CN114805261B - 苯并呋喃类lsd1抑制剂及其制备方法 - Google Patents
苯并呋喃类lsd1抑制剂及其制备方法 Download PDFInfo
- Publication number
- CN114805261B CN114805261B CN202110061056.8A CN202110061056A CN114805261B CN 114805261 B CN114805261 B CN 114805261B CN 202110061056 A CN202110061056 A CN 202110061056A CN 114805261 B CN114805261 B CN 114805261B
- Authority
- CN
- China
- Prior art keywords
- methyl
- benzonitrile
- benzofuran
- tolyl
- reaction
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360 preparation method Methods 0.000 title abstract description 41
- 229940123628 Lysine (K)-specific demethylase 1A inhibitor Drugs 0.000 title abstract description 12
- IANQTJSKSUMEQM-UHFFFAOYSA-N 1-benzofuran Chemical compound C1=CC=C2OC=CC2=C1 IANQTJSKSUMEQM-UHFFFAOYSA-N 0.000 title description 4
- 150000003839 salts Chemical class 0.000 claims abstract description 21
- 239000003112 inhibitor Substances 0.000 claims abstract description 8
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims abstract description 6
- 239000004472 Lysine Substances 0.000 claims abstract description 6
- -1 nitro, amino Chemical group 0.000 claims description 77
- 150000001875 compounds Chemical class 0.000 claims description 41
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzenecarbonitrile Natural products N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 claims description 22
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 15
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 7
- 125000001424 substituent group Chemical group 0.000 claims description 7
- 229910052760 oxygen Inorganic materials 0.000 claims description 6
- 229910052717 sulfur Inorganic materials 0.000 claims description 6
- 229910052736 halogen Inorganic materials 0.000 claims description 5
- 150000002367 halogens Chemical class 0.000 claims description 5
- 125000000623 heterocyclic group Chemical group 0.000 claims description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 5
- 125000005842 heteroatom Chemical group 0.000 claims description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 4
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 claims description 3
- 125000006569 (C5-C6) heterocyclic group Chemical group 0.000 claims description 3
- 101000615488 Homo sapiens Methyl-CpG-binding domain protein 2 Proteins 0.000 claims description 3
- 102100021299 Methyl-CpG-binding domain protein 2 Human genes 0.000 claims description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 3
- 125000001624 naphthyl group Chemical group 0.000 claims description 3
- 229910052757 nitrogen Inorganic materials 0.000 claims description 3
- PULTVXLQRCFDMC-UHFFFAOYSA-N 4-[3-[(3-aminopyrrolidin-1-yl)methyl]-6-(4-fluorophenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound NC1CN(CC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)F)C1=CC=C(C#N)C=C1 PULTVXLQRCFDMC-UHFFFAOYSA-N 0.000 claims description 2
- YNKYOTUZEIAWLY-UHFFFAOYSA-N 4-[3-[(3-aminopyrrolidin-1-yl)methyl]-6-[4-(trifluoromethyl)phenyl]-1-benzofuran-5-yl]benzonitrile Chemical compound NC1CN(CC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 YNKYOTUZEIAWLY-UHFFFAOYSA-N 0.000 claims description 2
- MQEXBLBDOHQHHG-DEOSSOPVSA-N 4-[3-[[(3S)-3-aminopiperidin-1-yl]methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound N[C@@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 MQEXBLBDOHQHHG-DEOSSOPVSA-N 0.000 claims description 2
- ZINQDMVPWVWQNM-UHFFFAOYSA-N 4-[3-[[methyl-[3-(methylamino)propyl]amino]methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound CN(CCCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 ZINQDMVPWVWQNM-UHFFFAOYSA-N 0.000 claims description 2
- LOVNJYFCBFUYEC-UHFFFAOYSA-N 4-[6-(4-methylphenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N1CC(CC1)NCC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 LOVNJYFCBFUYEC-UHFFFAOYSA-N 0.000 claims description 2
- JDCGRRWCOGNXCM-UHFFFAOYSA-N CN(CCCNC)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 Chemical compound CN(CCCNC)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 JDCGRRWCOGNXCM-UHFFFAOYSA-N 0.000 claims description 2
- IUFLRTBBADJCNH-UHFFFAOYSA-N CN(CCCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 Chemical compound CN(CCCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 IUFLRTBBADJCNH-UHFFFAOYSA-N 0.000 claims description 2
- VFTGGUDSNLJEBR-UHFFFAOYSA-N CN(CCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 Chemical compound CN(CCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 VFTGGUDSNLJEBR-UHFFFAOYSA-N 0.000 claims description 2
- XFTHBAGADCWUPX-HSZRJFAPSA-N N[C@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 Chemical compound N[C@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 XFTHBAGADCWUPX-HSZRJFAPSA-N 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 239000008194 pharmaceutical composition Substances 0.000 claims 3
- 238000004519 manufacturing process Methods 0.000 claims 1
- 238000003786 synthesis reaction Methods 0.000 abstract description 13
- 239000000203 mixture Substances 0.000 abstract description 10
- 229940079593 drug Drugs 0.000 abstract description 7
- 239000003814 drug Substances 0.000 abstract description 7
- 230000000694 effects Effects 0.000 abstract description 6
- 150000001907 coumarones Chemical class 0.000 abstract description 4
- 230000002401 inhibitory effect Effects 0.000 abstract description 3
- 150000001413 amino acids Chemical class 0.000 abstract 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 189
- 238000006243 chemical reaction Methods 0.000 description 109
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 87
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 84
- 235000019439 ethyl acetate Nutrition 0.000 description 64
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 62
- 239000000543 intermediate Substances 0.000 description 57
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 50
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 49
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 49
- 239000002904 solvent Substances 0.000 description 48
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 47
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 46
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 45
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 40
- 239000007787 solid Substances 0.000 description 39
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 38
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 33
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 33
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 27
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 27
- 230000002829 reductive effect Effects 0.000 description 26
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 24
- 150000007529 inorganic bases Chemical class 0.000 description 23
- 229910000027 potassium carbonate Inorganic materials 0.000 description 23
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 23
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 22
- 239000000243 solution Substances 0.000 description 22
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 21
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 21
- 101150003085 Pdcl gene Proteins 0.000 description 20
- 239000012046 mixed solvent Substances 0.000 description 20
- 239000011541 reaction mixture Substances 0.000 description 19
- 238000010898 silica gel chromatography Methods 0.000 description 19
- 229910000029 sodium carbonate Inorganic materials 0.000 description 19
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 18
- 238000010438 heat treatment Methods 0.000 description 17
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 16
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 16
- 239000002253 acid Substances 0.000 description 16
- 229910052763 palladium Inorganic materials 0.000 description 16
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 15
- 229910000024 caesium carbonate Inorganic materials 0.000 description 15
- 239000007810 chemical reaction solvent Substances 0.000 description 14
- 101001050886 Homo sapiens Lysine-specific histone demethylase 1A Proteins 0.000 description 13
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-diisopropylethylamine Substances CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 13
- 239000000460 chlorine Substances 0.000 description 13
- 239000011698 potassium fluoride Substances 0.000 description 12
- 235000003270 potassium fluoride Nutrition 0.000 description 12
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 11
- 102100024985 Lysine-specific histone demethylase 1A Human genes 0.000 description 10
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 10
- 239000002798 polar solvent Substances 0.000 description 10
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 10
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 9
- 239000011230 binding agent Substances 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 8
- 238000006069 Suzuki reaction reaction Methods 0.000 description 8
- 229910052786 argon Inorganic materials 0.000 description 8
- 239000004327 boric acid Substances 0.000 description 8
- AEDZKIACDBYJLQ-UHFFFAOYSA-N ethane-1,2-diol;hydrate Chemical compound O.OCCO AEDZKIACDBYJLQ-UHFFFAOYSA-N 0.000 description 8
- DQYBDCGIPTYXML-UHFFFAOYSA-N ethoxyethane;hydrate Chemical compound O.CCOCC DQYBDCGIPTYXML-UHFFFAOYSA-N 0.000 description 8
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 description 8
- 229910052808 lithium carbonate Inorganic materials 0.000 description 8
- 238000000034 method Methods 0.000 description 8
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 8
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 8
- UGOMMVLRQDMAQQ-UHFFFAOYSA-N xphos Chemical compound CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 UGOMMVLRQDMAQQ-UHFFFAOYSA-N 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 7
- 150000007530 organic bases Chemical class 0.000 description 7
- 239000001488 sodium phosphate Substances 0.000 description 7
- 229910000162 sodium phosphate Inorganic materials 0.000 description 7
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 239000007821 HATU Substances 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- BHIIGRBMZRSDRI-UHFFFAOYSA-N [chloro(phenoxy)phosphoryl]oxybenzene Chemical compound C=1C=CC=CC=1OP(=O)(Cl)OC1=CC=CC=C1 BHIIGRBMZRSDRI-UHFFFAOYSA-N 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 239000012043 crude product Substances 0.000 description 6
- ZWWWLCMDTZFSOO-UHFFFAOYSA-N diethoxyphosphorylformonitrile Chemical compound CCOP(=O)(C#N)OCC ZWWWLCMDTZFSOO-UHFFFAOYSA-N 0.000 description 6
- 238000002156 mixing Methods 0.000 description 6
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical group ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 6
- 239000002775 capsule Substances 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- CEBAHYWORUOILU-UHFFFAOYSA-N (4-cyanophenyl)boronic acid Chemical compound OB(O)C1=CC=C(C#N)C=C1 CEBAHYWORUOILU-UHFFFAOYSA-N 0.000 description 4
- BIWQNIMLAISTBV-UHFFFAOYSA-N (4-methylphenyl)boronic acid Chemical compound CC1=CC=C(B(O)O)C=C1 BIWQNIMLAISTBV-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 238000005917 acylation reaction Methods 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical class OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- VWWQXMAJTJZDQX-UYBVJOGSSA-N flavin adenine dinucleotide Chemical compound C1=NC2=C(N)N=CN=C2N1[C@@H]([C@H](O)[C@@H]1O)O[C@@H]1CO[P@](O)(=O)O[P@@](O)(=O)OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C2=NC(=O)NC(=O)C2=NC2=C1C=C(C)C(C)=C2 VWWQXMAJTJZDQX-UYBVJOGSSA-N 0.000 description 4
- 235000019162 flavin adenine dinucleotide Nutrition 0.000 description 4
- 239000011714 flavin adenine dinucleotide Substances 0.000 description 4
- 229940093632 flavin-adenine dinucleotide Drugs 0.000 description 4
- 238000000227 grinding Methods 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- 239000005457 ice water Substances 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 229910000160 potassium phosphate Inorganic materials 0.000 description 4
- 235000011009 potassium phosphates Nutrition 0.000 description 4
- 230000035484 reaction time Effects 0.000 description 4
- 238000011160 research Methods 0.000 description 4
- JPJALAQPGMAKDF-UHFFFAOYSA-N selenium dioxide Chemical compound O=[Se]=O JPJALAQPGMAKDF-UHFFFAOYSA-N 0.000 description 4
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- QBZXNCTYNYRTIJ-UHFFFAOYSA-N 3-chloro-1,3-oxazolidin-2-one Chemical compound ClN1CCOC1=O QBZXNCTYNYRTIJ-UHFFFAOYSA-N 0.000 description 3
- NDNUEQUTEGSZQQ-UHFFFAOYSA-N 4-[6-(4-methylphenyl)-3-(piperazin-1-ylmethyl)-1-benzofuran-5-yl]benzonitrile Chemical compound CC(C=C1)=CC=C1C(C(C(C=C1)=CC=C1C#N)=C1)=CC2=C1C(CN1CCNCC1)=CO2 NDNUEQUTEGSZQQ-UHFFFAOYSA-N 0.000 description 3
- SNAXHHZZWGGTDG-QFIPXVFZSA-N CC(C=C1)=CC=C1C(C=C1NC=C(C(N(CCC2)C[C@H]2N)=O)C1=C1)=C1C(C=C1)=CC=C1C#N Chemical compound CC(C=C1)=CC=C1C(C=C1NC=C(C(N(CCC2)C[C@H]2N)=O)C1=C1)=C1C(C=C1)=CC=C1C#N SNAXHHZZWGGTDG-QFIPXVFZSA-N 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108010033040 Histones Proteins 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical class CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000012317 TBTU Substances 0.000 description 3
- SORGEQQSQGNZFI-UHFFFAOYSA-N [azido(phenoxy)phosphoryl]oxybenzene Chemical compound C=1C=CC=CC=1OP(=O)(N=[N+]=[N-])OC1=CC=CC=C1 SORGEQQSQGNZFI-UHFFFAOYSA-N 0.000 description 3
- CLZISMQKJZCZDN-UHFFFAOYSA-N [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium Chemical compound C1=CC=C2N(OC(N(C)C)=[N+](C)C)N=NC2=C1 CLZISMQKJZCZDN-UHFFFAOYSA-N 0.000 description 3
- 229960000583 acetic acid Drugs 0.000 description 3
- 238000007171 acid catalysis Methods 0.000 description 3
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 3
- 150000001540 azides Chemical class 0.000 description 3
- 238000005893 bromination reaction Methods 0.000 description 3
- 239000007853 buffer solution Substances 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 238000006555 catalytic reaction Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- MKRTXPORKIRPDG-UHFFFAOYSA-N diphenylphosphoryl azide Chemical compound C=1C=CC=CC=1P(=O)(N=[N+]=[N-])C1=CC=CC=C1 MKRTXPORKIRPDG-UHFFFAOYSA-N 0.000 description 3
- 239000010408 film Substances 0.000 description 3
- 235000011187 glycerol Nutrition 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 125000006239 protecting group Chemical group 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000000829 suppository Substances 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- TXUICONDJPYNPY-UHFFFAOYSA-N (1,10,13-trimethyl-3-oxo-4,5,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl) heptanoate Chemical compound C1CC2CC(=O)C=C(C)C2(C)C2C1C1CCC(OC(=O)CCCCCC)C1(C)CC2 TXUICONDJPYNPY-UHFFFAOYSA-N 0.000 description 2
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 2
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 2
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- XXRCUYVCPSWGCC-UHFFFAOYSA-N Ethyl pyruvate Chemical compound CCOC(=O)C(C)=O XXRCUYVCPSWGCC-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 229910021626 Tin(II) chloride Inorganic materials 0.000 description 2
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 2
- 239000012346 acetyl chloride Substances 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 239000012300 argon atmosphere Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 239000012964 benzotriazole Substances 0.000 description 2
- WTEOIRVLGSZEPR-UHFFFAOYSA-N boron trifluoride Chemical compound FB(F)F WTEOIRVLGSZEPR-UHFFFAOYSA-N 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000006196 drop Substances 0.000 description 2
- PQJJJMRNHATNKG-UHFFFAOYSA-N ethyl bromoacetate Chemical compound CCOC(=O)CBr PQJJJMRNHATNKG-UHFFFAOYSA-N 0.000 description 2
- 229940117360 ethyl pyruvate Drugs 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000012362 glacial acetic acid Substances 0.000 description 2
- 238000005658 halogenation reaction Methods 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 239000013038 irreversible inhibitor Substances 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000000865 liniment Substances 0.000 description 2
- MKIJJIMOAABWGF-UHFFFAOYSA-N methyl 2-sulfanylacetate Chemical compound COC(=O)CS MKIJJIMOAABWGF-UHFFFAOYSA-N 0.000 description 2
- 230000011987 methylation Effects 0.000 description 2
- 238000007069 methylation reaction Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- GVWISOJSERXQBM-UHFFFAOYSA-N n-methylpropan-1-amine Chemical compound CCCNC GVWISOJSERXQBM-UHFFFAOYSA-N 0.000 description 2
- 238000010534 nucleophilic substitution reaction Methods 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- 235000010288 sodium nitrite Nutrition 0.000 description 2
- 239000001119 stannous chloride Substances 0.000 description 2
- 235000011150 stannous chloride Nutrition 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- AKEJUJNQAAGONA-UHFFFAOYSA-N sulfur trioxide Chemical compound O=S(=O)=O AKEJUJNQAAGONA-UHFFFAOYSA-N 0.000 description 2
- 238000001308 synthesis method Methods 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000002813 thiocarbonyl group Chemical group *C(*)=S 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 1
- JLTLCFYJJZDGIY-UHFFFAOYSA-N 1-(5-bromo-4-chloro-2-hydroxyphenyl)ethanone Chemical compound CC(=O)c1cc(Br)c(Cl)cc1O JLTLCFYJJZDGIY-UHFFFAOYSA-N 0.000 description 1
- NGLGKGRCTLFWND-UHFFFAOYSA-N 1-bromo-2-chloro-4-(dibromomethyl)-5-fluorobenzene Chemical compound BrC1=C(C=C(C(=C1)F)C(Br)Br)Cl NGLGKGRCTLFWND-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- RMTZLUPWTUGBFD-UHFFFAOYSA-N 4-[3-[(3-aminopyrrolidin-1-yl)methyl]-6-(3,5-difluorophenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound NC1CN(CC1)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC(=C1)F)F)C1=CC=C(C#N)C=C1 RMTZLUPWTUGBFD-UHFFFAOYSA-N 0.000 description 1
- XYRHFIZNXGOROH-UHFFFAOYSA-N 4-[3-[(3-aminopyrrolidin-1-yl)methyl]-6-(3-nitrophenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound NC1CN(CC1)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 XYRHFIZNXGOROH-UHFFFAOYSA-N 0.000 description 1
- GMTJDCDDDXPCMJ-UHFFFAOYSA-N 4-[3-[(3-aminopyrrolidin-1-yl)methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound CC(C=C1)=CC=C1C(C(C(C=C1)=CC=C1C#N)=C1)=CC2=C1C(CN(CC1)CC1N)=CO2 GMTJDCDDDXPCMJ-UHFFFAOYSA-N 0.000 description 1
- MLETXFGRTIAXND-UHFFFAOYSA-N 4-[3-[(4-aminopiperidin-1-yl)methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound CC(C=C1)=CC=C1C(C(C(C=C1)=CC=C1C#N)=C1)=CC2=C1C(CN(CC1)CCC1N)=CO2 MLETXFGRTIAXND-UHFFFAOYSA-N 0.000 description 1
- QNNYVTYUGPAGBK-UHFFFAOYSA-N 4-[3-[(pyrrolidin-3-ylamino)methyl]-6-[4-(trifluoromethyl)phenyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N1CC(CC1)NCC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C(F)(F)F)C1=CC=C(C#N)C=C1 QNNYVTYUGPAGBK-UHFFFAOYSA-N 0.000 description 1
- MQEXBLBDOHQHHG-XMMPIXPASA-N 4-[3-[[(3R)-3-aminopiperidin-1-yl]methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound N[C@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 MQEXBLBDOHQHHG-XMMPIXPASA-N 0.000 description 1
- NIMJPIJRCLKPFK-UHFFFAOYSA-N 4-[3-[[methyl-[2-(methylamino)ethyl]amino]methyl]-6-(4-methylphenyl)-1-benzofuran-5-yl]benzonitrile Chemical compound CN(CCNC)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 NIMJPIJRCLKPFK-UHFFFAOYSA-N 0.000 description 1
- YSBGZDHINUNSJV-UHFFFAOYSA-N 4-[6-(2-fluoro-4-methylphenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound FC1=C(C=CC(=C1)C)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 YSBGZDHINUNSJV-UHFFFAOYSA-N 0.000 description 1
- ZZKHTYXAJNRVRB-UHFFFAOYSA-N 4-[6-(3,5-dimethylphenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound CC=1C=C(C=C(C=1)C)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 ZZKHTYXAJNRVRB-UHFFFAOYSA-N 0.000 description 1
- XVEDRRFDYLVWDC-UHFFFAOYSA-N 4-[6-(3-fluorophenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound FC=1C=C(C=CC=1)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 XVEDRRFDYLVWDC-UHFFFAOYSA-N 0.000 description 1
- HOEYFJLKLPLYBY-UHFFFAOYSA-N 4-[6-(4-ethylphenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound C(C)C1=CC=C(C=C1)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 HOEYFJLKLPLYBY-UHFFFAOYSA-N 0.000 description 1
- XDYMMLDNURHRGR-UHFFFAOYSA-N 4-[6-(4-methylphenyl)-3-[(4-methylpiperazin-1-yl)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound CN1CCN(CC1)CC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 XDYMMLDNURHRGR-UHFFFAOYSA-N 0.000 description 1
- SULSXUGFPDVOEZ-UHFFFAOYSA-N 4-[6-(4-methylphenyl)-3-[(piperidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N1CC(CCC1)NCC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 SULSXUGFPDVOEZ-UHFFFAOYSA-N 0.000 description 1
- DGYQVPZDHUEMAO-UHFFFAOYSA-N 4-[6-(4-methylphenyl)-3-[(piperidin-4-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N1CCC(CC1)NCC1=COC2=C1C=C(C(=C2)C1=CC=C(C=C1)C)C1=CC=C(C#N)C=C1 DGYQVPZDHUEMAO-UHFFFAOYSA-N 0.000 description 1
- QJCOGKFIBGKNEC-UHFFFAOYSA-N 4-[6-(4-phenylphenyl)-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N#CC(C=C1)=CC=C1C(C(C(C=C1)=CC=C1C1=CC=CC=C1)=C1)=CC2=C1OC=C2CNC1CNCC1 QJCOGKFIBGKNEC-UHFFFAOYSA-N 0.000 description 1
- WPLSWIIRVXCXJV-UHFFFAOYSA-N 4-[6-naphthalen-2-yl-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound C1=C(C=CC2=CC=CC=C12)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 WPLSWIIRVXCXJV-UHFFFAOYSA-N 0.000 description 1
- SSRURWLLKPLYTE-UHFFFAOYSA-N 4-[6-phenyl-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound C1(=CC=CC=C1)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 SSRURWLLKPLYTE-UHFFFAOYSA-N 0.000 description 1
- KLKVWPPBTJXTKP-UHFFFAOYSA-N 4-[6-pyrimidin-5-yl-3-[(pyrrolidin-3-ylamino)methyl]-1-benzofuran-5-yl]benzonitrile Chemical compound N1=CN=CC(=C1)C1=CC2=C(C(=CO2)CNC2CNCC2)C=C1C1=CC=C(C#N)C=C1 KLKVWPPBTJXTKP-UHFFFAOYSA-N 0.000 description 1
- FQEYHIPPYOSPLF-UHFFFAOYSA-N 4-bromo-3-chlorophenol Chemical compound OC1=CC=C(Br)C(Cl)=C1 FQEYHIPPYOSPLF-UHFFFAOYSA-N 0.000 description 1
- AJGYCHJPYQRCMC-UHFFFAOYSA-N 4-bromo-5-chloro-2-fluorobenzaldehyde Chemical compound FC1=CC(Br)=C(Cl)C=C1C=O AJGYCHJPYQRCMC-UHFFFAOYSA-N 0.000 description 1
- UCINOBZMLCREGM-RNNUGBGQSA-N 4-n-[(1r,2s)-2-phenylcyclopropyl]cyclohexane-1,4-diamine;dihydrochloride Chemical compound Cl.Cl.C1CC(N)CCC1N[C@H]1[C@H](C=2C=CC=CC=2)C1 UCINOBZMLCREGM-RNNUGBGQSA-N 0.000 description 1
- KTVYKJCVHUVDPT-UHFFFAOYSA-N 5-bromo-6-chloro-1h-indole-3-carboxylic acid Chemical compound ClC1=C(Br)C=C2C(C(=O)O)=CNC2=C1 KTVYKJCVHUVDPT-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- 229910015900 BF3 Inorganic materials 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- QJEDVOZVZJUTTH-UHFFFAOYSA-N BrC=1C(=CC2=C(C(=CO2)CO)C=1)Cl Chemical compound BrC=1C(=CC2=C(C(=CO2)CO)C=1)Cl QJEDVOZVZJUTTH-UHFFFAOYSA-N 0.000 description 1
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 description 1
- HEQHLABBUNVJHZ-UHFFFAOYSA-N CNCCNCC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 Chemical compound CNCCNCC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 HEQHLABBUNVJHZ-UHFFFAOYSA-N 0.000 description 1
- FZAHPYKVDDXANG-UHFFFAOYSA-N COC(=O)c1cc2cc(Cl)c(Br)cc2s1 Chemical compound COC(=O)c1cc2cc(Cl)c(Br)cc2s1 FZAHPYKVDDXANG-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 102000008169 Co-Repressor Proteins Human genes 0.000 description 1
- 108010060434 Co-Repressor Proteins Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 101100477411 Dictyostelium discoideum set1 gene Proteins 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- LRULVYSBRWUVGR-FCHUYYIVSA-N GSK2879552 Chemical compound C1=CC(C(=O)O)=CC=C1CN1CCC(CN[C@H]2[C@@H](C2)C=2C=CC=CC=2)CC1 LRULVYSBRWUVGR-FCHUYYIVSA-N 0.000 description 1
- 108010074870 Histone Demethylases Proteins 0.000 description 1
- 102000008157 Histone Demethylases Human genes 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 239000002841 Lewis acid Substances 0.000 description 1
- KQKBMHGOHXOHTD-KKUQBAQOSA-N N-[(2S)-5-[[(1R,2S)-2-(4-fluorophenyl)cyclopropyl]amino]-1-(4-methylpiperazin-1-yl)-1-oxopentan-2-yl]-4-(triazol-1-yl)benzamide Chemical compound FC1=CC=C(C=C1)[C@H]1[C@@H](C1)NCCC[C@@H](C(=O)N1CCN(CC1)C)NC(C1=CC=C(C=C1)N1N=NC=C1)=O KQKBMHGOHXOHTD-KKUQBAQOSA-N 0.000 description 1
- PHSPJQZRQAJPPF-UHFFFAOYSA-N N-alpha-Methylhistamine Chemical compound CNCCC1=CN=CN1 PHSPJQZRQAJPPF-UHFFFAOYSA-N 0.000 description 1
- KCZLYPBVRRONFU-JOCHJYFZSA-N N[C@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 Chemical compound N[C@H]1CN(CCC1)CC1=COC2=C1C=C(C(=C2)C1=CC(=CC=C1)[N+](=O)[O-])C1=CC=C(C#N)C=C1 KCZLYPBVRRONFU-JOCHJYFZSA-N 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 108700025716 Tumor Suppressor Genes Proteins 0.000 description 1
- 102000044209 Tumor Suppressor Genes Human genes 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 230000031709 bromination Effects 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000017858 demethylation Effects 0.000 description 1
- 238000010520 demethylation reaction Methods 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- 125000003963 dichloro group Chemical group Cl* 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000007888 film coating Substances 0.000 description 1
- 238000009501 film coating Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 230000002140 halogenating effect Effects 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 150000007517 lewis acids Chemical class 0.000 description 1
- 229940040145 liniment Drugs 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 239000012982 microporous membrane Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- ZUHZZVMEUAUWHY-UHFFFAOYSA-N n,n-dimethylpropan-1-amine Chemical compound CCCN(C)C ZUHZZVMEUAUWHY-UHFFFAOYSA-N 0.000 description 1
- UPSFMJHZUCSEHU-JYGUBCOQSA-N n-[(2s,3r,4r,5s,6r)-2-[(2r,3s,4r,5r,6s)-5-acetamido-4-hydroxy-2-(hydroxymethyl)-6-(4-methyl-2-oxochromen-7-yl)oxyoxan-3-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide Chemical compound CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@H]1[C@H](O)[C@@H](NC(C)=O)[C@H](OC=2C=C3OC(=O)C=C(C)C3=CC=2)O[C@@H]1CO UPSFMJHZUCSEHU-JYGUBCOQSA-N 0.000 description 1
- YZMHQCWXYHARLS-UHFFFAOYSA-N naphthalene-1,2-disulfonic acid Chemical compound C1=CC=CC2=C(S(O)(=O)=O)C(S(=O)(=O)O)=CC=C21 YZMHQCWXYHARLS-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- UHZYTMXLRWXGPK-UHFFFAOYSA-N phosphorus pentachloride Chemical compound ClP(Cl)(Cl)(Cl)Cl UHZYTMXLRWXGPK-UHFFFAOYSA-N 0.000 description 1
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- RFIOZSIHFNEKFF-UHFFFAOYSA-M piperazine-1-carboxylate Chemical compound [O-]C(=O)N1CCNCC1 RFIOZSIHFNEKFF-UHFFFAOYSA-M 0.000 description 1
- 229920000137 polyphosphoric acid Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 238000006722 reduction reaction Methods 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- AKQXKEBCONUWCL-QMMMGPOBSA-N tert-butyl (3s)-3-aminopiperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC[C@H](N)C1 AKQXKEBCONUWCL-QMMMGPOBSA-N 0.000 description 1
- CMIBWIAICVBURI-UHFFFAOYSA-N tert-butyl 3-aminopyrrolidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCC(N)C1 CMIBWIAICVBURI-UHFFFAOYSA-N 0.000 description 1
- CWXPZXBSDSIRCS-UHFFFAOYSA-N tert-butyl piperazine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCNCC1 CWXPZXBSDSIRCS-UHFFFAOYSA-N 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- FEONEKOZSGPOFN-UHFFFAOYSA-K tribromoiron Chemical compound Br[Fe](Br)Br FEONEKOZSGPOFN-UHFFFAOYSA-K 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- XBBRLCXCBCZIOI-DLBZAZTESA-N vafidemstat Chemical compound O1C(N)=NN=C1CN[C@H]1[C@H](C=2C=CC(OCC=3C=CC=CC=3)=CC=2)C1 XBBRLCXCBCZIOI-DLBZAZTESA-N 0.000 description 1
- 229940099259 vaseline Drugs 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/78—Benzo [b] furans; Hydrogenated benzo [b] furans
- C07D307/79—Benzo [b] furans; Hydrogenated benzo [b] furans with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the hetero ring
- C07D307/81—Radicals substituted by nitrogen atoms not forming part of a nitro radical
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/06—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
本发明属于化学合成药物技术领域,涉及一类新型苯并呋喃类衍生物以及所述衍生物的药学可接受的盐、它们的制备方法及其作为赖氨酸特异性去甲基酶‑1(LSD1)抑制剂的用途。所述衍生物的药学可接受的盐和立体异构体的结构如通式(I)所示。其中,X、R1、R2、R3、W1和W2如权利要求和说明书所述。本发明所述的衍生物以及所述衍生物的药学可接受的盐、异构体或含有该衍生物的组合物具有明显的抑制氨酸特异性去甲基酶‑1的作用,可以用于制备LSD1抑制剂。
Description
技术领域
本发明属于化学合成药物技术领域,涉及一类新型苯并呋喃类衍生物以及所述衍生物的药学可接受的盐、它们的制备方法及其作为赖氨酸特异性去甲基酶-1(LSD1)抑制剂的用途。
背景技术
组蛋白赖氨酸去甲基化酶在开发表观遗传学领域的药物中占据着非常重要的地位,直到2004年,Shi Yang课题组第一次发现了组蛋白赖氨酸特异性去甲基化酶1,揭示了组蛋白甲基化过程是一个可逆、可控的过程(Cell 2004,119,941–953.)。研究发现LSD1在辅因子黄素腺嘌呤二核苷酸(FAD)的辅助下可以特异性地去除组蛋白赖氨酸的单甲基或双甲基,通过作用于甲基化的赖氨酸可以分别发挥基因转录激活和转录抑制的作用。进而调控下游信号通路,发挥调节作用。通过去除H3K4的双甲基化修饰,LSD1可以与转录共阻遏物诱导抑癌基因的沉默,从而促进肿瘤细胞的生长。研究者相继报道了LSD1作为肿瘤治疗靶标,其抑制剂与其他药物(如激酶抑制剂)联合用药可发挥更好的肿瘤治疗效果。因此,LSD1抑制剂的开发有利于抗肿瘤药物的研发,可以单独或者与其他药物组合用于癌症的治疗。
已报道的LSD1抑制剂有六百多个,其中尚无用于肿瘤治疗的上市LSD1抑制剂。目前处于临床研究阶段的LSD1抑制剂共有11个,可分为不可逆性抑制剂和可逆性抑制剂两类。不可逆性抑制剂可以与辅因子FAD形成共价结合,阻断FAD参与去甲基的循环,从而发挥LSD1抑制活性。目前处于临床研究阶段的代表性LSD1抑制剂ORY-1001、ORY-2001、GSK-2879552、IMG-7289均以反苯基环丙胺为结构母核的不可逆抑制剂。目前已公布结构的抑制剂中只有Utah大学原研、Salarius制药公司推进临床试验的Seclidemstat mesylate和Celgene制药公司开发的CC-90011作为可逆性LSD1抑制剂处于临床研究阶段,适应症均为肿瘤。
目前对于LSD1抑制剂的研发多数以反苯基环丙胺类化合物展开,开发更多新骨架LSD1抑制剂是目前抗肿瘤领域的研究热点。本发明所述通式Ⅰ化合物作为LSD1抑制剂,在体外酶水平表现出较好的活性。
发明内容
本发明的目的在于提供一种通式I所示的苯并呋喃类衍生物,以及所述衍生物的药学可接受的盐和立体异构体,用于制备LSD1抑制剂,
其中:
X=CH2或NR、O、S;R为H或C1-C6烷基;
R2为-CH2-、羰基或硫代羰基;
R3为取代或未取代的5-10元的杂环基或-N(R4)2,所述杂环基含有1-3个N、O或S的杂原子,所述的取代基为C1-C6烷基、C1-C6烷氧基、氨基、C1-C6烷基氨基;
每个R4独立地选自氢、C1~C7脂肪伯胺、C1~C7脂肪仲胺、C1~C7脂肪叔胺;
W1和W2独立地选自N、C-H、C-F、C-NH2、C-CH3;
R1为任选地为取代或未取代的5-10元芳基、5-10元杂芳基、C3-C6环烷基、C1-C6烷基、5-10元杂环基、所述的取代基为C1-C6烷基、C1-C6烷氧基、卤素、卤代C1-C6烷基、硝基、氨基、5-10元芳基。
本发明优选涉及通式(Ⅰ)所示的苯并呋喃类衍生物,及其药学上可接受的盐、立体异构体,
其中:
X=CH2或NR、O、S;R为H或C1-C6烷基;
R2为-CH2-、羰基或硫代羰基;
R3为取代或未取代的5-6元的杂环基或-N(R4)2,所述杂环基含有1-2个N杂原子,所述的取代基为C1-C6烷基、C1-C6烷氧基、氨基、C1-C6烷基氨基;
每个R4独立地选为丙胺、N-甲基丙胺、N,N-二甲基丙胺。
本发明优选涉及通式(Ⅰ)所示的衍生物,及其药学上可接受的盐、立体异构体,
其中,
R3为取代或未取代的如下基团:
本发明优选涉及通式(Ⅰ)所示的衍生物,及其药学上可接受的盐、立体异构体,
其中,
W1和W2独立地选自N、C-H、C-F、C-NH2、C-CH3;
本发明优选涉及通式(Ⅰ)所示的衍生物,及其药学上可接受的盐、立体异构体,
其中,
R1为任选地取代或未取代的5-6元芳基、5-6元杂芳基、C3-C6环烷基、C1-C6烷基、5-6元杂环基、所述的取代基为C1-C6烷基、C1-C6烷氧基、卤素、卤代C1-C6烷基、硝基、氨基、5-6元芳基;
优选地,R1为任选地取代或未取代的苯基、萘基,所述的取代基为C1-C6烷基、C1-C6烷氧基、卤素、卤代C1-C6烷基、硝基、氨基、苯基;
本发明通式(Ⅰ)化合物及其药学上可接受的盐优选以下化合物,但这些化合物并不意味着对本发明的任何限制:
4-(3-(哌嗪-1-基甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(R)-4-(3-((3-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((哌啶-4-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-氨基环己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((哌啶-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(S)-4-(3-((3-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((((1r,4r)-4-氨基环己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((2-氨基乙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(2-(甲氨基)乙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲氨基)丙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基丙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-氨基丁基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((5-氨基戊基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((6-氨基己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-甲基哌嗪-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(2-(甲胺基)乙基)氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲胺基)丙基)氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(3,5-二氟苯基)苯并呋喃-5-基)苯甲腈
(R)-4-(3-((3-氨基哌啶-1-基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(3-((2-(甲基氨基)乙基氨基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
(R)-4-(3-((3-氨基哌啶-1-基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(4-氟苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲氨基)丙基)氨基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(6-(萘-2-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(3,5-二甲基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(3-氟苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-([1,1'-联苯]-4-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(4-乙基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(2-氟-4-甲基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-苯基-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(1-甲基-1H-吲哚唑-5-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(3-((吡咯烷-3-基氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(6-(嘧啶-5-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
(S)-4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(R)-4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
5-(4-氰基苯基)-N-(哌啶-4-基甲基)-6-(对甲苯基)-1H-吲哚-3-甲酰胺
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-3-甲酰胺
(S)-4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)-1H-吲哚-5-基)苯甲腈
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基甲基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺
(S)-4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)苯并噻吩-5-基)苯甲腈
而且,按照本发明所属领域的一些通常方法,本发明中通式I的化合物可以与酸生成药学上可接受的盐。可药用加成盐包括无机酸和有机酸加成盐,与酸加成的盐是特别优选的酸为:盐酸、氢溴酸、硫酸、磷酸、甲磺酸、乙磺酸、对甲苯磺酸、苯磺酸、萘二磺酸、乙酸、丙酸、乳酸、三氟乙酸、马来酸、柠檬酸、富马酸、草酸、酒石酸、苯甲酸。
本发明中“卤素”是指氟、氯、溴或碘;“烷基”是指直链或支链的烷基;“芳杂环”是指含有一个或多个选自N、O、S杂原子的单环或多环的环状体系,该环状体系是指具有芳香性的,并且除去环状体系中的一个或不同位置的多个氢原子而得到的有机基团,如噻唑基,咪唑基、吡啶基、吡唑基、(1,2,3)-和(1,2,4)-三唑基、呋喃基、噻吩基、吡咯基,吲哚基,苯并噻唑基,噁唑基,异噁唑基,萘基,喹啉基,异喹啉基,苯并咪唑基,苯并噁唑基等。
下文中提供的实施例和制备例进一步阐明和举例说明本发明化合物及其制备方法。应当理解,下述实例和制备例的范围并不以任何方式限制本发明的范围。
按照本发明的式(I)化合物,可按照路线一、二、三或四的方法制备得来。
如路线一所示,其中应用的全部可变因数如权利要求中的定义。
起始原料A I与乙酰氯或乙酸酐反应得到中间体AII,反应条件a为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)做缚酸剂的情况下,于室温条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选碳酸钾做缚酸剂,乙腈做溶剂,25℃反应。中间体AII经重排反应得中间体A III,反应条件b可以为各种路易斯酸(如:三氯化铝、三氟化硼、三氧化硫和溴化铁,等)作为催化剂;反应溶剂可以是甲苯、N,N-二甲基甲酰胺、二甲基亚砜等或者无溶剂;催化条件优选三氯化铝、无溶剂、反应温度优选150℃。AIII与溴乙酸乙酯发生亲核取代反应得到中间体A IV,反应条件c为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)做缚酸剂的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选碳酸钾做缚酸剂,丙酮做溶剂,80℃反应。AIV与通过水解反应得到中间体A V,反应条件d为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选氢氧化钠做缚酸剂,甲醇/水(体积比为1:1)的混合溶剂做溶剂,70℃反应。中间体A V发生缩合反应得到中间体A VI,反应条件e为醋酸钠做碱,醋酐和冰乙酸做溶剂,120℃反应。中间体A VI发生氧化反应得到中间体A VII,反应条件f为二氧化硒做氧化剂,二氧六环做溶剂,100℃反应。中间体A VII与取代的芳基硼酸经铃木反应得中间体A VIII,反应条件g为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选PdCl2(dppf);无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选碳酸钠;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选110℃。中间体VIII与取代的芳基硼酸经铃木反应得中间体IX,反应条件h为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选Pd(OAc)2和X-phos;无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选磷酸钾;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选80℃。中间体A IX发生卤代反应得到中间体A X,反应条件i为二氯亚砜做卤代试剂,二氯做溶剂,25℃反应。中间体A X和取代的脂肪胺发生亲核取代反应得到中间体AXI,反应条件j为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)做缚酸剂的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选碳酸钾做缚酸剂,N,N-二甲基甲酰胺做溶剂,80℃反应。中间体A XI通过脱出Boc保护基反应得到目标产物,反应条件k为酸催化下,加热4–12小时;酸可以为三氟乙酸、盐酸/乙醇、盐酸/二氧六环和盐酸/乙酸乙酯等,优选盐酸/乙酸乙酯;溶剂可以为甲醇、乙醇、异丙醇、N,N-二甲基甲酰胺和二甲基亚砜等,优选乙酸乙酯;反应温度可以为25–80℃,优选25℃;反应时间优选4小时。
路线二所示,其中应用的全部可变因数如权利要求中的定义。
起始原料B1通过溴代反应得到中间体B2,溴代试剂可以是NBS、溴素等,可加适量催化剂,优选卤化试剂是NBS;反应溶剂可为四氢呋喃、乙醚、二氯甲烷、氯仿、甲苯、甲醇等,优选四氢呋喃;反应温度0℃-60℃。中间体B2通过酯化反应得到中间体B3,酰化条件可以是二氯亚砜、草酰氯、五氯化磷、三氯化磷、三氯氧磷、三溴化磷;反应溶剂可为四氢呋喃、乙醚、二氯甲烷、氯仿、甲苯、甲醇等,优选甲醇;反应温度0℃-60℃。中间体B3与取代的芳基硼酸经铃木反应得中间体B4,反应条件c为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选PdCl2(dppf);无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选碳酸钠;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选110℃。中间体B4与取代的芳基硼酸经铃木反应得中间体B5,反应条件d为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选Pd(OAc)2和X-phos;无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选磷酸钾;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选80℃。B5与通过水解反应得到中间体B6,反应条件e为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选氢氧化钠做缚酸剂,甲醇/水(体积比为1:1)的混合溶剂做溶剂,70℃反应。中间体B6和取代的脂肪胺发生酰化反应得到中间体B7,反应条件f:为O-(7-氮杂苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HATU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HBTU)、O-(5-氯苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HCTU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓四氟硼酸盐(TBTU)、O-(N-丁二酰亚胺基)-二(二甲胺基)碳鎓四氟硼酸盐(TSTU)、O-(N-endo-5-降莰烯-2,3-二碳二酰亚胺)-二(二甲胺基)碳鎓四氟硼酸盐(TNTU)、二苯基磷酰氯(DPP-Cl)、氰代磷酸二乙酯(DECP)、叠氮化磷酸二苯酯(DPPA、硫代二甲基磷酰基叠氮(MPTA)、二(2-氧-3-唑烷基)磷酰氯(BOP-Cl),2,4,6-三丙基-1,3,5,2,4,6-三氧三磷酸-2,4,6-三氧化物(T3P)等做缩合剂的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选N,N-二甲基甲酰胺做溶剂和HATU做缩合剂,40℃反应。中间体B7通过脱出Boc保护基反应得到目标产物,反应条件g为酸催化下,加热4–12小时;酸可以为三氟乙酸、盐酸/乙醇、盐酸/二氧六环和盐酸/乙酸乙酯等,优选盐酸/乙酸乙酯;溶剂可以为甲醇、乙醇、异丙醇、N,N-二甲基甲酰胺和二甲基亚砜等,优选乙酸乙酯;反应温度可以为25–80℃,优选25℃;反应时间优选4小时。
路线三所示,其中应用的全部可变因数如权利要求中的定义。
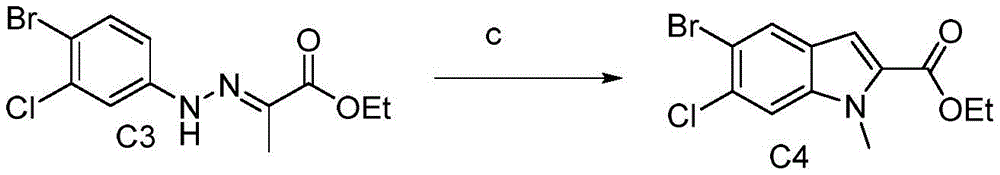
起始原料C1通过重氮化还原反应得到中间体C2,亚硝酸钠作为反应试剂,反应溶剂为浓盐酸,氯化亚锡作为还原剂,反应温度-10℃。中间体C2通过与丙酮酸乙酯缩合得到中间体C3,C3在多聚氧磷的条件下环合得到中间体C4.中间体C4与取代的芳基硼酸经铃木反应得中间体C5,反应条件d为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选PdCl2(dppf);无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选碳酸钠;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选110℃。中间体C5与取代的芳基硼酸经铃木反应得中间体C6,反应条件e为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选Pd(OAc)2和X-phos;无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选磷酸钾;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选80℃。C6与通过水解反应得到中间体C7,反应条件f为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选氢氧化钠做缚酸剂,甲醇/水(体积比为1:1)的混合溶剂做溶剂,70℃反应。中间体C7和取代的脂肪胺发生酰化反应得到中间体C8,反应条件g:为O-(7-氮杂苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HATU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HBTU)、O-(5-氯苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HCTU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓四氟硼酸盐(TBTU)、O-(N-丁二酰亚胺基)-二(二甲胺基)碳鎓四氟硼酸盐(TSTU)、O-(N-endo-5-降莰烯-2,3-二碳二酰亚胺)-二(二甲胺基)碳鎓四氟硼酸盐(TNTU)、二苯基磷酰氯(DPP-Cl)、氰代磷酸二乙酯(DECP)、叠氮化磷酸二苯酯(DPPA、硫代二甲基磷酰基叠氮(MPTA)、二(2-氧-3-唑烷基)磷酰氯(BOP-Cl),2,4,6-三丙基-1,3,5,2,4,6-三氧三磷酸-2,4,6-三氧化物(T3P)等做缩合剂的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选N,N-二甲基甲酰胺做溶剂和HATU做缩合剂,40℃反应。中间体C8通过脱出Boc保护基反应得到目标产物,反应条件h为酸催化下,加热4–12小时;酸可以为三氟乙酸、盐酸/乙醇、盐酸/二氧六环和盐酸/乙酸乙酯等,优选盐酸/乙酸乙酯;溶剂可以为甲醇、乙醇、异丙醇、N,N-二甲基甲酰胺和二甲基亚砜等,优选乙酸乙酯;反应温度可以为25–80℃,优选25℃;反应时间优选4小时。
路线四所示,其中应用的全部可变因数如权利要求中的定义。
起始原料D1通过溴代反应得到中间体D2,AIBN作为催化剂,反应溶剂为四氯化碳,反应温度80℃。中间体D2通过发生氧化反应得到中间体D3,D3在巯基乙酸甲酯的条件下环合得到中间体D4.D4与通过水解反应得到中间体D5,反应条件d为一种有机碱(如:三乙胺、N,N-二异丙基乙胺等)或无机碱(如:碳酸钠、碳酸钾、碳酸铯、磷酸钠、氢氧化钠、氢氧化钾等)的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选氢氧化钠做缚酸剂,甲醇/水(体积比为1:1)的混合溶剂做溶剂,70℃反应。中间体D5和取代的脂肪胺发生酰化反应得到中间体D6,反应条件e:为O-(7-氮杂苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HATU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HBTU)、O-(5-氯苯并三氮唑-1-基)-二(二甲胺基)碳鎓六氟磷酸盐(HCTU)、O-(苯并三氮唑-1-基)-二(二甲胺基)碳鎓四氟硼酸盐(TBTU)、O-(N-丁二酰亚胺基)-二(二甲胺基)碳鎓四氟硼酸盐(TSTU)、O-(N-endo-5-降莰烯-2,3-二碳二酰亚胺)-二(二甲胺基)碳鎓四氟硼酸盐(TNTU)、二苯基磷酰氯(DPP-Cl)、氰代磷酸二乙酯(DECP)、叠氮化磷酸二苯酯(DPPA、硫代二甲基磷酰基叠氮(MPTA)、二(2-氧-3-唑烷基)磷酰氯(BOP-Cl),2,4,6-三丙基-1,3,5,2,4,6-三氧三磷酸-2,4,6-三氧化物(T3P)等做缩合剂的情况下,于室温或加热条件下进行;反应溶剂可以是甲醇、乙醇、丙醇、丙酮、N,N-二甲基甲酰胺、二甲基亚砜等极性溶剂;优选N,N-二甲基甲酰胺做溶剂和HATU做缩合剂,40℃反应。中间体D6与取代的芳基硼酸经铃木反应得中间体D7,反应条件f为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选PdCl2(dppf);无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选碳酸钠;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选110℃。中间体D7与取代的芳基硼酸经铃木反应得中间体D8,反应条件g为钯配合物催化、无机碱和无氧条件下加热反应;零价钯配合物可以为Pd(PPh3)4、PdCl2、PdCl2(dppf)、Pd(OAc)2和Pd(PPh3)2Cl2等,优选Pd(OAc)2和X-phos;无机碱可以为碳酸钾、碳酸钠、碳酸锂、碳酸铯和氟化钾等,优选磷酸钾;溶剂可以为乙醇、1,4-二氧六环、四氢呋喃、甲苯、N,N-二甲基甲酰胺、二甲基亚砜、水和乙二醇二甲醚等,也可以是两种溶剂组成的混合溶剂,溶剂优选乙二醇二甲醚和水(体积比为10:1)的混合溶剂;反应温度可以为80–140℃,优选80℃。中间体D8通过脱出Boc保护基反应得到目标产物,反应条件h为酸催化下,加热4–12小时;酸可以为三氟乙酸、盐酸/乙醇、盐酸/二氧六环和盐酸/乙酸乙酯等,优选盐酸/乙酸乙酯;溶剂可以为甲醇、乙醇、异丙醇、N,N-二甲基甲酰胺和二甲基亚砜等,优选乙酸乙酯;反应温度可以为25–80℃,优选25℃;反应时间优选4小时。
具体实施方式:
实施例旨在阐述而不是限制本发明的范围。化合物的核磁共振氢谱用BrukerARX-400测定,质谱用Agilent 1100LC/MSD测定;所用试剂均为分析纯或化学纯。
表1.实施例结构式、化学名、相对分子质量
实施例1:4-(3-(哌嗪-1-基甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈的合成
步骤A-1:4-溴-3-氯苯乙酸酯的制备
将4-溴-3-氯苯酚(8.0g,38.6mmol)和碳酸钾(5.3g,38.6mmol)加入250mL反应瓶中,加入100mL乙腈,室温搅拌30min。将乙酰氯(3.0g,38.6mmol)滴加到反应液中,加毕,升温至60℃继续反应6h。冷却反应液至室温,将反应液减压浓缩,加入50mL乙酸乙酯溶解粗品。乙酸乙酯相依次水洗(10mL×3)、饱和食盐水洗(10mL×3),无水硫酸钠干燥后浓缩,白色粗品固体8.7g,收率90.0%。
步骤A-2:1-(5-溴-4-氯-2-羟基苯基)乙烷-1-酮的制备
将A II(8.7g,34.7mmol)和无水三氯化铝(1.7g,10.0mmol)加入100mL反应瓶中,150℃反应30min。将反应液加入40mL水、40mL乙酸乙酯,剧烈搅拌5min后。分出乙酸乙酯相,以无水硫酸钠干燥后浓缩得粗品,得白色固体7.8g,收率90.0%。
步骤A-3:2-(2-乙酰基-4-溴-5-氯苯氧基)乙酸乙酯的合成
将A III(7.8g,31.2mmol)、碳酸钾(4.3g,31.2mmol)和溴乙酸乙酯(5.2g,31.2mmol)加入250mL反应瓶,60℃反应4h。冷却反应液,旋干浓缩,40mL水,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,以无水硫酸钠干燥后浓缩得粗品,得黄色固体9.4g,收率90.0%。
步骤A-4:2-(2-乙酰基-4-溴-5-氯苯氧基)乙酸的制备
将A IV(9.4g,28.1mmol)、氢氧化钠(1.1g,28.1mmol)加入250mL反应瓶,加入40mL甲醇和40mL水,70℃反应2h。冷却反应液,将反应液浓缩,用1N的盐酸调节PH=2,析出白色固体,干燥,得粗品白色固体7.8g,收率90.0%。
步骤A-5:5-溴-6-氯-3-甲基苯并呋喃的合成
将A V(7.8g,25.3mmol)、醋酸钠(2.1g,25.3mmol)、冰乙酸50mL和醋酐50mL,120℃搅拌反应10h。将反应液倒入50mL冰水,用乙酸乙酯(40mL×3)萃取,合并乙酸乙酯并用饱和食盐水(10mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩,柱层析分离得白色固体3.5g,收率56.8%。
步骤A-6:(5-溴-6-氯苯并呋喃-3-基)甲醇的制备
将A VI(3.5g,14.4mmol)、二氧化硒(1.6g,14.4mmol)和二氧六环50mL加入100mL反应瓶,100℃反应40h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体2.5g,收率66.3%。
步骤A-7:4-(6-氯-3-(羟甲基)苯并呋喃-5-基)苯甲腈的合成
将A VII(2.5g,9.5mmol)、Pd(dppf)Cl2(0.4g,0.5mmol)、氟化钾(0.8g,14.4mmol)、4-氰基苯硼酸(1.4g,9.5mmol)和二氧六环50mL加入100mL反应瓶,在氩气保护下90℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.6g,收率60.3%。
步骤A-8:4-(3-(羟甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈的合成
将A VIII(1.6g,5.7mmol)、Pd(OAc)2(0.07g,0.3mmol)、X-phos(2.7g,5.7mmol)、4-甲基苯硼酸(1.4g,5.7mmol)、乙二醇二甲醚50mL和水5mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.0g,收率50.3%。
步骤A-9:4-(3-(氯甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈的制备
将A IX(1.0g,2.9mmol)、氯化亚砜(0.3g,2.9mmol)和二氯甲烷20mL加入100ml反应瓶中,室温反应3h。反应液冷却减压浓缩得油状物,用40ml乙酸乙酯溶解油状物,并依次用水和饱和食盐水洗涤。无水硫酸钠干燥后浓缩,经硅胶柱层析纯化得黄色油状液体0.9g,收率90.0%。
步骤A-10:4-((5-(4-氰基苯基)-6-(对甲苯基)苯并呋喃-3-基)甲基)哌嗪-1-羧酸叔丁酯的制备
将A X(0.1g,0.3mmol)、1-Boc-哌嗪(0.05g,0.3mmol)、碳酸钾(0.04g,0.3mmol)和DMF 5mL加入10mL反应瓶中,室温反应10h。反应液中加入20mL水,用40ml乙酸乙酯萃取,并依次用水和饱和食盐水洗涤。无水硫酸钠干燥后浓缩,经硅胶柱层析纯化得黄色油状液体80.0mg,收率52.5%。
步骤A-11:4-(3-(哌嗪-1-基甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈的制备
AX(80.0mg,0.15mmol)加入5ml 6N HCl/MeOH中,室温搅拌反应2h。减压浓缩溶剂,加入5ml水,用饱和氢氧化钠溶液调节PH=10。乙酸乙酯(10mL×3)萃取,合并乙酸乙酯并用饱和食盐水(10mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩,得橘红色固体55.7mg,收率86.8%。
实施例02-40的合成按照实施例01的合成方法得到。
实施例43:(S)-4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)-1H-吲哚-5-基)苯甲腈的合成
步骤B-1:5-溴-6-氯-1H-吲哚-3-羧酸的制备
将B1(5.0g,25.6mmol)、NBS(4.6g,25.6mmol)和四氯化碳50mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体3.0g,收率75.3%。
步骤B-2:5-溴-6-氯-1H-吲哚-3-羧酸乙酯的制备
将B2(5.0g,18.2mmol)、二氯亚砜(4.3g,36.4mmol)和乙醇50mL加入100mL反应瓶,在70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体3.0g,收率75.3%。
步骤B-3:6-氯-5-(4-氰基苯基)-1H-吲哚-3-羧酸乙酯的制备
将B3(2.9g,9.5mmol)、Pd(dppf)Cl2(0.4g,0.5mmol)、氟化钾(0.8g,14.4mmol)、4-氰基苯硼酸(1.4g,9.5mmol)和二氧六环50mL加入100mL反应瓶,在氩气保护下90℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.6g,收率60.3%。
步骤B-4:5-(4-氰基苯基)-6-(对甲苯基)-1H-吲哚-3-羧酸乙酯的制备
将B4(1.85g,5.7mmol)、Pd(OAc)2(0.07g,0.3mmol)、X-phos(2.7g,5.7mmol)、4-甲基苯硼酸(1.4g,5.7mmol)、乙二醇二甲醚50mL和水5mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.0g,收率50.3%。
步骤B-5:5-(4-氰基苯基)-6-(对甲苯基)-1H-吲哚-3-羧酸的制备
将B5(10.8g,28.1mmol)、氢氧化钠(1.1g,28.1mmol)加入250mL反应瓶,加入40mL甲醇和40mL水,70℃反应2h。冷却反应液,将反应液浓缩,用1N的盐酸调节PH=2,析出白色固体,干燥,得粗品白色固体7.8g,收率90.0%。
步骤B-6:叔丁基(1-(5-(4-氰基苯基)-6-(对甲苯基)吲哚-3-羰基)哌啶-3-基)氨基甲酸酯的制备
将中间体B6(0.35g,1mmol)、S-3氨基Boc-哌啶(0.20g,1.0mmol)、2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(0.38g,1.0mmol)加入10mL反应瓶,加入2mL无水DMF和0.26g N,N-二异丙基乙胺,室温反应12h。将反应液倒入20mL水中,用二氯甲烷10mL×3萃取,合并二氯甲烷相,用无水硫酸钠干燥,浓缩得粗品,硅胶柱层析分离纯化得0.33g白色固体,收率56.1%。
步骤B-7:(S)-4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)-1H-吲哚-5-基)苯甲腈的制备
B7(80.0mg,0.15mmol)加入5ml 6N HCl/MeOH中,室温搅拌反应2h。减压浓缩溶剂,加入5ml水,用饱和氢氧化钠溶液调节PH=10。乙酸乙酯(10mL×3)萃取,合并乙酸乙酯并用饱和食盐水(10mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩,得橘红色固体55.7mg,收率86.8%。
实施例41-42的合成按照实施例43的合成方法得到。
实施例45:5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺的合成
步骤C1:(4-溴-3-氯苯)肼盐酸盐的制备
C1(5.0g,24.2mmol)、亚硝酸钠(1.7g,24.2mmol)加入100mL反应瓶,加入20mL浓盐酸,-10℃反应2h。加入氯化亚锡(5.5g,24.2mmol),-10℃反应2h。过滤,干燥,减压浓缩,得淡黄色固体7.1g,收率86.8%。
步骤C2:(E)-2-(2-(4-溴-3-氯苯基)肼基内酯)丙酸乙酯的制备
C2(5.0g,19.4mmol)、丙酮酸乙酯(2.3g,19.4mmol)加入100mL反应瓶,加入20mL乙醇,室温下反应2h。倒入冰水中,析出固体过滤,干燥,,得淡黄色固体7.1g,收率86.8%。
步骤C3:5-溴-6-氯-1-甲基-1H-吲哚-2-羧酸乙酯的制备
C2(6.2g,19.4mmol)加入100mL反应瓶,加入20mL多聚磷酸,120℃下反应2h。倒入冰水中,析出固体过滤,干燥,得淡黄色固体3.5g,在加入碘甲烷(2.8g,19.4mmol),碳酸钾(2.7g,19.4mmol)和8mLDMF,40℃下反应2h。倒入冰水中,析出固体过滤,干燥,得淡黄色固体5.6g,收率66.8%。
步骤C4:6-氯-5-(4-氰基苯基)-1-甲基-1H-吲哚-2-羧酸乙酯的制备
将C4(2.9g,9.5mmol)、Pd(dppf)Cl2(0.4g,0.5mmol)、氟化钾(0.8g,14.4mmol)、4-氰基苯硼酸(1.4g,9.5mmol)和二氧六环50mL加入100mL反应瓶,在氩气保护下90℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.9g,收率60.3%。
步骤C5:5-(4-氰基苯基)-1-甲基-6-(对甲苯基)-1H-吲哚-2-羧酸乙酯的制备
将C5(1.9g,5.7mmol)、Pd(OAc)2(0.07g,0.3mmol)、X-phos(2.7g,5.7mmol)、4-甲基苯硼酸(1.4g,5.7mmol)、乙二醇二甲醚50mL和水5mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体1.2g,收率50.3%。
步骤C6:5-(4-氰基苯基)-1-甲基-6-(对甲苯基)-1H-吲哚-2-羧酸的制备
将C6(10.7g,28.1mmol)、氢氧化钠(1.1g,28.1mmol)加入250mL反应瓶,加入40mL甲醇和40mL水,70℃反应2h。冷却反应液,将反应液浓缩,用1N的盐酸调节PH=2,析出白色固体,干燥,得粗品白色固体7.8g,收率90.0%。
步骤C7:4-(5-(4-氰基苯基)-1-甲基-6-(对甲苯基)-1H-吲哚-2-甲酰胺基)哌啶-1-羧酸叔丁酯的制备
将中间体C7(0.35g,1mmol)、1-Boc-3-氨基吡咯烷(0.20g,1.0mmol)、2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(0.38g,1.0mmol)加入10mL反应瓶,加入2mL无水DMF和0.26g N,N-二异丙基乙胺,室温反应12h。将反应液倒入20mL水中,用二氯甲烷10mL×3萃取,合并二氯甲烷相,用无水硫酸钠干燥,浓缩得粗品,硅胶柱层析分离纯化得0.33g白色固体,收率56.1%。
步骤C8:5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺的制备
C8(80.0mg,0.15mmol)加入5ml 6N HCl/MeOH中,室温搅拌反应2h。减压浓缩溶剂,加入5ml水,用饱和氢氧化钠溶液调节PH=10。乙酸乙酯(10mL×3)萃取,合并乙酸乙酯并用饱和食盐水(10mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩,得橘红色固体55.7mg,收率86.8%。
实施例44的合成按照实施例45的合成方法得到。
实施例46:(S)-4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)苯并噻吩-5-基)苯甲腈的合成
步骤D1:1-溴-2-氯-4-(二溴甲基)-5-氟苯的制备
将D1(5.0g,25.6mmol)、NBS(9.2g,51.2mmol),0.5g AIBN和四氯化碳50mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色油状液体6.0g,收率90.3%。
步骤D2:4-溴-5-氯-2-氟苯甲醛的制备
将D2(9.8g,25.6mmol)和DMSO 20mL加入100mL反应瓶,100℃反应2h。冷却反应液,加入水100mL,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体6.0g,收率60.3%。
步骤D3:6-溴-5-氯苯并噻吩-2-羧酸甲酯的制备
将D3(3.7g,15.5mmol),巯基乙酸甲酯(1.6g,15.5mmol),碳酸钾(2.1g,15.5mmol)和DMF 20mL加入100mL反应瓶,40℃反应12h。冷却反应液,加入水100mL,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体6.0g,收率60.3%。
步骤D4:6-溴-5-氯苯并噻吩-2-羧酸的制备
将D4(8.6g,28.1mmol)、氢氧化钠(1.1g,28.1mmol)加入250mL反应瓶,加入40mL甲醇和40mL水,70℃反应2h。冷却反应液,将反应液浓缩,用1N的盐酸调节PH=2,析出白色固体,干燥,得粗品白色固体7.8g,收率90.0%。
步骤D5:叔丁基(R)-(1-(6-溴-5-氯苯并[b]噻吩-2-羰基)哌啶-3-基)氨基甲酸酯的制备
将中间体D5(0.4g,1.4mmol)、(S)-3氨基Boc-哌啶(0.42g,2.1mmol)、2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(0.78g,2.1mmol)加入10mL反应瓶,加入2mL无水DMF和0.26g N,N-二异丙基乙胺,室温反应12h。将反应液倒入20mL水中,用二氯甲烷10mL×3萃取,合并二氯甲烷相,用无水硫酸钠干燥,浓缩得粗品,硅胶柱层析分离纯化得0.45g黄色固体,收率85.0%。
步骤D6:叔丁基(R)-(1-(5-氯-6-(对甲苯基)苯并噻吩-2-羰基)哌啶-3-基)氨基甲酸酯的制备
将D6(0.9g,1.9mmol)、Pd(dppf)Cl2(0.1g,0.1mmol)、氟化钾(0.2g,2.8mmol)、4-甲基苯硼酸(0.2g,1.9mmol)和二氧六环50mL加入100mL反应瓶,在氩气保护下90℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体0.4g,收率60.3%。
步骤D7:叔丁基(R)-(1-(5-(4-氰基苯基)-6-(对甲苯基)苯并噻吩-2-羰基)哌啶-3-基)氨基甲酸酯的制备
将D7(0.4g,0.82mmol)、Pd(OAc)2(0.02g,0.08mmol)、X-phos(0.08g,0.17mmol)、4-氰基苯硼酸(0.36g,2.5mmol)、乙二醇二甲醚50mL和水5mL加入100mL反应瓶,在氩气保护下70℃反应10h。冷却反应液,浓缩反应液,乙酸乙酯(40mL×3)萃取,饱和食盐水(40mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩。经硅胶柱层析纯化得黄色固体0.15g,收率40.3%。
步骤D8:(R)-4-(2-(3-氨基哌啶-1-羰基)-6-(对甲苯基)苯并[b]噻吩-5-基)苯腈的制备
D8(80.0mg,0.15mmol)加入5ml 6N HCl/MeOH中,室温搅拌反应2h。减压浓缩溶剂,加入5ml水,用饱和氢氧化钠溶液调节PH=10。乙酸乙酯(10mL×3)萃取,合并乙酸乙酯并用饱和食盐水(10mL×3)洗涤。将乙酸乙酯相用无水硫酸钠干燥,减压浓缩,得橘红色固体55.7mg,收率86.8%。
本发明部分产物的体外药理测试
本发明采购Cayman公司的700120试剂盒对部分化合物的LSD1抑制活性进行检测,具体操作步骤如下:
(1)检测设置100%活性孔、背景孔、阳性对照孔和化合物孔。每组设三个复孔。
(2)100%活性孔:依次加入120μL LSD1 Buffer溶液、10μL溶液(与溶解化合物和阳性药相同成分的溶液)、20μL LSD1酶、20μL LSD1检测肽。
(3)测试孔和阳性对照孔:依次加入120μL LSD1 Buffer溶液、10μL待测化合物溶液、20μL LSD1酶、20μL LSD1检测肽。
(4)背景孔:依次加入140μL LSD1 Buffer溶液、10μL溶液(与溶解化合物和阳性药相同成分的溶液)、20μL LSD1酶。
(5)加溶液过程中将96孔板置于冰袋上降温,防止酶促反应的进行。加毕,避光,室温孵育30min。
(6)孵育30min后,依次向每个孔加入20μL辣根过氧化物酶溶液、10μL荧光底物溶液。避光,室温孵育10min。
(7)酶标仪530nM波长下激发,检测590nM的发射荧光的强弱。
抑制率%=(100%活性孔–样品孔)/100%活性孔*100
表2.实施例核磁氢谱、酶抑制活性数据
注:体外酶水平活性测试IC50被指定为在以下范围内:
A:<0.1μΜ
B:>0.1μΜ至<1μΜ
C:>1μΜ至<50μΜ
D:>50μΜ
本发明中通式I的化合物及其药学上可接受的盐或前药可单独施用,但通常是和药用载体混合物给予,所述药用载体的选择要根据所需用药途径和标准药物实践,下面分别用该类化合物的各种药物剂型,例如片剂、胶囊剂、注射剂、气雾剂、栓剂、膜剂、滴丸剂、外用搽剂和软膏剂的制备方法,说明其在制药领域中的新应用。
实施例49:片剂
用含有权利要求1中化合物的化合物(以实施例1化合物为例)10g,按照药剂学一般压片法加辅料20g混匀后,压制成100片,每片重300mg。
实施例50:胶囊剂
用含有权利要求1中化合物的化合物(以实施例2化合物为例)10g,按照药剂学胶囊剂的要求将辅料20g混匀后,装入空心胶囊,每个胶囊重300mg。
实施例51:注射剂
用含有权利要求1中化合物的化合物(以实施例3化合物为例)10g,按照药剂学常规方法,进行活性炭吸附,经0.65μm微孔滤膜过滤后,填入氮气罐制成水针制剂,每只装2mL,共灌装100瓶。
实施例52:气雾剂
用含有权利要求1中化合物的化合物(以实施例5化合物为例)10g,用适量丙二醇溶解后,加入蒸馏水及其他辐料后,制成500mL的澄清溶液即得。
实施例53:栓剂
用含有权利要求1中化合物的化合物(以实施例9化合物为例)10g,将之研细加入甘油适量,研匀后加入已熔化的甘油明胶,研磨均匀,倾入已涂润滑剂的模型中,制得栓剂50颗
实施例54:膜剂
用含有权利要求1中化合物的化合物(以实施例23化合物为例)10g,将聚乙烯醇、药用甘油、水等搅拌膨胀后加热溶解,80目筛网过滤,再将实施例18化合物加入到滤液中搅拌溶解,涂膜机制膜100片。
实施例55:滴丸剂
用含有权利要求1中化合物的化合物(以实施例30化合物为例)10g,与明胶等基质50g加热熔化混匀后,滴入低温液体石蜡中,共制得滴丸1000丸。
实施例56:外用搽剂
用含有权利要求1中化合物的化合物(以实施例35化合物为例)10g,按照常规药剂学方法与乳化剂等辅料2.5g混合研磨,再加蒸馏水至200mL制得。
实施例57:软膏剂
用含有权利要求1中化合物的化合物(以实施例40化合物为例)10g,研细后与凡士林等油性基质500g研匀制得。
尽管已经通过特定实施方案描述了本发明,但修改和等价变化对于精通此领域的技术人员而言是显见的,且它们都包含在本发明范围。
Claims (7)
3.权利要求1或2所述的通式(Ⅰ)所示的化合物,及其药学上可接受的盐、立体异构体,其中,X = NH、O、S、N-CH3。
4.如下的化合物,及其药学上可接受的盐、立体异构体,
4-(3-(哌嗪-1-基甲基)-6-(对甲苯基)苯并呋喃-5-基) 苯甲腈
4-(3-((4-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(R) -4-(3-((3-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((哌啶-4-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-氨基环己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((哌啶-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(S) -4-(3-((3-氨基哌啶-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((((1r,4r)-4-氨基环己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((2-氨基乙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(2-(甲氨基)乙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲氨基)丙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基丙基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-氨基丁基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((5-氨基戊基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((6-氨基己基)氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((4-甲基哌嗪-1-基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(2-(甲胺基)乙基)氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲胺基)丙基)氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(3,5-二氟苯基)苯并呋喃-5-基)苯甲腈
(R) -4-(3-((3-氨基哌啶-1-基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(3-((2-(甲基氨基)乙基氨基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
(R) -4-(3-((3-氨基哌啶-1-基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(3-((3-氨基吡咯烷-1-基)甲基)-6-(4-氟苯基)苯并呋喃-5-基)苯甲腈
4-(3-((甲基(3-(甲氨基)丙基)氨基)甲基)-6-(3-硝基苯基)苯并呋喃-5-基)苯甲腈
4-(6-(萘-2-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(3,5-二甲基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(3-氟苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-([1,1'-联苯]-4-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(4-乙基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(2-氟-4-甲基苯基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-苯基-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(6-(1-甲基-1H-吲哚唑-5-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
4-(3-((吡咯烷-3-基氨基)甲基)-6-(4-(三氟甲基)苯基)苯并呋喃-5-基)苯甲腈
4-(6-(嘧啶-5-基)-3-((吡咯烷-3-基氨基)甲基)苯并呋喃-5-基)苯甲腈
(S) -4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
(R) -4-(3-((吡咯烷-3-基氨基)甲基)-6-(对甲苯基)苯并呋喃-5-基)苯甲腈
5-(4-氰基苯基)-N-(哌啶-4-基甲基)-6-(对甲苯基)-1H-吲哚-3-甲酰胺
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-3-甲酰胺
(S) -4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)-1H-吲哚-5-基)苯甲腈
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基甲基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺
5-(4-氰基苯基)-1-甲基-N-(哌啶-4-基)-6-(对甲苯基)-1H-吲哚-2-甲酰胺
(S) -4-(3-(3-氨基哌啶-1-羰基)-6-(对甲苯基)苯并噻吩-5-基)苯甲腈。
5.药物组合物,包含权利要求1-4任何一项的化合物及其药学上可接受的盐、立体异构体和药学上可接受的载体。
6.药物组合物,包含权利要求1-4任何一项的化合物及其药学上可接受的盐、立体异构体和其它赖氨酸特异性去甲基化酶1抑制剂。
7.权利要求1-4任何一项所述的化合物及其药学上可接受的盐、立体异构体或权利要求5或6所述的药物组合物在制备赖氨酸特异性去甲基化酶1抑制剂中的应用。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110061056.8A CN114805261B (zh) | 2021-01-18 | 2021-01-18 | 苯并呋喃类lsd1抑制剂及其制备方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110061056.8A CN114805261B (zh) | 2021-01-18 | 2021-01-18 | 苯并呋喃类lsd1抑制剂及其制备方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114805261A CN114805261A (zh) | 2022-07-29 |
| CN114805261B true CN114805261B (zh) | 2023-03-21 |
Family
ID=82523697
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110061056.8A Active CN114805261B (zh) | 2021-01-18 | 2021-01-18 | 苯并呋喃类lsd1抑制剂及其制备方法 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114805261B (zh) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023217758A1 (en) | 2022-05-09 | 2023-11-16 | Oryzon Genomics, S.A. | Methods of treating malignant peripheral nerve sheath tumor (mpnst) using lsd1 inhibitors |
| WO2023217784A1 (en) | 2022-05-09 | 2023-11-16 | Oryzon Genomics, S.A. | Methods of treating nf1-mutant tumors using lsd1 inhibitors |
| WO2024110649A1 (en) | 2022-11-24 | 2024-05-30 | Oryzon Genomics, S.A. | Combinations of lsd1 inhibitors and menin inhibitors for treating cancer |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002074758A2 (en) * | 2001-03-16 | 2002-09-26 | Abbott Laboratories | Novel amines as histamine-3 receptor ligands and their therapeutic applications |
| CN112110936A (zh) * | 2019-06-20 | 2020-12-22 | 沈阳药科大学 | 四氢喹啉类衍生物及其制备方法和应用 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR080433A1 (es) * | 2010-03-02 | 2012-04-11 | Merck Sharp & Dohme | Derivados de benzofurancarboxamidas utiles para tratar o prevenir infecciones por vhc y composiciones farmaceuticas que los contienen. |
| US20170283397A1 (en) * | 2016-03-31 | 2017-10-05 | University Of Utah Research Foundation | Substituted 1-h-indol-3-yl-benzamide and 1, 1'-biphenyl analogs as histone demethylase inhibitors |
-
2021
- 2021-01-18 CN CN202110061056.8A patent/CN114805261B/zh active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002074758A2 (en) * | 2001-03-16 | 2002-09-26 | Abbott Laboratories | Novel amines as histamine-3 receptor ligands and their therapeutic applications |
| CN112110936A (zh) * | 2019-06-20 | 2020-12-22 | 沈阳药科大学 | 四氢喹啉类衍生物及其制备方法和应用 |
Non-Patent Citations (4)
| Title |
|---|
| Xiangyu Zhang et al..Design, synthesis and biological evaluation of novel benzofuran derivatives as potent LSD1 inhibitors.2021,第220卷第113501页. * |
| 周鑫鑫等.LSD1小分子抑制剂的合成及抗肿瘤活性研究.2017,第39卷(第8期),第823-829页. * |
| 王林等.吲哚类 LSD1 抑制剂的设计合成及酶抑制活性评价.2022,第32卷(第8期),第600-607页. * |
| 罗敏贤等.苯并呋喃类组胺H3受体拮抗剂的构效关系研究.2009,第26卷(第1期),第39-43页. * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN114805261A (zh) | 2022-07-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN114805261B (zh) | 苯并呋喃类lsd1抑制剂及其制备方法 | |
| CN113544128A (zh) | Kras-g12c抑制剂 | |
| CN112110936B (zh) | 四氢喹啉类衍生物及其制备方法和应用 | |
| TWI527800B (zh) | 作為聚(二磷酸腺苷酸-核醣)聚合酶(parp)之抑制劑之1-(芳基甲基)喹唑啉-2,4(1h,3h)-二酮及其應用 | |
| CN113354622B (zh) | 对苯二胺类lsd1抑制剂及其制备方法 | |
| WO2012089137A1 (zh) | 一种芳基脲类化合物、其中间体及其应用 | |
| WO2015007249A1 (zh) | N-烷基色胺酮衍生物及其制备方法和应用 | |
| WO2017016463A1 (zh) | Egfr抑制剂及其药学上可接受的盐和多晶型物及其应用 | |
| CN102639535B (zh) | 稠合杂芳基化合物及其制备方法和应用 | |
| KR20160063026A (ko) | 단백질 키나아제 저해제로 유용한 헤테로아릴아민 유도체 | |
| CN112094266A (zh) | 一种吡咯并吡啶酮类化合物、其制备方法、其组合物和用途 | |
| CN102108078B (zh) | 1,4-取代酞嗪类化合物及其制备方法和用途 | |
| TW201242955A (en) | Nitrogen-containing heterocyclic compound | |
| CN115322158A (zh) | 作为krasg12c蛋白抑制剂的取代喹唑啉类化合物 | |
| CN112125885A (zh) | 苯并吲哚类双功能分子衍生物及其制备方法与应用 | |
| CN106146515B (zh) | 新型激酶抑制剂的制备及应用 | |
| AU2012241442B2 (en) | 7-(heteroaryl-amino)-6,7,8,9-tetrahydropyrido(1,2-a)indol acetic acid derivatives and their use as prostaglandin D2 receptor modulators | |
| CN112979613B (zh) | 2-(1h-吡唑-3-基)吡啶衍生物及其制备方法和用途 | |
| CN114853680A (zh) | 芳香六元环并咪唑类衍生物及其制备方法和应用 | |
| CN109666022B (zh) | 三氮唑衍生物及其制备方法和用途 | |
| CN113880814B (zh) | 一种嘧啶胺类化合物及应用 | |
| WO2021259049A1 (zh) | 吲哚类衍生物及其制备方法和应用 | |
| CN111606888B (zh) | 吡咯类衍生物及其制备方法与应用 | |
| CN107793360B (zh) | 吲哚胺2,3-双加氧酶抑制剂与应用 | |
| CN113880813B (zh) | 一种2-氨基嘧啶类杂环化合物及应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |