US20090319195A1 - Method of monitoring and optimizing additive concentration in fuel ethanol - Google Patents
Method of monitoring and optimizing additive concentration in fuel ethanol Download PDFInfo
- Publication number
- US20090319195A1 US20090319195A1 US12/143,400 US14340008A US2009319195A1 US 20090319195 A1 US20090319195 A1 US 20090319195A1 US 14340008 A US14340008 A US 14340008A US 2009319195 A1 US2009319195 A1 US 2009319195A1
- Authority
- US
- United States
- Prior art keywords
- fuel ethanol
- additive composition
- corrosion inhibitor
- component
- fluorescent signal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 title claims abstract description 413
- 239000000446 fuel Substances 0.000 title claims abstract description 134
- 239000000654 additive Substances 0.000 title claims abstract description 121
- 230000000996 additive effect Effects 0.000 title claims abstract description 101
- 238000000034 method Methods 0.000 title claims abstract description 79
- 238000012544 monitoring process Methods 0.000 title claims abstract description 25
- 239000000203 mixture Substances 0.000 claims abstract description 113
- 238000005260 corrosion Methods 0.000 claims description 130
- 230000007797 corrosion Effects 0.000 claims description 130
- 239000003112 inhibitor Substances 0.000 claims description 128
- 239000003398 denaturant Substances 0.000 claims description 68
- 238000005259 measurement Methods 0.000 claims description 37
- 239000000700 radioactive tracer Substances 0.000 claims description 27
- 230000005284 excitation Effects 0.000 claims description 16
- 238000003860 storage Methods 0.000 claims description 5
- 150000001875 compounds Chemical class 0.000 claims description 4
- 230000008859 change Effects 0.000 claims description 2
- 239000003795 chemical substances by application Substances 0.000 claims description 2
- 238000004891 communication Methods 0.000 claims description 2
- 238000001212 derivatisation Methods 0.000 claims description 2
- 238000007865 diluting Methods 0.000 claims 2
- 230000009918 complex formation Effects 0.000 claims 1
- 238000007792 addition Methods 0.000 description 27
- 238000004519 manufacturing process Methods 0.000 description 18
- 238000009472 formulation Methods 0.000 description 13
- 239000000126 substance Substances 0.000 description 10
- 230000008901 benefit Effects 0.000 description 9
- DZNJMLVCIZGWSC-UHFFFAOYSA-N 3',6'-bis(diethylamino)spiro[2-benzofuran-3,9'-xanthene]-1-one Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(N(CC)CC)C=C1OC1=CC(N(CC)CC)=CC=C21 DZNJMLVCIZGWSC-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 230000001276 controlling effect Effects 0.000 description 6
- FEEONEKLFGDWHR-UHFFFAOYSA-N 1-[2-hydroxy-3-propyl-4-[4-[4-(2h-tetrazol-5-yl)phenoxy]butoxy]phenyl]ethanone Chemical compound C1=CC(C(C)=O)=C(O)C(CCC)=C1OCCCCOC1=CC=C(C2=NNN=N2)C=C1 FEEONEKLFGDWHR-UHFFFAOYSA-N 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 239000000356 contaminant Substances 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 3
- 235000014113 dietary fatty acids Nutrition 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 229930195729 fatty acid Natural products 0.000 description 3
- 239000000194 fatty acid Substances 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 150000004820 halides Chemical class 0.000 description 3
- 150000002430 hydrocarbons Chemical class 0.000 description 3
- LXNHXLLTXMVWPM-UHFFFAOYSA-N pyridoxine Chemical compound CC1=NC=C(CO)C(CO)=C1O LXNHXLLTXMVWPM-UHFFFAOYSA-N 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- FKJSFKCZZIXQIP-UHFFFAOYSA-N 2-bromo-1-(4-bromophenyl)ethanone Chemical group BrCC(=O)C1=CC=C(Br)C=C1 FKJSFKCZZIXQIP-UHFFFAOYSA-N 0.000 description 2
- RIOSJKSGNLGONI-UHFFFAOYSA-N 2-phenylbenzenesulfonic acid Chemical class OS(=O)(=O)C1=CC=CC=C1C1=CC=CC=C1 RIOSJKSGNLGONI-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- AUNGANRZJHBGPY-UHFFFAOYSA-N D-Lyxoflavin Natural products OCC(O)C(O)C(O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- WDECIBYCCFPHNR-UHFFFAOYSA-N chrysene Chemical compound C1=CC=CC2=CC=C3C4=CC=CC=C4C=CC3=C21 WDECIBYCCFPHNR-UHFFFAOYSA-N 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 229910021645 metal ion Inorganic materials 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical class C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 125000005575 polycyclic aromatic hydrocarbon group Chemical group 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- BBEAQIROQSPTKN-UHFFFAOYSA-N pyrene Chemical compound C1=CC=C2C=CC3=CC=CC4=CC=C1C2=C43 BBEAQIROQSPTKN-UHFFFAOYSA-N 0.000 description 2
- DLOBKMWCBFOUHP-UHFFFAOYSA-N pyrene-1-sulfonic acid Chemical class C1=C2C(S(=O)(=O)O)=CC=C(C=C3)C2=C2C3=CC=CC2=C1 DLOBKMWCBFOUHP-UHFFFAOYSA-N 0.000 description 2
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 2
- 229960002477 riboflavin Drugs 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 150000003460 sulfonic acids Chemical class 0.000 description 2
- 239000013638 trimer Substances 0.000 description 2
- 229940011671 vitamin b6 Drugs 0.000 description 2
- 239000008096 xylene Substances 0.000 description 2
- VIFBEEYZXDDZCT-UHFFFAOYSA-N 2-(2-phenylethenyl)benzenesulfonic acid Chemical class OS(=O)(=O)C1=CC=CC=C1C=CC1=CC=CC=C1 VIFBEEYZXDDZCT-UHFFFAOYSA-N 0.000 description 1
- CTENSLORRMFPDH-UHFFFAOYSA-N 4-(bromomethyl)-7-methoxychromen-2-one Chemical compound BrCC1=CC(=O)OC2=CC(OC)=CC=C21 CTENSLORRMFPDH-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 239000004258 Ethoxyquin Substances 0.000 description 1
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 229930003471 Vitamin B2 Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 239000012445 acidic reagent Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 1
- CUOZYXPNIFEQTK-UHFFFAOYSA-N benzenesulfonic acid;formaldehyde Chemical compound O=C.OS(=O)(=O)C1=CC=CC=C1 CUOZYXPNIFEQTK-UHFFFAOYSA-N 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 229960001948 caffeine Drugs 0.000 description 1
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 230000003467 diminishing effect Effects 0.000 description 1
- DECIPOUIJURFOJ-UHFFFAOYSA-N ethoxyquin Chemical compound N1C(C)(C)C=C(C)C2=CC(OCC)=CC=C21 DECIPOUIJURFOJ-UHFFFAOYSA-N 0.000 description 1
- 229940093500 ethoxyquin Drugs 0.000 description 1
- 235000019285 ethoxyquin Nutrition 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GVEPBJHOBDJJJI-UHFFFAOYSA-N fluoranthrene Natural products C1=CC(C2=CC=CC=C22)=C3C2=CC=CC3=C1 GVEPBJHOBDJJJI-UHFFFAOYSA-N 0.000 description 1
- 238000012921 fluorescence analysis Methods 0.000 description 1
- 238000002189 fluorescence spectrum Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- NVVZQXQBYZPMLJ-UHFFFAOYSA-N formaldehyde;naphthalene-1-sulfonic acid Chemical compound O=C.C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 NVVZQXQBYZPMLJ-UHFFFAOYSA-N 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical compound [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000000491 multivariate analysis Methods 0.000 description 1
- 239000003498 natural gas condensate Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 1
- CSHWQDPOILHKBI-UHFFFAOYSA-N peryrene Natural products C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 CSHWQDPOILHKBI-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 238000001782 photodegradation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- CZLSHVQVNDDHDQ-UHFFFAOYSA-N pyrene-1,3,6,8-tetrasulfonic acid Chemical compound C1=C2C(S(=O)(=O)O)=CC(S(O)(=O)=O)=C(C=C3)C2=C2C3=C(S(O)(=O)=O)C=C(S(O)(=O)=O)C2=C1 CZLSHVQVNDDHDQ-UHFFFAOYSA-N 0.000 description 1
- RADKZDMFGJYCBB-UHFFFAOYSA-N pyridoxal hydrochloride Natural products CC1=NC=C(CO)C(C=O)=C1O RADKZDMFGJYCBB-UHFFFAOYSA-N 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000002151 riboflavin Substances 0.000 description 1
- 235000019192 riboflavin Nutrition 0.000 description 1
- 238000012284 sample analysis method Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical class C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- MWOOGOJBHIARFG-UHFFFAOYSA-N vanillin Chemical compound COC1=CC(C=O)=CC=C1O MWOOGOJBHIARFG-UHFFFAOYSA-N 0.000 description 1
- FGQOOHJZONJGDT-UHFFFAOYSA-N vanillin Natural products COC1=CC(O)=CC(C=O)=C1 FGQOOHJZONJGDT-UHFFFAOYSA-N 0.000 description 1
- 235000012141 vanillin Nutrition 0.000 description 1
- 235000019164 vitamin B2 Nutrition 0.000 description 1
- 239000011716 vitamin B2 Substances 0.000 description 1
- 235000019158 vitamin B6 Nutrition 0.000 description 1
- 239000011726 vitamin B6 Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 150000003738 xylenes Chemical class 0.000 description 1
- 235000004835 α-tocopherol Nutrition 0.000 description 1
- 150000003772 α-tocopherols Chemical class 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/84—Systems specially adapted for particular applications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/62—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light
- G01N21/63—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light optically excited
- G01N21/64—Fluorescence; Phosphorescence
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/62—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light
- G01N21/63—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light optically excited
- G01N21/64—Fluorescence; Phosphorescence
- G01N21/6428—Measuring fluorescence of fluorescent products of reactions or of fluorochrome labelled reactive substances, e.g. measuring quenching effects, using measuring "optrodes"
- G01N21/643—Measuring fluorescence of fluorescent products of reactions or of fluorochrome labelled reactive substances, e.g. measuring quenching effects, using measuring "optrodes" non-biological material
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/26—Oils; Viscous liquids; Paints; Inks
- G01N33/28—Oils, i.e. hydrocarbon liquids
- G01N33/2835—Specific substances contained in the oils or fuels
- G01N33/2852—Alcohol in fuels
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2201/00—Features of devices classified in G01N21/00
- G01N2201/06—Illumination; Optics
- G01N2201/061—Sources
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/26—Oils; Viscous liquids; Paints; Inks
- G01N33/28—Oils, i.e. hydrocarbon liquids
- G01N33/2835—Specific substances contained in the oils or fuels
- G01N33/2882—Markers
Definitions
- This invention relates generally to methods of monitoring and/or controlling additive composition dosages in fuel ethanol. More specifically, the invention relates to monitoring and optimizing dosages of additive compositions including corrosion inhibitors, combinations of different corrosion inhibitors, denaturants, and mixtures of corrosion inhibitor(s) and denaturants in fuel ethanol. The invention has particular relevance to monitoring such dosages using fluorescence signals from one or more components in the additive composition.
- Fuel ethanol production in the U.S. increased by about 440% during the period from 1996 to 2007 (from 1.1 to 6.5 billion gallons per year) and world ethanol production reached about 13.1 billion gallons per year in 2007.
- Fuel ethanol plants under construction/expansion are expected to double current U.S. production capacity, and legislation has been passed that could increase fuel ethanol demand by more than 600% by 2022.
- the maximum specification range currently allowed in the U.S. for denaturant is typically about 1.96 to 4.76% by volume. Due to the cost differential between ethanol and denaturant, it is valuable for a fuel ethanol plant to have the ability to be as close as possible to the upper or lower edge of denaturant dosage specification range. When ethanol costs exceed denaturant costs, for instance, it is desirable for the fuel ethanol plant to be at the high dosage edge of denaturant specification range to keep production costs to a minimum. On the other hand, when denaturant costs more than ethanol, it is desirable for the fuel ethanol plant to be at the low dosage edge of denaturant specification range.
- This invention accordingly includes methods of monitoring and optimizing dosage of one or more fuel ethanol additives by measuring a fluorescent signal. Such measurements are taken, for example, from one or more components of an additive composition, a derivative of a component in the additive, and/or from an inert tracer used in conjunction with or as part of the additive to provide an indication of dosage concentration. It is contemplated that the described method may be applied to any additive for fuel ethanol. In a preferred embodiment, the method is applied to measuring and controlling dosages of denaturants and/or corrosion inhibitors. Such monitoring and control may be directed to additives present in or added to the fuel ethanol. Depending upon whether a denaturant or corrosion inhibitor is the traced additive (and the particular chemistry used), the chosen method of measuring the fluorescent signal may be different.
- An additional advantage of the invention is to enable fuel ethanol producers to include certificates of analysis with respect to additive dosage for each fuel ethanol shipment.
- a further advantage of the invention is to provide a versatile method of monitoring and controlling additive dosages in fuel ethanol that could be used in both a grab sample analysis scheme and/or adapted to online dosage control with datalogging capabilities.
- Another advantage of the invention is to provide a method of compensating for changes in fuel ethanol system characteristics by adjusting additive dosage.
- Yet another advantage of the invention is to provide methods of controlling additive dosages at fuel ethanol manufacturing plants to eliminate the possibility of out-of-specification product batches and prevent costly reworking of batches to achieve specification and/or government compliance.
- FIG. 1 shows the ability to measure intrinsic fluorescence of components in two different commercially available fuel ethanol corrosion inhibitors, illustrated as contour fluorescence spectra.
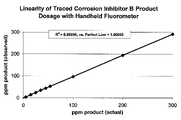
- FIG. 2 illustrates the linearity and predictability of fluorescence in a preferred embodiment where an inert fluorescent tracer was added in a corrosion inhibitor composition added to fuel ethanol, as explained in Example 8.
- the invention includes methods of monitoring, regulating, and/or optimizing the concentration of an additive composition in a fuel ethanol using a fluorescent signal generated from a component in the additive composition.
- the disclosed method of this invention is suitable for all manner of fuel ethanol production and is compatible with essentially all grades of fuel ethanol mixtures.
- the method is particularly well suited for use in conjunction with a variety of fuel ethanol additives.
- Application of the method begins in the production process where additives including denaturants and/or corrosion inhibitors are typically added, and may also be implemented at any stage of the packaging and shipping process.
- the described method is equally applicable to various sampling techniques including grab samples, sidestream and inline measurements, and measurements taken from a bulk container or vessel.
- the method in certain embodiments, may be combined with other utilities known in the ethanol industry.
- Representative utilities include sensors for measuring alcohol content in, for example, gasoline; sensors for determining fuel composition; individual alcohol concentration sensors (e.g., methanol, ethanol); alcohol/gasoline ratio sensors; dissolved or particulate contaminant sensors; other sensors based upon resistance, capacitance, spectroscopic absorbance, calorimetric measurements, and fluorescence; and mathematical tools for analyzing sensor/controller results (e.g., multivariate analysis, chemometrics, on/off dosage control, PID dosage control, the like, and combinations thereof).
- the additive composition typically has a corrosion inhibitor, denaturant, or a mixture of both.
- the additive may also be a neat product or a mixture of two or more additives. It should be appreciated that the additive composition may include any number of compounds or components. Although such additives are most commonly a corrosion inhibitor or denaturant (or combination), as explained above, the described method is equally suited to any additive composition in used in fuel ethanol. Executing the method involves adding a known amount of the additive composition to the fuel ethanol to create a treated fuel ethanol. The added amount is calculated to provide an optimum concentration range for the additive composition in the treated fuel ethanol.
- the intrinsic fluorescence of different batches of denaturants has significantly variable fluorescence intensities and peak shapes. Though technically feasible, it is not a preferred method of the invention to use the inherent or intrinsic fluorescence of denaturant additives for monitoring and optimizing the dosage of fuel ethanol additives unless the denaturant has a consistent chemical composition.
- the intrinsic fluorescence of corrosion inhibitor formulations is acceptable when properly implemented and is a preferred embodiment of the invention. Methods utilizing fluorescent tracers have equal efficacy in all types of additive formulations.
- the additive composition includes a corrosion inhibitor. It is contemplated that the described method is operable with any corrosion inhibitor used for fuel ethanol.
- a corrosion inhibitors containing compounds such as organic acid anhydrides; monomer, dimer, and/or trimer organic fatty acid mixtures; and tertiary organic amines may be used.
- Corrosion inhibitors also typically include a mixture of one or more of the following: organic (cyclohexyl-containing) amine; monomer, dimer, and/or trimer organic fatty acids including synthetics; organic acid anhydride; and organic solvents such as alcohol, xylenes, or other hydrocarbon-based solvent.
- the optimum concentration range for corrosion inhibitor products is typically in the ppm range (see Examples), although this range may be above or below the optimum target dosage for certain applications. It should be appreciated that the described method is applicable for use with any corrosion inhibiting composition.
- the additive composition includes a denaturant.
- Typical denaturants include condensates from natural gas condensate, which may include gasoline, methanol, straight-chain hydrocarbons, naphthenes, aromatics, and others. It should be appreciated that any denaturant known in the art may be used with the method of the invention.
- the additive composition includes at least one component that is either inherently capable of providing a fluorescent signal or capable of being chemically derivatized or functionalized to provide a fluorescent signal. Fluorescence behavior has been found to be markedly and unexpectedly different in ethanol than in aqueous, ethanol-free solutions or low polarity hydrocarbon-containing solutions. Intensive testing and experimentation was required to ascertain effective fluorescing molecules and moieties in ethanol-containing systems (see Example 2).
- a component that is normally a part of a conventional additive composition is inherently fluorescent.
- Such an additive is, for example, a corrosion inhibitor composition having a solvent containing an aromatic hydrocarbon.
- Xylene, other aromatic hydrocarbons, and functionalized aromatic hydrocarbons are inherently fluorescent and its fluorescent signal may be used as an analytical signal to determine the concentration of the additive composition in a treated fuel ethanol.
- FIG. 1 illustrates two different commercially used corrosion inhibitors that exhibit such inherent fluorescence and fluorescence excitation/emission wavelength combinations where the intrinsic fluorescence corrosion inhibitor is different from the intrinsic fluorescence of denaturant, contaminants, etc.
- a component that does not inherently provide an analytical signal but that is normally part of the additive composition can be chosen for modification, such as in a grab sample or sidestream taken from the system.
- This component is derivatized or functionalized with a moiety that imparts that ability to provide a fluorescent signal or a colorimetric signal.
- a compound in a corrosion inhibitor composition may chemically be derivatized with a fluorescent moiety or reacted to provide a calorimetric signal.
- a component of the corrosion inhibitor e.g., tertiary organic amine
- aromatic carbonyl chloride Ar—COCl
- Derivatization agents can be used to react with any component of the fuel ethanol corrosion inhibitor to utilize a fluorescent analysis technique. For example, 4-bromomethyl-7-methoxy-coumarin can react with carboxylic acids to form a fluorescent derivative (See W. Dunges, in “Analytical Chemistry,” vol. 49, p. 442, 1977).
- carboxylic acids e.g., dimer fatty acids
- a corrosion inhibitor may be reacted to produce a colorimetric signal.
- a representative carboxylic acid reagent is p-bromophenacyl bromide (PBPB). (See Durst et al., in “Analytical Chemistry,” vol. 47, p. 1747, 1975).
- an inert fluorescent tracer is included in the additive composition.
- a known proportion of the fluorescent tracer is added either simultaneously or sequentially with the additive composition.
- the inert fluorescent tracer is added first to the additive composition and the tracer-containing additive is then combined with the fuel ethanol or combined with another additive (e.g., traced corrosion inhibitor formulation combined with denaturant), which combination is added to the fuel ethanol.
- Effective inert fluorescent tracers include those substances that are chemically non-reactive with other components in the fuel ethanol and that do not significantly degrade with time. Such tracers should also be completely (or essentially completely) soluble in the additive formulation, mixtures of additives, and mixtures of additive(s) and fuel ethanol at all relevant levels of concentration and preferably the fluorescence intensity should be substantially proportional to its concentration and not significantly quenched or otherwise diminished by the fuel ethanol or other components in the fuel ethanol. Furthermore, the inert fluorescent tracer should not be appreciably or significantly affected by any other chemistry in fuel ethanol. The statement, “not appreciably or significantly affected,” means that an inert fluorescent compound generally has no more than about a 10% change in its fluorescent signal, under conditions normally encountered in fuel ethanol.
- Desired characteristics for an inert fluorescent tracer preferably include: fluorescence excitation/emission wavelengths that do not have significant overlap with light absorbing substances in the fuel ethanol, other additives, contaminants, etc.; high solubility in an additive (and combinations of additives) and additive(s) combination with fuel ethanol; excellent chemical stability; suitable fluorescence properties at manageable wavelengths (e.g., other additives in the fuel ethanol should not interfere with the fluorescence properties at those wavelengths) and excitation/emission wavelengths that are separate from other fluorescent components in the fuel ethanol and additive mixtures to prevent interference; chemical composition typically containing only C, H, N, O, and/or S (where S content of fuel ethanol ⁇ 15 ppm of total composition, and avoiding “S” if possible); and avoiding negative impacts on fuel properties.
- ideal inert fluorescent tracers would: not be significantly impacted by surrounding temperature or pressure; presence of water or other solvents; have acceptably low light absorbance; lack metal ions, phosphorous, and halides; not be impacted by changes in the composition of other additives or contaminants (e.g., butanol); should not adversely alter performance of additives, such as pH buffering ability of corrosion inhibitor; sufficiently burned when fuel ethanol mixtures are used in internal combustion engines; and not cause deposits, fouling, corrosion, etc. in downstream applications.
- additives such as pH buffering ability of corrosion inhibitor
- Representative inert fluorescent tracers that do not have metal ion/halide counterions or halide functional groups include fluorescein or fluorescein derivatives; rhodamine or rhodamine derivatives; naphthalene sulfonic acids (mono-, di-, tri-, etc.); pyrene sulfonic acids (mono-, di-, tri-, tetra-, etc.); stilbene derivatives containing sulfonic acids (including optical brighteners); biphenyl sulfonic acids; phenylalanine; tryptophan; tyrosine; vitamin B2 (riboflavin); vitamin B6 (pyridoxin); vitamin E ( ⁇ -tocopherols); ethoxyquin; caffeine; vanillin; naphthalene sulfonic acid formaldehyde condensation polymers; phenyl sulfonic acid formaldehyde condensates; lignin sulfonic acids;
- inert fluorescent tracers may be found in U.S. Pat. No. 6,966,213 B2, entitled “Rapid Method for Detecting Leaks of Hydraulic Fluids in Production Plants” and U.S. Pat. No. 7,169,236 B2, entitled “Method of Monitoring Membrane Cleaning Process.”
- These inert fluorescent tracers are either commercially available under the tradename TRASAR® from Nalco Company® (Naperville, Ill.) or may be synthesized using techniques known to persons of ordinary skill in the art of organic chemistry.
- PAH polyaromatic hydrocarbon
- a working curve for the particular fluorophore chosen should be developed. Similar curves can be readily created for any desired fluorophore when the fluorescence analysis conditions (for example, excitation and emission wavelength) are defined.
- the present fluorometric method requires the selection of an excitation wavelength to activate the fluorescence process and an emission wavelength at which fluorescence intensity is to be measured, which preferably is substantially free of interference from other species present in the fuel ethanol being monitored. Undesirable interference may be encountered when one or more other species have significant fluorescence emission or light absorbance at about the excitation/emission wavelengths selected for monitoring the chosen fluorophore.
- the excitation wavelength is chosen to also prevent photodegradation from occurring.
- the background fluorescent signal may be measured in the treated fuel ethanol at any point subsequent to adding the additive composition.
- “Treated fuel ethanol” refers to fuel ethanol including the additive composition as herein described.
- the fluorescent signal is acquired at one, two, or more points.
- the fluorescent signal is acquired online, either continuously or intermittently.
- Such online measurements may be analyzed in real-time or with a user-defined or other delay.
- online measurements may take place by using a side-stream, inline, or other suitable flow-through device.
- a sample of treated fuel ethanol is removed, either automatically or manually, and the fluorescent signal is acquired from the removed sample.
- the total or component concentration of the additive composition may be determined. Three possible scenarios exist for the outcome of this determination. The first is that the concentration of the additive composition is within the optimum concentration range. In this instance, no further action would be taken. In the event the determined concentration of the additive composition is higher than the optimum concentration range, the treated fuel ethanol would optionally be diluted with a known additional volume of fuel ethanol. The additional volume would be calculated to bring the concentration of the additive composition into the optimum concentration range. If the determined concentration of the additive composition is below the optimum concentration range, an additional amount of the additive composition would optionally be introduced into the treated fuel ethanol in an amount calculated to bring the concentration of the additive composition into the optimum concentration range. The method of the invention may optionally be repeated (e.g., in an iterative fashion) until the determined concentration of the additive composition is within the optimum concentration range (or another chosen concentration range, such as a user-selected concentration range).
- Fuel ethanol (usually approximately E95) is typically mixed with gasoline to form ethanol-containing gasolines, such as E10 and E85.
- an E10 formulation generally includes about 9.5 to 9.8% vol/vol ethanol, about 0.2% to 0.5% vol/vol denaturant, and about 90% vol/vol gasoline.
- the described method is equally applicable in such fuel ethanol compositions, including determining the total ethanol content in an alternative embodiment.
- a manual operator or an electronic device having components such as a processor, memory device, digital storage medium, cathode ray tube, liquid crystal display, plasma display, touch screen, or other monitor, and/or other components may be used to execute all or parts of the described method.
- the controller may be operable for integration with one or more application-specific integrated circuits, programs, computer-executable instructions, or algorithms, one or more hard-wired devices, wireless devices, and/or one or more mechanical devices.
- Some or all of the controller system functions may be at a central location, such as a network server, for communication over a local area network, wide area network, wireless network, Internet connection, microwave link, infrared link, and the like.
- a signal conditioner or system monitor may be included to facilitate signal-processing algorithms. It is also contemplated that any needed sensors, couplers, connectors, or other data measuring/transmitting/communicating equipment may be used to capture and transmit data.
- Dosage results in Table 1 are listed as ppm.
- the variability in dosage is given as ⁇ 3 SIGMA and as % deviation from average, which is based upon an assumption of a statistically normal distribution.
- the results from the 40 samples are that overall average dosage (51 ppm) is significantly below the recommended target dosage of 72 ppm.
- the average dosages in Table 1 are typically significantly below the recommended target dosage with many samples being much below (and some samples being significantly above) the recommended target dosage.
- Examples 2 to 5 illustrate the differences between current methods of adjusting additive dosages; direct manual measurement of traced corrosion inhibitor, either with or without providing a measurement for added denaturant (by tracer fluorescence); and automatic control of corrosion inhibitor dosage, either with or without providing a measurement for added denaturant, based on fluorescence measurements of traced corrosion inhibitor being added to fuel ethanol.
- fluorescence of tracer added to corrosion inhibitor product to measure corrosion inhibitor dosage could significantly improve accuracy and reduce variability.
- Manual adjustment of product dosage after measuring of corrosion inhibitor concentration would provide for improved additive dosage accuracy and reduced variability in final treated fuel ethanol.
- Online monitoring/control of corrosion inhibitor dosage would result in further improved accuracy and reduced variability in concentration levels.
- the predicted variability is shown as ⁇ 3 SIGMA and based on assumption that a statistically normal distribution would occur.
- a corrosion inhibitor may initially be added by the plant to series of batches of fuel ethanol using a “splash addition” method (standard industry practice).
- the estimated volume of corrosion inhibitor to be added is typically based on the estimated volume of fuel ethanol in the storage tank.
- Table 3 shows dosage of corrosion inhibitor during three phases of dosage monitoring and/or control.
- Batch numbers 1 to 5 illustrate dosage prior to any changes in corrosion inhibitor dosing procedure (i.e., manual addition with no measurement during addition of corrosion inhibitor); 6 to 10 show improved results with direct measurement of corrosion inhibitor (by inherent fluorescence) and manual addition/adjustment of corrosion inhibitor based on measurement of corrosion inhibitor; and 11 to 15 exemplify further improvement in results (average closer to target dosage and lower ⁇ SIGMA value) due to automatic measurement and dosage control of corrosion inhibitor dosage based on inherent fluorescence measurements of the corrosion inhibitor being added to fuel ethanol.
- the target dosage of corrosion inhibitor is 72 ppm in fuel ethanol mixture for this Example. Predicted variability is shown as ⁇ 3 SIGMA and based on assumption that a statistically normal distribution occurs.
- a small known amount of fluorescent tracer could be added into corrosion inhibitor formulation during its manufacture.
- Traced corrosion inhibitor may initially be added by the plant to series of batches of fuel ethanol using a “splash addition” method.
- Estimated volume of corrosion inhibitor to be added is typically based on estimated volume of fuel ethanol in storage tank. Results in Table 4 show theoretical dosage of traced corrosion inhibitor during three phases of dosage monitoring and/or control.
- Batch numbers 1 to 5 show results prior to any changes in corrosion inhibitor dosing procedure using a manual addition method and no measurement; 6 to 10 illustrate improved dosage using direct measurement of traced corrosion inhibitor (by tracer fluorescence) and manual addition/adjustment of corrosion inhibitor; and 11 to 15 exemplify further improvement in results due to automatic control of corrosion inhibitor dosage based on fluorescence measurements of traced corrosion inhibitor being added to fuel ethanol.
- Target dosage of traced corrosion inhibitor is 72 ppm in fuel ethanol mixture in this Example.
- the use of fluorescent traced corrosion inhibitor also allows a ready means to identify that the correct additive was mixed with the fuel ethanol. If the fluorescence signal of the traced corrosion inhibitor is absent or at significantly reduced level in the treated fuel ethanol, that measurement demonstrates: (i) an incorrect corrosion inhibitor product was used; (ii) that the treated fuel ethanol was diluted with an untraced corrosion inhibitor; (iii) that the batch of treated fuel ethanol was mixed with another batch of fuel ethanol that was treated with an untraced or incorrect corrosion inhibitor; or (iv) that batches of fuel ethanol that were correctly and incorrectly treated were mixed.
- Fluorescent tracer may be added to corrosion inhibitor and then the traced corrosion inhibitor may be mixed into denaturant at a prescribed dosage to provide monitoring and/or control of denaturant and traced corrosion inhibitor dosage.
- denaturant can typically be added from about 1.96% up to about 4.76% volume/volume (or about 1.63% to about 3.98% weight/weight) into fuel ethanol, depending on the locality of fuel ethanol manufacture. If the target dosage for corrosion inhibitor was 72 ppm (or 0.072% weight/weight) and denaturant was 2.20% volume/volume (1.83% weight/weight), the traced corrosion inhibitor may be added to denaturant in a ratio of 1 part traced corrosion inhibitor to 25.4 parts (by weight/weight) of denaturant. The mixture of denaturant and traced corrosion inhibitor may then be added to the fuel ethanol and the dosages of denaturant and corrosion inhibitor can both be monitored and/or controlled based on the fluorescent tracer signal.
- Results in Table 5A to 5C show theoretical dosage of traced corrosion inhibitor and denaturant during three phases of dosage monitoring and/or control: (A) prior to any changes in corrosion inhibitor and denaturant dosing procedure with manual dosage control, (B) with direct measurement of traced corrosion inhibitor and denaturant (by tracer fluorescence) and with manual corrosion inhibitor addition, and (C) automatic control of corrosion inhibitor and denaturant dosages based on fluorescence measurements of the traced corrosion inhibitor+denaturant mixture being added to fuel ethanol.
- Target dosage of corrosion inhibitor is typically 72 ppm and 2.20% volume/volume (or 1.84% weight/weight) denaturant to produce treated fuel ethanol.
- the target dosage for fluorescent traced corrosion inhibitor can be increased, the level of traced fluorescent corrosion inhibitor can be increased in its mixture with denaturant, the level of corrosion inhibitor can be adjusted.
- fluorescent tracer would be added to corrosion inhibitor and then the traced corrosion inhibitor mixed into denaturant at a prescribed dosage to provide monitoring and/or control of higher dosages of denaturant and traced corrosion inhibitor dosage.
- Current legal guidelines allow for a denaturant range from 1.96% up to 4.76% on a volume/volume basis (or 1.63% to 3.98% weight/weight) into fuel ethanol, depending on the locality of fuel ethanol manufacture.
- traced corrosion inhibitor would be added to denaturant in a ratio of 1 part traced corrosion inhibitor to 51.9 parts (by weight/weight) of denaturant.
- the mixture of denaturant and traced corrosion inhibitor would be added to the fuel ethanol and the dosages of denaturant and corrosion inhibitor would both be monitored and/or controlled based on the fluorescent tracer signal.
- Results in Tables 6A to 6C show dosage of traced corrosion inhibitor added to denaturant during three phases of dosage monitoring and/or control of addition of that mixture: (A) prior to any changes in corrosion inhibitor and denaturant dosing procedure with manual addition of corrosion inhibitor, (B) with direct measurement of traced corrosion inhibitor and denaturant (by tracer fluorescence) and with manual addition of corrosion inhibitor, and (C) automatic control of corrosion inhibitor and denaturant dosages based on fluorescence measurements of the traced corrosion inhibitor plus denaturant mixture being added to fuel ethanol.
- a hand-held fluorometer calibrated for use with Rhodamine B base (CAS No. 509-34-2) inert fluorescent tracer was tested. The test was performed with Nalco EC 5624A containing 0.008 wt % Rhodamine B base, which yields 4.32 ppb Rhodamine B base when the corrosion inhibitor composition was dosed the recommended treatment rate of 54 ppm. A calculated amount of the traced corrosion inhibitor composition was added to a volume of fuel ethanol to give a final concentration of 54 ppm the corrosion inhibitor. Ten samples were independently tested to ascertain repeatability. Results presented in Table 7 below. The average determined concentration of the corrosion inhibitor was 53.6 ppm ⁇ 0.8 ppm (at ⁇ 3 SIGMA), which translates to 4.29 ⁇ 0.06 ppb of Rhodamine B base.
- FIG. 2 illustrates the linearity and predictability of fluorescence where Rhodamine B base was used as the inert fluorescent tracer (about 0.006% wt/wt) in Corrosion Inhibitor B and added to fuel ethanol.
- Excitation wavelength was 540 nm and emission wavelength was 560 nm.
- Table 8 illustrates a nonexhaustive list various excitation and emission wavelength ranges that may be used in the method of the invention.
- the list encompasses all of the described fluorescent components of the additive composition including an inherent or intrinsic fluorescent component, an inert fluorescent tracer, or a component that is reacted to become fluorescent.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Physics & Mathematics (AREA)
- Pathology (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Optics & Photonics (AREA)
- Liquid Carbonaceous Fuels (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
Abstract
Description
- This invention relates generally to methods of monitoring and/or controlling additive composition dosages in fuel ethanol. More specifically, the invention relates to monitoring and optimizing dosages of additive compositions including corrosion inhibitors, combinations of different corrosion inhibitors, denaturants, and mixtures of corrosion inhibitor(s) and denaturants in fuel ethanol. The invention has particular relevance to monitoring such dosages using fluorescence signals from one or more components in the additive composition.
- Fuel ethanol production in the U.S. increased by about 440% during the period from 1996 to 2007 (from 1.1 to 6.5 billion gallons per year) and world ethanol production reached about 13.1 billion gallons per year in 2007. Fuel ethanol plants under construction/expansion are expected to double current U.S. production capacity, and legislation has been passed that could increase fuel ethanol demand by more than 600% by 2022.
- Two most commonly used types of additives in fuel ethanol include denaturants and corrosion inhibitors, the use of which is growing concomitantly with the growth in fuel ethanol production. Inaccurate dosing of such additives can create a multitude of problems, including noncompliance with ASTM D-4806. For example, underdosing of corrosion inhibitor can lead to corrosion problems, whereas overdosing wastes chemicals and causes higher production costs. High dosages of some fuel ethanol corrosion inhibitors have also been linked to increases in intake valve deposits, which can cause substantial engine operational issues.
- Inaccurate dosing of denaturant causes significant government regulatory and legal problems. Releasing inaccurately dosed batches of fuel ethanol would likewise violate ASTM D-4806. Both underdosing and overdosing of denaturant leads to out-of-specification results that in turn lead to higher production/shipping costs and delays due to rework of batches.
- The maximum specification range currently allowed in the U.S. for denaturant is typically about 1.96 to 4.76% by volume. Due to the cost differential between ethanol and denaturant, it is valuable for a fuel ethanol plant to have the ability to be as close as possible to the upper or lower edge of denaturant dosage specification range. When ethanol costs exceed denaturant costs, for instance, it is desirable for the fuel ethanol plant to be at the high dosage edge of denaturant specification range to keep production costs to a minimum. On the other hand, when denaturant costs more than ethanol, it is desirable for the fuel ethanol plant to be at the low dosage edge of denaturant specification range.
- To operate near either edge of the additive dosage specification range requires highly accurate and precise measuring/dosing of additive concentration. Presently, fuel ethanol plants tend to dose additives via splash blending and/or based on how “long” a chemical feed pump is “on” with a “constant flowrate assumed” or sometimes based on flowmeters or depth gauges. Even when such flowmeters are regularly and properly calibrated, proper dosage rates are not always achieved. Very rarely (if ever) is dosage of ethanol additives directly measured. Also, batch-to-batch variations and the complex chemical nature of ethanol additives increase difficulty of precisely and accurately measuring additive dosages with currently used methods.
- There thus exists an ongoing need to develop methods of accurately and efficiently monitoring and controlling additive concentrations in fuel ethanol production plants. Such methods would allow the fuel ethanol producer to easily minimize costs of production by adjusting formulations based upon raw material costs and to maximize the quality and value of the fuel ethanol product.
- This invention accordingly includes methods of monitoring and optimizing dosage of one or more fuel ethanol additives by measuring a fluorescent signal. Such measurements are taken, for example, from one or more components of an additive composition, a derivative of a component in the additive, and/or from an inert tracer used in conjunction with or as part of the additive to provide an indication of dosage concentration. It is contemplated that the described method may be applied to any additive for fuel ethanol. In a preferred embodiment, the method is applied to measuring and controlling dosages of denaturants and/or corrosion inhibitors. Such monitoring and control may be directed to additives present in or added to the fuel ethanol. Depending upon whether a denaturant or corrosion inhibitor is the traced additive (and the particular chemistry used), the chosen method of measuring the fluorescent signal may be different. Alternative methods of measuring additive concentrations include, for example, an additive having an intrinsically fluorescent component, adding an inert fluorescent tracer (monitoring/control on-line or by grab sample), or adding a fluorometric or colorimetric reagent that reacts with one of the components of the additive formulation (grab sample). Certain limitations and extensions of these alternatives are explained in more detail below.
- It is an advantage of the invention to provide an easy, accurate, and precise method to measure additive dosages in fuel ethanol and to definitively adjust the dosage setpoint as needed.
- It is another advantage of the invention to provide methods of controlling additive dosages at fuel ethanol manufacturing plants thereby significantly reducing operating costs by preventing inaccurate dosing of treatment chemicals.
- An additional advantage of the invention is to enable fuel ethanol producers to include certificates of analysis with respect to additive dosage for each fuel ethanol shipment.
- It is also an advantage of the invention to provide accurate measurements of additive dosages in fuel ethanol for compliance with government regulations.
- A further advantage of the invention is to provide a versatile method of monitoring and controlling additive dosages in fuel ethanol that could be used in both a grab sample analysis scheme and/or adapted to online dosage control with datalogging capabilities.
- Another advantage of the invention is to provide a method of compensating for changes in fuel ethanol system characteristics by adjusting additive dosage.
- Yet another advantage of the invention is to provide methods of controlling additive dosages at fuel ethanol manufacturing plants to eliminate the possibility of out-of-specification product batches and prevent costly reworking of batches to achieve specification and/or government compliance.
- Additional features and advantages are described herein, and will be apparent from, the following Detailed Description, Examples, and Figures.
-
FIG. 1 shows the ability to measure intrinsic fluorescence of components in two different commercially available fuel ethanol corrosion inhibitors, illustrated as contour fluorescence spectra. -
FIG. 2 illustrates the linearity and predictability of fluorescence in a preferred embodiment where an inert fluorescent tracer was added in a corrosion inhibitor composition added to fuel ethanol, as explained in Example 8. - In preferred embodiments, the invention includes methods of monitoring, regulating, and/or optimizing the concentration of an additive composition in a fuel ethanol using a fluorescent signal generated from a component in the additive composition. The disclosed method of this invention is suitable for all manner of fuel ethanol production and is compatible with essentially all grades of fuel ethanol mixtures. The method is particularly well suited for use in conjunction with a variety of fuel ethanol additives. Application of the method begins in the production process where additives including denaturants and/or corrosion inhibitors are typically added, and may also be implemented at any stage of the packaging and shipping process. The described method is equally applicable to various sampling techniques including grab samples, sidestream and inline measurements, and measurements taken from a bulk container or vessel.
- It should be appreciated that the method, in certain embodiments, may be combined with other utilities known in the ethanol industry. Representative utilities include sensors for measuring alcohol content in, for example, gasoline; sensors for determining fuel composition; individual alcohol concentration sensors (e.g., methanol, ethanol); alcohol/gasoline ratio sensors; dissolved or particulate contaminant sensors; other sensors based upon resistance, capacitance, spectroscopic absorbance, calorimetric measurements, and fluorescence; and mathematical tools for analyzing sensor/controller results (e.g., multivariate analysis, chemometrics, on/off dosage control, PID dosage control, the like, and combinations thereof).
- In addition to solvents, stabilizers, and other components, the additive composition typically has a corrosion inhibitor, denaturant, or a mixture of both. The additive may also be a neat product or a mixture of two or more additives. It should be appreciated that the additive composition may include any number of compounds or components. Although such additives are most commonly a corrosion inhibitor or denaturant (or combination), as explained above, the described method is equally suited to any additive composition in used in fuel ethanol. Executing the method involves adding a known amount of the additive composition to the fuel ethanol to create a treated fuel ethanol. The added amount is calculated to provide an optimum concentration range for the additive composition in the treated fuel ethanol.
- One limitation is that the intrinsic fluorescence of different batches of denaturants has significantly variable fluorescence intensities and peak shapes. Though technically feasible, it is not a preferred method of the invention to use the inherent or intrinsic fluorescence of denaturant additives for monitoring and optimizing the dosage of fuel ethanol additives unless the denaturant has a consistent chemical composition. The intrinsic fluorescence of corrosion inhibitor formulations, however, is acceptable when properly implemented and is a preferred embodiment of the invention. Methods utilizing fluorescent tracers have equal efficacy in all types of additive formulations.
- In an embodiment, the additive composition includes a corrosion inhibitor. It is contemplated that the described method is operable with any corrosion inhibitor used for fuel ethanol. For example, a corrosion inhibitors containing compounds such as organic acid anhydrides; monomer, dimer, and/or trimer organic fatty acid mixtures; and tertiary organic amines may be used. Corrosion inhibitors also typically include a mixture of one or more of the following: organic (cyclohexyl-containing) amine; monomer, dimer, and/or trimer organic fatty acids including synthetics; organic acid anhydride; and organic solvents such as alcohol, xylenes, or other hydrocarbon-based solvent. The optimum concentration range for corrosion inhibitor products is typically in the ppm range (see Examples), although this range may be above or below the optimum target dosage for certain applications. It should be appreciated that the described method is applicable for use with any corrosion inhibiting composition.
- In another embodiment, the additive composition includes a denaturant. Typical denaturants include condensates from natural gas condensate, which may include gasoline, methanol, straight-chain hydrocarbons, naphthenes, aromatics, and others. It should be appreciated that any denaturant known in the art may be used with the method of the invention.
- The additive composition includes at least one component that is either inherently capable of providing a fluorescent signal or capable of being chemically derivatized or functionalized to provide a fluorescent signal. Fluorescence behavior has been found to be markedly and unexpectedly different in ethanol than in aqueous, ethanol-free solutions or low polarity hydrocarbon-containing solutions. Intensive testing and experimentation was required to ascertain effective fluorescing molecules and moieties in ethanol-containing systems (see Example 2).
- In one embodiment, a component that is normally a part of a conventional additive composition is inherently fluorescent. Such an additive is, for example, a corrosion inhibitor composition having a solvent containing an aromatic hydrocarbon. Xylene, other aromatic hydrocarbons, and functionalized aromatic hydrocarbons are inherently fluorescent and its fluorescent signal may be used as an analytical signal to determine the concentration of the additive composition in a treated fuel ethanol.
FIG. 1 illustrates two different commercially used corrosion inhibitors that exhibit such inherent fluorescence and fluorescence excitation/emission wavelength combinations where the intrinsic fluorescence corrosion inhibitor is different from the intrinsic fluorescence of denaturant, contaminants, etc. - Alternatively, a component that does not inherently provide an analytical signal but that is normally part of the additive composition can be chosen for modification, such as in a grab sample or sidestream taken from the system. This component is derivatized or functionalized with a moiety that imparts that ability to provide a fluorescent signal or a colorimetric signal. A compound in a corrosion inhibitor composition may chemically be derivatized with a fluorescent moiety or reacted to provide a calorimetric signal. According to an embodiment, a component of the corrosion inhibitor (e.g., tertiary organic amine) may be reacted with aromatic carbonyl chloride (Ar—COCl) in a grab sample analysis method to measure the amount of corrosion inhibitor present in the fuel ethanol at any given point (See Coppex, L., Derivatives for HPLC analysis, November 1999 to February 2000). Derivatization agents can be used to react with any component of the fuel ethanol corrosion inhibitor to utilize a fluorescent analysis technique. For example, 4-bromomethyl-7-methoxy-coumarin can react with carboxylic acids to form a fluorescent derivative (See W. Dunges, in “Analytical Chemistry,” vol. 49, p. 442, 1977). In another example, carboxylic acids (e.g., dimer fatty acids) in a corrosion inhibitor may be reacted to produce a colorimetric signal. A representative carboxylic acid reagent is p-bromophenacyl bromide (PBPB). (See Durst et al., in “Analytical Chemistry,” vol. 47, p. 1747, 1975).
- In a preferred embodiment, an inert fluorescent tracer is included in the additive composition. A known proportion of the fluorescent tracer is added either simultaneously or sequentially with the additive composition. Preferably, the inert fluorescent tracer is added first to the additive composition and the tracer-containing additive is then combined with the fuel ethanol or combined with another additive (e.g., traced corrosion inhibitor formulation combined with denaturant), which combination is added to the fuel ethanol.
- Effective inert fluorescent tracers include those substances that are chemically non-reactive with other components in the fuel ethanol and that do not significantly degrade with time. Such tracers should also be completely (or essentially completely) soluble in the additive formulation, mixtures of additives, and mixtures of additive(s) and fuel ethanol at all relevant levels of concentration and preferably the fluorescence intensity should be substantially proportional to its concentration and not significantly quenched or otherwise diminished by the fuel ethanol or other components in the fuel ethanol. Furthermore, the inert fluorescent tracer should not be appreciably or significantly affected by any other chemistry in fuel ethanol. The statement, “not appreciably or significantly affected,” means that an inert fluorescent compound generally has no more than about a 10% change in its fluorescent signal, under conditions normally encountered in fuel ethanol.
- Desired characteristics for an inert fluorescent tracer preferably include: fluorescence excitation/emission wavelengths that do not have significant overlap with light absorbing substances in the fuel ethanol, other additives, contaminants, etc.; high solubility in an additive (and combinations of additives) and additive(s) combination with fuel ethanol; excellent chemical stability; suitable fluorescence properties at manageable wavelengths (e.g., other additives in the fuel ethanol should not interfere with the fluorescence properties at those wavelengths) and excitation/emission wavelengths that are separate from other fluorescent components in the fuel ethanol and additive mixtures to prevent interference; chemical composition typically containing only C, H, N, O, and/or S (where S content of fuel ethanol<15 ppm of total composition, and avoiding “S” if possible); and avoiding negative impacts on fuel properties.
- Furthermore, ideal inert fluorescent tracers would: not be significantly impacted by surrounding temperature or pressure; presence of water or other solvents; have acceptably low light absorbance; lack metal ions, phosphorous, and halides; not be impacted by changes in the composition of other additives or contaminants (e.g., butanol); should not adversely alter performance of additives, such as pH buffering ability of corrosion inhibitor; sufficiently burned when fuel ethanol mixtures are used in internal combustion engines; and not cause deposits, fouling, corrosion, etc. in downstream applications.
- Representative inert fluorescent tracers that do not have metal ion/halide counterions or halide functional groups include fluorescein or fluorescein derivatives; rhodamine or rhodamine derivatives; naphthalene sulfonic acids (mono-, di-, tri-, etc.); pyrene sulfonic acids (mono-, di-, tri-, tetra-, etc.); stilbene derivatives containing sulfonic acids (including optical brighteners); biphenyl sulfonic acids; phenylalanine; tryptophan; tyrosine; vitamin B2 (riboflavin); vitamin B6 (pyridoxin); vitamin E (α-tocopherols); ethoxyquin; caffeine; vanillin; naphthalene sulfonic acid formaldehyde condensation polymers; phenyl sulfonic acid formaldehyde condensates; lignin sulfonic acids; polycyclic aromatic hydrocarbons; aromatic (poly)cyclic hydrocarbons containing amine, phenol, sulfonic acid, carboxylic acid functionalities in any combination; (poly)heterocyclic aromatic hydrocarbons having N, O, or S; a polymer containing at least one of the following moieties: naphthalene sulfonic acids, pyrene sulfonic acids, biphenyl sulfonic acids, or stilbene sulfonic acids. Additional examples of such inert fluorescent tracers may be found in U.S. Pat. No. 6,966,213 B2, entitled “Rapid Method for Detecting Leaks of Hydraulic Fluids in Production Plants” and U.S. Pat. No. 7,169,236 B2, entitled “Method of Monitoring Membrane Cleaning Process.” These inert fluorescent tracers are either commercially available under the tradename TRASAR® from Nalco Company® (Naperville, Ill.) or may be synthesized using techniques known to persons of ordinary skill in the art of organic chemistry.
- It should be appreciated that the process of selecting an inert fluorescent tracer, such as a polyaromatic hydrocarbon (“PAH”), requires substantial experimentation to determine those PAHs suitable for use as an inert fluorescent tracer. It was unexpectedly found that some PAHs (containing solubilizing groups, such as sulfonates (e.g., 1,3,6,8-pyrene tetrasulfonic acid)) that are effective as fluorescent tracers in aqueous systems, the corresponding PAHs (e.g., pyrene) is unfavorable in fuel ethanol formulations due to weak or no fluorescence or encountered high background fluorescence from other PAHs such as naphthalene, chrysene, and certain other 2 to 4 aromatic ring PAHs. Anthracene and perylene, for example, showed strong fluorescence in fuel ethanol systems, 3 and 5 aromatic ring PAHs, respectively.
- Regardless of which of the described fluorescent methods is used, a working curve for the particular fluorophore chosen, such as that shown in
FIG. 2 , should be developed. Similar curves can be readily created for any desired fluorophore when the fluorescence analysis conditions (for example, excitation and emission wavelength) are defined. The present fluorometric method requires the selection of an excitation wavelength to activate the fluorescence process and an emission wavelength at which fluorescence intensity is to be measured, which preferably is substantially free of interference from other species present in the fuel ethanol being monitored. Undesirable interference may be encountered when one or more other species have significant fluorescence emission or light absorbance at about the excitation/emission wavelengths selected for monitoring the chosen fluorophore. The excitation wavelength is chosen to also prevent photodegradation from occurring. - The background fluorescent signal may be measured in the treated fuel ethanol at any point subsequent to adding the additive composition. “Treated fuel ethanol” refers to fuel ethanol including the additive composition as herein described.
- In alternative embodiments, the fluorescent signal is acquired at one, two, or more points.
- In a preferred embodiment, the fluorescent signal is acquired online, either continuously or intermittently. Such online measurements may be analyzed in real-time or with a user-defined or other delay. For example, online measurements may take place by using a side-stream, inline, or other suitable flow-through device.
- In another embodiment, a sample of treated fuel ethanol is removed, either automatically or manually, and the fluorescent signal is acquired from the removed sample.
- Based upon the fluorescent signal, the total or component concentration of the additive composition may be determined. Three possible scenarios exist for the outcome of this determination. The first is that the concentration of the additive composition is within the optimum concentration range. In this instance, no further action would be taken. In the event the determined concentration of the additive composition is higher than the optimum concentration range, the treated fuel ethanol would optionally be diluted with a known additional volume of fuel ethanol. The additional volume would be calculated to bring the concentration of the additive composition into the optimum concentration range. If the determined concentration of the additive composition is below the optimum concentration range, an additional amount of the additive composition would optionally be introduced into the treated fuel ethanol in an amount calculated to bring the concentration of the additive composition into the optimum concentration range. The method of the invention may optionally be repeated (e.g., in an iterative fashion) until the determined concentration of the additive composition is within the optimum concentration range (or another chosen concentration range, such as a user-selected concentration range).
- Fuel ethanol (usually approximately E95) is typically mixed with gasoline to form ethanol-containing gasolines, such as E10 and E85. For example, an E10 formulation generally includes about 9.5 to 9.8% vol/vol ethanol, about 0.2% to 0.5% vol/vol denaturant, and about 90% vol/vol gasoline. The described method is equally applicable in such fuel ethanol compositions, including determining the total ethanol content in an alternative embodiment.
- A manual operator or an electronic device having components such as a processor, memory device, digital storage medium, cathode ray tube, liquid crystal display, plasma display, touch screen, or other monitor, and/or other components may be used to execute all or parts of the described method. In certain instances, the controller may be operable for integration with one or more application-specific integrated circuits, programs, computer-executable instructions, or algorithms, one or more hard-wired devices, wireless devices, and/or one or more mechanical devices. Some or all of the controller system functions may be at a central location, such as a network server, for communication over a local area network, wide area network, wireless network, Internet connection, microwave link, infrared link, and the like. In addition, other components such as a signal conditioner or system monitor may be included to facilitate signal-processing algorithms. It is also contemplated that any needed sensors, couplers, connectors, or other data measuring/transmitting/communicating equipment may be used to capture and transmit data.
- The foregoing description may be better understood by reference to the following examples, which are intended for illustrative purposes and are not intended to limit the scope of the invention.
- To demonstrate corrosion inhibitor concentration variability, a series of 40 samples were collected from seven different fuel ethanol manufacturing plants (designated as “Source” in Table 1), where corrosion inhibitor was being dosed by manual addition, such as splash addition or other indirectly measured methods. The dosages of corrosion inhibitor were measured by fluorescence measurement of the inherent fluorescence of corrosion inhibitor formulation. Table 1 summarizes the average, maximum and minimum dosages, and variability in the corrosion inhibitor dosages measured due to all sources of variability. The recommended “target dosage” of corrosion inhibitor is often 72 ppm (weight/weight) or 20 pounds per thousand barrels of ethanol (“PTBE”) for several commercially used products listed in Renewal Fuels Association list of corrosion inhibitor products. Table 2 illustrates industry recommended treatment rates for several commercially available corrosion inhibitors, where 1 PTBE=3.59 ppm or 20 PTBE=72 ppm (see Renewable Fuels Association Memorandum, entitled “Corrosion Inhibitor in Fuel Ethanol, Industry Guidelines, Specifications, and Procedures,” published Sep. 10, 2007). Each product is a trademark of the respective owner.
- Dosage results in Table 1 are listed as ppm. The variability in dosage is given as ±3 SIGMA and as % deviation from average, which is based upon an assumption of a statistically normal distribution. The results from the 40 samples are that overall average dosage (51 ppm) is significantly below the recommended target dosage of 72 ppm. The average dosages in Table 1 are typically significantly below the recommended target dosage with many samples being much below (and some samples being significantly above) the recommended target dosage.
- The variability ±3 SIGMA or 99.7% probability that readings will occur in a range from “average+3 SIGMA” to “average−3 SIGMA” was unacceptably high in each case, indicating that dosage control was poor in the systems surveyed. The higher the ±3 SIGMA value (expressed as % of “Avg ppm”), the more variable the readings are and the poorer the dosage control. These results demonstrate the significant industry need for more accurate corrosion inhibitor dosage control than currently exists.
-
TABLE 1 Variability % Variability # of Max Min Avg (in ppm) as (±3 SIGMA) Source Samples ppm ppm ppm ±3 SIGMA relative to Avg A 4 29 20 24 ±14 ±58% B 7 55 4 23 ±59 ±256% C 14 82 27 52 ±55 ±106% D 3 176 53 94 ±212 ±225 E 2 100 84 92 N/A N/A F 3 150 45 89 ±55 ±62 G 1 30 N/A N/A N/A N/A H 6 19 89 49 ±77 ±157% Overall 40 176 4 51 ±106 ±208 -
TABLE 2 Dosage Dosage Additive (PTBE) (ppm) Innospec Octel DCI-11 20 72 Petrolite Tolad 3222 20 72 Petrolite Tolad 3224 13 47 Nalco 5403 30 108 ENDCOR FE-9730 20 72 (formerly Betz CAN 13) MidContinental MCC5011E 20 72 MidContinental MCC5011EW 27 97 US Water CorrPro 654 13 47 Nalco EC 5624A 15 54 Afton Chemical Bio Tec 9880 10 36 Lubrizol LZ 541 16 57 US Water CorrPro 656 13 47 - An independent method based on a tertiary amine component of the corrosion inhibitor formulation also confirmed a high level of product dosage variability with a significant number of samples much higher or much lower than the recommended target dosage rate.
- Examples 2 to 5 illustrate the differences between current methods of adjusting additive dosages; direct manual measurement of traced corrosion inhibitor, either with or without providing a measurement for added denaturant (by tracer fluorescence); and automatic control of corrosion inhibitor dosage, either with or without providing a measurement for added denaturant, based on fluorescence measurements of traced corrosion inhibitor being added to fuel ethanol. In each of these examples, it can be seen that fluorescence of tracer added to corrosion inhibitor product to measure corrosion inhibitor dosage could significantly improve accuracy and reduce variability. Manual adjustment of product dosage after measuring of corrosion inhibitor concentration would provide for improved additive dosage accuracy and reduced variability in final treated fuel ethanol. Online monitoring/control of corrosion inhibitor dosage would result in further improved accuracy and reduced variability in concentration levels. The predicted variability is shown as ±3 SIGMA and based on assumption that a statistically normal distribution would occur.
- To illustrate corrosion inhibitor dosage monitoring and/or control by inherent fluorescence of a component in an additive formulation, a corrosion inhibitor may initially be added by the plant to series of batches of fuel ethanol using a “splash addition” method (standard industry practice). The estimated volume of corrosion inhibitor to be added is typically based on the estimated volume of fuel ethanol in the storage tank. The theoretical results in Table 3 shows dosage of corrosion inhibitor during three phases of dosage monitoring and/or control.
Batch numbers 1 to 5 illustrate dosage prior to any changes in corrosion inhibitor dosing procedure (i.e., manual addition with no measurement during addition of corrosion inhibitor); 6 to 10 show improved results with direct measurement of corrosion inhibitor (by inherent fluorescence) and manual addition/adjustment of corrosion inhibitor based on measurement of corrosion inhibitor; and 11 to 15 exemplify further improvement in results (average closer to target dosage and lower ±SIGMA value) due to automatic measurement and dosage control of corrosion inhibitor dosage based on inherent fluorescence measurements of the corrosion inhibitor being added to fuel ethanol. The target dosage of corrosion inhibitor is 72 ppm in fuel ethanol mixture for this Example. Predicted variability is shown as ±3 SIGMA and based on assumption that a statistically normal distribution occurs. -
TABLE 3 Manual addition w/out Manual addition/ Automatic measurement adjustment with measurement and during addition measurement dosage control Dosage during addition Dosage Batch # (ppm) Batch # Dosage (ppm) Batch # (ppm) 1 50 6 77 11 74 2 32 7 79 12 75 3 100 8 76 13 72 4 71 9 71 14 74 5 25 10 67 15 70 Avg. ± 3 56 ± 92 Avg. ± 3 74 ± 15 Avg. ± 3 73 ± 6 SIGMA SIGMA SIGMA - To show corrosion inhibitor dosage monitoring and/or control by addition of a fluorescent tracer to an additive formulation, a small known amount of fluorescent tracer could be added into corrosion inhibitor formulation during its manufacture. Traced corrosion inhibitor may initially be added by the plant to series of batches of fuel ethanol using a “splash addition” method. Estimated volume of corrosion inhibitor to be added is typically based on estimated volume of fuel ethanol in storage tank. Results in Table 4 show theoretical dosage of traced corrosion inhibitor during three phases of dosage monitoring and/or control.
Batch numbers 1 to 5 show results prior to any changes in corrosion inhibitor dosing procedure using a manual addition method and no measurement; 6 to 10 illustrate improved dosage using direct measurement of traced corrosion inhibitor (by tracer fluorescence) and manual addition/adjustment of corrosion inhibitor; and 11 to 15 exemplify further improvement in results due to automatic control of corrosion inhibitor dosage based on fluorescence measurements of traced corrosion inhibitor being added to fuel ethanol. Target dosage of traced corrosion inhibitor is 72 ppm in fuel ethanol mixture in this Example. -
TABLE 4 Manual Manual addition w/out addition/adjustment measurement with measurement Automatic during during measurement and addition addition dosage control Dosage Dosage Dosage Batch # (ppm) Batch # (ppm) Batch # (ppm) 1 25 6 67 11 73 2 32 7 74 12 71 3 85 8 71 13 70 4 54 9 73 14 72 5 104 10 68 15 73 Avg. ± 3 60 ± 102 Avg. ± 3 71 ± 9 Avg. ± 3 72 ± 4 SIGMA SIGMA SIGMA - The use of fluorescent traced corrosion inhibitor also allows a ready means to identify that the correct additive was mixed with the fuel ethanol. If the fluorescence signal of the traced corrosion inhibitor is absent or at significantly reduced level in the treated fuel ethanol, that measurement demonstrates: (i) an incorrect corrosion inhibitor product was used; (ii) that the treated fuel ethanol was diluted with an untraced corrosion inhibitor; (iii) that the batch of treated fuel ethanol was mixed with another batch of fuel ethanol that was treated with an untraced or incorrect corrosion inhibitor; or (iv) that batches of fuel ethanol that were correctly and incorrectly treated were mixed.
- Fluorescent tracer may be added to corrosion inhibitor and then the traced corrosion inhibitor may be mixed into denaturant at a prescribed dosage to provide monitoring and/or control of denaturant and traced corrosion inhibitor dosage. Under current legal standards, denaturant can typically be added from about 1.96% up to about 4.76% volume/volume (or about 1.63% to about 3.98% weight/weight) into fuel ethanol, depending on the locality of fuel ethanol manufacture. If the target dosage for corrosion inhibitor was 72 ppm (or 0.072% weight/weight) and denaturant was 2.20% volume/volume (1.83% weight/weight), the traced corrosion inhibitor may be added to denaturant in a ratio of 1 part traced corrosion inhibitor to 25.4 parts (by weight/weight) of denaturant. The mixture of denaturant and traced corrosion inhibitor may then be added to the fuel ethanol and the dosages of denaturant and corrosion inhibitor can both be monitored and/or controlled based on the fluorescent tracer signal.
- Results in Table 5A to 5C show theoretical dosage of traced corrosion inhibitor and denaturant during three phases of dosage monitoring and/or control: (A) prior to any changes in corrosion inhibitor and denaturant dosing procedure with manual dosage control, (B) with direct measurement of traced corrosion inhibitor and denaturant (by tracer fluorescence) and with manual corrosion inhibitor addition, and (C) automatic control of corrosion inhibitor and denaturant dosages based on fluorescence measurements of the traced corrosion inhibitor+denaturant mixture being added to fuel ethanol. Target dosage of corrosion inhibitor is typically 72 ppm and 2.20% volume/volume (or 1.84% weight/weight) denaturant to produce treated fuel ethanol.
-
TABLE 5A Manual Addition with Measurement Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 1 61 1.86 2 92 2.81 3 105 3.21 4 70 2.14 5 127 3.88 Avg. ± 3 91 ± 80 2.78 ± 2.44 SIGMA -
TABLE 5B Traced Additive with Manual Addition/Adjustment During Measurment Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 6 77 2.35 7 79 2.41 8 75 2.29 9 71 2.17 10 70 2.14 Avg. ± 3 74 ± 12 2.27 ± 0.35 SIGMA -
TABLE 5C Automated Measurement and Dosage Control Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 11 74 2.26 12 73 2.23 13 70 2.14 14 72 2.20 15 70 2.14 Avg. ± 3 72 ± 5 2.19 ± 0.16 SIGMA - The results above demonstrate that using fluorescence of traced corrosion inhibitor plus denaturant mixture to measure corrosion inhibitor and denaturant dosages can significantly improve accuracy and reduce variability in concentration of both additives. For example, it can be seen that
Batch # 1 in Table 5A has a denaturant vol % that is less than specification range of 1.96% to 4.76%, with a concomitantly low inhibitor dosage and overall high average dosage of denaturant and corrosion inhibitor and high variability in dosage of those two additions. That batch of treated ethanol would require additional denaturant plus traced fluorescent corrosion inhibitor mixture to meet specifications and regulatory/legal requirements. - In order to measure and/or control higher dosages of denaturant, the target dosage for fluorescent traced corrosion inhibitor can be increased, the level of traced fluorescent corrosion inhibitor can be increased in its mixture with denaturant, the level of corrosion inhibitor can be adjusted. In this scenario, fluorescent tracer would be added to corrosion inhibitor and then the traced corrosion inhibitor mixed into denaturant at a prescribed dosage to provide monitoring and/or control of higher dosages of denaturant and traced corrosion inhibitor dosage. Current legal guidelines allow for a denaturant range from 1.96% up to 4.76% on a volume/volume basis (or 1.63% to 3.98% weight/weight) into fuel ethanol, depending on the locality of fuel ethanol manufacture. If the target dosage for corrosion inhibitor was 72 ppm (or 0.072% weight/weight) and denaturant was 4.50% volume/volume (3.74% weight/weight), then traced corrosion inhibitor would be added to denaturant in a ratio of 1 part traced corrosion inhibitor to 51.9 parts (by weight/weight) of denaturant. The mixture of denaturant and traced corrosion inhibitor would be added to the fuel ethanol and the dosages of denaturant and corrosion inhibitor would both be monitored and/or controlled based on the fluorescent tracer signal.
- Results in Tables 6A to 6C show dosage of traced corrosion inhibitor added to denaturant during three phases of dosage monitoring and/or control of addition of that mixture: (A) prior to any changes in corrosion inhibitor and denaturant dosing procedure with manual addition of corrosion inhibitor, (B) with direct measurement of traced corrosion inhibitor and denaturant (by tracer fluorescence) and with manual addition of corrosion inhibitor, and (C) automatic control of corrosion inhibitor and denaturant dosages based on fluorescence measurements of the traced corrosion inhibitor plus denaturant mixture being added to fuel ethanol.
-
TABLE 6A Manual Addition with Measurement Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 1 71 4.44 2 102 6.38 3 52 3.25 4 64 4.00 5 74 4.63 Avg. ± 3 73 ± 55 4.54 ± 3.47 SIGMA -
TABLE 6B Traced Additive with Manual Addition/Adjustment During Measurement Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 6 73 4.56 7 72 4.50 8 73 4.56 9 65 4.06 10 71 4.44 Avg. ± 3 71 ± 10 4.42 ± 0.63 SIGMA -
TABLE 6C Automatic Measurement and Dosage Control Batch # Corr. Inh. (ppm) Denat. (% vol/vol) 11 73 4.56 12 70 4.38 13 72 4.50 14 73 4.56 15 74 4.63 Avg. ± 3 72 ± 5 4.53 ± 0.28 SIGMA - It can be seen that the dosage for Batch #2 of Table 6A was outside of the 1.96% to 4.76% (volume/volume) specification and legal limit range for denaturant in fuel ethanol, as well as having a high corrosion inhibitor dosage. That batch of treated ethanol would require dilution with an additional volume of untreated fuel ethanol to meet specifications and regulatory/legal requirements.
- A hand-held fluorometer calibrated for use with Rhodamine B base (CAS No. 509-34-2) inert fluorescent tracer was tested. The test was performed with Nalco EC 5624A containing 0.008 wt % Rhodamine B base, which yields 4.32 ppb Rhodamine B base when the corrosion inhibitor composition was dosed the recommended treatment rate of 54 ppm. A calculated amount of the traced corrosion inhibitor composition was added to a volume of fuel ethanol to give a final concentration of 54 ppm the corrosion inhibitor. Ten samples were independently tested to ascertain repeatability. Results presented in Table 7 below. The average determined concentration of the corrosion inhibitor was 53.6 ppm±0.8 ppm (at ±3 SIGMA), which translates to 4.29±0.06 ppb of Rhodamine B base.
-
TABLE 7 Corrosion Inhibitor Sample # Dosage (ppm) Tracer (ppb) 1 53.9 4.31 2 53.8 4.30 3 53.4 4.27 4 54.0 4.32 5 53.5 4.28 6 53.7 4.30 7 53.2 4.26 8 53.7 4.30 9 53.3 4.26 10 53.5 4.28 Average ± 3 53.6 ± 0.8 4.29 ± 0.06 SIGMA (target = 54) (target = 4.32) -
FIG. 2 illustrates the linearity and predictability of fluorescence where Rhodamine B base was used as the inert fluorescent tracer (about 0.006% wt/wt) in Corrosion Inhibitor B and added to fuel ethanol. The test was conducted with a range of the corrosion inhibitor concentration from 0 to 300 ppm. Excellent linearity of response was observed R2=0.999, where 1.00=perfect linearity). Excitation wavelength was 540 nm and emission wavelength was 560 nm. - Table 8 below illustrates a nonexhaustive list various excitation and emission wavelength ranges that may be used in the method of the invention. The list encompasses all of the described fluorescent components of the additive composition including an inherent or intrinsic fluorescent component, an inert fluorescent tracer, or a component that is reacted to become fluorescent.
-
TABLE 8 Excitation Range Emission Range Ultraviolet light Ultraviolet light Ultraviolet light Visible light Visible light Visible light Visible light Near infrared light Near infrared light Near infrared light - It should be understood that various changes and modifications to the presently preferred embodiments described herein will be apparent to those skilled in the art. Such changes and modifications can be made without departing from the spirit and scope of the invention and without diminishing its intended advantages. It is therefore intended that such changes and modifications be covered by the appended claims.
Claims (19)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/143,400 US20090319195A1 (en) | 2008-06-20 | 2008-06-20 | Method of monitoring and optimizing additive concentration in fuel ethanol |
| ARP090102271A AR072270A1 (en) | 2008-06-20 | 2009-06-19 | METHOD OF MONITORING AND OPTIMIZATION OF THE CONCENTRATION OF ADDITIVES IN FUELS OF ETANOL |
| CA2724760A CA2724760C (en) | 2008-06-20 | 2009-06-19 | Method of monitoring and optimizing additive concentration in fuel ethanol |
| EP09767833.8A EP2304429B1 (en) | 2008-06-20 | 2009-06-19 | Method of monitoring and optimizing additive concentration in fuel ethanol |
| PCT/US2009/047964 WO2009155524A1 (en) | 2008-06-20 | 2009-06-19 | Method of monitoring and optimizing additive concentration in fuel ethanol |
| US14/679,575 US10436719B2 (en) | 2008-06-20 | 2015-04-06 | Method of monitoring and optimizing additive concentration in fuel ethanol |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/143,400 US20090319195A1 (en) | 2008-06-20 | 2008-06-20 | Method of monitoring and optimizing additive concentration in fuel ethanol |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/679,575 Continuation US10436719B2 (en) | 2008-06-20 | 2015-04-06 | Method of monitoring and optimizing additive concentration in fuel ethanol |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20090319195A1 true US20090319195A1 (en) | 2009-12-24 |
Family
ID=40983369
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/143,400 Abandoned US20090319195A1 (en) | 2008-06-20 | 2008-06-20 | Method of monitoring and optimizing additive concentration in fuel ethanol |
| US14/679,575 Active 2028-12-06 US10436719B2 (en) | 2008-06-20 | 2015-04-06 | Method of monitoring and optimizing additive concentration in fuel ethanol |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/679,575 Active 2028-12-06 US10436719B2 (en) | 2008-06-20 | 2015-04-06 | Method of monitoring and optimizing additive concentration in fuel ethanol |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US20090319195A1 (en) |
| EP (1) | EP2304429B1 (en) |
| AR (1) | AR072270A1 (en) |
| CA (1) | CA2724760C (en) |
| WO (1) | WO2009155524A1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090044532A1 (en) * | 2007-08-17 | 2009-02-19 | Gm Global Technology Operations, Inc. | Flexible fuel variable boost supercharged engine |
| WO2012050844A1 (en) * | 2010-09-28 | 2012-04-19 | Authentix, Inc. | Determining the quantity of a taggant in a liquid sample |
| FR2985316A1 (en) * | 2012-01-04 | 2013-07-05 | Rhodia Operations | Method for external diagnosis of malfunction of e.g. lubricant additive, additivation device in vehicle's diesel engine, involves analyzing variation between measured and theoretical additive contents with respect to maximum variation |
| WO2013150274A2 (en) | 2012-04-03 | 2013-10-10 | Formatex (Offshore) S.A.L. | A method of determining the suitability of a fuel for use in an engine and a composition for use in such a method |
| WO2013170119A1 (en) * | 2012-05-11 | 2013-11-14 | Knowflame, Inc. | System for measuring the concentration of an additive in a mixture |
| EP2758595A4 (en) * | 2011-09-23 | 2015-06-03 | Nalco Co | Fluorometric method for monitoring surface additives in a papermaking process |
| US20150355091A1 (en) * | 2010-09-28 | 2015-12-10 | Authentix, Inc. | Determining the Quantity of a Taggant in a Liquid Sample |
| US9541502B2 (en) | 2014-10-03 | 2017-01-10 | Formatex (Offshore) S.A.L. | Method of determining the suitability of a fuel for use in an engine and a composition for use in such a method |
| US20180320098A1 (en) * | 2015-11-30 | 2018-11-08 | Sabic Global Technologies B.V. | Process for enhancing gasoline octane boosters, gasoline boosters, and gasolines |
| EP3256693A4 (en) * | 2015-02-10 | 2018-12-19 | Ecolab USA Inc. | Corrosion inhibitors and kinetic hydrate inhibitors |
| US10436719B2 (en) | 2008-06-20 | 2019-10-08 | Ecolab Usa Inc. | Method of monitoring and optimizing additive concentration in fuel ethanol |
| WO2020229804A1 (en) | 2019-05-15 | 2020-11-19 | Innospec Limited | Compositions and methods and uses relating thereto |
| US10851725B2 (en) * | 2018-12-18 | 2020-12-01 | Caterpillar Inc. | Fuel content detection based on a measurement from a sensor and a model estimation of the measurement |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20220136968A1 (en) * | 2020-11-02 | 2022-05-05 | Afton Chemical Corporation | Methods of identifying a hydrocarbon fuel |
Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3413227A (en) * | 1963-12-06 | 1968-11-26 | Geigy Chem Corp | Compositions containing substituted benzotriazoles |
| US4406811A (en) * | 1980-01-16 | 1983-09-27 | Nalco Chemical Company | Composition and method for controlling corrosion in aqueous systems |
| US4426208A (en) * | 1981-11-02 | 1984-01-17 | Ethyl Corporation | Corrosion inhibitors for alcohol-based fuels |
| US4783314A (en) * | 1987-02-26 | 1988-11-08 | Nalco Chemical Company | Fluorescent tracers - chemical treatment monitors |
| US4994197A (en) * | 1989-12-18 | 1991-02-19 | Mobil Oil Corporation | Triazole compositions as fuel and lube additives |
| US5171450A (en) * | 1991-03-20 | 1992-12-15 | Nalco Chemical Company | Monitoring and dosage control of tagged polymers in cooling water systems |
| US5225679A (en) * | 1992-01-24 | 1993-07-06 | Boston Advanced Technologies, Inc. | Methods and apparatus for determining hydrocarbon fuel properties |
| US5278074A (en) * | 1992-04-22 | 1994-01-11 | Nalco Chemical Company | Method of monitoring and controlling corrosion inhibitor dosage in aqueous systems |
| US5702684A (en) * | 1994-05-02 | 1997-12-30 | Nalco Chemical Company | Method of use of compositions of biocides and fluorescent indicators to control microbial growth |
| US5843783A (en) * | 1994-11-04 | 1998-12-01 | Amoco Corporation | Tagging hydrocarbons for subsequent identification |
| US20010029701A1 (en) * | 1999-07-23 | 2001-10-18 | Quantum Energy Technologies | Sub-critical water-fuel composition and combustion system |
| US20030006385A1 (en) * | 2001-06-28 | 2003-01-09 | Banks Rodney H. | Mirror fluorometer |
| US20040157334A1 (en) * | 2002-08-12 | 2004-08-12 | Barashkov Nikolay N. | Method for determination of ethanol concentration in an aqueous solution containing an alcoholic beverage |
| US20050008532A1 (en) * | 2003-07-11 | 2005-01-13 | Jenkins Brian V. | Method of inhibiting corrosion of copper plated or metallized surfaces and circuitry during semiconductor manufacturing processes |
| US20050025660A1 (en) * | 2003-07-31 | 2005-02-03 | Hoots John E. | Method of tracing corrosive materials |
| US6966213B2 (en) * | 2004-01-28 | 2005-11-22 | Nalco Company | Rapid method for detecting leaks of hydraulic fluids in production plants |
| US6984340B1 (en) * | 1999-10-14 | 2006-01-10 | Brad-Chem Technology Limtied | Corrosion inhibiting formulations |
| US20060214112A1 (en) * | 2004-09-10 | 2006-09-28 | Bam Bundesanstalt Fuer Materialforschung Und - Pruefung | Method and kit for calibrating a photoluminescence measurement |
| US20090260454A1 (en) * | 2008-03-28 | 2009-10-22 | Young Paul R | Methods and compositions for inhibiting corrosion in non-aqueous, non-conductive liquids |
Family Cites Families (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5009799A (en) | 1988-02-16 | 1991-04-23 | Nalco Chemical Company | Inorganic acid solution viscosifier and corrosion inhibitor and method |
| US4992380A (en) | 1988-10-14 | 1991-02-12 | Nalco Chemical Company | Continuous on-stream monitoring of cooling tower water |
| US4964468A (en) | 1989-08-08 | 1990-10-23 | Nalco Chemical Company | Method of inhibiting corrosion |
| US5128419A (en) * | 1990-08-20 | 1992-07-07 | Nalco Chemical Company | Synthesis of tagged polymers by post-polymerization (trans) amidation reaction |
| US5358873A (en) * | 1992-07-27 | 1994-10-25 | Atlantic Richfield Company | Method for determining adulteration of gasolines |
| EP0703451A3 (en) * | 1994-09-26 | 1998-07-29 | Nalco Chemical Company | Monitoring process fluids in papermaking systems |
| US5744665A (en) | 1995-06-08 | 1998-04-28 | Exxon Production Research Company | Maleimide copolymers and method for inhibiting hydrate formation |
| ZA984976B (en) | 1997-06-11 | 1999-04-19 | Nalco Chemical Co | Solid-state fluorometer and methods of use therefore |
| US6866797B1 (en) | 2000-08-03 | 2005-03-15 | Bj Services Company | Corrosion inhibitors and methods of use |
| US6472219B1 (en) | 2000-08-31 | 2002-10-29 | Ondeo Nalco Company | Use of tracers to monitor application of treatment products to cut flowers |
| US7057050B2 (en) | 2003-04-11 | 2006-06-06 | Nalco Energy Services L.P. | Imidazoline corrosion inhibitors |
| GB0326928D0 (en) * | 2003-11-19 | 2003-12-24 | Johnson Matthey Plc | Apparatus and method for identifying a liquid product |
| US7311144B2 (en) | 2004-10-12 | 2007-12-25 | Greg Allen Conrad | Apparatus and method for increasing well production using surfactant injection |
| US20070204505A1 (en) * | 2006-03-02 | 2007-09-06 | Novus International Inc. | Gasoline fuel compositions having increased oxidative stability |
| US20080168708A1 (en) * | 2007-01-11 | 2008-07-17 | Cunningham Lawrence J | Method and compositions for reducing deposits in engines combusting ethanol-containing fuels and a corrosion inhibitor |
| US20080308770A1 (en) | 2007-06-14 | 2008-12-18 | Laxmikant Tiwari | Mono and bis-ester derivatives of pyridinium and quinolinium compounds as environmentally friendly corrosion inhibitors |
| US8551925B2 (en) | 2007-11-15 | 2013-10-08 | Nalco Company | Imidazoline-based heterocyclic foamers for downhole injection |
| US7951754B2 (en) | 2007-12-07 | 2011-05-31 | Nalco Company | Environmentally friendly bis-quaternary compounds for inhibiting corrosion and removing hydrocarbonaceous deposits in oil and gas applications |
| US20090319195A1 (en) | 2008-06-20 | 2009-12-24 | Hoots John E | Method of monitoring and optimizing additive concentration in fuel ethanol |
| US8105987B2 (en) | 2008-10-06 | 2012-01-31 | Nalco Company | Corrosion inhibitors for an aqueous medium |
| US8105988B2 (en) | 2008-10-06 | 2012-01-31 | Nalco Company | Corrosion inhibitors for a fluid |
| US8329620B2 (en) | 2008-10-06 | 2012-12-11 | Nalco Company | Compositions and methods for inhibiting the agglomeration of hydrates |
| US8334240B2 (en) | 2008-10-06 | 2012-12-18 | Nalco Company | Compositions and methods for inhibiting the agglomeration of hydrates in a process |
| US10392573B2 (en) | 2008-10-17 | 2019-08-27 | Ecolab Usa Inc. | Method of controlling gas hydrates in fluid systems |
| US8921478B2 (en) | 2008-10-17 | 2014-12-30 | Nalco Company | Method of controlling gas hydrates in fluid systems |
| US7989403B2 (en) | 2009-03-02 | 2011-08-02 | Nalco Company | Corrosion inhibitors containing amide surfactants for a fluid |
| US8288323B2 (en) | 2009-03-02 | 2012-10-16 | Nalco Company | Compositions containing amide surfactants and methods for inhibiting the formation of hydrate agglomerates |
| US8911615B2 (en) | 2010-04-08 | 2014-12-16 | William Marsh Rice University | Recovery and separation of crude oil and water from emulsions |
| US8618027B2 (en) | 2010-12-08 | 2013-12-31 | Nalco Company | Corrosion inhibitors for oil and gas applications |
| US8618025B2 (en) | 2010-12-16 | 2013-12-31 | Nalco Company | Composition and method for reducing hydrate agglomeration |
| US20150034319A1 (en) | 2013-07-31 | 2015-02-05 | Baker Hughes Incorporated | H2s scavengers with synergistic corrosion inhibition |
-
2008
- 2008-06-20 US US12/143,400 patent/US20090319195A1/en not_active Abandoned
-
2009
- 2009-06-19 AR ARP090102271A patent/AR072270A1/en active IP Right Grant
- 2009-06-19 EP EP09767833.8A patent/EP2304429B1/en active Active
- 2009-06-19 CA CA2724760A patent/CA2724760C/en active Active
- 2009-06-19 WO PCT/US2009/047964 patent/WO2009155524A1/en active Application Filing
-
2015
- 2015-04-06 US US14/679,575 patent/US10436719B2/en active Active
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3413227A (en) * | 1963-12-06 | 1968-11-26 | Geigy Chem Corp | Compositions containing substituted benzotriazoles |
| US4406811A (en) * | 1980-01-16 | 1983-09-27 | Nalco Chemical Company | Composition and method for controlling corrosion in aqueous systems |
| US4426208A (en) * | 1981-11-02 | 1984-01-17 | Ethyl Corporation | Corrosion inhibitors for alcohol-based fuels |
| US4783314A (en) * | 1987-02-26 | 1988-11-08 | Nalco Chemical Company | Fluorescent tracers - chemical treatment monitors |
| US4994197A (en) * | 1989-12-18 | 1991-02-19 | Mobil Oil Corporation | Triazole compositions as fuel and lube additives |
| US5171450A (en) * | 1991-03-20 | 1992-12-15 | Nalco Chemical Company | Monitoring and dosage control of tagged polymers in cooling water systems |
| US5225679A (en) * | 1992-01-24 | 1993-07-06 | Boston Advanced Technologies, Inc. | Methods and apparatus for determining hydrocarbon fuel properties |
| US5278074A (en) * | 1992-04-22 | 1994-01-11 | Nalco Chemical Company | Method of monitoring and controlling corrosion inhibitor dosage in aqueous systems |
| US5702684A (en) * | 1994-05-02 | 1997-12-30 | Nalco Chemical Company | Method of use of compositions of biocides and fluorescent indicators to control microbial growth |
| US5843783A (en) * | 1994-11-04 | 1998-12-01 | Amoco Corporation | Tagging hydrocarbons for subsequent identification |
| US20010029701A1 (en) * | 1999-07-23 | 2001-10-18 | Quantum Energy Technologies | Sub-critical water-fuel composition and combustion system |
| US6984340B1 (en) * | 1999-10-14 | 2006-01-10 | Brad-Chem Technology Limtied | Corrosion inhibiting formulations |
| US20030006385A1 (en) * | 2001-06-28 | 2003-01-09 | Banks Rodney H. | Mirror fluorometer |
| US20040157334A1 (en) * | 2002-08-12 | 2004-08-12 | Barashkov Nikolay N. | Method for determination of ethanol concentration in an aqueous solution containing an alcoholic beverage |
| US20050008532A1 (en) * | 2003-07-11 | 2005-01-13 | Jenkins Brian V. | Method of inhibiting corrosion of copper plated or metallized surfaces and circuitry during semiconductor manufacturing processes |
| US20050025660A1 (en) * | 2003-07-31 | 2005-02-03 | Hoots John E. | Method of tracing corrosive materials |
| US6966213B2 (en) * | 2004-01-28 | 2005-11-22 | Nalco Company | Rapid method for detecting leaks of hydraulic fluids in production plants |
| US20060214112A1 (en) * | 2004-09-10 | 2006-09-28 | Bam Bundesanstalt Fuer Materialforschung Und - Pruefung | Method and kit for calibrating a photoluminescence measurement |
| US20090260454A1 (en) * | 2008-03-28 | 2009-10-22 | Young Paul R | Methods and compositions for inhibiting corrosion in non-aqueous, non-conductive liquids |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8276549B2 (en) * | 2007-08-17 | 2012-10-02 | GM Global Technology Operations LLC | Flexible fuel variable boost supercharged engine |
| US20090044532A1 (en) * | 2007-08-17 | 2009-02-19 | Gm Global Technology Operations, Inc. | Flexible fuel variable boost supercharged engine |
| US10436719B2 (en) | 2008-06-20 | 2019-10-08 | Ecolab Usa Inc. | Method of monitoring and optimizing additive concentration in fuel ethanol |
| GB2497477B (en) * | 2010-09-28 | 2017-03-15 | Authentix Inc | Determining the quantity of a taggant in a liquid sample |
| WO2012050844A1 (en) * | 2010-09-28 | 2012-04-19 | Authentix, Inc. | Determining the quantity of a taggant in a liquid sample |
| GB2497477A (en) * | 2010-09-28 | 2013-06-12 | Authentix Inc | Determining the quantity of a taggant in a liquid sample |
| US9995681B2 (en) * | 2010-09-28 | 2018-06-12 | Authentix, Inc. | Determining the quantity of a taggant in a liquid sample |
| US20150355091A1 (en) * | 2010-09-28 | 2015-12-10 | Authentix, Inc. | Determining the Quantity of a Taggant in a Liquid Sample |
| EP2758595A4 (en) * | 2011-09-23 | 2015-06-03 | Nalco Co | Fluorometric method for monitoring surface additives in a papermaking process |
| FR2985316A1 (en) * | 2012-01-04 | 2013-07-05 | Rhodia Operations | Method for external diagnosis of malfunction of e.g. lubricant additive, additivation device in vehicle's diesel engine, involves analyzing variation between measured and theoretical additive contents with respect to maximum variation |
| WO2013150274A2 (en) | 2012-04-03 | 2013-10-10 | Formatex (Offshore) S.A.L. | A method of determining the suitability of a fuel for use in an engine and a composition for use in such a method |
| US9194858B2 (en) | 2012-05-11 | 2015-11-24 | Polaris Sensor Technologies, Inc. | System for measuring the concentration of an additive in a mixture |
| WO2013170119A1 (en) * | 2012-05-11 | 2013-11-14 | Knowflame, Inc. | System for measuring the concentration of an additive in a mixture |
| US9541502B2 (en) | 2014-10-03 | 2017-01-10 | Formatex (Offshore) S.A.L. | Method of determining the suitability of a fuel for use in an engine and a composition for use in such a method |
| EP3256693A4 (en) * | 2015-02-10 | 2018-12-19 | Ecolab USA Inc. | Corrosion inhibitors and kinetic hydrate inhibitors |
| US10466175B2 (en) | 2015-02-10 | 2019-11-05 | Ecolab Usa Inc. | Corrosion inhibitors and kinetic hydrate inhibitors |
| US11125690B2 (en) | 2015-02-10 | 2021-09-21 | Championx Usa Inc. | Corrosion inhibitors and kinetic hydrate inhibitors |
| US20180320098A1 (en) * | 2015-11-30 | 2018-11-08 | Sabic Global Technologies B.V. | Process for enhancing gasoline octane boosters, gasoline boosters, and gasolines |
| US10851725B2 (en) * | 2018-12-18 | 2020-12-01 | Caterpillar Inc. | Fuel content detection based on a measurement from a sensor and a model estimation of the measurement |
| WO2020229804A1 (en) | 2019-05-15 | 2020-11-19 | Innospec Limited | Compositions and methods and uses relating thereto |
| US11261389B2 (en) | 2019-05-15 | 2022-03-01 | Innospec Limited | Compositions and methods and uses relating thereto |
Also Published As
| Publication number | Publication date |
|---|---|
| AR072270A1 (en) | 2010-08-18 |
| US20150212007A1 (en) | 2015-07-30 |
| EP2304429B1 (en) | 2020-06-17 |
| CA2724760C (en) | 2018-02-27 |
| EP2304429A1 (en) | 2011-04-06 |
| CA2724760A1 (en) | 2009-12-23 |
| US10436719B2 (en) | 2019-10-08 |
| WO2009155524A1 (en) | 2009-12-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10436719B2 (en) | Method of monitoring and optimizing additive concentration in fuel ethanol | |
| CN103026207B (en) | The method of the chemicals in assessment produced fluid | |
| CN100390541C (en) | Methods for optimal usage and improved valuation of corrosive petroleum feedstocks and fractions | |
| BR112019015343B1 (en) | CONTROLLED BIODIESEL MIXING IN DISTILLATE STREAMS | |
| US20110069307A1 (en) | Dipyrromethenes and Azadipyrromethenes as Markers for Petroleum Products | |
| US20230324361A1 (en) | Elemental sulfur analysis in fluids | |
| CN103940770B (en) | Quantitative analysis method and determination method for emulsification performance of petroleum crude oil emulsification system | |
| US5397708A (en) | Method for detection of sulfides | |
| CN104964935A (en) | Oil-soluble identification agent and its application in fuel oil and fuel oil additives | |
| US20100101140A1 (en) | Method of monitoring and optimizing denaturant concentration in fuel ethanol | |
| Rossegger et al. | Lube Oil Consumption Measurement On Internal Combustion Engines | |
| CN111257251A (en) | Method for measuring potassium content | |
| US10351789B2 (en) | Method of improving the accuracy when quantifying fluorescence markers in fuels | |
| Pavlova et al. | Determination of total nitrogen content by different approaches in petroleum matrices | |
| US20200371028A1 (en) | Biocide detection | |
| CN1560607A (en) | Method for quickly detecting content of sodium content in juice and beverage | |
| RU2768892C2 (en) | Method for quantitative determination of multifunctional detergent additive in gasolines by infrared spectrometry with preliminary concentration | |
| US11474045B2 (en) | Method and device for the determination of film forming amines in a liquid | |
| Ibragimova et al. | Development of an Ultraviolet‐Spectrophotometric Method for Analysis of Esterquat‐Containing Flotation Collectors in Aqueous Solutions | |
| Lambert et al. | A review of oil-in-water monitoring techniques | |
| Lu et al. | Tracer Studies in Boiler Scale and Corrosion Control | |
| JPH0894520A (en) | Measuring method for cooling water system water treatment agent concentration and retaining water quantity by means of fluorescent material | |
| TO | CRC Report No. CM-136-15-1 | |
| CN116818726A (en) | Fluorescent instrument and method for measuring petroleum content in water | |
| Drobyshev et al. | X-ray fluorescence determination of sulfur and other elements in customs control of oil and liquid oil products |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: NALCO COMPANY, ILLINOIS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:HOOTS, JOHN E.;BUREMAN, PHILLIP E.;MYERS, CRIAG W.;REEL/FRAME:021129/0977;SIGNING DATES FROM 20080318 TO 20080619 |
|
| AS | Assignment |
Owner name: NALCO COMPANY, ILLINOIS Free format text: CORRECTIVE ASSIGNMENT TO CORRECT THE INVENTORS LISTED, BECAUSE OF A WRONGFUL SUBMISSION OF AN OLDER COPY OF THE ASSIGNMENT PREVIOUSLY RECORDED ON REEL 021129 FRAME 0977;ASSIGNORS:HOOTS, JOHN E.;BUREMAN, PHILLIP E.;MYERS, CRIAG W.;REEL/FRAME:021135/0256;SIGNING DATES FROM 20080619 TO 20080620 |
|
| AS | Assignment |
Owner name: BANK OF AMERICA, N.A., AS COLLATERAL AGENT,NEW YOR Free format text: SECURITY AGREEMENT;ASSIGNORS:NALCO COMPANY;CALGON LLC;NALCO ONE SOURCE LLC;AND OTHERS;REEL/FRAME:022703/0001 Effective date: 20090513 Owner name: BANK OF AMERICA, N.A., AS COLLATERAL AGENT, NEW YO Free format text: SECURITY AGREEMENT;ASSIGNORS:NALCO COMPANY;CALGON LLC;NALCO ONE SOURCE LLC;AND OTHERS;REEL/FRAME:022703/0001 Effective date: 20090513 |
|
| AS | Assignment |
Owner name: NALCO COMPANY, ILLINOIS Free format text: RELEASE BY SECURED PARTY;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:035771/0668 Effective date: 20111201 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION |
|
| AS | Assignment |
Owner name: NALCO COMPANY, ILLINOIS Free format text: RELEASE BY SECURED PARTY;ASSIGNOR:BANK OF AMERICA, N.A.;REEL/FRAME:041808/0713 Effective date: 20111201 |
|
| AS | Assignment |
Owner name: NALCO COMPANY LLC, DELAWARE Free format text: CHANGE OF NAME;ASSIGNOR:NALCO COMPANY;REEL/FRAME:041835/0903 Effective date: 20151229 Owner name: ECOLAB USA INC., MINNESOTA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:NALCO COMPANY LLC;CALGON CORPORATION;CALGON LLC;AND OTHERS;REEL/FRAME:041836/0437 Effective date: 20170227 |
|
| AS | Assignment |
Owner name: ECOLAB USA INC., MINNESOTA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:NALCO COMPANY;REEL/FRAME:042147/0420 Effective date: 20170227 |