JP2016214395A - Medicine packaging film - Google Patents
Medicine packaging film Download PDFInfo
- Publication number
- JP2016214395A JP2016214395A JP2015100443A JP2015100443A JP2016214395A JP 2016214395 A JP2016214395 A JP 2016214395A JP 2015100443 A JP2015100443 A JP 2015100443A JP 2015100443 A JP2015100443 A JP 2015100443A JP 2016214395 A JP2016214395 A JP 2016214395A
- Authority
- JP
- Japan
- Prior art keywords
- density
- polyethylene
- fluidity
- film
- linear low
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Landscapes
- Wrappers (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Laminated Bodies (AREA)
Abstract
Description
本発明は、高温下でも保存可能な切り裂き容易な3層構造の医薬品包装用フィルムに関する。 The present invention relates to a film for packaging pharmaceutical products having a three-layer structure that can be stored easily even at high temperatures.
内容物を封入するための包装用袋は熱などでフィルムを溶かして密閉している。
包装用袋を開封する場合には刃物を用いて開けることもあるが、道具が無い状況下では手で破って開けることがある。
しかし手で開封した場合きれいにフィルムを破くことは難しく、また開封した時に粉状、粒状の内容物が飛び出すおそれがあった。
The packaging bag for enclosing the contents is sealed by melting the film with heat or the like.
When opening a packaging bag, it may be opened using a blade, but it may be opened by hand when there is no tool.
However, it is difficult to tear the film cleanly when opened by hand, and there is a possibility that powdered and granular contents may pop out when opened.
上記課題を解決するために、袋の素材に工夫を施し引き裂き易くしたフィルムが開発されている。 In order to solve the above-mentioned problems, a film has been developed in which the material of the bag is devised to be easily torn.
特許文献1には、縦方向及び横方向の引き裂き性に優れた易引き裂き性フィルムが記載されている。 Patent Document 1 describes an easily tearable film having excellent longitudinal and lateral tearability.
上記特許文献1記載の包装袋は、引き裂きやすさは向上したが材料のガラス転移点が低く、夏場高温となる倉庫などの通気性が悪い場所で保管すると劣化してしまうことがあった。 The packaging bag described in Patent Document 1 has improved ease of tearing, but has a low glass transition point of the material, and may be deteriorated when stored in a place with poor air permeability such as a warehouse that is hot in summer.
本発明は、上記したような従来技術の問題点を解決すべくなされたものであって、引き裂き易く保存し易いフィルムを提供する。 The present invention has been made to solve the problems of the prior art as described above, and provides a film that is easy to tear and easy to store.
請求項1に係る発明は、密度が0.910〜0.930g/cm3で、190℃での流動性が0.5〜0.930g/10minの直鎖状低密度ポリエチレンからなる外層と、
密度が0.910〜0.930g/cm3で、190℃での流動性が0.5〜0.930g/10minの直鎖状低密度ポリエチレンと密度1.020g/cm3でガラス転移温度が100℃以上である環状ポリエチレンからなり、該環状ポリエチレンを40%以上60%未満の割合で含む中間層と、密度が0.910〜0.930g/cm3の低密度ポリエチレンまたは密度が0.910〜0.930g/cm3で、190℃での流動性が0.5〜0.930g/10minの直鎖状低密度ポリエチレンからなる内層の三層から構成される医薬品包装用多層フィルムに関する。
The invention according to claim 1 has an outer layer made of linear low density polyethylene having a density of 0.910 to 0.930 g / cm 3 and a fluidity at 190 ° C. of 0.5 to 0.930 g / 10 min.
Density of 0.910~0.930g / cm 3, a glass transition temperature fluidity at 190 ° C. is a linear low density polyethylene and a density 1.020g / cm 3 of 0.5~0.930g / 10min An intermediate layer comprising a cyclic polyethylene having a temperature of 100 ° C. or higher and containing the cyclic polyethylene in a proportion of 40% or more and less than 60%, and a low density polyethylene having a density of 0.910 to 0.930 g / cm 3 or a density of 0.910. The present invention relates to a multilayer film for pharmaceutical packaging composed of three inner layers composed of linear low-density polyethylene having a flowability at 190 ° C of 0.5 to 0.930 g / 10 min at ˜0.930 g / cm 3 .
請求項1に係る発明によれば、シール強度とカット性のバランスが優れており、引き裂き容易でかつ高温下でも保存が可能な医薬品包装用フィルムを提供する。 According to the first aspect of the present invention, there is provided a pharmaceutical packaging film that has an excellent balance between seal strength and cutability, is easy to tear and can be stored even at high temperatures.
以下、本発明に係る包装袋の好適な実施形態について説明する。 Hereinafter, preferred embodiments of the packaging bag according to the present invention will be described.
本発明の包装袋は外層(ラミネート層)、中間層(コア層)及び内層(シール層)からなる構成をとる。
外層は直鎖状低密度ポリエチレン(以下C4−LLDという)、中間層はC4−LLDと環状ポリオレフィン(以下COCという)及び内層C4−LLDまたはポリエチレン(以下LDPEという)から構成される。
なおC4−LLDは枝を有しない直鎖構造であるが、LDPEは分岐を有している分枝構造である。
The packaging bag of this invention takes the structure which consists of an outer layer (laminate layer), an intermediate | middle layer (core layer), and an inner layer (seal layer).
The outer layer is composed of linear low density polyethylene (hereinafter referred to as C4-LLD), and the intermediate layer is composed of C4-LLD and cyclic polyolefin (hereinafter referred to as COC) and inner layer C4-LLD or polyethylene (hereinafter referred to as LDPE).
C4-LLD is a linear structure having no branches, whereas LDPE is a branched structure having branches.
引き裂き容易性からC4−LLDの密度は0.910〜0.930g/cm3が好ましく、より好ましくは0.915〜0.925g/cm3がよい。
C4−LLDの流動性は190℃で0.5〜2.0g/10min、好ましくは0.915〜0.925g/10minが良い。
190℃とは一般的に樹脂の融解粘度を計測する時に用いる温度である。
この範囲の流動性を有することでフィルム原料の混合時にむらができず、フィルム加工安定性および外観が良好となる。
In view of ease of tearing, the density of C4-LLD is preferably 0.910 to 0.930 g / cm 3 , more preferably 0.915 to 0.925 g / cm 3 .
The fluidity of C4-LLD is 0.5 to 2.0 g / 10 min at 190 ° C., preferably 0.915 to 0.925 g / 10 min.
190 ° C. is a temperature generally used when measuring the melt viscosity of a resin.
By having fluidity within this range, unevenness cannot be produced when the film raw materials are mixed, and film processing stability and appearance are improved.
COCの密度は引き裂き容易性や夏場の高温下で保存しても品質に影響が出ないことから密度を1.020g/cm3、ガラス転移温度を100度以上にすることが好ましい。 The density of COC is preferably set to 1.020 g / cm 3 and the glass transition temperature to 100 ° C. or higher because it does not affect the quality even if it is stored at high temperatures in summer.
LDPEはヒートシール性から密度0.910〜0.930g/cm3のものが好ましい。 LDPE having a density of 0.910 to 0.930 g / cm 3 is preferable from the viewpoint of heat sealability.
本件発明は内層に静防性を付してもよい。
静防性を付すことで、静電気の発生を防ぐことができ、粉末状、顆粒状の内容物が包装材にくっつき取り出しにくくなることを防ぐ。
In the present invention, the inner layer may be provided with antistatic properties.
By adding antistatic properties, it is possible to prevent the generation of static electricity and prevent powdered and granular contents from sticking to the packaging material and becoming difficult to take out.
本件発明はフィルムのみで包装資材として使用しても良いが、他のフィルムとラミネートを行って、シーラントフィルムとして使用してもよい。 The present invention may be used as a packaging material only with a film, but may be used as a sealant film after being laminated with another film.
本件発明は上記構成の3層構造であるため引き裂き強度が低く引き裂き易く、縦方向、横方向への引き裂きが容易である。 Since the present invention has a three-layer structure having the above-described configuration, the tear strength is low and the tear is easy to tear, and tearing in the longitudinal and lateral directions is easy.
フィルムの厚さは50μm以下が良く、シール強度安定性の観点から30μm以上が好ましい。
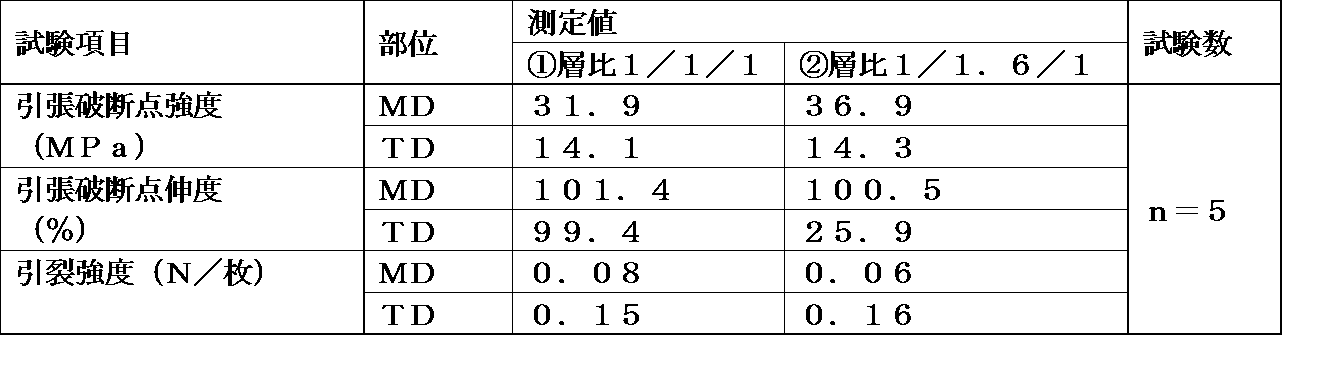
外層:中間層:内層の厚さの比率は1:1:1でも良く、1:1.6:1という比率にしてもよい。
本発明の各層の薄さの比を1:1:1のものと1:1.6:1の物とを比較した試験結果を下記表1に示す。
引張試験はJIS K−7127による方法を用いる
詳しくは一定の幅に切ったフィルムを、片方を固定し、もう片方を引張速度50mm/minで引張り、フィルムが切断した時の荷重を測定して行った。
引き裂き強度はJIS K‐7128に基づきエルメンドルフ引き裂き試験を行った。
下記の表から3層の厚さの比率は1:1.6:1の方が直角方向の引張破断点伸度が低く裂け易いことが分かった。
The thickness of the film is preferably 50 μm or less, and preferably 30 μm or more from the viewpoint of stability of seal strength.
The thickness ratio of the outer layer: intermediate layer: inner layer may be 1: 1: 1 or may be a ratio of 1: 1.6: 1.
Table 1 below shows the test results comparing the thickness ratio of each layer according to the present invention between 1: 1: 1 and 1: 1.6: 1.
The tensile test uses the method according to JIS K-7127. Specifically, a film cut to a certain width is fixed, one side is fixed, the other is pulled at a tensile speed of 50 mm / min, and the load when the film is cut is measured. It was.
As for the tear strength, an Elmendorf tear test was performed based on JIS K-7128.
From the table below, it was found that the ratio of the thickness of the three layers was 1: 1.6: 1 and the tensile elongation at break in the perpendicular direction was low and the film was easily torn.
本件発明は切り裂き容易な医薬品包装用多層フィルムであり、またガラス転移温度が100℃以上であるため夏場の高温下で保存しても品質に影響がない。 The present invention is a multi-layer film for packaging pharmaceuticals that is easy to tear, and has a glass transition temperature of 100 ° C. or higher, so that the quality is not affected even when stored at high temperatures in summer.
Claims (1)
密度が0.910〜0.930g/cm3で、190℃での流動性が0.5〜0.930g/10minの直鎖状低密度ポリエチレンと密度1.020g/cm3でガラス転移温度が100℃以上である環状ポリエチレンからなり、該環状ポリエチレンを40%以上60%未満の割合で含む中間層と、密度が0.910〜0.930g/cm3の低密度ポリエチレンまたは密度が0.910〜0.930g/cm3で、190℃での流動性が0.5〜0.930g/10minの直鎖状低密度ポリエチレンからなる内層の三層から構成される医薬品包装用多層フィルム。 An outer layer made of linear low density polyethylene having a density of 0.910 to 0.930 g / cm 3 and a fluidity at 190 ° C. of 0.5 to 0.930 g / 10 min;
Density of 0.910~0.930g / cm 3, a glass transition temperature fluidity at 190 ° C. is a linear low density polyethylene and a density 1.020g / cm 3 of 0.5~0.930g / 10min An intermediate layer comprising a cyclic polyethylene having a temperature of 100 ° C. or higher and containing the cyclic polyethylene in a proportion of 40% or more and less than 60%, and a low density polyethylene having a density of 0.910 to 0.930 g / cm 3 or a density of 0.910. A multilayer film for pharmaceutical packaging composed of three inner layers composed of linear low-density polyethylene having a fluidity at 190 ° C of 0.5 to 0.930 g / 10 min at ˜0.930 g / cm 3 .
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015100443A JP2016214395A (en) | 2015-05-15 | 2015-05-15 | Medicine packaging film |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015100443A JP2016214395A (en) | 2015-05-15 | 2015-05-15 | Medicine packaging film |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2016214395A true JP2016214395A (en) | 2016-12-22 |
Family
ID=57579531
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015100443A Pending JP2016214395A (en) | 2015-05-15 | 2015-05-15 | Medicine packaging film |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2016214395A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018153964A (en) * | 2017-03-16 | 2018-10-04 | 日本ポリエチレン株式会社 | Easily tearable multilayer film and packaging bag having heat resistance |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012000885A (en) * | 2010-06-17 | 2012-01-05 | Japan Polyethylene Corp | Easily tearable multilayered film and packaging material |
| JP2014529520A (en) * | 2011-08-23 | 2014-11-13 | フータマキ フィルムズ ジャーマニー ゲゼルシャフト ミット ベシュレンクテル ハフツング ウント コンパニー コマンディートゲゼルシャフト | Multilayer film with linear tear propagation |
-
2015
- 2015-05-15 JP JP2015100443A patent/JP2016214395A/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012000885A (en) * | 2010-06-17 | 2012-01-05 | Japan Polyethylene Corp | Easily tearable multilayered film and packaging material |
| JP2014529520A (en) * | 2011-08-23 | 2014-11-13 | フータマキ フィルムズ ジャーマニー ゲゼルシャフト ミット ベシュレンクテル ハフツング ウント コンパニー コマンディートゲゼルシャフト | Multilayer film with linear tear propagation |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018153964A (en) * | 2017-03-16 | 2018-10-04 | 日本ポリエチレン株式会社 | Easily tearable multilayer film and packaging bag having heat resistance |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| PH12021550401A1 (en) | Heat-sealable laminate, layered substrate, laminate for gas barrier intermediate layer, laminate for packaging material, and packaging material | |
| JP5262134B2 (en) | Barrier packaging material and packaging bag having straight cut characteristics | |
| TWI658932B (en) | Cover tape for packaging electronic parts | |
| BR102015011912A2 (en) | multi-layer film for reusable closure packaging having improved reseal | |
| JP2011025637A (en) | Co-extruded multi-layer film and packaging material comprising this film | |
| US20220348391A1 (en) | Packaging body | |
| JP2010005935A (en) | Composite film | |
| JP6667958B2 (en) | Tear directional sealant film and film laminate | |
| JP2016214395A (en) | Medicine packaging film | |
| JP2008155527A (en) | Laminate | |
| JP6150687B2 (en) | Multilayer sealant film | |
| JP2014148104A (en) | Lateral direction heat seal film and easy-to-open package using the same | |
| WO2016051566A1 (en) | Polyethylene-type thermally shrinkable multi-layer film for packaging use, packaged product, and method for packaging said packaged product | |
| CN104827722A (en) | Easy-to-tear-off aluminum foil composite film and preparation method thereof | |
| JP2014162162A (en) | Easily peelable multilayer film and easily peelable medical package | |
| JP6541017B2 (en) | Easy-to-open laminated film and packaging container | |
| JP2010280391A (en) | Self-standing packaging container, and method for manufacturing the same | |
| KR20160069545A (en) | Polypropylene multilayer film and medicine packaging film using the same | |
| JP6753119B2 (en) | Sealant adhesive and easy-to-peel film | |
| JP2019000991A (en) | Easily opening composite film | |
| JP4877062B2 (en) | Coextruded multilayer film and packaging material comprising the film | |
| JP2015048093A (en) | Packing bag | |
| JP7312344B1 (en) | Pillow packaging film | |
| JP7257777B2 (en) | Laminated films and packaging materials | |
| JP6727991B2 (en) | Melted film |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180110 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20181018 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20181017 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20190408 |