KR102102972B1 - High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds - Google Patents
High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds Download PDFInfo
- Publication number
- KR102102972B1 KR102102972B1 KR1020177035075A KR20177035075A KR102102972B1 KR 102102972 B1 KR102102972 B1 KR 102102972B1 KR 1020177035075 A KR1020177035075 A KR 1020177035075A KR 20177035075 A KR20177035075 A KR 20177035075A KR 102102972 B1 KR102102972 B1 KR 102102972B1
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- acid
- chemical formula
- hpp
- substituted
- Prior art date
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 85
- 239000004599 antimicrobial Substances 0.000 title abstract description 114
- 239000000203 mixture Substances 0.000 title abstract description 81
- 239000000651 prodrug Substances 0.000 title abstract description 12
- 229940002612 prodrug Drugs 0.000 title abstract description 12
- 230000000845 anti-microbial effect Effects 0.000 title description 9
- 230000035515 penetration Effects 0.000 title description 3
- -1 5-methyl-3-phenyl-2-isoxazoline-4-carboxamido Chemical group 0.000 claims description 128
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 35
- 239000002253 acid Substances 0.000 claims description 33
- 239000008194 pharmaceutical composition Substances 0.000 claims description 33
- 150000002148 esters Chemical class 0.000 claims description 32
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 19
- 208000015181 infectious disease Diseases 0.000 claims description 18
- VOUGEZYPVGAPBB-UHFFFAOYSA-N penicillin acid Natural products OC(=O)C=C(OC)C(=O)C(C)=C VOUGEZYPVGAPBB-UHFFFAOYSA-N 0.000 claims description 16
- 239000003937 drug carrier Substances 0.000 claims description 15
- 210000000056 organ Anatomy 0.000 claims description 15
- YCOFRPYSZKIPBQ-UHFFFAOYSA-N penicillic acid Natural products COC1=CC(=O)OC1(O)C(C)=C YCOFRPYSZKIPBQ-UHFFFAOYSA-N 0.000 claims description 15
- WNPVZANXRCPJPW-UHFFFAOYSA-N 5-[isocyano-(4-methylphenyl)sulfonylmethyl]-1,2,3-trimethoxybenzene Chemical compound COC1=C(OC)C(OC)=CC(C([N+]#[C-])S(=O)(=O)C=2C=CC(C)=CC=2)=C1 WNPVZANXRCPJPW-UHFFFAOYSA-N 0.000 claims description 14
- 230000001580 bacterial effect Effects 0.000 claims description 13
- 210000004072 lung Anatomy 0.000 claims description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 9
- 206010035664 Pneumonia Diseases 0.000 claims description 9
- 210000004556 brain Anatomy 0.000 claims description 9
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 claims description 8
- 235000000346 sugar Nutrition 0.000 claims description 8
- 206010057190 Respiratory tract infections Diseases 0.000 claims description 7
- 206010040047 Sepsis Diseases 0.000 claims description 7
- 210000003734 kidney Anatomy 0.000 claims description 7
- 210000002784 stomach Anatomy 0.000 claims description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 claims description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 6
- 210000004185 liver Anatomy 0.000 claims description 6
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 6
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 claims description 5
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 5
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 5
- 206010046306 Upper respiratory tract infection Diseases 0.000 claims description 5
- 239000007864 aqueous solution Substances 0.000 claims description 5
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 5
- 210000001508 eye Anatomy 0.000 claims description 5
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 5
- 230000000968 intestinal effect Effects 0.000 claims description 5
- 210000000214 mouth Anatomy 0.000 claims description 5
- 210000001331 nose Anatomy 0.000 claims description 5
- 208000020029 respiratory tract infectious disease Diseases 0.000 claims description 5
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 claims description 5
- WXTMDXOMEHJXQO-UHFFFAOYSA-N 2,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1O WXTMDXOMEHJXQO-UHFFFAOYSA-N 0.000 claims description 4
- 206010068172 Anal pruritus Diseases 0.000 claims description 4
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 claims description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 4
- 235000010233 benzoic acid Nutrition 0.000 claims description 4
- 210000001072 colon Anatomy 0.000 claims description 4
- 210000001198 duodenum Anatomy 0.000 claims description 4
- 210000000232 gallbladder Anatomy 0.000 claims description 4
- 239000000174 gluconic acid Substances 0.000 claims description 4
- 235000012208 gluconic acid Nutrition 0.000 claims description 4
- 210000002216 heart Anatomy 0.000 claims description 4
- TWBYWOBDOCUKOW-UHFFFAOYSA-N isonicotinic acid Chemical compound OC(=O)C1=CC=NC=C1 TWBYWOBDOCUKOW-UHFFFAOYSA-N 0.000 claims description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 claims description 4
- 210000000496 pancreas Anatomy 0.000 claims description 4
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 claims description 4
- 210000002105 tongue Anatomy 0.000 claims description 4
- 125000004182 2-chlorophenyl group Chemical group [H]C1=C([H])C(Cl)=C(*)C([H])=C1[H] 0.000 claims description 3
- 108010065152 Coagulase Proteins 0.000 claims description 3
- 206010012335 Dependence Diseases 0.000 claims description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 3
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 3
- 208000005119 Necrotizing Pneumonia Diseases 0.000 claims description 3
- 208000001388 Opportunistic Infections Diseases 0.000 claims description 3
- 208000032536 Pseudomonas Infections Diseases 0.000 claims description 3
- 230000001154 acute effect Effects 0.000 claims description 3
- 235000010323 ascorbic acid Nutrition 0.000 claims description 3
- 239000011668 ascorbic acid Substances 0.000 claims description 3
- 210000004204 blood vessel Anatomy 0.000 claims description 3
- 210000005069 ears Anatomy 0.000 claims description 3
- 206010014665 endocarditis Diseases 0.000 claims description 3
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 claims description 3
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 claims description 3
- 201000007119 infective endocarditis Diseases 0.000 claims description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 3
- 239000011713 pantothenic acid Substances 0.000 claims description 3
- 235000019161 pantothenic acid Nutrition 0.000 claims description 3
- 206010040872 skin infection Diseases 0.000 claims description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 claims description 3
- 208000019206 urinary tract infection Diseases 0.000 claims description 3
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 claims description 2
- 239000005711 Benzoic acid Substances 0.000 claims description 2
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 claims description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 2
- 206010024971 Lower respiratory tract infections Diseases 0.000 claims description 2
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 claims description 2
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 claims description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 2
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 2
- 150000001298 alcohols Chemical group 0.000 claims description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 2
- 229960005070 ascorbic acid Drugs 0.000 claims description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 claims description 2
- 229940092714 benzenesulfonic acid Drugs 0.000 claims description 2
- 235000015165 citric acid Nutrition 0.000 claims description 2
- 235000019253 formic acid Nutrition 0.000 claims description 2
- 239000001530 fumaric acid Substances 0.000 claims description 2
- 235000011087 fumaric acid Nutrition 0.000 claims description 2
- 229960005219 gentisic acid Drugs 0.000 claims description 2
- 239000004220 glutamic acid Substances 0.000 claims description 2
- 235000013922 glutamic acid Nutrition 0.000 claims description 2
- 239000004310 lactic acid Substances 0.000 claims description 2
- 235000014655 lactic acid Nutrition 0.000 claims description 2
- 239000011976 maleic acid Substances 0.000 claims description 2
- 229940098779 methanesulfonic acid Drugs 0.000 claims description 2
- 229910017604 nitric acid Inorganic materials 0.000 claims description 2
- WLJNZVDCPSBLRP-UHFFFAOYSA-N pamoic acid Chemical compound C1=CC=C2C(CC=3C4=CC=CC=C4C=C(C=3O)C(=O)O)=C(O)C(C(O)=O)=CC2=C1 WLJNZVDCPSBLRP-UHFFFAOYSA-N 0.000 claims description 2
- 229940055726 pantothenic acid Drugs 0.000 claims description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 claims description 2
- 229960004889 salicylic acid Drugs 0.000 claims description 2
- 239000011975 tartaric acid Substances 0.000 claims description 2
- 235000002906 tartaric acid Nutrition 0.000 claims description 2
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 claims description 2
- 208000035143 Bacterial infection Diseases 0.000 claims 2
- 208000022362 bacterial infectious disease Diseases 0.000 claims 2
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 claims 1
- 229940071870 hydroiodic acid Drugs 0.000 claims 1
- 230000000241 respiratory effect Effects 0.000 claims 1
- 239000001384 succinic acid Substances 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 35
- 229940079593 drug Drugs 0.000 abstract description 34
- 238000011282 treatment Methods 0.000 abstract description 29
- 230000004888 barrier function Effects 0.000 abstract description 23
- 239000002207 metabolite Substances 0.000 abstract description 11
- 230000035699 permeability Effects 0.000 abstract description 10
- 239000002359 drug metabolite Substances 0.000 abstract description 2
- 230000001575 pathological effect Effects 0.000 abstract description 2
- 239000000126 substance Substances 0.000 description 325
- 238000000034 method Methods 0.000 description 39
- 210000003491 skin Anatomy 0.000 description 37
- 125000000217 alkyl group Chemical group 0.000 description 36
- 239000000460 chlorine Substances 0.000 description 35
- 125000000753 cycloalkyl group Chemical group 0.000 description 28
- 239000010410 layer Substances 0.000 description 28
- 210000004027 cell Anatomy 0.000 description 27
- 239000003242 anti bacterial agent Substances 0.000 description 26
- 229910052799 carbon Inorganic materials 0.000 description 26
- 238000009472 formulation Methods 0.000 description 25
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 24
- 125000003118 aryl group Chemical group 0.000 description 22
- 235000002639 sodium chloride Nutrition 0.000 description 22
- 239000003795 chemical substances by application Substances 0.000 description 21
- 150000003839 salts Chemical class 0.000 description 20
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 19
- 229910052794 bromium Inorganic materials 0.000 description 19
- 229910052801 chlorine Inorganic materials 0.000 description 19
- 229910052731 fluorine Inorganic materials 0.000 description 19
- 125000001072 heteroaryl group Chemical group 0.000 description 19
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 18
- 229910052739 hydrogen Inorganic materials 0.000 description 18
- 229910052740 iodine Inorganic materials 0.000 description 18
- 229910052721 tungsten Inorganic materials 0.000 description 18
- 238000012360 testing method Methods 0.000 description 17
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 16
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 15
- 241000700605 Viruses Species 0.000 description 15
- 229940121375 antifungal agent Drugs 0.000 description 15
- 239000003429 antifungal agent Substances 0.000 description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 15
- 229910052760 oxygen Inorganic materials 0.000 description 15
- 239000011541 reaction mixture Substances 0.000 description 15
- 229910052717 sulfur Inorganic materials 0.000 description 15
- 241000894006 Bacteria Species 0.000 description 14
- 241000233866 Fungi Species 0.000 description 14
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 14
- 201000010099 disease Diseases 0.000 description 14
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 13
- 125000000304 alkynyl group Chemical group 0.000 description 13
- 229940088710 antibiotic agent Drugs 0.000 description 13
- 239000008363 phosphate buffer Substances 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 13
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 12
- 125000003342 alkenyl group Chemical group 0.000 description 12
- 238000001914 filtration Methods 0.000 description 12
- 239000003921 oil Substances 0.000 description 12
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- 125000003545 alkoxy group Chemical group 0.000 description 11
- 125000003282 alkyl amino group Chemical group 0.000 description 11
- 125000003277 amino group Chemical group 0.000 description 11
- 239000003782 beta lactam antibiotic agent Substances 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 244000005700 microbiome Species 0.000 description 11
- 235000019198 oils Nutrition 0.000 description 11
- 125000005010 perfluoroalkyl group Chemical group 0.000 description 11
- 239000002132 β-lactam antibiotic Substances 0.000 description 11
- 229940124586 β-lactam antibiotics Drugs 0.000 description 11
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 10
- 229960003350 isoniazid Drugs 0.000 description 10
- 239000000843 powder Substances 0.000 description 10
- 239000007787 solid Substances 0.000 description 10
- 239000000758 substrate Substances 0.000 description 10
- 238000007910 systemic administration Methods 0.000 description 10
- 241000196324 Embryophyta Species 0.000 description 9
- 206010049933 Hypophosphatasia Diseases 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- 150000001350 alkyl halides Chemical class 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 9
- 239000008280 blood Substances 0.000 description 9
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 9
- 230000002538 fungal effect Effects 0.000 description 9
- 150000002430 hydrocarbons Chemical group 0.000 description 9
- QRXWMOHMRWLFEY-UHFFFAOYSA-N isoniazide Chemical group NNC(=O)C1=CC=NC=C1 QRXWMOHMRWLFEY-UHFFFAOYSA-N 0.000 description 9
- 125000005647 linker group Chemical group 0.000 description 9
- 239000007788 liquid Substances 0.000 description 9
- 210000004379 membrane Anatomy 0.000 description 9
- 239000012528 membrane Substances 0.000 description 9
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 9
- MHWLWQUZZRMNGJ-UHFFFAOYSA-N nalidixic acid Chemical compound C1=C(C)N=C2N(CC)C=C(C(O)=O)C(=O)C2=C1 MHWLWQUZZRMNGJ-UHFFFAOYSA-N 0.000 description 9
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 9
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 8
- 125000004414 alkyl thio group Chemical group 0.000 description 8
- 150000003840 hydrochlorides Chemical class 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 229920001223 polyethylene glycol Polymers 0.000 description 8
- 229940124530 sulfonamide Drugs 0.000 description 8
- 239000003826 tablet Substances 0.000 description 8
- 230000000699 topical effect Effects 0.000 description 8
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 7
- 229930182555 Penicillin Natural products 0.000 description 7
- 229930195708 Penicillin V Natural products 0.000 description 7
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 7
- 229950008560 almecillin Drugs 0.000 description 7
- 230000000844 anti-bacterial effect Effects 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 210000002615 epidermis Anatomy 0.000 description 7
- 235000019441 ethanol Nutrition 0.000 description 7
- 229960003085 meticillin Drugs 0.000 description 7
- 229960000210 nalidixic acid Drugs 0.000 description 7
- 229910052757 nitrogen Inorganic materials 0.000 description 7
- 230000036407 pain Effects 0.000 description 7
- 229940049954 penicillin Drugs 0.000 description 7
- QULKGELYPOJSLP-WCABBAIRSA-N penicillin O Chemical compound OC(=O)[C@H]1C(C)(C)S[C@@H]2[C@H](NC(=O)CSCC=C)C(=O)N21 QULKGELYPOJSLP-WCABBAIRSA-N 0.000 description 7
- 229940056367 penicillin v Drugs 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- BPLBGHOLXOTWMN-MBNYWOFBSA-N phenoxymethylpenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)COC1=CC=CC=C1 BPLBGHOLXOTWMN-MBNYWOFBSA-N 0.000 description 7
- 238000007920 subcutaneous administration Methods 0.000 description 7
- 239000003981 vehicle Substances 0.000 description 7
- WZPBZJONDBGPKJ-VEHQQRBSSA-L 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfonatoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoate Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C([O-])=O)\C1=CSC(N)=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-L 0.000 description 6
- 241000193738 Bacillus anthracis Species 0.000 description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 229910019142 PO4 Inorganic materials 0.000 description 6
- 206010035148 Plague Diseases 0.000 description 6
- 239000002202 Polyethylene glycol Substances 0.000 description 6
- 230000009471 action Effects 0.000 description 6
- 239000000443 aerosol Substances 0.000 description 6
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 6
- 239000003443 antiviral agent Substances 0.000 description 6
- 229960003644 aztreonam Drugs 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 230000008499 blood brain barrier function Effects 0.000 description 6
- 210000001218 blood-brain barrier Anatomy 0.000 description 6
- 201000006824 bubonic plague Diseases 0.000 description 6
- 125000004432 carbon atom Chemical group C* 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 230000000813 microbial effect Effects 0.000 description 6
- 238000007911 parenteral administration Methods 0.000 description 6
- 229940056360 penicillin g Drugs 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 6
- 239000010452 phosphate Substances 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 150000003141 primary amines Chemical class 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 5
- NWTNSOCBLRIHKX-UHFFFAOYSA-N 2-(diethylamino)ethyl 2-methyloct-2-enoate hydrochloride Chemical compound Cl.C(C)N(CCOC(=O)C(C)=CCCCCC)CC NWTNSOCBLRIHKX-UHFFFAOYSA-N 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 5
- 229930186147 Cephalosporin Natural products 0.000 description 5
- 101150065749 Churc1 gene Proteins 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- 206010024229 Leprosy Diseases 0.000 description 5
- 241000699670 Mus sp. Species 0.000 description 5
- 229910004013 NO 2 Inorganic materials 0.000 description 5
- 102100038239 Protein Churchill Human genes 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- 238000009825 accumulation Methods 0.000 description 5
- 239000002775 capsule Substances 0.000 description 5
- 229940124587 cephalosporin Drugs 0.000 description 5
- 150000001780 cephalosporins Chemical class 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- 238000000921 elemental analysis Methods 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 235000011187 glycerol Nutrition 0.000 description 5
- 239000001257 hydrogen Substances 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 238000001990 intravenous administration Methods 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 150000002960 penicillins Chemical class 0.000 description 5
- 239000006187 pill Substances 0.000 description 5
- 229920000728 polyester Polymers 0.000 description 5
- 235000018102 proteins Nutrition 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 210000002345 respiratory system Anatomy 0.000 description 5
- 239000011734 sodium Substances 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- 239000012265 solid product Substances 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- FDDDEECHVMSUSB-UHFFFAOYSA-N sulfanilamide Chemical compound NC1=CC=C(S(N)(=O)=O)C=C1 FDDDEECHVMSUSB-UHFFFAOYSA-N 0.000 description 5
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 5
- 229960001940 sulfasalazine Drugs 0.000 description 5
- 150000003456 sulfonamides Chemical class 0.000 description 5
- 150000003952 β-lactams Chemical class 0.000 description 5
- NKBWMBRPILTCRD-UHFFFAOYSA-N 2-Methylheptanoic acid Chemical compound CCCCCC(C)C(O)=O NKBWMBRPILTCRD-UHFFFAOYSA-N 0.000 description 4
- WRYDGMWSKBGVHS-UHFFFAOYSA-N 2-bromo-n,n-diethylethanamine Chemical compound CCN(CC)CCBr WRYDGMWSKBGVHS-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
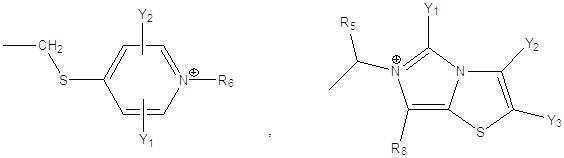
- 0 C*C(C1=C(*)CCC(C2NC(C(c3ccccc3)NC(*)=O)=O)N1C2=O)=O Chemical compound C*C(C1=C(*)CCC(C2NC(C(c3ccccc3)NC(*)=O)=O)N1C2=O)=O 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 4
- 206010037660 Pyrexia Diseases 0.000 description 4
- 206010039438 Salmonella Infections Diseases 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 229960000571 acetazolamide Drugs 0.000 description 4
- BZKPWHYZMXOIDC-UHFFFAOYSA-N acetazolamide Chemical compound CC(=O)NC1=NN=C(S(N)(=O)=O)S1 BZKPWHYZMXOIDC-UHFFFAOYSA-N 0.000 description 4
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 4
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 4
- 210000000481 breast Anatomy 0.000 description 4
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 125000004494 ethyl ester group Chemical group 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 238000004128 high performance liquid chromatography Methods 0.000 description 4
- 201000001371 inclusion conjunctivitis Diseases 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 210000004940 nucleus Anatomy 0.000 description 4
- UWYHMGVUTGAWSP-JKIFEVAISA-N oxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1 UWYHMGVUTGAWSP-JKIFEVAISA-N 0.000 description 4
- 229960001019 oxacillin Drugs 0.000 description 4
- 239000006072 paste Substances 0.000 description 4
- 235000019371 penicillin G benzathine Nutrition 0.000 description 4
- 229910052698 phosphorus Inorganic materials 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 206010039447 salmonellosis Diseases 0.000 description 4
- 150000003335 secondary amines Chemical class 0.000 description 4
- 235000017557 sodium bicarbonate Nutrition 0.000 description 4
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 229940032147 starch Drugs 0.000 description 4
- 150000008163 sugars Chemical class 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 239000000454 talc Substances 0.000 description 4
- 229910052623 talc Inorganic materials 0.000 description 4
- 235000012222 talc Nutrition 0.000 description 4
- 150000003512 tertiary amines Chemical group 0.000 description 4
- 239000001993 wax Substances 0.000 description 4
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 3
- PMWLDHRFQGGMTH-UHFFFAOYSA-N 2-(diethylamino)ethyl 7-[[2-(2-acetamido-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;hydrochloride Chemical compound Cl.O=C1N2C(C(=O)OCCN(CC)CC)=CCSC2C1NC(=O)C(=NOC)C1=CSC(NC(C)=O)=N1 PMWLDHRFQGGMTH-UHFFFAOYSA-N 0.000 description 3
- 229920001817 Agar Polymers 0.000 description 3
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 3
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 3
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 3
- HZZVJAQRINQKSD-UHFFFAOYSA-N Clavulanic acid Natural products OC(=O)C1C(=CCO)OC2CC(=O)N21 HZZVJAQRINQKSD-UHFFFAOYSA-N 0.000 description 3
- 206010012735 Diarrhoea Diseases 0.000 description 3
- 206010059866 Drug resistance Diseases 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 240000007472 Leucaena leucocephala Species 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 239000000232 Lipid Bilayer Substances 0.000 description 3
- 201000009906 Meningitis Diseases 0.000 description 3
- 241000985694 Polypodiopsida Species 0.000 description 3
- 241000592344 Spermatophyta Species 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- TYBKIVWJECMPFZ-UHFFFAOYSA-M [Na+].N1=CC(=CC=C1)OP([O-])(=O)CNC(=O)OCC1=CC=CC=C1 Chemical compound [Na+].N1=CC(=CC=C1)OP([O-])(=O)CNC(=O)OCC1=CC=CC=C1 TYBKIVWJECMPFZ-UHFFFAOYSA-M 0.000 description 3
- OOEAPCXQLFMNNV-UHFFFAOYSA-M [Na+].[N+](=O)([O-])C1=CC=C(C=C1)OP([O-])(=O)CNC(=O)OCC1=CC=CC=C1 Chemical compound [Na+].[N+](=O)([O-])C1=CC=C(C=C1)OP([O-])(=O)CNC(=O)OCC1=CC=CC=C1 OOEAPCXQLFMNNV-UHFFFAOYSA-M 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 235000010419 agar Nutrition 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 125000003368 amide group Chemical group 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 229960000723 ampicillin Drugs 0.000 description 3
- 239000003904 antiprotozoal agent Substances 0.000 description 3
- 235000012216 bentonite Nutrition 0.000 description 3
- 239000000440 bentonite Substances 0.000 description 3
- 229910000278 bentonite Inorganic materials 0.000 description 3
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 3
- 239000003781 beta lactamase inhibitor Substances 0.000 description 3
- 229940126813 beta-lactamase inhibitor Drugs 0.000 description 3
- 229940041011 carbapenems Drugs 0.000 description 3
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- JFPVXVDWJQMJEE-IZRZKJBUSA-N cefuroxime Chemical compound N([C@@H]1C(N2C(=C(COC(N)=O)CS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CC=CO1 JFPVXVDWJQMJEE-IZRZKJBUSA-N 0.000 description 3
- 229960001668 cefuroxime Drugs 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 229960003405 ciprofloxacin Drugs 0.000 description 3
- HZZVJAQRINQKSD-PBFISZAISA-N clavulanic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 HZZVJAQRINQKSD-PBFISZAISA-N 0.000 description 3
- 229960003324 clavulanic acid Drugs 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- YFAGHNZHGGCZAX-JKIFEVAISA-N dicloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(Cl)C=CC=C1Cl YFAGHNZHGGCZAX-JKIFEVAISA-N 0.000 description 3
- 229960001585 dicloxacillin Drugs 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 235000013355 food flavoring agent Nutrition 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- ZRALSGWEFCBTJO-UHFFFAOYSA-N guanidine group Chemical group NC(=N)N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 3
- 239000003701 inert diluent Substances 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 235000013336 milk Nutrition 0.000 description 3
- 239000008267 milk Substances 0.000 description 3
- 210000004080 milk Anatomy 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- 210000004877 mucosa Anatomy 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 235000008390 olive oil Nutrition 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 230000000149 penetrating effect Effects 0.000 description 3
- 102000013415 peroxidase activity proteins Human genes 0.000 description 3
- 108040007629 peroxidase activity proteins Proteins 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 239000003380 propellant Substances 0.000 description 3
- 150000007660 quinolones Chemical class 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 3
- 235000012239 silicon dioxide Nutrition 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 3
- 239000000829 suppository Substances 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 230000009885 systemic effect Effects 0.000 description 3
- LPQZKKCYTLCDGQ-WEDXCCLWSA-N tazobactam Chemical compound C([C@]1(C)S([C@H]2N(C(C2)=O)[C@H]1C(O)=O)(=O)=O)N1C=CN=N1 LPQZKKCYTLCDGQ-WEDXCCLWSA-N 0.000 description 3
- 229960003865 tazobactam Drugs 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 150000007970 thio esters Chemical class 0.000 description 3
- 150000003568 thioethers Chemical class 0.000 description 3
- 238000011200 topical administration Methods 0.000 description 3
- 201000008827 tuberculosis Diseases 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- SXKMQCSYTIDQTC-IBTYICNHSA-N (2s,5r)-3,3-dimethyl-4,4,7-trioxo-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;sodium Chemical compound [Na].O=S1(=O)C(C)(C)[C@H](C(O)=O)N2C(=O)C[C@H]21 SXKMQCSYTIDQTC-IBTYICNHSA-N 0.000 description 2
- JETQIUPBHQNHNZ-NJBDSQKTSA-N (2s,5r,6r)-3,3-dimethyl-7-oxo-6-[[(2r)-2-phenyl-2-sulfoacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Chemical compound C1([C@H](C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)S(O)(=O)=O)=CC=CC=C1 JETQIUPBHQNHNZ-NJBDSQKTSA-N 0.000 description 2
- QJVHTELASVOWBE-AGNWQMPPSA-N (2s,5r,6r)-6-[[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2r,3z,5r)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21.C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 QJVHTELASVOWBE-AGNWQMPPSA-N 0.000 description 2
- BSIMZHVOQZIAOY-SCSAIBSYSA-N 1-carbapenem-3-carboxylic acid Chemical compound OC(=O)C1=CC[C@@H]2CC(=O)N12 BSIMZHVOQZIAOY-SCSAIBSYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- FRPZMMHWLSIFAZ-UHFFFAOYSA-N 10-undecenoic acid Chemical compound OC(=O)CCCCCCCCC=C FRPZMMHWLSIFAZ-UHFFFAOYSA-N 0.000 description 2
- KZDCMKVLEYCGQX-UDPGNSCCSA-N 2-(diethylamino)ethyl 4-aminobenzoate;(2s,5r,6r)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;hydrate Chemical compound O.CCN(CC)CCOC(=O)C1=CC=C(N)C=C1.N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 KZDCMKVLEYCGQX-UDPGNSCCSA-N 0.000 description 2
- VHVPQPYKVGDNFY-DFMJLFEVSA-N 2-[(2r)-butan-2-yl]-4-[4-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one Chemical compound O=C1N([C@H](C)CC)N=CN1C1=CC=C(N2CCN(CC2)C=2C=CC(OC[C@@H]3O[C@](CN4N=CN=C4)(OC3)C=3C(=CC(Cl)=CC=3)Cl)=CC=2)C=C1 VHVPQPYKVGDNFY-DFMJLFEVSA-N 0.000 description 2
- XMDCQQRSDKDTNP-UHFFFAOYSA-N 2-bromo-n,n-diethylpropan-1-amine Chemical compound CCN(CC)CC(C)Br XMDCQQRSDKDTNP-UHFFFAOYSA-N 0.000 description 2
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical group CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- LBXHRAWDUMTPSE-AOOOYVTPSA-N 4-chloro-N-[(2S,6R)-2,6-dimethyl-1-piperidinyl]-3-sulfamoylbenzamide Chemical compound C[C@H]1CCC[C@@H](C)N1NC(=O)C1=CC=C(Cl)C(S(N)(=O)=O)=C1 LBXHRAWDUMTPSE-AOOOYVTPSA-N 0.000 description 2
- WUWFMDMBOJLQIV-UHFFFAOYSA-N 7-(3-aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid Chemical compound C1C(N)CCN1C(C(=C1)F)=NC2=C1C(=O)C(C(O)=O)=CN2C1=CC=C(F)C=C1F WUWFMDMBOJLQIV-UHFFFAOYSA-N 0.000 description 2
- MPORYQCGWFQFLA-ONPDANIMSA-N 7-[(7s)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1r,2s)-2-fluorocyclopropyl]-4-oxoquinoline-3-carboxylic acid;trihydrate Chemical compound O.O.O.C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1.C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 MPORYQCGWFQFLA-ONPDANIMSA-N 0.000 description 2
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Chemical compound FC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-UHFFFAOYSA-N 0.000 description 2
- 208000030507 AIDS Diseases 0.000 description 2
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 2
- 108010064760 Anidulafungin Proteins 0.000 description 2
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 description 2
- 206010003011 Appendicitis Diseases 0.000 description 2
- 241000203069 Archaea Species 0.000 description 2
- 206010053555 Arthritis bacterial Diseases 0.000 description 2
- 241000235349 Ascomycota Species 0.000 description 2
- 201000002909 Aspergillosis Diseases 0.000 description 2
- 208000036641 Aspergillus infections Diseases 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 208000004429 Bacillary Dysentery Diseases 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 206010006500 Brucellosis Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000222122 Candida albicans Species 0.000 description 2
- 206010007134 Candida infections Diseases 0.000 description 2
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 2
- 206010008631 Cholera Diseases 0.000 description 2
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 206010011409 Cross infection Diseases 0.000 description 2
- 201000007336 Cryptococcosis Diseases 0.000 description 2
- 241000221204 Cryptococcus neoformans Species 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- 108090000204 Dipeptidase 1 Proteins 0.000 description 2
- 108010049047 Echinocandins Proteins 0.000 description 2
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 2
- 208000004145 Endometritis Diseases 0.000 description 2
- 206010014896 Enterocolitis haemorrhagic Diseases 0.000 description 2
- UIOFUWFRIANQPC-JKIFEVAISA-N Floxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(F)C=CC=C1Cl UIOFUWFRIANQPC-JKIFEVAISA-N 0.000 description 2
- 206010016952 Food poisoning Diseases 0.000 description 2
- 208000019331 Foodborne disease Diseases 0.000 description 2
- 206010017533 Fungal infection Diseases 0.000 description 2
- 208000005577 Gastroenteritis Diseases 0.000 description 2
- 206010017982 Gastrointestinal necrosis Diseases 0.000 description 2
- 241000206672 Gelidium Species 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 206010018612 Gonorrhoea Diseases 0.000 description 2
- CTETYYAZBPJBHE-UHFFFAOYSA-N Haloprogin Chemical compound ClC1=CC(Cl)=C(OCC#CI)C=C1Cl CTETYYAZBPJBHE-UHFFFAOYSA-N 0.000 description 2
- 208000032759 Hemolytic-Uremic Syndrome Diseases 0.000 description 2
- 201000002563 Histoplasmosis Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 206010021531 Impetigo Diseases 0.000 description 2
- JUZNIMUFDBIJCM-ANEDZVCMSA-N Invanz Chemical compound O=C([C@H]1NC[C@H](C1)SC=1[C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)NC1=CC=CC(C(O)=O)=C1 JUZNIMUFDBIJCM-ANEDZVCMSA-N 0.000 description 2
- 208000007764 Legionnaires' Disease Diseases 0.000 description 2
- 206010024238 Leptospirosis Diseases 0.000 description 2
- GSDSWSVVBLHKDQ-JTQLQIEISA-N Levofloxacin Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-JTQLQIEISA-N 0.000 description 2
- 206010024641 Listeriosis Diseases 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- TYMRLRRVMHJFTF-UHFFFAOYSA-N Mafenide Chemical compound NCC1=CC=C(S(N)(=O)=O)C=C1 TYMRLRRVMHJFTF-UHFFFAOYSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- BYBLEWFAAKGYCD-UHFFFAOYSA-N Miconazole Chemical compound ClC1=CC(Cl)=CC=C1COC(C=1C(=CC(Cl)=CC=1)Cl)CN1C=NC=C1 BYBLEWFAAKGYCD-UHFFFAOYSA-N 0.000 description 2
- 241000204031 Mycoplasma Species 0.000 description 2
- 241001420836 Ophthalmitis Species 0.000 description 2
- 206010033078 Otitis media Diseases 0.000 description 2
- KYGZCKSPAKDVKC-UHFFFAOYSA-N Oxolinic acid Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC2=C1OCO2 KYGZCKSPAKDVKC-UHFFFAOYSA-N 0.000 description 2
- TYMABNNERDVXID-DLYFRVTGSA-N Panipenem Chemical compound C([C@@H]1[C@H](C(N1C=1C(O)=O)=O)[C@H](O)C)C=1S[C@H]1CCN(C(C)=N)C1 TYMABNNERDVXID-DLYFRVTGSA-N 0.000 description 2
- 241001537205 Paracoccidioides Species 0.000 description 2
- 208000008469 Peptic Ulcer Diseases 0.000 description 2
- 201000005702 Pertussis Diseases 0.000 description 2
- 201000007100 Pharyngitis Diseases 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 206010054161 Pontiac fever Diseases 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 206010036790 Productive cough Diseases 0.000 description 2
- PWNMXPDKBYZCOO-UHFFFAOYSA-N Prulifloxacin Chemical compound C1=C2N3C(C)SC3=C(C(O)=O)C(=O)C2=CC(F)=C1N(CC1)CCN1CC=1OC(=O)OC=1C PWNMXPDKBYZCOO-UHFFFAOYSA-N 0.000 description 2
- 241000287531 Psittacidae Species 0.000 description 2
- 206010037742 Rabies Diseases 0.000 description 2
- NJCJBUHJQLFDSW-UHFFFAOYSA-N Rufloxacin Chemical compound C1CN(C)CCN1C1=C(F)C=C2C(=O)C(C(O)=O)=CN3CCSC1=C32 NJCJBUHJQLFDSW-UHFFFAOYSA-N 0.000 description 2
- 206010039587 Scarlet Fever Diseases 0.000 description 2
- 206010040550 Shigella infections Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- NHUHCSRWZMLRLA-UHFFFAOYSA-N Sulfisoxazole Chemical compound CC1=NOC(NS(=O)(=O)C=2C=CC(N)=CC=2)=C1C NHUHCSRWZMLRLA-UHFFFAOYSA-N 0.000 description 2
- PJSFRIWCGOHTNF-UHFFFAOYSA-N Sulphormetoxin Chemical compound COC1=NC=NC(NS(=O)(=O)C=2C=CC(N)=CC=2)=C1OC PJSFRIWCGOHTNF-UHFFFAOYSA-N 0.000 description 2
- HMHVCUVYZFYAJI-UHFFFAOYSA-N Sultiame Chemical compound C1=CC(S(=O)(=O)N)=CC=C1N1S(=O)(=O)CCCC1 HMHVCUVYZFYAJI-UHFFFAOYSA-N 0.000 description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 2
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 description 2
- 235000011941 Tilia x europaea Nutrition 0.000 description 2
- 208000002474 Tinea Diseases 0.000 description 2
- 201000005485 Toxoplasmosis Diseases 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- 241000893966 Trichophyton verrucosum Species 0.000 description 2
- 208000037386 Typhoid Diseases 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 208000003152 Yellow Fever Diseases 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000010933 acylation Effects 0.000 description 2
- 238000005917 acylation reaction Methods 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- 229960003022 amoxicillin Drugs 0.000 description 2
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 2
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 2
- 229960003942 amphotericin b Drugs 0.000 description 2
- JHVAMHSQVVQIOT-MFAJLEFUSA-N anidulafungin Chemical compound C1=CC(OCCCCC)=CC=C1C1=CC=C(C=2C=CC(=CC=2)C(=O)N[C@@H]2C(N[C@H](C(=O)N3C[C@H](O)C[C@H]3C(=O)N[C@H](C(=O)N[C@H](C(=O)N3C[C@H](C)[C@H](O)[C@H]3C(=O)N[C@H](O)[C@H](O)C2)[C@@H](C)O)[C@H](O)[C@@H](O)C=2C=CC(O)=CC=2)[C@@H](C)O)=O)C=C1 JHVAMHSQVVQIOT-MFAJLEFUSA-N 0.000 description 2
- 229960003348 anidulafungin Drugs 0.000 description 2
- 230000000843 anti-fungal effect Effects 0.000 description 2
- 229940121357 antivirals Drugs 0.000 description 2
- 239000008365 aqueous carrier Substances 0.000 description 2
- 125000005200 aryloxy carbonyloxy group Chemical group 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 150000001559 benzoic acids Chemical class 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 102000005936 beta-Galactosidase Human genes 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- 102000006635 beta-lactamase Human genes 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 230000031018 biological processes and functions Effects 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 206010006451 bronchitis Diseases 0.000 description 2
- 239000006172 buffering agent Substances 0.000 description 2
- PTOJXIKSKSASRB-UHFFFAOYSA-O candicine Chemical compound C[N+](C)(C)CCC1=CC=C(O)C=C1 PTOJXIKSKSASRB-UHFFFAOYSA-O 0.000 description 2
- 201000003984 candidiasis Diseases 0.000 description 2
- KHAVLLBUVKBTBG-UHFFFAOYSA-N caproleic acid Natural products OC(=O)CCCCCCCC=C KHAVLLBUVKBTBG-UHFFFAOYSA-N 0.000 description 2
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 2
- 229960003669 carbenicillin Drugs 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000012876 carrier material Substances 0.000 description 2
- XIURVHNZVLADCM-IUODEOHRSA-N cefalotin Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC(=O)C)C(O)=O)C(=O)CC1=CC=CS1 XIURVHNZVLADCM-IUODEOHRSA-N 0.000 description 2
- 229960000603 cefalotin Drugs 0.000 description 2
- 229960001139 cefazolin Drugs 0.000 description 2
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 description 2
- 229960002100 cefepime Drugs 0.000 description 2
- HVFLCNVBZFFHBT-ZKDACBOMSA-O cefepime(1+) Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1C[N+]1(C)CCCC1 HVFLCNVBZFFHBT-ZKDACBOMSA-O 0.000 description 2
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 2
- 229960000590 celecoxib Drugs 0.000 description 2
- 210000002421 cell wall Anatomy 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- ZAIPMKNFIOOWCQ-UEKVPHQBSA-N cephalexin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=CC=C1 ZAIPMKNFIOOWCQ-UEKVPHQBSA-N 0.000 description 2
- 229940106164 cephalexin Drugs 0.000 description 2
- JQXXHWHPUNPDRT-YOPQJBRCSA-N chembl1332716 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CCN(C)CC1 JQXXHWHPUNPDRT-YOPQJBRCSA-N 0.000 description 2
- 210000000038 chest Anatomy 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 239000003593 chromogenic compound Substances 0.000 description 2
- QGPKADBNRMWEQR-UHFFFAOYSA-N clinafloxacin Chemical compound C1C(N)CCN1C1=C(F)C=C2C(=O)C(C(O)=O)=CN(C3CC3)C2=C1Cl QGPKADBNRMWEQR-UHFFFAOYSA-N 0.000 description 2
- 229950001320 clinafloxacin Drugs 0.000 description 2
- 229960004070 clopamide Drugs 0.000 description 2
- 229960003326 cloxacillin Drugs 0.000 description 2
- LQOLIRLGBULYKD-JKIFEVAISA-N cloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl LQOLIRLGBULYKD-JKIFEVAISA-N 0.000 description 2
- 229940110456 cocoa butter Drugs 0.000 description 2
- 235000019868 cocoa butter Nutrition 0.000 description 2
- 206010009887 colitis Diseases 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 125000000392 cycloalkenyl group Chemical group 0.000 description 2
- 201000003146 cystitis Diseases 0.000 description 2
- CJBJHOAVZSMMDJ-HEXNFIEUSA-N darunavir Chemical compound C([C@@H]([C@H](O)CN(CC(C)C)S(=O)(=O)C=1C=CC(N)=CC=1)NC(=O)O[C@@H]1[C@@H]2CCO[C@@H]2OC1)C1=CC=CC=C1 CJBJHOAVZSMMDJ-HEXNFIEUSA-N 0.000 description 2
- 229960005107 darunavir Drugs 0.000 description 2
- DYDCPNMLZGFQTM-UHFFFAOYSA-N delafloxacin Chemical compound C1=C(F)C(N)=NC(N2C3=C(Cl)C(N4CC(O)C4)=C(F)C=C3C(=O)C(C(O)=O)=C2)=C1F DYDCPNMLZGFQTM-UHFFFAOYSA-N 0.000 description 2
- 229950006412 delafloxacin Drugs 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 239000000032 diagnostic agent Substances 0.000 description 2
- 229940039227 diagnostic agent Drugs 0.000 description 2
- GJQPMPFPNINLKP-UHFFFAOYSA-N diclofenamide Chemical compound NS(=O)(=O)C1=CC(Cl)=C(Cl)C(S(N)(=O)=O)=C1 GJQPMPFPNINLKP-UHFFFAOYSA-N 0.000 description 2
- 229960005081 diclofenamide Drugs 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 206010013023 diphtheria Diseases 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- AVAACINZEOAHHE-VFZPANTDSA-N doripenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](CNS(N)(=O)=O)C1 AVAACINZEOAHHE-VFZPANTDSA-N 0.000 description 2
- 229960000895 doripenem Drugs 0.000 description 2
- 239000008298 dragée Substances 0.000 description 2
- 208000001848 dysentery Diseases 0.000 description 2
- 230000002500 effect on skin Effects 0.000 description 2
- IDYZIJYBMGIQMJ-UHFFFAOYSA-N enoxacin Chemical compound N1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 IDYZIJYBMGIQMJ-UHFFFAOYSA-N 0.000 description 2
- 229960002549 enoxacin Drugs 0.000 description 2
- RPBAFSBGYDKNRG-NJBDSQKTSA-N epicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CCC=CC1 RPBAFSBGYDKNRG-NJBDSQKTSA-N 0.000 description 2
- 229960002457 epicillin Drugs 0.000 description 2
- 229960002770 ertapenem Drugs 0.000 description 2
- MMXKVMNBHPAILY-UHFFFAOYSA-N ethyl laurate Chemical compound CCCCCCCCCCCC(=O)OCC MMXKVMNBHPAILY-UHFFFAOYSA-N 0.000 description 2
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 2
- 229940093471 ethyl oleate Drugs 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 210000003608 fece Anatomy 0.000 description 2
- 229960004273 floxacillin Drugs 0.000 description 2
- 208000024386 fungal infectious disease Diseases 0.000 description 2
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 2
- 229960003883 furosemide Drugs 0.000 description 2
- 229960001430 garenoxacin Drugs 0.000 description 2
- NJDRXTDGYFKORP-LLVKDONJSA-N garenoxacin Chemical compound N([C@@H](C1=CC=2)C)CC1=CC=2C(C=1OC(F)F)=CC=C(C(C(C(O)=O)=C2)=O)C=1N2C1CC1 NJDRXTDGYFKORP-LLVKDONJSA-N 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 206010017931 gastrointestinal anthrax Diseases 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 208000001786 gonorrhea Diseases 0.000 description 2
- 210000004209 hair Anatomy 0.000 description 2
- 229960001906 haloprogin Drugs 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- DXVUYOAEDJXBPY-NFFDBFGFSA-N hetacillin Chemical compound C1([C@@H]2C(=O)N(C(N2)(C)C)[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 DXVUYOAEDJXBPY-NFFDBFGFSA-N 0.000 description 2
- 229960003884 hetacillin Drugs 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 229960002003 hydrochlorothiazide Drugs 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- ZSKVGTPCRGIANV-ZXFLCMHBSA-N imipenem Chemical compound C1C(SCC\N=C\N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 ZSKVGTPCRGIANV-ZXFLCMHBSA-N 0.000 description 2
- 229960002182 imipenem Drugs 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- NDDAHWYSQHTHNT-UHFFFAOYSA-N indapamide Chemical compound CC1CC2=CC=CC=C2N1NC(=O)C1=CC=C(Cl)C(S(N)(=O)=O)=C1 NDDAHWYSQHTHNT-UHFFFAOYSA-N 0.000 description 2
- 229960004569 indapamide Drugs 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 206010022000 influenza Diseases 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 229960004130 itraconazole Drugs 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 229960003376 levofloxacin Drugs 0.000 description 2
- 239000004571 lime Substances 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 230000033001 locomotion Effects 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 229960003640 mafenide Drugs 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 201000004792 malaria Diseases 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 208000037941 meningococcal disease Diseases 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- FZECHKJQHUVANE-MCYUEQNJSA-N metampicillin Chemical compound C1([C@@H](N=C)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 FZECHKJQHUVANE-MCYUEQNJSA-N 0.000 description 2
- 229940101856 methampicillin Drugs 0.000 description 2
- 229960002509 miconazole Drugs 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- 229960003702 moxifloxacin Drugs 0.000 description 2
- FABPRXSRWADJSP-MEDUHNTESA-N moxifloxacin Chemical compound COC1=C(N2C[C@H]3NCCC[C@H]3C2)C(F)=CC(C(C(C(O)=O)=C2)=O)=C1N2C1CC1 FABPRXSRWADJSP-MEDUHNTESA-N 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- GPXLMGHLHQJAGZ-JTDSTZFVSA-N nafcillin Chemical compound C1=CC=CC2=C(C(=O)N[C@@H]3C(N4[C@H](C(C)(C)S[C@@H]43)C(O)=O)=O)C(OCC)=CC=C21 GPXLMGHLHQJAGZ-JTDSTZFVSA-N 0.000 description 2
- 229960000515 nafcillin Drugs 0.000 description 2
- 229960003255 natamycin Drugs 0.000 description 2
- 239000004311 natamycin Substances 0.000 description 2
- NCXMLFZGDNKEPB-FFPOYIOWSA-N natamycin Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C[C@@H](C)OC(=O)/C=C/[C@H]2O[C@@H]2C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 NCXMLFZGDNKEPB-FFPOYIOWSA-N 0.000 description 2
- 235000010298 natamycin Nutrition 0.000 description 2
- 229960001180 norfloxacin Drugs 0.000 description 2
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 2
- 238000011580 nude mouse model Methods 0.000 description 2
- 229960000988 nystatin Drugs 0.000 description 2
- VQOXZBDYSJBXMA-NQTDYLQESA-N nystatin A1 Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/CC/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 VQOXZBDYSJBXMA-NQTDYLQESA-N 0.000 description 2
- 229960001699 ofloxacin Drugs 0.000 description 2
- 229960003752 oseltamivir Drugs 0.000 description 2
- NENPYTRHICXVCS-YNEHKIRRSA-N oseltamivir acid Chemical compound CCC(CC)O[C@@H]1C=C(C(O)=O)C[C@H](N)[C@H]1NC(C)=O NENPYTRHICXVCS-YNEHKIRRSA-N 0.000 description 2
- 229960000321 oxolinic acid Drugs 0.000 description 2
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 2
- 229950011346 panipenem Drugs 0.000 description 2
- 208000010403 panophthalmitis Diseases 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 208000011906 peptic ulcer disease Diseases 0.000 description 2
- 239000000816 peptidomimetic Substances 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 229960002292 piperacillin Drugs 0.000 description 2
- WCMIIGXFCMNQDS-IDYPWDAWSA-M piperacillin sodium Chemical compound [Na+].O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC=CC=1)C(=O)N[C@@H]1C(=O)N2[C@@H](C([O-])=O)C(C)(C)S[C@@H]21 WCMIIGXFCMNQDS-IDYPWDAWSA-M 0.000 description 2
- RCIMBBZXSXFZBV-UHFFFAOYSA-N piromidic acid Chemical compound N1=C2N(CC)C=C(C(O)=O)C(=O)C2=CN=C1N1CCCC1 RCIMBBZXSXFZBV-UHFFFAOYSA-N 0.000 description 2
- 229960004444 piromidic acid Drugs 0.000 description 2
- 150000004291 polyenes Chemical class 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 229960001589 posaconazole Drugs 0.000 description 2
- RAGOYPUPXAKGKH-XAKZXMRKSA-N posaconazole Chemical compound O=C1N([C@H]([C@H](C)O)CC)N=CN1C1=CC=C(N2CCN(CC2)C=2C=CC(OC[C@H]3C[C@@](CN4N=CN=C4)(OC3)C=3C(=CC(F)=CC=3)F)=CC=2)C=C1 RAGOYPUPXAKGKH-XAKZXMRKSA-N 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- DBABZHXKTCFAPX-UHFFFAOYSA-N probenecid Chemical compound CCCN(CCC)S(=O)(=O)C1=CC=C(C(O)=O)C=C1 DBABZHXKTCFAPX-UHFFFAOYSA-N 0.000 description 2
- 229960003081 probenecid Drugs 0.000 description 2
- 239000001294 propane Substances 0.000 description 2
- 229960001224 prulifloxacin Drugs 0.000 description 2
- 229960005206 pyrazinamide Drugs 0.000 description 2
- IPEHBUMCGVEMRF-UHFFFAOYSA-N pyrazinecarboxamide Chemical compound NC(=O)C1=CN=CC=N1 IPEHBUMCGVEMRF-UHFFFAOYSA-N 0.000 description 2
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 2
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 2
- 201000003068 rheumatic fever Diseases 0.000 description 2
- 229960001225 rifampicin Drugs 0.000 description 2
- XBPZXDSZHPDXQU-UHFFFAOYSA-N rosoxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC=C1C1=CC=NC=C1 XBPZXDSZHPDXQU-UHFFFAOYSA-N 0.000 description 2
- 229960003889 rosoxacin Drugs 0.000 description 2
- 229960004062 rufloxacin Drugs 0.000 description 2
- 239000008159 sesame oil Substances 0.000 description 2
- 235000011803 sesame oil Nutrition 0.000 description 2
- 201000005113 shigellosis Diseases 0.000 description 2
- 229960003177 sitafloxacin Drugs 0.000 description 2
- 230000008591 skin barrier function Effects 0.000 description 2
- 208000019116 sleep disease Diseases 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 229960004954 sparfloxacin Drugs 0.000 description 2
- DZZWHBIBMUVIIW-DTORHVGOSA-N sparfloxacin Chemical compound C1[C@@H](C)N[C@@H](C)CN1C1=C(F)C(N)=C2C(=O)C(C(O)=O)=CN(C3CC3)C2=C1F DZZWHBIBMUVIIW-DTORHVGOSA-N 0.000 description 2
- 208000024794 sputum Diseases 0.000 description 2
- 210000003802 sputum Anatomy 0.000 description 2
- 239000008223 sterile water Substances 0.000 description 2
- 210000000434 stratum corneum Anatomy 0.000 description 2
- 229960004932 sulbenicillin Drugs 0.000 description 2
- SKIVFJLNDNKQPD-UHFFFAOYSA-N sulfacetamide Chemical compound CC(=O)NS(=O)(=O)C1=CC=C(N)C=C1 SKIVFJLNDNKQPD-UHFFFAOYSA-N 0.000 description 2
- 229960002673 sulfacetamide Drugs 0.000 description 2
- SEEPANYCNGTZFQ-UHFFFAOYSA-N sulfadiazine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=NC=CC=N1 SEEPANYCNGTZFQ-UHFFFAOYSA-N 0.000 description 2
- 229960004306 sulfadiazine Drugs 0.000 description 2
- ZZORFUFYDOWNEF-UHFFFAOYSA-N sulfadimethoxine Chemical compound COC1=NC(OC)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 ZZORFUFYDOWNEF-UHFFFAOYSA-N 0.000 description 2
- 229960000973 sulfadimethoxine Drugs 0.000 description 2
- 229960004673 sulfadoxine Drugs 0.000 description 2
- 229960000654 sulfafurazole Drugs 0.000 description 2
- 229960005404 sulfamethoxazole Drugs 0.000 description 2
- VLYWMPOKSSWJAL-UHFFFAOYSA-N sulfamethoxypyridazine Chemical compound N1=NC(OC)=CC=C1NS(=O)(=O)C1=CC=C(N)C=C1 VLYWMPOKSSWJAL-UHFFFAOYSA-N 0.000 description 2
- 229960004936 sulfamethoxypyridazine Drugs 0.000 description 2
- JLKIGFTWXXRPMT-UHFFFAOYSA-N sulphamethoxazole Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 JLKIGFTWXXRPMT-UHFFFAOYSA-N 0.000 description 2
- 229960002573 sultiame Drugs 0.000 description 2
- KQKPFRSPSRPDEB-UHFFFAOYSA-N sumatriptan Chemical compound CNS(=O)(=O)CC1=CC=C2NC=C(CCN(C)C)C2=C1 KQKPFRSPSRPDEB-UHFFFAOYSA-N 0.000 description 2
- 229960003708 sumatriptan Drugs 0.000 description 2
- 239000002344 surface layer Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 208000006379 syphilis Diseases 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- SOROUYSPFADXSN-SUWVAFIASA-N talampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(=O)OC2C3=CC=CC=C3C(=O)O2)(C)C)=CC=CC=C1 SOROUYSPFADXSN-SUWVAFIASA-N 0.000 description 2
- 229960002780 talampicillin Drugs 0.000 description 2
- BVCKFLJARNKCSS-DWPRYXJFSA-N temocillin Chemical compound N([C@]1(OC)C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C(C(O)=O)C=1C=CSC=1 BVCKFLJARNKCSS-DWPRYXJFSA-N 0.000 description 2
- 229960001114 temocillin Drugs 0.000 description 2
- VAMSVIZLXJOLHZ-QWFSEIHXSA-N tigemonam Chemical compound O=C1N(OS(O)(=O)=O)C(C)(C)[C@@H]1NC(=O)C(=N/OCC(O)=O)\C1=CSC(N)=N1 VAMSVIZLXJOLHZ-QWFSEIHXSA-N 0.000 description 2
- 229950010206 tigemonam Drugs 0.000 description 2
- FUSNMLFNXJSCDI-UHFFFAOYSA-N tolnaftate Chemical compound C=1C=C2C=CC=CC2=CC=1OC(=S)N(C)C1=CC=CC(C)=C1 FUSNMLFNXJSCDI-UHFFFAOYSA-N 0.000 description 2
- 229960004880 tolnaftate Drugs 0.000 description 2
- 229950008187 tosufloxacin Drugs 0.000 description 2
- 206010044325 trachoma Diseases 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 238000002834 transmittance Methods 0.000 description 2
- 229960000497 trovafloxacin Drugs 0.000 description 2
- WVPSKSLAZQPAKQ-CDMJZVDBSA-N trovafloxacin Chemical compound C([C@H]1[C@@H]([C@H]1C1)N)N1C(C(=CC=1C(=O)C(C(O)=O)=C2)F)=NC=1N2C1=CC=C(F)C=C1F WVPSKSLAZQPAKQ-CDMJZVDBSA-N 0.000 description 2
- 201000008297 typhoid fever Diseases 0.000 description 2
- 229960002703 undecylenic acid Drugs 0.000 description 2
- 208000000143 urethritis Diseases 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 229960004740 voriconazole Drugs 0.000 description 2
- BCEHBSKCWLPMDN-MGPLVRAMSA-N voriconazole Chemical compound C1([C@H](C)[C@](O)(CN2N=CN=C2)C=2C(=CC(F)=CC=2)F)=NC=NC=C1F BCEHBSKCWLPMDN-MGPLVRAMSA-N 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- ARAIBEBZBOPLMB-UFGQHTETSA-N zanamivir Chemical compound CC(=O)N[C@@H]1[C@@H](N=C(N)N)C=C(C(O)=O)O[C@H]1[C@H](O)[C@H](O)CO ARAIBEBZBOPLMB-UFGQHTETSA-N 0.000 description 2
- 229960001028 zanamivir Drugs 0.000 description 2
- UBQNRHZMVUUOMG-UHFFFAOYSA-N zonisamide Chemical compound C1=CC=C2C(CS(=O)(=O)N)=NOC2=C1 UBQNRHZMVUUOMG-UHFFFAOYSA-N 0.000 description 2
- 229960002911 zonisamide Drugs 0.000 description 2
- WKJGTOYAEQDNIA-IOOZKYRYSA-N (6r,7r)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydrate Chemical compound O.C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CS[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 WKJGTOYAEQDNIA-IOOZKYRYSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- CRSBERNSMYQZNG-UHFFFAOYSA-N 1-dodecene Chemical group CCCCCCCCCCC=C CRSBERNSMYQZNG-UHFFFAOYSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- KGLPWQKSKUVKMJ-UHFFFAOYSA-N 2,3-dihydrophthalazine-1,4-dione Chemical compound C1=CC=C2C(=O)NNC(=O)C2=C1 KGLPWQKSKUVKMJ-UHFFFAOYSA-N 0.000 description 1
- YYEMWBZRNYJJNO-UHFFFAOYSA-N 2-(diethylamino)ethyl 1-ethyl-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylate Chemical compound CC1=CC=C2C(=O)C(C(=O)OCCN(CC)CC)=CN(CC)C2=N1 YYEMWBZRNYJJNO-UHFFFAOYSA-N 0.000 description 1
- UIFLRRZFKGCXLV-UHFFFAOYSA-N 2-(diethylamino)ethyl 3-(carbamoyloxymethyl)-7-[[2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;hydrochloride Chemical compound Cl.O=C1N2C(C(=O)OCCN(CC)CC)=C(COC(N)=O)CSC2C1NC(=O)C(=NOC)C1=CC=CO1 UIFLRRZFKGCXLV-UHFFFAOYSA-N 0.000 description 1
- WHCNCMKMWANSED-UHFFFAOYSA-N 2-(diethylamino)ethyl 3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;hydrochloride Chemical compound Cl.CCN(CC)CCOC(=O)C1=C(COC(N)=O)CSC2N1C(=O)C2(OC)NC(=O)CC1=CC=CS1 WHCNCMKMWANSED-UHFFFAOYSA-N 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- DPBYCORQBMMFJZ-UHFFFAOYSA-N 20-episilicine Natural products O=C1CC2C(CC)CN(C)CC2CC2=C1NC1=CC=CC=C21 DPBYCORQBMMFJZ-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- HSVNKCAFGMZMHX-UHFFFAOYSA-N 3-(diethylamino)propyl 6-oxo-3-[[4-(pyridin-2-ylsulfamoyl)phenyl]hydrazinylidene]cyclohexa-1,4-diene-1-carboxylate Chemical compound C1=CC(=O)C(C(=O)OCCCN(CC)CC)=CC1=NNC1=CC=C(S(=O)(=O)NC=2N=CC=CC=2)C=C1 HSVNKCAFGMZMHX-UHFFFAOYSA-N 0.000 description 1
- ALKYHXVLJMQRLQ-UHFFFAOYSA-N 3-Hydroxy-2-naphthoate Chemical class C1=CC=C2C=C(O)C(C(=O)O)=CC2=C1 ALKYHXVLJMQRLQ-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-M 3-carboxy-2,3-dihydroxypropanoate Chemical compound OC(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-M 0.000 description 1
- OBWSOTREAMFOCQ-UHFFFAOYSA-N 4-(4-amino-3,5-dimethylphenyl)-2,6-dimethylaniline;hydrochloride Chemical compound Cl.CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 OBWSOTREAMFOCQ-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- XZKIHKMTEMTJQX-UHFFFAOYSA-N 4-Nitrophenyl Phosphate Chemical compound OP(O)(=O)OC1=CC=C([N+]([O-])=O)C=C1 XZKIHKMTEMTJQX-UHFFFAOYSA-N 0.000 description 1
- KWHPWBXOLZTZMJ-UHFFFAOYSA-N 4-ethylpiperidine Chemical compound CCC1CCNCC1 KWHPWBXOLZTZMJ-UHFFFAOYSA-N 0.000 description 1
- FUFZNHHSSMCXCZ-UHFFFAOYSA-N 5-piperidin-4-yl-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole Chemical compound FC(F)(F)C1=CC=CC(C=2N=C(ON=2)C2CCNCC2)=C1 FUFZNHHSSMCXCZ-UHFFFAOYSA-N 0.000 description 1
- BZTDTCNHAFUJOG-UHFFFAOYSA-N 6-carboxyfluorescein Chemical compound C12=CC=C(O)C=C2OC2=CC(O)=CC=C2C11OC(=O)C2=CC=C(C(=O)O)C=C21 BZTDTCNHAFUJOG-UHFFFAOYSA-N 0.000 description 1
- 102100031126 6-phosphogluconolactonase Human genes 0.000 description 1
- 108010029731 6-phosphogluconolactonase Proteins 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- CWJJDUAFBDSADP-UHFFFAOYSA-N 7-[(2-acetamido-2-phenylacetyl)amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Chemical compound C12SCC(Cl)=C(C(O)=O)N2C(=O)C1NC(=O)C(NC(=O)C)C1=CC=CC=C1 CWJJDUAFBDSADP-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000224489 Amoeba Species 0.000 description 1
- 241001403152 Appendicularia <angiosperm> Species 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 241000239223 Arachnida Species 0.000 description 1
- 241000238421 Arthropoda Species 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108090000363 Bacterial Luciferases Proteins 0.000 description 1
- 241000221198 Basidiomycota Species 0.000 description 1
- ZBJJDYGJCNTNTH-UHFFFAOYSA-N Betahistine mesilate Chemical group CS(O)(=O)=O.CS(O)(=O)=O.CNCCC1=CC=CC=N1 ZBJJDYGJCNTNTH-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 241000760366 Blastocladiomycota Species 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 239000004604 Blowing Agent Substances 0.000 description 1
- 241000737252 Botrychium Species 0.000 description 1
- 206010006261 Breast infections Diseases 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- ZTQSAGDEMFDKMZ-UHFFFAOYSA-N Butyraldehyde Chemical group CCCC=O ZTQSAGDEMFDKMZ-UHFFFAOYSA-N 0.000 description 1
- GLHBUDATGSOWEC-UHFFFAOYSA-N C(C)N(CC)COP(OC1=CC=C(C=C1)[N+](=O)[O-])(=O)CNC(=O)OCC1=CC=CC=C1 Chemical compound C(C)N(CC)COP(OC1=CC=C(C=C1)[N+](=O)[O-])(=O)CNC(=O)OCC1=CC=CC=C1 GLHBUDATGSOWEC-UHFFFAOYSA-N 0.000 description 1
- SRCDHAPUCVPQFI-UHFFFAOYSA-N C1=CC=C2NC(SC(=O)CCCN(C)C)=NC2=C1 Chemical compound C1=CC=C2NC(SC(=O)CCCN(C)C)=NC2=C1 SRCDHAPUCVPQFI-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 241000195628 Chlorophyta Species 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000218631 Coniferophyta Species 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 241000938605 Crocodylia Species 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 241000238424 Crustacea Species 0.000 description 1
- 229920000832 Cutin Polymers 0.000 description 1
- 241000592295 Cycadophyta Species 0.000 description 1
- 241000252233 Cyprinus carpio Species 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- MQJKPEGWNLWLTK-UHFFFAOYSA-N Dapsone Chemical compound C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=C1 MQJKPEGWNLWLTK-UHFFFAOYSA-N 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- 238000004435 EPR spectroscopy Methods 0.000 description 1
- 241000195952 Equisetaceae Species 0.000 description 1
- 241000758993 Equisetidae Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 108010015133 Galactose oxidase Proteins 0.000 description 1
- 235000011201 Ginkgo Nutrition 0.000 description 1
- 244000194101 Ginkgo biloba Species 0.000 description 1
- 235000008100 Ginkgo biloba Nutrition 0.000 description 1
- 241001583499 Glomeromycotina Species 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 108010018962 Glucosephosphate Dehydrogenase Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 1
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical group CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 1
- 241000737279 Isoetales Species 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 108010023244 Lactoperoxidase Proteins 0.000 description 1
- 102000045576 Lactoperoxidases Human genes 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 241000758962 Lycopodiopsida Species 0.000 description 1
- 241000195947 Lycopodium Species 0.000 description 1
- IPWKIXLWTCNBKN-UHFFFAOYSA-N Madelen Chemical compound CC1=NC=C([N+]([O-])=O)N1CC(O)CCl IPWKIXLWTCNBKN-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 241001646725 Mycobacterium tuberculosis H37Rv Species 0.000 description 1
- 108700035964 Mycobacterium tuberculosis HsaD Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- SCIFESDRCALIIM-UHFFFAOYSA-N N-Me-Phenylalanine Natural products CNC(C(O)=O)CC1=CC=CC=C1 SCIFESDRCALIIM-UHFFFAOYSA-N 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- SCIFESDRCALIIM-VIFPVBQESA-N N-methyl-L-phenylalanine Chemical compound C[NH2+][C@H](C([O-])=O)CC1=CC=CC=C1 SCIFESDRCALIIM-VIFPVBQESA-N 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 241000963650 Nymphon gracile Species 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 241000737286 Ophioglossum Species 0.000 description 1
- 208000005141 Otitis Diseases 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 239000004936 P-84 Substances 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 235000008331 Pinus X rigitaeda Nutrition 0.000 description 1
- 235000011613 Pinus brutia Nutrition 0.000 description 1
- 241000018646 Pinus brutia Species 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000195965 Psilotopsida Species 0.000 description 1
- 235000007959 Psilotum nudum Nutrition 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 241001415519 Thaliacea Species 0.000 description 1
- HJLSLZFTEKNLFI-UHFFFAOYSA-N Tinidazole Chemical compound CCS(=O)(=O)CCN1C(C)=NC=C1[N+]([O-])=O HJLSLZFTEKNLFI-UHFFFAOYSA-N 0.000 description 1
- 241000397921 Turbellaria Species 0.000 description 1
- 108010046334 Urease Proteins 0.000 description 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 1
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 108010093894 Xanthine oxidase Proteins 0.000 description 1
- 102100033220 Xanthine oxidase Human genes 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 241000758405 Zoopagomycotina Species 0.000 description 1
- MZVQCMJNVPIDEA-UHFFFAOYSA-N [CH2]CN(CC)CC Chemical group [CH2]CN(CC)CC MZVQCMJNVPIDEA-UHFFFAOYSA-N 0.000 description 1
- KPCZJLGGXRGYIE-UHFFFAOYSA-N [C]1=CC=CN=C1 Chemical group [C]1=CC=CN=C1 KPCZJLGGXRGYIE-UHFFFAOYSA-N 0.000 description 1
- WUNKRZNFNIYEPN-UHFFFAOYSA-N [[n-(benzyloxycarbonyl)amino]methyl]phosphate Chemical compound OP(O)(=O)CNC(=O)OCC1=CC=CC=C1 WUNKRZNFNIYEPN-UHFFFAOYSA-N 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 125000003668 acetyloxy group Chemical group [H]C([H])([H])C(=O)O[*] 0.000 description 1
- 125000004018 acid anhydride group Chemical group 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 125000000777 acyl halide group Chemical group 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 210000000436 anus Anatomy 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 210000000576 arachnoid Anatomy 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- JTWOMNBEOCYFNV-NFFDBFGFSA-N azlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCNC1=O JTWOMNBEOCYFNV-NFFDBFGFSA-N 0.000 description 1
- 229960003623 azlocillin Drugs 0.000 description 1
- 230000000721 bacterilogical effect Effects 0.000 description 1
- 239000000022 bacteriostatic agent Substances 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- RDHPKYGYEGBMSE-UHFFFAOYSA-N bromoethane Chemical compound CCBr RDHPKYGYEGBMSE-UHFFFAOYSA-N 0.000 description 1
- 210000005178 buccal mucosa Anatomy 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- XBDQGPFHYCDHEK-UHFFFAOYSA-N butyl 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylate Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)OCCCC)=CN1C1CC1 XBDQGPFHYCDHEK-UHFFFAOYSA-N 0.000 description 1
- BPZMTBOWVRYXDZ-MGPUTAFESA-N butyl 7-[(4as,7as)-1,2,3,4,4a,5,7,7a-octahydropyrrolo[3,4-b]pyridin-6-yl]-1-cyclopropyl-6-fluoro-8-methoxy-4-oxoquinoline-3-carboxylate Chemical compound C12=C(OC)C(N3C[C@H]4NCCC[C@H]4C3)=C(F)C=C2C(=O)C(C(=O)OCCCC)=CN1C1CC1 BPZMTBOWVRYXDZ-MGPUTAFESA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 235000011148 calcium chloride Nutrition 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- GCFBRXLSHGKWDP-XCGNWRKASA-N cefoperazone Chemical compound O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC(O)=CC=1)C(=O)N[C@@H]1C(=O)N2C(C(O)=O)=C(CSC=3N(N=NN=3)C)CS[C@@H]21 GCFBRXLSHGKWDP-XCGNWRKASA-N 0.000 description 1
- 229960004682 cefoperazone Drugs 0.000 description 1
- WYUSVOMTXWRGEK-HBWVYFAYSA-N cefpodoxime Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC)C(O)=O)C(=O)C(=N/OC)\C1=CSC(N)=N1 WYUSVOMTXWRGEK-HBWVYFAYSA-N 0.000 description 1
- 229960005090 cefpodoxime Drugs 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 150000005827 chlorofluoro hydrocarbons Chemical class 0.000 description 1
- 210000003763 chloroplast Anatomy 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000006547 cyclononyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 1
- 229960000860 dapsone Drugs 0.000 description 1
- 125000003493 decenyl group Chemical group [H]C([*])=C([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical class OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 125000005066 dodecenyl group Chemical group C(=CCCCCCCCCCC)* 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 229940000406 drug candidate Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 208000019258 ear infection Diseases 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 210000004696 endometrium Anatomy 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003038 endothelium Anatomy 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 210000001339 epidermal cell Anatomy 0.000 description 1
- 210000005175 epidermal keratinocyte Anatomy 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 235000021050 feed intake Nutrition 0.000 description 1
- 210000004996 female reproductive system Anatomy 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 229960001625 furazolidone Drugs 0.000 description 1
- PLHJDBGFXBMTGZ-WEVVVXLNSA-N furazolidone Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)OCC1 PLHJDBGFXBMTGZ-WEVVVXLNSA-N 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 210000001156 gastric mucosa Anatomy 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 239000005090 green fluorescent protein Substances 0.000 description 1
- 125000002795 guanidino group Chemical group C(N)(=N)N* 0.000 description 1
- 210000004919 hair shaft Anatomy 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000005980 hexynyl group Chemical group 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- CFUQBFQTFMOZBK-QUCCMNQESA-N ibazocine Chemical compound C12=CC(O)=CC=C2C[C@H]2N(CC=C(C)C)CC[C@]1(C)C2(C)C CFUQBFQTFMOZBK-QUCCMNQESA-N 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 244000000053 intestinal parasite Species 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 125000001977 isobenzofuranyl group Chemical group C=1(OC=C2C=CC=CC12)* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- TWBYWOBDOCUKOW-UHFFFAOYSA-M isonicotinate Chemical compound [O-]C(=O)C1=CC=NC=C1 TWBYWOBDOCUKOW-UHFFFAOYSA-M 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940057428 lactoperoxidase Drugs 0.000 description 1
- 210000000088 lip Anatomy 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 210000004995 male reproductive system Anatomy 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 229960000282 metronidazole Drugs 0.000 description 1
- VAOCPAMSLUNLGC-UHFFFAOYSA-N metronidazole Chemical compound CC1=NC=C([N+]([O-])=O)N1CCO VAOCPAMSLUNLGC-UHFFFAOYSA-N 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 108010029942 microperoxidase Proteins 0.000 description 1
- 210000001589 microsome Anatomy 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229940041009 monobactams Drugs 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229940051866 mouthwash Drugs 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- HEDOODBJFVUQMS-UHFFFAOYSA-N n-[2-(5-methoxy-1h-indol-3-yl)ethyl]-n-methylpropan-2-amine Chemical group COC1=CC=C2NC=C(CCN(C)C(C)C)C2=C1 HEDOODBJFVUQMS-UHFFFAOYSA-N 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 125000005187 nonenyl group Chemical group C(=CCCCCCCC)* 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004365 octenyl group Chemical group C(=CCCCCCC)* 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005069 octynyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C#C* 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 210000003463 organelle Anatomy 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 229960002313 ornidazole Drugs 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 229940014662 pantothenate Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 229960005065 paromomycin sulfate Drugs 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 230000037368 penetrate the skin Effects 0.000 description 1
- 229960004448 pentamidine Drugs 0.000 description 1
- XDRYMKDFEDOLFX-UHFFFAOYSA-N pentamidine Chemical compound C1=CC(C(=N)N)=CC=C1OCCCCCOC1=CC=C(C(N)=N)C=C1 XDRYMKDFEDOLFX-UHFFFAOYSA-N 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000005004 perfluoroethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- HCTVWSOKIJULET-LQDWTQKMSA-M phenoxymethylpenicillin potassium Chemical compound [K+].N([C@H]1[C@H]2SC([C@@H](N2C1=O)C([O-])=O)(C)C)C(=O)COC1=CC=CC=C1 HCTVWSOKIJULET-LQDWTQKMSA-M 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 210000002706 plastid Anatomy 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000307 polymer substrate Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 108010054624 red fluorescent protein Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- DSIJXEVOJMBKAM-UHFFFAOYSA-M sodium;2-methyloct-2-enoate Chemical compound [Na+].CCCCCC=C(C)C([O-])=O DSIJXEVOJMBKAM-UHFFFAOYSA-M 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 125000005017 substituted alkenyl group Chemical group 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- FKENQMMABCRJMK-RITPCOANSA-N sulbactam Chemical compound O=S1(=O)C(C)(C)[C@H](C(O)=O)N2C(=O)C[C@H]21 FKENQMMABCRJMK-RITPCOANSA-N 0.000 description 1
- 229960005256 sulbactam Drugs 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- RSPCKAHMRANGJZ-UHFFFAOYSA-N thiohydroxylamine Chemical compound SN RSPCKAHMRANGJZ-UHFFFAOYSA-N 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 229960005053 tinidazole Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 125000005065 undecenyl group Chemical group C(=CCCCCCCCCC)* 0.000 description 1
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940116269 uric acid Drugs 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 210000003934 vacuole Anatomy 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 108091005957 yellow fluorescent proteins Proteins 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 235000014692 zinc oxide Nutrition 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/16—Otologicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- A61P31/06—Antibacterial agents for tuberculosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/04—Amoebicides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
- A61P33/12—Schistosomicides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/72—Nitrogen atoms
- C07D213/76—Nitrogen atoms to which a second hetero atom is attached
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/20—Oxygen atoms
- C07D215/22—Oxygen atoms attached in position 2 or 4
- C07D215/233—Oxygen atoms attached in position 2 or 4 only one oxygen atom which is attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/46—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with acyclic hydrocarbon radicals or such radicals substituted by carbocyclic or heterocyclic rings, attached to the carboxamido radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/48—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical
- C07D499/50—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in beta-position to the carboxamido radical
- C07D499/52—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in beta-position to the carboxamido radical by oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/48—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical
- C07D499/58—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in alpha-position to the carboxamido radical
- C07D499/62—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in alpha-position to the carboxamido radical by sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/48—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical
- C07D499/58—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in alpha-position to the carboxamido radical
- C07D499/64—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in alpha-position to the carboxamido radical by nitrogen atoms
- C07D499/68—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with a carbon chain, substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, attached to the carboxamido radical substituted in alpha-position to the carboxamido radical by nitrogen atoms with aromatic rings as additional substituents on the carbon chain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/74—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with carbocyclic rings directly attached to the carboxamido radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/21—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a nitrogen atom directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D499/44—Compounds with an amino radical acylated by carboxylic acids, attached in position 6
- C07D499/76—Compounds with an amino radical acylated by carboxylic acids, attached in position 6 with hetero rings directly attached to the carboxamido radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D499/00—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D499/86—Heterocyclic compounds containing 4-thia-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. penicillins, penems; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with only atoms other than nitrogen atoms directly attached in position 6 and a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/20—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids
- C07D501/22—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids with radicals containing only hydrogen and carbon atoms, attached in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/20—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids
- C07D501/24—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids with hydrocarbon radicals, substituted by hetero atoms or hetero rings, attached in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/20—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids
- C07D501/24—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids with hydrocarbon radicals, substituted by hetero atoms or hetero rings, attached in position 3
- C07D501/26—Methylene radicals, substituted by oxygen atoms; Lactones thereof with the 2-carboxyl group
- C07D501/34—Methylene radicals, substituted by oxygen atoms; Lactones thereof with the 2-carboxyl group with the 7-amino radical acylated by carboxylic acids containing hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/20—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids
- C07D501/24—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids with hydrocarbon radicals, substituted by hetero atoms or hetero rings, attached in position 3
- C07D501/36—Methylene radicals, substituted by sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/59—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3 with hetero atoms directly attached in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D503/00—Heterocyclic compounds containing 4-oxa-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxapenicillins, clavulanic acid derivatives; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D503/10—Heterocyclic compounds containing 4-oxa-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxapenicillins, clavulanic acid derivatives; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D503/12—Heterocyclic compounds containing 4-oxa-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxapenicillins, clavulanic acid derivatives; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 unsubstituted in position 6
- C07D503/14—Heterocyclic compounds containing 4-oxa-1-azabicyclo [3.2.0] heptane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxapenicillins, clavulanic acid derivatives; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 unsubstituted in position 6 with hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, other than a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, attached in position 3
- C07D503/16—Radicals substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical
- C07D503/18—Radicals substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D505/00—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D505/10—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D505/12—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 substituted in position 7
- C07D505/14—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 substituted in position 7 with hetero atoms directly attached in position 7
- C07D505/16—Nitrogen atoms
- C07D505/18—Nitrogen atoms further acylated by radicals derived from carboxylic acids or by nitrogen or sulfur analogues thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D505/00—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D505/10—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2
- C07D505/12—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 substituted in position 7
- C07D505/14—Heterocyclic compounds containing 5-oxa-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. oxacephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring with a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, e.g. an ester or nitrile radical, directly attached in position 2 substituted in position 7 with hetero atoms directly attached in position 7
- C07D505/16—Nitrogen atoms
- C07D505/18—Nitrogen atoms further acylated by radicals derived from carboxylic acids or by nitrogen or sulfur analogues thereof
- C07D505/20—Nitrogen atoms further acylated by radicals derived from carboxylic acids or by nitrogen or sulfur analogues thereof with the acylating radicals further substituted by hetero atoms or by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/30—Phosphinic acids [R2P(=O)(OH)]; Thiophosphinic acids ; [R2P(=X1)(X2H) (X1, X2 are each independently O, S or Se)]
- C07F9/32—Esters thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/38—Phosphonic acids [RP(=O)(OH)2]; Thiophosphonic acids ; [RP(=X1)(X2H)2(X1, X2 are each independently O, S or Se)]
- C07F9/40—Esters thereof
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Virology (AREA)
- Molecular Biology (AREA)
- Pulmonology (AREA)
- Biochemistry (AREA)
- Urology & Nephrology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Dermatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Immunology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- AIDS & HIV (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Quinoline Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
Abstract
본 발명은 생체 장벽을 고투과 효능으로 통과할 수 있는, 항균제 및 항균제-관련 화합물의 새로운 고투과 조성물 (HPC) 또는 고투과 전구약물 (HPP)에 관한 것이다. HPC/HPP는 생체 장벽을 통과한 후 모 활성 약물 또는 약물 대사산물로 전환될 수 있으므로 모 약물 또는 대사산물로 치료될 수 있는 병태를 치료할 수 있다. 또한, HPP는 이의 모 약물이 표적 부위에 접근할 수 없거나 충분한 농도가 될 수 없는 부위에 도달할 수 있도록 하여 새로운 치료가 가능하다. HPP는 대상체에게 각종 투여 경로를 통해 투여할 수 있고, 예를 들면, 병태 작용 부위에 고 농도로 국소 전달될 수 있거나 생물학적 대상체에게 전신적으로 투여되어 빠른 속도로 대순환에 도입될 수 있다.The present invention relates to a new high permeability composition (HPC) or high permeation prodrug (HPP) of antimicrobial agents and antimicrobial agent-related compounds capable of crossing a biological barrier with high permeability. Since HPC / HPP crosses the bio-barrier and can be converted into a parent active drug or drug metabolite, it can treat conditions that can be treated with the parent drug or metabolite. In addition, HPP enables new treatment by allowing its parent drug to reach a site that cannot reach the target site or that cannot be at a sufficient concentration. HPP can be administered to a subject through a variety of routes of administration, for example, can be delivered locally at high concentrations to the site of pathological action, or can be administered systemically to a biological subject and introduced into the circulation at a rapid rate.
Description
본 출원은 2009년 6월 10일에 출원된 미국 특허출원 제12/482,373호의 일부 계속 출원이고 이에 대해 우선권을 주장하며, 이는 본원에 참고로 포함된다. 또한 본 출원은 2009년 6월 10일에 출원된 중국 특허출원 제200910141944X호에 대한 우선권을 주장하며, 이는 본원에 참고로 포함된다.This application is part of the United States Patent Application Serial No. 12 / 482,373, filed June 10, 2009 and claims priority to it, which is incorporated herein by reference. In addition, this application claims priority to Chinese Patent Application No. 200910141944X filed on June 10, 2009, which is incorporated herein by reference.
본 발명은 하나 이상의 생체 장벽(biological barrier)을 투과할 수 있는 약학적 조성물 분야에 관한 것이며, 항균제 및 항균제-관련 화합물에 의해 치료 가능한 인간 및 동물의 병태들 또는 질환들을 예방, 진단 및/또는 치료하기 위한 약학적 조성물 이용 방법에 관한 것이다. 또한 본 발명은 새로운 약물 후보들의 선별을 위한 약학적 조성물 이용 방법 및 생물학적 대상체에서 병태 진단을 위한 약학적 조성물 이용 방법에 관한 것이다.The present invention relates to the field of pharmaceutical compositions capable of penetrating one or more biological barriers, preventing, diagnosing and / or treating conditions and diseases of humans and animals treatable by antibacterial and antimicrobial-related compounds It relates to a method of using a pharmaceutical composition for. The present invention also relates to a method of using a pharmaceutical composition for screening new drug candidates and a method of using a pharmaceutical composition for diagnosing a condition in a biological subject.
항균제들은 세균, 진균, 또는 원충과 같은 미생물을 사멸시키거나 성장을 억제시킬뿐 아니라 바이러스를 파괴할 수 있는 물질이다. 항균제의 주요 종류들은 예를 들면 세균-관련 병태들을 치료하는 항생제, 바이러스-관련 병태들을 치료하는 항바이러스제, 진균-관련 병태들을 치료하는 항진균제 및 원충-관련 병태들을 치료하는 항원충제를 포함한다.Antibacterial agents are substances that can destroy microorganisms, as well as kill or inhibit growth of microorganisms such as bacteria, fungi, or protozoa. The main types of antimicrobial agents include, for example, antibiotics for treating bacterial-related conditions, antiviral agents for treating virus-related conditions, antifungal agents for treating fungal-related conditions, and antiprotozoal agents for treating protozoa-related conditions.
베타-락탐계 항생제는 분자구조에 4-원 고리 베타-락탐 핵을 가지는 항생제이다. 수십만 종류가 넘는 베타-락탐계 항생제들이 부분 또는 총 화학 합성으로 제조되었다. (L.A. Mitscher, et al., Antibiotic and Antimicrobial Drugs, in D.F. Smith, Ed., Handbook of Stereoisomers: Therapeutic Drugs, Boca Raton, FL, CRC Press, 1989; R.B. Morin and M. Gorman Eds., Chemistry and Biology of Beta-lactam Antibiotics, Volumes 1-3, New York, Academic Press, 1982; and A.L.Demain and N.A. Solomon, Eds., Antibiotics Containing the Beta-lactam Structure, Vols, 1 and 2, Handbook of experimental Pharmacology, vol. 67, New York, Springer, 1983). 베타-락탐계 항생제의 예로는 페니실린 유도체, 세팔로스포린, 모노박탐, 카바페넴, 베타-락타마제 억제제, 설폰아미드 및 퀴놀론을 포함한다.Beta-lactam antibiotics are antibiotics that have a 4-membered ring beta-lactam nucleus in their molecular structure. Over hundreds of thousands of beta-lactam antibiotics have been prepared by partial or total chemical synthesis. (LA Mitscher, et al., Antibiotic and Antimicrobial Drugs, in DF Smith, Ed., Handbook of Stereoisomers: Therapeutic Drugs, Boca Raton, FL, CRC Press, 1989; RB Morin and M. Gorman Eds., Chemistry and Biology of Beta-lactam Antibiotics, Volumes 1-3, New York, Academic Press, 1982; and ALDemain and NA Solomon, Eds., Antibiotics Containing the Beta-lactam Structure, Vols, 1 and 2, Handbook of experimental Pharmacology, vol. 67 , New York, Springer, 1983). Examples of beta-lactam antibiotics include penicillin derivatives, cephalosporins, monobactams, carbapenems, beta-lactamase inhibitors, sulfonamides and quinolones.
항균제 과다 사용으로, 시간 경과에 따른 병원체 돌열변이가 진행됨에 따라 약물 내성이 일반적이고 심각한 문제가 되었다. 따라서 새로운 항균제 개발은 시급하고도 도전적인 과제이다.With excessive use of antimicrobial agents, drug resistance has become a common and serious problem as pathogen mutations progress over time. Therefore, developing new antibacterial agents is an urgent and challenging task.
광범위한 항균제들은 정맥내 주입, 근육내 주사, 피하, 구강, 경구, 및 직장 경로로 투여된다. 경구 투여는 GI 관에서 항생제 흡수가 불량하다는 단점이 있다. 정맥내, 피하 및 근육내 경로는 통증뿐 아니라 숙련가에 의한 투여가 필요하며 바늘에 의한 손상, 감염 및 기타 외상과 같은 다른 위험들이 발생될 수 있다.Extensive antimicrobial agents are administered by intravenous infusion, intramuscular injection, subcutaneous, oral, oral, and rectal routes. Oral administration has the disadvantage of poor absorption of antibiotics in the GI tract. Intravenous, subcutaneous and intramuscular routes require pain, as well as administration by a skilled person, and other risks such as needle damage, infection, and other trauma may occur.
약물투여의 대안적 방법은 국소전달이다. 국소 약물전달은 여러 장점들이 있다. 본 방법으로 간 및 위장관에서의 일차통과 대사로 유발되는 비할성화를 피할 수 있다. 또한 전신노출 없이 의도된 작용 부위에 적합한 농도의 약물을 국소적으로 전달할 수 있다. 피셔만 (Fishman; Robert, 미국특허번호 제 7,052,715호)은 경구 투약과 연관된 추가적인 문제는 원위적 통증, 염증, 또는 감염 부위를 효과적으로 치료하기 위하여 혈류에서 달성되어야 할 농도 수준은 상당하여야 한다고 지적하였다. 때로 이러한 수준들은 약물들이 정확하게 특정 통증 또는 손상 부위에 전달되었을 경우 필요한 것보다 훨씬 높은 것이다. 대부분의 항균제들 경우, 국소적 투여로는 효과적인 치료 수준을 전달할 수 없다.An alternative method of drug administration is topical delivery. Topical drug delivery has several advantages. This method avoids the inactivation caused by primary passage metabolism in the liver and gastrointestinal tract. It is also possible to deliver a drug at a concentration suitable for the intended site of action without systemic exposure. Fisherman (Robert, U.S. Patent No. 7,052,715) pointed out that the additional problem associated with oral dosing is that the level of concentration that must be achieved in the bloodstream to effectively treat distal pain, inflammation, or the site of infection must be significant. Sometimes these levels are much higher than what is needed if the drugs are delivered exactly to a particular pain or injury site. For most antimicrobial agents, topical administration cannot deliver effective levels of treatment.
따라서, 최소한의 부작용으로 병태들을 예방, 감소 또는 치료하기 위하여, 병태 (예를 들면, 질환)의 작용 부위에 효율적으로 및 효과적으로 전달될 수 있는 신규한 조성물을 개발할 필요성이 있다.Accordingly, there is a need to develop novel compositions that can be efficiently and effectively delivered to the site of action of a condition (eg, a disease) in order to prevent, reduce or treat conditions with minimal side effects.
본 발명의 한 측면은 링커를 통해 이송 단위에 공유적으로 연결된 관능성 단위를 포함하는 고투과력 전구약물 (HPP) 또는 고투과력 조성물(HPC)에 관한 것이다. 본원에서 용어 ‘HPP’ 및 ‘HPC’는 단독으로 또는 함께 사용되고 달리 특별히 언급되지 않는 한 상호 교환적으로 사용된다.One aspect of the invention relates to a high permeability prodrug (HPP) or a high permeability composition (HPC) comprising functional units covalently linked to a transport unit through a linker. The terms 'HPP' and 'HPC' are used herein alone or together and are used interchangeably unless otherwise specified.
특정 양태에서, HPP 또는 HPC의 관능성 단위는 모약물의 잔기를 포함하는 포함하고, 여기서 모약물의 생물학적 대상체 내로의 효율적이고 효과적인 전달 및/또는 하나 이상의 생체 장벽을 통과하는 이송이 바람직하다.In certain embodiments, a functional unit of HPP or HPC comprises comprising a residue of the parent drug, where efficient and effective delivery of the parent drug to a biological subject and / or transport across one or more biological barriers is preferred.
특정 양태에서, 관능성 단위는 친수성, 친지성 또는 양친성(즉, 친수성 및 친지성)일 수 있다. 예를 들면, 상기 관능성 단위의 친지성은 내재되어 있거나 관능성 단위의 친수성 잔기를 친지성 잔기로 전환시켜 수득할 수 있다. 특정 양태에서, HPP 또는 HPC를 더욱 친지성으로 만들기 위하여 카복실기, 아미노기, 구아니딘기 또는 기타 관능성 단위의 친수성기는 알킬, 아릴, 또는 헤테로아릴 에스테르 또는 아미드기로 보호된다.In certain embodiments, functional units can be hydrophilic, lipophilic, or amphiphilic (ie, hydrophilic and lipophilic). For example, the lipophilicity of the functional unit is inherent or can be obtained by converting the hydrophilic residue of the functional unit to a lipophilic residue. In certain embodiments, the hydrophilic groups of carboxyl groups, amino groups, guanidine groups or other functional units are protected with alkyl, aryl, or heteroaryl ester or amide groups to make HPP or HPC more lipophilic.
특정 양태에서, HPP 또는 HPC의 관능성 단위는 항균제 또는 항균제-관련 화합물의 잔기를 포함한다. 항균제는 세균, 진균, 또는 원충과 같은 미생물을 사멸시키거나 성장을 억제할 수 있을 뿐 아니라 바이러스를 파괴할 수 있는 물질이다.In certain embodiments, the functional units of HPP or HPC include residues of an antimicrobial agent or antimicrobial agent-related compound. Antibacterial agents are substances that can kill microorganisms, such as bacteria, fungi, or protozoa, or inhibit growth as well as destroy viruses.
항균제-관련 화합물은 항균제 구조, 항균제 대사산물, 또는 HPP 또는 HPC가 하나 이상의 생체 장벽들을 투과한 후 항균제 또는 항균제 대사산물로 대사될 수 있는 제제를 포함하는 화합물이다. 항균제-관련 화합물은 항균제 또는 항균제 대사산물의 유사체 또는 모방체인 화합물, 또는 HPP 또는 HPC가 하나 이상의 생체 장벽들을 투과한 후 항균제 또는 항균제 대사산물의 유사체 또는 모방체로 대사될 수 있는 제제를 더욱 포함한다.An antimicrobial agent-related compound is a compound comprising an antimicrobial agent structure, an antimicrobial agent metabolite, or an agent that can be metabolized to an antimicrobial agent or an antimicrobial agent metabolite after HPP or HPC penetrates one or more biological barriers. The antimicrobial agent-related compound further includes a compound that is an analog or mimetic of the antimicrobial agent or antimicrobial agent metabolite, or an agent that can be metabolized to an analog or mimetic agent of the antimicrobial agent or antimicrobial agent metabolite after HPP or HPC penetrates one or more bio-barriers.
항균제의 예시로는 예를 들면 세균-관련 병태들을 치료하는 항생제, 바이러스-관련 병태들을 치료하는 항바이러스제, 진균-관련 병태들을 치료하는 항진균제 및 원충-관련 병태들을 치료하는 항원충제를 포함한다.Examples of antibacterial agents include, for example, antibiotics to treat bacterial-related conditions, antiviral agents to treat virus-related conditions, antifungal agents to treat fungal-related conditions, and protozoa to treat protozoa-related conditions.
항생제의 예로는 베타-락탐계 항생제, 설폰아미드 및 퀴놀론을 포함하지만 이에 국한되지 않는다. 베타-락탐계 항생제의 예로는 페니실린 유도체, 세팔로스포린, 페넴, 모노박탐, 카바페넴, 베타-락타마제 억제제 및 이의 배합물을 포함하나, 이에 제한되는 것은 아니다. 페니실린 유도체의 예로는 아미노페니실린 (예: 아목시실린, 암피실린 및 에피실린); 카복시페니실린 (예: 카베니실린, 티카실린 및 테모실린); 우레이도페니실린 (예: 아즐로실린, 피페라실린 및 메즐로실린); 메실리남, 술베니실린, 벤자틴 페니실린, 페니실린 G (벤질페니실린), 페니실린 V (페녹시메틸페니실린), 페니실린 O (알릴머캅토메틸페니실린), 프로카인 페니실린, 옥사실린, 메티실린, 나프실린, 클록사실린, 디클록사실린, 플루클록사실린, 피밤피실린, 헤타실린, 베캄피실린, 메탐피실린, 탈람피실린, 코-아목시클라브(아목시실린 및 클라불란산), 및 피페라실리온을 포함하나 이에 제한되는 것은 아니다. 세팔로스포린의 예로는 세팔렉신, 세팔로틴, 세파졸린, 세파클러, 세푸록심, 세파만돌, 세포테탄, 세폭시틴, 세포라니드, 세프트리악손, 세포탁심, 세프포독심 프록세틸, 세프타지딤, 세페핌, 세포페라존, 세프티족심, 세픽심 및 세프피롬을 포함하나 이에 제한되는 것은 아니다. 페넴의 예로는 파로페넴을 포함하나 이에 제한되는 것은 아니다. 모노박탐계의 예로는 아즈트레오남 및 티게모남을 포함하나 이에 제한되는 것은 아니다. 카바페넴의 예로는 바이아페넴, 도리페넴, 에르타페넴, 이미페넴, 레로페넴 및 파니페넴을 포함하나 이에 제한되는 것은 아니다. 베타-락타마제의 예로는 타조박탐 ([2S-(2알파,3베타,5알파)]-3-메틸-7-옥소-3-(1H-1,2,3-트리아졸-1-일메틸)-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨 염), 설박탐 (2S,5R)-3,3-디메틸-7-옥소-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨), 및 클라불란산((2R,5R,Z)-3-(2-히드록시에틸리덴)-7-옥소-4-옥사-1-아자-바이시클로[3.2.0]헵탄-2-카복시산)을 포함하나 이에 제한되는 것은 아니다. 항생제의 다른 예로는 [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(4-니트로페닐) 에스테르 나트륨 염, [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(3-피리디닐) 에스테르 나트륨 염, 설파닐아미드 (4-아미노벤젠설폰아미드), 설파살라진 (6-옥소-3-(2-[4-(N-피리딘-2-일설파모일)페닐]히드라조노)시클로헥사-1,4-디엔카복시산), 1-시클로프로필-6-플루오로-4-옥소-7-피페라진-1-일-퀴놀린-3-카복시산, 날리딕스산(1-에틸-7-메틸-4-옥소-[1,8]나프티리딘-3-카복시산)을 포함하나 이에 제한되는 것은 아니다.Examples of antibiotics include, but are not limited to, beta-lactam antibiotics, sulfonamides, and quinolones. Examples of beta-lactam antibiotics include, but are not limited to, penicillin derivatives, cephalosporins, peten, monobactam, carbapenem, beta-lactamase inhibitors, and combinations thereof. Examples of penicillin derivatives include aminopenicillins (eg amoxicillin, ampicillin and epicillin); Carboxypenicillins (eg, carbenicillin, ticacillin and temocillin); Ureidophenicillins (eg, azalocillin, piperacillin and mezzocillin); Mesylinam, sulbenicillin, benzatine penicillin, penicillin G (benzylpenicillin), penicillin V (phenoxymethylpenicillin), penicillin O (allylmercaptomethylpenicillin), procaine penicillin, oxacillin, methicillin, naphcillin , Cloxacillin, dicloxacillin, flucloxacillin, pibampicillin, hetacillin, becampicillin, methampicillin, talampicillin, co-amoxiclab (amoxicillin and clavulanic acid), and pipera Silion, but is not limited thereto. Examples of cephalosporins include cephalexin, cephalotin, cefazoline, cefacler, cefuroxime, cepamandol, cethetan, cepoxytin, celaranid, cetriaxone, cetaxam, cepodoxime proxetyl, ceftage Dim, cefepime, cepeferazone, ceftysim, sepicsim and cefipyrom. Examples of petite include, but are not limited to, paropenet. Examples of monobactam systems include, but are not limited to, aztreonam and tigemonam. Examples of carbapenems include, but are not limited to, baiapenem, doripenem, ertapenem, imipenem, leropenem and panipenem. Examples of beta-lactamase are tazobactam ([2S- (2alpha, 3beta, 5alpha)]-3-methyl-7-oxo-3- (1H-1,2,3-triazol-1-yl Methyl) -4-thia-1-azabicyclo [3.2.0] heptan-2-carboxylic acid 4,4-dioxide sodium salt), sulfactam (2S, 5R) -3,3-dimethyl-7-oxo- 4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid 4,4-dioxide sodium), and clavulanic acid ((2R, 5R, Z) -3- (2-hydroxyethyly Den) -7-oxo-4-oxa-1-aza-bicyclo [3.2.0] heptan-2-carboxylic acid). Other examples of antibiotics are [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- (4-nitrophenyl) ester sodium salt, [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- ( 3-pyridinyl) ester sodium salt, sulfanilamide (4-aminobenzenesulfonamide), sulfasalazine (6-oxo-3- (2- [4- (N-pyridin-2-ylsulfamoyl) phenyl] hydrazono ) Cyclohexa-1,4-dienecarboxylic acid), 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-quinoline-3-carboxylic acid, nalidixic acid (1-ethyl -7-methyl-4-oxo- [1,8] naphthyridine-3-carboxylic acid).
설폰아미드의 예로는 제한적이지 않지만 설파이소디미딘, 설파닐아미드, 설파디아진, 설피속사졸, 설파메톡사졸, 설파디메톡신, 설파메톡시피리다진, 설파세타미드, 설파독신, 아세타졸라미드, 부메타니드, 클로탈리돈, 클로파미드, 푸로세미드, 히드로클로로티아지드, 인다파미드, 메프루시드, 메토라존, 시파미드, 디클로로페나미드, 도졸라미드, 아세타졸라미드, 에톡스졸라미드, 설티암, 조니사미드, 마페니드, 셀레콕시브, 다루나비르, 프로베네시드, 설파살라진 및 수마트리프탄을 포함한다.Examples of sulfonamides include, but are not limited to, sulfisodimidine, sulfanilamide, sulfadiazine, sulfisoxazole, sulfamethoxazole, sulfadimethoxine, sulfamethoxypyridazine, sulfacetamide, sulfadoxine, acetazolamide , Bomethanid, clotalidone, clopamide, furosemide, hydrochlorothiazide, indapamide, meproside, metorazone, cypamide, dichlorophenamide, dozolamide, acetazolamide, e Toxzolamide, sulthiam, zonisamide, mafenide, celecoxib, darunavir, probenecid, sulfasalazine and sumatriptan.
퀴놀론의 예시로는 제한적이지 않지만 시녹사신, 플루메퀸, 날리딕스산, 옥소린산, 피로미드산, 피페미드산, 로속사신, 시프로플로삭신, 에녹사신, 플레녹사신, 로메플록사신, 나디플록사신, 노르플록사신, 오플록사신, 퍼플록사신, 루플록사신, 발로플록사신, 가티플록사신, 그레파플록사신, 레보플록사신, 목시플록사신, 파주플록사신, 스파르플록사신, 테마플록사신, 토수플록사신, 클리나플록사신, 게미플록사신, 시타플록사신, 트로바플록사신, 프룰리플록사신, 가레녹사신, 에시노플록사신, 델라플록사신 및 날리딕스산을 포함한다.Examples of quinolone are, but are not limited to, synoxacin, flumequin, nalidixic acid, oxolinic acid, pyromidic acid, pipemedic acid, rosoxacin, ciprofloxacin, enoxacin, plenoxacin, romefloxacin, nadi Phloxacin, Norfloxacin, Ofloxacin, Purpleoxacin, Rufloxacin, Vallofloxacin, Gattifloxacin, Grefafloxacin, Levofloxacin, Moxifloxacin, Pajufloxacin, Sparfloxacin, Themefloxacin , Tosufloxacin, clinafloxacin, hemifloxacin, sitafloxacin, trovafloxacin, prulifloxacin, garenoxacin, einofloxacin, delafloxacin, and nalidixic acid.
항바이러스의 예시로는 제한적이지 않지만 리팜피신, 자나미비르 및 오셀타미비르를 포함한다. 항진균제의 예로는 폴리엔 항진균제 (예를 들면, 나타마이신, 리모시딘, 필리핀, 니스타틴, 암포테리신 B, 칸디신), 이미다졸 항진균제 (예를 들면, 미코나졸, 케토노나졸, 클로트리마졸, 이트라코나졸, 이사부코나졸, 라부코나졸, 포사코나졸, 보리코나졸, 및 테코나졸), 티아졸 항진균제 (예를 들면, 아바푼긴), 알리아민 (예를 들면, 테비나핀, 아모롤핀, 나프티핀 및 부테나핀), 에키노칸딘 (예를 들면, 아니둘라푼긴, 카스포푼긴 및 미카푼긴) 및 기타 벤조산, 시클로피록스, 톨나프테이트, 운데실렌산, 플루시코신, 그리세오풀빈, 할로프로긴과 같은 기타 항진균제를 포함한다.Examples of antivirals include, but are not limited to, rifampicin, zanamivir and oseltamivir. Examples of antifungal agents include polyene antifungal agents (e.g., natamycin, limosidine, philippines, nystatin, amphotericin B, candicin), imidazole antifungal agents (e.g., miconazole, ketononazole, clotrie Mazol, itraconazole, isabuconazole, rabuconazole, posaconazole, voriconazole, and teconazole), thiazole antifungal agents (e.g., abapungin), aliamines (e.g., tebinafine, amo Rollpin, naphthypine and butenapine), echinocandin (e.g., anidulafungin, caspopungin and micapungin) and other benzoic acids, cyclopyrox, tolnaftate, undecylenic acid, flucosicocin, and Other antifungal agents, such as seofulbin and haloprogin.
항원충제는 엘로니틴, 푸라졸리돈, 멜라소프롤, 메트로니다졸, 오르니다졸, 파로모마이신 설페이트, 펜타미딘, 피리메타민, 및 티니다졸을 포함하지만 이에 국한되지 않는다.Antiprotozoal agents include, but are not limited to, ellonitine, furazolidone, melassoprole, metronidazole, ornidazole, paromomycin sulfate, pentamidine, pyrimetamine, and tinidazole.
특정 양태에서, HPP 또는 HPC의 이송 단위는 하나 이상의 생체 장벽들을 통하여 HPP 또는 HPC의 이송 또는 횡단을 촉진 또는 개선할 수 있는 양성자화 아민기를 포함한다. 특정 양태에서, 양성자화 아민기는 HPP 또는 HPC가 투과 통과하는 생체 장벽 pH에서 실질적으로 양성자화 될 수 있다. 특정 양태에서, 아민기는 가역적으로 양성자화 또는 탈양성자화 될 수 있다.In certain embodiments, the transport unit of HPP or HPC comprises a protonated amine group capable of promoting or improving transport or crossing of HPP or HPC through one or more bio-barriers. In certain embodiments, protonated amine groups can be substantially protonated at the bio-barrier pH through which HPP or HPC passes. In certain embodiments, the amine group can be reversibly protonated or deprotonated.
특정 양태에서, 링커는 HPP 이송 단위에 관능성 단위를 공유적으로 연결시키고 HPP가 하나 이상의 생체 장벽을 통과하여 투과한 후에 절단될 수 있는 결합을 포함한다. 절단될 수 있는 결합으로 예를 들면, 공유 결합, 에테르, 티오에테르, 아미드, 에스테르, 티오에스테르, 카보네이트, 카바메이트, 포스페이트 또는 옥심 결합을 포함한다In certain embodiments, the linker comprises a bond that can be cleaved after the functional unit is covalently linked to the HPP transport unit and the HPP has penetrated through one or more biological barriers. Cleavable bonds include, for example, covalent bonds, ether, thioether, amide, ester, thioester, carbonate, carbamate, phosphate or oxime bonds.
특정 양태에서, 항균제 또는 항균제-관련 화합물의 HPP 또는 HPC는 생리적 pH에서 양성자화 형태로 존재하는 하나 이상의 1급, 2급 또는 3급 아민기를 가진다. 특정 양태에서, HPP 또는 HPC는 생리적 pH에서 양성자화 형태로 존재하는 하나의 1급, 2급 또는 3급 아민기를 가진다.In certain embodiments, the HPP or HPC of the antimicrobial agent or antimicrobial agent-related compound has one or more primary, secondary or tertiary amine groups present in protonated form at physiological pH. In certain embodiments, HPP or HPC has one primary, secondary or tertiary amine group present in protonated form at physiological pH.
본 발명의 다른 측면은 항균제 또는 항균제-관련 화합물의 최소한 하나의 HPP 또는HPC 및 약학적으로 허용되는 담체를 포함하는 약학적 조성물에 관한 것이다.Another aspect of the invention relates to a pharmaceutical composition comprising at least one HPP or HPC of an antimicrobial agent or an antimicrobial agent-related compound and a pharmaceutically acceptable carrier.
본 발명의 다른 측면은 항균제 또는 항균제 -관련 화합물의 HPP 또는HPC를 이용한 생체 장벽 투과 방법에 관한 것이다.Another aspect of the present invention relates to a method for penetrating a bio-barrier using HPP or HPC of an antibacterial agent or an antibacterial agent-related compound.
본 발명의 다른 측면은 항균제 또는 항균제 -관련 화합물의 HPP 또는HPC를 이용한 생물학적 대상체의 병태의 개시, 진행 또는 완화를 진단하는 방법에 관한 것이다. 특정 양태에서, HPC (또는 HPP) 또는 이의 관능성 단위는 내재적으로 검출가능하다. 특정 양태에서, HPC 또는 HPC의 관능성 단위는 내재적으로 검출가능하거나, 검출 가능한 마커로 표지되거나, 검출가능한 마커와 결합된다.Another aspect of the invention relates to a method for diagnosing the onset, progression or alleviation of a condition in a biological subject using HPP or HPC of an antimicrobial agent or antimicrobial agent-related compound. In certain embodiments, HPC (or HPP) or functional units thereof are implicitly detectable. In certain embodiments, HPC or functional units of HPC are implicitly detectable, labeled with a detectable marker, or combined with a detectable marker.
본 발명의 다른 측면은 목적하는 특성을 가진 관능성 단위, 링커 또는 이송 단위를 선별하는 방법에 관한 것이다.Another aspect of the invention relates to a method for selecting functional units, linkers or transport units having desired properties.
본 발명의 다른 측면은 본 발명에 따른 조성물을 대상체에 투여함으로써 생물학적 대상체의 병태를 예방, 개선, 또는 치료하는 방법에 관한 것이다. 특정 양태에서, 본 방법은 치료적 유효량의 항균제 또는 항균제-관련 화합물 HPP, 또는 이의 약학적 조성물을 대상체에 투여함으로써 항균제 또는 항균제 -관련 화합물로 치료 가능한 대상체의 병태를 치료하는 것에 관한 것이다. 특정 양태에서, 본 방법에 의해 치료 가능한 병태는, 제한적이지 않지만, 통증, 손상, 및 미생물 관련 병태들을 포함한다. 미생물 관련 병태들은 세균, 진균, 원충 및 바이러스와 같은 미생물들에 의해 유발하는 병태들이다. 예를 들면, 세균에 의한 유발 병태들 (세균-관련 병태들), 원충에 의한 유발 병태들 (원충-관련 병태들), 진균에 의한 유발 병태들 (진균-관련 병태들), 및 바이러스에 의한 유발 병태들 (바이러스-관련 병태들). 세균-관련 병태들은, 예를 들면 감염 (간, 폐, 위장, 뇌, 신장, 심장, 귀, 눈, 코, 입, 혀, 결장, 췌장, 담낭, 십이지장, 직장위장, 결장직장, 장, 정맥, 호흡계통, 혈관, 항문직장 및 항문소양증, 호흡계 감염, 상부 호흡기 감염, 요로 감염, 원내감염, 슈도모나스 감염, 코아귤라제양성포도구균 감염 (예를 들면, 피부 감염, 중독증, 급성 감염성 심내막염, 패혈증, 괴사성 폐렴), 인공기관 이식 감염, 패혈증 및 폐렴 동반 기회감염), 흑사병 (예를 들면, 가래톳흑사병 및 폐렴흑사병), 탄저병 (예를 들면, 피부 탄저병, 폐 탄저병 및 위창자성 탄저병), 라임질환, 브루셀라증, 백일해, 급성장염, 호흡기 감염, 앵무새병, 비임균요도염, 트라코마, 신생아 봉입체결막염, 성병림프육아종, 거짓막대장염, 가스괴저, 식중독, 무산소균연조직염, 디프테리아, 설사, 유아 뇌수막염, 출혈성대장염, 용혈요독증후군, 야토병, 폐렴, 기관지염, 소화성궤양, 리지오넬라병, 폰티악열, 렙토스피라증, 리스테리아증, 나병, 결핵, 폐렴미코플라스마, 임질, 신생아안염, 화농성관절염, 수막알균병, 워터하우스-프리데릭센증후군, 록키산 홍반열, 장티푸스형 살모넬라증, 위장염 및 소장대장염 동반 살모넬라증, 세균이질, 시겔라증, 방광염, 수막염, 패혈증, 자궁내막염, 중이염, 굴염, 매독, 괴사근막염, 사슬알균성 인두염, 성홍열, 류마티스열, 농가진, 얕은연조직염, 산후열, 및 콜레라를 포함한다. 원충 관련 병태들은 예를 들면 말라리아, 수면병, 및 톡소포자충증을 포함한다. 진균 관련 병태들은 예를 들면 아스페르길루스증, 분아균증, 백선, 칸디다증, 콕시디오이데스진균증, 크립토코쿠스증, 히스토플라스마증, 파라콕시디오이데스, 스포로트릭스증, 및 털곰팡이증을 포함한다. 바이러스 관련 병태들은 예를 들면 인플루엔자, 황열병 및 AIDS를 포함한다.Another aspect of the invention relates to a method of preventing, improving, or treating a condition in a biological subject by administering a composition according to the invention to the subject. In certain embodiments, the method relates to treating a condition in a subject treatable with an antimicrobial agent or antimicrobial agent-related compound by administering to the subject a therapeutically effective amount of an antimicrobial agent or antimicrobial agent-related compound HPP, or a pharmaceutical composition thereof. In certain embodiments, conditions treatable by the present methods include, but are not limited to, pain, injury, and microbial related conditions. Microbial conditions are conditions caused by microorganisms such as bacteria, fungi, protozoa, and viruses. For example, conditions caused by bacteria (bacteria-related conditions), conditions caused by protozoa (protozoa-related conditions), conditions caused by fungi (fungal-related conditions), and caused by viruses Induced conditions (virus-related conditions). Bacterial-related conditions include, for example, infection (liver, lung, stomach, brain, kidney, heart, ear, eye, nose, mouth, tongue, colon, pancreas, gallbladder, duodenum, rectal stomach, colorectal, intestinal, venous , Respiratory system, blood vessels, rectal and anal pruritus, respiratory system infections, upper respiratory tract infections, urinary tract infections, in-hospital infections, pseudomonas infections, coagulase-positive aureus infections (e.g., skin infections, addictions, acute infective endocarditis, sepsis) , Necrotizing pneumonia), artificial organ transplant infection, sepsis and pneumonia, opportunistic infections), black plague (e.g. sputum black plague and pneumonia black plague), anthrax (e.g. skin anthrax, lung anthrax and gastrointestinal anthrax), lime Diseases, brucellosis, whooping cough, acute appendicitis, respiratory infections, parrot disease, non-gonococcal urethritis, trachoma, neonatal inclusion conjunctivitis, venereal lymphoma, granulomatous colitis, gastric necrosis, food poisoning, anaerobic vasculitis, diphtheria, diarrhea, infant brain Salt, hemorrhagic colitis, hemolytic uremic syndrome, rabies, pneumonia, bronchitis, peptic ulcer, ligonella disease, Pontiac fever, leptospirosis, listeriosis, leprosy, tuberculosis, pneumonia mycoplasma, gonorrhea, neonatal ophthalmitis, purulent arthritis, meningococcal disease , Waterhouse-Fredericsen Syndrome, Rocky Mountain Red Fever, Typhoid Salmonellosis, Gastroenteritis and Small Bowel Colitis Salmonellosis, Bacterial dysentery, Shigellosis, Cystitis, Meningitis, Sepsis, Endometritis, Otitis Media, Otitisitis, Syphilis, Necrotitis, Chain Include bacterial pharyngitis, scarlet fever, rheumatic fever, impetigo, shallow soft tissueitis, postpartum fever, and cholera. Protozoa related conditions include, for example, malaria, sleep disease, and toxoplasmosis. Fungal-related conditions include, for example, aspergillosis, feces, ringworm, candidiasis, coccidioidis mycosis, cryptococcosis, histoplasmosis, paracoccidioides, sporotrix, and hair fungus. Include symptoms. Virus-related conditions include, for example, influenza, yellow fever, and AIDS.
특정 양태에서, HPP 또는 HPC의 약학적 조성물은 다양한 경로, 예를 들면 제한적이지 않지만 경구, 장관, 구강, 비강, 국소, 직장, 질, 에어로졸, 경점막, 표피, 경피, 피부, 눈, 폐, 피하 및/또는 비경구 경로를 통해 생물학적 대상체에 투여된다. 특정한 바람직한 양태에서, HPC또는 HPC의 약학적 조성물은 경구, 경피, 국소, 피하 및/또는 비경구적으로 투여된다.In certain embodiments, the pharmaceutical composition of HPP or HPC may be various routes, including but not limited to oral, intestinal, oral, nasal, topical, rectal, vaginal, aerosol, transmucosal, epidermal, transdermal, skin, eye, lung, It is administered to a biological subject via subcutaneous and / or parenteral routes. In certain preferred embodiments, the HPC or pharmaceutical composition of HPC is administered orally, transdermally, topically, subcutaneously and / or parenterally.
본 발명의 이점에 의하면, 임의 특정 기작으로 제한시키고자 하는 의도 없이, HPP 또는 HPC의 치료적 유효량은 고농도로 병태 부위에 국소적으로 투여되어 전신투여보다 전체적으로 낮은 함량을 투여할 수 있다. 또한 본 발명의 이점은, 예를 들면 전신 투여의 회피 및 부작용 감소 (예를 들면, 주사 통증, 위장/신장 영향 및 다른 부작용들), 및 HPP, HPC 또는 활성 제제의 국소적 고농도로 인한 잠재적 새로운 치료를 포함한다. 본 발명의 추가적인 이점은, 예를 들면 HPP 또는HPC를 생물학적 대상체에 전신 투여하여 더욱 빠르고 더욱 효율적인 생체이용율, 유의하게 통과할 수 없었던 생체 장벽 (예를 들면, 혈관 뇌 장벽 및 혈유 장벽)의 투과를 얻는 것, 및 생체 장벽 통과 결과로 인한 새로운 처방을 포함한다.According to the advantages of the present invention, a therapeutically effective amount of HPP or HPC can be administered topically to the site of the disease at a high concentration, without intention to limit it to any particular mechanism, thereby allowing a lower overall content than systemic administration. The advantages of the present invention also include potential novelty due to, for example, avoidance of systemic administration and reduction of side effects (eg, injection pain, gastrointestinal / kidney effects and other side effects), and local high concentrations of HPP, HPC or active agents. Treatment. A further advantage of the present invention is faster and more efficient bioavailability, for example, systemic administration of HPP or HPC to biological subjects to allow for penetration of bio-barriers (e.g., vascular brain barriers and blood oil barriers) that were not able to pass significantly. And obtaining new prescriptions as a result of crossing the biological barrier.
도 1a1: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, 6-페녹시아세트아세트아미도페니실란산 2-디에틸아미노에틸 에스테르 염산염 (A), 알릴메르캅토메틸페니실린산 2-디메틸아미노에틸 에스테르 염산염 (B), 6-(2,6-디메톡시벤즈아미도)페니실린산 2-디프로필아미노에틸 에스테르 염산염 (C), 6-(5-메틸-3-페닐-2-이속사졸린-4-카복사미도)페니실린산 4-피페리딘에틸 에스테르 염산염 (D), 6-[3-(o-클로로페닐)-5-메틸-4-이속사졸카복사미도]페니실린산 3-피페리딘 에틸 에스테르 염산염 (E), 6-[3-(2,6-디클로로페닐)-5-메틸-4-이속사졸카복사미도]페니실린산 1-피페리딘에틸 에스테르 염산염 (F), 페니실린 V (G), 페니실린 O (H), 메티실린 (I), 옥사실린 (J), 클록사실린 (K), 및 디클록사실린 (L) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다.
도 1a2: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, 6-[D(-)-α-아미노페닐아세트아미도페니실린산 에틸 에스테르 염산염 (A), D-α-[(이미다졸리딘-2-온-1-일)카보닐아미노]벤질페니실린 2-피롤리딘메틸 에스테르 염산염 (B), 6R-[2-[3-(메틸설포닐)-2-옥소-1-이미다졸리딘카복사미도]-2-페닐아세트아미도]페니실린산 1-피롤리딘에틸 에스테르 염산염 (C), 6-D(-)-α-(4-에틸-2,3-디옥소-1-피페라지닐카보닐아미노)-α-페닐아세트아미도페니실린산 2-디에틸아미노에틸 에스테르 염산염 (D), 7-(2-티에닐아세트아미도)세팔로스포란산 2-디에틸아미노에틸 에스테르 염산염 (E), 암피실린 (F), 아즐로실린 (G), 메즐로실린 (H), 피페라실리온 (I), 및 세파로틴 (J) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다.
도 1a3: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, 7-[(히드록시페닐아세틸)아미노]-3-[[(1-메틸-1H-테트라졸-5-일)티오]메틸]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (A), 3-[[(아미노카보닐)옥시]메틸]-7-[[2-푸라닐(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1- 아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (B), 3-[[(아미노카보닐)옥시]메틸]-7-메톡시-8-옥소-7-[(2-티에닐아세틸)아미노]-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (C), 7-[[[2-(아세틸아미노메틸)페닐]아세틸]아미노]-3-[[[1-(에톡실카보닐메틸)-1H-테트라졸-5-일]티오]메틸]-8-옥소-5-티아-1- 아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (D), 7-[(아세틸아미노페닐아세틸)아미노]-3-클로로-8-옥소-5-티아-1- 아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (E), 세파만돌 (F), 세푸록심 (G), 세폭시틴 (H), 세포라니드 (I), 및 세파클로르 (J) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다..
도 1a4: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, 3-[(아세틸옥시)메틸]-7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (A), 7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (B), 7-[[[[(4-에틸-2,3-디옥소-1-피페라지닐)카보닐]아미노](4-아세톡시페닐)아세틸]아미노]-3-[[(1-메틸-1H-테트라졸-5-일)티오]메틸]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (C), 7-[2-(2-아세틸아미노-4-티아졸릴)-2-((Z)-메톡시이미노)아세트아미도]-3-(메톡시메틸)-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (D), 7-[2-(2-아세틸아미노-4-티아졸릴)-2-((Z)-에톡시카보닐메톡시)이미노]아세트아미도]-3-(비닐)-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (E), 세포탁심 (F), 세프티족심 (G), 세포페라존 (H), 세프포독심 프록세틸 (I), 및 세픽심 (J) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다.
도 1b: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, [2S-(2알파,3베타,5알파)]-3-메틸-7-옥소-3-(1H-1,2,3-트리아졸-1-일메틸)-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨염 (타조박탐, F), [2S-(2알파,3베타,5알파)]-3-메틸-7-옥소-3-(1H-1,2,3-트리아졸-1-일메틸)-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 1-피페리딘에틸 에스테르.HCl 염 (타조박탐-PEE, A), (2S,5R)-3,3-디메틸-7-옥소-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨 (설박탐, G), (2S,5R)-3,3-디메틸-7-옥소-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 N,N-디에틸아미노에틸 에스테르.HCl 염 (설박탐-DEE, B), (2R,5R,Z)-3-(2-히드록시에틸리덴)-7-옥소-4-옥사-1-아자-바이시클로[3.2.0]헵탄-2-카복시산 (클라불란산, H), (2R,5R,Z)-3-(2-히드록시에틸리덴)-7-옥소-4-옥사-1-아자-바이시클로[3.2.0]헵탄-2-카복시산 4-피페리딘에틸 에스테르.HCl 염 (클라불란산-PEE, C), [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(4-니트로페닐) 에스테르 나트륨염 (I) , [(N-벤질옥시카보닐아미노)메틸]-포스폰산 (4-니트로페닐)(N,N-디에틸아미노메틸) 에스테르.HCl 염 (D), [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(3-피리디닐) 에스테르 나트륨염 (J), 및 [(N-벤질옥시카보닐아미노)메틸]-포스폰산 (3-피리디닐)(1-피레리디닐) 에스테르.HCl 염 (E) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다.
도 1c: 프란츠 셀에서 단리 인간 피부 조직(n=5)을 통과하는, 4-아미노벤젠설폰아미드 (설파닐아미드, E), 4-(4-디메틸아미노부티릴)아미도벤젠설폰아미드.HCl 염 (DMAB-설파닐아미드, A), 6-옥소-3-(2-[4-(N-피리딘-2-일설파모일)페닐]히드라조노)시클로헥사-1,4-디엔카복시산, 6-옥소-3-(2-[4-(N-피리딘-2-일설파모일)페닐]히드라조노)시클로헥사-1,4-디엔카복시산 (설파살라진, F), 6-옥소-3-(2-[4-(N-피리딘-2-일설파모일)페닐]히드라조노)시클로헥사-1,4-디엔카복시산 N,N-디에틸아미노프로필 에스테르.HCl 염 (설파살라진-DEPE, B), 1-시클로프로필- 6-플루오로- 4-옥소- 7-피페라진- 1-일- 퀴놀린- 3-카복시산 (시프로플록사신, G), 1-시클로프로필- 6-플루오로- 4-옥소- 7-피페라진- 1-일- 퀴놀린- 3-카복시산 butyl 에스테르.HCl 염 (시프로플록사신-BE, C), 1-에틸-7-메틸-4-옥소-[1,8]나프티리딘-3-카복시산 (날리딕스산, H), 1-에틸-7-메틸-4-옥소-[1,8]나프티리딘-3-카복시산 N,N-디에틸아미노에틸 에스테르.HCl 염 (날리딕스산-DEE, D) 의 축적량. 각 경우에서, 비히클은 pH 7.4 인산 완충액(0.2 M) 이었다.1A1: 6-Phenoxyacetacetamidophenylanic acid 2-diethylaminoethyl ester hydrochloride (A), allylmercaptomethylpenicillin 2-, passing through isolated human skin tissue (n = 5) in Franz cells. Dimethylaminoethyl ester hydrochloride (B), 6- (2,6-dimethoxybenzamido) penicillic acid 2-dipropylaminoethyl ester hydrochloride (C), 6- (5-methyl-3-phenyl-2-isomer Sazoline-4-carboxamido) penicillic acid 4-piperidineethyl ester hydrochloride (D), 6- [3- (o-chlorophenyl) -5-methyl-4-isoxazolecarboxamido] penicillic acid 3 -Piperidine ethyl ester hydrochloride (E), 6- [3- (2,6-dichlorophenyl) -5-methyl-4-isoxazolcarboxamido] penicillic acid 1-piperidineethyl ester hydrochloride (F) , Accumulation amount of penicillin V (G), penicillin O (H), methicillin (I), oxacillin (J), coxacillin (K), and dicloxacillin (L). In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
1A2: 6- [D (-)-α-aminophenylacetamidophenicillic acid ethyl ester hydrochloride (A), D-α-[(already), passing through isolated human skin tissue (n = 5) in Franz cells Dazolidin-2-one-1-yl) carbonylamino] benzylpenicillin 2-pyrrolidinemethyl ester hydrochloride (B), 6R- [2- [3- (methylsulfonyl) -2-oxo-1- Imidazolidinecarboxamido] -2-phenylacetamido] penicillic acid 1-pyrrolidineethyl ester hydrochloride (C), 6-D (-)-α- (4-ethyl-2,3-dioxo- 1-piperazinylcarbonylamino) -α-phenylacetamidopenicillin 2-diethylaminoethyl ester hydrochloride (D), 7- (2-thienylacetamido) cephalosporic acid 2-diethyl Accumulation of aminoethyl ester hydrochloride (E), ampicillin (F), azlocillin (G), mezzocillin (H), piperacillion (I), and cepharotin (J). In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
1A3: 7-[(hydroxyphenylacetyl) amino] -3-[[(1-methyl-1H-tetrazol-5-yl) thio, passing through isolated human skin tissue (n = 5) in Franz cells ] Methyl] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (A), 3-[[(amino Carbonyl) oxy] methyl] -7-[[2-furanyl (methoxyimino) acetyl] amino] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene- 2-Carboxylic acid 2-diethylaminoethyl ester hydrochloride (B), 3-[[(aminocarbonyl) oxy] methyl] -7-methoxy-8-oxo-7-[(2-thienylacetyl) amino ] -5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (C), 7-[[[2- (acetylaminomethyl) Phenyl] acetyl] amino] -3-[[[1- (ethoxycarbonylcarbonyl) -1H-tetrazol-5-yl] thio] methyl] -8-oxo-5-thia-1-azabicyclo [ 4.2.0] Oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester Acidic acid (D), 7-[(acetylaminophenylacetyl) amino] -3-chloro-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2 -The accumulation amount of diethylaminoethyl ester hydrochloride (E), sefamandol (F), cefuroxime (G), cepoxytin (H), Sephoranid (I), and cefachlor (J). In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
1A4: 3-[(acetyloxy) methyl] -7-[[(2-acetylamino-4-thiazolyl) (methoxyimino) acetyl, passing through isolated human skin tissue (n = 5) in Franz cells ] Amino] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (A), 7-[[(2 -Acetylamino-4-thiazolyl) (methoxyimino) acetyl] amino] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-di Ethylaminoethyl ester hydrochloride (B), 7-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl) carbonyl] amino] (4-acetoxyphenyl) acetyl] amino]- 3-[[(1-methyl-1H-tetrazol-5-yl) thio] methyl] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxy Acid 2-diethylaminoethyl ester hydrochloride (C), 7- [2- (2-acetylamino-4-thiazolyl) -2-((Z) -methoxyimino) acetamido] -3- (meth Thoxymethyl) -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2- 2-Diethylaminoethyl ester hydrochloride (D), 7- [2- (2-acetylamino-4-thiazolyl) -2-((Z) -ethoxycarbonylmethoxy) imino] acetamido ] -3- (vinyl) -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (E), cell taksim (F), accumulation amount of ceftoxime (G), cefoperazone (H), cefpodoxime proxetyl (I), and sepicsim (J). In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
1B: [2S- (2alpha, 3beta, 5alpha)]-3-methyl-7-oxo-3- (1H-1,2, passing through isolated human skin tissue (n = 5) in Franz cells , 3-triazol-1-ylmethyl) -4-thia-1-azabicyclo [3.2.0] heptan-2-carboxylic acid 4,4-dioxide sodium salt (tazobactam, F), [2S- ( 2 alpha, 3 beta, 5 alpha)]-3-methyl-7-oxo-3- (1H-1,2,3-triazol-1-ylmethyl) -4-thia-1-azabicyclo [3.2 .0] heptane-2-carboxylic acid 4,4-dioxide 1-piperidineethyl ester.HCl salt (tazobactam-PEE, A), (2 S , 5 R ) -3,3-dimethyl-7-oxo -4-thia-1-azabicyclo [3.2.0] heptan-2-carboxylic acid 4,4-dioxide sodium (sulbactam, G), (2 S , 5 R ) -3,3-dimethyl-7- Oxo-4-thia-1-azabicyclo [3.2.0] heptan-2-carboxylic acid 4,4-dioxide N, N-diethylaminoethyl ester.HCl salt (sulbactam-DEE, B), (2 R , 5 R , Z ) -3- (2-hydroxyethylidene) -7-oxo-4-oxa-1-aza-bicyclo [3.2.0] heptan-2-carboxylic acid (clavulanic acid, H), (2 R , 5 R , Z ) -3- (2- Hydroxyethylidene) -7-oxo-4-oxa-1-aza-bicyclo [3.2.0] heptan-2-carboxylic acid 4-piperidineethyl ester.HCl salt (Clavulanic acid-PEE, C ), [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- (4-nitrophenyl) ester sodium salt (I), [(N-benzyloxycarbonylamino) methyl] -phosphonic acid (4- Nitrophenyl) (N, N-diethylaminomethyl) ester. HCl salt (D), [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- (3-pyridinyl) ester sodium salt (J) , And [(N-benzyloxycarbonylamino) methyl] -phosphonic acid (3-pyridinyl) (1-pyrrididinyl) ester. Accumulated amount of HCl salt (E). In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
Figure 1c: 4-aminobenzenesulfonamide (sulfanylamide, E), 4- (4-dimethylaminobutyryl) amidobenzenesulfonamide, passing through isolated human skin tissue (n = 5) in Franz cells. Salt (DMAB-sulfanylamide, A), 6-oxo-3- (2- [4- ( N -pyridin-2-ylsulfamoyl) phenyl] hydrazono) cyclohexa-1,4-dienecarboxylic acid, 6-oxo-3- (2- [4- ( N -pyridin-2-ylsulfamoyl) phenyl] hydrazono) cyclohexa-1,4-dienecarboxylic acid (sulfasalazine, F), 6-oxo-3- (2- [4- ( N -pyridin-2-ylsulphamoyl) phenyl] hydrazono) cyclohexa-1,4-dienecarboxylic acid N, N-diethylaminopropyl ester.HCl salt (sulfasalazine-DEPE, B ), 1-cyclopropyl-6-fluoro- 4-oxo-7-piperazine- 1-yl-quinoline- 3-carboxylic acid (ciprofloxacin, G), 1-cyclopropyl-6-fluoro- 4-oxo -7-piperazine- 1-yl-quinoline- 3-carboxylic acid butyl ester.HCl salt (ciprofloxacin-BE, C), 1-ethyl-7-methyl-4-oxo- [1,8] naphthy Dean-3-carboxylic acid (nalidixic acid, H), 1-ethyl-7-methyl-4-oxo- [1,8] naphthyridine-3-carboxylic acid N, N-diethylaminoethyl ester.HCl salt (Nalidic acid-DEE, D) accumulation. In each case, the vehicle was pH 7.4 phosphate buffer (0.2 M).
I. 고투과 전구약물 (HPP) 또는 고투과 조성물 (HPC) 화학식I. High permeation prodrug (HPP) or high permeation composition (HPC) formula
본 발명의 일 측면은 고투과 전구약물 (HPP) 또는 고투과 조성물 (HPC)에 관한 것이다. 본원에서 용어 "고투과 전구약물" 또는 "HPP" 또는 "고투과 조성물" 또는 "HPC"은 링커를 통하여 이송 단위에 공유 결합되는 관능성 단위를 언급한다.One aspect of the invention relates to a high permeation prodrug (HPP) or a high permeation composition (HPC). The term "high permeation prodrug" or "HPP" or "high permeation composition" or "HPC" herein refers to a functional unit that is covalently linked to a transport unit through a linker.
모 약물 잔기를 포함하는 HPP 또는 HPC의 관능성 단위는 다음 특성들을 가진다: 1) 모 약물, 또는 HPP/HPC는 생물학적 대상체에 전달되고/전달되거나, 모 약물의 생체 장벽을 가로지르는 이송이 바람직함, 2) HPP/HPC는 생체 장벽을 투과하거나 가로지를 수 있음, 3) HPP/HPC는 절단되어 모 약물의 잔기가 모 약물 또는 모 약물 대사산물로 전환될 수 있음.The functional unit of the HPP or HPC comprising the parent drug moiety has the following properties: 1) The parent drug, or HPP / HPC, is preferably delivered to a biological subject and / or transported across the biological barrier of the parent drug , 2) HPP / HPC can penetrate or cross the bio-barrier, 3) HPP / HPC can be cleaved and the residue of the parent drug can be converted to the parent drug or parent drug metabolite.
특정 양태에서, 관능성 단위는, 친수성, 친지성 또는 양친성(친수성 및 친지성)일 수 있다. 관능성 단위의 친지성 잔기는 내재되어 있거나, 관능성 단위의 하나 이상의 친수성 잔기를 친지성 잔기로 전환시킴으로써 수득될 수 있다. 예를 들면, 관능성 단위의 친지성 잔기는 관능성 단위의 하나 이상의 친수성 기를 유기 합성법을 통해 친지성 기로 전환시킴으로써 생성될 수 있다. 친수성 기의 예로는 카복실, 히드록실, 티올, 아미노, 포스페이트/포스포네이트, 구아니딘 및 카보닐을 포함하나, 이에 제한되는 것은 아니다. 이들 친수성 기의 변형에 의해 생성된 친지성 잔기들은, 제한적이지 않지만, 에테르, 티오에테르, 에스테르, 티오에스테르, 카보네이트, 카바메이트, 아미드, 포스페이트 및 옥심을 포함한다. 특정 양태에서, 관능성 단위는 아세틸화 또는 아실화 (알카노일화)에 의해 친지화된다. 특정 양태에서, 관능성 단위는 에스테르화에 의해 친지화된다.In certain embodiments, the functional units can be hydrophilic, lipophilic or amphiphilic (hydrophilic and lipophilic). The lipophilic residue of the functional unit is either inherent or can be obtained by converting one or more hydrophilic residues of the functional unit to lipophilic residues. For example, lipophilic moieties of functional units can be generated by converting one or more hydrophilic groups of the functional units to lipophilic groups through organic synthesis. Examples of hydrophilic groups include, but are not limited to, carboxyl, hydroxyl, thiol, amino, phosphate / phosphonate, guanidine and carbonyl. Lipophilic residues produced by modification of these hydrophilic groups include, but are not limited to, ether, thioether, ester, thioester, carbonate, carbamate, amide, phosphate and oxime. In certain embodiments, functional units are lipophilized by acetylation or acylation (alkanoylation). In certain embodiments, functional units are lipophilized by esterification.
특정 양태에서, HPP 또는 HPC의 모 약물은 항균제 및 항균제-관련 화합물로 이루어진 군에서 선택된다. 항균제 또는 항균제-관련 화합물의 잔기는 상기된 바와 같이 친지성 잔기로 더욱 전환될 수 있다.In certain embodiments, the parent drug of HPP or HPC is selected from the group consisting of antimicrobial agents and antimicrobial agent-related compounds. The residues of the antimicrobial agent or antimicrobial agent-related compound can be further converted to lipophilic residues as described above.
항균제들은 세균, 진균, 또는 원충과 같은 미생물을 사멸시키거나 성장을 억제시킬뿐 아니라 바이러스를 파괴하거나 성장을 억제할 수 있는 물질이다. 항균제의 주요 종류들은 예를 들면 세균-관련 병태들을 치료하는 항생제, 바이러스-관련 병태들을 치료하는 항바이러스제, 진균-관련 병태들을 치료하는 항진균제 및 원충-관련 병태들을 치료하는 항원충제를 포함한다.Antibacterial agents are substances capable of destroying or inhibiting viruses as well as killing or inhibiting growth of microorganisms such as bacteria, fungi, or protozoa. The main types of antimicrobial agents include, for example, antibiotics for treating bacterial-related conditions, antiviral agents for treating virus-related conditions, antifungal agents for treating fungal-related conditions, and antiprotozoal agents for treating protozoa-related conditions.
항균제-관련 화합물은 항균제 구조, 항균제 대사산물, 또는 HPP 또는 HPC가 하나 이상의 생체 장벽들을 투과한 후 항균제 또는 항균제 대사산물로 대사될 수 있는 제제를 포함하는 화합물이다. 항균제-관련 화합물은 항균제 또는 항균제 대사산물의 유사체 또는 모방체인 화합물, 또는 HPP 또는 HPC가 하나 이상의 생체 장벽들을 투과한 후 항균제 또는 항균제 대사산물의 유사체 또는 모방체로 대사될 수 있는 제제를 더욱 포함한다.An antimicrobial agent-related compound is a compound comprising an antimicrobial agent structure, an antimicrobial agent metabolite, or an agent that can be metabolized to an antimicrobial agent or an antimicrobial agent metabolite after HPP or HPC penetrates one or more biological barriers. The antimicrobial agent-related compound further includes a compound that is an analog or mimetic of the antimicrobial agent or antimicrobial agent metabolite, or an agent that can be metabolized to an analog or mimetic agent of the antimicrobial agent or antimicrobial agent metabolite after HPP or HPC penetrates one or more bio-barriers.
항균제의 예시로는 예를 들면 세균-관련 병태들을 치료하는 항생제, 바이러스-관련 병태들을 치료하는 항바이러스제, 진균-관련 병태들을 치료하는 항진균제 및 원충-관련 병태들을 치료하는 항원충제를 포함한다.Examples of antibacterial agents include, for example, antibiotics to treat bacterial-related conditions, antiviral agents to treat virus-related conditions, antifungal agents to treat fungal-related conditions, and protozoa to treat protozoa-related conditions.
항생제의 예로는 베타-락탐계 항생제, 설폰아미드 및 퀴놀론을 포함하지만 이에 국한되지 않는다. 베타-락탐 항생제는 본 분야에서 널리 알려져 있고 여러 병태들와 관련하여 사용된다. 본원에서 사용되는 바와 같이, 베타-락탐 항생제는 베타-락탐 핵을 가지는 화합물을 언급한다.Examples of antibiotics include, but are not limited to, beta-lactam antibiotics, sulfonamides, and quinolones. Beta-lactam antibiotics are well known in the art and used in connection with a number of conditions. As used herein, beta-lactam antibiotic refers to a compound having a beta-lactam nucleus.
베타-락탐계 항생제의 예로는 페니실린 유도체, 세팔로스포린, 페넴, 모노박탐, 카바페넴, 베타-락타마제 억제제 및 이의 배합물을 포함하나, 이에 제한되는 것은 아니다. 페니실린 유도체의 예로는 아미노페니실린 (예: 아목시실린, 암피실린 및 에피실린); 카복시페니실린 (예: 카베니실린, 티카실린 및 테모실린); 우레이도페니실린 (예: 아즐로실린, 피페라실린 및 메즐로실린); 메실리남, 술베니실린, 벤자틴 페니실린, 페니실린 G (벤질페니실린), 페니실린 V (페녹시메틸페니실린), 페니실린 O (알릴머캅토메틸페니실린), 프로카인 페니실린, 옥사실린, 메티실린, 나프실린, 클록사실린, 디클록사실린, 플루클록사실린, 피밤피실린, 헤타실린, 베캄피실린, 메탐피실린, 탈람피실린, 코-아목시클라브(아목시실린 및 클라불란산), 및 피페라실리온을 포함하나 이에 제한되는 것은 아니다. 세팔로스포린의 예로는 세팔렉신, 세팔로틴, 세파졸린, 세파클러, 세푸록심, 세파만돌, 세포테탄, 세폭시틴, 세포라니드, 세프트리악손, 세포탁심, 세프포독심 프록세틸, 세프타지딤, 세페핌, 세포페라존, 세프티족심, 세픽심 및 세프피롬을 포함하나 이에 제한되는 것은 아니다. 페넴의 예로는 파로페넴을 포함하나 이에 제한되는 것은 아니다. 모노박탐계의 예로는 아즈트레오남 및 티게모남을 포함하나 이에 제한되는 것은 아니다. 카바페넴의 예로는 바이아페넴, 도리페넴, 에르타페넴, 이미페넴, 레로페넴 및 파니페넴을 포함하나 이에 제한되는 것은 아니다. 베타-락타마제의 예로는 타조박탐 ([2S-(2알파, 3베타, 5알파)]-3-메틸-7-옥소-3-(1H-1,2,3-트리아졸-1-일메틸)-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨 염), 설박탐 (2S,5R)-3,3-디메틸-7-옥소-4-티아-1-아자바이시클로[3.2.0]헵탄-2-카복시산 4,4-디옥사이드 나트륨), 및 클라불란산((2R,5R,Z)-3-(2-히드록시에틸리덴)-7-옥소-4-옥사-1-아자-바이시클로[3.2.0]헵탄-2-카복시산)을 포함하나 이에 제한되는 것은 아니다. 항생제의 다른 예로는 [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(4-니트로페닐) 에스테르 나트륨 염, [(N-벤질옥시카보닐아미노)메틸]-포스폰산 모노-(3-피리디닐) 에스테르 나트륨 염, 설파닐아미드 (4-아미노벤젠설폰아미드), 설파살라진 (6-옥소-3-(2-[4-(N-피리딘-2-일설파모일)페닐]히드라조노)시클로헥사-1,4-디엔카복시산), 1-시클로프로필-6-플루오로-4-옥소-7-피페라진-1-일-퀴놀린-3-카복시산, 날리딕스산(1-에틸-7-메틸-4-옥소-[1,8]나프티리딘-3-카복시산)을 포함하나 이에 제한되는 것은 아니다.Examples of beta-lactam antibiotics include, but are not limited to, penicillin derivatives, cephalosporins, peten, monobactam, carbapenem, beta-lactamase inhibitors, and combinations thereof. Examples of penicillin derivatives include aminopenicillins (eg amoxicillin, ampicillin and epicillin); Carboxypenicillins (eg, carbenicillin, ticacillin and temocillin); Ureidophenicillins (eg, azalocillin, piperacillin and mezzocillin); Mesylinam, sulbenicillin, benzatine penicillin, penicillin G (benzylpenicillin), penicillin V (phenoxymethylpenicillin), penicillin O (allylmercaptomethylpenicillin), procaine penicillin, oxacillin, methicillin, naphcillin , Cloxacillin, dicloxacillin, flucloxacillin, pibampicillin, hetacillin, becampicillin, methampicillin, talampicillin, co-amoxiclab (amoxicillin and clavulanic acid), and pipera Silion, but is not limited thereto. Examples of cephalosporins include cephalexin, cephalotin, cefazoline, cefacler, cefuroxime, cepamandol, cethetan, cepoxytin, celaranid, cetriaxone, cetaxam, cepodoxime proxetyl, ceftage Dim, cefepime, cepeferazone, ceftysim, sepicsim and cefipyrom. Examples of petite include, but are not limited to, paropenet. Examples of monobactam systems include, but are not limited to, aztreonam and tigemonam. Examples of carbapenems include, but are not limited to, baiapenem, doripenem, ertapenem, imipenem, leropenem and panipenem. Examples of beta-lactamase are tazobactam ([2S- (2alpha, 3beta, 5alpha)]-3-methyl-7-oxo-3- (1H-1,2,3-triazol-1-yl Methyl) -4-thia-1-azabicyclo [3.2.0] heptan-2-carboxylic acid 4,4-dioxide sodium salt), sulfactam (2S, 5R) -3,3-dimethyl-7-oxo- 4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid 4,4-dioxide sodium), and clavulanic acid ((2R, 5R, Z) -3- (2-hydroxyethyly Den) -7-oxo-4-oxa-1-aza-bicyclo [3.2.0] heptan-2-carboxylic acid). Other examples of antibiotics are [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- (4-nitrophenyl) ester sodium salt, [(N-benzyloxycarbonylamino) methyl] -phosphonic acid mono- ( 3-pyridinyl) ester sodium salt, sulfanilamide (4-aminobenzenesulfonamide), sulfasalazine (6-oxo-3- (2- [4- (N-pyridin-2-ylsulfamoyl) phenyl] hydrazono ) Cyclohexa-1,4-dienecarboxylic acid), 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-quinoline-3-carboxylic acid, nalidixic acid (1-ethyl -7-methyl-4-oxo- [1,8] naphthyridine-3-carboxylic acid).
설폰아미드의 예로는 제한적이지 않지만 설파이소디미딘, 설파닐아미드, 설파디아진, 설피속사졸, 설파메톡사졸, 설파디메톡신, 설파메톡시피리다진, 설파세타미드, 설파독신, 아세타졸라미드, 부메타니드, 클로탈리돈, 클로파미드, 푸로세미드, 히드로클로로티아지드, 인다파미드, 메프루시드, 메토라존, 시파미드, 디클로로페나미드, 도졸라미드, 아세타졸라미드, 에톡스졸라미드, 설티암, 조니사미드, 마페니드, 셀레콕시브, 다루나비르, 프로베네시드, 설파살라진 및 수마트리프탄을 포함한다.Examples of sulfonamides include, but are not limited to, sulfisodimidine, sulfanilamide, sulfadiazine, sulfisoxazole, sulfamethoxazole, sulfadimethoxine, sulfamethoxypyridazine, sulfacetamide, sulfadoxine, acetazolamide , Bomethanid, clotalidone, clopamide, furosemide, hydrochlorothiazide, indapamide, meproside, metorazone, cypamide, dichlorophenamide, dozolamide, acetazolamide, e Toxzolamide, sulthiam, zonisamide, mafenide, celecoxib, darunavir, probenecid, sulfasalazine and sumatriptan.
퀴놀론의 예시로는 제한적이지 않지만 시녹사신, 플루메퀸, 날리딕스산, 옥소린산, 피로미드산, 피페미드산, 로속사신, 시프로플로삭신, 에녹사신, 플레녹사신, 로메플록사신, 나디플록사신, 노르플록사신, 오플록사신, 퍼플록사신, 루플록사신, 발로플록사신, 가티플록사신, 그레파플록사신, 레보플록사신, 목시플록사신, 파주플록사신, 스파르플록사신, 테마플록사신, 토수플록사신, 클리나플록사신, 게미플록사신, 시타플록사신, 트로바플록사신, 프룰리플록사신, 가레녹사신, 에시노플록사신, 델라플록사신 및 날리딕스산을 포함한다.Examples of quinolone are, but are not limited to, synoxacin, flumequin, nalidixic acid, oxolinic acid, pyromidic acid, pipemedic acid, rosoxacin, ciprofloxacin, enoxacin, plenoxacin, romefloxacin, nadi Phloxacin, Norfloxacin, Ofloxacin, Purpleoxacin, Rufloxacin, Vallofloxacin, Gattifloxacin, Grefafloxacin, Levofloxacin, Moxifloxacin, Pajufloxacin, Sparfloxacin, Themefloxacin , Tosufloxacin, clinafloxacin, hemifloxacin, sitafloxacin, trovafloxacin, prulifloxacin, garenoxacin, einofloxacin, delafloxacin, and nalidixic acid.
항바이러스의 예시로는 제한적이지 않지만 리팜피신, 자나미비르 및 오셀타미비르를 포함한다. 항진균제의 예로는 폴리엔 항진균제 (예를 들면, 나타마이신, 리모시딘, 필리핀, 니스타틴, 암포테리신 B, 칸디신), 이미다졸 항진균제 (예를 들면, 미코나졸, 케토노나졸, 클로트리마졸, 이트라코나졸, 이사부코나졸, 라부코나졸, 포사코나졸, 보리코나졸, 및 테코나졸), 티아졸 항진균제 (예를 들면, 아바푼긴), 알리아민 (예를 들면, 테비나핀, 아모롤핀, 나프티핀 및 부테나핀), 에키노칸딘 (예를 들면, 아니둘라푼긴, 카스포푼긴 및 미카푼긴) 및 기타 벤조산, 시클로피록스, 톨나프테이트, 운데실렌산, 플루시코신, 그리세오풀빈, 할로프로긴과 같은 기타 항진균제를 포함한다.Examples of antivirals include, but are not limited to, rifampicin, zanamivir and oseltamivir. Examples of antifungal agents include polyene antifungal agents (e.g., natamycin, limosidine, philippines, nystatin, amphotericin B, candicin), imidazole antifungal agents (e.g., miconazole, ketononazole, clotrie Mazol, itraconazole, isabuconazole, rabuconazole, posaconazole, voriconazole, and teconazole), thiazole antifungal agents (e.g., abapungin), aliamines (e.g., tebinafine, amo Rollpin, naphthypine and butenapine), echinocandin (e.g., anidulafungin, caspopungin and micapungin) and other benzoic acids, cyclopyrox, tolnaftate, undecylenic acid, flucosicocin, and Other antifungal agents, such as seofulbin and haloprogin.
특정 양태에서, 항균제 또는 항균제-관련 화합물의 HPP 관능성 단위는 입체이성질체 및 약학적으로 허용되는 염을 포함한 화학식 F-1구조를 가지는 잔기를 포함한다:In certain embodiments, the HPP functional unit of the antimicrobial agent or antimicrobial agent-related compound comprises a moiety having the structure F-1, including stereoisomers and pharmaceutically acceptable salts:
화학식 F-1Formula F-1
본 명세서에서 달리 특정되지 않으면, Y는 H, OH, NHCHO, NHC(=O)R6, OC(=O)CH3, OC(=O)R6, OCH3, OC2H5, OR6, CH3, C2H5, R6, CH3SO3, R6SO3 , NO2, CN, CF3, C2F5, C3F7, OCF3, OC2F5, OC3F7, F, Br, I, Cl, 및 치환된 및 비치환된 (substituted and unsubstituted) 알킬옥실로 이루어진 군에서 선택되고; Unless otherwise specified herein, Y is H, OH, NHCHO, NHC (= O) R 6 , OC (= O) CH 3 , OC (= O) R 6 , OCH 3 , OC 2 H 5 , OR 6 , CH 3 , C 2 H 5 , R 6 , CH 3 SO 3 , R 6 SO 3 , NO 2 , CN, CF 3 , C 2 F 5 , C 3 F 7 , OCF 3 , OC 2 F 5 , OC 3 F 7 , F, Br, I, Cl, and substituted and unsubstituted alkyloxyl;
는 화학식 NS-1, 화학식 NS-2, 화학식 NS-3, 화학식 NS-4 및 화학식 NS-5로 이루어진 군에서 선택되고: Is selected from the group consisting of Formula NS-1, Formula NS-2, Formula NS-3, Formula NS-4, and Formula NS-5:
화학식 NS-1 화학식 NS-2 화학식 NS-3 화학식 NS-4 화학식 NS-5; Formula NS-1 Formula NS-2 Formula NS-3 Formula NS-4 Formula NS-5;
X1은 H, OH, OCH3, OC2H5, OR6, C(=O)NH2, CH2OC(=O)NH2, CH2OC(=O)CH3 , CH2OC(=O)R6, OC(=O)CH3, OC(=O)R6, CH2OCH3, CH3, C2H5, R6, Cl, F, Br, I, HC=CHCH3, HC=CH2, CH2OCH3, CH2OR6, S(CH2)n-NHR7, 화학식 X1-1, 화학식 X1-2, 화학식 X1-3, 화학식 X1-4, 화학식 X1-5, 화학식 X1-6, 화학식 X1-7, 화학식 X1-8, 화학식 X1-9, 화학식 X1-10, 화학식 X1-11, 화학식 X1-12, 화학식 X1-13, 화학식 X1-14, 화학식 X1-15, 화학식 X1-16, 화학식 X1-17, 화학식 X1-18, 화학식 X1-19, 화학식 X1-20, 화학식 X1-21, 화학식 X1-22, 화학식 X1-23, 화학식 X1-24, 화학식 X1-25, 화학식 X1-26, 화학식 X1-27, 화학식 X1-28, 화학식 X1-29, 화학식 X1-30, 화학식 X1-31, 화학식 X1-32, 화학식 X1-33, 화학식 X1-34, 화학식 X1-35, 화학식 X1-36, 화학식 X1-37, 화학식 X1-38, 화학식 X1-39, 화학식 X1-40, 화학식 X1-41, 화학식 X1-42, 화학식 X1-43, 화학식 X1-44, 화학식 X1-45, 화학식 X1-46, 화학식 X1-47, 화학식 X1-48, 화학식 X1-49, 화학식 X1-50, 화학식 X1-51, 화학식 X1-52, 화학식 X1-53, 화학식 X1-54, 화학식 X1-55, 화학식 X1-56, 화학식 X1-57, 화학식 X1-58, 화학식 X1-59, 화학식 X1-60, 화학식 X1-61, 화학식 X1-62, 화학식 X1-63, 화학식 X1-64, 화학식 X1-65, 화학식 X1-66, 화학식 X1-67, 화학식 X1-68, 화학식 X1-69, 화학식 X1-70, 화학식 X1-71, 화학식 X1-72, 화학식 X1-73, 화학식 X17-4, 화학식 X1-75, 화학식 X1-76, 화학식 X1-77, 화학식 X1-78, 화학식 X1-79, 화학식 X1-80, 화학식 X1-81, 및 화학식 X1-82로 이루어진 군에서 선택되고:X 1 is H, OH, OCH 3 , OC 2 H 5 , OR 6 , C (= O) NH 2 , CH 2 OC (= O) NH 2 , CH 2 OC (= O) CH 3 , CH 2 OC ( = O) R 6, OC (= O) CH 3 , OC (= O) R 6 , CH 2 OCH 3 , CH 3 , C 2 H 5 , R 6, Cl, F, Br, I, HC = CHCH 3 , HC = CH 2 , CH 2 OCH 3 , CH 2 OR 6 , S (CH 2 ) n -NHR 7 , Formula X 1 -1, Formula X 1 -2, Formula X 1 -3, Formula X 1 -4, formula X 1 -5, -6 formula X 1, X formula 1 -7, -8 formula X 1, X formula 1 -9, the formula X 1 -10, 1 -11 the general formula X, formula (X) 1 -12, the formula X 1 -13, Formula X 1 -14, Formula X 1 -15, Formula X 1 -16, Formula X 1 -17, Formula X 1 -18, Formula X 1 -19, Formula X 1 -20, Formula X 1- 21, Formula X 1 -22, Formula X 1 -23, Formula X 1 -24, Formula X 1 -25, Formula X 1 -26, Formula X 1 -27, Formula X 1 -28, Formula X 1 -29, Formula X 1 -30, Formula X 1 -31, Formula X 1 -32, Formula X 1 -33, Formula X 1 -34, Formula X 1 -35, Formula X 1 -36, Formula X 1 -37, Formula X 1 -38, Formula X 1 -39, Formula X 1 -40, Tu Formula X 1 -41, Formula X 1 -42, Formula X 1 -43, Formula X 1 -44, Formula X 1 -45, Formula X 1 -46, Formula X 1 -47, Formula X 1 -48, Formula X 1 -49, Formula X 1 -50, Formula X 1 -51, Formula X 1 -52, Formula X 1 -53, Formula X 1 -54, Formula X 1 -55, Formula X 1 -56, Formula X 1- 57, Formula X 1 -58, Formula X 1 -59, Formula X 1 -60, Formula X 1 -61, Formula X 1 -62, Formula X 1 -63, Formula X 1 -64, Formula X 1 -65, Formula X 1 -66, Formula X 1 -67, Formula X 1 -68, Formula X 1 -69, Formula X 1 -70, Formula X 1 -71, Formula X 1 -72, Formula X 1 -73, Formula X 17 -4, Formula X 1 -75, Formula X 1 -76, Formula X 1 -77, Formula X 1 -78, Formula X 1 -79, Formula X 1 -80, Formula X 1 -81, and Formula X 1 Is selected from the group consisting of -82:
화학식 X1-1 화학식 X1-2 화학식 X1-3Formula X 1 -1 Formula X 1 -2 Formula X 1 -3
화학식 X1-4 화학식 X1-5Formula X Formula X 1 1 -4 -5
화학식 X1-6 화학식 X1-7 화학식 X1-8Formula X Formula X 1 -6 1 1 -7 -8 formula X
화학식 X1-9 화학식 X1-10Formula X 1 -9 Formula X 1 -10
화학식 X1-11 화학식 X1-12Formula X 1 -11 Formula X 1 -12
화학식 X1-13 화학식 X1-14Formula X 1 -13 Formula X 1 -14
화학식 X1-15 화학식 X1-16Formula X Formula X 1 -15 1 -16
화학식 X1-17 화학식 X1-18Formula X 1 -17 Formula X 1 -18
화학식 X1-19 화학식 X1-20Formula X 1 -19 Formula X 1 -20
화학식 X1-21 화학식 X1-22Formula X 1 -21 Formula X 1 -22
화학식 X1-23 화학식 X1-24Formula X 1 -23 Formula X 1 -24
화학식 X1-25 화학식 X1-26Formula X 1 -25 Formula X 1 -26
화학식 X1-27 화학식 X1-28Formula X 1 -27 Formula X 1 -28
화학식 X1-29 화학식 X1-30Formula X 1 -29 Formula X 1 -30
화학식 X1-31 화학식 X1-32Formula X 1 -31 Formula X 1 -32
화학식 X1-33 화학식 X1-34Formula X 1 -33 Formula X 1 -34
화학식 X1-35 화학식 X1-36Formula X 1 -35 Formula X 1 -36
화학식 X1-37 화학식 X1-38Formula X 1 -37 Formula X 1 -38
화학식 X1-39 화학식 X1-40Formula X 1 -39 Formula X 1 -40
화학식 X1-41 화학식 X1-42 화학식 X1-43Formula X 1 -41 Formula X 1 -42 Formula X 1 -43
화학식 X1-44 화학식 X1-45Formula X 1 -44 Formula X 1 -45
화학식 X1-46 화학식 X1-47Formula X 1 -46 Formula X 1 -47
화학식 X1-48 화학식 X1-49Formula X 1 -48 Formula X 1 -49
화학식 X1-50 화학식 X1-51Formula X 1 -50 Formula X 1 -51
화학식 X1-52 화학식 X1-53 화학식 X1-54 Formula X 1 -52 Formula X 1 -53 Formula X 1 -54
화학식 X1-55 화학식 X1-56 화학식 X1-57Formula X 1 -55 Formula X 1 -56 Formula X 1 -57
화학식 X1-58 화학식 X1-59Formula X 1 -58 Formula X 1 -59
화학식 X1-60 화학식 X1-61Formula X 1 -60 Formula X 1 -61
화학식 X1-62 화학식 X1-63 화학식 X1-64Formula X 1 -62 Formula X 1 -63 Formula X 1 -64
화학식 X1-65 화학식 X1-66Formula X 1 -65 Formula X 1 -66
화학식 X1-67 화학식 X1-68Formula X Formula X 1 -67 1 -68
화학식 X1-69 화학식 X1-70Formula X 1 -69 Formula X 1 -70
화학식 X1-71 화학식 X1-72Formula X 1 -71 Formula X 1 -72
화학식 X1-73 화학식 X1-74Formula X 1 -73 Formula X 1 -74
화학식 X1-75 화학식 X1-76Formula X 1 -75 Formula X 1 -76
화학식 X1-77 화학식 X1-78Formula X 1 -77 Formula X 1 -78
화학식 X1-79 화학식 X1-80Formula X 1 -79 Formula X 1 -80
화학식 X1-81 화학식 X1-82,Formula X Formula X 1 -81 1 -82,
Rs-는 Y와 함께 R6OCH2C(R5)=,또는 단독으로 R6OOCCH(NHR7)(CH2)nC(=O)NH-, R6OOCCH(NHR7)(CH2)nSC(=O)NH-, CF3SCH2C(=O)NH-, CF3CH2C(=O)NH-, CHF2SCH2C(=O)NH-, CH2FSCH2C(=O)NH-, NH2C(=O)CHFS-CH2C(=O)NH-, R7NHCH(C(=O)OW)CH2SCH2C(=O)NH-, R7NHCH(L1-L4-L2-W)CH2SCH2C(=O)NH-, CNCH2SCH2C(=O)NH-, CH3(CH2)nC(=O)NH-, R7N=CHNR7CH2CH2S-, R7N=C(NHR7)NHC(=O)-, R7N=C(NHR7)NHC(=O)CH2 , CH3C(Cl)=CHCH2SCH2C(=O)NH-, (CH3)2C(OR6)-, CNCH2C(=O)NH-, CNCH2CH2S-, R7HN=CH(NR7)CH2CH2S-, CH2=CHCH2SCH2C(=O)NH-, CH3CH(OH)-, CH3CH(OR8)-, CH3CH(Y1)-, (CH3)2CH-, CH3CH2-, CH3(CH2)nCH=CH(CH2)mC(=O)NH-, 화학식 Rs-1, 화학식 Rs-2, 화학식 Rs-3, 화학식 Rs-4, 화학식 Rs-5, 화학식 Rs-6, 화학식 Rs-7, 화학식 Rs-8, 화학식 Rs-9, 화학식 Rs-10, 화학식 Rs-11, 화학식 Rs-12, 화학식 Rs-13, 화학식 Rs-14, 화학식 Rs-15, 화학식 Rs-16, 화학식 Rs-17, 화학식 Rs-18, 화학식 Rs-19, 화학식 Rs-20, 화학식 Rs-21, 화학식 Rs-22, 화학식 Rs-23, 화학식 Rs-24, 화학식 Rs-25, 화학식 Rs-26, 화학식 Rs-27, 화학식 Rs-28, 화학식 Rs-29, 화학식 Rs-30, 화학식 Rs-31, 화학식 Rs-32, 화학식 Rs-33, 화학식 Rs-34, 화학식 Rs-35, 화학식 Rs-36, 화학식 Rs-37, 화학식 Rs-38, 화학식 Rs-39, 화학식 Rs-40, 화학식 Rs-41, 화학식 Rs-42, 화학식 Rs-43, 화학식 Rs-44, 화학식 Rs-45, 및 화학식 Rs-46으로 이루어진 군에서 선택되고:R s -is R 6 OCH 2 C (R 5 ) = with Y, or R 6 OOCCH (NHR 7 ) (CH 2 ) n C (= O) NH-, R 6 OOCCH (NHR 7 ) (CH alone 2 ) n SC (= O) NH-, CF 3 SCH 2 C (= O) NH-, CF 3 CH 2 C (= O) NH-, CHF 2 SCH 2 C (= O) NH-, CH 2 FSCH 2 C (= O) NH-, NH 2 C (= O) CHFS-CH 2 C (= O) NH-, R 7 NHCH (C (= O) OW) CH 2 SCH 2 C (= O) NH- , R 7 NHCH (L 1 -L 4 -L 2 -W) CH 2 SCH 2 C (= O) NH-, CNCH 2 SCH 2 C (= O) NH-, CH 3 (CH 2 ) n C (= O) NH-, R 7 N = CHNR 7 CH 2 CH 2 S-, R 7 N = C (NHR 7 ) NHC (= O)-, R 7 N = C (NHR 7 ) NHC (= O) CH 2 , CH 3 C (Cl) = CHCH 2 SCH 2 C (= O) NH-, (CH 3 ) 2 C (OR 6 )-, CNCH 2 C (= O) NH-, CNCH 2 CH 2 S-, R 7 HN = CH (NR 7 ) CH 2 CH 2 S-, CH 2 = CHCH 2 SCH 2 C (= O) NH-, CH 3 CH (OH)-, CH 3 CH (OR 8 )-, CH 3 CH (Y 1 )-, (CH 3 ) 2 CH-, CH 3 CH 2- , CH 3 (CH 2 ) n CH = CH (CH 2 ) m C (= O) NH-, formula Rs-1, formula Rs -2, Formula Rs-3, Formula Rs-4, Formula Rs-5, Formula Rs-6, Formula Rs-7, Formula Rs-8, Formula Rs-9, Formula Rs-10, Formula Rs-11, Formula Rs -12, Formula Rs-13, Formula Rs-14, Formula Rs-15, Formula Rs-16, Formula Rs-17, Formula Rs-18, Formula Rs-19, Formula Rs-20, Formula Rs-21, Formula Rs-22, Formula Rs-23, Formula Rs-24, Formula Rs-25, Formula Rs-26, Formula Rs-27, Formula Rs-28, Formula Rs-29, Formula Rs-30, Formula Rs-31, Formula Rs-32, Formula Rs-33, Formula Rs-34, Formula Rs-35, Formula Rs-36, Formula To Rs-37, formula Rs-38, formula Rs-39, formula Rs-40, formula Rs-41, formula Rs-42, formula Rs-43, formula Rs-44, formula Rs-45, and formula Rs-46 Is selected from the group consisting of:
화학식 Rs-1, 화학식 Rs-2,Formula Rs-1, Formula Rs-2,
화학식 Rs-3 화학식 Rs-4Formula Rs-3 Formula Rs-4
화학식 Rs-5 화학식 Rs-6Formula Rs-5 Formula Rs-6
화학식 Rs-7 화학식 Rs-8Formula Rs-7 Formula Rs-8
화학식 Rs-9, 화학식 Rs-10Formula Rs-9, Formula Rs-10
화학식 Rs-11 화학식 Rs-12Formula Rs-11 Formula Rs-12
화학식 Rs-13 화학식 Rs-14Formula Rs-13 Formula Rs-14
화학식 Rs-15 화학식 Rs-16Formula Rs-15 Formula Rs-16
화학식 Rs-17 화학식 Rs-18Formula Rs-17 Formula Rs-18
화학식 Rs-19 화학식 Rs-20Formula Rs-19 Formula Rs-20
화학식 Rs-21 화학식 Rs-22Formula Rs-21 Formula Rs-22
화학식 Rs-23 화학식 Rs-24Formula Rs-23 Formula Rs-24
화학식 Rs-25 화학식 Rs-26Formula Rs-25 Formula Rs-26
화학식 Rs-27 화학식 Rs-28Formula Rs-27 Formula Rs-28
화학식 Rs-29 화학식 Rs-30Formula Rs-29 Formula Rs-30
화학식 Rs-31 화학식 Rs-32Formula Rs-31 Formula Rs-32
화학식 Rs-33 화학식 Rs-34Formula Rs-33 Formula Rs-34
화학식 Rs-35 화학식 Rs-36Formula Rs-35 Formula Rs-36
화학식 Rs-37 화학식 Rs-38Formula Rs-37 Formula Rs-38
화학식 Rs-39 화학식 Rs-40Formula Rs-39 Formula Rs-40
화학식 Rs-41 화학식 Rs-42Formula Rs-41 Formula Rs-42
화학식 Rs-43 화학식 Rs-44Formula Rs-43 Formula Rs-44
화학식 Rs-45 화학식 Rs-46;Formula Rs-45 Formula Rs-46;
W는 H, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알킬옥시, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 양성자화 아민기, 약학적으로 허용되는 치환된 및 비치환된 1급 아민기, 화학식 Wa, 화학식 W-1, 화학식 W-2, 화학식 W-3, 화학식 W-4, 화학식 W-5, 화학식 W-6, 화학식 W-7, 화학식 W-8, 화학식 W-9, 화학식 W-10, 화학식 W-11, 화학식 W-12, 화학식 W-13, 화학식 W-14, 화학식 W-15, 화학식 W-16, 화학식 W-17 및 화학식 W-18로 이루어진 군에서 선택되고:W is H, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted alkyloxy, substituted and unsubstituted alkenyl, substituted And unsubstituted alkynyl, substituted and unsubstituted aryl, substituted and unsubstituted heteroaryl, protonated amine group, pharmaceutically acceptable substituted and unsubstituted primary amine group, formula Wa, formula W-1, Chemical Formula W-2, Chemical Formula W-3, Chemical Formula W-4, Chemical Formula W-5, Chemical Formula W-6, Chemical Formula W-7, Chemical Formula W-8, Chemical Formula W-9, Chemical Formula W-10, Chemical Formula W-11, Formula W-12, Formula W-13, Formula W-14, Formula W-15, Formula W-16, Formula W-17, and Formula W-18:
화학식 Wa 화학식 W-1 화학식 W-2 화학식 W-3Formula Wa Formula W-1 Formula W-2 Formula W-3
화학식 W-4 화학식 W-5 화학식 W-6Formula W-4 Formula W-5 Formula W-6
화학식 W-7 화학식 W-8 화학식 W-9Formula W-7 Formula W-8 Formula W-9
화학식 W-10 화학식 W-11 화학식 W-12Formula W-10 Formula W-11 Formula W-12
화학식 W-13 화학식 W-14 화학식 W-15Formula W-13 Formula W-14 Formula W-15
화학식 W-16 화학식 W-17 화학식 W-18; Chemical Formula W-16 Chemical Formula W-17 Chemical Formula W-18;
Z는 CH2, S, SO, SO2, NH, NR6, CHCH3, CHCH2CH3, CHR6, R6, -C(=O)-, 및 O로 이루어진 군에서 선택되고; Z is selected from the group consisting of CH 2 , S, SO, SO 2 , NH, NR 6 , CHCH 3 , CHCH 2 CH 3 , CHR 6 , R 6 , -C (= O)-, and O;
AA는 임의의 아미노산이고; AA is any amino acid;
각각의 m 및 n은 독립적으로 0 및 정수, 예를 들면 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, … 로 이루어진 군에서 선택되고; Each m and n is independently 0 and an integer, for example 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 , 18, 19, 20,… Is selected from the group consisting of;
HA는 없음, 및 약학적으로 허용되는 산, 예를 들면 염산염, 브롬화수소산염, 요오드화수소산염, 질산, 황산, 중황산, 인산, 아인산, 포스폰산, 이소니코틴산, 아세트산, 락트산, 살리실산, 시트르산, 타르타르산, 판토텐산, 바이타르타르산, 아스코르브산, 석신산, 말레산, 겐티신산, 푸마르산, 글루콘산, 글루카론산, 당산, 포름산, 벤조산, 글루탐산, 메탄설폰산, 에탄설폰산, 벤젠설폰산, p-톨루엔설폰산 및 파모인산으로 이루어진 군에서 선택되고;HA is free, and pharmaceutically acceptable acids such as hydrochloride, hydrobromide, hydroiodide, nitric acid, sulfuric acid, bisulfite, phosphoric acid, phosphorous acid, phosphonic acid, isonicotinic acid, acetic acid, lactic acid, salicylic acid, citric acid, Tartaric acid, pantothenic acid, bitartaric acid, ascorbic acid, succinic acid, maleic acid, gentisic acid, fumaric acid, gluconic acid, gluconic acid, sugar acid, formic acid, benzoic acid, glutamic acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, p- Toluenesulfonic acid and pamoic acid;
R은 없음, H, CH2C(=O)OR6 , 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬 할라이드, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 아릴, 및 치환된 및 비치환된 헤테로아릴로 이루어진 군에서 선택되고, 여기에서 R의 임의의 CH2는 O, S, P, NR6, 또는 임의의 기타 약학적으로 허용되는 기들로 추가로 대체될 수 있고;R is absent, H, CH 2 C (= O) OR 6 , substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted eggs Coxyl, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl halides, substituted and unsubstituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted aryl, and substituted Selected from the group consisting of substituted and unsubstituted heteroaryls, wherein any CH 2 of R can be further replaced by O, S, P, NR 6 , or any other pharmaceutically acceptable groups;
R1-R3은 독립적으로 H, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알킬옥실, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 아릴 및 치환된 및 비치환된 헤테로아릴 잔기들로 이루어진 군에서 선택되고;R 1 -R 3 are independently H, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted alkyloxyl, substituted and unsubstituted Selected from the group consisting of substituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted aryl and substituted and unsubstituted heteroaryl residues;
R5 및 R35는 독립적으로 H, C(=O)NH2, CH2CH2OR6 , CH2CH2N(CH3)2, CH2CH2N(CH2CH3)2, Cl, F, Br, I, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알킬옥실, 치환된 및 비치환된 시클로알킬옥실, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 치환된 및 비치환된 알킬카보닐, 치환된 및 비치환된 알킬아미노, -C(=O)-W, L1-L4-L2-W, 및 W로 이루어진 군에서 선택되고; R 5 and R 35 are independently H, C (= O) NH 2 , CH 2 CH 2 OR 6 , CH 2 CH 2 N (CH 3 ) 2 , CH 2 CH 2 N (CH 2 CH 3 ) 2, Cl , F, Br, I, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted alkyloxyl, substituted and unsubstituted cyclo Alkyloxyl, substituted and unsubstituted aryl, substituted and unsubstituted heteroaryl, substituted and unsubstituted alkylcarbonyl, substituted and unsubstituted alkylamino, -C (= O) -W, L 1 -L 4 -L 2 -W, and W;
R6, R36 및 R46은 독립적으로 H, F, Cl, Br, I, Na+, K+, C(=O)R5, 2-옥소-1-이미다졸리딘일, 페닐, 5-인다닐, 2,3-디히드로-1H-인덴-5-일, 4-히드록시-1,5-나프티리딘-3-일, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 알킬옥실, 치환된 및 비치환된 시클로알킬옥실, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, -C(=O)-W, -L1-L4-L2-W, 및 W로 이루어진 군에서 선택되고; R 6 , R 36 and R 46 are independently H, F, Cl, Br, I, Na + , K + , C (= O) R 5, 2-oxo-1-imidazolidinyl, phenyl, 5- Indanyl, 2,3-dihydro-1H-inden-5-yl, 4-hydroxy-1,5-naphthyridin-3-yl, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl , Substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted alkyloxyl, substituted and unsubstituted cycloalkyloxyl, substituted And unsubstituted aryl, substituted and unsubstituted heteroaryl, -C (= O) -W, -L 1 -L 4 -L 2 -W, and W;
R7 및 R37은 독립적으로 H, F, Cl, Br, I, CH3NHC(=O)CH2CH(NHR8)C(=O), R5N=C(NHR6)NHC(=O)-, C(=O)CH3, C(=O)R6, PO(OR5)OR6, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알킬옥실, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 치환된 및 비치환된 알킬카보닐, 치환된 및 비치환된 알킬아미노, L1-L4-L2-W, 및 C-(=O)-W로 이루어진 군에서 선택되고; R 7 and R 37 are independently H, F, Cl, Br, I, CH 3 NHC (= O) CH 2 CH (NHR 8 ) C (= O), R 5 N = C (NHR 6 ) NHC (= O)-, C (= O) CH 3 , C (= O) R 6 , PO (OR 5 ) OR 6 , substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted Heterocycloalkyl, substituted and unsubstituted alkyloxyl, substituted and unsubstituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted aryl, substituted and unsubstituted heteroaryl, substituted And selected from the group consisting of unsubstituted alkylcarbonyl, substituted and unsubstituted alkylamino, L 1 -L 4 -L 2 -W, and C-(= O) -W;
R8 및 R38은 독립적으로 H, F, Cl, Br, I, CH3, C2H5, CF3, CH2CH2F, CH2CH2Cl, CH2CH2Br, CH2CH2I, CH2CHF2, CH2CF3 , CH2F, CH2Cl, CH2Br, CH2I, CH2NR6R7, CH(NHR7)CH2C(=O)NH2, C3H7, C4H9, C5H11, R6, C(=O)R6, C(=O)NH2, CH2C(=O)NH2 , CH2OC(=O)NH2, PO(OR5)OR6, C(CH3)2C(=O)OR6, CH(CH3)C(=O)OR6 , CH2C(=O)OR6, C(=O)-W, L1-L4-L2-W, W, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 알킬아미노, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬 할라이드 및 치환된 및 비치환된 알킬카보닐로 이루어진 군에서 선택되고; R 8 and R 38 are independently H, F, Cl, Br, I, CH 3 , C 2 H 5 , CF 3 , CH 2 CH 2 F, CH 2 CH 2 Cl, CH 2 CH 2 Br, CH 2 CH 2 I, CH 2 CHF 2 , CH 2 CF 3 , CH 2 F , CH 2 Cl, CH 2 Br, CH 2 I, CH 2 NR 6 R 7 , CH (NHR 7 ) CH 2 C (= O) NH 2 , C 3 H 7 , C 4 H 9 , C 5 H 11 , R 6 , C (= O) R 6, C (= O) NH 2 , CH 2 C (= O) NH 2 , CH 2 OC (= O) NH 2 , PO (OR 5 ) OR 6 , C (CH 3 ) 2 C (= O) OR 6 , CH (CH 3 ) C (= O) OR 6 , CH 2 C (= O) OR 6 , C (= O) -W, L 1 -L 4 -L 2 -W, W, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted And unsubstituted heterocycloalkyl, substituted and unsubstituted alkoxyl, substituted and unsubstituted alkylamino, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl halides and substituted and Selected from the group consisting of unsubstituted alkylcarbonyl;
R11-R16은 독립적으로 없음, H, CH2C(=O)OR11 , 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬 할라이드, 치환된 및 비치환된 알케닐, 치환된 및 비치환된 알키닐, 치환된 및 비치환된 아릴, 및 치환된 및 비치환된 헤테로아릴로 이루어진 군에서 선택되고; R 11 -R 16 are independently absent, H, CH 2 C (= O) OR 11 , substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, substituted and unsubstituted heterocycloalkyl, substituted And unsubstituted alkoxyl, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl halides, substituted and unsubstituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted Substituted aryl, and substituted and unsubstituted heteroaryl;
X2는 없음, H, C(=O), C(=S), CH2(CH2)nOR8, Cl, F, Br, I, NO2, CN, CF3, C2F5, C3F7, OCF3, OC2F5, NH2, NHR6, CH3, C2H5, R6, C(=O)NH2, CH2OC(=O)NH2, CH2C(=O)OR5, CH2(CH2)nN(CH3)2, CH2(CH2)nSO3R5, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬, 치환된 및 비치환된 알킬티오, 치환된 및 비치환된 알킬아미노, 및 치환된 및 비치환된 알킬옥실로 이루어진 군에서 선택되고;X 2 is none, H, C (= O), C (= S), CH 2 (CH 2 ) n OR 8 , Cl, F, Br, I, NO 2 , CN, CF 3 , C 2 F 5 , C 3 F 7 , OCF 3 , OC 2 F 5 , NH 2 , NHR 6 , CH 3 , C 2 H 5 , R 6 , C (= O) NH 2 , CH 2 OC (= O) NH 2 , CH 2 C (= O) OR 5 , CH 2 (CH 2 ) n N (CH 3 ) 2 , CH 2 (CH 2 ) n SO 3 R 5 , substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl , Substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl, substituted and unsubstituted alkylthio, substituted and unsubstituted alkylamino, and substituted Selected from the group consisting of substituted and unsubstituted alkyloxyls;
X3은 없음, H, N3, SO3W, F, Cl, Br, OH, OCH3, OR6, CH3, R6, C(=O), C(=S), C(=O)OW, OW, L1-L4-L2-W, 및 I로 이루어진 군에서 선택되고;X 3 is absent, H, N 3 , SO 3 W, F, Cl, Br, OH, OCH 3 , OR 6 , CH 3 , R 6 , C (= O), C (= S), C (= O ) OW, OW, L 1 -L 4 -L 2 -W, and I;
X4는 없음, N, CH, 및 CY1로 이루어진 군에서 선택되고; X 4 is selected from the group consisting of none, N, CH, and CY 1 ;
X5 및 X35은 독립적으로 없음, C(=O), C(=S), OC(=O), CH2, CH, S, O 및 NR5로 이루어진 군에서 선택되고; X 5 and X 35 are independently selected from the group consisting of none, C (= O), C (= S), OC (= O), CH 2 , CH, S, O and NR 5 ;
Y1, Y31, Y2, Y32, Y3, 및 Y4는 독립적으로 H, OH, OW, OC(=O)W, L1-L4-L2-W, OC(=O)CH3, CH3, C2H5, C3H7, C4H9, R6, SO3R6, CH2OR6, CH2OC(=O)R6, CH2C(=O)OR8, OCH3, OC2H5, OR6, CH3SO2, R6SO2,CH3SO3, R6SO3, NO2, CN, CF3, OCF3, CH2(CH2)nNR5R6, CH2(CH2)nOR6, CH(C(=O)NH2)NHR6, CH2C(=O)NH2 , F, Br, I, Cl, CH=CHC(=O)NHCH2C(=O)OW, CH=CHC(=O)NHCH2L1-L4-L2-W, NR8C(=O)R5, SO2NR5R8, C(=O)R5, SR5, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 알킬티오, 치환된 및 비치환된 알킬아미노, 치환된 및 비치환된 퍼플루오로알킬, 치환된 및 비치환된 알킬 할라이드 및 치환된 및 비치환된 알킬카보닐로 이루어진 군에서 선택되고; Y 1 , Y 31 , Y 2 , Y 32 , Y 3 , and Y 4 are independently H, OH, OW, OC (= O) W, L 1 -L 4 -L 2 -W, OC (= O) CH 3 , CH 3 , C 2 H 5 , C 3 H 7 , C 4 H 9 , R 6 , SO 3 R 6 , CH 2 OR 6 , CH 2 OC (= O) R 6 , CH 2 C (= O ) OR 8 , OCH 3 , OC 2 H 5 , OR 6 , CH 3 SO 2 , R 6 SO 2 , CH 3 SO 3 , R 6 SO 3 , NO 2 , CN, CF 3 , OCF 3 , CH 2 (CH 2 ) n NR 5 R 6 , CH 2 (CH 2 ) n OR 6, CH (C (= O) NH 2 ) NHR 6 , CH 2 C (= O) NH 2 , F, Br, I, Cl, CH = CHC (= O) NHCH 2 C (= O) OW, CH = CHC (= O) NHCH 2 L 1 -L 4 -L 2 -W, NR 8 C (= O) R 5 , SO 2 NR 5 R 8 , C (= O) R 5 , SR 5 , substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkoxyl, substituted and unsubstituted alkylthio, substituted and unsubstituted alkylamino , Selected from the group consisting of substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl halides and substituted and unsubstituted alkylcarbonyl;
L1은 없음, O, S, -O-L3-, -S-L3-, -N(L3)-, -N(L3)-CH2-O, -N(L3)-CH2-N(L5)-, -O-CH2-O-, -O-CH(L3)-O, 및 -S-CH(L3)-O-로 이루어진 군에서 선택되고;L 1 is absent, O, S, -OL 3- , -SL 3- , -N (L 3 )-, -N (L 3 ) -CH 2 -O, -N (L 3 ) -CH 2 -N (L 5 )-, -O-CH 2 -O-, -O-CH (L 3 ) -O, and -S-CH (L 3 ) -O-;
L2는 없음, O, S, -O-L3-, -S-L3-, -N(L3)-, -N(L3)-CH2-O, -N(L3)-CH2-N(L5)-, -O-CH2-O-, -O-CH(L3)-O, -S-CH(L3)-O-, -O-L3-, -N-L3-, -S-L3-, -N(L3)-L5- 및 L3으로 이루어진 군에서 선택되고;L 2 is none, O, S, -OL 3- , -SL 3- , -N (L 3 )-, -N (L 3 ) -CH 2 -O, -N (L 3 ) -CH 2 -N (L 5 )-, -O-CH 2 -O-, -O-CH (L 3 ) -O, -S-CH (L 3 ) -O-, -OL 3- , -NL 3- , -SL 3- , -N (L 3 ) -L 5 -and L 3 ;
L4는 C=O, C=S, , 및 로 이루어진 군에서 선택되고;L 4 is C = O, C = S, , And Is selected from the group consisting of;
각각의 L1, L2, 및 L4, L3 및 L5 에 대하여 독립적으로 없음, H, CH2C(=O)OL6, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 알킬티오, 치환된 및 비치환된 알킬아미노, 치환된 및 비치환된 퍼플루오로알킬, 및 치환된 및 비치환된 알킬 할라이드로 이루어진 군에서 선택되고, 여기에서 임의의 탄소 또는 수소는 독립적으로 O, S, P, NL3, 또는 기타 임의의 약학적으로 허용되는 기들로 추가로 대체될 수 있고;Independently for each L 1 , L 2 , and L 4 , L 3 and L 5 , H, CH 2 C (= O) OL 6 , substituted and unsubstituted alkyl, substituted and unsubstituted cyclo Alkyl, substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted aryl, substituted and unsubstituted heteroaryl, substituted and unsubstituted alkoxyl, substituted and unsubstituted alkylthio, substituted and Selected from the group consisting of unsubstituted alkylamino, substituted and unsubstituted perfluoroalkyl, and substituted and unsubstituted alkyl halides, wherein any carbon or hydrogen is independently O, S, P, NL 3 , or any other pharmaceutically acceptable groups;
L6은 독립적으로 H, OH, Cl, F, Br, I, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 및 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 알킬티오, 치환된 및 비치환된 알킬아미노, 치환된 및 비치환된 퍼플루오로알킬, 및 치환된 및 비치환된 알킬 할라이드로 이루어진 군에서 선택되고, 여기에서 임의의 탄소 또는 수소는 독립적으로 O, S, N, P(O)OL6, CH=CH, C≡C, CHL6 , CL6L7, 아릴, 헤테로아릴, 또는 시클릭기들로 추가로 대체될 수 있고; L 6 is independently H, OH, Cl, F, Br, I, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, and substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted Aryl, substituted and unsubstituted heteroaryl, substituted and unsubstituted alkoxyl, substituted and unsubstituted alkylthio, substituted and unsubstituted alkylamino, substituted and unsubstituted perfluoroalkyl, And substituted and unsubstituted alkyl halides, wherein any carbon or hydrogen is independently O, S, N, P (O) OL 6 , CH = CH, C≡C, CHL 6 , CL 6 L 7 , aryl, heteroaryl, or cyclic groups may be further substituted;
L7은 독립적으로 H, OH, Cl, F, Br, I, 치환된 및 비치환된 알킬, 치환된 및 비치환된 시클로알킬, 및 치환된 및 비치환된 헤테로시클로알킬, 치환된 및 비치환된 아릴, 치환된 및 비치환된 헤테로아릴, 치환된 및 비치환된 알콕실, 치환된 및 비치환된 알킬티오, 치환된 및 비치환된 알킬아미노, 치환된 및 비치환된 퍼플루오로알킬, 및 치환된 및 비치환된 알킬 할라이드로 이루어진 군에서 선택되고, 여기에서 임의의 탄소 또는 수소는 독립적으로 O, S, N, P(O)OL6, CH=CH, C≡C, CHL6 , CL6L7, 아릴, 헤테로아릴, 또는 시클릭기들로 추가로 대체될 수 있고;L 7 is independently H, OH, Cl, F, Br, I, substituted and unsubstituted alkyl, substituted and unsubstituted cycloalkyl, and substituted and unsubstituted heterocycloalkyl, substituted and unsubstituted Aryl, substituted and unsubstituted heteroaryl, substituted and unsubstituted alkoxyl, substituted and unsubstituted alkylthio, substituted and unsubstituted alkylamino, substituted and unsubstituted perfluoroalkyl, And substituted and unsubstituted alkyl halides, wherein any carbon or hydrogen is independently O, S, N, P (O) OL 6 , CH = CH, C≡C, CHL 6 , CL 6 L 7 , aryl, heteroaryl, or cyclic groups may be further substituted;
임의의 CH2 기들은 O, S, 또는 NH로 추가로 대체될 수 있다.Any CH 2 groups can be further replaced by O, S, or NH.
특정 양태들에서, 항균제 또는 항균제-관련 화합물의 HPP 또는 HPC의 관능성 단위는 입체이성질체 및 약학적으로 허용되는 염을 포함한 화학식 FP-1, 화학식 FP-2, 화학식 FP-3, 화학식 FP-4, 화학식 FP-5, 화학식 FP-6, 화학식 FP-7, 화학식 FP-8, 화학식 FP-9, 화학식 FP-10, 화학식 FP-11, 화학식 FP-12, 화학식 FP-13, 화학식 FP-14, 화학식 FP-15, 화학식 FP-16, 화학식 FP-17, 화학식 FP-18, 화학식 FP-19, 화학식 FP-20, 화학식 FP-21, 화학식 FP-22, 화학식 FP-23, 화학식 FP-24, 화학식 FP-25, 화학식 FP-26, 화학식 FP-27, 화학식 FP-28, 화학식 FP-29, 화학식 FP-30, 화학식 FP-31, 화학식 FP-32, 화학식 FP-33, 화학식 FP-34, 화학식 FP-35, 화학식 FP-36, 화학식 FP-37, 화학식 FP-38, 화학식 FP-39, 화학식 FP-40, 화학식 FP-41, 화학식 FP-42, 화학식 FP-43, 화학식 FP-44, 화학식 FP-45, 화학식 FP-46, 화학식 FP-47, 화학식 FP-48, 화학식 FP-49, 화학식 FP-50, 화학식 FP-51, 화학식 FP-52, 화학식 FP-53, 화학식 FP-54, 화학식 FP-55, 화학식 FP-56, 화학식 FP-57, 화학식 FP-58, 화학식 FP-59, 화학식 FP-60, 화학식 FP-61, 화학식 FP-62, 화학식 FP-63, 화학식 FP-64, 화학식 FP-65, 화학식 FP-66, 화학식 FP-67, 화학식 FP-68, 화학식 FP-69, 화학식 FP-70, 화학식 FP-71, 화학식 FP-72, 화학식 FP-73, 화학식 FP-74, 화학식 FP-75, 화학식 FP-76, 화학식 FP-77, 화학식 FP-78, 화학식 FP-79, 화학식 FP-80, 화학식 FP-81, 화학식 FP-82, 화학식 FP-83, 화학식 FP-84, 화학식 FP-85, 화학식 FP-86, 화학식 FI-1, 화학식 FI-2, 화학식 FI-3, 화학식 FI-4, 화학식 FI-5, 화학식 FI-6, 화학식 FI-7, 화학식 FI-8, 화학식 FI-9, 화학식 FI-10, 화학식 FI-11, 화학식 FI-12, 화학식 FI-13, 화학식 FI-14, 화학식 FI-15, 화학식 FI-16, 화학식 FI-17, 화학식 FI-18, 화학식 FI-19, 화학식 FI-20, 화학식 FI-21, 화학식 FI-22, 화학식 FI-23, 화학식 FI-24, 화학식 FI-25, 화학식 FI-26, 화학식 FI-27, 화학식 FI-28, 화학식 FI-29, 화학식 FI-30, 화학식 FI-31, 화학식 FI-32, 화학식 FI-33, 화학식 FS-1, 화학식 FS-2, 화학식 FS-3, 화학식 FS-4, 화학식 FS-5, 화학식 FS-6, 화학식 FS-7, 화학식 FS-8, 화학식 FS-9, 화학식 FS-10, 화학식 FS-11, 화학식 FS-12, 화학식 FS-13, 화학식 FS-14, 화학식 FS-15, 화학식 FS-16, 화학식 FS-17, 화학식 FS-18, 화학식 FS-19, 화학식 FS-20, 화학식 FT-1, 화학식 FT-2, 화학식 FT-3, 화학식 FT-4, 화학식 FT-5, 화학식 FT-6, 화학식 FT-7, 화학식 FT-8, 화학식 FT-9, 화학식 FT-10, 화학식 FT-11, 화학식 FT-12, 화학식 FT-13, 화학식 FT-14, 화학식 FT-15, 및 화학식 FT-16으로 이루어진 군에서 선택되는 구조를 가지는 잔기를 포함하고:In certain embodiments, the functional unit of the HPP or HPC of the antimicrobial agent or antimicrobial agent-related compound is Formula FP-1, Formula FP-2, Formula FP-3, Formula FP-4, including stereoisomers and pharmaceutically acceptable salts. , Chemical Formula FP-5, Chemical Formula FP-6, Chemical Formula FP-7, Chemical Formula FP-8, Chemical Formula FP-9, Chemical Formula FP-10, Chemical Formula FP-11, Chemical Formula FP-12, Chemical Formula FP-13, Chemical Formula FP-14 , Chemical Formula FP-15, Chemical Formula FP-16, Chemical Formula FP-17, Chemical Formula FP-18, Chemical Formula FP-19, Chemical Formula FP-20, Chemical Formula FP-21, Chemical Formula FP-22, Chemical Formula FP-23, Chemical Formula FP-24 , Chemical Formula FP-25, Chemical Formula FP-26, Chemical Formula FP-27, Chemical Formula FP-28, Chemical Formula FP-29, Chemical Formula FP-30, Chemical Formula FP-31, Chemical Formula FP-32, Chemical Formula FP-33, Chemical Formula FP-34 , Formula FP-35, Formula FP-36, Formula FP-37, Formula FP-38, Formula FP-39, Formula FP-40, Formula FP-41, Formula FP-42, Formula FP-43, Formula FP-44 , Formula FP-45, Formula FP-46, Formula FP-47, Formula FP-48, Formula FP-49 , Formula FP-50, Formula FP-51, Formula FP-52, Formula FP-53, Formula FP-54, Formula FP-55, Formula FP-56, Formula FP-57, Formula FP-58, Formula FP-59 , Chemical Formula FP-60, Chemical Formula FP-61, Chemical Formula FP-62, Chemical Formula FP-63, Chemical Formula FP-64, Chemical Formula FP-65, Chemical Formula FP-66, Chemical Formula FP-67, Chemical Formula FP-68, Chemical Formula FP-69 , Chemical Formula FP-70, Chemical Formula FP-71, Chemical Formula FP-72, Chemical Formula FP-73, Chemical Formula FP-74, Chemical Formula FP-75, Chemical Formula FP-76, Chemical Formula FP-77, Chemical Formula FP-78, Chemical Formula FP-79 , Formula FP-80, Formula FP-81, Formula FP-82, Formula FP-83, Formula FP-84, Formula FP-85, Formula FP-86, Formula FI-1, Formula FI-2, Formula FI-3 , Formula FI-4, Formula FI-5, Formula FI-6, Formula FI-7, Formula FI-8, Formula FI-9, Formula FI-10, Formula FI-11, Formula FI-12, Formula FI-13 , Chemical Formula FI-14, Chemical Formula FI-15, Chemical Formula FI-16, Chemical Formula FI-17, Chemical Formula FI-18, Chemical Formula FI-19, Chemical Formula FI-20, Chemical Formula FI-21, Chemical Formula FI-22, Chemical Formula FI-23, Formula FI-24, Formula FI-25, Formula FI-26, Formula FI-27, Formula FI-28, Formula FI-29, Formula FI-30, Formula FI-31, Formula FI-32, Formula FI-33, Formula FS-1, Formula FS-2, Formula FS-3, Formula FS-4, Formula FS-5, Formula FS-6, Formula FS-7, Formula FS-8, Formula FS-9, Formula FS-10, Formula FS-11, Formula FS-12, Formula FS-13, Formula FS-14, Formula FS-15, Formula FS-16, Formula FS-17, Formula FS-18, Formula FS-19, Formula FS-20, Formula FT-1, Formula FT-2, Formula FT-3, Formula FT-4, Formula FT-5, Formula FT-6, Formula FT-7, Formula FT-8, Formula FT-9, Formula FT-10, Formula FT-11, Formula FT-12, Formula FT-13, Formula FT-14, Formula FT-15, and includes a residue having a structure selected from the group consisting of Formula FT-16:
화학식 FP-1 화학식 FP-2 Formula FP-1 Formula FP-2
화학식 FP-3 화학식 FP-4 Formula FP-3 Formula FP-4
화학식 FP-5 화학식 FP-6Formula FP-5 Formula FP-6
화학식 FP-7 화학식 FP-8 Formula FP-7 Formula FP-8
화학식 FP-9 화학식 FP-10 Formula FP-9 Formula FP-10
화학식 FP-11 화학식 FP-12Formula FP-11 Formula FP-12
화학식 FP-13 화학식 FP-14Formula FP-13 Formula FP-14
화학식 FP-15 화학식 FP-16Formula FP-15 Formula FP-16
화학식 FP-17 화학식 FP-18Formula FP-17 Formula FP-18
화학식 FP-19 화학식 FP-20Formula FP-19 Formula FP-20
화학식 FP-21 화학식 FP-22Formula FP-21 Formula FP-22
화학식 FP-23 화학식 FP-24Formula FP-23 Formula FP-24
화학식 FP-25 화학식 FP-26Formula FP-25 Formula FP-26
화학식 FP-27 화학식 FP-28Formula FP-27 Formula FP-28
화학식 FP-29 화학식 FP-30Formula FP-29 Formula FP-30
화학식 FP-31 화학식 FP-32Formula FP-31 Formula FP-32
화학식 FP-33 화학식 FP-34Formula FP-33 Formula FP-34
화학식 FP-35 화학식 FP-36Formula FP-35 Formula FP-36
화학식 FP-37 화학식 FP-38Formula FP-37 Formula FP-38
화학식 FP-39 화학식 FP-40Formula FP-39 Formula FP-40
화학식 FP-41 화학식 FP-42Formula FP-41 Formula FP-42
화학식 FP-43 화학식 FP-44Formula FP-43 Formula FP-44
화학식 FP-45 화학식 FP-46Formula FP-45 Formula FP-46
화학식 FP-47 화학식 FP-48Formula FP-47 Formula FP-48
화학식 FP-49 화학식 FP-50Formula FP-49 Formula FP-50
화학식 FP-51 화학식 FP-52Formula FP-51 Formula FP-52
화학식 FP-53 화학식 FP-54Formula FP-53 Formula FP-54
화학식 FP-55 화학식 FP-56Formula FP-55 Formula FP-56
화학식 FP-57 화학식 FP-58Formula FP-57 Formula FP-58
화학식 FP-59 화학식 FP-60Formula FP-59 Formula FP-60
화학식 FP-61 화학식 FP-62Formula FP-61 Formula FP-62
화학식 FP-63 화학식 FP-64Formula FP-63 Formula FP-64
화학식 FP-65 화학식 FP-66Formula FP-65 Formula FP-66
화학식 FP-67 화학식 FP-68Formula FP-67 Formula FP-68
화학식 FP-69 화학식 FP-70Formula FP-69 Formula FP-70
화학식 FP-71 화학식 FP-72Formula FP-71 Formula FP-72
화학식 FP-73 화학식 FP-74Formula FP-73 Formula FP-74
화학식 FP-75 화학식 FP-76Formula FP-75 Formula FP-76
화학식 FP-77 화학식 FP-78Formula FP-77 Formula FP-78
화학식 FP-79 화학식 FP-80Formula FP-79 Formula FP-80
화학식 FP-81 화학식 FP-82Formula FP-81 Formula FP-82
화학식 FP-83 화학식 FP-84Formula FP-83 Formula FP-84
화학식 FP-85 화학식 FP-86Formula FP-85 Formula FP-86
화학식 FI-1 화학식 FI-2 화학식 FI-3Formula FI-1 Formula FI-2 Formula FI-3
화학식 FI-4 화학식 FI-5 Formula FI-4 Formula FI-5
화학식 FI-6 화학식 FI-7Formula FI-6 Formula FI-7
화학식 FI-8 화학식 FI-9Formula FI-8 Formula FI-9
화학식 FI-10 화학식 FI-11Formula FI-10 Formula FI-11
화학식 FI-12 화학식 FI-13Formula FI-12 Formula FI-13
화학식 FI-14 화학식 FI-15Formula FI-14 Formula FI-15
화학식 FI-16 화학식 FI-17Formula FI-16 Formula FI-17
화학식 FI-18 화학식 FI-19Formula FI-18 Formula FI-19
화학식 FI-20 화학식 FI-21Formula FI-20 Formula FI-21
화학식 FI-22 화학식 FI-23Formula FI-22 Formula FI-23
화학식 FI-24 화학식 FI-25Formula FI-24 Formula FI-25
화학식 FI-26 화학식 FI-27Formula FI-26 Formula FI-27
화학식 FI-28 화학식 FI-29Formula FI-28 Formula FI-29
화학식 FI-30 화학식 FI-31Formula FI-30 Formula FI-31
화학식 FI-32 화학식 FI-33Formula FI-32 Formula FI-33
화학식 FS-1 화학식 FS-2Formula FS-1 Formula FS-2
화학식 FS-3 화학식 FS-4Formula FS-3 Formula FS-4
화학식 FS-5 화학식 FS-6Formula FS-5 Formula FS-6
화학식 FS-7 화학식 FS-8Formula FS-7 Formula FS-8
화학식 FS-9 화학식 FS-10Formula FS-9 Formula FS-10
화학식 FS-11 화학식 FS-12 화학식 FS-13Formula FS-11 Formula FS-12 Formula FS-13
화학식 FS-14 화학식 FS-15 화학식 FS-16Formula FS-14 Formula FS-15 Formula FS-16
화학식 FS-17 화학식 FS-18Formula FS-17 Formula FS-18
화학식 FS-19 화학식 FS-20Formula FS-19 Formula FS-20
화학식 FT-1 화학식 FT-2 화학식 FT-3Formula FT-1 Formula FT-2 Formula FT-3
화학식 FT-4 화학식 FT-5 화학식 FT-6Formula FT-4 Formula FT-5 Formula FT-6
화학식 FT-7 화학식 FT-8Formula FT-7 Formula FT-8
화학식 FT-9 화학식 FT-10Formula FT-9 Formula FT-10
화학식 FT-11 화학식 FT-12 화학식 FT-13Formula FT-11 Formula FT-12 Formula FT-13
화학식 FT-14 화학식 FT-15 화학식 FT-16Formula FT-14 Formula FT-15 Formula FT-16
여기에서:From here:
n, R5, R7, X5, X35, Y1, Y2, Y31, Y32, Y3, 및 Y4는 상기와 동일하게 정의되고; n, R 5 , R 7 , X 5 , X 35 , Y 1 , Y 2 , Y 31 , Y 32 , Y 3 , and Y 4 are defined as above;
L31은 상기 L1 와 동일하게 정의되고, L32은 상기 L2 와 동일하게 정의되고, L34는 상기 L4 와 동일하게 정의되고, 특정 양태들에서, -L1-L4-L2- 및 -L31-L34-L32-은 독립적으로 -O-, -X-, -O-X-, -N-X-, -S-X-, -X5-, -O-X5-, -N-X5-, -S-X5-, -O-X7-, -O-C(=O)-, -NH-C(=O)-, -C(=O)-, -C(=O)-O-, -C(=O)-N-, 및 C(=O)-X-로 이루어진 군에서 선택되고;L 31 is defined equal to L 1 , L 32 is defined equal to L 2 , L 34 is defined equal to L 4, and in certain embodiments, -L 1 -L 4 -L 2 -And -L 31 -L 34 -L 32 -are independently -O-, -X-, -OX-, -NX-, -SX-, -X 5- , -OX 5- , -NX 5- , -SX 5- , -OX 7- , -OC (= O)-, -NH-C (= O)-, -C (= O)-, -C (= O) -O-, -C (= O) -N-, and C (= O) -X-;
X는 없음, C(=O), OC(=O), CH2, CH, S, NH, NR6, 및 O로 이루어진 군에서 선택되고;X is selected from the group consisting of none, C (= O), OC (= O), CH 2 , CH, S, NH, NR 6 , and O;
X6, X36 및 X46은 독립적으로 없음, C(=O), C(=S), OC(=O), CH2, CH, S, O 및 NR5로 이루어진 군에서 선택되고;X 6 , X 36 and X 46 are independently selected from the group consisting of none, C (= O), C (= S), OC (= O), CH 2 , CH, S, O and NR 5 ;
X7은 없음, C(=O), C(=S), OC(=O), CH2, CH, S, O 및 NR5로 이루어진 군에서 선택된다.X 7 is selected from the group consisting of none, C (= O), C (= S), OC (= O), CH 2 , CH, S, O and NR 5 .
특정 양태들에서, 항균제 및 항균제-관련 화합물의 HPP의 관능성 단위는 입체이성질체 및 약학적으로 허용되는 염을 포함한 상기 정의된 화학식 F-1, 화학식 FP-1, 화학식 FP-2, 화학식 FP-3, 화학식 FP-4, 화학식 FP-5, 화학식 FP-6, 화학식 FP-7, 화학식 FP-8, 화학식 FP-9, 화학식 FP-10, 화학식 FP-11, 화학식 FP-12, 화학식 FP-13, 화학식 FP-14, 화학식 FP-15, 화학식 FP-16, 화학식 FP-17, 화학식 FP-18, 화학식 FP-19, 화학식 FP-20, 화학식 FP-21, 화학식 FP-22, 화학식 FP-23, 화학식 FP-24, 화학식 FP-25, 화학식 FP-26, 화학식 FP-27, 화학식 FP-28, 화학식 FP-29, 화학식 FP-30, 화학식 FP-31, 화학식 FP-32, 화학식 FP-33, 화학식 FP-34, 화학식 FP-35, 화학식 FP-36, 화학식 FP-37, 화학식 FP-38, 화학식 FP-39, 화학식 FP-40, 화학식 FP-41, 화학식 FP-42, 화학식 FP-43, 화학식 FP-44, 화학식 FP-45, 화학식 FP-46, 화학식 FP-47, 화학식 FP-48, 화학식 FP-49, 화학식 FP-50, 화학식 FP-51, 화학식 FP-52, 화학식 FP-53, 화학식 FP-54, 화학식 FP-55, 화학식 FP-56, 화학식 FP-57, 화학식 FP-58, 화학식 FP-59, 화학식 FP-60, 화학식 FP-61, 화학식 FP-62, 화학식 FP-63, 화학식 FP-64, 화학식 FP-65, 화학식 FP-66, 화학식 FP-67, 화학식 FP-68, 화학식 FP-69, 화학식 FP-70, 화학식 FP-71, 화학식 FP-72, 화학식 FP-73, 화학식 FP-74, 화학식 FP-75, 화학식 FP-76, 화학식 FP-77, 화학식 FP-78, 화학식 FP-79, 화학식 FP-80, 화학식 FP-81, 화학식 FP-82, 화학식 FP-83, 화학식 FP-84, 화학식 FP-85, 화학식 FP-86, 화학식 FI-1, 화학식 FI-2, 화학식 FI-3, 화학식 FI-4, 화학식 FI-5, 화학식 FI-6, 화학식 FI-7, 화학식 FI-8, 화학식 FI-9, 화학식 FI-10, 화학식 FI-11, 화학식 FI-12, 화학식 FI-13, 화학식 FI-14, 화학식 FI-15, 화학식 FI-16, 화학식 FI-17, 화학식 FI-18, 화학식 FI-19, 화학식 FI-20, 화학식 FI-21, 화학식 FI-22, 화학식 FI-23, 화학식 FI-24, 화학식 FI-25, 화학식 FI-26, 화학식 FI-27, 화학식 FI-28, 화학식 FI-29, 화학식 FI-30, 화학식 FI-31, 화학식 FI-32, 화학식 FI-33, 화학식 FS-1, 화학식 FS-2, 화학식 FS-3, 화학식 FS-4, 화학식 FS-5, 화학식 FS-6, 화학식 FS-7, 화학식 FS-8, 화학식 FS-9, 화학식 FS-10, 화학식 FS-11, 화학식 FS-12, 화학식 FS-13, 화학식 FS-14, 화학식 FS-15, 화학식 FS-16, 화학식 FS-17, 화학식 FS-18, 화학식 FS-19, 화학식 FS-20, 화학식 FT-1, 화학식 FT-2, 화학식 FT-3, 화학식 FT-4, 화학식 FT-5, 화학식 FT-6, 화학식 FT-7, 화학식 FT-8, 화학식 FT-9, 화학식 FT-10, 화학식 FT-11, 화학식 FT-12, 화학식 FT-13, 화학식 FT-14, 화학식 FT-15, 및 화학식 FT-16으로 이루어진 군에서 선택되는 구조를 가지는 잔기를 포함하고, 여기에서:In certain embodiments, the functional unit of the HPP of the antimicrobial agent and the antimicrobial agent-related compound is Formula F-1, Formula FP-1, Formula FP-2, Formula FP- as defined above, including stereoisomers and pharmaceutically acceptable salts. 3, Chemical Formula FP-4, Chemical Formula FP-5, Chemical Formula FP-6, Chemical Formula FP-7, Chemical Formula FP-8, Chemical Formula FP-9, Chemical Formula FP-10, Chemical Formula FP-11, Chemical Formula FP-12, Chemical Formula FP- 13, Chemical Formula FP-14, Chemical Formula FP-15, Chemical Formula FP-16, Chemical Formula FP-17, Chemical Formula FP-18, Chemical Formula FP-19, Chemical Formula FP-20, Chemical Formula FP-21, Chemical Formula FP-22, Chemical Formula FP- 23, Chemical Formula FP-24, Chemical Formula FP-25, Chemical Formula FP-26, Chemical Formula FP-27, Chemical Formula FP-28, Chemical Formula FP-29, Chemical Formula FP-30, Chemical Formula FP-31, Chemical Formula FP-32, Chemical Formula FP- 33, Chemical Formula FP-34, Chemical Formula FP-35, Chemical Formula FP-36, Chemical Formula FP-37, Chemical Formula FP-38, Chemical Formula FP-39, Chemical Formula FP-40, Chemical Formula FP-41, Chemical Formula FP-42, Chemical Formula FP- 43, Chemical Formula FP-44, Chemical Formula FP-45, Chemical Formula FP-46, Chemical Formula FP-47, Chemical Formula FP-4 8, Chemical Formula FP-49, Chemical Formula FP-50, Chemical Formula FP-51, Chemical Formula FP-52, Chemical Formula FP-53, Chemical Formula FP-54, Chemical Formula FP-55, Chemical Formula FP-56, Chemical Formula FP-57, Chemical Formula FP- 58, FP-59, FP-60, FP-61, FP-62, FP-63, FP-64, FP-65, FP-66, FP-67, FP-67 68, Chemical Formula FP-69, Chemical Formula FP-70, Chemical Formula FP-71, Chemical Formula FP-72, Chemical Formula FP-73, Chemical Formula FP-74, Chemical Formula FP-75, Chemical Formula FP-76, Chemical Formula FP-77, Chemical Formula FP- 78, Chemical Formula FP-79, Chemical Formula FP-80, Chemical Formula FP-81, Chemical Formula FP-82, Chemical Formula FP-83, Chemical Formula FP-84, Chemical Formula FP-85, Chemical Formula FP-86, Chemical Formula FI-1, Chemical Formula FI- 2, Chemical Formula FI-3, Chemical Formula FI-4, Chemical Formula FI-5, Chemical Formula FI-6, Chemical Formula FI-7, Chemical Formula FI-8, Chemical Formula FI-9, Chemical Formula FI-10, Chemical Formula FI-11, Chemical Formula FI- 12, Chemical Formula FI-13, Chemical Formula FI-14, Chemical Formula FI-15, Chemical Formula FI-16, Chemical Formula FI-17, Chemical Formula FI-18, Chemical Formula FI-19, Chemical Formula FI-20, Chemical Formula FI-21, Chemical Formula FI-22, Formula FI-23, Formula FI-24, Formula FI-25, Formula FI-26, Formula FI-27, Formula FI-28, Formula FI-29, Formula FI-30, Formula FI-31, Formula FI-32, Formula FI-33, Formula FS-1, Formula FS-2, Formula FS-3, Formula FS-4, Formula FS-5, Formula FS-6, Formula FS-7, Formula FS-8, Formula FS-9, Formula FS-10, Formula FS-11, Formula FS-12, Formula FS-13, Formula FS-14, Formula FS-15, Formula FS-16, Formula FS-17, Formula FS-18, Formula FS-19, Formula FS-20, Formula FT-1, Formula FT-2, Formula FT-3, Formula FT-4, Formula FT-5, Formula FT-6, Formula FT-7, Formula FT-8, Residues having a structure selected from the group consisting of Formula FT-9, Formula FT-10, Formula FT-11, Formula FT-12, Formula FT-13, Formula FT-14, Formula FT-15, and Formula FT-16 Including, here:
m = 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, …;m = 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20,… ;
n = 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, …;n = 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20,… ;
R1은 H, C1-C20 알킬, C1-C20 알킬옥실, C1-C20 알케닐, C1-C20 알키닐, 아릴, 및 헤테로아릴로 이루어진 군에서 선택되고; R 1 is selected from the group consisting of H, C 1 -C 20 alkyl, C 1 -C 20 alkyloxyl, C 1 -C 20 alkenyl, C 1 -C 20 alkynyl, aryl, and heteroaryl;
R2는 H, C1-C20 알킬, C1-C20 알킬옥시, C1-C20 알케닐, C1-C20 알키닐, 아릴, 및 헤테로아릴 잔기들로 이루어진 군에서 선택되고; R 2 is selected from the group consisting of H, C 1 -C 20 alkyl, C 1 -C 20 alkyloxy, C 1 -C 20 alkenyl, C 1 -C 20 alkynyl, aryl, and heteroaryl residues;
R3은 H, C1-C20 알킬, C1-C20 알킬옥시, C1-C20 알케닐, C1-C20 알키닐, 아릴 및 헤테로아릴 잔기들로 이루어진 군에서 선택되고; R 3 is selected from the group consisting of H, C 1 -C 20 alkyl, C 1 -C 20 alkyloxy, C 1 -C 20 alkenyl, C 1 -C 20 alkynyl, aryl and heteroaryl residues;
R5 및 R35은 독립적으로 H, -C(=O)NH2, CH2CH2OR6 , CH2CH2N(CH3)2, CH2CH2N(CH2CH3)2, CH2CH2OR6 , Cl, F, Br, I, C1-C20 알킬, C1-C20 시클로알킬, C1-C20 알킬옥실, C1-C20 시클로알킬옥실, C1-C20 알케닐, C1-C20 시클로알케닐, C1-C20 시클로알키닐, C1-C20 알키닐, 아릴, 헤테로아릴, C(=O)-W, 및 W로 이루어진 군에서 선택되고;R 5 and R 35 are independently H, -C (= O) NH 2 , CH 2 CH 2 OR 6 , CH 2 CH 2 N (CH 3 ) 2 , CH 2 CH 2 N (CH 2 CH 3 ) 2, CH 2 CH 2 OR 6 , Cl, F, Br, I, C 1 -C 20 alkyl, C 1 -C 20 cycloalkyl, C 1 -C 20 alkyloxyl, C 1 -C 20 cycloalkyloxyl, C 1- In the group consisting of C 20 alkenyl, C 1 -C 20 cycloalkenyl, C 1 -C 20 cycloalkynyl, C 1 -C 20 alkynyl, aryl, heteroaryl, C (= O) -W, and W Is selected;
R6, R36 및 R46은 독립적으로 H, F, Cl, Br, I, Na+, K+, C(=O)R5, 2-옥소-1-이미다졸리딘일, 페닐, 5-인다닐, 2,3-디히드로-1H-인덴-5-일, 4-히드록시-1,5-나프티리딘-3-일, C1-C12 알킬, C1-C12 시클로알킬, C1-C12 알킬옥실, C1-C12 시클로알킬옥실, C1-C12 알케닐, C1-C12 시클로알케닐, C1-C12 시클로알키닐, C1-C12 알키닐, 아릴, 헤테로아릴, C(=O)-W, 및 W로 이루어진 군에서 선택되고; R 6 , R 36 and R 46 are independently H, F, Cl, Br, I, Na + , K + , C (= O) R 5, 2-oxo-1-imidazolidinyl, phenyl, 5- Indanyl, 2,3-dihydro-1H-inden-5-yl, 4-hydroxy-1,5-naphthyridin-3-yl, C 1 -C 12 alkyl, C 1 -C 12 cycloalkyl, C 1 -C 12 alkyloxyl, C 1 -C 12 cycloalkyloxyl, C 1 -C 12 alkenyl, C 1 -C 12 cycloalkenyl, C 1 -C 12 cycloalkynyl, C 1 -C 12 alkynyl, Aryl, heteroaryl, C (= O) -W, and W;
R7 및 R37은 독립적으로 H, F, Cl, Br, I, CH3NHC(=O)CH2CH(NHR8)C(=O), R5N=C(NHR6)NHC(=O)-, C(=O)CH3, C(=O)R6, PO(OR5)OR6, C1-C20 알킬, C1-C20 알킬옥실, C1-C20 알케닐, C1-C20 알키닐, 아릴, 헤테로아릴, C(=O)-W, 및 W로 이루어진 군에서 선택되고; R 7 and R 37 are independently H, F, Cl, Br, I, CH 3 NHC (= O) CH 2 CH (NHR 8 ) C (= O), R 5 N = C (NHR 6 ) NHC (= O)-, C (= O) CH 3 , C (= O) R 6 , PO (OR 5 ) OR 6 , C 1 -C 20 alkyl, C 1 -C 20 alkyloxyl, C 1 -C 20 alkenyl , C 1 -C 20 alkynyl, aryl, heteroaryl, C (= O) -W, and W;
R8 및 R38은 독립적으로 H, F, Cl, Br, I, CH3, C2H5, CF3, CH2CH2F, CH2CH2Cl, CH2CH2Br, CH2CH2I, CH2CHF2, CH2CF3 , CH2F, CH2Cl, CH2Br, CH2I, CH2NR6R7, CH(NHR7)CH2C(=O)NH2, C3H7, C4H9, C5H11, R6, C(=O)R6, C(=O)NH2, CH2C(=O)NH2 , CH2OC(=O)NH2, PO(OR5)OR6, C(CH3)2C(=O)OR6, CH(CH3)C(=O)OR6 , CH2C(=O)OR6, C(=O)-W로 이루어진 군에서 선택되고; R 8 and R 38 are independently H, F, Cl, Br, I, CH 3 , C 2 H 5 , CF 3 , CH 2 CH 2 F, CH 2 CH 2 Cl, CH 2 CH 2 Br, CH 2 CH 2 I, CH 2 CHF 2 , CH 2 CF 3 , CH 2 F , CH 2 Cl, CH 2 Br, CH 2 I, CH 2 NR 6 R 7 , CH (NHR 7 ) CH 2 C (= O) NH 2 , C 3 H 7 , C 4 H 9 , C 5 H 11 , R 6 , C (= O) R 6, C (= O) NH 2 , CH 2 C (= O) NH 2 , CH 2 OC (= O) NH 2 , PO (OR 5 ) OR 6 , C (CH 3 ) 2 C (= O) OR 6 , CH (CH 3 ) C (= O) OR 6 , CH 2 C (= O) OR 6 , C (= O) -W;
X2는 없음, H, CH2(CH2)nOR8, Cl, F, Br, I, NO2, CN, CF3, C2F5, C3F7, OCF3, OC2F5, NH2, NHR6, CH3, C2H5, R6, C(=O)NH2, CH2OC(=O)NH2, CH2C(=O)OR5, CH2(CH2)nN(CH3)2, CH2(CH2)nSO3R5, C1-8 알킬, C1-8 알킬티오, C1-8 알킬아미노, 및 C1-8 알킬옥실로 이루어진 군에서 선택되고; X 2 is None, H, CH 2 (CH 2 ) n OR 8 , Cl, F, Br, I, NO 2 , CN, CF 3 , C 2 F 5 , C 3 F 7 , OCF 3 , OC 2 F 5 , NH 2 , NHR 6 , CH 3 , C 2 H 5 , R 6 , C (= O) NH 2 , CH 2 OC (= O) NH 2 , CH 2 C (= O) OR 5 , CH 2 (CH 2 ) n N (CH 3 ) 2 , CH 2 (CH 2 ) n SO 3 R 5 , C 1-8 alkyl, C 1-8 alkylthio, C 1-8 alkylamino, and C 1-8 alkyloxyl Selected from the group consisting of;
X3은 없음, H, N3, SO3W, F, Cl, Br, OH, OCH3, OR6, CH3, R6, C(=O)OW, OW, 및 I로 이루어진 군에서 선택되고;X 3 is none, selected from the group consisting of H, N 3 , SO 3 W, F, Cl, Br, OH, OCH 3 , OR 6 , CH 3 , R 6 , C (= O) OW, OW, and I Become;
X4는 없음, N, CH, 및 CY1로 이루어진 군에서 선택되고; X 4 is selected from the group consisting of none, N, CH, and CY 1 ;
X5 및 X35은 독립적으로 없음, C(=O), C(=S), OC(=O), CH2, CH, S, O 및 NR5로 이루어진 군에서 선택되고;X 5 and X 35 are independently selected from the group consisting of none, C (= O), C (= S), OC (= O), CH 2 , CH, S, O and NR 5 ;
각각의 Y1, Y31, Y2, Y32, Y3, 및 Y4는 독립적으로 H, OH, OW, OC(=O)W, OC(=O)CH3, CH3, C2H5, C3H7, C4H9,SO3R6, CH2OR6, CH2OC(=O)R6, CH2C(=O)OR8, OCH3, OC2H5, CH3SO2, R6SO2,R6SO3OR6, CH3SO3, R6SO3, NO2, CN, CF3, OCF3, CH=CHC(=O)NHCH2C(=O)OW, CH2(CH2)nNR5R6, CH2(CH2)nOR6 , CH(C(=O)NH2)NHR6, CH2C(=O)NH2 , F, Br, I, 및 Cl로 이루어진 군에서 선택되고; Each Y 1 , Y 31 , Y 2 , Y 32 , Y 3 , and Y 4 is independently H, OH, OW, OC (= O) W, OC (= O) CH 3 , CH 3 , C 2 H 5 , C 3 H 7 , C 4 H 9 , SO 3 R 6 , CH 2 OR 6 , CH 2 OC (= O) R 6 , CH 2 C (= O) OR 8 , OCH 3 , OC 2 H 5 , CH 3 SO 2 , R 6 SO 2 , R 6 SO 3 OR 6 , CH 3 SO 3 , R 6 SO 3 , NO 2 , CN, CF 3 , OCF 3 , CH = CHC (= O) NHCH 2 C (= O) OW, CH 2 (CH 2 ) n NR 5 R 6 , CH 2 (CH 2 ) n OR 6 , CH (C (= O) NH 2 ) NHR 6 , CH 2 C (= O) NH 2 , F , Br, I, and Cl;
Z, AA, HA, R, Rs, Y, R11-R16, X, L1, L2, L4, L31, L32, L34 및 W는 상기에서 정의된 바와 같고;Z, AA, HA, R, R s , Y, R 11 -R 16 , X, L 1 , L 2 , L 4 , L 31 , L 32 , L 34 and W are as defined above;
임의의 CH2 기들은 O, S, NR6 또는 임의의 기타 약학적으로 허용되는 기들로 치환될 수 있다.Any CH 2 groups can be substituted with O, S, NR 6 or any other pharmaceutically acceptable group.
본원에 사용되는, 용어 "약학적으로 허용되는 염"은 대상체에서 적용하기에 안전한 본 발명 화합물의 염을 의미한다. 약학적으로 허용되는 염은 본 발명 화합물에 존재하는 산성 또는 염기성 기의 염을 포함한다. 약학적으로 허용되는 산 부가 염은 염산염, 브롬화수소산염, 요오드화수소산염, 질산염, 황산염, 비설페이트, 포스페이트, 산 포스페이트, 이소니코티네이트, 아세테이트, 락테이트, 살리실레이트, 시트레이트, 타르트레이트, 판토테네이트, 비타르트레이트, 아스코르베이트, 석시네이트, 말레에이트, 겐티시네이트, 푸마레이트, 글루코네이트, 글루카로네이트, 사카레이트, 포르메이트, 벤조에이트, 글루타메이트, 메탄설포네이트, 에탄설포네이트, 벤젠설포네이트, p-톨루엔설포네이트 및 파모에이트 (즉, 1,11-메틸렌-비스-(2-히드록시-3-나프토에이트)) 염을 포함하나, 이에 한정되지 않는다. 본 발명의 특정 화합물은 각종 아미노산과의 약학적으로 허용되는 염을 형성할 수 있다. 적합한 염기 염은 알루미늄, 칼슘, 리튬, 마그네슘, 칼륨, 나트륨, 아연 및 디에탄올아민 염을 포함하나, 이에 한정되지 않는다. 약학적으로 허용되는 염에 대한 고찰을 위해서는 본원에 참조로 포함된 문헌 [BERGE ET AL., 66 J. PHARM. SCI. 1 - 19 (1977)]을 참조한다.As used herein, the term “pharmaceutically acceptable salt” refers to salts of the compounds of the present invention that are safe for application in a subject. Pharmaceutically acceptable salts include salts of acidic or basic groups present in the compounds of the invention. Pharmaceutically acceptable acid addition salts include hydrochloride, hydrobromide, hydroiodide, nitrate, sulfate, bisulfate, phosphate, acid phosphate, isonicotinate, acetate, lactate, salicylate, citrate, tartrate , Pantothenate, bitartrate, ascorbate, succinate, maleate, gentinate, fumarate, gluconate, glucaronate, saccharide, formate, benzoate, glutamate, methanesulfonate, ethanesulfo Nate, benzenesulfonate, p-toluenesulfonate and famoate (ie, 1,11-methylene-bis- (2-hydroxy-3-naphthoate)) salts. Certain compounds of the present invention can form pharmaceutically acceptable salts with various amino acids. Suitable base salts include, but are not limited to, aluminum, calcium, lithium, magnesium, potassium, sodium, zinc and diethanolamine salts. For a review of pharmaceutically acceptable salts, see BERGE ET AL., 66 J. PHARM. SCI. 1-19 (1977).
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알킬"은 포화된 알킬 기, 알케닐 기 및 알키닐 기를 포함하는, 분지되거나 분지되지 않은, 포화되거나 비포화된, 1가 또는 다가 탄화수소 기를 의미한다. 알킬의 예는 메틸, 에틸, 프로필, 이소프로필, 부틸, 이소부틸, t-부틸, 펜틸, 헥실, 헵틸, 옥틸, 노닐, 데실, 운데실, 도데실, 에테닐, 프로페닐, 부테닐, 이소부테닐, 펜테닐, 헥세닐, 헵테닐, 옥테닐, 노네닐, 데케닐, 운데세닐, 도데세닐, 에티닐, 프로피닐, 부티닐, 이소부티닐, 펜티닐, 헥시닐, 헵티닐, 옥티닐, 노니닐, 데시닐, 운데시닐, 도데시닐, 메틸렌, 에틸렌, 프로필렌, 이소프로필렌, 부틸렌, 이소부틸렌, t-부틸렌, 펜틸렌, 헥실렌, 헵틸렌, 옥틸렌, 노닐렌, 데실렌, 운데실렌 및 도데실렌을 포함하나, 이에 한정되지 않는다. 특정 양태에서, 탄화수소 기는, 탄소수가 1 내지 30이다. 특정 양태에서, 탄화수소 기는, 탄소수가 1 내지 20이다. 특정 양태에서, 탄화수소 기는, 탄소수가 1 내지 12이다.As used herein, unless specified otherwise, the term "alkyl" refers to a branched or unbranched, saturated or unsaturated, monovalent or polyvalent hydrocarbon group, including saturated alkyl groups, alkenyl groups and alkynyl groups. do. Examples of alkyl are methyl, ethyl, propyl, isopropyl, butyl, isobutyl, t-butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, ethenyl, propenyl, butenyl, isobu Tenyl, Fentenyl, Hexenyl, Heptenyl, Octenyl, Nonenyl, Decenyl, Undecenyl, Dodecenyl, Ethynyl, Propynyl, Butynyl, Isobutynyl, Fentynyl, Hexynyl, Heptynyl, Octynyl , Noninyl, desinyl, undecynyl, dodecinyl, methylene, ethylene, propylene, isopropylene, butylene, isobutylene, t-butylene, pentylene, hexylene, heptylene, octylene, nonylene , Dexylene, undecylene, and dodecylene. In certain embodiments, the hydrocarbon group has 1 to 30 carbon atoms. In certain embodiments, the hydrocarbon group has 1 to 20 carbon atoms. In certain embodiments, the hydrocarbon group has 1 to 12 carbon atoms.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "시클로알킬"은 적어도 하나의 환을 함유하고 방향족 환이 없는 알킬을 의미한다. 시클로알킬의 예는 시클로프로필, 시클로부틸, 시클로펜틸, 시클로헥실, 시클로헵틸, 시클로옥틸, 시클로노닐, 시클로데실, 시클로운데실 및 시클로도데실을 포함하나, 이에 한정되지 않는다. 특정 양태에서, 탄화수소 사슬은, 탄소수가 1 내지 30이다. 특정 양태에서, 탄화수소 기는, 탄소수가 1 내지 20이다. 특정 양태에서, 탄화수소 기는, 탄소수가 1 내지 12이다.As used herein, unless specified otherwise, the term "cycloalkyl" means alkyl containing at least one ring and free of aromatic rings. Examples of cycloalkyl include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclodecyl, cycloundecyl and cyclododecyl. In a specific embodiment, the hydrocarbon chain has 1 to 30 carbon atoms. In certain embodiments, the hydrocarbon group has 1 to 20 carbon atoms. In certain embodiments, the hydrocarbon group has 1 to 12 carbon atoms.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "헤테로시클로알킬"은, 적어도 하나의 환 원자가 비-탄소 원자인 시클로알킬을 의미한다. 비-탄소 환 원자의 예는 S, O 및 N을 포함하나, 이에 한정되지 않는다. As used herein, unless specified otherwise, the term “heterocycloalkyl” refers to cycloalkyl wherein at least one ring atom is a non-carbon atom. Examples of non-carbon ring atoms include, but are not limited to, S, O and N.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알콕실"은 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미하며, 이는 하나 이상의 산소 원자를 포함한다. 알콕실의 예는 -CH2-OH, -OCH3, -O-Re, -Re-OH, Re1-O-Re2-을 포함하지만, 이에 한정되지 않으며, 여기에서 Re, Re1 및 Re2는동일하거나 상이한 알킬, 시클로알킬 또는 헤테로시클로알킬일 수 있다As used herein, unless specified otherwise, the term “alkoxyl” refers to alkyl, cycloalkyl or heterocycloalkyl, which includes one or more oxygen atoms. Examples of alkoxyl include, but are not limited to, -CH 2 -OH, -OCH 3 , -OR e , -R e -OH, R e1 -OR e2- , where R e , R e1 And R e2 can be the same or different alkyl, cycloalkyl or heterocycloalkyl.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알킬 할라이드"는, 동일하거나 상이할 수 있는 하나 이상의 할로겐 원자를 함유하는 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미한다. 용어 "할로겐"은 불소, 염소, 브롬 또는 요오드를 의미한다. 알킬 할라이드의 예는 제한적이지 않지만 -Re-F, -Re-Cl, -Re-Br, -Re-I, -Re(F)-, -Re(Cl)-, -Re(Br)- 및 -Re(I)-을 포함하고, 여기에서 Re는 알킬, 시클로알킬 또는 헤테로시클로알킬이다.As used herein, unless specified otherwise, the term "alkyl halide" means alkyl, cycloalkyl or heterocycloalkyl containing one or more halogen atoms, which may be the same or different. The term "halogen" means fluorine, chlorine, bromine or iodine. Examples of the alkyl halide are not limited, but -R e -F, -R e -Cl, -R e -Br, -R e -I, -R e (F)-, -R e (Cl)-, -R e (Br)-and -R e (I)-, wherein R e is alkyl, cycloalkyl or heterocycloalkyl.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알킬티오"는, 하나 이상의 황 원자를 함유하는 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미한다. 알킬티오의 예는 제한적이지 않지만 -CH2-SH, -SCH3, -S-Re, -Re-SH, -Re1-S-Re2-을 포함하고, 여기에서 Re, Re1 및 Re2는 동일하거나 상이한 알킬, 시클로알킬 또는 헤테로시클로알킬이다.As used herein, unless specified otherwise, the term "alkylthio" means alkyl, cycloalkyl or heterocycloalkyl containing one or more sulfur atoms. Examples of alkylthio are not limited, but -CH 2 -SH, -SCH 3, -SR e, -R e -SH, -R e1 -SR e2 - , and where R e, R e1 include And R e2 is the same or different alkyl, cycloalkyl or heterocycloalkyl.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알킬아미노"는, 하나 이상의 질소 원자를 함유하는 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미한다. 알킬아미노의 예는 제한적이지 않지만 -CH2-NH, -NCH3, -N(Re1)-Re2, -N-Re, -Re-NH2, -Re1-N-Re2 및 -Re-N(Re1)-Re2 을 포함하고, 여기에서 Re, Re1 및 Re2는 동일하거나 상이한 알킬, 시클로알킬 또는 헤테로시클로알킬이다.As used herein, unless specified otherwise, the term "alkylamino" means alkyl, cycloalkyl or heterocycloalkyl containing one or more nitrogen atoms. Examples of alkylamino are not limited, but -CH 2 -NH, -NCH 3 , -N (R e1 ) -R e2 , -NR e , -R e -NH 2 , -R e1 -NR e2 and -R e- N (R e1 ) -R e2 , where R e , R e1 And R e2 is the same or different alkyl, cycloalkyl or heterocycloalkyl.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "알킬카보닐"은, 하나 이상의 카보닐 기를 함유하는 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미한다. 알킬카보닐 기의 예는 제한되지는 않지만 알데하이드 기(-Re-C(O)-H), 케톤기(-Re-C(O)-Re1), 카복실산 기(Re-C(=O)OH), 에스테르 기(-Re-C(=O)O-Re1), 카복사미드 (-Re-C(=O)O-N(Re1)Re2), 에논 기(-Re-C(O)-C(Re1)=C(Re2)Re3), 아실 할라이드 기(-Re-C(O)-Xh) 및 산 무수물 기(-Re-C(O)-O-C(O)-Re1)를 포함하고, 여기서, Re, Re1 , Re2 및 Re3은 동일하거나 상이한 알킬, 시클로알킬, 또는 헤테로시클로알킬이고; Xh는 할로겐이다.As used herein, unless specified otherwise, the term "alkylcarbonyl" means alkyl, cycloalkyl or heterocycloalkyl containing one or more carbonyl groups. Examples of the alkylcarbonyl group are not limited, but aldehyde groups (-R e -C (O) -H), ketone groups (-R e -C (O) -R e1 ), carboxylic acid groups (R e -C ( = O) OH), ester group (-R e -C (= O) OR e1 ), carboxamide (-R e -C (= O) ON (R e1 ) R e2 ), enone group (-R e -C (O) -C (R e1 ) = C (R e2 ) R e3 ), acyl halide group (-R e -C (O) -X h ) and acid anhydride group (-R e -C (O) -OC (O) -R e1 ), where R e , R e1 , R e2 And R e3 is the same or different alkyl, cycloalkyl, or heterocycloalkyl; X h is halogen.
본원에 사용되는, 달리 명시하지 않는 한, 용어 "퍼플루오로알킬"은, 하나 이상의 플루오로 기를 함유하는 알킬, 시클로알킬 또는 헤테로시클로알킬을 의미하며, 퍼플루오로메틸, 퍼플루오로에틸, 퍼플루오로프로필을 포함하나, 이에 한정되지 않는다. As used herein, unless specified otherwise, the term “perfluoroalkyl” means alkyl, cycloalkyl or heterocycloalkyl containing one or more fluoro groups, perfluoromethyl, perfluoroethyl, purple Luoropropyl, but is not limited thereto.
본원에 사용되는, 달리 명시하지 않는 한, 용어 '아릴"은 하나 이상의 방향족 환을 포함하는 화학 구조를 의미한다. 특정 양태에서, 환 원자는 모두 탄소이다. 특정 양태에서, 하나 이상의 환 원자는 비-탄소, 예를 들면, 산소, 질소 또는 황 ("헤테로아릴")이다. 아릴의 예는 페닐, 벤질, 나프탈레닐, 안트라세닐, 피리딜, 퀴노일, 이소퀴노일, 피라지닐, 퀴녹살리닐, 아크리디닐, 피리미디닐, 퀴나졸리닐, 피리다지닐, 신놀리닐, 이미다졸릴, 벤즈이미다졸릴, 푸리닐, 인돌릴, 푸라닐, 벤조푸라닐, 이소벤조푸라닐, 피롤릴, 인돌릴, 이소인돌릴, 티오페닐, 벤조티오페닐, 피라졸릴, 인다졸릴, 옥사졸릴, 벤족사졸릴, 이속사졸릴, 벤즈이속사졸릴, 티악솔릴, 구아니디노 및 벤조티아졸릴을 포함하나, 이에 한정되지 않는다.As used herein, unless specified otherwise, the term “aryl” refers to a chemical structure comprising one or more aromatic rings. In certain embodiments, all ring atoms are carbon. In certain embodiments, one or more ring atoms are non- -Carbon, eg oxygen, nitrogen or sulfur ("heteroaryl") Examples of aryl are phenyl, benzyl, naphthalenyl, anthracenyl, pyridyl, quinoyl, isoquinoyl, pyrazinyl, quinoxaly Neil, acridinyl, pyrimidinyl, quinazolinyl, pyridazinyl, cynolinyl, imidazolyl, benzimidazolyl, furinyl, indolyl, furanyl, benzofuranyl, isobenzofuranyl, pi Rollyl, indolyl, isoindolyl, thiophenyl, benzothiophenyl, pyrazolyl, indazolyl, oxazolyl, benzoxazolyl, isoxazolyl, benzisoxazolyl, thiaxolyl, guanidino and benzothiazolyl , But is not limited to this.
특정 양태에서, HPP의 이송 단위는 HPP를 하나 이상의 생체 장벽을 통해 이송하거나 횡단하는 것을 촉진 (예를 들면, 모 약물보다 > 약 20배, > 약 50배, > 약 100배, > 약 300배, > 약 500배, > 약 1,000배, > 약 10,000배 더 빠르게)할 수 있는 양성자성 아민기를 포함한다. 특정 양태에서, 양성자성 아민기는 실질적으로 생리학적 pH에서 양성자화된다. 특정 양태에서, 아민기는 가역적으로 양성자화되고 탈양성자화될 수 있다. 특정 양태에서, 최소한 하나의 자유 아미노기를 가지는 항균제 및 항균제-관련 화합물들에 있어서 관능성 단위는 또한 하나 이상의 이송 가능한 단위들을 함유할 수 있다.In certain embodiments, the transport unit of HPP facilitates transport or crossing of HPP through one or more bio-barriers (e.g.,> about 20 times,> about 50 times,> about 100 times,> about 300 times over the parent drug) ,> About 500 times,> about 1,000 times,> about 10,000 times faster). In certain embodiments, protic amine groups are protonated at substantially physiological pH. In certain embodiments, amine groups are reversibly protonated and deprotonated. In certain embodiments, functional units for antimicrobial and antimicrobial agent-related compounds having at least one free amino group can also contain one or more transportable units.
특정 양태에서, 양성자성 아민기는 약학적으로 허용되는 치환된 및 비치환된 1급 아민기, 약학적으로 허용되는 치환된 및 비치환된 2급 아민기, 및 약학적으로 허용되는 치환된 및 비치환된 3급 아민기로 이루어진 군으로부터 선택된다.In certain embodiments, protic amine groups are pharmaceutically acceptable substituted and unsubstituted primary amine groups, pharmaceutically acceptable substituted and unsubstituted secondary amine groups, and pharmaceutically acceptable substituted and unsubstituted It is selected from the group consisting of a substituted tertiary amine group.
특정 양태들에서, 양성자화 아민기은 입체이성질체 및 약학적으로 허용되는 염을 포함한 상기에서 정의된 약학적으로 허용되는 치환된 및 비치환된 1급 아민기, 화학식 W-1, 화학식 W-2, 화학식 W-3, 화학식 W-4, 화학식 W-5, 화학식 W-6, 화학식 W-7, 화학식 W-8, 화학식 W-9, 화학식 W-10, 화학식 W-11, 화학식 W-12, 화학식 W-13, 화학식 W-14, 화학식 W-15, 화학식 W-16, 화학식 W-17 및 화학식 W-18로 이루어진 군에서 선택된다.In certain embodiments, the protonated amine group is a pharmaceutically acceptable substituted and unsubstituted primary amine group as defined above, including the stereoisomers and pharmaceutically acceptable salts, Formula W-1, Formula W-2, Chemical Formula W-3, Chemical Formula W-4, Chemical Formula W-5, Chemical Formula W-6, Chemical Formula W-7, Chemical Formula W-8, Chemical Formula W-9, Chemical Formula W-10, Chemical Formula W-11, Chemical Formula W-12, Formula W-13, Formula W-14, Formula W-15, Formula W-16, Formula W-17, and Formula W-18.
특정 양태에서, HPP 의 관능성 단위 및 이송 단위를 공유결합으로 연결하는 링커는, HPP가 하나 이상의 BB를 통과한 후 절단될 수 있는 결합을 포함한다. 절단가능한 결합은 예를 들면 공유 결합, 에테르, 티오에테르, 아미드, 에스테르, 티오에스테르, 카보네이트, 카바메이트, 포스페이트 또는 옥심 결합을 포함한다. In certain embodiments, linkers that link the functional and transport units of HPP covalently include a bond that can be cleaved after HPP has passed through one or more BBs. Cleavable bonds include, for example, covalent bonds, ether, thioether, amide, ester, thioester, carbonate, carbamate, phosphate or oxime bonds.
특정 양태들에서, 항균제 및 항균제-관련 화합물의 HPP는 다음 화학식 L-1 및 이의 입체이성질체 및 약학적으로 허용되는 염을 가지고:In certain embodiments, the HPP of the antimicrobial agent and the antimicrobial agent-related compound has the formula L-1 and stereoisomers thereof and pharmaceutically acceptable salts thereof:
화학식 L-1Formula L-1
여기에서:From here:
F는 항균제 또는 항균제-관련 화합물의 HPP의 관능성 단위이다. F의 예로는 상기된 바와 같은 화학식 F-1, 화학식 FP-1, 화학식 FP-2, 화학식 FP-3, 화학식 FP-4, 화학식 FP-5, 화학식 FP-6, 화학식 FP-7, 화학식 FP-8, 화학식 FP-9, 화학식 FP-10, 화학식 FP-11, 화학식 FP-12, 화학식 FP-13, 화학식 FP-14, 화학식 FP-15, 화학식 FP-16, 화학식 FP-17, 화학식 FP-18, 화학식 FP-19, 화학식 FP-20, 화학식 FP-21, 화학식 FP-22, 화학식 FP-23, 화학식 FP-24, 화학식 FP-25, 화학식 FP-26, 화학식 FP-27, 화학식 FP-28, 화학식 FP-29, 화학식 FP-30, 화학식 FP-31, 화학식 FP-32, 화학식 FP-33, 화학식 FP-34, 화학식 FP-35, 화학식 FP-36, 화학식 FP-37, 화학식 FP-38, 화학식 FP-39, 화학식 FP-40, 화학식 FP-41, 화학식 FP-42, 화학식 FP-43, 화학식 FP-44, 화학식 FP-45, 화학식 FP-46, 화학식 FP-47, 화학식 FP-48, 화학식 FP-49, 화학식 FP-50, 화학식 FP-51, 화학식 FP-52, 화학식 FP-53, 화학식 FP-54, 화학식 FP-55, 화학식 FP-56, 화학식 FP-57, 화학식 FP-58, 화학식 FP-59, 화학식 FP-60, 화학식 FP-61, 화학식 FP-62, 화학식 FP-63, 화학식 FP-64, 화학식 FP-65, 화학식 FP-66, 화학식 FP-67, 화학식 FP-68, 화학식 FP-69, 화학식 FP-70, 화학식 FP-71, 화학식 FP-72, 화학식 FP-73, 화학식 FP-74, 화학식 FP-75, 화학식 FP-76, 화학식 FP-77, 화학식 FP-78, 화학식 FP-79, 화학식 FP-80, 화학식 FP-81, 화학식 FP-82, 화학식 FP-83, 화학식 FP-84, 화학식 FP-85, 화학식 FP-86, 화학식 FI-1, 화학식 FI-2, 화학식 FI-3, 화학식 FI-4, 화학식 FI-5, 화학식 FI-6, 화학식 FI-7, 화학식 FI-8, 화학식 FI-9, 화학식 FI-10, 화학식 FI-11, 화학식 FI-12, 화학식 FI-13, 화학식 FI-14, 화학식 FI-15, 화학식 FI-16, 화학식 FI-17, 화학식 FI-18, 화학식 FI-19, 화학식 FI-20, 화학식 FI-21, 화학식 FI-22, 화학식 FI-23, 화학식 FI-24, 화학식 FI-25, 화학식 FI-26, 화학식 FI-27, 화학식 FI-28, 화학식 FI-29, 화학식 FI-30, 화학식 FI-31, 화학식 FI-32, 화학식 FI-33, 화학식 FS-1, 화학식 FS-2, 화학식 FS-3, 화학식 FS-4, 화학식 FS-5, 화학식 FS-6, 화학식 FS-7, 화학식 FS-8, 화학식 FS-9, 화학식 FS-10, 화학식 FS-11, 화학식 FS-12, 화학식 FS-13, 화학식 FS-14, 화학식 FS-15, 화학식 FS-16, 화학식 FS-17, 화학식 FS-18, 화학식 FS-19, 화학식 FS-20, 화학식 FT-1, 화학식 FT-2, 화학식 FT-3, 화학식 FT-4, 화학식 FT-5, 화학식 FT-6, 화학식 FT-7, 화학식 FT-8, 화학식 FT-9, 화학식 FT-10, 화학식 FT-11, 화학식 FT-12, 화학식 FT-13, 화학식 FT-14, 화학식 FT-15, 및 화학식 FT-16을 포함하고; F is a functional unit of HPP of an antimicrobial agent or an antimicrobial agent-related compound. Examples of F are Formula F-1, Formula FP-1, Formula FP-2, Formula FP-3, Formula FP-4, Formula FP-5, Formula FP-6, Formula FP-7, Formula FP as described above. -8, Chemical Formula FP-9, Chemical Formula FP-10, Chemical Formula FP-11, Chemical Formula FP-12, Chemical Formula FP-13, Chemical Formula FP-14, Chemical Formula FP-15, Chemical Formula FP-16, Chemical Formula FP-17, Chemical Formula FP -18, Chemical Formula FP-19, Chemical Formula FP-20, Chemical Formula FP-21, Chemical Formula FP-22, Chemical Formula FP-23, Chemical Formula FP-24, Chemical Formula FP-25, Chemical Formula FP-26, Chemical Formula FP-27, Chemical Formula FP -28, Chemical Formula FP-29, Chemical Formula FP-30, Chemical Formula FP-31, Chemical Formula FP-32, Chemical Formula FP-33, Chemical Formula FP-34, Chemical Formula FP-35, Chemical Formula FP-36, Chemical Formula FP-37, Chemical Formula FP -38, Chemical Formula FP-39, Chemical Formula FP-40, Chemical Formula FP-41, Chemical Formula FP-42, Chemical Formula FP-43, Chemical Formula FP-44, Chemical Formula FP-45, Chemical Formula FP-46, Chemical Formula FP-47, Chemical Formula FP -48, Chemical Formula FP-49, Chemical Formula FP-50, Chemical Formula FP-51, Chemical Formula FP-52, Chemical Formula FP-53, Chemical Formula FP-54, Chemical Formula FP-55, Chemical Formula FP-56, Chemical Formula FP-57, Formula FP-58, Formula FP-59, Formula FP-60, Formula FP-61, Formula FP-62, Formula FP-63, Formula FP-64, Formula FP-65, Formula FP-66, Formula FP-67, Formula FP-68, Formula FP-69, Formula FP-70, Formula FP-71, Formula FP-72, Formula FP-73, Formula FP-74, Formula FP-75, Formula FP-76, Formula FP-77, Formula FP-78, Formula FP-79, Formula FP-80, Formula FP-81, Formula FP-82, Formula FP-83, Formula FP-84, Formula FP-85, Formula FP-86, Formula FI-1, Formula FI-2, Formula FI-3, Formula FI-4, Formula FI-5, Formula FI-6, Formula FI-7, Formula FI-8, Formula FI-9, Formula FI-10, Formula FI-11, Formula FI-12, Formula FI-13, Formula FI-14, Formula FI-15, Formula FI-16, Formula FI-17, Formula FI-18, Formula FI-19, Formula FI-20, Chemical Formula FI-21, Chemical Formula FI-22, Chemical Formula FI-23, Chemical Formula FI-24, Chemical Formula FI-25, Chemical Formula FI-26, Chemical Formula FI-27, Chemical Formula FI-28, Chemical Formula FI-29, Chemical Formula FI-30, Formula FI-31, Formula FI-32, Formula FI-33, Formula FS-1, Formula FS-2, Formula FS-3, Formula FS-4, Formula FS-5, Formula FS-6, Formula FS-7, Formula FS-8, Formula FS-9, Formula FS-10, Formula FS-11, Formula FS-12, Formula FS-13, Formula FS-14, Formula FS-15, Formula FS-16, Formula FS-17, Chemical Formula FS-18, Chemical Formula FS-19, Chemical Formula FS-20, Chemical Formula FT-1, Chemical Formula FT-2, Chemical Formula FT-3, Chemical Formula FT-4, Chemical Formula FT-5, Chemical Formula FT-6, Chemical Formula FT-7, FT-8, FT-9, FT-10, FT-11, FT-12, FT-13, FT-14, FT-15, and FT-16 Contains;
T는 항균제 또는 항균제-관련 화합물의 HPP의 이송 단위이다. 예를 들면, T는 양성자화 아민기, 약학적으로 허용되는 치환된 및 비치환된 1급 아민기, 약학적으로 허용되는 치환된 및 비치환된 2급 아민기, 및 약학적으로 허용되는 치환된 및 비치환된 3급 아민기, 상기에서 정의된 화학식 W-1, 화학식 W-2, 화학식 W-3, 화학식 W-4, 화학식 W-5, 화학식 W-6, 화학식 W-7, 화학식 W-8, 화학식 W-9, 화학식 W-10, 화학식 W-11, 화학식 W-12, 화학식 W-13, 화학식 W-14, 화학식 W-15, 화학식 W-16, 화학식 W-17 및 화학식 W-18로 이루어진 군에서 선택되고;T is the transport unit of the HPP of the antimicrobial agent or antimicrobial agent-related compound. For example, T is a protonated amine group, a pharmaceutically acceptable substituted and unsubstituted primary amine group, a pharmaceutically acceptable substituted and unsubstituted secondary amine group, and a pharmaceutically acceptable substitution. And unsubstituted tertiary amine groups, Formula W-1, Formula W-2, Formula W-3, Formula W-4, Formula W-5, Formula W-6, Formula W-7, Formula defined above. W-8, Formula W-9, Formula W-10, Formula W-11, Formula W-12, Formula W-13, Formula W-14, Formula W-15, Formula W-16, Formula W-17 and Formula W-18;
L1, L31, L2, L32, L4, 및 L34은 상기에서 정의된 바와 같고, 특정 양태들에서, -L1-L4-L2- 및 -L31-L34-L32-은 독립적으로 -O-, -X-, -O-X-, -N-X-, -S-X-, -X5-, -O-X5-, -N-X5-, -S-X5-, -O-X7-, -O-C(=O)-, -NH-C(=O)-, -C(=O)-, -C(=O)-O-, -C(=O)-N-, 및 C(=O)-X-로 이루어진 군에서 선택되고 여기에서 X, X5 및 X7은 상기에서 정의된다. L 1 , L 31 , L 2 , L 32 , L 4 , and L 34 are as defined above, and in certain embodiments, -L 1 -L 4 -L 2 -and -L 31 -L 34 -L 32 -is independently -O-, -X-, -OX-, -NX-, -SX-, -X 5- , -OX 5- , -NX 5- , -SX 5- , -OX 7- , -OC (= O)-, -NH-C (= O)-, -C (= O)-, -C (= O) -O-, -C (= O) -N-, and C (= O) -X-, wherein X, X 5 and X 7 are defined above.
특정 양태들에서, 항균제 또는 항균제-관련 화합물의 HPP 또는 HPC는 입체이성질체 및 약학적으로 허용되는 염을 포함한 화학식 P-1, 화학식 P-2, 화학식 P-3, 화학식 P-4, 화학식 P-5, 화학식 P-6, 화학식 P-7, 화학식 P-8, 화학식 P-9, 화학식 P-10, 화학식 P-11, 화학식 P-12, 화학식 P-13, 화학식 P-14, 화학식 P-15, 화학식 P-16, 화학식 P-17, 화학식 P-18, 화학식 P-19, 화학식 P-20, 화학식 P-21, 화학식 P-22, 화학식 P-23, 화학식 P-24, 화학식 P-25, 화학식 P-26, 화학식 P-27, 화학식 P-28, 화학식 P-29, 화학식 P-30, 화학식 P-31, 화학식 P-32, 화학식 P-33, 화학식 P-34, 화학식 P-35, 화학식 P-36, 화학식 P-37, 화학식 P-38, 화학식 P-39, 화학식 P-40, 화학식 P-41, 화학식 P-42, 화학식 P-43, 화학식 P-44, 화학식 P-45, 화학식 P-46, 화학식 P-47, 화학식 P-48, 화학식 P-49, 화학식 P-50, 화학식 P-51, 화학식 P-52, 화학식 P-53, 화학식 P-54, 화학식 P-55, 화학식 P-56, 화학식 P-57, 화학식 P-58, 화학식 P-59, 화학식 P-60, 화학식 P-61, 화학식 P-62, 화학식 P-63, 화학식 P-64, 화학식 P-65, 화학식 P-66, 화학식 P-67, 화학식 P-68, 화학식 P-69, 화학식 P-70, 화학식 P-71, 화학식 P-72, 화학식 P-73, 화학식 P-74, 화학식 P-75, 화학식 P-76, 화학식 P-77, 화학식 P-78, 화학식 P-79, 화학식 P-80, 화학식 P-81, 화학식 P-82, 화학식 P-83, 화학식 P-84, 화학식 P-85, 화학식 P-86, 화학식 I-1, 화학식 I-2, 화학식 I-3, 화학식 I-4, 화학식 I-5, 화학식 I-6, 화학식 I-7, 화학식 I-8, 화학식 I-9, 화학식 I-10, 화학식 I-11, 화학식 I-12, 화학식 I-13, 화학식 I-14, 화학식 I-15, 화학식 I-16, 화학식 I-17, 화학식 I-18, 화학식 I-19, 화학식 I-20, 화학식 I-21, 화학식 I-22, 화학식 I-23, 화학식 I-24, 화학식 I-25, 화학식 I-26, 화학식 I-27, 화학식 I-28, 화학식 I-29, 화학식 I-30, 화학식 I-31, 화학식 I-32, 화학식 I-33, 화학식 S-1, 화학식 S-2, 화학식 S-3, 화학식 S-4, 화학식 S-5, 화학식 S-6, 화학식 S-7, 화학식 S-8, 화학식 S-9, 화학식 S-10, 화학식 S-11, 화학식 S-12, 화학식 S-13, 화학식 S-14, 화학식 S-15, 화학식 S-16, 화학식 S-17, 화학식 S-18, 화학식 S-19, 화학식 S-20, 화학식 T-1, 화학식 T-2, 화학식 T-3, 화학식 T-4, 화학식 T-5, 화학식 T-6, 화학식 T-7, 화학식 T-8, 화학식 T-9, 화학식 T-10, 화학식 T-11, 화학식 T-12, 화학식 T-13, 화학식 T-14, 화학식 T-15, 및 화학식 T-16으로 이루어진 군에서 선택된 구조를 포함하고:In certain embodiments, the HPP or HPC of the antimicrobial agent or antimicrobial agent-related compound comprises Formula P-1, Formula P-2, Formula P-3, Formula P-4, Formula P-, including stereoisomers and pharmaceutically acceptable salts. 5, Chemical Formula P-6, Chemical Formula P-7, Chemical Formula P-8, Chemical Formula P-9, Chemical Formula P-10, Chemical Formula P-11, Chemical Formula P-12, Chemical Formula P-13, Chemical Formula P-14, Chemical Formula P- 15, Chemical Formula P-16, Chemical Formula P-17, Chemical Formula P-18, Chemical Formula P-19, Chemical Formula P-20, Chemical Formula P-21, Chemical Formula P-22, Chemical Formula P-23, Chemical Formula P-24, Chemical Formula P- 25, Chemical Formula P-26, Chemical Formula P-27, Chemical Formula P-28, Chemical Formula P-29, Chemical Formula P-30, Chemical Formula P-31, Chemical Formula P-32, Chemical Formula P-33, Chemical Formula P-34, Chemical Formula P- 35, Chemical Formula P-36, Chemical Formula P-37, Chemical Formula P-38, Chemical Formula P-39, Chemical Formula P-40, Chemical Formula P-41, Chemical Formula P-42, Chemical Formula P-43, Chemical Formula P-44, Chemical Formula P- 45, Chemical Formula P-46, Chemical Formula P-47, Chemical Formula P-48, Chemical Formula P-49, Chemical Formula P-50, Chemical Formula P-51, Chemical Formula P-52, Chemical Formula P-53, Chemical Formula P-54, Formula P-55, Formula P-56, Formula P-57, Formula P-58, Formula P-59, Formula P-60, Formula P-61, Formula P-62, Formula P-63, Chemical Formula P-64, Chemical Formula P-65, Chemical Formula P-66, Chemical Formula P-67, Chemical Formula P-68, Chemical Formula P-69, Chemical Formula P-70, Chemical Formula P-71, Chemical Formula P-72, Chemical Formula P-73, Chemical Formula P-74, Chemical Formula P-75, Chemical Formula P-76, Chemical Formula P-77, Chemical Formula P-78, Chemical Formula P-79, Chemical Formula P-80, Chemical Formula P-81, Chemical Formula P-82, Chemical Formula P-83, Formula P-84, Formula P-85, Formula P-86, Formula I-1, Formula I-2, Formula I-3, Formula I-4, Formula I-5, Formula I-6, Formula I-7, Formula I-8, Formula I-9, Formula I-10, Formula I-11, Formula I-12, Formula I-13, Formula I-14, Formula I-15, Formula I-16, Formula I-17, Formula I-18, Formula I-19, Formula I-20, Formula I-21, Formula I-22, Formula I-23, Formula I-24, Formula I-25, Formula I-26, Formula I-27, Formula I-28, Formula I-29, Formula I-30, Tu Formula I-31, Formula I-32, Formula I-33, Formula S-1, Formula S-2, Formula S-3, Formula S-4, Formula S-5, Formula S-6, Formula S-7, Formula S-8, Formula S-9, Formula S-10, Formula S-11, Formula S-12, Formula S-13, Formula S-14, Formula S-15, Formula S-16, Formula S-17, Formula S-18, Formula S-19, Formula S-20, Formula T-1, Formula T-2, Formula T-3, Formula T-4, Formula T-5, Formula T-6, Formula T-7, Formula T-8, Formula T-9, Formula T-10, Formula T-11, Formula T-12, Formula T-13, Formula T-14, Formula T-15, and Formula T-16. Structure includes:
여기에서: From here:
m, n, R1, R2, R5, R35, R6, R36, R46, R7, R8, R38, T, W, X, X2, X4, X5, X35, X6, X36, X46, X7, Y1, Y2, Y31, Y32, Y3, Y4, Z, AA, HA, R, Rs, 및 R11-R16은 상기에서 정의된 바와 같다.m, n, R 1 , R 2 , R 5 , R 35 , R 6 , R 36 , R 46 , R 7 , R 8 , R 38 , T, W, X, X 2 , X 4 , X 5 , X 35 , X 6 , X 36 , X 46 , X 7 , Y 1 , Y 2 , Y 31 , Y 32 , Y 3 , Y 4 , Z, AA, HA, R, R s , and R 11 -R 16 are As defined above.
II. HPP를 포함한 약학적 조성물II. Pharmaceutical composition including HPP
본 발명의 다른 측면은 최소한 하나의 항균제 또는 항균제-관련 화합물의 HPP 및 약학적으로 허용되는 담체를 포함하는 약학적 조성물에 관한 것이다.Another aspect of the invention relates to a pharmaceutical composition comprising HPP of at least one antimicrobial agent or antimicrobial agent-related compound and a pharmaceutically acceptable carrier.
본원에 사용되는 용어 "약학적으로 허용되는 담체"는 하나의 위치, 체액, 조직, 기관 (내부 또는 외부), 또는 신체의 부위로부터 또 다른 위치, 체액, 조직, 기관 또는 신체의 부위로 HPP를 운반하거나 이송하는데 관여하는, 액체 또는 고체 충전제, 희석제, 부형제, 용매 또는 캡슐화 물질과 같은 약학적으로-허용되는 물질, 조성물 또는 비히클을 의미한다.As used herein, the term "pharmaceutically acceptable carrier" refers to HPP from one location, body fluid, tissue, organ (internal or external), or part of the body to another location, body fluid, tissue, organ or body part. Means a pharmaceutically-acceptable material, composition or vehicle, such as liquid or solid fillers, diluents, excipients, solvents or encapsulating materials, involved in transporting or transporting.
각 담체는 제형의 다른 성분, 예를 들면, HPP와 상용되고 과도한 독성, 자극, 알레르기 반응, 면역원성 또는 기타 문제점 또는 합병증 없이 생물학적 대상체의 조직 또는 기관과 접촉하여 사용하기에 적합하고, 합리적인 이익/위험 비율에 부합한다는 측면에서 "약학적으로 허용되는" 것이다. Each carrier is compatible with other components of the formulation, e.g. HPP, and is suitable for use in contact with the tissues or organs of biological subjects without excessive toxicity, irritation, allergic reactions, immunogenicity or other problems or complications, and reasonable benefit / It is "pharmaceutically acceptable" in terms of conforming to the risk ratio.
약학적으로 허용되는 담체로서 제공될 수 있는 물질의 일부 예는: (1) 락토스, 글루코스 및 수크로스와 같은 당; (2) 옥수수 전분 및 감자 전분과 같은 전분; (3) 나트륨 카복시메틸 셀룰로스, 에틸 셀룰로스 및 셀룰로스 아세테이트와 같은 셀룰로스, 및 이의 유도체; (4) 분말 트래커캔스; (5) 맥아; (6) 젤라틴; (7) 활석; (8) 코코아 버터 및 좌제 왁스와 같은 부형제; (9) 땅콩유, 면화유, 홍화유, 참깨유, 올리브유, 옥수수유 및 대두유와 같은 오일; (10) 프로필렌 글리콜과 같은 글리콜; (11) 글리세린, 소르비톨, 만니톨 및 폴리에틸렌 글리콜과 같은 폴리올; (12) 에틸 올레에이트 및 에틸 라우레이트와 같은 에스테르; (13) 한천; (14) 수산화마그네슘 및 수산화알루미늄과 같은 완충제; (15) 알긴산; (16) 무균수; (17) 등장성 염수; (18) 링거액; (19) 에틸 알코올 및 프로판 알코올과 같은 알코올; (20) 인산염 완충액; 및 (21) 아세톤과 같이 약학적 제형에 사용되는 기타 무-독성의 상용성 물질을 포함한다. Some examples of substances that can serve as pharmaceutically acceptable carriers are: (1) sugars such as lactose, glucose and sucrose; (2) starch such as corn starch and potato starch; (3) cellulose, such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate, and derivatives thereof; (4) powder tracker cans; (5) malt; (6) gelatin; (7) talc; (8) excipients such as cocoa butter and suppository waxes; (9) oils such as peanut oil, cotton oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10) glycols such as propylene glycol; (11) polyols such as glycerin, sorbitol, mannitol and polyethylene glycol; (12) esters such as ethyl oleate and ethyl laurate; (13) agar; (14) buffering agents such as magnesium hydroxide and aluminum hydroxide; (15) alginic acid; (16) sterile water; (17) isotonic saline; (18) Ringer's solution; (19) alcohols such as ethyl alcohol and propane alcohol; (20) phosphate buffers; And (21) other non-toxic compatible substances used in pharmaceutical formulations such as acetone.
약학적 조성물은 pH 조절 및 완충제, 독성 조절제 등과 같이 적절한 생리학적 상태의 유지를 위해 필요한 약학적으로 허용되는 보조 물질, 예를 들면, 아세트산나트륨, 염화나트륨, 염화칼륨, 염화칼슘, 락트산나트륨 등을 함유할 수 있다The pharmaceutical composition may contain pharmaceutically acceptable auxiliary substances necessary for maintaining an appropriate physiological state, such as pH adjustment and buffering agent, toxic control agent, etc., for example, sodium acetate, sodium chloride, potassium chloride, calcium chloride, sodium lactate, and the like. have
하나의 양태에서, 약학적으로 허용되는 담체는 수성 담체, 예를 들면 완충 염수 등이다. 특정 양태에서, 약학적으로 허용되는 담체는 극성 용매, 예를 들면 아세톤 및 알코올이다In one embodiment, the pharmaceutically acceptable carrier is an aqueous carrier, such as buffered saline. In certain embodiments, pharmaceutically acceptable carriers are polar solvents, such as acetone and alcohol.
이들 제형에서 HPP의 농도는 광범위하게 변할 수 있으며 선택된 특정 투여 방식 및 생물학적 대상체의 요구에 따라 주로 유액 용적, 점도, 체중 등을 기준으로 선택될 것이다. 예를 들면, 농도는 0.0001중량% 내지 100중량%, 0.001중량% 내지 50중량%, 0.01중량% 내지 30중량%, 0.1중량% 내지 20중량%, 1중량% 내지 10중량%일 수 있다.The concentration of HPP in these formulations can vary widely and will be selected primarily based on emulsion volume, viscosity, body weight, etc., depending on the particular mode of administration chosen and the needs of the biological subject. For example, the concentration may be 0.0001 wt% to 100 wt%, 0.001 wt% to 50 wt%, 0.01 wt% to 30 wt%, 0.1 wt% to 20 wt%, 1 wt% to 10 wt%.
본 발명의 조성물은 예방학적, 치료학적 및/또는 위생학적 용도를 위해 투여될 수 있다. 이러한 투여는, 국소, 점막, 예를 들면 경구, 비강, 질내, 직장, 비경구, 경피, 피하, 근육내, 정맥내, 흡입을 통해, 안구 및 기타 편리한 경로일 수 있다. 약학적 조성물은 투여 방법에 따라 각종 단위 용량 형태로 투여될 수 있다. 예를 들면, 경구 투여에 적합한 단위 용량 형태는 분말, 정제, 환제, 캡슐제 및 함당정제를 포함하며 경피투여용으로는 용액, 현탁액 및 겔을 포함한다.The compositions of the present invention can be administered for prophylactic, therapeutic and / or hygienic uses. Such administration may be topical, mucosal, for example, oral, nasal, intravaginal, rectal, parenteral, transdermal, subcutaneous, intramuscular, intravenous, inhalation, ocular and other convenient routes. The pharmaceutical composition may be administered in various unit dosage forms depending on the method of administration. For example, unit dosage forms suitable for oral administration include powders, tablets, pills, capsules and saccharide tablets, and for transdermal administration solutions, suspensions and gels.
따라서, 경피, 경구, 및 정맥내 투여들을 위한 통상적인 약학적 조성물은 1일 대상체 당 약 10-10 g 내지 약 100 g, 약 10-10 g 내지 약 10-3 g, 약 10-9 g 내지 약 10-6 g, 약 10-6 g 내지 약 100 g, 약 0.001 g 내지 약 100 g, 약 0.01 g 내지 약 10 g, 또는 약 0.1 g 내지 약 1 g 일 수 있다. 1일 대상체 당 약 0.01 mg 내지 약 100 g까지의 투여량이 사용될 수 있다. 비경구 투여가능한 조성물을 제조하는 실제방법은 당해 분야의 숙련가에게 공지되어 있으며 명백할 것이고 문헌 (Remington: The Science, and Practice of Pharmacy 21st ed., Mack Publishing Company, Easton, Pa. (2005))과 같은 공개 문헌에 보다 상세히 기술되어 있다.Thus, conventional pharmaceutical compositions for transdermal, oral, and intravenous administrations range from about 10 -10 g to about 100 g, from about 10 -10 g to about 10 -3 g, from about 10 -9 g to 1 day subject About 10 -6 g, about 10 -6 g to about 100 g, about 0.001 g to about 100 g, about 0.01 g to about 10 g, or about 0.1 g to about 1 g. Dosages from about 0.01 mg to about 100 g per subject per day can be used. The actual method for preparing parenterally administrable compositions will be known and apparent to those skilled in the art and will be described in Remington: The Science, and Practice of Pharmacy 21 st ed., Mack Publishing Company, Easton, Pa. (2005). It is described in more detail in the public literature such as.
III. HPP 적용III. Apply HPP
i) 생체 장벽 투과 방법. i) Bio-barrier permeation method.
본 발명의 다른 측면은 생물학적 대상체의 하나 이상의 생체 장벽을 투과하는데 있어서 본 발명의 조성물을 이용하는 방법에 관한 것이다. 본 방법은 생물학적 대상체에게 항균제 또는 항균제-관련 화합물의HPP, 또는 이의 약학적 조성물을 투여하는 단계를 포함한다. 특정 양태에서, HPP는 모 약물보다 하나 이상의 생체 장벽에 대해 약 20배 이상 또는 초과, 50 배 이상 또는 초과, 약 100배 이상 또는 초과, 약 200배 이상 또는 초과, 약 300배 이상 또는 초과, 약 500배 이상 또는 초과, 약 1,000배 이상 또는 초과의 투과율을 나타낸다. Another aspect of the invention relates to methods of using the compositions of the invention in penetrating one or more biological barriers of a biological subject. The method comprises administering an antimicrobial agent or an HPP of an antimicrobial agent-related compound, or a pharmaceutical composition thereof, to a biological subject. In certain embodiments, HPP is at least about 20 times or more, at least 50 times or more, at least about 100 times or more, at least about 200 times or more, at least about 300 times or more, about at least one biological barrier to the parent drug It exhibits a transmittance of 500 times or more, or about 1,000 times or more.
본원에 사용되는, 용어 "생체 장벽"은 상이한 공간적 부위 또는 구획으로 환경을 분리하는 생물학적 층을 말하며, 여기서, 분리는 하나의 구획/부위로부터 다른 곳으로 물질 또는 성분을 통과, 투과, 또는 이동을 조절 (예를 들면, 제한, 한정, 향상, 또는 작용을 취하지 않음)할 수 있다. 본원에서 언급되는 상이한 공간적 부위 또는 구획은 동일하거나 상이한 화학적 또는 생물학적 환경(들)을 가질 수 있다. 본원에 언급되는 생물학적 층은 생물학적 막, 세포 층, 생물학적 구조, 대상체, 유기체, 기관 또는 체강의 내부 표면, 대상체, 유기체, 기관 또는 체강의 외부 표면, 또는 이의 임의적 조합 또는 다수를 포함하나, 이에 한정되지 않는다. As used herein, the term “biological barrier” refers to a biological layer that separates the environment into different spatial sites or compartments, where separation allows passage, permeation, or movement of a substance or component from one compartment / site to another. Can be adjusted (eg, not limited, limited, improved, or taken action). The different spatial sites or compartments referred to herein can have the same or different chemical or biological environment (s). Biological layers referred to herein include, but are not limited to, biological membranes, cell layers, biological structures, inner surfaces of a subject, organism, organ or body cavity, outer surfaces of a subject, organism, organ or body cavity, or any combination or multiple thereof. Does not work.
생물학적 막의 예는 지질 이층 구조, 진핵 세포 막, 원핵세포 막, 및 세포간 막 (예를 들면, 골지체의 막 또는 외피, 조면 및 활면 소포체 (ER), 리보솜, 액포, 소포, 리포좀, 미토콘드리아, 리소좀, 핵, 엽록체, 색소체, 퍼옥시좀 또는 미소체와 같은 핵 또는 세포소기관 막)을 포함한다. Examples of biological membranes include lipid bilayer structures, eukaryotic membranes, prokaryotic membranes, and intercellular membranes (e.g., Golgi membranes or envelopes, rough and smooth vesicles (ER), ribosomes, vacuoles, vesicles, liposomes, mitochondria, lysosomes , Nucleus, chloroplast, plastid, peroxysome or microsome such as nucleus or organelle membrane).
본원에서 언급되는 지질 이중층은 인지질 및 콜레스테롤을 포함하나, 이에 한정되지 않는 지질-부류 분자의 이중층이다. 특정 양태에서, 이중층용 지질은 극성 헤드기 및 비-극성 지방산 테일로 이루어진 양친성 분자이다. 이중층은, 이들의 탄화수소 테일이 서로 대면하여 소수성 작용에 의해 함께 유지되는 오일 코어를 형성하는 반면, 이들의 하전된 헤드는 막의 한쪽 면에서 수용액과 대면하도록 배열된 지질의 2개 층으로 구성된다. 다른 특정 양태에서, 지질 이중층은 하나 이상의 포매된 단백질 및/또는 당 분자(들)을 함유할 수 있다.Lipid bilayers referred to herein are bilayers of lipid-class molecules, including but not limited to phospholipids and cholesterol. In certain embodiments, the bilayer lipid is an amphiphilic molecule consisting of a polar head group and a non-polar fatty acid tail. The bilayers consist of two layers of lipids arranged so that their hydrocarbon tails face each other to form an oil core that is held together by hydrophobic action, while their charged heads are arranged to face an aqueous solution on one side of the membrane. In certain other embodiments, the lipid bilayer can contain one or more embedded protein and / or sugar molecule (s).
세포 층의 예는 진핵 세포의 내층 (예를 들면, 상피, 고유판(lamina propria), 및 평활근 또는 점막근육 (위장관내)), 원핵 세포의 내층 (예를 들면, 동일한 단백질 또는 당단백질로 구성된 2차원 구조 단일분자 층을 언급하는 표면층 또는 S-층, 상세하게는, S-층은 세균 및 고세균에서 일반적으로 발견된 세포 외피의 일부를 말함), 바이오필름 (자가-발달된 중합체성 기질내 봉입되고 살아있거나 불활성인 표면에 부착된 미생물의 구조화된 군집), 및 식물 세포 층 (예를 들면, 표피)을 포함한다. 세포는 정상 세포 또는 병리학적 세포 (예를 들면, 질환 세포, 암세포)일 수 있다.Examples of cell layers include the inner layer of eukaryotic cells (e.g., epithelium, lamina propria, and smooth or mucosal muscles (in the gastrointestinal tract)), the inner layer of prokaryotic cells (e.g., composed of the same protein or glycoprotein) Surface layer or S-layer, referring to a two-dimensional monolayer structure, specifically, the S-layer refers to the part of the cell envelope commonly found in bacteria and archaea), biofilms (in self-developed polymeric substrates) Structured populations of microorganisms enclosed and attached to a living or inactive surface), and plant cell layers (eg, epidermis). The cells can be normal cells or pathological cells (eg disease cells, cancer cells).
생물학적 구조의 예는 독소, 세균 및 바이러스의 도입에 대해 장벽, 예를 들면 혈유 장벽 및 혈액 뇌 장벽(BBB)을 제공하는 치밀하거나 폐색적인 이음부에 의해 밀봉된 구조를 포함한다. 특히, BBB는 내피의 투과불가능한 부류로 구성되며, 이는 이웃하는 내피 세포와 인접하고 있는 치밀한 이음부를 통한 물리적 장벽 및 유출 이송인자로 구성된 이송 장벽 둘 다를 나타낸다. 생물학적 구조는 또한 세포, 단백질 및 당 의 혼합물(예를 들면, 혈전)을 포함할 수 있다. Examples of biological structures include structures sealed by dense or occlusive joints that provide barriers to the introduction of toxins, bacteria and viruses, such as the blood oil barrier and the blood brain barrier (BBB). In particular, BBB consists of an impermeable class of endothelium, which represents both a physical barrier through the adjacent endothelial cells and a dense joint adjacent to it and a transport barrier consisting of effluent transport factors. Biological structures may also include mixtures of cells, proteins and sugars (eg, clots).
대상체, 유기체, 기관 또는 체강의 내부 표면의 예는 볼 점막, 식도 점막, 위 점막, 장 점막, 후각 점막, 구강 점막, 기관지 점막, 자궁 점막 및 자궁내막 (자궁의 점막, 꽃가루의 벽 내부 층 또는 포자의 내부 벽 층), 또는 이의 조합 또는 이의 다수를 포함한다.Examples of the inner surface of a subject, organism, organ or body cavity include the buccal mucosa, esophageal mucosa, gastric mucosa, intestinal mucosa, olfactory mucosa, oral mucosa, bronchial mucosa, uterine mucosa and endometrium (uterus mucosa, pollen wall inner layer or Spores' inner wall layer), or a combination thereof, or a plurality thereof.
대상체, 유기체, 기관 또는 체강의 외부 표면의 예는 모세혈관 (예를 들면, 심장 조직내 모세혈관), 피부와 연속된 점막성 막 (예를 들면, 비공, 입술, 귀, 생식기 부위 및 항문과 같은), 기관 (예를 들면 간, 폐, 위, 뇌, 신장, 심장, 귀, 눈, 코, 입, 혀, 결장, 췌장, 담낭, 십이지장, 직장위장, 결장직장, 장, 정맥, 호흡기계, 혈관, 항문직장 및 항문 소양증)의 외부 표면, 피부, 각피 (예를 들면, 표피 세포 또는 각질세포의 얕은 층(dead layer) 또는 동물의 모간(hair shaft)을 덮고 있는 중첩 세포(overlapping cell)의 표면층, 많은 무척추동물의 표피 외부의 다층화된 구조, 식물 각피 또는 중합체 큐틴 및/또는 큐탄), 꽃가루의 벽 외부 층 또는 포자의 외벽 층, 및 이의 조합 또는 이의 다수를 포함한다.Examples of the outer surface of a subject, organism, organ or body cavity include capillaries (e.g., capillaries in heart tissue), skin and continuous mucosal membranes (e.g., nostrils, lips, ears, genital areas and anus) Same), organs (e.g. liver, lungs, stomach, brain, kidneys, heart, ears, eyes, nose, mouth, tongue, colon, pancreas, gallbladder, duodenum, rectal stomach, colorectal, intestine, vein, respiratory system , Vascular, anal rectal and anal pruritus), the outer surface of the skin, the epidermis (e.g., a dead layer of epidermal cells or keratinocytes or an overlapping cell covering the animal's hair shaft) Surface layer, multi-layered structure outside the epidermis of many invertebrates, plant cuticle or polymer cutin and / or cutane), outer wall layer of pollen wall or outer wall layer of spores, and combinations thereof, or multiples thereof.
또한, 생체 장벽은 당 층, 단백질 층 또는 임의의 다른 생물학적 층, 또는 이의 조합 또는 다수를 추가로 포함한다. 예를 들면, 피부는 다수의 생물학적 층을 갖는 생체 장벽이다. 피부는 표피 층 (외부 표면), 진피 층 및 피하 층을 포함한다. 표피 층은 기본 세포 층, 가시 세포 층, 과립 세포 층, 및 각질층을 포함한 몇몇의 층들을 함유한다. 표피내 세포는 각질세포로 불리운다. 각질층 ("허니층(horny layer)")은 표피의 가장 외부층이며, 여기서, 이곳의 세포는 모양이 평편하고 비늘과 유사("비닐모양")하다. 이들 세포는 다량의 케라틴을함유하며 피부 표면에 촉감(tough) 및 방유 및 방수 특성을 부여하는 중첩된 세포로 정렬되어 있다.In addition, the bio-barrier further comprises a sugar layer, a protein layer or any other biological layer, or a combination or multiple thereof. For example, the skin is a biological barrier with multiple biological layers. The skin includes an epidermal layer (outer surface), a dermal layer and a subcutaneous layer. The epidermal layer contains several layers, including a basic cell layer, a visible cell layer, a granular cell layer, and a stratum corneum layer. Cells in the epidermis are called keratinocytes. The stratum corneum (“horny layer”) is the outermost layer of the epidermis, where the cells are flat in shape and resemble scales (“vinyl shaped”). These cells are arranged in overlapping cells that contain a large amount of keratin and impart tactile and oil and waterproof properties to the skin surface.
ii) 생물학적 대상체의 병태를 진단하는 방법. ii) A method of diagnosing the condition of a biological subject.
본 발명의 다른 측면은 생물학적 대상체에서 병태를 진단하는데 있어 조성물을 이용하는 방법에 관한 것이다. 본 방법은:Another aspect of the invention relates to a method of using the composition in diagnosing a condition in a biological subject. This method:
1) 항균제 또는 항균제-관련 화합물의 HPP 를 포함하는 조성물을 생물학적 대상체에게 투여하는 단계;1) administering a composition comprising HPP of an antimicrobial agent or an antimicrobial agent-related compound to a biological subject;
2) 생물학적 대상체에서 HPP, HPP의 관능성 단위 또는 이의 대사산물의 존재, 위치 또는 양을 검출하는 단계; 및2) detecting the presence, location or amount of HPP, a functional unit of HPP, or a metabolite thereof in a biological subject; And
3) 생물학적 대상체에서 병태를 측정하는 단계를 포함한다.3) measuring the condition in the biological subject.
특정 양태에서, HPP (또는 HPP로부터 절단된 제제)는 병태가 발생한 작용 부위에 응집된다. 특정 양태에서, HPP의 관능성 단위의 존재, 위치 또는 양을 또한 검출한다. 특정 양태에서, 관련 병태 (예를 들면, 감염)의 발병, 발달, 진행 또는 완화를 또한 측정한다. In certain embodiments, HPP (or an agent cleaved from HPP) aggregates at the site of action where the condition occurred. In certain embodiments, the presence, position or amount of functional units of HPP is also detected. In certain embodiments, the onset, development, progression or alleviation of related conditions (eg, infection) is also measured.
특정 양태에서, HPP는 검출가능한 제제로 표지되거나 이에 접합된다. 달리, HPP는 검출용 방사성동위원소를 포함하도록 제조된다. 일반적으로 다음 범주로 그룹화될 수 있는 다수의 검출가능한 제제가 이용가능하다:In certain embodiments, HPP is labeled or conjugated to a detectable agent. Alternatively, HPP is prepared to contain radioactive isotopes for detection. In general, a number of detectable agents that can be grouped into the following categories are available:
(a) 35S, 14C, 13C, 15N, 125I, 3H, 및 131I 와 같은 방사성동위원소. 진단제는 당해 분야에 공지된 기술을 사용하여 방사성동위원소로 표지하고 방사활성을 섬광 계수를 사용하여 측정할 수 있으며; 또한, 진단제는 탄소 및 질소 라벨에 대한 전자 상자성 공명에 대해 스핀 표지될 수 있다.(a) Radioactive isotopes such as 35 S, 14 C, 13 C, 15 N, 125 I, 3 H, and 131 I. Diagnostic agents can be labeled with radioactive isotopes using techniques known in the art and radioactivity can be measured using scintillation counting; In addition, diagnostic agents can be spin labeled for electron paramagnetic resonance for carbon and nitrogen labels.
(b) BODIPY, BODIPY 유사체, 희토류 킬레이트(유로퓸 킬레이트), 플루오레세인 및 이의 유도체, FITC, 5,6 카복시플루오레세인, 로다민 및 이의 유도체, 단실, 리싸민, 피코에리트린, 녹색 형광성 단백질, 황색 형광성 단백질, 적색 형광성 단백질 및 텍사스 레드와 같은 형광성 제제. 형광성은 형광계를 사용하여 정량화할 수 있다.(b) BODIPY, BODIPY analogs, rare earth chelates (Europium chelates), fluorescein and its derivatives, FITC, 5,6 carboxyfluorescein, rhodamine and its derivatives, dansyl, lysamine, picoerythrin, green fluorescent protein , Yellow fluorescent protein, red fluorescent protein and fluorescent agents such as Texas Red. Fluorescence can be quantified using a fluorimeter.
(c) 루시퍼라제 (예를 들면, 반딧불이 루시페라제 및 세균 루시퍼라제), 루시페린, 2,3-디하이드로프탈라진디온, 말산탈수소효소, 우레아제, 서양고추냉이 퍼옥시다제(HRPO)와 같은 퍼옥시다제, 알칼린 포스파타제, β-갈락토시다제, 글루코아밀라제, 리소자임, 당류 산화효소 (예를 들면, 글루코스 옥시다제, 갈락토즈 옥시다제 및 글루코스-6-포스페이트 탈수소효소), 헤테로사이클릭 옥시다제 (예를 들면, 요산분해효소 및 크산틴 옥시다제), 락토퍼옥시다제, 마이크로퍼옥시다제 등과 같은 각종 효소-기질 제제. 효소-기질 조합물의 예는 예를 들면, (i) 서양 고추냉이 퍼옥시다제(HRPO)와 기질로서 하이드로겐 퍼옥시다제, 여기서, 하이드로겐 퍼옥시다제는 염료 전구체 (예를 들면, 오르토페닐렌 디아민(OPD) 또는 3,3',5,5'-테트라메틸 벤지딘 하이드로클로라이드(TMB))를 산화시킨다; (ii) 알칼리성 포스파타제(AP)와 색원체 기질로서 니트로페닐 포스페이트; 및 (iii) β-D-갈락토시다제 (β-D-Gal)과 색원체 기질 (예를 들면, p-니트로페닐-β-D-갈락토시다제) 또는 형광성 기질 4-메틸움벨리페릴-β-D-갈락토시다제를 포함한다.(c) luciferase (e.g., firefly luciferase and bacterial luciferase), luciferin, 2,3-dihydrophthalazinedione, malic acid dehydrogenase, urease, horseradish peroxidase (HRPO) Peroxidase, alkaline phosphatase, β-galactosidase, glucoamylase, lysozyme, saccharide oxidase (e.g. glucose oxidase, galactose oxidase and glucose-6-phosphate dehydrogenase), heterocyclic oxy Various enzyme-substrate agents such as multidrugs (eg, uric acid degrading enzymes and xanthine oxidase), lactoperoxidase, microperoxidase, and the like. Examples of enzyme-substrate combinations are, for example, (i) horseradish peroxidase (HRPO) and hydrogen peroxidase as a substrate, where hydrogen peroxidase is a dye precursor (e.g., orthophenylene Oxidize diamine (OPD) or 3,3 ', 5,5'-tetramethyl benzidine hydrochloride (TMB); (ii) alkaline phosphatase (AP) and nitrophenyl phosphate as a chromogenic substrate; And (iii) β-D-galactosidase (β-D-Gal) and chromogenic substrate (eg, p-nitrophenyl-β-D-galactosidase) or fluorescent substrate 4-methylumbelly Peryl-β-D-galactosidase.
특정 양태에서, 본 발명의 HPP는 키트, 즉, 진단 검정을 수행하기 위한 지시사항과 함께 소정의 양의 시약의 포장된 조합으로 제공될 수 있다. HPP를 효소로 표지하는 경우, 키트는 효소 (예를 들면, 검출가능한 발색단 또는 형광단을 제공하는 기질 전구체)에게 필요한 기질 및 보조인자를 포함할 것이다. 또한, 안정화제, 완충제(예를 들면, 차단 완충제 또는 분해 완충제) 등과 같은 다른 첨가제가 포함될 수 있다. 각종 시약의 상대적인 양은 검정의 민감성을 실질적으로 최적화시키는 시약의 용액내 농도를 제공하기 위해 광범위하게 변할 수 있다. 특히, 시약은 용해시 적절한 농도를 갖는 시약 용액을 제공할 부형제를 포함하는, 일반적으로 동결건조된 무수 분말로서 제공될 수 있다.In certain embodiments, HPPs of the present invention may be provided in kits, ie, packaged combinations of reagents in predetermined amounts, along with instructions for performing a diagnostic assay. When HPP is labeled with an enzyme, the kit will contain the substrate and cofactors required for the enzyme (eg, a substrate precursor that provides a detectable chromophore or fluorophore). In addition, other additives may be included, such as stabilizers, buffers (eg, blocking buffers or degradation buffers). The relative amounts of various reagents can vary widely to provide concentrations in solution of reagents that substantially optimize the sensitivity of the assay. In particular, the reagent can be provided as an anhydrous powder, generally lyophilized, which contains an excipient that will provide a reagent solution with an appropriate concentration upon dissolution.
iii) 바람직한 특성의 기질을 선별하는 방법iii) Method of selecting a substrate with desirable properties
본 발명의 다른 측면은 바람직한 특성의 HPP 를 선별하는 방법에 관한 것이다.Another aspect of the invention relates to a method for selecting HPPs of desirable properties.
특정 양태에서, 본 방법은:In certain embodiments, the method comprises:
1) 시험 관능성 단위를 이송 단위에 링커를 통해 공유결합으로 연결시켜 시험 조성물을 형성시키는 단계 (또는 관능성 단위를 시험 이송 단위에 링커를 통해 공유결합으로 연결시키거나, 관능성 단위를 이송 단위에 시험 링커를 통해 공유결합으로 연결시키는 단계);1) forming a test composition by linking a test functional unit to a transport unit covalently via a linker (or linking a functional unit to a test transport unit covalently through a linker, or to transfer a functional unit to a transport unit) To the covalent link through a test linker);
2) 생물학적 대상체에게 시험 조성물을 투여하는 단계; 및2) administering the test composition to the biological subject; And
3) 시험 조성물이 바람직한 특성 또는 특징을 갖는지를 측정하는 단계를 포함한다.3) measuring whether the test composition has desirable properties or characteristics.
하나의 양태에서, 바람직한 특징은 예를 들면, 1) 고투과 조성물을 형성하거나 모 약물로 역 전환하는 시험 관능성 단위의 능력; 2) 시험 조성물의 투과 능력 및/또는 속도, 3) 시험 조성물의 효율성 및/또는 효능, 4) 시험 이송 단위의 이송 능력, 및 5) 시험 링커의 절단능을 포함할 수 있다.In one embodiment, preferred features are, for example, 1) the ability of a test functional unit to form a high permeable composition or back convert to a parent drug; 2) permeability and / or rate of the test composition, 3) efficiency and / or efficacy of the test composition, 4) transport ability of the test transport unit, and 5) cleavage of the test linker.
iv) 생물학적 대상체의 병태를 치료하는 방법iv) methods of treating a condition in a biological subject
본 발명의 다른 측면은 생물학적 대상체에서 병태를 치료하는데 있어 본 발명의 조성물을 이용하는 방법에 관한 것이다. 본 방법은 약학적 조성물을 생물학적 대상체에게 투여하는 단계를 포함한다.Another aspect of the invention relates to methods of using the compositions of the invention in treating a condition in a biological subject. The method includes administering the pharmaceutical composition to a biological subject.
본원에서 사용되는, 용어 "치료"는 치유, 완화, 억제 또는 예방을 의미한다. 본원에 사용되는 용어 "치료하다"는 치유하다, 완화시키다, 억제하다 또는 예방하는 것을 의미한다. 본원에 사용되는, 용어 "치료"는 치유, 완화, 억제 또는 예방을 의미한다.As used herein, the term "treatment" means healing, alleviation, inhibition or prevention. As used herein, the term “treat” means to heal, alleviate, inhibit or prevent. As used herein, the term “treatment” means healing, alleviation, inhibition or prevention.
본원에서 사용되는, 용어 “생물학적 시스템”, "생물학적 대상체" 또는 "대상체"는 기관, 함께 작업하여 특정 임무를 수행하는 기관의 그룹, 유기체 또는 유기체 군을 의미한다. 본원에서 사용되는, 용어 "유기체"는 다소 안정한 전체로서 기능하며 동물, 식물, 진균 또는 미생물과 같은 생명의 특성을 갖는 분자의 조립체를 의미한다. As used herein, the term “biological system”, “biological subject” or “subject” refers to an organ, group of organisms, or group of organisms that work together to perform a specific task. As used herein, the term “organic” means an assembly of molecules that function as a rather stable whole and have life properties such as animals, plants, fungi, or microorganisms.
본원에 사용되는, 용어 "동물"은 자발적인 운동으로 특징화된 진핵세포 유기체를 의미한다. 동물들의 예는 척추동물 (예를 들면, 사람, 포유동물, 조류, 파충류, 양서류, 어류, 마르시포브란키아타(marsipobranchiata) 및 척삭동물(leptocardia)),As used herein, the term "animal" refers to a eukaryotic organism characterized by spontaneous movement. Examples of animals are vertebrates (eg, humans, mammals, birds, reptiles, amphibians, fish, marsipobranchiata and leptocardia),
피남류 (예를 들면, 탈리아류(thaliacea), 아펜디큘라리아(appendicularia), 소르베라세아(sorberacea) 및 아스시디오이데아(ascidioidea)], 체절동물 (예를 들면, 곤충류, 다지류다족강, 말라카포다(malacapoda), 거미아강, 바다거미강, 퇴구강, 갑각류 및 환형동물), 게히레아(gehyrea) (아나르트로포다(anarthropoda)), 및 장내기생충 (예를 들면, 윤형동물)을 포함하나, 이에 한정되지 않는다. Pinnacles (e.g. thaliacea, appendicularia, sorberacea and ascidioidea), arthropods (e.g. insects, multi-family multifamily rivers) , Malacapoda, arachnid, sea spider river, mandibular, crustacean and annular), gehyrea (anarthropoda), and intestinal parasites (e.g., limpidus) It includes, but is not limited to.
본원에 사용되는, 용어 "식물"은 식물계에 속하는 유기체를 의미한다. 식물의 예는 종자식물, 선태식물, 양치식물(ferns), 페른 알리(fern ally)를 포함하나, 이에 한정되지 않는다. 종자식물의 예는 소철, 은행나무, 침엽수, 그네토피테(gnetophytes), 속씨식물을 포함하나, 이에 한정되지 않는다. 선태식물의 예는 우산이끼, 붕어마름 및 이끼를 포함하나, 이에 한정되지 않는다. 양치식물의 예는 오피오글로살레스(ophioglossales) (예를 들면, 아더스-통그스(adders-tongues), 고사리삼, 및 그레이프-페른(grape-ferns))을 포함하나, 이에 한정되지 않는다. 페른 알리의 예는 리콥시다(lycopsida) (예를 들면, 클럽모세스(clubmosses), 스파이크모세스(spikemosses) 및 퀼워트스(quillworts)), 솔잎란과 (예를 들면, 라이코포디오피타 (lycopodiophyta) 및 휘스크 페른(whisk ferns)) 및 이퀴세타케아에(equisetaceae) (예를 들면, 호르세타일스(horsetails))를 포함하나, 이에 한정되지 않는다.As used herein, the term "plant" means an organism belonging to the plant kingdom. Examples of plants include, but are not limited to, seed plants, native plants, ferns, and fern ally. Examples of seed plants include, but are not limited to cycads, ginkgo, conifers, gnetophytes, genus seed plants. Examples of selected plants include, but are not limited to, umbrella moss, carp dry and moss. Examples of ferns include, but are not limited to, opioglossales (e.g., adders-tongues, ferns, and grape-ferns). Examples of Fern Ali are lycopsida (e.g. clubmosses, spikeemosses and quillworts), pine needles (e.g. lycopodiophyta ) And whisk ferns and equisetaceae (eg, horsetails).
본원에 사용되는, 용어 "진균"은 진균계의 구성원인 진핵 유기체를 의미한다. 진균의 예는 호산균, 블라스토클라디오마이코타(blastocladiomycota), 네오칼리마스티고마이코타(neocallimastigomycota), 자이고마이코타(zygomycota), 글로머로마이코타(glomeromycota), 아스코마이코타(ascomycota) 및 바시디오마이코타(basidiomycota)를 포함하나, 이에 한정되지 않는다.As used herein, the term "fungus" refers to a eukaryotic organism that is a member of the fungal system. Examples of fungi include Eosinophils, Blastocladiomycota, Neocalistigomycota, Zygomycota, Glomeromycota, Ascomycota, and Ascomycota. Basidiomycota, but is not limited thereto.
본원에서 사용되는, 용어 "미생물"은 미세한 (예를 들면, 마이크로미터의 길이 규모) 유기체를 의미한다. 미생물의 예는 세균, 진균, 고세균류, 원생생물 및 미세 식물 (예를 들면, 녹색 조류) 및 미세 동물 (예를 들면, 플랑크톤, 플라나리아 및 아메바)를 포함하나, 이에 한정되지 않는다.As used herein, the term “microorganism” refers to a microscopic (eg, micrometer length scale) organism. Examples of microorganisms include, but are not limited to, bacteria, fungi, archaea, protozoa and micro plants (eg, green algae) and micro animals (eg, plankton, planaria and amoeba).
본 방법이 치료할 수 있는 병태의 일부 예는 HPP의 모 약물에 의해 치료될 수 있는 병태를 포함한다.Some examples of conditions the method can treat include conditions that can be treated by the parent drug of HPP.
v). 치료에 있어서항균제 및 항균제-관련 화합물의HPP및이의약학적조성물을 이용하는방법. v). A method of using HPP and a pharmaceutical composition of an antibacterial and antibacterial agent-related compound in treatment .
본 발명의 또 다른 측면은 항균제 및 항균제-관련 화합물의HPP또는 이의 약학적 조성물을 생물학적 시스템 또는 대상체에게 투여함으로써 생물학적 시스템 또는 대상체의 병태를 치료하는데 있어서 항균제 및 항균제-관련 화합물의HPP또는 이의 약학적 조성물을 이용하는 방법에 관한 것이다.In another aspect of the present invention, an HPP of an antimicrobial agent and an antimicrobial agent-related compound or a pharmaceutical thereof in treating a biological system or a subject's condition by administering an HPP of an antimicrobial agent and an antimicrobial agent-related compound or a pharmaceutical composition thereof to a biological system or subject It relates to a method of using the composition.
항균제 및 항균제-관련 화합물은 생물학적 대상체에서 다양한 생물학적 작용을 조절함에 이용될 수 있다. 이러한 생물학적 작용들과 관련된 병태들은 상응 항균제 또는 항균제-관련 화합물로 치료될 수 있고, 따라서 항균제 또는 항균제-관련 화합물의 HPP/HPC, 및 이의 약학적 조성물로 치료될 수 있다.Antimicrobial agents and antimicrobial agent-related compounds can be used to modulate various biological actions in biological subjects. Conditions associated with these biological actions can be treated with a corresponding antimicrobial agent or antimicrobial agent-related compound, and thus can be treated with HPP / HPC of an antimicrobial agent or antimicrobial agent-related compound, and pharmaceutical compositions thereof.
이러한 병태들은, 제한적이지 않지만, 통증, 손상, 및 미생물 관련 병태들을 포함한다. 미생물 관련 병태들은 세균, 진균, 원충 및 바이러스와 같은 미생물들에 의해 유발하는 병태들이다. 예를 들면, 세균에 의한 유발 병태들 (세균-관련 병태들), 원충에 의한 유발 병태들 (원충-관련 병태들), 진균에 의한 유발 병태들 (진균-관련 병태들), 및 바이러스에 의한 유발 병태들 (바이러스-관련 병태들). These conditions include, but are not limited to, pain, injury, and microbial related conditions. Microbial conditions are conditions caused by microorganisms such as bacteria, fungi, protozoa, and viruses. For example, conditions caused by bacteria (bacteria-related conditions), conditions caused by protozoa (protozoa-related conditions), conditions caused by fungi (fungal-related conditions), and caused by viruses Induced conditions (virus-related conditions).
세균-관련 병태들은, 예를 들면 감염 (간, 폐, 위장, 뇌, 신장, 심장, 귀, 눈, 코, 입, 혀, 결장, 췌장, 담낭, 십이지장, 직장위장, 결장직장, 장, 정맥, 호흡계통, 혈관, 항문직장 및 항문소양증, 호흡계 감염, 상부 호흡기 감염, 요로 감염, 원내감염, 슈도모나스 감염, 코아귤라제양성포도구균 감염 (예를 들면, 피부 감염, 중독증, 급성 감염성 심내막염, 패혈증, 괴사성 폐렴), 인공기관 이식 감염, 패혈증 및 폐렴 동반 기회감염), 흑사병 (예를 들면, 가래톳흑사병 및 폐렴흑사병), 탄저병 (예를 들면, 피부 탄저병, 폐 탄저병 및 위창자성 탄저병), 라임질환, 브루셀라증, 백일해, 급성장염, 호흡기 감염, 앵무새병, 비임균요도염, 트라코마, 신생아 봉입체결막염, 성병림프육아종, 거짓막대장염, 가스괴저, 식중독, 무산소균연조직염, 디프테리아, 설사, 유아 뇌수막염, 출혈성대장염, 용혈요독증후군, 야토병, 폐렴, 기관지염, 소화성궤양, 리지오넬라병, 폰티악열, 렙토스피라증, 리스테리아증, 나병, 결핵, 폐렴미코플라스마, 임질, 신생아안염, 화농성관절염, 수막알균병, 워터하우스-프리데릭센증후군, 록키산 홍반열, 장티푸스형 살모넬라증, 위장염 및 소장대장염 동반 살모넬라증, 세균이질, 시겔라증, 방광염, 수막염, 패혈증, 자궁내막염, 중이염, 굴염, 매독, 괴사근막염, 사슬알균성 인두염, 성홍열, 류마티스열, 농가진, 얕은연조직염, 산후열, 및 콜레라를 포함한다. Bacterial-related conditions include, for example, infection (liver, lung, stomach, brain, kidney, heart, ear, eye, nose, mouth, tongue, colon, pancreas, gallbladder, duodenum, rectal stomach, colorectal, intestinal, venous , Respiratory system, blood vessels, rectal and anal pruritus, respiratory system infections, upper respiratory tract infections, urinary tract infections, in-hospital infections, pseudomonas infections, coagulase-positive aureus infections (e.g., skin infections, addictions, acute infective endocarditis, sepsis) , Necrotizing pneumonia), artificial organ transplant infection, sepsis and pneumonia, opportunistic infections), black plague (e.g. sputum black plague and pneumonia black plague), anthrax (e.g. skin anthrax, lung anthrax and gastrointestinal anthrax), lime Diseases, brucellosis, whooping cough, acute appendicitis, respiratory infections, parrot disease, non-gonococcal urethritis, trachoma, neonatal inclusion conjunctivitis, venereal lymphoma, granulomatous colitis, gastric necrosis, food poisoning, anaerobic vasculitis, diphtheria, diarrhea, infant brain Salt, hemorrhagic colitis, hemolytic uremic syndrome, rabies, pneumonia, bronchitis, peptic ulcer, ligonella disease, Pontiac fever, leptospirosis, listeriosis, leprosy, tuberculosis, pneumonia mycoplasma, gonorrhea, neonatal ophthalmitis, purulent arthritis, meningococcal disease , Waterhouse-Fredericsen Syndrome, Rocky Mountain Red Fever, Typhoid Salmonellosis, Gastroenteritis and Small Bowel Colitis Salmonellosis, Bacterial dysentery, Shigellosis, Cystitis, Meningitis, Sepsis, Endometritis, Otitis Media, Otitisitis, Syphilis, Necrotitis, Chain Include bacterial pharyngitis, scarlet fever, rheumatic fever, impetigo, shallow soft tissueitis, postpartum fever, and cholera.
원충 관련 병태들은 예를 들면 말라리아, 수면병, 및 톡소포자충증을 포함한다. Protozoa related conditions include, for example, malaria, sleep disease, and toxoplasmosis.
진균 관련 병태들은 예를 들면 아스페르길루스증, 분아균증, 백선, 칸디다증, 콕시디오이데스진균증, 크립토코쿠스증, 히스토플라스마증, 파라콕시디오이데스, 스포로트릭스증, 및 털곰팡이증을 포함한다. Fungal-related conditions include, for example, aspergillosis, feces, ringworm, candidiasis, coccidioidis mycosis, cryptococcosis, histoplasmosis, paracoccidioides, sporotrix, and hair fungus. Include symptoms.
바이러스 관련 병태들은 예를 들면 인플루엔자, 황열병 및 AIDS를 포함한다.Virus-related conditions include, for example, influenza, yellow fever, and AIDS.
특정 양태에서, 항균제 또는 항균제-관련 화합물로 개선되거나 치료될 수 있는 대상체 병태를 치료하는 방법은 치료학적 유효량의 항균제 또는 항균제-관련 화합물의 HPP 또는 이의 약학적 조성물을 대상체에게 투여하는 것을 포함한다. In certain embodiments, a method of treating a subject condition that can be improved or treated with an antimicrobial agent or an antimicrobial agent-related compound comprises administering to a subject a therapeutically effective amount of an antimicrobial agent or HPP of an antimicrobial agent-related compound or a pharmaceutical composition thereof.
HPP 또는 이의 약학적 조성물은 생물학적 대상체에게 경구, 장내, 볼내, 비강내, 국소, 직장, 질내, 에어로졸, 경점막, 표피, 경피, 피부, 안과, 폐, 피하 및/또는 비경구 투여를 포함하나, 이에 한정되지 않는 당해 분야에 공지된 어떠한 투여 경로에 의해서도 투여될 수 있다. 약학적 조성물은 투여 방법에 따라 각종의 단위 용량 형태로 투여될 수 있다.HPP or pharmaceutical compositions thereof include oral, intestinal, intranasal, intranasal, topical, rectal, vaginal, aerosol, transmucosal, epidermal, transdermal, skin, ophthalmic, lung, subcutaneous and / or parenteral administration to biological subjects , It can be administered by any route of administration known in the art, but not limited to. The pharmaceutical composition may be administered in various unit dose forms depending on the method of administration.
비경구 투여는 정맥내, 근육내, 동맥내, 수막공간내, 관절낭내, 안와내(intraorbital), 심장내, 진피내, 복강내, 경기관, 피하, 피부 및, 동맥내, 피막하, 지주막하, 척주내 및/또는 흉골내 주사 및/또는 주입을 포함하나, 이에 한정되지 않는 일반적으로 주사와 관련된 투여 경로를 말한다.Parenteral administration is intravenous, intramuscular, intraarterial, intrameningral, intraarticular, intraorbital, intracardiac, intradermal, intraperitoneal, vascular, subcutaneous, dermal, and intraarterial, subcapsular, arachnoid Lower, refers to the route of administration generally associated with injection, including, but not limited to, intrathecal and / or intrasternal injection and / or infusion.
HPP 또는 이의 약학적 조성물은 각 투여 경로에 적합한 제형 또는 제제의 형태로 대상체에게 제공될 수 있다. 본 발명의 방법에서 유용한 제형은 하나 이상의 HPP, 이에 대한 하나 이상의 약학적으로 허용되는 담체, 및 임의의 기타 치료학적 성분을 포함한다. 제형들은 단위 용량 형태로 편리하게 제공될 수 있으며 제약 분야에 잘 공지된 임의의 방법으로도 제조될 수 있다. 담체 물질과 조합되어 단일 용량 형태를 생성할 수 있는 활성 성분의 양은 치료하는 대상체 및 특정 투여 방식에 따라 변할 것이다. 담체 물질과 결합되어 약학적으로 유효한 투여량을 생성할 수 있는 HPP의 양은 일반적으로 치료학적 효과를 생산하는 HPP의 양일 것이다. 일반적으로, 100중량% 중에서, HPP/HPC의 당해 양은 약 1% 내지 약 99%의 HPP, 바람직하게는 약 1% 내지 약 20%의 범위일 것이다.The HPP or pharmaceutical composition thereof can be provided to a subject in the form of a formulation or formulation suitable for each route of administration. Formulations useful in the methods of the present invention include one or more HPPs, one or more pharmaceutically acceptable carriers thereof, and any other therapeutic ingredient. The formulations can be conveniently provided in unit dose form and can be prepared by any method well known in the pharmaceutical arts. The amount of active ingredient that can be combined with a carrier material to produce a single dosage form will vary depending on the subject being treated and the particular mode of administration. The amount of HPP that can be combined with a carrier material to produce a pharmaceutically effective dosage will generally be that amount of HPP that produces a therapeutic effect. Generally, out of 100% by weight, this amount of HPP / HPC will range from about 1% to about 99% HPP, preferably from about 1% to about 20%.
이들 제형 또는 조성물을 제조하는 방법은 HPP와 하나 이상의 약학적으로 허용되는 담체 및, 임의로 하나 이상의 보조 성분을 결합하는 단계를 포함한다. 일반적으로, 제형은 HPP를 액체 담체, 또는 미분된 고체 담체, 또는 이들 둘 다와 균일하고 치밀하게 결합한 후, 경우에 따라 생성물로 성형시킴으로써 제조한다Methods of making these formulations or compositions include combining HPP with one or more pharmaceutically acceptable carriers, and optionally one or more accessory ingredients. Generally, formulations are prepared by uniformly and intimately binding the HPP with a liquid carrier, or a finely divided solid carrier, or both, and then, if necessary, molding into a product.
경구 투여에 적합한 제형은 캡슐제, 사쉐제, 환제, 정제, 함당정제 (풍미화 기재(flavored basis), 일반적으로 수크로스 및 아카시아 또는 트래커캔스 사용), 분말, 과립제의 형태, 또는 수성 또는 비-수성 액체 중 액제 또는 현탁제, 또는 수중유(oil-in-water) 또는 유중수(water-in-oil) 에멀젼, 또는 엘릭시르제 또는 시럽제, 또는 향정제 (젤라틴 및 글리세린과 같은 불활성 기재, 또는 수크로스 및 아카시아 사용) 및/또는 구강 세척액 등의 형태일 수 있으며, 각각은 활성 성분으로서 소정량의 HPP를 함유한다. 화합물은 또한 볼루스(bolus), 연약(electuary) 또는 페이스트로서 투여될 수 있다Formulations suitable for oral administration include capsules, sachets, pills, tablets, tablets (with flavored basis, usually using sucrose and acacia or trachacans), powders, granules, or aqueous or non- -Liquids or suspensions in aqueous liquids, or oil-in-water or water-in-oil emulsions, or elixirs or syrups, or flavoring agents (inert substrates such as gelatin and glycerin, or Sucrose and acacia) and / or mouthwash, etc., each containing a predetermined amount of HPP as an active ingredient. The compound can also be administered as a bolus, electuary or paste.
경구 투여용 고체 용량 형태 (예를 들면, 캡슐제, 정제, 환제, 당의제, 분말, 과립제 등)에서, HPP는 시트르산나트륨 또는 인산이칼슘과 같은 하나 이상의 약학적으로-허용되는 담체, 및/또는 다음 중의 어느 하나와 혼합된다: (1) 전분, 락토오스, 수크로스, 글루코스, 만니톨 및/또는 규산과 같은 충전제 또는 연장제; (2) 예를 들면, 카복시메틸셀룰로스, 알기네이트, 젤라틴, 폴리비닐 피롤리돈, 수크로스 및/또는 아카시아와 같은 결합제; (3) 글리세롤과 같은 보습제; (4) 한천-한천, 탄산칼슘, 감자 또는 타피오카 전분, 알긴산, 특정 실리케이트, 및 탄산나트륨과 같은 붕해제, (5) 파라핀과 같은 용해 지연제, (6) 4급 암모늄 화합물과 같은 흡수 촉진제; (7) 예를 들면, 아세틸 알코올 및 글리세롤 모노스테아레이트와 같은 습윤제; (8) 카올린 및 벤토나이트 점토와 같은 흡수제; (9) 활석, 스테아르산칼슘, 스테아르산마그네슘, 고체 폴리에틸렌 글리콜, 나트륨 라우릴 설페이트, 및 이의 혼합물과 같은 윤활제; 및 (10) 착색제. 캡슐제, 정제 및 환제의 경우에, 약학적 조성물은 또한 완충제를 포함할 수 있다. 유사한 유형의 고체 조성물은 또한 연질 및 경질-충전된 젤라틴 캡슐 속에 락토오스 또는 유당과 같은 이러한 부형제, 및 고분자량 폴리에틸렌 글리콜 등을 이용하여 충전제로 사용될 수 있다.In solid dosage forms for oral administration (eg, capsules, tablets, pills, dragees, powders, granules, etc.), HPP is one or more pharmaceutically-acceptable carriers such as sodium citrate or dicalcium phosphate, and / or Or mixed with any of the following: (1) fillers or extenders such as starch, lactose, sucrose, glucose, mannitol and / or silicic acid; (2) binders such as, for example, carboxymethylcellulose, alginate, gelatin, polyvinyl pyrrolidone, sucrose and / or acacia; (3) moisturizers such as glycerol; (4) agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and disintegrants such as sodium carbonate, (5) dissolution retarders such as paraffin, (6) absorption accelerators such as quaternary ammonium compounds; (7) humectants such as, for example, acetyl alcohol and glycerol monostearate; (8) absorbents such as kaolin and bentonite clay; (9) lubricants such as talc, calcium stearate, magnesium stearate, solid polyethylene glycol, sodium lauryl sulfate, and mixtures thereof; And (10) colorants. In the case of capsules, tablets and pills, the pharmaceutical composition may also include a buffer. Similar types of solid compositions can also be used as fillers using such excipients such as lactose or lactose in soft and hard-filled gelatin capsules, and high molecular weight polyethylene glycols.
정제는 임의로 하나 이상의 보조 성분과 함께 압착 또는 성형에 의해 제조할 수 있다. 압착된 정제는 결합제 (예를 들면, 젤라틴 또는 히드록시프로필메틸 셀룰로스), 윤활제, 불활성 희석제, 방부제, 붕해제 (예를 들면,나트륨 전분 글리콜레이트 또는 가교-결합된 나트륨 카복시메틸 셀룰로스), 표면-활성 또는 붕해제를 사용하여 제조할 수 있다. 성형된 정제는 적합한 기계내에서 불활성 액체 희석제로 습윤화시킨 분말화된 항균제 또는 펩티도미메틱(peptidomimetic)의 혼합물을 성형하여 제조할 수 있다. 정제, 및 당의제, 캡슐제, 환제 및 과립제와 같은 기타 고체 용량 형태는 장 피복물 및 약제-제형 분야에 잘 공지된 기타 피복물과 같은 피복물 및 쉘(shell)과 함께 임의로 스코어링(scoring)하거나 제조할 수 있다. 이들은 또한 예를 들면, 바람직한 방출 프로파일을 제공하기 위해 다양한 비로 히드록시프로필메틸 셀룰로스, 기타 중합체 기질, 리포좀 및/또는 미세구를 사용하여, HPP의 지연되거나 조절된 방출을 제공하도록 제형화될 수 있다. 이들은 예를 들면, 세균-보유 필터를 통한 여과에 의해, 또는 멸균수에 용해되거나 일부 다른 멸균 주사가능한 매질 속에 사용 직전에 용해시킬 수 있는 멸균 고체 조성물의 형태로 멸균화제를 혼입시킴으로써 멸균시킬 수 있다. 이들 조성물은 또한 임의로 진정화제(pacifying agent)를 함유할 수 있거나, 이들이 HPP(들)만을, 또는 바람직하게는 위장관의 특정 부위에서, 임의로 지연된 방식으로 우선적으로 방출하는 조성물일 수 있다. 사용될 수 있는 포매 조성물의 예는 중합체성 물질 및 왁스를 포함한다. HPP는 또한, 적절한 경우, 하나 이상의 위에서 기술한 성분과의 미세-봉입된 형태로 존재할 수 있다Tablets may be made by compression or molding, optionally with one or more accessory ingredients. Compressed tablets include binders (e.g. gelatin or hydroxypropylmethyl cellulose), lubricants, inert diluents, preservatives, disintegrants (e.g. sodium starch glycolate or cross-linked sodium carboxymethyl cellulose), surface- It can be prepared using active or disintegrating agents. Molded tablets can be made by molding a mixture of a powdered antimicrobial agent or peptidomimetic wetted with an inert liquid diluent in a suitable machine. Tablets and other solid dosage forms such as dragees, capsules, pills, and granules may be optionally scored or prepared with coatings and shells, such as enteric coatings and other coatings well known in the pharmaceutical-formulation art. You can. They can also be formulated to provide delayed or controlled release of HPP, using, for example, hydroxypropylmethyl cellulose, other polymer substrates, liposomes and / or microspheres in various ratios to provide the desired release profile. . They can be sterilized, for example, by filtration through a bacteria-retaining filter, or by incorporating a sterilizing agent in the form of a sterile solid composition that can be dissolved in sterile water or dissolved in some other sterile injectable medium immediately prior to use. . These compositions may also optionally contain a pacifying agent, or they may be a composition that preferentially releases only the HPP (s), or preferably in a specific region of the gastrointestinal tract, optionally in a delayed manner. Examples of embedding compositions that can be used include polymeric materials and waxes. HPP may also be present in micro-encapsulated form with one or more of the ingredients described above, where appropriate.
경구 투여용 액체 투여 형태는 약학적으로 허용되는 에멀젼, 미세에멀젼, 액제, 현탁제, 시럽제 및 엘릭시르제를 포함한다. HPP 외에, 액체 투여 형태는 예를 들면, 물 또는 기타 용매, 가용화제 및 유화제, 예를 들면, 에틸 알코올, 이소프로필 알코올, 에틸 카보네이트, 에틸 아세테이트, 벤질 알코올, 벤질 벤조에이트, 프로필렌 글리콜, 1,3-부틸렌 글리콜, 오일 (특히, 면화씨, 땅콩, 옥수수, 배(germ), 올리브, 캐스터 및 참깨 오일), 글리세롤, 테트라하이드로푸릴 알코올, 폴리에틸렌 글리콜 및 소르비탄의 지방산 에스테르, 및 이의 혼합물과 같이 당해 분야에서 일반적으로 사용된 불활성 희석제를 함유할 수 있다. 불활성 희석제 외에, 상기 경구 조성물은 또한 습윤제, 유화제 및 현탁화제, 감미제, 풍미제, 착색제, 향료 및 방부제와 같은 보조제를 포함할 수 있다.Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, microemulsions, liquids, suspensions, syrups and elixirs. In addition to HPP, liquid dosage forms are, for example, water or other solvents, solubilizers and emulsifiers, such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1, 3-butylene glycol, oil (especially cottonseed, peanut, corn, germ, olive, castor and sesame oil), fatty acid esters of glycerol, tetrahydrofuryl alcohol, polyethylene glycol and sorbitan, and mixtures thereof It may contain inert diluents commonly used in the art. In addition to inert diluents, the oral composition may also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening agents, flavoring agents, coloring agents, flavoring agents and preservatives.
현탁제는, HPP 외에, 예를 들면, 에톡실화 이소스테아릴 알코올, 폴리옥시에틸렌 소르비톨 및 소르비탄 에스테르, 미세결정성 셀룰로즈, 알루미늄 메타하이드록사이드, 벤토나이트, 한천-한천 및 트래거캔스 및 이의 혼합물과 같은 현탁화제를 함유할 수 있다.Suspension agents, in addition to HPP, for example, ethoxylated isostearyl alcohol, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth and mixtures thereof It may contain a suspending agent such as.
직장 또는 질 투여용 제형은 하나 이상의 HPP를 예를 들면, 코코아 버터, 폴리에틸렌 글리콜, 좌제 왁스 또는 살리실레이트를 포함하는 하나 이상의 적합한 비자극성 부형제 또는 담체와 혼합될 수 있거나, 실온에서 고체이지만 체온에서 액체이어서 직장 또는 질강 속에서 용융되어 활성제를 방출하는 좌제로서 제공될 수 있다. 질 투여에 적합한 제형은 또한 당해 분야에서 적절한 것으로 공지된 담체를 함유하는 페서리, 탐폰, 크림, 겔, 페이스트, 발포제 또는 스프레이 제형을 또한 포함한다.Formulations for rectal or vaginal administration may be mixed with one or more HPPs with one or more suitable non-irritating excipients or carriers including, for example, cocoa butter, polyethylene glycol, suppository waxes or salicylates, or solid at room temperature but at body temperature It can be a liquid and then served as a suppository that melts in the rectum or vaginal steel to release the active agent. Formulations suitable for vaginal administration also include pessary, tampon, cream, gel, paste, blowing agent or spray formulations containing carriers known to be suitable in the art.
HPP 조성물의 국소 또는 경피 또는 표피 또는 피부 투여용 제형은 산제, 스프레이제, 연고제, 페이스트제, 크림제, 로션제, 겔제, 액제, 펫치 및 흡입제를 포함한다. 활성 성분은 멸균 조건하에서 약학적으로 허용되는 담체, 및 필요할 수 있는 임의의 방부제, 완충제 또는 추진제와 혼합될 수 있다. 연고제, 페이스트제, 크림제 및 겔제는 HPP 조성물 외에, 동물성 및 식물성 지방, 오일, 왁스, 파라핀, 전분, 트라가칸트, 셀룰로스 유도체, 폴리에틸렌 글리콜, 실리콘, 벤토나이트, 규산, 활석 및 산화아연, 또는 이의 혼합물과 같은 부형제를 함유할 수 있다. 산제 및 분무제는 HPP 조성물 외에, 락토오스, 활석, 규산, 수산화알루미늄, 규산칼슘 및 폴리아미드 분말, 또는 이들 물질의 혼합물과 같은 부형제를 함유할 수 있다. 분무제는 클로로플루오로하이드로카본과 같은 통상 추진제, 및 부탄 및 프로판과 같은 휘발성의 치환되지 않은 탄화수소를 추가로 함유한다. 국소 또는 경피투여를 위한 최선의 제형은 순수한 물, 용액, 수성용액, 에탄올 수용액, 및 이소프로판올 수용액이다.Formulations for topical or transdermal or epidermal or skin administration of HPP compositions include powders, sprays, ointments, pastes, creams, lotions, gels, liquids, fetches and inhalants. The active ingredient can be mixed under sterile conditions with a pharmaceutically acceptable carrier, and any preservatives, buffers or propellants that may be needed. Ointments, pastes, creams and gels, in addition to HPP compositions, animal and vegetable fats, oils, waxes, paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols, silicones, bentonite, silicic acid, talc and zinc oxide, or the like Excipients such as mixtures. Powders and sprays may contain, in addition to the HPP composition, excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium silicate and polyamide powder, or mixtures of these materials. The nebulizer further contains conventional propellants such as chlorofluorohydrocarbons, and volatile unsubstituted hydrocarbons such as butane and propane. The best formulations for topical or transdermal administration are pure water, solutions, aqueous solutions, aqueous ethanol solutions, and aqueous isopropanol solutions.
HPP 또는 이의 약학적 조성물은 달리 에어로졸에 의해 투여될 수 있다. 이는 HPP를 함유하는 수성 에어로졸, 리포좀 제제 또는 고체 입자를 제조함으로써 달성할 수 있다. 비수성 (예를 들면, 플루오로카본 추진제) 현탁액을 사용할 수 있다. 초음파 분무기(sonic nebulizer)를 또한 사용할 수 있다. 수성 에어로졸제는 제제의 수용액 또는 현탁액을 통상의 약학적으로 허용되는 담체 및 안정화제와 함께 제형화하여 제조한다. 담체 및 안정화제는 특정 화합물의 요구도에 따라 변하지만, 통상적으로 비이온성 표면활성제 (트윈(Tween), 플루로닉 (Pluronic) 또는 폴리에틸렌 글리콜), 혈청 알부민과 같은 무해한 단백질, 소르비탄 에스테르, 올레산, 레시틴, 글리신과 같은 아미노산, 완충제, 염, 당 또는 당 알코올을 포함한다. 에어로졸제는 일반적으로 등장성 용액으로부터 제조한다.HPP or a pharmaceutical composition thereof can be administered by aerosol otherwise. This can be achieved by preparing an aqueous aerosol, liposome formulation or solid particles containing HPP. Non-aqueous (eg, fluorocarbon propellant) suspensions can be used. Sonic nebulizers can also be used. Aqueous aerosols are prepared by formulating an aqueous solution or suspension of the formulation together with a conventional pharmaceutically acceptable carrier and stabilizer. Carriers and stabilizers vary depending on the needs of the particular compound, but are typically nonionic surfactants (Tween, Pluronic or polyethylene glycol), harmless proteins such as serum albumin, sorbitan esters, oleic acid, Amino acids such as lecithin, glycine, buffers, salts, sugars or sugar alcohols. Aerosols are generally prepared from isotonic solutions.
경피 패치는 또한 HPP 조성물을 표적 부위로 전달하기 위해 사용될 수 있다. 이러한 제형은 제제를 적절한 매질 속에 용해시키거나 분산시켜 제조할 수 있다. Transdermal patches can also be used to deliver HPP compositions to target sites. Such formulations can be made by dissolving or dispersing the formulation in the proper medium.
흡수 증진제를 또한 사용하여 피부를 통과하는 펩티도미메틱의 유동을증가시킬 수 있다. 이러한 유동의 속도는 속도 조절 막을 제공하거나 중합체 기질 또는 겔 속에 펩티도미메틱를 분산시켜 조절할 수 있다.Absorption enhancers can also be used to increase the flow of peptidomimetic across the skin. The rate of this flow can be controlled by either providing a rate controlling membrane or dispersing the peptidomeric in a polymer matrix or gel.
안과용 제형, 안 연고제, 산제, 액제 등도 또한 본 발명의 범위 내에 있는 것으로 고려된다.Ophthalmic formulations, ointments, powders, liquids and the like are also contemplated as being within the scope of the present invention.
비경구 투여에 적합한 제형은 하나 이상의 약학적으로 허용되는 멸균 등장성 수성 또는 비수성 용액, 분산액, 현탁액 또는 에멀젼과 배합된 HPP/HPC, 또는 사용 직전에 멸균 주사가능한 용액 또는 분산액으로 재구성될 수 있고, 항산화제, 완충제, 세균정지제, 제형이 의도된 수용체의 혈액과 등장성이 되도록 하는 용질, 또는 현탁화제 또는 증점제를 함유할 수 있는 멸균 분말과 배합된 HPP/HPC를 포함할 수 있다.Formulations suitable for parenteral administration may be reconstituted with one or more pharmaceutically acceptable sterile isotonic aqueous or non-aqueous solutions, dispersions, suspensions or emulsions in combination with HPP / HPC, or sterile injectable solutions or dispersions immediately prior to use. , HPP / HPC in combination with antioxidants, buffers, bacteriostatic agents, solutes that render the formulation isotonic with the blood of the intended receptor, or sterile powders that may contain suspending or thickening agents.
비경구 투여에 적합한 제형에 사용될 수 있는 적합한 수성 및 비수성 담체의 예는 물, 에탄올, 폴리올(예를 들면, 글리세롤, 프로필렌 글리콜, 폴리에틸렌 글리콜 등) 및 이의 적합한 혼합물, 올리브 오일과 같은 식물성 오일, 에틸 올레에이트와 같은 주사가능한 유기 에스테르를 포함한다. 적절한 유동성은 예를 들면, 레시틴과 같은 피복 물질을 사용하고, 분산제의 경우에 요구된 입자 크기를 유지함으로써, 및 표면활성제를 사용함으로써 유지시킬 수 있다.Examples of suitable aqueous and non-aqueous carriers that can be used in formulations suitable for parenteral administration include water, ethanol, polyols (e.g. glycerol, propylene glycol, polyethylene glycol, etc.) and suitable mixtures thereof, vegetable oils such as olive oil, And injectable organic esters such as ethyl oleate. Proper fluidity can be maintained, for example, by using a coating material such as lecithin, maintaining the required particle size in the case of dispersants, and by using a surfactant.
비경구 투여에 적합한 제형은 또한 방부제, 습윤제, 유화제 및 분산제와 같은 보조제를 함유할 수 있다. 미생물의 작용의 예방은 각종 항세균제 및 항진균제, 예를 들면, 파라벤, 클로로부탄올, 페놀 소르브산 등을 혼입시켜 보장할 수 있다. 당, 염화나트륨 등과 같은 등장성 제제를 조성물내로 포함시키는 것이 바람직할 수도 있다. 또한, 주사가능한 약학적 형태의 흡수 연장은 알루미늄 모노스테아레이트 및 젤라틴과 같은 흡수를 지연시키는 제제를 혼입시켜 달성할 수 있다.Formulations suitable for parenteral administration may also contain adjuvants such as preservatives, wetting agents, emulsifying agents and dispersing agents. Prevention of the action of microorganisms can be ensured by incorporating various antibacterial and antifungal agents such as parabens, chlorobutanol, phenol sorbic acid, and the like. It may be desirable to include isotonic agents such as sugars, sodium chloride, and the like into the compositions. In addition, prolonged absorption of the injectable pharmaceutical form can be achieved by incorporating agents that delay absorption, such as aluminum monostearate and gelatin.
주사가능한 디포(depot) 형태는 HPP의 미세캡슐화 기질 또는 폴리락타이드-폴리글리콜라이드와 같은 생분해 가능한 중합체 속에 형성시켜 제조한다. 중합체에 대한 HPP의 비율, 및 사용된 특정 중합체의 특성에 따라서, 약물 방출 속도를 조절할 수 있다. 다른 생분해가능한 중합체의 예는 폴리(오르토에스테르) 및 폴리(무수물)을 포함한다. 디포 주사가능한 제형은 또한 HPP를 체 조직과 상용성인 리포좀 또는 미세유액 속에 포획시켜 제조한다.Injectable depot forms are made by forming into microencapsulating substrates of HPP or biodegradable polymers such as polylactide-polyglycolide. Depending on the ratio of HPP to polymer, and the nature of the particular polymer used, the rate of drug release can be controlled. Examples of other biodegradable polymers include poly (orthoesters) and poly (anhydrides). Depot injectable formulations are also prepared by entrapping HPP in liposomes or microemulsions that are compatible with body tissue.
특정 양태에서, 항균제 또는 항균제-관련 화합물의HPP, 또는 이의 약학적 조성물은 치료학적 유효 투여량으로 질환 또는 종양작용 부위에 전달된다. 약리학 분야에서 공지된 바와 같이, 대상 환자에서 치료의 효능 측면에서 가장 효과적인 결과를 수득할 HPP의 약학적 유효 투여량의 정확한 양은, 몇가지만 예를 들면, 특정 HPP의 활성, 특정한 특성, 약동학, 약력학 및 생체이용률, 대상체의 생리학적 병태 (인종, 연령, 성별, 체중, 식이, 질환 유형 및 단계, 일반적인 생리학적 조건, 의약의 제공된 용량 및 유형에 대한 반응성 포함), 제형 중 약제학적으로 허용되는 담체의 특성, 사용되는 투여 경로 및 횟수, 및 병원성 표적 미생물 유기체에 의해 유발된 질환의 중증도 또는 성향에 의존할 것이다. 그러나, 상기 가이드라인은 대상체의 모니터링 및 용량의 조절로 이루어진 통상의 실험을 더 이상 필요로 하지 않는 치료를 미세조정하기 위한 기준, 예를 들면, 투여의 최적 용량을 결정하기 위한 기준으로서 사용될 수 있다. Remington: The Science and Practice of Pharmacy (Gennaro ed. 20th edition, Williams & Wilkins PA, USA) (2000)).In certain embodiments, the HPP of an antimicrobial agent or antimicrobial agent-related compound, or pharmaceutical composition thereof, is delivered to a site of disease or oncology at a therapeutically effective dose. As is known in the field of pharmacology, the exact amount of a pharmaceutically effective dose of HPP that will yield the most effective results in terms of efficacy of treatment in a subject patient is, for example, several, for example, the activity of a particular HPP, specific properties, pharmacokinetics, pharmacodynamics And bioavailability, subject's physiological condition (including race, age, sex, weight, diet, disease type and stage, general physiological conditions, responsiveness to a given dose and type of medicament), pharmaceutically acceptable carrier in the formulation Will depend on the nature of, the route and frequency of administration used, and the severity or nature of the disease caused by the pathogenic target microbial organism. However, the guidelines can be used as criteria for fine-tuning treatments that no longer require routine experimentation consisting of monitoring and adjusting the subject's dose, for example, as a criterion for determining the optimal dose of administration. . Remington: The Science and Practice of Pharmacy (Gennaro ed. 20 th edition, Williams & Wilkins PA, USA) (2000).
IV. 장점IV. Advantages
항균제 (예를 들면, 항생제) 및 항균제-관련 화합물은 때로 친수성이며 피부막 장벽을 통과하기 어렵다. 항균제 또는 항균제-관련 화합물들이 경구로 섭취되면, 이들은 일차통과 대사에 의해 불활성될 수 있다. 주사의 경우, 항균제 및 항균제-관련 화합물들의 투여는 통증을 수반하며, 고가의 병원 방문을 빈번하게 필요로 한다.Antimicrobial agents (eg, antibiotics) and antimicrobial agent-related compounds are sometimes hydrophilic and difficult to cross the skin barrier. When antibacterial or antimicrobial agent-related compounds are taken orally, they can be inactivated by primary pass metabolism. In the case of injection, administration of antimicrobial agents and antimicrobial agents-related compounds is painful and frequently requires expensive hospital visits.
특정 양태에서, 본 발명의 HPP 또는HPC는 하나 이상의 생체 장벽을 통과할 수 있으므로, HPP 또는 HPC는 전신적 투여 (예를 들면, 경구 또는 비경구 투여)의 필요성 없이, 국부적으로 (예를 들면, 국소 또는 경피적으로) 투여되어, 병태가 발생한 부위에 도달하도록 투여될 수 있다. HPP 또는HPC의 국부 투여 및 투과는, 모 제제 또는 약물의 전신적 투여와 비교하여, HPP 또는HPC 가 훨씬 적은 양 또는 용량으로 제제 또는 약물의 국소 농도의 동일한 수준에 도달하도록 하거나; 다르게는, 전신 투여로 제공될 수 없거나, 전신 투여가 가능한 경우 전신 투여시 제제의 상당한 높은 용량을 요구하는 경우, 보다 높은 수준의 국소 농도에 도달하도록 한다. 분해되는 경우 HPP또는HPC 또는 이의 모 제제의 높은 국소 농도는 전신적으로 전달된 모 제제보다 효과적으로 또는 훨씬 신속하게 병태를 치료할 수 있고, 이전에 가능하지 않았거나 관측되지 않았을 새로운 병태를 치료할 수 있도록 한다. HPP 또는HPC 의 국소 투여는, 생물학적 대상체가 전신 투여로 고생할 가능성, 예를 들면, 제제의 전신적 노출과 관련된 부작용, 위장/신장 영향과 관련된 부작용을 감소시킬 수 있다. 또한, 국소 투여는 HPP/HPC 가 다수의 생체 장벽을 통과하여, 예를 들면,일반적인 순환을 통해 전신적으로 도달하도록 함으로써 전신적 투여 (예를 들면, 주사)에 대한 필요성을 피하고 비경구 주사와 관련된 동통을 제거하게 할 수 있다.In certain embodiments, the HPP or HPC of the present invention can cross one or more bio-barriers, such that HPP or HPC is administered locally (e.g., topically) without the need for systemic administration (e.g., oral or parenteral administration). Or transdermally) to reach the site where the condition occurred. Local administration and permeation of HPP or HPC allows HPP or HPC to reach the same level of local concentration of the agent or drug in a much smaller amount or dose compared to systemic administration of the parent agent or drug; Alternatively, a higher level of local concentration should be reached if systemic administration cannot be provided, or if systemic administration is possible, where a systemic administration requires a significant high dose of the formulation. The high local concentration of HPP or HPC or its parent agent when degraded is capable of treating the condition more effectively or much faster than the parent agent delivered systemically, and enabling treatment of new conditions that have not been previously possible or observed. Topical administration of HPP or HPC can reduce the likelihood of biological subjects suffering from systemic administration, e.g., side effects associated with systemic exposure of agents, and side effects associated with gastrointestinal / kidney effects. In addition, topical administration avoids the need for systemic administration (e.g., injection) and pain associated with parenteral injection by allowing HPP / HPC to cross multiple biological barriers, systemically, for example, through general circulation. Can be removed.
특정 양태에서, 본 발명에 따른 HPP/HPC 또는 약학적 조성물은 전신적으로 (예를 들면, 경구 또는 비경구적으로) 투여될 수 있다. HPP/HPC 또는 HPP/HPC의 활성제제 (예를 들면, 약물 또는 대사산물)은 모 제제보다 더 빠른 속도로 일반 순환계로 도입되어 병태의 작용 부위에 보다 신속하게 접근할 수 있다. 또한, HPP/HPC는 모 제제가 단독으로 투여되는 경우 투과되지 않았을 생체 장벽 (예를 들면, 혈액 뇌 장벽 및 혈유 장벽)을 통과함으로써 이전에 가능하거나 관측되지 않을 수 있던 병태의 새로운 치료를 제공한다.In certain embodiments, the HPP / HPC or pharmaceutical composition according to the present invention can be administered systemically (eg, orally or parenterally). Activators of HPP / HPC or HPP / HPC (eg, drugs or metabolites) can be introduced into the general circulatory system at a faster rate than the parent agent, allowing faster access to the site of action of the condition. In addition, HPP / HPC provides a new treatment for conditions that could not have been previously possible or observed by crossing bio-barriers (e.g., blood brain barrier and blood oil barrier) that would not have been penetrated if the parent agent was administered alone. .
예를 들면, 본 발명에서 항균제 또는 항균제-관련 화합물의HPP/HPC는 생체 장벽에 대해 투과율 (예를 들면, 항균제 또는 항균제-관련 화합물을 단독으로 투여하는 경우보다 > 약 10배, > 약 50배, >약 100배, >약 200배, >약 300배, >약 1000배 더 높음)을 입증하였다. 항균제 HPP/HPC를 제공받은 대상체로부터 부작용은 전혀 관측되지 않거나 거의 관측되지 않은 반면, 유사한 용량에서 모 항균제를 제공받은 대상체로부터는 부작용 (예를 들면, 오심)이 관측되었다.For example, in the present invention, the HPP / HPC of an antimicrobial agent or an antimicrobial agent-related compound has a permeability to a bio-barrier (e.g.,> about 10 times,> about 50 times than when an antimicrobial agent or an antimicrobial agent-related compound is administered alone). ,> About 100 times,> about 200 times,> about 300 times,> about 1000 times higher). No or little side effects were observed from subjects receiving the antimicrobial agent HPP / HPC, while side effects (eg, nausea) were observed from subjects receiving the parental antimicrobial agent at similar doses.
항균제 과다 사용으로, 시간 경과에 따른 병원체 돌열변이가 진행됨에 따라 약물 내성이 일반적이고 심각한 문제가 되었다. 본 발명에 의한 HPP/HPC 또는 약학적 조성물은 생체 장벽 예를 들면 생물막, 세균, 진균 및 기타 미생물들의 세포벽을 더 높은 속도로 투과할 수 있고 약물 내성을 극복할 수 있다.With excessive use of antimicrobial agents, drug resistance has become a common and serious problem as pathogen mutations progress over time. The HPP / HPC or pharmaceutical composition according to the present invention can penetrate bio-barriers such as biofilms, bacteria, fungi and other microbial cell walls at higher rates and overcome drug resistance.
V. 실시예V. Examples
하기 실시예는 청구된 발명을 보다 잘 설명하기 위해 제공되며 어떠한 방식으로도 본 발명의 범위를 제한하는 것으로 의도되지 않아야 한다.The following examples are provided to better illustrate the claimed invention and should not be intended to limit the scope of the invention in any way.
하기한 모든 구체적인 조성물, 물질 및 방법은 전체적으로 또는 부분적으로 본 발명의 범주내에 속한다. 이들 구체적인 조성물, 물질 및 방법은 본 발명을 제한하는 것으로 의도되지 않으며, 단지 본 발명의 범주내에 속하는 구체적인 양태를 나열하기 위한 것이다. 당해 분야의 숙련가는 창조적 능력을 발휘하는 실습 없이 및 본 발명의 범주로부터 벗어남이 없이 균등한 조성물, 물질 및 방법을 개발할 수 있다. 본 발명의 범위를 벗어나지 않으면서, 수많은 변형이 본원에 기술된 공정에서 이루어질 수 있다는 것이 이해될것이다. 이러한 변형은 본 발명의 범주 내에 포함된다는 것이 본 발명자들의 의도이다.All specific compositions, materials and methods described below are wholly or partly within the scope of this invention. These specific compositions, materials, and methods are not intended to limit the invention, but are only intended to list specific embodiments falling within the scope of the invention. Those skilled in the art can develop equivalent compositions, materials, and methods without practicing creative ability and without departing from the scope of the present invention. It will be understood that numerous modifications can be made in the processes described herein without departing from the scope of the invention. It is the intention of the inventors that such modifications are included within the scope of the present invention.
실시예 1. 모 약물로부터 HPP 제조.Example 1. Preparation of HPP from parent drug.
특정 양태들에서, 입체이성질체 및 약학적으로 허용되는 염을 포함한 다음 화학식 F-C을 가지는 모 화합물은: In certain embodiments, the parent compound having the formula F-C, including stereoisomers and pharmaceutically acceptable salts, is:
화학식 F-CFormula F-C
화학식 L-1을 가지는 HPP로 전환되며: Converted to HPP with formula L-1:
화학식 L-1Formula L-1
여기에서: From here:
F, L1, L2, 및 L4는 상기에서 정의되고;F, L1, L2, and L4 are defined above;
T는 항균제 또는 항균제-관련 화합물의 HPP의 이송 단위이다. 예를 들면, T는 상기에서 정의된 W 및 R6으로 이루어진 군에서 선택된다.T is the transport unit of the HPP of the antimicrobial agent or antimicrobial agent-related compound. For example, T is selected from the group consisting of W and R6 as defined above.
본 발명의 특정 양태들에서, 화학식 L-1을 가지는 HPP는 화학식 D를 가지는 모 화합물들 또는 모화합물들의 유도체 (예를 들면 모 화합물의 산 할라이드, 혼합 무수물, 등)와: In certain embodiments of the present invention, HPP having Formula L-1 is a parent compound having Formula D or a derivative of the parent compounds (eg, acid halide of the parent compound, mixed anhydride, etc.):
화학식 DFormula D
화학식 E의 화합물과의 반응으로 유기합정에 따라 제조되고 (경로 1): Prepared according to an organic crystal by reaction with a compound of Formula E (Path 1):
T-L2-HTL 2 -H
화학식 EFormula E
여기에서 WC는 OH, 할로겐, 알콕시카보닐 및 치환된 아릴옥시카보닐옥시로 이루어진 군에서 선택되고; Wherein W C is selected from the group consisting of OH, halogen, alkoxycarbonyl and substituted aryloxycarbonyloxy;
F, L1, L2, L4 및 T는 상기에서 정의된다.F, L 1 , L 2 , L 4 and T are defined above.
경로 1. 모 화합물 (I)로부터 HPP 제조.
특정 양태들에서, 화학식 L-1을 가지는 HPP는 상기와 같이 경로 1을 따라 제조되고, 여기에서 L4는 C=O이다. In certain embodiments, HPP having formula L-1 is prepared according to
특정 양태들에서, 입체이성질체 및 약학적으로 허용되는 염을 포함한 다음 화학식 F를 가지는 모 화합물은: In certain embodiments, the parent compound having Formula F, including stereoisomers and pharmaceutically acceptable salts:
화학식 F-NFormula F-N
다음 화학식 G를 가지는 화합물과 반응하여: Reacting with a compound having the formula G:
화학식 G Formula G
화학식 L의 HPP를 얻고: Obtain HPP of formula L:
화학식 L-1 Formula L-1
여기에서: From here:
F, L1, L2, 및 L4는 상기에서 정의되고;F, L1, L2, and L4 are defined above;
T는 항균제 또는 항균제-관련 화합물 HPP의 이송단위이다. 예를 들면, T는 상기에서 정의된 W 및 R6으로 이루어진 군에서 선택되고;T is the transport unit of the antimicrobial agent or antimicrobial agent-related compound HPP. For example, T is selected from the group consisting of W and R 6 as defined above;
M은 Na, K, 또는 기타 금속으로 이루어진 군에서 선택된다. WN은 OH, 할로겐, 알콕시카보닐 및 치환된 아릴옥시카보닐옥시로 이루어진 군에서 선택된다. (경로 2)M is selected from the group consisting of Na, K, or other metals. W N is selected from the group consisting of OH, halogen, alkoxycarbonyl and substituted aryloxycarbonyloxy. (Path 2)
경로 2. 모 화합물 (II)로부터 HPP 제조.
특정 양태에서, 화학식 L-1의 구조를 가지는 HPP는 이송 단위를 관능성 단위와 연결하기 전에 상기된 합성 경로의 하나에 따라 -C(=O)OH, -NH2, -OH, 또는 -SH 와 같이 원하지 않는 반응 부위를 보호하는 유기 합성에 의해 제조된다. 특정 양태에서, 수득된 보호 HPP는 부분적으로 또는 완전히 탈보호되어 각각 부분 보호 HPP 또는 탈보호 HPP에 이른다In certain embodiments, HPPs having the structure of Formula L-1 have -C (= O) OH, -NH 2 , -OH, or -SH depending on one of the synthetic routes described above before linking the transport unit with the functional unit. It is prepared by organic synthesis that protects unwanted reaction sites. In certain embodiments, the obtained protective HPP is partially or completely deprotected leading to partially protected HPP or deprotected HPP, respectively.
6- 페녹시아세트아세트아미도페니실란산 2- 디에틸아미노에틸 에스테르 염산염의 제조 Preparation of 6- phenoxyacetacetamidophenylanic acid 2 -diethylaminoethyl ester hydrochloride
39 g의 페니실린 V 칼륨염을 100 ml의 아세토니트릴에 용해하였다. 에틸 아세테이트 중의 39 g 2-브로모-N,N-디에틸에틸아민. HBr를 반응혼합물에 첨가하였다. 혼합물을 16 h 동안 RT에서 교반하였다. 39 g (0.15 mol)의 2-브로모-N,N-디에틸에틸아민.HBr 및 30g의 탄산수소나트륨을 반응혼합물에 첨가하였다. 혼합물을 다시 12 h 동안 RT에서 교반하였다. 여과시켜 고체를 제거하였다. in 50 ml 에테르 중의3.5g HCl을 교반하면서 반응혼합물에 첨가하였다. 여과하여 고체 생성물을 모았다. 건조한 후, 원하는 흡습성 생성물38g을 수득하였다 (78.2%). 원소분석: C22H32ClN3O5S; MW: 486.0. 계산치 % C: 54.37; H: 6.64; N: 8.65; Cl: 7.29; O: 16.46; S: 6.60; 실측치 % C: 54.32; H: 6.68; N: 8.61; Cl: 7.32; O: 16.51; S: 6.56.39 g of penicillin V potassium salt was dissolved in 100 ml of acetonitrile. 39 g 2-bromo-N, N-diethylethylamine in ethyl acetate. HBr was added to the reaction mixture. The mixture was stirred for 16 h at RT. 39 g (0.15 mol) of 2-bromo-N, N-diethylethylamine. HBr and 30 g of sodium hydrogen carbonate were added to the reaction mixture. The mixture was stirred again for 12 h at RT. The solid was removed by filtration. 3.5 g HCl in 50 ml ether was added to the reaction mixture with stirring. Filtration collected the solid product. After drying, 38 g of the desired hygroscopic product was obtained (78.2%). Elemental analysis: C 22 H 32 ClN 3 O 5 S; MW: 486.0. Calculated% C: 54.37; H: 6.64; N: 8.65; Cl: 7.29; O: 16.46; S: 6.60; Found% C: 54.32; H: 6.68; N: 8.61; Cl: 7.32; O: 16.51; S: 6.56.
6-(2,6- 디메톡시벤즈아미도 ) 페니실린산 2- 디에틸아미노에틸 에스테르 염산염의 제조 Preparation of 6- (2,6 -dimethoxybenzamido ) penicillic acid 2 -diethylaminoethyl ester hydrochloride
38g의 6-(2,6-디메톡시벤즈아미도)페니실린산을 300 ml의 클로로포름에 용해하였다. 20.6의 N, N'-디시클로헥실카보디이미드를 반응혼합물에 첨가하였다. 11.7g의 N,N-디메틸아미노에탄올 및 2g의 4-디메틸아미노피리딘을 반응혼합물에 첨가하였다. 혼합물을 10 시간 동안 RT에서 교반하였다. 여과하여 고체를 제거하였다. 클로로포름 용액을 5% NaHCO3 (2 x 100 ml) 및 물 (3 x 100 ml)로 세척하였다. 유기 용액을 무수 황산나트륨에서 건조하였다. 황산나트륨을 여과하여 제거하였다. 50ml 에테르 중의3.5g HCl을 교반하면서 반응혼합물에 첨가하였다. 고체 생성물을 여과하여 수집하였다. 건조한 후, 원하는 흡습성 생성물40g을 얻었다 (77.5%). 원소분석: C23H34ClN3O6S; MW: 516.05. 계산치 % C: 53.53; H: 6.64; N: 8.14; Cl: 6.87; O: 18.60; S: 6.21; 실측치 % C: 53.49; H: 6.68; N: 8.11; Cl: 6.90; O: 18.64; S:6.18.38 g of 6- (2,6-dimethoxybenzamido) penicillic acid was dissolved in 300 ml of chloroform. 20.6 of N, N'-dicyclohexylcarbodiimide was added to the reaction mixture. 11.7 g of N, N-dimethylaminoethanol and 2 g of 4-dimethylaminopyridine were added to the reaction mixture. The mixture was stirred at RT for 10 hours. The solid was removed by filtration. The chloroform solution was washed with 5% NaHCO 3 (2 × 100 ml) and water (3 × 100 ml). The organic solution was dried over anhydrous sodium sulfate. Sodium sulfate was removed by filtration. 3.5 g HCl in 50 ml ether was added to the reaction mixture with stirring. The solid product was collected by filtration. After drying, 40 g of the desired hygroscopic product was obtained (77.5%). Elemental analysis: C 23 H 34 ClN 3 O 6 S; MW: 516.05. Calculated% C: 53.53; H: 6.64; N: 8.14; Cl: 6.87; O: 18.60; S: 6.21; Found% C: 53.49; H: 6.68; N: 8.11; Cl: 6.90; O: 18.64; S: 6.18.
아세트아미도페닐아세트아미도페니실린산 2- 디에틸아미노프로필에스테르염산 염 제조 Preparation of acetamidophenyl acetamidopenicillic acid 2 -diethylaminopropyl ester hydrochloride
43g의 아세트아미도페닐아세트아미도페니실린산 나트륨염을 100 ml의 아세토니트릴에 용해하였다. 에틸 아세테이트 중의 40g 2-브로모-N,N-디에틸프로필아민.HBr을 반응혼합물에 첨가하였다. 혼합물을 16 h 동안 RT에서 교반하였다. 40g의 2-브로모-N,N-디에틸프로필아민.HBr 및 30g의 탄산수소나트륨을 반응혼합물에 첨가하였다. 혼합물을 다시 12 h 동안 RT에서 교반하였다. 여과하여 고체를 제거하였다. 50 ml 에테르 중의3.5g HCl을 교반하면서 반응혼합물에 첨가하였다. 고체 생성물을 여과하여 수집하였다. 건조한 후, 원하는 흡습성 생성물35g을 수득하였다. 원소분석: C26H33ClN4O5S; MW: 541.11. 계산치 % C: 55.49; H: 6.89; N: 10.35; Cl: 6.55; O: 14.78; S: 5.92; 실측치 % C: 55.44; H: 6.92; N: 10.32; Cl: 6.58; O: 14.82; S:5.92.43 g of acetamidophenylacetamidopenicillin sodium salt was dissolved in 100 ml of acetonitrile. 40 g 2-bromo-N, N-diethylpropylamine.HBr in ethyl acetate was added to the reaction mixture. The mixture was stirred for 16 h at RT. 40 g of 2-bromo-N, N-diethylpropylamine.HBr and 30 g of sodium hydrogen carbonate were added to the reaction mixture. The mixture was stirred again for 12 h at RT. The solid was removed by filtration. 3.5 g HCl in 50 ml ether was added to the reaction mixture with stirring. The solid product was collected by filtration. After drying, 35 g of the desired hygroscopic product was obtained. Elemental analysis: C 26 H 33 ClN 4 O 5 S; MW: 541.11. Calculated% C: 55.49; H: 6.89; N: 10.35; Cl: 6.55; O: 14.78; S: 5.92; Found% C: 55.44; H: 6.92; N: 10.32; Cl: 6.58; O: 14.82; S: 5.92.
6-(5- 메틸 -3-페닐-2- 이속사졸린 -4- 카복사미도 ) 페니실린산 4- 피페리딘에틸에 스테르염산염 제조 6- (5-methyl-2-isoxazolyl-4-carboxamide shown) penicillin acid 4-ethyl piperidine's blood in Terre hydrochloride prepared
50g의 6-(5-메틸-3-페닐-2-이속사졸린-4-카복사미도)페니실린산 나트륨염을 100ml의 아세토니트릴에 용해하였다. 에틸 아세테이트 중의38g 4-피페리딘에틸 브롬화물.HBr을 반응혼합물에 첨가하였다. 혼합물을 16 h 동안 RT에서 교반하였다. 38g의 4-피페리딘에틸 브롬화물.HBr 및 30g의 탄산수소나트륨을 반응혼합물에 첨가하였다. 혼합물을 다시 12 h 동안 RT에서 교반하였다. 여과하여 고체를 제거하였다. 50 ml 에테르 중의3.5g HCl을 교반하면서 반응혼합물에 첨가하였다. 고체 생성물을 여과하여 수집하였다. 건조한 후, 원하는 흡습성 생성물 30g을 얻었다. 원소분석: C26H33ClN4O5S; MW: 549.08. 계산치 % C: 56.88; H: 6.06; N: 10.20; Cl: 6.46; O: 14.57; S: 5.83; 실측치 % C: 56.85; H: 6.08; N: 10.19; Cl: 6.47; O: 14.59; S:5.82. 50 g of 6- (5-methyl-3-phenyl-2-isoxazoline-4-carboxamido) penicillic acid sodium salt was dissolved in 100 ml of acetonitrile. 38 g 4-piperidine ethyl bromide in ethyl acetate. HBr was added to the reaction mixture. The mixture was stirred for 16 h at RT. 38 g of 4-piperidineethyl bromide. HBr and 30 g of sodium hydrogen carbonate were added to the reaction mixture. The mixture was stirred again for 12 h at RT. The solid was removed by filtration. 3.5 g HCl in 50 ml ether was added to the reaction mixture with stirring. The solid product was collected by filtration. After drying, 30 g of the desired hygroscopic product was obtained. Elemental analysis: C 26 H 33 ClN 4 O 5 S; MW: 549.08. Calculated% C: 56.88; H: 6.06; N: 10.20; Cl: 6.46; O: 14.57; S: 5.83; Found% C: 56.85; H: 6.08; N: 10.19; Cl: 6.47; O: 14.59; S: 5.82.
3-[[( 아미노카보닐 ) 옥시 ] 메틸 ]-7- 메톡시 -8-옥소-7-[(2- 티에닐아세틸 )아미노] -5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸에스테르염산염 제조 3-[[( aminocarbonyl ) oxy ] methyl ] -7 -methoxy -8-oxo-7-[(2 -thienylacetyl ) amino] -5-thia-1-azabicyclo [4.2.0] Production of oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride
41g의 3-[[(아미노카보닐)옥시]메틸]-7-메톡시-8-옥소-7-[(2-티에닐아세틸)아미노]-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 나트륨염을 100 ml의 아세토니트릴에 용해하였다. 에틸 아세테이트 중의 35g 2-브로모-N,N-디에틸에틸아민. HBr을 반응혼합물에 첨가하였다. 혼합물을 16 h 동안 RT에서 교반하였다. 30g의 2-브로모-N,N-디에틸에틸아민.HBr 및 30g의 탄산수소나트륨을 반응혼합물에 첨가하였다. 혼합물을 다시 12 h 동안 RT에서 교반하였다. 여과하여 고체를 제거하였다. 50 ml 에테르 중의3.5g HCl을 교반하면서 반응혼합물에 첨가하였다. 고체 생성물을 여과하여 수집하였다. 건조한 후, 원하는 흡습성 생성물 30g을 얻었다. 원소분석: C22H31ClN4O7S2; MW: 563.08. 계산치 % C: 46.93; H: 5.55; N: 9.95; Cl: 6.30; O: 19.89; S: 11.39; 실측치 % C: 46.91; H: 5.57; N: 9.93; Cl: 6.32; O: 19.91; S: 11.36. 41 g 3-[[(aminocarbonyl) oxy] methyl] -7-methoxy-8-oxo-7-[(2-thienylacetyl) amino] -5-thia-1-azabicyclo [4.2. 0] Oct-2-ene-2-carboxylate sodium salt was dissolved in 100 ml of acetonitrile. 35 g 2-bromo-N, N-diethylethylamine in ethyl acetate. HBr was added to the reaction mixture. The mixture was stirred for 16 h at RT. 30 g of 2-bromo-N, N-diethylethylamine. HBr and 30 g of sodium hydrogen carbonate were added to the reaction mixture. The mixture was stirred again for 12 h at RT. The solid was removed by filtration. 3.5 g HCl in 50 ml ether was added to the reaction mixture with stirring. The solid product was collected by filtration. After drying, 30 g of the desired hygroscopic product was obtained. Elemental analysis: C 22 H 31 ClN 4 O 7 S 2 ; MW: 563.08. Calculated% C: 46.93; H: 5.55; N: 9.95; Cl: 6.30; O: 19.89; S: 11.39; Found% C: 46.91; H: 5.57; N: 9.93; Cl: 6.32; O: 19.91; S: 11.36.
기타 항균제 또는 항균제-관련 화합물의 HPP들도 유사한 방법으로 합성할 수 있다.HPPs of other antibacterial or antimicrobial agent-related compounds can also be synthesized in a similar manner.
실시예 2. 항균제 및 항균제- 간련 화합물들의 HPP들은 모 약물과 대비하여 더 높은 시험관내 인간 피부 통과 투과율을 보인다. Example 2. Antimicrobial and antibiotic - HPP of ganryeon compounds show higher in vitro human skin pass transmittance in comparison with the parent drug.
인간 피부를 통한 HPP및 모 약물의 투과율은 변경 프란즈 셀로 시험관 내에서 측정되었다. 프란즈 셀은 상부 시료 챔버 및 하부 수용 챔버의 2개의 챔버를 갖는다. 상부 및 수용체 챔버를 분리하는 인간 피부 조직 (360-400 um 두께)은 전방 또는 후방 대퇴부 영역으로부터 분리되었다. The permeability of HPP and parent drug through human skin was measured in vitro with modified Franz cells. The Franz cell has two chambers, an upper sample chamber and a lower receiving chamber. Human skin tissue (360-400 um thick) separating the upper and receptor chambers was separated from the anterior or posterior femoral region.
시험 화합물 (2 mL, 0.2 M 인산완충액에서10%, pH 7.4)을 프란츠 셀 시료 챔버에 넣었다. 수용 챔버는 10 ml의 0.2 M 인산완충액을 포함하고 600 rpm으로 교반되었다. 피부를 통과하는 시험 화합물 함량은 고성능 액체 크로마토그래피 (HPLC) 방법으로 측정되었다. 결과를 도 1a1, 1a2, 1a3, 1a4, 1b 및 1c에 도시하였다. 도 1a1, 1a2, 1a3 및 1a4 기울기로부터 계산된 시험 화합물의 겉보기 유동값 (flux value)들은 표 1a에 요약되었다. 도 1b 및 1c 기울기로부터 계산된 시험 화합물의 겉보기 유동값들은 표 1b 및1c에 각각 요약되었다.The test compound (2 mL, 10% in 0.2 M phosphate buffer, pH 7.4) was placed in a Franz cell sample chamber. The receiving chamber contained 10 ml of 0.2 M phosphate buffer and stirred at 600 rpm. The content of the test compound across the skin was determined by high performance liquid chromatography (HPLC). The results are shown in Figures 1a1, 1a2, 1a3, 1a4, 1b and 1c. The apparent flux values of the test compounds calculated from the slopes of FIGS. 1A1, 1A2, 1A3 and 1A4 are summarized in Table 1A. The apparent flow values of the test compounds calculated from the slopes of FIGS. 1B and 1C are summarized in Tables 1B and 1C, respectively.
본 방법에서 검출가능한 가장 낮은 겉보기 유동값은 1 ug /cm2/h이므로, 이와 동일하거나 더 낮은 겉보기 유동값을 보인 모 약물들은 피부 조직 투과에 대하여 검출될 수 없는 것으로 고려되었다. 겉보기 유동값이 < 1 ug /cm2/h인 모 화합물들 (예를 들면 페니실린 V, 페니실린 O)에 대하여, 이들의 HPP는 검출 가능한 겉보기 유동값을 가졌다. 겉보기 유동값이 > 1 ug /cm2/h인 모 화합물들에 대하여, 이들의 HPP는 더 높은 검출 가능한 겉보기 유동값을 가졌다. 따라서 항균제 또는 항균제-관련 화합물들의 HPP는 모 화합물들과 비교하여 더 높은 피부 조직 통과 투과율 (340-600 배 이상)을 보였다.Since the lowest detectable flow value detectable in this method is 1 ug / cm 2 / h, it was considered that parent drugs that exhibited the same or lower apparent flow value could not be detected for skin tissue penetration. For the parent compounds (eg penicillin V, penicillin O) with an apparent flow value <1 ug / cm 2 / h, their HPP had a detectable apparent flow value. For parent compounds with an apparent flow value> 1 ug / cm 2 / h, their HPP had a higher detectable apparent flow value. Therefore, the HPP of the antimicrobial agent or antimicrobial agent-related compounds showed a higher skin tissue transmissivity (340-600 times or more) compared to the parent compounds.
실시예 3. 피부 및/또는 혈관-뇌 장벽을 통과하는 HPP들의 생체 투과율Example 3. Biotransmittance of HPPs across the skin and / or blood-brain barrier
무손상 누드 마우스의피부 및 혈관-뇌 장벽을 통과하는 베타-락담 항생제 HPP들의 생체 투과율을 연구하였다. 1mL 이소프로판올 중의 6-(2,6-디메톡시벤즈아미도)페니실린산 2-디에틸아미노에틸 에스테르 염산염, 6-(5-메틸-3-페닐-2-이속사졸린-4-카복사미도)페니실린산 2-디에틸아미노에틸 에스테르 염산염, 6-[3-(o-클로로페닐)-5-메틸-4-이속사졸카복사미도]페니실린산 2-디에틸아미노에틸 에스테르 염산염, 메티실린, 옥사실린, 및 클록사실린의 20% 용액으로 이루어진 도너를 각각 누드 마우스의 등 10 cm2 면적에 적용하였다. 2 시간 후, 마우스를 죽였다. 5 ml의 메탄올을 1g의 균질 혈액, 간, 신장, 근육, 또는 뇌에 첨가하였다. 시료를 5분간 원심분리하고 HPLC로 분석하였다 (표 2). 모 약물 (메티실린, 옥사실린, 및 클록사실린)로만 처리된 마우스에서 어떠한 약물도 검출되지 않았다. 이러한 결과는 각각의 모 약물들은 피부를 통과할 수 없지만 이들 전구약물들은 높은 혈관-뇌 장벽 투과율을 가진다는 것을 보였다.The biotransmittance of beta-lactam antibiotic HPPs crossing the skin and blood vessel-brain barriers of intact nude mice was studied. 6- (2,6-dimethoxybenzamido) penicillin 2-diethylaminoethyl ester hydrochloride in 1mL isopropanol, 6- (5-methyl-3-phenyl-2-isoxazoline-4-carboxamido) Penicillic acid 2-diethylaminoethyl ester hydrochloride, 6- [3- (o-chlorophenyl) -5-methyl-4-isoxazolcarboxamido] penicillic acid 2-diethylaminoethyl ester hydrochloride, methicillin, oxa Donors consisting of a 20% solution of silicin and coxasilin were each applied to the back 10 cm 2 area of a nude mouse. After 2 hours, mice were killed. 5 ml of methanol was added to 1 g of homogeneous blood, liver, kidney, muscle, or brain. Samples were centrifuged for 5 minutes and analyzed by HPLC (Table 2). No drugs were detected in mice treated only with the parent drug (methicillin, oxacillin, and coxacillin). These results showed that each parent drug was unable to penetrate the skin, but these prodrugs had a high vascular-brain barrier permeability.
90마리의 수유 젖소들을 선발하였다. 10 ml의 pH 7.4 인산완충액 (0.2 M) 중의 500mg의 6-페녹시아세트아세트아미도페니실란산 2-디에틸아미노에틸 에스테르 염산염 (페니실린 V-DEE), 6-(2,6-디메톡시벤즈아미도)페니실린산 2-디에틸아미노에틸 에스테르 염산염 (메티실린-DEE), 또는 7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (세프티족심-DEE)을 1일 2회 유방 피부에 뿌렸다. 국소 적용1시간 후, 우유 시료들을 취하여 분석하였다 (표 3). 모 약물들 함량을 검출하였다. 이러한 결과들은 이들 전구약물들이 혈유 장벽에 매우 높은 투과율을 가진다는 것을 보인다. 혈유 또는 혈액-뇌 장벽에 대한 이러한 매우 높은 투과율로 인하여, 이들은 뇌, 유방, 전립선 및 기타 감염들 치료에 매우 유용하다.90 feeding cows were selected. 500 mg of 6-phenoxyacetamidophenicylic acid 2-diethylaminoethyl ester hydrochloride (penicillin V-DEE), 6- (2,6-dimethoxybenz) in 10 ml pH 7.4 phosphate buffer (0.2 M) Amido) penicillinic acid 2-diethylaminoethyl ester hydrochloride (methicillin-DEE), or 7-[[(2-acetylamino-4-thiazolyl) (methoxyimino) acetyl] amino] -8-oxo- 5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (ceftoxime-DEE) was applied to the breast skin twice a day. One hour after topical application, milk samples were taken and analyzed (Table 3). The content of the parent drugs was detected. These results show that these prodrugs have very high permeability to the blood oil barrier. Due to this very high permeability to the blood oil or blood-brain barrier, they are very useful in the treatment of brain, breast, prostate and other infections.
실시예 4. 항균제 또는 항균제-관련 화합물들의 HPP는 이들의 모 약물 보다 더 신속하게 세균의 세포벽을 통과한다. Example 4. HPP of antimicrobial agents or antimicrobial agent-related compounds is higher than their parent drug It passes through the cell wall of bacteria more quickly.
0.5 mmol의 시험 화합물 (6-페녹시아세트아세트아미도페니실란산 1-피페리딘에틸 에스테르 염산염 (페니실린 V-PEE), 페니실린 V, 6-(2,6-디메톡시벤즈아미도)페니실린산 2-피롤리딘메틸 에스테르 염산염 (메티실린-PME), 메티실린, 7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (세프티족심-DEE), 또는 세프티족심)을 100 ml의 대장균 현탁액에 첨가하고 3분 동안 교반하였다. 혼합물을 3000 rpm에서 원시분리하였다. 상등액을 버리고 펠렛을 pH 7.4 인산완충액으로 3회 세척하였다. 아세토니트릴 (100 ml)을 펠렛에 첨가하고 혼합물을 60 °C로 2분 동안 가열하였다. 아세토니트릴 용액을 모아 증발시켜 완전히 건조하였다. 시험 화합물 함량을 HPLC로 측정하였다. 결과는 표 4에 나타낸다.0.5 mmol of test compound (6-phenoxyacetacetamidophenylanic acid 1-piperidineethyl ester hydrochloride (penicillin V-PEE), penicillin V, 6- (2,6-dimethoxybenzamido) penicillic acid 2-pyrrolidinemethyl ester hydrochloride (methicillin-PME), methicillin, 7-[[(2-acetylamino-4-thiazolyl) (methoxyimino) acetyl] amino] -8-oxo-5-thia Add -1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (ceftoxime-DEE), or ceftoxime) to 100 ml of E. coli suspension And stirred for 3 minutes. The mixture was primitive separated at 3000 rpm. The supernatant was discarded and the pellet was washed three times with pH 7.4 phosphate buffer. Acetonitrile (100 ml) was added to the pellet and the mixture was heated to 60 ° C for 2 minutes. The acetonitrile solution was collected and evaporated to dryness. Test compound content was determined by HPLC. Table 4 shows the results.
실시예 5 HPP들의 이들 모 약물들로의 전환.Example 5 Conversion of HPPs to these parent drugs.
항균제 또는 항균제-관련 화합물들의HPP는 인간 혈장에서 양호한 수율로 모 항균제 또는 항균제-관련 화합물들로 전환하였다.The HPP of the antimicrobial agent or antimicrobial agent-related compounds was converted to the parent antimicrobial agent or antimicrobial agent-related compounds in good yield in human plasma.
항균제 또는 항균제-관련 화합물 (10 mg)의 HPP를 0.1 ml의 0.2M pH 7.4 인산완충액에 용해하였다. 37 ˚C로 예비가열된 1 ml의 인간혈장을 혼합물에 첨가하였다. 혼합물을 37 ˚C 수조에 두고, 매 2분 간격으로 0.2 ml의 시료를 채취하여 0.4ml 메탄올에 첨가하여 혈장단백질을 침전시켰다. 시료를 5분간 원심분리하고 HPLC로 분석하였다. 결과는 항균제 또는 항균제-관련 화합물의 대부분 HPP는 모 항균제 또는 항균제-관련 화합물들로 다시 전환된 것을 보였다 (표 5).HPP of antimicrobial agent or antimicrobial agent-related compound (10 mg) was dissolved in 0.1 ml of 0.2M pH 7.4 phosphate buffer. 1 ml of human plasma preheated to 37 ° C was added to the mixture. The mixture was placed in a 37 ° C water bath, and 0.2 ml samples were taken every 2 minutes, and added to 0.4 ml methanol to precipitate plasma proteins. Samples were centrifuged for 5 minutes and analyzed by HPLC. The results showed that most HPP of the antimicrobial agent or antimicrobial agent-related compound was converted back to the parent antimicrobial agent or antimicrobial agent-related compound (Table 5).
실시예 6. 항균제 또는 항균제-관련 화합물들의 HPP의 최소억제농도 (MIC) Example 6 Minimum Inhibitory Concentration (MIC) of HPP of Antibacterial or Antimicrobial Agent-Related Compounds
항균제들 및 이들의 전구약물들의 최소억제농도 (MIC)를 Jennifer M. Andrews, Journal of Antimicrobial Chemotherapy 48, suppl. S1, 5-16 (2001)에 따라 평가하였다. 결과 (표 6a-6c)는 항균제들의 HPP는 최소억제농도 (MIC)에 의하면 메티실린-내성 포도상구균 (MRSA)에서 베타-락탐 내성을 극복할 수 있었다는 것을 보였다.The minimum inhibitory concentration (MIC) of antimicrobial agents and their prodrugs was determined by Jennifer M. Andrews, Journal of Antimicrobial Chemotherapy 48, suppl. S1, 5-16 (2001). Results (Table 6a-6c) showed that the HPP of the antibacterial agents was able to overcome the beta-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA) according to the minimum inhibitory concentration (MIC).
실시예 7. 항균제 또는 항균제-관련 화합물들의 HPP의 항진균 활성Example 7. Antifungal activity of HPP of antibacterial or antimicrobial-related compounds
6-페녹시아세트아세트아미도페니실란산 2-디에틸아미노에틸 에스테르 염산염 (페니실린 V-DEE), 6-(2,6-디메톡시벤즈아미도)페니실린산 2-디에틸아미노에틸 에스테르 염산염 (메티실린-DEE), 및 7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (세프티족심-DEE)의 항진균 활성들을 Roether W. 등., Mykosen 27 (1), 14-28 (1984)에 따라 평가하였다. 결과는 아래 표 7에 나열하였다.6-Phenoxyacetamidophenicilanoic acid 2-diethylaminoethyl ester hydrochloride (penicillin V-DEE), 6- (2,6-dimethoxybenzamido) penicillic acid 2-diethylaminoethyl ester hydrochloride ( Methicillin-DEE), and 7-[[(2-acetylamino-4-thiazolyl) (methoxyimino) acetyl] amino] -8-oxo-5-thia-1-azabicyclo [4.2.0] Antifungal activities of oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (ceftoxime-DEE) were evaluated according to Roether W. et al., Mykosen 27 (1), 14-28 (1984) Did. The results are listed in Table 7 below.
실시예 8. 베타-락탐 항생제 또는 관련 화합물들의 HPP를 이용한 임상형 유방염 치료. Example 8 the beta-lactam clinical oil flame treatment using a HPP of antibiotics or related compounds.
90마리의 수유 젖소들을 선발하였다. 치료 전 시료에서 분리된 세균 종들이 17일 및 22일에 감염된 1/4에서 취한 시료들에서 보이지 않는다면 세균학적 치료가 달성된 것으로 고려하였다. 임상적 치료는 치료 하루 전 관찰되는 질환의 임상적 징후가 사라진 것으로, 즉 정상적인 사료섭취, 직장온도 < 39.0˚C, 양호한 전신상태, 유방부종 부재, 정상적인 우유 외관, 및 정상적인 우유 수율로 정하였다. 90 feeding cows were selected. Bacterial treatment was considered to have been achieved if bacterial species isolated from pre-treatment samples were not seen in samples taken from 1/4 infected on days 17 and 22. Clinical treatment was defined as the disappearance of clinical signs of the disease observed one day prior to treatment, ie, normal feed intake, rectal temperature <39.0 ° C, good systemic condition, absence of edema, normal milk appearance, and normal milk yield.
10ml의 pH7.4 인산완충액 (0.2M) 중의 500mg의 6-페녹시아세트아세트아미도페니실란산 2-디에틸아미노에틸 에스테르 염산염 (페니실린 V-DEE), 6-(2,6-디메톡시벤즈아미도)페니실린산 2-디에틸아미노에틸 에스테르 염산염 (메티실린-DEE), 또는 7-[[(2-아세틸아미노-4-티아졸릴)(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염 (세프티족심-DEE)을 1일 2회 유방 피부에 뿌렸다. 결과를 표 8a 및 8b에 나타낸다. 전구약물들은 매우 높은 임상적 치료율 및 세균학적 치료율을 보였다.500 mg of 6-phenoxyacetamidophenicylic acid 2-diethylaminoethyl ester hydrochloride (penicillin V-DEE), 6- (2,6-dimethoxybenz) in 10 ml of pH7.4 phosphate buffer (0.2M) Amido) penicillinic acid 2-diethylaminoethyl ester hydrochloride (methicillin-DEE), or 7-[[(2-acetylamino-4-thiazolyl) (methoxyimino) acetyl] amino] -8-oxo- 5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride (ceftoxime-DEE) was applied to the breast skin twice a day. The results are shown in Tables 8a and 8b. Prodrugs showed very high clinical and bacteriological rates.
실시예 9. 항균성 약물의 전구약물의 항-결핵균 활성.Example 9. Anti-TB activity of prodrugs of antimicrobial drugs.
6주령 암컷 마우스 (BALB/c mice)를 공기전염을 통해 2.21 ±0.15 x 103 CFU 결핵균 H37Rv을 이용하여 감염시켰다. 20일 후, 폐속의 CFU는 8.23±0.27 x107 CFU이었고, 치료를 개시하였다. 그룹 A는 비치료 그룹 (n=20), 그룹 B는 이소니아지드/목시폭사신/피라진아미드 (0.18/0.22/1.2mmol/kg, 경구 투여)로 45일 동안 치료된 그룹, 그룹 C는 이소니아지드/목시폭사신/피라진아미드 (0.18/0.22/1.2mmol/kg, 경구 투여)로 90일 동안 치료된 그룹, 그룹 D는 N-(N-메틸-페닐알라닐)이소니아지드 (프로-이소니아지드, N-메틸페닐알라닌 및 이소니아지드로 제조, 경피 투여)/ 1-시클로프로필-7-[(1S,6S)-2,8-디아자바이시클로 [4.3.0]논-8-일]-6-플루오로-8-메톡시-4-옥소-퀴놀린-3-카복시산 부틸 에스테르(프로-목시폭사신)/피라진산 N,N-디에틸아미노에틸 에스테르 (프로-피라진산, 0.18/0.22/1.2mmol/kg, 경피 투여)로 45일 동안 치료된 그룹, 그룹 E는 프로-이소니아지드/프로-목시폭사신/프로-피라진산 (0.18/0.22/1.2mmol/kg, 경피 투여)로 90일 동안 치료된 그룹, 그룹 F는 프로-이소니아지드/프로-목시폭사신/프로-피라진산 (0.06/0.07/0.4mmol/kg, 경피 투여)로 45일 동안 치료된 그룹, 및 그룹 G는 프로-이소니아지드/프로-목시폭사신/프로-피라진산 (0.06/0.07/0.4mmol/kg, 경피 투여)로 90 동안 치료된 그룹이었다. 치료가 끝난 후, 마우스를 치료없이 추가로 90동안 유지하고 치료를 나타내는 음성 폐 배양물 정도를 결정하기 위하여 죽였다. 결과는 전구약물들이 이들의 모 약물들보다 우수하였고 경피적으로 사용할 수 있다는 것을 보였다 (표 9a 및 9b).6-week-old female mice (BALB / c mice) were infected with 2.21 ± 0.15 x 10 3 CFU Mycobacterium tuberculosis H37Rv via air transmission. After 20 days, the CFU in the lung was 8.23 ± 0.27 × 10 7 CFU, and treatment was started. Group A is a non-treated group (n = 20), group B is isoniazid / moxioxacin / pyrazinamide (0.18 / 0.22 / 1.2mmol / kg, orally administered) for 45 days, group C is isoniazid / moxy Group treated for 90 days with oxacin / pyrazinamide (0.18 / 0.22 / 1.2mmol / kg, administered orally), group D is N- (N-methyl-phenylalanyl) isoniazide (pro-isoniazide, N-methylphenylalanine And isoniazid, transdermal administration) / 1-cyclopropyl-7-[(1S, 6S) -2,8-diazabicyclo [4.3.0] non-8-yl] -6-fluoro-8- Methoxy-4-oxo-quinoline-3-carboxylic acid butyl ester (pro-moxyoxacin) / pyrazine acid N, N-diethylaminoethyl ester (pro-pyrazine acid, 0.18 / 0.22 / 1.2mmol / kg, transdermal Group treated for 45 days with dosing), group E is group treated with pro-isoniazide / pro-moxyoxacin / pro-pyrazine acid (0.18 / 0.22 / 1.2mmol / kg, transdermal) for 90 days, group F The Pro-Isoniazid / F Group treated for 45 days with ro-moxyoxacin / pro-pyrazine acid (0.06 / 0.07 / 0.4mmol / kg, transdermal), and group G is pro-isoniazide / pro-moxyoxacin / pro-pyrazine acid ( 0.06 / 0.07 / 0.4mmol / kg, transdermally) for 90 years. After treatment, mice were maintained for an additional 90 days without treatment and killed to determine the extent of negative lung culture indicating treatment. The results showed that prodrugs were superior to their parent drugs and could be used percutaneously (Tables 9a and 9b).
실시예 10. 성인의 결핵 치료 (아동의 경우 투여 함량을 줄임).Example 10. Treatment of tuberculosis in adults (reduced dosage in children).
3ml의 물 중의 40mg의 N-(N-메틸-페닐알라닐)이소니아지드 (프로-이소니아지드)/ 50mg의 1-시클로프로필-7-[(1S,6S)-2,8-디아자바이시클로 [4.3.0]논-8-일]-6-플루오로-8-메톡시-4-옥소-퀴놀린-3-카복시산 부틸 에스테르(프로-목시폭사신)/40mg의 피라진산 N,N-디에틸아미노에틸 에스테르 (프로- 피라진산)를 환자 가슴 피부 또는 (감염 기관에 가까운) 신체 다른 피부에 매일 아침과 저녁에 (하루 2회) 90일 동안 또는 완쾌될 때까지 적용한다.40 mg of N- (N-methyl-phenylalanyl) isoniazid (pro-isoniazide) / 50 mg of 1-cyclopropyl-7-[(1S, 6S) -2,8-diazabicyclo [4.3 in 3 ml water .0] non-8-yl] -6-fluoro-8-methoxy-4-oxo-quinoline-3-carboxylic acid butyl ester (pro-moxyoxacin) / 40mg pyrazine acid N, N-diethyl Aminoethyl ester (pro-pyrazine acid) is applied to the patient's chest skin or other skin of the body (close to the infectious organ) every morning and evening (twice a day) for 90 days or until complete.
실시예 11. 성인의 나병 또는 한센병 (HD) 치료 ( 아동의 경우 투여 함량을 줄임). Example 11. Treatment of leprosy or Hansen's disease (HD) in adults ( reduced dosage in children ).
3ml의 물 중의 30mg의 4-디메틸아미노부티릴아미도페닐-4`-아미노페닐설폰 (프로-다프손)/ 50mg의 1-시클로프로필-7-[(1S,6S)-2,8-디아자바이시클로 [4.3.0]논-8-일]-6-플루오로-8-메톡시-4-옥소-퀴놀린-3-카복시산 부틸 에스테르(프로-목시폭사신)/ 15 mg의 2(4-디메틸아미노부티릴티오벤즈이미다졸을 환자 신체 감염 기관들에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 6 개월 동안 또는 완쾌될 때까지 적용한다.30mg 4-dimethylaminobutyrylamidophenyl-4`-aminophenylsulfone (pro-dapson) / 50mg 1-cyclopropyl-7-[(1S, 6S) -2,8-dia in 3 ml water Javaicyclo [4.3.0] non-8-yl] -6-fluoro-8-methoxy-4-oxo-quinoline-3-carboxylic acid butyl ester (pro-moxyoxacin) / 15 mg 2 ( 4-Dimethylaminobutyrylthiobenzimidazole is applied to the skin close to the patient's body infectious organs every morning and evening (twice a day) for 6 months or until complete relief.
실시예 12. 귀염증 치료. Example 12. Treatment of ear infections.
1ml의 물 중의 20mg의 6-페녹시아세트아세트아미도페니실란산 2-디에틸아미노에틸 에스테르 염산염을 환자의 감염된 귀에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.20 mg of 6-phenoxycetacetamidophenylanic acid 2-diethylaminoethyl ester hydrochloride in 1 ml of water will be applied to the skin close to the patient's infected ear every morning and evening (twice a day) for 2 weeks or to be cured. Apply until.
실시예 13. 성인의 하기도 감염 치료 (아동의 경우 투여 함량을 줄임). Example 13. Treatment of lower respiratory tract infections in adults (for children, reducing dosage).
3ml의 물 중의 80mg의 D-α-[(이미다졸리딘-2-온-1-일)카보닐아미노]벤질페니실린 2-피롤리딘메틸 에스테르 염산염을 환자의 목 및/또는 가슴에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.Skin close to the patient's neck and / or chest with 80 mg of D-α-[(imidazolidin-2-one-1-yl) carbonylamino] benzylpenicillin 2-pyrrolidinemethyl ester hydrochloride in 3 ml of water Apply every morning and evening (twice a day) for 2 weeks or until complete.
실시예 14. 성인의 상기도 감염 치료 (아동의 경우 투여 함량을 줄임). Example 14. Treatment of upper respiratory tract infections in adults (reduced dosage in children).
2ml의 물 중의 80mg의 6-D(-)-α-(4-에틸-2,3-디옥소-1-피페라지닐카보닐아미노)-α-페닐아세트아미도페니실린산 2-디에틸아미노에틸 에스테르 염산염을 환자의 목에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.80 mg of 6-D (-)-α- (4-ethyl-2,3-dioxo-1-piperazinylcarbonylamino) -α-phenylacetamidopenicillin 2-diethylamino in 2 ml of water Ethyl ester hydrochloride is applied to the skin close to the patient's neck every morning and evening (twice a day) for two weeks or until complete.
실시예 15. 성인의 상기도 감염 치료 (아동의 경우 투여 함량을 줄임).Example 15. Treatment of upper respiratory tract infections in adults (for children, reducing the dose).
2ml의 물 중의 30mg의 3-[[(아미노카보닐)옥시]메틸]-7-메톡시-8-옥소-7-[(2-티에닐아세틸)아미노]-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염을 환자의 입 또는 코속으로 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 뿌린다. 30 mg 3-[[(aminocarbonyl) oxy] methyl] -7-methoxy-8-oxo-7-[(2-thienylacetyl) amino] -5-thia-1-azabicycle in 2 ml of water Ro [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride into the patient's mouth or nose every morning and evening (twice a day) for 2 weeks or until complete Sprinkle.
실시예 16. 성인의 수막염 치료 (아동의 경우 투여 함량을 줄임). Example 16. Treatment of meningitis in adults (reduced dosage in children).
3ml의 물 중의 80mg의 6-D(-)-α-(4-에틸-2,3-디옥소-1-피페라지닐카보닐아미노)-α-페닐아세트아미도페니실린산 2-디에틸아미노에틸 에스테르 염산염을 환자의 목 및 머리에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.80 mg of 6-D (-)-α- (4-ethyl-2,3-dioxo-1-piperazinylcarbonylamino) -α-phenylacetamidopenicillin 2-diethylamino in 3 ml water Ethyl ester hydrochloride is applied to the skin close to the patient's neck and head every morning and evening (twice a day) for 2 weeks or until complete.
실시예 17. 설사성 질환 치료 (아동의 경우 투여 함량을 줄임).Example 17. Treatment of diarrhea disease (reduced dosage in children).
3ml의 물 중의 80mg의 7-(2-티에닐아세트아미도)세팔로스포란산 2-디에틸아미노에틸 에스테르 염산염을 환자의 배꼽에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.80mg of 7- (2-thienylacetamido) cephalosporic acid 2-diethylaminoethyl ester hydrochloride in 3 ml of water is applied to the skin close to the patient's belly every morning and evening (twice a day) 2 Apply for a week or until complete.
실시예 18. 유방 감염 치료.Example 18. Treatment of breast infections.
2ml의 물 중의 50mg의 7-[(히드록시페닐아세틸)아미노]-3-[[(1-메틸-1H-테트라졸-5-일)티오]메틸]-8-옥소-5-티아-1-아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염을 환자의 유방에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.50 mg of 7-[(hydroxyphenylacetyl) amino] -3-[[(1-methyl-1H-tetrazol-5-yl) thio] methyl] -8-oxo-5-thia-1 in 2 ml water -Azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride is applied to the skin close to the patient's breast every morning and evening (twice a day) for 2 weeks or Apply until complete.
실시예 19. 남성 또는 여성의 생식기관 감염 치료 ( 아동의 경우 투여 함량을 줄임). Example 19. Treatment of male or female reproductive system infections ( reduced dose in children ).
3ml의 물 중의 80mg의 3-[[(아미노카보닐)옥시]메틸]-7-[[2-푸라닐(메톡시이미노)아세틸]아미노]-8-옥소-5-티아-1- 아자바이시클로[4.2.0]옥트-2-엔-2-카복시산 2-디에틸아미노에틸 에스테르 염산염을 환자의 치골부에 가까운 피부에 매일 아침과 저녁에 (1일 2회) 2주 동안 또는 완쾌될 때까지 적용한다.80 mg 3-[[(aminocarbonyl) oxy] methyl] -7-[[2-furanyl (methoxyimino) acetyl] amino] -8-oxo-5-thia-1-azabicycline in 3 ml water Ro [4.2.0] oct-2-ene-2-carboxylic acid 2-diethylaminoethyl ester hydrochloride is applied to the skin close to the patient's pubic area every morning and evening (twice a day) for two weeks or to be cured. Apply until.
Claims (8)
6-[3-(o-클로로페닐)-5-메틸-4-이속사졸카복사미도]페니실린산 3-피페리딘에틸 에스테르 HA; 또는
6-[3-(2,6-디클로로페닐)-5-메틸-4-이속사졸카복사미도]페니실린산 1-피페리딘에틸 에스테르 HA이며,
여기서 HA는 없음, 염산, 브롬화수소산, 요오드화수소산, 질산, 황산, 중황산, 인산, 아인산, 포스폰산, 이소니코틴산, 아세트산, 락트산, 살리실산, 시트르산, 타르타르산, 판토텐산, 비타르타르산, 아스코르브산, 석신산, 말레산, 겐티신산, 푸마르산, 글루콘산, 글루카론산, 당산, 포름산, 벤조산, 글루탐산, 메탄설폰산, 에탄설폰산, 벤젠설폰산, p-톨루엔설폰산 및 파모인산으로 이루어진 군에서 선택되는 것인,
고투과 화합물 또는 이의 입체이성질체.6- (5-methyl-3-phenyl-2-isoxazoline-4-carboxamido) penicillic acid 4-piperidineethyl ester HA;
6- [3- (o-chlorophenyl) -5-methyl-4-isoxazolcarboxamido] penicillic acid 3-piperidineethyl ester HA; or
6- [3- (2,6-dichlorophenyl) -5-methyl-4-isoxazolcarboxamido] penicillic acid 1-piperidineethyl ester HA,
Where HA is absent, hydrochloric acid, hydrobromic acid, hydroiodic acid, nitric acid, sulfuric acid, bisulfuric acid, phosphoric acid, phosphorous acid, phosphonic acid, isonicotinic acid, acetic acid, lactic acid, salicylic acid, citric acid, tartaric acid, pantothenic acid, bitartaric acid, ascorbic acid, succinic acid Selected from the group consisting of, maleic acid, gentisic acid, fumaric acid, gluconic acid, gluconic acid, sugar acid, formic acid, benzoic acid, glutamic acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid and pamoic acid Being
Highly permeable compound or stereoisomer thereof.
생물학적 대상체에서
항문직장 및 항문소양증, 하부 호흡기 감염, 상부 호흡기 감염, 요로 감염, 세균성 원내감염, 슈도모나스 감염, 코아귤라제양성포도구균 감염, 피부 감염, 세균 중독증, 급성 감염성 심내막염, 괴사성 폐렴, 인공기관 이식 감염, 패혈증 및 폐렴 동반 기회감염, 및 간, 폐, 위장, 뇌, 신장, 심장, 귀, 눈, 코, 입, 혀, 결장, 췌장, 담낭, 십이지장, 직장위장, 결장직장, 장, 정맥, 호흡계통, 및 혈관으로 이루어진 군에서 선택된 기관에의 감염으로 이루어진 군으로부터 선택되는 세균 감염을 치료하기 위한 약학적 조성물. Comprising the high-permeability compound according to claim 2 and a pharmaceutically acceptable carrier,
In biological subjects
Anorectal and anal pruritus, lower respiratory tract infections, upper respiratory tract infections, urinary tract infections, bacterial infections, pseudomonas infections, coagulase-positive aureus infections, skin infections, bacterial addiction, acute infective endocarditis, necrotizing pneumonia, artificial organ transplant infections Opportunistic infections with sepsis and pneumonia, and liver, lungs, stomach, brain, kidneys, heart, ears, eyes, nose, mouth, tongue, colon, pancreas, gallbladder, duodenum, rectal gastrointestinal tract, colorectal, intestinal, venous, respiratory A pharmaceutical composition for treating a bacterial infection selected from the group consisting of an infection with a selected organ from the group consisting of the lineage and blood vessels.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/482,373 US20100040548A1 (en) | 2006-12-10 | 2009-06-10 | High penetration prodrug compositions of antimicrobials and antimicrobial-related compounds |
| US12/482,373 | 2009-06-10 | ||
| CN200910141944 | 2009-06-10 | ||
| CN200910141944.X | 2009-06-10 | ||
| PCT/CN2010/073743 WO2010142241A1 (en) | 2009-06-10 | 2010-06-10 | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127000567A Division KR20120034721A (en) | 2009-06-10 | 2010-06-10 | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20170137958A KR20170137958A (en) | 2017-12-13 |
| KR102102972B1 true KR102102972B1 (en) | 2020-04-22 |
Family
ID=43308429
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177035075A KR102102972B1 (en) | 2009-06-10 | 2010-06-10 | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds |
| KR1020127000567A KR20120034721A (en) | 2009-06-10 | 2010-06-10 | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127000567A KR20120034721A (en) | 2009-06-10 | 2010-06-10 | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP2440523A4 (en) |
| JP (3) | JP5916129B2 (en) |
| KR (2) | KR102102972B1 (en) |
| CN (1) | CN105566213B (en) |
| AU (2) | AU2010257905B2 (en) |
| BR (1) | BRPI1012905A8 (en) |
| CA (1) | CA2764376C (en) |
| HK (2) | HK1178537A1 (en) |
| MX (2) | MX366035B (en) |
| RU (2) | RU2016115906A (en) |
| WO (1) | WO2010142241A1 (en) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103945842A (en) | 2011-09-09 | 2014-07-23 | 丘比斯特药物股份有限公司 | Methods for treating intrapulmonary infections |
| PL2849738T3 (en) * | 2012-01-18 | 2024-05-13 | Techfields Pharma Co., Ltd. | High penetration prodrug compositions and pharmaceutical composition thereof for treatment of pulmonary conditions |
| US8809314B1 (en) | 2012-09-07 | 2014-08-19 | Cubist Pharmacueticals, Inc. | Cephalosporin compound |
| US8476425B1 (en) | 2012-09-27 | 2013-07-02 | Cubist Pharmaceuticals, Inc. | Tazobactam arginine compositions |
| WO2014139161A1 (en) * | 2013-03-15 | 2014-09-18 | Techfields Pharma Co., Ltd. | Novel high penetration drugs and their compositions thereof for treatment of parkinson diseases |
| US20140274997A1 (en) | 2013-03-15 | 2014-09-18 | Cubist Pharmaceuticals, Inc. | Cephalosporin pharmaceutical compositions |
| US20140274994A1 (en) | 2013-03-15 | 2014-09-18 | Cubist Pharmaceuticals, Inc. | Stabilizing ceftolozane |
| US9872906B2 (en) | 2013-03-15 | 2018-01-23 | Merck Sharp & Dohme Corp. | Ceftolozane antibiotic compositions |
| EP3043797B1 (en) | 2013-09-09 | 2020-04-08 | Merck Sharp & Dohme Corp. | Treating infections with ceftolozane/tazobactam in subjects having impaired renal function |
| US20150094293A1 (en) | 2013-09-27 | 2015-04-02 | Calixa Therapeutics, Inc. | Solid forms of ceftolozane |
| CN108396007B (en) * | 2018-03-15 | 2021-07-23 | 东北农业大学 | Method for in-vitro construction of three-dimensional model of blood and milk barrier of dairy cow |
| CN118556043A (en) * | 2022-01-17 | 2024-08-27 | 苏州泰飞尔医药有限公司 | Treatment of signs, symptoms and/or complications of viral, bacterial, protozoal and/or fungal infections by highly penetrating prodrugs |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2694031A (en) * | 1949-07-07 | 1954-11-09 | Leo Pharm Prod Ltd | Hydroiodides of amino esters of penicillin and oil composition thereof |
| BE790440A (en) * | 1971-10-23 | 1973-04-24 | Bayer Ag | |
| IT1048274B (en) * | 1974-03-02 | 1980-11-20 | Farmaceutici Italia | Acylamido-methyl penicillin and cephalosporin esters - for oral administration and subsequent in-tissue enzymic hydrolysis to parent antibiotic |
| GB1569421A (en) * | 1976-06-11 | 1980-06-18 | Beecham Group Ltd | Penicillin compositions |
| GB1578730A (en) * | 1976-06-24 | 1980-11-05 | Beecham Group Ltd | Esters of penicillin antibiotics |
| JPS5377083A (en) * | 1976-12-16 | 1978-07-08 | Asahi Chem Ind Co Ltd | Penicillins and their derivatives |
| US4215120A (en) * | 1977-07-14 | 1980-07-29 | Beecham Group Limited | Penicillin esters and their preparation |
| JPS5446800A (en) * | 1977-09-22 | 1979-04-12 | Sangyo Kagaku Kenkyu Kyokai | Antibacterial compound |
| JPS5646885A (en) * | 1979-09-27 | 1981-04-28 | Meiji Seika Kaisha Ltd | Novel cephamycin ester and its preparation |
| US4428935A (en) * | 1982-05-24 | 1984-01-31 | Pfizer Inc. | Penicillanic acid dioxide prodrug |
| DE4423915A1 (en) * | 1994-07-07 | 1996-01-11 | Carl Heinrich Dr Weischer | New tocopheryl esters of sulphasalazine or 5-amino-salicyclic acid |
| AU721819B2 (en) * | 1996-05-10 | 2000-07-13 | Pharmacia & Upjohn Company | Topical administration of antimicrobial agents for the treatment of systemic bacterial diseases |
| US5929086A (en) * | 1996-05-10 | 1999-07-27 | Pharmacia & Upjohn Company | Topical administration of antimicrobial agents for the treatment of systemic bacterial diseases |
| EP1792882A1 (en) * | 2005-12-02 | 2007-06-06 | SOLVAY (Société Anonyme) | Process for the manufacture of lightweight construction materials containing clay. |
| PL2121593T3 (en) * | 2006-12-10 | 2015-11-30 | Chongxi Yu | Transdermal delivery systems of beta-lactam antibiotics |
| WO2010028458A1 (en) * | 2008-09-12 | 2010-03-18 | Parnell Laboratories (Aust) Pty Ltd | Prodrugs of isoxazolyl penicillins and uses thereof |
| KR101946297B1 (en) * | 2008-12-04 | 2019-02-11 | 충시 위 | High Penetration Compositions and Their Applications |
-
2010
- 2010-06-10 JP JP2012514336A patent/JP5916129B2/en active Active
- 2010-06-10 RU RU2016115906A patent/RU2016115906A/en not_active Application Discontinuation
- 2010-06-10 KR KR1020177035075A patent/KR102102972B1/en active IP Right Grant
- 2010-06-10 EP EP10785754.2A patent/EP2440523A4/en not_active Withdrawn
- 2010-06-10 WO PCT/CN2010/073743 patent/WO2010142241A1/en active Application Filing
- 2010-06-10 CA CA2764376A patent/CA2764376C/en active Active
- 2010-06-10 MX MX2015003152A patent/MX366035B/en unknown
- 2010-06-10 KR KR1020127000567A patent/KR20120034721A/en active Application Filing
- 2010-06-10 MX MX2011013314A patent/MX338552B/en active IP Right Grant
- 2010-06-10 BR BRPI1012905A patent/BRPI1012905A8/en not_active Application Discontinuation
- 2010-06-10 AU AU2010257905A patent/AU2010257905B2/en not_active Ceased
- 2010-06-10 CN CN201610005542.7A patent/CN105566213B/en active Active
- 2010-06-10 RU RU2011152319/15A patent/RU2586287C2/en not_active IP Right Cessation
-
2013
- 2013-05-27 HK HK13106282.9A patent/HK1178537A1/en unknown
-
2015
- 2015-06-02 JP JP2015112700A patent/JP6256924B2/en active Active
-
2016
- 2016-06-23 HK HK16107313.7A patent/HK1219275A1/en unknown
-
2017
- 2017-02-09 AU AU2017200897A patent/AU2017200897B2/en not_active Ceased
- 2017-11-28 JP JP2017228121A patent/JP6707512B2/en active Active
Non-Patent Citations (1)
| Title |
|---|
| 국제특허공보 제2008-072032호(2008. 6. 19)* |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015163640A (en) | 2015-09-10 |
| RU2016115906A3 (en) | 2019-09-30 |
| HK1219275A1 (en) | 2017-03-31 |
| CA2764376A1 (en) | 2010-12-16 |
| EP2440523A1 (en) | 2012-04-18 |
| CN105566213B (en) | 2019-11-12 |
| AU2010257905B2 (en) | 2016-11-10 |
| JP5916129B2 (en) | 2016-05-11 |
| BRPI1012905A8 (en) | 2018-01-02 |
| HK1178537A1 (en) | 2014-05-09 |
| JP6256924B2 (en) | 2018-01-10 |
| RU2586287C2 (en) | 2016-06-10 |
| EP2440523A4 (en) | 2014-03-19 |
| MX338552B (en) | 2016-04-20 |
| KR20170137958A (en) | 2017-12-13 |
| AU2017200897A1 (en) | 2017-03-02 |
| JP2018048197A (en) | 2018-03-29 |
| WO2010142241A1 (en) | 2010-12-16 |
| MX366035B (en) | 2019-06-25 |
| JP2012529443A (en) | 2012-11-22 |
| AU2017200897B2 (en) | 2018-10-04 |
| CA2764376C (en) | 2021-05-04 |
| JP6707512B2 (en) | 2020-06-10 |
| AU2010257905A1 (en) | 2012-02-02 |
| BRPI1012905A2 (en) | 2017-09-12 |
| RU2016115906A (en) | 2018-11-28 |
| KR20120034721A (en) | 2012-04-12 |
| CN105566213A (en) | 2016-05-11 |
| MX2011013314A (en) | 2012-09-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102102972B1 (en) | High penetration compositions or prodrugs of antimicrobials and antimicrobial-related compounds | |
| US20210206782A1 (en) | High penetration prodrug compositions of antimicrobials and antimicrobial-related compounds | |
| US20230130980A1 (en) | High penetration prodrug compositions of antimicrobials and antimicrobial-related compounds | |
| AU2013262320B2 (en) | High penetration prodrug compositions and pharmaceutical composition thereof for treatment of pulmonary conditions | |
| CN102803211B (en) | Prodrug compositions of antimicrobials and antimicrobial-related compounds with high penetration | |
| TWI496577B (en) | High penetration prodrug compositions of antimicrobials and antimicrobial-related compounds | |
| JP5795343B2 (en) | Transdermal delivery system for β-lactam antibiotics | |
| CN104324028B (en) | The transdermal drug delivery system of beta-lactam antibiotic |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E90F | Notification of reason for final refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant |