KR102279196B1 - An electroluminescent compound and an electroluminescent device comprising the same - Google Patents
An electroluminescent compound and an electroluminescent device comprising the same Download PDFInfo
- Publication number
- KR102279196B1 KR102279196B1 KR1020160163809A KR20160163809A KR102279196B1 KR 102279196 B1 KR102279196 B1 KR 102279196B1 KR 1020160163809 A KR1020160163809 A KR 1020160163809A KR 20160163809 A KR20160163809 A KR 20160163809A KR 102279196 B1 KR102279196 B1 KR 102279196B1
- Authority
- KR
- South Korea
- Prior art keywords
- group
- mol
- substituted
- light emitting
- layer
- Prior art date
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 54
- 230000000903 blocking effect Effects 0.000 claims abstract description 21
- -1 dibenzofuranyl group Chemical group 0.000 claims description 46
- 239000011368 organic material Substances 0.000 claims description 36
- 238000002347 injection Methods 0.000 claims description 20
- 239000007924 injection Substances 0.000 claims description 20
- 125000001424 substituent group Chemical group 0.000 claims description 17
- 125000004432 carbon atom Chemical group C* 0.000 claims description 14
- 125000003118 aryl group Chemical group 0.000 claims description 13
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 claims description 12
- 230000005525 hole transport Effects 0.000 claims description 11
- 125000000623 heterocyclic group Chemical group 0.000 claims description 7
- 238000000034 method Methods 0.000 claims description 7
- 125000001072 heteroaryl group Chemical group 0.000 claims description 6
- 125000000217 alkyl group Chemical group 0.000 claims description 5
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 claims description 5
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 239000001257 hydrogen Substances 0.000 claims description 4
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 4
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 claims description 3
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 125000005549 heteroarylene group Chemical group 0.000 claims description 3
- 125000001624 naphthyl group Chemical group 0.000 claims description 3
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 claims description 3
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 claims description 3
- 229910052717 sulfur Inorganic materials 0.000 claims description 3
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 2
- TXCDCPKCNAJMEE-UHFFFAOYSA-N Dibenzofuran Natural products C1=CC=C2C3=CC=CC=C3OC2=C1 TXCDCPKCNAJMEE-UHFFFAOYSA-N 0.000 claims description 2
- 125000000732 arylene group Chemical group 0.000 claims description 2
- 125000005566 carbazolylene group Chemical group 0.000 claims description 2
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 2
- 229910052805 deuterium Inorganic materials 0.000 claims description 2
- 125000005509 dibenzothiophenyl group Chemical group 0.000 claims description 2
- 125000005567 fluorenylene group Chemical group 0.000 claims description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 2
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 2
- 125000004434 sulfur atom Chemical group 0.000 claims description 2
- 150000004826 dibenzofurans Chemical class 0.000 claims 1
- 125000001183 hydrocarbyl group Chemical group 0.000 claims 1
- 125000003003 spiro group Chemical group 0.000 claims 1
- 239000010410 layer Substances 0.000 description 129
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 93
- 238000006243 chemical reaction Methods 0.000 description 83
- 230000015572 biosynthetic process Effects 0.000 description 80
- 238000003786 synthesis reaction Methods 0.000 description 80
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 56
- 238000000746 purification Methods 0.000 description 49
- 238000000926 separation method Methods 0.000 description 45
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 42
- 239000000463 material Substances 0.000 description 39
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 34
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 26
- 239000003054 catalyst Substances 0.000 description 23
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 21
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 21
- 238000003756 stirring Methods 0.000 description 21
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 20
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 18
- 229910000027 potassium carbonate Inorganic materials 0.000 description 17
- 238000010992 reflux Methods 0.000 description 16
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 15
- 238000005160 1H NMR spectroscopy Methods 0.000 description 13
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 13
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 10
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 8
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 8
- DMVOXQPQNTYEKQ-UHFFFAOYSA-N biphenyl-4-amine Chemical group C1=CC(N)=CC=C1C1=CC=CC=C1 DMVOXQPQNTYEKQ-UHFFFAOYSA-N 0.000 description 8
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 5
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 5
- GUTJITRKAMCHSD-UHFFFAOYSA-N 9,9-dimethylfluoren-2-amine Chemical compound C1=C(N)C=C2C(C)(C)C3=CC=CC=C3C2=C1 GUTJITRKAMCHSD-UHFFFAOYSA-N 0.000 description 5
- 101150003085 Pdcl gene Proteins 0.000 description 5
- 235000011056 potassium acetate Nutrition 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- QCSLIRFWJPOENV-UHFFFAOYSA-N (2-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=CC=C1F QCSLIRFWJPOENV-UHFFFAOYSA-N 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 4
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 238000000151 deposition Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 3
- OOKAZRDERJMRCJ-KOUAFAAESA-N (3r)-7-[(1s,2s,4ar,6s,8s)-2,6-dimethyl-8-[(2s)-2-methylbutanoyl]oxy-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl]-3-hydroxy-5-oxoheptanoic acid Chemical compound C1=C[C@H](C)[C@H](CCC(=O)C[C@@H](O)CC(O)=O)C2[C@@H](OC(=O)[C@@H](C)CC)C[C@@H](C)C[C@@H]21 OOKAZRDERJMRCJ-KOUAFAAESA-N 0.000 description 3
- 0 *c1ccccc1 Chemical compound *c1ccccc1 0.000 description 3
- UCCUXODGPMAHRL-UHFFFAOYSA-N 1-bromo-4-iodobenzene Chemical compound BrC1=CC=C(I)C=C1 UCCUXODGPMAHRL-UHFFFAOYSA-N 0.000 description 3
- PEAOEILWTHNQKK-UHFFFAOYSA-N 2-bromo-4-iodophenol Chemical compound OC1=CC=C(I)C=C1Br PEAOEILWTHNQKK-UHFFFAOYSA-N 0.000 description 3
- VKLKXFOZNHEBSW-UHFFFAOYSA-N 5-[[3-[(4-morpholin-4-ylbenzoyl)amino]phenyl]methoxy]pyridine-3-carboxamide Chemical compound O1CCN(CC1)C1=CC=C(C(=O)NC=2C=C(COC=3C=NC=C(C(=O)N)C=3)C=CC=2)C=C1 VKLKXFOZNHEBSW-UHFFFAOYSA-N 0.000 description 3
- XFJBGINZIMNZBW-CRAIPNDOSA-N 5-chloro-2-[4-[(1r,2s)-2-[2-(5-methylsulfonylpyridin-2-yl)oxyethyl]cyclopropyl]piperidin-1-yl]pyrimidine Chemical compound N1=CC(S(=O)(=O)C)=CC=C1OCC[C@H]1[C@@H](C2CCN(CC2)C=2N=CC(Cl)=CN=2)C1 XFJBGINZIMNZBW-CRAIPNDOSA-N 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical group C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 3
- LJOOWESTVASNOG-UFJKPHDISA-N [(1s,3r,4ar,7s,8s,8as)-3-hydroxy-8-[2-[(4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl] (2s)-2-methylbutanoate Chemical compound C([C@H]1[C@@H](C)C=C[C@H]2C[C@@H](O)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)CC1C[C@@H](O)CC(=O)O1 LJOOWESTVASNOG-UFJKPHDISA-N 0.000 description 3
- 125000003545 alkoxy group Chemical group 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 239000010405 anode material Substances 0.000 description 3
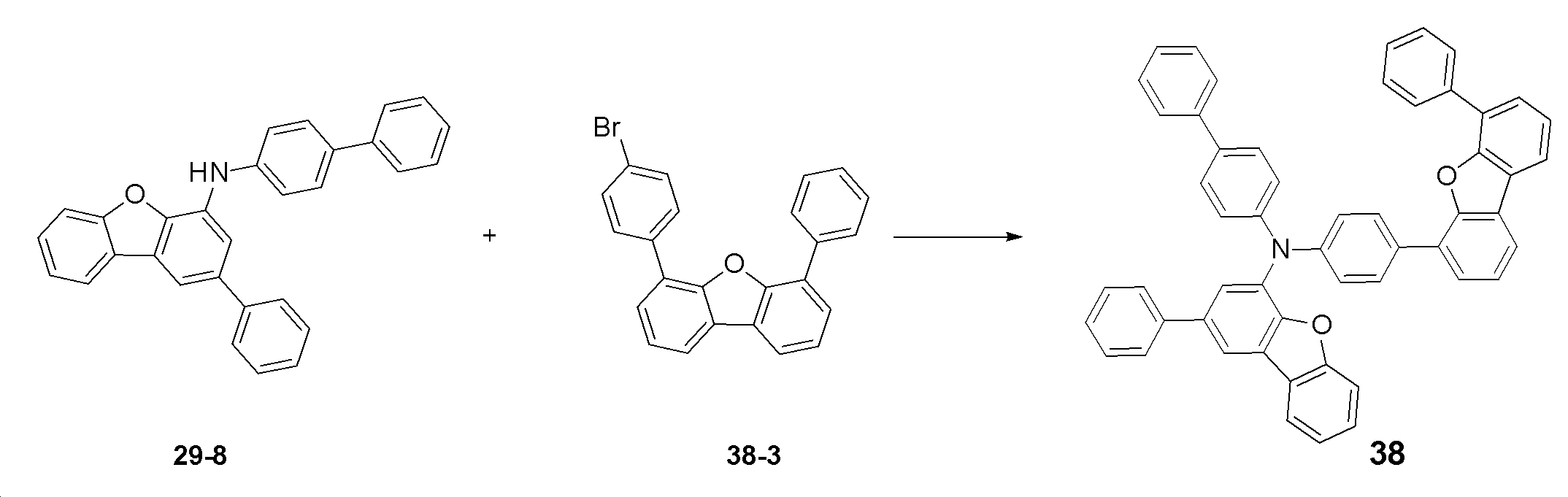
- 229940127204 compound 29 Drugs 0.000 description 3
- 229940127573 compound 38 Drugs 0.000 description 3
- 229920001940 conductive polymer Polymers 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 238000004770 highest occupied molecular orbital Methods 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 3
- 239000012044 organic layer Substances 0.000 description 3
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 3
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical group C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 2
- PKJBWOWQJHHAHG-UHFFFAOYSA-N 1-bromo-4-phenylbenzene Chemical group C1=CC(Br)=CC=C1C1=CC=CC=C1 PKJBWOWQJHHAHG-UHFFFAOYSA-N 0.000 description 2
- MYKQKWIPLZEVOW-UHFFFAOYSA-N 11h-benzo[a]carbazole Chemical group C1=CC2=CC=CC=C2C2=C1C1=CC=CC=C1N2 MYKQKWIPLZEVOW-UHFFFAOYSA-N 0.000 description 2
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical group C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 2
- MBHPOBSZPYEADG-UHFFFAOYSA-N 2-bromo-9,9-dimethylfluorene Chemical compound C1=C(Br)C=C2C(C)(C)C3=CC=CC=C3C2=C1 MBHPOBSZPYEADG-UHFFFAOYSA-N 0.000 description 2
- 125000006176 2-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(C([H])([H])*)C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000005916 2-methylpentyl group Chemical group 0.000 description 2
- LTBWKAYPXIIVPC-UHFFFAOYSA-N 3-bromo-9h-carbazole Chemical compound C1=CC=C2C3=CC(Br)=CC=C3NC2=C1 LTBWKAYPXIIVPC-UHFFFAOYSA-N 0.000 description 2
- UQEANKGXXSENNF-UHFFFAOYSA-N 4-bromo-1-fluoro-2-nitrobenzene Chemical compound [O-][N+](=O)C1=CC(Br)=CC=C1F UQEANKGXXSENNF-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 2
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 2
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical group C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 2
- 150000004982 aromatic amines Chemical class 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 125000006267 biphenyl group Chemical group 0.000 description 2
- 239000010406 cathode material Substances 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- IBHBKWKFFTZAHE-UHFFFAOYSA-N n-[4-[4-(n-naphthalen-1-ylanilino)phenyl]phenyl]-n-phenylnaphthalen-1-amine Chemical compound C1=CC=CC=C1N(C=1C2=CC=CC=C2C=CC=1)C1=CC=C(C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C3=CC=CC=C3C=CC=2)C=C1 IBHBKWKFFTZAHE-UHFFFAOYSA-N 0.000 description 2
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 125000002971 oxazolyl group Chemical group 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 238000005240 physical vapour deposition Methods 0.000 description 2
- 229920000553 poly(phenylenevinylene) Polymers 0.000 description 2
- 229920000767 polyaniline Polymers 0.000 description 2
- 125000003367 polycyclic group Chemical group 0.000 description 2
- 125000001725 pyrenyl group Chemical group 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 125000001973 tert-pentyl group Chemical group [H]C([H])([H])C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 2
- 125000001425 triazolyl group Chemical group 0.000 description 2
- 239000011787 zinc oxide Substances 0.000 description 2
- XPEIJWZLPWNNOK-UHFFFAOYSA-N (4-phenylphenyl)boronic acid Chemical compound C1=CC(B(O)O)=CC=C1C1=CC=CC=C1 XPEIJWZLPWNNOK-UHFFFAOYSA-N 0.000 description 1
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 1
- 125000000355 1,3-benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical group C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000006218 1-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006023 1-pentenyl group Chemical group 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- USMQLFCVCDEXAK-UHFFFAOYSA-N 2-bromo-4-chlorobenzoic acid Chemical compound OC(=O)C1=CC=C(Cl)C=C1Br USMQLFCVCDEXAK-UHFFFAOYSA-N 0.000 description 1
- YSXQTEHREKNJPU-UHFFFAOYSA-N 2-bromo-4-iodobenzenethiol Chemical compound Sc1ccc(I)cc1Br YSXQTEHREKNJPU-UHFFFAOYSA-N 0.000 description 1
- CRJISNQTZDMKQD-UHFFFAOYSA-N 2-bromodibenzofuran Chemical compound C1=CC=C2C3=CC(Br)=CC=C3OC2=C1 CRJISNQTZDMKQD-UHFFFAOYSA-N 0.000 description 1
- VADKRMSMGWJZCF-UHFFFAOYSA-N 2-bromophenol Chemical compound OC1=CC=CC=C1Br VADKRMSMGWJZCF-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000006024 2-pentenyl group Chemical group 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000006607 3,3-dimethylbutyloxy group Chemical group 0.000 description 1
- DMEVMYSQZPJFOK-UHFFFAOYSA-N 3,4,5,6,9,10-hexazatetracyclo[12.4.0.02,7.08,13]octadeca-1(18),2(7),3,5,8(13),9,11,14,16-nonaene Chemical group N1=NN=C2C3=CC=CC=C3C3=CC=NN=C3C2=N1 DMEVMYSQZPJFOK-UHFFFAOYSA-N 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000006027 3-methyl-1-butenyl group Chemical group 0.000 description 1
- XSJLDNSNUICSQC-UHFFFAOYSA-N 4,6-dibromodibenzofuran Chemical compound O1C2=C(Br)C=CC=C2C2=C1C(Br)=CC=C2 XSJLDNSNUICSQC-UHFFFAOYSA-N 0.000 description 1
- 125000004920 4-methyl-2-pentyl group Chemical group CC(CC(C)*)C 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 239000005725 8-Hydroxyquinoline Substances 0.000 description 1
- ROFVEXUMMXZLPA-UHFFFAOYSA-N Bipyridyl Chemical group N1=CC=CC=C1C1=CC=CC=N1 ROFVEXUMMXZLPA-UHFFFAOYSA-N 0.000 description 1
- WLCYPHBFQRFXEJ-UHFFFAOYSA-N Brc(cc1)ccc1-c1cccc2c1[o]c1c2cccc1-c1ccccc1 Chemical compound Brc(cc1)ccc1-c1cccc2c1[o]c1c2cccc1-c1ccccc1 WLCYPHBFQRFXEJ-UHFFFAOYSA-N 0.000 description 1
- KUBSCXXKQGDPPD-UHFFFAOYSA-N Brc(cc1c2c3cccc2)ccc1[n]3-c1ccccc1 Chemical compound Brc(cc1c2c3cccc2)ccc1[n]3-c1ccccc1 KUBSCXXKQGDPPD-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- XWLUUAONZCGUDG-UHFFFAOYSA-N C=[Br]c(cc1)ccc1-c1c2[o]c(cccc3)c3c2cc(-c2ccccc2)c1 Chemical compound C=[Br]c(cc1)ccc1-c1c2[o]c(cccc3)c3c2cc(-c2ccccc2)c1 XWLUUAONZCGUDG-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- MTIDYGLTAOZOGU-UHFFFAOYSA-N Cc(cc1Br)ccc1O Chemical compound Cc(cc1Br)ccc1O MTIDYGLTAOZOGU-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- PNRASSPCKQKOQT-UHFFFAOYSA-N Oc(c(Br)c1)ccc1-c(cc1)ccc1-c1ccccc1 Chemical compound Oc(c(Br)c1)ccc1-c(cc1)ccc1-c1ccccc1 PNRASSPCKQKOQT-UHFFFAOYSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- NRCMAYZCPIVABH-UHFFFAOYSA-N Quinacridone Chemical compound N1C2=CC=CC=C2C(=O)C2=C1C=C1C(=O)C3=CC=CC=C3NC1=C2 NRCMAYZCPIVABH-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229910006404 SnO 2 Inorganic materials 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- FCHZACFMGHJGEE-UHFFFAOYSA-N [O-][N+](c1cc(-c2ccccc2)ccc1F)=O Chemical compound [O-][N+](c1cc(-c2ccccc2)ccc1F)=O FCHZACFMGHJGEE-UHFFFAOYSA-N 0.000 description 1
- 125000004450 alkenylene group Chemical group 0.000 description 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 1
- 150000004056 anthraquinones Chemical class 0.000 description 1
- YCOXTKKNXUZSKD-UHFFFAOYSA-N as-o-xylenol Natural products CC1=CC=C(O)C=C1C YCOXTKKNXUZSKD-UHFFFAOYSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- WZJYKHNJTSNBHV-UHFFFAOYSA-N benzo[h]quinoline Chemical group C1=CN=C2C3=CC=CC=C3C=CC2=C1 WZJYKHNJTSNBHV-UHFFFAOYSA-N 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 description 1
- UFVXQDWNSAGPHN-UHFFFAOYSA-K bis[(2-methylquinolin-8-yl)oxy]-(4-phenylphenoxy)alumane Chemical compound [Al+3].C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC=C([O-])C2=NC(C)=CC=C21.C1=CC([O-])=CC=C1C1=CC=CC=C1 UFVXQDWNSAGPHN-UHFFFAOYSA-K 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- OHXPZWMUMBOXEH-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1N(c(cc1)ccc1-c1c2[o]c(c(-c3ccccc3)ccc3)c3c2ccc1)c1c2[o]c3ccccc3c2cc(-c2ccccc2)c1 Chemical compound c(cc1)ccc1-c(cc1)ccc1N(c(cc1)ccc1-c1c2[o]c(c(-c3ccccc3)ccc3)c3c2ccc1)c1c2[o]c3ccccc3c2cc(-c2ccccc2)c1 OHXPZWMUMBOXEH-UHFFFAOYSA-N 0.000 description 1
- ZWDMGMPIXBGJTJ-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1Nc1cc(-c2ccccc2)cc2c1[o]c1ccccc21 Chemical compound c(cc1)ccc1-c(cc1)ccc1Nc1cc(-c2ccccc2)cc2c1[o]c1ccccc21 ZWDMGMPIXBGJTJ-UHFFFAOYSA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 150000001716 carbazoles Chemical group 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 125000002676 chrysenyl group Chemical group C1(=CC=CC=2C3=CC=C4C=CC=CC4=C3C=CC12)* 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000004210 cyclohexylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004851 cyclopentylmethyl group Chemical group C1(CCCC1)C* 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- YSSSPARMOAYJTE-UHFFFAOYSA-N dibenzo-18-crown-6 Chemical compound O1CCOCCOC2=CC=CC=C2OCCOCCOC2=CC=CC=C21 YSSSPARMOAYJTE-UHFFFAOYSA-N 0.000 description 1
- IYYZUPMFVPLQIF-ALWQSETLSA-N dibenzothiophene Chemical group C1=CC=CC=2[34S]C3=C(C=21)C=CC=C3 IYYZUPMFVPLQIF-ALWQSETLSA-N 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- 239000002019 doping agent Substances 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- GVEPBJHOBDJJJI-UHFFFAOYSA-N fluoranthene Chemical group C1=CC(C2=CC=CC=C22)=C3C2=CC=CC3=C1 GVEPBJHOBDJJJI-UHFFFAOYSA-N 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000005283 ground state Effects 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 229910003437 indium oxide Inorganic materials 0.000 description 1
- PJXISJQVUVHSOJ-UHFFFAOYSA-N indium(iii) oxide Chemical compound [O-2].[O-2].[O-2].[In+3].[In+3] PJXISJQVUVHSOJ-UHFFFAOYSA-N 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 238000007641 inkjet printing Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- SNHMUERNLJLMHN-UHFFFAOYSA-N iodobenzene Chemical compound IC1=CC=CC=C1 SNHMUERNLJLMHN-UHFFFAOYSA-N 0.000 description 1
- 125000002510 isobutoxy group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])O* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000004491 isohexyl group Chemical group C(CCC(C)C)* 0.000 description 1
- 125000005921 isopentoxy group Chemical group 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 125000006606 n-butoxy group Chemical group 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000006610 n-decyloxy group Chemical group 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001298 n-hexoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000006609 n-nonyloxy group Chemical group 0.000 description 1
- 125000006608 n-octyloxy group Chemical group 0.000 description 1
- 125000003935 n-pentoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003506 n-propoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000007773 negative electrode material Substances 0.000 description 1
- 125000005484 neopentoxy group Chemical group 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical group C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 125000001820 oxy group Chemical group [*:1]O[*:2] 0.000 description 1
- 229960003540 oxyquinoline Drugs 0.000 description 1
- 125000003538 pentan-3-yl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- CSHWQDPOILHKBI-UHFFFAOYSA-N peryrene Natural products C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 CSHWQDPOILHKBI-UHFFFAOYSA-N 0.000 description 1
- 125000001484 phenothiazinyl group Chemical group C1(=CC=CC=2SC3=CC=CC=C3NC12)* 0.000 description 1
- ANRQGKOBLBYXFM-UHFFFAOYSA-M phenylmagnesium bromide Chemical compound Br[Mg]C1=CC=CC=C1 ANRQGKOBLBYXFM-UHFFFAOYSA-M 0.000 description 1
- 108091008695 photoreceptors Proteins 0.000 description 1
- 125000004592 phthalazinyl group Chemical group C1(=NN=CC2=CC=CC=C12)* 0.000 description 1
- 229920002098 polyfluorene Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 229920000123 polythiophene Polymers 0.000 description 1
- 239000007774 positive electrode material Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical group C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical compound C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- YYMBJDOZVAITBP-UHFFFAOYSA-N rubrene Chemical compound C1=CC=CC=C1C(C1=C(C=2C=CC=CC=2)C2=CC=CC=C2C(C=2C=CC=CC=2)=C11)=C(C=CC=C2)C2=C1C1=CC=CC=C1 YYMBJDOZVAITBP-UHFFFAOYSA-N 0.000 description 1
- 238000007650 screen-printing Methods 0.000 description 1
- 125000005920 sec-butoxy group Chemical group 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000003548 sec-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000004528 spin coating Methods 0.000 description 1
- 150000003413 spiro compounds Chemical class 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical group C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 1
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000005649 substituted arylene group Chemical group 0.000 description 1
- 238000010345 tape casting Methods 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000002207 thermal evaporation Methods 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000005580 triphenylene group Chemical group 0.000 description 1
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- YVTHLONGBIQYBO-UHFFFAOYSA-N zinc indium(3+) oxygen(2-) Chemical compound [O--].[Zn++].[In+3] YVTHLONGBIQYBO-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/91—Dibenzofurans; Hydrogenated dibenzofurans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/76—Dibenzothiophenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- H01L51/0073—
-
- H01L51/0074—
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6574—Polycyclic condensed heteroaromatic hydrocarbons comprising only oxygen in the heteroaromatic polycondensed ring system, e.g. cumarine dyes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6576—Polycyclic condensed heteroaromatic hydrocarbons comprising only sulfur in the heteroaromatic polycondensed ring system, e.g. benzothiophene
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1092—Heterocyclic compounds characterised by ligands containing sulfur as the only heteroatom
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
본 발명은 유기발광소자에 채용되는 유기발광 화합물에 관한 것으로서, 하기 [화학식 Ⅰ]로 표시되는 것을 특징으로 하고, 이를 전자 저지층에 채용하여 종래 소자에 비하여 발광 효율, 양자 효율 등 발광 특성이 현저히 우수한 유기발광소자를 구현할 수 있는 유기발광 화합물에 관한 것이다.
[화학식 Ⅰ]
The present invention relates to an organic light emitting compound employed in an organic light emitting device, characterized in that it is represented by the following [Formula I], and by employing it in an electron blocking layer, light emitting properties such as luminous efficiency and quantum efficiency are significantly improved compared to conventional devices It relates to an organic light emitting compound capable of implementing an excellent organic light emitting device.
[Formula Ⅰ]
Description
본 발명은 신규한 유기발광 화합물 및 이를 소자의 유기물층에 채용하여 발광 효율, 양자 효율 등 발광 특성이 현저히 우수한 소자를 구현할 수 있는 유기발광소자에 관한 것이다.The present invention relates to a novel organic light emitting compound and an organic light emitting device capable of implementing a device having remarkably excellent light emitting characteristics such as light emitting efficiency and quantum efficiency by employing the same in an organic material layer of the device.
유기 발광 현상이란 유기 물질을 이용하여 전기 에너지를 빛 에너지로 전환시켜주는 현상을 말한다. 유기 발광 현상을 이용하는 유기발광소자는 통상 양극과 음극 및 이 사이에 유기물층을 포함하는 구조를 가진다. 여기서 유기물층은 유기발광소자의 효율과 안정성을 높이기 위하여 각기 다른 물질로 구성된 다층의 구조로 이루어진 경우가 많으며, 예컨대 정공 주입층, 정공 수송층, 발광층, 전자 수송층, 전자 주입층, 정공 저지층, 전자 저지층 등으로 다양하게 이루어 질 수 있다. 이러한 유기 발광 소자의 구조에서 두 전극 사이에 전압을 걸어주게 되면 양극에서는 정공이, 음극에서는 전자가 유기물층에 주입되게 되고, 주입된 정공과 전자가 만났을 때 엑시톤(exciton)이 형성되며, 이 엑시톤이 다시 바닥상태로 떨어질 때 빛이 나게 된다. 이러한 유기 발광 소자는 자발광, 고휘도, 고효율, 낮은 구동 전압, 넓은 시야각, 높은 콘트라스트, 고속 응답성 등의 특성을 갖는 것으로 알려져 있다.The organic light emitting phenomenon refers to a phenomenon in which electric energy is converted into light energy using an organic material. An organic light emitting device using an organic light emitting phenomenon has a structure including an anode and a cathode and an organic material layer therebetween. Here, the organic material layer is often composed of a multilayer structure composed of different materials in order to increase the efficiency and stability of the organic light emitting device, for example, a hole injection layer, a hole transport layer, a light emitting layer, an electron transport layer, an electron injection layer, a hole blocking layer, an electron blocking layer. It can be made in various ways, such as layers. In the structure of the organic light emitting device, when a voltage is applied between the two electrodes, holes are injected into the organic material layer from the anode and electrons from the cathode are injected into the organic material layer. When the injected holes and electrons meet, excitons are formed, and the excitons When it falls back to the ground state, it lights up. Such an organic light emitting device is known to have characteristics such as self-luminescence, high luminance, high efficiency, low driving voltage, wide viewing angle, high contrast, and high-speed response.
유기발광소자에서 유기물층으로 사용되는 물질은 기능에 따라, 발광 물질과 전하 수송 물질, 정공 주입 물질, 정공 수송 물질, 전자 수송 물질, 전자 주입 물질, 정공 저지 물질 및 전자 저지 물질 등으로 분류될 수 있다. 또한, 발광 물질은 발광색에 따라 청색, 녹색, 적색 발광 물질과 보다 나은 천연색을 구현하기 위해 필요한 노란색 및 주황색 발광 물질로 구분될 수 있다.The material used as the organic material layer in the organic light emitting device may be classified into a light emitting material, a charge transport material, a hole injection material, a hole transport material, an electron transport material, an electron injection material, a hole blocking material and an electron blocking material according to their function. . In addition, the light emitting material may be divided into blue, green, and red light emitting materials and yellow and orange light emitting materials necessary to realize a better natural color according to the emission color.
유기발광소자가 전술한 우수한 특징들을 충분히 발휘하기 위해서는 소자 내 유기물층을 이루는 다양한 물질 등이 안정하고 효율적인 재료에 의하여 뒷받침되는 것이 선행되어야 하나, 아직까지 안정하고 효율적인 유기 발광 소자용 유기물층 재료의 개발이 충분히 이루어지지 않은 상태이다. 따라서 새로운 재료의 개발이 계속 요구되고 있으며, 이와 같은 재료 개발의 필요성은 다른 유기 전자 소자에서도 마찬가지이다.In order for an organic light emitting device to sufficiently exhibit the above-described excellent characteristics, it is necessary that the various materials constituting the organic material layer in the device be supported by stable and efficient materials, but the development of stable and efficient organic material layer materials for organic light emitting devices is still insufficient. has not been done Therefore, the development of new materials is continuously required, and the necessity of such material development is the same in other organic electronic devices.
본 발명은 유기발광소자의 유기층에 채용되어 발광 효율, 양자 효율 등의 우수한 발광 특성을 구현할 수 있는 신규한 유기발광 화합물과 이를 포함하는 유기발광소자를 제공하고자 한다.An object of the present invention is to provide a novel organic light emitting compound capable of implementing excellent light emitting properties such as luminous efficiency and quantum efficiency by being employed in an organic layer of an organic light emitting device and an organic light emitting device including the same.
본 발명은 상기 과제를 해결하기 위하여, 하기 [화학식 Ⅰ]로 표시되는 유기발광소자를 제공한다.The present invention provides an organic light emitting device represented by the following [Formula I] in order to solve the above problems.
[화학식 Ⅰ][Formula Ⅰ]
상기 [화학식 Ⅰ]의 구체적인 구조 및 치환기에 대해서는 후술한다.The specific structure and substituents of the [Formula I] will be described later.
또한, 본 발명은 제1 전극, 제2 전극, 및 상기 제1 전극과 제2 전극 사이에 배치된 1층 이상의 유기물층을 포함하는 유기발광소자로서, 상기 유기물층 중 1 층 이상은 상기 유기발광 화합물을 포함하는 유기발광소자를 제공한다.In addition, the present invention is an organic light emitting device comprising a first electrode, a second electrode, and at least one organic material layer disposed between the first electrode and the second electrode, wherein at least one layer of the organic material layer comprises the organic light emitting compound It provides an organic light emitting device comprising.
본 발명에 따른 유기발광 화합물을 전자 저지층에 채용한 소자는 종래 소자에 비하여 발광 효율, 양자 효율 등의 발광특성이 우수하여 다양한 디스플레이 소자에 유용하게 적용할 수 있다.The device employing the organic light emitting compound according to the present invention as an electron blocking layer has superior light emitting characteristics such as luminous efficiency and quantum efficiency compared to conventional devices, and thus can be usefully applied to various display devices.
이하, 본 발명을 보다 구체적으로 설명한다.Hereinafter, the present invention will be described in more detail.
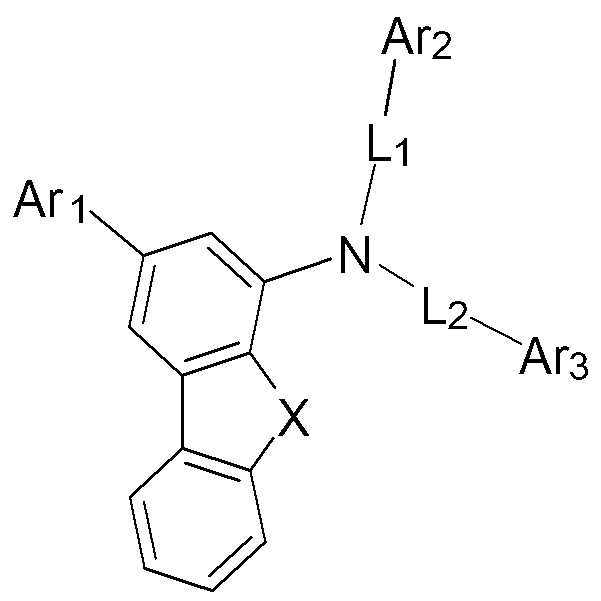
본 발명의 일 측면은 유기발광소자의 유기물층, 바람직하게는 전자 저지층에 채용되어 발광 효율, 양자 효율 등의 우수한 발광특성을 구현할 수 있는 유기발광 화합물에 관한 것으로서, 하기 [화학식 Ⅰ]로 표시되는 것을 특징으로 한다.One aspect of the present invention relates to an organic light emitting compound that is employed in an organic material layer, preferably an electron blocking layer, of an organic light emitting device to realize excellent light emitting characteristics such as luminous efficiency and quantum efficiency, and is represented by the following [Formula I] characterized in that
[화학식 Ⅰ][Formula Ⅰ]
상기 [화학식 Ⅰ]에서,In the [Formula I],
X는 CR1R2, O 또는 S이고, 상기 R1 내지 R2는 서로 동일하거나 상이하고, 각각 독립적으로 수소, 치환 또는 비치환된 탄소수 1 내지 7의 알킬기 및 치환 또는 비치환된 탄소수 6 내지 30의 아릴기 중에서 선택되며, 상기 R1 내지 R2는 서로 또는 인접한 치환기와 연결되어 치환 또는 비치환된 탄화수소 고리 또는 치환 또는 비치환된 헤테로고리를 형성할 수 있다.X is CR 1 R 2 , O or S, wherein R 1 to R 2 are the same as or different from each other, and each independently hydrogen, a substituted or unsubstituted C 1 to C 7 alkyl group, and a substituted or unsubstituted C 6 to C 6 It is selected from the aryl group of 30, wherein R 1 To R 2 may be connected to each other or adjacent substituents to form a substituted or unsubstituted hydrocarbon ring or a substituted or unsubstituted heterocycle.
L1 내지 L2는 서로 동일하거나 상이하고, 각각 독립적으로 단일결합이거나, 치환 또는 비치환된 아릴렌기, 치환 또는 비치환된 알케닐렌기, 치환 또는 비치환된 플루오레닐렌기, 치환 또는 비치환된 카바졸릴렌기, 또는 N, O 및 S 원자 중 1개 이상을 포함하는 치환 또는 비치환된 헤테로아릴렌기이다.L 1 To L 2 Are the same as or different from each other, and each independently is a single bond, a substituted or unsubstituted arylene group, a substituted or unsubstituted alkenylene group, a substituted or unsubstituted fluorenylene group, a substituted or unsubstituted a carbazolylene group, or a substituted or unsubstituted heteroarylene group containing at least one of N, O and S atoms.
Ar1 내지 Ar3는 서로 동일하거나 상이하고, 각각 독립적으로 수소, 치환 또는 비치환된 탄소수 6 내지 30의 아릴기, 치환 또는 비치환된 탄소수 3 내지 30의 헤테로아릴기, 치환 또는 비치환된 탄소수 3 내지 20의 시클로알킬이 하나 이상 융합된 치환 또는 비치환된 탄소수 6 내지 30의 아릴기, 치환 또는 비치환된 탄소수 3 내지 20의 시클로알킬이 하나 이상 융합된 치환 또는 비치환된 탄소수 3 내지 30의 헤테로아릴기, 치환 또는 비치환된 플루오레닐기 및 치환 또는 비치환된 카바졸기 중에서 선택되는 어느 하나이다.Ar 1 To Ar 3 are the same or different from each other, and each independently represents hydrogen, a substituted or unsubstituted C 6 to C 30 aryl group, a substituted or unsubstituted C 3 to C 30 heteroaryl group, or a substituted or unsubstituted C 6 C heteroaryl group. A substituted or unsubstituted aryl group having 6 to 30 carbon atoms in which one or more 3 to 20 cycloalkyl is fused, a substituted or unsubstituted C 3 to 30 aryl group having 3 to 20 carbon atoms fused to one or more substituted or unsubstituted cycloalkyl groups of a heteroaryl group, a substituted or unsubstituted fluorenyl group, and a substituted or unsubstituted carbazole group.
한편, 상기 치환 또는 비치환이란 수소, 중수소, 할로겐기, 시아노기, 니트로기, 히드록시기, 실릴기, 알킬기, 시클로알킬기, 알콕시기, 알케닐기, 아릴기 및 헤테로고리기로 이루어진 군에서 선택된 1 또는 2 이상의 치환기로 치환되거나, 상기 치환기 중 2 이상의 치환기가 연결된 치환기로 치환되거나, 또는 어떠한 치환기도 갖지 않는 것을 의미한다.Meanwhile, the substituted or unsubstituted means 1 or 2 selected from the group consisting of hydrogen, deuterium, a halogen group, a cyano group, a nitro group, a hydroxy group, a silyl group, an alkyl group, a cycloalkyl group, an alkoxy group, an alkenyl group, an aryl group, and a heterocyclic group It means that it is substituted with one or more substituents, is substituted with a substituent to which two or more of the substituents are connected, or does not have any substituents.
구체적인 예를 들면, 치환된 아릴렌기라 함은, 페닐기, 비페닐기, 나프탈렌기, 플루오레닐기, 파이레닐기, 페난트레닐기, 페릴렌기, 테트라세닐기. 안트라센닐기 등이 다른 치환기로 치환된 것을 의미한다.For specific examples, the substituted arylene group includes a phenyl group, a biphenyl group, a naphthalene group, a fluorenyl group, a pyrenyl group, a phenanthrenyl group, a perylene group, and a tetracenyl group. It means that the anthracenyl group is substituted with another substituent.
치환된 헤테로아릴렌기라 함은, 피리딜기, 티오페닐기, 트리아진기, 퀴놀린기, 페난트롤린기, 이미다졸기, 티아졸기, 옥사졸기, 카바졸기 및 이들의 축합헤테로고리기, 예컨대 벤즈퀴놀린기, 벤즈이미다졸기, 벤즈옥사졸기, 벤즈티아졸기, 벤즈카바졸기, 디벤조티오페닐기, 디벤조퓨란기 등이 다른 치환기로 치환된 것을 의미한다.The substituted heteroarylene group includes a pyridyl group, a thiophenyl group, a triazine group, a quinoline group, a phenanthroline group, an imidazole group, a thiazole group, an oxazole group, a carbazole group and a condensed heterocyclic group thereof, such as a benzquinoline group, It means that a benzimidazole group, a benzoxazole group, a benzthiazole group, a benzcarbazole group, a dibenzothiophenyl group, a dibenzofuran group, etc. are substituted with other substituents.
본 발명에 있어서, 상기 치환기들의 예시들에 대해서 아래에서 구체적으로 설명하나, 이에 한정되는 것은 아니다.In the present invention, examples of the substituents will be described in detail below, but the present invention is not limited thereto.
본 발명에 있어서, 상기 알킬기는 직쇄 또는 분지쇄일 수 있고, 탄소수는 특별히 한정되지 않으며, 구체적인 예로는 메틸기, 에틸기, 프로필기, n-프로필기, 이소프로필기, 부틸기, n-부틸기, 이소부틸기, tert-부틸기, sec-부틸기, 1-메틸-부틸기, 1-에틸-부틸기, 펜틸기, n-펜틸기, 이소펜틸기, 네오펜틸기, tert-펜틸기, 헥실기, n-헥실기, 1-메틸펜틸기, 2-메틸펜틸기, 4-메틸-2-펜틸기, 3,3-디메틸부틸기, 2-에틸부틸기, 헵틸기, n-헵틸기, 1-메틸헥실기, 시클로펜틸메틸기, 시클로헥틸메틸기, 옥틸기, n-옥틸기, tert-옥틸기, 1-메틸헵틸기, 2-에틸헥실기, 2-프로필펜틸기, n-노닐기, 2,2-디메틸헵틸기, 1-에틸-프로필기, 1,1-디메틸-프로필기, 이소헥실기, 2-메틸펜틸기, 4-메틸헥실기, 5-메틸헥실기 등이 있으나, 이들에 한정되지 않는다.In the present invention, the alkyl group may be linear or branched, and the number of carbon atoms is not particularly limited, and specific examples include a methyl group, an ethyl group, a propyl group, an n-propyl group, an isopropyl group, a butyl group, a n-butyl group, and an iso Butyl group, tert-butyl group, sec-butyl group, 1-methyl-butyl group, 1-ethyl-butyl group, pentyl group, n-pentyl group, isopentyl group, neopentyl group, tert-pentyl group, hexyl group , n-hexyl group, 1-methylpentyl group, 2-methylpentyl group, 4-methyl-2-pentyl group, 3,3-dimethylbutyl group, 2-ethylbutyl group, heptyl group, n-heptyl group, 1 -Methylhexyl group, cyclopentylmethyl group, cyclohexylmethyl group, octyl group, n-octyl group, tert-octyl group, 1-methylheptyl group, 2-ethylhexyl group, 2-propylpentyl group, n-nonyl group, 2 ,2-dimethylheptyl group, 1-ethyl-propyl group, 1,1-dimethyl-propyl group, isohexyl group, 2-methylpentyl group, 4-methylhexyl group, 5-methylhexyl group, etc., but these not limited
본 발명에 있어서, 알콕시기는 직쇄 또는 분지쇄일 수 있다. 알콕시기의 탄소수는 특별히 한정되지 않으나, 입체적 방해를 주지 않는 범위이면 바람직하고, 구체적으로, 메톡시기, 에톡시기, n-프로폭시기, 이소프로폭시기, i-프로필옥시기, n-부톡시기, 이소부톡시기, tert-부톡시기, sec-부톡시기, n-펜틸옥시기, 네오펜틸옥시기, 이소펜틸옥시기, n-헥실옥시기, 3,3-디메틸부틸옥시기, 2-에틸부틸옥시기, n-옥틸옥시기, n-노닐옥시기, n-데실옥시기, 벤질옥시기, p-메틸벤질옥시기 등이 될 수 있으나, 이에 한정되는 것은 아니다.In the present invention, the alkoxy group may be straight-chain or branched. The number of carbon atoms of the alkoxy group is not particularly limited, but is preferably within a range that does not interfere with stericity, and specifically, a methoxy group, an ethoxy group, n-propoxy group, isopropoxy group, i-propyloxy group, n-butoxy group , isobutoxy group, tert-butoxy group, sec-butoxy group, n-pentyloxy group, neopentyloxy group, isopentyloxy group, n-hexyloxy group, 3,3-dimethylbutyloxy group, 2-ethylbutyl It may be an oxy group, n-octyloxy group, n-nonyloxy group, n-decyloxy group, benzyloxy group, p-methylbenzyloxy group, and the like, but is not limited thereto.
본 발명에 있어서, 상기 알케닐기는 직쇄 또는 분지쇄일 수 있고, 탄소수는 특별히 한정되지 않으며, 구체적인 예로는 비닐기, 1-프로페닐기, 이소프로페닐기, 1-부테닐기, 2-부테닐기, 3-부테닐기, 1-펜테닐기, 2-펜테닐기, 3-펜테닐기, 3-메틸-1-부테닐기, 1,3-부타디에닐기, 알릴기, 1-페닐비닐-1-일기, 2-페닐비닐-1-일기, 2,2-디페닐비닐-1-일기, 2-페닐-2-(나프틸-1-일)비닐-1-일기, 2,2-비스(디페닐-1-일)비닐-1-일기, 스틸베닐기, 스티레닐기 등이 있으나 이들에 한정되지 않는다.In the present invention, the alkenyl group may be linear or branched, and the number of carbon atoms is not particularly limited, and specific examples include a vinyl group, 1-propenyl group, isopropenyl group, 1-butenyl group, 2-butenyl group, 3- Butenyl group, 1-pentenyl group, 2-pentenyl group, 3-pentenyl group, 3-methyl-1-butenyl group, 1,3-butadienyl group, allyl group, 1-phenylvinyl-1-yl group, 2-phenyl Vinyl-1-yl group, 2,2-diphenylvinyl-1-yl group, 2-phenyl-2-(naphthyl-1-yl)vinyl-1-yl group, 2,2-bis(diphenyl-1-yl) ) vinyl-1-yl group, stilbenyl group, styrenyl group, and the like, but is not limited thereto.
본 발명에 있어서, 아릴기는 단환식 또는 다환식일 수 있고, 탄소수는 특별히 한정되지 않으나 6 내지 30인 것이 바람직하다. 단환식 아릴기의 예로는 페닐기, 비페닐기, 터페닐기, 스틸벤기 등이 있고, 다환식 아릴기의 예로는 나프틸기, 안트라세닐기, 페난트레닐기, 파이레닐기, 페릴레닐기, 테트라세닐기, 크라이세닐기, 플루오레닐기, 아세나프타센닐기, 트리페닐렌기, 플루오안트렌(fluoranthrene)기 등이 있으나, 본 발명의 범위가 이들 예로만 한정되는 것은 아니다.In the present invention, the aryl group may be monocyclic or polycyclic, and the number of carbon atoms is not particularly limited, but is preferably 6 to 30. Examples of the monocyclic aryl group include a phenyl group, a biphenyl group, a terphenyl group, and a stilbene group, and examples of the polycyclic aryl group include a naphthyl group, an anthracenyl group, a phenanthrenyl group, a pyrenyl group, a perylenyl group, and a tetracenyl group. , chrysenyl group, fluorenyl group, acenaphthacenyl group, triphenylene group, fluoranthrene group, etc., but the scope of the present invention is not limited to these examples.
본 발명에 있어서, 헤테로고리기는 이종원자로 O, N 또는 S를 포함하는 헤테로고리기로서, 탄소수는 특별히 한정되지 않으나 탄소수 3 내지 30인 것이 바람직하다. 헤테로고리기의 예로는 티오펜기, 퓨란기, 피롤기, 이미다졸기, 티아졸기, 옥사졸기, 옥사디아졸기, 트리아졸기, 피리딜기, 비피리딜기, 피리미딜기, 트리아진기, 트리아졸기, 아크리딜기, 피리다진기, 피라지닐기, 퀴놀리닐기, 퀴나졸린기, 퀴녹살리닐기, 프탈라지닐기, 피리도 피리미디닐기, 피리도 피라지닐기, 피라지노 피라지닐기, 이소퀴놀린기, 인돌기, 카바졸기, 벤조옥사졸기, 벤조이미다졸기, 벤조티아졸기, 벤조카바졸기, 벤조티오펜기, 디벤조티오펜기, 벤조퓨라닐기, 디벤조퓨라닐기, 페난트롤린기, 티아졸릴기, 이소옥사졸릴기, 옥사디아졸릴기, 티아디아졸릴기, 벤조티아졸릴기, 페노티아지닐기 등이 있으나, 이들에만 한정되는 것은 아니다.In the present invention, the heterocyclic group is a heterocyclic group containing O, N or S as a heteroatom, and the number of carbon atoms is not particularly limited, but it is preferably from 3 to 30 carbon atoms. Examples of the heterocyclic group include a thiophene group, a furan group, a pyrrole group, an imidazole group, a thiazole group, an oxazole group, an oxadiazole group, a triazole group, a pyridyl group, a bipyridyl group, a pyrimidyl group, a triazine group, a triazole group, Acridyl group, pyridazine group, pyrazinyl group, quinolinyl group, quinazoline group, quinoxalinyl group, phthalazinyl group, pyrido pyrimidinyl group, pyrido pyrazinyl group, pyrazino pyrazinyl group, isoquinoline group , indole group, carbazole group, benzooxazole group, benzoimidazole group, benzothiazole group, benzocarbazole group, benzothiophene group, dibenzothiophene group, benzofuranyl group, dibenzofuranyl group, phenanthroline group, thiazolyl group, isoxazolyl group, oxadiazolyl group, thiadiazolyl group, benzothiazolyl group, phenothiazinyl group, and the like, but is not limited thereto.
본 발명에 있어서, 시클로알킬기는 특별히 한정되지 않으나, 탄소수 3 내지 20인 것이 바람직하며, 구체적으로 시클로프로필기 시클로부틸기 시클로펜틸기 3-메틸시클로펜틸기 2,3-디메틸시클로펜틸기, 시클로헥실기, 3-메틸시클로헥실기, 4-메틸시클로헥실기, 2,3-디메틸시클로헥실기, 3,4,5-트리메틸시클로헥실기, 4-tert-부틸시클로헥실기, 시클로헵틸기, 시클로옥틸기 등이 있으나, 이에 한정되지 않는다.In the present invention, the cycloalkyl group is not particularly limited, but preferably has 3 to 20 carbon atoms, and specifically, cyclopropyl group, cyclobutyl group, cyclopentyl group, 3-methylcyclopentyl group, 2,3-dimethylcyclopentyl group, cyclohex Sil group, 3-methylcyclohexyl group, 4-methylcyclohexyl group, 2,3-dimethylcyclohexyl group, 3,4,5-trimethylcyclohexyl group, 4-tert-butylcyclohexyl group, cycloheptyl group, cyclo There is an octyl group, and the like, but is not limited thereto.
본 발명에 있어서, 할로겐기의 예로는 불소, 염소, 브롬 또는 요오드가 있다.In the present invention, examples of the halogen group include fluorine, chlorine, bromine or iodine.
본 발명에 있어서, 플루오레닐기는 2개의 고리 유기화합물이 1개의 원자를 통하여 연결된 구조로서, 예로는 , 등이 있다.In the present invention, the fluorenyl group is a structure in which two ring organic compounds are connected through one atom, for example, , etc.
본 발명에 있어서, 플루오레닐기는 열린 플루오레닐기의 구조를 포함하며, 여기서 열린 플루오레닐기는 2개의 고리 유기화합물이 1개의 원자를 통하여 연결된 구조에서 한쪽 고리 화합물의 연결이 끊어진 상태의 구조로서, 예로는 , 등이 있다.In the present invention, the fluorenyl group includes a structure of an open fluorenyl group, wherein the open fluorenyl group is a structure in which one ring compound is disconnected in a structure in which two ring organic compounds are connected through one atom. , for example , etc.
본 발명에 있어서, 실릴기는 구체적으로 트리메틸실릴기, 트리에틸실릴기, t-부틸디메틸실릴기, 비닐디메틸실릴기, 프로필디메틸실릴기, 트리페닐실릴기, 디페닐실릴기, 페닐실릴기 등이 있으나 이에 한정되지 않는다.In the present invention, the silyl group specifically includes a trimethylsilyl group, a triethylsilyl group, a t-butyldimethylsilyl group, a vinyldimethylsilyl group, a propyldimethylsilyl group, a triphenylsilyl group, a diphenylsilyl group, a phenylsilyl group, and the like. However, the present invention is not limited thereto.
상기 [화학식 Ⅰ]로 표시되는 본 발명에 따른 유기발광 화합물은 그 구조적 특이성으로 인하여 유기발광소자의 유기물층으로 사용될 수 있고, 보다 구체적으로 유기물층의 전자 저지층 물질로 사용될 수 있다.The organic light emitting compound according to the present invention represented by the above [Formula I] may be used as an organic material layer of an organic light emitting device due to its structural specificity, and more specifically, it may be used as an electron blocking layer material of the organic material layer.
본 발명에 따른 [화학식 Ⅰ]로 표시되는 화합물의 바람직한 구체예로는 하기 화합물들이 있으나, 이들에만 한정되는 것은 아니다.Preferred examples of the compound represented by [Formula I] according to the present invention include the following compounds, but are not limited thereto.
상기와 같은 구조의 코어 구조에 다양한 치환기를 도입함으로써 도입된 치환기의 고유 특성을 갖는 유기발광 화합물을 합성할 수 있으며, 이를 통하여 유기발광소자의 다양한 유기물층에서 요구하는 조건들을 충족시키는 물질을 제조할 수 있다. 본 발명의 화합물은 유기발광소자의 통상의 제조방법에 따라 소자에 적용할 수 있다.By introducing various substituents into the core structure of the above structure, it is possible to synthesize an organic light emitting compound having the intrinsic properties of the introduced substituent, and through this, a material satisfying the conditions required by various organic material layers of the organic light emitting device can be manufactured. have. The compound of the present invention can be applied to a device according to a conventional method for manufacturing an organic light emitting device.
본 발명의 하나의 실시예에 따른 유기발광소자는 제1 전극과 제2 전극 및 이 사이에 배치된 유기물층을 포함하는 구조로 이루어질 수 있으며, 본 발명에 따른 유기발광 화합물을 소자의 유기물층에 사용한다는 것을 제외하고는 통상의 소자의 제조 방법 및 재료를 사용하여 제조될 수 있다.The organic light emitting device according to an embodiment of the present invention may have a structure including a first electrode and a second electrode and an organic material layer disposed therebetween, and the organic light emitting compound according to the present invention is used for the organic material layer of the device. Except for that, it may be manufactured using a conventional device manufacturing method and material.
본 발명에 따른 유기발광소자의 유기물층은 단층 구조로 이루어질 수도 있으나, 2층 이상의 유기물층이 적층된 다층 구조로 이루어질 수 있다. 예컨대, 정공 주입층, 정공 수송층, 전자 저지층, 정공 저지층, 발광층, 전자 수송층, 전자 주입층뿐만 아니라 다양한 기능을 갖는 층을 포함하는 구조를 가질 수 있다. 그러나, 이에 한정되지 않고 더 적은 수의 유기물층을 포함할 수도 있다.The organic material layer of the organic light emitting device according to the present invention may have a single-layer structure, but may have a multi-layer structure in which two or more organic material layers are stacked. For example, it may have a structure including a hole injection layer, a hole transport layer, an electron blocking layer, a hole blocking layer, a light emitting layer, an electron transport layer, an electron injection layer, as well as layers having various functions. However, the present invention is not limited thereto and may include a smaller number of organic material layers.
따라서, 본 발명에 따른 유기발광소자에서, 상기 층들 중 1층 이상이 상기 [화학식 Ⅰ]로 표시되는 화합물을 포함할 수 있다.Accordingly, in the organic light emitting device according to the present invention, one or more of the layers may include the compound represented by the [Formula I].
또한, 본 발명에 따른 유기발광소자는 스퍼터링(sputtering)이나 전자빔 증발(e-beam evaporation)과 같은 PVD(physical vapor deposition) 방법을 이용하여, 기판 상에 금속 또는 전도성을 가지는 금속 산화물 또는 이들의 합금을 증착시켜 양극을 형성하고, 그 위에 정공 주입층, 정공 수송층, 전자 저지층, 발광층, 전자 수송층을 포함하는 유기물층을 형성한 후, 그 위에 음극으로 사용할 수 있는 물질을 증착시킴으로써 제조될 수 있다.In addition, the organic light emitting device according to the present invention uses a PVD (physical vapor deposition) method, such as sputtering or e-beam evaporation, to form a metal or a conductive metal oxide or an alloy thereof on a substrate. It can be prepared by depositing an anode to form an anode, forming an organic material layer including a hole injection layer, a hole transport layer, an electron blocking layer, a light emitting layer, and an electron transport layer thereon, and then depositing a material that can be used as a cathode thereon.
이와 같은 방법 외에도, 기판 상에 음극 물질부터 유기물층, 양극 물질을 차례로 증착시켜 유기발광소자를 만들 수도 있다. 상기 유기물층은 정공 주입층, 정공 수송층, 전자 저지층, 발광층 및 전자 수송층 등을 포함하는 다층 구조일 수도 있으나, 이에 한정되지 않고 단층 구조일 수 있다. 또한, 상기 유기물층은 다양한 고분자 소재를 사용하여 증착법이 아닌 솔벤트 프로세스(solvent process), 예컨대 스핀 코팅, 딥 코팅, 닥터 블레이딩, 스크린 프린팅, 잉크젯 프린팅 또는 열 전사법 등의 방법에 의하여 더 적은 수의 층으로 제조할 수 있다.In addition to this method, an organic light emitting diode may be manufactured by sequentially depositing a cathode material, an organic material layer, and an anode material on a substrate. The organic material layer may have a multilayer structure including a hole injection layer, a hole transport layer, an electron blocking layer, a light emitting layer, and an electron transport layer, but is not limited thereto and may have a single layer structure. In addition, the organic layer is formed using a variety of polymer materials by a solvent process rather than a deposition method, such as spin coating, dip coating, doctor blading, screen printing, inkjet printing, or a thermal transfer method. It can be made in layers.
양극 물질로는 통상 유기물층으로 정공주입이 원활할 수 있도록 일함수가 큰 물질이 바람직하다. 본 발명에서 사용될 수 있는 양극 물질의 구체적인 예로는 바나듐, 크롬, 구리, 아연, 금과 같은 금속 또는 이들의 합금, 아연 산화물, 인듐 산화물, 인듐 주석 산화물(ITO), 인듐 아연 산화물(IZO)과 같은 금속 산화물, ZnO:Al 또는 SnO2:Sb와 같은 금속과 산화물의 조합, 폴리(3-메틸티오펜), 폴리[3,4-(에틸렌-1,2-디옥시)티오펜](PEDT), 폴리피롤 및 폴리아닐린과 같은 전도성 고분자 등이 있으나, 이들에만 한정되는 것은 아니다.As the anode material, a material having a large work function is generally preferred so that holes can be smoothly injected into the organic material layer. Specific examples of the anode material that can be used in the present invention include metals such as vanadium, chromium, copper, zinc, gold, or alloys thereof, zinc oxide, indium oxide, indium tin oxide (ITO), indium zinc oxide (IZO), etc. Metal oxides, combinations of metals and oxides such as ZnO:Al or SnO 2 :Sb, poly(3-methylthiophene), poly[3,4-(ethylene-1,2-dioxy)thiophene] (PEDT) , a conductive polymer such as polypyrrole and polyaniline, but is not limited thereto.
음극 물질로는 통상 유기물층으로 전자 주입이 용이하도록 일함수가 작은 물질인 것이 바람직하다. 음극 물질의 구체적인 예로는 마그네슘, 칼슘, 나트륨, 칼륨, 타이타늄, 인듐, 이트륨, 리튬, 가돌리늄, 알루미늄, 은, 주석 및 납과 같은 금속 또는 이들의 합금, LiF/Al 또는 LiO2/Al과 같은 다층 구조 물질 등이 있으나, 이들에만 한정되는 것은 아니다.The cathode material is preferably a material having a small work function to facilitate electron injection into the organic layer. Specific examples of the negative electrode material include metals such as magnesium, calcium, sodium, potassium, titanium, indium, yttrium, lithium, gadolinium, aluminum, silver, tin and lead or alloys thereof, and multilayers such as LiF/Al or LiO 2 /Al Structural materials and the like, but are not limited thereto.
정공 주입 물질로는 낮은 전압에서 양극으로부터 정공을 잘 주입받을 수 있는 물질로서, 정공 주입 물질의 HOMO(highest occupied molecular orbital)가 양극 물질의 일함수와 주변 유기물층의 HOMO 사이인 것이 바람직하다. 정공 주입 물질의 구체적인 예로는 금속 포피린(porphyrine), 올리고티오펜, 아릴아민 계열의 유기물, 헥사니트릴 헥사아자트리페닐렌, 퀴나크리돈(quinacridone) 계열의 유기물, 페릴렌(perylene) 계열의 유기물, 안트라퀴논 및 폴리아닐린과 폴리티오펜 계열의 전도성 고분자 등이 있으나, 이들에만 한정되는 것은 아니다.The hole injection material is a material capable of well injecting holes from the anode at a low voltage, and it is preferable that the highest occupied molecular orbital (HOMO) of the hole injection material is between the work function of the positive electrode material and the HOMO of the surrounding organic material layer. Specific examples of the hole injection material include metal porphyrine, oligothiophene, arylamine-based organic material, hexanitrile hexaazatriphenylene, quinacridone-based organic material, perylene-based organic material, anthraquinone, polyaniline, and polythiophene-based conductive polymers, but is not limited thereto.
정공 수송 물질로는 양극이나 정공 주입층으로부터 정공을 수송 받아 발광층으로 옮겨줄 수 있는 물질로 정공에 대한 이동성이 큰 물질이 적합하다. 구체적인 예로는 아릴아민 계열의 유기물, 전도성 고분자, 및 공액 부분과 비공액 부분이 함께 있는 블록 공중합체 등이 있으나, 이들에만 한정되는 것은 아니다.As the hole transport material, a material capable of transporting holes from the anode or the hole injection layer to the light emitting layer is suitable, and a material having high hole mobility is suitable. Specific examples include, but are not limited to, an arylamine-based organic material, a conductive polymer, and a block copolymer having a conjugated portion and a non-conjugated portion together.
발광 물질로는 정공 수송층과 전자 수송층으로부터 정공과 전자를 각각 수송받아 결합시킴으로써 가시광선 영역의 빛을 낼 수 있는 물질로서, 형광이나 인광에 대한 양자효율이 좋은 물질이 바람직하다. 구체적인 예로는 8-히드록시-퀴놀린 알루미늄 착물(Alq3), 카르바졸 계열 화합물, 이량체화 스티릴(dimerized styryl) 화합물, BAlq, 10-히드록시벤조 퀴놀린-금속 화합물, 벤족사졸, 벤즈티아졸 및 벤즈이미다졸 계열의 화합물, 폴리(p-페닐렌비닐렌)(PPV) 계열의 고분자, 스피로(spiro) 화합물, 폴리플루오렌, 루브렌 등이 있으나, 이들에만 한정되는 것은 아니다.The light emitting material is a material capable of emitting light in the visible ray region by receiving and combining holes and electrons from the hole transport layer and the electron transport layer, respectively, and a material having good quantum efficiency for fluorescence or phosphorescence is preferable. Specific examples include 8-hydroxy-quinoline aluminum complex (Alq 3 ), carbazole-based compounds, dimerized styryl compounds, BAlq, 10-hydroxybenzoquinoline-metal compounds, benzoxazole, benzthiazole and Benzimidazole-based compounds, poly(p-phenylenevinylene) (PPV)-based polymers, spiro compounds, polyfluorene, rubrene, and the like, but are not limited thereto.
전자 수송 물질로는 음극으로부터 전자를 잘 주입 받아 발광층으로 옮겨줄 수 있는 물질로서, 전자에 대한 이동성이 큰 물질이 적합하다. 구체적인 예로는 8-히드록시퀴놀린의 Al 착물, Alq3를 포함한 착물, 유기 라디칼 화합물, 히드록시플라본-금속 착물 등이 있으나, 이들에만 한정되는 것은 아니다.As the electron transport material, a material capable of receiving electrons from the cathode and transferring them to the light emitting layer is suitable, and a material having high electron mobility is suitable. Specific examples include, but are not limited to, an Al complex of 8-hydroxyquinoline , a complex including Alq 3 , an organic radical compound, and a hydroxyflavone-metal complex.
본 발명에 따른 유기발광소자는 사용되는 재료에 따라 전면 발광형, 후면 발광형 또는 양면 발광형일 수 있다.The organic light emitting device according to the present invention may be a top emission type, a back emission type, or a double side emission type depending on the material used.
또한, 본 발명에 따른 유기발광 화합물은 유기 태양 전지, 유기 감광체, 유기 트랜지스터 등을 비롯한 유기전자소자에서도 유기발광소자에 적용되는 것과 유사한 원리로 작용할 수 있다.In addition, the organic light emitting compound according to the present invention can act on a principle similar to that applied to the organic light emitting device in organic electronic devices including organic solar cells, organic photoreceptors, organic transistors, and the like.
이하, 본 발명의 이해를 돕기 위하여 바람직한 실시예를 제시한다. 그러나, 하기의 실시예는 본 발명을 예시하기 위한 것이며, 이에 의하여 본 발명의 범위가 한정되는 것은 아니다.Hereinafter, preferred examples are presented to help the understanding of the present invention. However, the following examples are intended to illustrate the present invention, and the scope of the present invention is not limited thereby.
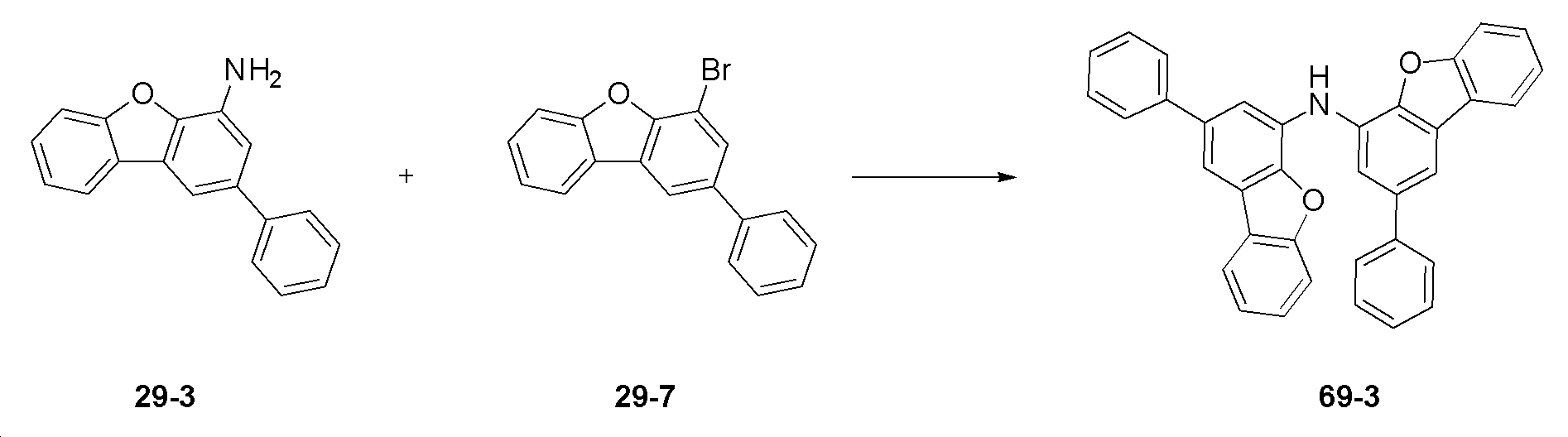
합성예Synthesis example 1 : 화합물 29의 합성 1: Synthesis of compound 29
(1) 중간체 29-1의 합성(1) Synthesis of Intermediate 29-1
4-bromo-1-fluoro-2-nitrobenzene (20 g, 0.091 mol, sigma aldrich), phenylboronic acid (13.30 g, 0.109 mol, sigma aldrich), potassium carbonate (25.13 g, 0.182 mol, sigma aldrich), Pd(PPh3)4 (3.15 g, 0.0027 mol, sigma aldrich), Toluene 220 mL, Ethanol 40 mL 넣고 5시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 29-1>을 17.6 g (수율 89%) 수득하였다.4-bromo-1-fluoro-2-nitrobenzene (20 g, 0.091 mol, sigma aldrich), phenylboronic acid (13.30 g, 0.109 mol, sigma aldrich), potassium carbonate (25.13 g, 0.182 mol, sigma aldrich), Pd ( PPh 3 ) 4 (3.15 g, 0.0027 mol, sigma aldrich), 220 mL of Toluene, and 40 mL of Ethanol were added, and the reaction was stirred under reflux for 5 hours. After completion of the reaction, 17.6 g (yield 89%) of <Intermediate 29-1> was obtained through column purification (N-HEXANE: MC) after layer separation using H 2 O: MC.
(2) 중간체 29-2의 합성 (2) Synthesis of Intermediate 29-2
중간체 29-1 (15 g, 0.069 mol, sigma aldrich), 2-bromophenol (11.95 g, 0.069 mol, sigma aldrich), potassium carbonate (19.09 g, 0.138 mol, sigma aldrich)에 dimethylformamide 200 mL 넣고 90 ℃에서 12시간 동안 교반 후 촉매 Pd(OAc)2 (0.78 g, 0.0035 mol, sigma aldrich), triphenylphosphin e (1.81 g, 0.007mol, sigma Aldrich)을 추가 투입하고 90 ℃에서 2.5시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA )하여 <중간체 29-2>를 15.8 g (수율 79%) 수득하였다.Add 200 mL of dimethylformamide to Intermediate 29-1 (15 g, 0.069 mol, sigma aldrich), 2-bromophenol (11.95 g, 0.069 mol, sigma aldrich), potassium carbonate (19.09 g, 0.138 mol, sigma aldrich) at 90 ° C. After stirring for an hour, the catalyst Pd(OAc) 2 (0.78 g, 0.0035 mol, sigma aldrich), triphenylphosphin e (1.81 g, 0.007 mol, sigma Aldrich) was further added, and the reaction was stirred at 90 ° C. for 2.5 hours. After completion of the reaction, 15.8 g (yield 79%) of <Intermediate 29-2> was obtained by column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
(3) 중간체 29-3의 합성 (3) Synthesis of Intermediate 29-3
중간체 29-2 (15 g, 0.052 mol)을 Ethanol : H2O(4:1)에 넣고 그 뒤에 iron powder(14.48 g, 0.26 mol, sigma Aldrich)와 염산을 넣고 2시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE:EA)하여 <중간체 29-3>을 10 g (수율 74%) 수득하였다.Intermediate 29-2 (15 g, 0.052 mol) was added to Ethanol: H 2 O (4:1), then iron powder (14.48 g, 0.26 mol, sigma Aldrich) and hydrochloric acid were added, followed by reaction under reflux stirring for 2 hours. . After completion of the reaction, 10 g (yield 74%) of <Intermediate 29-3> was obtained by performing column purification (N-HEXANE:EA) after layer separation in H 2 O:EA.
(4) 중간체 29-4의 합성 (4) Synthesis of intermediate 29-4
2-bromo-4-iodophenol (20 g, 0.067 mol, yurui), phenylboronic acid (9.79 g, 0.080 mol, sigma aldrich), potassium carbonate (18.50 g, 0.134 mol, sigma aldrich), Pd(PPh3)4 (3.87 g, 0.0033 mol, sigma aldrich), Toluene 220 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 29-4>를 14.1 g (수율84.6%) 수득하였다.2-bromo-4-iodophenol (20 g, 0.067 mol, yurui), phenylboronic acid (9.79 g, 0.080 mol, sigma aldrich), potassium carbonate (18.50 g, 0.134 mol, sigma aldrich), Pd(PPh 3 ) 4 ( 3.87 g, 0.0033 mol, sigma aldrich), 220 mL of Toluene, and 40 mL of Ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, 14.1 g (yield 84.6%) of <Intermediate 29-4> was obtained by performing column purification (N-HEXANE: MC) after layer separation using H 2 O:MC.
(5) 중간체 29-5의 합성 (5) Synthesis of intermediate 29-5
중간체 29-4 (14 g, 0.056 mol), 2-fluorophenylboronic acid (9.44 g, 0.067 mol, sigma aldrich), potassium carbonate (15.54 g, 0.112 mol, sigma aldrich), Pd(PPh3)4 (3.25 g, 0.0028 mol, sigma aldrich), Toluene 200 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 29-5>를 13.2 g (수율 88.9%) 수득하였다.Intermediate 29-4 (14 g, 0.056 mol), 2-fluorophenylboronic acid (9.44 g, 0.067 mol, sigma aldrich), potassium carbonate (15.54 g, 0.112 mol, sigma aldrich), Pd(PPh 3 ) 4 (3.25 g, 0.0028 mol, sigma aldrich), 200 mL of toluene, and 40 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, 13.2 g of <Intermediate 29-5> was obtained (yield 88.9%) by column purification (N-HEXANE: MC) after layer separation using H 2 O: MC.
(6) 중간체 29-6의 합성 (6) Synthesis of intermediate 29-6
중간체 29-5 (13 g, 0.050 mol), N-Bromosuccinimide (8.76 g, 0.050 mol, sigma aldrich), Dimethylformamide 150 mL 넣고 60 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 1M NaOH 수용액을 넣고 교반시켜 여과한 후 건조하여 <중간체 29-6>을 10.2 g (수율 60%) 수득하였다.Intermediate 29-5 (13 g, 0.050 mol), N-Bromosuccinimide (8.76 g, 0.050 mol, sigma aldrich), and 150 mL of dimethylformamide were added and reacted by stirring at 60 °C for 12 hours. After completion of the reaction, 1M aqueous NaOH solution was added, stirred, filtered, and dried to obtain 10.2 g (yield: 60%) of <Intermediate 29-6>.
(7) 중간체 29-7의 합성 (7) Synthesis of intermediate 29-7
중간체 29-6 (10 g, 0.030 mol), NaH (1.4 g, 0.058 mol, sigma aldrich), Dimethyl sulfoxide 100 mL 넣고 140 ℃에서 5시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 29-7>을 3 g (수율 31.9%) 수득하였다.Intermediate 29-6 (10 g, 0.030 mol), NaH (1.4 g, 0.058 mol, sigma aldrich), 100 mL of dimethyl sulfoxide were added, and the reaction was stirred at 140 °C for 5 hours. After completion of the reaction , layer separation was performed on H 2 O:MC, and column purification (N-HEXANE:MC) was performed to obtain 3 g (yield 31.9%) of <Intermediate 29-7>.
(8) 중간체 29-8의 합성 (8) Synthesis of intermediate 29-8
중간체 29-3 (10 g, 0.039 mol), 4-bromobiphenyl (9.89 g, 0.042 mol, sigma aldrich), Sodium tert-butoxide (7.41 g, 0.077 mol, sigma aldrich), 촉매 Pd(dba)2 (1.11 g, 0.0019 mol, sigma aldrich), tri-tert-Bu-phosphine(0.78 g, 0.004 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 4시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 <중간체 29-8>을 12.4 g (수율 78%) 수득하였다.Intermediate 29-3 (10 g, 0.039 mol), 4-bromobiphenyl (9.89 g, 0.042 mol, sigma aldrich), Sodium tert-butoxide (7.41 g, 0.077 mol, sigma aldrich), catalyst Pd(dba) 2 (1.11 g) , 0.0019 mol, sigma aldrich), tri-tert-Bu-phosphine (0.78 g, 0.004 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 4 hours to react. After completion of the reaction, 12.4 g (yield 78%) of <Intermediate 29-8> was obtained by performing column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
(9) 화합물 29의 합성 (9) Synthesis of compound 29
중간체 29-8 (10 g, 0.024 mol), 중간체 29-7 (9.42 g, 0.029 mol), Sodium tert-butoxide (4.67 g, 0.049 mol, sigma aldrich), 촉매 Pd(dba)2 (0.70 g, 0.0012 mol, sigma aldrich), tri-tert-Bu-phosphine (0.49 g, 0.0024 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 4시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 29를 11.7 g (수율 73.6%) 수득하였다.Intermediate 29-8 (10 g, 0.024 mol), Intermediate 29-7 (9.42 g, 0.029 mol), Sodium tert-butoxide (4.67 g, 0.049 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.70 g, 0.0012) mol, sigma aldrich), tri-tert-Bu-phosphine (0.49 g, 0.0024 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 4 hours to react. After completion of the reaction, 11.7 g (yield 73.6%) of compound 29 was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.89/d, 7.66/d, 7.57/s, 7.54/d, 7.38/m, 7.32/m, 7.07/s, 6.69/d) 3H(7.41/m) 6H(7.52/d, 7.51/m)H-NMR (200 MHz, CDCl3): δ ppm, 2H (7.89/d, 7.66/d, 7.57/s, 7.54/d, 7.38/m, 7.32/m, 7.07/s, 6.69/d) 3H (7.41/ m) 6H (7.52/d, 7.51/m)
LC/MS: m/z=653[(M+1)+]LC/MS: m/z=653 [(M+1) + ]
합성예Synthesis example 2 : 화합물 38의 합성 2: Synthesis of compound 38
(1) 중간체 38-1의 합성 (1) Synthesis of Intermediate 38-1
4,6-dibromodibenzofuran (20 g, 0.061 mol, Yurui), phenylboronic acid (8.98 g, 0.074 mol, sigma aldrich), potassium carbonate (16.96 g, 0.123 mol, sigma aldrich), Pd(PPh3)4 (3.54 g, 0.0031 mol, sigma aldrich), THF 250 mL, H2O 50 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 38-1>을 14.3 g (수율 72%) 수득하였다.4,6-dibromodibenzofuran (20 g, 0.061 mol, Yurui), phenylboronic acid (8.98 g, 0.074 mol, sigma aldrich), potassium carbonate (16.96 g, 0.123 mol, sigma aldrich), Pd(PPh 3 ) 4 (3.54 g) , 0.0031 mol, sigma aldrich), THF 250 mL, H 2 O 50 mL, and reacted by stirring under reflux for 4 hours. After completion of the reaction, 14.3 g (yield 72%) of <Intermediate 38-1> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(2) 중간체 38-2의 합성 (2) Synthesis of Intermediate 38-2
중간체 38-1 (14 g, 0.043 mol), Bis(pinacolato)dibron (14.3 g, 0.056 mol, sigma aldrich), potassium acetate (8.50 g, 0.087 mol, sigma aldrich), PdCl2(dppf) (0.95 g, 0.0013 mol, sigma aldrich), 1,4-Dioxane 200 mL 넣고 95 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O 넣고 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 38-2>을 11.3 g (수율 70.5%) 수득하였다.Intermediate 38-1 (14 g, 0.043 mol), Bis(pinacolato)dibron (14.3 g, 0.056 mol, sigma aldrich), potassium acetate (8.50 g, 0.087 mol, sigma aldrich), PdCl 2 (dppf) (0.95 g, 0.0013 mol, sigma aldrich) and 1,4-Dioxane (200 mL) were added, and the reaction was stirred at 95 °C for 12 hours. After completion of the reaction, H 2 O was added, the layers were separated, and column purification (N-HEXANE: MC) was performed to obtain 11.3 g (yield 70.5%) of <Intermediate 38-2>.
(3) 중간체 38-3의 합성 (3) Synthesis of Intermediate 38-3
1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), 중간체 38-2 (15.71 g, 0.042 mol, sigma aldrich), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd(PPh3)4 (2.04 g, 0.0018 mol, sigma aldrich), THF 250 mL, H2O 50 mL 넣고 12시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 38-3>을 11.6 g (수율 82%) 수득하였다.1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), intermediate 38-2 (15.71 g, 0.042 mol, sigma aldrich), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd (PPh 3) ) 4 (2.04 g, 0.0018 mol, sigma aldrich), 250 mL of THF, and 50 mL of H 2 O were added, and the reaction was stirred under reflux for 12 hours. After completion of the reaction, 11.6 g (yield 82%) of <Intermediate 38-3> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(4) 화합물 38의 합성 (4) Synthesis of compound 38
중간체 29-8 (10 g, 0.024 mol), 중간체 38-3 (11.64 g, 0.03 mol, sigma Aldrich), Sodium tert-butoxide (4.67 g, 0.049 mol, sigma aldrich), 촉매 Pd(dba)2 (0.70 g, 0.0012 mol, sigma aldrich), tri-tert-Bu-phosphine(0.49 g, 0.0024 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 38을 13.2 g (수율 74.4%) 수득하였다.Intermediate 29-8 (10 g, 0.024 mol), Intermediate 38-3 (11.64 g, 0.03 mol, sigma Aldrich), Sodium tert-butoxide (4.67 g, 0.049 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.70) g, 0.0012 mol, sigma aldrich), tri-tert-Bu-phosphine (0.49 g, 0.0024 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 8 hours. After the reaction was completed, 13.2 g of compound 38 (yield 74.4%) was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.66/d, 7.57/s, 7.32/m, 7.07/s) 2H(7.85/d, 7.81/d) 3H(7.41/m, 7.38/m) 4H(7.54/d, 6.69/d) 6H(7.52/d, 7.51/m)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.66/d, 7.57/s, 7.32/m, 7.07/s) 2H (7.85/d, 7.81/d) 3H (7.41/m, 7.38/m) 4H (7.54/d, 6.69/d) 6H (7.52/d, 7.51/m)
LC/MS: m/z=729[(M+1)+]LC/MS: m/z=729 [(M+1) + ]
합성예Synthesis example 3 : 화합물 48 합성 3: Synthesis of compound 48
(1) 중간체 48-1의 합성 (1) Synthesis of Intermediate 48-1
2-bromo-4-chlorobenzoic acid (20 g, 0.085 mol, sigma aldrich), iodomethane (18.08 g, 0.127 mol, sigma aldrich), Sodium bicarbonate (14.27 g, 0.17 mol, sigma aldrich)에 dimethylformamide 200 mL를 넣고 60 ℃에서 24시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 <중간체 48-1>을 12.4 g (수율 79%) 수득하였다.Add 200 mL of dimethylformamide to 2-bromo-4-chlorobenzoic acid (20 g, 0.085 mol, sigma aldrich), iodomethane (18.08 g, 0.127 mol, sigma aldrich), sodium bicarbonate (14.27 g, 0.17 mol, sigma aldrich) 60 The reaction was stirred at ℃ for 24 hours. After completion of the reaction, 12.4 g (yield 79%) of <Intermediate 48-1> was obtained by performing column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
(2) 중간체 48-2의 합성 (2) Synthesis of Intermediate 48-2
중간체 48-1 (12 g, 0.048 mol)에 THF 150 mL 넣고 0 ℃에서 교반하면서 phenyl magnesium bromide(13.08 g, 0.072mol, sigma aldrich) 를 천천히 넣어주고 상온에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 <중간체 48-2>를 11.2 g (수율 78.8%) 수득하였다.150 mL of THF was added to Intermediate 48-1 (12 g, 0.048 mol), and phenyl magnesium bromide (13.08 g, 0.072 mol, sigma aldrich) was slowly added thereto while stirring at 0 °C, followed by stirring at room temperature for 12 hours to react. After completion of the reaction, 11.2 g (yield 78.8%) of <Intermediate 48-2> was obtained by column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
(3) 중간체 48-3의 합성 (3) Synthesis of Intermediate 48-3
중간체 48-2 (20 g, 0.068 mol), silver oxide (23.52 g, 0.102 mol, sigma aldrich), potassium carbonate (23.38 g, 0.169 mol, sigma aldrich), 촉매 Pd(OAc)2 (0.61 g, 0.0011 mol, sigma aldrich)에 trifluoroacetic acid(TFA)를 넣고 140 ℃에서 24시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 <중간체 48-3>을 12.6 g (수율 77%) 수득하였다.Intermediate 48-2 (20 g, 0.068 mol), silver oxide (23.52 g, 0.102 mol, sigma aldrich), potassium carbonate (23.38 g, 0.169 mol, sigma aldrich), catalyst Pd(OAc) 2 (0.61 g, 0.0011 mol) , sigma aldrich) was added with trifluoroacetic acid (TFA), and the reaction was stirred at 140 °C for 24 hours. After completion of the reaction, 12.6 g (yield 77%) of <Intermediate 48-3> was obtained by performing column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
(4) 중간체 48-4의 합성 (4) Synthesis of Intermediate 48-4
중간체 48-3 (20 g, 0.068 mol), sodium borohydride (149.07 g, 0.341mol, sigma aldrich), anhydrous aluminum chloride (3.87 g, 0.102 mol, sigma aldrich), tetrahydrofuran 300 mL를 넣고 2시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 <중간체 48-3>을 13 g (수율 68%) 수득하였다.Intermediate 48-3 (20 g, 0.068 mol), sodium borohydride (149.07 g, 0.341 mol, sigma aldrich), anhydrous aluminum chloride (3.87 g, 0.102 mol, sigma aldrich), 300 mL of tetrahydrofuran were added and stirred under reflux for 2 hours. reacted. After completion of the reaction , layer separation was performed on H 2 O:EA, followed by column purification (N-HEXANE:EA) to obtain 13 g (yield 68%) of <Intermediate 48-3>.
(5) 중간체 48-5의 합성 (5) Synthesis of intermediate 48-5
중간체 48-4 (15 g, 0.054 mol), potassium tertbutoxide (18.06 g, 0.161 mol, sigma aldrich)를 tetrahydrofuran 200 mL를 넣고 0 ℃ 10분 동안 교반시킨 후 iodomethane(22.85 g, 0.161 mol, sigma aldrich)를 넣고 상온에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 Hexane을 넣고 교반 후 여과하고 컬럼정제 (N-HEXANE : EA)하여 <중간체 48-5>을 11 g (수율 66.7%) 수득하였다.Intermediate 48-4 (15 g, 0.054 mol), potassium tertbutoxide (18.06 g, 0.161 mol, sigma aldrich) was added to 200 mL of tetrahydrofuran and stirred at 0 °C for 10 minutes, followed by iodomethane (22.85 g, 0.161 mol, sigma aldrich). and stirred at room temperature for 12 hours to react. After completion of the reaction, hexane was added, stirred, filtered, and column purified (N-HEXANE: EA) to obtain 11 g (yield 66.7%) of <Intermediate 48-5>.
(6) 중간체 48-6의 합성 (6) Synthesis of intermediate 48-6
중간체 48-5 (15 g, 0.049 mol), 4-aminobiphenyl (9.90 g, 0.059 mol, sigma Aldrich), Sodium tert-butoxide (9.37 g, 0.098 mol, sigma aldrich), 촉매 Pd(dba)2 (1.40 g, 0.0024 mol, sigma aldrich), tri-tert-Bu-phosphine (0.99 g, 0.0049 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 48-6>을 15.8 g (수율 82%) 수득하였다.Intermediate 48-5 (15 g, 0.049 mol), 4-aminobiphenyl (9.90 g, 0.059 mol, sigma Aldrich), Sodium tert-butoxide (9.37 g, 0.098 mol, sigma aldrich), catalyst Pd(dba) 2 (1.40 g) , 0.0024 mol, sigma aldrich), tri-tert-Bu-phosphine (0.99 g, 0.0049 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 6 hours. After completion of the reaction, 15.8 g (yield 82%) of <Intermediate 48-6> was obtained by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
(7) 중간체 48-7의 합성 (7) Synthesis of intermediate 48-7
중간체 48-6 (15 g, 0.038 mol), phenylboronic acid (5.54 g, 0.046 mol, sigma aldrich), potassium carbonate (10.47 g, 0.076 mol, sigma aldrich), Pd(PPh3)4 (2.19 g, 0.0019 mol, sigma aldrich), Toluene 200 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 48-7>을 13.3 g (수율 80%) 수득하였다.Intermediate 48-6 (15 g, 0.038 mol), phenylboronic acid (5.54 g, 0.046 mol, sigma aldrich), potassium carbonate (10.47 g, 0.076 mol, sigma aldrich), Pd(PPh 3 ) 4 (2.19 g, 0.0019 mol) , sigma aldrich), toluene 200 mL, and ethanol 40 mL, and reacted by stirring under reflux for 4 hours. After completion of the reaction, 13.3 g (yield 80%) of <Intermediate 48-7> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(8) 화합물 48의 합성 (8) Synthesis of compound 48
중간체 48-5 (10 g, 0.023 mol), 중간체 38-3 (10.95 g, 0.027 mol), Sodium tert-butoxide (4.39 g, 0.046 mol, sigma aldrich), 촉매 Pd(dba)2 (0.66 g, 0.0011 mol, sigma aldrich), tri-tert-Bu-phosphine (0.46 g, 0.0023 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 48을 13.2 g (수율 76%) 수득하였다.Intermediate 48-5 (10 g, 0.023 mol), Intermediate 38-3 (10.95 g, 0.027 mol), Sodium tert-butoxide (4.39 g, 0.046 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.66 g, 0.0011) mol, sigma aldrich), tri-tert-Bu-phosphine (0.46 g, 0.0023 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 6 hours to react. After completion of the reaction, 13.2 g (yield 76%) of compound 48 was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.87/d, 7.55/d, 7.42/s, 7.28/m, 6.70/s) 2H(7.85/d, 7.81/d) 3H(7.41/m, 7.38/m) 4H(7.54/d, 6.69/d) 6H(7.52/d, 7.51/m, 1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.87/d, 7.55/d, 7.42/s, 7.28/m, 6.70/s) 2H (7.85/d, 7.81/d) 3H (7.41/m, 7.38/m) 4H (7.54/d, 6.69/d) 6H (7.52/d, 7.51/m, 1.72/s)
LC/MS: m/z=755[(M+1)+]LC/MS: m/z=755[(M+1) + ]
합성예Synthesis example 4 : 화합물 58의 합성 4: Synthesis of compound 58
(1) 중간체 58-1의 합성 (1) Synthesis of Intermediate 58-1
2-bromo-4-iodobenzenethiol (15 g, 0.048 mol, yurui), phenylboronic acid (6.99 g, 0.057 mol, sigma aldrich), potassium carbonate (13.21 g, 0.096 mol, sigma aldrich), Pd(PPh3)4 (2.76 g, 0.0024 mol, sigma aldrich), Toluene 200 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 58-1>을 10g (수율 79%) 수득하였다.2-bromo-4-iodobenzenethiol (15 g, 0.048 mol, yurui), phenylboronic acid (6.99 g, 0.057 mol, sigma aldrich), potassium carbonate (13.21 g, 0.096 mol, sigma aldrich), Pd(PPh 3 ) 4 ( 2.76 g, 0.0024 mol, sigma aldrich), 200 mL of toluene, and 40 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, the layers were separated using H 2 O: MC, and then column purification (N-HEXANE: MC) was performed to obtain 10 g (yield 79%) of <Intermediate 58-1>.
(2) 중간체 58-2의 합성 (2) Synthesis of Intermediate 58-2
중간체 58-1 (15 g, 0.057 mol), 2-fluorophenylboronic acid (9.50 g, 0.068 mol, sigma aldrich), potassium carbonate (15.64 g, 0.113 mol, sigma aldrich), Pd(PPh3)4 (3.27 g, 0.0028 mol, sigma aldrich), Toluene 200 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 58-2>을 12.7 g (수율 80%) 수득하였다.Intermediate 58-1 (15 g, 0.057 mol), 2-fluorophenylboronic acid (9.50 g, 0.068 mol, sigma aldrich), potassium carbonate (15.64 g, 0.113 mol, sigma aldrich), Pd(PPh 3 ) 4 (3.27 g, 0.0028 mol, sigma aldrich), 200 mL of toluene, and 40 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, 12.7 g (yield 80%) of <Intermediate 58-2> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(3) 중간체 58-3의 합성 (3) Synthesis of Intermediate 58-3
중간체 58-2 (10 g, 0.0360 mol), N-Bromosuccinimide (6.35 g, 0.036 mol, sigma aldrich), Dimethylformamide 150 mL 넣고 60 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 1M NaOH 수용액을 넣고 교반시켜 여과한 후 건조하여 <중간체 58-3>을 9.7 g (수율 75.7%) 수득하였다.Intermediate 58-2 (10 g, 0.0360 mol), N-Bromosuccinimide (6.35 g, 0.036 mol, sigma aldrich), and 150 mL of Dimethylformamide were added and reacted by stirring at 60 °C for 12 hours. After completion of the reaction, 1M aqueous NaOH solution was added, stirred, filtered, and dried to obtain 9.7 g (yield 75.7%) of <Intermediate 58-3>.
(4) 중간체 58-4의 합성 (4) Synthesis of intermediate 58-4
중간체 29-6 (10 g, 0.028 mol), NaH (1.0 g, 0.042 mol, sigma aldrich), Dimethyl sulfoxide 100 mL 넣고 140 ℃에서 5시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 58-4>를 3.3 g (수율 35%) 수득하였다.Intermediate 29-6 (10 g, 0.028 mol), NaH (1.0 g, 0.042 mol, sigma aldrich), and 100 mL of dimethyl sulfoxide were added, and the reaction was stirred at 140 °C for 5 hours. After completion of the reaction , layer separation was performed on H 2 O: MC, and column purification (N-HEXANE: MC) was performed to obtain 3.3 g (yield 35%) of <Intermediate 58-4>.
(5) 중간체 58-5의 합성 (5) Synthesis of intermediate 58-5
중간체 58-4 (10 g, 0.029 mol), 4-aminobiphenyl (5.97 g, 0.035 mol, sigma aldrich), Sodium tert-butoxide (5.65 g, 0.059 mol, sigma aldrich), 촉매 Pd(dba)2 (0.85 g, 0.0015 mol, sigma aldrich), tri-tert-Bu-phosphine(0.60 g, 0.0029 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 58-5>를 10 g (수율 79.5%) 수득하였다.Intermediate 58-4 (10 g, 0.029 mol), 4-aminobiphenyl (5.97 g, 0.035 mol, sigma aldrich), Sodium tert-butoxide (5.65 g, 0.059 mol, sigma aldrich), catalyst Pd(dba) 2 (0.85 g , 0.0015 mol, sigma aldrich), tri-tert-Bu-phosphine (0.60 g, 0.0029 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 6 hours. After completion of the reaction, 10 g (yield 79.5%) of <Intermediate 58-5> was obtained by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
(6) 화합물 58의 합성 (6) Synthesis of compound 58
중간체 58-5 (10 g, 0.023 mol), 중간체 38-3 (11.21 g, 0.028 mol), Sodium tert-butoxide (4.5 g, 0.047 mol, sigma aldrich), 촉매 Pd(dba)2 (0.67 g, 0.0012 mol, sigma aldrich), tri-tert-Bu-phosphine (0.47 g, 0.0023 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 58을 13.9 g (수율 79.7%) 수득하였다.Intermediate 58-5 (10 g, 0.023 mol), Intermediate 38-3 (11.21 g, 0.028 mol), Sodium tert-butoxide (4.5 g, 0.047 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.67 g, 0.0012) mol, sigma aldrich), tri-tert-Bu-phosphine (0.47 g, 0.0023 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 6 hours to react. After the reaction was completed, 13.9 g of compound 58 (yield 79.7%) was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.45/d, 7.98/d, 7.50/m, 7.36/s, 7.15/s) 2H(7.85/d, 7.81/d, 7.38/m) 3H(7.41/m) 4H(7.54/d, 6.69/d) 6H(7.51/m) 7H(7.52/m)H-NMR (200 MHz, CDCl3): δ ppm, 1H (8.45/d, 7.98/d, 7.50/m, 7.36/s, 7.15/s) 2H (7.85/d, 7.81/d, 7.38/m) 3H ( 7.41/m) 4H (7.54/d, 6.69/d) 6H (7.51/m) 7H (7.52/m)
LC/MS: m/z=745[(M+1)+]LC/MS: m/z=745[(M+1) + ]
합성예Synthesis example 5 : 화합물 69의 합성 5: Synthesis of compound 69
(1) 중간체 69-1의 합성 (1) Synthesis of Intermediate 69-1
2-bromodibenzofuran (15 g, 0.061 mol, sigma aldrich), Bis(pinacolato)dibron (13.58 g, 0.079 mol, sigma aldrich), potassium acetate (11.92 g, 0.121 mol, sigma aldrich), PdCl2(dppf) (1.33 g, 0.0018 mol, sigma aldrich), 1,4-Dioxane 200 mL 넣고 95 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O 넣고 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 69-1>을 12.5 g (수율 70%) 수득하였다.2-bromodibenzofuran (15 g, 0.061 mol, sigma aldrich), Bis(pinacolato)dibron (13.58 g, 0.079 mol, sigma aldrich), potassium acetate (11.92 g, 0.121 mol, sigma aldrich), PdCl 2 (dppf) (1.33) g, 0.0018 mol, sigma aldrich), and 1,4-Dioxane (200 mL) were added, and the reaction was stirred at 95 °C for 12 hours. After completion of the reaction , 12.5 g (yield 70%) of <Intermediate 69-1> was obtained by adding H 2 O, performing layer separation, and performing column purification (N-HEXANE: MC).
(2) 중간체 69-2의 합성 (2) Synthesis of Intermediate 69-2
1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), 중간체 69-1 (13.52 g, 0.046 mol), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd(PPh3)4 (2.04 g, 0.0018 mol, sigma aldrich), THF 250 mL, H2O 50 mL 넣고 12시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 69-2>를 9.6 g (수율 84%) 수득하였다.1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), Intermediate 69-1 (13.52 g, 0.046 mol), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd(PPh 3 ) 4 ( 2.04 g, 0.0018 mol, sigma aldrich), 250 mL of THF, and 50 mL of H 2 O were added, and the reaction was stirred under reflux for 12 hours. After completion of the reaction, layer separation was performed using H 2 O: MC, and column purification (N-HEXANE: MC) was performed to obtain 9.6 g (yield 84%) of <Intermediate 69-2>.
(3) 중간체 69-3의 합성 (3) Synthesis of Intermediate 69-3
중간체 29-3 (10 g, 0.039 mol), 중간체 29-7 (14.96 g, 0.046 mol), Sodium tert-butoxide (7.41 g, 0.077 mol, sigma aldrich), 촉매 Pd(dba)2 (1.11 g, 0.0019 mol, sigma aldrich), tri-tert-Bu-phosphine (0.78 g, 0.0039 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 69-3>을 15.3 g (수율 79%) 수득하였다.Intermediate 29-3 (10 g, 0.039 mol), Intermediate 29-7 (14.96 g, 0.046 mol), Sodium tert-butoxide (7.41 g, 0.077 mol, sigma aldrich), Catalyst Pd(dba) 2 (1.11 g, 0.0019) mol, sigma aldrich), tri-tert-Bu-phosphine (0.78 g, 0.0039 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 6 hours to react. After completion of the reaction, 15.3 g (yield 79%) of <Intermediate 69-3> was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
(4) 화합물 69의 합성 (4) Synthesis of compound 69
중간체 69-3 (10 g, 0.020 mol), 중간체 69-2 (7.73 g, 0.024 mol), Sodium tert-butoxide (3.83 g, 0.040 mol, sigma aldrich), 촉매 Pd(dba)2 (0.57 g, 0.0010 mol, sigma aldrich), tri-tert-Bu-phosphine (0.40 g, 0.0020 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 69를 11.7 g (수율 78.9%) 수득하였다.Intermediate 69-3 (10 g, 0.020 mol), Intermediate 69-2 (7.73 g, 0.024 mol), Sodium tert-butoxide (3.83 g, 0.040 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.57 g, 0.0010) mol, sigma aldrich), tri-tert-Bu-phosphine (0.40 g, 0.0020 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 12 hours to react. After the reaction was completed, 11.7 g of compound 69 was obtained (yield 78.9%) by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.85/d, 7.81/d) 2H(7.57/s, 7.54/d, 7.41/m, 7.07/s, 6.69/d) 3H(7.89/d, 7.66/d, 7.32/m) 4H(7.52/d, 7.51/m, 7.38/m)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.85/d, 7.81/d) 2H (7.57/s, 7.54/d, 7.41/m, 7.07/s, 6.69/d) 3H (7.89/d, 7.66/d, 7.32/m) 4H (7.52/d, 7.51/m, 7.38/m)
LC/MS: m/z=743[(M+1)+]LC/MS: m/z=743[(M+1) + ]
합성예Synthesis example 6 : 화합물 75의 합성 6: Synthesis of compound 75
(1) 중간체 75-1의 합성 (1) Synthesis of Intermediate 75-1
중간체 29-7 (15 g, 0.046 mol), Bis(pinacolato)dibron (10.38 g, 0.060 mol, sigma aldrich), potassium acetate (9.11 g, 0.093 mol, sigma aldrich), PdCl2(dppf) (1.02 g, 0.0014 mol, sigma aldrich), 1,4-Dioxane 200 mL 넣고 95 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O 넣고 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 75-1>을 12.6 g (수율 73%) 수득하였다.Intermediate 29-7 (15 g, 0.046 mol), Bis(pinacolato)dibron (10.38 g, 0.060 mol, sigma aldrich), potassium acetate (9.11 g, 0.093 mol, sigma aldrich), PdCl 2 (dppf) (1.02 g, 0.0014 mol, sigma aldrich) and 1,4-Dioxane (200 mL) were added, and the reaction was stirred at 95 °C for 12 hours. After completion of the reaction, H 2 O was added, and the layers were separated and then purified by column (N-HEXANE: MC) to obtain 12.6 g (yield 73%) of <Intermediate 75-1>.
(2) 중간체 75-2의 합성 (2) Synthesis of Intermediate 75-2
1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), 중간체 75-1 (15.71 g, 0.042 mol), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd(PPh3)4 (2.04 g, 0.0018 mol, sigma aldrich), THF 150 mL, H2O 30 mL 넣고 6시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 75-2>를 11.7 g (수율 83%) 수득하였다.1-bromo-4-iodobenzene (10 g, 0.035 mol, sigma aldrich), intermediate 75-1 (15.71 g, 0.042 mol), potassium carbonate (9.77 g, 0.071 mol, sigma aldrich), Pd(PPh 3 ) 4 ( 2.04 g, 0.0018 mol, sigma aldrich), 150 mL of THF, and 30 mL of H 2 O were added, and the reaction was stirred under reflux for 6 hours. After completion of the reaction, 11.7 g (yield 83%) of <Intermediate 75-2> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(3) 중간체 75-3의 합성 (3) Synthesis of intermediate 75-3
9,9-dimethyl-9H-fluoren-2-amine (20 g, 0.096 mol, Yurui), 4-bromobiphenyl (22.28 g, 0.096 mol, sigma aldrich), Sodium tert-butoxide (27.55 g, 0.28 mol, sigma aldrich), 촉매 Pd(dba)2 (2.75 g, 0.005 mol, sigma aldrich), tri-tert-Bu-phosphine(1.93 g, 0.01 mol, sigma aldrich)에 Toluene 200 mL를 넣고 100 ℃에서 3시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE:MC)하여 <중간체 75-3>을 28 g (수율 81%) 수득하였다.9,9-dimethyl-9H-fluoren-2-amine (20 g, 0.096 mol, Yurui), 4-bromobiphenyl (22.28 g, 0.096 mol, sigma aldrich), Sodium tert-butoxide (27.55 g, 0.28 mol, sigma aldrich) ), catalyst Pd(dba) 2 (2.75 g, 0.005 mol, sigma aldrich), tri-tert-Bu-phosphine (1.93 g, 0.01 mol, sigma aldrich) into 200 mL of Toluene and stirred at 100 °C for 3 hours. reacted. After completion of the reaction , the layer was separated on H 2 O: MC and then column purified (N-HEXANE:MC) to obtain 28 g (yield 81%) of <Intermediate 75-3>.
(4) 화합물 75의 합성 (4) Synthesis of compound 75
중간체 75-2 (10 g, 0.025 mol), 중간체 75-3 (10.86 g, 0.030 mol), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 75을 13.7 g (수율 75%) 수득하였다.Intermediate 75-2 (10 g, 0.025 mol), Intermediate 75-3 (10.86 g, 0.030 mol), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.72 g, 0.0013) mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 8 hours to react. After the reaction was completed, 13.7 g of compound 75 was obtained (yield 75%) by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.87/d, 7.67/s, 7.66/d, 7.63/s, 7.62/d, 7.55/d, 7.32/m, 7.28/m, 6.75/s, 6.58/d) 2H(7.41/m, 7.38/m) 4H(7.54/d, 7.52/d, 7.51/m, 6.69/d) 6H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.87/d, 7.67/s, 7.66/d, 7.63/s, 7.62/d, 7.55/d, 7.32/m, 7.28/m, 6.75/s, 6.58/d) 2H (7.41/m, 7.38/m) 4H (7.54/d, 7.52/d, 7.51/m, 6.69/d) 6H (1.72/s)
LC/MS: m/z=679[(M+1)+]LC/MS: m/z=679[(M+1) + ]
합성예Synthesis example 7 : 화합물 86의 합성 7: Synthesis of compound 86
(1) 중간체 86-1의 합성 (1) Synthesis of Intermediate 86-1
중간체 75-2 (10 g, 0.025 mol), 4-aminobiphenyl (5.09 g, 0.030 mol, sigma aldrich), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine(0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 86-1>을 9.6 g (수율 78.6%) 수득하였다.Intermediate 75-2 (10 g, 0.025 mol), 4-aminobiphenyl (5.09 g, 0.030 mol, sigma aldrich), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), catalyst Pd(dba) 2 (0.72 g) , 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 6 hours. After completion of the reaction , layer separation was performed on H 2 O:MC, followed by column purification (N-HEXANE:MC) to obtain 9.6 g of <Intermediate 86-1> (yield 78.6%).
(2) 화합물 86의 합성 (2) Synthesis of compound 86
중간체 38-3 (10 g, 0.025 mol), 중간체 86-1 (14.65 g, 0.030 mol), Sodium tert-butoxide (4.81 g, 0.051 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 86을 16.5 g (수율 81.7%) 수득하였다.Intermediate 38-3 (10 g, 0.025 mol), Intermediate 86-1 (14.65 g, 0.030 mol), Sodium tert-butoxide (4.81 g, 0.051 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.72 g, 0.0013) mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 8 hours to react. After the reaction was completed, 16.5 g of compound 86 was obtained (yield: 81.7%) by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.67/s, 7.66/d, 7.63/s, 7.32/m) 2H(7.85/d, 7.81/d) 3H(7.41/m, 7.38/m) 6H(7.54/d, 7.52/d, 7.51/m, 6.69/d)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.67/s, 7.66/d, 7.63/s, 7.32/m) 2H (7.85/d, 7.81/d) 3H (7.41/m, 7.38/m) 6H (7.54/d, 7.52/d, 7.51/m, 6.69/d)
LC/MS: m/z=805[(M+1)+]LC/MS: m/z=805[(M+1) + ]
합성예Synthesis example 8 : 화합물 96의 합성 8: Synthesis of compound 96
2-amino-9,9-dimethylfluorene (10 g, 0.025 mol, TCI), 중간체 75-3 (14.80 g, 0.030 mol), Sodium tert-butoxide (4.86 g, 0.051 mol, sigma aldrich), 촉매 Pd(dba)2 (0.73 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine(0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 96을 16.4 g (수율 81%) 수득하였다.2-amino-9,9-dimethylfluorene (10 g, 0.025 mol, TCI), intermediate 75-3 (14.80 g, 0.030 mol), sodium tert-butoxide (4.86 g, 0.051 mol, sigma aldrich), catalyst Pd (dba ) 2 (0.73 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene and reacted by stirring at 100 °C for 8 hours. After completion of the reaction, 16.4 g (yield 81%) of compound 96 was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.87/d, 7.67/s, 7.66/d, 7.63/s, 7.62/d, 7.55/d, 7.32/m, 7.28/m, 6.75/s, 6.58/d) 2H(7.75/d, 7.41/m, 7.38/m, 7.35/d, 7.19/m, 7.16/m) 4H(7.54/d, 7.52/d, 7.51/m, 6.69/d)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.87/d, 7.67/s, 7.66/d, 7.63/s, 7.62/d, 7.55/d, 7.32/m, 7.28/m, 6.75/s, 6.58/d) 2H (7.75/d, 7.41/m, 7.38/m, 7.35/d, 7.19/m, 7.16/m) 4H (7.54/d, 7.52/d, 7.51/m, 6.69/ d)
LC/MS: m/z=801[(M+1)+]LC/MS: m/z=801[(M+1) + ]
합성예Synthesis example 9 : 화합물 111의 합성 9: Synthesis of compound 111
(1) 중간체 111-1의 합성 (1) Synthesis of intermediate 111-1
중간체 75-2 (10 g, 0.025 mol), 2-amino-9,9-dimethylfluorene (6.29 g, 0.030 mol, TCI), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine(0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 4시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 111-1>을 10.6 g (수율 80%) 수득하였다.Intermediate 75-2 (10 g, 0.025 mol), 2-amino-9,9-dimethylfluorene (6.29 g, 0.030 mol, TCI), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), Catalyst Pd (dba ) 2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 4 hours. After completion of the reaction, 10.6 g (yield 80%) of <Intermediate 111-1> was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
(2) 화합물 111의 합성 (2) Synthesis of compound 111
중간체 111-1 (10 g, 0.019 mol), 2-bromo-9,9-dimethylfluorene (6.21 g, 0.023 mol, sigma aldrich), Sodium tert-butoxide (3.64 g, 0.038 mol, sigma aldrich), 촉매 Pd(dba)2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine(0.38 g, 0.0019 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 111을 10.8 g (수율 79%) 수득하였다.Intermediate 111-1 (10 g, 0.019 mol), 2-bromo-9,9-dimethylfluorene (6.21 g, 0.023 mol, sigma aldrich), Sodium tert-butoxide (3.64 g, 0.038 mol, sigma aldrich), catalyst Pd ( 150 mL of Toluene was added to dba) 2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine (0.38 g, 0.0019 mol, sigma aldrich), and the reaction was stirred at 100 °C for 8 hours. After completion of the reaction, 10.8 g (yield 79%) of compound 111 was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.67/s, 7.66/d, 7.63/s, 7.41/m, 7.32/m) 2H(7.87/d, 7.62/d, 7.55/d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 6.75/s, 6.69/d, 6.58/d) 3H(7.38/m) 12H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.67/s, 7.66/d, 7.63/s, 7.41/m, 7.32/m) 2H (7.87/d, 7.62/d, 7.55/ d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 6.75/s, 6.69/d, 6.58/d) 3H (7.38/m) 12H (1.72/s)
LC/MS: m/z=719[(M+1)+]LC/MS: m/z=719[(M+1) + ]
합성예Synthesis example 10 : 화합물 126의 합성 10: Synthesis of compound 126
(1) 중간체 126-1의 합성 (1) Synthesis of Intermediate 126-1
2-bromo-9,9-dimethylfluorene (15 g, 0.055 mol, sigma aldrich), Bis(pinacolato)dibron (18.13 g, 0.071 mol, sigma aldrich), potassium acetate (10.78 g, 0.110 mol, sigma aldrich), PdCl2(dppf) (1.21 g, 0.0016 mol, sigma aldrich), 1,4-Dioxane 200 mL 넣고 95 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O 넣고 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-1>을 12.3 g (수율 70%) 수득하였다.2-bromo-9,9-dimethylfluorene (15 g, 0.055 mol, sigma aldrich), Bis(pinacolato)dibron (18.13 g, 0.071 mol, sigma aldrich), potassium acetate (10.78 g, 0.110 mol, sigma aldrich), PdCl 2 (dppf) (1.21 g, 0.0016 mol, sigma aldrich), 200 mL of 1,4-Dioxane was added, and the reaction was stirred at 95 °C for 12 hours. After completion of the reaction, H 2 O was added, the layers were separated, and column purification was performed (N-HEXANE: MC) to obtain 12.3 g (yield 70%) of <Intermediate 126-1>.
(2) 중간체 126-2의 합성 (2) Synthesis of Intermediate 126-2
2-bromo-4-iodophenol (10 g, 0.034 mol, yurui), 중간체 126-1 (12.86 g, 0.040 mol), potassium carbonate (9.25 g, 0.067 mol, sigma aldrich), Pd(PPh3)4 (1.93 g, 0.0017 mol, sigma aldrich), THF 150 mL, H2O 30 mL 넣고 5시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-2>를 10 g (수율 82%) 수득하였다.2-bromo-4-iodophenol (10 g, 0.034 mol, yurui), intermediate 126-1 (12.86 g, 0.040 mol), potassium carbonate (9.25 g, 0.067 mol, sigma aldrich), Pd(PPh 3 ) 4 (1.93) g, 0.0017 mol, sigma aldrich), THF 150 mL, H 2 O 30 mL, and reacted by stirring under reflux for 5 hours. After completion of the reaction, layer separation was performed using H 2 O: MC, and column purification (N-HEXANE: MC) was performed to obtain 10 g (yield 82%) of <Intermediate 126-2>.
(3) 중간체 126-3의 합성 (3) Synthesis of Intermediate 126-3
중간체 126-2 (15 g, 0.041 mol), 2-fluorophenylboronic acid (6.90 g, 0.049 mol, sigma aldrich), potassium carbonate (11.35 g, 0.082 mol, sigma aldrich), Pd(PPh3)4 (2.37 g, 0.0021 mol, sigma aldrich), Toluene 200 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-3>을 12.2 g (수율 78%) 수득하였다.Intermediate 126-2 (15 g, 0.041 mol), 2-fluorophenylboronic acid (6.90 g, 0.049 mol, sigma aldrich), potassium carbonate (11.35 g, 0.082 mol, sigma aldrich), Pd(PPh 3 ) 4 (2.37 g, 0.0021 mol, sigma aldrich), 200 mL of toluene, and 40 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, 12.2 g (yield 78%) of <Intermediate 126-3> was obtained through column purification (N-HEXANE: MC) after layer separation using H 2 O: MC.
(4) 중간체 126-4의 합성 (4) Synthesis of Intermediate 126-4
중간체 126-4 (15 g, 0.050 mol), N-Bromosuccinimide (7.72 g, 0.043 mol, sigma aldrich), Dimethylformamide 200 mL 넣고 60 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 1M NaOH 수용액을 넣고 교반시켜 여과한 후 건조하여 <중간체 126-4>를 13 g (수율 71.8%) 수득하였다.Intermediate 126-4 (15 g, 0.050 mol), N-Bromosuccinimide (7.72 g, 0.043 mol, sigma aldrich), and 200 mL of dimethylformamide were added and reacted by stirring at 60 °C for 12 hours. After completion of the reaction, 1M aqueous NaOH solution was added, stirred, filtered, and dried to obtain 13 g (yield 71.8%) of <Intermediate 126-4>.
(5) 중간체 126-5의 합성 (5) Synthesis of Intermediate 126-5
중간체 126-4 (15 g, 0.033 mol), NaH (1.18 g, 0.049 mol, sigma aldrich), Dimethyl sulfoxide 200 mL 넣고 140 ℃에서 5시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-5>를 5.6 g (수율 39%) 수득하였다.Intermediate 126-4 (15 g, 0.033 mol), NaH (1.18 g, 0.049 mol, sigma aldrich), 200 mL of dimethyl sulfoxide was added, and the reaction was stirred at 140 °C for 5 hours. After completion of the reaction , layer separation was performed on H 2 O: MC, followed by column purification (N-HEXANE: MC) to obtain 5.6 g (yield 39%) of <Intermediate 126-5>.
(6) 중간체 126-6의 합성 (6) Synthesis of Intermediate 126-6
중간체 126-5 (15 g, 0.034 mol), 4-aminobiphenyl (6.93 g, 0.041 mol, sigma Aldrich), Sodium tert-butoxide (6.56 g, 0.068 mol, sigma aldrich), 촉매 Pd(dba)2 (0.98 g, 0.0017 mol, sigma aldrich), tri-tert-Bu-phosphine (0.68 g, 0.0034 mol, sigma aldrich)에 Toluene 200 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-6>을 14.5 g (수율 80.5%) 수득하였다.Intermediate 126-5 (15 g, 0.034 mol), 4-aminobiphenyl (6.93 g, 0.041 mol, sigma Aldrich), Sodium tert-butoxide (6.56 g, 0.068 mol, sigma aldrich), catalyst Pd(dba) 2 (0.98 g) , 0.0017 mol, sigma aldrich), tri-tert-Bu-phosphine (0.68 g, 0.0034 mol, sigma aldrich) was added with 200 mL of toluene and stirred at 100 °C for 6 hours to react. After completion of the reaction, 14.5 g (yield 80.5%) of <Intermediate 126-6> was obtained by column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
(7) 중간체 126-7의 합성 (7) Synthesis of Intermediate 126-7
3-bromo-9H-carbazole (20 g, 0.081 mol, sigma aldrich), iodobenzene (41.4 g, 0.203 mol, sigma aldrich), potassium carbonate (33.69 g, 0.244 mol, sigma aldrich), Cu (10.33 g, 0.162 mol, sigma aldrich), dibenzo-18-crown-6 (2.93 g, 0.008 mol, sigma Aldrich), Dimethylformamide 150 mL 넣고 150 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE)하여 <중간체 126-7>을 24 g (수율 93%) 수득하였다.3-bromo-9H-carbazole (20 g, 0.081 mol, sigma aldrich), iodobenzene (41.4 g, 0.203 mol, sigma aldrich), potassium carbonate (33.69 g, 0.244 mol, sigma aldrich), Cu (10.33 g, 0.162 mol) , sigma aldrich), dibenzo-18-crown-6 (2.93 g, 0.008 mol, sigma Aldrich), and 150 mL of dimethylformamide were added and reacted by stirring at 150 °C for 12 hours. After the reaction was completed , the layers were separated in H 2 O:MC and then column purified (N-HEXANE) to obtain 24 g (yield 93%) of <Intermediate 126-7>.
(8) 중간체 126-8의 합성 (8) Synthesis of Intermediate 126-8
중간체 126-7 (36.86 g, 0.114 mol), Bis(pinacolato)dibron (34.86 g, 0.137 mol, sigma aldrich), potassium acetate (33.68 g, 0.343 mol, sigma aldrich), PdCl2(dppf) (2.488 g, 0.0034 mol, sigma aldrich), 1,4-Dioxane 400 mL 넣고 95 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : Toluene에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-8>을 19 g (수율 40%) 수득하였다.Intermediate 126-7 (36.86 g, 0.114 mol), Bis(pinacolato)dibron (34.86 g, 0.137 mol, sigma aldrich), potassium acetate (33.68 g, 0.343 mol, sigma aldrich), PdCl 2 (dppf) (2.488 g, 0.0034 mol, sigma aldrich) and 1,4-Dioxane (400 mL) were added, and the reaction was stirred at 95 °C for 12 hours. After the reaction was completed , the layers were separated in H 2 O: Toluene, and then column purification (N-HEXANE: MC) was performed to obtain 19 g (yield 40%) of <Intermediate 126-8>.
(9) 중간체 126-9의 합성 (9) Synthesis of Intermediate 126-9
중간체 126-8 (17 g, 0.046 mol), 1-boromo-4-iodobenzene (15.68 g, 0.055 mol, sigma aldrich), sodium hydroxide (5.54 g, 0.138 mol, sigma aldrich), Pd(PPh3)4 (3.26 g, 0.0028 mol, sigma aldrich), THF 200 mL, 물 60 mL 넣고 60 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 126-9>를 16.9 g (수율 92%) 수득하였다.Intermediate 126-8 (17 g, 0.046 mol), 1-boromo-4-iodobenzene (15.68 g, 0.055 mol, sigma aldrich), sodium hydroxide (5.54 g, 0.138 mol, sigma aldrich), Pd(PPh 3 ) 4 ( 3.26 g, 0.0028 mol, sigma aldrich), 200 mL of THF, and 60 mL of water were added, and the reaction was stirred at 60 °C for 12 hours. After completion of the reaction, 16.9 g (yield 92%) of <Intermediate 126-9> was obtained by performing layer separation using H 2 O: MC and column purification (N-HEXANE: MC).
(10) 화합물 126의 합성 (10) Synthesis of compound 126
중간체 126-9 (10 g, 0.025 mol), 중간체 126-6 (15.9 g, 0.030 mol), Sodium tert-butoxide (4.83 g, 0.050 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 6시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 126을 17 g (수율 80%) 수득하였다.Intermediate 126-9 (10 g, 0.025 mol), Intermediate 126-6 (15.9 g, 0.030 mol), Sodium tert-butoxide (4.83 g, 0.050 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.72 g, 0.0013) mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene, followed by stirring at 100 °C for 6 hours. After completion of the reaction , layer separation was performed on H 2 O: MC, followed by column purification (N-HEXANE: MC) to obtain 17 g of compound 126 (yield 80%).
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.55/d, 7.94/d, 7.93/d, 7.89/d, 7.69/d, 7.66/d, 7.63/d, 7.57/s, 7.55/d, 7.45/m, 7.41/m, 7.33/m, 7.32/m, 7.28/m, 7.25/m, 7.07/s) 2H(7.87/d, 7.77/d, 7.58/m, 7.52/d, 7.51/m, 7.50/d, 7.38/m) 4H(7.54/d, 6.69/d) 6H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (8.55/d, 7.94/d, 7.93/d, 7.89/d, 7.69/d, 7.66/d, 7.63/d, 7.57/s, 7.55/d, 7.45/m, 7.41/m, 7.33/m, 7.32/m, 7.28/m, 7.25/m, 7.07/s) 2H (7.87/d, 7.77/d, 7.58/m, 7.52/d, 7.51/m, 7.50/d, 7.38/m) 4H (7.54/d, 6.69/d) 6H (1.72/s)
LC/MS: m/z=844[(M+1)+]LC/MS: m/z=844 [(M+1) + ]
합성예Synthesis example 11 : 화합물 133의 합성 11: Synthesis of compound 133
중간체 126-6 (10 g, 0.019 mol), 2-amino-9,9-dimethylfluorene (8.99 g, 0.023 mol, TCI), Sodium tert-butoxide (3.65 g, 0.038 mol, sigma aldrich), 촉매 Pd(dba)2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine(0.38 g, 0.0019 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 133을 13 g (수율 81%) 수득하였다.Intermediate 126-6 (10 g, 0.019 mol), 2-amino-9,9-dimethylfluorene (8.99 g, 0.023 mol, TCI), Sodium tert-butoxide (3.65 g, 0.038 mol, sigma aldrich), Catalyst Pd (dba ) 2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine (0.38 g, 0.0019 mol, sigma aldrich) was added with 150 mL of Toluene, followed by stirring at 100 °C for 8 hours. After completion of the reaction , layer separation was performed on H 2 O: MC, followed by column purification (N-HEXANE: MC) to obtain 13 g of compound 133 (yield 81%).
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.93/d, 7.89/d, 7.77/s, 7.66/d, 7.63/d, 7.62/d, 7.57/s, 7.41/m, 7.32/m, 7.07/s, 6.75/s, 6.58/d) 2H(7.87/d, 7.55/d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 7.26/m, 6.69/d) 3H(7.38/m) 4H(7.33/m, 7.11/d) 6H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.93/d, 7.89/d, 7.77/s, 7.66/d, 7.63/d, 7.62/d, 7.57/s, 7.41/m, 7.32/m, 7.07/s, 6.75/s, 6.58/d) 2H (7.87/d, 7.55/d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 7.26/m, 6.69/d) 3H (7.38/d) m) 4H (7.33/m, 7.11/d) 6H (1.72/s)
LC/MS: m/z=844[(M+1)+]LC/MS: m/z=844 [(M+1) + ]
합성예Synthesis example 12 : 화합물 134의 합성 12: Synthesis of compound 134
중간체 126-6 (10 g, 0.019 mol), 2-amino-9,9-dimethylfluorene (6.21 g, 0.023 mol, TCI), Sodium tert-butoxide (3.64 g, 0.038 mol, sigma aldrich), 촉매 Pd(dba)2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine(0.38 g, 0.0019 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 8시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 화합물 134을 11.3 g (수율 82.8%) 수득하였다.Intermediate 126-6 (10 g, 0.019 mol), 2-amino-9,9-dimethylfluorene (6.21 g, 0.023 mol, TCI), Sodium tert-butoxide (3.64 g, 0.038 mol, sigma aldrich), Catalyst Pd (dba ) 2 (0.54 g, 0.0009 mol, sigma aldrich), tri-tert-Bu-phosphine (0.38 g, 0.0019 mol, sigma aldrich) was added with 150 mL of Toluene, followed by stirring at 100 °C for 8 hours. After completion of the reaction, 11.3 g (yield 82.8%) of compound 134 was obtained by performing column purification (N-HEXANE: MC) after layer separation in H 2 O:MC.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.93/d, 7.89/d, 7.77/s, 7.66/d, 7.63/d, 7.62/d, 7.57/s, 7.41/m, 7.32/m, 7.07/s, 6.75/s, 6.58/d) 2H(7.87/d, 7.55/d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 6.69/d) 3H(7.38/m) 12H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.93/d, 7.89/d, 7.77/s, 7.66/d, 7.63/d, 7.62/d, 7.57/s, 7.41/m, 7.32/m, 7.07/s, 6.75/s, 6.58/d) 2H (7.87/d, 7.55/d, 7.54/d, 7.52/d, 7.51/m, 7.28/m, 6.69/d) 3H (7.38/m) 12H ( 1.72/s)
LC/MS: m/z=719[(M+1)+]LC/MS: m/z=719[(M+1) + ]
합성예Synthesis example 13 : 화합물 146의 합성 13: Synthesis of compound 146
(1) 중간체 146-1의 합성 (1) Synthesis of Intermediate 146-1
2-bromo-4-iodophenol (20 g, 0.067 mol, yurui), 4-biphenylboronic acid (15.90 g, 0.080 mol, sigma aldrich), potassium carbonate (18.50 g, 0.134 mol, sigma aldrich), Pd(PPh3)4 (3.87 g, 0.0033 mol, sigma aldrich), Toluene 250 mL, Ethanol 50 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 146-1>을 17.4 g (수율 80%) 수득하였다.2-bromo-4-iodophenol (20 g, 0.067 mol, yurui), 4-biphenylboronic acid (15.90 g, 0.080 mol, sigma aldrich), potassium carbonate (18.50 g, 0.134 mol, sigma aldrich), Pd(PPh 3 ) 4 (3.87 g, 0.0033 mol, sigma aldrich), 250 mL of toluene, and 50 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, 17.4 g (yield 80%) of <Intermediate 146-1> was obtained by performing column purification (N-HEXANE: MC) after layer separation using H 2 O: MC.
(2) 중간체 146-2의 합성 (2) Synthesis of Intermediate 146-2
중간체 146-1 (20 g, 0.062 mol), 2-fluorophenylboronic acid (10.33 g, 0.074 mol, sigma aldrich), potassium carbonate (17.00 g, 0.123 mol, sigma aldrich), Pd(PPh3)4 (3.55 g, 0.0031 mol, sigma aldrich), Toluene 250 mL, Ethanol 40 mL 넣고 4시간 동안 환류 교반하여 반응시켰다. 반응 종료 후 H2O : MC를 이용하여 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 146-2>를 17 g (수율 81%) 수득하였다.Intermediate 146-1 (20 g, 0.062 mol), 2-fluorophenylboronic acid (10.33 g, 0.074 mol, sigma aldrich), potassium carbonate (17.00 g, 0.123 mol, sigma aldrich), Pd(PPh 3 ) 4 (3.55 g, 0.0031 mol, sigma aldrich), 250 mL of toluene, and 40 mL of ethanol were added, and the reaction was stirred under reflux for 4 hours. After completion of the reaction, layer separation was performed using H 2 O: MC, followed by column purification (N-HEXANE: MC) to obtain 17 g (yield 81%) of <Intermediate 146-2>.
(3) 중간체 146-3의 합성 (3) Synthesis of intermediate 146-3
중간체 146-2 (15 g, 0.044 mol), N-Bromosuccinimide (8.63 g, 0.049 mol, sigma aldrich), Dimethylformamide 200 mL 넣고 60 ℃에서 12시간 동안 교반하여 반응시켰다. 반응 종료 후 1M NaOH 수용액을 넣고 교반시켜 여과한 후 건조하여 <중간체 146-3>을 13.5 g (수율 73%) 수득하였다.Intermediate 146-2 (15 g, 0.044 mol), N-Bromosuccinimide (8.63 g, 0.049 mol, sigma aldrich), and 200 mL of Dimethylformamide were added and reacted by stirring at 60 °C for 12 hours. After completion of the reaction, 1M aqueous NaOH solution was added, stirred, filtered, and dried to obtain 13.5 g (yield 73%) of <Intermediate 146-3>.
(4) 중간체 146-4의 합성 (4) Synthesis of Intermediate 146-4
중간체 146-4 (15 g, 0.036 mol), NaH (1.03 g, 0.043 mol, sigma aldrich), Dimethyl sulfoxide 200 mL 넣고 140 ℃에서 5시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : MC에 층분리를 한 후 컬럼정제 (N-HEXANE : MC)하여 <중간체 146-4>을 5.7 g (수율 40%) 수득하였다.Intermediate 146-4 (15 g, 0.036 mol), NaH (1.03 g, 0.043 mol, sigma aldrich), 200 mL of dimethyl sulfoxide was added, and the reaction was stirred at 140 °C for 5 hours. After completion of the reaction , layer separation was performed on H 2 O:MC, and column purification (N-HEXANE:MC) was performed to obtain 5.7 g (yield 40%) of <Intermediate 146-4>.
(5) 화합물 146의 합성 (5) Synthesis of compound 146
중간체 146-4 (10 g, 0.025 mol), 중간체 75-3 (10.86 g, 0.042 mol), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), 촉매 Pd(dba)2 (0.72 g, 0.0013 mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich)에 Toluene 150 mL를 넣고 100 ℃에서 4시간 동안 교반하여 반응시켰다. 반응 종료 후 H2O : EA에 층분리를 한 후 컬럼정제 (N-HEXANE : EA)하여 화합물 146을 13.9 g (수율 81.6%) 수득하였다.Intermediate 146-4 (10 g, 0.025 mol), Intermediate 75-3 (10.86 g, 0.042 mol), Sodium tert-butoxide (4.81 g, 0.050 mol, sigma aldrich), Catalyst Pd(dba) 2 (0.72 g, 0.0013) mol, sigma aldrich), tri-tert-Bu-phosphine (0.51 g, 0.0025 mol, sigma aldrich) was added with 150 mL of toluene and stirred at 100 °C for 4 hours to react. After the reaction was completed, 13.9 g of compound 146 was obtained (yield: 81.6%) by column purification (N-HEXANE: EA) after layer separation in H 2 O:EA.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.89/d, 7.87/d, 7.66/d, 7.62/d, 7.57/s, 7.55/d, 7.32/m, 7.28/m, 7.07/s, 6.75/s, 6.58/d) 2H(7.54/d, 7.41/m, 7.38/m, 6.69/d) 4H(7.52/d, 7.51/m, 7.25/d) 6H(1.72/s)H-NMR (200 MHz, CDCl3): δ ppm, 1H (7.89/d, 7.87/d, 7.66/d, 7.62/d, 7.57/s, 7.55/d, 7.32/m, 7.28/m, 7.07/s, 6.75/s, 6.58/d) 2H (7.54/d, 7.41/m, 7.38/m, 6.69/d) 4H (7.52/d, 7.51/m, 7.25/d) 6H (1.72/s)
LC/MS: m/z=679[(M+1)+]LC/MS: m/z=679[(M+1) + ]
소자 실시예device embodiment
본 발명에 따른 실시예에서, ITO 투명 전극은 25 mm × 25 mm × 0.7 mm의 유리 기판 위에, ITO 투명 전극이 부착된 ITO 유리 기판을 이용하여, 발광 면적이 2 mm × 2 mm 크기가 되도록 패터닝한 후 세정하였다. 기판을 진공 챔버에 장착한 후 베이스 압력이 1 × 10-6 torr가 되도록 한 후 유기물을 상기 ITO 위에 하기 구조로 유기물과 금속을 증착하였다.In an embodiment according to the present invention, the ITO transparent electrode is patterned so that the light emitting area is 2 mm × 2 mm on a glass substrate of 25 mm × 25 mm × 0.7 mm, using an ITO glass substrate to which an ITO transparent electrode is attached. After that, it was washed. After the substrate was mounted in a vacuum chamber, the base pressure was set to 1 × 10 -6 torr, and the organic material and the metal were deposited on the ITO in the following structure.
소자 실시예 1 내지 15Device Examples 1 to 15
본 발명에 따른 [화학식 Ⅰ]로 구현되는 화합물을 전자저지층의 화합물로 하여, 하기와 같은 소자 구조를 갖는 청색 발광 유기전계발광소자를 제조하여, 발광 효율을 포함한 발광 특성을 측정하였다.By using the compound implemented by [Formula I] according to the present invention as the compound of the electron blocking layer, a blue light emitting organic electroluminescent device having the following device structure was manufactured, and luminescent properties including luminous efficiency were measured.
ITO / 정공주입층(HAT_CN 5 nm) / 정공수송층(a-NPB 100 nm) / 전자저지층(10 nm)/ 발광층 (20 nm) / 전자수송층 (201:Liq 30 nm) / LiF(1 nm) / Al (100 nm)ITO / hole injection layer (HAT_CN 5 nm) / hole transport layer (a-NPB 100 nm) / electron blocking layer (10 nm) / light emitting layer (20 nm) / electron transport layer (201:Liq 30 nm) / LiF (1 nm) / Al (100 nm)
ITO 투명 전극에 정공주입층을 형성하기 위해 [HAT_CN]을 이용하여 정공주입층의 두께를 5 nm로 진공 열증착 방법으로 형성하고, 이후 정공수송층을 α-NPB를 사용하여 성막하였다. 전자 저지층은 본 발명으로 구현되는 화학식 29, 38, 48, 58, 69, 75, 86, 96, 111, 126, 133, 134, 146, 155, 176을 사용하여 10 nm의 두께로 성막하였다. 또한, 발광층에는 호스트 화합물로는 [BH1]을 사용하고, 도판트 화합물로 [BD1]을 사용하여 두께가 20 nm 정도가 되도록 성막하였으며, 추가로 전자 수송층(하기 [201] 화합물 Liq 50% 도핑) 30 nm 및 LiF 1nm 및 알루미늄 100 nm를 증착법으로 성막하여, 유기전계발광소자를 제조하였다.To form a hole injection layer on the ITO transparent electrode, [HAT_CN] was used to form a hole injection layer with a thickness of 5 nm by vacuum thermal evaporation, and then a hole transport layer was formed using α-NPB. The electron blocking layer was formed to a thickness of 10 nm using Chemical Formulas 29, 38, 48, 58, 69, 75, 86, 96, 111, 126, 133, 134, 146, 155, and 176 implemented in the present invention. In addition, the light emitting layer was formed to have a thickness of about 20 nm using [BH1] as a host compound and [BD1] as a dopant compound, and additionally an electron transport layer (doped with 50% of the compound Liq below [201]) 30 nm and 1 nm of LiF and 100 nm of aluminum were deposited by vapor deposition to prepare an organic electroluminescent device.
소자 비교예 1Device Comparative Example 1
소자 비교예 1를 위한 유기전계발광소자는 상기 실시예 1의 소자구조에서 전자저지층을 사용하지 않는 것을 제외하고 동일하게 제작하였다.The organic electroluminescent device for Device Comparative Example 1 was manufactured in the same manner except that the electron blocking layer was not used in the device structure of Example 1.
실험예 1 : 소자 실시예 1 내지 15의 발광 특성Experimental Example 1: Light emitting properties of Device Examples 1 to 15
상기 실시예에 따라 제조된 유기전계발광소자는 Source meter (Model 237, Keithley)와 휘도계 (PR-650, Photo Research)를 이용하여 전압, 전류 및 발광 효율을 측정하였고, 전류 밀도 10 mA/㎠가 되는 전압을 "구동 전압"으로 정의하여 비교하였다. 결과는 하기 [표 1]과 같다.The organic electroluminescent device manufactured according to the above example was measured for voltage, current and luminous efficiency using a source meter (Model 237, Keithley) and a luminance meter (PR-650, Photo Research), and a current density of 10 mA/cm 2 The resulting voltage was defined as "driving voltage" and compared. The results are shown in [Table 1] below.
상기 [표 1]에 나타낸 결과를 살펴보면, 먼저, 본 발명에 따른 [화학식 Ⅰ]로 구현되는 화합물을 전자저지층 화합물로 채용한 소자의 경우, 종래 소자(비교예)에 비하여 발광 효율, 양자 효율 등 발광 특성이 현저히 우수함을 확인할 수 있다.Looking at the results shown in [Table 1], first, in the case of a device employing the compound implemented by [Formula I] according to the present invention as an electron blocking layer compound, the luminous efficiency and quantum efficiency compared to the conventional device (Comparative Example) It can be seen that the light emission characteristics are remarkably excellent.
[HAT_CN] [α-NPB] [BH1] [BD1] [201][HAT_CN] [α-NPB] [BH1] [BD1] [201]
Claims (8)
[화학식 Ⅰ]
상기 [화학식 Ⅰ]에서,
X는 CR1R2, O 또는 S이고,
상기 R1 내지 R2는 서로 동일하거나 상이하고, 각각 독립적으로 수소, 치환 또는 비치환된 탄소수 1 내지 7의 알킬기 및 치환 또는 비치환된 탄소수 6 내지 30의 아릴기 중에서 선택되는 어느 하나이며,
상기 R1 내지 R2는 서로 또는 인접한 치환기와 연결되어 치환 또는 비치환된 탄화수소 고리 또는 치환 또는 비치환된 헤테로고리를 형성할 수 있고,
L1 내지 L2는 서로 동일하거나 상이하고, 각각 독립적으로 단일결합이거나, 아릴렌기, 플루오레닐렌기, 카바졸릴렌기, 또는 N, O 및 S 원자 중 1개 이상을 포함하는 헤테로아릴렌기이며,
Ar1은 치환 또는 비치환된 탄소수 6 내지 30의 아릴기 (단, 나프틸기, 안트라센일기, 페난트렌일기는 제외함) 및 치환 또는 비치환된 플루오레닐기 중에서 선택되는 어느 하나이고,
Ar2 및 Ar3는 서로 동일하거나 상이하고, 각각 독립적으로 치환 또는 비치환된 탄소수 6 내지 30의 아릴기, 치환 또는 비치환된 탄소수 3 내지 30의 헤테로아릴기, 치환 또는 비치환된 플루오레닐기 및 치환 또는 비치환된 카바졸기 중에서 선택되는 어느 하나이며, 상기 Ar2 및 Ar3 중에서 적어도 하나는 치환 또는 비치환된 플루오레닐기, 치환 또는 비치환된 카바졸기, 치환 또는 비치환된 디벤조퓨란일기 및 치환 또는 비치환된 디벤조티오펜일기 중에서 선택되는 어느 하나이고,
단, X가 O이면서 상기 Ar2 및 Ar3 중에서 어느 하나가 치환 또는 비치환된 디벤조퓨란일기 경우에, (ⅰ) Ar2 및 Ar3 중 나머지 하나는 치환 또는 비치환된 탄소수 6 내지 30의 아릴기, 치환 또는 비치환된 탄소수 3 내지 30의 헤테로아릴기, 치환 또는 비치환된 플루오레닐기 (단, 스피로 구조의 플루오레닐기는 제외함) 및 치환 또는 비치환된 카바졸기 중에서 선택되는 어느 하되는 어느 하나이거나; (ⅱ) L1 내지 L2 중 적어도 하나는 단일결합이 아닌 것을 특징으로 하며,
상기 치환 또는 비치환이란 중수소, 할로겐기, 시아노기, 니트로기, 실릴기, 알킬기, 시클로알킬기, 아릴기 및 헤테로아릴기로 이루어진 군에서 선택된 1 또는 2 이상의 치환기로 치환되거나, 상기 치환기 중 2 이상의 치환기가 연결된 치환기로 치환되거나, 또는 어떠한 치환기도 갖지 않는 것을 의미한다.An organic light emitting compound represented by the following [Formula I]:
[Formula Ⅰ]
In the [Formula I],
X is CR 1 R 2 , O or S;
Wherein R 1 To R 2 are the same or different from each other, and each independently is any one selected from hydrogen, a substituted or unsubstituted C 1 to C 7 alkyl group, and a substituted or unsubstituted C 6 to C 30 aryl group,
The R 1 To R 2 may be connected to each other or an adjacent substituent to form a substituted or unsubstituted hydrocarbon ring or a substituted or unsubstituted heterocycle,
L 1 to L 2 are the same as or different from each other, and each independently is a single bond, an arylene group, a fluorenylene group, a carbazolylene group, or a heteroarylene group including at least one of N, O and S atoms,
Ar 1 is any one selected from a substituted or unsubstituted aryl group having 6 to 30 carbon atoms (except for a naphthyl group, an anthracenyl group, and a phenanthrenyl group) and a substituted or unsubstituted fluorenyl group,
Ar 2 and Ar 3 are the same as or different from each other, and are each independently a substituted or unsubstituted aryl group having 6 to 30 carbon atoms, a substituted or unsubstituted heteroaryl group having 3 to 30 carbon atoms, or a substituted or unsubstituted fluorenyl group and a substituted or unsubstituted carbazole group, wherein at least one of Ar 2 and Ar 3 is a substituted or unsubstituted fluorenyl group, a substituted or unsubstituted carbazole group, or a substituted or unsubstituted dibenzofuran. Any one selected from a diyl group and a substituted or unsubstituted dibenzothiophenyl group,
However, when X is O and any one of Ar 2 and Ar 3 is a substituted or unsubstituted dibenzofuranyl group, (i) the other one of Ar 2 and Ar 3 is substituted or unsubstituted having 6 to 30 carbon atoms Any selected from an aryl group, a substituted or unsubstituted heteroaryl group having 3 to 30 carbon atoms, a substituted or unsubstituted fluorenyl group (provided that the spiro structure fluorenyl group is excluded) and a substituted or unsubstituted carbazole group any one of the following; (ii) at least one of L 1 to L 2 is not a single bond,
The substituted or unsubstituted means deuterium, a halogen group, a cyano group, a nitro group, a silyl group, an alkyl group, a cycloalkyl group, an aryl group and a heteroaryl group substituted with one or two or more substituents selected from the group consisting of, or two or more substituents among the substituents It means that is substituted with a linked substituent, or does not have any substituents.
상기 [화학식 Ⅰ]로 표시되는 화합물은 하기 화합물로 이루어진 군으로부터 선택되는 어느 하나인 것을 특징으로 하는 유기발광 화합물:
According to claim 1,
The compound represented by the [Formula I] is an organic light emitting compound, characterized in that any one selected from the group consisting of the following compounds:
상기 유기물층 중 1 층 이상은 제1항의 [화학식 Ⅰ]로 표시되는 유기발광 화합물을 포함하는 것인 유기발광소자.An organic light emitting device comprising a first electrode, a second electrode, and at least one organic material layer disposed between the first electrode and the second electrode,
At least one layer of the organic material layer is an organic light emitting device comprising the organic light emitting compound represented by the [Formula I] of claim 1.
상기 유기물층은 정공 주입층, 정공 수송층, 전자 저지층, 발광층, 전자 수송층 및 전자 주입층 중에서 1층 이상을 포함하고,
상기 층들 중 1층 이상이 상기 [화학식 Ⅰ]로 표시되는 유기발광 화합물을 포함하는 것을 특징으로 하는 유기발광소자.4. The method of claim 3,
The organic material layer includes at least one layer from among a hole injection layer, a hole transport layer, an electron blocking layer, a light emitting layer, an electron transport layer, and an electron injection layer,
At least one of the layers comprises an organic light emitting compound represented by the [Formula I].
상기 전자 저지층이 상기 [화학식 Ⅰ]로 표시되는 유기발광 화합물을 포함하는 것을 특징으로 하는 유기발광소자.5. The method of claim 4,
The organic light emitting device, characterized in that the electron blocking layer comprises an organic light emitting compound represented by the [Formula I].
상기 유기물층에 적색, 녹색 또는 청색 발광을 하는 유기 발광층을 하나 이상을 더 포함하여 백색 발광을 하는 것을 특징으로 하는 유기발광소자.4. The method of claim 3,
An organic light emitting device, characterized in that the organic material layer further comprises at least one organic light emitting layer emitting red, green or blue light to emit white light.
An organic light-emitting compound, characterized in that any one selected from the group consisting of the following compounds:
상기 유기물층은 정공 주입층, 정공 수송층, 전자 저지층, 발광층, 전자 수송층 및 전자 주입층 중에서 1층 이상을 포함하고,
상기 전자 저지층이 상기 [화학식 Ⅰ]로 표시되는 유기발광 화합물을 포함하는 것을 특징으로 하는 유기발광소자.An organic light emitting device comprising a first electrode, a second electrode, and at least one organic material layer disposed between the first electrode and the second electrode,
The organic material layer includes at least one layer from among a hole injection layer, a hole transport layer, an electron blocking layer, a light emitting layer, an electron transport layer, and an electron injection layer,
The organic light emitting device, characterized in that the electron blocking layer comprises an organic light emitting compound represented by the [Formula I].
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020160163809A KR102279196B1 (en) | 2016-12-02 | 2016-12-02 | An electroluminescent compound and an electroluminescent device comprising the same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020160163809A KR102279196B1 (en) | 2016-12-02 | 2016-12-02 | An electroluminescent compound and an electroluminescent device comprising the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20180063707A KR20180063707A (en) | 2018-06-12 |
| KR102279196B1 true KR102279196B1 (en) | 2021-07-19 |
Family
ID=62622576
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020160163809A KR102279196B1 (en) | 2016-12-02 | 2016-12-02 | An electroluminescent compound and an electroluminescent device comprising the same |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR102279196B1 (en) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111278803B (en) * | 2018-01-25 | 2023-01-17 | 株式会社Lg化学 | Compound and organic light emitting device including the same |
| KR102559633B1 (en) * | 2018-05-31 | 2023-08-02 | 주식회사 동진쎄미켐 | Novel compound and organic electroluminescent divice including the same |
| JP7351713B2 (en) * | 2018-11-30 | 2023-09-27 | 関東化学株式会社 | 2-substituted fluorene compound, hole transport material containing the compound, and organic electronic device containing the compound in the hole transport layer |
| KR20200090091A (en) * | 2019-01-18 | 2020-07-28 | 롬엔드하스전자재료코리아유한회사 | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| US20220144855A1 (en) * | 2019-01-18 | 2022-05-12 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| CN112334456B (en) | 2019-06-28 | 2023-08-15 | 出光兴产株式会社 | Compound, material for organic electroluminescent element, and electronic device |
| KR20210084715A (en) * | 2019-12-27 | 2021-07-08 | 엘티소재주식회사 | Organic light emitting device and composition for organic layer of organic light emitting device |
| CN113563316B (en) * | 2020-04-28 | 2024-07-05 | 江苏三月科技股份有限公司 | Aromatic amine derivative and application thereof |
| WO2022260117A1 (en) * | 2021-06-10 | 2022-12-15 | 出光興産株式会社 | Organic electroluminescent element, organic electroluminescent display device, and electronic equipment |
| KR20240019325A (en) * | 2021-06-10 | 2024-02-14 | 이데미쓰 고산 가부시키가이샤 | Organic electroluminescent devices, organic electroluminescent display devices and electronic devices |
| CN117024387B (en) * | 2023-10-09 | 2024-02-02 | 吉林奥来德光电材料股份有限公司 | Light-emitting auxiliary material, preparation method thereof and organic electroluminescent device |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101535606B1 (en) * | 2015-01-29 | 2015-07-09 | 덕산네오룩스 주식회사 | Compound for organic electric element, organic electric element comprising the same and electronic device thereof |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102188300B1 (en) * | 2014-02-19 | 2020-12-08 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| KR102329806B1 (en) * | 2014-11-04 | 2021-11-22 | 덕산네오룩스 주식회사 | Display device using a composition for organic electronic element, and an organic electronic element thereof |
| KR102292572B1 (en) * | 2014-11-07 | 2021-08-24 | 덕산네오룩스 주식회사 | Display device using a composition for organic electronic element, and an organic electronic element thereof |
| KR101694492B1 (en) * | 2014-11-12 | 2017-01-11 | (주)위델소재 | Amine compound and organic electroluminescent device using the same |
| KR102342493B1 (en) * | 2014-11-19 | 2021-12-23 | 덕산네오룩스 주식회사 | Display device using a composition for organic electronic element, and an organic electronic element thereof |
| KR102293436B1 (en) * | 2014-11-19 | 2021-08-25 | 덕산네오룩스 주식회사 | Display device using a composition for organic electronic element, and an organic electronic element thereof |
| KR20160127429A (en) * | 2015-04-27 | 2016-11-04 | (주)피엔에이치테크 | An electroluminescent compound and an electroluminescent device comprising the same |
-
2016
- 2016-12-02 KR KR1020160163809A patent/KR102279196B1/en active IP Right Grant
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101535606B1 (en) * | 2015-01-29 | 2015-07-09 | 덕산네오룩스 주식회사 | Compound for organic electric element, organic electric element comprising the same and electronic device thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20180063707A (en) | 2018-06-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102279196B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20230033661A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102210267B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20180096458A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102192692B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102665302B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20230025838A (en) | Novel compound and organic light emitting device comprising the same | |
| KR20180063709A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102220659B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102170558B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102316570B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20190027545A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102171941B1 (en) | Novel compound and organic light emitting device comprising same | |
| KR102054069B1 (en) | Hetero cyclic compound and organic light emitting device comprising the same | |
| KR20210007121A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102233678B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102251836B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20220017368A (en) | Novel compound and organic light emitting device comprising the same | |
| KR101985650B1 (en) | Double spiro structured compound and organic light emitting device comprising the same | |
| KR102232409B1 (en) | Compound and organic light emitting device comprising the same | |
| KR20210072660A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102438617B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20210048018A (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR102498512B1 (en) | An electroluminescent compound and an electroluminescent device comprising the same | |
| KR20220017367A (en) | Novel compound and organic light emitting device comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| E902 | Notification of reason for refusal | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant |