JP4234418B2 - Manganese nickel composite hydroxide particles - Google Patents
Manganese nickel composite hydroxide particles Download PDFInfo
- Publication number
- JP4234418B2 JP4234418B2 JP2002379506A JP2002379506A JP4234418B2 JP 4234418 B2 JP4234418 B2 JP 4234418B2 JP 2002379506 A JP2002379506 A JP 2002379506A JP 2002379506 A JP2002379506 A JP 2002379506A JP 4234418 B2 JP4234418 B2 JP 4234418B2
- Authority
- JP
- Japan
- Prior art keywords
- manganese
- nickel
- composite hydroxide
- lithium
- particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
Description
【0001】
【発明の属する技術分野】
本発明は、実質的にマンガン:ニッケルが1:1(以下、「マンガン:ニッケル(1:1)」とする)である複合水酸化物粒子に関する。
【0002】
【従来の技術】
近年のコードレスおよびポータブルなAV機器およびパソコンなどの普及にともない、より小型、より軽量およびより高エネルギー密度の電池への要望が強まっている。特に、リチウム二次電池は、高エネルギー密度を有する電池であることから、次世代の主力電池として期待され、その潜在的市場規模も大きい。
従来、いわゆる共沈殿法を利用して、実質的にマンガン:ニッケルが1:1である複合水酸化物(Ni1/2Mn1/2(OH)2)の粒子が知られており、これを用いた実質的にマンガン:ニッケルが1:1であるリチウムマンガンニッケル複合酸化物が製造され、非水電解質電池用正極活物質として使用できることが知られている(例えば、特許文献1参照。)。しかしながらその電池性能についてはさらに改善の余地があり、さらに充放電特性等の種々の望ましい特性を有するものが強く要望されている。
【特許文献1】
特開2002−42813号
【0003】
【課題を解決するための手段】
本発明者はかかる要望を踏まえ、前記マンガン:ニッケル(1:1)であるリチウムマンガンニッケル複合酸化物を用いた非水電解質電池用正極活物質をさらに改良する目的で鋭意研究し、リチウムマンガンニッケル複合酸化物の燒成に使用するマンガン:ニッケル(1:1)複合水酸化物粒子の形状を制御するすることにより、それを用いた非水電解質二次電池(例えばリチウムイオン二次電池)が非常に優れた充放電特性(サイクル数、エネルギー密度等)を有することを見出し本発明を完成するに至った。
【0004】
すなわち、本発明にかかるリチウムマンガンニッケル複合水酸化物粒子は、マンガンとニッケルの原子比が実質的に1:1である、形式的には(Mn1/2Ni1/ 2)(OH)2なる組成式で表されるものであり、その粒子が特徴ある形状を有するものである。
【0005】
特に本発明にかかるリチウムマンガンニッケル複合水酸化物粒子は、その平均粒径が5〜20μm(好ましくは5〜15μm)、タップ密度が0.6〜1.4g/ml、バルク密度が0.4〜1.0g/mlのものである。また比表面積が20〜100m2/g(好ましくは20〜55m2/g)であることを特徴とする。
さらに、その2次粒子表面および内部の構造は、電子顕微鏡による表面観察に基づけば、1次粒子によるひだ状壁により網状を形成し、そのひだ状壁で囲まれた空間が比較的大きいことを特徴とする。
【0006】
またその2次粒子表面および内部の構造は、共沈澱法において中和反応の過程の酸化状態により大きく依存することを特徴とする。これはマンガンイオンの一部が酸化される条件で製造することにより大きく依存することを意味する。硫酸イオンの測定によればかかる含有硫酸根が0.25〜0.45重量%であることを特徴とする。またX線回折においても15≦2θ≦25にあるピークの最大強度(I0)と、30≦2θ≦40にあるピークの最大強度(I1)との比(I0/I1)が、1〜6であることを特徴とする。
【0007】
本発明はさらに、前記記載のマンガンニッケル複合水酸化物粒子と水酸化リチウムを焼成して得られる、実質的にマンガン:ニッケルが1:1であるリチウムマンガンニッケル複合酸化物をも含むものである。また、それを正極活性物質成分として含有することを特徴とするリチウムイオン二次電池をも含むものである。
また、本発明は、前記記載のマンガンニッケル複合水酸化物粒子、リチウムマンガンニッケル複合酸化物、リチウムイオン二次電池の製造方法をも含むものである。
【0008】
特に本発明にかかる製造方法は、pH9〜13の水溶液中で、錯化剤の存在下、マンガンとニッケルの原子比が実質的に1:1であるマンガン塩とニッケル塩の混合水溶液を、アルカリ溶液と適当な攪拌条件下で反応させて生じる粒子を共沈殿させることにより、マンガンとニッケルの原子比が実質的に1:1で均一に混合されたリチウムマンガンニッケル複合水酸化物をえる方法において、マンガンイオンの酸化の程度を一定の範囲に制御することを特徴とする。
以下、本発明を、発明の実施の形態に即して詳細に説明する。
【0009】
【発明の実施の形態】
マンガンニッケル複合水酸化物粒子の製造
まず本発明に係るリチウムマンガンニッケル複合水酸化物の製造方法を以下説明する。すなわち好ましくは以下のようにいわゆる共沈殿法を用いて、かつマンガンイオンの酸化の程度を一定の範囲に制御する条件で行うことで得ることができる。
すなわちここでいう共沈殿法とは、適当な範囲のpH(例えば9〜13)の水溶液中で、錯化剤の存在下、マンガンとニッケルの原子比が実質的に1:1であるマンガン塩とニッケル塩の混合水溶液を、アルカリ溶液と適当な攪拌条件下で反応させて生じる粒子を共沈殿させることにより、リチウムマンガンニッケル複合水酸化物をえるものである。かかる共沈殿法によりマンガンとニッケルの原子比が実質的に1:1で均一に混合された好ましい粒子径を有する粒子を得ることができる。
【0010】
ここで、使用可能なマンガン塩は特に制限はなく水溶液中で生成するマンガンイオンが錯化剤と錯体を形成可能なものであればよい。具体的には硫酸マンガン、硝酸マンガン、塩化マンガンが挙げられる。同様に使用可能なニッケル塩は、水溶液中で生成するニッケルイオンが錯化剤と錯体を形成可能なものであればよく特に制限はない。具体的には硫酸ニッケル、硝酸ニッケル、塩化ニッケルが挙げられる。本発明においてマンガンとニッケルの原子比が実質的に1:1とは、それぞれプラスマイナス10%程度の範囲であれば含まれる。またこの値は種々の金属分析方法(例えば原子吸光法)により正確に測定することができる。
【0011】
水溶液のpH値は、pH9〜13の範囲が好ましく、反応中必要ならばアルカリ金属水酸化物(例えば水酸化ナトリウム、水酸化カリウム)を添加することによりこの範囲に維持することができる。
また、錯化剤は、水溶液中でマンガンイオンおよびニッケルイオンと錯体を形成可能なものであり、例えばアンモニウムイオン供給体(塩化アンモニウム、炭酸アンモニウム、弗化アンモニウム等)、ヒドラジン、エチレンジアミン四酢酸、ニトリト三酢酸、ウラシル二酢酸、グリシンが挙げられる。
【0012】
また、ここで意味するマンガンイオンの酸化の程度を一定の範囲に制御する条件とは、反応溶液中にバブリングする雰囲気用ガスの窒素に適当量の空気(酸素)を混合することにより制御することである。
マンガンイオンは前記共沈殿法の条件では通常非常に酸化され易く、複雑なマンガン酸化物を与える。一般にこのマンガン酸化物を含有したマンガンニッケル複合水酸化物粒子は、それを原料とした場合に電池の特性が悪くなる。従って、本発明では、できるだけマンガン酸化物の生成を抑制することが必要である。
【0013】
一方、前記共沈殿法において、ほぼ完全にマンガンイオンの酸化を抑制する条件を設定することが可能である。例えば、溶液中の溶存酸素を除去するための試薬(例えばヒドラジン)を加え、さらに酸素を実質的に除いた不活性ガス雰囲気下で反応させる方法である。この場合、得られるマンガンニッケル複合水酸化物粒子のマンガンイオンはほぼ完全に2価を保持する。しかしながら本発明者は、前記共沈殿法においてほぼ完全にマンガンイオンの酸化を抑制する条件下で得られるマンガンニッケル複合水酸化物粒子の形状が大きく異なることを見出した。この条件では、1次粒子が非常に密に積み重なり高い密度の2次粒子を形成し、その表面および内部には実質的に網目のような構造が見られないという特徴を有する。本発明者は、この形状の場合これを原料として水酸化リチウムと焼成して、2次電池材料としてのリチウムマンガンニッケル複合酸化物を得る工程において、溶融状態のリチウムイオンが十分に、均一に、そのマンガンニッケル複合酸化物の結晶の中に取りこまれないことを見出した。
【0014】
これらの知見から、溶融状態のリチウムイオンを十分に、均一に、そのマンガンニッケル複合酸化物の結晶の中に取りこませるためには、マンガンニッケル複合水酸化物粒子の形状が重要であり、好ましい網目構造に制御することが必要となる。このために、本発明においては、好ましい網目構造に制御するために共沈澱反応条件をマンガンイオンは酸化され得るが、不必要な量のマンガン酸化物が生成しない条件下で共沈澱を行う。
【0015】
酸化条件、程度については特に制限はない。好ましくは、溶存酸素はあらかじめ還元剤で除去しておく。還元剤としては、共沈澱を適当な錯化剤の存在下行うものであることから、錯化剤としても還元剤としても作用するヒドラジンの使用が好ましい。ヒドラジンは反応終了後においても約10%程度残留させる程度添加することが好ましい。酸化剤についても特に制限はないが、共沈澱法によりスラリーが得られること、適当な攪拌が必要なことから、溶液中に吹きこむ不活性ガスに含めることが好ましい。このために空気、酸素、その他の酸化性ガス(塩素など)の使用が可能である。
【0016】
マンガンイオンの酸化の程度は種々の方法で評価することができる。目視によれば、酸化の程度が大きい場合、生成する酸化マンガンにより粒子が黒灰色となる。一方ほぼ酸化を抑制した場合、薄青緑色となる。従って、その中間の酸化の程度も評価可能である。さらに従来公知の酸化還元滴定方法などにより定量的にも酸化の程度を評価することができる。また、酸化されたマンガンイオンが対アニオンとして硫酸イオンを含む場合、その硫酸イオンの簡便な分析法により間接的に酸化の程度が評価できる。
【0017】
マンガンニッケル複合水酸化物粒子の形状
前記共沈澱法により得られる粒子リチウムマンガンニッケル複合水酸化物粒子の2次粒子径は、共沈澱法において適用されるpHや反応装置に依存する。通常公知のpHや反応装置を使用することにより平均粒径が5〜20μmのものが得られる。この粒子はより小さい粒径の1次粒子が集まって、大きな2次粒子を形成する。
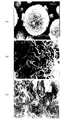
また、電子顕微鏡観察によりその表面、および内部の構造を容易に見分けることができる。酸化を実質的に抑制した条件で得られる粒子は、その1次粒子が強く密に集まり2次粒子を形成する。マンガンの酸化が進むと、粒子の表面および内部の構造は大きく変化する。1次粒子が集まりひだ状態の壁を形成し、これが集まり網目状態の2次粒子を形成する。従って、大きなひだ状の壁で仕切られたスポンジ状の構造を呈する。この構造は、酸化の程度により変化する。特に網目構造で囲まれた空間のサイズの大きさが変化する。酸化がある程度すすむと、むしろこの網目構造はより密になり、囲まれた空間のサイズの大きさが小さくなる。以下に説明する実施例では、このような各条件下での得られた粒子の形状の電子顕微鏡写真を示した。
【0018】
上で説明した2次粒子の表面および内部の構造の違いは、具体的には、タップ密度(TD)、バルク密度(BD)、比表面(BET)に顕著に表れている。酸化の程度が低いほど2次粒子はより1次粒子が強く集まった高い密度の構造を示す。一方これに比較して、酸化が適当な範囲である場合タップ密度(TD)もバルク密度(BD)もほぼ半分となり、粒子内部に空間が存在することが示される。また、この構造状に相違は、比表面に大きく影響し、酸化程度が適当な場合、酸化されていない場合に比較して約2倍となる。
【0019】
リチウムマンガンニッケル複合酸化物
リチウムマンガンニッケル複合酸化物は、上で説明した複合水酸化物粒子と、この複合水酸化物粒子のマンガンとニッケルの合計の原子比とリチウムの原子比が実質的に1:1となるように、リチウム化合物と混合し、得られる混合物を少なくとも850℃以上で、空気気流中焼成加熱して得られるものである。かかる焼成条件および焼成に使用する過熱炉については特に制限はなく、従来公知の
LiMn2O4やLiNiO2の合成に用いられる焼成装置が好ましく使用できる。焼成の際の雰囲気は通常の大気雰囲気が好ましい。
【0020】
使用可能なリチウム化合物としては特に制限はないが、例えば水酸化リチウム、炭酸リチウム、硝酸リチウム、酸化リチウムが挙げられる。特に水酸化リチウムの使用が好ましい。マンガンニッケル(1:1)複合酸化物とリチウム化合物とのモル比は、実質的に1:1である。ここでマンガンニッケル複合酸化物とリチウム化合物とのモル比が実質的に1:1とは、それぞれプラスマイナス10%程度の範囲であれば含まれる。またこれらの値は種々の金属分析方法(例えば原子吸光法)により正確に測定することができる。焼成する前にこれらを十分混合しておくことが好ましい。
【0021】
非水電解質二次電池
本発明のリチウムイオン二次電池は、前記リチウムマンガンニッケル複合酸化物を正極活性物質成分として含有することを特徴とするリチウムイオン二次電池である。その基本的構造については何ら制限はない。通常公知の形状、材料を使用して種々のタイプの電池を構成することができる。また、本発明にかかるリチウムマンガンニッケル複合酸化物を正極活性物質成分として含有することから、かかる電池は図8〜13に示すように、4V付近に極めて平坦でかつ低い分極を特徴とする充放電特性を有し、極めて高い初期容量(200mAh/g程度)を有する。
【0022】
【実施例】
以下本発明を実施例に即して説明するが、本発明はこれらの実施例に限定されるものではない。
実施例1
攪拌機とオーバーフローパイプを備えた15Lの円筒形反応槽に水を13L入れた後、pHが12.2(40℃測定)になるまで32%水酸化ナトリウム水溶液を加え、窒素ガスを0.3L/分の流量にて反応槽内にバブリングさせながら、温度を50℃に保持し一定速度にて攪拌を行った。次にNi:Mnの原子比が1:1となるように混合した1.7mol/L硫酸ニッケル水溶液と1.1mol/L硫酸マンガン水溶液の混合液に2.8mol/L硫酸アンモニウム水溶液を混合水溶液容量に対して5%(v/v)加え、さらにこの混合溶液中の溶存酸素を除去する目的で4wt%ヒドラジン水溶液を混合水溶液量に対して1.3%(v/v)加え、10cc/分の流量にて反応槽に滴下した。さらに反応槽内の溶液がpH12.2になるように32%水酸化ナトリウム水溶液を断続的に加えニッケルマンガン複合水酸化物粒子を形成させた。反応槽内が定常状態になった後、オーバーフローパイプよりニッケルマンガン複合水酸化物粒子を連続的に採取し水洗後、濾過し100℃にて15時間乾燥し乾燥粉末であるニッケルマンガン複合水酸化物を得た。得られたニッケルマンガン複合水酸化物を試料aとした。
【0023】
実施例2
密閉型の反応槽を用い、pHを12.0(40℃測定)にした以外は実施例1と同様の条件にてニッケルマンガン複合水酸化物を製造し、得られたニッケルマンガン複合水酸化物を試料bとした。
【0024】
実施例3
窒素ガス流量を1.0L/分、純空気を0.1L/分の流量にてバブリングした以外は実施例2と同様の条件にてニッケルマンガン複合水酸化物を製造し、得られたニッケルマンガン複合水酸化物を試料cとした。
【0025】
実施例4
窒素ガスを0.5L/分にてバブリングし、pHを11.7(40℃測定)にした以外は実施例1と同様の条件にてニッケルマンガン複合水酸化物を製造し、得られたニッケルマンガン複合水酸化物を試料dとした。
【0026】
実施例5
窒素ガスを0.5L/分の流量にてバブリングし、pHを11.3(40℃測定)にした以外は実施例2と同様の条件にてニッケルマンガン複合水酸化物を製造し、得られたニッケルマンガン複合水酸化物を試料eとした。
【0027】
実施例6
窒素ガスを1.0L/分の流量にてバブリングし、pHを11.9(40℃測定)にした以外は実施例2と同様の条件にてニッケルマンガン複合水酸化物を製造し、得られたニッケルマンガン複合水酸化物を試料fとした。
各実施例で得られた粒子の特性値を以下の表1にまとめた。
【0028】
【表1】
【0029】
また、各実施例1〜6で得られた粒子(a〜f)の電子顕微鏡写真をそれぞれ図1〜6に示した。中和反応時での酸化の程度により、2次粒子の表面および内部の構造が大きく依存することが分かる。
【0030】
また、各実施例1〜6で得られた粒子(a〜f)のX線回折の結果をそれぞれ図7に示した。中和反応時での酸化の程度が、2つのピークの強度比により簡便に評価できることが分かる。また表2には、X線回折において15≦2θ≦25にあるピークの最大強度(I0)と、30≦2θ≦40にあるピークの最大強度(I1)との比(I0/I1)をまとめた。
【0031】
【表2】
【0032】
また、各実施例1〜6で得られた粒子(a〜f)を用いたリチウムイオン二次電池充放電曲線をそれぞれ図8〜13に示した。例えば初期容量が極めて大きく、優れた電池特性を示すことが分かる。
【0033】
【発明の効果】
本発明にかかる製造方法により、実質的にマンガン:ニッケルが1:1である複合水酸化物粒子であって、平均粒径が5〜15μm、タップ密度が0.6〜1.4g/ml、バルク密度が0.4〜1.0g/ml、比表面積が20〜55m2/g、含有硫酸根が0.25〜0.45重量%であり、かつX線回折において15≦2θ≦25にあるピークの最大強度(I0)と、30≦2θ≦40にあるピークの最大強度(I1)との比(I0/I1)が、1〜6であることを特徴とする、マンガンニッケル複合水酸化物粒子を得る。かかる粒子から製造されるリチウムマンガンニッケル複合酸化物を正極活性物質成分として含有することで非常に優れた電池特性を示すリチウムイオン二次電池を得ることができる。
【図面の簡単な説明】
【図1】実施例1で得られた粒子(a)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍、(C)断面20000倍。
【図2】実施例2で得られた粒子(b)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍、(C)断面20000倍。
【図3】実施例3で得られた粒子(c)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍。
【図4】実施例4で得られた粒子(d)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍、(C)断面20000倍。
【図5】実施例5で得られた粒子(e)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍、(C)断面20000倍。
【図6】実施例6で得られた粒子(f)の電子顕微鏡写真である。(A)表面5000倍、(B)表面20000倍、(C)断面20000倍。
【図7】各実施例で得られた各試料(a)〜(f)のX線回折の結果を示す。
【図8】実施例1で得られた粒子(a)を用いてたリチウムイオン二次電池の充放電曲線を示した。
【図9】実施例2で得られた粒子(b)を用いてたリチウムイオン二次電池の充放電曲線を示した。
【図10】実施例3で得られた粒子(c)を用いてたリチウムイオン二次電池の充放電曲線を示した。
【図11】実施例4で得られた粒子(d)を用いてたリチウムイオン二次電池の充放電曲線を示した。
【図12】実施例5で得られた粒子(e)を用いてたリチウムイオン二次電池の充放電曲線を示した。
【図13】実施例6で得られた粒子(f)を用いてたリチウムイオン二次電池の充放電曲線を示した。[0001]
BACKGROUND OF THE INVENTION
The present invention relates to composite hydroxide particles substantially having a manganese: nickel ratio of 1: 1 (hereinafter referred to as “manganese: nickel (1: 1)”).
[0002]
[Prior art]
With the recent spread of cordless and portable AV devices and personal computers, there is an increasing demand for smaller, lighter and higher energy density batteries. In particular, since the lithium secondary battery is a battery having a high energy density, it is expected as a next-generation main battery and has a large potential market scale.
Conventionally, composite hydroxide (Ni 1/2 Mn 1/2 (OH) 2 ) particles having a manganese: nickel ratio of 1: 1 are known by using a so-called coprecipitation method. It is known that a lithium manganese nickel composite oxide having a manganese: nickel ratio of 1: 1 is substantially produced and can be used as a positive electrode active material for a nonaqueous electrolyte battery (see, for example, Patent Document 1). . However, there is room for further improvement in battery performance, and there is a strong demand for batteries having various desirable characteristics such as charge / discharge characteristics.
[Patent Document 1]
JP 2002-42813 A
[Means for Solving the Problems]
Based on this demand, the present inventor has intensively studied for the purpose of further improving the positive electrode active material for a non-aqueous electrolyte battery using the lithium manganese nickel composite oxide of manganese: nickel (1: 1). By controlling the shape of the manganese: nickel (1: 1) composite hydroxide particles used to form the composite oxide, a non-aqueous electrolyte secondary battery (for example, a lithium ion secondary battery) using the same can be obtained. The inventors have found that it has very excellent charge / discharge characteristics (cycle number, energy density, etc.) and have completed the present invention.
[0004]
That is, lithium-manganese-nickel composite hydroxide particles according to the present invention, the atomic ratio of manganese to nickel substantially 1: 1, formally (Mn 1/2 Ni 1/2) (OH) 2 And the particles have a characteristic shape.
[0005]
In particular, the lithium manganese nickel composite hydroxide particles according to the present invention have an average particle size of 5 to 20 μm (preferably 5 to 15 μm), a tap density of 0.6 to 1.4 g / ml, and a bulk density of 0.4. -1.0 g / ml. The specific surface area is 20 to 100 m 2 / g (preferably 20 to 55 m 2 / g).
Furthermore, based on surface observation with an electron microscope, the surface of the secondary particles and the internal structure are formed by a pleated wall of primary particles, and the space surrounded by the pleated walls is relatively large. Features.
[0006]
In addition, the structure of the surface and the interior of the secondary particles largely depends on the oxidation state during the neutralization reaction in the coprecipitation method. This means that it depends greatly by producing it under the condition that a part of manganese ion is oxidized. According to the measurement of sulfate ion, the content of the sulfate group is 0.25 to 0.45% by weight. In X-ray diffraction, the ratio (I 0 / I 1 ) between the maximum intensity (I 0 ) of the peak at 15 ≦ 2θ ≦ 25 and the maximum intensity (I 1 ) of the peak at 30 ≦ 2θ ≦ 40 is 1-6.
[0007]
The present invention further includes a lithium manganese nickel composite oxide obtained by firing the above-described manganese nickel composite hydroxide particles and lithium hydroxide, and substantially having a manganese: nickel ratio of 1: 1. In addition, a lithium ion secondary battery including the positive electrode active material component is also included.
Moreover, this invention also includes the manufacturing method of the said manganese nickel composite hydroxide particle, lithium manganese nickel composite oxide, and a lithium ion secondary battery.
[0008]
In particular, in the production method according to the present invention, a mixed aqueous solution of a manganese salt and a nickel salt in which an atomic ratio of manganese to nickel is substantially 1: 1 in an aqueous solution having a pH of 9 to 13 in the presence of a complexing agent is alkalinized. In a method of obtaining lithium manganese nickel composite hydroxide in which the atomic ratio of manganese to nickel is substantially uniformly mixed by coprecipitation of particles produced by reacting with a solution under appropriate stirring conditions In addition, the degree of oxidation of manganese ions is controlled within a certain range.
Hereinafter, the present invention will be described in detail according to the embodiments of the invention.
[0009]
DETAILED DESCRIPTION OF THE INVENTION
Production of manganese nickel composite hydroxide particles First, a method for producing a lithium manganese nickel composite hydroxide according to the present invention will be described below. That is, it can be preferably obtained by using a so-called coprecipitation method as described below and under the condition that the degree of oxidation of manganese ions is controlled within a certain range.
That is, the coprecipitation method referred to here is a manganese salt in which the atomic ratio of manganese to nickel is substantially 1: 1 in the presence of a complexing agent in an aqueous solution having an appropriate pH range (for example, 9 to 13). Lithium manganese nickel composite hydroxide is obtained by coprecipitation of particles produced by reacting a mixed aqueous solution of nickel and a nickel salt with an alkali solution under appropriate stirring conditions. By such a coprecipitation method, particles having a preferable particle diameter in which the atomic ratio of manganese and nickel is substantially uniformly mixed at 1: 1 can be obtained.
[0010]
Here, the usable manganese salt is not particularly limited as long as manganese ions generated in an aqueous solution can form a complex with a complexing agent. Specific examples include manganese sulfate, manganese nitrate, and manganese chloride. Similarly, the usable nickel salt is not particularly limited as long as nickel ions generated in an aqueous solution can form a complex with a complexing agent. Specific examples include nickel sulfate, nickel nitrate, and nickel chloride. In the present invention, the atomic ratio of manganese to nickel is substantially 1: 1 as long as it is in the range of about plus or
[0011]
The pH value of the aqueous solution is preferably in the range of pH 9 to 13, and can be maintained in this range by adding an alkali metal hydroxide (for example, sodium hydroxide, potassium hydroxide) if necessary during the reaction.
The complexing agent can form a complex with manganese ions and nickel ions in an aqueous solution. For example, an ammonium ion supplier (ammonium chloride, ammonium carbonate, ammonium fluoride, etc.), hydrazine, ethylenediaminetetraacetic acid, nitrite Examples include triacetic acid, uracil diacetic acid, and glycine.
[0012]
In addition, the condition for controlling the degree of oxidation of manganese ions within a certain range, as used herein, is to control by mixing an appropriate amount of air (oxygen) with nitrogen as an atmosphere gas to be bubbled into the reaction solution. It is.
Manganese ions are usually very oxidizable under the conditions of the coprecipitation method, giving complex manganese oxides. In general, manganese nickel composite hydroxide particles containing manganese oxide have poor battery characteristics when used as a raw material. Therefore, in the present invention, it is necessary to suppress the production of manganese oxide as much as possible.
[0013]
On the other hand, in the coprecipitation method, it is possible to set conditions for suppressing oxidation of manganese ions almost completely. For example, there is a method in which a reagent (for example, hydrazine) for removing dissolved oxygen in a solution is added and the reaction is performed in an inert gas atmosphere from which oxygen is substantially removed. In this case, the manganese ions of the resulting manganese nickel composite hydroxide particles are almost completely divalent. However, the present inventor has found that the shape of the manganese nickel composite hydroxide particles obtained under the above-mentioned coprecipitation method under the condition of suppressing the oxidation of manganese ions almost completely differs greatly. Under this condition, the primary particles are stacked very densely to form secondary particles having a high density, and the surface and the inside thereof are substantially free of a network-like structure. In the process of obtaining the lithium manganese nickel composite oxide as the secondary battery material by firing with lithium hydroxide as a raw material in the case of this shape, the inventor sufficiently and uniformly melted lithium ions. It was found that the manganese nickel composite oxide could not be incorporated into the crystal.
[0014]
From these findings, the shape of the manganese nickel composite hydroxide particles is important and preferable in order to incorporate the molten lithium ions sufficiently and uniformly into the crystal of the manganese nickel composite oxide. It is necessary to control the network structure. For this reason, in the present invention, manganese ions can be oxidized under the coprecipitation reaction conditions in order to control the preferred network structure, but the coprecipitation is carried out under conditions where unnecessary amounts of manganese oxide are not formed.
[0015]
There are no particular restrictions on the oxidation conditions and degree. Preferably, dissolved oxygen is previously removed with a reducing agent. As the reducing agent, coprecipitation is performed in the presence of an appropriate complexing agent, and therefore, hydrazine that acts as both a complexing agent and a reducing agent is preferably used. It is preferable to add hydrazine to the extent that about 10% remains even after completion of the reaction. The oxidizing agent is not particularly limited, but is preferably included in an inert gas blown into the solution because a slurry is obtained by a coprecipitation method and appropriate stirring is required. For this purpose, it is possible to use air, oxygen, and other oxidizing gases (such as chlorine).
[0016]
The degree of oxidation of manganese ions can be evaluated by various methods. Visually, when the degree of oxidation is large, the produced manganese oxide makes the particles blackish gray. On the other hand, when oxidation is substantially suppressed, the color becomes light blue-green. Therefore, the intermediate degree of oxidation can be evaluated. Furthermore, the degree of oxidation can be quantitatively evaluated by a conventionally known oxidation-reduction titration method or the like. Moreover, when the oxidized manganese ion contains a sulfate ion as a counter anion, the degree of oxidation can be indirectly evaluated by a simple analysis method of the sulfate ion.
[0017]
Shape of manganese nickel composite hydroxide particles The particle size obtained by the coprecipitation method The secondary particle size of lithium manganese nickel composite hydroxide particles depends on the pH applied in the coprecipitation method and the reactor. . Usually, those having an average particle diameter of 5 to 20 μm can be obtained by using a known pH or reaction apparatus. The particles are gathered by smaller primary particles to form larger secondary particles.
In addition, the surface and the internal structure can be easily distinguished by electron microscope observation. Particles obtained under conditions in which oxidation is substantially suppressed, the primary particles gather strongly and densely to form secondary particles. As the oxidation of manganese proceeds, the surface and internal structure of the particles change significantly. Primary particles gather to form a pleated wall, which gathers to form a meshed secondary particle. Therefore, it exhibits a sponge-like structure partitioned by large pleated walls. This structure changes depending on the degree of oxidation. In particular, the size of the space surrounded by the network structure changes. When the oxidation proceeds to some extent, the network structure becomes more dense and the size of the enclosed space becomes smaller. In the examples described below, electron micrographs of the shape of the obtained particles under each of these conditions were shown.
[0018]
Specifically, the difference in the structure of the surface and the interior of the secondary particles described above is remarkably shown in the tap density (TD), bulk density (BD), and specific surface (BET). The lower the degree of oxidation, the higher the secondary particles have a higher density structure in which the primary particles are gathered more strongly. On the other hand, when the oxidation is in an appropriate range, the tap density (TD) and bulk density (BD) are almost halved, indicating that there is a space inside the particle. Further, the difference in the structure greatly affects the specific surface, and when the degree of oxidation is appropriate, the difference is about twice as large as that in the case of not being oxidized.
[0019]
Lithium manganese nickel composite oxide The lithium manganese nickel composite oxide is composed of the composite hydroxide particles described above, the total atomic ratio of manganese and nickel of the composite hydroxide particles, and the atomic ratio of lithium. It is obtained by mixing with a lithium compound so as to be substantially 1: 1 and baking and heating the resulting mixture at a temperature of at least 850 ° C. in an air stream. There is no particular limitation on heating furnace for use in such firing conditions and firing, sintering apparatus can be preferably used for use in conventional synthesis of LiMn 2 O 4 and LiNiO 2. The atmosphere during firing is preferably a normal air atmosphere.
[0020]
Although there is no restriction | limiting in particular as a lithium compound which can be used, For example, lithium hydroxide, lithium carbonate, lithium nitrate, lithium oxide is mentioned. In particular, the use of lithium hydroxide is preferred. The molar ratio of the manganese nickel (1: 1) composite oxide to the lithium compound is substantially 1: 1. Here, the molar ratio between the manganese nickel composite oxide and the lithium compound being substantially 1: 1 is included in the range of about plus or minus 10%. These values can be accurately measured by various metal analysis methods (for example, atomic absorption method). It is preferable to sufficiently mix them before firing.
[0021]
Non-aqueous electrolyte secondary battery The lithium ion secondary battery of the present invention is a lithium ion secondary battery comprising the lithium manganese nickel composite oxide as a positive electrode active material component. There are no restrictions on its basic structure. Various types of batteries can be constructed using generally known shapes and materials. Further, since the lithium manganese nickel composite oxide according to the present invention is contained as a positive electrode active material component, such a battery is charge / discharge characterized by extremely flat and low polarization in the vicinity of 4 V, as shown in FIGS. It has characteristics and has a very high initial capacity (about 200 mAh / g).
[0022]
【Example】
The present invention will be described below with reference to examples, but the present invention is not limited to these examples.
Example 1
After 13 L of water was put into a 15 L cylindrical reaction vessel equipped with a stirrer and an overflow pipe, a 32% sodium hydroxide aqueous solution was added until the pH reached 12.2 (measured at 40 ° C.), and nitrogen gas was supplied at 0.3 L / While bubbling into the reaction vessel at a flow rate of minutes, the temperature was maintained at 50 ° C. and stirring was performed at a constant speed. Next, 2.8 mol / L ammonium sulfate aqueous solution is mixed with a 1.7 mol / L nickel sulfate aqueous solution and a 1.1 mol / L manganese sulfate aqueous solution mixed so that the atomic ratio of Ni: Mn is 1: 1. 5% (v / v) was added to the solution, and 4% by weight of hydrazine aqueous solution was added 1.3% (v / v) with respect to the amount of the mixed solution for the purpose of removing dissolved oxygen in the mixed solution. It was dripped at the reaction tank with the flow volume of. Further, a 32% sodium hydroxide aqueous solution was intermittently added so that the solution in the reaction vessel had a pH of 12.2 to form nickel manganese composite hydroxide particles. After the reaction vessel is in a steady state, the nickel manganese composite hydroxide particles are continuously collected from the overflow pipe, washed with water, filtered, dried at 100 ° C. for 15 hours, and dried as a nickel manganese composite hydroxide. Got. The obtained nickel manganese composite hydroxide was used as sample a.
[0023]
Example 2
A nickel manganese composite hydroxide was produced under the same conditions as in Example 1 except that a closed reaction vessel was used and the pH was set to 12.0 (measured at 40 ° C.). The resulting nickel manganese composite hydroxide was obtained. Was designated as sample b.
[0024]
Example 3
A nickel manganese composite hydroxide was produced under the same conditions as in Example 2 except that bubbling was performed at a nitrogen gas flow rate of 1.0 L / min and pure air at a flow rate of 0.1 L / min. The composite hydroxide was used as sample c.
[0025]
Example 4
Nickel-manganese composite hydroxide was produced under the same conditions as in Example 1 except that nitrogen gas was bubbled at 0.5 L / min and the pH was 11.7 (measured at 40 ° C.). Manganese composite hydroxide was used as sample d.
[0026]
Example 5
A nickel manganese composite hydroxide was produced under the same conditions as in Example 2 except that bubbling of nitrogen gas was performed at a flow rate of 0.5 L / min and the pH was set to 11.3 (measured at 40 ° C.). Nickel-manganese composite hydroxide was used as sample e.
[0027]
Example 6
A nickel manganese composite hydroxide was produced under the same conditions as in Example 2 except that bubbling of nitrogen gas was performed at a flow rate of 1.0 L / min and the pH was 11.9 (measured at 40 ° C.). The nickel manganese composite hydroxide was designated as sample f.
The characteristic values of the particles obtained in each example are summarized in Table 1 below.
[0028]
[Table 1]
[0029]
Moreover, the electron micrograph of the particle | grains (af) obtained in each Example 1-6 was shown to FIGS. 1-6, respectively. It can be seen that the structure of the surface and the interior of the secondary particles greatly depend on the degree of oxidation during the neutralization reaction.
[0030]
Moreover, the result of the X-ray diffraction of the particle | grains (af) obtained in each Example 1-6 was shown in FIG. 7, respectively. It can be seen that the degree of oxidation during the neutralization reaction can be easily evaluated by the intensity ratio of the two peaks. Table 2 also shows the ratio (I 0 / I) of the maximum intensity (I 0 ) of the peak at 15 ≦ 2θ ≦ 25 to the maximum intensity (I 1 ) of the peak at 30 ≦ 2θ ≦ 40 in X-ray diffraction. 1 ) is summarized.
[0031]
[Table 2]
[0032]
Moreover, the lithium ion secondary battery charging / discharging curve using the particle | grains (af) obtained in each Examples 1-6 was shown to FIGS. 8-13, respectively. For example, it can be seen that the initial capacity is extremely large and exhibits excellent battery characteristics.
[0033]
【The invention's effect】
According to the production method of the present invention, the composite hydroxide particles are substantially 1: 1 of manganese: nickel, the average particle diameter is 5 to 15 μm, the tap density is 0.6 to 1.4 g / ml, The bulk density is 0.4 to 1.0 g / ml, the specific surface area is 20 to 55 m 2 / g, the contained sulfate radical is 0.25 to 0.45% by weight, and 15 ≦ 2θ ≦ 25 in X-ray diffraction. Manganese characterized in that the ratio (I 0 / I 1 ) between the maximum intensity (I 0 ) of a certain peak and the maximum intensity (I 1 ) of a peak at 30 ≦ 2θ ≦ 40 is 1-6 Nickel composite hydroxide particles are obtained. By including a lithium manganese nickel composite oxide produced from such particles as a positive electrode active material component, a lithium ion secondary battery exhibiting very excellent battery characteristics can be obtained.
[Brief description of the drawings]
1 is an electron micrograph of particles (a) obtained in Example 1. FIG. (A) Surface 5000 times, (B) Surface 20000 times, (C) Cross section 20000 times.
2 is an electron micrograph of particles (b) obtained in Example 2. FIG. (A) Surface 5000 times, (B) Surface 20000 times, (C) Cross section 20000 times.
3 is an electron micrograph of particles (c) obtained in Example 3. FIG. (A) Surface 5000 times, (B) Surface 20000 times.
4 is an electron micrograph of particles (d) obtained in Example 4. FIG. (A) Surface 5000 times, (B) Surface 20000 times, (C) Cross section 20000 times.
5 is an electron micrograph of particles (e) obtained in Example 5. FIG. (A) Surface 5000 times, (B) Surface 20000 times, (C) Cross section 20000 times.
6 is an electron micrograph of particles (f) obtained in Example 6. FIG. (A) Surface 5000 times, (B) Surface 20000 times, (C) Cross section 20000 times.
FIG. 7 shows the results of X-ray diffraction of samples (a) to (f) obtained in each example.
8 shows a charge / discharge curve of a lithium ion secondary battery using the particles (a) obtained in Example 1. FIG.
9 shows a charge / discharge curve of a lithium ion secondary battery using the particles (b) obtained in Example 2. FIG.
10 shows a charge / discharge curve of a lithium ion secondary battery using the particles (c) obtained in Example 3. FIG.
11 shows a charge / discharge curve of a lithium ion secondary battery using the particles (d) obtained in Example 4. FIG.
12 shows a charge / discharge curve of a lithium ion secondary battery using the particles (e) obtained in Example 5. FIG.
13 shows a charge / discharge curve of a lithium ion secondary battery using the particles (f) obtained in Example 6. FIG.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002379506A JP4234418B2 (en) | 2002-12-27 | 2002-12-27 | Manganese nickel composite hydroxide particles |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002379506A JP4234418B2 (en) | 2002-12-27 | 2002-12-27 | Manganese nickel composite hydroxide particles |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004210560A JP2004210560A (en) | 2004-07-29 |
| JP4234418B2 true JP4234418B2 (en) | 2009-03-04 |
Family
ID=32815992
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002379506A Expired - Fee Related JP4234418B2 (en) | 2002-12-27 | 2002-12-27 | Manganese nickel composite hydroxide particles |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4234418B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7240484B2 (en) | 2019-03-29 | 2023-03-15 | 三井化学株式会社 | Ink raw materials for textile printing |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4595475B2 (en) * | 2004-10-01 | 2010-12-08 | 住友金属鉱山株式会社 | Positive electrode active material for non-aqueous electrolyte secondary battery, non-aqueous electrolyte secondary battery using the same, and method for producing the same |
| US10017875B2 (en) | 2011-03-28 | 2018-07-10 | Sumitomo Metal Mining Co., Ltd. | Nickel manganese composite hydroxide particles and manufacturing method thereof, cathode active material for a non-aqueous electrolyte secondary battery and manufacturing method thereof, and a non-aqueous electrolyte secondary battery |
| KR101696524B1 (en) | 2011-06-07 | 2017-01-13 | 스미토모 긴조쿠 고잔 가부시키가이샤 | Nickel composite hydroxide and process for producing same, positive active material for nonaqueous-electrolyte secondary battery and process for producing same, and nonaqueous-electrolyte secondary battery |
| JP4894969B1 (en) | 2011-06-07 | 2012-03-14 | 住友金属鉱山株式会社 | Nickel-manganese composite hydroxide particles and production method thereof, positive electrode active material for non-aqueous electrolyte secondary battery and production method thereof, and non-aqueous electrolyte secondary battery |
| JP6055967B2 (en) * | 2012-05-10 | 2017-01-11 | 株式会社田中化学研究所 | Positive electrode active material and method for producing the same, positive electrode active material precursor, positive electrode for lithium secondary battery, and lithium secondary battery |
| KR101525000B1 (en) * | 2012-09-24 | 2015-06-03 | 한국화학연구원 | Method for preparing nickel-manganese complex hydroxides for cathode materials in lithium batteries, nickel-manganese complex hydroxides prepared by the method and cathode materials in lithium batteries comprising the same |
| JP6241349B2 (en) | 2014-03-28 | 2017-12-06 | 住友金属鉱山株式会社 | Precursor of positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same, and positive electrode active material for non-aqueous electrolyte secondary battery and method for producing the same |
| JP6583418B2 (en) | 2015-08-24 | 2019-10-02 | 住友金属鉱山株式会社 | Manganese nickel composite hydroxide and method for producing the same, lithium manganese nickel composite oxide and method for producing the same, and non-aqueous electrolyte secondary battery |
| JP6582824B2 (en) | 2015-09-30 | 2019-10-02 | 住友金属鉱山株式会社 | Nickel-manganese-containing composite hydroxide and method for producing the same |
| JP6970671B2 (en) * | 2016-07-28 | 2021-11-24 | 住友化学株式会社 | Method for manufacturing lithium nickel composite oxide |
| CN115385395A (en) * | 2017-07-12 | 2022-11-25 | 住友金属矿山株式会社 | Metal composite hydroxide, positive electrode active material for nonaqueous electrolyte secondary battery, and nonaqueous electrolyte secondary battery using same |
| KR102023063B1 (en) | 2017-12-15 | 2019-09-19 | 주식회사 포스코 | Manufacturing method of positive active material precursor for secondary battery and manufacturing apparatus using the same |
-
2002
- 2002-12-27 JP JP2002379506A patent/JP4234418B2/en not_active Expired - Fee Related
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7240484B2 (en) | 2019-03-29 | 2023-03-15 | 三井化学株式会社 | Ink raw materials for textile printing |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2004210560A (en) | 2004-07-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10326133B2 (en) | Methods of making inorganic compounds | |
| JP5817143B2 (en) | Positive electrode active material precursor particle powder, positive electrode active material particle powder, and non-aqueous electrolyte secondary battery | |
| JP3489685B2 (en) | Lithium manganate, method for producing the same, and lithium battery using the same | |
| JP4826147B2 (en) | Aluminum-containing nickel hydroxide particles and method for producing the same | |
| JP5021892B2 (en) | Precursor for positive electrode material of lithium ion secondary battery, method for producing the same, and method for producing positive electrode material using the same | |
| JP5678482B2 (en) | Manganese oxide and method for producing the same | |
| JP6237330B2 (en) | Precursor of positive electrode active material for nonaqueous electrolyte secondary battery and method for producing the same, and method for producing positive electrode active material for nonaqueous electrolyte secondary battery | |
| JP2015191848A (en) | Precursor of positive electrode active material for nonaqueous electrolyte secondary batteries and manufacturing method thereof, and positive electrode active material for nonaqueous electrolyte secondary batteries and manufacturing method thereof | |
| JP2007123255A (en) | Lithium transition metal complex oxide, its manufacturing method, and lithium cell made by using the same | |
| JP2003059490A (en) | Positive active material for nonaqueous electrolyte secondary battery and its manufacturing method | |
| JP2007091573A (en) | Lithium-nickel-manganese-cobalt multiple oxide, method for producing the same, and application of the multiple oxide | |
| JP4234418B2 (en) | Manganese nickel composite hydroxide particles | |
| JP7118129B2 (en) | METHOD FOR PRODUCING POSITIVE ACTIVE MATERIAL FOR LITHIUM-ION BATTERY | |
| JP6303279B2 (en) | Positive electrode active material particle powder, method for producing the same, and nonaqueous electrolyte secondary battery | |
| JP7064685B2 (en) | A method for producing a nickel-manganese composite hydroxide and a method for producing a positive electrode active material for a non-aqueous electrolyte secondary battery. | |
| JP6237229B2 (en) | Precursor of positive electrode active material for nonaqueous electrolyte secondary battery and method for producing the same, and positive electrode active material for nonaqueous electrolyte secondary battery and method for producing the same | |
| JP2019164981A (en) | Manufacturing method of positive electrode active material precursor for lithium ion secondary battery, manufacturing method of intermediate of positive electrode active material for lithium ion secondary battery, and manufacturing method of positive electrode active material for lithium ion secondary battery, including combination thereof | |
| JP4602689B2 (en) | Positive electrode material for lithium ion secondary battery | |
| JP2001114521A (en) | Trimanganese tetraoxide and method for its production | |
| JP2021160970A (en) | Spinel lithium manganate and method for producing the same and applications thereof | |
| JP6876600B2 (en) | Precursor of positive electrode active material for non-aqueous electrolyte secondary battery and its manufacturing method, and positive electrode active material for non-aqueous electrolyte secondary battery and its manufacturing method | |
| JP2005272213A (en) | Method for producing lithium-cobalt oxide | |
| JP2014205617A (en) | Manganese oxide and method for producing lithium manganate using the same | |
| JP6123391B2 (en) | Trimanganese tetraoxide and method for producing the same | |
| JP2021134100A (en) | Spinel-type lithium manganate and method for producing the same, and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20051004 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20080331 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080930 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081016 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20081111 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20081211 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20111219 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4234418 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121219 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121219 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131219 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |