted alicyclic group, substituted or unsubstituted carbocyclic aromatic cyclic group or substituted or unsubstituted heterocyclic group; and X 1 and X 2 each independently represent an oxygen atom, sulfur atom or dicyanomethylene group.
- the following compound (see JP-A-2000-173774) can also be favorably used for the electron transporting compound.
- R 1 , R 2 , R 3 and R 4 which may be mutually the same or different, each represent an aromatic hydrocarbon group or fused aromatic hydrocarbon group represented by the following formula.
- R 5 , R 6 , R 7 , R 8 and R 9 which may be mutually the same or different, each represent a hydrogen atom, a saturated or unsaturated alkoxyl group, alkyl group, amino group or alkylamino group. At least one of R 5 , R 6 , R 7 , R 8 and R 9 represents a saturated or unsaturated alkoxyl group, alkyl group, amino group or alkylamino group.
- a polymer compound containing the nitrogen-containing heterocyclic group or a nitrogen-containing heterocyclic derivative may be used for the electron transporting compound.
- the electron transporting layer preferably contains at least one of nitrogen-containing heterocycle derivatives respectively represented by the following formulae (201) to (203).
- R represents a hydrogen atom, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms;
- n is an integer in a range of 0 to 4.
- R 1 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, ⁇ nret>> or alkoxy group having 1 to 20 carbon atoms;
- R 2 and R 3 each independently represent a hydrogen atom, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or a substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms;
- L represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridinylene group, substituted or unsubstituted quinolylene group, or substituted or unsubstituted fluorenylene group;
- Ar 1 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridinylene group, substituted or unsubstituted quinolyl group.
- Ar e represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms; and
- Ar 3 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms or group represented by —Ar 1 —Ar 2 (Ar 1 and Ar 2 may be the same as the above).
- R represents a hydrogen atom, a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms.
- a thickness of the electron injecting layer or the electron transporting layer is not specifically limited, the thickness is preferably 1 nm to 100 nm.
- the electron injecting layer preferably contains an inorganic compound such as an insulator or a semiconductor in addition to the nitrogen-containing cyclic derivative.
- an insulator or a semiconductor when contained in the electron injecting layer, can effectively prevent a current leak, thereby enhancing electron capability of the electron injecting layer.
- the insulator it is preferable to use at least one metal compound selected from the group consisting of an alkali metal chalcogenide, an alkali earth metal chalcogenide, a halogenide of alkali metal and a halogenide of alkali earth metal.
- the electron injecting layer from the alkali metal chalcogenide or the like, the electron injecting capability can preferably be further enhanced.
- preferable examples of the alkali metal chalcogenide are Li 2 O, K 2 O, Na 2 S, Na 2 Se and Na 2 O
- preferable example of the alkali earth metal chalcogenide are CaO, BaO, SrO, BeO, BaS and CaSe.
- halogenide of the alkali metal are LiF, NaF, KF, LiCl, KCl and NaCl.
- halogenide of the alkali earth metal are fluorides such as CaF 2 , BaF 2 , SrF 2 , MgF 2 and BeF 2 , and halogenides other than the fluoride.
- Examples of the semiconductor are one of or a combination of two or more of an oxide, a nitride or an oxidized nitride containing at least one element selected from Ba, Ca, Sr, Yb, Al, Ga, In, Li, Na, Cd, Mg, Si, Ta, Sb and Zn.
- An inorganic compound for forming the electron injecting layer is preferably a microcrystalline or amorphous semiconductor film. When the electron injecting layer is formed of such insulator film, more uniform thin film can be formed, thereby reducing pixel defects such as a dark spot.
- Examples of such an inorganic compound are the above-described alkali metal chalcogenide, alkali earth metal chalcogenide, halogenide of the alkali metal and halogenide of the alkali earth metal.
- a thickness thereof is preferably in a range of approximately 0.1 nm to 15 nm.
- the electron injecting layer in this exemplary embodiment may preferably contain the above-described reduction-causing dopant.
- the hole injecting layer or the hole transporting layer may contain an aromatic amine compound such as an aromatic amine derivative represented by the following (I).
- Ar 1 to Ar 4 each represent a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 50 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms, or a group formed by combining the aromatic hydrocarbon group or the fused aromatic hydrocarbon group with the aromatic heterocyclic group or fused aromatic heterocyclic group.
- Examples of the compound represented by the formula (I) are shown below. However, the compound represented by the formula (I) is not limited thereto.
- Aromatic amine represented by the following (II) can also be preferably used for forming the hole injecting layer or the hole transporting layer.
- Ar 1 to Ar 3 each represent the same as Ar 1 to Ar 4 of the above (I).
- Examples of the compound represented by the general formula (II) are shown below. However, the compound represented by the formula (II) is not limited thereto.
- a method of forming each of the layers in the organic EL device according to this exemplary embodiment is not particularly limited. Conventionally-known methods such as vacuum deposition and spin coating may be employed for forming the layers.
- the organic thin-film layer containing the compound represented by the formula (1), which is used in the organic EL device according to this exemplary embodiment, may be formed by a conventional coating method such as vacuum deposition, molecular beam epitaxy (MBE method) and coating methods using a solution such as a dipping, spin coating, casting, bar coating, and roll coating.
- MBE method molecular beam epitaxy
- each organic layer of the organic EL device is not particularly limited, the thickness is generally preferably in a range of several nanometers to 1 ⁇ m because an excessively-thinned film likely entails defects such as a pin hole while an excessively-thickened film requires high voltage to be applied and deteriorates efficiency.
- the organic EL device according to the second exemplary embodiment is different in that the emitting layer includes the first host material, the second host material and the phosphorescent material.
- the first host material is the biscarbazole derivative according to the exemplary embodiment represented by the formulae (1) to (3).
- the organic-EL-device material represented by the formulae (1) to (3) has a biscarbazole skeleton having an excellent hole transporting capability and a heterocyclic skeleton having an excellent electron transporting capability, which leads to a bi-polar performance sufficient for functioning as a single host.
- a luminous efficiency and a lifetime of the multilayered organic EL device depend on a carrier balance of an entire organic EL device.
- Main factors for controlling the carrier balance are carrier transporting capability of each of the organic layers and carrier injecting capability in the interfacial region of separate organic layers.
- the second host material is suitably selected as a cohost and used in the emitting layer.
- the cathode When a material having a poor electron injecting capability (e.g., metal chelate complex) is used as the cathode, a carrier balance in the emitting layer becomes shifted toward the cathode. For improving such a disadvantage, it is preferable to select a material having a high electron transporting capability as the second host material.
- the host material of this exemplary embodiment is preferably represented by a formula (5) or (6).
- Cz represents a substituted or unsubstituted arylcarbazolyl group or carbazolylaryl group
- a 3 represents a group represented by a formula (7A) below.
- a and b each represent an integer of 1 to 3.
- M 1 and M 2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M 1 and M 2 may be the same or different;
- L 5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- An arylcarbazolyl group means a carbazolyl group having at least one aryl group or heteroaryl group as a substituent, in which a position where the aryl group or heteroaryl group is substituted does not matter.
- Ar represents an aryl group or heteroaryl group.
- * represents a position where another group is bonded.
- a carbazolylaryl group means an aryl group having at least one carbazolyl group as a substituent, in which a position where the aryl group is substituted does not matter.
- Ar represents an aryl group. * represents a position where another group is bonded.
- a substituted arylcarbazolyl group means the arylcarbazolyl group having at least one substituent irrespective of a substitution position.
- a substituted carbazolylaryl group means the carbazolylaryl group having at least one substituent irrespective of a substitution position.
- a and b each represent an integer of 1 to 3.
- An aryl group in the arylcarbazolyl group or carbazolylaryl group preferably has 6 to 30 carbon atoms.
- Examples of the aryl group are a phenyl group, naphthyl group, anthryl group, phenanthryl group, naphthacenyl group, pyrenyl group, fluorenyl group, biphenyl group and terphenyl group, among of which a phenyl group, naphthyl group, biphenyl group and terphenyl group are preferable.
- heteroaryl group in the arylcarbazolyl group are groups formed based on rings of pyridine, pyrimidine, pyrazine, triazine, aziridine, azaindolizine, indolizine, imidazoles, indole, isoindole, indazole, purine, pteridine, d-carboline, naphthyridine, quinoxaline, terpyridine, bipyridine, acridine, phenanthroline, phenazine and imidazopyridine, among which rings of pyridine, terpyridine, pyrimidine, imidazopyridine and triazine are preferable.
- a in the formulae (5) and (6) is a group represented by the formula (7A).
- M 1 and M 2 each independently represent a substituted or unsubstituted nitrogen-containing heterocyclic group having 2 to 40 ring carbon atoms. M 1 and M 2 may be the same or different.
- Examples of the nitrogen-containing heterocyclic ring in the arylcarbazolyl group are groups formed based on rings of pyridine, pyrimidine, pyrazine, triazine, aziridine, azaindolizine, indolizine, imidazoles, indole, isoindole, indazole, purine, pteridine, â-carboline, naphthyridine, quinoxaline, terpyridine, bipyridine, acridine, phenanthroline, phenazine and imidazopyridine, among which rings of pyridine, terpyridine, pyrimidine, imidazopyridine and triazine are preferable.
- L 5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Examples of the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms are a phenyl group, biphenyl group, terphenyl group, naphthyl group, anthranil group, phenanthryl group, pyrenyl group, crycenyl group, fluoranthenyl group and perfluoroaryl group, fluorenyl group, and 9,9-dimethylfluorenyl group, among which a phenyl group, biphenyl group, terphenyl group and perfluoroaryl group are preferable.
- Examples of the cycloalkylene group having 5 to 30 carbon atoms are cyclopentyl group, cyclohexylene group, and cyclohepthylene group, among which a cyclohexylene group is preferable.
- aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms are 1-pyrrolyl group, 2-pyrrolyl group, 3-pyrrolyl group, pyrazinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group
- Examples of the substituents for Cz, M 1 and M 2 in the formulae (5), (6) and (7A) are a halogen atom such as chlorine, bromine and fluorine, carbazole group, hydroxyl group, substituted or unsubstituted amino group, nitro group, cyano group, silyl group, trifluoromethyl group, carbonyl group, carboxyl group, substituted or unsubstituted alkyl group, substituted or unsubstituted alkenyl group, substituted or unsubstituted arylalkyl group, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group, substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group, substituted or unsubstituted aralkyl group, substituted or unsubstituted aryloxy group, and substituted or unsubstituted alkyloxy group.
- a halogen atom such as chlorine, bromine and flu
- a fluorine atom, methyl group, perfluorophenylene group, phenyl group, naphthyl group, pyridyl group, pyrazil group, pyrimidyl group, adamantyl group, benzyl group, cyano group and silyl group are preferable.
- Bonding patterns of the compound represented by the formula (5) or (6) are shown in Table 1 below in accordance with values of a and b.
- Bonding patterns of the compound represented by the formula (7A) are shown in Tables 2 and 3 below in accordance with values of c, d and e.
- Cz bonded to A may be bonded to any one of M 1 , L 5 and M 2 of the formula (7A) representing A.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is preferably a substituted or unsubstituted arylcarbazolyl group, more preferably phenylcarbozolyl group. Moreover, an aryl site of the arylcarbazolyl group is preferably substituted by a carbazolyl group.
- the compound represented by the formula (5) or (6) in this exemplary embodiment has triplet energy gap of 2.5 eV to 3.3 eV, preferably 2.5 eV to 3.2 eV.
- the compound represented by the formula (5) or (6) in this exemplary embodiment has singlet energy gap of 2.8 eV to 3.8 eV, preferably 2.9 eV to 3.7 eV.
- An organic EL device according to a third exemplary embodiment is different from the organic EL device according to the second exemplary embodiment in that a material having a poor electron capability is used as the second material.
- the second host material of this exemplary embodiment is preferably a compound in which A 3 is a group represented by the following formula (7B) in the formula (5) or (6).
- M 3 and M 4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 6 to 40 ring carbon atoms; M 3 and M 4 may be the same or different; L 6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 6 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- M 3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L 6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- Cz is preferably a substituted or unsubstituted arylcarbazolyl group, more preferably phenylcarbozolyl group. Moreover, an aryl site of the arylcarbazolyl group is preferably substituted by a carbazolyl group.
- R 101 to R 106 each independently represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to
- R 101 to R 106 is a substituted or unsubstituted 9-carbazolyl group, substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms, or -L-9-carbazolyl group;
- L represents a substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group
- Xa represents a sulfur atom, oxygen atom or N—R 108 ;
- R 108 represents the same as R 101 to R 106 .
- substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms are shown below (in which any substituent is omitted), but the substituted or unsubstituted azacarbazolyl group is not limited thereto.
- halogen atom examples include fluorine, chlorine, bromine, and iodine.
- Examples of the substituted or unsubstituted alkyl group having 1 to 40 carbon atoms include a methyl group, an ethyl group, a propyl group, an isopropyl group, an n-butyl group, an s-butyl group, an isobutyl group, a t-butyl group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-nonyl group, an n-decyl group, an n-undecyl group, an n-dodecyl group, an n-tridecyl group, an n-tetradecyl group, an n-pentadecyl group, an n-hexadecyl group, an n-heptadecyl group, an n-octadecyl group, a n
- Examples of the substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms include a cyclopentyl group, cyclohexyl group, cyclooctyl group, and 3,5,5,5-tetramethylcyclohexyl group.
- a cyclohexyl group, cyclooctyl group, and 3,5-tetramethylcyclohexyl group are preferable.
- the cycloalkyl group (excluding a substituent) preferably has 3 to 12 carbon atoms.
- Examples of the substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms are a 1-pyroryl group, 2-pyroryl group, 3-pyroryl group, pyrazinyl group, 2-pyridinyl group, 1-imidazolyl, 2-imidazolyl, 1-pyrazolyl, 1-indolidinyl, 2-indolidinyl, 3-indolidinyl, 5-indolidinyl, 6-indolidinyl, 7-indolidinyl, 8-indolidinyl, 2-imidazopyridinyl, 3-imidazopyridinyl, 5-imidazopyridinyl, 6-imidazopyridinyl, 7-imidazopyridinyl, 8-imidazopyridinyl, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indoly
- the heterocyclic group is preferably a 2-pyridinyl group, 1-indolidinyl, 2-indolidinyl, 3-indolidinyl, 5-indolidinyl, 6-indolidinyl, 7-indolidinyl, 8-indolidinyl, 2-imidazopyridinyl, 3-imidazopyridinyl, 5-imidazopyridinyl, 6-imidazopyridinyl, 7-imidazopyridinyl, 8-imidazopyridinyl, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group,
- the substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms is a group represented by —OY.
- Y are the same as those described in relation to the alkyl group. Preferred examples are also the same.
- Examples of the substituted or unsubstituted aryl group having 6 to 40 carbon atoms are a phenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, o-tolyl group, m-tolyl group, p-tolyl group, p-t-butylphenyl group, p-(2-phenylpropyl)phenyl group, 4′-methylbiphenylyl group, 4′′-t-butyl-p-terphenyl-4-yl group, o-cumenyl group,
- the substituted or unsubstituted aryl group is preferably a phenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, p-tolyl group, 3,4-xylyl group, m-quarter-phenyl-2-yl group, 1-naphtyl group, 2-naphtyl group, 1-phenanthrenyl group, 2-phenanthrenyl group, 3-phenanthrenyl group, 4-phenanthrenyl group, 9-phenanthrenyl group, 1-triphenylenyl group, 2-triphenylenyl group, 3-triphenylenyl group, 4-triphenylenyl group, 1-chrysenyl group, 2-chrysenyl group, 3-chrysenyl group,
- the substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms is a group represented by —OAr.
- Ar are the same as those described in relation to the aryl group. Preferred examples are also the same.
- Examples of the substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms are a benzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenylisopropyl group, 2-phenylisopropyl group, phenyl-t-butyl group, á-naphthylmethyl group, 1-á-naphthylethyl group, 2-á-naphthylethyl group, 1-á-naphthylisopropyl group, 2-á-naphthylisopropyl group, â-naphthylmethyl group, 1-â-naphthylethyl group, 2-â-naphthylethyl group, 1-â-naphthylisopropyl group, 2-â-naphthylisopropyl group, 1-pyrorylmethyl group, 2-(1-pyro
- a benzyl group preferred are a benzyl group, a p-cyanobenzyl group, m-cyanobenzyl group, o-cyanobenzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenylisopropyl group, and 2-phenylisopropyl group.
- An alkyl portion of the aralkyl group preferably has 1 to 8 carbon atoms.
- An aryl portion thereof (including heteroaryl) preferably has 6 to 18 carbon atoms.
- the substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, the substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, and the substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms each are represented by —NQ 1 Q 2 .
- Examples of Q 1 and Q 2 each are independently the same as those described in relation to the alkyl group, aryl group and aralkyl group. Preferred examples are also the same.
- the substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms is represented by —COAr 2 .
- Ar 2 are the same as those described in relation to the aryl group. Preferred examples are also the same.
- the substituted or unsubstituted arylthio group having 6 to 20 carbon atoms is exemplified by a group obtained by replacing an oxygen atom of the aryloxy group represented by —OAr with a sulfur atom. Preferred examples are also the same.
- the substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms is exemplified by a halogenated alkyl group in which at least one hydrogen atom of the alkyl group is substituted by a halogen atom. Preferred examples are also the same.
- the compound represented by the general formula (8) preferably has triplet energy gap of 2.2 eV to 3.2 eV. Specific examples of the formula (8) are shown below.
- An organic EL device according to a fourth exemplary embodiment is different from the organic EL devices according to the second and third exemplary embodiments in being a red phosphorescent device.
- the compound according to this exemplary embodiment is not disclosed as a phosphorescent host material of which color is specified.
- the compound since having a high resistance against oxidation and reduction, the compound is also applicable to a red phosphorescent device.
- a red phosphorescent device a hydrocarbon material, which exhibits a small triplet energy and broad electron clouds compared with a green phosphorescent material, can be used.
- the hydrocarbon material is difficult to be used as a green phosphorescent material because of its small triplet energy, the hydrocarbon material is highly appropriate as a red phosphorescent host material because of its high oxidation and reduction. Accordingly, by using the hydrocarbon material as the second host material, a red phosphorescent device can become highly efficient.
- the second host material is preferably a compound selected from the group consisting of polycyclic aromatic compounds represented by formulae (9A), (9B) and (9C) below.
- Ar 101 , Ar 102 , Ar 103 , Ra and Rb represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms.
- Ar 101 , Ar 102 , Ar 103 , Ra and Rb preferably represent a polycyclic aromatic skeleton selected from a substituted or unsubstituted benzene ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted chrysene ring, substituted or unsubstituted fluoranthene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzophenanthrene ring, substituted or unsubstituted dibenzophenanthrene ring, substituted or unsubstituted triphenylene ring, substituted or unsubstituted benzo[a]triphenylene ring, substituted or unsubstituted benzochrysene ring, substituted or unsubstituted benzo[b]fluoranthene ring, substituted or unsubstituted fluorene ring and substituted or
- Ra and Rb are not aryl groups; and Ar 101 , Ar 102 , Ar 103 , Ra and Rb are not substituted or unsubstituted benzene ring at the same time.
- Ra and Rb are preferably selected from the group consisting of a substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzo[c]phenanthrene ring and substituted or unsubstituted fluoranthene ring.
- the polycyclic aromatic skeleton of the polycyclic aromatic compound may be substituted.
- Examples of the substituent for the polycyclic aromatic skeleton are a halogen atom, hydroxyl group, substituted or unsubstituted amino group, nitro group, cyano group, substituted or unsubstituted alkyl group, substituted or unsubstituted alkenyl group, substituted or unsubstituted cycloalkyl group, substituted or unsubstituted alkoxy group, substituted or unsubstituted aromatic hydrocarbon group, substituted or unsubstituted aromatic heterocyclic group, substituted or unsubstituted aralkyl group, substituted or unsubstituted aryloxy group, substituted or unsubstituted alkoxycarbonyl group, and carboxyl group.
- Preferred examples of the aromatic hydrocarbon group are naphthalene, phenanthrene, fluorene, chrysene, fluoranthene and triphenylene.
- the substituents may form a ring.
- the polycyclic aromatic skeleton is preferably any one selected from the group consisting of compounds represented by formulae (9-1) to (9-4) below.
- Ar 1 to Ar 5 each represent a substituted or unsubstituted fused ring structure having 4 to 16 ring carbon atoms.
- Examples of the compound represented by the formula (9-1) are elementary substances or derivatives of substituted or unsubstituted phenanthrene and chrysene.
- Examples of the compound represented by the formula (9-2) are elementary substances or derivatives of substituted or unsubstituted acenaphthylene, acenaphthene and fluoranthene.
- Examples of the compound represented by the formula (9-3) are elementary substances or derivatives of substituted or unsubstituted benzofluoranthene.
- Examples of the compound represented by the formula (9-4) are elementary substances or derivatives of substituted or unsubstituted benzofluoranthene.
- the naphthalene derivative is exemplified by a formula (9-5) below.
- R 1 to R 8 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the naphthalene derivative is exemplified by a formula (9-6) below.
- R 1 to R 10 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the chrysene derivative is exemplified by a formula (9-7) below.
- R 1 to R 12 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the polyaromatic skeleton is preferably benzo[c]phenanthrene or its derivative.
- the benzo[c]phenanthrene derivative is exemplified by a formula (9-8) below.
- R 1 to R 9 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the polycyclic aromatic skeleton is preferably benzo[c]chrysene or its derivative.
- the benzo[c]phenanthrene derivative is exemplified by a formula (9-9) below.
- R 1 to R 11 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the polycyclic aromatic skeleton is preferably dibenzo[c,g]phenanthrene represented by a formula (9-10) below or its derivative.
- the polycyclic aromatic skeleton is preferably fluoranthene or its derivative.
- the fluoranthene derivative is exemplified by a formula (9-11) below.
- X 12 to X 21 each represent a hydrogen atom; halogen atom; linear, branched or cyclic alkyl group; linear, branched or cyclic alkoxy group; substituted or unsubstituted aryl group; or substituted or unsubstituted heteroaryl group.
- the polycyclic aromatic skeleton is preferably triphenylene or its derivative.
- the triphenylene derivative is exemplified by a formula (9-12) below.
- R 1 to R 6 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- the polycyclic aromatic compound may be represented by a formula (9-13) below.
- Ra and Rb represent the same as Ra and Rb in the formulae (9A) to (9C).
- the single or plural substituents are an alkyl group having 1 to 20 carbon atoms, haloalkyl group having 1 to 20 carbon atoms, cycloalkyl group having 5 to 18 carbon atoms, silyl group having 3 to 20 carbon atoms, cyano group or halogen atom, while substituents for the naphthalene rings other than Ra and Rb are further allowed to be an aryl group having 6 to 22 carbon atoms.
- Ra and Rb each preferably represent a group selected from fluorene ring, phenanthrene ring, triphenylene ring, benzophenanthrene ring, dibenzophenanthrene ring, benzotriphenylene ring, fluoranthene ring, benzochrysene ring, benzo[b]fluoranthene ring and picene ring.
- the second host material is preferably a monoamine derivative represented by any one of formulae (10) to (12) below.
- Ar 111 , Ar 112 and Ar 113 each are a substituted or unsubstituted aryl group or heteroaryl group.
- the aryl group has 6 to 50 ring carbon atoms (preferably 6 to 30 ring carbon atoms, more preferably 6 to 20 ring carbon atoms).
- the aryl group are a phenyl group, naphthyl group, phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, acenaphthenyl group, and diazaphenanthrenyl group.
- a phenyl group or naphthyl group is preferable.
- the heteroaryl group has 5 to 50 ring atoms (preferably 6 to 30 ring atoms, more preferably 6 to 20 ring atoms).
- Examples of the heteroaryl group are a pyrimidyl group and diazaphenanthrenyl group.
- At least one of Ar 111 , Ar 112 and Ar 113 is preferably a fused aromatic hydrocarbon group selected from a phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, and diazaphenanthrenyl group.
- a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable.
- the fused aromatic hydrocarbon group is unsubstituted.
- Ar 111 and Ar 112 each are preferably a phenyl group or naphthyl group, and Ar 113 is preferably a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- Ar 114 , Ar 115 and Ar 117 each are a substituted or unsubstituted aryl group or heteroaryl group.
- aryl group or heteroaryl group are the same as those defined as the aryl group or heteroaryl group for Ar 111 , among which a phenyl group or naphthyl group is preferable.
- Ar 116 is a substituted or unsubstituted arylene group or heteroarylene group.
- the arylene group has 6 to 50 ring carbon atoms (preferably 6 to 30 ring carbon atoms, more preferably 6 to 20 ring carbon atoms).
- the arylene group are a phenylene group, naphthylene group, phenanthrenylene group, naphthacenylene group, pyrenylene group, biphenylene group, terphenylenylene group, benzophenanthrenylene group, dibenzophenanthrenylene group, benzochrysenylene group, dibenzochrysenylene group, fluoranthenylene group, benzofluoranthenylene group, triphenylenylene group, benzotriphenylenylene group, dibenzotriphenylenylene group, picenylene group, benzopicenylene group, and dibenzopicenylene group.
- a phenylene group or naphthylene group is preferable.
- the heteroaryl group has 5 to 50 ring atoms (preferably 6 to 30 ring atoms, more preferably 6 to 20 ring atoms).
- Examples of the heteroaryl group are a pyridylene group, pyrimidylene group, dibenzofuranylene group, and dibenzothiophenylene group.
- Ar 117 is preferably a fused aromatic hydrocarbon group selected from a phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, and dibenzopicenyl group.
- a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable.
- the fused aromatic hydrocarbon group is unsubstituted.
- Ar 114 and Ar 115 each are a phenyl group or naphthyl group
- Ar 116 is a phenyl group or naphthyl group
- Ar 117 is a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- Ar 118 , Ar 119 and Ar 121 are a substituted or unsubstituted aryl group or heteroaryl group.
- aryl group or heteroaryl group are the same as those defined as the aryl group or heteroaryl group for Ar 111 and are preferably a phenyl group.
- Ar 120 is a substituted or unsubstituted arylene group or heteroarylene group and the same as those defined as the arylene group or heteroarylene group for Ar 116 .
- Ar 120 is preferably a phenylene group or naphthylene group.
- n is an integer of 2 to 5, preferably 2 to 4, more preferably 2 to 3.
- Ar 120 may be mutually the same or different.
- Ar 121 is preferably a fused aromatic hydrocarbon group selected from a phenyl group, naphthyl group, phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, and diazaphenanthrenyl group.
- a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable.
- Ar 118 and Ar 119 each are preferably a phenyl group or naphthyl group;
- Ar 120 is preferably a phenylene group or naphthylene group;
- Ar 121 is preferably a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- the substituent(s) is preferably an alkyl group having 1 to 20 carbon atoms, haloalkyl group having 1 to 20 carbon atoms, cycloalkyl group having 3 to 18 carbon atoms, aryl group having 6 to 30 ring carbon atoms, silyl group having 3 to 20 carbon atoms, cyano group, and halogen atom.
- alkyl group examples include a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, 1-methylpropyl group and 1-propylbutyl group.
- Examples of the aryl group are the same as those for Ar 101 .
- the haloalkyl group is exemplified by a 2,2,2-trifluoroethyl group.
- cycloalkyl group examples include a cyclopropyl group, cyclobutyl group, cyclopentyl group, cyclohexyl group and cyclooctyl group.
- silyl group examples are a trimethylsilyl group and triethylsilyl group.
- halogen atom examples include fluorine, chlorine, bromine, and iodine.
- the hydrogen atom of the monoamine derivatives represented by the formulae (10) to (12) includes light hydrogen and deuterium.
- Carbon atoms forming a ring (ring carbon atoms) mean carbon atoms forming a saturated ring, unsaturated ring, or aromatic ring.
- “Atoms forming a ring (ring atoms)” mean carbon atoms and hetero atoms forming a ring including a saturated ring, unsaturated ring, or aromatic ring.
- an aromatic amine compound is used as the second host material.
- aromatic amine compound is preferably a compound represented by the formula (13) or (14).
- X 3 represents a substituted or unsubstituted arylene group having 10 to 40 ring carbon atoms
- a 3 to A 6 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- a 7 to A 9 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- the second host material represented by the formula (13) or (14) is preferably represented by formulae (15) to (19).
- a 10 to A 19 each represent a substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 40 carbon atoms, substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic amino group, or substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic heterocyclic group;
- a 10 , A 13 , A 15 and A 17 are adapted to be respectively bonded to A 11 , A 14 , A 16 and A 18 to form a ring;
- X 4 to X 9 represent a single bond or a linking group having 1 to 30 carbon atoms
- Y 6 to Y 24 represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted alkenyl group having 2 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted alkylsilyl group having 3 to 20 carbon atoms, substituted or unsubstituted arylsilyl group having 8 to 40 carbon atoms, substituted or unsubstituted aralkylsilyl group having 8 to
- X A , X B , X C , X D , X E each represent a sulfur atom, an oxygen atom or a monoaryl-substituted nitrogen atom.
- An organic EL device preferably contains a metal complex as the second host material.
- the metal complex is preferably represented by a formula (20) below.
- ligands L 11 , L 12 and L 13 are independently selected from a structure represented by a formula (21) below; M 11 is a divalent metal; and Q 11 is a monovalent anion induced from inorganic or organic acids.
- Xb is O, S or Se
- a-ring is oxazole, thiazole, imidazoles, oxadiazole, thiadiazole, benzooxazole, benzothiazole, benzoimidazole, pyridine, or quinoline
- R 121 to R 124 are independently hydrogen, an alkyl group having 1 to 5 carbon atoms, halogen, silyl group or aryl group having 6 to 20 carbon atoms, which may be bonded to an adjacent substituent via alkylene or alkenylene to form a fused ring.
- the pyridine and quinoline may be bonded to R1 to form a fused ring.
- the a-ring and the aryl group for R 121 to R 124 may be further substituted by a C1-C5 alkyl group, halogen, C1-C5 alkyl group having a halogen substituent, phenyl group, naphthyl group, silyl group, or amino group.
- the ligands L 11 , L 12 and L 13 are independently selected from the following structures.
- X and R 1 to R 4 represent the same as Xb and R 121 to R 124 in the formula (21); Y is O, S or NR 21 ; Z is CH or N; R 11 to R 16 are independently hydrogen, a C1-C5 alkyl group, halogen, C1-C5 alkyl group having a halogen substituent, phenyl group, naphthyl group, silyl group, or amino group; and R 11 to R 14 may be bonded to an adjacent substituent via alkylene or alkenylene to form a fused ring.
- the ligands L 11 , L 12 and L 13 of the compound may be the same and can be selected from the following structures.
- X is O, S or Se
- R 2 , R 3 , R 12 and R 13 are independently hydrogen, methyl, ethyl, n-propyl, isopropyl, fluorine, chlorine, trifluoromethyl, phenyl, naphthyl, fluorenyl, trimethylsilyl, triphenylsilyl, t-butyldimethylsilyl, dimethylamine, diethylamine, or diphenylamine.
- phenyl, naphthyl, fluorenyl are further substituted by fluorine, chlorine, trimethylsilyl, triphenylsilyl, t-butyldimethylsilyl, dimethylamine, diethylamine, or diphenylamine
- the metal complex is preferably a zinc complex. Examples of such a preferable zinc complex are shown below.
- the second host material may be compounds represented by formulae (22) to (24) below.
- X 101 to X 108 are a nitrogen atom or C—Ar 131 .
- Ar 131 represent a hydrogen atom, a fluorine atom, a cyano group, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted
- Adjacent ones of X 101 to X 108 may be bonded to each other to form a ring structure.
- B 1 and B 2 represent a group represented by a formula (25A) or (25B) below.
- M 1 and M 2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M 1 and M 2 may be the same or different;
- L 5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- M 3 and M 4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 2 to 40 ring carbon atoms; M 3 and M 4 may be the same or different; L 6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- the formulae (25A) and (7A) are respectively the same as the formulae (25B) and (7B).
- M 1 to M 4 and L 5 to L 6 are the same as those described in relation to the formulae (7A) and (7B).
- the second host material may be compounds represented by the above formula (1) and having a different structure from that of the first host material.
- the emitting layer may also preferably contain an assistance material for assisting injection of charges.
- the emitting layer is formed of a host material that exhibits a wide energy gap, a difference in ionization potential (Ip) between the host material and the hole injecting/transporting layer etc. becomes so large that injection of the holes into the emitting layer becomes difficult, which may cause a rise in a driving voltage required for providing sufficient luminance.
- Ip ionization potential
- introducing a hole-injectable/transportable assistance material for assisting injection of charges in the emitting layer can contribute to facilitation of the injection of the holes into the emitting layer and to reduction of the driving voltage.
- the assistance material for assisting the injection of charges for instance, a typical hole injecting/transporting material or the like can be used.
- the assistance material for assisting the injection of charges are a triazole derivative, oxadiazole derivative, imidazoles derivative, polyarylalkane derivative, pyrazoline derivative, pyrazolone derivative, phenylenediamine derivative, arylamine derivative, amino-substituted chalcone derivative, oxazole derivative, styrylanthracene derivative, fluorenone derivative, hydrazone derivative, silazane derivative, polysilane copolymer, aniline copolymer, and conductive polymer oligomer (particularly, a thiophene oligomer).
- the hole injecting material is exemplified by the above.
- the hole injecting material is preferably a porphyrin compound, aromatic tertiary amine compound and styryl amine compound, particularly preferably aromatic tertiary amine compound.
- NPD 4,4′-bis(N-(1-naphthyl)-N-phenylamino)biphenyl
- MTDATA 4,4′,4′′-tris(N-(3-methylphenyl)-N-phenylamino)triphenylamine
- a hexaazatriphenylene derivative and the like may be also preferably used as the hole injecting material.
- inorganic compounds such as p-type Si and p-type SiC can also be used as the hole-injecting material.
- Example(s) the invention will be described in further detail by exemplifying Example(s) and Comparison(s). However, the invention is not limited by the description of Example(s).
- the intermediate body 1-1 (20 g, 69.7 mmol) and benzamidine hydrochloride (10.8 g, 69.7 mmol) were added to ethanole (300 mL). Sodium hydroxide (5.6 g, 140 mmol) was further added thereto and heated to reflux at room temperature for 8 hours. A precipitated solid was separated by filtration. Then, the obtained solid was washed with hexane. A white solid intermediate body 1-2 (10.3 g, a yield rate 38%) was obtained.
- Carbazole (15 g, 92.6 mmol) was added to ethanol (70 mL). Sulfuric acid (6 mL), water (3 mL), HIO 4 .2H 2 O (8.2 g, 35.9 mmol) and I 2 (9.1 g, 35.9 mmol) were added thereto and stirred at room temperature for 4 hours. Water was added to the reaction solution and a precipitated solid was separated by filtration. Then, the obtained solid was washed with methanol. By dissolving the obtained solid in heated toluene for recrystallization, an intermediate body 1-3 (5.1 g, a yield rate 18.8%) was obtained.
- N-phenylcarbazolyl-3-boronic acid 2.0 g, 7.0 mmol
- the intermediate body 1-3 (2.05 g, 7.0 mmol)
- Pd(PPh 3 ) 4 (0.15 g, 0.14 mmol)
- toluene 20 mL
- an aqueous solution of 2M sodium carbonate (10.5 mL)
- the intermediate body 1-2 (2.28 g, 5.88 mmol), the intermediate body 1-4 (2.4 g, 5.88 mmol), CuI (0.56 g, 2.9 mmol), tripotassium phosphate (2.5 g, 11.8 mmol), anhydrous dioxane (30 mL) and cyclohexane diamine (0.33 g, 2.9 mmol) were added together in sequential order, and stirred at 100 degrees C. for 8 hours.
- the intermediate body 1-1 (6.49 g, 22.6 mmol), phenacylpyridinium bromide (12.7 g, 45.6 mmol), ammonium acetate (45 g), acetic acid (200 mL), and N,N-dimethylformamide (200 mL) were heated to reflux and stirred for 8 hours.
- the reaction solution was put into an ice water and a precipitated solid was separated by filtration. Then, the obtained solid was washed with methanol. The obtained solid was refined by silica-gel column chromatography (dissolving solvent: hexane/methylene chloride), whereby an intermediate body 2-1 (3.7 g, a yield rate 42%) was obtained.
- the intermediate body 2-1 (2.81 g, 7.3 mmol), the intermediate body 1-4 (3.0 g, 7.3 mmol), Cut (1.4 g, 7.3 mmol), tripotassium phosphate (2.3 g, 11 mmol), anhydrous dioxane (30 mL) and cyclohexane diamine (0.84 g, 7.3 mmol) were added together in sequential order, and stirred at 100 degrees C. for 8 hours.
- intermediate body 3-1 (8 g, 29.9 mmol), p-chlorophenylboronic acid (5.1 g, 32.9 mmol), tetrakis(triphenylphosphine)palladium (0.63 g, 0.6 mmol), toluene (60 mL) and an aqueous solution of 2M sodium carbonate (30 mL) were added together in sequential order, and heated to reflux for 8 hours.
- the intermediate body 3-2 (6.5 g, 18.9 mmol), the intermediate body 1-4 (7.7 g, 18.9 mmol), palladium acetate (0.085 g, 0.38 mmol), sodium t-butoxide (2.72 g, 28.4 mmol), anhydrous toluene (60 mL), and tri-t-butyl phosphine (0.077 g, 0.38 mmol) were sequentially mixed, and stirred at 90 degrees C. for 8 hours.
- 3,3′-dicarbazolyl was synthesized by a method disclosed in WO2006-25186.
- the intermediate body 3-1 (1.0 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours.
- the intermediate body 6-1 (1.5 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours.
- an intermediate body 7-1 was firstly synthesized by applying a method described in a document (J. Bergman, A. Brynolf, B. Elman and E. Vuorinen, Tetrahedron, 42, 3697-3706(1986)). Specifically, to a three-necked flask (500 ml), 1M tetrahydrofuran solution of phenylmagnesium bromide (100 ml, 100 mmol) was added. Dry ether (100 ml) was further added and heated to reflux in an oil bath at 45 degrees C.
- the intermediate body 7-1 (1.4 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 7 (2.0 g, a yield of 75%) was obtained.
- the intermediate body 8-1 (1.4 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 8 (1.9 g, a yield of 71%) was obtained.
- a glass substrate (size: 25 mm ⁇ 75 mm ⁇ 1.1 mm) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV (Ultraviolet)/ozone-cleaned for 30 minutes.
- the glass substrate having the transparent electrode was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, and a hole injecting layer was initially formed by depositing a compound A onto the substrate to be 40 nm thick to cover a surface of the glass substrate where a transparent electrode line was provided. Next, a compound B was deposited onto the hole injecting layer to be 20 nm thick, and a hole transporting layer was obtained.
- a phosphorescent-emitting layer was obtained by co-depositing the compound 1 used as a phosphorescent host material and Ir(Ph-ppy) 3 used as a phosphorescent dopant material onto the hole transporting layer to be 40 nm thick.
- the concentration of Ir(Ph-ppy) 3 was 20 mass %.
- a 30-nm-thick compound C, 1-nm-thick LiF and 80-nm-thick metal Al are sequentially layered to obtain a cathode.
- LiF which is an electron injectable electrode, was formed at a speed of 1 ⁇ /min.
- Example 1 the compounds 2 to 6 below were used in place of the compound 1 to manufacture organic EL devices 2 to 6.
- the organic EL devices according respectively to Comparisons 1 to 4 were formed in the same manner as in Example 1 except that the following comparative compounds D to G were respectively used as a host material in place of the compound 1 in Example 1.
- the organic EL devices manufactured in Examples 1 to 6 and Comparisons 1 to 4 were driven by direct-current electricity to emit light, where luminescent performance was evaluated and time elapsed until an initial luminescence intensity of 20,000 cd/m 2 was reduced to the half was measured. The results are shown in Table 4.
- Table 4 shows that the compounds of the invention used in Examples 1 to 6 have a significantly long luminance half-life and a high luminous efficiency while being capable of low-voltage drive compared with those of Comparisons 1 to 4.
- a glass substrate (size: 25 mm ⁇ 75 mm ⁇ 1.1 mm thick) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV/ozone-cleaned for 30 minutes.
- the glass substrate having the transparent electrode line was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, so that the following electron accepting compound (C-1) was deposited to form a 5-nm thick C-1 film on a surface of the glass substrate where the transparent electrode line was provided so as to cover the transparent electrode.
- the following aromatic amine derivative (X1) was deposited as a first hole transporting material to form a 50-nm thick first hole transporting layer.
- the following aromatic amine derivative (X2) was deposited as a second hole transporting material to form a 60-nm thick second hole transporting layer.
- the compound 1 obtained in Synthesis Example 1 was deposited to form a 45-nm thick emitting layer.
- the following compound (D3) was co-deposited as a phosphorescent material.
- a concentration of the compound D3 was 8.0 mass %. This co-deposited film serves as the emitting layer.
- the ET1 film serves as the electron transporting layer.
- a 1-nm thick film of LiF was formed as an electron-injecting electrode (cathode) at a film-forming speed of 0.1 A/min.
- Metal (Al) was deposited on the LiF film to form an 80-nm thick metal cathode, thereby providing an organic electroluminescence device.
- the organic EL devices according to Examples 8 to 14 were manufactured in the same manner as that in Example 7 except that the compounds 2 to 8 were used in place of the compound 1 as materials for the emitting layer.
- luminous efficiency was measured when the device was driven by DC constant current at the initial luminescence of 2000 cd/m 2 at the room temperature, and the time elapsed until a half-life of emission was measured when the device was driven by DC constant current at the initial luminescence of 5000 cd/m 2 at the room temperature. The results are shown in Table 5.
- the organic EL devices according to Comparisons 5 and 7 were manufactured in the same manner as that in Example 7 except that the comparative compounds D and F were used in place of the compound 1 as materials for the emitting layer.
- luminous efficiency was measured when the device was driven by DC constant current at the initial luminescence of 2000 cd/m 2 at the room temperature, and the time elapsed until a half-life of emission was measured when the device was driven by DC constant current at the initial luminescence of 5000 cd/m 2 at the room temperature. The results are shown in Table 5.
- the table 5 shows that the compounds of the invention also function as a red phosphorescent host material.
- the organic EL devices according respectively to Examples 15 to 18 and Comparison 7 were formed in the same manner as in Example 1 except that the materials, a thickness of each of the layers and a concentration of each of the emitting materials were changed as shown in Table 6.
- Table 6 figures in parentheses without a unit indicate a thickness of each of the layers (unit: nm).
- a structure of HT-7 is shown below.
- Table 7 shows physical properties of the host materials used in Examples 15 to 18 and Comparison 7.
- a method for measuring each of the physical properties is as follows.
- Ip Ionization Potential
- Ionization potential was measured in the atmosphere by using a photoelectron spectrometer (AC-1 manufactured by Riken Keiki Co., Ltd.). Specifically, ionization potential was measured by irradiating the materials with light and measuring the amount of electrons generated by charge separation at that time.
- Affinity was calculated based on measurement values of ionization potential Ip and energy gap Eg.
- a calculation equitation is as follows.
- the energy gap was measured from an absorption end of absorption spectrum of benzene. Specifically, the absorption spectrum is measured with a commercially available ultraviolet-visible spectrophotometer, and the energy gap is calculated from a wavelength at which the absorption spectrum appears.
- the optical energy gap S1 (also referred to as singlet energy) is a difference between a conduction level and a valence level.
- the optical energy gap was obtained by converting into energy a wavelength value at an intersection of a long-wavelength-side tangent line in an absorbing spectrum of a toluene-diluted solution of each material and a base line in the absorbing spectrum (zero absorption).
- the triplet energy gap T1 of the material may be exemplarily defined based on the phosphorescence spectrum.
- the triplet energy gap T1 was defined as follows in Examples.
- the sample for phosphorescence measurement was put into a quartz cell, cooled to 77K and irradiated with exciting light, so that a wavelength of phosphorescence radiated therefrom was measured.
- a tangent line is drawn to be tangent to a rising section adjacent to short-wavelength of the obtained phosphorescence spectrum, a wavelength value at an intersection of the tangent line and a base line is converted into energy value, and the converted energy value is defined as the triplet energy gap T1.
- a measurement machine F-4500 manufactured by Hitachi was used.
- Example 15 ITO(70)/Compound A(30)/HT-7(20)/PH1 + Ir(ppy) 3 (40, 90% + 10%)/ET2(30)/Liq(1)/Al(80)
- Example 16 ITO(70)/Compound A(30)/HT-7(20)/PH2 + Ir(ppy) 3 (40, 90% + 10%)/ET2(30)/LiF(1)/Al(80)
- Example 17 ITO(70)/Compound A(30)/HT-7(20)/PH3 + Ir(ppy) 3 (40, 90% + 10%)/ET2(30)/LiF(1)/Al(80)
- Example 18 ITO(130)/Compound A(50)/PH2 + Ir(piq) 3 (30, 92% + 8%)/ET2(30)/LiF(1)/Al(80) Comparison 7 ITO(70)/Compound A(30)/HT-7(20)/PH5 + Ir(ppy) 3 (40, 90% +
- a glass substrate (size: 25 mm ⁇ 75 mm ⁇ 1.1 mm) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV (Ultraviolet)/ozone-cleaned for 30 minutes.
- the glass substrate having the transparent electrode was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, and a hole injecting layer was initially formed by depositing a compound A onto the substrate to be 40 nm thick to cover a surface of the glass substrate where a transparent electrode line was provided. Next, a compound B was deposited onto the hole injecting layer to be 20 nm thick, and a hole transporting layer was obtained.
- a phosphorescent-emitting layer was obtained by co-depositing the compound 3 used as the first phosphorescent host material, Ir(Ph-ppy) 3 used as a phosphorescent dopant material, and HT-5 used as the following second host material onto the hole transporting layer to be 40 nm thick.
- the concentration of Ir(Ph-ppy) 3 was 20 mass % and the concentration of HT-5 was 10 mass %.
- a 30-nm-thick compound C, 1-nm-thick LiF and 80-nm-thick metal Al are sequentially layered to obtain a cathode.
- LiF which is an electron injectable electrode, was formed at a speed of 1 ⁇ /min.
- Example 19 HT-9 was used in place of HT-5 to manufacture an organic EL device 6 .
- Example 19 the compound D was used in place of the compound 3 to manufacture an organic EL device.
- luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m 2 was reduced to the half was measured. The results are shown in Table 9.
- HT-9 was used in place of HT-5 to manufacture an organic EL device.
- luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m 2 was reduced to the half was measured. The results are shown in Table 9.
- Table 9 shows that the compounds of the invention used in Examples 19 and 20 have a significantly long luminance half-life and a significantly high luminous efficiency as compared with those in Comparisons 8 and 9.
- the organic EL devices according respectively to Examples 21 to 26 were formed in the same manner as in Example 19 except that the materials, a thickness of each of the layers and a concentration of each of the emitting materials were changed as shown in Table 10.
- Table 7 above and Table 11 below show the chemical formulae and physical properties of the host material used in Examples 21 to 26.
- Example 21 ITO(70)/Compound A(30)/HT-7(20)/PH1 + PH5 + Ir(ppy) 3 (40, 45% + 45% + 10%)/ET2(30)/Liq(1)/Al(80)
- Example 22 ITO(70)/Compound A(30)/HT-7(20)/PH2 + PH6 + Ir(ppy) 3 (40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80)
- Example 23 ITO(70)/Compound A(30)/HT-7(20)/PH3 + PH6 + Ir(ppy) 3 (40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80)
- Example 24 ITO(70)/Compound A(30)/HT-7(20)/PH3 + PH7 + Ir(ppy) 3 (40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al)
- FIG. 2(A) shows an energy diagram in the emitting layer in Example 21.
- FIG. 2(B) shows an energy diagram in the emitting layer in Example 23.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
A biscarbazole derivative of the invention is represented by a formula (1) below.
In the formula (1): A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; X1 and X2 each are a linking group; Y1 to Y4 each represent a substituent; p and q represent an integer of 1 to 4; and r and s represent an integer of 1 to 3.
Description
- This application is a Continuation of U.S. Ser. No. 13/372,954 filed Feb. 14, 2012, which is a Continuation of U.S. Ser. No. 13/091,036, filed Apr. 20, 2011, which claims priority from Japanese Patent Application No. 2010-097317, filed Apr. 20, 2010, Japanese Patent Application No. 2010-291138, filed Dec. 27, 2010, U.S. Provisional Application No. 61/353,047, filed Jun. 9, 2010, and U.S. Provisional Application No. 61/433,084, filed Jan. 14, 2011, the entire contents of which are expressly incorporated by reference herein.
- 1. Field of the Invention
- The present invention relates to a biscarbazole derivative, a material for an organic electroluminescence device, and an organic electroluminescence device using those.
- 2. Description of Related Art
- A known organic electroluminescence device includes an organic thin-film layer between an anode and a cathode, the organic thin-film layer including an emitting layer, and emits light using exciton energy generated by a recombination of holes and electrons injected into the emitting layer (see Patent Literature 1: WO2003-080760, Patent Literature 2: Japanese Patent No.: 3139321, Patent Literature 3: Japanese Patent No.: 4357781, Patent Literature 4: JP-A-2003-151774, Patent Literature 5: JP-A-2008-135498, Patent Literature 6: JP-A-2009-21336, Patent Literature 7: JP-A-2008-214307).
- Such an organic EL device, which has the advantages as a self-emitting device, is expected to serve as an emitting device excellent in luminous efficiency, image quality, power consumption and thin design.
- In forming the emitting layer, a doping method, according to which an emitting material (dopant) is doped to a host, has been known as a usable method.
- The emitting layer formed by the doping method can efficiently generate excitons from electric charges injected into the host. With the exciton energy generated by the excitons being transferred to the dopant, the dopant can emit light with high efficiency.
- Recently, in order to improve performance of the organic electroluminescence device (hereinafter, occasionally referred to as an organic EL device), a doping method has been further studied to find a suitable host material.
- Such a host material is disclosed in, for instance,
Patent Literatures 1 to 7.Patent Literatures 1 to 7 disclose a compound including a carbazole skeleton and a nitrogen-containing aromatic ring in the same molecule and a compound including a plurality of carbazole skeletons in the same molecule, as shown in the following compounds I to VIII. - The compounds I and II disclosed in
Patent Literature 1 each have a structure in which a carbazole skeleton is bonded to a benzene ring and an electron-deficient nitrogen-containing hetero aromatic ring structure. A carbazole skeleton, which is represented by polyvinyl carbazole, has been known as a main skeleton of a hole transporting material. In contrast, the electron-deficient nitrogen-containing hetero aromatic ring structure has been known as a structure having a high electron transporting capability. In other words, the compounds I and II disclosed inPatent Literature 1 are materials for balancing charge transportation by combining a hole transporting skeleton and an electron transporting skeleton. - The compound I has only a single carbazole skeleton and lacks a hole transporting capability, so that a favorable luminescence property cannot be obtained. The compound II has two carbazolyl groups that are branched to left and right relative to a bond axis of a pyrimidine ring and a benzene ring (two conjugated aromatic ring). Accordingly, an overlapping margin of the carbazole skeleton between molecules is impaired, so that a hole transporting capability is insufficient and a re-bonding position of charges is likely to be closer to the anode. Consequently, favorable luminescence property and lifetime property cannot be obtained.
- In order to enlarge the overlapping margin between the molecules and exhibit a sufficient hole transporting capability, it has been proposed to incorporate a structure in which carbazole skeletons are linked in the molecules. For instance, the compounds III to VI disclosed in
Patent Literatures 2 to 5 have a structure in which two carbazole skeletons are linked. However, since none of the compounds III to VI has an electron-deficient nitrogen-containing hetero aromatic ring structure, adjustment of carrier balance between holes and electrons is difficult, so that a favorable luminescence property cannot be obtained. - The compound VII disclosed in
Patent Literature 6 has an electron-deficient nitrogen-containing hetero aromatic ring structure and a carbazole-linking structure. However, two carbazole skeletons are bonded to a carbon atom at 3-position by a nitrogen atom. In this structure, the two carbazole skeletons are twisted to each other to lose flatness. Accordingly, the overlapping margin between the molecules becomes small and a hole transporting capability becomes insufficient, so that favorable luminescence property and lifetime property cannot be obtained. - The compound VIII disclosed in Patent Literature 7 has a structure in which a bipyridyl group (a nitrogen-containing aromatic heterocyclic group) is bonded to a benzene ring of a carbazole skeleton. The compound is not disclosed as a phosphorescent host material although being used as a material for an electron transporting layer. However, since the compound is considered to exhibit a high electron transporting capability, when used as a host material, the compound provides a poor carrier balance within the emitting layer and fails to exhibit a favorable luminescence property.
- Accordingly, an object of the invention is to provide a novel biscarbazole derivative having a hole transporting capability and an electron transporting capability and exhibiting an excellent carrier balance, a material for an organic EL device (hereinafter, referred to as an organic-EL-device material) and a phosphorescent and long-life organic EL device using those.
- After dedicated study to achieve the above object, the inventors found that a compound including two carbazolyl groups and a nitrogen-containing heterocyclic group effectively works for optimizing a carrier balance in the emitting layer of an organic EL device, and achieved the invention.
- Specifically, a biscarbazole derivative according to an aspect of the invention is represented by a formula (1) below. Herein, “hydrogen” is meant to also include deuterium.
- In the formula (1): A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 carbon atoms forming a ring (hereinafter, referred to as ring carbon atoms);
- A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- X1 and X2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- Y1 to Y4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- adjacent ones of Y1 to Y4 may be bonded to each other to form a ring structure;
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- when p and q are an integer of 2 to 4 and r and s are an integer of 2 to 3, a plurality of Y1 to Y4 may be the same or different.
- When Y1 to Y4 are bonded to each other to form a ring structure, the ring structure is exemplified by structures represented by the following formulae.
- The biscarbazole derivative according to the aspect of the invention is preferably represented by a formula (2) below.
- In the formula (2), A1, A2, X1, X2, Y1 to Y4, p, q, r and s represent the same as A1, A2, X1, X2, Y1 to Y4, p, q, r and s in the formula (1).
- Moreover, in the biscarbazole derivative according to the above aspect of the invention, A1 in the formula (1A) or (1B) is preferably selected from the group consisting of a substituted or unsubstituted pyridine ring, substituted or unsubstituted pyrimidine ring and substituted or unsubstituted triazine ring, more preferably selected from a substituted or unsubstituted pyrimidine ring or substituted or unsubstituted triazine ring, particularly preferably a substituted or unsubstituted pyrimidine ring.
- The biscarbazole derivative according to the aspect of the invention is preferably represented by a formula (3) below in the formula (2)
- In the formula (3): A2, X1, Y1 to Y4, p, q, r and s represent the same as A2, X1, Y1 to Y4, p, q, r and s of the formula (1); Y5 represent the same as Y1 to Y4 of the formula (1); t represents an integer from 1 to 3; and when t is an integer of 2 to 3, a plurality of Y5 may be the same or different.
- Moreover, in the biscarbazole derivative according to the aspect of the invention represented by the formula (1) or (2), A1 is preferably a substituted or unsubstituted quinazoline ring.
- The organic-EL-device material according to another aspect of the invention contains the above biscarbazole derivative.
- The organic EL device according to still another aspect of the invention includes: a cathode; an anode; and a plurality of organic thin-film layers provided between the cathode and the anode, and the organic thin-film layer including an emitting layer, in which at least one layer of the organic thin-film layers contains the above-described organic-EL-device material.
- In the organic EL device according to the above aspect of the invention, the emitting layer preferably contains the organic-EL-device material according to the aspect of the invention as a host material.
- Also preferably in the organic EL device according to the above aspect of the invention, the emitting layer includes a phosphorescent material.
- The phosphorescent material is preferably an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- The organic EL device according to further aspect of the invention includes: a cathode; an anode; and a plurality of organic thin-film layers provided between a cathode and an anode, and the organic thin-film layer includes an emitting layer, in which at least one of the organic thin-film layers is the emitting layer containing a first host material, a second host material and a phosphorescent material providing phosphorescence, the first host material being a compound represented by a formula (4) below.
- In the formula (4): A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
-
- A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- X1 and X2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- Y1 to Y4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- adjacent ones of Y1 to Y4 may be bonded to each other to form a ring structure;
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- when p and q are an integer of 2 to 4 and r and s are an integer of 2 to 3, a plurality of Y1 to Y4 may be the same or different.
- When Y1 to Y4 are bonded to each other to form a ring structure, the ring structure is exemplified by the same structures as ones listed when Y1 to Y4 are bonded to each other to form a ring structure in the formula (1).
- In the organic EL device according to the above aspect of the invention, the second host material is preferably represented by either one of a formula (5) or (6) below.
-
(Cz−)aA3 (5) -
Cz(−A3)b (6) - In the formula (5) or (6): Cz represents a substituted or unsubstituted arylcarbazolyl group or carbazolylaryl group;
- A3 represents a group represented by a formula (7A) or (7B) below; and
- a and b each represent an integer of 1 to 3.
-
(M1)c−(L5)d−(M2)e (7A) - In the formula (7A): M1 and M2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M1 and M2 may be the same or different;
- L5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
-
(M3)c−(L6)d−(M4)e (7B) - In the formula (7B): M3 and M4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 6 to 40 ring carbon atoms; M3 and M4 may be the same or different;
- L6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
- In the organic EL device according to the above aspect of the invention, the second host material is preferably represented by a formula (8) below.
- In the formula (8): R101 to R106 each independently represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms, substituted or unsubstituted arylthio group having 6 to 20 carbon atoms, substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms or cyano group;
- at least one of R101 to R106 is a substituted or unsubstituted 9-carbazolyl group, substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms, or -L-9-carbazolyl group;
- L represents a substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms, substituted or unsubstituted arylthio group having 6 to 20 carbon atoms, or substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms;
- Xa represents a sulfur atom, oxygen atom or N—R108; and
- R108 represents the same as R101 to R106.
- In the organic EL device according to the aspect of the invention, the second host material is preferably a compound selected from the group consisting of polycyclic aromatic compounds represented by formulae (9A), (9B) and (9C) below.
-
Ra—Ar101—Rb (9A) -
Ra—Ar101—Ar102—Rb (9B) -
Ra—Ar101—Ar102—Ar103—Rb (9C) - In the formulae (9A) to (9C): Ar101, Ar102, Ar103, Ra and Rb represent a polycyclic aromatic skeleton having 6 to 60 ring carbon atoms selected from a substituted or unsubstituted benzene ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted chrysene ring, substituted or unsubstituted fluoranthene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzophenanthrene ring, substituted or unsubstituted dibenzophenanthrene ring, substituted or unsubstituted triphenylene ring, substituted or unsubstituted benzo[a]triphenylene ring, substituted or unsubstituted benzochrysene ring, substituted or unsubstituted benzo[b]fluoranthene ring, substituted or unsubstituted fluorene ring and substituted or unsubstituted picene ring.
- Moreover, in the organic EL device according to the aspect of the invention, in the formulae (9A) to (9C), either one or both of Ra and Rb are preferably selected from the group consisting of a substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzo[c]phenanthrene ring and substituted or unsubstituted fluoranthene ring.
- In the organic EL device according to the above aspect of the invention, the second host material is preferably a monoamine derivative represented by any one of formulae (10) to (12) below.
- Ar111, Ar112 and Ar113 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar114, Ar115 and Ar117 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar116 is a substituted or unsubstituted arylene group or heteroarylene group.
- Ar118, Ar119 and Ar121 are a substituted or unsubstituted aryl group or heteroaryl group.
- Ar120 is a substituted or unsubstituted arylene group or heteroarylene group.
- n is an integer of 2 to 5: when n is 2 or more, Ar120 may be the same or different.
- In the organic EL device according to the aspect of the invention, the second host material is represented by a formula (13) or (14) below.
- In the formula (14): X3 represents a substituted or unsubstituted arylene group having 10 to 40 ring carbon atoms; and A3 to A6 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- In the formula (14), A7 to A9 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- In the organic EL device according to the above aspect of the invention, the second host material is more preferably represented by any one of formulae (15) to (19) below.
- In the formulae (15) to (19): A10 to A19 each represent a substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 40 carbon atoms, substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic amino group, or substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic heterocyclic group;
- A10, A13, A15 and A17 are adapted to be respectively bonded to A11, A14, A16 and A18 to form a ring;
- X4 to X9 represent a single bond or a linking group having 1 to 30 carbon atoms;
- Y6 to Y24 represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted alkenyl group having 2 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted alkylsilyl group having 3 to 20 carbon atoms, substituted or unsubstituted arylsilyl group having 8 to 40 carbon atoms, substituted or unsubstituted aralkylsilyl group having 8 to 40 carbon atoms, or substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms; and
- XA, XB, XC, XD, XE each represent a sulfur atom, an oxygen atom or a monoaryl-substituted nitrogen atom.
- In the organic EL device according to the above aspect of the invention, it is preferable that the emitting layer includes a host material and a phosphorescent material, the phosphorescent material being an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- In the organic EL device according to the above aspect of the invention, it is preferable that an electron injecting layer is provided between the cathode and the emitting layer and includes a nitrogen-containing cyclic derivative.
- In the organic EL device according to the above aspect of the invention, it is preferable that an electron transporting layer is provided between the cathode and the emitting layer and includes the above-described organic-EL-device material.
- In the organic EL device according to the above aspect of the invention, it is preferable that a reduction-causing dopant is present at an interfacial region between the cathode and the organic thin-film layer.
- The organic EL device according to the above aspect of the invention preferably includes a cathode, an anode, and an organic layer between the cathode and the anode, in which the organic layer include the biscarbazole derivative represented by the formulae (1) to (3).
- In the organic electroluminescence device according to the above aspect of the invention, the organic layer preferably includes a phosphorescent material, the phosphorescent material being an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
- According to the above aspect of the invention, since the biscarbazole derivative is used as the organic-EL-device material, a long-life organic electroluminescence device can be provided. Moreover, the organic-EL-device material is effective as organic-electron-device material for an organic solar cell, an organic semiconductor laser, a sensor using an organic substance and an organic TFT.
-
FIG. 1 schematically shows an exemplary arrangement of an organic electroluminescence device according to an exemplary embodiment of the invention. -
FIG. 2A shows an energy diagram of an emitting layer in an organic EL device according to Examples of the invention. -
FIG. 2B shows an energy diagram of an emitting layer in an organic EL device according to Examples of the invention. - The invention will be described below in detail.
- First of all, arrangement(s) of an organic EL device will be described below.
- The followings are representative arrangement examples of an organic EL device:
- (1) anode/emitting layer/cathode;
(2) anode/hole injecting layer/emitting layer/cathode;
(3) anode/emitting layer/electron injecting•transporting layer/cathode;
(4) anode/hole injecting layer/emitting layer/electron injecting•transporting layer/cathode;
(5) anode/organic semiconductor layer/emitting layer/cathode;
(6) anode/organic semiconductor layer/electron blocking layer/emitting layer/cathode;
(7) anode/organic semiconductor layer/emitting layer/adhesion improving layer/cathode;
(8) anode/hole injecting•transporting layer/emitting layer/electron injecting•transporting layer/cathode;
(9) anode/insulating layer/emitting layer/insulating layer/cathode;
(10) anode/inorganic semiconductor layer/insulating layer/emitting layer/insulating layer/cathode;
(11) anode/organic semiconductor layer/insulating layer/emitting layer/insulating layer/cathode;
(12) anode/insulating layer/hole injecting•transporting layer/emitting layer/insulating layer/cathode; and
(13) anode/insulating layer/hole injecting•transporting layer/emitting layer/electron injecting•transporting layer/cathode. - While the arrangement (8) is preferably used among the above, the arrangement of the invention is not limited to the above arrangements.
-
FIG. 1 schematically shows an exemplary arrangement of an organic EL device according to a first exemplary embodiment of the invention. - The
organic EL device 1 includes atransparent substrate 2, ananode 3, acathode 4 and an organic thin-film layer 10 positioned between theanode 3 and thecathode 4. - The organic thin-
film layer 10 includes a phosphorescent-emittinglayer 5 containing a phosphorescent host (a host material) and a phosphorescent dopant (a phosphorescent material). A layer such as a hole injecting/transportinglayer 6 may be provided between the phosphorescent-emittinglayer 5 and theanode 3 while a layer such as an electron injecting/transporting layer 7 may be provided between the phosphorescent-emittinglayer 5 and thecathode 4. - In addition, an electron blocking layer may be provided to the phosphorescent-emitting
layer 5 adjacent to theanode 3 while a hole blocking layer may be provided to the phosphorescent-emittinglayer 5 adjacent to thecathode 4. - With this arrangement, electrons and holes can be trapped in the phosphorescent-emitting
layer 5, thereby enhancing probability of exciton generation in the phosphorescent-emittinglayer 5. - It should be noted that a “fluorescent host” and a “phosphorescent host” herein respectively mean a host combined with a fluorescent dopant and a host combined with a phosphorescent dopant, and that a distinction between the fluorescent host and phosphorescent host is not unambiguously derived only from a molecular structure of the host in a limited manner.
- In other words, the fluorescent host herein means a material for forming a fluorescent-emitting layer containing a fluorescent dopant, and does not mean a host that is only usable as a host of a fluorescent material.
- Likewise, the phosphorescent host herein means a material for forming a phosphorescent-emitting layer containing a phosphorescent dopant, and does not mean a host that is only usable as a host of a phosphorescent material.
- It should be noted that the “hole injecting/transporting layer” herein means “at least either one of a hole injecting layer and a hole transporting layer” while the “electron injecting/transporting layer” herein means “at least either one of an electron injecting layer and an electron transporting layer.”
- The organic EL device according to this exemplary embodiment is formed on a light-transmissive substrate. The light-transmissive plate, which supports the organic EL device, is preferably a smoothly-shaped substrate that transmits 50% or more of light in a visible region of 400 nm to 700 nm.
- Specifically, a glass plate, a polymer plate, and the like are preferable.
- For the glass plate, materials such as soda-lime glass, barium/strontium-containing glass, lead glass, aluminosilicate glass, borosilicate glass, barium borosilicate glass and quartz can be used.
- For the polymer plate, materials such as polycarbonate, acryl, polyethylene terephthalate, polyether sulfide and polysulfone can be used.
- The anode of the organic EL device is used for injecting holes into the hole injecting layer, the hole transporting layer or the emitting layer. It is effective that the anode has a work function of 4.5 eV or more.
- Exemplary materials for the anode are alloys of indium-tin oxide (ITO), tin oxide (NESA), indium zinc oxide, gold, silver, platinum and copper.
- The anode may be made by forming a thin film from these electrode materials through methods such as vapor deposition and sputtering.
- When light from the emitting layer is to be emitted through the anode as in this embodiment, the anode preferably transmits more than 10% of the light in the visible region. Sheet resistance of the anode is preferably several hundreds Ω/square or lower. Although depending on the material of the anode, thickness of the anode is typically in a range of 10 nm to 1 μm, and preferably in a range of 10 to 200 nm.
- The cathode is preferably formed of a material with smaller work function in order to inject electrons into the electron injecting layer, the electron transporting layer and the emitting layer.
- Although a material for the cathode is subject to no specific limitation, examples of the material are indium, aluminum, magnesium, alloy of magnesium and indium, alloy of magnesium and aluminum, alloy of aluminum and lithium, alloy of aluminum, scandium and lithium, alloy of magnesium and silver and the like.
- Like the anode, the cathode may be made by forming a thin film from the above materials through a method such as vapor deposition or sputtering. In addition, the light may be emitted through the cathode.
- The emitting layer of the organic EL device is an organic thin-film layer having a function for providing conditions for recombination of the electrons and the holes to emit light.
- Injectability of the holes may differ from that of the electrons and transporting capabilities of the hole and the electrons (represented by mobilities of the holes and the electrons) may differ from each other.
- As a method of forming the emitting layer, known methods such as vapor deposition, spin coating and an LB method may be employed.
- The emitting layer is preferably a molecular deposit film.
- The molecular deposit film means a thin film formed by depositing a material compound in gas phase or a film formed by solidifying a material compound in a solution state or in liquid phase. The molecular deposit film is typically distinguished from a thin film formed by the LB method (molecular accumulation film) by differences in aggregation structures, higher order structures and functional differences arising therefrom.
- As disclosed in JP-A-57-51781, the emitting layer can be formed from a thin film formed by spin coating or the like, the thin film being formed from a solution prepared by dissolving a binder (e.g. a resin) and a material compound in a solvent.
- An organic EL device according to this exemplary embodiment includes: a cathode; an anode; and a single or a plurality of organic thin-film layers provided between the cathode and the anode, in which the organic thin-film layer(s) includes at least one emitting layer, and at least one of the organic thin-film layers includes at least one phosphorescent material and, as an organic-EL-device material, at least one biscarbazole derivative according to this exemplary embodiment (described later). It is also preferable that at least one emitting layer includes the biscarbazole derivative as the organic-EL-device material according to this exemplary embodiment and at least one phosphorescent material.
- The biscarbazole derivative according to this exemplary embodiment is represented by a formula (1) below.
- In the formula (1): A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms;
- X1 and X2 each are a linking group and independently represent a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- Y1 to Y4 independently represent a hydrogen atom, fluorine atom, cyano group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms;
- adjacent ones of Y1 to Y4 may be bonded to each other to form a ring structure;
- p and q represent an integer of 1 to 4; r and s represent an integer of 1 to 3; and
- when p and q are an integer of 2 to 4 and r and s are an integer of 2 to 3, a plurality of Y1 to Y4 may be the same or different.
- When Y1 to Y4 are bonded to each other to form a ring structure, the ring structure is exemplified by structures represented by the following formulae.
- The biscarbazole derivative represented by the formula (1) is more preferably represented by a formula (2) below.
- In the formula (2), A1, A2, X1, X2, Y1 to Y4, p, q, r and s are the same as those in the formula (1).
- In the formula (2), A1 and A2 are preferably a nitrogen-containing heterocyclic group at the same time. More preferably, A1 and A2 are a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- Moreover, A1 in the formula (2) is preferably selected from the group consisting of a substituted or unsubstituted pyridine ring, substituted or unsubstituted pyrimidine ring and substituted or unsubstituted triazine ring, more preferably selected from a substituted or unsubstituted pyrimidine ring or substituted or unsubstituted triazine ring.
- A1 in the formula (2) is further preferably a substituted or unsubstituted pyrimidine ring and is particularly preferably represented by a formula (3) below.
- In the formula (3), A2, X1, Y1 to Y4, p, q, r and s are the same as those in the formula (1); Y5 represents the same as Y1 to Y4 of the formula (1); t represents an integer from 1 to 3; and when t is an integer of 2 to 3, a plurality of Y5 may be the same or different.
- In the formula (3), A2 is preferably a nitrogen-containing heterocyclic group. More preferably, A2 is a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- In the formula (1) or (2), A1 is preferably a substituted or unsubstituted quinazoline ring.
- In the formulae (1) to (3), X1 is preferably a single bond or a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 30 ring carbon atoms, more preferably a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 30 ring carbon atoms, particularly preferably a benzene ring or naphthalene ring.
- When X1 is a substituted or unsubstituted benzene ring in the formulae (1) to (3), A1 and the carbazolyl group, which are bonded to X1, are preferably in meta positions or para positions. Particularly preferably, X1 is unsubstituted para-phenylene.
- In the formulae (1) and (2), the pyridine ring, pyrimidine ring and triazine ring are more preferably represented by the following formulae. In the formulae, Y and Y′ represent a substituent. Examples of the substituent are the same groups as those represented by Y1 to Y4 as described above. Y and Y′ may be the same or different. Preferred examples thereof are the substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, and the substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms. In the following formulae, * represents a bonding position to X1 or X2.
- In the formulae (1) and (2), the quinazoline ring is represented by the following formula. Y represents a substituent. u represents an integer of 1 to 5. When u is an integer of 2 to 5, a plurality of Y may be the same or different. As the substituent Y, the same groups as those for the above Y1 to Y4 are usable, among which preferred examples thereof are the substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, and the substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms. Also in the following formulae, * represents a bonding position to X1 or X2.
- In the formulae (1) to (3), the alkyl group, alkoxy group, haloalkyl group, haloalkoxy group and alkylsilyl group, which are represented by Y1 to Y5, may be linear, branched or cyclic.
- In the formulae (1) to (3), examples of the alkyl group having 1 to 20 carbon atoms are a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, n-nonyl group, n-decyl group, n-undecyl group, n-dodecyl group, n-tridecyl group, n-tetradecyl group, n-pentadecyl group, n-hexadecyl group, n-heptadecyl group, n-octadecyl group, neo-pentyl group, 1-methylpentyl group, 2-methylpentyl group, 1-pentylhexyl group, 1-butylpentyl group, 1-heptyloctyl group, 3-methylpentyl group, cyclopentyl group, cyclohexyl group, cycloheptyl group, cyclooctyl group and 3,5-tetramethylcyclohexyl group. Examples of the alkyl group having 1 to 10 carbon atoms are a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, cyclopentyl group, cyclohexyl group and cycloheptyl group.
- As the alkoxy group having 1 to 20 carbon atoms, an alkoxy group having 1 to 6 carbon atoms is preferable and specific examples thereof are a methoxy group, ethoxy group, propoxy group, butoxy group, pentyloxy group, and hexyloxy group.
- The haloalkyl group having 1 to 20 carbon atoms is exemplified by an haloalkyl group provided by substituting the alkyl group having 1 to 20 carbon atoms with one or more halogen atoms. Preferred one of the halogen atoms is fluorine. The haloalkyl group is exemplified by a trifluoromethyl group and a 2,2,2-trifluoroethyl group.
- The haloalkoxy group having 1 to 20 carbon atoms is exemplified by a haloalkoxy group provided by substituting the alkoxy group having 1 to 20 carbon atoms with one or more halogen atoms.
- Examples of the alkylsilyl group having 1 to 10 carbon atoms are a trimethylsilyl group, triethylsilyl group, tributylsilyl group, dimethylethylsilyl group, dimethylisopropylsilyl group, dimethylpropylsilyl group, dimethylbutylsilyl group, dimethyl-tertiary-butylsilyl group and diethylisopropylsilyl group.
- Examples of the arylsilyl group having 6 to 30 carbon atoms are a phenyldimethylsilyl group, diphenylmethylsilyl group, diphenyl-tertiary-butylsilyl group and triphenylsilyl group.
- Examples of the aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms are a pyroryl group, pyrazinyl group, pyridinyl group, indolyl group, isoindolyl group, furyl group, benzofuranyl group, isobenzofuranyl group, dibenzofuranyl group, dibenzothiophenyl group, quinolyl group, isoquinolyl group, quinoxalinyl group, carbazolyl group, phenantridinyl group, acridinyl group, phenanthrolinyl group, thienyl group and a group formed from a pyridine ring, pyrazine ring, pyrimidine ring, pyridazine ring, triazine ring, indol ring, quinoline ring, acridine ring, pirrolidine ring, dioxane ring, piperidine ring, morpholine ring, piperadine ring, carbazole ring, furan ring, thiophene ring, oxazole ring, oxadiazole ring, benzooxazole ring, thiazole ring, thiadiazole ring, benzothiazole ring, triazole ring, imidazole ring, benzoimidazole ring, pyrane ring and dibenzofuran ring. Among the above, the aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 10 ring carbon atoms is preferable.
- Examples of the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms are a phenyl group, naphthyl group, phenanthryl group, biphenyl group, terphenyl group, quarterphenyl group, fluoranthenyl group, triphenylenyl group, phenanthrenyl group, pyrenyl group, chrysenyl group, fluorenyl group, and 9,9-dimethylfluorenyl group. Among the above, the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 20 ring carbon atoms is preferable.
- When A1, A2, X1, X2 and Y1 to Y5 of the formulae (1) to (3) each have one or more substituents, the substituents are preferably a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkoxy group having 1 to 20 carbon atoms; linear, branched or cyclic haloalkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkylsilyl group having 1 to 10 carbon atoms; arylsilyl group having 6 to 30 ring carbon atoms; cyano group; halogen atom; aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms; or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- Examples of the linear, branched or cyclic alkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkoxy group having 1 to 20 carbon atoms; linear, branched or cyclic haloalkyl group having 1 to 20 carbon atoms; linear, branched or cyclic alkylsilyl group having 1 to 10 carbon atoms; arylsilyl group having 6 to 30 ring carbon atoms; aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms; and aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 ring carbon atoms are the above-described groups. The halogen atom is exemplified by a fluorine atom.
- Examples of compounds for the biscarbazole derivative according to this exemplary embodiment represented by the formulae (1) to (3) are as follows.
- An organic-EL-device material according to this exemplary embodiment contains the above biscarbazole derivative.
- The organic-EL-device material according to this exemplary embodiment includes the biscarbazole derivative represented by any one of the above formulae (1) to (3).
- The organic EL device according to this exemplary embodiment includes a cathode, an anode, and an organic layer between the cathode and the anode, in which the organic layer include the biscarbazole derivative any one of the above formulae (1) to (3).
- In the organic EL device according to this exemplary embodiment, the emitting layer may preferably contain the organic-EL-device material according to this exemplary embodiment.
- The organic EL device according to this exemplary embodiment may preferably contain the electron injecting/transporting layer, in which the electron injecting/transporting layer may preferably contain the organic-EL-device material according to this exemplary embodiment.
- The organic EL device according to this exemplary embodiment may preferably contain at least one of the electron injecting/transporting layer and the hole blocking layer that contains the organic-EL-device material according to this exemplary embodiment.
- The organic EL device according to this exemplary embodiment may preferably include the hole transporting layer (hole injecting layer) that contains the organic-EL-device material according to this exemplary embodiment.
- According to this exemplary embodiment, the phosphorescent material preferably contains a metal complex, and the metal complex preferably has a metal atom selected from Ir, Pt, Os, Au, Cu, Re and Ru, and a ligand. Particularly, the ligand preferably has an ortho-metal bond.
- The phosphorescent material is preferably a compound containing a metal selected from iridium (Ir), osmium (Os) and platinum (Pt) because such a compound, which exhibits high phosphorescence quantum yield, can further enhance external quantum efficiency of the emitting device. The phosphorescent material is more preferably a metal complex such as an iridium complex, osmium complex or platinum complex, among which an iridium complex and platinum complex are more preferable and ortho metalation of an iridium complex is the most preferable.
- Examples of such a preferable metal complex are shown below.
- According to this exemplary embodiment, at least one of the phosphorescent material contained in the emitting layer preferably emits light with the maximum wavelength of 450 nm to 720 nm.
- By doping the phosphorescent material (phosphorescent dopant) having such an emission wavelength to the specific host material used in this exemplary embodiment so as to form the emitting layer, the organic EL device can exhibit high efficiency.
- In the organic EL device according to this exemplary embodiment, a reduction-causing dopant may be preferably contained in an interfacial region between the cathode and the organic thin-film layer.
- With this arrangement, the organic EL device can emit light with enhanced luminance intensity and have a longer lifetime.
- The reduction-causing dopant may be at least one compound selected from an alkali metal, alkali metal complex, alkali metal compound, alkali earth metal, alkali earth metal complex, alkali earth metal compound, rare-earth metal, rare-earth metal complex, rare-earth metal compound and the like.
- Examples of the alkali metal are Na (work function: 2.36 eV), K (work function: 2.28 eV), Rb (work function: 2.16 eV), Cs (work function: 1.95 eV) and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable. Among the above, the reduction-causing dopant is preferably K, Rb or Cs, more preferably Rb or Cs, the most preferably Cs.
- Examples of the alkali earth metal are Ca (work function: 2.9 eV), Sr (work function: 2.0 to 2.5 eV), Ba (work function: 2.52 eV) and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable.
- Examples of the rare-earth metal are Sc, Y, Ce, Tb, Yb and the like, among which a substance having a work function of 2.9 eV or less is particularly preferable.
- Since the above preferable metals have particularly high reducibility, addition of a relatively small amount of the metals to an electron injecting zone can enhance luminance intensity and lifetime of the organic EL device.
- Examples of the alkali metal compound include an alkali oxide such as Li2O, Cs2O and K2O, and an alkali halide such as LiF, NaF, CsF and KF. LiF, Li2O, and NaF are preferable.
- Examples of the alkali earth metal compound include BaO, SrO, CaO and their mixture such as BaxSr1-xO (0<x<1) and BaxCa1-xO (0<x<1). BaO, SrO, and CaO are preferable.
- Examples of the rare earth metal compound include YbF3, ScF3, ScO3, Y2O3, Ce2O3, GdF3 and TbF3. YbF3, ScF3, and TbF3 are preferable.
- The alkali metal complex, alkali earth metal complex and rare earth metal complex are not specifically limited as long as they contain at least one metal ion of an alkali metal ion, an alkali earth metal ion and a rare earth metal ion. A ligand for each of the complexes is preferably quinolinol, benzoquinolinol, acridinol, phenanthridinol, hydroxyphenyl oxazole, hydroxyphenyl thiazole, hydroxydiaryl oxadiazole, hydroxydiaryl thiadiazole, hydroxyphenyl pyridine, hydroxyphenyl benzoimidazole, hydroxybenzo triazole, hydroxy fluborane, bipyridyl, phenanthroline, phthalocyanine, porphyrin, cyclopentadiene, β-diketones, azomethines, or a derivative thereof, but the ligand is not limited thereto.
- The reduction-causing dopant is added to preferably form a layer or an island pattern in the interfacial region. The layer of the reduction-causing dopant or the island pattern of the reduction-causing dopant is preferably formed by depositing the reduction-causing dopant by resistance heating deposition while an emitting material for forming the interfacial region or an organic substance as an electron-injecting material are simultaneously deposited, so that the reduction-causing dopant is dispersed in the organic substance. Dispersion concentration at which the reduction-causing dopant is dispersed in the organic substance is a mole ratio (organic substance to reduction-causing dopant) of 100:1 to 1:100, preferably 5:1 to 1:5.
- When the reduction-causing dopant forms the layer, the emitting material or the electron injecting material for forming the organic layer of the interfacial region is initially layered, and the reduction-causing dopant is subsequently deposited singularly thereon by resistance heating deposition to form a preferably 0.1 to 15 nm-thick layer.
- When the reduction-causing dopant forms the island pattern, the emitting material or the electron injecting material for forming the organic layer of the interfacial region is initially formed in an island shape, and the reduction-causing dopant is subsequently deposited singularly thereon by resistance heating deposition to form a preferably 0.05 to 1 nm-thick island shape.
- A ratio of the main component to the reduction-causing dopant in the organic EL device according to this exemplary embodiment is preferably a mole ratio (main component to reduction-causing dopant) of 5:1 to 1:5, more preferably 2:1 to 1:2.
- The electron injecting layer or the electron transporting layer, which aids injection of the electrons into the emitting layer, has a large electron mobility. The electron injecting layer is provided for adjusting energy level, by which, for instance, sudden changes of the energy level can be reduced.
- The organic EL device according to this exemplary embodiment preferably includes the electron injecting layer between the emitting layer and the cathode, and the electron injecting layer preferably contains a nitrogen-containing cyclic derivative as the main component. The electron injecting layer may serve as the electron transporting layer.
- It should be noted that “as the main component” means that the nitrogen-containing cyclic derivative is contained in the electron injecting layer at a content of 50 mass % or more.
- A preferable example of an electron transporting material for forming the electron injecting layer is an aromatic heterocyclic compound having in the molecule at least one heteroatom. Particularly, a nitrogen-containing cyclic derivative is preferable. The nitrogen-containing cyclic derivative is preferably an aromatic ring having a nitrogen-containing six-membered or five-membered ring skeleton, or a fused aromatic cyclic compound having a nitrogen-containing six-membered or five-membered ring skeleton.
- A preferable example of the nitrogen-containing cyclic derivative is a nitrogen-containing cyclic metal chelate complex represented by the following formula (A).
- R2 to R7 in the formula (A) each independently represent a hydrogen atom, a halogen atom, an oxy group, an amino group, a hydrocarbon group having 1 to 40 carbon atoms, an alkoxy group, an aryloxy group, an alkoxycarbonyl group, or an aromatic heterocyclic group. These groups may be substituted or unsubstituted.
- Examples of the halogen atom include fluorine, chlorine, bromine, and iodine. In addition, examples of the substituted or unsubstituted amino group include an alkylamino group, an arylamino group, and an aralkylamino group.
- The alkoxycarbonyl group is represented by —COOY′. Examples of Y′ are the same as the examples of the alkyl group. The alkylamino group and the aralkylamino group are represented by —NQ1Q2. Examples for each of Q1 and Q2 are the same as the examples described in relation to the alkyl group and the aralkyl group, and preferable examples for each of Q1 and Q2 are also the same as those described in relation to the alkyl group and the aralkyl group. Either one of Q1 and Q2 may be a hydrogen atom.
- The arylamino group is represented by —NAr1Ar2. Examples for each of Ar1 and Are are the same as the examples described in relation to the non-fused aromatic hydrocarbon group and the fused aromatic hydrocarbon group. Either one of Ar1 and Ar2 may be a hydrogen atom.
- M represents aluminum (Al), gallium (Ga) or indium (In), among which In is preferable.
- L in the formula (A) represents a group represented by the following formula (A′) or the following formula (A″).
- In the formula (A′), R8 to R12 each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 40 carbon atoms. Adjacent groups may form a cyclic structure. In the formula (A″), R13 to R27 each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 40 carbon atoms. Adjacent groups may form a cyclic structure.
- Examples of the hydrocarbon group having 1 to 40 carbon atoms represented by each of R8 to R12 and R13 to R27 in the formulae (A′) and (A″) are the same as those of R2 to R7 in the formula (A).
- Examples of a divalent group formed when an adjacent set of R8 to R12 and R13 to R27 forms a cyclic structure are a tetramethylene group, a pentamethylene group, a hexamethylene group, a diphenylmethane-2,2′-diyl group, a diphenylethane-3,3′-diyl group, a diphenylpropane-4,4′-diyl group and the like.
- Moreover, in this exemplary embodiment, the electron transporting layer may contain the biscarbazole derivatives represented by the formulae (1) to (3) (or the formulae (4) to (6)).
- As an electron transporting compound for the electron injecting layer or the electron transporting layer, 8-hydroxyquinoline or a metal complex of its derivative, an oxadiazole derivative and a nitrogen-containing heterocyclic derivative are preferable. An example of the 8-hydroxyquinoline or the metal complex of its derivative is a metal chelate oxinoid compound containing a chelate of oxine (typically 8-quinolinol or 8-hydroxyquinoline). For instance, tris(8-quinolinol) aluminum can be used. Examples of the oxadiazole derivative are as follows.
- In the formula, Ar17, Ar18, Ar19, Ar21, Ar22 and Ar25 each represent a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms. Ar17, Ar19 and Ar22 may be the same as or different from Ar18, Ar21 and Ar25 respectively. Examples of the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms are a phenyl group, biphenyl group, anthranil group, perylenyl group and pyrenyl group. Examples of the substituent therefor are an alkyl group having 1 to 10 carbon atoms, alkoxy group having 1 to 10 carbon atoms and cyano group.
- Ar20, Ar23 and Ar24 each represent a substituted or unsubstituted divalent aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms. Ar23 and Ar24 may be mutually the same or different.
- Examples of the divalent aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms are a phenylene group, naphthylene group, biphenylene group, anthranylene group, perylenylene group and pyrenylene group. Examples of the substituent therefor are an alkyl group having 1 to 10 carbon atoms, alkoxy group having 1 to 10 carbon atoms and cyano group.
- Such an electron transport compound is preferably an electron transport compound that can be favorably formed into a thin film(s). Examples of the electron transporting compounds are as follows.
- An example of the nitrogen-containing heterocyclic derivative as the electron transporting compound is a nitrogen-containing compound that is not a metal complex, the derivative being formed of an organic compound represented by one of the following general formulae. Examples of the nitrogen-containing heterocyclic derivative are a five-membered ring or six-membered ring derivative having a skeleton represented by the following formula (A) and a derivative having a structure represented by the following formula (B).
- In the formula (B), X represents a carbon atom or a nitrogen atom. Z1 and Z2 each independently represent a group of atoms capable of forming a nitrogen-containing heterocycle.
- Preferably, the nitrogen-containing heterocyclic derivative is an organic compound having a nitrogen-containing aromatic polycyclic group having a five-membered ring or six-membered ring. When the nitrogen-containing heterocyclic derivative includes such nitrogen-containing aromatic polycyclic series having plural nitrogen atoms, the nitrogen-containing heterocyclic derivative may be a nitrogen-containing aromatic polycyclic organic compound having a skeleton formed by a combination of the skeletons respectively represented by the formulae (A) and (B), or by a combination of the skeletons respectively represented by the formulae (A) and (C).
- A nitrogen-containing group of the nitrogen-containing aromatic polycyclic organic compound is selected from nitrogen-containing heterocyclic groups respectively represented by the following general formulae.
- In the formulae: R represents an aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms; aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms; alkyl group having 1 to 20 carbon atoms or alkoxy group having 1 to 20 carbon atoms; and n represents an integer in a range of 0 to 5. When n is an integer of 2 or more, plural R may be mutually the same or different.
- A preferable specific compound is a nitrogen-containing heterocyclic derivative represented by the following formula.
-
HAr-L1-Ar1—Ar2 - In the formula: HAr represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 40 ring carbon atoms; L1 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms; Ar1 represents a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 40 ring carbon atoms; and Ar2 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms.
- HAr is exemplarily selected from the following group.
- L1 is exemplarily selected from the following group.
- Ar1 is exemplarily selected from the following arylanthranil groups.
- In the formulae: R1 to R14 each independently represent a hydrogen atom, halogen atom, alkyl group having 1 to 20 carbon atoms, alkoxy group having 1 to 20 carbon atoms, aryloxy group having 6 to 40 ring carbon atoms, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms; and Ar3 represents aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 40 ring carbon atoms, or aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms.
- All of R1 to R8 of a nitrogen-containing heterocyclic derivative may be hydrogen atoms.
- Ar2 is exemplarily selected from the following group.
- Other than the above, the following compound (see JP-A-9-3448) can be favorably used for the nitrogen-containing aromatic polycyclic organic compound as the electron transporting compound.
- In the formula: R1 to R4 each independently represent a hydrogen atom, substituted or unsubstituted aliphatic group, substituted or unsubstituted alicyclic group, substituted or unsubstituted carbocyclic aromatic cyclic group or substituted or unsubstituted heterocyclic group; and X1 and X2 each independently represent an oxygen atom, sulfur atom or dicyanomethylene group.
- The following compound (see JP-A-2000-173774) can also be favorably used for the electron transporting compound.
- In the formula, R1, R2, R3 and R4, which may be mutually the same or different, each represent an aromatic hydrocarbon group or fused aromatic hydrocarbon group represented by the following formula.
- In the formula, R5, R6, R7, R8 and R9, which may be mutually the same or different, each represent a hydrogen atom, a saturated or unsaturated alkoxyl group, alkyl group, amino group or alkylamino group. At least one of R5, R6, R7, R8 and R9 represents a saturated or unsaturated alkoxyl group, alkyl group, amino group or alkylamino group.
- A polymer compound containing the nitrogen-containing heterocyclic group or a nitrogen-containing heterocyclic derivative may be used for the electron transporting compound.
- The electron transporting layer preferably contains at least one of nitrogen-containing heterocycle derivatives respectively represented by the following formulae (201) to (203).
- In the formulae (201) to (203): R represents a hydrogen atom, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms;
- n is an integer in a range of 0 to 4;
- R1 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, <<nret>> or alkoxy group having 1 to 20 carbon atoms;
- R2 and R3 each independently represent a hydrogen atom, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or a substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms;
- L represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridinylene group, substituted or unsubstituted quinolylene group, or substituted or unsubstituted fluorenylene group;
- Ar1 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridinylene group, substituted or unsubstituted quinolyl group. Are represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms; and
- Ar3 represents a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms or group represented by —Ar1—Ar2 (Ar1 and Ar2 may be the same as the above).
- In the formulae (201) to (203), R represents a hydrogen atom, a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 60 ring carbon atoms, substituted or unsubstituted pyridyl group, substituted or unsubstituted quinolyl group, substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, or substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms.
- Although a thickness of the electron injecting layer or the electron transporting layer is not specifically limited, the thickness is preferably 1 nm to 100 nm.
- The electron injecting layer preferably contains an inorganic compound such as an insulator or a semiconductor in addition to the nitrogen-containing cyclic derivative. Such an insulator or a semiconductor, when contained in the electron injecting layer, can effectively prevent a current leak, thereby enhancing electron capability of the electron injecting layer.
- As the insulator, it is preferable to use at least one metal compound selected from the group consisting of an alkali metal chalcogenide, an alkali earth metal chalcogenide, a halogenide of alkali metal and a halogenide of alkali earth metal. By forming the electron injecting layer from the alkali metal chalcogenide or the like, the electron injecting capability can preferably be further enhanced. Specifically, preferable examples of the alkali metal chalcogenide are Li2O, K2O, Na2S, Na2Se and Na2O, while preferable example of the alkali earth metal chalcogenide are CaO, BaO, SrO, BeO, BaS and CaSe. Preferable examples of the halogenide of the alkali metal are LiF, NaF, KF, LiCl, KCl and NaCl. Preferable examples of the halogenide of the alkali earth metal are fluorides such as CaF2, BaF2, SrF2, MgF2 and BeF2, and halogenides other than the fluoride.
- Examples of the semiconductor are one of or a combination of two or more of an oxide, a nitride or an oxidized nitride containing at least one element selected from Ba, Ca, Sr, Yb, Al, Ga, In, Li, Na, Cd, Mg, Si, Ta, Sb and Zn. An inorganic compound for forming the electron injecting layer is preferably a microcrystalline or amorphous semiconductor film. When the electron injecting layer is formed of such insulator film, more uniform thin film can be formed, thereby reducing pixel defects such as a dark spot. Examples of such an inorganic compound are the above-described alkali metal chalcogenide, alkali earth metal chalcogenide, halogenide of the alkali metal and halogenide of the alkali earth metal.
- When the electron injecting layer contains such an insulator or such a semiconductor, a thickness thereof is preferably in a range of approximately 0.1 nm to 15 nm. The electron injecting layer in this exemplary embodiment may preferably contain the above-described reduction-causing dopant.
- The hole injecting layer or the hole transporting layer (including the hole injecting/transporting layer) may contain an aromatic amine compound such as an aromatic amine derivative represented by the following (I).
- In the above (I), Ar1 to Ar4 each represent a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 50 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 40 ring carbon atoms, or a group formed by combining the aromatic hydrocarbon group or the fused aromatic hydrocarbon group with the aromatic heterocyclic group or fused aromatic heterocyclic group.
- Examples of the compound represented by the formula (I) are shown below. However, the compound represented by the formula (I) is not limited thereto.
- Aromatic amine represented by the following (II) can also be preferably used for forming the hole injecting layer or the hole transporting layer.
- In the above (II), Ar1 to Ar3 each represent the same as Ar1 to Ar4 of the above (I). Examples of the compound represented by the general formula (II) are shown below. However, the compound represented by the formula (II) is not limited thereto.
- A method of forming each of the layers in the organic EL device according to this exemplary embodiment is not particularly limited. Conventionally-known methods such as vacuum deposition and spin coating may be employed for forming the layers. The organic thin-film layer containing the compound represented by the formula (1), which is used in the organic EL device according to this exemplary embodiment, may be formed by a conventional coating method such as vacuum deposition, molecular beam epitaxy (MBE method) and coating methods using a solution such as a dipping, spin coating, casting, bar coating, and roll coating.
- Although the thickness of each organic layer of the organic EL device according to this exemplary embodiment is not particularly limited, the thickness is generally preferably in a range of several nanometers to 1 μm because an excessively-thinned film likely entails defects such as a pin hole while an excessively-thickened film requires high voltage to be applied and deteriorates efficiency.
- Next, an organic EL device according to a second exemplary embodiment will be described below.
- The organic EL device according to the second exemplary embodiment is different in that the emitting layer includes the first host material, the second host material and the phosphorescent material. In this case, the first host material is the biscarbazole derivative according to the exemplary embodiment represented by the formulae (1) to (3).
- The organic-EL-device material represented by the formulae (1) to (3) has a biscarbazole skeleton having an excellent hole transporting capability and a heterocyclic skeleton having an excellent electron transporting capability, which leads to a bi-polar performance sufficient for functioning as a single host. However, a luminous efficiency and a lifetime of the multilayered organic EL device depend on a carrier balance of an entire organic EL device. Main factors for controlling the carrier balance are carrier transporting capability of each of the organic layers and carrier injecting capability in the interfacial region of separate organic layers. In order to balance the carrier injecting capability to neighboring layers in the emitting layer (recombination region), it is preferable to adjust the carrier balance not by a single host material but by a plurality of host materials. Specifically, it is preferable that, in addition to the first host material, the second host material is suitably selected as a cohost and used in the emitting layer.
- When a material having a poor electron injecting capability (e.g., metal chelate complex) is used as the cathode, a carrier balance in the emitting layer becomes shifted toward the cathode. For improving such a disadvantage, it is preferable to select a material having a high electron transporting capability as the second host material. Specifically, the host material of this exemplary embodiment is preferably represented by a formula (5) or (6).
-
(Cz−)aA3 (5) -
Cz(−A3)b (6) - In the formula (5) or (6): Cz represents a substituted or unsubstituted arylcarbazolyl group or carbazolylaryl group;
- A3 represents a group represented by a formula (7A) below; and
- a and b each represent an integer of 1 to 3.
-
(M1)c−(L5)d−(M2)e (7A) - In the formula (7A): M1 and M2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M1 and M2 may be the same or different;
- L5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
- Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- An arylcarbazolyl group means a carbazolyl group having at least one aryl group or heteroaryl group as a substituent, in which a position where the aryl group or heteroaryl group is substituted does not matter.
- Specific examples are as follows. In the following chemical formulae, Ar represents an aryl group or heteroaryl group. * represents a position where another group is bonded.
- A carbazolylaryl group means an aryl group having at least one carbazolyl group as a substituent, in which a position where the aryl group is substituted does not matter.
- Specific examples are as follows. In the following chemical formulae, Ar represents an aryl group. * represents a position where another group is bonded.
- A substituted arylcarbazolyl group means the arylcarbazolyl group having at least one substituent irrespective of a substitution position. A substituted carbazolylaryl group means the carbazolylaryl group having at least one substituent irrespective of a substitution position.
- In the formulae (5) and (6), a and b each represent an integer of 1 to 3.
- An aryl group in the arylcarbazolyl group or carbazolylaryl group preferably has 6 to 30 carbon atoms. Examples of the aryl group are a phenyl group, naphthyl group, anthryl group, phenanthryl group, naphthacenyl group, pyrenyl group, fluorenyl group, biphenyl group and terphenyl group, among of which a phenyl group, naphthyl group, biphenyl group and terphenyl group are preferable.
- Examples of the heteroaryl group in the arylcarbazolyl group are groups formed based on rings of pyridine, pyrimidine, pyrazine, triazine, aziridine, azaindolizine, indolizine, imidazoles, indole, isoindole, indazole, purine, pteridine, d-carboline, naphthyridine, quinoxaline, terpyridine, bipyridine, acridine, phenanthroline, phenazine and imidazopyridine, among which rings of pyridine, terpyridine, pyrimidine, imidazopyridine and triazine are preferable.
- A in the formulae (5) and (6) is a group represented by the formula (7A).
- In the formula (7A), M1 and M2 each independently represent a substituted or unsubstituted nitrogen-containing heterocyclic group having 2 to 40 ring carbon atoms. M1 and M2 may be the same or different.
- Examples of the nitrogen-containing heterocyclic ring in the arylcarbazolyl group are groups formed based on rings of pyridine, pyrimidine, pyrazine, triazine, aziridine, azaindolizine, indolizine, imidazoles, indole, isoindole, indazole, purine, pteridine, â-carboline, naphthyridine, quinoxaline, terpyridine, bipyridine, acridine, phenanthroline, phenazine and imidazopyridine, among which rings of pyridine, terpyridine, pyrimidine, imidazopyridine and triazine are preferable.
- L5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, or substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
- Examples of the aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms are a phenyl group, biphenyl group, terphenyl group, naphthyl group, anthranil group, phenanthryl group, pyrenyl group, crycenyl group, fluoranthenyl group and perfluoroaryl group, fluorenyl group, and 9,9-dimethylfluorenyl group, among which a phenyl group, biphenyl group, terphenyl group and perfluoroaryl group are preferable.
- Examples of the cycloalkylene group having 5 to 30 carbon atoms are cyclopentyl group, cyclohexylene group, and cyclohepthylene group, among which a cyclohexylene group is preferable.
- Examples of the aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms are 1-pyrrolyl group, 2-pyrrolyl group, 3-pyrrolyl group, pyrazinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofuranyl group, 1-isobenzofuranyl group, 3-isobenzofuranyl group, 4-isobenzofuranyl group, 5-isobenzofuranyl group, 6-isobenzofuranyl group, 7-isobenzofuranyl group, 2-quinolyl group, 3-quinolyl group, 4-quinolyl group, 5-quinolyl group, 6-quinolyl group, 7-quinolyl group, 8-quinolyl group, 1-isoquinolyl group, 3-isoquinolyl group, 4-isoquinolyl group, 5-isoquinolyl group, 6-isoquinolyl group, 7-isoquinolyl group, 8-isoquinolyl group, 2-quinoxalinyl group, 5-quinoxalinyl group, 6-quinoxalinyl group, 1-carbazolyl group, 2-carbazolyl group, 3-carbazolyl group, 4-carbazolyl group, 9-carbazolyl group, 1-phenanthridinyl group, 2-phenanthridinyl group, 3-phenanthridinyl group, 4-phenanthridinyl group, 6-phenanthridinyl group, 7-phenanthridinyl group, 8-phenanthridinyl group, 9-phenanthridinyl group, 10-phenanthridinyl group, 1-acridinyl group, 2-acridinyl group, 3-acridinyl group, 4-acridinyl group, 9-acridinyl group, 1,7-phenanthrolin-2-yl group, 1,7-phenanthrolin-3-yl group, 1,7-phenanthrolin-4-yl group, 1,7-phenanthrolin-5-yl group, 1,7-phenanthrolin-6-yl group, 1,7-phenanthrolin-8-yl group, 1,7-phenanthrolin-9-yl group, 1,7-phenanthrolin-10-yl group, 1,8-phenanthrolin-2-yl group, 1,8-phenanthrolin-3-yl group, 1,8-phenanthrolin-4-yl group, 1,8-phenanthrolin-5-yl group, 1,8-phenanthrolin-6-yl group, 1,8-phenanthrolin-7-yl group, 1,8-phenanthrolin-9-yl group, 1,8-phenanthrolin-10-yl group, 1,9-phenanthrolin-2-yl group, 1,9-phenanthrolin-3-yl group, 1,9-phenanthrolin-4-yl group, 1,9-phenanthrolin-5-yl group, 1,9-phenanthrolin-6-yl group, 1,9-phenanthrolin-7-yl group, 1,9-phenanthrolin-8-yl group, 1,9-phenanthrolin-10-yl group, 1,10-phenanthrolin-2-yl group, 1,10-phenanthrolin-3-yl group, 1,10-phenanthrolin-4-yl group, 1,10-phenanthirolin-5-yl group, 2,9-phenanthrolin-1-yl group, 2,9-phenanthrolin-3-yl group, 2,9-phenanthrolin-4-yl group, 2,9-phenanthrolin-5-yl group, 2,9-phenanthrolin-6-yl group, 2,9-phenanthrolin-7-yl group, 2,9-phenanthrolin-8-yl group, 2,9-phenanthrolin-10-yl group, 2,8-phenanthrolin-1-yl group, 2,8-phenanthrolin-3-yl group, 2,8-phenanthrolin-4-yl group, 2,8-phenanthrolin-5-yl group, 2,8-phenanthrolin-6-yl group, 2,8-phenanthrolin-7-yl group, 2,8-phenanthrolin-9-yl group, 2,8-phenanthrolin-10-yl group, 2,7-phenanthrolin-1-yl group, 2,7-phenanthrolin-3-yl group, 2,7-phenanthrolin-4-yl group, 2,7-phenanthrolin-5-yl group, 2,7-phenanthrolin-6-yl group, 2,7-phenanthrolin-8-yl group, 2,7-phenanthrolin-9-yl group, 2,7-phenanthrolin-10-yl group, 1-phenazinyl group, 2-phenazinyl group, 1-phenothiazinyl group, 2-phenothiazinyl group, 3-phenothiazinyl group, 4-phenothiazinyl group, 10-phenothiazinyl group, 1-phenoxazinyl group, 2-phenoxazinyl group, 3-phenoxazinyl group, 4-phenoxazinyl group, 10-phenoxazinyl group, 2-oxazolyl group, 4-oxazolyl group, 5-oxazolyl group, 2-oxadiazolyl group, 5-oxadiazolyl group, 3-furazanyl group, 2-thienyl group, 3-thienyl group, 2-methylpyrrol-1-yl group, 2-methylpyrrol-3-yl group, 2-methylpyrrol-4-yl group, 2-methylpyrrol-5-yl group, 3-methylpyrrol-1-yl group, 3-methylpyrrol-2-yl group, 3-methylpyrrol-4-yl group, 3-methylpyrrol-5-yl group, 2-t-butylpyrrol-4-yl group, 3-(2-phenylpropyl)pyrrol-1-yl group, 2-methyl-1-indolyl group, 4-methyl-1-indolyl group, 2-methyl-3-indolyl group, 4-methyl-3-indolyl group, 2-t-butyl-1-indolyl group, 4-t-butyl-1-indolyl group, 2-t-butyl-3-indolyl group, and 4-t-butyl-3-indolyl group, among which a pyridinyl group and quinolyl group are preferable.
- Examples of the substituents for Cz, M1 and M2 in the formulae (5), (6) and (7A) are a halogen atom such as chlorine, bromine and fluorine, carbazole group, hydroxyl group, substituted or unsubstituted amino group, nitro group, cyano group, silyl group, trifluoromethyl group, carbonyl group, carboxyl group, substituted or unsubstituted alkyl group, substituted or unsubstituted alkenyl group, substituted or unsubstituted arylalkyl group, substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group, substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group, substituted or unsubstituted aralkyl group, substituted or unsubstituted aryloxy group, and substituted or unsubstituted alkyloxy group. Among these, a fluorine atom, methyl group, perfluorophenylene group, phenyl group, naphthyl group, pyridyl group, pyrazil group, pyrimidyl group, adamantyl group, benzyl group, cyano group and silyl group are preferable.
- Bonding patterns of the compound represented by the formula (5) or (6) are shown in Table 1 below in accordance with values of a and b.
- Bonding patterns of the compound represented by the formula (7A) are shown in Tables 2 and 3 below in accordance with values of c, d and e.
-
TABLE 2 No c d e Bonding Patterns (1) 0 1 1 L5—M2 (2) 0 1 2 L5—M2—M2, M2—L5—M2 (3) 0 2 1 L5—L5—M2, L5—M2—L5 (4) 0 2 2 L5—L5—M2—M2, M2—L5—L5—M2, (5) 1 1 0 the same as [1](M2 is replaced with M1) (6) 1 1 1 M1—L5—M2 (7) 1 1 2 (8) 1 2 0 the same as [3] (M2 is replaced with M1) (9) 1 2 1 M1—L5—L5—M2, L5—M1—L5—M2, L5—M1—L5—M2 (10) 1 2 2 M1—L5—L5—M2—M2, M2—L5—M1—L5—M2, (11) 2 1 0 the same as [2] (M2 is replaced with M1) (12) 2 1 1 the same as [7] (M2 is replaced with M1) (13) 2 1 2 - Cz bonded to A may be bonded to any one of M1, L5 and M2 of the formula (7A) representing A.
- For instance, when a=b=1 and Cz-A3-Cz are given in the formula (5) or (6) and [6] (c=d=e=1) of Table 2 is given in the formula (A), three bonding patterns of Cz-M1-L5-M2, M1-L5(Cz)-M2, and M1-L5-M2-Cz are listed.
- Moreover, for instance, when a=2 and Cz-A3-Cz are given in the formula (5) and [7] (c=d=1,e=2) Table 2 is given in the formula (7A), the following bonding patterns are listed.
- In the bonding patterns of the formulae (5), (6) and (7A) and exemplary combinations of the groups as described above, compounds represented by [1] to [4] below are preferable.
- [1] a=1 is given in the formula (5) and c=1 and d=0 are given in the formula (7A).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7A): M1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [2] a=2 is given in the formula (5) and c=1 and e=0 are given in the formula (7A).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7A): M1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [3] a=1 is given in the formula (5) and c=2 and e=0 are given in the formula (7A).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7A): M1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [4] b=2 is given in the formula (6) and c=d=1 is given in the formula (7A).
- In the formula (6), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7A): M1 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L5 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- In the formulae (5) and (6), Cz is preferably a substituted or unsubstituted arylcarbazolyl group, more preferably phenylcarbozolyl group. Moreover, an aryl site of the arylcarbazolyl group is preferably substituted by a carbazolyl group.
- Specific examples of the compound represented by the formula (5) according to this exemplary embodiment are shown below, but the compound represented by the formula (25) is not limited thereto.
- Specific examples of the compound represented by the formula (6) are shown below, but the compound represented by the formula (6) is not limited thereto.
- The compound represented by the formula (5) or (6) in this exemplary embodiment has triplet energy gap of 2.5 eV to 3.3 eV, preferably 2.5 eV to 3.2 eV.
- The compound represented by the formula (5) or (6) in this exemplary embodiment has singlet energy gap of 2.8 eV to 3.8 eV, preferably 2.9 eV to 3.7 eV.
- An organic EL device according to a third exemplary embodiment is different from the organic EL device according to the second exemplary embodiment in that a material having a poor electron capability is used as the second material.
- When a material having an excellent electron injecting capability from the electrode (e.g., LiF) is used as the cathode, a carrier balance in the emitting layer becomes shifted toward the anode. For improving such a disadvantage, it is preferable to select a material having a poor electron injecting capability as the second host material. Specifically, the second host material of this exemplary embodiment is preferably a compound in which A3 is a group represented by the following formula (7B) in the formula (5) or (6).
-
(M3)c−(L6)d−(M4)e (7B) - In the formula (7B): M3 and M4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 6 to 40 ring carbon atoms; M3 and M4 may be the same or different; L6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
- In the formula (7B), as the aromatic hydrocarbon group for M3 and M4 and the aromatic hydrocarbon group, fused aromatic hydrocarbon group and cycloalkylene group for L6, those represented by the formula (26A) can be used. As bonding patterns of the groups represented by the formula (27B), the same bonding patterns as those of the formula (7A) can be used. Specifically, in the bonding patterns of the formula (7A), M1, L5 and M2 may be respectively replaced with M3, L6 and M4.
- In the bonding patterns of the formulae (5), (6) and (7B) and exemplary combinations of the groups as described above, compounds represented by [5] to [8] below are preferable.
- [5] a=1 is given in the formula (5) and c=1 and d=0 are given in the formula (7B).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7B): M3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L6 is a substituted or unsubstituted aryl group or aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [6] a=2 is given in the formula (5) and c=1 and e=0 are given in the formula (7B).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7B): M3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [7] a=1 is given in the formula (5) and c=2 and e=0 are given in the formula (7B).
- In the formula (5), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7B): M3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- [8] b=2 is given in the formula (6) and c=d=1 is given in the formula (7B).
- In the formula (6), Cz is a substituted or unsubstituted arylcarbazolyl group or substituted or unsubstituted carbazolylaryl group.
- In the formula (7B): M3 is a substituted or unsubstituted nitrogen-containing six-membered or seven-membered hetero ring having 4 to 5 ring carbon atoms, substituted or unsubstituted nitrogen-containing five-membered hetero ring having 2 to 4 ring carbon atoms, substituted or unsubstituted nitrogen-containing hetero ring having 8 to 11 ring carbon atoms, substituted or unsubstituted imidazopyridinyl ring; and L6 is a substituted or unsubstituted aromatic hydrocarbon group or fused aromatic hydrocarbon group having 6 to 30 carbon atoms and substituted or unsubstituted aromatic heterocyclic group or fused aromatic heterocyclic group having 2 to 30 carbon atoms.
- In the formulae (5) and (6), Cz is preferably a substituted or unsubstituted arylcarbazolyl group, more preferably phenylcarbozolyl group. Moreover, an aryl site of the arylcarbazolyl group is preferably substituted by a carbazolyl group.
- Examples of the compound in which A3 is a group represented by the following formula (7B) in the formula (5) or (6) are listed below.
- As the second host material of this exemplary embodiment, a compound represented by a formula (8) below may be used.
- In the formula (8): R101 to R106 each independently represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms, substituted or unsubstituted arylthio group having 6 to 20 carbon atoms, substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms or cyano group;
- at least one of R101 to R106 is a substituted or unsubstituted 9-carbazolyl group, substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms, or -L-9-carbazolyl group;
- L represents a substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms, substituted or unsubstituted arylthio group having 6 to 20 carbon atoms, or substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms;
- Xa represents a sulfur atom, oxygen atom or N—R108; and
- R108 represents the same as R101 to R106.
- Specific examples of the substituted or unsubstituted azacarbazolyl group having 2 to 5 nitrogen atoms are shown below (in which any substituent is omitted), but the substituted or unsubstituted azacarbazolyl group is not limited thereto.
- Examples of the halogen atom include fluorine, chlorine, bromine, and iodine.
- Examples of the substituted or unsubstituted alkyl group having 1 to 40 carbon atoms include a methyl group, an ethyl group, a propyl group, an isopropyl group, an n-butyl group, an s-butyl group, an isobutyl group, a t-butyl group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-nonyl group, an n-decyl group, an n-undecyl group, an n-dodecyl group, an n-tridecyl group, an n-tetradecyl group, an n-pentadecyl group, an n-hexadecyl group, an n-heptadecyl group, an n-octadecyl group, a neopentyl group, a 1-methylpentyl group, a 2-methylpentyl group, a 1-pentylhexyl group, a 1-butylpentyl group, a 1-heptyloctyl group, a 3-methylpentyl group, a hydroxymethyl group, a 1-hydroxyethyl group, a 2-hydroxyethyl group, a 2-hydroxyisobutyl group, a 1,2-dihydroxyethyl group, a 1,3-dihydroxyisopropyl group, a 2,3-dihydroxy-t-butyl group, a 1,2,3-trihydroxypropyl group, a chloromethyl group, a 1-chloroethyl group, a 2-chloroethyl group, a 2-chloroisobutyl group, a 1,2-dichloroethyl group, a 1,3-dichloroisopropyl group, a 2,3-dichloro-t-butyl group, a 1,2,3-trichloropropyl group, a bromomethyl group, a 1-bromoethyl group, a 2-bromoethyl group, a 2-bromoisobutyl group, a 1,2-dibromoethyl group, a 1,3-dibromoisopropyl group, a 2,3-dibromo-t-butyl group, a 1,2,3-tribromopropyl group, an iodomethyl group, a 1-iodoethyl group, a 2-iodoethyl group, a 2-iodoisobutyl group, a 1,2-diiodoethyl group, a 1,3-diiodoisopropyl group, a 2,3-diiodo-t-butyl group, a 1,2,3-triiodopropyl group, an aminomethyl group, a 1-aminoethyl group, a 2-aminoethyl group, a 2-aminoisobutyl group, a 1,2-diaminoethyl group, a 1,3-diaminoisopropyl group, a 2,3-diamino-t-butyl group, a 1,2,3-triaminopropyl group, a cyanomethyl group, a 1-cyanoethyl group, a 2-cyanoethyl group, a 2-cyanoisobutyl group, a 1,2-dicyanoethyl group, a 1,3-dicyanoisopropyl group, a 2,3-dicyano-t-butyl group, a 1,2,3-tricyanopropyl group, a nitromethyl group, a 1-nitroethyl group, a 2-nitroethyl group, a 1,2-dinitroethyl group, a 2,3-dinitro-t-butyl group, and a 1,2,3-trinitropropyl group, among of which a methyl group, an ethyl group, a propyl group, an isopropyl group, an n-butyl group, an s-butyl group, an isobutyl group, a t-butyl group, an n-pentyl group, an n-hexyl group, an n-heptyl group, an n-octyl group, an n-nonyl group, an n-decyl group, an n-undecyl group, an n-dodecyl group, an n-tridecyl group, an n-tetradecyl group, an n-pentadecyl group, an n-hexadecyl group, an n-heptadecyl group, an n-octadecyl group, a neopentyl group, a 1-methylpentyl group, a 1-pentylhexyl group, a 1-butylpentyl group, a 1-heptyloctyl group are preferable. The alkyl group (excluding a substituent) preferably has 1 to 10 carbon atoms.
- Examples of the substituted or unsubstituted cycloalkyl group having 3 to 15 carbon atoms include a cyclopentyl group, cyclohexyl group, cyclooctyl group, and 3,5,5,5-tetramethylcyclohexyl group. A cyclohexyl group, cyclooctyl group, and 3,5-tetramethylcyclohexyl group are preferable. The cycloalkyl group (excluding a substituent) preferably has 3 to 12 carbon atoms.
- Examples of the substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms are a 1-pyroryl group, 2-pyroryl group, 3-pyroryl group, pyrazinyl group, 2-pyridinyl group, 1-imidazolyl, 2-imidazolyl, 1-pyrazolyl, 1-indolidinyl, 2-indolidinyl, 3-indolidinyl, 5-indolidinyl, 6-indolidinyl, 7-indolidinyl, 8-indolidinyl, 2-imidazopyridinyl, 3-imidazopyridinyl, 5-imidazopyridinyl, 6-imidazopyridinyl, 7-imidazopyridinyl, 8-imidazopyridinyl, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofuranyl group, 1-isobenzofuranyl group, 3-isobenzofuranyl group, 4-isobenzofuranyl group, 5-isobenzofuranyl group, 6-isobenzofuranyl group, 7-isobenzofuranyl group, 2-quinolyl group, 3-quinolyl group, 4-quinolyl group, 5-quinolyl group, 6-quinolyl group, 7-quinolyl group, 8-quinolyl group, 1-isoquinolyl group, 3-isoquinolyl group, 4-isoquinolyl group, 5-isoquinolyl group, 6-isoquinolyl group, 7-isoquinolyl group, 8-isoquinolyl group, 2-quinoxalinyl group, 5-quinoxalinyl group, 6-quinoxalinyl group, 1-carbazolyl group, 2-carbazolyl group, 3-carbazolyl group, 4-carbazolyl group, 9-carbazolyl group, azacarbazolyl-1-yl, azacarbazolyl-2-yl, azacarbazolyl-3-yl, azacarbazolyl-4-yl, azacarbazolyl-5-yl, azacarbazolyl-6-yl, azacarbazolyl-7-yl, azacarbazolyl-8-yl, azacarbazolyl-9-yl, 1-phenanthrydinyl group, 2-phenanthrydinyl group, 3-phenanthrydinyl group, 4-phenanthrydinyl group, 6-phenanthrydinyl group, 7-phenanthrydinyl group, 8-phenanthrydinyl group, 9-phenanthrydinyl group, 10-phenanthrydinyl group, 1-acridinyl group, 2-acridinyl group, 3-acridinyl group, 4-acridinyl group, 9-acridinyl group, 1,7-phenanthroline-2-yl group, 1,7-phenanthroline-3-yl group, 1,7-phenanthroline-4-yl group, 1,7-phenanthroline-5-yl group, 1,7-phenanthroline-6-yl group, 1,7-phenanthroline-8-yl group, 1,7-phenanthroline-9-yl group, 1,7-phenanthroline-10-yl group, 1,8-phenanthroline-2-yl group, 1,8-phenanthroline-3-yl group, 1,8-phenanthroline-4-yl group, 1,8-phenanthroline-5-yl group, 1,8-phenanthroline-6-yl group, 1,8-phenanthroline-7-yl group, 1,8-phenanthroline-9-yl group, 1,8-phenanthroline-10-yl group, 1,9-phenanthroline-2-yl group, 1,9-phenanthroline-3-yl group, 1,9-phenanthroline-4-yl group, 1,9-phenanthroline-5-yl group, 1,9-phenanthroline-6-yl group, 1,9-phenanthroline-7-yl group, 1,9-phenanthroline-8-yl group, 1,9-phenanthroline-10-yl group, 1,10-phenanthroline-2-yl group, 1,10-phenanthroline-3-yl group, 1,10-phenanthroline-4-yl group, 1,10-phenanthroline-5-yl group, 2,9-phenanthroline-1-yl group, 2,9-phenanthroline-3-yl group, 2,9-phenanthroline-4-yl group, 2,9-phenanthroline-5-yl group, 2,9-phenanthroline-6-yl group, 2,9-phenanthroline-7-yl group, 2,9-phenanthroline-8-yl group, 2,9-phenanthroline-10-yl group, 2,8-phenanthroline-1-yl group, 2,8-phenanthroline-3-yl group, 2,8-phenanthroline-4-yl group, 2,8-phenanthroline-5-yl group, 2,8-phenanthroline-6-yl group, 2,8-phenanthroline-7-yl group, 2,8-phenanthroline-9-yl group, 2,8-phenanthroline-10-yl group, 2,7-phenanthroline-1-yl group, 2,7-phenanthroline-3-yl group, 2,7-phenanthroline-4-yl group, 2,7-phenanthroline-5-yl group, 2,7-phenanthroline-6-yl group, 2,7-phenanthroline-8-yl group, 2,7-phenanthroline-9-yl group, 2,7-phenanthroline-10-yl group, 1-phenazinyl group, 2-phenazinyl group, 1-phenothiazinyl group, 2-phenothiazinyl group, 3-phenothiazinyl group, 4-phenothiazinyl group, 10-phenothiazinyl group, 1-phenoxazinyl group, 2-phenoxazinyl group, 3-phenoxazinyl group, 4-phenoxazinyl group, 10-phenoxazinyl group, 2-oxazolyl group, 4-oxazolyl group, 5-oxazolyl group, 2-oxadiazolyl group, 5-oxadiazolyl group, 3-furazanyl group, 2-thienyl group, 3-thienyl group, 2-methylpyrrole-1-yl group, 2-methylpyrrole-3-yl group, 2-methylpyrrole-4-yl group, 2-methylpyrrole-5-yl group, 3-methylpyrrole-1-yl group, 3-methylpyrrole-2-yl group, 3-methylpyrrole-4-yl group, 3-methylpyrrole-5-yl group, 2-t-butylpyrrole-4-yl group, 3-(2-phenylpropyl)pyrrole-1-yl group, 2-methyl-1-indolyl group, 4-methyl-1-indolyl group, 2-methyl-3-indolyl group, 4-methyl-3-indolyl group, 2-t-butyl-1-indolyl group, 4-t-butyl-1-indolyl group, 2-t-butyl-3-indolyl group, 4-t-butyl-3-indolyl group, 1-dibenzofuranyl group, 2-dibenzofuranyl group, 3-dibenzofuranyl group, 4-dibenzofuranyl group, 1-dibenzothiophenyl group, 2-dibenzothiophenyl group, 3-dibenzothiophenyl group, 4-dibenzothiophenyl group, 1-silafluorenyl group, 2-silafluorenyl group, 3-silafluorenyl group, 4-silafluorenyl group, 1-germafluorenyl group, 2-germafluorenyl group, 3-germafluorenyl group and 4-germafluorenyl group.
- Among the above, the heterocyclic group is preferably a 2-pyridinyl group, 1-indolidinyl, 2-indolidinyl, 3-indolidinyl, 5-indolidinyl, 6-indolidinyl, 7-indolidinyl, 8-indolidinyl, 2-imidazopyridinyl, 3-imidazopyridinyl, 5-imidazopyridinyl, 6-imidazopyridinyl, 7-imidazopyridinyl, 8-imidazopyridinyl, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 9-carbazolyl group, 1-dibenzofuranyl group, 2-dibenzofuranyl group, 3-dibenzofuranyl group, 4-dibenzofuranyl group, 1-dibenzothiophenyl group, 2-dibenzothiophenyl group, 3-dibenzothiophenyl group, 4-dibenzothiophenyl group, 1-silafluorenyl group, 2-silafluorenyl group, 3-silafluorenyl group, 4-silafluorenyl group, 1-germafluorenyl group, 2-germafluorenyl group, 3-germafluorenyl group, 4-germafluorenyl group, azacarbazolyl-1-yl group, azacarbazolyl-2-yl group, azacarbazolyl-3-yl group, azacarbazolyl-4-yl group, azacarbazolyl-5-yl group, azacarbazolyl-6-yl group, azacarbazolyl-7-yl group, azacarbazolyl-8-yl group, and azacarbazolyl-9-yl group. The heterocyclic group (excluding a substituent) preferably has 3 to 14 carbon atoms.
- The substituted or unsubstituted alkoxy group having 1 to 40 carbon atoms is a group represented by —OY. Examples of Y are the same as those described in relation to the alkyl group. Preferred examples are also the same.
- Examples of the substituted or unsubstituted aryl group having 6 to 40 carbon atoms (including a fused aromatic hydrocarbon group and a ring assembly aromatic hydrocarbon group) are a phenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, o-tolyl group, m-tolyl group, p-tolyl group, p-t-butylphenyl group, p-(2-phenylpropyl)phenyl group, 4′-methylbiphenylyl group, 4″-t-butyl-p-terphenyl-4-yl group, o-cumenyl group, m-cumenyl group, p-cumenyl group, 2,3-xylyl group, 3,4-xylyl group, 2,5-xylyl group, mesityl group and m-quarter-phenyl group. Among the above, the substituted or unsubstituted aryl group is preferably a phenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, p-tolyl group, 3,4-xylyl group, m-quarter-phenyl-2-yl group, 1-naphtyl group, 2-naphtyl group, 1-phenanthrenyl group, 2-phenanthrenyl group, 3-phenanthrenyl group, 4-phenanthrenyl group, 9-phenanthrenyl group, 1-triphenylenyl group, 2-triphenylenyl group, 3-triphenylenyl group, 4-triphenylenyl group, 1-chrysenyl group, 2-chrysenyl group, 3-chrysenyl group, 4-chrysenyl group, 5-chrysenyl group, and 6-chrysenyl group. The aryl group (excluding a substituent) preferably has 6 to 24 carbon atoms. The aryl group preferably further includes a 9-carbazolyl group as a substituent.
- The substituted or unsubstituted aryloxy group having 6 to 20 carbon atoms is a group represented by —OAr. Examples of Ar are the same as those described in relation to the aryl group. Preferred examples are also the same.
- Examples of the substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms are a benzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenylisopropyl group, 2-phenylisopropyl group, phenyl-t-butyl group, á-naphthylmethyl group, 1-á-naphthylethyl group, 2-á-naphthylethyl group, 1-á-naphthylisopropyl group, 2-á-naphthylisopropyl group, â-naphthylmethyl group, 1-â-naphthylethyl group, 2-â-naphthylethyl group, 1-â-naphthylisopropyl group, 2-â-naphthylisopropyl group, 1-pyrorylmethyl group, 2-(1-pyroryl)ethyl group, p-methylbenzyl group, m-methylbenzyl group, o-methylbenzyl group, p-chlorobenzyl group, m-chlorobenzyl group, o-chlorobenzyl group, p-bromobenzyl group, m-bromobenzyl group, o-bromobenzyl group, p-iodobenzyl group, m-iodobenzyl group, o-iodobenzyl group, p-hydroxybenzyl group, m-hydroxybenzyl group, o-hydroxybenzyl group, p-aminobenzyl group, m-aminobenzyl group, o-aminobenzyl group, p-nitrobenzyl group, m-nitrobenzyl group, o-nitrobenzyl group, p-cyanobenzyl group, m-cyanobenzyl group, o-cyanobenzyl group, 1-hydroxy-2-phenylisopropyl group, 1-chloro-2-phenylisopropyl group and the like. Among these, preferred are a benzyl group, a p-cyanobenzyl group, m-cyanobenzyl group, o-cyanobenzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenylisopropyl group, and 2-phenylisopropyl group. An alkyl portion of the aralkyl group preferably has 1 to 8 carbon atoms. An aryl portion thereof (including heteroaryl) preferably has 6 to 18 carbon atoms.
- The substituted or unsubstituted arylamino group having 6 to 40 carbon atoms, the substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, and the substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms each are represented by —NQ1Q2. Examples of Q1 and Q2 each are independently the same as those described in relation to the alkyl group, aryl group and aralkyl group. Preferred examples are also the same.
- The substituted or unsubstituted arylcarbonyl group having 7 to 40 carbon atoms is represented by —COAr2. Examples of Ar2 are the same as those described in relation to the aryl group. Preferred examples are also the same.
- The substituted or unsubstituted arylthio group having 6 to 20 carbon atoms is exemplified by a group obtained by replacing an oxygen atom of the aryloxy group represented by —OAr with a sulfur atom. Preferred examples are also the same.
- The substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms is exemplified by a halogenated alkyl group in which at least one hydrogen atom of the alkyl group is substituted by a halogen atom. Preferred examples are also the same.
- The compound represented by the general formula (8) preferably has triplet energy gap of 2.2 eV to 3.2 eV. Specific examples of the formula (8) are shown below.
- An organic EL device according to a fourth exemplary embodiment is different from the organic EL devices according to the second and third exemplary embodiments in being a red phosphorescent device.
- The compound according to this exemplary embodiment is not disclosed as a phosphorescent host material of which color is specified. However, since having a high resistance against oxidation and reduction, the compound is also applicable to a red phosphorescent device. As a red phosphorescent device, a hydrocarbon material, which exhibits a small triplet energy and broad electron clouds compared with a green phosphorescent material, can be used. Although the hydrocarbon material is difficult to be used as a green phosphorescent material because of its small triplet energy, the hydrocarbon material is highly appropriate as a red phosphorescent host material because of its high oxidation and reduction. Accordingly, by using the hydrocarbon material as the second host material, a red phosphorescent device can become highly efficient.
- The second host material is preferably a compound selected from the group consisting of polycyclic aromatic compounds represented by formulae (9A), (9B) and (9C) below.
-
Ra—Ar101—Rb (9A) -
Ra—Ar101-Ar102—Rb (9B) -
Ra—Ar101—Ar102—Ar103—Rb (9C) - In the formulae (9A) to (9C), Ar101, Ar102, Ar103, Ra and Rb represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms.
- Ar101, Ar102, Ar103, Ra and Rb preferably represent a polycyclic aromatic skeleton selected from a substituted or unsubstituted benzene ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted chrysene ring, substituted or unsubstituted fluoranthene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzophenanthrene ring, substituted or unsubstituted dibenzophenanthrene ring, substituted or unsubstituted triphenylene ring, substituted or unsubstituted benzo[a]triphenylene ring, substituted or unsubstituted benzochrysene ring, substituted or unsubstituted benzo[b]fluoranthene ring, substituted or unsubstituted fluorene ring and substituted or unsubstituted picene ring.
- It is further preferable that substituents for Ra and Rb are not aryl groups; and Ar101, Ar102, Ar103, Ra and Rb are not substituted or unsubstituted benzene ring at the same time.
- Moreover, in the formulae (9A) to (9C), either one or both of Ra and Rb are preferably selected from the group consisting of a substituted or unsubstituted phenanthrene ring, substituted or unsubstituted benzo[c]phenanthrene ring and substituted or unsubstituted fluoranthene ring.
- The polycyclic aromatic skeleton of the polycyclic aromatic compound may be substituted.
- Examples of the substituent for the polycyclic aromatic skeleton are a halogen atom, hydroxyl group, substituted or unsubstituted amino group, nitro group, cyano group, substituted or unsubstituted alkyl group, substituted or unsubstituted alkenyl group, substituted or unsubstituted cycloalkyl group, substituted or unsubstituted alkoxy group, substituted or unsubstituted aromatic hydrocarbon group, substituted or unsubstituted aromatic heterocyclic group, substituted or unsubstituted aralkyl group, substituted or unsubstituted aryloxy group, substituted or unsubstituted alkoxycarbonyl group, and carboxyl group. Preferred examples of the aromatic hydrocarbon group are naphthalene, phenanthrene, fluorene, chrysene, fluoranthene and triphenylene.
- When the polycyclic aromatic skeleton has a plurality of substituents, the substituents may form a ring.
- The polycyclic aromatic skeleton is preferably any one selected from the group consisting of compounds represented by formulae (9-1) to (9-4) below.
- In the formulae (9-1) to (9-4), Ar1 to Ar5 each represent a substituted or unsubstituted fused ring structure having 4 to 16 ring carbon atoms.
- Examples of the compound represented by the formula (9-1) are elementary substances or derivatives of substituted or unsubstituted phenanthrene and chrysene.
- Examples of the compound represented by the formula (9-2) are elementary substances or derivatives of substituted or unsubstituted acenaphthylene, acenaphthene and fluoranthene.
- Examples of the compound represented by the formula (9-3) are elementary substances or derivatives of substituted or unsubstituted benzofluoranthene.
- Examples of the compound represented by the formula (9-4) are elementary substances or derivatives of substituted or unsubstituted benzofluoranthene.
- The naphthalene derivative is exemplified by a formula (9-5) below.
- In the formula (9-5), R1 to R8 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The naphthalene derivative is exemplified by a formula (9-6) below.
- In the formula (9-6), R1 to R10 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The chrysene derivative is exemplified by a formula (9-7) below.
- In the formula (9-7), R1 to R12 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The polyaromatic skeleton is preferably benzo[c]phenanthrene or its derivative. The benzo[c]phenanthrene derivative is exemplified by a formula (9-8) below.
- In the formula (9-8), R1 to R9 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The polycyclic aromatic skeleton is preferably benzo[c]chrysene or its derivative. The benzo[c]phenanthrene derivative is exemplified by a formula (9-9) below.
- In the formula (9-9), R1 to R11 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The polycyclic aromatic skeleton is preferably dibenzo[c,g]phenanthrene represented by a formula (9-10) below or its derivative.
- The polycyclic aromatic skeleton is preferably fluoranthene or its derivative. The fluoranthene derivative is exemplified by a formula (9-11) below.
- In the formula (9-11), X12 to X21 each represent a hydrogen atom; halogen atom; linear, branched or cyclic alkyl group; linear, branched or cyclic alkoxy group; substituted or unsubstituted aryl group; or substituted or unsubstituted heteroaryl group.
- The polycyclic aromatic skeleton is preferably triphenylene or its derivative. The triphenylene derivative is exemplified by a formula (9-12) below.
- In the formula (9-12), R1 to R6 each independently represent a hydrogen atom, or a substituent consisting of one of or a combination of two or more of substituted or unsubstituted aryl group having 5 to 30 ring carbon atoms, branched or linear alkyl group having 1 to 30 carbon atoms and substituted or unsubstituted cycloalkyl group having 3 to 20 carbon atoms.
- The polycyclic aromatic compound may be represented by a formula (9-13) below.
- In the formula (9-13), Ra and Rb represent the same as Ra and Rb in the formulae (9A) to (9C). When Ra, Rb and the naphthalene ring have a single or plural substituent(s), the single or plural substituents) are an alkyl group having 1 to 20 carbon atoms, haloalkyl group having 1 to 20 carbon atoms, cycloalkyl group having 5 to 18 carbon atoms, silyl group having 3 to 20 carbon atoms, cyano group or halogen atom, while substituents for the naphthalene rings other than Ra and Rb are further allowed to be an aryl group having 6 to 22 carbon atoms.
- In the formula (9-13), Ra and Rb each preferably represent a group selected from fluorene ring, phenanthrene ring, triphenylene ring, benzophenanthrene ring, dibenzophenanthrene ring, benzotriphenylene ring, fluoranthene ring, benzochrysene ring, benzo[b]fluoranthene ring and picene ring.
- The second host material is preferably a monoamine derivative represented by any one of formulae (10) to (12) below.
- In the formula (10), Ar111, Ar112 and Ar113 each are a substituted or unsubstituted aryl group or heteroaryl group.
- The aryl group has 6 to 50 ring carbon atoms (preferably 6 to 30 ring carbon atoms, more preferably 6 to 20 ring carbon atoms). Examples of the aryl group are a phenyl group, naphthyl group, phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, acenaphthenyl group, and diazaphenanthrenyl group. Among the above, a phenyl group or naphthyl group is preferable.
- The heteroaryl group has 5 to 50 ring atoms (preferably 6 to 30 ring atoms, more preferably 6 to 20 ring atoms). Examples of the heteroaryl group are a pyrimidyl group and diazaphenanthrenyl group.
- At least one of Ar111, Ar112 and Ar113 is preferably a fused aromatic hydrocarbon group selected from a phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, and diazaphenanthrenyl group. Among the above, a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable. Preferably, the fused aromatic hydrocarbon group is unsubstituted.
- In the monoamine derivative represented by the formula (10), Ar111 and Ar112 each are preferably a phenyl group or naphthyl group, and Ar113 is preferably a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- In the formula (11), Ar114, Ar115 and Ar117 each are a substituted or unsubstituted aryl group or heteroaryl group.
- Examples of the aryl group or heteroaryl group are the same as those defined as the aryl group or heteroaryl group for Ar111, among which a phenyl group or naphthyl group is preferable.
- Ar116 is a substituted or unsubstituted arylene group or heteroarylene group.
- The arylene group has 6 to 50 ring carbon atoms (preferably 6 to 30 ring carbon atoms, more preferably 6 to 20 ring carbon atoms). Examples of the arylene group are a phenylene group, naphthylene group, phenanthrenylene group, naphthacenylene group, pyrenylene group, biphenylene group, terphenylenylene group, benzophenanthrenylene group, dibenzophenanthrenylene group, benzochrysenylene group, dibenzochrysenylene group, fluoranthenylene group, benzofluoranthenylene group, triphenylenylene group, benzotriphenylenylene group, dibenzotriphenylenylene group, picenylene group, benzopicenylene group, and dibenzopicenylene group. Among the above, a phenylene group or naphthylene group is preferable.
- The heteroaryl group has 5 to 50 ring atoms (preferably 6 to 30 ring atoms, more preferably 6 to 20 ring atoms). Examples of the heteroaryl group are a pyridylene group, pyrimidylene group, dibenzofuranylene group, and dibenzothiophenylene group.
- Ar117 is preferably a fused aromatic hydrocarbon group selected from a phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, and dibenzopicenyl group. Among the above, a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable. Preferably, the fused aromatic hydrocarbon group is unsubstituted.
- In the monoamine derivative of the formula (11), more preferably, Ar114 and Ar115 each are a phenyl group or naphthyl group, Ar116 is a phenyl group or naphthyl group, and Ar117 is a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- In the formula (12), Ar118, Ar119 and Ar121 are a substituted or unsubstituted aryl group or heteroaryl group.
- Examples of the aryl group or heteroaryl group are the same as those defined as the aryl group or heteroaryl group for Ar111 and are preferably a phenyl group.
- Ar120 is a substituted or unsubstituted arylene group or heteroarylene group and the same as those defined as the arylene group or heteroarylene group for Ar116.
- Ar120 is preferably a phenylene group or naphthylene group.
- n is an integer of 2 to 5, preferably 2 to 4, more preferably 2 to 3. When n is 2 or more, Ar120 may be mutually the same or different.
- Ar121 is preferably a fused aromatic hydrocarbon group selected from a phenyl group, naphthyl group, phenanthrenyl group, benzophenanthrenyl group, dibenzophenanthrenyl group, benzochrysenyl group, dibenzochrysenyl group, fluoranthenyl group, benzofluoranthenyl group, triphenylenyl group, benzotriphenylenyl group, dibenzotriphenylenyl group, picenyl group, benzopicenyl group, dibenzopicenyl group, phenalenyl group, and diazaphenanthrenyl group. Among the above, a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group is more preferable.
- In the exemplary embodiment, for the second host material in the formula (12), Ar118 and Ar119 each are preferably a phenyl group or naphthyl group; Ar120 is preferably a phenylene group or naphthylene group; and Ar121 is preferably a benzochrysenyl group, triphenylenyl group, or phenanthrenyl group.
- When Ar101 to Ar121 have substituent(s), the substituent(s) is preferably an alkyl group having 1 to 20 carbon atoms, haloalkyl group having 1 to 20 carbon atoms, cycloalkyl group having 3 to 18 carbon atoms, aryl group having 6 to 30 ring carbon atoms, silyl group having 3 to 20 carbon atoms, cyano group, and halogen atom.
- Examples of the alkyl group are a methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, 1-methylpropyl group and 1-propylbutyl group.
- Examples of the aryl group are the same as those for Ar101.
- The haloalkyl group is exemplified by a 2,2,2-trifluoroethyl group.
- Examples of the cycloalkyl group are a cyclopropyl group, cyclobutyl group, cyclopentyl group, cyclohexyl group and cyclooctyl group.
- Examples of the silyl group are a trimethylsilyl group and triethylsilyl group.
- Examples of the halogen atom are fluorine, chlorine, bromine, and iodine.
- When the monoamine derivatives represented by the formulae (10) to (12) do not have a substituent, it is meant that a hydrogen atom is substituted. The hydrogen atom of the monoamine derivatives represented by the formulae (10) to (12) includes light hydrogen and deuterium. “Carbon atoms forming a ring (ring carbon atoms)” mean carbon atoms forming a saturated ring, unsaturated ring, or aromatic ring. “Atoms forming a ring (ring atoms)” mean carbon atoms and hetero atoms forming a ring including a saturated ring, unsaturated ring, or aromatic ring.
- Specific examples of the monoamine derivatives represented by the formula (10) are shown below.
- Specific examples of the monoamine derivatives represented by the formula (11) are shown below.
- Specific examples of the monoamine derivatives represented by the formula (12) are shown below.
- In an organic EL device according to a sixth exemplary embodiment, an aromatic amine compound is used as the second host material.
- An example of the aromatic amine compound is preferably a compound represented by the formula (13) or (14).
- In the formula (13): X3 represents a substituted or unsubstituted arylene group having 10 to 40 ring carbon atoms; and
- A3 to A6 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- In the formula (14), A7 to A9 represent a substituted or unsubstituted aryl group having 6 to 60 ring carbon atoms, or heteroaryl group having 6 to 60 ring atoms.
- The second host material represented by the formula (13) or (14) is preferably represented by formulae (15) to (19).
- In the formulae (15) to (19): A10 to A19 each represent a substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 40 carbon atoms, substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic amino group, or substituted or unsubstituted aryl group having 8 to 40 carbon atoms bonded with an aromatic heterocyclic group;
- A10, A13, A15 and A17 are adapted to be respectively bonded to A11, A14, A16 and A18 to form a ring;
- X4 to X9 represent a single bond or a linking group having 1 to 30 carbon atoms;
- Y6 to Y24 represent a hydrogen atom, halogen atom, substituted or unsubstituted alkyl group having 1 to 40 carbon atoms, substituted or unsubstituted heterocyclic group having 3 to 20 carbon atoms, substituted or unsubstituted aryl group having 6 to 40 carbon atoms, substituted or unsubstituted aralkyl group having 7 to 20 carbon atoms, substituted or unsubstituted alkenyl group having 2 to 40 carbon atoms, substituted or unsubstituted alkylamino group having 1 to 40 carbon atoms, substituted or unsubstituted aralkylamino group having 7 to 60 carbon atoms, substituted or unsubstituted alkylsilyl group having 3 to 20 carbon atoms, substituted or unsubstituted arylsilyl group having 8 to 40 carbon atoms, substituted or unsubstituted aralkylsilyl group having 8 to 40 carbon atoms, or substituted or unsubstituted halogenated alkyl group having 1 to 40 carbon atoms; and
- XA, XB, XC, XD, XE each represent a sulfur atom, an oxygen atom or a monoaryl-substituted nitrogen atom.
- Examples of compounds represented by the formulae (13), (14), and (15) to (19) are as follows.
- An organic EL device according to a seventh exemplary embodiment preferably contains a metal complex as the second host material.
- The metal complex is preferably represented by a formula (20) below.
-
L11L12L13M11 2Q11 (20) - In the formula: ligands L11, L12 and L13 are independently selected from a structure represented by a formula (21) below; M11 is a divalent metal; and Q11 is a monovalent anion induced from inorganic or organic acids.
- In the ligands: Xb is O, S or Se; a-ring is oxazole, thiazole, imidazoles, oxadiazole, thiadiazole, benzooxazole, benzothiazole, benzoimidazole, pyridine, or quinoline; R121 to R124 are independently hydrogen, an alkyl group having 1 to 5 carbon atoms, halogen, silyl group or aryl group having 6 to 20 carbon atoms, which may be bonded to an adjacent substituent via alkylene or alkenylene to form a fused ring.
- The pyridine and quinoline may be bonded to R1 to form a fused ring.
- The a-ring and the aryl group for R121 to R124 may be further substituted by a C1-C5 alkyl group, halogen, C1-C5 alkyl group having a halogen substituent, phenyl group, naphthyl group, silyl group, or amino group.
- The ligands L11, L12 and L13 are independently selected from the following structures.
- In the ligands: X and R1 to R4 represent the same as Xb and R121 to R124 in the formula (21); Y is O, S or NR21; Z is CH or N; R11 to R16 are independently hydrogen, a C1-C5 alkyl group, halogen, C1-C5 alkyl group having a halogen substituent, phenyl group, naphthyl group, silyl group, or amino group; and R11 to R14 may be bonded to an adjacent substituent via alkylene or alkenylene to form a fused ring.
- The ligands L11, L12 and L13 of the compound may be the same and can be selected from the following structures.
- In the ligands: X is O, S or Se; R2, R3, R12 and R13 are independently hydrogen, methyl, ethyl, n-propyl, isopropyl, fluorine, chlorine, trifluoromethyl, phenyl, naphthyl, fluorenyl, trimethylsilyl, triphenylsilyl, t-butyldimethylsilyl, dimethylamine, diethylamine, or diphenylamine.
- The phenyl, naphthyl, fluorenyl are further substituted by fluorine, chlorine, trimethylsilyl, triphenylsilyl, t-butyldimethylsilyl, dimethylamine, diethylamine, or diphenylamine
- Furthermore, in this exemplary embodiment, the metal complex is preferably a zinc complex. Examples of such a preferable zinc complex are shown below.
- The second host material may be compounds represented by formulae (22) to (24) below.
- In the formulae (22) to (24): X101 to X108 are a nitrogen atom or C—Ar131.
- Ar131 represent a hydrogen atom, a fluorine atom, a cyano group, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, substituted or unsubstituted alkylsilyl having 1 to 10 carbon atoms, substituted or unsubstituted arylsilyl having 6 to 30 carbon atoms, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 ring carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms.
- Adjacent ones of X101 to X108 may be bonded to each other to form a ring structure.
- B1 and B2 represent a group represented by a formula (25A) or (25B) below.
-
(M1)c−(L5)d−(M2)e (5) - In the formula (25A): M1 and M2 each independently represent a substituted or unsubstituted nitrogen-containing aromatic heterocyclic ring or nitrogen-containing fused aromatic heterocyclic ring having 2 to 40 ring carbon atoms; M1 and M2 may be the same or different;
- L5 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms, substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, or substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
-
(M3)c−(L6)d−(M4)e (25B) - In the formula (25B): M3 and M4 each independently represent a substituted or unsubstituted aromatic hydrocarbon group having 2 to 40 ring carbon atoms; M3 and M4 may be the same or different; L6 represents a single bond, substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, substituted or unsubstituted fused aromatic hydrocarbon group having 6 to 30 carbon atoms, or substituted or unsubstituted cycloalkylene group having 5 to 30 carbon atoms;
- c represents an integer of 0 to 2; d represents an integer of 1 to 2; e represents an integer of 0 to 2; and c+e represents 1 or more.
- The formulae (25A) and (7A) are respectively the same as the formulae (25B) and (7B). M1 to M4 and L5 to L6 are the same as those described in relation to the formulae (7A) and (7B).
- Specific examples of compounds represented by the formulae (22) to (24) are shown.
- The second host material may be compounds represented by the above formula (1) and having a different structure from that of the first host material.
- It should be noted that the invention is not limited to the above description but may include any modification as long as such modification stays within a scope and a spirit of the invention.
- For instance, the following is a preferable example of such modification made to the invention.
- In the invention, the emitting layer may also preferably contain an assistance material for assisting injection of charges.
- When the emitting layer is formed of a host material that exhibits a wide energy gap, a difference in ionization potential (Ip) between the host material and the hole injecting/transporting layer etc. becomes so large that injection of the holes into the emitting layer becomes difficult, which may cause a rise in a driving voltage required for providing sufficient luminance.
- In the above instance, introducing a hole-injectable/transportable assistance material for assisting injection of charges in the emitting layer can contribute to facilitation of the injection of the holes into the emitting layer and to reduction of the driving voltage.
- As the assistance material for assisting the injection of charges, for instance, a typical hole injecting/transporting material or the like can be used.
- Specific examples of the assistance material for assisting the injection of charges are a triazole derivative, oxadiazole derivative, imidazoles derivative, polyarylalkane derivative, pyrazoline derivative, pyrazolone derivative, phenylenediamine derivative, arylamine derivative, amino-substituted chalcone derivative, oxazole derivative, styrylanthracene derivative, fluorenone derivative, hydrazone derivative, silazane derivative, polysilane copolymer, aniline copolymer, and conductive polymer oligomer (particularly, a thiophene oligomer).
- The hole injecting material is exemplified by the above. The hole injecting material is preferably a porphyrin compound, aromatic tertiary amine compound and styryl amine compound, particularly preferably aromatic tertiary amine compound.
- In addition, 4,4′-bis(N-(1-naphthyl)-N-phenylamino)biphenyl (hereinafter, abbreviated as NPD) having two fused aromatic rings in a molecule, or 4,4′,4″-tris(N-(3-methylphenyl)-N-phenylamino)triphenylamine (hereinafter, abbreviated as MTDATA) in which three triphenylamine units are bonded in a starburst form as disclosed and the like may also be used.
- Moreover, a hexaazatriphenylene derivative and the like may be also preferably used as the hole injecting material.
- Alternatively, inorganic compounds such as p-type Si and p-type SiC can also be used as the hole-injecting material.
- Next, the invention will be described in further detail by exemplifying Example(s) and Comparison(s). However, the invention is not limited by the description of Example(s).
- A synthesis scheme is shown below.
- 4-bromobenzaldehyde (25 g, 135 mmol) and acetophenone (16.2 g, 135 mmol) were added to ethanole (200 mL). An aqueous solution of 3M potassium hydrate (60 mL) was further added thereto and stirred at room temperature for 7 hours. A precipitated solid was separated by filtration. Then, the obtained solid was washed with methanol. A white solid intermediate body 1-1 (28.3 g, a yield rate 73%) was obtained.
- The intermediate body 1-1 (20 g, 69.7 mmol) and benzamidine hydrochloride (10.8 g, 69.7 mmol) were added to ethanole (300 mL). Sodium hydroxide (5.6 g, 140 mmol) was further added thereto and heated to reflux at room temperature for 8 hours. A precipitated solid was separated by filtration. Then, the obtained solid was washed with hexane. A white solid intermediate body 1-2 (10.3 g, a yield rate 38%) was obtained.
- Carbazole (15 g, 92.6 mmol) was added to ethanol (70 mL). Sulfuric acid (6 mL), water (3 mL), HIO4.2H2O (8.2 g, 35.9 mmol) and I2 (9.1 g, 35.9 mmol) were added thereto and stirred at room temperature for 4 hours. Water was added to the reaction solution and a precipitated solid was separated by filtration. Then, the obtained solid was washed with methanol. By dissolving the obtained solid in heated toluene for recrystallization, an intermediate body 1-3 (5.1 g, a yield rate 18.8%) was obtained.
- Under an argon gas atmosphere, N-phenylcarbazolyl-3-boronic acid (2.0 g, 7.0 mmol), the intermediate body 1-3 (2.05 g, 7.0 mmol), Pd(PPh3)4 (0.15 g, 0.14 mmol), toluene (20 mL) and an aqueous solution of 2M sodium carbonate (10.5 mL) were added together, and stirred at 80 degrees C. for 7 hours. Water was added to the reaction solution to precipitate solid. Then, the obtained solid was washed with methanol. By washing the obtained solid by heated toluene, an intermediate body 1-4 (2.43 g, a yield rate 84%) was obtained.
- Under an argon gas atmosphere, to a three-necked flask, the intermediate body 1-2 (2.28 g, 5.88 mmol), the intermediate body 1-4 (2.4 g, 5.88 mmol), CuI (0.56 g, 2.9 mmol), tripotassium phosphate (2.5 g, 11.8 mmol), anhydrous dioxane (30 mL) and cyclohexane diamine (0.33 g, 2.9 mmol) were added together in sequential order, and stirred at 100 degrees C. for 8 hours.
- Water was added to the reaction solution to precipitate solid. Then, the obtained solid was washed with hexane, followed by methanol. The obtained solid was refined by silica-gel column chromatography, whereby a white solid compound 1 (2.7 g, a yield rate 65%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 714 while a calculated molecular weight was 714.
- The intermediate body 1-1 (6.49 g, 22.6 mmol), phenacylpyridinium bromide (12.7 g, 45.6 mmol), ammonium acetate (45 g), acetic acid (200 mL), and N,N-dimethylformamide (200 mL) were heated to reflux and stirred for 8 hours.
- The reaction solution was put into an ice water and a precipitated solid was separated by filtration. Then, the obtained solid was washed with methanol. The obtained solid was refined by silica-gel column chromatography (dissolving solvent: hexane/methylene chloride), whereby an intermediate body 2-1 (3.7 g, a yield rate 42%) was obtained.
- Subsequently, under an argon gas atmosphere, to a three-necked flask, the intermediate body 2-1 (2.81 g, 7.3 mmol), the intermediate body 1-4 (3.0 g, 7.3 mmol), Cut (1.4 g, 7.3 mmol), tripotassium phosphate (2.3 g, 11 mmol), anhydrous dioxane (30 mL) and cyclohexane diamine (0.84 g, 7.3 mmol) were added together in sequential order, and stirred at 100 degrees C. for 8 hours.
- Water was added to the reaction solution to precipitate solid. Then, the obtained solid was washed with hexane, followed by methanol. The obtained solid was refined by silica-gel column chromatography, whereby a compound 2 (3.4 g, a yield rate 65%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 713 while a calculated molecular weight was 713.
- A synthesis scheme of the
compound 2 is shown below. - Under a nitrogen gas atmosphere, trichloropyrimidine (8 g, 43.4 mmol), phenylboronic acid (11.6 g, 95.4 mmol), tetrakis(triphenylphosphine)palladium (1.83 g, 1.74 mmol), toluene (300 mL) and an aqueous solution of 2M sodium carbonate (130 mL) were added together in sequential order, and heated to reflux for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby an intermediate body 3-1 (8.2 g, a yield of 71%) was obtained.
- Subsequently, under a nitrogen gas atmosphere, intermediate body 3-1 (8 g, 29.9 mmol), p-chlorophenylboronic acid (5.1 g, 32.9 mmol), tetrakis(triphenylphosphine)palladium (0.63 g, 0.6 mmol), toluene (60 mL) and an aqueous solution of 2M sodium carbonate (30 mL) were added together in sequential order, and heated to reflux for 8 hours.
- After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby an intermediate body 3-2 (7.0 g, a yield of 68%) was obtained.
- Under an argon gas atmosphere, the intermediate body 3-2 (6.5 g, 18.9 mmol), the intermediate body 1-4 (7.7 g, 18.9 mmol), palladium acetate (0.085 g, 0.38 mmol), sodium t-butoxide (2.72 g, 28.4 mmol), anhydrous toluene (60 mL), and tri-t-butyl phosphine (0.077 g, 0.38 mmol) were sequentially mixed, and stirred at 90 degrees C. for 8 hours.
- After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 3 (7.8 g, a yield of 58%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 715 while a calculated molecular weight was 715.
- A synthesis scheme of the
compound 3 is shown below. - 3,3′-dicarbazolyl was synthesized by a method disclosed in WO2006-25186.
- Under an argon gas atmosphere, to a three-necked flask, 3,3′-dicarbazolyl (2.4 g, 7.3 mmol), the intermediate body 1-2 (5.6 g, 14.6 mmol), Cut (1.4 g, 7.3 mmol), tripotassium phosphate (6.4 g, 30 mmol), anhydrous dioxane (50 mL) and cyclohexane diamine (0.84 g, 7.3 mmol) were added together in sequential order, and stirred at 100 degrees C. for 8 hours.
- Water was added to the reaction solution to precipitate solid. Then, the obtained solid was washed with hexane, followed by methanol. The obtained solid was refined by silica-gel column chromatography, whereby a compound 4 (3.1 g, a yield rate 45%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 945 while a calculated molecular weight was 945.
- A synthesis scheme of the
compound 4 is shown below. - Under an argon gas atmosphere, the intermediate body 3-1 (1.0 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours.
- After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 5 (1.8 g, a yield of 74%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 639 while a calculated molecular weight was 639.
- A synthesis scheme of the
compound 5 is shown below. - 3-bromobenzaldehyde (18.4 g, 100 mmol) was dissolved in dimethylformamide (300 mL). Benzamidine hydrochloride (31.2 g, 200 mmol) and potassium carbonate (41 g, 300 mmol) were added thereto and heated to reflux at 80 degrees C. for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby an intermediate body 6-1 (12 g, a yield of 32%) was obtained.
- Under an argon gas atmosphere, the intermediate body 6-1 (1.5 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours.
- After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 6 (2.3 g, a yield of 82%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 715 while a calculated molecular weight was 715.
- A synthesis scheme of the
compound 6 is shown below. - For synthesis of Compound 7, an intermediate body 7-1 was firstly synthesized by applying a method described in a document (J. Bergman, A. Brynolf, B. Elman and E. Vuorinen, Tetrahedron, 42, 3697-3706(1986)). Specifically, to a three-necked flask (500 ml), 1M tetrahydrofuran solution of phenylmagnesium bromide (100 ml, 100 mmol) was added. Dry ether (100 ml) was further added and heated to reflux in an oil bath at 45 degrees C. A dry ether solution (50 ml) of 2-cyanoaniline (5.91 g, 50 mmol) was dropped in for 30 minutes After refluxed for another 1.5 hours, the reaction solution was cooled down to 0 degree C. in an ice water bath. Subsequently, a dry ether solution (100 ml) of 4-bromobenzoate chloride (13.2 g, 60 mmol) was dropped in the reaction solution for 10 minutes and heated to reflux for 2 hours in a 45-degree-C oil bath. After reaction, the reaction solution was cooled down to 0 degree C. in an ice water bath. A saturated ammonium chloride aqueous solution was added. A precipitated solid was separated by filtration. Then, the obtained was washed with a small amount of methanol and vacuum-dried to obtain an intermediate body 7-1 (10.8 g, a yield of 60%).
- Subsequently, under a nitrogen atmosphere, the intermediate body 7-1 (1.4 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 7 (2.0 g, a yield of 75%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 688 while a calculated molecular weight was 688.
- A synthesis scheme of the compound 7 is shown below.
- Specifically, to a three-necked flask (500 ml), 1M tetrahydrofuran solution of phenylmagnesium bromide (100 ml, 100 mmol) was added. Dry ether (100 ml) was further added and heated to reflux in an oil bath at 45 degrees C. A dry ether solution (50 ml) of 2-cyanoaniline (5.91 g, 50 mmol) was dropped in for 30 minutes After refluxed for another 1.5 hours, the reaction solution was cooled down to 0 degree C. in an ice water bath. Subsequently, a dry ether solution (100 ml) of 3-bromobenzoate chloride (13.2 g, 60 mmol) was dropped in the reaction solution for 10 minutes and heated to reflux for 2 hours in a 45-degree-C oil bath. After reaction, the reaction solution was cooled down to 0 degree C. in an ice water bath. A saturated ammonium chloride aqueous solution was added. A precipitated solid was separated by filtration. Then, the obtained was washed with a small amount of methanol and vacuum-dried to obtain an intermediate body 8-1 (8.5 g, a yield of 47%).
- Subsequently, under a nitrogen atmosphere, the intermediate body 8-1 (1.4 g, 3.9 mmol), the intermediate body 1-4 (1.6 g, 3.9 mmol), tris(dibenzylideneacetone)dipalladium (0.071 g, 0.078 mmol), tri-t-butylphosphonium tetrafluoroborate (0.091 g, 0.31 mmol), sodium t-butoxide (0.53 g, 5.5 mmol), and anhydrous toluene (20 mL) were sequentially mixed, and heated to reflux for 8 hours. After the reaction solution was cooled down to the room temperature, an organic layer was removed and an organic solvent was distilled away under reduced pressure. The obtained residue was refined by silica-gel column chromatography, whereby a compound 8 (1.9 g, a yield of 71%) was obtained.
- FD-MS analysis consequently showed that m/e was equal to 688 while a calculated molecular weight was 688.
- A synthesis scheme of the compound 8 is shown below.
- A glass substrate (size: 25 mm×75 mm×1.1 mm) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV (Ultraviolet)/ozone-cleaned for 30 minutes.
- After the glass substrate having the transparent electrode was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, and a hole injecting layer was initially formed by depositing a compound A onto the substrate to be 40 nm thick to cover a surface of the glass substrate where a transparent electrode line was provided. Next, a compound B was deposited onto the hole injecting layer to be 20 nm thick, and a hole transporting layer was obtained.
- A phosphorescent-emitting layer was obtained by co-depositing the
compound 1 used as a phosphorescent host material and Ir(Ph-ppy)3 used as a phosphorescent dopant material onto the hole transporting layer to be 40 nm thick. The concentration of Ir(Ph-ppy)3 was 20 mass %. - Subsequently, a 30-nm-thick compound C, 1-nm-thick LiF and 80-nm-thick metal Al are sequentially layered to obtain a cathode. LiF, which is an electron injectable electrode, was formed at a speed of 1 Å/min.
- In Example 1, the
compounds 2 to 6 below were used in place of thecompound 1 to manufactureorganic EL devices 2 to 6. - The organic EL devices according respectively to
Comparisons 1 to 4 were formed in the same manner as in Example 1 except that the following comparative compounds D to G were respectively used as a host material in place of thecompound 1 in Example 1. - The organic EL devices manufactured in Examples 1 to 6 and
Comparisons 1 to 4 were driven by direct-current electricity to emit light, where luminescent performance was evaluated and time elapsed until an initial luminescence intensity of 20,000 cd/m2 was reduced to the half was measured. The results are shown in Table 4. -
TABLE 4 Luminous Efficiency Voltage (V) (cd/A) @1 Luminance Host Material @1 mA/cm2 mA/cm2 half-life (hrs) Example 1 Compound 14.0 61 950 Example 2 Compound 24.1 63 750 Example 3 Compound 34.0 65 730 Example 4 Compound 44.3 62 670 Example 5 Compound 54.1 61 1100 Example 6 Compound 64.3 64 1000 Comparison 1Compound D 4.2 38 310 Comparison 2Compound E 4.5 54 450 Comparison 3Compound F 5.1 50 210 Comparison 4Compound G 4.6 48 350 - Table 4 shows that the compounds of the invention used in Examples 1 to 6 have a significantly long luminance half-life and a high luminous efficiency while being capable of low-voltage drive compared with those of
Comparisons 1 to 4. - In
Comparison 1, since the compound D has a single carbazolyl group and is poor in hole transporting performance, luminance half-life is short. InComparison 2, although having two carbazolyl groups, the compound E has a poor hole transporting performance and a short luminance half-life, presumably because of small overlapping margin between the molecules. InComparison 3, since the compound F has a nitrogen-containing heterocyclic ring only in a carbazolyl group, electrons are difficult to be injected, so that the compound F has a low luminous efficiency and a short luminance half-life. InComparison 4, although having two carbazolyl groups, the compound G has a poor hole transporting capability and a short luminance half-life, presumably because of small overlapping margin between the molecules. - A glass substrate (size: 25 mm×75 mm×1.1 mm thick) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV/ozone-cleaned for 30 minutes.
- After the glass substrate having the transparent electrode line was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, so that the following electron accepting compound (C-1) was deposited to form a 5-nm thick C-1 film on a surface of the glass substrate where the transparent electrode line was provided so as to cover the transparent electrode. On the C-1 film, the following aromatic amine derivative (X1) was deposited as a first hole transporting material to form a 50-nm thick first hole transporting layer. After film formation of the first hole transporting layer, the following aromatic amine derivative (X2) was deposited as a second hole transporting material to form a 60-nm thick second hole transporting layer.
- Further on the second hole transporting layer, the
compound 1 obtained in Synthesis Example 1 was deposited to form a 45-nm thick emitting layer. Simultaneously, the following compound (D3) was co-deposited as a phosphorescent material. A concentration of the compound D3 was 8.0 mass %. This co-deposited film serves as the emitting layer. - After the film formation of the emitting layer, a 30-nm thick film of the following compound (ET2) was formed. The ET1 film serves as the electron transporting layer.
- Next, a 1-nm thick film of LiF was formed as an electron-injecting electrode (cathode) at a film-forming speed of 0.1 A/min. Metal (Al) was deposited on the LiF film to form an 80-nm thick metal cathode, thereby providing an organic electroluminescence device.
- For each of the obtained organic EL devices, luminous efficiency was measured when the device was driven by DC constant current at the initial luminescence of 2000 cd/m2 at the room temperature, and the time elapsed until a half-life of emission was measured when the device was driven by DC constant current at the initial luminescence of 5000 cd/m2 at the room temperature. The results are shown in Table 5.
- The organic EL devices according to Examples 8 to 14 were manufactured in the same manner as that in Example 7 except that the
compounds 2 to 8 were used in place of thecompound 1 as materials for the emitting layer. For each of the obtained organic EL devices, luminous efficiency was measured when the device was driven by DC constant current at the initial luminescence of 2000 cd/m2 at the room temperature, and the time elapsed until a half-life of emission was measured when the device was driven by DC constant current at the initial luminescence of 5000 cd/m2 at the room temperature. The results are shown in Table 5. - The organic EL devices according to
Comparisons 5 and 7 were manufactured in the same manner as that in Example 7 except that the comparative compounds D and F were used in place of thecompound 1 as materials for the emitting layer. For each of the obtained organic EL devices, luminous efficiency was measured when the device was driven by DC constant current at the initial luminescence of 2000 cd/m2 at the room temperature, and the time elapsed until a half-life of emission was measured when the device was driven by DC constant current at the initial luminescence of 5000 cd/m2 at the room temperature. The results are shown in Table 5. -
TABLE 5 Luminous Efficiency Luminance Host Material Voltage (V) (cd/A) half-life (hrs) Example 7 Compound 14.1 11 400 Example 8 Compound 24.3 10 350 Example 9 Compound 34.2 12 540 Example 10 Compound 44.4 12 350 Example 11 Compound 54.1 12 450 Example 12 Compound 64.2 12 450 Example 13 Compound 7 4.2 11 400 Example 14 Compound 8 4.1 10 350 Comparison 5Compound D 4.1 7 200 Comparison 6Compound F 5.2 6.5 220 - The table 5 shows that the compounds of the invention also function as a red phosphorescent host material.
- The organic EL devices according respectively to Examples 15 to 18 and Comparison 7 were formed in the same manner as in Example 1 except that the materials, a thickness of each of the layers and a concentration of each of the emitting materials were changed as shown in Table 6. In Table 6, figures in parentheses without a unit indicate a thickness of each of the layers (unit: nm). A structure of HT-7 is shown below.
- Table 7 shows physical properties of the host materials used in Examples 15 to 18 and Comparison 7.
- A method for measuring each of the physical properties is as follows.
- Ionization potential was measured in the atmosphere by using a photoelectron spectrometer (AC-1 manufactured by Riken Keiki Co., Ltd.). Specifically, ionization potential was measured by irradiating the materials with light and measuring the amount of electrons generated by charge separation at that time.
- Affinity was calculated based on measurement values of ionization potential Ip and energy gap Eg. A calculation equitation is as follows.
-
Af=Ip−Eg - The energy gap was measured from an absorption end of absorption spectrum of benzene. Specifically, the absorption spectrum is measured with a commercially available ultraviolet-visible spectrophotometer, and the energy gap is calculated from a wavelength at which the absorption spectrum appears.
- The optical energy gap S1 (also referred to as singlet energy) is a difference between a conduction level and a valence level. The optical energy gap was obtained by converting into energy a wavelength value at an intersection of a long-wavelength-side tangent line in an absorbing spectrum of a toluene-diluted solution of each material and a base line in the absorbing spectrum (zero absorption).
- The triplet energy gap T1 of the material may be exemplarily defined based on the phosphorescence spectrum. The triplet energy gap T1 was defined as follows in Examples.
- Specifically, each material was dissolved in an EPA solvent (diethylether:isopentane:ethanol=5:5:2 in volume ratio) with a concentration of 10 μmol/L, thereby forming a sample for phosphorescence measurement.
- Then, the sample for phosphorescence measurement was put into a quartz cell, cooled to 77K and irradiated with exciting light, so that a wavelength of phosphorescence radiated therefrom was measured.
- A tangent line is drawn to be tangent to a rising section adjacent to short-wavelength of the obtained phosphorescence spectrum, a wavelength value at an intersection of the tangent line and a base line is converted into energy value, and the converted energy value is defined as the triplet energy gap T1.
- For the measurement, a measurement machine F-4500 (manufactured by Hitachi) was used.
- Voltage was applied to the organic EL device so that a current density becomes 10 mA/cm2, and a voltage value (V) at that time was measured. Moreover, current efficiency (L/J) (cd/A), power efficiency (lm/W) and life-time (hrs) were measured.
- As for life-time, after the device was driven by constant current at the initial luminescence, an elapsed time when the luminescence reached 90% of the initial luminescence (LT90) or the luminescence reached 50% of the initial luminescence (LT50) was measured.
- The results are shown in Table 8.
-
TABLE 6 Arrangement of Organic EL Device Example 15 ITO(70)/Compound A(30)/HT-7(20)/PH1 + Ir(ppy)3(40, 90% + 10%)/ET2(30)/Liq(1)/Al(80) Example 16 ITO(70)/Compound A(30)/HT-7(20)/PH2 + Ir(ppy)3(40, 90% + 10%)/ET2(30)/LiF(1)/Al(80) Example 17 ITO(70)/Compound A(30)/HT-7(20)/PH3 + Ir(ppy)3(40, 90% + 10%)/ET2(30)/LiF(1)/Al(80) Example 18 ITO(130)/Compound A(50)/PH2 + Ir(piq)3(30, 92% + 8%)/ET2(30)/LiF(1)/Al(80) Comparison 7 ITO(70)/Compound A(30)/HT-7(20)/PH5 + Ir(ppy)3(40, 90% + 10%)/ET2(30)/Liq(1)/Al(80) -
TABLE 8 Current Host Voltage density luminescence material (V) (mA/cm2) (nit) L/J (cd/A) η (lm/W) LT90 (hrs) LT50 (hrs) Example 15 PH1 3.00 1 494 49.4 51.7 250 — @3000 cd/m2 Example 16 PH2 2.85 1 637 63.7 70.3 — 500 @10000 cd/m2 Example 17 PH3 2.67 1 572 57.2 67.3 — 800 @10000 cd/m2 Example 18 PH2 3.54 1 85 8.5 7.5 — 1000 @10000 cd/m2 Comparison 7 PH5 2.72 1 492 49.2 56.7 90 — @3000 cd/m2 - A glass substrate (size: 25 mm×75 mm×1.1 mm) having an ITO transparent electrode (manufactured by GEOMATEC Co., Ltd.) was ultrasonic-cleaned in isopropyl alcohol for five minutes, and then UV (Ultraviolet)/ozone-cleaned for 30 minutes.
- After the glass substrate having the transparent electrode was cleaned, the glass substrate was mounted on a substrate holder of a vacuum deposition apparatus, and a hole injecting layer was initially formed by depositing a compound A onto the substrate to be 40 nm thick to cover a surface of the glass substrate where a transparent electrode line was provided. Next, a compound B was deposited onto the hole injecting layer to be 20 nm thick, and a hole transporting layer was obtained.
- A phosphorescent-emitting layer was obtained by co-depositing the
compound 3 used as the first phosphorescent host material, Ir(Ph-ppy)3 used as a phosphorescent dopant material, and HT-5 used as the following second host material onto the hole transporting layer to be 40 nm thick. The concentration of Ir(Ph-ppy)3 was 20 mass % and the concentration of HT-5 was 10 mass %. - Subsequently, a 30-nm-thick compound C, 1-nm-thick LiF and 80-nm-thick metal Al are sequentially layered to obtain a cathode. LiF, which is an electron injectable electrode, was formed at a speed of 1 Å/min.
- With respect to the organic EL devices 19, luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m2 was reduced to the half was measured. The results are shown in Table 9.
- In Example 19, HT-9 was used in place of HT-5 to manufacture an
organic EL device 6. - With respect to the organic EL devices 20, luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m2 was reduced to the half was measured. The results are shown in Table 9.
- In Example 19, the compound D was used in place of the
compound 3 to manufacture an organic EL device. With respect to the organic EL devices, luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m2 was reduced to the half was measured. The results are shown in Table 9. - In Comparison 8, HT-9 was used in place of HT-5 to manufacture an organic EL device. With respect to the organic EL devices, luminescent performance was evaluated in the same manner as in Example 1 and time elapsed until an initial luminescence intensity of 20,000 cd/m2 was reduced to the half was measured. The results are shown in Table 9.
-
TABLE 9 Voltage Luminous (V) Efficiency First/Second @1 mA/ (cd/A) Luminance host materials cm2 @1 mA/cm2 half-life (hrs) Example 19 Compound 3.9 72 800 3/HT-5 Example 20 Compound 3.9 69 800 3/HT-9 Comparison 8 Compound 4.0 40 400 D/HT-5 Comparison 9 Compound 4.1 41 380 D/HT-9 - Table 9 shows that the compounds of the invention used in Examples 19 and 20 have a significantly long luminance half-life and a significantly high luminous efficiency as compared with those in Comparisons 8 and 9.
- The organic EL devices according respectively to Examples 21 to 26 were formed in the same manner as in Example 19 except that the materials, a thickness of each of the layers and a concentration of each of the emitting materials were changed as shown in Table 10.
- Table 7 above and Table 11 below show the chemical formulae and physical properties of the host material used in Examples 21 to 26.
-
TABLE 10 Arrangement of Organic EL Device Example 21 ITO(70)/Compound A(30)/HT-7(20)/PH1 + PH5 + Ir(ppy)3(40, 45% + 45% + 10%)/ET2(30)/Liq(1)/Al(80) Example 22 ITO(70)/Compound A(30)/HT-7(20)/PH2 + PH6 + Ir(ppy)3(40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80) Example 23 ITO(70)/Compound A(30)/HT-7(20)/PH3 + PH6 + Ir(ppy)3(40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80) Example 24 ITO(70)/Compound A(30)/HT-7(20)/PH3 + PH7 + Ir(ppy)3(40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80) Example 25 ITO(130)/Compound A(50)/PH2 + PH10 + Ir(piq)3(30, 50% + 42% + 8%)/ET2(30)/LiF(1)/Al(80) Example 26 ITO(70)/Compound A(30)/HT-7(20)/PH1 + PH3 + Ir(ppy)3(40, 45% + 45% + 10%)/ET2(30)/LiF(1)/Al(80) - The organic EL devices according to Examples 21 to 26 were evaluated in the same manner as in Examples 15 to 18. The results are shown in Table 12.
-
FIG. 2(A) shows an energy diagram in the emitting layer in Example 21.FIG. 2(B) shows an energy diagram in the emitting layer in Example 23. -
TABLE 12 Current Host Voltage density luminescence material (V) (mA/cm2) (nit) L/J (cd/A) η (lm/W) LT90 (hrs) LT50 (hrs) Example 21 PH1/PH5 2.83 1 517 51.7 57.5 600 — @3000 cd/m2 Example 22 PH2/PH6 3.39 1 673 67.3 62.4 — 900 @10000 cd/m2 Example 23 PH3/PH6 3.10 1 726 72.6 73.6 — 1000 @10000 cd/m2 Example 24 PH3/PH7 3.08 1 888 88.8 90.6 40 — @20000 cd/m2 Example 25 PH2/PH10 3.88 1 92 9.2 7.4 — 1500 @10000 cd/m2 Example 26 PH2/PH3 2.92 1 752 75.2 80.9 — 750 @10000 cd/m3 - In comparison between Table 8 and Table 12, it has been found that the organic EL devices according to Examples 21 to 26, in which the first and second host materials are used, have a long life-time and improved luminous efficiency as compared with the organic EL devices in which a single host material is used.
Claims (11)
1-27. (canceled)
28. A compound represented by a formula (1) below:
wherein A1 and A2 each mutually independently represent a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms,
X1 and X2 are each a linking group and independently represent a single bond, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, a substituted or unsubstituted fused aromatic hydrocarbon group having 10 to 30 ring carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms, or a substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms,
Y1, Y2, Y3 and Y4 each independently represent a hydrogen atom, a fluorine atom, a cyano group, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 20 carbon atoms, a substituted or unsubstituted haloalkyl group having 1 to 20 carbon atoms, a substituted or unsubstituted haloalkoxy group having 1 to 20 carbon atoms, a substituted or unsubstituted alkylsilyl group having 1 to 10 carbon atoms, a substituted or unsubstituted arylsilyl group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, a substituted or unsubstituted fused aromatic hydrocarbon group having 10 to 30 ring carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 ring carbon atoms; or a substituted or unsubstituted fused aromatic heterocyclic group having 2 to 30 ring carbon atoms,
p and q represent 0 or 2, p and q being not simultaneously 0;
r and s represent an integer of 1 to 3,
when p=2, a plurality of Y1 may be mutually the same or different,
when q=2, a plurality of Y2 may be mutually the same or different,
when r=2 or 3, a plurality of Y3 may be mutually the same or different,
when s=2 or 3, a plurality of Y4 may be mutually the same or different, when p is 2, a single pair of adjacent ones of Y1 are mutually bonded to form a ring structure, and
when q is 2, a single pair of adjacent ones of Y2 are mutually bonded to form a ring structure.
31. The compound according to claim 30 , wherein A1 is a substituted or unsubstituted nitrogen-containing compound having 1 to 30 ring carbon atoms.
32. The compound according to claim 28 , wherein one of A1 and A2 is a substituted or unsubstituted pyridine ring, a substituted or unsubstituted pyrimidine ring, a substituted or unsubstituted triazine ring, or a substituted or unsubstituted quinazoline ring.
33. An organic-EL-device material comprising the compound according to claim 28 .
34. An organic electroluminescence device, comprising:
a cathode;
an anode; and
a plurality of organic thin-film layers comprising an emitting layer, at least one layer of the organic thin-film layers comprising the organic-EL-device material according to claim 33 .
35. The organic electroluminescence device according to claim 34 , wherein the emitting layer comprises the organic-EL-device material as a host material.
36. The organic electroluminescence device according to claim 34 , wherein the emitting layer comprises a phosphorescent material.
37. The organic electroluminescence device according to claim 34 , wherein the phosphorescent material is an ortho-metalated complex of a metal atom selected from iridium (Ir), osmium (Os) and platinum (Pt).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/692,010 US20150228912A1 (en) | 2010-04-20 | 2015-04-21 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010097317 | 2010-04-20 | ||
| JP2010-097317 | 2010-04-20 | ||
| US35304710P | 2010-06-09 | 2010-06-09 | |
| JP2010291138 | 2010-12-27 | ||
| JP2010-291138 | 2010-12-27 | ||
| US201161433084P | 2011-01-14 | 2011-01-14 | |
| US13/091,036 US8652654B2 (en) | 2010-04-20 | 2011-04-20 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/372,954 US8865323B2 (en) | 2010-04-20 | 2012-02-14 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US14/492,395 US10193077B2 (en) | 2010-04-20 | 2014-09-22 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US14/692,010 US20150228912A1 (en) | 2010-04-20 | 2015-04-21 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/492,395 Continuation US10193077B2 (en) | 2010-04-20 | 2014-09-22 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20150228912A1 true US20150228912A1 (en) | 2015-08-13 |
Family
ID=44834201
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/091,071 Active US8877352B2 (en) | 2010-04-20 | 2011-04-20 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/091,036 Active US8652654B2 (en) | 2010-04-20 | 2011-04-20 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/372,954 Active US8865323B2 (en) | 2010-04-20 | 2012-02-14 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/372,943 Active US8940414B2 (en) | 2010-04-20 | 2012-02-14 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US14/492,395 Active 2034-03-08 US10193077B2 (en) | 2010-04-20 | 2014-09-22 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US14/692,010 Abandoned US20150228912A1 (en) | 2010-04-20 | 2015-04-21 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Family Applications Before (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/091,071 Active US8877352B2 (en) | 2010-04-20 | 2011-04-20 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/091,036 Active US8652654B2 (en) | 2010-04-20 | 2011-04-20 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/372,954 Active US8865323B2 (en) | 2010-04-20 | 2012-02-14 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US13/372,943 Active US8940414B2 (en) | 2010-04-20 | 2012-02-14 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US14/492,395 Active 2034-03-08 US10193077B2 (en) | 2010-04-20 | 2014-09-22 | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Country Status (7)
| Country | Link |
|---|---|
| US (6) | US8877352B2 (en) |
| EP (2) | EP2415769B1 (en) |
| JP (4) | JP5074627B2 (en) |
| KR (3) | KR101421365B1 (en) |
| CN (3) | CN104592206B (en) |
| TW (2) | TWI477580B (en) |
| WO (2) | WO2011132684A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9287512B2 (en) | 2011-03-08 | 2016-03-15 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds, layers and organic electroluminescent device using the same |
| US10069076B2 (en) | 2012-08-03 | 2018-09-04 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, and lighting device |
| US10326079B2 (en) | 2015-04-27 | 2019-06-18 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| EP3588599A1 (en) | 2018-06-26 | 2020-01-01 | Idemitsu Kosan Co., Ltd. | Composition, organic-electroluminescence-device material, composition film, organic electroluminescence device, and electronic device |
| US10693094B2 (en) | 2015-09-30 | 2020-06-23 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, display device, electronic device, and lighting device |
| US11552256B2 (en) | 2014-07-25 | 2023-01-10 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, lighting device, and organic compound |
| US11812626B2 (en) | 2011-02-16 | 2023-11-07 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element |
| US11925041B2 (en) | 2015-09-30 | 2024-03-05 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, display device, electronic device, and lighting device |
| US12029059B2 (en) | 2012-08-10 | 2024-07-02 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, display device, electronic device, and lighting device |
Families Citing this family (231)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2518045A1 (en) * | 2006-11-24 | 2012-10-31 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent element using the same |
| WO2009066600A1 (en) * | 2007-11-19 | 2009-05-28 | Idemitsu Kosan Co., Ltd. | Monobenzochrysene derivative, organic electroluminescent device material containing the same, and organic electroluminescent device using the organic electroluminescent device material |
| KR101506999B1 (en) | 2009-11-03 | 2015-03-31 | 제일모직 주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| US8828561B2 (en) | 2009-11-03 | 2014-09-09 | Cheil Industries, Inc. | Compound for organic photoelectric device and organic photoelectric device including the same |
| WO2011132684A1 (en) | 2010-04-20 | 2011-10-27 | 出光興産株式会社 | Bis-carbazole derivative, material for organic electroluminescent element and organic electroluminescent element using same |
| KR20110122051A (en) | 2010-05-03 | 2011-11-09 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| KR101420318B1 (en) * | 2010-06-17 | 2014-07-16 | 이-레이 옵토일렉트로닉스 테크놀로지 컴퍼니 리미티드 | Compound for organic electroluminescent device and organic electroluminescent device having the same |
| JP5821635B2 (en) * | 2010-06-24 | 2015-11-24 | 東レ株式会社 | Light emitting device material and light emitting device |
| JP6007467B2 (en) * | 2010-07-27 | 2016-10-12 | コニカミノルタ株式会社 | Organic electroluminescence element material, organic electroluminescence element, |
| JP2013539206A (en) * | 2010-07-30 | 2013-10-17 | ローム・アンド・ハース・エレクトロニック・マテリアルズ・コリア・リミテッド | Electroluminescent device using electroluminescent compound as luminescent material |
| KR101753172B1 (en) | 2010-08-20 | 2017-07-04 | 유니버셜 디스플레이 코포레이션 | Bicarbazole compounds for oleds |
| KR101477614B1 (en) * | 2010-09-17 | 2014-12-31 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| KR101478000B1 (en) * | 2010-12-21 | 2015-01-05 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| JP2012156499A (en) * | 2011-01-05 | 2012-08-16 | Idemitsu Kosan Co Ltd | Organic electroluminescent element |
| JPWO2012099219A1 (en) * | 2011-01-20 | 2014-06-30 | 出光興産株式会社 | Organic electroluminescence device |
| US10147888B2 (en) * | 2011-02-07 | 2018-12-04 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative and organic electroluminescent element using same |
| WO2012108389A1 (en) | 2011-02-07 | 2012-08-16 | 出光興産株式会社 | Biscarbazole derivative and organic electroluminescent element using same |
| JP5964328B2 (en) * | 2011-02-11 | 2016-08-03 | ユニバーサル ディスプレイ コーポレイション | ORGANIC LIGHT EMITTING DEVICE AND MATERIAL FOR USE IN THE ORGANIC LIGHT EMITTING DEVICE |
| KR101427611B1 (en) * | 2011-03-08 | 2014-08-11 | 롬엔드하스전자재료코리아유한회사 | Novel compounds for organic electronic material and organic electroluminescence device using the same |
| CN103430344B (en) | 2011-03-14 | 2016-03-23 | 东丽株式会社 | Light emitting element material and light-emitting component |
| EP2690093A4 (en) * | 2011-03-24 | 2014-08-13 | Idemitsu Kosan Co | Bis-carbazole derivative and organic electroluminescent element using same |
| KR20120109744A (en) * | 2011-03-25 | 2012-10-09 | 롬엔드하스전자재료코리아유한회사 | Novel compounds for organic electronic material and organic electroluminescence device using the same |
| TWI552406B (en) * | 2011-03-25 | 2016-10-01 | 出光興產股份有限公司 | Organic electroluminescence device |
| JP6197265B2 (en) * | 2011-03-28 | 2017-09-20 | 東レ株式会社 | Light emitting device material and light emitting device |
| KR102159895B1 (en) * | 2011-04-07 | 2020-09-24 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Light-emitting element |
| CN106025099B (en) | 2011-04-12 | 2018-09-07 | 精工爱普生株式会社 | Light-emitting component, light-emitting device, authentication device and electronic equipment |
| KR20120116269A (en) * | 2011-04-12 | 2012-10-22 | 롬엔드하스전자재료코리아유한회사 | Novel compounds for organic electronic material and organic electroluminescence device using the same |
| JP5765034B2 (en) | 2011-04-18 | 2015-08-19 | セイコーエプソン株式会社 | Thiadiazole compounds, compounds for light emitting devices, light emitting devices, light emitting devices, authentication devices, and electronic devices |
| KR101971895B1 (en) | 2011-05-12 | 2019-04-25 | 도레이 카부시키가이샤 | Light-emitting element material and light-emitting element |
| KR101443756B1 (en) * | 2011-05-26 | 2014-09-23 | 제일모직 주식회사 | Compound for organic OPTOELECTRONIC device, ORGANIC LIGHT EMITTING DIODE INCLUDING THE SAME and DISPLAY INCLUDING THE organic LIGHT EMITTING DIODE |
| KR101354638B1 (en) | 2011-06-20 | 2014-01-22 | 제일모직주식회사 | MATERIAL for organic OPTOELECTRONIC device, ORGANIC LIGHT EMITTING DIODE INCLUDING THE SAME and DISPLAY INCLUDING THE organic LIGHT EMITTING DIODE |
| JP6034005B2 (en) * | 2011-08-03 | 2016-11-30 | 出光興産株式会社 | Biscarbazole derivative and organic electroluminescence device using the same |
| KR20130018547A (en) | 2011-08-09 | 2013-02-25 | 세이코 엡슨 가부시키가이샤 | Thiadiazole, light-emitting element, light-emitting apparatus, authentication apparatus, and electronic device |
| JP5790279B2 (en) | 2011-08-09 | 2015-10-07 | セイコーエプソン株式会社 | LIGHT EMITTING ELEMENT, LIGHT EMITTING DEVICE, AND ELECTRONIC DEVICE |
| KR102059328B1 (en) * | 2011-09-09 | 2020-02-20 | 이데미쓰 고산 가부시키가이샤 | Organic electroluminescence element |
| KR20140092332A (en) * | 2011-10-21 | 2014-07-23 | 이데미쓰 고산 가부시키가이샤 | Organic electroluminescence element and material for organic electroluminescence element |
| CN103380508B (en) | 2011-11-22 | 2017-09-08 | 出光兴产株式会社 | Aromatic heterocyclic derivative, material for organic electroluminescent element, and organic electroluminescent element |
| KR101497124B1 (en) * | 2011-11-28 | 2015-03-06 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| JP2013116975A (en) * | 2011-12-02 | 2013-06-13 | Kyushu Univ | Delayed fluorescent material, organic light emitting device, and compound |
| JP5898683B2 (en) * | 2011-12-05 | 2016-04-06 | 出光興産株式会社 | Material for organic electroluminescence device and organic electroluminescence device |
| WO2013084885A1 (en) * | 2011-12-05 | 2013-06-13 | 出光興産株式会社 | Organic electroluminescent element |
| US9530969B2 (en) | 2011-12-05 | 2016-12-27 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescence device and organic electroluminescence device |
| CN103159745B (en) * | 2011-12-16 | 2016-01-20 | 昱镭光电科技股份有限公司 | For compound and the OLED of OLED |
| KR20130074765A (en) * | 2011-12-26 | 2013-07-04 | 제일모직주식회사 | Compound for organic optoelectronic device, organic light emitting diode including the same and display including the organic light emitting diode |
| KR101474796B1 (en) * | 2011-12-26 | 2014-12-19 | 제일모직주식회사 | Compound for organic optoelectronic device, organic light emitting diode including the same and display including the organic light emitting diode |
| JP5970811B2 (en) | 2011-12-28 | 2016-08-17 | セイコーエプソン株式会社 | LIGHT EMITTING ELEMENT, LIGHT EMITTING DEVICE, AND ELECTRONIC DEVICE |
| TWI470776B (en) * | 2011-12-29 | 2015-01-21 | Ind Tech Res Inst | Light detecting array structure and light detecting module |
| KR101507004B1 (en) * | 2011-12-29 | 2015-03-30 | 제일모직 주식회사 | Compound for organic optoelectronic device, organic light emitting diode including the same and display including the organic light emitting diode |
| KR20130078437A (en) * | 2011-12-30 | 2013-07-10 | 제일모직주식회사 | Compound for organic optoelectronic device, organic light emitting diode including the same and display including the organic light emitting diode |
| KR101498278B1 (en) * | 2012-01-18 | 2015-03-06 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| WO2013112557A1 (en) | 2012-01-26 | 2013-08-01 | Universal Display Corporation | Phosphorescent organic light emitting devices having a hole transporting cohost material in the emissive region |
| US9324952B2 (en) | 2012-02-28 | 2016-04-26 | Seiko Epson Corporation | Thiadiazole, compound for light-emitting elements, light-emitting element, light-emitting apparatus, authentication apparatus, and electronic device |
| US9130174B2 (en) * | 2012-02-29 | 2015-09-08 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescence device and organic electroluminescence device |
| JP2015135836A (en) | 2012-03-29 | 2015-07-27 | 出光興産株式会社 | Organic electroluminescent element and material for organic electroluminescent element |
| WO2013145923A1 (en) * | 2012-03-30 | 2013-10-03 | 出光興産株式会社 | Organic electroluminescent element |
| TWI585091B (en) | 2012-03-30 | 2017-06-01 | 新日鐵住金化學股份有限公司 | Organic electroluminescent elements |
| KR20130114785A (en) * | 2012-04-10 | 2013-10-21 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device containing the same |
| KR20130121479A (en) * | 2012-04-27 | 2013-11-06 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device comprising the same |
| WO2013168688A1 (en) * | 2012-05-10 | 2013-11-14 | コニカミノルタ株式会社 | Organic electroluminescence element, illumination device, and display device |
| US9985232B2 (en) * | 2012-05-17 | 2018-05-29 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative host materials for OLED emissive region |
| JP2015167150A (en) | 2012-05-28 | 2015-09-24 | 出光興産株式会社 | Organic electroluminescent element |
| JP5925308B2 (en) * | 2012-06-01 | 2016-05-25 | 出光興産株式会社 | Organic electroluminescence device |
| KR101513006B1 (en) * | 2012-06-13 | 2015-04-17 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescence compounds and organic electroluminescence device containing the same |
| JP6100368B2 (en) * | 2012-06-14 | 2017-03-22 | ユニバーサル ディスプレイ コーポレイション | Biscarbazole derivative host material and green light emitter for OLED light emitting region |
| CN104364344A (en) * | 2012-06-14 | 2015-02-18 | 通用显示公司 | Biscarbazole derivative host materials and red emitters for OLED emissive regions |
| CN104507927A (en) * | 2012-06-18 | 2015-04-08 | 东曹株式会社 | Cyclic azine compound, method for producing same, and organic electroluminescent element containing same |
| TWI564297B (en) * | 2012-06-18 | 2017-01-01 | Tosoh Corp | Ring A compound, a method for producing the same, and an organic electroluminescent device containing the same |
| KR101507423B1 (en) * | 2012-06-22 | 2015-04-08 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof |
| KR101431645B1 (en) * | 2012-06-26 | 2014-08-20 | 롬엔드하스전자재료코리아유한회사 | Light-Emitting Layer and Organic Electroluminescence Device comprising the same |
| WO2014007565A1 (en) | 2012-07-04 | 2014-01-09 | 제일모직 주식회사 | Compound for organic optoelectric device, organic optoelectric device comprising same, and display apparatus comprising organic optoelectric device |
| JP2015524610A (en) * | 2012-07-04 | 2015-08-24 | サムスン エスディアイ カンパニー,リミテッド | Composition for organic light emitting device, organic light emitting layer containing the same, and organic light emitting device |
| KR101946891B1 (en) * | 2012-07-05 | 2019-02-12 | 도레이 카부시키가이샤 | Light emitting elment material and light emitting element |
| WO2014007022A1 (en) * | 2012-07-06 | 2014-01-09 | 東レ株式会社 | Light emitting element material and light emitting element |
| JP5959970B2 (en) | 2012-07-20 | 2016-08-02 | 出光興産株式会社 | Organic electroluminescence device |
| JP6299223B2 (en) * | 2012-07-25 | 2018-03-28 | 東レ株式会社 | Light emitting device material and light emitting device |
| JP6312960B2 (en) * | 2012-08-03 | 2018-04-18 | 株式会社半導体エネルギー研究所 | Light emitting element, light emitting device, electronic device, lighting device, and heterocyclic compound |
| CN104603107B (en) * | 2012-09-07 | 2020-11-17 | 出光兴产株式会社 | Novel aromatic heterocyclic derivative, material for organic electroluminescent element, material solution for organic electroluminescent element, and organic electroluminescent element |
| US10957870B2 (en) | 2012-09-07 | 2021-03-23 | Universal Display Corporation | Organic light emitting device |
| US20140103316A1 (en) * | 2012-10-12 | 2014-04-17 | Industry-Academic Cooperation Foundation Gyeongsang National University | Organometallic complex and organic light-emitting device including the same |
| KR101973973B1 (en) * | 2012-10-12 | 2019-08-23 | 도레이 카부시키가이샤 | Fluoranthene derivative, luminescent element material containing same, and luminescent element |
| CN103772416B (en) | 2012-10-18 | 2018-01-19 | 精工爱普生株式会社 | Thiadiazoles system compound, light-emitting component compound, light-emitting component, light-emitting device, authentication device and electronic equipment |
| US20140131665A1 (en) * | 2012-11-12 | 2014-05-15 | Universal Display Corporation | Organic Electroluminescent Device With Delayed Fluorescence |
| JP2014103243A (en) | 2012-11-20 | 2014-06-05 | Samsung Display Co Ltd | Organic el material containing azacarbazole derivative having carbazolyl group, and organic el element using the same |
| KR101468402B1 (en) * | 2012-11-21 | 2014-12-03 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescence compounds and organic electroluminescence device containing the same |
| JP6133583B2 (en) * | 2012-12-03 | 2017-05-24 | 出光興産株式会社 | Organic electroluminescence device |
| KR102087950B1 (en) | 2012-12-04 | 2020-03-12 | 삼성디스플레이 주식회사 | Heterocyclic compound and organic light emitting device comprising the same |
| JP6307687B2 (en) | 2012-12-05 | 2018-04-11 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | AMINE DERIVATIVE, ORGANIC LIGHT EMITTING MATERIAL AND ORGANIC ELECTROLUMINESCENT DEVICE USING THE SAME |
| US9209411B2 (en) | 2012-12-07 | 2015-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10361378B2 (en) | 2012-12-17 | 2019-07-23 | Nippon Steel Chemical & Material Co., Ltd. | Organic electroluminescent device |
| KR102061571B1 (en) * | 2012-12-24 | 2020-01-02 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof |
| KR102055685B1 (en) * | 2012-12-27 | 2019-12-16 | 삼성디스플레이 주식회사 | Organic light emitting diode comprising the same |
| KR101820865B1 (en) | 2013-01-17 | 2018-01-22 | 삼성전자주식회사 | MATERIAL FOR ORGANIC OPTOELECTRONIC DEVICE, ORGANIC LiGHT EMITTING DIODE INCLUDING THE SAME AND DISPLAY INCLUDING THE ORGANIC LiGHT EMITTING DIODE |
| WO2014122937A1 (en) * | 2013-02-08 | 2014-08-14 | ソニー株式会社 | Organic electroluminescence element |
| KR102052071B1 (en) | 2013-02-21 | 2019-12-05 | 삼성디스플레이 주식회사 | Organic light emitting device |
| KR20140126610A (en) * | 2013-04-23 | 2014-10-31 | 삼성디스플레이 주식회사 | Organic light emitting diode |
| JP6376727B2 (en) * | 2013-04-26 | 2018-08-22 | 出光興産株式会社 | Organic electroluminescence device and electronic device |
| CN105073763B (en) | 2013-04-29 | 2019-03-01 | Udc 爱尔兰有限责任公司 | Transient metal complex with carbene ligands and its purposes in OLED |
| US10840454B2 (en) | 2013-06-14 | 2020-11-17 | Samsung Display Co., Ltd. | Organic light-emitting devices |
| KR102052076B1 (en) | 2013-06-14 | 2019-12-05 | 삼성디스플레이 주식회사 | Organic light-emitting diode |
| KR102188028B1 (en) | 2013-06-18 | 2020-12-08 | 삼성디스플레이 주식회사 | Organic light emitting device |
| WO2015031242A1 (en) * | 2013-08-25 | 2015-03-05 | Molaire Consulting Llc | Molecular glass mixtures for organic electronics applications |
| US10593886B2 (en) | 2013-08-25 | 2020-03-17 | Molecular Glasses, Inc. | OLED devices with improved lifetime using non-crystallizable molecular glass mixture hosts |
| US10461269B2 (en) | 2013-12-20 | 2019-10-29 | Molecular Glasses, Inc. | Crosslinkable, /polymerizable and combinations thereof charge-transporting molecular glass mixtures, luminescent molecular glass mixtures, or combinations thereof for organic light emitting diodes and other organic electronics and photonics applications and method of making same |
| CN103467447B (en) * | 2013-09-04 | 2015-10-28 | 吉林奥来德光电材料股份有限公司 | One class electroluminescent organic material and applying in the devices |
| KR102180085B1 (en) * | 2013-09-12 | 2020-11-17 | 덕산네오룩스 주식회사 | Organic electronic element using a compound for organic electronic element, and an electronic device thereof |
| KR20150035317A (en) | 2013-09-27 | 2015-04-06 | 삼성디스플레이 주식회사 | Organic light-emitting devices |
| KR101779110B1 (en) | 2013-10-11 | 2017-09-18 | 제일모직 주식회사 | Organic optoelectric device and display device |
| KR102140006B1 (en) * | 2013-10-11 | 2020-07-31 | 에스에프씨 주식회사 | An electroluminescent compound and an electroluminescent device comprising the same |
| US11101433B2 (en) | 2013-11-12 | 2021-08-24 | Kyulux, Inc. | Light-emitting material, and delayed fluorescent emitter and organic light-emitting device using same |
| WO2015072520A1 (en) | 2013-11-13 | 2015-05-21 | 出光興産株式会社 | Compound, material for organic electroluminescent element, organic electroluminescent element, and electronic device |
| TWI540129B (en) | 2013-11-22 | 2016-07-01 | Dic股份有限公司 | Material for organic electroluminescence device and application thereof, organic electroluminescence device and application thereof |
| CN103664909B (en) | 2013-12-10 | 2015-02-25 | 京东方科技集团股份有限公司 | Bicarbazole derivative, preparation method, application and organic light-emitting device |
| KR102235596B1 (en) * | 2013-12-12 | 2021-04-05 | 삼성디스플레이 주식회사 | Organic light-emitting devices |
| KR20150071624A (en) * | 2013-12-18 | 2015-06-26 | 롬엔드하스전자재료코리아유한회사 | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| KR102201319B1 (en) * | 2014-01-14 | 2021-01-11 | 삼성전자주식회사 | Condensed cyclic compound and organic light emitting device including the same |
| CN104795503B (en) | 2014-01-16 | 2018-07-20 | 三星显示有限公司 | Organic light emitting apparatus |
| KR102177213B1 (en) | 2014-01-20 | 2020-11-11 | 삼성디스플레이 주식회사 | Organic light-emitting devices |
| KR101829745B1 (en) * | 2014-01-24 | 2018-02-19 | 삼성에스디아이 주식회사 | Organic compound and composition and organic optoelectric device and display device |
| EP2907815B1 (en) | 2014-02-12 | 2023-12-06 | Samsung Electronics Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| KR20150099192A (en) * | 2014-02-21 | 2015-08-31 | 삼성에스디아이 주식회사 | Organic optoelectric device and display device |
| KR20160132025A (en) * | 2014-03-12 | 2016-11-16 | 이데미쓰 고산 가부시키가이샤 | Composition, compound, material for organic electroluminescence element, ink composition, organic electroluminescence element, and electronic device |
| WO2015148327A2 (en) * | 2014-03-25 | 2015-10-01 | Molaire Consulting Llc | Pi-conjugated semiconductive organic glass mixtures for oled and oeds |
| KR101754715B1 (en) * | 2014-04-08 | 2017-07-10 | 롬엔드하스전자재료코리아유한회사 | Multi-component host material and organic electroluminescence device comprising the same |
| KR101773363B1 (en) * | 2014-04-09 | 2017-08-31 | 제일모직 주식회사 | Organic compound and composition and organic optoelectric device and display device |
| JP6378106B2 (en) | 2014-04-16 | 2018-08-22 | 出光興産株式会社 | Compound, organic electroluminescence device and electronic device |
| KR102491209B1 (en) * | 2014-04-29 | 2023-01-26 | 롬엔드하스전자재료코리아유한회사 | Multi-Component Host Material and Organic Electroluminescent Device Comprising the Same |
| WO2015167259A1 (en) * | 2014-04-29 | 2015-11-05 | Rohm And Haas Electronic Materials Korea Ltd. | Multi-component host material and organic electroluminescent device comprising the same |
| KR102395933B1 (en) * | 2014-05-07 | 2022-05-11 | 롬엔드하스전자재료코리아유한회사 | Multi-component host material and organic electroluminescent device comprising the same |
| KR20150130928A (en) * | 2014-05-14 | 2015-11-24 | 롬엔드하스전자재료코리아유한회사 | Multi-component host material and organic electroluminescent device comprising the same |
| KR101897039B1 (en) * | 2014-05-22 | 2018-09-10 | 제일모직 주식회사 | Organic compound and composition and organic optoelectric device and display device |
| KR20150135123A (en) * | 2014-05-23 | 2015-12-02 | 롬엔드하스전자재료코리아유한회사 | Multi-Component Host Material and an Organic Electroluminescence Device Comprising the Same |
| KR102300023B1 (en) * | 2014-06-13 | 2021-09-09 | 삼성디스플레이 주식회사 | Organic light-emitting devices and method for manufacturing the same |
| KR102308903B1 (en) * | 2014-06-17 | 2021-10-06 | 삼성디스플레이 주식회사 | Organic light emitting device |
| DE102014008722B4 (en) | 2014-06-18 | 2024-08-22 | Merck Patent Gmbh | Compositions for electronic devices, formulation containing them, use of the composition, use of the formulation and organic electronic device containing the composition |
| KR102273047B1 (en) | 2014-06-30 | 2021-07-06 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| KR102502306B1 (en) * | 2014-07-22 | 2023-02-23 | 롬엔드하스전자재료코리아유한회사 | Organic Electroluminescence Device |
| WO2016013875A1 (en) * | 2014-07-22 | 2016-01-28 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device |
| JP5875742B1 (en) * | 2014-07-25 | 2016-03-02 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| KR102241847B1 (en) * | 2014-07-29 | 2021-04-20 | 삼성디스플레이 주식회사 | Organic light emitting device |
| CN107074896B (en) | 2014-08-08 | 2021-04-13 | Udc 爱尔兰有限责任公司 | Electroluminescent imidazoquinoxaline carbene metal complexes |
| JP6788335B2 (en) | 2014-08-11 | 2020-11-25 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | Monoamine materials for organic electroluminescence devices and organic electroluminescence devices using them |
| KR101835501B1 (en) | 2014-08-13 | 2018-03-07 | 삼성에스디아이 주식회사 | Organic optoelectric device and display device |
| JP2017212024A (en) * | 2014-08-28 | 2017-11-30 | 出光興産株式会社 | Organic electroluminescent device and electronic equipment |
| KR101493482B1 (en) | 2014-08-29 | 2015-02-16 | 덕산네오룩스 주식회사 | Organic electronic element using a compound for organic electronic element, and an electronic device thereo |
| KR101785153B1 (en) * | 2014-09-05 | 2017-10-17 | 주식회사 엘지화학 | Hetero-cyclic compound and organic light emitting device comprising the same |
| KR102304720B1 (en) * | 2014-09-19 | 2021-09-27 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| KR102288348B1 (en) | 2014-09-24 | 2021-08-11 | 삼성디스플레이 주식회사 | Organic light-emitting devices |
| KR102321379B1 (en) | 2014-09-24 | 2021-11-04 | 삼성디스플레이 주식회사 | Organic light-emitting devices |
| KR102304719B1 (en) | 2014-10-01 | 2021-09-27 | 삼성디스플레이 주식회사 | Condensed-cyclic compound and organic light emitting diode comprising the same |
| KR101595697B1 (en) | 2014-10-14 | 2016-02-26 | 주식회사 엘지화학 | Multicyclic compound including nitrogen and organic light emitting device using the same |
| KR101818581B1 (en) * | 2014-10-31 | 2018-01-15 | 삼성에스디아이 주식회사 | Organic optoelectric device and display device |
| KR20160052443A (en) * | 2014-11-04 | 2016-05-12 | 롬엔드하스전자재료코리아유한회사 | A Novel Combination of a Host Compound and a Dopant Compound and an Organic Electroluminescent Device Comprising the Same |
| KR102352287B1 (en) | 2014-11-07 | 2022-01-18 | 삼성디스플레이 주식회사 | Organic light emitting device |
| KR102417122B1 (en) | 2014-11-12 | 2022-07-06 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| KR102285388B1 (en) * | 2014-11-13 | 2021-08-04 | 삼성디스플레이 주식회사 | Organic light emitting device |
| JP2016100364A (en) | 2014-11-18 | 2016-05-30 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | Material for organic electroluminescent element and organic electroluminescent element using the same |
| KR102363259B1 (en) | 2014-12-02 | 2022-02-16 | 삼성디스플레이 주식회사 | Organic light emitting device |
| KR101818579B1 (en) * | 2014-12-09 | 2018-01-15 | 삼성에스디아이 주식회사 | Organic optoelectric device and display device |
| KR102490882B1 (en) | 2014-12-31 | 2023-01-25 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| CN104710978A (en) * | 2015-02-11 | 2015-06-17 | 深圳市前海安测信息技术有限公司 | Organic electroluminescent material and preparation method thereof |
| KR102338908B1 (en) * | 2015-03-03 | 2021-12-14 | 삼성디스플레이 주식회사 | An organic light emitting device |
| US11672167B2 (en) | 2015-03-13 | 2023-06-06 | Samsung Electronics Co., Ltd. | Organometallic compound and organic light-emitting device including the same |
| KR102429871B1 (en) | 2015-03-13 | 2022-08-05 | 삼성전자주식회사 | Organometallic compound and organic light emitting device including the same |
| US20180254426A1 (en) | 2015-03-30 | 2018-09-06 | Nippon Steel & Sumikin Chemical Co., Ltd. | Organic electroluminescent element |
| CN107592860B (en) | 2015-04-24 | 2020-11-03 | 三星Sdi株式会社 | Organic compound, composition and organic photodiode |
| KR101929860B1 (en) * | 2015-05-15 | 2018-12-17 | 삼성에스디아이 주식회사 | Organic optoelectronic device and display device |
| CN106328816B (en) * | 2015-06-16 | 2018-11-13 | 昆山国显光电有限公司 | A kind of organic electroluminescence device and preparation method thereof |
| US20170047532A1 (en) | 2015-08-13 | 2017-02-16 | Samsung Electronics Co., Ltd. | Organometallic compound, organic light-emitting device including the organometallic compound, and diagnosis composition including the organometallic compound |
| EP3133078B1 (en) | 2015-08-18 | 2019-01-30 | Samsung Electronics Co., Ltd. | Organometallic compound and organic light-emitting device including the same |
| KR102510392B1 (en) | 2015-09-10 | 2023-03-16 | 삼성디스플레이 주식회사 | Carbazole-based compound and organic light-emitting device including the same |
| WO2017043652A1 (en) * | 2015-09-11 | 2017-03-16 | 出光興産株式会社 | Organic electroluminescent element, illumination device, display device, and mixed material |
| JP2018206785A (en) * | 2015-09-18 | 2018-12-27 | 出光興産株式会社 | Organic electroluminescent element |
| EP3353828A4 (en) * | 2015-09-21 | 2019-03-20 | Molecular Glasses, Inc. | Isomeric and asymmetric molecular glass mixtures for oled and other organic electronics and photonics applications |
| EP3356369B1 (en) | 2015-10-01 | 2022-05-04 | Idemitsu Kosan Co., Ltd | Benzimidazolo[1,2-a]benzimidazole carrying triazine groups for organic light emitting diodes |
| WO2017086629A1 (en) * | 2015-11-17 | 2017-05-26 | Rohm And Haas Electronic Materials Korea Ltd. | A plurality of host materials and organic electroluminescent device comprising the same |
| KR20170057825A (en) * | 2015-11-17 | 2017-05-25 | 롬엔드하스전자재료코리아유한회사 | A plurality of host materials and organic electroluminescent device comprising the same |
| KR102399570B1 (en) | 2015-11-26 | 2022-05-19 | 삼성디스플레이 주식회사 | Organic light emitting device |
| CN106883187A (en) * | 2015-12-16 | 2017-06-23 | 上海大学 | A kind of quinazoline derivant and its preparation method and application |
| KR102684614B1 (en) | 2015-12-21 | 2024-07-15 | 유디씨 아일랜드 리미티드 | Transition metal complexes with tripodal ligands and the use thereof in oleds |
| US11910707B2 (en) | 2015-12-23 | 2024-02-20 | Samsung Display Co., Ltd. | Organic light-emitting device |
| KR102396293B1 (en) * | 2015-12-29 | 2022-05-11 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| CN105576139B (en) * | 2016-01-06 | 2017-11-07 | 京东方科技集团股份有限公司 | A kind of quanta point electroluminescent diode and preparation method thereof, display |
| CN107068888B (en) * | 2016-04-25 | 2018-12-28 | 中节能万润股份有限公司 | A kind of organic electroluminescence device containing ketone and heterocyclic nitrogen compound and its application |
| KR102698887B1 (en) | 2016-05-04 | 2024-08-28 | 삼성디스플레이 주식회사 | An organic light emitting device |
| KR20170127101A (en) * | 2016-05-10 | 2017-11-21 | 삼성디스플레이 주식회사 | Organic light emitting device |
| KR20170141987A (en) * | 2016-06-16 | 2017-12-27 | 에스에프씨 주식회사 | An electroluminescent compound and an electroluminescent device comprising the same |
| KR102054276B1 (en) | 2016-06-29 | 2019-12-10 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| KR102027961B1 (en) * | 2016-06-29 | 2019-10-02 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| KR102050000B1 (en) | 2016-07-12 | 2019-11-28 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| WO2018021714A1 (en) * | 2016-07-26 | 2018-02-01 | 주식회사 엘지화학 | Organic light emitting element |
| KR102054277B1 (en) | 2016-07-29 | 2019-12-10 | 삼성에스디아이 주식회사 | Composition for organic optoelectronic device and organic optoelectronic device and display device |
| US10741769B2 (en) | 2016-10-14 | 2020-08-11 | Semiconductor Energy Laboratory Co., Ltd. | Organic compound, light-emitting element, light-emitting device, electronic device, and lighting device |
| CN110168048B (en) | 2017-01-05 | 2022-10-21 | 三星Sdi株式会社 | Organic photoelectric device, compound and composition used for same, and display device |
| US11283027B1 (en) | 2017-03-03 | 2022-03-22 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US10892425B1 (en) | 2017-03-03 | 2021-01-12 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| JP7124818B2 (en) * | 2017-03-21 | 2022-08-24 | コニカミノルタ株式会社 | organic electroluminescence element |
| JP6317499B2 (en) * | 2017-03-22 | 2018-04-25 | 出光興産株式会社 | Organic electroluminescence device |
| KR101943428B1 (en) * | 2017-03-24 | 2019-01-30 | 엘티소재주식회사 | Organic light emitting device and composition for organic layer of organic light emitting device |
| WO2018174679A1 (en) * | 2017-03-24 | 2018-09-27 | 희성소재(주) | Organic light emitting element and composition for organic material layer in organic light emitting element |
| WO2018174681A1 (en) * | 2017-03-24 | 2018-09-27 | 희성소재(주) | Organic light emitting element and composition for organic material layer in organic light emitting element |
| JP2018163975A (en) | 2017-03-24 | 2018-10-18 | 出光興産株式会社 | Composition, material for organic electroluminescent element, composition film, organic electroluminescence element, and electronic device |
| KR20180108425A (en) * | 2017-03-24 | 2018-10-04 | 희성소재 (주) | Heterocyclic compound and organic light emitting device comprising the same |
| JP6846256B2 (en) * | 2017-03-29 | 2021-03-24 | 日鉄ケミカル&マテリアル株式会社 | Materials for organic electroluminescent devices and organic electroluminescent devices using them |
| US11594685B2 (en) | 2017-03-30 | 2023-02-28 | Lg Chem, Ltd. | Organic light emitting device |
| KR20180137772A (en) | 2017-06-19 | 2018-12-28 | 삼성에스디아이 주식회사 | Organic optoelectric device and display device |
| US10547014B2 (en) | 2017-06-23 | 2020-01-28 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| JP7226806B2 (en) | 2017-06-23 | 2023-02-21 | 株式会社Kyulux | Composition for use in organic light-emitting diodes |
| KR102641048B1 (en) | 2017-06-26 | 2024-02-27 | 메르크 파텐트 게엠베하 | Method for preparing substituted nitrogen-containing heterocycles |
| US11069860B2 (en) | 2017-08-21 | 2021-07-20 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| JP2019036697A (en) | 2017-08-21 | 2019-03-07 | 出光興産株式会社 | Organic electroluminescent element, electronic device, composition, material for organic electroluminescent device, and composition film |
| US11444250B2 (en) | 2017-12-05 | 2022-09-13 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US11575088B2 (en) | 2017-12-22 | 2023-02-07 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US10644249B2 (en) | 2017-12-22 | 2020-05-05 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| TWI706946B (en) * | 2017-12-29 | 2020-10-11 | 昱鐳光電科技股份有限公司 | Carbazole-substituted triazine compounds and organic electroluminescent devices using the same |
| US11542260B2 (en) | 2018-01-31 | 2023-01-03 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US11104669B2 (en) | 2018-02-02 | 2021-08-31 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US11608333B2 (en) | 2018-03-20 | 2023-03-21 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US11498914B2 (en) | 2018-03-30 | 2022-11-15 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| US11778904B2 (en) | 2018-05-09 | 2023-10-03 | Kyulux, Inc. | Composition of matter for use in organic light-emitting diodes |
| KR102290362B1 (en) * | 2018-06-19 | 2021-08-19 | 엘티소재주식회사 | Heterocyclic compound, organic light emitting device comprising the same, manufacturing method of the same and composition for organic layer of organic light emitting device |
| TWI664267B (en) * | 2018-08-17 | 2019-07-01 | Yuan Ze University | Tricarbazole-n-benzimidazole bipolar materials and organic light emitting diodes |
| KR102704883B1 (en) * | 2018-10-01 | 2024-09-09 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| KR102101354B1 (en) * | 2018-10-02 | 2020-04-17 | 엘티소재주식회사 | Heterocyclic compound, organic light emitting device comprising same, composition for organic layer of organic light emitting device and manufacturing method of organic light emitting device |
| CN109516947A (en) * | 2018-12-29 | 2019-03-26 | 江苏广域化学有限公司 | A method of N- phenylcarbazole compounds are synthesized with sulfur dioxide compound of fluorene class |
| CN113950475A (en) * | 2019-06-18 | 2022-01-18 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| KR102516811B1 (en) * | 2019-10-23 | 2023-03-31 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display device |
| KR102288206B1 (en) * | 2019-11-06 | 2021-08-11 | 엘티소재주식회사 | Heterocyclic compound, organic light emitting device comprising same, composition for organic layer of organic light emitting device and manufacturing method of organic light emitting device |
| KR102587497B1 (en) * | 2022-05-06 | 2023-10-11 | 솔루스첨단소재 주식회사 | Composition for organic electroluminescent device and organic electroluminescent device comprising the same |
| KR102643046B1 (en) * | 2022-05-27 | 2024-03-04 | 솔루스첨단소재 주식회사 | Composition for organic electroluminescent device and organic electroluminescent device comprising the same |
| KR102577795B1 (en) * | 2022-06-10 | 2023-09-12 | 솔루스첨단소재 주식회사 | Homogeneous organic complexs for organic electroluminescent device and organic electroluminescent device comprising the same |
| CN115637147A (en) * | 2022-10-27 | 2023-01-24 | 京东方科技集团股份有限公司 | Light-emitting material and light-emitting device |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11144866A (en) * | 1997-11-05 | 1999-05-28 | Toray Ind Inc | Electroluminescent element |
| US20110266528A1 (en) * | 2010-04-06 | 2011-11-03 | Basf Se | Substituted carbazole derivatives and use thereof in organic electronics |
| US8865323B2 (en) * | 2010-04-20 | 2014-10-21 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Family Cites Families (122)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH584268A5 (en) | 1973-10-29 | 1977-01-31 | Ciba Geigy Ag | |
| US4356429A (en) | 1980-07-17 | 1982-10-26 | Eastman Kodak Company | Organic electroluminescent cell |
| JPS62127376A (en) | 1985-11-27 | 1987-06-09 | Nitto Electric Ind Co Ltd | Surface protecting film |
| JPH0574627A (en) | 1991-09-11 | 1993-03-26 | Toshiba Corp | Low temperature structure body |
| JP3139321B2 (en) | 1994-03-31 | 2001-02-26 | 東レ株式会社 | Light emitting element |
| JP3704748B2 (en) | 1995-06-23 | 2005-10-12 | 東洋インキ製造株式会社 | Electron transport material for organic electroluminescence device and organic electroluminescence device using the same |
| JP2000173774A (en) | 1998-12-09 | 2000-06-23 | Sony Corp | Organic electric field light-emitting element |
| EP2278637B2 (en) | 1999-12-01 | 2021-06-09 | The Trustees of Princeton University | Complexes of form L2MX |
| JP3390391B2 (en) * | 1999-12-16 | 2003-03-24 | エヌイーシーアクセステクニカ株式会社 | Recording paper feed support |
| US6645645B1 (en) * | 2000-05-30 | 2003-11-11 | The Trustees Of Princeton University | Phosphorescent organic light emitting devices |
| JP2003151774A (en) | 2001-11-14 | 2003-05-23 | Toray Ind Inc | Light emitting element |
| WO2003078541A1 (en) * | 2002-03-15 | 2003-09-25 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescent devices and organic electroluminescent devices made by using the same |
| CN100366703C (en) * | 2002-03-22 | 2008-02-06 | 出光兴产株式会社 | Material for organic electroluminescent device and organic electroluminescent device using the same |
| WO2003080760A1 (en) * | 2002-03-22 | 2003-10-02 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescent devices and organic electroluminescent devices made by using the same |
| KR101178006B1 (en) * | 2002-07-19 | 2012-08-28 | 이데미쓰 고산 가부시키가이샤 | Organic electroluminescent devices and organic luminescent medium |
| JP4427947B2 (en) | 2002-11-18 | 2010-03-10 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| JP4078960B2 (en) * | 2002-11-19 | 2008-04-23 | コニカミノルタホールディングス株式会社 | Organic electroluminescence device and display device thereof |
| JP4265219B2 (en) | 2003-01-06 | 2009-05-20 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| EP1589789B1 (en) | 2003-01-24 | 2015-07-01 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| JP4305046B2 (en) | 2003-05-14 | 2009-07-29 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, lighting device and display device |
| JP4408367B2 (en) | 2003-12-22 | 2010-02-03 | 富士フイルム株式会社 | Organic electroluminescence device |
| JP4992183B2 (en) | 2004-02-10 | 2012-08-08 | 三菱化学株式会社 | Luminescent layer forming material and organic electroluminescent device |
| JP2005340121A (en) | 2004-05-31 | 2005-12-08 | Konica Minolta Holdings Inc | Organic electroluminescent element, lighting system, and display |
| US7329722B2 (en) | 2004-08-30 | 2008-02-12 | Samsung Electronics Co., Ltd | Polymeric charge transport materials having carbazolyl repeating units |
| JP4541809B2 (en) | 2004-09-08 | 2010-09-08 | キヤノン株式会社 | Organic compound and organic light emitting device |
| JP2006131519A (en) | 2004-11-04 | 2006-05-25 | Idemitsu Kosan Co Ltd | Condensed ring-containing compound and organic electroluminescent element using the same |
| US20060105202A1 (en) | 2004-11-17 | 2006-05-18 | Fuji Photo Film Co., Ltd. | Organic electroluminescent device |
| US20090242876A1 (en) | 2004-12-08 | 2009-10-01 | Koninklijke Philips Electronics, N.V. | Carbazole compounds |
| US7597967B2 (en) | 2004-12-17 | 2009-10-06 | Eastman Kodak Company | Phosphorescent OLEDs with exciton blocking layer |
| JP5050344B2 (en) | 2004-12-24 | 2012-10-17 | パイオニア株式会社 | Organic compounds, charge transport materials, and organic electroluminescent devices |
| CN1687066A (en) * | 2005-03-24 | 2005-10-26 | 复旦大学 | Conjugated polymer material with lateral group possessing function of guanine, preparing process and application |
| JP4701818B2 (en) * | 2005-04-28 | 2011-06-15 | Jsr株式会社 | Triazine compound, composition for organic electroluminescence device, and organic electroluminescence device |
| JP5261887B2 (en) | 2005-05-17 | 2013-08-14 | 三菱化学株式会社 | Monoamine compound, charge transport material, and organic electroluminescence device |
| CN102633820B (en) | 2005-12-01 | 2015-01-21 | 新日铁住金化学株式会社 | Compound for organic electroluminescent element and organic electroluminescent element |
| WO2007069740A1 (en) | 2005-12-16 | 2007-06-21 | Pioneer Corporation | Organic electroluminescent device |
| JP5181676B2 (en) * | 2006-01-05 | 2013-04-10 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, display device and lighting device |
| JP2007194241A (en) * | 2006-01-17 | 2007-08-02 | Toray Ind Inc | Light emitting device |
| WO2007099802A1 (en) * | 2006-02-23 | 2007-09-07 | Idemitsu Kosan Co., Ltd. | Red organic electroluminescence element |
| JP5055818B2 (en) * | 2006-04-19 | 2012-10-24 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| JP5233081B2 (en) * | 2006-05-17 | 2013-07-10 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| JP5555972B2 (en) | 2006-05-17 | 2014-07-23 | 三菱化学株式会社 | Organic electroluminescence device |
| EP2518045A1 (en) * | 2006-11-24 | 2012-10-31 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent element using the same |
| JP2008135498A (en) | 2006-11-28 | 2008-06-12 | Toray Ind Inc | Light emitting element |
| EP2437326A3 (en) | 2006-12-13 | 2013-11-13 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, display device and lighting device |
| JP5176343B2 (en) | 2007-03-07 | 2013-04-03 | Jnc株式会社 | Electron transport material and organic electroluminescent device using the same |
| US8541111B2 (en) * | 2007-05-21 | 2013-09-24 | Sony Corporation | Organic electroluminescent device and display apparatus |
| US8242488B2 (en) | 2007-05-30 | 2012-08-14 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, display device, and illuminating device |
| TW200916434A (en) * | 2007-06-07 | 2009-04-16 | Idemitsu Kosan Co | Aromatic amine derivatives and organic electroluminescent device |
| WO2008156105A1 (en) * | 2007-06-21 | 2008-12-24 | Konica Minolta Holdings, Inc. | Organic electroluminescence element material, organic electroluminescence element, display device and illuminating device |
| US8330350B2 (en) | 2007-07-07 | 2012-12-11 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and material for organic electroluminescence device |
| US8154195B2 (en) | 2007-07-07 | 2012-04-10 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and material for organic electroluminescence device |
| JP5115061B2 (en) | 2007-07-09 | 2013-01-09 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, display device and lighting device |
| EP2170990B1 (en) * | 2007-07-11 | 2016-10-05 | 3M Innovative Properties Company | Methods for melt-processing thermoplastic fluoropolymers |
| JP5194596B2 (en) | 2007-07-11 | 2013-05-08 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, display device and lighting device |
| KR100904070B1 (en) | 2007-07-18 | 2009-06-23 | 제일모직주식회사 | Compounds for organic electroluminescent device |
| WO2009020095A1 (en) * | 2007-08-06 | 2009-02-12 | Idemitsu Kosan Co., Ltd. | Aromatic amine derivative and organic electroluminescent device using the same |
| KR20090024998A (en) | 2007-09-05 | 2009-03-10 | 제일모직주식회사 | Material for organic photoelectric device comprising electron transporting unit and hole transporting unit, and organic photoelectric device thereby |
| WO2009060742A1 (en) | 2007-11-08 | 2009-05-14 | Konica Minolta Holdings, Inc. | Organic electroluminescent device, display device, and illuminating device |
| JP2009114369A (en) | 2007-11-08 | 2009-05-28 | Konica Minolta Holdings Inc | Organic electroluminescent material, organic electroluminescent element, display and illuminator |
| JP5304653B2 (en) | 2007-11-26 | 2013-10-02 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| US7867675B2 (en) | 2007-12-20 | 2011-01-11 | Xerox Corporation | Nitrogen heterocyclics in photoconductor charge transport layer |
| CN101314635B (en) * | 2008-05-06 | 2010-12-22 | 武汉大学 | Polymer containing carbazole and oxdiazole unit, and uses thereof |
| EP2460866B1 (en) | 2008-05-13 | 2019-12-11 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, display device and lighting device |
| JP5609641B2 (en) * | 2008-07-10 | 2014-10-22 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| JP5338184B2 (en) | 2008-08-06 | 2013-11-13 | コニカミノルタ株式会社 | Organic electroluminescence element, display device, lighting device |
| KR100958641B1 (en) | 2008-08-18 | 2010-05-20 | 삼성모바일디스플레이주식회사 | An organic light emitting diode employing a layer for improving a light efficiency |
| US9196845B2 (en) | 2008-08-22 | 2015-11-24 | Lg Chem, Ltd. | Material for organic electronic device, and organic electronic device using same |
| JPWO2010044342A1 (en) * | 2008-10-15 | 2012-03-15 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT, METHOD FOR PRODUCING ORGANIC ELECTROLUMINESCENT ELEMENT, WHITE ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| JP5577579B2 (en) | 2008-10-20 | 2014-08-27 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT, ORGANIC ELECTROLUMINESCENT MATERIAL, DISPLAY DEVICE AND LIGHTING DEVICE |
| KR101506919B1 (en) | 2008-10-31 | 2015-03-30 | 롬엔드하스전자재료코리아유한회사 | Novel compounds for organic electronic material and organic electronic device using the same |
| JP5493333B2 (en) | 2008-11-05 | 2014-05-14 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT, WHITE ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| JP5707665B2 (en) * | 2008-12-03 | 2015-04-30 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENCE ELEMENT, LIGHTING DEVICE AND DISPLAY DEVICE HAVING THE ELEMENT |
| KR101288557B1 (en) | 2008-12-24 | 2013-07-22 | 제일모직주식회사 | Novel compound for organic photoelectric device and organic photoelectric device including the same |
| KR20100079458A (en) | 2008-12-31 | 2010-07-08 | 덕산하이메탈(주) | Bis-carbazole chemiclal and organic electroric element using the same, terminal thererof |
| JP5549228B2 (en) | 2009-01-09 | 2014-07-16 | 三菱化学株式会社 | Organic EL device and organic light emitting device |
| WO2010079678A1 (en) | 2009-01-09 | 2010-07-15 | コニカミノルタホールディングス株式会社 | Organic electroluminescent element, display device, and lighting device |
| EP2394980B1 (en) | 2009-02-09 | 2016-12-21 | Hodogaya Chemical Co., Ltd. | Aromatic amino compound manufacturing method |
| JP2010212676A (en) | 2009-02-13 | 2010-09-24 | Mitsubishi Chemicals Corp | Organic light emitting device |
| JP5509634B2 (en) * | 2009-03-16 | 2014-06-04 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE, LIGHTING DEVICE, AND ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL |
| US8247575B2 (en) * | 2009-03-20 | 2012-08-21 | Semiconductor Energy Laboratory Co., Ltd. | Carbazole derivative with heteroaromatic ring, and light-emitting element, light-emitting device, and electronic device using carbazole derivative with heteroaromatic ring |
| KR101603070B1 (en) | 2009-03-31 | 2016-03-14 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| KR101172052B1 (en) | 2009-05-08 | 2012-08-07 | 덕산하이메탈(주) | Compound and Organic Electronic Element Using the Same, and Terminal Thereof |
| JP5629980B2 (en) * | 2009-05-22 | 2014-11-26 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| DE102009023155A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR101311934B1 (en) | 2009-06-08 | 2013-09-26 | 제일모직주식회사 | Composition for organic photoelectric device and organic photoelectric device using the same |
| KR101649950B1 (en) | 2009-06-08 | 2016-08-23 | 에스에프씨 주식회사 | Heteroarylamine derivatives and organoelectroluminescent device using the same |
| JP5697856B2 (en) * | 2009-06-24 | 2015-04-08 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT, WHITE ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| KR20110013220A (en) | 2009-07-31 | 2011-02-09 | 다우어드밴스드디스플레이머티리얼 유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| JP4590020B1 (en) | 2009-07-31 | 2010-12-01 | 富士フイルム株式会社 | Charge transport material and organic electroluminescent device |
| JP4474493B1 (en) | 2009-07-31 | 2010-06-02 | 富士フイルム株式会社 | Organic electroluminescence device |
| KR101789708B1 (en) | 2009-07-31 | 2017-10-25 | 유디씨 아일랜드 리미티드 | Organic electroluminescent element |
| KR101859346B1 (en) | 2009-07-31 | 2018-05-17 | 유디씨 아일랜드 리미티드 | Organic electroluminescent element |
| JP5499890B2 (en) * | 2009-08-05 | 2014-05-21 | コニカミノルタ株式会社 | Organic electroluminescence device and method for manufacturing the same |
| KR101431644B1 (en) * | 2009-08-10 | 2014-08-21 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| KR101108154B1 (en) | 2009-08-10 | 2012-02-08 | 삼성모바일디스플레이주식회사 | A condensed-cyclic compound and an organic light emitting diode employing an organic layer comprising the same |
| JP5784608B2 (en) | 2009-09-16 | 2015-09-24 | メルク パテント ゲーエムベーハー | Formulations for electronic device manufacturing |
| WO2011037429A2 (en) | 2009-09-28 | 2011-03-31 | 덕산하이메탈(주) | Compounds having 5-membered aryl-ring-condensed heterocyclic derivatives, organic electronic device using the compounds, and terminal comprising the organic electronic device |
| DE102009048791A1 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR20110041727A (en) | 2009-10-16 | 2011-04-22 | 에스에프씨 주식회사 | Carbazole derivatives and organoelectroluminescent device using the same |
| US9306175B2 (en) | 2009-10-23 | 2016-04-05 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| US9306174B2 (en) | 2009-10-23 | 2016-04-05 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| DE102009051172A1 (en) | 2009-10-29 | 2011-05-05 | Merck Patent Gmbh | Materials for electronic devices |
| KR101506999B1 (en) | 2009-11-03 | 2015-03-31 | 제일모직 주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| US8828561B2 (en) | 2009-11-03 | 2014-09-09 | Cheil Industries, Inc. | Compound for organic photoelectric device and organic photoelectric device including the same |
| DE102009053382A1 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| WO2011108707A1 (en) | 2010-03-05 | 2011-09-09 | 出光興産株式会社 | Material for organic electroluminescent element, and organic electroluminescent element using same |
| DE102010010481A1 (en) | 2010-03-06 | 2011-09-08 | Merck Patent Gmbh | Organic electroluminescent device |
| KR20110102055A (en) | 2010-03-10 | 2011-09-16 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| KR20110105285A (en) | 2010-03-18 | 2011-09-26 | 에스에프씨 주식회사 | Triazine compound and organic electroluminescent devices comprising the same |
| CN102823015B (en) | 2010-03-31 | 2014-05-14 | 出光兴产株式会社 | Material for organic electroluminescent element and organic electroluminescent element using same |
| US8227801B2 (en) * | 2010-04-26 | 2012-07-24 | Universal Display Corporation | Bicarbzole containing compounds for OLEDs |
| KR101420318B1 (en) | 2010-06-17 | 2014-07-16 | 이-레이 옵토일렉트로닉스 테크놀로지 컴퍼니 리미티드 | Compound for organic electroluminescent device and organic electroluminescent device having the same |
| US9382206B2 (en) * | 2010-08-31 | 2016-07-05 | Idemitsu Kosan Co., Ltd. | Nitrogen-containing aromatic heterocyclic derivative and organic electroluminescence device using the same |
| CN103270032B (en) * | 2010-12-20 | 2016-05-04 | 出光兴产株式会社 | Aromatic heterocyclic derivative and organic electroluminescent element using same |
| KR101867115B1 (en) * | 2010-12-28 | 2018-07-19 | 이데미쓰 고산 가부시키가이샤 | Material for organic electroluminescent elements, and organic electroluminescent element using same |
| JP5964328B2 (en) | 2011-02-11 | 2016-08-03 | ユニバーサル ディスプレイ コーポレイション | ORGANIC LIGHT EMITTING DEVICE AND MATERIAL FOR USE IN THE ORGANIC LIGHT EMITTING DEVICE |
| JP5839027B2 (en) * | 2011-02-22 | 2016-01-06 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENCE ELEMENT, ITS MANUFACTURING METHOD, LIGHTING DEVICE, AND DISPLAY DEVICE |
| JP5747555B2 (en) * | 2011-02-24 | 2015-07-15 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| US9640775B2 (en) * | 2011-03-04 | 2017-05-02 | Konica Minolta, Inc. | Organic electroluminescence element |
| EP2690093A4 (en) * | 2011-03-24 | 2014-08-13 | Idemitsu Kosan Co | Bis-carbazole derivative and organic electroluminescent element using same |
| JP2013051014A (en) | 2011-08-31 | 2013-03-14 | Renesas Electronics Corp | Optical disk device and semiconductor device |
| JP2015135836A (en) * | 2012-03-29 | 2015-07-27 | 出光興産株式会社 | Organic electroluminescent element and material for organic electroluminescent element |
-
2011
- 2011-04-19 WO PCT/JP2011/059658 patent/WO2011132684A1/en active Application Filing
- 2011-04-19 JP JP2011542615A patent/JP5074627B2/en active Active
- 2011-04-19 KR KR1020127028022A patent/KR101421365B1/en active IP Right Grant
- 2011-04-19 WO PCT/JP2011/059657 patent/WO2011132683A1/en active Application Filing
- 2011-04-19 KR KR1020117025041A patent/KR20120057561A/en not_active Application Discontinuation
- 2011-04-19 KR KR1020117029561A patent/KR101441177B1/en active IP Right Grant
- 2011-04-19 CN CN201410718443.4A patent/CN104592206B/en active Active
- 2011-04-19 EP EP11763575.5A patent/EP2415769B1/en active Active
- 2011-04-19 CN CN201180001965.7A patent/CN102421772B/en active Active
- 2011-04-19 CN CN2011800021657A patent/CN102439004A/en active Pending
- 2011-04-19 JP JP2011533888A patent/JP5562970B2/en active Active
- 2011-04-19 EP EP11770992.3A patent/EP2423209B1/en active Active
- 2011-04-20 US US13/091,071 patent/US8877352B2/en active Active
- 2011-04-20 TW TW100113712A patent/TWI477580B/en active
- 2011-04-20 TW TW100113709A patent/TW201141989A/en unknown
- 2011-04-20 US US13/091,036 patent/US8652654B2/en active Active
-
2012
- 2012-02-14 US US13/372,954 patent/US8865323B2/en active Active
- 2012-02-14 US US13/372,943 patent/US8940414B2/en active Active
- 2012-08-23 JP JP2012184401A patent/JP5405630B2/en active Active
-
2013
- 2013-09-02 JP JP2013181656A patent/JP5390728B1/en active Active
-
2014
- 2014-09-22 US US14/492,395 patent/US10193077B2/en active Active
-
2015
- 2015-04-21 US US14/692,010 patent/US20150228912A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11144866A (en) * | 1997-11-05 | 1999-05-28 | Toray Ind Inc | Electroluminescent element |
| US20110266528A1 (en) * | 2010-04-06 | 2011-11-03 | Basf Se | Substituted carbazole derivatives and use thereof in organic electronics |
| US8865323B2 (en) * | 2010-04-20 | 2014-10-21 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US8877352B2 (en) * | 2010-04-20 | 2014-11-04 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
| US8940414B2 (en) * | 2010-04-20 | 2015-01-27 | Idemitsu Kosan Co., Ltd. | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same |
Non-Patent Citations (2)
| Title |
|---|
| Machine translation of JP 11-144866 A (05-1999). * |
| U.S. Provisional Application No. 61/321,177 (filed April 06, 2010). * |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11812626B2 (en) | 2011-02-16 | 2023-11-07 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element |
| US9287512B2 (en) | 2011-03-08 | 2016-03-15 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds, layers and organic electroluminescent device using the same |
| US11043637B2 (en) | 2012-08-03 | 2021-06-22 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, and lighting device |
| US10897012B2 (en) | 2012-08-03 | 2021-01-19 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, and lighting device |
| US10069076B2 (en) | 2012-08-03 | 2018-09-04 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, and lighting device |
| US11968889B2 (en) | 2012-08-03 | 2024-04-23 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, and lighting device |
| US12029059B2 (en) | 2012-08-10 | 2024-07-02 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, display device, electronic device, and lighting device |
| US11552256B2 (en) | 2014-07-25 | 2023-01-10 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, lighting device, and organic compound |
| US11800799B2 (en) | 2014-07-25 | 2023-10-24 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, lighting device, and organic compound |
| US12127479B2 (en) | 2014-07-25 | 2024-10-22 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, light-emitting device, electronic device, lighting device, and organic compound |
| US10326079B2 (en) | 2015-04-27 | 2019-06-18 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| US10693094B2 (en) | 2015-09-30 | 2020-06-23 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, display device, electronic device, and lighting device |
| US11925041B2 (en) | 2015-09-30 | 2024-03-05 | Semiconductor Energy Laboratory Co., Ltd. | Light-emitting element, display device, electronic device, and lighting device |
| EP3588599A1 (en) | 2018-06-26 | 2020-01-01 | Idemitsu Kosan Co., Ltd. | Composition, organic-electroluminescence-device material, composition film, organic electroluminescence device, and electronic device |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10193077B2 (en) | Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same | |
| US8940412B2 (en) | Material for organic electroluminescent element, and organic electroluminescent element | |
| JP5753873B2 (en) | Material for organic electroluminescence device and organic electroluminescence device using the same | |
| JP6129075B2 (en) | Biscarbazole derivative and organic electroluminescence device using the same | |
| US9209410B2 (en) | Material for organic electroluminescence device and organic electroluminescence device utilizing the same | |
| US20150194622A1 (en) | Biscarbazole derivative host materials and red emitter for oled emissive region | |
| JP6034196B2 (en) | Material for organic electroluminescence device and organic electroluminescence device using the same | |
| US9126887B2 (en) | Organic electroluminescent element material and organic electroluminescent element comprising same | |
| WO2013146942A1 (en) | Novel compound, material for organic electroluminescence element, and organic electroluminescence element | |
| JP2015135836A (en) | Organic electroluminescent element and material for organic electroluminescent element | |
| US20120007059A1 (en) | Organic electroluminescence element and compound |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |