TWI754619B - Composition for forming resin thin film - Google Patents
Composition for forming resin thin film Download PDFInfo
- Publication number
- TWI754619B TWI754619B TW105131993A TW105131993A TWI754619B TW I754619 B TWI754619 B TW I754619B TW 105131993 A TW105131993 A TW 105131993A TW 105131993 A TW105131993 A TW 105131993A TW I754619 B TWI754619 B TW I754619B
- Authority
- TW
- Taiwan
- Prior art keywords
- resin film
- formula
- composition
- polyimide
- same
- Prior art date
Links
- 0 Cc(c(*)c1)cc(C2(*)C(F)(F)F)c1Oc1c2cc(C)c(*)c1 Chemical compound Cc(c(*)c1)cc(C2(*)C(F)(F)F)c1Oc1c2cc(C)c(*)c1 0.000 description 8
- YUAYHGTVPRAFMU-UHFFFAOYSA-N Cc(c(C)c(c(Oc1c(c(C)c2C)F)c3Oc1c2F)F)c3F Chemical compound Cc(c(C)c(c(Oc1c(c(C)c2C)F)c3Oc1c2F)F)c3F YUAYHGTVPRAFMU-UHFFFAOYSA-N 0.000 description 1
- PJJVQODGNFEFBK-UHFFFAOYSA-N Cc(c(OC(F)(F)F)c1C)c(C)c(OC(F)(F)F)c1I Chemical compound Cc(c(OC(F)(F)F)c1C)c(C)c(OC(F)(F)F)c1I PJJVQODGNFEFBK-UHFFFAOYSA-N 0.000 description 1
- IKZZLUBYOKOJOQ-UHFFFAOYSA-N O=C(OC1=O)[BrH]1(C(O1)=O)C1=O Chemical compound O=C(OC1=O)[BrH]1(C(O1)=O)C1=O IKZZLUBYOKOJOQ-UHFFFAOYSA-N 0.000 description 1
- BEGJYJRHGGXXRK-UHFFFAOYSA-N O=C([B]1(C(O2)=O)[IH]C2=O)OC1=O Chemical compound O=C([B]1(C(O2)=O)[IH]C2=O)OC1=O BEGJYJRHGGXXRK-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L79/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen with or without oxygen or carbon only, not provided for in groups C08L61/00 - C08L77/00
- C08L79/04—Polycondensates having nitrogen-containing heterocyclic rings in the main chain; Polyhydrazides; Polyamide acids or similar polyimide precursors
- C08L79/08—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
- C08G73/1039—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors comprising halogen-containing substituents
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/34—Silicon-containing compounds
- C08K3/36—Silica
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0016—Plasticisers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K2201/00—Specific properties of additives
- C08K2201/011—Nanostructured additives
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Macromolecular Compounds Obtained By Forming Nitrogen-Containing Linkages In General (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
本發明之目的以提供一種樹脂薄膜形成用組成物,其可賦予不僅是耐熱性及耐溶劑性為優異,亦具有所謂延遲為低之特徵之樹脂薄膜,特以適合作為可撓性裝置之基板之樹脂薄膜。 An object of the present invention is to provide a composition for forming a resin film, which can impart not only excellent heat resistance and solvent resistance but also a resin film with a feature of low retardation, which is particularly suitable as a substrate for flexible devices resin film.
一種樹脂薄膜形成用組成物,其係包含聚醯亞胺、二氧化矽粒子、交聯劑及有機溶劑,該二氧化矽粒子係從藉由氮氣吸附法所測定的比表面積值算出的平均粒徑為100nm以下,以及由該樹脂薄膜形成用組成物所形成的樹脂薄膜。 A composition for forming a resin film comprising polyimide, silica particles, a crosslinking agent and an organic solvent, the silica particles being an average particle size calculated from a specific surface area value measured by a nitrogen gas adsorption method A diameter of 100 nm or less, and a resin film formed from the composition for forming a resin film.
Description
本發明為關於樹脂薄膜形成用組成物,更具體而言,特別是關於用來形成適合於可撓性顯示器基板等之顯示器基板用途之樹脂薄膜之組成物。 The present invention relates to a composition for forming a resin film, more specifically, a composition for forming a resin film suitable for display substrate applications such as flexible display substrates.
近年來,隨著液晶顯示器或有機電致發光顯示器等之電子元件(electronics)之急速進展,變得要求裝置之薄型化或輕量化,進而為可撓性化。 In recent years, with the rapid development of electronic components (electronics) such as liquid crystal displays and organic electroluminescence displays, thinning and weight reduction of devices, and further flexibility, have become required.
該等裝置係於玻璃基板上形成各種的電子元件,例如薄膜電晶體或透明電極等,藉由將此玻璃材料取代成為柔軟且輕量的樹脂材料,可謀求裝置本身之薄型化或輕量化、可撓性化。然後,作為如此般樹脂材料之候選,以聚醯亞胺備受矚目,以往已有各種有關聚醯亞胺薄膜之報告(參考例如專利文獻1、2)。 In these devices, various electronic components, such as thin film transistors or transparent electrodes, are formed on a glass substrate. Flexibility. Then, as a candidate for such a resin material, polyimide has been attracting attention, and there have been various reports on polyimide films in the past (for example, refer to Patent Documents 1 and 2).
[專利文獻1]日本特開昭60-188427號公報 [Patent Document 1] Japanese Patent Laid-Open No. 60-188427
[專利文獻2]日本特開昭58-208322號公報 [Patent Document 2] Japanese Patent Laid-Open No. 58-208322
[專利文獻3]日本特開2015-63655號公報 [Patent Document 3] Japanese Patent Laid-Open No. 2015-63655
[專利文獻4]國際公開2011/149018號說明書 [Patent Document 4] International Publication No. 2011/149018
[專利文獻5]美國專利申請公開第2011/130495號說明書 [Patent Document 5] US Patent Application Publication No. 2011/130495
[專利文獻6]國際公開2012/129422號說明書 [Patent Document 6] International Publication No. 2012/129422
然而,將聚醯亞胺樹脂材料使用作為顯示器之基板時,不僅要求該樹脂材料為透明性或柔軟性優異,作為要求性能之一係以延遲(Retardation)為低之材料為宜,且亦被如此地要求著。此外,在顯示器之製造步驟若將其他的構件等設置於基板上時,亦有被曝露於溶劑之情形,故對於基板用途的聚醯亞胺樹脂材料而言亦要求著耐溶劑性(專利文獻3)。 However, when a polyimide resin material is used as a substrate of a display, not only is the resin material required to be excellent in transparency or flexibility, but one of the required properties is a material with low retardation, and it is also required to have low retardation. so requested. In addition, when other components are provided on the substrate in the manufacturing process of the display, it may be exposed to the solvent, so the solvent resistance is also required for the polyimide resin material for the substrate (Patent Document 1). 3).
尚,所謂的延遲(相位差)係指雙折射(正交的2個折射率之差)與膜厚之積,但此數值特別是厚度方向之延遲,係對於影響視野角特性而言為重要的數值。已知大的延遲值可能會成為導致顯示器之顯示品質降低之原因(參考例如專利文獻4)。 Furthermore, the so-called retardation (retardation) refers to the product of birefringence (the difference between two orthogonal refractive indices) and the film thickness, but this value, especially the retardation in the thickness direction, is important for affecting the viewing angle characteristics. value of . It is known that a large delay value may be a cause of deterioration of the display quality of the display (refer to, for example, Patent Document 4).
本發明為有鑑於如此般情事之發明,目的以提供一種樹脂薄膜形成用組成物,其可賦予不僅是耐熱性 及柔軟性為優異,亦具有所謂延遲為低之特徵,進而透明性亦為優異,亦具有耐溶劑性的適合於作為顯示器基板的基底薄膜,特以具有作為可撓性顯示器基板的基底薄膜為優異的性能的樹脂薄膜。 The present invention has been made in view of such circumstances, and an object of the present invention is to provide a composition for forming a resin film capable of imparting not only heat resistance It has excellent flexibility, low retardation, excellent transparency, and solvent resistance. Excellent performance resin film.
本發明入為了達成上述目的經重複深入研究之結果發現:於聚醯亞胺中調配二氧化矽與交聯劑而成的樹脂薄膜,其耐熱性優異、延遲為低,進而亦具有所謂柔軟性及溶劑耐性為優異之特徵;以及,藉由將該二氧化矽之調配量設為指定之範圍,可實現耐熱性為優異、延遲為低、柔軟性為優異,進而透明性亦為優異的樹脂薄膜,而完成本發明。 In the present invention, as a result of repeated and in-depth research in order to achieve the above-mentioned object, it was found that a resin film prepared by blending silicon dioxide and a cross-linking agent in polyimide has excellent heat resistance, low retardation, and also has so-called flexibility. and solvent resistance are excellent; and, by setting the amount of the silica to be formulated in a specified range, a resin excellent in heat resistance, low retardation, excellent flexibility, and furthermore excellent in transparency can be realized film, and the present invention was completed.
尚,專利文獻5及6中揭示包含交聯劑的組成物。然而專利文獻5及6皆未記載有關藉由本發明之構成所能得到之特定功效之教示內容,亦未有啟示該等內容之記載。 Furthermore, Patent Documents 5 and 6 disclose compositions containing a crosslinking agent. However, Patent Documents 5 and 6 do not describe teaching contents about specific effects that can be obtained by the constitution of the present invention, nor do they disclose such contents.
即,作為本發明之第1觀點為關於一種樹脂薄膜形成用組成物,其係包含聚醯亞胺、二氧化矽粒子、交聯劑、及有機溶劑,該二氧化矽粒子係從藉由氮氣吸附法所測定的比表面積值算出的平均粒徑為100nm以下,該交聯劑係僅由氫原子、碳原子、氮原子及氧原子所構成之化合物並具有2以上選自由羥基、環氧基及碳原子數1~5之烷氧基所成之群之基,且由具有環狀構造之化合物所成。 That is, as a first aspect of the present invention, it relates to a composition for forming a resin film comprising a polyimide, silica particles, a crosslinking agent, and an organic solvent, the silica particles being prepared from a The average particle size calculated from the specific surface area value measured by the adsorption method is 100 nm or less, and the crosslinking agent is a compound composed only of hydrogen atoms, carbon atoms, nitrogen atoms and oxygen atoms, and has 2 or more selected from the group consisting of hydroxyl groups, epoxy groups and a group of alkoxy groups with 1 to 5 carbon atoms, and a compound with a cyclic structure.
作為第2觀點為關於前述第1觀點記載之樹脂薄膜形成用組成物,其中,前述聚醯亞胺係使包含脂環式四羧酸二酐的四羧酸二酐成分、與包含含氟芳香族二胺的二胺成分反應,並將所得到的聚醯胺酸醯亞胺化而得到的聚醯亞胺。 As a second aspect, the composition for forming a resin film according to the first aspect, wherein the polyimide-based tetracarboxylic dianhydride component containing an alicyclic tetracarboxylic dianhydride and a fluorine-containing aromatic A polyimide obtained by reacting a diamine component of a family of diamines and imidizing the obtained polyamide acid.
作為第3觀點為關於前述第2觀點記載之樹脂薄膜形成用組成物,其中,前述脂環式四羧酸二酐包含式(C1)所表示的四羧酸二酐,
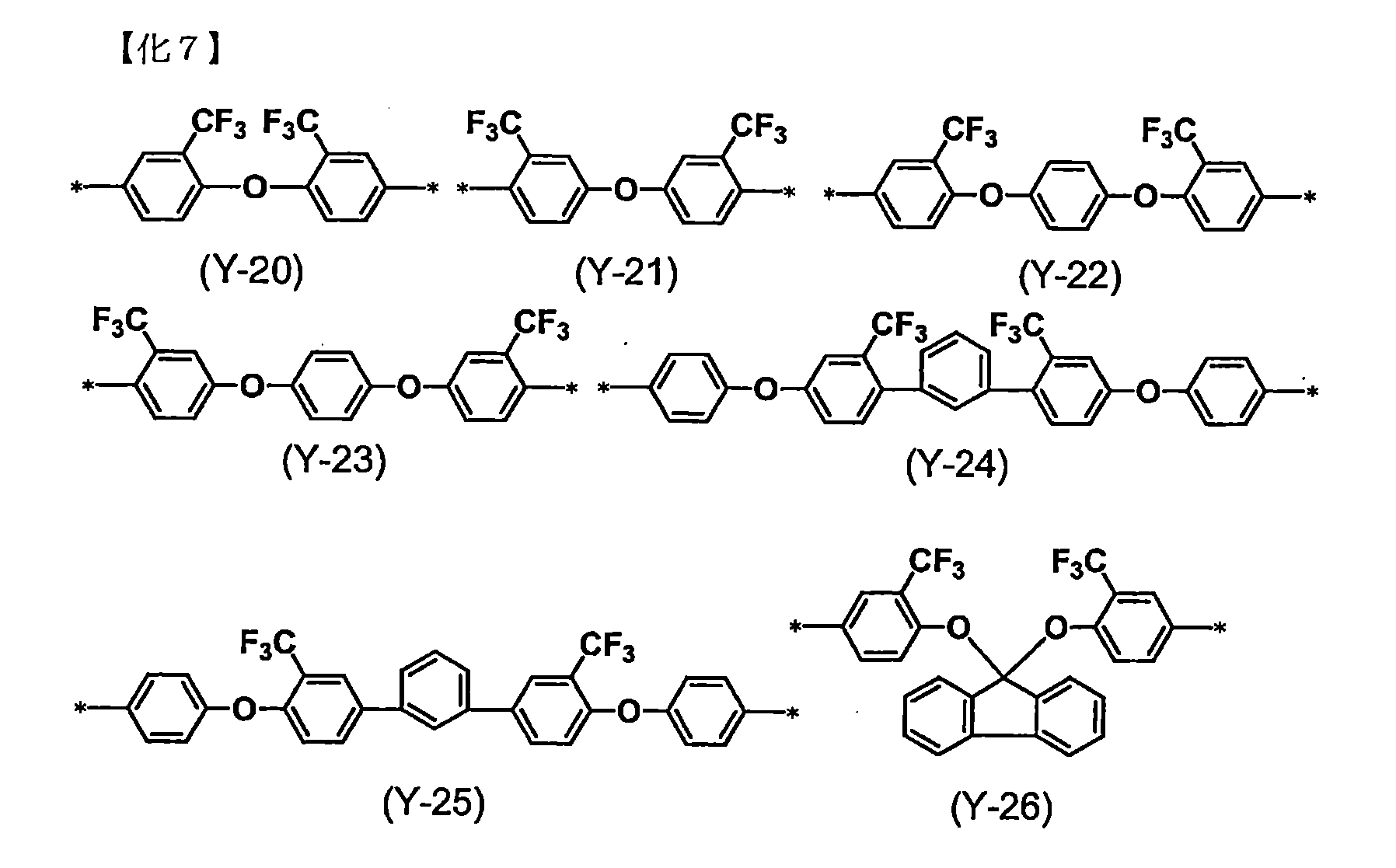
作為第4觀點為關於前述第2觀點或第3觀點之樹脂薄膜形成用組成物,其中,前述含氟芳香族二胺包含式(A1)所表示的二胺,
【化3】H2N-B2-NH2 (A1)(式中,B2表示由式(Y-1)~(Y-34)所成之群所選出的二價基),
作為第5觀點為關於前述第1觀點至第4觀點中任一項之樹脂薄膜形成用組成物,其中,前述聚醯亞胺與前述二氧化矽粒子之質量比為7:3~3:7。 As a fifth aspect, the composition for forming a resin film according to any one of the first aspect to the fourth aspect, wherein the mass ratio of the polyimide to the silica particles is 7:3 to 3:7 .
作為第6觀點為關於前述第1觀點至第5觀點中任一 項之樹脂薄膜形成用組成物,其中,前述二氧化矽粒子之平均粒徑為60nm以下。 As the sixth viewpoint, any one of the aforementioned first viewpoint to fifth viewpoint The composition for forming a resin film according to the item, wherein the average particle diameter of the silica particles is 60 nm or less.
作為第7觀點為關於一種樹脂薄膜,其係由前述第1觀點至第6觀點中任一項之樹脂薄膜形成用組成物所形成。 A seventh aspect relates to a resin film formed from the composition for forming a resin film according to any one of the first to sixth aspects.
藉由本發明相關的樹脂薄膜形成用組成物,可再現性良好地形成具有低線膨脹係數、耐熱性為優異、且具有高透明性與低延遲,進而柔軟性及溶劑耐性為優異的樹脂薄膜。 The resin film-forming composition according to the present invention can reproducibly form a resin film having a low coefficient of linear expansion, excellent heat resistance, high transparency and low retardation, and excellent flexibility and solvent resistance.
又,由於本發明相關的樹脂薄膜展現出低線膨脹係數、高透明性(高光線透射率、低黃色度)、低延遲,進而柔軟性及溶劑耐性亦為優異,故可適合作為可撓性裝置(特別是可撓性顯示器)之基板來使用。 In addition, since the resin film according to the present invention exhibits a low coefficient of linear expansion, high transparency (high light transmittance, low yellowness), and low retardation, and furthermore is excellent in flexibility and solvent resistance, it can be suitably used as a flexible It is used as a substrate of a device (especially a flexible display).
如此般地本發明相關的樹脂薄膜形成用組成物及樹脂薄膜,係可充分對應於要求高柔軟性、低線膨脹係數、高透明性(高光線透射率、低黃色度)、低延遲等之特性的可撓性裝置用基板(特別是可撓性顯示器用基板)之領域中之進展。 In this way, the composition for forming a resin film and the resin film according to the present invention can sufficiently respond to the requirements of high flexibility, low coefficient of linear expansion, high transparency (high light transmittance, low yellowness), low retardation, and the like. Progress in the field of flexible device substrates (especially flexible display substrates) with specific characteristics.
以下,對於本發明進行詳細說明。 Hereinafter, the present invention will be described in detail.
本發明的樹脂薄膜形成用組成物係含有下述特定的聚醯亞胺、二氧化矽粒子、交聯劑及有機溶劑。 The resin film-forming composition of the present invention contains the following specific polyimide, silica particles, a crosslinking agent, and an organic solvent.
本發明中使用的聚醯亞胺係較佳為使包含脂環式四羧酸二酐的四羧酸二酐成分與包含含氟芳香族二胺的二胺成分反應所得到的聚醯胺酸醯亞胺化而得到的聚醯亞胺。 The polyimide type used in the present invention is preferably a polyamide acid obtained by reacting a tetracarboxylic dianhydride component containing an alicyclic tetracarboxylic dianhydride and a diamine component containing a fluorine-containing aromatic diamine Polyimide obtained by imidization.
其中、前述脂環式四羧酸二酐,其較佳為包含下述式(C1)所表示的四羧酸二酐;前述含氟芳香族二胺,其較佳為包含下述式(A1)所表示的二胺。 Among them, the aforementioned alicyclic tetracarboxylic dianhydride preferably includes a tetracarboxylic dianhydride represented by the following formula (C1); the aforementioned fluorine-containing aromatic diamine preferably includes the following formula (A1) ) represented by the diamine.
【化11】H2N-B2-NH2 (A1)(式中,B2係表示由式(Y-1)~(Y-34)所成之群所選出的二價基)。 [Chemical 11] H 2 NB 2 -NH 2 (A1) (wherein, B 2 represents a divalent group selected from the group of formulae (Y-1) to (Y-34)).
上述式(C1)所表示的四羧酸二酐之中,式中的B1較佳為式(X-1)、(X-4)、(X-6)、(X-7)所表示的化合物。 Among the tetracarboxylic dianhydrides represented by the above formula (C1), B 1 in the formula is preferably represented by the formulae (X-1), (X-4), (X-6), and (X-7) compound of.
又,上述(A1)所表示的二胺之中,式中的B2較佳為式(Y-12)、(Y-13)所表示的化合物。 Moreover, among the diamines represented by the above (A1), B 2 in the formula is preferably a compound represented by the formulae (Y-12) and (Y-13).
作為適合之例為使上述式(C1)所表示的四羧酸二酐與上述式(A1)所表示的二胺反應,將所得到的聚醯胺酸醯亞胺化後而得到的聚醯亞胺,其係包含後述式(2)所表示的單體單位。 A suitable example is a polyamide obtained by reacting a tetracarboxylic dianhydride represented by the above formula (C1) with a diamine represented by the above formula (A1), and imidizing the obtained polyamide acid. The imine contains a monomer unit represented by the following formula (2).
為了得到具有本發明之目的之低線膨脹係數、低延遲及高透明性之特性、且柔軟性為優異的樹脂薄膜,相對於四羧酸二酐成分之全莫耳數,脂環式四羧酸二酐例如上述式(C1)所表示的四羧酸二酐以90莫耳%以上為較佳,95莫耳%以上為又較佳,特別以全部(100莫耳%)為上述式式(C1)所表示的四羧酸二酐為最適合。 In order to obtain a resin film having the characteristics of low linear expansion coefficient, low retardation, and high transparency, which are the objects of the present invention, and excellent in flexibility, alicyclic tetracarboxylic The acid dianhydride such as the tetracarboxylic dianhydride represented by the above formula (C1) is preferably more than 90 mol%, more preferably 95 mol% or more, especially the whole (100 mol%) is the above formula The tetracarboxylic dianhydride represented by (C1) is the most suitable.
又,相同地為了得到上述低線膨脹係數、低延遲及高透明性之特性、且柔軟性為優異的樹脂薄膜,相對於二胺成分之全莫耳數,含氟芳香族二胺例如式(A1)所表示的二胺以90莫耳%以上為較佳,95莫耳%以上為又較佳。又,二胺成分之全部(100莫耳%)亦可為上述式(A1)所表示的二胺。 Also, in order to obtain a resin film having the above-mentioned characteristics of low linear expansion coefficient, low retardation and high transparency, and excellent flexibility, a fluorine-containing aromatic diamine such as the formula ( The diamine represented by A1) is preferably more than 90 mol%, and more preferably 95 mol% or more. Moreover, the diamine represented by the said formula (A1) may be all (100 mol%) of diamine components.
作為適合的樣態之一例,本發明中使用的聚醯亞胺係包含下述式(2)所表示的單體單位。 As an example of a suitable aspect, the polyimide type|system|group used by this invention contains the monomer unit represented by following formula (2).
作為上述式(2)所表示的單體單位,係以式(2-1)或式(2-2)所表示者為較佳,以式(2-1)所表示者為又較佳。 As a monomer unit represented by said formula (2), what is represented by formula (2-1) or formula (2-2) is preferable, and what is represented by formula (2-1) is more preferable.
本發明的聚醯亞胺,除了由包含前述之式(C1)所表示的四羧酸二酐的脂環式四羧酸二酐成分、與包含式(A1)所表示的二胺的二胺成分所衍生的單體單位以外,亦可包含其他的單體單位。此其他的單體單位之含有比例,在不損及由本發明的樹脂薄膜形成用組成物所形成的樹脂薄膜之特性之範圍內可任意決定。該比例,相對於由包含前述之式(C1)所表示的四羧酸二酐的脂環式四羧酸 二酐成分、與包含式(A1)所表示的二胺的二胺成分所衍生的單體單位之總莫耳數,以未滿20莫耳%為較佳,未滿10莫耳%為又較佳,未滿5莫耳%為又更佳。 The polyimide of the present invention is composed of an alicyclic tetracarboxylic dianhydride component containing the tetracarboxylic dianhydride represented by the aforementioned formula (C1), and a diamine containing the diamine represented by the formula (A1). In addition to the monomer units derived from the components, other monomer units may be included. The content ratio of the other monomer units can be arbitrarily determined within a range that does not impair the properties of the resin film formed from the composition for forming a resin film of the present invention. This ratio is relative to the alicyclic tetracarboxylic acid containing the tetracarboxylic dianhydride represented by the aforementioned formula (C1). The total number of moles of monomer units derived from the dianhydride component and the diamine component containing the diamine represented by the formula (A1) is preferably less than 20 mol %, and less than 10 mol %. More preferably, less than 5 mol % is still more preferable.
作為如此般地其他的單體單位,可舉例如式(3)所表示的具有其他的聚醯亞胺構造的單體單位,但並不限定於此。 Such other monomer units include, for example, monomer units having other polyimide structures represented by formula (3), but are not limited thereto.
式(3)中,A係表示四價有機基,較佳為下述式(A-1)~(A-4)中任一者所表示的四價基。又,上述式(3)中,B係表示2價有機基,較佳為式(B-1)~(B-11)中任一者所表示的二價基。各式中,*表示鍵結鍵。尚,式(3)中,若A為下述式(A-1)~(A-4)中任一者所表示的四價基時,B亦可為前述之式(Y-1)~(Y-34)中任一者所表示的二價基。或式(3)中,若B為下述式(B-1)~(B-11)中任一者所表示的二價基時,A亦可為前述之式(X-1)~(X-12)中任一者所表示的四價基。 In formula (3), A represents a tetravalent organic group, preferably a tetravalent group represented by any one of the following formulae (A-1) to (A-4). Moreover, in said Formula (3), B represents a divalent organic group, Preferably it is a divalent group represented by any one of Formulas (B-1)-(B-11). In each formula, * represents a bond bond. Still, in formula (3), if A is a tetravalent group represented by any one of the following formulas (A-1) to (A-4), B may also be the aforementioned formula (Y-1) to The divalent group represented by any one of (Y-34). Or in formula (3), when B is a divalent group represented by any one of the following formulae (B-1) to (B-11), A may also be the aforementioned formula (X-1) to ( The tetravalent group represented by any one of X-12).
本發明的聚醯亞胺中若包含式(3)所表示的單體單位時,A及B例如亦可僅包含由下述式所例示之基之中僅一種所構成的單體單位,A及B之至少一方亦可包含由下述所例示之二種以上之基所選出的二種以上的單體單位。 When the polyimide of the present invention contains the monomer unit represented by the formula (3), A and B may contain, for example, only a monomer unit composed of only one of the groups exemplified by the following formula, A At least one of B and B may contain two or more monomer units selected from two or more groups exemplified below.
尚,本發明中使用的聚醯亞胺中,各單體單位係以任意順序鍵結。 Furthermore, in the polyimide used in the present invention, the monomer units are bonded in an arbitrary order.
又,本發明中使用的聚醯亞胺,除了由包含前述之式(C1)所表示的四羧酸二酐的脂環式四羧酸二酐成 分、與包含式(A1)所表示的二胺的二胺成分所衍生的單體單位以外,若具有上述式(3)所表示的其他的單體單位時,含有各單體單位的聚醯亞胺,係藉由使作為四羧酸二酐成分之上述式(C1)所表示的四羧酸二酐以外的下述式(5)所表示的四羧酸二酐、與作為二胺成分之上述(A1)所表示的二胺以外的下述式(6)所表示的二胺在有機溶劑中聚合,並將所得到的聚醯胺酸醯亞胺化而得到。 In addition, the polyimide used in the present invention is composed of an alicyclic tetracarboxylic dianhydride containing the tetracarboxylic dianhydride represented by the aforementioned formula (C1). In addition to the monomer unit derived from the diamine component containing the diamine represented by the formula (A1), if there is another monomer unit represented by the above formula (3), the polyamide containing each monomer unit The imine is obtained by combining a tetracarboxylic dianhydride represented by the following formula (5) other than the tetracarboxylic dianhydride represented by the above formula (C1) as a tetracarboxylic dianhydride component, and a diamine component The diamine represented by the following formula (6) other than the diamine represented by the above (A1) is obtained by polymerizing in an organic solvent and imidizing the obtained polyamide.
上述式(5)中的A及式(6)中的B,係與前述之式(3)中的A及B分別表示相同意思。 A in the above formula (5) and B in the formula (6) have the same meanings as A and B in the above-mentioned formula (3), respectively.
具體而言,作為式(5)所表示的四羧酸二酐,可舉例苯均四酸二酐、3,3’,4,4’-聯苯四羧酸二酐、3,3’,4,4’-二苯甲酮四羧酸二酐、3,3’,4,4’-二苯基醚四羧酸二酐、3,3’,4,4’-二苯基碸四羧酸二酐、4,4’-(六氟亞異丙基)二鄰苯二甲酸二酐、11,11-雙(三氟甲基)-1H-二氟[3,4-b:3’,4’-i]二苯并哌喃-1,3,7,9-(11H-四酮)、6,6’-雙(三氟甲基)-[5,5’-二異苯并呋喃]-1,1’,3,3’-四酮、4,6,10,12-四氟二呋喃并[3,4-b:3’,4’-i]二苯并[b,e][1,4]戴奧辛-1,3,7,9-四酮、4,8-雙(三氟甲氧基)苯并[1,2-c:4,5-c’]二呋喃-1,3,5,7-四酮、N,N’-[2,2’-雙(三氟甲基)聯苯-4,4’-二基]雙(1,3-二 氧-1,3-二氫異苯并呋喃-5-羧醯胺)等之芳香族四羧酸;1,2-二甲基-1,2,3,4-環丁烷四羧酸二酐、1,2,3,4-四甲基-1,2,3,4-環丁烷四羧酸二酐、1,2,3,4-環戊烷四羧酸二酐、1,2,3,4-環己烷四羧酸二酐、3,4-二羧基-1,2,3,4-四氫-1-萘琥珀酸二酐等之脂環式四羧酸二酐;1,2,3,4-丁烷四羧酸二酐等之脂肪族四羧酸二酐,但不限定於該等。 Specifically, as the tetracarboxylic dianhydride represented by the formula (5), pyromellitic dianhydride, 3,3',4,4'-biphenyltetracarboxylic dianhydride, 3,3', 4,4'-benzophenone tetracarboxylic dianhydride, 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride, 3,3',4,4'-diphenyl tetracarboxylic acid Carboxylic dianhydride, 4,4'-(hexafluoroisopropylidene)diphthalic dianhydride, 11,11-bis(trifluoromethyl)-1H-difluoro[3,4-b:3 ',4'-i]Dibenzopyran-1,3,7,9-(11H-tetraone), 6,6'-bis(trifluoromethyl)-[5,5'-diisophenyl furan]-1,1',3,3'-tetraone, 4,6,10,12-tetrafluorodifuro[3,4-b:3',4'-i]dibenzo[b ,e][1,4]dioxin-1,3,7,9-tetraone, 4,8-bis(trifluoromethoxy)benzo[1,2-c:4,5-c']di Furan-1,3,5,7-tetraone, N,N'-[2,2'-bis(trifluoromethyl)biphenyl-4,4'-diyl]bis(1,3-diyl) Aromatic tetracarboxylic acids such as oxygen-1,3-dihydroisobenzofuran-5-carboxamide); 1,2-dimethyl-1,2,3,4-cyclobutanetetracarboxylic acid anhydride, 1,2,3,4-tetramethyl-1,2,3,4-cyclobutanetetracarboxylic dianhydride, 1,2,3,4-cyclopentanetetracarboxylic dianhydride, 1, Alicyclic tetracarboxylic dianhydride such as 2,3,4-cyclohexanetetracarboxylic dianhydride, 3,4-dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic dianhydride, etc. ; Aliphatic tetracarboxylic dianhydrides such as 1,2,3,4-butane tetracarboxylic dianhydride, etc., but not limited to these.
該等之中,式(5)中的A以前述式(A-1)~(A-4)中任一者所表示的四價基的四羧酸二酐為較佳,即,可舉例11,11-雙(三氟甲基)-1H-二氟[3,4-b:3’,4’-i]二苯并哌喃-1,3,7,9-(11H-四酮)、6,6’-雙(三氟甲基)-[5,5’-二異苯并呋喃]-1,1’,3,3’-四酮、4,6,10,12-四氟二呋喃并[3,4-b:3’,4’-i]二苯并[b,e][1,4]戴奧辛-1,3,7,9-四酮、4,8-雙(三氟甲氧基)苯并[1,2-c:4,5-c’]二呋喃-1,3,5,7-四酮為較佳的化合物。 Among these, A in the formula (5) is preferably a tetravalent tetracarboxylic dianhydride represented by any one of the aforementioned formulas (A-1) to (A-4), that is, for example 11,11-Bis(trifluoromethyl)-1H-difluoro[3,4-b:3',4'-i]dibenzopyran-1,3,7,9-(11H-tetraone ), 6,6'-bis(trifluoromethyl)-[5,5'-diisobenzofuran]-1,1',3,3'-tetraone, 4,6,10,12-tetrakis Fluorodifuro[3,4-b:3',4'-i]dibenzo[b,e][1,4]dioxin-1,3,7,9-tetraone, 4,8-bis (Trifluoromethoxy)benzo[1,2-c:4,5-c']difuran-1,3,5,7-tetraone is a preferred compound.
又,作為式(6)所表示的二胺,可舉例如2-(三氟甲基)苯-1,4-二胺、5-(三氟甲基)苯-1,3-二胺、5-(三氟甲基)苯-1,2-二胺、2,5-雙(三氟甲基)-苯-1,4-二胺、2,3-雙(三氟甲基)-苯-1,4-二胺、2,6-雙(三氟甲基)-苯-1,4-二胺、3,5-雙(三氟甲基)-苯-1,2-二胺、肆(三氟甲基)-1,4-苯二胺、2-(三氟甲基)-1,3-苯二胺、4-(三氟甲基)-1,3-苯二胺、2-甲氧基-1,4-苯二胺、2,5-二甲氧基-1,4-苯二胺、2-羥基-1,4-苯二胺、2,5-二羥基-1,4-苯二胺、2-氟苯-1,4-二胺、2,5-二氟苯-1,4-二胺、2-氯苯-1,4-二胺、2,5-二氯苯-1,4-二胺、2,3,5,6-四氟苯-1,4-二胺、4,4’-(全氟丙烷-2,2-二基)雙苯胺、4,4’-氧基雙[3-(三氟甲基)苯胺]、1,4-雙(4- 胺基苯氧基)苯、1,3’-雙(4-胺基苯氧基)苯、1,4-雙(3-胺基苯氧基)苯、聯苯胺、2-甲基聯苯胺、3-甲基聯苯胺、2-(三氟甲基)聯苯胺、3-(三氟甲基)聯苯胺、2,2’-二甲基聯苯胺(m-聯甲苯胺)、3,3’-二甲基聯苯胺(o-聯甲苯胺)、2,3’-二甲基聯苯胺、2,2’-二甲氧基聯苯胺、3,3’-二甲氧基聯苯胺、2,3’-二甲氧基聯苯胺、2,2’-二羥基聯苯胺、3,3’-二羥基聯苯胺、2,3’-二羥基聯苯胺、2,2’-二氟聯苯胺、3,3’-二氟聯苯胺、2,3,-二氟聯苯胺、2,2’-二氯聯苯胺、3,3’-二氯聯苯胺、2,3’-二氯聯苯胺、4,4’-二胺基苯甲醯苯胺、4-胺基苯基-4’-胺基苯甲酸酯、八氟聯苯胺、2,2’,5,5’-四甲基聯苯胺、3,3’,5,5’-四甲基聯苯胺、2,2’,5,5’-肆(三氟甲基)聯苯胺、3,3’,5,5’-肆(三氟甲基)聯苯胺、2,2’,5,5’-四氯聯苯胺、4,4’-雙(4-胺基苯氧基)聯苯、4,4’-雙(3-胺基苯氧基)聯苯、4,4’-{[3,3”-雙(三氟甲基)-(1,1’:3’,1”-聯三苯)-4,4”-二基]-雙(氧基)}雙苯胺、4,4’-{[(全氟丙烷-2,2-二基)雙(4,1-伸苯基)]雙(氧基)}雙苯胺、1-(4-胺基苯基)-2,3-二氫-1,3,3-三甲基-1H-茚-5(或6)胺等之芳香族二胺;4,4’-亞甲基雙(環己基胺)、4,4’-亞甲基雙(3-甲基環己基胺)、異佛酮二胺、反式-1,4-環己二胺、順式-1,4-環己二胺、1,4-環己烷雙(甲基胺)、2,5-雙(胺基甲基)雙環[2.2.1]庚烷、2,6-雙(胺基甲基)雙環[2.2.1]庚烷、3,8-雙(胺基甲基)三環[5.2.1.0]癸烷、1,3-二胺基金剛烷、2,2-雙(4-胺基環己基)丙烷、2,2-雙(4-胺基環己基)六氟丙烷、1,3-丙二胺、1,4-四亞甲基二胺、1,5-五亞甲基 二胺、1,6-六亞甲基二胺、1,7-七亞甲基二胺、1,8-八亞甲基二胺、1,9-九亞甲基二胺等之脂肪族二胺,但不限定於該等。 Moreover, as the diamine represented by formula (6), for example, 2-(trifluoromethyl)benzene-1,4-diamine, 5-(trifluoromethyl)benzene-1,3-diamine, 5-(Trifluoromethyl)benzene-1,2-diamine, 2,5-bis(trifluoromethyl)-benzene-1,4-diamine, 2,3-bis(trifluoromethyl)- Benzene-1,4-diamine, 2,6-bis(trifluoromethyl)-benzene-1,4-diamine, 3,5-bis(trifluoromethyl)-benzene-1,2-diamine , 4-(trifluoromethyl)-1,4-phenylenediamine, 2-(trifluoromethyl)-1,3-phenylenediamine, 4-(trifluoromethyl)-1,3-phenylenediamine , 2-methoxy-1,4-phenylenediamine, 2,5-dimethoxy-1,4-phenylenediamine, 2-hydroxy-1,4-phenylenediamine, 2,5-dihydroxy -1,4-phenylenediamine, 2-fluorobenzene-1,4-diamine, 2,5-difluorobenzene-1,4-diamine, 2-chlorobenzene-1,4-diamine, 2, 5-Dichlorobenzene-1,4-diamine, 2,3,5,6-tetrafluorobenzene-1,4-diamine, 4,4'-(perfluoropropane-2,2-diyl)bis Aniline, 4,4'-oxybis[3-(trifluoromethyl)aniline], 1,4-bis(4- Aminophenoxy)benzene, 1,3'-bis(4-aminophenoxy)benzene, 1,4-bis(3-aminophenoxy)benzene, benzidine, 2-methylbenzidine , 3-methylbenzidine, 2-(trifluoromethyl)benzidine, 3-(trifluoromethyl)benzidine, 2,2'-dimethylbenzidine (m-benzidine), 3, 3'-Dimethylbenzidine (o-tolidine), 2,3'-dimethylbenzidine, 2,2'-dimethoxybenzidine, 3,3'-dimethoxybenzidine , 2,3'-dimethoxybenzidine, 2,2'-dihydroxybenzidine, 3,3'-dihydroxybenzidine, 2,3'-dihydroxybenzidine, 2,2'-difluoro Benzidine, 3,3'-difluorobenzidine, 2,3'-difluorobenzidine, 2,2'-dichlorobenzidine, 3,3'-dichlorobenzidine, 2,3'-dichlorobenzidine Benzidine, 4,4'-diaminobenzylaniline, 4-aminophenyl-4'-aminobenzoate, octafluorobenzidine, 2,2',5,5'-tetramethyl benzidine, 3,3',5,5'-tetramethylbenzidine, 2,2',5,5'-tetra(trifluoromethyl)benzidine, 3,3',5,5'- Four (trifluoromethyl)benzidine, 2,2',5,5'-tetrachlorobenzidine, 4,4'-bis(4-aminophenoxy)biphenyl, 4,4'-bis( 3-Aminophenoxy)biphenyl, 4,4'-{[3,3"-bis(trifluoromethyl)-(1,1':3',1"-bitriphenyl)-4, 4"-diyl]-bis(oxy)}dianiline, 4,4'-{[(perfluoropropane-2,2-diyl)bis(4,1-phenylene)]bis(oxy) )} dianiline, 1-(4-aminophenyl)-2,3-dihydro-1,3,3-trimethyl-1H-indene-5(or 6)amine and other aromatic diamines; 4,4'-methylenebis(cyclohexylamine), 4,4'-methylenebis(3-methylcyclohexylamine), isophoronediamine, trans-1,4-cyclohexanedi Amine, cis-1,4-cyclohexanediamine, 1,4-cyclohexanebis(methylamine), 2,5-bis(aminomethyl)bicyclo[2.2.1]heptane, 2, 6-Bis(aminomethyl)bicyclo[2.2.1]heptane, 3,8-bis(aminomethyl)tricyclo[5.2.1.0]decane, 1,3-diaminoadamantane, 2 , 2-bis(4-aminocyclohexyl)propane, 2,2-bis(4-aminocyclohexyl)hexafluoropropane, 1,3-propanediamine, 1,4-tetramethylenediamine, 1,5-Pentamethylene Aliphatic diamine, 1,6-hexamethylenediamine, 1,7-heptamethylenediamine, 1,8-octamethylenediamine, 1,9-nonamethylenediamine, etc. Diamines, but not limited to these.
該等之中,式(6)中的B以前述式(B-1)~(B-11)中任一者所表示的二價基的芳香族二胺為較佳,即可舉例2,2’-雙(三氟甲氧基)-(1,1’-聯苯)-4,4’-二胺[別名:2,2’-二甲氧基聯苯胺]、4,4’-(全氟丙烷-2,2-二基)雙苯胺、2,5-雙(三氟甲基)苯-1,4-二胺、2-(三氟甲基)苯-1,4-二胺、2-氟苯-1,4-二胺、4,4’-氧基雙[3-(三氟甲基)苯胺]、2,2’,3,3’,5,5’,6,6’-八氟[1,1’-聯苯]-4,4’-二胺[別名:八氟聯苯胺]、2,3,5,6-四氟苯-1,4-二胺、4,4’-{[3,3”-雙(三氟甲基)-(1,1’:3’,1”-聯三苯)-4,4”-二基]-雙(氧基)}雙苯胺、4,4’-{[(全氟丙烷-2,2-二基)雙(4,1-伸苯基)]雙(氧基)}雙苯胺、1-(4-胺基苯基)-2,3-二氫-1,3,3-三甲基-1H-茚-5(或6)胺作為較佳的二胺。 Among these, B in the formula (6) is preferably a divalent aromatic diamine represented by any one of the aforementioned formulas (B-1) to (B-11), such as 2, 2'-bis(trifluoromethoxy)-(1,1'-biphenyl)-4,4'-diamine [alias: 2,2'-dimethoxybenzidine], 4,4'- (Perfluoropropane-2,2-diyl)dianiline, 2,5-bis(trifluoromethyl)benzene-1,4-diamine, 2-(trifluoromethyl)benzene-1,4-diamine Amine, 2-fluorobenzene-1,4-diamine, 4,4'-oxybis[3-(trifluoromethyl)aniline], 2,2',3,3',5,5',6 ,6'-Octafluoro[1,1'-biphenyl]-4,4'-diamine [alias: octafluorobenzidine], 2,3,5,6-tetrafluorobenzene-1,4-diamine , 4,4'-{[3,3"-bis(trifluoromethyl)-(1,1':3',1"-triphenyl)-4,4"-diyl]-bis(oxygen) base)}dianiline, 4,4'-{[(perfluoropropane-2,2-diyl)bis(4,1-phenylene)]bis(oxy)}dianiline, 1-(4- Aminophenyl)-2,3-dihydro-1,3,3-trimethyl-1H-indene-5(or 6)amine is a preferred diamine.
本發明中使用的聚醯亞胺,如前述般,使上述式(C1)所表示的包含脂環式四羧酸二酐的四羧酸二酐成分、與上述式(A1)所表示的包含含氟芳香族二胺的二胺成分反應,並將所得到的聚醯胺酸醯亞胺化而得到。 As described above, the polyimide used in the present invention is composed of a tetracarboxylic dianhydride component containing an alicyclic tetracarboxylic dianhydride represented by the above formula (C1) and a tetracarboxylic dianhydride component represented by the above formula (A1) containing The diamine component of a fluorine-containing aromatic diamine is reacted, and the obtained polyamic acid is obtained by imidization.
由上述二成分轉換至聚醯胺酸之反應,以在有機溶劑中可相對地較容易進行,且就不生成副產物之點而言為有利。 The conversion reaction from the above two components to polyamic acid is advantageous in that it can be carried out relatively easily in an organic solvent, and by-products are not generated.
該等四羧酸二酐成分與二胺成分之反應中,考量聚醯胺酸、進而藉由之後的醯亞胺化所得到的聚醯亞胺之分子量等,可適當設定二胺成分的置入比(莫耳比),相對於二胺成分1通常可將四羧酸二酐成分設為0,8~1.2左右,例如0.9~1.1左右,較佳為0.95~1.02左右。與通常的縮聚合反應為相同地,若此莫耳比越接近1.0時,所生成的聚醯胺酸之分子量則會變得越大。 In the reaction between these tetracarboxylic dianhydride components and the diamine component, the position of the diamine component can be appropriately set in consideration of the molecular weight of the polyamic acid and the polyimide obtained by subsequent imidization. The ratio (molar ratio) of the tetracarboxylic dianhydride component relative to the diamine component 1 is usually about 0.8 to 1.2, for example, about 0.9 to 1.1, preferably about 0.95 to 1.02. As in the general polycondensation reaction, as the molar ratio is closer to 1.0, the molecular weight of the produced polyamic acid becomes larger.
上述四羧酸二酐成分與二胺成分之反應之際所使用的有機溶劑,只要是對於反應不會造成不良影響,又可使所生成的聚醯胺酸溶解者即可,未特別限定。以下舉例該具體例。 The organic solvent used in the reaction of the tetracarboxylic dianhydride component and the diamine component is not particularly limited as long as it does not adversely affect the reaction and can dissolve the produced polyamide. This specific example is illustrated below.
例如、m-甲酚、2-吡咯啶酮、N-甲基-2-吡咯啶酮、N-乙基-2-吡咯啶酮、N-乙烯基-2-吡咯啶酮、N,N-二甲基甲醯胺、N,N-二甲基乙醯胺、3-甲氧基-N,N-二甲基丙醯胺、3-乙氧基-N,N-二甲基丙醯胺、3-丙氧基-N,N-二甲基丙醯胺、3-異丙氧基-N,N-二甲基丙醯胺、3-丁氧基-N,N-二甲基丙醯胺、3-sec-丁氧基-N,N-二甲基丙醯胺、3-tert-丁氧基-N,N-二甲基丙醯胺、γ-丁內酯、N-甲基己內醯胺、二甲基亞碸、四甲基脲、吡啶、二甲基碸、異丙醇、甲氧基甲基戊醇、二戊烯、乙基戊基酮、甲基壬基酮、甲基乙基酮、甲基異戊基酮、甲基異丙基酮、甲基賽珞蘇、乙基賽珞蘇、乙酸甲基賽珞蘇、乙酸乙基賽珞蘇、丁基卡必醇、乙基卡必醇、乙二醇、乙二醇單乙酸酯、乙二醇單異丙基醚、乙二醇單丁基醚、丙二醇、丙二醇單乙酸酯、 丙二醇單甲基醚、丙二醇-tert-丁基醚、二丙二醇單甲基醚、二乙二醇、二乙二醇單乙酸酯、二乙二醇二甲基醚、二丙二醇單乙酸酯單甲基醚、二丙二醇單甲基醚、二丙二醇單乙基醚、二丙二醇單乙酸酯單乙基醚、二丙二醇單丙基醚、二丙二醇單乙酸酯單丙基醚、3-甲基-3-甲氧基丁基乙酸酯、三丙二醇甲基醚、3-甲基-3-甲氧基丁醇、二異丙基醚、乙基異丁基醚、二異丁烯、乙酸戊酯、丁酸丁酯、丁基醚、二異丁酮、甲基環己烯、丙基醚、二己基醚、二噁烷、n-己烷、n-戊烷、n-辛烷、二乙基醚、環己酮、碳酸伸乙酯、碳酸伸丙酯、乳酸甲酯、乳酸乙酯、乙酸甲酯、乙酸乙酯、酢酸n-丁酯、乙酸丙二醇單乙基醚、丙酮酸甲酯、丙酮酸乙酯、3-甲氧基丙酸甲酯、3-乙氧基丙酸甲基乙酯、3-甲氧基丙酸乙酯、3-乙氧基丙酸、3-甲氧基丙酸、3-甲氧基丙酸丙酯、3-甲氧基丙酸丁酯、二乙二醇二甲醚、4-羥基-4-甲基-2-戊酮等,但不限定於該等。此等可單獨使用,或亦可組合2種以上使用。 For example, m-cresol, 2-pyrrolidone, N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone, N-vinyl-2-pyrrolidone, N,N- Dimethylformamide, N,N-dimethylacetamide, 3-methoxy-N,N-dimethylpropionamide, 3-ethoxy-N,N-dimethylpropionamide Amine, 3-Propoxy-N,N-Dimethylpropionamide, 3-Isopropoxy-N,N-Dimethylpropionamide, 3-Butoxy-N,N-Dimethyl Propionamide, 3-sec-butoxy-N,N-dimethylpropionamide, 3-tert-butoxy-N,N-dimethylpropionamide, γ-butyrolactone, N- Methyl caprolactam, dimethyl sulfoxide, tetramethyl urea, pyridine, dimethyl sulfoxide, isopropanol, methoxymethyl pentanol, dipentene, ethyl amyl ketone, methyl nonyl methyl ketone, methyl ethyl ketone, methyl isoamyl ketone, methyl isopropyl ketone, methyl cylosu base carbitol, ethyl carbitol, ethylene glycol, ethylene glycol monoacetate, ethylene glycol monoisopropyl ether, ethylene glycol monobutyl ether, propylene glycol, propylene glycol monoacetate, Propylene Glycol Monomethyl Ether, Propylene Glycol-tert-Butyl Ether, Dipropylene Glycol Monomethyl Ether, Diethylene Glycol, Diethylene Glycol Monoacetate, Diethylene Glycol Dimethyl Ether, Dipropylene Glycol Monoacetate Monomethyl ether, dipropylene glycol monomethyl ether, dipropylene glycol monoethyl ether, dipropylene glycol monoacetate monoethyl ether, dipropylene glycol monopropyl ether, dipropylene glycol monoacetate monopropyl ether, 3- Methyl-3-methoxybutyl acetate, tripropylene glycol methyl ether, 3-methyl-3-methoxybutanol, diisopropyl ether, ethyl isobutyl ether, diisobutylene, acetic acid Amyl ester, butyl butyrate, butyl ether, diisobutyl ketone, methylcyclohexene, propyl ether, dihexyl ether, dioxane, n-hexane, n-pentane, n-octane, Diethyl ether, cyclohexanone, ethylidene carbonate, propylidene carbonate, methyl lactate, ethyl lactate, methyl acetate, ethyl acetate, n-butyl anhydride, propylene glycol monoethyl ether acetate, pyruvic acid methyl ester, ethyl pyruvate, methyl 3-methoxypropionate, methyl ethyl 3-ethoxypropionate, ethyl 3-methoxypropionate, 3-ethoxypropionic acid, 3- Methoxypropionic acid, propyl 3-methoxypropionate, butyl 3-methoxypropionate, diethylene glycol dimethyl ether, 4-hydroxy-4-methyl-2-pentanone, etc., but Not limited to these. These may be used alone or in combination of two or more.
進而,即使是不能溶解聚醯胺酸的溶劑,只要是在生成的聚醯胺酸不會析出的範圍內,亦可與上述溶劑混合來使用。又,因為有機溶劑中的水分不但會阻礙聚合反應,且進而成為使生成的聚醯胺酸水解之原因,故以使用已盡可能脫水乾燥後的有機溶劑為較佳。 Furthermore, even if it is a solvent incapable of dissolving polyamic acid, as long as it is a range in which the produced polyamic acid does not precipitate, you may mix and use it with the said solvent. In addition, the moisture in the organic solvent not only inhibits the polymerization reaction, but also causes the hydrolysis of the produced polyamic acid. Therefore, it is preferable to use an organic solvent that has been dehydrated and dried as much as possible.
作為使上述四羧酸二酐成分與二胺成分在有機溶劑中反應之方法,可舉例如下方法:攪拌使二胺成分分散或溶解於有機溶劑中而得到分散液或溶液,於該分散 液或溶液中直接添加四羧酸二酐成分、或添加有機溶劑為分散或溶解有四羧酸二酐成分者之方法;相反地,於有機溶劑中為分散或溶解有四羧酸二酐成分的分散液或溶液中添加二胺成分之方法;又將四羧酸二酐成分與二胺化合物成分交替添加之方法等,可使用該等中任一之方法。 As a method of reacting the above-mentioned tetracarboxylic dianhydride component and diamine component in an organic solvent, a method of dispersing or dissolving the diamine component in an organic solvent by stirring to obtain a dispersion or solution, A method of directly adding a tetracarboxylic dianhydride component to a liquid or solution, or adding an organic solvent to disperse or dissolve the tetracarboxylic dianhydride component; on the contrary, to disperse or dissolve the tetracarboxylic dianhydride component in an organic solvent The method of adding the diamine component to the dispersion liquid or solution of the above; the method of adding the tetracarboxylic dianhydride component and the diamine compound component alternately, etc., any of these methods can be used.
又,四羧酸二酐成分及/或二胺成分為由多種的化合物所成時,可以在預先混合的狀態下使其反應,亦可使其各別地依序反應,進而可使各別地反應而得到的低分子量體混合反應而得到高分子量體。 Moreover, when the tetracarboxylic dianhydride component and/or the diamine component are composed of a plurality of compounds, they may be reacted in a state of being mixed in advance, or may be reacted in sequence separately, and further The low-molecular-weight body obtained by the direct reaction is mixed and reacted to obtain a high-molecular-weight body.
上述之聚醯胺酸合成時之溫度係適宜設定在上述使用之溶劑之融點至沸點為止之範圍內即可,例如可選擇-20℃~150℃之任意之溫度,如-5℃~100℃,通常為0~100℃左右,較佳為0~70℃左右。 The temperature during the synthesis of the above-mentioned polyamic acid should be appropriately set within the range from the melting point to the boiling point of the solvent used above. °C is usually about 0 to 100 °C, preferably about 0 to 70 °C.
反應時間係取決於反應溫度或原料物質之反應性,而無法一概地予以規定,但通常為1~100小時左右。 The reaction time depends on the reaction temperature and the reactivity of the raw material, and cannot be determined uniformly, but is usually about 1 to 100 hours.
又,反應可在任意濃度下進行,若濃度過低時,難以得到高分子量的聚合物;若濃度過高時,反應液之黏性會變得過高而難以均勻的攪拌,故在四羧酸二酐成分與二胺成分之反應溶液中之合計濃度,較佳為1~50質量%,又較佳為5~40質量%。亦可於反應初期以高濃度來進行,之後再追加有機溶劑。 In addition, the reaction can be carried out at any concentration. If the concentration is too low, it will be difficult to obtain a polymer with high molecular weight; if the concentration is too high, the viscosity of the reaction solution will become too high and it will be difficult to stir uniformly. The total concentration in the reaction solution of the acid dianhydride component and the diamine component is preferably 1 to 50% by mass, and more preferably 5 to 40% by mass. It is also possible to carry out the reaction at a high concentration in the initial stage, and then add an organic solvent.
作為使聚醯胺酸醯亞胺化之方法,可舉例直接加熱聚 醯胺酸的溶液的熱醯亞胺化、在聚醯胺酸的溶液中添加觸媒的觸媒醯亞胺化。 As a method of imidizing the polyamide, direct heating of the polyamide can be exemplified. Thermal imidization of a solution of aramidic acid and catalytic imidization of a solution of polyamide acid by adding a catalyst.
在溶液中使聚醯胺酸熱醯亞胺化時,溫度為100℃~400℃,較佳為120℃~250℃,以將藉由醯亞胺化反應所生成的水排出至體系外,同時進行醯亞胺化為較佳。 When thermal imidization of the polyamic acid in the solution is performed, the temperature is 100°C to 400°C, preferably 120°C to 250°C, so as to discharge the water generated by the imidization reaction to the outside of the system, Simultaneous imidization is preferably performed.
聚醯胺酸的化學(觸媒)醯亞胺化,係可藉由在聚醯胺酸的溶液中添加鹼性觸媒與酸酐,在-20~250℃、較佳為0~180℃之溫度條件下予以系內攪拌來進行。 The chemical (catalyst) imidization of polyamic acid can be carried out by adding alkaline catalyst and acid anhydride to the solution of polyamic acid at -20~250℃, preferably 0~180℃. It is carried out by stirring in the system under temperature conditions.
鹼性觸媒之量,係聚醯胺酸之醯胺酸基的0.5~30莫耳倍,較佳為1.5~20莫耳倍,酸酐之量,係聚醯胺酸之醯胺酸基的1~50莫耳倍,較佳為2~30莫耳倍。 The amount of the alkaline catalyst is 0.5~30 mole times of the amide group of the polyamide acid, preferably 1.5~20 mole times, and the amount of the acid anhydride is the amount of the amide group of the polyamide acid. 1~50 mole times, preferably 2~30 mole times.
作為鹼性觸媒,可舉例吡啶、三乙基胺、三甲基胺、三丁基胺、三辛基胺、1-乙基哌啶等,其中吡啶具有對於使反應進行而言為適度的鹼性,故為較佳。 Examples of the basic catalyst include pyridine, triethylamine, trimethylamine, tributylamine, trioctylamine, 1-ethylpiperidine, and the like, wherein pyridine has an appropriate amount for the reaction to proceed. Alkaline, so it is better.
作為酸酐,可舉例乙酸酐、偏苯三甲酸酐、苯均四酸二酐等,其中若使用乙酸酐時,易於進行反應結束後的純化,故為較佳。 Examples of the acid anhydride include acetic anhydride, trimellitic anhydride, pyromellitic dianhydride, and the like. Among them, when acetic anhydride is used, purification after the completion of the reaction is easy, and is therefore preferred.
採用觸媒醯亞胺化之醯亞胺化率,可藉由調節觸媒量與反應溫度、反應時間來控制。 The imidization rate of the imidization using the catalyst can be controlled by adjusting the amount of the catalyst, the reaction temperature and the reaction time.
本發明中使用的聚醯亞胺樹脂中,醯胺酸基之脫水閉環率(醯亞胺化率)沒有一定是100%,可因應用途或目的而任意調整來使用。特佳為50%以上。 In the polyimide resin used in the present invention, the dehydration ring closure rate (imidation rate) of the amide acid group is not necessarily 100%, and can be adjusted arbitrarily according to the application or purpose. Excellent is more than 50%.
本發明中,在將上述反應溶液過濾後,可直接使用該濾液、或亦可於稀釋或濃縮,並對此調配後述之 二氧化矽等使其成為樹脂薄膜形成用組成物。如此般地若經由過濾時,不僅可減低雜質(其係使所得到的樹脂薄膜的耐熱性、柔軟性或線膨脹係數特性惡化之原因)之混入,亦可效率良好地得到樹脂薄膜形成用組成物。 In the present invention, after filtering the above-mentioned reaction solution, the filtrate can be used directly, or it can also be diluted or concentrated, and the preparation will be described later. Silicon dioxide or the like makes it a composition for forming a resin film. Through filtration in this way, not only can the contamination of impurities (which cause deterioration of the heat resistance, flexibility, or linear expansion coefficient characteristics of the obtained resin film) be reduced, but also the composition for forming a resin film can be efficiently obtained. thing.
又,本發明中使用的聚醯亞胺,考量樹脂薄膜之強度、形成樹脂薄膜之際之作業性、樹脂薄膜之均勻性等,藉由凝膠滲透色譜法(GPC)的聚苯乙烯換算而得的重量平均分子量(Mw)以5,000至200,000為較佳。 The polyimide used in the present invention is determined in terms of polystyrene by gel permeation chromatography (GPC) in consideration of the strength of the resin film, workability when forming the resin film, and uniformity of the resin film. The obtained weight average molecular weight (Mw) is preferably 5,000 to 200,000.
從聚醯胺酸及聚醯亞胺的反應溶液中回收聚合物成分並使用時,只要將反應溶液投入至弱溶劑中使其沉澱即可。作為用於沉澱的弱溶劑,可舉例甲醇、丙酮、己烷、丁基賽珞蘇、庚烷、甲基乙基酮、甲基異丁酮、乙醇、甲苯、苯、異丙醇、水等。投入至弱溶劑中而使其沉澱的聚合物可在濾過回收後,在常壓或減壓下於常溫或加熱來進行乾燥。 When the polymer component is recovered from the reaction solution of polyimide and polyimide and used, the reaction solution may be thrown into a weak solvent and precipitated. Examples of the weak solvent for precipitation include methanol, acetone, hexane, butyl cylosol, heptane, methyl ethyl ketone, methyl isobutyl ketone, ethanol, toluene, benzene, isopropanol, water, and the like. . The polymer which is thrown into the weak solvent and precipitated can be collected by filtration, and then dried at normal temperature or by heating under normal pressure or reduced pressure.
又,若重複使沉澱回收的聚合物再溶解至有機溶劑中,並再沉澱回收的操作2~10次,則可減少聚合物中的雜質。作為此時的弱溶劑,若使用例如醇類、酮類、烴等3種類以上的弱溶劑時,則可更進一步提高純化的效率故為較佳。 In addition, if the operation of re-dissolving the polymer recovered by precipitation in an organic solvent and then reprecipitating and recovering is repeated 2 to 10 times, impurities in the polymer can be reduced. As the weak solvent at this time, it is preferable to use three or more types of weak solvents, such as alcohols, ketones, and hydrocarbons, because the efficiency of purification can be further improved.
再沉澱回收步驟中,使樹脂成分溶解的有機溶劑未特別限定。作為具體例,可舉例N,N-二甲基甲醯 胺、N,N-二甲基乙醯胺、N-甲基-2-吡咯啶酮、N-甲基己內醯胺、2-吡咯啶酮、N-乙基吡咯啶酮、N-乙烯基吡咯啶酮、二甲基亞碸、四甲基脲、吡啶、二甲基碸、γ-丁內酯、1,3-二甲基-咪唑啉酮、二戊烯、乙基戊基酮、甲基壬基酮、甲基乙基酮、甲基異戊基酮、甲基異丙基酮、環己酮、碳酸伸乙酯、碳酸伸丙酯、二乙二醇二甲醚、4-羥基-4-甲基-2-戊酮等。該等的溶劑亦可混合2種類以上來使用。 In the reprecipitation recovery step, the organic solvent for dissolving the resin component is not particularly limited. Specific examples include N,N-dimethylformamide Amine, N,N-dimethylacetamide, N-methyl-2-pyrrolidone, N-methylcaprolactam, 2-pyrrolidone, N-ethylpyrrolidone, N-vinyl pyrrolidone, dimethylsulfoxide, tetramethylurea, pyridine, dimethylsulfoxide, gamma-butyrolactone, 1,3-dimethyl-imidazolidinone, dipentene, ethyl amyl ketone , methyl nonyl ketone, methyl ethyl ketone, methyl isoamyl ketone, methyl isopropyl ketone, cyclohexanone, ethylene carbonate, propyl carbonate, diethylene glycol dimethyl ether, 4 -Hydroxy-4-methyl-2-pentanone, etc. These solvents may be used by mixing two or more types.
本發明中使用的二氧化矽(矽石)未特別限定,粒子形態的二氧化矽,例如平均粒徑為100nm以下,例如5nm~100nm,較佳為5nm~55nm,就可再現性良好地得到更高透明之薄膜之觀點而言,較佳為5nm~50nm,又較佳為5nm~45nm,更較佳為5nm~35nm,又更佳為5nm~30nm。 The silica (silica) used in the present invention is not particularly limited, and silica in particle form, for example, with an average particle diameter of 100 nm or less, for example, 5 nm to 100 nm, preferably 5 nm to 55 nm, can be obtained with good reproducibility. From the viewpoint of a higher transparent film, it is preferably 5 nm to 50 nm, more preferably 5 nm to 45 nm, more preferably 5 nm to 35 nm, and still more preferably 5 nm to 30 nm.
本發明中所謂的二氧化矽粒子之平均粒徑,係使用二氧化矽粒子並從藉由氮氣吸附法所測定的比表面積值所算出的平均粒徑值。 The average particle diameter of the silica particles in the present invention is the average particle diameter value calculated from the specific surface area value measured by the nitrogen gas adsorption method using the silica particles.
特以本發明可適合使用具有上述平均粒徑之值之膠態矽石(colloidal silica),作為該膠態矽石,可使用矽石溶膠。作為矽石溶膠,可使用將矽酸鈉水溶液作為原料並藉由周知的方法所製造的水性矽石溶膠及該水性矽石溶膠之分散媒之水取代成為有機溶劑而得到的有機矽石溶 膠。 In particular, colloidal silica having the above-mentioned average particle diameter can be suitably used in the present invention, and as the colloidal silica, a silica sol can be used. As the silica sol, an aqueous silica sol produced by a known method using an aqueous sodium silicate solution as a raw material, and an organosilica sol obtained by replacing the water as a dispersing medium of the aqueous silica sol with an organic solvent can be used. glue.
又,亦可使用矽酸甲酯或矽酸乙酯等之烷氧基矽烷在醇等之有機溶劑中,並在觸媒(例如氨、有機胺化合物、氫氧化鈉等之鹼觸媒)之存在下予以水解、縮合所得到的矽石溶膠、或將該矽石溶膠取代成為其他的有機溶劑而得到的有機矽石溶膠。 In addition, alkoxysilanes such as methyl silicate or ethyl silicate can also be used in organic solvents such as alcohols and in catalysts (such as ammonia, organic amine compounds, alkali catalysts such as sodium hydroxide, etc.). A silica sol obtained by hydrolysis and condensation in the presence of the silica sol, or an organosilica sol obtained by substituting the silica sol with another organic solvent.
該等之中,本發明係以使用分散媒為有機溶劑之有機矽石溶膠為較佳。 Among these, in the present invention, it is preferable to use an organosilicon sol in which the dispersing medium is an organic solvent.
作為上述之有機矽石溶膠中之有機溶劑之例,可舉例甲醇、乙醇、異丙醇等之低階醇;N,N-二甲基甲醯胺、N,N-二甲基乙醯胺等之直鏈醯胺類;N-甲基-2-吡咯啶酮等之環狀醯胺類;γ-丁內酯等之醚類;乙基賽珞蘇、乙二醇等之二醇類、乙腈等。該取代係可藉由蒸餾法、超過濾法等之通常的方法來進行。 As examples of the organic solvent in the above-mentioned organosilicon sol, lower alcohols such as methanol, ethanol, isopropanol, etc.; N,N-dimethylformamide, N,N-dimethylacetamide Linear amides such as N-methyl-2-pyrrolidone; cyclic amides such as N-methyl-2-pyrrolidone; ethers such as γ-butyrolactone; diols such as ethyl cylosoline and ethylene glycol , acetonitrile, etc. The substitution can be carried out by ordinary methods such as distillation and ultrafiltration.
上述之有機矽石溶膠之黏度係以20℃下、0.6mPa‧s~100mPa‧s左右。 The viscosity of the above-mentioned organosilicon sol is about 0.6mPa·s~100mPa·s at 20℃.
作為上述有機矽石溶膠之市售品之例,可舉例如商品名MA-ST-S(甲醇分散矽石溶膠、日產化學工業(股)製)、商品名MT-ST(甲醇分散矽石溶膠、日產化學工業(股)製)、商品名MA-ST-UP(甲醇分散矽石溶膠、日產化學工業(股)製)、商品名MA-ST-M(甲醇分散矽石溶膠、日產化學工業(股)製)、商品名MA-ST-L(甲醇分散矽石溶膠、日產化學工業(股)製)、商品名IPA-ST-S(異丙醇分散矽石溶膠、日產化學工業(股)製)、商品名IPA-ST(異丙醇 分散矽石溶膠、日產化學工業(股)製)、商品名IPA-ST-UP(異丙醇分散矽石溶膠、日產化學工業(股)製)、商品名IPA-ST-L(異丙醇分散矽石溶膠、日產化學工業(股)製)、商品名IPA-ST-ZL(異丙醇分散矽石溶膠、日產化學工業(股)製)、商品名NPC-ST-30(n-丙基賽珞蘇分散矽石溶膠、日產化學工業(股)製)、商品名PGM-ST(1-甲氧基-2-丙醇分散矽石溶膠、日產化學工業(股)製)、商品名DMAC-ST(二甲基乙醯胺分散矽石溶膠、日產化學工業(股)製)、商品名XBA-ST(二甲苯‧n-丁醇混合溶劑分散矽石溶膠、日產化學工業(股)製)、商品名EAC-ST(乙酸乙酯分散矽石溶膠、日產化學工業(股)製)、商品名PMA-ST(丙二醇單甲基醚乙酸酯分散矽石溶膠、日產化學工業(股)製)、商品名MEK-ST(甲基乙基酮分散矽石溶膠、日產化學工業(股)製)、商品名MEK-ST-UP(甲基乙基酮分散矽石溶膠、日產化學工業(股)製)、商品名MEK-ST-L(甲基乙基酮分散矽石溶膠、日產化學工業(股)製)及商品名MIBK-ST(甲基異丁酮分散矽石溶膠、日產化學工業(股)製)等,但不限定於該等。 Examples of commercial products of the above-mentioned organosilica sol include trade name MA-ST-S (methanol-dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name MT-ST (methanol-dispersed silica sol), and trade name. , Nissan Chemical Industry Co., Ltd.), trade name MA-ST-UP (methanol-dispersed silica sol, Nissan Chemical Industry Co., Ltd.), trade name MA-ST-M (methanol-dispersed silica sol, Nissan Chemical Industry Co., Ltd.) (stock), trade name MA-ST-L (methanol-dispersed silica sol, Nissan Chemical Industry Co., Ltd.), trade name IPA-ST-S (isopropanol-dispersed silica sol, Nissan Chemical Industry (stock) ), trade name IPA-ST (isopropyl alcohol Dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name IPA-ST-UP (isopropanol-dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name IPA-ST-L (isopropyl alcohol) Dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name IPA-ST-ZL (isopropanol-dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name NPC-ST-30 (n-propane Kisailuosu dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name PGM-ST (1-methoxy-2-propanol-dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name DMAC-ST (dimethylacetamide-dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name XBA-ST (xylene-n-butanol mixed solvent-dispersed silica sol, Nissan Chemical Industry Co., Ltd.) made), trade name EAC-ST (ethyl acetate dispersed silica sol, manufactured by Nissan Chemical Industry Co., Ltd.), trade name PMA-ST (propylene glycol monomethyl ether acetate dispersed silica sol, Nissan Chemical Industry Co., Ltd. ), trade name MEK-ST (Methyl Ethyl Ketone Dispersed Silica Sol, Nissan Chemical Industry Co., Ltd.), trade name MEK-ST-UP (Methyl Ethyl Ketone Dispersed Silica Sol, Nissan Chemical Industry Co., Ltd.) (stock), trade name MEK-ST-L (methyl ethyl ketone disperse silica sol, Nissan Chemical Industry Co., Ltd.) and trade name MIBK-ST (methyl isobutyl ketone disperse silica sol, Nissan Chemical industry (stock) system), etc., but not limited to these.
本發明中的二氧化矽,作為例如有機矽石溶膠來使用並舉例如上述製品般的二氧化矽,亦可混合二種以上來使用。 The silica in the present invention may be used as, for example, an organosilicon sol, and may be used as a mixture of two or more of the above-mentioned products.
本發明中使用的交聯劑係僅由氫原子、碳原子、氮原 子及氧原子所構成之化合物並具有2以上選自由羥基、環氧基及碳原子數1~5之烷氧基所成之群之基,且由具有環構造之化合物所成的交聯劑。於此,所謂“僅由氫原子、碳原子、氮原子及氧原子所構成”,係指僅由選自由上述4原子所成之群之原子所構成之意,即,不單是包含上述4原子之全部且僅由該等原子所構成,亦可為僅由上述4原子中之3原子(例如氫原子、碳原子及氧原子等)所構成。藉由使用如此般之交聯劑,不僅可再現性良好地得到耐溶劑性為優異的樹脂薄膜,亦可實現保存安定性為更加改善的樹脂組成物。 The crosslinking agent used in the present invention is composed of only hydrogen atoms, carbon atoms and nitrogen atoms. A compound composed of a proton and an oxygen atom and having 2 or more groups selected from the group consisting of a hydroxyl group, an epoxy group, and an alkoxy group having 1 to 5 carbon atoms, and a crosslinking agent composed of a compound having a ring structure . Here, "consisting only of hydrogen atoms, carbon atoms, nitrogen atoms, and oxygen atoms" means consisting of only atoms selected from the group of the above-mentioned four atoms, that is, not only including the above-mentioned four atoms All and only of these atoms may be composed of only 3 of the above-mentioned 4 atoms (for example, a hydrogen atom, a carbon atom, an oxygen atom, etc.). By using such a crosslinking agent, not only a resin film excellent in solvent resistance can be obtained with good reproducibility, but also a resin composition with further improved storage stability can be realized.
其中,交聯劑中每之一化合物的羥基、環氧基及碳原子數1~5之烷氧基之合計數,就再現性良好地實現所得到的樹脂薄膜的耐溶劑性之觀點而言,較佳為3以上,就再現性良好實現所得到的樹脂薄膜的柔軟性之觀點而言,較佳為10以下,又較佳為8以下,又更佳為6以下。 Among them, the total number of hydroxyl groups, epoxy groups, and alkoxy groups having 1 to 5 carbon atoms in each compound in the crosslinking agent is from the viewpoint of realizing the solvent resistance of the obtained resin film with good reproducibility. , is preferably 3 or more, and is preferably 10 or less, more preferably 8 or less, still more preferably 6 or less, from the viewpoint of achieving good reproducibility and flexibility of the obtained resin film.
作為交聯劑所具有的環構造之具體例,可舉例苯等之芳基環、吡啶、吡嗪、嘧啶、嗒、1,3,5-三嗪等之含氮原子雜芳基環、環戊烷、環己烷、環庚烷等之環烷烴環、哌啶、哌嗪、六氫嘧啶、六氫嗒、六氫-1,3,5-三嗪等之環狀胺等。 Specific examples of the ring structure of the crosslinking agent include aryl rings such as benzene, pyridine, pyrazine, pyrimidine, pyridine, etc. , 1,3,5-triazine and other nitrogen-containing heteroaryl rings, cyclopentane, cyclohexane, cycloheptane and other cycloalkane rings, piperidine, piperazine, hexahydropyrimidine, hexahydropyridine , cyclic amines such as hexahydro-1,3,5-triazine, etc.
交聯劑中之每一化合物之環構造的數目只要是1以上即可未特別限定,但就確保交聯劑之對於溶劑之溶解性並得到平坦性為高的樹脂薄膜之觀點而言,以1或2為較佳。 The number of ring structures per compound in the crosslinking agent is not particularly limited as long as it is 1 or more, but from the viewpoint of securing the solubility of the crosslinking agent in the solvent and obtaining a resin film with high flatness, 1 or 2 is preferred.
尚,若環構造存在2以上時,環構造彼此可縮合,亦可介隔著亞甲基、伸乙基、三伸乙基、丙烷-2,2-二基等之碳原子數1~5之烷烴-二基等之連結基來使環構造彼此鍵結。 In addition, if there are 2 or more ring structures, the ring structures can be condensed with each other, and the number of carbon atoms such as methylene, ethylidene, triphenylene, and propane-2,2-diyl can be 1~5. A linking group such as an alkane-diyl group is used to bond the ring structures to each other.
交聯劑之分子量,只要是具有交聯能力、且能溶解於使用的溶劑中即可未特別限定,但若考慮所得到的樹脂薄膜的溶劑耐性、交聯劑本身之對於有機溶劑的溶解性、取得容易性或價格等時,較佳為100~500左右,又較佳為150~400左右。 The molecular weight of the cross-linking agent is not particularly limited as long as it has cross-linking ability and can be dissolved in the solvent used, but the solvent resistance of the obtained resin film and the solubility of the cross-linking agent itself in organic solvents are considered. , In terms of availability or price, it is preferably about 100 to 500, and more preferably about 150 to 400.
交聯劑係進而亦可具有能由酮基、酯基(鍵結)等、氫原子、碳原子、氮原子及氧原子衍生之基。 The crosslinking agent may further have a group which can be derived from a ketone group, an ester group (bonding), etc., a hydrogen atom, a carbon atom, a nitrogen atom, and an oxygen atom.
作為交聯劑,作為較佳例子,可舉例下述式(K1)~(K5)所表示的化合物,分別作為式(K4)之較佳樣態之1,可舉例式(K4-1)所表示的化合物;作為式(K5)之較佳樣態之1,可舉例式(5-1)所表示的化合物。 As a cross-linking agent, as a preferred example, the compounds represented by the following formulae (K1) to (K5) can be exemplified, respectively as one of the preferred forms of the formula (K4), and the compounds represented by the formula (K4-1) can be exemplified respectively. The compound represented by the formula (K5) can be exemplified by the compound represented by the formula (5-1).
上述式中,各A1及A2係相互獨立表示亞甲基、伸乙基、三伸乙基、丙烷-2,2-二基等之碳原子數1~5之烷烴-二基,其中作為A1係以亞甲基、伸乙基為較佳,亞甲基為又較佳,作為A2係以亞甲基、丙烷-2,2-二基為較佳。 In the above formula, each of A 1 and A 2 independently represents an alkane-diyl group having 1 to 5 carbon atoms such as methylene, ethylidene, trimethylene, propane-2,2-diyl, etc., wherein As A 1 series, methylene group and ethylidene group are preferred, methylene group is more preferred, and as A 2 series, methylene group and propane-2,2-diyl group are preferred.
各X係相互獨立表示羥基、環氧基(氧雜-環丙基)、或甲氧基、乙氧基、1-丙氧基、異丙氧基、1-丁氧基、t-丁氧基等之碳原子數1~5之烷氧基。 Each X series independently represents hydroxyl, epoxy (oxa-cyclopropyl), or methoxy, ethoxy, 1-propoxy, isopropoxy, 1-butoxy, t-butoxy Alkoxy groups having 1 to 5 carbon atoms such as radicals.
其中,若考慮交聯劑的取得容易性、價格等時,X係式(K1)及(K5)中以環氧基為較佳,式(K2)及(K3)中以碳原子數1~5之烷氧基為較佳,式(K4)中以羥基為較佳。 Among them, considering the availability and price of the cross-linking agent, epoxy groups are preferred in the X-series formulas (K1) and (K5), and 1 to 1 to carbon atoms are preferred in the formulas (K2) and (K3). The alkoxy group of 5 is preferred, and the hydroxyl group in formula (K4) is preferred.
式(K4)中,各n係表示與苯環鍵結的-(A1-X) 基的數目,且相互獨立為1~5的整數,但較佳為2~3,又較佳為3。 In the formula (K4), each n represents the number of -(A 1 -X) groups bonded to the benzene ring, and independently of each other is an integer of 1 to 5, but preferably 2 to 3, and preferably 3 .
各化合物中,各A1係以全部為相同之基為較佳,各X係以全部為相同之基為較佳。 In each compound, it is preferable that all A 1 series are the same group, and each X series is preferably all the same group.
上述式(K1)~(K5)所表示的化合物可藉由下述般而得到:將具有與該等各化合物中的環構造為相同環構造之芳基化合物、雜芳基化合物、環狀胺等之骨架化合物、與環氧烷基鹵化物化合物、烷氧基鹵化物化合物等,經碳-碳偶合反應或N-烷基化反應,或將產物之烷氧基部位水解。 The compounds represented by the above formulae (K1) to (K5) can be obtained by combining aryl compounds, heteroaryl compounds, and cyclic amines having the same ring structure as those in these compounds. The skeleton compound, etc., with epoxy alkyl halide compound, alkoxy halide compound, etc., undergo carbon-carbon coupling reaction or N-alkylation reaction, or hydrolyze the alkoxy group of the product.
交聯劑係可使用市售品、亦可使用以周知的合成方法所合成者。 As a crosslinking agent, a commercial item may be used, and what was synthesize|combined by a well-known synthesis method may be used.
作為市售品,可舉例CYMEL(註冊商標)300、同301、同303LF,同303ULF、同304、同350、同3745、同X W 3106、同MM-100、同323、同325、同327、同328、同385、同370、同373、同380、同1116、同1130、同1133、同1141、同1161、同1168、同3020、同202、同203、同1156、同MB-94、同MB-96、同MB-98、同247-10、同651、同658、同683、同688、同1158、同MB-14、同MI-12-I、同MI-97-IX、同U-65、同UM-15、同U-80、同U-21-511、同U-21-510、同U-216-8、同U-227-8、同U-1050-10、同U-1052-8、同U-1054、同U-610、同U-640、同UB-24-BX、同UB-26-BX、同UB-90-BX、同UB-25-BE、同UB-30-B、同U- 662、同U-663、同U-1051、同UI-19-I、同UI-19-IE、同UI-21-E、同U1-27-EI、同U-38-I、同UI-20-E同659、同1123、同1125、同5010、同1170、同1172、同NF3041、同NF2000等(以上,allnex公司製);TEPIC(註冊商標)V、同S、同HP、同L、同PAS、同VL、同UC(以上,日產化學工業(股)製)、TM-BIP-A(旭有機材工業(股)製)、1,3,4,6-肆(甲氧基甲基)乙炔脲(以下,簡稱TMG)(東京化成工業(股)製)、4,4’-亞甲基雙(N,N-二縮水甘油基苯胺)(Aldrich公司製)等。 Examples of commercial products include CYMEL (registered trademark) 300, the same 301, the same 303LF, the same 303ULF, the same 304, the same 350, the same 3745, the same XW 3106, the same MM-100, the same 323, the same 325, the same 327, Same as 328, same as 385, same as 370, same as 373, same as 380, same as 1116, same as 1130, same as 1133, same as 1141, same as 1161, same as 1168, same as 3020, same as 202, same as 203, same as 1156, same as MB-94, Same as MB-96, Same as MB-98, Same as 247-10, Same as 651, Same as 658, Same as 683, Same as 688, Same as 1158, Same as MB-14, Same as MI-12-I, Same as MI-97-IX, Same as U-65, same as UM-15, same as U-80, same as U-21-511, same as U-21-510, same as U-216-8, same as U-227-8, same as U-1050-10, same as U-1050-10 U-1052-8, same as U-1054, same as U-610, same as U-640, same as UB-24-BX, same as UB-26-BX, same as UB-90-BX, same as UB-25-BE, same as UB-30-B, same as U- 662, same as U-663, same as U-1051, same as UI-19-I, same as UI-19-IE, same as UI-21-E, same as U1-27-EI, same as U-38-I, same as UI- 20-E Same as 659, same as 1123, same as 1125, same as 5010, same as 1170, same as 1172, same as NF3041, same as NF2000 etc. , Same as PAS, Same as VL, Same as UC (above, Nissan Chemical Industry Co., Ltd.), TM-BIP-A (Asahi Organic Materials Industry Co., Ltd.), 1,3,4,6-4 (Methoxy Methyl)acetylene carbamide (hereinafter abbreviated as TMG) (manufactured by Tokyo Chemical Industry Co., Ltd.), 4,4'-methylenebis(N,N-diglycidylaniline) (manufactured by Aldrich Corporation), and the like.
以下,作為交聯劑可舉例較佳的具體例,但並非限定於該等。 Hereinafter, although preferable specific examples of the crosslinking agent are given, it is not limited to these.
交聯劑之調配比例,相對於前述聚醯亞胺及前述二氧化矽之合計質量為0.1~200質量%,較佳為0.2~100質量%。 The mixing ratio of the crosslinking agent is 0.1 to 200 mass %, preferably 0.2 to 100 mass %, with respect to the total mass of the polyimide and the silicon dioxide.
本發明的樹脂薄膜形成用組成物除了前述聚醯亞胺及二氧化矽以外可包含有機溶劑。該有機溶劑未特別限定,可舉例如與用於上述聚醯胺酸及聚醯亞胺之調製時的反應溶劑之具體例為相同者。更具體而言,可舉例N,N-二甲基甲醯胺、N,N-二甲基乙醯胺、N-甲基-2-吡咯啶酮、1,3-二甲基-2-咪唑啉酮、N-乙基-2-吡咯啶酮、γ-丁內酯等。尚,有機溶劑係可單獨1種使用、亦可組合2種以上來使用。 The resin film-forming composition of the present invention may contain an organic solvent in addition to the aforementioned polyimide and silica. Although this organic solvent is not specifically limited, For example, it is the same as the specific example of the reaction solvent used for the preparation of the said polyamic acid and polyimide. More specifically, N,N-dimethylformamide, N,N-dimethylacetamide, N-methyl-2-pyrrolidone, 1,3-dimethyl-2- Imidazolidinone, N-ethyl-2-pyrrolidone, γ-butyrolactone, etc. In addition, an organic solvent system may be used individually by 1 type, and may be used in combination of 2 or more types.
該等之中,若考慮可再現性良好地得到平坦性為高的樹脂薄膜時,以N,N-二甲基乙醯胺、N-甲基-2-吡咯啶酮、γ-丁內酯為較佳。 Among these, in consideration of obtaining a resin film with high flatness with good reproducibility, N,N-dimethylacetamide, N-methyl-2-pyrrolidone, γ-butyrolactone is better.
本發明係含有前述聚醯亞胺、二氧化矽與有機溶劑的樹脂薄膜形成用組成物。於此,本發明的樹脂薄膜形成用組成物為均勻且未確認到相分離者。 The present invention is a composition for forming a resin film containing the aforementioned polyimide, silica and an organic solvent. Here, the composition for forming a resin film of the present invention is uniform and phase separation is not confirmed.
本發明的樹脂薄膜形成用組成物中,前述聚醯亞胺與前述二氧化矽之調配比,以質量比,聚醯亞胺:二氧化矽=10:1~1:10為較佳,又較佳為8:2~2:8、例如7:3~3:7。 In the composition for forming a resin film of the present invention, the blending ratio of the aforementioned polyimide and the aforementioned silicon dioxide is preferably polyimide:silicon dioxide=10:1~1:10 in terms of mass ratio. Preferably it is 8:2~2:8, for example, 7:3~3:7.
本發明的樹脂薄膜形成用組成物中,交聯劑之調配比例,相對於前述聚醯亞胺及前述二氧化矽之合計質量為 0.1~200質量%,較佳為0.2~100質量%。 In the composition for forming a resin film of the present invention, the mixing ratio of the crosslinking agent relative to the total mass of the polyimide and the silicon dioxide is: 0.1 to 200 mass %, preferably 0.2 to 100 mass %.
又,本發明的樹脂薄膜形成用組成物中之固形物含量之調配量,通常為0.5~30質量%左右,較佳為5~25質量%左右。若固形物含量濃度未滿0.5質量%時,在製作樹脂薄膜方面的製膜效率會變低,又,由於樹脂薄膜形成用組成物之黏度變低,故難以得到表面為均勻的塗膜。又,若固形物含量濃度超過30質量%時,樹脂薄膜形成用組成物之黏度會變得過高,依然有成膜效率之惡化或欠缺塗膜之表面均勻性之虞。尚,於此所謂的固形物含量,係意味著有機溶劑以外之成分之總質量,即使是液狀的單體等亦設定作為固形物而包含於重量中。 Moreover, the compounding quantity of the solid content in the composition for resin film formation of this invention is about 0.5-30 mass % normally, Preferably it is about 5-25 mass %. If the solid content concentration is less than 0.5 mass %, the film-forming efficiency in the production of the resin film will be low, and the viscosity of the resin film-forming composition will be low, making it difficult to obtain a uniform coating film on the surface. Moreover, when the solid content concentration exceeds 30 mass %, the viscosity of the composition for resin film formation will become too high, and there exists a possibility that film-forming efficiency may deteriorate or the surface uniformity of a coating film may be lacking. Incidentally, the solid content as used herein means the total mass of components other than the organic solvent, and even liquid monomers are included in the weight as solids.
尚,樹脂薄膜形成用組成物之黏度,係考量所製作的樹脂薄膜之厚度等而予以適當設定,若特別以可再現性良好地得到厚度5~50μm左右的樹脂薄膜為目的時,通常在25℃下為500~50,000mPa‧s左右,較佳為1,000~20,000mPa‧s左右。 In addition, the viscosity of the composition for forming a resin film is appropriately set in consideration of the thickness of the resin film to be produced, etc. In particular, if the purpose of obtaining a resin film with a thickness of about 5 to 50 μm with good reproducibility is especially, it is usually 25 μm. At ℃, it is about 500~50,000mPa·s, preferably about 1,000~20,000mPa·s.
為了賦予加工特性或各種機能性,本發明的樹脂薄膜形成用組成物中亦可調配其他各式各樣的有機或無機的低分子或高分子化合物。可使用例如、觸媒、消泡劑、調平劑、界面活性劑、染料、可塑劑、微粒子、偶合劑、增感劑等。例如,就降低樹脂薄膜之延遲或線膨脹係數之目的下,可添加觸媒。尚,除了前述聚醯亞胺、二氧化矽及有機溶劑以外,進而包含觸媒的樹脂薄膜形成用組成物亦可設定為本發明的對象。 Various other organic or inorganic low-molecular or high-molecular compounds may be blended in the composition for forming a resin film of the present invention in order to impart processing properties and various functionalities. For example, catalysts, antifoaming agents, leveling agents, surfactants, dyes, plasticizers, fine particles, coupling agents, sensitizers and the like can be used. For example, a catalyst may be added for the purpose of reducing the retardation or linear expansion coefficient of the resin film. Furthermore, in addition to the aforementioned polyimide, silica, and organic solvent, a composition for forming a resin thin film that further includes a catalyst can also be set as the object of the present invention.
本發明的樹脂薄膜形成用組成物,可藉由將以上述之方法所得到的聚醯亞胺以及二氧化矽溶解於上述之有機溶劑中而得到,亦可將二氧化矽添加至聚醯亞胺之調製後的反應溶液中,並依所希望進而在添加前述有機溶劑而得到。 The composition for forming a resin film of the present invention can be obtained by dissolving the polyimide and silica obtained by the above-mentioned method in the above-mentioned organic solvent, or by adding silica to the polyimide It can be obtained by further adding the above-mentioned organic solvent to the reaction solution after the preparation of the amine as desired.
藉由將以上所說明的本發明的樹脂薄膜形成用組成物塗佈至基材並進行乾燥‧加熱,使有機溶劑除去,而可得到具有高耐熱性、高透明性、適度的柔軟性、與適度的線膨脹係數,且延遲為小的樹脂薄膜。 By applying the above-described composition for forming a resin film of the present invention to a substrate, drying and heating, and removing the organic solvent, it is possible to obtain high heat resistance, high transparency, moderate flexibility, and A moderate coefficient of linear expansion, and the retardation is a small resin film.
然後,上述樹脂薄膜,即含有上述聚醯亞胺、與上述無機矽石化合物的樹脂薄膜,亦為本發明的對象。進而,除了前述聚醯亞胺及二氧化矽以外,進而包含觸媒的樹脂薄膜亦為本發明的對象。 Then, the above-mentioned resin film, that is, the resin film containing the above-mentioned polyimide and the above-mentioned inorganic silica compound is also the object of the present invention. Furthermore, in addition to the aforementioned polyimide and silica, a resin film further including a catalyst is also the object of the present invention.
作為樹脂薄膜之製造中所使用的基材,可舉例如塑膠(聚碳酸酯、聚甲基丙烯酸酯、聚苯乙烯、聚酯、聚烯烴、環氧、三聚氰胺、三乙醯纖維素、ABS、AS、降莰烯系樹脂等)、金屬、不鏽鋼(SUS)、木材、紙、玻璃、矽晶圓、石板(slate)等。 As the base material used in the production of the resin film, for example, plastics (polycarbonate, polymethacrylate, polystyrene, polyester, polyolefin, epoxy, melamine, triacetate cellulose, ABS, AS, norbornene-based resin, etc.), metal, stainless steel (SUS), wood, paper, glass, silicon wafer, slate, etc.
特以,在作為電子裝置之基板材料來予以適用時,就可利用既有設備之觀點而言,適用之基材以玻璃、矽晶圓為較佳,又,由於所得到的樹脂薄膜為展現良好的剝離性,故以玻璃為更佳。尚,作為適用之基材之線膨脹係 數,就塗佈後的基材之翹曲之觀點而言,以30ppm/℃以下為較佳,20ppm/℃以下為更佳。 In particular, when it is applied as a substrate material for electronic devices, from the viewpoint of utilizing existing equipment, the applicable substrates are preferably glass and silicon wafers. Moreover, since the obtained resin film is used as a display material. Good peelability, so glass is better. still, as the linear expansion system of the applicable substrate From the viewpoint of the warpage of the substrate after coating, it is preferably 30 ppm/°C or lower, and more preferably 20 ppm/°C or lower.
樹脂薄膜形成用組成物之對於基材之塗佈法,未特別限定,但可舉例如澆鑄塗佈法、旋轉塗佈法、刮刀塗佈法、浸漬塗佈法、輥塗佈法、棒塗佈法、模具塗佈法、噴墨法、印刷法(凸版、凹版、平版、網版印刷等)等,可因應目的而適當使用。 The method for coating the substrate with the composition for forming a resin film is not particularly limited, and examples include cast coating, spin coating, blade coating, dip coating, roll coating, and bar coating. A cloth method, a die coating method, an ink jet method, a printing method (relief, gravure, lithography, screen printing, etc.) and the like can be appropriately used according to the purpose.
加熱溫度以300℃以下為較佳。若超過300℃時,所得到的樹脂薄膜會變脆,特別是有無法得到適合於顯示器基板用途之樹脂薄膜之情形。 The heating temperature is preferably 300°C or lower. When it exceeds 300 degreeC, the obtained resin film may become brittle, and the resin film suitable for a display board|substrate application especially may not be obtained.
又,若考量所得到的樹脂薄膜之耐熱性與線膨脹係數特性時,以將塗佈後的樹脂薄膜形成用組成物以40℃~100℃加熱5分鐘~2小時後,直接階段性的使加熱溫度上昇,以最終成為超過175℃~280℃並加熱30分~2小時為宜。如此般地,以藉由使溶劑乾燥之階段與促進分子配向之階段之2階段以上之溫度來進行加熱,可使展現出低熱膨脹特性。 In addition, considering the heat resistance and linear expansion coefficient characteristics of the obtained resin film, after heating the coated composition for forming a resin film at 40° C. to 100° C. for 5 minutes to 2 hours, it is directly applied in stages. The heating temperature rises, and it is preferable that the temperature finally exceeds 175°C to 280°C and heating is performed for 30 minutes to 2 hours. In this way, it is possible to exhibit low thermal expansion characteristics by heating at a temperature of two or more stages of the stage of drying the solvent and the stage of promoting molecular alignment.
特別是將塗佈後的樹脂薄膜形成用組成物以40℃~100℃加熱5分鐘~2小時後,以超過100℃~175℃加熱5分鐘~2小時,接著,以超過175℃超~280℃加熱5分~2小時為較佳。 In particular, the coated resin film forming composition is heated at 40°C to 100°C for 5 minutes to 2 hours, then heated at over 100°C to 175°C for 5 minutes to 2 hours, and then heated at over 175°C to 280°C ℃ heating for 5 minutes to 2 hours is preferred.
加熱中所使用的器具,可舉例如加熱板、烘箱等。加熱環境可為空氣下,亦可為氮等之惰性氣體下;又,可為常壓下,亦可為減壓下;又,在加熱之各階段中,亦可適 用不同之壓力。 As the apparatus used for heating, a hot plate, an oven, etc. are mentioned, for example. The heating environment can be under air, or under inert gas such as nitrogen; also, it can be under normal pressure or under reduced pressure; and, in each stage of heating, it can also be suitable. Use different pressures.
樹脂薄膜之厚度,特別是作為可撓性顯示器用基板使用時,通常為1~60μm左右,較佳為5~50μm左右,以調整加熱前的塗膜之厚度,使形成所希望厚度的樹脂薄膜。 The thickness of the resin film, especially when used as a substrate for a flexible display, is usually about 1 to 60 μm, preferably about 5 to 50 μm, so that the thickness of the coating film before heating can be adjusted to form a resin film of the desired thickness .
尚,作為將如此般所形成的樹脂薄膜從基材予以剝離之方法,未特別限定,可舉例使該樹脂薄膜連同基材一起冷卻,對薄膜置入裂縫並使其剝離之方法或透過輥施予張力並使其剝離之方法等。 The method for peeling the resin film formed in this way from the substrate is not particularly limited, and examples include cooling the resin film together with the substrate, inserting cracks into the film and peeling the film, or applying a roll to the film. A method of pretensioning and peeling it off, etc.
以如此般所得到的本發明較佳一樣態相關的樹脂薄膜,其係可實現在波長400nm之光透射率為75%以上的所謂的高透明性。 The thus-obtained resin film related to a preferred state of the present invention can achieve so-called high transparency with a light transmittance of 75% or more at a wavelength of 400 nm.
更,該樹脂薄膜,例如在50℃至200℃之線膨脹係數為60ppm/℃以下,特以具有10ppm/℃至35ppm/℃的所謂的低值,加熱時之尺寸安定性為優異者。 Furthermore, the resin film, for example, has a linear expansion coefficient of 60 ppm/°C or less at 50°C to 200°C, particularly a so-called low value of 10 ppm/°C to 35 ppm/°C, and is excellent in dimensional stability during heating.
特別是,該樹脂薄膜係以590nm作為入射光之波長之情形時,面內延遲R0以及厚度方向延遲Rth皆為非常小之值為優點,前述面內延遲R0係以雙折射(面內正交的2個折射率之差)與膜厚之積所表示者;前述厚度方向延遲Rth係以從厚度方向之斷面觀察時,對於2個雙折射(面內的2個折射率與厚度方向之折射率之分別的差)乘以分別的膜厚,並將所得到的2個相位差以作為平均值表示者。本發明的樹脂薄膜,若平均膜厚若大約15μm~40μm時,厚度方向之延遲Rth為未滿700nm,例如300nm以下,例 如1nm~300nm,面內延遲R0為未滿4,例如0.1~3.9,雙折射△n為未滿0.01,例如0.0003~0.009之具有所謂非常低之值。 In particular, when the wavelength of the incident light is 590 nm, the in-plane retardation R 0 and the thickness-direction retardation R th are both very small. Represented by the product of the difference between the two orthogonal refractive indices) and the film thickness; the above-mentioned thickness-direction retardation R th is observed from a cross-section in the thickness direction, for two birefringences (two refractive indices in the plane) The difference between the refractive indices in the thickness direction) is multiplied by the respective film thickness, and the obtained two retardations are expressed as an average value. In the resin film of the present invention, if the average film thickness is about 15 μm to 40 μm, the retardation R th in the thickness direction is less than 700 nm, such as 300 nm or less, such as 1 nm to 300 nm, and the in-plane retardation R 0 is less than 4, such as 0.1~ 3.9, the birefringence Δn is less than 0.01, for example, 0.0003 to 0.009 has a so-called very low value.
以上所說明的本發明的樹脂薄膜,由於具有上述之特性,故可滿足作為可撓性顯示器基板的基底薄膜所必需的各種條件,特以可適合使用作為可撓性顯示器基板的基底薄膜。 Since the resin film of the present invention described above has the above-mentioned properties, it can satisfy various conditions required as a base film of a flexible display substrate, and can be suitably used as a base film of a flexible display substrate.
以下列舉實施例來更詳細地說明本發明,但本發明並不受限於該等中。尚,使用的試劑的簡稱以及使用的裝置及其條件係如同以下般。 Hereinafter, the present invention will be described in more detail by way of examples, but the present invention is not limited to these. Furthermore, the abbreviations of the reagents used, the apparatuses used, and their conditions are as follows.
裝置:昭和電工(股)製、Showdex GPC-101 Installation: Showa Denko Co., Ltd., Showdex GPC-101
管柱:KD803及KD805 Column: KD803 and KD805
管柱溫度:50℃ Column temperature: 50℃
溶出溶劑:DMF、流量:1.5ml/分 Dissolution solvent: DMF, flow rate: 1.5ml/min
檢量線:標準聚苯乙烯 Calibration Line: Standard Polystyrene
CBDA:1,2,3,4-環丁烷四羧酸二酐 CBDA: 1,2,3,4-cyclobutanetetracarboxylic dianhydride
BODAxx:雙環[2,2,2]辛烷-2,3,5,6-四羧酸二酐 BODAxx: Bicyclo[2,2,2]octane-2,3,5,6-tetracarboxylic dianhydride
TFMB:2,2’-雙(三氟甲基)聯苯胺 TFMB: 2,2'-bis(trifluoromethyl)benzidine
NMP:N-甲基-2-吡咯啶酮 NMP: N-methyl-2-pyrrolidone
GBL:γ-丁內酯 GBL: gamma-butyrolactone
於具有氮注入/排出口、且裝配有Dean-Stark裝置與機械攪拌器的250mL的三頸反應燒瓶內,加入TFMB12.8092g(0.04mol)、γ-丁內酯(GBL)60.85g並開始攪拌。二胺(TFMB)完全地溶解於溶劑中後,加入BODAxx 5.001g(0.02莫耳)及GBL 13.04g,藉由在氮環境下以90℃加熱20分鐘。之後,加入CBDA 3.922g(0.02mol)與GBL(γ-丁內酯)13.04g,藉由在氮環境下使其反應20分鐘。將1-乙基哌啶0.87g(0.0076mol)加入於反應物中,使其昇溫至溫度180℃並保持7小時。以固形物含量濃度(去除有機溶劑的成分之濃度)成為10質量%之方式,於反應混合物中加入GBL來稀釋。之後,將稀釋的反應混合物加入於甲醇760g中並攪拌30分鐘,然後藉由過濾來回收過濾物。將該程序重複3次。 Into a 250 mL three-necked reaction flask equipped with a nitrogen injection/discharge port and equipped with a Dean-Stark device and a mechanical stirrer, TFMB 12.8092 g (0.04 mol) and γ-butyrolactone (GBL) 60.85 g were added and stirring was started . After the diamine (TFMB) was completely dissolved in the solvent, BODAxx 5.001 g (0.02 mol) and GBL 13.04 g were added, by heating at 90° C. for 20 minutes under nitrogen atmosphere. After that, 3.922 g (0.02 mol) of CBDA and 13.04 g of GBL (γ-butyrolactone) were added and reacted under nitrogen atmosphere for 20 minutes. 0.87 g (0.0076 mol) of 1-ethylpiperidine was added to the reactant, and the temperature was raised to 180° C. and kept for 7 hours. GBL was added to the reaction mixture to dilute so that the solid content concentration (the concentration of the components excluding the organic solvent) would be 10% by mass. After that, the diluted reaction mixture was added to 760 g of methanol and stirred for 30 minutes, and the filtrate was recovered by filtration. This procedure was repeated 3 times.
最後,將所得到的過濾物在真空烘箱下以150℃乾燥8小時,可得到作為目的之聚醯亞胺(I)18.77g(收率86.4%、Mw:205,321、Mn:77,087)。 Finally, the obtained filtrate was dried in a vacuum oven at 150° C. for 8 hours to obtain 18.77 g of the intended polyimide (I) (yield 86.4%, Mw: 205,321, Mn: 77,087).
於1000mL的圓底燒瓶中,加入日產化學工業(股)製甲醇分散矽石溶膠:MA-ST-M 350g(矽石固形物含量濃度:40.4質量%)與γ-丁內酯419g。然後,將該燒瓶與真空蒸發器連接並使燒瓶內減壓,藉由浸漬於約35℃的溫水浴中20~50分鐘,可得到溶劑從甲醇被取代成為γ-丁內酯的矽石溶膠(GBL-M)約560.3g(矽石固形物含量濃度:25.25質量%)。 In a 1000 mL round-bottomed flask, 350 g of methanol-dispersed silica sol: MA-ST-M (silica solid content concentration: 40.4 mass %) and 419 g of γ-butyrolactone manufactured by Nissan Chemical Industry Co., Ltd. were added. Then, the flask was connected to a vacuum evaporator, the inside of the flask was depressurized, and the silica sol in which the solvent was substituted from methanol to γ-butyrolactone was obtained by immersing it in a warm water bath at about 35° C. for 20 to 50 minutes. (GBL-M) about 560.3 g (silica solid content concentration: 25.25 mass %).
尚,上述矽石溶膠中,從藉由氮氣吸附法所測定的比表面積值所算出的平均粒徑係22nm。尚,具體而言,使用YUASA-IONICS公司製、比表面積測定裝置MONOSORB MS-16測定矽石溶膠之乾燥粉末之比表面積,並將所測定的比表面積S(m2/g)以D(nm)=2720/S之式算出平均一次粒徑。 Furthermore, in the above silica sol, the average particle diameter calculated from the specific surface area value measured by the nitrogen adsorption method is 22 nm. More specifically, the specific surface area of the dry powder of silica sol was measured using a specific surface area measuring device MONOSORB MS-16 manufactured by YUASA-IONICS, and the measured specific surface area S (m 2 /g) was expressed as D (nm). )=2720/S to calculate the average primary particle size.
在室溫下,將合成例所調製的粉末聚醯亞胺(I)1g溶解於GBL中,並使用5μm的過濾器緩慢地將所得到的溶液進行加壓過濾,來調製固形物含量濃度為8質量%的溶液(聚醯亞胺溶液(I))。於該聚醯亞胺(I)溶液中,加入調製例所調製的GBL-M矽石溶膠(矽石固形物含量濃度:25.25質量%)9.24g與Cymel 303(純度100%)1.428g,並攪拌一晚可得到樹脂薄膜形成用組成物。 At room temperature, 1 g of the powdered polyimide (I) prepared in the synthesis example was dissolved in GBL, and the resulting solution was gradually subjected to pressure filtration using a 5 μm filter to prepare a solid content concentration of 8 mass % solution (polyimide solution (I)). To this polyimide (I) solution, 9.24 g of GBL-M silica sol (concentration of silica solid content: 25.25 mass %) prepared in the preparation example and 1.428 g of Cymel 303 (purity 100%) were added, and The resin film-forming composition can be obtained by stirring overnight.
除了使用1,3,4,6-肆(甲氧基甲基)乙炔脲(TMG)(純度99%)1.442g來替代Cymel 303 1.428g以外,與實施例1-1以以相同方法而得到樹脂薄膜形成用組成物。 Obtained in the same manner as in Example 1-1, except that 1,3,4,6-tetra(methoxymethyl)acetylene carbamide (TMG) (purity 99%) 1.442g was used instead of Cymel 303 1.428g A composition for forming a resin film.
除了使用GBL-M矽石溶膠3.96g與TEPIC-L(純度99%)0.866g來替代GBL-M矽石溶膠9.24g與Cymel 303 1.428g以外,與實施例1-1以相同方法而得到樹脂薄膜形成用組成物。 A resin was obtained in the same manner as in Example 1-1, except that 3.96 g of GBL-M silica sol and 0.866 g of TEPIC-L (purity 99%) were used instead of 9.24 g of GBL-M silica sol and 1.428 g of Cymel 303 Composition for thin film formation.
除了使用Cymel 303(純度100%)0.594g與TM-BIP-A(純度98%)0.0396g來替代Cymel 303 1.428g以外,與實施例1-1以相同方法而得到樹脂薄膜形成用組成物。 A resin film-forming composition was obtained in the same manner as in Example 1-1, except that 0.594 g of Cymel 303 (purity 100%) and 0.0396 g of TM-BIP-A (purity 98%) were used instead of 1.428 g of Cymel 303.
除了不使用Cymel 303 1.428g以外,與實施例1-1以相同方法而得到樹脂薄膜形成用組成物。 A composition for forming a resin film was obtained in the same manner as in Example 1-1 except that 1.428 g of Cymel 303 was not used.
將實施例1-1所得到的樹脂薄膜形成用組成物塗佈於玻璃基板上,在大氣下將塗膜依序以50℃下30分鐘、 140℃下30分鐘、200℃下60分鐘,接著在氮環境下以280℃下60分鐘來依序加熱可得到樹脂薄膜。 The resin film-forming composition obtained in Example 1-1 was coated on a glass substrate, and the coating film was sequentially heated at 50° C. for 30 minutes under the atmosphere, followed by: A resin film can be obtained by sequentially heating at 140°C for 30 minutes, 200°C for 60 minutes, and then at 280°C for 60 minutes in a nitrogen atmosphere.
將所得到的薄膜以機械性切斷並剝離,提供於之後之評估。 The resulting film was mechanically cut and peeled for subsequent evaluation.
除了使用實施例1-2~1-4及比較例1-A所得到的樹脂薄膜形成用組成物,來替代實施例1-1所得到的樹脂薄膜形成用組成物以外,藉由與上述以相同方法而得到各樹脂薄膜。 The resin film-forming compositions obtained in Examples 1-2 to 1-4 and Comparative Example 1-A were used instead of the resin film-forming compositions obtained in Example 1-1. Each resin film was obtained by the same method.
有關上述之程序所製作的各樹脂薄膜(評估試料)的耐熱性及光學特性,即於50℃至200℃之線膨脹係數(CTE)、5%重量減少溫度(Td5%)、光線透射率(T308nm、T400nm、T550nm)及CIE b*值(黃色評估)、延遲(Rth、R0)以及雙折射(△n),依據下述程序分別來進行評估。將結果表示於表1。 The heat resistance and optical properties of each resin film (evaluation sample) produced by the above procedure, namely coefficient of linear expansion (CTE) at 50°C to 200°C, 5% weight reduction temperature (Td 5% ), light transmittance (T 308 nm , T 400 nm , T 550 nm ) and CIE b * value (yellow evaluation), retardation (R th , R 0 ) and birefringence (Δn) were evaluated according to the following procedures, respectively. The results are shown in Table 1.
使用TA Instruments公司製TMA Q400,將薄膜裁切成寬5mm、長度16mm之尺寸,首先,以10℃/min昇溫並加熱至50至300℃為止(第一加熱),接著,以10℃/min降溫並冷卻至50℃後,以10℃/min昇溫並加熱至50至420℃為止(第二加熱)之際,藉由測定第二加熱之50℃至 200℃之線膨脹係數(CTE[ppm/℃])之值而可求得。尚,第一加熱、冷卻及第二加熱之全程為施加荷重0.05N。 Using TMA Q400 manufactured by TA Instruments, the film was cut into a size of 5 mm in width and 16 mm in length. First, the temperature was increased at 10°C/min and heated to 50 to 300°C (first heating), and then the temperature was increased at 10°C/min. After cooling and cooling to 50°C, the temperature was increased at 10°C/min and heated to 50 to 420°C (second heating), by measuring the second heating from 50°C to 50°C. It can be obtained from the value of the coefficient of linear expansion (CTE [ppm/°C]) at 200°C. Furthermore, the whole process of the first heating, cooling and the second heating is an applied load of 0.05N.
5%重量減少溫度(Td5%[℃])係使用TA Instruments公司製TGA Q500,藉由測定於氮中將薄膜約5至10mg以10℃/min昇溫到50至800℃為止而可求得。 The 5% weight loss temperature (Td 5% [°C]) can be obtained by measuring about 5 to 10 mg of the film in nitrogen using TGA Q500 manufactured by TA Instruments, Inc. at a temperature of 50 to 800°C at 10°C/min. .
波長308nm、400nm及550nm之光線透射率(T308nm、T400nm、T550nm[%])及CIE b值(CIE b*)係使用日本電色工業(股)製SA4000分光計藉由室溫下將空氣作為參考來進行測定。 Light transmittance (T 308 nm , T 400 nm , T 550 nm [%]) and CIE b value (CIE b * ) at wavelengths of 308 nm, 400 nm and 550 nm were measured at room temperature using SA4000 spectrometer manufactured by Nippon Denshoku Industries Co., Ltd. The measurement is performed using air as a reference.
使用王子計測機器(股)製、KOBURA 2100ADH,藉由室溫下來測定厚度方向延遲(Rth)及面內延遲(R0)。 The thickness direction retardation (R th ) and the in-plane retardation (R 0 ) were measured at room temperature using KOBURA 2100ADH manufactured by Oji Scientific Instruments.
尚,厚度方向延遲(Rth)及面內延遲(R0)係依據以下之式所算出。 Furthermore, the retardation in the thickness direction (R th ) and the in-plane retardation (R 0 ) were calculated according to the following equations.
R0=(Nx-Ny)×d=△Nxy×d R 0 =(Nx-Ny)×d=△Nxy×d
Rth=[(Nx+Ny)/2-Nz]×d=[(△Nxz×d)+(△Nyz×d)/2 R th =[(Nx+Ny)/2-Nz]×d=[(△Nxz×d)+(△Nyz×d)/2
Nx、Ny:面內正交的2個折射率(Nx>Ny亦將Nx稱為慢軸(slow axis)、將Ny稱為快軸(fast axis)) Nx, Ny: two refractive indices orthogonal to each other in the plane (Nx>Ny, Nx is also referred to as the slow axis (slow axis), Ny is referred to as the fast axis (fast axis))
Nz:相對於面而言為厚度(垂直)方向(垂直)之折射率 Nz: Refractive index in the thickness (vertical) direction (vertical) with respect to the plane
d:膜厚 d: film thickness
△Nxy:面內的2個折射率之差(Nx-Ny)(雙折射) △Nxy: Difference between two refractive indices in the plane (Nx-Ny) (birefringence)
△Nxz:面內的折射率Nx與厚度方向的折射率Nz之差(雙折射) ΔNxz: Difference between the refractive index Nx in the plane and the refractive index Nz in the thickness direction (birefringence)
△Nyz:面內的折射率Ny與厚度方向的折射率Nz之差(雙折射) ΔNyz: Difference between the refractive index Ny in the plane and the refractive index Nz in the thickness direction (birefringence)
所得到的薄膜的膜厚係藉由(股)Teclock製厚度計來進行測定。 The film thickness of the obtained thin film was measured with the thickness gauge made by (stock) Teclock.
使用藉由前述之<4)延遲>所得到的厚度方向延遲(Rth)之值,並依據以下之式來算出。 Using the value of the retardation (R th ) in the thickness direction obtained by the above-mentioned <4) retardation>, it was calculated according to the following formula.
△N=[Rth/d(薄膜膜厚)]/1000 △N=[R th /d(film thickness)]/1000
藉由在室溫下,將實施例2-1所得到的薄膜(3cm×3cm的長方形薄膜)浸漬於60至70℃的試驗溶劑中3至5分鐘。作為試驗溶劑,使用TOK106(東京應化工業(股)製)、NMP、溶劑A(NMP:PGME:去離子水:四氫糠醇=30質量%:30質量%:20質量%:20質量%)、或溶劑B(NMP:PGME:去離子水:四氫糠醇=50質量%:10質量%:20質量%:20質量%)。 By immersing the film obtained in Example 2-1 (a rectangular film of 3 cm×3 cm) in a test solvent at 60 to 70° C. for 3 to 5 minutes at room temperature. As a test solvent, TOK106 (manufactured by Tokyo Oka Kogyo Co., Ltd.), NMP, and solvent A (NMP: PGME: deionized water: tetrahydrofurfuryl alcohol=30 mass %: 30 mass %: 20 mass %: 20 mass %) were used , or solvent B (NMP: PGME: deionized water: tetrahydrofurfuryl alcohol=50 mass %: 10 mass %: 20 mass %: 20 mass %).
溶劑試驗後,用去離子水洗淨薄膜,並將水滴藉由200℃的大氣烘箱使其乾燥10分鐘。 After the solvent test, the film was washed with deionized water, and the water droplets were allowed to dry in an atmospheric oven at 200°C for 10 minutes.
於本試驗前後測定薄膜質量,並算出其變化率並作為質量減少[%]來進行評估(負值係反映質量減少)。又,藉 由目視來觀察本試驗前後之薄膜外觀。 The film quality was measured before and after this test, and the change rate was calculated and evaluated as a mass reduction [%] (a negative value reflects a mass reduction). again, borrow The appearance of the film before and after this test was visually observed.
使用實施例2-2~2-4及比較例2-A所得到的薄膜,並實施與實施例3-1相同的溶劑浸漬試驗。尚實施例3-3及實施例3-4作為試驗溶劑僅使用TOK106來實施試驗。 Using the thin films obtained in Examples 2-2 to 2-4 and Comparative Example 2-A, the same solvent immersion test as in Example 3-1 was implemented. In addition, in Example 3-3 and Example 3-4, only TOK106 was used as the test solvent to carry out the test.
分別將藉由各樹脂薄膜形成用組成物所得到的樹脂薄膜的耐熱性及光學特性之評估表示於表1、溶劑浸漬試驗之結果表示於表2。 Table 1 shows the evaluation of the heat resistance and optical properties of the resin films obtained from the respective resin film-forming compositions, and Table 2 shows the results of the solvent immersion test, respectively.
[表2]
如表1及表2所表示般,本發明的樹脂薄膜係具有線膨脹係數[ppm/℃](50~200℃)為低(未滿35ppm/℃)、光線透射率[%]為高,進而耐熱性也為改善、且黃色度(CIE b*)亦為低之結果。進而厚度方向延遲Rth將為未滿300nm的極低值、且雙折射△n也為未滿0.01的極低值,又對於各種溶劑具有溶劑耐性。 As shown in Tables 1 and 2, the resin film of the present invention has a low linear expansion coefficient [ppm/°C] (50 to 200°C) (less than 35 ppm/°C) and a high light transmittance [%], Furthermore, the heat resistance was also improved, and the yellowness (CIE b * ) was also low. Furthermore, the thickness direction retardation R th is an extremely low value of less than 300 nm, the birefringence Δn is also an extremely low value of less than 0.01, and it has solvent resistance to various solvents.
另一方面,比較例之樹脂薄膜,雖具有與實施例相同的耐熱性及光學特性,但耐溶劑性卻為差之結果。 On the other hand, the resin film of the comparative example had the same heat resistance and optical properties as the examples, but the solvent resistance was poor.
如此般地使用二胺來製造本發明的樹脂薄膜形成用組成物的樹脂薄膜,係具有低線膨脹係數、高透明性(高光線透射率、低黃色度)、低延遲、優異的溶劑耐性之特性,即可滿足作為可撓性顯示器基板的基底薄膜所必需的 條件,故可期待特別適合使用作為可撓性顯示器基板的基底薄膜。 In this way, the resin film of the composition for forming a resin film of the present invention produced by using diamine has a low coefficient of linear expansion, high transparency (high light transmittance, low yellowness), low retardation, and excellent solvent resistance. characteristics, which can satisfy the necessary base film for flexible display substrates Therefore, it is expected to be particularly suitable for use as a base film of a flexible display substrate.
Claims (3)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015194707 | 2015-09-30 | ||
| JP2015-194707 | 2015-09-30 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW201730275A TW201730275A (en) | 2017-09-01 |
| TWI754619B true TWI754619B (en) | 2022-02-11 |
Family
ID=58424097
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW105131993A TWI754619B (en) | 2015-09-30 | 2016-09-30 | Composition for forming resin thin film |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6990354B2 (en) |
| KR (1) | KR102599925B1 (en) |
| CN (1) | CN108137924B (en) |
| TW (1) | TWI754619B (en) |
| WO (1) | WO2017057741A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102604658B1 (en) * | 2017-06-08 | 2023-11-21 | 닛산 가가쿠 가부시키가이샤 | Manufacturing method of substrate for flexible device |
| JP6711467B2 (en) * | 2017-09-13 | 2020-06-17 | 三菱瓦斯化学株式会社 | Polyimide, polyimide varnish, and polyimide film |
| KR102023130B1 (en) * | 2017-11-09 | 2019-09-19 | 스미또모 가가꾸 가부시키가이샤 | Optical film |
| JP6554599B1 (en) * | 2017-11-09 | 2019-07-31 | 住友化学株式会社 | Optical film |
| JP7449867B2 (en) * | 2018-09-20 | 2024-03-14 | 住友精化株式会社 | Electrodeposition paint and insulation coating |
| KR102245533B1 (en) * | 2020-11-02 | 2021-04-28 | 주식회사 지게차코리아 | Vehicle rim and its manufacturing method |
| WO2024005021A1 (en) * | 2022-06-30 | 2024-01-04 | 富士フイルム株式会社 | Composition, transfer film, method for producing laminate, laminate, and method for producing semiconductor package |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005041936A (en) * | 2003-07-24 | 2005-02-17 | Toray Ind Inc | Thermosetting resin composition and electronic part using the same |
| JP2013197441A (en) * | 2012-03-22 | 2013-09-30 | Toray Ind Inc | Method of manufacturing circuit board with cured material layer |
| TW201422718A (en) * | 2012-09-27 | 2014-06-16 | Mitsubishi Gas Chemical Co | Polyimide resin composition |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS58208322A (en) | 1982-05-31 | 1983-12-05 | Japan Synthetic Rubber Co Ltd | Polyimide compound |
| JPS60188427A (en) | 1984-03-09 | 1985-09-25 | Nissan Chem Ind Ltd | Novel polyimide resin and its production |
| JP4374840B2 (en) * | 2002-08-30 | 2009-12-02 | 東レ株式会社 | Positive photosensitive resin composition, semiconductor element manufacturing method, and semiconductor device |
| WO2006118105A1 (en) * | 2005-04-28 | 2006-11-09 | Ni Material Co., Ltd. | Thermosetting resin composition |
| JP5087923B2 (en) * | 2005-06-30 | 2012-12-05 | 東レ株式会社 | Photosensitive resin composition and pattern forming method |
| TWI342323B (en) * | 2007-01-22 | 2011-05-21 | Chang Chun Plastics Co Ltd | Thermoset resin modified polyimide resin composition |
| CN102325819B (en) * | 2009-02-21 | 2014-11-12 | 索尼化学&信息部件株式会社 | Starting liquid for forming protective film, protective film, and wired substrate having protective film |
| TWI434883B (en) | 2009-11-27 | 2014-04-21 | Ind Tech Res Inst | Organic/inorganic hybrid material and fabrication method thereof |
| TWI430024B (en) * | 2010-08-05 | 2014-03-11 | Asahi Kasei E Materials Corp | A photosensitive resin composition, a method for manufacturing a hardened bump pattern, and a semiconductor device |
| KR101341038B1 (en) | 2011-05-20 | 2013-12-11 | (주)에버켐텍 | Hydrophilic coating composition and entry sheets with same |
| WO2013077365A1 (en) * | 2011-11-25 | 2013-05-30 | 日産化学工業株式会社 | Resin composition for display substrates |
| CN104114606A (en) * | 2012-03-05 | 2014-10-22 | 日产化学工业株式会社 | Polyamic acid and polyimide |
| TWI471360B (en) * | 2012-12-26 | 2015-02-01 | Ind Tech Res Inst | Photosensitive polyimide and negative type photo-resist composition containing the same |
| US9395628B2 (en) * | 2013-02-25 | 2016-07-19 | Nissan Chemical Industries, Ltd. | Resist underlayer film-forming composition containing aryl sulfonate salt having hydroxyl group |
| JP6415107B2 (en) | 2013-08-27 | 2018-10-31 | 日本合成化学工業株式会社 | Resin molded body and its use |
-
2016
- 2016-09-30 JP JP2017543651A patent/JP6990354B2/en active Active
- 2016-09-30 TW TW105131993A patent/TWI754619B/en active
- 2016-09-30 CN CN201680056917.0A patent/CN108137924B/en active Active
- 2016-09-30 KR KR1020187010600A patent/KR102599925B1/en active IP Right Grant
- 2016-09-30 WO PCT/JP2016/079145 patent/WO2017057741A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005041936A (en) * | 2003-07-24 | 2005-02-17 | Toray Ind Inc | Thermosetting resin composition and electronic part using the same |
| JP2013197441A (en) * | 2012-03-22 | 2013-09-30 | Toray Ind Inc | Method of manufacturing circuit board with cured material layer |
| TW201422718A (en) * | 2012-09-27 | 2014-06-16 | Mitsubishi Gas Chemical Co | Polyimide resin composition |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201730275A (en) | 2017-09-01 |
| KR20180063137A (en) | 2018-06-11 |
| CN108137924A (en) | 2018-06-08 |
| JP6990354B2 (en) | 2022-01-12 |
| JPWO2017057741A1 (en) | 2018-07-19 |
| WO2017057741A1 (en) | 2017-04-06 |
| CN108137924B (en) | 2021-08-13 |
| KR102599925B1 (en) | 2023-11-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI705993B (en) | Production method for resin thin film and composition for forming resin thin film | |
| TWI754619B (en) | Composition for forming resin thin film | |
| TWI742204B (en) | Composition for forming flexible device substrate | |
| JP7116366B2 (en) | Method for manufacturing substrate for flexible device | |
| KR102701544B1 (en) | Hybrid resin composition | |
| TWI758357B (en) | Composition for forming flexible device substrate | |
| TWI775739B (en) | Composition for forming flexible device substrate |