JP6195817B2 - 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム - Google Patents
脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム Download PDFInfo
- Publication number
- JP6195817B2 JP6195817B2 JP2014234876A JP2014234876A JP6195817B2 JP 6195817 B2 JP6195817 B2 JP 6195817B2 JP 2014234876 A JP2014234876 A JP 2014234876A JP 2014234876 A JP2014234876 A JP 2014234876A JP 6195817 B2 JP6195817 B2 JP 6195817B2
- Authority
- JP
- Japan
- Prior art keywords
- occlusion
- anchor
- implantable device
- aneurysm
- shape
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 230000007547 defect Effects 0.000 title claims description 59
- 238000000034 method Methods 0.000 title description 57
- 210000004204 blood vessel Anatomy 0.000 claims description 57
- 239000000463 material Substances 0.000 claims description 57
- 239000012528 membrane Substances 0.000 claims description 30
- 230000003014 reinforcing effect Effects 0.000 claims description 18
- 230000002093 peripheral effect Effects 0.000 claims description 7
- 230000002787 reinforcement Effects 0.000 claims description 6
- 230000001131 transforming effect Effects 0.000 claims 2
- 206010002329 Aneurysm Diseases 0.000 description 135
- 210000001519 tissue Anatomy 0.000 description 35
- 230000008439 repair process Effects 0.000 description 32
- 239000000126 substance Substances 0.000 description 32
- 229910001000 nickel titanium Inorganic materials 0.000 description 25
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 24
- 229910045601 alloy Inorganic materials 0.000 description 23
- 239000000956 alloy Substances 0.000 description 23
- 239000010409 thin film Substances 0.000 description 22
- 239000003550 marker Substances 0.000 description 14
- 230000007246 mechanism Effects 0.000 description 12
- 230000002792 vascular Effects 0.000 description 12
- 230000003073 embolic effect Effects 0.000 description 11
- -1 polytetrafluoroethylene copolymer Polymers 0.000 description 11
- 230000004913 activation Effects 0.000 description 9
- 210000004027 cell Anatomy 0.000 description 9
- 238000009434 installation Methods 0.000 description 9
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 9
- 238000011282 treatment Methods 0.000 description 9
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 8
- 229910052804 chromium Inorganic materials 0.000 description 8
- 239000011651 chromium Substances 0.000 description 8
- 230000001788 irregular Effects 0.000 description 8
- 229920000642 polymer Polymers 0.000 description 8
- 230000017531 blood circulation Effects 0.000 description 7
- 238000000576 coating method Methods 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 208000005189 Embolism Diseases 0.000 description 4
- 239000000853 adhesive Substances 0.000 description 4
- 230000001070 adhesive effect Effects 0.000 description 4
- 239000000560 biocompatible material Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 230000010261 cell growth Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 229920001577 copolymer Polymers 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 230000003511 endothelial effect Effects 0.000 description 4
- 208000025339 heart septal defect Diseases 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000004544 sputter deposition Methods 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 238000003466 welding Methods 0.000 description 4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 206010053648 Vascular occlusion Diseases 0.000 description 3
- 229920000249 biocompatible polymer Polymers 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 238000005530 etching Methods 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000007769 metal material Substances 0.000 description 3
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 3
- 239000004810 polytetrafluoroethylene Substances 0.000 description 3
- 239000004814 polyurethane Substances 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 230000008733 trauma Effects 0.000 description 3
- 230000007556 vascular defect Effects 0.000 description 3
- 208000021331 vascular occlusion disease Diseases 0.000 description 3
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- 101100269850 Caenorhabditis elegans mask-1 gene Proteins 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 229920004934 Dacron® Polymers 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 2
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000008065 acid anhydrides Chemical class 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- 208000034158 bleeding Diseases 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 229920002678 cellulose Chemical class 0.000 description 2
- 239000001913 cellulose Chemical class 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 230000010102 embolization Effects 0.000 description 2
- 210000003038 endothelium Anatomy 0.000 description 2
- 229920000295 expanded polytetrafluoroethylene Polymers 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 239000003292 glue Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229920000669 heparin Polymers 0.000 description 2
- 229960002897 heparin Drugs 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000001453 nonthrombogenic effect Effects 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 239000005014 poly(hydroxyalkanoate) Substances 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 229920002627 poly(phosphazenes) Polymers 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 239000005020 polyethylene terephthalate Substances 0.000 description 2
- 229920000903 polyhydroxyalkanoate Polymers 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 239000012781 shape memory material Substances 0.000 description 2
- 239000002210 silicon-based material Substances 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 210000003625 skull Anatomy 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- 229920001897 terpolymer Polymers 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000000472 traumatic effect Effects 0.000 description 2
- YFHICDDUDORKJB-UHFFFAOYSA-N trimethylene carbonate Chemical compound O=C1OCCCO1 YFHICDDUDORKJB-UHFFFAOYSA-N 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 229920008347 Cellulose acetate propionate Polymers 0.000 description 1
- 206010008111 Cerebral haemorrhage Diseases 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 229920002567 Chondroitin Polymers 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- 229920001651 Cyanoacrylate Polymers 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 229920000544 Gore-Tex Polymers 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- MWCLLHOVUTZFKS-UHFFFAOYSA-N Methyl cyanoacrylate Chemical compound COC(=O)C(=C)C#N MWCLLHOVUTZFKS-UHFFFAOYSA-N 0.000 description 1
- 229920001410 Microfiber Polymers 0.000 description 1
- 229910000990 Ni alloy Inorganic materials 0.000 description 1
- 229920002201 Oxidized cellulose Polymers 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 102000001938 Plasminogen Activators Human genes 0.000 description 1
- 108010001014 Plasminogen Activators Proteins 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 208000009443 Vascular Malformations Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 210000000013 bile duct Anatomy 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical class C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- DLGJWSVWTWEWBJ-HGGSSLSASA-N chondroitin Chemical compound CC(O)=N[C@@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1OC1[C@H](O)[C@H](O)C=C(C(O)=O)O1 DLGJWSVWTWEWBJ-HGGSSLSASA-N 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 210000004177 elastic tissue Anatomy 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000010291 electrical method Methods 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 210000001105 femoral artery Anatomy 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- XUCNUKMRBVNAPB-UHFFFAOYSA-N fluoroethene Chemical compound FC=C XUCNUKMRBVNAPB-UHFFFAOYSA-N 0.000 description 1
- 229920002313 fluoropolymer Polymers 0.000 description 1
- 239000004811 fluoropolymer Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 150000001261 hydroxy acids Chemical class 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000007373 indentation Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 208000020658 intracerebral hemorrhage Diseases 0.000 description 1
- 238000012977 invasive surgical procedure Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000011866 long-term treatment Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 239000003658 microfiber Substances 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 229940107304 oxidized cellulose Drugs 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 229940127126 plasminogen activator Drugs 0.000 description 1
- 229920001308 poly(aminoacid) Polymers 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920002721 polycyanoacrylate Polymers 0.000 description 1
- 239000000622 polydioxanone Substances 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920006149 polyester-amide block copolymer Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920001855 polyketal Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 239000012857 radioactive material Substances 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 150000003377 silicon compounds Chemical class 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229910002070 thin film alloy Inorganic materials 0.000 description 1
- 230000002885 thrombogenetic effect Effects 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000007723 transport mechanism Effects 0.000 description 1
- 230000006496 vascular abnormality Effects 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
Images
Landscapes
- Surgical Instruments (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Description
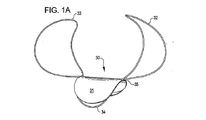
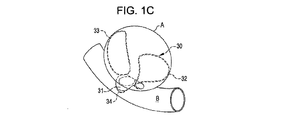
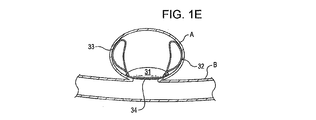
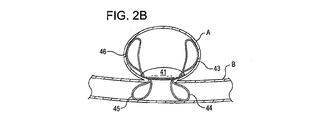
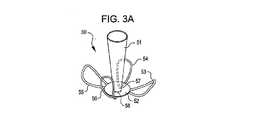
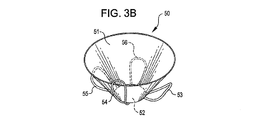
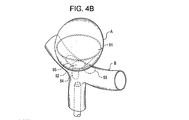
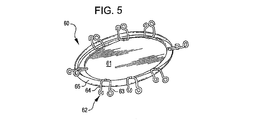
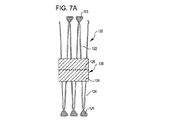
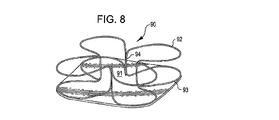
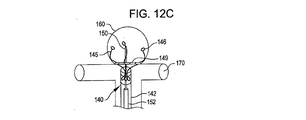
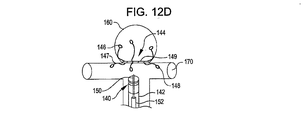
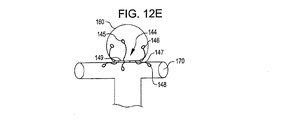
31、41、51、61 閉塞構造体
32、34、37、38、43、44、45、46 アンカー構造体
84、85、145、147 アンカーアーム
126 襟構造体
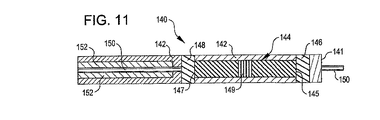
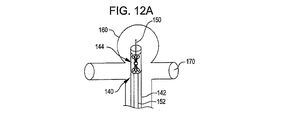
140 搬送システム
142 搬送カテーテル
150 ガイドワイヤ
152 プッシャー
A 動脈瘤
B 血管
Claims (15)
- 対象とする組織欠損の腔内を修復し、小さな直径の形状を有する搬送状態からより大きな直径の形状を有する展開状態へと変形することのできる植え込み型デバイスであって、
前記デバイスが前記展開状態であるときに前記腔内を実質的に覆う寸法の閉塞構造体であって、中央部および周辺部を有する閉塞構造体と、
前記閉塞構造体に結合されて、前記対象とする組織欠損において前記植え込み型デバイスを固定するように構成される複数のアンカー構造体と、
前記閉塞構造体の前記周辺部に近接して前記腔内に少なくとも部分的に突出するように構成される補強構造体と
を備え、
前記補強構造体は、前記閉塞構造体の前記周辺部に結合される襟構造体を含み、
前記複数のアンカー構造体のそれぞれは、前記襟構造体の外面に取り付けられた構造体支持中間体と、前記閉塞構造体の面の両側において前記腔内及び血管内に突出した部分とを有し、且つ前記腔内及び血管内に突出した部分のそれぞれが前記構造体支持中間体によって環状に結合している、植え込み型デバイス。 - 前記補強構造体は、前記閉塞構造体の前記周辺部に結合されるフレームワークを含んでいる、請求項1に記載の植え込み型デバイス。
- 前記補強構造体は、植え込み型デバイスが展開状態のときに、前記襟構造体は、略円筒形状を有する、請求項1に記載の植え込み型デバイス。
- 前記襟構造体は、展開状態の間、前記閉塞構造体が配置される面と略直角の面内に拡張する、請求項3に記載の植え込み型デバイス。
- 前記襟構造体は、略可撓性メンブレンをさらに含む、請求項3に記載の植え込み型デバイス。
- 前記襟構造体は、前記植え込み型デバイスが展開状態にあるときに、前記腔内の外側に向かって前記植え込み型デバイスの中央から離れるように前記閉塞構造体の面に対して角度を付けてもしくは湾曲して血管壁と接触する裾部を備える、請求項3に記載の植え込み型デバイス。
- 前記裾部は、前記閉塞構造体の直径よりも大きな直径を有する、請求項6に記載の植え込み型デバイス。
- 前記閉塞構造体は、他のデバイスの通過を容易にする少なくとも1つの開口部を有する、請求項1に記載の植え込み型デバイス。
- 前記アンカー構造体は、非外傷性である、請求項1に記載の植え込み型デバイス。
- 対象とする組織欠損の開口部または腔内を修復し、小さな直径の形状を有する搬送状態からより大きな直径の形状を有する展開状態へと変形することのできる植え込み型デバイスであって、
前記デバイスが前記展開状態であるときに前記開口部または腔内を実質的に覆う寸法の閉塞構造体と、
前記閉塞構造体に結合されて前記対象とする組織欠損において前記植え込み型デバイスを固定するように構成される複数のアンカー構造体であって、前記閉塞構造体から離れる方向に少なくとも部分的に突出して、前記植え込み型デバイスが展開状態であるときに略円筒構造をなすアンカー構造体と、
前記閉塞構造体の周辺部に近接して前記腔内に少なくとも部分的に突出するように構成される補強構造体と
を備え、
前記補強構造体は、前記閉塞構造体の前記周辺部に結合される襟構造体を含み、
前記複数のアンカー構造体のそれぞれは、前記襟構造体の外面に取り付けられた構造体支持中間体と、前記閉塞構造体の面の両側において前記腔内及び血管内に突出した部分とを有し、且つ前記腔内及び血管内に突出した部分のそれぞれが前記構造体支持中間体によって環状に結合している、植え込み型デバイス。 - 各アンカー構造体は、前記植え込み型デバイスが展開状態であるときに、半径方向に外側に曲がる、請求項10に記載の植え込み型デバイス。
- 前記アンカー構造体の少なくとも一部は、略平坦な断面形状を有する、請求項10に記載の植え込み型デバイス。
- 前記アンカー構造体は、前記植え込み型デバイスが展開状態にある間、実質的に互いにジグザグ状に設けられる、請求項10に記載の植え込み型デバイス。
- 各アンカー構造体は、略円形、略楕円形、略曲線、略多角形、略三角形、およびこれらを組み合わせた形状のグループから選択される1つの形状を有する、請求項10に記載の植え込み型デバイス。
- 前記アンカー構造体は、少なくとも一部が形状変更可能な材料から構成される、請求項10に記載の植え込み型デバイス。
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US72805205P | 2005-10-19 | 2005-10-19 | |
| US60/728,052 | 2005-10-19 | ||
| US11/324,827 | 2006-01-03 | ||
| US11/324,827 US8545530B2 (en) | 2005-10-19 | 2006-01-03 | Implantable aneurysm closure systems and methods |
| US74740006P | 2006-05-16 | 2006-05-16 | |
| US60/747,400 | 2006-05-16 | ||
| US80320006P | 2006-05-25 | 2006-05-25 | |
| US60/803,200 | 2006-05-25 | ||
| US82373006P | 2006-08-28 | 2006-08-28 | |
| US60/823,730 | 2006-08-28 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013098487A Division JP5785215B2 (ja) | 2005-10-19 | 2013-05-08 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2015083139A JP2015083139A (ja) | 2015-04-30 |
| JP6195817B2 true JP6195817B2 (ja) | 2017-09-13 |
Family
ID=48878414
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013098487A Expired - Fee Related JP5785215B2 (ja) | 2005-10-19 | 2013-05-08 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
| JP2013098488A Expired - Fee Related JP5955266B2 (ja) | 2005-10-19 | 2013-05-08 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
| JP2014234876A Expired - Fee Related JP6195817B2 (ja) | 2005-10-19 | 2014-11-19 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013098487A Expired - Fee Related JP5785215B2 (ja) | 2005-10-19 | 2013-05-08 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
| JP2013098488A Expired - Fee Related JP5955266B2 (ja) | 2005-10-19 | 2013-05-08 | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム |
Country Status (3)
| Country | Link |
|---|---|
| JP (3) | JP5785215B2 (ja) |
| CN (2) | CN103381101B (ja) |
| HK (1) | HK1134421A1 (ja) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3305214A3 (en) | 2009-09-04 | 2018-07-04 | Pulsar Vascular, Inc. | Systems for enclosing an anatomical opening |
| US10624647B2 (en) | 2011-06-03 | 2020-04-21 | Pulsar Vascular, Inc. | Aneurysm devices with additional anchoring mechanisms and associated systems and methods |
| CA2837717C (en) | 2011-06-03 | 2019-07-09 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening, including shock absorbing aneurysm devices |
| ES2809210T3 (es) | 2011-10-05 | 2021-03-03 | Pulsar Vascular Inc | Sistemas y dispositivos para envolver una abertura anatómica |
| WO2013169380A1 (en) | 2012-05-10 | 2013-11-14 | Pulsar Vascular, Inc. | Coil-tipped aneurysm devices |
| WO2016137997A1 (en) * | 2015-02-25 | 2016-09-01 | Galaxy Therapeutics, Llc | System for and method of treating aneurysms |
| CN106139291A (zh) * | 2015-04-20 | 2016-11-23 | 浦易(上海)生物技术有限公司 | 一种鼻窦冲洗导管 |
| CN108882959A (zh) * | 2016-01-15 | 2018-11-23 | Tva医疗公司 | 用于形成瘘管的装置和方法 |
| US20180049859A1 (en) * | 2016-08-16 | 2018-02-22 | Spartan Micro, Inc. | Intravascular flow diversion devices |
| EP3908208A4 (en) * | 2019-03-15 | 2022-10-19 | Sequent Medical, Inc. | FILAMENTARY DEVICES WITH A FLEXIBLE JOINT FOR THE TREATMENT OF VASCULAR ABNORMALITIES |
| EP3908354A4 (en) * | 2019-03-15 | 2023-04-26 | Sequent Medical, Inc. | FIBROUS DEVICES FOR TREATMENT OF VASCULAR DEFECTS |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2298637A1 (en) * | 1997-08-04 | 1999-02-11 | Jennifer J. Mccrory | Occlusion system for aneurysm repair |
| FR2767671B1 (fr) * | 1997-08-27 | 1999-11-26 | Ethnor | Dispositif obturateur prothetique pour l'obturation de canaux herniaires |
| EP1685808B1 (en) * | 1998-01-30 | 2016-09-14 | St.Jude Medical ATG, Inc. | Device for use in closing septal defects and an installation assembly for such device |
| US6022369A (en) * | 1998-02-13 | 2000-02-08 | Precision Vascular Systems, Inc. | Wire device with detachable end |
| US6015424A (en) * | 1998-04-28 | 2000-01-18 | Microvention, Inc. | Apparatus and method for vascular embolization |
| US6613074B1 (en) * | 1999-03-10 | 2003-09-02 | Cordis Corporation | Endovascular aneurysm embolization device |
| US6551303B1 (en) * | 1999-10-27 | 2003-04-22 | Atritech, Inc. | Barrier device for ostium of left atrial appendage |
| JP2005508201A (ja) * | 2001-03-08 | 2005-03-31 | アトリテック, インコーポレイテッド | 心房フィルターインプラント |
| US20030181922A1 (en) * | 2002-03-20 | 2003-09-25 | Spiration, Inc. | Removable anchored lung volume reduction devices and methods |
| US20030195553A1 (en) * | 2002-04-12 | 2003-10-16 | Scimed Life Systems, Inc. | System and method for retaining vaso-occlusive devices within an aneurysm |
| US8075585B2 (en) * | 2002-08-29 | 2011-12-13 | Stryker Corporation | Device and method for treatment of a vascular defect |
| JP2009512515A (ja) * | 2005-10-19 | 2009-03-26 | パルサー バスキュラー インコーポレイテッド | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム。 |
-
2006
- 2006-10-18 CN CN201310108702.7A patent/CN103381101B/zh not_active Expired - Fee Related
- 2006-10-18 CN CN 201310106823 patent/CN103230290A/zh active Pending
-
2010
- 2010-01-08 HK HK10100192.4A patent/HK1134421A1/xx not_active IP Right Cessation
-
2013
- 2013-05-08 JP JP2013098487A patent/JP5785215B2/ja not_active Expired - Fee Related
- 2013-05-08 JP JP2013098488A patent/JP5955266B2/ja not_active Expired - Fee Related
-
2014
- 2014-11-19 JP JP2014234876A patent/JP6195817B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015083139A (ja) | 2015-04-30 |
| JP2013226419A (ja) | 2013-11-07 |
| CN103381101B (zh) | 2017-12-01 |
| JP2013208440A (ja) | 2013-10-10 |
| JP5785215B2 (ja) | 2015-09-24 |
| HK1134421A1 (en) | 2010-04-30 |
| JP5955266B2 (ja) | 2016-07-20 |
| CN103230290A (zh) | 2013-08-07 |
| CN103381101A (zh) | 2013-11-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6195817B2 (ja) | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム | |
| JP2009512515A (ja) | 脈管内をクリッピングし、腔内および組織欠損を修復するための方法およびシステム。 | |
| CA3012247C (en) | Methods and systems for endovascularly clipping and repairing lumen and tissue defects | |
| KR101652804B1 (ko) | 생리적 구멍 또는 공동을 지지하거나 또는 폐쇄하기 위한 시스템과 방법 | |
| US7232461B2 (en) | Neck covering device for an aneurysm | |
| EP1316293A2 (en) | Aneurysm embolic device with an occlusive member | |
| EP1312312A1 (en) | Aneurysm neck cover for sealing an aneurysm | |
| AU2014200427B2 (en) | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20151020 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20151021 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160120 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160415 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161004 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20161228 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170223 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20170330 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20170330 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20170424 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170718 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170816 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6195817 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |