WO2021101273A2 - 조절 t 세포 배양용 조성물 및 이의 용도 - Google Patents
조절 t 세포 배양용 조성물 및 이의 용도 Download PDFInfo
- Publication number
- WO2021101273A2 WO2021101273A2 PCT/KR2020/016382 KR2020016382W WO2021101273A2 WO 2021101273 A2 WO2021101273 A2 WO 2021101273A2 KR 2020016382 W KR2020016382 W KR 2020016382W WO 2021101273 A2 WO2021101273 A2 WO 2021101273A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- regulatory
- cell
- seq
- variant
- Prior art date
Links
- 210000003289 regulatory T cell Anatomy 0.000 title claims abstract description 103
- 239000000203 mixture Substances 0.000 title claims description 67
- 238000012258 culturing Methods 0.000 title claims description 23
- 108010002350 Interleukin-2 Proteins 0.000 claims abstract description 153
- 102000000588 Interleukin-2 Human genes 0.000 claims abstract description 153
- 210000004027 cell Anatomy 0.000 claims abstract description 126
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 95
- 102000037865 fusion proteins Human genes 0.000 claims abstract description 92
- 108020001507 fusion proteins Proteins 0.000 claims abstract description 92
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 claims abstract description 62
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 claims abstract description 62
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 claims abstract description 62
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 claims abstract description 53
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 claims abstract description 50
- 238000000034 method Methods 0.000 claims abstract description 44
- 239000012634 fragment Substances 0.000 claims abstract description 39
- 239000013636 protein dimer Substances 0.000 claims abstract description 38
- 150000001413 amino acids Chemical class 0.000 claims description 90
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 79
- 238000004113 cell culture Methods 0.000 claims description 18
- 230000006052 T cell proliferation Effects 0.000 claims description 17
- 201000010099 disease Diseases 0.000 claims description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 12
- 230000001404 mediated effect Effects 0.000 claims description 9
- 229940088594 vitamin Drugs 0.000 claims description 9
- 229930003231 vitamin Natural products 0.000 claims description 9
- 235000013343 vitamin Nutrition 0.000 claims description 9
- 239000011782 vitamin Substances 0.000 claims description 9
- 239000004480 active ingredient Substances 0.000 claims description 7
- 230000001105 regulatory effect Effects 0.000 claims description 7
- 235000000346 sugar Nutrition 0.000 claims description 7
- 150000003839 salts Chemical class 0.000 claims description 6
- 210000000601 blood cell Anatomy 0.000 claims description 5
- 150000008163 sugars Chemical class 0.000 claims description 3
- 230000035755 proliferation Effects 0.000 abstract description 9
- 230000004083 survival effect Effects 0.000 abstract description 9
- 239000006143 cell culture medium Substances 0.000 abstract description 8
- 238000012136 culture method Methods 0.000 abstract description 4
- 230000002062 proliferating effect Effects 0.000 abstract description 3
- 239000003814 drug Substances 0.000 abstract 1
- 229940124597 therapeutic agent Drugs 0.000 abstract 1
- 235000001014 amino acid Nutrition 0.000 description 103
- 229940024606 amino acid Drugs 0.000 description 89
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 88
- 239000002609 medium Substances 0.000 description 41
- 239000000872 buffer Substances 0.000 description 34
- 239000000539 dimer Substances 0.000 description 27
- 108700004922 F42A Proteins 0.000 description 24
- 102220495631 Putative uncharacterized protein LOC645739_F42A_mutation Human genes 0.000 description 24
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 24
- 102220596838 Non-structural maintenance of chromosomes element 1 homolog_R38A_mutation Human genes 0.000 description 23
- 239000000654 additive Substances 0.000 description 22
- 108090000623 proteins and genes Proteins 0.000 description 21
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 20
- 238000001542 size-exclusion chromatography Methods 0.000 description 18
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 18
- 102000003814 Interleukin-10 Human genes 0.000 description 17
- 108090000174 Interleukin-10 Proteins 0.000 description 17
- 102220505697 Palmitoyl-protein thioesterase 1_Y45F_mutation Human genes 0.000 description 17
- 102220610852 Thialysine N-epsilon-acetyltransferase_L72G_mutation Human genes 0.000 description 17
- 238000006467 substitution reaction Methods 0.000 description 17
- 239000006228 supernatant Substances 0.000 description 17
- 239000012980 RPMI-1640 medium Substances 0.000 description 16
- 239000011324 bead Substances 0.000 description 16
- 239000004615 ingredient Substances 0.000 description 16
- 238000002360 preparation method Methods 0.000 description 16
- 235000018102 proteins Nutrition 0.000 description 16
- 102000004169 proteins and genes Human genes 0.000 description 16
- 230000008569 process Effects 0.000 description 13
- 108090000765 processed proteins & peptides Proteins 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 108060003951 Immunoglobulin Proteins 0.000 description 10
- 230000003833 cell viability Effects 0.000 description 10
- 102000018358 immunoglobulin Human genes 0.000 description 10
- 238000005457 optimization Methods 0.000 description 10
- 230000028327 secretion Effects 0.000 description 10
- 239000011780 sodium chloride Substances 0.000 description 10
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 9
- 229930182555 Penicillin Natural products 0.000 description 9
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 9
- 239000000427 antigen Substances 0.000 description 9
- 229940049954 penicillin Drugs 0.000 description 9
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 9
- 229960005322 streptomycin Drugs 0.000 description 9
- 102000036639 antigens Human genes 0.000 description 8
- 108091007433 antigens Proteins 0.000 description 8
- 229940076144 interleukin-10 Drugs 0.000 description 8
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 8
- 238000005406 washing Methods 0.000 description 8
- 108010076504 Protein Sorting Signals Proteins 0.000 description 7
- 238000004458 analytical method Methods 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 238000000926 separation method Methods 0.000 description 7
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 7
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 6
- 239000004475 Arginine Substances 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 6
- 108090000695 Cytokines Proteins 0.000 description 6
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 6
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 6
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 6
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 6
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 6
- 230000000996 additive effect Effects 0.000 description 6
- 229960003767 alanine Drugs 0.000 description 6
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 6
- 235000009697 arginine Nutrition 0.000 description 6
- 229960003121 arginine Drugs 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 229960002989 glutamic acid Drugs 0.000 description 6
- 239000001963 growth medium Substances 0.000 description 6
- 210000002443 helper t lymphocyte Anatomy 0.000 description 6
- 229960003136 leucine Drugs 0.000 description 6
- 210000003071 memory t lymphocyte Anatomy 0.000 description 6
- 239000002773 nucleotide Substances 0.000 description 6
- 125000003729 nucleotide group Chemical group 0.000 description 6
- 229960004441 tyrosine Drugs 0.000 description 6
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 5
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 5
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 5
- 229930182816 L-glutamine Natural products 0.000 description 5
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 5
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 5
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 5
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 229910002091 carbon monoxide Inorganic materials 0.000 description 5
- 238000005119 centrifugation Methods 0.000 description 5
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 5
- 238000012790 confirmation Methods 0.000 description 5
- 239000012091 fetal bovine serum Substances 0.000 description 5
- 235000013922 glutamic acid Nutrition 0.000 description 5
- 239000004220 glutamic acid Substances 0.000 description 5
- 229940089468 hydroxyethylpiperazine ethane sulfonic acid Drugs 0.000 description 5
- 210000002602 induced regulatory T cell Anatomy 0.000 description 5
- 238000002826 magnetic-activated cell sorting Methods 0.000 description 5
- 210000002501 natural regulatory T cell Anatomy 0.000 description 5
- 229960005190 phenylalanine Drugs 0.000 description 5
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 5
- 235000008729 phenylalanine Nutrition 0.000 description 5
- 239000002157 polynucleotide Substances 0.000 description 5
- 102000040430 polynucleotide Human genes 0.000 description 5
- 108091033319 polynucleotide Proteins 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 5
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 4
- 208000023275 Autoimmune disease Diseases 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 108010074328 Interferon-gamma Proteins 0.000 description 4
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 4
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 4
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- 108091008874 T cell receptors Proteins 0.000 description 4
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 4
- 238000002835 absorbance Methods 0.000 description 4
- 235000004279 alanine Nutrition 0.000 description 4
- 230000027455 binding Effects 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 235000018417 cysteine Nutrition 0.000 description 4
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 4
- 230000028993 immune response Effects 0.000 description 4
- 230000001506 immunosuppresive effect Effects 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 210000000581 natural killer T-cell Anatomy 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 229960001153 serine Drugs 0.000 description 4
- 229940054269 sodium pyruvate Drugs 0.000 description 4
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000011534 wash buffer Substances 0.000 description 4
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 108050003558 Interleukin-17 Proteins 0.000 description 3
- 102000013691 Interleukin-17 Human genes 0.000 description 3
- 102000015696 Interleukins Human genes 0.000 description 3
- 108010063738 Interleukins Proteins 0.000 description 3
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 3
- 239000012515 MabSelect SuRe Substances 0.000 description 3
- 239000004473 Threonine Substances 0.000 description 3
- 239000007983 Tris buffer Substances 0.000 description 3
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- 210000003719 b-lymphocyte Anatomy 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 3
- 229960002433 cysteine Drugs 0.000 description 3
- 238000000502 dialysis Methods 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000003797 essential amino acid Substances 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 229960002885 histidine Drugs 0.000 description 3
- 102000054189 human CD80 Human genes 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 229910017053 inorganic salt Inorganic materials 0.000 description 3
- 229960004452 methionine Drugs 0.000 description 3
- 229960002429 proline Drugs 0.000 description 3
- 239000012562 protein A resin Substances 0.000 description 3
- 239000008213 purified water Substances 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229960002898 threonine Drugs 0.000 description 3
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 3
- 229960004295 valine Drugs 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- PWKSKIMOESPYIA-UHFFFAOYSA-N 2-acetamido-3-sulfanylpropanoic acid Chemical compound CC(=O)NC(CS)C(O)=O PWKSKIMOESPYIA-UHFFFAOYSA-N 0.000 description 2
- CFKMVGJGLGKFKI-UHFFFAOYSA-N 4-chloro-m-cresol Chemical compound CC1=CC(O)=CC=C1Cl CFKMVGJGLGKFKI-UHFFFAOYSA-N 0.000 description 2
- OLXZPDWKRNYJJZ-UHFFFAOYSA-N 5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol Chemical compound C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(CO)O1 OLXZPDWKRNYJJZ-UHFFFAOYSA-N 0.000 description 2
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 2
- 210000001239 CD8-positive, alpha-beta cytotoxic T lymphocyte Anatomy 0.000 description 2
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical group [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- 238000008157 ELISA kit Methods 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 2
- 101001033233 Homo sapiens Interleukin-10 Proteins 0.000 description 2
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 2
- 102100037850 Interferon gamma Human genes 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 102000004388 Interleukin-4 Human genes 0.000 description 2
- 108090000978 Interleukin-4 Proteins 0.000 description 2
- QNAYBMKLOCPYGJ-UWTATZPHSA-N L-Alanine Natural products C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 description 2
- FFEARJCKVFRZRR-UHFFFAOYSA-N L-Methionine Natural products CSCCC(N)C(O)=O FFEARJCKVFRZRR-UHFFFAOYSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 2
- 229930064664 L-arginine Natural products 0.000 description 2
- 235000014852 L-arginine Nutrition 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- 229930195722 L-methionine Natural products 0.000 description 2
- 229930182821 L-proline Natural products 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 2
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 2
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 239000002313 adhesive film Substances 0.000 description 2
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 2
- 208000004631 alopecia areata Diseases 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 208000002399 aphthous stomatitis Diseases 0.000 description 2
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 2
- 229960005070 ascorbic acid Drugs 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 229960005261 aspartic acid Drugs 0.000 description 2
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- LVXHNCUCBXIIPE-UHFFFAOYSA-L disodium;hydrogen phosphate;hydrate Chemical compound O.[Na+].[Na+].OP([O-])([O-])=O LVXHNCUCBXIIPE-UHFFFAOYSA-L 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 229960002743 glutamine Drugs 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 102000052620 human IL10 Human genes 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- 231100000053 low toxicity Toxicity 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000011325 microbead Substances 0.000 description 2
- 229960003966 nicotinamide Drugs 0.000 description 2
- 235000005152 nicotinamide Nutrition 0.000 description 2
- 239000011570 nicotinamide Substances 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 230000008823 permeabilization Effects 0.000 description 2
- 239000001103 potassium chloride Substances 0.000 description 2
- 235000011164 potassium chloride Nutrition 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- ZUFQODAHGAHPFQ-UHFFFAOYSA-N pyridoxine hydrochloride Chemical compound Cl.CC1=NC=C(CO)C(CO)=C1O ZUFQODAHGAHPFQ-UHFFFAOYSA-N 0.000 description 2
- 229960004172 pyridoxine hydrochloride Drugs 0.000 description 2
- 235000019171 pyridoxine hydrochloride Nutrition 0.000 description 2
- 239000011764 pyridoxine hydrochloride Substances 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 229930002330 retinoic acid Natural products 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000010265 sodium sulphite Nutrition 0.000 description 2
- 238000009331 sowing Methods 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 208000003265 stomatitis Diseases 0.000 description 2
- 239000012089 stop solution Substances 0.000 description 2
- WPLOVIFNBMNBPD-ATHMIXSHSA-N subtilin Chemical compound CC1SCC(NC2=O)C(=O)NC(CC(N)=O)C(=O)NC(C(=O)NC(CCCCN)C(=O)NC(C(C)CC)C(=O)NC(=C)C(=O)NC(CCCCN)C(O)=O)CSC(C)C2NC(=O)C(CC(C)C)NC(=O)C1NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C1NC(=O)C(=C/C)/NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C2NC(=O)CNC(=O)C3CCCN3C(=O)C(NC(=O)C3NC(=O)C(CC(C)C)NC(=O)C(=C)NC(=O)C(CCC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)CC=4C5=CC=CC=C5NC=4)CSC3)C(C)SC2)C(C)C)C(C)SC1)CC1=CC=CC=C1 WPLOVIFNBMNBPD-ATHMIXSHSA-N 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- DPJRMOMPQZCRJU-UHFFFAOYSA-M thiamine hydrochloride Chemical compound Cl.[Cl-].CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N DPJRMOMPQZCRJU-UHFFFAOYSA-M 0.000 description 2
- 229960000344 thiamine hydrochloride Drugs 0.000 description 2
- 235000019190 thiamine hydrochloride Nutrition 0.000 description 2
- 239000011747 thiamine hydrochloride Substances 0.000 description 2
- 210000001541 thymus gland Anatomy 0.000 description 2
- 229960001727 tretinoin Drugs 0.000 description 2
- 229960004799 tryptophan Drugs 0.000 description 2
- 150000003722 vitamin derivatives Chemical class 0.000 description 2
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 2
- -1 膨-aminobutyric acid Chemical compound 0.000 description 2
- JTSYPLOSGCFYBZ-SECBINFHSA-N (2S)-2-amino-3-(4-hydroxyphenyl)-2,3,3-triiodopropanoic acid Chemical compound IC([C@](N)(C(=O)O)I)(C1=CC=C(C=C1)O)I JTSYPLOSGCFYBZ-SECBINFHSA-N 0.000 description 1
- PBTPTBMYJPCXRQ-MGMRMFRLSA-N (2r)-2-[(1s)-1,2-dihydroxyethyl]-3,4-dihydroxy-2h-furan-5-one;hexadecanoic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O.CCCCCCCCCCCCCCCC(O)=O PBTPTBMYJPCXRQ-MGMRMFRLSA-N 0.000 description 1
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 1
- YKBGVTZYEHREMT-KVQBGUIXSA-N 2'-deoxyguanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 YKBGVTZYEHREMT-KVQBGUIXSA-N 0.000 description 1
- YKBGVTZYEHREMT-UHFFFAOYSA-N 2'-deoxyguanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1CC(O)C(CO)O1 YKBGVTZYEHREMT-UHFFFAOYSA-N 0.000 description 1
- SPSPIUSUWPLVKD-UHFFFAOYSA-N 2,3-dibutyl-6-methylphenol Chemical compound CCCCC1=CC=C(C)C(O)=C1CCCC SPSPIUSUWPLVKD-UHFFFAOYSA-N 0.000 description 1
- UTIMIHJZXIJZKZ-UHFFFAOYSA-N 2-amino-3-(2,4-dihydroxyphenyl)propanoic acid Chemical compound OC(=O)C(N)CC1=CC=C(O)C=C1O UTIMIHJZXIJZKZ-UHFFFAOYSA-N 0.000 description 1
- QDGAVODICPCDMU-UHFFFAOYSA-N 2-amino-3-[3-[bis(2-chloroethyl)amino]phenyl]propanoic acid Chemical compound OC(=O)C(N)CC1=CC=CC(N(CCCl)CCCl)=C1 QDGAVODICPCDMU-UHFFFAOYSA-N 0.000 description 1
- 239000001763 2-hydroxyethyl(trimethyl)azanium Substances 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- QULIOZDJZXKLNY-UHFFFAOYSA-N 3,4,5-trihydroxy-2-propylbenzoic acid Chemical compound CCCC1=C(O)C(O)=C(O)C=C1C(O)=O QULIOZDJZXKLNY-UHFFFAOYSA-N 0.000 description 1
- MOMKYJPSVWEWPM-UHFFFAOYSA-N 4-(chloromethyl)-2-(4-methylphenyl)-1,3-thiazole Chemical compound C1=CC(C)=CC=C1C1=NC(CCl)=CS1 MOMKYJPSVWEWPM-UHFFFAOYSA-N 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 208000033116 Asbestos intoxication Diseases 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 206010050245 Autoimmune thrombocytopenia Diseases 0.000 description 1
- 208000009137 Behcet syndrome Diseases 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- QRMPKOFEUHIBNM-UHFFFAOYSA-N CC1CCC(C)CC1 Chemical compound CC1CCC(C)CC1 QRMPKOFEUHIBNM-UHFFFAOYSA-N 0.000 description 1
- 235000019743 Choline chloride Nutrition 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- AUNGANRZJHBGPY-UHFFFAOYSA-N D-Lyxoflavin Natural products OCC(O)C(O)C(O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-UHFFFAOYSA-N 0.000 description 1
- CKLJMWTZIZZHCS-UHFFFAOYSA-N D-OH-Asp Natural products OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 description 1
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 description 1
- CIWBSHSKHKDKBQ-DUZGATOHSA-N D-araboascorbic acid Natural products OC[C@@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-DUZGATOHSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 1
- XUIIKFGFIJCVMT-GFCCVEGCSA-N D-thyroxine Chemical compound IC1=CC(C[C@@H](N)C(O)=O)=CC(I)=C1OC1=CC(I)=C(O)C(I)=C1 XUIIKFGFIJCVMT-GFCCVEGCSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- 208000026019 Fanconi renotubular syndrome Diseases 0.000 description 1
- 201000006328 Fanconi syndrome Diseases 0.000 description 1
- 229910016870 Fe(NO3)3-9H2O Inorganic materials 0.000 description 1
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 208000001640 Fibromyalgia Diseases 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 102100027581 Forkhead box protein P3 Human genes 0.000 description 1
- 101710088098 Forkhead box protein P3 Proteins 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000007465 Giant cell arteritis Diseases 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- 102000001398 Granzyme Human genes 0.000 description 1
- 108060005986 Granzyme Proteins 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 208000002972 Hepatolenticular Degeneration Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 206010020850 Hyperthyroidism Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000016844 Immunoglobulin-like domains Human genes 0.000 description 1
- 108050006430 Immunoglobulin-like domains Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 102000000704 Interleukin-7 Human genes 0.000 description 1
- CKLJMWTZIZZHCS-UWTATZPHSA-N L-Aspartic acid Natural products OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- 239000002211 L-ascorbic acid Substances 0.000 description 1
- 235000000069 L-ascorbic acid Nutrition 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- 229930182844 L-isoleucine Natural products 0.000 description 1
- 239000004395 L-leucine Substances 0.000 description 1
- 235000019454 L-leucine Nutrition 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 241000721454 Pemphigus Species 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 206010065159 Polychondritis Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- 241000978776 Senegalia senegal Species 0.000 description 1
- 201000010001 Silicosis Diseases 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 206010061372 Streptococcal infection Diseases 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 210000000447 Th1 cell Anatomy 0.000 description 1
- 210000000068 Th17 cell Anatomy 0.000 description 1
- 210000004241 Th2 cell Anatomy 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 1
- 206010043781 Thyroiditis chronic Diseases 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 229930003779 Vitamin B12 Natural products 0.000 description 1
- 208000018839 Wilson disease Diseases 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 229940087168 alpha tocopherol Drugs 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229940124277 aminobutyric acid Drugs 0.000 description 1
- 208000007502 anemia Diseases 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- LNTHITQWFMADLM-UHFFFAOYSA-N anhydrous gallic acid Natural products OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 206010003441 asbestosis Diseases 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000005441 aurora Substances 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 102220363900 c.182A>C Human genes 0.000 description 1
- FAPWYRCQGJNNSJ-UBKPKTQASA-L calcium D-pantothenic acid Chemical compound [Ca+2].OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O.OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O FAPWYRCQGJNNSJ-UBKPKTQASA-L 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229960002242 chlorocresol Drugs 0.000 description 1
- SGMZJAMFUVOLNK-UHFFFAOYSA-M choline chloride Chemical compound [Cl-].C[N+](C)(C)CCO SGMZJAMFUVOLNK-UHFFFAOYSA-M 0.000 description 1
- 229960003178 choline chloride Drugs 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- AGVAZMGAQJOSFJ-WZHZPDAFSA-M cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].N#[C-].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP(O)(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O AGVAZMGAQJOSFJ-WZHZPDAFSA-M 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- JZCCFEFSEZPSOG-UHFFFAOYSA-L copper(II) sulfate pentahydrate Chemical compound O.O.O.O.O.[Cu+2].[O-]S([O-])(=O)=O JZCCFEFSEZPSOG-UHFFFAOYSA-L 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- 229940013361 cresol Drugs 0.000 description 1
- 150000001945 cysteines Chemical class 0.000 description 1
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- VGONTNSXDCQUGY-UHFFFAOYSA-N desoxyinosine Natural products C1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 VGONTNSXDCQUGY-UHFFFAOYSA-N 0.000 description 1
- 229940119744 dextran 40 Drugs 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 201000002491 encephalomyelitis Diseases 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 235000010350 erythorbic acid Nutrition 0.000 description 1
- 239000004318 erythorbic acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- DNJIEGIFACGWOD-UHFFFAOYSA-N ethyl mercaptane Natural products CCS DNJIEGIFACGWOD-UHFFFAOYSA-N 0.000 description 1
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 230000033581 fucosylation Effects 0.000 description 1
- 229940074391 gallic acid Drugs 0.000 description 1
- 235000004515 gallic acid Nutrition 0.000 description 1
- 210000004475 gamma-delta t lymphocyte Anatomy 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 235000004554 glutamine Nutrition 0.000 description 1
- 229960002449 glycine Drugs 0.000 description 1
- 229940029575 guanosine Drugs 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 230000004727 humoral immunity Effects 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229940028885 interleukin-4 Drugs 0.000 description 1
- 229940100994 interleukin-7 Drugs 0.000 description 1
- SZQUEWJRBJDHSM-UHFFFAOYSA-N iron(3+);trinitrate;nonahydrate Chemical compound O.O.O.O.O.O.O.O.O.[Fe+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O SZQUEWJRBJDHSM-UHFFFAOYSA-N 0.000 description 1
- XNCMOUSLNOHBKY-UHFFFAOYSA-H iron(3+);trisulfate;heptahydrate Chemical compound O.O.O.O.O.O.O.[Fe+3].[Fe+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O XNCMOUSLNOHBKY-UHFFFAOYSA-H 0.000 description 1
- 229940026239 isoascorbic acid Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- AGBQKNBQESQNJD-UHFFFAOYSA-M lipoate Chemical compound [O-]C(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-M 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- 235000018977 lysine Nutrition 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 201000006292 polyarteritis nodosa Diseases 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 208000005987 polymyositis Diseases 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 229960002477 riboflavin Drugs 0.000 description 1
- 235000019192 riboflavin Nutrition 0.000 description 1
- 239000002151 riboflavin Substances 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000015424 sodium Nutrition 0.000 description 1
- IFGCUJZIWBUILZ-UHFFFAOYSA-N sodium 2-[[2-[[hydroxy-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyphosphoryl]amino]-4-methylpentanoyl]amino]-3-(1H-indol-3-yl)propanoic acid Chemical compound [Na+].C=1NC2=CC=CC=C2C=1CC(C(O)=O)NC(=O)C(CC(C)C)NP(O)(=O)OC1OC(C)C(O)C(O)C1O IFGCUJZIWBUILZ-UHFFFAOYSA-N 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- 229940079827 sodium hydrogen sulfite Drugs 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 235000019983 sodium metaphosphate Nutrition 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 206010043207 temporal arteritis Diseases 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 229940034208 thyroxine Drugs 0.000 description 1
- XUIIKFGFIJCVMT-UHFFFAOYSA-N thyroxine-binding globulin Natural products IC1=CC(CC([NH3+])C([O-])=O)=CC(I)=C1OC1=CC(I)=C(O)C(I)=C1 XUIIKFGFIJCVMT-UHFFFAOYSA-N 0.000 description 1
- 229960000984 tocofersolan Drugs 0.000 description 1
- 229940042585 tocopherol acetate Drugs 0.000 description 1
- 239000012929 tonicity agent Substances 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 235000019163 vitamin B12 Nutrition 0.000 description 1
- 239000011715 vitamin B12 Substances 0.000 description 1
- RZLVQBNCHSJZPX-UHFFFAOYSA-L zinc sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Zn+2].[O-]S([O-])(=O)=O RZLVQBNCHSJZPX-UHFFFAOYSA-L 0.000 description 1
- 239000002076 α-tocopherol Substances 0.000 description 1
- 235000004835 α-tocopherol Nutrition 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0637—Immunosuppressive T lymphocytes, e.g. regulatory T cells or Treg
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2302—Interleukin-2 (IL-2)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/50—Cell markers; Cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/50—Cell markers; Cell surface determinants
- C12N2501/51—B7 molecules, e.g. CD80, CD86, CD28 (ligand), CD152 (ligand)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/998—Proteins not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/999—Small molecules not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/11—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from blood or immune system cells
- C12N2506/115—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from blood or immune system cells from monocytes, from macrophages
Definitions
- the present invention relates to a composition for culturing T cells and a method for culturing control T cells using the same.
- T cells play a central role in cell-mediated immunity.
- T cells can be differentiated from other lymphocytes, including B cells, by receptors present on the surface of T cells, such as T cell receptors (TCRs).
- T cells are memory T cells (TM) including helper T cells (TH cells), cytotoxic T cells (TC cells, or CTL), central memory T cells (TCM cells) and effector memory T cells (TEM cells).
- TM memory T cells
- TH cells helper T cells
- T cells cytotoxic T cells
- TCM cells central memory T cells
- TEM cells effector memory T cells
- Cells natural killer T cells (NKT cells), gamma delta T cells ( ⁇ T cells), and regulatory T cells (Treg cells).
- Treg regulatory T cells
- Treg cells are T cells that act to suppress the immune response of other cells.
- Treg cells suppress T cell-mediated immunity during the immune response and play a role in suppressing self-reactive T cells that have escaped during the negative selection process of the thymus.
- Treg cells can be largely divided into natural regulatory T cells (nTreg) and induced regulatory T cells (iTreg).
- Natural regulatory T cells known as CD4+CD25+FoxP3+ regulatory T cells arise in the thymus.
- Induced regulatory T cells share a number of properties with naturally occurring Treg cells, but the characteristic of conversion from CD4+CD25-FoxP3- T cells to CD4+CD25+FoxP3+ regulatory T cells is known as a representative difference.
- regulatory T cells can secrete IL-10, TGF- ⁇ , and IL-35, which are known as immunosuppressive cytokines (H Nishikawa et al., Int. J. Cancer, 2010, 127: 759- 767). Regulatory T cells that secrete immunosuppressive cytokines secrete IL-10 and induce antigen-specific T cells that cause autoimmune diseases to immune tolerant antigen presenting cells, thereby inducing immunological resistance. Research to prove that is in progress (S. Karumuthil-Melethil et, al., Diabetes, 2015, 64:1341-1357).
- the present inventors studied a method for effectively culturing regulatory T cells, by confirming that a novel fusion protein dimer containing IL-2 protein and CD80 protein in one molecule can effectively proliferate regulatory T cells.
- the present invention has been completed.
- composition or medium for culturing a regulated T cell comprising an IL-2 protein or a variant thereof and a fusion protein dimer including a CD80 protein or a fragment thereof as an active ingredient.
- a fusion protein dimer comprising an IL-2 protein or a variant thereof and a CD80 protein or a fragment thereof.
- composition comprising, as an active ingredient, regulatory T cells cultured in a medium containing an IL-2 protein or a variant thereof and a fusion protein dimer comprising a CD80 protein or a fragment thereof.
- the culture composition of the present invention When the culture composition of the present invention is used, not only T cells can be effectively proliferated, but in particular, regulatory T cells can be effectively proliferated. In particular, it was confirmed that the survival rate of regulatory T cells significantly increased compared to the conventional culture method using IL-2. In addition, it was confirmed that the amount of Foxp3+ expression was increased in the obtained regulatory T cells. Therefore, this proliferation method can be utilized in the field of cell therapy using regulatory T cells.
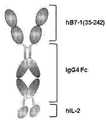
- Figure 1a shows a schematic diagram of the fusion protein dimer used in the present invention.
- Fig. 1b is a confirmation of the obtained fusion protein dimer (GI-101) by SDS-PAGE.
- Figure 1c shows the content of the fusion protein dimer (GI-101) according to the absorbance.
- 1D is an analysis of the obtained fusion protein dimer (GI-101) by size exclusion chromatography (SEC).
- Figure 2a shows the obtained hCD80-Fc fusion protein dimer confirmed by SDS-PAGE.
- 2B is an analysis of the obtained hCD80-Fc fusion protein dimer by size exclusion chromatography (SEC).
- Figure 3a shows the obtained Fc-IL2v2 fusion protein dimer confirmed by SDS-PAGE.
- 3B is an analysis of the obtained Fc-IL2v2 fusion protein dimer by size exclusion chromatography (SEC).
- Figure 3c shows the obtained Fc-IL2wt fusion protein dimer confirmed by SDS-PAGE.
- 3D is an analysis of the obtained Fc-IL2wt fusion protein dimer by size exclusion chromatography (SEC).
- Figure 4a shows the obtained hCD80-Fc-IL2wt fusion protein dimer confirmed by SDS-PAGE.
- 4B is an analysis of the obtained hCD80-Fc-IL2wt fusion protein dimer by size exclusion chromatography (SEC).
- FIG. 5 shows a schematic diagram of a method of culturing Treg cells using a fusion protein dimer.
- FIG. 6 shows the number of regulatory T cells cultured in a composition containing RPMI1640 medium.
- FIG. 7 shows the survival rate of regulatory T cells cultured in a composition containing RPMI1640 medium.
- FIG. 10 shows the IL-10 secretion ability of regulatory T cells cultured using a composition containing RPMI1640 medium.
- 11 shows the IL-10 secretion ability of regulatory T cells cultured using a composition containing TexMACS medium.
- 12A and 12B show the number of regulatory T cells proliferated when cultured in a composition including RPMI1640 medium in the optimization process.
- 13A and 13B show the survival rate of regulatory T cells when cultured in a composition including RPMI1640 medium in the optimization process.
- 14A and 14B show the number of regulatory T cells when cultured in a composition including TexMACS medium in the optimization process.
- 15A and 15B show the survival rate of regulatory T cells when cultured in a composition including TexMACS medium in the optimization process.
- 16A to 16C show the results of FACS analysis of the characteristics of cells cultured in a composition including RPMI1640 medium in the optimization process.
- 17A to 17C show the number of regulatory T cells expressing Foxp3 when cultured in a composition including RPMI1640 medium in the optimization process.
- 18A to 18C show the results of FACS analysis of the characteristics of cells cultured in a composition including a TexMACS medium in the optimization process.
- 19A to 19C show the number of regulatory T cells expressing Foxp3 when cultured in a composition including TexMACS medium in the optimization process.
- 21 shows the IL-10 secretion ability of regulatory T cells cultured in a composition including TexMACS medium in the optimization process.
- compositions and medium for regulatory T cell proliferation Composition and medium for regulatory T cell proliferation
- composition for regulated T cell proliferation comprising, as an active ingredient, a fusion protein dimer including an IL-2 protein or a variant thereof and a CD80 protein or a fragment thereof.
- a medium for regulatory T cell proliferation comprising the fusion protein dimer as an active ingredient.
- the medium for proliferation of the regulatory T cells may be a medium to which a fusion protein dimer including the IL-2 protein or a variant thereof and a CD80 protein or a fragment thereof is added to a medium for culturing T cells.
- the T cell culture medium may contain any one selected from the group consisting of amino acids, sugars, inorganic salts, and vitamins.
- the T cell culture medium may contain all of amino acids, sugars, inorganic salts, and vitamins.
- the medium may further include Fetal Bovine Serum (FBS), Hydroxyethyl Piperazine Ethane Sulfonic Acid (HEPES), protein, carbohydrate, mercaptoethanol, and growth factors.
- the medium for culturing the regulated T cells may further contain retinoic acid.
- the medium for proliferation of the regulatory T cells may include the basic components of Table 3 or Table 4 below.
- the term "cell culture medium” refers to a medium used for culturing cells, and specifically refers to a medium for culturing regulatory T cells, more specifically CD4 + CD25 + CD127- cells. . Contains components required by cells for cell growth and survival in vitro , or contains components that aid in cell growth and survival. Specifically, the ingredients may be vitamins, essential or non-essential amino acids, and trace elements.
- the medium may be a medium used for culturing cells, preferably eukaryotic cells, more preferably regulatory T cells, even more preferably CD4 + CD25 + CD127- T cells or CD4 + CD25 + Foxp3 + T cells.
- the cell culture medium according to the present invention is composed of an amino acid component, a vitamin component, an inorganic salt component, other components and purified water,
- the amino acid component is glycine, L-alanine, L-valine, L-leucine, L-isoleucine, L-threonine, L-serine, L-cysteine, L-methionine, L-aspartic acid, L-asparagine, L -Glutamic acid, L-glutamine, L-lysine, L-arginine, L-histidine, L-phenylalanine, L-tyrosine, L-tryptophan, L-proline, ⁇ -alanine, ⁇ -aminobutyric acid, ornithine, citrulline, homo It is at least any one amino acid selected from the group consisting of serine, triiodotyrosine, thyroxine, and dioxyphenylalanine, or a combination thereof, and preferably, glycine, L-alanine, L-arginine, L-cysteine, L-glutamine, L -
- the vitamin component is at least one selected from the group consisting of biotin, D-calcium pantothenate, folic acid, niacinamide, pyridoxine hydrochloride, riboflavin, thiamine hydrochloride, vitamin B12, choline chloride, i-inositol and ascorbic acid. Vitamins or combinations thereof, preferably at least one or more vitamins selected from the group consisting of i-inositol, thiamine hydrochloride, niacinamide and pyridoxine hydrochloride, or combinations thereof,
- the inorganic salt component is calcium chloride (CaCl 2) (anhydride), copper sulfate pentahydrate (CuSO 4 -5H 2 O), ferric sulfate hepta-hydrate (FeSO 4 -7H 2 O), magnesium chloride (anhydrous), magnesium sulfate ( MgSO 4 ) (anhydrous), potassium chloride (KCl), sodium chloride (NaCl), disodium hydrogen phosphate (Na 2 HPO 4 ), sodium hydrogen phosphate monohydrate (NaH 2 PO 4 -H 2 O), zinc sulfate heptahydrate (ZnSO 4 -7H 2 O), ferric nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) and sodium hydrogen carbonate (NaHCO 3 ).
- CaCl 2 2 calcium chloride
- CuSO 4 -5H 2 O copper sulfate pentahydrate
- FeSO 4 -7H 2 O ferric sulfate hepta-hydrate
- the other ingredients are D-glucose (dextrose), sodium pyruvate, hypoxanthine Na, thymidine, linoleic acid, lipoic acid, adenosine, cytidine, guanosine, uridine, 2'-deoxyadenosine, 2 It is at least any one other component selected from the group consisting of'-deoxycytidine HCl and 2'-deoxyguanosine, or a combination thereof, preferably sodium pyruvate.
- Purified water is used to dissolve the amino acids, vitamins, inorganic salts and other components, and may be obtained through one or more distillation, or purified water through a filter.
- growth factors or cytokines may be further included in the cell culture medium according to the present invention.
- growth factor IGF, bFGF, TGF, HGF, EGF, VEGF, or PDGF may be used alone or in two or more, but is not particularly limited thereto.
- cytokine IL-1, IL-4, IL-6, IFN- ⁇ , IL-10, or IL-17 may be used alone or in two or more, but is not particularly limited thereto.
- T cell refers to one of the lymphocytes responsible for antigen-specific adaptive immunity. T cells are classified into naive T cells that have not yet met the antigen, mature T cells that have met the antigen, and memory T cells. At this time, the mature effect T cells include helper T cells, cytotoxic T cells, and natural killer T cells.
- helper T cell refers to a cell that promotes humoral immunity by regulating the differentiation and activation of other white blood cells. Because it has a CD4 protein on the cell surface, it is also called a CD4+ T cell. Helper T cells can be further classified into Th1, Th2, Th17, and Treg according to their detailed function. Th1 cells secrete interferon-gamma (IFN- ⁇ ) and tumor necrosis factor beta (TNF- ⁇ ) to induce endosomes and lysosomes to fuse inside macrophages to form endolysosomes. do. Meanwhile, Th2 cells secrete several types of interleukin (IL) to allow B cells to differentiate into plasma cells. Th17 cells secrete interleukin-17 (IL-17) to gather neutrophils.
- IFN- ⁇ interferon-gamma
- TNF- ⁇ tumor necrosis factor beta
- regulatory T cell includes natural regulatory T cells (nTreg) or induced regulatory T cells (iTreg).
- Regulatory T cells herein include CD4+CD25+ T cells, CD4+CD25+CD127low/- T cells or CD4+CD25+Foxp3+ T cells.
- the regulatory T cells maintain immunity homeostasis by suppressing the immune response and block autoimmune reactions and the like.
- cytotoxic T cells refers to cells that kill virus-infected cells or tumor cells by secreting cytotoxic substances such as granzyme or perforin. It is also called a CD8 T cell because it has a CD8 protein on the cell surface. In contrast to helper T cells, it mediates cellular immunity to eliminate virus and cancer cells.
- Natural killer T cells refers to one of effective T cells distributed in a small proportion compared to helper T cells and cytotoxic T cells. Natural killer T cells have the same T cell receptor (TCR) on the cell surface as T cells, but also have natural killer cell-specific molecules such as NK1.1. Naturally killer T cells secrete gamma interferon and interleukin-4 to regulate the immune response.
- TCR T cell receptor
- memory T cells refers to T cells that recognize antigens, which survive for a long time after undergoing differentiation and selection process, and are then rapidly activated when the antigen re-invades later to function as effective T cells. It refers to a cell with potential ability.
- naive T cells meet antigens, cells in an activated state, or effective T cells, are influenced by interleukin-7 and interleukin-15 to differentiate into long-lived memory T cells.
- the fusion protein dimer including the IL-2 protein or a variant thereof and a CD80 protein or a fragment thereof may be included in the culture medium from 1 nM to 2,000 nM.
- the dimer may be included in 1 nM to 1,000 nM, or 1 nM to 500 nM.
- the dimer may be included in 2 nM to 300 nM, 5 nM to 100 nM, 10 nM to 80 nM, 20 nM to 70 nM, or 40 nM to 50 nM.
- the fusion protein dimer may be included in 1 nM, 3.2 nM, 10 nM, 50 nM in the medium.
- Fusion protein dimer comprising IL-2 protein or variant thereof and CD80 protein or fragment thereof
- IL-2 or "Interleukin-2"
- mammals such as primates (eg humans) and rodents (eg mice and rats).
- rodents eg mice and rats
- the IL-2 may be obtained from animal cells, but includes those obtained from recombinant cells capable of producing IL-2.
- the IL-2 may be wild-type IL-2 or a variant thereof.
- IL-2 or a variant thereof is collectively referred to as "IL-2 protein” or "IL-2 polypeptide".
- IL-2, IL-2 protein, IL-2 polypeptide, and IL-2 variants specifically bind to, for example, IL-2 receptors. This specific binding can be confirmed through methods known to those skilled in the art.
- the IL-2 may have the amino acid sequence of SEQ ID NO: 35 or SEQ ID NO: 36.
- the IL-2 may be in a mature form. Specifically, the matured IL-2 may not include a signal sequence, and may have an amino acid sequence of SEQ ID NO: 10.
- the IL-2 may be used as a concept including a fragment in which a part of the N-terminus or C-terminus of wild-type IL-2 is deleted (truncated).
- the IL-2 fragment is 1, 2, 3, 4, 5, 6, 7, 8 consecutively from the N-terminus of the protein having the amino acid sequence of SEQ ID NO: 35 or SEQ ID NO: 36. Pcs, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 or It may be a form in which 25 amino acids have been deleted.
- the fragment of IL-2 is 1, 2, 3, 4, 5, 6, 7, 8 consecutively from the C-terminus of the protein having the amino acid sequence of SEQ ID NO: 35 or SEQ ID NO: 36 , 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 or 25 It may be a form in which dog amino acids have been deleted.
- the term "IL-2 variant” refers to a form in which a part of an amino acid of a full-length IL-2 or a fragment of IL-2 is substituted. That is, the IL-2 variant may have an amino acid sequence different from that of wild-type IL-2 or a fragment thereof. However, the IL-2 variant may have an activity equal to or similar to wild-type IL-2.
- IL-2 activity may mean specifically binding to the IL-2 receptor, for example, and this specific binding can be measured through a method known to those skilled in the art.
- the IL-2 variant may be one in which a part of the amino acid of wild-type IL-2 is substituted.
- the IL-2 variant by amino acid substitution at least one of the 38th, 42nd, 45th, 61st, and 72nd amino acids in the amino acid sequence of SEQ ID NO: 10 may be substituted.
- the IL-2 variant may be one in which at least one of the 38th, 42nd, 45th, 61st, or 72nd amino acids in the amino acid sequence of SEQ ID NO: 10 is substituted with another amino acid.
- IL-2 when IL-2 is a form in which a part of the N-terminal portion of the amino acid sequence of SEQ ID NO: 35 is deleted, an amino acid at a position corresponding to that of the amino acid sequence of SEQ ID NO: 10 may be substituted with another amino acid.
- the variant of IL-2 is among the 58th, 62nd, 65th, 81st or 92nd amino acids in the amino acid sequence of SEQ ID NO: 35.
- At least one of them may be substituted with another amino acid.
- These correspond to the 38th, 42nd, 45th, 61st and 72nd amino acid residues of the amino acid sequence of SEQ ID NO: 10, respectively.
- 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 amino acids may be substituted. have.
- from 1 to 5 amino acids may be substituted.
- the IL-2 variant may be in a form in which two amino acids are substituted. Specifically, the IL-2 variant may be one in which the 38th and 42nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may have the 38th and 45th amino acids substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may have the 38th and 61st amino acids substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which the 42nd and 45th amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 42nd and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 42nd and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 45th and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which the 45th and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 61st and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be in a form in which three amino acids are substituted. Specifically, the IL-2 variant may be one in which the 38th, 42nd, and 45th amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 42nd, and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 42nd, and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which the 38th, 45th, and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 45th, and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 61st, and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 42nd, 45th, and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which the 42nd, 45th, and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 45th, 61st, and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be in a form in which four amino acids are substituted. Specifically, the IL-2 variant may be one in which the 38th, 42nd, 45th and 61st amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 42nd, 45th and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10. In addition, in one embodiment, the IL-2 variant may be one in which the 38th, 45th, 61st and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which the 38th, 42nd, 61st and 72nd amino acids are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may be one in which amino acids 42, 45, 61, and 72 are substituted in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may have a form in which five amino acids are substituted. Specifically, in the IL-2 variant, all of the 38th, 42nd, 45th, 61st and 72nd amino acids in the amino acid sequence of SEQ ID NO: 10 may be substituted with other amino acids.
- the "other amino acids" introduced by the substitution are alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, and glutamine. , Histidine, isoleucine, leucine, lysine, methionine, phenyl alanine, proline, serine, threonine, tryptophan ( tryptophan), tyrosine, and valine.
- the 38th in the amino acid sequence of SEQ ID NO: 10 cannot be substituted with arginine
- the 42nd cannot be substituted with phenylalanine
- the 45th cannot be substituted with tyrosine.
- the 61st position cannot be substituted with glutamic acid
- the 72nd position cannot be substituted with leucine.
- arginine which is the 38th amino acid in the amino acid sequence of SEQ ID NO: 10 may be substituted with an amino acid other than arginine.
- arginine which is the 38th amino acid in the amino acid sequence of SEQ ID NO: 10 may be substituted with alanine (R38A).
- phenylalanine which is the 42nd amino acid in the amino acid sequence of SEQ ID NO: 10
- amino acids other than phenylalanine Preferably, in the amino acid substitution of the IL-2 variant, phenylalanine, which is the 42nd amino acid in the amino acid sequence of SEQ ID NO: 10, may be substituted with alanine (F42A).
- tyrosine which is the 45th amino acid in the amino acid sequence of SEQ ID NO: 10 may be substituted with an amino acid other than tyrosine.
- tyrosine which is the 45th amino acid in the amino acid sequence of SEQ ID NO: 10 may be substituted with alanine (Y45A).
- glutamic acid which is the 61st amino acid in the amino acid sequence of SEQ ID NO: 10
- glutamic acid which is the 61st amino acid in the amino acid sequence of SEQ ID NO: 10
- glutamic acid which is the 61st amino acid in the amino acid sequence of SEQ ID NO: 10
- glutamic acid which is the 61st amino acid in the amino acid sequence of SEQ ID NO: 10
- glutamic acid which is the 61st amino acid in the amino acid sequence of SEQ ID NO: 10
- E61A arginine
- leucine which is the 72nd amino acid in the amino acid sequence of SEQ ID NO: 10
- an amino acid other than leucine Preferably, in the amino acid substitution of the IL-2 variant, leucine, which is the 72nd amino acid in the amino acid sequence of SEQ ID NO: 10, may be substituted with glycine (L72G).
- the IL-2 variant may have at least one substitution selected from the group consisting of R38A, F42A, Y45A, E61R, and L72G in the amino acid sequence of SEQ ID NO: 10.
- the IL-2 variant may have amino acid substitutions at two, three, four, or five positions at positions selected from the group consisting of R38A, F42A, Y45A, E61R and L72G.
- the IL-2 variant may have a form in which two amino acids are substituted. Specifically, the IL-2 variant may have been substituted with R38A and F42A. In addition, in one embodiment, the IL-2 variant may be substituted with R38A and Y45A. In addition, in one embodiment, the IL-2 variant may be substituted with R38A and E61R. In addition, in one embodiment, the IL-2 variant may be substituted with R38A and L72G. In addition, in one embodiment, the IL-2 variant may be substituted with F42A and Y45A. In addition, in one embodiment, the IL-2 mutant may have been substituted with F42A and E61R. In addition, in one embodiment, the IL-2 mutant may have been substituted with F42A and L72G. In addition, in one embodiment, the IL-2 mutant may have been substituted with E61R and L72G. In addition, in one embodiment, the IL-2 mutant may have been substituted with E61R and L72G.
- the IL-2 variant may be in a form in which three amino acids are substituted. Specifically, the IL-2 variant may have been substituted with R38A, F42A and Y45A. In addition, in one embodiment, the IL-2 variant may have been substituted with R38A, F42A, and E61R. In addition, in one embodiment, the IL-2 variant may have been substituted with R38A, F42A, and L72G. In addition, in one embodiment, the IL-2 variant may have been substituted with R38A, Y45A, or E61R. In addition, in one embodiment, the IL-2 variant may have been substituted with R38A, Y45A, and L72G.
- the IL-2 mutant may have been substituted with F42A, Y45A, and E61R.
- the IL-2 variant may have been substituted with F42A, Y45A, and L72G.
- the IL-2 variant may have been substituted with F42A, E61R, and L72G.
- the IL-2 variant may have been substituted with Y45A, E61R, and L72G.
- the IL-2 variant may be in a form in which four amino acids are substituted. Specifically, the IL-2 variant may have been substituted with R38A, F42A, Y45A and E61R. In addition, in one embodiment, the IL-2 variant may be substituted with R38A, F42A, Y45A, and L72G. In addition, in one embodiment, the IL-2 variant may have been substituted with R38A, F42A, E61R, and L72G. In addition, in one embodiment, the IL-2 variant may be substituted with R38A, Y45A, E61R, and L72G. In addition, in one embodiment, the IL-2 mutant may have been substituted with F42A, Y45A, E61R, and L72G.
- the IL-2 variant may have been substituted with R38A, F42A, Y45A, E61R and L72G.
- one specific example of the IL-2 variant may be a substitution of any one combination selected from the following (a) to (d) combinations in the amino acid sequence of SEQ ID NO: 10:
- IL-2 when IL-2 has the amino acid sequence of SEQ ID NO: 35, it may have an amino acid substitution at a position complementary to SEQ ID NO: 10. In addition, even when IL-2 is a fragment of the amino acid sequence of SEQ ID NO: 35, an amino acid at a position complementary to SEQ ID NO: 10 may be substituted.
- the variant of IL-2 may have an amino acid sequence of SEQ ID NO: 6, 22, 23 or 24.
- the IL-2 variant may be characterized in that it has low toxicity in vivo.
- the low toxicity in vivo may be a side effect caused by binding of IL-2 to the alpha chain (IL-2R ⁇ ) of the IL-2 receptor.
- IL-2R ⁇ alpha chain
- Various IL-2 variants have been developed in order to improve the side effects caused by the IL-2 and IL-2R ⁇ binding, and such IL-2 variants can be used those disclosed in U.S. Patent No. 5,229,109 and Korean Patent No. 10-1667096.
- the mutant of IL-2 described in the present application has low binding power to the alpha chain (IL-2R ⁇ ) of the IL-2 receptor, and thus has a lower toxicity in vivo than that of wild-type IL-2.
- CD80 is also called “B7-1", and is a membrane protein present in dendritic cells, activated B cells, and monocytes. CD80 provides co-stimulatory signals essential for T cell activation and survival. CD80 is known as a ligand for two different proteins, CD28 and CTLA-4, present on the surface of T cells. CD80 is composed of 288 amino acids, and may specifically have the amino acid sequence of SEQ ID NO: 11.
- CD80 protein refers to a full-length CD80 or CD80 fragment.
- CD80 fragment means a truncated form of CD80.
- the CD80 fragment may be the extracellular domain of CD80.
- amino acids 1 to 34 from the N-terminus, which is a signal sequence of CD80 may be excluded.
- one specific example of the CD80 fragment may be a protein composed of amino acids 35 to 288 of SEQ ID NO: 11.
- a specific example of the CD80 fragment may be a protein composed of amino acids 35 to 242 of SEQ ID NO: 11.
- a specific example of the CD80 fragment may be a protein consisting of amino acids 35 to 232 of SEQ ID NO: 11.
- a specific example of the CD80 fragment may be a protein composed of amino acids 35 to 139 of SEQ ID NO: 11.
- a specific example of the CD80 fragment may be a protein consisting of amino acids 142 to 242 of SEQ ID NO: 11.
- the CD80 fragment may have an amino acid sequence of SEQ ID NO: 2.
- the IL-2 protein and the CD80 protein may be bound by a linker or a carrier.
- the IL-2 or a variant thereof and the CD80(B7-1) or fragment thereof may be bound by a linker or a carrier.
- the linker and the carrier may be used interchangeably.
- the linker connects the two proteins.
- One embodiment of the linker may include 1 to 50 amino acids, albumin or fragments thereof, or the Fc domain of an immunoglobulin.
- the Fc domain of the immunoglobulin includes the heavy chain constant region 2 (CH2) and the heavy chain constant region 3 (CH3) of the immunoglobulin, and includes the variable region of the heavy and light chain and the light chain constant region 1 (CH1) of the immunoglobulin. It means a protein that doesn't.
- the immunoglobulin may be IgG, IgA, IgE, IgD or IgM, preferably IgG4.
- the Fc domain of wild-type immunoglobulin G4 may have an amino acid sequence of SEQ ID NO: 4.
- the Fc domain of the immunoglobulin may be a wild-type Fc domain as well as an Fc domain variant.
- the term "Fc domain variant" used herein is different from the glycosylation pattern of the wild-type Fc domain, increased sugar chains compared to the wild-type Fc domain, decreased sugar chains compared to the wild-type Fc domain, or a sugar chain has been removed. (deglycosylated) form.
- an aglycosylated Fc domain is also included.
- the Fc domain or variant may have a sialic acid, fucosylation, or glycosylation whose content is controlled through culture conditions or genetic manipulation of the host.
- the Fc domain variant may be an immunoglobulin in a form in which an Fc region of IgG, IgA, IgE, IgD or IgM is mixed.
- the Fc domain variant may have a form in which some amino acids of the Fc domain are substituted with other amino acids.
- One specific example of the Fc domain variant may have an amino acid sequence of SEQ ID NO: 12.
- the fusion protein may have a structure in which CD80 and IL-2 proteins are linked or IL-2 and CD80 are linked to the N-terminus and C-terminus of the Fc domain as a linker (or carrier) (FIG. 1A).
- the link between the N-terminus or C-terminus of the Fc domain and CD-80 or IL-2 may be optionally made by a linker peptide.
- the fusion protein may be composed of the following structural formula (I) or (II):
- the N' is the N-terminus of the fusion protein
- X is a CD80 protein
- Y is an IL-2 protein
- linker (1) and linker (2) are peptide linkers
- n and m are independently O or 1.
- the fusion protein may be composed of structural formula (I).
- the IL-2 protein is as described above.
- the CD80 protein is as described above.
- the IL-2 protein may be an IL-2 variant in which one to five amino acids are substituted compared to wild-type IL-2.
- the CD80 protein may be a fragment in which up to about 34 amino acid residues are successively deleted (truncated) from the N-terminus or C-terminus of wild-type CD80.
- the CD80 protein may be an extracellular immunoglobulin-like domain having activity to bind to T-cell surface receptors CTLA-4 and CD28.
- the fusion protein may have an amino acid sequence of SEQ ID NO: 9, 26, 28 or 30.
- the fusion protein is 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93% with respect to the amino acid sequence of SEQ ID NO: 9, 26, 28 or 30 , 94%, 95%, 96%, 97%, 98%, 99% or 100% sequence identity.
- the identity may be determined through, for example, percent homology, homology comparison software such as BlastN software of the National Center of Biotechnology Information (NCBI).
- a peptide linker (1) may be included between the CD80 protein and the Fc domain.
- the peptide linker (1) may consist of 5 to 80 contiguous amino acids, 20 to 60 contiguous amino acids, or 25 to 50 contiguous amino acids, or 30 to 40 amino acids. In one embodiment, the peptide linker (1) may consist of 30 amino acids.
- the peptide linker 1 may include at least one cysteine. Specifically, it may contain one, two or three cysteines.
- the peptide linker (1) may be derived from a hinge of an immunoglobulin. In one embodiment, the peptide linker (1) may be a peptide linker consisting of the amino acid sequence of SEQ ID NO: 3.
- the peptide linker (2) may consist of 1 to 50 contiguous amino acids, or 3 to 30 contiguous amino acids, or 5 to 15 amino acids.
- the peptide linker (2) may be (G4S)n (here, n is an integer of 1 to 10). In this case, n in (G4S)n may be 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10.
- the peptide linker (2) may be a peptide linker consisting of the amino acid sequence of SEQ ID NO: 5.
- Another aspect of the present invention provides a dimer in which two fusion proteins including the IL-2 protein and the CD80 protein are bound.
- the fusion protein comprising IL-2 or a variant thereof and CD80 or a fragment thereof is as described above.
- the bond between the fusion proteins constituting the dimer may be made by a disulfide bond by a cysteine present in the linker, but is not limited thereto.
- the fusion proteins constituting the dimer may be the same, but may be different fusion proteins.
- the dimer may be a homodimer.
- An embodiment of the fusion protein constituting the dimer may be a protein having the amino acid sequence of SEQ ID NO: 9.
- a regulatory T cell comprising culturing CD4 + CD25 + CD127- T cells in a medium containing IL-2 protein or a variant thereof and a fusion protein dimer comprising a CD80 protein or fragment thereof. It provides a way to cultivate.
- the CD4 + CD25 + CD127- T cells may be obtained from blood cells.
- CD4 + CD25 + CD127- T cells may be isolated from PBMC (peripheral blood mononuclear cells).
- the CD4+CD25+CD127- T cells may be obtained by specifically proliferating CD4+CD25+CD127- T cells in blood cells.
- the CD4+CD25+CD127- T cells can be obtained from CD4+ cells after CD4-T cells are removed from PBMCs.
- CD4+ T cells may be isolated using anti-CD4 antibodies, and in one embodiment, they were isolated using beads to which anti-CD4 antibodies were bound.
- CD25+ T cells can be isolated using an anti-CD25+ antibody.
- regulatory T cells can be isolated by isolating T cells that are CD4+, CD25+ and CD127-.
- the medium may be a conventionally used medium. Preferably, it may be a medium optimized for CD4 + CD25 + CD127- T cells. In one embodiment, as disclosed in Tables 3 and 4, it may be a medium in which FBS, HEPES, L-Glutamine, and 2-Mercaptoethanol are added to RPMI1640 medium or TexMACS medium. In addition, the medium may further contain retinoic acid. In addition, the medium may further contain penicillin and/or streptomycin.
- control CD4+ T cells may be cultured in the medium for 1 to 30 days and 2 to 20 days. In addition, it can be cultured for 3 to 10 days, and can be cultured for 4 to 6 days.
- the CD4 + CD25 + CD127- T cells culturing CD4 + T cells may be obtained through the step of culturing CD25+ T cells.
- the CD4+ T cells and CD25+ T cells may be obtained from blood cells, respectively.
- each of the CD4+ T cells and CD25+ T cells may be isolated from PBMC (peripheral blood mononuclear cells) or may be obtained by specifically proliferating CD4+ T cells or CD25+ T cells from blood cells.
- PBMC peripheral blood mononuclear cells
- CD4+ T cells or CD25+ T cells can be obtained after removing CD4- T cells or CD25- T cells from PBMCs.
- CD4+ T cells may be isolated using anti-CD4 antibodies, and in one embodiment, they were isolated using beads to which anti-CD4 antibodies were bound.
- CD25+ T cells can be isolated using an anti-CD25+ antibody.
- regulatory T cells can be isolated by isolating T cells, which are CD4+, CD25+ and CD127-, from CD4+ T cells or CD25+ T cells.
- composition for treating a regulated T cell mediated disease comprising the regulatory T cell obtained by the above-described method as an active ingredient.
- the regulatory T cells obtained by the above culture method may have an increased expression level of Foxp3+.
- Foxp3 is a protein also called scurfin.
- the protein is a protein involved in the regulatory mechanism pathway of regulatory T cells, and is known as a marker of regulatory T cells.
- the regulatory T cells obtained above may be CD4+CD25+CD127-Foxp3+ T cells.
- regulatory T cell mediated disease refers to a disease induced by abnormality or deficiency of regulatory T cells, and may be specifically characterized as an inflammatory disease or an autoimmune disease.
- the inflammatory disease is lupus, Sjogren's syndrome, rheumatoid arthritis, fibromyositis, scleroderma, ankylosing spondylitis, Behcet's disease, Aphtha stomatitis, Gillian Barre syndrome, alopecia areata, dermatitis, Crohn's disease, colitis, nodular It may be characterized in that at least one selected from the group consisting of polyarteritis, recurrent polychondritis, and autoimmune thrombocytopenia.
- the autoimmune disease is rheumatoid arthritis, systemic scleroderma, insulin-dependent childhood diabetes by pancreatic cell antibodies, alopecia areata, psoriasis, pemphigus, asthma, aphtha stomatitis, chronic thyroiditis, some acquired aplastic aplasticity.
- Anemia primary cirrhosis, ulcerative colitis, Becce's disease, Crohn's disease, silicosis, asbestosis, IgA kidney disease, glomerulonephritis after streptococcal infection, Sjogren's syndrome, Gillian-Barre syndrome, dermatitis, multiple myositis, multiple sclerosis, Autoimmune hemolytic anemia, autoimmune encephalomyelitis, myasthenia gravis, Graves' hyperthyroidism, polyarteritis nodosa, ankylosing spondylitis, fibromyalitis, temporal arteritis, Wilson's disease, Fanconi syndrome, multiple myeloma, and systemic lupus erythematosus. It can be characterized by the above.
- composition of the present invention may contain a pharmaceutically acceptable carrier and/or additive.
- a pharmaceutically acceptable carrier and/or additive for example, sterile water, physiological saline, conventional buffers (phosphoric acid, citric acid, other organic acids, etc.), stabilizers, salts, antioxidants, surfactants, suspending agents, isotonic agents, or preservatives.
- organic substances such as biopolymers, inorganic substances such as hydroxyapatite, specifically collagen matrix, polylactic acid polymer or copolymer, polyethylene glycol polymer or copolymer and chemical derivatives thereof, and mixtures thereof can be used. However, it is not limited thereto.
- dextran 40 for example, dextran 40, methylcellulose, gelatin, sodium sulfite, sodium metasulfate, and the like may be used.
- the antioxidant include erythorbic acid, dibutylhydroxytoluene, butylhydroxyanisole, ⁇ -tocopherol, tocopherol acetate, L-ascorbic acid and salts thereof, L-ascorbic acid palmitate, L-ascorb Chelating agents such as acid stearate, sodium hydrogen sulfite, sodium sulfite, triamyl gallic acid, propyl gallic acid or sodium ethylenediamine tetraacetate (EDTA), sodium pyrophosphate, sodium metaphosphate, and the like can be used.
- EDTA ethylenediamine tetraacetate
- the suspending agent may be, for example, methylcellulose, polysorbate 80, hydroxyethylcellulose, gum arabic, tragantmal, sodium carboxymethyl cellulose, polyoxyethylene sorbitan monolaurate, and the like.
- the tonicity agent for example, D-mannitol, sorbitol, and the like may be used.
- the preservative for example, methyl paraoxybenzoate, ethyl paraoxybenzoate, sorbic acid, phenol, cresol, chlorocresol, and the like can be used.
- a method for treating a regulatory T cell mediated disease comprising administering the regulatory T cells to an individual having a regulatory T cell mediated disease.
- regulatory T cells and regulatory T cell mediated diseases are as described above.
- a fusion protein comprising a human CD80 fragment, an Fc domain and an IL-2 variant, a signal peptide (SEQ ID NO: 1), a CD80 fragment (SEQ ID NO: 2), an Ig hinge (SEQ ID NO: 3) to which a linker is bound, Fc A fusion protein comprising a domain (SEQ ID NO: 4), a linker (SEQ ID NO: 5) and two amino acid-substituted IL-2 variants (2M) (R38A, F42A) (SEQ ID NO: 6) in this order from the N-terminus
- a polynucleotide containing the encoding nucleotide sequence (SEQ ID NO: 8) was synthesized through the Invitrogen GeneArt Gene Synthesis service of ThermoFisher Scientific, and loaded into the pcDNA3_4 vector.
- the vector was introduced into CHO cells (Expi-CHO TM ) to express the fusion protein of SEQ ID NO: 9. After the vector was introduced, the culture medium was collected and cultured for 7 days in an environment at 37° C., 125 RPM, and 8% CO 2 to purify the fusion protein.
- the purified fusion protein dimer was named "GI-101".
- the fusion protein was bound under the conditions of 25 mM Tris, 25 mM NaCl, and pH 7.4. Then, it was eluted with 100 mM NaCl and 100 mM acetic acid at pH 3. After putting 20% of 1 M Tris-HCl of pH 9 into the collection tube, the fusion protein was collected. The collected fusion protein was changed by dialysis with PBS buffer for 16 hours.
- a high concentration fusion protein was obtained by measuring absorbance at a wavelength of 280 nm over time using size exclusion chromatography using a TSKgel G3000SWXL column (TOSOH Bioscience).
- the separated and purified fusion protein was subjected to SDS-PAGE under reducing (R) or non-reducing (NR) conditions, and stained with coomassie blue to confirm its purity (Fig. 1b).
- Fig. 1c When detected using NanoDrop, it was confirmed that the fusion protein was contained at a concentration of 2.78 mg/ml.
- the results analyzed using size exclusion chromatography are as shown in FIG. 1D.
- a signal peptide SEQ ID NO: 1
- Ig hinge SEQ ID NO: 38
- Fc domain SEQ ID NO: 4
- linker SEQ ID NO: 5
- ThermoFisher a polynucleotide containing the nucleotide sequence (SEQ ID NO: 45) encoding the fusion protein containing the amino acid-substituted IL-2 variant (2M) (R38A, F42A) (SEQ ID NO: 6) from the N-terminus in this order
- the polynucleotide was synthesized through Scientific's Invitrogen GeneArt Gene Synthesis service and loaded into the pcDNA3_4 vector.
- the vector was introduced into CHO cells (Expi-CHOTM) to express the fusion protein of SEQ ID NO: 44. After the vector was introduced, the culture medium was collected and cultured for 7 days in an environment at 37° C., 125 RPM, and 8% CO 2 to purify the fusion protein dimer.
- the purified fusion protein dimer was named "Fc-IL2v2".
- the purification and collection of the fusion protein were performed in the same manner as in Preparation Example 1.
- the separated and purified fusion protein was subjected to SDS-PAGE under reducing (R) or non-reducing (NR) conditions, and stained with Comasi Blue to confirm its purity (FIG. 3A ). As a result, it was confirmed that the fusion protein forms a dimer.
- the results analyzed using size exclusion chromatography are as shown in FIG. 3B.
- a fusion protein comprising an Fc domain and wild-type IL-2, signal peptide (SEQ ID NO: 1), Ig hinge (SEQ ID NO: 38), Fc domain (SEQ ID NO: 4), linker (SEQ ID NO: 5) and wild-type IL PcDNA3_4 by synthesizing a polynucleotide containing a nucleotide sequence (SEQ ID NO: 43) encoding a fusion protein containing -2 (SEQ ID NO: 43) in this order from the N-terminus through the Invitrogen GeneArt Gene Synthesis service of ThermoFisher Scientific Loaded into the vector.
- the vector was introduced into CHO cells (Expi-CHOTM) to express the fusion protein of SEQ ID NO: 42. After the vector was introduced, the culture medium was collected and cultured for 7 days in an environment at 37° C., 125 RPM, and 8% CO 2 to purify the fusion protein dimer.
- the purified fusion protein dimer was named "Fc-IL2wt”.
- the purification and collection of the fusion protein were performed in the same manner as in Preparation Example 1.
- the separated and purified fusion protein was subjected to SDS-PAGE under reducing (R) or non-reducing (NR) conditions, and stained with Comasi Blue to confirm its purity (FIG. 3C). As a result, it was confirmed that the fusion protein forms a dimer.
- the results analyzed using size exclusion chromatography are as shown in FIG. 3D.
- a signal peptide SEQ ID NO: 1
- a CD80 fragment SEQ ID NO: 2
- an Ig hinge to which a linker is bound SEQ ID NO: 3
- Poly comprising an Fc domain (SEQ ID NO: 4), a linker (SEQ ID NO: 5) and IL-2 wild type (SEQ ID NO: 10) from the N-terminus to the nucleotide sequence (SEQ ID NO: 41) encoding a fusion protein containing in this order
- Nucleotides were synthesized by ThermoFisher Scientific's Invitrogen GeneArt Gene Synthesis service and loaded into pcDNA3_4 vector.
- the vector was introduced into CHO cells (Expi-CHO TM ) to express the fusion protein of SEQ ID NO: 46. After the vector was introduced, the culture medium was collected and cultured for 7 days in an environment at 37° C., 125 RPM, and 8% CO 2 to purify the fusion protein dimer.
- the purified fusion protein dimer was named "hCD80-Fc-IL2wt".
- the fusion protein was bound under the conditions of 25 mM Tris, 25 mM NaCl, and pH 7.4. Then, it was eluted with 100 mM NaCl and 100 mM acetic acid at pH 3. After putting 20% of 1M Tris-HCl of pH 9 into the collection tube, the fusion protein was collected. The collected fusion protein was changed by dialysis with PBS buffer for 16 hours.
- a high concentration fusion protein was obtained by measuring absorbance at a wavelength of 280 nm over time using size exclusion chromatography using a TSKgel G3000SWXL column (TOSOH Bioscience).
- the separated and purified fusion protein was subjected to SDS-PAGE under reducing (R) or non-reducing (NR) conditions, and stained with coomassie blue to confirm its purity (FIG. 4A).
- R reducing
- NR non-reducing
- FIG. 4B results analyzed using size exclusion chromatography are as shown in FIG. 4B.
- a signal peptide SEQ ID NO: 1
- a CD80 fragment SEQ ID NO: 2
- a linker-bound Ig hinge SEQ ID NO: 3
- an Fc domain SEQ ID NO: 4
- the vector was introduced into CHO cells (Expi-CHO TM ) to express the fusion protein of SEQ ID NO: 40. After the vector was introduced, the culture medium was collected and cultured for 7 days in an environment at 37° C., 125 RPM, and 8% CO 2 to purify the fusion protein dimer.
- the purified fusion protein dimer was named "hCD80-Fc".
- the fusion protein was bound under the conditions of 25 mM Tris, 25 mM NaCl, and pH 7.4. Then, it was eluted with 100 mM NaCl and 100 mM acetic acid at pH 3. After putting 20% of 1 M Tris-HCl of pH 9 into the collection tube, the fusion protein was collected. The collected fusion protein was changed by dialysis with PBS buffer for 16 hours.
- a high concentration fusion protein was obtained by measuring absorbance at a wavelength of 280 nm over time using size exclusion chromatography using a TSKgel G3000SWXL column (TOSOH Bioscience).
- the separated and purified fusion protein was subjected to SDS-PAGE under reducing (R) or non-reducing (NR) conditions, and stained with coomassie blue to confirm its purity (FIG. 2A).
- R reducing
- NR non-reducing
- FIG. 2B the analysis results using size exclusion chromatography are as shown in FIG. 2B.
- Preparation Example 1 Composition for cultivation for culturing control T cells
- a medium for culturing CD4+ cells was prepared in the following composition. At this time, after preparing the medium of the basic component, the additive components GI-101, GI-101WT, hCD80-Fc, Fc-IL-2v2 or Fc-IL-2wt were added before use.
- CD4 + CD25 + CD127 - cell culture medium was prepared.
- Example 1 Confirmation of the degree of regulatory T cell proliferation of fusion protein dimers including IL-2 protein and CD80 protein
- Example 1.1 Preparation of beads for stimulation of regulatory T cell proliferation
- beads for stimulating regulatory T cell proliferation using MACS GMP ExpAct Treg Kit (Cat#:170-076-119) (Miltenyi Biotec, Bergisch Gladbach, Germany) bead) was prepared. Specifically, transfer the reagent in the MACS GMP ExpAct Treg Kit containing beads for proliferation stimulation of regulatory T cells to a new tube, wait for 1 minute in a magnet, and remove the supernatant to bead (bead) was separated. At this time, the reagent in the MACS GMP ExpAct Treg Kit was 1 ⁇ L per 2 ⁇ 10 5 cells. After separation of the beads, 0.5 mL to 1 mL of the CD4+ cell culture composition of Table 1 or Table 2 (including only the basic components) not containing any additional components was added to release the beads.
- the number of cells of the purchased PBMC (Cat#: SER-PBMC-200-F) (Zen-Bio. Inc, NC 27709, USA) was measured. Then, it centrifuged for 10 minutes at 300xg. Then, after removing the supernatant buffer, 80 ⁇ L of MACs buffer per 1 ⁇ 10 7 cell number was added to release the cell pellet. Thereafter, 20 ⁇ L of CD4 MicroBeads (Cat#: 130-045-101) (Miltenyi Biotec, Bergisch Gladbach, Germany) per 1 ⁇ 10 7 cell number was dispensed, tapped, and sufficiently mixed. Then, it was reacted at 4°C to 8°C for 15 minutes.
- MACs buffer For washing, 10 mL of MACs buffer was added and centrifuged at 300 ⁇ g for 10 minutes. Then, after removing the supernatant, 500 ⁇ L of MACs buffer per 1 ⁇ 10 8 cell number was added to release the cell pellet. Thereafter, after preparing the LS column, 3 mL of MACs buffer was flowed. The cell suspension prepared above was passed through an LS column (Cat#: 130-042-401) (Miltenyi Biotec, Bergisch Gladbach, Germany). 3 mL of MACs buffer was flowed 3 times so that the cells attached to the LS column could be sufficiently washed.
- the LS column was separated from a magnet stand, and then 3 mL of MACs buffer was added and pressure was applied with a piston to recover CD4+ cells. Then, it centrifuged for 5 minutes at 300xg. Then, the number of cells was measured after removing the supernatant.
- control T cell culture solution containing beads prepared in Example 1.1 was inoculated onto the CD4+ T cells isolated above.
- the CD4+ cells prepared in Example 1.2 were seeded at 1 ⁇ 10 7 cells/mL, and GI-101 (50 nM), GI-101_WT (50 nM), and CD80-Fc dimers as additives.
- Cells of Table 1 or Table 2 each containing a body (50 nM) + Fc-IL-2v2 dimer (50 nM) or CD80-Fc dimer (50 nM) + Fc-IL-2wt dimer (50 nM) CD4+ cells were cultured under the conditions of the culture composition.
- the cells in the 6-well plate showed more than 80% confluency, they were subcultured into 25T flasks.
- the cells in the 25T flask showed more than 80% confluency
- cells were obtained when the cells in the 75T flask showed more than 80% confluency.
- the CD4+ cells recovered in Example 1.3 were centrifuged for 5 minutes at 1,300 rpm, 4°C.
- Example 1.4 The CD4 + CD25 + CD127- cells isolated in Example 1.4 were seeded in a 48-well plate at 3 ⁇ 10 5 cells/mL, and at the same time, 2 ⁇ 10 to stimulate the proliferation of regulatory T cells as in Example 1.1. Beads were isolated from 1 ⁇ L MACS GMP ExpAct Treg Kit (Cat#:170-076-119) (Miltenyi Biotec, Bergisch Gladbach, Germany) per 5 cell count. After dissolving the isolated beads in the cell culture composition (0.5 mL to 1 mL) of Table 3 or Table 4 (including only the basic components) containing no additional components, CD4 + CD25 + CD127- obtained in Example 1.4 The cells were added to the seeded well plate.
- GI-101 50 nM
- GI-101_WT 50 nM
- CD80-Fc 50 nM + Fc- CD4 + CD25 + CD127- cells were cultured under the conditions of the cell culture composition of Table 3 or Table 4 each containing IL-2wt (50 nM).
- control T cell proliferation in the control T cell culture medium composition including the RPMI1640 medium are shown in Tables 5 and 6, and the cell viability is shown in Tables 6 and 7.
- control T cell proliferation results in the culture composition containing the TexMACS medium are shown in Tables 7 and 8, and the cell viability in Tables 8 and 9.
- Example 1.6 Confirmation of the ability to secrete immunosuppressive cytokines: interleukin-10
- Example 1.5 In order to evaluate the IL-10 secretion ability of the regulatory T cells obtained in Example 1.5, the cell number was adjusted to 1 ⁇ 10 6 cells/mL, and then the culture supernatant was obtained, followed by ELISA (enzyme-linked immunosorbent assay) analysis. Performed.
- the interleukin-10 secretion ability of cells cultured from the composition containing RPMI1640 medium is as shown in FIG. 10
- the interleukin-10 secretion ability of the cells cultured from the composition containing TexMACS medium is as shown in FIG. 11. 10 and 11, it was confirmed that interleukin-10 was highly expressed from regulatory T cells in which GI-101 was added to the culture composition.
- the number of cells of the purchased PBMC (Cat#: SER-PBMC-200-F) (Zen-Bio. Inc, NC 27709, USA) was measured. Then, it centrifuged for 10 minutes at 300xg. Thereafter, after removing the supernatant buffer, 80 ⁇ L of MACs buffer per 1 ⁇ 10 7 cell number was added to release the cell pellet. Thereafter, 20 ⁇ L of CD4 MicroBeads (Cat#: 130-045-101) (Miltenyi Biotec, Bergisch Gladbach, Germany) per 1 ⁇ 10 7 cell number was dispensed, tapped, and sufficiently mixed. Then, it was reacted at 4°C to 8°C for 15 minutes.
- MACs buffer For washing, 10 mL of MACs buffer was added and centrifuged at 300 ⁇ g for 10 minutes. Then, after removing the supernatant, 500 ⁇ L of MACs buffer per 1 ⁇ 10 8 cell number was added to release the cell pellet. Thereafter, after preparing the LS Column, 3 mL of MACs buffer was flowed. The cell suspension prepared above was passed through an LS column (Cat#: 130-042-401) (Miltenyi Biotec, Bergisch Gladbach, Germany). 3 mL of MACs buffer was flowed 3 times so that the cells attached to the LS column could be sufficiently washed.
- the LS column was separated from a magnet stand, and then 3 mL of MACs buffer was added and pressure was applied with a piston to recover CD4+ cells. Then, it centrifuged for 5 minutes at 300xg. After centrifugation, the supernatant was removed and the number of cells was measured.
- Example 1.1 In order to stimulate the proliferation of regulatory T cells in the isolated CD4+ cells, 0.5 mL to 1 mL of the CD4+ cell culture composition of Table 1 or Table 2 (including only the basic component) containing no additional components was added to the above Example 1.1. As described above, the prepared beads were released, and the CD4+ cells isolated from the PBMC were inoculated.
- the CD4+ cells prepared in Example 2.1 were seeded at 1 ⁇ 10 7 cells/mL, and GI-101 (3.2 nM/50 nM) and GI-101_WT (3.2 nM/50 nM) as additives. ), CD80-Fc dimer (3.2 nM/50 nM) + Fc-IL-2v2 dimer (3.2 nM/50 nM) or CD80-Fc dimer (3.2 nM/50 nM) + Fc-IL-2wt dimer CD4+ was cultured under the conditions of the cell culture composition of Table 1 or Table 2 each containing (3.2 nM/50 nM).
- the CD4+ cells recovered in Example 2.2 were centrifuged for 5 minutes at 1,300 rpm, 4°C. In addition, the supernatant was removed, 1 mL of an Fc block (biolegend, cat#422302) diluted 1:200 in FACS buffer was added, and left on ice for 10 minutes. After that, 50 ul CD4-Pacific Blue (BioLegend, cat#317429), 50 ul CD25-PE/Cy7 (BioLegend, cat#356108), and 50 ul CD127-PE (BD, cat#557938) were added to the second sample. It was left on ice for 20 minutes.
- Example 2.3 All CD4 + CD25 + CD127- cells isolated in Example 2.3 were seeded in a 24-well plate, and at the same time, 1 ⁇ L MACS GMP per 2 ⁇ 10 5 cells number to stimulate the proliferation of regulatory T cells as in Example 2.1.
- Cell culture in Table 3 or Table 4 (basic ingredients only) without additional ingredients by separating beads from ExpAct Treg Kit (Cat#:170-076-119) (Miltenyi Biotec, Bergisch Gladbach, Germany) Beads were released into the composition (0.5 mL to 1 mL). Then, the CD4+CD25+CD127- cells were added to the seeded well plate.
- GI-101 (3.2 nM/50 nM), GI-101_WT (3.2 nM/50 nM), CD80-Fc dimer (3.2 nM/50 nM) + Fc-IL-2v2 dimer (3.2 nM / 50 nM) or CD80-Fc dimer (3.2 nM / 50 nM) + Fc-IL-2wt dimer (3.2 nM / 50 nM) containing CD4 + under the conditions of the cell culture composition of Table 3 or Table 4, respectively CD25+CD127- cells were cultured. When the cells in the 24-well plate showed more than 80% confluency, they were subcultured into a 12-well plate.
- the cells in the 12-well plate showed more than 80% confluency, they were subcultured into a 6-well plate.
- the cells in the 6-well plate showed more than 80% confluence, they were subcultured into 25T flasks.
- the cells in the 25T flask showed more than 80% confluency, they were subcultured into the 75T flask.
- the cell proliferation results in the culture composition containing RPMI1640 medium are shown in Table 9, Figs. 12a and 12b, and the cell viability is shown in Table 10, Figs. 13a and 13b.
- the cell proliferation results in the culture composition containing the TexMACS medium are shown in Table 11, FIGS. 14A and 14B, and the cell viability in Table 12, FIGS. 15A and 15B.
- the CD4+CD25+CD127- cells recovered in Example 2.4 were centrifuged for 5 minutes at 1,300 rpm, 4°C. In addition, the supernatant was removed, and 100 ul of the Fc block (biolegend, cat#422302) diluted 1:200 in FACS buffer was added and left on ice for 10 minutes, and an additional 2.5 ul CD4-PerCP-Cy5.5 (eBioscience, cat #45-0048-42), 2.5 ul CD25-APC/Cy7 (BD, cat#557753), 2.5 ul CD127-Brilliant Violet 785 (Biolegend, cat#351330) and left on ice for 20 minutes.
- FACS analysis results for the cell characteristics cultured from the RPMI1640 medium-containing composition are as shown in Figs. 16A to 16C, and the number of T cells expressing CD4 + CD25 + Foxp3 + confirmed in the culture conditions is shown in Table 13, Figs. Same as 17c.
- the FACS analysis results for the cell characteristics cultured from the composition containing TexMACS medium are as shown in Figs. 18A to 18C, and the number of T cells expressing CD4 + CD25 + Foxp3 + confirmed in the culture conditions is Table 14 and Figs. 19A to 19C Is the same as
- Example 2.4 In order to evaluate the IL-10 secretion ability of the regulatory T cells obtained in Example 2.4, the cells were cultured at 1 ⁇ 10 6 cells/mL, and then the culture supernatant was obtained and analyzed by ELISA (enzyme-linked immunosorbent assay). Was performed.
- the interleukin-10 secretion ability of cells cultured from the composition containing RPMI1640 medium is shown in FIG. 20
- the interleukin-10 secretion ability of cells cultured from the composition containing TexMACS medium is shown in FIG. 20 and 21, it was confirmed that interleukin-10 was highly expressed from regulatory T cells in which GI-101 was added to the culture composition.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Cell Biology (AREA)
- Hematology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Rheumatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Developmental Biology & Embryology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pain & Pain Management (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
| 성분(Component) | 용량 | 최종 농도 | |||
| 성분명 | 제조사 | Cat.# | |||
| 기본 성분 | RPMI1640 medium | Welgene | LM 011-01 | to 500 mL | |
| FBS | Hyclone | SH30084.03 | 50 mL | 10% | |
| HEPES | Welgene | BB 001-01 | 5 mL | 10 mM | |
| Penicillin & Streptomycin | Welgene | LS 202-02 | 5 mL | Penicillin: 100 U/ml Streptomycin: 100 μg/ml |
|
| Sodium pyruvate | Welgene | LS 013-01 | 5 mL | 1 mM | |
| MEM Non-Essential Amino Acids Solution | GIBCO | 11140050 | 5 mL | 1 mM | |
| L-Glutamine | GIBCO | 25030149 | 5 mL | 2 mM | |
| 2-Mercaptoethanol | GIBCO | 21985-023 | 0.5 mL | 55 μM | |
| 첨가 성분 | GI-101 | GI-Innovation | - | 사용 전 즉시 첨가 |
3.2 nM, 50 nM |
| GI-101_WT | GI-Cell | - | 3.2 nM, 50 nM | ||
| hCD80-Fc | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2v2 | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2wt | GI-Cell | - | 3.2 nM, 50 nM | ||
| 성분(Component) | 용량 | 최종 농도 | |||
| 성분명 | 제조사 | Cat.# | |||
| 기본 성분 | TexMACS medium | Miltenyi Biotec | 170-076-307 | to 1000 mL | |
| Human AB serum | Sigma | H4522 | 50 mL | 5% | |
| Penicillin& Streptomycin | Welgene | LS 202-02 | 10 mL | 100 U/ml (Penicillin) 및 100 μg/ml (Streptomycin) |
|
| 첨가 성분 | GI-101 | GI-Innovation | - | 사용 전 즉시 첨가 |
3.2 nM, 50 nM |
| GI-101_WT | GI-Cell | - | 3.2 nM, 50 nM | ||
| hCD80-Fc | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2v2 | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2wt | GI-Cell | - | 3.2 nM, 50 nM | ||
| 성분(Component) | 용량 | 최종 농도 | |||
| 성분명 | 제조사 | Cat.# | |||
| 기본 성분 | RPMI1640 medium | Welgene | LM 011-01 | to 500 mL | |
| FBS | Hyclone | SH30084.03 | 50 ml | 10% | |
| HEPES | Welgene | BB 001-01 | 5 mL | 10 mM | |
| Penicillin & Streptomycin | Welgene | LS 202-02 | 5 mL | Penicillin: 100 U/ml Streptomycin: 100 μg/ml |
|
| Sodium pyruvate | Welgene | LS 013-01 | 5 mL | 1 mM | |
| MEM Non-Essential Amino Acids Solution | GIBCO | 11140050 | 5 mL | 1 mM | |
| L-Glutamine | GIBCO | 25030149 | 5 mL | 2 mM | |
| 2-Mercaptoethanol | GIBCO | 21985-023 | 0.5 mL | 55 μM | |
| 첨가 성분 | GI-101 | GI-Innovation | - | 사용 전 즉시 첨가 |
3.2 nM, 50 nM |
| GI-101_WT | GI-Cell | - | 3.2 nM, 50 nM | ||
| hCD80-Fc | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2v2 | GI-Innovation | - | 3.2 nM, 50 nM | ||
| Fc-IL-2wt | GI-Cell | - | 3.2 nM, 50 nM | ||
| 성분(Component) | 용량 | 최종 농도 | |||
| 성분명 | 제조사 | Cat.# | |||
| 기본 성분 | TexMACS medium | Miltenyi Biotec | 170-076-307 | to 1000 mL | |
| Human AB serum | Sigma | H4522 | 50 mL | 5% | |
| Penicillin& Streptomycin | Welgene | LS 202-02 | 10 mL | 100 U/ml (Penicillin) 및 100 ㎍/ml (Streptomycin) |
|
| 첨가 성분 | GI-101 | GI-Innovation | - | 사용 전 즉시 첨가 |
3.2 nM, 50 nM |
| GI-101_WT | GI-Cell | - | 3.2 nM, 50 nM | ||
| hCD80-Fc | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2v2 | GI-Cell | - | 3.2 nM, 50 nM | ||
| Fc-IL-2wt | GI-Cell | - | 3.2 nM, 50 nM | ||
| 총 세포수(×106) | |||||||
| 배양 조성물 첨가물질 | CD4+CD25+CD127- 세포수 | ||||||
| 0일 (파종) |
3일 | 5일 | 7일 | 9일 | 12일 | ||
| 50nM | GI-101 | 0.3 | 0.26 | 1.3 | 1.6 | 3 | 11.7 |
| GI-101 WT | 0.3 | 0.23 | 1.3 | 1.5 | 2.1 | 8.4 | |
| hCD80-Fc+Fc-IL-2_v2 | 0.3 | 0.25 | 1 | 1.4 | 3.2 | 10.3 | |
| hCD80-Fc+Fc-IL-2_wt | 0.3 | 0.3 | 1.2 | 1.5 | 1.9 | 9.36 | |
| 배양 조성물 첨가물질 | CD4+CD25+CD127- FACS 세포 생존률 | |||||
| 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 50nM | GI-101 | 71 | 91 | 93 | 94 | 91 |
| GI-101 WT | 70 | 94 | 94 | 96 | 92 | |
| hCD80-Fc+Fc-IL-2_v2 | 58 | 90 | 90 | 95 | 92 | |
| hCD80-Fc+Fc-IL-2_wt | 64 | 94 | 93 | 96 | 90 | |
| 총 세포수(×106) | |||||||
| 배양 조성물 첨가물질 | CD4+CD25+CD127- 세포수 | ||||||
| 0일 (파종) |
3일 | 5일 | 7일 | 9일 | 12일 | ||
| 50nM | GI-101 | 0.3 | 0.8 | 2.9 | 3 | 8.4 | 10.6 |
| GI-101 WT | 0.3 | 0.5 | 1.1 | 0.8 | 1.8 | 2 | |
| hCD80-Fc+Fc-IL-2_v2 | 0.3 | 0.7 | 2.7 | 2.4 | 8 | 9.6 | |
| hCD80-Fc+Fc-IL-2_wt | 0.3 | 0.7 | 2.9 | 2.4 | 5.4 | 7.3 | |
| 배양 조성물 첨가물질 | CD4+CD25+CD127- FACS 세포 생존률 | |||||
| 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 50nM | GI-101 | 92 | 88 | 95 | 93 | 88 |
| GI-101 WT | 91 | 73 | 88 | 89 | 90 | |
| hCD80-Fc+Fc-IL-2_v2 | 88 | 88 | 94 | 94 | 89 | |
| hCD80-Fc+Fc-IL-2_wt | 89 | 90 | 94 | 93 | 88 | |
| 총 세포수(×106) | ||||||||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포수 | ||||||||
| 0일 | 3일 | 6일 | 0일 | 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 3.2nM | GI-101 | 10 | 12.1 | 18.8 | 0.5 | 4.9 | 32 | 58 | 98 | 420 |
| GI-101 WT | 10 | 13.1 | 12 | 0.6 | 2.77 | 30 | 45 | 70 | 300 | |
| hCD80-Fc+ Fc-IL-2_v2 |
10 | 12.1 | 14.1 | 0.4 | 1.69 | 16 | 74 | 80 | 255 | |
| hCD80-Fc+ Fc-IL-2_wt |
10 | 11.1 | 13.5 | 0.3 | 1.07 | 6.4 | 63 | 88 | 196 | |
| 50nM | GI-101 | 10 | 21 | 51.9 | 1.8 | 2.7 | 29 | 47 | 71 | 380 |
| GI-101 WT | 10 | 23 | 45.8 | 0.8 | 2.6 | 22 | 64 | 65 | 206 | |
| hCD80-Fc+ Fc-IL-2_v2 |
10 | 16.5 | 37.3 | 0.89 | 0.9 | 7.7 | 24 | 41 | 108 | |
| hCD80-Fc+ Fc-IL-2_wt |
10 | 18.9 | 49.3 | 1.1 | 2.4 | 10 | 38 | 52 | 200 | |
| 세포 생존률(%) | ||||||||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포 생존률 | ||||||||
| 0일 | 3일 | 6일 | 0일 | 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 3.2nM | GI-101 | 92 | 96.5 | 96 | 92 | 93 | 90 | 93 | 92 | 93 |
| GI-101 WT | 92 | 96 | 95 | 89 | 92 | 89 | 93 | 92 | 92 | |
| hCD80-Fc+ Fc-IL-2_v2 |
92 | 95 | 95 | 90 | 92 | 91 | 93 | 90 | 93 | |
| hCD80-Fc+ Fc-IL-2_wt |
92 | 95 | 94 | 90 | 91 | 90 | 92 | 91 | 92 | |
| 50nM | GI-101 | 92 | 96.5 | 96 | 92 | 94 | 93 | 92 | 94 | 94 |
| GI-101 WT | 92 | 96 | 95 | 90 | 92 | 94 | 92 | 93 | 93 | |
| hCD80-Fc+ Fc-IL-2_v2 |
92 | 95 | 95 | 90 | 92 | 93 | 94 | 91 | 94 | |
| hCD80-Fc+ Fc-IL-2_wt |
92 | 95 | 94 | 91 | 92 | 92 | 93 | 92 | 94 | |
| 총 세포수(×106) | ||||||||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포수 | ||||||||
| 0일 | 3일 | 6일 | 0일 | 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 3.2nM | GI-101 | 10 | 35 | 64 | 5.8 | 5.2 | 30 | 30 | 92 | 220 |
| GI-101 WT | 10 | 27 | 57 | 5 | 2.14 | 46 | 16 | 74 | 200 | |
| hCD80-Fc+ Fc-IL-2_v2 |
10 | 24 | 50 | 5.48 | 3.45 | 16 | 16 | 50 | 170 | |
| hCD80-Fc+ Fc-IL-2_wt |
10 | 25 | 55 | 3.5 | 2.7 | 15 | 15 | 42 | 72 | |
| 50nM | GI-101 | 10 | 30 | 68 | 6.2 | 5.7 | 30.6 | 30 | 240 | 500 |
| GI-101 WT | 10 | 28 | 60 | 5.4 | 5 | 21 | 20 | 276 | 370 | |
| hCD80-Fc+ Fc-IL-2_v2 |
10 | 23.5 | 48 | 5 | 4.2 | 13.3 | 13.3 | 113 | 150 | |
| hCD80-Fc+ Fc-IL-2_wt |
10 | 24 | 54 | 3.73 | 3.8 | 19 | 19.6 | 248 | 220 | |
| 세포 생존률(%) | ||||||||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포 생존률 | ||||||||
| 0일 | 3일 | 6일 | 0일 | 3일 | 5일 | 7일 | 9일 | 12일 | ||
| 3.2nM | GI-101 | 94 | 93 | 96 | 92 | 93 | 97 | 94 | 92 | 96 |
| GI-101 WT | 94 | 93 | 95 | 90 | 92 | 93 | 93 | 92 | 94 | |
| hCD80-Fc+ Fc-IL-2_v2 |
94 | 95 | 95 | 90 | 92 | 95 | 93 | 96 | 95 | |
| hCD80-Fc+ Fc-IL-2_wt |
94 | 94 | 94 | 91 | 91 | 90 | 92 | 91 | 95 | |
| 50nM | GI-101 | 94 | 96.5 | 96 | 92 | 94 | 96 | 96 | 94 | 98 |
| GI-101 WT | 94 | 96 | 95 | 90 | 92 | 94 | 92 | 96 | 93 | |
| hCD80-Fc+ Fc-IL-2_v2 |
94 | 95 | 95 | 91 | 88 | 93 | 94 | 91 | 96 | |
| hCD80-Fc+ Fc-IL-2_wt |
94 | 95 | 94 | 93 | 92 | 92 | 93 | 92 | 94 | |
| 총 세포수(×106) | |||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포 수 | |||
| 0일 | 6일 | 6일 | 12일 | ||
| 3.2nM | GI-101 | 0.25 | 2.1 | 33.3 | 61.3 |
| GI-101 WT | 0.25 | 0.97 | 18.9 | 30 | |
| hCD80-Fc+ Fc-IL-2_v2 |
0.25 | 0.99 | 2.96 | 67 | |
| hCD80-Fc+ Fc-IL-2_wt |
0.25 | 1.08 | 7.56 | 18 | |
| 50nM | GI-101 | 0.25 | 7.27 | 27.7 | 98.8 |
| GI-101 WT | 0.25 | 2.75 | 28.1 | 30.3 | |
| hCD80-Fc+ Fc-IL-2_v2 |
0.25 | 2.35 | 1.2 | 11.2 | |
| hCD80-Fc+ Fc-IL-2_wt |
0.25 | 1.97 | 3.3 | 20 | |
| 총 세포수(×106) | |||||
| 배양 조성물 첨가물질 | CD4+ 세포 | CD4+CD25+CD127- FACS 분리 후 세포 수 | |||
| 0일 | 6일 | 6일 | 12일 | ||
| 3.2nM | GI-101 | 0.3 | 38.4 | 18 | 118.8 |
| GI-101 WT | 0.3 | 35.3 | 26.7 | 83.4 | |
| hCD80-Fc+Fc-IL-2_v2 | 0.3 | 8.2 | 5.4 | 56.1 | |
| hCD80-Fc+Fc-IL-2_wt | 0.3 | 8.7 | 5.25 | 30.2 | |
| 50nM | GI-101 | 0.3 | 43.1 | 20.8 | 353.5 |
| GI-101 WT | 0.3 | 38.2 | 13.6 | 189.8 | |
| hCD80-Fc+Fc-IL-2_v2 | 0.3 | 7.7 | 5.3 | 18 | |
| hCD80-Fc+Fc-IL-2_wt | 0.3 | 10.5 | 7.2 | 28.6 | |
Claims (17)
- IL-2 단백질 또는 이의 변이체 및 CD80 단백질 또는 이의 단편을 포함하는 융합단백질 이량체를 유효성분으로 포함하는 조절 T 세포 증식용 조성물.
- 제1항에 있어서,상기 IL-2 단백질 또는 이의 변이체 및 CD80 단백질 또는 이의 단편은 링커에 의해 결합된 것인, 조절 T 세포 증식용 조성물.
- 제1항에 있어서,상기 IL-2 단백질은 서열번호 10의 아미노산 서열을 갖는 것인, 조절 T 세포 증식용 조성물.
- 제1항에 있어서,상기 CD80은 서열번호 11의 아미노산 서열을 갖는 것인, 조절 T 세포 증식용 조성물.
- 제1항에 있어서,상기 융합단백질은 서열번호 9의 아미노산 서열을 갖는 것인, 조절 T 세포 증식용 조성물.
- 제1항에 있어서,상기 조절 T 세포는 CD4+CD25+CD127- 세포인 것인, 조절 T 세포 증식용 조성물.
- IL-2 단백질 또는 이의 변이체 및 CD80 단백질 또는 이의 단편을 포함하는 융합단백질 이량체를 포함하는 조절 T 세포 증식용 배지.
- 제7항에 있어서,CD4+ T 세포 배양용 배지를 더 포함하는 것인, 조절 T 세포 증식용 배지.
- 제8항에 있어서,상기 CD4+ T 세포 배양용 배지는 아미노산(amino acids), 당류(sugars), 무기염(inorganic salts) 및 비타민을 포함하는, 조절 T 세포 증식용 배지.
- IL-2 단백질 또는 이의 변이체 및 CD80 단백질 또는 이의 단편을 포함하는 융합단백질 이량체가 포함된 배지에서 CD4+CD25+CD127- T 세포를 배양하는 단계를 포함하는 조절 T 세포 배양 방법.
- 제10항에 있어서,상기 CD4+CD25+CD127- T 세포는 혈액세포에서 수득된 것인, 조절 T 세포 수득 방법.
- 제10항에 있어서,1일 내지 20일 배양하는 것인, 조절 T 세포 수득 방법.
- 제10항에 있어서,상기 CD4+CD25+CD127- T 세포는 CD4+ T 세포를 배양하는 단계; 또는 CD25+ T 세포를 배양하는 단계를 통해 수득된 것인, 조절 T 세포 수득 방법.
- 제10항 내지 제13항 중 어느 하나의 방법에 의해서 수득된 조절 T 세포.
- 제14항에 있어서,상기 조절 T 세포는 Foxp3+ 발현량이 증가된 것인, 조절 T 세포.
- 제14항의 조절 T 세포를 유효성분으로 포함하는 조절 T 세포 매개성 질환 치료용 조성물.
- 제14항의 조절 T 세포를 조절 T 세포 매개성 질환을 가지고 있는 개체에 투여하는 단계를 포함하는 조절 T 세포 매개성 질환 치료 방법.
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2020387479A AU2020387479B2 (en) | 2019-11-20 | 2020-11-19 | Composition for culturing regulatory T cells and use thereof |
| JP2022529596A JP7425195B2 (ja) | 2019-11-20 | 2020-11-19 | 調節t細胞培養用組成物及びその用途 |
| EP20890419.3A EP4063490A4 (en) | 2019-11-20 | 2020-11-19 | COMPOSITION FOR THE CULTURE OF REGULATORY T LYMPHOCYTES AND THEIR USE |
| CA3152351A CA3152351A1 (en) | 2019-11-20 | 2020-11-19 | Composition for culturing regulatory t cells and use thereof |
| BR112022008129A BR112022008129A2 (pt) | 2019-11-20 | 2020-11-19 | Composição da cultura de células t reguladoras e uso da mesma |
| IL291909A IL291909A (en) | 2019-11-20 | 2020-11-19 | The composition of T regulatory cell culture and its use |
| MX2022006000A MX2022006000A (es) | 2019-11-20 | 2020-11-19 | Composicion de cultivo de linfocitos t reguladores y su uso. |
| CN202080078640.8A CN114901807B (zh) | 2019-11-20 | 2020-11-19 | 调节性t细胞培养组合物及其用途 |
| CN202310807404.0A CN117004564A (zh) | 2019-11-20 | 2020-11-19 | 调节性t细胞培养组合物及其用途 |
| US17/743,181 US11702633B2 (en) | 2019-11-20 | 2022-05-12 | Composition for culturing regulatory T cells and use thereof |
| US18/317,199 US20230407253A1 (en) | 2019-11-20 | 2023-05-15 | Composition for culturing regulatory t cells and use thereof |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR10-2019-0149779 | 2019-11-20 | ||
| KR20190149779 | 2019-11-20 | ||
| KR20200033229 | 2020-03-18 | ||
| KR10-2020-0033229 | 2020-03-18 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/743,181 Continuation US11702633B2 (en) | 2019-11-20 | 2022-05-12 | Composition for culturing regulatory T cells and use thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/743,181 Continuation US11702633B2 (en) | 2019-11-20 | 2022-05-12 | Composition for culturing regulatory T cells and use thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2021101273A2 true WO2021101273A2 (ko) | 2021-05-27 |
| WO2021101273A3 WO2021101273A3 (ko) | 2021-07-15 |
Family
ID=75980980
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2020/016382 WO2021101273A2 (ko) | 2019-11-20 | 2020-11-19 | 조절 t 세포 배양용 조성물 및 이의 용도 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US11702633B2 (ko) |
| EP (1) | EP4063490A4 (ko) |
| JP (1) | JP7425195B2 (ko) |
| KR (1) | KR102325857B1 (ko) |
| CN (2) | CN114901807B (ko) |
| AU (1) | AU2020387479B2 (ko) |
| BR (1) | BR112022008129A2 (ko) |
| CA (1) | CA3152351A1 (ko) |
| IL (1) | IL291909A (ko) |
| MX (1) | MX2022006000A (ko) |
| TW (1) | TWI812901B (ko) |
| WO (1) | WO2021101273A2 (ko) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3157592A1 (en) * | 2019-11-20 | 2021-05-27 | Myoung Ho Jang | Composition for culturing natural killer cells and method for preparing natural killer cells by using same |
| KR20240053223A (ko) | 2022-10-17 | 2024-04-24 | 주식회사 이뮤니스바이오 | Treg 세포 분화 및 활성화를 이용한 IBD치료용 약제학적 조성물 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5229109A (en) | 1992-04-14 | 1993-07-20 | Board Of Regents, The University Of Texas System | Low toxicity interleukin-2 analogues for use in immunotherapy |
| KR101667096B1 (ko) | 2011-02-10 | 2016-10-18 | 로슈 글리카트 아게 | 돌연변이 인터루킨-2 폴리펩티드 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009521409A (ja) * | 2005-12-08 | 2009-06-04 | ユニバーシティー オブ ルーイビル リサーチ ファンデーション,インコーポレーテッド | 制御性t細胞を増殖する方法と組成物 |
| CN101426533A (zh) * | 2005-12-08 | 2009-05-06 | 路易斯维尔大学研究基金会有限公司 | 免疫刺激组合物和方法 |
| US10273281B2 (en) * | 2015-11-02 | 2019-04-30 | Five Prime Therapeutics, Inc. | CD80 extracellular domain polypeptides and their use in cancer treatment |
| CN116903741A (zh) * | 2016-06-20 | 2023-10-20 | 科马布有限公司 | 特异性结合于pd-l1的抗体或其抗原结合片段及其用途 |
| CN117003887A (zh) * | 2017-04-03 | 2023-11-07 | 豪夫迈·罗氏有限公司 | 抗pd-1抗体与突变体il-2或与il-15的免疫缀合物 |
| CN110392392B (zh) | 2018-04-16 | 2021-07-09 | 华为技术有限公司 | 通信方法、通信装置及可读存储介质 |
-
2020
- 2020-11-19 WO PCT/KR2020/016382 patent/WO2021101273A2/ko active Application Filing
- 2020-11-19 BR BR112022008129A patent/BR112022008129A2/pt unknown
- 2020-11-19 IL IL291909A patent/IL291909A/en unknown
- 2020-11-19 AU AU2020387479A patent/AU2020387479B2/en active Active
- 2020-11-19 JP JP2022529596A patent/JP7425195B2/ja active Active
- 2020-11-19 CN CN202080078640.8A patent/CN114901807B/zh active Active
- 2020-11-19 CN CN202310807404.0A patent/CN117004564A/zh not_active Withdrawn
- 2020-11-19 EP EP20890419.3A patent/EP4063490A4/en active Pending
- 2020-11-19 MX MX2022006000A patent/MX2022006000A/es unknown
- 2020-11-19 KR KR1020200155385A patent/KR102325857B1/ko active IP Right Grant
- 2020-11-19 CA CA3152351A patent/CA3152351A1/en active Pending
- 2020-11-20 TW TW109140867A patent/TWI812901B/zh active
-
2022
- 2022-05-12 US US17/743,181 patent/US11702633B2/en active Active
-
2023
- 2023-05-15 US US18/317,199 patent/US20230407253A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5229109A (en) | 1992-04-14 | 1993-07-20 | Board Of Regents, The University Of Texas System | Low toxicity interleukin-2 analogues for use in immunotherapy |
| KR101667096B1 (ko) | 2011-02-10 | 2016-10-18 | 로슈 글리카트 아게 | 돌연변이 인터루킨-2 폴리펩티드 |
Non-Patent Citations (5)
| Title |
|---|
| H NISHIKAWA, INT. J. CANCER, vol. 127, 2010, pages 759 - 767 |
| R K GERSHONK KONDO, IMMUNOLOGY, vol. 18, 1970, pages 723 - 37 |
| S SAKAGUCHI, J IMMUNOL, vol. 155, 1995, pages 1151 - 1164 |
| S. KARUMUTHIL-MELETHIL, DIABETES, vol. 64, 2015, pages 1341 - 1357 |
| See also references of EP4063490A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2020387479A1 (en) | 2022-05-26 |
| KR102325857B9 (ko) | 2021-12-07 |
| EP4063490A2 (en) | 2022-09-28 |
| JP2022547352A (ja) | 2022-11-11 |
| US20220290102A1 (en) | 2022-09-15 |
| JP7425195B2 (ja) | 2024-01-30 |
| EP4063490A4 (en) | 2023-08-23 |
| CA3152351A1 (en) | 2021-05-27 |
| CN114901807A (zh) | 2022-08-12 |
| CN117004564A (zh) | 2023-11-07 |
| BR112022008129A2 (pt) | 2022-08-23 |
| AU2020387479B2 (en) | 2024-06-06 |
| WO2021101273A3 (ko) | 2021-07-15 |
| KR102325857B1 (ko) | 2021-11-12 |
| TW202132562A (zh) | 2021-09-01 |
| US11702633B2 (en) | 2023-07-18 |
| TWI812901B (zh) | 2023-08-21 |
| MX2022006000A (es) | 2022-06-17 |
| IL291909A (en) | 2022-06-01 |
| CN114901807B (zh) | 2023-07-11 |
| US20230407253A1 (en) | 2023-12-21 |
| KR20210061950A (ko) | 2021-05-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2018217064A2 (ko) | 형질전환된 t세포를 이용한 자연살해세포의 배양방법 | |
| US20230407253A1 (en) | Composition for culturing regulatory t cells and use thereof | |
| WO2016209021A1 (ko) | 자연살해세포의 증식 방법 및 자연살해세포 증식용 조성물 | |
| WO2016048107A1 (ko) | 인터페론-감마 또는 인터류킨-1베타를 처리한 줄기세포 또는 그 배양물을 포함하는 면역질환 또는 염증질환의 예방 또는 치료용 약학조성물 | |
| WO2020101361A1 (ko) | 형질전환된 t세포를 이용한 제대혈 유래 자연살해세포의 배양방법 | |
| WO2023219414A1 (ko) | 인터루킨21 변이체, 이를 포함하는 융합단백질 및 이의 용도 | |
| WO2017146538A1 (ko) | 조절 t 세포 매개성 질환의 예방 또는 치료용 약학적 조성물 | |
| WO2021101270A1 (ko) | 자연살해세포 배양용 조성물 및 이를 이용한 자연살해세포 제조방법 | |
| WO2022080912A1 (ko) | IgM 영역을 이용한 융합단백질 플랫폼 | |
| WO2021101271A1 (ko) | T 세포 배양용 배지 조성물 및 이를 이용한 t 세포의 배양 방법 | |
| WO2022255793A1 (ko) | 배양보조세포를 포함하는 자연살해세포 증식용 조성물 | |
| WO2017003153A1 (ko) | 제대혈 단핵세포 또는 이로부터 유래된 세포로부터 자연살해세포를 제조하는 방법 | |
| WO2016117960A1 (ko) | 면역질환 치료 효능을 갖는 grim19이 과발현된 중간엽줄기세포 및 이의 용도 | |
| WO2021107635A1 (ko) | Il-2 단백질 및 cd80 단백질을 포함하는 융합단백질 및 nk 세포를 포함하는 항암 치료용 조성물 | |
| WO2023128733A1 (ko) | 감마-델타 t 세포의 증식 배양 방법 | |
| WO2023277639A1 (ko) | 자연살해세포 배양용 배지 및 이를 이용한 자연살해세포 대량증식방법 | |
| RU2807802C1 (ru) | Композиция для культивирования регуляторных т-клеток и ее применение | |
| WO2021187911A1 (ko) | 제대혈 혈장 유래의 엑소좀 또는 이의 모방체 및 이의 약학적 용도 | |
| WO2022045849A1 (ko) | Il15 단백질, il15 수용체 알파 단백질, fc 도메인 및 il2 단백질을 포함하는 융합단백질 및 이의 용도 | |
| WO2024076030A1 (ko) | 혈소판의 제조 방법 | |
| WO2023167575A1 (ko) | 저면역원성 줄기세포, 줄기세포로부터 분화되거나 유래된 저면역원성 세포 및 이의 제조방법 | |
| WO2024117781A1 (ko) | Cd137 세포외 도메인을 포함하는 융합단백질, 이를 발현하는 면역세포 및 이의 용도 | |
| WO2022220648A1 (ko) | Hla-dr 특이적 키메라 항원 수용체 및 이의 용도 | |
| WO2013109104A1 (ko) | 인간 혈액 유래 혈구 세포괴를 포함하는 약학적 조성물 | |
| WO2019112327A2 (ko) | 세포독성 t 세포 생성방법 및 이의 용도 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20890419 Country of ref document: EP Kind code of ref document: A2 |
|
| ENP | Entry into the national phase |
Ref document number: 3152351 Country of ref document: CA |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112022008129 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 2022529596 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2020387479 Country of ref document: AU Date of ref document: 20201119 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2022113174 Country of ref document: RU |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2020890419 Country of ref document: EP Effective date: 20220620 |
|
| ENP | Entry into the national phase |
Ref document number: 112022008129 Country of ref document: BR Kind code of ref document: A2 Effective date: 20220428 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 522432641 Country of ref document: SA |