JP3903900B2 - Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability - Google Patents
Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability Download PDFInfo
- Publication number
- JP3903900B2 JP3903900B2 JP2002306343A JP2002306343A JP3903900B2 JP 3903900 B2 JP3903900 B2 JP 3903900B2 JP 2002306343 A JP2002306343 A JP 2002306343A JP 2002306343 A JP2002306343 A JP 2002306343A JP 3903900 B2 JP3903900 B2 JP 3903900B2
- Authority
- JP
- Japan
- Prior art keywords
- corrosion resistance
- zinc phosphate
- steel sheet
- compound
- paint adhesion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Landscapes
- Laminated Bodies (AREA)
- Chemical Treatment Of Metals (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
Description
【0001】
【発明が属する技術分野】
本発明は、家電製品、配電盤、電話交換機パネル、自動車部品、建材などの素材として好適な非クロム系リン酸亜鉛処理鋼板に関するものである。
【0002】
【従来の技術】
リン酸亜鉛処理鋼板は、一時防錆性を確保する目的と塗装下地として使用された場合においても十分な耐食性を確保する目的から、リン酸亜鉛処理した後にクロメートシーリングすることが一般的であるが、このクロメートシーリング処理は環境規制物質である6価クロムを用いることから、6価クロムを用いない非クロム系シーリング処理技術の開発が望まれている。
【0003】
リン酸亜鉛処理した後にクロムフリーの処理を施した表面処理鋼板としては、亜鉛系めっき鋼板表面にリン酸亜鉛処理皮膜を有し、その上層に有機樹脂とチオカルボニル基含有化合物又はバナジン酸化合物を含む皮膜を有する非クロム系処理亜鉛系めっき鋼板(特許文献1)、亜鉛系めっき鋼板などの表面に、Niを1〜20mg/m2析出させ、その上に付着量が0.2〜3g/m2のリン酸亜鉛処理皮膜を形成し、さらにその上に非クロム系防錆顔料を10〜60mass%含む下塗り塗膜、上塗り塗膜を順次形成した非クロム系塗装金属板(特許文献2)などが知られている。
【0004】
【特許文献1】
特開2000−248367号公報
【特許文献2】
特開2001−081578号公報
【0005】
【発明が解決しようとする課題】
しかし、これら従来の表面処理鋼板は、耐食性が不十分であったり、製造コストが高いなどの難点がある。
したがって本発明の目的は、シーリング処理皮膜中に6価クロムを含むことなく、従来のクロメートシーリング処理材に匹敵する優れた耐食性や塗料密着性を有するとともに、優れた加工性を有し、しかも安価に製造することができる非クロム系リン酸亜鉛処理鋼板を提供することにある。
【0006】
【課題を解決するための手段】
本発明者らは、クロメートシーリング処理材が施されたリン酸亜鉛処理鋼板と同等の性能を、クロムを全く含有しない液状組成物によるシーリング処理により達成するために、液状組成物の組成について鋭意検討を行った。その結果、銅化合物と特定の金属化合物を含む水系の液状組成物でシーリング処理を行うことにより、上記性能に加えてクロメートシーリング処理材に匹敵する優れた性能(耐食性及び塗料密着性)が得られること、また、この液状組成物にさらに特定の樹脂化合物を添加することにより、上記性能に加えて優れた加工性が得られることを見出した。
【0007】
本発明は、このような知見に基づきなされたもので、その特徴は以下のとおりである。
【0008】
[ 1 ] 亜鉛含有めっき鋼板の表面に、付着量(Zn3(PO4)2換算量)が0.1〜5g/m2のリン酸亜鉛処理皮膜を有し、その上層に、銅化合物(A)と、チタン化合物及びジルコニウム化合物の中から選ばれる少なくとも1種の金属化合物(B)と、下記(1)式の化学構造を有するビスフェノールAと下記(2)式の化学構造を有するアミン類とホルムアルデヒドとの重縮合樹脂化合物であって、数平均分子量が400〜10000、1ベンゼン環当たりのアミノ基の置換数の平均値が0.2〜1.0である重縮合樹脂化合物(C)と、水とを含む液状組成物を塗布し、乾燥させて得られたシーリング処理皮膜を有することを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
【化3】
【化4】
但し、式中、R1、及びR2は、それぞれ互いに独立に水素原子、炭素数1〜10のアルキル基又は炭素数1〜10のヒドロキシアルキル基を表す。
【0009】
[ 2 ] 上記[ 1 ]の非クロム系リン酸亜鉛処理鋼板において、液状組成物に含まれる重縮合樹脂化合物(C)の固形分の含有量が、銅化合物(A)と金属化合物(B)の合計含有量の10〜300mass%であることを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
[ 3 ] 上記[1]又は[ 2 ]の非クロム系リン酸亜鉛処理鋼板において、液状組成物に含まれる銅化合物(A)が2価の銅化合物であり、且つその陰イオン成分が硝酸、硫酸、炭酸、ピロリン酸の中から選ばれる少なくとも1種の無機酸であることを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
[ 4 ] 上記[1]〜[ 3 ]のいずれかの非クロム系リン酸亜鉛処理鋼板において、液状組成物に含まれる銅化合物(A)の銅イオンの含有量が、金属化合物(B)の含有量の10〜200mass%であることを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
【0010】
[ 5 ] 上記[1]〜[ 4 ]のいずれかの非クロム系リン酸亜鉛処理鋼板において、液状組成物に含まれる金属化合物(B)がジルコニウム化合物であることを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
[ 6 ] 上記[1]〜[ 5 ]のいずれかの非クロム系リン酸亜鉛処理鋼板において、シーリング処理皮膜中のチタン又は/及びジルコニウムの合計付着量が1〜100mg/m2であることを特徴とする、耐食性、塗料密着性及び加工性に優れた非クロム系リン酸亜鉛処理鋼板。
【0011】
【発明の実施の形態】
本発明のリン酸亜鉛処理鋼板は、亜鉛含有めっき鋼板を素材鋼板とし、そのめっき皮膜表面に、下層側からリン酸亜鉛処理皮膜と特定の組成物によるシーリング処理皮膜を順次形成したものである。
本発明のリン酸亜鉛処理鋼板の下地鋼板となる亜鉛含有めっき鋼板に特別な制限はなく、純亜鉛めっき鋼板、亜鉛合金めっき鋼板のいずれでもよい。例えば、亜鉛めっき鋼板、Zn−Niめっき鋼板、Zn−Feめっき鋼板、(電気めっき、合金化溶融亜鉛めっき)、Zn−Crめっき鋼板、Zn−Mnめっき鋼板、Zn−Coめっき鋼板、Zn−Co−Cr合金めっき鋼板、Zn−Cr−Niめっき鋼板、Zn−Cr−Feめっき鋼板、Zn−A1めっき鋼板(例えば、Zn−5%A1合金めっき鋼板、Zn−55%A1合金めっき鋼板)、さらにはこれらのめっきに金属酸化物、ポリマーなどを分散した亜鉛系複合めっき鋼板(例えば、Zn−SiO2分散めっき鋼板)などを用いることができる。また、上記のようなめっきのうち、同種または異種のものを2層以上めっきした複層めっき鋼板を用いることもできる。また、めっき鋼板としては鋼板面に予めNiなどの薄目付けのめっきを施し、その上に上記のような各種めっきを施したものであってもよい。めっき方法についても、電解法、溶融法、気相法のうち、実施可能ないずれの方法も採用することができる。さらに、めっきの黒変を防止する目的で、めっき皮膜中にNi、Co、Feの1種以上を1〜2000ppm程度析出させたり、或いはめっき表面にNi、Co、Feの1種以上を含むアルカリ又は酸性水溶液により表面調整処理を施し、それらの元素を析出させるようにしてもよい。また、めっき付着量についても特に制限はない。
【0012】
リン酸亜鉛処理皮膜の付着量(Zn3(PO4)2換算量)は0.1〜5g/m2とする。この付着量が0.1g/m2未満では、リン酸亜鉛処理皮膜が本来有する防錆性、塗装密着性が発現しない。一方、付着量が5g/m2を超えると、リン酸亜鉛処理皮膜がめっき表面を完全に覆いつくしてしまうため、この皮膜が加工時に割れて加工後の性能が低下するので好ましくない。また、上記の観点から、リン酸亜鉛処理皮膜のより好ましい付着量は0.5〜2g/m2である。
リン酸亜鉛処理皮膜を形成するための処理方法などに特別な制限はなく、通常の結晶性リン酸亜鉛皮膜を形成するための公知の処理方法でよい。
【0013】
リン酸亜鉛処理皮膜の上層に形成するシーリング処理皮膜は6価クロムを含まない皮膜であり、この皮膜は6価クロムを含まない特定の水系液状組成物を塗布し、乾燥することにより形成する。通常、液状組成物を塗布した後は水洗することなく乾燥を行うが、水洗した後に乾燥を行ってもよい。
リン酸亜鉛処理皮膜上にシーリング処理皮膜を形成するための液状組成物は、銅化合物(A)と、チタン化合物及びジルコニウム化合物の中から選ばれる少なくとも1種の金属化合物(B)と、水とを含む組成物であり、水に上記各化合物(A),(B)などを添加することにより調製できる。
【0014】
上記銅化合物(A)の種類に特別な制限はないが、例えば、硫酸銅、硝酸銅、炭酸銅などの酸素酸塩やピロリン酸銅などの有機酸塩などが挙げられ、これらの1種又は2種以上を用いることができる。また、これらの中でも乾燥時に対イオンが残存する銅化合物は、その対イオン自体が塗装密着性に悪影響を及ぼすことがあるためあまり好ましくなく、したがって、この観点からは硝酸銅、炭酸銅などのような対イオンが昇華する銅化合物を用いることが好ましい。本発明において、銅化合物(A)を含む組成物で皮膜を形成することにより優れた耐食性や塗料密着性が得られるのは、銅化合物が有していると推定される有機高分子のドナー原子を含む官能基に配位してキレートを形成する作用などによるものであると考えられる。
【0015】
上記金属化合物(B)のうち、チタン化合物としては、例えば、硫酸チタン、酸化チタン、シュウ酸カリウムチタン、フッ化カリウムチタン、フッ化チタン水素酸、四塩化チタンなどの無機塩や有機チタネートなどが挙げられ、これらの1種又は2種以上を用いることができる。また、ジルコニウム化合物としては、例えば、酸化ジルコニウム、硝酸酸化ジルコニウム、オキシ塩化ジルコニウム、珪酸ジルコニウム、硝酸酸化ジルコニウム、フッ化カリウムジルコニウム、フッ化ジルコニウム水素酸、炭酸ジルコニウムアンモニウムや有機ジルコネートなどが挙げられ、これらの1種又は2種以上を用いることができる。また、酸化チタンや酸化ジルコニウムなどは、高分子化したコロイド状のものであってもよい。
【0016】
本発明において、銅化合物(A)とともに上記特定の金属化合物(B)を含む組成物で皮膜を形成することにより優れた耐食性や塗膜密着性が得られるのは、特定の金属化合物(B)が亜鉛含有めっき上で強固に成膜することにより耐食性が高められるとともに、この特定の金属化合物(B)に銅化合物(A)をめっき表面に固定化させる働きがあることによるものと考えられる。
また、金属化合物(B)としては、成膜性の観点からジルコニウム化合物が特に好ましい。
【0017】
液状組成物に含まれる銅化合物(A)の銅イオンの含有量は、金属化合物(B)の含有量の10〜200mass%とすることが好ましい。銅化合物(A)の銅イオンの含有量が金属化合物(B)の含有量の10mass%未満では、銅と塗膜との相互作用が十分に得られなくなり、一方、200mass%を超えると、下地表面を銅が被覆する割合が増加して自然電位の低い下地表面の面積が減少することになるため、アノード電流が下地金属に集中して局部電池形成による腐食が進行しやすくなる。金属化合物(B)の含有量に対する銅化合物(A)の銅イオンのより好ましい含有量は10〜100mass%である。
【0018】
シーリング処理皮膜中でのジルコニウム又は/及びチタンの合計付着量は1〜100mg/m2とすることが好ましい。皮膜中でのジルコニウム又は/及びチタンの合計付着量が1mg/m2未満では、下地表面をそれらの金属が被覆する割合が少ないため、十分に優れた耐食性が得られなくなる。一方、合計付着量が100mg/m2を超えると固く脆い皮膜が形成され、加工性が低下するので好ましくない。ジルコニウム又は/及びチタンのより好ましい付着量は1〜50mg/m2である。
【0019】
本発明では、上記液状組成物中に、さらに下記の重縮合樹脂化合物(C)を配合することにより、特に優れた加工性が得られる。
この重縮合樹脂化合物(C)は、下記(1)式の化学構造を有するビスフェノールAと、下記(2)式の化学構造を有するアミン類と、ホルムアルデヒドとの重縮合樹脂化合物であり、数平均分子量が400〜10000、1ベンゼン環当たりのアミノ基の置換数の平均値が0.2〜1.0である重縮合樹脂化合物である。
【化5】
【化6】
但し、式中、R1、及びR2は、それぞれ互いに独立に水素原子、炭素数1〜10のアルキル基又は炭素数1〜10のヒドロキシアルキル基を表す。
【0020】
本発明において、このような特定の重縮合樹脂化合物(C)を含む組成物で皮膜を形成することにより優れた加工性が得られるのは、鋼板が加工された際に重縮合樹脂化合物(C)が伸びや歪みに追従して伸縮するためであると考えられる。
ここで、重縮合樹脂化合物(C)の1ベンゼン環当たりのアミノ基の置換数の平均値が0.2未満であると、得られる重合体の基体表面への密着性が不十分となるため、十分な塗料密着性が得られない。また、その平均値が1.0を超えると、得られる重合体の親水性が大きくなり、耐食性が不十分となる。
上記(2)式中のR1、R2は、それぞれ互いに独立に水素原子、炭素数が1〜10のアルキル基又は炭素数が1〜10のヒドロキシアルキル基を表すが、これらの炭素数が11以上になると液状組成物の成膜性が低下するため、耐食性、塗料密着性、加工性などが不十分になる。
【0021】
液状組成物中での重縮合樹脂化合物(C)の固形分の含有量は、銅化合物(A)と金属化合物(B)の合計含有量の10〜300mass%とすることが好ましい。重縮合樹脂化合物(C)の固形分の含有量が、銅化合物(A)と金属化合物(B)の合計含有量の10mass%未満では、リン酸亜鉛処理鋼板が深絞りしごき加工などのような高加工を受けた場合、皮膜が高加工に対して十分に追従できず、塗料密着性の向上効果が十分でなくなるので好ましくない。一方、重縮合樹脂化合物(C)の固形分の含有量が、銅化合物(A)と金属化合物(B)の合計含有量の300mass%を超えると、塗料密着性に効果がある銅化合物(A)が重縮合樹脂化合物(C)に封印されてしまい、上塗り樹脂に対するキレート効果が発現しなくなるため塗料密着性が低下し、また、同じく金属化合物(B)も封印されることになるため耐食性も低下する。銅化合物(A)と金属化合物(B)の合計含有量に対する重縮合樹脂化合物(C)の固形分のより好ましい含有量は10〜100mass%である。
【0022】
液状組成物には、以上述べた成分以外に各種添加剤を添加することができ、例えば、濡れ性向上剤として、エタノール、2−プロパノール、セロソルブなどの水溶性溶剤や市販の界面活性剤を添加してもよいし、また、液状組成物のpHを調整する目的でアンモニアや硝酸、フッ酸などの無機酸を添加してもよい。
液状組成物を塗布する方法としては、ロールコータ−法、スプレー法、浸漬法などの任意の方法を使用することができる。
液状組成物を塗布した後の乾燥温度は50〜200℃とすることが好ましい。乾燥温度が50℃未満では皮膜の乾燥が不十分であるため水分が残留し、塗料密着性が不十分となる。一方、200℃を超えるとシーリング処理皮膜にクラックが生じるため塗料密着性が劣り、且つ製造上のコスト面で不利となる。このような観点から、より好ましい乾燥温度は60〜120℃である。
【0023】
【実施例】
[実施例1]
試験材を以下の条件で作製した。
(1)供試材(素材めっき鋼板)
以下に示す市販の亜鉛系めっき鋼板を使用した。なお、各供試材のサイズは200mm×300mmとした。
・電気亜鉛めっき鋼板(EG)
板厚0.8mm,めっき目付量=20/20(g/m2)
・溶融亜鉛めっき鋼板(GI)
板厚0.8mm,めっき目付量=60/60(g/m2)
【0024】
(2)リン酸亜鉛処理
上記供試材を表面調整剤で表面調整した後、リン酸亜鉛処理を行った。上記表面調整では、通常使用されるチタンコロイド系表面調整剤「プレパレンZ」(商品名、日本パーカライジング(株)製)を1g/Lに希釈した処理液(室温)中に供試材を10秒間浸漬した。また、上記リン酸亜鉛処理では、リン酸亜鉛処理液「パルボンド3312」(商品名、日本パーカライジング(株)製)を55g/Lに希釈した処理液(50℃)中に供試材を5〜8秒間浸漬した。
(3)シーリング処理
上記リン酸亜鉛処理を施した供試材に表1に示す液状組成物をロールコーターで塗布し、水洗することなく乾燥させ、シーリング処理皮膜を形成させた。
【0025】
このようにして得られた試験材について、以下に示す試験方法で耐食性、塗料密着性及び擦り傷部耐食性を評価した。
(a)耐食性
試験材から70mm×150mmのサンプルを切り出し、裏面及び端面部をテープシールした後、JIS−Z−2371による塩水噴霧試験を72時間行い、白錆発生状況を観察し、下記基準により評価した。
◎:白錆の発生なし
○:白錆発生面積が全面積の5%未満
△:白錆発生面積が全面積の5%以上、20%未満
×:白錆発生面積が全面積の20%以上
【0026】
(b)塗料密着性
試験材にメラミンアルキッド系塗料(商品名「グリミン」、新東塗料社製)を乾燥膜厚が25μmになるように塗布し、120℃で30分間焼き付けた後、80℃の温水中に2時間浸漬し、その後、4時間放置した。この試験片の塗膜面に1mm間隔の碁盤目を100個刻み、この部分に粘着テープを貼着した後剥離させ、剥離しなかった碁盤目の塗膜の個数に基づき、下記基準で評価した。
◎:塗膜の剥離なし(塗膜残個数100%)
○:塗膜残存個数90%以上
△:塗膜残存個数50%以上、90%未満
×:塗膜残存個数50%未満
【0027】
(c)擦り傷部耐食性
試験材にメラミンアルキッド系塗料(商品名「グリミン」、新東塗料社製)を乾燥膜厚が25μmになるように塗布し、120℃で30分間焼き付けた後、塗膜面に対角線状にカッターナイフでカットを入れ、JIS−Z−2371による塩水噴霧試験を120時間行った。この試験片のカット部に粘着テープを貼着した後剥離させ、カット部からの片側最大剥離幅を測定した。評価基準は以下の通りである。
◎:剥離幅2mm未満
○:剥離幅2mm以上、4mm未満
△:剥離幅4mm以上、8mm未満
×:剥離幅8mm以上
【0028】
表2に各試験材の皮膜構成とシーリング処理条件を、表3に上記試験結果を示す。これによれば、参考例1〜8は平面部耐食性、擦り傷部耐食性及び塗料密着性が何れも良好であり、比較例4のクロメートシーリング処理材とほぼ同等の性能が得られている。これに対し、比較例1〜3では、平面部耐食性、擦り傷部耐食性及び塗料密着性のすべてを満足するものは得られていない。
【0029】
【表1】
【0030】
【表2】
【0031】
【表3】
【0032】
[実施例2]
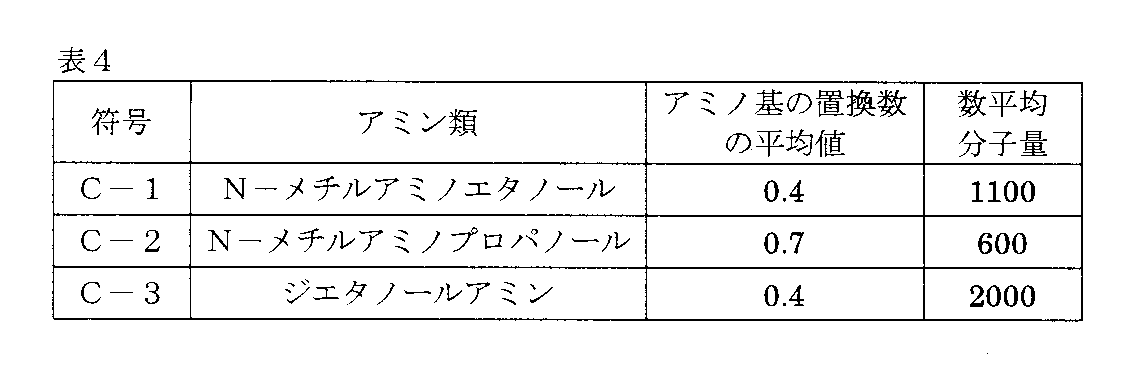
使用した供試材、表面調整及びリン酸亜鉛処理条件は実施例1と同様とした。液状組成物に配合する表4に記載の重縮合性樹脂化合物を、下記の製造法によって合成した。
・重縮合性樹脂化合物C−1
攪拌装置、還流冷却機及び温度計を取り付けた1000mlの三口セパラブルフラスコにビスフェノールAを1モル(228g)、触媒としてp−トルエンスルホン酸0.3gを仕込み、内部温度を100℃まで上げ、37%ホルムアルデヒド水溶液0.85モル(69g)を1時間かけて添加し、100℃で2時間還流反応を行った。一旦、反応容器を水冷放置し、上層に分離する水層の濁りがなくなってから、デカンテーションして水層を除去し、さらに170〜175℃になるまで加熱攪拌して、未反応分、水分を除去した(Step1)。
次いで、100℃以下まで温度を下げ、ブチルセロソルブ234gを添加して重縮合物を完全に溶解させた後、純水234gを加え系内の温度が50℃まで下がったところで、N−メチルアミノエタノール1モル(75g)を添加し、これに37%ホルムアルデヒド水溶液1モル(81.1g)を50℃で約1時間かけて滴下した。さらに80℃まで温度を上げ約3時間攪拌しながら反応を続け(Step2)、重縮合性樹脂化合物C−1を得た。
【0033】
・重縮合性樹脂化合物C−2
上記Step1で用いるホルムアルデヒドが0.7モル(56.8g)、上記Step2で用いるホルムアルデヒドが1.4モル(113.5g)、用いるアミン化合物がN−メチルアミノプロパノール1.4モル(124.6g)であること以外は、上記C−1の製造法と同様の条件で重縮合性樹脂化合物C−2を製造した。
・重縮合性樹脂化合物C−3
上記Step1で用いるホルムアルデヒドが1モル(81.1g)、同じくStep1での還流反応時間が4時間、上記Step2で用いるアミン化合物がジエタノールアミンであること以外は、上記C−1の製造法と同様の条件で重縮合性樹脂化合物C−3を製造した。
【0034】
以上のようにして得られた重縮合性樹脂化合物を用いて表5に示す液状組成物を調整した。
リン酸亜鉛処理を施した供試材に、表5に記載の液状組成物をロールコーターで塗布し、水洗することなく乾燥させ、シーリング処理皮膜を形成させた。
このようにして得られた試験材について、実施例1と同様の試験方法で耐食性、塗料密着性及び擦り傷部耐食性を評価するとともに、下記の試験方法で加工性を評価した。
【0035】
(d)加工性
試験材にメラミンアルキッド系塗料(商品名「グリミン」、新東塗料社製)を乾燥膜厚が25μmになるように塗布し、120℃で30分間焼き付けた後、塗膜面に1mm間隔の碁盤目を100個刻んだ。この試験片に対してエリクセン試験機を用いて5mm張り出し加工を施した後、前記碁盤目を刻んだ部分に粘着テープを貼着した後剥離させ、剥離しなかった碁盤目の塗膜の個数に基づき、下記基準で評価した。
◎:塗膜の剥離なし(塗膜残個数100%)
○:塗膜残存個数90%以上
△:塗膜残存個数50%以上、90%未満
×:塗膜残存個数50%未満
【0036】
表6に各試験材の皮膜構成とシーリング処理条件を、表7に上記試験結果を示す。これによれば発明例1〜8は平面部耐食性、擦り傷部耐食性、塗料密着性及び加工性が何れも良好であり、比較例4のクロメートシーリング処理材とほぼ同等の性能が得られている。これに対し、本発明条件外の表面処理剤を用いた比較例1〜3では、平面部耐食性、擦り傷部耐食性、塗料密着性及び加工性のすべてを満足するものは得られていない。
【0037】
【表4】
【0038】
【表5】
【0039】
【表6】
【0040】
【表7】
【0041】
【発明の効果】
以上述べたように、本発明のリン酸亜鉛処理鋼板は、クロメートシーリング処理材に匹敵する優れた耐食性と塗料密着性を有するとともに、特に優れた加工性を有する。 [0001]
[Technical field to which the invention belongs]
The present invention relates to a non-chromium-based zinc phosphate-treated steel sheet suitable as a material for home appliances, switchboards, telephone switchboard panels, automobile parts, building materials, and the like.
[0002]
[Prior art]
In general, zinc phosphate-treated steel sheets are chromate-sealed after zinc phosphate treatment for the purpose of ensuring temporary rust prevention and ensuring sufficient corrosion resistance even when used as a coating base. Since this chromate sealing treatment uses hexavalent chromium, which is an environmentally regulated substance, development of a non-chromium sealing treatment technique that does not use hexavalent chromium is desired.
[0003]
As the surface treated steel sheet was subjected to a treatment of chromium-free after treated with zinc phosphate, zinc-plated steel sheet surface to have a zinc phosphate coating, an organic resin and a thiocarbonyl group-containing compound or vanadate compound thereon 1-20 mg / m 2 of Ni is deposited on the surface of a non-chromium-treated zinc-plated steel sheet (Patent Document 1), a zinc-plated steel sheet, etc., having a coating containing a coating amount of 0.2-3 g. / M 2 zinc phosphate-treated film, and a non-chromic coated metal sheet on which an undercoating film and an overcoating film containing 10 to 60 mass% of a non-chromium anticorrosive pigment are further formed (Patent Document 2) ) Etc. are known.
[0004]
[Patent Document 1]
JP 2000-248367 A [Patent Document 2]
Japanese Patent Application Laid-Open No. 2001-081578
[Problems to be solved by the invention]
However, these conventional surface-treated steel sheets have drawbacks such as insufficient corrosion resistance and high manufacturing costs.
An object of the present invention, therefore, without containing hexavalent chromium in a sealing treatment coating, as well as have a good corrosion resistance and paint adhesion comparable to conventional chromate sealing treatment material has excellent processability, moreover The object is to provide a non-chromium zinc phosphate-treated steel sheet that can be manufactured at low cost .
[0006]
[Means for Solving the Problems]
In order to achieve performance equivalent to that of a zinc phosphate-treated steel sheet to which a chromate sealing treatment material has been applied, the present inventors have conducted intensive studies on the composition of the liquid composition in order to achieve a sealing treatment with a liquid composition containing no chromium. Went. As a result, by performing sealing treatment with an aqueous liquid composition containing a copper compound and a specific metal compound, excellent performance (corrosion resistance and paint adhesion) comparable to the chromate sealing material can be obtained in addition to the above performance. In addition, it has been found that by adding a specific resin compound to the liquid composition, excellent processability can be obtained in addition to the above performance.
[0007]
The present invention has been made based on such findings, and the features thereof are as follows .
[0008]
[ 1 ] The surface of the zinc-containing plated steel sheet has a zinc phosphate-treated film having an adhesion amount (Zn 3 (PO 4 ) 2 equivalent) of 0.1 to 5 g / m 2 , and a copper compound ( A), at least one metal compound (B) selected from titanium compounds and zirconium compounds, bisphenol A having the chemical structure of the following formula (1), and amines having the chemical structure of the following formula (2) Polycondensation resin compound (C) having a number average molecular weight of 400 to 10,000 and an average number of amino group substitutions per benzene ring of 0.2 to 1.0 And a non-chromium zinc phosphate-treated steel sheet excellent in corrosion resistance , paint adhesion and workability , comprising a sealing treatment film obtained by applying a liquid composition containing water and drying.
[Chemical 3]
[Formula 4]
However, in formula, R1 and R2 respectively independently represent a hydrogen atom, a C1-C10 alkyl group, or a C1-C10 hydroxyalkyl group.
[0009]
[ 2 ] In the non-chromium zinc phosphate treated steel sheet of [ 1 ] , the solid content of the polycondensation resin compound (C) contained in the liquid composition is such that the copper compound (A) and the metal compound (B) A non-chromium zinc phosphate-treated steel sheet excellent in corrosion resistance , paint adhesion and workability , characterized by being 10 to 300 mass% of the total content of.
[ 3 ] In the non-chromium zinc phosphate-treated steel sheet according to [1] or [ 2 ], the copper compound (A) contained in the liquid composition is a divalent copper compound, and the anion component is nitric acid, A non-chromium zinc phosphate-treated steel sheet excellent in corrosion resistance , paint adhesion and workability , characterized in that it is at least one inorganic acid selected from sulfuric acid, carbonic acid and pyrophosphoric acid.
[ 4 ] In the non-chromium zinc phosphate-treated steel sheet according to any one of [1] to [ 3 ] , the copper ion content of the copper compound (A) contained in the liquid composition is that of the metal compound (B). A non-chromium zinc phosphate-treated steel sheet excellent in corrosion resistance , paint adhesion and workability , characterized by being 10 to 200 mass% of the content.
[0010]
[5] In any one of the chromium-free zinc phosphate-treated steel sheet of [1] to [4], wherein the metal compound contained in the liquid composition (B) is a zirconium compound, corrosion resistance, paint Non-chromium zinc phosphate-treated steel sheet with excellent adhesion and workability .
[ 6 ] In the non-chromium zinc phosphate-treated steel sheet according to any one of [1] to [ 5 ] , the total adhesion amount of titanium and / or zirconium in the sealing film is 1 to 100 mg / m 2. A non-chromium zinc phosphate treated steel sheet with excellent corrosion resistance , paint adhesion and processability .
[0011]
DETAILED DESCRIPTION OF THE INVENTION
The zinc phosphate-treated steel sheet of the present invention uses a zinc-containing plated steel sheet as a raw steel sheet, and a zinc phosphate-treated film and a sealing composition film with a specific composition are sequentially formed on the surface of the plated film from the lower layer side.
There is no special restriction | limiting in the zinc containing plated steel plate used as the base steel plate of the zinc phosphate treatment steel plate of this invention, Any of a pure zinc plating steel plate and a zinc alloy plating steel plate may be sufficient. For example, galvanized steel sheet, Zn-Ni plated steel sheet, Zn-Fe plated steel sheet, (electroplating, alloyed hot dip galvanizing), Zn-Cr plated steel sheet, Zn-Mn plated steel sheet, Zn-Co plated steel sheet, Zn-Co -Cr alloy plated steel sheet, Zn-Cr-Ni plated steel sheet, Zn-Cr-Fe plated steel sheet, Zn-A1 plated steel sheet (for example, Zn-5% A1 alloy plated steel sheet, Zn-55% A1 alloy plated steel sheet), and In these platings, a zinc-based composite plated steel sheet (for example, a Zn—SiO 2 dispersion plated steel sheet) in which a metal oxide, a polymer, or the like is dispersed can be used. In addition, among the above-described plating, a multi-layer plated steel sheet in which two or more layers of the same type or different types are plated can also be used. Moreover, as a plated steel plate, the steel plate surface may be plated in advance with a thin plate such as Ni, and the above-described various types of plating may be performed thereon. As the plating method, any feasible method among an electrolytic method, a melting method, and a vapor phase method can be employed. Furthermore, for the purpose of preventing blackening of the plating, about 1 to 2000 ppm of one or more of Ni, Co and Fe is deposited in the plating film, or an alkali containing one or more of Ni, Co and Fe on the plating surface. Or you may make it surface-treat with an acidic aqueous solution and precipitate those elements. Moreover, there is no restriction | limiting in particular also about the amount of plating adhesion.
[0012]
The adhesion amount (Zn 3 (PO 4 ) 2 equivalent amount) of the zinc phosphate-treated film is 0.1 to 5 g / m 2 . When the adhesion amount is less than 0.1 g / m 2 , the rust prevention and coating adhesion inherent in the zinc phosphate-treated film are not exhibited. On the other hand, if the adhesion amount exceeds 5 g / m 2 , the zinc phosphate-treated film completely covers the plating surface, which is not preferable because this film is cracked during processing and the performance after processing decreases. Further, from the above viewpoint, the more preferable adhesion amount of the zinc phosphate-treated film is 0.5 to 2 g / m 2 .
There is no particular limitation on the treatment method for forming the zinc phosphate-treated film, and a known treatment method for forming a normal crystalline zinc phosphate film may be used.
[0013]
The sealing treatment film formed on the upper layer of the zinc phosphate treatment film is a film not containing hexavalent chromium, and this film is formed by applying a specific aqueous liquid composition not containing hexavalent chromium and drying. Usually, after apply | coating a liquid composition, it dries without washing with water, However, You may dry after washing with water.
A liquid composition for forming a sealing treatment film on a zinc phosphate treatment film comprises a copper compound (A), at least one metal compound (B) selected from a titanium compound and a zirconium compound, water, And can be prepared by adding each of the above compounds (A), (B) and the like to water.
[0014]
Although there is no special restriction | limiting in the kind of the said copper compound (A), For example, organic acid salts, such as oxygen acid salts, such as copper sulfate, copper nitrate, copper carbonate, copper pyrophosphate, etc. are mentioned, These 1 type or Two or more kinds can be used. Of these, copper compounds in which counterions remain upon drying are not so preferred because the counterions themselves may adversely affect paint adhesion. Therefore, from this point of view, such as copper nitrate and copper carbonate are not preferred. It is preferable to use a copper compound that sublimes a counter ion. In the present invention, excellent corrosion resistance and paint adhesion can be obtained by forming a film with a composition containing a copper compound (A). The organic polymer donor atom is estimated to have a copper compound. This is considered to be due to the action of forming a chelate by coordination with a functional group containing
[0015]
Among the metal compounds (B), examples of titanium compounds include inorganic salts such as titanium sulfate, titanium oxide, potassium titanium oxalate, potassium titanium fluoride, titanium hydrofluoric acid, and titanium tetrachloride, and organic titanates. 1 type or 2 types or more can be used. Examples of zirconium compounds include zirconium oxide, zirconium nitrate oxide, zirconium oxychloride, zirconium silicate, zirconium nitrate oxide, potassium zirconium fluoride, zirconium hydrofluoric acid, ammonium zirconium carbonate, and organic zirconate. 1 type (s) or 2 or more types can be used. Further, titanium oxide, zirconium oxide, and the like may be a polymerized colloidal material.
[0016]
In the present invention, excellent corrosion resistance and coating film adhesion can be obtained by forming a film with a composition containing the specific metal compound (B) together with the copper compound (A). The specific metal compound (B) It is considered that this is due to the fact that the specific metal compound (B) has a function of immobilizing the copper compound (A) on the plating surface while the film is firmly formed on the zinc-containing plating.
In addition, as the metal compound (B), a zirconium compound is particularly preferable from the viewpoint of film formability.
[0017]
The copper ion content of the copper compound (A) contained in the liquid composition is preferably 10 to 200 mass% of the content of the metal compound (B). When the copper ion content of the copper compound (A) is less than 10 mass% of the content of the metal compound (B), sufficient interaction between copper and the coating film cannot be obtained, while when it exceeds 200 mass%, Since the ratio of covering the surface with copper increases and the area of the base surface having a low natural potential decreases, the anode current concentrates on the base metal, and corrosion due to local cell formation is likely to proceed. The more preferable content of the copper ion of the copper compound (A) with respect to the content of the metal compound (B) is 10 to 100 mass%.
[0018]
The total adhesion amount of zirconium and / or titanium in the sealing treatment film is preferably 1 to 100 mg / m 2 . When the total adhesion amount of zirconium and / or titanium in the film is less than 1 mg / m 2 , the metal surface is covered with a small proportion of these metals, and thus sufficiently excellent corrosion resistance cannot be obtained. On the other hand, if the total adhesion amount exceeds 100 mg / m 2 , a hard and brittle film is formed and workability is lowered, which is not preferable. A more preferable adhesion amount of zirconium and / or titanium is 1 to 50 mg / m 2 .
[0019]
In the present invention, particularly excellent processability can be obtained by further blending the following polycondensation resin compound (C) into the liquid composition.
This polycondensation resin compound (C) is a polycondensation resin compound of bisphenol A having the chemical structure of the following formula (1), amines having the chemical structure of the following formula (2), and formaldehyde, and the number average It is a polycondensation resin compound having a molecular weight of 400 to 10,000 and an average value of the number of amino group substitutions per benzene ring of 0.2 to 1.0.
[Chemical formula 5]
[Chemical 6]
However, in formula, R1 and R2 respectively independently represent a hydrogen atom, a C1-C10 alkyl group, or a C1-C10 hydroxyalkyl group.
[0020]
In the present invention, excellent workability can be obtained by forming a film with a composition containing such a specific polycondensation resin compound (C) when the steel sheet is processed. This is considered to be due to expansion and contraction following the elongation and distortion.
Here, if the average value of the number of amino group substitutions per benzene ring of the polycondensation resin compound (C) is less than 0.2, the resulting polymer has insufficient adhesion to the substrate surface. Insufficient paint adhesion is obtained. Moreover, when the average value exceeds 1.0, the hydrophilic property of the polymer obtained will become large and corrosion resistance will become inadequate.
R1 and R2 in the formula (2) each independently represent a hydrogen atom, an alkyl group having 1 to 10 carbon atoms, or a hydroxyalkyl group having 1 to 10 carbon atoms, and these carbon numbers are 11 or more. When it becomes, since the film formability of a liquid composition will fall, corrosion resistance, paint adhesion, workability, etc. will become inadequate.
[0021]
The solid content of the polycondensation resin compound (C) in the liquid composition is preferably 10 to 300 mass% of the total content of the copper compound (A) and the metal compound (B). When the solid content of the polycondensation resin compound (C) is less than 10 mass% of the total content of the copper compound (A) and the metal compound (B), the zinc phosphate-treated steel sheet is deep drawn and ironed. When subjected to high processing, the coating cannot sufficiently follow high processing, and the effect of improving paint adhesion is not sufficient, which is not preferable. On the other hand, if the solid content of the polycondensation resin compound (C) exceeds 300 mass% of the total content of the copper compound (A) and the metal compound (B), the copper compound (A ) Is sealed in the polycondensation resin compound (C), and the chelate effect on the topcoat resin is not expressed, so that the adhesion of the paint is lowered, and the metal compound (B) is also sealed, so that the corrosion resistance is also good. descend. The more preferable content of the solid content of the polycondensation resin compound (C) with respect to the total content of the copper compound (A) and the metal compound (B) is 10 to 100 mass%.
[0022]
In addition to the components described above, various additives can be added to the liquid composition. For example, water-soluble solvents such as ethanol, 2-propanol and cellosolve and commercially available surfactants are added as wettability improvers. Alternatively, an inorganic acid such as ammonia, nitric acid, or hydrofluoric acid may be added for the purpose of adjusting the pH of the liquid composition.
As a method for applying the liquid composition, any method such as a roll coater method, a spray method, and an immersion method can be used.
The drying temperature after applying the liquid composition is preferably 50 to 200 ° C. If the drying temperature is less than 50 ° C., the film is not sufficiently dried, so that moisture remains and the paint adhesion is insufficient. On the other hand, when the temperature exceeds 200 ° C., cracks occur in the sealing film, resulting in poor paint adhesion and disadvantages in manufacturing costs. From such a viewpoint, a more preferable drying temperature is 60 to 120 ° C.
[0023]
【Example】
[Example 1]
Test materials were produced under the following conditions.
(1) Test material (material plated steel sheet)
Commercially available galvanized steel sheets shown below were used. The size of each test material was 200 mm × 300 mm.
・ Electrogalvanized steel sheet (EG)
Plate thickness 0.8mm, plating basis weight = 20/20 (g / m 2 )
・ Hot galvanized steel sheet (GI)
Plate thickness 0.8mm, plating basis weight = 60/60 (g / m 2 )
[0024]
(2) Zinc Phosphate Treatment After the surface of the above test material was surface-adjusted with a surface conditioner, zinc phosphate treatment was performed. In the above surface adjustment, the test material is placed in a treatment liquid (room temperature) diluted with 1 g / L of a commonly used titanium colloidal surface conditioner “preparene Z” (trade name, manufactured by Nippon Parkerizing Co., Ltd.) for 10 seconds. Soaked. In addition, in the above zinc phosphate treatment, the test material was placed in a treatment solution (50 ° C.) in which a zinc phosphate treatment solution “Palbond 3312” (trade name, manufactured by Nippon Parkerizing Co., Ltd.) was diluted to 55 g / L. Soaked for 8 seconds.
(3) Sealing treatment The liquid composition shown in Table 1 was applied to the specimen subjected to the zinc phosphate treatment with a roll coater and dried without washing with water to form a sealing treatment film.
[0025]
The test materials thus obtained were evaluated for corrosion resistance, paint adhesion and scratched portion corrosion resistance by the following test methods.
(a) After cutting out a 70 mm × 150 mm sample from the corrosion resistance test material and tape-sealing the back and end surfaces, a salt spray test according to JIS-Z-2371 was conducted for 72 hours, and the occurrence of white rust was observed. evaluated.
◎: No white rust occurrence ○: White rust occurrence area is less than 5% of the total area △: White rust occurrence area is less than 5% of the total area, less than 20% ×: White rust occurrence area is more than 20% of the total area [0026]
(b) A melamine alkyd paint (trade name “Grimin”, manufactured by Shinto Paint Co., Ltd.) was applied to the paint adhesion test material so that the dry film thickness was 25 μm, baked at 120 ° C. for 30 minutes, and then 80 ° C. In hot water for 2 hours and then left for 4 hours. The grid surface of this test piece was cut into 100 squares with a 1 mm interval, and the adhesive tape was attached to this part and then peeled off. Based on the number of cross-cut paint films that were not peeled, the following criteria were evaluated. .
A: No peeling of coating film (100% remaining coating film number)
○: Remaining coating film number 90% or more Δ: Remaining coating film number 50% or more, less than 90% ×: Remaining coating film number less than 50%
(c) A melamine alkyd paint (trade name “Grimin”, manufactured by Shinto Paint Co., Ltd.) was applied to the scratch corrosion resistance test material so that the dry film thickness was 25 μm, and baked at 120 ° C. for 30 minutes, and then the coating film The surface was cut diagonally with a cutter knife, and a salt spray test according to JIS-Z-2371 was performed for 120 hours. After sticking an adhesive tape to the cut part of this test piece, it was made to peel, and the one-side maximum peeling width from a cut part was measured. The evaluation criteria are as follows.
A: Peel width less than 2 mm B: Peel width 2 mm or more, less than 4 mm Δ: Peel width 4 mm or more, less than 8 mm X: Peel width 8 mm or more
Table 2 shows the coating composition and sealing treatment conditions of each test material, and Table 3 shows the test results. According to this, each of Reference Examples 1 to 8 has good flat part corrosion resistance, scratch part corrosion resistance, and paint adhesion, and almost the same performance as the chromate sealing material of Comparative Example 4 is obtained. In contrast, in the specific Comparative Examples 1 to 3, it has not been obtained which satisfies all of the planar portion corrosion resistance, abrasion portion corrosion resistance and paint adhesion.
[0029]
[Table 1]
[0030]
[Table 2]
[0031]
[Table 3]
[0032]
[Example 2]
The used test material, surface adjustment and zinc phosphate treatment conditions were the same as in Example 1. The polycondensable resin compounds shown in Table 4 to be blended in the liquid composition were synthesized by the following production method.
・ Polycondensable resin compound C-1
A 1000 ml three-necked separable flask equipped with a stirrer, a reflux condenser and a thermometer was charged with 1 mol (228 g) of bisphenol A and 0.3 g of p-toluenesulfonic acid as a catalyst, and the internal temperature was raised to 100 ° C. A 0.85 mol% formaldehyde aqueous solution (69 g) was added over 1 hour, and a reflux reaction was performed at 100 ° C. for 2 hours. Once the reaction vessel is left in the water-cooled state, the turbidity of the aqueous layer separated into the upper layer disappears, and then the aqueous layer is removed by decantation, and further heated and stirred until the temperature reaches 170 to 175 ° C. Was removed (Step 1).
Next, the temperature was lowered to 100 ° C. or lower, and 234 g of butyl cellosolve was added to completely dissolve the polycondensate. Then, 234 g of pure water was added, and when the temperature in the system was lowered to 50 ° C., N-methylaminoethanol 1 Mole (75 g) was added, and 1 mol (81.1 g) of a 37% aqueous formaldehyde solution was added dropwise thereto at 50 ° C. over about 1 hour. Furthermore, the temperature was raised to 80 ° C. and the reaction was continued with stirring for about 3 hours (Step 2) to obtain a polycondensable resin compound C-1.
[0033]
・ Polycondensable resin compound C-2
The formaldehyde used in Step 1 is 0.7 mol (56.8 g), the formaldehyde used in Step 2 is 1.4 mol (113.5 g), and the amine compound used is N-methylaminopropanol 1.4 mol (124.6 g). A polycondensable resin compound C-2 was produced under the same conditions as in the production method of C-1 except that the above.
・ Polycondensable resin compound C-3
The same conditions as in the production method of C-1, except that 1 mol of formaldehyde used in Step 1 is 8 mol, the reflux reaction time in Step 1 is 4 hours, and the amine compound used in Step 2 is diethanolamine. The polycondensable resin compound C-3 was produced.
[0034]
A liquid composition shown in Table 5 was prepared using the polycondensable resin compound obtained as described above.
The liquid composition described in Table 5 was applied to a test material subjected to zinc phosphate treatment with a roll coater and dried without washing with water to form a sealing treatment film.
The test material thus obtained was evaluated for corrosion resistance, paint adhesion and scratched portion corrosion resistance by the same test method as in Example 1, and processability was evaluated by the following test method.
[0035]
(d) A melamine alkyd paint (trade name “Grimin”, manufactured by Shinto Paint Co., Ltd.) was applied to the workability test material so that the dry film thickness was 25 μm and baked at 120 ° C. for 30 minutes, and then the coating surface 100 square grids with 1 mm intervals were cut into each. The test piece was subjected to an extension process of 5 mm using an Erichsen testing machine, and then the adhesive tape was applied to the part of the grid that had been cut off, and then peeled off. Based on the following criteria.
A: No peeling of coating film (100% remaining coating film number)
○: Remaining coating film number 90% or more Δ: Remaining coating film number 50% or more, less than 90% ×: Remaining coating film number less than 50%
Table 6 shows the coating composition and sealing treatment conditions of each test material, and Table 7 shows the test results. According to this, the inventive examples 1 to 8 are all excellent in the corrosion resistance of the plane portion, the scratch portion corrosion resistance, the paint adhesion and the workability, and almost the same performance as the chromate sealing material of Comparative Example 4 is obtained. On the other hand, in Comparative Examples 1 to 3 using the surface treating agent outside the conditions of the present invention, those satisfying all of the flat surface corrosion resistance, the scratched portion corrosion resistance, the paint adhesion and the workability have not been obtained.
[0037]
[Table 4]
[0038]
[Table 5]
[0039]
[Table 6]
[0040]
[Table 7]
[0041]
【The invention's effect】
As described above, the zinc phosphate-treated steel sheet of the present invention has excellent corrosion resistance and paint adhesion comparable to chromate sealing materials, and particularly excellent workability.
Claims (6)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002306343A JP3903900B2 (en) | 2002-10-21 | 2002-10-21 | Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002306343A JP3903900B2 (en) | 2002-10-21 | 2002-10-21 | Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004143475A JP2004143475A (en) | 2004-05-20 |
| JP3903900B2 true JP3903900B2 (en) | 2007-04-11 |
Family
ID=32453149
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002306343A Expired - Fee Related JP3903900B2 (en) | 2002-10-21 | 2002-10-21 | Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP3903900B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100908162B1 (en) * | 2004-08-20 | 2009-07-16 | 제이에프이 스틸 가부시키가이샤 | Phosphated Galvanized Steel Sheet |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5085741B2 (en) | 2008-10-08 | 2012-11-28 | 新日本製鐵株式会社 | Metal material with excellent corrosion resistance |
| JP6515389B2 (en) * | 2015-10-09 | 2019-05-22 | 日本製鉄株式会社 | Sliding member and method of manufacturing the same |
| CN109111587B (en) * | 2018-08-20 | 2023-05-26 | 浙江汇锋智造科技有限公司 | Preparation method and device of polymer membrane material for flame-retardant and corrosion-resistant environmental engineering |
-
2002
- 2002-10-21 JP JP2002306343A patent/JP3903900B2/en not_active Expired - Fee Related
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100908162B1 (en) * | 2004-08-20 | 2009-07-16 | 제이에프이 스틸 가부시키가이샤 | Phosphated Galvanized Steel Sheet |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2004143475A (en) | 2004-05-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3883831B2 (en) | Surface-treated steel sheet with excellent white rust resistance and method for producing the same | |
| JP3898302B2 (en) | Surface treatment agent composition for metal material and treatment method | |
| JP5274560B2 (en) | Chemical treatment solution and treatment method for coating base of steel material | |
| TWI550099B (en) | Galvanized steel sheet containing aluminum and its manufacturing method | |
| KR100999328B1 (en) | Surface-treated steel sheet | |
| WO2010001861A1 (en) | Chemical conversion liquid for metal structure and surface treating method | |
| GB2091591A (en) | Surface treated steel sheets for paint coating | |
| JP3967519B2 (en) | Zn-Mg electroplated metal plate and method for producing the same | |
| JP3851482B2 (en) | Galvanized steel sheet with excellent white rust resistance and coating adhesion | |
| JP4509425B2 (en) | Paint surface treatment agent, surface treatment method, metal material, processing method, and metal product | |
| JP3967796B2 (en) | Surface-treated metal material | |
| JP3911160B2 (en) | Phosphate-treated galvanized steel sheet with excellent corrosion resistance and paintability | |
| JP3903900B2 (en) | Non-chromium zinc phosphate treated steel plate with excellent corrosion resistance, paint adhesion and workability | |
| JP3923419B2 (en) | Non-chromium treatment of non-chromium steel sheet | |
| JP4207536B2 (en) | Surface treatment metal plate and surface treatment agent | |
| JPWO2002061175A1 (en) | Surface treatment agent for metal material and surface treatment method | |
| JP3962123B2 (en) | Organic surface treatment metal plate and organic metal surface treatment liquid | |
| JP4966480B2 (en) | Post-treatment method for zinc phosphate-based treatment material excellent in corrosion resistance and top coatability and post-treated zinc phosphate-based treatment material | |
| JP5000802B2 (en) | Inorganic film-forming coating agent, inorganic film-forming method, inorganic film-coated aluminum material and inorganic film-coated steel material obtained by using the same | |
| JP7169409B1 (en) | Hexavalent chromium-free aqueous surface treatment liquid, surface treated metal and surface treatment method | |
| JP3900070B2 (en) | Non-chromic treatment of galvanized steel sheet | |
| JP3892642B2 (en) | Surface-treated steel sheet and manufacturing method thereof | |
| EP4209616A1 (en) | Surface treated steel sheet for organic resin coating and manufacturing method thereof; organic resin coated steel sheet and manufacturing method thereof | |
| JPH09228067A (en) | Surface treated steel sheet excellent in resistance against environmental pollution and corrosion | |
| JP3149760B2 (en) | Manufacturing method of galvanized steel sheet with excellent white rust resistance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050909 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20060914 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20060926 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20061124 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20061219 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20070101 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 3903900 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110119 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120119 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130119 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130119 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140119 Year of fee payment: 7 |
|
| LAPS | Cancellation because of no payment of annual fees |