WO2012117763A1 - Silica dispersion composition - Google Patents
Silica dispersion composition Download PDFInfo
- Publication number
- WO2012117763A1 WO2012117763A1 PCT/JP2012/051154 JP2012051154W WO2012117763A1 WO 2012117763 A1 WO2012117763 A1 WO 2012117763A1 JP 2012051154 W JP2012051154 W JP 2012051154W WO 2012117763 A1 WO2012117763 A1 WO 2012117763A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- silica
- dispersion composition
- polyurethane resin
- mass
- Prior art date
Links
- AYJGDLVQTQYBFM-UHFFFAOYSA-N OC12OC1CCC2 Chemical compound OC12OC1CCC2 AYJGDLVQTQYBFM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/08—Processes
- C08G18/0804—Manufacture of polymers containing ionic or ionogenic groups
- C08G18/0833—Manufacture of polymers containing ionic or ionogenic groups containing cationic or cationogenic groups together with anionic or anionogenic groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/32—Polyhydroxy compounds; Polyamines; Hydroxyamines
- C08G18/3203—Polyhydroxy compounds
- C08G18/3206—Polyhydroxy compounds aliphatic
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/32—Polyhydroxy compounds; Polyamines; Hydroxyamines
- C08G18/3271—Hydroxyamines
- C08G18/3275—Hydroxyamines containing two hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/34—Carboxylic acids; Esters thereof with monohydroxyl compounds
- C08G18/348—Hydroxycarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/42—Polycondensates having carboxylic or carbonic ester groups in the main chain
- C08G18/4266—Polycondensates having carboxylic or carbonic ester groups in the main chain prepared from hydroxycarboxylic acids and/or lactones
- C08G18/4269—Lactones
- C08G18/4277—Caprolactone and/or substituted caprolactone
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/65—Low-molecular-weight compounds having active hydrogen with high-molecular-weight compounds having active hydrogen
- C08G18/66—Compounds of groups C08G18/42, C08G18/48, or C08G18/52
- C08G18/6633—Compounds of group C08G18/42
- C08G18/6637—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38
- C08G18/6648—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3225 or C08G18/3271 and/or polyamines of C08G18/38
- C08G18/6655—Compounds of group C08G18/42 with compounds of group C08G18/32 or polyamines of C08G18/38 with compounds of group C08G18/3225 or C08G18/3271 and/or polyamines of C08G18/38 with compounds of group C08G18/3271
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/67—Unsaturated compounds having active hydrogen
- C08G18/671—Unsaturated compounds having only one group containing active hydrogen
- C08G18/672—Esters of acrylic or alkyl acrylic acid having only one group containing active hydrogen
- C08G18/673—Esters of acrylic or alkyl acrylic acid having only one group containing active hydrogen containing two or more acrylate or alkylacrylate ester groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/73—Polyisocyanates or polyisothiocyanates acyclic
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/76—Polyisocyanates or polyisothiocyanates cyclic aromatic
- C08G18/7657—Polyisocyanates or polyisothiocyanates cyclic aromatic containing two or more aromatic rings
- C08G18/7664—Polyisocyanates or polyisothiocyanates cyclic aromatic containing two or more aromatic rings containing alkylene polyphenyl groups
- C08G18/7671—Polyisocyanates or polyisothiocyanates cyclic aromatic containing two or more aromatic rings containing alkylene polyphenyl groups containing only one alkylene bisphenyl group
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/0047—Photosensitive materials characterised by additives for obtaining a metallic or ceramic pattern, e.g. by firing
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/027—Non-macromolecular photopolymerisable compounds having carbon-to-carbon double bonds, e.g. ethylenic compounds
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03F—PHOTOMECHANICAL PRODUCTION OF TEXTURED OR PATTERNED SURFACES, e.g. FOR PRINTING, FOR PROCESSING OF SEMICONDUCTOR DEVICES; MATERIALS THEREFOR; ORIGINALS THEREFOR; APPARATUS SPECIALLY ADAPTED THEREFOR
- G03F7/00—Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefor, e.g. comprising photoresists; Apparatus specially adapted therefor
- G03F7/004—Photosensitive materials
- G03F7/038—Macromolecular compounds which are rendered insoluble or differentially wettable
- G03F7/0388—Macromolecular compounds which are rendered insoluble or differentially wettable with ethylenic or acetylenic bands in the side chains of the photopolymer
Definitions
- the present invention relates to a silica dispersion composition suitably used for solder resist and the like.
- a photosensitive film in which a photosensitive layer is formed by applying a silica dispersion composition on a support and drying it has been used.
- a method for forming a permanent pattern such as a solder resist for example, a photosensitive film is laminated on a substrate such as a copper-clad laminate on which a permanent pattern is formed to form a laminate, and the photosensitive layer in the laminate is formed.
- a method of forming a permanent pattern by performing exposure on the substrate, developing the photosensitive layer after the exposure to form a pattern, and then performing a curing process or the like.
- a pigment dispersant which is a polyurethane resin having one or more nonionic polar molecular chains, (B) a low polar molecular chain, and (C) an active energy ray-curable unsaturated group.
- this proposal relates to a dispersant for carbon black that is suitably used for photoresists that form images by photolithography, and is suitable for solder resists that can be used for semiconductor package substrates with a small L / S (line space).
- the proposed pigment dispersant has a graft chain modified to a basic functional group, the amine value is lowered, silica dispersibility is lowered, melt viscosity is increased, and transferability is inferior. There's a problem.
- silica dispersion composition capable of obtaining a high-performance cured film promptly.
- the present invention can obtain a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulating property, and resolution of an exposed portion, and a semiconductor package substrate having a small L / S (line space).
- An object of the present invention is to provide a silica dispersion composition suitable for a solder resist that can cope with the above.
- the present inventors have made extensive studies, and as a result, contain a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent,
- the silica dispersant preferably has a graft chain in at least one of a side chain and a terminal, and has an amine value of 0.65 mmol / g or more, so that silica fine particles can be highly filled, melt viscosity, and developability. It is possible to obtain a high-performance cured film that has an excellent insulating property and an excellent resolution of the exposed portion, and has a small L / S (line space). It has been found that a silica dispersion composition suitable for a solder resist that can be applied to a semiconductor package substrate can be obtained.
- the silica dispersion composition of the present invention comprises a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent,
- the amine value of the silica dispersant is 0.65 mmol / g or more.
- a silica dispersion composition suitable for a solder resist that can be used for a semiconductor package substrate having a small (line space) can be provided.
- the silica dispersion composition of the present invention contains a silica dispersant, silica fine particles, and a thermal crosslinking agent, and contains a binder, a polymerizable compound, a photopolymerization initiator, and other components as necessary. It becomes.
- the silica dispersant is a dispersant comprising a polyurethane resin having at least an acidic group and a basic group in one molecule, and preferably having a graft chain and a polymerizable group.
- the silica dispersant is made of a polyurethane resin whose main chain is polyurethane, and is bonded to the main chain or bonded to a side chain bonded to the main chain, or a molecular chain constituting the main chain As above, it has an acidic group and a basic group, and preferably has a graft chain and a polymerizable group.
- the acidic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a carboxyl group, a sulfo group, a phosphonyl group, and —COCH 2 CO—R (where R is a carbon number of 1 to 10).
- -CONHCO-R wherein R represents a hydrocarbon group having 1 to 10 carbon atoms
- -COCH 2 CN a phenolic hydroxyl group
- -R F CH 2 OH where R F is A perfluoroalkyl group
- R F represents a perfluoroalkyl group
- a carboxyl group is particularly preferable from the viewpoint of developability.
- the basic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a primary amino group, a secondary amino group, a tertiary amino group, and a nitrogen-containing heterocycle. However, since the primary amino group and the secondary amino group react with the isocyanate and are taken into the main chain, a tertiary amino group and a nitrogen-containing heterocyclic ring are preferable, and the tertiary amino group is particularly preferable. preferable. Examples of the tertiary amino group include a dimethylamino group and a diethylamino group.
- nitrogen-containing heterocycle examples include 2-pyrrolidone, N-methyl-2-pyrrolidone, 1,3-dimethylimidazolidinone, ⁇ -caprolactam, ⁇ -butyrolactone, and the like.
- the graft chain preferably has at least one of a side chain and a terminal of the polyurethane resin.
- the graft chain include polyester, polymethyl methacrylate, polyethylene oxide, and polystyrene.
- a graft chain having a polyester moiety at the terminal of the polyurethane resin is preferable from the viewpoint of resolution.
- the degree of polymerization of the graft chain is preferably 50 or less, more preferably 5 to 30.
- the content of the graft is preferably 10% by mass to 60% by mass and more preferably 20% by mass to 50% by mass with respect to the entire polyurethane resin.
- polymerizable (crosslinkable) groups There is no restriction
- the polyurethane resin constituting the silica dispersant is a polyurethane resin obtained by reacting an isocyanate compound with a compound having a hydroxyl group or an active hydrogen atom, specifically, a diol compound having an acidic group and a basic group. It is obtained by reacting a diol compound having a diisocyanate compound.
- the diol compound having an acidic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 3,5-dihydroxybenzoic acid, 2,2-bis (hydroxymethyl) propionic acid, 2, 2-bis (2-hydroxyethyl) propionic acid, 2,2-bis (3-hydroxypropyl) propionic acid, bis (hydroxymethyl) acetic acid, bis (4-hydroxyphenyl) acetic acid, 2,2-bis (hydroxymethyl) ) Butyric acid, 4,4-bis (4-hydroxyphenyl) pentanoic acid, tartaric acid, N, N-dihydroxyethylglycine, N, N-bis (2-hydroxyethyl) -3-carboxy-propionamide and the like. These may be used individually by 1 type and may use 2 or more types together.
- the diol compound having a basic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 3- (dimethylamino) -1,2-propanediol. Unlike the graft chain having an amino group, the diol compound having a basic group has a high amine content in the monomer, so that the amine value of the silica dispersant can be increased.
- the diisocyanate compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 2,4-tolylene diisocyanate, dimer of 2,4-tolylene diisocyanate, and 2,6-tolylene diene. Range isocyanate, p-xylylene diisocyanate, m-xylylene diisocyanate, 4,4'-diphenylmethane diisocyanate (MDI), 1,5-naphthylene diisocyanate, 3,3'-dimethylbiphenyl-4,4'-diisocyanate, etc.
- MDI 4,4'-diphenylmethane diisocyanate
- Aromatic diisocyanate compounds aliphatic diisocyanate compounds such as hexamethylene diisocyanate, trimethylhexamethylene diisocyanate, lysine diisocyanate, dimer acid diisocyanate; isophorone diisocyanate, 4,4′-methyle Alicyclic diisocyanate compounds such as bis (cyclohexyl isocyanate), methylcyclohexane-2,4 (or 2,6) diisocyanate, 1,3- (isocyanatomethyl) cyclohexane; 1 mol of 1,3-butylene glycol and tolylene diisocyanate 2
- a diisocyanate compound which is a reaction product of a diol such as an adduct with a mole and a diisocyanate; These may be used individually by 1 type and may use 2 or more types together.
- 4,4'-diphenylmethane diisocyanate (MDI) is particularly preferable from the viewpoint of hard
- a terminal blocking agent When synthesizing the polyurethane resin of the present invention, a terminal blocking agent may be added.
- the end capping agent include monoalcohol compounds, monoisocyanate compounds, polyesters having alcohol at one end, and polyethylene oxides having alcohol at one end. These may be used individually by 1 type and may use 2 or more types together.
- silica dispersant an appropriately synthesized one or a commercially available product may be used.
- the amine value of the silica dispersant is 0.65 mmol / g or more, preferably 0.75 mmol / g or more, and more preferably 0.9 mmol / g or more. If the amine value is less than 0.65 mmol / g, the melt viscosity may increase and the embedding property may deteriorate.
- the amine value is measured, for example, by weighing a sample in a beaker, adding acetic acid, stirring and dissolving, adjusting the measurement temperature to 25 ° C., and then adding 0.1N perchloric acid acetic acid as a titration reagent. And can be determined by titrating with a titration apparatus.
- the acid value of the silica dispersant is preferably 0.3 mmol / g or more, and more preferably 0.45 mmol / g or more. If the acid value is less than 0.3 mmol / g, developability may deteriorate and a residue may be generated in an unexposed area.
- silica fine particles There is no restriction
- the average particle diameter (d50) of the silica fine particles is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.2 ⁇ m to 3.0 ⁇ m, more preferably 0.3 ⁇ m to 2.5 ⁇ m, 0.5 ⁇ m to 2.5 ⁇ m is particularly preferable.
- the average particle diameter (d50) of the silica particles is less than 0.2 ⁇ m, the coating viscosity may be increased, and when it exceeds 3.0 ⁇ m, smoothness may not be maintained.
- the average particle diameter (d50) of the silica particles is within the particularly preferable range, it is advantageous in terms of coating viscosity, smoothness of the cured film and heat resistance.
- D 50 the average particle size of the silica particles
- D 50 the integration
- D 50 the integration
- D 50 the size distribution analysis method
- the content of the silica fine particles in the silica dispersion composition is preferably 16% by mass to 80% by mass, and more preferably 25% by mass to 70% by mass. When the content is less than 16% by mass, impact resistance may be inferior, and when it exceeds 80% by mass, dispersibility may be insufficient.
- thermo crosslinking agent There is no restriction
- the epoxy resin is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0095] of JP-A-2007-2030, and JP-A-2010-72340. Examples include the compounds described in paragraph [0130].
- the polyfunctional oxetane compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include the compounds described in paragraph [0096] of JP-A-2007-2030.
- the content of the thermal crosslinking agent in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 1% by mass to 50% by mass, and 2% by mass to 40% by mass. Is more preferable, and 3% by mass to 30% by mass is particularly preferable.
- the content is less than 1% by mass, heat resistance may be inferior, and when it exceeds 50% by mass, developability and crack resistance may be inferior.
- a cured film can be produced with good sensitivity, and the formed cured film is advantageous in that both heat resistance and crack resistance can be achieved.
- the other thermal crosslinking agent can be added separately from the epoxy resin and the polyfunctional oxetane compound.
- the other thermal crosslinking agent is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0098] to [0100] of JP-A-2007-2030. Can be mentioned.
- the binder is not particularly limited and may be appropriately selected depending on the intended purpose.
- acid-modified vinyl group-containing polyurethane resin, unsaturated group-containing polycarboxylic acid resin, acid-modified vinyl group-containing epoxy resin, unsaturated Examples thereof include a resin containing a group and a carboxyl group, and a polyimide precursor.
- the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the purpose.
- a polyurethane resin having an ethylenically unsaturated bond (vinyl group) in the side chain ii) A polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule.
- Polyurethane resin having vinyl groups in the side chain--- The urethane resin having a vinyl group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose.
- the side chain is represented by the following general formulas (1) to (3). The thing which has at least 1 among functional groups is mentioned.
- R 1 is not particularly limited and may be appropriately selected depending on the intended purpose.
- examples thereof include a hydrogen atom and an alkyl group which may have a substituent. Among these, a hydrogen atom and a methyl group are preferable in terms of high radical reactivity.
- the R 2 and R 3 are not particularly limited and may be appropriately selected depending on the purpose.
- each of R 2 and R 3 is independently a hydrogen atom, a halogen atom, an amino group, a carboxyl group, an alkoxycarbonyl group, a sulfo group.
- a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
- X represents an oxygen atom, a sulfur atom, or —N (R 12 ) —
- R 12 represents a hydrogen atom or a monovalent organic group.
- R 12 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group which may have a substituent.
- a hydrogen atom, a methyl group, an ethyl group, and an isopropyl group are preferable because of high radical reactivity.
- the substituent that can be introduced is not particularly limited and may be appropriately selected depending on the intended purpose.
- examples thereof include an alkyl group, an alkenyl group, an alkynyl group, an aryl group, an alkoxy group, an aryloxy group, and a halogen atom.
- R 4 to R 8 are not particularly limited and may be appropriately selected depending on the intended purpose.
- An arylsulfonyl group etc. are mentioned.
- a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
- Examples of the substituent that can be introduced include the same as those in the general formula (1).
- Y represents an oxygen atom, a sulfur atom, or —N (R 12 ) —.
- Said R ⁇ 12 > is synonymous with the case of R ⁇ 12 > of the said General formula (1), and its preferable example is also the same.
- R 9 is not particularly limited and may be appropriately selected depending on the purpose.
- examples thereof include a hydrogen atom or an alkyl group which may have a substituent. Among these, a hydrogen atom and a methyl group are preferable in terms of high radical reactivity.
- R 10 and R 11 are not particularly limited and may be appropriately selected depending on the intended purpose.
- a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
- Z represents an oxygen atom, a sulfur atom, —N (R 13 ) —, or an optionally substituted phenylene group.
- R 13 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group which may have a substituent. Among these, a methyl group, an ethyl group, and an isopropyl group are preferable because of high radical reactivity.
- the urethane resin having an ethylenically unsaturated bond in the side chain includes at least one diisocyanate compound represented by the following general formula (4) and at least one diol compound represented by the following general formula (5):
- At least one of the diisocyanate compound represented by the general formula (4) and the diol compound represented by the general formula (5) is a group represented by the general formulas (1) to (3). If at least one of them is present, a polyurethane resin in which the groups represented by the above general formulas (1) to (3) are introduced into the side chain as a reaction product of the diisocyanate compound and the diol compound is provided. Generated. According to such a method, a polyurethane resin in which the groups represented by the general formulas (1) to (3) are introduced into the side chain can be easily used, rather than replacing and introducing a desired side chain after the reaction of the polyurethane resin. Can be manufactured.
- the diisocyanate compound represented by the general formula (4) is not particularly limited and can be appropriately selected depending on the purpose.
- a triisocyanate compound and a monofunctional alcohol having an unsaturated group Or the product obtained by addition-reacting with 1 equivalent of monofunctional amine compounds is mentioned.
- the triisocyanate compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0034] to [0035] of JP-A-2005-250438. Is mentioned.
- the diol compound represented by the general formula (5) is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include polyether diol compounds, polyester diol compounds, and polycarbonate diol compounds.
- the monofunctional alcohol having an unsaturated group or the monofunctional amine compound is not particularly limited and may be appropriately selected depending on the intended purpose.
- paragraphs of JP-A-2005-250438 Examples thereof include compounds described in [0037] to [0040].
- the method for introducing an unsaturated group into the side chain of the polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose.
- a method using a diisocyanate compound containing is preferable.
- diisocyanate compounds that can be obtained include compounds having an unsaturated group in the side chain described in paragraphs [0042] to [0049] of JP-A-2005-250438.
- the polyurethane resin having an ethylenically unsaturated bond in the side chain is a diisocyanate containing the unsaturated group from the viewpoint of improving compatibility with other components in the polymerizable composition and improving storage stability.
- Diisocyanate compounds other than the compounds can also be copolymerized.
- the diisocyanate compound to be copolymerized is not particularly limited and may be appropriately selected depending on the intended purpose.
- it is a diisocyanate compound represented by the following general formula (6).
- OCN-L 1 -NCO General formula (6) L 1 represents an aliphatic or aromatic hydrocarbon group which may have a substituent. If necessary, L 1 may have another functional group that does not react with an isocyanate group, for example, an ester, urethane, amide, or ureido group.
- the diisocyanate compound represented by the general formula (6) is not particularly limited and may be appropriately selected depending on the intended purpose.
- Aromatic diisocyanate compounds such as' -diisocyanate; aliphatic diisocyanate compounds such as hexamethylene diisocyanate, trimethylhexamethylene diisocyanate, lysine diisocyanate, dimer diisocyanate; isophorone diisocyanate, 4,4 -Alicyclic diisocyanate compounds such as methylenebis (cyclohexyl
- a method for introducing an unsaturated group into the side chain of the polyurethane resin in addition to the above-described method, a method using a diol compound containing an unsaturated group in the side chain as a raw material for producing a polyurethane resin is also preferable.
- the diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the purpose. For example, a commercially available product such as trimethylolpropane monoallyl ether may be used.
- a compound such as a halogenated diol compound, a triol compound or an aminodiol compound and a compound containing an unsaturated group such as a carboxylic acid, acid chloride, isocyanate, alcohol, amine, thiol or halogenated alkyl compound. It may be a compound that is easily produced.
- the diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0057] to [0060] of JP-A-2005-250438 And the compounds described in paragraphs [0064] to [0066] of JP-A-2005-250438 represented by the following general formula (G).
- R 1 to R 3 each independently represents a hydrogen atom or a monovalent organic group
- A represents a divalent organic residue
- X represents an oxygen atom or a sulfur atom.
- —N (R 12 ) — wherein R 12 represents a hydrogen atom or a monovalent organic group.
- R 1 ⁇ R 3 and X in the general formula (G) said a general formula (1) the same meaning as R 1 ⁇ R 3 and X in preferred embodiments versa.
- the effect of suppressing the excessive molecular movement of the polymer main chain caused by the secondary alcohol having a large steric hindrance can be reduced. It is thought that improvement can be achieved.
- the polyurethane resin having an ethylenically unsaturated bond in the side chain is unsaturated in the side chain from the viewpoint of improving compatibility with other components in the polymerizable composition and improving storage stability, for example.
- a diol compound other than a diol compound containing a group can be copolymerized.
- diol compounds other than the diol compound containing an unsaturated group in the said side chain For example, a polyether diol compound, a polyester diol compound, a polycarbonate diol compound etc. can be selected. Is mentioned.
- the polyether diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0068] to [0076] of JP-A-2005-250438. It is done.
- the polyester diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include paragraphs [0077] to [0079] and paragraphs [0083] to [0085] of JP-A-2005-250438. No. 1-No. 8 and no. 13-No. 18 and the like.
- the polycarbonate diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in the paragraphs [0080] to [0081] and paragraph [0084] of JP-A-2005-250438, No. 9-No. 12 and the like.
- the diol compound which has a substituent which does not react with an isocyanate group other than the diol compound mentioned above can also be used together.
- the diol compound having a substituent that does not react with the isocyanate group is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0087] to [0088] of JP-A-2005-250438 And the compounds described.
- a diol compound having a carboxyl group can be used in combination with the diol compound described above.
- Examples of the diol compound having a carboxyl group include those represented by the following formulas (X) to (Z).
- R 15 represents a hydrogen atom, a substituent (for example, a halogen atom such as a cyano group, a nitro group, —F, —Cl, —Br, —I, etc., —CONH 2 , —COOR 16 , —OR 16 , —NHCONHR 16 , —NHCOOR 16 , —NHCOR 16 , —OCONHR 16 (wherein R 16 is an alkyl group having 1 to 10 carbon atoms or an aralkyl group having 7 to 15 carbon atoms) As long as it represents an alkyl group, an aralkyl group, an aryl group, an alkoxy group, or an aryloxy group, which may have a group, depending on the purpose.
- a substituent for example, a halogen atom such as a cyano group, a nitro group, —F, —Cl, —Br, —I, etc., —CONH 2
- L 9 , L 10 and L 11 may be the same or different, and each may be a single bond, a substituent (for example, alkyl, aralkyl, aryl, alkoxy, As long as it represents a divalent aliphatic or aromatic hydrocarbon group that may have a divalent aliphatic or aromatic hydrocarbon group, and may be appropriately selected depending on the purpose.
- an alkylene group having 1 to 20 carbon atoms and an arylene group having 6 to 15 carbon atoms are preferable, and an alkylene group having 1 to 8 carbon atoms is more preferable.
- the L 9 to L 11 may have other functional groups that do not react with isocyanate groups, such as carbonyl, ester, urethane, amide, ureido, and ether groups.
- a ring may be formed by two or three of R 15 , L 7 , L 8 and L 9 .
- Ar is not particularly limited as long as it represents a trivalent aromatic hydrocarbon group which may have a substituent, and can be appropriately selected according to the purpose. However, an aromatic group having 6 to 15 carbon atoms is preferred.
- the diol compound having a carboxyl group represented by the formulas (X) to (Z) is not particularly limited and may be appropriately selected depending on the intended purpose.
- the polyurethane resin having an ethylenically unsaturated bond group in the side chain is a resin having a carboxyl group in the side chain.
- the compound which ring-opened tetracarboxylic dianhydride with the diol compound other than the diol compound mentioned above can also be used together.
- the compound obtained by ring-opening the tetracarboxylic dianhydride with a diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, paragraph [0095] to JP 2005-250438 A And the compounds described in [0101].
- polyurethane resin having an ethylenically unsaturated bond in the side chain those having an unsaturated group in the polymer terminal and main chain are also preferably used.
- a plate material having excellent printing durability can be provided.
- an unsaturated group it is especially preferable to have an unsaturated group from the ease of a crosslinking reaction.
- Examples of the method for introducing an unsaturated group at the polymer terminal include the following methods. That is, in the step of synthesizing the polyurethane resin having an ethylenically unsaturated bond in the side chain as described above, in the step of treating with the residual isocyanate group at the polymer end and the alcohol or amine, the alcohol having an unsaturated group. Alternatively, amines or the like may be used. Specific examples of such a compound include the same compounds as those exemplified above as the monofunctional alcohol or monofunctional amine compound having an unsaturated group.
- the unsaturated group is preferably introduced into the polymer side chain rather than the polymer terminal from the viewpoint that the introduction amount can be easily controlled and the introduction amount can be increased, and that the crosslinking reaction efficiency is improved.
- the ethylenically unsaturated bond group to be introduced is not particularly limited and may be appropriately selected depending on the intended purpose. From the viewpoint of forming a crosslinked cured film, a methacryloyl group, an acryloyl group, and a styryl group are preferable, and methacryloyl Group and acryloyl group are more preferable, and methacryloyl group is particularly preferable in terms of both the formability of the crosslinked cured film and the raw storage stability.
- the amount of methacryloyl group introduced is not particularly limited and may be appropriately selected depending on the intended purpose.
- the vinyl group equivalent is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol. / G to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable.
- a method for introducing an unsaturated group into the main chain there is a method of using a diol compound having an unsaturated group in the main chain direction for the synthesis of a polyurethane resin.
- the diol compound having an unsaturated group in the main chain direction is not particularly limited and may be appropriately selected depending on the intended purpose. For example, cis-2-butene-1,4-diol, trans-2-butene 1,4-diol, polybutadiene diol and the like.
- the polyurethane resin having an ethylenically unsaturated bond in the side chain can be used in combination with an alkali-soluble polymer containing a polyurethane resin having a structure different from that of the specific polyurethane resin.
- the polyurethane resin having an ethylenically unsaturated bond in the side chain can be used in combination with a polyurethane resin containing an aromatic group in the main chain and / or side chain.
- polyurethane resin (i) having an ethylenically unsaturated bond in the side chain include, for example, P-1 to P— shown in paragraphs [0293] to [0310] of JP-A-2005-250438. And 31 polymers. Among these, polymers of P-27 and P-28 shown in paragraphs [0308] and [0309] are preferable.
- polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule--
- the polyurethane resin is a polyurethane resin obtained by reacting a carboxyl group-containing polyurethane having a diisocyanate and a carboxylic acid group-containing diol as essential components with a compound having an epoxy group and a vinyl group in the molecule.
- diol component a low molecular diol having a weight average molecular weight of 300 or less and a low molecular diol having a weight average molecular weight of 500 or more may be added as a copolymer component.
- the polyurethane resin By using the polyurethane resin, it is excellent in stable dispersibility with an inorganic filler, crack resistance and impact resistance, so that heat resistance, moist heat resistance, adhesion, mechanical properties and electrical properties are improved.
- the polyurethane resin includes a divalent aliphatic or aromatic hydrocarbon diisocyanate which may have a substituent, a COOH group and two OH groups via any one of a C atom and an N atom.
- a reaction product comprising a carboxylic acid-containing diol as an essential component, which is obtained by reacting the obtained reaction product with a compound having an epoxy group and a vinyl group in the molecule via a —COO— bond It may be.
- the polyurethane resin includes at least one selected from diisocyanates represented by the following general formula (I) and carboxylic acid group-containing diols represented by the following general formulas (II-1) to (II-3): And at least one selected from polymer diols having a weight average molecular weight in the range of 800 to 3,000 represented by the following general formulas (III-1) to (III-5) according to the purpose:
- R 1 may have a substituent (for example, any of an alkyl group, an aralkyl group, an aryl group, an alkoxy group, and a halogeno group is preferable). Represents an aromatic or aromatic hydrocarbon. If necessary, R 1 may have any other functional group that does not react with an isocyanate group, such as an ester group, a urethane group, an amide group, or a ureido group.
- a substituent for example, any of an alkyl group, an aralkyl group, an aryl group, an alkoxy group, and a halogeno group is preferable.
- R 1 may have any other functional group that does not react with an isocyanate group, such as an ester group, a urethane group, an amide group, or a ureido group.
- R 2 represents a hydrogen atom, a substituent (for example, a cyano group, a nitro group, a halogen atom (—F, —Cl, —Br, —I), —CONH 2 , —COOR. 6 , —OR 6 , —NHCONHR 6 , —NHCOOR 6 , —NHCOR 6 , —OCONHR 6 , —CONHR 6 (where R 6 is an alkyl group having 1 to 10 carbon atoms and an aralkyl group having 7 to 15 carbon atoms) Or an alkyl group, an aralkyl group, an aryl group, an alkoxy group, or an aryloxy group.
- a substituent for example, a cyano group, a nitro group, a halogen atom (—F, —Cl, —Br, —I), —CONH 2 , —COOR. 6 , —OR 6 , —NH
- R 3 , R 4 and R 5 may be the same or different from each other, and may be a single bond, a substituent (for example, an alkyl group or an aralkyl group).
- An aryl group, an alkoxy group, and a halogeno group are preferable).
- an alkylene group having 1 to 20 carbon atoms and an arylene group having 6 to 15 carbon atoms are preferable, and an alkylene group having 1 to 8 carbon atoms is more preferable.
- any one of other functional groups that do not react with the isocyanate group in the R 3 , R 4 and R 5 for example, any one of a carbonyl group, an ester group, a urethane group, an amide group, a ureido group, and an ether group. You may have. In addition, you may form a ring by 2 or 3 of said R ⁇ 2 >, R ⁇ 3 >, R ⁇ 4 > and R ⁇ 5 >.
- Ar represents a trivalent aromatic hydrocarbon which may have a substituent, and is preferably an aromatic group having 6 to 15 carbon atoms.
- R 7 , R 8 , R 9 , R 10 and R 11 may be the same or different from each other.
- R 7 , R 9 , R 10 and R 11 are each preferably an alkylene group having 2 to 20 carbon atoms or an arylene group having 6 to 15 carbon atoms, and an alkylene or carbon having 2 to 10 carbon atoms Several to 10 arylene groups are more preferred.
- R 8 represents an alkylene group having 1 to 20 carbon atoms or an arylene group having 6 to 15 carbon atoms, and an alkylene group having 1 to 10 carbon atoms or an arylene group having 6 to 10 carbon atoms is More preferred.
- R 7 , R 8 , R 9 , R 10 and R 11 other functional groups that do not react with isocyanate groups, such as ether groups, carbonyl groups, ester groups, cyano groups, olefin groups, urethane groups , An amide group, a ureido group, or a halogen atom.
- R 12 represents a hydrogen atom, an alkyl group, an aryl group, an aralkyl group, a cyano group, or a halogen atom.
- a hydrogen atom, an alkyl group having 1 to 10 carbon atoms, an aryl group having 6 to 15 carbon atoms, an aralkyl having 7 to 15 carbon atoms, a cyano group, or a halogen atom is preferable, and a hydrogen atom or one carbon atom is preferable. More preferred are ⁇ 6 alkyl and aryl groups having 6 to 10 carbon atoms.
- R 12 may have other functional groups that do not react with isocyanate groups, such as alkoxy groups, carbonyl groups, olefin groups, ester groups, or halogen atoms.
- R 13 represents an aryl group or a cyano group, preferably an aryl group or a cyano group having 6 to 10 carbon atoms.
- m represents an integer of 2 to 4.
- n 1 , n 2 , n 3 , n 4 and n 5 each represents an integer of 2 or more, and an integer of 2 to 100 is preferable.
- n 6 represents 0 or an integer of 2 or more, preferably 0 or an integer of 2 to 100.
- R 14 represents a hydrogen atom or a methyl group
- R 15 represents an alkylene group having 1 to 10 carbon atoms
- R 16 represents a carbon atom. This represents a hydrocarbon group having a number of 1 to 10.
- p represents 0 or an integer of 1 to 10.
- the polyurethane resin may further be copolymerized with a low molecular weight diol containing no carboxylic acid group as a fifth component, and the low molecular weight diol may be any of the above general formulas (III-1) to (III-5).
- the weight average molecular weight is 500 or less.
- the low molecular weight diol containing no carboxylic acid group can be added as long as the alkali solubility is not lowered and the elastic modulus of the cured film can be kept sufficiently low.
- the polyurethane resin in particular, at least one selected from diisocyanates represented by the general formula (I) and carboxylic acid group-containing diols represented by the general formulas (II-1) to (II-3) And at least one selected from polymer diols having a weight average molecular weight in the range of 800 to 3,000 represented by the general formulas (III-1) to (III-5) according to the purpose
- the reaction product of the general formulas (III-1) to (III-5) with the low molecular weight diol containing no carboxylic acid group having a weight average molecular weight of 500 or less is further added to the general formulas (IV-1) to (IV).
- IV-16 which is obtained by reacting a compound having one epoxy group and at least one (meth) acrylic group in the molecule represented by any one of the molecules, and has an acid value of 20 mgKOH / g to 120 mgK
- An alkali-soluble photocrosslinkable polyurethane resin that is OH / g is preferred.
- the polyurethane resin is synthesized by adding the above-mentioned diisocyanate compound and diol compound to an aprotic solvent, and adding a known catalyst having an activity corresponding to the reactivity thereof, followed by heating.
- the molar ratio (Ma: Mb) of the diisocyanate and diol compound used in the synthesis is not particularly limited and can be appropriately selected according to the purpose, preferably 1: 1 to 1.2: 1, and alcohols Alternatively, by treating with an amine or the like, a product having a desired physical property such as molecular weight or viscosity is synthesized in a form in which no isocyanate group remains finally.
- the amount of the ethylenically unsaturated bond introduced into the polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose.
- the vinyl group equivalent is 0.05 mmol / g to 3.0 mmol / g.
- 0.5 mmol / g to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable.
- the polyurethane resin having an ethylenically unsaturated bond in the side chain preferably has a carboxyl group introduced in the side chain together with the ethylenically unsaturated bond group.
- the acid value is preferably 20 mgKOH / g to 120 mgKOH / g, more preferably 30 mgKOH / g to 110 mgKOH / g, and particularly preferably 35 mgKOH / g to 100 mgKOH / g.
- the molecular weight of the polyurethane resin having an ethylenically unsaturated bond in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2,000 to 50,000 in terms of weight average molecular weight. 30,000 to 30,000 is more preferable, and 5,000 to 30,000 is particularly preferable. In particular, when the composition is used for a photosensitive solder resist, it is excellent in dispersibility with an inorganic filler, excellent in crack resistance and heat resistance, and excellent in developability of non-image areas with an alkaline developer.
- the content of the acid-modified vinyl group-containing polyurethane resin contained in the total solid content of the silica dispersion composition or the like is preferably 2% by mass to 30% by mass, and more preferably 5% by mass to 25% by mass.
- the content is less than 2% by mass, a sufficiently low elastic modulus at a high temperature of the cured film may not be obtained, and when it exceeds 30% by mass, the developability deterioration and the toughness of the cured film are reduced. May happen.
- the diisocyanate compound and the diol compound are synthesized in an aprotic solvent by adding a known catalyst having an activity corresponding to each reactivity and heating.
- the molar ratio of the diisocyanate and diol compound to be used is preferably 0.8: 1 to 1.2: 1. If an isocyanate group remains at the end of the polymer, the molar ratio can be reduced by treatment with alcohols or amines. It is synthesized in such a way that no isocyanate groups remain entangled.
- the diisocyanate compound represented by the general formula (I) is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0021] of JP-A-2007-2030, Etc.
- the high molecular weight diol compound represented by the general formulas (III-1) to (III-5) is not particularly limited and may be appropriately selected depending on the intended purpose. For example, as disclosed in JP-A-2007-2030 Examples thereof include compounds described in paragraphs [0022] to [0046].
- diol compound having a carboxyl group represented by the general formulas (II-1) to (II-3) is not particularly limited and may be appropriately selected depending on the intended purpose. And the compounds described in paragraph [0047] of the publication No. 2030.
- the carboxylic acid group-free low molecular weight diol is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0048] of JP-A-2007-2030. It is done.
- the copolymerization amount of the carboxylic acid group-free diol is preferably 95 mol% or less, more preferably 80% or less, and particularly preferably 50% or less in the low molecular weight diol. When the copolymerization amount exceeds 95 mol%, a urethane resin having good developability may not be obtained.
- polyurethane resin obtained by reacting the above (ii) carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule include, for example, paragraph [0314] of JP-A-2007-2030.
- glycidyl acrylate as an epoxy group- and vinyl group-containing compound is replaced with glycidyl methacrylate, 3,4-epoxycyclohexylmethyl acrylate (trade name: Cyclomer A400, Daicel). Chemical Co., Ltd.), 3,4-epoxycyclohexylmethyl methacrylate (trade name: Cyclomer M400, manufactured by Daicel Chemical Industries), and the like.
- the content of the acid-modified vinyl group-containing polyurethane resin in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 5% by mass to 80% by mass, and preferably 20% by mass. Is more preferably from 75 to 75% by weight, particularly preferably from 30 to 70% by weight. If the content is less than 5% by mass, good crack resistance may not be maintained, and if it exceeds 80% by mass, the heat resistance may fail. On the other hand, when the content is within the particularly preferable range, it is advantageous in terms of both good crack resistance and heat resistance.
- the weight average molecular weight of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2,000 to 60,000, and preferably 2,000 to 50,000. More preferably, 2,000 to 30,000 is particularly preferable. When the weight average molecular weight is less than 2,000, a sufficiently low elastic modulus at a high temperature of the cured film may not be obtained, and when it exceeds 60,000, coating suitability and developability may be deteriorated. .
- the weight average molecular weight is determined by using, for example, a high-speed GPC apparatus (manufactured by Toyo Soda Co., Ltd., HLC-802A), a 0.5 mass% THF solution as a sample solution, and a column of one TSKgel HZM-M. 200 ⁇ L of sample is injected, eluted with the THF solution, and measured at 25 ° C. with a refractive index detector or UV detector (detection wavelength 254 nm). Next, the weight average molecular weight can be determined from the molecular weight distribution curve calibrated with standard polystyrene.
- the acid value of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose. 35 mg KOH / g to 100 mg KOH / g is particularly preferable. If the acid value is less than 20 mgKOH / g, the developability may be insufficient, and if it exceeds 120 mgKOH / g, the development speed may be too high, and the development control may be difficult.
- the said acid value can be measured based on JISK0070, for example. However, if the sample does not dissolve, dioxane or tetrahydrofuran is used as the solvent.
- the vinyl group equivalent of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol / g to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable.
- the vinyl group equivalent can be calculated
- the bromine number can be measured according to, for example, JIS K2605.
- the acid-modified vinyl group-containing epoxy resin is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include those described in Japanese Patent No. 4127010 (Japanese Patent Laid-Open No. 2004-133060). Can be mentioned.
- the polymerizable compound is not particularly limited and may be appropriately selected depending on the intended purpose.
- the compound has at least one addition-polymerizable group in the molecule and has a boiling point of 100 ° C. or higher at normal pressure.
- at least one selected from monomers having a (meth) acryl group is preferable.
- polyethyleneglycol mono (meth) acrylate polypropylene glycol mono (meth) acrylate, phenoxyethyl (meth) ) Monofunctional acrylate such as acrylate; Monofunctional methacrylate; Polyethylene glycol di (meth) acrylate, Polypropylene glycol di (meth) acrylate, Trimethylolethane triacrylate, Trimethylolpropane triacrylate, Trimethylolpropane diacrylate, Neopentylglycol di (Meth) acrylate, pentaerythritol tetra (meth) acrylate, pentaerythritol tri (meth) acrylate, dipentaerythritol hex (Meth) acrylate, dipentaerythritol penta (meth) acrylate,
- trimethylolpropane tri (meth) acrylate pentaerythritol tetra (meth) acrylate, dipentaerythritol hexa (meth) acrylate, and dipentaerythritol penta (meth) acrylate are particularly preferable.
- the content of the polymerizable compound in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2% by mass to 50% by mass, and 3% by mass to 40% by mass. Is more preferable, and 4% by mass to 35% by mass is particularly preferable.
- the content is less than 2% by mass, pattern formation may not be possible, and when it exceeds 50% by mass, crack resistance may be inferior.
- the content is within the particularly preferable range, it is advantageous in that both good pattern formation and crack resistance can be achieved.
- the photopolymerization initiator is not particularly limited as long as it has the ability to initiate polymerization of the polymerizable compound, and can be appropriately selected according to the purpose.
- a halogenated hydrocarbon derivative for example, a triazine skeleton
- those having an oxadiazole skeleton phosphine oxide, hexaarylbiimidazole, oxime derivatives, organic peroxides, thio compounds, ketone compounds, aromatic onium salts, ketoxime ethers, and the like.
- the halogenated hydrocarbon compound having a triazine skeleton is not particularly limited and may be appropriately selected depending on the intended purpose.
- compounds described in British Patent No. 1388492 compounds described in JP-A-53-133428
- German Patent No. 3337024 Compounds, F.I. C. J. Schaefer et al. Org. Chem.
- halogenated hydrocarbon compound having an oxadiazole skeleton examples include the compounds described in US Pat. No. 4,221,976.
- the oxime derivative is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0085] of JP-A-2007-2030.
- the ketone compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0087] of JP-A-2007-2030.
- the photopolymerization initiator other than the above is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0086] of JP-A-2007-2030. It is done.
- a sensitizer can be added for the purpose of adjusting exposure sensitivity and photosensitive wavelength in exposure to the photosensitive layer described later.
- the sensitizer can be appropriately selected by a visible light, an ultraviolet laser, a visible laser or the like as a light irradiation means described later.
- the sensitizer is excited by active energy rays and interacts with other substances (eg, radical generator, acid generator, etc.) (eg, energy transfer, electron transfer, etc.), thereby causing radicals, acids, etc. It is possible to generate a useful group of
- the sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0089] of JP-A-2007-2030.
- the combination of the photopolymerization initiator and the sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose.
- an electron transfer type initiation system described in JP-A-2001-305734 [ (1) Electron donating initiator and sensitizing dye, (2) Electron accepting initiator and sensitizing dye, (3) Electron donating initiator, sensitizing dye and electron accepting initiator (ternary initiation system) ] Etc. are mentioned.
- the content of the sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose. It is preferably 0.05% by mass to 30% by mass with respect to all components in the silica dispersion composition. 0.1 mass% to 20 mass% is more preferable, and 0.2 mass% to 10 mass% is particularly preferable. When the content is less than 0.05% by mass, the sensitivity to active energy rays decreases, the exposure process takes time, and the productivity may be reduced.
- the sensitizer may be precipitated from the photosensitive layer.
- the said photoinitiator may be used individually by 1 type, and may use 2 or more types together.
- Particularly preferred examples of the photopolymerization initiator include composite light in which phosphine oxides, the ⁇ -aminoalkyl ketones, the halogenated hydrocarbon compound having the triazine skeleton and an amine compound as a sensitizer described later are combined. Examples thereof include an initiator, a hexaarylbiimidazole compound, and titanocene.
- the content of the photopolymerization initiator in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.5% by mass to 20% by mass, and preferably 0.5% by mass. % To 15% by mass is more preferable, and 1% to 10% by mass is particularly preferable. When the content is less than 0.5% by mass, the exposed portion tends to be eluted during development, and when it exceeds 20% by mass, the heat resistance may be lowered. On the other hand, when the content is within the particularly preferable range, it is advantageous in that a good pattern can be formed and heat resistance is also improved.
- the other components are not particularly limited and may be appropriately selected depending on the purpose.
- thermoplastic elastomers, fillers, thermosetting accelerators, thermal polymerization inhibitors, plasticizers, colorants (color pigments or Dyes) and the like, and further adhesion promoters to the substrate surface, or other auxiliary agents (for example, conductive particles, fillers, antifoaming agents, flame retardants, leveling agents, peeling accelerators, antioxidants). , Fragrance, surface tension adjusting agent, chain transfer agent, etc.) may be used in combination.
- properties such as the stability, photographic properties, and film properties of the intended photosensitive film can be adjusted.
- thermoplastic elastomer there is no restriction
- These elastomers are composed of a hard segment component and a soft segment component. In general, the former contributes to heat resistance and strength, and the latter contributes to flexibility and toughness.
- the thermoplastic elastomer is described in paragraphs [0197] to [0207] of JP-A-2007-199532.
- the filler is described in detail in, for example, paragraphs [0098] to [0099] of JP-A-2008-250074.
- the thermal polymerization inhibitor is described in detail, for example, in paragraphs [0101] to [0102] of JP-A-2008-250074.
- the thermosetting accelerator is described in detail, for example, in paragraph [0093] of JP-A-2008-250074.
- the plasticizer is described in detail, for example, in paragraphs [0103] to [0104] of JP-A-2008-250074.
- the colorant is described in detail, for example, in paragraphs [0105] to [0106] of JP-A-2008-250074.
- the adhesion promoter is described in detail, for example, in paragraphs [0107] to [0109] of JP-A-2008-250074.
- the silica dispersion composition of the present invention can be used as a liquid resist by coating and drying on a substrate on which a conductor wiring is formed, but is particularly useful for producing a photosensitive film.
- the photosensitive film has at least a support and a photosensitive layer, preferably has a protective film, and further, if necessary, a cushion layer, an oxygen barrier layer (hereinafter abbreviated as PC layer). ) And other layers.
- PC layer oxygen barrier layer
- the PC layer, the photosensitive layer, and the protective film are provided in this order on the support, and the cushion layer, the PC layer, the photosensitive layer, and the protection are provided on the support.
- the form etc. which have a film in this order are mentioned.
- the photosensitive layer may be a single layer or a plurality of layers.
- the photosensitive layer is formed from the silica dispersion composition of the present invention.
- the melt viscosity at 70 ° C. of the photosensitive layer is preferably 1.4 ⁇ 10 3 Pa ⁇ s or less, more preferably 1.0 ⁇ 10 3 Pa ⁇ s or less, and 6.0 ⁇ 10 2 Pa ⁇ s or less. Further preferred.
- the melt viscosity at 70 ° C. of the photosensitive layer exceeds 1.4 ⁇ 10 3 Pa ⁇ s, the embedding property may be deteriorated, and when the melt viscosity at 70 ° C. is in a more preferable range, the embedding property is deteriorated. Is advantageous in that it is sufficiently obtained.
- the melt viscosity at 30 ° C. of the photosensitive layer is preferably 1.0 ⁇ 10 4 Pa ⁇ s or more, more preferably 1.3 ⁇ 10 4 Pa ⁇ s or more, and 3.0 ⁇ 10 4 Pa ⁇ s or more. Further preferred. If the melt viscosity at 30 ° C. of the photosensitive layer is less than 1.0 ⁇ 10 4 Pa ⁇ s, edge fusion may be deteriorated, and if the melt viscosity at 30 ° C. is in a more preferable range, embedding is performed. It is advantageous in that it can be compatible with edge fusion.
- the melt viscosity of the photosensitive layer is measured by, for example, a melt viscosity measuring device such as a rheometer VAR-1000 type (manufactured by Rheological Co., Ltd.), Vibron DD-III type (manufactured by Toyo Baldwin Co., Ltd.) or the like. Can be measured.
- a melt viscosity measuring device such as a rheometer VAR-1000 type (manufactured by Rheological Co., Ltd.), Vibron DD-III type (manufactured by Toyo Baldwin Co., Ltd.) or the like. Can be measured.
- the permanent pattern used in the present invention is obtained by the permanent pattern forming method.
- the permanent pattern is described in paragraphs [0128] to [0283] of Japanese Patent Application Laid-Open No. 2007-2030.
- the printed board used in the present invention has at least a base and a permanent pattern formed by the permanent pattern forming method, and further has other configurations appropriately selected as necessary.
- Synthesis Example 4 ⁇ Synthesis of Silica Dispersant P-2>
- Synthesis Example 3 the same procedure as in Synthesis Example 3 was performed, except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 6.79 g and 2.21 g of neopentyl glycol was changed to 1.19 g.
- a silica dispersant P-2 solution solid content: 20% by mass having a mass ratio of components represented by the following formula was synthesized.
- CL represents a caprolactone and a number represents mass ratio.
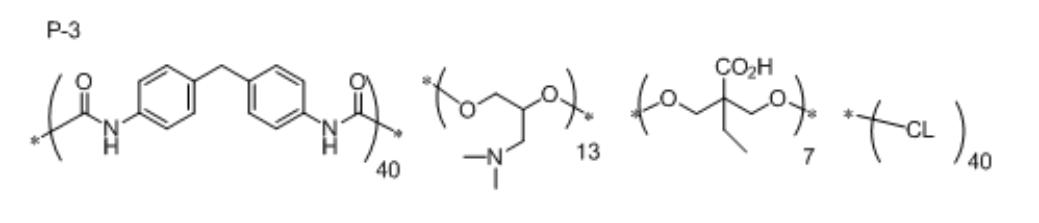
- Synthesis Example 5 ⁇ Synthesis of Silica Dispersant P-3>
- the components represented by the following formulas were used in the same manner as in Synthesis Example 3 except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 8.15 g and neopentyl glycol was not added.
- a silica dispersant P-3 solution (solid content: 20% by mass) having a mass ratio of 5% was synthesized.
- CL represents a caprolactone and a number represents mass ratio.
- Synthesis Example 9 ⁇ Synthesis of Silica Dispersant P-7>
- Synthesis Example 3 the same procedure as in Synthesis Example 3 was performed except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 3.84 g and 2.21 g of neopentyl glycol was changed to 3.77 g.
- a silica dispersant P-7 solution solid content 20% by mass having a mass ratio of the components represented by the following formula was synthesized.
- CL represents a caprolactone and a number represents mass ratio.
- light acrylate 130A methoxypolyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd., bromine number 34.9Br / 100 g
- light acrylate NP-4EA nonylphenyl polyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd. 17 0.7 parts by mass was added, the temperature was raised to 80 ° C., and the reaction was performed for 4 hours.
- the mixture was cooled to 60 ° C., 60 parts by weight of castor oil (diol HS-2G-150R, manufactured by Toyokuni Seiyaku), 163 parts by weight of Kuraray polyester diol P-2050 (number average molecular weight 2,066, manufactured by Kuraray Co., Ltd.), 29.1 parts by weight of methylol butanoic acid, 49.5 parts by weight of 1,6-hexamethylene diisocyanate, 76.4 parts by weight of isophorone diisocyanate, and 83 parts by weight of propylene glycol monomethyl ether acetate were added and reacted at 60 ° C. for 5 hours. It was.
- castor oil diol HS-2G-150R, manufactured by Toyokuni Seiyaku
- Kuraray polyester diol P-2050 number average molecular weight 2,066, manufactured by Kuraray Co., Ltd.
- 29.1 parts by weight of methylol butanoic acid 49.5 parts by weight
- ⁇ Measurement of acid value> 0.7 g of each silica dispersant was weighed in a 100 mL beaker, 60 mL of a solution of tetrahydrofuran (THF) / water 5/1 (volume ratio) was added, and dissolved by stirring. After adjusting measurement temperature to 25 degreeC, it titrated with the titration apparatus using 0.1N NaOH aqueous solution as a titration reagent, and the acid value was measured.
- THF tetrahydrofuran
- the silica dispersant of P-5 is made of a polyurethane resin having no basic group.
- the silica dispersant of P-6 is made of a polyurethane resin having no acidic group.
- Example 1 Composition of silica dispersion composition solution- -Polyurethane binder solution of Synthesis Example 1 (solid content 45% by mass) ... 25.3 parts by mass-Color pigment: HELIOGEN BLUE D7086 (manufactured by BASF) ... 0.02 parts by mass-Color pigment: Pariotol Yellow D0960 (Manufactured by BASF) 0.005 parts by mass Dispersant: Silica dispersant P-1 solution (solid content 20% by mass) 1.51 parts by mass Polymerizable compound: DCP-A (Kyoeisha Chemical) 4.67 parts by mass ⁇ Initiator: Irgacure 907 (manufactured by BASF) ...
- a silica dispersion composition solution having the above composition is applied onto a polyethylene terephthalate film (16FB50, manufactured by Toray Industries, Inc.) having a thickness of 16 ⁇ m as a support, and dried to form a photosensitive layer having a thickness of 30 ⁇ m on the support. Formed.
- a 20 ⁇ m-thick polypropylene film manufactured by Oji Specialty Paper Co., Ltd., Alphan E-200 was laminated as a protective layer to produce a photosensitive film.
- the substrate was prepared by subjecting a surface of a copper clad laminate (no through hole, copper thickness: 12 ⁇ m) to chemical polishing.
- a vacuum laminator manufactured by Nichigo Morton Co., Ltd., VP130 was used on the copper-clad laminate while peeling off the protective film from the photosensitive film so that the photosensitive layer of the photosensitive film was in contact with the copper-clad laminate.
- a photosensitive laminate was prepared in which the copper-clad laminate, the photosensitive layer, and the polyethylene terephthalate film (support) were laminated in this order.
- the pressure bonding conditions were a vacuum drawing time of 40 seconds, a pressure bonding temperature of 70 ° C., a pressure bonding pressure of 0.2 MPa, and a pressure application time of 10 seconds.
- melt viscosity was measured on condition of the following using rheometer * VAR-1000 type (made by Rheological Co., Ltd.). -Measurement conditions of melt viscosity- Melt viscoelasticity was measured using a plate having a diameter of 20 mm at a strain of 0.005 and a frequency of 1 Hz. The temperature range was 25 ° C. to 85 ° C., and the measurement was performed at a rate of temperature increase of 5 ° C./min. In addition, the melt viscosity in Table 2 shows a value at 70 ° C.
- the polyethylene terephthalate film (support) was peeled off from the photosensitive laminate.
- a 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper clad laminate at a spray pressure of 0.15 MPa.
- the time required until the layer was dissolved and removed was measured, and this was taken as the shortest development time.
- a 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper-clad laminate at a spray pressure of 0.15 MPa for a time twice as short as the shortest developing time, thereby uncured regions.
- developability (development time, residue) was evaluated according to the following criteria.
- -Resolution Observe the surface of the obtained copper-clad laminate with a cured resin pattern with an optical microscope, there is no residue at the bottom of the round hole of the pattern, there is no abnormality such as blistering / peeling of the pattern part, and space can be formed
- the smallest round hole pattern diameter was measured, and the resolution was evaluated according to the following criteria.
- [Development evaluation criteria] -About development time, it evaluated by the said shortest development time. -Residues were visually evaluated according to the following criteria.
- ⁇ No residue ⁇ : Residue is slightly seen on the wall and bottom ⁇ : Residue is clearly seen [Evaluation criteria for resolution] ⁇ : A round hole with a diameter of 90 ⁇ m or less can be resolved, and the resolution is excellent. ⁇ : A round hole with a diameter of 200 ⁇ m or less can be resolved, and the resolution is slightly inferior. , Poor resolution
- the photosensitive layer was irradiated with ultraviolet rays with an energy amount of 1 J / cm 2 using an ultraviolet irradiation device manufactured by Oak Manufacturing. Further, the photosensitive layer was heat-treated at 150 ° C. for 60 minutes to obtain an evaluation substrate on which a solder resist was formed. After connecting a shield wire made of polytetrafluoroethylene to these comb electrodes by Sn / Pb solder so that a voltage is applied between the comb electrodes of the evaluation laminate after heating, 50 V is applied to the evaluation laminate. With the voltage applied, the laminate for evaluation was allowed to stand in a super accelerated high-temperature high-humidity life test (HAST) bath at 130 ° C. and 85% RH for 200 hours.
- HAST high-temperature high-humidity life test
- TCT Thermal shock resistance
- appearance such as cracks and peeling was evaluated by a temperature cycle test (TCT).
- TCT uses a gas-phase cold heat tester, and the electronic component module is left in the gas phase at ⁇ 55 ° C. and 125 ° C. for 30 minutes each, and this is regarded as one cycle under the conditions of 1,000 cycles and 1,500 cycles.
- the thermal shock resistance was evaluated according to the following criteria. ⁇ Evaluation criteria ⁇ ⁇ : No crack occurred ⁇ : Shallow crack occurred ⁇ : Deep crack occurred
- Example 2 to 6 and Comparative Examples 1 to 4 In Example 1, as shown in Table 2, Examples 2 to 6 and Example 1 were performed in the same manner as Example 1 except that the silica dispersant P-1 was replaced with silica dispersants P-2 to P-10. The photosensitive films, photosensitive laminates, and permanent patterns of Comparative Examples 1 to 4 were produced. About each obtained photosensitive film and photosensitive laminated body, it carried out similarly to Example 1, and melt viscosity, embedding property, developability (unexposed part), resolution (lower part of exposure), insulation, and thermal shock Sex (TCT) was evaluated. The results are shown in Table 2.
- the aspect of the present invention is as follows.
- ⁇ 1> comprising a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent;
- the silica dispersion composition is characterized in that the amine value of the silica dispersant is 0.65 mmol / g or more.
- ⁇ 2> The silica dispersion composition according to ⁇ 1>, wherein the basic group is a tertiary amino group.
- ⁇ 3> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 2>, wherein the acidic group is a carboxyl group.
- ⁇ 4> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 3>, wherein the polyurethane resin has a graft chain in at least one of a side chain and a terminal.
- ⁇ 5> The silica dispersion composition according to ⁇ 4>, wherein the polyurethane resin has a polyester graft chain at a terminal thereof.
- ⁇ 6> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 5>, wherein the acid value of the silica dispersant is 0.3 mmol / g or more.
- ⁇ 7> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 6>, wherein the polyurethane resin further has a polymerizable group.
- ⁇ 8> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 7>, which is for a solder resist.
- ⁇ 9> The silica dispersion composition according to any one of ⁇ 1> to ⁇ 8>, further including a binder, a polymerizable compound, and a photopolymerization initiator.
- ⁇ 10> The silica dispersion composition according to ⁇ 9>, wherein the binder is an acid-modified vinyl group-containing polyurethane resin.
- the binder is an acid-modified vinyl group-containing epoxy resin.
- the binder is a resin containing an unsaturated group and a carboxyl group.
- the binder is a polyimide precursor.
- a photosensitive film comprising a photosensitive layer containing the silica dispersion composition according to any one of ⁇ 1> to ⁇ 14> on a support.
- a photosensitive laminate comprising a photosensitive layer containing the silica dispersion composition according to any one of ⁇ 1> to ⁇ 14> on a substrate.
- a method for forming a permanent pattern comprising at least exposing a photosensitive layer formed of the silica dispersion composition according to any one of ⁇ 1> to ⁇ 14>.
- a printed circuit board wherein a permanent pattern is formed by the method for forming a permanent pattern according to ⁇ 17>.
- the silica-dispersed composition of the present invention can be used suitably for a solder resist because a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulation, and resolution of an exposed portion can be obtained. be able to.
- the film made of the silica dispersion composition of the present invention includes various patterns such as a protective film, an interlayer insulating film, and a permanent pattern such as a solder resist pattern, BGA (ball grid array), CSP (chip size package), TCP (tape) It can be suitably used for the formation of semiconductor packages such as carrier packages), the manufacture of liquid crystal structural members such as color filters, pillars, ribs, spacers, partition walls, holograms, micromachines, and proofs. It can be suitably used for the formation of a semiconductor package for permanent pattern formation, BGA (ball grid array), CSP (chip size package), TCP (tape carrier package) and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Ceramic Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Materials For Photolithography (AREA)
- Polyurethanes Or Polyureas (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Non-Metallic Protective Coatings For Printed Circuits (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
Provided is a silica dispersion composition containing a silica dispersant made of a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal cross-linking agent, the amine value of the silica dispersant being 0.65 mmol/g or higher.
Description
本発明は、ソルダーレジスト等に好適に用いられるシリカ分散組成物に関する。
The present invention relates to a silica dispersion composition suitably used for solder resist and the like.
従来より、ソルダーレジスト等の永久パターンを形成するに際して、支持体上にシリカ分散組成物を塗布し、乾燥することにより感光層を形成させた感光性フィルムが用いられてきている。ソルダーレジスト等の永久パターンを形成する方法としては、例えば、永久パターンが形成される銅張積層板等の基体上に、感光性フィルムを積層させて積層体を形成し、該積層体における感光層に対して露光を行い、該露光後、感光層を現像してパターンを形成させ、その後硬化処理等を行うことにより永久パターンを形成する方法等が知られている。
Conventionally, when forming a permanent pattern such as a solder resist, a photosensitive film in which a photosensitive layer is formed by applying a silica dispersion composition on a support and drying it has been used. As a method for forming a permanent pattern such as a solder resist, for example, a photosensitive film is laminated on a substrate such as a copper-clad laminate on which a permanent pattern is formed to form a laminate, and the photosensitive layer in the laminate is formed. There is known a method of forming a permanent pattern by performing exposure on the substrate, developing the photosensitive layer after the exposure to form a pattern, and then performing a curing process or the like.
最近、デバイスの微細化に伴って、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジストの開発が進められている。そこで、耐衝撃性を向上させるため、シリカ微粒子の高充填化が行われているが、現在までのところ、L/S(ラインスペース)の小さいところで解像性と耐衝撃性を両立したソルダーレジスト組成物は提供されていない。これは、シリカ微粒子を高充填すると、溶融粘度が高くなり転写性が低下してしまうこと、シリカ微粒子は現像性基を有していないので、現像性が低下してしまうからである。
Recently, with the miniaturization of devices, the development of solder resists that can be applied to semiconductor package substrates with a small L / S (line space) has been underway. Therefore, in order to improve impact resistance, silica fine particles have been highly filled, but so far, solder resists that have both resolution and impact resistance at a small L / S (line space). No composition is provided. This is because when the silica fine particles are highly filled, the melt viscosity becomes high and the transferability is lowered, and the silica fine particles do not have a developable group, so the developability is lowered.
関連する先行技術文献として、例えば、(A1)酸性官能基及び酸性官能基の中和塩基のうちの1種又は2種と、(A2)塩基性官能基、塩基性官能基の中和塩基及びノニオン系極性分子鎖のうちの1種又は2種以上と、(B)低極性分子鎖と、(C)活性エネルギー線硬化性不飽和基とを有するポリウレタン樹脂である顔料分散剤が提案されている(特許文献1参照)。
しかし、この提案は、フォトリソグラフィ法により画像形成するフォトレジストに好適に用いられるカーボンブラックの分散剤に関するものであり、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジスト用に適正化されたものではない。また、この提案の顔料分散剤は、塩基性官能基にグラフト鎖を修飾しているので、アミン価が低くなってしまい、シリカ分散性が低下し、溶融粘度が高くなって転写性が劣るという問題がある。 Related prior art documents include, for example, (A1) one or two of an acidic functional group and a neutralized base of an acidic functional group, and (A2) a basic functional group, a neutralized base of a basic functional group, and A pigment dispersant is proposed which is a polyurethane resin having one or more nonionic polar molecular chains, (B) a low polar molecular chain, and (C) an active energy ray-curable unsaturated group. (See Patent Document 1).
However, this proposal relates to a dispersant for carbon black that is suitably used for photoresists that form images by photolithography, and is suitable for solder resists that can be used for semiconductor package substrates with a small L / S (line space). It has not been converted into one. In addition, since the proposed pigment dispersant has a graft chain modified to a basic functional group, the amine value is lowered, silica dispersibility is lowered, melt viscosity is increased, and transferability is inferior. There's a problem.
しかし、この提案は、フォトリソグラフィ法により画像形成するフォトレジストに好適に用いられるカーボンブラックの分散剤に関するものであり、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジスト用に適正化されたものではない。また、この提案の顔料分散剤は、塩基性官能基にグラフト鎖を修飾しているので、アミン価が低くなってしまい、シリカ分散性が低下し、溶融粘度が高くなって転写性が劣るという問題がある。 Related prior art documents include, for example, (A1) one or two of an acidic functional group and a neutralized base of an acidic functional group, and (A2) a basic functional group, a neutralized base of a basic functional group, and A pigment dispersant is proposed which is a polyurethane resin having one or more nonionic polar molecular chains, (B) a low polar molecular chain, and (C) an active energy ray-curable unsaturated group. (See Patent Document 1).
However, this proposal relates to a dispersant for carbon black that is suitably used for photoresists that form images by photolithography, and is suitable for solder resists that can be used for semiconductor package substrates with a small L / S (line space). It has not been converted into one. In addition, since the proposed pigment dispersant has a graft chain modified to a basic functional group, the amine value is lowered, silica dispersibility is lowered, melt viscosity is increased, and transferability is inferior. There's a problem.
したがって、シリカ微粒子の高充填化と、溶融粘度及び解像性の向上との両立を図ることができ、埋め込み性、耐熱衝撃性、現像性、絶縁性、及び露光部の解像性に優れた高性能な硬化膜を得ることができるシリカ分散組成物の速やかな提供が望まれているのが現状である
Therefore, it is possible to achieve both high packing of silica fine particles and improvement in melt viscosity and resolution, and excellent embedding properties, thermal shock resistance, developability, insulating properties, and resolution of exposed areas. At present, it is desired to provide a silica dispersion composition capable of obtaining a high-performance cured film promptly.
本発明は、埋め込み性、耐熱衝撃性、現像性、絶縁性、及び露光部の解像性に優れた高性能な硬化膜を得ることができ、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジスト用などに好適なシリカ分散組成物を提供することを目的とする。
INDUSTRIAL APPLICABILITY The present invention can obtain a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulating property, and resolution of an exposed portion, and a semiconductor package substrate having a small L / S (line space). An object of the present invention is to provide a silica dispersion composition suitable for a solder resist that can cope with the above.
前記課題を解決するため、本発明者が鋭意検討を重ねた結果、酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、前記シリカ分散剤が、好ましくは側鎖及び末端の少なくともいずれかにグラフト鎖を有し、アミン価を0.65mmol/g以上とすることにより、シリカ微粒子の高充填化と、溶融粘度及び現像性の向上との両立を図ることができ、予想外に、絶縁性に優れ、なおかつ露光部の解像性に優れた高性能な硬化膜を得ることができ、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジスト用に好適なシリカ分散組成物が得られることを知見した。
In order to solve the above-mentioned problems, the present inventors have made extensive studies, and as a result, contain a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent, The silica dispersant preferably has a graft chain in at least one of a side chain and a terminal, and has an amine value of 0.65 mmol / g or more, so that silica fine particles can be highly filled, melt viscosity, and developability. It is possible to obtain a high-performance cured film that has an excellent insulating property and an excellent resolution of the exposed portion, and has a small L / S (line space). It has been found that a silica dispersion composition suitable for a solder resist that can be applied to a semiconductor package substrate can be obtained.
本発明は、本発明者による前記知見に基づくものであり、前記課題を解決するための手段としては、以下の通りである。即ち、
本発明のシリカ分散組成物は、酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、
前記シリカ分散剤のアミン価が0.65mmol/g以上であることを特徴とする。 This invention is based on the said knowledge by this inventor, and as a means for solving the said subject, it is as follows. That is,
The silica dispersion composition of the present invention comprises a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent,
The amine value of the silica dispersant is 0.65 mmol / g or more.
本発明のシリカ分散組成物は、酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、
前記シリカ分散剤のアミン価が0.65mmol/g以上であることを特徴とする。 This invention is based on the said knowledge by this inventor, and as a means for solving the said subject, it is as follows. That is,
The silica dispersion composition of the present invention comprises a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent,
The amine value of the silica dispersant is 0.65 mmol / g or more.
本発明によると、従来における諸問題を解決でき、埋め込み性、耐熱衝撃性、現像性、絶縁性、及び露光部の解像性に優れた高性能な硬化膜を得ることができ、L/S(ラインスペース)の小さい半導体パッケージ基板に対応できるソルダーレジスト用などに好適なシリカ分散組成物を提供することができる。
According to the present invention, conventional problems can be solved, and a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulation, and resolution of an exposed portion can be obtained. A silica dispersion composition suitable for a solder resist that can be used for a semiconductor package substrate having a small (line space) can be provided.
(シリカ分散組成物)
本発明のシリカ分散組成物は、シリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、バインダー、重合性化合物、光重合開始剤、更に必要に応じてその他の成分を含有してなる。 (Silica dispersion composition)
The silica dispersion composition of the present invention contains a silica dispersant, silica fine particles, and a thermal crosslinking agent, and contains a binder, a polymerizable compound, a photopolymerization initiator, and other components as necessary. It becomes.
本発明のシリカ分散組成物は、シリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、バインダー、重合性化合物、光重合開始剤、更に必要に応じてその他の成分を含有してなる。 (Silica dispersion composition)
The silica dispersion composition of the present invention contains a silica dispersant, silica fine particles, and a thermal crosslinking agent, and contains a binder, a polymerizable compound, a photopolymerization initiator, and other components as necessary. It becomes.
<シリカ分散剤>
前記シリカ分散剤は、1分子中に酸性基及び塩基性基を少なくとも有し、好ましくは、グラフト鎖、重合性基を有するポリウレタン樹脂からなる分散剤である。
前記シリカ分散剤は、主鎖がポリウレタンであるポリウレタン樹脂からなり、前記主鎖に結合して、又は前記主鎖に結合している側鎖に結合して、あるいは前記主鎖を構成する分子鎖として、酸性基及び塩基性基を有し、好ましくは、グラフト鎖、重合性基を有する。 <Silica dispersant>
The silica dispersant is a dispersant comprising a polyurethane resin having at least an acidic group and a basic group in one molecule, and preferably having a graft chain and a polymerizable group.
The silica dispersant is made of a polyurethane resin whose main chain is polyurethane, and is bonded to the main chain or bonded to a side chain bonded to the main chain, or a molecular chain constituting the main chain As above, it has an acidic group and a basic group, and preferably has a graft chain and a polymerizable group.
前記シリカ分散剤は、1分子中に酸性基及び塩基性基を少なくとも有し、好ましくは、グラフト鎖、重合性基を有するポリウレタン樹脂からなる分散剤である。
前記シリカ分散剤は、主鎖がポリウレタンであるポリウレタン樹脂からなり、前記主鎖に結合して、又は前記主鎖に結合している側鎖に結合して、あるいは前記主鎖を構成する分子鎖として、酸性基及び塩基性基を有し、好ましくは、グラフト鎖、重合性基を有する。 <Silica dispersant>
The silica dispersant is a dispersant comprising a polyurethane resin having at least an acidic group and a basic group in one molecule, and preferably having a graft chain and a polymerizable group.
The silica dispersant is made of a polyurethane resin whose main chain is polyurethane, and is bonded to the main chain or bonded to a side chain bonded to the main chain, or a molecular chain constituting the main chain As above, it has an acidic group and a basic group, and preferably has a graft chain and a polymerizable group.
-酸性基-
前記酸性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、カルボキシル基、スルホ基、ホスホニル基、-COCH2CO-R(ただし、Rは炭素数1~10の炭化水素基を表す)、-CONHCO-R(ただし、Rは炭素数1~10の炭化水素基を表す)、-COCH2CN、フェノール性水酸基、-RFCH2OH(ただし、RFはペルフルオロアルキル基を表す)、-(RF)2CHOH(ただし、RFはペルフルオロアルキル基を表す)、スルホンアミド基などが挙げられる。これらの中でも、現像性の点で、カルボキシル基が特に好ましい。 -Acid group-
The acidic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a carboxyl group, a sulfo group, a phosphonyl group, and —COCH 2 CO—R (where R is a carbon number of 1 to 10). -CONHCO-R (wherein R represents a hydrocarbon group having 1 to 10 carbon atoms), -COCH 2 CN, a phenolic hydroxyl group, -R F CH 2 OH (where R F is A perfluoroalkyl group), — (R F ) 2 CHOH (wherein R F represents a perfluoroalkyl group), and a sulfonamide group. Among these, a carboxyl group is particularly preferable from the viewpoint of developability.
前記酸性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、カルボキシル基、スルホ基、ホスホニル基、-COCH2CO-R(ただし、Rは炭素数1~10の炭化水素基を表す)、-CONHCO-R(ただし、Rは炭素数1~10の炭化水素基を表す)、-COCH2CN、フェノール性水酸基、-RFCH2OH(ただし、RFはペルフルオロアルキル基を表す)、-(RF)2CHOH(ただし、RFはペルフルオロアルキル基を表す)、スルホンアミド基などが挙げられる。これらの中でも、現像性の点で、カルボキシル基が特に好ましい。 -Acid group-
The acidic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a carboxyl group, a sulfo group, a phosphonyl group, and —COCH 2 CO—R (where R is a carbon number of 1 to 10). -CONHCO-R (wherein R represents a hydrocarbon group having 1 to 10 carbon atoms), -COCH 2 CN, a phenolic hydroxyl group, -R F CH 2 OH (where R F is A perfluoroalkyl group), — (R F ) 2 CHOH (wherein R F represents a perfluoroalkyl group), and a sulfonamide group. Among these, a carboxyl group is particularly preferable from the viewpoint of developability.
-塩基性基-
前記塩基性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、第1級アミノ基、第2級アミノ基、第3級アミノ基、含窒素ヘテロ環などが挙げられるが、第1級アミノ基及び第2級アミノ基はイソシアネートと反応し、主鎖にとりこまれてしまうため、第3級アミノ基、含窒素へテロ環が好ましく、第3級アミノ基が特に好ましい。
前記第3級アミノ基としては、例えば、ジメチルアミノ基、ジエチルアミノ基などが挙げられる。
前記含窒素ヘテロ環としては、例えば、2-ピロリドン、N-メチル-2-ピロリドン、1,3-ジメチルイミイダゾリジノン、ε-カプロラクタム、γ-ブチロラクトンなどが挙げられる。
本発明においては、前記塩基性基と前記酸性基を併有することが、現像性だけでなく、絶縁性の点でも好ましい。 -Basic group-
The basic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a primary amino group, a secondary amino group, a tertiary amino group, and a nitrogen-containing heterocycle. However, since the primary amino group and the secondary amino group react with the isocyanate and are taken into the main chain, a tertiary amino group and a nitrogen-containing heterocyclic ring are preferable, and the tertiary amino group is particularly preferable. preferable.
Examples of the tertiary amino group include a dimethylamino group and a diethylamino group.
Examples of the nitrogen-containing heterocycle include 2-pyrrolidone, N-methyl-2-pyrrolidone, 1,3-dimethylimidazolidinone, ε-caprolactam, γ-butyrolactone, and the like.
In the present invention, it is preferable to have both the basic group and the acidic group from the viewpoint of insulation as well as developability.
前記塩基性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、第1級アミノ基、第2級アミノ基、第3級アミノ基、含窒素ヘテロ環などが挙げられるが、第1級アミノ基及び第2級アミノ基はイソシアネートと反応し、主鎖にとりこまれてしまうため、第3級アミノ基、含窒素へテロ環が好ましく、第3級アミノ基が特に好ましい。
前記第3級アミノ基としては、例えば、ジメチルアミノ基、ジエチルアミノ基などが挙げられる。
前記含窒素ヘテロ環としては、例えば、2-ピロリドン、N-メチル-2-ピロリドン、1,3-ジメチルイミイダゾリジノン、ε-カプロラクタム、γ-ブチロラクトンなどが挙げられる。
本発明においては、前記塩基性基と前記酸性基を併有することが、現像性だけでなく、絶縁性の点でも好ましい。 -Basic group-
The basic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a primary amino group, a secondary amino group, a tertiary amino group, and a nitrogen-containing heterocycle. However, since the primary amino group and the secondary amino group react with the isocyanate and are taken into the main chain, a tertiary amino group and a nitrogen-containing heterocyclic ring are preferable, and the tertiary amino group is particularly preferable. preferable.
Examples of the tertiary amino group include a dimethylamino group and a diethylamino group.
Examples of the nitrogen-containing heterocycle include 2-pyrrolidone, N-methyl-2-pyrrolidone, 1,3-dimethylimidazolidinone, ε-caprolactam, γ-butyrolactone, and the like.
In the present invention, it is preferable to have both the basic group and the acidic group from the viewpoint of insulation as well as developability.
-グラフト鎖-
前記グラフト鎖としては、ポリウレタン樹脂の側鎖及び末端の少なくともいずれかに有することが好ましい。
前記グラフト鎖としては、例えばポリエステル、ポリメチルメタクリレート、ポリエチレンオキシド、ポリスチレンなどが挙げられる。これらの中でも、ポリウレタン樹脂の末端にポリエステル部位を有するグラフト鎖(ポリエステルグラフト鎖)であることが、解像性の点で好ましい。
前記グラフト鎖の鎖長は、重合度が、50以下が好ましく、5~30がより好ましい。
前記グラフトの含有量は、ポリウレタン樹脂全体に対し10質量%~60質量%が好ましく、20質量%~50質量%がより好ましい。 -Graft chain-
The graft chain preferably has at least one of a side chain and a terminal of the polyurethane resin.
Examples of the graft chain include polyester, polymethyl methacrylate, polyethylene oxide, and polystyrene. Among these, a graft chain having a polyester moiety at the terminal of the polyurethane resin (polyester graft chain) is preferable from the viewpoint of resolution.
The degree of polymerization of the graft chain is preferably 50 or less, more preferably 5 to 30.
The content of the graft is preferably 10% by mass to 60% by mass and more preferably 20% by mass to 50% by mass with respect to the entire polyurethane resin.
前記グラフト鎖としては、ポリウレタン樹脂の側鎖及び末端の少なくともいずれかに有することが好ましい。
前記グラフト鎖としては、例えばポリエステル、ポリメチルメタクリレート、ポリエチレンオキシド、ポリスチレンなどが挙げられる。これらの中でも、ポリウレタン樹脂の末端にポリエステル部位を有するグラフト鎖(ポリエステルグラフト鎖)であることが、解像性の点で好ましい。
前記グラフト鎖の鎖長は、重合度が、50以下が好ましく、5~30がより好ましい。
前記グラフトの含有量は、ポリウレタン樹脂全体に対し10質量%~60質量%が好ましく、20質量%~50質量%がより好ましい。 -Graft chain-
The graft chain preferably has at least one of a side chain and a terminal of the polyurethane resin.
Examples of the graft chain include polyester, polymethyl methacrylate, polyethylene oxide, and polystyrene. Among these, a graft chain having a polyester moiety at the terminal of the polyurethane resin (polyester graft chain) is preferable from the viewpoint of resolution.
The degree of polymerization of the graft chain is preferably 50 or less, more preferably 5 to 30.
The content of the graft is preferably 10% by mass to 60% by mass and more preferably 20% by mass to 50% by mass with respect to the entire polyurethane resin.
-重合性(架橋性)基-
前記重合性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ビニル基、アクリロイル基、メタアクリロイル基、エポキシ基、オキセタニル基などが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。 -Polymerizable (crosslinkable) groups-
There is no restriction | limiting in particular as said polymeric group, According to the objective, it can select suitably, For example, a vinyl group, an acryloyl group, a methacryloyl group, an epoxy group, an oxetanyl group etc. are mentioned. These may be used individually by 1 type and may use 2 or more types together.
前記重合性基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ビニル基、アクリロイル基、メタアクリロイル基、エポキシ基、オキセタニル基などが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。 -Polymerizable (crosslinkable) groups-
There is no restriction | limiting in particular as said polymeric group, According to the objective, it can select suitably, For example, a vinyl group, an acryloyl group, a methacryloyl group, an epoxy group, an oxetanyl group etc. are mentioned. These may be used individually by 1 type and may use 2 or more types together.
-ポリウレタン樹脂骨格-
前記シリカ分散剤を構成するポリウレタン樹脂は、イソシアネート化合物と水酸基又は活性水素原子を有する化合物とを反応させて得られるポリウレタン樹脂であり、具体的には、酸性基を有するジオール化合物と、塩基性基を有するジオール化合物と、ジイソシアネート化合物とを反応させて得られる。 -Polyurethane resin skeleton-
The polyurethane resin constituting the silica dispersant is a polyurethane resin obtained by reacting an isocyanate compound with a compound having a hydroxyl group or an active hydrogen atom, specifically, a diol compound having an acidic group and a basic group. It is obtained by reacting a diol compound having a diisocyanate compound.
前記シリカ分散剤を構成するポリウレタン樹脂は、イソシアネート化合物と水酸基又は活性水素原子を有する化合物とを反応させて得られるポリウレタン樹脂であり、具体的には、酸性基を有するジオール化合物と、塩基性基を有するジオール化合物と、ジイソシアネート化合物とを反応させて得られる。 -Polyurethane resin skeleton-
The polyurethane resin constituting the silica dispersant is a polyurethane resin obtained by reacting an isocyanate compound with a compound having a hydroxyl group or an active hydrogen atom, specifically, a diol compound having an acidic group and a basic group. It is obtained by reacting a diol compound having a diisocyanate compound.
前記酸性基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、3,5-ジヒドロキシ安息香酸、2,2-ビス(ヒドロキシメチル)プロピオン酸、2,2-ビス(2-ヒドロキシエチル)プロピオン酸、2,2-ビス(3-ヒドロキシプロピル)プロピオン酸、ビス(ヒドロキシメチル)酢酸、ビス(4-ヒドロキシフェニル)酢酸、2,2-ビス(ヒドロキシメチル)酪酸、4,4-ビス(4-ヒドロキシフェニル)ペンタン酸、酒石酸、N,N-ジヒドロキシエチルグリシン、N,N―ビス(2-ヒドロキシエチル)-3-カルボキシ-プロピオンアミドなどが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。
The diol compound having an acidic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 3,5-dihydroxybenzoic acid, 2,2-bis (hydroxymethyl) propionic acid, 2, 2-bis (2-hydroxyethyl) propionic acid, 2,2-bis (3-hydroxypropyl) propionic acid, bis (hydroxymethyl) acetic acid, bis (4-hydroxyphenyl) acetic acid, 2,2-bis (hydroxymethyl) ) Butyric acid, 4,4-bis (4-hydroxyphenyl) pentanoic acid, tartaric acid, N, N-dihydroxyethylglycine, N, N-bis (2-hydroxyethyl) -3-carboxy-propionamide and the like. These may be used individually by 1 type and may use 2 or more types together.
前記塩基性基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、3-(ジメチルアミノ)-1,2-プロパンジオールなどが挙げられる。前記塩基性基を有するジオール化合物は、アミノ基を有するグラフト鎖と異なり、モノマー中のアミン含率が高いのでシリカ分散剤のアミン価を高くできる。
The diol compound having a basic group is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 3- (dimethylamino) -1,2-propanediol. Unlike the graft chain having an amino group, the diol compound having a basic group has a high amine content in the monomer, so that the amine value of the silica dispersant can be increased.
前記ジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、2,4-トリレンジイソシアネート、2,4-トリレンジイソシアネートの二量体、2,6-トリレンジレンジイソシアネート、p-キシリレンジイソシアネート、m-キシリレンジイソシアネート、4,4’-ジフェニルメタンジイソシアネート(MDI)、1,5-ナフチレンジイソシアネート、3,3’-ジメチルビフェニル-4,4’-ジイソシアネート等のような芳香族ジイソシアネート化合物;ヘキサメチレンジイソシアネート、トリメチルヘキサメチレンジイソシアネート、リジンジイソシアネート、ダイマー酸ジイソシアネート等の脂肪族ジイソシアネート化合物;イソホロンジイソシアネート、4,4’-メチレンビス(シクロヘキシルイソシアネート)、メチルシクロヘキサン-2,4(又は2,6)ジイソシアネート、1,3-(イソシアネートメチル)シクロヘキサン等の脂環族ジイソシアネート化合物;1,3-ブチレングリコール1モルとトリレンジイソシアネート2モルとの付加体等のジオールとジイソシアネートとの反応物であるジイソシアネート化合物;などが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。これらの中でも、硬度の観点から4,4’-ジフェニルメタンジイソシアネート(MDI)が特に好ましい。
The diisocyanate compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include 2,4-tolylene diisocyanate, dimer of 2,4-tolylene diisocyanate, and 2,6-tolylene diene. Range isocyanate, p-xylylene diisocyanate, m-xylylene diisocyanate, 4,4'-diphenylmethane diisocyanate (MDI), 1,5-naphthylene diisocyanate, 3,3'-dimethylbiphenyl-4,4'-diisocyanate, etc. Aromatic diisocyanate compounds; aliphatic diisocyanate compounds such as hexamethylene diisocyanate, trimethylhexamethylene diisocyanate, lysine diisocyanate, dimer acid diisocyanate; isophorone diisocyanate, 4,4′-methyle Alicyclic diisocyanate compounds such as bis (cyclohexyl isocyanate), methylcyclohexane-2,4 (or 2,6) diisocyanate, 1,3- (isocyanatomethyl) cyclohexane; 1 mol of 1,3-butylene glycol and tolylene diisocyanate 2 A diisocyanate compound which is a reaction product of a diol such as an adduct with a mole and a diisocyanate; These may be used individually by 1 type and may use 2 or more types together. Among these, 4,4'-diphenylmethane diisocyanate (MDI) is particularly preferable from the viewpoint of hardness.
本発明のポリウレタン樹脂を合成する際には末端封止剤を添加してもよい。前記末端封止剤としては、例えば、モノアルコール化合物、モノイソシアネート化合物、片末端にアルコールを有するポリエステル、片末端にアルコールを有するポリエチレンオキシドなどが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。
When synthesizing the polyurethane resin of the present invention, a terminal blocking agent may be added. Examples of the end capping agent include monoalcohol compounds, monoisocyanate compounds, polyesters having alcohol at one end, and polyethylene oxides having alcohol at one end. These may be used individually by 1 type and may use 2 or more types together.
前記シリカ分散剤としては、適宜合成したものを使用してもよいし、市販品を使用してもよい。
As the silica dispersant, an appropriately synthesized one or a commercially available product may be used.
前記シリカ分散剤のアミン価は、0.65mmol/g以上であり、0.75mmol/g以上が好ましく、0.9mmol/g以上がより好ましい。前記アミン価が、0.65mmol/g未満であると、溶融粘度が上昇し、埋め込み性が悪化することがある。
ここで、前記アミン価の測定は、例えば、試料をビーカーに秤とり、酢酸を加え、撹拌して溶解させて、測定温度を25℃に調整後、滴定試薬として0.1N過塩素酸酢酸を用いて、滴定装置で滴定することにより、求めることができる。 The amine value of the silica dispersant is 0.65 mmol / g or more, preferably 0.75 mmol / g or more, and more preferably 0.9 mmol / g or more. If the amine value is less than 0.65 mmol / g, the melt viscosity may increase and the embedding property may deteriorate.
Here, the amine value is measured, for example, by weighing a sample in a beaker, adding acetic acid, stirring and dissolving, adjusting the measurement temperature to 25 ° C., and then adding 0.1N perchloric acid acetic acid as a titration reagent. And can be determined by titrating with a titration apparatus.
ここで、前記アミン価の測定は、例えば、試料をビーカーに秤とり、酢酸を加え、撹拌して溶解させて、測定温度を25℃に調整後、滴定試薬として0.1N過塩素酸酢酸を用いて、滴定装置で滴定することにより、求めることができる。 The amine value of the silica dispersant is 0.65 mmol / g or more, preferably 0.75 mmol / g or more, and more preferably 0.9 mmol / g or more. If the amine value is less than 0.65 mmol / g, the melt viscosity may increase and the embedding property may deteriorate.
Here, the amine value is measured, for example, by weighing a sample in a beaker, adding acetic acid, stirring and dissolving, adjusting the measurement temperature to 25 ° C., and then adding 0.1N perchloric acid acetic acid as a titration reagent. And can be determined by titrating with a titration apparatus.
前記シリカ分散剤の酸価は、0.3mmol/g以上が好ましく、0.45mmol/g以上がより好ましい。前記酸価が、0.3mmol/g未満であると、現像性が悪化し、未露光部に残渣が発生することがある。
ここで、前記酸価の測定は、例えば、試料をビーカーに秤とり、テトラヒドロフラン(THF)/水=5/1(体積比)の溶液を加え、撹拌して溶解させて、測定温度を25℃に調整した後、滴定試薬として0.1NのNaOH水溶液を用いて、滴定装置で滴定することにより、酸価を求めることができる。 The acid value of the silica dispersant is preferably 0.3 mmol / g or more, and more preferably 0.45 mmol / g or more. If the acid value is less than 0.3 mmol / g, developability may deteriorate and a residue may be generated in an unexposed area.
Here, for the measurement of the acid value, for example, a sample is weighed in a beaker, a solution of tetrahydrofuran (THF) / water = 5/1 (volume ratio) is added, stirred and dissolved, and the measurement temperature is 25 ° C. Then, the acid value can be determined by titrating with a titration apparatus using a 0.1N NaOH aqueous solution as a titration reagent.
ここで、前記酸価の測定は、例えば、試料をビーカーに秤とり、テトラヒドロフラン(THF)/水=5/1(体積比)の溶液を加え、撹拌して溶解させて、測定温度を25℃に調整した後、滴定試薬として0.1NのNaOH水溶液を用いて、滴定装置で滴定することにより、酸価を求めることができる。 The acid value of the silica dispersant is preferably 0.3 mmol / g or more, and more preferably 0.45 mmol / g or more. If the acid value is less than 0.3 mmol / g, developability may deteriorate and a residue may be generated in an unexposed area.
Here, for the measurement of the acid value, for example, a sample is weighed in a beaker, a solution of tetrahydrofuran (THF) / water = 5/1 (volume ratio) is added, stirred and dissolved, and the measurement temperature is 25 ° C. Then, the acid value can be determined by titrating with a titration apparatus using a 0.1N NaOH aqueous solution as a titration reagent.
<シリカ微粒子>
前記シリカ微粒子におけるシリカとしては、特に制限はなく、目的に応じて適宜選択でき、例えば、気相法シリカ、結晶性シリカ、溶融シリカなどが挙げられる。
前記シリカ微粒子としては、適宜合成したものを使用してもよいし、市販品を使用してもよい。該市販品としては、例えば、SO-C2(アドマテックス社製)などが挙げられる。 <Silica fine particles>
There is no restriction | limiting in particular as a silica in the said silica fine particle, According to the objective, it can select suitably, For example, vapor phase method silica, crystalline silica, fused silica etc. are mentioned.
As the silica fine particles, those appropriately synthesized may be used, or commercially available products may be used. Examples of the commercially available product include SO-C2 (manufactured by Admatechs).
前記シリカ微粒子におけるシリカとしては、特に制限はなく、目的に応じて適宜選択でき、例えば、気相法シリカ、結晶性シリカ、溶融シリカなどが挙げられる。
前記シリカ微粒子としては、適宜合成したものを使用してもよいし、市販品を使用してもよい。該市販品としては、例えば、SO-C2(アドマテックス社製)などが挙げられる。 <Silica fine particles>
There is no restriction | limiting in particular as a silica in the said silica fine particle, According to the objective, it can select suitably, For example, vapor phase method silica, crystalline silica, fused silica etc. are mentioned.
As the silica fine particles, those appropriately synthesized may be used, or commercially available products may be used. Examples of the commercially available product include SO-C2 (manufactured by Admatechs).
前記シリカ微粒子の平均粒径(d50)は、特に制限はなく、目的に応じて適宜選択することができるが、0.2μm~3.0μmが好ましく、0.3μm~2.5μmがより好ましく、0.5μm~2.5μmが特に好ましい。
前記シリカ粒子の平均粒径(d50)が、0.2μm未満であると、塗布粘度が高くなってしまうことがあり、3.0μmを超えると、平滑性を維持することができないことがある。一方、前記シリカ粒子の平均粒径(d50)が、前記特に好ましい範囲内であると、塗布粘度と硬化膜の平滑性及び耐熱性の点で有利である。
ここで、前記シリカ粒子の平均粒径(d50)は、積算(累積)重量百分率で表したときの積算値50%の粒度で定義されるもので、d50(D50)などと定義されるものであり、例えば、ダイナミック光散乱光度計(商品名:DLS7000、大塚電子社製)を用いて、測定原理を動的光散乱法として、サイズ分布解析手法をキュムラント法、ヒストグラム法などにより、測定することができる。 The average particle diameter (d50) of the silica fine particles is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.2 μm to 3.0 μm, more preferably 0.3 μm to 2.5 μm, 0.5 μm to 2.5 μm is particularly preferable.
When the average particle diameter (d50) of the silica particles is less than 0.2 μm, the coating viscosity may be increased, and when it exceeds 3.0 μm, smoothness may not be maintained. On the other hand, when the average particle diameter (d50) of the silica particles is within the particularly preferable range, it is advantageous in terms of coating viscosity, smoothness of the cured film and heat resistance.
The average particle size of the silica particles (d50), the integration (accumulation) intended to be defined by the integrated value of 50% particle size when expressed in weight percentage, d50 (D 50) such as those defined For example, using a dynamic light scattering photometer (trade name: DLS7000, manufactured by Otsuka Electronics Co., Ltd.), the measurement principle is the dynamic light scattering method, and the size distribution analysis method is measured by the cumulant method, the histogram method, or the like. be able to.
前記シリカ粒子の平均粒径(d50)が、0.2μm未満であると、塗布粘度が高くなってしまうことがあり、3.0μmを超えると、平滑性を維持することができないことがある。一方、前記シリカ粒子の平均粒径(d50)が、前記特に好ましい範囲内であると、塗布粘度と硬化膜の平滑性及び耐熱性の点で有利である。
ここで、前記シリカ粒子の平均粒径(d50)は、積算(累積)重量百分率で表したときの積算値50%の粒度で定義されるもので、d50(D50)などと定義されるものであり、例えば、ダイナミック光散乱光度計(商品名:DLS7000、大塚電子社製)を用いて、測定原理を動的光散乱法として、サイズ分布解析手法をキュムラント法、ヒストグラム法などにより、測定することができる。 The average particle diameter (d50) of the silica fine particles is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.2 μm to 3.0 μm, more preferably 0.3 μm to 2.5 μm, 0.5 μm to 2.5 μm is particularly preferable.
When the average particle diameter (d50) of the silica particles is less than 0.2 μm, the coating viscosity may be increased, and when it exceeds 3.0 μm, smoothness may not be maintained. On the other hand, when the average particle diameter (d50) of the silica particles is within the particularly preferable range, it is advantageous in terms of coating viscosity, smoothness of the cured film and heat resistance.
The average particle size of the silica particles (d50), the integration (accumulation) intended to be defined by the integrated value of 50% particle size when expressed in weight percentage, d50 (D 50) such as those defined For example, using a dynamic light scattering photometer (trade name: DLS7000, manufactured by Otsuka Electronics Co., Ltd.), the measurement principle is the dynamic light scattering method, and the size distribution analysis method is measured by the cumulant method, the histogram method, or the like. be able to.
前記シリカ微粒子の前記シリカ分散組成物における含有量は、16質量%~80質量%が好ましく、25質量%~70質量%がより好ましい。
前記含有量が、16質量%未満であると、耐衝撃性が劣ることがあり、80質量%を超えると、分散性が不十分となることがある。 The content of the silica fine particles in the silica dispersion composition is preferably 16% by mass to 80% by mass, and more preferably 25% by mass to 70% by mass.
When the content is less than 16% by mass, impact resistance may be inferior, and when it exceeds 80% by mass, dispersibility may be insufficient.
前記含有量が、16質量%未満であると、耐衝撃性が劣ることがあり、80質量%を超えると、分散性が不十分となることがある。 The content of the silica fine particles in the silica dispersion composition is preferably 16% by mass to 80% by mass, and more preferably 25% by mass to 70% by mass.
When the content is less than 16% by mass, impact resistance may be inferior, and when it exceeds 80% by mass, dispersibility may be insufficient.
<熱架橋剤>
前記熱架橋剤としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、エポキシ樹脂、多官能オキセタン化合物、などが挙げられる。
これらの中でも、1分子内に少なくとも2つのオキシラン基を有するエポキシ樹脂化合物、1分子内に少なくとも2つのオキセタニル基を有するオキセタン化合物が好ましい。 <Thermal crosslinking agent>
There is no restriction | limiting in particular as said thermal crosslinking agent, According to the objective, it can select suitably, For example, an epoxy resin, a polyfunctional oxetane compound, etc. are mentioned.
Among these, an epoxy resin compound having at least two oxirane groups in one molecule and an oxetane compound having at least two oxetanyl groups in one molecule are preferable.
前記熱架橋剤としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、エポキシ樹脂、多官能オキセタン化合物、などが挙げられる。
これらの中でも、1分子内に少なくとも2つのオキシラン基を有するエポキシ樹脂化合物、1分子内に少なくとも2つのオキセタニル基を有するオキセタン化合物が好ましい。 <Thermal crosslinking agent>
There is no restriction | limiting in particular as said thermal crosslinking agent, According to the objective, it can select suitably, For example, an epoxy resin, a polyfunctional oxetane compound, etc. are mentioned.
Among these, an epoxy resin compound having at least two oxirane groups in one molecule and an oxetane compound having at least two oxetanyl groups in one molecule are preferable.
前記エポキシ樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0095〕に記載された化合物、特開2010-72340号公報の段落〔0130〕に記載された化合物などが挙げられる。
The epoxy resin is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0095] of JP-A-2007-2030, and JP-A-2010-72340. Examples include the compounds described in paragraph [0130].
前記多官能オキセタン化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0096〕に記載された化合物などが挙げられる。
The polyfunctional oxetane compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include the compounds described in paragraph [0096] of JP-A-2007-2030.
前記熱架橋剤の前記シリカ分散組成物における含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、1質量%~50質量%が好ましく、2質量%~40質量%がより好ましく、3質量%~30質量%が特に好ましい。
前記含有量が、1質量%未満であると、耐熱性が劣ることがあり、50質量%を超えると、現像性及び耐クラック性が劣ることがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好な感度で硬化膜が作製でき、形成された硬化膜も、耐熱性と耐クラック性とを両立できる点で有利である。 The content of the thermal crosslinking agent in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 1% by mass to 50% by mass, and 2% by mass to 40% by mass. Is more preferable, and 3% by mass to 30% by mass is particularly preferable.
When the content is less than 1% by mass, heat resistance may be inferior, and when it exceeds 50% by mass, developability and crack resistance may be inferior. On the other hand, when the content is within the particularly preferable range, a cured film can be produced with good sensitivity, and the formed cured film is advantageous in that both heat resistance and crack resistance can be achieved.
前記含有量が、1質量%未満であると、耐熱性が劣ることがあり、50質量%を超えると、現像性及び耐クラック性が劣ることがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好な感度で硬化膜が作製でき、形成された硬化膜も、耐熱性と耐クラック性とを両立できる点で有利である。 The content of the thermal crosslinking agent in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 1% by mass to 50% by mass, and 2% by mass to 40% by mass. Is more preferable, and 3% by mass to 30% by mass is particularly preferable.
When the content is less than 1% by mass, heat resistance may be inferior, and when it exceeds 50% by mass, developability and crack resistance may be inferior. On the other hand, when the content is within the particularly preferable range, a cured film can be produced with good sensitivity, and the formed cured film is advantageous in that both heat resistance and crack resistance can be achieved.
前記その他の熱架橋剤としては、前記エポキシ樹脂及び前記多官能オキセタン化合物とは別に添加することができる。前記その他の熱架橋剤としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0098〕~〔0100〕に記載された化合物などが挙げられる。
The other thermal crosslinking agent can be added separately from the epoxy resin and the polyfunctional oxetane compound. The other thermal crosslinking agent is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0098] to [0100] of JP-A-2007-2030. Can be mentioned.
<<バインダー>>
前記バインダーとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、酸変性ビニル基含有ポリウレタン樹脂、不飽和基含有ポリカルボン酸樹脂、酸変性ビニル基含有エポキシ樹脂、不飽和基及びカルボキシル基を含有する樹脂、ポリイミド前駆体などが挙げられる。 << Binder >>
The binder is not particularly limited and may be appropriately selected depending on the intended purpose. For example, acid-modified vinyl group-containing polyurethane resin, unsaturated group-containing polycarboxylic acid resin, acid-modified vinyl group-containing epoxy resin, unsaturated Examples thereof include a resin containing a group and a carboxyl group, and a polyimide precursor.
前記バインダーとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、酸変性ビニル基含有ポリウレタン樹脂、不飽和基含有ポリカルボン酸樹脂、酸変性ビニル基含有エポキシ樹脂、不飽和基及びカルボキシル基を含有する樹脂、ポリイミド前駆体などが挙げられる。 << Binder >>
The binder is not particularly limited and may be appropriately selected depending on the intended purpose. For example, acid-modified vinyl group-containing polyurethane resin, unsaturated group-containing polycarboxylic acid resin, acid-modified vinyl group-containing epoxy resin, unsaturated Examples thereof include a resin containing a group and a carboxyl group, and a polyimide precursor.
-酸変性ビニル基含有ポリウレタン樹脂-
前記酸変性ビニル基含有ポリウレタン樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、(i)側鎖にエチレン性不飽和結合(ビニル基)を有するポリウレタン樹脂、(ii)カルボキシル基含有ポリウレタンと分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂などが挙げられる。 -Acid-modified vinyl group-containing polyurethane resin-
The acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the purpose. For example, (i) a polyurethane resin having an ethylenically unsaturated bond (vinyl group) in the side chain, ii) A polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule.
前記酸変性ビニル基含有ポリウレタン樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、(i)側鎖にエチレン性不飽和結合(ビニル基)を有するポリウレタン樹脂、(ii)カルボキシル基含有ポリウレタンと分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂などが挙げられる。 -Acid-modified vinyl group-containing polyurethane resin-
The acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the purpose. For example, (i) a polyurethane resin having an ethylenically unsaturated bond (vinyl group) in the side chain, ii) A polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule.
--(i)側鎖にビニル基を有するポリウレタン樹脂--
前記側鎖にビニル基を有するウレタン樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、その側鎖に、下記一般式(1)~(3)で表される官能基のうち少なくとも1つを有するものが挙げられる。
-(I) Polyurethane resin having vinyl groups in the side chain--
The urethane resin having a vinyl group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose. For example, the side chain is represented by the following general formulas (1) to (3). The thing which has at least 1 among functional groups is mentioned.
前記側鎖にビニル基を有するウレタン樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、その側鎖に、下記一般式(1)~(3)で表される官能基のうち少なくとも1つを有するものが挙げられる。
The urethane resin having a vinyl group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose. For example, the side chain is represented by the following general formulas (1) to (3). The thing which has at least 1 among functional groups is mentioned.
前記一般式(1)において、前記R1としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、水素原子、置換基を有してもよいアルキル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、メチル基が好ましい。また、前記R2及びR3としては、特に制限はなく、目的に応じて適宜選択することができ、それぞれ独立に、例えば、水素原子、ハロゲン原子、アミノ基、カルボキシル基、アルコキシカルボニル基、スルホ基、ニトロ基、シアノ基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基、置換基を有してもよいアルコキシ基、置換基を有してもよいアリールオキシ基、置換基を有してもよいアルキルアミノ基、置換基を有してもよいアリールアミノ基、置換基を有してもよいアルキルスルホニル基、置換基を有してもよいアリールスルホニル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、カルボキシル基、アルコキシカルボニル基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基が好ましい。
前記一般式(1)中、Xは、酸素原子、硫黄原子、又は-N(R12)-を表し、前記R12は、水素原子、又は1価の有機基を表す。前記R12としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、置換基を有してもよいアルキル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、メチル基、エチル基、イソプロピル基が好ましい。
ここで、導入し得る前記置換基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、アルキル基、アルケニル基、アルキニル基、アリール基、アルコキシ基、アリーロキシ基、ハロゲン原子、アミノ基、アルキルアミノ基、アリールアミノ基、カルボキシル基、アルコキシカルボニル基、スルホ基、ニトロ基、シアノ基、アミド基、アルキルスルホニル基、アリールスルホニル基などが挙げられる。 In the general formula (1), R 1 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a hydrogen atom and an alkyl group which may have a substituent. Among these, a hydrogen atom and a methyl group are preferable in terms of high radical reactivity. The R 2 and R 3 are not particularly limited and may be appropriately selected depending on the purpose. For example, each of R 2 and R 3 is independently a hydrogen atom, a halogen atom, an amino group, a carboxyl group, an alkoxycarbonyl group, a sulfo group. Group, nitro group, cyano group, alkyl group which may have a substituent, aryl group which may have a substituent, alkoxy group which may have a substituent, aryl which may have a substituent An oxy group, an alkylamino group which may have a substituent, an arylamino group which may have a substituent, an alkylsulfonyl group which may have a substituent, an arylsulfonyl group which may have a substituent Etc. Among these, a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
In the general formula (1), X represents an oxygen atom, a sulfur atom, or —N (R 12 ) —, and R 12 represents a hydrogen atom or a monovalent organic group. R 12 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group which may have a substituent. Among these, a hydrogen atom, a methyl group, an ethyl group, and an isopropyl group are preferable because of high radical reactivity.
Here, the substituent that can be introduced is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group, an alkenyl group, an alkynyl group, an aryl group, an alkoxy group, an aryloxy group, and a halogen atom. Amino group, alkylamino group, arylamino group, carboxyl group, alkoxycarbonyl group, sulfo group, nitro group, cyano group, amide group, alkylsulfonyl group, arylsulfonyl group and the like.
前記一般式(1)中、Xは、酸素原子、硫黄原子、又は-N(R12)-を表し、前記R12は、水素原子、又は1価の有機基を表す。前記R12としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、置換基を有してもよいアルキル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、メチル基、エチル基、イソプロピル基が好ましい。
ここで、導入し得る前記置換基としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、アルキル基、アルケニル基、アルキニル基、アリール基、アルコキシ基、アリーロキシ基、ハロゲン原子、アミノ基、アルキルアミノ基、アリールアミノ基、カルボキシル基、アルコキシカルボニル基、スルホ基、ニトロ基、シアノ基、アミド基、アルキルスルホニル基、アリールスルホニル基などが挙げられる。 In the general formula (1), R 1 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include a hydrogen atom and an alkyl group which may have a substituent. Among these, a hydrogen atom and a methyl group are preferable in terms of high radical reactivity. The R 2 and R 3 are not particularly limited and may be appropriately selected depending on the purpose. For example, each of R 2 and R 3 is independently a hydrogen atom, a halogen atom, an amino group, a carboxyl group, an alkoxycarbonyl group, a sulfo group. Group, nitro group, cyano group, alkyl group which may have a substituent, aryl group which may have a substituent, alkoxy group which may have a substituent, aryl which may have a substituent An oxy group, an alkylamino group which may have a substituent, an arylamino group which may have a substituent, an alkylsulfonyl group which may have a substituent, an arylsulfonyl group which may have a substituent Etc. Among these, a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
In the general formula (1), X represents an oxygen atom, a sulfur atom, or —N (R 12 ) —, and R 12 represents a hydrogen atom or a monovalent organic group. R 12 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group which may have a substituent. Among these, a hydrogen atom, a methyl group, an ethyl group, and an isopropyl group are preferable because of high radical reactivity.
Here, the substituent that can be introduced is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group, an alkenyl group, an alkynyl group, an aryl group, an alkoxy group, an aryloxy group, and a halogen atom. Amino group, alkylamino group, arylamino group, carboxyl group, alkoxycarbonyl group, sulfo group, nitro group, cyano group, amide group, alkylsulfonyl group, arylsulfonyl group and the like.
前記一般式(2)において、R4~R8としては、特に制限はなく、目的に応じて適宜選択することができ、水素原子、ハロゲン原子、アミノ基、ジアルキルアミノ基、カルボキシル基、アルコキシカルボニル基、スルホ基、ニトロ基、シアノ基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基、置換基を有してもよいアルコキシ基、置換基を有してもよいアリールオキシ基、置換基を有してもよいアルキルアミノ基、置換基を有してもよいアリールアミノ基、置換基を有してもよいアルキルスルホニル基、置換基を有してもよいアリールスルホニル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、カルボキシル基、アルコキシカルボニル基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基が好ましい。
In the general formula (2), R 4 to R 8 are not particularly limited and may be appropriately selected depending on the intended purpose. The hydrogen atom, halogen atom, amino group, dialkylamino group, carboxyl group, alkoxycarbonyl A group, a sulfo group, a nitro group, a cyano group, an alkyl group which may have a substituent, an aryl group which may have a substituent, an alkoxy group which may have a substituent, and a substituent. An aryloxy group which may have a substituent, an alkylamino group which may have a substituent, an arylamino group which may have a substituent, an alkylsulfonyl group which may have a substituent, and a substituent. An arylsulfonyl group etc. are mentioned. Among these, a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
導入し得る置換基としては、前記一般式(1)と同様のものが挙げられる。また、Yは、酸素原子、硫黄原子、又は-N(R12)-を表す。前記R12は、前記一般式(1)のR12の場合と同義であり、好ましい例も同様である。
Examples of the substituent that can be introduced include the same as those in the general formula (1). Y represents an oxygen atom, a sulfur atom, or —N (R 12 ) —. Said R < 12 > is synonymous with the case of R < 12 > of the said General formula (1), and its preferable example is also the same.
前記一般式(3)において、前記R9としては、特に制限はなく、目的に応じて適宜選択することができ、水素原子又は置換基を有してもよいアルキル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、メチル基が好ましい。前記一般式(3)中、前記R10及びR11としては、特に制限はなく、目的に応じて適宜選択することができ、水素原子、ハロゲン原子、アミノ基、ジアルキルアミノ基、カルボキシル基、アルコキシカルボニル基、スルホ基、ニトロ基、シアノ基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基、置換基を有してもよいアルコキシ基、置換基を有してもよいアリールオキシ基、置換基を有してもよいアルキルアミノ基、置換基を有してもよいアリールアミノ基、置換基を有してもよいアルキルスルホニル基、置換基を有してもよいアリールスルホニル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、水素原子、カルボキシル基、アルコキシカルボニル基、置換基を有してもよいアルキル基、置換基を有してもよいアリール基が好ましい。
In the general formula (3), R 9 is not particularly limited and may be appropriately selected depending on the purpose. Examples thereof include a hydrogen atom or an alkyl group which may have a substituent. Among these, a hydrogen atom and a methyl group are preferable in terms of high radical reactivity. In the general formula (3), R 10 and R 11 are not particularly limited and may be appropriately selected depending on the intended purpose. A hydrogen atom, a halogen atom, an amino group, a dialkylamino group, a carboxyl group, an alkoxy group A carbonyl group, a sulfo group, a nitro group, a cyano group, an alkyl group which may have a substituent, an aryl group which may have a substituent, an alkoxy group which may have a substituent, and a substituent An aryloxy group which may have a substituent, an alkylamino group which may have a substituent, an arylamino group which may have a substituent, an alkylsulfonyl group which may have a substituent, and a substituent. And a good arylsulfonyl group. Among these, a hydrogen atom, a carboxyl group, an alkoxycarbonyl group, an alkyl group which may have a substituent, and an aryl group which may have a substituent are preferable because of high radical reactivity.
ここで、導入し得る置換基としては、前記一般式(1)と同様のものが挙げられる。また、Zは、酸素原子、硫黄原子、-N(R13)-、又は置換基を有してもよいフェニレン基を表す。前記R13としては、特に制限はなく、目的に応じて適宜選択することができ、置換基を有してもよいアルキル基などが挙げられる。これらの中でも、ラジカル反応性が高い点で、メチル基、エチル基、イソプロピル基が好ましい。
Here, examples of the substituent that can be introduced include the same as those in the general formula (1). Z represents an oxygen atom, a sulfur atom, —N (R 13 ) —, or an optionally substituted phenylene group. R 13 is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include an alkyl group which may have a substituent. Among these, a methyl group, an ethyl group, and an isopropyl group are preferable because of high radical reactivity.
前記側鎖にエチレン性不飽和結合を有するウレタン樹脂は、下記一般式(4)で表されるジイソシアネート化合物の少なくとも1種と、下記一般式(5)で表されるジオール化合物の少なくとも1種と、の反応生成物で表される構造単位を基本骨格とするポリウレタン樹脂である。
The urethane resin having an ethylenically unsaturated bond in the side chain includes at least one diisocyanate compound represented by the following general formula (4) and at least one diol compound represented by the following general formula (5): A polyurethane resin having a structural unit represented by the reaction product as a basic skeleton.
OCN-X0-NCO ・・・一般式(4)
HO-Y0-OH ・・・一般式(5)
ただし、前記一般式(4)及び(5)中、X0、Y0は、それぞれ独立に2価の有機残基を表す。 OCN-X 0 -NCO General formula (4)
HO—Y 0 —OH —General formula (5)
However, in the general formulas (4) and (5), X 0 and Y 0 each independently represent a divalent organic residue.
HO-Y0-OH ・・・一般式(5)
ただし、前記一般式(4)及び(5)中、X0、Y0は、それぞれ独立に2価の有機残基を表す。 OCN-X 0 -NCO General formula (4)
HO—Y 0 —OH —General formula (5)
However, in the general formulas (4) and (5), X 0 and Y 0 each independently represent a divalent organic residue.
前記一般式(4)で表されるジイソシアネート化合物、又は、前記一般式(5)で表されるジオール化合物の少なくともどちらか一方が、前記一般式(1)~(3)で表される基のうち少なくとも1つを有していれば、当該ジイソシアネート化合物と当該ジオール化合物との反応生成物として、側鎖に前記一般式(1)~(3)で表される基が導入されたポリウレタン樹脂が生成される。かかる方法によれば、ポリウレタン樹脂の反応生成後に所望の側鎖を置換、導入するよりも、側鎖に前記一般式(1)~(3)で表される基が導入されたポリウレタン樹脂を容易に製造することができる。
At least one of the diisocyanate compound represented by the general formula (4) and the diol compound represented by the general formula (5) is a group represented by the general formulas (1) to (3). If at least one of them is present, a polyurethane resin in which the groups represented by the above general formulas (1) to (3) are introduced into the side chain as a reaction product of the diisocyanate compound and the diol compound is provided. Generated. According to such a method, a polyurethane resin in which the groups represented by the general formulas (1) to (3) are introduced into the side chain can be easily used, rather than replacing and introducing a desired side chain after the reaction of the polyurethane resin. Can be manufactured.
前記一般式(4)で表されるジイソシアネート化合物としては、特に制限されるものではなく、目的に応じて適宜選択することができ、例えば、トリイソシアネート化合物と、不飽和基を有する単官能のアルコール又は単官能のアミン化合物1当量とを付加反応させて得られる生成物、などが挙げられる。
前記トリイソシアネート化合物としては、特に制限されるものではなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0034〕~〔0035〕に記載された化合物などが挙げられる。 The diisocyanate compound represented by the general formula (4) is not particularly limited and can be appropriately selected depending on the purpose. For example, a triisocyanate compound and a monofunctional alcohol having an unsaturated group Or the product obtained by addition-reacting with 1 equivalent of monofunctional amine compounds is mentioned.
The triisocyanate compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0034] to [0035] of JP-A-2005-250438. Is mentioned.
前記トリイソシアネート化合物としては、特に制限されるものではなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0034〕~〔0035〕に記載された化合物などが挙げられる。 The diisocyanate compound represented by the general formula (4) is not particularly limited and can be appropriately selected depending on the purpose. For example, a triisocyanate compound and a monofunctional alcohol having an unsaturated group Or the product obtained by addition-reacting with 1 equivalent of monofunctional amine compounds is mentioned.
The triisocyanate compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0034] to [0035] of JP-A-2005-250438. Is mentioned.
前記一般式(5)で表されるジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ポリエーテルジオール化合物、ポリエステルジオール化合物、ポリカーボネートジオール化合物などが挙げられる。
The diol compound represented by the general formula (5) is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include polyether diol compounds, polyester diol compounds, and polycarbonate diol compounds.
前記不飽和基を有する単官能のアルコール又は前記単官能のアミン化合物としては、特に制限されるものではなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0037〕~〔0040〕に記載された化合物などが挙げられる。
The monofunctional alcohol having an unsaturated group or the monofunctional amine compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, paragraphs of JP-A-2005-250438 Examples thereof include compounds described in [0037] to [0040].
ここで、前記ポリウレタン樹脂の側鎖に不飽和基を導入する方法としては、特に制限はなく、目的に応じて適宜選択することができるが、ポリウレタン樹脂製造の原料として、側鎖に不飽和基を含有するジイソシアネート化合物を用いる方法が好ましい。前記ジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することができ、トリイソシアネート化合物と不飽和基を有する単官能のアルコール又は単官能のアミン化合物1当量とを付加反応させることにより得ることできるジイソシアネート化合物であって、例えば、特開2005-250438号公報の段落〔0042〕~〔0049〕に記載された側鎖に不飽和基を有する化合物などが挙げられる。
Here, the method for introducing an unsaturated group into the side chain of the polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose. A method using a diisocyanate compound containing is preferable. There is no restriction | limiting in particular as said diisocyanate compound, According to the objective, it can select suitably, By carrying out addition reaction of the monoisocyanate which has a triisocyanate compound, and a monofunctional amine compound or monofunctional amine compound. Examples of diisocyanate compounds that can be obtained include compounds having an unsaturated group in the side chain described in paragraphs [0042] to [0049] of JP-A-2005-250438.
前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂は、重合性組成物中の他の成分との相溶性を向上させ、保存安定性を向上させるといった観点から、前記不飽和基を含有するジイソシアネート化合物以外のジイソシアネート化合物を共重合させることもできる。
The polyurethane resin having an ethylenically unsaturated bond in the side chain is a diisocyanate containing the unsaturated group from the viewpoint of improving compatibility with other components in the polymerizable composition and improving storage stability. Diisocyanate compounds other than the compounds can also be copolymerized.
前記共重合させるジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することでき、例えば、下記一般式(6)で表されるジイソシアネート化合物である。
OCN-L1-NCO ・・・一般式(6)
ただし、前記一般式(6)中、L1は、置換基を有していてもよい2価の脂肪族又は芳香族炭化水素基を表す。必要に応じて、L1は、イソシアネート基と反応しない他の官能基、例えば、エステル、ウレタン、アミド、ウレイド基を有していてもよい。 The diisocyanate compound to be copolymerized is not particularly limited and may be appropriately selected depending on the intended purpose. For example, it is a diisocyanate compound represented by the following general formula (6).
OCN-L 1 -NCO General formula (6)
However, in the general formula (6), L 1 represents an aliphatic or aromatic hydrocarbon group which may have a substituent. If necessary, L 1 may have another functional group that does not react with an isocyanate group, for example, an ester, urethane, amide, or ureido group.
OCN-L1-NCO ・・・一般式(6)
ただし、前記一般式(6)中、L1は、置換基を有していてもよい2価の脂肪族又は芳香族炭化水素基を表す。必要に応じて、L1は、イソシアネート基と反応しない他の官能基、例えば、エステル、ウレタン、アミド、ウレイド基を有していてもよい。 The diisocyanate compound to be copolymerized is not particularly limited and may be appropriately selected depending on the intended purpose. For example, it is a diisocyanate compound represented by the following general formula (6).
OCN-L 1 -NCO General formula (6)
However, in the general formula (6), L 1 represents an aliphatic or aromatic hydrocarbon group which may have a substituent. If necessary, L 1 may have another functional group that does not react with an isocyanate group, for example, an ester, urethane, amide, or ureido group.
前記一般式(6)で表されるジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することでき、例えば、2,4-トリレンジイソシアネート、2,4-トリレンジイソシアネートの二量体、2,6-トリレンジレンジイソシアネート、p-キシリレンジイソシアネート、m-キシリレンジイソシアネート、4,4’-ジフェニルメタンジイソシアネート、1,5-ナフチレンジイソシアネート、3,3’-ジメチルビフェニル-4,4’-ジイソシアネート等のような芳香族ジイソシアネート化合物;ヘキサメチレンジイソシアネート、トリメチルヘキサメチレンジイソシアネート、リジンジイソシアネート、ダイマー酸ジイソシアネート等の脂肪族ジイソシアネート化合物;イソホロンジイソシアネート、4,4’-メチレンビス(シクロヘキシルイソシアネート)、メチルシクロヘキサン-2,4(又は2,6)ジイソシアネート、1,3-(イソシアネートメチル)シクロヘキサン等の脂環族ジイソシアネート化合物;1,3-ブチレングリコール1モルとトリレンジイソシアネート2モルとの付加体等のジオールとジイソシアネートとの反応物であるジイソシアネート化合物;などが挙げられる。
The diisocyanate compound represented by the general formula (6) is not particularly limited and may be appropriately selected depending on the intended purpose. For example, dimer of 2,4-tolylene diisocyanate and 2,4-tolylene diisocyanate 2,6-tolylene diisocyanate, p-xylylene diisocyanate, m-xylylene diisocyanate, 4,4'-diphenylmethane diisocyanate, 1,5-naphthylene diisocyanate, 3,3'-dimethylbiphenyl-4,4 Aromatic diisocyanate compounds such as' -diisocyanate; aliphatic diisocyanate compounds such as hexamethylene diisocyanate, trimethylhexamethylene diisocyanate, lysine diisocyanate, dimer diisocyanate; isophorone diisocyanate, 4,4 -Alicyclic diisocyanate compounds such as methylenebis (cyclohexyl isocyanate), methylcyclohexane-2,4 (or 2,6) diisocyanate, 1,3- (isocyanatomethyl) cyclohexane; 1 mol of 1,3-butylene glycol and tolylene diisocyanate And a diisocyanate compound that is a reaction product of a diol such as an adduct with 2 mol and a diisocyanate.
ここで、前記ポリウレタン樹脂の側鎖に不飽和基を導入する方法としては、前述の方法の他に、ポリウレタン樹脂製造の原料として、側鎖に不飽和基を含有するジオール化合物を用いる方法も好ましい。前記側鎖に不飽和基を含有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、トリメチロールプロパンモノアリルエーテルのように市販されているものでもよいし、ハロゲン化ジオール化合物、トリオール化合物、アミノジオール化合物等の化合物と、不飽和基を含有する、カルボン酸、酸塩化物、イソシアネート、アルコール、アミン、チオール、ハロゲン化アルキル化合物等の化合物との反応により容易に製造される化合物であってもよい。前記側鎖に不飽和基を含有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0057〕~〔0060〕に記載された化合物、下記一般式(G)で表される特開2005-250438号公報の段落〔0064〕~〔0066〕に記載された化合物などが挙げられる。これらの中でも、下記一般式(G)で表される特開2005-250438号公報の段落〔0064〕~〔0066〕に記載された化合物が特に好ましい。
ただし、前記一般式(G)中、R1~R3は、それぞれ独立に水素原子又は1価の有機基を表し、Aは2価の有機残基を表し、Xは、酸素原子、硫黄原子、又は-N(R12)-を表し、前記R12は、水素原子、又は1価の有機基を表す。
なお、前記一般式(G)におけるR1~R3及びXは、前記一般式(1)におけるR1~R3及びXと同義であり、好ましい態様もまた同様である。
前記一般式(G)で表されるジオール化合物に由来するポリウレタン樹脂を用いることにより、立体障害の大きい2級アルコールに起因するポリマー主鎖の過剰な分子運動を抑制効果により、層の被膜強度の向上が達成できるものと考えられる。 Here, as a method for introducing an unsaturated group into the side chain of the polyurethane resin, in addition to the above-described method, a method using a diol compound containing an unsaturated group in the side chain as a raw material for producing a polyurethane resin is also preferable. . The diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the purpose. For example, a commercially available product such as trimethylolpropane monoallyl ether may be used. By reaction with a compound such as a halogenated diol compound, a triol compound or an aminodiol compound and a compound containing an unsaturated group such as a carboxylic acid, acid chloride, isocyanate, alcohol, amine, thiol or halogenated alkyl compound. It may be a compound that is easily produced. The diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0057] to [0060] of JP-A-2005-250438 And the compounds described in paragraphs [0064] to [0066] of JP-A-2005-250438 represented by the following general formula (G). Among these, compounds described in paragraphs [0064] to [0066] of JP-A-2005-250438 represented by the following general formula (G) are particularly preferable.
In the general formula (G), R 1 to R 3 each independently represents a hydrogen atom or a monovalent organic group, A represents a divalent organic residue, and X represents an oxygen atom or a sulfur atom. Or —N (R 12 ) —, wherein R 12 represents a hydrogen atom or a monovalent organic group.
Incidentally, R 1 ~ R 3 and X in the general formula (G), said a general formula (1) the same meaning as R 1 ~ R 3 and X in preferred embodiments versa.
By using the polyurethane resin derived from the diol compound represented by the general formula (G), the effect of suppressing the excessive molecular movement of the polymer main chain caused by the secondary alcohol having a large steric hindrance can be reduced. It is thought that improvement can be achieved.
なお、前記一般式(G)におけるR1~R3及びXは、前記一般式(1)におけるR1~R3及びXと同義であり、好ましい態様もまた同様である。
前記一般式(G)で表されるジオール化合物に由来するポリウレタン樹脂を用いることにより、立体障害の大きい2級アルコールに起因するポリマー主鎖の過剰な分子運動を抑制効果により、層の被膜強度の向上が達成できるものと考えられる。 Here, as a method for introducing an unsaturated group into the side chain of the polyurethane resin, in addition to the above-described method, a method using a diol compound containing an unsaturated group in the side chain as a raw material for producing a polyurethane resin is also preferable. . The diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the purpose. For example, a commercially available product such as trimethylolpropane monoallyl ether may be used. By reaction with a compound such as a halogenated diol compound, a triol compound or an aminodiol compound and a compound containing an unsaturated group such as a carboxylic acid, acid chloride, isocyanate, alcohol, amine, thiol or halogenated alkyl compound. It may be a compound that is easily produced. The diol compound containing an unsaturated group in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0057] to [0060] of JP-A-2005-250438 And the compounds described in paragraphs [0064] to [0066] of JP-A-2005-250438 represented by the following general formula (G). Among these, compounds described in paragraphs [0064] to [0066] of JP-A-2005-250438 represented by the following general formula (G) are particularly preferable.
Incidentally, R 1 ~ R 3 and X in the general formula (G), said a general formula (1) the same meaning as R 1 ~ R 3 and X in preferred embodiments versa.
By using the polyurethane resin derived from the diol compound represented by the general formula (G), the effect of suppressing the excessive molecular movement of the polymer main chain caused by the secondary alcohol having a large steric hindrance can be reduced. It is thought that improvement can be achieved.
前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂は、例えば、重合性組成物中の他の成分との相溶性を向上させ、保存安定性を向上させるといった観点から、前記側鎖に不飽和基を含有するジオール化合物以外のジオール化合物を共重合させることができる。
前記側鎖に不飽和基を含有するジオール化合物以外のジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ポリエーテルジオール化合物、ポリエステルジオール化合物、ポリカーボネートジオール化合物などが挙げられる。 The polyurethane resin having an ethylenically unsaturated bond in the side chain is unsaturated in the side chain from the viewpoint of improving compatibility with other components in the polymerizable composition and improving storage stability, for example. A diol compound other than a diol compound containing a group can be copolymerized.
There is no restriction | limiting in particular as diol compounds other than the diol compound containing an unsaturated group in the said side chain, For example, a polyether diol compound, a polyester diol compound, a polycarbonate diol compound etc. can be selected. Is mentioned.
前記側鎖に不飽和基を含有するジオール化合物以外のジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ポリエーテルジオール化合物、ポリエステルジオール化合物、ポリカーボネートジオール化合物などが挙げられる。 The polyurethane resin having an ethylenically unsaturated bond in the side chain is unsaturated in the side chain from the viewpoint of improving compatibility with other components in the polymerizable composition and improving storage stability, for example. A diol compound other than a diol compound containing a group can be copolymerized.
There is no restriction | limiting in particular as diol compounds other than the diol compound containing an unsaturated group in the said side chain, For example, a polyether diol compound, a polyester diol compound, a polycarbonate diol compound etc. can be selected. Is mentioned.
前記ポリエーテルジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0068〕~〔0076〕に記載された化合物などが挙げられる。
The polyether diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraphs [0068] to [0076] of JP-A-2005-250438. It is done.
前記ポリエステルジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0077〕~〔0079〕、段落〔0083〕~〔0085〕におけるNo.1~No.8及びNo.13~No.18に記載された化合物などが挙げられる。
The polyester diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include paragraphs [0077] to [0079] and paragraphs [0083] to [0085] of JP-A-2005-250438. No. 1-No. 8 and no. 13-No. 18 and the like.
前記ポリカーボネートジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0080〕~〔0081〕及び段落〔0084〕におけるNo.9~No.12に記載された化合物などが挙げられる。
The polycarbonate diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in the paragraphs [0080] to [0081] and paragraph [0084] of JP-A-2005-250438, No. 9-No. 12 and the like.
また、前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の合成には、上述したジオール化合物の他に、イソシアネート基と反応しない置換基を有するジオール化合物を併用することもできる。
前記イソシアネート基と反応しない置換基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0087〕~〔0088〕に記載された化合物などが挙げられる。 Moreover, in the synthesis | combination of the polyurethane resin which has an ethylenically unsaturated bond in the said side chain, the diol compound which has a substituent which does not react with an isocyanate group other than the diol compound mentioned above can also be used together.
The diol compound having a substituent that does not react with the isocyanate group is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0087] to [0088] of JP-A-2005-250438 And the compounds described.
前記イソシアネート基と反応しない置換基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0087〕~〔0088〕に記載された化合物などが挙げられる。 Moreover, in the synthesis | combination of the polyurethane resin which has an ethylenically unsaturated bond in the said side chain, the diol compound which has a substituent which does not react with an isocyanate group other than the diol compound mentioned above can also be used together.
The diol compound having a substituent that does not react with the isocyanate group is not particularly limited and may be appropriately selected depending on the intended purpose. For example, in paragraphs [0087] to [0088] of JP-A-2005-250438 And the compounds described.
更に、前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の合成には、上述したジオール化合物の他に、カルボキシル基を有するジオール化合物を併用することもできる。前記カルボキシル基を有するジオール化合物としては、例えば、以下の式(X)~(Z)に示すものが含まれる。
Furthermore, in the synthesis of a polyurethane resin having an ethylenically unsaturated bond in the side chain, a diol compound having a carboxyl group can be used in combination with the diol compound described above. Examples of the diol compound having a carboxyl group include those represented by the following formulas (X) to (Z).
前記式(X)~(Z)中、R15としては、水素原子、置換基(例えば、シアノ基、ニトロ基、-F、-Cl、-Br、-I等のハロゲン原子、-CONH2、-COOR16、-OR16、-NHCONHR16、-NHCOOR16、-NHCOR16、-OCONHR16(ここで、前記R16は、炭素数1~10のアルキル基、又は炭素数7~15のアラルキル基を表す。)などの各基が含まれる。)を有していてもよいアルキル基、アラルキル基、アリール基、アルコキシ基、アリーロキシ基を表すものである限り、特に制限はなく、目的に応じて適宜選択することができるが、水素原子、炭素数1~8個のアルキル基、炭素数6~15個のアリール基が好ましい。前記式(X)~(Z)中、L9、L10、L11は、それぞれ同一でもよいし、相違していてもよく、単結合、置換基(例えば、アルキル、アラルキル、アリール、アルコキシ、ハロゲノの各基が好ましい。)を有していてもよい2価の脂肪族又は芳香族炭化水素基を表すものである限り、特に制限はなく、目的に応じて適宜選択することができるが、炭素数1~20個のアルキレン基、炭素数6~15個のアリーレン基が好ましく、炭素数1~8個のアルキレン基がより好ましい。また必要に応じ、前記L9~L11中にイソシアネート基と反応しない他の官能基、例えば、カルボニル、エステル、ウレタン、アミド、ウレイド、エーテル基を有していてもよい。なお、前記R15、L7、L8、L9のうちの2個又は3個で環を形成してもよい。
前記式(Y)中、Arとしては、置換基を有していてもよい三価の芳香族炭化水素基を表すものである限り、特に制限はなく、目的に応じて適宜選択することができるが、炭素数6~15個の芳香族基が好ましい。 In the formulas (X) to (Z), R 15 represents a hydrogen atom, a substituent (for example, a halogen atom such as a cyano group, a nitro group, —F, —Cl, —Br, —I, etc., —CONH 2 , —COOR 16 , —OR 16 , —NHCONHR 16 , —NHCOOR 16 , —NHCOR 16 , —OCONHR 16 (wherein R 16 is an alkyl group having 1 to 10 carbon atoms or an aralkyl group having 7 to 15 carbon atoms) As long as it represents an alkyl group, an aralkyl group, an aryl group, an alkoxy group, or an aryloxy group, which may have a group, depending on the purpose. A hydrogen atom, an alkyl group having 1 to 8 carbon atoms, and an aryl group having 6 to 15 carbon atoms are preferable. In the above formulas (X) to (Z), L 9 , L 10 and L 11 may be the same or different, and each may be a single bond, a substituent (for example, alkyl, aralkyl, aryl, alkoxy, As long as it represents a divalent aliphatic or aromatic hydrocarbon group that may have a divalent aliphatic or aromatic hydrocarbon group, and may be appropriately selected depending on the purpose. An alkylene group having 1 to 20 carbon atoms and an arylene group having 6 to 15 carbon atoms are preferable, and an alkylene group having 1 to 8 carbon atoms is more preferable. If necessary, the L 9 to L 11 may have other functional groups that do not react with isocyanate groups, such as carbonyl, ester, urethane, amide, ureido, and ether groups. A ring may be formed by two or three of R 15 , L 7 , L 8 and L 9 .
In the formula (Y), Ar is not particularly limited as long as it represents a trivalent aromatic hydrocarbon group which may have a substituent, and can be appropriately selected according to the purpose. However, an aromatic group having 6 to 15 carbon atoms is preferred.
前記式(Y)中、Arとしては、置換基を有していてもよい三価の芳香族炭化水素基を表すものである限り、特に制限はなく、目的に応じて適宜選択することができるが、炭素数6~15個の芳香族基が好ましい。 In the formulas (X) to (Z), R 15 represents a hydrogen atom, a substituent (for example, a halogen atom such as a cyano group, a nitro group, —F, —Cl, —Br, —I, etc., —CONH 2 , —COOR 16 , —OR 16 , —NHCONHR 16 , —NHCOOR 16 , —NHCOR 16 , —OCONHR 16 (wherein R 16 is an alkyl group having 1 to 10 carbon atoms or an aralkyl group having 7 to 15 carbon atoms) As long as it represents an alkyl group, an aralkyl group, an aryl group, an alkoxy group, or an aryloxy group, which may have a group, depending on the purpose. A hydrogen atom, an alkyl group having 1 to 8 carbon atoms, and an aryl group having 6 to 15 carbon atoms are preferable. In the above formulas (X) to (Z), L 9 , L 10 and L 11 may be the same or different, and each may be a single bond, a substituent (for example, alkyl, aralkyl, aryl, alkoxy, As long as it represents a divalent aliphatic or aromatic hydrocarbon group that may have a divalent aliphatic or aromatic hydrocarbon group, and may be appropriately selected depending on the purpose. An alkylene group having 1 to 20 carbon atoms and an arylene group having 6 to 15 carbon atoms are preferable, and an alkylene group having 1 to 8 carbon atoms is more preferable. If necessary, the L 9 to L 11 may have other functional groups that do not react with isocyanate groups, such as carbonyl, ester, urethane, amide, ureido, and ether groups. A ring may be formed by two or three of R 15 , L 7 , L 8 and L 9 .
In the formula (Y), Ar is not particularly limited as long as it represents a trivalent aromatic hydrocarbon group which may have a substituent, and can be appropriately selected according to the purpose. However, an aromatic group having 6 to 15 carbon atoms is preferred.
前記式(X)~(Z)で表されるカルボキシル基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、3,5-ジヒドロキシ安息香酸、2,2-ビス(ヒドロキシメチル)プロピオン酸、2,2-ビス(2-ヒドロキシエチル)プロピオン酸、2,2-ビス(3-ヒドロキシプロピル)プロピオン酸、ビス(ヒドロキシメチル)酢酸、ビス(4-ヒドロキシフェニル)酢酸、2,2-ビス(ヒドロキシメチル)酪酸、4,4-ビス(4-ヒドロキシフェニル)ペンタン酸、酒石酸、N,N-ジヒドロキシエチルグリシン、N,N―ビス(2-ヒドロキシエチル)-3-カルボキシ-プロピオンアミドなどが挙げられる。
The diol compound having a carboxyl group represented by the formulas (X) to (Z) is not particularly limited and may be appropriately selected depending on the intended purpose. For example, 3,5-dihydroxybenzoic acid, 2, 2-bis (hydroxymethyl) propionic acid, 2,2-bis (2-hydroxyethyl) propionic acid, 2,2-bis (3-hydroxypropyl) propionic acid, bis (hydroxymethyl) acetic acid, bis (4-hydroxy Phenyl) acetic acid, 2,2-bis (hydroxymethyl) butyric acid, 4,4-bis (4-hydroxyphenyl) pentanoic acid, tartaric acid, N, N-dihydroxyethylglycine, N, N-bis (2-hydroxyethyl) -3-Carboxy-propionamide and the like.
このようなカルボキシル基の存在により、ポリウレタン樹脂に水素結合性とアルカリ可溶性といった特性を付与できるため好ましい。より具体的には、前記側鎖にエチレン性不飽和結合基を有するポリウレタン樹脂が、更に側鎖にカルボキシル基を有する樹脂である。
It is preferable because the presence of such a carboxyl group can impart properties such as hydrogen bonding and alkali solubility to the polyurethane resin. More specifically, the polyurethane resin having an ethylenically unsaturated bond group in the side chain is a resin having a carboxyl group in the side chain.
また、側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の合成には、上述したジオール化合物の他に、テトラカルボン酸二無水物をジオール化合物で開環させた化合物を併用することもできる。
前記テトラカルボン酸二無水物をジオール化合物で開環させた化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0095〕~〔0101〕に記載された化合物などが挙げられる。 Moreover, in the synthesis | combination of the polyurethane resin which has an ethylenically unsaturated bond in a side chain, the compound which ring-opened tetracarboxylic dianhydride with the diol compound other than the diol compound mentioned above can also be used together.
The compound obtained by ring-opening the tetracarboxylic dianhydride with a diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, paragraph [0095] to JP 2005-250438 A And the compounds described in [0101].
前記テトラカルボン酸二無水物をジオール化合物で開環させた化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2005-250438号公報の段落〔0095〕~〔0101〕に記載された化合物などが挙げられる。 Moreover, in the synthesis | combination of the polyurethane resin which has an ethylenically unsaturated bond in a side chain, the compound which ring-opened tetracarboxylic dianhydride with the diol compound other than the diol compound mentioned above can also be used together.
The compound obtained by ring-opening the tetracarboxylic dianhydride with a diol compound is not particularly limited and may be appropriately selected depending on the intended purpose. For example, paragraph [0095] to JP 2005-250438 A And the compounds described in [0101].
また、前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂としては、ポリマー末端、主鎖に不飽和基を有するものも好適に使用される。ポリマー末端、主鎖に不飽和基を有することにより、更に、シリカ分散組成物と側鎖にエチレン性不飽和結合を有するポリウレタン樹脂との間、又は側鎖にエチレン性不飽和結合を有するポリウレタン樹脂間で架橋反応性が向上し、光硬化物強度が増す。その結果、側鎖にエチレン性不飽和結合を有するポリウレタン樹脂を平版印刷版に使用した際、耐刷力に優れる版材を与えることができる。ここで、不飽和基としては、架橋反応の起こり易さから、不飽和基を有することが特に好ましい。
Further, as the polyurethane resin having an ethylenically unsaturated bond in the side chain, those having an unsaturated group in the polymer terminal and main chain are also preferably used. Polyurethane resin having an ethylenically unsaturated bond in the side chain, or between the silica dispersion composition and the polyurethane resin having an ethylenically unsaturated bond in the side chain, by having an unsaturated group at the polymer terminal and main chain Crosslinking reactivity is improved, and the strength of the photocured product is increased. As a result, when a polyurethane resin having an ethylenically unsaturated bond in the side chain is used for a lithographic printing plate, a plate material having excellent printing durability can be provided. Here, as an unsaturated group, it is especially preferable to have an unsaturated group from the ease of a crosslinking reaction.
ポリマー末端に不飽和基を導入する方法としては、以下に示す方法がある。即ち、上述した側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の合成の工程での、ポリマー末端の残存イソシアネート基と、アルコール類又はアミン類等で処理する工程において、不飽和基を有するアルコール類又はアミン類等を用いればよい。このような化合物としては、具体的には、先に、不飽和基を有する単官能のアルコール又は単官能のアミン化合物として挙げられた例示化合物と同様のものを挙げることができる。
なお、不飽和基は、導入量の制御が容易で導入量を増やすことができ、また、架橋反応効率が向上するといった観点から、ポリマー末端よりもポリマー側鎖に導入されることが好ましい。
導入されるエチレン性不飽和結合基としては、特に制限はなく、目的に応じて適宜選択することができるが、架橋硬化膜形成性の点で、メタクリロイル基、アクリロイル基、スチリル基が好ましく、メタクリロイル基、アクリロイル基がより好ましく、架橋硬化膜の形成性と生保存性との両立の点で、メタクリロイル基が特に好ましい。
また、メタクリロイル基の導入量としては、特に制限はなく、目的に応じて適宜選択することができるが、ビニル基当量としては、0.05mmol/g~3.0mmol/gが好ましく、0.5mmol/g~2.7mmol/gがより好ましく、0.75mmol/g~2.4mmol/gが特に好ましい。 Examples of the method for introducing an unsaturated group at the polymer terminal include the following methods. That is, in the step of synthesizing the polyurethane resin having an ethylenically unsaturated bond in the side chain as described above, in the step of treating with the residual isocyanate group at the polymer end and the alcohol or amine, the alcohol having an unsaturated group. Alternatively, amines or the like may be used. Specific examples of such a compound include the same compounds as those exemplified above as the monofunctional alcohol or monofunctional amine compound having an unsaturated group.
The unsaturated group is preferably introduced into the polymer side chain rather than the polymer terminal from the viewpoint that the introduction amount can be easily controlled and the introduction amount can be increased, and that the crosslinking reaction efficiency is improved.
The ethylenically unsaturated bond group to be introduced is not particularly limited and may be appropriately selected depending on the intended purpose. From the viewpoint of forming a crosslinked cured film, a methacryloyl group, an acryloyl group, and a styryl group are preferable, and methacryloyl Group and acryloyl group are more preferable, and methacryloyl group is particularly preferable in terms of both the formability of the crosslinked cured film and the raw storage stability.
The amount of methacryloyl group introduced is not particularly limited and may be appropriately selected depending on the intended purpose. The vinyl group equivalent is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol. / G to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable.
なお、不飽和基は、導入量の制御が容易で導入量を増やすことができ、また、架橋反応効率が向上するといった観点から、ポリマー末端よりもポリマー側鎖に導入されることが好ましい。
導入されるエチレン性不飽和結合基としては、特に制限はなく、目的に応じて適宜選択することができるが、架橋硬化膜形成性の点で、メタクリロイル基、アクリロイル基、スチリル基が好ましく、メタクリロイル基、アクリロイル基がより好ましく、架橋硬化膜の形成性と生保存性との両立の点で、メタクリロイル基が特に好ましい。
また、メタクリロイル基の導入量としては、特に制限はなく、目的に応じて適宜選択することができるが、ビニル基当量としては、0.05mmol/g~3.0mmol/gが好ましく、0.5mmol/g~2.7mmol/gがより好ましく、0.75mmol/g~2.4mmol/gが特に好ましい。 Examples of the method for introducing an unsaturated group at the polymer terminal include the following methods. That is, in the step of synthesizing the polyurethane resin having an ethylenically unsaturated bond in the side chain as described above, in the step of treating with the residual isocyanate group at the polymer end and the alcohol or amine, the alcohol having an unsaturated group. Alternatively, amines or the like may be used. Specific examples of such a compound include the same compounds as those exemplified above as the monofunctional alcohol or monofunctional amine compound having an unsaturated group.
The unsaturated group is preferably introduced into the polymer side chain rather than the polymer terminal from the viewpoint that the introduction amount can be easily controlled and the introduction amount can be increased, and that the crosslinking reaction efficiency is improved.
The ethylenically unsaturated bond group to be introduced is not particularly limited and may be appropriately selected depending on the intended purpose. From the viewpoint of forming a crosslinked cured film, a methacryloyl group, an acryloyl group, and a styryl group are preferable, and methacryloyl Group and acryloyl group are more preferable, and methacryloyl group is particularly preferable in terms of both the formability of the crosslinked cured film and the raw storage stability.
The amount of methacryloyl group introduced is not particularly limited and may be appropriately selected depending on the intended purpose. The vinyl group equivalent is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol. / G to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable.
主鎖に不飽和基を導入する方法としては、主鎖方向に不飽和基を有するジオール化合物をポリウレタン樹脂の合成に用いる方法がある。前記主鎖方向に不飽和基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、cis-2-ブテン-1,4-ジオール、trans-2-ブテン-1,4-ジオール、ポリブタジエンジオールなどが挙げられる。
As a method for introducing an unsaturated group into the main chain, there is a method of using a diol compound having an unsaturated group in the main chain direction for the synthesis of a polyurethane resin. The diol compound having an unsaturated group in the main chain direction is not particularly limited and may be appropriately selected depending on the intended purpose. For example, cis-2-butene-1,4-diol, trans-2-butene 1,4-diol, polybutadiene diol and the like.
前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂は、該特定ポリウレタン樹脂とは異なる構造を有するポリウレタン樹脂を含むアルカリ可溶性高分子を併用することも可能である。例えば、前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂は、は、主鎖及び/又は側鎖に芳香族基を含有したポリウレタン樹脂を併用することが可能である。
The polyurethane resin having an ethylenically unsaturated bond in the side chain can be used in combination with an alkali-soluble polymer containing a polyurethane resin having a structure different from that of the specific polyurethane resin. For example, the polyurethane resin having an ethylenically unsaturated bond in the side chain can be used in combination with a polyurethane resin containing an aromatic group in the main chain and / or side chain.
前記(i)側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の具体例としては、例えば、特開2005-250438号公報の段落〔0293〕~〔0310〕に示されたP-1~P-31のポリマーなどが挙げられる。これらの中でも、段落〔0308〕及び〔0309〕に示されたP-27及びP-28のポリマーが好ましい。
Specific examples of the polyurethane resin (i) having an ethylenically unsaturated bond in the side chain include, for example, P-1 to P— shown in paragraphs [0293] to [0310] of JP-A-2005-250438. And 31 polymers. Among these, polymers of P-27 and P-28 shown in paragraphs [0308] and [0309] are preferable.
--(ii)カルボキシル基含有ポリウレタンと分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂--
前記ポリウレタン樹脂は、ジイソシアネートと、カルボン酸基含有ジオールとを必須成分とするカルボキシル基含有ポリウレタンと、分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂である。目的に応じて、ジオール成分として、重量平均分子量300以下の低分子ジオール、重量平均分子量500以上の低分子ジオールを共重合成分として加えてもよい。
前記ポリウレタン樹脂を用いることにより、無機充填剤との安定した分散性及び耐クラック性及び耐衝撃性に優れることから、耐熱性、耐湿熱性、密着性、機械特性、電気特性が向上する。
また、前記ポリウレタン樹脂としては、置換基を有していてもよい二価の脂肪族及び芳香族炭化水素のジイソシアネートと、C原子及びN原子のいずれかを介してCOOH基と2つのOH基を有するカルボン酸含有ジオールとを必須成分とした反応物であって、得られた反応物と、-COO-結合を介して分子中にエポキシ基とビニル基を有する化合物とを反応して得られるものであってもよい。
また、前記ポリウレタン樹脂としては、下記一般式(I)で示されるジイソシアネートと、下記一般式(II-1)~(II-3)で示されるカルボン酸基含有ジオールから選ばれた少なくとも1種とを必須成分とし、目的に応じて下記一般式(III-1)~(III-5)で示される重量平均分子量が800~3,000の範囲にある高分子ジオールから選ばれた少なくとも1種との反応物であって、得られた反応物と、下記一般式(IV-1)~(IV-16)で示される分子中にエポキシ基とビニル基を有する化合物とを反応して得られるものであってもよい。 -(Ii) Polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule--
The polyurethane resin is a polyurethane resin obtained by reacting a carboxyl group-containing polyurethane having a diisocyanate and a carboxylic acid group-containing diol as essential components with a compound having an epoxy group and a vinyl group in the molecule. Depending on the purpose, as the diol component, a low molecular diol having a weight average molecular weight of 300 or less and a low molecular diol having a weight average molecular weight of 500 or more may be added as a copolymer component.
By using the polyurethane resin, it is excellent in stable dispersibility with an inorganic filler, crack resistance and impact resistance, so that heat resistance, moist heat resistance, adhesion, mechanical properties and electrical properties are improved.
The polyurethane resin includes a divalent aliphatic or aromatic hydrocarbon diisocyanate which may have a substituent, a COOH group and two OH groups via any one of a C atom and an N atom. A reaction product comprising a carboxylic acid-containing diol as an essential component, which is obtained by reacting the obtained reaction product with a compound having an epoxy group and a vinyl group in the molecule via a —COO— bond It may be.
Further, the polyurethane resin includes at least one selected from diisocyanates represented by the following general formula (I) and carboxylic acid group-containing diols represented by the following general formulas (II-1) to (II-3): And at least one selected from polymer diols having a weight average molecular weight in the range of 800 to 3,000 represented by the following general formulas (III-1) to (III-5) according to the purpose: A reaction product obtained by reacting the obtained reaction product with a compound having an epoxy group and a vinyl group in a molecule represented by the following general formulas (IV-1) to (IV-16): It may be.
前記ポリウレタン樹脂は、ジイソシアネートと、カルボン酸基含有ジオールとを必須成分とするカルボキシル基含有ポリウレタンと、分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂である。目的に応じて、ジオール成分として、重量平均分子量300以下の低分子ジオール、重量平均分子量500以上の低分子ジオールを共重合成分として加えてもよい。
前記ポリウレタン樹脂を用いることにより、無機充填剤との安定した分散性及び耐クラック性及び耐衝撃性に優れることから、耐熱性、耐湿熱性、密着性、機械特性、電気特性が向上する。
また、前記ポリウレタン樹脂としては、置換基を有していてもよい二価の脂肪族及び芳香族炭化水素のジイソシアネートと、C原子及びN原子のいずれかを介してCOOH基と2つのOH基を有するカルボン酸含有ジオールとを必須成分とした反応物であって、得られた反応物と、-COO-結合を介して分子中にエポキシ基とビニル基を有する化合物とを反応して得られるものであってもよい。
また、前記ポリウレタン樹脂としては、下記一般式(I)で示されるジイソシアネートと、下記一般式(II-1)~(II-3)で示されるカルボン酸基含有ジオールから選ばれた少なくとも1種とを必須成分とし、目的に応じて下記一般式(III-1)~(III-5)で示される重量平均分子量が800~3,000の範囲にある高分子ジオールから選ばれた少なくとも1種との反応物であって、得られた反応物と、下記一般式(IV-1)~(IV-16)で示される分子中にエポキシ基とビニル基を有する化合物とを反応して得られるものであってもよい。 -(Ii) Polyurethane resin obtained by reacting a carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule--
The polyurethane resin is a polyurethane resin obtained by reacting a carboxyl group-containing polyurethane having a diisocyanate and a carboxylic acid group-containing diol as essential components with a compound having an epoxy group and a vinyl group in the molecule. Depending on the purpose, as the diol component, a low molecular diol having a weight average molecular weight of 300 or less and a low molecular diol having a weight average molecular weight of 500 or more may be added as a copolymer component.
By using the polyurethane resin, it is excellent in stable dispersibility with an inorganic filler, crack resistance and impact resistance, so that heat resistance, moist heat resistance, adhesion, mechanical properties and electrical properties are improved.
The polyurethane resin includes a divalent aliphatic or aromatic hydrocarbon diisocyanate which may have a substituent, a COOH group and two OH groups via any one of a C atom and an N atom. A reaction product comprising a carboxylic acid-containing diol as an essential component, which is obtained by reacting the obtained reaction product with a compound having an epoxy group and a vinyl group in the molecule via a —COO— bond It may be.
Further, the polyurethane resin includes at least one selected from diisocyanates represented by the following general formula (I) and carboxylic acid group-containing diols represented by the following general formulas (II-1) to (II-3): And at least one selected from polymer diols having a weight average molecular weight in the range of 800 to 3,000 represented by the following general formulas (III-1) to (III-5) according to the purpose: A reaction product obtained by reacting the obtained reaction product with a compound having an epoxy group and a vinyl group in a molecule represented by the following general formulas (IV-1) to (IV-16): It may be.
前記一般式(II-1)中、R2は、水素原子、置換基(例えば、シアノ基、ニトロ基、ハロゲン原子(-F、-Cl、-Br、-I)、-CONH2、-COOR6、-OR6、-NHCONHR6、-NHCOOR6、-NHCOR6、-OCONHR6、-CONHR6(ここで、R6は、炭素数1~10のアルキル基、炭素数7~15のアラルキル基のいずれかを表す)、などの各基が含まれる)を有していてもよいアルキル基、アラルキル基、アリール基、アルコキシ基、又はアリーロキシ基を表す。これらの中でも、水素原子、炭素数1個~3個のアルキル基、炭素数6個~15個のアリール基が好ましい。
前記一般式(II-1)及び(II-2)中、R3、R4及びR5は、それぞれ同一でも相異していてもよく、単結合、置換基(例えば、アルキル基、アラルキル基、アリール基、アルコキシ基、ハロゲノ基の各基が好ましい)を有していてもよい二価の脂肪族又は芳香族炭化水素を表す。これらの中でも、炭素数1~20個のアルキレン基、炭素数6~15個のアリーレン基が好ましく、炭素数1~8個のアルキレン基が更に好ましい。また、必要に応じて、前記R3、R4及びR5中にイソシアネート基と反応しない他の官能基、例えば、カルボニル基、エステル基、ウレタン基、アミド基、ウレイド基、エーテル基のいずれかを有していてもよい。なお、前記R2、R3、R4及びR5のうちの2個又は3個で環を形成してもよい。Arは置換基を有していてもよい三価の芳香族炭化水素を表し、炭素数6個~15個の芳香族基が好ましい。
In the general formula (II-1), R 2 represents a hydrogen atom, a substituent (for example, a cyano group, a nitro group, a halogen atom (—F, —Cl, —Br, —I), —CONH 2 , —COOR. 6 , —OR 6 , —NHCONHR 6 , —NHCOOR 6 , —NHCOR 6 , —OCONHR 6 , —CONHR 6 (where R 6 is an alkyl group having 1 to 10 carbon atoms and an aralkyl group having 7 to 15 carbon atoms) Or an alkyl group, an aralkyl group, an aryl group, an alkoxy group, or an aryloxy group. Among these, a hydrogen atom, an alkyl group having 1 to 3 carbon atoms, and an aryl group having 6 to 15 carbon atoms are preferable.
In the general formulas (II-1) and (II-2), R 3 , R 4 and R 5 may be the same or different from each other, and may be a single bond, a substituent (for example, an alkyl group or an aralkyl group). , An aryl group, an alkoxy group, and a halogeno group are preferable). Among these, an alkylene group having 1 to 20 carbon atoms and an arylene group having 6 to 15 carbon atoms are preferable, and an alkylene group having 1 to 8 carbon atoms is more preferable. Further, if necessary, any one of other functional groups that do not react with the isocyanate group in the R 3 , R 4 and R 5 , for example, any one of a carbonyl group, an ester group, a urethane group, an amide group, a ureido group, and an ether group. You may have. In addition, you may form a ring by 2 or 3 of said R < 2 >, R < 3 >, R < 4 > and R < 5 >. Ar represents a trivalent aromatic hydrocarbon which may have a substituent, and is preferably an aromatic group having 6 to 15 carbon atoms.
前記一般式(III-5)中、R13は、アリール基又はシアノ基を表し、炭素数6個~10個のアリール基又はシアノ基が好ましい。前記一般式(III-4)中、mは、2~4の整数を表す。前記一般式(III-1)~(III-5)中、n1、n2、n3、n4及びn5は、それぞれ2以上の整数を表し、2~100の整数が好ましい。前記一般式(III-5)中、n6は、0又は2以上の整数を示し、0又は2~100の整数が好ましい。
In the general formula (III-5), R 13 represents an aryl group or a cyano group, preferably an aryl group or a cyano group having 6 to 10 carbon atoms. In the general formula (III-4), m represents an integer of 2 to 4. In the general formulas (III-1) to (III-5), n 1 , n 2 , n 3 , n 4 and n 5 each represents an integer of 2 or more, and an integer of 2 to 100 is preferable. In the general formula (III-5), n 6 represents 0 or an integer of 2 or more, preferably 0 or an integer of 2 to 100.
また、前記ポリウレタン樹脂は、更に第5成分として、カルボン酸基非含有の低分子量ジオールを共重合させてもよく、該低分子量ジオールとしては、前記一般式(III-1)~(III-5)で表され、重量平均分子量が500以下のものである。該カルボン酸基非含有低分子量ジオールは、アルカリ溶解性が低下しない限り、また、硬化膜の弾性率が十分低く保つことができる範囲で添加することができる。
The polyurethane resin may further be copolymerized with a low molecular weight diol containing no carboxylic acid group as a fifth component, and the low molecular weight diol may be any of the above general formulas (III-1) to (III-5). The weight average molecular weight is 500 or less. The low molecular weight diol containing no carboxylic acid group can be added as long as the alkali solubility is not lowered and the elastic modulus of the cured film can be kept sufficiently low.
前記ポリウレタン樹脂としては、特に、前記一般式(I)で示されるジイソシアネートと、前記一般式(II-1)~(II-3)で示されるカルボン酸基含有ジオールから選ばれた少なくとも1種とを必須成分とし、目的に応じて、前記一般式(III-1)~(III-5)で示される重量平均分子量が800~3,000の範囲にある高分子ジオールから選ばれた少なくとも1種、前記一般式(III-1)~(III-5)で示される重量平均分子量が500以下のカルボン酸基非含有の低分子量ジオールとの反応物に、更に一般式(IV-1)~(IV-16)のいずれかで示される分子中に1個のエポキシ基と少なくとも1個の(メタ)アクリル基を有する化合物を反応して得られる、酸価が20mgKOH/g~120mgKOH/gであるアルカリ可溶性光架橋性ポリウレタン樹脂が好適である。
As the polyurethane resin, in particular, at least one selected from diisocyanates represented by the general formula (I) and carboxylic acid group-containing diols represented by the general formulas (II-1) to (II-3) And at least one selected from polymer diols having a weight average molecular weight in the range of 800 to 3,000 represented by the general formulas (III-1) to (III-5) according to the purpose In addition, the reaction product of the general formulas (III-1) to (III-5) with the low molecular weight diol containing no carboxylic acid group having a weight average molecular weight of 500 or less is further added to the general formulas (IV-1) to (IV). IV-16), which is obtained by reacting a compound having one epoxy group and at least one (meth) acrylic group in the molecule represented by any one of the molecules, and has an acid value of 20 mgKOH / g to 120 mgK An alkali-soluble photocrosslinkable polyurethane resin that is OH / g is preferred.
前記ポリウレタン樹脂は、上記ジイソシアネート化合物及びジオール化合物を、非プロトン性溶媒中、それぞれの反応性に応じた活性の公知の触媒を添加し、加熱することにより合成される。合成に使用されるジイソシアネート及びジオール化合物のモル比(Ma:Mb)としては、特に制限はなく、目的に応じて適宜選択することができ、1:1~1.2:1が好ましく、アルコール類又はアミン類等で処理することにより、分子量あるいは粘度といった所望の物性の生成物が、最終的にイソシアネート基が残存しない形で合成される。
The polyurethane resin is synthesized by adding the above-mentioned diisocyanate compound and diol compound to an aprotic solvent, and adding a known catalyst having an activity corresponding to the reactivity thereof, followed by heating. The molar ratio (Ma: Mb) of the diisocyanate and diol compound used in the synthesis is not particularly limited and can be appropriately selected according to the purpose, preferably 1: 1 to 1.2: 1, and alcohols Alternatively, by treating with an amine or the like, a product having a desired physical property such as molecular weight or viscosity is synthesized in a form in which no isocyanate group remains finally.
前記ポリウレタン樹脂におけるエチレン性不飽和結合の導入量としては、特に制限はなく、目的に応じて適宜選択することができるが、ビニル基当量としては、0.05mmol/g~3.0mmol/gが好ましく、0.5mmol/g~2.7mmol/gがより好ましく、0.75mmol/g~2.4mmol/gが特に好ましい。更に、前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂には、前記エチレン性不飽和結合基とともに、側鎖にカルボキシル基が導入されていることが好ましい。前記酸価としては、20mgKOH/g~120mgKOH/gが好ましく、30mgKOH/g~110mgKOH/gがより好ましく、35mgKOH/g~100mgKOH/gが特に好ましい。
The amount of the ethylenically unsaturated bond introduced into the polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose. The vinyl group equivalent is 0.05 mmol / g to 3.0 mmol / g. Preferably, 0.5 mmol / g to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable. Furthermore, the polyurethane resin having an ethylenically unsaturated bond in the side chain preferably has a carboxyl group introduced in the side chain together with the ethylenically unsaturated bond group. The acid value is preferably 20 mgKOH / g to 120 mgKOH / g, more preferably 30 mgKOH / g to 110 mgKOH / g, and particularly preferably 35 mgKOH / g to 100 mgKOH / g.
前記側鎖にエチレン性不飽和結合を有するポリウレタン樹脂の分子量としては、特に制限はなく、目的に応じて適宜選択することができるが、重量平均分子量で2,000~50,000が好ましく、3,000~30,000がより好ましく、5,000~30,000が特に好ましい。特に、前記組成物を感光性ソルダーレジストに用いた場合には、無機充填剤との分散性に優れ、クラック耐性と耐熱性にも優れ、アルカリ性現像液による非画像部の現像性に優れる。
The molecular weight of the polyurethane resin having an ethylenically unsaturated bond in the side chain is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2,000 to 50,000 in terms of weight average molecular weight. 30,000 to 30,000 is more preferable, and 5,000 to 30,000 is particularly preferable. In particular, when the composition is used for a photosensitive solder resist, it is excellent in dispersibility with an inorganic filler, excellent in crack resistance and heat resistance, and excellent in developability of non-image areas with an alkaline developer.
これらの高分子化合物は、単独で用いてもよいし、2種以上を併用してもよい。シリカ分散組成物などの全固形分中に含まれる、前記酸変性ビニル基含有ポリウレタン樹脂の含有量としては、2質量%~30質量%が好ましく、5質量%~25質量%がより好ましい。前記含有量が、2質量%未満であると、硬化膜の高温時の十分な低弾性率が得られないことがあり、30質量%を超えると、現像性劣化及び硬化膜の強靱性低下が起きることがある。
These polymer compounds may be used alone or in combination of two or more. The content of the acid-modified vinyl group-containing polyurethane resin contained in the total solid content of the silica dispersion composition or the like is preferably 2% by mass to 30% by mass, and more preferably 5% by mass to 25% by mass. When the content is less than 2% by mass, a sufficiently low elastic modulus at a high temperature of the cured film may not be obtained, and when it exceeds 30% by mass, the developability deterioration and the toughness of the cured film are reduced. May happen.
-カルボキシル基含有ポリウレタンと分子中にエポキシ基とビニル基を有する化合物とを反応して得られるポリウレタン樹脂の合成法-
前記ポリウレタン樹脂の合成方法としては、上記ジイソシアネート化合物及びジオール化合物を非プロトン性溶媒中、それぞれの反応性に応じた活性の公知な触媒を添加し、加熱することにより合成される。使用するジイソシアネート及びジオール化合物のモル比は好ましくは、0.8:1~1.2:1であり、ポリマー末端にイソシアネート基が残存した場合、アルコール類又はアミン類等で処理することにより、最絡的にイソシアネート基が残存しない形で合成される。 -Synthesis of polyurethane resin obtained by reacting carboxyl group-containing polyurethane with compound having epoxy group and vinyl group in molecule-
As a method for synthesizing the polyurethane resin, the diisocyanate compound and the diol compound are synthesized in an aprotic solvent by adding a known catalyst having an activity corresponding to each reactivity and heating. The molar ratio of the diisocyanate and diol compound to be used is preferably 0.8: 1 to 1.2: 1. If an isocyanate group remains at the end of the polymer, the molar ratio can be reduced by treatment with alcohols or amines. It is synthesized in such a way that no isocyanate groups remain entangled.
前記ポリウレタン樹脂の合成方法としては、上記ジイソシアネート化合物及びジオール化合物を非プロトン性溶媒中、それぞれの反応性に応じた活性の公知な触媒を添加し、加熱することにより合成される。使用するジイソシアネート及びジオール化合物のモル比は好ましくは、0.8:1~1.2:1であり、ポリマー末端にイソシアネート基が残存した場合、アルコール類又はアミン類等で処理することにより、最絡的にイソシアネート基が残存しない形で合成される。 -Synthesis of polyurethane resin obtained by reacting carboxyl group-containing polyurethane with compound having epoxy group and vinyl group in molecule-
As a method for synthesizing the polyurethane resin, the diisocyanate compound and the diol compound are synthesized in an aprotic solvent by adding a known catalyst having an activity corresponding to each reactivity and heating. The molar ratio of the diisocyanate and diol compound to be used is preferably 0.8: 1 to 1.2: 1. If an isocyanate group remains at the end of the polymer, the molar ratio can be reduced by treatment with alcohols or amines. It is synthesized in such a way that no isocyanate groups remain entangled.
--ジイソシアネート--
前記一般式(I)で示されるジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0021〕に記載された化合物、などが挙げられる。 --Diisocyanate--
The diisocyanate compound represented by the general formula (I) is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0021] of JP-A-2007-2030, Etc.
前記一般式(I)で示されるジイソシアネート化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0021〕に記載された化合物、などが挙げられる。 --Diisocyanate--
The diisocyanate compound represented by the general formula (I) is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0021] of JP-A-2007-2030, Etc.
--高分子量ジオール--
前記一般式(III-1)~(III-5)で示される高分子量ジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0022〕~〔0046〕に記載された化合物などが挙げられる。 --High molecular weight diol--
The high molecular weight diol compound represented by the general formulas (III-1) to (III-5) is not particularly limited and may be appropriately selected depending on the intended purpose. For example, as disclosed in JP-A-2007-2030 Examples thereof include compounds described in paragraphs [0022] to [0046].
前記一般式(III-1)~(III-5)で示される高分子量ジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0022〕~〔0046〕に記載された化合物などが挙げられる。 --High molecular weight diol--
The high molecular weight diol compound represented by the general formulas (III-1) to (III-5) is not particularly limited and may be appropriately selected depending on the intended purpose. For example, as disclosed in JP-A-2007-2030 Examples thereof include compounds described in paragraphs [0022] to [0046].
--カルボン酸基含有ジオール--
また、前記一般式(II-1)~(II-3)で表されるカルボキシル基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0047〕に記載された化合物などが挙げられる。 --Carboxylic acid group-containing diol--
In addition, the diol compound having a carboxyl group represented by the general formulas (II-1) to (II-3) is not particularly limited and may be appropriately selected depending on the intended purpose. And the compounds described in paragraph [0047] of the publication No. 2030.
また、前記一般式(II-1)~(II-3)で表されるカルボキシル基を有するジオール化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0047〕に記載された化合物などが挙げられる。 --Carboxylic acid group-containing diol--
In addition, the diol compound having a carboxyl group represented by the general formulas (II-1) to (II-3) is not particularly limited and may be appropriately selected depending on the intended purpose. And the compounds described in paragraph [0047] of the publication No. 2030.
--カルボン酸基非含有低分子量ジオール--
前記カルボン酸基非含有低分子量ジオールとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0048〕に記載された化合物などが挙げられる。
前記カルボン酸基非含有ジオールの共重合量としては、低分子量ジオール中の95モル%以下が好ましく、80%以下がより好ましく、50%以下が特に好ましい。前記共重合量が、95モル%を超えると現像性のよいウレタン樹脂が得られないことがある。 --Low molecular weight diol containing no carboxylic acid groups--
The carboxylic acid group-free low molecular weight diol is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0048] of JP-A-2007-2030. It is done.
The copolymerization amount of the carboxylic acid group-free diol is preferably 95 mol% or less, more preferably 80% or less, and particularly preferably 50% or less in the low molecular weight diol. When the copolymerization amount exceeds 95 mol%, a urethane resin having good developability may not be obtained.
前記カルボン酸基非含有低分子量ジオールとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0048〕に記載された化合物などが挙げられる。
前記カルボン酸基非含有ジオールの共重合量としては、低分子量ジオール中の95モル%以下が好ましく、80%以下がより好ましく、50%以下が特に好ましい。前記共重合量が、95モル%を超えると現像性のよいウレタン樹脂が得られないことがある。 --Low molecular weight diol containing no carboxylic acid groups--
The carboxylic acid group-free low molecular weight diol is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0048] of JP-A-2007-2030. It is done.
The copolymerization amount of the carboxylic acid group-free diol is preferably 95 mol% or less, more preferably 80% or less, and particularly preferably 50% or less in the low molecular weight diol. When the copolymerization amount exceeds 95 mol%, a urethane resin having good developability may not be obtained.
前記(ii)カルボキシル基含有ポリウレタンと分子中にエポキシ基とビニル基を有する化合物とを反応させて得られるポリウレタン樹脂の具体例としては、例えば、特開2007-2030号公報の段落〔0314〕~〔0315〕に示されたU1~U4、U6~U11のポリマーにおけるエポキシ基及びビニル基含有化合物としてのグリシジルアクリレートを、グリシジルメタクリレート、3,4-エポキシシクロヘキシルメチルアクリレート(商品名:サイクロマーA400、ダイセル化学社製)、3,4-エポキシシクロヘキシルメチルメタクリレート(商品名:サイクロマーM400、ダイセル化学社製)に代えたポリマーなどが挙げられる。
Specific examples of the polyurethane resin obtained by reacting the above (ii) carboxyl group-containing polyurethane with a compound having an epoxy group and a vinyl group in the molecule include, for example, paragraph [0314] of JP-A-2007-2030. In the polymers of U1 to U4 and U6 to U11 shown in [0315], glycidyl acrylate as an epoxy group- and vinyl group-containing compound is replaced with glycidyl methacrylate, 3,4-epoxycyclohexylmethyl acrylate (trade name: Cyclomer A400, Daicel). Chemical Co., Ltd.), 3,4-epoxycyclohexylmethyl methacrylate (trade name: Cyclomer M400, manufactured by Daicel Chemical Industries), and the like.
--酸変性ビニル基含有ポリウレタン樹脂の含有量--
前記酸変性ビニル基含有ポリウレタン樹脂の前記シリカ分散組成物における含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、5質量%~80質量%が好ましく、20質量%~75質量%がより好ましく、30質量%~70質量%が特に好ましい。
前記含有量が、5質量%未満であると、耐クラック性が良好に保つことができないことがあり、80質量%を超えると、耐熱性が破綻をきたすことがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好な耐クラック性と耐熱性の両立の点で有利である。 -Content of acid-modified vinyl group-containing polyurethane resin-
The content of the acid-modified vinyl group-containing polyurethane resin in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 5% by mass to 80% by mass, and preferably 20% by mass. Is more preferably from 75 to 75% by weight, particularly preferably from 30 to 70% by weight.
If the content is less than 5% by mass, good crack resistance may not be maintained, and if it exceeds 80% by mass, the heat resistance may fail. On the other hand, when the content is within the particularly preferable range, it is advantageous in terms of both good crack resistance and heat resistance.
前記酸変性ビニル基含有ポリウレタン樹脂の前記シリカ分散組成物における含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、5質量%~80質量%が好ましく、20質量%~75質量%がより好ましく、30質量%~70質量%が特に好ましい。
前記含有量が、5質量%未満であると、耐クラック性が良好に保つことができないことがあり、80質量%を超えると、耐熱性が破綻をきたすことがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好な耐クラック性と耐熱性の両立の点で有利である。 -Content of acid-modified vinyl group-containing polyurethane resin-
The content of the acid-modified vinyl group-containing polyurethane resin in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 5% by mass to 80% by mass, and preferably 20% by mass. Is more preferably from 75 to 75% by weight, particularly preferably from 30 to 70% by weight.
If the content is less than 5% by mass, good crack resistance may not be maintained, and if it exceeds 80% by mass, the heat resistance may fail. On the other hand, when the content is within the particularly preferable range, it is advantageous in terms of both good crack resistance and heat resistance.
前記酸変性ビニル基含有ポリウレタン樹脂の重量平均分子量としては、特に制限はなく、目的に応じて適宜選択することができるが、2,000~60,000が好ましく、2,000~50,000がより好ましく、2,000~30,000が特に好ましい。前記重量平均分子量が2,000未満であると、硬化膜の高温時の十分な低弾性率が得られないことがあり、60,000を超えると、塗布適性及び現像性が悪化することがある。
ここで、前記重量平均分子量は、例えば、高速GPC装置(東洋曹達社製、HLC-802A)を使用して、0.5質量%のTHF溶液を試料溶液とし、カラムはTSKgel HZM-M 1本を使用し、200μLの試料を注入し、前記THF溶液で溶離して、25℃で屈折率検出器あるいはUV検出器(検出波長254nm)により測定することができる。次に、標準ポリスチレンで較正した分子量分布曲線より重量平均分子量を求めることができる。 The weight average molecular weight of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2,000 to 60,000, and preferably 2,000 to 50,000. More preferably, 2,000 to 30,000 is particularly preferable. When the weight average molecular weight is less than 2,000, a sufficiently low elastic modulus at a high temperature of the cured film may not be obtained, and when it exceeds 60,000, coating suitability and developability may be deteriorated. .
Here, the weight average molecular weight is determined by using, for example, a high-speed GPC apparatus (manufactured by Toyo Soda Co., Ltd., HLC-802A), a 0.5 mass% THF solution as a sample solution, and a column of one TSKgel HZM-M. 200 μL of sample is injected, eluted with the THF solution, and measured at 25 ° C. with a refractive index detector or UV detector (detection wavelength 254 nm). Next, the weight average molecular weight can be determined from the molecular weight distribution curve calibrated with standard polystyrene.
ここで、前記重量平均分子量は、例えば、高速GPC装置(東洋曹達社製、HLC-802A)を使用して、0.5質量%のTHF溶液を試料溶液とし、カラムはTSKgel HZM-M 1本を使用し、200μLの試料を注入し、前記THF溶液で溶離して、25℃で屈折率検出器あるいはUV検出器(検出波長254nm)により測定することができる。次に、標準ポリスチレンで較正した分子量分布曲線より重量平均分子量を求めることができる。 The weight average molecular weight of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2,000 to 60,000, and preferably 2,000 to 50,000. More preferably, 2,000 to 30,000 is particularly preferable. When the weight average molecular weight is less than 2,000, a sufficiently low elastic modulus at a high temperature of the cured film may not be obtained, and when it exceeds 60,000, coating suitability and developability may be deteriorated. .
Here, the weight average molecular weight is determined by using, for example, a high-speed GPC apparatus (manufactured by Toyo Soda Co., Ltd., HLC-802A), a 0.5 mass% THF solution as a sample solution, and a column of one TSKgel HZM-M. 200 μL of sample is injected, eluted with the THF solution, and measured at 25 ° C. with a refractive index detector or UV detector (detection wavelength 254 nm). Next, the weight average molecular weight can be determined from the molecular weight distribution curve calibrated with standard polystyrene.
前記酸変性ビニル基含有ポリウレタン樹脂の酸価としては、特に制限はなく、目的に応じて適宜選択することができるが、20mgKOH/g~120mgKOH/gが好ましく、30mgKOH/g~110mgKOH/gがより好ましく、35mgKOH/g~100mgKOH/gが特に好ましい。前記酸価が、20mgKOH/g未満であると、現像性が不十分となることがあり、120mgKOH/gを超えると、現像速度が高すぎるため現像のコントロールが難しくなることがある。
ここで、前記酸価は、例えば、JIS K0070に準拠して測定することができる。ただし、サンプルが溶解しない場合は、溶媒としてジオキサン又はテトラヒドロフランなどを使用する。 The acid value of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose. 35 mg KOH / g to 100 mg KOH / g is particularly preferable. If the acid value is less than 20 mgKOH / g, the developability may be insufficient, and if it exceeds 120 mgKOH / g, the development speed may be too high, and the development control may be difficult.
Here, the said acid value can be measured based on JISK0070, for example. However, if the sample does not dissolve, dioxane or tetrahydrofuran is used as the solvent.
ここで、前記酸価は、例えば、JIS K0070に準拠して測定することができる。ただし、サンプルが溶解しない場合は、溶媒としてジオキサン又はテトラヒドロフランなどを使用する。 The acid value of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose. 35 mg KOH / g to 100 mg KOH / g is particularly preferable. If the acid value is less than 20 mgKOH / g, the developability may be insufficient, and if it exceeds 120 mgKOH / g, the development speed may be too high, and the development control may be difficult.
Here, the said acid value can be measured based on JISK0070, for example. However, if the sample does not dissolve, dioxane or tetrahydrofuran is used as the solvent.
前記酸変性ビニル基含有ポリウレタン樹脂のビニル基当量としては、特に制限はなく、目的に応じて適宜選択することができるが、0.05mmol/g~3.0mmol/gが好ましく、0.5mmol/g~2.7mmol/gがより好ましく、0.75mmol/g~2.4mmol/gが特に好ましい。前記ビニル基当量が、0.05mmol/g未満であると、硬化膜の耐熱性が劣ることがあり、3.0mmol/gを超えると、耐クラック性が悪化することがある。
ここで、前記ビニル基当量は、例えば、臭素価を測定することにより求めることができる。なお、前記臭素価は、例えば、JIS K2605に準拠して測定することができる。 The vinyl group equivalent of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol / g to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable. When the vinyl group equivalent is less than 0.05 mmol / g, the heat resistance of the cured film may be inferior, and when it exceeds 3.0 mmol / g, the crack resistance may be deteriorated.
Here, the said vinyl group equivalent can be calculated | required by measuring a bromine number, for example. The bromine number can be measured according to, for example, JIS K2605.
ここで、前記ビニル基当量は、例えば、臭素価を測定することにより求めることができる。なお、前記臭素価は、例えば、JIS K2605に準拠して測定することができる。 The vinyl group equivalent of the acid-modified vinyl group-containing polyurethane resin is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.05 mmol / g to 3.0 mmol / g, preferably 0.5 mmol / g to 2.7 mmol / g is more preferable, and 0.75 mmol / g to 2.4 mmol / g is particularly preferable. When the vinyl group equivalent is less than 0.05 mmol / g, the heat resistance of the cured film may be inferior, and when it exceeds 3.0 mmol / g, the crack resistance may be deteriorated.
Here, the said vinyl group equivalent can be calculated | required by measuring a bromine number, for example. The bromine number can be measured according to, for example, JIS K2605.
-不飽和基含有ポリカルボン酸樹脂-
前記不飽和基含有ポリカルボン酸樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特許第2877659号公報に記載されたものなどが挙げられる。 -Unsaturated group-containing polycarboxylic acid resin-
There is no restriction | limiting in particular as said unsaturated group containing polycarboxylic acid resin, According to the objective, it can select suitably, For example, what was described in patent 2877659 etc. is mentioned.
前記不飽和基含有ポリカルボン酸樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特許第2877659号公報に記載されたものなどが挙げられる。 -Unsaturated group-containing polycarboxylic acid resin-
There is no restriction | limiting in particular as said unsaturated group containing polycarboxylic acid resin, According to the objective, it can select suitably, For example, what was described in patent 2877659 etc. is mentioned.
-酸変性ビニル基含有エポキシ樹脂-
前記酸変性ビニル基含有エポキシ樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特許第4127010号公報(特開2004-133060号公報)に記載されたものなどが挙げられる。 -Acid-modified vinyl group-containing epoxy resin-
The acid-modified vinyl group-containing epoxy resin is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include those described in Japanese Patent No. 4127010 (Japanese Patent Laid-Open No. 2004-133060). Can be mentioned.
前記酸変性ビニル基含有エポキシ樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特許第4127010号公報(特開2004-133060号公報)に記載されたものなどが挙げられる。 -Acid-modified vinyl group-containing epoxy resin-
The acid-modified vinyl group-containing epoxy resin is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include those described in Japanese Patent No. 4127010 (Japanese Patent Laid-Open No. 2004-133060). Can be mentioned.
-不飽和基及びカルボキシル基を含有する樹脂-
前記不飽和基及びカルボキシル基を含有する樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、国際公開第2004/034147号パンフレットに記載されたものなどが挙げられる。 -Resins containing unsaturated groups and carboxyl groups-
There is no restriction | limiting in particular as resin which contains the said unsaturated group and a carboxyl group, According to the objective, it can select suitably, For example, what was described in the international publication 2004/034147 pamphlet etc. are mentioned.
前記不飽和基及びカルボキシル基を含有する樹脂としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、国際公開第2004/034147号パンフレットに記載されたものなどが挙げられる。 -Resins containing unsaturated groups and carboxyl groups-
There is no restriction | limiting in particular as resin which contains the said unsaturated group and a carboxyl group, According to the objective, it can select suitably, For example, what was described in the international publication 2004/034147 pamphlet etc. are mentioned.
-ポリイミド前駆体-
前記ポリイミド前駆体としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2010-6946号公報に記載されたものなどが挙げられる。 -Polyimide precursor-
There is no restriction | limiting in particular as said polyimide precursor, According to the objective, it can select suitably, For example, what was described in Unexamined-Japanese-Patent No. 2010-6946 etc. is mentioned.
前記ポリイミド前駆体としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2010-6946号公報に記載されたものなどが挙げられる。 -Polyimide precursor-
There is no restriction | limiting in particular as said polyimide precursor, According to the objective, it can select suitably, For example, what was described in Unexamined-Japanese-Patent No. 2010-6946 etc. is mentioned.
<<重合性化合物>>
前記重合性化合物としては、特に制限はなく、目的に応じて適宜選択することができ、分子中に少なくとも1個の付加重合可能な基を有し、沸点が常圧で100℃以上である化合物が好ましく、例えば、(メタ)アクリル基を有するモノマーから選択される少なくとも1種が好適に挙げられる。 << polymerizable compound >>
The polymerizable compound is not particularly limited and may be appropriately selected depending on the intended purpose. The compound has at least one addition-polymerizable group in the molecule and has a boiling point of 100 ° C. or higher at normal pressure. For example, at least one selected from monomers having a (meth) acryl group is preferable.
前記重合性化合物としては、特に制限はなく、目的に応じて適宜選択することができ、分子中に少なくとも1個の付加重合可能な基を有し、沸点が常圧で100℃以上である化合物が好ましく、例えば、(メタ)アクリル基を有するモノマーから選択される少なくとも1種が好適に挙げられる。 << polymerizable compound >>
The polymerizable compound is not particularly limited and may be appropriately selected depending on the intended purpose. The compound has at least one addition-polymerizable group in the molecule and has a boiling point of 100 ° C. or higher at normal pressure. For example, at least one selected from monomers having a (meth) acryl group is preferable.
前記(メタ)アクリル基を有するモノマーとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、ポリエチレングリコールモノ(メタ)アクリレート、ポリプロピレングリコールモノ(メタ)アクリレート、フェノキシエチル(メタ)アクリレート等の単官能アクリレート;単官能メタクリレート;ポリエチレングリコールジ(メタ)アクリレート、ポリプロピレングリコールジ(メタ)アクリレート、トリメチロールエタントリアクリレート、トリメチロールプロパントリアクリレート、トリメチロールプロパンジアクリレート、ネオペンチルグリコールジ(メタ)アクリレート、ペンタエリトリトールテトラ(メタ)アクリレート、ペンタエリトリトールトリ(メタ)アクリレート、ジペンタエリトリトールヘキサ(メタ)アクリレート、ジペンタエリトリトールペンタ(メタ)アクリレート、ヘキサンジオールジ(メタ)アクリレート、トリメチロールプロパントリ(アクリロイルオキシプロピル)エーテル、トリ(アクリロイルオキシエチル)イソシアヌレート、トリ(アクリロイルオキシエチル)シアヌレート、グリセリントリ(メタ)アクリレート、トリシクロデカンジメタノール(メタ)アクリレート、トリメチロールプロパン、グリセリン、ビスフェノール等の多官能アルコールに、エチレンオキサイド、プロピレンオキサイドを付加反応した後で(メタ)アクリレート化したもの、特公昭48-41708号公報、特公昭50-6034号公報、特開昭51-37193号公報等の各公報に記載されているウレタンアクリレート類;特開昭48-64183号公報、特公昭49-43191号公報、特公昭52-30490号公報等の各公報に記載されているポリエステルアクリレート類;エポキシ樹脂と(メタ)アクリル酸の反応生成物であるエポキシアクリレート類等の多官能アクリレート及びメタクリレートなどが挙げられる。これらは、1種単独で使用してもよいし、2種以上を併用してもよい。これらの中でも、トリメチロールプロパントリ(メタ)アクリレート、ペンタエリトリトールテトラ(メタ)アクリレート、ジペンタエリトリトールヘキサ(メタ)アクリレート、ジペンタエリトリトールペンタ(メタ)アクリレートが特に好ましい。
There is no restriction | limiting in particular as a monomer which has the said (meth) acryl group, According to the objective, it can select suitably, For example, polyethyleneglycol mono (meth) acrylate, polypropylene glycol mono (meth) acrylate, phenoxyethyl (meth) ) Monofunctional acrylate such as acrylate; Monofunctional methacrylate; Polyethylene glycol di (meth) acrylate, Polypropylene glycol di (meth) acrylate, Trimethylolethane triacrylate, Trimethylolpropane triacrylate, Trimethylolpropane diacrylate, Neopentylglycol di (Meth) acrylate, pentaerythritol tetra (meth) acrylate, pentaerythritol tri (meth) acrylate, dipentaerythritol hex (Meth) acrylate, dipentaerythritol penta (meth) acrylate, hexanediol di (meth) acrylate, trimethylolpropane tri (acryloyloxypropyl) ether, tri (acryloyloxyethyl) isocyanurate, tri (acryloyloxyethyl) cyanurate, (Meth) acrylate after addition reaction of ethylene oxide and propylene oxide to polyfunctional alcohols such as glycerin tri (meth) acrylate, tricyclodecane dimethanol (meth) acrylate, trimethylolpropane, glycerin, bisphenol, Urethane acrylates described in JP-B-48-41708, JP-B-50-6034, JP-A-51-37193, etc .; Polyester acrylates described in publications such as JP-B-48-64183, JP-B-49-43191, JP-B-52-30490, etc .; Epoxy acrylate which is a reaction product of epoxy resin and (meth) acrylic acid And other polyfunctional acrylates and methacrylates. These may be used individually by 1 type and may use 2 or more types together. Among these, trimethylolpropane tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexa (meth) acrylate, and dipentaerythritol penta (meth) acrylate are particularly preferable.
前記重合性化合物の前記シリカ分散組成物における含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、2質量%~50質量%が好ましく、3質量%~40質量%がより好ましく、4質量%~35質量%が特に好ましい。
前記含有量が、2質量%未満であると、パターン形成ができないことがあり、50質量%を超えると、耐クラック性が劣ることがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好なパターン形成と耐クラック性とを両立できる点で有利である。 The content of the polymerizable compound in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2% by mass to 50% by mass, and 3% by mass to 40% by mass. Is more preferable, and 4% by mass to 35% by mass is particularly preferable.
When the content is less than 2% by mass, pattern formation may not be possible, and when it exceeds 50% by mass, crack resistance may be inferior. On the other hand, when the content is within the particularly preferable range, it is advantageous in that both good pattern formation and crack resistance can be achieved.
前記含有量が、2質量%未満であると、パターン形成ができないことがあり、50質量%を超えると、耐クラック性が劣ることがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好なパターン形成と耐クラック性とを両立できる点で有利である。 The content of the polymerizable compound in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 2% by mass to 50% by mass, and 3% by mass to 40% by mass. Is more preferable, and 4% by mass to 35% by mass is particularly preferable.
When the content is less than 2% by mass, pattern formation may not be possible, and when it exceeds 50% by mass, crack resistance may be inferior. On the other hand, when the content is within the particularly preferable range, it is advantageous in that both good pattern formation and crack resistance can be achieved.
<<光重合開始剤>>
前記光重合開始剤としては、前記重合性化合物の重合を開始する能力を有する限り、特に制限はなく、目的に応じて適宜選択することができ、例えば、ハロゲン化炭化水素誘導体(例えば、トリアジン骨格を有するもの、オキサジアゾール骨格を有するもの)、ホスフィンオキサイド、ヘキサアリールビイミダゾール、オキシム誘導体、有機過酸化物、チオ化合物、ケトン化合物、芳香族オニウム塩、ケトオキシムエーテルなどが挙げられる。 << photopolymerization initiator >>
The photopolymerization initiator is not particularly limited as long as it has the ability to initiate polymerization of the polymerizable compound, and can be appropriately selected according to the purpose. For example, a halogenated hydrocarbon derivative (for example, a triazine skeleton) And those having an oxadiazole skeleton), phosphine oxide, hexaarylbiimidazole, oxime derivatives, organic peroxides, thio compounds, ketone compounds, aromatic onium salts, ketoxime ethers, and the like.
前記光重合開始剤としては、前記重合性化合物の重合を開始する能力を有する限り、特に制限はなく、目的に応じて適宜選択することができ、例えば、ハロゲン化炭化水素誘導体(例えば、トリアジン骨格を有するもの、オキサジアゾール骨格を有するもの)、ホスフィンオキサイド、ヘキサアリールビイミダゾール、オキシム誘導体、有機過酸化物、チオ化合物、ケトン化合物、芳香族オニウム塩、ケトオキシムエーテルなどが挙げられる。 << photopolymerization initiator >>
The photopolymerization initiator is not particularly limited as long as it has the ability to initiate polymerization of the polymerizable compound, and can be appropriately selected according to the purpose. For example, a halogenated hydrocarbon derivative (for example, a triazine skeleton) And those having an oxadiazole skeleton), phosphine oxide, hexaarylbiimidazole, oxime derivatives, organic peroxides, thio compounds, ketone compounds, aromatic onium salts, ketoxime ethers, and the like.
前記トリアジン骨格を有するハロゲン化炭化水素化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、若林ら著、Bull.Chem.Soc.Japan,42、2924(1969)に記載された化合物、英国特許1388492号明細書に記載された化合物、特開昭53-133428号公報に記載された化合物、独国特許3337024号明細書に記載された化合物、F.C.SchaeferなどによるJ.Org.Chem.;29、1527(1964)に記載された化合物、特開昭62-58241号公報に記載された化合物、特開平5-281728号公報に記載された化合物、特開平5-34920号公報に記載された化合物などが挙げられ、前記オキサジアゾール骨格を有するハロゲン化炭化水素化合物としては、例えば、米国特許第4212976号明細書に記載された化合物などが挙げられる。
The halogenated hydrocarbon compound having a triazine skeleton is not particularly limited and may be appropriately selected depending on the intended purpose. For example, Wakabayashi et al., Bull. Chem. Soc. Japan, 42, 2924 (1969), compounds described in British Patent No. 1388492, compounds described in JP-A-53-133428, German Patent No. 3337024 Compounds, F.I. C. J. Schaefer et al. Org. Chem. 29, 1527 (1964), a compound described in JP-A-62-258241, a compound described in JP-A-5-281728, and a compound described in JP-A-5-34920 Examples of the halogenated hydrocarbon compound having an oxadiazole skeleton include the compounds described in US Pat. No. 4,221,976.
前記オキシム誘導体としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0085〕に記載された化合物などが挙げられる。
The oxime derivative is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0085] of JP-A-2007-2030.
前記ケトン化合物としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0087〕に記載された化合物などが挙げられる。
The ketone compound is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0087] of JP-A-2007-2030.
また、上記以外の光重合開始剤としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0086〕に記載された化合物などが挙げられる。
The photopolymerization initiator other than the above is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0086] of JP-A-2007-2030. It is done.
また、後述する感光層への露光における露光感度及び感光波長を調整する目的で、前記光重合開始剤に加えて、増感剤を添加することが可能である。
前記増感剤は、後述する光照射手段としての可視光線、紫外光レーザ、可視光レーザなどにより適宜選択することができる。
前記増感剤は、活性エネルギー線により励起状態となり、他の物質(例えば、ラジカル発生剤、酸発生剤など)と相互作用(例えば、エネルギー移動、電子移動など)することにより、ラジカル、酸などの有用基を発生することが可能である。 In addition to the photopolymerization initiator, a sensitizer can be added for the purpose of adjusting exposure sensitivity and photosensitive wavelength in exposure to the photosensitive layer described later.
The sensitizer can be appropriately selected by a visible light, an ultraviolet laser, a visible laser or the like as a light irradiation means described later.
The sensitizer is excited by active energy rays and interacts with other substances (eg, radical generator, acid generator, etc.) (eg, energy transfer, electron transfer, etc.), thereby causing radicals, acids, etc. It is possible to generate a useful group of
前記増感剤は、後述する光照射手段としての可視光線、紫外光レーザ、可視光レーザなどにより適宜選択することができる。
前記増感剤は、活性エネルギー線により励起状態となり、他の物質(例えば、ラジカル発生剤、酸発生剤など)と相互作用(例えば、エネルギー移動、電子移動など)することにより、ラジカル、酸などの有用基を発生することが可能である。 In addition to the photopolymerization initiator, a sensitizer can be added for the purpose of adjusting exposure sensitivity and photosensitive wavelength in exposure to the photosensitive layer described later.
The sensitizer can be appropriately selected by a visible light, an ultraviolet laser, a visible laser or the like as a light irradiation means described later.
The sensitizer is excited by active energy rays and interacts with other substances (eg, radical generator, acid generator, etc.) (eg, energy transfer, electron transfer, etc.), thereby causing radicals, acids, etc. It is possible to generate a useful group of
前記増感剤としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2007-2030号公報の段落〔0089〕に記載された化合物などが挙げられる。
The sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose. Examples thereof include compounds described in paragraph [0089] of JP-A-2007-2030.
前記光重合開始剤と前記増感剤との組合せとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、特開2001-305734号公報に記載の電子移動型開始系[(1)電子供与型開始剤及び増感色素、(2)電子受容型開始剤及び増感色素、(3)電子供与型開始剤、増感色素及び電子受容型開始剤(三元開始系)]などの組合せが挙げられる。
The combination of the photopolymerization initiator and the sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose. For example, an electron transfer type initiation system described in JP-A-2001-305734 [ (1) Electron donating initiator and sensitizing dye, (2) Electron accepting initiator and sensitizing dye, (3) Electron donating initiator, sensitizing dye and electron accepting initiator (ternary initiation system) ] Etc. are mentioned.
前記増感剤の含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、前記シリカ分散組成物中の全成分に対し、0.05質量%~30質量%が好ましく、0.1質量%~20質量%がより好ましく、0.2質量%~10質量%が特に好ましい。前記含有量が、0.05質量%未満であると、活性エネルギー線への感度が低下し、露光プロセスに時間がかかり、生産性が低下することがあり、30質量%を超えると、保存時に前記感光層から前記増感剤が析出してしまうことがある。
The content of the sensitizer is not particularly limited and may be appropriately selected depending on the intended purpose. It is preferably 0.05% by mass to 30% by mass with respect to all components in the silica dispersion composition. 0.1 mass% to 20 mass% is more preferable, and 0.2 mass% to 10 mass% is particularly preferable. When the content is less than 0.05% by mass, the sensitivity to active energy rays decreases, the exposure process takes time, and the productivity may be reduced. The sensitizer may be precipitated from the photosensitive layer.
前記光重合開始剤は、1種単独で使用してもよく、2種以上を併用してもよい。
前記光重合開始剤の特に好ましい例としては、ホスフィンオキサイド類、前記α-アミノアルキルケトン類、前記トリアジン骨格を有するハロゲン化炭化水素化合物と後述する増感剤としてのアミン化合物とを組合せた複合光開始剤、ヘキサアリールビイミダゾール化合物、あるいは、チタノセンなどが挙げられる。 The said photoinitiator may be used individually by 1 type, and may use 2 or more types together.
Particularly preferred examples of the photopolymerization initiator include composite light in which phosphine oxides, the α-aminoalkyl ketones, the halogenated hydrocarbon compound having the triazine skeleton and an amine compound as a sensitizer described later are combined. Examples thereof include an initiator, a hexaarylbiimidazole compound, and titanocene.
前記光重合開始剤の特に好ましい例としては、ホスフィンオキサイド類、前記α-アミノアルキルケトン類、前記トリアジン骨格を有するハロゲン化炭化水素化合物と後述する増感剤としてのアミン化合物とを組合せた複合光開始剤、ヘキサアリールビイミダゾール化合物、あるいは、チタノセンなどが挙げられる。 The said photoinitiator may be used individually by 1 type, and may use 2 or more types together.
Particularly preferred examples of the photopolymerization initiator include composite light in which phosphine oxides, the α-aminoalkyl ketones, the halogenated hydrocarbon compound having the triazine skeleton and an amine compound as a sensitizer described later are combined. Examples thereof include an initiator, a hexaarylbiimidazole compound, and titanocene.
前記光重合開始剤の前記シリカ分散組成物における含有量としては、特に制限はなく、目的に応じて適宜選択することができるが、0.5質量%~20質量%が好ましく、0.5質量%~15質量%がより好ましく、1質量%~10質量%が特に好ましい。
前記含有量が、0.5質量%未満であると、露光部が現像中に溶出する傾向があり、20質量%を超えると、耐熱性が低下することがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好なパターン形成ができ、耐熱性も良好になる点で有利である。 The content of the photopolymerization initiator in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.5% by mass to 20% by mass, and preferably 0.5% by mass. % To 15% by mass is more preferable, and 1% to 10% by mass is particularly preferable.
When the content is less than 0.5% by mass, the exposed portion tends to be eluted during development, and when it exceeds 20% by mass, the heat resistance may be lowered. On the other hand, when the content is within the particularly preferable range, it is advantageous in that a good pattern can be formed and heat resistance is also improved.
前記含有量が、0.5質量%未満であると、露光部が現像中に溶出する傾向があり、20質量%を超えると、耐熱性が低下することがある。一方、前記含有量が、前記特に好ましい範囲内であると、良好なパターン形成ができ、耐熱性も良好になる点で有利である。 The content of the photopolymerization initiator in the silica dispersion composition is not particularly limited and may be appropriately selected depending on the intended purpose, but is preferably 0.5% by mass to 20% by mass, and preferably 0.5% by mass. % To 15% by mass is more preferable, and 1% to 10% by mass is particularly preferable.
When the content is less than 0.5% by mass, the exposed portion tends to be eluted during development, and when it exceeds 20% by mass, the heat resistance may be lowered. On the other hand, when the content is within the particularly preferable range, it is advantageous in that a good pattern can be formed and heat resistance is also improved.
<<その他の成分>>
前記その他の成分としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、熱可塑性エラストマー、フィラー、熱硬化促進剤、熱重合禁止剤、可塑剤、着色剤(着色顔料あるいは染料)などが挙げられ、更に基材表面への密着促進剤、又はその他の助剤類(例えば、導電性粒子、充填剤、消泡剤、難燃剤、レベリング剤、剥離促進剤、酸化防止剤、香料、表面張力調整剤、連鎖移動剤など)を併用してもよい。
これらの成分を適宜含有させることにより、目的とする感光性フィルムの安定性、写真性、膜物性などの性質を調整することができる。 << Other ingredients >>
The other components are not particularly limited and may be appropriately selected depending on the purpose. For example, thermoplastic elastomers, fillers, thermosetting accelerators, thermal polymerization inhibitors, plasticizers, colorants (color pigments or Dyes) and the like, and further adhesion promoters to the substrate surface, or other auxiliary agents (for example, conductive particles, fillers, antifoaming agents, flame retardants, leveling agents, peeling accelerators, antioxidants). , Fragrance, surface tension adjusting agent, chain transfer agent, etc.) may be used in combination.
By appropriately containing these components, properties such as the stability, photographic properties, and film properties of the intended photosensitive film can be adjusted.
前記その他の成分としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、熱可塑性エラストマー、フィラー、熱硬化促進剤、熱重合禁止剤、可塑剤、着色剤(着色顔料あるいは染料)などが挙げられ、更に基材表面への密着促進剤、又はその他の助剤類(例えば、導電性粒子、充填剤、消泡剤、難燃剤、レベリング剤、剥離促進剤、酸化防止剤、香料、表面張力調整剤、連鎖移動剤など)を併用してもよい。
これらの成分を適宜含有させることにより、目的とする感光性フィルムの安定性、写真性、膜物性などの性質を調整することができる。 << Other ingredients >>
The other components are not particularly limited and may be appropriately selected depending on the purpose. For example, thermoplastic elastomers, fillers, thermosetting accelerators, thermal polymerization inhibitors, plasticizers, colorants (color pigments or Dyes) and the like, and further adhesion promoters to the substrate surface, or other auxiliary agents (for example, conductive particles, fillers, antifoaming agents, flame retardants, leveling agents, peeling accelerators, antioxidants). , Fragrance, surface tension adjusting agent, chain transfer agent, etc.) may be used in combination.
By appropriately containing these components, properties such as the stability, photographic properties, and film properties of the intended photosensitive film can be adjusted.
前記熱可塑性エラストマーとしては、特に制限はなく、目的に応じて適宜選択することができ、例えば、スチレン系エラストマー、オレフィン系エラストマー、ウレタン系エラストマー、ポリエステル系エラストマー、ポリアミド系エラストマー、アクリル系エラストマー、シリコーン系エラストマーなどが挙げられる。
これらのエラストマーは、ハードセグメント成分とソフトセグメント成分から成り立っており、一般に前者が耐熱性、強度に、後者が柔軟性、強靭性に寄与している。
前記熱可塑性エラストマーとしては、特開2007-199532号公報の段落〔0197〕~〔0207〕に記載されている。
前記フィラーについては、例えば、特開2008-250074号公報の段落〔0098〕~〔0099〕に詳細に記載されている。
前記熱重合禁止剤については、例えば、特開2008-250074号公報の段落〔0101〕~〔0102〕に詳細に記載されている。
前記熱硬化促進剤については、例えば、特開2008-250074号公報の段落〔0093〕に詳細に記載されている。
前記可塑剤については、例えば、特開2008-250074号公報の段落〔0103〕~〔0104〕に詳細に記載されている。
前記着色剤については、例えば、特開2008-250074号公報の段落〔0105〕~〔0106〕に詳細に記載されている。
前記密着促進剤については、例えば、特開2008-250074号公報の段落〔0107〕~〔0109〕に詳細に記載されている。 There is no restriction | limiting in particular as said thermoplastic elastomer, According to the objective, it can select suitably, For example, styrene-type elastomer, olefin-type elastomer, urethane-type elastomer, polyester-type elastomer, polyamide-type elastomer, acrylic-type elastomer, silicone Based elastomers and the like.
These elastomers are composed of a hard segment component and a soft segment component. In general, the former contributes to heat resistance and strength, and the latter contributes to flexibility and toughness.
The thermoplastic elastomer is described in paragraphs [0197] to [0207] of JP-A-2007-199532.
The filler is described in detail in, for example, paragraphs [0098] to [0099] of JP-A-2008-250074.
The thermal polymerization inhibitor is described in detail, for example, in paragraphs [0101] to [0102] of JP-A-2008-250074.
The thermosetting accelerator is described in detail, for example, in paragraph [0093] of JP-A-2008-250074.
The plasticizer is described in detail, for example, in paragraphs [0103] to [0104] of JP-A-2008-250074.
The colorant is described in detail, for example, in paragraphs [0105] to [0106] of JP-A-2008-250074.
The adhesion promoter is described in detail, for example, in paragraphs [0107] to [0109] of JP-A-2008-250074.
これらのエラストマーは、ハードセグメント成分とソフトセグメント成分から成り立っており、一般に前者が耐熱性、強度に、後者が柔軟性、強靭性に寄与している。
前記熱可塑性エラストマーとしては、特開2007-199532号公報の段落〔0197〕~〔0207〕に記載されている。
前記フィラーについては、例えば、特開2008-250074号公報の段落〔0098〕~〔0099〕に詳細に記載されている。
前記熱重合禁止剤については、例えば、特開2008-250074号公報の段落〔0101〕~〔0102〕に詳細に記載されている。
前記熱硬化促進剤については、例えば、特開2008-250074号公報の段落〔0093〕に詳細に記載されている。
前記可塑剤については、例えば、特開2008-250074号公報の段落〔0103〕~〔0104〕に詳細に記載されている。
前記着色剤については、例えば、特開2008-250074号公報の段落〔0105〕~〔0106〕に詳細に記載されている。
前記密着促進剤については、例えば、特開2008-250074号公報の段落〔0107〕~〔0109〕に詳細に記載されている。 There is no restriction | limiting in particular as said thermoplastic elastomer, According to the objective, it can select suitably, For example, styrene-type elastomer, olefin-type elastomer, urethane-type elastomer, polyester-type elastomer, polyamide-type elastomer, acrylic-type elastomer, silicone Based elastomers and the like.
These elastomers are composed of a hard segment component and a soft segment component. In general, the former contributes to heat resistance and strength, and the latter contributes to flexibility and toughness.
The thermoplastic elastomer is described in paragraphs [0197] to [0207] of JP-A-2007-199532.
The filler is described in detail in, for example, paragraphs [0098] to [0099] of JP-A-2008-250074.
The thermal polymerization inhibitor is described in detail, for example, in paragraphs [0101] to [0102] of JP-A-2008-250074.
The thermosetting accelerator is described in detail, for example, in paragraph [0093] of JP-A-2008-250074.
The plasticizer is described in detail, for example, in paragraphs [0103] to [0104] of JP-A-2008-250074.
The colorant is described in detail, for example, in paragraphs [0105] to [0106] of JP-A-2008-250074.
The adhesion promoter is described in detail, for example, in paragraphs [0107] to [0109] of JP-A-2008-250074.
(感光性フィルム)
本発明のシリカ分散組成物は、導体配線の形成された基板上に塗布乾燥することにより液状レジストとしても使用可能であるが、感光性フィルムの製造に特に有用である。
前記感光性フィルムは、少なくとも支持体と、感光層とを有してなり、好ましくは保護フィルムを有してなり、更に必要に応じて、クッション層、酸素遮断層(以下、PC層と省略する)などのその他の層を有してなる。
前記感光性フィルムの形態としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、前記支持体上に、前記感光層、及び前記保護膜フィルムをこの順に有してなる形態、前記支持体上に、前記PC層、前記感光性層、及び前記保護フィルムをこの順に有してなる形態、前記支持体上に、前記クッション層、前記PC層、前記感光層、及び前記保護フィルムをこの順に有してなる形態などが挙げられる。なお、前記感光層は、単層であってもよいし、複数層であってもよい。 (Photosensitive film)
The silica dispersion composition of the present invention can be used as a liquid resist by coating and drying on a substrate on which a conductor wiring is formed, but is particularly useful for producing a photosensitive film.
The photosensitive film has at least a support and a photosensitive layer, preferably has a protective film, and further, if necessary, a cushion layer, an oxygen barrier layer (hereinafter abbreviated as PC layer). ) And other layers.
There is no restriction | limiting in particular as a form of the said photosensitive film, According to the objective, it can select suitably, For example, the form which has the said photosensitive layer and the said protective film in this order on the said support body. The PC layer, the photosensitive layer, and the protective film are provided in this order on the support, and the cushion layer, the PC layer, the photosensitive layer, and the protection are provided on the support. The form etc. which have a film in this order are mentioned. The photosensitive layer may be a single layer or a plurality of layers.
本発明のシリカ分散組成物は、導体配線の形成された基板上に塗布乾燥することにより液状レジストとしても使用可能であるが、感光性フィルムの製造に特に有用である。
前記感光性フィルムは、少なくとも支持体と、感光層とを有してなり、好ましくは保護フィルムを有してなり、更に必要に応じて、クッション層、酸素遮断層(以下、PC層と省略する)などのその他の層を有してなる。
前記感光性フィルムの形態としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、前記支持体上に、前記感光層、及び前記保護膜フィルムをこの順に有してなる形態、前記支持体上に、前記PC層、前記感光性層、及び前記保護フィルムをこの順に有してなる形態、前記支持体上に、前記クッション層、前記PC層、前記感光層、及び前記保護フィルムをこの順に有してなる形態などが挙げられる。なお、前記感光層は、単層であってもよいし、複数層であってもよい。 (Photosensitive film)
The silica dispersion composition of the present invention can be used as a liquid resist by coating and drying on a substrate on which a conductor wiring is formed, but is particularly useful for producing a photosensitive film.
The photosensitive film has at least a support and a photosensitive layer, preferably has a protective film, and further, if necessary, a cushion layer, an oxygen barrier layer (hereinafter abbreviated as PC layer). ) And other layers.
There is no restriction | limiting in particular as a form of the said photosensitive film, According to the objective, it can select suitably, For example, the form which has the said photosensitive layer and the said protective film in this order on the said support body. The PC layer, the photosensitive layer, and the protective film are provided in this order on the support, and the cushion layer, the PC layer, the photosensitive layer, and the protection are provided on the support. The form etc. which have a film in this order are mentioned. The photosensitive layer may be a single layer or a plurality of layers.
<感光層>
前記感光層は、本発明の前記シリカ分散組成物から形成される。
前記感光層の70℃における溶融粘度としては、1.4×103Pa・s以下が好ましく、1.0×103Pa・s以下がより好ましく、6.0×102Pa・s以下が更に好ましい。
前記感光層の70℃における溶融粘度が、1.4×103Pa・sを超えると、埋め込み性が悪化することがあり、前記70℃における溶融粘度が、より好ましい範囲であると、埋め込み性が充分得られる点で有利である。
前記感光層の30℃における溶融粘度としては、1.0×104Pa・s以上が好ましく、1.3×104Pa・s以上がより好ましく、3.0×104Pa・s以上が更に好ましい。
前記感光層の30℃における溶融粘度が、1.0×104Pa・s未満であると、エッジヒュージョンが悪化することがあり、前記30℃における溶融粘度が、より好ましい範囲であると、埋め込み性とエッジヒュージョンを両立できる点で有利である。 <Photosensitive layer>
The photosensitive layer is formed from the silica dispersion composition of the present invention.
The melt viscosity at 70 ° C. of the photosensitive layer is preferably 1.4 × 10 3 Pa · s or less, more preferably 1.0 × 10 3 Pa · s or less, and 6.0 × 10 2 Pa · s or less. Further preferred.
When the melt viscosity at 70 ° C. of the photosensitive layer exceeds 1.4 × 10 3 Pa · s, the embedding property may be deteriorated, and when the melt viscosity at 70 ° C. is in a more preferable range, the embedding property is deteriorated. Is advantageous in that it is sufficiently obtained.
The melt viscosity at 30 ° C. of the photosensitive layer is preferably 1.0 × 10 4 Pa · s or more, more preferably 1.3 × 10 4 Pa · s or more, and 3.0 × 10 4 Pa · s or more. Further preferred.
If the melt viscosity at 30 ° C. of the photosensitive layer is less than 1.0 × 10 4 Pa · s, edge fusion may be deteriorated, and if the melt viscosity at 30 ° C. is in a more preferable range, embedding is performed. It is advantageous in that it can be compatible with edge fusion.
前記感光層は、本発明の前記シリカ分散組成物から形成される。
前記感光層の70℃における溶融粘度としては、1.4×103Pa・s以下が好ましく、1.0×103Pa・s以下がより好ましく、6.0×102Pa・s以下が更に好ましい。
前記感光層の70℃における溶融粘度が、1.4×103Pa・sを超えると、埋め込み性が悪化することがあり、前記70℃における溶融粘度が、より好ましい範囲であると、埋め込み性が充分得られる点で有利である。
前記感光層の30℃における溶融粘度としては、1.0×104Pa・s以上が好ましく、1.3×104Pa・s以上がより好ましく、3.0×104Pa・s以上が更に好ましい。
前記感光層の30℃における溶融粘度が、1.0×104Pa・s未満であると、エッジヒュージョンが悪化することがあり、前記30℃における溶融粘度が、より好ましい範囲であると、埋め込み性とエッジヒュージョンを両立できる点で有利である。 <Photosensitive layer>
The photosensitive layer is formed from the silica dispersion composition of the present invention.
The melt viscosity at 70 ° C. of the photosensitive layer is preferably 1.4 × 10 3 Pa · s or less, more preferably 1.0 × 10 3 Pa · s or less, and 6.0 × 10 2 Pa · s or less. Further preferred.
When the melt viscosity at 70 ° C. of the photosensitive layer exceeds 1.4 × 10 3 Pa · s, the embedding property may be deteriorated, and when the melt viscosity at 70 ° C. is in a more preferable range, the embedding property is deteriorated. Is advantageous in that it is sufficiently obtained.
The melt viscosity at 30 ° C. of the photosensitive layer is preferably 1.0 × 10 4 Pa · s or more, more preferably 1.3 × 10 4 Pa · s or more, and 3.0 × 10 4 Pa · s or more. Further preferred.
If the melt viscosity at 30 ° C. of the photosensitive layer is less than 1.0 × 10 4 Pa · s, edge fusion may be deteriorated, and if the melt viscosity at 30 ° C. is in a more preferable range, embedding is performed. It is advantageous in that it can be compatible with edge fusion.
ここで、前記感光層の溶融粘度の測定は、例えば、レオメーター・VAR-1000型(レオロジカル株式会社製)、バイブロン・DD-III型(東洋ボールドウイン株式会社製)などの溶融粘度測定装置を用いて測定することができる。
Here, the melt viscosity of the photosensitive layer is measured by, for example, a melt viscosity measuring device such as a rheometer VAR-1000 type (manufactured by Rheological Co., Ltd.), Vibron DD-III type (manufactured by Toyo Baldwin Co., Ltd.) or the like. Can be measured.
<永久パターン及び永久パターン形成方法>
本発明で用いられる永久パターンは、前記永久パターン形成方法により得られる。
前記永久パターンとしては、特開2007-2030号公報の段落〔0128〕~〔0283〕に記載されている。 <Permanent pattern and permanent pattern forming method>
The permanent pattern used in the present invention is obtained by the permanent pattern forming method.
The permanent pattern is described in paragraphs [0128] to [0283] of Japanese Patent Application Laid-Open No. 2007-2030.
本発明で用いられる永久パターンは、前記永久パターン形成方法により得られる。
前記永久パターンとしては、特開2007-2030号公報の段落〔0128〕~〔0283〕に記載されている。 <Permanent pattern and permanent pattern forming method>
The permanent pattern used in the present invention is obtained by the permanent pattern forming method.
The permanent pattern is described in paragraphs [0128] to [0283] of Japanese Patent Application Laid-Open No. 2007-2030.
<プリント基板>
本発明で用いられるプリント基板は、少なくとも基体と、前記永久パターン形成方法により形成された永久パターンとを有してなり、更に必要に応じて適宜選択した、その他の構成を有してなる。
その他の構成としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、前記基体と前記永久パターン間に、更に絶縁層が設けられたビルドアップ基板などが挙げられる。 <Printed circuit board>
The printed board used in the present invention has at least a base and a permanent pattern formed by the permanent pattern forming method, and further has other configurations appropriately selected as necessary.
There is no restriction | limiting in particular as another structure, According to the objective, it can select suitably, For example, the buildup board | substrate etc. in which the insulating layer was further provided between the said base | substrate and the said permanent pattern are mentioned.
本発明で用いられるプリント基板は、少なくとも基体と、前記永久パターン形成方法により形成された永久パターンとを有してなり、更に必要に応じて適宜選択した、その他の構成を有してなる。
その他の構成としては、特に制限はなく、目的に応じて適宜選択することができ、例えば、前記基体と前記永久パターン間に、更に絶縁層が設けられたビルドアップ基板などが挙げられる。 <Printed circuit board>
The printed board used in the present invention has at least a base and a permanent pattern formed by the permanent pattern forming method, and further has other configurations appropriately selected as necessary.
There is no restriction | limiting in particular as another structure, According to the objective, it can select suitably, For example, the buildup board | substrate etc. in which the insulating layer was further provided between the said base | substrate and the said permanent pattern are mentioned.
以下、本発明の実施例を説明するが、本発明は、これらの実施例に何ら限定されるものではない。
Examples of the present invention will be described below, but the present invention is not limited to these examples.
(合成例1)
-ポリウレタンバインダーの合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、2,2-ビス(ヒドロキシメチル)プロピオン酸(DMPA)17.71g(0.132モル)、及びグリセロールモノメタクリレート(GLM)34.6g(0.216モル)をプロピレングリコールモノメチルエーテルモノアセテート140mLに溶解した。これに、4,4’-ジフェニルメタンジイソシアネート(MDI)60.06g(0.24モル)、ヘキサメチレンジイソシアネート(HDI)10.09g(0.01モル)、2,6-ジ-t-ブチルヒドロキシトルエン0.2g、及び触媒として商品名:ネオスタンU-600(日東化成株式会社製)0.4gを添加し、75℃にて、5時間加熱撹拌した。その後、メチルアルコール32.04mLにて希釈して30分間撹拌し、272gの酸変性ビニル基含有ポリウレタンバインダー溶液(固形分45質量%)を合成した。 (Synthesis Example 1)
-Synthesis of polyurethane binder-
In a 500 mL 3-neck round bottom flask equipped with a condenser and stirrer, 17.71 g (0.132 mol) of 2,2-bis (hydroxymethyl) propionic acid (DMPA) and glycerol monomethacrylate (GLM) 34.6 g (0.216 mol) was dissolved in 140 mL of propylene glycol monomethyl ether monoacetate. To this, 60.06 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI), 10.09 g (0.01 mol) of hexamethylene diisocyanate (HDI), 2,6-di-t-butylhydroxytoluene 0.2 g, and 0.4 g of a trade name: Neostan U-600 (manufactured by Nitto Kasei Co., Ltd.) as a catalyst were added, and the mixture was stirred at 75 ° C. for 5 hours. Thereafter, the mixture was diluted with 32.04 mL of methyl alcohol and stirred for 30 minutes to synthesize 272 g of an acid-modified vinyl group-containing polyurethane binder solution (solid content: 45% by mass).
-ポリウレタンバインダーの合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、2,2-ビス(ヒドロキシメチル)プロピオン酸(DMPA)17.71g(0.132モル)、及びグリセロールモノメタクリレート(GLM)34.6g(0.216モル)をプロピレングリコールモノメチルエーテルモノアセテート140mLに溶解した。これに、4,4’-ジフェニルメタンジイソシアネート(MDI)60.06g(0.24モル)、ヘキサメチレンジイソシアネート(HDI)10.09g(0.01モル)、2,6-ジ-t-ブチルヒドロキシトルエン0.2g、及び触媒として商品名:ネオスタンU-600(日東化成株式会社製)0.4gを添加し、75℃にて、5時間加熱撹拌した。その後、メチルアルコール32.04mLにて希釈して30分間撹拌し、272gの酸変性ビニル基含有ポリウレタンバインダー溶液(固形分45質量%)を合成した。 (Synthesis Example 1)
-Synthesis of polyurethane binder-
In a 500 mL 3-neck round bottom flask equipped with a condenser and stirrer, 17.71 g (0.132 mol) of 2,2-bis (hydroxymethyl) propionic acid (DMPA) and glycerol monomethacrylate (GLM) 34.6 g (0.216 mol) was dissolved in 140 mL of propylene glycol monomethyl ether monoacetate. To this, 60.06 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI), 10.09 g (0.01 mol) of hexamethylene diisocyanate (HDI), 2,6-di-t-butylhydroxytoluene 0.2 g, and 0.4 g of a trade name: Neostan U-600 (manufactured by Nitto Kasei Co., Ltd.) as a catalyst were added, and the mixture was stirred at 75 ° C. for 5 hours. Thereafter, the mixture was diluted with 32.04 mL of methyl alcohol and stirred for 30 minutes to synthesize 272 g of an acid-modified vinyl group-containing polyurethane binder solution (solid content: 45% by mass).
(合成例2)
<中間体ポリエステルの合成>
ε-カプロラクトン200g、2-エチルヘキサノール22.8g、及びモノブチルスズオキシド0.1gを500mLの3つ口丸底フラスコ内に入れ、90℃で4時間撹拌し、110℃で4時間撹拌し、中間体ポリエステル樹脂を合成した。得られた中間体ポリエステル樹脂の数平均分子量(Mn)は2,100であった。 (Synthesis Example 2)
<Synthesis of intermediate polyester>
200 g of ε-caprolactone, 22.8 g of 2-ethylhexanol, and 0.1 g of monobutyltin oxide were placed in a 500 mL three-necked round bottom flask, stirred at 90 ° C. for 4 hours, and stirred at 110 ° C. for 4 hours. Body polyester resin was synthesized. The number average molecular weight (Mn) of the obtained intermediate polyester resin was 2,100.
<中間体ポリエステルの合成>
ε-カプロラクトン200g、2-エチルヘキサノール22.8g、及びモノブチルスズオキシド0.1gを500mLの3つ口丸底フラスコ内に入れ、90℃で4時間撹拌し、110℃で4時間撹拌し、中間体ポリエステル樹脂を合成した。得られた中間体ポリエステル樹脂の数平均分子量(Mn)は2,100であった。 (Synthesis Example 2)
<Synthesis of intermediate polyester>
200 g of ε-caprolactone, 22.8 g of 2-ethylhexanol, and 0.1 g of monobutyltin oxide were placed in a 500 mL three-necked round bottom flask, stirred at 90 ° C. for 4 hours, and stirred at 110 ° C. for 4 hours. Body polyester resin was synthesized. The number average molecular weight (Mn) of the obtained intermediate polyester resin was 2,100.
(合成例3)
<シリカ分散剤P-1の合成>
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール5.62g、ネオペンチルグリコール2.21g、2,2-ビス(ヒドロキシメチル)酪酸4.68g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-1溶液(固形分20質量%)を得た。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 3)
<Synthesis of Silica Dispersant P-1>
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 5.52 g of 3- (dimethylamino) -1,2-propanediol, 2.21 g of neopentyl glycol, 2,2-bis (hydroxy) 4.68 g of methyl) butyric acid, 25.43 g of the intermediate polyester resin, and 0.19 g of 2,6-di-t-butylhydroxytoluene were dissolved in 73 mL of cyclohexanone. To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-1 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-1の合成>
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール5.62g、ネオペンチルグリコール2.21g、2,2-ビス(ヒドロキシメチル)酪酸4.68g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-1溶液(固形分20質量%)を得た。
<Synthesis of Silica Dispersant P-1>
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 5.52 g of 3- (dimethylamino) -1,2-propanediol, 2.21 g of neopentyl glycol, 2,2-bis (hydroxy) 4.68 g of methyl) butyric acid, 25.43 g of the intermediate polyester resin, and 0.19 g of 2,6-di-t-butylhydroxytoluene were dissolved in 73 mL of cyclohexanone. To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-1 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
(合成例4)
<シリカ分散剤P-2の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを6.79gに変え、ネオペンチルグリコール2.21gを1.19gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-2溶液(固形分20質量%)を合成した。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 4)
<Synthesis of Silica Dispersant P-2>
In Synthesis Example 3, the same procedure as in Synthesis Example 3 was performed, except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 6.79 g and 2.21 g of neopentyl glycol was changed to 1.19 g. Thus, a silica dispersant P-2 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula was synthesized.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-2の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを6.79gに変え、ネオペンチルグリコール2.21gを1.19gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-2溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-2>
In Synthesis Example 3, the same procedure as in Synthesis Example 3 was performed, except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 6.79 g and 2.21 g of neopentyl glycol was changed to 1.19 g. Thus, a silica dispersant P-2 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula was synthesized.
(合成例5)
<シリカ分散剤P-3の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを8.15gに変え、ネオペンチルグリコールを添加しない以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-3溶液(固形分20質量%)を合成した。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 5)
<Synthesis of Silica Dispersant P-3>
In Synthesis Example 3, the components represented by the following formulas were used in the same manner as in Synthesis Example 3 except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 8.15 g and neopentyl glycol was not added. A silica dispersant P-3 solution (solid content: 20% by mass) having a mass ratio of 5% was synthesized.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-3の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを8.15gに変え、ネオペンチルグリコールを添加しない以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-3溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-3>
In Synthesis Example 3, the components represented by the following formulas were used in the same manner as in Synthesis Example 3 except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 8.15 g and neopentyl glycol was not added. A silica dispersant P-3 solution (solid content: 20% by mass) having a mass ratio of 5% was synthesized.
(合成例6)
<シリカ分散剤P-4の合成>
合成例3において、中間体ポリエステル樹脂25.43gをリンゴ酸ジメチルエステル4.91gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-4溶液(固形分20質量%)を合成した。
ただし、前記式中、数字は質量比を表す。
(Synthesis Example 6)
<Synthesis of Silica Dispersant P-4>
In the same manner as in Synthesis Example 3 except that 25.43 g of the intermediate polyester resin was changed to 4.91 g of malic acid dimethyl ester in Synthesis Example 3, the silica dispersant P-4 solution having the mass ratio of the components shown in the following formula (Solid content 20% by mass) was synthesized.
However, in the said formula, a number represents mass ratio.
<シリカ分散剤P-4の合成>
合成例3において、中間体ポリエステル樹脂25.43gをリンゴ酸ジメチルエステル4.91gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-4溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-4>
In the same manner as in Synthesis Example 3 except that 25.43 g of the intermediate polyester resin was changed to 4.91 g of malic acid dimethyl ester in Synthesis Example 3, the silica dispersant P-4 solution having the mass ratio of the components shown in the following formula (Solid content 20% by mass) was synthesized.
(合成例7)
<シリカ分散剤P-5の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオールを添加せず、ネオペンチルグリコール2.21gを7.19gに変え、2,2-ビス(ヒドロキシメチル)酪酸4.68gを4.59gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-5溶液(固形分20質量%)を合成した。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 7)
<Synthesis of Silica Dispersant P-5>
In Synthesis Example 3, 3- (dimethylamino) -1,2-propanediol was not added, neopentyl glycol 2.21 g was changed to 7.19 g, and 2,2-bis (hydroxymethyl) butyric acid 4.68 g A silica dispersant P-5 solution (solid content: 20% by mass) was synthesized in the same manner as in Synthesis Example 3 except that the amount was changed to 4.59 g.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-5の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオールを添加せず、ネオペンチルグリコール2.21gを7.19gに変え、2,2-ビス(ヒドロキシメチル)酪酸4.68gを4.59gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-5溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-5>
In Synthesis Example 3, 3- (dimethylamino) -1,2-propanediol was not added, neopentyl glycol 2.21 g was changed to 7.19 g, and 2,2-bis (hydroxymethyl) butyric acid 4.68 g A silica dispersant P-5 solution (solid content: 20% by mass) was synthesized in the same manner as in Synthesis Example 3 except that the amount was changed to 4.59 g.
(合成例8)
<シリカ分散剤P-6の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを8.34gに変え、ネオペンチルグリコール2.21gを5.21gに変え、2,2-ビス(ヒドロキシメチル)酪酸を添加しない以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-6溶液(固形分20質量%)を合成した。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 8)
<Synthesis of Silica Dispersant P-6>
In Synthesis Example 3, 3- (dimethylamino) -1,2-propanediol (5.62 g) was changed to 8.34 g, neopentyl glycol (2.21 g) was changed to 5.21 g, and 2,2-bis (hydroxymethyl) A silica dispersant P-6 solution (solid content 20% by mass) was synthesized in the same manner as in Synthesis Example 3, except that butyric acid was not added.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-6の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを8.34gに変え、ネオペンチルグリコール2.21gを5.21gに変え、2,2-ビス(ヒドロキシメチル)酪酸を添加しない以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-6溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-6>
In Synthesis Example 3, 3- (dimethylamino) -1,2-propanediol (5.62 g) was changed to 8.34 g, neopentyl glycol (2.21 g) was changed to 5.21 g, and 2,2-bis (hydroxymethyl) A silica dispersant P-6 solution (solid content 20% by mass) was synthesized in the same manner as in Synthesis Example 3, except that butyric acid was not added.
(合成例9)
<シリカ分散剤P-7の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを3.84gに変え、ネオペンチルグリコール2.21gを3.77gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-7溶液(固形分20質量%)を合成した。
ただし、前記式中、CLはカプロラクトン、数字は質量比を表す。
(Synthesis Example 9)
<Synthesis of Silica Dispersant P-7>
In Synthesis Example 3, the same procedure as in Synthesis Example 3 was performed except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 3.84 g and 2.21 g of neopentyl glycol was changed to 3.77 g. Thus, a silica dispersant P-7 solution (solid content 20% by mass) having a mass ratio of the components represented by the following formula was synthesized.
However, in said formula, CL represents a caprolactone and a number represents mass ratio.
<シリカ分散剤P-7の合成>
合成例3において、3-(ジメチルアミノ)-1,2-プロパンジオール5.62gを3.84gに変え、ネオペンチルグリコール2.21gを3.77gに変えた以外は、合成例3と同様にして、下記式に示す成分の質量比のシリカ分散剤P-7溶液(固形分20質量%)を合成した。
<Synthesis of Silica Dispersant P-7>
In Synthesis Example 3, the same procedure as in Synthesis Example 3 was performed except that 5.52 g of 3- (dimethylamino) -1,2-propanediol was changed to 3.84 g and 2.21 g of neopentyl glycol was changed to 3.77 g. Thus, a silica dispersant P-7 solution (solid content 20% by mass) having a mass ratio of the components represented by the following formula was synthesized.
(合成例10)
-シリカ分散剤P-8の合成-
特開2010-7059号公報の実施例2を再現したシリカ分散剤を合成した。
撹拌、乾燥窒素、吹き込み管、及び冷却管付きのフラスコに、N-ジエタノールアミン19.6質量部、プロピレングリコールモノメチルエーテルアセテート250質量部、及びカレンズMOI(昭和電工株式会社製)7.6質量部を、撹拌しながら25℃で加えて、40℃まで昇温し、40℃で2時間撹拌した。
次いで、ライトアクリレート130A(メトキシポリオキシエチレンエーテルアクリレート、共栄社化学社製、臭素価34.9Br/100g)45質量部、ライトアクリレートNP-4EA(ノニルフェニルポリオキシエチレンエーテルアクリレート、共栄社化学社製)17.7質量部を加え、80℃に昇温して、4時間反応させた。次いで、60℃まで冷却し、ひまし油(ジオールHS-2G-150R、豊国製油社製)60質量部、クラレポリエステルジオールP-2050(数平均分子量2,066、株式会社クラレ製)163質量部、ジメチロールブタン酸29.1質量部、1,6-ヘキサメチレンジイソシアネート49.5質量部、イソホロンジイソシアネート76.4質量部、及びプロピレングリコールモノメチルエーテルアセテート83質量部を加えて、60℃で5時間反応させた。
次いで、分子中に水酸基と(メタ)アクリロイル基を有するライトアクリレートG-201P(共栄社化学社製)23.4質量部、N-ジメチルアミノエタノール9.1質量部、ジブチル錫ラウリレート0.1質量部、メトキノン0.3質量部、及びプロピレングリコールモノメチルエーテルアセテート167質量部を加え、70℃で5時間反応させて、シリカ分散剤P-8を得た。 (Synthesis Example 10)
-Synthesis of Silica Dispersant P-8-
A silica dispersant that reproduces Example 2 of JP 2010-7059 A was synthesized.
In a flask equipped with stirring, dry nitrogen, blowing tube, and cooling tube, 19.6 parts by mass of N-diethanolamine, 250 parts by mass of propylene glycol monomethyl ether acetate, and 7.6 parts by mass of Karenz MOI (manufactured by Showa Denko KK). The mixture was added at 25 ° C. with stirring, the temperature was raised to 40 ° C., and the mixture was stirred at 40 ° C. for 2 hours.
Next, 45 parts by mass of light acrylate 130A (methoxypolyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd., bromine number 34.9Br / 100 g), light acrylate NP-4EA (nonylphenyl polyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd.) 17 0.7 parts by mass was added, the temperature was raised to 80 ° C., and the reaction was performed for 4 hours. Next, the mixture was cooled to 60 ° C., 60 parts by weight of castor oil (diol HS-2G-150R, manufactured by Toyokuni Seiyaku), 163 parts by weight of Kuraray polyester diol P-2050 (number average molecular weight 2,066, manufactured by Kuraray Co., Ltd.), 29.1 parts by weight of methylol butanoic acid, 49.5 parts by weight of 1,6-hexamethylene diisocyanate, 76.4 parts by weight of isophorone diisocyanate, and 83 parts by weight of propylene glycol monomethyl ether acetate were added and reacted at 60 ° C. for 5 hours. It was.
Subsequently, 23.4 parts by mass of light acrylate G-201P (produced by Kyoeisha Chemical Co., Ltd.) having a hydroxyl group and a (meth) acryloyl group in the molecule, 9.1 parts by mass of N-dimethylaminoethanol, 0.1 parts by mass of dibutyltin laurate Then, 0.3 part by mass of methoquinone and 167 parts by mass of propylene glycol monomethyl ether acetate were added and reacted at 70 ° C. for 5 hours to obtain silica dispersant P-8.
-シリカ分散剤P-8の合成-
特開2010-7059号公報の実施例2を再現したシリカ分散剤を合成した。
撹拌、乾燥窒素、吹き込み管、及び冷却管付きのフラスコに、N-ジエタノールアミン19.6質量部、プロピレングリコールモノメチルエーテルアセテート250質量部、及びカレンズMOI(昭和電工株式会社製)7.6質量部を、撹拌しながら25℃で加えて、40℃まで昇温し、40℃で2時間撹拌した。
次いで、ライトアクリレート130A(メトキシポリオキシエチレンエーテルアクリレート、共栄社化学社製、臭素価34.9Br/100g)45質量部、ライトアクリレートNP-4EA(ノニルフェニルポリオキシエチレンエーテルアクリレート、共栄社化学社製)17.7質量部を加え、80℃に昇温して、4時間反応させた。次いで、60℃まで冷却し、ひまし油(ジオールHS-2G-150R、豊国製油社製)60質量部、クラレポリエステルジオールP-2050(数平均分子量2,066、株式会社クラレ製)163質量部、ジメチロールブタン酸29.1質量部、1,6-ヘキサメチレンジイソシアネート49.5質量部、イソホロンジイソシアネート76.4質量部、及びプロピレングリコールモノメチルエーテルアセテート83質量部を加えて、60℃で5時間反応させた。
次いで、分子中に水酸基と(メタ)アクリロイル基を有するライトアクリレートG-201P(共栄社化学社製)23.4質量部、N-ジメチルアミノエタノール9.1質量部、ジブチル錫ラウリレート0.1質量部、メトキノン0.3質量部、及びプロピレングリコールモノメチルエーテルアセテート167質量部を加え、70℃で5時間反応させて、シリカ分散剤P-8を得た。 (Synthesis Example 10)
-Synthesis of Silica Dispersant P-8-
A silica dispersant that reproduces Example 2 of JP 2010-7059 A was synthesized.
In a flask equipped with stirring, dry nitrogen, blowing tube, and cooling tube, 19.6 parts by mass of N-diethanolamine, 250 parts by mass of propylene glycol monomethyl ether acetate, and 7.6 parts by mass of Karenz MOI (manufactured by Showa Denko KK). The mixture was added at 25 ° C. with stirring, the temperature was raised to 40 ° C., and the mixture was stirred at 40 ° C. for 2 hours.
Next, 45 parts by mass of light acrylate 130A (methoxypolyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd., bromine number 34.9Br / 100 g), light acrylate NP-4EA (nonylphenyl polyoxyethylene ether acrylate, manufactured by Kyoeisha Chemical Co., Ltd.) 17 0.7 parts by mass was added, the temperature was raised to 80 ° C., and the reaction was performed for 4 hours. Next, the mixture was cooled to 60 ° C., 60 parts by weight of castor oil (diol HS-2G-150R, manufactured by Toyokuni Seiyaku), 163 parts by weight of Kuraray polyester diol P-2050 (number average molecular weight 2,066, manufactured by Kuraray Co., Ltd.), 29.1 parts by weight of methylol butanoic acid, 49.5 parts by weight of 1,6-hexamethylene diisocyanate, 76.4 parts by weight of isophorone diisocyanate, and 83 parts by weight of propylene glycol monomethyl ether acetate were added and reacted at 60 ° C. for 5 hours. It was.
Subsequently, 23.4 parts by mass of light acrylate G-201P (produced by Kyoeisha Chemical Co., Ltd.) having a hydroxyl group and a (meth) acryloyl group in the molecule, 9.1 parts by mass of N-dimethylaminoethanol, 0.1 parts by mass of dibutyltin laurate Then, 0.3 part by mass of methoquinone and 167 parts by mass of propylene glycol monomethyl ether acetate were added and reacted at 70 ° C. for 5 hours to obtain silica dispersant P-8.
(合成例11)
-シリカ分散剤P-9の合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール4.84g、ネオペンチルグリコール2.9g、2,2-ビス(ヒドロキシメチル)酪酸4.68g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-9溶液(固形分20質量%)を得た。
(Synthesis Example 11)
-Synthesis of Silica Dispersant P-9-
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 4.84 g of 3- (dimethylamino) -1,2-propanediol, 2.9 g of neopentyl glycol, 2,2-bis (hydroxy) 4.68 g of methyl) butyric acid, 25.43 g of the intermediate polyester resin, and 0.19 g of 2,6-di-t-butylhydroxytoluene were dissolved in 73 mL of cyclohexanone. To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-9 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
-シリカ分散剤P-9の合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール4.84g、ネオペンチルグリコール2.9g、2,2-ビス(ヒドロキシメチル)酪酸4.68g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-9溶液(固形分20質量%)を得た。
-Synthesis of Silica Dispersant P-9-
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 4.84 g of 3- (dimethylamino) -1,2-propanediol, 2.9 g of neopentyl glycol, 2,2-bis (hydroxy) 4.68 g of methyl) butyric acid, 25.43 g of the intermediate polyester resin, and 0.19 g of 2,6-di-t-butylhydroxytoluene were dissolved in 73 mL of cyclohexanone. To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-9 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
(合成例12)
-シリカ分散剤P-10の合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール5.62g、ネオペンチルグリコール4.71g、2,2-ビス(ヒドロキシメチル)酪酸2.31g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-10溶液(固形分20質量%)を得た。
(Synthesis Example 12)
-Synthesis of Silica Dispersant P-10-
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 5.52 g of 3- (dimethylamino) -1,2-propanediol, 4.71 g of neopentyl glycol, 2,2-bis (hydroxy) Methyl) butyric acid (2.31 g), the intermediate polyester resin (25.43 g), and 2,6-di-t-butylhydroxytoluene (0.19 g) were dissolved in cyclohexanone (73 mL). To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-10 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
-シリカ分散剤P-10の合成-
コンデンサー、及び撹拌機を備えた500mLの3つ口丸底フラスコ内に、3-(ジメチルアミノ)-1,2-プロパンジオール5.62g、ネオペンチルグリコール4.71g、2,2-ビス(ヒドロキシメチル)酪酸2.31g、前記中間体ポリエステル樹脂25.43g、及び2,6-ジ-t-ブチルヒドロキシトルエン0.19gをシクロヘキサノン73mLに溶解した。これに、シクロヘキサノン73mLに溶解した4,4’-ジフェニルメタンジイソシアネート(MDI)25.03g(0.24モル)を添加し、75℃にて、5時間加熱撹拌した。その後、シクロヘキサノン100mLで希釈して、下記式に示す成分の質量比のシリカ分散剤P-10溶液(固形分20質量%)を得た。
-Synthesis of Silica Dispersant P-10-
In a 500 mL three-necked round bottom flask equipped with a condenser and a stirrer, 5.52 g of 3- (dimethylamino) -1,2-propanediol, 4.71 g of neopentyl glycol, 2,2-bis (hydroxy) Methyl) butyric acid (2.31 g), the intermediate polyester resin (25.43 g), and 2,6-di-t-butylhydroxytoluene (0.19 g) were dissolved in cyclohexanone (73 mL). To this was added 25.03 g (0.24 mol) of 4,4′-diphenylmethane diisocyanate (MDI) dissolved in 73 mL of cyclohexanone, and the mixture was stirred with heating at 75 ° C. for 5 hours. Thereafter, the resultant was diluted with 100 mL of cyclohexanone to obtain a silica dispersant P-10 solution (solid content: 20% by mass) having a mass ratio of components represented by the following formula.
次に、得られたシリカ分散剤P-1~P-10について、以下のようにして、アミン価、及び酸価を測定した。末端封止ポリエステルグラフト鎖の有無と共に、結果を表1に示す。
Next, the amine value and the acid value of the obtained silica dispersants P-1 to P-10 were measured as follows. The results are shown in Table 1, along with the presence or absence of end-capped polyester graft chains.
<アミン価の測定方法>
各シリカ分散剤0.7gを100mLビーカーに秤とり、酢酸60mLを加え、撹拌して溶解させた。測定温度を25℃に調整後、滴定試薬として0.1N過塩素酸酢酸を用いて、滴定装置で滴定し、アミン価を測定した。 <Method of measuring amine value>
0.7 g of each silica dispersant was weighed into a 100 mL beaker, and 60 mL of acetic acid was added and dissolved by stirring. After adjusting measurement temperature to 25 degreeC, it titrated with the titration apparatus using 0.1N perchloric acid acetic acid as a titration reagent, and measured the amine titer.
各シリカ分散剤0.7gを100mLビーカーに秤とり、酢酸60mLを加え、撹拌して溶解させた。測定温度を25℃に調整後、滴定試薬として0.1N過塩素酸酢酸を用いて、滴定装置で滴定し、アミン価を測定した。 <Method of measuring amine value>
0.7 g of each silica dispersant was weighed into a 100 mL beaker, and 60 mL of acetic acid was added and dissolved by stirring. After adjusting measurement temperature to 25 degreeC, it titrated with the titration apparatus using 0.1N perchloric acid acetic acid as a titration reagent, and measured the amine titer.
<酸価の測定>
各シリカ分散剤0.7gを100mLビーカーに秤とり、テトラヒドロフラン(THF)/水=5/1(体積比)の溶液60mLを加え、撹拌して溶解させた。測定温度を25℃に調整した後、滴定試薬として0.1NのNaOH水溶液を用いて、滴定装置で滴定し、酸価を測定した。 <Measurement of acid value>
0.7 g of each silica dispersant was weighed in a 100 mL beaker, 60 mL of a solution of tetrahydrofuran (THF) / water = 5/1 (volume ratio) was added, and dissolved by stirring. After adjusting measurement temperature to 25 degreeC, it titrated with the titration apparatus using 0.1N NaOH aqueous solution as a titration reagent, and the acid value was measured.
各シリカ分散剤0.7gを100mLビーカーに秤とり、テトラヒドロフラン(THF)/水=5/1(体積比)の溶液60mLを加え、撹拌して溶解させた。測定温度を25℃に調整した後、滴定試薬として0.1NのNaOH水溶液を用いて、滴定装置で滴定し、酸価を測定した。 <Measurement of acid value>
0.7 g of each silica dispersant was weighed in a 100 mL beaker, 60 mL of a solution of tetrahydrofuran (THF) / water = 5/1 (volume ratio) was added, and dissolved by stirring. After adjusting measurement temperature to 25 degreeC, it titrated with the titration apparatus using 0.1N NaOH aqueous solution as a titration reagent, and the acid value was measured.
*P-6のシリカ分散剤は、酸性基を有さないポリウレタン樹脂からなる。
* The silica dispersant of P-6 is made of a polyurethane resin having no acidic group.
(実施例1)
-シリカ分散組成物溶液の組成-
・合成例1のポリウレタンバインダー溶液(固形分45質量%)・・・25.3質量部
・着色顔料:HELIOGEN BLUE D7086(BASF社製)・・・0.02質量部
・着色顔料:Pariotol Yellow D0960(BASF社製)・・・0.005質量部
・分散剤:シリカ分散剤P-1溶液(固形分20質量%)・・・1.51質量部
・重合性化合物:DCP-A(共栄社化学株式会社製)・・・4.67質量部
・開始剤:イルガキュア907(BASF社製)・・・0.51質量部
・増感剤:DETX-S(日本化薬株式会社製)・・・0.005質量部
・反応助剤:EAB-F(保土ヶ谷化学化株式会社製)・・・0.017質量部
・硬化剤:メラミン(和光純薬工業株式会社製)・・・0.14質量部
・熱架橋剤:エポトートYDF-170(新日鐵化学株式会社製)・・・2.56質量部
・シリカ微粒子:SO-C2(アドマテックス社製)・・・21.5質量部
・イオントラップ剤:IXE-6107(東亞合成株式会社製)・・・0.7質量部
・塗布助剤:メガファックF-780F(DIC株式会社製、30質量%メチルエチルケトン溶液)・・・0.23質量部
・エラストマー:エスぺル1612(日立化成工業株式会社製)・・・1.09質量部
・シクロヘキサノン(溶媒)・・・41.7質量部 Example 1
-Composition of silica dispersion composition solution-
-Polyurethane binder solution of Synthesis Example 1 (solid content 45% by mass) ... 25.3 parts by mass-Color pigment: HELIOGEN BLUE D7086 (manufactured by BASF) ... 0.02 parts by mass-Color pigment: Pariotol Yellow D0960 (Manufactured by BASF) 0.005 parts by mass Dispersant: Silica dispersant P-1 solution (solid content 20% by mass) 1.51 parts by mass Polymerizable compound: DCP-A (Kyoeisha Chemical) 4.67 parts by mass ・ Initiator: Irgacure 907 (manufactured by BASF) ... 0.51 parts by mass ・ Sensitizer: DETX-S (manufactured by Nippon Kayaku Co., Ltd.) 0.005 parts by mass-Reaction aid: EAB-F (Hodogaya Chemical Co., Ltd.) ... 0.017 parts by mass-Curing agent: Melamine (Wako Pure Chemical Industries, Ltd.) ... 0.14 parts by mass Department Thermal crosslinking agent: Epototo YDF-170 (manufactured by Nippon Steel Chemical Co., Ltd.) 2.56 parts by mass Silica fine particles: SO-C2 (manufactured by Admatechs) 21.5 parts by mass Ion trap agent : IXE-6107 (manufactured by Toagosei Co., Ltd.) 0.7 parts by mass Application aid: MegaFac F-780F (manufactured by DIC, 30 mass% methyl ethyl ketone solution) 0.23 parts by mass Elastomer: Esper 1612 (manufactured by Hitachi Chemical Co., Ltd.) ... 1.09 parts by mass Cyclohexanone (solvent) ... 41.7 parts by mass
-シリカ分散組成物溶液の組成-
・合成例1のポリウレタンバインダー溶液(固形分45質量%)・・・25.3質量部
・着色顔料:HELIOGEN BLUE D7086(BASF社製)・・・0.02質量部
・着色顔料:Pariotol Yellow D0960(BASF社製)・・・0.005質量部
・分散剤:シリカ分散剤P-1溶液(固形分20質量%)・・・1.51質量部
・重合性化合物:DCP-A(共栄社化学株式会社製)・・・4.67質量部
・開始剤:イルガキュア907(BASF社製)・・・0.51質量部
・増感剤:DETX-S(日本化薬株式会社製)・・・0.005質量部
・反応助剤:EAB-F(保土ヶ谷化学化株式会社製)・・・0.017質量部
・硬化剤:メラミン(和光純薬工業株式会社製)・・・0.14質量部
・熱架橋剤:エポトートYDF-170(新日鐵化学株式会社製)・・・2.56質量部
・シリカ微粒子:SO-C2(アドマテックス社製)・・・21.5質量部
・イオントラップ剤:IXE-6107(東亞合成株式会社製)・・・0.7質量部
・塗布助剤:メガファックF-780F(DIC株式会社製、30質量%メチルエチルケトン溶液)・・・0.23質量部
・エラストマー:エスぺル1612(日立化成工業株式会社製)・・・1.09質量部
・シクロヘキサノン(溶媒)・・・41.7質量部 Example 1
-Composition of silica dispersion composition solution-
-Polyurethane binder solution of Synthesis Example 1 (solid content 45% by mass) ... 25.3 parts by mass-Color pigment: HELIOGEN BLUE D7086 (manufactured by BASF) ... 0.02 parts by mass-Color pigment: Pariotol Yellow D0960 (Manufactured by BASF) 0.005 parts by mass Dispersant: Silica dispersant P-1 solution (solid content 20% by mass) 1.51 parts by mass Polymerizable compound: DCP-A (Kyoeisha Chemical) 4.67 parts by mass ・ Initiator: Irgacure 907 (manufactured by BASF) ... 0.51 parts by mass ・ Sensitizer: DETX-S (manufactured by Nippon Kayaku Co., Ltd.) 0.005 parts by mass-Reaction aid: EAB-F (Hodogaya Chemical Co., Ltd.) ... 0.017 parts by mass-Curing agent: Melamine (Wako Pure Chemical Industries, Ltd.) ... 0.14 parts by mass Department Thermal crosslinking agent: Epototo YDF-170 (manufactured by Nippon Steel Chemical Co., Ltd.) 2.56 parts by mass Silica fine particles: SO-C2 (manufactured by Admatechs) 21.5 parts by mass Ion trap agent : IXE-6107 (manufactured by Toagosei Co., Ltd.) 0.7 parts by mass Application aid: MegaFac F-780F (manufactured by DIC, 30 mass% methyl ethyl ketone solution) 0.23 parts by mass Elastomer: Esper 1612 (manufactured by Hitachi Chemical Co., Ltd.) ... 1.09 parts by mass Cyclohexanone (solvent) ... 41.7 parts by mass
-感光性フィルムの製造-
支持体としての厚み16μmのポリエチレンテレフタレートフィルム(東レ株式会社製、16FB50)上に、上記の組成からなるシリカ分散組成物溶液を塗布し、乾燥させて、前記支持体上に厚み30μmの感光層を形成した。前記感光層上に、保護層として厚み20μmのポリプロピレンフィルム(王子特殊紙株式会社製、アルファンE-200)を積層し、感光性フィルムを製造した。 -Production of photosensitive film-
A silica dispersion composition solution having the above composition is applied onto a polyethylene terephthalate film (16FB50, manufactured by Toray Industries, Inc.) having a thickness of 16 μm as a support, and dried to form a photosensitive layer having a thickness of 30 μm on the support. Formed. On the photosensitive layer, a 20 μm-thick polypropylene film (manufactured by Oji Specialty Paper Co., Ltd., Alphan E-200) was laminated as a protective layer to produce a photosensitive film.
支持体としての厚み16μmのポリエチレンテレフタレートフィルム(東レ株式会社製、16FB50)上に、上記の組成からなるシリカ分散組成物溶液を塗布し、乾燥させて、前記支持体上に厚み30μmの感光層を形成した。前記感光層上に、保護層として厚み20μmのポリプロピレンフィルム(王子特殊紙株式会社製、アルファンE-200)を積層し、感光性フィルムを製造した。 -Production of photosensitive film-
A silica dispersion composition solution having the above composition is applied onto a polyethylene terephthalate film (16FB50, manufactured by Toray Industries, Inc.) having a thickness of 16 μm as a support, and dried to form a photosensitive layer having a thickness of 30 μm on the support. Formed. On the photosensitive layer, a 20 μm-thick polypropylene film (manufactured by Oji Specialty Paper Co., Ltd., Alphan E-200) was laminated as a protective layer to produce a photosensitive film.
-基体への積層-
前記基体として、銅張積層板(スルーホールなし、銅厚み12μm)の表面に化学研磨処理を施して調製した。該銅張積層板上に、前記感光性フィルムの感光層が前記銅張積層板に接するようにして前記感光性フィルムにおける保護フィルムを剥がしながら、真空ラミネータ(ニチゴーモートン株式会社製、VP130)を用いて積層させ、前記銅張積層板と、前記感光層と、前記ポリエチレンテレフタレートフィルム(支持体)とがこの順に積層された感光性積層体を調製した。
圧着条件は、真空引きの時間40秒、圧着温度70℃、圧着圧力0.2MPa、加圧時間10秒とした。 -Lamination on substrate-
The substrate was prepared by subjecting a surface of a copper clad laminate (no through hole, copper thickness: 12 μm) to chemical polishing. A vacuum laminator (manufactured by Nichigo Morton Co., Ltd., VP130) was used on the copper-clad laminate while peeling off the protective film from the photosensitive film so that the photosensitive layer of the photosensitive film was in contact with the copper-clad laminate. Thus, a photosensitive laminate was prepared in which the copper-clad laminate, the photosensitive layer, and the polyethylene terephthalate film (support) were laminated in this order.
The pressure bonding conditions were a vacuum drawing time of 40 seconds, a pressure bonding temperature of 70 ° C., a pressure bonding pressure of 0.2 MPa, and a pressure application time of 10 seconds.
前記基体として、銅張積層板(スルーホールなし、銅厚み12μm)の表面に化学研磨処理を施して調製した。該銅張積層板上に、前記感光性フィルムの感光層が前記銅張積層板に接するようにして前記感光性フィルムにおける保護フィルムを剥がしながら、真空ラミネータ(ニチゴーモートン株式会社製、VP130)を用いて積層させ、前記銅張積層板と、前記感光層と、前記ポリエチレンテレフタレートフィルム(支持体)とがこの順に積層された感光性積層体を調製した。
圧着条件は、真空引きの時間40秒、圧着温度70℃、圧着圧力0.2MPa、加圧時間10秒とした。 -Lamination on substrate-
The substrate was prepared by subjecting a surface of a copper clad laminate (no through hole, copper thickness: 12 μm) to chemical polishing. A vacuum laminator (manufactured by Nichigo Morton Co., Ltd., VP130) was used on the copper-clad laminate while peeling off the protective film from the photosensitive film so that the photosensitive layer of the photosensitive film was in contact with the copper-clad laminate. Thus, a photosensitive laminate was prepared in which the copper-clad laminate, the photosensitive layer, and the polyethylene terephthalate film (support) were laminated in this order.
The pressure bonding conditions were a vacuum drawing time of 40 seconds, a pressure bonding temperature of 70 ° C., a pressure bonding pressure of 0.2 MPa, and a pressure application time of 10 seconds.
得られた各感光性フィルム及び感光性積層体について、以下のようにして、溶融粘度、埋め込み性、現像性(未露光部)、解像性(露光下部)、絶縁性、及び耐熱衝撃性(TCT)の評価を行った。結果を表2に示す。
About each obtained photosensitive film and photosensitive laminated body, melt viscosity, embedding property, developability (unexposed part), resolution (lower part of exposure), insulation, and thermal shock resistance ( TCT) was evaluated. The results are shown in Table 2.
<溶融粘度の測定>
各感光性フィルムについて、レオメーター・VAR-1000型(レオロジカル株式会社製)を用いて、下記条件により溶融粘度の測定を行った。
-溶融粘度の測定条件-
直径20mmのプレートを用い歪0.005、周波数1Hzで溶融粘弾性を測定した。温度範囲を25℃~85℃とし、5℃/分の昇温速度で測定を行った。なお、表2中の溶融粘度は、70℃での値を示す。 <Measurement of melt viscosity>
About each photosensitive film, melt viscosity was measured on condition of the following using rheometer * VAR-1000 type (made by Rheological Co., Ltd.).
-Measurement conditions of melt viscosity-
Melt viscoelasticity was measured using a plate having a diameter of 20 mm at a strain of 0.005 and a frequency of 1 Hz. The temperature range was 25 ° C. to 85 ° C., and the measurement was performed at a rate of temperature increase of 5 ° C./min. In addition, the melt viscosity in Table 2 shows a value at 70 ° C.
各感光性フィルムについて、レオメーター・VAR-1000型(レオロジカル株式会社製)を用いて、下記条件により溶融粘度の測定を行った。
-溶融粘度の測定条件-
直径20mmのプレートを用い歪0.005、周波数1Hzで溶融粘弾性を測定した。温度範囲を25℃~85℃とし、5℃/分の昇温速度で測定を行った。なお、表2中の溶融粘度は、70℃での値を示す。 <Measurement of melt viscosity>
About each photosensitive film, melt viscosity was measured on condition of the following using rheometer * VAR-1000 type (made by Rheological Co., Ltd.).
-Measurement conditions of melt viscosity-
Melt viscoelasticity was measured using a plate having a diameter of 20 mm at a strain of 0.005 and a frequency of 1 Hz. The temperature range was 25 ° C. to 85 ° C., and the measurement was performed at a rate of temperature increase of 5 ° C./min. In addition, the melt viscosity in Table 2 shows a value at 70 ° C.
<埋め込み性の評価>
L/S(ライン/スペース)=50μm/50μmの配線パターン間への感光層の埋め込み状態を、光学顕微鏡を用いて50倍~200倍の倍率で観察し、下記基準に基づいて評価した。
〔評価基準〕
○:感光性フィルムが、前記パターン回路とベースフイルムとの段差を埋め込み、前記感光性フィルムと前記回路付き銅張り積層板との間に隙間ができていない場合
△:前記感光性フィルムと上記回路付き銅張り積層板との間に隙間が生じている場合、又はパターン回路と感光性積層体との間に空気の泡等が生じている場合
×:溶融粘度が高すぎてラミネートできない場合 <Evaluation of embeddability>
The embedded state of the photosensitive layer between the wiring patterns of L / S (line / space) = 50 μm / 50 μm was observed at a magnification of 50 to 200 times using an optical microscope and evaluated based on the following criteria.
〔Evaluation criteria〕
◯: When the photosensitive film embeds a step between the pattern circuit and the base film, and no gap is formed between the photosensitive film and the copper-clad laminate with circuit. Δ: The photosensitive film and the circuit. When there is a gap between the coated copper-clad laminate or when air bubbles are generated between the pattern circuit and the photosensitive laminate ×: When the melt viscosity is too high to laminate
L/S(ライン/スペース)=50μm/50μmの配線パターン間への感光層の埋め込み状態を、光学顕微鏡を用いて50倍~200倍の倍率で観察し、下記基準に基づいて評価した。
〔評価基準〕
○:感光性フィルムが、前記パターン回路とベースフイルムとの段差を埋め込み、前記感光性フィルムと前記回路付き銅張り積層板との間に隙間ができていない場合
△:前記感光性フィルムと上記回路付き銅張り積層板との間に隙間が生じている場合、又はパターン回路と感光性積層体との間に空気の泡等が生じている場合
×:溶融粘度が高すぎてラミネートできない場合 <Evaluation of embeddability>
The embedded state of the photosensitive layer between the wiring patterns of L / S (line / space) = 50 μm / 50 μm was observed at a magnification of 50 to 200 times using an optical microscope and evaluated based on the following criteria.
〔Evaluation criteria〕
◯: When the photosensitive film embeds a step between the pattern circuit and the base film, and no gap is formed between the photosensitive film and the copper-clad laminate with circuit. Δ: The photosensitive film and the circuit. When there is a gap between the coated copper-clad laminate or when air bubbles are generated between the pattern circuit and the photosensitive laminate ×: When the melt viscosity is too high to laminate
<現像性及び解像性の評価>
-現像性(現像時間、残渣)-
作製した各感光性積層体を室温(23℃)で55%RHにて10分間静置した。得られた感光性積層体のポリエチレンテレフタレートフィルム(支持体)上から、回路基板用露光機(EXM-1172、オーク製作所製)を用いて、直径50μm~200μmの丸穴パターンを有するフォトマスク越しに40mJ/cm2で露光を行った。
この際の露光量は、前記感度の評価における前記感光性積層体の感光層を硬化させるために必要な光エネルギー量である。
室温にて10分間静置した後、前記感光性積層体からポリエチレンテレフタレートフィルム(支持体)を剥がし取った。前記銅張積層板上の感光層の全面に、現像液として30℃の1質量%炭酸ナトリウム水溶液をスプレー圧0.15MPaにてスプレーし、炭酸ナトリウム水溶液のスプレー開始から銅張積層板上の感光層が溶解除去されるまでに要した時間を測定し、これを最短現像時間とした。
次に、銅張積層板上の感光層の全面に、現像液として30℃の1質量%炭酸ナトリウム水溶液をスプレー圧0.15MPaにて前記最短現像時間の2倍の時間スプレーし、未硬化領域を溶解除去した。以上により、現像性(現像時間、残渣)を下記基準で評価した。
-解像性-
得られた硬化樹脂パターン付き銅張積層板の表面を光学顕微鏡で観察し、パターンの丸穴底部に残渣が無いこと、パターン部の捲くれ・剥がれなどの異常が無いこと、かつスペース形成可能な最小の丸穴パターン直径を測定し、下記基準で解像性を評価した。
〔現像性の評価基準〕
・現像時間については、前記最短現像時間で評価した。
・残渣については、目視により以下の基準で評価した。
○:残渣なし
△:若干壁及び底面に残渣が見られる
×:残渣が明確に見られる
〔解像性の評価基準〕
○:直径90μm以下の丸穴が解像可能で、解像性に優れている
△:直径200μm以下の丸穴が解像可能で、解像性がやや劣る
×:丸穴が解像不可で、解像性が劣る <Evaluation of developability and resolution>
-Developability (development time, residue)-
Each of the produced photosensitive laminates was allowed to stand at 55% RH for 10 minutes at room temperature (23 ° C.). From the polyethylene terephthalate film (support) of the obtained photosensitive laminate, using a circuit board exposure machine (EXM-1172, manufactured by Oak Manufacturing Co., Ltd.), through a photomask having a round hole pattern with a diameter of 50 μm to 200 μm. Exposure was performed at 40 mJ / cm 2 .
The exposure amount at this time is the amount of light energy necessary for curing the photosensitive layer of the photosensitive laminate in the sensitivity evaluation.
After standing at room temperature for 10 minutes, the polyethylene terephthalate film (support) was peeled off from the photosensitive laminate. A 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper clad laminate at a spray pressure of 0.15 MPa. The time required until the layer was dissolved and removed was measured, and this was taken as the shortest development time.
Next, a 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper-clad laminate at a spray pressure of 0.15 MPa for a time twice as short as the shortest developing time, thereby uncured regions. Was dissolved and removed. As described above, developability (development time, residue) was evaluated according to the following criteria.
-Resolution-
Observe the surface of the obtained copper-clad laminate with a cured resin pattern with an optical microscope, there is no residue at the bottom of the round hole of the pattern, there is no abnormality such as blistering / peeling of the pattern part, and space can be formed The smallest round hole pattern diameter was measured, and the resolution was evaluated according to the following criteria.
[Development evaluation criteria]
-About development time, it evaluated by the said shortest development time.
-Residues were visually evaluated according to the following criteria.
○: No residue △: Residue is slightly seen on the wall and bottom ×: Residue is clearly seen [Evaluation criteria for resolution]
○: A round hole with a diameter of 90 μm or less can be resolved, and the resolution is excellent. Δ: A round hole with a diameter of 200 μm or less can be resolved, and the resolution is slightly inferior. , Poor resolution
-現像性(現像時間、残渣)-
作製した各感光性積層体を室温(23℃)で55%RHにて10分間静置した。得られた感光性積層体のポリエチレンテレフタレートフィルム(支持体)上から、回路基板用露光機(EXM-1172、オーク製作所製)を用いて、直径50μm~200μmの丸穴パターンを有するフォトマスク越しに40mJ/cm2で露光を行った。
この際の露光量は、前記感度の評価における前記感光性積層体の感光層を硬化させるために必要な光エネルギー量である。
室温にて10分間静置した後、前記感光性積層体からポリエチレンテレフタレートフィルム(支持体)を剥がし取った。前記銅張積層板上の感光層の全面に、現像液として30℃の1質量%炭酸ナトリウム水溶液をスプレー圧0.15MPaにてスプレーし、炭酸ナトリウム水溶液のスプレー開始から銅張積層板上の感光層が溶解除去されるまでに要した時間を測定し、これを最短現像時間とした。
次に、銅張積層板上の感光層の全面に、現像液として30℃の1質量%炭酸ナトリウム水溶液をスプレー圧0.15MPaにて前記最短現像時間の2倍の時間スプレーし、未硬化領域を溶解除去した。以上により、現像性(現像時間、残渣)を下記基準で評価した。
-解像性-
得られた硬化樹脂パターン付き銅張積層板の表面を光学顕微鏡で観察し、パターンの丸穴底部に残渣が無いこと、パターン部の捲くれ・剥がれなどの異常が無いこと、かつスペース形成可能な最小の丸穴パターン直径を測定し、下記基準で解像性を評価した。
〔現像性の評価基準〕
・現像時間については、前記最短現像時間で評価した。
・残渣については、目視により以下の基準で評価した。
○:残渣なし
△:若干壁及び底面に残渣が見られる
×:残渣が明確に見られる
〔解像性の評価基準〕
○:直径90μm以下の丸穴が解像可能で、解像性に優れている
△:直径200μm以下の丸穴が解像可能で、解像性がやや劣る
×:丸穴が解像不可で、解像性が劣る <Evaluation of developability and resolution>
-Developability (development time, residue)-
Each of the produced photosensitive laminates was allowed to stand at 55% RH for 10 minutes at room temperature (23 ° C.). From the polyethylene terephthalate film (support) of the obtained photosensitive laminate, using a circuit board exposure machine (EXM-1172, manufactured by Oak Manufacturing Co., Ltd.), through a photomask having a round hole pattern with a diameter of 50 μm to 200 μm. Exposure was performed at 40 mJ / cm 2 .
The exposure amount at this time is the amount of light energy necessary for curing the photosensitive layer of the photosensitive laminate in the sensitivity evaluation.
After standing at room temperature for 10 minutes, the polyethylene terephthalate film (support) was peeled off from the photosensitive laminate. A 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper clad laminate at a spray pressure of 0.15 MPa. The time required until the layer was dissolved and removed was measured, and this was taken as the shortest development time.
Next, a 1 mass% sodium carbonate aqueous solution at 30 ° C. is sprayed as a developing solution over the entire surface of the photosensitive layer on the copper-clad laminate at a spray pressure of 0.15 MPa for a time twice as short as the shortest developing time, thereby uncured regions. Was dissolved and removed. As described above, developability (development time, residue) was evaluated according to the following criteria.
-Resolution-
Observe the surface of the obtained copper-clad laminate with a cured resin pattern with an optical microscope, there is no residue at the bottom of the round hole of the pattern, there is no abnormality such as blistering / peeling of the pattern part, and space can be formed The smallest round hole pattern diameter was measured, and the resolution was evaluated according to the following criteria.
[Development evaluation criteria]
-About development time, it evaluated by the said shortest development time.
-Residues were visually evaluated according to the following criteria.
○: No residue △: Residue is slightly seen on the wall and bottom ×: Residue is clearly seen [Evaluation criteria for resolution]
○: A round hole with a diameter of 90 μm or less can be resolved, and the resolution is excellent. Δ: A round hole with a diameter of 200 μm or less can be resolved, and the resolution is slightly inferior. , Poor resolution
<絶縁性(HAST)>
12μm厚の銅箔をガラスエポキシ基材に積層したプリント基板の銅箔にエッチングを施して、ライン幅/スペース幅が50μm/50μmであり、互いのラインが接触しておらず、互いに対向した同一面上の櫛形電極を得た。この基板の櫛形電極上にソルダーレジスト層を定法にて形成し、最適露光量(40mJ/cm2)で露光を行った。次いで、常温で1時間静置した後、30℃の1質量%炭酸ナトリウム水溶液にて20秒間スプレー現像を行った。続いて、オーク製作所製紫外線照射装置を使用して1J/cm2のエネルギー量で感光層に対する紫外線照射を行った。更に感光層を150℃で60分間加熱処理を行うことにより、ソルダーレジストを形成した評価用基板を得た。
加熱後の評価用積層体の櫛形電極間に電圧が印加されるように、ポリテトラフルオロエチレン製のシールド線をSn/Pbはんだによりそれらの櫛形電極に接続した後、評価用積層体に50Vの電圧を印加した状態で、該評価用積層体を130℃で85%RHの超加速高温高湿寿命試験(HAST)槽内に200時間静置した。その後の評価用積層体のソルダーレジストのマイグレーションの発生程度を100倍の金属顕微鏡により観察した。
〔評価基準〕
○:マイグレーションの発生が確認できず、絶縁性に優れる。
△:マイグレーションの発生が確認され、絶縁性にやや劣る。
×:電極間が短絡し、絶縁性に劣る。 <Insulation (HAST)>
Etching was performed on the copper foil of a printed circuit board in which a 12 μm thick copper foil was laminated on a glass epoxy base material, the line width / space width was 50 μm / 50 μm, the lines were not in contact with each other, and the same facing each other A comb electrode on the surface was obtained. A solder resist layer was formed on the comb-shaped electrode of this substrate by a conventional method, and exposure was performed with an optimum exposure amount (40 mJ / cm 2 ). Subsequently, after leaving still at room temperature for 1 hour, spray development was performed for 20 second in 1 mass% sodium carbonate aqueous solution of 30 degreeC. Subsequently, the photosensitive layer was irradiated with ultraviolet rays with an energy amount of 1 J / cm 2 using an ultraviolet irradiation device manufactured by Oak Manufacturing. Further, the photosensitive layer was heat-treated at 150 ° C. for 60 minutes to obtain an evaluation substrate on which a solder resist was formed.
After connecting a shield wire made of polytetrafluoroethylene to these comb electrodes by Sn / Pb solder so that a voltage is applied between the comb electrodes of the evaluation laminate after heating, 50 V is applied to the evaluation laminate. With the voltage applied, the laminate for evaluation was allowed to stand in a super accelerated high-temperature high-humidity life test (HAST) bath at 130 ° C. and 85% RH for 200 hours. Thereafter, the degree of migration of the solder resist in the laminate for evaluation was observed with a 100-fold metal microscope.
〔Evaluation criteria〕
○: The occurrence of migration cannot be confirmed, and the insulation is excellent.
(Triangle | delta): Generation | occurrence | production of migration is confirmed and it is somewhat inferior to insulation.
X: The electrodes are short-circuited and insulative.
12μm厚の銅箔をガラスエポキシ基材に積層したプリント基板の銅箔にエッチングを施して、ライン幅/スペース幅が50μm/50μmであり、互いのラインが接触しておらず、互いに対向した同一面上の櫛形電極を得た。この基板の櫛形電極上にソルダーレジスト層を定法にて形成し、最適露光量(40mJ/cm2)で露光を行った。次いで、常温で1時間静置した後、30℃の1質量%炭酸ナトリウム水溶液にて20秒間スプレー現像を行った。続いて、オーク製作所製紫外線照射装置を使用して1J/cm2のエネルギー量で感光層に対する紫外線照射を行った。更に感光層を150℃で60分間加熱処理を行うことにより、ソルダーレジストを形成した評価用基板を得た。
加熱後の評価用積層体の櫛形電極間に電圧が印加されるように、ポリテトラフルオロエチレン製のシールド線をSn/Pbはんだによりそれらの櫛形電極に接続した後、評価用積層体に50Vの電圧を印加した状態で、該評価用積層体を130℃で85%RHの超加速高温高湿寿命試験(HAST)槽内に200時間静置した。その後の評価用積層体のソルダーレジストのマイグレーションの発生程度を100倍の金属顕微鏡により観察した。
〔評価基準〕
○:マイグレーションの発生が確認できず、絶縁性に優れる。
△:マイグレーションの発生が確認され、絶縁性にやや劣る。
×:電極間が短絡し、絶縁性に劣る。 <Insulation (HAST)>
Etching was performed on the copper foil of a printed circuit board in which a 12 μm thick copper foil was laminated on a glass epoxy base material, the line width / space width was 50 μm / 50 μm, the lines were not in contact with each other, and the same facing each other A comb electrode on the surface was obtained. A solder resist layer was formed on the comb-shaped electrode of this substrate by a conventional method, and exposure was performed with an optimum exposure amount (40 mJ / cm 2 ). Subsequently, after leaving still at room temperature for 1 hour, spray development was performed for 20 second in 1 mass% sodium carbonate aqueous solution of 30 degreeC. Subsequently, the photosensitive layer was irradiated with ultraviolet rays with an energy amount of 1 J / cm 2 using an ultraviolet irradiation device manufactured by Oak Manufacturing. Further, the photosensitive layer was heat-treated at 150 ° C. for 60 minutes to obtain an evaluation substrate on which a solder resist was formed.
After connecting a shield wire made of polytetrafluoroethylene to these comb electrodes by Sn / Pb solder so that a voltage is applied between the comb electrodes of the evaluation laminate after heating, 50 V is applied to the evaluation laminate. With the voltage applied, the laminate for evaluation was allowed to stand in a super accelerated high-temperature high-humidity life test (HAST) bath at 130 ° C. and 85% RH for 200 hours. Thereafter, the degree of migration of the solder resist in the laminate for evaluation was observed with a 100-fold metal microscope.
〔Evaluation criteria〕
○: The occurrence of migration cannot be confirmed, and the insulation is excellent.
(Triangle | delta): Generation | occurrence | production of migration is confirmed and it is somewhat inferior to insulation.
X: The electrodes are short-circuited and insulative.
<耐熱衝撃性(耐クラック性)(TCT)>
信頼性試験項目として、温度サイクル試験(TCT)によりクラック及び剥れ等の外観を評価した。TCTは気相冷熱試験機を用い、電子部品モジュールを温度が-55℃及び125℃の気相中に各30分間放置し、これを1サイクルとして1,000サイクル及び1,500サイクルの条件で行い、以下の基準で耐熱衝撃性を評価した。
〔評価基準〕
○:クラック発生無し
△:浅いクラック発生有り
×:深いクラック発生有り <Thermal shock resistance (crack resistance) (TCT)>
As reliability test items, appearance such as cracks and peeling was evaluated by a temperature cycle test (TCT). TCT uses a gas-phase cold heat tester, and the electronic component module is left in the gas phase at −55 ° C. and 125 ° C. for 30 minutes each, and this is regarded as one cycle under the conditions of 1,000 cycles and 1,500 cycles. The thermal shock resistance was evaluated according to the following criteria.
〔Evaluation criteria〕
○: No crack occurred △: Shallow crack occurred ×: Deep crack occurred
信頼性試験項目として、温度サイクル試験(TCT)によりクラック及び剥れ等の外観を評価した。TCTは気相冷熱試験機を用い、電子部品モジュールを温度が-55℃及び125℃の気相中に各30分間放置し、これを1サイクルとして1,000サイクル及び1,500サイクルの条件で行い、以下の基準で耐熱衝撃性を評価した。
〔評価基準〕
○:クラック発生無し
△:浅いクラック発生有り
×:深いクラック発生有り <Thermal shock resistance (crack resistance) (TCT)>
As reliability test items, appearance such as cracks and peeling was evaluated by a temperature cycle test (TCT). TCT uses a gas-phase cold heat tester, and the electronic component module is left in the gas phase at −55 ° C. and 125 ° C. for 30 minutes each, and this is regarded as one cycle under the conditions of 1,000 cycles and 1,500 cycles. The thermal shock resistance was evaluated according to the following criteria.
〔Evaluation criteria〕
○: No crack occurred △: Shallow crack occurred ×: Deep crack occurred
(実施例2~6及び比較例1~4)
実施例1において、表2に示すように、シリカ分散剤P-1を、シリカ分散剤P-2~P-10に代えた以外は、実施例1と同様にして、実施例2~6及び比較例1~4の感光性フィルム、感光性積層体、及び永久パターンを製造した。
得られた各感光性フィルム及び感光性積層体について、実施例1と同様にして、溶融粘度、埋め込み性、現像性(未露光部)、解像性(露光下部)、絶縁性、及び耐熱衝撃性(TCT)の評価を行った。結果を表2に示す。 (Examples 2 to 6 and Comparative Examples 1 to 4)
In Example 1, as shown in Table 2, Examples 2 to 6 and Example 1 were performed in the same manner as Example 1 except that the silica dispersant P-1 was replaced with silica dispersants P-2 to P-10. The photosensitive films, photosensitive laminates, and permanent patterns of Comparative Examples 1 to 4 were produced.
About each obtained photosensitive film and photosensitive laminated body, it carried out similarly to Example 1, and melt viscosity, embedding property, developability (unexposed part), resolution (lower part of exposure), insulation, and thermal shock Sex (TCT) was evaluated. The results are shown in Table 2.
実施例1において、表2に示すように、シリカ分散剤P-1を、シリカ分散剤P-2~P-10に代えた以外は、実施例1と同様にして、実施例2~6及び比較例1~4の感光性フィルム、感光性積層体、及び永久パターンを製造した。
得られた各感光性フィルム及び感光性積層体について、実施例1と同様にして、溶融粘度、埋め込み性、現像性(未露光部)、解像性(露光下部)、絶縁性、及び耐熱衝撃性(TCT)の評価を行った。結果を表2に示す。 (Examples 2 to 6 and Comparative Examples 1 to 4)
In Example 1, as shown in Table 2, Examples 2 to 6 and Example 1 were performed in the same manner as Example 1 except that the silica dispersant P-1 was replaced with silica dispersants P-2 to P-10. The photosensitive films, photosensitive laminates, and permanent patterns of Comparative Examples 1 to 4 were produced.
About each obtained photosensitive film and photosensitive laminated body, it carried out similarly to Example 1, and melt viscosity, embedding property, developability (unexposed part), resolution (lower part of exposure), insulation, and thermal shock Sex (TCT) was evaluated. The results are shown in Table 2.
本発明の態様としては、以下のとおりである。
<1> 酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、
前記シリカ分散剤のアミン価が0.65mmol/g以上であることを特徴とするシリカ分散組成物である。
<2> 塩基性基が第3級アミノ基である前記<1>に記載のシリカ分散組成物である。
<3> 酸性基がカルボキシル基である前記<1>から<2>のいずれかに記載のシリカ分散組成物である。
<4> ポリウレタン樹脂が、側鎖及び末端の少なくともいずれかにグラフト鎖を有する前記<1>から<3>のいずれかに記載のシリカ分散組成物である。
<5> ポリウレタン樹脂の末端にポリエステルグラフト鎖を有する前記<4>に記載のシリカ分散組成物である。
<6> シリカ分散剤の酸価が0.3mmol/g以上である前記<1>から<5>のいずれかに記載のシリカ分散組成物である。
<7> ポリウレタン樹脂が、更に、重合性基を有する前記<1>から<6>のいずれかに記載のシリカ分散組成物である。
<8> ソルダーレジスト用である前記<1>から<7>のいずれかに記載のシリカ分散組成物である。
<9> 更にバインダー、重合性化合物、及び光重合開始剤を含有する前記<1>から<8>のいずれかに記載のシリカ分散組成物である。
<10> バインダーが、酸変性ビニル基含有ポリウレタン樹脂である前記<9>に記載のシリカ分散組成物である。
<11> バインダーが、不飽和基含有ポリカルボン酸樹脂である前記<9>に記載のシリカ分散組成物である。
<12> バインダーが、酸変性ビニル基含有エポキシ樹脂である前記<9>に記載のシリカ分散組成物である。
<13> バインダーが、不飽和基及びカルボキシル基を含有する樹脂である前記<9>に記載のシリカ分散組成物である。
<14> バインダーが、ポリイミド前駆体である前記<9>に記載のシリカ分散組成物である。
<15> 前記<1>から<14>のいずれかに記載のシリカ分散組成物を含む感光層を支持体上に有してなることを特徴とする感光性フィルムである。
<16> 基体上に、前記<1>から<14>のいずれかに記載のシリカ分散組成物を含む感光層を有することを特徴とする感光性積層体である。
<17> 前記<1>から<14>のいずれかに記載のシリカ分散組成物により形成された感光層に対して露光を行うことを少なくとも含むことを特徴とする永久パターン形成方法である。
<18> 前記<17>に記載の永久パターン形成方法により永久パターンが形成されることを特徴とするプリント基板である。 The aspect of the present invention is as follows.
<1> comprising a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent;
The silica dispersion composition is characterized in that the amine value of the silica dispersant is 0.65 mmol / g or more.
<2> The silica dispersion composition according to <1>, wherein the basic group is a tertiary amino group.
<3> The silica dispersion composition according to any one of <1> to <2>, wherein the acidic group is a carboxyl group.
<4> The silica dispersion composition according to any one of <1> to <3>, wherein the polyurethane resin has a graft chain in at least one of a side chain and a terminal.
<5> The silica dispersion composition according to <4>, wherein the polyurethane resin has a polyester graft chain at a terminal thereof.
<6> The silica dispersion composition according to any one of <1> to <5>, wherein the acid value of the silica dispersant is 0.3 mmol / g or more.
<7> The silica dispersion composition according to any one of <1> to <6>, wherein the polyurethane resin further has a polymerizable group.
<8> The silica dispersion composition according to any one of <1> to <7>, which is for a solder resist.
<9> The silica dispersion composition according to any one of <1> to <8>, further including a binder, a polymerizable compound, and a photopolymerization initiator.
<10> The silica dispersion composition according to <9>, wherein the binder is an acid-modified vinyl group-containing polyurethane resin.
<11> The silica dispersion composition according to <9>, wherein the binder is an unsaturated group-containing polycarboxylic acid resin.
<12> The silica dispersion composition according to <9>, wherein the binder is an acid-modified vinyl group-containing epoxy resin.
<13> The silica dispersion composition according to <9>, wherein the binder is a resin containing an unsaturated group and a carboxyl group.
<14> The silica dispersion composition according to <9>, wherein the binder is a polyimide precursor.
<15> A photosensitive film comprising a photosensitive layer containing the silica dispersion composition according to any one of <1> to <14> on a support.
<16> A photosensitive laminate comprising a photosensitive layer containing the silica dispersion composition according to any one of <1> to <14> on a substrate.
<17> A method for forming a permanent pattern, comprising at least exposing a photosensitive layer formed of the silica dispersion composition according to any one of <1> to <14>.
<18> A printed circuit board wherein a permanent pattern is formed by the method for forming a permanent pattern according to <17>.
<1> 酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、
前記シリカ分散剤のアミン価が0.65mmol/g以上であることを特徴とするシリカ分散組成物である。
<2> 塩基性基が第3級アミノ基である前記<1>に記載のシリカ分散組成物である。
<3> 酸性基がカルボキシル基である前記<1>から<2>のいずれかに記載のシリカ分散組成物である。
<4> ポリウレタン樹脂が、側鎖及び末端の少なくともいずれかにグラフト鎖を有する前記<1>から<3>のいずれかに記載のシリカ分散組成物である。
<5> ポリウレタン樹脂の末端にポリエステルグラフト鎖を有する前記<4>に記載のシリカ分散組成物である。
<6> シリカ分散剤の酸価が0.3mmol/g以上である前記<1>から<5>のいずれかに記載のシリカ分散組成物である。
<7> ポリウレタン樹脂が、更に、重合性基を有する前記<1>から<6>のいずれかに記載のシリカ分散組成物である。
<8> ソルダーレジスト用である前記<1>から<7>のいずれかに記載のシリカ分散組成物である。
<9> 更にバインダー、重合性化合物、及び光重合開始剤を含有する前記<1>から<8>のいずれかに記載のシリカ分散組成物である。
<10> バインダーが、酸変性ビニル基含有ポリウレタン樹脂である前記<9>に記載のシリカ分散組成物である。
<11> バインダーが、不飽和基含有ポリカルボン酸樹脂である前記<9>に記載のシリカ分散組成物である。
<12> バインダーが、酸変性ビニル基含有エポキシ樹脂である前記<9>に記載のシリカ分散組成物である。
<13> バインダーが、不飽和基及びカルボキシル基を含有する樹脂である前記<9>に記載のシリカ分散組成物である。
<14> バインダーが、ポリイミド前駆体である前記<9>に記載のシリカ分散組成物である。
<15> 前記<1>から<14>のいずれかに記載のシリカ分散組成物を含む感光層を支持体上に有してなることを特徴とする感光性フィルムである。
<16> 基体上に、前記<1>から<14>のいずれかに記載のシリカ分散組成物を含む感光層を有することを特徴とする感光性積層体である。
<17> 前記<1>から<14>のいずれかに記載のシリカ分散組成物により形成された感光層に対して露光を行うことを少なくとも含むことを特徴とする永久パターン形成方法である。
<18> 前記<17>に記載の永久パターン形成方法により永久パターンが形成されることを特徴とするプリント基板である。 The aspect of the present invention is as follows.
<1> comprising a silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent;
The silica dispersion composition is characterized in that the amine value of the silica dispersant is 0.65 mmol / g or more.
<2> The silica dispersion composition according to <1>, wherein the basic group is a tertiary amino group.
<3> The silica dispersion composition according to any one of <1> to <2>, wherein the acidic group is a carboxyl group.
<4> The silica dispersion composition according to any one of <1> to <3>, wherein the polyurethane resin has a graft chain in at least one of a side chain and a terminal.
<5> The silica dispersion composition according to <4>, wherein the polyurethane resin has a polyester graft chain at a terminal thereof.
<6> The silica dispersion composition according to any one of <1> to <5>, wherein the acid value of the silica dispersant is 0.3 mmol / g or more.
<7> The silica dispersion composition according to any one of <1> to <6>, wherein the polyurethane resin further has a polymerizable group.
<8> The silica dispersion composition according to any one of <1> to <7>, which is for a solder resist.
<9> The silica dispersion composition according to any one of <1> to <8>, further including a binder, a polymerizable compound, and a photopolymerization initiator.
<10> The silica dispersion composition according to <9>, wherein the binder is an acid-modified vinyl group-containing polyurethane resin.
<11> The silica dispersion composition according to <9>, wherein the binder is an unsaturated group-containing polycarboxylic acid resin.
<12> The silica dispersion composition according to <9>, wherein the binder is an acid-modified vinyl group-containing epoxy resin.
<13> The silica dispersion composition according to <9>, wherein the binder is a resin containing an unsaturated group and a carboxyl group.
<14> The silica dispersion composition according to <9>, wherein the binder is a polyimide precursor.
<15> A photosensitive film comprising a photosensitive layer containing the silica dispersion composition according to any one of <1> to <14> on a support.
<16> A photosensitive laminate comprising a photosensitive layer containing the silica dispersion composition according to any one of <1> to <14> on a substrate.
<17> A method for forming a permanent pattern, comprising at least exposing a photosensitive layer formed of the silica dispersion composition according to any one of <1> to <14>.
<18> A printed circuit board wherein a permanent pattern is formed by the method for forming a permanent pattern according to <17>.
本発明のシリカ分散組成物は、埋め込み性、耐熱衝撃性、現像性、絶縁性、及び露光部の解像性に優れた高性能な硬化膜を得ることができるので、ソルダーレジストに好適に用いることができる。
本発明のシリカ分散組成物からなるフィルムは、保護膜、層間絶縁膜、及びソルダーレジストパターン等の永久パターン等の各種パターン形成、BGA(ボールグリッドアレイ)、CSP(チップサイズパッケージ)、TCP(テープキャリアパッケージ)等の半導体パッケージ形成用、カラーフィルタ、柱材、リブ材、スペーサー、隔壁等の液晶構造部材の製造、ホログラム、マイクロマシン、プルーフの製造等に好適に用いることができ、特にプリント基板の永久パターン形成用、BGA(ボールグリッドアレイ)、CSP(チップサイズパッケージ)、TCP(テープキャリアパッケージ)等の半導体パッケージの形成に好適に用いることができる。 The silica-dispersed composition of the present invention can be used suitably for a solder resist because a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulation, and resolution of an exposed portion can be obtained. be able to.
The film made of the silica dispersion composition of the present invention includes various patterns such as a protective film, an interlayer insulating film, and a permanent pattern such as a solder resist pattern, BGA (ball grid array), CSP (chip size package), TCP (tape) It can be suitably used for the formation of semiconductor packages such as carrier packages), the manufacture of liquid crystal structural members such as color filters, pillars, ribs, spacers, partition walls, holograms, micromachines, and proofs. It can be suitably used for the formation of a semiconductor package for permanent pattern formation, BGA (ball grid array), CSP (chip size package), TCP (tape carrier package) and the like.
本発明のシリカ分散組成物からなるフィルムは、保護膜、層間絶縁膜、及びソルダーレジストパターン等の永久パターン等の各種パターン形成、BGA(ボールグリッドアレイ)、CSP(チップサイズパッケージ)、TCP(テープキャリアパッケージ)等の半導体パッケージ形成用、カラーフィルタ、柱材、リブ材、スペーサー、隔壁等の液晶構造部材の製造、ホログラム、マイクロマシン、プルーフの製造等に好適に用いることができ、特にプリント基板の永久パターン形成用、BGA(ボールグリッドアレイ)、CSP(チップサイズパッケージ)、TCP(テープキャリアパッケージ)等の半導体パッケージの形成に好適に用いることができる。 The silica-dispersed composition of the present invention can be used suitably for a solder resist because a high-performance cured film excellent in embedding property, thermal shock resistance, developability, insulation, and resolution of an exposed portion can be obtained. be able to.
The film made of the silica dispersion composition of the present invention includes various patterns such as a protective film, an interlayer insulating film, and a permanent pattern such as a solder resist pattern, BGA (ball grid array), CSP (chip size package), TCP (tape) It can be suitably used for the formation of semiconductor packages such as carrier packages), the manufacture of liquid crystal structural members such as color filters, pillars, ribs, spacers, partition walls, holograms, micromachines, and proofs. It can be suitably used for the formation of a semiconductor package for permanent pattern formation, BGA (ball grid array), CSP (chip size package), TCP (tape carrier package) and the like.
Claims (12)
- 酸性基及び塩基性基を少なくとも有するポリウレタン樹脂からなるシリカ分散剤と、シリカ微粒子と、熱架橋剤とを含有してなり、
前記シリカ分散剤のアミン価が0.65mmol/g以上であることを特徴とするシリカ分散組成物。 A silica dispersant comprising a polyurethane resin having at least an acidic group and a basic group, silica fine particles, and a thermal crosslinking agent;
A silica dispersion composition, wherein the silica dispersant has an amine value of 0.65 mmol / g or more. - 塩基性基が第3級アミノ基である請求項1に記載のシリカ分散組成物。 The silica dispersion composition according to claim 1, wherein the basic group is a tertiary amino group.
- 酸性基がカルボキシル基である請求項1から2のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to claim 1, wherein the acidic group is a carboxyl group.
- ポリウレタン樹脂が、側鎖及び末端の少なくともいずれかにグラフト鎖を有する請求項1から3のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to any one of claims 1 to 3, wherein the polyurethane resin has a graft chain in at least one of a side chain and a terminal.
- ポリウレタン樹脂の末端にポリエステルグラフト鎖を有する請求項4に記載のシリカ分散組成物。 The silica dispersion composition according to claim 4, which has a polyester graft chain at the end of the polyurethane resin.
- シリカ分散剤の酸価が0.3mmol/g以上である請求項1から5のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to any one of claims 1 to 5, wherein the silica dispersant has an acid value of 0.3 mmol / g or more.
- ポリウレタン樹脂が、更に、重合性基を有する請求項1から6のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to any one of claims 1 to 6, wherein the polyurethane resin further has a polymerizable group.
- ソルダーレジスト用である請求項1から7のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to any one of claims 1 to 7, which is for a solder resist.
- 更にバインダー、重合性化合物、及び光重合開始剤を含有する請求項1から8のいずれかに記載のシリカ分散組成物。 The silica dispersion composition according to any one of claims 1 to 8, further comprising a binder, a polymerizable compound, and a photopolymerization initiator.
- バインダーが、酸変性ビニル基含有ポリウレタン樹脂である請求項9に記載のシリカ分散組成物。 The silica dispersion composition according to claim 9, wherein the binder is an acid-modified vinyl group-containing polyurethane resin.
- 請求項1から10のいずれかに記載のシリカ分散組成物を含む感光層を支持体上に有してなることを特徴とする感光性フィルム。 A photosensitive film comprising a photosensitive layer containing the silica dispersion composition according to any one of claims 1 to 10 on a support.
- 請求項1から10のいずれかに記載のシリカ分散組成物により形成された感光層に対して露光を行うことを少なくとも含むことを特徴とする永久パターン形成方法。 A method for forming a permanent pattern, comprising at least exposing a photosensitive layer formed of the silica dispersion composition according to any one of claims 1 to 10.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-046642 | 2011-03-03 | ||
| JP2011046642 | 2011-03-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012117763A1 true WO2012117763A1 (en) | 2012-09-07 |
Family
ID=46757705
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/051154 WO2012117763A1 (en) | 2011-03-03 | 2012-01-20 | Silica dispersion composition |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2012193341A (en) |
| WO (1) | WO2012117763A1 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106489100B (en) * | 2014-07-11 | 2020-04-03 | 三菱化学株式会社 | Photosensitive resin composition, cured product, black matrix, and image display device |
| WO2018151161A1 (en) * | 2017-02-17 | 2018-08-23 | 富士フイルム株式会社 | Solid electrolyte composition, solid electrolyte-containing sheet and method for producing same, all-solid secondary battery and method for producing same, and polymer and non-aqueous solvent dispersion thereof |
| WO2020004503A1 (en) * | 2018-06-27 | 2020-01-02 | 富士フイルム株式会社 | Positive lithographic printing plate precursor, method for manufacturing lithographic printing plate, and positive photosensitive resin composition |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6123143A (en) * | 1984-07-12 | 1986-01-31 | Dainippon Ink & Chem Inc | Image forming material developable by peeling |
| JP2005513300A (en) * | 2001-12-21 | 2005-05-12 | アクゾ ノーベル エヌ.ブイ. | Aqueous silica-containing composition and paper manufacturing method |
| JP2009169383A (en) * | 2007-06-22 | 2009-07-30 | Ricoh Co Ltd | Toner, developer, toner container, image forming method, image forming device, and process cartridge |
| JP2010037427A (en) * | 2008-08-05 | 2010-02-18 | Tokai Carbon Co Ltd | Pigment-dispersed photosensitive resin composition |
| WO2010068871A1 (en) * | 2008-12-12 | 2010-06-17 | E. I. Du Pont De Nemours And Company | Amphoteric poyurethane dispersants and their use in inkjet inks |
| CN101747487A (en) * | 2009-12-16 | 2010-06-23 | 江南大学 | Preparation method of ultraviolet cured silica sol modified aqueous urethane acrylate dispersoid |
| CN101845217A (en) * | 2010-05-11 | 2010-09-29 | 陕西科技大学 | Preparation method of water-based polyurethane/nano-silicon dioxide composite emulsion |
-
2012
- 2012-01-20 WO PCT/JP2012/051154 patent/WO2012117763A1/en active Application Filing
- 2012-01-24 JP JP2012011873A patent/JP2012193341A/en active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6123143A (en) * | 1984-07-12 | 1986-01-31 | Dainippon Ink & Chem Inc | Image forming material developable by peeling |
| JP2005513300A (en) * | 2001-12-21 | 2005-05-12 | アクゾ ノーベル エヌ.ブイ. | Aqueous silica-containing composition and paper manufacturing method |
| JP2009169383A (en) * | 2007-06-22 | 2009-07-30 | Ricoh Co Ltd | Toner, developer, toner container, image forming method, image forming device, and process cartridge |
| JP2010037427A (en) * | 2008-08-05 | 2010-02-18 | Tokai Carbon Co Ltd | Pigment-dispersed photosensitive resin composition |
| WO2010068871A1 (en) * | 2008-12-12 | 2010-06-17 | E. I. Du Pont De Nemours And Company | Amphoteric poyurethane dispersants and their use in inkjet inks |
| CN101747487A (en) * | 2009-12-16 | 2010-06-23 | 江南大学 | Preparation method of ultraviolet cured silica sol modified aqueous urethane acrylate dispersoid |
| CN101845217A (en) * | 2010-05-11 | 2010-09-29 | 陕西科技大学 | Preparation method of water-based polyurethane/nano-silicon dioxide composite emulsion |
Non-Patent Citations (2)
| Title |
|---|
| ANJIE DONG ET AL.: "Properties of amphoteric polyurethane waterborne dispersions II. Macromolecular self-assembly behavior", JOURNAL OF COLLOID AND INTERFACE SCIENCE, vol. 226, no. 2, 2003, pages 276 - 281 * |
| YONG QIAO ET AL.: "Stabilized Micelles of Amphoteric Polyurethane Formed by Thermoresponsive Micellization in HC1 Aqueous Solution", LANGMUIR, vol. 24, no. 7, 2008, pages 3122 - 3126 * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2012193341A (en) | 2012-10-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012029785A1 (en) | Photosensitive composition, and photosensitive film, permanent pattern, permanent pattern formation method and printed substrate | |
| WO2012029786A1 (en) | Photosensitive composition, as well as photosensitive film, permanent pattern, method for forming permanent pattern, and printed substrate | |
| WO2012117786A1 (en) | Photosensitive composition, photosensitive solder-resist composition, photosensitive solder-resist film, permanent pattern, method for forming same, and printed circuit board | |
| WO2011040299A1 (en) | Photosensitive composition, photosensitive solder resist composition, photosensitive solder resist film, and permanent pattern, method for forming same, and printed substrate | |
| WO2013027646A1 (en) | Photosensitive resin composition, and photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed substrate using same | |
| US20120183776A1 (en) | Curable composition, curable film, curable laminate, method for forming a permanent pattern, and printed substrate | |
| WO2011136286A1 (en) | Photosensitive composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2012073589A (en) | Photosensitive composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2012198361A (en) | Photosensitive composition, photosensitive film, method for forming permanent pattern, permanent pattern, and printed board | |
| JP2011175254A (en) | Photosensitive composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2012185459A (en) | Photosensitive composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2013041227A (en) | Photosensitive resin composition, and photosensitive film, photosensitive laminate, method for forming permanent pattern and printed board using the composition | |
| WO2012117763A1 (en) | Silica dispersion composition | |
| JP2013057755A (en) | Photosensitive resin composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2011197310A (en) | Curable composition, and curable film, curable laminate, permanent pattern forming method and printed board using the same | |
| WO2013008631A1 (en) | Photosensitive composition | |
| WO2012029481A1 (en) | Photosensitive composition, photosensitive film, photosensitive laminate, permanent pattern casting method, and print substrate | |
| JP2013041225A (en) | Photosensitive resin composition, and photosensitive film, photosensitive laminate, method for forming permanent pattern and printed board using the composition | |
| JP2013029781A (en) | Photosensitive resin composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| JP2012078462A (en) | Photosensitive resin composition, photosensitive solder resist composition and photosensitive solder resist film, and permanent pattern and method for forming the same and print circuit board | |
| JP2012078463A (en) | Photosensitive resin composition, photosensitive solder resist composition and photosensitive solder resist film, and permanent pattern and method for forming the same and print circuit board | |
| WO2012169385A1 (en) | Composition, photosensitive film, photosensitive laminate, method for forming permanent pattern, and printed board | |
| KR101660574B1 (en) | Photosensitive composition, photosensitive film, photosensitive laminate, permanent pattern formation method, and printed substrate | |
| JP2013020094A (en) | Photosensitive composition, photosensitive dry film, photosensitive laminate, flexible wiring board, and permanent pattern formation method | |
| WO2012132491A1 (en) | Photosensitive composition, photosensitive laminate, permanent pattern forming method, and printed board |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12751843 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12751843 Country of ref document: EP Kind code of ref document: A1 |