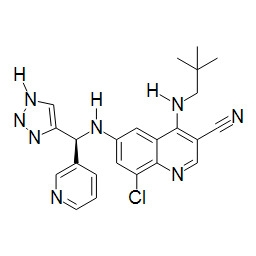
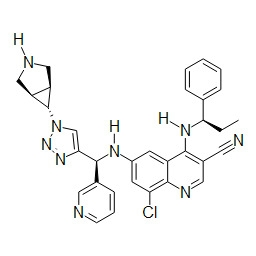
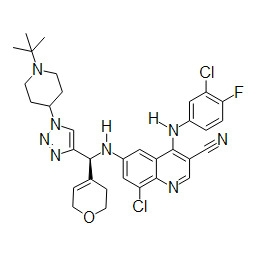
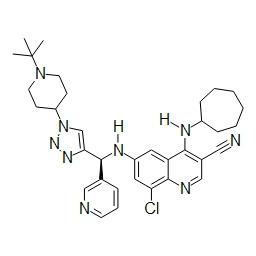
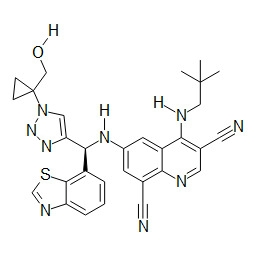
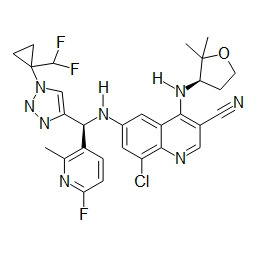
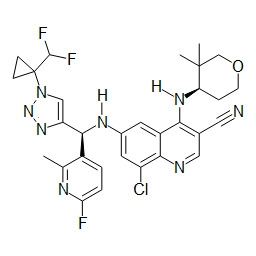
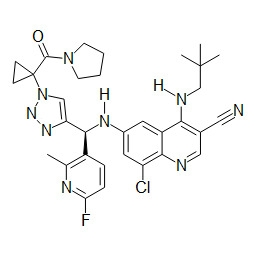
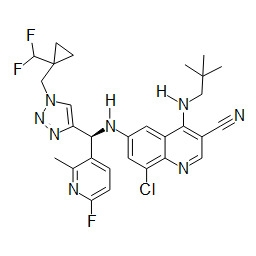
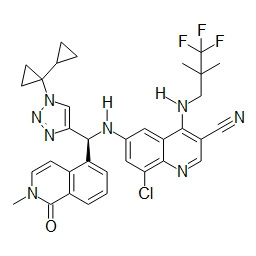
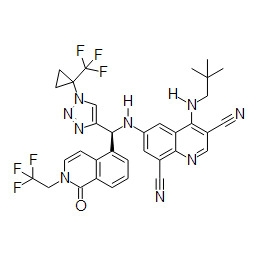
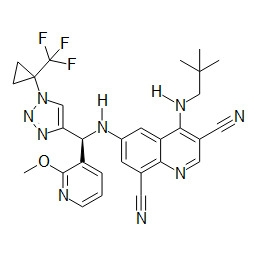
TWI748539B - Cot調節劑及其使用方法 - Google Patents
Cot調節劑及其使用方法 Download PDFInfo
- Publication number
- TWI748539B TWI748539B TW109122003A TW109122003A TWI748539B TW I748539 B TWI748539 B TW I748539B TW 109122003 A TW109122003 A TW 109122003A TW 109122003 A TW109122003 A TW 109122003A TW I748539 B TWI748539 B TW I748539B
- Authority
- TW
- Taiwan
- Prior art keywords
- alkyl
- group
- heteroaryl
- aryl
- cycloalkyl
- Prior art date
Links
- 238000000034 method Methods 0.000 title abstract description 67
- 125000006545 (C1-C9) alkyl group Chemical group 0.000 claims description 546
- 125000000623 heterocyclic group Chemical group 0.000 claims description 522
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 claims description 463
- 125000001072 heteroaryl group Chemical group 0.000 claims description 463
- 125000003118 aryl group Chemical group 0.000 claims description 434
- 125000006647 (C3-C15) cycloalkyl group Chemical group 0.000 claims description 339
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 217
- -1 -N 3 Chemical group 0.000 claims description 211
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 209
- 150000001875 compounds Chemical class 0.000 claims description 206
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 claims description 199
- 125000005843 halogen group Chemical group 0.000 claims description 143
- 229910052739 hydrogen Inorganic materials 0.000 claims description 118
- 239000001257 hydrogen Substances 0.000 claims description 118
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 111
- 239000000203 mixture Substances 0.000 claims description 111
- 125000000217 alkyl group Chemical group 0.000 claims description 104
- 125000001424 substituent group Chemical group 0.000 claims description 98
- 150000003839 salts Chemical class 0.000 claims description 74
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 73
- 150000002431 hydrogen Chemical class 0.000 claims description 71
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 59
- 201000010099 disease Diseases 0.000 claims description 52
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 51
- 238000006467 substitution reaction Methods 0.000 claims description 46
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 44
- 125000001188 haloalkyl group Chemical group 0.000 claims description 43
- 125000003342 alkenyl group Chemical group 0.000 claims description 39
- 125000000304 alkynyl group Chemical group 0.000 claims description 36
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 36
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 31
- 238000011282 treatment Methods 0.000 claims description 29
- 229910052757 nitrogen Inorganic materials 0.000 claims description 28
- 125000005842 heteroatom Chemical group 0.000 claims description 27
- 125000001820 oxy group Chemical group [*:1]O[*:2] 0.000 claims description 27
- 239000003814 drug Substances 0.000 claims description 19
- 230000001404 mediated effect Effects 0.000 claims description 15
- 208000011231 Crohn disease Diseases 0.000 claims description 13
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 13
- 125000004414 alkyl thio group Chemical group 0.000 claims description 13
- 125000000468 ketone group Chemical group 0.000 claims description 13
- 229910052717 sulfur Inorganic materials 0.000 claims description 13
- 239000011593 sulfur Substances 0.000 claims description 13
- 125000000446 sulfanediyl group Chemical group *S* 0.000 claims description 12
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 11
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 11
- 206010009900 Colitis ulcerative Diseases 0.000 claims description 10
- 201000006704 Ulcerative Colitis Diseases 0.000 claims description 10
- 229910052799 carbon Inorganic materials 0.000 claims description 10
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 6
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 5
- 201000002510 thyroid cancer Diseases 0.000 claims description 5
- 229940079593 drug Drugs 0.000 claims description 4
- 150000001335 aliphatic alkanes Chemical class 0.000 claims description 3
- LMBFAGIMSUYTBN-MPZNNTNKSA-N teixobactin Chemical compound C([C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H]1C(N[C@@H](C)C(=O)N[C@@H](C[C@@H]2NC(=N)NC2)C(=O)N[C@H](C(=O)O[C@H]1C)[C@@H](C)CC)=O)NC)C1=CC=CC=C1 LMBFAGIMSUYTBN-MPZNNTNKSA-N 0.000 claims description 2
- 150000001924 cycloalkanes Chemical group 0.000 claims 1
- 206010028980 Neoplasm Diseases 0.000 abstract description 13
- 238000004519 manufacturing process Methods 0.000 abstract description 13
- 201000011510 cancer Diseases 0.000 abstract description 5
- 210000001685 thyroid gland Anatomy 0.000 abstract description 2
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 72
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 51
- 239000000243 solution Substances 0.000 description 36
- 125000004432 carbon atom Chemical group C* 0.000 description 34
- 235000019439 ethyl acetate Nutrition 0.000 description 34
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 33
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 32
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 32
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 30
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 27
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 23
- 208000024891 symptom Diseases 0.000 description 23
- 238000006243 chemical reaction Methods 0.000 description 22
- 229940002612 prodrug Drugs 0.000 description 22
- 239000000651 prodrug Substances 0.000 description 22
- 239000000047 product Substances 0.000 description 22
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 22
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 20
- 239000002585 base Substances 0.000 description 19
- 230000015572 biosynthetic process Effects 0.000 description 19
- 230000000694 effects Effects 0.000 description 19
- 230000008569 process Effects 0.000 description 19
- 239000011541 reaction mixture Substances 0.000 description 19
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 239000003795 chemical substances by application Substances 0.000 description 18
- 238000003786 synthesis reaction Methods 0.000 description 18
- 239000012267 brine Substances 0.000 description 17
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 17
- 239000002904 solvent Substances 0.000 description 16
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 16
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 15
- 239000003480 eluent Substances 0.000 description 14
- 239000003112 inhibitor Substances 0.000 description 14
- 150000002576 ketones Chemical group 0.000 description 14
- 150000001412 amines Chemical class 0.000 description 13
- 125000004429 atom Chemical group 0.000 description 13
- 239000012074 organic phase Substances 0.000 description 13
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 12
- 239000000460 chlorine Substances 0.000 description 12
- 201000006417 multiple sclerosis Diseases 0.000 description 12
- 239000008194 pharmaceutical composition Substances 0.000 description 12
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 12
- 239000007858 starting material Substances 0.000 description 12
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 11
- QTGVXQVMKCJZTR-UHFFFAOYSA-N benzyl 4-(4-methylphenyl)sulfonyloxypiperidine-1-carboxylate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OC1CCN(C(=O)OCC=2C=CC=CC=2)CC1 QTGVXQVMKCJZTR-UHFFFAOYSA-N 0.000 description 11
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 11
- 229910052805 deuterium Inorganic materials 0.000 description 11
- 238000003818 flash chromatography Methods 0.000 description 11
- 125000004404 heteroalkyl group Chemical group 0.000 description 11
- KBOPZPXVLCULAV-UHFFFAOYSA-N mesalamine Chemical compound NC1=CC=C(O)C(C(O)=O)=C1 KBOPZPXVLCULAV-UHFFFAOYSA-N 0.000 description 11
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 description 10
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium on carbon Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 10
- 239000004480 active ingredient Substances 0.000 description 10
- 238000004458 analytical method Methods 0.000 description 10
- UHOVQNZJYSORNB-UHFFFAOYSA-N benzene Substances C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 10
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 10
- 230000002829 reductive effect Effects 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- 239000000725 suspension Substances 0.000 description 10
- 229940124597 therapeutic agent Drugs 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 101000990902 Homo sapiens Matrix metalloproteinase-9 Proteins 0.000 description 9
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 9
- 102100040247 Tumor necrosis factor Human genes 0.000 description 9
- 239000002253 acid Substances 0.000 description 9
- 230000009266 disease activity Effects 0.000 description 9
- 150000002367 halogens Chemical class 0.000 description 9
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 9
- 239000000546 pharmaceutical excipient Substances 0.000 description 9
- 206010039073 rheumatoid arthritis Diseases 0.000 description 9
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 8
- KALZAFFKKBWHEP-UHFFFAOYSA-N C(=O)OCC1=CC=CC=C1.C(C)OC(C=1N=NN(C1)C1CCNCC1)OCC Chemical compound C(=O)OCC1=CC=CC=C1.C(C)OC(C=1N=NN(C1)C1CCNCC1)OCC KALZAFFKKBWHEP-UHFFFAOYSA-N 0.000 description 8
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- 239000012141 concentrate Substances 0.000 description 8
- 235000008504 concentrate Nutrition 0.000 description 8
- 125000004122 cyclic group Chemical group 0.000 description 8
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 8
- 229910052736 halogen Inorganic materials 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 125000000547 substituted alkyl group Chemical group 0.000 description 8
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 7
- 102000011011 Sphingosine 1-phosphate receptors Human genes 0.000 description 7
- 108050001083 Sphingosine 1-phosphate receptors Proteins 0.000 description 7
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 7
- 125000003545 alkoxy group Chemical group 0.000 description 7
- HYHAMKPNWDJSEP-UHFFFAOYSA-N benzyl 4-azidopiperidine-1-carboxylate Chemical compound C1CC(N=[N+]=[N-])CCN1C(=O)OCC1=CC=CC=C1 HYHAMKPNWDJSEP-UHFFFAOYSA-N 0.000 description 7
- 125000002619 bicyclic group Chemical group 0.000 description 7
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 7
- 229910052801 chlorine Inorganic materials 0.000 description 7
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 6
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 241000124008 Mammalia Species 0.000 description 6
- 102100026907 Mitogen-activated protein kinase kinase kinase 8 Human genes 0.000 description 6
- 241000124033 Salix Species 0.000 description 6
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 6
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 239000003124 biologic agent Substances 0.000 description 6
- 206010012601 diabetes mellitus Diseases 0.000 description 6
- 208000035475 disorder Diseases 0.000 description 6
- 239000002158 endotoxin Substances 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 6
- 150000002466 imines Chemical class 0.000 description 6
- 229920006008 lipopolysaccharide Polymers 0.000 description 6
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 6
- 150000003384 small molecules Chemical class 0.000 description 6
- 150000003431 steroids Chemical class 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 125000003107 substituted aryl group Chemical group 0.000 description 6
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 6
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 6
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 6
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- 206010019728 Hepatitis alcoholic Diseases 0.000 description 5
- 208000003456 Juvenile Arthritis Diseases 0.000 description 5
- SUAKHGWARZSWIH-UHFFFAOYSA-N N,N‐diethylformamide Chemical compound CCN(CC)C=O SUAKHGWARZSWIH-UHFFFAOYSA-N 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 208000002353 alcoholic hepatitis Diseases 0.000 description 5
- 150000001408 amides Chemical class 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- JKIUUDJOCYHIGY-UHFFFAOYSA-N benzyl 4-hydroxypiperidine-1-carboxylate Chemical compound C1CC(O)CCN1C(=O)OCC1=CC=CC=C1 JKIUUDJOCYHIGY-UHFFFAOYSA-N 0.000 description 5
- 125000002618 bicyclic heterocycle group Chemical group 0.000 description 5
- 125000003636 chemical group Chemical group 0.000 description 5
- 238000001914 filtration Methods 0.000 description 5
- 235000019000 fluorine Nutrition 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 208000027866 inflammatory disease Diseases 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 239000012044 organic layer Substances 0.000 description 5
- 239000003960 organic solvent Substances 0.000 description 5
- 125000006299 oxetan-3-yl group Chemical group [H]C1([H])OC([H])([H])C1([H])* 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 5
- 238000010791 quenching Methods 0.000 description 5
- 125000005017 substituted alkenyl group Chemical group 0.000 description 5
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 5
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 4
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 4
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 4
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical class N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 4
- 201000005569 Gout Diseases 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 4
- 208000002193 Pain Diseases 0.000 description 4
- 239000004793 Polystyrene Substances 0.000 description 4
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 4
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 4
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 4
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 4
- 150000001299 aldehydes Chemical class 0.000 description 4
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 4
- 150000001721 carbon Chemical group 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 238000004587 chromatography analysis Methods 0.000 description 4
- 206010009887 colitis Diseases 0.000 description 4
- 238000002425 crystallisation Methods 0.000 description 4
- 230000008025 crystallization Effects 0.000 description 4
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 229910052731 fluorine Inorganic materials 0.000 description 4
- 239000011737 fluorine Substances 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 229910052740 iodine Inorganic materials 0.000 description 4
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 4
- 239000007937 lozenge Substances 0.000 description 4
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical compound O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 230000002503 metabolic effect Effects 0.000 description 4
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 4
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- 239000006187 pill Substances 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 125000006239 protecting group Chemical group 0.000 description 4
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- 229910052938 sodium sulfate Inorganic materials 0.000 description 4
- 235000011152 sodium sulphate Nutrition 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 4
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 3
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 3
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 3
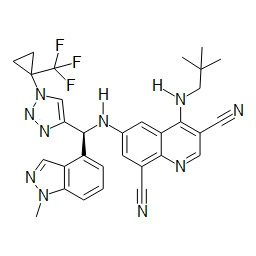
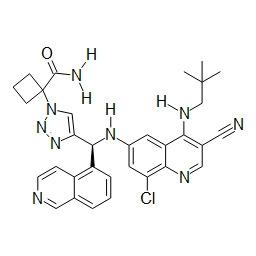
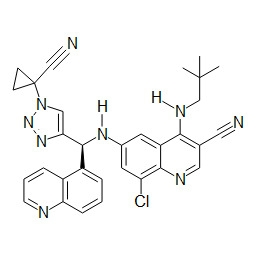
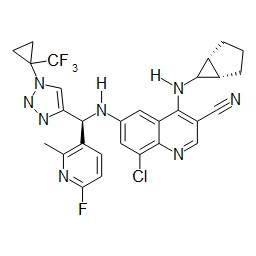
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 3
- 101150015280 Cel gene Proteins 0.000 description 3
- 101150050349 FFAR2 gene Proteins 0.000 description 3
- 102100040133 Free fatty acid receptor 2 Human genes 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 102000013264 Interleukin-23 Human genes 0.000 description 3
- 108010065637 Interleukin-23 Proteins 0.000 description 3
- 102000042838 JAK family Human genes 0.000 description 3
- 108091082332 JAK family Proteins 0.000 description 3
- 208000008839 Kidney Neoplasms Diseases 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 102100030608 Mothers against decapentaplegic homolog 7 Human genes 0.000 description 3
- 238000005481 NMR spectroscopy Methods 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 229910019213 POCl3 Inorganic materials 0.000 description 3
- 101100272976 Panax ginseng CYP716A53v2 gene Proteins 0.000 description 3
- 201000004681 Psoriasis Diseases 0.000 description 3
- 206010038389 Renal cancer Diseases 0.000 description 3
- 101700026522 SMAD7 Proteins 0.000 description 3
- 206010039491 Sarcoma Diseases 0.000 description 3
- 206010040047 Sepsis Diseases 0.000 description 3
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 3
- 102000008235 Toll-Like Receptor 9 Human genes 0.000 description 3
- 108010060818 Toll-Like Receptor 9 Proteins 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 208000036142 Viral infection Diseases 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 125000004644 alkyl sulfinyl group Chemical group 0.000 description 3
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 3
- 208000007502 anemia Diseases 0.000 description 3
- 206010003246 arthritis Diseases 0.000 description 3
- 150000001540 azides Chemical class 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 3
- 229910052794 bromium Inorganic materials 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 238000009833 condensation Methods 0.000 description 3
- 230000005494 condensation Effects 0.000 description 3
- 239000003246 corticosteroid Substances 0.000 description 3
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 3
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 3
- 239000012973 diazabicyclooctane Substances 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- 239000012458 free base Substances 0.000 description 3
- 206010022000 influenza Diseases 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000011630 iodine Substances 0.000 description 3
- 201000010982 kidney cancer Diseases 0.000 description 3
- 201000001441 melanoma Diseases 0.000 description 3
- 229960004963 mesalazine Drugs 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- RIJLVEAXPNLDTC-UHFFFAOYSA-N n-[5-[4-[(1,1-dioxo-1,4-thiazinan-4-yl)methyl]phenyl]-[1,2,4]triazolo[1,5-a]pyridin-2-yl]cyclopropanecarboxamide Chemical compound C1CC1C(=O)NC(=NN12)N=C1C=CC=C2C(C=C1)=CC=C1CN1CCS(=O)(=O)CC1 RIJLVEAXPNLDTC-UHFFFAOYSA-N 0.000 description 3
- 125000004433 nitrogen atom Chemical group N* 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 125000003226 pyrazolyl group Chemical group 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 238000010898 silica gel chromatography Methods 0.000 description 3
- 239000012453 solvate Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- 230000009385 viral infection Effects 0.000 description 3
- RRKODOZNUZCUBN-CCAGOZQPSA-N (1z,3z)-cycloocta-1,3-diene Chemical compound C1CC\C=C/C=C\C1 RRKODOZNUZCUBN-CCAGOZQPSA-N 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 2
- GRXZDXCWBKCRMW-UHFFFAOYSA-N 1,3-benzothiazole-7-carbaldehyde Chemical compound O=CC1=CC=CC2=C1SC=N2 GRXZDXCWBKCRMW-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- ZCJAYDKWZAWMPR-UHFFFAOYSA-N 1-chloro-2-fluorobenzene Chemical group FC1=CC=CC=C1Cl ZCJAYDKWZAWMPR-UHFFFAOYSA-N 0.000 description 2
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 2
- MKGXYADVRDCKBY-UHFFFAOYSA-N 2,3-dihydro-1h-isoindole-4-carbaldehyde Chemical compound O=CC1=CC=CC2=C1CNC2 MKGXYADVRDCKBY-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- LSONWRHLFZYHIN-UHFFFAOYSA-N 2-[(4-phenoxyphenyl)sulfonylmethyl]thiirane Chemical compound C=1C=C(OC=2C=CC=CC=2)C=CC=1S(=O)(=O)CC1CS1 LSONWRHLFZYHIN-UHFFFAOYSA-N 0.000 description 2
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 2
- 125000004777 2-fluoroethyl group Chemical group [H]C([H])(F)C([H])([H])* 0.000 description 2
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 2
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 2
- 125000004179 3-chlorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(Cl)=C1[H] 0.000 description 2
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 2
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 2
- 125000002373 5 membered heterocyclic group Chemical group 0.000 description 2
- XRVDGNKRPOAQTN-FQEVSTJZSA-N 5-[3-[(1s)-1-(2-hydroxyethylamino)-2,3-dihydro-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxybenzonitrile Chemical group C1=C(C#N)C(OC(C)C)=CC=C1C1=NC(C=2C=3CC[C@@H](C=3C=CC=2)NCCO)=NO1 XRVDGNKRPOAQTN-FQEVSTJZSA-N 0.000 description 2
- 125000004070 6 membered heterocyclic group Chemical group 0.000 description 2
- PZPNGWWKCSJKOS-UHFFFAOYSA-N 6-fluoropyridine-3-carbaldehyde Chemical compound FC1=CC=C(C=O)C=N1 PZPNGWWKCSJKOS-UHFFFAOYSA-N 0.000 description 2
- 208000004998 Abdominal Pain Diseases 0.000 description 2
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 2
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 102100022718 Atypical chemokine receptor 2 Human genes 0.000 description 2
- 206010005949 Bone cancer Diseases 0.000 description 2
- 208000018084 Bone neoplasm Diseases 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 206010006811 Bursitis Diseases 0.000 description 2
- DUWLWUSJWKBLEO-MNOVXSKESA-N C(C1=CC=CC=C1)[C@H]1[C@@H](C1)C#N Chemical compound C(C1=CC=CC=C1)[C@H]1[C@@H](C1)C#N DUWLWUSJWKBLEO-MNOVXSKESA-N 0.000 description 2
- 102100036848 C-C motif chemokine 20 Human genes 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- 206010008342 Cervix carcinoma Diseases 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- 206010012735 Diarrhoea Diseases 0.000 description 2
- 101100296720 Dictyostelium discoideum Pde4 gene Proteins 0.000 description 2
- 108010052167 Dihydroorotate Dehydrogenase Proteins 0.000 description 2
- 102100032823 Dihydroorotate dehydrogenase (quinone), mitochondrial Human genes 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 102000001301 EGF receptor Human genes 0.000 description 2
- 201000009273 Endometriosis Diseases 0.000 description 2
- 201000001342 Fallopian tube cancer Diseases 0.000 description 2
- 208000013452 Fallopian tube neoplasm Diseases 0.000 description 2
- 102100020997 Fractalkine Human genes 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 101001066288 Gallus gallus GATA-binding factor 3 Proteins 0.000 description 2
- 206010018429 Glucose tolerance impaired Diseases 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 206010019233 Headaches Diseases 0.000 description 2
- 101000678892 Homo sapiens Atypical chemokine receptor 2 Proteins 0.000 description 2
- 101000716070 Homo sapiens C-C chemokine receptor type 9 Proteins 0.000 description 2
- 101000713099 Homo sapiens C-C motif chemokine 20 Proteins 0.000 description 2
- 101000854520 Homo sapiens Fractalkine Proteins 0.000 description 2
- 238000006736 Huisgen cycloaddition reaction Methods 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- 208000004044 Hypesthesia Diseases 0.000 description 2
- 102100025323 Integrin alpha-1 Human genes 0.000 description 2
- 108010041341 Integrin alpha1 Proteins 0.000 description 2
- 108010055795 Integrin alpha1beta1 Proteins 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- 229940122245 Janus kinase inhibitor Drugs 0.000 description 2
- 102000001291 MAP Kinase Kinase Kinase Human genes 0.000 description 2
- 108060006687 MAP kinase kinase kinase Proteins 0.000 description 2
- 239000007993 MOPS buffer Substances 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 101150010110 Map3k8 gene Proteins 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- 238000007126 N-alkylation reaction Methods 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- CKMOQBVBEGCJGW-LLIZZRELSA-L OC1=CC=C(C=C1C(=O)O[Na])\N=N\C1=CC=C(C=C1)C(=O)NCCC(=O)O[Na] Chemical compound OC1=CC=C(C=C1C(=O)O[Na])\N=N\C1=CC=C(C=C1)C(=O)NCCC(=O)O[Na] CKMOQBVBEGCJGW-LLIZZRELSA-L 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 208000018737 Parkinson disease Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 101100082610 Plasmodium falciparum (isolate 3D7) PDEdelta gene Proteins 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 206010038063 Rectal haemorrhage Diseases 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 2
- 239000004012 Tofacitinib Substances 0.000 description 2
- 206010052779 Transplant rejections Diseases 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- 206010046431 Urethral cancer Diseases 0.000 description 2
- 206010046458 Urethral neoplasms Diseases 0.000 description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 2
- 206010047741 Vulval cancer Diseases 0.000 description 2
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- CTCBPRXHVPZNHB-VQFZJOCSSA-N [[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate;(2r,3r,4s,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O.C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O CTCBPRXHVPZNHB-VQFZJOCSSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 239000003429 antifungal agent Substances 0.000 description 2
- 229940121375 antifungal agent Drugs 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- FFBHFFJDDLITSX-UHFFFAOYSA-N benzyl N-[2-hydroxy-4-(3-oxomorpholin-4-yl)phenyl]carbamate Chemical compound OC1=C(NC(=O)OCC2=CC=CC=C2)C=CC(=C1)N1CCOCC1=O FFBHFFJDDLITSX-UHFFFAOYSA-N 0.000 description 2
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 229960004436 budesonide Drugs 0.000 description 2
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- SKOLWUPSYHWYAM-UHFFFAOYSA-N carbonodithioic O,S-acid Chemical compound SC(S)=O SKOLWUPSYHWYAM-UHFFFAOYSA-N 0.000 description 2
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 201000007455 central nervous system cancer Diseases 0.000 description 2
- 201000010881 cervical cancer Diseases 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 208000019069 chronic childhood arthritis Diseases 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 208000008609 collagenous colitis Diseases 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 2
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 2
- 230000003412 degenerative effect Effects 0.000 description 2
- NKLCNNUWBJBICK-UHFFFAOYSA-N dess–martin periodinane Chemical compound C1=CC=C2I(OC(=O)C)(OC(C)=O)(OC(C)=O)OC(=O)C2=C1 NKLCNNUWBJBICK-UHFFFAOYSA-N 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- 125000000664 diazo group Chemical group [N-]=[N+]=[*] 0.000 description 2
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 2
- 230000036267 drug metabolism Effects 0.000 description 2
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- ZSWFCLXCOIISFI-UHFFFAOYSA-N endo-cyclopentadiene Natural products C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 2
- 239000012055 enteric layer Substances 0.000 description 2
- 208000010227 enterocolitis Diseases 0.000 description 2
- 230000029142 excretion Effects 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 125000004438 haloalkoxy group Chemical group 0.000 description 2
- 231100000869 headache Toxicity 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 150000004677 hydrates Chemical class 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 2
- 238000005984 hydrogenation reaction Methods 0.000 description 2
- 208000034783 hypoesthesia Diseases 0.000 description 2
- 125000001841 imino group Chemical group [H]N=* 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 239000011261 inert gas Substances 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 238000007689 inspection Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 108010044426 integrins Proteins 0.000 description 2
- 102000006495 integrins Human genes 0.000 description 2
- 208000002551 irritable bowel syndrome Diseases 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- 125000000842 isoxazolyl group Chemical group 0.000 description 2
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 208000019423 liver disease Diseases 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 208000020984 malignant renal pelvis neoplasm Diseases 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 239000003607 modifier Substances 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- XYURSCOGYWBRDR-UHFFFAOYSA-N n-diazoimidazole-1-sulfonamide;hydrochloride Chemical compound Cl.[N-]=[N+]=NS(=O)(=O)N1C=CN=C1 XYURSCOGYWBRDR-UHFFFAOYSA-N 0.000 description 2
- XBXCNNQPRYLIDE-UHFFFAOYSA-M n-tert-butylcarbamate Chemical compound CC(C)(C)NC([O-])=O XBXCNNQPRYLIDE-UHFFFAOYSA-M 0.000 description 2
- 239000006199 nebulizer Substances 0.000 description 2
- 208000004296 neuralgia Diseases 0.000 description 2
- 125000006574 non-aromatic ring group Chemical group 0.000 description 2
- 231100000862 numbness Toxicity 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 229950008141 ozanimod Drugs 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 125000003367 polycyclic group Chemical group 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 238000002600 positron emission tomography Methods 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 201000009104 prediabetes syndrome Diseases 0.000 description 2
- 229960005205 prednisolone Drugs 0.000 description 2
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- KCXFHTAICRTXLI-UHFFFAOYSA-N propane-1-sulfonic acid Chemical compound CCCS(O)(=O)=O KCXFHTAICRTXLI-UHFFFAOYSA-N 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 125000006514 pyridin-2-ylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 201000007444 renal pelvis carcinoma Diseases 0.000 description 2
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000007363 ring formation reaction Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000002603 single-photon emission computed tomography Methods 0.000 description 2
- 229910000104 sodium hydride Inorganic materials 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 210000002784 stomach Anatomy 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 2
- 230000000153 supplemental effect Effects 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- FBWNMEQMRUMQSO-UHFFFAOYSA-N tergitol NP-9 Chemical compound CCCCCCCCCC1=CC=C(OCCOCCOCCOCCOCCOCCOCCOCCOCCO)C=C1 FBWNMEQMRUMQSO-UHFFFAOYSA-N 0.000 description 2
- DYHSDKLCOJIUFX-UHFFFAOYSA-N tert-butoxycarbonyl anhydride Chemical compound CC(C)(C)OC(=O)OC(=O)OC(C)(C)C DYHSDKLCOJIUFX-UHFFFAOYSA-N 0.000 description 2
- RUPAXCPQAAOIPB-UHFFFAOYSA-N tert-butyl formate Chemical group CC(C)(C)OC=O RUPAXCPQAAOIPB-UHFFFAOYSA-N 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000001981 tert-butyldimethylsilyl group Chemical group [H]C([H])([H])[Si]([H])(C([H])([H])[H])[*]C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 2
- 150000003573 thiols Chemical class 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- QERYCTSHXKAMIS-UHFFFAOYSA-M thiophene-2-carboxylate Chemical compound [O-]C(=O)C1=CC=CS1 QERYCTSHXKAMIS-UHFFFAOYSA-M 0.000 description 2
- 238000002877 time resolved fluorescence resonance energy transfer Methods 0.000 description 2
- JMXKSZRRTHPKDL-UHFFFAOYSA-N titanium ethoxide Chemical compound [Ti+4].CC[O-].CC[O-].CC[O-].CC[O-] JMXKSZRRTHPKDL-UHFFFAOYSA-N 0.000 description 2
- UJLAWZDWDVHWOW-YPMHNXCESA-N tofacitinib Chemical compound C[C@@H]1CCN(C(=O)CC#N)C[C@@H]1N(C)C1=NC=NC2=C1C=CN2 UJLAWZDWDVHWOW-YPMHNXCESA-N 0.000 description 2
- 229960001350 tofacitinib Drugs 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 125000002088 tosyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])S(*)(=O)=O 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 150000003852 triazoles Chemical class 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 125000001889 triflyl group Chemical group FC(F)(F)S(*)(=O)=O 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 229940046728 tumor necrosis factor alpha inhibitor Drugs 0.000 description 2
- 239000002451 tumor necrosis factor inhibitor Substances 0.000 description 2
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 2
- 208000037965 uterine sarcoma Diseases 0.000 description 2
- 201000005102 vulva cancer Diseases 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- 208000016261 weight loss Diseases 0.000 description 2
- XYQCVKKUOLJPGU-HBNTYKKESA-N (1R,2R)-2-[(S)-amino(phenyl)methyl]cyclopropane-1-carbonitrile Chemical compound N[C@@H]([C@H]1[C@@H](C1)C#N)C1=CC=CC=C1 XYQCVKKUOLJPGU-HBNTYKKESA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- ZZMYWYZPNZRYPX-SANMLTNESA-N (2r)-2-amino-2-[5-[4-[2-(4-phenylphenyl)ethoxy]-3-(trifluoromethyl)phenyl]-1h-imidazol-2-yl]propan-1-ol Chemical compound N1C([C@@](N)(CO)C)=NC=C1C(C=C1C(F)(F)F)=CC=C1OCCC1=CC=C(C=2C=CC=CC=2)C=C1 ZZMYWYZPNZRYPX-SANMLTNESA-N 0.000 description 1
- KSELABKNBIUMGG-YGBAREPYSA-N (2z,3ar,4r,5r,6as)-3,3-difluoro-4-[(e,3r,4r)-3-hydroxy-4-(3-methylphenyl)pent-1-enyl]-2-[4-(2h-tetrazol-5-yl)butylidene]-4,5,6,6a-tetrahydro-3ah-cyclopenta[b]furan-5-ol Chemical compound O([C@H]1C[C@@H](O)[C@@H]([C@H]1C1(F)F)/C=C/[C@@H](O)[C@H](C)C=2C=C(C)C=CC=2)\C1=C/CCCC=1N=NNN=1 KSELABKNBIUMGG-YGBAREPYSA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 description 1
- 125000006653 (C1-C20) heteroaryl group Chemical group 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 1
- 125000006649 (C2-C20) alkynyl group Chemical group 0.000 description 1
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 description 1
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 description 1
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 description 1
- 125000006652 (C3-C12) cycloalkyl group Chemical group 0.000 description 1
- 125000006654 (C3-C12) heteroaryl group Chemical group 0.000 description 1
- 125000006651 (C3-C20) cycloalkyl group Chemical group 0.000 description 1
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 1
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 1
- 125000006655 (C3-C8) heteroaryl group Chemical group 0.000 description 1
- PFABGJWLRSXPDV-FGBSKUFISA-N (R)-N-[(S)-[(1R,2R)-2-cyanocyclopropyl]-phenylmethyl]-2-methylpropane-2-sulfinamide Chemical compound C(#N)[C@H]1[C@@H](C1)[C@H](N[S@](=O)C(C)(C)C)C1=CC=CC=C1 PFABGJWLRSXPDV-FGBSKUFISA-N 0.000 description 1
- PSQGBBLJWNJCHX-XEKQOVJPSA-N (R)-N-[[(1R,2R)-2-cyanocyclopropyl]-phenylmethylidene]-2-methylpropane-2-sulfinamide Chemical compound C(#N)[C@H]1[C@@H](C1)C(=N[S@](=O)C(C)(C)C)C1=CC=CC=C1 PSQGBBLJWNJCHX-XEKQOVJPSA-N 0.000 description 1
- PSQGBBLJWNJCHX-QHRIQVFBSA-N (R)-N-[[(1S,2S)-2-cyanocyclopropyl]-phenylmethylidene]-2-methylpropane-2-sulfinamide Chemical compound C(#N)[C@@H]1[C@H](C1)C(=N[S@](=O)C(C)(C)C)C1=CC=CC=C1 PSQGBBLJWNJCHX-QHRIQVFBSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- ODIGIKRIUKFKHP-UHFFFAOYSA-N (n-propan-2-yloxycarbonylanilino) acetate Chemical compound CC(C)OC(=O)N(OC(C)=O)C1=CC=CC=C1 ODIGIKRIUKFKHP-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 125000004605 1,2,3,4-tetrahydroisoquinolinyl group Chemical group C1(NCCC2=CC=CC=C12)* 0.000 description 1
- 125000005926 1,2-dimethylbutyloxy group Chemical group 0.000 description 1
- KAWZXBGHYZRUQA-UHFFFAOYSA-N 1,3-benzothiazol-7-ylmethanol Chemical compound OCC1=CC=CC2=C1SC=N2 KAWZXBGHYZRUQA-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- CBRJPFGIXUFMTM-WDEREUQCSA-N 1-[(2S,5R)-2-methyl-5-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)piperidin-1-yl]prop-2-en-1-one Chemical compound N1=CN=C(C2=C1NC=C2)N[C@@H]2CC[C@@H](N(C2)C(C=C)=O)C CBRJPFGIXUFMTM-WDEREUQCSA-N 0.000 description 1
- GXHFOEOQFJGWSJ-UHFFFAOYSA-N 1-chloro-2-cyclopropyloxy-3-nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC(Cl)=C1OC1CC1 GXHFOEOQFJGWSJ-UHFFFAOYSA-N 0.000 description 1
- RBAHXNSORRGCQA-UHFFFAOYSA-N 1-chloro-2-fluoro-3-nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC(Cl)=C1F RBAHXNSORRGCQA-UHFFFAOYSA-N 0.000 description 1
- 125000004066 1-hydroxyethyl group Chemical group [H]OC([H])([*])C([H])([H])[H] 0.000 description 1
- 125000006432 1-methyl cyclopropyl group Chemical group [H]C([H])([H])C1(*)C([H])([H])C1([H])[H] 0.000 description 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical group C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 1
- 125000004778 2,2-difluoroethyl group Chemical group [H]C([H])(*)C([H])(F)F 0.000 description 1
- LATZVDXOTDYECD-UFTFXDLESA-N 2,3-dihydroxybutanedioic acid (3S,4R)-3-ethyl-4-(1,5,7,10-tetrazatricyclo[7.3.0.02,6]dodeca-2(6),3,7,9,11-pentaen-12-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide tetrahydrate Chemical compound O.O.O.O.OC(C(O)C(O)=O)C(O)=O.CC[C@@H]1CN(C[C@@H]1c1cnc2cnc3[nH]ccc3n12)C(=O)NCC(F)(F)F LATZVDXOTDYECD-UFTFXDLESA-N 0.000 description 1
- 125000006508 2,6-difluorobenzyl group Chemical group [H]C1=C([H])C(F)=C(C(F)=C1[H])C([H])([H])* 0.000 description 1
- MOMFXATYAINJML-UHFFFAOYSA-N 2-Acetylthiazole Chemical group CC(=O)C1=NC=CS1 MOMFXATYAINJML-UHFFFAOYSA-N 0.000 description 1
- XPRDUGXOWVXZLL-UHFFFAOYSA-N 2-[[2-fluoro-4-(3-methoxyphenyl)phenyl]carbamoyl]cyclopentene-1-carboxylic acid Chemical compound COC1=CC=CC(C=2C=C(F)C(NC(=O)C=3CCCC=3C(O)=O)=CC=2)=C1 XPRDUGXOWVXZLL-UHFFFAOYSA-N 0.000 description 1
- JVCPIJKPAKAIIP-UHFFFAOYSA-N 2-amino-2-[2-[4-heptoxy-3-(trifluoromethyl)phenyl]ethyl]propane-1,3-diol Chemical compound CCCCCCCOC1=CC=C(CCC(N)(CO)CO)C=C1C(F)(F)F JVCPIJKPAKAIIP-UHFFFAOYSA-N 0.000 description 1
- LOCWBQIWHWIRGN-UHFFFAOYSA-N 2-chloro-4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1Cl LOCWBQIWHWIRGN-UHFFFAOYSA-N 0.000 description 1
- MNNZINNZIQVULG-UHFFFAOYSA-N 2-chloroethylbenzene Chemical compound ClCCC1=CC=CC=C1 MNNZINNZIQVULG-UHFFFAOYSA-N 0.000 description 1
- CESUXLKAADQNTB-SSDOTTSWSA-N 2-methylpropane-2-sulfinamide Chemical compound CC(C)(C)[S@](N)=O CESUXLKAADQNTB-SSDOTTSWSA-N 0.000 description 1
- CESUXLKAADQNTB-ZETCQYMHSA-N 2-methylpropane-2-sulfinamide Chemical compound CC(C)(C)[S@@](N)=O CESUXLKAADQNTB-ZETCQYMHSA-N 0.000 description 1
- RGUXEWWHSQGVRZ-UHFFFAOYSA-N 3,3-diethoxyprop-1-yne Chemical compound CCOC(C#C)OCC RGUXEWWHSQGVRZ-UHFFFAOYSA-N 0.000 description 1
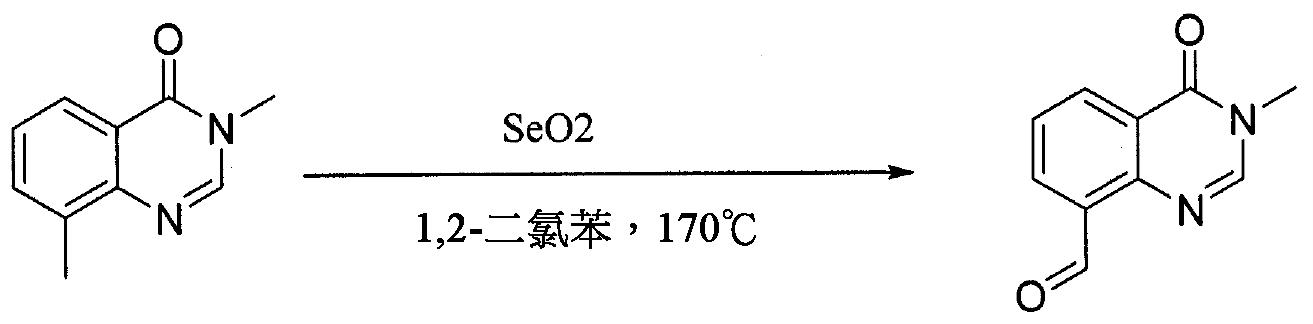
- IJDQJMMCEREAOJ-UHFFFAOYSA-N 3,8-dimethylquinazolin-4-one Chemical compound N1=CN(C)C(=O)C2=C1C(C)=CC=C2 IJDQJMMCEREAOJ-UHFFFAOYSA-N 0.000 description 1
- XZYXCQXTKOYHGK-UHFFFAOYSA-N 3-(2-hydroxy-1-methylindol-3-yl)indol-2-one Chemical compound Cn1c(O)c(C2=c3ccccc3=NC2=O)c2ccccc12 XZYXCQXTKOYHGK-UHFFFAOYSA-N 0.000 description 1
- VJXPRLZGWQAQEX-UHFFFAOYSA-N 3-(aminomethyl)oxetan-3-ol Chemical compound NCC1(O)COC1 VJXPRLZGWQAQEX-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 125000005917 3-methylpentyl group Chemical group 0.000 description 1
- IUZIMWDWXWFSKZ-UHFFFAOYSA-N 3-oxo-1,2-dihydroisoindole-4-carbaldehyde Chemical compound O=CC1=CC=CC2=C1C(=O)NC2 IUZIMWDWXWFSKZ-UHFFFAOYSA-N 0.000 description 1
- MPJQECCTXQJBOS-UHFFFAOYSA-N 4,8-dichloro-6-nitroquinoline-3-carbonitrile Chemical compound N1=CC(C#N)=C(Cl)C2=CC([N+](=O)[O-])=CC(Cl)=C21 MPJQECCTXQJBOS-UHFFFAOYSA-N 0.000 description 1
- MPMKMQHJHDHPBE-RUZDIDTESA-N 4-[[(2r)-1-(1-benzothiophene-3-carbonyl)-2-methylazetidine-2-carbonyl]-[(3-chlorophenyl)methyl]amino]butanoic acid Chemical compound O=C([C@@]1(N(CC1)C(=O)C=1C2=CC=CC=C2SC=1)C)N(CCCC(O)=O)CC1=CC=CC(Cl)=C1 MPMKMQHJHDHPBE-RUZDIDTESA-N 0.000 description 1
- HNVZVGCFIFFWMV-UHFFFAOYSA-N 4-bromo-2-methyl-3h-isoindol-1-one Chemical compound O=C1N(C)CC2=C1C=CC=C2Br HNVZVGCFIFFWMV-UHFFFAOYSA-N 0.000 description 1
- LYPAFUINURXJSG-AWEZNQCLSA-N 5-benzyl-n-[(3s)-5-methyl-4-oxo-2,3-dihydro-1,5-benzoxazepin-3-yl]-1h-1,2,4-triazole-3-carboxamide Chemical compound N([C@H]1COC2=CC=CC=C2N(C1=O)C)C(=O)C(N=1)=NNC=1CC1=CC=CC=C1 LYPAFUINURXJSG-AWEZNQCLSA-N 0.000 description 1
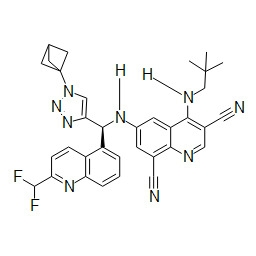
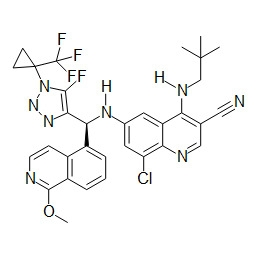
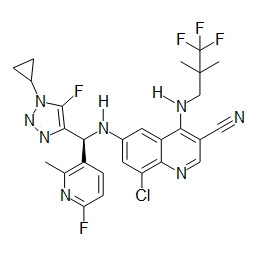
- ZDCQBTVFRULIPR-QFIPXVFZSA-N 5-bromo-8-chloro-6-[[(S)-[1-[1-(difluoromethyl)cyclopropyl]-5-fluorotriazol-4-yl]-(6-fluoro-2-methylpyridin-3-yl)methyl]amino]-4-(2,2-dimethylpropylamino)quinoline-3-carbonitrile Chemical compound BrC1=C2C(=C(C=NC2=C(C=C1N[C@@H](C=1C(=NC(=CC=1)F)C)C=1N=NN(C=1F)C1(CC1)C(F)F)Cl)C#N)NCC(C)(C)C ZDCQBTVFRULIPR-QFIPXVFZSA-N 0.000 description 1
- BNVPFDRNGHMRJS-UHFFFAOYSA-N 5-cyano-n-[2-(4,4-dimethylcyclohexen-1-yl)-6-(2,2,6,6-tetramethyloxan-4-yl)pyridin-3-yl]-1h-imidazole-2-carboxamide Chemical compound C1C(C)(C)CCC(C=2C(=CC=C(N=2)C2CC(C)(C)OC(C)(C)C2)NC(=O)C=2NC=C(N=2)C#N)=C1 BNVPFDRNGHMRJS-UHFFFAOYSA-N 0.000 description 1
- MJZJYWCQPMNPRM-UHFFFAOYSA-N 6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,6-dihydro-1,3,5-triazine-2,4-diamine Chemical compound CC1(C)N=C(N)N=C(N)N1OCCCOC1=CC(Cl)=C(Cl)C=C1Cl MJZJYWCQPMNPRM-UHFFFAOYSA-N 0.000 description 1
- IAGIOFJILQXGIA-UHFFFAOYSA-N 6-[(2-methylpropan-2-yl)oxycarbonyl]-5,7-dihydro-4h-thieno[2,3-c]pyridine-3-carboxylic acid Chemical compound C1N(C(=O)OC(C)(C)C)CCC2=C1SC=C2C(O)=O IAGIOFJILQXGIA-UHFFFAOYSA-N 0.000 description 1
- BLLSRSWUYXEXNF-UHFFFAOYSA-N 6-chloro-2-methylpyridine-3-carbaldehyde Chemical compound CC1=NC(Cl)=CC=C1C=O BLLSRSWUYXEXNF-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- RPISMGBJCPUIMP-UHFFFAOYSA-N 8-chloro-6-nitro-4-oxo-1h-quinoline-3-carbonitrile Chemical compound C1=C([N+]([O-])=O)C=C2C(O)=C(C#N)C=NC2=C1Cl RPISMGBJCPUIMP-UHFFFAOYSA-N 0.000 description 1
- IRBAWVGZNJIROV-SFHVURJKSA-N 9-(2-cyclopropylethynyl)-2-[[(2s)-1,4-dioxan-2-yl]methoxy]-6,7-dihydropyrimido[6,1-a]isoquinolin-4-one Chemical compound C1=C2C3=CC=C(C#CC4CC4)C=C3CCN2C(=O)N=C1OC[C@@H]1COCCO1 IRBAWVGZNJIROV-SFHVURJKSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 1
- 206010000830 Acute leukaemia Diseases 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 208000007848 Alcoholism Diseases 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical class [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 208000006820 Arthralgia Diseases 0.000 description 1
- 206010003267 Arthritis reactive Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- IVRMZWNICZWHMI-UHFFFAOYSA-N Azide Chemical class [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 201000009144 Bartter disease type 3 Diseases 0.000 description 1
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 1
- 208000027496 Behcet disease Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010006143 Brain stem glioma Diseases 0.000 description 1
- SZPOMQKYFJGSGI-UHFFFAOYSA-N C(C)(C)(C)OC(=O)NC=1SC2=C(N=1)C=CC=C2C(=O)OCC Chemical compound C(C)(C)(C)OC(=O)NC=1SC2=C(N=1)C=CC=C2C(=O)OCC SZPOMQKYFJGSGI-UHFFFAOYSA-N 0.000 description 1
- 102100025248 C-X-C motif chemokine 10 Human genes 0.000 description 1
- AQFLVLHRZFLDDV-JBTOUSLDSA-N C1(=CC=CC=C1)[C@@](C(C([2H])([2H])[2H])([2H])[2H])(N[2H])[2H] Chemical compound C1(=CC=CC=C1)[C@@](C(C([2H])([2H])[2H])([2H])[2H])(N[2H])[2H] AQFLVLHRZFLDDV-JBTOUSLDSA-N 0.000 description 1
- 125000003358 C2-C20 alkenyl group Chemical group 0.000 description 1
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 description 1
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 description 1
- 125000000041 C6-C10 aryl group Chemical group 0.000 description 1
- WGEWYYPHYMGJNT-HLHYUOOASA-N CC(C)C[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(=O)N)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N1)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](N)CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)C(=O)O Chemical compound CC(C)C[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(=O)N)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N1)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](N)CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)C(=O)O WGEWYYPHYMGJNT-HLHYUOOASA-N 0.000 description 1
- 102100031974 CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,3-sialyltransferase 4 Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 208000000094 Chronic Pain Diseases 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 206010009657 Clostridium difficile colitis Diseases 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- 206010009895 Colitis ischaemic Diseases 0.000 description 1
- 206010056979 Colitis microscopic Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 206010010741 Conjunctivitis Diseases 0.000 description 1
- VMQMZMRVKUZKQL-UHFFFAOYSA-N Cu+ Chemical compound [Cu+] VMQMZMRVKUZKQL-UHFFFAOYSA-N 0.000 description 1
- QPLDLSVMHZLSFG-UHFFFAOYSA-N CuO Inorganic materials [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 208000005156 Dehydration Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 208000005171 Dysmenorrhea Diseases 0.000 description 1
- 206010013935 Dysmenorrhoea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 206010014824 Endotoxic shock Diseases 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 208000004232 Enteritis Diseases 0.000 description 1
- 206010058838 Enterocolitis infectious Diseases 0.000 description 1
- 102100023688 Eotaxin Human genes 0.000 description 1
- 101710139422 Eotaxin Proteins 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 208000006168 Ewing Sarcoma Diseases 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- 201000006107 Familial adenomatous polyposis Diseases 0.000 description 1
- 229910017344 Fe2 O3 Inorganic materials 0.000 description 1
- 208000009849 Female Genital Neoplasms Diseases 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 206010016717 Fistula Diseases 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 229940125821 GLPG1205 Drugs 0.000 description 1
- 229940126656 GS-4224 Drugs 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- 208000002705 Glucose Intolerance Diseases 0.000 description 1
- 206010018634 Gouty Arthritis Diseases 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 208000000616 Hemoptysis Diseases 0.000 description 1
- 206010019663 Hepatic failure Diseases 0.000 description 1
- 208000007514 Herpes zoster Diseases 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000858088 Homo sapiens C-X-C motif chemokine 10 Proteins 0.000 description 1
- 101000703754 Homo sapiens CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,3-sialyltransferase 4 Proteins 0.000 description 1
- 101000916644 Homo sapiens Macrophage colony-stimulating factor 1 receptor Proteins 0.000 description 1
- 101001055091 Homo sapiens Mitogen-activated protein kinase kinase kinase 8 Proteins 0.000 description 1
- 101000984753 Homo sapiens Serine/threonine-protein kinase B-raf Proteins 0.000 description 1
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- LELOWRISYMNNSU-UHFFFAOYSA-N Hydrocyanic acid Natural products N#C LELOWRISYMNNSU-UHFFFAOYSA-N 0.000 description 1
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 206010022489 Insulin Resistance Diseases 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 206010061252 Intraocular melanoma Diseases 0.000 description 1
- 206010023126 Jaundice Diseases 0.000 description 1
- 208000012659 Joint disease Diseases 0.000 description 1
- 206010023232 Joint swelling Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 229910010082 LiAlH Inorganic materials 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010067125 Liver injury Diseases 0.000 description 1
- 208000008930 Low Back Pain Diseases 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000005777 Lupus Nephritis Diseases 0.000 description 1
- 208000008771 Lymphadenopathy Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 102100028198 Macrophage colony-stimulating factor 1 receptor Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 description 1
- 208000001145 Metabolic Syndrome Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 101710164353 Mitogen-activated protein kinase kinase kinase 8 Proteins 0.000 description 1
- 206010028116 Mucosal inflammation Diseases 0.000 description 1
- 201000010927 Mucositis Diseases 0.000 description 1
- 206010028391 Musculoskeletal Pain Diseases 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- 201000002481 Myositis Diseases 0.000 description 1
- 235000009421 Myristica fragrans Nutrition 0.000 description 1
- JLTDJTHDQAWBAV-UHFFFAOYSA-N N,N-dimethylaniline Chemical compound CN(C)C1=CC=CC=C1 JLTDJTHDQAWBAV-UHFFFAOYSA-N 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- IQTSJNPHVPZWQH-UHFFFAOYSA-N N1=CNCC2=CC=CC(=C12)C=O.[O] Chemical compound N1=CNCC2=CC=CC(=C12)C=O.[O] IQTSJNPHVPZWQH-UHFFFAOYSA-N 0.000 description 1
- NWEHXVBCRQXJFC-UHFFFAOYSA-N N1C=C(C=CC1)C=O.[O] Chemical compound N1C=C(C=CC1)C=O.[O] NWEHXVBCRQXJFC-UHFFFAOYSA-N 0.000 description 1
- JADHNBVMSQXMTF-UHFFFAOYSA-N N1C=C(CC2=CC=CC=C12)C#N.[O] Chemical compound N1C=C(CC2=CC=CC=C12)C#N.[O] JADHNBVMSQXMTF-UHFFFAOYSA-N 0.000 description 1
- JIRAMTWSJGQNAT-UHFFFAOYSA-N N1N=NC=C1.N1CCCCC1 Chemical compound N1N=NC=C1.N1CCCCC1 JIRAMTWSJGQNAT-UHFFFAOYSA-N 0.000 description 1
- 229910004809 Na2 SO4 Inorganic materials 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 206010028836 Neck pain Diseases 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 208000036110 Neuroinflammatory disease Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- IHGLEKUZSYTZLP-UHFFFAOYSA-N OCC1=CC=CC=2N=C(SC=21)NC(OC(C)(C)C)=O Chemical compound OCC1=CC=CC=2N=C(SC=21)NC(OC(C)(C)C)=O IHGLEKUZSYTZLP-UHFFFAOYSA-N 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 201000010133 Oligodendroglioma Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- BYPFEZZEUUWMEJ-UHFFFAOYSA-N Pentoxifylline Chemical compound O=C1N(CCCCC(=O)C)C(=O)N(C)C2=C1N(C)C=N2 BYPFEZZEUUWMEJ-UHFFFAOYSA-N 0.000 description 1
- 206010034719 Personality change Diseases 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 206010034972 Photosensitivity reaction Diseases 0.000 description 1
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 1
- 201000005746 Pituitary adenoma Diseases 0.000 description 1
- 206010061538 Pituitary tumour benign Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000002389 Pouchitis Diseases 0.000 description 1
- 208000001280 Prediabetic State Diseases 0.000 description 1
- 206010036774 Proctitis Diseases 0.000 description 1
- 208000003100 Pseudomembranous Enterocolitis Diseases 0.000 description 1
- 206010037128 Pseudomembranous colitis Diseases 0.000 description 1
- 208000020410 Psoriasis-related juvenile idiopathic arthritis Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 208000012322 Raynaud phenomenon Diseases 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 229910006124 SOCl2 Inorganic materials 0.000 description 1
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 1
- 206010039705 Scleritis Diseases 0.000 description 1
- 229940124639 Selective inhibitor Drugs 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 description 1
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 206010049416 Short-bowel syndrome Diseases 0.000 description 1
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- 208000010040 Sprains and Strains Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 241001222723 Sterna Species 0.000 description 1
- PJANXHGTPQOBST-VAWYXSNFSA-N Stilbene Chemical group C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 1
- 208000007107 Stomach Ulcer Diseases 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 1
- 229910021626 Tin(II) chloride Inorganic materials 0.000 description 1
- 206010062129 Tongue neoplasm Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 102100033438 Tyrosine-protein kinase JAK1 Human genes 0.000 description 1
- 101710112793 Tyrosine-protein kinase JAK1 Proteins 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 201000005969 Uveal melanoma Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- IXYNFLOLUBKHQU-FZCWJHTDSA-N [(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-3-[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[(2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [(2R,3S,5R)-2-[[[(2R,3S,5R)-2-[[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-2-[[[(2R,3S,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-2-(hydroxymethyl)oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]-5-(6-aminopurin-9-yl)oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]-5-(6-aminopurin-9-yl)oxolan-3-yl] hydrogen phosphate Chemical compound Cc1cn([C@H]2C[C@H](OP(O)(=O)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=O)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=O)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=O)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=S)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=S)OC[C@H]3O[C@H](C[C@@H]3OP(O)(=S)OC[C@H]3O[C@H](C[C@@H]3O)n3ccc(N)nc3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)[C@@H](COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=S)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=S)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=S)O[C@H]3C[C@@H](O[C@@H]3CO)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c3nc(N)[nH]c4=O)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cnc4c3nc(N)[nH]c4=O)O2)c(=O)[nH]c1=O IXYNFLOLUBKHQU-FZCWJHTDSA-N 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 229950008347 abrilumab Drugs 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 229940081735 acetylcellulose Drugs 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 208000030002 adult glioblastoma Diseases 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 206010001584 alcohol abuse Diseases 0.000 description 1
- 208000025746 alcohol use disease Diseases 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 230000003113 alkalizing effect Effects 0.000 description 1
- 125000002355 alkine group Chemical group 0.000 description 1
- 150000003973 alkyl amines Chemical class 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- 230000029936 alkylation Effects 0.000 description 1
- 238000005804 alkylation reaction Methods 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 125000005466 alkylenyl group Chemical group 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 238000005576 amination reaction Methods 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 229940020544 apriso Drugs 0.000 description 1
- 239000011260 aqueous acid Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 125000000732 arylene group Chemical group 0.000 description 1
- 229940072224 asacol Drugs 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 125000005873 benzo[d]thiazolyl group Chemical group 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 229950010015 bertilimumab Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 235000001465 calcium Nutrition 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 229960001714 calcium phosphate Drugs 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- 229960003340 calcium silicate Drugs 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 229940072225 canasa Drugs 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- QHMBSVQNZZTUGM-ZWKOTPCHSA-N cannabidiol Chemical compound OC1=CC(CCCCC)=CC(O)=C1[C@H]1[C@H](C(C)=C)CCC(C)=C1 QHMBSVQNZZTUGM-ZWKOTPCHSA-N 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 150000001722 carbon compounds Chemical class 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 229940082638 cardiac stimulant phosphodiesterase inhibitors Drugs 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 230000009920 chelation Effects 0.000 description 1
- DREIJXJRTLTGJC-ZLBJMMTISA-N chembl3137308 Chemical compound C([C@H]1C[C@@](O)(C2)C3)C2C[C@H]3[C@H]1NC1=C2C=CNC2=NC=C1C(=O)N DREIJXJRTLTGJC-ZLBJMMTISA-N 0.000 description 1
- 201000008240 chemical colitis Diseases 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000012320 chlorinating reagent Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- 208000019902 chronic diarrheal disease Diseases 0.000 description 1
- 208000024207 chronic leukemia Diseases 0.000 description 1
- 229940090100 cimzia Drugs 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 208000029664 classic familial adenomatous polyposis Diseases 0.000 description 1
- 208000035850 clinical syndrome Diseases 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 229940112505 colazal Drugs 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical class [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 1
- OPQARKPSCNTWTJ-UHFFFAOYSA-L copper(ii) acetate Chemical compound [Cu+2].CC([O-])=O.CC([O-])=O OPQARKPSCNTWTJ-UHFFFAOYSA-L 0.000 description 1
- HFKYIDIFIFHBCJ-UHFFFAOYSA-N copper;pentahydrate Chemical compound O.O.O.O.O.[Cu+2] HFKYIDIFIFHBCJ-UHFFFAOYSA-N 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 208000030381 cutaneous melanoma Diseases 0.000 description 1
- CPPKAGUPTKIMNP-UHFFFAOYSA-N cyanogen fluoride Chemical compound FC#N CPPKAGUPTKIMNP-UHFFFAOYSA-N 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- YPHMISFOHDHNIV-FSZOTQKASA-N cycloheximide Chemical compound C1[C@@H](C)C[C@H](C)C(=O)[C@@H]1[C@H](O)CC1CC(=O)NC(=O)C1 YPHMISFOHDHNIV-FSZOTQKASA-N 0.000 description 1
- 125000006639 cyclohexyl carbonyl group Chemical group 0.000 description 1
- YOXHCYXIAVIFCZ-UHFFFAOYSA-N cyclopropanol Chemical compound OC1CC1 YOXHCYXIAVIFCZ-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 229940113965 delzicol Drugs 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- HUMNYLRZRPPJDN-RAMDWTOOSA-N deuterio(phenyl)methanone Chemical compound [2H]C(=O)C1=CC=CC=C1 HUMNYLRZRPPJDN-RAMDWTOOSA-N 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 125000005265 dialkylamine group Chemical group 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- GGSUCNLOZRCGPQ-UHFFFAOYSA-N diethylaniline Chemical compound CCN(CC)C1=CC=CC=C1 GGSUCNLOZRCGPQ-UHFFFAOYSA-N 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 125000004982 dihaloalkyl group Chemical group 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 201000008243 diversion colitis Diseases 0.000 description 1
- 208000007784 diverticulitis Diseases 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000002183 duodenal effect Effects 0.000 description 1
- 210000001198 duodenum Anatomy 0.000 description 1
- 229950010217 eldelumab Drugs 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 201000002491 encephalomyelitis Diseases 0.000 description 1
- 210000000750 endocrine system Anatomy 0.000 description 1
- 238000001861 endoscopic biopsy Methods 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940095399 enema Drugs 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 239000002662 enteric coated tablet Substances 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 229940002671 entocort Drugs 0.000 description 1
- 229940104788 entyvio Drugs 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 150000002148 esters Chemical group 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- VIRFIEKKLULPKS-FPLPWBNLSA-N ethyl (z)-3-(2-chloro-4-nitroanilino)-2-cyanoprop-2-enoate Chemical compound CCOC(=O)C(\C#N)=C/NC1=CC=C([N+]([O-])=O)C=C1Cl VIRFIEKKLULPKS-FPLPWBNLSA-N 0.000 description 1
- JJJDYTDZPDRXCF-UHFFFAOYSA-N ethyl 2-amino-1,3-benzothiazole-7-carboxylate Chemical compound CCOC(=O)C1=CC=CC2=C1SC(N)=N2 JJJDYTDZPDRXCF-UHFFFAOYSA-N 0.000 description 1
- 125000006125 ethylsulfonyl group Chemical group 0.000 description 1
- 229950004912 etrolizumab Drugs 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000005562 fading Methods 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 229950006663 filgotinib Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 230000003890 fistula Effects 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- WBJINCZRORDGAQ-UHFFFAOYSA-N formic acid ethyl ester Natural products CCOC=O WBJINCZRORDGAQ-UHFFFAOYSA-N 0.000 description 1
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 201000005917 gastric ulcer Diseases 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 229940014259 gelatin Drugs 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 230000008570 general process Effects 0.000 description 1
- 208000004104 gestational diabetes Diseases 0.000 description 1
- 229940001908 giazo Drugs 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 125000003827 glycol group Chemical group 0.000 description 1
- 229960001743 golimumab Drugs 0.000 description 1
- 125000002795 guanidino group Chemical group C(N)(=N)N* 0.000 description 1
- 208000024963 hair loss Diseases 0.000 description 1
- 230000003676 hair loss Effects 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 230000002008 hemorrhagic effect Effects 0.000 description 1
- 231100000753 hepatic injury Toxicity 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 125000004366 heterocycloalkenyl group Chemical group 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 229940048921 humira Drugs 0.000 description 1
- 125000000717 hydrazino group Chemical group [H]N([*])N([H])[H] 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 208000009326 ileitis Diseases 0.000 description 1
- 201000008254 ileocolitis Diseases 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000012606 in vitro cell culture Methods 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000003387 indolinyl group Chemical group N1(CCC2=CC=CC=C12)* 0.000 description 1
- 208000027139 infectious colitis Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 229960000598 infliximab Drugs 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229940125798 integrin inhibitor Drugs 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 201000004614 iritis Diseases 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 201000008222 ischemic colitis Diseases 0.000 description 1
- 125000004594 isoindolinyl group Chemical group C1(NCC2=CC=CC=C12)* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- FMKOJHQHASLBPH-UHFFFAOYSA-N isopropyl iodide Chemical compound CC(C)I FMKOJHQHASLBPH-UHFFFAOYSA-N 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 230000005445 isotope effect Effects 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- 201000008242 jejunoileitis Diseases 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000010983 kinetics study Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229940013926 lialda Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000011344 liquid material Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 1
- 210000005229 liver cell Anatomy 0.000 description 1
- 231100000835 liver failure Toxicity 0.000 description 1
- 208000007903 liver failure Diseases 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 208000004341 lymphocytic colitis Diseases 0.000 description 1
- 239000001115 mace Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- LROBJRRFCPYLIT-UHFFFAOYSA-M magnesium;ethyne;bromide Chemical compound [Mg+2].[Br-].[C-]#C LROBJRRFCPYLIT-UHFFFAOYSA-M 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 230000001071 malnutrition Effects 0.000 description 1
- 235000000824 malnutrition Nutrition 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- MMHHPKCJJIFLBQ-QFIPXVFZSA-N methyl (2s)-2-[(2,6-dichlorobenzoyl)amino]-3-[4-[6-(dimethylamino)-1-methyl-2,4-dioxoquinazolin-3-yl]phenyl]propanoate Chemical compound N([C@@H](CC=1C=CC(=CC=1)N1C(C2=CC(=CC=C2N(C)C1=O)N(C)C)=O)C(=O)OC)C(=O)C1=C(Cl)C=CC=C1Cl MMHHPKCJJIFLBQ-QFIPXVFZSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 208000008275 microscopic colitis Diseases 0.000 description 1
- ABASMUXUCSQFKC-PKEKLUKKSA-N mongersen Chemical compound CC1=CN([C@H]2C[C@H](OP(=S)(O)OC[C@H]3O[C@H](C[C@@H]3OP(=S)(O)OC[C@H]4O[C@H](C[C@@H]4OP(=S)(O)OC[C@H]5O[C@H](C[C@@H]5OP(=S)(O)OC[C@H]6O[C@H](C[C@@H]6OP(=S)(O)OC[C@H]7O[C@H](C[C@@H]7O)N8C=CC(=NC8=O)N)n9cnc%10C(=O)NC(=Nc9%10)N)n%11cnc%12c(N)ncnc%11%12)N%13C=CC(=NC%13=O)N)n%14cnc%15C(=O)NC(=Nc%14%15)N)[C@@H](COP(=S)(O)O[C@H]%16C[C@@H](O[C@@H]%16COP(=S)(O)O[C@H]%17C[C@@H](O[C@@H]%17COP(=S)(O)O[C@H]%18C[C@@H](O[C@@H]%18COP(=S)(O)O[C@H]%19C[C@@H](O[C@@H]%19COP(=S)(O)O[C@H]%20C[C@@H](O[C@@H]%20COP(=S)(O)O[C@H]%21C[C@@H](O[C@@H]%21COP(=S)(O)O[C@H]%22C[C@@H](O[C@@H]%22COP(=S)(O)O[C@H]%23C[C@@H](O[C@@H]%23COP(=S)(O)O[C@H]%24C[C@@H](O[C@@H]%24COP(=S)(O)O[C@H]%25C[C@@H](O[C@@H]%25COP(=S)(O)O[C@H]%26C[C@@H](O[C@@H]%26COP(=S)(O)O[C@H]%27C[C@@H](O[C@@H]%27COP(=S)(O)O[C@H]%28C[C@@H](O[C@@H]%28COP(=S)(O)O[C@H]%29C[C@@H](O[C@@H]%29COP(=S)(O)O[C@H]%30C[C@@H](O[C@@H]%30CO)n%31cnc%32C(=O)NC(=Nc%31%32)N)N%33C=C(C)C(=O)NC%33=O)N%34C=C(C)C(=NC%34=O)N)n%35cnc%36C(=O)NC(=Nc%35%36)N)N%37C=CC(=NC%37=O)N)N%38C=CC(=NC%38=O)N)N%39C=CC(=NC%39=O)N)N%40C=CC(=NC%40=O)N)N%41C=C(C)C(=O)NC%41=O)N%42C=C(C)C(=O)NC%42=O)N%43C=CC(=NC%43=O)N)N%44C=C(C)C(=O)NC%44=O)N%45C=CC(=NC%45=O)N)N%46C=CC(=NC%46=O)N)N%47C=CC(=NC%47=O)N)O2)C(=O)N=C1N ABASMUXUCSQFKC-PKEKLUKKSA-N 0.000 description 1
- 229950002917 mongersen Drugs 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 208000018962 mouth sore Diseases 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 210000001087 myotubule Anatomy 0.000 description 1
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229960005027 natalizumab Drugs 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 201000002120 neuroendocrine carcinoma Diseases 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- GNRSAWUEBMWBQH-UHFFFAOYSA-N nickel(II) oxide Inorganic materials [Ni]=O GNRSAWUEBMWBQH-UHFFFAOYSA-N 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000005935 nucleophilic addition reaction Methods 0.000 description 1
- 238000007339 nucleophilic aromatic substitution reaction Methods 0.000 description 1
- 230000000269 nucleophilic effect Effects 0.000 description 1
- 238000010534 nucleophilic substitution reaction Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 208000015380 nutritional deficiency disease Diseases 0.000 description 1
- 201000002575 ocular melanoma Diseases 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000006548 oncogenic transformation Effects 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 238000006053 organic reaction Methods 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940072223 pentasa Drugs 0.000 description 1
- 229960001476 pentoxifylline Drugs 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 125000003170 phenylsulfonyl group Chemical group C1(=CC=CC=C1)S(=O)(=O)* 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 239000002571 phosphodiesterase inhibitor Substances 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 238000006303 photolysis reaction Methods 0.000 description 1
- 230000015843 photosynthesis, light reaction Effects 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 208000021310 pituitary gland adenoma Diseases 0.000 description 1
- JAMNHZBIQDNHMM-UHFFFAOYSA-N pivalonitrile Chemical compound CC(C)(C)C#N JAMNHZBIQDNHMM-UHFFFAOYSA-N 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- LJCNRYVRMXRIQR-OLXYHTOASA-L potassium sodium L-tartrate Chemical compound [Na+].[K+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O LJCNRYVRMXRIQR-OLXYHTOASA-L 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- WDXARTMCIRVMAE-UHFFFAOYSA-N quinoline-2-carbonitrile Chemical group C1=CC=CC2=NC(C#N)=CC=C21 WDXARTMCIRVMAE-UHFFFAOYSA-N 0.000 description 1
- 150000003248 quinolines Chemical group 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 208000002574 reactive arthritis Diseases 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 238000006722 reduction reaction Methods 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- AOPOCGPBAIARAV-OTBJXLELSA-N resolvin E1 Chemical compound CC[C@@H](O)\C=C\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O AOPOCGPBAIARAV-OTBJXLELSA-N 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 206010039083 rhinitis Diseases 0.000 description 1
- 230000033764 rhythmic process Effects 0.000 description 1
- 229950007943 risankizumab Drugs 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- JPJALAQPGMAKDF-UHFFFAOYSA-N selenium dioxide Chemical compound O=[Se]=O JPJALAQPGMAKDF-UHFFFAOYSA-N 0.000 description 1
- 239000012056 semi-solid material Substances 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 229940068638 simponi Drugs 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 201000003708 skin melanoma Diseases 0.000 description 1
- 231100000046 skin rash Toxicity 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000012354 sodium borodeuteride Substances 0.000 description 1
- 239000012312 sodium hydride Substances 0.000 description 1
- 235000011006 sodium potassium tartrate Nutrition 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 125000003003 spiro group Chemical group 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 230000000707 stereoselective effect Effects 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical group C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 1
- 235000021286 stilbenes Nutrition 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 229940066769 systemic antihistamines substituted alkylamines Drugs 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- MLGCXEBRWGEOQX-UHFFFAOYSA-N tetradifon Chemical compound C1=CC(Cl)=CC=C1S(=O)(=O)C1=CC(Cl)=C(Cl)C=C1Cl MLGCXEBRWGEOQX-UHFFFAOYSA-N 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- AXZWODMDQAVCJE-UHFFFAOYSA-L tin(II) chloride (anhydrous) Chemical compound [Cl-].[Cl-].[Sn+2] AXZWODMDQAVCJE-UHFFFAOYSA-L 0.000 description 1
- 125000005147 toluenesulfonyl group Chemical group C=1(C(=CC=CC1)S(=O)(=O)*)C 0.000 description 1
- 201000006134 tongue cancer Diseases 0.000 description 1
- 208000004371 toothache Diseases 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 238000006276 transfer reaction Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 125000005270 trialkylamine group Chemical group 0.000 description 1
- 125000005259 triarylamine group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 125000004385 trihaloalkyl group Chemical group 0.000 description 1
- RKBCYCFRFCNLTO-UHFFFAOYSA-N triisopropylamine Chemical compound CC(C)N(C(C)C)C(C)C RKBCYCFRFCNLTO-UHFFFAOYSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 230000006433 tumor necrosis factor production Effects 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 1
- 229940079023 tysabri Drugs 0.000 description 1
- 229940082189 uceris Drugs 0.000 description 1
- 231100000397 ulcer Toxicity 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 201000000360 urethra cancer Diseases 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 229960003824 ustekinumab Drugs 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 229960004914 vedolizumab Drugs 0.000 description 1
- 229950010644 vidofludimus Drugs 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- XLOMVQKBTHCTTD-UHFFFAOYSA-N zinc oxide Inorganic materials [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/38—Nitrogen atoms
- C07D215/42—Nitrogen atoms attached in position 4
- C07D215/44—Nitrogen atoms attached in position 4 with aryl radicals attached to said nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/02—Stomatological preparations, e.g. drugs for caries, aphtae, periodontitis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/06—Anti-spasmodics, e.g. drugs for colics, esophagic dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/06—Antiarrhythmics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B59/00—Introduction of isotopes of elements into organic compounds ; Labelled organic compounds per se
- C07B59/002—Heterocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/38—Nitrogen atoms
- C07D215/42—Nitrogen atoms attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/05—Isotopically modified compounds, e.g. labelled
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Diabetes (AREA)
- Neurology (AREA)
- Physical Education & Sports Medicine (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Hematology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Dermatology (AREA)
- Obesity (AREA)
- Pulmonology (AREA)
- Hospice & Palliative Care (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Endocrinology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Urology & Nephrology (AREA)
- Nutrition Science (AREA)
- Emergency Medicine (AREA)
- Gastroenterology & Hepatology (AREA)
- Otolaryngology (AREA)
- Ophthalmology & Optometry (AREA)
Abstract
本發明大體上係關於Cot (大阪甲狀腺癌)之調節劑及其使用及製造方法。
Description
本發明大體上係關於Cot (大阪甲狀腺癌,cancer Osaka thyroid)之調節劑及其使用及製造方法。
Cot (大阪甲狀腺癌)蛋白質為絲胺酸/蘇胺酸激酶,其為MAP激酶激酶激酶(MAP3K)家族之成員。其亦稱為「Tpl2」(腫瘤進展基因座)、「MAP3K8」(有絲分裂原活化之蛋白激酶激酶激酶8)或「EST」(尤文氏肉瘤轉型體,Ewing sarcoma transformant)。Cot藉由其在細胞中之致癌轉型活性來加以鑑別,且已展示其調控致癌及發炎路徑。
已知Cot在MEK-ERK路徑中上游,且對LPS誘導之腫瘤壞死因子-α (TNF-α)產生為至關重要的。已展示Cot涉及TNFα之產生及信號傳導兩者。TNFα為促炎性細胞激素,且在發炎性疾病中起重要作用,該等發炎性疾病諸如類風濕性關節炎(RA)、多發性硬化症(MS)、發炎性腸病(IBD)、糖尿病、敗血症、牛皮癬、調控異常之TNFα表現及移植排斥。
因此,調節Cot之表現或活性的藥劑及方法可適用於預防或治療此類疾病。
本發明提供調節Cot之表現或活性的化合物。本發明亦提供組合物(包括醫藥組合物)、包括該等化合物之套組、及使用(或投與)及製造該等化合物之方法。本文中所提供之化合物適用於治療由Cot介導之疾病、病症或病狀。本發明亦提供用於治療之化合物。本發明進一步提供用於治療由Cot介導之疾病、病症或病狀之方法中的化合物。此外,本發明提供該等化合物之用途,其用於製造用於治療由Cot介導(或至少部分地由Cot介導)之疾病、病症或病狀的藥劑。
在一個態樣中,提供一種具有式I結構之化合物:I
其中
R1
為氫、-O-R7
、-N(R8
)(R9
)、-C(O)-R7
、-S(O)2
-R7
、-C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基及雜芳基可視情況經一至四個Z1
取代;
R2
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z2
取代;
或R1
及R2
與其所連接之氮一起以形成雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z2
取代;
R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z3
取代;
R4
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z4
取代;
R5
為氫、鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z5
取代;
R6
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z6
取代;
各R7
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z7
取代;
R8
及R9
在每次出現時獨立地為氫、-S(O)2
R10
、-C(O)-R10
、-C(O)O-R10
、-C(O)N(R10
)(R11
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z8
取代;
R10
及R11
在每次出現時獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基,
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基視情況經一至四個Z1b
取代;
各Z1
、Z2
、Z3
、Z4
、Z5
、Z6
、Z7
及Z8
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;
各R15
獨立地為鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);及
m為0、1或2;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
一些實施例提供一種使用(或投與)式I化合物或通篇所描述其他式之化合物的方法,其用於在哺乳動物(尤其人類)中治療能夠藉由Cot調節劑治療之疾病或病狀。
在某些實施例中,本發明提供醫藥組合物,其包含治療有效量之本發明化合物(例如式I化合物或通篇所描述其他式之化合物)及至少一種醫藥學上可接受之賦形劑。
相關申請案之交叉參考
本申請案根據35 U.S.C. 119(e)主張2015年7月6日提交之美國臨時申請案第62/189,158號及2015年12月17日提交之美國臨時申請案第62/269,060號的權益,其中各臨時申請案之內容以全文引用之方式併入本文中。 定義及一般參數
以下描述闡述例示性方法、參數及其類似者。然而,應認識到,此描述並不意欲作為本發明之範疇的限制,而是替代地作為例示性實施例之描述而提供。
如本說明書中所用,以下字語、片語及符號一般意欲具有如在下文中所闡述之含義,但使用其之上下文另有說明之情況除外。
不在兩個字母或符號之間的短劃(「-」)用於指示取代基之連接點。舉例而言,-C(O)NH2
經由碳原子連接。在化學基團之前端或末端之短劃為出於方便之目的;可在存在或不存在一或多個短劃之情況下描繪化學基團而不丟失其普通含義。穿過結構中之線所畫之波浪線指示基團之連接點。除非在化學上或在結構上需要,否則化學基團所書寫或命名之次序不指示或暗示方向性。
前綴「Cu-v
」指示以下基團具有u至v個碳原子。舉例而言,「C1-6
烷基」指示該烷基具有1至6個碳原子。
本文中提及「約」某一值或參數包括(且描述)針對該值或參數本身之實施例。在某些實施例中,術語「約」包括指示量±10%。在其他實施例中,術語「約」包括指示量±5%。在某些其他實施例中,術語「約」包括指示量±1%。此外,術語「約X」包括「X」之描述。此外,除非上下文另外明確指示,否則單數形式「一(a/an)」及「該(the)」包括複數個提及物。因此,例如提及「該化合物」包括複數種此類化合物且提及「該分析」包括提及一或多個分析及其熟習此項技術者已知之等效物。
「烷基」係指非分支鏈或分支鏈飽和烴鏈。如本文中所用,烷基具有1至20個碳原子(亦即C1-20
烷基)、1至8個碳原子(亦即C1-8
烷基)、1至6個碳原子(亦即C1-6
烷基)或1至4個碳原子(亦即C1-4
烷基)。烷基之實例包括甲基、乙基、丙基、異丙基、正丁基、第二丁基、異丁基、第三丁基、戊基、2-戊基、異戊基、新戊基、己基、2-己基、3-己基及3-甲基戊基。當具有特定數目之碳的烷基殘基藉由化學名稱命名或藉由分子式鑑別時,可涵蓋具有該數目之碳的所有位置異構體;因此,舉例而言,「丁基」包括正丁基(亦即-(CH2
)3
CH3
)、第二丁基(亦即-CH(CH3
)CH2
CH3
)、異丁基(亦即-CH2
CH(CH3
)2
)及第三丁基(亦即-C(CH3
)3
);且「丙基」包括正丙基(亦即-(CH2
)2
CH3
)及異丙基(亦即-CH(CH3
)2
)。
「烯基」係指含有至少一個碳-碳雙鍵且具有2至20個碳原子(亦即C2-20
烯基)、2至8個碳原子(亦即C2-8
烯基)、2至6個碳原子(亦即C2-6
烯基)或2至4個碳原子(亦即C2-4
烯基)之烷基。烯基之實例包括乙烯基、丙烯基、丁二烯基(包括1,2-丁二烯基及1,3-丁二烯基)。
「炔基」係指含有至少一個碳-碳參鍵且具有2至20個碳原子(亦即C2-20
炔基)、2至8個碳原子(亦即C2-8
炔基)、2至6個碳原子(亦即C2-6
炔基)或2至4個碳原子(亦即C2-4
炔基)之烷基。術語「炔基」亦包括具有一個參鍵及一個雙鍵之彼等基團。
「烷氧基」係指基團「烷基-O-」。烷氧基之實例包括甲氧基、乙氧基、正丙氧基、異丙氧基、正丁氧基、第三丁氧基、第二丁氧基、正戊氧基、正己氧基及1,2-二甲基丁氧基。
「鹵烷氧基」係指如上文所定義之烷氧基,其中一或多個氫原子經鹵素置換。
「烷基硫基」係指基團「烷基-S-」。
「醯基」係指基團-C(O)R,其中R為氫、烷基、環烷基、雜環基、芳基、雜烷基或雜芳基;如本文中所定義,其中之每一者可視情況經取代。醯基之實例包括甲醯基、乙醯基、環己基羰基、環己基甲基-羰基及苯甲醯基。
「醯胺基」係指以下兩者:係指基團-C(O)NRy
Rz
之「C-醯胺基」基團及係指基團-NRy
C(O)Rz
之「N-醯胺基」基團,其中Ry
及Rz
獨立地選自由氫、烷基、芳基、鹵烷基或雜芳基組成之群;其中之每一者可視情況經取代。
「胺基」係指基團-NRy
Rz
,其中Ry
及Rz
獨立地選自由氫、烷基、鹵烷基、芳基或雜芳基組成之群;其中之每一者可視情況經取代。
「甲脒基」係指-C(NH)(NH2
)。
「芳基」係指具有單個環(例如單環)或多個環(例如雙環或三環) (包括稠合系統)之芳族碳環基。如本文中所用,芳基具有6至20個環碳原子(亦即C6-20
芳基)、6至12個碳環原子(亦即C6-12
芳基)或6至10個碳環原子(亦即C6-10
芳基)。芳基之實例包括苯基、萘基、茀基及蒽基。然而,芳基無論如何不涵蓋下文所定義之雜芳基或與其重疊。若一或多個芳基與雜芳基稠合,則所得環系統為雜芳基。若一或多個芳基與雜環基稠合,則所得環系統為雜環基。
「疊氮基」係指-N3
。
「胺甲醯基」係指以下兩者:係指基團-O-C(O)NRy
Rz
之「O-胺甲醯基」基團及係指基團-NRy
C(O)ORz
之「N-胺甲醯基」基團,其中Ry
及Rz
獨立地選自由氫、烷基、芳基、鹵烷基或雜芳基組成之群;其中之每一者可視情況經取代。
「羧基」係指-C(O)OH。
「羧基酯」係指-OC(O)R及-C(O)OR兩者,其中R為氫、烷基、環烷基、雜環基、芳基、雜烷基或雜芳基;如本文中所定義,其中之每一者可視情況經取代。
「氰基」或「甲腈」係指基團-CN。
「環烷基」係指具有單個環或多個環(包括稠合、橋連及螺環系統)之飽和或部分不飽和環烷基。術語「環烷基」包括環烯基(亦即具有至少一個雙鍵之環基)。如本文中所用,環烷基具有3至20個環碳原子(亦即C3-20
環烷基)、3至12個環碳原子(亦即C3-12
環烷基)、3至10個環碳原子(亦即C3-10
環烷基)、3至8個環碳原子(亦即C3-8
環烷基)或3至6個環碳原子(亦即C3-6
環烷基)。環烷基之實例包括環丙基、環丁基、環戊基及環己基。
「胍基」係指-NHC(NH)(NH2
)。
「肼基」係指-NHNH2
。
「亞胺基」係指基團-C(NR)R,其中各R為烷基、環烷基、雜環基、芳基、雜烷基或雜芳基;如本文中所定義,其中之每一者可視情況經取代。
「鹵素」或「鹵基」包括氟、氯、溴及碘。「鹵烷基」係指如上文所定義之非分支鏈或分支鏈烷基,其中一或多個氫原子經鹵素置換。舉例而言,在殘基經超過一個鹵素取代之情況下,其可藉由使用對應於所連接之鹵素部分之數目的前綴來提及。二鹵烷基及三鹵烷基係指經兩個(「二」)或三個(「三」)鹵基取代之烷基,該等鹵基可為(但並非必須為)相同鹵素。鹵烷基之實例包括二氟甲基(-CHF2
)及三氟甲基(-CF3
)。
「雜烷基」係指其中碳原子(及任何相關氫原子)中之一或多者各自獨立地經相同或不同雜原子基團置換之烷基。術語「雜烷基」包括具有碳及雜原子之非分支鏈或分支鏈飽和鏈。舉例而言,1、2或3個碳原子可獨立地經相同或不同雜原子基團置換。雜原子基團包括(但不限於)-NR-、-O-、-S-、-S(O)-、-S(O)2
-及其類似基團,其中R為H、烷基、芳基、環烷基、雜烷基、雜芳基或雜環基,其中之每一者可視情況經取代。雜烷基之實例包括-OCH3
、-CH2
OCH3
、-SCH3
、-CH2
SCH3
、-NRCH3
及-CH2
NRCH3
,其中R為氫、烷基、芳基、芳烷基、雜烷基或雜芳基,其中之每一者可視情況經取代。如本文中所用,雜烷基包括1至10個碳原子、1至8個碳原子或1至4個碳原子;以及1至3個雜原子、1至2個雜原子或1個雜原子。
「雜芳基」係指具有單個環、多個環或多個稠合環之芳族基,其中一或多個環雜原子獨立地選自氮、氧及硫。如本文中所用,雜芳基包括1至20個環碳原子(亦即C1-20
雜芳基)、3至12個環碳原子(亦即C3-12
雜芳基)或3至8碳環原子(亦即C3-8
雜芳基);及獨立地選自氮、氧及硫之1至5個雜原子、1至4個雜原子、1至3個環雜原子、1至2個環雜原子或1個環雜原子雜芳基之實例包括嘧啶基、嘌呤基、吡啶基、噠嗪基、苯并噻唑基及吡唑基。稠合-雜芳基環之實例包括(但不限於)苯并[d]噻唑基、喹啉基、異喹啉基、苯并[b]噻吩基、吲唑基、苯并[d]咪唑基、吡唑并[1,5-a]吡啶基及咪唑并[1,5-a]吡啶基,其中該雜芳基可經由稠合系統之任一環結合。不論與分子其餘部分之連接如何(亦即,經由稠合環中之任一者),具有單個或多個稠合環、含有至少一個雜原子之任何芳族環均視為雜芳基。雜芳基不涵蓋如上文所定義之芳基或與其重疊。
「雜環基」係指具有一或多個獨立地選自氮、氧及硫之環雜原子的飽和或不飽和環烷基。術語「雜環基」包括雜環烯基(亦即具有至少一個雙鍵之雜環基)、橋連-雜環基、稠合-雜環基及螺-雜環基。雜環基可為單環或多環,其中多環可為稠合環、橋連環或螺環。不論連接如何(亦即,可經由碳原子或雜原子結合),含有至少一個雜原子之任何非芳族環均視為雜環基。此外,不論與分子其餘部分之連接如何,術語雜環基意欲涵蓋含有至少一個雜原子之任何非芳族環,該環可與芳基或雜芳基環稠合。如本文中所用,雜環基具有2至20個環碳原子(亦即C2-20
雜環基)、2至12個環碳原子(亦即C2-12
雜環基)、2至10個環碳原子(亦即C2-10
雜環基)、2至8個環碳原子(亦即C2-8
雜環基)、3至12個環碳原子(亦即C3-12
雜環基)、3至8個環碳原子(亦即C3-8
雜環基)或3至6個環碳原子(亦即C3-6
雜環基);具有獨立地選自氮、硫或氧之1至5個環雜原子、1至4個環雜原子、1至3個環雜原子、1至2個環雜原子或1個環雜原子。雜環基可含有一或多個側氧基及/或硫(酮)基。雜環基之實例包括吡咯啶基、哌啶基、哌嗪基、氧雜環丁基、二氧戊環基、氮雜環丁基及嗎啉基。如本文中所用,術語「橋連-雜環基」係指在雜環基之兩個不相鄰原子處連接的四員至十員環狀部分,其中一或多個(例如1或2個)四員至十員環狀部分具有至少一個雜原子,其中各雜原子獨立地選自氮、氧及硫。如本文中所用,橋連-雜環基包括雙環及三環之環系統。如本文中亦使用,術語「螺-雜環基」係指其中三員至十員雜環基具有一或多個額外環之環系統,其中該一或多個額外環為三員至十員環烷基或三員至十員雜環基,其中該一或多個額外環之單個原子亦為該三員至十員雜環基之原子。螺-雜環基環之實例包括雙環及三環之環系統,諸如2-氧雜-7-氮雜螺[3.5]壬基、2-氧雜-6-氮雜螺[3.4]辛基及6-氧雜-1-氮雜螺[3.3]庚基。稠合-雜環基環之實例包括(但不限於)1,2,3,4-四氫異喹啉基、1-側氧基-1,2,3,4-四氫異喹啉基、1-側氧基-1,2-二氫異喹啉基、4,5,6,7-四氫噻吩并[2,3-c]吡啶基、吲哚啉基及異吲哚啉基,其中該雜環基可經由稠合系統之任一環結合。
「羥基(Hydroxy或hydroxyl)」係指基團-OH。「羥烷基」係指如上文所定義之非分支鏈或分支鏈烷基,其中一或多個氫原子經羥基置換。
「側氧基」係指基團(=O)或(O)。
「硝基」係指基團-NO2
。
「磺醯基」係指基團-S(O)2
R,其中R為烷基、鹵烷基、雜環基、環烷基、雜芳基或芳基。磺醯基之實例為甲基磺醯基、乙基磺醯基、苯磺醯基及甲苯磺醯基。
「烷基磺醯基」係指基團-S(O)2
R,其中R為烷基。
「烷基亞磺醯基」係指基團-S(O)R,其中R為烷基。
「硫氰酸酯」-SCN。
「硫醇」係指基團-SR,其中R為烷基、鹵烷基、雜環基、環烷基、雜芳基或芳基。
「硫(酮)基」或「硫酮」係指基團(=S)或(S)。
可使用某些常用替代性化學名稱。舉例而言,諸如二價「烷基」、二價「芳基」等二價基團亦可分別稱為「伸烷基(alkylene或alkylenyl)」、「伸芳基(arylene或arylenyl)」。此外,除非另外明確指示,否則在本文中將基團組合稱為一個部分(例如芳烷基)之情況下,最後提及之基團含有該部分連接至分子其餘部分之原子。
術語「視情況選用之」或「視情況地」意謂隨後所描述之事件或情形可發生或可不發生,且該描述包括其中該事件或情形發生之情況及其中該事件或情形不發生之情況。此外,術語「視情況經取代」係指指定原子或基團上之任何一或多個氫原子可經除氫以外之部分置換或可不經置換。
化合物中之一些以互變異構體之形式存在。互變異構體彼此處於平衡。舉例而言,含醯胺化合物可與亞胺酸互變異構體平衡存在。不論展示何種互變異構體且不論互變異構體之間的平衡性質如何,一般技術者將化合物均理解為包含醯胺及亞胺酸互變異構體兩者。因此,含醯胺化合物理解為包括其亞胺酸互變異構體。同樣,含亞胺酸化合物理解為包括其醯胺互變異構體。
本文中所給出之任何式或結構亦意欲表示化合物的未經標記之形式以及經同位素標記之形式。經同位素標記之化合物具有由本文中所給出之式所描繪的結構,其例外之處在於一或多個原子經具有所選原子質量或質量數之原子置換。可併入本發明之化合物中的同位素之實例包括氫、碳、氮、氧、磷、氟及氯之同位素,諸如(但不限於)2
H (氘, D)、3
H (氚)、11
C、13
C、14
C、15
N、18
F、31
P、32
P、35
S、36
Cl及125
I。本發明的經各種同位素標記之化合物,例如將諸如3
H、13
C及14
C之放射性同位素併入至其中的彼等化合物。此類經同位素標記之化合物可適用於代謝研究、反應動力學研究、偵測或成像技術(諸如正電子發射斷層攝影法(PET)或單光子發射電腦斷層攝影法(SPECT),包括藥物或受質組織分佈分析)或用於患者之放射性治療。
本發明亦包括式I化合物之「氘化類似物」,其中連接至碳原子之1至n個氫經氘置換。其中氮為分子中氫之數目。此類化合物展現增加之代謝抗性,且因此適用於當向哺乳動物(尤其人類)投與時增加任何式I化合物之半衰期。參見例如,Foster, 「Deuterium Isotope Effects in Studies of Drug Metabolism」, Trends Pharmacol. Sci. 5(12):524-527 (1984)。此類化合物藉由此項技術中熟知之手段來合成,舉例而言,藉由採用其中一或多個氫已經氘置換之起始物質。
本發明的經氘標記或取代之治療性化合物可具有經改良之DMPK (藥物代謝及藥物動力學)特性,該等特性與分佈、代謝及排泄(ADME)相關。用較重同位素(諸如氘)取代可得到由更大代謝穩定性而產生之某些治療性優點,例如增加之活體內半衰期、降低之劑量需求及/或治療指數改良。經18
F標記之化合物可適用於PET或SPECT研究。本發明的經同位素標記之化合物及其前藥一般可藉由進行流程中所揭示之程序或下文所描述之或實例及製備,藉由用可容易獲得的經同位素標記之試劑取代非同位素標記之試劑來製備。應理解,在此上下文中,氘視為式I化合物之取代基。
可藉由同位素增濃因子來界定此類較重同位素(尤其氘)之濃度。在本發明之化合物中,未具體指定為特定同位素之任何原子意欲表示彼原子之任何穩定同位素。除非另外陳述,否則當位置被具體指定為「H」或「氫」時,應理解該位置在其天然豐度同位素組成中具有氫。因此,在本發明之化合物中,具體指定為氘(D)之任何原子意欲表示氘。
在許多情況下,本發明之化合物能夠藉助於胺基及/或羧基或與其類似之基團的存在而形成酸鹽及/或鹼鹽。
亦提供本文中所描述之化合物的醫藥學上可接受之鹽、水合物、溶劑合物、互變異構形式、多晶型物及前藥。「醫藥學上可接受」或「生理學上可接受」係指化合物、鹽、組合物、劑型及其他物質適用於製備適合於獸醫學或人類醫藥使用之醫藥組合物。
所給出之化合物的術語「醫藥學上可接受之鹽」係指保留所給出之化合物的生物學有效性及特性且在生物學上或其他方面並非不合需要的鹽。「醫藥學上可接受之鹽」或「生理學上可接受之鹽」包括例如與無機酸所成之鹽及與有機酸所成之鹽。另外,若本文中所描述之化合物以酸加成鹽形式獲得,則可藉由使酸鹽之溶液鹼化來獲得游離鹼。反之,若產物為游離鹼,則可根據自鹼化合物製備酸加成鹽之習知程序,藉由將該游離鹼溶解於適合之有機溶劑中且用酸處理該溶液來產生加成鹽,尤其醫藥學上可接受之加成鹽。熟習此項技術者將認識到可用於製備無毒性醫藥學上可接受之加成鹽的各種合成方法。醫藥學上可接受之酸加成鹽可由無機酸及有機酸製備。衍生自無機酸之鹽包括鹽酸、氫溴酸、硫酸、硝酸、磷酸及其類似者之鹽。衍生自有機酸之鹽包括乙酸、丙酸、乙醇酸、丙酮酸、草酸、蘋果酸、丙二酸、丁二酸、順丁烯二酸、反丁烯二酸、酒石酸、檸檬酸、苯甲酸、肉桂酸、杏仁酸、甲磺酸、乙磺酸、對甲苯磺酸、水楊酸及其類似者之鹽。同樣,醫藥學上可接受之鹼加成鹽可由無機鹼及有機鹼製備。僅舉例而言,衍生自無機鹼之鹽包括鈉鹽、鉀鹽、鋰鹽、銨鹽、鈣鹽以及鎂鹽。衍生自有機鹼之鹽包括(但不限於)一級、二級及三級胺之鹽,該等胺諸如烷基胺(亦即NH2
(烷基))、二烷基胺(亦即HN(烷基)2
)、三烷基胺(亦即N(烷基)3
)、經取代之烷基胺(亦即NH2
(經取代之烷基))、二(經取代之烷基)胺(亦即HN(經取代之烷基)2
)、三(經取代之烷基)胺(亦即N(經取代之烷基)3
)、烯基胺(亦即NH2
(烯基))、二烯基胺(亦即HN(烯基)2
)、三烯基胺(亦即N(烯基)3
)、經取代之烯基胺(亦即NH2
(經取代之烯基))、二(經取代之烯基)胺(亦即HN(經取代之烯基)2
)、三(經取代之烯基)胺(亦即N(經取代之烯基)3
、單-、二-或三-環烷基胺(亦即NH2
(環烷基)、HN(環烷基)2
、N(環烷基)3
)、單-、二-或三-芳基胺 (亦即NH2
(芳基)、HN(芳基)2
、N(芳基)3
)或混合胺等。僅舉例而言,適合之胺的具體實例包括異丙胺、三甲基胺、二乙基胺、三(異丙基)胺、三(正丙基)胺、乙醇胺、2-二甲胺基乙醇、哌嗪、哌啶、嗎啉、N-乙基哌啶及其類似胺。
術語「經取代」意謂指定原子或基團上之任何一或多個氫原子經一或多個除氫以外之取代基置換,其限制條件為不超過指定原子之正常價。一或多個取代基包括(但不限於)烷基、烯基、炔基、烷氧基、醯基、胺基、醯胺基、甲脒基、芳基、疊氮基、胺甲醯基、羧基、羧基酯、氰基、胍基、鹵基、鹵烷基、鹵烷氧基、雜烷基、雜芳基、雜環基、羥基、肼基、亞胺基、側氧基、硝基、烷基亞磺醯基、磺酸、烷基磺醯基、硫氰酸酯、硫醇、硫酮或其組合。藉由無限地用所附接之其他取代基定義取代基(例如經取代之芳基具有經取代之烷基,該經取代之烷基本身經一經取代之芳基取代,該經取代之芳基進一步經一經取代之雜烷基取代等等)達成之聚合物或類似無限結構不意欲包含在本文中。除非另外指出,否則本文中所描述之化合物中的連續置換之最大數目為三。舉例而言,用兩個其他經取代之芳基連續取代經取代之芳基限於經(經(經取代之芳基)取代之芳基)取代之芳基。類似地,以上定義不意欲包括不允許之取代模式(例如,經5個氟取代之甲基或具有兩個相鄰氧環原子之雜芳基)。此類不許可之取代模式為熟習此項技術者所熟知。當用於修飾化學基團時,術語「經取代」可描述本文中所定義之其他化學基團。除非另外規定,否則在基團描述為視情況經取代之情況下,該基團之任何取代基本身未經取代。舉例而言,在一些實施例中,術語「經取代之烷基」係指具有一或多個取代基之烷基,該等取代基包括羥基、鹵基、烷氧基、環烷基、雜環基、芳基及雜芳基。在其他實施例中,該一或多個取代基可進一步經鹵基、烷基、鹵烷基、羥基、烷氧基、環烷基、雜環基、芳基或雜芳基取代,其中之每一者均經取代。在其他實施例中,取代基可進一步經鹵基、烷基、鹵烷基、烷氧基、羥基、環烷基、雜環基、芳基或雜芳基取代,其中之每一者均未經取代。
如本文中所用,「醫藥學上可接受之載劑」或「醫藥學上可接受之賦形劑」包括任何及所有溶劑、分散介質、包衣、抗細菌及抗真菌劑、等滲及吸收延遲劑及其類似物。此類介質及藥劑在醫藥學上活性之物質中的用途為此項技術中熟知的。除非任何習知介質或試劑與活性成分不相容,否則考慮將其用於治療性組合物中。亦可將補充活性成分併入組合物中。
如本文中所用,「醫藥學上可接受之載劑」或「醫藥學上可接受之賦形劑」包括任何及所有溶劑、分散介質、包衣、抗細菌及抗真菌劑、等滲及吸收延遲劑及其類似物。此類介質及藥劑在醫藥學上活性之物質中的用途為此項技術中熟知的。除非任何習知介質或試劑與活性成分不相容,否則考慮將其用於治療性組合物中。亦可將補充活性成分併入組合物中。
「溶劑合物」藉由溶劑與化合物之相互相用形成。亦提供本文中所描述之化合物之鹽的溶劑合物。亦提供本文中所描述之化合物之水合物。縮寫及首字母縮寫詞之列表
化合物
本文提供充當Cot之調節劑的化合物。在一個態樣中,提供一種具有式I結構之化合物:I
其中
R1
為氫、-O-R7
、-N(R8
)(R9
)、-C(O)-R7
、-S(O)2
-R7
、-C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基及雜芳基可視情況經一至四個Z1
取代;
R2
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z2
取代;
或R1
及R2
與其所連接之氮一起以形成雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z2
取代;
R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z3
取代;
R4
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z4
取代;
R5
為氫、鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z5
取代;
R6
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z6
取代;
各R7
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z7
取代;
R8
及R9
在每次出現時獨立地為氫、-S(O)2
R10
、-C(O)-R10
、-C(O)O-R10
、-C(O)N(R10
)(R11
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z8
取代;
R10
及R11
在每次出現時獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基,
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基視情況經一至四個Z1b
取代;
各Z1
、Z2
、Z3
、Z4
、Z5
、Z6
、Z7
及Z8
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;
各R15
獨立地為鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);及
m為0、1或2;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在另一態樣中,提供一種具有式I結構之化合物:I
其中
R1
為氫、-O-R7
、-N(R8
)(R9
)、-C(O)-R7
、-S(O)2
-R7
、-C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、雜環基、芳基及雜芳基可視情況經一至四個Z1
取代;
R2
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z2
取代;
或R1
及R2
與其所連接之氮一起以形成雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z2
取代;
R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z3
取代;
R4
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一至四個Z4
取代;
R5
為氫、鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z5
取代;
R6
為氫、-C(O)-R7
、-C(O)O-R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z6
取代;
各R7
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z7
取代;
R8
及R9
在每次出現時獨立地為氫、-S(O)2
R10
、-C(O)-R10
、-C(O)O-R10
、-C(O)N(R10
)(R11
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z8
取代;
R10
及R11
在每次出現時獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基,
其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基及雜芳基視情況經一至四個Z1b
取代;
各Z1
、Z2
、Z3
、Z4
、Z5
、Z6
、Z7
及Z8
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、C1-8
羥烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-C(O)N(R12
)-S(O)2
R12
、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;
各R15
獨立地為鹵基、-CN、-NO2
、-O-R7
、-N(R8
)(R9
)、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-OC(O)-R7
、-C(O)O-R7
、-OC(O)O-R7
、-OC(O)N(R10
)(R11
)、-C(O)N(R7
)2
、-N(R7
)C(O)(R7
)、C1-9
烷基、C2-6
烯基、C2-6
炔基、C1-9
烷基硫基、C1-6
鹵烷基、C3-15
環烷基、芳基、雜環基或雜芳基;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);
m為0、1或2;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在某些實施例中,式I化合物由式IA表示:IA
其中R1
-R6
、R15
及m如本文中所描述。
在某些實施例中,式I化合物由式IB表示:IB
其中R1
-R6
、R15
及m如本文中所描述。
在某些實施例中,m為0。在某些實施例中,R2
為氫。
在某些實施例中,提供式II化合物:II
其中R1
、R3
、R4
、R5
及R6
如本文中所定義。
在某些實施例中,提供式IIA化合物:IIA
其中R1
、R3
、R4
、R5
及R6
如本文中所定義。
在某些實施例中,提供式III化合物:III
其中R1
、R4
、R5
及R6
如本文中所定義,
W、X及Y各自獨立地為N或C;
n為1、2或3;
各Z3
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、C1-8
羥烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-C(O)N(R12
)-S(O)2
R12
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在某些實施例中,提供式IIIA化合物:IIIA
其中R1
、R4
、R5
及R6
如本文中所定義,
W、X及Y各自獨立地為N或C;
n為1、2或3;
各Z3
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在某些實施例中,提供式IIIA化合物:IIIA
其中R1
、R4
、R5
及R6
如請求項1中所定義,
W、X及Y各自獨立地為N或C;
n為1、2或3;
各Z3
獨立地為氫、側氧基、鹵基、-NO2
、-N3
、-CN、硫(酮)基、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-N(R12
)C(O)-R12
、-N(R12
)C(O)O-R12
、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-NR12
S(O)2
N(R13
)(R14
)、-NR12
S(O)2
O(R12
)、-OC(O)R12
、-OC(O)-N(R13
)(R14
)、-P(O)(OR12
)2
、-OP(O)(OR12
)2
、-CH2
P(O)(OR12
)2
、-OCH2
P(O)(OR12
)2
、-C(O)OCH2
P(O)(OR12
)2
、-P(O)(R12
)(OR12
)、-OP(O)(R12
)(OR12
)、-CH2
P(O)(R12
)(OR12
)、-OCH2
P(O)(R12
)(OR12
)、-C(O)OCH2
P(O)(R12
)(OR12
)、-P(O)(N(R12
)2
)2
、-OP(O)(N(R12
)2
)2
、-CH2
P(O)(N(R12
)2
)2
、-OCH2
P(O)(N(R12
)2
)2
、-C(O)OCH2
P(O)(N(R12
)2
)2
、-P(O)(N(R12
)2
)(OR12
)、-OP(O)(N(R12
)2
)(OR12
)、-CH2
P(O)(N(R12
)2
)(OR12
)、-OCH2
P(O)(N(R12
)2
)(OR12
)、-C(O)OCH2
P(O)(N(R12
)2
)(OR12
)、-P(O)(R12
)(N(R12
)2
)、-OP(O)(R12
)(N(R12
)2
)、-CH2
P(O)(R12
)(N(R12
)2
)、-OCH2
P(O)(R12
)(N(R12
)2
)、-C(O)OCH2
P(O)(R12
)(N(R12
)2
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、鹵烷基、芳基、雜芳基或雜環基視情況經一至四個Z1a
基團取代;
各Z1a
獨立地為側氧基、鹵基、硫(酮)基、-NO2
、-CN、-N3
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、C1-8
羥烷基、芳基、雜芳基、雜環基、-O-R12
、-C(O)R12
、-C(O)O-R12
、-C(O)N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-C(O)N(R12
)-S(O)2
R12
、-N(R12
)-C(O)R12
、-N(R12
)C(O)O(R12
)、-N(R12
)C(O)N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R12
)S(O)2
-N(R13
)(R14
)、-N(R12
)S(O)2
O(R12
)、-OC(O)R12
、-OC(O)OR12
、-OC(O)-N(R13
)(R14
)、-Si(R12
)3
、-S-R12
、-S(O)R12
、-S(O)(NH)R12
、-S(O)2
R12
或-S(O)2
N(R13
)(R14
);
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基,
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;
R13
及R14
在每次出現時各自獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代,或R13
及R14
與其所連接之氮一起形成雜環基,其中該雜環基視情況經一至四個Z1b
基團取代;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基);
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在某些實施例中,W為N,X為N-Z3
,且Y為C-Z3
。在某些實施例中,W為C-Z3
,X為N-Z3
,且Y為C-Z3
。
在某些實施例中,式I化合物由式IV表示:IV
其中R1
、R3
、R5
、R6
及Z4
如本文中所定義,q為0、1、2、3或4,環A為5員或6員環烷基、雜環基或雜芳基環,及環B為6員環烷基、雜環基或雜芳基環,其限制條件為在環A或環B中存在至少一個雜原子以使得R4
為視情況經取代之雙環雜環基或視情況經取代之雙環雜芳基。在上文中,波浪線指示與分子其餘部分之連接點,其中連接可經由視情況經取代之雙環雜環基或視情況經取代之雙環雜芳基的任一環(亦即環A或環B)。在一些實施例中,環A及/或環B包含側氧基(=O)。
在某些實施例中,提供式IVA化合物:IVA
其中R1
、R3
、R5
、R6
、Z4
、q、環A及環B如本文中所定義。
在某些實施例中,提供式V化合物:V
其中W、X、Y、R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義。
在某些實施例中,提供式VA化合物:VA
其中W、X、Y、R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義。
在某些實施例中,式I化合物由式VI表示:VI
其中R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義,且Z9
為氫、鹵基、-CN或-O-R12
。
在某些實施例中,式I化合物由式VIA表示:VIA
其中R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義,且Z9
為氫、鹵基、-CN或-O-R12
。
在某些實施例中,式I化合物由式VII表示:VII
其中R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義。
在某些實施例中,式I化合物由式VIIA表示:VIIA
其中R1
、R5
、R6
、Z3
、Z4
、q、n、環A及環B如本文中所定義。
在某些實施例中,式I化合物由式VIII或式IX表示:VIIIIX
其中Z3
、R1
、R4
、R5
及R6
如本文中所定義,且Z9
為氫、鹵基、-CN或-O-R12
。
在某些實施例中,式I化合物由式VIIIA或式IXA表示:VIIIAIXA
其中Z3
、R1
、R4
、R5
及R6
如本文中所定義,且Z9
為氫、鹵基、-CN或-O-R12
。
在某些實施例中,式I化合物由式X或式XI表示:XXI
其中Z3
、R1
、R4
、R5
及R6
如本文中所定義。
在某些實施例中,式I化合物由式XA或式XIA表示:XAXIA
其中Z3
、R1
、R4
、R5
及R6
如本文中所定義。
在某些實施例中,R6
為氫。
在某些實施例中,Z3
為氫、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-OC(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-O(C1-9
烷基)、-C(O)N(C1-9
烷基)2
、C1-9
烷基及雜環基。
在某些實施例中,Z3
為氫、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、C3-15
環烷基、芳基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-OC(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、雜環基或芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-O(C1-9
烷基)、-C(O)N(C1-9
烷基)2
、C1-9
烷基及雜環基。
在某些實施例中,Z3
為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-OC(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、雜環基或芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-O(C1-9
烷基)、-C(O)N(C1-9
烷基)2
、C1-9
烷基及雜環基。
在某些實施例中,Z3
為氫或視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:-CN、鹵基、-O-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、雜環基及雜芳基。
在某些實施例中,Z3
為C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-OC(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、芳基、雜環基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-O(C1-9
烷基)、-C(O)N(C1-9
烷基)2
、C1-9
烷基及雜環基。
在某些實施例中,Z3
為C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C3-15
環烷基、雜環基或芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-OC(O)-R12
、-C(O)O-R12
、-C(O)-N(R13
)(R14
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、雜環基或芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-O(C1-9
烷基)、-C(O)N(C1-9
烷基)2
、C1-9
烷基及雜環基。
在某些實施例中,Z3
為氫或視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:-CN、鹵基、-O-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-C(O)N(R12
)-S(O)2
R12
、C1-9
烷基、雜環基、芳基及雜芳基。
在某些實施例中,Z3
為氫或視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:-CN、鹵基、-O-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、雜環基及雜芳基。
在某些實施例中,Z3
為C3-15
環烷基、雜環基、芳基或雜芳基;且該C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、C1-8
鹵烷基、C1-8
羥烷基、C3-15
環烷基、雜環基及雜芳基。
在某些實施例中,Z3
為C3-15
環烷基、雜環基、芳基或雜芳基;且該C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、雜環基及雜芳基。
在某些實施例中,Z3
為氫、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、C3-15
環烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:側氧基、-CN、鹵基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-OC(O)-R12
、-C(O)-N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、-C(O)N(R12
)-S(O)2
R12
、C1-9
烷基、C1-8
鹵烷基、C1-8
羥烷基、C3-15
環烷基、芳基、雜環基及雜芳基;
Z9
為氫;
R1
為C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、雜環基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、-S(O)2
R12
、C1-9
烷基、C1-9
鹵烷基、雜環基及芳基,其中該C3-15
環烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9
烷基及C1-9
鹵烷基;
R4
為雜環基或雜芳基;
其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基;
R5
為-CN、鹵基、-O-R7
或-S(O)2
R7
;
R6
為氫;
各R7
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
各R12
獨立地為氫、C1-9
烷基或雜環基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;及
各R13
及R14
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物或氘化類似物。
在某些實施例中,Z3
為氫、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)O-R12
、-OC(O)-R12
、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、雜環基及雜芳基;
R1
為C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、雜環基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、C1-9
烷基及芳基;
R4
為雜環基或雜芳基;
其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、C1-9
烷基、C1-9
鹵烷基及雜環基;
R5
為-CN、鹵基或-O-R7
;
R6
為氫;
各R7
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
各R12
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;及
各R13
及R14
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。
在某些實施例中,Z3
為氫、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、C3-15
環烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:側氧基、-CN、鹵基、-O-R12
、-C(O)-R12
、-C(O)O-R12
、-OC(O)-R12
、-C(O)-N(R13
)(R14
)、-N(R12
)S(O)2
(R12
)、-N(R13
)(R14
)、-N(R13
)2
(R14
)+
、C1-9
烷基、C3-15
環烷基、芳基、雜環基及雜芳基;
其中該C1-9
烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:鹵基及羥基。
R1
為C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;
其中該C1-9
烷基、雜環基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、C1-9
烷基及芳基;
R4
為雜環基或雜芳基;
其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基;
R5
為-CN、鹵基、-O-R7
或-S(O)2
R7
;
R6
為氫;
各R7
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
各R12
獨立地為氫、C1-9
烷基或雜環基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;及
各R13
及R14
獨立地為氫或C1-9
烷基;
其中該C1-9
烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9
烷基)及芳基;
或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物或氘化類似物。
在某些實施例中,Z3
為視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C3-15
環烷基:-CN、鹵基、-C(O)-R12
、-OC(O)-R12
、-C(O)N(R13
)(R14
)、C1-9
烷基、C1-8
鹵烷基、C1-8
羥烷基、C3-15
環烷基及雜芳基。
在某些實施例中,Z3
為視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的雜環基:-O-R12
、-C(O)O-R12
、C1-9
烷基、C1-8
鹵烷基、C1-8
羥烷基及雜環基。
在某些實施例中,R1
為C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;且該C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、C1-9
烷基及芳基。
在某些實施例中,R1
為-O-R7
、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;且該C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、-S(O)2
R12
、C1-9
烷基、C1-9
鹵烷基、C3-15
環烷基、雜環基及芳基,其中該C3-15
環烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9
烷基及C1-9
鹵烷基。
在某些實施例中,R1
為-O-R7
、C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基;且該C1-9
烷基、C3-15
環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、C1-9
烷基、C3-15
環烷基及芳基。在某些實施例中,R1
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:鹵基、-CN、-O-R12
、-S(O)2
R12
、C3-15
環烷基、雜環基及芳基,其中該C3-15
環烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9
烷基及C1-9
鹵烷基。
在某些實施例中,R1
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:鹵基、-CN、-O-R12
、C1-9
烷基及芳基。
在某些實施例中,R1
為C3-15
環烷基、雜環基或雜芳基;
其中該C3-15
環烷基、雜環基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-N(R13
)(R14
)、-NH-C(O)O-R12
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、雜環基、芳基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-N(R13
)(R14
)、C1-9
烷基、C3-15
環烷基及芳基。
在某些實施例中,R1
為C3-15
環烷基、雜環基或雜芳基,其中該C3-15
環烷基、雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12
、C1-9
烷基及芳基。
在某些實施例中,R1
為雜環基或雜芳基,其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基及C1-9
烷基。
在某些實施例中,R1
為芳基;
其中該芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-N(R13
)(R14
)、-NH-C(O)O-R12
、-S(O)2
-R12
、-Si(R12
)3
、C1-9
烷基、C3-15
環烷基、雜環基、芳基及雜芳基;且
其中該C1-9
烷基、C3-15
環烷基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-N(R13
)(R14
)、C1-9
烷基、C3-15
環烷基及芳基。
在某些實施例中,R1
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的芳基:鹵基、-CN、-O-R7
、C1-9
烷基及芳基。
在某些實施例中,R1
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的芳基:鹵基、-O-R7
及C1-9
烷基。
在一個實施例中,R1
為(1S,3S,5S,7S)-金剛烷-2-基、(R)-1-苯基乙基、(R)-1-苯基丙基、(R)-1-苯基丙基-1,2,2,3,3,3-d6、(R)-1-苯基丙基-2,2,3,3,3-d5、(R)-2-氰基-1-苯基乙基、(R)-2-羥基-1-苯基乙基、(R)-2-羥基-2-甲基-1-苯基丙基、(R)-2-甲氧基-1-苯基乙基、(R)-3-氰基-1-苯基丙基、(R)-3-氟-1-苯基丙基、(R)-3-羥基-1-苯基丙基、(S)-1-苯基丙基-2,2,3,3,3-d5、(S)-2-氰基-1-苯基乙基、(S)-2-羥基-1-苯基乙基、(S)-2-羥基-2-甲基-1-苯基丙基、(S)-3-氰基-1-苯基丙基、(S)-3-羥基-1-苯基丙基、1-苯基丙基-2,2,3,3,3-d5、2-氰基-1-苯基乙基、3,3-二甲基四氫-2H-哌喃-4-基、3,4-二氯-2-氟苯基、3,4-二氟苯基、3-氯-2,6-二氟苯基、3-氯-2-氟苯基、3-氯-2-甲氧基苯基、3-氯-4-氟苯基、3-氯-4-甲氧基苯基、3-氯苯基、3-氰基-1-苯基丙基、5,6-二氟吡啶-3-基、5-氯-6-氟吡啶-3-基、5-氯吡啶-3-基、環庚基、環己基、新戊基、新戊基-1,1-d2、(1-(二氟甲基)環丙基)甲基、(1-甲基環丁基)甲基、(1R,5S)-雙環[3.1.0]己-6-基、(1R,5S,6r)-3-氧雜雙環[3.1.0]己-6-基、(R)-2,2-二甲基四氫呋喃-3-基、(R)-3,3-二甲基丁-2-基、(R)-3,3-二甲基四氫-2H-哌喃-4-基、(R)-環丙基(苯基)甲基、(S)-2,2-二甲基四氫呋喃-3-基、(S)-3,3-二甲基四氫-2H-哌喃-4-基、2,2-二甲基丙基-1,1-d2、2,2-二甲基四氫呋喃-3-基、2-氰基-2-甲基丙基、2-甲基-2-苯基丙基、3-氯-2,2-二甲基丙基、3-氰基-2,2-二甲基丙基、3-羥基-2,2-二甲基丙基、第三丁氧基或四氫-2H-哌喃-4-基。
在一個實施例中,R1
為(1S,3S,5S,7S)-金剛烷-2-基、(R)-1-苯基乙基、(R)-1-苯基丙基、(R)-1-苯基丙基-1,2,2,3,3,3-d6、(R)-1-苯基丙基-2,2,3,3,3-d5、(R)-2-氰基-1-苯基乙基、(R)-2-羥基-1-苯基乙基、(R)-2-羥基-2-甲基-1-苯基丙基、(R)-2-甲氧基-1-苯基乙基、(R)-3-氰基-1-苯基丙基、(R)-3-氟-1-苯基丙基、(R)-3-羥基-1-苯基丙基、(S)-1-苯基丙基-2,2,3,3,3-d5、(S)-2-氰基-1-苯基乙基、(S)-2-羥基-1-苯基乙基、(S)-2-羥基-2-甲基-1-苯基丙基、(S)-3-氰基-1-苯基丙基、(S)-3-羥基-1-苯基丙基、1-苯基丙基-2,2,3,3,3-d5、2-氰基-1-苯基乙基、3,3-二甲基四氫-2H-哌喃-4-基、3,4-二氯-2-氟苯基、3,4-二氟苯基、3-氯-2,6-二氟苯基、3-氯-2-氟苯基、3-氯-2-甲氧基苯基、3-氯-4-氟苯基、3-氯-4-甲氧基苯基、3-氯苯基、3-氰基-1-苯基丙基、5,6-二氟吡啶-3-基、5-氯-6-氟吡啶-3-基、5-氯吡啶-3-y、環庚基、環己基、新戊基、新戊基-1,1-d2或四氫-2H-哌喃-4-基。
在另一個實施例中,R1
為(R)-1-苯基乙基、(R)-1-苯基丙基、3,4-二氯-2-氟苯基、3-氯-2-氟苯基、3-氯-4-氟苯基、5,6-二氟吡啶-3-基或新戊基。
在一個實施例中,R2
為氫。在一個實施例中,R2
為C1
-6
烷基。在一個實施例中,R2
為甲基。
在一個實施例中,R1
及R2
與其所連接之氮原子一起形成雜環基或雜環基。在某些實施例中,R1
及R2
與其所連接之氮一起以形成雜環基或雜芳基,其中該雜環基可視情況經一至三個C1-9
烷基取代。在某些實施例中,R1
及R2
與其所連接之氮原子一起形成視情況經取代之吡唑基。在某些實施例中,R1
及R2
與其所連接之氮原子一起形成3,3-二甲基哌啶-1-基。
在一個實施例中,R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一或多個選自由以下各者組成之群的取代基(亦即Z3
)取代:(1R,5S,6r)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基、(1R,5S,6s)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基、(1R,5S,6s)-3-氮雜雙環[3.1.0]己-6-基、(3-羥基氧雜環丁-3-基)甲基、(R)-1,1,1-三氟丙-2-基、(R)-1-乙基吡咯啶-3-基、(R)-吡咯啶-3-基、(S)-1-氟丙-2-基、1-((苯甲氧基)羰基)哌啶-4-基、1-((苯甲氧基)羰基)吡咯啶-4-基、1-((第三丁氧基)羰基)甲基、1-((第三丁氧基)羰基)哌啶-4-基、氧雜環丁-3-基、1-(氧雜環丁-3-基)哌啶-4-基、1-(第三丁基)哌啶-4-基、1,1-二氟-2-羥基乙基、1-乙基哌啶-4-基、1-丙基哌啶-4-基、2-(2-羥基乙氧基)乙基、2-(2-甲氧基乙氧基)乙基、2-(二乙基(甲基)銨基)乙基、2-(二甲胺基)乙基、2-(哌啶-1-基)乙基、2,2,2-三氟乙基、2,2,6,6-四甲基哌啶-4-基、2-胺基乙基、2-氟乙基、2-羥基乙基、2-甲氧基乙基、2-(N-嗎啉基)乙基、3-(二甲胺基)丙基、3-(吡咯啶-1-基)丙基、羧甲基、氰基甲基、環戊基、環丙基、氫、異丙基、甲基、氧雜環丁-3-基、苯基、哌啶-4-基、吡啶-2-基甲基、吡啶-3-基、(1R,2S)-2-氟環丙基、[1,1'-雙(環丙)]-1-基、1-(二氟甲基)環丙基、1-(氟甲基)環丙基、1-(羥基甲基)環丙基、1-(嗎啉-4-羰基)環丙-1-基、1-(吡啶-4-基)環丙基、1-(吡咯啶-1-羰基)環丙-1-基、1-(三氟甲基)環丙基、1,1,1-三氟-2-甲基丙-2-基、1,1-二氟-2-甲基丙-2-基、1-胺甲醯基環丁-1-基、1-胺甲醯基環丙-1-基、1-羧基環丙基、1-氰基環丁基、1-氰基環丙基、1-氟-2-甲基丙-2-基、1-甲基環丙基、1-N,N-二甲基胺甲醯基環丙-1-基、2-(甲基磺醯胺基)-2-側氧基乙基、2,2-二氟乙基、2,6-二氟benzyl、3-(羥基甲基)氧雜環丁-3-基、3-(三氟甲基)氧雜環丁-3-基、3,3-二氟-1-(羧基)環丁-1-基、3,3-二氟環丁基、雙環[1.1.1]戊-1-基、氯、氰基、氟、碘或第三丁基。
在一個實施例中,R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一或多個選自由以下各者組成之群的取代基(亦即Z3
)取代:(1R,5S,6r)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基、(1R,5S,6s)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基、(1R,5S,6s)-3-氮雜雙環[3.1.0]己-6-基、(3-羥基氧雜環丁-3-基)甲基、(R)-1,1,1-三氟丙-2-基、(R)-1-乙基吡咯啶-3-基、(R)-吡咯啶-3-基、(S)-1-氟丙-2-基、1-((苯甲氧基)羰基)哌啶-4-基、1-((苯甲氧基)羰基)吡咯啶-4-基、1-((第三丁氧基)羰基)甲基、1-((第三丁氧基)羰基)哌啶-4-基、氧雜環丁-3-基、1-(氧雜環丁-3-基)哌啶-4-基、1-(第三丁基)哌啶-4-基、1,1-二氟-2-羥基乙基、1-乙基哌啶-4-基、1-丙基哌啶-4-基、2-(2-羥基乙氧基)乙基、2-(2-甲氧基乙氧基)乙基、2-(二乙基(甲基)銨基)乙基、2-(二甲胺基)乙基、2-(哌啶-1-基)乙基、2,2,2-三氟乙基、2,2,6,6-四甲基哌啶-4-基、2-胺基乙基、2-氟乙基、2-羥基乙基、2-甲氧基乙基、2-(N-嗎啉基)乙基、3-(二甲胺基)丙基、3-(吡咯啶-1-基)丙基、羧甲基、氰基甲基、環戊基、環丙基、氫、異丙基、甲基、氧雜環丁-3-基、苯基、苯基、哌啶-4-基、吡啶-2-基甲基、吡啶-3-基或第三丁基。
在另一個實施例中,R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一或多個選自由以下各者組成之群的取代基(亦即Z3
)取代:氫、異丙基、甲基、氧雜環丁-3-基、1-(第三丁基)哌啶-4-基、1-乙基哌啶-4-基、環丙基、1-(三氟甲基)環丙基、1-(二氟甲基)環丙基、1-(氟甲基)環丙基、1-氰基環丙基或哌啶-4-基。
在另一個實施例中,R3
為雜環基或雜芳基,其中各雜環基或雜芳基視情況經一或多個選自由以下各者組成之群的取代基(亦即Z3
)取代:氫、異丙基、甲基、氧雜環丁-3-基、1-(第三丁基)哌啶-4-基、1-乙基哌啶-4-基、環丙基或哌啶-4-基。
在一個實施例中,R3
為三唑基、吡唑基、異噁唑基、異噁唑基、噁唑基、吡嗪基、吡啶基、嘧啶基、咪唑基、噻二唑基、四唑基或噁二唑基,其中各自視情況由一或多個如本文中所描述之Z3
基團取代。在一個實施例中,R3
為視情況經取代之三唑(例如1H-1,2,3-三唑基)。
在某些實施例中,R3
為經一或多個選自由以下各者組成之群之取代基取代的三唑:1-(苯甲氧基羰基)哌啶-4-基、1-(第三丁基)哌啶-4-基、1-乙基哌啶-4-基、環丙基、異丙基、甲基及哌啶-4-基。
在一個實施例中,R4
為雜環基或雜芳基;且該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12
、-C(O)-R12
、C1-6
烷基、C1-6
鹵烷基及雜環基。
在某些實施例中,R4
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜芳基:-CN、鹵基、-O-R12
、-C(O)-R12
、C1-9
烷基、C1-9
鹵烷基及雜環基。
在某些實施例中,R4
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜環基:-CN、鹵基、-O-R12
、-C(O)-R12
、C1-9
烷基、C1-9
鹵烷基及雜環基。
在某些實施例中,R4
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜芳基:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基。
在某些實施例中,R4
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜環基:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基。
在某些實施例中,R4
為視情況經取代之雙環雜環基或視情況經取代之雙環雜芳基。在某些實施例中,R4
為,其中Z4
如本文中所定義,q為0、1、2、3或4,環A為5員或6員環烷基、雜環基或雜芳基環,及環B為6員環烷基、雜環基或雜芳基環,其限制條件為在環A或環B中存在至少一個雜原子以使得R4
為視情況經取代之雙環雜環基或視情況經取代之雙環雜芳基。在上文中,波浪線指示與分子其餘部分之連接點,其中連接可經由視情況經取代之雙環雜環基或視情況經取代之雙環雜芳基的任一環(亦即環A或環B)。在一些實施例中,環A及/或環B包含側氧基(=O)。
在某些實施例中,R4
為視情況經取代之雙環雜芳基。在某些實施例中,R4
為選自由以下各者組成之群的視情況經取代之雙環雜芳基:、、及,其中Z4
如本文中所定義,q為0、1、2、3或4,及環A為5員或6員雜環基或雜芳基環。在一些實施例中,環A包含側氧基(=O)。
R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、或,其中Z4
如本文中所定義,且q為0、1、2、3或4。
在某些實施例中,R4
為、、、、、、或,其中Z4
如本文中所定義,且q為0、1、2、3或4。
在某些實施例中,式I化合物由式XII表示:XII
其中,q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義,環A為5員或6員雜環基或雜芳基,且Z9
為氫、鹵基、-CN或-O-R12
。在某些實施例中,式I化合物由式XIIA表示:XIIA
其中,q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義,環A為5員或6員雜環基或雜芳基,且Z9
為氫、鹵基、-CN或-O-R12
。
在某些實施例中,式I化合物由式XIII表示:XIII
其中,q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義,且環A為5員或6員雜環基或雜芳基。在某些實施例中,式I化合物由式XIIIA表示:XIIIA
其中,q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義,且環A為5員或6員雜環基或雜芳基。
在某些實施例中,式I化合物由式XIIIB表示:XIIIB
其中q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義。
在某些實施例中,式I化合物由式XIIIC表示:XIIIC
其中q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義。
在某些實施例中,式I化合物由式XIIID表示:XIIID
其中q、Z3
、R1
、Z4
、R5
及R6
如本文中所定義。
在某些實施例中,各Z4
獨立地選自由以下各者組成之群:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基。在一些實施例中,各Z4
獨立地選自由以下各者組成之群:-CN、鹵基、-O-R12
及C1-9
烷基。
在某些實施例中,R4
為視情況經取代之單環雜芳基。在某些實施例中,R4
為、或,其中Z4
如本文中所定義,且q為0、1、2、3或4。在某些實施例中,R4
為、或,其中Z4
如本文中所定義。在某些實施例中,式I化合物由式XIV表示:XIV
其中Z3
、R1
、Z4
、R5
及R6
如本文中所定義。在某些實施例中,式I化合物由式XIVA表示:XIVA
其中Z3
、R1
、Z4
、R5
及R6
如本文中所定義。
在某些實施例中,Z3
為 或
在某些實施例中,各Z4
獨立地選自由以下各者組成之群:-CN、鹵基、-O-R12
、-C(O)-R12
、-N(R13
)(R14
)、C1-9
烷基、C1-9
鹵烷基及雜環基。在一些實施例中,各Z4
獨立地選自由以下各者組成之群:-CN、鹵基、-O-R12
及C1-9
烷基。在式IX或式X化合物之某些實施例中,R1
為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的C1-9
烷基:鹵基、-CN、-O-R12
、C1-9
烷基及芳基。在式IX或式X化合物之某些實施例中,R6
為氫。在式IX或式X化合物之某些實施例中,R5
為鹵基或氰基。
在一個實施例中,R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、或。
在一個實施例中,R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、 、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、或。
在一個實施例中,R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、 、、、、、、、、、、或。
在一個實施例中,R4
為、、、、、、、、、或。
在一個實施例中,R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、或。
在某些實施例中,R4
為、、、、、、、、、、、、、、、、、、、、、、、、、、、、、、或。
在某些實施例中,R5
為氫、鹵基、-CN、-O-R7
、-S(O)-R7
、-S(O)2
R7
、-S(O)2
N(R7
)2
、-C(O)R7
、-C(O)N(R7
)2
、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜環基或雜芳基;其中各C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z5
取代。
在某些實施例中,R5
為氫、鹵基、-CN、-C(O)R7
或雜芳基。在一個實施例中,R5
為-CN、鹵基或-O-R7
。在某些實施例中,R5
為氫、鹵基、-CN、-C(O)R7
、-O-R7
、-S(O)2
R7
或雜芳基。在一個實施例中,R5
為鹵基。
在某些實施例中,R5
為1H-吡唑-4-基、1-羥基乙基、1-甲基-1H-吡唑-4-基、4-(乙醯胺基)苯基、6-氟吡啶-3-基、甲基乙醯基、溴、氯、氰基、環丙基、二甲胺基羰基、乙炔基、氟、碘、甲氧基、甲基、羥基、苯基、吡啶-3-基、吡啶-4-基、嘧啶-5-基、乙醯基、甲基磺醯基或三氟甲基。在一個實施例中,R5
為氯。
在一個實施例中,m為0。在另一個實施例中,m為1。
一般而言,本文中所例示之具體化合物使用ChemBioDraw Ultra來命名。然而,應理解,可使用其他名稱來鑑別相同結構之化合物。特定言之,化合物亦可使用在化學技術中公認之其他命名系統及符號命名,包括例如化學摘要服務社(Chemical Abstract Service;CAS)及國際純化學與應用化學聯合會(International Union of Pure and Applied Chemistry;IUPAC)。其他化合物或基團可用常見名稱或系統性或非系統性名稱命名。
在某些實施例中,提供本文中所描述之化合物或其醫藥學上可接受之鹽或混合物的光學異構體、外消旋體或其其他混合物。在彼等情形中,單一對映異構體或非對映異構體(亦即光學活性形式)可藉由不對稱合成或藉由解析來獲得。解析可例如藉由習知方法實現,諸如在存在解析劑情況下之結晶,或使用例如對掌性高效液相層析(HPLC)管柱之層析。
本文中所提供的包括本文中所描述之化合物或其醫藥學上可接受之鹽、異構體或混合物的組合物可包括外消旋混合物、或含有對映異構體過量之一種對映異構體的混合物、或單一非對映異構體或非對映異構體混合物。此等化合物之所有此類異構形式明確地包括在本文中,如同具體地且分別地列舉每一種異構形式一樣。
本文亦提供一種組合物,其包含本文中所描述之化合物或其醫藥學上可接受之鹽之對映異構體(或非對映異構體)的混合物。在一些實施例中,組合物包含化合物之單一對映異構體,且基本上不含另一對映異構體。在某些實施例中,式I化合物(或如本文中所描述之另一個式的化合物)含有一或多個額外立體對稱原子(例如在R1
及/或R3
處)。在此類情況下,組合物可含有非對映異構體之混合物。在一些實施例中,組合物包含化合物之單一對映異構體,且基本上不含(亦即,具有小於或約40%、30%、25%、20%、15%、10%、5%、1%、0.05%或0.01%)一或多種非對映異構體。
因此,在某些實施例中,提供一種組合物,其包含式IA或其醫藥學上可接受之鹽及式IB或其醫藥學上可接受之鹽的混合物。IAIB
其中m、R1
、R2
、R3
、R4
、R5
、R6
及R15
如本文中所定義。
在一個實施例中,混合物為外消旋混合物。在其他實施例中,組合物包含式IA或其醫藥學上可接受之鹽及式IB或其醫藥學上可接受之鹽的混合物,其中式IA存在超過式IB或其醫藥學上可接受之鹽。在某些實施例中,提供基本上不含式IB,具有小於或約40%、30%、25%、20%、15%、10%、5%、1%0.05%或0.01%之式IB化合物的組合物。
在某些實施例中,本文提供包含式I化合物立體異構體之混合物的組合物:I
其中該混合物以至少約3:1之比率包含式IA及式IB化合物:IAIB
其中m、R1
、R2
、R3
、R4
、R5
、R6
及R15
如本文中所定義。
式IA中所描繪之R4
基團的立體化學可以替代性方式表示,其限制條件為其所連接之碳原子的組態不改變。舉例而言,式1A化合物可描繪於下文所示之式IA等效表示中之任一者中。
在其他實施例中,混合物分別以至少或約3:1、至少或約4:1、至少或約5:1、至少或約6:1、至少或約7:1、至少或約8:1、至少或約9:1、至少或約10:1、至少或約11:1、至少或約12:1、至少或約20:1、至少或約30:1、至少或約40:1、至少或約80:1、至少或約160:1、或至少或約320:1之莫耳比包含式IA及式IB化合物。
在某些實施例中,亦提供本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物的螯合物、非共價複合物及其混合物。「螯合物」藉由使化合物在兩個(或超過兩個)點處配位至金屬離子來形成。「非共價複合物」藉由化合物與另一分子之相互作用形成,其中在該化合物與該分子之間不形成共價鍵。舉例而言,複合可經由凡得瓦爾力(van der Waals)相互作用、氫鍵結及靜電相互作用(亦稱作離子鍵結)發生。
在某些實施例中,提供本文中所描述之化合物的前藥。「前藥」係指當向生物學系統投與時,由於自發化學反應、酶催化化學反應、光解及/或代謝化學反應而產生原料藥或活性成分的任何化合物。因此,前藥為治療學上之活性化合物的經共價修飾之類似物或潛伏形式。前藥之非限制性實例包括酯部分、四級銨部分、二醇部分及其類似部分。
在某些實施例中,提供式I、式IA、式IB、式II、式IIA、式III、式IIIA、式IV、式IVA、式V、式VA、式VI、式VIA、式VII、式VIIA、式VIII、式VIIIA、式IX、式IXA、式X、式XA,XI、式XIA、式XII、式XIIA、式XIII、式XIIIA、式XIV或式XIVA化合物,其中R6
為、、、、、、、、、、、、、、、、、、、、、、、或;
其中各R12
獨立地為氫、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、芳基、雜芳基或雜環基;
其中任何烷基、烯基、炔基、環烷基、芳基、雜芳基或雜環基視情況經一至四個Z1b
基團取代;及
各Z1b
獨立地為側氧基、硫(酮)基、羥基、鹵基、-NO2
、-N3
、-CN、C1-9
烷基、C2-6
烯基、C2-6
炔基、C3-15
環烷基、C1-8
鹵烷基、芳基、雜芳基、雜環基、-O(C1-9
烷基)、-O(C2-6
烯基)、-O(C2-6
炔基)、-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)、-NH2
、-NH(C1-9
烷基)、-NH(C2-6
烯基)、-NH(C2-6
炔基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-N(C2-6
烯基)2
、-N(C2-6
炔基)2
、-N(C3-15
環烷基)2
、-N(C1-8
鹵烷基)2
、-N(芳基)2
、-N(雜芳基)2
、-N(雜環基)2
、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C2-6
烯基)、-N(C1-9
烷基)(C2-6
炔基)、-N(C1-9
烷基)(C3-15
環烷基)、-N(C1-9
烷基)(C1-8
鹵烷基)、-N(C1-9
烷基)(芳基)、-N(C1-9
烷基)(雜芳基)、-N(C1-9
烷基)(雜環基)、-C(O)(C1-9
烷基)、-C(O)(C2-6
烯基)、-C(O)(C2-6
炔基)、-C(O)(C3-15
環烷基)、-C(O)(C1-8
鹵烷基)、-C(O)(芳基)、-C(O)(雜芳基)、-C(O)(雜環基)、-C(O)O(C1-9
烷基)、-C(O)O(C2-6
烯基)、-C(O)O(C2-6
炔基)、-C(O)O(C3-15
環烷基)、-C(O)O(C1-8
鹵烷基)、-C(O)O(芳基)、-C(O)O(雜芳基)、-C(O)O(雜環基)、-C(O)NH2
、-C(O)NH(C1-9
烷基)、-C(O)NH(C2-6
烯基)、-C(O)NH(C2-6
炔基)、-C(O)NH(C3-15
環烷基)、-C(O)NH(C1-8
鹵烷基)、-C(O)NH(芳基)、-C(O)NH(雜芳基)、-C(O)NH(雜環基)、-C(O)N(C1-9
烷基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C2-6
烯基)2
、-C(O)N(C2-6
炔基)2
、-C(O)N(C3-15
環烷基)2
、-C(O)N(C1-8
鹵烷基)2
、-C(O)N(芳基)2
、-C(O)N(雜芳基)2
、-C(O)N(雜環基)2
、-NHC(O)(C1-9
烷基)、-NHC(O)(C2-6
烯基)、-NHC(O)(C2-6
炔基)、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
烯基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-NHC(O)NH(C2-6
烯基)、-NHC(O)NH(C2-6
炔基)、-NHC(O)NH(C3-15
環烷基)、-NHC(O)NH(C1-8
鹵烷基)、-NHC(O)NH(芳基)、-NHC(O)NH(雜芳基)、-NHC(O)NH(雜環基)、-SH、-S(C1-9
烷基)、-S(C2-6
烯基)、-S(C2-6
炔基)、-S(C3-15
環烷基)、-S(C1-8
鹵烷基)、-S(芳基)、-S(雜芳基)、-S(雜環基)、-NHS(O)(C1-9
烷基)、-N(C1-9
烷基)(S(O)(C1-9
烷基)、-S(O)N(C1-9
烷基)2
、-S(O)(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、-S(O)(C2-6
烯基)、-S(O)(C2-6
炔基)、-S(O)(C3-15
環烷基)、-S(O)(C1-8
鹵烷基)、-S(O)(芳基)、-S(O)(雜芳基)、-S(O)(雜環基)、-S(O)2
(C1-9
烷基)、-S(O)2
(C2-6
烯基)、-S(O)2
(C2-6
炔基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)或-S(O)2
N(C1-9
烷基)2
;
其中任何烷基、環烷基、芳基、雜芳基或雜環基視情況經一至四個以下基團取代:鹵基、C1-9
烷基、C1-8
鹵烷基、-OH、-NH2
、-NH(C1-9
烷基)、-NH(C3-15
環烷基)、-NH(C1-8
鹵烷基)、-NH(芳基)、-NH(雜芳基)、-NH(雜環基)、-N(C1-9
烷基)2
、-N(C3-15
環烷基)2
、-NHC(O)(C3-15
環烷基)、-NHC(O)(C1-8
鹵烷基)、-NHC(O)(芳基)、-NHC(O)(雜芳基)、-NHC(O)(雜環基)、-NHC(O)O(C1-9
烷基)、-NHC(O)O(C2-6
炔基)、-NHC(O)O(C3-15
環烷基)、-NHC(O)O(C1-8
鹵烷基)、-NHC(O)O(芳基)、-NHC(O)O(雜芳基)、-NHC(O)O(雜環基)、-NHC(O)NH(C1-9
烷基)、-S(O)(NH)(C1-9
烷基)、S(O)2
(C1-9
烷基)、-S(O)2
(C3-15
環烷基)、-S(O)2
(C1-8
鹵烷基)、-S(O)2
(芳基)、-S(O)2
(雜芳基)、-S(O)2
(雜環基)、-S(O)2
NH(C1-9
烷基)、-S(O)2
N(C1-9
烷基)2 、
-O(C3-15
環烷基)、-O(C1-8
鹵烷基)、-O(芳基)、-O(雜芳基)、-O(雜環基)或-O(C1-9
烷基)。
在某些實施例中,R6
為,且各R12
獨立地如本文中所定義。
在某些實施例中,R6
為。
R6
亦包括所有個別立體異構體及其混合物,包括(但不限於)在磷原子處之對掌性,諸如在上文所示之例示性部分中。
本文亦提供本文中所描述之化合物的活體內代謝產物。此類產物可例如由所投與化合物之氧化、還原、水解、醯胺化、酯化及其類似作用產生,主要由於酶促過程產生。 化合物之治療性用途
「治療(treatment)或(treating)」為用於獲得有益或所要結果(包括臨床結果)之途徑。有益或所要臨床結果可包括以下各者中之一或多者:a)抑制疾病或病狀(例如,減少由該疾病或病狀產生之一或多種症狀及/或減輕該疾病或病狀之程度);b)減緩或停止與該疾病或病狀相關之一或多種臨床症狀的發展(例如,使該疾病或病狀穩定、預防或延遲該疾病或病狀之惡化或進展及/或預防或延遲該疾病或病狀之擴散(例如轉移));及/或c)減輕該疾病,亦即使臨床症狀消退(例如,改善疾病病況、提供該疾病或病狀之部分或總體緩解、增強另一種藥物療法之作用、延遲該疾病之進展、提高生命品質及/或延長存活期)。
「預防(prevention或preventing)」意謂疾病或病狀之促使該疾病或病狀之臨床症狀不發展的任何治療。在一些實施例中,化合物可向具有該疾病或病狀之風險或具有該疾病或病狀之家族史的個體(包括人類)投與。
「個體」係指已成為或將成為治療、觀察或實驗之對象的動物,諸如哺乳動物(包括人類)。本文中所描述之方法可適用於人類療法及/或獸醫學應用。在一些實施例中,個體為哺乳動物。在一個實施例中,個體為人類。
本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物的術語「治療有效量」或「有效量」意謂當向個體投與時足以實現治療以提供治療效益(諸如改善症狀或減緩疾病進展)的量。舉例而言,治療有效量可為足以響應於Cot活性之抑制而減少疾病或病狀之症狀的量。治療有效量可視待治療之個體及疾病或病狀、個體之體重及年齡、疾病或病狀之嚴重程度及投藥方式而變化,其可容易地由一般技術者確定。
術語「抑制」指示生物活動或過程之基線活動減少。「抑制Cot活性」或其變化形式係指Cot之活性直接或間接響應於本申請案之化合物的存在,相對於在無本申請案之化合物存在下之Cot活性而減少。「抑制Cot」係指Cot活性直接或間接響應於本文中所描述之化合物的存在,相對於在無本文中所描述之化合物存在下之Cot活性而減少。在一些實施例中,可在治療之前的同一個體或未接受該治療之其他個體中比較Cot活性之抑制。
本文中所描述之方法可應用於活體內或離體細胞群體。「活體內」意謂在活的個體內,如在動物或人類內。在此上下文中,可在個體中在治療學上使用本文中所描述之方法。「離體」意謂在活的個體外部。離體細胞群體之實例包括活體外細胞培養物及生物樣品,包括自個體獲得之體液或組織樣品。此類樣品可藉由此項技術中熟知之方法獲得。例示性生物流體樣品包括血液、腦脊髓液、尿液及唾液。例示性組織樣品包括腫瘤及其生檢。在此上下文中,本文中所描述之化合物及組合物可用於多種目的,包括治療性及實驗目的。舉例而言,可離體使用本文中所描述之化合物及組合物來確定對所給出之適應症、細胞類型、個體及其他參數而投與Cot抑制劑的最佳時程及/或劑量。自此類用法搜集之資訊可用於實驗目的或臨床中以設定活體內治療方案。本文中所描述之化合物及組合物可能適合之其他離體用途描述於下文中或將對熟習此項技術者變得顯而易見。所選化合物可進一步經表徵以檢查在人類或非人類個體中之安全性或耐受劑量。此類特性可使用熟習此項技術者通常已知之方法檢查。
本文中所揭示之化合物適用於治療由Cot介導之疾病或病狀。由Cot介導之疾病或病狀的非限制性實例包括(但不限於)癌症、糖尿病及發炎性疾病,諸如類風濕性關節炎(RA)、多發性硬化症(MS)、發炎性腸病(IBD)、敗血症、牛皮癬、調控異常之TNF表現及移植排斥。
在其他實施例中,提供用於減輕由Cot介導之疾病或病症之症狀的方法。在一些實施例中,該方法包括鑑別具有由Cot介導之疾病或病症之症狀的哺乳動物,及向該哺乳動物提供有效改善症狀(亦即,減輕該症狀之嚴重性)之量的如本文中所描述之化合物。
在一些實施例中,由Cot介導之疾病或病狀為實體腫瘤。在特定實施例中,實體腫瘤來自胰臟癌、膀胱癌、結腸直腸癌、乳癌、前列腺癌、腎癌、肝細胞癌、肺癌、卵巢癌、子宮頸癌、胃癌、食道癌、頭頸癌、黑素瘤、神經內分泌癌、CNS癌症、腦瘤(例如神經膠質瘤、退行性少突神經膠質瘤、成人多形性膠質母細胞瘤及成人退行性星形細胞瘤)、骨癌或軟組織肉瘤。在一些實施例中,實體腫瘤來自非小細胞肺癌、小細胞肺癌、結腸癌、CNS癌症、黑素瘤、卵巢癌、腎癌、前列腺癌或乳癌。
在一些實施例中,由Cot介導之疾病或病狀為糖尿病,其包括由胰島素產生及葡萄糖耐受性異常表徵之任何代謝病症。在一些實施例中,糖尿病包括1型及2型糖尿病、妊娠期糖尿病、前驅糖尿病、胰島素抗性、代謝症候群、空腹血糖異常及葡萄糖耐量異常。1型糖尿病亦稱為胰島素依賴性糖尿病(IDDM)。2型亦稱為非胰島素依賴型糖尿病(NIDDM)。
在一些實施例中,由Cot介導之疾病或病狀為發炎性疾病或LPS誘導之內毒素休克。在一些實施例中,該疾病為自體免疫疾病。在特定實施例中,自體免疫疾病為全身性紅斑狼瘡(SLE)、重症肌無力、類風濕性關節炎(RA)、急性瀰漫性腦脊髓炎、特發性血小板減少性紫癜、多發性硬化症(MS)、發炎性腸病(IBD)、敗血症、牛皮癬、休格連氏症候群、自體免疫溶血性貧血、哮喘或慢性阻塞性肺病(COPD)、僵直性脊椎炎、急性痛風及僵直性脊椎炎、反應性關節炎、單關節性關節炎、骨關節炎、痛風性關節炎、幼年期關節炎、幼年期發作型類風濕性關節炎、幼年期類風濕性關節炎或牛皮癬性關節炎。在其他實施例中,該疾病為炎症。在又其他實施例中,該疾病為過度或破壞性免疫反應,諸如哮喘、類風濕性關節炎、多發性硬化症、慢性阻塞性肺病(COPD)及狼瘡。
在一些實施例中,由Cot介導之疾病或病狀為發炎性腸病(IBD)。如本文中所用之術語「發炎性腸病」或「IBD」為描述胃腸道之發炎性病症的集合術語,其最常見形式為潰瘍性結腸炎及克羅恩氏病(Crohn's disease)。可用本發明之化合物、組合物及方法治療的IBD之其他形式包括改道性結腸炎、缺血性結腸炎、感染性結腸炎、化學性結腸炎、顯微性結腸炎(包括膠原性結腸炎及淋巴細胞性結腸炎)、非典型結腸炎、偽膜性結腸炎、突發性結腸炎、自閉性小腸結腸炎、不確定性結腸炎、貝賽特氏病(Behçet's disease)、胃與十二指腸CD、空腸迴腸炎、迴腸炎、迴腸結腸炎、克羅恩氏(肉芽腫性)結腸炎、腸激躁症候群、黏膜炎、放射誘導腸炎、短腸症候群、乳糜瀉、胃潰瘍、憩室炎、貯存袋炎、直腸炎及慢性腹瀉。
治療或預防IBD亦包括減輕或減少IBD之一或多種症狀。如本文中所用,術語「IBD之症狀」係指所偵測之症狀,諸如腹痛、腹瀉、直腸出血、體重減輕、發熱、食慾不振,及其他更嚴重併發症,諸如脫水、貧血及營養不良。對多種此類症狀進行定量分析(例如體重減輕、發熱、貧血等)。一些症狀容易由血液測試(例如貧血)或偵測血液存在之測試(例如直腸出血)來確定。術語「其中該症狀減少」係指可偵測症狀之定性或定量減少,包括(但不限於)對疾病恢復速率(例如體重增加速率)之可偵測影響。通常藉助於黏膜之內窺鏡觀察及內窺鏡生檢樣本之病理性檢查來確定診斷。
IBD之病程變化,且常常與疾病緩解及疾病惡化之間歇性時間段相關聯。已描述表徵IBD之疾病活性及嚴重性以及患有IBD之個體對治療之反應的各種方法。根據本發明方法之治療一般可適用於患有具任何疾病活性水準或程度之IBD的個體。
在一些實施例中,藉由投與本文中所描述之組合物之化合物來治療的疾病或病狀包括急性痛風及僵直性脊椎炎、過敏性病症、阿茲海默氏病(Alzheimer's disease)、肌肉萎縮性側索硬化(ALS)、肌肉萎縮性側索硬化及多發性硬化症、動脈粥樣硬化、細菌感染、骨癌疼痛及歸因於子宮內膜異位之疼痛、耐BRAF性黑色素瘤、腦幹神經膠質瘤或垂體腺瘤、灼傷、滑囊炎、肛門區癌、內分泌系統癌、腎臟或尿管癌(例如腎細胞癌、腎盂癌)、陰莖癌、小腸癌、甲狀腺癌、尿道癌、血癌(諸如急性骨髓性白血病)、舌癌、子宮頸癌、子宮內膜癌、輸卵管癌、腎盂癌、陰道癌或外陰癌、慢性骨髓性白血病、慢性或急性白血病、慢性疼痛、典型巴特症候群(classic Bartter syndrome)、感冒、結膜炎、冠心病、皮膚或眼內黑色素瘤、皮炎、痛經、濕疹、子宮內膜異位、家族性腺瘤性息肉病、肌肉纖維疼痛、真菌感染、痛風、婦科腫瘤、子宮肉瘤、輸卵管癌、頭痛、嗜血性關節病、帕金森氏病(Parkinson's disease)、AIDS、帶狀疱疹、霍奇金氏病(Hodgkin's disease)、亨廷頓氏症(Huntington's)、前列腺素E過多症候群、流感、虹膜炎、幼年期關節炎、幼年期發作型類風濕性關節炎、幼年期類風濕性關節炎、下背及頸部疼痛、淋巴球淋巴瘤、肌筋膜病症、肌炎、神經痛、神經退化性病症(諸如阿茲海默氏病)、神經發炎性病症、神經痛、外陰癌、帕金森氏病、兒童惡性疾病、肺纖維化、直腸癌、鼻炎、類肉瘤病、軟組織肉瘤、鞏膜炎、皮膚癌、兒童實體腫瘤、脊椎軸腫瘤、扭傷及拉傷、胃癌、中風、亞急性及慢性肌肉骨骼痛症候群(諸如滑囊炎)、手術或牙科手術、與流感或其他病毒感染相關聯之症狀、關節膜炎、牙痛、潰瘍、子宮癌、子宮肉瘤、葡萄膜炎、脈管炎、病毒感染、病毒感染(例如流感)及傷口癒合。
適用於評定患有潰瘍性結腸炎之個體中疾病活性的準則可見於例如Truelove等人 (1955) Br Med J 2:1041-1048。使用此等準則,可將患有IBD之個體中的疾病活性表徵為輕度疾病活性或嚴重疾病活性。不滿足嚴重疾病活性之所有準則且超過輕度疾病活性準則的個體分類為具有中度疾病活性。
本發明所揭示之治療方法亦可應用於疾病過程中任何時間點處。在某些實施例中,該方法在緩解(亦即無活性疾病)時間段期間應用於患有IBD之個體。在此類實施例中,本發明方法藉由延長緩解時間段(例如延長無活性疾病之時段)或藉由預防、減少或延遲活動性疾病發作來提供益處。在其他實施例中,方法可在活動性疾病時段期間應用於患有IBD之個體。此類方法藉由減少活動性疾病時段之持續時間、減少或減輕IBD之一或多種症狀或治療IBD來提供益處。
已描述用於在臨床實踐中測定治療IBD之功效的量測,且其包括例如以下:症狀控制;瘺閉合術;所需皮質類固醇療法程度;及生活品質改良。健康相關生活品質(HRQL)可使用發炎性腸病調查表(IBDQ)來評定,該調查表在臨床實踐中廣泛用於評定患有IBD之個體的生活品質。(參見Guyatt等人 (1989) Gastroenterology 96:804-810。) 在一些實施例中,疾病或病狀為免疫介導性肝損傷、疾病或病狀。Tpl2可介導免疫相關之肝病或病狀。(Vyrla等人, The Journal of Immunology, 2016, 196;Perugorria等人, Hepatology, 2013;57:1238-1249)
在一些實施例中,由Cot介導之疾病或病狀為酒精性肝炎。酒精性肝炎為由在長期及主動酒精濫用之個體中出現之黃疸及肝臟衰竭表徵的臨床症候群。(參見Akriviadis E.等人, Ann Gastroenterol. 2016年4月-6月; 29(2): 236-237)。酒精性肝炎可引起肝臟細胞之硬化及纖維化。糖皮質激素(例如潑尼龍(prednisolone))及磷酸二酯酶抑制劑(例如配妥西菲林(pentoxifylline))可用於治療酒精性肝炎。本文中之化合物可以單獨治療形式或與目前用於酒精性肝炎之治療組合使用。
在一些實施例中,由Cot介導之疾病或病狀為全身性紅斑狼瘡(SLE)、狼瘡性腎炎、狼瘡相關或其他自體免疫病症或SLE症狀。全身性紅斑狼瘡之症狀包括關節疼痛、關節腫脹、關節炎、疲乏、脫髮、口瘡、淋巴結腫脹、日光敏感、皮疹、頭痛、麻木、發麻、癲癇、視力問題、人格變化、腹痛、噁心、嘔吐、心臟節律異常、咳血及呼吸困難、皮膚顏色有斑及雷諾氏現象(Raynaud's phenomenon)。
前述反應準則中任一者之改良具體由本發明之方法提供。 組合療法
在一個實施例中,本文中所揭示之化合物可與一或多種正用於及/或研發用於治療發炎性病症(例如IBD)的額外治療劑組合使用。該一或多種額外治療劑可為α4β7抑制劑、類固醇、MMP-9抗體、S1P1促效劑、TNF生物製劑或其任何組合。
在一些實施例中,一或多種額外治療劑可為α4β7整合素抑制劑,或抑制α4β7整合素之表現及/或活性的藥劑。抑制劑可為小分子或生物製劑。舉例而言,α4β7整合素抑制劑可為那他珠單抗(natalizumab)或維多珠單抗(vedolizumab)。
在一些實施例中,一或多種額外治療劑可為類固醇,包括(但不限於)皮質類固醇。皮質類固醇可藉由各種途徑投與,包括靜脈內(亦即,甲潑尼龍(methylprednisolone)、氫皮質酮),經口(亦即,潑尼松(prednisone)、潑尼龍(prednisolone)、布地奈德(budesonide)、地塞米松(dexamethasone))或局部(亦即,灌腸、栓劑或泡沫製劑)。
在一些實施例中,一或多種額外治療劑可為MMP9抑制劑,或抑制MMP9之表現及/或活性的藥劑。MMP9之代表性蛋白質序列為GenBank寄存編號NP_004985。抑制劑可為小分子或生物製劑。舉例而言,Gu等人,The Journal of Neuroscience,
25(27): 6401-6408 (2005)揭示特異性MMP9抑制劑,SB-3CT (CAS 292605-14-2)。此外,亦已展現siRNA、反義RNA及抗體抑制MMP9之表現或活性,且其處於本發明之範疇內。在一個實施例中,MMP9抑制劑為單株抗MMP9抗體。在一些實施例中,一或多種額外治療劑包括MMP9抑制劑及核苷類似物,諸如吉西他濱(gemcitabine)。
在一些實施例中,一或多種額外治療劑可為神經鞘胺醇1-磷酸酯受體(S1P1)抑制劑,或抑制S1P1之表現及/或活性的藥劑。抑制劑可為小分子或生物製劑。舉例而言,S1P1抑制劑可為RPC1063。
在一些實施例中,一或多種額外治療劑可為TNF抑制劑,或抑制TNF之表現及/或活性的藥劑。抑制劑可為小分子或生物製劑。舉例而言,TNF抑制劑可為戈利木單抗(golimumab)。
在一些實施例中,一或多種額外治療劑正用於及/或研發用於治療潰瘍性結腸炎(UC)及/或克羅恩氏病(CD)。藥劑可為生物製劑或小分子。在一些實施例中,藥劑為S1P1、IL-6、CX3CL1、DHODH、α4、β7、JAK、TNF、CB、IL-12/IL-23、CCL20、TLR9、MAdCAM、CCR9、CXCL10、Smad7、PDE4、MC、VLA-1、GC、GATA-3、嗜酸性粒細胞趨化因子(Eotaxin)、FFA2、LIGHT、FMS、MMP9、CD40、類固醇、5-ASA、Immunomod、STAT3及/或EP4之調節劑(例如促效劑或拮抗劑)。
正用於及/或研發用於治療潰瘍性結腸炎(UC)之藥劑的非限制性實例包括GSK3050002 (CCL20調節劑,由GSK生產)、GS-5745 (MMP9調節劑,由Gilead生產)、AVX-470 (TNF調節劑,由Avaxia生產)、柏替木單抗(Bertilimumab) (嗜酸性粒細胞趨化因子調節劑,由Immune Pharma生產)、Simponi (TNF調節劑,由Johnson & Johnson and Merck生產)、RX-10001 (由Resolvyx生產)、IBD-98 (5-ASA調節劑,由Holy Stone生產)、SP-333 (GC調節劑,由Synergy生產)、KAG-308 (EP4調節劑,由Kaken生產)、SB012 (GATA-3調節劑,由Sterna生產)、AJM300 (α4調節劑,由Ajinomoto生產)、BL-7040 (TLR9調節劑,由BiolineRx生產)、TAK-114 (SAT3調節劑,由Takeda生產)、CyCol (由Sigmoid生產)、GWP-42003 (CB調節劑,由GW Pharma生產)、ASP3291 (MC調節劑,由Drais生產)、GLPG0974 (FFA2調節劑,由Galapagos生產)、奧紮尼莫(Ozanimod) (S1P1調節劑,由Receptos生產)、ASP015K (JAK調節劑,由Astellas生產)、阿普司特(Apremilast) (PDE4調節劑,由Celgene生產)、Zoenasa (由Altheus生產)、Kappaproct (TLR9調節劑,由InDex生產)、磷脂醯膽鹼(由Dr Falk/Lipid Tx生產)、托法替尼(Tofacitinib) (JAk調節劑,由Pfizer生產)、Cortment (類固醇調節劑,由Ferring生產)、Uceris (Steroid調節劑,由Salix生產)及5-ASA調節劑,諸如Delzicol (由Actavis生產)、Canasa (由Aptalis生產)、Asacol (由Actavis生產)、Pentasa (由Shire/Ferring生產)、Lialda (由Shire生產)、Mezavant (由Shire生產)、Apriso (由Salix生產)、Colazal (由Salix生產)、Giazo (由Salix生產)及Salofalk (由Dr Falk生產)。正用於及/或研發用於治療克羅恩氏病(CD)之藥劑的非限制性實例包括FFP102 (CD40調節劑,由Fast Forward生產)、E6011 (CX3CL1調節劑,由Eisai生產)、PF-06480605 (由Pfizer生產)、QBECO SSI (Immunomod調節劑,由Qu Biologics生產)、PDA-001 (由Celgene生產)、BI 655066 (IL-12/IL-23調節劑,由Boehringer生產)、TNFα人體細胞因子(TNFα kinoid) (TNF調節劑,由Neovacs生產)、AMG 139/MEDI-2070 (IL-12/IL-23調節劑,由AstraZeneca生產)、PF-04236921 (IL-6調節劑,由Pfizer生產)、Tysabri (β7調節劑,由Biogen Idec在美國銷售)、Cimzia (由UCB在美國銷售)、JNJ-40346527 (FMS調節劑,由J&J生產)、SGX-203 (類固醇調節劑,由Solgenix生產)、CyCron (由Sigmoid生產)、CCX507 (CCR9調節劑,由ChemoCentryx生產)、MT1303 (S1P1調節劑,由Mitsubishi生產)、6-MP (由Teva生產)、ABT-494 (JAk調節劑,由Abbvie生產)、Tofacitinib (JAk調節劑,由Pfizer生產)、GLPG0634 (JAk調節劑,由Galapagos生產)、TRK-170 (β7調節劑,由Toray生產)、Mongersen (Smad7調節劑,由Celgene生產)、RHB-104 (由Redhill生產)、Rifaxmin EIR (由Salix生產)、Budenofalk (由Dr Falk生產)及Entocort (由AstraZeneca生產)。
正用於及/或研發用於治療潰瘍性結腸炎(UC)及克羅恩氏病(CD)之藥劑的非限制性實例包括PF-06410293 (由Pfizer生產)、SAN-300 (VLA-1調節劑,由Salix生產)、SAR252067 (LIGHT調節劑,由Sanofi生產)、PF-00547659 (MAdCAM調節劑,由Pfizer生產)、艾德魯單抗(Eldelumab) (Smad7調節劑,由BMS生產)、AMG 181/ MEDI-7183 (β7調節劑,由Amgen/AstraZeneca生產)、艾托珠單抗(Etrolizumab) (β7調節劑,由Roche生產)、優特克單抗(Ustekinumab) (IL-12/IL-23調節劑,由J&J生產)、雷米卡德(Remicade) (TNF調節劑,由J&J and Merck生產)、Entyvio (β7調節劑,由Takeda生產)、Humira (TNF調節劑,由Abbvie生產)、英利昔單抗(Infliximab) (由Celtrion生產)、PF-06651600 (由Pfizer生產)、GSK2982772 (由GSK生產)、GLPG1205 (FFA2調節劑,由Galapagos生產)、AG014 (由Intrexon生產)及Vidofludimus (DHODH調節劑,由4SC生產)
在一些實施例中,一或多種額外治療劑可為JAK抑制劑,尤其JAK-1選擇性抑制劑。抑制劑可為小分子或生物製劑。舉例而言,JAK抑制劑可為菲戈替尼(Filgotinib)、GLPG0634 (JAK調節劑,由Galápagos生產)。 套組
本文亦提供套組,其包括式I化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物,及適合之包裝。在一個實施例中,套組進一步包括使用說明書。在一個態樣中,套組包括式I化合物(或本文中所描述之任何其他式的化合物)或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物,及在治療本文中所描述之適應症(包括疾病或病狀)中使用該等化合物的標籤及/或使用說明書。
本文亦提供製品,其包括處於適合之容器中的本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物。該容器可為小瓶、廣口瓶、安瓿、預裝載注射器及靜脈內袋。 醫藥組合物及投藥模式
本文中所提供之化合物化合物通常以醫藥組合物形式投與。因此,本文亦提供醫藥組合物,其含有本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物中之一或多者及一或多種選自載劑、佐劑及賦形劑的醫藥學上可接受之媒劑。適合之醫藥學上可接受之媒劑可包括例如惰性固體稀釋劑及填充劑、稀釋劑(包括無菌水溶液及各種有機溶劑)、穿透增強劑、增溶劑及佐劑。此類組合物以醫藥技術中熟知之方式製備。參見例如Remington's Pharmaceutical Sciences, Mace Publishing Co., Philadelphia, Pa. 第17版 (1985);及Modern Pharmaceutics, Marcel Dekker, Inc. 第3版 (G.S. Banker及C.T. Rhodes編)。
醫藥組合物可以單一劑量或多劑量形式投與。醫藥組合物可藉由各種方法投與,包括例如經直腸、經頰、鼻內及經皮途徑。在某些實施例中,醫藥組合物可藉由動脈內注射、靜脈內、腹膜內、非經腸、肌肉內、皮下、經口、局部或以吸入劑形式投與。
一種投藥模式為非經腸,例如藉由注射。本文中所描述之醫藥組合物可併入以用於藉由注射投與的形式包括例如水性或油性懸浮液,或與芝麻油、玉米油、棉籽油或花生油之乳液,以及酏劑、甘露糖醇、右旋糖或無菌水溶液及類似醫藥媒劑。
經口投藥可為用於投與本文中所描述之化合物化合物的另一個途徑。舉例而言,可經由膠囊或包覆腸溶包衣錠劑投與。在製造包括至少一種本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物的醫藥組合物中,活性成分通常由賦形劑稀釋及/或密封於可呈膠囊、藥囊、紙張或其他容器形式之此類載劑內。當賦形劑用作稀釋劑時,其可呈固體、半固體或液體材料形式,其充當活性成分之媒劑、載劑或介質。因此,組合物可呈以下形式:錠劑、丸劑、散劑、口含錠、藥囊、扁囊劑、酏劑、懸浮液、乳液、溶液、糖漿、氣霧劑(呈固體形式或於液體介質中)、含有例如高達10重量%之活性化合物之軟膏、軟及硬明膠膠囊、無菌可注射溶液及無菌封裝粉末。
適合賦形劑之一些實例包括乳糖、右旋糖、蔗糖、山梨糖醇、甘露糖醇、澱粉、阿拉伯膠、磷酸鈣、海藻酸鹽、黃蓍、明膠、矽酸鈣、微晶纖維素、聚乙烯吡咯啶酮、纖維素、無菌水、糖漿及甲基纖維素。調配物可另外包括:潤滑劑,諸如滑石、硬脂酸鎂及礦物油;濕潤劑;乳化劑及懸浮劑;防腐劑,諸如羥基苯甲酸甲酯及羥基苯甲酸丙酯;甜味劑;及調味劑。
可藉由採用此項技術中已知之程序來調配包括至少一種本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物的醫藥組合物以便在向個體投與之後提供活性成分之快速、持續或延遲釋放。用於經口投藥之控制釋放藥物遞送系統包括含有經聚合物塗佈之儲集囊或藥物-聚合物基質調配物的滲透泵系統及溶解系統。控制釋放系統之實例在美國專利第3,845,770號、第4,326,525號、第4,902,514號及第5,616,345號中給出。另一種用於本文中所揭示之方法中的調配物採用經皮遞送裝置(「貼片」)。此類經皮貼片可用於提供本文中所描述之化合物以控制量連續或非連續輸注。用於遞送藥劑之經皮貼片的構造及用法為此項技術中所熟知。參見例如美國專利第5,023,252號、第4,992,445號及第5,001,139號。此類貼片可經構造以連續、脈衝式或按需求遞送醫藥劑。
對於製備固體組合物(諸如錠劑),可將主活性成分與醫藥賦形劑混合以形成固體預調配組合物,該組合物含有本文中所描述之化合物或其醫藥學上可接受之鹽、互變異構體、立體異構體、立體異構體混合物、前藥或氘化類似物的均勻混合物。當提及此等預調配組合物為均勻組合物時,活性成分可均勻分散在整個組合物中,以使得該組合物可容易地再分成同等有效之單位劑型,諸如錠劑、丸劑及膠囊。
本文中所描述之化合物的錠劑或丸劑可經包衣包覆或以其他方式混配以得到提供長作用時間或保護免受胃之酸性條件影響之優勢的劑型。舉例而言,錠劑或丸劑可包括內部劑量及外部劑量組分,後者呈前者上之包膜形式。兩種組分可由腸溶層隔開,該腸溶層用以防止在胃中崩解且允許內部組分完整進入十二指腸或釋放延遲。各種材料可用於此類腸溶層或腸溶包衣,此類材料包括多種聚合酸及聚合酸與諸如蟲膠、鯨蠟醇及乙酸纖維素之材料的混合物。
用於吸入或吹入之組合物可包括於醫藥學上可接受之水性或有機溶劑或其混合物中之溶液及懸浮液以及粉末。液體或固體組合物可含有如本文中所描述之適合的醫藥學上可接受之賦形劑。在一些實施例中,為局部或全身性作用,藉由經口或經鼻呼吸道途徑投與組合物。在其他實施例中,於醫藥學上可接受之溶劑中之組合物可藉由使用惰性氣體進行霧化。霧化溶液可直接自霧化裝置吸入或霧化裝置可連接至面罩托或間歇性正壓呼吸機。可以恰當方式自遞送該調配物之裝置較佳地經口或經鼻投與溶液、懸浮或粉末組合物。 給藥
本申請案之化合物用於任何特定個體的具體劑量水準將視多種因素而定,包括所採用具體化合物之活性、年齡、體重、一般健康狀況、性別、膳食、投藥時間、投藥途徑及排泄速率、藥物組合及進行治療之個體中特定疾病之嚴重性。舉例而言,劑量可表示為每公斤個體體重的本文中所描述之化合物之毫克數(mg/kg)。介於約0.1與150 mg/kg之間的劑量可為恰當的。在一些實施例中,介於約0.1與100 mg/kg之間可為恰當的。在其他實施例中,介於0.5與60 mg/kg之間的劑量可為恰當的。當在大小廣泛不同之個體之間調整劑量時,諸如當在兒童及成人兩者中使用藥物時或當將諸如犬之非人類個體中之有效劑量轉換成適合於人類個體之劑量時所發生的,根據個體之體重標準化為特別適用的。
日劑量亦可描述為每劑量或每天投與的本文中所描述之化合物之總量。式I化合物之日劑量可在約1 mg與4,000 mg之間,在每天約2,000至4,000 mg之間,在每天約1至2,000 mg之間,在每天約1至1,000 mg之間,在每天約10至500 mg之間,在每天約20至500 mg之間,在每天約50至300 mg之間,在每天約75至200 mg之間或在每天約15至150 mg之間。
當經口投與時,人類個體之每日總劑量可在1 mg與1,000 mg之間,在每天約1,000-2,000 mg之間,在每天約10-500 mg之間,在每天約50-300 mg之間,在每天約75-200 mg之間或在每天約100-150 mg之間。
本申請案之化合物或其組合物可使用上文所描述的任何適合之模式每天投與一次、兩次、三次或四次。另外,用化合物投與或治療可持續多天;舉例而言,對一個治療週期,共同治療將持續至少7天、14天或28天。治療週期在癌症化學療法中為熟知的,且常常與介於週期之間的約1至28天、通常約7天或約14天之休息期交替。在其他實施例中,治療週期亦可為連續的。
在一個特定實施例中,該方法包含向個體投與約1至800 mg本文中所描述之化合物的初始日劑量,及遞增地增加劑量直至達成臨床功效為止。可使用約5、10、25、50或100 mg之增量來增加劑量。劑量可每天、每隔一天、每週兩次或每週一次地增加。 式 I 化合物之合成
化合物可使用本文中所揭示之方法及鑒於本文中之揭示內容及此項技術中熟知之方法將顯而易見的其常規修改來製備。除本文中之教示內容之外,亦可使用習知且熟知之合成方法。本文中所描述之典型化合物的合成可如以下實例中所描述來實現。若可獲得,則試劑可在商業上購買,例如購自Sigma Aldrich或其他化學供應商。通用合成
本文中所描述之化合物的典型實施例可使用下文所描述之一般反應流程來合成。鑒於本文中之描述將顯而易見的,可藉由用具有類似結構之其他物質取代起始物質來改變通用流程,從而產生對應不同的產物。隨後為合成描述,用於提供可如何改變起始物質來提供對應產物之數個實例。鑒於已限定取代基之所要產物,必需起始物質一般可藉由檢查來確定。起始物質通常自商業來源獲得或使用公開方法合成。為合成作為本發明中所描述之實施例的化合物,待合成之化合物的結構之檢查將提供各取代基之身分。鑒於本文中之實例,最終產物之鑑別一般將使得必需起始物質之身分藉由簡單檢查方法而變得顯而易見。一般而言,本文中所描述之化合物在室溫及室壓下通常為穩定且可分離的。合成反應參數
本發明之化合物可使用例如以下通用方法及程序來由可容易獲得之起始物質製備。應瞭解,當給定典型或較佳製程條件(亦即反應溫度、時間、反應物之莫耳比率、溶劑、壓力等)時,除非另外陳述,否則亦可使用其他製程條件。最佳反應條件可隨所用特定反應物或溶劑而變化,但此類條件可由熟習此項技術者藉由常規最佳化程序來確定。
另外,如熟習此項技術者將顯而易見的,可能必需習知保護基來防止某些官能基經歷非所要反應。適用於各種官能基之保護基以及適用於保護特定官能基及使特定官能基脫保護之條件在此項技術中已為熟知的。舉例而言,諸多保護基描述於T. W. Greene及G. M. Wuts (1999) Protecting Groups in Organic Synthesis, 第3版, Wiley, New York及其所引用之參考文獻中。
此外,本發明之化合物可含有一或多個對掌性中心。因此,必要時,此類化合物可製備或分離為純立體異構體,亦即製備或分離為個別對映異構體或非對映異構體,或製備或分離為立體異構體增濃混合物。除非另外指示,否則所有此類立體異構體(及增濃混合物)均包括於本發明之範疇內。純立體異構體(或增濃混合物)可使用例如此項技術中熟知之光學活性起始物質或立體選擇性試劑來製備。或者,此類化合物之外消旋混合物可使用例如對掌性管柱層析、對掌性解析劑及其類似物來分離。
以下反應之起始物質為一般已知之化合物或可藉由已知程序或其明顯修改來製備。舉例而言,許多起始物質可購自商業供應商,諸如Aldrich Chemical Co. (Milwaukee, Wisconsin, USA)、Bachem (Torrance, California, USA)、Emka-Chemce或Sigma (St. Louis, Missouri, USA)。其他起始物質可藉由描述於標準參考文本中之程序或其明顯修改來製備,該等標準參考文本諸如Fieser and Fieser's Reagents for Organic Synthesis, 第1-15卷(John Wiley及Sons, 1991)、Rodd's Chemistry of Carbon Compounds, 第1-5卷及增刊(埃塞維爾科學 出版社(Elsevier Science Publishers), 1989) organic Reactions, 第1-40卷(John Wiley及Sons, 1991)、March's Advanced Organic Chemistry, (John Wiley及Sons, 第5版, 2001),及Larock's Comprehensive Organic Transformations (VCH Publishers Inc., 1989)。
術語「溶劑」一般係指在與其結合描述之反應條件下惰性的溶劑(包括例如苯、甲苯、乙腈、四氫呋喃(THF)、二甲基甲醯胺(DMF)、氯仿、二氯甲烷(methylene chloride/dichloromethane)、乙醚、甲醇及其類似物)。除非相反地說明,否則溶劑為惰性有機溶劑,且反應可在惰性氣體(較佳地,氬氣或氮氣)下進行。
術語「足量(q.s.)」意謂添加足以達成所陳述之功能(例如使溶液達到所要體積(亦即100%))之量。
式I化合物可藉由以下來製備:首先提供經取代之喹啉核心,及視情況視需要進一步修飾該核心以提供本文中所揭示之取代基。流程 1
展示製備喹啉核心以提供式1 -e
化合物,其中m、R5
及R15
如本文中所定義,或為可使用標準反應條件向其轉化之官能基。流程 1
在流程 1
中,使經適合取代之1-a
及1-b
於適合之溶劑(例如DMF等)中,在催化劑(例如Cs2
CO3
等)存在下在高溫(例如約40℃-50℃)下縮合以提供1-c
。隨後在熱環化條件(亦即約250℃)下或在微波條件下將化合物1 -c
轉化成1-d
。在高溫(例如約110℃-120℃)下,在鹼(例如吡啶、二甲基苯胺、二乙基苯胺等)或催化劑(例如DMF、DEF等)存在下且在適合之溶劑(例如氯苯、CH3
CN等)中或無溶劑條件(亦即純淨的)下使用適合之氯化劑(例如POCl3
、SOCl2
等)達成1-d
之氯化以提供1-e
。流程 2
展示式化合物2-c
及2-d
之合成,其中m、R1
、R2
、R5
及R15
如本文中所定義。流程 2
在流程 2
中,使1-e
與適合之胺在標準親核芳族取代條件下,在鹼(例如NEt3
等)存在下且在高溫(例如150℃)下反應以獲得2-a
。藉由使2-a
與適合之氰化劑(例如CuCN、Zn(CN)2
等)在催化劑(例如鈀、鎳、銅等)存在下反應來提供其中R5
及/或R15
為氰基之式I化合物。隨後經由分別使化合物2-a
或2-b
之硝基還原(使用例如Fe、SnCl2
等)來提供化合物2-c
及2-d
。流程 3
展示化合物3-d
及3-e
之合成,其中R4
如本文中所定義。流程 3
在流程3中,藉由以下來提供氘化3-c
:用含氘化物之還原劑(例如NaBD4
)使經適合取代之醛3-a
,繼而在標準氧化條件(例如MnO2
、Fe2
O3
、NiO、CuO、ZnO、ZrO2
、La2
O3
、Sm2
O3
、Eu2
O3
、Yb2
O3
等)下將3-b
氧化為對應的醛3-c
。在兩個步驟中藉由以下來獲得化合物3 -d
:使3-c
與乙炔基格林納反應,繼而用乙酸酐在鹼(例如吡啶、TEA等)存在下使所得醇醯化。在類似之兩步製程中藉由以下來獲得化合物3 -e
:使經適合取代之醛3-a
與乙炔基格林納反應,繼而用乙酸酐使所得醇醯化。流程 4
展示受適合保護之式4-b
疊氮化合物的合成,其中Lg為離去基且Z3
如本文中所定義。流程 4
在流程 4
中,用重氮基轉移劑(例如咪唑-1-磺醯基疊氮化物鹽酸鹽)處理經適合取代之胺4 -a
,得到對應的4-b
。或者,可在兩個步驟中藉由以下自醇4-c
獲得之4-b
:將羥基部分轉化成適合之離去基(Lg) (例如TsO-、MsO-、NsO-、TfO-等),繼而用疊氮化物進行親核置換。流程 5
展示式5-c
中間化合物之合成,其中R50
為烷基且Z3
如本文中所定義。流程 5
在流程 5
中,藉由使用標準1,3-偶極環加成條件使4-b
與5-a
反應來獲得經適合取代之三唑5-b
。在標準羰基脫保護條件(例如水性酸)下將縮醛5-b
轉化成對應的醛5-c
。流程 6
展示例示性式I化合物之通用合成,其中Z3
、m、R1
、R2
、R4
、R5
及R15
如本文中所定義。流程 6
在流程 6
中,可經由以下來提供式6-c
化合物:用3-d
(或3-e
)使胺2-d
N-烷基化,繼而在標準1,3-偶極環加成條件下與疊氮化物4-b
環化。分離式6-a
之異構體以得到式6-b
化合物可使用標準對掌性分離/解析技術(例如對掌性層析,結晶等)來進行。或者,式6-b
化合物可經由使用對掌性金屬錯合物(例如[Cu(CH3
CN)4
]PF6
、CuOTf·苯、Cu(OAc)2
或Cu(I)I等,與對掌性配位體一起)用3-d
(或3-e
)對2-d
進行對映選擇性N-烷基化來提供。適合之反應條件及例示性對掌性配位體/錯合物可見於文獻中(參見例如Detz等人 Angew. Chem. Int. Ed. 2008, 47, 3777-3780)。使化合物6-c
與疊氮化物4-b
在標準1,3-偶極環加成條件下接觸提供化合物6-b
。6-c
可或可不在添加化合物4 -b
之前經分離。流程 7
展示式I化合物經由亞胺形成及後續親核加成之替代性合成,其中Z3
、m、R1
、R2
、R3
、R4
、R5
及R15
如本文中所定義。流程 7
在流程 7
中,使胺2-d
與醛7-a
在標準亞胺形成條件下反應以得到對應的亞胺7-b
。隨後使化合物7-b
與格林納試劑7-c
反應以提供式I。或者,可使2-d
與醛7-d
反應以得到亞胺7-e
,隨後使其與乙炔基格林納反應以提供化合物7-f
。隨後如流程 6
中所示,可在標準1,3-偶極環加成條件下用4-b
將化合物7-f
轉化成化合物7-g
。此外,解析式I或化合物7-g
之異構體可使用標準對掌性分離/解析條件(例如對掌性層析、結晶等)來進行。流程 8
展示式I化合物之另一個替代性通用合成,其中m、R1
、R2
、R3
、R4
、R5
及R15
如本文中所定義。流程 8
在流程 8
中,使胺2-d
與經恰當取代之8-a
在親核取代條件下在鹼存在下反應以提供式I化合物,其中Lg為適合之離去基,諸如鹵離子(例如氟、氯、溴、碘)或活化醇(例如AcO-、TsO-、TfO-、MsO-等)。或者,使胺2-d
與酮8-b
反應以提供8-c
,隨後使其還原以提供式I化合物。解析式I異構體可使用標準對掌性分離/解析條件(例如對掌性層析、結晶等)來進行。實例
包括以下實例以展現本發明之具體實施例。熟習此項技術者應瞭解,以下實例中所揭示之技術代表在實踐本發明中良好運行之技術,且因此可視為構成其實踐之具體模式。然而,根據本發明,熟習此項技術者應瞭解,在不背離本發明之精神及範疇的情況下可對所揭示之特定實施例作出許多改變且仍獲得相同或相似結果。縮寫及首字母縮寫詞之列表
中間物: 氰基喹啉核心之實例合成:
將2-氯-4-硝基苯胺(1當量)、(Z)-2-氰基-3-乙氧基丙烯酸乙酯(1.3當量)及Cs2
CO3
(1.3當量)於DMF中之混合物在45℃下加熱隔夜。在冷卻至室溫之後,將混合物傾入水中。過濾所形成之固體,且用水洗滌,且乾燥,得到呈固體狀之標題化合物,其不經進一步純化即用於下一步驟。1
H NMR (DMSO-d 6
, 300 MHz): δ 11.28 (d,J
= 12.9 Hz, 1H), 8.84 (d,J
= 12.9 Hz, 1H), 8.42 (d,J
= 2.4 Hz, 1H), 8.26-8.22 (m, 1H), 8.02 (d,J
= 9.3 Hz, 1H), 4.27 (q,J
= 7.2 Hz, 2H), 1.27 (t,J
= 7.2 Hz, 3H)。合成 8- 氯 - 6- 硝基 -4- 側氧基 -1,4- 二氫喹啉 -3- 甲腈
在氮氣下,在加熱套中用沙浴將(Z)-3-((2-氯-4-硝基苯基)胺基)-2-氰基丙烯酸乙酯於二苯醚中之懸浮液加熱至回流,持續24小時。在冷卻至室溫之後,將反應混合物傾入己烷中,且攪拌2小時。過濾混合物,且用己烷洗滌濾餅兩次,得到呈棕色固體狀之標題化合物。1
H NMR (DMSO-d 6
, 300 MHz): δ 12.86 (br s, 1H), 8.73-8.71 (m, 3H)。合成 4,8- 二氯 -6- 硝基喹啉 -3- 甲腈
將8-氯-6-硝基-4-側氧基-1,4-二氫喹啉-3-甲腈及五滴DMF於POCl3
中之懸浮液在115℃下加熱隔夜。使棕色透明溶液冷卻至室溫,且移除過量POCl3
。將殘餘物溶解於DCM中,用飽和NaHCO3
、鹽水洗滌,且經Na2
SO4
乾燥。過濾溶液且濃縮,得到粗產物。用己烷及EtOAc濕磨殘餘物,得到呈棕色固體狀之標題化合物。1
H NMR (DMSO-d 6
, 300 MHz): δ 9.50 (s, 1H), 8.98 (d,J
= 2.4 Hz, 1H), 8.89 (d,J
= 2.4 Hz, 1H)。實例乙酸炔酯 乙酸 1-(6- 氟吡啶 -3- 基 ) 丙 -2- 炔 -1- 酯 :
將6-氟菸鹼醛(300 mg,2.40 mmol)溶解於THF (15 mL)中,且使其達到0℃。緩慢添加溴化乙炔基鎂(於THF中之0.5 M溶液,5.76 mL,2.88 mmol),且使所得溶液攪拌30分鐘。隨後添加乙酸酐(0.45 mL,4.80 mmol),移除冷浴,且歷經2小時使反應混合物升溫至室溫。藉由添加飽和NH4
Cl水溶液(5 mL)來使反應內容物淬滅,將其傾入水(5 mL)中,且用EtOAc (3 × 15 mL)萃取。用鹽水(10 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到所要產物。
將乙酸1-(1-側氧基-1,2-二氫異喹啉-5-基)丙-2-炔-1-酯(200 mg,0.83 mmol)溶解於DMF (2 mL)中,其後添加碳酸銫(405 mg,1.2 mmol)及2-碘丙烷(211 mg,1.2 mmol),且將所得混合物在25℃下在環境氛圍下攪拌隔夜。將反應混合物傾入水(3 mL)中,且用EtOAc (3 × 5 mL)萃取。經MgSO4
乾燥有機層,過濾,濃縮,且經由矽膠層析(溶離劑:EtOAc/己烷)來純化,得到N-烷基化產物。註釋:可對前述1-側氧基-1,2-二氫異喹啉-5-甲醛進行相同烷基化方案。用於乙酸炔酯合成之實例醛 6- 氟菸鹼 - 醛 -α-D :
在室溫下將6-氟菸鹼醛(1.14 g,9.11 mmol)溶解於MeOH (8 mL)中。隨後一次性添加NaBD4
(458 mg,10.9 mmol),且攪拌反應混合物20分鐘。小心地用水(5 mL)使反應混合物淬滅,且用EtOAc (3 × 15 mL)萃取。用鹽水(5 mL)洗滌經合併之有機層,經MgSO4
乾燥,且濃縮,得到粗物質醇,其不經進一步純化即繼續使用。將粗物質醇再溶解於DCM (40 mL)中,且在室溫下添加氧化錳(IV) (19.9 g,281 mmol)。在2小時之後,經由矽藻土墊過濾反應混合物,用DCM及EtOAc沖洗。隨後濃縮濾液,得到氘併入為大約95%之所要產物。 2- 乙醯基 -3- 側氧基異吲哚啉 -4- 甲醛:
在室溫下將3-側氧基異吲哚啉-4-甲醛(300 mg,1.86 mmol)溶解於THF (5 mL)中。添加乙酸酐(0.53 mL,5.59 mmol)及DMAP (45 mg,0.37 mmol),且攪拌反應混合物隔夜。藉由添加飽和NH4
Cl水溶液(3 mL)來使反應內容物淬滅,將其傾入水(3 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到所要產物。(7- 甲醯基苯并 [d] 噻唑 -2- 基 ) 胺基甲酸第三丁酯 2-(( 第三丁氧基羰基 ) 胺基 ) 苯并 [d] 噻唑 -7- 甲酸乙酯 :
將2-胺基苯并[d]噻唑-7-甲酸乙酯(300 mg,1.35 mmol)、二碳酸二第三丁酯(0.34 mL,1.49 mmol)及DMAP (181 mg,1.49 mmol)溶解於DCM (10 mL)中,且在室溫下攪拌3小時。隨後將反應混合物傾入水(10 mL)中,且用DCM (2 × 20 mL)萃取。經MgSO4
乾燥經合併之有機萃取物,濃縮,且藉由急驟層析(溶離劑:EtOAc/己烷)來純化,得到所要產物。(7-( 羥基甲基 ) 苯并 [d] 噻唑 -2- 基 ) 胺基甲酸第三丁酯 :
將2-((第三丁氧基羰基)胺基)苯并[d]噻唑-7-甲酸乙酯(204 mg,0.63 mmol)溶解於THF (7 mL)中,且使其達到0℃。分批添加LiAlH4
(72 mg,1.90 mmol),且使反應混合物攪拌90分鐘。在0℃下小心地用水(5 mL)使反應混合物淬滅,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮,得到所要產物,其不經進一步純化即使用。(7- 甲醯基苯并 [d] 噻唑 -2- 基 ) 胺基甲酸第三丁酯 :
將(7-(羥甲基)苯并[d]噻唑-2-基)胺基甲酸第三丁酯(177 mg,0.63 mmol)溶解於DCM (5 mL)中,其後在室溫下添加戴斯-馬丁高碘烷(Dess-Martin periodinane) (321 mg,0.76 mmol)。在30分鐘之後,藉由添加飽和Na2
SO3
水溶液(3 mL)來使反應內容物淬滅,且劇烈攪拌5分鐘。隨後將反應混合物傾入飽和NaHCO3
水溶液(5 mL)中,且用EtOAc (3 × 15 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮,得到所要醛,其不經進一步純化即使用。 2- 甲基 -1- 側氧基異吲哚啉 -4- 甲醛 :
向4-溴-2-甲基異吲哚啉-1-酮(200 mg,0.89 mmol)於THF (3 mL)中之溶液中,在-78℃下向溶液中添加n-BuLi (0.78 mL,1.95 mmol)。在30分鐘之後,向溶液中添加DMF (0.273 mL,3.57 mmol)。在1小時之後,使反應物升溫。用EtOAc稀釋,且用鹽水洗滌。經硫酸鈉乾燥有機層,且濃縮。藉由矽膠層析(溶離劑:EtOAc/己烷)來純化產物,在自水/MeCN凍乾之後得到產物。 1- 甲基 -6- 側氧基 -1,6- 二氫吡啶 -3- 甲醛 :
將6-氯-2-甲基菸鹼醛(1.0 g,6.43 mmol)於濃HCl (3 mL)中之溶液加熱至90℃,持續隔夜。使其冷卻,且將其傾入冰水(20 mL)中。過濾且經真空乾燥。不經進一步純化即使用。
向6-側氧基-1,6-二氫吡啶-3-甲醛(300 mg,2.19 mmol)於DMF中之懸浮液中,在冰浴條件下向懸浮液中添加氫化鈉(96 mg,2.4 mmol)。向懸浮液中添加碘甲烷(0.15 mL,2.4 mmol)。隨後將其攪拌隔夜。用EtOAc稀釋,且用鹽水洗滌。乾燥且濃縮有機層。不經進一步純化即使用。 3- 甲基 -4- 側氧基 -3,4- 二氫喹唑啉 -8- 甲醛 :
將3,8-二甲基喹唑啉-4(3H)-酮(300 mg,2 mmol) (根據Organic and Biomolecular Chemistry, 2011 , 第9卷, 第17期 第6089 - 6099頁製備)及二氧化硒(955 mg,9 mmol)於1,2-二氯苯(1270 mg,9 mmol)中之懸浮液加熱至170℃,持續隔夜。經MgSO4
乾燥有機層,過濾,濃縮,且經由矽膠層析(溶離劑:EtOAc/己烷)來純化,得到標題化合物。實例胺 (2,2- 二甲基丙基 -1,1-d2) 胺鹽酸鹽 :
在室溫下將LiAlD4
(252 mg,6.02 mmol)懸浮於Et2
O (10 mL)中。隨後以Et2
O (6 mL)中之溶液的形式緩慢添加三甲基乙腈(0.67 mL,6.02 mmol),從而將溫度保持在低於回流。在30分鐘之後,藉由小心緩慢添加水來使反應混合物淬滅,直至氣體逸出停止為止。隨後添加飽和羅謝爾鹽(Rochelle's salt)水溶液(50 mL),且將所得溶液劇烈攪拌2小時。隨後分離各相,且用Et2
O (3 × 30 mL)萃取水相。用鹽水(15 mL)洗滌經合併之有機相,經MgSO4
乾燥,且過濾。向於乙醚中之產物溶液中添加HCl (於乙醚中之1.0 M溶液,15 mL,15 mmol),其後藉由過濾來收集新形成之鹽酸鹽。(R)-1- 苯基丙 -1- 胺 -d7 埃爾曼輔助縮合 (Ellman auxiliary condensation) :
將(S)-(-)-2-甲基-2-丙烷亞磺醯胺(862 mg,7.12 mmol)溶解於DCM (15 mL)中。隨後添加PPTS (81 mg,0.32 mmol)、MgSO4
(3.89 g,32.3 mmol)及苯甲醛-d,且使所得混合物在室溫下攪拌4小時。在用DCM沖洗的情況下經由矽藻土過濾反應混合物,濃縮,且藉由急驟層析(溶離劑:EtOAc/己烷)來純化,得到所要產物。格林納形成及向亞磺醯亞胺中添加:
向鎂屑(426 mg,17.5 mmol)於無水THF (7 mL)中之懸浮液中添加呈無水THF (2 mL)中之溶液形式的乙基溴-d5 (1.00 g,8.77 mmol),且在室溫下攪拌2小時。熱產生及褪色指示格林納試劑成功形成,得到EtMgBr-d5於THF中之大約1.0 M溶液。在-78℃下向亞磺醯亞胺(752 mg,3.58 mmol)於DCM (10 mL)中之溶液中逐滴添加EtMgBr-d5 (於THF中之1.0 M溶液,7.2 mL,7.2 mmol)。在於-78℃下攪拌3小時之後,使反應混合物升溫至室溫,持續隔夜。藉由添加飽和NH4
Cl水溶液(5 mL)來使反應內容物淬滅,將其傾入水(5 mL)中,且用EtOAc (3 × 30 mL)萃取。用鹽水(15 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到所要產物。輔助移除:
在室溫下將起始物質(451 mg,1.84 mmol)溶解於MeOH (0.9 mL)中。添加HCl (於二噁烷中之4.0 M溶液,0.92 mL,3.69 mmol),且攪拌溶液30分鐘。用Et2
O (20 mL)稀釋反應混合物,且藉由過濾來收集所得沈澱,得到呈鹽酸鹽形式之所要產物。(1R,2R)-2-((S)- 胺基 ( 苯基 ) 甲基 ) 環丙烷甲腈 2- 苯甲醯基 環丙烷甲腈 :
在室溫下將苯甲醯甲基氯(10.0 g,64.7 mmol)及DABCO (7.26 g,64.7 mmol)溶解於THF (200 mL)及DMSO (50 mL)中,且攪拌30分鐘。隨後添加Na2
CO3
(10.3 g,97.0 mmol)及丙烯腈(8.48 mL,129.4 mmol),將所得混合物加熱至90℃,持續隔夜。藉由添加飽和NH4
Cl水溶液(40 mL)來使反應內容物淬滅,將其傾入水(20 mL)中,且用EtOAc (3 × 150 mL)萃取。用鹽水(40 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到分開的反-2-苯甲醯基環丙烷甲腈(5.91 g,53%)及順-2-苯甲醯基環丙烷甲腈及呈外消旋混合物形式之兩者。(R)-N-(((1S,2S)-2- 氰基環丙基 )( 苯基 ) 亞甲基 )-2- 甲基丙烷 -2- 亞磺醯胺及 (R)-N-(((1R,2R)-2- 氰基環丙基 )( 苯基 ) 亞甲基 )-2- 甲基丙烷 -2- 亞磺醯胺 :
組合外消旋反-2-苯甲醯基環丙烷甲腈(1.00 g,5.84 mmol)、(R)-(+)-2-甲基-2-丙烷亞磺醯胺(2.12 g,17.5 mmol)及乙醇鈦(IV) (7.35 mL,35.1 mmol),且將其加熱至85℃,持續3小時。使反應混合物冷卻至室溫,用EtOAc (100 mL)稀釋,繼而用水(5 mL)稀釋,且使其攪拌30分鐘。經由過濾移除白色沈澱,且用鹽水洗滌濾液,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到呈純對映異構體形式之(R)-N-(((1R,2R)-2-氰基環丙基)(苯基)亞甲基)-2-甲基丙烷-2-亞磺醯胺及(R)-N-(((1S,2S)-2-氰基環丙基)(苯基)亞甲基)-2-甲基丙烷-2-亞磺醯胺。(R)-N-((S)-((1R,2R)-2- 氰基環丙基 )( 苯基 ) 甲基 )-2- 甲基丙烷 -2- 亞磺醯胺 :
將(R)-N-(((1R,2R)-2-氰基環丙基)(苯基)亞甲基)-2-甲基丙烷-2-亞磺醯胺(250 mg,0.91 mmol)溶解於THF中,且使其達到-78℃。一次性添加NaBH4
(70.0 mg,1.85 mmol),且使反應混合物緩慢升溫至室溫。在達到室溫後,用水(2 mL)使反應內容物淬滅,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到呈純對映異構體形式之(R)-N-((R)-((1R,2R)-2-氰基環丙基)(苯基)甲基)-2-甲基丙烷-2-亞磺醯胺(56 mg,22%)及(R)-N-((S)-((1R,2R)-2-氰基環丙基)(苯基)甲基)-2-甲基丙烷-2-亞磺醯胺。(1R,2R)-2-((S)- 胺基 ( 苯基 ) 甲基 ) 環丙烷甲腈 :
在室溫下將(R)-N-((S)-((1R,2R)-2-氰基環丙基)(苯基)甲基)-2-甲基丙烷-2-亞磺醯胺(143 mg,0.52 mmol)溶解於MeOH (0.5 mL)中。添加HCl (於二噁烷中之4.0 M溶液,0.26 mL,1.04 mmol),且攪拌溶液30分鐘。用Et2
O (20 mL)稀釋反應混合物,且藉由過濾來收集所得沈澱,得到呈鹽酸鹽形式之所要產物。3- 氯 - 2- 環丙氧基苯胺 1- 氯 -2- 環丙氧基 -3- 硝基苯:
向NaH (於礦物油中之60%分散液,319 mg,7.98 mmol)於THF (10 mL)中之溶液中緩慢添加環丙醇(0.35 mL,5.58 mmol)。在攪拌15分鐘之後,添加1-氯-2-氟-3-硝基苯(700 mg,3.99 mmol),且使所得溶液加熱至75℃,持續1小時。使反應混合物冷卻至室溫,用水(5 mL)使其淬滅,且用EtOAc (3 × 15 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由急驟層析(溶離劑:EtOAc/己烷)來純化粗殘餘物,得到所要產物。3- 氯 -2- 環丙氧基苯胺 :
在室溫下將1-氯-2-環丙氧基-3-硝基苯(420 mg,1.97 mmol)溶解於EtOH (8 mL)中隨後添加鐵(549 mg,9.83 mmol)、CaCl2
(327 mg,2.95 mmol)及水(1 mL),且將所得混合物加熱至75℃,持續3小時。藉由在用MeOH及EtOAc沖洗的情況下過濾來移除固體,濃縮濾液,且隨後再溶解於EtOAc (100 mL)中。用飽和NaHCO3
(2 × 20 mL)水溶液、鹽水(20 mL)洗滌有機相,經MgSO4
乾燥,且濃縮,得到產物,其不經進一步純化即使用。重氮基轉移反應 及疊氮化物產生
向1H
-咪唑-1-磺醯基疊氮化物鹽酸鹽(129.5 mg,0.62 mmol)、碳酸鉀(136 mg,0.99 mmol)及五水合硫酸銅(II) (12.3 mg,0.049 mmol)於甲醇(1.0 mL)中之懸浮液中添加3-(胺基甲基)氧雜環丁-3-醇(50 mg,0.49 mmol)。將藍色混合物在室溫下攪拌16小時,且不經點擊化學(Click chemistry) (實例4)中之處理即使用。參考文獻:E. D. Goddard等人, Org. Lett., 2007, 第3797頁。哌啶 - 三唑醛 4-( 甲苯磺醯基氧基 ) 哌啶 -1- 甲酸 苯甲 酯 (2)
將4-羥基哌啶-1-甲酸苯甲酯(1) (17.2 g,73.1 mmol)及對甲苯磺醯氯(15.3 g,80.4 mmol)溶解於吡啶(50 mL)中,且在室溫下攪拌。在23小時之後,在減壓下移除吡啶,且將殘餘物溶解於EtOAc (300 mL)中。用水(2 × 150 mL)及飽和氯化銨(100 mL)洗滌有機相,經硫酸鈉乾燥,且在減壓下移除溶劑。對殘餘物進行急驟層析(溶離劑:乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑,得到4-(甲苯磺醯基氧基)哌啶-1-甲酸苯甲酯(2
)。4- 疊氮基哌啶 -1- 甲酸 苯甲 酯 (3)
向4-(甲苯磺醯基氧基)哌啶-1-甲酸苯甲酯(2
) (12.4 g,31.8 mmol)於二甲基甲醯胺(100 mL)中之溶液中添加疊氮化鈉(2.48 g,38.2 mmol)。將混合物在90℃下加熱30分鐘。使混合物冷卻,且用乙酸乙酯(250 mL)稀釋,且用水(2 × 15 mL)、5%氯化鋰水溶液(10 mL)及鹽水(10 mL)洗滌。經硫酸鈉乾燥有機相,且濃縮(不至乾燥),得到所要物質。所有物質用於下一步驟中。4-(4-( 二乙氧基甲基 )-1H-1,2,3- 三唑 -1- 基 ) 哌啶 -1- 甲酸 苯甲 酯 (4)
向4-疊氮基哌啶-1-甲酸苯甲酯(3
) (8.2 g,31.5 mmol)、3,3-二乙氧基丙-1-炔(4.44 g,34.6 mmol)及飽和硫酸銅(II) (8 mL)於四氫呋喃(100 mL)中之溶液中添加銅粉末(2.0 g,31.5 mmol)。在17小時之後,經由矽藻土墊過濾混合物。在減壓下移除溶劑,且使殘餘物溶於乙酸乙酯(200 mL)中。用鹽水(3 × 100 mL)洗滌有機相,經硫酸鈉乾燥,且濃縮。對殘餘物進行矽膠急驟層析(溶離劑:乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑,得到4-(4-(二乙氧基甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸苯甲酯(4
)。4-(4- 甲醯基 -1H-1,2,3- 三唑 -1- 基 ) 哌啶 -1- 甲酸 苯甲 酯 (5)
向4-(4-(二乙氧基甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸苯甲酯(4
) (429 mg,1.1 mmol)於四氫呋喃(4 mL)及水(2 mL)中之溶液中添加鹽酸水溶液(1 M,2.2 mL,2.2 mmol)。在減壓下移除有機溶劑。用乙腈(2 mL)稀釋水性混合物,且對其進行凍乾。3- 甲醯基 -4,7- 二氫噻吩并 [2,3-c] 吡啶 -6(5H
)- 甲酸 第三丁 酯
向6-(第三丁氧基羰基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-甲酸(1.00 g,3.53 mmol)及六氟磷酸N-[(二甲胺基)-1H-1,2,3-三唑并-[4,5-b]吡啶-1-基亞甲基]-N-甲基甲銨N-氧化物(1.62 g,4.24 mmol)於二甲基甲醯胺(15 mL)中之溶液中添加N,N
-二異丙基乙胺(1.53 mL,8.82 mmol)。在2分鐘之後,添加N
,O-
二甲基羥胺(413 mg,4.24 mmol)。在16小時之後,用乙酸乙酯(75 mL)稀釋反應物,且用水(2 × 25 mL)、飽和氯化銨(2 × 25 mL)及鹽水(25 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑,得到3-(甲氧基(甲基)胺甲醯基)-4,7-二氫噻吩并[2,3-c
]吡啶-6(5H
)-甲酸第三丁酯。
在-78℃下在氬氣氛圍下向3-(甲氧基(甲基)胺甲醯基)-4,7-二氫噻吩并[2,3-c
]吡啶-6(5H
)-甲酸第三丁酯(1.03 g,3.15 mmol)於四氫呋喃(20 mL)中之溶液中逐滴添加二異丁基氫化鋁於四氫呋喃中之溶液(4.42 mL,1.0 M,4.42 mmol)。在於-78℃下5小時之後,反應40%完成。逐滴添加二異丁基氫化鋁於四氫呋喃中之溶液(3.15 mL,1.0 M,3.15 mmol)。在30分鐘之後,在-78℃下用飽和氯化銨(20 mL)使反應淬滅,且使其升溫至室溫。將有機相與水(20 mL)及乙酸乙酯(75 mL)一起震盪(導致凝膠形成)。添加鹽酸(2 N,5 mL),且經由矽藻土墊過濾來移除固體。用飽和碳酸氫鈉(25 mL)及鹽水(25 mL)洗滌有機相。經硫酸鈉乾燥有機相,且濃縮。對殘餘物進行急驟層析(0%-50%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑,得到3-甲醯基-4,7-二氫噻吩并[2,3-c]吡啶-6(5H)-甲酸第三丁酯。化合物實例 實例 1
程序1 8- 氯 -4-( 新戊基胺基 )-6- 硝基喹啉 -3- 甲腈 :
在微波條件下將含4,8-二氯-6-硝基喹啉-3-甲腈(615 mg,2.29 mmol)、新戊胺(220 mg,0.25 mmol)及三乙胺(278 mg,2.75 mmol)之異丙醇(4 mL)在150℃下加熱45分鐘。使反應物冷卻至室溫。添加水,且經由過濾收集所得沈澱。粗產物不經進一步純化即用於下一步驟中。
ES/MS 319.1 (M+H+
)。此轉化之替代性反應條件 :
將含4,8-二氯-6-硝基喹啉-3-甲腈(3000 mg,11.2 mmol)、新戊胺(1073 mg,12.3 mmol)及三乙胺(1246 mg,12.3 mmol)之異丙醇(60 mL)在80℃下加熱4小時。使反應物冷卻至室溫。移除溶劑,且經由矽膠層析(溶離劑:EtOAc/己烷)來純化粗反應產物,從而得到產物。
ES/MS (M+H+
) 319.1。6- 胺基 -8- 氯 -4-( 新戊基胺基 ) 喹啉 -3- 甲腈 :
將8-氯-4-(新戊基胺基)-6-硝基喹啉-3-甲腈(699 mg,2.2 mmol)、氯化鈣(483.6 mg,3.28 mmol)、鐵粉(612.3 mg,10.96 mmol)在乙醇(22 mL)/水(2.2 mL)中在60℃下加熱1小時。使反應物冷卻至室溫,且經由過濾移除固體。用EtOAc洗滌固體,且用碳酸氫鈉水溶液、鹽水洗滌經合併之有機層,且經硫酸鈉乾燥。過濾及蒸發所有揮發物得到產物。
ES/MS 289.1 (M+H+
)。使用氯化錫之替代性還原條件 :
將8-氯-4-(新戊基胺基)-6-硝基喹啉-3-甲腈(2,000 mg,6.2 mmol)及氯化錫(7079 mg,31.3 mmol)在70℃下加熱4小時。添加更多氯化錫(2832 mg,12.6 mmol)。在5小時之後,反應完成。使反應物冷卻至室溫。在減壓下移除一半乙醇。將混合物添加至NaHCO3
(200 mL)中,且用EtOAc (500 mL)稀釋。用鹽水(200 mL)洗滌有機相,且經硫酸鈉乾燥。在減壓下移除溶劑,得到所要物質。
1H NMR (400 MHz, DMSO-d6) δ 8.19 (s, 1H), 7.32 (d, J = 2.1 Hz, 1H), 7.29 (t, J = 7.3 Hz, 1H), 7.18 (d, J = 2.3 Hz, 1H), 5.74 (s, 2H), 3.66 (d, J = 6.6 Hz, 2H), 0.96 (s, 9H)。
ES/MS 289.1 (M+H+
)。(S)-8- 氯 -6-(((1- 環丙基 -1H-1,2,3- 三唑 -4- 基 )(4- 氟 -3- 吡啶基 )- 甲基 -d) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈 :
在MeOH (3.5 mL)中對6-胺基-8-氯-4-(新戊基胺基)喹啉-3-甲腈(75 mg,0.26 mmol)、CuI (3.6 mg,0.019 mmol)及2,6-雙((4S,5R)-4,5-二苯基-4,5-二氫噁唑-2-基)吡啶[噁唑啉配位體] (9.9 mg,0.019 mmol)進行音波處理持續約1分鐘。添加乙酸炔酯(44.4 mg,0.23 mmol)及二-異丙基乙胺(29.4 mg,0.229 mmol),且將反應物在室溫下攪拌隔夜。添加第三丁基疊氮化物(45 mg,0.454 mmol),且將反應物在室溫下再攪拌24小時。在真空中移除溶劑,且經由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗物質,得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, DMSO-d6) δ 8.37 (m, 2H), 8.17 (s, 1H), 8.05 (m, 1H), 7.79 (brs, 1H), 7.62 (d, J = 2.2 Hz, 1H), 7.51 (br s, 1H), 7.15 (m, 2H), 4.03 (m, 1H), 3.44 (dd, J = 13.9 / 5.5 Hz, 1H), 1.59 (s, 9H), 0.88 (s, 9H)。
ES/MS 522.2 (M+H+
)。實例 2
程序28- 氯 -6-(((S)-(1- 異丙基 -1H-1,2,3- 三唑 -4- 基 )(2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 (R)-8- 氯 -6- 硝基 -4-((1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 :
在微波條件下將含4,8-二氯-6-硝基喹啉-3-甲腈(200 mg,0.75 mmol)、(R)-乙基苯甲胺(121 mg,0.895 mmol)之異丙醇(3 mL)在150℃下加熱45分鐘。使反應物冷卻至室溫。添加水及EtOAc。用EtOAc萃取水層,且經硫酸鈉乾燥經合併之有機層。過濾及蒸發溶劑得到粗產物,其不經進一步純化即用於下一步驟中。
ES/MS 367.1 (M+H+
)。6- 胺基 -8- 氯 -4-((1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 :
將(R)-6-胺基-8-氯-4-((1-苯基丙基)胺基)喹啉-3-甲腈(287 mg,0.78 mmol)、氯化鈣(172.6 mg,1.17 mmol)、鐵粉(218.5 mg,3.91 mmol)在乙醇(5 mL)/水(0.5 mL)中在60℃下加熱1小時。使反應物冷卻至室溫,且經由過濾移除固體。用EtOAc洗滌固體,且用碳酸氫鈉水溶液、鹽水洗滌經合併之有機層,且經硫酸鈉乾燥。過濾及蒸發所有揮發物得到產物。
ES/MS 337.1 (M+H+
)。8- 氯 -6-(((R)-1-(2- 甲基吡啶 -3- 基 ) 丙 -2- 炔 -1- 基 ) 胺基 )-4-(((R)-1- 苯基乙基 ) 胺基 ) 喹啉 -3- 甲腈 :
在MeOH (3.0 mL)中對CuI (2.0 mg,0.01 mmol)及2,6-雙((4S,5R)-4,5-二苯基-4,5-二氫噁唑-2-基)吡啶[噁唑啉配位體] (6.9 mg,0.013 mmol)進行音波處理持續5分鐘。添加含乙酸1-(2-甲基吡啶-3-基)丙-2-炔-1-酯(50 mg,0.27 mmol)之MeOH (1 mL)、(R)-6-胺基-8-氯-4-((1-苯基丙基)胺基)喹啉-3-甲腈(75 mg,0.223 mmol)及二-異丙基乙胺(34 mg,0.27 mmol),且在室溫下攪拌反應物持續隔夜。藉由矽膠層析(20%-100% EtOAc/己烷)來純化粗反應產物,得到產物。
ES/MS 465.99 (M+H+
)。8- 氯 -6-(((S)-(1- 異丙基 -1H-1,2,3- 三唑 -4- 基 )(2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈
:在室溫下將8-氯-6-(((R)-1-(2-甲基吡啶-3-基)丙-2-炔-1-基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈(45 mg,0.10 mmol)溶解於THF (0.5 mL)中,且添加噻吩甲酸銅(I) (5.7 mg,0.030 mmol)。添加2-疊氮基丙烷(10 mg,0.120 mmol),且將反應物在室溫下攪拌4小時。將反應物分配於碳酸氫鈉水溶液與EtOAc之間。用EtOAc萃取水層,且用飽和碳酸氫鈉溶液洗滌經合併之層,且經硫酸鈉乾燥。過濾及蒸發溶劑得到粗物質。用RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗物質,得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, CD3
OD) δ 8.61 (m, 1H), 8.37 (m, 1H), 8.29 (m, 1H), 8.05 (m, 1H), 7.73 (m, 1H), 7.60 (s, 1H), 7.32 (m, 5H), 7.14 (m, 1H), 6.46 (s, 1H), 5.64 (m, 1H), 4.88 (m, 1H), 2.83 (s, 3H), 2.17 - 2.02 (m, 2H), 1.56 (d, 6H), 0.97 (m, 3H)。
ES/MS 551.09 (M+H+
)。實例 3
程序38- 氯 -6-(((S)-(1- 環丙基 -1H-1,2,3- 三唑 -4- 基 )(2,6- 二氟吡啶 -3- 基 ) 甲基 -d)) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 8- 氯 -6-(((S)-(1- 環丙基 -1H-1,2,3- 三唑 -4- 基 )(2,6- 二氟吡啶 -3- 基 ) 甲基 -d)) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈
:在MeOH (3.0 mL)中對CuI (2.0 mg,0.01 mmol)及2,6-雙((4S,5R)-4,5-二苯基-4,5-二氫噁唑-2-基)吡啶[噁唑啉配位體] (4.6 mg,0.009 mmol)進行音波處理持續約5分鐘。添加含乙酸炔酯(79 mg,0.37 mmol)之MeOH (1 mL)、(R)-6-胺基-8-氯-4-((1-苯基丙基)胺基)喹啉-3-甲腈(50 mg,0.148 mmol)及二-異丙基乙胺(23 mg,0.18 mmol),且將反應物在室溫下攪拌隔夜。粗反應混合物直接用於下一步驟。
ES/MS: 489.19 (M+H+
)。
向反應混合物中添加環丙基疊氮化物(16 mg,0.192 mmol)。在於室溫下1小時之後,過濾混合物,且隨後藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化,得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, CD3
OD) δ 8.43 (m, 1H), 8.05 (m, 1H), 8.01 (m, 1H), 7.64 (m, 1H), 7.42 - 7.25 (m, 6H), 6.98 (m, 1H), 5.80 - 5.66 (m, 1H), 3.97 - 3.84 (m, 1H), 2.25 - 2.01 (m, 2H), 1.28 - 1.11 (m, 4H), 1.01 (m, 3H)。
ES/MS: 572.24 (M+H+
)。實例 4
程序4 8- 氯 -6-(((S)-(1-((3- 羥基氧雜環丁 -3- 基 ) 甲基 )-1H-1,2,3- 三唑 -4- 基 )( 吡啶 -3- 基 ) 甲基 ) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 :
向3-(疊氮基甲基)氧雜環丁-3-醇(0.049 mmol)之儲備溶液中添加8-氯-4-(((R)-1-苯基丙基)胺基)-6-(((R)-1-(吡啶-3-基)丙-2-炔-1-基)胺基)喹啉-3-甲腈(20 mg,0.046 mmol)、銅粉末(15 mg,0.23 mmol)、乙酸(118 μL,1.8 mmol)及飽和硫酸銅(II)水溶液(0.1 mL)及THF (3 mL)。在2小時之後,反應完成,且在真空中移除揮發物。將粗物質分配於乙酸乙酯(15 mL)與水之間。用飽和碳酸氫鈉、鹽水洗滌有機層,經Na2
SO4
乾燥,且在過濾之後濃縮。使殘餘物溶於含2滴TFA之水(1 mL)及MeOH (1 mL)中,且對其進行RP-HPLC (溶離劑:水/MeCN *0.1% TFA)。合併含有所要產物之溶離份,且對其進行凍乾,得到所要化合物。1
H NMR (400 MHz, DMSO-d6) δ 8.84 (dd, J = 14.0, 2.2 Hz, 1H), 8.64 - 8.52 (m, 1H), 8.23 (d, J = 2.1 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 6.7 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.46 (d, J = 9.5 Hz, 1H), 7.43 - 7.38 (m, 1H), 7.38 - 7.31 (m, 2H), 7.28 - 7.21 (m, 2H), 7.21 - 7.15 (m, 3H), 6.48 (d, J = 6.9 Hz, 1H), 5.48 (q, J = 7.7 Hz, 1H), 4.68 (d, J = 2.0 Hz, 2H), 4.50 (dd, J = 6.2, 4.5 Hz, 3H), 4.41 (dd, J = 6.7, 3.4 Hz, 2H), 2.12 (dt, J = 14.5, 7.4 Hz, 1H), 2.04 - 1.78 (m, 1H), 0.94 (t, J = 7.3 Hz, 3H)。
ES/MS 581.2 (M+H+
)。實例 5
程序5 (R)-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-6-((1-(2,5- 二氯噻吩 -3- 基 ) 丙 -2- 炔 -1- 基 ) 胺基 ) 喹啉 -3- 甲腈
:在MeOH (1 mL)中對CuI (4.1 mg,0.022 mmol)及2,6-雙((4S,5R)-4,5-二苯基-4,5-二氫噁唑-2-基)吡啶[噁唑啉配位體] (13.5 mg,0.026 mmol)進行音波處理持續約5分鐘。添加額外MeOH (4 mL)。添加乙酸炔酯(150.7 mg,0.61 mmol)、6-胺基-8-氯-4-((4-氯-3-氟苯基)胺基)喹啉-3-甲腈(150 mg,0.43 mmol)及二-異丙基乙胺(67 mg,0.52 mmol),且將反應物在-15℃下攪拌4天。在真空中移除溶劑。經由矽膠層析(溶離劑:EtOAc/己烷)來純化粗反應產物,得到產物。1
H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.45 (s, 1H), 7.54 (dd, J = 6.4, 2.4 Hz, 2H), 7.43 (t, J = 9.0 Hz, 1H), 7.38 (d, J = 2.3 Hz, 1H), 7.30 (d, J = 4.3 Hz, 1H), 7.27 (s, 1H), 7.11 (d, J = 8.6 Hz, 1H), 5.54 (dd, J = 8.6, 2.2 Hz, 1H), 3.51 (d, J = 2.2 Hz, 1H)。
ES/MS 534.9 (M+H+
)。(S)-4-(4-(((8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-3- 氰基喹啉 -6- 基 ) 胺基 )(2,5- 二氯噻吩 -3- 基 ) 甲基 )-1H-1,2,3- 三唑 -1- 基 ) 哌啶 -1- 甲酸 第三丁 酯
:將(R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((1-(2,5-二氯噻吩-3-基)丙-2-炔-1-基)胺基)喹啉-3-甲腈(174 mg,0.324 mmol)及N-
Boc-哌啶-4-疊氮化物(73.4 mg,0.324 mmol)溶解於THF (5 mL)中。添加噻吩甲酸銅(6.2 mg,0.032 mmol),且將反應物在室溫下攪拌16小時。在減壓下移除揮發物,且藉由矽膠層析(溶離劑:EtOAc/己烷)來純化殘餘物。合併含有產物之溶離份,且在減壓下移除溶劑,得到產物。1
H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.47 (s, 1H), 8.16 (s, 1H), 7.60 (d, J = 2.2 Hz, 1H), 7.40 - 7.33 (m, 2H), 7.27 (d, J = 8.3 Hz, 1H), 7.14 (dd, J = 7.1, 2.0 Hz, 2H), 7.10 (s, 1H), 5.96 (d, J = 8.2 Hz, 1H), 4.72 - 4.59 (m, 1H), 4.01 (q, J = 9.1, 8.1 Hz, 3H), 2.86 (d, J = 17.5 Hz, 2H), 1.96 (d, J = 5.0 Hz, 3H), 1.77 (qd, J = 12.2, 4.4 Hz, 2H), 1.39 (s, 9H)。
ES/MS 762.9 (M+H+
)。
或者,環加成可以一鍋方式使用由N-烷基化提供之Cu(I)來進行。(S)-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-6-(((2,5- 二氯噻吩 -3- 基 )(1-( 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 ) 喹啉 -3- 甲腈
:將(S)-4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(2,5-二氯噻吩-3-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯(157 mg,0.206 mmol)懸浮於DCM (0.5 mL)中。添加含HCl之二噁烷(5 mL;4 M),且將反應物在室溫下攪拌30分鐘。在減壓下移除溶劑。對殘餘物進行急驟層析(溶離劑:(含20% MeOH之EtOAc)/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於EtOAc/飽和碳酸氫鈉水溶液中。分離有機層,且經硫酸鈉乾燥。過濾及在真空中蒸發溶劑得到產物。1
H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.45 (s, 1H), 8.10 (s, 1H), 7.59 (d, J = 2.1 Hz, 1H), 7.37 (t, J = 8.9 Hz, 2H), 7.26 (d, J = 8.1 Hz, 1H), 7.13 (d, J = 10.0 Hz, 3H), 5.96 (d, J = 8.2 Hz, 1H), 4.48 (tt, J = 11.8, 4.3 Hz, 1H), 3.75 - 3.62 (m, 1H), 3.55 (s, 1H), 3.46 (dq, J = 9.7, 5.2 Hz, 1H), 2.99 (d, J = 12.2 Hz, 2H), 2.55 (td, J = 12.5, 2.4 Hz, 2H), 1.92 (dd, J = 11.9, 3.7 Hz, 2H), 1.75 (t, J = 12.0 Hz, 3H)。
ES/MS 662.1 (M+H+
)。(S)-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-6-(((2,5- 二氯噻吩 -3- 基 )(1-(1- 乙基哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 ) 喹啉 -3- 甲腈
:將(S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2,5-二氯噻吩-3-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈(138 mg,0.208 mmol)溶解於THF (3 mL)及二氯乙烷(3 mL)中。添加乙醛(91.8 mg,2.08 mmol)及三乙醯氧基硼氫化鈉(176 mg,0.833 mmol),且將反應物在室溫下攪拌1小時。用EtOAc稀釋反應物,且用碳酸氫鈉水溶液、鹽水洗滌,且經硫酸鈉乾燥。過濾粗物質,且在真空中移除揮發物,且經由矽膠層析(溶離劑:含MeOH (20%)之EtOAc/己烷)來純化粗物質,得到產物。1
H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.47 (s, 1H), 8.14 (s, 1H), 7.60 (d, J = 2.3 Hz, 1H), 7.41 - 7.34 (m, 2H), 7.27 (d, J = 8.2 Hz, 1H), 7.15 (dd, J = 9.1, 3.3 Hz, 2H), 7.12 (s, 1H), 5.96 (d, J = 8.1 Hz, 1H), 4.50 - 4.32 (m, 1H), 3.00 - 2.83 (m, 2H), 2.42 - 2.25 (m, 2H), 2.18 - 1.81 (m, 6H), 0.98 (t, J = 7.2 Hz, 3H)。
ES/MS 689.9 (M+H+
)。實例 6
程序6 4-(4-(((8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-3- 氰基喹啉 -6- 基 ) 胺基 )(3- 吡啶基 ) 甲基 )-1H-1,2,3- 三唑 -1- 基 ) 哌啶 -1- 甲酸 苯甲 酯
:在回流下加熱6-胺基-8-氯-4-((4-氯-3-氟苯基)胺基)喹啉-3-甲腈(159 mg,0.51 mmol)、醛(176 mg,0.51 mmol)及pTSA (9.6 mg,0.05 mmol)於甲苯(12 mL)中之懸浮液(配備有希克曼蒸餾頭(Hickman still)之50 mL RBF)。在4小時之後,在減壓下移除溶劑。將固體溶解於甲基-THF中,且在-10℃下逐滴添加溴化3-吡啶基鎂(2.03 mmol;8.1 mL,0.25-M Me-THF)。在130分鐘之後,用飽和NH4
Cl (3 mL)使反應淬滅。分離各層,且用EtOAc (15 mL)萃取水相。用鹽水(5 mL)洗滌經合併之有機層,經硫酸鈉乾燥,且濃縮。對殘餘物進行矽膠急驟層析(溶離劑:EtOAc/己烷)。合併含有產物之溶離份,且移除溶劑,得到產物。8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-6-(((1-(1- 乙基哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )( 吡啶 -3- 基 ) 甲基 ) 胺基 ) 喹啉 -3- 甲腈
:在氫氣氛圍下攪拌含4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(3-吡啶基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸苯甲酯(57 mg,0.079 mmol)、乙醛(34.7 mg,0.79 mmol)及Pd -C (25 mg,10%)之EtOH (3 mL)/EtOAc (2 mL)。在43小時之後,過濾反應物,且在真空中移除揮發物,且經由RP-HPLC (溶離劑:水/MeCN 0.1%TFA)來純化粗物質,得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, DMSO-d 6
) δ 9.41 (s, 1H), 8.74 (d,J
= 2.2 Hz, 1H), 8.54 (dd,J
= 5.0, 1.5 Hz, 1H), 8.41 (s, 1H), 8.13 (s, 1H), 8.00 (d,J
= 8.0 Hz, 1H), 7.67 (d,J
= 2.2 Hz, 1H), 7.49 (tt,J
= 6.8, 3.7 Hz, 3H), 7.42 (t,J
= 9.0 Hz, 1H), 7.26 (d,J
= 2.5 Hz, 1H), 7.25 - 7.21 (m, 1H), 6.20 (d,J
= 7.8 Hz, 2H), 4.79 - 4.69 (m, 1H), 3.61 (d,J
= 12.4 Hz, 2H), 3.26 - 2.98 (m, 4H), 2.34 (d,J
= 13.9 Hz, 2H), 2.15 (q,J
= 12.5, 11.6 Hz, 2H), 1.22 (t,J
= 7.3 Hz, 3H)。
ES/MS: 616.1 (M+H+
)。
在已進行C6N-
烷基化之後,可經由恰當手段(例如使用對掌性固定相之層析、結晶學)將此序列之化合物分離成相應立體異構體。
在無反應搭配物存在下移除保護基得到對應的未烷基化之胺衍生物。實例 7
程序7 8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 )-6-(( 吡啶 -2- 基 ( 吡啶 -3- 基 ) 甲基 ) 胺基 ) 喹啉 -3- 甲腈 :
向6-胺基-8-氯-4-((4-氯-3-氟苯基)胺基)喹啉-3-甲腈(50 mg,0.144 mmol)及吡啶-2-基(吡啶-3-基)甲酮(27 mg,0.144 mmol)於DCM (1 mL)中之懸浮液中添加三乙胺(35 mg,0.346 mmol),繼而添加含TiCl4
之DCM (0.086 mmol/0.086 mL)。將反應物在室溫下攪拌隔夜。用MeOH (2 mL)對其進行稀釋,且添加硼氫化鈉(16 mg,0.432 mmol)。攪拌反應物2小時,隨後用水稀釋,且用1 M NaOH處理,直至達至pH約13為止。經由過濾移除固體,且用DCM洗滌。經硫酸鈉乾燥經合併之有機層,過濾,且濃縮。藉由矽膠層析(溶離劑:EtOAc/己烷)來純化產物,在自水/MeCN凍乾之後得到產物。1
H NMR (400 MHz, DMSO-d6) δ 9.42 (s, 1H), 8.66 (dd, J = 2.4, 0.8 Hz, 1H), 8.56 (ddd, J = 4.9, 1.8, 0.9 Hz, 1H), 8.47 - 8.39 (m, 2H), 7.87 - 7.75 (m, 3H), 7.61 - 7.19 (m, 8H), 6.08 (d, J = 8.7 Hz, 1H)。
ES/MS: 515.1 (M+H+
)。實例 8
程序8 8- 氯 -4-((5,6- 二氟吡啶 -3- 基 ) 胺基 )-6- 硝基喹啉 -3- 甲腈
:將含4,8-二氯-6-硝基喹啉-3-甲腈(1.4 g,5.22 mmol)、2,6-二氟吡啶-3-胺(755 mg,5.74 mmol)及吡啶鹽酸鹽(1.8 g,15.6 mmol)之異丙醇(40 mL)在70℃下加熱隔夜。使反應物冷卻至室溫。添加水,且經由過濾收集所得沈澱。粗產物不經進一步純化即用於下一步驟中。ES/MS 362.0 (M+H+
)。6- 胺基 -8- 氯 -4-((5,6- 二氟吡啶 -3- 基 ) 胺基 ) 喹啉 -3- 甲腈
:由8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-硝基喹啉-3-甲腈經由通用程序1之步驟2製造。ES/MS 332.0 (M+H+
)。
(S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈):由6-胺基-8-氯-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈藉由通用程序1之步驟3製造。ES/MS 532.1 (M+H+
)。實例 9
程序9 (S)-6-(( 苯并 [d] 噻唑 -7- 基 (1- 環丙基 -1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3,8- 二甲腈 ()
:在微波小瓶中向(S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-溴-4-(新戊基胺基)喹啉-3-甲腈(38 mg,0.064 mmol)及CuCN (41 mg,0.46 mmol)中添加DMF (2 mL)。在微波中將小瓶加熱至200℃,持續15分鐘,且使其冷卻至室溫。將反應混合物傾入水(4 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, 甲醇-d 4
) δ 9.20 (s, 1H), 8.34 (s, 1H), 8.05 (dd,J
= 7.3, 1.9 Hz, 1H), 7.86 (d,J
= 2.5 Hz, 1H), 7.82 (s, 1H), 7.64 - 7.54 (m, 2H), 7.16 (d,J
= 2.6 Hz, 1H), 6.34 (s, 1H), 3.97 (d,J
= 13.7 Hz, 1H), 3.91 - 3.80 (m, 1H), 3.49 (d,J
= 13.7 Hz, 1H), 1.23 - 1.07 (m, 4H), 0.81 (s, 9H). ES/MS 534.1 (M+H+
)。8 氰基之替代性引入 6- 胺基 -4-( 新戊基胺基 ) 喹啉 -3,8- 二甲腈 :
向6-胺基-8-溴-4-(新戊基胺基)喹啉-3-甲腈(500 mg,1.5 mmol)於NMP (20 mL)中之溶液中添加固體Zn(CN)2
(211 mg,1.8 mmol)及Pd(PPh)4
(35 mg,0.03 mmol)。藉由使氬氣鼓泡通過來使所得混合物脫氣5分鐘。密封反應容器,隨後加熱至120℃,持續16小時。使反應混合物冷卻,隨後直接加載至二氧化矽管柱上,得到純腈。ES/MS 280.3 (M+H+
)。
根據本文檔中所概述之程序進一步細加工(elaboration)成最終化合物實例 10
程序10 (S)-6-(( 苯并 [d] 噻唑 -6- 基 (1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 ) -8- 氯 - 4-((3- 氯 - 4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈
:將(R)-6-((1-(苯并[d]噻唑-6-基)丙-2-炔-1-基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(40.0 mg,0.077 mmol)溶解於THF (2 mL)中。添加噻吩-2-甲酸銅(I) (4.4 mg,0.023 mmol)及特戊酸疊氮基甲氧酯(0.018 mL,0.12 mmol),且將所得溶液在室溫下攪拌30分鐘。將反應內容物傾入飽和NaHCO3
水溶液(5 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機層,經MgSO4
乾燥,且濃縮。隨後將所得粗殘餘物溶解於MeOH (2 mL)中。添加NaOH (1.0 M水溶液,0.17 mL,0.17 mmol),且使反應物在室溫下攪拌30分鐘。隨後添加HCl (1.0 M水溶液,0.17 mL,0.17 mmol),將所得溶液傾入水(5 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機層,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來經純化所得粗殘餘物,得到呈三氟乙酸鹽形式之產物。
ES/MS 561.0 (M+H+
)。實例 11
程序118- 氯 -6-[[(S)-(1- 環丙基三唑 -4- 基 )- 氘 -(6- 氟吡啶 -3- 基 ) 甲基 ] 胺基 ]-4-[[(1R)-3- 氟 -1- 苯基丙基 ] 胺基 ] 喹啉 -3- 甲腈 (R)-8- 氯 -4-((3- 羥基 -1- 苯基丙基 ) 胺基 )-6- 硝基喹啉 -3- 甲腈 :
在微波條件下將含4,8-二氯-6-硝基喹啉-3-甲腈(400 mg,1.49 mmol)、(R)-3-胺基-3-苯基丙-1-醇(270.76 mg,1.79 mmol)之異丙醇(1.5 mL)在150℃下加熱45分鐘。使反應物冷卻至室溫。添加水及Et2
O。用Et2
O萃取水層,且經硫酸鈉乾燥經合併之有機層。過濾及蒸發溶劑得到粗產物,其不經進一步純化即用於下一步驟中。
ES/MS 383.1 (M+H+
)。(R)-8- 氯 -4-((3- 氟 -1- 苯基丙基 ) 胺基 )-6- 硝基喹啉 -3- 甲腈 :
在室溫下用deoxofluor® (0.6 mL)處理(R)-8-氯-4-((3-羥基-1-苯基丙基)胺基)-6-硝基喹啉-3-甲腈(100 mg,0.26 mmol)持續16小時。使反應混合物在冰浴中冷卻,小心地用飽和碳酸氫鈉溶液淬滅,隨後用乙酸乙酯萃取。經硫酸鈉乾燥有機相,過濾且濃縮,得到粗產物(115 mg),其不經進一步純化即使用。
ES/MS 385.1 (M+H+
)。(R)-6- 胺基 -8- 氯 -4-((3- 氟 -1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈 :
將(R)-8-氯-4-((3-氟-1-苯基丙基)胺基)-6-硝基喹啉-3-甲腈(115 mg,0.3 mmol)、二水合氯化鈣(66 mg,0.45 mmol)及鐵粉(83 mg,1.49 mmol)在乙醇(3 mL)/水(0.3 mL)中在60℃下加熱12小時。使反應物冷卻至室溫,且經由過濾移除固體。用EtOAc洗滌固體,且用碳酸氫鈉水溶液、鹽水洗滌經合併之有機層,且經硫酸鈉乾燥。過濾及蒸發所有揮發物得到產物。
ES/MS 355.0 (M+H+
)。8-
氯-6-(((S)-(1-
環丙基-1H-1,2,3-
三唑-4-基)(4- 氟苯基 )
甲基)胺基)-4-(((R)-3-
氟-1-
苯基丙基) 胺基 )
喹啉-3-
甲腈:在MeOH (2.0 mL)中對(R)-6-胺基-8-氯-4-((3-氟-1-苯基丙基)胺基)喹啉-3-甲腈(50 mg,0.14 mmol)、CuI (1.4 mg,0.05當量)及2,6-雙((4S,5R)-4,5-二苯基-4,5-二氫噁唑-2-基)吡啶[噁唑啉配位體] (4.4 mg,0.06當量)進行音波處理持續約1分鐘。添加乙酸炔酯(68 mg,0.35 mmol)及二-異丙基乙胺(22 mg,0.17 mmol),且攪拌反應物隔夜。添加環丙基疊氮化物(16 mg),且將反應物在室溫下再攪拌16小時。在真空中移除溶劑,且藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗物質,得到呈三氟乙酸鹽形式之產物。
ES/MS 572.0 (M+H+
)。實例 12
程序12 (S)-8- 氯 - 4-((3- 氯 - 4- 氟苯基 ) 胺基 )-6-(( 吲哚啉 -4- 基 (1- 異丙基 -1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 ) 喹啉 -3- 甲腈
:將(S)-4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)吲哚啉-1-甲酸第三丁酯(45 mg,0.065 mmol)溶解於DCM及三氟乙酸中,且在室溫下攪拌。在30分鐘之後,將反應混合物濃縮至乾燥,且藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化殘餘物,得到呈三氟乙酸鹽形式之產物。
ES/MS 586.9 (M+H+
)。實例 13
程序13 (S)-6-(((1-(1-( 第三丁基 ) 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )(2- 乙基異吲哚啉 -4- 基 ) 甲基 ) 胺基 )-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈
:在室溫下將(S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(80.0 mg,0.12 mmol)溶解於MeOH (3 mL)及AcOH (1 mL)中。隨後添加乙醛(0.066 mL,1.17 mmol)及聚合物結合之PS-CNBH3
(467 mg,1.17 mmol),且在室溫下攪拌反應物。在1小時之後,添加額外乙醛(0.066 mL,1.17 mmol)及聚合物結合之PS-CNBH3
(467 mg,1.17 mmol)。在再1小時之後,經由真空過濾移除PS-CNBH3
,將濾液濃縮至乾燥。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來經純化所得粗殘餘物,得到呈三氟乙酸鹽形式之產物。
ES/MS 712.1 (M+H+
).實例 14
程序14 (S)-6-(((1-(1-( 第三丁基 ) 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )(2-( 氧雜環丁 -3- 基 ) 異吲哚啉 -4- 基 ) 甲基 ) 胺基 )-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈
:將(S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(15 mg,0.022 mmol)溶解於THF及DCE之1:1混合物中,其後添加氧雜環丁酮(0.007 mL,0.11 mmol)及三乙醯氧基硼氫化鈉(23.2 mg,0.11 mmol)。在1.5小時之後,將反應混合物傾入飽和NaHCO3
水溶液中,且用EtOAc萃取。用鹽水洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。
ES/MS 740.0 (M+H+
)。實例 15
程序15 (S)-6-(((1-(1-( 第三丁基 ) 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )(2-(2- 羥基乙醯基 ) 異吲哚啉 -4- 基 ) 甲基 ) 胺基 )-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈
:將(S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(10.0 mg,0.015 mmol)溶解於DMF (1 mL)中,其後在室溫下添加乙醇酸(5.6 mg,0.073 mmol)、二異丙基乙胺(0.008 mL,0.044 mmol)及HATU (7.1 mg,0.022 mmL)。攪拌反應混合物20分鐘,此時將其傾入水(4 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。
ES/MS 742.1 (M+H+
)。實例 16
程序16 8- 氯 -6-(((S)-(1-(1,1- 二氟 -2- 羥乙基 )-1H
-1,2,3- 三唑 -4- 基 )( 吡啶 -3- 基 ) 甲基 ) 胺基 )-4-(((R
)-1- 苯基丙基 ) 胺基 ) 喹啉 -3- 甲腈
向8-氯-4-(((R
)-1-苯基丙基)胺基)-6-(((R
)-1-(吡啶-3-基)丙-2-炔-1-基)胺基)喹啉-3-甲腈(130 mg,0.26 mmol)及噻吩-2-甲酸銅(I) (4.9 mg,0.026 mmol)於THF (2 mL)中之溶液中添加2-疊氮基-2,2-二氟乙酸乙酯(47 mg,0.29 mmol)。在1小時之後,在減壓下移除溶劑。使殘餘物溶於甲醇(6 mL)中,且向溶液中添加硼氫化鈉(19.5 mg,0.52 mmol)。在1小時之後,用水使反應物淬滅,且用乙酸乙酯(3 × 10 mL)萃取。用鹽水洗滌經合併之有機相,經硫酸鈉乾燥,且濃縮。對殘餘物進行急驟層析(0%-100% (含20%甲醇之乙酸乙酯)/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含兩滴三氟乙酸之甲醇/水中,且將其付諸含0.1 %三氟乙酸之乙腈/水溶離的預備之HPLC。合併含有產物之溶離份,且對其進行凍乾,得到8-氯-6-(((S
)-(1-(1,1-二氟-2-羥乙基)-1H
-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R
)-1-苯基丙基)胺基)喹啉-3-甲腈。1
H NMR (400 MHz, DMSO-d6) δ 8.92 - 8.83 (m, 1H), 8.67 (d, J = 9.5 Hz, 1H), 8.65 - 8.58 (m, 1H), 8.24 (d, J = 3.3 Hz, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.64 - 7.56 (m, 2H), 7.52 (d, J = 8.3 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.39 - 7.29 (m, 2H), 7.29 - 7.16 (m, 6H), 6.54 (d, J = 7.8 Hz, 1H), 5.47 (q, J = 7.6 Hz, 1H), 4.32 (t, J = 12.0 Hz, 2H), 2.11 (m, 1H), 2.04 - 1.83 (m, 1H), 0.93 (t, J = 7.3 Hz, 3H)。
ES/MS 575.1 (M + H+
)。實例 17
程序17(S)-6-(((1-(1-( 第三丁基 ) 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )(6- 異丙氧基吡啶 -3- 基 ) 甲基 ) 胺基 )-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈 :
在0℃下向i
PrOH (2 mL)中添加NaH (於礦物油中之60%分散液,26.6 mg,0.66 mmol)持續20分鐘。隨後向新形成之醇鹽中添加含(S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(22 mg,0.033 mmol)之DMF (0.5 mL)。移除冷浴,且將所得溶液加熱至70℃,持續1小時。由水(1 mL)使反應混合物淬滅,且用EtOAc (3 × 8 mL)萃取。隨後用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。實例 18
程序18(S)-6-(((1-(1-( 第三丁基 ) 哌啶 -4- 基 )-1H-1,2,3- 三唑 -4- 基 )( 四氫 -2H- 哌喃 -4- 基 ) 甲基 ) 胺基 )-8- 氯 -4-((3- 氯 -4- 氟苯基 ) 胺基 ) 喹啉 -3- 甲腈 :
合併(S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(3,6-二氫-2H-哌喃-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(20.0 mg,0.023 mmol)、10% Pd/C (2.5 mg,0.002 mmol)及EtOH (1.5 mL),且使H2
鼓泡通過反應混合物持續5分鐘。使反應混合物在1 atm H2
下攪拌隔夜,其後在用EtOAc及EtOH沖洗的情況下經由矽藻土對其進行過濾。隨後濃縮濾液,且藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化,得到呈三氟乙酸鹽形式之產物。實例 19
程序19(S)-6-(((1- 環丙基 -1H-1,2,3- 三唑 -4- 基 )(3- 側氧基異吲哚啉 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3,8- 二甲腈 :
在室溫下將(S)-6-(((2-乙醯基-3-側氧基異吲哚啉-4-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈(34 mg,0.059 mmol)溶解於MeOH (2 mL)中。添加NaOH (1.0 M水溶液,0.30 mL,0.30 mmol),且攪拌反應物30分鐘。添加HCl (1.0 M水溶液,0.30 mL,0.30 mmol),其後將反應混合物傾入水(3 mL)中,且用EtOAc (3 × 8 mL)萃取。用鹽水(5 mL)洗滌經合併之有機相,經MgSO4
乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。實例 20
程序208- 氯 -4-(3- 氯 -4- 氟苯胺基 )-6-[[(S)-[1-(1- 乙基哌啶 -4- 基 ) 三唑 -4- 基 ]-(1,3- 噻唑 -4- 基 ) 甲基 ] 胺基 ] 喹啉 -3- 甲腈
按如下用於最終步驟之條件製備標題化合物:將(S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈(74.31 mg,0.13 mmol)溶解於1.20 mL 3:1 2-甲基四氫呋喃:乙酸中,且用乙醛(10.52 µl,0.19 mmol)及PS-BH3
CN (聚苯乙烯負載之氰基硼氫化物,58 mg,2.28 mmol/g)處理。攪拌混合物隔夜。添加額外乙醛及0.1 mL甲醇,且反應在一小時內完成。過濾樹脂,且濃縮所得濾液,將其溶解於二氯甲烷中,用飽和碳酸氫鈉洗滌,經硫酸鈉乾燥,過濾,且濃縮。使用RP-HPLC (溶離劑:水/MeCN *0.1% TFA)純化得到呈三氟乙酸鹽形式之產物。1
H NMR (400 MHz, CD3
OD) δ 9.02 (m, 1H), 8.46 (s, 1H), 8.01 (m, 1H), 7.68 (m, 1H), 7.60 (m, 1H), 7.52 (m, 1H), 7.33 (m, 4H), 6.31 (s, 1H), 3.75 (m, 2H), 3.25 - 3.13 (m, 3H), 2.45 (m, 2H), 2.36 (m, 2H), 2.25 - 2.01 (m, 2H), 1.37 (m, 3H),
ES/MS 622.0 (M+H+
)實例 21
程序218- 氯 -4-(3- 氯 -4- 氟苯胺基 )-6-[[(S)-(1- 丙 -2- 基三唑 -4- 基 )-[5-( 吡咯啶 -1- 羰基 ) 吡啶 -3- 基 ] 甲基 ] 胺基 ] 喹啉 -3- 甲腈
使(S)-6-(((5-溴吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈(200 mg,0.32 mmol)、吡咯啶(580.6 mg,8.16 mmol)及二氯1,1-雙(二苯膦基)二茂鐵鈀(II)二氯甲烷(269.1 mg,0.32 mmol)於DMF (1.2 ml)中之混合物脫氣且用一氧化碳吹掃兩次,隨後在80℃下加熱5小時。使溶液冷卻,且將其傾入水中,隨後用乙酸乙酯萃取。經硫酸鈉乾燥經合併之有機層,過濾,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗產物,得到呈三氟乙酸鹽形式之產物。
ES/MS 644.1 (M+H+
)實例 22
程序22 6-(((S)-(6- 氟吡啶 -3- 基 )(1- 甲基 -1H-1,2,3- 三唑 -4- 基 ) 甲基 -d) 胺基 )-4-(((R)-1- 苯基丙基 ) 胺基 ) 喹啉 -3,8- 二甲腈
:向6-(((R)-(6-氟吡啶-3-基)(1-((三甲基矽烷基)甲基)-1H-1,2,3-三唑-4-基)氘甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈(150 毫克,0.25 mmol)於THF (5 mL)中之攪拌溶液中添加TBAF (0.38 mL,0.38 mmol)於THF中之1.0 M溶液。攪拌所得溶液2小時,隨後濃縮,得到粗物質。HPLC純化得到標題化合物。1
H NMR (400 MHz, DMSO-d6) δ 8.49 - 8.38 (m, 1H), 8.28 (d, J = 5.1 Hz, 1H), 8.13 - 8.02 (m, 2H), 7.84 (t, J = 2.4 Hz, 1H), 7.65 - 7.52 (m, 2H), 7.42 - 7.31 (m, 1H), 7.31 - 7.15 (m, 4H), 5.49 (q, J = 7.7 Hz, 1H), 4.04 (s, 3H), 3.20 - 3.11 (m, 1H), 2.20 - 2.05 (m, 1H), 2.05 - 1.85 (m, 1H), 1.63 - 1.50 (m, 1H), 1.37 - 1.20 (m, 1H), 0.99 - 0.83 (m, 3H)。
ES/MS 519.2 (M+H+
)。實例 23
程序23
將CuI (2.6 mg,0.014 mmol)及配位體(8.7 mg,0.017 mmol)懸浮於MeOH (1 mL)中,且在氬氣下音波處理5分鐘。添加剩餘MeOH,繼而在室溫下按以下順序添加乙酸酯(70.3 mg,0.36 mmol)及胺(80 mg,0.27 mmol)及DIPEA (43 mg,0.33 mmol)。在14小時之後,N-烷基化反應完成。蒸發及矽膠純化(溶離劑:EtOAc/己烷)得到95 mg N-烷基化產物。使物質溶於THF (2 mL)中。添加疊氮化物儲備液(1毫升/1當量)、Cu及CuSO4
。在室溫下攪拌1小時。用EtOAc稀釋,用NaHCO3
、鹽水洗滌,且經硫酸鈉乾燥。過濾、蒸發及經由RP-HPLC (溶離劑:水/MeCN *0.1 TFA)純化得到呈TFA鹽形式之產物。1
H NMR (400 MHz, 甲醇-d4) δ 8.79 (s, 1H), 8.49 (s, 1H), 8.00 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.26 (d, J = 2.3 Hz, 1H), 6.31 (s, 1H), 5.91 (t, J = 54.8 Hz, 1H), 4.09 (d, J = 13.9 Hz, 1H), 3.93 (d, J = 14.0 Hz, 1H), 2.56 (s, 3H), 1.50 (m, 4H), 1.05 (s, 9H)。實例 24 程序 24 :
在二甲基乙醯胺(1 mL)中組合SM (38 mg,0.05 mmol)、Zn (0.4 mg,0.007 mmol)、PddppfCl2
(0.8 mg,0.001 mmol)及Zn(CN)2
(7.1 mg,0.061 mmol),且脫氣2分鐘。在微波反應器中將混合物在200℃加熱20分鐘。過濾混合物,且經由RP-HPLC來純化。合併產物溶離份,且對其進行凍乾,得到呈TFA鹽形式之所要化合物。1
H NMR (400 MHz, 甲醇-d4) δ 8.78 (s, 1H), 8.37 (s, 1H), 8.02 (s, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 6.32 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 3.96 (d, J = 13.9 Hz, 1H), 3.80 (d, J = 13.9 Hz, 1H), 2.56 (s, 3H), 1.50 (m, 4H), 1.02 (s, 9H)。實例 25 程序 25 :
在二甲基乙醯胺(0.7 mL)中組合SM (0.04 g,0.06 mmol)、Zn粉末(0.006 g,0.09 mmol)、Pd(dppf)Cl2
(0.009 g,0.012 mmol)及Zn(CN)2
(0.021 g,0.18 mmol),且脫氣1分鐘。在微波反應器中將混合物在200℃加熱15分鐘。過濾混合物,且經由RP-HPLC來純化。合併產物溶離份,且對其進行凍乾,得到呈TFA鹽形式之所要化合物。1
H NMR (400 MHz, 甲醇-d4) δ 9.61 (s, 1H), 8.69 (d, J = 6.1 Hz, 1H), 8.33 (s, 1H), 8.23 (s, 1H), 8.17 (d, J = 7.6 Hz, 1H), 8.16 (d, J = 6.1 Hz, 1H), 8.02 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.91 (s, 1H), 3.82 (d, J = 13.8 Hz, 1H), 3.42 (d, J = 13.7 Hz, 1H), 1.79 - 1.55 (m, 4H), 0.62 (s, 9H)。實例 26 程序 26 :
向(S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈(TFA鹽,24 mg,0.04 mmol)中添加二甲基胺(於MeOH中之2 M溶液,0.9 mL)。將溶液加熱至100℃ (外部溫度,µW),持續8小時。濃縮所得溶液,經由製備型HPLC (Gemini管柱,10%-42% MeCN/H2
O/0.1% TFA)來純化,且凍乾,得到呈對應TFA鹽形式之產物。1
H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 8.06 (dd, J = 5.4, 1.8 Hz, 1H), 7.92 (s, 1H), 7.81 (dd, J = 7.6, 1.8 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 7.02 (dd, J = 7.6, 5.4 Hz, 1H), 4.10 (d, J = 14.0 Hz, 1H), 3.78 (ddd, J = 11.4, 7.1, 4.2 Hz, 1H), 3.63 (d, J = 14.0 Hz, 1H), 2.97 (s, 6H), 1.12 - 1.02 (m, 4H), 0.91 (s, 9H)。實例 27 程序 27 :
在MeOH (10 mL)中對碘化銅(I) (16.5 mg,0.09 mmol)及雙噁唑啉配位體(54.2 mg)進行音波處理持續5分鐘。使混合物冷卻至0℃。添加乙酸炔酯(687 mg,3.3 mmol)於MeOH (7 mL)中之溶液,繼而添加喹啉(500 mg,1.73 mmol)及二-異丙基乙胺(268.5 mg,2.08 mmol)。繼續在0℃下攪拌。在起始物質消耗之後,使反應體積減小,且經由矽膠層析(溶離劑:EtOAc/己烷)來純化粗物質,得到產物。1
H NMR (400 MHz, 乙腈-d3) δ 8.34 (s, 1H), 8.14 (t, J = 8.2 Hz, 1H), 7.43 (d, J = 2.3 Hz, 1H), 6.95 - 6.82 (m, 2H), 5.98 (t, J = 6.4 Hz, 1H), 5.64 (dd, J = 7.1, 2.2 Hz, 1H), 5.52 (d, J = 7.2 Hz, 1H), 3.81 (dd, J = 13.4, 6.7 Hz, 1H), 3.67 (dd, J = 13.4, 6.0 Hz, 1H), 2.88 (d, J = 2.2 Hz, 1H), 2.56 (s, 3H), 2.23 (s, 1H), 1.01 (s, 9H)。
ES/MS m/z: 436.2。
將炔起始物質(1.6 g,3.67 mmol)溶解於MeTHF (16 mL)中,且添加MTBE中之疊氮化物溶液(0.5 M,7.34 mL)及噻吩甲酸銅(I) (24 mg,0.18 mmol),且繼續在室溫下攪拌。在SM消耗之後,用EtOAc稀釋反應物,且用碳酸氫鈉水溶液洗滌,且經硫酸鈉乾燥。過濾及蒸發溶劑得到粗物質,其經由矽膠層析(溶離劑. EtOAc/己烷)來純化,得到產物。1
H NMR (400 MHz, 氯仿-d) δ 8.45 (s, 1H), 7.93 (t, J = 8.1 Hz, 1H), 7.41 (s, 1H), 7.35 (d, J = 2.3 Hz, 1H), 6.80 (dd, J = 8.4, 3.2 Hz, 1H), 6.25 (s, 1H), 5.93 (s, 1H), 5.91 (t, J = 56.0 Hz, 1H), 5.27 (s, 1H), 3.57 (m, 2H), 2.58 (s, 3H), 1.55 - 1.50 (m, 4H), 0.94 (s, 9H)。
ES/MS m/z: 569.6。實例 28 程序 28 : (S
)-8- 氯 -6-(((1- 環丙基 -5- 碘 -1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
向(R
)-8-氯-6-((1-(6-氟-2-甲基吡啶-3-基)丙-2-炔-1-基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(394 mg,0.906 mmol)、環丙基疊氮化物79.1 mg 0.906 mmol)及三乙胺(151.6 μL,1.09 mmol)於四氫呋喃(15 mL)中之溶液中添加碘化銅(I) (172.5 mg,0.906 mmol)及一氯化碘(147 mg,0.906 mmol)。在16小時之後,用乙酸乙酯(50 mL)稀釋反應物,且用水(25 mL)及鹽水(25 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。將物質與乙酸乙酯(5 mL)混合,且藉由過濾來分離固體,得到(S
)-8-氯-6-(((1-環丙基-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 29 程序 29 : (S
)-8- 氯 -6-(((1,5- 二環丙基 -1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
在微波小瓶中向(S
)-8-氯-6-(((1-環丙基-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(100 mg,0.155 mmol)、環丙基酸(20 mg,0.223 mmol)、肆(三苯基膦)鈀(0) (35.8 mg,0.031 mmol)及碳酸鉀(42.8 mg,0.310 mmol)中添加1,4-二噁烷(4.0 mL)及水(0.5 mL)。在微波反應器中將反應物在130℃下加熱20分鐘。用乙酸乙酯(10 mL)稀釋混合物,且用鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含2滴三氟乙酸之甲醇(1 mL)及水(0.5 mL)中,且將其付諸製備型HPLC。合併潔淨溶離份,且對其進行凍乾,得到(S
)-8-氯-6-(((1,5-二環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 30 程序 30 : (S
)-8- 氯 -6-(((1- 環丙基 -5- 氟 -1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
在微波小瓶中向(S
)-8-氯-6-(((1-環丙基-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(50 mg,0.078 mmol)及氟化鉀(22.5 mg,0.388 mmol)中添加乙腈(1.0 mL)及水(1.0 mL)。密封小瓶,且在微波反應器中將反應物在180℃下加熱12分鐘。用乙酸乙酯(10 mL)稀釋反應物,且用鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含2滴三氟乙酸之甲醇(1 mL)及水(0.5 mL)中,且將其付諸製備型HPLC。合併潔淨溶離份,且對其進行凍乾,得到(S
)-8-氯-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 31 程序 31 : (S
)-8- 氯 -6-(((1- 環丙基 -5- 甲氧基 -1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
向(S
)-8-氯-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(42.6 mg,0.079 mmol)於四氫呋喃(2.0 mL)中之溶液中添加甲醇鈉(26 μL,0.119 mmol,THF中25%純)。將溶液在90℃下加熱30分鐘,且用2滴乙酸使反應淬滅。用乙酸乙酯(15 mL)稀釋溶液,且用飽和碳酸氫鈉(5 mL)及鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含2滴三氟乙酸之甲醇(1 mL)及水(0.5 mL)中,且將其付諸製備型HPLC。合併含有產物之潔淨溶離份,且對其進行凍乾,得到(S
)-8-氯-6-(((1-環丙基-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 32 程序 32
:
將醛(223 mg,1.4 mmol)溶解於乙腈(8 mL)中。添加三乙胺(0.29 mL,2.1 mmol)及DMAP (34 mg,0.28 mmol),繼而添加Boc2
O (365 mg,1.7 mmol),且攪拌所得混合物2分鐘。在完成後,直接濃縮反應內容物,隨後經由矽膠層析(EtOAc/己烷)來純化,得到產物。
使新形成之物質溶於THF (15 mL)中,且使其達到0℃。逐滴添加溴化乙炔基鎂(於THF中之0.5 M溶液,3.7 mL,1.8 mmol),且攪拌所得溶液30分鐘,此時添加乙酸酐(0.29 mL,3.1 mmol),且歷經1小時使反應內容物升溫至室溫。藉由添加飽和NH4
Cl水溶液來使反應淬滅,且用EtOAc萃取。用鹽水洗滌有機層,經硫酸鎂乾燥,過濾且濃縮,得到粗乙酸炔丙酯,其不經進一步純化即使用。
將CuI (6.6 mg,0.035 mmol)及配位體(22 mg,0.042 mmol)懸浮於MeOH (5 mL)中,且在氬氣下進行音波處理持續5分鐘。在室溫下按以下順序添加呈MeOH (2 mL)中溶液形式之乙酸酯(136 mg,0.42 mmol)、胺(100 mg,0.35 mmol)及DIPEA (54 mg,0.42 mmol)。在2小時之後,直接濃縮反應混合物,且在矽膠(EtOAc/己烷)上純化,得到烷基化產物。
使物質溶於THF (2 mL)中。添加疊氮化物儲備液(1毫升/1當量)、Cu及CuSO4,且將所得混合物在室溫下攪拌30分鐘。用EtOAc稀釋,用水、鹽水洗滌,且經硫酸鎂乾燥。過濾及濃縮得到粗產物,隨後將其在1 mL 1:1 DCM:三氟乙酸混合物中攪拌30分鐘。藉由旋轉蒸發來移除DCM及TFA,其後用RP-HPLC (溶離劑:水/MeCN *0.1 TFA)來純化粗殘餘物,得到呈TFA鹽形式之產物。實例 33 程序 33 :
在室溫下用DCM中之疊氮化物溶液(25重量%,80 mg,0.138 mmol)及噻吩甲酸銅(I) (1 mg)處理炔起始物質(50 mg,0.115 mmol)於氘化甲醇(CD3
OD,2 mL)中之溶液。在1小時之後,用EtOAc稀釋反應物,且用碳酸氫鈉水溶液洗滌,且經硫酸鈉乾燥。過濾及蒸發溶劑得到粗物質,其經由逆相HPLC來純化,得到潔淨產物。1
H NMR (400 MHz, 乙腈-d3) δ 8.44 (s, 1H), 7.82 (t, J = 8.2 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 6.84 (dd, J = 8.4, 3.2 Hz, 1H), 6.78 (s, 1H), 6.17 (s, 1H), 3.83 (m, 2H), 1.78 - 1.65 (m, 2H), 1.65 (m, 2H), 0.95 (s, 9H)。
ES/MS m/z: 588.31。實例 34 程序 34
將(S)-8-碘-4-(新戊基胺基)-6-((喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈(61 mg,0.09 mmol)及氰化亞銅(23.53 mg,0.26 mmol)於DMF (2 mL)中之混合物於微波中在135℃下加熱15分鐘。用Si-硫醇處理溶液,過濾,且藉由逆相HPLC來純化,得到呈雙三氟乙酸鹽形式之(S)-4-(新戊基胺基)-6-((喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈。1
H NMR (400 MHz, 甲醇-d4) δ 9.11 (s, 1H), 9.04 (d, J = 8.7 Hz, 1H), 8.30 (s, 1H), 8.21 (s, 1H), 8.18 - 8.10 (m, 1H), 7.99 - 7.90 (m, 1H), 7.90 - 7.82 (m, 2H), 7.80 (s, 1H), 7.14 - 7.08 (m, 1H), 6.94 (s, 1H), 3.79 (d, J = 13.8 Hz, 1H), 3.48 (d, J = 13.8 Hz, 1H), 1.75 - 1.56 (m, 4H), 0.66 (s, 9H)。
ES/MS m/z: 596.35。實例 35 程序 35 :
向(S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲酸(25 mg,0.044 mmol)、99%六氟磷酸2-(7-氮雜-1H-苯并三唑-1-基)-1,1,3,3-四甲(HATU) (17.27 mg,0.05 mmol)及2 M二甲基胺溶液(44.4 µl,0.053 mmol)於二甲基甲醯胺(1 mL)中之混合物中添加N-乙基二異丙胺(15.47 µl,0.09 mmol)。在3小時之後,在減壓下移除一半溶劑。用甲醇(0.75 mL)、水(0.5 mL)及TFA (50 μL)稀釋溶液。將此溶液付製備型HPLC。合併含有產物之更潔淨溶離份,且對其進行凍乾,得到所要化合物。使經凍乾之固體溶於甲醇(0.5 mL)中,且在甲醇沖洗(5 mL)的情況下使其穿過碳酸酯樹脂。在減壓下移除溶劑,且使殘餘物溶於含TFA (0.02 mL)之ACN (1 mL)及水(1 mL)中,且對其進行凍乾,得到(S
)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H
-1,2,3-三唑-1-基)-N, N-二甲基環丙烷-1-甲醯胺。實例 36 程序 36 :
在微波反應器中將(S
)-8-氯-6-(((1-環丙基-5-碘-1H-
1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(33 mg,0.051 mmol)、氰化銅(I) (13.7 mg,0.15 mmol)於二甲基甲醯胺(1 mL)中之溶液在200℃下加熱20分鐘。用乙酸乙酯(10 mL)稀釋混合物,且用5%氯化鋰(2 × 5 mL)及鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100% EtOAc/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含2滴TFA之水(0.5 ml)及甲醇(1 mL)中,且將其付諸製備型HPLC。合併潔淨溶離份,且對其進行凍乾,得到(S
)-8-氯-6-(((5-氰基-1-環丙基-1H
-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 37 程序 37 :
用氬氣使(S
)-3-(((3-氰基-8-碘-4-(新戊基胺基)喹啉-6-基)胺基)(1-環丙基-1H
-1,2,3-三唑-4-基)甲基)-4,7-二氫噻吩并[2,3-c]吡啶-6(5H
)-甲酸第三丁酯(60.3 mg,0.082 mmol)、肆(三苯基膦)鈀(0) (7.55 mg,0.01 mmol)及氰化鋅(23.96 mg,0.20 mmol)之溶液脫氣10分鐘,在密封小瓶中在100℃下加熱混合物。在36小時之後,用乙酸乙酯(20 ml)稀釋反應物,且用5%氯化鋰(2 × 5 mL)及鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100% (20%甲醇/乙酸乙酯)/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑,得到(S
)-3-((1-環丙基-1H
-1,2,3-三唑-4-基)((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-4,7-二氫噻吩并[2,3-c]吡啶-6(5H
)-甲酸第三丁酯。
向(S
)-3-((1-環丙基-1H
-1,2,3-三唑-4-基)((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-4,7-二氫噻吩并[2,3-c]吡啶-6(5H
)-甲酸第三丁酯(50.2 mg,0.079 mmol)於硝基甲烷(5 mL)中之溶液中添加溴化鋅(88.6 mg,0.39 mmol)。在50分鐘之後,在減壓下移除溶劑。將殘餘物分配於乙酸乙酯(20 mL)與飽和碳酸氫鈉(10 mL)之間。形成固體,藉由過濾來對其進行移除。用飽和碳酸氫鈉(10 mL)及鹽水(10 mL)來洗滌有機相。經硫酸鈉乾燥有機相,且在減壓下移除溶劑,得到(S
)-6-(((1-環丙基-1H
-1,2,3-三唑-4-基)(4,5,6,7-四氫噻吩并[2,3-c
]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈。
向(S
)-6-(((1-環丙基-1H
-1,2,3-三唑-4-基)(4,5,6,7-四氫噻吩并[2,3-c
]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈(32.5 mg,0.060 mmol)及三乙醯氧基硼氫化鈉(128.1 mg,0.60 mmol)於四氫呋喃(2 mL)、二氯乙烷(2 mL)中之混合物中添加3-氧雜環丁酮(38.75 µl,0.6 mmol),且在40℃下加熱16小時。用乙酸乙酯(10 mL)稀釋混合物,且用飽和碳酸氫鈉(2 × 5 mL)及鹽水(5 mL)洗滌。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-100% (含20%甲醇之乙酸乙酯)/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於含2滴TFA之甲醇(0.5 mL)/水(0.5 mL)中,且將其付諸製備型HPLC(用0%-100%含0.05%三氟乙酸之乙腈與水溶離)。合併潔淨溶離份,且對其進行凍乾,得到(S
)-6-(((1-環丙基-1H
-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c
]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈。實例 38 程序 38 : (S)-6-(( 苯并 [d] 噻唑 -7- 基 (1- 環丙基 -1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 第三丁氧基胺基 ) 喹啉 -3,8- 二甲腈
:向含(S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(第三丁氧基胺基)-8-碘代喹啉-3-甲腈(59 mg,0.093 mmol)之
N-甲基-2-吡咯啶酮(1 mL)中添加氰化鋅(27 mg,0.232 mmol)及肆(三苯基膦)鈀(9 mg,0.007 mmol)。用氮氣使反應混合物脫氣5分鐘,隨後在100℃下攪拌隔夜。隨後使反應物達到室溫,且用水及EtOAc稀釋。用EtOAc再一次萃取水層。用水、鹽水洗滌經合併之有機物,乾燥(Na2
SO4
),且濃縮,得到粗產物,其藉由HPLC (溶離劑:水/MeCN *0.1% TFA)來進行純化,得到標題產物。ES/MS 536.20 (M+H+
)。實例 39 程序 39 : (S)-6-(( 苯并 [d] 噻唑 -7- 基 (1-(1-( 三氟甲基 ) 環丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-8-( 甲基磺醯基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:向(S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-溴-4-(新戊基胺基)喹啉-3-甲腈(31 mg,0.047 mmol)、(L)-脯氨酸(1.1 mg,0.009 mmol)、Cu(I)I (1 mg,0.005 mmol)、甲基磺酸鈉(5.8 mg,0.057 mmol)及Cs2
CO3
(15 mg,0.047 mmol)中添加DMSO (0.8 mL)。將
反應混合物置放在氮氣氛圍下,
在110℃下攪拌隔夜。隨後使反應物達到室溫,且用水及EtOAc稀釋。用EtOAc再一次萃取水層。用水、鹽水洗滌經合併之有機物,乾燥(Na2
SO4
),且濃縮,得到粗產物,其藉由HPLC (溶離劑:水/MeCN *0.1% TFA)來進行純化,得到標題產物。ES/MS 655.7 (M+H+
)。實例 40 程序 40 : 6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(1-(1-( 三氟甲基 ) 環丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-8-(3- 羥基 -3- 甲基丁 -1- 炔 -1- 基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:將6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-碘-4-(新戊基胺基)喹啉-3-甲腈(30 mg,0.044 mmol)、碘化銅(0.84 mg,0.004 mg)及2-甲基-3-丁炔-2-醇(18.6 mg,0.22 mmol)溶解於Me-THF中。隨後向混合物中添加雙(三苯膦)二氯化鈀(II)(3.1 mg,0.004 mmol),繼而添加二乙胺(0.05 ml,0.44 mmol)。將反應物加熱至80℃,持續一小時,隨後用EtOAc及鹽水稀釋,保留有機層,經硫酸鈉乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。實例 41 程序 41 : (S)-8- 氯 -6-(((1-(1-( 二氟甲基 ) 環丙基 )-1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-5- 氟 -4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:將(S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(100 mg,0.18 mmol)溶解於ACN中。向攪拌混合物中添加Selectfluor (31.8 mg,0.176 mmol)。在20分鐘之後中止反應,用EtOAc及水稀釋。經硫酸鈉乾燥有機層,且濃縮。藉由矽膠層析(溶離劑:EtOAc/己烷)來純化產物,在自水/MeCN凍乾之後得到產物。實例 42 程序 42 : (S
)-5- 溴 -8- 氯 -6-(((1-(1-( 二氟甲基 ) 環丙基 )-5- 氟 -1H
-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:在0℃下向(S
)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H
-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(26 mg,0.044 mmol)於乙腈(1 mL)中之溶液中添加N
-溴代丁二醯亞胺(7.8 mg,0.044 mmol)在於室溫下24小時之後,
添加三氟乙酸(4滴),且用水稀釋混合物。將黃色溶液付諸製備型HPLC。合併含有產物之溶離份,且對溶劑進行減壓,得到呈TFA鹽形式之(S
)-5-溴-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H
-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。實例 43 程序 43 : 1- 疊氮基雙環 [1.1.1] 戊烷
:歷經1分鐘向雙環[1.1.1]戊-1-胺鹽酸鹽(150 mg,1.25 mmol)及1,8-二氮雜雙環[5.4.0]十一-7-烯(420 mg,4.2 mmol)於乙腈(3 mL)中之溶液中逐滴添加六氟磷酸2-疊氮基-1,3-二甲基咪唑鎓(429 mg,1.5 mmol)於乙腈(2 mL)中之溶液。在於室溫下16小時之後,將反應物在40℃下加熱3小時。假設反應完成,且按原樣添加至點擊反應中。 (S
)-6-(((1-( 雙環 [1.1.1] 戊 -1- 基 )-1H
-1,2,3- 三唑 -4- 基 )(2- 甲基 -1- 側氧基 -1,2- 二氫異喹啉 -5- 基 ) 甲基 ) 胺基 )-8- 氯 -4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:組合2-甲基四氫呋喃(12 mL)、銅粉末(394 mg,6.2 mmol)及(R
)-8-氯-6-((1-(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)丙-2-炔-1-基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(500 mg,1.03 mmol)。添加飽和硫酸銅(II) (0.6 mL),繼而添加乙酸(236 μL,4.13 mmol)。添加1-疊氮基雙環[1.1.1]戊烷(137 mg,1.25 mmol)於乙腈(5 mL)中之溶液(來自上文之反應混合物)。在1小時之後,
藉由過濾移除固體。用乙酸乙酯(50 mL)及飽和氯化銨(50 mL)分配混合物。形成具有淺色固體之乳液。經由矽藻土過濾移除固體。用飽和氯化銨(50 mL)、飽和碳酸氫鈉(4 × 50 mL)及鹽水(50 mL)洗滌有機相。經硫酸鈉乾燥有機相,且在減壓下移除溶劑。對殘餘物進行急驟層析(0%-70%乙酸乙酯/己烷)。合併含有產物之溶離份,且在減壓下移除溶劑。使殘餘物溶於乙腈(15 mL)及水(15 mL)中,且對其進行凍乾,得到(S
)-6-(((1-(雙環[1.1.1]戊-1-基)-1H
-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈。實例 44 程序 44 : (S)-8- 氯 -6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(4,5,6,7- 四氫 -[1,2,3] 三唑并 [1,5-a] 吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:用氬氣吹掃溴烷(54 mg,0.09 mmol)於4.4 mL甲苯中之混合物持續45分鐘。將其加熱至回流,且隨後添加參(三甲基矽烷基)矽烷(43.74 mg,0.18 mmol),繼而逐滴添加含98% 2,2'-偶氮二異丁腈(1.44 mg,0.01 mmol)之0.44 mL甲苯。在於回流下加熱16小時之後,添加另一部分之矽烷,且再繼續加熱4小時。濃縮混合物,且藉由RP-HPLC來純化,得到呈三氟乙酸鹽形式之產物。實例 45 程序 45 : (S)-8- 氯 -6-(((6,7- 二氫 -5H-[1,2,3] 三唑并 [5,1-b][1,3] 噁嗪 -3- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈及 (S)-8- 氯 -6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(1-(3- 羥基丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:將(S)-8-氯-6-(((5-氟-1-(3-羥基丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(37 mg,0.07 mmol,如對實例23所製備)溶解於DMF中,且在冰水浴中冷卻。添加氫化鈉於礦物油中之60%分散液(5.6 mg,0.23 mmol),使NaH及混合物升溫至室溫。在1小時之後,藉由UPLC-MS確認反應完成,且反應物含有脫鹵素以及未環化產物。藉RP HPLC由純化15分鐘(10%-49%)得到獨立地呈對應三氟乙酸鹽形式之兩種產物。(S)-8- 氯 -6-(((6,7- 二氫 -5H-[1,2,3] 三唑并 [5,1-b][1,3] 噁嗪 -3- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:ES/MS m/z: 535.34。1
H NMR (400 MHz, 乙腈-d3) δ 8.42 (s, 1H), 7.92 (m, 1H), 7.48 (s, 1H), 6.79 (m, 1H), 6.70 (s, 1H), 5.97 (s, 1H), 4.35 (m, 4H), 3.90 (m, 1H), 3.68 (m, 1H), 2.50 (s, 3H), 2.23 (m, 2H), 0.94 (s, 9H)。(S)-8- 氯 -6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(1-(3- 羥基丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:ES/MS m/z: 537.27。1
H NMR (400 MHz, 乙腈-d3) δ 8.41 (s, 1H), 7.84 (m, 1H), 7.66 (m, 1H), 7.48 (m, 1H), 6.82 (m, 1H), 6.74 (m, 1H), 6.15 (m, 1H), 4.42 (m, 2H), 3.76 (m, 2H), 3.48 (m, 2H), 2.51 (s, 3H), 1.98 (m, 2H), 0.94 (s, 9H)。實例 46 程序 46 : (S)-6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(1-(1-( 三氟甲基 ) 環丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 )-8-( 嘧啶 -5- 基 ) 喹啉 -3- 甲腈
:將6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-碘-4-(新戊基胺基)喹啉-3-甲腈(20 mg,0.03 mmol)、碳酸鉀(0.03 mL,0.06 mmol)及嘧啶-5-基酸(5.3 mg,0.045 mmol)溶解於DME中。隨後向混合物中添加雙(三苯膦)二氯化鈀(II) (1.0 mg,0.002 mmol)。在微波反應器中將反應物加熱至110℃,持續5分鐘,隨後用EtOAc及鹽水稀釋,保留有機層,經硫酸鈉乾燥,且濃縮。藉由RP-HPLC (溶離劑:水/MeCN *0.1% TFA)來純化粗殘餘物,得到呈三氟乙酸鹽形式之產物。實例 47 程序 47 : (S)-8- 氯 -6-(((6- 氟 -2- 甲基吡啶 -3- 基 )(5- 碘 -1-(1- 甲基環丙基 )-1H-1,2,3- 三唑 -4- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
:向含(R)-8-氯-6-((1-(6-氟-2-甲基吡啶-3-基)丙-2-炔-1-基)胺基)-4-(新戊基胺基)喹啉-3-甲腈(100 mg,0.23 mmol)之MeTHF (2 mL)中添加碘化銅(I)(4 mg,0.02 mmol)及N-碘嗎啉氫碘酸鹽(95 mg,0.28 mmol)。將溶液在室溫下攪拌5小時。經由碳酸酯樹脂過濾所得溶液,且濃縮,得到粗物質(S)-8-氯-6-((1-(6-氟-2-甲基吡啶-3-基)-3-碘丙-2-炔-1-基)胺基)-4-(新戊基胺基)喹啉-3-甲腈。
向(S)-8-氯-6-((1-(6-氟-2-甲基吡啶-3-基)-3-碘丙-2-炔-1-基)胺基)-4-(新戊基胺基)喹啉-3-甲腈於MeTHF (2 mL)中之溶液中添加三乙胺(0.05 mL,0.36 mmol)、
碘化銅(I) (4 mg,0.02 mmol)及1-疊氮基-1-甲基環丙烷(0.5 mL,於MTBE中之0.5 M溶液,0.25 mmol)。所得溶液在室溫下攪拌3天,且隨後用碳酸氫鹽水溶液洗滌。用EtOAc (2×)反萃取水層,且經Na2
SO4
乾燥經合併之有機層,且濃縮。藉由反相HPLC (10%-60%含0.1% TFA之MeCN/H2
O)來純化粗殘餘物,得到呈TFA鹽形式之產物。將產物溶解於EtOAc中,
且用碳酸氫鹽水溶液洗滌。用EtOAc (2×)反萃取水層,且經Na2
SO4
乾燥經合併之有機層,且濃縮。藉由正相層析(10%-50% EtOAc/CH2
Cl2
)來純化粗殘餘物,得到產物。1
H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 1.1 Hz, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.6, 2.7 Hz, 1H), 6.83 (d, J = 2.3 Hz, 1H), 6.07 (s, 1H), 4.13 (d, J = 13.9 Hz, 1H), 3.70 (d, J = 13.9 Hz, 1H), 2.40 (s, 3H), 1.64 (s, 3H), 1.44 - 1.31 (m, 2H), 1.22 (t, J = 2.0 Hz, 2H), 0.89 (s, 9H)。
ES/MS: 659.255 (M+H+)。實例 48 程序 48
: (S)-8-
氯-6-(((6-
氟-2-
甲基吡啶-3- 基 )(1-(1-( 三氟甲基 )
環丙基)-1H-1,2,3-
三唑-4-基) 甲基 )( 甲基 )
胺基)-4-(
新戊基胺基)
喹啉-3-
甲腈:在0℃ (外部)下向6-胺基-8-氯-4-(新戊基胺基)喹啉-3-甲腈(1 g,3.46 mmol)於H2
O (35 mL)及H2
SO4
(1.8 mL)中之漿料中逐滴添加1.5 M NaNO2
水溶液(2.8 ml)。將
所得溶液在0℃下攪拌1.5小時,接著添加含碘化鉀(1.2 g,7.23 mmol)之H2
O (15 mL)。將
所得漿料在室溫下劇烈攪拌18小時。用NaOH (2 M)中和漿料,過濾,且用H2
O洗滌兩次。將所得濾液溶解於EtOAc中,
且用NaCl水溶液洗滌。用EtOAc反萃取水層,且經MgSO4
乾燥經合併之有機層,且濃縮。藉由SiO2
層析(5%-25%-100% EtOAc/Hex,20% MeOH/EtOAc洗滌)來純化粗物質,得到所要產物。1
H NMR (400 MHz, DMSO-d6) δ 8.82 (d, J = 1.7 Hz, 1H), 8.55 (s, 1H), 8.25 (d, J = 1.5 Hz, 1H), 8.19 (t, J = 7.0 Hz, 1H), 3.72 (d, J = 6.8 Hz, 2H), 0.96 (s, 9H)。
ES/MS: 400.428 (M+H+)。
向(S,E)-2-甲基-N-(3-(三甲基矽烷基)丙-2-炔-1-亞基)丙烷-2-亞磺醯胺(0.5 g,2.18 mmol)於THF (7.5 mL)中之溶液中添加2-噻吩甲酸銅(I) (50 mg,0.26 mmol)、2,6-二甲基吡啶(1.3 ml,11.16 mmol)及環丙基疊氮化物(於MTBE中之17%溶液,1 ml,7.82 mmol)。將所得溶液在40℃ (外部)下攪拌18小時,且隨後用EtOAc稀釋。用H2
O洗滌溶液,且用NH4
Cl水溶液洗滌兩次。用EtOAc反萃取水層,且經MgSO4
乾燥經合併之有機層,且濃縮。藉由SiO2
層析(15%-50% EtOAc/CH2
Cl2
)來純化粗殘餘物,得到所要產物。1
H NMR (400 MHz, 氯仿-d) δ 8.78 (d, J = 1.2 Hz, 1H), 8.26 (s, 1H), 1.82 - 1.75 (m, 2H), 1.72 (dt, J = 8.0, 4.9 Hz, 2H), 1.26 (d, J = 1.2 Hz, 9H)。
ES/MS: 309.100 (M+H+)。
在-78℃ (外部)下向3-溴-6-氟-2-甲基吡啶(370 mg,1.95 mmol)於MeTHF (7.5 mL)中之溶液中逐滴添加正丁基鋰溶液(於己烷中之2.5 M溶液,1.25 ml),且將反應物在-78℃下攪拌1.5小時。向黃色/橙色溶液中添加含(S,E)-2-甲基-N-((1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)亞甲基)丙烷-2-亞磺醯胺(200 mg,0.65 mmol)之MeTHF (2 mL),且使所得溶液升溫至室溫,持續2小時。用50% NH4
Cl稀釋反應物,且用EtOAc萃取兩次。經Na2
SO4
乾燥經合併之有機層,且濃縮。藉由SiO2
層析(25%-60% EtOAc (5% MeOH)/CH2
Cl2
)來純化粗物質,得到呈單一異構體形式之所要產物。1
H NMR (400 MHz, 氯仿-d) δ 7.91 - 7.81 (m, 1H), 7.52 (s, 1H), 6.81 (dd, J = 8.5, 3.3 Hz, 1H), 5.93 (d, J = 3.4 Hz, 1H), 4.41 (d, J = 3.5 Hz, 1H), 2.55 (s, 3H), 1.74 - 1.59 (m, 4H), 1.24 (d, J = 0.8 Hz, 9H)。
ES/MS: 420.099 (M+H+)。
使(S)-N-((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)-2-甲基丙烷-2-亞磺醯胺(0.1 g,0.25 mmol)於TH F(3 mL)中之溶液冷卻至0℃。添加氫化鈉(於礦物油中之60%分散液,0.01 g,0.29 mmol),且攪拌30分鐘,接著添加碘甲烷(0.02 mL,0.32 mmol)。將所得溶液在室溫下攪拌24小時,且用EtOAc稀釋。用50%NH4
Cl洗滌溶液,且用EtOAc反萃取水溶液。經Na2
SO4
乾燥合併之有機層,且濃縮。藉由SiO2
層析(20%-50%-60% EtOAc (5% MeOH)/CH2
Cl2
)來純化粗殘餘物,得到所要產物。1
H NMR (400 MHz, 氯仿-d) δ 7.95 (t, J = 8.2 Hz, 1H), 7.78 (s, 1H), 6.83 (dd, J = 8.5, 3.3 Hz, 1H), 5.99 (s, 1H), 2.58 (s, 3H), 2.46 (s, 3H), 1.63 (d, J = 62.7 Hz, 4H), 1.17 (s, 9H)。
ES/MS: 433.820 (M+H+)。
向(S)-N-((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)-N,2-二甲基丙烷-2-亞磺醯胺(0.07 g,0.17 mmol)於MeOH (1 mL)中之溶液中添加含4 M HCl之
二噁烷(0.45 ml)。將所得溶液在室溫下攪拌2小時,且濃縮。用EtOAc稀釋粗殘餘物,且用碳酸氫鹽水溶液洗滌。用EtOAc 反萃取水層,且經Na2
SO4
乾燥合併之有機層,且濃縮。將粗物質胺溶解於CH2
Cl2
及甲苯之1:1混合物中,且濃縮至乾燥,得到所要產物。
ES/MS: 329.872 (M+H+)。
向(S)-1-(6-氟-2-甲基吡啶-3-基)-N-甲基-1-(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲胺(0.05 g,0.17 mmol)於甲苯(3.5 mL)中之溶液中添加8-氯-6-碘-4-(新戊基胺基)喹啉-3-甲腈(0.07 g,0.17 mmol)、2-(二-第三丁基膦基)聯二苯(0.02 g,0.07 mmol)及參(二苯亞甲基丙酮)二鈀(O) (0.03 g,0.03 mmol)。用氬氣使漿料脫氣5分鐘,且添加95%第三丁醇鉀(0.06 g,0.5 mmol)。將所得漿料加熱至80℃ (外部),持續2小時。隨後用EtOAc稀釋反應混合物,且用碳酸氫鹽水溶液洗滌。反萃取水層,及濃縮所得有機層。隨後藉由反相HPLC (10%-70%含0.1%TFA之MeCN/H2
O)來純化粗油狀物。藉由反相HPLC (10%-65%含0.1%TFA之MeCN/H2
O)來第二次純化產物,得到呈TFA鹽形式之所要產物。1
H NMR (400 MHz, 甲醇-d4) δ 8.55 (d, J = 1.6 Hz, 1H), 8.32 (s, 1H), 7.88 (d, J = 2.6 Hz, 1H), 7.59 (t, J = 8.1 Hz, 1H), 7.40 (d, J = 2.6 Hz, 1H), 6.92 (dd, J = 8.5, 2.8 Hz, 1H), 6.76 (s, 1H), 4.09 (d, J = 14.1 Hz, 1H), 3.96 (d, J = 14.1 Hz, 1H), 2.97 (s, 3H), 2.35 (s, 3H), 1.80 - 1.70 (m, 4H), 1.02 (d, J = 3.2 Hz, 9H)。
ES/MS: 601.367 (M+H+)。實例 49 , 程序 49 : (S)-8- 乙醯基 -6-(((1-(1-( 二氟甲基 ) 環丙基 )-1H-1,2,3- 三唑 -4- 基 )(6- 氟 -2- 甲基吡啶 -3- 基 ) 甲基 ) 胺基 )-4-( 新戊基胺基 ) 喹啉 -3- 甲腈
向含(S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-碘-4-(新戊基胺基)喹啉-3-甲腈(37 mg,0.056 mmol)及雙(三苯基膦)二氯化鈀(II)
(5 mg,0.006 mmol)之甲苯(1 mL)中添加三丁基(2-乙氧基烯丙基)錫烷(23 mg,0.062 mmol)。用氮氣吹掃反應混合物,且在100℃下加熱隔夜。在冷卻至室溫之後,添加2 N HCl (1 mL),且攪拌混合物2小時。用水稀釋反應物,且用EtOAc萃取三次。用水及鹽水洗滌經合併之有機物,且乾燥(Na2
SO4
)。濃縮濾液,得到粗物質,藉由HPLC (溶離劑:水/MeCN *0.1% TFA)來對其純化兩次,得到產物。
根據本文中所描述(及在實例/程序下之表1中所指示)之實例及程序,按需要使用恰當起始物質及恰當保護基化學製備以下化合物。表 1
所選化合物之質子NMR資料展示於下文表2中。表 2
生物學分析
以下實例(實例40至42)描述用於量測某些測試化合物針對TNFα、Cot (亦稱為Tpl2)及EGFR之活性的生物學分析。如表3中所概述,測試化合物為Cot之有效抑制劑。實例 40 : 基於 Cot 單核球 TNFα 細胞之分析
使冷凍保存之人類單核球(Stem Cell Technologies)解凍,在RPMI中用含有10% FBS之Glutamax (10mM HEPES、1×青-鏈黴素(Pen-Strep)、55 µM β-巰基乙醇、1 mM丙酮酸鈉)培養基稀釋至0.125 × 10^6個細胞/毫升,且在37℃下復原2小時。隨後將細胞懸浮液以5,000個細胞/孔之密度接種至黑色384孔Greiner透明底部培養盤上。培養盤經測試化合物預點樣,且在DMSO中連續稀釋,其中使用Echo 550聲學液體分配器(Labcyte®)遞送200奈升/孔以得到最終DMSO濃度0.5%。在37℃下用化合物處理經接種之細胞持續1小時。隨後用50 pg/ml LPS (Sigma)刺激細胞,從而排除用於未經刺激之細胞對照孔的培養盤外側欄。將細胞在37℃下再培育4小時。隨後自培養基旋轉出細胞,且取得5 μl樣品且使用TR-FRET人類TNFα偵測系統(CisBio)來分析總TNFα含量。此系統利用兩種經標記之抗體(穴狀化合物(cryptate)及XL665),其結合於TNFα分子之兩個不同抗原決定基且產生與樣品中TNFα之濃度成比例的FRET信號。50:50混合偵測抗體,且將5 µL分配至各孔中。用透明密封件覆蓋培養盤,且在室溫下培育隔夜。在此日早晨,使用Envision 2103多標記讀取器(珀金埃爾默)用分別處於340 nm/615 nm/665 nm下之激發/發射/FRET發射來讀取培養盤。處於615 nm及665 nm發射波長下之螢光強度表示為比率(665 nm/615 nm)。對照百分比如下計算:
對照% = 100 × (比率樣品
-比率0% 刺激
)/(比率100% 刺激
-比率0% 刺激
)
其中未經刺激之細胞(0%刺激為陰性對照,且經刺激之細胞(100%刺激)用作陽性對照。實例 41 : 高通量 Cot 生物化學分析
使用KinEASE (Cisbio) (時差式螢光共振能量轉移(TR-FRET)免疫分析)來量測人類Cot酶活性。在此分析中,Cot催化經XL665標記之肽受質的磷酸化。銪結合(conjugate)之磷酸-酪胺酸特異抗體結合(bind)所得經磷酸化之肽。在2步終點分析中,藉由TR-FRET用銪作為供體及XL665作為受體來量化經磷酸化之肽的形成。經純化之重組人類Cot催化域(30-397個胺基酸)購自Carna Biosciences。簡言之,使用Echo 550聲學液體分配器(Labcyte®)將在DMSO中連續稀釋之測試化合物遞送至Proxy白色、較小體積之384孔培養盤中。使用Multi-Flo (Bio-Tek Instruments)將Cot酶及受質分配於分析盤中。標準5 μL反應混合物在反應緩衝液(10 mM MOPS (pH 7.0)、0.02% NaN3
、0.5 mg/mL BSA、10 mM MgOAc、1 mM DTT、0.025% NP-40、1.5%甘油)及0.1% DMSO中含有400 µM ATP、1 µM STK3肽、5 nM Cot。在於室溫下培育2.5小時之後,添加5 µL終止及偵測溶液(1:200經銪穴狀化合物標記之抗磷酸化肽抗體溶液,及於含有足夠EDTA之50 mM Hepes (pH 7.0)偵測緩衝液中之125 nM鏈黴親和素-XL665示蹤劑)。隨後在室溫下再對該培養盤培育120分鐘,且使用Envision 2103多標記讀取器(PerkinElmer)用分別處於340 nm/615 nm/665 nm下之激發/發射/FRET發射來讀取。處於615 nm及665 nm發射波長下之螢光強度表示為比率(665 nm/615 nm)。抑制百分比如下計算:
抑制% = 100 × (比率樣品
-比率0% 抑制
)/(比率100% 抑制
-比率0% 抑制
)
其中0.1% DMSO (0%抑制)為陰性對照,且100 µM比較實例1 (100%抑制)用作陽性對照。實例 42 : 高通量 EGFR 生物化學分析
使用KinEASE (Cisbio) (時差式螢光共振能量轉移(TR-FRET)免疫分析)來量測EGFR活性。在此分析中,EGFR催化經XL665標記之通用酪胺酸激酶肽受質的磷酸化。銪結合(conjugate)之磷酸-酪胺酸特異抗體結合(bind)所得經磷酸化之肽。藉由TR-FRET用銪作為供體及XL665作為受體來量化經磷酸化之肽的形成。在兩個主要步驟中進行分析。第一步驟為激酶反應步驟,且第二步驟為使用TR-FRET試劑之偵測步驟。簡言之,使用Echo 550聲學液體分配器(Labcyte®)將在DMSO中1:3連續稀釋之測試化合物遞送至Corning白色、較小體積、非結合之384孔培養盤中。使用Multi-Flo (Bio-Tek Instruments)將EGFR酶(來自Carna Biosciences之人類EGFR,細胞質域[669-1210],目錄號08-115)及受質TK受質-生物素(包括於Cisbio HTRF KinEASE-TK套組中,目錄號62TK0PEJ)分配至分析盤中。標準10 µL反應混合物在反應緩衝液(10 mM MOPs (pH 7.0)、1.5%甘油、0.5 mg/ml BSA、10 mM乙酸鎂、1 mM DTT、0.025% NP-40)中含有6 µM ATP (1×Km)或12 µM ATP (2×Km)、1 µM生物素標記肽、0.3 nM EGFR (用於1×Km ATP)或0.1 nM EGFR (用於2×Km ATP)。在於室溫下培育60分鐘之後,添加10 µL終止及偵測溶液(1:400經銪穴狀化合物標記之抗磷酸化肽抗體溶液,及於含有足夠EDTA之50 mM Hepes (pH 7.0)偵測緩衝液中之125 nM鏈黴親和素-XL665示蹤劑)。隨後在室溫下再對該培養盤培育超過60分鐘,且使用Envision 2103多標記讀取器(PerkinElmer)用分別處於340 nm/615 nm/665 nm下之激發/發射/FRET發射來讀取。處於615 nm及665 nm發射波長下之螢光強度表示為比率(665 nm/615 nm)。抑制百分比如下計算:
抑制% = 100 × (比率樣品
-比率0% 抑制
)/(比率100% 抑制
-比率0% 抑制
)
其中0.05% DMSO (0%抑制)為陰性對照,且100 µM星形孢菌素(Staurosporine)及吉非替尼(Gefitinib) (100%抑制)用作陽性對照。
如表3中所示,式I化合物為Cot 大阪甲狀腺癌)之抑制劑。表 3
表4及表5中之資料展示,本文中所揭示之化合物為大阪甲狀腺癌(Cot)之有效抑制劑。另外,所主張之化合物不為顯著EGFR配位體。表 4
表 5
| 縮寫 | 含義 |
| ℃ | 攝氏度 |
| Ac | 乙醯基 |
| aq. | 水性 |
| ATP | 三磷酸腺苷 |
| BOC | 第三丁氧基羰基 |
| br | 寬峰 |
| BSA | 牛血清白蛋白 |
| Cbz | 羧基苯甲基 |
| COD | 環辛二烯 |
| COPD | 慢性阻塞性肺病 |
| Cot | 大阪甲狀腺癌 |
| Cp | 環戊二烯基 |
| d | 二重峰 |
| DABCO | 1,4-二氮雜雙環[2.2.2]辛烷 |
| DBU | 1,8-二氮雜雙環[5.4.0]十一-7-烯 |
| DCE | 二氯乙烯 |
| DCM | 二氯甲烷 |
| dd | 雙二重峰 |
| DEF | N, N-二乙基甲醯胺 |
| DMF | 二甲基甲醯胺 |
| DMSO | 二甲亞碸 |
| dppf | 1,1'-雙(二苯膦基)二茂鐵 |
| dt | 二重峰-三重峰 |
| DTT | 二硫蘇糖醇 |
| EC50 | 半最大有效濃度 |
| EGFR | 表皮生長因子受體 |
| eq | 當量 |
| ES/MS | 電噴霧質譜 |
| Et | 乙基 |
| FBS | 胎牛血清 |
| g | 公克 |
| HEPES | 2-[4-(2-羥基乙基)哌嗪-1-基]乙磺酸 |
| HPLC | 高效液相層析 |
| hrs | 小時 |
| Hz | 赫茲 |
| IBD | 發炎性腸病 |
| i-pr | 異丙基 |
| J | 耦合常數(MHz) |
| Kg/kg | 公斤 |
| LCMS | 液相層析-質譜 |
| LPS | 脂多醣 |
| M | 莫耳濃度 |
| m | 多重峰 |
| M+ | 質量峰 |
| M+H+ | 質量峰加氫 |
| Me | 甲基 |
| mg | 毫克 |
| MHz | 兆赫茲 |
| min | 分鐘 |
| ml/mL | 毫升 |
| mM | 毫莫耳濃度 |
| mmol | 毫莫耳 |
| MOPS | 3-(N-嗎啉基)丙烷-1-磺酸 |
| MS | 質譜 |
| Ms | 甲磺醯基 |
| nBu/Bu | 丁基 |
| nL | 奈升 |
| nm | 奈米 |
| NMR | 核磁共振 |
| NP-40 | 壬基苯氧基聚乙氧基乙醇 |
| Ns | 硝基苯磺醯基 |
| Pd-C/ Pd/C | 鈀/碳 |
| pg | 皮克 |
| Ph | 苯基 |
| PPTS | 對甲苯磺酸吡啶鎓 |
| PS | 聚苯乙烯 |
| p-TSOH/ pTSA | 對甲苯磺酸 |
| q | 四重峰 |
| q.s. | 足以達成所陳述功能之量 |
| RBF | 圓底燒瓶 |
| RP | 逆相 |
| RPMI | 洛斯維公園紀念所培養基(Roswell Park Memorial Institute medium) |
| rt | 室溫 |
| s | 單重峰 |
| sat. | 飽和 |
| t | 三重峰 |
| TBAF | 氟化四正丁基銨 |
| TBS | 第三丁基二甲基矽烷基 |
| t-Bu | 第三丁基 |
| TC | 噻吩-2-甲酸酯 |
| TEA | 三乙醇胺 |
| Tf | 三氟甲磺醯基 |
| TFA | 三氟乙酸 |
| THF | 四氫呋喃 |
| Tpl2 | 腫瘤進展基因座2 |
| TR-FRET | 時差式螢光能量傳遞 |
| Ts | 甲苯磺醯基 |
| δ | 化學位移(ppm) |
| μL/ μl | 微升 |
| μM | 微莫耳濃度 |
| 縮寫 | 含義 |
| ℃ | 攝氏度 |
| Ac | 乙醯基 |
| aq. | 水性 |
| ATP | 三磷酸腺苷 |
| BOC | 第三丁氧基羰基 |
| br | 寬峰 |
| BSA | 牛血清白蛋白 |
| Cbz | 羧基苯甲基 |
| COD | 環辛二烯 |
| COPD | 慢性阻塞性肺病 |
| Cp | 環戊二烯基 |
| d | 二重峰 |
| DABCO | 1,4-二氮雜雙環[2.2.2]辛烷 |
| DBU | 1,8-二氮雜雙環[5.4.0]十一-7-烯 |
| DCE | 二氯乙烯 |
| DCM | 二氯甲烷 |
| dd | 雙二重峰 |
| DEF | N, N-二乙基甲醯胺 |
| DMF | 二甲基甲醯胺 |
| DMSO | 二甲亞碸 |
| dppf | 1,1'-雙(二苯膦基)二茂鐵 |
| dt | 二重峰-三重峰 |
| DTT | 二硫蘇糖醇 |
| EC50 | 半最大有效濃度 |
| EGFR | 表皮生長因子受體 |
| eq | 當量 |
| ES/MS | 電噴霧質譜 |
| Et | 乙基 |
| FBS | 胎牛血清 |
| g | 公克 |
| HEPES | 2-[4-(2-羥基乙基)哌嗪-1-基]乙磺酸 |
| HPLC | 高效液相層析 |
| hrs | 小時 |
| Hz | 赫茲 |
| IBD | 發炎性腸病 |
| i-pr | 異丙基 |
| J | 耦合常數(MHz) |
| Kg/kg | 公斤 |
| LCMS | 液相層析-質譜 |
| LPS | 脂多醣 |
| M | 莫耳濃度 |
| m | 多重峰 |
| M+ | 質量峰 |
| M+H+ | 質量峰加氫 |
| Me | 甲基 |
| mg | 毫克 |
| MHz | 兆赫茲 |
| min | 分鐘 |
| ml/mL | 毫升 |
| mM | 毫莫耳濃度 |
| mmol | 毫莫耳 |
| MOPS | 3-(N-嗎啉基)丙烷-1-磺酸 |
| MS | 質譜 |
| Ms | 甲磺醯基 |
| nBu/Bu | 丁基 |
| nL | 奈升 |
| nm | 奈米 |
| NMR | 核磁共振 |
| NP-40 | 壬基苯氧基聚乙氧基乙醇 |
| Ns | 硝基苯磺醯基 |
| Pd-C/ Pd/C | 鈀/碳 |
| pg | 皮克 |
| Ph | 苯基 |
| PPTS | 對甲苯磺酸吡啶鎓 |
| PS | 聚苯乙烯 |
| p-TSOH/ pTSA | 對甲苯磺酸 |
| q | 四重峰 |
| q.s. | 足以達成所陳述功能之量 |
| RBF | 圓底燒瓶 |
| RP | 逆相 |
| RPMI | 洛斯維公園紀念所培養基(Roswell Park Memorial Institute medium) |
| rt | 室溫 |
| s | 單重峰 |
| sat. | 飽和 |
| t | 三重峰 |
| TBAF | 氟化四正丁基銨 |
| TBS | 第三丁基二甲基矽烷基 |
| t-Bu | 第三丁基 |
| TC | 噻吩-2-甲酸酯 |
| TEA | 三乙醇胺 |
| Tf | 三氟甲磺醯基 |
| TFA | 三氟乙酸 |
| THF | 四氫呋喃 |
| Tpl-2 | 腫瘤進展基因座2 |
| TR-FRET | 時差式螢光能量傳遞 |
| Ts | 甲苯磺醯基 |
| δ | 化學位移(ppm) |
| μL/ μl | 微升 |
| μM | 微莫耳濃度 |
| 化合物 | 結構 | 名稱 | 實例程序 | ES/MS m/z |
| 1 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS 522.2 (M+H+) | |
| 2 | 8-氯-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.09 (M+H+) | |
| 3 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2,6-二氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 572.24 (M+H+) | |
| 4 | 8-氯-6-(((S)-(1-((3-羥基氧雜環丁-3-基)甲基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | ES/MS 581.1 (M + H+). | |
| 5 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2,5-二氯噻吩-3-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 688.9 (M + H+) | |
| 6 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 7 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吡啶-2-基(吡啶-4-基)甲基)胺基)喹啉-3-甲腈 | 7 | ES/MS 515.0 (M+H+) | |
| 8 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 532.1 (M+H+) | |
| 9 | (S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9a | ES/MS 534.1 (M+H+) | |
| 10 | (S)-6-((苯并[d]噻唑-6-基(1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 10 | ES/MS 561.0 (M+H+) | |
| 11 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-3-氟-1-苯基丙基)胺基)喹啉-3-甲腈 | 11 | 572 | |
| 12 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吲哚啉-4-基(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 586.9 (M+H+) | |
| 13 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-乙基異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 13 | ES/MS 712.1 (M+H+) | |
| 14 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-(氧雜環丁-3-基)異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 14 | ES/MS 740.0 (M+H+) | |
| 15 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-(2-羥基乙醯基)異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 15 | ES/MS 742.1 (M+H+) | |
| 16 | 8-氯-6-(((S)-(1-(1,1-二氟-2-羥基乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 16 | ES/MS 575.1 (M + H+) | |
| 17 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-異丙氧基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 17 | ES/MS 702.0 (M+H+) | |
| 18 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(四氫-2H-哌喃-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 18 | ES/MS 651.1 (M+H+) | |
| 19 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(3-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 19 | ES/MS 532.2 (M+H+) | |
| 20 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 622.0 (M+H+) | |
| 21 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(5-(吡咯啶-1-羰基)吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 21 | ES/MS 644.1 (M+H+) | |
| 22 | 6-(((S)-(6-氟吡啶-3-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 22 | ES/MS 519.2 (M+H+) | |
| 23 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 12 | ES/MS 518.2 (M+H+) | |
| 24 | (S)-8-溴-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 12 | ES/MS 571.1 (M+H+) | |
| 25 | (S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-溴-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 587.2 (M+H+) | |
| 26 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-(氧雜環丁-3-基)異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 14 | ES/MS 583.1 (M+H+) | |
| 27 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 12 | ES/MS 527.0 (M+H+) | |
| 28 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 600.0 (M+H+) | |
| 29 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吲哚啉-4-基(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 572.1 (M+H+) | |
| 30 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吲哚啉-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 530.1 (M+H+) | |
| 31 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((異吲哚啉-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 530.0 (M+H+) | |
| 32 | (S)-6-((苯并[d]噻唑-7-基(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 601.9 (M+H+) | |
| 33 | (S)-6-((苯并[d]噻唑-7-基(1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 545.9 (M+H+) | |
| 34 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-(氧雜環丁-3-基)-1,2,3,4-四氫異喹啉-5-基)甲基)胺基)喹啉-3-甲腈 | 13 | ES/MS 657.3 (M+H+) | |
| 35 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-(氧雜環丁-3-基)-1,2,3,4-四氫異喹啉-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 13 | ES/MS 754.2 (M+H+) | |
| 36 | 6-(((S)-(1-(第三丁基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 12 | ES/MS 591.2 (M+H+) | |
| 37 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-((異吲哚啉-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 579.0 (M+H+) | |
| 38 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基-3-側氧基異吲哚啉-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 614.9 (M+H+) | |
| 39 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基-3-側氧基異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 712.1 (M+H+) | |
| 40 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(3-側氧基異吲哚啉-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 601.0 (M+H+) | |
| 41 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(3-側氧基異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 698.1 (M+H+) | |
| 42 | (S)-6-(((1H-苯并[d]咪唑-4-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 586.0 (M+H+) | |
| 43 | (S)-6-((苯并[d]噻唑-4-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 700.5 (M+H+) | |
| 44 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-(氧雜環丁-3-基)異吲哚啉-4-基)甲基)胺基)喹啉-3-甲腈 | 14 | ES/MS 644.0 (M+H+) | |
| 45 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-(2-羥基乙醯基)異吲哚啉-4-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 15 | ES/MS 645.3 (M+H+) | |
| 46 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-(2-羥基乙醯基)異吲哚啉-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 15 | ES/MS 603.0 (M+H+) | |
| 47 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-(氧雜環丁-3-基)異吲哚啉-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 14 | ES/MS 601.1 (M+H+) | |
| 48 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((異吲哚啉-4-基(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 587.0 (M+H+) | |
| 49 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-乙基-1,2,3,4-四氫異喹啉-7-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 13 | ES/MS 628.9 (M+H+) | |
| 50 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(1,2,3,4-四氫異喹啉-7-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 601.1 (M+H+) | |
| 51 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吲哚啉-6-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 12 | ES/MS 684.2 (M+H+) | |
| 52 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 700.0 (M+H+) | |
| 53 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-乙基異吲哚啉-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 13 | ES/MS 573.1 (M+H+) | |
| 54 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-乙基異吲哚啉-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 13 | ES/MS 573.1 (M+H+) | |
| 55 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((異吲哚啉-5-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 545.0 (M+H+) | |
| 56 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((異吲哚啉-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 545.0 (M+H+) | |
| 57 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(異吲哚啉-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 12 | ES/MS 684.2 (M+H+) | |
| 58 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-乙基異吲哚啉-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 13 | ES/MS 712.0 (M+H+) | |
| 59 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 12 | ES/MS 684.0 (M+H+) | |
| 60 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吲哚啉-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 12 | ES/MS 684.2 (M+H+) | |
| 61 | (S)-6-((苯并[d]噻唑-6-基(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 603.0 (M+H+) | |
| 62 | (S)-6-((苯并[d]噻唑-5-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 700.0 (M+H+) | |
| 63 | (S)-6-((苯并[d]噻唑-6-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 700.0 (M+H+) | |
| 64 | (S)-6-((苯并[b]噻吩-5-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 698.9 (M+H+) | |
| 65 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(喹啉-7-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 666.1 (M+H+) | |
| 66 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9a | ES/MS 602.2 (M+H+) | |
| 67 | (S)-6-((苯并[d]噻唑-7-基(1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9a | ES/MS 559.1 (M+H+) | |
| 68 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)喹啉-3-甲腈 | 12 | ES/MS 600.0 (M+H+) | |
| 69 | 6-(((S)-苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 616.1 (M+H+) | |
| 70 | (S)-6-(((2-胺基苯并[d]噻唑-7-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-溴-4-(新戊基胺基)喹啉-3-甲腈 | 12 | ES/MS 602.1 (M+H+) | |
| 71 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | 579.1 | |
| 72 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | 593.2 | |
| 73 | 8-氯-6-(((2-氯吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-3-氰基-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | 595.1 | |
| 74 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | 574.1 | |
| 75 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | 560.1 | |
| 76 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9a | ES/MS 532.1 (M+H+) | |
| 77 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基-3-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9a | ES/MS 546.2 (M+H+) | |
| 78 | (S)-8-溴-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(3-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 585.1 (M+H+) | |
| 79 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 12 | ES/MS 518.6 (M+H+) | |
| 80 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 5 | ES/MS 603.2 (M + H+) | |
| 81 | (R)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 5 | ES/MS 603.2 (M + H+) | |
| 82 | 8-氯-4-((3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-((1R,5S,6r)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基)-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 773.1 (M + H+) | |
| 83 | 8-氯-4-((3-氰基-1-苯基丙基)胺基)-6-(((R)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 676.1 (M + H+) | |
| 84 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 676.1 (M + H+) | |
| 85 | (S)-2-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟吡啶-3-基)甲基-d)-1H-1,2,3-三唑-1-基)乙酸 | 1 | ES/MS 524.2 (M+H+) | |
| 86 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.2 (M+H+) | |
| 87 | 8-氯-4-(((S)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.2 (M+H+) | |
| 88 | 8-氯-4-(((S)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.2 (M+H+) | |
| 89 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.2 (M+H+) | |
| 90 | 8-氯-4-((3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 676.1 (M + H+) | |
| 91 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 5 | ES/MS 603.2 (M + H+) | |
| 92 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-((3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 548.2 (M+H+) | |
| 93 | 8-氯-4-((3-氰基-1-苯基丙基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.2 (M+H+) | |
| 94 | (S)-8-氯-4-((3-氯-2-甲氧基苯基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 8 | ES/MS 576.1 (M+H+) | |
| 95 | 4-(((1S,3S,5S,7S)-金剛烷-2-基)胺基)-8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 1 | ES/MS 570.2 (M+H+) | |
| 96 | (S)-8-氯-4-((3-氯-4-甲氧基苯基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 8 | ES/MS 576.2 (M+H+) | |
| 97 | 6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-甲氧基-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 550.1 (M+H+) | |
| 98 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-甲氧基-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 502.3 (M+H+) | |
| 99 | (S)-6-(((6-氟吡啶-3-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 22 | ES/MS 471.3 (M+H+) | |
| 100 | 4-(((R)-2-氰基-1-苯基乙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3,8-二甲腈 | 1 | ES/MS 556.2 (M+H+) | |
| 101 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 556.2 (M+H+) | |
| 102 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 508.3 (M+H+) | |
| 103 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2,6-二氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 524.3 (M+H+) | |
| 104 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 506.4 (M+H+) | |
| 105 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(2-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 554.2 (M+H+) | |
| 106 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基-2,2,3,3,3-d5)胺基)喹啉-3-甲腈 | 2 | ES/MS 559.2 (M+H+) | |
| 107 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((S)-1-苯基丙基-2,2,3,3,3-d5)胺基)喹啉-3-甲腈 | 2 | ES/MS 559.2 (M+H+) | |
| 108 | 8-氯-6-(((R)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基-2,2,3,3,3-d5)胺基)喹啉-3-甲腈 | 2 | ES/MS 559.1 (M+H+) | |
| 109 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基-1,2,2,3,3,3-d6)胺基)喹啉-3-甲腈 | 2 | ES/MS 560.2 (M+H+) | |
| 110 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 568.2 (M+H+) | |
| 111 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 520.3 (M+H+) | |
| 112 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-((2,2-二甲基丙基-1,1-d2)胺基)喹啉-3-甲腈 | 2 | ES/MS 508.3 (M+H+) | |
| 113 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(5-氟-2-甲基吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 568.2 (M+H+) | |
| 114 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(5-氟-2-甲基吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS 520.2 (M+H+) | |
| 115 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-((1-苯基丙基-2,2,3,3,3-d5)胺基)喹啉-3-甲腈 | 2 | ES/MS 559.3 (M+H+) | |
| 116 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 1 | ES/MS 597.2 (M+H+) | |
| 117 | 6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 1 | ES/MS 545.2 (M+H+) | |
| 118 | 6-(((S)-(1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 3 | ES/MS 570.3 (M+H+) | |
| 119 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-氟-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS 490.2 (M+H+) | |
| 120 | 6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-8-氟-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 1 | ES/MS 538.2 (M+H+) | |
| 121 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-3-羥基-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 570.1 (M+H+) | |
| 122 | 8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 584.3 (M+H+) | |
| 123 | 8-氯-6-(((4-氰基噻吩-2-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 566.0 (M+H+) | |
| 124 | 8-氯-6-(((2-氯吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 570.5 (M+H+) | |
| 125 | 8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 536.9 (M+H+) | |
| 126 | 8-氯-6-(((2-氯吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 522.4 (M+H+) | |
| 127 | (S)-8-氯-4-((3-氯-2,6-二氟苯基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 582.2 (M+H+) | |
| 128 | 8-氯-4-(((S)-2-氰基-1-苯基乙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 565.1 (M+H+) | |
| 129 | 8-氯-4-(((R)-2-氰基-1-苯基乙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 565.1 (M+H+) | |
| 130 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基-d)胺基)-4-(((R)-3-羥基-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 552.1 (M+H+) | |
| 131 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 501.2 (M+H+) | |
| 132 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | ES/MS: 502.16 (M+H+) | |
| 133 | (S)-8-氯-6-(((4-氯吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 563.9 (M+H+) | |
| 134 | 8-氯-6-(((S)-(6-氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 514.1 (M+H+) | |
| 135 | (S)-8-氯-6-(((6-氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 10 | ES/MS 466.1 (M+H+) | |
| 136 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((咪唑并[1,5-a]吡啶-8-基(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 586.0 (M+H+) | |
| 137 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(5-甲基噻唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 664.1 (M+H+) | |
| 138 | (S)-8-氯-4-(新戊基胺基)-6-((吡啶-3-基(1-(吡啶-3-基)-1H-1,2,3-三唑-4-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 525.2 (M+H+) | |
| 139 | (S)-8-氯-4-((5-氯-6-氟吡啶-3-基)胺基)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 564.9 (M+H+) | |
| 140 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 549.1 (M+H+) | |
| 141 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 488.1 (M+H+) | |
| 142 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | ES/MS 506.1 (M+H+) | |
| 143 | (S)-8-氯-6-(((5-氰基吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 556.1 (M + H+) | |
| 144 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS: 550.1 (M+H+) | |
| 145 | 8-氯-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 510.0 (M+H+) | |
| 146 | (S)-8-氯-6-(((5-氯吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 564.0 (M+H+) | |
| 147 | 8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基-d)胺基)喹啉-3-甲腈 | 10 | ES/MS 505.0 (M+H+) | |
| 148 | 8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基-d)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 545.1 (M+H+) | |
| 149 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2,6-二氟吡啶-3-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 566.0 (M+H+) | |
| 150 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 1 | ES/MS 543.9 (M+H+) | |
| 151 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((2-氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 508.1 (M+H+) | |
| 152 | (S)-8-氯-6-(((5-氯吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 524.0 (M+H+) | |
| 153 | (S)-8-氯-6-(((4-氯吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 524.0 (M+H+) | |
| 154 | (S)-8-氯-6-(((2,6-二氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 526.0 (M+H+) | |
| 155 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 554.1 (M+H+) | |
| 156 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((3,6-二氫-2H-哌喃-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 495.1 (M+H+) | |
| 157 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(吡唑并[1,5-a]吡啶-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 586.2 (M+H+) | |
| 158 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基-d)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 530.9 (M+H+) | |
| 159 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基-d)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 536.1 (M+H+) | |
| 160 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-苯基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 565.9 (M+H+) | |
| 161 | (S)-8-氯-6-(((2-氯吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 524.0 (M+H+) | |
| 162 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-苯基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基-d)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.1 (M+H+) | |
| 163 | (S)-8-氯-6-(((5,6-二氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 526.0 (M+H+) | |
| 164 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基-d)胺基)喹啉-3-甲腈 | 10 | ES/MS 491.1 (M+H+) | |
| 165 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-甲基-1H-吡唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 493.1 (M+H+) | |
| 166 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((6-氟吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 508.0 (M+H+) | |
| 167 | (S)-8-氯-4-(新戊基胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 447.2 (M+H+) | |
| 168 | (S)-8-氯-4-(新戊基胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 503.2 (M+H+) | |
| 169 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 10 | ES/MS 538.1 (M+H+) | |
| 170 | (R)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((4-甲基噻唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 510.0 (M+H+) | |
| 171 | 8-氯-6-(((S)-(2-甲基吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.1 (M+H+) | |
| 172 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((2-甲基吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 560.0 (M+H+) | |
| 173 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 504.0 (M+H+) | |
| 174 | 8-氯-6-(((S)-(1-((1R,5S,6s)-3-(氧雜環丁-3-基)-3-氮雜雙環[3.1.0]己-6-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | ES/MS 632.2 (M + H+) | |
| 175 | 8-氯-6-(((S)-(1-(氰基甲基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 534.1 (M + H+) | |
| 176 | 8-氯-4-(((R)-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1-(3-(吡咯啶-1-基)丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 606.3 (M+H+) | |
| 177 | 8-氯-6-(((S)-(1-(2-氟乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 1 | ES/MS 541.2 (M+H+) | |
| 178 | 8-氯-4-(((S)-3-羥基-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.1 (M+H+) | |
| 179 | 8-氯-4-(((R)-3-羥基-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.1 (M+H+) | |
| 180 | 6-(((S)-(1-((1R,5S,6s)-3-氮雜雙環[3.1.0]己-6-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | ES/MS 576.2 (M + H+) | |
| 181 | 8-氯-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | ES/MS 555.1 (M + H+) | |
| 182 | 8-氯-4-(((S)-3-羥基-1-苯基丙基)胺基)-6-(((S)-(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.1 (M+H+) | |
| 183 | 8-氯-4-(((R)-3-羥基-1-苯基丙基)胺基)-6-(((S)-(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.1 (M+H+) | |
| 184 | 8-氯-4-(((S)-3-羥基-1-苯基丙基)胺基)-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.1 (M+H+) | |
| 185 | 8-氯-4-(((R)-3-羥基-1-苯基丙基)胺基)-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.1 (M+H+) | |
| 186 | 8-氯-4-(((S)-2-羥基-2-甲基-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 525.1 (M+H+) | |
| 187 | 8-氯-4-(((S)-2-羥基-2-甲基-1-苯基丙基)胺基)-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.1 (M+H+) | |
| 188 | 8-氯-4-(((R)-2-羥基-2-甲基-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 525.1 (M+H+) | |
| 189 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-(2-氟乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 536.1 (M+H+) | |
| 190 | 8-氯-4-(((R)-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1-((R)-1,1,1-三氟丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | ES/MS 591.1 (M + H+) | |
| 191 | 8-氯-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | ES/MS 535.1 (M + H+) | |
| 192 | 6-(((S)-(1-(第三丁基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.2 (M + H+) | |
| 193 | 8-氯-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 537.1 (M + H+) | |
| 194 | 8-氯-6-(((S)-(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.1 (M + H+) | |
| 195 | 8-氯-4-(((R)-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1-(2,2,2-三氟乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 577.1 (M + H+) | |
| 196 | (S)-8-氯-4-((3-氯苯基)胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 543.1 (M+H+) | |
| 197 | 8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 4 | ES/MS 549.9 (M+H+) | |
| 198 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 8 | ES/MS 546.2 (M+H+) | |
| 199 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 546.1 (M+H+) | |
| 200 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吡啶-3-基(1-(3-(吡咯啶-1-基)丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 601.2 (M+H+) | |
| 201 | (S)-8-氯-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-((四氫-2H-哌喃-4-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 517.1 (M+H+) | |
| 202 | 8-氯-4-(((R)-2-羥基-2-甲基-1-苯基丙基)胺基)-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.2 (M+H+) | |
| 203 | 8-氯-4-(((R)-2-甲氧基-1-苯基乙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.0 (M+H+) | |
| 204 | 8-氯-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-2-甲氧基-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.3 (M+H+) | |
| 205 | 8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-(((S)-吡啶-3-基(1-((R)-1,1,1-三氟丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | ES/MS 585.9 (M+H+) | |
| 206 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | ES/MS 529.9 (M+H+) | |
| 207 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 490.1 (M+H+) | |
| 208 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 8 | ES/MS 629.3 (M+H+) | |
| 209 | (S)-8-氯-4-((5,6-二氟吡啶-3-基)胺基)-6-((吡啶-3-基(1-(2,2,2-三氟乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 572.0 (M+H+) | |
| 210 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((3,6-二氫-2H-哌喃-4-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 599.8 (M+H+) | |
| 211 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((3,6-二氫-2H-哌喃-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 544.0 (M+H+) | |
| 212 | 2-(4-((S)-((8-氯-3-氰基-4-(((R)-1-苯基丙基)胺基)喹啉-6-基)胺基)(吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)-N,N-二乙基-N-甲基乙-1-銨 | 1 | ||
| 213 | 8-氯-6-(((S)-(1-環戊基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 1 | ES/MS 563.2 (M+H+) | |
| 214 | 8-氯-4-(((R)-2-羥基-1-苯基乙基)胺基)-6-(((S)-噁唑-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 487.1 (M+H+) | |
| 215 | 8-氯-4-(((S)-2-羥基-1-苯基乙基)胺基)-6-(((S)-噁唑-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 487.0 (M+H+) | |
| 216 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((1-甲基-1H-吡唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 542.0 (M+H+) | |
| 217 | (R)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((4-甲基噻唑-5-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 614.8 (M+H+) | |
| 218 | 8-氯-4-(((S)-2-羥基-1-苯基乙基)胺基)-6-(((S)-(2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.1 (M+H+) | |
| 219 | 8-氯-4-(((R)-2-羥基-1-苯基乙基)胺基)-6-(((S)-(2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.1 (M+H+) | |
| 220 | 8-氯-6-(((S)-(2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 509.0 (M+H+) | |
| 221 | 6-(((S)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 648.2 (M+H+) | |
| 222 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 655.4 (M + H+) | |
| 223 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)喹啉-3-甲腈 | 5 | ES/MS 794.3 (M + H+) | |
| 224 | (S)-8-氯-4-((3-氯苯基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 501.0 (M+H+) | |
| 225 | (S)-8-氯-4-((3-氯苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 543.1 (M+H+) | |
| 226 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 640.1 (M+H+) | |
| 227 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 553.0 (M+H+) | |
| 228 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(5-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 561.1 (M+H+) | |
| 229 | 8-氯-6-(((S)-(2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 495.0 (M+H+) | |
| 230 | 8-氯-6-(((S)-(1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 537.1 (M+H+) | |
| 231 | 6-(((S)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 634.1 (M+H+) | |
| 232 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 595.0 (M+H+) | |
| 233 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 692.0 (M+H+) | |
| 234 | (R)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((4-甲基噻唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 558.9 (M+H+) | |
| 235 | (R)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 600.8 (M+H+) | |
| 236 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 697.9 (M+H+) | |
| 237 | 6-(((S)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 5 | ES/MS 736.2 (M + H+) | |
| 238 | 8-氯-6-(((S)-(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 597.1 (M + H+) | |
| 239 | 8-氯-6-(((R)-(1-異丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 543.1 (M+H+) | |
| 240 | 6-(((R)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-8-氯-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 2 | ES/MS 640.1 (M+H+) | |
| 241 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-2-氟苯基)胺基)喹啉-3-甲腈 | 5 | ES/MS 760.1 (M + H+) | |
| 242 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-((噁唑-4-基(1-(1-(氧雜環丁-3-基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 668.0 (M + H+) | |
| 243 | 8-氯-6-(((R)-(4-甲基噻唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 10 | ES/MS 501.0 (M+H+) | |
| 244 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((3,6-二氫-2H-哌喃-4-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.9 (M+H+) | |
| 245 | (S)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 561.2 (M+H+) | |
| 246 | (S)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-(((6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 621.5 (M + H+) | |
| 247 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-2-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 658.1 (M+H+) | |
| 248 | (S)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 519.2 (M+H+) | |
| 249 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((5-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 519.0 (M+H+) | |
| 250 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(5-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 658.1 (M+H+) | |
| 251 | (R)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-(((4-甲基噻唑-5-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 525.1 (M+H+) | |
| 252 | (R)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.1 (M+H+) | |
| 253 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-8-氯-4-((3-氯-2-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 664.2 (M+H+) | |
| 254 | 8-氯-4-(((R)-1-苯基丙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 495.2 (M+H+) | |
| 255 | 8-氯-4-(((R)-1-苯基乙基)胺基)-6-(((S)-吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 481.2 (M+H+) | |
| 256 | (S)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 539.1 (M+H+) | |
| 257 | (S)-8-氯-4-((3-氯-2-氟苯基)胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 505.1 (M+H+) | |
| 258 | 6-(((S)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 1 | ES/MS 634.3 (M+H+) | |
| 259 | 6-(((S)-(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-1-苯基乙基)胺基)喹啉-3-甲腈 | 1 | ES/MS 620.3 (M+H+) | |
| 260 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((3,4-二氯-2-氟苯基)胺基)喹啉-3-甲腈 | 8 | ES/MS 678.1 (M + H+). | |
| 261 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2,4-二甲基噻唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 678.0 (M+H+) | |
| 262 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2,5-二甲基噁唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 662.0 (M+H+) | |
| 263 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(5-甲基噁唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 648.3 (M+H+) | |
| 264 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(3,6-二氫-2H-哌喃-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 649.1 (M+H+) | |
| 265 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4-甲基噁唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 648.2 (M+H+) | |
| 266 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-咪唑-4-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 508.1 (M+H+) | |
| 267 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-咪唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 647.1 (M+H+) | |
| 268 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡唑-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 647.0 (M+H+) | |
| 269 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 621.1 (M + H+) | |
| 270 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-乙基-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 593.1 (M + H+) | |
| 271 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 519.0 (M+H+) | |
| 272 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-甲基吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 519.0 (M+H+) | |
| 273 | (S)-特戊酸(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(6-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)甲酯 | 10 | ES/MS 633.1 (M+H+) | |
| 274 | (S)-特戊酸(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)甲酯 | 10 | ES/MS 633.0 (M+H+) | |
| 275 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 565.1 (M + H+) | |
| 276 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((噻唑-4-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.0 (M+H+) | |
| 277 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((噻唑-5-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 511.1 (M+H+) | |
| 278 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-乙基-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 649.1 (M + H+). | |
| 279 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 621.0 (M + H+) | |
| 280 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(6-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 561.2 (M+H+) | |
| 281 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 658.1 (M+H+) | |
| 282 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 561.2 (M+H+) | |
| 283 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 658.1 (M+H+) | |
| 284 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((5-氯吡啶-3-基)胺基)喹啉-3-甲腈 | 8 | ES/MS 629.2 (M + H+). | |
| 285 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((3,4-二氟苯基)胺基)喹啉-3-甲腈 | 8 | ES/MS 628.2 (M+H+) | |
| 286 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-2-氟苯基)胺基)喹啉-3-甲腈 | 8 | ES/MS 644.2 (M+H+) | |
| 287 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(環己基胺基)喹啉-3-甲腈 | 1 | ES/MS 598.3 (M+H+) | |
| 288 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-(環庚基胺基)喹啉-3-甲腈 | 1 | ES/MS 612.3 (M+H+) | |
| 289 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-氯-4-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 692.2 (M+H+) | |
| 290 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-甲氧基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 674.2 (M+H+) | |
| 291 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-異丙氧基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 702.1 (M+H+) | |
| 292 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(5,6-二氟吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 680.2 (M+H+) | |
| 293 | (S)-6-(((5-溴-2-氯吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 661.2 (M+H+) | |
| 294 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 595.6 (M+H+) | |
| 295 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2,6-二氯吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 617.1 (M+H+) | |
| 296 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4,6-二氯吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 617.1 (M+H+) | |
| 297 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-氯吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 581.2 (M+H+) | |
| 298 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(噁唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 537.1 (M+H+) | |
| 299 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(噁唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 634.1 (M+H+) | |
| 300 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-(N-嗎啉基)噻唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 735.1 (M + H+) | |
| 301 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(2-(N-嗎啉基)噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 638.0 (M + H+) | |
| 302 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-(N-嗎啉基)噻唑-4-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 652.0 (M + H+) | |
| 303 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.0 (M+H+) | |
| 304 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 550.1 (M+H+) | |
| 305 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 647.1 (M+H+) | |
| 306 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 566.9 (M+H+) | |
| 307 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 664.0 (M+H+) | |
| 308 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吡啶-3-基(1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 10 | ES/MS 505.1 (M+H+) | |
| 309 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吡啶-3-基(1-(2,2,6,6-四甲基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 644.2 (M+H+) | |
| 310 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(噁唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 551.1 (M+H+) | |
| 311 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-咪唑-5-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 550.1 (M+H+) | |
| 312 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(噁唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 537.0 (M+H+) | |
| 313 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 505.1 (M+H+) | |
| 314 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-(氧雜環丁-3-基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 644.2 (M+H+) | |
| 315 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-氯吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 680.1 (M+H+) | |
| 316 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(吡啶-2-基甲基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 601.9 (M + H+) | |
| 317 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(吡啶-2-基甲基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 626.0 (M + H+) | |
| 318 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-氯-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 692.2 (M+H+) | |
| 319 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-氯吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 595.0 (M+H+) | |
| 320 | (S)-6-(((5-溴-2-氯吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 675.0 (M+H+) | |
| 321 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 609.1 (M+H+) | |
| 322 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2,6-二氯吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 631.0 (M+H+) | |
| 323 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4,6-二氯吡啶-3-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 631.0 (M+H+) | |
| 324 | (S)-6-(((5-溴-2-氯吡啶-3-基)(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 758.1 (M+H+) | |
| 325 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2,6-二氯吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 714.2 (M+H+) | |
| 326 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(4,6-二氯吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 714.1 (M+H+) | |
| 327 | (S)-6-(((5-溴-2-氯吡啶-3-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 663.0 (M+H+) | |
| 328 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 1 | ES/MS 597.0 (M+H+) | |
| 329 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2,6-二氯吡啶-3-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 619.0 (M+H+) | |
| 330 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4,6-二氯吡啶-3-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 619.0 (M+H+) | |
| 331 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((2-氯吡啶-3-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | ES/MS 583.1 (M+H+) | |
| 332 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(2-(N-嗎啉基)乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 648.1 (M + H+) | |
| 333 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 579.0 (M + H+) | |
| 334 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(哌啶-1-基)乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 616.2 (M+H+) | |
| 335 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(N-嗎啉基)乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 570.2 (M+H+) | |
| 336 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 549.1 (M+H+) | |
| 337 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(2-甲氧基乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 593.0 (M + H+) | |
| 338 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 577.0 (M + H+) | |
| 339 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 591.0 (M + H+) | |
| 340 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(2-甲氧基乙氧基)乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 607.2 (M+H+) | |
| 341 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(2-羥基乙氧基)乙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 593.2 (M+H+) | |
| 342 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-咪唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 647.0 (M+H+) | |
| 343 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 344 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-乙基-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1-異丙基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 635.0 (M + H+) | |
| 345 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(6-甲基-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 620.9 (M + H+) | |
| 346 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 606.9 (M + H+) | |
| 347 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 662.1 (M+H+) | |
| 348 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(噁唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 634.1 (M+H+) | |
| 349 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(5-氯苯并[b]噻吩-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 735.0 (M+H+) | |
| 350 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)-8-氯-4-((3,4-二氯苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 665.9 (M+H+) | |
| 351 | (S)-6-((苯并[b]噻吩-3-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 699.7 (M+H+) | |
| 352 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(N-嗎啉基)乙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 624.1 (M + H+) | |
| 353 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 650.1 (M+H+) | |
| 354 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 594.0 (M+H+) | |
| 355 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-(二甲胺基)乙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 581.9 (M + H+) | |
| 356 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-羥基乙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 555.0 (M + H+) | |
| 357 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-異丙基-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.0 (M + H+) | |
| 358 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(2-甲氧基乙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 569.0 (M + H+) | |
| 359 | (S)-6-(((1-(2-胺基乙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 553.9 (M + H+) | |
| 360 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(3-(二甲胺基)丙基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 596.0 (M + H+) | |
| 361 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.0 (M + H+) | |
| 362 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 616.1 (M+H+) | |
| 363 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 567.0 (M + H+) | |
| 364 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(氰基甲基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | ES/MS 550.0 (M + H+) | |
| 365 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(5-(三氟甲基)噻吩-2-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 689.1 (M + H+) | |
| 366 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(嘧啶-5-基)甲基)胺基)喹啉-3-甲腈 | 7 | ES/MS 617.1 (M + H+). | |
| 367 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(嘧啶-2-基)甲基)胺基)喹啉-3-甲腈 | 7 | ES/MS 617.2 (M+H+) | |
| 368 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(乙基((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 8 | ES/MS 644.2 (M + H+). | |
| 369 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 8 | ES/MS 644.2 (M+H+) | |
| 370 | (R)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-5-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 2 | ES/MS 650.1 (M+H+) | |
| 371 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氯噻吩-3-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 655.3 (M + H+). | |
| 372 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氰基噻吩-2-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 646.2 (M + H+) | |
| 373 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-丙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 636.1 (M+H+) | |
| 374 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(乙基((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 622.1 (M+H+) | |
| 375 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 594.1 (M+H+) | |
| 376 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吡啶-2-基(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 7 | ES/MS 515.1 (M+H+) | |
| 377 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-((二(吡啶-2-基)甲基)胺基)喹啉-3-甲腈 | 7 | ES/MS 515.1 (M+H+) | |
| 378 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻唑-5-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 621.9 (M+H+) | |
| 379 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-氯吡啶-3-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 650.0 (M+H+) | |
| 380 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻吩-2-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 620.9 (M+H+) | |
| 381 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氯噻吩-2-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 654.9 (M + H+) | |
| 382 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((5-氯噻吩-2-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 654.9 (M + H+) | |
| 383 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((6-氯吡啶-3-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 624.0 (M+H+) | |
| 384 | (R)-4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(噻唑-5-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯 | 5 | ES/MS 694.0 (M+H+) | |
| 385 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((4-氯噻吩-2-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 627.0 (M + H+) | |
| 386 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((5-氯噻吩-2-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 3 | ES/MS 628.9 (M + H+) | |
| 387 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((3-氯噻吩-2-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 626.9 (M + H+) | |
| 388 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻吩-2-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 593.0 (M+H+) | |
| 389 | (R)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((3-氯噻吩-2-基)(1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 655.0 (M + H+) | |
| 390 | (S)-4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(6-氯吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯 | 5 | ES/MS 723.2 (M+H+) | |
| 391 | (R)-4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(噻吩-2-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯 | 5 | ES/MS 694.2 (M+H+) | |
| 392 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻吩-3-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 621.2 (M+H+) | |
| 393 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(噻吩-3-基)甲基)胺基)喹啉-3-甲腈 | 5 | ES/MS 593.2 (M+H+) | |
| 394 | (S)-4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(噻吩-3-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯 | 5 | ES/MS 694.0 (M+H+) | |
| 395 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 396 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(1-乙基哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 20 | ES/MS 620.1 (M+H+) | |
| 397 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-1,2,3-三唑-4-基)(1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 6 | ES/MS 592.0 (M+H+) | |
| 398 | 4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(1-甲基-1H-1,2,3-三唑-4-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸第三丁酯 | 6 | ES/MS 693.2 (M+H+) | |
| 399 | 6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-((3-氯-4-氟苯基)胺基)喹啉-3-甲腈 | 6 | ES/MS 648.3 (M+H+) | |
| 400 | (3R)-3-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)吡咯啶-1-甲酸苯甲酯 | 6 | ||
| 401 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-((吡啶-3-基(1-((R)-吡咯啶-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 402 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-((R)-1-乙基吡咯啶-3-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 403 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-咪唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ES/MS 518.1 (M + H+) | |
| 404 | 4-(4-(((8-氯-4-((3-氯-4-氟苯基)胺基)-3-氰基喹啉-6-基)胺基)(吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)哌啶-1-甲酸苯甲酯 | 6 | ||
| 405 | 8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-(哌啶-4-基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 406 | (S)-8-氯-4-((3-氯-4-氟苯基)胺基)-6-(((1-甲基-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 6 | ||
| 407 | (S)-(苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)(3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基甲酸(膦醯氧基)甲酯 | |||
| 408 | (S)-1-(4-(苯并[d]噻唑-7-基((8-溴-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 4 | 631 | |
| 409 | (S)-1-(4-(苯并[d]噻唑-7-基((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 9 | 577.10 | |
| 410 | (R)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 534.20 | |
| 411 | 6-(((S)-苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 9 | 582.10 | |
| 412 | 6-(((S)-苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 9 | 650.20 | |
| 413 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基-d)胺基)喹啉-3-甲腈 | 4 | 661.26 | |
| 414 | 4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)喹啉-3,8-二甲腈 | 37 | 667.30 | |
| 415 | (S)-6-((苯并[d]噻唑-7-基(1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 568.30 | |
| 416 | (S)-1-(4-(苯并[d]噻唑-7-基((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 4 | 586.50 | |
| 417 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 611.70 | |
| 418 | (S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 543.30 | |
| 419 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 642.30 | |
| 420 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 633.20 | |
| 421 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 593.40 | |
| 422 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(吡唑并[1,5-a]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 526.40 | |
| 423 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 540.30 | |
| 424 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 584.30 | |
| 425 | (S)-2-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟吡啶-3-基)甲基-d)-1H-1,2,3-三唑-1-基)-N-(甲基磺醯基)乙醯胺 | 1 | 600.99 | |
| 426 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基-2H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 540.30 | |
| 427 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基苯并[d]噻唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 548.20 | |
| 428 | 6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 12 | 566.20 | |
| 429 | (S)-4-(新戊基胺基)-6-(((6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 37 | 662.40 | |
| 430 | (S)-6-(((2-甲基苯并[d]噻唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 616.30 | |
| 431 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((S)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 23 | 592.12 | |
| 432 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 587.27 | |
| 433 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-(氧雜環丁-3-基)-4,5,6,7-四氫噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 37 | 594.20 | |
| 434 | (S)-6-((苯并[d]噻唑-7-基(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 557.40 | |
| 435 | (S)-6-((苯并[d]噻唑-7-基(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 548.20 | |
| 436 | (S)-8-氯-6-(((2-氯-6-氟吡啶-3-基)(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 539.57 | |
| 437 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 537.25 | |
| 438 | 8-氯-4-(((R)-3-氰基-1-苯基丙基)胺基)-6-(((R)-(1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 23 | 592.12 | |
| 439 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-溴-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 655.05 | |
| 440 | 8-氯-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 4 | 635.45 | |
| 441 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 566.30 | |
| 442 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-(甲基磺醯基)-4-(新戊基胺基)喹啉-3-甲腈 | 39 | 655.65 | |
| 443 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 537.15 | |
| 444 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(吡唑并[1,5-a]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 567.20 | |
| 445 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 12 | 577.20 | |
| 446 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(咪唑并[1,5-a]吡啶-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 526.30 | |
| 447 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 569.60 | |
| 448 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 526.30 | |
| 449 | (S)-6-((苯并[d]噻唑-4-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 543.30 | |
| 450 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(喹啉-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 537.46 | |
| 451 | (S)-6-((異吲哚啉-4-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 12 | 586.10 | |
| 452 | 6-(((S)-苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3,8-二甲腈 | 34 | 644.10 | |
| 453 | (S)-8-乙醯基-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 14 | 619.48 | |
| 454 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 510.66 | |
| 455 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氟-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 595.2 | |
| 456 | (S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(第三丁氧基胺基)喹啉-3,8-二甲腈 | 38 | 536.20 | |
| 457 | (S)-6-((苯并[d]噻唑-7-基(1-環丙基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(第三丁氧基胺基)-8-碘喹啉-3-甲腈 | 1 | 637.10 | |
| 458 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(羥甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 573.2 | |
| 459 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-苯并[d]咪唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 540.2 | |
| 460 | (S)-6-((苯并[d]噻唑-7-基(1-(2,2,2-三氟乙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 576.1 | |
| 461 | (S)-8-氯-4-(新戊基胺基)-6-((喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 23 | 605.10 | |
| 462 | (S)-8-氯-6-((異喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 605.19 | |
| 463 | (S)-1-(4-(苯并[d]噻唑-7-基((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 4 | 600.2 | |
| 464 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(吡啶-4-基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 611.1 | |
| 465 | (S)-6-((苯并[d]噻唑-7-基(1-(1-(羥甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 564.2 | |
| 466 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2,7-啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 538.3 | |
| 467 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 528.13 | |
| 468 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 528.15 | |
| 469 | (S)-4-(新戊基胺基)-6-((喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 34 | 596.35 | |
| 470 | (S)-6-((異喹啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 596.27 | |
| 471 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 578.24 | |
| 472 | (S)-6-(((1H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 585.2 | |
| 473 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 560.2 | |
| 474 | 6-(((S)-苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((R)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3,8-二甲腈 | 34 | 644.20 | |
| 475 | 6-(((S)-苯并[d]噻唑-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((S)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3,8-二甲腈 | 34 | 644.20 | |
| 476 | (S)-8-氯-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 544.2 | |
| 477 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 4 | 562.3 | |
| 478 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 4 | 576.3 | |
| 479 | (S)-8-氯-6-(((1-(1-氰基環丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 558.2 | |
| 480 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 533.3 | |
| 481 | (S)-1-(4-(苯并[d]噻唑-7-基((3-氰基-8-氟-4-(新戊基胺基)喹啉-6-基)胺基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 4 | 584.20 | |
| 482 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(羥甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 549.18 | |
| 483 | (S)-8-氯-6-(((1-甲基-1H-苯并[d]咪唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.5 | |
| 484 | (S)-6-(((1-甲基-1H-苯并[d]咪唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 599.3 | |
| 485 | (S)-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氟-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 528.2 | |
| 486 | (S)-1-(4-(((3-氰基-8-氟-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 4 | 546.3 | |
| 487 | (S)-1-(4-(((3-氰基-8-氟-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 4 | 560.3 | |
| 488 | (S)-8-氟-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 517.3 | |
| 489 | (S)-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 535.2 | |
| 490 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 24 | 553.3 | |
| 491 | (S)-6-(((1-(1-氰基環丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 549.3 | |
| 492 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 524.2 | |
| 493 | (S)-8-氯-4-(新戊基胺基)-6-((吡唑并[1,5-b]噠嗪-3-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | 595.3 | |
| 494 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 24 | 567.3 | |
| 495 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 551.30 | |
| 496 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 590.5 | |
| 497 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-2H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 590.4 | |
| 498 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-3-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 605.3 | |
| 499 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-苯并[d]咪唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 590.3 | |
| 500 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 32 | 576.2 | |
| 501 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((2,2-二甲基丙基-1,1-d2)胺基)喹啉-3-甲腈 | 4 | 571.3 | |
| 502 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((1-甲基環丁基)甲基)胺基)喹啉-3-甲腈 | 4 | 581.3 | |
| 503 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 542.30 | |
| 504 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(異喹啉-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 537.13 | |
| 505 | 8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 556.14 | |
| 506 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 537.08 | |
| 507 | 8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 552.18 | |
| 508 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 587.11 | |
| 509 | (R)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 569.60 | |
| 510 | 8-氯-6-(((2-氯吡啶-3-基)(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 572.11 | |
| 511 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((2,2-二甲基四氫呋喃-3-基)胺基)喹啉-3-甲腈 | 23 | 597.25 | |
| 512 | (S)-8-氯-4-((3-氰基-2,2-二甲基丙基)胺基)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 23 | 594.19 | |
| 513 | (S)-8-氟-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 571.20 | |
| 514 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氟-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 553.30 | |
| 515 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-2,2-二甲基四氫呋喃-3-基)胺基)喹啉-3-甲腈 | 23 | 597.24 | |
| 516 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((S)-2,2-二甲基四氫呋喃-3-基)胺基)喹啉-3-甲腈 | 23 | 597.17 | |
| 517 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲酸 | 4 | 563.1 | |
| 518 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 556.3 | |
| 519 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 540.3 | |
| 520 | (S)-8-氯-6-(((1-甲基-1H-苯并[d]咪唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.3 | |
| 521 | (S)-8-氯-6-(((1-甲基-1H-吲唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.3 | |
| 522 | (S)-8-氯-6-(((1-甲基-1H-苯并[d][1,2,3]三唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 609.3 | |
| 523 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-苯并[d]咪唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 34 | 581.2 | |
| 524 | (S)-8-氯-6-(((3-甲基-1H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.4 | |
| 525 | (S)-8-氯-6-(((1-甲基-1H-吲唑-4-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 554.3 | |
| 526 | (S)-6-(((1-甲基-1H-吲唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.5 | |
| 527 | (S)-6-(((1-甲基-1H-苯并[d][1,2,3]三唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 600.5 | |
| 528 | (S)-6-(((3-甲基-1H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.3 | |
| 529 | (S)-6-(((1-甲基-1H-吲唑-4-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 545.3 | |
| 530 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 535.4 | |
| 531 | (S)-8-氯-6-(((1-(1-氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 553.2 | |
| 532 | (S)-8-氯-6-(((1-(2,2-二氟乙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 543.2 | |
| 533 | (S)-8-氯-6-((異喹啉-8-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 605.36 | |
| 534 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3-羥基-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 1 | 603.17 | |
| 535 | (S)-6-((異喹啉-8-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.14 | |
| 536 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 587.23 | |
| 537 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 587.21 | |
| 538 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(吡唑并[1,5-a]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 576.3 | |
| 539 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3-甲腈 | 4 | 611.3 | |
| 540 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((S)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3-甲腈 | 4 | 611.2 | |
| 541 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1,1,1-三氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 589.3 | |
| 542 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-苯并[d][1,2,3]三唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 591.7 | |
| 543 | (S)-8-氯-6-(((1-甲基-1H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.5 | |
| 544 | (S)-8-氯-6-(((2-甲基-2H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.7 | |
| 545 | (S)-8-氯-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 571.3 | |
| 546 | 8-氯-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 539.4 | |
| 547 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 559.6 | |
| 548 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-(三氟甲基)氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 603.4 | |
| 549 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基-5-d)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 33 | 588.31 | |
| 550 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.28 | |
| 551 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.2 | |
| 552 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.19 | |
| 553 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)-N,N-二甲基環丙烷-1-甲醯胺 | 35 | 590.2 | |
| 554 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(吡咯啶-1-羰基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 35 | 616.2 | |
| 555 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(嗎啉-4-羰基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 35 | 632.3 | |
| 556 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(3-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 32 | 590.4 | |
| 557 | (S)-6-(((1-甲基-1H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.3 | |
| 558 | (S)-6-(((2-甲基-2H-吲唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.2 | |
| 559 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-苯并[d][1,2,3]三唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 582.2 | |
| 560 | (S)-8-氯-4-(新戊基胺基)-6-((吡唑并[1,5-a]吡啶-4-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | 594.3 | |
| 561 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(異喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 4 | 580.2 | |
| 562 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(異喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 4 | 594.2 | |
| 563 | (S)-8-氯-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 562.1 | |
| 564 | (S)-8-氯-6-((異喹啉-5-基(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 551.2 | |
| 565 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 23 | 580.17 | |
| 566 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 23 | 594.21 | |
| 567 | (S)-8-氯-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 562.22 | |
| 568 | (S)-8-氯-6-(((1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 551.25 | |
| 569 | (S)-6-(((1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 560.3 | |
| 570 | (S)-8-氯-6-(((2-甲基-2H-吲唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.2 | |
| 571 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(3-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 581.2 | |
| 572 | (S)-4-(新戊基胺基)-6-((吡唑并[1,5-a]吡啶-4-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 585.2 | |
| 573 | (S)-6-(((2-甲基-2H-吲唑-7-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.2 | |
| 574 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基-5-d)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 579.17 | |
| 575 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 24 | 571.18 | |
| 576 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 24 | 585.10 | |
| 577 | (S)-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 553.12 | |
| 578 | (S)-6-(((1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 542.17 | |
| 579 | 8-氯-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-((1R,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 537.3 | |
| 580 | (S)-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 553.2 | |
| 581 | (S)-6-((異喹啉-5-基(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 542.2 | |
| 582 | 8-氯-4-(((R)-3,3-二甲基丁-2-基)胺基)-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 23 | 601.25 | |
| 583 | 4-(((R)-3,3-二甲基丁-2-基)胺基)-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 592.30 | |
| 584 | (S)-8-氯-6-(((1-(1,1-二氟-2-羥基乙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 559.3 | |
| 585 | (S)-4-(環己基胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 590.30 | |
| 586 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(異喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丙烷-1-甲醯胺 | 24 | 571.3 | |
| 587 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(異喹啉-5-基)甲基)-1H-1,2,3-三唑-1-基)環丁烷-1-甲醯胺 | 24 | 585.2 | |
| 588 | (S)-8-氯-4-(環己基胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 599.32 | |
| 589 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 526.3 | |
| 590 | (S)-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 562.2 | |
| 591 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1,1,1-三氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 580.2 | |
| 592 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-乙基-6-氟吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 583.25 | |
| 593 | (S)-8-氯-6-(((2-乙基-6-氟吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 601.42 | |
| 594 | (S)-8-氯-6-(((1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-8-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 569.3 | |
| 595 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-甲氧基-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 583.20 | |
| 596 | (S)-6-(((1-(1-氰基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-甲氧基-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 540.2 | |
| 597 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-乙基-6-氟吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 574.23 | |
| 598 | (S)-6-(((2-乙基-6-氟吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 592.39 | |
| 599 | (S)-8-氯-6-((咪唑并[1,5-a]吡啶-8-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 594.1 | |
| 600 | (S)-8-氯-6-(((2-乙基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 583.3 | |
| 601 | (S)-6-(((1-(2,6-二氟苯甲基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 596.3 | |
| 602 | (S)-6-(((2-乙基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 574.2 | |
| 603 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 590.3 | |
| 604 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-乙基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 565.2 | |
| 605 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 581.2 | |
| 606 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-乙基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 556.3 | |
| 607 | 4-(((1R,5S)-雙環[3.1.0]己-6-基)胺基)-8-氯-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 597.26 | |
| 608 | 4-(((1R,5S,6r)-3-氧雜雙環[3.1.0]己-6-基)胺基)-8-氯-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 599.20 | |
| 609 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 531.4 | |
| 610 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(羥甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 540.3 | |
| 611 | 8-氯-4-(((R)-環丙基(苯基)甲基)胺基)-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 647.18 | |
| 612 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((2-甲基-2-苯基丙基)胺基)喹啉-3-甲腈 | 1 | 649.23 | |
| 613 | 4-(((R)-環丙基(苯基)甲基)胺基)-6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 638.33 | |
| 614 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((2-甲基-2-苯基丙基)胺基)喹啉-3,8-二甲腈 | 25 | 640.30 | |
| 615 | (S)-4-((2-氰基-2-甲基丙基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 589.3 | |
| 616 | (S)-8-氯-6-(((8-氟異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 623.747 | |
| 617 | (S)-8-氯-6-(((8-氟喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 623.685 | |
| 618 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 635.4 | |
| 619 | (R)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 557.4 | |
| 620 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(5-甲基噻唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 557.7 | |
| 621 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 550.3 | |
| 622 | 6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-((1R,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 528.3 | |
| 623 | 6-(((S)-(6-氟-2-甲基吡啶-3-基)(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 530.3 | |
| 624 | (S)-1-(4-(((8-氯-3-氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)-3,3-二氟環丁烷-1-甲酸 | 4 | 613.3 | |
| 625 | (S)-6-(((1-(1-氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 544.5 | |
| 626 | (S)-6-(((8-氟異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 614.453 | |
| 627 | (S)-6-(((8-氟喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 614.444 | |
| 628 | (S)-8-氯-6-((異喹啉-4-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 605.604 | |
| 629 | (S)-8-氯-4-(新戊基胺基)-6-((喹啉-3-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 27 | 605.197 | |
| 630 | (R)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(4-甲基噁唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 541.3 | |
| 631 | (S)-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 626.2 | |
| 632 | (S)-8-氯-6-(((4-甲氧基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 635.3 | |
| 633 | (S)-8-氯-4-((2-氰基-2-甲基丙基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 598.34 | |
| 634 | (S)-6-(((1-(2,2-二氟乙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 534.4 | |
| 635 | (S)-8-氯-4-((3-氯-2,2-二甲基丙基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 621.07 | |
| 636 | (S)-8-氯-4-(((1-(二氟甲基)環丙基)甲基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 1 | 621.06 | |
| 637 | (S)-4-((3-氯-2,2-二甲基丙基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 612.14 | |
| 638 | (S)-4-(((1-(二氟甲基)環丙基)甲基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 25 | 612.22 | |
| 639 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2,5-二甲基噁唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 555.3 | |
| 640 | (R)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.4 | |
| 641 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(5-甲基噻唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.4 | |
| 642 | (S)-1-(4-(((3,8-二氰基-4-(新戊基胺基)喹啉-6-基)胺基)(6-氟-2-甲基吡啶-3-基)甲基)-1H-1,2,3-三唑-1-基)-N,N-二甲基環丙烷-1-甲醯胺 | 24 | 581.2 | |
| 643 | (S)-8-氯-6-(((1-環丙基-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 645.50 | |
| 644 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-(三氟甲基)氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 594.4 | |
| 645 | (S)-8-氯-6-(((1-(3,3-二氟環丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 569.4 | |
| 646 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 545.7 | |
| 647 | (S)-8-氯-6-(((1-環丙基-5-甲基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 29 | 533.3 | |
| 648 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-(羥甲基)氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 565.4 | |
| 649 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 536.3 | |
| 650 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-(二甲胺基)吡啶-3-基)甲基-d)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 26 | 531.226 | |
| 651 | (S)-8-氯-6-(((8-氯異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 639.953 | |
| 652 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 605.334 | |
| 653 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 605.337 | |
| 654 | (S)-8-氯-6-(((1-(2,6-二氟苯甲基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 605.3 | |
| 655 | (S)-8-氯-6-(((1-(2,6-二氟苯甲基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 626.2 | |
| 656 | (S)-6-(((1-(2,6-二氟苯甲基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 9 | 617.3 | |
| 657 | (R)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(4-甲基噁唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 532.4 | |
| 658 | (S)-8-氯-6-(((1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.7 | |
| 658 | (S)-8-氯-6-(((1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 608.7 | |
| 659 | (S)-8-氯-6-(((4-甲基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 619.51 | |
| 660 | (S)-8-氯-6-(((4-甲基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 619.64 | |
| 661 | (S)-6-(((1-(1,1-二氟-2-羥基乙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 550.2 | |
| 662 | (S)-8-氯-6-(((5-氯-1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 553.4 | |
| 663 | (S)-8-氯-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 537.4 | |
| 664 | (S)-6-(((1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 599.5 | |
| 665 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 540.3 | |
| 666 | (S)-8-氯-6-(((1-甲基-1H-吡唑-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 558.4 | |
| 667 | (S)-6-(((4-甲基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 610.41 | |
| 668 | (S)-6-(((4-甲基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 610.51 | |
| 669 | 6-(((S)-(1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(((R)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3-甲腈 | 4 | 601.3 | |
| 670 | (S)-6-(((8-氰基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 25 | 621.690 | |
| 671 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.477 | |
| 672 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.148 | |
| 673 | (S)-6-((異喹啉-4-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.253 | |
| 674 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2,5-二甲基噁唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 546.4 | |
| 675 | 6-(((S)-(1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-3,3-二甲基四氫-2H-哌喃-4-基)胺基)喹啉-3,8-二甲腈 | 24 | 592.4 | |
| 676 | (S)-8-氯-4-(新戊基胺基)-6-(((1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | 621.3 | |
| 677 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 531.4 | |
| 678 | (S)-6-(((1-甲基-1H-吡唑-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 549.2 | |
| 679 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-(二甲胺基)吡啶-3-基)甲基-d)胺基)-4-((2,2-二甲基丙基-1,1-d2)胺基)喹啉-3-甲腈 | 26 | 533.309 | |
| 680 | (S)-6-(((5-氯-1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 30 | 544.2 | |
| 681 | (S)-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 30 | 528.1 | |
| 682 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 577.2 | |
| 683 | (S)-8-氯-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 589.2 | |
| 684 | (S)-8-氯-6-(((1-(1-氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 571.6 | |
| 685 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 563.2 | |
| 686 | (S)-8-氯-6-(((5-氰基-1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 36 | 544.6 | |
| 687 | (S)-8-氯-6-(((2,6-二甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 583.307 | |
| 688 | (S)-8-氯-6-(((2-氟-6-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 587.622 | |
| 689 | (S)-6-(((2,6-二甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 574.505 | |
| 690 | (S)-6-(((2-氟-6-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.467 | |
| 691 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 577.37 | |
| 692 | 8-氯-6-(((S)-(1-((1R,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 555.07 | |
| 693 | (S)-8-氯-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 589.14 | |
| 694 | (S)-8-氯-6-(((1-(1-氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 571.11 | |
| 695 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 563.36 | |
| 696 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 568.31 | |
| 697 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 554.30 | |
| 698 | (S)-8-氯-4-(新戊基胺基)-6-((喹啉-7-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 27 | 605.770 | |
| 699 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 587.720 | |
| 700 | (S)-4-(新戊基胺基)-6-(((1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 612.2 | |
| 701 | (R)-8-氯-6-(((4-甲基噻唑-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 575.4 | |
| 702 | (R)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 547.6 | |
| 703 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 568.2 | |
| 704 | 6-(((S)-(1-((1S,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 546.2 | |
| 705 | (S)-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 580.2 | |
| 706 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 554.2 | |
| 707 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 695.3 | |
| 708 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.151 | |
| 709 | (S)-8-氯-6-(((8-氯喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 639.263 | |
| 710 | (S)-6-(((8-氰基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 25 | 621.265 | |
| 704 | 6-(((S)-(1-((1S,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 546.2 | |
| 705 | (S)-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 580.2 | |
| 706 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 554.2 | |
| 707 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 695.3 | |
| 708 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.151 | |
| 709 | (S)-8-氯-6-(((8-氯喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 639.263 | |
| 710 | (S)-6-(((8-氰基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 25 | 621.265 | |
| 711 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-(甲基-d3)-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 43 | 596.32 | |
| 712 | (S)-8-氯-6-(((3-氰基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 9 | 630.28 | |
| 713 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 43 | 593.40 | |
| 714 | (S)-6-(((1-(1-(第三丁基)哌啶-4-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 1 | 666.65 | |
| 715 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 589.34 | |
| 716 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 519.25 | |
| 717 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2,6-二甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 564.16 | |
| 718 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 585.60 | |
| 719 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 599.17 (M+H+) | |
| 720 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 587.1 (M+H+) | |
| 721 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 641.1 (M+H+) | |
| 722 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((2-甲基-2-(甲基磺醯基)丙基)胺基)喹啉-3-甲腈 | 2 | 651.22 (M+H+) | |
| 723 | 8-氯-6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 2 | 617.21 (M+H+) | |
| 724 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 671.2 (M+H+) | |
| 725 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基-d)胺基)-4-((2,2-二甲基丙基-1,1-d2)胺基)喹啉-3-甲腈 | 2 | 613 (M+H+) | |
| 726 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 657.4 (M+H+) | |
| 727 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 621.95 (M+H+) | |
| 728 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(3-甲基-4-側氧基-3,4-二氫喹唑啉-8-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 594.19 (M+H+) | |
| 729 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-甲氧基-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 581.44 (M+H+) | |
| 730 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(((1-(三氟甲基)環丁基)甲基)胺基)喹啉-3-甲腈 | 2 | 683.41 (M+H+) | |
| 731 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((1-(三氟甲基)環丁基)甲基)胺基)喹啉-3-甲腈 | 2 | 635.12 (M+H+) | |
| 732 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1,2-二甲基-6-側氧基-1,6-二氫吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 581.15 (M+H+) | |
| 733 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((2,2,3,3-四甲基環丙基)甲基)胺基)喹啉-3-甲腈 | 2 | 609.29 (M+H+) | |
| 734 | (S)-8-氯-4-(新戊基胺基)-6-((喹喏啉-5-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | 606.17 (M+H+) | |
| 735 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲哚-4-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 566 (M+H+) | |
| 736 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲哚-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 589.38 (M+H+) | |
| 737 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基異吲哚啉-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 605.2 (M+H+) | |
| 738 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 543.20 | |
| 739 | (S)-4-((雙環[1.1.1]戊-1-基甲基)胺基)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)喹啉-3-甲腈 | 2 | 579.30 | |
| 740 | (S)-4-((雙環[1.1.1]戊-1-基甲基)胺基)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | 597.30 | |
| 741 | (S)-8-氯-4-(新戊基胺基)-6-((噻吩并[2,3-c]吡啶-3-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 2 | 611.20 | |
| 742 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 2 | 612.10 | |
| 743 | (S)-8-氯-6-(((2-(2,2-二氟乙基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 667.40 | |
| 744 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 616.20 | |
| 745 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 567.40 | |
| 746 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3-羥基-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 2 | 585.30 | |
| 747 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 2 | 583.50 | |
| 748 | 8-氯-6-(((S)-(1-((1R,2R)-2-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 548.30 | |
| 749 | (S)-8-氯-6-(((1-(2,2-二氟丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 557.32 (M+H+) | |
| 750 | (S)-8-氯-6-(((1-(2,2-二氟丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 571.77 (M+H+) | |
| 751 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-((1-(三氟甲基)環丙基)甲基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 601.47 (M+H+) | |
| 752 | (S)-8-氯-6-(((1-((1-(二氟甲基)環丙基)甲基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 583.42 (M+H+) | |
| 753 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(2,2,2-三氟乙基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 601.39 (M+H+) | |
| 754 | (S)-8-氯-6-(((1-(1-乙基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 547.5 (M+H+) | |
| 755 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(3,3,3-三氟-2,2-二甲基丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 603.43 (M+H+) | |
| 756 | (S)-8-氯-6-(((1-(1-乙基環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 595.1 (M+H+) | |
| 757 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-異丙基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 561.40 | |
| 758 | (S)-8-氯-6-(((1-(2-環丙基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 561.50 | |
| 759 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-甲基氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 549.20 | |
| 760 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(3-甲基氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 597.20 | |
| 761 | (S)-6-(((1-(1-(第三丁基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 575.40 | |
| 762 | (S)-8-氯-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 557.37 | |
| 763 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 613.30 | |
| 764 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-((1-甲基環丙基)甲基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 547.40 | |
| 765 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 603.30 | |
| 766 | 8-氯-6-(((1S)-(6-氟-2-甲基吡啶-3-基)(1-(螺[2.2]戊-1-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 545.30 | |
| 767 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 590.20 | |
| 768 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-((3-羥基-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 609.23 | |
| 769 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-乙基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 607.30 | |
| 770 | (S)-8-氯-6-(((2-(2,2-二氟乙基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 684 | |
| 771 | (S)-8-氯-4-(新戊基胺基)-6-(((1-側氧基-2-(2,2,2-三氟乙基)-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 4 | 702.10 | |
| 772 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-異丙基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 621.40 | |
| 773 | (S)-8-氯-6-(((2-乙基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 595.30 | |
| 774 | (S)-8-氯-6-(((2-異丙基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 609.40 | |
| 775 | (S)-8-氯-6-(((1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 599.30 | |
| 776 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 581.30 | |
| 777 | (S)-8-氯-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 605.30 | |
| 778 | 8-氯-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 587.30 | |
| 779 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 607.30 | |
| 780 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 689.20 | |
| 781 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 635.20 | |
| 782 | (S)-8-氯-6-(((2-甲氧基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 585.40 | |
| 783 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 661.20 | |
| 784 | 8-氯-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 641.10 | |
| 785 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 4 | 647.20 | |
| 786 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲基-2-側氧基-1,2-二氫喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 593.30 | |
| 787 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 579.20 | |
| 788 | (S)-8-氯-6-(((2-(二氟甲基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 671.50 | |
| 789 | (S)-8-氯-6-(((1-甲基-2-側氧基-1,2-二氫喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 4 | 635.40 | |
| 790 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 22 | 541.20 | |
| 791 | 8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 22 | 493.10 | |
| 792 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(((3-甲基氧雜環丁-3-基)甲基)胺基)喹啉-3-甲腈 | 23 | 601.34 | |
| 793 | (S)-8-氯-6-(((6-氯吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 589.39 | |
| 794 | (S)-8-氯-6-(((6-氯-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 602.22 | |
| 795 | (S)-8-氯-6-(((6-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 569.27 | |
| 796 | (S)-8-氯-6-(((1-環丁基-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 581.21 | |
| 797 | (S)-8-氯-6-(((1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 23 | 605.14 | |
| 798 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 23 | 631.09 | |
| 799 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 23 | 617.18 | |
| 800 | (S)-8-氯-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丁基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 595.23 | |
| 801 | 6-(((1S)-(1-(第二丁基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 583.28 | |
| 802 | 8-氯-6-(((S)-(1-((S)-1-甲氧基丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 599.36 | |
| 803 | 8-氯-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 557.28 | |
| 804 | (S)-8-氯-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 575.19 | |
| 805 | (S)-8-氯-6-(((6-甲氧基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 585.20 | |
| 806 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(5-甲基-1,3,4-噁二唑-2-基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 23 | 601.30 | |
| 807 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 584.40 | |
| 808 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 558.32 | |
| 809 | (S)-6-(((1-甲氧基異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 572.32 | |
| 810 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 608.43 | |
| 811 | (S)-6-(((2-甲氧基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 625.17 | |
| 812 | (S)-6-(((1-甲氧基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 625.28 | |
| 813 | (S)-6-(((8-氟喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 614.13 | |
| 814 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 572.26 | |
| 815 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 650.48 | |
| 816 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 626.49 | |
| 817 | (S)-6-(((1-氰基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 621.60 | |
| 818 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-7-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.15 | |
| 819 | (S)-6-(((1-甲氧基異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 626.22 | |
| 820 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 638.16 | |
| 821 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 662.50 | |
| 822 | (S)-6-((咪唑并[1,2-a]吡啶-6-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 584.11 | |
| 823 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 598.63 | |
| 824 | 6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.43 | |
| 825 | (S)-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.67 | |
| 826 | (S)-6-(((6-氰基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 571.27 | |
| 827 | (S)-6-(((1-(第三丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 580.56 | |
| 828 | (S)-6-(((6-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 560.12 | |
| 829 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2,6-二甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 555.26 | |
| 830 | (S)-6-(((6-氰基-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 585.55 | |
| 831 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((1-(三氟甲基)環丁基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 626.37 (M+H+) | |
| 832 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 614.2 (M+H+) | |
| 833 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 590.5 (M+H+) | |
| 834 | 6-(((S)-(1-((1R,2R)-2-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 560.31 (M+H+) | |
| 835 | 6-(((S)-(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 548.30 | |
| 836 | (S)-6-(((1-(2,2-二氟丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.30 | |
| 837 | (S)-6-(((1-(2,2-二氟丁基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 562.35 (M+H+) | |
| 838 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 662.5 (M+H+) | |
| 839 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-((1-(三氟甲基)環丙基)甲基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 592.3 (M+H+) | |
| 840 | (S)-6-(((1-((1-(二氟甲基)環丙基)甲基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 574.35 (M+H+) | |
| 841 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 613 (M+H+) | |
| 842 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 629.7 (M+H+) | |
| 843 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 630.95 (M+H+) | |
| 844 | (S)-6-(((1-甲基-2-側氧基-1,2-二氫吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 575.53 (M+H+) | |
| 845 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((2,2,3,3-四甲基環丙基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 600.4 (M+H+) | |
| 846 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 578.2 (M+H+) | |
| 847 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吲哚-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 580.12 (M+H+) | |
| 848 | (S)-4-((雙環[1.1.1]戊-1-基甲基)胺基)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 588.20 | |
| 849 | (S)-4-((雙環[1.1.1]戊-1-基甲基)胺基)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 570.20 | |
| 850 | (S)-6-(((1-(2-環丙基丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 552.50 | |
| 851 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-異丙基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 552.60 | |
| 852 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-甲基氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 540.30 | |
| 853 | (S)-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(3-甲基氧雜環丁-3-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 588.20 | |
| 854 | 6-(((S)-(5-氟-1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.20 | |
| 855 | (S)-4-(新戊基胺基)-6-((噻吩并[2,3-c]吡啶-3-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 602.10 | |
| 856 | (S)-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(噻吩并[2,3-c]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 534.30 | |
| 857 | (S)-6-(((1,5-二環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 550.29 | |
| 858 | 6-(((S)-(5-氟-1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 602.10 | |
| 859 | (S)-6-(((1-環丙基-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 540.20 | |
| 860 | (S)-6-(((1-(1-(第三丁基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 566.40 | |
| 861 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 608.30 | |
| 862 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 622.10 | |
| 863 | (S)-6-(((5-氟-1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 596.20 | |
| 864 | (S)-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 582.10 | |
| 865 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 568.10 | |
| 866 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-((1-甲基環丙基)甲基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 538.30 | |
| 867 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 604.20 | |
| 868 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 566.20 | |
| 869 | (S)-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.20 | |
| 870 | (S)-6-(((2-(2,2-二氟乙基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 658.30 | |
| 871 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 607.10 | |
| 872 | 6-(((1S)-(6-氟-2-甲基吡啶-3-基)(1-(螺[2.2]戊-1-基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 535.20 | |
| 873 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲基-1H-吡咯并[2,3-b]吡啶-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 581.20 | |
| 874 | (S)-6-(((2-(2,2-二氟乙基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 674.90 | |
| 875 | (S)-4-(新戊基胺基)-6-(((1-側氧基-2-(2,2,2-三氟乙基)-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3,8-二甲腈 | 24 | 692.90 | |
| 876 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 594.30 | |
| 877 | 6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 578.30 | |
| 878 | (S)-6-(((1-(1-(氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 590.20 | |
| 879 | (S)-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 572.20 | |
| 880 | (S)-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 596.20 | |
| 881 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 598.20 | |
| 882 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 652.20 | |
| 883 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 638.10 | |
| 884 | (S)-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 626.20 | |
| 885 | (S)-6-(((2-甲氧基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 576.30 | |
| 886 | (S)-6-(((2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 680.20 | |
| 887 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲基-2-側氧基-1,2-二氫喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 584.20 | |
| 888 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 570.10 | |
| 889 | (S)-6-(((2-(二氟甲基)-1-側氧基-1,2-二氫異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 662.20 | |
| 890 | (S)-6-(((1-甲基-2-側氧基-1,2-二氫喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 626.20 | |
| 891 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 584.20 | |
| 892 | 6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 548.28 | |
| 893 | (S)-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 566.30 | |
| 894 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 632.07 | |
| 895 | (S)-6-(((1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 596.11 | |
| 896 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 622.08 | |
| 897 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3,8-二甲腈 | 24 | 608.11 | |
| 898 | (S)-6-(((1-(1,1-二氟-2-甲基丙-2-基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 580.22 | |
| 899 | 6-(((S)-(1-((1R,2S)-2-氟環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 546.22 | |
| 900 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-甲基喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 568.41 | |
| 901 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(2-(二氟甲基)喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 604.13 | |
| 902 | (S)-6-(((1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(2-甲基喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 556.29 | |
| 903 | (R)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(4-甲基噻唑-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 538.20 | |
| 904 | (R)-6-(((4-甲基噻唑-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 566.20 | |
| 905 | (S)-6-(((6-甲氧基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 576.20 | |
| 906 | (S)-6-(((5-氟-1-甲基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 502.20 | |
| 907 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(5-甲基-1,3,4-噁二唑-2-基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 592.30 | |
| 908 | 6-(((6-氟-2-甲基吡啶-3-基)(5-甲氧基-1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 24 | 514.30 | |
| 909 | (S)-6-(((3-氰基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 25 | 621.46 | |
| 910 | (S)-6-(((8-氰基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 25 | 621.27 | |
| 911 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 593.45 | |
| 912 | (S)-8-氯-6-(((1-環丙基-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 567.24 | |
| 913 | (S)-8-氯-6-(((1-甲氧基異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 581.30 | |
| 914 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 617.30 | |
| 915 | (S)-8-氯-6-(((3-氯異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 639.70 | |
| 916 | (S)-8-氯-6-(((1-氯異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 639.98 | |
| 917 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 635.09 | |
| 918 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 659.22 | |
| 919 | (S)-8-氯-6-(((8-氟喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 623.24 | |
| 920 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(8-氟喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 581.34 | |
| 921 | (S)-8-氯-6-(((8-氯喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 639.26 | |
| 922 | (S)-8-氯-6-(((5-氟-1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 653.66 | |
| 923 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 635.20 | |
| 924 | (S)-8-氯-6-(((5-碘-1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 761.55 | |
| 925 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-碘-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 743.10 | |
| 926 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 661.42 | |
| 927 | (S)-8-氯-6-(((1-甲氧基異喹啉-5-基)(1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 635.42 | |
| 928 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 647.40 | |
| 929 | (S)-8-氯-6-(((1-甲氧基異喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 635.90 | |
| 930 | (S)-8-氯-6-(((2-甲氧基喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 635.10 | |
| 931 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 607.63 | |
| 932 | 8-氯-6-(((S)-(1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 587.81 | |
| 933 | (S)-8-氯-6-(((1-(1,3-二氟丙-2-基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 605.85 | |
| 934 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(1-甲氧基異喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 671.31 | |
| 935 | (S)-8-氯-6-((咪唑并[1,2-a]吡啶-6-基(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 594.10 | |
| 936 | (S)-8-氯-6-(((1-甲基-2-側氧基-1,2-二氫吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 584.7 (M+H+) | |
| 937 | (S)-8-氯-6-(((3-氟喹啉-5-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 623.12 | |
| 938 | (S)-8-氯-6-(((2-氯-3-甲基吡啶-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 603.13 | |
| 939 | (S)-6-(((1-(雙環[2.2.2]辛-1-基)-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 635.21 | |
| 940 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 27 | 641.10 | |
| 941 | (S)-8-氯-6-(((2-甲氧基吡啶-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 27 | 585.30 | |
| 942 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 28 | 749.23 (M+H+);748.25 (M+H+) | |
| 943 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 685.20 | |
| 944 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 671.10 | |
| 945 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-碘-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 28 | 695.20 | |
| 946 | (S)-8-氯-6-(((1,5-二環丙基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 29 | EXP-15-AN3588 | |
| 947 | (S)-8-氯-6-(((5-氟-1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 551.64 | |
| 948 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 641 (M+H+) | |
| 949 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 577.10 | |
| 950 | 8-氯-6-(((S)-(5-氟-1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 | 30 | 653.40 | |
| 951 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 563.40 | |
| 952 | 8-氯-6-(((S)-(5-氟-1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 557.30 | |
| 953 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((5,6-二氟吡啶-3-基)胺基)喹啉-3-甲腈 | 30 | 630.10 | |
| 954 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 30 | 554.40 | |
| 955 | 8-氯-6-(((S)-(5-氟-1-((S)-1-氟丙-2-基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 611.20 | |
| 956 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 617.40 | |
| 957 | (S)-6-(((1-([1,1'-聯(環丙)]-1-基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 631.40 | |
| 958 | (S)-8-氯-6-(((1-環丙基-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 591.10 | |
| 959 | (S)-8-氯-6-(((5-氟-1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((3,3,3-三氟-2,2-二甲基丙基)胺基)喹啉-3-甲腈 | 30 | 605.20 | |
| 960 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 587.10 | |
| 961 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-氟-1H-1,2,3-三唑-4-基)(2-甲基-1-側氧基-1,2-二氫異喹啉-5-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 611.30 | |
| 962 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(喹啉-5-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 605.09 | |
| 963 | (S)-8-氯-6-(((5-氟-1-甲基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 30 | 511.10 | |
| 964 | (S)-6-(((1-(1-(二氟甲基)環丙基)-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3,8-二甲腈 | 31 | 590.20 | |
| 965 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 599.30 | |
| 966 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-甲氧基-1H-1,2,3-三唑-4-基)(6-甲氧基-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 611.20 | |
| 967 | (S)-8-氯-6-(((1-環丙基-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 549.28 | |
| 968 | (S)-6-(((1-(雙環[1.1.1]戊-1-基)-5-甲氧基-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-8-氯-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 575.20 | |
| 969 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(5-甲氧基-1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 523.20 | |
| 970 | 8-氯-6-(((5-甲氧基-1-甲基-1H-1,2,3-三唑-4-基)(6-甲氧基-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 535.20 | |
| 971 | 8-氯-6-(((6-氟-2-甲基吡啶-3-基)(5-甲氧基-1-甲基-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 31 | 523.20 | |
| 972 | (S)-8-氯-4-(新戊基胺基)-6-(((2-(三氟甲基)-1H-苯并[d]咪唑-4-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)喹啉-3-甲腈 | 32 | 662.14 | |
| 973 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基-5-d)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-((2,2-二甲基丙基-1,1-d2)胺基)喹啉-3-甲腈 | 33 | 572.36 (M+H+) | |
| 974 | (S)-8-氯-6-(((5-氰基-1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 36 | 594.40 | |
| 975 | (S)-5-溴-8-氯-6-(((1-(1-(二氟甲基)環丙基)-5-氟-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 42 | 665.30 | |
| 976 | (S)-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)-8-(嘧啶-5-基)喹啉-3-甲腈 | 46 | 613.3 (M+H+) | |
| 977 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(5-碘-1-(1-甲基環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 47 | 659.26 | |
| 978 | 8-氯-6-(((6-甲氧基-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 48 | 599.25 | |
| 979 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)(甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 48 | 601.37 | |
| 980 | (S)-6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-(3-甲基-3-(甲基磺醯基)丁-1-炔-1-基)-4-(新戊基胺基)喹啉-3-甲腈 | 40 | 697.29 | |
| 981 | (S)-8-乙醯基-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 49 | 577.20 | |
| 982 | 6-(((6-氟-2-甲基吡啶-3-基)(1-(1-(三氟甲基)環丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-8-(3-羥基-3-甲基丁-1-炔-1-基)-4-(新戊基胺基)喹啉-3-甲腈 | 40 | 635.29 | |
| 983 | (S)-8-氯-6-(((1-(1-(二氟甲基)環丙基)-1H-1,2,3-三唑-4-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-5-氟-4-(新戊基胺基)喹啉-3-甲腈 | 41 | 587.45 | |
| 984 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(4,5,6,7-四氫-[1,2,3]三唑并[1,5-a]吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 44 | 534.22 | |
| 985 | (S)-8-氯-6-(((6,7-二氫-5H-[1,2,3]三唑并[5,1-b][1,3]噁嗪-3-基)(6-氟-2-甲基吡啶-3-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 45 | 535.34 | |
| 986 | (S)-8-氯-6-(((6-氟-2-甲基吡啶-3-基)(1-(3-羥基丙基)-1H-1,2,3-三唑-4-基)甲基)胺基)-4-(新戊基胺基)喹啉-3-甲腈 | 46 | 537.27 | |
| 987 | 8-氯-6-(((S)-(1-((1-羥基環丁基)甲基)-1H-1,2,3-三唑-4-基)(吡啶-3-基)甲基)胺基)-4-(((R)-1-苯基丙基)胺基)喹啉-3-甲腈 |
| 化合物 | 1 H-NMR |
| 1 | 1H NMR (400 MHz, DMSO-d6) δ 8.37 (m, 2H), 8.17 (s, 1H), 8.05 (m, 1H), 7.79 (brs, 1H), 7.62 (d, J = 2.2 Hz, 1H), 7.51 (br s, 1H), 7.15 (m, 2H), 4.03 (m, 1H), 3.44 (dd, J = 13.9 / 5.5 Hz, 1H), 1.59 (s, 9H), 0.88 (s, 9H). |
| 2 | 1H NMR (400 MHz, CD3OD) δ 8.61 (m, 1H), 8.37 (m, 1H), 8.29 (m, 1H), 8.05 (m, 1H), 7.73 (m, 1H), 7.60 (s, 1H), 7.32 (m, 5H), 7.14 (m, 1H), 6.46 (s, 1H), 5.64 (m, 1H), 4.88 (m, 1H), 2.83 (s, 3H), 2.17 - 2.02 (m, 2H), 1.56 (d, 6H), 0.97 (m, 3H). |
| 3 | 1H NMR (400 MHz, CD3OD) δ 8.43 (m, 1H), 8.05 (m, 1H), 8.01 (m, 1H), 7.64 (m, 1H), 7.42 - 7.25 (m, 6H), 6.98 (m, 1H), 5.80 - 5.66 (m, 1H), 3.97 - 3.84 (m, 1H), 2.25 - 2.01 (m, 2H), 1.28 - 1.11 (m, 4H), 1.01 (m, 3H). |
| 4 | 1H NMR (400 MHz, DMSO-d6) δ 8.84 (dd, J = 14.0, 2.2 Hz, 1H), 8.64 - 8.52 (m, 1H), 8.23 (d, J = 2.1 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 6.7 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.46 (d, J = 9.5 Hz, 1H), 7.43 - 7.38 (m, 1H), 7.38 - 7.31 (m, 2H), 7.28 - 7.21 (m, 2H), 7.21 - 7.15 (m, 3H), 6.48 (d, J = 6.9 Hz, 1H), 5.48 (q, J = 7.7 Hz, 1H), 5.35 (s, 0H), 4.68 (d, J = 2.0 Hz, 2H), 4.50 (dd, J = 6.2, 4.5 Hz, 3H), 4.41 (dd, J = 6.7, 3.4 Hz, 2H), 2.12 (dt, J = 14.5, 7.4 Hz, 1H), 2.04 - 1.78 (m, 1H), 0.94 (t, J = 7.3 Hz, 3H), 0.85 (t, J = 7.2 Hz, 1H). |
| 5 | 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.47 (s, 1H), 8.14 (s, 1H), 7.60 (d, J = 2.3 Hz, 1H), 7.41 - 7.34 (m, 2H), 7.27 (d, J = 8.2 Hz, 1H), 7.15 (dd, J = 9.1, 3.3 Hz, 2H), 7.12 (s, 1H), 5.96 (d, J = 8.1 Hz, 1H), 4.50 - 4.32 (m, 1H), 3.00 - 2.83 (m, 2H), 2.42 - 2.25 (m, 2H), 2.18 - 1.81 (m, 6H), 0.98 (t, J = 7.2 Hz, 3H). |
| 6 | 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.74 (d, J = 2.2 Hz, 1H), 8.54 (dd, J = 5.0, 1.5 Hz, 1H), 8.41 (s, 1H), 8.13 (s, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.59 - 7.36 (m, 3H), 7.33 - 7.17 (m, 2H), 6.20 (d, J = 8.0 Hz, 1H), 4.83 - 4.67 (m, 1H), 3.61 (d, J = 12.4 Hz, 2H), 3.28 - 2.97 (m, 2H), 2.34 (d, J = 13.8 Hz, 2H), 2.17 (m, 2H), 1.22 (t, J = 7.3 Hz, 2H). |
| 9 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.34 (s, 1H), 8.05 (dd, J = 7.3, 1.9 Hz, 1H), 7.86 (d, J = 2.5 Hz, 1H), 7.82 (s, 1H), 7.64 - 7.54 (m, 2H), 7.16 (d, J = 2.6 Hz, 1H), 6.34 (s, 1H), 3.97 (d, J = 13.7 Hz, 1H), 3.91 - 3.80 (m, 1H), 3.49 (d, J = 13.7 Hz, 1H), 1.23 - 1.07 (m, 4H), 0.81 (s, 9H) |
| 16 | 1H NMR (400 MHz, DMSO-d6) δ 8.92 - 8.83 (m, 1H), 8.67 (d, J = 9.5 Hz, 1H), 8.65 - 8.58 (m, 1H), 8.24 (d, J = 3.3 Hz, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.64 - 7.56 (m, 2H), 7.52 (d, J = 8.3 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.39 - 7.29 (m, 2H), 7.29 - 7.16 (m, 6H), 6.54 (d, J = 7.8 Hz, 1H), 5.47 (q, J = 7.6 Hz, 1H), 4.32 (t, J = 12.0 Hz, 2H), 2.11 (m, 1H), 2.04 - 1.83 (m, 1H), 0.93 (t, J = 7.3 Hz, 3H). |
| 20 | 1H NMR (400 MHz, CD3OD) δ 9.02 (m, 1H), 8.46 (s, 1H), 8.01 (m, 1H), 7.68 (m, 1H), 7.60 (m, 1H), 7.52 (m, 1H), 7.33 (m, 4H), 6.31 (s, 1H), 3.75 (m, 2H), 3.25 - 3.13 (m, 3H), 2.45 (m, 2H), 2.36 (m, 2H), 2.25 - 2.01 (m, 2H), 1.37 (m, 3H), |
| 22 | 1H NMR (400 MHz, DMSO-d6) δ 8.49 - 8.38 (m, 1H), 8.28 (d, J = 5.1 Hz, 1H), 8.13 - 8.02 (m, 2H), 7.84 (t, J = 2.4 Hz, 1H), 7.65 - 7.52 (m, 2H), 7.42 - 7.31 (m, 1H), 7.31 - 7.15 (m, 4H), 5.49 (q, J = 7.7 Hz, 1H), 4.04 (s, 3H), 3.20 - 3.11 (m, 1H), 2.20 - 2.05 (m, 1H), 2.05 - 1.85 (m, 1H), 1.63 - 1.50 (m, 1H), 1.37 - 1.20 (m, 1H), 0.99 - 0.83 (m, 3H) |
| 23 | 1H NMR (400 MHz, 甲醇-d4) δ 8.19 (s, 1H), 7.86 (s, 1H), 7.62 (d, J = 2.5 Hz, 1H), 7.43 - 7.24 (m, 3H), 7.13 (d, J = 2.6 Hz, 1H), 6.05 (s, 1H), 3.86 - 3.63 (m, 4H), 3.56 (d, J = 13.9 Hz, 1H), 3.39 - 3.29 (m, 1H), 3.15 - 3.04 (m, 1H), 1.13 - 1.04 (m, 4H), 0.83 (s, 9H) |
| 25 | 1H NMR (400 MHz, 氯仿-d) δ 8.97 (s, 1H), 8.41 (s, 1H), 8.14 (d, J = 8.1 Hz, 1H), 7.75 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.58 (t, J = 7.7 Hz, 1H), 7.15 (s, 1H), 6.50 (br s, 1H), 6.09 (br s, 1H), 3.67 (m, 1H), 3.59 (br s, 2H), 3.53 - 3.45 (m, 1H), 1.23 - 1.17 (m, 2H), 1.17 - 1.08 (m, 2H), 0.84 (s, 9H) |
| 27 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 7.95 (s, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.48 (dd, J = 7.1, 1.7 Hz, 1H), 7.45 - 7.37 (m, 2H), 7.05 (d, J = 2.3 Hz, 1H), 6.08 (s, 1H), 4.86 (d, J = 14.7 Hz, 1H), 4.57 (d, J = 1.7 Hz, 2H), 4.51 (d, J = 14.7 Hz, 1H), 4.12 (d, J = 13.9 Hz, 1H), 3.94 - 3.84 (m, 1H), 3.73 (d, J = 13.9 Hz, 1H), 1.25 - 1.13 (m, 4H), 0.95 (s, 9H) |
| 36 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.08 (s, 1H), 7.62 (d, J = 2.2 Hz, 1H), 7.58 - 7.50 (m, 1H), 7.43 - 7.34 (m, 2H), 7.37 - 7.22 (m, 6H), 6.27 (s, 1H), 5.68 (t, J = 7.3 Hz, 1H), 4.92 (d, J = 14.4 Hz, 1H), 4.61 - 4.49 (m, 3H), 2.27 - 2.19 (m, 1H), 2.12 - 2.02 (m, 1H), 1.67 (s, 9H), 1.01 (t, J = 7.4 Hz, 2H) |
| 61 | 1H NMR (400 MHz, 甲醇-d4) δ 9.24 (s, 1H), 8.49 (s, 1H), 8.18 (d, J = 1.7 Hz, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.78 (s, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.64 (dd, J = 8.4, 1.8 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.39 - 7.30 (m, 2H), 7.26 (d, J = 2.4 Hz, 1H), 6.22 (s, 1H), 4.79 (m, 1H), 1.52 (dd, J = 6.7, 2.3 Hz, 6H) |
| 62 | 1H NMR (400 MHz, 甲醇-d4) δ 9.26 (s, 1H), 8.45 (s, 1H), 8.15 (s, 1H), 8.08 (d, J = 8.2 Hz, 1H), 7.84 (s, 1H), 7.71 (d, J = 2.4 Hz, 1H), 7.64 - 7.56 (m, 1H), 7.47 (dd, J = 6.5, 2.4 Hz, 1H), 7.35 - 7.21 (m, 3H), 6.24 (s, 1H), 4.89 - 4.71 (m, 1H), 3.79 (d, J = 12.7 Hz, 2H), 3.20 (dd, J = 13.7, 10.8 Hz, 2H), 2.42 (m, 4H), 1.45 (s, 9H) |
| 63 | 1H NMR (400 MHz, 甲醇-d4) δ 9.25 (s, 1H), 8.46 (s, 1H), 8.18 (d, J = 1.8 Hz, 1H), 8.06 (d, J = 8.6 Hz, 1H), 7.85 (s, 1H), 7.71 (d, J = 2.4 Hz, 1H), 7.64 (dd, J = 8.6, 1.8 Hz, 1H), 7.47 (dd, J = 6.4, 2.5 Hz, 1H), 7.35 - 7.20 (m, 3H), 6.21 (s, 1H), 4.83 - 4.73 (m, 1H), 3.79 (d, J = 12.6 Hz, 2H), 3.26 - 3.15 (m, 2H), 2.48 - 2.35 (m, 4H), 1.45 (s, 9H) |
| 64 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 7.95 - 7.84 (m, 2H), 7.77 (s, 1H), 7.69 (d, J = 2.4 Hz, 1H), 7.59 (d, J = 5.5 Hz, 1H), 7.50 - 7.39 (m, 2H), 7.35 - 7.19 (m, 4H), 6.14 (s, 1H), 4.83 - 4.68 (m, 1H), 3.78 (d, J = 12.5 Hz, 2H), 3.20 (t, J = 12.6 Hz, 2H), 2.52 - 2.30 (m, 4H), 1.45 (s, 9H) |
| 71 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 8.13 - 8.07 (m, 1H), 7.95 - 7.85 (m, 2H), 7.55 (m, 1H), 7.36 - 7.18 (m, 7H), 5.91 - 5.79 (m, 1H), 3.82 m, 1H), 2.59 - 2.46 (m, 2H), 2.50 - 2.22 (m, 2H), 1.20 - 1.02 (m, 4H). |
| 72 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 7.92 (m, 1H), 7.85 - 7.72 (m, 1H), 7.60 (m, 1H), 7.49 - 7.28 (m, 5H), 7.27 - 7.14 (m, 1H), 6.88 (m, 1H), 5.92 (m, 1H), 3.90 (m, 1H), 2.66 - 2.50 (m, 5H), 2.43 (m, 2H), 1.28 - 1.10 (m, 4H). |
| 73 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 - 8.33 (m, 2H), 8.01 - 7.87 (m, 2H), 7.60 (m, 1H), 7.46 - 7.27 (m, 6H), 7.25 (m, 1H), 5.98 - 5.86 (m, 1H), 3.92 (m, 1H), 2.67 - 2.33 (m, 4H), 1.29 - 1.12 (m, 4H). |
| 74 | 1H NMR (400 MHz, 甲醇-d4) δ 8.64 (m, 1H), 8.39 (m, 1H), 8.07 (s, 1H), 7.79 (m, 1H), 7.60 (m, 1H), 7.41 - 7.29 (m, 6H), 6.46 (s, 1H), 5.86 (m, 1H), 3.93 (m, 1H), 2.63 (m, 2H), 2.43 (m, 2H), 1.29 - 1.13 (m, 4H). |
| 75 | 1H NMR (400 MHz, 甲醇-d4) δ 8.83 (s, 1H), 8.70 (s, 1H), 8.43 (m, 1H), 8.38 - 8.28 (m, 1H), 8.03 (m, 1H), 7.78 (m, 1H), 7.70 - 7.62 (m, 1H), 7.49 - 7.29 (m, 5H), 6.43 (m, 1H), 6.00 - 5.89 (m, 1H), 2.66-1.98 (m, 4H), 1.25 - 1.13 (m, 4H). |
| 90 | 1H NMR (400 MHz, DMSO-d6) δ 8.21 (m, 1H), 8.09 (m, 1H), 7.60 (m, 1H), 7.55 - 7.48 (m, 1H), 7.41 - 7.33 (m, 3H), 7.31 (d, J = 5.0 Hz, 2H), 7.29 - 7.22 (m, 1H), 6.27 (d, J = 3.4 Hz, 1H), 5.66 (dd, J = 8.9, 5.5 Hz, 1H), 4.82 - 4.64 (m, 4H), 4.56 - 4.41 (m, 1H), 3.96 (qd, J = 7.2, 4.2 Hz, 1H), 3.00 (m, 1H), 2.82 (m, 1H), 2.62 (dd, J = 14.7, 7.5 Hz, 2H), 2.40 (dt, J = 15.1, 7.4 Hz, 0H), 2.31 (d, J = 5.7 Hz, 0H), 2.29 - 2.17 (m, 1H), 1.16 - 1.11 (m, 4H), 1.11 (s, 1H). |
| 91 | 1H NMR (400 MHz, DMSO-d6) δ 8.30 (s, 1H), 8.05 (s, 1H), 7.59 (d, J = 2.2 Hz, 1H), 7.43 (s, 1H), 7.13 (s, 1H), 6.11 (s, 1H), 4.73 (dt, J = 8.0, 4.4 Hz, 4H), 4.59 - 4.45 (m, 1H), 3.93 (tt, J = 7.6, 4.4 Hz, 1H), 3.00 (s, 1H), 2.77 (d, J = 15.6 Hz, 1H), 1.11 (td, J = 2.8, 1.7 Hz, 3H), 1.09 (t, J = 2.0 Hz, 1H), 0.89 (s, 9H). |
| 92 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (m, 1H), 8.29 (m, 1H), 8.06 (m, 1H), 7.98 (m, 1H), 7.58 (m, 1H), 7.34 (m, 1H), 7.16 (m, 1H), 7.01 (m, 1H), 6.61 (m, 1H), 4.32 (tt, m, 1H), 3.93 (m, 2H), 3.40 (m, 2H), 3.06 (m, 1H), 2.02 - 1.67 (m, 1H), 1.20 - 1.02 (m, 4H), 0.91 m, 3H), 0.54 (m, 3H) |
| 99 | 1H NMR (400 MHz, DMSO-d6) δ 8.38 - 8.30 (m, 2H), 8.08 - 7.98 (m, 2H), 7.82 (d, J = 2.4 Hz, 1H), 7.55 (t, J = 6.9 Hz, 2H), 7.41 (d, J = 2.5 Hz, 1H), 7.16 (dd, J = 8.5, 2.7 Hz, 1H), 4.02 (s, 4H), 3.42 (dd, J = 14.0, 5.5 Hz, 1H), 0.85 (s, 9H) |
| 100 | 1H NMR (400 MHz, DMSO-d6) δ 8.45 (dd, J = 2.3, 1.1 Hz, 1H), 8.42 - 8.34 (m, 1H), 8.20 - 8.05 (m, 2H), 7.97 - 7.84 (m, 2H), 7.68 - 7.58 (m, 2H), 7.50 - 7.25 (m, 5H), 7.23 - 7.12 (m, 1H), 6.05 - 5.90 (m, 1H), 4.03 - 3.92 (m, 1H), 3.42 - 3.17 (m, 2H), 1.23 - 1.05 (m, 4H) |
| 101 | 1H NMR (400 MHz, 甲醇-d4) δ 8.87 (d, J = 5.4 Hz, 1H), 8.47 (d, J = 4.4 Hz, 1H), 7.97 (d, J = 6.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.50 - 7.27 (m, 6H), 5.78 (t, J = 7.2 Hz, 1H), 3.96 - 3.84 (m, 1H), 2.43 (s, 3H), 2.28 - 2.04 (m, 2H), 1.27 - 1.11 (m, 4H), 1.02 (t, J = 7.3 Hz, 3H). |
| 102 | 1H NMR (400 MHz, 甲醇-d4) δ 8.86 (s, 1H), 8.52 (s, 1H), 7.96 (s, 1H), 7.62 (m, 1H), 7.12 (m, 1H), 4.03 (m 1H), 3.95 - 3.85 (m, 2H), 2.45 (s, 3H), 1.26 - 1.07 (m, 4H), 0.99 (s, 9H). |
| 103 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (m, 1H), 8.11 - 7.98 (m, 2H), 7.65 (m, 1H), 7.11 - 7.04 (m, 1H), 6.98 (m, 1H), 4.08 (m, 1H), 3.96 - 3.78 (m, 2H), 1.27 - 1.11 (m, 4H), 0.97 (s, 9H). |
| 106 | 1H NMR (400 MHz, 甲醇-d4) δ 8.40 (s, 1H), 8.33 (d, J = 2.6 Hz, 1H), 8.01 (ddd, J = 8.5, 7.5, 2.6 Hz, 1H), 7.93 (s, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.39 - 7.23 (m, 6H), 7.11 - 7.03 (m, 1H), 5.73 (s, 1H), 3.95 - 3.85 (m, 1H), 1.28 - 1.11 (m, 4H) |
| 109 | 1H NMR (400 MHz, 甲醇-d4) δ 8.42 (s, 1H), 8.33 (d, J = 2.6 Hz, 1H), 8.00 (ddd, J = 8.5, 7.5, 2.6 Hz, 1H), 7.93 (s, 1H), 7.64 (t, J = 2.1 Hz, 1H), 7.42 - 7.25 (m, 6H), 7.07 (dd, J = 8.5, 2.6 Hz, 1H), 3.90 (m, 1H), 1.26 - 1.11 (m, 4H) |
| 110 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (m, 1H), 7.92 (m, 1H), 7.79 (m, 1H), 7.62 (m, 1H), 7.43 - 7.26 (m, 5H), 7.21 - 7.11 (m, 1H), 6.87 (m, 1H), 5.73 (m, 1H), 3.89 (m, 1H), 2.54 (s, 3H), 2.25 - 2.10 (m, 1H), 2.08 (m, 1H), 1.27 - 1.09 (m, 4H), 0.98 (m, 3H). |
| 111 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.93 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.6, 2.7 Hz, 1H), 4.02 (m, 1H), 3.94 - 3.83 (m, 2H), 2.51 (s, 3H), 1.25 - 1.10 (m, 4H), 0.94 (s, 9H). |
| 112 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.30 (dt, J = 2.6, 0.8 Hz, 1H), 8.00 (ddd, J = 8.5, 7.5, 2.6 Hz, 1H), 7.92 (s, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.12 - 7.04 (m, 2H), 3.89 (m, 1H), 1.25 - 1.10 (m, 4H), 0.97 (s, 9H) |
| 116 | 1H NMR (400 MHz, DMSO-d6) δ 8.37 - 8.30 (m, 2H), 8.13 (s, 1H), 8.03 (td, J = 8.2, 2.6 Hz, 1H), 7.82 (d, J = 2.3 Hz, 1H), 7.61 - 7.50 (m, 2H), 7.38 (d, J = 2.5 Hz, 1H), 7.19 - 7.11 (m, 1H), 4.01 - 3.91 (m, 2H), 3.42 (dd, J = 14.0, 5.5 Hz, 1H), 1.19 - 1.04 (m, 4H), 0.85 (s, 9H) |
| 117 | 1H NMR (400 MHz, DMSO-d6) δ 8.53 - 8.38 (m, 1H), 8.28 (d, J = 5.7 Hz, 1H), 8.20 - 8.03 (m, 2H), 7.83 (t, J = 2.5 Hz, 1H), 7.63 - 7.52 (m, 3H), 7.42 - 7.14 (m, 6H), 5.54 - 5.44 (m, 1H), 4.03 - 3.93 (m, 1H), 2.21 - 2.04 (m, 1H), 2.05 - 1.84 (m, 1H), 1.25 - 1.05 (m, 4H), 1.04 - 0.80 (m, 3H) |
| 119 | 1H NMR (400 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.44 - 8.29 (m, 2H), 8.14 (s, 1H), 8.04 (td, J = 8.2, 2.6 Hz, 1H), 7.72 (s, 1H), 7.43 - 7.26 (m, 2H), 7.21 - 7.12 (m, 1H), 7.08 (d, J = 2.2 Hz, 1H), 4.10 (dd, J = 14.0, 8.0 Hz, 1H), 4.01 - 3.90 (m, 1H), 3.59 - 3.49 (m, 1H), 1.19 - 1.04 (m, 4H), 0.89 (s, 9H) |
| 120 | 1H NMR (400 MHz, DMSO-d6) δ 8.48 - 8.38 (m, 1H), 8.37 - 8.30 (m, 1H), 8.18 - 8.03 (m, 2H), 7.76 (d, J = 8.7 Hz, 1H), 7.55 (s, 1H), 7.43 - 7.14 (m, 8H), 5.56 (q, J = 8.1 Hz, 1H), 4.03 - 3.92 (m, 1H), 2.23 - 2.05 (m, 1H), 1.98 (ddt, J = 20.7, 13.7, 7.0 Hz, 1H), 1.21 - 1.05 (m, 4H), 1.00 - 0.83 (m, 3H) |
| 125 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.91 (s, 1H), 7.69 - 7.59 (m, 2H), 7.26 (m, 1H), 6.85 (m, 1H), 4.03 (d, J = 13.9 Hz, 1H), 3.95 - 3.84 (m, 1H), 3.78 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 1.26 - 1.10 (m, 4H), 0.92 (s, 9H). |
| 126 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.35 (dd, J = 4.8, 1.9 Hz, 1H), 7.96 (s, 1H), 7.88 (dd, J = 7.7, 1.9 Hz, 1H), 7.61 (d, J = 2.3 Hz, 1H), 7.39 (m, 1H), 6.96 (d, J = 2.3 Hz, 1H), 4.02 (m, 1H), 3.96 - 3.78 (m, 2H), 1.27 - 1.07 (m, 4H), 0.96 (s, 9H). |
| 131 | 1H NMR (400 MHz, 甲醇-d4) δ 8.61 (m, 1H), 8.47 (s, 1H), 8.26 (m, 1H), 8.04 (s, 1H), 7.71 (m, 1H), 7.62 (m, 1H), 6.96 (m, 1H), 6.33 (s, 1H), 4.03 - 3.83 (m, 3H), 2.73 (s, 3H), 1.27 - 1.12 (m, 4H), 0.93 (s, 9H). |
| 132 | 1H NMR (400 MHz, 甲醇-d4) δ 8.62 (m, 1H), 8.48 (s, 1H), 8.28 (m, 1H), 8.05 (s, 1H), 7.73 (m, 1H), 7.63 (d, J = 2.4 Hz, 1H), 6.99 (d, J = 2.4 Hz, 1H), 4.03 - 3.80 (m, 3H), 2.74 (s, 3H), 1.27 - 1.12 (m, 4H), 0.94 (s, 9H). |
| 142 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.30 (d, J = 2.5 Hz, 1H), 8.00 (ddd, J = 8.5, 7.5, 2.6 Hz, 1H), 7.92 (s, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.09 (d, J = 2.4 Hz, 1H), 7.08 (dd, J = 8.4, 2.4 Hz, 1H), 4.13 (d, J = 14.0 Hz, 1H), 3.89 (m, 1H), 3.82 (d, J = 14.0 Hz, 1H), 1.25 - 1.10 (m, 4H), 0.98 (s, 9H) |
| 143 | 1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 1H), 8.93 (d, J = 2.1 Hz, 1H), 8.91 (d, J = 1.9 Hz, 1H), 8.48 (s, 1H), 8.33 (t, J = 2.1 Hz, 1H), 8.14 (s, 1H), 7.99 (d, J = 12.5 Hz, 2H), 7.67 (d, J = 2.3 Hz, 1H), 7.49 (s, 1H), 7.17 (d, J = 2.4 Hz, 1H), 4.00 - 3.89 (m, 1H), 1.16 - 1.10 (m, 2H), 1.10 - 1.04 (m, 2H). |
| 144 | 1H NMR (400 MHz, 甲醇-d4) δ 8.61 (m, 1H), 8.38 (m, 1H), 8.29 (m, 1H), 8.04 (m, 1H), 7.71 (m, 1H), 7.59 (m, 1H), 7.38 - 7.27 (m, 5H), 7.18 (m, 1H), 5.66 (m, 1H), 3.92 (m, 1H), 2.77 (s, 3H), 2.28 - 1.95 (m, 2H), 1.25 - 1.07 (m, 4H), 1.03 - 0.92 (m, 3H). |
| 155 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (d, J = 1.0 Hz, 1H), 8.33 (d, J = 2.5 Hz, 1H), 8.00 (ddd, J = 8.5, 7.6, 2.6 Hz, 1H), 7.94 (s, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.41 - 7.25 (m, 6H), 7.07 (ddd, J = 8.5, 2.7, 0.7 Hz, 1H), 5.77 (t, J = 7.2 Hz, 1H), 3.95 - 3.83 (m, 1H), 2.26 - 2.03 (m, 2H), 1.26 - 1.11 (m, 4H), 1.03 (t, J = 7.3 Hz, 3H) |
| 174 | 1H NMR (400 MHz, DMSO-d6) δ 8.86 - 8.82 (m, 1H), 8.59 (dd, J = 5.0, 1.6 Hz, 1H), 8.22 (d, J = 2.3 Hz, 1H), 8.18 (d, J = 5.4 Hz, 1H), 8.06 (d, J = 2.0 Hz, 1H), 8.04 (d, J = 2.3 Hz, 1H), 7.58 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 8.0, 4.9 Hz, 1H), 7.37 (dt, J = 9.7, 3.8 Hz, 3H), 7.35 - 7.28 (m, 1H), 7.27 - 7.21 (m, 2H), 7.21 - 7.15 (m, 3H), 6.41 (t, J = 8.6 Hz, 1H), 5.46 (q, J = 7.7 Hz, 1H), 4.72 (t, J = 7.4 Hz, 2H), 4.58 (dd, J = 7.8, 5.1 Hz, 2H), 4.42 - 4.29 (m, 1H), 4.17 (s, 1H), 3.77 (3, 1H), 3.25 (m, 1H), 2.70 - 2.55 (m, 2H), 2.18 - 2.03 (m, 1H), 2.03 - 1.82 (m, 1H), 0.93 (t, J = 7.3 Hz, 3H). |
| 175 | 1H NMR (400 MHz, DMSO-d6) δ 8.87 - 8.79 (m, 1H), 8.64 - 8.54 (m, 1H), 8.23 (s, 2H), 8.08 (d, J = 7.8 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.57 - 7.53 (m, 1H), 7.47 (d, J = 8.4 Hz, 1H), 7.40 (d, J = 8.6 Hz, 1H), 7.37 - 7.31 (m, 2H), 7.29 - 7.21 (m, 2H), 7.19 (dt, J = 4.5, 3.3 Hz, 2H), 6.49 (d, J = 7.8 Hz, 1H), 5.79 (d, J = 2.0 Hz, 2H), 5.46 (q, J = 7.5 Hz, 1H), 5.35 (s, 0H), 2.23 - 2.06 (m, 1H), 2.02 - 1.84 (m, 1H), 0.93 (t, J = 7.2 Hz, 3H). |
| 176 | 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.90 (s, 1H), 8.65 (s, 1H), 8.25 (s, 1H), 8.23 - 8.09 (m, 2H), 7.62 (dd, J = 3.7, 2.1 Hz, 2H), 7.47 (d, J = 8.6 Hz, 1H), 7.42 - 7.33 (m, 2H), 7.30 - 7.16 (m, 4H), 6.50 (d, J = 6.5 Hz, 1H), 5.49 (q, J = 7.7 Hz, 1H), 5.43 - 5.32 (m, 1H), 4.46 (t, J = 7.0 Hz, 2H), 3.55 (m, 2H), 3.22 - 3.09 (m, 2H), 3.03 - 2.90 (m, 2H), 2.27 - 2.07 (m, 3H), 2.07 - 1.77 (m, 5H), 0.95 (t, J = 7.3 Hz, 3H) |
| 177 | 1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.67 (s, 1H), 8.28 - 8.13 (m, 3H), 7.68 (s, 1H), 7.60 (dd, J = 4.8, 2.1 Hz, 1H), 7.56 - 7.43 (m, 2H), 7.40 - 7.28 (m, 2H), 7.28 - 7.12 (m, 5H), 6.50 (d, J = 5.9 Hz, 1H), 5.47 (q, J = 7.7 Hz, 1H), 4.86 (dd, J = 5.2, 4.1 Hz, 1H), 4.78 - 4.63 (m, 3H), 2.11 (dt, J = 13.6, 7.5 Hz, 1H), 1.91 (ddp, J = 20.8, 14.0, 7.3 Hz, 1H), 0.97 - 0.77 (m, 3H) |
| 181 | 1H NMR (400 MHz, DMSO-d6) δ 8.88 (dd, J = 10.1, 2.1 Hz, 1H), 8.64 (ddd, J = 15.1, 5.2, 1.5 Hz, 1H), 8.25 (s, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.22 (s, 2H), 8.20 (s, 1H), 7.66 (dd, J = 8.1, 5.2 Hz, 1H), 7.60 (dd, J = 4.5, 2.1 Hz, 1H), 7.49 (s, 1H), 7.43 (d, J = 8.9 Hz, 1H), 7.40 - 7.34 (m, 1H), 7.33 (s, 1H), 7.28 - 7.20 (m, 3H), 7.18 (dt, J = 8.0, 1.8 Hz, 2H), 6.50 (d, J = 5.7 Hz, 1H), 5.48 (q, J = 7.6 Hz, 1H), 5.05 (dtd, J = 18.1, 7.0, 4.2 Hz, 1H), 4.83 - 4.72 (m, 1H), 4.72 - 4.59 (m, 1H), 2.21 - 2.06 (m, 1H), 2.04 - 1.85 (m, 1H), 1.49 (d, J = 8.0 Hz, 2H), 0.94 (t, J = 7.3 Hz, 3H). |
| 189 | 1H NMR (400 MHz, DMSO-d6) δ 9.61 (s, 1H), 8.80 (s, 3H), 8.49 (s, 1H), 8.30 (d, J = 7.9 Hz, 1H), 8.17 (s, 1H), 8.09 - 7.98 (m, 2H), 7.81 (s, 1H), 7.72 (d, J = 2.2 Hz, 1H), 7.65 (d, J = 8.5 Hz, 1H), 7.28 (d, J = 2.4 Hz, 1H), 6.33 (d, J = 6.4 Hz, 1H), 4.87 (dd, J = 5.2, 4.0 Hz, 1H), 4.79 - 4.63 (m, 3H) |
| 190 | 1H NMR (400 MHz, DMSO-d6) δ 8.84 (dd, J = 14.0, 2.2 Hz, 1H), 8.64 - 8.52 (m, 1H), 8.23 (d, J = 2.1 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 6.7 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.46 (d, J = 9.5 Hz, 1H), 7.43 - 7.38 (m, 1H), 7.38 - 7.31 (m, 2H), 7.28 - 7.21 (m, 2H), 7.21 - 7.15 (m, 3H), 6.48 (d, J = 6.9 Hz, 1H), 5.48 (q, J = 7.7 Hz, 1H), 5.35 (s, 0H), 4.68 (d, J = 2.0 Hz, 2H), 4.50 (dd, J = 6.2, 4.5 Hz, 3H), 4.41 (dd, J = 6.7, 3.4 Hz, 2H), 2.12 (dt, J = 14.5, 7.4 Hz, 1H), 2.04 - 1.78 (m, 1H), 0.94 (t, J = 7.3 Hz, 3H), 0.85 (t, J = 7.2 Hz, 1H). |
| 191 | 1H NMR (400 MHz, DMSO-d6) δ 8.88 (s, 1H), 8.67 (s, 1H), 8.24 (d, J = 1.9 Hz, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.17 (d, J = 6.2 Hz, 1H), 7.67 (dt, J = 7.5, 3.2 Hz, 1H), 7.59 (dd, J = 4.1, 2.1 Hz, 1H), 7.44 (t, J = 8.1 Hz, 2H), 7.40 - 7.30 (m, 1H), 7.29 (d, J = 2.3 Hz, 1H), 7.26 - 7.20 (m, 2H), 7.20 - 7.13 (m, 3H), 6.45 (d, J = 4.9 Hz, 1H), 5.47 (q, J = 7.6 Hz, 1H), 4.03 - 3.92 (m, 1H), 2.18 - 2.02 (m, 1H), 2.02 - 1.82 (m, 1H), 1.18 - 1.13 (m, 2H), 1.13 - 1.10 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). |
| 192 | 1H NMR (400 MHz, DMSO-d6) δ 8.89 (dd, J = 10.6, 2.1 Hz, 1H), 8.65 (ddd, J = 15.0, 5.2, 1.5 Hz, 1H), 8.24 (dd, J = 6.1, 3.6 Hz, 3H), 7.72 - 7.65 (m, 1H), 7.60 (dd, J = 4.3, 2.1 Hz, 1H), 7.52 - 7.38 (m, 2H), 7.30 (d, J = 2.3 Hz, 1H), 7.28 - 7.19 (m, 3H), 7.16 (dq, J = 5.6, 1.6 Hz, 2H), 6.47 (m, 1H), 5.49 (q, J = 7.6 Hz, 1H), 5.35 (q, J = 7.5 Hz, 0H), 2.11 (dq, J = 15.3, 7.6 Hz, 1H), 1.92 (ddq, J = 21.0, 14.1, 7.2 Hz, 1H), 1.59 (d, J = 1.0 Hz, 9H), 0.94 (t, J = 7.3 Hz, 3H). |
| 193 | 1H NMR (400 MHz, DMSO-d6) δ 8.87 (d, J = 11.9 Hz, 1H), 8.64 (d, J = 5.1 Hz, 1H), 8.23 (d, J = 2.6 Hz, 1H), 8.21 - 8.13 (m, 2H), 7.63 (dd, J = 8.0, 5.1 Hz, 1H), 7.60 (dd, J = 4.0, 2.2 Hz, 1H), 7.41 (dd, J = 15.7, 7.3 Hz, 2H), 7.38 - 7.33 (m, 1H), 7.33 - 7.27 (m, 1H), 7.27 - 7.20 (m, 2H), 7.20 - 7.14 (m, 2H), 6.48 (s, 1H), 5.48 (q, J = 7.6 Hz, 1H), 4.80 (h, J = 6.7 Hz, 1H), 2.11 (dq, J = 14.8, 7.3 Hz, 1H), 2.00 - 1.85 (m, 1H), 1.47 (d, J = 8.0 Hz, 6H), 0.94 (t, J = 7.3 Hz, 3H). |
| 194 | 1H NMR (400 MHz, DMSO-d6) δ 8.93 - 8.83 (m, 1H), 8.64 (ddd, J = 14.7, 5.1, 1.5 Hz, 1H), 8.36 (d, J = 7.0 Hz, 1H), 8.25 (d, J = 1.5 Hz, 1H), 8.23 - 8.16 (m, 1H), 7.65 (dd, J = 8.0, 5.1 Hz, 1H), 7.60 (dd, J = 4.0, 2.2 Hz, 1H), 7.44 (d, J = 8.8 Hz, 1H), 7.41 - 7.33 (m, 1H), 7.32 (d, J = 2.3 Hz, 1H), 7.29 - 7.20 (m, 2H), 7.20 - 7.14 (m, 2H), 6.51 (d, J = 5.6 Hz, 1H), 5.83 (tt, J = 7.6, 6.0 Hz, 1H), 5.52 - 5.44 (m, 1H), 4.99 (ddd, J = 7.7, 6.8, 0.8 Hz, 2H), 4.88 (m, 2H), 2.18 - 2.04 (m, 1H), 2.04 - 1.84 (m, 1H), 0.93 (t, J = 7.2 Hz, 2H). |
| 195 | 1H NMR (400 MHz, DMSO-d6) δ 8.86 - 8.83 (m, 1H), 8.60 (dd, J = 5.0, 1.6 Hz, 1H), 8.26 - 8.21 (m, 1H), 8.20 (s, 1H), 8.10 - 8.04 (m, 1H), 7.59 (d, J = 2.3 Hz, 1H), 7.56 (dd, J = 8.0, 5.0 Hz, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.45 - 7.37 (m, 1H), 7.37 - 7.31 (m, 2H), 7.26 - 7.16 (m, 6H), 6.55 - 6.44 (m, 2H), 5.60 - 5.43 (m, 4H), 2.18 - 2.03 (m, 1H), 2.03 - 1.83 (m, 1H), 0.89 (t, J = 7.3 Hz, 3H). |
| 212 | 1H NMR (400 MHz, DMSO-d6) δ 8.46 (d, J = 27.8 Hz, 2H), 8.28 (d, J = 7.1 Hz, 2H), 7.78 - 7.08 (m, 6H), 6.79 (s, 4H), 5.59 - 5.33 (m, 1H), 4.92 (t, J = 6.8 Hz, 2H), 3.79 (t, J = 6.9 Hz, 2H), 3.68 - 3.56 (m, 1H), 3.49 - 3.27 (m, 4H), 3.22 - 3.06 (m, 1H), 2.99 (d, J = 2.4 Hz, 3H), 2.22 - 2.06 (m, 1H), 2.06 - 1.82 (m, 1H), 1.29 - 1.23 (m, 3H), 1.17 (t, J = 7.1 Hz, 3H), 0.99 - 0.80 (m, 3H) |
| 213 | 1H NMR (400 MHz, DMSO-d6) δ 8.31 - 8.16 (m, 3H), 7.72 (s, 1H), 7.61 (dd, J = 4.6, 2.1 Hz, 1H), 7.53 - 7.43 (m, 2H), 7.43 - 7.13 (m, 7H), 6.55 - 6.44 (m, 1H), 5.49 (q, J = 7.6 Hz, 1H), 5.04 - 4.92 (m, 1H), 2.24 - 2.05 (m, 3H), 2.05 - 1.60 (m, 8H), 1.29 - 1.20 (m, 1H), 0.95 (t, J = 7.3 Hz, 3H) |
| 221 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.20 (m, 1H), 7.90 (s, 1H), 7.74 (m, 1H), 7.66 (m, 1H), 7.52 (m, 1H), 7.45 - 7.32 (m, 2H), 7.32 (m, 1H), 7.27 - 7.20 (m, 2H), 6.82 (d, J = 8.7 Hz, 1H), 6.05 (s, 1H), 4.91 - 4.77 (m, 1H), 3.89 (s, 3H), 3.80 (m, 3H), 3.22 (m, 3H), 2.50 - 2.37 (m, 4H), 1.46 (s, 9H), 1.43 (m, 3H). |
| 223 | 1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 1H), 9.18 (s, 1H), 8.44 (s, 1H), 8.07 (s, 1H), 7.68 (d, J = 3.0 Hz, 1H), 7.62 - 7.50 (m, 1H), 7.47 - 7.19 (m, 3H), 6.10 (d, J = 8.1 Hz, 1H), 4.75 (m, 5H), 3.62 (m, 8H), 3.21 - 3.03 (m, 2H), 2.77 (m, 1H), 2.44 - 2.13 (m, 4H), 1.46 - 1.15 (m, 9H). |
| 237 | 1H NMR (400 MHz, DMSO-d6) δ 9.21 (s, 1H), 8.21 (d, J = 2.0 Hz, 1H), 8.14 (s, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.47 (d, J = 8.2 Hz, 2H), 7.40 (s, 2H), 7.38 - 7.11 (m, 4H), 6.36 (d, J = 7.8 Hz, 1H), 5.81 - 5.70 (m, 1H), 4.90 - 4.78 (m, 1H), 4.70 (m, 4H), 3.68 (d, J = 12.3 Hz, 3H), 3.14 (d, J = 12.0 Hz, 3H), 2.44 - 2.24 (m, 5H), 1.68 (d, J = 6.7 Hz, 3H), 1.36 (s, 9H). |
| 238 | 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 2.3 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.63 (dd, J = 5.4, 2.1 Hz, 1H), 7.58 (d, J = 8.3 Hz, 1H), 7.55 (s, 1H), 7.41 (s, 1H), 7.34 - 7.26 (m, 4H), 7.23 (ddd, J = 8.6, 5.2, 2.3 Hz, 2H), 6.38 (d, J = 6.3 Hz, 1H), 5.76 (q, J = 7.1 Hz, 1H), 4.79 - 4.65 (m, 4H), 4.54 - 4.40 (m, 1H), 3.07 - 2.70 (m, 3H), 1.69 (d, J = 6.6 Hz, 3H). |
| 241 | 1H NMR (400 MHz, DMSO-d6) δ 9.44 (d, J = 4.9 Hz, 1H), 9.16 (s, 1H), 8.41 (s, 1H), 8.08 (s, 1H), 7.67 (s, 1H), 7.51 (t, J = 7.5 Hz, 1H), 7.44 - 7.30 (m, 3H), 7.30 - 7.22 (m, 2H), 6.12 (d, J = 8.3 Hz, 1H), 4.92 (s, 0H), 4.88 - 4.76 (m, 1H), 4.71 (s, 3H), 3.65 (d, J = 12.0 Hz, 2H), 3.23 - 3.06 (m, 2H), 2.82 (s, 1H), 2.43 - 2.30 (m, 2H), 2.25 (d, J = 13.8 Hz, 2H), 1.35 (s, 9H). |
| 253 | 1H NMR (400 MHz, 甲醇-d4) δ 8.87 (s, 1H), 8.49 (s, 1H), 8.00 (s, 1H), 7.63 (d, J = 2.4 Hz, 1H), 7.47 (t, J = 6.7 Hz, 1H), 7.35 - 7.18 (m, 3H), 6.34 (s, 1H), 4.84 - 4.76 (m, 1H), 3.80 (d, J = 12.4 Hz, 2H), 3.27 - 3.16 (m, 2H), 2.53 - 2.36 (m, 4H), 2.42 (s, 3H), 1.46 (s, 9H) |
| 258 | 1H NMR (400 MHz, DMSO-d6) δ 9.17 (s, 1H), 8.88 (d, J = 11.8 Hz, 1H), 8.63 (d, J = 15.9 Hz, 1H), 8.37 - 8.05 (m, 3H), 7.68 - 7.10 (m, 10H), 6.49 (d, J = 7.0 Hz, 1H), 5.53 - 5.30 (m, 1H), 4.95 - 4.74 (m, 1H), 3.69 (d, J = 11.8 Hz, 2H), 3.15 (q, J = 11.6 Hz, 2H), 2.46 - 2.20 (m, 4H), 2.14 (dt, J = 14.4, 7.4 Hz, 1H), 1.94 (tt, J = 13.8, 7.5 Hz, 1H), 1.37 (s, 9H), 1.10 - 0.76 (m, 3H) |
| 264 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.03 (s, 1H), 7.64 (d, J = 2.4 Hz, 1H), 7.59 - 7.53 (m, 1H), 7.37 - 7.34 (m, 2H), 7.23 (d, J = 2.4 Hz, 1H), 5.85 (s, 1H), 5.37 (s, 1H), 4.84 - 4.74 (m, 1H), 4.14 (s, 2H), 3.81 (d, J = 12.4 Hz, 2H), 3.77 (t, J = 5.6 Hz, 2H), 3.42 (s, 1H), 3.30 - 3.16 (m, 2H), 2.50 - 2.35 (m, 4H), 2.18 (d, J = 17.4 Hz, 1H), 2.06 (d, J = 17.5 Hz, 1H), 1.47 (s, 9H) |
| 275 | 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 9.02 (d, J = 15.9 Hz, 2H), 8.40 (s, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.50 (dd, J = 6.6, 2.7 Hz, 1H), 7.43 (t, J = 9.0 Hz, 1H), 7.31 (s, 1H), 7.29 (d, J = 2.3 Hz, 1H), 7.28 - 7.20 (m, 2H), 6.11 (d, J = 8.6 Hz, 1H), 4.31 (d, J = 5.2 Hz, 2H), 3.33 (s, 2H), 2.88 - 2.72 (m, 1H), 2.72 - 2.56 (m, 1H). |
| 279 | 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 9.03 (s, 2H), 8.40 (s, 1H), 8.21 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.50 (dd, J = 6.5, 2.7 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.32 (s, 1H), 7.30 (t, J = 1.4 Hz, 1H), 7.29 - 7.24 (m, 1H), 7.22 (d, J = 9.6 Hz, 2H), 6.11 (d, J = 8.6 Hz, 1H), 5.81 (tt, J = 7.6, 6.0 Hz, 1H), 5.04 - 4.95 (m, 2H), 4.86 (ddd, J = 6.8, 6.0, 0.7 Hz, 2H), 4.32 (m, 2H), 3.34 (m, 2H), 2.89 - 2.68 (m, 3H). |
| 282 | 1H NMR (400 MHz, 甲醇-d4) δ 8.66 (m, 1H), 8.49 (m, 2H), 8.09 (s, 1H), 7.87 (m, 1H), 7.68 (m, 1H), 7.40 (m, 1H), 7.29 - 7.19 (m, 2H), 7.06 (m, 1H), 6.31 (s, 1H), 4.97 - 4.86 (m, 1H), 2.69 (s,3H), 1.56 (m, 6H). |
| 283 | 1H NMR (400 MHz, 甲醇-d4) δ 8.66 (m, 1H), 8.45 (m, 2H), 8.17 (s, 1H), 7.84 (m, 1H), 7.67 (m, 1H), 7.39 (m, 1H), 7.38 - 7.19 (m, 2H), 7.09 (m, 1H), 6.34 (s, 1H), 4.97 - 4.86 (m, 1H), 3.80 (m, 2H), 3.23 (m, 2H), 2.70 (s,3H), 2.48 - 2.41 (m, 4H), 1.46 (s, 9H). |
| 301 | 1H NMR (400 MHz, DMSO-d6) δ 9.65 (s, 1H), 8.46 (s, 1H), 8.05 (s, 1H), 7.74 (d, J = 2.2 Hz, 1H), 7.59 (dd, J = 6.6, 2.6 Hz, 1H), 7.50 (t, J = 9.0 Hz, 1H), 7.42 (d, J = 2.4 Hz, 1H), 7.34 (ddd, J = 8.8, 4.2, 2.6 Hz, 1H), 7.20 (d, J = 22.3 Hz, 1H), 6.78 (s, 1H), 6.07 (s, 1H), 4.83 (p, J = 6.7 Hz, 1H), 3.70 (t, J = 4.7 Hz, 4H), 3.35 (dd, J = 6.1, 3.7 Hz, 4H), 1.49 (d, J = 8.0 Hz,65H). |
| 302 | 1H NMR (400 MHz, DMSO-d6) δ 9.68 (s, 1H), 8.47 (s, 1H), 8.23 (s, 1H), 7.74 (d, J = 2.2 Hz, 1H), 7.60 (dd, J = 6.6, 2.6 Hz, 1H), 7.51 (t, J = 9.0 Hz, 1H), 7.44 (d, J = 2.4 Hz, 1H), 7.35 (ddd, J = 8.6, 4.3, 2.6 Hz, 1H), 6.80 (s, 1H), 6.12 (s, 1H), 5.94 - 5.83 (m, 1H), 5.02 (t, J = 7.3 Hz, 2H), 4.92 (dt, J = 9.8, 6.6 Hz, 2H), 3.70 (t, J = 4.7 Hz, 4H), 3.35 (dd, J = 6.0, 3.7 Hz, 4H). |
| 306 | 1H NMR (400 MHz, 甲醇-d4) δ 8.89 (s, 1H), 8.54 (s, 1H), 7.96 (s, 1H), 7.67 (d, J = 2.4 Hz, 1H), 7.63 - 7.55 (m, 1H), 7.40 - 7.30 (m, 3H), 6.35 (s, 1H), 4.83 (m, 1H), 2.40 (s, 3H), 1.54 (dd, J = 6.7, 0.7 Hz, 6H) |
| 307 | 1H NMR (400 MHz, 甲醇-d4) δ 8.85 (s, 1H), 8.49 (s, 1H), 7.99 (s, 1H), 7.63 (d, J = 2.4 Hz, 1H), 7.47 (dd, J = 6.4, 2.5 Hz, 1H), 7.35 - 7.24 (m, 2H), 7.23 (d, J = 2.4 Hz, 1H), 6.32 (s, 1H), 4.82 - 4.75 (m, 1H), 3.81 (d, J = 12.7 Hz, 2H), 3.32 - 3.17 (m, 2H), 2.51 - 2.38 (m, 4h), 2.40 (s, 3H), 1.46 (s, 9H) |
| 308 | 1H NMR (400 MHz, DMSO-d6) δ 9.48 (s, 1H), 8.93 - 8.80 (m, 1H), 8.67 (dd, J = 5.2, 1.5 Hz, 1H), 8.44 (s, 1H), 8.24 (d, J = 8.1 Hz, 1H), 7.94 (s, 1H), 7.82 - 7.65 (m, 2H), 7.58 (d, J = 8.8 Hz, 1H), 7.53 (dd, J = 6.6, 2.6 Hz, 1H), 7.45 (t, J = 9.0 Hz, 1H), 7.34 - 7.24 (m, 2H), 6.32 (d, J = 7.4 Hz, 1H) |
| 310 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.23 (s, 1H), 8.03 (s, 1H), 7.90 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.63 (dd, J = 6.6, 2.4 Hz, 1H), 7.46 - 7.34 (m, 3H), 6.11 (s, 1H), 1.65 (s, 9H) |
| 311 | 1H NMR (400 MHz, 甲醇-d4) δ 8.96 (s, 1H), 8.44 (s, 1H), 8.16 (s, 1H), 7.63 (d, J = 2.4 Hz, 1H), 7.45 (d, J = 6.7 Hz, 1H), 7.41 (s, 1H), 7.35 - 7.18 (m, 3H), 6.33 (s, 1H), 4.89 - 4.80 (m, 1H), 3.84 (s, 3H), 1.56 (d, J = 6.7 Hz, 6H) |
| 312 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.23 (s, 1H), 7.98 (s, 1H), 7.90 (s, 1H), 7.69 (d, J = 2.4 Hz, 1H), 7.62 (dd, J = 6.4, 2.4 Hz, 1H), 7.43 - 7.33 (m, 3H), 6.11 (s, 1H), 4.86 - 4.77 (m, 1H), 1.55 (d, J = 6.8 Hz, 6H) |
| 316 | 1H NMR (400 MHz, DMSO-d6) δ 9.61 (s, 1H), 9.11 (d, J = 1.9 Hz, 1H), 8.59 - 8.55 (m, 1H), 8.45 (s, 1H), 8.14 (s, 1H), 7.83 (td, J = 7.6, 1.8 Hz, 1H), 7.74 (d, J = 2.1 Hz, 1H), 7.70 (d, J = 2.0 Hz, 1H), 7.65 - 7.59 (m, 1H), 7.57 - 7.44 (m, 2H), 7.43 - 7.32 (m, 2H), 7.30 (d, J = 7.8 Hz, 1H), 6.43 (s, 1H), 5.74 (s, 2H). |
| 317 | 1H NMR (400 MHz, DMSO-d6) δ 9.53 (s, 1H), 8.56 (d, J = 5.0 Hz, 1H), 8.51 (d, J = 1.5 Hz, 1H), 8.49 (d, J = 2.0 Hz, 1H), 8.27 (d, J = 1.7 Hz, 1H), 7.85 (td, J = 7.8, 2.0 Hz, 1H), 7.71 (d, J = 2.3 Hz, 1H), 7.67 - 7.56 (m, 2H), 7.54 - 7.43 (m, 3H), 7.43 - 7.29 (m, 3H), 6.50 (d, J = 8.0 Hz, 1H), 5.77 (d, J = 1.9 Hz, 2H). |
| 331 | 1H NMR (400 MHz, 甲醇-d4) δ 8.55 (m, 1H), 8.35 (m, 1H), 7.92 (s, 1H), 7.89 (m, 1H), 7.68 (m, 1H), 7.57 (m, 1H), 7.41 (m, 1H), 7.36 (m, 2H), 7.26 (m, 1H), 6.32 (s, 1H), 4.48 (m, 2H), 3.92 (m, 2H). |
| 332 | 1H NMR (400 MHz, DMSO-d6) δ 8.50 (s, 1H), 8.48 (d, J = 1.1 Hz, 1H), 8.22 (d, J = 1.5 Hz, 1H), 7.72 (d, J = 2.3 Hz, 1H), 7.57 (dd, J = 6.5, 2.7 Hz, 1H), 7.53 - 7.43 (m, 2H), 7.41 (d, J = 1.3 Hz, 1H), 7.33 (dt, J = 7.6, 3.4 Hz, 1H), 6.49 (s, 1H), 4.84 (t, J = 6.7 Hz, 2H), 3.83 (s, 4H), 3.70 (t, J = 6.7 Hz, 2H), 3.24 (d, J = 37.5 Hz, 4H). |
| 333 | 1H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.50 (d, J = 1.4 Hz, 1H), 8.48 (s, 1H), 8.14 (s, 1H), 7.72 (d, J = 2.2 Hz, 1H), 7.60 (dd, J = 6.8, 2.8 Hz, 2H), 7.56 - 7.46 (m, 2H), 7.41 (s, 1H), 7.40 - 7.32 (m, 1H), 6.48 (d, J = 8.4 Hz, 1H), 4.45 (t, J = 5.3 Hz, 2H), 3.81 (t, J = 5.3 Hz, 2H). |
| 334 | 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.67 (s, 1H), 8.48 (d, J = 4.7 Hz, 1H), 8.40 (s, 1H), 7.98 (d, J = 0.5 Hz, 1H), 7.86 - 7.80 (m, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.51 (dd, J = 6.6, 2.6 Hz, 1H), 7.48 - 7.42 (m, 2H), 7.41 - 7.36 (m, 1H), 7.32 (d, J = 2.4 Hz, 1H), 7.26 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 6.29 - 6.07 (m, 1H), 4.42 (t, J = 6.3 Hz, 2H), 2.62 (t, J = 6.4 Hz, 2H), 2.29 (s, 4H), 1.48 - 1.21 (m, 6H) |
| 336 | 1H NMR (400 MHz, DMSO-d6) δ 9.42 (s, 1H), 8.69 (d, J = 2.3 Hz, 1H), 8.46 (dd, J = 4.8, 1.6 Hz, 1H), 8.40 (s, 1H), 8.02 (s, 1H), 7.88 - 7.80 (m, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.53 (dd, J = 6.6, 2.7 Hz, 1H), 7.49 (d, J = 9.1 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.37 (ddd, J = 7.9, 4.8, 0.8 Hz, 1H), 7.32 (d, J = 2.4 Hz, 1H), 7.28 (dd, J = 7.9, 4.4 Hz, 1H), 6.19 (d, J = 9.0 Hz, 1H), 5.09 - 4.99 (m, 1H), 4.38 (t, J = 5.3 Hz, 2H), 3.82 - 3.68 (m, 2H) |
| 337 | 1H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.50 (d, J = 1.5 Hz, 1H), 8.48 (s, 1H), 8.13 (s, 1H), 7.72 (d, J = 2.2 Hz, 1H), 7.65 - 7.58 (m, 2H), 7.53 - 7.45 (m, 2H), 7.43 (d, J = 1.4 Hz, 1H), 7.34 (dt, J = 8.6, 3.4 Hz, 1H), 6.48 (d, J = 8.3 Hz, 1H), 4.57 (t, J = 5.1 Hz, 2H), 3.76 (t, J = 5.1 Hz, 2H), 3.24 (s, 2H). |
| 338 | 1H NMR (400 MHz, DMSO-d6) δ 9.59 (s, 1H), 8.50 (d, J = 2.2 Hz, 2H), 8.22 (d, J = 1.6 Hz, 1H), 7.72 (d, J = 2.2 Hz, 1H), 7.61 (dt, J = 6.5, 3.9 Hz, 2H), 7.50 (dd, J = 9.8, 8.2 Hz, 1H), 7.46 (dd, J = 4.2, 2.1 Hz, 2H), 7.35 (ddd, J = 8.6, 4.3, 2.5 Hz, 1H), 6.46 (q, J = 2.4 Hz, 1H), 4.86 (p, J = 6.7 Hz, 1H), 1.52 (d, J = 8.0 Hz, 6H). |
| 339 | 1H NMR (400 MHz, DMSO-d6) δ 9.53 (s, 1H), 8.50 (d, J = 1.4 Hz, 1H), 8.48 (s, 1H), 8.39 (s, 1H), 7.71 (d, J = 2.2 Hz, 1H), 7.66 - 7.55 (m, 2H), 7.54 - 7.42 (m, 3H), 7.34 (ddd, J = 8.6, 4.1, 2.4 Hz, 1H), 6.49 (d, J = 7.9 Hz, 1H), 5.88 (tt, J = 7.3, 6.1 Hz, 1H), 5.03 (t, J = 7.3 Hz, 2H), 4.93 (q, J = 6.3 Hz, 2H). |
| 340 | 1H NMR (400 MHz, DMSO-d6) δ 9.42 (s, 1H), 8.71 - 8.64 (m, 1H), 8.47 (dd, J = 4.8, 1.6 Hz, 1H), 8.40 (s, 1H), 7.99 (d, J = 0.5 Hz, 1H), 7.84 (dt, J = 7.9, 1.9 Hz, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 6.6, 2.6 Hz, 1H), 7.50 - 7.41 (m, 2H), 7.38 (ddd, J = 7.9, 4.8, 0.8 Hz, 1H), 7.31 (d, J = 2.4 Hz, 1H), 7.27 (ddd, J = 8.9, 4.2, 2.7 Hz, 1H), 6.18 (d, J = 8.9 Hz, 1H), 4.50 (t, J = 5.2 Hz, 2H), 3.77 (dd, J = 5.6, 4.8 Hz, 2H), 3.53 - 3.41 (m, 2H), 3.36 - 3.24 (m, 2H), 3.13 (s, 3H) |
| 342 | 1H NMR (400 MHz, 甲醇-d4) δ 8.97 (s, 1H), 8.45 (s, 1H), 8.26 (s, 1H), 7.64 (d, J = 2.4 Hz, 1H), 7.51 - 7.39 (m, 2H), 7.39 - 7.25 (m, 3H), 6.36 (s, 1H), 4.92 - 4.85 (m, 1H), 3.88 (s, 3H), 3.80 (d, J = 12.6 Hz, 2H), 3.29 - 3.18 (m, 2H), 2.47 (m, 4H), 1.46 (s, 9H) |
| 345 | 1H NMR (400 MHz, DMSO-d6) δ 9.92 (s, 1H), 9.40 (d, J = 8.6 Hz, 1H), 8.40 (d, J = 3.4 Hz, 1H), 8.04 (d, J = 11.0 Hz, 1H), 7.66 (dd, J = 10.6, 2.2 Hz, 1H), 7.54 - 7.39 (m, 2H), 7.34 (s, 1H), 7.31 - 7.18 (m, 3H), 6.08 (dd, J = 29.6, 8.6 Hz, 1H), 4.78 (p, J = 6.7 Hz, 1H), 4.57 (d, J = 15.6 Hz, 1H), 4.27 (s, 1H), 3.62 (s, 1H), 3.27 (d, J = 12.0 Hz, 1H), 2.93 (s, 1H), 2.86 (d, J = 4.4 Hz, 3H), 2.80 (s, 1H), 1.45 (d, J = 8.0 Hz, 6H). |
| 346 | 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.01 (s, 1H), 7.65 (d, J = 2.4 Hz, 1H), 7.49 (dd, J = 6.6, 2.7 Hz, 1H), 7.43 (t, J = 9.0 Hz, 1H), 7.31 (s, 1H), 7.28 - 7.21 (m, 2H), 6.05 (s, 1H), 4.76 (p, J = 6.7 Hz, 1H), 4.29 (s, 2H), 3.31 (dq, J = 22.6, 6.6 Hz, 2H), 2.87 - 2.74 (m, 1H), 2.75 - 2.62 (m, 1H), 1.43 (d, J = 8.0 Hz, 6H). |
| 347 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 8.28 (s, 1H), 8.02 (s, 1H), 7.96 (s, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.47 (dd, J = 6.4, 2.5 Hz, 1H), 7.43 - 7.24 (m, 2H), 7.20 (d, J = 2.3 Hz, 1H), 7.09 (dd, J = 8.6, 2.4 Hz, 1H), 6.15 (s, 1H), 4.79 (d, J = 11.9 Hz, 1H), 3.80 (d, J = 12.6 Hz, 2H), 3.22 (t, J = 12.5 Hz, 2H), 2.55 - 2.32 (m, 4H), 1.46 (s, 9H) |
| 348 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 8.25 - 8.20 (m, 1H), 8.03 (s, 1H), 7.92 (s, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.53 - 7.46 (m, 1H), 7.38 - 7.28 (m, 3H), 6.11 (s, 1H), 4.84 - 4.73 (m, 1H), 3.81 (d, J = 12.1 Hz, 2H), 3.23 (t, J = 13.2 Hz, 2H), 2.51 - 2.35 (m, 4H), 1.46 (s, 9H) |
| 349 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 7.95 - 7.85 (m, 2H), 7.72 (d, J = 2.0 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.59 (s, 1H), 7.43 (dd, J = 6.7, 2.3 Hz, 1H), 7.35 (dd, J = 8.6, 2.0 Hz, 1H), 7.31 - 7.18 (m, 3H), 6.38 (s, 1H), 4.82 - 4.69 (m, 1H), 3.79 (d, J = 12.6 Hz, 2H), 3.20 (t, J = 12.5 Hz, 2H), 2.57 - 2.29 (m, 5H), 1.45 (s, 9H) |
| 350 | 1H NMR (400 MHz, 甲醇-d4) δ 9.01 (d, J = 1.9 Hz, 1H), 8.49 (s, 1H), 7.95 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.62 - 7.52 (m, 2H), 7.50 - 7.39 (m, 1H), 7.31 - 7.17 (m, 2H), 6.27 (s, 1H), 4.83 - 4.74 (m, 1H), 3.80 (d, J = 12.5 Hz, 2H), 3.22 (t, J = 12.7 Hz, 2H), 2.74 - 2.14 (m, 4H), 1.46 (s, 9H) |
| 351 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.95 - 7.86 (m, 2H), 7.75 - 7.65 (m, 2H), 7.55 - 7.46 (m, 2H), 7.40 - 7.26 (m, 5H), 6.45 (s, 1H), 4.79 (m, 1H), 3.78 (d, J = 12.6 Hz, 2H), 3.25 - 3.14 (m, 2H), 2.47 - 2.31 (m, 4H), 1.45 (s, 9H) |
| 352 | 1H NMR (400 MHz, DMSO-d6) δ 9.06 (q, J = 1.8 Hz, 1H), 8.39 (s, 1H), 8.08 (s, 1H), 7.69 (dd, J = 2.5, 1.2 Hz, 1H), 7.65 (d, J = 2.0 Hz, 1H), 7.56 - 7.51 (m, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.39 (t, J = 1.9 Hz, 1H), 7.28 (m, 1H), 6.36 (s, 1H), 4.76 (t, J = 6.8 Hz, 2H), 3.76 (m, 4H), 3.63 (t, J = 6.7 Hz, 2H), 3.17 (m, 4H). |
| 353 | 1H NMR (400 MHz, 甲醇-d4) δ 9.02 (d, J = 2.0 Hz, 1H), 8.46 (s, 1H), 7.98 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.65 - 7.50 (m, 2H), 7.38 - 7.30 (m, 3H), 6.32 (s, 1H), 4.79 (m, 1H), 3.80 (d, J = 12.6 Hz, 2H), 3.28 - 3.17 (m, 2H), 2.49 (d, J = 13.0 Hz, 2H), 2.40 (d, J = 12.4 Hz, 2H), 1.46 (s, 9H) |
| 354 | 1H NMR (400 MHz, 甲醇-d4) δ 9.03 (d, J = 1.9 Hz, 1H), 8.46 (s, 1H), 7.97 (s, 1H), 7.68 (d, J = 2.4 Hz, 1H), 7.60 (d, J = 1.9 Hz, 1H), 7.53 (dd, J = 5.7, 1.9 Hz, 1H), 7.37 - 7.30 (m, 3H), 6.31 (s, 1H), 4.86 - 4.80 (m, 1H), 3.60 - 3.50 (m, 2H), 3.22 (t, J = 11.0 Hz, 2H), 2.40 (d, J = 14.4 Hz, 2H), 2.34 - 2.24 (m, 2H) |
| 355 | 1H NMR (400 MHz, DMSO-d6) δ 10.02 (s, 1H), 9.68 (s, 1H), 9.08 (s, 1H), 8.40 (s, 1H), 8.11 (s, 1H), 7.75 - 7.65 (m, 2H), 7.55 (dd, J = 6.6, 2.6 Hz, 1H), 7.51 - 7.39 (m, 2H), 7.30 (ddd, J = 8.8, 4.2, 2.6 Hz, 1H), 6.39 (s, 1H), 4.78 (t, J = 6.6 Hz, 2H), 3.59 (dd, J = 7.9, 5.3 Hz, 2H), 2.77 (s, 7H). |
| 356 | 1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 1H), 9.05 (d, J = 1.9 Hz, 1H), 8.38 (s, 1H), 7.96 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.62 (d, J = 2.0 Hz, 1H), 7.55 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.29 (ddd, J = 8.8, 4.3, 2.7 Hz, 1H), 6.40 - 6.33 (m, 1H), 4.36 (t, J = 5.4 Hz, 3H), 3.73 (t, J = 5.4 Hz, 3H). |
| 357 | 1H NMR (400 MHz, DMSO-d6) δ 9.54 (d, J = 2.1 Hz, 1H), 9.05 (d, J = 1.9 Hz, 1H), 8.39 (s, 1H), 8.01 (s, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.62 (d, J = 1.9 Hz, 1H), 7.53 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.39 (d, J = 2.3 Hz, 1H), 7.28 (ddd, J = 8.9, 4.2, 2.7 Hz, 1H), 6.33 (s, 1H), 4.77 (p, J = 6.7 Hz, 1H), 1.44 (d, J = 1.9 Hz, 3H), 1.43 (d, J = 1.9 Hz, 3H). |
| 358 | 1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 1H), 9.05 (d, J = 1.9 Hz, 1H), 8.38 (s, 1H), 7.95 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.63 (d, J = 2.0 Hz, 1H), 7.54 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.41 (d, J = 2.3 Hz, 1H), 7.29 (ddd, J = 8.9, 4.3, 2.7 Hz, 1H), 6.35 (s, 1H), 4.49 (t, J = 5.1 Hz, 2H), 3.68 (t, J = 5.2 Hz, 2H), 3.18 (s, 4H). |
| 359 | 1H NMR (400 MHz, DMSO-d6) δ 9.48 (s, 1H), 9.07 (d, J = 2.0 Hz, 1H), 8.38 (s, 1H), 8.08 (s, 1H), 7.93 (s, 3H), 7.69 (d, J = 2.2 Hz, 1H), 7.64 (d, J = 2.0 Hz, 1H), 7.53 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.41 (d, J = 2.4 Hz, 1H), 7.28 (ddd, J = 8.9, 4.3, 2.7 Hz, 2H), 6.37 (s, 1H), 4.56 (t, J = 6.3 Hz, 2H), 3.39 - 3.25 (m, 2H). |
| 360 | 1H NMR (400 MHz, DMSO-d6) δ 9.47 (s, 1H), 9.37 (d, J = 10.0 Hz, 1H), 9.06 (d, J = 1.9 Hz, 1H), 8.38 (s, 1H), 8.03 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.63 (d, J = 2.0 Hz, 1H), 7.52 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.39 (d, J = 2.3 Hz, 1H), 7.37 - 7.25 (m, 2H), 6.35 (d, J = 7.3 Hz, 1H), 4.40 (t, J = 7.1 Hz, 2H), 3.14 - 2.97 (m, 2H), 2.75 (s, 3H), 2.74 (s, 3H), 2.15 (p, J = 7.3 Hz, 2H). |
| 361 | 1H NMR (400 MHz, DMSO-d6) δ 9.59 (s, 1H), 9.04 (d, J = 1.9 Hz, 1H), 8.40 (s, 1H), 8.07 (s, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.63 (dd, J = 2.1, 0.6 Hz, 1H), 7.55 (dd, J = 6.6, 2.6 Hz, 1H), 7.45 (t, J = 9.0 Hz, 1H), 7.39 (d, J = 2.4 Hz, 1H), 7.29 (m, 1H), 6.33 (s, 1H), 1.55 (s, 9H). |
| 362 | 1H NMR (400 MHz, DMSO-d6) δ 9.44 - 9.36 (m, 1H), 8.75 - 8.65 (m, 1H), 8.46 (dd, J = 4.8, 1.6 Hz, 1H), 8.40 (s, 1H), 8.13 (s, 1H), 7.92 - 7.80 (m, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.50 (dd, J = 6.6, 2.6 Hz, 1H), 7.49 - 7.40 (m, 2H), 7.37 (ddd, J = 7.9, 4.8, 0.8 Hz, 1H), 7.29 - 7.23 (m, 2H), 6.13 (d, J = 8.5 Hz, 1H), 4.53 - 4.37 (m, 1H), 2.92 (d, J = 10.6 Hz, 2H), 2.33 (t, J = 7.1 Hz, 2H), 2.09 - 1.86 (m, 6H), 1.00 (t, J = 7.2 Hz, 3H). |
| 363 | 1H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H), 9.06 (d, J = 2.0 Hz, 1H), 8.38 (s, 1H), 8.20 (s, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.64 (d, J = 1.9 Hz, 1H), 7.54 (dd, J = 6.6, 2.6 Hz, 1H), 7.44 (t, J = 9.0 Hz, 1H), 7.40 (d, J = 2.3 Hz, 1H), 7.28 (ddd, J = 8.9, 4.3, 2.7 Hz, 1H), 6.37 (s, 1H), 5.81 (tt, J = 7.6, 6.1 Hz, 1H), 4.96 (t, J = 7.3 Hz, 2H), 4.85 (q, J = 6.6 Hz, 2H). |
| 364 | 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H), 9.08 (d, J = 2.0 Hz, 1H), 8.38 (s, 1H), 8.16 (s, 1H), 7.68 (dd, J = 3.9, 2.1 Hz, 2H), 7.55 (dd, J = 6.7, 2.7 Hz, 1H), 7.49 - 7.40 (m, 2H), 7.29 (ddd, J = 8.6, 4.3, 2.7 Hz, 1H), 6.39 (s, 1H), 5.77 (s, 2H). |
| 365 | 1H NMR (400 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.42 (s, 1H), 8.19 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.57 - 7.49 (m, 3H), 7.45 - 7.37 (m, 2H), 7.26 (ddd, J = 8.8, 4.2, 2.6 Hz, 1H), 7.16 (dt, J = 3.8, 1.1 Hz, 1H), 6.43 (d, J = 8.6 Hz, 1H), 4.53 - 4.39 (m, 1H), 2.91 (d, J = 10.8 Hz, 2H), 2.33 (q, J = 6.9 Hz, 2H), 2.13 - 1.98 (m, 4H), 1.93 (dq, J = 11.7, 4.0, 3.5 Hz, 2H), 0.98 (t, J = 7.2 Hz, 3H). |
| 369 | 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.70 (s, 1H), 8.56 - 8.30 (m, 2H), 8.14 (s, 1H), 7.87 (dt, J = 8.0, 1.9 Hz, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.61 - 7.31 (m, 4H), 7.25 (qd, J = 4.1, 2.6 Hz, 2H), 6.12 (d, J = 8.4 Hz, 1H), 4.60 - 4.28 (m, 1H), 3.05 (d, J = 10.9 Hz, 2H), 2.27 - 1.75 (m, 6H), 1.02 (s, 9H) |
| 370 | 1H NMR (400 MHz, DMSO-d6) δ 9.46 (s, 1H), 9.03 (d, J = 0.8 Hz, 1H), 8.95 (s, 1H), 8.44 (s, 1H), 8.16 (s, 1H), 7.87 (t, J = 0.8 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.60 - 7.41 (m, 4H), 7.31 (dt, J = 8.8, 3.7 Hz, 1H), 6.51 (d, J = 8.7 Hz, 1H), 4.88 - 4.77 (m, 1H), 3.67 (d, J = 12.1 Hz, 2H), 3.20 - 3.09 (m, 2H), 2.39 (d, J = 13.8 Hz, 2H), 2.25 (t, J = 12.8 Hz, 2H), 1.36 (s, 9H), 1.30 (s, 1H) |
| 371 | 1H NMR (400 MHz, DMSO-d6) δ 9.45 (s, 1H), 9.26 (s, 1H), 8.42 (s, 1H), 8.03 (s, 1H), 7.64 (d, J = 2.2 Hz, 1H), 7.61 (d, J = 3.4 Hz, 1H), 7.48 - 7.42 (m, 2H), 7.39 (t, J = 9.0 Hz, 1H), 7.26 - 7.14 (m, 3H), 6.01 (d, J = 7.2 Hz, 1H), 4.72 (ddt, J = 11.8, 8.1, 4.3 Hz, 1H), 3.59 (d, J = 12.3 Hz, 2H), 3.24 - 2.97 (m, 4H), 2.35 - 2.28 (m, 2H), 2.26 - 2.06 (m, 2H), 1.22 (t, J = 7.3 Hz, 3H). |
| 372 | 1H NMR (400 MHz, DMSO-d6) δ 9.47 (s, 2H), 8.44 (d, J = 1.4 Hz, 1H), 8.43 (s, 1H), 8.17 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.58 - 7.47 (m, 2H), 7.47 - 7.37 (m, 4H), 7.27 (ddd, J = 8.9, 4.2, 2.7 Hz, 1H), 6.42 (d, J = 7.4 Hz, 1H), 4.77 (tt, J = 11.8, 4.1 Hz, 1H), 3.73 - 3.57 (m, 2H), 3.12 (tdd, J = 23.8, 18.1, 9.9 Hz, 4H), 2.42 - 2.28 (m, 2H), 2.28 - 2.12 (m, 2H), 1.23 (t, J = 7.2 Hz, 3H). |
| 373 | 1H NMR (400 MHz, DMSO-d6) δ 9.53 (s, 3H), 9.03 (dd, J = 3.0, 0.8 Hz, 1H), 8.43 (s, 1H), 8.20 (s, 2H), 7.88 (t, J = 0.8 Hz, 2H), 7.66 (d, J = 2.1 Hz, 2H), 7.61 - 7.42 (m, 8H), 7.32 (ddd, J = 8.9, 5.6, 3.0 Hz, 2H), 6.54 (d, J = 8.4 Hz, 2H), 4.84 - 4.72 (m, 2H), 3.63 (d, J = 12.3 Hz, 4H), 3.43 (s, 1H), 3.17 - 3.00 (m, 8H), 2.36 (d, J = 14.6 Hz, 4H), 2.23 (q, J = 12.8 Hz, 5H), 1.67 (ddt, J = 15.8, 11.1, 7.5 Hz, 4H), 0.92 (td, J = 7.3, 5.5 Hz, 6H). |
| 375 | 1H NMR (400 MHz, DMSO-d6) δ 9.46 (s, 1H), 9.02 (d, J = 0.8 Hz, 1H), 8.65 (s, 1H), 8.43 (s, 1H), 8.18 (s, 1H), 7.87 (t, J = 0.8 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.57 (dd, J = 6.6, 2.6 Hz, 1H), 7.52 - 7.40 (m, 3H), 7.35 - 7.26 (m, 1H), 6.51 (d, J = 8.6 Hz, 1H), 4.86 - 4.76 (m, 1H), 3.41 (d, J = 13.0 Hz, 2H), 3.12 - 3.02 (m, 2H), 2.28 (d, J = 13.5 Hz, 2H), 2.12 (d, J = 12.5 Hz, 2H) |
| 381 | 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 9.29 (s, 1H), 8.42 (s, 1H), 8.13 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 6.6, 2.6 Hz, 1H), 7.46 (t, J = 1.5 Hz, 1H), 7.43 (t, J = 9.0 Hz, 2H), 7.39 (d, J = 2.4 Hz, 1H), 7.27 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 7.04 - 6.97 (m, 1H), 6.35 (d, J = 8.3 Hz, 1H), 4.76 (tt, J = 11.9, 4.1 Hz, 1H), 3.62 (d, J = 12.4 Hz, 2H), 3.26 - 2.99 (m, 4H), 2.44 - 2.29 (m, 2H), 2.18 (ddt, J = 22.1, 13.3, 7.4 Hz, 2H), 1.23 (t, J = 7.3 Hz, 3H). |
| 382 | 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 9.20 (s, 1H), 8.42 (s, 1H), 8.26 (s, 0H), 8.13 (s, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.51 (dd, J = 6.6, 2.6 Hz, 1H), 7.48 - 7.37 (m, 4H), 7.26 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 6.95 (d, J = 3.8 Hz, 1H), 6.90 (dd, J = 3.9, 0.9 Hz, 1H), 6.31 (d, J = 8.4 Hz, 1H), 4.84 - 4.66 (m, 1H), 3.62 (d, J = 12.7 Hz, 2H), 3.24 - 2.98 (m, 4H), 2.36 (d, J = 13.3 Hz, 2H), 2.17 (dd, J = 14.6, 11.1 Hz, 2H), 1.23 (t, J = 7.3 Hz, 3H). |
| 385 | 1H NMR (400 MHz, DMSO-d6) δ 9.42 (s, 1H), 8.64 (s, 1H), 8.42 (s, 2H), 8.12 (s, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.51 (dd, J = 6.6, 2.6 Hz, 1H), 7.47 - 7.40 (m, 3H), 7.39 (d, J = 2.4 Hz, 1H), 7.26 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 7.00 (dd, J = 1.6, 0.9 Hz, 1H), 6.35 (d, J = 8.4 Hz, 1H), 4.79 (tt, J = 11.0, 4.0 Hz, 1H), 3.39 (d, J = 13.0 Hz, 2H), 3.06 (q, J = 11.9 Hz, 2H), 2.26 (d, J = 13.2 Hz, 2H), 2.19 - 2.01 (m, 2H). |
| 386 | 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.65 (s, 1H), 8.42 (s, 2H), 8.12 (s, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 6.6, 2.6 Hz, 1H), 7.47 - 7.40 (m, 2H), 7.38 (d, J = 2.4 Hz, 1H), 7.26 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 6.95 (d, J = 3.8 Hz, 1H), 6.90 (dd, J = 3.8, 0.9 Hz, 1H), 6.31 (d, J = 8.1 Hz, 1H), 4.78 (ddt, J = 11.0, 8.1, 4.1 Hz, 1H), 3.39 (d, J = 12.7 Hz, 2H), 3.07 (q, J = 11.8 Hz, 2H), 2.26 (d, J = 13.4 Hz, 2H), 2.20 - 2.02 (m, 2H). |
| 387 | 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.61 (s, 1H), 8.47 (s, 1H), 8.37 (d, J = 18.2 Hz, 1H), 8.07 (s, 1H), 7.64 (d, J = 2.2 Hz, 1H), 7.56 (d, J = 5.4 Hz, 1H), 7.44 - 7.32 (m, 4H), 7.19 - 7.08 (m, 3H), 6.99 (d, J = 5.4 Hz, 1H), 6.22 (d, J = 7.2 Hz, 1H), 4.76 (td, J = 11.2, 5.4 Hz, 1H), 3.37 (d, J = 13.4 Hz, 2H), 3.06 (t, J = 11.6 Hz, 2H), 2.22 (d, J = 13.7 Hz, 2H), 2.09 (t, J = 12.2 Hz, 2H). |
| 389 | 1H NMR (400 MHz, DMSO-d6) δ 9.46 (s, 1H), 9.33 (s, 1H), 8.47 (s, 1H), 8.09 (s, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.56 (dd, J = 5.3, 2.1 Hz, 1H), 7.42 - 7.33 (m, 4H), 7.13 (ddd, J = 8.7, 3.7, 2.3 Hz, 3H), 7.01 - 6.96 (m, 1H), 6.22 (d, J = 6.6 Hz, 1H), 4.74 (ddt, J = 11.8, 8.2, 4.1 Hz, 1H), 3.60 (d, J = 12.3 Hz, 2H), 3.15 (qt, J = 11.5, 5.3 Hz, 3H), 3.04 (dd, J = 13.2, 10.0 Hz, 2H), 2.41 - 2.28 (m, 3H), 2.24 - 2.09 (m, 3H), 1.22 (t, J = 7.3 Hz, 3H). |
| 400 | 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.80 (s, 1H), 8.60 (d, J = 5.1 Hz, 1H), 8.41 (s, 1H), 8.25 - 8.06 (m, 2H), 7.74 - 7.57 (m, 2H), 7.56 - 7.14 (m, 8H), 6.86 (d, J = 6.5 Hz, 1H), 6.23 (d, J = 6.9 Hz, 1H), 5.25 (s, 1H), 5.04 (t, J = 9.8 Hz, 2H), 3.96 - 3.76 (m, 1H), 3.67 (d, J = 10.2 Hz, 1H), 3.59 - 3.37 (m, 2H), 2.47 - 2.21 (m, 2H). |
| 401 | 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 9.12 (brs, 2H), 8.74 (d, J = 2.2 Hz, 1H), 8.53 (dd, J = 4.9, 1.6 Hz, 1H), 8.41 (s, 1H), 8.21 (d, J = 6.3 Hz, 1H), 7.98 (d, J = 7.9 Hz, 1H), 7.66 (dd, J = 2.4, 1.3 Hz, 1H), 7.56 - 7.36 (m, 4H), 7.35 - 7.18 (m, 2H), 6.24 (d, J = 8.2 Hz, 1H), 5.49 - 5.31 (m, 1H), 3.75 - 3.56 (m, 2H), 3.36 (t, J = 6.9 Hz, 2H), 2.48 (m, 1H), 2.36 - 2.19 (m, 1H). |
| 402 | 1H NMR (400 MHz, DMSO-d6) δ 10.01 (brs, 1H), 9.40 (s, 1H), 8.71 (d, J = 2.2 Hz, 1H), 8.51 (dd, J = 5.0, 1.5 Hz, 1H), 8.41 (s, 1H), 8.21 (dd, J = 14.2, 7.1 Hz, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.66 (t, J = 2.1 Hz, 1H), 7.57 - 7.36 (m, 4H), 7.34 - 7.18 (m, 2H), 6.21 (d, J = 8.0 Hz, 1H), 5.58 - 5.32 (m, 1H), 3.95 - 3.49 (m, 3H), 3.38 - 3.14 (m, 3H), 2.80 - 2.62 (m, 1H), 2.42 - 2.19 (m, 1H), 1.21 (t, J = 7.2 Hz, 3H). |
| 403 | 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 8.94 (s, 1H), 8.74 (dd, J = 2.3, 0.8 Hz, 1H), 8.62 (dd, J = 4.9, 1.6 Hz, 1H), 8.44 (s, 1H), 7.97 (dt, J = 8.0, 1.9 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.56 (ddd, J = 8.0, 4.9, 0.8 Hz, 1H), 7.47 (dd, J = 6.6, 2.7 Hz, 2H), 7.44 - 7.38 (m, 2H), 7.26 (d, J = 2.4 Hz, 1H), 7.23 (ddd, J = 8.8, 4.2, 2.7 Hz, 1H), 6.13 (d, J = 7.9 Hz, 1H), 3.76 (s, 3H). |
| 404 | 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.76 (s, 1H), 8.63 - 8.48 (m, 1H), 8.40 (s, 1H), 8.16 (s, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.61 - 7.13 (m, 10H), 6.87 (s, 2H), 6.19 (d, J = 7.7 Hz, 1H), 5.07 (s, 2H), 4.71 (m, 1H), 4.07 (d, J = 13.1 Hz, 2H), 3.00 (m, 2H), 2.02 (d, J = 12.5 Hz, 2H), 1.92 - 1.66 (m, 2H). |
| 405 | 1H NMR (400 MHz, DMSO-d6) δ 9.46 (s, 1H), 8.81 - 8.64 (m, 2H), 8.54 (dd, J = 5.0, 1.6 Hz, 1H), 8.41 (s, 2H), 8.14 (s, 1H), 8.02 (d, J = 8.1 Hz, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.58 - 7.36 (m, 4H), 7.32 - 7.16 (m, 2H), 6.22 (d, J = 7.9 Hz, 1H), 4.87 - 4.66 (m, 1H), 3.38 (d, J = 12.9 Hz, 2H), 3.06 (q, J = 12.0 Hz, 2H), 2.33 - 2.18 (m, 2H), 2.18 - 1.98 (m, 2H). |
| 408 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.43 (s, 1H), 8.09 - 8.02 (m, 1H), 7.98 (s, 1H), 7.89 (d, J = 2.3 Hz, 1H), 7.67 (d, J = 7.2 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.38 (s, 1H), 4.21 (d, J = 13.9 Hz, 1H), 3.53 (d, J = 13.9 Hz, 1H), 1.87 - 1.74 (m, 2H), 1.65 - 1.50 (m, 2H), 0.85 (s, 9H). |
| 409 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.33 (s, 1H), 8.04 (d, J = 7.9 Hz, 1H), 8.00 (s, 1H), 7.84 (d, J = 2.5 Hz, 1H), 7.68 (d, J = 7.3 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.15 (d, J = 2.5 Hz, 1H), 6.37 (s, 1H), 4.00 (d, J = 13.7 Hz, 1H), 3.42 (d, J = 13.7 Hz, 1H), 1.87 - 1.74 (m, 2H), 1.62 - 1.52 (m, 2H), 0.80 (s, 9H). |
| 410 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.35 (s, 1H), 8.04 (dd, J = 7.4, 1.8 Hz, 1H), 7.87 (d, J = 2.5 Hz, 1H), 7.82 (s, 1H), 7.64 - 7.53 (m, 2H), 7.17 (d, J = 2.5 Hz, 1H), 6.34 (s, 1H), 3.98 (d, J = 13.7 Hz, 1H), 3.97 - 3.80 (m, 1H), 3.50 (d, J = 13.7 Hz, 1H), 1.23 - 1.07 (m, 4H), 0.81 (s, 9H). |
| 411 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.23 (s, 1H), 8.10 - 8.03 (m, 1H), 7.86 (d, J = 2.5 Hz, 1H), 7.83 (s, 1H), 7.69 (d, J = 7.3 Hz, 1H), 7.65 - 7.54 (m, 1H), 7.36 - 7.29 (m, 1H), 7.21 - 7.11 (m, 2H), 6.92 (d, J = 6.8 Hz, 2H), 6.46 (s, 1H), 5.48 (t, J = 7.1 Hz, 1H), 3.90 - 3.84 (m, 1H), 2.05 (dt, J = 14.4, 7.2 Hz, 1H), 1.84 (dt, J = 13.9, 7.1 Hz, 1H), 1.22 - 1.10 (m, 4H), 0.94 (t, J = 7.3 Hz, 3H). |
| 412 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.24 (s, 1H), 8.12 (s, 1H), 8.07 (dd, J = 8.1, 1.1 Hz, 1H), 7.88 (d, J = 2.6 Hz, 1H), 7.71 (d, J = 7.3 Hz, 1H), 7.62 - 7.55 (m, 1H), 7.38 - 7.30 (m, 2H), 7.23 - 7.10 (m, 2H), 6.96 - 6.89 (m, 2H), 6.53 (s, 1H), 5.49 (t, J = 7.0 Hz, 1H), 2.06 (dt, J = 14.1, 7.1 Hz, 1H), 1.85 (dt, J = 14.1, 7.3 Hz, 1H), 1.75 - 1.62 (m, 4H), 0.95 (t, J = 7.3 Hz, 3H). |
| 413 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 8.21 (s, 1H), 8.05 (m, 1H), 7.81 (t, J = 8.1 Hz, 2H), 7.62 (d, J = 2.4 Hz, 1H), 7.44 - 7.29 (m, 5H), 7.24 (m, 1H), 6.97 - 6.85 (m, 2H), 5.95 (m, 1H), 3.47 (m, 1H), 2.67 - 2.41 (m, 7H), 1.80 (m, 4H). |
| 414 | 1H NMR (400 MHz, 甲醇-d4) δ 8.26 (s, 1H), 7.99 (s, 1H), 7.73 (m, 1H), 7.57 (m, 1H), 7.42 (s, 1H), 7.40 - 7.27 (m, 5H) , 6.19 (s, 1H), 5.76 (dd, J = 8.6, 5.5 Hz, 1H), 4.90 (td, J = 7.6, 3.1 Hz, 2H), 4.78 (m, 2H), 4.55 - 4.35 (m, 5H), 3.92 (m, 1H), 2.91 (m, 1H), 2.63 (m, 2H), 2.44 (m, 1H), 2.36 (m, 1H), 1.22 (m, 2H), 1.17 (m, 2H). |
| 415 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.47 (s, 1H), 8.14 (d, J = 0.6 Hz, 1H), 8.06 (dd, J = 7.4, 1.8 Hz, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.65 - 7.55 (m, 2H), 7.01 (d, J = 2.3 Hz, 1H), 6.40 (s, 1H), 4.17 (d, J = 13.9 Hz, 1H), 3.60 (d, J = 13.8 Hz, 1H), 2.02 - 1.83 (m, 4H), 0.86 (s, 9H). |
| 416 | 1H NMR (400 MHz, 甲醇-d4) δ 9.20 (s, 1H), 8.49 (s, 1H), 8.05 (dd, J = 8.0, 1.1 Hz, 1H), 7.98 (s, 1H), 7.72 (d, J = 2.3 Hz, 1H), 7.67 (d, J = 7.1 Hz, 1H), 7.59 (dd, J = 8.1, 7.4 Hz, 1H), 7.01 (d, J = 2.3 Hz, 1H), 6.39 (s, 1H), 4.24 (d, J = 13.9 Hz, 1H), 3.55 (d, J = 13.9 Hz, 1H), 1.88 - 1.74 (m, 2H), 1.65 - 1.50 (m, 2H), 0.86 (s, 9H). |
| 417 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.47 (s, 1H), 8.10 (s, 1H), 8.06 (dd, J = 7.3, 1.9 Hz, 1H), 7.74 (d, J = 2.3 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.02 (d, J = 2.3 Hz, 1H), 6.41 (s, 1H), 4.18 (d, J = 13.8 Hz, 1H), 3.60 (d, J = 13.8 Hz, 1H), 1.79 - 1.59 (m, 4H), 0.86 (s, 9H). |
| 418 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.47 (s, 1H), 8.05 (dd, J = 6.7, 2.5 Hz, 1H), 7.80 (s, 1H), 7.72 (d, J = 2.3 Hz, 1H), 7.63 - 7.54 (m, 2H), 6.99 (d, J = 2.4 Hz, 1H), 6.35 (s, 1H), 4.17 (d, J = 13.9 Hz, 1H), 3.86 (ddd, J = 11.4, 7.3, 4.0 Hz, 1H), 3.60 (d, J = 13.8 Hz, 1H), 1.23 - 1.08 (m, 4H), 0.85 (s, 9H). |
| 419 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.45 (s, 1H), 8.09 - 8.02 (m, 1H), 7.83 (s, 1H), 7.72 (d, J = 2.3 Hz, 1H), 7.64 (d, J = 7.4 Hz, 1H), 7.62 - 7.55 (m, 1H), 7.00 (d, J = 2.4 Hz, 1H), 6.39 (s, 1H), 4.77 (td, J = 11.4, 5.5 Hz, 1H), 4.18 (d, J = 13.8 Hz, 1H), 3.79 (d, J = 12.6 Hz, 2H), 3.55 (d, J = 13.8 Hz, 1H), 3.20 (t, J = 12.0 Hz, 2H), 2.47 - 2.37 (m, 4H), 1.45 (s, 9H), 0.84 (s, 9H). |
| 420 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.28 (s, 1H), 8.06 (dd, J = 7.7, 1.5 Hz, 1H), 7.82 (d, J = 2.2 Hz, 2H), 7.67 - 7.55 (m, 2H), 7.15 (d, J = 2.5 Hz, 1H), 6.39 (d, J = 4.6 Hz, 1H), 4.79 - 4.71 (m, 1H), 3.90 (d, J = 13.7 Hz, 1H), 3.80 (d, J = 12.5 Hz, 2H), 3.43 (d, J = 13.7 Hz, 1H), 3.24 - 3.15 (m, 2H), 2.51 - 2.34 (m, 4H), 1.45 (s, 9H), 0.79 (s, 9H). |
| 421 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.47 (s, 1H), 8.06 (dd, J = 7.5, 1.8 Hz, 1H), 7.96 (s, 1H), 7.74 (d, J = 2.2 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.02 (d, J = 2.3 Hz, 1H), 6.40 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.18 (d, J = 13.9 Hz, 1H), 3.60 (d, J = 13.9 Hz, 1H), 1.54 - 1.46 (m, 4H), 0.86 (s, 9H). |
| 422 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 5.8 Hz, 2H), 7.95 (d, J = 2.4 Hz, 1H), 7.90 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.27 (dd, J = 7.0, 1.0 Hz, 1H), 7.00 (d, J = 2.3 Hz, 1H), 6.86 (t, J = 7.0 Hz, 1H), 6.61 (dd, J = 2.4, 0.9 Hz, 1H), 6.33 (s, 1H), 4.10 (d, J = 13.9 Hz, 1H), 3.87 (tt, J = 7.5, 4.1 Hz, 1H), 3.68 (d, J = 13.8 Hz, 1H), 1.24 - 1.08 (m, 4H), 0.83 (s, 9H). |
| 423 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.09 (d, J = 0.9 Hz, 1H), 7.75 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 8.5 Hz, 1H), 7.39 (dd, J = 8.5, 7.1 Hz, 1H), 7.26 (d, J = 7.0 Hz, 1H), 7.01 (d, J = 2.3 Hz, 1H), 6.43 (s, 1H), 4.15 (d, J = 13.9 Hz, 1H), 4.05 (s, 3H), 3.90 - 3.79 (m, 1H), 3.64 (d, J = 13.9 Hz, 1H), 1.22 - 1.06 (m, 4H), 0.84 (s, 9H). |
| 424 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.35 (s, 1H), 8.05 (dd, J = 7.7, 1.6 Hz, 1H), 7.97 (s, 1H), 7.88 (d, J = 2.5 Hz, 1H), 7.66 - 7.54 (m, 2H), 7.19 (d, J = 2.5 Hz, 1H), 6.39 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 3.99 (d, J = 13.7 Hz, 1H), 3.49 (d, J = 13.8 Hz, 1H), 1.51 - 1.48 (m, 4H), 0.82 (s, 9H). |
| 425 | 1H NMR (400 MHz, DMSO-d6) δ 8.40 - 8.35 (m, 2H), 8.08 (d, J = 0.8 Hz, 1H), 8.05 (dd, J = 8.2, 2.6 Hz, 1H), 7.85 (s, 1H), 7.66 - 7.62 (m, 1H), 7.58 (s, 1H), 7.23 - 7.17 (m, 2H), 5.32 (s, 2H), 4.04 (dd, J = 14.1, 8.0 Hz, 1H), 3.48 (dd, J = 14.1, 5.6 Hz, 1H), 3.27 (d, J = 0.7 Hz, 3H), 0.88 (s, 9H). |
| 426 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.25 (s, 1H), 7.76 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.54 (dt, J = 8.6, 0.9 Hz, 1H), 7.25 (dd, J = 8.6, 6.9 Hz, 1H), 7.18 (d, J = 6.8 Hz, 1H), 7.00 (d, J = 2.3 Hz, 1H), 6.32 (s, 1H), 4.17 (s, 3H), 4.15 (d, J = 14.7 Hz, 1H), 3.89 - 3.79 (m, 1H), 3.63 (d, J = 13.8 Hz, 1H), 1.21 - 1.05 (m, 4H), 0.84 (s, 9H). |
| 427 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 7.85 (dd, J = 6.2, 2.8 Hz, 2H), 7.80 (s, 1H), 7.56 - 7.44 (m, 2H), 7.14 (d, J = 2.5 Hz, 1H), 6.26 (s, 1H), 3.98 (d, J = 13.7 Hz, 1H), 3.91 - 3.81 (m, 1H), 3.47 (d, J = 13.7 Hz, 1H), 2.78 (s, 3H), 1.26 - 1.06 (m, 4H), 0.82 (s, 9H). |
| 428 | 1H NMR (400 MHz, 甲醇-d4) δ 8.22 (s, 1H), 7.99 (s, 1H), 7.75 (d, J = 2.5 Hz, 1H), 7.63 - 7.56 (m, 1H), 7.44 - 7.39 (m, 2H), 7.37 (d, J = 2.5 Hz, 1H), 7.34 (d, J = 4.4 Hz, 1H), 7.31 - 7.21 (m, 2H), 7.15 (dd, J = 7.2, 2.5 Hz, 2H), 6.20 (s, 1H), 5.51 (t, J = 7.0 Hz, 1H), 4.62 - 4.48 (m, 4H), 3.94 - 3.88 (m, 1H), 2.20 - 1.89 (m, 2H), 1.23 - 1.17 (m, 4H), 0.99 (t, J = 7.4 Hz, 3H). |
| 429 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (s, 1H), 8.25 (s, 1H), 7.72 (d, J = 2.5 Hz, 1H), 7.39 (s, 1H), 7.35 (d, J = 2.5 Hz, 1H), 6.15 (s, 1H), 4.91 (m, 2H), 4.80 (m, 2H), 4.62 - 4.46 (m, 2H), 4.39 (m, 1H), 3.86 (d, J = 13.9 Hz, 1H), 3.75 (d, J = 13.9 Hz, 1H), 3.52 (s, 1H), 3.48 - 3.36 (m, 1H), 3.23 - 3.11 (m, 1H), 3.00 - 2.94 (m, 1H), 1.78 (m, 2H), 1.68 (3, 2H), 0.98 (s, 9H). |
| 430 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 8.09 (s, 1H), 7.86 (td, J = 3.3, 2.2 Hz, 2H), 7.58 - 7.46 (m, 2H), 7.17 (d, J = 2.5 Hz, 1H), 6.33 (s, 1H), 3.99 (d, J = 13.8 Hz, 1H), 3.47 (d, J = 13.7 Hz, 1H), 2.77 (s, 3H), 1.79 - 1.57 (m, 4H), 0.83 (s, 9H). |
| 431 | 1H NMR (400 MHz, 氯仿-d) δ 8.42 (s, 1H), 7.88 (m, 1H), 7.48 - 7.28 (m, 6H), 6.81 (m, 1H), 6.76 (s, 1H), 5.97 (s, 1H), 5.67 (m, 1H), 5.30 (s, 2H), 3.73 (m, 1H), 2.57 (s, 3H), 2.57 - 2.47 (m, 1H), 2.33 (m, 1H), 1.57 (s, 2H), 0.92 - 0.81 (m, 4H). |
| 432 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 8.20 (s, 1H), 7.79 (m, 1H), 7.61 (m, 1H), 6.88 (m, 2H), 6.24 (s, 1H), 3.88 (m, 2H), 3.62 (m, 2H), 2.51 (s, 3H), 1.86 - 1.56 (m, 4H), 0.92 (s, 9H). |
| 433 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (s, 1H), 7.96 (s, 1H), 7.71 (d, J = 2.5 Hz, 1H), 7.38 (s, 1H), 7.31 (d, J = 2.5 Hz, 1H), 6.07 (s, 1H), 4.92 (m, 2H), 4.82 - 4.78 (m, 2H), 4.64 - 4.47 (m, 2H), 4.40 (m, 1H), 3.90 (m, 1H), 3.85 (d, J = 14.1 Hz, 1H), 3.76 (d, J = 13.9 Hz, 1H), 3.52 (m, 1H), 3.42 (m, 1H), 3.16 - 3.13 (m, 1H), 3.02 - 2.88 (m, 1H), 1.24 - 1.13 (m, 4H), 0.98 (s, 9H). |
| 434 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.46 (s, 1H), 8.05 (dd, J = 7.0, 2.2 Hz, 1H), 7.87 (s, 1H), 7.72 (d, J = 2.3 Hz, 1H), 7.64 - 7.54 (m, 2H), 6.98 (d, J = 2.3 Hz, 1H), 6.35 (s, 1H), 4.17 (d, J = 13.8 Hz, 1H), 3.58 (d, J = 13.8 Hz, 1H), 1.63 (s, 3H), 1.32 - 1.25 (m, 2H), 1.08 - 1.00 (m, 2H), 0.84 (s, 9H). |
| 435 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.35 (s, 1H), 8.05 (dd, J = 7.5, 1.7 Hz, 1H), 7.89 (s, 1H), 7.87 (d, J = 2.5 Hz, 1H), 7.65 - 7.54 (m, 2H), 7.16 (d, J = 2.5 Hz, 1H), 6.35 (s, 1H), 4.00 (d, J = 13.7 Hz, 1H), 3.49 (d, J = 13.8 Hz, 1H), 1.63 (s, 3H), 1.31 - 1.25 (m, 2H), 1.08 - 0.99 (m, 2H), 0.81 (s, 9H). |
| 436 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.02 - 7.93 (m, 2H), 7.62 (m, 1H), 7.07 (m, 1H), 6.96 (m, 1H), 6.31 (s, 1H), 4.12 - 3.98 (m, 1H), 3.95 - 3.80 (m, 1H), 3.39 (m, 1H), 1.29 - 1.10 (m, 2H), 1.03 (s, 2H), 0.97 (s, 9H). 4.94 - 4.85 (m, 4H), 4.87 (s, 21H), |
| 437 | 1H NMR (400 MHz, 甲醇-d4) δ 9.01 (m, 1H), 8.83 (d, J = 8.6 Hz, 1H), 8.49 (s, 1H), 8.10 (d, J = 8.6 Hz, 1H), 7.90 - 7.81 (m, 2H), 7.81 - 7.65 (m, 4H), 6.92 (d, J = 2.3 Hz, 1H), 6.84 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.86 (m, 1H), 3.62 (d, J = 13.9 Hz, 1H), 1.22 - 1.07 (m, 4H), 0.70 (s, 9H). |
| 438 | 1H NMR (400 MHz, 氯仿-d) δ 8.40 (s, 1H), 7.90 (m, 1H), 7.43 - 7.26 (m, 5H), 7.22 (m, 2H), 6.78 (m, 1H), 6.61 (s, 1H), 6.00 (s, 1H), 5.69 (m, 1H), 5.30 (s, 2H), 3.75 (m, 3.8 Hz, 1H), 2.65 (s, 3H), 2.64 - 2.49 (m, 1H) 2.44 (s, 2H), 2.33 (s, 1H), 0.92 - 0.81 (m, 4H). |
| 440 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (m, 1H), 8.21 (s, 1H), 7.82 (m, 1H), 7.64 (m, 1H), 7.40 - 7.25 (m, 5H), 7.13 (m, 1H), 6.93 - 6.83 (m, 1H), 6.37 (m, 1H), 5.79 - 5.64 (m, 1H), 2.53 (m, 3H), 2.23 - 2.09 (m, 1H), 2.07 (m, 1H), 1.81 - 1.64 (m, 4H), 1.04 - 0.91 (m, 3H) |
| 441 | 1H NMR (400 MHz, DMSO-d6) δ 9.32 (s, 1H), 8.30 (d, J = 1.5 Hz, 1H), 8.16 (s, 1H), 8.00 (dd, J = 8.1, 1.2 Hz, 1H), 7.92 (d, J = 2.3 Hz, 1H), 7.69 - 7.57 (m, 2H), 7.50 (td, J = 7.8, 1.5 Hz, 1H), 7.43 - 7.30 (m, 2H), 6.46 (d, J = 5.8 Hz, 1H), 4.72 (s, 1H), 4.60 (s, 1H), 3.87 (dd, J = 13.8, 8.1 Hz, 1H), 3.34 (dd, J = 13.8, 5.0 Hz, 1H), 1.41 - 1.27 (m, 4H), 0.75 (s, 9H). |
| 443 | 1H NMR (400 MHz, 甲醇-d4) δ 9.75 (s, 1H), 8.59 (m, 1H), 8.46 (s, 1H), 8.26 m, 1H), 8.13 (m, 1H), 8.01 - 7.86 (m, 3H), 7.68 (m, 1H), 6.96 (m, 2H), 4.05 - 3.96 (m, 1H), 3.87 (m, 1H), 3.63 (m, 1H), 1.37 (m, 1H), 1.21 - 1.07 (m, 4H), 0.72 (s, 9H). |
| 444 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (d, J = 7.0 Hz, 1H), 8.37 (s, 1H), 8.06 (s, 1H), 7.95 (d, J = 2.4 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.29 (dt, J = 7.0, 1.0 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.87 (t, J = 7.0 Hz, 1H), 6.64 (dd, J = 2.4, 0.9 Hz, 1H), 6.38 (s, 1H), 5.93 (t, J = 54.8 Hz, 1H), 3.93 (d, J = 13.8 Hz, 1H), 3.54 (d, J = 13.7 Hz, 1H), 1.52 - 1.48 (m, 4H), 0.78 (s, 9H). |
| 445 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 8.13 (s, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.50 (d, J = 7.1 Hz, 1H), 7.48 - 7.36 (m, 2H), 7.04 (d, J = 2.3 Hz, 1H), 6.12 (s, 1H), 5.94 (t, J = 54.6 Hz, 1H), 4.92 (d, J = 14.6 Hz, 1H), 4.56 (s, 2H), 4.55 (d, J = 14.7 Hz, 1H), 4.12 (d, J = 13.9 Hz, 1H), 3.69 (d, J = 13.9 Hz, 1H), 1.58 - 1.50 (m, 4H), 0.96 (s, 9H). |
| 446 | 1H NMR (400 MHz, 甲醇-d4) δ 9.13 (s, 1H), 8.46 (s, 1H), 8.39 (d, J = 7.1 Hz, 1H), 8.04 (s, 1H), 7.80 (s, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.20 (d, J = 6.9 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H), 6.98 (t, J = 7.0 Hz, 1H), 6.32 (s, 1H), 4.08 (d, J = 13.9 Hz, 1H), 3.89 (ddd, J = 11.5, 7.4, 4.1 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 1.25 - 1.10 (m, 4H), 0.87 (s, 9H). |
| 447 | 1H NMR (400 MHz, 氯仿-d) δ 8.45 (s, 1H), 7.93 (t, J = 8.1 Hz, 1H), 7.41 (s, 1H), 7.35 (d, J = 2.3 Hz, 1H), 6.80 (dd, J = 8.4, 3.2 Hz, 1H), 6.25 (s, 1H), 5.93 (s, 1H), 5.91 (t, J = 56.0 Hz, 1H), 5.27 (s, 1H), 3.57 (m, 2H), 2.58 (s, 3H), 1.55 - 1.50 (m, 4H), 0.94 (s, 9H). |
| 448 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.13 (s, 1H), 7.74 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.51 (d, J = 8.5 Hz, 1H), 7.35 (dd, J = 8.6, 7.0 Hz, 1H), 7.23 (d, J = 7.0 Hz, 1H), 7.02 (d, J = 2.3 Hz, 1H), 6.44 (s, 1H), 4.13 (d, J = 13.9 Hz, 1H), 3.90 - 3.79 (m, 1H), 3.69 - 3.60 (m, 1H), 1.22 - 1.06 (m, 4H), 0.84 (s, 9H). |
| 449 | 1H NMR (400 MHz, 甲醇-d4) δ 9.29 (s, 1H), 8.44 (s, 1H), 8.06 (d, J = 8.1 Hz, 1H), 7.89 (s, 1H), 7.69 (s, 1H), 7.62 (d, J = 7.5 Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 7.11 (s, 1H), 6.93 (s, 1H), 4.04 (d, J = 13.8 Hz, 1H), 3.91 - 3.77 (m, 1H), 3.73 (d, J = 13.8 Hz, 1H), 1.24 - 1.00 (m, 4H), 0.87 (s, 8H). |
| 450 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (dd, J = 4.3, 1.8 Hz, 1H), 8.47 (s, 1H), 8.37 (dd, J = 8.3, 1.8 Hz, 1H), 7.93 (dd, J = 8.1, 1.4 Hz, 1H), 7.86 (dd, J = 7.2, 1.3 Hz, 1H), 7.76 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.62 - 7.53 (m, 2H), 7.27 (s, 1H), 7.10 (d, J = 2.2 Hz, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.86 - 3.70 (m, 2H), 1.19 - 1.04 (m, 4H), 0.80 (s, 9H). |
| 451 | 1H NMR (400 MHz, 甲醇-d4) δ 8.28 (s, 2H), 7.74 (d, J = 2.5 Hz, 1H), 7.53 (d, J = 7.4 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 7.3 Hz, 1H), 7.21 (d, J = 2.5 Hz, 1H), 6.13 (s, 1H), 4.99 (d, J = 14.6 Hz, 1H), 4.59 (d, J = 14.8 Hz, 1H), 4.56 (s, 2H), 3.97 (d, J = 13.8 Hz, 1H), 3.55 (d, J = 13.8 Hz, 1H), 1.85 - 1.74 (m, 2H), 1.70 - 1.61 (m, 2H), 0.93 (s, 9H). |
| 452 | 1H NMR (400 MHz, 甲醇-d4) δ 8.86 (d, J = 15.0 Hz, 2H), 8.01 (d, J = 11.5 Hz, 2H), 7.85 (s, 1H), 7.77 - 7.64 (m, 3H), 7.56 (d, J = 2.5 Hz, 1H), 7.50 - 7.41 (m, 1H), 7.34 - 7.19 (m, 3H), 7.12 (d, J = 7.4 Hz, 1H), 6.94 (d, J = 2.5 Hz, 1H), 6.70 (d, J = 2.5 Hz, 1H), 6.12 (s, 1H), 6.07 (s, 1H), 4.58 - 4.42 (m, 3H), 4.20 (dd, J = 10.0, 5.8 Hz, 1H), 4.02 (dd, J = 11.4, 4.5 Hz, 1H), 3.62 (dt, J = 15.5, 7.9 Hz, 2H), 3.20 - 3.07 (m, 2H), 3.02 (d, J = 11.6 Hz, 1H), 2.94 (p, J = 1.7 Hz, 14H), 2.89 - 2.81 (m, 1H), 2.75 (d, J = 11.6 Hz, 1H), 1.66 - 1.22 (m, 11H), 0.66 (s, 3H), 0.43 (s, 3H), 0.29 (s, 3H), -0.00 (s, 3H). |
| 453 | 1H NMR (400 MHz, DMSO-d6) δ 9.36 (d, J = 1.3 Hz, 1H), 8.42 (s, 2H), 8.04 (dd, J = 8.0, 1.2 Hz, 1H), 7.80 (s, 1H), 7.62 (d, J = 7.4 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.29 (s, 1H), 6.49 (d, J = 6.2 Hz, 1H), 3.97 (m, 2H), 2.69 (d, J = 1.3 Hz, 3H), 1.71 (d, J = 35.6 Hz, 4H), 0.79 (d, J = 1.3 Hz, 9H). |
| 454 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.92 (s, 1H), 7.84 - 7.70 (m, 2H), 7.09 (m,1H), 6.87 (m, 1H), 6.19 (s, 1H), 3.96 - 3.80 (m, 2H), 3.72 (m, 1H), 2.51 (s, 3H), 1.34 - 0.94 (m, 4H), 0.89 (s, 9H). |
| 455 | 1H NMR (400 MHz, DMSO-d6) δ 9.32 (s, 1H), 8.35 (m, 2H), 8.00 (d, J = 8.0 Hz, 1H), 7.67 - 7.54 (m, 2H), 7.49 (t, J = 7.7 Hz, 1H), 7.35 (d, J = 12.8 Hz, 1H), 6.94 (s, 1H), 6.44 (d, J = 6.1 Hz, 1H), 3.89 (dd, J = 13.7, 7.8 Hz, 1H), 3.43 (dd, J = 13.7, 5.4 Hz, 1H), 1.72 (m, 2H), 1.64 (m, 2H), 0.77 (s, 9H). |
| 456 | 1H NMR (400 MHz, DMSO-d6) δ 11.05 (s, 1H), 9.34 (d, J = 1.4 Hz, 1H), 8.07 (s, 1H), 8.01 (d, J = 8.2 Hz, 1H), 7.61 (s, 2H), 7.58 - 7.52 (m, 1H), 7.36 (s, 3H), 7.25 (s, 1H), 6.01 (d, J = 13.4 Hz, 1H), 3.94 (s, 1H), 1.13 (s, 13H). |
| 458 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.48 (s, 1H), 8.05 (dd, J = 7.9, 1.4 Hz, 1H), 7.86 (s, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.60 (dt, J = 15.3, 7.3 Hz, 2H), 7.01 (d, J = 2.3 Hz, 1H), 6.37 (s, 1H), 4.21 (d, J = 13.9 Hz, 1H), 3.74 (d, J = 1.8 Hz, 2H), 3.57 (d, J = 13.9 Hz, 1H), 1.34 - 1.25 (m, 2H), 1.21 - 1.13 (m, 2H), 0.86 (s, 9H). |
| 459 | 1H NMR (400 MHz, 甲醇-d4) δ 9.18 (s, 1H), 8.48 (s, 1H), 7.96 (s, 1H), 7.82 (d, J = 8.3 Hz, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.53 (t, J = 7.9 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 7.01 (d, J = 2.3 Hz, 1H), 6.84 (s, 1H), 4.22 (s, 3H), 3.95 - 3.85 (m, 1H), 3.89 (s, 2H), 1.27 - 1.10 (m, 4H), 0.84 (s, 9H). |
| 460 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.33 (s, 1H), 8.06 (dd, J = 7.6, 1.5 Hz, 1H), 7.90 (s, 1H), 7.88 (d, J = 2.5 Hz, 1H), 7.67 - 7.55 (m, 2H), 7.19 (d, J = 2.5 Hz, 1H), 6.42 (s, 1H), 5.26 (qd, J = 8.7, 2.5 Hz, 2H), 3.99 (d, J = 13.7 Hz, 1H), 3.46 (d, J = 13.7 Hz, 1H), 0.80 (s, 9H). |
| 461 | 1H NMR (400 MHz, 甲醇-d4) δ 8.99 (s, 1H), 8.77 (m, 1H), 8.47 (s, 1H), 8.16 - 8.05 (m, 2H), 7.87 - 7.78 (m, 1H), 7.78 - 7.66 (m, 3H), 6.97 - 6.88 (m, 2H), 4.00 (d, J = 13.9 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 1.76 - 1.58 (m, 4H), 0.70 (s, 9H). |
| 462 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 m, 3H), 8.23 (m, 1H), 8.11 (m, 1H), 7.96 m, 1H), 7.63 (m, 1H), 7.50 (m, 1H), 7.43 (s, 1H), 7.27 m, 1H), 6.45 (s, 1H), 4.08 (d, J = 14.0 Hz, 1H), 3.83 (d, J = 14.0 Hz, 1H), 1.31 - 1.08 (m, 4H), 0.98 (s, 9H). |
| 463 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.49 (s, 1H), 8.06 (dd, J = 8.0, 1.3 Hz, 1H), 7.87 (s, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.67 - 7.54 (m, 2H), 7.02 (d, J = 2.3 Hz, 1H), 6.41 (s, 1H), 4.22 (d, J = 13.9 Hz, 1H), 3.59 (d, J = 13.9 Hz, 1H), 2.98 - 2.86 (m, 2H), 2.86 - 2.73 (m, 2H), 2.13 - 1.96 (m, 2H), 0.86 (s, 9H). |
| 464 | 1H NMR (400 MHz, 甲醇-d4) δ 9.22 (s, 1H), 8.59 (d, J = 6.0 Hz, 2H), 8.30 (s, 1H), 8.12 (s, 1H), 8.06 (dd, J = 8.1, 1.1 Hz, 1H), 7.85 (d, J = 2.5 Hz, 1H), 7.69 (d, J = 7.4 Hz, 1H), 7.61 (dd, J = 8.1, 7.5 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 7.14 (d, J = 6.1 Hz, 2H), 6.45 (s, 1H), 3.94 (d, J = 13.7 Hz, 1H), 3.41 (d, J = 13.7 Hz, 1H), 2.17 - 1.94 (m, 4H), 0.79 (s, 9H). |
| 465 | 1H NMR (400 MHz, 甲醇-d4) δ 9.21 (s, 1H), 8.34 (s, 1H), 8.08 - 8.01 (m, 1H), 7.88 (s, 1H), 7.87 (d, J = 2.4 Hz, 1H), 7.67 - 7.62 (m, 1H), 7.62 - 7.55 (m, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.37 (s, 1H), 4.01 (d, J = 13.7 Hz, 1H), 3.74 (d, J = 1.9 Hz, 2H), 3.46 (d, J = 13.8 Hz, 1H), 1.32 - 1.27 (m, 2H), 1.22 - 1.14 (m, 2H), 0.81 (s, 9H). |
| 466 | 1H NMR (400 MHz, 甲醇-d4) δ 9.60 (s, 1H), 9.53 (s, 1H), 8.76 (d, J = 6.1 Hz, 1H), 8.72 (s, 1H), 8.51 (s, 1H), 8.01 (d, J = 6.2 Hz, 1H), 7.96 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.01 (d, J = 2.3 Hz, 1H), 6.76 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.96 - 3.76 (m, 1H), 3.70 (d, J = 13.9 Hz, 1H), 1.24 - 1.11 (m, 4H), 0.76 (s, 9H). |
| 467 | 1H NMR (400 MHz, 乙腈-d3) δ 9.08 (d, J = 4.6 Hz, 1H), 8.97 (d, J = 8.6 Hz, 1H), 8.36 (s, 1H), 8.24 (d, J = 8.2 Hz, 1H), 7.87 (s, 1H), 7.83 - 7.70 (m, 2H), 7.65 (s, 1H), 6.90 (d, J = 2.5 Hz, 1H), 6.77 (s, 1H), 5.74 (s, 1H), 3.65 (dd, J = 13.3, 6.8 Hz, 1H), 3.48 (dd, J = 13.5, 5.3 Hz, 1H), 1.29 (s, 1H), 1.10 m, 4H), 0.68 (s, 9H). |
| 468 | 1H NMR (400 MHz, 乙腈-d3) δ 8.35 (s, 1H), 8.10 (s, 2H), 7.96 (s, 1H), 7.88 (s, 1H), 7.76 (s, 1H), 6.94 (s, 1H), 6.88 (s, 1H), 5.93 (s, 1H), 3.84 - 3.71 (m, 2H), 3.39 (d, J = 13.9 Hz, 1H), 1.21 - 0.98 (m, 4H), 0.65 (s, 9H). |
| 469 | 1H NMR (400 MHz, 甲醇-d4) δ 9.11 (s, 1H), 9.04 (d, J = 8.7 Hz, 1H), 8.30 (s, 1H), 8.21 (s, 1H), 8.18 - 8.10 (m, 1H), 7.99 - 7.90 (m, 1H), 7.90 - 7.82 (m, 2H), 7.80 (s, 1H), 7.14 - 7.08 (m, 1H), 6.94 (s, 1H), 3.79 (d, J = 13.8 Hz, 1H), 3.48 (d, J = 13.8 Hz, 1H), 1.75 - 1.56 (m, 4H), 0.66 (s, 9H). |
| 470 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (d, J = 7.5 Hz, 2H), 8.19 (d, J = 8.0 Hz, 1H), 8.06 (t, J = 7.7 Hz, 1H), 8.00 (d, J = 7.3 Hz, 1H), 7.81 (d, J = 2.4 Hz, 1H), 7.15 (s, 1H), 7.00 (s, 1H), 3.82 (d, J = 13.7 Hz, 1H), 3.48 (d, J = 13.8 Hz, 1H), 1.82 - 1.53 (m, 4H), 0.68 (s, 9H). |
| 471 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 8.20 (s, 1H), 7.84 - 7.74 (m, 3H), 7.11 - 7.06 (m, 1H), 6.91 - 6.84 (m, 1H), 6.25 (s, 1H), 3.85 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.80 - 1.65 (m, 4H), 0.88 (s, 9H). |
| 472 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (s, 1H), 8.16 (s, 1H), 8.04 (s, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.51 (d, J = 8.4 Hz, 1H), 7.40 - 7.31 (m, 1H), 7.26 (d, J = 7.1 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.50 (s, 1H), 3.88 (d, J = 13.6 Hz, 1H), 3.51 (d, J = 13.7 Hz, 1H), 1.79 - 1.54 (m, 4H), 0.79 (s, 9H). |
| 473 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 8.05 (s, 1H), 7.84 - 7.75 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.24 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.88 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.53 (s, 4H), 0.89 (s, 9H). |
| 474 | 1H NMR (400 MHz, 甲醇-d4) δ 8.86 (s, 1H), 7.96 (s, 1H), 7.72 (s, 1H), 7.70 (d, 1H), 7.55 (d, J = 2.6 Hz, 1H), 7.33 (d, J = 7.3 Hz, 1H), 7.25 (t, J = 7.7 Hz, 1H), 6.69 (d, J = 2.6 Hz, 1H), 6.09 (s, 1H), 3.96 (dd, J = 11.1, 4.9 Hz, 1H), 3.68 - 3.60 (m, 1H), 3.23 - 3.12 (m, 1H), 3.03 (d, J = 11.5 Hz, 1H), 2.96 (p, J = 1.7 Hz, 21H), 2.77 (d, J = 11.5 Hz, 1H), 1.64 - 1.47 (m, 2H), 1.40 - 1.21 (m, 4H), 0.29 (s, 3H), 0.00 (s, 3H). |
| 475 | 1H NMR (400 MHz, 甲醇-d4) δ 9.24 (s, 1H), 8.36 (s, 1H), 8.21 (s, 1H), 8.10 (d, J = 8.3 Hz, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 7.4 Hz, 1H), 7.26 (d, J = 2.6 Hz, 1H), 6.48 (s, 1H), 4.56 - 4.47 (m, 1H), 3.95 (d, J = 11.4 Hz, 1H), 3.50 (d, J = 11.5 Hz, 2H), 3.22 (d, J = 11.7 Hz, 1H), 1.86 - 1.77 (m, 2H), 1.76 - 1.59 (m, 4H), 1.01 (s, 3H), 0.78 (s, 3H). |
| 476 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.23 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.2 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.3, 2.8 Hz, 1H), 6.24 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.85 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 2.04 - 1.97 (m, 2H), 1.97 - 1.91 (m, 2H), 0.94 (s, 9H). |
| 477 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.05 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.61 (d, J = 2.2 Hz, 1H), 6.89 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 2.7 Hz, 1H), 6.20 (s, 1H), 4.11 (d, J = 13.9 Hz, 1H), 3.76 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 1.89 - 1.80 (m, 2H), 1.66 - 1.59 (m, 2H), 0.94 (s, 9H). |
| 478 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 7.95 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.2 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.6, 2.7 Hz, 1H), 6.23 (s, 1H), 4.08 (d, J = 13.9 Hz, 1H), 3.82 (d, J = 13.9 Hz, 1H), 3.00 - 2.90 (m, 1H), 2.90 - 2.80 (m, 1H), 2.51 (s, 3H), 2.16 - 1.98 (m, 1H), 0.94 (s, 9H). |
| 479 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.22 (s, 1H), 7.80 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.89 (dd, J = 8.4, 2.8 Hz, 1H), 6.27 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.87 (d, J = 13.9 Hz, 1H), 3.09 - 2.95 (m, 3H), 2.53 (s, 3H), 2.33 (m, 1H), 2.24 - 2.10 (m, 1H), 0.94 (s, 9H). |
| 480 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.96 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.2 Hz, 1H), 6.88 (d, J = 2.3 Hz, 2H), 6.86 (d, J = 2.8 Hz, 1H), 6.18 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.83 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.66 (s, 3H), 1.35 - 1.29 (m, 2H), 1.09 - 1.03 (m, 2H), 0.93 (s, 9H). |
| 481 | 1H NMR (400 MHz, DMSO-d6) δ 9.31 (s, 1H), 8.38 (s, 1H), 8.11 - 7.92 (m, 2H), 7.78 (s, 1H), 7.64 (dd, J = 12.6, 6.8 Hz, 2H), 7.55 - 7.28 (m, 4H), 6.95 (s, 1H), 6.43 (d, J = 5.8 Hz, 1H), 3.97 (dd, J = 13.4, 8.5 Hz, 1H), 3.38 (dd, J = 13.7, 5.2 Hz, 1H), 2.90 - 2.71 (m, 2H), 2.73 - 2.57 (m, 2H), 1.87 (q, J = 8.3 Hz, 2H), 0.78 (s, 9H). |
| 482 | 1H NMR (400 MHz, 乙腈-d3) δ 8.45 (s, 1H), 7.85 (t, J = 8.2 Hz, 1H), 7.79 (s, 1H), 7.50 (d, J = 2.3 Hz, 1H), 6.87 - 6.80 (m, 2H), 6.15 (s, 1H), 3.90 (dd, J = 13.5, 6.2 Hz, 1H), 3.81 (dd, J = 13.5, 5.6 Hz, 1H), 3.72 (s, 2H), 2.52 (s, 3H), 1.29 (s, 1H), 1.19 - 1.13 (m, 2H), 0.96 (s, 9H). |
| 483 | 1H NMR (400 MHz, 甲醇-d4) δ 9.13 (s, 1H), 8.49 (s, 1H), 8.25 (s, 1H), 7.82 (d, J = 8.1 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 2.3 Hz, 1H), 6.89 (s, 1H), 4.22 (s, 3H), 3.89 (d, J = 2.5 Hz, 2H), 1.82 - 1.66 (m, 4H), 0.85 (s, 9H). |
| 484 | 1H NMR (400 MHz, 甲醇-d4) δ 9.29 (s, 1H), 8.31 (d, J = 0.6 Hz, 1H), 8.28 (s, 1H), 7.83 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 2.4 Hz, 1H), 7.57 (t, J = 7.9 Hz, 1H), 7.44 (d, J = 7.8 Hz, 1H), 7.20 (d, J = 2.5 Hz, 1H), 6.89 (s, 1H), 4.27 (s, 3H), 3.72 (q, J = 13.9 Hz, 2H), 1.81 - 1.65 (m, 4H), 0.81 (s, 9H). |
| 485 | 1H NMR (400 MHz, 甲醇-d4) δ 8.56 (s, 1H), 8.23 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.32 (dd, J = 12.3, 2.2 Hz, 1H), 6.95 - 6.75 (m, 2H), 6.24 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.87 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 2.04 - 1.88 (m, 4H), 0.95 (s, 9H). |
| 486 | 1H NMR (400 MHz, 甲醇-d4) δ 8.54 (s, 1H), 8.05 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.29 (dd, J = 12.5, 2.1 Hz, 1H), 6.92 - 6.82 (m, 1H), 6.78 (d, J = 2.1 Hz, 1H), 6.20 (s, 1H), 4.10 (d, J = 13.9 Hz, 1H), 3.76 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 1.88 - 1.77 (m, 2H), 1.62 (td, J = 4.2, 1.9 Hz, 2H), 0.94 (s, 9H). |
| 487 | 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.01 (s, 1H), 7.81 (m, 2H), 7.50 - 7.22 (m, 4H), 6.92 (dd, J = 8.5, 3.0 Hz, 1H), 6.82 (d, J = 2.1 Hz, 1H), 6.22 (d, J = 6.1 Hz, 1H), 3.94 (dd, J = 13.8, 8.0 Hz, 1H), 3.43 (dd, J = 13.7, 5.1 Hz, 1H), 2.88 - 2.74 (m, 1H), 2.68 (dd, J = 18.4, 8.4 Hz, 2H), 2.46 (s, 3H), 1.99 - 1.76 (m, 2H), 0.81 (s, 9H). |
| 488 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (s, 1H), 8.06 (s, 1H), 7.77 (t, J = 8.3 Hz, 1H), 7.65 (s, 1H), 7.38 (d, J = 7.1 Hz, 1H), 7.25 (d, J = 12.3 Hz, 1H), 6.91 (dd, J = 8.5, 3.0 Hz, 1H), 6.83 - 6.71 (m, 1H), 6.17 (d, J = 6.0 Hz, 1H), 3.95 - 3.82 (m, 1H), 3.45 (dd, J = 13.8, 5.2 Hz, 1H), 2.42 (s, 3H), 1.60 (s, 3H), 1.32 - 1.18 (m, 2H), 1.06 - 0.94 (m, 2H), 0.80 (s, 9H). |
| 489 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.23 (s, 1H), 7.83 - 7.75 (m, 2H), 7.10 (d, J = 2.6 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.24 (s, 1H), 3.87 (d, J = 13.8 Hz, 1H), 3.71 (d, J = 13.8 Hz, 1H), 2.52 (s, 3H), 2.04 - 1.96 (m, 2H), 1.94 (m, 2H), 0.90 (s, 9H). |
| 490 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.07 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.75 (d, J = 2.5 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 6.85 (m, 1H), 6.20 (s, 1H), 3.94 (d, J = 13.8 Hz, 1H), 3.59 (d, J = 13.8 Hz, 1H), 2.53 (s, 3H), 1.83 (m, 2H), 1.62 (m, 2H), 0.89 (s, 9H). |
| 491 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.23 (s, 1H), 7.86 - 7.76 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.27 (s, 1H), 3.86 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.8 Hz, 1H), 3.10 - 2.91 (m, 3H), 2.54 (s, 3H), 2.33 (m, 1H), 2.21 - 2.12 (m, 1H), 0.90 (s, 9H). |
| 492 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.97 (s, 1H), 7.83 - 7.74 (m, 2H), 7.08 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.6, 2.8 Hz, 1H), 6.19 (s, 1H), 3.87 (d, J = 13.9 Hz, 1H), 3.71 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.66 (s, 3H), 1.35 - 1.28 (m, 2H), 1.10 - 1.01 (m, 2H), 0.89 (s, 9H). |
| 493 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (dd, J = 4.5, 1.9 Hz, 1H), 8.21 (s, 1H), 8.20 (s, 1H), 8.17 (dd, J = 9.1, 1.9 Hz, 1H), 7.99 (s, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.14 (dd, J = 9.1, 4.4 Hz, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.45 (s, 1H), 3.77 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.78 - 1.61 (m, 4H), 0.87 (s, 9H). |
| 494 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 7.96 (s, 1H), 7.83 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.0 Hz, 1H), 7.10 (s, 1H), 6.91 - 6.81 (m, 1H), 6.23 (s, 1H), 3.93 (d, J = 13.8 Hz, 1H), 3.67 (d, J = 13.8 Hz, 1H), 2.94 (m, 2H), 2.90 - 2.78 (m, 1H), 2.52 (s, 3H), 2.17 - 1.99 (m, 2H), 0.90 (s, 9H). |
| 495 | 1H NMR (400 MHz, DMSO-d6) δ 8.49 (s, 1H), 8.22 (s, 1H), 8.14 (s, 1H), 7.80 (t, J = 8.3 Hz, 1H), 7.67 (d, J = 2.1 Hz, 1H), 7.57 (s, 1H), 7.07 - 6.90 (m, 2H), 6.25 (s, 1H), 4.75 (s, 1H), 4.62 (s, 1H), 3.99 (dd, J = 13.8, 7.9 Hz, 1H), 3.51 (dd, J = 13.8, 5.3 Hz, 1H), 2.45 (s, 3H), 1.42 (dd, J = 8.2, 4.5 Hz, 2H), 1.33 (s, 2H), 0.82 (s, 9H).;1H NMR (400 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.13 (s, 1H), 7.80 (t, J = 8.3 Hz, 1H), 7.61 (d, J = 2.2 Hz, 1H), 7.47 (s, 1H), 7.36 (d, J = 7.4 Hz, 1H), 6.94 (dd, J = 8.5, 3.0 Hz, 1H), 6.91 (s, 1H), 6.23 (d, J = 6.7 Hz, 1H), 4.75 (s, 1H), 4.63 (s, 1H), 3.88 (dd, J = 13.8, 8.1 Hz, 1H), 3.40 (dd, J = 14.0, 4.8 Hz, 1H), 2.44 (s, 3H), 1.42 (s, 2H), 1.33 (s, 2H), 0.79 (d, J = 1.1 Hz, 9H). |
| 496 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.09 (d, J = 1.0 Hz, 1H), 7.89 (s, 1H), 7.70 (d, J = 2.2 Hz, 1H), 7.56 - 7.49 (m, 1H), 7.39 (dd, J = 8.5, 7.1 Hz, 1H), 7.28 (d, J = 7.1 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 6.48 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 4.15 (d, J = 13.9 Hz, 1H), 4.05 (s, 3H), 3.64 (d, J = 13.9 Hz, 1H), 1.48 (d, J = 9.0 Hz, 4H), 0.85 (s, 9H). |
| 497 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.25 (s, 1H), 7.91 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.25 (dd, J = 8.5, 6.9 Hz, 1H), 7.20 (d, J = 6.6 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H), 6.37 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.17 (s, 3H), 4.16 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 1.52 - 1.45 (m, 4H), 0.85 (s, 9H). |
| 498 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.96 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.64 - 7.59 (m, 1H), 7.56 (t, J = 7.5 Hz, 1H), 7.53 - 7.48 (m, 1H), 7.34 (d, J = 2.3 Hz, 1H), 7.22 (s, 1H), 5.93 (t, J = 54.8 Hz, 1H), 4.49 (d, J = 3.6 Hz, 2H), 4.05 (d, J = 14.0 Hz, 1H), 3.93 (d, J = 14.0 Hz, 1H), 3.20 (s, 3H), 1.51 (s, 4H), 1.01 (s, 9H). |
| 499 | 1H NMR (400 MHz, 甲醇-d4) δ 9.05 (s, 1H), 8.46 (s, 1H), 8.08 (s, 1H), 7.80 (d, J = 7.9 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.39 (d, J = 7.6 Hz, 1H), 6.99 (d, J = 2.3 Hz, 1H), 6.86 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.20 (s, 3H), 3.86 (d, J = 1.7 Hz, 2H), 1.54 (s, 4H), 0.84 (s, 9H). |
| 500 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.13 (d, J = 1.0 Hz, 1H), 7.88 (s, 1H), 7.71 (d, J = 2.3 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.35 (dd, J = 8.4, 7.0 Hz, 1H), 7.25 (d, J = 7.0 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 6.49 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 4.15 (d, J = 13.9 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.61 - 1.37 (m, 4H), 0.86 (s, 9H). |
| 501 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.05 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.23 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 2.50 (s, 3H), 1.53 (s, 4H), 0.93 (s, 9H). |
| 502 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.05 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.98 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.25 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.12 (d, J = 14.2 Hz, 1H), 3.90 (d, J = 14.3 Hz, 1H), 2.50 (s, 3H), 2.03 - 1.90 (m, 1H), 1.76 - 1.66 (m, 3H), 1.54 (s, 4H), 1.19 (s, 3H). |
| 503 | 1H NMR (400 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.14 (s, 1H), 7.87 - 7.74 (m, 2H), 7.49 - 7.38 (m, 2H), 7.20 (d, J = 2.5 Hz, 1H), 6.93 (dd, J = 8.3, 3.1 Hz, 1H), 6.24 (d, J = 7.2 Hz, 1H), 4.74 (s, 1H), 4.62 (s, 1H), 3.85 (dd, J = 13.8, 8.1 Hz, 1H), 3.40 (dd, J = 13.8, 5.1 Hz, 1H), 2.44 (s, 3H), 1.42 (s, 2H), 1.33 (s, 2H), 0.79 (s, 9H). |
| 504 | 1H NMR (400 MHz, 乙腈-d3) δ 10.01 (s, 1H), 8.60 (d, J = 6.3 Hz, 1H), 8.41 (s, 1H), 8.30 (d, J = 6.3 Hz, 1H), 8.13 (d, J = 7.9 Hz, 1H), 8.07 - 7.96 (m, 2H), 7.83 (s, 1H), 7.58 (d, J = 2.2 Hz, 1H), 6.89 (s, 1H), 6.81 (d, J = 2.3 Hz, 1H), 3.95 - 3.75 (m, 2H), 3.51 (d, J = 13.3 Hz, 1H), 1.17 - 1.07 (m, 4H), 0.69 (s, 9H). |
| 505 | 1H NMR (400 MHz, 乙腈-d3) δ 8.46 (s, 1H), 8.18 (m, 1H), 8.04 - 7.96 (m, 1H), 7.97 (s, 1H), 7.57 (d, J = 2.3 Hz, 1H), 7.33 - 7.22 (m, 2H), 6.96 (d, J = 2.3 Hz, 1H), 6.29 (s, 1H), 5.89 (t, J = 54.6 Hz, 1H), 3.98 (dd, J = 13.9, 6.3 Hz, 1H), 3.78 (dd, J = 13.8, 5.3 Hz, 1H), 1.52 (d, J = 1.2 Hz, 4H), 0.97 (s, 9H). |
| 506 | 1H NMR (400 MHz, 乙腈-d3) δ 8.88 (s, 1H), 8.64 (d, J = 5.2 Hz, 1H), 8.42 (s, 1H), 8.25 (d, J = 8.2 Hz, 1H), 7.97 (s, 1H), 7.70 - 7.62 (m, 1H), 7.55 (d, J = 2.3 Hz, 1H), 6.98 (d, J = 2.3 Hz, 1H), 6.28 (s, 1H), 6.04 - 5.74 (m, 1H), 3.93 (m, 1H), 3.71 m, 1H), 1.52 (s, 4H), 0.96 (s, 9H). |
| 507 | 1H NMR (400 MHz, 乙腈-d3) δ 8.68 (dd, J = 5.8, 1.5 Hz, 1H), 8.46 (s, 1H), 8.41 (dd, J = 8.1, 1.5 Hz, 1H), 7.97 (s, 1H), 7.74 (dd, J = 8.1, 5.8 Hz, 1H), 7.51 (d, J = 2.3 Hz, 1H), 7.38 (s, 1H), 7.00 (d, J = 2.4 Hz, 1H), 6.40 (s, 1H), 5.89 (t, J = 54.6 Hz, 1H), 3.95 - 3.79 (m, 2H), 2.79 (s, 3H), 1.53 (d, J = 2.8 Hz, 4H), 0.96 (s, 9H). |
| 508 | 1H NMR (400 MHz, 乙腈-d3) δ 9.07 (d, J = 4.8 Hz, 1H), 8.94 (d, J = 8.6 Hz, 1H), 8.44 (m, 1H), 8.27 - 8.20 (m, 1H), 7.92 - 7.81 (m, 3H), 7.76 (m, 1H), 7.56 (m, 1H), 6.85 - 6.76 (m, 2H), 6.67 (s, 1H), 5.85 (t, J = 54.6 Hz, 1H), 3.81 (m, 1H), 3.60 (m, 1H), 1.48 (m, 4H), 0.72 (s, 9H). |
| 509 | 1H NMR (400 MHz, 氯仿-d) δ 8.42 (s, 1H), 7.93 (t, J = 8.1 Hz, 1H), 7.42 - 7.35 (m, 2H), 6.78 (m, 1H), 6.16 (s, 1H), 5.92 (d, J = 3.1 Hz, 1H), 5.90 (t, J = 55.9 Hz, 1H), 5.51 (d, J = 3.1 Hz, 1H), 5.29 (s, 2H), 4.99 (s, 1H), 3.55 (m, 2H), 2.57 (s, 3H), 1.53 - 1.50 (m, 4H), 0.92 (s, 9H). |
| 510 | 1H NMR (400 MHz, 乙腈-d3) δ 8.43 (s, 1H), 8.36 (m, 1H), 7.94 (s, 1H), 7.97 - 7.90 (m, 1H), 7.51 (m, 1H), 7.36 (m, 1H), 6.80 (m, 1H), 3.82 (m, 1H), 1.52 (s, 4H), 0.98 (s, 9H). |
| 511 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (d, J = 1.2 Hz, 1H), 8.19 - 8.13 (m, 1H), 7.82 (dt, J = 15.9, 8.2 Hz, 1H), 7.61 (dd, J = 26.8, 2.1 Hz, 1H), 7.43 - 7.28 (m, 1H), 7.09 (s, 1H), 7.02 - 6.87 (m, 2H), 6.34 (dd, J = 25.4, 7.3 Hz, 1H), 6.29 - 5.98 (m, 1H), 4.68 (d, J = 18.2 Hz, 1H), 3.92 (s, 1H), 3.78 (d, J = 6.9 Hz, 1H), 2.45 (s, 3H), 2.50 (m, 2H), 1.50 (d, J = 4.5 Hz, 4H), 1.22 (d, J = 1.2 Hz, 3H), 1.14 (d, J = 1.2 Hz, 3H), 1.09 - 1.06 (m, 3H), 0.80 (s, 3H). |
| 512 | 1H NMR (400 MHz, DMSO-d6) δ 8.36 (s, 1H), 8.18 (s, 1H), 7.82 (t, J = 8.2 Hz, 1H), 7.63 (dd, J = 2.3, 1.0 Hz, 1H), 7.50 - 7.34 (m, 2H), 7.00 - 6.90 (m, 2H), 6.29 - 5.97 (m, 2H), 3.92 (dd, J = 14.0, 7.9 Hz, 1H), 3.59 (dd, J = 14.0, 4.9 Hz, 1H), 2.47 (s, 2H), 2.45 (s, 3H), 1.51 (s, 4H), 0.90 (d, J = 8.0 Hz, 6H). |
| 513 | 1H NMR (400 MHz, 甲醇-d4) δ 8.56 (d, J = 1.9 Hz, 1H), 8.20 (d, J = 1.7 Hz, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.34 (ddd, J = 12.3, 2.3, 1.2 Hz, 1H), 6.95 - 6.77 (m, 2H), 6.25 (s, 1H), 4.04 (dd, J = 13.9, 1.3 Hz, 1H), 3.87 (dd, J = 13.9, 2.1 Hz, 1H), 2.50 (s, 3H), 1.83 - 1.60 (m, 4H), 0.95 (d, J = 1.0 Hz, 9H). |
| 514 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 8.05 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.32 (dd, J = 12.4, 2.2 Hz, 1H), 6.94 - 6.75 (m, 2H), 6.23 (s, 1H), 5.94 (t, J = 54.6 Hz, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.54 (s, 4H), 0.94 (s, 9H). |
| 517 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.03 (s, 1H), 7.80 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.89 - 6.84 (m, 1H), 6.21 (s, 1H), 4.05 (d, J = 14.0 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.90 (m, 2H), 1.77 - 1.68 (m, 2H), 0.94 (s, 9H). |
| 518 | 1H NMR (400 MHz, DMSO-d6) δ 8.39 - 8.30 (m, 2H), 8.26 (d, J = 1.1 Hz, 1H), 8.05 (td, J = 8.3, 2.5 Hz, 1H), 7.66 - 7.57 (m, 2H), 7.47 (s, 1H), 7.20 - 7.12 (m, 2H), 6.29 - 5.94 (m, 1H), 4.00 (dd, J = 13.9, 8.0 Hz, 1H), 3.45 (dd, J = 13.8, 5.4 Hz, 1H), 1.51 (s, 4H), 0.87 (d, J = 1.1 Hz, 9H). |
| 519 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.22 (d, J = 5.0 Hz, 1H), 7.78 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.39 (d, J = 3.6 Hz, 1H), 7.23 (d, J = 5.1 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.60 (d, J = 3.5 Hz, 1H), 6.44 (s, 1H), 4.12 (d, J = 13.8 Hz, 1H), 3.86 (s, 3H), 3.60 (d, J = 13.8 Hz, 1H), 1.24 - 1.05 (m, 4H), 0.81 (s, 9H). |
| 520 | 1H NMR (400 MHz, 甲醇-d4) δ 8.88 (s, 1H), 8.47 (s, 1H), 8.12 (s, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.61 - 7.47 (m, 2H), 7.15 (d, J = 2.3 Hz, 1H), 6.65 (s, 1H), 4.15 (d, J = 13.9 Hz, 1H), 4.04 (s, 3H), 3.69 (d, J = 14.0 Hz, 1H), 1.79 - 1.56 (m, 4H), 0.88 (s, 9H). |
| 521 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.25 (s, 1H), 7.98 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.69 - 7.63 (m, 1H), 7.37 (d, J = 6.9 Hz, 1H), 7.10 (d, J = 2.3 Hz, 1H), 7.05 (dd, J = 8.4, 6.9 Hz, 1H), 6.64 (s, 1H), 4.22 (s, 3H), 4.15 (d, J = 13.9 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 1.76 - 1.52 (m, 4H), 0.83 (s, 9H). |
| 522 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.17 (s, 1H), 7.98 (dd, J = 5.3, 4.0 Hz, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.39 - 7.31 (m, 2H), 6.97 (d, J = 2.4 Hz, 1H), 6.82 (s, 1H), 4.39 (s, 3H), 3.93 (d, J = 13.9 Hz, 1H), 3.78 (d, J = 13.9 Hz, 1H), 1.79 - 1.63 (m, 4H), 0.78 (s, 9H). |
| 523 | 1H NMR (400 MHz, 甲醇-d4) δ 9.25 (s, 1H), 8.31 (s, 1H), 8.12 (s, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.57 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 7.6 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.87 (s, 1H), 5.94 (t, J = 54.6 Hz, 1H), 4.26 (s, 3H), 3.71 (d, J = 1.2 Hz, 2H), 1.54 (s, 4H), 0.81 (s, 9H). |
| 524 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.95 (s, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.28 (dd, J = 8.4, 7.2 Hz, 1H), 6.97 (d, J = 7.1 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.70 (s, 1H), 3.96 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.8 Hz, 1H), 2.52 (s, 3H), 1.84 - 1.56 (m, 4H), 0.77 (s, 9H). |
| 525 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (d, J = 1.4 Hz, 1H), 8.25 (d, J = 1.0 Hz, 1H), 8.07 (s, 1H), 7.80 (s, 1H), 7.68 (d, J = 2.1 Hz, 1H), 7.59 (s, 1H), 7.50 (dt, J = 7.7, 1.2 Hz, 1H), 7.34 - 7.23 (m, 2H), 7.14 (d, J = 2.2 Hz, 1H), 6.46 (s, 1H), 5.19 (s, 1H), 3.98 (s, 3H), 3.40 (dd, J = 13.8, 5.3 Hz, 1H), 1.59 (s, 3H), 1.26 - 1.18 (m, 2H), 1.04 - 0.96 (m, 2H), 0.81 (s, 9H). |
| 526 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 8.24 (s, 1H), 8.00 (s, 1H), 7.84 (d, J = 2.5 Hz, 1H), 7.70 - 7.63 (m, 1H), 7.38 (d, J = 6.9 Hz, 1H), 7.24 (d, J = 2.5 Hz, 1H), 7.05 (dd, J = 8.4, 7.0 Hz, 1H), 6.62 (s, 1H), 4.22 (s, 3H), 3.94 (d, J = 13.8 Hz, 1H), 3.55 (d, J = 13.7 Hz, 1H), 1.72 - 1.58 (m, 4H), 0.78 (s, 9H). |
| 527 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.17 (s, 1H), 7.97 (t, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.36 (d, J = 4.8 Hz, 2H), 7.16 (d, J = 2.6 Hz, 1H), 6.83 (s, 1H), 4.41 (s, 3H), 3.76 (d, J = 13.9 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.78 - 1.62 (m, 4H), 0.74 (s, 9H). |
| 528 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 7.95 (s, 1H), 7.80 (d, J = 2.5 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.29 (dd, J = 8.4, 7.2 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 7.01 (d, J = 7.2 Hz, 1H), 6.70 (s, 1H), 3.76 (d, J = 13.6 Hz, 1H), 3.57 (d, J = 13.5 Hz, 1H), 2.54 (s, 3H), 1.78 - 1.56 (m, 4H), 0.72 (s, 9H). |
| 529 | 1H NMR (400 MHz, DMSO-d6) δ 8.30 - 8.22 (m, 2H), 8.08 (d, J = 0.4 Hz, 1H), 7.88 (d, J = 2.3 Hz, 1H), 7.57 (s, 1H), 7.50 (ddd, J = 7.4, 1.9, 1.0 Hz, 1H), 7.43 - 7.34 (m, 2H), 7.34 - 7.23 (m, 2H), 6.48 (s, 1H), 3.98 (s, 3H), 3.91 (dd, J = 13.8, 8.0 Hz, 1H), 3.35 (dd, J = 13.8, 5.2 Hz, 1H), 1.59 (s, 3H), 1.27 - 1.18 (m, 2H), 1.05 - 0.96 (m, 2H), 0.79 (s, 9H). |
| 530 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.95 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.20 (s, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.85 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.64 (s, 9H), 0.93 (s, 9H). |
| 531 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.98 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.22 (s, 1H), 4.63 (d, J = 47.1 Hz, 2H), 4.03 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.67 (s, 6H), 0.93 (s, 9H). |
| 532 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.92 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.27 (tt, J = 56 Hz, J = 3.3 Hz, 1H), 6.25 (s, 1H), 4.95 - 4.86 (m, 2H), 4.04 (d, J = 13.9 Hz, 1H), 3.83 (d, J = 14.0 Hz, 1H), 2.50 (s, 3H), 0.94 (s, 9H). |
| 533 | 1H NMR (400 MHz, 乙腈-d3) δ 9.88 (s, 1H), 8.51 (s, 1H), 8.22 (m, 1H), 8.12 (s, 1H), 8.03 - 7.92 (m, 1H), 7.87 (m, 3H), 7.46 (m, 1H), 6.95 (s, 1H), 6.62 (s, 1H), 6.22 (s, 1H), 3.71 (s, 2H), 3.35 (m, 1H), 1.57 -1.45 (m, 4H), 0.50 (s, 9H). |
| 534 | 1H NMR (400 MHz, 乙腈-d3) δ 8.43 (s, 1H), 8.31 (s, 1H), 7.97 (s, 1H), 7.84 (m, 1H), 7.44 (m, 1H), 6.88 - 6.75 (m, 2H), 6.09 (s, 1H), 3.89 (m, 2H), 3.62 - 3.51 (m, 2H), 2.51 (s, 3H), 1.73 (s, 1H), 1.78 - 1.65 (m, 1H), 1.65 (s, 2H), 0.98 (s, 9H). |
| 535 | 1H NMR (400 MHz, 乙腈-d3) δ 10.37 (s, 1H), 8.56 (d, J = 6.5 Hz, 1H), 8.38 (d, J = 6.6 Hz, 1H), 8.18 - 8.06 (m, 1H), 8.08 - 7.97 (m, 3H), 7.92 (m, 1H), 7.74 (d, J = 2.4 Hz, 1H), 7.53 (s, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.29 (s, 1H), 3.85 - 3.73 (m, 1H), 3.27 (m, 1H), 1.77 - 1.60 (m, 3H), 1.56 - 1.47 (m, 1H), 0.42 (s, 9H). |
| 536 | 1H NMR (400 MHz, 乙腈-d3) δ 9.50 (s, 1H), 8.83 - 8.59 (m, 2H), 8.48 (d, J = 6.7 Hz, 1H), 8.31 - 8.08 (m, 3H), 7.87 - 7.66 (m, 2H), 7.50 (d, J = 2.1 Hz, 1H), 7.26 (s, 1H), 7.08 (d, J = 2.3 Hz, 1H), 6.69 (s, 1H), 5.73 (t, J = 54.5 Hz, 1H), 3.95 (dd, J = 13.8, 8.1 Hz, 1H), 3.55 (dd, J = 13.8, 5.1 Hz, 1H), 1.48 - 1.17 (m, 4H), 0.50 (s, 9H). |
| 537 | 1H NMR (400 MHz, 乙腈-d3) δ 9.07 (d, J = 4.8 Hz, 1H), 8.94 (d, J = 8.6 Hz, 1H), 8.44 (m, 1H), 8.27 - 8.20 (m, 1H), 7.92 - 7.81 (m, 3H), 7.76 (m, 1H), 7.56 (m, 1H), 6.85 - 6.76 (m, 2H), 6.67 (s, 1H), 5.85 (t, J = 54.6 Hz, 1H), 3.81 (m, 1H), 3.60 (m, 1H), 1.48 (m, 4H), 0.72 (s, 9H). |
| 538 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 7.0 Hz, 1H), 8.49 (s, 1H), 8.05 (s, 1H), 7.95 (d, J = 2.4 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.27 (d, J = 7.0 Hz, 1H), 7.02 (d, J = 2.3 Hz, 1H), 6.86 (t, J = 7.0 Hz, 1H), 6.62 (dd, J = 2.5, 1.0 Hz, 1H), 6.38 (s, 1H), 4.09 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 13.9 Hz, 1H), 1.58 - 1.44 (m, 4H), 0.84 (s, 9H). |
| 539 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.03 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 6.89 - 6.82 (m, 1H), 6.75 (d, J = 2.2 Hz, 1H), 6.23 (s, 1H), 5.96 (t, J = 54.7 Hz, 1H), 4.59 (dd, J = 11.5, 4.3 Hz, 1H), 4.09 - 4.01 (m, 1H), 3.58 - 3.46 (m, 2H), 3.21 (d, J = 11.7 Hz, 1H), 2.43 (s, 3H), 2.21 - 1.99 (m, 1H), 1.94 (d, J = 13.1 Hz, 1H), 1.55 (s, 4H), 0.90 (s, 3H), 0.55 (s, 3H). |
| 540 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 8.09 (s, 1H), 7.66 - 7.58 (m, 2H), 7.09 (d, J = 2.3 Hz, 1H), 6.94 - 6.86 (m, 1H), 6.34 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.76 (dd, J = 11.7, 4.4 Hz, 1H), 4.06 (dd, J = 11.8, 4.7 Hz, 1H), 3.60 - 3.49 (m, 2H), 3.25 (d, J = 11.7 Hz, 1H), 2.59 (s, 3H), 2.18 - 2.05 (m, 1H), 1.92 (d, J = 12.6 Hz, 1H), 1.58 - 1.48 (m, 4H), 1.05 (s, 3H), 0.74 (s, 3H). |
| 541 | 1H NMR (400 MHz, 甲醇-d4) δ 8.21 (s, 1H), 8.19 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.50 (d, J = 2.4 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.22 (s, 1H), 3.81 (d, J = 13.7 Hz, 1H), 3.59 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.97 - 1.90 (m, 6H), 0.87 (s, 9H). |
| 542 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.01 (s, 1H), 8.00 - 7.91 (m, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.45 - 7.28 (m, 2H), 6.95 (d, J = 2.3 Hz, 1H), 6.80 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.38 (s, 3H), 3.95 (d, J = 13.9 Hz, 1H), 3.77 (d, J = 13.9 Hz, 1H), 1.61 - 1.42 (m, 4H), 0.77 (s, 9H). |
| 543 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.09 (d, J = 1.0 Hz, 1H), 8.01 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 8.5, 7.1 Hz, 1H), 7.27 (d, J = 7.0 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H), 6.49 (s, 1H), 4.14 (d, J = 13.8 Hz, 1H), 4.05 (s, 3H), 3.64 (d, J = 13.9 Hz, 1H), 1.77 - 1.55 (m, 4H), 0.85 (s, 9H). |
| 544 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.25 (s, 1H), 8.03 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.25 (dd, J = 8.6, 6.9 Hz, 1H), 7.19 (d, J = 6.8 Hz, 1H), 7.02 (d, J = 2.2 Hz, 1H), 6.37 (s, 1H), 4.17 (s, 3H), 4.14 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 1.74 - 1.59 (m, 4H), 0.84 (s, 9H). |
| 545 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.05 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.2 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.4, 2.7 Hz, 1H), 6.24 (s, 1H), 6.12 (t, J = 56.0 Hz, 1H), 4.03 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.76 (s, 6H), 0.94 (s, 9H). |
| 546 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 7.94 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.23 (s, 1H), 5.10 - 4.93 (m, 1H), 4.77 - 4.72 (m, 1H), 4.64 - 4.60 (m, 1H), 4.05 (d, J = 14.0 Hz, 1H), 3.85 (d, J = 14.0 Hz, 1H), 2.50 (s, 3H), 1.57 (d, J = 7.1, 3H), 0.94 (s, 9H). |
| 547 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.02 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.2 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.20 (s, 1H), 4.06 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.55 (m, 1H), 1.27 - 1.20 (m, 2H), 1.03 (m, 2H), 0.94 (s, 9H), 0.57 - 0.48 (m, 2H), 0.37 - 0.30 (m, 2H). |
| 548 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.27 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 6.97 (d, J = 2.3 Hz, 1H), 6.90 (dd, J = 8.5, 2.8 Hz, 1H), 6.30 (s, 1H), 5.28 - 5.21 (m, 2H), 5.18 (d, J = 8.3 Hz, 2H), 4.03 (d, J = 14.0 Hz, 1H), 3.87 (d, J = 14.0 Hz, 1H), 2.52 (s, 3H), 0.94 (s, 9H). |
| 549 | 1H NMR (400 MHz, 乙腈-d3) δ 8.44 (s, 1H), 7.82 (t, J = 8.2 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 6.84 (dd, J = 8.4, 3.2 Hz, 1H), 6.78 (s, 1H), 6.17 (s, 1H), 3.83 (m, 2H), 1.78 - 1.65 (m, 2H), 1.65 (m, 2H), 0.95 (s, 9H). |
| 550 | 1H NMR (400 MHz, 乙腈-d3) δ 9.14 - 9.06 (m, 2H), 8.37 (s, 1H), 8.32 - 8.25 (m, 1H), 8.00 - 7.81 (m, 4H), 7.74 (d, J = 2.5 Hz, 1H), 6.91 (d, J = 2.5 Hz, 1H), 6.82 (s, 1H), 5.85 (t, J = 54.6 Hz, 1H), 5.80 (s, 1H), 3.68 m, 1H), 3.48 (m, 1H), 1.53 - 1.43 (m, 4H), 0.69 (s, 9H). |
| 551 | 1H NMR (400 MHz, 乙腈-d3) δ 9.65 (m, 1H), 9.12 - 9.01 (m, 2H), 8.46 (s, 1H), 8.42 (m, 1H), 8.31 (s, 1H), 8.06 - 7.96 (m, 2H), 7.94 (m, 1H), 7.86 (s, 1H), 7.64 (m, 1H), 7.50 (s, 1H), 7.23 (m, 1H), 6.88 (s, 1H), 5.83 (t, J = 54.6 Hz, 1H), 4.09 (m, 1H), 3.64 (m, 1H), 1.53 - 1.32 (m, 4H), 0.58 (s, 9H). |
| 552 | 1H NMR (400 MHz, 乙腈-d3) δ 9.98 (s, 1H), 8.60 (d, J = 6.3 Hz, 1H), 8.38 - 8.29 (m, 2H), 8.15 (d, J = 8.0 Hz, 1H), 8.09 - 7.98 (m, 3H), 7.76 (d, J = 2.5 Hz, 1H), 6.95 - 6.88 (m, 2H), 6.30 (s, 1H), 5.88 (t, J = 54.6 Hz, 1H), 3.76 (m, 1H), 3.39 (m, 1H), 1.56 - 1.42 (m, 4H), 0.65 (m, 9H). |
| 553 | 1H NMR (400 MHz, 甲醇-d4) δ 8.20 (s, 1H), 8.00 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.47 (d, J = 2.4 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.19 (s, 1H), 3.82 (d, J = 13.7 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 2.93 (s, 6H), 2.51 (s, 3H), 1.74 - 1.62 (m, 4H), 0.88 (s, 9H). |
| 554 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.07 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.23 (s, 1H), 4.09 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 14.0 Hz, 1H), 3.39 (m, 2H), 3.08 (m, 2H), 2.50 (s, 3H), 1.74 (m, 6H), 1.63 (m, 2H), 0.95 (s, 9H). |
| 555 | 1H NMR (400 MHz, 甲醇-d4) δ 8.20 (s, 1H), 8.02 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.48 (d, J = 2.4 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.77 (d, J = 2.4 Hz, 1H), 6.21 (s, 1H), 3.84 (d, J = 13.7 Hz, 1H), 3.62 - 3.52 (m, 5H), 3.40 (m, 4H), 2.53 (s, 3H), 1.77 - 1.67 (m, 2H), 1.65 (m, 2H), 0.89 (s, 9H). |
| 556 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.81 (s, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.49 - 7.42 (m, 1H), 7.28 (dd, J = 8.4, 7.2 Hz, 1H), 6.97 (d, J = 7.2 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 6.70 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 3.99 (d, J = 13.8 Hz, 1H), 3.73 (d, J = 13.8 Hz, 1H), 2.52 (s, 3H), 1.50 (s, 4H), 0.77 (s, 9H). |
| 557 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (s, 1H), 8.11 (d, J = 1.0 Hz, 1H), 8.04 (s, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 8.5, 7.1 Hz, 1H), 7.29 (d, J = 7.1 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.49 (s, 1H), 4.05 (s, 3H), 3.94 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.7 Hz, 1H), 1.76 - 1.58 (m, 4H), 0.79 (s, 9H). |
| 558 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (d, J = 0.6 Hz, 1H), 8.29 (s, 1H), 8.06 (s, 1H), 7.85 (d, J = 2.4 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.26 (dd, J = 8.6, 6.9 Hz, 1H), 7.23 - 7.18 (m, 2H), 6.37 (s, 1H), 4.17 (s, 3H), 3.99 (d, J = 13.7 Hz, 1H), 3.53 (d, J = 13.7 Hz, 1H), 1.77 - 1.59 (m, 4H), 0.80 (s, 9H). |
| 559 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.02 (s, 1H), 7.99 - 7.94 (m, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.40 - 7.33 (m, 2H), 7.14 (d, J = 2.5 Hz, 1H), 6.81 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 4.40 (s, 3H), 3.77 (d, J = 13.9 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 1.55 - 1.49 (m, 4H), 0.72 (s, 9H). |
| 560 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (d, J = 7.0 Hz, 1H), 8.50 (s, 1H), 8.19 (s, 1H), 7.96 (d, J = 2.5 Hz, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.28 (dt, J = 7.0, 0.9 Hz, 1H), 7.03 (d, J = 2.4 Hz, 1H), 6.87 (t, J = 7.0 Hz, 1H), 6.61 (dd, J = 2.4, 0.9 Hz, 1H), 6.39 (s, 1H), 4.10 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 13.9 Hz, 1H), 1.79 - 1.57 (m, 4H), 0.83 (s, 9H). |
| 561 | 1H NMR (400 MHz, 甲醇-d4) δ 9.64 (s, 1H), 8.56 (d, J = 6.6 Hz, 1H), 8.48 (s, 1H), 8.41 (d, J = 6.6 Hz, 1H), 8.36 (d, J = 8.3 Hz, 1H), 8.18 (d, J = 7.3 Hz, 1H), 8.07 (s, 1H), 7.88 (dd, J = 8.3, 7.4 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.87 (s, 1H), 4.05 (d, J = 13.9 Hz, 1H), 3.59 (d, J = 13.9 Hz, 1H), 1.88 - 1.75 (m, 2H), 1.64 - 1.52 (m, 2H), 0.72 (s, 9H). |
| 562 | 1H NMR (400 MHz, 甲醇-d4) δ 9.59 (s, 1H), 8.55 (d, J = 6.5 Hz, 1H), 8.46 (s, 1H), 8.38 - 8.29 (m, 2H), 8.09 (d, J = 7.3 Hz, 1H), 7.96 (s, 1H), 7.85 (t, J = 7.8 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.87 (s, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 2.99 - 2.86 (m, 1H), 2.81 (m,1H), 2.59 (m, 1H), 2.40 - 2.24 (m, 1H), 2.18 - 1.95 (m, 2H), 0.72 (s, 9H). |
| 563 | 1H NMR (400 MHz, 甲醇-d4) δ 9.59 (s, 1H), 8.56 (d, J = 6.5 Hz, 1H), 8.47 (s, 1H), 8.37 - 8.29 (m, 2H), 8.23 (s, 1H), 8.06 (d, J = 7.3 Hz, 1H), 7.85 (t, J = 7.8 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 6.97 (d, J = 2.3 Hz, 1H), 6.89 (s, 1H), 3.98 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 13.9 Hz, 1H), 2.01 - 1.94 (m, 2H), 1.94 - 1.83 (m, 2H), 0.73 (s, 9H). |
| 564 | 1H NMR (400 MHz, 甲醇-d4) δ 9.55 (s, 1H), 8.54 (d, J = 6.5 Hz, 1H), 8.45 (s, 1H), 8.28 (m, 2H), 8.03 (d, J = 7.3 Hz, 1H), 7.95 (s, 1H), 7.82 (t, J = 7.8 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 6.90 (d, J = 2.4 Hz, 1H), 6.82 (s, 1H), 3.97 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.9 Hz, 1H), 1.63 (s, 3H), 1.29 (m, 2H), 1.08 - 1.01 (m, 2H), 0.71 (s, 9H). |
| 565 | 1H NMR (400 MHz, 乙腈-d3) δ 9.13 - 8.99 (m, 2H), 8.43 (s, 1H), 8.31 - 8.24 (m, 1H), 7.97 - 7.87 (m, 2H), 7.86 - 7.77 (m, 2H), 7.56 (m, 1H), 7.01 (s, 1H), 6.91 - 6.81 (m, 2H), 5.97 (s, 1H), 5.66 (s, 1H), 3.88 (m, 1H), 3.60 (m, 1H), 1.94 (m, 1H) 1.73 (m, 1H), 1.56 - 1.41 (m, 2H), 0.73 (s, 9H). |
| 566 | 1H NMR (400 MHz, 乙腈-d3) δ 9.14 - 9.05 (m, 2H), 8.44 (s, 1H), 8.29 (m, 1H), 7.98 - 7.76 (m, 4H), 7.56 (m, 1H), 7.13 (s, 1H), 6.91 - 6.83 (m, 2H), 6.58 (s, 1H), 5.96 (s, 1H), 3.90 (m, 1H), 3.61 (m, 1H), 2.93 - 2.80 (m, 2H), 2.79 - 2.64 (m, 2H), 2.12 - 1.96 (m, 2H), 0.73 (s, 9H). |
| 567 | 1H NMR (400 MHz, 乙腈-d3) δ 9.09 (dd, J = 4.8, 1.5 Hz, 1H), 8.96 (d, J = 8.5 Hz, 1H), 8.43 (s, 1H), 8.27 (d, J = 8.2 Hz, 1H), 7.95 - 7.83 (m, 3H), 7.80 (dd, J = 8.7, 4.8 Hz, 1H), 7.55 (d, J = 2.3 Hz, 1H), 6.93 (s, 1H), 6.87 - 6.79 (m, 2H), 6.42 (s, 1H), 3.85 (dd, J = 13.7, 6.6 Hz, 1H), 3.63 (dd, J = 13.4, 5.1 Hz, 1H), 1.97 - 1.76 (m, 4H), 0.73 (s, 9H). |
| 568 | 1H NMR (400 MHz, 乙腈-d3) δ 9.09 (dd, J = 4.8, 1.5 Hz, 1H), 9.01 (d, J = 8.8 Hz, 1H), 8.43 (s, 1H), 8.30 - 8.22 (m, 1H), 7.93 - 7.86 (m, 2H), 7.90 - 7.76 (m, 1H), 7.73 (s, 1H), 7.55 (d, J = 2.2 Hz, 1H), 6.89 (m, 1H), 6.80 (s, 2H), 3.84 (dd, J = 13.6, 6.7 Hz, 1H), 3.61 (dd, J = 13.6, 5.0 Hz, 1H), 1.60 (s, 3H), 1.28 - 1.19 (m, 2H), 1.05 - 0.98 (m, 2H), 0.72 (s, 9H). |
| 569 | 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 8.57 (d, J = 6.0 Hz, 1H), 8.31 (s, 1H), 8.13 (d, J = 5.7 Hz, 3H), 7.89 - 7.83 (m, 2H), 7.69 (t, J = 7.7 Hz, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.31 - 7.19 (m, 2H), 6.90 (d, J = 7.3 Hz, 1H), 4.71 (s, 1H), 4.59 (s, 1H), 3.70 (dd, J = 13.7, 7.6 Hz, 1H), 3.42 - 3.33 (m, 1H), 1.41 - 1.27 (m, 4H), 0.58 (s, 9H). |
| 570 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 8.25 (s, 1H), 7.98 (s, 1H), 7.72 - 7.64 (m, 2H), 7.37 (d, J = 6.9 Hz, 1H), 7.11 - 7.01 (m, 2H), 6.63 (s, 1H), 4.22 (s, 3H), 4.12 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 13.9 Hz, 1H), 1.80 - 1.50 (m, 2H), 1.28 (s, 2H), 0.82 (s, 9H). |
| 571 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.81 (s, 1H), 7.48 - 7.41 (m, 1H), 7.29 (dd, J = 8.5, 7.1 Hz, 1H), 7.05 (d, J = 2.5 Hz, 1H), 7.00 (d, J = 7.1 Hz, 1H), 6.69 (s, 1H), 3.80 (d, J = 13.6 Hz, 1H), 3.58 (d, J = 13.6 Hz, 1H), 2.54 (s, 3H), 1.50 (d, J = 2.3 Hz, 4H), 0.72 (s, 9H). |
| 572 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 7.0 Hz, 1H), 8.37 (s, 1H), 8.20 (s, 1H), 7.96 (d, J = 2.4 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.29 (d, J = 7.1 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 6.87 (t, J = 7.0 Hz, 1H), 6.63 (dd, J = 2.5, 1.0 Hz, 1H), 6.39 (s, 1H), 3.92 (d, J = 13.8 Hz, 1H), 3.53 (d, J = 13.7 Hz, 1H), 1.79 - 1.57 (m, 4H), 0.78 (s, 9H). |
| 573 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.25 (s, 1H), 8.00 (s, 1H), 7.85 (d, J = 2.5 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.38 (d, J = 7.0 Hz, 1H), 7.26 (d, J = 2.5 Hz, 1H), 7.05 (dd, J = 8.4, 7.0 Hz, 1H), 6.63 (s, 1H), 4.23 (s, 3H), 3.98 (d, J = 13.8 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 1.73 - 1.58 (m, 4H), 0.79 (s, 9H). |
| 574 | 1H NMR (400 MHz, 乙腈-d3) δ 8.41 (s, 1H), 7.81 (t, J = 8.2 Hz, 1H), 7.68 (d, J = 2.4 Hz, 1H), 6.97 (d, J = 2.4 Hz, 1H), 6.84 (dd, J = 8.4, 3.1 Hz, 1H), 6.41 (s, 1H), 6.17 (s, 1H), 3.84 - 3.69 (m, 2H), 2.50 (s, 3H), 1.79 - 1.61 (m, 4H), 0.94 (s, 9H). |
| 575 | 1H NMR (400 MHz, 乙腈-d3) δ 9.27 - 9.20 (m, 1H), 9.17 - 9.10 (m, 1H), 8.40 - 8.29 (m, 2H), 8.05 - 7.92 (m, 3H), 7.85 (s, 1H), 7.75 (d, J = 2.5 Hz, 1H), 6.93 (d, J = 2.5 Hz, 1H), 6.83 (s, 1H), 5.92 (s, 1H), 5.62 (s, 1H), 3.78 - 3.68 (m, 1H), 3.52 - 3.42 (m, 1H), 1.76 - 1.71 (m, 2H), 1.57 - 1.37 (m, 2H), 0.69 (s, 9H). |
| 576 | 1H NMR (400 MHz, 氯仿-d) δ 8.91 (m, 1H), 8.55 (m, 1H), 8.33 (s, 1H), 8.08 (m, 1H), 7.70 (m, 2H), 7.56 - 7.44 (m, 2H), 7.31 (s, 1H), 7.22 (s, 2H), 6.60 (m, 1H), 6.50 (m, 1H), 3.45 (m, 2H), 2.87-2.67 (m, 2H), 2.15 - 1.81 (m, 2H), 0.57 (s, 9H). |
| 577 | 1H NMR (400 MHz, 乙腈-d3) δ 9.14 - 9.03 (m, 2H), 8.37 (s, 1H), 8.30 (d, J = 8.3 Hz, 1H), 8.00 - 7.84 (m, 4H), 7.73 (d, J = 2.4 Hz, 1H), 6.91 (d, J = 2.5 Hz, 1H), 6.82 (s, 1H), 5.82 (s, 1H), 3.68 (dd, J = 13.3, 6.7 Hz, 1H), 3.49 (dd, J = 13.4, 5.2 Hz, 1H), 1.97 - 1.79 (m, 4H), 0.69 (s, 9H). |
| 578 | 1H NMR (400 MHz, 乙腈-d3) δ 9.18 - 9.08 (m, 2H), 8.33 (d, J = 22.8 Hz, 2H), 8.01 - 7.86 (m, 3H), 7.73 (t, J = 1.2 Hz, 2H), 6.90 (d, J = 2.5 Hz, 1H), 6.79 (s, 1H), 6.21 (s, 1H), 5.83 (s, 1H), 3.68 (dd, J = 13.3, 6.8 Hz, 1H), 3.48 (dd, J = 13.4, 5.0 Hz, 1H), 1.60 (s, 3H), 1.25 (s, 2H), 1.05 - 0.98 (m, 2H), 0.68 (s, 9H). |
| 579 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.94 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.91 (d, J = 2.4 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.22 (s, 1H), 5.98 - 4.79 (m, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.95 - 3.81 (m, 2H), 2.50 (s, 3H), 2.02 - 1.89 (m, 1H), 1.67 - 1.55 (m, 1H), 0.93 (s, 9H). |
| 580 | 1H NMR (400 MHz, 甲醇-d4) δ 9.74 (s, 1H), 8.60 (d, J = 6.5 Hz, 1H), 8.53 (d, J = 6.8 Hz, 1H), 8.43 (d, J = 8.3 Hz, 1H), 8.31 (s, 1H), 8.29 (s, 1H), 8.20 (d, J = 7.4 Hz, 1H), 7.95 (dd, J = 8.3, 7.4 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.14 (d, J = 2.5 Hz, 1H), 6.91 (s, 1H), 3.79 (d, J = 13.8 Hz, 1H), 3.51 (d, J = 13.8 Hz, 1H), 2.05 - 1.94 (m, 2H), 1.94 - 1.86 (m, 2H), 0.69 (s, 9H). |
| 581 | 1H NMR (400 MHz, 甲醇-d4) δ 9.73 (s, 1H), 8.59 (d, J = 6.7 Hz, 1H), 8.53 (d, J = 6.7 Hz, 1H), 8.42 (d, J = 8.3 Hz, 1H), 8.30 (s, 1H), 8.19 (d, J = 7.3 Hz, 1H), 8.04 (s, 1H), 7.95 (dd, J = 8.3, 7.4 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.10 (d, J = 2.5 Hz, 1H), 6.86 (s, 1H), 3.78 (d, J = 13.8 Hz, 1H), 3.50 (d, J = 13.8 Hz, 1H), 1.64 (s, 3H), 1.34 - 1.25 (m, 2H), 1.09 - 1.00 (m, 2H), 0.68 (s, 9H). |
| 582 | 1H NMR (400 MHz, DMSO-d6) δ 8.40 - 8.34 (m, 2H), 7.81 (t, J = 8.3 Hz, 1H), 7.68 - 7.62 (m, 1H), 7.43 (d, J = 7.0 Hz, 1H), 6.90 (dd, J = 8.4, 3.1 Hz, 1H), 6.77 (d, J = 2.4 Hz, 1H), 6.30 (dd, J = 21.8, 6.2 Hz, 1H), 4.48 - 4.20 (m, 1H), 2.39 (s, 3H), 1.83 - 1.59 (m, 4H), 1.26 (dd, J = 19.5, 6.6 Hz, 4H), 0.85 (s, 3H), 0.67 (s, 9H) |
| 583 | 1H NMR (400 MHz, DMSO-d6) δ 8.37 (dd, J = 14.4, 8.7 Hz, 2H), 7.89 (d, J = 2.3 Hz, 1H), 7.84 - 7.76 (m, 1H), 7.52 (d, J = 7.0 Hz, 1H), 7.35 - 7.29 (m, 1H), 7.05 (d, J = 2.5 Hz, 1H), 6.93 - 6.83 (m, 2H), 6.70 (d, J = 10.5 Hz, 0H), 6.28 (d, J = 6.5 Hz, 1H), 4.24 (dd, J = 10.5, 6.6 Hz, 1H), 2.39 (s, 3H), 1.81 - 1.60 (m, 4H), 1.26 (dd, J = 17.3, 6.6 Hz, 4H), 0.85 (s, 3H), 0.67 (s, 9H). |
| 584 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 8.33 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 6.99 (d, J = 2.3 Hz, 1H), 6.89 (dd, J = 8.5, 2.8 Hz, 1H), 6.31 (s, 1H), 4.38 (ddd, J = 11.7, 10.7, 1.5 Hz, 2H), 4.00 (d, J = 14.0 Hz, 1H), 3.91 (d, J = 14.0 Hz, 1H), 2.53 (s, 3H), 0.95 (s, 9H). |
| 585 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (d, J = 9.9 Hz, 2H), 7.86 (t, J = 8.2 Hz, 1H), 7.80 (d, J = 2.3 Hz, 1H), 7.49 - 7.32 (m, 2H), 7.24 (d, J = 8.5 Hz, 1H), 7.01 - 6.94 (m, 1H), 6.35 (d, J = 7.8 Hz, 1H), 4.17 (s, 1H), 2.06 (s, 1H), 1.89 (d, J = 12.0 Hz, 1H), 1.82 - 1.58 (m, 8H), 1.54 - 1.12 (m, 6H). |
| 586 | 1H NMR (400 MHz, 甲醇-d4) δ 9.76 (s, 1H), 8.63 - 8.55 (m, 2H), 8.44 (d, J = 8.2 Hz, 1H), 8.29 (m, 2H), 8.13 (s, 1H), 7.96 (dd, J = 8.3, 7.4 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.10 (d, J = 2.5 Hz, 1H), 6.89 (s, 1H), 3.84 (d, J = 13.8 Hz, 1H), 3.44 (d, J = 13.8 Hz, 1H), 1.86 - 1.77 (m, 2H), 1.63 - 1.52 (m, 2H), 0.67 (s, 9H). |
| 587 | 1H NMR (400 MHz, 甲醇-d4) δ 9.74 (s, 1H), 8.63 - 8.57 (m, 1H), 8.55 (m, 1H), 8.42 (d, J = 8.3 Hz, 1H), 8.30 (s, 1H), 8.23 (d, J = 7.3 Hz, 1H), 8.03 (s, 1H), 7.95 (dd, J = 8.3, 7.4 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.13 (d, J = 2.5 Hz, 1H), 6.90 (s, 1H), 3.82 (d, J = 13.8 Hz, 1H), 3.48 (d, J = 13.8 Hz, 1H), 2.93 (m, 2H), 2.81 (m, 2H), 2.16 - 1.95 (m, 2H), 0.69 (s, 9H). |
| 588 | 1H NMR (400 MHz, DMSO-d6) δ 8.37 (s, 1H), 8.36 (s, 1H), 7.87 (t, J = 8.3 Hz, 1H), 7.58 (d, J = 2.2 Hz, 1H), 7.48 - 7.37 (m, 2H), 7.08 (d, J = 2.3 Hz, 1H), 6.98 (dd, J = 8.4, 3.0 Hz, 1H), 6.34 (d, J = 7.2 Hz, 1H), 4.20 (s, 0H), 2.52 (s, 3H), 2.07 (d, J = 10.4 Hz, 1H), 1.89 (d, J = 12.1 Hz, 1H), 1.84 - 1.60 (m, 8H), 1.54 - 1.27 (m, 4H), 1.27 - 1.14 (m, 1H). |
| 589 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 7.95 (s, 1H), 7.84 - 7.76 (m, 2H), 7.08 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.20 (s, 1H), 3.89 (d, J = 13.9 Hz, 1H), 3.71 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.64 (s, 9H), 0.90 (s, 9H). |
| 590 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 8.05 (s, 1H), 7.83 - 7.75 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 6.12 (t, J = 55.5 Hz, 1H), 3.90 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.76 (d, J = 1.6 Hz, 6H), 0.90 (s, 9H). |
| 591 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 8.21 (s, 1H), 7.83 - 7.75 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.4, 2.7 Hz, 1H), 6.25 (s, 1H), 3.89 (d, J = 13.9 Hz, 1H), 3.71 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.94 (s, 6H), 0.90 (s, 9H). |
| 592 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.05 (s, 1H), 7.76 (m, 1H), 7.60 (d, J = 2.2 Hz, 1H), 6.96 (d, J = 2.3 Hz, 1H), 6.86 (m, 1H), 6.30 (s, 1H), 5.92 (t, J = 54.6 Hz, 1H), 3.92 (m, 2H), 2.85 (dt, J = 15.3, 7.5 Hz, 2H), 1.53 (s, 4H), 1.15 (t, J = 7.5 Hz, 3H), 0.94 (s, 9H). |
| 593 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.21 (s, 1H), 7.76 (s, 0H), 7.61 (d, J = 2.3 Hz, 1H), 6.98 (d, J = 2.3 Hz, 1H), 6.86 (m, 1H), 6.31 (s, 1H), 3.93 (m, 2H), 2.94 - 2.70 (m, 2H), 1.84 - 1.54 (m, 4H), 1.15 (t, J = 7.5 Hz, 3H), 0.94 (s, 9H). |
| 594 | 1H NMR (400 MHz, DMSO-d6) δ 9.76 (s, 1H), 8.61 (d, J = 6.0 Hz, 1H), 8.28 (s, 1H), 8.21 (s, 1H), 8.12 (s, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.91 - 7.79 (m, 2H), 7.67 (d, J = 2.2 Hz, 1H), 7.58 (d, J = 7.3 Hz, 1H), 7.14 (s, 1H), 7.04 (d, J = 6.8 Hz, 1H), 6.95 (s, 1H), 4.72 (s, 1H), 4.60 (s, 1H), 3.83 - 3.70 (m, 1H), 3.26 (dd, J = 13.7, 4.9 Hz, 1H), 1.42 - 1.27 (m, 4H), 0.99 (s, 1H), 0.51 (s, 9H). |
| 595 | 1H NMR (400 MHz, 甲醇-d4) δ 8.42 (s, 1H), 8.19 (s, 1H), 7.80 (t, J = 8.1 Hz, 1H), 7.06 (d, J = 1.9 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.56 (d, J = 1.9 Hz, 1H), 6.26 (s, 1H), 4.04 (m, 4H), 3.83 (d, J = 14.0 Hz, 1H), 2.51 (s, 3H), 1.83 - 1.60 (m, 4H), 0.93 (s, 9H). |
| 596 | 1H NMR (400 MHz, 甲醇-d4) δ 8.42 (s, 1H), 8.22 (s, 1H), 7.80 (t, J = 8.1 Hz, 1H), 7.05 (d, J = 1.9 Hz, 1H), 6.95 - 6.80 (m, 1H), 6.55 (d, J = 1.9 Hz, 1H), 6.25 (s, 1H), 4.04 (m, 4H), 3.84 (d, J = 14.0 Hz, 1H), 2.52 (s, 3H), 2.08 - 1.84 (m, 4H), 0.93 (s, 9H). |
| 597 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.06 (s, 1H), 7.84 - 7.67 (m, 2H), 7.14 (d, J = 2.5 Hz, 1H), 6.86 (m, 1H), 6.30 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 3.90 - 3.68 (m, 2H), 2.96 - 2.76 (m, 2H), 1.58 - 1.44 (m, 4H), 1.15 (t, J = 7.5 Hz, 3H), 0.90 (s, 9H). |
| 598 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.21 (s, 1H), 7.85 - 7.74 (m, 2H), 7.14 (d, J = 2.6 Hz, 1H), 6.86 (m, 1H), 6.31 (s, 1H), 3.78 (d, J = 2.6 Hz, 2H), 2.86 (m, 2H), 1.84 - 1.54 (m, 4H), 1.15 (t, J = 7.5 Hz, 3H), 0.90 (s, 9H). |
| 599 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.28 - 8.13 (m, 3H), 7.53 (d, J = 2.3 Hz, 1H), 7.44 (s, 1H), 6.97 (d, J = 6.7 Hz, 1H), 6.81 (d, J = 2.4 Hz, 1H), 6.67 (t, J = 6.9 Hz, 1H), 6.29 (s, 1H), 3.81 (d, J = 13.6 Hz, 1H), 3.46 (d, J = 13.6 Hz, 1H), 1.78 - 1.58 (m, 4H), 0.77 (s, 9H). |
| 600 | 1H NMR (400 MHz, 甲醇-d4) δ 8.69 (dd, J = 5.6, 1.6 Hz, 1H), 8.51 (s, 1H), 8.38 (s, 1H), 8.34 (dd, J = 8.0, 1.5 Hz, 1H), 7.77 (dd, J = 8.1, 5.6 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.15 (d, J = 2.3 Hz, 1H), 6.50 (s, 1H), 3.96 (d, J = 1.8 Hz, 2H), 3.14 (q, J = 7.6 Hz, 2H), 1.82 - 1.60 (m, 4H), 1.21 (t, J = 7.6 Hz, 3H), 0.97 (s, 9H). |
| 601 | 1H NMR (400 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.04 (s, 1H), 7.84 - 7.73 (m, 2H), 7.57 - 7.35 (m, 3H), 7.25 - 7.07 (m, 3H), 6.93 (dd, J = 8.4, 3.0 Hz, 1H), 6.25 (d, J = 7.0 Hz, 1H), 5.64 (s, 2H), 3.84 (dd, J = 13.8, 8.0 Hz, 1H), 3.40 (dd, J = 13.8, 5.3 Hz, 1H), 2.44 (s, 3H), 0.79 (s, 9H). |
| 602 | 1H NMR (400 MHz, 甲醇-d4) δ 8.70 (dd, J = 5.7, 1.5 Hz, 1H), 8.42 (d, J = 8.5 Hz, 1H), 8.39 (s, 1H), 8.32 (s, 1H), 7.82 (dd, J = 8.1, 5.7 Hz, 1H), 7.73 (d, J = 2.5 Hz, 1H), 7.24 (d, J = 2.5 Hz, 1H), 6.48 (s, 1H), 3.77 (s, 2H), 3.17 (q, J = 7.6 Hz, 2H), 1.82 - 1.64 (m, 4H), 1.22 (t, J = 7.6 Hz, 3H), 0.92 (s, 9H). |
| 603 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.04 (s, 1H), 7.93 (s, 1H), 7.75 (dd, J = 8.1, 1.1 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.19 (d, J = 7.2 Hz, 1H), 7.13 - 7.04 (m, 1H), 6.97 (d, J = 2.3 Hz, 1H), 6.84 (s, 1H), 5.92 (t, J = 54.6 Hz, 1H), 4.17 (s, 3H), 3.87 (d, J = 2.9 Hz, 2H), 1.52 (s, 4H), 0.80 (s, 9H). |
| 604 | 1H NMR (400 MHz, 甲醇-d4) δ 8.63 (dd, J = 5.5, 1.5 Hz, 1H), 8.48 (s, 1H), 8.21 (d, J = 5.4 Hz, 2H), 7.66 (dd, J = 8.0, 5.4 Hz, 1H), 7.61 (d, J = 2.3 Hz, 1H), 7.04 (d, J = 2.4 Hz, 1H), 6.44 (s, 1H), 5.93 (t, J = 54.4 Hz, 1H), 3.92 (d, J = 2.5 Hz, 2H), 3.09 (q, J = 7.6 Hz, 2H), 1.57 - 1.51 (m, 4H), 1.19 (t, J = 7.6 Hz, 3H), 0.95 (s, 9H). |
| 605 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.04 (s, 1H), 7.92 (s, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.75 (dd, J = 7.9, 1.0 Hz, 1H), 7.20 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 2.6 Hz, 1H), 7.08 (t, J = 7.6 Hz, 1H), 6.84 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 4.19 (s, 3H), 3.73 (s, 1H), 1.51 (s, 4H), 0.78 (s, 9H). |
| 606 | 1H NMR (400 MHz, 甲醇-d4) δ 8.69 (dd, J = 5.7, 1.5 Hz, 1H), 8.47 - 8.40 (m, 1H), 8.32 (s, 1H), 8.25 (s, 1H), 7.82 (dd, J = 8.1, 5.7 Hz, 1H), 7.72 (d, J = 2.5 Hz, 1H), 7.23 (d, J = 2.5 Hz, 1H), 6.47 (s, 1H), 5.94 (t, J = 54.5 Hz, 1H), 3.84 - 3.70 (m, 2H), 3.16 (q, J = 7.6 Hz, 2H), 1.61 - 1.51 (m, 4H), 1.22 (t, J = 7.6 Hz, 3H), 0.92 (s, 9H). |
| 607 | 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.34 (s, 1H), 8.04 (s, 1H), 7.86 - 7.79 (m, 1H), 7.56 (d, J = 2.2 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H), 7.12 (d, J = 2.4 Hz, 1H), 7.00 (dd, J = 8.4, 3.1 Hz, 1H), 6.29 (d, J = 7.8 Hz, 1H), 2.82 (s, 1H), 2.49 (s, 3H), 2.10 (dt, J = 12.5, 7.7 Hz, 2H), 1.79 - 1.65 (m, 5H), 1.64 - 1.54 (m, 1H), 1.50 (d, J = 2.4 Hz, 2H), 1.05 (m, J = 11.7 Hz, 1H). |
| 608 | 1H NMR (400 MHz, DMSO-d6) δ 8.38 (d, J = 4.0 Hz, 1H), 8.34 (d, J = 0.6 Hz, 1H), 8.07 (s, 1H), 7.84 (t, J = 8.3 Hz, 1H), 7.57 (d, J = 2.2 Hz, 1H), 7.42 - 7.16 (m, 1H), 7.12 (d, J = 2.4 Hz, 1H), 7.01 (dd, J = 8.4, 3.1 Hz, 1H), 6.28 (d, J = 7.9 Hz, 1H), 4.11 (t, J = 8.2 Hz, 2H), 3.68 (td, J = 8.7, 2.9 Hz, 2H), 2.89 - 2.82 (m, 1H), 2.48 (s, 3H), 2.03 - 1.92 (m, 2H), 1.80 - 1.65 (m, 4H). |
| 609 | 1H NMR (400 MHz, DMSO-d6) δ 8.31 - 8.22 (m, 2H), 8.01 (s, 1H), 7.87 (d, J = 2.4 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.51 (ddd, J = 7.0, 2.4, 1.0 Hz, 1H), 7.41 - 7.32 (m, 2H), 7.33 - 7.23 (m, 2H), 6.48 (d, J = 6.9 Hz, 1H), 3.98 (s, 3H), 3.98 - 3.86 (m, 2H), 3.34 (dd, J = 13.8, 5.2 Hz, 1H), 1.18 - 1.01 (m, 4H), 0.79 (s, 9H). |
| 610 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 7.93 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.75 (d, J = 2.5 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.94 - 6.77 (m, 1H), 6.19 (s, 1H), 3.87 (d, J = 13.8 Hz, 1H), 3.77 (d, J = 4.5 Hz, 2H), 3.63 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.32 (m, 2H), 1.20 (s, 2H), 0.88 (s, 9H). |
| 611 | 1H NMR (400 MHz, DMSO-d6) δ 8.45 - 8.37 (m, 1H), 8.26 (d, J = 10.1 Hz, 1H), 8.02 - 7.83 (m, 2H), 7.64 (t, J = 1.9 Hz, 1H), 7.50 - 7.41 (m, 1H), 7.39 - 7.32 (m, 1H), 7.31 - 7.16 (m, 3H), 7.10 (dd, J = 6.8, 2.9 Hz, 1H), 7.00 (dd, J = 8.4, 3.0 Hz, 1H), 6.47 (d, J = 7.3 Hz, 1H), 5.02 - 4.70 (m, 1H), 2.56 (d, J = 8.3 Hz, 3H), 1.81 - 1.65 (m, 5H), 1.39 (dt, J = 8.7, 4.7 Hz, 1H), 0.72 - 0.57 (m, 2H), 0.57 - 0.29 (m, 2H). |
| 612 | 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H), 8.20 (s, 1H), 7.83 (t, J = 8.3 Hz, 1H), 7.62 (d, J = 2.2 Hz, 1H), 7.55 (s, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.18 - 6.99 (m, 6H), 6.89 (d, J = 2.3 Hz, 1H), 6.26 (d, J = 7.1 Hz, 1H), 4.29 (dd, J = 13.9, 8.3 Hz, 1H), 3.64 (dd, J = 13.9, 5.1 Hz, 1H), 2.47 (s, 3H), 1.85 - 1.60 (m, 4H), 1.19 (d, J = 41.4 Hz, 6H). |
| 613 | 1H NMR (400 MHz, DMSO-d6) δ 8.43 - 8.38 (m, 1H), 8.27 (d, J = 0.9 Hz, 1H), 7.96 - 7.79 (m, 3H), 7.59 - 7.52 (m, 2H), 7.48 - 7.24 (m, 1H), 7.24 - 7.18 (m, 2H), 7.10 (dd, J = 6.7, 3.0 Hz, 1H), 7.00 (dd, J = 8.4, 3.2 Hz, 1H), 6.47 (d, J = 7.9 Hz, 1H), 4.92 (t, J = 8.1 Hz, 1H), 4.75 (t, J = 8.6 Hz, 0H), 2.55 (d, J = 8.7 Hz, 3H), 1.83 - 1.65 (m, 5H), 1.37 (td, J = 8.6, 8.2, 4.5 Hz, 1H), 0.76 - 0.28 (m, 3H). |
| 614 | 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H), 8.19 (s, 1H), 7.88 - 7.79 (m, 2H), 7.49 (t, J = 7.0 Hz, 2H), 7.19 (d, J = 2.5 Hz, 1H), 7.17 - 6.97 (m, 6H), 6.29 (d, J = 6.6 Hz, 1H), 4.27 (dd, J = 14.0, 8.3 Hz, 1H), 3.63 (dd, J = 13.9, 5.1 Hz, 1H), 2.47 (s, 3H), 1.83 - 1.63 (m, 4H), 1.19 (d, J = 42.4 Hz, 6H). |
| 615 | 1H NMR (400 MHz, DMSO-d6) δ 8.40 (d, J = 2.4 Hz, 2H), 7.99 (t, J = 7.1 Hz, 1H), 7.86 (d, J = 2.3 Hz, 1H), 7.80 (t, J = 8.3 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 7.34 (d, J = 2.5 Hz, 1H), 6.98 (dd, J = 8.4, 3.0 Hz, 1H), 6.27 (d, J = 7.5 Hz, 1H), 4.19 (dd, J = 14.7, 7.6 Hz, 1H), 3.85 (dd, J = 14.7, 6.4 Hz, 1H), 2.46 (s, 3H), 1.81 - 1.63 (m, 4H), 1.29 (d, J = 18.6 Hz, 6H). |
| 616 | 1H NMR (400 MHz, 甲醇-d4) δ 9.56 (s, 1H), 8.59 (d, J = 6.2 Hz, 1H), 8.48 (s, 1H), 8.14 (s, 1H), 8.04 (d, J = 6.3 Hz, 1H), 7.84 (dd, J = 8.3, 5.4 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.44 - 7.35 (m, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.80 (s, 1H), 3.98 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 14.0 Hz, 1H), 1.77 - 1.55 (m, 3H), 0.73 (s, 10H). |
| 617 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (d, J = 4.3 Hz, 1H), 8.59 (d, J = 8.8 Hz, 1H), 8.48 (s, 1H), 8.10 (s, 1H), 7.69 (d, J = 2.2 Hz, 1H), 7.64 (ddd, J = 16.5, 8.5, 4.6 Hz, 2H), 6.91 (d, J = 2.4 Hz, 1H), 6.81 (s, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.63 (d, J = 13.9 Hz, 1H), 1.76 - 1.52 (m, 3H), 0.70 (s, 11H). |
| 618 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.38 - 8.31 (m, 1H), 8.06 (s, 1H), 7.73 (dd, J = 8.0, 1.0 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.53 - 7.44 (m, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.86 (d, J = 2.3 Hz, 1H), 6.82 (d, J = 7.6 Hz, 1H), 6.60 (s, 1H), 4.02 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.79 - 1.59 (m, 4H), 0.78 (s, 9H). |
| 619 | 1H NMR (400 MHz, 甲醇-d4) δ 8.87 (s, 1H), 8.54 (s, 1H), 8.08 (s, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.17 (d, J = 2.3 Hz, 1H), 6.42 (s, 1H), 5.94 (t, J = 54.6 Hz, 1H), 4.07 (d, J = 13.9 Hz, 1H), 3.91 (d, J = 14.0 Hz, 1H), 2.44 (s, 3H), 1.54 (m, 4H), 1.00 (s, 9H). |
| 620 | 1H NMR (400 MHz, 甲醇-d4) δ 8.79 (s, 1H), 8.49 (s, 1H), 8.00 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.26 (d, J = 2.3 Hz, 1H), 6.31 (s, 1H), 5.91 (t, J = 54.8 Hz, 1H), 4.09 (d, J = 13.9 Hz, 1H), 3.93 (d, J = 14.0 Hz, 1H), 2.56 (s, 3H), 1.50 (m, 4H), 1.05 (s, 9H). |
| 621 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.01 (s, 1H), 7.80 (t, J = 8.3 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.07 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.20 (s, 1H), 3.87 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.54 (m, 1H), 1.28 - 1.19 (m, 2H), 1.09 - 0.98 (m, 2H), 0.89 (s, 9H), 0.58 - 0.46 (m, 2H), 0.38 - 0.29 (m, 2H). |
| 622 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 7.93 (s, 1H), 7.81 - 7.74 (m, 2H), 7.67 - 7.60 (m, 1H), 7.56 (dd, J = 7.1, 3.3 Hz, 1H), 7.10 (d, J = 2.6 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.22 (s, 1H), 4.98 -4.78 (m, 1H), 3.92 - 3.83 (m, 2H), 3.74 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 2.01 - 1.88 (m, 1H), 1.60 (m, 1H), 0.90 (s, 9H). |
| 623 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 7.94 (s, 1H), 7.82 - 7.75 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.6, 2.8 Hz, 1H), 6.23 (s, 1H), 5.01 (m, 1H), 4.76 - 4.71 (m, 1H), 4.66 - 4.60 (m, 1H), 3.90 (d, J = 13.9 Hz, 1H), 3.72 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.57 (d, J = 7.1 Hz, 3H), 0.90 (s, 9H). |
| 625 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.98 (s, 1H), 7.83 - 7.76 (m, 2H), 7.08 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.6, 2.7 Hz, 1H), 6.22 (s, 1H), 4.62 (d, J = 47.1 Hz, 2H), 3.88 (d, J = 13.8 Hz, 1H), 3.70 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.67 (s, 6H), 0.89 (s, 9H). |
| 626 | 1H NMR (400 MHz, 甲醇-d4) δ 9.66 (s, 1H), 8.62 (d, J = 6.4 Hz, 1H), 8.32 (s, 1H), 8.24 (d, J = 6.4 Hz, 1H), 8.19 (s, 1H), 7.96 (dd, J = 8.2, 5.2 Hz, 1H), 7.80 (d, J = 2.5 Hz, 1H), 7.47 (dd, J = 9.8, 8.3 Hz, 1H), 7.11 (d, J = 2.5 Hz, 1H), 6.83 (s, 1H), 3.78 (d, J = 13.7 Hz, 1H), 3.53 (d, J = 13.7 Hz, 1H), 1.79 - 1.68 (m, 2H), 1.64 (t, J = 9.7 Hz, 2H), 0.69 (s, 10H). |
| 627 | 1H NMR (400 MHz, 甲醇-d4) δ 8.95 (dd, J = 4.4, 1.4 Hz, 1H), 8.70 - 8.61 (m, 1H), 8.32 (s, 1H), 8.12 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.66 (ddd, J = 13.4, 8.4, 4.5 Hz, 2H), 7.48 (dd, J = 10.3, 8.2 Hz, 1H), 7.07 (d, J = 2.5 Hz, 1H), 6.82 (s, 1H), 3.78 (d, J = 13.7 Hz, 1H), 3.48 (d, J = 13.7 Hz, 1H), 1.78 - 1.54 (m, 4H), 0.64 (s, 10H). |
| 628 | 1H NMR (400 MHz, 甲醇-d4) δ 9.49 (s, 1H), 8.51 (s, 1H), 8.47 (s, 1H), 8.37 (d, J = 8.1 Hz, 1H), 8.26 (s, 1H), 8.24 (d, J = 8.6 Hz, 1H), 8.02 (ddd, J = 8.5, 7.0, 1.3 Hz, 1H), 7.90 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.02 (d, J = 2.3 Hz, 1H), 6.94 (s, 1H), 3.93 (d, J = 13.9 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 1.78 - 1.58 (m, 4H), 0.73 (s, 9H). |
| 629 | 1H NMR (400 MHz, 甲醇-d4) δ 9.36 (d, J = 2.2 Hz, 1H), 9.06 - 9.02 (m, 1H), 8.48 (d, J = 2.1 Hz, 2H), 8.15 - 8.09 (m, 2H), 7.94 (ddd, J = 8.4, 6.9, 1.4 Hz, 2H), 7.77 - 7.71 (m, 2H), 7.52 (d, J = 2.3 Hz, 1H), 7.27 (d, J = 2.3 Hz, 1H), 4.05 (s, 2H), 3.80 (t, J = 6.3 Hz, 2H), 3.60 (t, J = 6.3 Hz, 2H), 1.78 - 1.63 (m, 1H), 1.10 (d, J = 2.1 Hz, 9H). |
| 630 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.13 (m, 2H), 7.65 (d, J = 2.3 Hz, 1H), 7.30 (d, J = 2.3 Hz, 1H), 6.36 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.11 - 3.89 (m, 2H), 2.15 (s, 3H), 1.53 (s, 4H), 1.04 (s, 9H). |
| 631 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (d, J = 7.9 Hz, 1H), 8.34 (s, 1H), 8.08 (s, 1H), 7.80 (d, J = 2.5 Hz, 1H), 7.77 - 7.69 (m, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.89 - 6.81 (m, 1H), 6.60 (s, 1H), 3.83 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.54 (d, J = 13.7 Hz, 1H), 1.78 - 1.59 (m, 4H), 0.73 (s, 9H). |
| 632 | 1H NMR (400 MHz, 甲醇-d4) δ 9.03 (d, J = 6.7 Hz, 1H), 8.46 (s, 1H), 8.15 (s, 1H), 8.13 - 7.98 (m, 3H), 7.65 (d, J = 2.2 Hz, 1H), 7.50 (d, J = 6.7 Hz, 1H), 7.12 (s, 1H), 6.77 (s, 1H), 4.09 (s, 3H), 4.06 (d, J = 14.0 Hz, 1H), 3.51 (d, J = 14.0 Hz, 1H), 1.84 - 1.55 (m, 4H), 0.69 (s, 9H). |
| 633 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (d, J = 6.9 Hz, 2H), 7.92 (t, J = 7.0 Hz, 1H), 7.79 (t, J = 8.3 Hz, 1H), 7.60 (d, J = 2.2 Hz, 1H), 7.36 (d, J = 7.8 Hz, 1H), 7.02 (d, J = 2.3 Hz, 1H), 6.99 - 6.92 (m, 1H), 6.24 (d, J = 7.0 Hz, 1H), 4.20 (dd, J = 14.7, 7.6 Hz, 1H), 3.81 (dd, J = 14.7, 6.3 Hz, 1H), 2.44 (s, 3H), 1.77 - 1.62 (m, 4H), 1.29 (s, 3H), 1.23 (s, 3H). |
| 634 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 7.92 (s, 1H), 7.84 - 7.75 (m, 2H), 7.13 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.43 - 6.09 (m, 2H), 4.88 (ddd, J = 14.9, 3.4, 1.1 Hz, 2H), 3.89 (d, J = 13.8 Hz, 1H), 3.70 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 0.90 (s, 9H). |
| 635 | 1H NMR (400 MHz, DMSO-d6) δ 8.38 (d, J = 3.9 Hz, 2H), 7.80 (t, J = 8.3 Hz, 1H), 7.64 (d, J = 2.2 Hz, 1H), 7.40 (t, J = 8.2 Hz, 1H), 6.99 - 6.87 (m, 2H), 6.24 (d, J = 6.5 Hz, 1H), 3.97 (dd, J = 13.9, 8.1 Hz, 1H), 3.61 (dd, J = 13.9, 4.8 Hz, 1H), 3.52 - 3.33 (m, 2H), 2.44 (s, 3H), 1.81 - 1.64 (m, 3H), 0.85 (d, J = 3.2 Hz, 6H). |
| 636 | 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 2.2 Hz, 2H), 7.81 (t, J = 8.3 Hz, 1H), 7.61 (d, J = 2.2 Hz, 1H), 7.49 - 7.35 (m, 2H), 7.02 - 6.90 (m, 2H), 6.26 (d, J = 7.0 Hz, 1H), 5.73 (t, J = 56.1 Hz, 1H), 4.30 (dd, J = 14.6, 7.1 Hz, 1H), 3.85 (dd, J = 14.6, 4.9 Hz, 1H), 2.48 (s, 3H), 1.80 - 1.62 (m, 3H), 0.81 - 0.62 (m, 4H). |
| 637 | 1H NMR (400 MHz, DMSO-d6) δ 8.39 (d, J = 7.2 Hz, 2H), 7.86 (d, J = 2.4 Hz, 1H), 7.80 (t, J = 8.2 Hz, 1H), 7.50 (d, J = 7.3 Hz, 1H), 7.19 (s, 1H), 6.99 - 6.90 (m, 1H), 6.25 (d, J = 7.3 Hz, 1H), 4.00 - 3.86 (m, 1H), 3.47 (d, J = 11.0 Hz, 3H), 2.44 (s, 3H), 1.84 - 1.60 (m, 3H), 0.85 (d, J = 3.5 Hz, 6H). |
| 638 | 1H NMR (400 MHz, DMSO-d6) δ 8.38 (s, 2H), 7.87 - 7.77 (m, 2H), 7.48 (d, J = 7.8 Hz, 1H), 7.38 (t, J = 6.2 Hz, 1H), 7.25 (d, J = 2.5 Hz, 1H), 6.97 (dd, J = 8.5, 3.0 Hz, 1H), 6.28 (d, J = 7.3 Hz, 1H), 5.73 (t, J = 56.1 Hz, 1H), 4.31 - 4.22 (m, 1H), 3.84 (dd, J = 14.6, 4.9 Hz, 1H), 2.48 (s, 3H), 1.83 - 1.59 (m, 4H), 0.80 - 0.62 (m, 4H). |
| 639 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.05 (s, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.22 (s, 1H), 6.11 - 5.74 (m, 2H), 4.05 (d, J = 14.0 Hz, 1H), 3.95 (d, J = 14.1 Hz, 1H), 2.38 (s, 3H), 2.26 (s, 3H), 1.52 (s, 4H), 1.04 (s, 9H). |
| 640 | 1H NMR (400 MHz, 甲醇-d4) δ 8.92 (s, 1H), 8.41 (s, 1H), 8.11 (s, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 6.43 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.93 (d, J = 13.9 Hz, 1H), 3.77 (d, J = 13.9 Hz, 1H), 2.45 (s, 3H), 1.53 (m, 4H), 0.97 (s, 9H). |
| 641 | 1H NMR (400 MHz, 甲醇-d4) δ 8.78 (s, 1H), 8.37 (s, 1H), 8.02 (s, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 6.32 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 3.96 (d, J = 13.9 Hz, 1H), 3.80 (d, J = 13.9 Hz, 1H), 2.56 (s, 3H), 1.50 (m, 4H), 1.02 (s, 9H). |
| 642 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 8.01 (s, 1H), 7.85 - 7.74 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.23 (s, 1H), 3.92 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 2.95 (s, 6H), 2.50 (s, 3H), 1.68 (m, 2H), 1.66 (m, 2H), 0.91 (s, 9H). |
| 643 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.90 - 6.83 (m, 1H), 6.81 (d, J = 2.3 Hz, 1H), 6.08 (s, 1H), 4.05 (d, J = 13.9 Hz, 1H), 3.78 - 3.68 (m, 2H), 2.43 (s, 3H), 1.33 - 1.23 (m, 4H), 0.90 (s, 9H). |
| 644 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 8.26 (s, 1H), 7.85 - 7.76 (m, 2H), 7.12 (d, J = 2.5 Hz, 1H), 6.89 (dd, J = 8.6, 2.7 Hz, 1H), 6.30 (s, 1H), 5.24 (m, 2H), 5.18 (m, 2H), 3.85 (d, J = 13.9 Hz, 1H), 3.70 (d, J = 13.8 Hz, 1H), 2.53 (s, 3H), 0.89 (s, 9H). |
| 645 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.97 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 6.90 - 6.85 (m, 1H), 6.22 (s, 1H), 5.08 (q, J = 6.7, 6.1 Hz, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.82 (d, J = 13.9 Hz, 1H), 3.29 - 3.16 (m, 4H), 2.51 (s, 3H), 0.93 (s, 9H). |
| 646 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.91 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.89 (d, J = 2.2 Hz, 2H), 6.86 (d, J = 2.8 Hz, 1H), 6.20 (s, 1H), 3.99 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.69 (s, 1H), 2.51 (s, 3H), 2.37 (s, 6H), 0.92 (s, 9H). |
| 647 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.61 (d, J = 2.2 Hz, 1H), 6.85 (dd, J = 8.6, 2.7 Hz, 1H), 6.80 (d, J = 2.3 Hz, 1H), 6.11 (s, 1H), 4.05 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.9 Hz, 1H), 3.69 - 3.58 (m, 1H), 2.42 (s, 3H), 2.34 (s, 3H), 1.29 - 1.15 (m, 4H), 0.90 (s, 9H). |
| 648 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.99 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 5.08 (d, J = 7.1 Hz, 1H), 5.03 (d, J = 7.1 Hz, 1H), 4.84 - 4.82 (m, 12), 4.14 (s, 2H), 4.04 (d, J = 13.9 Hz, 1H), 3.83 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 0.94 (s, 9H). |
| 649 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.91 (s, 1H), 7.83 - 7.72 (m, 2H), 7.08 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.21 (s, 1H), 3.85 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.8 Hz, 1H), 2.69 (s, 1H), 2.52 (s, 3H), 2.37 (s, 6H), 0.89 (s, 9H). |
| 650 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 8.06 (dd, J = 5.4, 1.8 Hz, 1H), 7.92 (s, 1H), 7.81 (dd, J = 7.6, 1.8 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 7.02 (dd, J = 7.6, 5.4 Hz, 1H), 4.10 (d, J = 14.0 Hz, 1H), 3.78 (ddd, J = 11.4, 7.1, 4.2 Hz, 1H), 3.63 (d, J = 14.0 Hz, 1H), 2.97 (s, 6H), 1.12 - 1.02 (m, 4H), 0.91 (s, 9H). |
| 651 | 1H NMR (400 MHz, 甲醇-d4) δ 9.72 (s, 1H), 8.61 (d, J = 6.1 Hz, 1H), 8.50 (s, 1H), 8.18 (s, 1H), 8.09 (d, J = 6.1 Hz, 1H), 7.88 - 7.75 (m, 2H), 7.70 (dd, J = 7.9, 2.3 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.83 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 1.78 - 1.54 (m, 4H), 0.70 (s, 9H). |
| 652 | 1H NMR (400 MHz, 甲醇-d4) δ 9.56 (s, 1H), 8.59 (d, J = 6.2 Hz, 1H), 8.48 (s, 1H), 8.05 (d, J = 6.2 Hz, 1H), 8.01 (s, 1H), 7.84 (dd, J = 8.2, 5.1 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.39 (dd, J = 9.9, 8.2 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.79 (s, 1H), 5.91 (t, J = 54.6 Hz, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.50 (d, J = 4.1 Hz, 4H), 0.72 (s, 9H). |
| 653 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (dd, J = 4.3, 1.4 Hz, 1H), 8.60 (dt, J = 8.9, 1.5 Hz, 1H), 8.49 (s, 1H), 7.97 (s, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.64 (ddd, J = 18.1, 8.4, 4.5 Hz, 2H), 7.47 (dd, J = 10.3, 8.2 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.80 (s, 1H), 5.90 (t, J = 54.7 Hz, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 1.55 - 1.42 (m, 4H), 0.70 (s, 9H). |
| 654 | 1H NMR (400 MHz, DMSO-d6) δ 8.32 (s, 1H), 8.03 (s, 1H), 7.78 (t, J = 8.3 Hz, 1H), 7.61 - 7.40 (m, 2H), 7.34 (d, J = 7.4 Hz, 2H), 7.23 - 7.13 (m, 2H), 6.98 - 6.89 (m, 1H), 6.24 (d, J = 6.8 Hz, 1H), 5.63 (s, 2H), 4.11 (s, 1H), 3.88 (dd, J = 13.8, 8.0 Hz, 1H), 3.40 (dd, J = 13.7, 5.2 Hz, 1H), 2.43 (s, 3H), 0.78 (s, 9H). |
| 655 | 1H NMR (400 MHz, DMSO-d6) δ 8.31 - 8.21 (m, 2H), 7.99 (s, 1H), 7.62 (d, J = 2.1 Hz, 1H), 7.55 - 7.46 (m, 2H), 7.34 - 7.23 (m, 3H), 7.22 - 7.11 (m, 3H), 6.49 (d, J = 6.8 Hz, 1H), 5.62 (s, 2H), 4.15 (s, 1H), 4.04 - 3.93 (m, 4H), 3.34 (dd, J = 13.8, 5.1 Hz, 1H), 0.80 (s, 9H). |
| 656 | 1H NMR (400 MHz, DMSO-d6) δ 8.30 - 8.20 (m, 2H), 8.00 (s, 1H), 7.85 (d, J = 2.3 Hz, 1H), 7.61 (d, J = 7.7 Hz, 1H), 7.56 - 7.35 (m, 4H), 7.34 - 7.23 (m, 2H), 7.22 - 7.11 (m, 1H), 6.51 (d, J = 7.0 Hz, 1H), 5.62 (s, 2H), 4.13 (s, 1H), 3.98 (s, 3H), 3.99 - 3.88 (m, 1H), 3.32 (dd, J = 13.8, 5.1 Hz, 1H), 0.79 (s, 9H). |
| 657 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.13 (m, 2H), 7.78 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 2.5 Hz, 1H), 6.36 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.86 (m, 2H), 2.16 (s, 3H), 1.52 (m, 4H), 1.01 (s, 9H). |
| 658 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.22 (d, J = 5.1 Hz, 1H), 8.05 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.40 (d, J = 3.6 Hz, 1H), 7.23 (d, J = 5.1 Hz, 1H), 6.98 (d, J = 2.3 Hz, 1H), 6.59 (d, J = 3.6 Hz, 1H), 6.50 (s, 1H), 4.13 (d, J = 13.9 Hz, 1H), 3.86 (s, 3H), 3.60 (d, J = 13.9 Hz, 1H), 1.75 - 1.57 (m, 4H), 0.82 (s, 9H). |
| 659 | 1H NMR (400 MHz, 甲醇-d4) δ 9.54 (s, 1H), 8.48 (s, 1H), 8.41 (d, J = 8.3 Hz, 2H), 8.18 (s, 1H), 8.05 (d, J = 7.2 Hz, 1H), 7.87 (t, J = 7.8 Hz, 1H), 7.63 (d, J = 2.2 Hz, 1H), 7.16 (s, 1H), 6.98 (d, J = 2.3 Hz, 1H), 5.13 - 4.50 (m, 21H), 3.93 (d, J = 14.0 Hz, 1H), 3.83 (d, J = 13.9 Hz, 1H), 3.01 (s, 3H), 1.79 - 1.64 (m, 4H), 0.82 (s, 9H). |
| 660 | 1H NMR (400 MHz, 甲醇-d4) δ 8.97 (d, J = 5.4 Hz, 1H), 8.48 (d, J = 8.4 Hz, 2H), 8.28 (s, 1H), 8.19 (d, J = 1.2 Hz, 2H), 7.85 (dd, J = 5.4, 1.0 Hz, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.17 (d, J = 2.3 Hz, 1H), 6.47 (s, 1H), 4.95 - 4.81 (m, 3H), 4.11 (d, J = 14.0 Hz, 1H), 3.72 (d, J = 14.0 Hz, 1H), 2.92 (d, J = 0.9 Hz, 3H), 1.81 - 1.64 (m, 4H), 0.84 (s, 9H). |
| 661 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 8.34 (s, 1H), 7.84 - 7.75 (m, 2H), 7.15 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.31 (s, 1H), 4.38 (t, J = 10.8, 2H), 3.86 (d, J = 13.9 Hz, 1H), 3.75 (d, J = 13.9 Hz, 1H), 2.54 (s, 3H), 0.90 (s, 9H). |
| 662 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.87 (m, 2H), 6.17 (s, 1H), 4.03 (d, J = 13.8 Hz, 1H), 3.79 (d, J = 13.9 Hz, 1H), 3.72 (m, 1H), 2.47 (s, 3H), 1.34 - 1.18 (m, 3H), 0.92 (s, 9H). |
| 663 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.94 - 6.83 (m, 2H), 6.17 (s, 1H), 4.03 (d, J = 13.9 Hz, 1H), 3.79 (d, J = 13.9 Hz, 1H), 3.71 - 3.63 (m, 1H), 2.51 (s, 3H), 1.32 - 1.17 (m, 4H), 0.93 (s, 9H). |
| 664 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 8.27 (d, J = 5.2 Hz, 1H), 8.14 (s, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 3.6 Hz, 1H), 7.34 (d, J = 5.3 Hz, 1H), 7.15 (d, J = 2.5 Hz, 1H), 6.70 (d, J = 3.6 Hz, 1H), 6.54 (s, 1H), 3.95 (d, J = 13.8 Hz, 1H), 3.89 (s, 3H), 3.47 (d, J = 13.7 Hz, 1H), 1.78 - 1.52 (m, 4H), 0.77 (s, 9H). |
| 665 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.08 (s, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.41 (d, J = 2.0 Hz, 1H), 7.18 (d, J = 2.3 Hz, 1H), 6.29 (s, 1H), 6.16 (d, J = 2.0 Hz, 1H), 5.93 (t, J = 54.7 Hz, 1H), 4.08 - 3.90 (m, 2H), 3.85 (s, 3H), 1.54 (s, 4H), 1.02 (s, 9H). |
| 666 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.23 (s, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.41 (d, J = 2.0 Hz, 1H), 7.17 (d, J = 2.3 Hz, 1H), 6.31 (s, 1H), 6.15 (d, J = 2.0 Hz, 1H), 3.97 (q, J = 14.0 Hz, 2H), 3.85 (s, 3H), 1.84 - 1.60 (m, 4H), 1.01 (s, 9H). |
| 667 | 1H NMR (400 MHz, 甲醇-d4) δ 9.61 (s, 1H), 8.44 (d, J = 6.7 Hz, 2H), 8.30 (s, 1H), 8.20 (s, 1H), 8.13 (d, J = 6.5 Hz, 1H), 7.96 - 7.87 (m, 1H), 7.74 (d, J = 2.5 Hz, 1H), 7.16 (s, 1H), 7.11 (d, J = 2.5 Hz, 1H), 3.74 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 3.04 (s, 3H), 1.74 (s, 2H), 1.66 (s, 2H), 0.76 (s, 9H). |
| 668 | 1H NMR (400 MHz, 甲醇-d4) δ 8.99 (d, J = 5.5 Hz, 1H), 8.53 (s, 1H), 8.35 - 8.15 (m, 4H), 7.90 (d, J = 5.6 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.27 (d, J = 2.5 Hz, 1H), 6.45 (s, 1H), 3.90 (t, J = 13.5 Hz, 1H), 3.52 (d, J = 13.9 Hz, 1H), 2.95 (s, 3H), 1.76 (s, 2H), 1.67 (s, 2H), 0.78 (s, 9H). |
| 669 | 1H NMR (400 MHz, 甲醇-d4) δ 8.15 (s, 1H), 7.66 (s, 1H), 7.55 (t, J = 8.1 Hz, 1H), 7.32 (d, J = 2.3 Hz, 1H), 6.52 (dd, J = 8.5, 2.7 Hz, 1H), 6.36 (d, J = 2.4 Hz, 1H), 5.85 (s, 1H), 4.20 (dd, J = 11.6, 4.3 Hz, 1H), 3.71 (dd, J = 11.8, 4.7 Hz, 1H), 3.26 - 3.14 (m, 1H), 3.15 (d, J = 11.5 Hz, 1H), 2.86 (d, J = 11.7 Hz, 1H), 2.09 (s, 3H), 1.82 - 1.66 (m, 1H), 1.59 (d, J = 13.2 Hz, 1H), 1.23 (ddd, J = 13.2, 8.2, 5.0 Hz, 1H), 1.05 - 0.79 (m, 2H), 0.79 - 0.61 (m, 2H), 0.53 (s, 3H), 0.25 - 0.18 (m, 2H), 0.18 (s, 3H), 0.10 - -0.05 (m, 2H). |
| 670 | 1H NMR (400 MHz, 甲醇-d4) δ 9.61 (s, 1H), 8.69 (d, J = 6.1 Hz, 1H), 8.33 (s, 1H), 8.23 (s, 1H), 8.17 (d, J = 7.6 Hz, 1H), 8.16 (d, J = 6.1 Hz, 1H), 8.02 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.91 (s, 1H), 3.82 (d, J = 13.8 Hz, 1H), 3.42 (d, J = 13.7 Hz, 1H), 1.79 - 1.55 (m, 4H), 0.62 (s, 9H). |
| 671 | 1H NMR (400 MHz, 甲醇-d4) δ 8.95 (dd, J = 4.3, 1.4 Hz, 1H), 8.70 - 8.63 (m, 1H), 8.35 (s, 1H), 7.99 (s, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.67 (ddd, J = 15.5, 8.4, 4.6 Hz, 2H), 7.48 (dd, J = 10.3, 8.2 Hz, 1H), 7.08 (d, J = 2.5 Hz, 1H), 6.81 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 3.82 (d, J = 13.7 Hz, 1H), 3.49 (d, J = 13.7 Hz, 1H), 1.55 - 1.42 (m, 4H), 0.65 (s, 9H). |
| 672 | 1H NMR (400 MHz, 甲醇-d4) δ 9.56 (s, 1H), 8.59 (d, J = 6.2 Hz, 1H), 8.29 (s, 1H), 8.11 (d, J = 6.2 Hz, 1H), 8.02 (s, 1H), 7.87 (dd, J = 8.2, 5.2 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.39 (dd, J = 10.0, 8.2 Hz, 1H), 7.05 (d, J = 2.5 Hz, 1H), 6.79 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 3.76 (d, J = 13.7 Hz, 1H), 3.44 (s, 1H), 1.50 (d, J = 3.2 Hz, 3H), 1.31 (t, J = 7.4 Hz, 1H), 0.65 (s, 9H). |
| 673 | 1H NMR (400 MHz, 甲醇-d4) δ 9.64 (s, 1H), 8.58 (s, 1H), 8.49 (d, J = 8.1 Hz, 1H), 8.38 - 8.27 (m, 3H), 8.01 (d, J = 7.7 Hz, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.15 (d, J = 2.5 Hz, 1H), 6.98 (s, 1H), 3.74 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.9 Hz, 1H), 1.80 - 1.59 (m, 4H), 0.66 (s, 10H). |
| 674 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 8.06 (s, 1H), 7.80 (d, J = 2.5 Hz, 1H), 7.42 (d, J = 2.5 Hz, 1H), 6.14 - 5.73 (m, 2H), 3.93 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.37 (s, 3H), 2.26 (s, 3H), 1.51 (m, 4H), 1.01 (s, 9H). |
| 675 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.99 (s, 1H), 7.90 (t, J = 8.1 Hz, 1H), 7.80 (d, J = 2.5 Hz, 1H), 6.90 (d, J = 2.5 Hz, 1H), 6.85 (dd, J = 8.4, 2.7 Hz, 1H), 6.19 (s, 1H), 4.35 (dd, J = 11.1, 4.9 Hz, 1H), 4.11 - 3.95 (m, 1H), 3.53 (td, J = 11.6, 3.3 Hz, 1H), 3.49 - 3.42 (m, 1H), 3.18 (d, J = 11.6 Hz, 1H), 2.44 (s, 3H), 2.04 - 1.84 (m, 2H), 1.57 (ddd, J = 13.2, 8.4, 5.0 Hz, 1H), 1.33 - 1.17 (m, 2H), 1.12 - 0.97 (m, 2H), 0.86 (s, 3H), 0.62 - 0.51 (m, 2H), 0.49 (s, 3H), 0.36 (dt, J = 6.4, 4.8 Hz, 2H). |
| 676 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.37 - 8.29 (m, 1H), 8.06 (s, 1H), 7.75 (dd, J = 7.6, 1.3 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.80 (d, J = 7.5 Hz, 1H), 6.61 (s, 1H), 4.05 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.8 Hz, 1H), 1.79 - 1.55 (m, 4H), 0.80 (s, 9H). |
| 677 | 1H NMR (400 MHz, 甲醇-d4) δ 8.40 (s, 1H), 8.09 (s, 1H), 7.80 (d, J = 2.4 Hz, 1H), 7.39 (m, 2H), 6.31 (s, 1H), 6.15 (d, J = 2.0 Hz, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.86 (d, J = 3.9 Hz, 4H), 1.53 (s, 4H), 0.98 (s, 9H). |
| 678 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.22 (s, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.38 (m, 2H), 6.32 (s, 1H), 6.14 (d, J = 2.0 Hz, 1H), 3.92 - 3.73 (m, 5H), 1.81 - 1.60 (m, 4H), 0.97 (s, 9H). |
| 679 | 1H NMR (400 MHz, 甲醇-d4) δ 8.17 (s, 1H), 8.09 (dd, J = 2.5, 0.7 Hz, 1H), 7.83 (s, 1H), 7.57 (dd, J = 8.9, 2.5 Hz, 1H), 7.47 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 2.4 Hz, 1H), 6.65 (dd, J = 8.9, 0.8 Hz, 1H), 3.93 - 3.81 (m, 1H), 3.04 (s, 6H), 2.03 (s, 1H), 1.21 - 1.10 (m, 4H), 0.91 (s, 9H). |
| 680 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.85 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.91 - 6.83 (m, 1H), 6.18 (s, 1H), 3.89 (d, J = 13.8 Hz, 1H), 3.77 - 3.68 (m, 1H), 3.63 (d, J = 13.8 Hz, 1H), 2.47 (s, 3H), 1.31 - 1.21 (m, 4H), 0.88 (s, 9H). |
| 681 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.18 (s, 1H), 3.90 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.32 - 1.15 (m, 4H), 0.90 (s, 9H). |
| 682 | 1H NMR (400 MHz, 甲醇-d4) δ 9.01 (dd, J = 4.6, 1.4 Hz, 1H), 8.85 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 8.10 (d, J = 8.5 Hz, 1H), 7.96 (s, 1H), 7.90 - 7.82 (m, 1H), 7.81 - 7.73 (m, 2H), 7.69 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.86 (s, 1H), 4.03 (d, J = 13.9 Hz, 1H), 3.61 (d, J = 13.9 Hz, 1H), 1.56 - 1.44 (m, 1H), 1.24 - 1.13 (m, 2H), 0.99 (m, 2H), 0.70 (s, 9H), 0.53 - 0.42 (m, 2H), 0.29 (m, 2H). |
| 683 | 1H NMR (400 MHz, 甲醇-d4) δ 8.99 (d, J = 4.4 Hz, 1H), 8.80 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.99 (s, 1H), 7.88 - 7.81 (m, 1H), 7.78 - 7.70 (m, 2H), 7.69 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.89 (s, 1H), 6.09 (t, J = 55.6 Hz, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.64 (d, J = 13.9 Hz, 1H), 1.77 - 1.67 (m, 6H), 0.71 (s, 9H). |
| 684 | 1H NMR (400 MHz, 甲醇-d4) δ 9.03 (dd, J = 4.7, 1.5 Hz, 1H), 8.88 (d, J = 8.7 Hz, 1H), 8.49 (s, 1H), 8.11 (d, J = 8.6 Hz, 1H), 7.95 (s, 1H), 7.88 (dd, J = 8.5, 7.3 Hz, 1H), 7.82 - 7.75 (m, 2H), 7.69 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.89 (s, 1H), 4.60 (d, J = 47.1 Hz, 2H), 4.02 (d, J = 13.9 Hz, 1H), 3.64 (d, J = 13.9 Hz, 1H), 1.64 (s, 6H), 0.71 (s, 9H). |
| 685 | 1H NMR (400 MHz, 甲醇-d4) δ 9.00 (dd, J = 4.6, 1.4 Hz, 1H), 8.81 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.89 - 7.81 (m, 2H), 7.78 - 7.70 (m, 2H), 7.68 (d, J = 2.3 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.86 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 2.67 (s, 1H), 2.34 (s, 6H), 0.69 (s, 9H). |
| 686 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.98 (d, J = 2.3 Hz, 1H), 6.94 - 6.85 (m, 1H), 6.36 (s, 1H), 4.00 - 3.92 (m, 2H), 3.89 (d, J = 14.0 Hz, 1H), 2.54 (s, 3H), 1.47 - 1.23 (m, 4H), 0.94 (s, 9H). |
| 687 | 1H NMR (400 MHz, 甲醇-d4) δ 8.42 (s, 1H), 8.37 (s, 1H), 8.27 (d, J = 8.2 Hz, 1H), 7.66 (d, J = 8.3 Hz, 1H), 7.60 (d, J = 2.3 Hz, 1H), 6.99 (d, J = 2.3 Hz, 1H), 6.38 (s, 1H), 3.96 (d, J = 14.0 Hz, 1H), 3.81 (d, J = 14.0 Hz, 1H), 2.75 (s, 3H), 2.72 (s, 3H), 1.84 - 1.74 (m, 2H), 1.70 (d, J = 13.9 Hz, 2H), 0.94 (s, 9H). |
| 688 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.26 (s, 1H), 7.82 (dd, J = 10.0, 7.6 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.17 (dd, J = 7.7, 1.5 Hz, 1H), 7.07 (d, J = 2.3 Hz, 1H), 6.30 (s, 1H), 4.10 (d, J = 14.0 Hz, 1H), 3.78 (d, J = 14.0 Hz, 1H), 2.45 (s, 3H), 1.81 - 1.71 (m, 2H), 1.72 - 1.62 (m, 2H), 0.96 (s, 9H). |
| 689 | 1H NMR (400 MHz, 甲醇-d4) δ 8.40 (s, 1H), 8.36 (d, J = 8.3 Hz, 1H), 8.31 (s, 1H), 7.77 - 7.66 (m, 2H), 7.20 (d, J = 2.5 Hz, 1H), 6.39 (s, 1H), 3.85 (d, J = 13.9 Hz, 1H), 3.69 (d, J = 14.0 Hz, 1H), 2.78 (s, 3H), 2.74 (s, 3H), 1.83 - 1.74 (m, 2H), 1.71 (d, J = 14.7 Hz, 2H), 0.92 (s, 9H). |
| 690 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.27 (s, 1H), 7.87 - 7.76 (m, 2H), 7.26 (dd, J = 2.5, 0.6 Hz, 1H), 7.16 (dd, J = 7.7, 1.5 Hz, 1H), 6.31 (s, 1H), 3.98 (d, J = 13.9 Hz, 1H), 3.65 (d, J = 13.9 Hz, 1H), 2.45 (s, 3H), 1.80 - 1.71 (m, 2H), 1.71 - 1.62 (m, 2H), 0.92 (s, 9H). |
| 691 | 1H NMR (400 MHz, 甲醇-d4) δ 9.37 (s, 1H), 8.28 (m, 1H), 8.20 (s, 1H), 8.14 - 8.05 (m, 2H), 7.87 - 7.75 (m, 2H), 7.60 (m, 1H), 7.40 (m, 1H), 6.69 (m, 1H), 6.58 (s, 1H), 3.73 (m, 1H), 3.36 (m, 1H), 3.07 (s, 1H), 2.93 (s, 1H), 1.23 (m, 1H), 0.90 (m, 2H), 0.74 - 0.67 (m, 2H), 0.44 (s, 9H), 0.20 (m, 2H), 0.02 (m, 2H). |
| 692 | 1H NMR (400 MHz, 甲醇-d4) δ 9.59 (s, 1H), 8.56 (m, 1H), 8.48 (s, 1H), 8.32 (m, 2H), 8.05 (d, J = 7.3 Hz, 1H), 7.96 (d, J = 2.4 Hz, 1H), 7.85 (t, J = 7.8 Hz, 1H), 7.69 (d, J = 2.3 Hz, 1H), 6.97 (m, 1H), 6.88 (s, 1H), 3.98 (m, 1H), 3.94 - 3.76 (m, 1H), 3.68 (m, 1H), 2.01 - 1.88 (m, 1H), 1.61 (m, 1H), 0.74 (s, 9H). |
| 693 | 1H NMR (400 MHz, 甲醇-d4) δ 9.54 (s, 1H), 8.54 (m, 1H), 8.46 (m, 1H), 8.28 (m, 2H), 8.08 - 7.98 (m, 2H), 7.86 - 7.78 (m, 1H), 7.67 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.88 (s, 1H), 6.10 (t, J = 55.6 Hz, 1H), 4.04 - 3.92 (m, 2H), 3.64 (m, 1H), 1.73 (s, 6H), 0.71 (s, 9H). |
| 694 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 8.27 M, 1H), 8.00 (M, 2H), 7.80 (s, 0H), 7.68 (s, 1H), 6.89 (M, 2H), 4.98 - 4.78 (m, 26H), 4.73 - 4.63 (m, 2H), 4.57 (s, 1H), 3.96 (d, J = 13.7 Hz, 1H), 3.62 (d, J = 13.7 Hz, 1H), 1.67 (s, 6H), 0.71 (s, 9H). |
| 695 | 1H NMR (400 MHz, 甲醇-d4) δ 9.63 (s, 1H), 8.58 (d, J = 6.5 Hz, 1H), 8.49 (s, 1H), 8.36 (d, J = 6.7 Hz, 2H), 8.09 (m, 1H), 7.96 (s, 1H), 7.92 - 7.83 (m, 1H), 7.68 (d, J = 2.3 Hz, 1H), 6.96 (d, J = 2.3 Hz, 1H), 6.88 (s, 1H), 4.06 - 3.96 (m, 1H), 3.67 (m, 1H), 3.36 (s, 1H), 2.70 (s, 1H), 2.36 (s, 6H), 1.12 (s, 1H), 0.73 (s, 9H). |
| 696 | 1H NMR (400 MHz, 甲醇-d4) δ 9.66 (s, 1H), 8.57 (d, J = 6.7 Hz, 1H), 8.45 (d, J = 6.7 Hz, 1H), 8.36 (m, 1H), 8.28 (s, 1H), 8.14 (m, 1H), 8.07 (s, 1H), 7.90 (m, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.86 (s, 1H), 3.78 (d, J = 13.8 Hz, 1H), 3.48 (d, J = 13.8 Hz, 1H), 1.20 (s, 2H), 1.00 (s, 2H), 0.67 (s, 9H), 0.53 - 0.46 (m, 2H), 0.30 (d, J = 5.2 Hz, 2H). |
| 697 | 1H NMR (400 MHz, 甲醇-d4) δ 9.66 (s, 1H), 8.57 (d, J = 6.6 Hz, 1H), 8.45 (d, J = 6.6 Hz, 1H), 8.37 (m, 1H), 8.28 (s, 1H), 8.13 (m, 1H), 7.99 - 7.86 (m, 2H), 7.77 (d, J = 2.5 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.87 (s, 1H), 3.76 (d, J = 13.7 Hz, 1H), 3.49 (d, J = 13.7 Hz, 1H), 2.68 (s, 1H), 2.35 (s, 6H), 0.66 (s, 9H). |
| 698 | 1H NMR (400 MHz, 甲醇-d4) δ 8.90 (dd, J = 4.6, 1.6 Hz, 1H), 8.53 (d, J = 8.3 Hz, 1H), 8.45 (s, 1H), 8.16 (s, 1H), 8.15 - 8.13 (m, 1H), 8.06 (d, J = 8.6 Hz, 1H), 7.81 - 7.76 (m, 1H), 7.74 (d, J = 2.3 Hz, 1H), 7.65 (dd, J = 8.3, 4.5 Hz, 1H), 7.07 (d, J = 2.3 Hz, 1H), 6.38 (s, 1H), 4.10 (d, J = 14.0 Hz, 1H), 3.63 (d, J = 14.0 Hz, 1H), 1.78 - 1.69 (m, 2H), 1.66 (s, 2H), 0.77 (s, 9H). |
| 699 | 1H NMR (400 MHz, 甲醇-d4) δ 8.92 (dd, J = 4.7, 1.6 Hz, 1H), 8.61 - 8.56 (m, 1H), 8.46 (s, 1H), 8.15 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 8.03 (s, 1H), 7.81 (dd, J = 8.5, 1.7 Hz, 1H), 7.74 (d, J = 2.3 Hz, 1H), 7.69 (dd, J = 8.4, 4.6 Hz, 1H), 7.08 (d, J = 2.3 Hz, 1H), 6.38 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.11 (d, J = 14.0 Hz, 1H), 3.64 (d, J = 14.0 Hz, 1H), 1.52 (d, J = 2.4 Hz, 4H), 0.78 (s, 9H). |
| 700 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (d, J = 7.6 Hz, 1H), 8.32 (s, 1H), 8.07 (s, 1H), 7.80 (d, J = 2.5 Hz, 1H), 7.76 (dd, J = 7.6, 1.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.84 (d, J = 7.5 Hz, 1H), 6.61 (s, 1H), 3.81 (d, J = 13.6 Hz, 1H), 3.53 (d, J = 13.6 Hz, 1H), 1.80 - 1.54 (m, 4H), 0.74 (s, 9H). |
| 701 | 1H NMR (400 MHz, 甲醇-d4) δ 8.86 (s, 1H), 8.51 (s, 1H), 8.24 (s, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.14 (d, J = 2.4 Hz, 1H), 6.42 (s, 1H), 4.05 (d, J = 14.0 Hz, 1H), 3.87 (d, J = 13.9 Hz, 1H), 2.44 (s, 3H), 1.87 - 1.57 (m, 4H), 0.99 (s, 9H). |
| 702 | 1H NMR (400 MHz, 甲醇-d4) δ 8.85 (s, 1H), 8.50 (s, 1H), 8.06 (s, 1H), 7.61 (d, J = 2.3 Hz, 1H), 7.11 (d, J = 2.3 Hz, 1H), 6.37 (s, 1H), 4.03 (d, J = 14.0 Hz, 1H), 3.87 (d, J = 13.9 Hz, 1H), 2.44 (s, 3H), 1.61 - 1.47 (m, 1H), 1.23 (d, J = 2.7 Hz, 2H), 0.99 (m, 11H), 0.53 (dd, J = 8.1, 1.8 Hz, 2H), 0.40 - 0.27 (m, 2H). |
| 703 | 1H NMR (400 MHz, 甲醇-d4) δ 9.11 - 9.02 (m, 2H), 8.30 (s, 1H), 8.13 (d, J = 8.5 Hz, 1H), 8.04 (s, 1H), 8.00 - 7.92 (m, 1H), 7.87 (m, 2H), 7.80 (d, J = 2.5 Hz, 1H), 7.08 (d, J = 2.5 Hz, 1H), 6.88 (s, 1H), 3.80 (d, J = 13.7 Hz, 1H), 3.46 (d, J = 13.8 Hz, 1H), 1.51 (m, 1H), 1.20 (m, 2H), 1.05 - 0.93 (m, 2H), 0.65 (s, 9H), 0.56 - 0.43 (m, 2H), 0.30 (m, 2H). |
| 704 | 1H NMR (400 MHz, 甲醇-d4) δ 9.07 (d, J = 4.8 Hz, 1H), 9.01 (d, J = 8.7 Hz, 1H), 8.30 (s, 1H), 8.12 (d, J = 8.5 Hz, 1H), 8.00 - 7.90 (m, 2H), 7.90 - 7.82 (m, 2H), 7.79 (d, J = 2.5 Hz, 1H), 7.10 (d, J = 2.7 Hz, 1H), 6.90 (s, 1H), 4.95 - 4.79 (m, 1H), 3.86 (m, 1H), 3.78 (d, J = 13.7 Hz, 1H), 3.54 - 3.45 (m, 1H), 2.05 - 1.84 (m, 1H), 1.68 - 1.51 (m, 1H), 0.66 (m, 9H) |
| 705 | 1H NMR (400 MHz, 甲醇-d4) δ 9.06 (d, J = 4.7 Hz, 1H), 8.99 (d, J = 8.6 Hz, 1H), 8.29 (s, 1H), 8.11 (d, J = 8.5 Hz, 1H), 8.06 (s, 1H), 7.98 - 7.87 (m, 1H), 7.87 - 7.73 (m, 3H), 7.09 (d, J = 2.6 Hz, 1H), 6.91 (s, 1H), 6.09 (t, J = 55.5 Hz, 1H), 3.78 (d, J = 13.8 Hz, 1H), 3.47 (d, J = 13.8 Hz, 1H), 1.73 (s, 6H), 0.65 (s, 9H). |
| 706 | 1H NMR (400 MHz, 甲醇-d4) δ 9.07 (d, J = 4.7 Hz, 1H), 9.03 (d, J = 8.9 Hz, 1H), 8.30 (s, 1H), 8.12 (d, J = 8.5 Hz, 1H), 7.98 - 7.91 (m, 2H), 7.84 (m, 2H), 7.79 (d, J = 2.5 Hz, 1H), 7.08 (d, J = 2.5 Hz, 1H), 6.89 (s, 1H), 3.78 (d, J = 13.8 Hz, 1H), 3.46 (d, J = 13.7 Hz, 1H), 2.67 (s, 1H), 2.35 (s, 6H), 0.64 (s, 9H). |
| 707 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.83 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.90 - 6.81 (m, 2H), 6.14 - 5.81 (m, 2H), 4.12 (d, J = 13.9 Hz, 1H), 3.69 (d, J = 13.9 Hz, 1H), 2.41 (s, 3H), 1.70 (m, 2H), 1.62 (m, 2H), 0.90 (s, 9H). |
| 708 | 1H NMR (400 MHz, 甲醇-d4) δ 9.02 (dd, J = 5.0, 1.6 Hz, 1H), 8.84 (d, J = 8.3 Hz, 1H), 8.29 (s, 1H), 8.22 (s, 1H), 8.19 (s, 1H), 8.12 (s, 1H), 7.93 (dd, J = 8.7, 1.7 Hz, 1H), 7.89 - 7.81 (m, 2H), 7.22 (d, J = 2.5 Hz, 1H), 6.41 (s, 1H), 5.95 (t, J = 54.6 Hz, 1H), 3.91 (d, J = 13.9 Hz, 1H), 3.47 (d, J = 13.9 Hz, 1H), 1.54 (s, 4H), 0.72 (s, 9H). |
| 709 | 1H NMR (400 MHz, 甲醇-d4) δ 8.99 (dd, J = 4.3, 1.5 Hz, 1H), 8.60 (dd, J = 8.7, 1.5 Hz, 1H), 8.50 (s, 1H), 8.12 (s, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.66 (dd, J = 8.7, 4.3 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.83 (s, 1H), 4.03 (d, J = 13.8 Hz, 1H), 3.58 (d, J = 13.9 Hz, 1H), 1.76 - 1.55 (m, 4H), 0.67 (s, 9H). |
| 710 | 1H NMR (400 MHz, 甲醇-d4) δ 9.05 (dd, J = 4.2, 1.5 Hz, 1H), 8.67 (dd, J = 8.8, 1.6 Hz, 1H), 8.31 (s, 1H), 8.21 (d, J = 7.5 Hz, 2H), 7.84 - 7.79 (m, 2H), 7.69 (dd, J = 8.7, 4.2 Hz, 1H), 6.99 (d, J = 2.5 Hz, 1H), 6.92 (s, 1H), 3.81 (d, J = 13.7 Hz, 1H), 3.37 (d, J = 13.7 Hz, 1H), 1.77 - 1.55 (m, 4H), 0.58 (s, 10H). |
| 711 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (d, J = 3.3 Hz, 1H), 8.35 (d, J = 7.9 Hz, 1H), 7.75 (t, J = 4.3 Hz, 2H), 7.65 (d, J = 3.3 Hz, 1H), 7.54 - 7.45 (m, 1H), 7.40 (m, 1H), 6.83 (m, 2H), 6.56 (d, J = 3.2 Hz, 1H), 4.01 (m, 1H), 3.75 - 3.66 (m, 1H), 2.69 (d, J = 3.3 Hz, 1H), 2.36 (d, J = 3.3 Hz, 6H), 0.80 (s, 9H). |
| 712 | 1H NMR (400 MHz, 甲醇-d4) δ 9.17 (s, 1H), 8.26 (s, 1H), 8.14 (s, 1H), 8.12 (d, J = 8.3 Hz, 1H), 8.09 (s, 1H), 7.88 (d, J = 7.2 Hz, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.68 - 7.62 (m, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.78 (s, 1H), 3.73 (d, J = 13.6 Hz, 1H), 3.54 - 3.44 (m, 3H), 1.76 - 1.58 (m, 3H), 0.64 (s, 9H). |
| 713 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (d, J = 1.1 Hz, 1H), 8.33 (d, J = 8.1 Hz, 1H), 7.78 (s, 1H), 7.72 (d, J = 7.5 Hz, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 6.81 (d, J = 7.3 Hz, 2H), 6.56 (s, 1H), 4.04 (d, J = 13.8 Hz, 1H), 3.64 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 2.67 (s, 1H), 2.35 (s, 6H), 0.76 (s, 9H). |
| 714 | 1H NMR (400 MHz, 乙腈-d3) δ 8.40 (s, 1H), 8.30 (d, J = 8.1 Hz, 1H), 7.82 - 7.75 (m, 2H), 7.53 (d, J = 2.2 Hz, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 6.60 (s, 1H), 6.48 (s, 1H), 6.24 (s, 2H), 3.77 - 3.56 (m, 5H), 3.54 (s, 3H), 3.04 (m, 2H), 2.35 (s, 2H), 1.42 (s, 9H), 0.80 (s, 9H). |
| 715 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 7.93 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.60 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.5, 2.8 Hz, 1H), 6.80 (d, J = 2.3 Hz, 1H), 6.17 (s, 1H), 4.30 (d, J = 15.0 Hz, 1H), 4.14 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.64 (s, 9H), 1.12 (d, J = 2.2 Hz, 6H). |
| 716 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 0.7 Hz, 1H), 7.91 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.94 - 6.84 (m, 2H), 6.18 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.94 - 3.79 (m, 2H), 2.50 (s, 3H), 1.27 - 1.10 (m, 4H), 0.93 (s, 9H). |
| 717 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (d, J = 1.0 Hz, 1H), 8.31 (d, J = 8.3 Hz, 1H), 8.25 (s, 1H), 7.68 (d, J = 8.3 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.03 (d, J = 2.3 Hz, 1H), 6.38 (s, 1H), 5.94 (t, J = 54.5 Hz, 1H), 3.99 (d, J = 14.0 Hz, 1H), 3.84 (d, J = 14.0 Hz, 1H), 2.75 (d, J = 7.7 Hz, 6H), 1.56 (s, 4H), 0.95 (d, J = 1.1 Hz, 9H). |
| 718 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.06 (s, 1H), 7.71 - 7.58 (m, 2H), 7.26 (d, J = 8.2 Hz, 1H), 6.87 (d, J = 2.3 Hz, 1H), 6.20 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.76 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.54 (d, J = 1.1 Hz, 4H), 0.92 (s, 9H). |
| 719 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.90 (s, 1H), 7.74 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.91 - 6.78 (m, 2H), 6.18 (s, 1H), 4.41 - 4.14 (m, 2H), 2.69 (s, 1H), 2.51 (s, 3H), 2.37 (s, 6H), 1.14 (d, J = 3.3 Hz, 6H). |
| 720 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 7.95 (s, 1H), 7.74 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.94 - 6.80 (m, 2H), 6.17 (s, 1H), 4.34 (d, J = 15.0 Hz, 1H), 4.20 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.65 (s, 3H), 1.31 (d, J = 5.2 Hz, 2H), 1.15 (d, J = 2.2 Hz, 6H), 1.09 - 1.00 (m, 2H). |
| 721 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 8.18 (s, 1H), 7.76 (t, J = 8.2 Hz, 1H), 7.61 (d, J = 2.3 Hz, 1H), 6.95 - 6.74 (m, 2H), 6.22 (s, 1H), 4.29 (d, J = 14.9 Hz, 1H), 4.13 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.84 - 1.59 (m, 4H), 1.13 (d, J = 2.1 Hz, 6H). |
| 722 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.20 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.93 - 6.77 (m, 1H), 6.62 (d, J = 2.3 Hz, 1H), 6.11 (s, 1H), 4.53 - 4.20 (m, 2H), 3.04 (s, 3H), 2.46 (s, 3H), 1.87 - 1.57 (m, 4H), 1.48 (s, 3H), 1.38 (s, 3H). |
| 723 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (d, J = 3.3 Hz, 1H), 8.05 (d, J = 3.6 Hz, 1H), 7.81 (q, J = 8.0 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.46 - 7.21 (m, 5H), 7.14 (dd, J = 25.5, 2.3 Hz, 1H), 6.97 - 6.77 (m, 1H), 6.35 (d, J = 5.8 Hz, 1H), 5.94 (td, J = 54.7, 8.7 Hz, 1H), 5.70 (dt, J = 20.5, 7.2 Hz, 1H), 2.53 (d, J = 5.7 Hz, 3H), 2.12 (dh, J = 29.2, 7.2 Hz, 2H), 1.53 (d, J = 6.7 Hz, 4H), 0.97 (dt, J = 14.5, 7.3 Hz, 3H). |
| 724 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.35 (d, J = 8.1 Hz, 1H), 7.91 (s, 1H), 7.74 - 7.61 (m, 2H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.94 - 6.74 (m, 2H), 6.57 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.33 (d, J = 14.8 Hz, 1H), 4.06 (d, J = 14.8 Hz, 1H), 1.58 - 1.40 (m, 4H), 1.01 (d, J = 17.0 Hz, 6H). |
| 725 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.04 (s, 1H), 7.85 - 7.70 (m, 2H), 7.04 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.21 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.21 (d, J = 14.9 Hz, 1H), 4.05 (d, J = 14.9 Hz, 1H), 2.51 (s, 3H), 1.53 (t, J = 2.3 Hz, 4H), 1.10 (d, J = 2.4 Hz, 6H). |
| 726 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (d, J = 1.3 Hz, 1H), 8.33 (d, J = 8.1 Hz, 1H), 7.92 (s, 1H), 7.79 - 7.63 (m, 2H), 7.48 (t, J = 7.8 Hz, 1H), 7.23 (d, J = 7.5 Hz, 1H), 6.85 (d, J = 2.2 Hz, 1H), 6.79 (d, J = 7.6 Hz, 1H), 6.58 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.36 (d, J = 14.8 Hz, 1H), 4.05 (d, J = 14.8 Hz, 1H), 1.50 (d, J = 5.1 Hz, 4H), 1.01 (d, J = 10.6 Hz, 6H). |
| 727 | 1H NMR (400 MHz, 甲醇-d4) δ 8.25 (s, 1H), 8.02 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.51 (d, J = 2.4 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.19 (s, 1H), 5.95 (t, J = 54.8 Hz, 1H), 4.19 (d, J = 14.8 Hz, 1H), 3.99 (d, J = 14.8 Hz, 1H), 2.50 (s, 3H), 1.53 (q, J = 2.1 Hz, 4H), 1.07 (d, J = 2.3 Hz, 6H). |
| 728 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 8.31 (s, 1H), 8.23 (dd, J = 7.9, 1.5 Hz, 1H), 7.92 - 7.86 (m, 1H), 7.77 (s, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 6.98 (d, J = 2.4 Hz, 2H), 4.03 (d, J = 13.8 Hz, 1H), 3.73 (d, J = 13.9 Hz, 1H), 3.59 (s, 3H), 2.66 (s, 1H), 2.34 (s, 6H), 0.86 (s, 9H). |
| 729 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.96 (s, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.51 (d, J = 8.6 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.61 (d, J = 8.6 Hz, 1H), 6.17 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.89 (s, 4H), 2.48 (s, 3H), 1.52 (s, 4H), 0.94 (s, 9H). |
| 730 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.33 (d, J = 8.1 Hz, 1H), 7.89 (s, 1H), 7.72 (dd, J = 7.2, 1.2 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 6.89 - 6.71 (m, 2H), 6.56 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.52 (d, J = 14.9 Hz, 1H), 4.09 (d, J = 14.8 Hz, 1H), 3.61 (s, 3H), 2.20 - 2.04 (m, 2H), 2.04 - 1.76 (m, 3H), 1.68 (d, J = 8.8 Hz, 1H), 1.50 (t, J = 3.8 Hz, 4H). |
| 731 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.03 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.94 - 6.74 (m, 2H), 6.21 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.55 (d, J = 15.0 Hz, 1H), 4.28 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 2.32 (ddd, J = 12.5, 9.3, 6.6 Hz, 2H), 2.19 - 1.77 (m, 4H), 1.64 - 1.46 (m, 4H). |
| 732 | 1H NMR (400 MHz, 甲醇-d4) δ 8.21 (d, J = 12.2 Hz, 1H), 8.05 (s, 1H), 7.51 - 7.34 (m, 2H), 6.79 (d, J = 2.4 Hz, 1H), 6.43 (dd, J = 15.8, 9.5 Hz, 1H), 6.08 (d, J = 9.5 Hz, 1H), 3.80 (d, J = 13.8 Hz, 1H), 3.70 - 3.54 (m, 4H), 2.48 (s, 3H), 1.52 (s, 4H), 0.90 (d, J = 2.4 Hz, 9H). |
| 733 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.05 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.61 (d, J = 2.2 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.26 (s, 1H), 5.94 (t, J = 54.6 Hz, 1H), 4.10 (dd, J = 14.6, 7.1 Hz, 1H), 3.97 (dd, J = 14.6, 7.3 Hz, 1H), 2.50 (s, 3H), 1.54 (s, 4H), 1.11 (d, J = 3.7 Hz, 6H), 1.02 (d, J = 3.4 Hz, 6H), 0.79 (t, J = 7.2 Hz, 1H). |
| 734 | 1H NMR (400 MHz, 甲醇-d4) δ 8.92 (s, 2H), 8.45 (s, 1H), 8.21 - 8.02 (m, 2H), 7.97 - 7.88 (m, 1H), 7.83 (dd, J = 8.4, 7.3 Hz, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.31 (s, 1H), 7.06 (d, J = 2.3 Hz, 1H), 3.99 (d, J = 13.8 Hz, 1H), 3.69 (d, J = 13.9 Hz, 1H), 1.79 - 1.48 (m, 4H), 0.78 (s, 9H). |
| 735 | 1H NMR (400 MHz, 甲醇-d4) δ 8.12 (s, 1H), 7.60 (d, J = 3.1 Hz, 1H), 7.47 (dd, J = 7.2, 2.3 Hz, 1H), 7.29 (dt, J = 7.9, 1.0 Hz, 1H), 7.22 - 7.03 (m, 3H), 6.65 - 6.49 (m, 2H), 6.28 (s, 1H), 3.76 (s, 3H), 3.67 - 3.57 (m, 1H), 3.53 - 3.41 (m, 1H), 2.60 (s, 1H), 2.29 (s, 6H), 0.73 (d, J = 1.4 Hz, 9H). |
| 736 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 7.80 - 7.60 (m, 2H), 7.36 (p, J = 3.8 Hz, 1H), 7.24 - 7.08 (m, 3H), 6.96 (d, J = 2.2 Hz, 1H), 6.54 (dd, J = 3.2, 0.9 Hz, 1H), 6.39 (s, 1H), 5.90 (t, J = 54.8 Hz, 1H), 4.05 (d, J = 13.7 Hz, 1H), 3.80 (s, 3H), 3.65 (d, J = 13.8 Hz, 1H), 1.58 - 1.35 (m, 4H), 0.84 (s, 9H). |
| 737 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 8.04 (s, 1H), 7.80 - 7.57 (m, 3H), 7.50 (t, J = 7.6 Hz, 1H), 7.43 - 7.20 (m, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.21 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.69 (d, J = 18.0 Hz, 1H), 4.33 (d, J = 18.0 Hz, 1H), 4.13 (d, J = 13.8 Hz, 1H), 3.70 - 3.56 (m, 1H), 3.15 (s, 3H), 1.53 (d, J = 3.2 Hz, 4H), 0.85 (s, 9H). |
| 738 | 1H NMR (400 MHz, 甲醇-d4) δ 9.57 (s, 1H), 8.58 (d, J = 6.4 Hz, 1H), 8.50 (s, 1H), 8.44 (d, J = 0.9 Hz, 1H), 8.24 (d, J = 6.4 Hz, 1H), 8.06 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.18 (d, J = 2.3 Hz, 1H), 6.61 (s, 1H), 4.01 (d, J = 14.0 Hz, 1H), 3.90 (tt, J = 7.4, 4.0 Hz, 1H), 3.82 (d, J = 14.0 Hz, 1H), 1.24 - 1.13 (m, 4H), 0.88 (s, 9H). |
| 739 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.06 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 6.89 (dd, J = 8.4, 2.8 Hz, 1H), 6.25 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.12 (m, 2H), 2.52 (s, 1H), 2.51 (s, 3H), 1.80 (s, 6H), 1.54 (s, 4H). |
| 740 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.20 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 6.89 (dd, J = 8.5, 2.8 Hz, 1H), 6.26 (s, 1H), 4.12 (s, 2H), 2.52 (s, 1H), 2.51 (s, 3H), 1.80 (s, 6H), 1.78 - 1.72 (m, 2H), 1.72 - 1.65 (m, 2H). |
| 741 | 1H NMR (400 MHz, 甲醇-d4) δ 9.52 (s, 1H), 8.56 (d, J = 6.3 Hz, 1H), 8.48 (s, 1H), 8.38 (d, J = 1.0 Hz, 1H), 8.32 (s, 1H), 8.20 (d, J = 6.3 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.17 (d, J = 2.3 Hz, 1H), 6.65 (s, 1H), 4.00 (d, J = 14.0 Hz, 1H), 3.80 (d, J = 14.0 Hz, 1H), 1.81 - 1.71 (m, 2H), 1.69 (m, 2H), 0.87 (s, 9H). |
| 742 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 8.04 (s, 1H), 7.98 (t, J = 2.1 Hz, 1H), 7.87 - 7.75 (m, 2H), 7.67 (d, J = 2.3 Hz, 1H), 7.10 (d, J = 2.4 Hz, 1H), 6.88 (dd, J = 8.4, 2.7 Hz, 1H), 6.17 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 2.45 (s, 3H), 1.53 (s, 4H). |
| 743 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.35 (d, J = 8.4 Hz, 1H), 7.93 (s, 1H), 7.76 (d, J = 7.3 Hz, 1H), 7.67 (d, J = 1.9 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 6.86 (d, J = 2.2 Hz, 1H), 6.83 (d, J = 7.9 Hz, 1H), 6.60 (s, 1H), 6.23 (tt, J = 56.0, 4.1 Hz, 1H), 5.92 (t, J = 54.6 Hz, 1H), 4.56 - 4.43 (m, 1H), 4.44 - 4.28 (m, 1H), 4.04 (d, J = 13.8 Hz, 1H), 3.64 (d, J = 13.8 Hz, 1H), 1.62 - 1.38 (m, 4H), 0.78 (s, 9H). |
| 744 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.34 (d, J = 8.1 Hz, 1H), 7.91 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.84 (d, J = 2.3 Hz, 1H), 6.82 (d, J = 7.8 Hz, 1H), 6.58 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.02 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.57 - 1.42 (m, 4H), 0.78 (s, 9H). |
| 745 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.33 (d, J = 8.1 Hz, 1H), 7.77 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 6.84 (d, J = 2.3 Hz, 1H), 6.81 (d, J = 7.7 Hz, 1H), 6.54 (s, 1H), 4.04 (d, J = 13.8 Hz, 1H), 3.91 - 3.81 (m, 1H), 3.66 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.22 - 1.08 (m, 4H), 0.78 (s, 9H). |
| 746 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.06 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.3, 2.8 Hz, 1H), 6.72 (d, J = 2.2 Hz, 1H), 6.09 (s, 1H), 5.95 (t, J = 54.6 Hz, 1H), 4.09 - 3.95 (m, 2H), 3.65 - 3.53 (m, 2H), 2.49 (s, 3H), 1.54 (s, 4H), 1.01 (s, 3H), 0.98 (s, 3H). |
| 747 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.39 - 8.28 (m, 1H), 7.80 (s, 1H), 7.73 (dd, J = 7.5, 1.3 Hz, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.89 - 6.73 (m, 2H), 6.55 (s, 1H), 4.03 (d, J = 13.8 Hz, 1H), 3.63 (m, 4H), 1.61 (s, 9H), 0.77 (s, 9H). |
| 748 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.90 (s, 1H), 7.83 - 7.73 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.25 (s, 1H), 3.88 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.65 (t, J = 18.7 Hz, 3H), 0.89 (s, 9H). |
| 749 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (d, J = 1.3 Hz, 1H), 7.90 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.1 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.25 (s, 1H), 4.94 - 4.82 (m, 1H), 4.03 (d, J = 14.2 Hz, 1H), 3.81 (d, J = 14.0 Hz, 1H), 2.50 (s, 3H), 1.66 (t, J = 18.7 Hz, 3H), 0.93 (s, 9H). |
| 750 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.89 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.25 (s, 1H), 4.90 (dd, J = 13.7, 2.8 Hz, 2H), 4.02 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.92 (td, J = 16.9, 8.3 Hz, 2H), 1.05 (t, J = 7.5 Hz, 3H), 0.93 (s, 9H). |
| 751 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.92 (s, 1H), 7.74 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.96 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 4.64 (d, J = 1.3 Hz, 2H), 4.01 (d, J = 13.9 Hz, 1H), 3.87 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.14 (s, 4H), 0.94 (s, 9H). |
| 752 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.88 (s, 1H), 7.75 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.22 (s, 1H), 5.59 (t, J = 56.0 Hz, 1H), 4.55 (s, 2H), 4.02 (d, J = 13.9 Hz, 1H), 3.86 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 0.94 (s, 14H). |
| 753 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.01 (s, 1H), 7.72 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.4, 2.8 Hz, 1H), 6.20 (s, 1H), 3.99 (d, J = 14.0 Hz, 1H), 3.86 (d, J = 14.0 Hz, 1H), 2.86 (q, J = 10.3 Hz, 2H), 2.48 (s, 3H), 1.52 - 1.23 (m, 4H), 0.93 (s, 9H). |
| 754 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.95 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.00 - 6.78 (m, 2H), 6.19 (s, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.83 (d, J = 14.0 Hz, 1H), 2.49 (s, 3H), 1.92 - 1.74 (m, 2H), 1.31 - 1.17 (m, 2H), 1.06 (d, J = 1.8 Hz, 2H), 0.93 (s, 9H), 0.82 (t, J = 7.4 Hz, 3H). |
| 755 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.88 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.4, 2.8 Hz, 1H), 6.23 (s, 1H), 4.55 (s, 2H), 4.01 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.17 (d, J = 6.7 Hz, 6H), 0.94 (s, 9H). |
| 756 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.33 (d, J = 8.3 Hz, 1H), 7.80 (s, 1H), 7.76 - 7.68 (m, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 6.88 - 6.75 (m, 2H), 6.54 (s, 1H), 4.01 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 3.60 (s, 3H), 1.83 (q, J = 7.4 Hz, 2H), 1.21 (d, J = 5.5 Hz, 2H), 1.13 - 0.97 (m, 2H), 0.78 (d, J = 7.6 Hz, 12H). |
| 757 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.91 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.21 (s, 1H), 4.03 (d, J = 13.9 Hz, 1H), 3.85 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.65 (p, J = 6.8 Hz, 1H), 1.24 - 1.15 (m, 2H), 1.15 - 1.08 (m, 2H), 0.94 (s, 9H), 0.93 - 0.87 (m, 6H). |
| 758 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.97 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.90 - 6.87 (m, 1H), 6.87 - 6.85 (m, 1H), 6.20 (s, 1H), 4.02 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.57 (s, 3H), 1.56 (s, 3H), 1.39 - 1.31 (m, 1H), 0.93 (s, 9H), 0.56 - 0.49 (m, 2H), 0.46 - 0.38 (m, 2H). |
| 759 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.07 (s, 1H), 7.80 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.95 - 6.84 (m, 2H), 6.24 (s, 1H), 5.09 (t, J = 6.3 Hz, 2H), 4.75 (dd, J = 6.8, 2.3 Hz, 2H), 4.02 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.92 (s, 3H), 0.93 (s, 9H). |
| 760 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 7.92 (s, 1H), 7.75 (dd, J = 7.5, 1.0 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.87 - 6.80 (m, 2H), 6.58 (s, 1H), 5.06 (dd, J = 6.8, 4.2 Hz, 2H), 4.76 - 4.69 (m, 2H), 4.02 (d, J = 13.8 Hz, 1H), 3.64 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.88 (s, 3H), 0.77 (s, 9H). |
| 761 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.90 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.6, 2.8 Hz, 1H), 6.20 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.85 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.28 (m, 2H), 1.08 (m, 2H), 0.94 (s, 9H), 0.88 (s, 9H). |
| 762 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.02 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.25 (s, 1H), 5.39 - 5.21 (m, 1H), 5.05 - 4.91 (m, 2H), 4.84 (s, 2H), 4.02 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 0.93 (s, 9H). |
| 763 | 1H NMR (400 MHz, 甲醇-d4) δ 8.54 (s, 1H), 8.00 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.88 (d, J = 2.8 Hz, 1H), 6.86 (d, J = 2.2 Hz, 2H), 6.18 (s, 1H), 4.37 (d, J = 15.0 Hz, 1H), 4.18 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.59 - 1.49 (m, 1H), 1.30 - 1.19 (m, 2H), 1.15 (s, 3H), 1.15 (s, 3H), 1.08 - 0.96 (m, 2H), 0.57 - 0.48 (m, 2H), 0.37 - 0.27 (m, 2H). |
| 764 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.87 (s, 1H), 7.79 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.22 (s, 1H), 4.23 (d, J = 3.1 Hz, 2H), 4.04 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 0.96 (s, 3H), 0.93 (s, 9H), 0.71 - 0.66 (m, 2H), 0.43 (m, 2H). |
| 765 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.33 (d, J = 7.9 Hz, 1H), 7.90 (s, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.7 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 6.84 (d, J = 2.2 Hz, 1H), 6.81 (d, J = 7.5 Hz, 1H), 6.59 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.02 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 1.51 (s, 4H), 0.79 (s, 9H). |
| 766 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.84 (s, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.90 (d, J = 2.2 Hz, 1H), 6.87 (d, J = 8.3 Hz, 1H), 6.19 (s, 1H), 4.21 - 4.14 (m, 1H), 4.02 (d, J = 14.0 Hz, 1H), 3.83 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.75 - 1.67 (m, 2H), 1.17 - 1.12 (m, 1H), 1.06 - 0.95 (m, 1H), 0.95 - 0.91 (m, 1H), 0.93 (s, 9H), 0.90 - 0.74 (m, 1H). |
| 767 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.22 (d, J = 5.0 Hz, 1H), 7.94 (s, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.40 (d, J = 3.5 Hz, 1H), 7.25 (d, J = 5.2 Hz, 1H), 6.99 (d, J = 2.3 Hz, 1H), 6.62 (d, J = 3.6 Hz, 1H), 6.50 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 4.14 (d, J = 13.8 Hz, 1H), 3.86 (s, 3H), 3.60 (d, J = 13.8 Hz, 1H), 1.56 - 1.39 (m, 4H), 0.82 (s, 9H). |
| 768 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.34 (d, J = 8.2 Hz, 1H), 7.79 (s, 1H), 7.74 - 7.67 (m, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 7.6 Hz, 1H), 6.70 (d, J = 2.3 Hz, 1H), 6.42 (s, 1H), 4.01 (d, J = 13.3 Hz, 1H), 3.90 (d, J = 13.4 Hz, 1H), 3.61 (s, 3H), 3.48 (d, J = 10.5 Hz, 1H), 3.40 (d, J = 10.5 Hz, 1H), 2.68 (s, 1H), 2.35 (s, 6H), 0.91 (s, 3H), 0.81 (s, 3H). |
| 769 | 1H NMR (400 MHz, 甲醇-d4) δ 8.44 (s, 1H), 8.33 (d, J = 8.2 Hz, 1H), 7.75 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 6.83 (d, J = 7.8 Hz, 1H), 6.80 - 6.75 (m, 1H), 6.55 (s, 1H), 4.15 - 4.03 (m, 2H), 4.00 (d, J = 13.8 Hz, 1H), 3.59 (d, J = 13.7 Hz, 1H), 2.67 (s, 1H), 2.34 (s, 6H), 1.36 (t, J = 7.1 Hz, 3H), 0.74 (s, 9H). |
| 770 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.39 - 8.32 (m, 1H), 8.06 (s, 1H), 7.80 - 7.73 (m, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.39 (d, J = 7.8 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 6.82 (d, J = 7.8 Hz, 1H), 6.61 (s, 1H), 6.39 - 6.05 (m, 1H), 4.55 - 4.32 (m, 2H), 4.04 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.75 - 1.56 (m, 4H), 0.78 (s, 9H). |
| 771 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.36 (d, J = 8.1 Hz, 1H), 8.07 (s, 1H), 7.81 - 7.73 (m, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 6.83 (d, J = 7.8 Hz, 1H), 6.61 (s, 1H), 5.03 - 4.84 (m, 1H), 4.81 - 4.66 (m, 1H), 4.06 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.9 Hz, 1H), 1.84 - 1.53 (m, 4H), 0.78 (s, 9H). |
| 772 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.34 (d, J = 8.1 Hz, 1H), 7.75 (s, 1H), 7.73 (d, J = 7.4 Hz, 1H), 7.65 (d, J = 2.2 Hz, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.46 (t, J = 7.8 Hz, 1H), 6.87 (d, J = 7.9 Hz, 1H), 6.79 (d, J = 2.3 Hz, 1H), 6.56 (s, 1H), 5.29 (p, J = 6.8 Hz, 1H), 4.04 (d, J = 13.7 Hz, 1H), 3.58 (d, J = 13.8 Hz, 1H), 2.67 (s, 1H), 2.34 (s, 6H), 1.42 (d, J = 6.8 Hz, 3H), 1.40 (d, J = 6.8 Hz, 3H), 0.73 (s, 9H). |
| 773 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.37 - 8.30 (m, 1H), 7.82 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 6.83 (d, J = 7.8 Hz, 1H), 6.80 (d, J = 2.3 Hz, 1H), 6.54 (s, 1H), 4.17 - 3.98 (m, 2H), 4.03 (d, J = 13.8 Hz, 1H), 3.61 (d, J = 13.8 Hz, 1H), 1.62 (s, 3H), 1.36 (t, J = 7.1 Hz, 3H), 1.32 - 1.22 (m, 2H), 1.06 - 1.00 (m, 2H), 0.75 (s, 9H). |
| 774 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 8.38 - 8.30 (m, 1H), 7.81 (s, 1H), 7.73 (d, J = 7.3 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 6.88 (d, J = 7.8 Hz, 1H), 6.76 (d, J = 2.3 Hz, 1H), 6.54 (s, 1H), 5.36 - 5.22 (m, 1H), 4.01 (d, J = 13.8 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 1.62 (s, 3H), 1.42 (d, J = 6.9 Hz, 3H), 1.40 (d, J = 6.9 Hz, 3H), 1.32 - 1.25 (m, 2H), 1.07 - 0.99 (m, 2H), 0.73 (s, 9H). |
| 775 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (d, J = 1.2 Hz, 1H), 8.34 (d, J = 8.1 Hz, 1H), 7.88 (s, 1H), 7.73 (d, J = 7.4 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.87 - 6.79 (m, 2H), 6.57 (s, 1H), 4.59 (d, J = 48.4 Hz, 2H), 4.05 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.46 - 1.29 (m, 4H), 0.77 (s, 9H). |
| 776 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.33 (d, J = 8.1 Hz, 1H), 7.83 (s, 1H), 7.72 (d, J = 7.4 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.85 - 6.77 (m, 2H), 6.54 (s, 1H), 4.06 (d, J = 13.9 Hz, 1H), 3.64 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 1.62 (s, 3H), 1.30 - 1.26 (m, 2H), 1.07 - 1.01 (m, 2H), 0.76 (s, 9H). |
| 777 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.34 (d, J = 8.2 Hz, 1H), 7.88 (s, 1H), 7.77 - 7.70 (m, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 6.85 (d, J = 2.2 Hz, 1H), 6.82 (d, J = 7.6 Hz, 1H), 6.61 (s, 1H), 5.24 (t, J = 19.1 Hz, 1H), 5.02 - 4.91 (m, 2H), 4.87 - 4.69 (m, 2H), 4.05 (d, J = 13.8 Hz, 1H), 3.64 (d, J = 13.7 Hz, 1H), 3.61 (s, 3H), 0.77 (s, 9H). |
| 778 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.34 (d, J = 8.2 Hz, 1H), 7.81 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.67 (d, J = 2.2 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 6.81 (d, J = 7.7 Hz, 1H), 6.58 (s, 1H), 5.05 - 4.91 (m, 1H), 4.67 (d, J = 46.9 Hz, 1H), 4.66 (d, J = 47.0 Hz, 1H), 4.06 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 1.53 (dd, J = 7.1, 1.2 Hz, 3H), 0.77 (s, 9H). |
| 779 | 1H NMR (400 MHz, 甲醇-d4) δ 8.18 (s, 1H), 8.04 (d, J = 8.0 Hz, 1H), 7.58 (s, 1H), 7.44 (d, J = 7.3 Hz, 1H), 7.36 (d, J = 2.1 Hz, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.12 (d, J = 7.7 Hz, 1H), 6.55 - 6.48 (m, 2H), 6.25 (s, 1H), 3.76 (d, J = 13.6 Hz, 1H), 3.33 (d, J = 14.0 Hz, 1H), 3.31 (s, 3H), 1.26 - 1.17 (m, 1H), 0.98 - 0.81 (m, 2H), 0.79 - 0.62 (m, 2H), 0.47 (s, 9H), 0.27 - 0.13 (m, 2H), 0.12 - -0.09 (m, 2H). |
| 780 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 8.35 (d, J = 8.3 Hz, 1H), 8.07 (s, 1H), 7.72 - 7.68 (m, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 6.80 (d, J = 7.6 Hz, 1H), 6.59 (s, 1H), 4.34 (d, J = 14.9 Hz, 1H), 4.07 (d, J = 14.9 Hz, 1H), 3.61 (s, 3H), 1.85 - 1.55 (m, 4H), 1.02 (d, J = 16.4 Hz, 6H). |
| 781 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 8.34 (d, J = 8.3 Hz, 1H), 7.84 (s, 1H), 7.72 - 7.67 (m, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.80 (d, J = 7.5 Hz, 1H), 6.53 (s, 1H), 4.35 (d, J = 14.9 Hz, 1H), 4.07 (d, J = 14.9 Hz, 1H), 3.61 (s, 3H), 1.63 (s, 3H), 1.32 - 1.25 (m, 2H), 1.06 - 1.01 (m, 5H), 0.99 (s, 3H). |
| 782 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.14 (s, 1H), 8.12 (dd, J = 5.0, 1.9 Hz, 1H), 7.68 (dd, J = 7.3, 1.9 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.3 Hz, 1H), 6.95 (dd, J = 7.4, 5.0 Hz, 1H), 6.29 (s, 1H), 4.05 (d, J = 13.9 Hz, 1H), 3.94 (s, 3H), 3.85 (d, J = 13.9 Hz, 1H), 1.82 - 1.59 (m, 4H), 0.97 (s, 9H). |
| 783 | 1H NMR (400 MHz, 甲醇-d4) δ 8.18 (s, 1H), 8.08 - 8.00 (m, 1H), 7.57 (s, 1H), 7.46 - 7.38 (m, 1H), 7.35 (d, J = 2.3 Hz, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 6.54 - 6.46 (m, 2H), 6.23 (s, 1H), 4.03 (d, J = 14.8 Hz, 1H), 3.71 (d, J = 14.9 Hz, 1H), 3.31 (s, 3H), 1.21 (ddd, J = 13.1, 8.1, 4.9 Hz, 1H), 0.97 - 0.79 (m, 2H), 0.69 (d, J = 18.4 Hz, 6H), 0.71 - 0.68 (m, 2H), 0.25 - 0.15 (m, 2H), 0.01 (t, J = 5.0 Hz, 2H). |
| 784 | 1H NMR (400 MHz, 甲醇-d4) δ 9.85 (s, 1H), 9.71 (d, J = 7.9 Hz, 1H), 9.17 (s, 1H), 9.07 (d, J = 7.5 Hz, 1H), 9.02 (s, 1H), 8.85 (t, J = 8.0 Hz, 1H), 8.78 (d, J = 7.6 Hz, 1H), 8.18 (s, 2H), 7.93 (s, 1H), 6.46 - 6.27 (m, 1H), 6.03 (dd, J = 46.9, 5.4 Hz, 2H), 5.70 (d, J = 14.8 Hz, 1H), 5.40 (d, J = 14.8 Hz, 1H), 4.98 (s, 3H), 2.90 (d, J = 7.0 Hz, 3H), 2.36 (d, J = 17.8 Hz, 6H). |
| 785 | 1H NMR (400 MHz, 甲醇-d4) δ 9.84 (s, 1H), 9.70 (d, J = 8.3 Hz, 1H), 9.14 (s, 1H), 9.05 (d, J = 7.5 Hz, 1H), 9.00 (d, J = 2.2 Hz, 1H), 8.84 (t, J = 7.8 Hz, 1H), 8.78 (d, J = 7.6 Hz, 1H), 8.28 - 8.05 (m, 2H), 7.90 (s, 1H), 5.66 (d, J = 14.9 Hz, 1H), 5.41 (d, J = 14.8 Hz, 1H), 4.97 (s, 3H), 4.03 (s, 1H), 3.71 (s, 6H), 2.36 (d, J = 18.1 Hz, 6H). |
| 786 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.17 (d, J = 9.9 Hz, 1H), 7.78 (s, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.64 - 7.59 (m, 2H), 7.40 - 7.33 (m, 1H), 6.83 (d, J = 2.3 Hz, 1H), 6.71 (d, J = 9.9 Hz, 1H), 6.65 (s, 1H), 4.07 (d, J = 13.9 Hz, 1H), 3.77 (s, 3H), 3.61 (d, J = 13.9 Hz, 1H), 2.67 (s, 1H), 2.35 (s, 6H), 0.77 (s, 9H). |
| 787 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.32 (d, J = 8.1 Hz, 1H), 7.77 - 7.72 (m, 2H), 7.65 (d, J = 2.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 6.81 (dd, J = 5.0, 2.6 Hz, 2H), 6.56 (s, 1H), 4.01 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 2.67 (s, 1H), 2.35 (s, 6H), 0.77 (s, 9H). |
| 788 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.36 (d, J = 8.0 Hz, 1H), 8.11 (s, 1H), 7.91 (t, J = 60.1 Hz, 1H), 7.83 - 7.79 (m, 1H), 7.69 (d, J = 2.3 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 6.92 (d, J = 8.1 Hz, 1H), 6.86 (d, J = 2.3 Hz, 1H), 6.62 (s, 1H), 4.07 (d, J = 13.8 Hz, 1H), 3.64 (d, J = 13.8 Hz, 1H), 1.85 - 1.51 (m, 4H), 0.79 (s, 9H). |
| 789 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.17 (d, J = 9.8 Hz, 1H), 8.08 (s, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.64 (s, 1H), 7.63 (d, J = 2.4 Hz, 1H), 7.37 (dd, J = 5.5, 3.1 Hz, 1H), 6.87 (d, J = 2.3 Hz, 1H), 6.72 (d, J = 9.8 Hz, 1H), 6.70 (s, 1H), 4.08 (d, J = 13.9 Hz, 1H), 3.78 (s, 3H), 3.61 (d, J = 13.8 Hz, 1H), 1.77 - 1.59 (m, 4H), 0.77 (s, 9H). |
| 790 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.40 - 8.26 (m, 1H), 7.74 (dd, J = 7.6, 1.2 Hz, 1H), 7.66 (d, J = 2.0 Hz, 2H), 7.47 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 6.88 - 6.75 (m, 2H), 6.54 (s, 1H), 4.03 (m, 4H), 3.71 - 3.55 (m, 4H), 0.77 (s, 9H). |
| 791 | 1H NMR (400 MHz, 甲醇-d4) δ 8.20 (s, 1H), 7.89 - 7.70 (m, 2H), 7.48 (d, J = 2.4 Hz, 1H), 6.86 (dd, J = 8.4, 2.7 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.16 (s, 1H), 4.07 (s, 3H), 3.79 (d, J = 13.7 Hz, 1H), 3.59 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 0.86 (s, 9H). |
| 792 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (d, J = 1.0 Hz, 1H), 8.19 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 4.53 (dd, J = 12.1, 6.4 Hz, 2H), 4.43 (t, J = 6.1 Hz, 2H), 4.12 (s, 2H), 2.50 (s, 3H), 1.81 - 1.56 (m, 4H), 1.39 (s, 3H). |
| 793 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (d, J = 3.1 Hz, 2H), 8.23 (s, 1H), 7.89 (dd, J = 8.3, 2.6 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.47 (d, J = 8.3 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.20 (s, 1H), 4.15 (d, J = 14.0 Hz, 1H), 3.73 (d, J = 14.0 Hz, 1H), 1.80 - 1.72 (m, 2H), 1.69 (d, J = 9.1 Hz, 2H), 0.96 (s, 9H). |
| 794 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.21 (s, 1H), 7.70 - 7.60 (m, 2H), 7.27 (d, J = 8.2 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.22 (s, 1H), 4.05 (d, J = 13.9 Hz, 1H), 3.77 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.81 - 1.61 (m, 4H), 0.92 (s, 9H). |
| 795 | 1H NMR (400 MHz, 甲醇-d4) δ 8.71 (d, J = 2.2 Hz, 1H), 8.48 (s, 1H), 8.31 (s, 1H), 8.28 (dd, J = 8.3, 2.3 Hz, 1H), 7.71 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.16 (d, J = 2.3 Hz, 1H), 6.33 (s, 1H), 4.09 (d, J = 14.0 Hz, 1H), 3.81 (d, J = 14.0 Hz, 1H), 2.69 (s, 3H), 1.80 - 1.73 (m, 2H), 1.72 - 1.62 (m, 2H), 0.97 (s, 9H). |
| 796 | 1H NMR (400 MHz, 乙腈-d3) δ 8.39 (s, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 7.2 Hz, 1H), 7.66 (s, 1H), 7.53 (d, J = 2.3 Hz, 1H), 7.45 (t, J = 7.8 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 6.80 (d, J = 7.7 Hz, 1H), 6.60 (s, 1H), 6.46 (s, 1H), 5.07 - 4.98 (m, 1H), 3.77 - 3.69 (m, 1H), 3.62 (m, 1H), 3.54 (s, 3H), 2.54 - 2.44 (m, 4H), 1.89 (m, 2H), 0.79 (s, 9H). |
| 797 | 1H NMR (400 MHz, 甲醇-d4) δ 9.03 (d, J = 4.5 Hz, 1H), 8.87 (d, J = 8.6 Hz, 1H), 8.45 (s, 1H), 8.10 (d, J = 8.5 Hz, 1H), 7.95 - 7.83 (m, 2H), 7.82 - 7.73 (m, 2H), 7.66 (d, J = 2.3 Hz, 1H), 6.85 - 6.79 (m, 2H), 4.30 (d, J = 14.9 Hz, 1H), 3.93 (d, J = 14.9 Hz, 1H), 1.62 (s, 3H), 1.30 (d, J = 13.3 Hz, 2H), 1.03 (s, 2H), 0.94 (s, 3H), 0.88 (s, 3H). |
| 798 | 1H NMR (400 MHz, 甲醇-d4) δ 9.02 (d, J = 4.9 Hz, 1H), 8.85 (d, J = 8.4 Hz, 1H), 8.44 (s, 1H), 8.09 (d, J = 8.6 Hz, 1H), 7.96 (s, 1H), 7.87 (t, J = 7.9 Hz, 1H), 7.81 - 7.72 (m, 2H), 7.67 (d, J = 2.3 Hz, 1H), 6.86 - 6.79 (m, 2H), 4.31 (d, J = 14.8 Hz, 1H), 3.91 (d, J = 14.9 Hz, 1H), 1.18 (s, 2H), 0.99 (s, 2H), 0.94 (s, 3H), 0.88 (s, 3H), 0.49 (d, J = 7.5 Hz, 2H), 0.29 (d, J = 5.1 Hz, 2H). |
| 799 | 1H NMR (400 MHz, 甲醇-d4) δ 9.05 (d, J = 4.6 Hz, 1H), 8.92 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 8.12 (d, J = 8.6 Hz, 1H), 7.94 - 7.85 (m, 2H), 7.85 - 7.76 (m, 2H), 7.67 (d, J = 2.3 Hz, 1H), 6.88 - 6.81 (m, 2H), 4.31 (d, J = 14.9 Hz, 1H), 3.96 (d, J = 14.9 Hz, 1H), 2.67 (s, 1H), 2.34 (s, 6H), 0.95 (s, 3H), 0.90 (s, 3H). |
| 800 | (1H NMR (400 MHz, 乙腈-d3) δ 8.38 (s, 1H), 8.30 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 7.5 Hz, 1H), 7.65 (s, 1H), 7.52 (d, J = 2.2 Hz, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 6.82 (d, J = 7.7 Hz, 1H), 6.58 (s, 1H), 6.46 (s, 1H), 3.77 - 3.67 (m, 1H), 3.61 (m, 1H), 3.54 (s, 3H), 2.68 (d, J = 10.2 Hz, 2H), 2.5, (m, 2H), 2.27 (s, 2H), 1.69 (s, 3H), 0.78 (s, 9H). |
| 801 | 1H NMR (400 MHz, 乙腈-d3) δ 8.40 (s, 1H), 8.31 (d, J = 7.9 Hz, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.56 (dd, J = 21.4, 1.8 Hz, 2H), 7.45 (t, J = 7.8 Hz, 1H), 7.30 (d, J = 7.7 Hz, 1H), 6.81 - 6.74 (m, 1H), 6.61 (s, 1H), 6.47 (s, 1H), 3.84 - 3.68 (m, 1H), 3.63 (m, 1H), 3.54 (s, 3H), 1.86 - 1.78 (m, 2H), 1.46 (dd, J = 6.8, 3.3 Hz, 3H), 0.79 (d, J = 1.1 Hz, 9H), 0.78 - 0.69 (m, 3H). |
| 802 | 1H NMR (400 MHz, 乙腈-d3) δ 8.39 (s, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.83 - 7.76 (m, 1H), 7.63 (s, 1H), 7.54 (d, J = 2.3 Hz, 1H), 7.45 (t, J = 7.8 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.61 (s, 1H), 6.47 (s, 1H), 6.24 (s, 1H), 4.79 (m, 1H), 3.76 (m, 1H), 3.71 - 3.53 (m, 3H), 3.54 (s, 3H), 3.20 (s, 3H), 1.45 (d, J = 6.9 Hz, 3H), 0.80 (s, 9H). |
| 803 | 1H NMR (400 MHz, 甲醇-d4) δ 8.98 (d, J = 4.6 Hz, 1H), 8.78 (d, J = 8.6 Hz, 1H), 8.46 (s, 1H), 8.08 (d, J = 8.4 Hz, 1H), 7.89 - 7.79 (m, 2H), 7.79 - 7.65 (m, 3H), 6.95 - 6.85 (m, 2H), 4.96 (dd, J = 12.2, 5.9 Hz, 1H), 4.71 (d, J = 5.4 Hz, 1H), 4.60 (d, J = 5.4 Hz, 1H), 4.00 (d, J = 13.8 Hz, 1H), 3.61 (d, J = 13.9 Hz, 1H), 1.52 (dd, J = 7.1, 1.3 Hz, 3H), 0.69 (s, 9H). |
| 804 | 1H NMR (400 MHz, 甲醇-d4) δ 8.98 (d, J = 4.5 Hz, 1H), 8.78 (d, J = 8.7 Hz, 1H), 8.46 (s, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.94 (s, 1H), 7.88 - 7.79 (m, 1H), 7.79 - 7.66 (m, 3H), 6.96 - 6.88 (m, 2H), 5.22 (s, 1H), 4.99 - 4.84 (m, 3H), 4.84 - 4.73 (m, 1H), 4.00 (d, J = 13.9 Hz, 1H), 3.61 (d, J = 13.8 Hz, 1H), 0.69 (s, 9H). |
| 805 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.22 (s, 1H), 8.13 (s, 1H), 7.74 (dd, J = 8.7, 2.5 Hz, 1H), 7.65 (d, J = 2.1 Hz, 1H), 7.10 (s, 1H), 6.81 (d, J = 8.7 Hz, 1H), 6.10 (s, 1H), 4.12 (d, J = 14.0 Hz, 1H), 3.89 (s, 3H), 3.79 (d, J = 14.0 Hz, 1H), 1.83 - 1.56 (m, 4H), 0.98 (s, 9H). |
| 806 | 1H NMR (400 MHz, 甲醇-d4) δ 8.54 (s, 1H), 8.20 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.96 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.7, 2.8 Hz, 1H), 6.25 (s, 1H), 4.12 (d, J = 13.9 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 2.46 (s, 3H), 2.06 - 1.93 (m, 4H), 0.94 (s, 9H). |
| 807 | 1H NMR (400 MHz, 甲醇-d4) δ 8.27 (s, 1H), 8.23 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 6.2 Hz, 1H), 7.75 (d, J = 2.5 Hz, 1H), 7.72 (s, 2H), 7.51 (d, J = 7.8 Hz, 1H), 7.49 - 7.42 (m, 2H), 6.93 (d, J = 2.5 Hz, 1H), 6.66 (s, 1H), 4.09 (s, 3H), 3.71 (d, J = 13.5 Hz, 1H), 3.44 (d, J = 13.6 Hz, 1H), 2.30 (s, 6H), 0.61 (s, 9H). |
| 808 | 1H NMR (400 MHz, 甲醇-d4) δ 8.28 (s, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.95 (dd, J = 6.3, 1.4 Hz, 1H), 7.77 - 7.70 (m, 4H), 7.53 - 7.44 (m, 2H), 6.94 (d, J = 2.4 Hz, 1H), 6.64 (s, 1H), 4.09 (d, J = 1.4 Hz, 3H), 3.85 - 3.77 (m, 0H), 3.72 (d, J = 13.4 Hz, 1H), 3.45 (d, J = 13.2 Hz, 1H), 1.16 - 1.04 (m, 4H), 0.62 (d, J = 1.4 Hz, 9H). |
| 809 | 1H NMR (400 MHz, 甲醇-d4) δ 8.26 (s, 1H), 8.22 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 6.3 Hz, 1H), 7.79 (s, 1H), 7.75 (d, J = 2.3 Hz, 1H), 7.73 (s, 1H), 7.54 - 7.43 (m, 2H), 6.92 (d, J = 2.5 Hz, 1H), 6.64 (s, 1H), 4.09 (d, J = 1.0 Hz, 3H), 3.71 (d, J = 13.4 Hz, 1H), 3.44 (d, J = 13.8 Hz, 1H), 1.58 (s, 3H), 1.24 (s, 3H), 1.01 - 0.96 (m, 2H), 0.61 (d, J = 1.0 Hz, 8H). |
| 810 | 1H NMR (400 MHz, 甲醇-d4) δ 8.29 (d, J = 1.4 Hz, 1H), 8.23 (d, J = 8.3 Hz, 1H), 7.98 - 7.93 (m, 1H), 7.87 (s, 1H), 7.79 - 7.76 (m, 1H), 7.74 (d, J = 7.3 Hz, 1H), 7.54 - 7.44 (m, 3H), 6.98 (d, J = 2.5 Hz, 1H), 6.69 (s, 1H), 5.88 (t, J = 55.0 Hz, 1H), 4.09 (d, J = 1.2 Hz, 3H), 3.75 (d, J = 13.6 Hz, 1H), 3.47 (d, J = 13.5 Hz, 1H), 1.46 (d, J = 1.2 Hz, 2H), 0.63 (d, J = 1.1 Hz, 10H). |
| 811 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (d, J = 9.2 Hz, 1H), 8.36 (s, 1H), 8.04 (s, 1H), 7.82 (s, 1H), 7.82 (d, J = 10.3 Hz, 1H), 7.59 (t, J = 8.5, 7.4 Hz, 1H), 7.43 (d, J = 7.3 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 7.00 (d, J = 9.2 Hz, 1H), 6.78 (s, 1H), 4.06 (d, J = 0.6 Hz, 3H), 3.83 (d, J = 13.7 Hz, 1H), 3.55 (d, J = 13.7 Hz, 1H), 1.76 - 1.56 (m, 5H), 0.69 (s, 9H). |
| 812 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 8.07 (s, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.83 (d, J = 2.5 Hz, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.49 (d, J = 6.4 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 6.75 (s, 1H), 4.13 (s, 3H), 3.82 (d, J = 13.7 Hz, 1H), 3.52 (d, J = 13.7 Hz, 1H), 1.77 - 1.55 (m, 4H), 0.66 (s, 9H). |
| 814 | 1H NMR (400 MHz, 甲醇-d4) δ 8.95 (dd, J = 4.4, 1.4 Hz, 1H), 8.67 (d, J = 8.8 Hz, 1H), 8.35 (s, 1H), 7.86 (s, 1H), 7.81 (d, J = 2.4 Hz, 1H), 7.69 (dd, J = 8.7, 4.3 Hz, 1H), 7.64 (dd, J = 8.2, 4.8 Hz, 1H), 7.48 (dd, J = 10.3, 8.2 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.78 (s, 1H), 3.82 (d, J = 13.7 Hz, 1H), 3.49 (d, J = 13.8 Hz, 1H), 2.66 (s, 1H), 2.34 (s, 6H), 0.64 (s, 9H). |
| 815 | 1H NMR (400 MHz, 甲醇-d4) δ 8.96 (d, J = 4.3 Hz, 1H), 8.68 (d, J = 8.8 Hz, 1H), 8.35 (s, 1H), 7.99 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.71 (dd, J = 8.7, 4.4 Hz, 1H), 7.63 (dd, J = 8.2, 4.8 Hz, 1H), 7.50 (dd, J = 10.0, 8.5 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 6.78 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 4.16 (d, J = 14.8 Hz, 1H), 3.86 (d, J = 14.8 Hz, 1H), 1.49 (d, J = 5.4 Hz, 4H), 0.90 (s, 3H), 0.85 (s, 3H). |
| 816 | 1H NMR (400 MHz, 甲醇-d4) δ 8.95 (d, J = 4.3 Hz, 1H), 8.66 (d, J = 8.7 Hz, 1H), 8.34 (s, 1H), 7.84 (s, 1H), 7.79 (d, J = 2.4 Hz, 1H), 7.69 (dd, J = 8.8, 4.3 Hz, 1H), 7.61 (dd, J = 8.2, 4.8 Hz, 1H), 7.52 - 7.44 (m, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.75 (s, 1H), 4.14 (d, J = 14.8 Hz, 1H), 3.85 (d, J = 14.8 Hz, 1H), 2.66 (s, 1H), 2.34 (s, 6H), 2.02 (s, 1H), 0.88 (s, 3H), 0.83 (s, 3H). |
| 817 | 1H NMR (400 MHz, 甲醇-d4) δ 8.67 (d, J = 5.9 Hz, 1H), 8.34 (d, J = 8.5 Hz, 1H), 8.30 (s, 1H), 8.29 (s, 1H), 8.16 (s, 1H), 7.98 (d, J = 7.2 Hz, 1H), 7.89 - 7.83 (m, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.88 (s, 1H), 3.80 (d, J = 13.7 Hz, 1H), 3.43 (d, J = 13.7 Hz, 1H), 1.76 - 1.56 (m, 4H), 0.62 (s, 9H). |
| 818 | 1H NMR (400 MHz, 甲醇-d4) δ 9.02 (dd, J = 5.0, 1.6 Hz, 1H), 8.84 (d, J = 8.3 Hz, 1H), 8.29 (s, 1H), 8.22 (s, 1H), 8.19 (s, 1H), 8.12 (s, 1H), 7.93 (dd, J = 8.7, 1.7 Hz, 1H), 7.89 - 7.81 (m, 2H), 7.22 (d, J = 2.5 Hz, 1H), 6.41 (s, 1H), 5.95 (t, J = 54.6 Hz, 1H), 3.91 (d, J = 13.9 Hz, 1H), 3.47 (d, J = 13.9 Hz, 1H), 1.54 (s, 4H), 0.72 (s, 9H). |
| 819 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (s, 1H), 8.26 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 6.2 Hz, 1H), 7.83 (s, 1H), 7.78 (d, J = 2.4 Hz, 1H), 7.73 (d, J = 7.2 Hz, 1H), 7.53 (t, J = 7.9 Hz, 1H), 7.48 (d, J = 6.3 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.66 (s, 1H), 4.12 (d, J = 14.8 Hz, 1H), 4.12 (s, 3H), 3.85 (d, J = 14.7 Hz, 1H), 1.62 (s, 3H), 1.27 (d, J = 5.6 Hz, 2H), 1.07 - 0.99 (m, 2H), 0.84 (d, J = 13.7 Hz, 6H). |
| 820 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (s, 1H), 8.26 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.78 (d, J = 2.4 Hz, 1H), 7.76 (s, 1H), 7.72 (d, J = 7.3 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.48 (d, J = 6.2 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.67 (s, 1H), 4.10 (d, J = 12.8 Hz, 4H), 3.86 (d, J = 14.6 Hz, 1H), 2.66 (s, 1H), 2.34 (s, 6H), 0.84 (d, J = 14.2 Hz, 6H). |
| 821 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.3 Hz, 1H), 7.91 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.73 (dd, J = 7.4, 1.2 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.48 (d, J = 6.3 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.71 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 4.13 (s, 4H), 3.89 (d, J = 14.7 Hz, 1H), 1.49 (d, J = 3.5 Hz, 4H), 0.87 (d, J = 12.6 Hz, 6H). |
| 822 | 1H NMR (400 MHz, 甲醇-d4) δ 8.91 (s, 1H), 8.38 (s, 1H), 8.30 (s, 1H), 8.18 (d, J = 1.8 Hz, 1H), 8.05 (d, J = 2.1 Hz, 1H), 8.02 (d, J = 2.0 Hz, 0H), 7.96 (s, 1H), 7.96 - 7.91 (m, 1H), 7.80 (d, J = 2.6 Hz, 1H), 7.28 (d, J = 2.5 Hz, 1H), 6.31 (s, 1H), 3.90 (d, J = 14.0 Hz, 1H), 3.57 (d, J = 14.0 Hz, 1H), 1.78 (s, 2H), 1.68 (s, 2H), 0.83 (s, 10H). |
| 823 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.86 (s, 1H), 7.82 (d, J = 2.4 Hz, 1H), 7.78 (d, J = 7.4 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 6.3 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.69 (s, 1H), 4.12 (s, 3H), 3.81 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.7 Hz, 1H), 1.49 (ddd, J = 13.2, 8.4, 5.0 Hz, 1H), 1.25 - 1.14 (m, 2H), 1.02 - 0.94 (m, 2H), 0.66 (s, 9H), 0.52 - 0.42 (m, 2H), 0.28 (q, J = 5.3 Hz, 2H). |
| 824 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 6.2 Hz, 1H), 7.82 (d, J = 2.4 Hz, 1H), 7.79 (s, 1H), 7.77 (d, J = 7.3 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 6.3 Hz, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.72 (s, 1H), 5.03 - 4.90 (m, 1H), 4.71 (d, J = 5.4 Hz, 1H), 4.59 (d, J = 5.4 Hz, 1H), 4.12 (s, 3H), 3.81 (d, J = 13.7 Hz, 1H), 3.51 (d, J = 13.7 Hz, 1H), 1.51 (dd, J = 7.0, 1.3 Hz, 3H), 0.66 (s, 9H), 0.09 (s, 1H). |
| 825 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.3 Hz, 1H), 7.87 (s, 1H), 7.83 (d, J = 2.4 Hz, 1H), 7.78 (d, J = 7.3 Hz, 1H), 7.58 (d, J = 7.1 Hz, 0H), 7.54 (dd, J = 8.3, 7.3 Hz, 1H), 7.50 (d, J = 6.3 Hz, 1H), 7.03 (d, J = 2.5 Hz, 1H), 6.75 (s, 1H), 5.29 - 5.14 (m, 1H), 4.98 - 4.86 (m, 2H), 4.83 - 4.74 (m, 1H), 4.68 (s, 0H), 4.30 (s, 0H), 4.12 (s, 3H), 3.81 (d, J = 13.6 Hz, 1H), 3.51 (d, J = 13.7 Hz, 1H), 0.66 (s, 9H). |
| 826 | 1H NMR (400 MHz, 甲醇-d4) δ 8.83 (d, J = 2.2 Hz, 1H), 8.33 (s, 1H), 8.29 (s, 1H), 8.07 (dd, J = 8.1, 2.3 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.21 (d, J = 2.6 Hz, 1H), 6.29 (s, 1H), 4.00 (d, J = 14.0 Hz, 1H), 3.57 (d, J = 14.0 Hz, 1H), 1.80 - 1.72 (m, 2H), 1.69 (d, J = 9.2 Hz, 2H), 0.90 (s, 9H). |
| 827 | 1H NMR (400 MHz, 甲醇-d4) δ 8.41 (s, 1H), 7.96 (s, 1H), 7.82 - 7.71 (m, 2H), 7.04 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.19 (s, 1H), 4.25 (d, J = 15.0 Hz, 1H), 4.08 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.64 (s, 9H), 1.10 (d, J = 1.9 Hz, 6H). |
| 828 | 1H NMR (400 MHz, 甲醇-d4) δ 8.79 (d, J = 2.1 Hz, 1H), 8.48 (dd, J = 8.4, 2.2 Hz, 1H), 8.37 (s, 1H), 8.31 (s, 1H), 7.86 (d, J = 8.4 Hz, 1H), 7.76 (dd, J = 2.6, 1.2 Hz, 1H), 7.32 (d, J = 2.5 Hz, 1H), 6.37 (s, 1H), 3.96 - 3.81 (m, 1H), 3.66 (d, J = 13.9 Hz, 1H), 2.75 (s, 3H), 1.82 - 1.74 (m, 2H), 1.69 (d, J = 5.5 Hz, 2H), 1.07 (s, 2H), 0.94 (s, 9H). |
| 829 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (d, J = 8.3 Hz, 1H), 8.29 (d, J = 15.4 Hz, 2H), 7.76 - 7.64 (m, 2H), 7.20 (d, J = 2.5 Hz, 1H), 6.37 (s, 1H), 5.95 (t, J = 54.5 Hz, 1H), 3.85 (d, J = 13.9 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 2.76 (d, J = 12.5 Hz, 6H), 1.56 (d, J = 2.5 Hz, 4H), 0.92 (s, 9H). |
| 830 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (s, 1H), 8.26 (s, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.29 (s, 1H), 3.89 (d, J = 13.8 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 2.59 (s, 3H), 1.80 - 1.63 (m, 4H), 0.86 (s, 9H). |
| 831 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.04 (s, 1H), 7.82 - 7.70 (m, 2H), 7.01 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.20 (s, 1H), 5.95 (t, J = 54.8 Hz, 1H), 4.42 (d, J = 14.9 Hz, 1H), 4.15 (d, J = 14.9 Hz, 1H), 2.49 (s, 3H), 2.41 - 2.20 (m, 2H), 2.15 - 1.79 (m, 3H), 1.53 (s, 5H). |
| 832 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.05 (s, 1H), 7.85 - 7.68 (m, 2H), 7.05 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.22 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 5.48 (s, 1H), 4.22 (d, J = 14.9 Hz, 1H), 4.06 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.52 (t, J = 1.7 Hz, 4H), 1.10 (d, J = 2.5 Hz, 6H). |
| 833 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.90 (s, 1H), 7.82 - 7.68 (m, 2H), 7.02 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.6, 2.7 Hz, 1H), 6.18 (s, 1H), 4.25 - 4.00 (m, 2H), 2.68 (s, 1H), 2.51 (s, 3H), 2.37 (s, 6H), 1.09 (d, J = 3.3 Hz, 6H). |
| 834 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 7.97 (s, 1H), 7.84 - 7.68 (m, 2H), 7.07 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.20 (s, 1H), 5.98 (tt, J = 56.8, 3.4 Hz, 1H), 4.17 (dt, J = 8.2, 4.0 Hz, 1H), 3.85 (d, J = 13.7 Hz, 1H), 3.69 (d, J = 13.8 Hz, 1H), 2.52 (s, 3H), 2.26 (ddq, J = 10.8, 7.4, 3.8 Hz, 1H), 1.66 (q, J = 10.4, 8.7 Hz, 1H), 1.52 - 1.43 (m, 1H), 0.89 (s, 9H). |
| 835 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.90 (s, 1H), 7.83 - 7.73 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.25 (s, 1H), 3.88 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.65 (t, J = 18.7 Hz, 3H), 0.89 (s, 9H). |
| 836 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.90 (s, 1H), 7.83 - 7.73 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.25 (s, 1H), 3.88 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.65 (t, J = 18.7 Hz, 3H), 0.89 (s, 9H). |
| 837 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.90 (s, 1H), 7.84 - 7.75 (m, 2H), 7.18 - 7.08 (m, 1H), 6.88 (dd, J = 8.4, 2.8 Hz, 1H), 6.25 (s, 1H), 3.90 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 2.01 - 1.77 (m, 2H), 1.05 (t, J = 7.5 Hz, 3H), 0.89 (s, 9H). |
| 838 | 1H NMR (400 MHz, DMSO-d6) δ 8.35 (s, 1H), 8.18 (d, J = 7.9 Hz, 1H), 8.09 (s, 1H), 7.84 (d, J = 2.3 Hz, 1H), 7.69 - 7.58 (m, 1H), 7.58 - 7.37 (m, 4H), 7.24 (s, 1H), 6.72 (d, J = 7.7 Hz, 1H), 6.60 (d, J = 7.4 Hz, 1H), 6.08 (t, J = 54.1 Hz, 1H), 4.07 (dd, J = 14.7, 7.6 Hz, 1H), 3.86 (dd, J = 14.6, 6.1 Hz, 1H), 3.48 (s, 3H), 1.45 (t, J = 4.4 Hz, 4H), 0.88 (d, J = 18.4 Hz, 6H). |
| 839 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.92 (s, 1H), 7.86 - 7.67 (m, 2H), 7.13 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.24 (s, 1H), 4.64 (d, J = 1.1 Hz, 2H), 3.86 (d, J = 13.8 Hz, 1H), 3.72 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 1.13 (s, 4H), 0.90 (s, 9H). |
| 840 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.88 (s, 1H), 7.82 - 7.68 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.22 (s, 1H), 5.59 (t, J = 56.0 Hz, 1H), 4.55 (s, 2H), 3.86 (d, J = 13.8 Hz, 1H), 3.71 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 0.90 (s, 14H). |
| 841 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.04 (s, 1H), 7.85 - 7.70 (m, 2H), 7.04 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.21 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 4.21 (d, J = 14.9 Hz, 1H), 4.05 (d, J = 14.9 Hz, 1H), 2.51 (s, 3H), 1.53 (t, J = 2.3 Hz, 4H), 1.10 (d, J = 2.4 Hz, 6H). |
| 842 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (d, J = 6.9 Hz, 2H), 7.94 (s, 1H), 7.85 - 7.64 (m, 2H), 7.49 (t, J = 7.8 Hz, 1H), 7.23 (d, J = 7.5 Hz, 1H), 7.00 (d, J = 2.4 Hz, 1H), 6.83 (d, J = 7.5 Hz, 1H), 6.58 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 4.15 (d, J = 14.7 Hz, 1H), 3.91 (d, J = 14.8 Hz, 1H), 1.50 (s, 4H), 0.94 (d, J = 11.1 Hz, 6H). |
| 843 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.19 (s, 1H), 7.87 - 7.66 (m, 2H), 7.04 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.23 (s, 1H), 4.19 (d, J = 14.9 Hz, 1H), 4.04 (d, J = 14.9 Hz, 1H), 2.51 (s, 3H), 1.81 - 1.59 (m, 4H), 1.09 (d, J = 1.9 Hz, 6H). |
| 844 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.21 (s, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.66 (dt, J = 6.5, 2.1 Hz, 2H), 7.25 (d, J = 2.5 Hz, 1H), 6.38 (t, J = 6.9 Hz, 1H), 6.18 (s, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.64 (d, J = 13.9 Hz, 1H), 3.59 (s, 3H), 1.81 - 1.54 (m, 4H), 0.96 (s, 9H). |
| 845 | 1H NMR (400 MHz, 甲醇-d4) δ 8.41 (s, 1H), 8.06 (s, 1H), 7.84 - 7.66 (m, 2H), 7.23 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.27 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.99 (dd, J = 14.4, 7.2 Hz, 1H), 3.86 (dd, J = 14.4, 7.4 Hz, 1H), 2.50 (s, 3H), 1.53 (s, 4H), 1.09 (d, J = 3.9 Hz, 6H), 1.00 (d, J = 1.7 Hz, 6H), 0.73 (d, J = 7.3 Hz, 1H). |
| 846 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.96 (s, 1H), 7.83 - 7.65 (m, 2H), 7.01 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.4, 2.8 Hz, 1H), 6.16 (s, 1H), 4.20 (d, J = 14.9 Hz, 1H), 4.07 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.65 (s, 3H), 1.41 - 1.24 (m, 2H), 1.07 (dd, J = 17.4, 1.8 Hz, 8H). |
| 847 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (s, 1H), 7.88 - 7.64 (m, 2H), 7.35 (dt, J = 7.5, 3.7 Hz, 1H), 7.27 - 6.97 (m, 4H), 6.57 (dd, J = 3.2, 0.9 Hz, 1H), 6.38 (s, 1H), 5.90 (t, J = 54.8 Hz, 1H), 5.48 (s, 1H), 3.96 - 3.69 (m, 4H), 3.53 (d, J = 13.5 Hz, 1H), 1.61 - 1.32 (m, 4H), 0.79 (s, 9H). |
| 848 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 8.20 (s, 1H), 7.81 - 7.72 (m, 2H), 7.22 (d, J = 2.5 Hz, 1H), 6.89 (dd, J = 8.5, 2.8 Hz, 1H), 6.26 (s, 1H), 4.04 - 3.91 (m, 2H), 2.52 (s, 3H), 2.49 (s, 1H), 1.74 (m, 8H), 1.69 (d, J = 8.0 Hz, 2H). |
| 849 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.06 (s, 1H), 7.83 - 7.71 (m, 2H), 7.22 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.25 (s, 1H), 5.94 (t, J = 54.7 Hz, 1H), 3.97 (m, 2H), 2.52 (s, 3H), 2.49 (s, 1H), 1.74 (s, 6H), 1.53 (s, 4H). |
| 850 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (d, J = 0.8 Hz, 1H), 8.00 (s, 1H), 7.85 - 7.77 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.89 (dd, J = 8.4, 2.7 Hz, 1H), 6.22 (s, 1H), 3.90 (d, J = 13.8 Hz, 1H), 3.71 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 1.58 (s, 3H), 1.57 (s, 3H), 1.43 - 1.31 (m, 1H), 0.91 (s, 9H), 0.58 - 0.48 (m, 2H), 0.47 - 0.37 (m, 2H). |
| 851 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 7.91 (s, 1H), 7.83 - 7.75 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.89 (dd, J = 8.5, 2.8 Hz, 1H), 6.22 (s, 1H), 3.88 (d, J = 13.9 Hz, 1H), 3.73 (d, J = 13.9 Hz, 1H), 2.52 (s, 3H), 1.66 (p, J = 6.9 Hz, 1H), 1.28 - 1.16 (m, 2H), 1.17 - 1.04 (m, 2H), 0.97 - 0.85 (m, 15H). |
| 852 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 8.07 (s, 1H), 7.85 - 7.75 (m, 2H), 7.12 - 7.06 (m, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 5.10 (t, J = 6.5 Hz, 2H), 4.75 (dd, J = 6.9, 2.0 Hz, 2H), 3.86 (d, J = 13.8 Hz, 1H), 3.70 (d, J = 13.8 Hz, 1H), 2.52 (s, 3H), 1.92 (s, 3H), 0.89 (s, 9H). |
| 853 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 - 8.29 (m, 2H), 7.95 (s, 1H), 7.81 - 7.73 (m, 2H), 7.53 - 7.38 (m, 2H), 6.99 (d, J = 2.5 Hz, 1H), 6.87 (d, J = 7.5 Hz, 1H), 6.58 (s, 1H), 5.07 (dd, J = 6.9, 3.0 Hz, 2H), 4.72 (d, J = 6.9 Hz, 2H), 3.80 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.52 (d, J = 13.6 Hz, 1H), 1.89 (s, 3H), 0.72 (s, 9H). |
| 854 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (d, J = 1.4 Hz, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.79 (d, J = 2.4 Hz, 1H), 7.10 (d, J = 2.4 Hz, 1H), 6.92 - 6.85 (m, 1H), 6.22 (s, 1H), 4.81 - 4.55 (m, 3H), 3.92 (d, J = 13.9 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 2.50 (s, 3H), 1.58 (d, 3H), 0.89 (s, 9H). |
| 855 | 1H NMR (400 MHz, 甲醇-d4) δ 9.59 (s, 1H), 8.59 (d, J = 6.4 Hz, 1H), 8.47 (s, 1H), 8.34 (s, 1H), 8.33 - 8.30 (m, 2H), 7.79 (d, J = 2.5 Hz, 1H), 7.30 (d, J = 2.5 Hz, 1H), 6.66 (s, 1H), 3.83 (d, J = 13.9 Hz, 1H), 3.61 (d, J = 13.9 Hz, 1H), 1.81 - 1.72 (m, 2H), 1.72 - 1.61 (m, 2H), 0.82 (s, 9H). |
| 856 | 1H NMR (400 MHz, 甲醇-d4) δ 9.59 (s, 1H), 8.58 (d, J = 6.4 Hz, 1H), 8.46 (s, 1H), 8.33 - 8.27 (m, 2H), 8.06 (s, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.27 (d, J = 2.6 Hz, 1H), 6.59 (d, J = 1.0 Hz, 1H), 3.97 - 3.87 (m, 1H), 3.82 (d, J = 13.8 Hz, 1H), 3.62 (d, J = 13.9 Hz, 1H), 1.29 - 1.12 (m, 4H), 0.81 (s, 9H). |
| 857 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.83 (dd, J = 8.5, 2.6 Hz, 1H), 6.09 (s, 1H), 4.03 (d, J = 13.8 Hz, 1H), 3.84 (tt, J = 7.4, 3.9 Hz, 1H), 3.51 (d, J = 13.8 Hz, 1H), 2.38 (s, 3H), 1.87 (tt, J = 8.5, 5.4 Hz, 1H), 1.34 - 1.26 (m, 2H), 1.26 - 1.19 (m, 2H), 1.07 (ddd, J = 8.5, 3.4, 1.6 Hz, 2H), 0.98 - 0.90 (m, 1H), 0.85 (s, 9H), 0.83 - 0.76 (m, 1H). |
| 858 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.05 (d, J = 2.6 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.19 (s, 1H), 4.89 (s, 1H), 4.80 - 4.69 (m, 1H), 4.69 - 4.56 (m, 1H), 4.25 (d, J = 14.8 Hz, 1H), 3.99 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.61 - 1.54 (m, 3H), 1.10 (s, 3H), 1.09 (s, 3H). |
| 859 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 7.88 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.13 (s, 1H), 3.97 (d, J = 13.8 Hz, 1H), 3.93 (s, 3H), 3.68 - 3.61 (m, 1H), 3.53 (d, J = 13.8 Hz, 1H), 2.45 (s, 3H), 1.29 - 1.21 (m, 2H), 1.21 - 1.13 (m, 2H), 0.87 (s, 9H). |
| 860 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.90 (s, 1H), 7.83 - 7.70 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.21 (s, 1H), 3.85 (d, J = 13.9 Hz, 1H), 3.71 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.32 - 1.22 (m, 2H), 1.12 - 1.02 (m, 2H), 0.90 (s, 9H), 0.88 (s, 9H). |
| 861 | 1H NMR (400 MHz, 甲醇-d4) δ 8.38 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.16 (s, 1H), 4.23 (d, J = 14.9 Hz, 1H), 4.03 (d, J = 14.9 Hz, 1H), 2.72 (s, 1H), 2.51 (s, 3H), 2.43 (s, 6H), 1.11 (s, 3H), 1.10 (s, 3H). |
| 862 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.03 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.16 (s, 1H), 4.28 (d, J = 14.9 Hz, 1H), 3.97 (d, J = 14.9 Hz, 1H), 2.49 (s, 3H), 1.54 - 1.44 (m, 1H), 1.28 - 1.18 (m, 2H), 1.10 (s, 4H), 1.10 - 1.05 (m, 5H), 0.57 - 0.45 (m, 2H), 0.36 - 0.25 (m, 2H). |
| 863 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.85 (t, J = 8.1 Hz, 1H), 7.76 (d, J = 2.5 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.3, 2.7 Hz, 1H), 6.15 (s, 1H), 4.26 (d, J = 14.9 Hz, 1H), 3.97 (d, J = 14.9 Hz, 1H), 2.49 (s, 3H), 1.60 (s, 3H), 1.41 - 1.28 (m, 2H), 1.14 - 1.05 (m, 6H). |
| 864 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.85 (t, J = 8.1 Hz, 1H), 7.76 (d, J = 2.5 Hz, 1H), 7.02 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.15 (s, 1H), 4.23 (d, J = 14.8 Hz, 1H), 4.00 (d, J = 14.9 Hz, 1H), 3.72 - 3.58 (m, 1H), 2.51 (s, 3H), 1.31 - 1.15 (m, 3H), 1.10 (s, 3H), 1.09 (s, 3H). |
| 865 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.88 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.08 (d, J = 2.6 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.19 (s, 1H), 3.90 (d, J = 13.8 Hz, 1H), 3.61 (d, J = 13.9 Hz, 1H), 2.50 (s, 3H), 1.54 - 1.42 (m, 1H), 1.27 - 1.22 (m, 2H), 1.10 - 1.05 (m, 2H), 0.89 (s, 9H), 0.52 - 0.47 (m, 2H), 0.34 - 0.28 (m, 2H). |
| 866 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 (s, 1H), 7.87 (s, 1H), 7.84 - 7.76 (m, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.23 (s, 1H), 4.23 (m, 2H), 3.86 (s, 1H), 3.68 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 0.96 (s, 3H), 0.90 (s, 9H), 0.68 (s, 2H), 0.43 (s, 2H). |
| 867 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.00 (s, 1H), 7.83 - 7.72 (m, 2H), 7.02 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.4, 2.7 Hz, 1H), 6.18 (s, 1H), 4.22 (d, J = 14.9 Hz, 1H), 4.03 (d, J = 14.9 Hz, 1H), 2.50 (s, 3H), 1.62 - 1.47 (m, 1H), 1.32 - 1.17 (m, 2H), 1.10 (s, 3H), 1.09 (s, 3H), 1.06 - 0.97 (m, 2H), 0.58 - 0.46 (m, 2H), 0.38 - 0.28 (m, 2H). |
| 868 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 6.99 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.4, 2.7 Hz, 1H), 6.14 (s, 1H), 3.96 (d, J = 13.8 Hz, 1H), 3.91 (s, 3H), 3.51 (d, J = 13.8 Hz, 1H), 2.69 (s, 1H), 2.44 (s, 3H), 2.43 (s, 6H), 0.86 (s, 9H). |
| 869 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (s, 1H), 8.02 (s, 1H), 7.85 - 7.74 (m, 2H), 7.11 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.8 Hz, 1H), 6.26 (s, 1H), 5.37 - 5.17 (m, 1H), 5.05 - 4.90 (m, 2H), 4.84 (m, 2H), 3.89 (d, J = 13.8 Hz, 1H), 3.69 (d, J = 13.8 Hz, 1H), 2.51 (s, 3H), 0.89 (s, 9H). |
| 870 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 8.34 (d, 1H), 7.94 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.77 (d, J = 7.5 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.03 (d, J = 2.5 Hz, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.60 (s, 1H), 6.25 (tt, J = 56.0, 4.1 Hz, 1H), 5.92 (t, J = 54.7 Hz, 1H), 4.58 - 4.30 (m, 2H), 3.85 (d, J = 13.7 Hz, 1H), 3.52 (d, J = 13.7 Hz, 1H), 1.53 - 1.47 (m, 4H), 0.73 (s, 9H). |
| 871 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (d, J = 8.2 Hz, 1H), 8.30 (s, 1H), 7.92 (s, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.98 (d, J = 2.5 Hz, 1H), 6.87 (d, J = 7.6 Hz, 1H), 6.58 (s, 1H), 5.92 (t, J = 54.8 Hz, 1H), 3.79 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.50 (d, J = 13.6 Hz, 1H), 1.50 (s, 4H), 0.72 (s, 9H). |
| 872 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 7.84 (s, 1H), 7.78 (t, J = 8.0 Hz, 1H), 7.76 (d, J = 2.3 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.6, 2.7 Hz, 1H), 6.19 (s, 1H), 4.17 (dd, J = 7.0, 3.4 Hz, 1H), 3.84 (d, J = 14.0 Hz, 1H), 3.68 (d, J = 14.0 Hz, 1H), 2.51 (s, 3H), 1.70 (q, J = 6.0 Hz, 2H), 1.20 - 1.09 (m, 1H), 1.06 - 0.90 (m, 2H), 0.88 (s, 9H), 0.87 - 0.76 (m, 1H). |
| 873 | 1H NMR (400 MHz, 甲醇-d4) δ 8.31 (s, 1H), 8.25 (d, J = 5.2 Hz, 1H), 7.98 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.43 (d, J = 3.6 Hz, 1H), 7.31 (d, J = 5.2 Hz, 1H), 7.12 (d, J = 2.5 Hz, 1H), 6.68 (d, J = 3.6 Hz, 1H), 6.51 (s, 1H), 5.92 (t, J = 54.7 Hz, 1H), 3.90 (d, J = 13.7 Hz, 1H), 3.88 (s, 3H), 3.45 (d, J = 13.7 Hz, 1H), 1.50 (br s, 4H), 0.76 (s, 9H). |
| 874 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (d, J = 8.0 Hz, 1H), 8.34 (s, 1H), 8.09 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.04 (d, J = 2.6 Hz, 1H), 6.85 (d, J = 7.9 Hz, 1H), 6.62 (s, 1H), 6.42 - 6.06 (m, 1H), 4.55 - 4.33 (m, 2H), 3.85 (d, J = 13.7 Hz, 1H), 3.53 (d, J = 13.6 Hz, 1H), 1.75 - 1.59 (m, 4H), 0.73 (s, 9H). |
| 875 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (d, J = 8.0 Hz, 1H), 8.33 (s, 1H), 8.09 (s, 1H), 7.81 (d, J = 2.5 Hz, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.03 (d, J = 2.5 Hz, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.62 (s, 1H), 5.02 - 4.91 (m, 1H), 4.81 - 4.65 (m, 1H), 3.85 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.8 Hz, 1H), 1.78 - 1.61 (m, 4H), 0.71 (s, 9H). |
| 876 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (d, J = 7.8 Hz, 1H), 8.32 (s, 1H), 7.92 (s, 1H), 7.80 (d, J = 2.4 Hz, 1H), 7.77 (d, J = 7.5 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 6.99 (d, J = 2.5 Hz, 1H), 6.85 (d, J = 7.6 Hz, 1H), 6.59 (s, 1H), 5.93 (t, J = 54.7 Hz, 1H), 3.81 (d, J = 13.6 Hz, 1H), 3.51 (d, J = 13.6 Hz, 1H), 1.50 (s, 4H), 0.73 (s, 9H). |
| 877 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (d, J = 8.1 Hz, 1H), 8.31 (s, 1H), 7.82 (s, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.74 (d, J = 7.5 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 6.99 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.58 (s, 1H), 5.05 - 4.91 (m, 1H), 4.67 (d, J = 46.9 Hz, 1H), 4.66 (d, J = 46.9 Hz, 1H), 3.82 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.50 (d, J = 13.6 Hz, 1H), 1.56 - 1.49 (m, 3H), 0.71 (s, 9H). |
| 878 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 - 8.30 (m, 2H), 7.89 (s, 1H), 7.80 (d, J = 2.4 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 6.99 (s, 1H), 6.86 (d, J = 7.6 Hz, 1H), 6.57 (s, 1H), 4.59 (d, J = 48.6 Hz, 2H), 3.84 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.52 (d, J = 13.6 Hz, 1H), 1.53 - 1.26 (m, 4H), 0.72 (s, 9H). |
| 879 | 1H NMR (400 MHz, 甲醇-d4) δ 8.36 - 8.29 (m, 2H), 7.85 (s, 1H), 7.78 (d, J = 2.4 Hz, 1H), 7.74 (d, J = 7.6 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 6.96 (d, J = 2.4 Hz, 1H), 6.85 (d, J = 7.6 Hz, 1H), 6.54 (s, 1H), 3.81 (d, J = 13.7 Hz, 1H), 3.61 (d, J = 1.2 Hz, 3H), 3.51 (d, J = 13.7 Hz, 1H), 1.63 (s, 3H), 1.36 - 1.22 (m, 2H), 1.09 - 0.96 (m, 2H), 0.71 (s, 9H). |
| 880 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 - 8.30 (m, 2H), 7.89 (s, 1H), 7.82 (d, J = 2.4 Hz, 1H), 7.75 (d, J = 7.4 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.86 (d, J = 7.7 Hz, 1H), 6.61 (s, 1H), 5.26 - 5.21 (m, 1H), 5.03 - 4.92 (m, 2H), 4.86 - 4.76 (m, 2H), 3.86 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.52 (d, J = 13.7 Hz, 1H), 0.72 (s, 9H). |
| 881 | 1H NMR (400 MHz, 甲醇-d4) δ 8.07 - 8.00 (m, 2H), 7.60 (s, 1H), 7.51 (d, J = 2.4 Hz, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 6.70 (s, 1H), 6.56 (d, J = 7.8 Hz, 1H), 6.26 (s, 1H), 3.56 (d, J = 13.5 Hz, 1H), 3.31 (d, J = 1.3 Hz, 3H), 3.23 (d, J = 13.6 Hz, 1H), 1.25 - 1.16 (m, 1H), 0.95 - 0.83 (m, 2H), 0.75 - 0.65 (m, 2H), 0.43 (s, 9H), 0.24 - 0.14 (m, 2H), 0.05 - -0.06 (m, 2H). |
| 882 | 1H NMR (400 MHz, 甲醇-d4) δ 8.05 (d, J = 8.1 Hz, 2H), 7.61 (s, 1H), 7.51 (d, J = 2.4 Hz, 1H), 7.47 - 7.40 (m, 1H), 7.20 (t, J = 7.8 Hz, 1H), 7.13 (d, J = 7.7 Hz, 1H), 6.69 (d, J = 2.5 Hz, 1H), 6.56 (d, J = 7.6 Hz, 1H), 6.25 (s, 1H), 3.89 (d, J = 14.7 Hz, 1H), 3.64 (d, J = 14.7 Hz, 1H), 3.33 (s, 3H), 1.30 - 1.16 (m, 1H), 0.96 - 0.88 (m, 2H), 0.75 - 0.68 (m, 2H), 0.69 (s, 3H), 0.64 (s, 3H), 0.21 (ddd, J = 8.2, 6.1, 4.5 Hz, 2H), 0.08 - -0.02 (m, 2H). |
| 883 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 8.34 (d, J = 8.2 Hz, 1H), 7.79 (s, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.69 (d, J = 7.1 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 6.97 (d, J = 2.5 Hz, 1H), 6.84 (d, J = 7.5 Hz, 1H), 6.54 (s, 1H), 4.13 (d, J = 14.8 Hz, 1H), 3.94 (d, J = 14.8 Hz, 1H), 3.61 (s, 3H), 2.67 (s, 1H), 2.35 (s, 6H), 0.94 (d, J = 19.8 Hz, 6H). |
| 884 | 1H NMR (400 MHz, 甲醇-d4) δ 8.34 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 7.85 (s, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.70 (d, J = 6.8 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.7 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.83 (d, J = 7.4 Hz, 1H), 6.52 (s, 1H), 4.15 (d, J = 14.7 Hz, 1H), 3.93 (d, J = 14.7 Hz, 1H), 3.61 (s, 3H), 1.63 (s, 3H), 1.36 - 1.24 (m, 2H), 1.07 - 1.01 (m, 2H), 0.94 (d, J = 19.6 Hz, 6H). |
| 885 | 1H NMR (400 MHz, 甲醇-d4) δ 8.40 (s, 1H), 8.14 (s, 1H), 8.11 (dd, J = 5.1, 1.9 Hz, 1H), 7.82 (d, J = 2.4 Hz, 1H), 7.69 (dd, J = 7.3, 1.8 Hz, 1H), 7.25 (d, J = 2.5 Hz, 1H), 6.95 (dd, J = 7.4, 5.0 Hz, 1H), 6.29 (s, 1H), 3.94 (d, J = 13.9 Hz, 1H), 3.94 (s, 3H), 3.74 (d, J = 13.9 Hz, 1H), 1.83 - 1.57 (m, 4H), 0.94 (s, 9H). |
| 886 | 1H NMR (400 MHz, 甲醇-d4) δ 8.25 (d, J = 7.3 Hz, 2H), 7.98 (s, 1H), 7.69 (d, J = 2.5 Hz, 1H), 7.64 - 7.57 (m, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 6.91 (d, J = 2.5 Hz, 1H), 6.74 (d, J = 7.7 Hz, 1H), 6.49 (s, 1H), 4.05 (d, J = 14.7 Hz, 1H), 3.85 (d, J = 14.7 Hz, 1H), 3.52 (s, 3H), 1.73 - 1.49 (m, 4H), 0.88 (s, 3H), 0.84 (s, 3H). |
| 887 | 1H NMR (400 MHz, 甲醇-d4) δ 8.23 (s, 1H), 8.12 (d, J = 9.9 Hz, 1H), 7.71 (s, 1H), 7.70 (d, J = 2.5 Hz, 1H), 7.55 - 7.49 (m, 2H), 7.30 (q, J = 4.8 Hz, 1H), 6.89 (d, J = 2.5 Hz, 1H), 6.62 (d, J = 9.9 Hz, 1H), 6.57 (s, 1H), 3.79 (d, J = 13.7 Hz, 1H), 3.68 (s, 3H), 3.37 (d, J = 13.7 Hz, 1H), 2.58 (s, 1H), 2.26 (s, 6H), 0.61 (s, 9H). |
| 888 | 1H NMR (400 MHz, 甲醇-d4) δ 8.23 (s, 1H), 8.22 (d, J = 8.0 Hz, 1H), 7.71 - 7.68 (m, 2H), 7.66 (d, J = 7.4 Hz, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.13 (d, J = 7.5 Hz, 1H), 6.87 (d, J = 2.5 Hz, 1H), 6.75 (d, J = 7.6 Hz, 1H), 6.47 (s, 1H), 3.72 (d, J = 13.6 Hz, 1H), 3.41 (d, J = 13.6 Hz, 1H), 2.58 (s, 1H), 2.26 (s, 6H), 0.63 (s, 9H). |
| 889 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 - 8.33 (m, 1H), 8.33 (s, 1H), 8.12 (s, 1H), 7.91 (t, J = 60.0 Hz, 1H), 7.87 - 7.79 (m, 2H), 7.54 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.96 (d, J = 8.1 Hz, 1H), 6.62 (s, 1H), 3.86 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.7 Hz, 1H), 1.85 - 1.55 (m, 4H), 0.73 (s, 9H). |
| 890 | 1H NMR (400 MHz, 甲醇-d4) δ 8.22 (s, 1H), 8.12 (d, J = 9.9 Hz, 1H), 8.00 (s, 1H), 7.72 (d, J = 2.5 Hz, 1H), 7.53 (d, J = 4.4 Hz, 2H), 7.30 (t, J = 4.3 Hz, 1H), 6.92 (d, J = 2.5 Hz, 1H), 6.63 (d, J = 10.1 Hz, 1H), 6.61 (s, 1H), 3.79 (d, J = 13.7 Hz, 1H), 3.68 (s, 3H), 3.37 (d, J = 13.7 Hz, 1H), 1.74 - 1.45 (m, 4H), 0.62 (s, 9H). |
| 891 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 - 8.30 (m, 2H), 7.79 (d, J = 1.9 Hz, 2H), 7.76 - 7.67 (m, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 6.97 (d, J = 2.5 Hz, 1H), 6.85 (d, J = 7.6 Hz, 1H), 6.56 (s, 1H), 3.82 (d, J = 13.6 Hz, 1H), 3.61 (s, 3H), 3.52 (d, J = 13.7 Hz, 1H), 2.67 (s, 1H), 2.35 (d, J = 1.1 Hz, 6H), 0.71 (s, 9H). |
| 892 | 1H NMR (400 MHz, 甲醇-d4) δ 9.09 - 8.97 (m, 2H), 8.29 (s, 1H), 8.12 (d, J = 8.5 Hz, 1H), 7.97 - 7.88 (m, 2H), 7.88 - 7.76 (m, 3H), 7.09 (d, J = 2.4 Hz, 1H), 6.90 (s, 1H), 4.72 (d, J = 5.5 Hz, 1H), 4.60 (d, J = 5.8 Hz, 1H), 3.78 (d, J = 13.8 Hz, 1H), 3.46 (d, J = 13.8 Hz, 1H), 1.53 (d, J = 7.4 Hz, 3H), 0.65 (s, 9H). |
| 893 | 1H NMR (400 MHz, 甲醇-d4) δ 9.12 - 9.04 (m, 2H), 8.29 (s, 1H), 8.14 (d, J = 8.5 Hz, 1H), 8.03 (s, 1H), 8.00 - 7.91 (m, 1H), 7.87 (m, 2H), 7.80 (d, J = 2.5 Hz, 1H), 7.11 (d, J = 2.5 Hz, 1H), 6.94 (s, 1H), 5.00 - 4.74 (m, 4H), 3.80 (d, J = 13.8 Hz, 1H), 3.47 (d, J = 13.8 Hz, 1H), 0.66 (s, 9H). |
| 894 | 1H NMR (400 MHz, 乙腈-d3) δ 9.15 - 9.07 (m, 2H), 8.41 (s, 1H), 8.30 (d, J = 8.2 Hz, 1H), 8.02 - 7.81 (m, 4H), 7.76 (d, J = 2.4 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 6.81 (s, 1H), 6.25 (s, 1H), 5.90 (s, 2H), 5.85 (t, J = 54.6 Hz, 1H), 4.09 (dd, J = 14.7, 7.4 Hz, 1H), 3.86 (dd, J = 14.7, 5.8 Hz, 1H), 1.51 - 1.43 (m, 4H), 0.94 (d, J = 21.8 Hz, 6H). |
| 895 | 1H NMR (400 MHz, 乙腈-d3) δ 9.13 (t, J = 7.7 Hz, 2H), 8.41 (s, 1H), 8.30 (d, J = 8.1 Hz, 1H), 8.02 - 7.87 (m, 3H), 7.78 - 7.70 (m, 2H), 6.85 (s, 1H), 6.77 (s, 1H), 5.88 (s, 1H), 4.10 (dd, J = 14.5, 7.2 Hz, 1H), 3.91 - 3.81 (m, 1H), 1.60 (s, 3H), 1.25 (s, 2H), 1.01 (d, J = 1.9 Hz, 4H), 0.93 (d, J = 22.7 Hz, 6H). |
| 896 | 1H NMR (400 MHz, 乙腈-d3) δ 9.11 (s, 1H), 9.01 (d, J = 8.6 Hz, 1H), 8.39 (s, 1H), 8.26 (d, J = 8.0 Hz, 1H), 7.89 (s, 1H), 7.82 (d, J = 9.0 Hz, 2H), 7.74 (s, 1H), 6.85 (s, 1H), 5.88 (s, 1H), 4.09 (dd, J = 14.6, 7.3 Hz, 1H), 3.85 (dd, J = 14.6, 5.7 Hz, 1H), 3.01 (s, 1H), 2.87 (s, 1H), 2.01 (s, 1H), 1.16 (d, J = 4.5 Hz, 2H), 0.95 (s, 5H), 0.90 (s, 3H), 0.47 (dd, J = 7.4, 5.7 Hz, 2H), 0.26 (d, J = 5.4 Hz, 2H). |
| 897 | 1H NMR (400 MHz, 乙腈-d3) δ 9.09 (dd, J = 14.8, 6.8 Hz, 2H), 8.40 (s, 1H), 8.29 (d, J = 8.2 Hz, 1H), 7.99 - 7.83 (m, 3H), 7.75 (d, J = 2.4 Hz, 1H), 7.65 (s, 1H), 6.87 (s, 1H), 6.78 (s, 1H), 5.89 (s, 1H), 4.08 (dd, J = 14.7, 7.2 Hz, 1H), 3.87 (dd, J = 14.7, 5.8 Hz, 1H), 2.66 (s, 1H), 2.32 (s, 6H), 0.93 (d, J = 21.2 Hz, 6H). |
| 898 | 1H NMR (400 MHz, 乙腈-d3) δ 9.61 (s, 1H), 8.60 (d, J = 6.7 Hz, 1H), 8.49 (d, J = 6.7 Hz, 1H), 8.38 - 8.33 (m, 2H), 8.19 (d, J = 7.3 Hz, 1H), 7.92 - 7.86 (m, 1H), 7.86 (s, 1H), 7.74 (d, J = 2.4 Hz, 1H), 6.90 (d, J = 2.4 Hz, 1H), 6.81 (s, 1H), 6.06 (t, J = 55.5 Hz, 1H), 5.80 (s, 1H), 3.68 (m, 1H), 3.47 (m, 1H), 1.70 (m, 6H), 0.68 (s,9H). |
| 899 | 1H NMR (400 MHz, 乙腈-d3) δ 9.13 - 9.06 (m, 2H), 8.37 (s, 1H), 8.28 (d, J = 8.3 Hz, 1H), 8.00 - 7.89 (m, 2H), 7.87 (dd, J = 8.7, 5.1 Hz, 1H), 7.74 (d, J = 2.4 Hz, 2H), 6.91 (d, J = 2.5 Hz, 1H), 6.82 (s, 1H), 5.79 (s, 1H), 4.86 (ddd, J = 64.1, 8.9, 5.4 Hz, 1H), 3.78 (dt, J = 10.2, 5.4 Hz, 1H), 3.68 (dd, J = 13.4, 6.6 Hz, 1H), 3.48 (dd, J = 13.4, 5.0 Hz, 1H), 1.94 - 1.79 (m, 1H), 1.64 - 1.49 (m, 1H), 0.69 (s, 9H). |
| 900 | 1H NMR (400 MHz, 乙腈-d3) δ 9.16 (d, J = 8.9 Hz, 1H), 8.35 (d, J = 7.5 Hz, 2H), 8.03 - 7.90 (m, 2H), 7.82 (d, J = 8.9 Hz, 1H), 7.75 - 7.69 (m, 2H), 6.98 (d, J = 2.4 Hz, 1H), 6.81 (s, 1H), 6.26 (s, 1H), 6.00 (s, 1H), 3.70 (dd, J = 13.5, 6.1 Hz, 1H), 3.52 (dd, J = 13.7, 4.6 Hz, 1H), 3.00 (s, 4H), 2.67 (s, 1H), 2.33 (s, 6H), 0.72 (s, 9H). |
| 901 | 1H NMR (400 MHz, 乙腈-d3) δ 9.06 (d, J = 4.8 Hz, 1H), 8.94 (d, J = 8.9 Hz, 1H), 8.42 (s, 1H), 8.30 - 8.16 (m, 1H), 7.89 - 7.83 (m, 2H), 7.77 (dd, J = 8.7, 4.7 Hz, 2H), 7.68 - 7.60 (m, 1H), 7.59 (s, 1H), 7.38 (t, J = 52.2 Hz, 1H), 6.96 (s, 1H), 6.94 - 6.87 (m, 1H), 6.23 (s, 1H), 5.74 (s, 1H), 3.80 - 3.27 (m, 2H), 2.31 (d, J = 6.4 Hz, 6H), 1.03 (s, 1H), 0.56 (s, 9H). |
| 902 | 1H NMR (400 MHz, 乙腈-d3) δ 9.14 - 9.07 (m, 1H), 8.37 (s, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.00 - 7.90 (m, 2H), 7.80 (d, J = 8.9 Hz, 1H), 7.76 (s, 1H), 7.72 (d, J = 2.5 Hz, 1H), 6.92 (d, J = 2.5 Hz, 1H), 6.76 (s, 1H), 6.20 (s, 1H), 5.83 (s, 1H), 3.70 (dd, J = 13.3, 7.0 Hz, 1H), 3.50 (dd, J = 13.4, 5.3 Hz, 1H), 2.99 (s, 3H), 1.61 (s, 3H), 1.25 (s, 2H), 1.06 - 0.99 (m, 2H), 0.72 (s, 9H). |
| 903 | 1H NMR (400 MHz, 甲醇-d4) δ 8.89 (s, 1H), 8.37 (s, 1H), 8.07 (s, 1H), 7.76 (d, J = 2.5 Hz, 1H), 7.31 (d, J = 2.5 Hz, 1H), 6.38 (s, 1H), 3.89 (d, J = 13.8 Hz, 1H), 3.75 (d, J = 13.9 Hz, 1H), 2.46 (s, 3H), 1.62 - 1.48 (m, 1H), 1.23 (m, 2H), 1.03 (m, 2H), 0.96 (s, 9H), 0.53 (dd, J = 8.3, 1.8 Hz, 2H), 0.40 - 0.27 (m, 2H). |
| 904 | 1H NMR (400 MHz, 甲醇-d4) δ 8.91 (s, 1H), 8.41 (s, 1H), 8.25 (s, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 6.44 (s, 1H), 3.92 (d, J = 13.9 Hz, 1H), 3.77 (d, J = 13.9 Hz, 1H), 2.46 (s, 3H), 1.82 - 1.63 (m, 4H), 0.96 (s, 9H). |
| 905 | 1H NMR (400 MHz, 甲醇-d4) δ 8.30 (s, 1H), 8.23 (d, J = 2.5 Hz, 1H), 8.14 (s, 1H), 7.75 (m, 2H), 7.24 (d, J = 2.5 Hz, 1H), 6.81 (d, J = 8.6 Hz, 1H), 6.10 (s, 1H), 4.01 - 3.84 (m, 4H), 3.64 (d, J = 13.9 Hz, 1H), 1.79 - 1.59 (m, 4H), 0.93 (s, 9H). |
| 906 | 1H NMR (400 MHz, 甲醇-d4) δ 8.27 (s, 1H), 7.88 (t, J = 8.1 Hz, 1H), 7.73 (d, J = 2.5 Hz, 1H), 7.05 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.18 (s, 1H), 3.95 (d, J = 1.2 Hz, 3H), 3.84 (d, J = 13.8 Hz, 1H), 3.58 (d, J = 13.7 Hz, 1H), 2.52 (s, 3H), 0.87 (s, 9H). |
| 907 | 1H NMR (400 MHz, 甲醇-d4) δ 8.35 (s, 1H), 8.20 (s, 1H), 7.85 (t, J = 8.1 Hz, 1H), 7.76 (d, J = 2.5 Hz, 1H), 7.10 (d, J = 2.5 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.24 (s, 1H), 3.92 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 2.53 (s, 3H), 2.45 (s, 3H), 2.00 (d, J = 3.8 Hz, 4H), 0.89 (s, 9H). |
| 908 | 1H NMR (400 MHz, 甲醇-d4) δ 8.32 (s, 1H), 7.88 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.5 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.86 (dd, J = 8.6, 2.6 Hz, 1H), 6.15 (s, 1H), 4.04 - 3.83 (m, 7H), 3.50 (d, J = 13.8 Hz, 1H), 2.45 (s, 3H), 0.86 (s, 9H). |
| 909 | 1H NMR (400 MHz, 甲醇-d4) δ 9.40 (d, J = 0.9 Hz, 1H), 8.59 (s, 1H), 8.30 (s, 1H), 8.21 (d, J = 8.7 Hz, 1H), 8.20 (s, 1H), 8.02 (d, J = 7.3 Hz, 1H), 7.85 (t, J = 7.8 Hz, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 6.86 (s, 1H), 3.79 (d, J = 13.7 Hz, 1H), 3.51 - 3.44 (m, 1H), 3.34 (s, 2H), 1.77 - 1.59 (m, 4H), 0.65 (s, 9H). |
| 910 | 1H NMR (400 MHz, 甲醇-d4) δ 9.05 (dd, J = 4.2, 1.5 Hz, 1H), 8.67 (dd, J = 8.8, 1.6 Hz, 1H), 8.31 (s, 1H), 8.21 (d, J = 7.5 Hz, 2H), 7.84 - 7.79 (m, 2H), 7.69 (dd, J = 8.7, 4.2 Hz, 1H), 6.99 (d, J = 2.5 Hz, 1H), 6.92 (s, 1H), 3.81 (d, J = 13.7 Hz, 1H), 3.37 (d, J = 13.7 Hz, 1H), 1.77 - 1.55 (m, 4H), 0.58 (s, 10H). |
| 911 | 1H NMR (400 MHz, 甲醇-d4) δ 8.43 (s, 1H), 8.23 (s, 1H), 7.95 (s, 1H), 7.70 (s, 2H), 7.62 (s, 1H), 7.49 (s, 1H), 7.42 (s, 1H), 6.80 (s, 1H), 6.66 (s, 1H), 4.08 (d, J = 5.0 Hz, 4H), 2.62 (s, 1H), 2.30 (s, 8H), 0.67 (s, 9H). |
| 912 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (d, J = 1.4 Hz, 1H), 8.23 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 6.2 Hz, 1H), 7.71 (d, J = 5.7 Hz, 2H), 7.60 (s, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.43 (d, J = 6.3 Hz, 1H), 6.77 (s, 1H), 6.63 (s, 1H), 4.08 (t, J = 0.9 Hz, 3H), 3.90 (d, J = 13.9 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 1.18 - 0.99 (m, 5H), 0.66 (d, J = 1.0 Hz, 10H). |
| 913 | 1H NMR (400 MHz, 甲醇-d4) δ 8.39 (d, J = 1.2 Hz, 1H), 8.23 (d, J = 8.3 Hz, 1H), 7.95 (dd, J = 6.2, 1.0 Hz, 1H), 7.77 (d, J = 0.9 Hz, 1H), 7.71 (d, J = 7.3 Hz, 1H), 7.62 - 7.59 (m, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.43 (d, J = 6.3 Hz, 1H), 6.76 (d, J = 2.3 Hz, 1H), 6.64 (s, 1H), 4.08 (d, J = 1.1 Hz, 3H), 3.90 (d, J = 13.5 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 1.58 (d, J = 0.7 Hz, 3H), 1.28 - 1.19 (m, 2H), 1.03 - 0.93 (m, 2H), 0.66 (d, J = 1.1 Hz, 9H). |
| 914 | 1H NMR (400 MHz, 甲醇-d4) δ 8.41 (d, J = 1.9 Hz, 1H), 8.27 - 8.20 (m, 1H), 7.96 (dd, J = 6.2, 1.8 Hz, 1H), 7.85 (s, 1H), 7.71 (d, J = 7.3 Hz, 1H), 7.62 (dd, J = 2.6, 1.6 Hz, 1H), 7.54 - 7.46 (m, 1H), 7.43 (d, J = 6.3 Hz, 1H), 6.82 (d, J = 2.2 Hz, 1H), 6.69 (s, 1H), 5.87 (t, J = 54.7 Hz, 1H), 4.09 (d, J = 1.9 Hz, 3H), 3.92 (d, J = 14.2 Hz, 1H), 3.60 (d, J = 13.4 Hz, 1H), 1.46 (s, 3H), 0.68 (d, J = 1.7 Hz, 10H). |
| 915 | 1H NMR (400 MHz, 甲醇-d4) δ 9.18 (s, 1H), 8.45 (s, 1H), 8.15 (s, 1H), 8.12 (s, 1H), 8.03 (s, 1H), 7.84 (d, J = 7.2 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.70 - 7.61 (m, 2H), 6.90 (d, J = 2.3 Hz, 1H), 6.77 (s, 1H), 3.95 (d, J = 13.8 Hz, 1H), 3.68 (d, J = 13.8 Hz, 1H), 1.78 - 1.56 (m, 4H), 0.73 (s, 9H). |
| 916 | 1H NMR (400 MHz, 甲醇-d4) δ 8.45 (s, 1H), 8.41 (d, J = 8.5 Hz, 1H), 8.27 (d, J = 6.0 Hz, 1H), 8.11 (s, 1H), 7.96 (d, J = 6.1 Hz, 1H), 7.94 - 7.88 (m, 1H), 7.74 (dd, J = 8.5, 7.3 Hz, 1H), 7.68 (d, J = 2.2 Hz, 1H), 6.83 (d, J = 2.9 Hz, 2H), 3.99 (d, J = 13.8 Hz, 1H), 3.56 (d, J = 13.8 Hz, 1H), 3.34 (s, 1H), 1.77 - 1.54 (m, 4H), 0.67 (s, 10H). |
| 918 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (d, J = 4.4 Hz, 1H), 8.59 (d, J = 8.7 Hz, 1H), 8.51 (s, 1H), 7.97 (s, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.68 - 7.64 (m, 1H), 7.59 (dd, J = 8.2, 4.9 Hz, 1H), 7.51 - 7.42 (m, 1H), 6.87 (d, J = 2.3 Hz, 1H), 6.77 (s, 1H), 5.91 (t, J = 54.7 Hz, 1H), 4.32 (d, J = 14.9 Hz, 1H), 3.99 (d, J = 14.9 Hz, 1H), 1.49 (s, 3H), 0.95 (s, 3H), 0.92 (s, 3H). |
| 919 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (d, J = 4.2 Hz, 1H), 8.59 (d, J = 8.8 Hz, 1H), 8.49 (s, 1H), 7.88 (s, 1H), 7.69 - 7.63 (m, 2H), 7.59 (dd, J = 8.2, 4.9 Hz, 1H), 7.49 - 7.42 (m, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.72 (s, 1H), 4.32 (d, J = 14.9 Hz, 1H), 3.97 (d, J = 14.9 Hz, 1H), 1.61 (s, 3H), 1.32 - 1.23 (m, 2H), 1.03 (s, 2H), 0.93 (s, 3H), 0.90 (s, 3H). |
| 920 | 1H NMR (400 MHz, 甲醇-d4) δ 8.94 (d, J = 4.2 Hz, 1H), 8.59 (d, J = 8.8 Hz, 1H), 8.49 (s, 1H), 7.82 (s, 1H), 7.67 (d, J = 2.5 Hz, 1H), 7.65 (d, J = 4.4 Hz, 1H), 7.61 (dd, J = 8.2, 4.9 Hz, 1H), 7.52 - 7.40 (m, 1H), 6.87 (s, 1H), 6.76 (s, 1H), 4.01 (d, J = 13.9 Hz, 1H), 3.61 (d, J = 13.9 Hz, 1H), 2.66 (s, 1H), 2.33 (s, 6H), 0.68 (s, 9H). |
| 921 | 1H NMR (400 MHz, 甲醇-d4) δ 8.99 (dd, J = 4.3, 1.5 Hz, 1H), 8.60 (dd, J = 8.7, 1.5 Hz, 1H), 8.50 (s, 1H), 8.12 (s, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.66 (dd, J = 8.7, 4.3 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.83 (s, 1H), 4.03 (d, J = 13.8 Hz, 1H), 3.58 (d, J = 13.9 Hz, 1H), 1.76 - 1.55 (m, 4H), 0.67 (s, 9H). |
| 922 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.28 (d, J = 8.3 Hz, 1H), 8.01 (d, J = 6.2 Hz, 1H), 7.81 (d, J = 7.3 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.54 (dd, J = 8.3, 7.4 Hz, 1H), 7.45 (d, J = 6.2 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.75 (s, 1H), 4.12 (s, 3H), 3.97 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 1.83 - 1.62 (m, 5H), 0.72 (s, 10H). |
| 923 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.28 (d, J = 8.3 Hz, 1H), 8.01 (d, J = 6.2 Hz, 1H), 7.80 (d, J = 7.1 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.45 (d, J = 6.3 Hz, 1H), 6.91 (d, J = 2.3 Hz, 1H), 6.73 (s, 1H), 5.85 (t, J = 54.2 Hz, 1H), 4.12 (s, 3H), 3.98 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 1.61 - 1.47 (m, 4H), 0.73 (s, 8H), 0.70 (s, 1H). |
| 924 | 1H NMR (400 MHz, 甲醇-d4) δ 8.25 (d, J = 8.4 Hz, 1H), 8.18 (s, 1H), 7.96 (d, J = 6.3 Hz, 1H), 7.85 (d, J = 7.3 Hz, 1H), 7.56 (s, 0H), 7.53 (q, J = 2.8 Hz, 2H), 7.38 (d, J = 6.3 Hz, 1H), 6.63 (s, 1H), 6.58 (s, 1H), 4.11 (s, 3H), 3.70 (d, J = 13.5 Hz, 1H), 3.38 (d, J = 13.5 Hz, 1H), 2.98 (s, 1H), 2.85 (d, J = 0.8 Hz, 1H), 1.89 (s, 2H), 1.74 (s, 2H), 1.28 (s, 1H), 0.96 - 0.84 (m, 0H), 0.61 (s, 9H). |
| 925 | 1H NMR (400 MHz, 甲醇-d4) δ 8.25 (d, J = 8.3 Hz, 1H), 8.19 (s, 1H), 7.96 (d, J = 6.2 Hz, 1H), 7.85 (d, J = 7.5 Hz, 1H), 7.58 - 7.48 (m, 2H), 7.39 (d, J = 6.2 Hz, 1H), 6.62 (s, 1H), 6.57 (s, 1H), 5.99 (t, J = 55.0 Hz, 1H), 4.11 (s, 3H), 3.71 (d, J = 13.4 Hz, 1H), 3.38 (d, J = 13.4 Hz, 1H), 1.68 (s, 2H), 1.58 (s, 2H), 1.28 (s, 1H), 0.62 (s, 9H). |
| 926 | 1H NMR (400 MHz, 甲醇-d4) δ 8.29 (s, 1H), 8.01 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 6.2 Hz, 1H), 7.60 (s, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.42 (d, J = 2.2 Hz, 1H), 7.30 - 7.24 (m, 1H), 7.17 (d, J = 6.3 Hz, 1H), 6.60 (d, J = 2.3 Hz, 1H), 6.42 (s, 1H), 4.12 (d, J = 14.8 Hz, 1H), 3.85 (s, 3H), 3.75 (d, J = 14.9 Hz, 1H), 1.23 (ddd, J = 13.2, 8.3, 5.0 Hz, 1H), 0.96 - 0.87 (m, 2H), 0.75 - 0.70 (m, 2H), 0.67 (d, J = 8.3 Hz, 6H), 0.25 - 0.17 (m, 2H), 0.02 (dt, J = 5.7, 4.5 Hz, 2H). |
| 927 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.81 (s, 1H), 7.71 (dd, J = 7.3, 1.2 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.56 - 7.50 (m, 1H), 7.44 (d, J = 6.3 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.66 (s, 1H), 4.32 (d, J = 14.8 Hz, 1H), 4.12 (s, 3H), 4.01 (d, J = 14.8 Hz, 1H), 1.61 (s, 3H), 1.27 (d, J = 4.9 Hz, 2H), 1.05 - 1.00 (m, 2H), 0.93 (d, J = 9.1 Hz, 6H). |
| 928 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.3 Hz, 1H), 7.75 (s, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.56 - 7.49 (m, 1H), 7.44 (dd, J = 6.3, 0.9 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.68 (s, 1H), 4.30 (d, J = 14.8 Hz, 1H), 4.12 (s, 3H), 4.02 (d, J = 14.8 Hz, 1H), 2.66 (s, 1H), 2.34 (s, 6H), 0.93 (d, J = 10.0 Hz, 6H). |
| 929 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.28 (d, J = 8.3 Hz, 1H), 8.04 (s, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.78 - 7.72 (m, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.54 (dd, J = 8.2, 7.4 Hz, 1H), 7.49 - 7.42 (m, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.75 (s, 1H), 4.12 (s, 3H), 3.98 (d, J = 13.8 Hz, 1H), 3.66 (d, J = 13.8 Hz, 1H), 1.76 - 1.54 (m, 4H), 0.72 (s, 9H). |
| 930 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.39 - 8.32 (m, 1H), 8.01 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.62 - 7.55 (m, 1H), 7.42 (d, J = 7.3 Hz, 1H), 6.98 (d, J = 9.2 Hz, 1H), 6.92 (d, J = 2.3 Hz, 1H), 6.77 (s, 1H), 4.05 (s, 3H), 4.00 (d, J = 13.9 Hz, 1H), 3.67 (d, J = 13.8 Hz, 1H), 1.77 - 1.55 (m, 4H), 0.74 (s, 9H). |
| 931 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.27 (d, J = 8.4 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.84 (s, 1H), 7.75 (dd, J = 7.3, 1.1 Hz, 1H), 7.67 (d, J = 2.3 Hz, 1H), 7.53 (dd, J = 8.3, 7.4 Hz, 1H), 7.45 (d, J = 6.2 Hz, 1H), 6.86 (d, J = 2.3 Hz, 1H), 6.69 (s, 1H), 4.12 (s, 3H), 4.01 (d, J = 13.8 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H), 1.49 (ddd, J = 13.2, 8.3, 5.0 Hz, 1H), 1.23 - 1.13 (m, 2H), 1.03 - 0.94 (m, 2H), 0.71 (s, 9H), 0.52 - 0.44 (m, 2H), 0.32 - 0.25 (m, 2H). |
| 932 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.78 (s, 1H), 7.76 - 7.72 (m, 1H), 7.68 (d, J = 2.2 Hz, 1H), 7.53 (dd, J = 8.4, 7.3 Hz, 1H), 7.45 (dd, J = 6.3, 0.9 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 6.72 (s, 1H), 5.03 - 4.90 (m, 1H), 4.71 (d, J = 5.4 Hz, 1H), 4.62 - 4.56 (m, 1H), 4.12 (s, 3H), 4.01 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 1.51 (dd, J = 7.1, 1.3 Hz, 3H), 0.72 (s, 9H). |
| 933 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 8.28 (d, J = 8.1 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.85 (s, 1H), 7.75 (d, J = 7.2 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.54 (dd, J = 8.4, 7.3 Hz, 1H), 7.46 (dd, J = 6.3, 0.9 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 6.75 (s, 1H), 5.31 - 5.12 (m, 1H), 4.99 - 4.86 (m, 2H), 4.83 - 4.71 (m, 2H), 4.01 (d, J = 13.8 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 0.72 (s, 11H). |
| 934 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.27 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 6.2 Hz, 1H), 7.89 (s, 1H), 7.71 (dd, J = 7.3, 1.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.53 (dd, J = 8.3, 7.4 Hz, 1H), 7.45 (d, J = 6.3 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.71 (s, 1H), 5.91 (t, J = 54.8 Hz, 1H), 4.28 (d, J = 14.7 Hz, 1H), 4.12 (s, 3H), 4.00 (d, J = 14.7 Hz, 1H), 2.02 (s, 1H), 1.49 (d, J = 4.5 Hz, 4H), 0.92 (d, J = 10.6 Hz, 6H). |
| 935 | 1H NMR (400 MHz, 甲醇-d4) δ 8.91 (s, 1H), 8.39 (s, 1H), 8.36 (s, 1H), 8.18 (d, J = 2.1 Hz, 1H), 8.04 (t, J = 2.2 Hz, 1H), 8.02 (d, J = 1.6 Hz, 1H), 7.94 (d, J = 9.4 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 7.10 (d, J = 2.3 Hz, 1H), 6.33 (s, 1H), 4.01 (d, J = 14.0 Hz, 1H), 3.75 - 3.61 (m, 1H), 2.03 (s, 1H), 1.82 - 1.73 (m, 2H), 1.68 (s, 2H), 1.10 (s, 0H), 0.87 (s, 9H). |
| 936 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.20 (s, 1H), 7.79 - 7.58 (m, 3H), 7.08 (d, J = 2.3 Hz, 1H), 6.39 (t, J = 6.9 Hz, 1H), 6.19 (s, 1H), 4.17 (d, J = 14.0 Hz, 1H), 3.77 (d, J = 14.0 Hz, 1H), 3.59 (s, 3H), 1.81 - 1.57 (m, 4H), 0.99 (s, 9H). |
| 937 | 1H NMR (400 MHz, 甲醇-d4) δ 8.88 (d, 1H), 8.45 (s, 1H), 8.25 (dd, 1H), 8.11 (s, 1H), 8.10 - 8.02 (m, 1H), 7.73 - 7.66 (m, 3H), 6.90 (d, 1H), 6.76 (s, 1H), 4.02 - 3.93 (m, 1H), 3.63 (d, 1H), 1.77 - 1.56 (m, 4H), 0.71 (s, 9H). |
| 938 | 1H NMR (400 MHz, 氯仿-d) δ 8.99 - 8.81 (m, 1H), 8.23 (d, 1H), 8.14 (s, 1H), 7.66 - 7.58 (m, 1H), 7.43 (d, 1H), 7.39 - 7.28 (m, 2H), 6.34 (s, 1H), 6.08 (s, 1H), 4.00 (d, 1H), 3.64 (d, 1H), 2.47 (d, 3H), 1.80 - 1.61 (m, 4H), 0.91 (d, 9H). |
| 939 | 1H NMR (400 MHz, 乙腈-d3) δ 8.44 (s, 1H), 8.30 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 7.5 Hz, 1H), 7.63 (s, 1H), 7.54 (s, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 7.7 Hz, 2H), 6.66 (s, 1H), 6.46 (s, 1H), 3.84 (dd, J = 13.5, 5.9 Hz, 1H), 3.68 (dd, J = 13.2, 4.7 Hz, 1H), 3.54 (s, 3H), 2.08 - 1.99 (m, 6H), 1.79 (m, 6H), 1.72 (m, 1H), 0.81 (s, 9H). |
| 940 | 1H NMR (400 MHz, 甲醇-d4) δ 9.06 (d, J = 4.7 Hz, 1H), 8.93 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 8.16 - 8.08 (m, 1H), 8.02 (s, 1H), 7.95 - 7.86 (m, 1H), 7.81 (t, J = 7.3 Hz, 2H), 7.68 (d, J = 2.3 Hz, 1H), 6.91 - 6.84 (m, 2H), 5.91 (t, J = 54.7 Hz, 1H), 4.32 (d, J = 15.0 Hz, 1H), 3.96 (d, J = 14.9 Hz, 1H), 1.50 (s, 4H), 0.96 (s, 3H), 0.91 (s, 3H). |
| 941 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 8.13 (m, 2H), 7.68 (d, J = 2.1 Hz, 1H), 7.05 (s, 2H), 6.91 (s, 1H), 6.10 (s, 1H), 4.22 (d, J = 13.9 Hz, 1H), 3.88 s, 3H), 3.68 (d, J = 13.9 Hz, 1H), 1.72 (d, J = 31.5 Hz, 4H), 0.97 (d, J = 1.0 Hz, 9H). |
| 942 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (d, J = 1.3 Hz, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.64 (dd, J = 2.3, 1.1 Hz, 1H), 6.97 - 6.82 (m, 1H), 6.77 (d, J = 2.3 Hz, 1H), 6.17 - 5.79 (m, 2H), 4.44 (d, J = 15.0 Hz, 1H), 4.01 (d, J = 14.9 Hz, 1H), 2.40 (s, 3H), 1.78 - 1.56 (m, 4H), 1.10 (d, J = 3.3 Hz, 6H). |
| 943 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 12.6, 2.6 Hz, 2H), 6.08 (s, 1H), 4.12 (d, J = 13.9 Hz, 1H), 3.69 (d, J = 13.9 Hz, 1H), 2.40 (s, 3H), 1.66 - 1.55 (m, 1H), 1.35 - 1.25 (m, 2H), 1.22 - 1.13 (m, 2H), 0.90 (s, 9H), 0.55 - 0.46 (m, 2H), 0.43 - 0.32 (m, 2H). |
| 944 | 1H NMR (400 MHz, 甲醇-d4) δ 8.23 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.51 (d, J = 2.4 Hz, 1H), 6.93 - 6.82 (m, 1H), 6.67 (d, J = 2.4 Hz, 1H), 6.03 (s, 1H), 3.88 (d, J = 13.7 Hz, 1H), 3.50 (d, J = 13.6 Hz, 1H), 2.73 (s, 1H), 2.61 (s, 6H), 2.42 (s, 3H), 0.85 (s, 9H). |
| 945 | 1H NMR (400 MHz, 甲醇-d4) δ 8.26 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 6.86 (dd, J = 8.5, 2.6 Hz, 1H), 6.71 (s, 1H), 6.17 - 5.81 (m, 2H), 3.95 (d, J = 13.8 Hz, 1H), 3.49 (d, J = 13.7 Hz, 1H), 2.39 (s, 3H), 1.69 (m, 2H), 1.65 - 1.56 (m, 2H), 0.85 (s, 9H). |
| 946 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.61 (d, J = 2.3 Hz, 1H), 6.83 (d, J = 6.3 Hz, 1H), 6.74 (d, J = 2.3 Hz, 1H), 6.08 (s, 1H), 4.14 (d, J = 13.8 Hz, 1H), 3.89 - 3.80 (m, 1H), 3.60 (d, J = 13.8 Hz, 1H), 2.37 (s, 3H), 1.91 - 1.83 (m, 1H), 1.35 - 1.26 (m, 2H), 1.27 - 1.19 (m, 2H), 1.07 (dd, J = 8.7, 2.9 Hz, 2H), 0.91 (s, 1H), 0.87 (s, 9H), 0.84 - 0.77 (m, 1H). |
| 947 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.2 Hz, 1H), 6.88 (q, J = 3.1 Hz, 2H), 6.17 (s, 1H), 4.05 (d, J = 13.9 Hz, 1H), 3.75 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.60 (s, 3H), 1.36 - 1.30 (m, 2H), 1.15 - 1.07 (m, 2H), 0.92 (s, 9H). |
| 948 | 1H NMR (400 MHz, 甲醇-d4) δ 8.23 (d, J = 1.2 Hz, 1H), 7.88 (t, J = 8.1 Hz, 1H), 7.51 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.70 (d, J = 2.4 Hz, 1H), 6.17 (s, 1H), 5.90 (t, J = 54.3 Hz, 1H), 4.23 (d, J = 14.8 Hz, 1H), 3.92 (d, J = 14.8 Hz, 1H), 2.49 (s, 3H), 1.66 - 1.43 (m, 4H), 1.07 (d, J = 5.3 Hz, 6H). |
| 949 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.1, 2.5 Hz, 2H), 6.18 (s, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.74 (d, J = 13.9 Hz, 1H), 2.49 (s, 3H), 1.55 - 1.42 (m, 1H), 1.28 - 1.20 (m, 2H), 1.11 - 1.03 (m, 2H), 0.92 (s, 10H), 0.57 - 0.46 (m, 2H), 0.35 - 0.28 (m, 2H). |
| 950 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.42 - 7.26 (m, 5H), 7.16 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.36 (s, 1H), 5.78 (t, J = 7.2 Hz, 1H), 2.52 (s, 3H), 2.14 (m, 2H), 1.81 (m, 2H), 1.74 (m, 2H), 1.02 (t, J = 7.3 Hz, 3H). |
| 951 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (d, J = 0.8 Hz, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.65 (dd, J = 2.3, 0.8 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.90 (dd, J = 8.4, 2.8 Hz, 1H), 6.19 (s, 1H), 4.06 (d, J = 13.9 Hz, 1H), 3.83 (d, J = 13.9 Hz, 1H), 2.75 (s, 1H), 2.52 (s, 3H), 2.45 (s, 6H), 0.96 (s, 9H). |
| 952 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 0.9 Hz, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.66 (dd, J = 2.3, 0.9 Hz, 1H), 6.95 (d, J = 2.3 Hz, 1H), 6.90 (dd, J = 8.6, 2.8 Hz, 1H), 6.23 (s, 1H), 4.98 - 4.87 (m, 0H), 4.82 - 4.58 (m, 2H), 4.14 - 4.02 (m, 1H), 3.81 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.66 - 1.55 (m, 3H), 0.95 (d, J = 0.9 Hz, 9H). |
| 953 | 1H NMR (400 MHz, 甲醇-d4) δ 8.52 (s, 1H), 7.92 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.78 - 7.72 (m, 1H), 7.65 (d, J = 2.3 Hz, 1H), 7.03 (d, J = 2.4 Hz, 1H), 6.89 (dd, J = 8.5, 2.8 Hz, 1H), 6.12 (s, 1H), 5.88 (t, J = 54.2 Hz, 1H), 2.45 (s, 3H), 1.56 (m, 4H). |
| 954 | 1H NMR (400 MHz, 甲醇-d4) δ 8.37 (d, J = 1.5 Hz, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.77 (d, J = 2.3 Hz, 1H), 7.09 (d, J = 2.4 Hz, 1H), 6.92 - 6.84 (m, 1H), 6.18 (s, 1H), 3.90 (d, J = 13.9 Hz, 1H), 3.65 (d, J = 13.8 Hz, 1H), 2.73 (s, 1H), 2.51 (s, 3H), 2.43 (s, 6H), 0.89 (s, 9H). |
| 955 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.83 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 9.0, 2.6 Hz, 2H), 6.19 (s, 1H), 4.99 - 4.88 (m, 0H), 4.80 - 4.68 (m, 1H), 4.68 - 4.52 (m, 1H), 4.37 (d, J = 14.9 Hz, 1H), 4.13 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.59 (d, J = 1.4 Hz, 2H), 1.57 (d, J = 1.4 Hz, 1H), 1.15 (s, 3H), 1.14 (s, 3H). |
| 956 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.83 (t, J = 8.1 Hz, 1H), 7.62 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 6.15 (s, 1H), 4.34 (d, J = 15.0 Hz, 1H), 4.16 (d, J = 15.0 Hz, 1H), 2.72 (s, 1H), 2.51 (s, 3H), 2.43 (s, 6H), 1.15 (s, 3H), 1.15 (s, 3H). |
| 957 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.89 (d, J = 2.8 Hz, 1H), 6.86 (d, J = 2.2 Hz, 2H), 6.17 (s, 1H), 4.41 (d, J = 15.0 Hz, 1H), 4.12 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.55 - 1.44 (m, 1H), 1.30 - 1.19 (m, 2H), 1.16 (s, 3H), 1.15 (s, 3H), 1.08 (m, 2H), 0.58 - 0.45 (m, 2H), 0.38 - 0.28 (m, 2H). |
| 958 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.61 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.4, 2.8 Hz, 1H), 6.83 (d, J = 2.3 Hz, 1H), 6.15 (s, 1H), 4.34 (d, J = 15.0 Hz, 1H), 4.13 (d, J = 15.0 Hz, 1H), 3.71 - 3.60 (m, 1H), 2.50 (s, 3H), 1.31 - 1.16 (m, 3H), 1.15 (s, 4H), 1.14 (s, 3H). |
| 959 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (s, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.87 (dd, J = 8.5, 2.8 Hz, 1H), 6.84 (d, J = 2.3 Hz, 1H), 6.15 (s, 1H), 4.39 (d, J = 15.0 Hz, 1H), 4.12 (d, J = 15.0 Hz, 1H), 2.49 (s, 3H), 1.60 (s, 3H), 1.40 - 1.29 (m, 2H), 1.15 (s, 3H), 1.14 (s, 3H), 1.12 - 1.06 (m, 2H). |
| 960 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.87 (t, J = 8.1 Hz, 1H), 7.64 (d, J = 2.3 Hz, 1H), 6.94 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.4, 2.8 Hz, 1H), 6.22 (s, 1H), 5.88 (t, J = 54.2 Hz, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.79 (d, J = 14.0 Hz, 1H), 2.50 (s, 3H), 1.58 (m, 4H), 0.94 (s, 9H). |
| 961 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 8.34 (dd, J = 8.1, 1.2 Hz, 1H), 7.81 - 7.74 (m, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 6.83 (s, 1H), 6.82 (d, J = 4.5 Hz, 1H), 6.54 (s, 1H), 4.01 (d, J = 13.8 Hz, 1H), 3.62 (d, J = 13.8 Hz, 1H), 3.61 (s, 3H), 2.71 (s, 1H), 2.41 (s, 6H), 0.76 (s, 9H). |
| 962 | 1H NMR (400 MHz, 乙腈-d3) δ 8.99 - 8.93 (m, 1H), 8.80 (d, J = 8.7 Hz, 1H), 8.31 (s, 1H), 8.12 (d, J = 7.5 Hz, 1H), 7.83 - 7.72 (m, 2H), 7.66 (dd, J = 8.7, 4.7 Hz, 1H), 7.44 (d, J = 2.2 Hz, 1H), 6.69 (d, J = 3.0 Hz, 2H), 6.60 (s, 1H), 6.30 (s, 1H), 5.69 (t, J = 54.1 Hz, 1H), 3.71 (dd, J = 13.5, 6.6 Hz, 1H), 3.47 (dd, J = 13.5, 4.7 Hz, 1H), 1.88 - 1.82 (m, 3H), 1.43 (m, 4H), 0.60 (s, 9H). |
| 963 | 1H NMR (400 MHz, 甲醇-d4) δ 8.22 (s, 1H), 7.89 (t, J = 8.1 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.5, 2.7 Hz, 1H), 6.74 (d, J = 2.4 Hz, 1H), 6.15 (s, 1H), 3.94 (d, J = 1.2 Hz, 3H), 3.84 (d, J = 13.7 Hz, 1H), 3.56 (d, J = 13.7 Hz, 1H), 2.51 (s, 3H), 0.87 (s, 9H). |
| 964 | 1H NMR (400 MHz, 甲醇-d4) δ 8.33 (s, 1H), 7.89 (t, J = 8.2 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 6.98 (d, J = 2.5 Hz, 1H), 6.91 - 6.81 (m, 1H), 6.18 (s, 1H), 5.96 (t, J = 55.1 Hz, 1H), 4.06 (s, 3H), 3.99 (d, J = 13.7 Hz, 1H), 3.45 (d, J = 13.8 Hz, 1H), 2.42 (s, 3H), 1.54 (m, 2H), 1.50 (m, 2H), 0.85 (s, 9H). |
| 965 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.66 (d, J = 2.3 Hz, 1H), 6.85 (m, 2H), 6.18 (s, 1H), 5.95 (t, J = 55.0 Hz, 1H), 4.17 (d, J = 13.9 Hz, 1H), 4.03 (s, 3H), 3.64 (d, J = 13.9 Hz, 1H), 2.43 (s, 3H), 1.60 - 1.53 (m, 2H), 1.53 - 1.44 (m, 2H), 0.91 (s, 9H). |
| 966 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.64 (d, J = 2.3 Hz, 1H), 7.62 (d, J = 8.6 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.63 (d, J = 8.6 Hz, 1H), 6.14 (s, 1H), 5.94 (t, J = 55.0 Hz, 1H), 4.13 (d, J = 13.9 Hz, 1H), 3.97 (s, 3H), 3.90 (s, 3H), 3.73 (d, J = 13.9 Hz, 1H), 2.43 (s, 3H), 1.61 - 1.52 (m, 2H), 1.52 - 1.42 (m, 2H), 0.93 (s, 9H). |
| 967 | 1H NMR (400 MHz, 甲醇-d4) δ 8.48 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.4, 2.7 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.12 (s, 1H), 4.11 (d, J = 13.9 Hz, 1H), 3.91 (s, 3H), 3.69 (d, J = 13.9 Hz, 1H), 3.66 - 3.61 (m, 1H), 2.45 (s, 3H), 1.31 - 1.20 (m, 1H), 1.20 - 1.12 (m, 2H), 0.91 (s, 9H). |
| 968 | 1H NMR (400 MHz, 甲醇-d4) δ 8.49 (s, 1H), 7.85 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.5, 2.8 Hz, 1H), 6.82 (d, J = 2.3 Hz, 1H), 6.13 (s, 1H), 4.11 (d, J = 13.8 Hz, 1H), 3.88 (s, 3H), 3.69 (d, J = 13.9 Hz, 1H), 2.70 (s, 1H), 2.44 (s, 3H), 2.43 (s, 6H), 0.91 (s, 9H). |
| 969 | 1H NMR (400 MHz, 甲醇-d4) δ 8.20 (s, 1H), 7.90 (t, J = 8.1 Hz, 1H), 7.50 (d, J = 2.3 Hz, 1H), 6.85 (dd, J = 8.4, 3.0 Hz, 1H), 6.65 (d, J = 2.4 Hz, 1H), 6.12 (s, 1H), 3.97 - 3.84 (m, 7H), 3.43 (d, J = 13.7 Hz, 1H), 2.44 (s, 3H), 0.84 (s, 9H). |
| 970 | 1H NMR (400 MHz, 甲醇-d4) δ 8.50 (s, 1H), 7.71 - 7.53 (m, 2H), 6.86 (d, J = 2.3 Hz, 1H), 6.62 (d, J = 8.5 Hz, 1H), 4.07 (d, J = 13.8 Hz, 1H), 3.88 (m, 7H), 3.81 (s, 3H), 2.45 (s, 3H), 0.93 (s, 9H). |
| 971 | 1H NMR (400 MHz, 甲醇-d4) δ 8.46 (s, 1H), 7.86 (t, J = 8.1 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.5, 2.8 Hz, 1H), 6.81 (d, J = 2.3 Hz, 1H), 6.14 (s, 1H), 4.10 (d, J = 13.9 Hz, 1H), 3.88 (m, 6H), 3.66 (d, J = 13.8 Hz, 1H), 2.45 (s, 3H), 0.90 (s, 9H). |
| 972 | 1H NMR (400 MHz, 甲醇-d4) δ 8.42 (s, 1H), 8.03 (s, 1H), 7.69 (d, 1H), 7.61 (d, 1H), 7.49 - 7.38 (m, 2H), 7.07 (s, 1H), 6.75 (s, 1H), 4.06 (d, 1H), 3.67 (d, 1H), 2.85 - 2.74 (m, 1H), 1.74 - 1.57 (m, 4H), 0.83 (s, 9H). |
| 973 | 1H NMR (400 MHz, DMSO-d6) δ 8.23 (s, 1H), 7.78 (t, J = 8.3 Hz, 1H), 7.57 (d, J = 2.4 Hz, 1H), 6.98 - 6.75 (m, 2H), 6.27 - 5.84 (m, 2H), 2.42 (s, 3H), 1.47 (d, J = 3.4 Hz, 4H), 0.76 (s, 9H). |
| 974 | 1H NMR (400 MHz, 甲醇-d4) δ 8.53 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.68 (d, J = 2.3 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H), 6.91 (dd, J = 8.5, 2.8 Hz, 1H), 6.43 (s, 1H), 5.92 (t, J = 53.7 Hz, 1H), 3.95 (s, 2H), 2.54 (s, 3H), 1.81 - 1.62 (m, 4H), 0.95 (s, 9H). |
| 975 | 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1H), 7.91 (t, J = 8.3 Hz, 1H), 7.73 (t, J = 5.5 Hz, 1H), 7.49 (s, 1H), 7.00 (dd, J = 8.5, 3.1 Hz, 1H), 6.47 (s, 2H), 6.06 (t, J = 53.4 Hz, 1H), 3.61 (d, J = 5.5 Hz, 2H), 2.50 (s, 3H), 1.56 (m, 4H), 1.05 (s, 9H). |
| 976 | 1H NMR (400 MHz, 甲醇-d4) δ 9.31 (s, 1H), 8.94 (s, 2H), 8.38 (s, 1H), 8.06 (s, 1H), 7.82 (t, J = 8.1 Hz, 1H), 7.46 (d, J = 2.4 Hz, 1H), 7.08 (d, J = 2.4 Hz, 1H), 6.87 (dd, J = 8.5, 2.7 Hz, 1H), 6.29 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.04 (d, J = 13.9 Hz, 1H), 3.84 (d, J = 13.9 Hz, 1H), 2.51 (s, 3H), 1.53 (s, 4H), 0.95 (s, 9H). |
| 977 | 1H NMR (400 MHz, 甲醇-d4) δ 8.51 (d, J = 1.1 Hz, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.65 (d, J = 2.3 Hz, 1H), 6.86 (dd, J = 8.6, 2.7 Hz, 1H), 6.83 (d, J = 2.3 Hz, 1H), 6.07 (s, 1H), 4.13 (d, J = 13.9 Hz, 1H), 3.70 (d, J = 13.9 Hz, 1H), 2.40 (s, 3H), 1.64 (s, 3H), 1.44 - 1.31 (m, 2H), 1.22 (t, J = 2.0 Hz, 2H), 0.89 (s, 9H). |
| 978 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 8.11 (s, 1H), 7.62 (d, J = 2.3 Hz, 1H), 7.51 (d, J = 8.5 Hz, 1H), 6.96 - 6.89 (m, 1H), 6.60 (d, J = 8.6 Hz, 1H), 6.19 (s, 1H), 3.98 (d, J = 13.9 Hz, 1H), 3.88 (d, J = 6.4 Hz, 4H), 2.48 (s, 3H), 1.73 (d, J = 5.6 Hz, 2H), 1.66 (s, 2H), 0.94 (s, 9H). |
| 979 | 1H NMR (400 MHz, 甲醇-d4) δ 8.55 (d, J = 1.6 Hz, 1H), 8.32 (s, 1H), 7.88 (d, J = 2.6 Hz, 1H), 7.59 (t, J = 8.1 Hz, 1H), 7.40 (d, J = 2.6 Hz, 1H), 6.92 (dd, J = 8.5, 2.8 Hz, 1H), 6.76 (s, 1H), 4.09 (d, J = 14.1 Hz, 1H), 3.96 (d, J = 14.1 Hz, 1H), 2.97 (s, 3H), 2.35 (s, 3H), 1.80 - 1.70 (m, 4H), 1.02 (d, J = 3.2 Hz, 9H). |
| 980 | 1H NMR (400 MHz, 氯仿-d) δ 8.36 (s, 1H), 7.91 (t, 1H), 7.48 (s, 1H), 7.33 (d, 1H), 6.79 (dd, 1H), 6.40 (s, 1H), 5.94 (s, 1H), 5.20 (s, 1H), 3.56 (t, 2H), 3.33 (s, 3H), 2.59 (s, 3H), 1.79 (d, 6H), 1.77 - 1.70 (m, 1H), 1.68 - 1.50 (m, 4H) 0.94 (s, 9H). |
| 981 | 1H NMR (400 MHz, 甲醇-d4) δ 8.56 (s, 1H), 8.29 (d, J = 2.4 Hz, 1H), 8.08 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 7.18 (d, J = 2.3 Hz, 1H), 6.88 (dd, J = 8.5, 2.7 Hz, 1H), 6.29 (s, 1H), 5.93 (t, J = 54.6 Hz, 1H), 4.05 (d, J = 14.0 Hz, 1H), 3.87 (d, J = 14.0 Hz, 1H), 2.77 (s, 3H), 2.52 (s, 3H), 1.54 (s, 4H), 0.94 (s, 9H). |
| 982 | 1H NMR (400 MHz, 氯仿-d) δ 10.32 (s, 1H), 9.20 (s, 1H),8.30 - 8.20 (m, 1H), 8.13 (s, 1H), 7.90 (t, 1H), 7.19 (s, 1H), 6.82 - 6.65 (m, 2H), 6.24 (s, 1H), 4.17 - 4.02 (m, 2H), 2.64 (s, 3H), 1.78 (s, 3H), 1.77 (s, 3H), 1.72 - 1.63 (m, 4H), 1.09 (s, 9H). |
| 983 | 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.17 (t, 1H), 8.01 (s, 1H),7.98 - 7.93 (m, 1H); 7.06 (dd, 1H), 6.70 (s, 1H),6.52 - 6.43 (m, 1H), 6.09 (t, 1H), 3.67 (qd, 2H), 2.43 (s, 3H), 1.52 - 1.40 (m, 4H), 0.95 (s, 9H). |
| 984 | 1H NMR (400 MHz, 甲醇-d4) δ 8.47 (s, 1H), 7.85 (s, 1H), 7.77 (m, 1H), 7.62 (s, 1H), 6.87 (m, 2H), 6.20 (s, 1H), 4.38 (t, J = 7.4 Hz, 2H), 4.01 (d, J = 13.9 Hz, 1H), 3.80 (d, J = 13.7 Hz, 1H), 2.50 (m, 5H), 1.89 - 1.80 (m, 2H), 1.29 (m, 2H), 0.92 (s, 11H). |
| 985 | 1H NMR (400 MHz, 乙腈-d3) δ 8.41 (s, 1H), 7.84 (m, 1H), 7.66 (m, 1H), 7.48 (m, 1H), 6.82 (m, 1H), 6.74 (m, 1H), 6.15 (m, 1H), 4.42 (m, 2H), 3.76 (m, 2H), 3.48 (m, 2H), 2.51 (s, 3H), 1.98 (m, 2H), 0.94 (s, 9H). |
| 986 | 1H NMR (400 MHz, 乙腈-d3) δ 8.42 (s, 1H), 7.92 (m, 1H), 7.48 (s, 1H), 6.79 (m, 1H), 6.70 (s, 1H), 5.97 (s, 1H), 4.35 (m, 4H), 3.90 (m, 1H), 3.68 (m, 1H), 2.50 (s, 3H), 2.23 (m, 2H), 0.94 (s, 9H). |
| 化合物 | IC50 HTRF (nM) | EC50 TNF (nM) |
| 1 | 8 | 218 |
| 2 | 2 | 89 |
| 3 | 8 | 314 |
| 4 | 2 | 497 |
| 5 | 9 | 258 |
| 7 | 380 | >1000 |
| 8 | 7 | 302 |
| 9 | 1 | 20 |
| 10 | 21 | 283 |
| 11 | 4 | 154 |
| 12 | 27 | 214 |
| 13 | 11 | 765 |
| 14 | 7 | 196 |
| 15 | 2 | 545 |
| 16 | 2 | 115 |
| 17 | 53 | 1388 |
| 18 | 28 | 399 |
| 19 | 2 | 156 |
| 20 | 2 | 69 |
| 21 | 14 | 2035 |
| 22 | 2 | 89 |
| 23 | 4 | 69 |
| 24 | 12 | 185 |
| 25 | 2 | 57 |
| 26 | 6 | 113 |
| 27 | 2 | 62 |
| 28 | 12 | 195 |
| 29 | 17 | 498 |
| 30 | 34 | 1322 |
| 31 | 5 | 5069 |
| 32 | 7 | 546 |
| 33 | 11 | 630 |
| 34 | 8 | 112 |
| 35 | 9 | 166 |
| 36 | 3 | 67 |
| 37 | 16 | 2638 |
| 38 | 25 | 952 |
| 39 | 11 | 295 |
| 40 | 6 | 249 |
| 41 | 8 | 137 |
| 42 | 135 | 1926 |
| 43 | 33 | 915 |
| 44 | 9 | 129 |
| 45 | 2 | 178 |
| 46 | 3 | 1102 |
| 47 | 25 | 1068 |
| 48 | 2 | 87 |
| 49 | 118 | 16684 |
| 50 | 70 | 3534 |
| 51 | 55 | 1556 |
| 52 | 4 | 88 |
| 53 | 81 | 4018 |
| 54 | 11 | 652 |
| 55 | 58 | 10816 |
| 56 | 6 | 2521 |
| 57 | 60 | 5990 |
| 58 | 72 | 2982 |
| 59 | 2 | 892 |
| 60 | 12 | 131 |
| 61 | 11 | 238 |
| 62 | 10 | 216 |
| 63 | 5 | 257 |
| 64 | 42 | 2652 |
| 65 | 6 | 538 |
| 66 | 3 | 53 |
| 67 | 1 | 32 |
| 68 | 1 | 51 |
| 69 | 2 | 33 |
| 70 | 19 | 804 |
| 71 | 2 | 36 |
| 72 | 1 | 11 |
| 73 | 2 | 33 |
| 74 | 1 | 14 |
| 75 | 2 | 79 |
| 76 | 1 | 981 |
| 77 | 3 | 154 |
| 78 | 3 | 332 |
| 79 | 1 | 203 |
| 80 | 1 | 23 |
| 81 | 400 | >1000 |
| 82 | 2 | 151 |
| 83 | 942 | 1000 |
| 84 | 1 | 10 |
| 85 | 4 | >1000 |
| 86 | 2 | 37 |
| 87 | 25 | 590 |
| 88 | 1161 | >1000 |
| 89 | 520 | >1000 |
| 90 | 1 | 18 |
| 91 | 2 | 52 |
| 92 | 6 | 65 |
| 93 | 7 | 74 |
| 94 | 2 | 130 |
| 95 | 10 | 445 |
| 96 | 11 | 173 |
| 97 | 20 | 358 |
| 98 | 49 | 821 |
| 99 | 3 | 143 |
| 100 | 3 | 142 |
| 101 | 5 | 348 |
| 102 | 3 | 461 |
| 103 | 3 | 133 |
| 104 | 1 | 99 |
| 105 | 3 | 144 |
| 106 | 2 | 76 |
| 107 | 182 | >10000 |
| 108 | 913 | >10000 |
| 109 | 2 | 54 |
| 110 | 2 | 56 |
| 111 | 2 | 77 |
| 112 | 2 | 61 |
| 113 | 13 | 253 |
| 114 | 10 | 233 |
| 115 | 5 | 119 |
| 116 | 3 | 144 |
| 117 | 3 | 94 |
| 118 | 6 | 150 |
| 119 | 4 | 99 |
| 120 | 5 | 137 |
| 121 | 7 | 171 |
| 122 | 6 | 240 |
| 123 | 6 | 193 |
| 124 | 8 | 376 |
| 125 | 3 | 78 |
| 126 | 3 | 146 |
| 127 | 16 | 194 |
| 128 | 698 | >10000 |
| 129 | 2 | 81 |
| 130 | 6 | 147 |
| 131 | 2 | 49 |
| 132 | 4 | 97 |
| 133 | 25 | 978 |
| 134 | 6 | 197 |
| 135 | 23 | 660 |
| 136 | 10 | 291 |
| 137 | 15 | 235 |
| 138 | 3 | 141 |
| 139 | 2 | 92 |
| 140 | 3 | 183 |
| 141 | 2 | 121 |
| 142 | 2 | 66 |
| 143 | 16 | 898 |
| 144 | 2 | 77 |
| 145 | 4 | 221 |
| 146 | 10 | 370 |
| 147 | 12 | 1139 |
| 148 | 5 | 174 |
| 149 | 8 | 381 |
| 150 | 4 | 154 |
| 151 | 8 | 1305 |
| 152 | 10 | 904 |
| 153 | 44 | 3759 |
| 154 | 26 | 1667 |
| 155 | 2 | 79 |
| 156 | 8 | 1191 |
| 157 | 5 | 127 |
| 159 | 2 | 95 |
| 160 | 11 | 484 |
| 161 | 12 | 1668 |
| 162 | 7 | 429 |
| 163 | 67 | 4142 |
| 164 | 5 | 1802 |
| 165 | 9 | 2368 |
| 166 | 6 | 1425 |
| 167 | 4 | 506 |
| 168 | 6 | 394 |
| 169 | 14 | 1217 |
| 170 | 6 | 1262 |
| 171 | 10 | 320 |
| 172 | 10 | 832 |
| 173 | 7 | 1112 |
| 174 | 2 | 54 |
| 175 | 3 | 73 |
| 176 | 2 | 31 |
| 177 | 2 | 79 |
| 178 | 7 | 931 |
| 179 | 265 | >10000 |
| 180 | 1 | 104 |
| 181 | 2 | 92 |
| 182 | 13 | 1041 |
| 183 | 357 | >10000 |
| 184 | 8 | 387 |
| 185 | 305 | 9745 |
| 186 | 118 | 6618 |
| 187 | 67 | 2114 |
| 188 | >10000 | >10000 |
| 189 | 8 | 1061 |
| 190 | 9 | 238 |
| 191 | 2 | 104 |
| 192 | 3 | 186 |
| 193 | 2 | 107 |
| 194 | 2 | 111 |
| 195 | 4 | 132 |
| 196 | 6 | 668 |
| 197 | 9 | 599 |
| 198 | 17 | 982 |
| 199 | 9 | 2522 |
| 200 | 7 | 558 |
| 201 | 56 | 5595 |
| 202 | >10000 | >10000 |
| 203 | 6923 | 9089 |
| 204 | 8513 | >10000 |
| 205 | 72 | 2048 |
| 206 | 3 | 211 |
| 207 | 5 | 931 |
| 208 | 12 | 465 |
| 209 | 23 | 941 |
| 210 | 10 | 424 |
| 211 | 16 | 544 |
| 212 | 44 | 9151 |
| 213 | 3 | 103 |
| 214 | >10000 | >10000 |
| 215 | 258 | >10000 |
| 216 | 38 | 1187 |
| 217 | 12 | 264 |
| 218 | 26 | 2711 |
| 219 | 4449 | >10000 |
| 220 | 4 | 348 |
| 221 | 2 | 28 |
| 222 | 19 | 739 |
| 223 | 7 | 94 |
| 224 | 12 | 2628 |
| 225 | 10 | 1488 |
| 226 | 8 | 880 |
| 227 | 44 | 6419 |
| 228 | 18 | 2307 |
| 229 | 12 | 1467 |
| 230 | 9 | 252 |
| 231 | 8 | 230 |
| 232 | 15 | 346 |
| 233 | 8 | 114 |
| 234 | 21 | 504 |
| 235 | 17 | 370 |
| 236 | 9 | 172 |
| 237 | 1 | 29 |
| 238 | 3 | 901 |
| 239 | 16 | 928 |
| 240 | 16 | 631 |
| 241 | 2 | 32 |
| 242 | 143 | 5801 |
| 243 | 41 | 9492 |
| 244 | 4 | 328 |
| 245 | 2 | 125 |
| 246 | 6 | 652 |
| 247 | 2 | 102 |
| 248 | 4 | 398 |
| 249 | 12 | 332 |
| 250 | 5 | 127 |
| 251 | 5 | 347 |
| 252 | 6 | 119 |
| 253 | 4 | 66 |
| 254 | 3 | 230 |
| 255 | 10 | 766 |
| 256 | 16 | 341 |
| 257 | 6 | 212 |
| 258 | 2 | 33 |
| 259 | 6 | 158 |
| 260 | 6 | 126 |
| 261 | 14 | 344 |
| 262 | 11 | 130 |
| 263 | 13 | 242 |
| 264 | 2 | 70 |
| 265 | 14 | 426 |
| 266 | 37 | 28752 |
| 267 | 30 | 5120 |
| 268 | 12 | 234 |
| 269 | 6 | 326 |
| 270 | 4 | 666 |
| 271 | 9 | 826 |
| 272 | 5 | 297 |
| 273 | 51 | 1564 |
| 274 | 12 | 370 |
| 275 | 7 | 2334 |
| 276 | 6 | 789 |
| 277 | 7 | 923 |
| 278 | 3 | 269 |
| 279 | 3 | 457 |
| 280 | 16 | 811 |
| 281 | 13 | 575 |
| 282 | 3 | 87 |
| 283 | 3 | 50 |
| 284 | 14 | 1305 |
| 285 | 8 | 219 |
| 286 | 4 | 121 |
| 287 | 20 | 373 |
| 288 | 26 | 1058 |
| 289 | 37 | 837 |
| 290 | 12 | 185 |
| 291 | 35 | 478 |
| 292 | 17 | 327 |
| 294 | 9 | 166 |
| 295 | 23 | 565 |
| 296 | 80 | 1104 |
| 297 | 9 | 200 |
| 298 | 39 | 1503 |
| 299 | 18 | 739 |
| 300 | 7 | 165 |
| 301 | 14 | 414 |
| 302 | 10 | 472 |
| 303 | 14 | 868 |
| 304 | 6 | 234 |
| 305 | 3 | 84 |
| 306 | 6 | 202 |
| 307 | 4 | 60 |
| 308 | 4 | 286 |
| 309 | 8 | 181 |
| 310 | 40 | 2342 |
| 312 | 13 | 1235 |
| 313 | 5 | 373 |
| 314 | 15 | 302 |
| 315 | 4 | 132 |
| 316 | 6 | 298 |
| 317 | 16 | 251 |
| 318 | 8 | 271 |
| 319 | 5 | 165 |
| 321 | 10 | 212 |
| 322 | 33 | 369 |
| 323 | 86 | 666 |
| 325 | 20 | 650 |
| 326 | 34 | 3753 |
| 328 | 4 | 272 |
| 331 | 3 | 173 |
| 334 | 4 | 82 |
| 335 | 10 | 405 |
| 339 | 13 | 1010 |
| 340 | 8 | 328 |
| 341 | 6 | 843 |
| 342 | 38 | 6094 |
| 344 | 3 | 151 |
| 345 | 3 | 73 |
| 346 | 3 | 208 |
| 347 | 4 | 278 |
| 348 | 13 | 444 |
| 349 | 41 | 718 |
| 350 | 26 | 4511 |
| 351 | 10 | 744 |
| 352 | 12 | 565 |
| 353 | 4 | 184 |
| 354 | 3 | 983 |
| 355 | 5 | 194 |
| 357 | 5 | 235 |
| 358 | 3 | 130 |
| 359 | 3 | 729 |
| 360 | 4 | 151 |
| 361 | 13 | 746 |
| 362 | 1 | 46 |
| 363 | 4 | 257 |
| 364 | 7 | 241 |
| 366 | 5 | 336 |
| 367 | 13 | 338 |
| 368 | 649 | 6296 |
| 369 | 3 | 89 |
| 370 | 8 | 232 |
| 371 | 2 | 173 |
| 372 | 2 | 81 |
| 373 | 3 | 81 |
| 374 | 37 | 480 |
| 375 | 4 | 1511 |
| 376 | 182 | >1000 |
| 377 | 398 | >1000 |
| 378 | 2 | 168 |
| 379 | 6 | 179 |
| 380 | 14 | 609 |
| 381 | 7 | 303 |
| 382 | 14 | 768 |
| 389 | 11 | 328 |
| 395 | 4 | |
| 403 | 9 | 290 |
| 405 | 2 | 1044 |
| 406 | 3 | 102 |
| 6 | 2 | |
| 293 | 32 | |
| 311 | 30 | |
| 320 | 60 | |
| 324 | 10 | |
| 327 | 15 | |
| 329 | 14 | |
| 330 | 59 | |
| 332 | 65 | |
| 333 | 3 | |
| 336 | 2 | |
| 337 | 16 | |
| 338 | 13 | |
| 343 | 504 | |
| 356 | 2 | |
| 365 | 38 | |
| 384 | 153 | |
| 385 | 14 | |
| 386 | 36 | |
| 387 | 18 | |
| 388 | 10 | |
| 390 | 343 | |
| 391 | 282 | |
| 392 | 3 | |
| 393 | 5 | |
| 394 | 147 | |
| 396 | 43 | |
| 397 | 46 | |
| 398 | 435 | |
| 399 | 58 | |
| 400 | 119 | |
| 401 | 10 | |
| 402 | 19 | |
| 404 | 232 | |
| 408 | 1 | 88 |
| 409 | 1 | 559 |
| 410 | 113 | 1000 |
| 411 | 5 | 76 |
| 412 | 7 | 157 |
| 413 | 4 | 44 |
| 414 | 1 | 35 |
| 415 | 3 | 67 |
| 416 | 2 | 124 |
| 417 | 9 | 218 |
| 418 | 2 | 45 |
| 419 | 3 | 49 |
| 420 | 2 | 25 |
| 421 | 4 | 100 |
| 422 | 2 | 57 |
| 423 | 3 | 71 |
| 424 | 2 | 37 |
| 425 | 7 | 1000 |
| 426 | 2 | 95 |
| 427 | 2 | 41 |
| 428 | 1 | 69 |
| 429 | 2 | 56 |
| 430 | 5 | 214 |
| 431 | 1 | 21 |
| 432 | 6 | 89 |
| 433 | 1 | 65 |
| 434 | 2 | 61 |
| 435 | 1 | 24 |
| 436 | 5 | 289 |
| 437 | 2 | 82 |
| 438 | 473 | 1000 |
| 439 | 13 | 299 |
| 440 | 7 | 171 |
| 441 | 2 | 26 |
| 442 | 56 | 2686 |
| 443 | 1 | 19 |
| 444 | 2 | 33 |
| 445 | 2 | 71 |
| 446 | 3 | 156 |
| 447 | 2 | 40 |
| 448 | 2 | 44 |
| 449 | 19 | 407 |
| 450 | 20 | 356 |
| 451 | 3 | 138 |
| 452 | 4 | 73 |
| 453 | 9 | 184 |
| 454 | 2 | 32 |
| 455 | 29 | 222 |
| 456 | 51 | 1000 |
| 457 | 315 | 1000 |
| 458 | 4 | 87 |
| 459 | 3 | 50 |
| 460 | 5 | 156 |
| 461 | 17 | 154 |
| 462 | 162 | 8766 |
| 463 | 2 | 63 |
| 464 | 1 | 16 |
| 465 | 2 | 155 |
| 466 | 28 | 926 |
| 467 | 2 | 47 |
| 468 | 1 | 18 |
| 469 | 4 | 69 |
| 470 | 2 | 46 |
| 471 | 3 | 45 |
| 472 | 4 | 78 |
| 473 | 2 | 26 |
| 474 | 2 | 26 |
| 475 | 20 | 258 |
| 476 | 3 | 71 |
| 477 | 2 | 185 |
| 478 | 1 | 77 |
| 479 | 5 | 78 |
| 480 | 2 | 60 |
| 481 | 6 | 391 |
| 482 | 3 | 86 |
| 483 | 7 | 108 |
| 484 | 3 | 73 |
| 485 | 3 | 29 |
| 486 | 3 | 991 |
| 487 | 1 | 245 |
| 488 | 3 | 79 |
| 489 | 2 | 129 |
| 490 | 1 | 1000 |
| 491 | 3 | 40 |
| 492 | 2 | 21 |
| 493 | 31 | 1000 |
| 494 | 1 | 444 |
| 495 | 3 | 50 |
| 496 | 6 | 467 |
| 497 | 6 | 379 |
| 498 | 19 | 689 |
| 499 | 3 | 97 |
| 500 | 8 | 321 |
| 501 | 3 | 55 |
| 502 | 6 | 206 |
| 503 | 2 | 45 |
| 504 | 1 | 38 |
| 505 | 5 | 277 |
| 506 | 8 | 528 |
| 507 | 2 | 87 |
| 508 | 4 | 96 |
| 509 | 267 | 1000 |
| 510 | 5 | 222 |
| 511 | 6 | 133 |
| 512 | 10 | 418 |
| 513 | 9 | 154 |
| 514 | 4 | 77 |
| 515 | 199 | 1000 |
| 516 | 2 | 34 |
| 517 | 3 | 1000 |
| 518 | 7 | 207 |
| 519 | 3 | 158 |
| 520 | 123 | 926 |
| 521 | 27 | 256 |
| 522 | 5 | 52 |
| 523 | 2 | 63 |
| 524 | 12 | 77 |
| 525 | 4 | 164 |
| 526 | 5 | 73 |
| 527 | 3 | 78 |
| 528 | 3 | 60 |
| 529 | 2 | 50 |
| 530 | 3 | 70 |
| 531 | 3 | 70 |
| 532 | 3 | 78 |
| 533 | 3 | 96 |
| 534 | 20 | 394 |
| 535 | 2 | 47 |
| 536 | 3 | 51 |
| 537 | 2 | 75 |
| 538 | 5 | 109 |
| 539 | 1 | 12 |
| 540 | 50 | 760 |
| 541 | 6 | 180 |
| 542 | 2 | 40 |
| 543 | 13 | 422 |
| 544 | 8 | 210 |
| 545 | 4 | 108 |
| 546 | 2 | 42 |
| 547 | 2 | 33 |
| 548 | 8 | 124 |
| 549 | 4 | 68 |
| 550 | 2 | 29 |
| 551 | 2 | 27 |
| 552 | 2 | 26 |
| 553 | 3 | 64 |
| 554 | 5 | 151 |
| 555 | 7 | 208 |
| 556 | 8 | 120 |
| 557 | 5 | 117 |
| 558 | 3 | 72 |
| 559 | 2 | 42 |
| 560 | 11 | 287 |
| 561 | 2 | 433 |
| 562 | 2 | 231 |
| 563 | 2 | 63 |
| 564 | 3 | 69 |
| 565 | 2 | 306 |
| 566 | 2 | 190 |
| 567 | 2 | 66 |
| 568 | 5 | 170 |
| 569 | 7 | 171 |
| 570 | 184 | 1000 |
| 571 | 2 | 77 |
| 572 | 4 | 79 |
| 573 | 66 | 751 |
| 574 | 4 | 86 |
| 575 | 1 | 974 |
| 576 | 1 | 433 |
| 577 | 1 | 49 |
| 578 | 2 | 32 |
| 579 | 2 | 24 |
| 580 | 2 | 84 |
| 581 | 2 | 32 |
| 582 | 14 | 184 |
| 583 | 4 | 121 |
| 584 | 2 | 53 |
| 585 | 37 | 838 |
| 586 | 2 | 1000 |
| 587 | 1 | 489 |
| 588 | 55 | 629 |
| 589 | 2 | 34 |
| 590 | 3 | 57 |
| 591 | 5 | 106 |
| 592 | 5 | 78 |
| 593 | 8 | 298 |
| 594 | 2 | 33 |
| 595 | 209 | 1000 |
| 596 | 79 | 826 |
| 597 | 3 | 150 |
| 598 | 5 | 198 |
| 599 | 13 | 316 |
| 600 | 5 | 113 |
| 601 | 4 | 32 |
| 602 | 3 | 69 |
| 603 | 7 | 107 |
| 604 | 3 | 71 |
| 605 | 3 | 30 |
| 606 | 2 | 27 |
| 607 | 21 | 225 |
| 608 | 42 | 314 |
| 609 | 2 | 46 |
| 610 | 2 | 199 |
| 611 | 20 | 229 |
| 612 | 11 | 171 |
| 613 | 13 | 144 |
| 614 | 9 | 154 |
| 615 | 33 | 588 |
| 616 | 12 | 104 |
| 617 | 9 | 77 |
| 618 | 3 | 37 |
| 619 | 7 | 189 |
| 620 | 102 | 1000 |
| 621 | 1 | 12 |
| 622 | 1 | 24 |
| 623 | 1 | 27 |
| 624 | 11 | 1000 |
| 625 | 2 | 26 |
| 626 | 5 | 35 |
| 627 | 4 | 46 |
| 628 | 450 | 1000 |
| 629 | 181 | 1000 |
| 630 | 68 | 1000 |
| 631 | 2 | 32 |
| 632 | 380 | 1000 |
| 633 | 28 | 267 |
| 634 | 2 | 43 |
| 635 | 3 | 60 |
| 636 | 69 | 720 |
| 637 | 3 | 85 |
| 638 | 58 | 858 |
| 639 | 58 | 806 |
| 640 | 3 | 65 |
| 641 | 55 | 811 |
| 642 | 5 | 182 |
| 643 | 5 | 1000 |
| 644 | 7 | 88 |
| 645 | 11 | 66 |
| 646 | 3 | 40 |
| 647 | 3 | 133 |
| 648 | 3 | 114 |
| 649 | 2 | 14 |
| 650 | 179 | 1000 |
| 651 | 14 | 135 |
| 652 | 4 | 40 |
| 653 | 4 | 37 |
| 654 | 3 | 36 |
| 655 | 12 | 104 |
| 656 | 4 | 86 |
| 657 | 37 | 806 |
| 658 | 29 | 374 |
| 659 | 41 | 279 |
| 660 | 43 | 839 |
| 661 | 2 | 103 |
| 662 | 6 | 335 |
| 663 | 3 | 62 |
| 664 | 12 | 154 |
| 665 | 6 | 198 |
| 666 | 13 | 246 |
| 667 | 10 | 71 |
| 668 | 13 | 408 |
| 669 | 1 | 7 |
| 670 | 5 | 102 |
| 671 | 2 | 32 |
| 672 | 2 | 22 |
| 673 | 142 | 808 |
| 674 | 22 | 301 |
| 675 | 1 | 7 |
| 676 | 6 | 112 |
| 677 | 4 | 133 |
| 678 | 5 | 131 |
| 679 | 13 | 710 |
| 680 | 3 | 98 |
| 681 | 2 | 26 |
| 682 | 7 | 66 |
| 683 | 35 | 283 |
| 684 | 14 | 101 |
| 685 | 4 | 39 |
| 686 | 4 | 77 |
| 687 | 15 | 178 |
| 688 | 30 | 437 |
| 689 | 9 | 81 |
| 690 | 21 | 250 |
| 691 | 6 | 66 |
| 692 | 3 | 38 |
| 693 | 15 | 141 |
| 694 | 7 | 84 |
| 695 | 3 | 39 |
| 696 | 2 | 14 |
| 697 | 1 | 11 |
| 698 | 27 | 684 |
| 699 | 12 | 386 |
| 700 | 2 | 179 |
| 701 | 10 | 147 |
| 702 | 4 | 188 |
| 703 | 2 | 22 |
| 704 | 1 | 23 |
| 705 | 6 | 61 |
| 706 | 2 | 14 |
| 707 | 16 | 230 |
| 708 | 6 | 176 |
| 709 | 17 | 187 |
| 710 | 5 | 148 |
| 711 | 2 | 32 |
| 713 | 2 | 21 |
| 714 | 6 | 57 |
| 715 | 3 | 91 |
| 716 | 2 | 54 |
| 717 | 8 | 100 |
| 718 | 9 | 112 |
| 719 | 2 | 24 |
| 720 | 2 | 35 |
| 721 | 6 | 109 |
| 722 | 43 | 403 |
| 723 | 4 | 84 |
| 724 | 3 | 44 |
| 725 | 3 | 56 |
| 726 | 4 | 127 |
| 727 | 3 | 48 |
| 728 | 7 | 154 |
| 729 | 5 | 108 |
| 730 | 10 | 89 |
| 731 | 11 | 179 |
| 732 | 11 | 779 |
| 733 | 42 | 835 |
| 734 | 41 | 313 |
| 735 | 47 | 478 |
| 736 | 117 | 645 |
| 737 | 3 | 91 |
| 738 | 2 | 40 |
| 739 | 21 | 254 |
| 740 | 45 | 311 |
| 741 | 6 | 120 |
| 742 | 17 | 199 |
| 743 | 5 | 116 |
| 744 | 3 | 33 |
| 745 | 2 | 28 |
| 746 | 13 | 178 |
| 747 | 4 | 36 |
| 748 | 5 | 47 |
| 749 | 6 | 66 |
| 750 | 11 | 194 |
| 751 | 15 | 230 |
| 752 | 5 | 57 |
| 753 | 5 | 59 |
| 754 | 3 | 31 |
| 755 | 25 | 365 |
| 756 | 3 | 26 |
| 757 | 5 | 60 |
| 758 | 4 | 50 |
| 759 | 2 | 42 |
| 760 | 2 | 58 |
| 761 | 6 | 69 |
| 762 | 3 | 50 |
| 763 | 3 | 40 |
| 764 | 4 | 46 |
| 765 | 4 | 131 |
| 766 | 2 | 30 |
| 767 | 18 | 209 |
| 768 | 6 | 86 |
| 769 | 2 | 28 |
| 770 | 4 | 108 |
| 771 | 4 | 293 |
| 772 | 2 | 33 |
| 773 | 2 | 35 |
| 774 | 2 | 40 |
| 775 | 3 | 27 |
| 776 | 2 | 24 |
| 777 | 5 | 46 |
| 778 | 4 | 30 |
| 779 | 3 | 26 |
| 780 | 5 | 52 |
| 781 | 2 | 22 |
| 782 | 23 | 177 |
| 783 | 2 | 23 |
| 784 | 3 | 51 |
| 785 | 2 | 23 |
| 786 | 5 | 47 |
| 787 | 2 | 65 |
| 788 | 23 | 208 |
| 789 | 13 | 156 |
| 790 | 5 | 82 |
| 791 | 22 | 242 |
| 792 | 89 | 843 |
| 793 | 35 | 782 |
| 794 | 15 | 130 |
| 795 | 34 | 530 |
| 796 | 2 | 31 |
| 797 | 3 | 77 |
| 798 | 7 | 90 |
| 799 | 4 | 46 |
| 800 | 2 | 25 |
| 801 | 3 | 31 |
| 802 | 4 | 36 |
| 803 | 7 | 97 |
| 804 | 9 | 137 |
| 805 | 28 | 778 |
| 806 | 3 | 28 |
| 807 | 3 | 31 |
| 808 | 3 | 29 |
| 809 | 3 | 32 |
| 810 | 4 | 54 |
| 811 | 11 | 333 |
| 812 | 5 | 68 |
| 813 | 2 | 24 |
| 814 | 2 | 16 |
| 815 | 3 | 39 |
| 816 | 1 | 23 |
| 817 | 10 | 116 |
| 818 | 6 | 176 |
| 819 | 2 | 28 |
| 820 | 3 | 33 |
| 821 | 3 | 60 |
| 822 | 10 | 687 |
| 823 | 4 | 53 |
| 824 | 5 | 59 |
| 825 | 7 | 113 |
| 826 | 51 | 1000 |
| 827 | 2 | 38 |
| 828 | 22 | 253 |
| 829 | 4 | 66 |
| 830 | 16 | 384 |
| 831 | 8 | 202 |
| 832 | 2 | 44 |
| 833 | 2 | 22 |
| 834 | 5 | 68 |
| 835 | 4 | 53 |
| 836 | 4 | 52 |
| 837 | 7 | 121 |
| 838 | 3 | 84 |
| 839 | 6 | 94 |
| 840 | 4 | 52 |
| 841 | 2 | 41 |
| 842 | 3 | 563 |
| 843 | 4 | 104 |
| 844 | 13 | 417 |
| 845 | 20 | 1000 |
| 846 | 2 | 17 |
| 847 | 31 | 296 |
| 848 | 31 | 247 |
| 849 | 16 | 261 |
| 850 | 6 | 65 |
| 851 | 3 | 21 |
| 852 | 2 | 58 |
| 853 | 1 | 98 |
| 854 | 4 | 17 |
| 855 | 2 | 53 |
| 856 | 1 | 29 |
| 857 | 4 | 180 |
| 858 | 7 | 44 |
| 859 | 2 | 53 |
| 860 | 3 | 31 |
| 861 | 7 | 40 |
| 862 | 8 | 49 |
| 863 | 2 | 35 |
| 864 | 2 | 37 |
| 865 | 4 | 23 |
| 866 | 2 | 18 |
| 867 | 3 | 27 |
| 868 | 2 | 70 |
| 869 | 2 | 37 |
| 870 | 2 | 169 |
| 871 | 1 | 32 |
| 872 | 1 | 15 |
| 873 | 5 | 97 |
| 874 | 3 | 73 |
| 875 | 2 | 166 |
| 876 | 2 | 385 |
| 877 | 2 | 34 |
| 878 | 2 | 31 |
| 879 | 1 | 13 |
| 880 | 3 | 80 |
| 881 | 2 | 13 |
| 882 | 2 | 18 |
| 883 | 1 | 13 |
| 884 | 1 | 19 |
| 885 | 10 | 132 |
| 886 | 3 | 30 |
| 887 | 3 | 33 |
| 888 | 2 | 165 |
| 889 | 9 | 104 |
| 890 | 6 | 68 |
| 891 | 1 | 12 |
| 892 | 2 | 44 |
| 893 | 4 | 99 |
| 894 | 3 | 54 |
| 895 | 3 | 41 |
| 896 | 3 | 30 |
| 897 | 2 | 18 |
| 898 | 5 | 56 |
| 899 | 2 | 29 |
| 900 | 3 | 30 |
| 901 | 484 | 1000 |
| 902 | 3 | 36 |
| 903 | 3 | 36 |
| 904 | 5 | 78 |
| 905 | 15 | 212 |
| 906 | 4 | 39 |
| 907 | 4 | 51 |
| 908 | 12 | 238 |
| 909 | 8 | 116 |
| 910 | 5 | 148 |
| 911 | 7 | 85 |
| 912 | 6 | 78 |
| 913 | 6 | 86 |
| 914 | 9 | 116 |
| 915 | 44 | 324 |
| 916 | 13 | 269 |
| 917 | 3 | 31 |
| 918 | 3 | 42 |
| 919 | 2 | 49 |
| 920 | 3 | 28 |
| 921 | 17 | 187 |
| 922 | 11 | 225 |
| 923 | 16 | 258 |
| 926 | 13 | 174 |
| 927 | 6 | 124 |
| 928 | 6 | 99 |
| 929 | 11 | 157 |
| 930 | 33 | 504 |
| 931 | 10 | 134 |
| 932 | 14 | 205 |
| 933 | 29 | 247 |
| 934 | 7 | 81 |
| 935 | 24 | 424 |
| 936 | 18 | 287 |
| 937 | 23 | 277 |
| 938 | 26 | 223 |
| 939 | 18 | 104 |
| 940 | 7 | 86 |
| 941 | 64 | 804 |
| 945 | 17 | 142 |
| 946 | 5 | 153 |
| 947 | 3 | 39 |
| 948 | 5 | 99 |
| 949 | 4 | 34 |
| 950 | 17 | 260 |
| 951 | 9 | 61 |
| 952 | 4 | 36 |
| 953 | 36 | 353 |
| 954 | 7 | 16 |
| 955 | 6 | 50 |
| 956 | 7 | 44 |
| 957 | 5 | 54 |
| 958 | 2 | 56 |
| 959 | 3 | 41 |
| 960 | 7 | 55 |
| 961 | 3 | 48 |
| 962 | 13 | 135 |
| 963 | 4 | 51 |
| 964 | 3 | 105 |
| 965 | 4 | 78 |
| 966 | 15 | 191 |
| 967 | 2 | 94 |
| 968 | 2 | 94 |
| 969 | 8 | 258 |
| 970 | 34 | 722 |
| 971 | 11 | 301 |
| 972 | 1817 | 1000 |
| 973 | 3 | 34 |
| 974 | 11 | 60 |
| 975 | 1231 | 1000 |
| 976 | 21 | 276 |
| 978 | 9 | 303 |
| 979 | 244 | 1000 |
| 980 | 1000 | 1000 |
| 981 | 4 | 20 |
| 982 | 1000 | 1000 |
| 983 | 9644 | 1000 |
| 984 | 10 | 169 |
| 985 | 13 | 126 |
| 986 | 5 | 141 |
| 化合物 | 化合物結構 | IC50 / EC50 [nM] | EGFR (IC50 ) [nM] |
| 比較實例1 | 4 / 310 | 357 | |
| 比較實例2 | 62 / NA | NA | |
| 362 | 1 / 46 | 3984 |
| 化合物 | 化合物結構 | IC50 / EC50 [nM] | EGFR (IC50 ) [nM] |
| 比較實例3 | 67 / >10000 | > 10000 | |
| 比較實例4 | > 10000 / Na | > 10000 | |
| 395 | 4 / NA | NA | |
| 406 | 3 / 102 | > 10000 |
Claims (42)
- 一種式I化合物或其醫藥學上可接受之鹽、立體異構體、或立體異構體混合物之用途,其係用以製備用於在人類患者中治療由大阪甲狀腺癌(Cot)介導之疾病或病狀之藥物,
- 如請求項1之用途,其中R2為氫。
- 如請求項1或2之用途,其中m為0。
- 如請求項1之用途,其中該化合物或其醫藥學上可接受之鹽、立體異 構體、或立體異構體混合物為式IIIA化合物,
- 如請求項1、2及4至6中任一項之用途,其中R5為氫、鹵基、-CN、O-R7、-S(O)-R7、-S(O)2R7、-SO2N(R7)2、-C(O)R7、-C(O)N(R7)2、C1-9烷基、C2-6烯基、C2-6炔基、C3-15環烷基、芳基、雜環基或雜芳基;其中各C1-9烷基、C2-6烯基、C2-6炔基、C3-15環烷基、芳基、雜環基及雜芳基可視情況經一至四個Z5取代。
- 如請求項1、2及4至6中任一項之用途,其中R5為氫、鹵基、-CN、-C(O)R7、-O-R7、-S(O)2R7或雜芳基。
- 如請求項1、2及4至6中任一項之用途,其中R6為氫。
- 如請求項1、2及4至6中任一項之用途,其中R1為-O-R7、C1-9烷基、C3-15環烷基、雜環基、芳基或雜芳基;且該C1-9烷基、C3-15環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12、-S(O)2R12、C1-9烷基、C1-9鹵烷基、C3-15環烷基、雜環基及芳基,其中該C3-15環烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9烷基及C1-9鹵烷基。
- 如請求項1、2及4至6中任一項之用途,其中R1為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的C1-9烷基:鹵基、-CN、-O-R12、-S(O)2R12、C3-15環烷基、雜環基及芳基,其中該C3-15環烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9烷基及C1-9鹵烷基。
- 如請求項6之用途,其中W為N,X為N-Z3,且Y為C-Z3。
- 如請求項1、2及4至6中任一項之用途,其中R1為C3-15環烷基、雜環基或雜芳基,其中該C3-15環烷基、雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12、C1-9烷基及芳基。
- 如請求項1、2及4至6中任一項之用途,其中R1為雜環基或雜芳基,其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基及C1-9烷基。
- 如請求項1、2及4至6中任一項之用途,其中R1為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的芳基:鹵基、-CN、-O-R7、C1-9烷基及芳基。
- 如請求項1、2及4至6中任一項之用途,其中R1為視情況經一至三個 獨立地選自由以下各者組成之群之取代基取代的芳基:鹵基、-O-R7及C1-9烷基。
- 如請求項6或12之用途,其中Z3為氫或視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C1-9烷基:-CN、鹵基、-O-R12、-C(O)O-R12、-OC(O)-R12、-N(R13)(R14)、-N(R13)2(R14)+、-C(O)N(R12)-S(O)2R12、C1-9烷基、雜環基、芳基及雜芳基。
- 如請求項6或12之用途,其中Z3為C3-15環烷基、雜環基、芳基或雜芳基;且該C3-15環烷基、雜環基、芳基或雜芳基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12、-C(O)-R12、-C(O)O-R12、-OC(O)-R12、-N(R13)(R14)、-N(R13)2(R14)+、C1-9烷基、C1-8鹵烷基、C1-8羥烷基、C3-15環烷基、雜環基及雜芳基。
- 如請求項19之用途,其中:Z3為氫、C1-9烷基、C3-15環烷基、雜環基、芳基或雜芳基;其中該C1-9烷基、C3-15環烷基或雜環基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:側氧基、-CN、鹵基、-O-R12、-C(O)-R12、-C(O)O-R12、-OC(O)-R12、-C(O)-N(R13)(R14)、-N(R12)S(O)2(R12)、-N(R13)(R14)、-N(R13)2(R14)+、-C(O)N(R12)-S(O)2R12、C1-9烷基、C1-8鹵烷基、C1-8羥烷基、C3-15環烷基、芳基、雜環基及雜芳基;Z9為氫;R1為C1-9烷基、C3-15環烷基、雜環基、芳基或雜芳基;其中該C1-9烷基、雜環基、芳基或雜芳基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:鹵基、-CN、-O-R12、-S(O)2R12、C1-9烷基、C1-9鹵烷基、雜環基及芳基,其中該C3-15環烷基可視情況經一至四個獨立地選自由以下各者組成之群的取代基取代:C1-9烷基及C1-9鹵烷基;R4為雜環基或雜芳基;其中該雜環基或雜芳基視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:-CN、鹵基、-O-R12、-C(O)-R12、-N(R13)(R14)、C1-9烷基、C1-9鹵烷基及雜環基;R5為-CN、鹵基、-O-R7或-S(O)2R7;R6為氫;各R7獨立地為氫或C1-9烷基;其中該C1-9烷基可視情況經一至三個獨立地選自由以下各者組 成之群的取代基取代:羥基、鹵基、-O(C1-9烷基)及芳基;各R12獨立地為氫、C1-9烷基或雜環基;其中該C1-9烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9烷基)及芳基;及各R13及R14獨立地為氫或C1-9烷基;其中該C1-9烷基可視情況經一至三個獨立地選自由以下各者組成之群的取代基取代:羥基、鹵基、-O(C1-9烷基)及芳基。
- 如請求項20之用途,其中Z3為視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的C3-15環烷基:-CN、鹵基、-C(O)-R12、-OC(O)-R12、-C(O)N(R13)(R14)、C1-9烷基、C1-8鹵烷基、C1-8羥烷基、C3-15環烷基及雜芳基。
- 如請求項20之用途,其中Z3為視情況經一至四個獨立地選自由以下各者組成之群之取代基取代的雜環基:-O-R12、-C(O)O-R12、C1-9烷基、C1-8鹵烷基、C1-8羥烷基及雜環基。
- 如請求項19或20之用途,其中R5為氰基或鹵基。
- 如請求項19或20之用途,其中R6為氫。
- 如請求項1、2、4至6、12及19至22中任一項之用途,其中R4為雜環基或雜芳基;且該雜環基或雜芳基視情況經一至三個獨立地選自由以下各 者組成之群的取代基取代:-CN、鹵基、-O-R12、-C(O)-R12、-N(R13)(R14)、C1-9烷基、C1-9鹵烷基及雜環基。
- 如請求項25之用途,其中R4為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜芳基:-CN、鹵基、-O-R12、-C(O)-R12、-N(R13)(R14)、C1-9烷基、C1-9鹵烷基及雜環基。
- 如請求項25之用途,其中R4為視情況經一至三個獨立地選自由以下各者組成之群之取代基取代的雜環基:-CN、鹵基、-O-R12、-C(O)-R12、-N(R13)(R14)、C1-9烷基、C1-9鹵烷基及雜環基。
- 如請求項19之用途,其中各Z4獨立地選自由以下各者組成之群:-CN、鹵基、-O-R12、-C(O)-R12、-N(R13)(R14)、C1-9烷基、C1-9鹵烷基及雜環基。
- 如請求項1、2、4至6、12、19至22、28及32至40中任一項之用途,其中該疾病或病狀係潰瘍性結腸炎。
- 如請求項1、2、4至6、12、19至22、28及32至40中任一項之用途,其中該疾病或病狀係克羅恩氏病。
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562189158P | 2015-07-06 | 2015-07-06 | |
| US62/189,158 | 2015-07-06 | ||
| US201562269060P | 2015-12-17 | 2015-12-17 | |
| US62/269,060 | 2015-12-17 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW202114673A TW202114673A (zh) | 2021-04-16 |
| TWI748539B true TWI748539B (zh) | 2021-12-01 |
Family
ID=56411943
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW109122003A TWI748539B (zh) | 2015-07-06 | 2016-07-05 | Cot調節劑及其使用方法 |
| TW107117905A TWI699361B (zh) | 2015-07-06 | 2016-07-05 | Cot調節劑及其使用方法 |
| TW105121281A TWI634112B (zh) | 2015-07-06 | 2016-07-05 | Cot調節劑及其使用方法 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW107117905A TWI699361B (zh) | 2015-07-06 | 2016-07-05 | Cot調節劑及其使用方法 |
| TW105121281A TWI634112B (zh) | 2015-07-06 | 2016-07-05 | Cot調節劑及其使用方法 |
Country Status (40)
| Country | Link |
|---|---|
| US (7) | US20170008905A1 (zh) |
| EP (3) | EP3896064A1 (zh) |
| JP (6) | JP6430060B2 (zh) |
| KR (5) | KR102073641B1 (zh) |
| CN (3) | CN109879859B (zh) |
| AU (5) | AU2016290820B2 (zh) |
| BR (1) | BR102016015656B1 (zh) |
| CA (1) | CA2971640C (zh) |
| CL (1) | CL2017003356A1 (zh) |
| CO (1) | CO2017013351A2 (zh) |
| CR (1) | CR20170599A (zh) |
| CU (1) | CU20170172A7 (zh) |
| CY (1) | CY1121750T1 (zh) |
| DK (1) | DK3191470T3 (zh) |
| DO (1) | DOP2017000311A (zh) |
| EA (1) | EA036788B1 (zh) |
| EC (1) | ECSP17084635A (zh) |
| ES (2) | ES2734713T3 (zh) |
| HR (1) | HRP20190853T1 (zh) |
| HU (1) | HUE043310T2 (zh) |
| IL (4) | IL293770B2 (zh) |
| LT (1) | LT3191470T (zh) |
| MA (1) | MA39422B1 (zh) |
| ME (1) | ME03425B (zh) |
| MX (1) | MX370984B (zh) |
| MY (1) | MY196173A (zh) |
| NZ (2) | NZ750707A (zh) |
| PE (1) | PE20180462A1 (zh) |
| PH (2) | PH12018500031A1 (zh) |
| PL (2) | PL3456717T3 (zh) |
| PT (2) | PT3456717T (zh) |
| RS (1) | RS58639B1 (zh) |
| SA (1) | SA517381350B1 (zh) |
| SG (1) | SG11201702041PA (zh) |
| SI (2) | SI3456717T1 (zh) |
| SV (1) | SV2017005605A (zh) |
| TW (3) | TWI748539B (zh) |
| UA (1) | UA123010C2 (zh) |
| UY (1) | UY36771A (zh) |
| WO (1) | WO2017007689A1 (zh) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2840095A1 (en) | 2011-07-06 | 2013-01-10 | Gilead Sciences, Inc. | Compounds for the treatment of hiv |
| WO2016057931A1 (en) | 2014-10-10 | 2016-04-14 | The Research Foundation For The State University Of New York | Trifluoromethoxylation of arenes via intramolecular trifluoromethoxy group migration |
| KR20200022527A (ko) | 2015-07-06 | 2020-03-03 | 길리애드 사이언시즈, 인코포레이티드 | Cot 조정제로서의 6-아미노-퀴놀린-3-카르보니트릴 |
| CA2971640C (en) * | 2015-07-06 | 2020-09-22 | Gilead Sciences, Inc. | Cot modulators and methods of use thereof |
| JP6776378B2 (ja) | 2016-06-30 | 2020-10-28 | ギリアード サイエンシーズ, インコーポレイテッド | Cotモジュレーターとしての4,6−ジアミノキナゾリン類およびその使用方法 |
| MA42795B1 (fr) * | 2016-08-19 | 2019-08-30 | Gilead Sciences Inc | Composés thérapeutiques utiles pour le traitement prophylactique ou thérapeutique d'une infection par le virus du vih |
| EA036921B1 (ru) * | 2017-02-10 | 2021-01-15 | Джилид Сайэнс, Инк. | Терапевтические соединения, которые можно применять для профилактического или терапевтического лечения инфекции вирусом вич |
| CN108794516A (zh) * | 2017-04-26 | 2018-11-13 | 上海时莱生物技术有限公司 | 硼酸和硼酸酯类化合物及其制备方法和用途 |
| AR112413A1 (es) | 2017-08-17 | 2019-10-23 | Gilead Sciences Inc | Formas sólidas de un inhibidor de la cápside del vih |
| AR112412A1 (es) | 2017-08-17 | 2019-10-23 | Gilead Sciences Inc | Formas de sal de colina de un inhibidor de la cápside del vih |
| WO2019065516A1 (ja) * | 2017-09-26 | 2019-04-04 | 日本曹達株式会社 | キノリン化合物および農園芸用殺菌剤 |
| JP7083398B2 (ja) | 2018-02-15 | 2022-06-10 | ギリアード サイエンシーズ, インコーポレイテッド | ピリジン誘導体およびhiv感染を処置するためのその使用 |
| CN116854630A (zh) | 2018-02-16 | 2023-10-10 | 吉利德科学公司 | 用于制备可用于治疗逆转录病毒科病毒感染的治疗性化合物的方法和中间体 |
| WO2019168874A1 (en) | 2018-02-27 | 2019-09-06 | The Research Foundation For The State University Of New York | Difluoromethoxylation and trifluoromethoxylation compositions and methods for synthesizing same |
| CN110294660B (zh) * | 2018-03-23 | 2021-09-24 | 中国农业大学 | 一种含碳碳三键和/或碳氮三键的不饱和有机化合物的还原氘化方法 |
| CN108658996A (zh) * | 2018-06-25 | 2018-10-16 | 中国药科大学 | 一种氘代Fiduxosin的制备方法 |
| CA3103522C (en) | 2018-07-16 | 2023-11-21 | Gilead Sciences, Inc. | Capsid inhibitors for the treatment of hiv |
| TW202235416A (zh) * | 2019-06-14 | 2022-09-16 | 美商基利科學股份有限公司 | Cot 調節劑及其使用方法 |
| PL4021904T3 (pl) | 2019-08-29 | 2024-05-13 | Idorsia Pharmaceuticals Ltd | Pochodne alfa-d-galaktopiranozydu |
| CN114727999A (zh) | 2019-11-26 | 2022-07-08 | 吉利德科学公司 | 用于预防hiv的衣壳抑制剂 |
| US11655237B2 (en) | 2020-03-30 | 2023-05-23 | Gilead Sciences, Inc. | Solid forms of a Cot inhibitor compound |
| US11845737B2 (en) | 2020-04-02 | 2023-12-19 | Gilead Sciences, Inc. | Process for preparing a Cot inhibitor compound |
| AU2021296607B2 (en) | 2020-06-25 | 2024-07-25 | Gilead Sciences, Inc. | Capsid inhibitors for the treatment of HIV |
| MX2023010241A (es) | 2021-03-03 | 2023-09-12 | Idorsia Pharmaceuticals Ltd | Derivados de alfa-d-galatopiranosido triazolil-metil sustituidos. |
| TW202304435A (zh) * | 2021-06-04 | 2023-02-01 | 美商基利科學股份有限公司 | 治療nash之方法 |
| JP2024521965A (ja) * | 2021-06-11 | 2024-06-04 | ニューロナセント インコーポレイテッド | 複素環タイプの親油性低分子治療薬の脂質製剤のための方法および組成物 |
| TW202342448A (zh) | 2021-12-03 | 2023-11-01 | 美商基利科學股份有限公司 | 用於hiv病毒感染之治療性化合物 |
| CA3235937A1 (en) | 2021-12-03 | 2023-06-08 | Gilead Sciences, Inc. | Therapeutic compounds for hiv virus infection |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998043960A1 (en) * | 1997-04-03 | 1998-10-08 | American Cyanamid Company | Substituted 3-cyano quinolines |
Family Cites Families (68)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3930837A (en) | 1971-12-31 | 1976-01-06 | Ici Australia Limited | 3-chloro-5-acetamidaisoquinoline as a herbicide |
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US4151298A (en) | 1977-01-10 | 1979-04-24 | Ciba-Geigy Corporation | Anthelmintic compositions |
| US4326525A (en) | 1980-10-14 | 1982-04-27 | Alza Corporation | Osmotic device that improves delivery properties of agent in situ |
| US5364620A (en) | 1983-12-22 | 1994-11-15 | Elan Corporation, Plc | Controlled absorption diltiazem formulation for once daily administration |
| US5023252A (en) | 1985-12-04 | 1991-06-11 | Conrex Pharmaceutical Corporation | Transdermal and trans-membrane delivery of drugs |
| US4992445A (en) | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5001139A (en) | 1987-06-12 | 1991-03-19 | American Cyanamid Company | Enchancers for the transdermal flux of nivadipine |
| US4902514A (en) | 1988-07-21 | 1990-02-20 | Alza Corporation | Dosage form for administering nilvadipine for treating cardiovascular symptoms |
| JP2634438B2 (ja) | 1988-07-26 | 1997-07-23 | 三井東圧化学株式会社 | 不斉ビスオキサゾリルピリジン誘導体およびその製造方法 |
| DE4014171A1 (de) | 1990-05-03 | 1991-11-07 | Basf Ag | Cyanochinolinverbindungen |
| GB9310700D0 (en) | 1993-05-24 | 1993-07-07 | Zeneca Ltd | Novel composition |
| AU734678B2 (en) | 1997-08-28 | 2001-06-21 | Nissan Chemical Industries Ltd. | Industrial antibacterial and antifungal agents, algicides and agents for preventing adhesion of organisms containing a cyanoacrylate compound |
| ATE360634T1 (de) | 1998-07-10 | 2007-05-15 | Massachusetts Inst Technology | Ligande für metalle und verbesserte metall- katalysierte verfahren, die darauf basieren |
| BRPI9914164B8 (pt) | 1998-09-29 | 2021-05-25 | American Cyanamid Co | compostos de 3-ciano quinolina |
| GEP20074230B (en) | 2002-03-20 | 2007-11-12 | Bristol Myers Squibb Co | Phosphate prodrugs of fluorooxindoles |
| ES2297386T3 (es) | 2003-03-03 | 2008-05-01 | F. Hoffmann-La Roche Ag | Tetrahidroisoquinolinas 2,5-sustituidas para uso como moduladores de 5-ht6. |
| CA2535385A1 (en) | 2003-08-19 | 2005-03-03 | Wyeth Holdings Corporation | Process for the preparation of 4-amino-3-quinolinecarbonitriles |
| US7399865B2 (en) | 2003-09-15 | 2008-07-15 | Wyeth | Protein tyrosine kinase enzyme inhibitors |
| TW200529846A (en) * | 2004-02-20 | 2005-09-16 | Wyeth Corp | 3-quinolinecarbonitrile protein kinase inhibitors |
| GB0420722D0 (en) | 2004-09-17 | 2004-10-20 | Addex Pharmaceuticals Sa | Novel allosteric modulators |
| MX2007014258A (es) | 2005-05-18 | 2008-01-22 | Wyeth Corp | Inhibidores de 4,6-diamino-[1,7]naftiridin-3-carbonitrilo de la tpl2 cinasa y metodos de fabricacion y uso de los mismos. |
| AU2006247520A1 (en) * | 2005-05-18 | 2006-11-23 | Wyeth | 3-cyanoquinoline inhibitors of TPL2 kinase and methods of making and using the same |
| CN101365684A (zh) | 2005-10-28 | 2009-02-11 | 艾博特公司 | 抑制trpv1受体的吲唑衍生物 |
| WO2007117465A2 (en) | 2006-03-31 | 2007-10-18 | Abbott Laboratories | Indazole compounds |
| EP2086960B1 (en) | 2006-11-09 | 2014-03-05 | Probiodrug AG | Novel inhibitors of glutaminyl cyclase |
| CN101583601B (zh) | 2007-01-17 | 2013-06-05 | 香港科技大学 | 作为褪黑素受体mt1和mt2的亚型选择性激动剂的异喹诺酮化合物 |
| GB0820856D0 (en) | 2008-11-14 | 2008-12-24 | Univ Leuven Kath | Novel inhibitors of flavivirus replication |
| TWI598347B (zh) | 2009-07-13 | 2017-09-11 | 基利科學股份有限公司 | 調節細胞凋亡信號之激酶的抑制劑 |
| GB0922302D0 (en) * | 2009-12-22 | 2010-02-03 | Imp Innovations Ltd | Compounds |
| ES2714875T3 (es) | 2010-03-09 | 2019-05-30 | Dana Farber Cancer Inst Inc | Métodos de diagnóstico y tratamiento del cáncer en pacientes que presentan o desarrollan resistencia a una primera terapia del cáncer |
| US9187458B2 (en) * | 2010-06-09 | 2015-11-17 | Tianjin Hemay Bio-Tech Co., Ltd. | Cyanoquinoline derivatives |
| US9453021B2 (en) | 2011-05-10 | 2016-09-27 | Kyowa Hakko Kirin Co., Ltd. | Pyrimidodiazepinone compound |
| US9173395B2 (en) | 2011-07-04 | 2015-11-03 | Bayer Intellectual Property Gmbh | Use of substituted isoquinolinones, isoquinolindiones, isoquinolintriones and dihydroisoquinolinones or in each case salts thereof as active agents against abiotic stress in plants |
| EP2545964A1 (en) | 2011-07-13 | 2013-01-16 | Phenex Pharmaceuticals AG | Novel FXR (NR1H4) binding and activity modulating compounds |
| MX339763B (es) | 2011-10-27 | 2016-06-07 | Taisho Pharmaceutical Co Ltd | Derivado de azol. |
| CA2855372C (en) | 2011-11-11 | 2022-03-22 | Nimbus Apollo, Inc. | 2,4-dioxo-thieno[2,3-d]pyrimidinyl derivatives and pharmaceutical compositions thereof used as acc inhibitors |
| UY34573A (es) | 2012-01-27 | 2013-06-28 | Gilead Sciences Inc | Inhibidor de la quinasa que regula la señal de la apoptosis |
| CN103483363B (zh) | 2012-06-13 | 2016-12-21 | 上海赛迦化工产品有限公司 | 多样性的手性氨基硼酸及其制备方法和应用 |
| CN105142621A (zh) | 2012-10-24 | 2015-12-09 | 国家健康科学研究所 | 用于预防或治疗糖尿病和促进β-细胞存活的TPL2激酶抑制剂 |
| CN103408572B (zh) | 2013-07-12 | 2015-12-02 | 上海工程技术大学 | 手性氨基硼酸衍生物及其制备方法和应用 |
| KR102380609B1 (ko) | 2013-12-12 | 2022-03-30 | 리커리엄 아이피 홀딩스, 엘엘씨 | 바이사이클릭 알킬 화합물 및 합성 |
| WO2015134710A1 (en) | 2014-03-07 | 2015-09-11 | Kalyra Pharmaceuticals, Inc. | Propellane derivates and synthesis |
| WO2016007966A2 (en) * | 2014-07-11 | 2016-01-14 | Northwestern University | 2-imidazolyl-pyrimidine scaffolds as potent and selective inhibitors of neuronal nitric oxide synthase |
| EP3193855B1 (en) | 2014-09-17 | 2021-05-19 | Recurium IP Holdings, LLC | Bicyclic compounds |
| ES2731602T3 (es) | 2014-09-24 | 2019-11-18 | Gilead Sciences Inc | Métodos para tratar la enfermedad hepática |
| PL3237404T3 (pl) | 2014-12-23 | 2021-05-04 | Gilead Sciences, Inc. | Procesy wytwarzania inhibitorów ask1 |
| MA41252A (fr) | 2014-12-23 | 2017-10-31 | Gilead Sciences Inc | Formes solides d'un inhibiteur d'ask 1 |
| JP2018501276A (ja) | 2015-01-09 | 2018-01-18 | ギリアド アポロ, エルエルシー | 非アルコール性脂肪肝疾患の処置のためのacc阻害剤併用療法 |
| CN107111844B (zh) | 2015-01-16 | 2021-07-13 | 3M创新有限公司 | 用于选择网格动作以改善网格结果的系统和方法 |
| KR20200022527A (ko) | 2015-07-06 | 2020-03-03 | 길리애드 사이언시즈, 인코포레이티드 | Cot 조정제로서의 6-아미노-퀴놀린-3-카르보니트릴 |
| CA2971640C (en) | 2015-07-06 | 2020-09-22 | Gilead Sciences, Inc. | Cot modulators and methods of use thereof |
| JP6804540B2 (ja) | 2015-12-31 | 2020-12-23 | 成都先導薬物開発股▲フン▼有限公司Hitgen Inc. | スルファミド誘導体およびその製造方法と応用 |
| KR102700008B1 (ko) | 2016-03-02 | 2024-08-29 | 길리어드 아폴로, 엘엘씨 | 티에노피리미딘디온 acc 억제제의 고체 형태 및 그의 제조 방법 |
| WO2017221944A1 (ja) | 2016-06-21 | 2017-12-28 | パナソニックヘルスケアホールディングス株式会社 | カタラーゼ阻害剤及びカタラーゼ阻害剤を用いるアナライトの測定方法 |
| JP6776378B2 (ja) | 2016-06-30 | 2020-10-28 | ギリアード サイエンシーズ, インコーポレイテッド | Cotモジュレーターとしての4,6−ジアミノキナゾリン類およびその使用方法 |
| CN106512014A (zh) | 2016-10-27 | 2017-03-22 | 武汉大学 | 肿瘤进展位点2在治疗脂肪肝和ⅱ型糖尿病中的功能和应用 |
| WO2018161022A1 (en) | 2017-03-03 | 2018-09-07 | Gilead Sciences, Inc. | Processes for preparing acc inhibitors and solid forms thereof |
| EP3600309B1 (en) | 2017-03-28 | 2022-06-22 | Gilead Sciences, Inc. | Therapeutic combinations for treating liver diseases |
| EP3609496A1 (en) | 2017-04-12 | 2020-02-19 | Gilead Sciences, Inc. | Methods of treating liver disease |
| AU2018345817B2 (en) | 2017-10-06 | 2021-10-28 | Gilead Sciences, Inc. | Combination therapy comprising an ACC inhibitor |
| CN118388473A (zh) | 2019-02-19 | 2024-07-26 | 吉利德科学公司 | Fxr激动剂的固体形式 |
| US20220143153A1 (en) | 2019-03-08 | 2022-05-12 | The Regents Of The University Of California | Compositions and methods for treating acne |
| TW202235416A (zh) | 2019-06-14 | 2022-09-16 | 美商基利科學股份有限公司 | Cot 調節劑及其使用方法 |
| AR119594A1 (es) | 2019-08-09 | 2021-12-29 | Gilead Sciences Inc | Derivados de tienopirimidina como inhibidores acc y usos de los mismos |
| US11655237B2 (en) | 2020-03-30 | 2023-05-23 | Gilead Sciences, Inc. | Solid forms of a Cot inhibitor compound |
| US11845737B2 (en) | 2020-04-02 | 2023-12-19 | Gilead Sciences, Inc. | Process for preparing a Cot inhibitor compound |
| TW202304435A (zh) | 2021-06-04 | 2023-02-01 | 美商基利科學股份有限公司 | 治療nash之方法 |
-
2016
- 2016-06-30 CA CA2971640A patent/CA2971640C/en active Active
- 2016-06-30 KR KR1020197011792A patent/KR102073641B1/ko active IP Right Grant
- 2016-06-30 EP EP21162393.9A patent/EP3896064A1/en active Pending
- 2016-06-30 PL PL18186568T patent/PL3456717T3/pl unknown
- 2016-06-30 CN CN201910292757.5A patent/CN109879859B/zh active Active
- 2016-06-30 MX MX2017004737A patent/MX370984B/es active IP Right Grant
- 2016-06-30 RS RS20190498A patent/RS58639B1/sr unknown
- 2016-06-30 CU CUP2017000172A patent/CU20170172A7/xx unknown
- 2016-06-30 AU AU2016290820A patent/AU2016290820B2/en active Active
- 2016-06-30 PT PT181865684T patent/PT3456717T/pt unknown
- 2016-06-30 MA MA39422A patent/MA39422B1/fr unknown
- 2016-06-30 WO PCT/US2016/040520 patent/WO2017007689A1/en active Application Filing
- 2016-06-30 PL PL16738976T patent/PL3191470T3/pl unknown
- 2016-06-30 KR KR1020187003221A patent/KR101974793B1/ko active IP Right Grant
- 2016-06-30 SG SG11201702041PA patent/SG11201702041PA/en unknown
- 2016-06-30 ES ES16738976T patent/ES2734713T3/es active Active
- 2016-06-30 PT PT16738976T patent/PT3191470T/pt unknown
- 2016-06-30 EA EA201792613A patent/EA036788B1/ru unknown
- 2016-06-30 JP JP2018500585A patent/JP6430060B2/ja active Active
- 2016-06-30 CN CN201680050602.5A patent/CN107922390B/zh active Active
- 2016-06-30 IL IL293770A patent/IL293770B2/en unknown
- 2016-06-30 NZ NZ750707A patent/NZ750707A/en unknown
- 2016-06-30 EP EP18186568.4A patent/EP3456717B1/en active Active
- 2016-06-30 EP EP16738976.6A patent/EP3191470B1/en active Active
- 2016-06-30 CN CN202210008753.1A patent/CN114380799B/zh active Active
- 2016-06-30 KR KR1020247000058A patent/KR20240008398A/ko not_active Application Discontinuation
- 2016-06-30 UA UAA201712984A patent/UA123010C2/uk unknown
- 2016-06-30 KR KR1020227031078A patent/KR20220129667A/ko not_active Application Discontinuation
- 2016-06-30 HU HUE16738976A patent/HUE043310T2/hu unknown
- 2016-06-30 CR CR20170599A patent/CR20170599A/es unknown
- 2016-06-30 DK DK16738976.6T patent/DK3191470T3/en active
- 2016-06-30 PE PE2017002804A patent/PE20180462A1/es unknown
- 2016-06-30 US US15/199,534 patent/US20170008905A1/en not_active Abandoned
- 2016-06-30 LT LTEP16738976.6T patent/LT3191470T/lt unknown
- 2016-06-30 MY MYPI2018700007A patent/MY196173A/en unknown
- 2016-06-30 IL IL274568A patent/IL274568B/en unknown
- 2016-06-30 ES ES18186568T patent/ES2872076T3/es active Active
- 2016-06-30 SI SI201631197T patent/SI3456717T1/sl unknown
- 2016-06-30 ME MEP-2019-117A patent/ME03425B/me unknown
- 2016-06-30 KR KR1020207002878A patent/KR102443575B1/ko active IP Right Grant
- 2016-06-30 NZ NZ738525A patent/NZ738525A/en unknown
- 2016-06-30 SI SI201630202T patent/SI3191470T1/sl unknown
- 2016-07-04 BR BR102016015656-4A patent/BR102016015656B1/pt active IP Right Grant
- 2016-07-05 TW TW109122003A patent/TWI748539B/zh active
- 2016-07-05 TW TW107117905A patent/TWI699361B/zh active
- 2016-07-05 TW TW105121281A patent/TWI634112B/zh active
- 2016-07-06 UY UY0001036771A patent/UY36771A/es not_active Application Discontinuation
-
2017
- 2017-02-09 US US15/429,086 patent/US9878995B2/en active Active
- 2017-04-18 SA SA517381350A patent/SA517381350B1/ar unknown
- 2017-12-20 IL IL256433A patent/IL256433B/en active IP Right Grant
- 2017-12-22 CO CONC2017/0013351A patent/CO2017013351A2/es unknown
- 2017-12-22 CL CL2017003356A patent/CL2017003356A1/es unknown
- 2017-12-22 SV SV2017005605A patent/SV2017005605A/es unknown
- 2017-12-22 EC ECIEPI201784635A patent/ECSP17084635A/es unknown
- 2017-12-26 DO DO2017000311A patent/DOP2017000311A/es unknown
-
2018
- 2018-01-03 PH PH12018500031A patent/PH12018500031A1/en unknown
- 2018-02-07 US US15/891,163 patent/US20180237455A1/en not_active Abandoned
- 2018-09-13 JP JP2018171794A patent/JP6781221B2/ja active Active
-
2019
- 2019-04-23 US US16/391,673 patent/US20190248807A1/en not_active Abandoned
- 2019-05-03 AU AU2019203122A patent/AU2019203122B2/en active Active
- 2019-05-08 HR HRP20190853TT patent/HRP20190853T1/hr unknown
- 2019-05-20 CY CY20191100535T patent/CY1121750T1/el unknown
- 2019-05-29 IL IL266995A patent/IL266995B/en active IP Right Grant
- 2019-07-26 JP JP2019137759A patent/JP6906021B2/ja active Active
- 2019-12-17 US US16/717,074 patent/US11066414B2/en active Active
-
2020
- 2020-09-06 PH PH12020551397A patent/PH12020551397A1/en unknown
- 2020-10-20 AU AU2020257055A patent/AU2020257055B2/en active Active
- 2020-12-16 JP JP2020208330A patent/JP7138155B2/ja active Active
-
2021
- 2021-05-11 US US17/317,041 patent/US11905299B2/en active Active
-
2022
- 2022-04-11 JP JP2022065168A patent/JP2022082816A/ja not_active Withdrawn
- 2022-06-10 AU AU2022204050A patent/AU2022204050B2/en active Active
-
2023
- 2023-12-11 US US18/534,800 patent/US20240254137A1/en active Pending
-
2024
- 2024-05-17 JP JP2024081151A patent/JP2024100865A/ja active Pending
- 2024-07-17 AU AU2024204902A patent/AU2024204902A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998043960A1 (en) * | 1997-04-03 | 1998-10-08 | American Cyanamid Company | Substituted 3-cyano quinolines |
Non-Patent Citations (5)
| Title |
|---|
| Green et al, J. Med. Chem., 2007,50, p4728-4745 * |
| Kaila et al, Bioorganic & Medicinal Chemistry, 2007, 15, p6425-6442 |
| Kaila et al, Bioorganic & Medicinal Chemistry, 2007, 15, p6425-6442; * |
| Wu et al, Bioorganic & Medicinal Chemistry Letters, 2009, 19, p3485-3488 |
| Wu et al, Bioorganic & Medicinal Chemistry Letters, 2009, 19, p3485-3488; * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11905299B2 (en) | Cot modulators and methods of use thereof | |
| US10316017B2 (en) | COT modulators and methods of use thereof | |
| OA19638A (en) | Cot modulators and methods of use thereof. |