WO2016063364A1 - 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 - Google Patents
細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 Download PDFInfo
- Publication number
- WO2016063364A1 WO2016063364A1 PCT/JP2014/078033 JP2014078033W WO2016063364A1 WO 2016063364 A1 WO2016063364 A1 WO 2016063364A1 JP 2014078033 W JP2014078033 W JP 2014078033W WO 2016063364 A1 WO2016063364 A1 WO 2016063364A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cell
- scattered light
- calibration curve
- concentration
- cells
- Prior art date
Links
- 230000007246 mechanism Effects 0.000 title claims abstract description 67
- 238000004113 cell culture Methods 0.000 title claims abstract description 57
- 238000000034 method Methods 0.000 title claims abstract description 13
- 210000004027 cell Anatomy 0.000 claims abstract description 479
- 239000006285 cell suspension Substances 0.000 claims abstract description 134
- 238000011088 calibration curve Methods 0.000 claims abstract description 103
- 238000002834 transmittance Methods 0.000 claims abstract description 79
- 210000004748 cultured cell Anatomy 0.000 claims abstract description 28
- 230000004083 survival effect Effects 0.000 claims abstract description 18
- 239000007788 liquid Substances 0.000 claims abstract description 16
- 230000001678 irradiating effect Effects 0.000 claims abstract description 8
- 230000003833 cell viability Effects 0.000 claims description 83
- 238000005259 measurement Methods 0.000 claims description 74
- 239000002245 particle Substances 0.000 claims description 41
- 230000003287 optical effect Effects 0.000 claims description 18
- 230000035699 permeability Effects 0.000 claims description 11
- 238000000691 measurement method Methods 0.000 claims description 7
- 230000001419 dependent effect Effects 0.000 abstract 1
- 230000000694 effects Effects 0.000 description 46
- 239000000243 solution Substances 0.000 description 30
- 238000012545 processing Methods 0.000 description 29
- 238000010586 diagram Methods 0.000 description 13
- 238000001514 detection method Methods 0.000 description 11
- 238000010899 nucleation Methods 0.000 description 10
- 238000001228 spectrum Methods 0.000 description 10
- 230000007423 decrease Effects 0.000 description 7
- 239000004816 latex Substances 0.000 description 7
- 229920000126 latex Polymers 0.000 description 7
- 238000010186 staining Methods 0.000 description 7
- 230000008569 process Effects 0.000 description 6
- 239000006143 cell culture medium Substances 0.000 description 5
- 238000010008 shearing Methods 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- 206010009944 Colon cancer Diseases 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 238000002659 cell therapy Methods 0.000 description 4
- 208000029742 colonic neoplasm Diseases 0.000 description 4
- 238000012258 culturing Methods 0.000 description 4
- 239000006185 dispersion Substances 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 230000001172 regenerating effect Effects 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 238000010409 ironing Methods 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 210000001612 chondrocyte Anatomy 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 2
- 239000007793 ph indicator Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- 238000003026 viability measurement method Methods 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 1
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 101710162629 Trypsin inhibitor Proteins 0.000 description 1
- 229940122618 Trypsin inhibitor Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 238000003705 background correction Methods 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000001772 blood platelet Anatomy 0.000 description 1
- 239000001045 blue dye Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 206010061592 cardiac fibrillation Diseases 0.000 description 1
- 239000012930 cell culture fluid Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 239000006059 cover glass Substances 0.000 description 1
- 239000012531 culture fluid Substances 0.000 description 1
- -1 dead cells Substances 0.000 description 1
- 108010007093 dispase Proteins 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 230000002600 fibrillogenic effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000006249 magnetic particle Substances 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 210000004683 skeletal myoblast Anatomy 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 239000002753 trypsin inhibitor Substances 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M41/00—Means for regulation, monitoring, measurement or control, e.g. flow regulation
- C12M41/46—Means for regulation, monitoring, measurement or control, e.g. flow regulation of cellular or enzymatic activity or functionality, e.g. cell viability
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/0006—Controlling or regulating processes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M41/00—Means for regulation, monitoring, measurement or control, e.g. flow regulation
- C12M41/48—Automatic or computerized control
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M47/00—Means for after-treatment of the produced biomass or of the fermentation or metabolic products, e.g. storage of biomass
- C12M47/02—Separating microorganisms from the culture medium; Concentration of biomass
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M47/00—Means for after-treatment of the produced biomass or of the fermentation or metabolic products, e.g. storage of biomass
- C12M47/04—Cell isolation or sorting
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials
- G01N15/06—Investigating concentration of particle suspensions
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/17—Systems in which incident light is modified in accordance with the properties of the material investigated
- G01N21/47—Scattering, i.e. diffuse reflection
- G01N21/49—Scattering, i.e. diffuse reflection within a body or fluid
- G01N21/53—Scattering, i.e. diffuse reflection within a body or fluid within a flowing fluid, e.g. smoke
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/4833—Physical analysis of biological material of solid biological material, e.g. tissue samples, cell cultures
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials
- G01N15/01—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials specially adapted for biological cells, e.g. blood cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials
- G01N15/06—Investigating concentration of particle suspensions
- G01N15/075—Investigating concentration of particle suspensions by optical means
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2201/00—Features of devices classified in G01N21/00
- G01N2201/12—Circuits of general importance; Signal processing
- G01N2201/127—Calibration; base line adjustment; drift compensation
Definitions
- the present invention relates to a cell measurement mechanism, an automatic cell culture apparatus having the same, and a cell measurement method.
- Patent Document 2 discloses a method for identifying and quantifying red blood cells, white blood cells, and platelets in whole blood by using a laser light source having a plurality of wavelengths and a plurality of in-flow optical measurements (flow cytometers). Yes.
- the accuracy is improved as compared with the conventional counting with a hemocytometer.
- the reliability of the counting results was extremely reduced in the concentration region where the cell concentration of the cell suspension was 1 ⁇ 10 5 cells / mL or less and 5 ⁇ 10 6 cells / mL or more.
- the cell culture device is applied to regenerative medicine or cell therapy, it is necessary to measure a specimen having a cell concentration of 1 ⁇ 10 5 cells / mL or less.
- the cell counter according to Patent Document 1 cannot be applied with low measurement accuracy in this concentration region.
- the cell viability is determined by virtue of the contrast of the cell outline.
- cells with reduced activity hereinafter referred to as low activity viable cells
- the present invention provides a cell measurement mechanism capable of performing at least cell viability measurement with high accuracy and speed without depending on the skill level of an operator and without staining cultured cells, and a cell culture apparatus having the same And providing a cell counting method.
- the cell measurement mechanism of the present invention includes a flow path through which a cell suspension flows, a liquid driving unit for feeding the cell suspension in the flow path, and a flow path in the flow path.
- the cell culture device of the present invention includes an expansion culture mechanism for culturing and proliferating cells, and peeling the proliferated cells, and a flow path for allowing a cell suspension containing the cells detached by the expansion culture mechanism to flow.
- a liquid drive unit for feeding the cell suspension in the flow path, and the forward scattered light intensity and transmittance obtained by irradiating the cell suspension flowing in the flow path with light from a light source.
- a cell measuring mechanism having a calculation unit that calculates at least the cell viability in the cell suspension based on the side scattered light intensity.
- the cell measurement method of the present invention is a cell measurement method for obtaining at least the cell viability in a cell suspension, A step of irradiating irradiation light from a light source from a direction orthogonal to the flow of the cell suspension and a forward scattered light intensity scattered from the cell suspension are measured for the cell suspension flowing through the flow cell.
- a cell measurement mechanism capable of executing at least measurement of cell viability with high accuracy and speed without depending on the skill level of an operator and without staining cultured cells, and cell culture having the same An apparatus and a cell measurement method can be provided.
- FIG. 4 is a spectrum diagram showing the relationship between the wavelength of irradiation light from a light source and the intensity of side scattered light in a culture solution in Caco-2 cell suspension and cell viability. It is a figure which shows the relationship between the cell viability in a Caco-2 living cell suspension, and a side scattered light intensity, and is explanatory drawing of a calibration curve (3).
- the present inventors in the case of cultured cells (passage cells etc.) used in regenerative medicine or cell therapy, depend on differences in cell activity or culture conditions (passage culture environment etc.) for each patient or subject. , Dead cells with a small particle size, dying cells, or live cells with low activity are mixed. Since these are different in size from active living cells, for example, an accurate cell count or only by scattered light obtained by irradiating a living cell suspension flowing through a flow cell with irradiation light from a light source. It was found that it was difficult to determine the cell viability.
- the present inventors use the forward scattered light intensity and transmittance, or the forward scattered light intensity and transmittance, and the side scattered light intensity, thereby the above-mentioned various cells having different sizes, that is, active living organisms. It has been found that it is possible to identify cells, low-activity live cells and dead cells, and to obtain the number of cells or cell viability with high accuracy and speed.
- FIG. 1 shows an overall configuration diagram of a cell culture device according to an embodiment of the present invention.
- the flow path through which the cell suspension flows is shown by a solid line, and the signal line for transmitting and receiving a control signal or a measurement signal is shown by a dotted line.
- the cell culture device 1 is configured to culture and proliferate cells, and an expanded culture mechanism 15 that peels off the proliferated cells, a cell measurement mechanism 16 that disperses and measures the cells detached by the expanded culture mechanism 15, and a cell measurement mechanism 16.
- a cell seeding mechanism 17 for feeding the dispersed cell suspension to the expansion culture mechanism 15 and a control unit 18 are configured.
- control unit 18 cooperates with the cell measurement mechanism 16, and based on the measured forward scattered light intensity, transmittance, and side scattered light intensity, the cell viability in the cell suspension, etc. Has a function to obtain the value by calculation.
- the control unit 18 constitutes a part of the cell measurement mechanism 16.
- the control unit 18 also has a function of controlling the expansion culture mechanism 15, the cell measurement mechanism 16, and the cell seeding mechanism 17.
- the control part 18 demonstrates as an example the structure which has a function which calculates
- a control calculation unit having the above function may be provided in the cell measurement mechanism 16, or a control calculation unit may be arranged in the measurement unit 6 described later.
- Cells to be cultured in the expansion culture vessel 2 constituting the expansion culture mechanism 15 are introduced from the cell supply unit 10 using a liquid driving unit, for example, a syringe pump.
- An appropriate amount of cell culture solution is introduced from the culture solution supply unit 3 by a liquid driving unit, for example, a squeeze pump 7, flows through the three-way valve 8 and the flow path, and is supplied to the expansion culture vessel 2.
- a liquid driving unit for example, a squeeze pump 7
- the cell is shaken so that the cells cultured in the expansion culture vessel 2 have a uniform concentration in the introduced cell culture solution, and then left to stand for several days.
- the cells to be cultured are cultured for several days in the expansion culture vessel 2 under appropriate conditions in a CO 2 incubator.
- a microscope is installed to enable observation of the growth state of the cultured cells.
- a washing liquid into the expansion culture container 2 from the cell washing liquid supply unit 11 via a syringe pump
- a cell culture liquid, dead cells, garbage, or the like having a long residence time is pushed out.
- the pushed cell culture solution containing dead cells is discharged out of the closed system of the cell culture device 1 as waste fluid 14 via the squeezing pump 7.
- the cell detachment solution supply unit 12 contains a proteolytic enzyme such as trypsin, collagenase, dispase and the like. These proteolytic enzymes are introduced into the expansion culture vessel 2 and left for a certain period of time.
- the sample introduction unit 4 collects the cultured cells peeled from the bottom surface of the expansion culture vessel 2 via the ironing pump 7 and the three-way valve 8. At this time, if there is a large amount of cultured cell residue on the bottom surface of the expansion culture vessel 2, it is washed with the cell culture solution introduced from the culture solution supply unit 3 and then collected in the sample introduction unit 4, thereby culturing. It is possible to improve the cell recovery rate.
- the cultured cells collected in the sample supply unit 4 are introduced as a cell suspension into the circulation channel of the cell measurement mechanism 16 via a liquid drive unit such as a syringe pump (not shown) and the three-way valve 8. .
- the cell measurement mechanism 16 includes a dispersion unit 5, an iron pump 7 as a liquid drive unit, and a measurement unit 6.
- the dispersion unit 5, the squeezing pump 7, and the measurement unit 6 are connected by a circulation channel.
- the aggregation property differs depending on the cell type to be cultured. If a highly aggregated cell type is cultured, the cultured cells contained in the cell suspension introduced into the circulation channel of the cell measurement mechanism 16 (hereinafter simply referred to as “cell aggregation mechanism”).
- the cell suspension (Referred to as cells) will be lumped and flow through the circulation channel. Therefore, after being dispersed from the lump by the squeezing pump 7 as a liquid driving unit via the three-way valve 8, the cell suspension is introduced into the measuring unit 6.
- the dispersion part 5 is formed, for example, by providing a narrowing part in which the diameter of the flow path rapidly decreases or a partition plate such as an orifice in the flow path. When the cell suspension flows through these narrowed portions or orifices, the massive cells are dispersed by shearing force (shear stress).
- the cell measurement mechanism 16 may be configured.
- the cell seeding mechanism 17 has a cell connected via a three-way valve 8 to a flow path having one end connected to the expansion culture vessel 2 and the other end connected to a circulation flow path in the cell measurement mechanism 16 via the three-way valve 8.
- a seeding sample adjustment unit 9 is provided.
- the cell seeding sample adjustment unit 9 is arranged to adjust the cell concentration in the cell suspension flowing through the circulation channel in the cell measurement mechanism 16. That is, the expansion culture container is driven via a flow path in which one end is connected to the expansion culture container 2 by driving the ironing pump 7 that is a liquid driving unit so that the cell concentration in the cell suspension becomes a desired cell concentration.
- the cell suspension containing the cells detached from the bottom surface of 2 is taken into the cell seeding sample preparation unit 9 through the three-way valve 8.
- the peristaltic pump 7 is driven to introduce a desired amount of cell culture fluid from the culture fluid supply unit 3 through the three-way valve 8 into the cell seeding sample preparation unit 9 and mix with the already taken-up cell suspension. Dilute. Then, the diluted cell suspension is sent to the circulation channel of the cell measurement mechanism 16, and the forward scattered light intensity, the transmittance, and the side scattered light intensity are measured by the measurement unit 6 described later in detail. .
- FIG. 2 shows a microscopic image of Caco-2 cells, which is a human colon cancer cell line.
- the microscopic image shown in FIG. 2 is an image of Caco-2 cells obtained under the following conditions. Caco-2 cells were cultured to 100% confluence or higher, and the cells floating in the culture medium were collected without detaching the adhered cells. Most of the cultured cells in this state have decreased activity, some of the cells are detached, and many dead cells are floating in the cell culture medium.
- FIG. 2 An image taken with an objective lens 20 times using an inverted microscope after dropping this sample on a slide glass and fixing with a cover glass is a microscope image shown in FIG.
- viable cells (Vc) 19 that are still active
- viable cells (Sc) 20 that have low activity
- dead cells (Dc) 21 that do not have activity and die are observed.
- the living cells (Vc) 19 shine white and have a large particle size
- the dead cells (Dc) 21 are generally colored black and are no longer particulate.
- the low activity live cell (Sc) 20 is in a state intermediate between the live cell (Vc) 19 and the dead cell (Dc) 21, but the particle size is smaller than that of the live cell (Vc) 19. I understand.
- the live cells (Vc), low-activity live cells (Sc), and dead cells (Dc) shown in FIG. 2 are Caco-2 cells.
- the particle size of living cells (Vc) is about 10 ⁇ m for NIH / 3T3 cells, about 14 ⁇ m for the above human colon cancer cell line (Caco-2 cells) or human oral mucosal epithelial cells, and about about 20 ⁇ m for human skeletal myoblasts. 20 ⁇ m.
- human mesenchymal stem cells are about 10-50 ⁇ m and human chondrocytes are about 10 ⁇ m.
- the measurement unit 6 in the present embodiment includes a flow cell 23 that flows through the cell suspension therein, a light source 22 that is arranged to be orthogonal to the flow direction of the cell suspension that flows through the flow cell 23, and the flow cell 23.
- a transmittance detector 25 is provided so as to face the sandwiched light source 22, that is, so that the light receiving surface of the detector faces the optical axis of the irradiation light emitted from the light source.
- the measurement unit 6 is on the side of the transmittance detector 25 and has a forward scattered light detector 24 arranged at a predetermined angle ⁇ (forward scattering detection angle) with respect to the optical axis of the irradiated light, and the light of the irradiated light.
- a side scattered light detector 26 is arranged on an axis perpendicular to the axis, the axis passes through the approximate center of the flow cell 23, and is arranged at a predetermined distance from the flow cell 23.
- the light source 22 for example, a laser light source, an LED light source, a tungsten lamp, a xenon lamp, or the like used in a spectrophotometer, a scattering photometer, a fluorophotometer, a flow cytometer, a particle size distribution measuring device, or the like can be used. .
- the color inside the cell changes to dark in the process of changing from a high active state to a low active state, that is, from a living cell (Vc) to a dead cell (Dc). Therefore, as the content of dead cells (Dc) in the cell suspension increases, the amount of light transmitted through the cell suspension decreases. Therefore, the concentration (CDc) of dead cells (Dc) can be obtained by measuring the transmittance with the transmittance detector 25. In the transmittance measurement, absorption of all organic components in the cell suspension overlaps in the vicinity of ultraviolet light, making it difficult to accurately evaluate only dead cells (Dc). Further, a pH indicator such as phenol red is added to the cell culture solution introduced from the culture solution supply unit 3 shown in FIG.
- the cell culture solution is colored yellow to red, and the transmittance is greatly reduced by the influence of the color of the cell culture solution at wavelengths in the visible light region. If these factors are taken into consideration and the transmittance is detected by the transmittance detector 25 in the long wavelength region, the concentration (CDc) of dead cells (Dc) in the living cell suspension is stabilized without being affected by interference components. Can be measured.
- the cell granule density and the internal structure differ between the living cell (Vc) and the dead cell (Dc).
- Side scattered light is light that is detected at an angle of 90 ° with respect to the optical axis of the light source 22 as described above, and reflects the density and form of the particles. Therefore, the difference in the side scattered light intensity that is scattered by irradiating the intracellular substance with the irradiation light reflects the cell viability.
- a gentle peak derived from the organic component of the cytoplasm is detected in the ultraviolet region (near 230 nm to 310 nm). Affected by the color of the cell culture. Therefore, it is desirable to measure the side scattered light depending on the intracellular particles on the short wavelength side in the ultraviolet region.
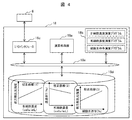
- FIG. 4 shows a schematic configuration diagram of the control unit 18 shown in FIG.
- the control unit 18 cooperates with the cell measurement mechanism 16, and based on the measured forward scattered light intensity, transmittance, and side scattered light intensity, The case where it has the function which calculates
- the control unit 18 includes a calculation processing unit 18a, a calculation program storage unit 18b, an I / O interface 18c, and a calibration curve database (DB), and these are connected to each other via an internal bus 18e.
- the I / O interface 18c comprises the measurement unit 6 described above, the forward scattered light intensity measured by the forward scattered light detector 24, the transmittance measured by the transmittance detector 25, and the side scattered light. The intensity of the side scattered light measured by the detector 26 can be captured, and the emission command (irradiation timing, etc.) of the irradiation light can be transmitted to the light source 22 constituting the measurement unit 6.
- the calculation program storage unit 18b stores a live cell concentration calculation program, a dead cell concentration calculation program, and a cell viability calculation program.
- the calibration curve database 18d stores in advance a calibration curve (1) used for calculation of the living cell concentration (CVc) indicating the relationship between the living cell concentration (CVc) and the forward scattered light intensity.
- a calibration curve (2) used for calculating the dead cell concentration (CDc) indicating the relationship between the dead cell concentration (CDc) and the transmittance T is also stored in advance.
- a calibration curve (3) used for calculating the cell viability indicating the relationship between the cell viability and the side scattered light intensity is also stored in advance.
- a live cell concentration calculation program, a dead cell concentration calculation program, and a cell viability calculation program may be incorporated into one program and stored.
- the arithmetic processing unit 18a is realized by a processor such as one CPU or a plurality of CPUs connected in parallel. Specific processing by the arithmetic processing unit 18a will be described later in the embodiment.
- the arithmetic processing unit 18a reads the live cell concentration calculation program from the calculation program storage unit 18b, acquires the forward scattered light intensity acquired from the I / O interface 18c via the internal bus 18e, and executes the live cell concentration calculation program. . Then, with reference to the calibration curve database 18d, the live cell concentration (CVc) in the live cell suspension is obtained using the calibration curve (1).
- the arithmetic processing unit 18a reads the dead cell concentration calculation program from the calculation program storage unit 18b, acquires the transmittance T acquired from the I / O interface 18c via the internal bus 18e, and executes the dead cell concentration calculation program. To do. Then, referring to the calibration curve database 18d, the dead cell concentration (CDc) in the live cell suspension is obtained using the calibration curve (2). Further, when the low-activity live cells (Sc) contained in the cell suspension are infinitely small and can be ignored, cell viability is determined using the above-mentioned live cell concentration (CVc) and dead cell concentration (CDc). Find the rate.
- the arithmetic processing unit 18a determines the living cell concentration (CVcc). ) And dead cell concentration (CDc), and then the following processing is performed.
- the arithmetic processing unit 18a reads the cell survival rate calculation program from the calculation program storage unit 18b, acquires the side scattered light intensity acquired from the I / O interface 18c via the internal bus 18e, and executes the cell survival rate calculation program. To do.
- the calibration curve database 18d using the calibration curve (3), the cell viability is further determined based on the living cell concentration (CVc) and the dead cell concentration (CDc).
- control unit 18 uses the forward scattered light intensity, the transmittance T and / or the side scattered light intensity measured by the measuring unit 6 and also stores the calibration curve (1) stored in the calibration curve database 18d in advance.
- the cell viability in the living cell suspension is obtained, so that at least the cell viability is measured without depending on the skill level of the operator and without staining the cultured cells. It becomes possible to execute with high accuracy and speed.
- the cell culture device 1 of the present embodiment obtains the living cell concentration (CVc) and the dead cell concentration (CDc), it can also obtain the number of living cells (Vc) and the number of dead cells (Dc). Is possible.
- the threshold value for low-activity living cells (Sc), by setting a predetermined threshold value in advance to the forward scattered light intensity obtained from the forward scattered light detector 24 and the transmittance T obtained from the transmittance detector 25, It is also possible to determine the low-activity viable cell concentration (CSc) and the number of low-activity viable cells (Sc).
- an optimal threshold value can be obtained by preparing a standard sample with a known concentration in advance and measuring the forward scattered light intensity and the transmittance T.
- FIG. 5 is a diagram showing the relationship between the viable cell concentration in the Caco-2 cell suspension and the forward scattered light intensity, and is an explanatory diagram of the calibration curve (1).
- the calibration curve (1) shown in FIG. 5 is obtained when the forward scattering angle ⁇ shown in FIG. 3 is 20 °.
- a standard sample in which the viable cell (Vc) has a live cell concentration (CVc) of 100% and a plurality of standard samples having different viable cell concentrations (CVc) are prepared in the Caco-2 cell suspension.
- the forward scattering detection angle ⁇ is adjusted to 20 °
- standard samples having different living cell concentrations (CVc) are passed through the flow cell 23, and the forward scattered light intensity is measured by the forward scattering detector 24.
- the live cell concentration (CVc) is plotted on the horizontal axis, and the forward scattered light intensity measured on the vertical axis is plotted.
- the plotted measured values of the forward scattered light intensity at each living cell concentration (CVc) are approximated by a straight line to create a calibration curve shown in FIG.
- FIG. 4 a calibration curve database shown in FIG. 4 as a calibration curve (1) 18d.
- a plurality of living cell concentrations (CVc) are prepared using the live cells (Vc) of Caco-2 cells as standard samples.
- the present invention is not limited to this.
- latex particles having the same particle size as the live cells (Vc) of Caco-2 cells are prepared, and the latex particles are mixed as standard samples in cell culture solutions at different concentrations.
- the calibration curve (1) may be created by measuring the light intensity.
- FIG. 6 is a spectrum diagram showing the relationship between the wavelength of light irradiated from the light source and the transmittance T at each dead cell concentration in the Caco-2 cell suspension.
- Caco-2 cells are introduced into a culture vessel, and the cells floating in the cell culture solution are collected without adhering to the bottom surface of the culture vessel, and allowed to stand for 10 minutes after stirring. Thereafter, the vicinity of the liquid surface is collected, and the collected cells are measured with a cell counter to obtain dead cells (Dc) with 83% having a particle diameter of about 5 ⁇ m.
- the dead cell concentration (CDc) is 1.5 to 6.0 ⁇ 10 5 cells / mL
- the live cell concentration (CVc) is 1.8 ⁇ 10 6 cells / mL.
- a cell suspension standard sample was prepared, and a transmittance T spectrum at each dead cell concentration (CDc) was measured. As a result, as shown in FIG.
- the horizontal axis represents the wavelength of the light emitted from the light source
- the vertical axis represents the transmittance T
- the dead cell concentration (CDc) is 0 cells / mL, 1.5 ⁇ 10 5 cells / Spectra were obtained at mL, 3.0 ⁇ 10 5 cells / mL, and 6.0 ⁇ 10 5 cells / mL.
- the transmittance T is greatly reduced in any dead cell concentration (CDc) sample in a wavelength range of 400 nm or less and a wavelength range of 450 to 600 nm. This is because absorption by the cytoplasm occurs at a wavelength of 400 nm or less, and absorption occurs by coloring the cell culture medium by adding a pH indicator as described above in the wavelength range of 450 to 600 nm.
- the optimum wavelength range ⁇ of 650 to 750 nm is suitable as a wavelength range in which the absorption of irradiation light by cells that are black outside this wavelength range, that is, dead cells (Dc), can be measured. Table 1 below shows the results of obtaining the transmittance T at a wavelength of 700 nm within the optimum wavelength range ⁇ .
- the permeability T is 100.6%, and the dead cell concentration (CDc) is 1.5 ⁇ 10 5 cells / mL. Then, the permeability T is 96.5%, and the dead cell concentration (CDc) is 3.0 ⁇ 10 5 cells / mL, and the permeability T is 93.8% and the dead cell concentration (CDc) is 6.0 ⁇ 10 5 cells. As for / mL, the transmittance T was 89.3%.
- FIG. 7 shows the relationship between the dead cell concentration (CDc) and the permeability T in the Caco-2 medium.
- FIG. 8 shows the relationship between the dead cell concentration (CDc) and the transmittance T in the live Caco-2 cell suspension. 7 and 8, as described above, the wavelength of the irradiation light is 700 nm, the transmittance T of a standard sample having a dead cell concentration (CDc) of 0 cells / mL is used as a baseline, and each dead cell concentration (CDc The difference from the transmittance T of the standard sample was obtained, and a calibration curve was created.
- FIG. 9 is a spectrum diagram showing the relationship between the wavelength of the irradiation light from the light source and the side scattered light intensity in the cell culture solution and the cell viability in the Caco-2 cell suspension.
- the side scattered light intensity is 17.48, and when the cell viability is 25%, the side scattered light intensity is 19.76.
- the rate was 40%, the side scattered light intensity was 24.31, and when the cell viability was 70%, the side scattered light intensity was 32.85%.
- the side scattered light intensity is measured in advance by the side scattered light detector 26, and the measured value is used as a baseline in the storage unit ( (Not shown).
- FIG. 10 shows the relationship between the cell viability and the side scattered light intensity in the live Caco-2 cell suspension.
- the side scattered light intensity of the standard sample with each cell viability is set to the baseline using the side scattered light intensity of the standard sample having the irradiation light wavelength of 280 nm and the cell viability of 0% (only cell culture medium).
- a difference from the intensity is obtained, a calibration curve is created, and stored in the calibration curve database 18d shown in FIG. 4 as a calibration curve (3).
- the side scattered light intensity is proportional to the cell viability.
- the cell measurement mechanism 16 and the cell culture device 1 of the present embodiment at least the measurement of the cell viability is highly accurate without depending on the skill level of the operator and without staining the cultured cells. And it becomes possible to execute quickly.
- the viable cell concentration (CVc), dead cell concentration (CDc), live cell (Vc) number, and dead cell (Dc) number are also possible. Furthermore, for the low activity live cells (Sc), the low activity live cell concentration (CSc) and the number of the low activity live cells (Sc) can also be obtained.
- the cell culture device 1 of the present embodiment is the same as the configuration shown in FIG. 1 described above, the measurement unit 6 configuring the cell measurement mechanism 16 is the same as the configuration shown in FIG. 3 described above, and the control unit The configuration of 18 is the same as the configuration shown in FIG. 4 described above, and redundant description is omitted.
- the control unit 18 cooperates with the cell measurement mechanism 16 to calculate the cell viability in the cell suspension based on the measured forward scattered light intensity, transmittance, and side scattered light intensity.
- the present invention is not limited to this.
- a control calculation unit having the above function may be provided in the cell measurement mechanism 16, and a control calculation unit is provided in the measurement unit 6 described later. It is good also as a structure to distribute.
- a human colon cancer cell line (Caco-2 cell) is used as an example of cultured cells, and the angle formed with the optical axis of the light source 22 shown in FIG. 3, that is, the forward scatter detection angle ⁇ is set to 20 ° in advance.
- the forward scatter detection angle ⁇ depends on the particle diameter of the living cell (Vc) of the cell type.
- the optimum forward scatter detection angle ⁇ for measurement of other cell tumors other than Caco-2 cells can be adjusted within a range of about 5 ° to 45 °.
- step S102 the arithmetic processing unit 18a takes in the forward scattered light detection intensity measured by the forward scattered light detector 24 constituting the measuring unit 6 via the I / O interface 18c and the internal bus 18e.
- the detected intensity of the forward scattered light that is taken in is cultured and expanded in the expansion culture mechanism 15, and the viable cell suspension containing the Caco-2 cells after detachment is passed through the flow cell 23, This is the intensity of the forward scattered light scattered forward by the irradiation light.
- step S103 the arithmetic processing unit 18a accesses the calibration curve database 18d, and a calibration curve (CVc) indicating the relationship between the viable cell concentration (CVc) in the Caco-2 cell suspension and the forward scattered light intensity shown in FIG. Refer to 1).
- the arithmetic processing unit 18a calculates the living cell concentration (CVc) by extracting the living cell concentration (CVc) corresponding to the measured forward scattered light intensity by using the calibration curve (1) (step) S104).
- step S105 the arithmetic processing unit 18a takes in the transmittance T measured by the transmittance detector 25 constituting the measuring unit 6 via the I / O interface 18c and the internal bus 18e. Then, the arithmetic processing unit 18a accesses the calibration curve database 18d again, and the calibration curve (2) showing the relationship between the dead cell concentration (CDc) and the transmittance T in the Caco-2 cell suspension shown in FIG. Is referred to (step S106). The arithmetic processing unit 18a calculates the dead cell concentration (CDc) by extracting the dead cell concentration (CDc) corresponding to the measured transmittance T using the calibration curve (2) (step S107). ).
- the forward scattered light intensity is a relative signal
- the transmittance T is a signal that can be handled as an absolute value. This is because, similarly to the creation of the calibration curve (2) described above, only the cell culture solution (equivalent to 0% cell viability) is passed through the flow cell 23 in advance during the measurement, and the transmittance measured at that time is measured. T is stored as a baseline. This is because the difference between the transmittance T obtained when the living cell suspension is passed through the flow cell 23 and the baseline, that is, the decrease in the transmittance T is output.
- the transmittance T obtained from the transmittance detector 24 is a signal after background correction (removal of background noise) by reference light.
- step S108 the arithmetic processing unit 18a calculates (CVc / (CVc + CDc)) using the living cell concentration (CVc) obtained in step S104 and the dead cell concentration (CDc) obtained in step S107. Determine the cell viability in the Caco-2 cell suspension.
- Caco-2 cells which are cultured cells, are resistant to shearing force (shear stress), can easily maintain a high cell viability, and are less affected by decreased activity due to shearing force. This is because it can be assumed that the viable cell concentration (CVc), dead cell concentration (CDc), and low-activity viable cell concentration (CSc) in the Caco-2 cell suspension have the following relationship because they are cells.
- CVc viable cell concentration
- CDc dead cell concentration
- CSc low-activity viable cell concentration
- the present embodiment it is possible to perform at least the measurement of the cell viability with high accuracy and speed without depending on the skill level of the operator and without staining the cultured cells.
- FIG. 12 shows a cell viability calculation flowchart of Example 2, which is another example of the present invention.
- the present embodiment is different from the first embodiment in that the side scattered light intensity measured by the side scattered light detector 26 constituting the measuring unit 6 is used for calculating the cell viability.
- the description of the same configuration as that of the first embodiment will be omitted.
- step S101 the arithmetic processing unit 18a receives a cell viability calculation program from the calculation program storage unit 18b in addition to the live cell concentration calculation program and the dead cell concentration calculation program. Read through. Subsequent steps S102 to S107 are executed in the same manner as in the first embodiment.
- the arithmetic processing unit 18a uses the side scattered light intensity measured by the side scattered light detector 26 constituting the measurement unit 6 as the I / O interface 18c and the internal The data is taken in via the bus 18e (step S109).
- step S111 the arithmetic processing unit 18a uses the living cell concentration (CVc) obtained in step S104 and the dead cell concentration (CDc) obtained in step S107 to refer to y of the calibration curve (3) referred to. Correct the intercept. Specifically, a cell suspension having a cell viability of about 100% is prepared in advance by using a cell type of an actual sample (here, Caco-2 cells). Then, the relationship between the living cell concentration (CVc) and the side scattered light intensity in the cell suspension is measured by the forward scattered light detector 24 and the side scattered light detector 26 and obtained. A cell suspension with a cell viability of about 100% can be obtained by removing dust and small dead cells in the suspension by centrifugation.
- CVc living cell concentration
- CDc dead cell concentration
- the maximum concentration is obtained from the viable cell concentration (CVc) and the dead cell concentration (CDc) obtained in steps S104 and S107.
- the side scattered light intensity at the cell viability is determined.
- the y intercept of the calibration curve (3) is corrected using the obtained side scattered light intensity value.
- step S112 the arithmetic processing unit 18a calculates the cell survival rate by extracting the cell survival rate corresponding to the measured side scattered light intensity using the corrected calibration curve (3). Is performed (step S112).
- the calibration curve (3) whose y-intercept has been corrected in step S111 is stored in place of the calibration curve (3) already stored in the calibration curve database 18d. That is, the corrected calibration curve (3) is updated and stored.
- Caco-2 cells which are cultured cells, are resistant to shearing force (shear stress), can easily maintain a high cell viability, and depend on shearing force. Since the cells are less affected by the decrease in activity, the viable cell concentration (CVc), dead cell concentration (CDc), and low active cell concentration (CSc) in the Caco-2 cell suspension have the following relationship: Assumed.
- the cell survival rate can be measured with higher accuracy by correcting the calibration curve (3) and calculating the cell survival rate.
- FIG. 13 shows a cell viability calculation flowchart of Example 3, which is another example of the present invention.
- Example 1 and Example 2 the case where the concentration of low-activity living cells (CSc) in the living cell suspension is negligibly low, that is, the case where CDc ⁇ (CSc + CDc) can be assumed has been described.
- the present embodiment is different from the first and second embodiments in that the cell survival rate in the live cell suspension when CDc ⁇ (CSc + CDc) cannot be assumed is calculated.
- the description of the same configurations as those in the first and second embodiments will be omitted.
- the particle diameter of low-activity live cells (Sc) in a living cell suspension sample is smaller than that of living cells (Vc) and larger than that of dead cells (Dc). Therefore, even if the particle size of the low activity live cell (Sc) is almost the same as the particle size of the dead cell (Dc), the calibration curve (1) used to calculate the live cell concentration (CVc). Will not be affected. However, if there are many low-activity living cells (Sc) having a size close to the particle size of the living cells (Vc) in the living cell suspension, the slope of the calibration curve (1) is affected. Will be affected.
- the arithmetic processing unit 18a executes steps S101 to S107 in the same manner as in the first embodiment. Thereafter, the arithmetic processing unit 18a uses the viable cell concentration (Cc) obtained in step S104 and the dead cell concentration (CDc) obtained in step S107 to reduce the viable cell concentration (CSc) and the total number of cells. That is, the number of live cells (Vc) + the number of dead cells (Dc) + the number of live cells (Sc) with low activity is calculated (step S201).
- step S202 the arithmetic processing unit 18a calculates the following (step S202). ((CVc + CSc + CDc) ⁇ CDc) / (CVc + CSc + CDc) Then, the calculation result is compared with CVc / (CVc + CDc) (similar to step S108 shown in FIG. 11). A large difference in the comparison results reveals that there are many low-activity live cells (Sc) having a size close to the particle size of the live cells (Vc) in the live cell suspension. Further, it can be seen that in step S104, the living cell concentration (CVc) calculated using the calibration curve (1) is not a normal value. Therefore, the process proceeds to a process for correcting the calibration curve (1).
- step S203 the living cell suspension is allowed to stand for a certain time ( ⁇ t).
- ⁇ t some of the low activity live cells (Sc) become dead cells (Dc)
- Dc dead cells
- the number of low activity live cells (Sc) is calculated after the passage of ⁇ t from the number at time t A before leaving ⁇ t. to reduce to the number at the time t B. Further, the number of dead cells (Dc) increases from the number at time t A before being left at ⁇ t to the number at time t B after ⁇ t has elapsed.
- latex particles smaller than the particle diameter of live cells (Vc) are prepared in advance, and these latex particles are used as pseudo low-activity live cells (Sc) as standard samples used when the calibration curve (1) is created. Added. Then, the influence on the relationship between the live cell concentration (CVc) and the forward scattered light intensity, which is the calibration curve (1) stored in the calibration curve database 18d, is obtained. Then, a calibration curve when latex particles having different concentrations are added to the standard sample is prepared and newly stored in the calibration curve database 18d, so that a large number of low-activity living cells (Sc) are present in the living cell suspension. When it exists, it is good also as a calibration curve used for calculation of a living cell density
- the particle size of the latex particles to be used is desirably ⁇ several ⁇ m of the particle size of the living cells (Vc), and it is desirable to use latex particles having at least one particle size.
- Caco-2 cells were shown as an example of cultured cells, but other cell types such as NIH / 3T3 cells, human oral mucosal epithelial cells, human skeletal muscle myoblasts, humans The same applies to the case of culturing various cells such as mesenchymal stem cells or human chondrocytes.
- this invention is not limited to the above-mentioned Example, Various modifications are included.
- the above-described embodiments have been described in detail for easy understanding of the present invention, and are not necessarily limited to those having all the configurations described.
- a part of the configuration of one embodiment can be replaced with the configuration of another embodiment, and the configuration of another embodiment can be added to the configuration of one embodiment.
- Calibration curve database 18e ... Internal bus, 19 ... Live cells , 20 ... low active living cells, 21 ... dead cells, 22 ... light source, 23 ... flow cell, 24 ... forward scattered light detector, 25 ... transmittance detector, 26 ... side scattered light detector
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Immunology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Sustainable Development (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Computer Hardware Design (AREA)
- Tropical Medicine & Parasitology (AREA)
- Optics & Photonics (AREA)
- Biophysics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
一方、再生医療あるいは細胞治療を目的とし細胞培養をおこなう場合、安全性が確認できていないため治療に供する細胞を染色することはできない。従って、色素で細胞を染色することを必須とする特許文献1による細胞計測方法では、このような細胞培養に適用することは困難である。また、上述のとおり特許文献2による細胞計測方法では、計測に多大の時間を要するため、細胞培養装置に適用することは困難となる。
そこで本発明は、作業者の熟練度に依ることなく、また、培養細胞を染色することなく、少なくとも細胞生存率の計測を高精度かつ迅速に実行し得る細胞計測機構及びそれを有する細胞培養装置、並びに細胞計測方法を提供することにある。
フローセル内を通流する細胞懸濁液に対し、前記細胞懸濁液の流れに直交する方向より光源から照射光を照射する工程と、前記細胞懸濁液より散乱する前方散乱光強度を測定する工程と、前記細胞懸濁液を透過する前記照射光の透過率を測定する工程と、前記測定された前方散乱光強度及び予め記憶された生細胞濃度と前記前方散乱光強度との関係を示す第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度を求める工程と、前記測定された前記透過率及び予め記憶された死細胞濃度と前記透過率との関係を示す第2の校正曲線に基づき、前記細胞懸濁液中の死細胞濃度を求める工程と、前記求めた生細胞濃度及び死細胞濃度に基づき、前記細胞懸濁液中の細胞生存率を求める工程と、を有することを特徴とする。
<生細胞濃度(CVc)と前方散乱光強度との関係を示す、校正曲線(1)>
図5は、Caco-2細胞懸濁液中の生細胞濃度と前方散乱光強度の関係を示す図であり、校正曲線(1)の説明図である。図5に示す校正曲線(1)は、図3に示す前方散乱角度θが20°とした場合のものである。
<死細胞胞濃度(CDc)と透過率Tとの関係を示す、校正曲線(2)>
図6は、Caco-2細胞懸濁液中の各死細胞濃度における、光源からの照射光の波長と透過率Tとの関係を示すスペクトル図である。
<細胞生存率と側方散乱光強度との関係を示す、校正曲線(3)>
図9は、Caco-2細胞懸濁液中の細胞培養液及び各細胞生存率における、光源からの照射光の波長と側方散乱光強度との関係を示すスペクトル図である。
CDc≒(CSc+CDc)
以上のとおり、本実施例では、生細胞懸濁液に光源より照射光を照射することにより得得られる、前方散乱光検出強度及び透過率に基づき、生細胞懸濁液に未知の濃度で含まれる生細胞、すなわち細胞生存率を得ることができる。なお、細胞生存率の他、生細胞濃度(CVc)、死細胞濃度(CDc)、生細胞(Vc)数及び死細胞(Dc)数も得ることができる。
CDc≒(CSc+CDc)
本実施例によれば、実施例1の効果に加え、校正曲線(3)を補正し細胞生存率を算出することにより、更に細胞生存率を高精度に計測することが可能となる。
((CVc+CSc+CDc)-CDc)/(CVc+CSc+CDc)
そして、上記算出結果と、CVc/(CVc+CDc)(図11に示すステップS108と同様)とを比較する。比較結果が大きく異なることで、生細胞(Vc)の粒子径に近い大きさの低活性の生細胞(Sc)が、生細胞懸濁液中に多数存在することが判明する。また、ステップS104にて、校正曲線(1)を用いて算出された生細胞濃度(CVc)が正常な値でないことがわかる。そこで校正曲線(1)を補正するための処理に移行する。
(CVc+CSc)/(CVc+CSc+CDc)
本実施例によれば、実施例1の効果に加え、生細胞懸濁液中に低活性の生細胞(Sc)が多数存在する場合においても、細胞生存率を高精度に求めることが可能となる。
Claims (15)
- 細胞懸濁液を通流させる流路と、
前記流路内の細胞懸濁液を送液する液体駆動部と、
前記流路内で流動中の細胞懸濁液に光源より照射光を照射し、得られる前方散乱光強度並びに透過率及び/又は側方散乱光強度に基づき、少なくとも前記細胞懸濁液中の細胞生存率を求める演算部を有することを特徴とする細胞計測機構。 - 請求項1に記載の細胞計測機構において、
前記演算部は、
生細胞濃度と前記前方散乱光強度との関係を示す第1の校正曲線と、死細胞濃度と前記透過率との関係を示す第2の校正曲線と、細胞生存率と前記側方散乱光強度との関係を示す第3の校正曲線と、を予め格納する校正曲線データベースを有することを特徴とする細胞計測機構。 - 請求項2に記載の細胞計測機構において、
前記細胞懸濁液を通流するフローセルと、
前記フローセルを通流する細胞懸濁液の流れの方向に対し直交するよう配される光源と、
前記フローセルを挟み前記光源と対向し、前記光源からの照射光の光軸上に配される透過率検出器と、
前記透過率検出器側であって、前記照射光の光軸に対し前記生細胞の粒子径に応じて所定の角度をもって配される前方散乱光検出器と、
前記細胞懸濁液の流れの方向及び前記照射光の光軸の双方と直交するよう配される側方散乱光検出器と、を有する測定部を備えることを特徴とする細胞計測機構。 - 請求項3に記載の細胞計測機構において、
前記演算部は、
前記前方散乱光検出器により測定される前方散乱光強度及び前記第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度を求め、
前記透過率検出器により測定される透過率及び前記第2の校正曲線に基づき、前記細胞懸濁液中の死細胞濃度を求め、
前記求めた生細胞濃度及び死細胞濃度に基づき、前記細胞懸濁液中の細胞生存率を求めることを特徴とする細胞計測機構。 - 請求項3に記載の細胞計測機構において、
前記前方散乱光検出器は、前記照射光の光軸に対し約5°~45°の角度範囲内に配され、
前記演算部は、前記前方散乱光検出器により測定される前方散乱光強度及び前記第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度又は生細胞数を求めることを特徴とする細胞計測機構。 - 請求項3に記載の細胞計測機構において、
細胞培養液のみを前記フローセルに通流したときに測定される透過率を予めベースラインとして格納し、培養細胞が前記細胞培養液に含まれる前記細胞懸濁液を前記フローセルに通流したときに測定される透過率を、前記ベースラインとの差分として出力することを特徴とする細胞計測機構。 - 請求項3に記載の細胞計測機構において、
前記演算部は、
前記前方散乱光検出器により測定される前方散乱光強度及び前記第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度を求め、
前記透過率検出器により測定される透過率及び前記第2の校正曲線に基づき、前記死細胞濃度を求め、
前記求めた生細胞濃度及び死細胞濃度に基づき、前記第3の校正曲線を補正し、
補正後の第3の校正曲線及び前記側方散乱光検出器により測定される側方散乱光強度に基づき、前記細胞懸濁液中の細胞生存率を求めることを特徴とする細胞計測機構。 - 細胞を培養し増殖させ、増殖させた細胞を剥離する拡大培養機構と、
前記拡大培養機構により剥離された細胞を含む細胞懸濁液を通流させる流路と、
前記流路内の細胞懸濁液を送液する液体駆動部と、
前記流路内で流動中の細胞懸濁液に光源より照射光を照射し、得られる前方散乱光強度並びに透過率及び/又は側方散乱光強度に基づき、少なくとも前記細胞懸濁液中の細胞生存率を算出する演算部を有する細胞計測機構と、を備えることを特徴とする細胞培養装置。 - 請求項8に記載の細胞培養装置において、
前記演算部は、生細胞濃度と前記前方散乱光強度との関係を示す第1の校正曲線と、死細胞濃度と前記透過率との関係を示す第2の校正曲線と、細胞生存率と前記側方散乱光強度との関係を示す第3の校正曲線と、を予め格納する校正曲線データベースを有することを特徴とする細胞培養装置。 - 請求項9に記載の細胞培養装置において、
前記細胞計測機構は、
前記細胞懸濁液を通流するフローセルと、
前記フローセルを通流する細胞懸濁液の流れの方向に対し直交するよう配される光源と、
前記フローセルを挟み前記光源と対向し、前記光源からの照射光の光軸上に配される透過率検出器と、
前記透過率検出器側であって、前記照射光の光軸に対し前記生細胞の粒子径に応じて所定の角度をもって配される前方散乱光検出器と、
前記細胞懸濁液の流れの方向及び前記照射光の光軸の双方と直交するよう配される側方散乱光検出器と、を有する測定部を備えることを特徴とする細胞培養装置。 - 請求項10に記載の細胞培養装置において、
前記前方散乱光検出器は、前記照射光の光軸に対し約5°~45°の角度範囲内に配され、
前記演算部は、前記前方散乱光検出器により測定される前方散乱光強度及び前記第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度又は生細胞数を求めることを特徴とする細胞培養装置。 - 請求項10に記載の細胞培養装置において、
細胞培養液のみを前記フローセルに通流したときに測定される透過率を予めベースラインとして格納し、培養細胞が前記細胞培養液に含まれる前記細胞懸濁液を前記フローセルに通流したときに測定される透過率を、前記ベースラインとの差分として出力することを特徴とする細胞培養装置。 - 請求項10に記載の細胞培養装置において、
前記演算部は、
前記前方散乱光検出器により測定される前方散乱光強度及び前記第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度を求め、
前記透過率検出器により測定される透過率及び前記第2の校正曲線に基づき、前記死細胞濃度を求め、
前記求めた生細胞濃度及び死細胞濃度に基づき、前記第3の校正曲線を補正し、
補正後の第3の校正曲線及び前記側方散乱光検出器により測定される側方散乱光強度に基づき、前記細胞懸濁液中の細胞生存率を求めることを特徴とする細胞培養装置。 - 細胞懸濁液中の少なくとも細胞生存率を求める細胞計測方法であって、
フローセル内を通流する細胞懸濁液に対し、前記細胞懸濁液の流れに直交する方向より光源から照射光を照射する工程と、
前記細胞懸濁液より散乱する前方散乱光強度を測定する工程と、
前記細胞懸濁液を透過する前記照射光の透過率を測定する工程と、
前記測定された前方散乱光強度及び予め記憶された生細胞濃度と前記前方散乱光強度との関係を示す第1の校正曲線に基づき、前記細胞懸濁液中の生細胞濃度を求める工程と、
前記測定された前記透過率及び予め記憶された死細胞濃度と前記透過率との関係を示す第2の校正曲線に基づき、前記細胞懸濁液中の死細胞濃度を求める工程と、
前記求めた生細胞濃度及び死細胞濃度に基づき、前記細胞懸濁液中の細胞生存率を求める工程と、を有することを特徴とする細胞計測方法。 - 請求項14に記載の細胞計測方法において、
前記細胞懸濁液より、前記照射光の光軸及び前記細胞懸濁液の流れの方向に直交する方向へ散乱する側方散乱光強度を測定する工程と、
前記求めた生細胞濃度及び死細胞濃度に基づき、予め記憶された細胞生存率と前記側方散乱光強度との関係を示す第3の校正曲線を補正する工程と、
前記補正された第3の校正曲線及び前記側方散乱光強度に基づき、前記細胞懸濁液中の細胞生存率を求める工程と、を更に備えることを特徴とする細胞計測方法。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201480082245.1A CN107075437B (zh) | 2014-10-22 | 2014-10-22 | 细胞测量机构和具有该细胞测量机构的细胞培养装置以及细胞测量方法 |
| US15/518,041 US10456767B2 (en) | 2014-10-22 | 2014-10-22 | Cytometric mechanism, cell culture device comprising same, and cytometric method |
| PCT/JP2014/078033 WO2016063364A1 (ja) | 2014-10-22 | 2014-10-22 | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
| JP2016554992A JP6367351B2 (ja) | 2014-10-22 | 2014-10-22 | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2014/078033 WO2016063364A1 (ja) | 2014-10-22 | 2014-10-22 | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016063364A1 true WO2016063364A1 (ja) | 2016-04-28 |
Family
ID=55760437
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/078033 WO2016063364A1 (ja) | 2014-10-22 | 2014-10-22 | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US10456767B2 (ja) |
| JP (1) | JP6367351B2 (ja) |
| CN (1) | CN107075437B (ja) |
| WO (1) | WO2016063364A1 (ja) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108728344A (zh) * | 2017-04-19 | 2018-11-02 | 阿自倍尔株式会社 | 细胞生存率判定装置以及细胞生存率判定方法 |
| CN108801991A (zh) * | 2017-04-26 | 2018-11-13 | 阿自倍尔株式会社 | 细胞生存率判定装置 |
| JP2020525800A (ja) * | 2017-07-05 | 2020-08-27 | サウジ アラビアン オイル カンパニー | ガスフローラインにおける黒色粉末濃度の光学的検知 |
| WO2021049117A1 (ja) * | 2019-09-12 | 2021-03-18 | シンフォニアテクノロジー株式会社 | 細胞培養装置 |
| WO2023022091A1 (ja) * | 2021-08-16 | 2023-02-23 | 株式会社ニコン | 解析システム、観察容器、解析方法およびプログラム |
| WO2023171252A1 (ja) * | 2022-03-08 | 2023-09-14 | キヤノン株式会社 | 培養装置、細胞密度測定装置、および細胞密度測定方法 |
| WO2023189395A1 (ja) * | 2022-03-28 | 2023-10-05 | テルモ株式会社 | 細胞培養装置及び校正方法 |
| WO2024181472A1 (ja) * | 2023-02-28 | 2024-09-06 | テルモ株式会社 | 推定装置及び推定方法 |
| WO2024181471A1 (ja) * | 2023-02-28 | 2024-09-06 | テルモ株式会社 | 細胞移送方法及び細胞移送装置 |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016063364A1 (ja) * | 2014-10-22 | 2016-04-28 | 株式会社日立ハイテクノロジーズ | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
| EP3505613B1 (en) * | 2017-12-27 | 2024-07-03 | Industrial Technology Research Institute | Cell culture module, cell culture system and cell culture method |
| CN108318436B (zh) * | 2018-02-06 | 2021-05-04 | 迈克医疗电子有限公司 | 反应曲线生成方法、装置及光学检测系统 |
| CN114514309B (zh) * | 2019-09-26 | 2024-05-24 | 京瓷株式会社 | 细胞检测装置和细胞检测方法 |
| US11268891B2 (en) * | 2020-06-17 | 2022-03-08 | Kidde Technologies, Inc. | Fire extinguishing agent concentration measuring system and method |
| US20220349873A1 (en) * | 2021-04-30 | 2022-11-03 | Avita Medical, Inc. | Cell suspension composition with therapeutic potential and related methods and systems for identifying same |
| US20240085306A1 (en) * | 2021-09-29 | 2024-03-14 | Nitto Boseki Co., Ltd. | Method for enriching cells or cell nuclei |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02500297A (ja) * | 1986-08-12 | 1990-02-01 | アンダーソン,ジェフリー,イー. | 単核白血球免疫システムの免疫調節状態の自動的評価のための方法及び装置 |
| JPH03503808A (ja) * | 1989-05-01 | 1991-08-22 | クールター コーポレーション | 粒子検出分類装置及び方法 |
| JP2006220423A (ja) * | 2005-02-08 | 2006-08-24 | Japan Science & Technology Agency | ゲル電極付セルソーターチップ |
| JP2012507008A (ja) * | 2008-10-24 | 2012-03-22 | ユニヴァーシティー オブ ノートル ダム デュ ラック | 懸濁している粒子の情報を得る方法及び装置 |
| JP2012143231A (ja) * | 2010-12-22 | 2012-08-02 | Toyama Univ | 非球体細胞の生死活性判定方法及び判定装置 |
| JP2013522644A (ja) * | 2010-03-24 | 2013-06-13 | ベックマン コールター, インコーポレイテッド | 血液サンプルを分析するための方法およびシステム |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH549796A (de) * | 1971-03-29 | 1974-05-31 | Sigrist Willy | Verfahren zur messung von in einer fluessigkeit suspendierten stoffen und einrichtung zur ausfuehrung des verfahrens. |
| US4286876A (en) * | 1979-01-02 | 1981-09-01 | Coulter Electronics, Inc. | Apparatus and method for measuring scattering of light in particle detection systems |
| US4595291A (en) * | 1982-10-15 | 1986-06-17 | Tokyo Shibaura Denki Kabushiki Kaisha | Particle diameter measuring device |
| US4676641A (en) * | 1986-01-08 | 1987-06-30 | Coulter Electronics Of New England, Inc. | System for measuring the size distribution of particles dispersed in a fluid |
| JP2635126B2 (ja) * | 1988-09-30 | 1997-07-30 | 東亜医用電子株式会社 | 核の分葉指数を求めるための粒子分析装置及び方法 |
| US4953978A (en) * | 1989-03-03 | 1990-09-04 | Coulter Electronics Of New England, Inc. | Particle size analysis utilizing polarization intensity differential scattering |
| US5812419A (en) * | 1994-08-01 | 1998-09-22 | Abbott Laboratories | Fully automated analysis method with optical system for blood cell analyzer |
| GB9818348D0 (en) * | 1998-08-22 | 1998-10-14 | Malvern Instr Ltd | Improvements relating to the measurement of particle size distribution |
| JP3393817B2 (ja) * | 1998-10-16 | 2003-04-07 | 株式会社堀場製作所 | 粒径分布測定装置 |
| US6507400B1 (en) * | 1999-02-27 | 2003-01-14 | Mwi, Inc. | Optical system for multi-part differential particle discrimination and an apparatus using the same |
| US6646742B1 (en) * | 2000-02-19 | 2003-11-11 | Mwi, Inc. | Optical device and method for multi-angle laser light scatter |
| US6859276B2 (en) * | 2003-01-24 | 2005-02-22 | Coulter International Corp. | Extracted polarization intensity differential scattering for particle characterization |
| DK2305832T3 (da) * | 2003-03-28 | 2022-05-23 | Inguran Llc | Fremgangsmåde til tilvejebringelse af kønssorteret dyresæd |
| US7745221B2 (en) * | 2003-08-28 | 2010-06-29 | Celula, Inc. | Methods and apparatus for sorting cells using an optical switch in a microfluidic channel network |
| US8634072B2 (en) * | 2004-03-06 | 2014-01-21 | Michael Trainer | Methods and apparatus for determining characteristics of particles |
| EP2522982B1 (en) * | 2007-08-15 | 2015-09-16 | Malvern Instruments Limited | Broad-Range Spectrometer |
| JP5323829B2 (ja) | 2008-06-27 | 2013-10-23 | 古河電気工業株式会社 | 細胞の識別およびソーティング方法およびその装置 |
| WO2010035775A1 (ja) * | 2008-09-26 | 2010-04-01 | 株式会社堀場製作所 | 粒子物性測定装置 |
| US8906309B2 (en) | 2009-04-27 | 2014-12-09 | Abbott Laboratories | Method for discriminating red blood cells from white blood cells by using forward scattering from a laser in an automated hematology analyzer |
| US9001200B2 (en) | 2010-01-12 | 2015-04-07 | Bio-Rad Laboratories, Inc. | Cell characterization using multiple focus planes |
| GB2494733A (en) * | 2011-09-14 | 2013-03-20 | Malvern Instr Ltd | Measuring particle size distribution by light scattering |
| WO2014034275A1 (ja) * | 2012-08-30 | 2014-03-06 | 株式会社 日立ハイテクノロジーズ | 核酸分析装置 |
| JP2014148636A (ja) * | 2013-02-04 | 2014-08-21 | Polyplastics Co | 樹脂組成物及びインサート成形品 |
| JP6322711B2 (ja) * | 2014-07-22 | 2018-05-09 | 株式会社日立ハイテクノロジーズ | 細胞数濃度調整装置およびそれを用いた自動継代培養システム |
| WO2016063364A1 (ja) * | 2014-10-22 | 2016-04-28 | 株式会社日立ハイテクノロジーズ | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 |
-
2014
- 2014-10-22 WO PCT/JP2014/078033 patent/WO2016063364A1/ja active Application Filing
- 2014-10-22 CN CN201480082245.1A patent/CN107075437B/zh active Active
- 2014-10-22 JP JP2016554992A patent/JP6367351B2/ja active Active
- 2014-10-22 US US15/518,041 patent/US10456767B2/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02500297A (ja) * | 1986-08-12 | 1990-02-01 | アンダーソン,ジェフリー,イー. | 単核白血球免疫システムの免疫調節状態の自動的評価のための方法及び装置 |
| JPH03503808A (ja) * | 1989-05-01 | 1991-08-22 | クールター コーポレーション | 粒子検出分類装置及び方法 |
| JP2006220423A (ja) * | 2005-02-08 | 2006-08-24 | Japan Science & Technology Agency | ゲル電極付セルソーターチップ |
| JP2012507008A (ja) * | 2008-10-24 | 2012-03-22 | ユニヴァーシティー オブ ノートル ダム デュ ラック | 懸濁している粒子の情報を得る方法及び装置 |
| JP2013522644A (ja) * | 2010-03-24 | 2013-06-13 | ベックマン コールター, インコーポレイテッド | 血液サンプルを分析するための方法およびシステム |
| JP2012143231A (ja) * | 2010-12-22 | 2012-08-02 | Toyama Univ | 非球体細胞の生死活性判定方法及び判定装置 |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108728344A (zh) * | 2017-04-19 | 2018-11-02 | 阿自倍尔株式会社 | 细胞生存率判定装置以及细胞生存率判定方法 |
| CN108801991A (zh) * | 2017-04-26 | 2018-11-13 | 阿自倍尔株式会社 | 细胞生存率判定装置 |
| JP2020525800A (ja) * | 2017-07-05 | 2020-08-27 | サウジ アラビアン オイル カンパニー | ガスフローラインにおける黒色粉末濃度の光学的検知 |
| WO2021049117A1 (ja) * | 2019-09-12 | 2021-03-18 | シンフォニアテクノロジー株式会社 | 細胞培養装置 |
| WO2023022091A1 (ja) * | 2021-08-16 | 2023-02-23 | 株式会社ニコン | 解析システム、観察容器、解析方法およびプログラム |
| WO2023171252A1 (ja) * | 2022-03-08 | 2023-09-14 | キヤノン株式会社 | 培養装置、細胞密度測定装置、および細胞密度測定方法 |
| WO2023189395A1 (ja) * | 2022-03-28 | 2023-10-05 | テルモ株式会社 | 細胞培養装置及び校正方法 |
| WO2024181472A1 (ja) * | 2023-02-28 | 2024-09-06 | テルモ株式会社 | 推定装置及び推定方法 |
| WO2024181471A1 (ja) * | 2023-02-28 | 2024-09-06 | テルモ株式会社 | 細胞移送方法及び細胞移送装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107075437B (zh) | 2019-12-03 |
| JPWO2016063364A1 (ja) | 2017-06-22 |
| US10456767B2 (en) | 2019-10-29 |
| JP6367351B2 (ja) | 2018-08-08 |
| CN107075437A (zh) | 2017-08-18 |
| US20170306287A1 (en) | 2017-10-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6367351B2 (ja) | 細胞計測機構及びそれを有する細胞培養装置並びに細胞計測方法 | |
| Sivandzade et al. | Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe | |
| Sharma et al. | Micro-flow imaging: flow microscopy applied to sub-visible particulate analysis in protein formulations | |
| Brito et al. | Andrology laboratory review: Evaluation of sperm concentration | |
| US10858621B2 (en) | Cell dispersion measurement mechanism, and cell subculture system utilizing same | |
| EP1161671B1 (en) | Determination of sperm concentration and viability for artificial insemination | |
| CA2927638C (en) | Methods and compositions for assessing spermatozoa in a semen sample | |
| CN101008641A (zh) | 幼稚白细胞分析用试剂及试剂盒 | |
| CN102089427B (zh) | 细胞分散方法、细胞分散剂及细胞测定方法 | |
| JP2014209090A (ja) | 細胞分析方法及び装置 | |
| US9017996B2 (en) | Bacteria analyzer, method for analyzing bacteria, and a computer program product | |
| Pierce et al. | Outcomes from a cell viability workshop: fit-for-purpose considerations for cell viability measurements for cellular therapeutic products | |
| JP2024096909A (ja) | 生細胞数計測方法および生細胞数計測装置 | |
| Corradini et al. | Methods for characterization/manipulation of human corneal stem cells and their applications in regenerative medicine | |
| CN104897630B (zh) | 一种检测人精子活力的方法 | |
| WO2019035462A1 (ja) | 細胞培養容器において培養される多能性幹細胞の未分化状態を位置特異的に判定する方法、多能性幹細胞の継代培養方法およびそれら方法に使用される装置 | |
| KR102118533B1 (ko) | 세포 생존율 판정 장치 | |
| KR102110288B1 (ko) | 세포 생존율 판정 장치 및 세포 생존율 판정 방법 | |
| JP2021092470A (ja) | スペクトル測定装置およびスペクトル測定方法 | |
| JPH02281131A (ja) | 微生物細胞の生死判別装置 | |
| TW202116999A (zh) | 細胞培養監測裝置及細胞培養系統 | |
| Dadgar et al. | Development of robust cell therapy manufacturing processes largely depends on the choice of cell counting method considering cell type, precision, and accuracy requirements | |
| US20100075370A1 (en) | Method for determination of cell viability by using flow cytometry with fixed volume acquisition | |
| Hawkins | A systematic review of the use of flow cytometry to quantify sperm sample quality in ruminants | |
| CN210736776U (zh) | 一种培养检测仪 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14904480 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2016554992 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15518041 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14904480 Country of ref document: EP Kind code of ref document: A1 |