CN116138287A - Complex enzyme preparation and preparation method thereof - Google Patents
Complex enzyme preparation and preparation method thereof Download PDFInfo
- Publication number
- CN116138287A CN116138287A CN202310051459.3A CN202310051459A CN116138287A CN 116138287 A CN116138287 A CN 116138287A CN 202310051459 A CN202310051459 A CN 202310051459A CN 116138287 A CN116138287 A CN 116138287A
- Authority
- CN
- China
- Prior art keywords
- catalase
- solution
- enzyme preparation
- amylase
- parts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000002360 preparation method Methods 0.000 title claims abstract description 111
- 102000004190 Enzymes Human genes 0.000 title claims abstract description 59
- 108090000790 Enzymes Proteins 0.000 title claims abstract description 59
- 102000016938 Catalase Human genes 0.000 claims abstract description 115
- 108010053835 Catalase Proteins 0.000 claims abstract description 115
- 229940105657 catalase Drugs 0.000 claims abstract description 115
- 229940088598 enzyme Drugs 0.000 claims abstract description 58
- 239000003094 microcapsule Substances 0.000 claims abstract description 55
- 102000004139 alpha-Amylases Human genes 0.000 claims abstract description 28
- 108090000637 alpha-Amylases Proteins 0.000 claims abstract description 28
- 229940024171 alpha-amylase Drugs 0.000 claims abstract description 28
- 102000004882 Lipase Human genes 0.000 claims abstract description 26
- 108090001060 Lipase Proteins 0.000 claims abstract description 26
- 235000013312 flour Nutrition 0.000 claims abstract description 25
- 239000004367 Lipase Substances 0.000 claims abstract description 22
- 235000019421 lipase Nutrition 0.000 claims abstract description 22
- 150000001875 compounds Chemical class 0.000 claims abstract description 18
- 108010015776 Glucose oxidase Proteins 0.000 claims abstract description 14
- 239000004366 Glucose oxidase Substances 0.000 claims abstract description 14
- 229940116332 glucose oxidase Drugs 0.000 claims abstract description 14
- 235000019420 glucose oxidase Nutrition 0.000 claims abstract description 14
- 241000209140 Triticum Species 0.000 claims abstract description 12
- 235000021307 Triticum Nutrition 0.000 claims abstract description 12
- 108010068370 Glutens Proteins 0.000 claims abstract description 11
- 235000021312 gluten Nutrition 0.000 claims abstract description 11
- 101710121765 Endo-1,4-beta-xylanase Proteins 0.000 claims abstract description 9
- 108010061330 glucan 1,4-alpha-maltohydrolase Proteins 0.000 claims abstract description 9
- 239000002994 raw material Substances 0.000 claims abstract description 8
- 239000000243 solution Substances 0.000 claims description 64
- 239000005995 Aluminium silicate Substances 0.000 claims description 45
- 235000012211 aluminium silicate Nutrition 0.000 claims description 45
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 claims description 45
- 229920001661 Chitosan Polymers 0.000 claims description 38
- 238000002156 mixing Methods 0.000 claims description 36
- 238000003756 stirring Methods 0.000 claims description 30
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 claims description 22
- 229940072056 alginate Drugs 0.000 claims description 22
- 235000010443 alginic acid Nutrition 0.000 claims description 22
- 229920000615 alginic acid Polymers 0.000 claims description 22
- 239000002245 particle Substances 0.000 claims description 21
- 235000010410 calcium alginate Nutrition 0.000 claims description 20
- 239000000648 calcium alginate Substances 0.000 claims description 20
- 229960002681 calcium alginate Drugs 0.000 claims description 20
- OKHHGHGGPDJQHR-YMOPUZKJSA-L calcium;(2s,3s,4s,5s,6r)-6-[(2r,3s,4r,5s,6r)-2-carboxy-6-[(2r,3s,4r,5s,6r)-2-carboxylato-4,5,6-trihydroxyoxan-3-yl]oxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylate Chemical compound [Ca+2].O[C@@H]1[C@H](O)[C@H](O)O[C@@H](C([O-])=O)[C@H]1O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[C@H]2[C@H]([C@@H](O)[C@H](O)[C@H](O2)C([O-])=O)O)[C@H](C(O)=O)O1 OKHHGHGGPDJQHR-YMOPUZKJSA-L 0.000 claims description 20
- 239000011259 mixed solution Substances 0.000 claims description 19
- 239000000843 powder Substances 0.000 claims description 19
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 17
- 238000005406 washing Methods 0.000 claims description 15
- 239000000203 mixture Substances 0.000 claims description 14
- 239000007974 sodium acetate buffer Substances 0.000 claims description 12
- 238000000034 method Methods 0.000 claims description 10
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 claims description 8
- 239000001110 calcium chloride Substances 0.000 claims description 8
- 229910001628 calcium chloride Inorganic materials 0.000 claims description 8
- 238000001035 drying Methods 0.000 claims description 8
- 238000004108 freeze drying Methods 0.000 claims description 7
- 230000002538 fungal effect Effects 0.000 claims description 7
- 235000011187 glycerol Nutrition 0.000 claims description 7
- 239000004005 microsphere Substances 0.000 claims description 7
- 230000001580 bacterial effect Effects 0.000 claims description 6
- ZIIUUSVHCHPIQD-UHFFFAOYSA-N 2,4,6-trimethyl-N-[3-(trifluoromethyl)phenyl]benzenesulfonamide Chemical compound CC1=CC(C)=CC(C)=C1S(=O)(=O)NC1=CC=CC(C(F)(F)F)=C1 ZIIUUSVHCHPIQD-UHFFFAOYSA-N 0.000 claims description 4
- 102000015439 Phospholipases Human genes 0.000 claims description 4
- 108010064785 Phospholipases Proteins 0.000 claims description 4
- 239000007788 liquid Substances 0.000 claims description 4
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 claims description 2
- 229930182830 galactose Natural products 0.000 claims description 2
- 238000000926 separation method Methods 0.000 claims description 2
- 239000002131 composite material Substances 0.000 claims 1
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 abstract description 65
- 239000000049 pigment Substances 0.000 abstract description 13
- 238000012545 processing Methods 0.000 abstract description 4
- 239000003607 modifier Substances 0.000 abstract 1
- 230000000694 effects Effects 0.000 description 35
- 230000000052 comparative effect Effects 0.000 description 32
- 239000000706 filtrate Substances 0.000 description 27
- 238000011068 loading method Methods 0.000 description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- 230000014759 maintenance of location Effects 0.000 description 12
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 8
- 235000008429 bread Nutrition 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 238000010025 steaming Methods 0.000 description 8
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- UPYKUZBSLRQECL-UKMVMLAPSA-N Lycopene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1C(=C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=C)CCCC2(C)C UPYKUZBSLRQECL-UKMVMLAPSA-N 0.000 description 6
- 150000001746 carotenes Chemical class 0.000 description 6
- 235000005473 carotenes Nutrition 0.000 description 6
- 238000001914 filtration Methods 0.000 description 6
- KBPHJBAIARWVSC-RGZFRNHPSA-N lutein Chemical compound C([C@H](O)CC=1C)C(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\[C@H]1C(C)=C[C@H](O)CC1(C)C KBPHJBAIARWVSC-RGZFRNHPSA-N 0.000 description 6
- 229960005375 lutein Drugs 0.000 description 6
- ORAKUVXRZWMARG-WZLJTJAWSA-N lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C ORAKUVXRZWMARG-WZLJTJAWSA-N 0.000 description 6
- 235000012680 lutein Nutrition 0.000 description 6
- 239000001656 lutein Substances 0.000 description 6
- KBPHJBAIARWVSC-XQIHNALSSA-N trans-lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C KBPHJBAIARWVSC-XQIHNALSSA-N 0.000 description 6
- NCYCYZXNIZJOKI-UHFFFAOYSA-N vitamin A aldehyde Natural products O=CC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-UHFFFAOYSA-N 0.000 description 6
- FJHBOVDFOQMZRV-XQIHNALSSA-N xanthophyll Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C=C(C)C(O)CC2(C)C FJHBOVDFOQMZRV-XQIHNALSSA-N 0.000 description 6
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 5
- 239000012467 final product Substances 0.000 description 5
- 235000010413 sodium alginate Nutrition 0.000 description 5
- 239000000661 sodium alginate Substances 0.000 description 5
- 229940005550 sodium alginate Drugs 0.000 description 5
- 229960000583 acetic acid Drugs 0.000 description 4
- 230000006196 deacetylation Effects 0.000 description 4
- 238000003381 deacetylation reaction Methods 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- 239000012362 glacial acetic acid Substances 0.000 description 4
- 239000004519 grease Substances 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 239000002775 capsule Substances 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- LDVVTQMJQSCDMK-UHFFFAOYSA-N 1,3-dihydroxypropan-2-yl formate Chemical compound OCC(CO)OC=O LDVVTQMJQSCDMK-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 108010093096 Immobilized Enzymes Proteins 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 239000013043 chemical agent Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 230000002000 scavenging effect Effects 0.000 description 2
- DNISEZBAYYIQFB-PHDIDXHHSA-N (2r,3r)-2,3-diacetyloxybutanedioic acid Chemical compound CC(=O)O[C@@H](C(O)=O)[C@H](C(O)=O)OC(C)=O DNISEZBAYYIQFB-PHDIDXHHSA-N 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000012970 cakes Nutrition 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 229940079919 digestives enzyme preparation Drugs 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000008157 edible vegetable oil Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- -1 enzyme preparations Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 235000010037 flour treatment agent Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000021552 granulated sugar Nutrition 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 235000015927 pasta Nutrition 0.000 description 1
- 238000011056 performance test Methods 0.000 description 1
- 238000005375 photometry Methods 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A21—BAKING; EDIBLE DOUGHS
- A21D—TREATMENT, e.g. PRESERVATION, OF FLOUR OR DOUGH, e.g. BY ADDITION OF MATERIALS; BAKING; BAKERY PRODUCTS; PRESERVATION THEREOF
- A21D8/00—Methods for preparing or baking dough
- A21D8/02—Methods for preparing dough; Treating dough prior to baking
- A21D8/04—Methods for preparing dough; Treating dough prior to baking treating dough with microorganisms or enzymes
- A21D8/042—Methods for preparing dough; Treating dough prior to baking treating dough with microorganisms or enzymes with enzymes
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Microbiology (AREA)
- Engineering & Computer Science (AREA)
- Food Science & Technology (AREA)
- Enzymes And Modification Thereof (AREA)
Abstract
The application relates to the field of flour processing modifier, and in particular discloses a compound enzyme preparation and a preparation method thereof, wherein the compound enzyme preparation comprises the following raw materials in parts by weight: 92-97 parts of high gluten wheat flour; 0.02-1 part of maltogenic amylase; 0.05-1.5 parts of xylanase; 0.05-1.2 parts of alpha-amylase; 0.5-1.5 parts of lipase; 0.5-2 parts of glucose oxidase; 0.5-1 part of catalase microcapsule. The compound enzyme preparation can reduce pigment in flour products, reduce residual hydrogen peroxide, enhance extensibility of dough and increase the volume of products.
Description
Technical Field
The invention relates to the field of flour processing improvers, in particular to a compound enzyme preparation and a preparation method thereof.
Background
Flour improvers are materials used to improve the quality of flour products, to improve the rheological properties and mechanical properties of the dough, and are generally formulated into compositions which include, inter alia, oxidizing agents, reducing agents, enzyme preparations, emulsifiers and the like. The enzyme preparation is one kind of matter extracted from organism and with enzyme characteristic and has the main effect of catalyzing various chemical reactions in food processing process to improve food processing process.
The related art discloses a bread improver which comprises the following raw material components in parts by weight: 20 to 65 parts of monoglyceride, 3 to 12 parts of diacetyl tartaric acid monoglyceride, 0.1 to 0.8 part of lipase, 0.03 to 0.12 part of pectase, 0.02 to 0.15 part of ascorbic acid and 10 to 45 parts of wheat gluten.
In view of the above related art, when cake, bread and pasta are processed by using flour and enzyme preparation, natural pigments such as carotene and lutein are contained in the flour and edible oil, which can affect the color of the product, and if chemical agents are added, the natural pigments are removed, and chemical agent residues are caused.
Disclosure of Invention
In order to reduce pigments in flour products and reduce residual amounts of chemical reagents, the application provides a compound enzyme preparation and a preparation method thereof.
In a first aspect, the present application provides a compound enzyme preparation, which adopts the following technical scheme:
a compound enzyme preparation is prepared from the following raw materials in parts by weight:
92-97 parts of high gluten wheat flour;
0.02-1 part of maltogenic amylase;
0.05-1.5 parts of xylanase;
0.05-1.2 parts of alpha-amylase;
0.5-1.5 parts of lipase;
0.5-2 parts of glucose oxidase;
0.5-1 part of catalase microcapsule.
By adopting the technical scheme, the lipase can hydrolyze fat to generate fatty acid, the fatty acid is oxidized into hydrogen peroxide by oxygen and oxidizing substances in the dough, and the hydrogen peroxide oxidizes the carotene, lutein and other pigments in flour and grease, so that the content of natural pigments is reduced, and the whiteness is increased; the glucose oxidase reacts with glucose in wheat flour to generate hydrogen peroxide, and the hydrogen peroxide can oxidize plant pigments such as carotene, lutein and the like in flour and grease, so that the content of natural pigments is further reduced, and the whiteness is increased; because the lipase and the glucose oxidase can generate hydrogen peroxide after participating in the reaction, residual hydrogen peroxide can damage human cells, and oxygen can be generated after the hydrogen peroxide is decomposed to lead to the oxidation of food, the residual hydrogen peroxide needs to be removed; the catalase microcapsule wraps catalase in, during dough mixing and proofing, catalase cannot be lost or participate in reaction, when dough is in the early stage of baking or steaming, part of the catalase microcapsule can be damaged to release the catalase, through holes in the capsule wall of the part of the catalase microcapsule become large, hydrogen peroxide with small molecular weight can enter from the through holes in the capsule wall and be decomposed into water and oxygen after contacting with the catalase, the water and the oxygen are discharged through the through holes in the capsule wall, the catalase microcapsule can remove residual hydrogen peroxide, the generated oxygen can form a new hole structure during discharging, the porosity is increased, the air holding capacity of the dough is increased, a small amount of generated water is uniformly distributed in the dough, the dough can be softened, the extensibility of the dough is enhanced, the product volume is increased, and the catalase can be deactivated in the high-temperature baking or steaming stage without affecting the characteristics of a final product.
Alternatively, the catalase microcapsule comprises calcium alginate particles and a chitosan shell, wherein the calcium alginate particles are adsorbed with kaolin and catalase.
By adopting the technical scheme, the calcium alginate is nontoxic, low in cost and resistant to microbial attack, but has the defects of low mechanical strength, large pore diameter and enzyme leakage; the alginate and calcium ions are crosslinked to form calcium alginate gel, so that the calcium alginate gel can absorb and wrap catalase, and kaolin is used as a porous supporting material, so that the leakage probability of the catalase can be reduced, and the mechanical strength and heat resistance of the microcapsule are improved; the chitosan shell is wrapped on the surface of calcium alginate particles, so that the surface of the calcium alginate particles is protected, the catalase is controlled not to participate in the reaction of decomposing hydrogen peroxide basically in the early stage, the chitosan shell of part of the microcapsules is destroyed in the early stage of baking or steaming, part of the hydrogen peroxide enters the microcapsules from the through holes of the microcapsules, the hydrogen peroxide is decomposed into water and oxygen after contacting with the catalase, and the catalase is deactivated after the temperature is continuously increased in the later stage of baking or steaming.
Optionally, the preparation method of the catalase microcapsule comprises the following steps:
s1, dissolving kaolin in 0.05-0.15mol/L sodium acetate buffer solution, uniformly mixing to obtain kaolin solution, adding alginate powder, uniformly mixing, adding glycerol, and uniformly mixing to obtain mixed solution;
s2, uniformly mixing a catalase solution and a mixed solution, adding calcium chloride with the addition amount of 1.5-2.5mol/L, after hardening, carrying out solid-liquid separation, collecting microspheres, washing with a sodium acetate buffer solution, and drying to obtain enzyme-carrying particles;
s3, adding enzyme-carrying particles into the chitosan solution, uniformly stirring, centrifuging, and freeze-drying to obtain the catalase microcapsule.
By adopting the technical scheme, intermolecular hydrogen bonds and electrostatic force exist between the alginate and the kaolin, calcium chloride and the alginate are crosslinked to form gel so as to wrap catalase, and as the calcium alginate has porosity and adsorptivity, a large amount of catalase can be adsorbed on the surface and inside the meshes of the calcium alginate, the loading efficiency and the immobilization yield of the catalase can be improved by the kaolin, and chitosan is alkaline polysaccharide which has a large amount of positive charges in an acidic environment and can be polymerized with the calcium alginate with negative charges so as to wrap enzyme-carrying particles; the freeze drying process has low temperature, and compared with other high temperature drying processes, the freeze drying process can reduce the deactivation probability of the catalase and maintain higher activity of the catalase.
Optionally, the mass concentration of the kaolin solution in the S1 is 1.5-2.5% W/V.
By adopting the technical scheme, the kaolin can obtain better enzyme loading efficiency and enzyme immobilization yield when the mass concentration is within the mass concentration range.
Optionally, the mass ratio of the alginate powder to the kaolin in the S1 is 1 (0.9-1.1).
By adopting the technical scheme, the concentration of the alginate can influence the immobilization yield, and under the proportion, the loading efficiency and the immobilization yield of the catalase are higher.
Optionally, the mass concentration of the catalase solution in the S2 is 2.5-3% W/V.
By adopting the technical scheme, the mass concentration of the catalase solution is too low, and the catalase carried in the catalase microcapsule is too little to remove residual hydrogen peroxide; the mass concentration of the catalase solution is too high, and part of catalase is difficult to be completely adsorbed, so that waste is caused.
Optionally, the pH of the chitosan solution in the step S3 is 3.5-4, and the mass concentration of the chitosan is 2.5-3% W/V.
By adopting the technical scheme, the chitosan can better wrap the enzyme-carrying particles in the pH range; the mass concentration of chitosan is too low, so that enzyme-carrying particles are difficult to be completely wrapped; the mass concentration of chitosan is too high, the viscosity is too high, and the chitosan is difficult to stir uniformly.
Optionally, the lipase is selected from at least one of triglyceride lipase, phospholipase and galactose lipase.
By adopting the technical scheme, the lipase can oxidize carotene and lutein and reduce the content of natural pigment, but the type of lipase is required to be selected according to the formula and the process of the flour product, so that the dough property is improved.
Optionally, the alpha-amylase is selected from at least one of a fungal alpha-amylase and a bacterial alpha-amylase.
By adopting the technical scheme, the fungal alpha-amylase has poor heat stability, and most of the fungal alpha-amylase is deactivated before starch starts to gelatinize, so that excessive dextrin in the final product is not generated to cause stickiness; the bacterial alpha-amylase has better heat resistance, is not easy to inactivate in the bread baking process, but can lead to stickiness of the final product, so that the type of the alpha-amylase needs to be selected according to the characteristics of the product, and the fungal alpha-amylase and the bacterial alpha-amylase can be compounded for use.
In a second aspect, the present application provides a preparation method of a compound enzyme preparation, which adopts the following technical scheme:
the preparation method of the compound enzyme preparation comprises the following steps:
uniformly mixing high gluten wheat flour and lipase to obtain a first mixture;
uniformly mixing the maltogenic amylase, xylanase, alpha-amylase, glucose oxidase and catalase microcapsules to obtain a second mixture;
and thirdly, uniformly mixing the first mixture and the second mixture to obtain the compound enzyme preparation.
By adopting the technical scheme, lipase and glucose oxidase can participate in the reaction to oxidize the carotene, lutein and other pigments in the flour and the grease, so that the content of natural pigments is reduced, and the whiteness is increased; the catalase microcapsule not only can remove residual hydrogen peroxide, but also can increase the air holding capacity of the dough, and a small amount of generated water is uniformly distributed in the dough, so that the dough can be softened, the extensibility of the dough is enhanced, the volume of the product is increased, and the catalase can be deactivated in a high-temperature baking or steaming stage and does not influence the characteristics of the final product.
In summary, the present application has the following beneficial effects:
1. because the lipase, the glucose oxidase and the catalase are compounded in the application, the lipase and the glucose oxidase can participate in the reaction to oxidize the carotene, lutein and other pigments in the flour and the grease, so that the content of natural pigments is reduced, and the whiteness is increased; the catalase microcapsule not only can remove residual hydrogen peroxide, but also can increase the air holding capacity of the dough, and a small amount of generated water is uniformly distributed in the dough, so that the dough can be softened, the extensibility of the dough is enhanced, the volume of the product is increased, and the catalase can be deactivated in a high-temperature baking or steaming stage and does not influence the characteristics of the final product.
2. The support material of the kaolin microcapsule is preferably adopted, so that the leakage probability of catalase can be reduced, and the mechanical strength and heat resistance of the microcapsule are improved; the chitosan shell is wrapped on the surface of calcium alginate particles, so that the surface of the calcium alginate particles is protected, the catalase is controlled not to participate in the reaction of decomposing hydrogen peroxide basically in the early stage, the chitosan shell of part of the microcapsules is destroyed in the early stage of baking or steaming, part of the hydrogen peroxide enters the microcapsules from the through holes of the microcapsules, the hydrogen peroxide is decomposed into water and oxygen after contacting with the catalase, and the catalase is deactivated after the temperature is continuously increased in the later stage of baking or steaming.
Detailed Description
The present application is described in further detail below with reference to examples.
Preparation example of catalase microcapsule
Preparation example 1
The preparation method of the catalase microcapsule comprises the following steps:
s1, dissolving 1.5g of food-grade kaolin in a sodium acetate buffer solution with the molar concentration of 0.05mol/L, pH of 5, uniformly mixing and stirring to obtain a kaolin solution with the mass concentration of 1.5% W/V, adding alginate powder which is sodium alginate, uniformly mixing and stirring the alginate powder and the kaolin in a mass ratio of 1:1.1, adding 2.5mL of glycerin, and uniformly mixing and stirring to obtain a mixed solution;
s2, mixing 100mL of catalase solution with the mass concentration of 2.5% W/V with the mixed solution, stirring uniformly to obtain an enzyme mixed solution, adding calcium chloride, hardening for 3 hours, filtering to obtain a first filtrate for standby, collecting microspheres, washing 3 times with sodium acetate buffer with the molar concentration of 0.05mol/L, pH of 5, washing the liquid for standby, drying to obtain enzyme-carrying particles, and storing at 4 ℃;
s3, adding chitosan with the molecular weight of 450000 and the deacetylation degree of 91% into glacial acetic acid water solution with the mass concentration of 1% W/V to generate transparent chitosan solution with the concentration of 2.5% W/V, pH of 3.5, adding enzyme-carrying particles into 200mL of chitosan solution, uniformly stirring, centrifuging to obtain a second filtrate for standby, and freeze-drying to obtain catalase microcapsules, and combining the second filtrate with the first filtrate and the washing solution to obtain total filtrate.
Wherein 1% w/V in the present application means 1g solute per 100mL solution.
Preparation example 2
The difference from preparation example 1 is that the mass concentration of the kaolin solution is 2% w/V.
Preparation example 3
The difference from preparation example 1 is that the mass concentration of the kaolin solution is 2.5% w/V.
Preparation example 4
The difference from preparation example 1 is that the mass concentration of the kaolin solution is 1% w/V.
Preparation example 5
The difference from preparation example 1 is that the mass concentration of the kaolin solution is 3% w/V.
Preparation example 6
The difference from preparation example 2 is that the mass ratio of the alginate powder to the kaolin is 1:1.
Preparation example 7
The difference from preparation example 2 is that the mass ratio of the alginate powder to the kaolin is 1:0.9.
Preparation example 8
The difference from preparation example 2 is that the mass ratio of the alginate powder to the kaolin is 1:0.5.
Preparation example 9
The difference from preparation example 2 is that the mass ratio of the alginate powder to the kaolin is 1:1.5.
Preparation example 10
The difference from preparation example 6 is that the mass concentration of the catalase solution in S2 was 2.8% W/V.
PREPARATION EXAMPLE 11
The difference from preparation example 6 is that the mass concentration of the catalase solution in S2 was 3% W/V.
Preparation example 12
The difference from preparation example 6 is that the mass concentration of the catalase solution in S2 was 2% W/V.
Preparation example 13
The difference from preparation example 6 is that the mass concentration of the catalase solution in S2 was 3.5% W/V.
PREPARATION EXAMPLE 14
The difference from preparation example 10 is that the catalase microcapsule was prepared by the following steps:
s1, dissolving 1.5g of food-grade kaolin in a sodium acetate buffer solution with the molar concentration of 0.15mol/L, pH of 5, uniformly mixing and stirring to obtain a kaolin solution with the mass concentration of 1.5% W/V, adding alginate powder which is sodium alginate, wherein the mass ratio of the alginate powder to the kaolin is 1:1.1, uniformly mixing and stirring, adding 3mL of glycerol, and uniformly mixing and stirring to obtain a mixed solution;
s2, mixing 100mL of catalase solution with the mass concentration of 2.5% W/V with the mixed solution, stirring uniformly to obtain an enzyme mixed solution, adding calcium chloride, hardening for 3 hours, filtering to obtain a first filtrate for standby, collecting microspheres, washing for 4 times with sodium acetate buffer with the molar concentration of 0.05mol/L, pH of 5, washing for standby, drying to obtain enzyme-carrying particles, and storing at the temperature of 4 ℃;
s3, adding chitosan with the molecular weight of 450000 and the deacetylation degree of 91% into glacial acetic acid water solution with the mass concentration of 1% W/V to generate transparent chitosan solution with the concentration of 3% W/V, pH of 4, adding enzyme-carrying particles into 200mL of chitosan solution, uniformly stirring, centrifuging to obtain second filtrate for standby, freeze-drying to obtain catalase microcapsules, and combining the second filtrate with the first filtrate and the washing solution to obtain total filtrate.
Comparative preparation example 1
The preparation method of the catalase microcapsule comprises the following steps:
s1, adding chitosan with the molecular weight of 450000 and the deacetylation degree of 91% into glacial acetic acid water solution with the mass concentration of 1% W/V to generate transparent chitosan solution with the concentration of 2.5% W/V, pH of 3.5, adding alginate powder which is sodium alginate, mixing and stirring uniformly, adding 2.5mL of glycerin, mixing and stirring uniformly to obtain mixed solution;
s2, mixing 100mL of catalase solution with the mass concentration of 2.5% W/V with the mixed solution, stirring uniformly to obtain an enzyme mixed solution, adding calcium chloride, hardening for 3 hours, filtering to obtain a first filtrate for standby, collecting microspheres, washing 3 times with sodium acetate buffer with the molar concentration of 0.05mol/L, pH of 5, drying to obtain catalase microcapsules, and combining the first filtrate and the washing solution to obtain a total filtrate for standby.
Comparative preparation example 2
The preparation method of the catalase microcapsule comprises the following steps:
s1, adding chitosan with the molecular weight of 450000 and the deacetylation degree of 91% into glacial acetic acid water solution with the mass concentration of 1% W/V to generate transparent chitosan solution with the concentration of 2.5% W/V, pH of 3.5, adding alginate powder which is sodium alginate, mixing and stirring uniformly, adding 2.5mL of glycerin, mixing and stirring uniformly to obtain mixed solution;
s2, mixing 100mL of catalase solution with the mass concentration of 2.5% W/V with the mixed solution, stirring uniformly to obtain an enzyme mixed solution, adding calcium chloride, hardening for 3 hours, filtering to obtain a first filtrate for standby, collecting microspheres, washing 3 times with sodium acetate buffer with the molar concentration of 0.05mol/L, pH of 5, washing the liquid for standby, drying to obtain enzyme-carrying particles, and storing at 4 ℃;
and S3, adding the enzyme-carrying particles into 200mL of chitosan solution, uniformly stirring, centrifuging, obtaining a second filtrate for standby, freeze-drying to obtain catalase microcapsules, and combining the second filtrate with the first filtrate and the washing solution to obtain a total filtrate.
Comparative preparation example 3
The preparation method of the catalase microcapsule comprises the following steps:
s1, dissolving 1.5g of food-grade kaolin in a sodium acetate buffer solution with the molar concentration of 0.05mol/L, pH of 5, uniformly mixing and stirring to obtain a kaolin solution with the mass concentration of 1.5% W/V, adding alginate powder which is sodium alginate, uniformly mixing and stirring the alginate powder and the kaolin in a mass ratio of 1:1.1, adding 2.5mL of glycerin, and uniformly mixing and stirring to obtain a mixed solution;
s2, mixing 100mL of catalase solution with the mass concentration of 2.5% W/V with the mixed solution, stirring uniformly to obtain an enzyme mixed solution, adding calcium chloride, hardening for 3 hours, filtering to obtain a first filtrate for standby, collecting microspheres, washing 3 times with sodium acetate buffer with the molar concentration of 0.05mol/L, pH of 5, drying to obtain catalase microcapsules, and combining the first filtrate and the washing solution to obtain a total filtrate for standby.
Examples
Example 1
A compound enzyme preparation is prepared from the following raw materials in parts by weight:
92g of high gluten wheat flour;
1g of maltogenic amylase;
1.5g of xylanase;
alpha-amylase 0.05g, the alpha-amylase being a fungal alpha-amylase;
1.5g of lipase, which is triglyceride lipase;
glucose oxidase 0.5g;
catalase microcapsule 1g, catalase microcapsule prepared in preparation example 1;
the preparation method of the compound enzyme preparation comprises the following steps:
step one, mixing and stirring high gluten wheat flour and lipase uniformly to obtain a first mixture;
step two, uniformly mixing and stirring the maltogenic amylase, xylanase, alpha-amylase, glucose oxidase and catalase microcapsules to obtain a second mixture;
and thirdly, uniformly mixing and stirring the first mixture and the second mixture to obtain the compound enzyme preparation.
Examples 2 to 14
The difference from example 1 is that catalase microcapsules were prepared in sequence from preparation examples 2 to 14.
Example 15
The difference with example 1 is that the compound enzyme preparation consists of the following raw materials in parts by weight:
95g of high gluten wheat flour;
0.5g of maltogenic amylase;
xylanase 0.8g;
alpha-amylase 0.6g, alpha-amylase being a bacterial alpha-amylase;
1g of lipase, which is a phospholipase;
glucose oxidase 1.2g;
catalase microcapsule 0.7g.
Example 16
The difference with example 1 is that the compound enzyme preparation consists of the following raw materials in parts by weight:
97g of high gluten wheat flour;
0.02g of maltogenic amylase;
xylanase 0.05g;
alpha-amylase 1.2g, the alpha-amylase consists of fungal alpha-amylase and bacterial alpha-amylase in a mass ratio of 2:1; 0.5g of lipase, wherein the lipase consists of triglyceride lipase and phospholipase in a mass ratio of 1:1;
glucose oxidase 2g;
catalase microcapsule 0.5g.
Comparative example
Comparative example 1
The difference from example 1 is that the catalase microgel was replaced with an equal weight of high gluten wheat flour.
Comparative example 2
The difference from example 1 is that the catalase microcapsule was replaced with an equal weight of catalase.
Comparative examples 3 to 5
The difference from example 1 is that catalase microcapsules were prepared in turn from comparative preparation examples 1 to 3.
Performance test
Detection method
(1) The loading efficiencies of the calculated preparations 1 to 14 and comparative preparations 1 to 3, respectively, are recorded in table 1; the initial protein concentration of the catalase solution and the protein concentration in the total filtrate were measured by Lowry method using Bovine Serum Albumin (BSA) as a standard, and the loading efficiency (%) = (C) i V i -C f V f )/C i V i *100, wherein C i Is the initial protein concentration (mg/mL) of the catalase solution, vi is the initial volume (mL) of the catalase solution, C f Is the protein concentration (mg/mL), V in the total filtrate f Is the volume (mL) of total filtrate.
(2) The immobilization yields of preparation examples 1 to 14 and comparative preparation examples 1 to 3, respectively, were measured and calculated and recorded in Table 1; immobilization yield (%) =a imm /A free *100%,A imm The specific activity of immobilized enzyme of catalase microcapsule, A free The specific activity of free enzyme in the total filtrate is respectively tested by an ultraviolet absorption method, and the enzyme activity of the immobilized catalase in the catalase microcapsule and the free enzyme activity in the total filtrate are converted into the specific activity of the immobilized enzyme and the specific activity of the free enzyme, wherein the specific activity of the enzyme is the unit number of the enzyme activity in each milliliter of catalase solution per milliliter of catalase solution.
(3) The catalase microcapsules in preparation examples 1-14 and comparative preparation examples 1-3 were crushed by a high-pressure homogenizer, catalase therein was extracted, the activity of the extracted catalase was tested, and the enzyme activity retention rate was calculated, wherein the enzyme activity retention rate (%) =1 g of the catalase after extraction of the catalase microcapsules per 1g of the catalase immobilized by the catalase microcapsules was 100%.
(4) Bread was made using the catalase microcapsules of examples 2, 6, 10, 15, 16 and comparative examples 1-5 in this order, and the bread formulation was as follows: 500g of bread flour, 15g of milk powder, 5g of yeast, 100g of white granulated sugar, 6g of salt, 50g of whole egg, 250g of water, 60g of cream and 2.5g of catalase microcapsule, and the preparation method of the bread comprises the following steps: putting all the raw materials into a stirrer, stirring at 200rpm for 3 min, 500rpm for 1 min, stirring at 200rpm for 3 min until the dough is ripe, dividing the dough into 60g, standing for 10 min, rubbing into a round shape, fermenting at 36-38deg.C and humidity of 80-85% for 120 min, baking at 180deg.C for 12 min, and cooling to bread center temperature of 30-40deg.C;
the testing method comprises the following steps: crushing 10g of bread sample, adding the crushed bread sample into 500mL of distilled water, uniformly stirring, soaking for 30min, and filtering to obtain filtrate as a sample to be detected; the content of hydrogen peroxide in the sample to be tested was measured by fluorescence photometry and is recorded in table 2.
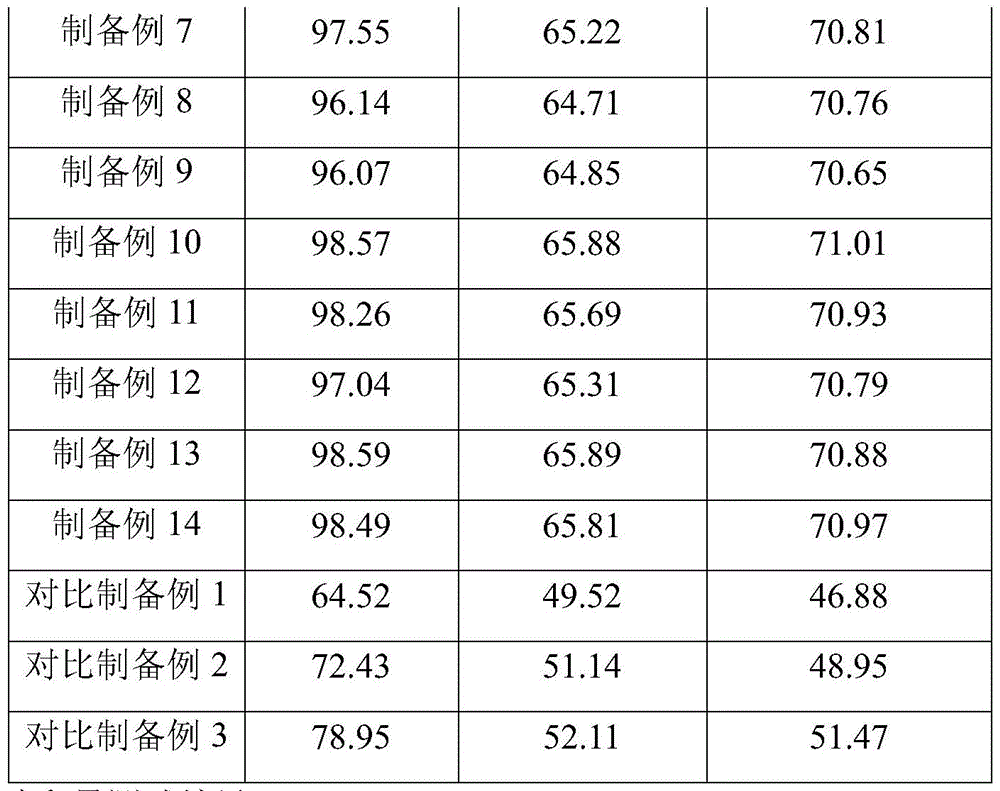
TABLE 1 results of the test for loading efficiency, immobilization yield and enzyme Activity maintenance
TABLE 2 Hydrogen peroxide residual amount test results
As can be seen from the combination of preparation examples 1-14 and comparative preparation examples 1-3 and the combination of table 1, in comparative preparation example 1, only calcium alginate is used for immobilizing catalase, the loading efficiency, immobilization yield and enzyme activity retention rate are the lowest, and in comparative preparation example 2, a layer of chitosan shell is wrapped on the basis of comparative preparation example 1, the loading efficiency, immobilization yield and enzyme activity retention rate are all greatly improved, which indicates that the chitosan shell can protect catalase and reduce enzyme loss, so that immobilized catalase still maintains higher activity; the comparative preparation example 3 is added with kaolin on the basis of the comparative preparation example 1, the loading efficiency, the immobilization yield and the enzyme activity retention rate are greatly improved, and the kaolin is higher than the comparative preparation example 2, which shows that the kaolin can be matched with calcium alginate to improve the loading efficiency and the immobilization yield of the catalase, and the immobilized catalase keeps higher activity, and the comprehensive effect is better than that of the chitosan shell; according to preparation example 1, on the basis of comparative preparation example 1, kaolin is added, chitosan shells are also wrapped, the loading efficiency, the immobilization yield and the enzyme activity retention rate are greatly improved, and the improvement values of the loading efficiency, the immobilization yield and the enzyme activity retention rate are larger than the sum of the improvement values of comparative preparation examples 2 and 3, so that the synergistic effect is achieved by adopting the kaolin, calcium alginate and chitosan to fix catalase in a matched manner, and unexpected technical effects are obtained. Preparation examples 2 to 5 each changed the mass concentration of the kaolin solution, wherein preparation example 4 showed a relatively high immobilization yield although the mass concentration of the kaolin solution was low, but the loading efficiency was relatively low, so that the overall effect of preparation example 2 was the best, and the loading efficiency, immobilization yield and enzyme activity retention rate of preparation examples 4 and 5 were relatively low, indicating that the mass concentration of the kaolin solution was preferably 1.5 to 2.5% w/V; preparation examples 6-9 the mass ratio of alginate powder to kaolin was changed based on preparation example 2, wherein the comprehensive effect of preparation example 6 was the best, and the loading efficiency, immobilization yield and enzyme activity retention rate of preparation examples 8 and 9 were lower, indicating that the mass ratio of alginate powder to kaolin was preferably 1 (0.9-1.1); preparation examples 10 to 13 respectively changed the mass concentration of the catalase solution on the basis of preparation example 6, wherein the comprehensive effect of preparation example 10 is the best, the loading efficiency, immobilization yield and enzyme activity retention rate of preparation example 12 are lower, the comprehensive effect of preparation example 13 is not obviously improved, but rather the cost is higher, therefore, the mass concentration of the catalase solution is preferably 2.5-3% w/V; preparation example 14 changed part of the preparation parameters, and the loading efficiency, immobilization yield and enzyme activity retention rate were all changed, which means that the parameters of each step had a certain influence on the loading efficiency, immobilization yield and enzyme activity retention rate.
As can be seen from the combination of examples 2, 6, 10, 15, 16 and comparative examples 1 to 5 and from table 2, comparative example 1 has a hydrogen peroxide residue of 350ppm without adding catalase, whereas the hydrogen peroxide residue in the food generally requires no more than 100ppm, comparative example 2 has a direct addition of catalase, and the hydrogen peroxide residue is reduced but still higher, indicating that the direct addition of catalase has little effect on scavenging residual hydrogen peroxide; comparative example 3, in which only calcium alginate was used to immobilize catalase, the residual hydrogen peroxide amount was greatly reduced, and was lower than comparative example 2, indicating that the use of calcium alginate to immobilize catalase was advantageous for scavenging residual hydrogen peroxide; comparative example 4, which is based on comparative example 3, is coated with a layer of chitosan shell, the residual hydrogen peroxide amount is greatly reduced, and is lower than comparative example 3, which shows that the use of chitosan shell can reduce the loss of catalase, and is beneficial to the removal of residual hydrogen peroxide; the comparative example 5, which is based on the comparative example 3, has a significantly reduced residual hydrogen peroxide amount, and is lower than the comparative example 4, shows that the kaolin keeps the immobilized catalase with higher activity, the effect of removing the residual hydrogen peroxide is better than that of the chitosan shell, and examples 2, 6, 10, 15 and 16 use catalase microcapsules with both kaolin and chitosan shell, and the residual hydrogen peroxide amount is lower than 100ppm, and show that the residual hydrogen peroxide can be effectively removed by using catalase microcapsules compounded by kaolin, calcium alginate and chitosan.
The present embodiment is merely illustrative of the present application and is not intended to be limiting, and those skilled in the art, after having read the present specification, may make modifications to the present embodiment without creative contribution as required, but is protected by patent laws within the scope of the claims of the present application.
Claims (10)
1. A compound enzyme preparation, which is characterized in that: the composite material consists of the following raw materials in parts by weight:
92-97 parts of high gluten wheat flour;
0.02-1 part of maltogenic amylase;
0.05-1.5 parts of xylanase;
0.05-1.2 parts of alpha-amylase;
0.5-1.5 parts of lipase;
0.5-2 parts of glucose oxidase;
0.5-1 part of catalase microcapsule.
2. A complex enzyme preparation according to claim 1, characterized in that: the catalase microcapsule comprises calcium alginate particles and a chitosan shell, wherein kaolin and catalase are adsorbed on the calcium alginate particles.
3. A complex enzyme preparation according to claim 2, characterized in that: the preparation method of the catalase microcapsule comprises the following steps:
s1, dissolving kaolin in 0.05-0.15mol/L sodium acetate buffer solution, uniformly mixing to obtain kaolin solution, adding alginate powder, uniformly mixing, adding glycerol, and uniformly mixing to obtain mixed solution;
s2, uniformly mixing a catalase solution and a mixed solution, adding calcium chloride with the addition amount of 1.5-2.5mol/L, after hardening, carrying out solid-liquid separation, collecting microspheres, washing with a sodium acetate buffer solution, and drying to obtain enzyme-carrying particles;
s3, adding enzyme-carrying particles into the chitosan solution, uniformly stirring, centrifuging, and freeze-drying to obtain the catalase microcapsule.
4. A complex enzyme preparation according to claim 3, characterized in that: the mass concentration of the kaolin solution in the S1 is 1.5-2.5% W/V.
5. A complex enzyme preparation according to claim 3, characterized in that: the mass ratio of the alginate powder to the kaolin in the S1 is 1 (0.9-1.1).
6. A complex enzyme preparation according to claim 3, characterized in that: the mass concentration of the catalase solution in the S2 is 2.5-3% W/V.
7. A complex enzyme preparation according to claim 3, characterized in that: the pH value of the chitosan solution in the S3 is 3.5-4, and the mass concentration of the chitosan is 2.5-3%W/V.
8. A complex enzyme preparation according to claim 1, characterized in that: the lipase is at least one selected from the group consisting of triglyceride lipase, phospholipase and galactose lipase.
9. A complex enzyme preparation according to claim 1, characterized in that: the alpha-amylase is at least one selected from fungal alpha-amylase and bacterial alpha-amylase.
10. The method for preparing the compound enzyme preparation according to any one of claims 1 to 9, characterized in that: the method comprises the following steps:
uniformly mixing high gluten wheat flour and lipase to obtain a first mixture;
uniformly mixing the maltogenic amylase, xylanase, alpha-amylase, glucose oxidase and catalase microcapsules to obtain a second mixture;
and thirdly, uniformly mixing the first mixture and the second mixture to obtain the compound enzyme preparation.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310051459.3A CN116138287A (en) | 2023-02-02 | 2023-02-02 | Complex enzyme preparation and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310051459.3A CN116138287A (en) | 2023-02-02 | 2023-02-02 | Complex enzyme preparation and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116138287A true CN116138287A (en) | 2023-05-23 |
Family
ID=86350245
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310051459.3A Pending CN116138287A (en) | 2023-02-02 | 2023-02-02 | Complex enzyme preparation and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116138287A (en) |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0468731A1 (en) * | 1990-07-26 | 1992-01-29 | Oriental Yeast Co., Ltd. | Bread improver and method of producing bread |
| CN101358186A (en) * | 2008-09-19 | 2009-02-04 | 哈尔滨理工大学 | Method for fixing nitrile hydratase strain by sodium alginate-chitosan microcapsules |
| CN106070416A (en) * | 2016-07-28 | 2016-11-09 | 柳州中品科技有限公司 | A kind of bread flour biological modification agent |
| CN110200036A (en) * | 2019-06-11 | 2019-09-06 | 青岛品品好食品发展有限公司 | A kind of bread improver and preparation method thereof |
| CN111073878A (en) * | 2019-12-31 | 2020-04-28 | 惠民县邦德生物科技有限公司 | Preparation method of compound enzyme preparation |
| US20200205431A1 (en) * | 2017-06-15 | 2020-07-02 | Dsm Ip Assets B.V. | Frozen enzyme pellets |
| CN114287456A (en) * | 2021-12-31 | 2022-04-08 | 武汉市仟吉食品有限公司 | Compound enzyme preparation and clean label bread |
| CN115530200A (en) * | 2022-10-10 | 2022-12-30 | 上海早苗食品有限公司 | Bread improver and preparation method and application thereof |
-
2023
- 2023-02-02 CN CN202310051459.3A patent/CN116138287A/en active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0468731A1 (en) * | 1990-07-26 | 1992-01-29 | Oriental Yeast Co., Ltd. | Bread improver and method of producing bread |
| CN101358186A (en) * | 2008-09-19 | 2009-02-04 | 哈尔滨理工大学 | Method for fixing nitrile hydratase strain by sodium alginate-chitosan microcapsules |
| CN106070416A (en) * | 2016-07-28 | 2016-11-09 | 柳州中品科技有限公司 | A kind of bread flour biological modification agent |
| US20200205431A1 (en) * | 2017-06-15 | 2020-07-02 | Dsm Ip Assets B.V. | Frozen enzyme pellets |
| CN110200036A (en) * | 2019-06-11 | 2019-09-06 | 青岛品品好食品发展有限公司 | A kind of bread improver and preparation method thereof |
| CN111073878A (en) * | 2019-12-31 | 2020-04-28 | 惠民县邦德生物科技有限公司 | Preparation method of compound enzyme preparation |
| CN114287456A (en) * | 2021-12-31 | 2022-04-08 | 武汉市仟吉食品有限公司 | Compound enzyme preparation and clean label bread |
| CN115530200A (en) * | 2022-10-10 | 2022-12-30 | 上海早苗食品有限公司 | Bread improver and preparation method and application thereof |
Non-Patent Citations (2)
| Title |
|---|
| 刘持标等: "微胶囊固定化过氧化氢酶的制取及对H2O2的分解作用", 生物化学杂志, vol. 13, no. 4, 31 December 1997 (1997-12-31), pages 478 - 482 * |
| 黄家岭等: "细菌脂肪酶的固定化研究", 安徽农业科学, vol. 37, no. 25, 31 December 2009 (2009-12-31), pages 11849 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3055693B2 (en) | Anti-aging process and drugs | |
| US5716654A (en) | Dry yeast compositions and processes for preparing the same | |
| EP0833563B2 (en) | A method of improving the properties of a flour dough | |
| USRE43341E1 (en) | Method of improving the properties of a flour dough, a flour dough improving composition and improved food products | |
| RU2182424C2 (en) | Method of reducing syrup content in cooled dough compositions (versions), method of inhibition of fermentative decomposition of arabinoxylans in cooled compositions, dough content, baked product | |
| ES2554107T3 (en) | A method to improve the rheological properties of a refined flour dough | |
| CN116138287A (en) | Complex enzyme preparation and preparation method thereof | |
| EP1613176B1 (en) | Method of improving the hydration of pasta and preparation of pasta products | |
| CN110003534B (en) | Method for increasing SDS and RS content in starch | |
| EP0999752B1 (en) | A composition comprising an enzyme having galactose oxidase activity and use thereof | |
| D'Souza et al. | Removal of glucose from egg prior to spray drying by fermentation with immobilized yeast cells | |
| JPH0675461B2 (en) | Oil and fat composition for improving flour dough | |
| JPS63185931A (en) | Removal of blood serum cholesterol rise promoting factor from wheat bran | |
| CN114431271A (en) | Special lipoxygenase for bread and preparation method thereof | |
| AU742362B2 (en) | Dry yeast compositions | |
| AU710097B2 (en) | Oat extract and process for producing it | |
| CN114190480A (en) | Superoxide dismutase microcapsule and preparation method and application thereof | |
| JPH0626516B2 (en) | Chitosan soy sauce manufacturing method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |