CN1520297A - 包含复合药物(codrugs)的缓释药物传递系统 - Google Patents
包含复合药物(codrugs)的缓释药物传递系统 Download PDFInfo
- Publication number
- CN1520297A CN1520297A CNA028129563A CN02812956A CN1520297A CN 1520297 A CN1520297 A CN 1520297A CN A028129563 A CNA028129563 A CN A028129563A CN 02812956 A CN02812956 A CN 02812956A CN 1520297 A CN1520297 A CN 1520297A
- Authority
- CN
- China
- Prior art keywords
- prodrug
- therapeutic activity
- activity form
- polymer
- slow releasing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/32—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. carbomers, poly(meth)acrylates, or polyvinyl pyrrolidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/55—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound the modifying agent being also a pharmacologically or therapeutically active agent, i.e. the entire conjugate being a codrug, i.e. a dimer, oligomer or polymer of pharmacologically or therapeutically active compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/554—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound the modifying agent being a steroid plant sterol, glycyrrhetic acid, enoxolone or bile acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L17/00—Materials for surgical sutures or for ligaturing blood vessels ; Materials for prostheses or catheters
- A61L17/005—Materials for surgical sutures or for ligaturing blood vessels ; Materials for prostheses or catheters containing a biologically active substance, e.g. a medicament or a biocide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/14—Materials characterised by their function or physical properties, e.g. lubricating compositions
- A61L29/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/10—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7007—Drug-containing films, membranes or sheets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
- A61L2300/406—Antibiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/41—Anti-inflammatory agents, e.g. NSAIDs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/45—Mixtures of two or more drugs, e.g. synergistic mixtures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Surgery (AREA)
- Molecular Biology (AREA)
- Vascular Medicine (AREA)
- Inorganic Chemistry (AREA)
- Heart & Thoracic Surgery (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Ophthalmology & Optometry (AREA)
- Neurosurgery (AREA)
- Tropical Medicine & Parasitology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Dermatology (AREA)
- Materials Engineering (AREA)
- Botany (AREA)
- Virology (AREA)
- Immunology (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Materials For Medical Uses (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
本发明公开了一种缓释系统,其包括一种聚合物和分散于该聚合物中的溶解度低于约1mg/ml的前体药物。有利地是该聚合物对于该前体药物而言可渗透的,并且对于该前体药物从该聚合物中的释放速率而言不是释放速率的限制因素。这使得可以通过在延长的时间内的缓释速率动力学来改善药物在体内在手术附近的传递,而同时不需要复杂的制造方法。
Description
技术领域
本发明一般涉及一种传递药物的改良系统。本发明特别是涉及一种以聚合物为基础的缓释药物传递系统和用该传递系统传递药物的方法。
背景技术
在药学领域中长期以来一直都承认缓释的可取性。已经提出了许多以聚合物为基础的系统来完成缓释的目标。这些系统一般依赖于聚合物的降解或通过聚合物的扩散来作为控制释放的手段。
可植入的药物传递系统提了一种用于替代口服给药、胃肠外给药、栓剂、和局部给药方式的有吸引力选择对象。例如,与口服、胃肠外和栓剂的给药方式相比,可植入的药物传递提供了比其它给药方式的定位性更强的定位性。因此,在临床医生希望得到更局部化的治疗药物作用的情况中可植入的药物传递装置尤其可取。此外,可植入的药物传递装置将药物直接传递到所需作用部位的能力使得临床医生可以使用吸收相对较差或在生物学液体中不稳定的药物,其常常具有更大的优点。可植入的药物传递装置使得可以在所需的作用部位获得治疗剂量,而同时维持低或可忽略的全身水平。因此,可植入的药物传递装置在所讨论药物有毒或清除性差或既有毒而且清除性也差的情况中尤其有吸引力。
与局部给药方式相比,可植入的药物传递系统具有可以被皮下应用的优点。其可以通过手术被植入并因此可以局部传递药物,并且在长时间内具有高浓度。相比较而言,药物的局部应用一般仅限于表皮,并且必需定期重复应用以将药物的浓度维持在其治疗有效的范围内。通过经皮途径如通过经皮贴剂进行的传递具有全身传递药物的缺点。
尽管可植入的药物传递装置具有明显的优点,但可植入的装置仍然有一些需要进一步改进的地方。例如,需要可以以恒定的速率释放药物的简单的药物传递装置。因为其难以装配并且应用不便,所以现有技术解决这些问题的努力仅取得了有限的成功。
因此,需要不需要复杂的制造过程的可以在体内长期内提供缓释药物传递的改良的药物传递装置。
现代的手术方法使用各种和大量通常被放置在体内并被长期留在体内的装置。该类装置非限制性地包括缝合线、斯滕特固定模、手术螺钉、关节修复物、人工瓣膜、板、起搏器等等。随着时间的流逝已经证明该类装置十分有用,但是仍然有一些与被植入的手术装置有关的问题。例如,在血管手术后,斯滕特固定模、人工瓣膜、并且在某种程度上甚至缝合线都可能引起再狭窄问题。因此,常常必需与植入的手术装置联合进行全身给药,其增加了手术后出血的危险。手术植入物有时会受到免疫反应或排斥。所以,有时必需放弃手术植入治疗或与某些手术植入物联合使用免疫抑制药物。在努力避免全身治疗方面通常有使用在控制速率的生物可降解聚合物中的药物的报道。该类系统随着聚合物被侵蚀而有计划的释放药物。这严重限制了药物和聚合物的选择。
因此,需要一种能在长期内将具有抗再狭窄或免疫抑制活性的药物以在药物治疗有效浓度范围内的缓释浓度传递到手术移植物附近的改良的药物传递装置。
已经进行了许多努力来降低患者在手术期间与病原微生物的接触,尽管如此,手术装置的植入仍涉及向体内引入一种可能会传染给患者各种病毒和/或细菌的外来物。因此,手术操作常常导致感染,该感染是患者在一般情况下不易患有的,并且其可危害或取消植入治疗的有效性。因此,在植入治疗时通常附加地进行抗生素、皮质类固醇和/或抗病毒药的给药以对感染进行预防或治疗。但是,该类抗菌剂组合物的全身给药常导致不希望出现的副作用。
因此,需要一种能在长期内将具有抗菌活性的药物以在该药物治疗有效浓度的范围内的缓释浓度传递到手术植入物附近的改良的药物传递装置。
手术植入物常常导致其它有害的副作用如疼痛和肿胀。通常用抗炎剂和止痛剂来对手术植入的患者进行常规治疗,所说的抗炎剂和止痛剂如甾体抗炎剂、非一甾体抗炎剂(NSAIDs),如阿司匹林、cefacoxib、rofecoxib、或消炎痛,其它止痛剂,如扑热息痛、以及鸦片制剂类。因为一些手术后的患者会发烧,所以通常用解热剂,如阿司匹林、布洛芬、萘普生、或扑热息痛来对该类患者进行治疗。患者常表现出对某些NSAIDs、甾类物质和鸦片制剂类的耐受性差。此外,一些NSAIDs可作为血液稀释剂和抗凝剂,其可增加手术后出血的危险。
因此,需要一种能在长期内将具有抗炎、镇痛和/或解热活性的药物以在该药物治疗有效浓度范围内的缓释浓度传递到手术植入物附近的改良的药物传递装置。
本发明的概述
本发明的某些实施方案提供了一种包含聚合物基质和分散在该聚合物内的前体药物的缓释系统,其中所说的前体药物具有A-L-B的通式,其中:A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分。在某些实施方案中,连接L在体液中被水解。在其它实施方案中,连接L被酶促裂解。可以使用的连接的实例包括一种或多种选自酯、酰胺、氨基甲酸酯、碳酸酯、环状缩酮、硫代酸酯、硫代酰胺、硫代氨基甲酸酯、硫代碳酸酯、黄原酸酯和磷酸酯的可水解基团。
本发明的其它实施方案提供了一种包含聚合物基质和分散在该聚合物内的前体药物的缓释制剂,其中所说的前体药物具有A∷B的通式,其中A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;∷表示A和B之间的在生理学条件下离解产生所说的A的治疗活性形式的离子键;和B表示一种当与A离子键合时产生一种溶解度低于A的治疗活性形式的前体药物的部分。
在某些优选的实施方案中,A的治疗活性形式在水中的溶解度高于1mg/ml,而该前体药物在水中的溶解度低于1mg/ml,并且更优选地低于0.1mg/ml、0.01mg/ml或甚至低于0.001mg/ml。
在某些优选的实施方案中,A的治疗活性形式在水中的溶解度比所说的前体药物高10倍并且其在水中的溶解度比所说的前体药物至少高100、1000或甚至10000倍。
在某些优选的实施方案中,当配置于生物流体(如血清、滑液、脑脊髓液、淋巴液、尿,等等)中时,该缓释制剂可以在至少24小时内提供A的治疗活性形式的持续释放,并且在释放期间,在聚合物外的流体中的前体药物的浓度低于A治疗活性形式浓度的10%,并且甚至更优选地低于A治疗活性形式浓度的5%、1%或甚至0.1%。
在某些优选的实施方案中,A的治疗活性形式的logP值比该前体药物的logP值低至少1个logP单位,并且更优选地比该前体药物的logP值低至少2、3或甚至4个logP单位。
在某些优选的实施方案中,其连接形式的前体药物产生临床响应的ED50比A的治疗活性形式的ED50高至少10倍,并且更优选地比A的治疗活性形式的ED50高至少100、1000或甚至10000倍。即,在许多实施方案中,前体药物本身不就产生临床响应而言是惰性的。
在某些实施方案中,B是一种疏水性脂族部分。
在一些情况中,B是一旦所说连接物L裂解或所说离子键解离则产生具有治疗活性形式的药物部分,并且可以是与A相同或不同的药物。
在其它实施方案中,在从该前体药物上裂解下来后,B是一种生物学惰性部分。
在许多优选的实施方案中,治疗有效量的A的治疗活性形式从该聚合物基质上释放的持续时间为至少24小时,并且更优选地为至少72小时、100、250、500或甚至750小时。在某些实施方案中,A的治疗活性形式从该聚合物基质上释放的持续时间为至少一周,更优选两周或更优选地为至少三周。在某些实施方案中,A的治疗活性形式从该聚合物基质上释放的持续时间为至少一个月,更优选地为两个月或甚至更优选地为六个月。
在某些实施方案中,该前体药物的ED50比A的治疗活性形式的ED50高至少10倍。在优选的实施方案中,该前体药物的ED50比A的治疗活性形式的ED50高至少100倍,或更优选地高至少1000倍。
在一些实施方案中,A的治疗活性形式在水中的溶解比所说的前体药物高至少10倍。在优选的实施方案中,A的治疗活性形式在水中的溶解比所说的前体药物高至少100倍,或更优选地高至少1000倍。
A(和任选的B)部分可以选自如下的药物,如免疫响应改性剂、抗增生剂、皮质类固醇、抑制血管的(angiostatic)甾类、抗寄生虫药、治疗青光眼的药物、抗生素、反义化合物、分化调节剂、抗病毒药、抗癌药、以及非甾体抗炎药。
在某些实施方案中,该聚合物基质是不可生物侵蚀的,而在其它实施方案中其是可生物侵蚀的。不可生物侵蚀的聚合物基质的实例可以有聚氨酯、聚硅氧烷、聚(乙烯-共-醋酸乙烯酯)、聚乙烯醇、以及它们的衍生物和共聚物。
可生物侵蚀的聚合物基质的实例可以是聚酐、聚乳酸、聚乙醇酸、聚原酸酯、聚烷基氰基丙烯酸酯、以及它们的衍生物和共聚物。
在某些实施方案中,选择聚合物基质的条件是其能降低位于该基质中的前体药物和周围浴液体中的蛋白质组分之间的相互作用,例如,通过形成一种具有可将蛋白质排除在该内部基质外的物理(孔大小等等)和/或化学(离子化基团、疏水性等等)特性的基质来实现该目的,例如可以将大于100kD的蛋白,并且更优选地可以将尺度大于50kD、25kD、10kD或甚至5kD的蛋白质排除在该内部基质外。
在某些实施方案中,该聚合物基质就A的治疗活性形式从该基质中释放的速率而言,基本不是释放速率的限制因素。
在其它实施方案中,该聚合物基质影响释放速率。例如,该基质可以被衍生以具有使该前体药物的螯合作用优于单体(A和B)释放的电荷或疏水性特性。同样,该聚合物基质也可以影响该水解反应的pH依赖性、或可产生一种不同于体液浴的pH的微环境,从而使得该前体药物在该基质中的水解和/或溶解度与周围液体中的水解和/或溶解度不同。该类聚合物可以以该类方式通过不同电子、疏水性或与该前体药物的化学相互作用而影响释放速率、以及该前体药物的水解速率。
在某些实施方案中,A或B的至少一个是抗肿瘤剂。抗肿瘤剂的实例包括蒽环素类(anthracyclines)、长春花属生物碱类、嘌呤类似物、嘧啶类似物、嘧啶生物合成的抑制剂、和/或烷化剂。抗肿瘤药的实例包括5-氟尿嘧啶(5FU)、5′-脱氧-5-氟尿苷5-氟尿苷、2′-脱氧-5-氟尿苷、氟胞嘧啶、5-三氟甲基-2′-脱氧尿苷、阿糖氧基(arabinoxyl)胞嘧啶、环胞苷、5-氮杂-2′-脱氧胞苷、阿拉伯糖基5-氮杂胞嘧啶、6-氮杂胞苷、N-膦酰基乙酰基-L-天门冬氨酸、吡唑呋喃菌素、6-氮杂尿苷、阿扎立平、3-去氮杂尿苷、阿拉伯糖基胞嘧啶、环胞苷、5-氮杂-2′-脱氧胞苷、阿拉伯糖基5-氮杂胞嘧啶、6-氮杂胞苷、克拉屈滨、6-巯基嘌呤、喷司他丁、6-硫鸟嘌呤、和磷酸氟达拉滨。
在某些优选的实施方案中,该抗肿瘤药是氟化的嘧啶,并且更优选地是5-氟尿嘧啶,例如,在某些实施方案中A优选地是5-氟尿嘧啶。
在某些实施方案中,A或B的至少一个是抗炎剂,用于解释性地如,非甾体抗炎药(双氯酚酸、非诺洛芬、氟比洛芬、布洛芬、酮洛芬、酮咯酸、nahumstone、萘普生、吡罗昔康等等)或糖皮质激素(如aclometasone、倍氯米松、倍他米松、布地奈德、氯倍他索、氯倍他松、可的松、地萘德、去羟米松、二氟拉松、氟米松、氟尼缩松、氟轻松、肤轻松、氟可龙、氟泼尼定、氟氢缩松、氟替卡松、氢化可的松、醋丙甲泼松龙、mometasone furdate、强的松龙、强的松和rofleponide)。
在某些优选的实施方案中,A是抗肿瘤的氟化嘧啶,如5-氟尿嘧啶,和B是抗炎剂,如氟轻松、曲安奈德、双氯酚酸、或萘普生。
在一些实施方案中,该前体药物选自与氟轻松共价结合的5FU(III)、与萘普生共价结合的5FU(IV)、和与双氯酚酸共价结合的5FU(V)。前体药物的实例包括:
5FU-氟轻松(III),
5FU-萘普生(IV),和
5FU-双氯酚酸(V)。
本发明另一方面涉及被涂布的药物装置。例如,在某些实施方案中,本发明提供了一种具有粘附在至少一个表面上的涂层的药物装置,其中所说的涂层包括主题聚合物基质和低溶解度的前体药物。该类涂层可被应用到手术器具如螺钉、板、衬垫、缝合线、假体锚凹、平头钉、U型钉、电导线、瓣膜、膜。该装置可以是导管、可植入的血管进入端口、血液储袋、血道、中央静脉导管、动脉导管、血管移植物、主动脉内气囊泵、心脏瓣膜、心血管缝合线、人造心脏、起搏器、心室助泵、体外装置、血液过滤器、血液透析装置、血灌注(hemoperfasion)装置、血浆去除装置、适于在血管中使用的过滤器。
在一个优选的实施方案中,该涂层可以被应用到血管斯滕特固定模上。在某些情况中,特别是在该斯滕特固定模是可扩张的斯滕特固定模的情况中,该涂层是柔软的以适应该斯滕特固定模的压缩和扩张状态。
在某些实施方案中,可归因于该前体药物的涂层的重量为每cm2被所说聚合物基质所涂布表面约0.05mg至约50mg前体药物,并且更优选地为5至25mg/cm2。
在某些实施方案中,该涂层的厚度为5微米至100微米。
在某些实施方案中,存在于该涂层中的前体药物的数量为该涂层重量的5%至70%重量,并且更优选地为25%至50%重量。
本发明的另一方面还提供了一种对患者的管腔内组织进行处理的方法。一般而言,该方法包括的步骤有:
(a)提供一种具有内表面和外表面的斯滕特固定模,所说的斯滕特固定模在其内表面、外表面的至少一个部分上或在内表面和内表面上都具有一种涂层;所说的涂层包含溶解或分散于生物学上可耐受的聚合物中的低溶解度的药学前体药物;
(b)将该斯滕特固定模放置在适当的管腔内组织部位上;和
(c)将该斯滕特固定模扩展开。
本发明另一方面涉及一种用于在体内将药物从药物装置的表面定位进行传递的涂层组合物。
该组合物包含一种聚合物基质和如上所述的低溶解度的前体药物。该涂层组合物可以以用于通过喷雾和/或将装置在该组合物中进行浸渍来进行应用到医学装置的表面上的液体或混悬液的形式被提供。在其它实施方案中,该涂层组合物是以粉末形式被提供的,一旦添加溶剂,其可以被重组成可通过喷雾和/或将装置在该组合物中进行浸渍来进行应用到医学装置表面的液体或混悬液的形式。
本发明另一方面涉及一种用于将药物传递给患者的可注射组合物。该组合物包括一种聚合物基质和如上所述的低溶解度的前体药物,并且其可以以适于通过针注射来进行传递的液体或混悬液形式被提供。
通过下面的详细说明,本发明另外的优点对本领域技术人员而言将会变得显而易见,其中仅表示了本发明的优选实施方案并通过完成本发明的最好实施方式的举例说明对其进行了描述。将意识到本发明能有其它和不同的实施方案,并且可以在各种方面对一些细节进行修改,所有这些都不会脱离本发明的范围。因此,附图和说明应当被认为本质上是要进行说明而不是要进行限制。
附图的简要说明
图1是前体药物从本发明的聚合物-前体药物分散体释放的时间-依赖性图。
图2是前体药物从本发明的聚合物-前体药物分散体释放的时间-依赖性图。
图3是本发明未展开的斯滕特固定模的侧面平面图。
图4是本发明已展开的斯滕特固定模的侧面平面图。
图5是TC-112从PVA涂布的载玻片上释放到pH 7.4的缓冲剂中的释放曲线。
图6是TC-112从硅氧烷-涂布的玻璃片上释放到pH 7.4的缓冲剂中的释放曲线。
图7是5-氟尿嘧啶(5FU)和曲安奈德(TA)从进行了涂布的插入物上的释放曲线。
图8是5-氟尿嘧啶(5FU)和曲安奈德(TA)从进行了涂布的插入物上的释放曲线。
图9说明了高剂量涂布的斯滕特固定模的体外释放方式。
图10表示了进行比较的移植的斯滕特固定模和未植入的斯滕特固定模之间药物释放曲线。
图11A和11B表示了γ辐射和血浆处理对药物释放的影响。组B:用血浆进行处理,用γ辐射进行处理。组C:不用血浆进行处理,用γ辐射进行处理。组D:用血浆进行处理,不用γ辐射进行处理。组F:不用血浆,不用γ辐射。
进行本发明的最好方式
本发明的详细说明
I.
定义
这里所用的术语“活性”指的是治疗或药理学活性。
术语“ED50”指的是产生50%最大响应或作用的药物剂量。
术语“IC50”指的是将生物学活性抑制50%的药物剂量。
术语“LD50”指的是有50%试验个体死亡的药物剂量。
术语“治疗指数”指的是用LD50/ED50来定义的药物治疗指数。
被本发明的方法所治疗的“患者”或“个体”可指的是人或非人的动物。
“生理学条件”描述了生物体内的条件,即体内条件。生理学条件包括体腔和器官的酸性和碱性环境、酶裂解、代谢、和其它生物学过程,并且优选地指的是脊椎动物如哺乳动物的生理学条件。
“LogP”指的是P(分配系数)的对数。P是测量该物质是如何在脂(油)和水之间进行分配的一种尺度。P本身是一个常数。其被定义为中性分子形式的化合物在水相中的浓度与化合物在不可溶混的溶剂中的浓度的比。
分配系数,P=[有机]/[水],其中[]=浓度
LogP=log10(分配系数)=log10P
实际上,LogP值将随着其测量条件和所选择的分配溶剂而变化。等于1的LogP值意味着有机相中的化合物浓度比水相中的浓度高10倍。logP值增加1表明与水相中的化合物相比,有机相中的化合物浓度增加了10倍。因此,logP值为3的化合物在水中的溶解比logP值为4的化合物高10倍并且logP值为3的化合物在水中的溶解度比logP值为5的化合物高100倍。一般认为logP值为7-10的化合物是低溶解度的化合物。
II.
实施方案实例
本发明提供了一种可以提供各种释放曲线的药物传递系统,例如,其可提供各种剂量和/或各种时间长度。因此,本发明满足了可提供可控的药物时间释放动力学,特别是可将所需的药物活性定位于所需部位附近并同时避免了与现有技术中的装置有关的并发症的可插入、可注射、可吸入、或可植入的药物传递系统的需要。
本发明的系统包括一种聚合物和分散在该聚合物中的具有低溶解度的前体药物。该聚合物对于该前体药物而言是可渗透的,并且基本不会限制该前体药物从该聚合物中释放的释放速率,并且提供了药物的持续释放。
一旦被给药,该系统就会持续将该前体药物供给到所需的活性部位,但并不一定需要另外向这些区域进行侵入性地渗入。作为替代,该系统留在体内并作为向受感染区域持续提供前体药物的来源。本发明的系统使得可以在特定的数天、数周、数月(例如约3个月至约6个月)或数年(例如约1年至约20年,如约5年至约10年)的时期内长期释放药物直至该前体药物被用尽。
管腔内的医学装置包含该缓释的药物传递涂层。本发明的斯滕特固定模涂层可以通过常规的涂布法而被应用到该斯滕特固定模上,其中所说的常规涂布法如浸渗涂布、喷涂和浸涂。
在一个实施方案中,管腔内的医学装置包含具有内腔表面和相对的沿着纵向斯滕特固定模轴延伸的外表面的放射状伸延的可扩展的管状斯滕特固定模。该斯滕特固定模可包括永久的可植入的斯滕特固定模、可植入的接枝斯滕特固定模、或临时的斯滕特固定模,其中所说的临时的斯滕特固定模被定义为在脉管内可扩展的并且其后可从该脉管中抽出的斯滕特固定模。该斯滕特固定模结构可包括螺旋斯滕特固定模、记忆性螺旋斯滕特固定模、镍钛合金斯滕特固定模、网孔斯滕特固定模、骨架斯滕特固定模、套管斯滕特固定模、可渗透的斯滕特固定模、具有温度传感器的斯滕特固定模、多孔的斯滕特固定模等等。该斯滕特固定模可以用常规方法来进行展开,如可以通过可膨胀的气囊导管、通过自动展开机理(在从导管中释放出来后)、或通过其它适宜方法来展开。该放射状伸延的可扩展的管状斯滕特固定模可以是一种接枝斯滕特固定模,其中所说的接枝斯滕特固定模是一种在一种移植物内部或外部具有斯滕特固定模的复合装置。该移植物可以是一种血管移植物,如ePTFE移植物、生物学移植物、或织制的移植物。
该药物组合可以通过许多方法被混入到或附着到该斯滕特固定模上。在一个实例性实施方案中,该药物组合被直接混入到聚合物基质中并被喷洒到该斯滕特固定模的外表面上。随着时间的流逝,该药物组合从该聚合物基质上洗脱下来并进入到周围组织中。该药物组合优选地在该斯滕特固定模上保留至少三天到高至约六个月,并且更优选地保留七至三十天。
该前体药物缓慢地溶解于生理性流体中,但是一旦溶解于生理性流体中则相对迅速地解离成至少一种药物学活性化合物。在一些实施方案中,该前体药物的溶解速度为约0.001μg/天至约10μg/天。在某些实施方案中,该前体药物具有约0.01至约1μg/天的溶解速率。在特定的实施方案中,该前体药物具有约0.1μg/天的溶解速率。
该低溶解度的药学前体药物被混入到一种生理学可相容(即,生理学可耐受的)聚合物载体中。在本发明的一些实施方案中,该低溶解度的前体药物是以分散在该聚合物载体中的许多颗粒的形式存在的。在该类情况中,优选地该低溶解度的药学前体药物在聚合物载体中相对不溶,不管该低溶解度的药学前体药物相对于该聚合物载体而言具有怎样有限的溶解度系数,其仍然在本发明的范围内。在两种情况的任何一种情况中,该低溶解度药学前体药物的聚合物载体溶解度应当是该前体药物将分散在该聚合物载体的各处而同时仍然基本为颗粒形式的溶解度。
在本发明的一些实施方案中,该低溶解度的药学前体药物被溶解在该聚合物载体内。在该类情况中,优选地该聚合物载体是可作为该相对疏水的低溶解度药学前体药物的良好溶剂的相对而言是相对非极性的或疏水的聚合物。在该类情况中,在该聚合物载体中的低溶解度药学前体药物的溶解度应当是该前体药物将完全溶解于该聚合物载体中的溶解度,药物将均匀地分布在该聚合物载体内。
本发明的聚合物包括任何生物学可耐受的聚合物,该聚合物对于该前体药物而言是可渗透的并且同时具有一种不是该前体药物从该聚合物中释放的释放速率的主要速率决定因素的渗透性。
在本发明的一些实施方案中,该聚合物是不可生物侵蚀的。用于本发明的不可生物侵蚀的聚合物的实例包括聚(乙烯-共-醋酸乙烯酯)(EVA)、聚乙烯醇和聚氨酯,如聚碳酸酯-基础的聚氨酯。在本发明的其它实施方案中,该聚合物是可生物侵蚀的。用于本发明的可生物侵蚀的聚合物的实例包括聚酐、聚乳酸、聚乙醇酸、聚原酸酯、聚烷基氰基丙烯酸酯或它们的衍生物和共聚物。如下面详细描述的那样,熟练技术人员将意识到选择生物侵蚀性的聚合物还是选择非生物侵蚀性的聚合物将取决于该系统最后的物理形式。聚合物的其它实例包括聚硅氧烷和得自透明质酸的聚合物。熟练技术人员将明白本发明的聚合物是在适于赋予其一种使其不是该低溶解度前体药物从该聚合物中释放的主要速率决定因素的渗透性的条件下来进行制备的。
此外,适宜的聚合物包括可以与体液和哺乳动物组织在生物学上相容并在该聚合物将与其进行接触的体液中基本不溶的天然存在的(胶原、透明质酸等等)或合成的材料。此外,适宜的聚合物基本可以阻止该分散/混悬于该聚合物中的低溶解度的前体药物和体液中的蛋白质组分之间的相互作用。因为聚合物的溶解或与蛋白质组分的相互作用将影响药物释放的持久性,所以在某些情况中避免使用迅速溶解的聚合物或在体液中具有高溶解度的聚合物或使得该低溶解度的前体药物与蛋白质组分相互作用的聚合物。
其它适宜的聚合物包括聚丙烯、聚酯、聚乙烯醋酸乙烯酯(PVA或EVA)、聚氧化乙烯(PEO)、聚氧化丙烯、聚羧酸、聚烷基丙烯酸酯、纤维素醚、硅酮、聚(dl-丙交酯-共乙交酯)、各种Eudragrits(例如,NE30D、RS PO和RL PO)、聚烷基-烷基丙烯酸酯共聚物、聚酯-聚氨酯嵌段共聚物、聚醚-聚氨酯嵌段共聚物、聚二噁酮、聚-(β-羟基丁酸酯)、聚乳酸(PLA)、聚己酸内酯、聚乙醇酸、和PEO-PLA共聚物。
本发明的涂层可以通过将一种或多种适宜的单体和适宜的低溶解度的药学前体药物进行混合,然后将该单体聚合形成该聚合物系统而形成的。因此,该前体药物被溶解或分散于该聚合物中。在其它实施方案中,该前体药物被混到一种液态聚合物或聚合物分散体中,然后将该聚合物进一步加工成本发明的涂层。适宜的进一步加工包括与适宜交联前体药物进行交联、该液态聚合物或聚合物分散体的进一步聚合、与适宜单体的共聚、与适宜聚合物嵌段的嵌段共聚等等。该进一步的加工可将该药物捕获在该聚合物中从而使得该药物被混悬或分散在该聚合物载体中。
可以与该药物组合联合使用任何数目不可侵蚀的聚合物。在这种应用中可用于进行涂布的成膜聚合物是可被吸收或不可被吸收的并且必需是可生物相容的以将对脉管壁的刺激降低到最低的程度。根据所需的释放速率或所需聚合物的稳定程度,该聚合物是生物学稳定的或生物学上可吸收的,但是优选生物可吸收的聚合物,这是因为其与生物学稳定的聚合物不同,其在被植入后不会长期存在,从而不会造成任何不利的慢性局部反应。此外,生物可吸收的聚合物不存在在过长的时间内其会由于可以使该涂层移动的生物环境压力而造成该斯滕特固定模和涂层之间的粘合力下降并且甚至在该斯滕特固定模被包埋在组织中后也会产生进一步的问题的风险。
可用于本发明的适宜的可生物吸收的成膜聚合物包括选自脂族聚酯、聚(氨基酸)、(醚-酯类)共聚物、聚亚烷基草酸酯、聚酰胺类、聚(亚氨基碳酸酯)、聚原酸酯、聚草酸酯类(polyoxaesters)、聚酰氨基酯类、包含酰氨基的聚草酸酯类、聚(酸酐)、含磷氮链聚合物、生物分子和其混合物的聚合物。对于本发明的目的而言,脂族聚酯包括丙交酯(其包括乳酸d-、1-和内消旋型丙交酯)、ε-己内酯、乙交酯(包括乙醇酸)、羟基丁酸酯、羟基戊酸酯、对-二噁酮、三亚甲基碳酸酯(以及其烷基衍生物)、1,4-二氧杂环庚烷-2-酮、1,5-二氧杂环庚烷-2-酮、6,6-二甲基-1,4-二噁烷-2-酮的均聚物和共聚物以及其聚合物混合物。用于本发明目的的聚(亚氨基碳酸酯)包括如Kemnitzer和Kohn,在“可生物降解的聚合物手册”,Domb,Kost和Wisemen编辑,HardwoodAcademic Press,1997,251-272页中所描述的物质。用于本发明目的的(醚-酯类)共聚物包括这些由Cohn在Journal of BiomaterialsResearch,第22卷,993-1009页,1988和Younes and Cohn,PolymerPreprints(ACS Division of Polymer Chemistry)第30卷(1),498页,1989中所描述共聚的酯-醚类(例如PEO/PLA)。用于本发明目的的聚亚烷基草酸酯包括US 4,208,511;4,141,087;4,130,639;4,140,678;4,105,034;和4,205,399中所述的物质(其在这里被引入作为参考)。含磷氮链聚合物、由L-丙交酯、D,L-丙交酯、乳酸、乙交酯、乙醇酸、对-二噁酮、三亚甲基碳酸酯和ε-己内酯制得的以二-、三-和更高次序的混合单体为基础的聚合物,如由Allcock在TheEncyclopedia of Polymer Science,第13卷,31-41页,WileyIntersciences,John Wiley & Sons,1988和由Vandorpe,Schacht,Dejardin and Lemmouchi在可生物降解的聚合物手册,Domb,Kost和Wisemen编辑,Hardwood Academic Press,1997,161-182页中所描述的物质(其在这里被引入作为参考)。聚酐得自形式为HOOC-C6H4-O-(CH2)m-O-C6H4-COOH的二酸,其中m是从2至8的整数,以及其与具有高至12个碳原子的脂族α-ω二酸的共聚物。聚草酸酯类聚草酰胺和包含胺和/或酰氨基基团的聚草酸酯类在US5,464,929;5,595,751;5,597,579;5,607,687;5,618,552;5,620,698;5,645,850;5,648,088;5,698,213和5,700,583中的一篇或几篇中进行了描述(其在这里被引入作为参考)。聚原酸酯类如这些由Heller在可生物降解的聚合物手册,Domb,Kost和Wisemen编辑,HardwoodAcademic Press,1997,99-118页(其在这里被引入作为参考)中所描述的物质。用于本发明目的的成膜聚合生物分子包括在人体内可以被酶降解或在人体内水解不稳定的天然存在的材料,如纤维蛋白、纤维蛋白原、胶原、弹性蛋白、和可吸收的生物可相容的多糖如壳聚糖、淀粉、脂肪酸(及其酯)、葡萄糖基(glucoso)-聚糖类和透明质酸。
还可以使用具有相对低的、慢的组织响应的适宜的生物学稳定的成膜聚合物如聚氨酯类、硅酮、聚(甲基)丙烯酸酯类、聚酯类、聚环氧烷类(聚环氧乙烷)、聚乙烯醇类、聚乙二醇类和聚乙烯吡咯烷酮,以及水凝胶类,如这些由交联聚乙烯吡咯烷酮和聚酯形成的物质。如果其可以被溶解、固化或聚合在该斯滕特固定模上,则也可以使用其它聚合物。这些物质包括聚烯烃类、聚异丁烯和乙烯-α烯烃共聚物;丙烯酸类聚合物(包括甲基丙烯酸酯)和共聚物、乙烯基卤化物聚合物和共聚物,如聚氯乙稀;聚乙烯基醚,如聚乙烯基甲基醚;聚亚乙烯基卤化物如聚偏二氟乙烯和聚偏二氯乙烯;聚丙烯腈,聚乙烯基酮;聚乙烯基芳族化合物如聚苯乙烯;聚乙烯基酯类如聚醋酸乙烯酯;乙烯基单体彼此或其与烯烃类的共聚物,如乙烯-甲基丙烯酸甲酯共聚物、丙烯腈苯乙烯共聚物、ABS树脂和乙烯-醋酸乙烯酯共聚物;聚酰胺,如Nylon 66和聚己内酰胺;醇酸树脂;聚碳酸酯;聚氧化甲烯;聚酰亚胺类;聚醚类;环氧树脂类,聚氨酯类;人造丝;人造丝-三醋酸酯、纤维素、醋酸纤维素、醋酸丁酸纤维素;玻璃纸;硝酸纤维素;丙酸纤维素;纤维素醚类(即羧甲基纤维素和羟烷基纤维素);以及它们的组合。用于本申请目的的聚酰胺类还包括形式为-NH-(CH2)n-CO-和NH-(CH2)x-NH-CO-(CH2)y-CO的聚酰胺,其中n优选地是从6至13的整数;x是从6至12的整数;和y是从4至16的整数。上面所列出的物质是用于进行说明而不是用于进行限制。
用于进行涂布的聚合物可以是具有足够高分子量以使其不是蜡状或发粘的物质的成膜聚合物。该聚合物还应能粘附到该斯滕特固定模上并且在沉积到该斯滕特固定模上后在被血液动力学应力移位方面不易变形。该聚合物的分子量应足够高以提供足够的韧性从而使得该聚合物在进行操作或该斯滕特固定模的展开期间不会被擦掉并且在该斯滕特固定模膨胀的过程中必需不会破裂。在某些实施方案中,该聚合物具有高于40℃的熔化温度,其熔化温度优选地高于约45℃,更优选地高于50℃并且最优选地高于55℃。
涂层可以通过将一种或多种治疗性前体药物与该涂层聚合物在涂层混合物中进行混合来进行制备。该治疗性前体药物可以以液体、分割地很细的固体、或任何其它适宜的物理形式存在。该混合物可以包括或不包括一种或多种添加剂,例如无毒的辅助物质如稀释剂、载体、赋形剂、稳定剂等等。其它适宜的添加剂可以与该聚合物和药学活性的前体药物或化合物一起进行制备。例如,可以向生物可相容的疏水性涂层中加入选自上面所列的生物可相容的成膜聚合物的亲水性聚合物以改变释放曲线(或可以向亲水性涂层中加入疏水性聚合物以改变释放曲线)。一个实例是向脂族聚酯涂层中添加选自聚氧化乙烯、聚乙烯吡咯烷酮、聚乙二醇、羧甲基纤维素、羟甲基纤维素及其组合物的亲水性聚合物以改变释放曲线。可以通过对该治疗性前体药物的体外和/或体内释放曲线进行监测来决定适宜的相对量。
该涂层的厚度可以决定该活性药物(活性药物类)或前体药物从该基质中洗脱出来的速率。实际上,该活性药物(活性药物类)或前体药物是通过经由该聚合物基质进行扩散而从基质中被洗脱出来的。聚合物是可渗透的,从而使得固体、液体和气体可以从其中逸出。该聚合物基质的总厚度在一微米至约二十微米或更厚的范围内。注意在将该聚合物基质附着于该医学装置上之前进行底层和金属表面处理是很重要的。例如,可以用酸清洗、强碱(碱)清洗、盐化和聚对亚苯基二甲基沉积作为所述的全过程的一部分。
在某些实施方案中,可以使用复合涂层。例如,各种涂层可以在前体药物浓度、前体药物特性(活性成分、连接物等等)、聚合物基质的特性(组成、孔隙率等等)和/或其它药物或释放改性剂的存在情况方面有所不同。
为了进一步进行说明,可以以许多方式将聚(乙烯-共-醋酸乙烯酯)、聚甲基丙烯酸丁酯和药物组合溶液混入到该斯滕特固定模中或该斯滕特固定模上。例如,该溶液可以被喷洒到该斯滕特固定模上或该斯滕特固定模可以被浸入到该溶液中。其它方法包括旋涂和RF等离子聚合。在一个实例性实施方案中,该溶液被喷洒到该斯滕特固定模上,然后被进行干燥。在另一个实例性实施方案中,该溶液被荷电成一种极性而该斯滕特固定模被荷电成相反的极性。通过这种方式,该溶液和斯滕特固定模将彼此吸引。在使用这种类型的喷涂方法时,可以降低浪费并可以更精确地对该涂层的厚度进行控制。
在另一个实例性实施方案中,该药物组合或其它治疗性前体药物可以被混入到一种包含一定数量的第一部分和一定数量的与第一部分共聚的第二部分的成膜聚氟共聚物中,从而产生聚氟共聚物;其中所说的的一部分选自聚合的偏二氟乙烯和聚合的四氟乙烯,所说的第二部分不同于第一部分,并能为该聚氟共聚物提供韧性或弹性,其中的一部分和第二部分的相对数量能有效提供由其而产生的具有用于处理该可植入的医学装置的性质的涂层和膜。
在本发明的一个实施方案中,本发明管腔内医学装置的可扩展的管状斯滕特固定模的外表面包含本发明的涂层。该具有涂层的斯滕特固定模的外表面是与组织进行接触的表面并且是生物可相容的。“进行了涂布的缓释药物传递系统表面”是“被涂布的表面”的同义词,该表面被本发明的缓释药物传递系统所涂布、覆盖或浸渍。
在一个供选择的实施方案中,本发明管腔内医学装置的放射状延伸的可扩展的管状斯滕特固定模的内腔表面或整个表面(即内表面和外表面)具有被涂布的表面。具有本发明缓释药物传递系统涂层的内腔表面也是与液体相接触的表面,并且其是可生物相容和可与血液相容的。
US 5,773,019、6,001,386和6,051,576公开了可植入的控制释放的装置和药物,其在这里都被全部引入作为参考。本发明用于制备一种具有被涂布的表面的斯滕特固定模的方法包括将一种涂层沉积在该斯滕特固定模上,例如通过浸涂或喷涂来进行制备。在对该斯滕特固定模的一侧进行涂布的情况中,仅仅将该被进行涂布的表面进行浸渍或喷洒。被处理的表面可以是该管腔内医学装置内腔表面、外表面、或内表面和外表面的全部或一部分。该斯滕特固定模可以由多孔的材料制成以使得更多的沉积物或涂层进入到多个微孔中或可应用的斯滕特固定模表面上,其中所说的微孔的大小优选地为约100微米或更小。
可以通过对用于对该医学装置进行涂布的药学前体药物进行选择来解决与再狭窄(restinosis)和新内膜增生的治疗有关的问题。在本发明某些优选的实施方案中,所选择的药学前体药物是一种低溶解度部分并包含至少两种药学活性的化合物。该药学活性的化合物可以是相同或不同类型的化学药品,并且可以根据需要在该化合物的相对活性和其它药动学性质的基础上以等摩尔或不等摩尔的浓度来形成以提供最佳的治疗。药物组合,特别是在使用复合药物的情况中的药物组合本身在生理学液体如血液和血浆中可有利地相对不溶,并且具有当溶解于生理学液体中时可以重新产生任何或所有的药学活性化合物的性质。换言之,就该低溶解度的前体药物溶解于生理学液体中的程度而言,其一旦溶解则迅速有效地被转化成组成其的药学活性化合物。因此,该药学前体药物的低溶解度确保了该前体药物能持续存在于管腔内的损害附近。该低溶解度的药学前体药物可迅速转化成其组成的药学活性化合物确保了药学活性化合物在所治疗的损害位置附近的稳定的受控的给药。
适宜的第一种药学活性化合物的实例包括免疫响应改性剂如环胞菌素A和FK 506、皮质类固醇如地塞米松、氟轻松和曲安奈德、抑制血管的甾类物质如三羟基甾类物质、包括环丙沙星在内的抗生素、分化调节剂如类维生素A类物质(例如,反式-视黄酸、顺式-视黄酸以及类似物)、抗癌剂/抗增剂前体药物如5-氟尿嘧啶(5FU)和BCNU、以及非甾体抗炎药前体药物如萘普生、双氯酚酸、消炎痛和氟比洛芬。
在本发明的一些实施方案中,优选的第一药学活性化合物是5FU。
5-氟尿嘧啶(5FU).
适宜的第二药学活性化合物的实例包括免疫响应改性剂如环胞菌素A和FK 506、皮质类固醇如地塞米松、氟轻松和曲安奈德、抑制血管的甾类物质如三羟基甾类物质、包括环丙沙星在内的抗生素、分化调节剂如类维生素A类物质(例如,反式-视黄酸、顺式-视黄酸以及类似物)、抗癌剂/抗增生剂前体药物如5-氟尿嘧啶(5FU)和BCNU、以及非甾体抗炎药前体药物如萘普生、双氯酚酸、消炎痛和氟比洛芬。
在本发明的一些实施方案中,第二药学活性化合物选自氟轻松、曲安奈德、双氯酚酸、以及萘普生。
曲安奈德 双氯酚酸
萘普生
本发明低溶解度药学活性的前体药物可包含另外的药学活性化合物的残基。该类另外的药学活性化合物包括免疫响应改性剂如环胞菌素A和FK 506、皮质类固醇如地塞米松、氟轻松和曲安奈德、抑制血管的甾类物质如三羟基甾类物质、包括环丙沙星在内的抗生素、分化调节剂如类维生素A类物质(例如,反式-视黄酸、顺式-视黄酸以及类似物)、抗癌剂/抗增生剂前体药物如5-氟尿嘧啶(5FU)和BCNU、以及非甾体抗炎药前体药物如萘普生、双氯酚酸、消炎痛和氟比洛芬。
在某些实施方案中,该低溶解度的药学前体药物包含一种至少两种药学活性化合物部分,所说的两种药学活性化合物部分通过一种连接物、离子键结合、或混合结合形式被共价键合、连接。
在本发明的一些实施方案中,该第一和第二药学活性化合物直接彼此共价结合。在第一和第二药学活性化合物和通过一种共价键彼此直接结合的情况中,该键可以通过在各活性化合物上活性基团间形成适宜的共价连接来形成。例如,第一药学活性化合物上的酸性基团可以于第二药学活性化合物上的胺、酸或醇缩合从而分别形成相应的酰胺、酸酐或酯。
除羧酸、胺和羟基基团外,可在药学活性部分之间形成连接的其它适宜的活性基团包括磺酰基、巯基、和氢卤酸以及羧酸的酸酐衍生物。
在其它实施方案中,药学活性化合物可以通过一种中间连接物彼此共价相连。该连接物有利地具有两个活性基团,所说两个活性基团中的一个可以与第一药学活性化合物上的活性基团互补,而另一个可以与第二药学活性化合物上的活性基团互补。例如,在所说的第一和第二药学活性化合物都具有游离羟基的情况中,该连接物可以适宜地是一种二酸,其将可以与两种化合物进行反应从而在两个残基之间形成一种二醚键。除羧酸、胺和羟基基团外,可以在药学活性部分之间形成连接的其它适宜活性基团包括磺酰基、巯基、和氢卤酸以及羧酸的酸酐衍生物。
将适宜的连接物列于下面的表1中。
表1
| 第一药学活性化合物活性基团 | 第二药学活性化合物活性基团 | 适宜连接物 |
| 胺 | 胺 | 二酸 |
| 胺 | 羟基 | 二酸 |
| 羟基 | 胺 | 二酸 |
| 羟基 | 羟基 | 二酸 |
| 酸 | 酸 | 二胺 |
| 酸 | 羟基 | 氨基酸、羟烷基酸、巯基烷基酸 |
| 酸 | 胺 | 氨基酸、羟烷基酸、巯基烷基酸 |
适宜的二酸连接物包括草酸、丙二酸、琥珀酸、戊二酸、己二酸、庚二酸、辛二酸、壬二酸、癸二酸、马来酸、富马酸、酒石酸、邻苯二甲酸、间苯二甲酸、和对苯二甲酸类。虽然被称为二酸,但本领域技术人员应当意识到在某些情况中,在连接复前体药物(reprodrug)时优选相应的酰基卤或酸酐(单侧或双侧)。一种优选的酸酐是琥珀酸酐。另一种优选的酸酐是马来酸酐。本领域技术人员可以使用其它酸酐和/或酰基卤来获得良好的作用。
适宜的氨基酸包括γ-丁酸、2-氨基乙酸、3-氨基丙酸、4-氨基丁酸、5-氨基戊酸、6-氨基己酸、丙氨酸、精氨酸、天冬酰胺、天门冬氨酸、半胱氨酸、谷氨酸、谷酰胺、甘氨酸、组氨酸、异亮氨酸、亮氨酸、赖氨酸、蛋氨酸、苯丙氨酸、脯氨酸、丝氨酸、苏氨酸、色氨酸、酪氨酸、和缬氨酸。在将其用作连接基团之前,该适宜的氨基酸的酸基团又可以被转化成酸酐或酰基卤形式。
适宜的二胺包括1,2-二氨基乙烷、1,3-二氨基丙烷、1,4-二氨基丁烷、1,5-二氨基戊烷、1,6-二氨基己烷。
适宜的氨基醇包括2-羟基-1-氨基乙烷、3-羟基-1-氨基乙烷、4-羟基-1-氨基丁烷、5-羟基-1-氨基戊烷、6-羟基-1-氨基己烷。
适宜的羟基烷基酸包括2-羟基乙酸、3-羟基丙酸、4-羟基丁酸、5-羟基戊酸、5-羟基己酸。
本领域技术人员将意识到可以对具有适宜活性基团的第一和第二药学部分(和存在或不存在的第三等药学部分)进行筛选,并且通过将其与适宜的连接物进行匹配,在本发明的范围内可以制备出许多宽的一套本发明的化合物。
优选的低溶解度的药学活性前体药物的实例包括与氟轻松共价结合的5FU,与双氯酚酸共价结合的5FU,和与萘普生共价结合的5FU。解释性的实例包括如下的物质:
5FU-氟轻松(通过草酸酯连接物)。
5FU-萘普生
5FU-双氯酚酸
其它复合药物的实例包括如下的物质:
5-TC-70.1(氟轻松与5-FU通过甲醛连接相连的复合药物)
5-TC-63.1(萘普生与氟尿苷通过含氧酸连接相连的复合药物)
3-TC-112(萘普生与5-FU通过甲醛连接相连的复合药物)
G-427.1(曲安奈德与5-FU的直接相连的复合药物)
TC-32(曲安奈德与5-FU通过甲醛连接相连的复合药物)
通过不同连接将第一和第二药学活性化合物相连的一些复合药物的实例包括:
氟尿苷与双氯酚酸的复合药物(1∶1)
氟尿苷与双氯酚酸的复合药物(1∶2)
氟尿苷与氟轻松的复合药物(1∶1)
氟尿苷与氟轻松的复合药物(1∶1)
氟尿苷与氟轻松的复合药物(1∶1)
氟尿苷与萘普生的复合药物(1∶1)
氟尿苷与萘普生的复合药物(1∶2)
在其它实施方案中,所说的第一和第二药学活性化合物可以结合形成一种盐。例如,第一药学活性化合物可以是一种酸,并且第二药学活性化合物可以是一种碱,如胺。作为一个特定的实例,该第一药学活性化合物可以是双氯酚酸或萘普生(酸类),和该第二药学活性化合物可以是环丙沙星(一种碱)。双氯酚酸和环丙沙星的组合物例如将形成如下的盐:
环丙沙星-双氯酚酸
对于以所需方式,例如在一些实施方案中以恒定或基本为线性的方式传递前体药物的本发明的系统而言,药物的溶解度和聚合物的渗透性必需是平衡的以使得该聚合物的渗透性在药物的传递中不是决定速率的主要因素。因此,该前体药物的释放速率基本是该前体药物在周围水性介质中溶解的速率。这种释放速率几乎基本上与时间成线性(所谓的零级动力学)。
本发明的系统可以通过将一种或多种适宜的单体和适宜的低溶解度的药学前体药物进行混合,然后将单体聚合形成该聚合体系来形成。这样,该前体药物就被溶解或分散于该聚合物中。在其它实施方案中,该前体药物被混合到一种液态聚合物或聚合物分散体中,然后对该聚合物进行进一步的处理从而形成本发明的系统。适宜的进一步的处理包括与适宜的交联前体药物的交联、该液态聚合物或聚合物分散体的进一步聚合、与适宜单体的共聚、与适宜聚合物段的嵌段共聚等等。该进一步的处理可将该药物捕获在该聚合物中从而使得药物被混悬或分散于该聚合物基质中。
在本发明的一些实施方案中,可以将用于形成聚合物的单体与本发明低溶解度的化合物相结合并将其混合以制备本发明化合物在单体溶液中的均匀的分散体。然后,根据常规的涂布法将该分散体应用到斯滕特固定模上,之后,用常规引发剂如UV线来开始交联过程。在本发明的其它实施方案中,将该聚合物组合物与本发明低溶解度的化合物相结合以形成一种分散体。然后将该分散体应用到斯滕特固定模上,并将该聚合物进行交联从而形成一种固体涂层。在本发明的其它实施方案中,将聚合物和本发明低溶解度的化合物与适宜的溶剂进行合并从而形成一种分散体,然后以常规方式将其应用到斯滕特固定模上。然后用常规方法如热蒸发来除去溶剂,结果,(一起形成缓释的药物传递系统的)该聚合物和本发明低溶解度的药物以涂层形式留在该斯滕特固定模上。在将本发明低溶解度的药学化合物溶解于该聚合物组合物中的情况中可以使用类似的方法。
在本发明的一些实施方案中,该系统包含一种相对坚硬的聚合物。在其它实施方案中,该系统包含一种柔软并有延展性的聚合物。还是在其它实施方案中,该系统包括一种具有粘着性的聚合物。正如在下面详细讨论的那样,根据该系统特定的最终物理形式,该聚合物的硬度、弹性、粘着性、以及其它特性可以在很宽的范围内变化。
本发明该系统的实施方案可以有许多不同的形式。在一些实施方案中,该系统包括低溶解度的前体药物,即混悬或分散于该聚合物中的前体药物。在某些其它实施方案中,该系统包括前体药物和半固态或凝胶形式的聚合物,其适于通过注射器被注射到体内。在本发明的其它实施方案中,该系统包括前体药物和柔软-可变形的聚合物,其适于通过适宜的手术方法被插入或植入到体内。还是在本发明另一些实施方案中,该系统包括硬的固态聚合物,其适于通过适宜的手术方法被插入或植入到体内。在本发明另外的实施方案中,该系统包含具有混悬或溶解于其中的低溶解度前体药物的适于吸入的聚合物。在另外的实施方案中,该系统包含具有混悬或分散于其中的前体药物的聚合物,其中所说的前体药物和聚合物的混合物可在手术器具上形成一种涂层,其中所说的手术器具如螺钉、斯滕特固定模、起搏器等等。在本发明特定的实施方案中,该装置由一种硬固体聚合物所组成,其被成形成手术器具如手术螺钉、板、斯滕特固定模等等或其一部分的形式。在本发明的其它实施方案中,该系统包括一种聚合物,该聚合物是具有分散或混悬于其中的药物的缝合线的形式。
在本发明的一些实施方案中,提供了一种包含具有表面如外表面的底物和在该外表面上的涂层的医学装置。该涂层包含一种聚合物和分散在该聚合物中的具有低溶解度的前体药物,其中所说的聚合物对于该前体药物而言是可渗透的并且对于该前体药物从聚合物中的释放速率而言基本不是释放速率的限制因素。在本发明的某些实施方案中,该装置包含混悬或分散于适宜聚合物中的前体药物,其中该前体药物和聚合物被涂布到整个底物例如手术器具上。可以通过喷涂或浸涂来完成该类涂布。
在本发明的其它实施方案中,该装置包含一种前体药物和聚合物混悬液或分散体,其中所说的聚合物是坚硬的,并且形成被插入或植入到体内的装置的组成部分。例如,在本发明的特定实施方案中,该装置是用混悬或分散于在该聚合物中的前体药物进行了涂布的手术螺钉、斯滕特固定模、起搏器等等。在本发明另一个特定的实施方案中,其中混悬有前体药物的该聚合物形成一种手术螺钉的尖端、帽或其一部分。在本发明的其它实施方案中,其中混悬或分散有前体药物的该聚合物被涂布到一种手术器具如手术管(如结肠造口术、腹膜灌洗、导管和静脉内管)上。还是在本发明另一个实施方案中,该装置是具有聚合物和涂布于其上的前体药物(例如抗凝剂的前体药物如肝素或其复合药物)的静脉内的针。
正如上面所讨论的那样,本发明的装置包含可生物侵蚀或不可生物侵蚀的聚合物。选择可生物侵蚀还是不可生物侵蚀的聚合物是以该体系或装置所需的最终应用为基础而作出的。在本发明的一些实施方案中,该聚合物有利地是可生物侵蚀的。例如,在该系统是手术可植入装置如螺钉、斯滕特固定模、起博器等等上的涂层的情况中,该聚合物有利地是可生物侵蚀的。本发明其中该聚合物有利地是生物可侵蚀性聚合物的其它实施方案包括的装置是在聚合物中的前体药物的可植入、可吸入或可注射的混悬液或分散体,其中不使用另外的部件(如螺钉或锚凹)。
在本发明一些其中该聚合物的可渗透性和生物侵蚀性差的实施方案中,该聚合物生物侵蚀的速率有利地远远慢于药物的释放速率,从而使得在药物被释放后相当长的时期内该聚合物仍然留在原位,但是其最终会被生物侵蚀并被吸收到周围组织中。例如,在该装置是包含混悬或分散于一种生物可侵蚀的聚合物中的药物的生物可侵蚀的缝合线的情况中,该聚合物的生物侵蚀速率有利地慢到足以使药物可以在约3至14天的时间内以线性方式进行释放,而该缝合线将持续存在约三周至约六个月的时间。本发明的类似装置包括包含混悬或分散于一种生物可侵蚀聚合物中的前体药物的手术用U型钉。
在本发明的其它实施方案中,该聚合物生物侵蚀的速率有利地与药物释放速率是相同的级数。例如,在该系统包含混悬或分散于被涂布到手术器具上的聚合物中的前体药物的情况中,该聚合物有利地以可以使得随着时间的流逝,直接与周围机体组织接触的前体药物的表面积基本保持恒定的速率被生物侵蚀,其中所说的手术器具如矫形用螺钉、斯滕特固定模、起搏器、或不可生物侵蚀的缝合线。
在本发明的一些实施方案中,该聚合物是不可生物侵蚀的,或者仅以比该低溶解度的药学前体药物的溶解速率低的速率被生物侵蚀,并且颗粒的直径是使得当该涂层被应用到斯滕特固定模上时,该颗粒的表面暴露于周围组织的直径。在该类实施方案中,该低溶解度前体药物的溶解与该颗粒所暴露的表面积成比例。
在本发明的其它实施方案中,该聚合物载体对于周围组织例如血浆中的水而言是可渗透的。在该类情况中,水溶液可以渗透到该聚合物中,从而接触到该低溶解度的药学前体药物。可以通过多种变量来对溶出速率进行控制,其中所说的变量如聚合物的渗透性、该低溶解度药学前体药物的溶解度、生理学液体的pH、离子浓度、以及蛋白质组成等等。但是,在某些实施方案中,可以对渗透性进行调节以使得主要通过该低溶解度药学前体药物在周围液相中的溶解度来对溶出速率进行控制,或在一些情况中几乎完全是通过这种方法来对溶出速率进行控制的。
在本发明的一些实施方案中,该聚合物不可生物侵蚀的。不可生物侵蚀的聚合物在该系统包括将被涂布到手术器具或形成该手术器具的组成部分的聚合物的情况中尤其有用,其中所说的手术器具适于被永久或半永久地插入或植入到体内。其中该聚合物有利地形成手术装置上的永久涂层的装置实例包括矫形用螺钉、斯滕特固定模、关节修复物、人工瓣膜、永久的缝合线、起搏器等等。
本发明的手术系统是以适于发挥所需疗效的方式被使用的。例如,在本发明的一些实施方案中,给药方式有利地是注射给药。在该类情况中,该系统是一种液体,通过将该系统吸入到注射器的管内并通过针将其注射到所需部位来将该系统引入到所需的部位。该类给药方式适于肌内注射,例如杀微生物剂缓释制剂的肌内注射,其中所说的杀微生物剂包括抗生素、抗病毒剂、和甾类物质。在需要激素类物质缓释治效的情况中也可以使用这种给药方式,其中所说的激素类物质如甲状腺药物、控制生育的前体药物、用于雌激素治疗的雌激素等等。熟练的临床医师将意识到这种给药方式适于各种治疗环境,并且将可以通过对特定聚合物和系统的药物进行调整以获得所需疗效。
在给药方式是注射给药的本发明的实施方案中,该体系有利地是混悬或分散于粘性聚合物载体中的相对非极性的药物。在该类情况中,该系统是一种非极性药物在液态聚合物载体中的稳定的混悬液或分散体。该聚合物载体有利地是不可生物侵蚀的或将以低于药物扩散到周围组织中的速率被生物侵蚀。在该类情况中,相对于周围组织而言,该系统适当地留在原地,防止药物过早地释放到周围组织中。
在本发明的其它实施方案中,该系统是混悬或分散于一种液态聚合物中的相对非极性的液体。在该类情况中,该系统进一步包含可以使得该相对非极性的药物在该聚合物内维持稳定的分散状态的乳化剂。该聚合物载体有利地是不可生物侵蚀的或者以低于药物扩散速率的速率被生物侵蚀,从而使得在药物释放的整个时期,相对于周围组织而言该系统都能维持药物的定位。
本发明系统的确切性质将取决于所需的治疗应用、被混入到该系统中的药物在生理条件下的物理状态等等。
在本发明的一些实施方案中,本发明的系统有利地是适于植入,例如皮下植入等的一定形状和形式的固体装置。在本发明的一些实施方案中,该系统是伸延的卵形体形状,前体药物是非极性的药物,如激素,并且该聚合物是一种固体聚合物,该聚合物的渗透性是使得其不是限制药物释放速率的主要速率决定因素的渗透性。在本发明的特定实施方案中,该聚合物是可生物侵蚀的。在本发明的其它实施方案中,该聚合物是不可生物侵蚀的。
在其中该装置包含一种底物和位于该底物上的涂层的本发明实施方案中,如在螺钉、斯滕特固定模、起搏器、关节修复物等等情况中,该装置基本以相关现有技术中手术器具的方式被应用。例如,包含用含有混悬或分散于聚合物中的低溶解度的前体药物的组合物进行涂布的螺钉的本发明装置以与现有技术中螺钉的使用方式相同的方式被拧到骨中,其中所说的低溶解度的前体药物如抗生物或FU-萘普生。然后,本发明的螺钉以持续时间的方式将药物缓释到环绕着该装置的组织如肌肉、骨、血液等等中,从而提供了治疗益处,如抗菌、抗炎和抗病毒作用。
在本说明书和所附的权利要求中所用的“缓释”指的是通过速率动力学进行释放,对于药物的释放速率而言,聚合物的渗透性不是限速因素。
在其中该装置是一种前体药物和聚合物已经被作为组成部分混入到其中的手术器具的本发明的实施方案中,该聚合物有利地是一种具有适于该装置的特定应用的物理性质的固体。例如,在该装置是缝合线的情况中,该聚合物将具有适用于特定手术情况的强度和生物可侵蚀性。在该装置是螺钉、斯滕特固定模等等的情况中,该聚合物有利地是一种可形成该手术器具的至少一部分的坚硬固体。在本发明的特定实施方案中,如在该系统是关节修复物的一部分的情况中,该聚合物有利地是不可生物侵蚀的,并且在药物已经被释放到周围组织中之后仍然留在原位。在本发明的其它实施方案中,如在生物可侵蚀的缝合线的情况中,该聚合物在基本释放了所有的前体药物后被生物侵蚀。
虽然在涉及再狭窄和经皮管腔内(transluminal)冠状血管成形术后相关并发症的治疗时将对本发明实例性的实施方案进行描述,但是注意到可以用药物/药物组合的局部传递来治疗使用任何数量的医学装置的各种情况或增强该装置的功能和/或寿命是很重要的。例如,在白内障手术后被放置以恢复视力的眼内透镜常常受到所形成的继发性白内障的损害。继发性白内障常常是细胞在透镜表面的过度生长的结果并且可能可以通过将药物或一些药物与该装置联用来将其最小化。常常由于组织向内生长或蛋白质物质在该装置内、上和该装置周围的积聚而不能使用的其它医学装置如用于脑积水的分流器、透析移植物、结肠开口术的袋连接装置、耳朵导液管、用于心房脉冲产生器和可植入的去纤颤器的导线也可以由该装置-药物组合法而获益。
当与适当的前体药物或复合药物联用时,用于改善组织或器官的结构和功能的装置也表现出受益情况。例如,可能可以通过将其与前体药物如骨形态形成蛋白联用来改善用于增加被移植装置稳定性的矫形用装置的骨一体化。同样,用这种药物-装置组合法使得其它手术装置、缝合线、U型钉、吻合装置、椎盘、骨钉、缝合线锚凹、止血屏障、夹子、螺钉、板、回形针、脉管植入物、组织粘合剂和密封剂、组织支架、各种类型的敷料、骨替代物、管腔内装置、以及血管支持器也可以提供增强的患者受益效果。实际上,任何类型的医学装置都可以以一些方式用前体药物或复合药物来进行涂布,其治疗效果优于只使用该装置或药学前体药物所获得的治疗效果。
可以用主题装置来传递的药物有,例如:抗增生/抗有丝分裂的前体药物,包括天然产物如长春花属生物碱类(即长春碱、长春新碱、和维诺利宾)、紫杉醇、epidipodophyllotoxins(即依托泊苷、替尼泊苷)、抗生素(更生霉素(放线菌素D)柔红霉素、阿霉素和伊达比星)、蒽环素类、米托蒽醌、博莱霉素、普卡霉素(光辉霉素)和丝裂霉素、酶类(全身代谢L-天冬酰胺并剥夺了细胞合成其自身的天冬酰胺能力的L-天门冬酰胺酶);抗血小板的前体药物;抗增生/抗有丝分裂的烷化剂类前体药物如氮芥类(恩比兴、环磷酰胺以及类似物、美法仑、苯丁酸氮芥)、氮丙啶类和甲基蜜胺类(六甲蜜胺和噻替派)、烷基磺酸酯-白消安、亚硝基脲(卡莫司汀(BCNU)和类似物、链脲霉素)、trazenes-达卡巴嗪(DTIC);抗增生/抗有丝分裂的抗代谢物如叶酸类似物(甲氨蝶呤)、嘧啶类似物(氟尿嘧啶、氟尿苷、和阿糖胞苷)、嘌呤类似物和相关的抑制剂(巯基嘌呤、硫鸟嘌呤、喷司他丁和2-氯脱氧腺苷克拉屈滨);铂配位络合物(顺铂、卡铂)、甲基苄肼、羟基脲、米托坦、氨鲁米特;激素类物质(即雌激素);抗凝剂(肝素、合成的肝素盐类和其它凝血酶抑制剂);溶解纤维蛋白的前体药物(如组织纤溶酶原活化剂、链激酶和尿激酶)、阿司匹林、双密达莫、噻氯匹定、氯吡格雷、阿昔单抗;抗转移剂;抗分泌剂(breveldin);抗炎剂:如肾上腺皮质类固醇物质(皮质醇、可的松、氟氢可的松、强的松、强的松龙、6U-甲泼松龙、去炎松、倍他米松、和地塞米松)、非载体类前体药物(水杨酸衍生物,即阿司匹林;对-氨基酚衍生物即acetominophen;吲哚和茚乙酸类物质(消炎痛、舒林酸和依托度酸(etodalac))、杂芳基乙酸类物质(托美丁、双氯酚酸、和酮咯酸),芳基丙酸类物质(布洛芬和衍生物)、邻氨基苯甲酸类物质(甲灭酸、以及甲氯灭酸)、烯醇酸类(吡罗昔康、替诺西康、保泰松和oxyphenthatrazone)、萘丁美酮、金化合物(金诺芬、金硫葡萄糖、硫代苹果酸金钠);免疫抑制剂(环胞菌素、他克莫司(FK-506)、西罗莫司(雷帕霉素)、硫唑嘌呤、霉酚酸莫非替克);生血管的前体药物:血管内皮生长因子(VEGF)、成纤维细胞生长因子(FGF);血管紧张素受体阻滞剂;一氧化氮供体;反义寡聚核苷酸及其组合;细胞循环抑制剂、mTOR抑制剂和生长因子信号转导激酶抑制剂。
在某些实施方案中,该前体药物是用阿片物质来形成的。阿片类物质的实例包括吗啡衍生物,如阿扑吗啡、丁丙诺啡、可待因、二氢可待因、二氢埃托啡、二丙诺啡、埃托啡、氢可酮、氢吗啡酮、羟甲左吗南、哌替啶、美托酮、邻-甲基纳曲酮、吗啡、纳洛酮、纳曲酮、去甲吗啡、氧可酮、和氧吗啡酮。在其它实施方案中,该阿片物质是一种芬太尼衍生物,其可被衍生化成前体药物,如β-羟基-3-甲基芬太尼。
在涉及该低溶解度的药学前体药物时所用的术语“低溶解度”指的是该药学前体药物在生物流体如血浆、淋巴液、腹膜液等等中的溶解度。低溶解度一般指的是该药学前体药物在pH为约5至约8的水性溶液,特别是生理性溶液如血液、血浆等等中仅十分微溶。本发明一些低溶解度的前体药物具有小于约1mg/ml,的溶解度,其溶解度小于约100μg/ml,优选地小于约20μg/ml,更优选地小于约15μg/ml,并且更优选地小于约10μg/ml。除非特别说明,否则溶解度是指在25℃下在水中的溶解度,例如其是用在1995 USP中所描述的方法来进行测量的。这包括微溶(约10mg/ml至约1mg/ml),十分微溶(约1mg/ml至约0.1mg/ml)的化合物和特别不溶或不溶的化合物(小于约0.01mg/ml)。
用于本发明的适宜的前体药物包括下列物质的前体药物:免疫响应改性剂如环胞菌素A和FK 506、皮质类固醇如地塞米松和曲安奈德、抑制血管的甾类物质如三羟基甾类物质、抗寄生虫的前体药物如阿托伐醌、治疗青光眼的前体药物如利尿酸、包括环丙沙星在内的抗生素、分化调节剂如类维生素A类物质(例如,反式-视黄酸、顺式-视黄酸及类似物)、包括高分子量的低(10-链节)反义化合物在内的抗病毒的前体药物、抗癌剂前体药物如BCNU、非甾体抗炎药前体药物如消炎痛和氟比洛芬、和包含通过可逆的共价或离子键相连的至少两种化合物的共轭物的前体药物,其中所说的键在体内的所需部位能裂解产生每个化合物的活性形式。在本发明的实施方案中,该前体药物在水性介质中相对不溶,其中所说的水性介质包括生理性流体如血清、粘液、腹膜液、边缘液等等。还是在本发明的实施方案中,适宜的前体药物包括亲水性药物的亲脂性衍生物类药物,其在可接近的生理条件下易于转化成其亲水性的药物形式。可以参考任何标准药学教科书中用于获得药物的低溶解度形式的方法。在这方面,本发明尤其适用于迄今为止由于其固有的低溶解性而没有被广泛应用的前体药物或仅在以油为基础或以其它脂类物质为基础的传递载体中被有限应用的前体药物。在某些实施方案中,本发明提供了一种用于植入到血管腔内,特别是管腔内的损害附近以维持管腔的开放性的管腔内医学装置,其中所说的管腔内的损害如动脉粥样硬化性损害。本发明特别是提供了一种辐射状延伸的可扩展的管状斯滕特固定模,其具有内腔表面和相对的沿着纵向斯滕特固定模轴延伸的外表面,该斯滕特固定模在其内表面或外表面的至少一部分上具有涂层。由斯滕特固定模进行的药物组合的局部传递具有如下的优点;即,防止了脉管的反冲并通过该斯滕特固定模的支持作用对其进行了重塑,并且预防了新内膜增生或再狭窄的多种因素,以及降低了炎症和血栓形成。这种药物向斯滕特固定模固定的冠状动脉的局部给药还具有另外的治疗益处。例如,使用局部传递可以获得比全身给药更高的组织浓度。此外,使用局部传递在维持较高组织浓度的同时可以获得比全身给药低的全身毒性。使用由斯滕特固定模进行的局部传递还可以获得一种比全身给药的方法更简单的病人顺从性更好的简单方法。组合药物治疗的另一个益处是可以降低各治疗药物、前体药物或化合物的剂量,从而限制了它们的毒性,并且同时仍然降低了再狭窄、炎症和血栓形成。因此,以斯滕特固定模为基础的局部治疗是一种改善抗再狭窄、抗炎、抗血栓形成药物、前体药物或化合物的治疗比率(功效/毒性)的方法。
在经皮腔内冠状血管成形术后可以使用各种不同的斯滕特固定模。虽然根据本发明可以使用任何数目的斯滕特固定模,但是为了简单起见,在本发明的实例性实施方案中将对有限数目的斯滕特固定模进行描述。熟练技术人员将意识到在与本发明有关的情况中可以使用任何数量的斯滕特固定模。此外,如上所述的那样,还可以使用其它医学装置。
常用的斯滕特固定模是一种留在管腔内以减轻阻塞的管状结构。斯滕特固定模通常以未扩张的形式被插入到腔内,然后自动膨胀或在原位上在第二种装置的帮助下进行膨胀。一种典型的膨胀方法是通过使用一种装有导管的血管成形术囊来进行的,所说的囊在狭窄的脉管或体腔内被膨胀以切断或破裂与该脉管的壁组分有关的梗塞,从而获得被扩大的腔。
本发明的斯滕特固定模可以用许多方法来进行制造。例如,该斯滕特固定模可以由一种空心或成形的不锈钢管来进行制造,所说的不锈钢管可以用激光、电冲压机(electric discharge milling)、化学侵蚀或其它手段来进行机械加工。将斯滕特固定模插入到体内并以未膨胀的形式放置到所需部位。在一个实例性实施方案中,可以通过一种气囊导管来完成其在血管内的膨胀,其中该斯滕特固定模的最终直径是所用气囊导管直径的函数。
应当意识到本发明的斯滕特固定模可以被包含于一种形状记忆材料中,其中所说的形状记忆材料包括例如适宜的镍钛合金或不锈钢。
由不锈钢管形成的结构可以是通过以预定方式来使该不锈钢管成形而形成的自动膨胀的结构,例如可以将其扭曲成辫状构型。在这种实施方案中,在已经形成该斯滕特固定模后,可以将其压缩以使其所占空间足够小从而可以通过插入工具将其插入到血管或其它组织中,其中所说的插入工具包括适宜的导管、或柔软的杆。
一旦从该导管中被排出,则该斯滕特固定模可以膨胀形成所需的构型,其中该膨胀可以自动完成或可以通过压力、温度的改变或电刺激而被引发。
不管该斯滕特固定模的设计如何,其优选地具有以足够的特性和足够的浓度被应用从而可在损害区域提供有效剂量的药物组合剂量。在这一点上,在涂层中的“储库大小”优选地是足以在所需部位并以所需数量使用该药物组合剂量的大小。
在供选择的实例性实施方案中,可以以治疗剂量用药物/药物组合来对该斯滕特固定模的整个内和外表面进行涂布。但是,重要的是要注意到涂布技术可以根据该药物组合来进行变化。该涂布技术也可以根据该包含斯滕特固定模或其它管腔内医学装置的材料来进行变化。
图3和4描述了本发明一种管腔内装置(斯滕特固定模)的实施方案。
图3表示了一种优选的未展开状态的具有用缓释药物传递系统进行了涂布的表面的辐射状延伸的可扩展的管状斯滕特固定模13的侧面平面图。如图3所示,在未展开状态下,该斯滕特固定模13具有其沿径向方向的外边界14A、14B。该斯滕特固定模13的内腔表面15、外表面16、或整个表面都可以用缓释药物传递系统进行涂布或包含一种缓释的药物传递系统。在血管扩展操作中,该内腔表面15与体液如血液相接触,同时,当该斯滕特固定模13被展开以支撑并扩大该生物学脉管或管道时,外表面16与组织相接触。
在一个供选择的实施方案中,当斯滕特固定模被展开时,可以用连接两个或多个相邻部件或该斯滕特固定模结构13的圈的可存在或不存在的增强丝17来将该斯滕特固定模锁定和/或维持在其扩张状态。这种增强丝17可以由镍钛合金或其它高强度的材料制成。一种镍钛合金装置众所周知地具有预成形的形状和所说镍钛合金装置能恢复成其预定形状的转化温度。一种用本发明进行了表面涂布的斯滕特固定模13对患者的管腔内组织进行处理的方法包括将可放射状扩展的管状斯滕特固定模进行折叠和该折叠的斯滕特固定模从患者体的收回。将可放射状扩展的管状斯滕特固定模进行折叠的操作可以通过升高温度使得增强丝17反转成其伸直状态或其它适宜状态从而造成该斯滕特固定模13的折叠以将所说的斯滕特固定模从患者体内取出来完成。
图4表示处于展开状态的具有涂布的斯滕特固定模表面的缓释药物传递系统的放射状伸展的可扩展的管状斯滕特固定模的全貌图。如图4所示,该位于展开状态的斯滕特固定模13具有径向方向的外边界24A、24B。该斯滕特固定模13的内腔表面14、外表面16或其整个表面都可以用该缓释药物传递系统进行涂布或可包含该缓释的药物传递系统。在血管扩展操作中,其内腔表面15与体液如血液相接触,而当该斯滕特固定模13展开以支持和扩展该生理学脉管时,其外表面16与组织相接触。可以用增强丝17来将扩展的斯滕特固定模维持在其扩张状态,使其作为永久性的斯滕特固定模或临时的斯滕特固定模。在作为临时性斯滕特固定模的表面进行了涂布的斯滕特固定模13的情况中,该增强丝17可具有使该扩张的斯滕特固定模折叠的能力。
斯滕特固定模的展开可以通过传递导管上的囊来完成或通过在预加应力的斯滕特固定模从传递脉管中时放出来时自动扩张来完成。用于斯滕特固定模的扩张的传递导管和方法对于本领域技术人员而言是众所周知的。该可扩展的斯滕特固定模13可以是可自动扩张的斯滕特固定模、囊-扩张的斯滕特固定模、或可扩张-可缩回的斯滕特固定模。
该可扩展的斯滕特固定模可以由记忆线圈、网眼材料等等组成。
III.
实施例
通过参考下面的实施例可以对本发明进行更充分的理解。
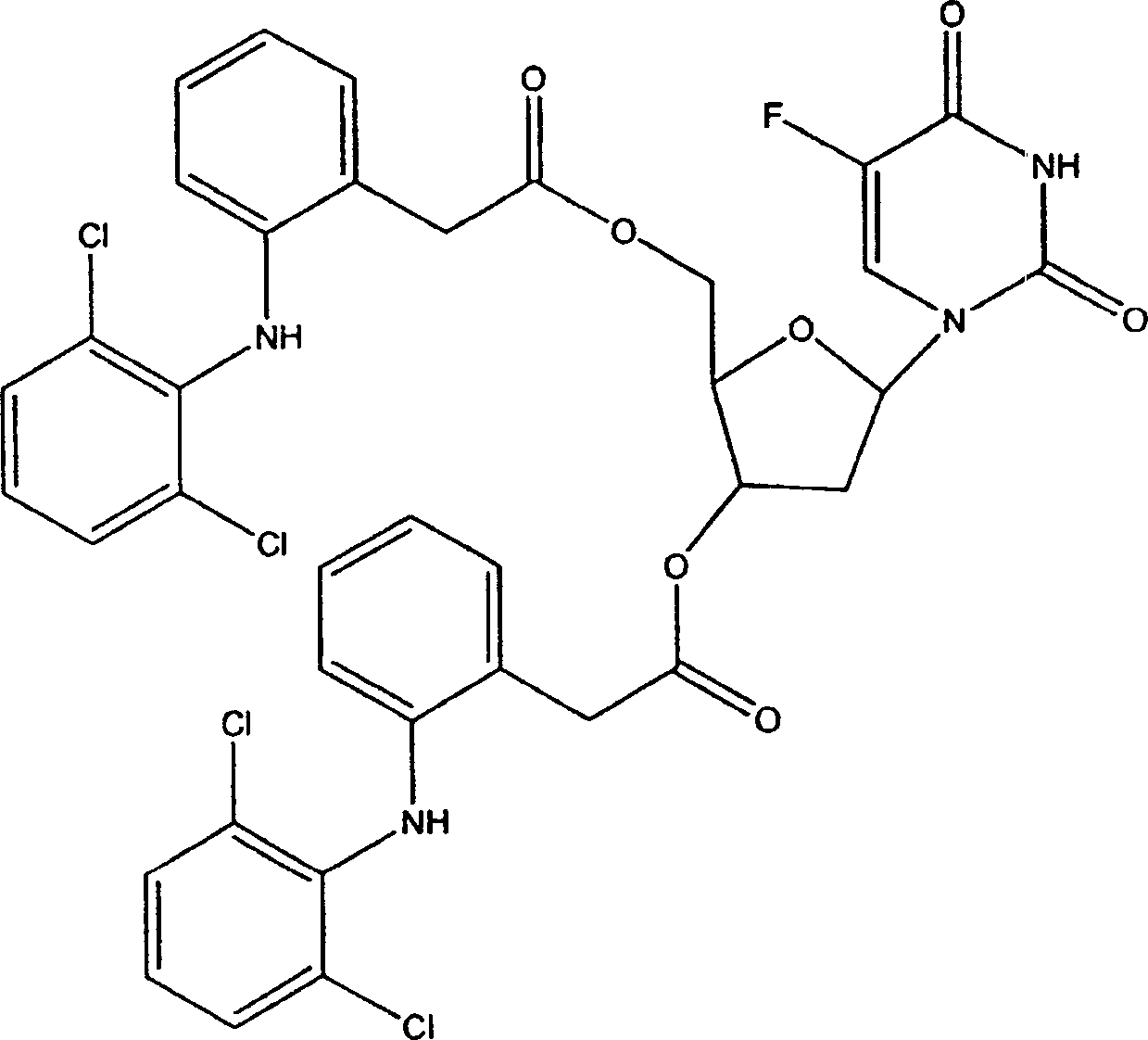
包含通过可逆的共价键相连的5-氟尿嘧啶和萘普生的共轭物的前体药物TC-112和包含5-氟尿嘧啶和氟轻松的共轭物的前体药物G.531.1是根据US 6,051,576中所描述的方法来进行制备的。将这些化合物的结构复制如下:
5-氟尿嘧啶(5-FU)
萘普生
用下面的实施例来对所公开的本发明进行说明。该实施例不是要进行限制,熟练技术人员将意识到其它实施方案也在所公开的本发明的范围内。
实施例1
向20gm 10%(w/v)水性聚(乙烯醇)(PVA)溶液中分散80.5mg前体药物TC-112。然后将5块玻璃片用这种TC-112/PVA混悬液进行浸涂,然后将其风干。然后将该涂布和风干再重复进行四次。在结束时在各玻璃片上涂布了约100mg TC-112/PVA。然后,将进行了涂布的玻璃片在135℃下热处理5小时。在冷却至室温后,将该玻璃片分别放置在20ml 0.1M mol磷酸盐缓冲剂(pH 7.4,37℃)中进行释放试验。每天取样并在各次取样时将整个缓释介质用新鲜介质进行替换。通过反相HPLC来测定释放到介质中的药物和TC-112。TC-112在pH 7.4的缓冲剂中的半衰期为456分钟,在血清中为14分钟。
结果如图1所示,其表明了TC-112从PVA涂布的玻璃片上的总的累积释放。曲线的斜率表明TC-112的释放为10μg/天。数据既表示了化合物TC-112的完整形式又表明了其组分。
实施例2
将12.0gm硅氧烷部分A(Med-6810A)与1.2gm硅氧烷部分B(Med-6810B)进行混合,将其在超声波仪中脱气10分钟,然后用水泵进行处理。将41.2mg(TC-112)分散在这种脱气的硅氧烷中,再对其进行脱气。将0.2gm该混合物涂在玻璃片的一个表面上。然后将该玻璃片(一共5片)放在烘箱中并将其在105℃下加热20分钟以使其固化。在将其从烘箱中取出并冷却至室温后,将0.2gm该混合物涂敷在各玻璃片另一个未进行涂布的表面上。然后将该进行了涂布的玻璃片再次在105℃下热处理20分钟。在冷却至室温后,将各玻璃片分别放置在20ml 0.1M磷酸盐缓冲剂(pH 7.4,37℃)中来进行释放试验。每天取样,并在各次取样时间将整个缓释介质用新鲜介质进行替换。用HPLC对释放到介质中的药物(5FU和TA)和TC-112进行测定。
硅氧烷涂层的总TC-112释放的计算如下。萘普生的分子量是230.3,5-氟尿嘧啶的分子量是130.1,而得自着两种药物的本发明的化合物(TC-112)具有372.4的分子量。对于要探测的萘普生的xmg而言,这意味着有x*372.4/230.3mg的TC-112被水解。所释放的总TC-112等于在释放介质中所检测到的TC-112和被水解的TC-112的总和。例如,在最高至第6天时,检测到43.9mg萘普生,这意味着71.0(43.9*372.4/230.3)mg TC-112被水解,同时,在缓冲剂中检测到51.4mg TC-112,因此,在最高到第6天时一共有112.4mg(51.4加71.0)TC-112被释放。
结果如图2所示,其表明了TC-112从硅氧烷涂布的玻璃片上的总的累积释放。该曲线的斜率表明TC-112的释放为13.3μg/天。该数据又表示了完整的本发明的化合物以及本发明化合物的组成部分。斜率的相似性证明该聚合物对药物的释放几乎没有影响。
实施例3
通过将两种分散体一起进行混合来制备都是在二甲基乙酰胺(DMAC)(1∶10,w/w)中的包含0.3gm Chronoflex C (65D)的3.3gmChronoflex C(65D)(Lot# CTB-G25B-1234)分散体和包含0.2gmChronoflex C(55D)的2.2gm Chronoflex C(55D)(Lot# CTB-121B-1265)分散体的混合物。向这种混合物中加入6.0gm四氢呋喃(HPLC级)并将其进行混合。最后的混合物不是澄清的溶液。然后向其中加入101.5mg 5-氟尿嘧啶(5FU)和曲安奈德(TA)的复合药物(该复合药物被定义为“TC-32”)并将其溶解于该聚合物溶液中。
然后通过浸渍将十个(10)HPLC插入物用该聚合物/TC-32溶液进行涂布,然后将其在环境温度下风干。将该涂布和风干过程重复四(4)次(一共5次),直至向各插入物上一共使用了约10mg聚合物/TC-32。然后将该插入物在80℃的烘箱中放置2小时以除去残余的溶剂。
将该插入物分别放置在位于玻璃管中的20ml 0.1m磷酸盐缓冲剂,pH 7.4中并开始在37℃下监测化合物从该插入物上的释放。每天取样,并在各次取样时间将整个介质用新鲜介质进行替换。用HPLC来测定释放到介质中的药物。因为TC-32在缓冲剂中的半衰期短,所以在该释放介质中没有检测到TC-32;仅能检测到一定数量的母体药物、5-FU和TA。释放曲线如图7所示。
实施例4
向5.0gm进行着搅拌的二甲基乙酰胺(DMAC)中加入300mgChronof1ex C(65D)(Lot# CTB-G25B-1234)和200mg ChronoflexC(55D)(Lot# CTB-121B-1265)。将聚合物缓慢地溶解于DMAC(约4小时)中。然后向该聚合物分散体中加入5.0gm THF。该混合物不是一种澄清的溶液。然后向其中加入100.9mg TC-32并将其溶解于该混合物中。
然后通过浸渍将三个(3)由Guidant Corp提供的斯滕特固定模用该聚合物/TC-32溶液进行涂布,然后将其在环境温度下风干。将该涂布和风干过程重复几次直至向该斯滕特固定模上一共使用了约2.0mg的聚合物/TC-32。将该进行了涂布的斯滕特固定模在一种生物学安全的小室内在环境温度下风干一整夜。然后将该斯滕特固定模在80℃下真空干燥2小时以除去残余的溶剂。然后将其分别放置于位于玻璃管中的5.0ml 0.1m磷酸盐缓冲剂,pH 7.4中,开始在37℃下监测化合物从该斯滕特固定模的释放。每天取样,并在各次取样时将整个介质用新鲜介质进行替换。用HPLC对介质中的药物释放进行测定。释放曲线如图8所示。在释放介质中没有检测到TC-32。
实施例5
首先将聚氨酯(PU)溶解于四氢呋喃中。将生物学可逆的5-FU和TA的共轭物溶解于这种溶液中并将所得的溶液喷涂到冠状的由Guidant制造的Tetra斯滕特固定模上。在风干后,将斯滕特固定模在50℃下真空干燥2小时以除去溶剂残余物,然后将其进行血浆处理和γ-辐射。向斯滕特固定模应用两种不同水平的药物填充量:80ug低剂量(13%)和600ug高剂量(60%)。在37℃下,通过将进行了涂布的斯滕特固定模(扩张开的)放在0.1M磷酸盐缓冲剂(pH 7.4)中来在体外对释放速率进行测定。定期从该缓冲液中取样来进行HPLC分析,并对该缓冲液进行替换以避免任何饱和效应。
图9所示的结果说明了高剂量涂布的斯滕特固定模的体外释放模式。该模式遵从一种假对数模式,在10周内释放了约70%。在负载了高剂量和低剂量的斯滕特固定模中都观察到了相似的模式。在试验的所有期间TA和5-FU一直是以等摩尔的方式进行释放的。在释放介质中没有检测到5-FU/TA的复合药物。
实施例6
将聚氨酯(1.008gm)添加到50.0gm四氢呋喃(THF)中。将该混合物搅拌一整夜以使该聚合物溶解。将5.0gm的聚合物溶液用10.0gmTHF进行稀释。向该聚合物溶液中加入150.2mg 5-氟尿嘧啶(5FU)和曲安奈德(TA)的复合药物(该复合药物被定义为“TC-32”)并使其溶解。该涂布液是用60%复合药物填充量来进行制备的。还制备负载了13%复合药物的涂布溶液。将裸露的斯滕特固定模(Tetra,Guidant,Lot# 1092154,13mm Tetra)用异丙醇进行洗涤,风干,然后通过使用精密喷枪用该涂布溶液对其进行喷涂。重复进行涂布直至向每一斯滕特固定模上一共使用了约1.0mg的涂层。将进行了涂布的斯滕特固定模在50℃下真空干燥2小时以除去溶剂残余物,然后将其进行血浆处理和γ-辐射。
在两组中对该复合物药物涂布的斯滕特固定模进行试验。将组一的斯滕特固定模各自放置到包含5.0ml 0.1M磷酸盐缓冲剂(pH 7.4)的玻璃管中。定期取样并用HPLC对该缓冲剂中的复合药物浓度进行检验。在每次取样后将整个释放介质进行替换。
将组二的斯滕特固定模放置在体内。在研究的第1天将进行了TC-32涂布的斯滕特固定模植入到三只普通猪的左前方下行(LAD)冠状动脉中。在研究的第5天收集该斯滕特固定模并如组一斯滕特固定模所述的那样将其放置在0.1M磷酸盐缓冲剂中。用HPLC对释放到该基质中的各药物的数量进行测量。在释放介质中检测不到完整的复合药物。
结果如图10所示,其表明了扩张的斯滕特固定模和未植入的斯滕特固定模之间药物释放曲线的比较。扩张的和植入前的斯滕特固定模的释放方式都表明可以通过体外释放方式来对体内释放进行预测。
实施例7
十四只(14)家猪在三个心外膜冠状动脉(LAD、LCX和RCA)中的任何一个中接受三个(3)扩展到最大程度的斯滕特固定模。给一些动物仅使用对照斯滕特固定模,包括在Cross Sail Rx囊传递系统(对照)上的未加涂饰的Bare Metal Tetra冠状动脉斯滕特固定模,或CrossSail Rx囊传递系统(对照)上的PU涂布的Tetra冠状动脉斯滕特固定模。给其它动物使用以低剂量((80μg TA+5FU(13%)))或高剂量(600μg TA+5FU(60%))涂布了药物的斯滕特固定模。将该斯滕特固定模移植到动物的动脉中。将各斯滕特固定模垫付到动脉中的所需部位,用一种膨胀装置使其扩张。对该膨胀装置的压力进行选择以得到一种使动脉比例为1.1-1.2∶1的囊。
在28天后,手术切除直接与该斯滕特固定模直接相邻的动脉部分,并将其嵌入到甲基丙烯酸酯树脂中。切下组织学上5-μm的部分并将其用维尔赫夫氏弹性蛋白和苏木精和曙红染色剂进行染色,并测量各切下部分的厚度。高剂量和低剂量药物涂布的斯滕特固定模的结果被列于下表中。低剂量和高剂量实验组第28天的响应表明由于TA和5FU从该聚合物涂布的Tetra斯滕特固定模上的共同释放而使得内膜厚度显著降低。
ζp=0.0008,未加涂饰的金属对低剂量,p=0.03聚合物对低剂量
§p=0.002未加涂饰的金属对低剂量,p=0.04聚合物对低剂量
ζp=0.02未加涂饰的金属对高剂量,p=0.07聚合物对高剂量
实施例8
图11A和11B表示了γ辐射和血浆处理对药物释放的影响。在血浆处理和γ辐射后,用膨胀导管(3.0mm的囊大小,20mm长)将该斯滕特固定模进行膨胀并将其各自放到包含5.0ml 0.1M磷酸盐缓冲剂(pH 7.4)的玻璃管中。定期取样并在每次取样后将整个释放介质进行替换。用HPLC对释放到介质中的各药物的数量进行测量。在释放介质中没有检测到完整的复合药物。
实施例9:涂布实施例A
将通过将Eudragit NE30D水性分散体进行蒸发和风干而获得的1.0gm EMM(聚(丙烯酸乙酯和甲基丙烯酸甲酯)共聚物)加入到9.0gm丙酮中。向这种分散体中加入51.5mg 5-氟尿嘧啶和氟轻松的复合药物(G.531.1)并在搅拌后使其溶解。通过将其在该复合药物/聚合物溶液中进行浸渍,然后进行风干而将10个HPLC插入物用该复合药物/共聚物进行涂布。将该涂布过程重复进行几次直至在个玻璃管上涂布了约30mg的复合药物/聚合物。然后将进行了涂布的插入物各自放置在10.0ml 0.1M磷酸盐缓冲剂(pH 7.4,37℃)中进行释放试验。每天取样并在各次取样时将整个释放介质用新鲜介质进行替换。用HPLC对释放到介质中的药物和复合药物进行测定。
实施例10:涂布实施例B
称取441.8mg聚(乙烯-共-醋酸乙烯酯)(EVA)并将其转移到15.0m1 THF中。EVA慢慢胀大,然后通过超声和磁力搅拌而使其部分溶解于THF中。向其中加入88.2mg复合药物(TC32)并使其溶解于该聚合物溶液中。然后通过浸渍将9个HPLC插入物用聚合物/复合药物溶液进行涂布,然后将其在环境温度下风干。将该涂布和风干重复进行几次,直至向各插入物上一共使用约10mg聚合物/复合药物。然后,将该插入物在50℃的烘箱中放置1小时以除去溶剂的残余物。在完成涂布之前和之后对插入物的重量和直径进行检查并进行记录。然后,将进行了涂布的插入物分别放置在10.0ml 0.1M磷酸盐缓冲剂(pH7.4,37℃)中进行释放试验。每天取样,并在各次取样时将整个释放介质用新鲜介质进行替换。用HPLC对释放到介质中的药物和复合药物进行测量。
上面的说明和实施例是要对本发明的一些实施方案进行说明而不是要对其进行任何限制。可以对本发明的系统、装置和方法进行各种修改和变化而不会脱离本发明的主旨或范围对于本领域技术人员而言是显而易见的。这里所列举的所有专利和公开物都被以其全文引入本文作为参考。
Claims (66)
1.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的具有A-L-B的通式的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;
其中A的治疗活性形式在水中的溶解度高于1mg/ml,而该前体药物在水中的溶解度低于1mg/ml。
2.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的具有A∷B的通式的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
∷表示A和B之间的在生理学条件下离解产生所说的A的治疗活性形式的离子键;
B表示一种当与A离子键合时产生溶解度低于A的治疗活性形式的前体药物的部分;和
其中A的治疗活性形式在水中的溶解度高于1mg/ml,而该前体药物在水中的溶解度低于1mg/ml。
3.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的具有A-L-B的通式的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;
其中,当被配置在生物流体中时,所说的缓释制剂在至少24小时的期间内提供了A的治疗活性形式的缓释,并且在释放期间,该前体药物在聚合物外的流体中的浓度小于A的治疗活性形式浓度的10%。
4.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的具有A∷B的通式的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
∷表示A和B之间的在生理学条件下离解产生所说的A的治疗活性形式的离子键;
B表示一种当与A离子键合时产生一种溶解度低于A的治疗活性形式的前体药物的部分;和
其中,当配置于生物流体中时,所说的缓释制剂可在至少24小时的期间内提供A的治疗活性形式的缓释,并且在释放期间,该前体药物在聚合物外的流体中的浓度低于A的治疗活性形式浓度的10%。
5.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的通式为A-L-B的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时产生一种溶解度低于A的治疗活性形式的前体药物的部分;
其中A的治疗活性形式具有比前体药物的logP值低至少1个logP单位的logP值。
6.一种缓释制剂,其包含聚合物基质和分散于该聚合物中的通式为A∷B的前体药物,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
∷表示A和B之间的在生理学条件下离解产生所说的A的治疗活性形式的离子键;
B表示一种当与A离子键合时产生一种溶解度低于A的治疗活性形式的前体药物的部分;和
其中A的治疗活性形式具有比该前体药物的logP值低至少1个logP单位的logP值。
7.如权利要求1或2所述的缓释制剂,其中所说的前体药物在水中的溶解度小于100μg/ml。
8.如权利要求1-6中任意一项所述的缓释制剂,其中所说的B是一种疏水性的脂族部分。
9.如权利要求1-6中任意一项所述的缓释制剂,其中所说的B是一旦所说的连接物L裂解或所说的离子键离解就产生的具有治疗活性形式的药物部分。
10.如权利要求9所述的缓释制剂,其中所说的A和B是相同的药物部分。
11.如权利要求9所述的缓释制剂,其中所说的A和B是不同的药物部分。
12.如权利要求1-6中任意一项所述的缓释制剂,其中所说的B在从该前体药物上裂解下来后是一种生物学惰性部分。
13.如权利要求1-6中任意一项所述的缓释制剂,其中所说的A选自免疫响应改性剂、抗增生剂、皮质类固醇、抑制血管的甾类物质、抗寄生虫药、治疗青光眼的药物、抗生素、反义化合物、分化调节剂、抗病毒药、抗癌药、和非甾体抗炎药。
14.如权利要求9所述的缓释制剂,其中所说的B选自免疫响应改性剂、抗增生剂、皮质类固醇、抑制血管的甾类物质、抗寄生虫药、治疗青光眼的药物、抗生素、反义化合物、分化调节剂、抗病毒药、抗癌药、和非甾体抗炎药。
15.如权利要求1-6中任意一项所述的缓释制剂,其中所说的A的治疗活性形式从该聚合物基质中释放的持续时间为至少24小时。
16.如权利要求9所述的缓释制剂,其中所说的A是5-氟尿嘧啶(5FU)并且B是萘普生。
17.如权利要求1-6中任意一项或权利要求9所述的缓释制剂,其中所说的A或B中的至少一个是抗肿瘤剂。
18.如权利要求17所述的缓释制剂,其中所说的抗肿瘤剂选自蒽环素类、长春花属生物碱类、嘌呤类似物、嘧啶类似物、嘧啶生物合成的抑制剂、和烷化剂。
19.如权利要求17所述的缓释制剂,其中所说的抗肿瘤药是氟化的嘧啶。
20.如权利要求17所述的缓释制剂,其中所说的抗肿瘤药选自5-氟尿嘧啶(5FU)、5′-脱氧-5-氟尿苷5-氟尿苷、2′-脱氧-5-氟尿苷、氟胞嘧啶、5-三氟甲基-2′-脱氧尿苷、阿糖氧基胞嘧啶、环胞苷、5-氮杂-2′-脱氧胞苷、阿拉伯糖基5-氮杂胞嘧啶、6-氮杂胞苷、N-膦酰基乙酰基-L-天门冬氨酸、吡唑呋喃菌素、6-氮杂尿苷、阿扎立平、和3-去氮杂尿苷。
21.如权利要求17所述的缓释制剂,其中所说的抗肿瘤药是选自阿拉伯糖基胞嘧啶、环胞苷、5-氮杂-2′-脱氧胞苷、阿拉伯糖基5-氮杂胞嘧啶、和6-氮杂胞苷的嘧啶核苷类似物。
22.如权利要求17所述的缓释制剂,其中所说的抗肿瘤药选自克拉屈滨、6-巯基嘌呤、喷司他丁、6-硫鸟嘌呤、和磷酸氟达拉滨。
23.如权利要求1-6中任意一项所述的缓释制剂,其中所说的A的治疗活性形式是5-氟尿嘧啶。
24.如权利要求1-6中任意一项或权利要求9所述的缓释制剂,其中A或B中的至少一个是抗炎剂。
25.如权利要求24所述的缓释制剂,其中所说的抗炎剂是非甾体抗炎剂。
26.如权利要求25所述的缓释制剂,其中所说的抗炎剂选自双氯酚酸、非诺洛芬、氟比洛芬、布洛芬、酮洛芬、酮咯酸、nahumstone、萘普生和吡罗昔康。
27.如权利要求24所述的缓释制剂,其中所说的抗炎剂是糖皮质激素。
28.如权利要求27所述的缓释制剂,其中所说的糖皮质激素选自aclometasone、倍氯米松、倍他米松、布地奈德、氯倍他索、氯倍他松、可的松、地萘德、去羟米松、二氟拉松、氟米松、氟尼缩松、氟轻松、肤轻松、氟可龙、氟泼尼定、氟氢缩松、氟替卡松、氢化可的松、醋丙甲泼松龙、mometasone furdate、强的松龙、强的松和rofleponide。
29.如权利要求9所述的缓释制剂,其中所说的B的治疗活性形式选自氟轻松、曲安奈德、双氯酚酸、和萘普生。
30.如权利要求1所述的缓释制剂,其中所说的连接L在体液中被水解。
31.如权利要求1所述的缓释制剂,其中所说的连接L包括一种或多种选自酯、酰胺、氨基甲酸酯、碳酸酯、环状缩酮、硫代酸酯、硫代酰胺、硫代氨基甲酸酯、硫代碳酸酯、黄原酸酯和磷酸酯的可水解的基团。
32.如权利要求1所述的缓释制剂,其中所说的连接L被酶促裂解。
33.如权利要求1所述的缓释制剂,其中所说的前体药物在其连接形式时产生所说临床响应的ED50比A的治疗活性形式的ED50高至少10倍。
34.如权利要求1所述的缓释制剂,其中所说的前体药物在其连接形式时具有比A的治疗活性形式的ED50高至少1000倍的产生所说临床响应的ED50。
35.如权利要求1所述的缓释制剂,其中所说的A的治疗活性形式在水中的溶解度比所说的前体药物高至少10倍。
36.如权利要求29所述的缓释制剂,其中所说的前体药物选自与氟轻松共价结合的5FU、与萘普生共价结合的5FU、和与双氯酚酸纳共价结合的5FU。
38.如权利要求1-6中任意一项所述的缓释制剂,其中所说的聚合物是不可生物侵蚀的。
39.如权利要求38所述的缓释制剂,其中所说的不可生物侵蚀的聚合物选自聚氨酯、聚硅氧烷、聚(乙烯-共-醋酸乙烯酯)、聚乙烯醇、以及它们的衍生物和共聚物。
40.如权利要求1-6中任意一项所述的缓释制剂,其中所说的聚合物是可生物侵蚀的。
41.如权利要求40所述的缓释制剂,其中所说的可生物侵蚀的聚合物选自聚酐、聚乳酸、聚乙醇酸、聚原酸酯、聚烷基氰基丙烯酸酯、以及它们的衍生物和共聚物。
42.如权利要求1-6中任意一项所述的缓释制剂,其中所说的聚合物将该前体药物保持在特定的生理学部位并防止了该前体药物的分解。
43.如权利要求1-6中任意一项所述的缓释制剂,其中所说的聚合物降低了聚合物中的前体药物和周围浴液体中的蛋白质组分之间的相互作用。
44.如权利要求1-6中任意一项所述的缓释制剂,其中所说的系统适于被注射或植入到体内。
45.一种医学装置,其包含:
(i)一种具有表面的底物;和,
(ii)一种粘附到该表面上的涂层,所说的涂层包含具有分散于其中的低溶解度前体药物的聚合物基质,其中所说的低溶解度的前体药物可由通式A-L-B来进行表示,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分。
46.如权利要求45所述的装置,其中所说的聚合物基质对于A的治疗活性形式从该涂层中的释放速率而言基本不是释放速率的限制因素。
47.如权利要求45所述的装置,其中所说的底物是选自螺钉、板、衬垫、缝合线、假体锚凹、平头钉、U型钉、电导线、瓣膜、和膜的手术器具。
48.如权利要求45所述的装置,其选自导管、可植入的血管进入端口、血液储袋、血道、中央静脉导管、动脉导管、血管移植物、主动脉内的囊泵、心脏瓣膜、心血管缝合线、人造心脏、起搏器、心室助泵、体外装置、血液过滤器、血液透析装置、血灌注装置、血浆去除装置、和适于在血管内展开的过滤器。
49.如权利要求45所述的装置,其是一种血管斯滕特固定模。
50.如权利要求49所述的装置,其上一种可扩展的斯滕特固定模,并且所说的涂层是柔软的,能适应所说的可扩展的斯滕特固定模的压缩和扩张状态。
51.如权利要求45所述的装置,其中可归因于该前体药物的该涂层的重量为每cm2被所说聚合物基质涂布的表面约0.05mg至约50mg前体药物。
52.如权利要求45所述的装置,其中所说涂层的厚度为5微米至100微米。
53.如权利要求45所述的装置,其中所说的前体药物的存在量为该涂层重量的5%至70%重量。
54.一种进行了涂布的装置组合,其包含用于植入到患者体内的医学装置,所说的医学装置具有一个或多个被权利要求1-6中任意一项所述的聚合物制剂涂布的表面,该涂布是以当被植入到患者体内时,使得该被涂布的表面在一定时期内能释放A的治疗活性形式的方式进行的。
55.如权利要求54所述的进行了涂布的装置,其中所说的装置是一种具有内腔表面和相对的沿着纵向斯滕特固定模轴延伸的外表面的放射状延伸的可扩展的管状斯滕特固定模。
56.一种具有至少一种可插入或可植入到患者体内的部分的斯滕特固定模,其中该部分具有适于与机体组织相接触的表面并且其中该表面的至少一部分被一种用于将至少一种生物学活性物质进行释放的涂层所涂布,该涂层包含具有分散于其中的低溶解度前体药物的聚合物基质,其中所说的低溶解度的前体药物是由通式A-L-B所表示的,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分。
57.一种用一种缓释系统进行了涂布的管腔内的医学装置,所说的缓释系统包含一种生物学可耐受的聚合物和分散于该聚合物中的低溶解度的前体药物,所说的装置具有内表面和外表面;所说的装置具有应用到该内表面、外表面、或内表面和外表面的至少一部分上的所说系统。
58.一种用于对患者的管腔内组织进行处理的方法,该方法包含的步骤有:
(a)提供一种具有内表面和外表面的斯滕特固定模,所说的斯滕特固定模在其内表面、外表面、或内表面和外表面的至少一部分上具有一种涂层;所说的涂层包含溶解或分散于一种生物学可耐受的聚合物中的低溶解度的药物的前体药物;
(b)将该斯滕特固定模定位在适宜的管腔内的组织部位;和
(c)将该斯滕特固定模展开。
59.一种用于将药物从医学装置表面定位传递到体内的涂层组合物,该组合物包含一种具有分散于其中的低溶解度前体药物的聚合物基质,其中所说的低溶解度的前体药物是由通式A-L-B表示的,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;和
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;
该涂层组合物是以适于通过喷涂和/或将该装置浸渍在所说的组合物中而被应用到所说医学装置表面的液体或混悬液形式被提供的。
60.一种用于将药物从医学装置表面定位传递到体内的涂层组合物,该组合物包含一种具有分散于其中的低溶解度前体药物的聚合物基质,其中所说的低溶解度的前体药物是由通式A-L-B表示的,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;
该涂层组合物是以粉状形式被提供的,并且一旦添加一种溶剂,则其可以被重组成用于通过喷涂和/或将该装置浸渍到所说组合物中而被应用到所说医学装置表面的液体或混悬液形式。
61.用于将药物传递给患者的可注射组合物,该组合物包含一种具有分散于其中的低溶解度前体药物的聚合物基质,其中所说的低溶解度前体药物是由通式A-L-B表示的,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;
该组合物是以适于通过一种针注射进行传递的液体或混悬液的形式被提供的。
62.一种制造一种缓释系统的方法,其包含将聚合物基质与治疗有效量的低溶解度的前体药物进行混合,其中
(i)所说的低溶解度的前体药物是由通式A-L-B表示的,其中
A表示一种具有在患者体内产生临床响应的治疗活性形式的药物部分;
L表示一种将A和B连接形成一种前体药物的共价连接物,所说的连接物在生理条件下被裂解从而产生所说的A的治疗活性形式;
B表示一种当与A相连时可产生溶解度低于A的治疗活性形式的前体药物的部分;和
(ii)该聚合物基质对于A的治疗活性形式而言是可渗透的,并且对于A的治疗活性形式从该聚合物基质中的释放速率而言基本不是释放速率的限制因素。
63.如权利要求62所述的方法,其进一步包含将聚合物基质和前体药物的混合物应用到一种手术器具表面上的步骤。
64.一种对哺乳动物生物体进行处理以获得所需的局部或全身生理学或药理学作用的方法,其包括:将治疗有效量的如权利要求1-6中任意一项所述的缓释制剂给予哺乳动物。
65.如权利要求1-6中任意一项所述的缓释系统在制造用一种A的治疗活性形式的持续剂量方案对患者进行治疗的药物中的应用。
66.如权利要求5或6所述的缓释制剂,其中所说的A的治疗活性形式具有比该前体药物的logP值低至少2个logP单位的logP值。
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28634301P | 2001-04-26 | 2001-04-26 | |
| US60/286,343 | 2001-04-26 | ||
| US32242801P | 2001-09-17 | 2001-09-17 | |
| US60/322,428 | 2001-09-17 | ||
| US37276102P | 2002-04-15 | 2002-04-15 | |
| US60/372,761 | 2002-04-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1520297A true CN1520297A (zh) | 2004-08-11 |
Family
ID=27403607
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA028129563A Pending CN1520297A (zh) | 2001-04-26 | 2002-04-26 | 包含复合药物(codrugs)的缓释药物传递系统 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US20030039689A1 (zh) |
| EP (3) | EP2033648A3 (zh) |
| JP (2) | JP2004536799A (zh) |
| KR (1) | KR20040005936A (zh) |
| CN (1) | CN1520297A (zh) |
| AU (1) | AU2002259045B2 (zh) |
| BR (1) | BR0209198A (zh) |
| CA (1) | CA2444894C (zh) |
| IL (1) | IL158527A0 (zh) |
| MX (1) | MXPA03009727A (zh) |
| NZ (1) | NZ528994A (zh) |
| WO (1) | WO2002087586A1 (zh) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104582782A (zh) * | 2012-08-20 | 2015-04-29 | 奥雷制药有限公司 | 用于制备药物递送制剂的方法 |
| CN107205956A (zh) * | 2014-10-30 | 2017-09-26 | 纺织品技术股份有限公司 | 输送系统 |
| CN114699563A (zh) * | 2022-02-22 | 2022-07-05 | 中国医科大学附属盛京医院 | 一种负载型聚醚型聚氨酯薄膜、制备方法及其应用 |
Families Citing this family (736)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7266725B2 (en) * | 2001-09-03 | 2007-09-04 | Pact Xpp Technologies Ag | Method for debugging reconfigurable architectures |
| US6576636B2 (en) | 1996-05-22 | 2003-06-10 | Protarga, Inc. | Method of treating a liver disorder with fatty acid-antiviral agent conjugates |
| EP1032605B1 (en) * | 1997-09-10 | 2007-11-14 | Rutgers, The State University | Polyanhydrides with therapeutically useful degradation products |
| US7985415B2 (en) | 1997-09-10 | 2011-07-26 | Rutgers, The State University Of New Jersey | Medical devices employing novel polymers |
| US20030158220A1 (en) * | 1997-11-03 | 2003-08-21 | Foss Joseph F. | Use of methylnaltrexone and related compounds to treat chronic opioid use side effects |
| US7208010B2 (en) | 2000-10-16 | 2007-04-24 | Conor Medsystems, Inc. | Expandable medical device for delivery of beneficial agent |
| US20040254635A1 (en) | 1998-03-30 | 2004-12-16 | Shanley John F. | Expandable medical device for delivery of beneficial agent |
| US6241762B1 (en) | 1998-03-30 | 2001-06-05 | Conor Medsystems, Inc. | Expandable medical device with ductile hinges |
| US20020188037A1 (en) * | 1999-04-15 | 2002-12-12 | Chudzik Stephen J. | Method and system for providing bioactive agent release coating |
| EP1555036B1 (en) * | 1998-04-27 | 2010-05-05 | Surmodics Inc. | Bioactive agent release coating |
| US20070032853A1 (en) * | 2002-03-27 | 2007-02-08 | Hossainy Syed F | 40-O-(2-hydroxy)ethyl-rapamycin coated stent |
| US7807211B2 (en) * | 1999-09-03 | 2010-10-05 | Advanced Cardiovascular Systems, Inc. | Thermal treatment of an implantable medical device |
| US20050238686A1 (en) * | 1999-12-23 | 2005-10-27 | Advanced Cardiovascular Systems, Inc. | Coating for implantable devices and a method of forming the same |
| US6375972B1 (en) | 2000-04-26 | 2002-04-23 | Control Delivery Systems, Inc. | Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof |
| US6953560B1 (en) | 2000-09-28 | 2005-10-11 | Advanced Cardiovascular Systems, Inc. | Barriers for polymer-coated implantable medical devices and methods for making the same |
| EP1328213B1 (en) | 2000-10-16 | 2005-07-27 | Conor Medsystems, Inc. | Expandable medical device for delivery of beneficial agent |
| US7807210B1 (en) | 2000-10-31 | 2010-10-05 | Advanced Cardiovascular Systems, Inc. | Hemocompatible polymers on hydrophobic porous polymers |
| GB0100761D0 (en) * | 2001-01-11 | 2001-02-21 | Biocompatibles Ltd | Drug delivery from stents |
| US20040073294A1 (en) | 2002-09-20 | 2004-04-15 | Conor Medsystems, Inc. | Method and apparatus for loading a beneficial agent into an expandable medical device |
| US6613753B2 (en) * | 2001-02-21 | 2003-09-02 | Supergen, Inc. | Restore cancer-suppressing functions to neoplastic cells through DNA hypomethylation |
| US8552054B2 (en) | 2001-03-23 | 2013-10-08 | Luitpold Pharmaceuticals, Inc. | Fatty amine drug conjugates |
| JP4634694B2 (ja) | 2001-03-23 | 2011-02-16 | ルイトポルド・ファーマシューティカルズ・インコーポレーテッド | 脂肪アルコール薬物複合体 |
| US6780424B2 (en) * | 2001-03-30 | 2004-08-24 | Charles David Claude | Controlled morphologies in polymer drug for release of drugs from polymer films |
| US6905669B2 (en) * | 2001-04-24 | 2005-06-14 | Supergen, Inc. | Compositions and methods for reestablishing gene transcription through inhibition of DNA methylation and histone deacetylase |
| US20040022853A1 (en) * | 2001-04-26 | 2004-02-05 | Control Delivery Systems, Inc. | Polymer-based, sustained release drug delivery system |
| US7923055B2 (en) * | 2001-05-11 | 2011-04-12 | Exogenesis Corporation | Method of manufacturing a drug delivery system |
| US6743462B1 (en) * | 2001-05-31 | 2004-06-01 | Advanced Cardiovascular Systems, Inc. | Apparatus and method for coating implantable devices |
| US8741378B1 (en) | 2001-06-27 | 2014-06-03 | Advanced Cardiovascular Systems, Inc. | Methods of coating an implantable device |
| US6695920B1 (en) * | 2001-06-27 | 2004-02-24 | Advanced Cardiovascular Systems, Inc. | Mandrel for supporting a stent and a method of using the mandrel to coat a stent |
| US8303651B1 (en) * | 2001-09-07 | 2012-11-06 | Advanced Cardiovascular Systems, Inc. | Polymeric coating for reducing the rate of release of a therapeutic substance from a stent |
| IN2014DN10834A (zh) | 2001-09-17 | 2015-09-04 | Psivida Inc | |
| US20030229390A1 (en) * | 2001-09-17 | 2003-12-11 | Control Delivery Systems, Inc. | On-stent delivery of pyrimidines and purine analogs |
| US6753071B1 (en) * | 2001-09-27 | 2004-06-22 | Advanced Cardiovascular Systems, Inc. | Rate-reducing membrane for release of an agent |
| US20030073961A1 (en) * | 2001-09-28 | 2003-04-17 | Happ Dorrie M. | Medical device containing light-protected therapeutic agent and a method for fabricating thereof |
| WO2003049804A2 (en) * | 2001-12-10 | 2003-06-19 | Control Delivery Systems Inc. | Treatment of genitourinary tract disorders |
| WO2003061626A1 (en) * | 2002-01-18 | 2003-07-31 | Control Delivery Systems, Inc. | Polymeric gel system for the controlled delivery of codrugs |
| US6998391B2 (en) * | 2002-02-07 | 2006-02-14 | Supergen.Inc. | Method for treating diseases associated with abnormal kinase activity |
| US20030147813A1 (en) * | 2002-02-07 | 2003-08-07 | John Lyons | Method for treating chronic myelogenous leukemia |
| US7264822B2 (en) * | 2002-04-03 | 2007-09-04 | Poly-Med, Inc. | Conjugated drug-polymer coated stent |
| US7041308B2 (en) * | 2002-04-18 | 2006-05-09 | Poly-Med, Inc. | Drug-polymer coated stents with segmented homochain copolyesters |
| US8871241B2 (en) * | 2002-05-07 | 2014-10-28 | Psivida Us, Inc. | Injectable sustained release delivery devices |
| US8313760B2 (en) | 2002-05-24 | 2012-11-20 | Angiotech International Ag | Compositions and methods for coating medical implants |
| CN100341589C (zh) | 2002-05-24 | 2007-10-10 | 血管技术国际股份公司 | 用于涂覆医用植入物的组合物和方法 |
| ES2665999T3 (es) * | 2002-05-31 | 2018-04-30 | Titan Pharmaceuticals, Inc. | Dispositivo polimérico implantable para la liberación sostenida de buprenorfina |
| US6982253B2 (en) | 2002-06-05 | 2006-01-03 | Supergen, Inc. | Liquid formulation of decitabine and use of the same |
| US7097850B2 (en) * | 2002-06-18 | 2006-08-29 | Surmodics, Inc. | Bioactive agent release coating and controlled humidity method |
| US20030232087A1 (en) * | 2002-06-18 | 2003-12-18 | Lawin Laurie R. | Bioactive agent release coating with aromatic poly(meth)acrylates |
| US7396539B1 (en) * | 2002-06-21 | 2008-07-08 | Advanced Cardiovascular Systems, Inc. | Stent coatings with engineered drug release rate |
| US7056523B1 (en) | 2002-06-21 | 2006-06-06 | Advanced Cardiovascular Systems, Inc. | Implantable medical devices incorporating chemically conjugated polymers and oligomers of L-arginine |
| US7217426B1 (en) | 2002-06-21 | 2007-05-15 | Advanced Cardiovascular Systems, Inc. | Coatings containing polycationic peptides for cardiovascular therapy |
| US8506617B1 (en) | 2002-06-21 | 2013-08-13 | Advanced Cardiovascular Systems, Inc. | Micronized peptide coated stent |
| US7033602B1 (en) | 2002-06-21 | 2006-04-25 | Advanced Cardiovascular Systems, Inc. | Polycationic peptide coatings and methods of coating implantable medical devices |
| US7794743B2 (en) * | 2002-06-21 | 2010-09-14 | Advanced Cardiovascular Systems, Inc. | Polycationic peptide coatings and methods of making the same |
| US20040063805A1 (en) * | 2002-09-19 | 2004-04-01 | Pacetti Stephen D. | Coatings for implantable medical devices and methods for fabrication thereof |
| CA2503150A1 (en) * | 2002-10-31 | 2004-05-21 | Supergen, Inc. | Pharmaceutical formulations targeting specific regions of the gastrointestinal tract |
| EP2338538A1 (en) * | 2002-11-08 | 2011-06-29 | Conor Medsystems, Inc. | Method and apparatus for reducing tissue damage after ischemic injury |
| US6896965B1 (en) * | 2002-11-12 | 2005-05-24 | Advanced Cardiovascular Systems, Inc. | Rate limiting barriers for implantable devices |
| DK1572154T3 (da) * | 2002-11-18 | 2012-05-14 | Univ Rutgers | Medicinske indretninger, der anvender nye polymerer |
| US7776926B1 (en) * | 2002-12-11 | 2010-08-17 | Advanced Cardiovascular Systems, Inc. | Biocompatible coating for implantable medical devices |
| US7758880B2 (en) | 2002-12-11 | 2010-07-20 | Advanced Cardiovascular Systems, Inc. | Biocompatible polyacrylate compositions for medical applications |
| US7074276B1 (en) * | 2002-12-12 | 2006-07-11 | Advanced Cardiovascular Systems, Inc. | Clamp mandrel fixture and a method of using the same to minimize coating defects |
| US20060002968A1 (en) * | 2004-06-30 | 2006-01-05 | Gordon Stewart | Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders |
| US7758881B2 (en) * | 2004-06-30 | 2010-07-20 | Advanced Cardiovascular Systems, Inc. | Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device |
| US8435550B2 (en) * | 2002-12-16 | 2013-05-07 | Abbot Cardiovascular Systems Inc. | Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device |
| US7063884B2 (en) * | 2003-02-26 | 2006-06-20 | Advanced Cardiovascular Systems, Inc. | Stent coating |
| US20080051866A1 (en) * | 2003-02-26 | 2008-02-28 | Chao Chin Chen | Drug delivery devices and methods |
| AU2004228017C1 (en) | 2003-03-31 | 2010-06-03 | Titan Pharmaceuticals, Inc. | Implantable polymeric device for sustained release of dopamine agonist |
| WO2004091623A1 (en) * | 2003-04-08 | 2004-10-28 | Progenics Pharmaceuticals. Inc. | Pharmaceutical formulations containing methylnaltrexone |
| US8791171B2 (en) * | 2003-05-01 | 2014-07-29 | Abbott Cardiovascular Systems Inc. | Biodegradable coatings for implantable medical devices |
| US8246974B2 (en) * | 2003-05-02 | 2012-08-21 | Surmodics, Inc. | Medical devices and methods for producing the same |
| US7279174B2 (en) * | 2003-05-08 | 2007-10-09 | Advanced Cardiovascular Systems, Inc. | Stent coatings comprising hydrophilic additives |
| US20070084897A1 (en) | 2003-05-20 | 2007-04-19 | Shelton Frederick E Iv | Articulating surgical stapling instrument incorporating a two-piece e-beam firing mechanism |
| US9060770B2 (en) | 2003-05-20 | 2015-06-23 | Ethicon Endo-Surgery, Inc. | Robotically-driven surgical instrument with E-beam driver |
| EP1633403A2 (en) * | 2003-05-21 | 2006-03-15 | Control Delivery Systems, Inc. | Codrugs of diclofenac |
| CA2525393A1 (en) * | 2003-05-28 | 2004-12-23 | Conor Medsystems, Inc. | Methods of delivering anti-restenotic agents from a stent |
| US20050031668A1 (en) * | 2003-05-30 | 2005-02-10 | Patel Rajesh A. | Implantable polymeric device for sustained release of nalmefene |
| WO2005009480A2 (en) * | 2003-06-11 | 2005-02-03 | Control Delivery Systems, Inc. | Implants containing codrugs |
| US20050118344A1 (en) | 2003-12-01 | 2005-06-02 | Pacetti Stephen D. | Temperature controlled crimping |
| SI1635875T1 (sl) | 2003-06-26 | 2009-04-30 | Psivida Inc | Sistem za dajanje zdravila, ki se gelira in situ |
| US20050009910A1 (en) * | 2003-07-10 | 2005-01-13 | Allergan, Inc. | Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug |
| US20050037992A1 (en) * | 2003-07-22 | 2005-02-17 | John Lyons | Composition and method for treating neurological disorders |
| US7785512B1 (en) | 2003-07-31 | 2010-08-31 | Advanced Cardiovascular Systems, Inc. | Method and system of controlled temperature mixing and molding of polymers with active agents for implantable medical devices |
| US7357940B2 (en) * | 2003-07-31 | 2008-04-15 | Boston Scientific Scimed, Inc. | Implantable or insertable medical devices containing graft copolymer for controlled delivery of therapeutic agents |
| US20050054612A1 (en) * | 2003-09-08 | 2005-03-10 | Monahan Sean D. | Delivery by labile hydrophobic modification of drugs |
| US20050059682A1 (en) * | 2003-09-12 | 2005-03-17 | Supergen, Inc., A Delaware Corporation | Compositions and methods for treatment of cancer |
| US7198675B2 (en) | 2003-09-30 | 2007-04-03 | Advanced Cardiovascular Systems | Stent mandrel fixture and method for selectively coating surfaces of a stent |
| US7261946B2 (en) | 2003-11-14 | 2007-08-28 | Advanced Cardiovascular Systems, Inc. | Block copolymers of acrylates and methacrylates with fluoroalkenes |
| US9114198B2 (en) * | 2003-11-19 | 2015-08-25 | Advanced Cardiovascular Systems, Inc. | Biologically beneficial coatings for implantable devices containing fluorinated polymers and methods for fabricating the same |
| US8192752B2 (en) * | 2003-11-21 | 2012-06-05 | Advanced Cardiovascular Systems, Inc. | Coatings for implantable devices including biologically erodable polyesters and methods for fabricating the same |
| US7220816B2 (en) * | 2003-12-16 | 2007-05-22 | Advanced Cardiovascular Systems, Inc. | Biologically absorbable coatings for implantable devices based on poly(ester amides) and methods for fabricating the same |
| US7435788B2 (en) * | 2003-12-19 | 2008-10-14 | Advanced Cardiovascular Systems, Inc. | Biobeneficial polyamide/polyethylene glycol polymers for use with drug eluting stents |
| US8652502B2 (en) * | 2003-12-19 | 2014-02-18 | Cordis Corporation | Local vascular delivery of trichostatin A alone or in combination with sirolimus to prevent restenosis following vascular injury |
| US8685431B2 (en) * | 2004-03-16 | 2014-04-01 | Advanced Cardiovascular Systems, Inc. | Biologically absorbable coatings for implantable devices based on copolymers having ester bonds and methods for fabricating the same |
| US20050208093A1 (en) * | 2004-03-22 | 2005-09-22 | Thierry Glauser | Phosphoryl choline coating compositions |
| US8778014B1 (en) | 2004-03-31 | 2014-07-15 | Advanced Cardiovascular Systems, Inc. | Coatings for preventing balloon damage to polymer coated stents |
| US20060083772A1 (en) * | 2004-04-06 | 2006-04-20 | Dewitt David M | Coating compositions for bioactive agents |
| WO2005099787A1 (en) * | 2004-04-06 | 2005-10-27 | Surmodics, Inc. | Coating compositions for bioactive agents |
| US7820732B2 (en) | 2004-04-30 | 2010-10-26 | Advanced Cardiovascular Systems, Inc. | Methods for modulating thermal and mechanical properties of coatings on implantable devices |
| US20050244461A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Controlled release drug delivery systems and methods for treatment of an eye |
| US8293890B2 (en) | 2004-04-30 | 2012-10-23 | Advanced Cardiovascular Systems, Inc. | Hyaluronic acid based copolymers |
| US9561309B2 (en) * | 2004-05-27 | 2017-02-07 | Advanced Cardiovascular Systems, Inc. | Antifouling heparin coatings |
| WO2005120578A2 (en) * | 2004-06-07 | 2005-12-22 | California Institute Of Technology | Biodegradable drug-polymer delivery system |
| US7563780B1 (en) * | 2004-06-18 | 2009-07-21 | Advanced Cardiovascular Systems, Inc. | Heparin prodrugs and drug delivery stents formed therefrom |
| US20050287184A1 (en) * | 2004-06-29 | 2005-12-29 | Hossainy Syed F A | Drug-delivery stent formulations for restenosis and vulnerable plaque |
| US8709469B2 (en) | 2004-06-30 | 2014-04-29 | Abbott Cardiovascular Systems Inc. | Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device |
| US7968085B2 (en) * | 2004-07-05 | 2011-06-28 | Ascendis Pharma A/S | Hydrogel formulations |
| US11998198B2 (en) | 2004-07-28 | 2024-06-04 | Cilag Gmbh International | Surgical stapling instrument incorporating a two-piece E-beam firing mechanism |
| US9072535B2 (en) | 2011-05-27 | 2015-07-07 | Ethicon Endo-Surgery, Inc. | Surgical stapling instruments with rotatable staple deployment arrangements |
| US11890012B2 (en) | 2004-07-28 | 2024-02-06 | Cilag Gmbh International | Staple cartridge comprising cartridge body and attached support |
| US8215531B2 (en) | 2004-07-28 | 2012-07-10 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument having a medical substance dispenser |
| US8357391B2 (en) | 2004-07-30 | 2013-01-22 | Advanced Cardiovascular Systems, Inc. | Coatings for implantable devices comprising poly (hydroxy-alkanoates) and diacid linkages |
| US7494665B1 (en) * | 2004-07-30 | 2009-02-24 | Advanced Cardiovascular Systems, Inc. | Polymers containing siloxane monomers |
| US7648727B2 (en) | 2004-08-26 | 2010-01-19 | Advanced Cardiovascular Systems, Inc. | Methods for manufacturing a coated stent-balloon assembly |
| US7244443B2 (en) * | 2004-08-31 | 2007-07-17 | Advanced Cardiovascular Systems, Inc. | Polymers of fluorinated monomers and hydrophilic monomers |
| US7063720B2 (en) * | 2004-09-14 | 2006-06-20 | The Wallace Enterprises, Inc. | Covered stent with controlled therapeutic agent diffusion |
| US8691277B2 (en) * | 2004-09-21 | 2014-04-08 | Shandong Luye Pharmaceutical Co., Ltd. | Long acting sustained-release formulation containing dopamine receptor agonist and the preparation method thereof |
| US8110211B2 (en) * | 2004-09-22 | 2012-02-07 | Advanced Cardiovascular Systems, Inc. | Medicated coatings for implantable medical devices including polyacrylates |
| US9011831B2 (en) * | 2004-09-30 | 2015-04-21 | Advanced Cardiovascular Systems, Inc. | Methacrylate copolymers for medical devices |
| US7166680B2 (en) * | 2004-10-06 | 2007-01-23 | Advanced Cardiovascular Systems, Inc. | Blends of poly(ester amide) polymers |
| US8603634B2 (en) * | 2004-10-27 | 2013-12-10 | Abbott Cardiovascular Systems Inc. | End-capped poly(ester amide) copolymers |
| US20060089485A1 (en) * | 2004-10-27 | 2006-04-27 | Desnoyer Jessica R | End-capped poly(ester amide) copolymers |
| US20060095122A1 (en) * | 2004-10-29 | 2006-05-04 | Advanced Cardiovascular Systems, Inc. | Implantable devices comprising biologically absorbable star polymers and methods for fabricating the same |
| US7390497B2 (en) * | 2004-10-29 | 2008-06-24 | Advanced Cardiovascular Systems, Inc. | Poly(ester amide) filler blends for modulation of coating properties |
| US20060105941A1 (en) * | 2004-11-12 | 2006-05-18 | Allergan, Inc. | Mixed antibiotic codrugs |
| US7214759B2 (en) * | 2004-11-24 | 2007-05-08 | Advanced Cardiovascular Systems, Inc. | Biologically absorbable coatings for implantable devices based on polyesters and methods for fabricating the same |
| US8609123B2 (en) * | 2004-11-29 | 2013-12-17 | Advanced Cardiovascular Systems, Inc. | Derivatized poly(ester amide) as a biobeneficial coating |
| US20060115449A1 (en) * | 2004-11-30 | 2006-06-01 | Advanced Cardiovascular Systems, Inc. | Bioabsorbable, biobeneficial, tyrosine-based polymers for use in drug eluting stent coatings |
| US7892592B1 (en) | 2004-11-30 | 2011-02-22 | Advanced Cardiovascular Systems, Inc. | Coating abluminal surfaces of stents and other implantable medical devices |
| US20060128654A1 (en) * | 2004-12-10 | 2006-06-15 | Chunlin Tang | Pharmaceutical formulation of cytidine analogs and derivatives |
| US20060128653A1 (en) * | 2004-12-10 | 2006-06-15 | Chunlin Tang | Pharmaceutical formulation of decitabine |
| EP1841808B1 (en) * | 2004-12-22 | 2009-01-21 | California Institute Of Technology | Degradable p0lymers and methods of preparation thereof |
| US7604818B2 (en) * | 2004-12-22 | 2009-10-20 | Advanced Cardiovascular Systems, Inc. | Polymers of fluorinated monomers and hydrocarbon monomers |
| US7419504B2 (en) * | 2004-12-27 | 2008-09-02 | Advanced Cardiovascular Systems, Inc. | Poly(ester amide) block copolymers |
| US8007775B2 (en) * | 2004-12-30 | 2011-08-30 | Advanced Cardiovascular Systems, Inc. | Polymers containing poly(hydroxyalkanoates) and agents for use with medical articles and methods of fabricating the same |
| US7202325B2 (en) * | 2005-01-14 | 2007-04-10 | Advanced Cardiovascular Systems, Inc. | Poly(hydroxyalkanoate-co-ester amides) and agents for use with medical articles |
| US9662325B2 (en) | 2005-03-07 | 2017-05-30 | The University Of Chicago | Use of opioid antagonists to attenuate endothelial cell proliferation and migration |
| US8518962B2 (en) | 2005-03-07 | 2013-08-27 | The University Of Chicago | Use of opioid antagonists |
| MX2007010833A (es) * | 2005-03-07 | 2009-02-17 | Univ Chicago | Uso de antagonistas opioides para atenuar proliferacion y migracion de células endoteliales. |
| US8524731B2 (en) | 2005-03-07 | 2013-09-03 | The University Of Chicago | Use of opioid antagonists to attenuate endothelial cell proliferation and migration |
| US7250416B2 (en) * | 2005-03-11 | 2007-07-31 | Supergen, Inc. | Azacytosine analogs and derivatives |
| US9381279B2 (en) | 2005-03-24 | 2016-07-05 | Abbott Cardiovascular Systems Inc. | Implantable devices formed on non-fouling methacrylate or acrylate polymers |
| US7700659B2 (en) * | 2005-03-24 | 2010-04-20 | Advanced Cardiovascular Systems, Inc. | Implantable devices formed of non-fouling methacrylate or acrylate polymers |
| EP2298319A1 (en) * | 2005-04-04 | 2011-03-23 | Sinexus, Inc. | Device and methods for treating paranasal sinus conditions |
| US7795467B1 (en) | 2005-04-26 | 2010-09-14 | Advanced Cardiovascular Systems, Inc. | Bioabsorbable, biobeneficial polyurethanes for use in medical devices |
| US8778375B2 (en) | 2005-04-29 | 2014-07-15 | Advanced Cardiovascular Systems, Inc. | Amorphous poly(D,L-lactide) coating |
| US8002730B2 (en) | 2005-04-29 | 2011-08-23 | Medtronic, Inc. | Anti-thrombogenic venous shunt system and method |
| AR057035A1 (es) * | 2005-05-25 | 2007-11-14 | Progenics Pharm Inc | SíNTESIS DE (R)-N-METILNALTREXONA, COMPOSICIONES FARMACÉUTICAS Y USOS |
| AR057325A1 (es) * | 2005-05-25 | 2007-11-28 | Progenics Pharm Inc | Sintesis de (s)-n-metilnaltrexona, composiciones farmaceuticas y usos |
| US7823533B2 (en) * | 2005-06-30 | 2010-11-02 | Advanced Cardiovascular Systems, Inc. | Stent fixture and method for reducing coating defects |
| US8021676B2 (en) | 2005-07-08 | 2011-09-20 | Advanced Cardiovascular Systems, Inc. | Functionalized chemically inert polymers for coatings |
| US7785647B2 (en) * | 2005-07-25 | 2010-08-31 | Advanced Cardiovascular Systems, Inc. | Methods of providing antioxidants to a drug containing product |
| US7735449B1 (en) | 2005-07-28 | 2010-06-15 | Advanced Cardiovascular Systems, Inc. | Stent fixture having rounded support structures and method for use thereof |
| US11484312B2 (en) | 2005-08-31 | 2022-11-01 | Cilag Gmbh International | Staple cartridge comprising a staple driver arrangement |
| US9237891B2 (en) | 2005-08-31 | 2016-01-19 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical stapling devices that produce formed staples having different lengths |
| US7934630B2 (en) | 2005-08-31 | 2011-05-03 | Ethicon Endo-Surgery, Inc. | Staple cartridges for forming staples having differing formed staple heights |
| US20070194079A1 (en) | 2005-08-31 | 2007-08-23 | Hueil Joseph C | Surgical stapling device with staple drivers of different height |
| US11246590B2 (en) | 2005-08-31 | 2022-02-15 | Cilag Gmbh International | Staple cartridge including staple drivers having different unfired heights |
| US7669746B2 (en) | 2005-08-31 | 2010-03-02 | Ethicon Endo-Surgery, Inc. | Staple cartridges for forming staples having differing formed staple heights |
| US10159482B2 (en) | 2005-08-31 | 2018-12-25 | Ethicon Llc | Fastener cartridge assembly comprising a fixed anvil and different staple heights |
| US7700567B2 (en) | 2005-09-29 | 2010-04-20 | Supergen, Inc. | Oligonucleotide analogues incorporating 5-aza-cytosine therein |
| US20070117776A1 (en) * | 2005-11-04 | 2007-05-24 | John Lyons | Low Dose Therapy Of DNA Methylation Inhibitors |
| US20070105792A1 (en) * | 2005-11-04 | 2007-05-10 | Dimartino Jorge F | Administration Of DNA Methylation Inhibitors For Treating Epigenetic Diseases |
| US20070106317A1 (en) | 2005-11-09 | 2007-05-10 | Shelton Frederick E Iv | Hydraulically and electrically actuated articulation joints for surgical instruments |
| EP1951332A1 (en) * | 2005-11-10 | 2008-08-06 | Schering Aktiengesellschaft | Reduction of restenosis |
| US20070128246A1 (en) * | 2005-12-06 | 2007-06-07 | Hossainy Syed F A | Solventless method for forming a coating |
| US20070135909A1 (en) * | 2005-12-08 | 2007-06-14 | Desnoyer Jessica R | Adhesion polymers to improve stent retention |
| US20070142905A1 (en) * | 2005-12-16 | 2007-06-21 | Medtronic Vascular, Inc. | Medical devices to treat or inhibit restenosis |
| US7976891B1 (en) | 2005-12-16 | 2011-07-12 | Advanced Cardiovascular Systems, Inc. | Abluminal stent coating apparatus and method of using focused acoustic energy |
| US7867547B2 (en) | 2005-12-19 | 2011-01-11 | Advanced Cardiovascular Systems, Inc. | Selectively coating luminal surfaces of stents |
| US20070156230A1 (en) | 2006-01-04 | 2007-07-05 | Dugan Stephen R | Stents with radiopaque markers |
| US8186555B2 (en) | 2006-01-31 | 2012-05-29 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting and fastening instrument with mechanical closure system |
| US8820603B2 (en) | 2006-01-31 | 2014-09-02 | Ethicon Endo-Surgery, Inc. | Accessing data stored in a memory of a surgical instrument |
| US7845537B2 (en) | 2006-01-31 | 2010-12-07 | Ethicon Endo-Surgery, Inc. | Surgical instrument having recording capabilities |
| US8708213B2 (en) | 2006-01-31 | 2014-04-29 | Ethicon Endo-Surgery, Inc. | Surgical instrument having a feedback system |
| US11278279B2 (en) | 2006-01-31 | 2022-03-22 | Cilag Gmbh International | Surgical instrument assembly |
| US7753904B2 (en) | 2006-01-31 | 2010-07-13 | Ethicon Endo-Surgery, Inc. | Endoscopic surgical instrument with a handle that can articulate with respect to the shaft |
| US11224427B2 (en) | 2006-01-31 | 2022-01-18 | Cilag Gmbh International | Surgical stapling system including a console and retraction assembly |
| US9861359B2 (en) | 2006-01-31 | 2018-01-09 | Ethicon Llc | Powered surgical instruments with firing system lockout arrangements |
| US20110024477A1 (en) | 2009-02-06 | 2011-02-03 | Hall Steven G | Driven Surgical Stapler Improvements |
| US11793518B2 (en) | 2006-01-31 | 2023-10-24 | Cilag Gmbh International | Powered surgical instruments with firing system lockout arrangements |
| US20120292367A1 (en) | 2006-01-31 | 2012-11-22 | Ethicon Endo-Surgery, Inc. | Robotically-controlled end effector |
| US20110295295A1 (en) | 2006-01-31 | 2011-12-01 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical instrument having recording capabilities |
| US20110006101A1 (en) | 2009-02-06 | 2011-01-13 | EthiconEndo-Surgery, Inc. | Motor driven surgical fastener device with cutting member lockout arrangements |
| CN101378766B (zh) * | 2006-02-01 | 2013-05-01 | 株式会社三养生物制药 | 用于抑制粘连的组合物 |
| US20070185569A1 (en) * | 2006-02-06 | 2007-08-09 | Soonkap Hahn | Drug eluting stent coating with extended duration of drug release |
| US20070196428A1 (en) * | 2006-02-17 | 2007-08-23 | Thierry Glauser | Nitric oxide generating medical devices |
| US7601383B2 (en) * | 2006-02-28 | 2009-10-13 | Advanced Cardiovascular Systems, Inc. | Coating construct containing poly (vinyl alcohol) |
| US7713637B2 (en) * | 2006-03-03 | 2010-05-11 | Advanced Cardiovascular Systems, Inc. | Coating containing PEGylated hyaluronic acid and a PEGylated non-hyaluronic acid polymer |
| US8992422B2 (en) | 2006-03-23 | 2015-03-31 | Ethicon Endo-Surgery, Inc. | Robotically-controlled endoscopic accessory channel |
| US8236010B2 (en) | 2006-03-23 | 2012-08-07 | Ethicon Endo-Surgery, Inc. | Surgical fastener and cutter with mimicking end effector |
| US20070225799A1 (en) * | 2006-03-24 | 2007-09-27 | Medtronic Vascular, Inc. | Stent, intraluminal stent delivery system, and method of treating a vascular condition |
| US20070231363A1 (en) * | 2006-03-29 | 2007-10-04 | Yung-Ming Chen | Coatings formed from stimulus-sensitive material |
| US20080082036A1 (en) * | 2006-04-25 | 2008-04-03 | Medtronic, Inc. | Cerebrospinal fluid shunt having long term anti-occlusion agent delivery |
| US20070259101A1 (en) * | 2006-05-02 | 2007-11-08 | Kleiner Lothar W | Microporous coating on medical devices |
| US8304012B2 (en) * | 2006-05-04 | 2012-11-06 | Advanced Cardiovascular Systems, Inc. | Method for drying a stent |
| US7985441B1 (en) | 2006-05-04 | 2011-07-26 | Yiwen Tang | Purification of polymers for coating applications |
| US8003156B2 (en) | 2006-05-04 | 2011-08-23 | Advanced Cardiovascular Systems, Inc. | Rotatable support elements for stents |
| US7775178B2 (en) * | 2006-05-26 | 2010-08-17 | Advanced Cardiovascular Systems, Inc. | Stent coating apparatus and method |
| US20130325104A1 (en) | 2006-05-26 | 2013-12-05 | Abbott Cardiovascular Systems Inc. | Stents With Radiopaque Markers |
| US8568764B2 (en) | 2006-05-31 | 2013-10-29 | Advanced Cardiovascular Systems, Inc. | Methods of forming coating layers for medical devices utilizing flash vaporization |
| US9561351B2 (en) | 2006-05-31 | 2017-02-07 | Advanced Cardiovascular Systems, Inc. | Drug delivery spiral coil construct |
| US8703167B2 (en) | 2006-06-05 | 2014-04-22 | Advanced Cardiovascular Systems, Inc. | Coatings for implantable medical devices for controlled release of a hydrophilic drug and a hydrophobic drug |
| US20080124372A1 (en) * | 2006-06-06 | 2008-05-29 | Hossainy Syed F A | Morphology profiles for control of agent release rates from polymer matrices |
| US20070286882A1 (en) * | 2006-06-09 | 2007-12-13 | Yiwen Tang | Solvent systems for coating medical devices |
| US8778376B2 (en) | 2006-06-09 | 2014-07-15 | Advanced Cardiovascular Systems, Inc. | Copolymer comprising elastin pentapeptide block and hydrophilic block, and medical device and method of treating |
| US8603530B2 (en) | 2006-06-14 | 2013-12-10 | Abbott Cardiovascular Systems Inc. | Nanoshell therapy |
| US8114150B2 (en) | 2006-06-14 | 2012-02-14 | Advanced Cardiovascular Systems, Inc. | RGD peptide attached to bioabsorbable stents |
| US20080095918A1 (en) * | 2006-06-14 | 2008-04-24 | Kleiner Lothar W | Coating construct with enhanced interfacial compatibility |
| US8048448B2 (en) | 2006-06-15 | 2011-11-01 | Abbott Cardiovascular Systems Inc. | Nanoshells for drug delivery |
| US8535372B1 (en) | 2006-06-16 | 2013-09-17 | Abbott Cardiovascular Systems Inc. | Bioabsorbable stent with prohealing layer |
| US8017237B2 (en) | 2006-06-23 | 2011-09-13 | Abbott Cardiovascular Systems, Inc. | Nanoshells on polymers |
| US8128688B2 (en) * | 2006-06-27 | 2012-03-06 | Abbott Cardiovascular Systems Inc. | Carbon coating on an implantable device |
| US8322455B2 (en) | 2006-06-27 | 2012-12-04 | Ethicon Endo-Surgery, Inc. | Manually driven surgical cutting and fastening instrument |
| US8956640B2 (en) * | 2006-06-29 | 2015-02-17 | Advanced Cardiovascular Systems, Inc. | Block copolymers including a methoxyethyl methacrylate midblock |
| US20080008736A1 (en) * | 2006-07-06 | 2008-01-10 | Thierry Glauser | Random copolymers of methacrylates and acrylates |
| US9028859B2 (en) * | 2006-07-07 | 2015-05-12 | Advanced Cardiovascular Systems, Inc. | Phase-separated block copolymer coatings for implantable medical devices |
| US7823263B2 (en) | 2006-07-11 | 2010-11-02 | Abbott Cardiovascular Systems Inc. | Method of removing stent islands from a stent |
| US8016879B2 (en) * | 2006-08-01 | 2011-09-13 | Abbott Cardiovascular Systems Inc. | Drug delivery after biodegradation of the stent scaffolding |
| US20080091262A1 (en) * | 2006-10-17 | 2008-04-17 | Gale David C | Drug delivery after biodegradation of the stent scaffolding |
| US8703169B1 (en) | 2006-08-15 | 2014-04-22 | Abbott Cardiovascular Systems Inc. | Implantable device having a coating comprising carrageenan and a biostable polymer |
| TW200817048A (en) * | 2006-09-08 | 2008-04-16 | Wyeth Corp | Dry powder compound formulations and uses thereof |
| US10130359B2 (en) | 2006-09-29 | 2018-11-20 | Ethicon Llc | Method for forming a staple |
| US10568652B2 (en) | 2006-09-29 | 2020-02-25 | Ethicon Llc | Surgical staples having attached drivers of different heights and stapling instruments for deploying the same |
| US8220690B2 (en) | 2006-09-29 | 2012-07-17 | Ethicon Endo-Surgery, Inc. | Connected surgical staples and stapling instruments for deploying the same |
| US11980366B2 (en) | 2006-10-03 | 2024-05-14 | Cilag Gmbh International | Surgical instrument |
| JP5557373B2 (ja) | 2006-11-21 | 2014-07-23 | アボット ラボラトリーズ | 薬剤溶出性コーティングにおけるテトラフルオロエチレン、ヘキサフルオロプロピレン、及びフッ化ビニリデンのターポリマーの使用 |
| US20080118541A1 (en) * | 2006-11-21 | 2008-05-22 | Abbott Laboratories | Use of a terpolymer of tetrafluoroethylene, hexafluoropropylene, and vinylidene fluoride in drug eluting coatings on medical devices |
| US7713541B1 (en) | 2006-11-21 | 2010-05-11 | Abbott Cardiovascular Systems Inc. | Zwitterionic terpolymers, method of making and use on medical devices |
| EP2101834B1 (en) * | 2006-12-01 | 2015-03-25 | Wake Forest University Health Sciences | Medical devices incorporating collagen inhibitors |
| US8597673B2 (en) * | 2006-12-13 | 2013-12-03 | Advanced Cardiovascular Systems, Inc. | Coating of fast absorption or dissolution |
| US8017141B2 (en) | 2006-12-15 | 2011-09-13 | Advanced Cardiovascular Systems, Inc. | Coatings of acrylamide-based copolymers |
| US8632535B2 (en) | 2007-01-10 | 2014-01-21 | Ethicon Endo-Surgery, Inc. | Interlock and surgical instrument including same |
| US11291441B2 (en) | 2007-01-10 | 2022-04-05 | Cilag Gmbh International | Surgical instrument with wireless communication between control unit and remote sensor |
| US8652120B2 (en) | 2007-01-10 | 2014-02-18 | Ethicon Endo-Surgery, Inc. | Surgical instrument with wireless communication between control unit and sensor transponders |
| US8684253B2 (en) | 2007-01-10 | 2014-04-01 | Ethicon Endo-Surgery, Inc. | Surgical instrument with wireless communication between a control unit of a robotic system and remote sensor |
| US20080169333A1 (en) | 2007-01-11 | 2008-07-17 | Shelton Frederick E | Surgical stapler end effector with tapered distal end |
| US11039836B2 (en) | 2007-01-11 | 2021-06-22 | Cilag Gmbh International | Staple cartridge for use with a surgical stapling instrument |
| GB0713463D0 (en) | 2007-07-11 | 2007-08-22 | Btg Int Ltd | Modulators of hypoxia inducible factor-1 and related uses |
| US7438209B1 (en) | 2007-03-15 | 2008-10-21 | Ethicon Endo-Surgery, Inc. | Surgical stapling instruments having a releasable staple-forming pocket |
| US8893946B2 (en) | 2007-03-28 | 2014-11-25 | Ethicon Endo-Surgery, Inc. | Laparoscopic tissue thickness and clamp load measuring devices |
| SI2565195T1 (sl) | 2007-03-29 | 2015-09-30 | Wyeth Llc | Periferalni opioidni receptorji in antagonisti in njih uporaba |
| AU2008233129B2 (en) * | 2007-03-29 | 2014-03-20 | Progenics Pharmaceuticals, Inc. | Peripheral opioid receptor antagonists and uses thereof |
| MX2009010550A (es) * | 2007-03-29 | 2009-12-14 | Progenics Pharm Inc | Formas de cristal de bromuro de (r)-n-metilnaltrexona y uso de las mismas. |
| US20080286332A1 (en) | 2007-05-14 | 2008-11-20 | Pacetti Stephen D | Implantable medical devices with a topcoat layer of phosphoryl choline acrylate polymer for reduced thrombosis, and improved mechanical properties |
| US8147769B1 (en) | 2007-05-16 | 2012-04-03 | Abbott Cardiovascular Systems Inc. | Stent and delivery system with reduced chemical degradation |
| US8084077B2 (en) * | 2007-05-25 | 2011-12-27 | Abbott Laboratories | One-step phosphorylcholine-linked polymer coating and drug loading of stent |
| US9056155B1 (en) | 2007-05-29 | 2015-06-16 | Abbott Cardiovascular Systems Inc. | Coatings having an elastic primer layer |
| US8931682B2 (en) | 2007-06-04 | 2015-01-13 | Ethicon Endo-Surgery, Inc. | Robotically-controlled shaft based rotary drive systems for surgical instruments |
| US11672531B2 (en) | 2007-06-04 | 2023-06-13 | Cilag Gmbh International | Rotary drive systems for surgical instruments |
| US20080317805A1 (en) * | 2007-06-19 | 2008-12-25 | Mckay William F | Locally administrated low doses of corticosteroids |
| US7753245B2 (en) | 2007-06-22 | 2010-07-13 | Ethicon Endo-Surgery, Inc. | Surgical stapling instruments |
| US8408439B2 (en) | 2007-06-22 | 2013-04-02 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument with an articulatable end effector |
| US8109904B1 (en) | 2007-06-25 | 2012-02-07 | Abbott Cardiovascular Systems Inc. | Drug delivery medical devices |
| US8048441B2 (en) | 2007-06-25 | 2011-11-01 | Abbott Cardiovascular Systems, Inc. | Nanobead releasing medical devices |
| US7901452B2 (en) * | 2007-06-27 | 2011-03-08 | Abbott Cardiovascular Systems Inc. | Method to fabricate a stent having selected morphology to reduce restenosis |
| US11849941B2 (en) | 2007-06-29 | 2023-12-26 | Cilag Gmbh International | Staple cartridge having staple cavities extending at a transverse angle relative to a longitudinal cartridge axis |
| US7955381B1 (en) | 2007-06-29 | 2011-06-07 | Advanced Cardiovascular Systems, Inc. | Polymer-bioceramic composite implantable medical device with different types of bioceramic particles |
| US20090041845A1 (en) * | 2007-08-08 | 2009-02-12 | Lothar Walter Kleiner | Implantable medical devices having thin absorbable coatings |
| KR101581480B1 (ko) * | 2008-02-06 | 2015-12-30 | 프로제닉스 파머슈티컬스, 인코포레이티드 | (r),(r)-2,2'-비스-메틸날트렉손의 제조법 및 용도 |
| US7905381B2 (en) | 2008-09-19 | 2011-03-15 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument with cutting member arrangement |
| US8561870B2 (en) | 2008-02-13 | 2013-10-22 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument |
| US8657174B2 (en) | 2008-02-14 | 2014-02-25 | Ethicon Endo-Surgery, Inc. | Motorized surgical cutting and fastening instrument having handle based power source |
| US8636736B2 (en) | 2008-02-14 | 2014-01-28 | Ethicon Endo-Surgery, Inc. | Motorized surgical cutting and fastening instrument |
| US9179912B2 (en) | 2008-02-14 | 2015-11-10 | Ethicon Endo-Surgery, Inc. | Robotically-controlled motorized surgical cutting and fastening instrument |
| US11986183B2 (en) | 2008-02-14 | 2024-05-21 | Cilag Gmbh International | Surgical cutting and fastening instrument comprising a plurality of sensors to measure an electrical parameter |
| US7819298B2 (en) | 2008-02-14 | 2010-10-26 | Ethicon Endo-Surgery, Inc. | Surgical stapling apparatus with control features operable with one hand |
| BRPI0901282A2 (pt) | 2008-02-14 | 2009-11-17 | Ethicon Endo Surgery Inc | instrumento cirúrgico de corte e fixação dotado de eletrodos de rf |
| US7866527B2 (en) | 2008-02-14 | 2011-01-11 | Ethicon Endo-Surgery, Inc. | Surgical stapling apparatus with interlockable firing system |
| US8573465B2 (en) | 2008-02-14 | 2013-11-05 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical end effector system with rotary actuated closure systems |
| US8758391B2 (en) | 2008-02-14 | 2014-06-24 | Ethicon Endo-Surgery, Inc. | Interchangeable tools for surgical instruments |
| US11272927B2 (en) | 2008-02-15 | 2022-03-15 | Cilag Gmbh International | Layer arrangements for surgical staple cartridges |
| US9615826B2 (en) | 2010-09-30 | 2017-04-11 | Ethicon Endo-Surgery, Llc | Multiple thickness implantable layers for surgical stapling devices |
| US20090206131A1 (en) | 2008-02-15 | 2009-08-20 | Ethicon Endo-Surgery, Inc. | End effector coupling arrangements for a surgical cutting and stapling instrument |
| AU2009225434B2 (en) | 2008-03-21 | 2014-05-22 | The University Of Chicago | Treatment with opioid antagonists and mTOR inhibitors |
| US8883768B2 (en) * | 2008-04-18 | 2014-11-11 | Warsaw Orthopedic, Inc. | Fluocinolone implants to protect against undesirable bone and cartilage destruction |
| US8202531B2 (en) * | 2008-07-23 | 2012-06-19 | Warsaw Orthopedic, Inc. | Drug depots having one or more anchoring members |
| PL3476312T3 (pl) | 2008-09-19 | 2024-03-11 | Ethicon Llc | Stapler chirurgiczny z urządzeniem do dopasowania wysokości zszywek |
| US8210411B2 (en) | 2008-09-23 | 2012-07-03 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting instrument |
| US9005230B2 (en) | 2008-09-23 | 2015-04-14 | Ethicon Endo-Surgery, Inc. | Motorized surgical instrument |
| US11648005B2 (en) | 2008-09-23 | 2023-05-16 | Cilag Gmbh International | Robotically-controlled motorized surgical instrument with an end effector |
| US9386983B2 (en) | 2008-09-23 | 2016-07-12 | Ethicon Endo-Surgery, Llc | Robotically-controlled motorized surgical instrument |
| CA2676881C (en) * | 2008-09-30 | 2017-04-25 | Wyeth | Peripheral opioid receptor antagonists and uses thereof |
| US8608045B2 (en) | 2008-10-10 | 2013-12-17 | Ethicon Endo-Sugery, Inc. | Powered surgical cutting and stapling apparatus with manually retractable firing system |
| US8517239B2 (en) | 2009-02-05 | 2013-08-27 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument comprising a magnetic element driver |
| US8444036B2 (en) | 2009-02-06 | 2013-05-21 | Ethicon Endo-Surgery, Inc. | Motor driven surgical fastener device with mechanisms for adjusting a tissue gap within the end effector |
| WO2010090940A1 (en) | 2009-02-06 | 2010-08-12 | Ethicon Endo-Surgery, Inc. | Driven surgical stapler improvements |
| JP5819733B2 (ja) * | 2009-02-12 | 2015-11-24 | ストライカー コーポレイションStryker Corporation | 障害および疾患に対する全身治療のためのTGF−βスーパーファミリーメンバー含有タンパク質の末梢投与 |
| WO2010093941A2 (en) * | 2009-02-12 | 2010-08-19 | Stryker Corporation | COMPOSITIONS AND METHODS FOR MINIMALLY-INVASIVE SYSTEMIC DELIVERY OF PROTEINS INCLUDING TGF-β SUPERFAMILY MEMBERS |
| US20100249783A1 (en) * | 2009-03-24 | 2010-09-30 | Warsaw Orthopedic, Inc. | Drug-eluting implant cover |
| US9414864B2 (en) | 2009-04-15 | 2016-08-16 | Warsaw Orthopedic, Inc. | Anterior spinal plate with preformed drug-eluting device affixed thereto |
| US9078712B2 (en) * | 2009-04-15 | 2015-07-14 | Warsaw Orthopedic, Inc. | Preformed drug-eluting device to be affixed to an anterior spinal plate |
| US8851354B2 (en) | 2009-12-24 | 2014-10-07 | Ethicon Endo-Surgery, Inc. | Surgical cutting instrument that analyzes tissue thickness |
| US8220688B2 (en) | 2009-12-24 | 2012-07-17 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting instrument with electric actuator directional control assembly |
| US8608046B2 (en) | 2010-01-07 | 2013-12-17 | Ethicon Endo-Surgery, Inc. | Test device for a surgical tool |
| US8808353B2 (en) | 2010-01-30 | 2014-08-19 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds having a low crossing profile |
| US8568471B2 (en) | 2010-01-30 | 2013-10-29 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds |
| US8685433B2 (en) | 2010-03-31 | 2014-04-01 | Abbott Cardiovascular Systems Inc. | Absorbable coating for implantable device |
| US8783543B2 (en) | 2010-07-30 | 2014-07-22 | Ethicon Endo-Surgery, Inc. | Tissue acquisition arrangements and methods for surgical stapling devices |
| US8360296B2 (en) | 2010-09-09 | 2013-01-29 | Ethicon Endo-Surgery, Inc. | Surgical stapling head assembly with firing lockout for a surgical stapler |
| US9289212B2 (en) | 2010-09-17 | 2016-03-22 | Ethicon Endo-Surgery, Inc. | Surgical instruments and batteries for surgical instruments |
| US8632525B2 (en) | 2010-09-17 | 2014-01-21 | Ethicon Endo-Surgery, Inc. | Power control arrangements for surgical instruments and batteries |
| US8733613B2 (en) | 2010-09-29 | 2014-05-27 | Ethicon Endo-Surgery, Inc. | Staple cartridge |
| US9232941B2 (en) | 2010-09-30 | 2016-01-12 | Ethicon Endo-Surgery, Inc. | Tissue thickness compensator comprising a reservoir |
| US8657176B2 (en) | 2010-09-30 | 2014-02-25 | Ethicon Endo-Surgery, Inc. | Tissue thickness compensator for a surgical stapler |
| US10945731B2 (en) | 2010-09-30 | 2021-03-16 | Ethicon Llc | Tissue thickness compensator comprising controlled release and expansion |
| US11298125B2 (en) | 2010-09-30 | 2022-04-12 | Cilag Gmbh International | Tissue stapler having a thickness compensator |
| US9314246B2 (en) | 2010-09-30 | 2016-04-19 | Ethicon Endo-Surgery, Llc | Tissue stapler having a thickness compensator incorporating an anti-inflammatory agent |
| US9364233B2 (en) | 2010-09-30 | 2016-06-14 | Ethicon Endo-Surgery, Llc | Tissue thickness compensators for circular surgical staplers |
| US11925354B2 (en) | 2010-09-30 | 2024-03-12 | Cilag Gmbh International | Staple cartridge comprising staples positioned within a compressible portion thereof |
| US9055941B2 (en) | 2011-09-23 | 2015-06-16 | Ethicon Endo-Surgery, Inc. | Staple cartridge including collapsible deck |
| US9277919B2 (en) | 2010-09-30 | 2016-03-08 | Ethicon Endo-Surgery, Llc | Tissue thickness compensator comprising fibers to produce a resilient load |
| US11812965B2 (en) | 2010-09-30 | 2023-11-14 | Cilag Gmbh International | Layer of material for a surgical end effector |
| US9332974B2 (en) | 2010-09-30 | 2016-05-10 | Ethicon Endo-Surgery, Llc | Layered tissue thickness compensator |
| US9301752B2 (en) | 2010-09-30 | 2016-04-05 | Ethicon Endo-Surgery, Llc | Tissue thickness compensator comprising a plurality of capsules |
| US9386988B2 (en) | 2010-09-30 | 2016-07-12 | Ethicon End-Surgery, LLC | Retainer assembly including a tissue thickness compensator |
| US9241714B2 (en) | 2011-04-29 | 2016-01-26 | Ethicon Endo-Surgery, Inc. | Tissue thickness compensator and method for making the same |
| US9307989B2 (en) | 2012-03-28 | 2016-04-12 | Ethicon Endo-Surgery, Llc | Tissue stapler having a thickness compensator incorportating a hydrophobic agent |
| US10213198B2 (en) | 2010-09-30 | 2019-02-26 | Ethicon Llc | Actuator for releasing a tissue thickness compensator from a fastener cartridge |
| US9629814B2 (en) | 2010-09-30 | 2017-04-25 | Ethicon Endo-Surgery, Llc | Tissue thickness compensator configured to redistribute compressive forces |
| US9220501B2 (en) | 2010-09-30 | 2015-12-29 | Ethicon Endo-Surgery, Inc. | Tissue thickness compensators |
| JP5902180B2 (ja) | 2010-09-30 | 2016-04-13 | エシコン・エンド−サージェリィ・インコーポレイテッドEthicon Endo−Surgery,Inc. | 保持マトリックス及び位置合わせマトリックスを含む、締結システム |
| US9301753B2 (en) | 2010-09-30 | 2016-04-05 | Ethicon Endo-Surgery, Llc | Expandable tissue thickness compensator |
| US8695866B2 (en) | 2010-10-01 | 2014-04-15 | Ethicon Endo-Surgery, Inc. | Surgical instrument having a power control circuit |
| US8632462B2 (en) | 2011-03-14 | 2014-01-21 | Ethicon Endo-Surgery, Inc. | Trans-rectum universal ports |
| CA2834649C (en) | 2011-04-29 | 2021-02-16 | Ethicon Endo-Surgery, Inc. | Staple cartridge comprising staples positioned within a compressible portion thereof |
| US11207064B2 (en) | 2011-05-27 | 2021-12-28 | Cilag Gmbh International | Automated end effector component reloading system for use with a robotic system |
| US8726483B2 (en) | 2011-07-29 | 2014-05-20 | Abbott Cardiovascular Systems Inc. | Methods for uniform crimping and deployment of a polymer scaffold |
| HUE042327T2 (hu) | 2011-08-30 | 2019-06-28 | Astex Pharmaceuticals Inc | Decitabin-származék készítmények |
| US9050084B2 (en) | 2011-09-23 | 2015-06-09 | Ethicon Endo-Surgery, Inc. | Staple cartridge including collapsible deck arrangement |
| US9044230B2 (en) | 2012-02-13 | 2015-06-02 | Ethicon Endo-Surgery, Inc. | Surgical cutting and fastening instrument with apparatus for determining cartridge and firing motion status |
| WO2013130619A1 (en) * | 2012-02-27 | 2013-09-06 | O-Ray Pharma, Inc. | Solid drug implants for intracochlear delivery of therapeutics for the treatment of otic disorders |
| US9078653B2 (en) | 2012-03-26 | 2015-07-14 | Ethicon Endo-Surgery, Inc. | Surgical stapling device with lockout system for preventing actuation in the absence of an installed staple cartridge |
| RU2644272C2 (ru) | 2012-03-28 | 2018-02-08 | Этикон Эндо-Серджери, Инк. | Узел ограничения, включающий компенсатор толщины ткани |
| MX350846B (es) | 2012-03-28 | 2017-09-22 | Ethicon Endo Surgery Inc | Compensador de grosor de tejido que comprende cápsulas que definen un ambiente de baja presión. |
| BR112014024102B1 (pt) | 2012-03-28 | 2022-03-03 | Ethicon Endo-Surgery, Inc | Conjunto de cartucho de prendedores para um instrumento cirúrgico, e conjunto de atuador de extremidade para um instrumento cirúrgico |
| US9198662B2 (en) | 2012-03-28 | 2015-12-01 | Ethicon Endo-Surgery, Inc. | Tissue thickness compensator having improved visibility |
| US9827401B2 (en) | 2012-06-01 | 2017-11-28 | Surmodics, Inc. | Apparatus and methods for coating medical devices |
| US9308355B2 (en) | 2012-06-01 | 2016-04-12 | Surmodies, Inc. | Apparatus and methods for coating medical devices |
| US9101358B2 (en) | 2012-06-15 | 2015-08-11 | Ethicon Endo-Surgery, Inc. | Articulatable surgical instrument comprising a firing drive |
| US11202631B2 (en) | 2012-06-28 | 2021-12-21 | Cilag Gmbh International | Stapling assembly comprising a firing lockout |
| US9649111B2 (en) | 2012-06-28 | 2017-05-16 | Ethicon Endo-Surgery, Llc | Replaceable clip cartridge for a clip applier |
| US9028494B2 (en) | 2012-06-28 | 2015-05-12 | Ethicon Endo-Surgery, Inc. | Interchangeable end effector coupling arrangement |
| US9289256B2 (en) | 2012-06-28 | 2016-03-22 | Ethicon Endo-Surgery, Llc | Surgical end effectors having angled tissue-contacting surfaces |
| US9101385B2 (en) | 2012-06-28 | 2015-08-11 | Ethicon Endo-Surgery, Inc. | Electrode connections for rotary driven surgical tools |
| US20140005718A1 (en) | 2012-06-28 | 2014-01-02 | Ethicon Endo-Surgery, Inc. | Multi-functional powered surgical device with external dissection features |
| US9119657B2 (en) | 2012-06-28 | 2015-09-01 | Ethicon Endo-Surgery, Inc. | Rotary actuatable closure arrangement for surgical end effector |
| BR112014032776B1 (pt) | 2012-06-28 | 2021-09-08 | Ethicon Endo-Surgery, Inc | Sistema de instrumento cirúrgico e kit cirúrgico para uso com um sistema de instrumento cirúrgico |
| US9125662B2 (en) | 2012-06-28 | 2015-09-08 | Ethicon Endo-Surgery, Inc. | Multi-axis articulating and rotating surgical tools |
| CN104487005B (zh) | 2012-06-28 | 2017-09-08 | 伊西康内外科公司 | 空夹仓闭锁件 |
| US9561038B2 (en) | 2012-06-28 | 2017-02-07 | Ethicon Endo-Surgery, Llc | Interchangeable clip applier |
| US9364230B2 (en) | 2012-06-28 | 2016-06-14 | Ethicon Endo-Surgery, Llc | Surgical stapling instruments with rotary joint assemblies |
| US20140001231A1 (en) | 2012-06-28 | 2014-01-02 | Ethicon Endo-Surgery, Inc. | Firing system lockout arrangements for surgical instruments |
| US9072536B2 (en) | 2012-06-28 | 2015-07-07 | Ethicon Endo-Surgery, Inc. | Differential locking arrangements for rotary powered surgical instruments |
| US9700310B2 (en) | 2013-08-23 | 2017-07-11 | Ethicon Llc | Firing member retraction devices for powered surgical instruments |
| US9386985B2 (en) | 2012-10-15 | 2016-07-12 | Ethicon Endo-Surgery, Llc | Surgical cutting instrument |
| US11090468B2 (en) | 2012-10-25 | 2021-08-17 | Surmodics, Inc. | Apparatus and methods for coating medical devices |
| US9386984B2 (en) | 2013-02-08 | 2016-07-12 | Ethicon Endo-Surgery, Llc | Staple cartridge comprising a releasable cover |
| US20140249557A1 (en) | 2013-03-01 | 2014-09-04 | Ethicon Endo-Surgery, Inc. | Thumbwheel switch arrangements for surgical instruments |
| MX364729B (es) | 2013-03-01 | 2019-05-06 | Ethicon Endo Surgery Inc | Instrumento quirúrgico con una parada suave. |
| MX368026B (es) | 2013-03-01 | 2019-09-12 | Ethicon Endo Surgery Inc | Instrumento quirúrgico articulable con vías conductoras para la comunicación de la señal. |
| JP2016510750A (ja) | 2013-03-08 | 2016-04-11 | アラーガン、インコーポレイテッドAllergan,Incorporated | ステロイド薬と直接結合した抗生物質共役体 |
| KR20150125663A (ko) * | 2013-03-08 | 2015-11-09 | 알러간, 인코포레이티드 | 사이클로스포린 a 스테로이드 켤레물 |
| US20140263552A1 (en) | 2013-03-13 | 2014-09-18 | Ethicon Endo-Surgery, Inc. | Staple cartridge tissue thickness sensor system |
| US9629623B2 (en) | 2013-03-14 | 2017-04-25 | Ethicon Endo-Surgery, Llc | Drive system lockout arrangements for modular surgical instruments |
| US9629629B2 (en) | 2013-03-14 | 2017-04-25 | Ethicon Endo-Surgey, LLC | Control systems for surgical instruments |
| CN105377319B (zh) * | 2013-03-15 | 2022-01-28 | 波纹疗法公司 | 用于药物释放的化合物和组合物 |
| US10010655B2 (en) * | 2013-03-15 | 2018-07-03 | Abbott Cardiovascular Systems Inc. | Drug delivery device, method, and system for administration of dual antiplatelet therapy |
| US9332984B2 (en) | 2013-03-27 | 2016-05-10 | Ethicon Endo-Surgery, Llc | Fastener cartridge assemblies |
| US9795384B2 (en) | 2013-03-27 | 2017-10-24 | Ethicon Llc | Fastener cartridge comprising a tissue thickness compensator and a gap setting element |
| US9572577B2 (en) | 2013-03-27 | 2017-02-21 | Ethicon Endo-Surgery, Llc | Fastener cartridge comprising a tissue thickness compensator including openings therein |
| BR112015026109B1 (pt) | 2013-04-16 | 2022-02-22 | Ethicon Endo-Surgery, Inc | Instrumento cirúrgico |
| US10149680B2 (en) | 2013-04-16 | 2018-12-11 | Ethicon Llc | Surgical instrument comprising a gap setting system |
| US9574644B2 (en) | 2013-05-30 | 2017-02-21 | Ethicon Endo-Surgery, Llc | Power module for use with a surgical instrument |
| US10391490B2 (en) * | 2013-05-31 | 2019-08-27 | Celsee Diagnostics, Inc. | System and method for isolating and analyzing cells |
| BR112016003329B1 (pt) | 2013-08-23 | 2021-12-21 | Ethicon Endo-Surgery, Llc | Instrumento cirúrgico |
| US9585662B2 (en) | 2013-12-23 | 2017-03-07 | Ethicon Endo-Surgery, Llc | Fastener cartridge comprising an extendable firing member |
| US20150173756A1 (en) | 2013-12-23 | 2015-06-25 | Ethicon Endo-Surgery, Inc. | Surgical cutting and stapling methods |
| US9724092B2 (en) | 2013-12-23 | 2017-08-08 | Ethicon Llc | Modular surgical instruments |
| US9681870B2 (en) | 2013-12-23 | 2017-06-20 | Ethicon Llc | Articulatable surgical instruments with separate and distinct closing and firing systems |
| US9839428B2 (en) | 2013-12-23 | 2017-12-12 | Ethicon Llc | Surgical cutting and stapling instruments with independent jaw control features |
| US9642620B2 (en) | 2013-12-23 | 2017-05-09 | Ethicon Endo-Surgery, Llc | Surgical cutting and stapling instruments with articulatable end effectors |
| US9962161B2 (en) | 2014-02-12 | 2018-05-08 | Ethicon Llc | Deliverable surgical instrument |
| US9839422B2 (en) | 2014-02-24 | 2017-12-12 | Ethicon Llc | Implantable layers and methods for altering implantable layers for use with surgical fastening instruments |
| JP6462004B2 (ja) | 2014-02-24 | 2019-01-30 | エシコン エルエルシー | 発射部材ロックアウトを備える締結システム |
| US9750499B2 (en) | 2014-03-26 | 2017-09-05 | Ethicon Llc | Surgical stapling instrument system |
| US9820738B2 (en) | 2014-03-26 | 2017-11-21 | Ethicon Llc | Surgical instrument comprising interactive systems |
| US9913642B2 (en) | 2014-03-26 | 2018-03-13 | Ethicon Llc | Surgical instrument comprising a sensor system |
| BR112016021943B1 (pt) | 2014-03-26 | 2022-06-14 | Ethicon Endo-Surgery, Llc | Instrumento cirúrgico para uso por um operador em um procedimento cirúrgico |
| US9826977B2 (en) | 2014-03-26 | 2017-11-28 | Ethicon Llc | Sterilization verification circuit |
| US10542988B2 (en) | 2014-04-16 | 2020-01-28 | Ethicon Llc | End effector comprising an anvil including projections extending therefrom |
| US9801627B2 (en) | 2014-09-26 | 2017-10-31 | Ethicon Llc | Fastener cartridge for creating a flexible staple line |
| JP6636452B2 (ja) | 2014-04-16 | 2020-01-29 | エシコン エルエルシーEthicon LLC | 異なる構成を有する延在部を含む締結具カートリッジ |
| US20150297225A1 (en) | 2014-04-16 | 2015-10-22 | Ethicon Endo-Surgery, Inc. | Fastener cartridges including extensions having different configurations |
| CN106456158B (zh) | 2014-04-16 | 2019-02-05 | 伊西康内外科有限责任公司 | 包括非一致紧固件的紧固件仓 |
| BR112016023807B1 (pt) | 2014-04-16 | 2022-07-12 | Ethicon Endo-Surgery, Llc | Conjunto de cartucho de prendedores para uso com um instrumento cirúrgico |
| US10045781B2 (en) | 2014-06-13 | 2018-08-14 | Ethicon Llc | Closure lockout systems for surgical instruments |
| US11311294B2 (en) | 2014-09-05 | 2022-04-26 | Cilag Gmbh International | Powered medical device including measurement of closure state of jaws |
| US10111679B2 (en) | 2014-09-05 | 2018-10-30 | Ethicon Llc | Circuitry and sensors for powered medical device |
| BR112017004361B1 (pt) | 2014-09-05 | 2023-04-11 | Ethicon Llc | Sistema eletrônico para um instrumento cirúrgico |
| US10105142B2 (en) | 2014-09-18 | 2018-10-23 | Ethicon Llc | Surgical stapler with plurality of cutting elements |
| MX2017003960A (es) | 2014-09-26 | 2017-12-04 | Ethicon Llc | Refuerzos de grapas quirúrgicas y materiales auxiliares. |
| US11523821B2 (en) | 2014-09-26 | 2022-12-13 | Cilag Gmbh International | Method for creating a flexible staple line |
| US10076325B2 (en) | 2014-10-13 | 2018-09-18 | Ethicon Llc | Surgical stapling apparatus comprising a tissue stop |
| US9924944B2 (en) | 2014-10-16 | 2018-03-27 | Ethicon Llc | Staple cartridge comprising an adjunct material |
| US11141153B2 (en) | 2014-10-29 | 2021-10-12 | Cilag Gmbh International | Staple cartridges comprising driver arrangements |
| US10517594B2 (en) | 2014-10-29 | 2019-12-31 | Ethicon Llc | Cartridge assemblies for surgical staplers |
| US9844376B2 (en) | 2014-11-06 | 2017-12-19 | Ethicon Llc | Staple cartridge comprising a releasable adjunct material |
| US10736636B2 (en) | 2014-12-10 | 2020-08-11 | Ethicon Llc | Articulatable surgical instrument system |
| US9968355B2 (en) | 2014-12-18 | 2018-05-15 | Ethicon Llc | Surgical instruments with articulatable end effectors and improved firing beam support arrangements |
| US10188385B2 (en) | 2014-12-18 | 2019-01-29 | Ethicon Llc | Surgical instrument system comprising lockable systems |
| US9844375B2 (en) | 2014-12-18 | 2017-12-19 | Ethicon Llc | Drive arrangements for articulatable surgical instruments |
| US10085748B2 (en) | 2014-12-18 | 2018-10-02 | Ethicon Llc | Locking arrangements for detachable shaft assemblies with articulatable surgical end effectors |
| US9844374B2 (en) | 2014-12-18 | 2017-12-19 | Ethicon Llc | Surgical instrument systems comprising an articulatable end effector and means for adjusting the firing stroke of a firing member |
| US9987000B2 (en) | 2014-12-18 | 2018-06-05 | Ethicon Llc | Surgical instrument assembly comprising a flexible articulation system |
| RU2703684C2 (ru) | 2014-12-18 | 2019-10-21 | ЭТИКОН ЭНДО-СЕРДЖЕРИ, ЭлЭлСи | Хирургический инструмент с упором, который выполнен с возможностью избирательного перемещения относительно кассеты со скобами вокруг дискретной неподвижной оси |
| US10117649B2 (en) | 2014-12-18 | 2018-11-06 | Ethicon Llc | Surgical instrument assembly comprising a lockable articulation system |
| US9999527B2 (en) | 2015-02-11 | 2018-06-19 | Abbott Cardiovascular Systems Inc. | Scaffolds having radiopaque markers |
| US10226250B2 (en) | 2015-02-27 | 2019-03-12 | Ethicon Llc | Modular stapling assembly |
| US11154301B2 (en) | 2015-02-27 | 2021-10-26 | Cilag Gmbh International | Modular stapling assembly |
| US10180463B2 (en) | 2015-02-27 | 2019-01-15 | Ethicon Llc | Surgical apparatus configured to assess whether a performance parameter of the surgical apparatus is within an acceptable performance band |
| US20160249910A1 (en) | 2015-02-27 | 2016-09-01 | Ethicon Endo-Surgery, Llc | Surgical charging system that charges and/or conditions one or more batteries |
| US9895148B2 (en) | 2015-03-06 | 2018-02-20 | Ethicon Endo-Surgery, Llc | Monitoring speed control and precision incrementing of motor for powered surgical instruments |
| US10245033B2 (en) | 2015-03-06 | 2019-04-02 | Ethicon Llc | Surgical instrument comprising a lockable battery housing |
| US10052044B2 (en) | 2015-03-06 | 2018-08-21 | Ethicon Llc | Time dependent evaluation of sensor data to determine stability, creep, and viscoelastic elements of measures |
| US10045776B2 (en) | 2015-03-06 | 2018-08-14 | Ethicon Llc | Control techniques and sub-processor contained within modular shaft with select control processing from handle |
| US10687806B2 (en) | 2015-03-06 | 2020-06-23 | Ethicon Llc | Adaptive tissue compression techniques to adjust closure rates for multiple tissue types |
| US9808246B2 (en) | 2015-03-06 | 2017-11-07 | Ethicon Endo-Surgery, Llc | Method of operating a powered surgical instrument |
| JP2020121162A (ja) | 2015-03-06 | 2020-08-13 | エシコン エルエルシーEthicon LLC | 測定の安定性要素、クリープ要素、及び粘弾性要素を決定するためのセンサデータの時間依存性評価 |
| US9924961B2 (en) | 2015-03-06 | 2018-03-27 | Ethicon Endo-Surgery, Llc | Interactive feedback system for powered surgical instruments |
| US10441279B2 (en) | 2015-03-06 | 2019-10-15 | Ethicon Llc | Multiple level thresholds to modify operation of powered surgical instruments |
| US10617412B2 (en) | 2015-03-06 | 2020-04-14 | Ethicon Llc | System for detecting the mis-insertion of a staple cartridge into a surgical stapler |
| US9993248B2 (en) | 2015-03-06 | 2018-06-12 | Ethicon Endo-Surgery, Llc | Smart sensors with local signal processing |
| US9901342B2 (en) | 2015-03-06 | 2018-02-27 | Ethicon Endo-Surgery, Llc | Signal and power communication system positioned on a rotatable shaft |
| US10433844B2 (en) | 2015-03-31 | 2019-10-08 | Ethicon Llc | Surgical instrument with selectively disengageable threaded drive systems |
| US10449055B2 (en) | 2015-04-23 | 2019-10-22 | Disc Fix L.L.C. | Systems and methods for treatment of intervertebral disc derangements |
| US9700443B2 (en) | 2015-06-12 | 2017-07-11 | Abbott Cardiovascular Systems Inc. | Methods for attaching a radiopaque marker to a scaffold |
| US10335149B2 (en) | 2015-06-18 | 2019-07-02 | Ethicon Llc | Articulatable surgical instruments with composite firing beam structures with center firing support member for articulation support |
| KR20180024010A (ko) | 2015-07-02 | 2018-03-07 | 오쓰까 세이야꾸 가부시키가이샤 | 동결건조된 약제학적 조성물 |
| US10617418B2 (en) | 2015-08-17 | 2020-04-14 | Ethicon Llc | Implantable layers for a surgical instrument |
| US10080806B2 (en) | 2015-08-19 | 2018-09-25 | International Business Machines Corporation | Sulfur-containing polymers from hexahydrotriazine and dithiol precursors as a carrier for active agents |
| US10357251B2 (en) | 2015-08-26 | 2019-07-23 | Ethicon Llc | Surgical staples comprising hardness variations for improved fastening of tissue |
| MX2022009705A (es) | 2015-08-26 | 2022-11-07 | Ethicon Llc | Metodo para formar una grapa contra un yunque de un instrumento de engrapado quirurgico. |
| BR112018003693B1 (pt) | 2015-08-26 | 2022-11-22 | Ethicon Llc | Cartucho de grampos cirúrgicos para uso com um instrumento de grampeamento cirúrgico |
| MX2022006191A (es) | 2015-09-02 | 2022-06-16 | Ethicon Llc | Configuraciones de grapas quirurgicas con superficies de leva situadas entre porciones que soportan grapas quirurgicas. |
| US10357252B2 (en) | 2015-09-02 | 2019-07-23 | Ethicon Llc | Surgical staple configurations with camming surfaces located between portions supporting surgical staples |
| US10076326B2 (en) | 2015-09-23 | 2018-09-18 | Ethicon Llc | Surgical stapler having current mirror-based motor control |
| US10105139B2 (en) | 2015-09-23 | 2018-10-23 | Ethicon Llc | Surgical stapler having downstream current-based motor control |
| US10238386B2 (en) | 2015-09-23 | 2019-03-26 | Ethicon Llc | Surgical stapler having motor control based on an electrical parameter related to a motor current |
| US10085751B2 (en) | 2015-09-23 | 2018-10-02 | Ethicon Llc | Surgical stapler having temperature-based motor control |
| US10327769B2 (en) | 2015-09-23 | 2019-06-25 | Ethicon Llc | Surgical stapler having motor control based on a drive system component |
| US10363036B2 (en) | 2015-09-23 | 2019-07-30 | Ethicon Llc | Surgical stapler having force-based motor control |
| US10299878B2 (en) | 2015-09-25 | 2019-05-28 | Ethicon Llc | Implantable adjunct systems for determining adjunct skew |
| US11890015B2 (en) | 2015-09-30 | 2024-02-06 | Cilag Gmbh International | Compressible adjunct with crossing spacer fibers |
| US10433846B2 (en) | 2015-09-30 | 2019-10-08 | Ethicon Llc | Compressible adjunct with crossing spacer fibers |
| US10307160B2 (en) | 2015-09-30 | 2019-06-04 | Ethicon Llc | Compressible adjunct assemblies with attachment layers |
| US10980539B2 (en) | 2015-09-30 | 2021-04-20 | Ethicon Llc | Implantable adjunct comprising bonded layers |
| US9550863B1 (en) | 2015-10-05 | 2017-01-24 | International Business Machines Corporation | Polymers from stabilized imines |
| US9534084B1 (en) | 2015-11-02 | 2017-01-03 | International Business Machines Corporation | High molecular weight polythioaminals from a single monomer |
| US9862802B2 (en) | 2015-11-30 | 2018-01-09 | International Business Machines Corporation | Poly(thioaminal) probe based lithography |
| US10265068B2 (en) | 2015-12-30 | 2019-04-23 | Ethicon Llc | Surgical instruments with separable motors and motor control circuits |
| US10292704B2 (en) | 2015-12-30 | 2019-05-21 | Ethicon Llc | Mechanisms for compensating for battery pack failure in powered surgical instruments |
| US10368865B2 (en) | 2015-12-30 | 2019-08-06 | Ethicon Llc | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| BR112018016098B1 (pt) | 2016-02-09 | 2023-02-23 | Ethicon Llc | Instrumento cirúrgico |
| US10245030B2 (en) | 2016-02-09 | 2019-04-02 | Ethicon Llc | Surgical instruments with tensioning arrangements for cable driven articulation systems |
| US11213293B2 (en) | 2016-02-09 | 2022-01-04 | Cilag Gmbh International | Articulatable surgical instruments with single articulation link arrangements |
| US11224426B2 (en) | 2016-02-12 | 2022-01-18 | Cilag Gmbh International | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10448948B2 (en) | 2016-02-12 | 2019-10-22 | Ethicon Llc | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10258331B2 (en) | 2016-02-12 | 2019-04-16 | Ethicon Llc | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10617413B2 (en) | 2016-04-01 | 2020-04-14 | Ethicon Llc | Closure system arrangements for surgical cutting and stapling devices with separate and distinct firing shafts |
| US11064997B2 (en) | 2016-04-01 | 2021-07-20 | Cilag Gmbh International | Surgical stapling instrument |
| US11284890B2 (en) | 2016-04-01 | 2022-03-29 | Cilag Gmbh International | Circular stapling system comprising an incisable tissue support |
| US10342543B2 (en) | 2016-04-01 | 2019-07-09 | Ethicon Llc | Surgical stapling system comprising a shiftable transmission |
| US10357246B2 (en) | 2016-04-01 | 2019-07-23 | Ethicon Llc | Rotary powered surgical instrument with manually actuatable bailout system |
| US11179150B2 (en) | 2016-04-15 | 2021-11-23 | Cilag Gmbh International | Systems and methods for controlling a surgical stapling and cutting instrument |
| US10405859B2 (en) | 2016-04-15 | 2019-09-10 | Ethicon Llc | Surgical instrument with adjustable stop/start control during a firing motion |
| US10426467B2 (en) | 2016-04-15 | 2019-10-01 | Ethicon Llc | Surgical instrument with detection sensors |
| US10335145B2 (en) | 2016-04-15 | 2019-07-02 | Ethicon Llc | Modular surgical instrument with configurable operating mode |
| US10492783B2 (en) | 2016-04-15 | 2019-12-03 | Ethicon, Llc | Surgical instrument with improved stop/start control during a firing motion |
| US10828028B2 (en) | 2016-04-15 | 2020-11-10 | Ethicon Llc | Surgical instrument with multiple program responses during a firing motion |
| US10456137B2 (en) | 2016-04-15 | 2019-10-29 | Ethicon Llc | Staple formation detection mechanisms |
| US10357247B2 (en) | 2016-04-15 | 2019-07-23 | Ethicon Llc | Surgical instrument with multiple program responses during a firing motion |
| US11607239B2 (en) | 2016-04-15 | 2023-03-21 | Cilag Gmbh International | Systems and methods for controlling a surgical stapling and cutting instrument |
| US11317917B2 (en) | 2016-04-18 | 2022-05-03 | Cilag Gmbh International | Surgical stapling system comprising a lockable firing assembly |
| US20170296173A1 (en) | 2016-04-18 | 2017-10-19 | Ethicon Endo-Surgery, Llc | Method for operating a surgical instrument |
| US10368867B2 (en) | 2016-04-18 | 2019-08-06 | Ethicon Llc | Surgical instrument comprising a lockout |
| US10893863B2 (en) | 2016-06-24 | 2021-01-19 | Ethicon Llc | Staple cartridge comprising offset longitudinal staple rows |
| JP6957532B2 (ja) | 2016-06-24 | 2021-11-02 | エシコン エルエルシーEthicon LLC | ワイヤステープル及び打ち抜き加工ステープルを含むステープルカートリッジ |
| USD847989S1 (en) | 2016-06-24 | 2019-05-07 | Ethicon Llc | Surgical fastener cartridge |
| USD826405S1 (en) | 2016-06-24 | 2018-08-21 | Ethicon Llc | Surgical fastener |
| USD850617S1 (en) | 2016-06-24 | 2019-06-04 | Ethicon Llc | Surgical fastener cartridge |
| US10687810B2 (en) | 2016-12-21 | 2020-06-23 | Ethicon Llc | Stepped staple cartridge with tissue retention and gap setting features |
| US10499914B2 (en) | 2016-12-21 | 2019-12-10 | Ethicon Llc | Staple forming pocket arrangements |
| US20180168648A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Durability features for end effectors and firing assemblies of surgical stapling instruments |
| US20180168625A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Surgical stapling instruments with smart staple cartridges |
| US10993715B2 (en) | 2016-12-21 | 2021-05-04 | Ethicon Llc | Staple cartridge comprising staples with different clamping breadths |
| US10426471B2 (en) | 2016-12-21 | 2019-10-01 | Ethicon Llc | Surgical instrument with multiple failure response modes |
| JP6983893B2 (ja) | 2016-12-21 | 2021-12-17 | エシコン エルエルシーEthicon LLC | 外科用エンドエフェクタ及び交換式ツールアセンブリのためのロックアウト構成 |
| US11134942B2 (en) | 2016-12-21 | 2021-10-05 | Cilag Gmbh International | Surgical stapling instruments and staple-forming anvils |
| US10617414B2 (en) | 2016-12-21 | 2020-04-14 | Ethicon Llc | Closure member arrangements for surgical instruments |
| US10813638B2 (en) | 2016-12-21 | 2020-10-27 | Ethicon Llc | Surgical end effectors with expandable tissue stop arrangements |
| US20180168619A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Surgical stapling systems |
| US10945727B2 (en) | 2016-12-21 | 2021-03-16 | Ethicon Llc | Staple cartridge with deformable driver retention features |
| CN110114014B (zh) | 2016-12-21 | 2022-08-09 | 爱惜康有限责任公司 | 包括端部执行器闭锁件和击发组件闭锁件的外科器械系统 |
| US11419606B2 (en) | 2016-12-21 | 2022-08-23 | Cilag Gmbh International | Shaft assembly comprising a clutch configured to adapt the output of a rotary firing member to two different systems |
| US20180168615A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Method of deforming staples from two different types of staple cartridges with the same surgical stapling instrument |
| US10667811B2 (en) | 2016-12-21 | 2020-06-02 | Ethicon Llc | Surgical stapling instruments and staple-forming anvils |
| US10610224B2 (en) | 2016-12-21 | 2020-04-07 | Ethicon Llc | Lockout arrangements for surgical end effectors and replaceable tool assemblies |
| US11191539B2 (en) | 2016-12-21 | 2021-12-07 | Cilag Gmbh International | Shaft assembly comprising a manually-operable retraction system for use with a motorized surgical instrument system |
| BR112019011947A2 (pt) | 2016-12-21 | 2019-10-29 | Ethicon Llc | sistemas de grampeamento cirúrgico |
| US20180168608A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Surgical instrument system comprising an end effector lockout and a firing assembly lockout |
| US10856868B2 (en) | 2016-12-21 | 2020-12-08 | Ethicon Llc | Firing member pin configurations |
| US11090048B2 (en) | 2016-12-21 | 2021-08-17 | Cilag Gmbh International | Method for resetting a fuse of a surgical instrument shaft |
| US10881401B2 (en) | 2016-12-21 | 2021-01-05 | Ethicon Llc | Staple firing member comprising a missing cartridge and/or spent cartridge lockout |
| US11684367B2 (en) | 2016-12-21 | 2023-06-27 | Cilag Gmbh International | Stepped assembly having and end-of-life indicator |
| JP7010956B2 (ja) | 2016-12-21 | 2022-01-26 | エシコン エルエルシー | 組織をステープル留めする方法 |
| US10327767B2 (en) | 2017-06-20 | 2019-06-25 | Ethicon Llc | Control of motor velocity of a surgical stapling and cutting instrument based on angle of articulation |
| US10779820B2 (en) | 2017-06-20 | 2020-09-22 | Ethicon Llc | Systems and methods for controlling motor speed according to user input for a surgical instrument |
| US10646220B2 (en) | 2017-06-20 | 2020-05-12 | Ethicon Llc | Systems and methods for controlling displacement member velocity for a surgical instrument |
| US10624633B2 (en) | 2017-06-20 | 2020-04-21 | Ethicon Llc | Systems and methods for controlling motor velocity of a surgical stapling and cutting instrument |
| US11517325B2 (en) | 2017-06-20 | 2022-12-06 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured displacement distance traveled over a specified time interval |
| US10307170B2 (en) | 2017-06-20 | 2019-06-04 | Ethicon Llc | Method for closed loop control of motor velocity of a surgical stapling and cutting instrument |
| USD879809S1 (en) | 2017-06-20 | 2020-03-31 | Ethicon Llc | Display panel with changeable graphical user interface |
| US10888321B2 (en) | 2017-06-20 | 2021-01-12 | Ethicon Llc | Systems and methods for controlling velocity of a displacement member of a surgical stapling and cutting instrument |
| US10881399B2 (en) | 2017-06-20 | 2021-01-05 | Ethicon Llc | Techniques for adaptive control of motor velocity of a surgical stapling and cutting instrument |
| USD879808S1 (en) | 2017-06-20 | 2020-03-31 | Ethicon Llc | Display panel with graphical user interface |
| US10813639B2 (en) | 2017-06-20 | 2020-10-27 | Ethicon Llc | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on system conditions |
| US10390841B2 (en) | 2017-06-20 | 2019-08-27 | Ethicon Llc | Control of motor velocity of a surgical stapling and cutting instrument based on angle of articulation |
| US10881396B2 (en) | 2017-06-20 | 2021-01-05 | Ethicon Llc | Surgical instrument with variable duration trigger arrangement |
| US10980537B2 (en) | 2017-06-20 | 2021-04-20 | Ethicon Llc | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured time over a specified number of shaft rotations |
| US11071554B2 (en) | 2017-06-20 | 2021-07-27 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on magnitude of velocity error measurements |
| US11653914B2 (en) | 2017-06-20 | 2023-05-23 | Cilag Gmbh International | Systems and methods for controlling motor velocity of a surgical stapling and cutting instrument according to articulation angle of end effector |
| USD890784S1 (en) | 2017-06-20 | 2020-07-21 | Ethicon Llc | Display panel with changeable graphical user interface |
| US11382638B2 (en) | 2017-06-20 | 2022-07-12 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured time over a specified displacement distance |
| US10368864B2 (en) | 2017-06-20 | 2019-08-06 | Ethicon Llc | Systems and methods for controlling displaying motor velocity for a surgical instrument |
| US11090046B2 (en) | 2017-06-20 | 2021-08-17 | Cilag Gmbh International | Systems and methods for controlling displacement member motion of a surgical stapling and cutting instrument |
| US10772629B2 (en) | 2017-06-27 | 2020-09-15 | Ethicon Llc | Surgical anvil arrangements |
| US10856869B2 (en) | 2017-06-27 | 2020-12-08 | Ethicon Llc | Surgical anvil arrangements |
| US11324503B2 (en) | 2017-06-27 | 2022-05-10 | Cilag Gmbh International | Surgical firing member arrangements |
| US11266405B2 (en) | 2017-06-27 | 2022-03-08 | Cilag Gmbh International | Surgical anvil manufacturing methods |
| US10993716B2 (en) | 2017-06-27 | 2021-05-04 | Ethicon Llc | Surgical anvil arrangements |
| US20180368844A1 (en) | 2017-06-27 | 2018-12-27 | Ethicon Llc | Staple forming pocket arrangements |
| US11564686B2 (en) | 2017-06-28 | 2023-01-31 | Cilag Gmbh International | Surgical shaft assemblies with flexible interfaces |
| US10716614B2 (en) | 2017-06-28 | 2020-07-21 | Ethicon Llc | Surgical shaft assemblies with slip ring assemblies with increased contact pressure |
| USD851762S1 (en) | 2017-06-28 | 2019-06-18 | Ethicon Llc | Anvil |
| USD869655S1 (en) | 2017-06-28 | 2019-12-10 | Ethicon Llc | Surgical fastener cartridge |
| US10211586B2 (en) | 2017-06-28 | 2019-02-19 | Ethicon Llc | Surgical shaft assemblies with watertight housings |
| USD906355S1 (en) | 2017-06-28 | 2020-12-29 | Ethicon Llc | Display screen or portion thereof with a graphical user interface for a surgical instrument |
| EP4070740A1 (en) | 2017-06-28 | 2022-10-12 | Cilag GmbH International | Surgical instrument comprising selectively actuatable rotatable couplers |
| US11020114B2 (en) | 2017-06-28 | 2021-06-01 | Cilag Gmbh International | Surgical instruments with articulatable end effector with axially shortened articulation joint configurations |
| US10765427B2 (en) | 2017-06-28 | 2020-09-08 | Ethicon Llc | Method for articulating a surgical instrument |
| US11259805B2 (en) | 2017-06-28 | 2022-03-01 | Cilag Gmbh International | Surgical instrument comprising firing member supports |
| US11000279B2 (en) | 2017-06-28 | 2021-05-11 | Ethicon Llc | Surgical instrument comprising an articulation system ratio |
| US11246592B2 (en) | 2017-06-28 | 2022-02-15 | Cilag Gmbh International | Surgical instrument comprising an articulation system lockable to a frame |
| US10903685B2 (en) | 2017-06-28 | 2021-01-26 | Ethicon Llc | Surgical shaft assemblies with slip ring assemblies forming capacitive channels |
| USD854151S1 (en) | 2017-06-28 | 2019-07-16 | Ethicon Llc | Surgical instrument shaft |
| US10932772B2 (en) | 2017-06-29 | 2021-03-02 | Ethicon Llc | Methods for closed loop velocity control for robotic surgical instrument |
| US11007022B2 (en) | 2017-06-29 | 2021-05-18 | Ethicon Llc | Closed loop velocity control techniques based on sensed tissue parameters for robotic surgical instrument |
| US10898183B2 (en) | 2017-06-29 | 2021-01-26 | Ethicon Llc | Robotic surgical instrument with closed loop feedback techniques for advancement of closure member during firing |
| US10258418B2 (en) | 2017-06-29 | 2019-04-16 | Ethicon Llc | System for controlling articulation forces |
| US10398434B2 (en) | 2017-06-29 | 2019-09-03 | Ethicon Llc | Closed loop velocity control of closure member for robotic surgical instrument |
| US20200146995A1 (en) * | 2017-06-30 | 2020-05-14 | Otomagnetics, Inc. | Magnetic nanoparticles for targeted delivery |
| US11304695B2 (en) | 2017-08-03 | 2022-04-19 | Cilag Gmbh International | Surgical system shaft interconnection |
| US11974742B2 (en) | 2017-08-03 | 2024-05-07 | Cilag Gmbh International | Surgical system comprising an articulation bailout |
| US11471155B2 (en) | 2017-08-03 | 2022-10-18 | Cilag Gmbh International | Surgical system bailout |
| JP2020529409A (ja) | 2017-08-03 | 2020-10-08 | 大塚製薬株式会社 | 薬物化合物およびその精製方法 |
| US11944300B2 (en) | 2017-08-03 | 2024-04-02 | Cilag Gmbh International | Method for operating a surgical system bailout |
| US10743872B2 (en) | 2017-09-29 | 2020-08-18 | Ethicon Llc | System and methods for controlling a display of a surgical instrument |
| US10765429B2 (en) | 2017-09-29 | 2020-09-08 | Ethicon Llc | Systems and methods for providing alerts according to the operational state of a surgical instrument |
| US10729501B2 (en) | 2017-09-29 | 2020-08-04 | Ethicon Llc | Systems and methods for language selection of a surgical instrument |
| USD907648S1 (en) | 2017-09-29 | 2021-01-12 | Ethicon Llc | Display screen or portion thereof with animated graphical user interface |
| USD917500S1 (en) | 2017-09-29 | 2021-04-27 | Ethicon Llc | Display screen or portion thereof with graphical user interface |
| US10796471B2 (en) | 2017-09-29 | 2020-10-06 | Ethicon Llc | Systems and methods of displaying a knife position for a surgical instrument |
| USD907647S1 (en) | 2017-09-29 | 2021-01-12 | Ethicon Llc | Display screen or portion thereof with animated graphical user interface |
| US11399829B2 (en) | 2017-09-29 | 2022-08-02 | Cilag Gmbh International | Systems and methods of initiating a power shutdown mode for a surgical instrument |
| US11090075B2 (en) | 2017-10-30 | 2021-08-17 | Cilag Gmbh International | Articulation features for surgical end effector |
| US11134944B2 (en) | 2017-10-30 | 2021-10-05 | Cilag Gmbh International | Surgical stapler knife motion controls |
| US10779903B2 (en) | 2017-10-31 | 2020-09-22 | Ethicon Llc | Positive shaft rotation lock activated by jaw closure |
| US10842490B2 (en) | 2017-10-31 | 2020-11-24 | Ethicon Llc | Cartridge body design with force reduction based on firing completion |
| US10743875B2 (en) | 2017-12-15 | 2020-08-18 | Ethicon Llc | Surgical end effectors with jaw stiffener arrangements configured to permit monitoring of firing member |
| US10966718B2 (en) | 2017-12-15 | 2021-04-06 | Ethicon Llc | Dynamic clamping assemblies with improved wear characteristics for use in connection with electromechanical surgical instruments |
| US10743874B2 (en) | 2017-12-15 | 2020-08-18 | Ethicon Llc | Sealed adapters for use with electromechanical surgical instruments |
| US11033267B2 (en) | 2017-12-15 | 2021-06-15 | Ethicon Llc | Systems and methods of controlling a clamping member firing rate of a surgical instrument |
| US10779826B2 (en) | 2017-12-15 | 2020-09-22 | Ethicon Llc | Methods of operating surgical end effectors |
| US10687813B2 (en) | 2017-12-15 | 2020-06-23 | Ethicon Llc | Adapters with firing stroke sensing arrangements for use in connection with electromechanical surgical instruments |
| US10779825B2 (en) | 2017-12-15 | 2020-09-22 | Ethicon Llc | Adapters with end effector position sensing and control arrangements for use in connection with electromechanical surgical instruments |
| US10869666B2 (en) | 2017-12-15 | 2020-12-22 | Ethicon Llc | Adapters with control systems for controlling multiple motors of an electromechanical surgical instrument |
| US11006955B2 (en) | 2017-12-15 | 2021-05-18 | Ethicon Llc | End effectors with positive jaw opening features for use with adapters for electromechanical surgical instruments |
| US11071543B2 (en) | 2017-12-15 | 2021-07-27 | Cilag Gmbh International | Surgical end effectors with clamping assemblies configured to increase jaw aperture ranges |
| US11197670B2 (en) | 2017-12-15 | 2021-12-14 | Cilag Gmbh International | Surgical end effectors with pivotal jaws configured to touch at their respective distal ends when fully closed |
| US10828033B2 (en) | 2017-12-15 | 2020-11-10 | Ethicon Llc | Handheld electromechanical surgical instruments with improved motor control arrangements for positioning components of an adapter coupled thereto |
| US11045270B2 (en) | 2017-12-19 | 2021-06-29 | Cilag Gmbh International | Robotic attachment comprising exterior drive actuator |
| US10716565B2 (en) | 2017-12-19 | 2020-07-21 | Ethicon Llc | Surgical instruments with dual articulation drivers |
| USD910847S1 (en) | 2017-12-19 | 2021-02-16 | Ethicon Llc | Surgical instrument assembly |
| US10835330B2 (en) | 2017-12-19 | 2020-11-17 | Ethicon Llc | Method for determining the position of a rotatable jaw of a surgical instrument attachment assembly |
| US10729509B2 (en) | 2017-12-19 | 2020-08-04 | Ethicon Llc | Surgical instrument comprising closure and firing locking mechanism |
| US11020112B2 (en) | 2017-12-19 | 2021-06-01 | Ethicon Llc | Surgical tools configured for interchangeable use with different controller interfaces |
| US11076853B2 (en) | 2017-12-21 | 2021-08-03 | Cilag Gmbh International | Systems and methods of displaying a knife position during transection for a surgical instrument |
| US11129680B2 (en) | 2017-12-21 | 2021-09-28 | Cilag Gmbh International | Surgical instrument comprising a projector |
| US11311290B2 (en) | 2017-12-21 | 2022-04-26 | Cilag Gmbh International | Surgical instrument comprising an end effector dampener |
| US10682134B2 (en) | 2017-12-21 | 2020-06-16 | Ethicon Llc | Continuous use self-propelled stapling instrument |
| WO2019148291A1 (en) | 2018-02-02 | 2019-08-08 | Interface Biologics, Inc. | Ocular inserts comprising a covalently linked steroid dimer |
| US11253256B2 (en) | 2018-08-20 | 2022-02-22 | Cilag Gmbh International | Articulatable motor powered surgical instruments with dedicated articulation motor arrangements |
| US11039834B2 (en) | 2018-08-20 | 2021-06-22 | Cilag Gmbh International | Surgical stapler anvils with staple directing protrusions and tissue stability features |
| USD914878S1 (en) | 2018-08-20 | 2021-03-30 | Ethicon Llc | Surgical instrument anvil |
| US10842492B2 (en) | 2018-08-20 | 2020-11-24 | Ethicon Llc | Powered articulatable surgical instruments with clutching and locking arrangements for linking an articulation drive system to a firing drive system |
| US10779821B2 (en) | 2018-08-20 | 2020-09-22 | Ethicon Llc | Surgical stapler anvils with tissue stop features configured to avoid tissue pinch |
| US11324501B2 (en) | 2018-08-20 | 2022-05-10 | Cilag Gmbh International | Surgical stapling devices with improved closure members |
| US10912559B2 (en) | 2018-08-20 | 2021-02-09 | Ethicon Llc | Reinforced deformable anvil tip for surgical stapler anvil |
| US11045192B2 (en) | 2018-08-20 | 2021-06-29 | Cilag Gmbh International | Fabricating techniques for surgical stapler anvils |
| US11291440B2 (en) | 2018-08-20 | 2022-04-05 | Cilag Gmbh International | Method for operating a powered articulatable surgical instrument |
| US11083458B2 (en) | 2018-08-20 | 2021-08-10 | Cilag Gmbh International | Powered surgical instruments with clutching arrangements to convert linear drive motions to rotary drive motions |
| US10856870B2 (en) | 2018-08-20 | 2020-12-08 | Ethicon Llc | Switching arrangements for motor powered articulatable surgical instruments |
| US11207065B2 (en) | 2018-08-20 | 2021-12-28 | Cilag Gmbh International | Method for fabricating surgical stapler anvils |
| WO2020112816A1 (en) | 2018-11-29 | 2020-06-04 | Surmodics, Inc. | Apparatus and methods for coating medical devices |
| CN111494307A (zh) * | 2019-01-30 | 2020-08-07 | 北京普德康利医药科技发展有限公司 | 一种氟比洛芬注射液 |
| US11147551B2 (en) | 2019-03-25 | 2021-10-19 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11696761B2 (en) | 2019-03-25 | 2023-07-11 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11147553B2 (en) | 2019-03-25 | 2021-10-19 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11172929B2 (en) | 2019-03-25 | 2021-11-16 | Cilag Gmbh International | Articulation drive arrangements for surgical systems |
| US11426251B2 (en) | 2019-04-30 | 2022-08-30 | Cilag Gmbh International | Articulation directional lights on a surgical instrument |
| US11648009B2 (en) | 2019-04-30 | 2023-05-16 | Cilag Gmbh International | Rotatable jaw tip for a surgical instrument |
| US11903581B2 (en) | 2019-04-30 | 2024-02-20 | Cilag Gmbh International | Methods for stapling tissue using a surgical instrument |
| US11471157B2 (en) | 2019-04-30 | 2022-10-18 | Cilag Gmbh International | Articulation control mapping for a surgical instrument |
| US11452528B2 (en) | 2019-04-30 | 2022-09-27 | Cilag Gmbh International | Articulation actuators for a surgical instrument |
| US11432816B2 (en) | 2019-04-30 | 2022-09-06 | Cilag Gmbh International | Articulation pin for a surgical instrument |
| US11253254B2 (en) | 2019-04-30 | 2022-02-22 | Cilag Gmbh International | Shaft rotation actuator on a surgical instrument |
| US11819590B2 (en) | 2019-05-13 | 2023-11-21 | Surmodics, Inc. | Apparatus and methods for coating medical devices |
| US11051807B2 (en) | 2019-06-28 | 2021-07-06 | Cilag Gmbh International | Packaging assembly including a particulate trap |
| US11478241B2 (en) | 2019-06-28 | 2022-10-25 | Cilag Gmbh International | Staple cartridge including projections |
| US11684434B2 (en) | 2019-06-28 | 2023-06-27 | Cilag Gmbh International | Surgical RFID assemblies for instrument operational setting control |
| US11259803B2 (en) | 2019-06-28 | 2022-03-01 | Cilag Gmbh International | Surgical stapling system having an information encryption protocol |
| US11638587B2 (en) | 2019-06-28 | 2023-05-02 | Cilag Gmbh International | RFID identification systems for surgical instruments |
| US11497492B2 (en) | 2019-06-28 | 2022-11-15 | Cilag Gmbh International | Surgical instrument including an articulation lock |
| US11426167B2 (en) | 2019-06-28 | 2022-08-30 | Cilag Gmbh International | Mechanisms for proper anvil attachment surgical stapling head assembly |
| US12004740B2 (en) | 2019-06-28 | 2024-06-11 | Cilag Gmbh International | Surgical stapling system having an information decryption protocol |
| US11291451B2 (en) | 2019-06-28 | 2022-04-05 | Cilag Gmbh International | Surgical instrument with battery compatibility verification functionality |
| US11771419B2 (en) | 2019-06-28 | 2023-10-03 | Cilag Gmbh International | Packaging for a replaceable component of a surgical stapling system |
| US11523822B2 (en) | 2019-06-28 | 2022-12-13 | Cilag Gmbh International | Battery pack including a circuit interrupter |
| US11298132B2 (en) | 2019-06-28 | 2022-04-12 | Cilag GmbH Inlernational | Staple cartridge including a honeycomb extension |
| US11464601B2 (en) | 2019-06-28 | 2022-10-11 | Cilag Gmbh International | Surgical instrument comprising an RFID system for tracking a movable component |
| US11219455B2 (en) | 2019-06-28 | 2022-01-11 | Cilag Gmbh International | Surgical instrument including a lockout key |
| US11627959B2 (en) | 2019-06-28 | 2023-04-18 | Cilag Gmbh International | Surgical instruments including manual and powered system lockouts |
| US11229437B2 (en) | 2019-06-28 | 2022-01-25 | Cilag Gmbh International | Method for authenticating the compatibility of a staple cartridge with a surgical instrument |
| US11660163B2 (en) | 2019-06-28 | 2023-05-30 | Cilag Gmbh International | Surgical system with RFID tags for updating motor assembly parameters |
| US11553971B2 (en) | 2019-06-28 | 2023-01-17 | Cilag Gmbh International | Surgical RFID assemblies for display and communication |
| US11246678B2 (en) | 2019-06-28 | 2022-02-15 | Cilag Gmbh International | Surgical stapling system having a frangible RFID tag |
| US11224497B2 (en) | 2019-06-28 | 2022-01-18 | Cilag Gmbh International | Surgical systems with multiple RFID tags |
| US11376098B2 (en) | 2019-06-28 | 2022-07-05 | Cilag Gmbh International | Surgical instrument system comprising an RFID system |
| US11298127B2 (en) | 2019-06-28 | 2022-04-12 | Cilag GmbH Interational | Surgical stapling system having a lockout mechanism for an incompatible cartridge |
| US11399837B2 (en) | 2019-06-28 | 2022-08-02 | Cilag Gmbh International | Mechanisms for motor control adjustments of a motorized surgical instrument |
| US11291447B2 (en) | 2019-12-19 | 2022-04-05 | Cilag Gmbh International | Stapling instrument comprising independent jaw closing and staple firing systems |
| US11504122B2 (en) | 2019-12-19 | 2022-11-22 | Cilag Gmbh International | Surgical instrument comprising a nested firing member |
| US11844520B2 (en) | 2019-12-19 | 2023-12-19 | Cilag Gmbh International | Staple cartridge comprising driver retention members |
| US11446029B2 (en) | 2019-12-19 | 2022-09-20 | Cilag Gmbh International | Staple cartridge comprising projections extending from a curved deck surface |
| US11559304B2 (en) | 2019-12-19 | 2023-01-24 | Cilag Gmbh International | Surgical instrument comprising a rapid closure mechanism |
| US11701111B2 (en) | 2019-12-19 | 2023-07-18 | Cilag Gmbh International | Method for operating a surgical stapling instrument |
| US11576672B2 (en) | 2019-12-19 | 2023-02-14 | Cilag Gmbh International | Surgical instrument comprising a closure system including a closure member and an opening member driven by a drive screw |
| US11607219B2 (en) | 2019-12-19 | 2023-03-21 | Cilag Gmbh International | Staple cartridge comprising a detachable tissue cutting knife |
| US11529137B2 (en) | 2019-12-19 | 2022-12-20 | Cilag Gmbh International | Staple cartridge comprising driver retention members |
| US11911032B2 (en) | 2019-12-19 | 2024-02-27 | Cilag Gmbh International | Staple cartridge comprising a seating cam |
| US11304696B2 (en) | 2019-12-19 | 2022-04-19 | Cilag Gmbh International | Surgical instrument comprising a powered articulation system |
| US11529139B2 (en) | 2019-12-19 | 2022-12-20 | Cilag Gmbh International | Motor driven surgical instrument |
| US11931033B2 (en) | 2019-12-19 | 2024-03-19 | Cilag Gmbh International | Staple cartridge comprising a latch lockout |
| US11464512B2 (en) | 2019-12-19 | 2022-10-11 | Cilag Gmbh International | Staple cartridge comprising a curved deck surface |
| US12035913B2 (en) | 2019-12-19 | 2024-07-16 | Cilag Gmbh International | Staple cartridge comprising a deployable knife |
| US11234698B2 (en) | 2019-12-19 | 2022-02-01 | Cilag Gmbh International | Stapling system comprising a clamp lockout and a firing lockout |
| CA3176134A1 (en) | 2020-05-01 | 2021-11-04 | Ripple Therapeutics Corporation | Heterodimer compositions and methods for the treatment of ocular disorders |
| USD975278S1 (en) | 2020-06-02 | 2023-01-10 | Cilag Gmbh International | Staple cartridge |
| USD966512S1 (en) | 2020-06-02 | 2022-10-11 | Cilag Gmbh International | Staple cartridge |
| USD974560S1 (en) | 2020-06-02 | 2023-01-03 | Cilag Gmbh International | Staple cartridge |
| USD976401S1 (en) | 2020-06-02 | 2023-01-24 | Cilag Gmbh International | Staple cartridge |
| USD975851S1 (en) | 2020-06-02 | 2023-01-17 | Cilag Gmbh International | Staple cartridge |
| USD967421S1 (en) | 2020-06-02 | 2022-10-18 | Cilag Gmbh International | Staple cartridge |
| USD975850S1 (en) | 2020-06-02 | 2023-01-17 | Cilag Gmbh International | Staple cartridge |
| US11857182B2 (en) | 2020-07-28 | 2024-01-02 | Cilag Gmbh International | Surgical instruments with combination function articulation joint arrangements |
| US11717289B2 (en) | 2020-10-29 | 2023-08-08 | Cilag Gmbh International | Surgical instrument comprising an indicator which indicates that an articulation drive is actuatable |
| US11779330B2 (en) | 2020-10-29 | 2023-10-10 | Cilag Gmbh International | Surgical instrument comprising a jaw alignment system |
| USD980425S1 (en) | 2020-10-29 | 2023-03-07 | Cilag Gmbh International | Surgical instrument assembly |
| US11452526B2 (en) | 2020-10-29 | 2022-09-27 | Cilag Gmbh International | Surgical instrument comprising a staged voltage regulation start-up system |
| US11931025B2 (en) | 2020-10-29 | 2024-03-19 | Cilag Gmbh International | Surgical instrument comprising a releasable closure drive lock |
| US11534259B2 (en) | 2020-10-29 | 2022-12-27 | Cilag Gmbh International | Surgical instrument comprising an articulation indicator |
| US11517390B2 (en) | 2020-10-29 | 2022-12-06 | Cilag Gmbh International | Surgical instrument comprising a limited travel switch |
| US11844518B2 (en) | 2020-10-29 | 2023-12-19 | Cilag Gmbh International | Method for operating a surgical instrument |
| US11896217B2 (en) | 2020-10-29 | 2024-02-13 | Cilag Gmbh International | Surgical instrument comprising an articulation lock |
| US12053175B2 (en) | 2020-10-29 | 2024-08-06 | Cilag Gmbh International | Surgical instrument comprising a stowed closure actuator stop |
| US11617577B2 (en) | 2020-10-29 | 2023-04-04 | Cilag Gmbh International | Surgical instrument comprising a sensor configured to sense whether an articulation drive of the surgical instrument is actuatable |
| USD1013170S1 (en) | 2020-10-29 | 2024-01-30 | Cilag Gmbh International | Surgical instrument assembly |
| US11944296B2 (en) | 2020-12-02 | 2024-04-02 | Cilag Gmbh International | Powered surgical instruments with external connectors |
| US11744581B2 (en) | 2020-12-02 | 2023-09-05 | Cilag Gmbh International | Powered surgical instruments with multi-phase tissue treatment |
| US11890010B2 (en) | 2020-12-02 | 2024-02-06 | Cllag GmbH International | Dual-sided reinforced reload for surgical instruments |
| US11653920B2 (en) | 2020-12-02 | 2023-05-23 | Cilag Gmbh International | Powered surgical instruments with communication interfaces through sterile barrier |
| US11653915B2 (en) | 2020-12-02 | 2023-05-23 | Cilag Gmbh International | Surgical instruments with sled location detection and adjustment features |
| US11849943B2 (en) | 2020-12-02 | 2023-12-26 | Cilag Gmbh International | Surgical instrument with cartridge release mechanisms |
| US11627960B2 (en) | 2020-12-02 | 2023-04-18 | Cilag Gmbh International | Powered surgical instruments with smart reload with separately attachable exteriorly mounted wiring connections |
| US11678882B2 (en) | 2020-12-02 | 2023-06-20 | Cilag Gmbh International | Surgical instruments with interactive features to remedy incidental sled movements |
| US11737751B2 (en) | 2020-12-02 | 2023-08-29 | Cilag Gmbh International | Devices and methods of managing energy dissipated within sterile barriers of surgical instrument housings |
| US11696757B2 (en) | 2021-02-26 | 2023-07-11 | Cilag Gmbh International | Monitoring of internal systems to detect and track cartridge motion status |
| US11749877B2 (en) | 2021-02-26 | 2023-09-05 | Cilag Gmbh International | Stapling instrument comprising a signal antenna |
| US11950779B2 (en) | 2021-02-26 | 2024-04-09 | Cilag Gmbh International | Method of powering and communicating with a staple cartridge |
| US11730473B2 (en) | 2021-02-26 | 2023-08-22 | Cilag Gmbh International | Monitoring of manufacturing life-cycle |
| US11980362B2 (en) | 2021-02-26 | 2024-05-14 | Cilag Gmbh International | Surgical instrument system comprising a power transfer coil |
| US11925349B2 (en) | 2021-02-26 | 2024-03-12 | Cilag Gmbh International | Adjustment to transfer parameters to improve available power |
| US11812964B2 (en) | 2021-02-26 | 2023-11-14 | Cilag Gmbh International | Staple cartridge comprising a power management circuit |
| US12108951B2 (en) | 2021-02-26 | 2024-10-08 | Cilag Gmbh International | Staple cartridge comprising a sensing array and a temperature control system |
| US11751869B2 (en) | 2021-02-26 | 2023-09-12 | Cilag Gmbh International | Monitoring of multiple sensors over time to detect moving characteristics of tissue |
| US11723657B2 (en) | 2021-02-26 | 2023-08-15 | Cilag Gmbh International | Adjustable communication based on available bandwidth and power capacity |
| US11950777B2 (en) | 2021-02-26 | 2024-04-09 | Cilag Gmbh International | Staple cartridge comprising an information access control system |
| US11793514B2 (en) | 2021-02-26 | 2023-10-24 | Cilag Gmbh International | Staple cartridge comprising sensor array which may be embedded in cartridge body |
| US11701113B2 (en) | 2021-02-26 | 2023-07-18 | Cilag Gmbh International | Stapling instrument comprising a separate power antenna and a data transfer antenna |
| US11744583B2 (en) | 2021-02-26 | 2023-09-05 | Cilag Gmbh International | Distal communication array to tune frequency of RF systems |
| US11759202B2 (en) | 2021-03-22 | 2023-09-19 | Cilag Gmbh International | Staple cartridge comprising an implantable layer |
| US11806011B2 (en) | 2021-03-22 | 2023-11-07 | Cilag Gmbh International | Stapling instrument comprising tissue compression systems |
| US11826012B2 (en) | 2021-03-22 | 2023-11-28 | Cilag Gmbh International | Stapling instrument comprising a pulsed motor-driven firing rack |
| US11826042B2 (en) | 2021-03-22 | 2023-11-28 | Cilag Gmbh International | Surgical instrument comprising a firing drive including a selectable leverage mechanism |
| US11723658B2 (en) | 2021-03-22 | 2023-08-15 | Cilag Gmbh International | Staple cartridge comprising a firing lockout |
| US11717291B2 (en) | 2021-03-22 | 2023-08-08 | Cilag Gmbh International | Staple cartridge comprising staples configured to apply different tissue compression |
| US11737749B2 (en) | 2021-03-22 | 2023-08-29 | Cilag Gmbh International | Surgical stapling instrument comprising a retraction system |
| US11786239B2 (en) | 2021-03-24 | 2023-10-17 | Cilag Gmbh International | Surgical instrument articulation joint arrangements comprising multiple moving linkage features |
| US11786243B2 (en) | 2021-03-24 | 2023-10-17 | Cilag Gmbh International | Firing members having flexible portions for adapting to a load during a surgical firing stroke |
| US11896218B2 (en) | 2021-03-24 | 2024-02-13 | Cilag Gmbh International | Method of using a powered stapling device |
| US11849944B2 (en) | 2021-03-24 | 2023-12-26 | Cilag Gmbh International | Drivers for fastener cartridge assemblies having rotary drive screws |
| US11857183B2 (en) | 2021-03-24 | 2024-01-02 | Cilag Gmbh International | Stapling assembly components having metal substrates and plastic bodies |
| US11744603B2 (en) | 2021-03-24 | 2023-09-05 | Cilag Gmbh International | Multi-axis pivot joints for surgical instruments and methods for manufacturing same |
| US11944336B2 (en) | 2021-03-24 | 2024-04-02 | Cilag Gmbh International | Joint arrangements for multi-planar alignment and support of operational drive shafts in articulatable surgical instruments |
| US11849945B2 (en) | 2021-03-24 | 2023-12-26 | Cilag Gmbh International | Rotary-driven surgical stapling assembly comprising eccentrically driven firing member |
| US11903582B2 (en) | 2021-03-24 | 2024-02-20 | Cilag Gmbh International | Leveraging surfaces for cartridge installation |
| US11832816B2 (en) | 2021-03-24 | 2023-12-05 | Cilag Gmbh International | Surgical stapling assembly comprising nonplanar staples and planar staples |
| US12102323B2 (en) | 2021-03-24 | 2024-10-01 | Cilag Gmbh International | Rotary-driven surgical stapling assembly comprising a floatable component |
| US11896219B2 (en) | 2021-03-24 | 2024-02-13 | Cilag Gmbh International | Mating features between drivers and underside of a cartridge deck |
| US11793516B2 (en) | 2021-03-24 | 2023-10-24 | Cilag Gmbh International | Surgical staple cartridge comprising longitudinal support beam |
| US20220378426A1 (en) | 2021-05-28 | 2022-12-01 | Cilag Gmbh International | Stapling instrument comprising a mounted shaft orientation sensor |
| US11980363B2 (en) | 2021-10-18 | 2024-05-14 | Cilag Gmbh International | Row-to-row staple array variations |
| US11877745B2 (en) | 2021-10-18 | 2024-01-23 | Cilag Gmbh International | Surgical stapling assembly having longitudinally-repeating staple leg clusters |
| US11957337B2 (en) | 2021-10-18 | 2024-04-16 | Cilag Gmbh International | Surgical stapling assembly with offset ramped drive surfaces |
| US12089841B2 (en) | 2021-10-28 | 2024-09-17 | Cilag CmbH International | Staple cartridge identification systems |
| US11937816B2 (en) | 2021-10-28 | 2024-03-26 | Cilag Gmbh International | Electrical lead arrangements for surgical instruments |
Family Cites Families (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3832252A (en) * | 1970-09-29 | 1974-08-27 | T Higuchi | Method of making a drug-delivery device |
| GB1509979A (en) * | 1975-11-28 | 1978-05-10 | Fisons Ltd | Pharmaceutical compositions containing aspirin or indomethacin |
| US4141087A (en) | 1977-01-19 | 1979-02-27 | Ethicon, Inc. | Isomorphic copolyoxalates and sutures thereof |
| US4208511A (en) | 1977-01-19 | 1980-06-17 | Ethicon, Inc. | Isomorphic copolyoxalates and sutures thereof |
| US4105034A (en) | 1977-06-10 | 1978-08-08 | Ethicon, Inc. | Poly(alkylene oxalate) absorbable coating for sutures |
| US4205399A (en) | 1977-06-13 | 1980-06-03 | Ethicon, Inc. | Synthetic absorbable surgical devices of poly(alkylene oxalates) |
| US4140678A (en) | 1977-06-13 | 1979-02-20 | Ethicon, Inc. | Synthetic absorbable surgical devices of poly(alkylene oxalates) |
| US4130639A (en) | 1977-09-28 | 1978-12-19 | Ethicon, Inc. | Absorbable pharmaceutical compositions based on isomorphic copolyoxalates |
| US4300557A (en) * | 1980-01-07 | 1981-11-17 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Method for treating intraocular malignancies |
| US4309996A (en) * | 1980-04-28 | 1982-01-12 | Alza Corporation | System with microporous releasing diffusor |
| US4309776A (en) * | 1980-05-13 | 1982-01-12 | Ramon Berguer | Intravascular implantation device and method of using the same |
| AU8762982A (en) * | 1981-07-02 | 1983-02-02 | Walton, A.G. | Glycosaminoglycan drug complexes |
| GB8403138D0 (en) * | 1984-02-07 | 1984-03-14 | Graham N B | Sustained release of active ingredient |
| US4588525A (en) * | 1984-02-27 | 1986-05-13 | Molecular Biosystems, Inc. | Prodrug compounds for dermal application |
| US4649047A (en) * | 1985-03-19 | 1987-03-10 | University Of Georgia Research Foundation, Inc. | Ophthalmic treatment by topical administration of cyclosporin |
| US4650803A (en) * | 1985-12-06 | 1987-03-17 | University Of Kansas | Prodrugs of rapamycin |
| US5057301A (en) * | 1988-04-06 | 1991-10-15 | Neorx Corporation | Modified cellular substrates used as linkers for increased cell retention of diagnostic and therapeutic agents |
| US4923699A (en) * | 1988-06-03 | 1990-05-08 | Kaufman Herbert E | Eye treatment suspension |
| US5194581A (en) * | 1989-03-09 | 1993-03-16 | Leong Kam W | Biodegradable poly(phosphoesters) |
| CH679207A5 (zh) * | 1989-07-28 | 1992-01-15 | Debiopharm Sa | |
| US5439688A (en) * | 1989-07-28 | 1995-08-08 | Debio Recherche Pharmaceutique S.A. | Process for preparing a pharmaceutical composition |
| US5091185A (en) * | 1990-06-20 | 1992-02-25 | Monsanto Company | Coated veterinary implants |
| US5130126A (en) * | 1990-07-09 | 1992-07-14 | Nippon Oil & Fats Co., Ltd. | Polymer-drug conjugate and a method of producing it |
| EP0492903A1 (en) * | 1990-12-21 | 1992-07-01 | MERCK SHARP & DOHME LTD. | Substituted pyrazines, pyrimidines and pyridazines for use in the treatment of glaucoma |
| US5104877A (en) * | 1991-02-25 | 1992-04-14 | Abbott Laboratories | Psoriasis treatment |
| US5176907A (en) * | 1991-08-13 | 1993-01-05 | The Johns Hopkins University School Of Medicine | Biocompatible and biodegradable poly (phosphoester-urethanes) |
| US5464650A (en) * | 1993-04-26 | 1995-11-07 | Medtronic, Inc. | Intravascular stent and method |
| US5994341A (en) * | 1993-07-19 | 1999-11-30 | Angiogenesis Technologies, Inc. | Anti-angiogenic Compositions and methods for the treatment of arthritis |
| CA2178592C (en) * | 1993-12-09 | 2009-07-28 | Jurgen Engel | Long-acting injection suspensions and a process for their preparation |
| US6051576A (en) * | 1994-01-28 | 2000-04-18 | University Of Kentucky Research Foundation | Means to achieve sustained release of synergistic drugs by conjugation |
| EP0740650B1 (en) * | 1994-01-28 | 2004-05-26 | University Of Kentucky Research Foundation | Codrugs as a method of controlled drug delivery |
| US5698213A (en) | 1995-03-06 | 1997-12-16 | Ethicon, Inc. | Hydrogels of absorbable polyoxaesters |
| US5700583A (en) | 1995-03-06 | 1997-12-23 | Ethicon, Inc. | Hydrogels of absorbable polyoxaesters containing amines or amido groups |
| US5618552A (en) | 1995-03-06 | 1997-04-08 | Ethicon, Inc. | Absorbable polyoxaesters |
| US5597579A (en) | 1995-03-06 | 1997-01-28 | Ethicon, Inc. | Blends of absorbable polyoxaamides |
| US5464929A (en) | 1995-03-06 | 1995-11-07 | Ethicon, Inc. | Absorbable polyoxaesters |
| US5607687A (en) | 1995-03-06 | 1997-03-04 | Ethicon, Inc. | Polymer blends containing absorbable polyoxaesters |
| US5648088A (en) | 1995-03-06 | 1997-07-15 | Ethicon, Inc. | Blends of absorbable polyoxaesters containing amines and/or amide groups |
| US5595751A (en) | 1995-03-06 | 1997-01-21 | Ethicon, Inc. | Absorbable polyoxaesters containing amines and/or amido groups |
| US5837313A (en) * | 1995-04-19 | 1998-11-17 | Schneider (Usa) Inc | Drug release stent coating process |
| US5569429A (en) * | 1995-05-05 | 1996-10-29 | Randcastle Extrusion Systems, Inc. | Dynamic seal and sealing method |
| CA2228118A1 (en) * | 1995-07-28 | 1997-02-13 | Focal, Inc. | Multiblock biodegradable hydrogels for use as controlled release agents for drugs delivery and tissue treatments agents |
| US5773019A (en) * | 1995-09-27 | 1998-06-30 | The University Of Kentucky Research Foundation | Implantable controlled release device to deliver drugs directly to an internal portion of the body |
| US5843172A (en) * | 1997-04-15 | 1998-12-01 | Advanced Cardiovascular Systems, Inc. | Porous medicated stent |
| US6240616B1 (en) * | 1997-04-15 | 2001-06-05 | Advanced Cardiovascular Systems, Inc. | Method of manufacturing a medicated porous metal prosthesis |
| EP0989864A1 (en) * | 1997-06-11 | 2000-04-05 | The School Of Pharmacy, University Of London | Pharmaceutical compositions containing antibody-enzyme conjugates in combination with prodrugs |
| US6159488A (en) * | 1997-08-14 | 2000-12-12 | Agricultural Research Org. Ministry Of Agriculture (Gov.) | Intracoronary stents containing quinazolinone derivatives |
| US5972027A (en) * | 1997-09-30 | 1999-10-26 | Scimed Life Systems, Inc | Porous stent drug delivery system |
| US5976169A (en) * | 1997-12-16 | 1999-11-02 | Cardiovasc, Inc. | Stent with silver coating and method |
| US6368658B1 (en) * | 1999-04-19 | 2002-04-09 | Scimed Life Systems, Inc. | Coating medical devices using air suspension |
| AU4941400A (en) * | 1999-05-27 | 2001-01-22 | Biocompatibles Limited | Local drug delivery |
| US6375972B1 (en) * | 2000-04-26 | 2002-04-23 | Control Delivery Systems, Inc. | Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof |
| US20030049689A1 (en) * | 2000-12-14 | 2003-03-13 | Cynthia Edwards | Multifunctional polypeptides |
| US20040022853A1 (en) * | 2001-04-26 | 2004-02-05 | Control Delivery Systems, Inc. | Polymer-based, sustained release drug delivery system |
| ATE506064T1 (de) * | 2001-06-05 | 2011-05-15 | Control Delivery Systems | Analgetika mit verzögerter wirkstoffabgabe |
| IN2014DN10834A (zh) * | 2001-09-17 | 2015-09-04 | Psivida Inc | |
| US20030118528A1 (en) * | 2001-11-19 | 2003-06-26 | Walters Kenneth A. | Topical delivery of codrugs |
-
2002
- 2002-04-26 CN CNA028129563A patent/CN1520297A/zh active Pending
- 2002-04-26 WO PCT/US2002/013385 patent/WO2002087586A1/en active Application Filing
- 2002-04-26 US US10/134,033 patent/US20030039689A1/en not_active Abandoned
- 2002-04-26 IL IL15852702A patent/IL158527A0/xx unknown
- 2002-04-26 JP JP2002584931A patent/JP2004536799A/ja active Pending
- 2002-04-26 MX MXPA03009727A patent/MXPA03009727A/es active IP Right Grant
- 2002-04-26 EP EP08161433A patent/EP2033648A3/en not_active Withdrawn
- 2002-04-26 CA CA2444894A patent/CA2444894C/en not_active Expired - Fee Related
- 2002-04-26 AU AU2002259045A patent/AU2002259045B2/en not_active Ceased
- 2002-04-26 EP EP10185074A patent/EP2283845A1/en not_active Withdrawn
- 2002-04-26 BR BR0209198-4A patent/BR0209198A/pt not_active Application Discontinuation
- 2002-04-26 KR KR10-2003-7014032A patent/KR20040005936A/ko not_active Application Discontinuation
- 2002-04-26 NZ NZ528994A patent/NZ528994A/en not_active IP Right Cessation
- 2002-04-26 EP EP02729028A patent/EP1383504A1/en not_active Ceased
-
2010
- 2010-12-03 JP JP2010270741A patent/JP2011052015A/ja active Pending
-
2011
- 2011-07-19 US US13/185,664 patent/US20120016467A1/en not_active Abandoned
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104582782A (zh) * | 2012-08-20 | 2015-04-29 | 奥雷制药有限公司 | 用于制备药物递送制剂的方法 |
| CN104582782B (zh) * | 2012-08-20 | 2018-11-27 | 奥雷制药有限公司 | 用于制备药物递送制剂的方法 |
| CN107205956A (zh) * | 2014-10-30 | 2017-09-26 | 纺织品技术股份有限公司 | 输送系统 |
| US10799464B2 (en) | 2014-10-30 | 2020-10-13 | Textile-Based Delivery, Inc. | Delivery systems |
| US11633366B2 (en) | 2014-10-30 | 2023-04-25 | Textile-Based Delivery, Inc. | Delivery systems |
| US11690808B2 (en) | 2014-10-30 | 2023-07-04 | Textile-Based Delivery, Inc. | Delivery systems |
| CN114699563A (zh) * | 2022-02-22 | 2022-07-05 | 中国医科大学附属盛京医院 | 一种负载型聚醚型聚氨酯薄膜、制备方法及其应用 |
| CN114699563B (zh) * | 2022-02-22 | 2024-02-02 | 中国医科大学附属盛京医院 | 一种负载型聚醚型聚氨酯薄膜、制备方法及其应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20030039689A1 (en) | 2003-02-27 |
| MXPA03009727A (es) | 2004-01-29 |
| WO2002087586A1 (en) | 2002-11-07 |
| JP2004536799A (ja) | 2004-12-09 |
| CA2444894C (en) | 2013-06-25 |
| EP2033648A3 (en) | 2009-06-03 |
| EP2033648A2 (en) | 2009-03-11 |
| KR20040005936A (ko) | 2004-01-16 |
| BR0209198A (pt) | 2004-06-08 |
| EP2283845A1 (en) | 2011-02-16 |
| AU2002259045B2 (en) | 2008-05-22 |
| CA2444894A1 (en) | 2002-11-07 |
| US20120016467A1 (en) | 2012-01-19 |
| IL158527A0 (en) | 2004-05-12 |
| NZ528994A (en) | 2006-02-24 |
| JP2011052015A (ja) | 2011-03-17 |
| EP1383504A1 (en) | 2004-01-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1520297A (zh) | 包含复合药物(codrugs)的缓释药物传递系统 | |
| CN1589166A (zh) | 包被有持续释放药物递送系统的支架及其使用方法 | |
| US20140037746A1 (en) | Polymer-based, sustained release drug delivery system | |
| CN101146514B (zh) | 用于整合入医疗装置的疏水性改善的药物 | |
| JP5153340B2 (ja) | 薬剤放出制御組成物および薬剤放出性医療器具 | |
| CN1748653A (zh) | 药物输送装置 | |
| CN1672746A (zh) | 局部血管递送panzem 与雷帕霉素的组合以防止血管损伤后的再狭窄 | |
| CN1754536A (zh) | 用于治疗cad的西罗莫司及其衍生物的溶液制剂 | |
| US20030229390A1 (en) | On-stent delivery of pyrimidines and purine analogs | |
| CN1735402A (zh) | 含大环三烯化合物的聚合物组合物 | |
| US20030158598A1 (en) | System for sustained-release delivery of anti-inflammatory agents from a coated medical device | |
| CN1669537A (zh) | 制滴菌素a单用或与西罗莫司联用经局部血管递送预防血管损伤后的再狭窄 | |
| AU2002259045A1 (en) | Sustained release drug delivery system containing codrugs | |
| CN1672745A (zh) | 局部血管递送依托泊苷与雷帕霉素的组合以防止血管损伤后的再狭窄 | |
| JP2008519771A (ja) | 安定化されたポリマー送達系 | |
| JP2006502135A (ja) | 活性薬剤デリバリーの、システム、医学用装置、及び方法 | |
| CN1964748A (zh) | 用于生物活性剂的涂料组合物 | |
| AU2002339938A1 (en) | Stent coated with a sustained-release drug delivery | |
| CN1679573A (zh) | 局部血管递送托泊替康与雷帕霉素的组合以防止血管损伤后的再狭窄 | |
| JP5907658B2 (ja) | 自己消失性コーティング | |
| CN1950116A (zh) | 用于制备生物相容性表面的组合物和方法 | |
| JP2008535563A (ja) | 生物活性剤のためのコーティング組成物 | |
| CN1565663A (zh) | 药物改性心血管支架 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1068544 Country of ref document: HK |
|
| C12 | Rejection of a patent application after its publication | ||
| RJ01 | Rejection of invention patent application after publication | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1068544 Country of ref document: HK |