CN113501766B - 一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 - Google Patents
一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 Download PDFInfo
- Publication number
- CN113501766B CN113501766B CN202110770331.3A CN202110770331A CN113501766B CN 113501766 B CN113501766 B CN 113501766B CN 202110770331 A CN202110770331 A CN 202110770331A CN 113501766 B CN113501766 B CN 113501766B
- Authority
- CN
- China
- Prior art keywords
- reaction
- mmol
- nmr
- cdcl
- yield
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title claims abstract description 56
- BZKFMUIJRXWWQK-UHFFFAOYSA-N Cyclopentenone Chemical class O=C1CCC=C1 BZKFMUIJRXWWQK-UHFFFAOYSA-N 0.000 title claims abstract description 11
- 238000011914 asymmetric synthesis Methods 0.000 title claims abstract description 9
- 238000006243 chemical reaction Methods 0.000 claims abstract description 62
- -1 furan alkene Chemical class 0.000 claims abstract description 61
- 239000007848 Bronsted acid Substances 0.000 claims abstract description 10
- 239000002994 raw material Substances 0.000 claims abstract description 7
- 239000002841 Lewis acid Substances 0.000 claims abstract description 6
- 150000007517 lewis acids Chemical class 0.000 claims abstract description 6
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims abstract description 6
- 238000006462 rearrangement reaction Methods 0.000 claims abstract description 3
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 claims description 69
- 230000035484 reaction time Effects 0.000 claims description 31
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 claims description 25
- 239000011941 photocatalyst Substances 0.000 claims description 17
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 claims description 13
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 8
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 8
- 150000004820 halides Chemical class 0.000 claims description 6
- 239000003960 organic solvent Substances 0.000 claims description 6
- 229910000027 potassium carbonate Inorganic materials 0.000 claims description 6
- XSVCYDUEICANRJ-UHFFFAOYSA-K dysprosium(3+);trifluoromethanesulfonate Chemical compound [Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F XSVCYDUEICANRJ-UHFFFAOYSA-K 0.000 claims description 4
- 229910052736 halogen Inorganic materials 0.000 claims description 4
- 150000002367 halogens Chemical class 0.000 claims description 4
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 4
- 239000000126 substance Substances 0.000 claims description 4
- 125000001424 substituent group Chemical group 0.000 claims description 4
- 239000002904 solvent Substances 0.000 claims description 3
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 claims description 2
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 claims description 2
- 239000003513 alkali Substances 0.000 claims description 2
- 238000005576 amination reaction Methods 0.000 claims description 2
- 239000012300 argon atmosphere Substances 0.000 claims description 2
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 claims description 2
- 150000002148 esters Chemical class 0.000 claims description 2
- 230000001404 mediated effect Effects 0.000 claims description 2
- 150000003254 radicals Chemical class 0.000 claims description 2
- 239000000376 reactant Substances 0.000 claims description 2
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 2
- LTVOKYUPTHZZQH-UHFFFAOYSA-N difluoromethane Chemical group F[C]F LTVOKYUPTHZZQH-UHFFFAOYSA-N 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 4
- 125000000217 alkyl group Chemical group 0.000 abstract description 3
- PQIOSYKVBBWRRI-UHFFFAOYSA-N methylphosphonyl difluoride Chemical group CP(F)(F)=O PQIOSYKVBBWRRI-UHFFFAOYSA-N 0.000 abstract description 3
- 238000003786 synthesis reaction Methods 0.000 abstract description 3
- 230000015572 biosynthetic process Effects 0.000 abstract description 2
- 229940079593 drug Drugs 0.000 abstract description 2
- 238000007171 acid catalysis Methods 0.000 abstract 1
- 238000006555 catalytic reaction Methods 0.000 abstract 1
- 238000005481 NMR spectroscopy Methods 0.000 description 121
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 102
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 102
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 72
- 238000004128 high performance liquid chromatography Methods 0.000 description 36
- 239000003208 petroleum Substances 0.000 description 35
- 239000000047 product Substances 0.000 description 35
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 34
- 229960001701 chloroform Drugs 0.000 description 34
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 30
- 238000010898 silica gel chromatography Methods 0.000 description 30
- GGYYSOXWFYFYOJ-UHFFFAOYSA-N 2-(1-phenylethenyl)furan Chemical compound C=1C=CC=CC=1C(=C)C1=CC=CO1 GGYYSOXWFYFYOJ-UHFFFAOYSA-N 0.000 description 25
- 239000003054 catalyst Substances 0.000 description 24
- 239000007810 chemical reaction solvent Substances 0.000 description 24
- IRSJDVYTJUCXRV-UHFFFAOYSA-N ethyl 2-bromo-2,2-difluoroacetate Chemical compound CCOC(=O)C(F)(F)Br IRSJDVYTJUCXRV-UHFFFAOYSA-N 0.000 description 24
- AKCRQHGQIJBRMN-UHFFFAOYSA-N 2-chloroaniline Chemical compound NC1=CC=CC=C1Cl AKCRQHGQIJBRMN-UHFFFAOYSA-N 0.000 description 22
- 239000007858 starting material Substances 0.000 description 22
- 238000002955 isolation Methods 0.000 description 20
- 238000000746 purification Methods 0.000 description 18
- 238000000926 separation method Methods 0.000 description 16
- 229910052692 Dysprosium Inorganic materials 0.000 description 15
- 229910052786 argon Inorganic materials 0.000 description 15
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 13
- 239000002585 base Substances 0.000 description 11
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 239000011541 reaction mixture Substances 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 8
- 238000004809 thin layer chromatography Methods 0.000 description 7
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 6
- 238000004440 column chromatography Methods 0.000 description 6
- 239000000741 silica gel Substances 0.000 description 6
- 229910002027 silica gel Inorganic materials 0.000 description 6
- 238000001291 vacuum drying Methods 0.000 description 5
- LKUDPHPHKOZXCD-UHFFFAOYSA-N 1,3,5-trimethoxybenzene Chemical compound COC1=CC(OC)=CC(OC)=C1 LKUDPHPHKOZXCD-UHFFFAOYSA-N 0.000 description 4
- PUDDYSBKCDKATP-UHFFFAOYSA-N methyl 2-amino-5-fluorobenzoate Chemical compound COC(=O)C1=CC(F)=CC=C1N PUDDYSBKCDKATP-UHFFFAOYSA-N 0.000 description 4
- VAMXMNNIEUEQDV-UHFFFAOYSA-N methyl anthranilate Chemical compound COC(=O)C1=CC=CC=C1N VAMXMNNIEUEQDV-UHFFFAOYSA-N 0.000 description 4
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000012512 characterization method Methods 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 239000012265 solid product Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000012043 crude product Substances 0.000 description 2
- 238000003818 flash chromatography Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- MAOBFOXLCJIFLV-UHFFFAOYSA-N (2-aminophenyl)-phenylmethanone Chemical compound NC1=CC=CC=C1C(=O)C1=CC=CC=C1 MAOBFOXLCJIFLV-UHFFFAOYSA-N 0.000 description 1
- QRADKVYIJIAENZ-UHFFFAOYSA-N 1-[[bromo(difluoro)methyl]-ethoxyphosphoryl]oxyethane Chemical compound CCOP(=O)(C(F)(F)Br)OCC QRADKVYIJIAENZ-UHFFFAOYSA-N 0.000 description 1
- YKGXVGQPQTWJSA-UHFFFAOYSA-N 2-(1-naphthalen-2-ylethenyl)furan Chemical compound C=C(C1=CC=CO1)C1=CC2=CC=CC=C2C=C1 YKGXVGQPQTWJSA-UHFFFAOYSA-N 0.000 description 1
- ITKIUHZTEFXXQN-UHFFFAOYSA-N 2-[1-(2-methoxyphenyl)ethenyl]furan Chemical compound COC1=CC=CC=C1C(=C)C1=CC=CO1 ITKIUHZTEFXXQN-UHFFFAOYSA-N 0.000 description 1
- XADZBHKNFIIWJC-UHFFFAOYSA-N 2-[1-(3-methoxyphenyl)ethenyl]furan Chemical compound COC1=CC=CC(C(C2=CC=CO2)=C)=C1 XADZBHKNFIIWJC-UHFFFAOYSA-N 0.000 description 1
- FGQBOWXXOVOPGL-UHFFFAOYSA-N 2-[1-(3-methylphenyl)ethenyl]furan Chemical compound CC1=CC(C(C2=CC=CO2)=C)=CC=C1 FGQBOWXXOVOPGL-UHFFFAOYSA-N 0.000 description 1
- ZZWTWSUJONTHPQ-UHFFFAOYSA-N 2-[1-(4-bromophenyl)ethenyl]furan Chemical compound C=C(C1=CC=CO1)C(C=C1)=CC=C1Br ZZWTWSUJONTHPQ-UHFFFAOYSA-N 0.000 description 1
- OXKHLMRPEWRGAY-UHFFFAOYSA-N 2-[1-(4-chlorophenyl)ethenyl]furan Chemical compound C=C(C1=CC=CO1)C(C=C1)=CC=C1Cl OXKHLMRPEWRGAY-UHFFFAOYSA-N 0.000 description 1
- PEMKKSNCBQHJHH-UHFFFAOYSA-N 2-[1-(4-fluorophenyl)ethenyl]furan Chemical compound C=C(C1=CC=CO1)C(C=C1)=CC=C1F PEMKKSNCBQHJHH-UHFFFAOYSA-N 0.000 description 1
- QNQKWMWERGQPRU-UHFFFAOYSA-N 2-[1-(4-methoxyphenyl)ethenyl]furan Chemical compound COC1=CC=C(C=C1)C(=C)C=1OC=CC=1 QNQKWMWERGQPRU-UHFFFAOYSA-N 0.000 description 1
- NZAQSMTYBKZQGZ-UHFFFAOYSA-N 2-[1-(4-methylphenyl)ethenyl]furan Chemical compound CC(C=C1)=CC=C1C(C1=CC=CO1)=C NZAQSMTYBKZQGZ-UHFFFAOYSA-N 0.000 description 1
- YUYRTLJANZZWAC-UHFFFAOYSA-N 2-bromo-2,2-difluoro-1-(4-methoxyphenyl)ethanone Chemical compound BrC(C(=O)C1=CC=C(C=C1)OC)(F)F YUYRTLJANZZWAC-UHFFFAOYSA-N 0.000 description 1
- QRVPSDIABPAOTN-UHFFFAOYSA-N 2-bromo-2,2-difluoro-1-phenylethanone Chemical compound FC(F)(Br)C(=O)C1=CC=CC=C1 QRVPSDIABPAOTN-UHFFFAOYSA-N 0.000 description 1
- LYWHFGULCWQURX-UHFFFAOYSA-N 2-bromo-2,2-difluoro-1-piperidin-1-ylethanone Chemical compound FC(F)(Br)C(=O)N1CCCCC1 LYWHFGULCWQURX-UHFFFAOYSA-N 0.000 description 1
- BYNRYSMTUCTWQO-UHFFFAOYSA-N 2-bromo-2,2-difluoro-n-phenylacetamide Chemical compound FC(F)(Br)C(=O)NC1=CC=CC=C1 BYNRYSMTUCTWQO-UHFFFAOYSA-N 0.000 description 1
- PSUBGTBDOZWLQV-UHFFFAOYSA-N 2-bromo-N-cyclohexyl-2,2-difluoroacetamide Chemical compound FC(F)(Br)C(=O)NC1CCCCC1 PSUBGTBDOZWLQV-UHFFFAOYSA-N 0.000 description 1
- BOHBJODHDSWYFG-UHFFFAOYSA-N 2-bromo-n,n-diethyl-2,2-difluoroacetamide Chemical compound CCN(CC)C(=O)C(F)(F)Br BOHBJODHDSWYFG-UHFFFAOYSA-N 0.000 description 1
- AOPBDRUWRLBSDB-UHFFFAOYSA-N 2-bromoaniline Chemical compound NC1=CC=CC=C1Br AOPBDRUWRLBSDB-UHFFFAOYSA-N 0.000 description 1
- PNPCRKVUWYDDST-UHFFFAOYSA-N 3-chloroaniline Chemical compound NC1=CC=CC(Cl)=C1 PNPCRKVUWYDDST-UHFFFAOYSA-N 0.000 description 1
- QSNSCYSYFYORTR-UHFFFAOYSA-N 4-chloroaniline Chemical compound NC1=CC=C(Cl)C=C1 QSNSCYSYFYORTR-UHFFFAOYSA-N 0.000 description 1
- ODGIMMLDVSWADK-UHFFFAOYSA-N 4-trifluoromethylaniline Chemical compound NC1=CC=C(C(F)(F)F)C=C1 ODGIMMLDVSWADK-UHFFFAOYSA-N 0.000 description 1
- 241000662429 Fenerbahce Species 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical compound CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000007805 chemical reaction reactant Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 125000004177 diethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 238000004896 high resolution mass spectrometry Methods 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 239000008204 material by function Substances 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- CEKCJQBZVNIMLD-UHFFFAOYSA-N methyl 2-amino-4-methoxybenzoate Chemical compound COC(=O)C1=CC=C(OC)C=C1N CEKCJQBZVNIMLD-UHFFFAOYSA-N 0.000 description 1
- VAQBJVZNPBNHGC-UHFFFAOYSA-N methyl 2-amino-4-methylbenzoate Chemical compound COC(=O)C1=CC=C(C)C=C1N VAQBJVZNPBNHGC-UHFFFAOYSA-N 0.000 description 1
- IGHVUURTQGBABT-UHFFFAOYSA-N methyl 2-amino-5-chlorobenzoate Chemical compound COC(=O)C1=CC(Cl)=CC=C1N IGHVUURTQGBABT-UHFFFAOYSA-N 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- FVNLXKRZPJQKCA-UHFFFAOYSA-N n-benzyl-2-bromo-2,2-difluoroacetamide Chemical compound FC(F)(Br)C(=O)NCC1=CC=CC=C1 FVNLXKRZPJQKCA-UHFFFAOYSA-N 0.000 description 1
- UTUYWZJPVLDHJJ-UHFFFAOYSA-N n-methyl-4-(trifluoromethyl)aniline Chemical compound CNC1=CC=C(C(F)(F)F)C=C1 UTUYWZJPVLDHJJ-UHFFFAOYSA-N 0.000 description 1
- GTDQGKWDWVUKTI-UHFFFAOYSA-N o-aminoacetophenone Chemical compound CC(=O)C1=CC=CC=C1N GTDQGKWDWVUKTI-UHFFFAOYSA-N 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000000575 pesticide Substances 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C227/00—Preparation of compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C227/14—Preparation of compounds containing amino and carboxyl groups bound to the same carbon skeleton from compounds containing already amino and carboxyl groups or derivatives thereof
- C07C227/16—Preparation of compounds containing amino and carboxyl groups bound to the same carbon skeleton from compounds containing already amino and carboxyl groups or derivatives thereof by reactions not involving the amino or carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B53/00—Asymmetric syntheses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C221/00—Preparation of compounds containing amino groups and doubly-bound oxygen atoms bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C231/00—Preparation of carboxylic acid amides
- C07C231/12—Preparation of carboxylic acid amides by reactions not involving the formation of carboxamide groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/16—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms
- C07D295/18—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms acylated on ring nitrogen atoms by radicals derived from carboxylic acids, or sulfur or nitrogen analogues thereof
- C07D295/182—Radicals derived from carboxylic acids
- C07D295/185—Radicals derived from carboxylic acids from aliphatic carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/56—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D307/66—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/38—Phosphonic acids [RP(=O)(OH)2]; Thiophosphonic acids ; [RP(=X1)(X2H)2(X1, X2 are each independently O, S or Se)]
- C07F9/40—Esters thereof
- C07F9/4003—Esters thereof the acid moiety containing a substituent or a structure which is considered as characteristic
- C07F9/4056—Esters of arylalkanephosphonic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/28—Phosphorus compounds with one or more P—C bonds
- C07F9/38—Phosphonic acids [RP(=O)(OH)2]; Thiophosphonic acids ; [RP(=X1)(X2H)2(X1, X2 are each independently O, S or Se)]
- C07F9/40—Esters thereof
- C07F9/4071—Esters thereof the ester moiety containing a substituent or a structure which is considered as characteristic
- C07F9/4075—Esters with hydroxyalkyl compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/06—Systems containing only non-condensed rings with a five-membered ring
- C07C2601/10—Systems containing only non-condensed rings with a five-membered ring the ring being unsaturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
本发明提供了一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法。它通过组合使用可见光催化、手性布朗斯特酸和非手性路易斯酸催化,以呋喃烯烃、二氟烷基卤化物和芳胺类化合物为原料进行不对称三组分氮杂‑Piancatelli重排反应,合成含二氟烷基的手性多官能化环戊烯酮衍生物。该方法反应条件温和、收率高、对映选择性和非对映选择性优良,在药物合成方面具有潜在的应用价值。
Description
技术领域
本发明属于有机化学中的不对称合成领域,具体涉及一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法。
背景技术
由于氟原子独特的性质,含氟有机化合物被广泛应用于医药、农药、功能材料和生命科学等领域。二氟烷基的引入可以显著提高分子的代谢稳定性和口服生物利用度,已成为药物研发的一种重要手段。然而,目前在作为有机合成重要中间体的环戊烯酮侧链引入二氟烷基的方法尚无相关文献报道。因此,开发一种简单、高效的方法实现含二氟烷基的多官能化环戊烯酮衍生物的不对称合成具有重要意义。
发明内容
本发明的目的在于提供一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法。
为实现上述目的,本发明采用的技术方案包括以下步骤:
步骤S1以呋喃烯烃、芳胺类化合物和二氟烷基卤化物为原料在可见光下进行自由基介导的三组分二氟烷基化胺化反应,其反应式如下:
步骤S2取中间物IV,加入手性布朗斯特酸(B*H)和非手性路易斯酸(LA),在有机溶剂中进行不对称重排反应得到产物V,其反应式如下:
其中
R为苯基、2-萘基或者含有取代基的苯环,苯环上的取代基为甲基、甲氧基或卤素中的一种;
R1为H或者甲基;
Ar为连有吸电子或供电子的不同基团的苯环,所述不同基团为甲基、甲氧基、卤素、酯基、三氟甲基、苯甲酰基或者乙酰基中的一种或者两种。
RF为连有不同基团的二氟亚甲基,其原料二氟烷基卤化物选自下列化合物结构式中的一种:
步骤S1中所述的光催化剂为mer-Ir(ppy)3,碱为碳酸钾,反应温度为室温;
步骤S2中所述的非手性路易斯酸为三氟甲磺酸镝,反应温度为室温;
步骤S2中所述的手性布朗斯特酸结构式如下:
所述的技术方案实施操作包括以下:
步骤S1:将光催化剂mer-Ir(ppy)3和K2CO3置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入乙腈。然后依次加入呋喃烯烃、芳胺类化合物和二氟烷基卤化物。将反应混合物用氩气脱气5分钟,然后在蓝色LED灯照射下室温反应,用TLC监测反应。当TLC显示芳胺类化合物完全转化时(t1),将反应混合物经硅藻土过滤,用二氯甲烷洗涤并浓缩,得到的粗产品中间物IV直接用于下一步。
步骤S2:将Dy(OTf)3和布朗斯特酸A1置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入三氯甲烷并在室温下搅拌1分钟。随后,将步骤1所得中间物IV溶解在三氯甲烷并加入反应的混合物中,室温下反应。待反应完成(t2),经快速柱层析后,使用1,3,5-三甲氧基苯作为内标,进行1H NMR表征以确定产物的核磁产率和dr值。粗产物通过硅胶柱层析进一步纯化,得到产物的主要非对映异构体及其相应的分离产率。ee值通过液相色谱仪(HPLC)进行手性分析确定。
本发明所述的制备方法中,步骤S1反应中的呋喃烯烃、芳胺类化合物和二氟烷基卤化物的用量比为0.24mmol:0.20mmol:0.40mmol。
本发明所述的制备方法中,步骤S1反应中的mer-Ir(ppy)3的摩尔用量为芳胺类化合物的0.33%。
本发明所述的制备方法中,步骤S1反应中的碳酸钾和芳胺类化合物的用量比为0.4mmol:0.20mmol。
本发明所述的制备方法中,步骤S1反应中的反应时间t1为10-96h。
本发明所述的制备方法中,步骤S1反应中的有机溶剂为乙腈,芳胺类化合物的物质的量与乙腈的体积之比为0.1mmol:2mL,反应在氩气氛围中进行。
本发明所述的制备方法中,步骤S1反应中可见光由功率为3W、波长为460-465nm的蓝色LED灯提供,所述蓝灯和反应物之间的距离为4cm。
本发明所述的制备方法中,步骤S2反应中的Dy(OTf)3、手性布朗斯特酸A1摩尔用量分别为芳胺类化合物的0.33%、1%。
本发明所述的制备方法中,步骤S2反应中的有机溶剂为三氯甲烷,溶剂用量为2mL,反应时间t2为3-120h。
本发明提供的这种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法,其反应条件温和,对映选择性和非对映选择性优良,化学收率高,在药物合成方面具有潜在的实际应用价值。
具体实施方式:
本发明的任一实施方案中的监控方法是:薄层层析法。
结构确证技术手段均为本领域技术人员知晓的通用技术手段:核磁共振技术,高分辨质谱。
下面结合实施例将对本发明作进一步的说明,但并不因此而限制本发明。
实施例1
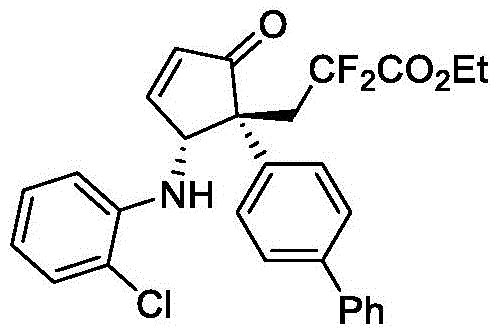
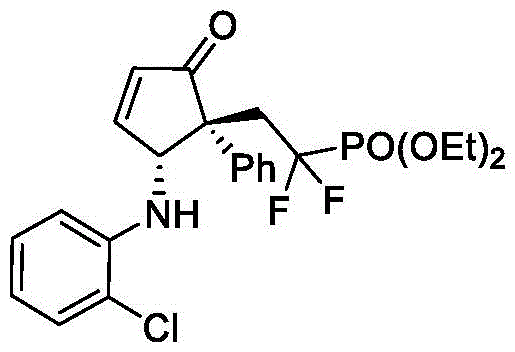
本发明的3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-1)的合成路线:
步骤1:将光催化剂mer-Ir(ppy)3(1.3mg,0.002mmol)和K2CO3(55.3mg,0.4mmol,2.0eq)置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入2mL乙腈。然后依次加入2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、邻氯苯胺(0.20mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.40mmol,2.0eq)。将反应混合物用氩气脱气5分钟,然后在功率为3W的蓝色LED灯照射下室温反应,用TLC监测反应。当TLC显示芳胺类化合物完全转化时(t1=10h),将反应混合物经硅藻土过滤,用二氯甲烷洗涤并浓缩,得到的粗产品中间物IV直接用于下一步。
步骤2:将Dy(OTf)3(0.4mg,0.0066mol)和布朗斯特酸A1(1.8mg,0.002mmol)。置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入三氯甲烷(0.5mL)并在室温下搅拌1分钟。随后,将步骤1所得中间物IV溶解在三氯甲烷(1.5mL)并加入反应的混合物中,室温下反应。待反应完成(t2=48h),经快速柱层析后,使用1,3,5-三甲氧基苯作为内标,进行1H NMR表征以确定产物的核磁产率和dr值。粗产物通过硅胶柱层析进一步纯化(石油醚/乙酸乙酯=10/1–8/1),得到3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯。核磁收率77%,分离收率72%。ee值通过HPLC进行分析确定。
所得结构式1的表征数据:
黄色油状,核磁收率77%,分离收率72%,18/1dr。HPLC分析得94%ee(FLM ChiralINB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=14.1min,tr(minor)=11.0min;[α]D 20=–98.7(c=0.82,in CHCl3).1H NMR(500MHz,CDCl3)δ7.63–7.56(m,1H),7.30–7.26(m,3H),7.21–7.13(m,2H),7.08–7.02(m,2H),6.90(d,J=8.2Hz,1H),6.65(t,J=7.6Hz,1H),6.59(d,J=5.7Hz,1H),5.44(d,J=10.3Hz,1H),4.31(q,J=7.1Hz,2H),3.84(d,J=10.4Hz,1H),3.28–3.00(m,2H),1.34(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.4,163.5(t,J=32.8),161.7,142.0,138.3,134.6,129.5,128.8,128.1,128.0,127.7,119.9,118.3,115.5(t,J=251.8Hz),111.5,63.4,59.8(t,J=6.3Hz),57.4,39.0(t,J=21.7Hz),13.9.19F NMR(377MHz,CDCl3)δ-95.8(d,J=262.2Hz),-102.2(d,J=262.3Hz).HRMS(ESI)m/z calcd.For C22H20ClF2NNaO3[M+Na]+442.0992,found 442.1010.
实施例2
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(4-甲氧基苯基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-2):
以2-(1-(4-甲氧基苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=16h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–4/1),得到黄色油状的产物V-2,核磁收率60%,分离收率57%,>20/1dr。HPLC分析得94%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=15.3min,tr(minor)=12.6min;[α]D 20=–101.6(c=0.88,in CHCl3).1H NMR(500MHz,CDCl3)δ7.61–7.56(m,1H),7.22–7.13(m,2H),6.98(d,J=7.3Hz,2H),6.90(d,J=8.1Hz,1H),6.80(d,J=7.3Hz,2H),6.66(t,J=7.6Hz,1H),6.59–6.56(m,1H),5.39(d,J=10.2Hz,1H),4.30(q,J=7.3,5.0Hz,2H),3.87(d,J=11.4Hz,1H),3.77(s,3H),3.20–2.95(m,2H),1.34(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.4,163.7(t,J=32.1),161.6,159.1,142.1,134.4,130.2,129.5,128.9,128.0,119.9,118.2,115.2(t,J=260.4Hz),114.2,111.6,63.4,59.7–59.2(m),56.8,55.4,39.1(t,J=22.4Hz),13.9.19F NMR(377MHz,CDCl3)δ-95.9(d,J=262.2Hz),-102.5(d,J=262.3Hz).HRMS(ESI)m/z calcd.For C23H22ClF2NNaO4[M+Na]+472.1098,found 472.1104.
实施例3
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(4-甲基苯基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-3):
以2-(1-(4-甲基苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=16h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–6/1),得到黄色油状的产物V-3,核磁收率54%,分离收率52%,>20/1dr。HPLC分析得94%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=11.0min,tr(minor)=8.9min;[α]D 20=–92.9(c=0.90,in CHCl3).1H NMR(500MHz,CDCl3)δ7.59–7.56(m,1H),7.20–7.13(m,2H),6.97(d,J=8.7Hz,2H),6.90(d,J=8.2Hz,1H),6.80(d,J=8.6Hz,2H),6.65(t,J=7.6Hz,1H),6.58–6.54(m,1H),5.39(d,J=10.3Hz,1H),4.29(q,J=7.1Hz,2H),3.87(d,J=10.3Hz,1H),3.76(s,3H),3.25–2.94(m,2H),1.34(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.4,163.5(t,J=29.2Hz),161.7,159.1,142.0,134.4,130.2,129.5,128.9,128.0,119.9,118.2,115.4(t,J=223.9Hz),114.2,111.5,63.4,59.9–59.7(m),56.8,55.4,39.1(t,J=22.0Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=262.5Hz),-102.4(d,J=262.1Hz).HRMS(ESI)m/z calcd.For C23H22ClF2NNaO3[M+Na]+456.1148,found456.1154.
实施例4
3-((1R,2R)-1-((1,1’-联苯)-4-基)-2-((氯苯基)氨基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-4):
以2-(1-((1,1’-联苯)-4-基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=10h,t2=34h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色固体产物V-4,核磁收率54%,分离收率51%,>20/1dr。HPLC分析得93%ee(Chiralcel AD-H,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=13.9min,tr(minor)=18.4min;[α]D 20=–26.9(c=0.90,in CHCl3);mp=123–125℃.1HNMR(500MHz,CDCl3)δ7.65–7.60(m,1H),7.52(d,J=7.4Hz,2H),7.48(d,J=8.2Hz,2H),7.43(t,J=7.5Hz,2H),7.35(t,J=7.2Hz,1H),7.19(t,J=7.8Hz,1H),7.14(d,J=7.9Hz,1H),7.10(d,J=8.2Hz,2H),6.93(d,J=8.2Hz,1H),6.66(t,J=7.6Hz,1H),6.61(d,J=5.7Hz,1H),5.48(d,J=10.0Hz,1H),4.32(q,J=7.1Hz,2H),3.90(d,J=10.5Hz,1H),3.30–3.01(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.4,163.6(t,J=31.6Hz),161.9,142.0,140.9,140.3,137.2,134.6,129.6,128.7,128.0,127.6,127.4,127.1,120.0,118.4,115.6(t,J=253.2Hz),111.6,63.5,60.0(t,J=3.6Hz),57.3,39.0(t,J=21.9Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=262.6Hz),-102.3(d,J=262.7Hz).HRMS(ESI)m/z calcd.For C28H24ClF2NNaO3[M+Na]+518.1305,found 518.1312.
实施例5
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(4-氟苯基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-5):
以2-(1-(4-氟苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=10h,t2=11h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–5/1),得到黄色油状的产物V-5,核磁收率80%,分离收率74%,19/1dr。HPLC分析得96%ee(FLM Chiral NS,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=8.3min,tr(minor)=10.3min;[α]D 20=–105.1(c=0.92,in CHCl3).1H NMR(500MHz,CDCl3)δ7.63–7.58(m,1H),7.21–7.14(m,2H),7.04–6.99(m,2H),6.95(t,J=8.5Hz,2H),6.91(d,J=8.1Hz,1H),6.67(t,J=7.6Hz,1H),6.60–6.56(m,1H),5.48–5.35(m,1H),4.31(q,J=7.1Hz,2H),3.79(br,1H),3.20–2.99(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.2,163.4(t,J=32.4Hz),162.1(d,J=248.7Hz),161.8,141.8,134.1(d,J=3.1Hz),134.1,129.6(d,J=2.4Hz),129.5,128.1,119.9,118.5,115.6(d,J=21.5Hz),115.5(t,J=253.7Hz),111.6,63.5,60.0(t,J=3.0Hz),56.9,39.4(t,J=22.2Hz),13.9.19F NMR(564MHz,CDCl3)δ-96.1(ddd,J=263.0,24.7,14.0Hz),-102.2(ddd,J=263.0,19.9,12.7Hz),-113.9–-113.9(m).HRMS(ESI)m/z calcd.For C22H19ClF3NNaO3[M+Na]+460.0898,found 460.0903.
实施例6
3-((1R,2R)-1-(4-氯苯基)-2-((2-氯苯基)氨基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-6):
以2-(1-(4-氯苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=16h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色油状的产物V-6,核磁收率39%,分离收率34%,13/1dr。HPLC分析得91%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=15.7min,tr(minor)=12.6min;[α]D 20=–77.3(c=0.64,in CHCl3).1H NMR(500MHz,CDCl3)δ7.61–7.60(m,1H),7.23(d,J=8.0Hz,2H),7.18(t,J=8.4Hz,2H),6.97(d,J=7.9Hz,2H),6.91(d,J=8.0Hz,1H),6.68(t,J=7.5Hz,1H),6.58(d,J=5.2Hz,1H),5.45(d,J=10.7Hz,1H),4.31(q,J=6.9Hz,2H),3.80(d,J=10.6Hz,1H),3.22–2.96(m,2H),1.35(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ206.9,163.3(t,J=32.3Hz),161.8,141.8,136.8,134.5,134.1,130.4,129.6,129.1,128.8,128.3,128.1,127.8,120.0,118.6,116.0(t,J=252.8Hz),111.6,60.0(t,J=3.8Hz),57.1,39.2(t,J=22.1Hz),13.9.19F NMR(564MHz,CDCl3)δ-96.0(d,J=262.9Hz),-102.2(d,J=263.3Hz).HRMS(ESI)m/z calcd.For C22H19Cl2F2NNaO3[M+Na]+476.0602,found 476.0608.
实施例7
3-((1R,2R)-1-(4-溴苯基)-2-((2-氯苯基)氨基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-7):
以2-(1-(4-溴苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=10h,t2=11h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色油状的产物V-7,核磁收率74%,分离收率63%,9/1dr。HPLC分析得94%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=15.6min,tr(minor)=13.0min;[α]D 20=–93.8(c=0.88,in CHCl3).1H NMR(500MHz,CDCl3)δ7.63–7.58(m,1H),7.33–7.28(m,3H),7.21(t,J=7.6Hz,1H),7.10–7.03(m,2H),6.87(d,J=8.0Hz,1H),6.62–6.56(m,2H),5.42(d,J=10.2Hz,1H),4.31(q,J=6.6Hz,2H),3.86(d,J=10.1Hz,1H),3.31–2.97(m,2H),1.35(t,J=6.9Hz,3H).13C NMR(126MHz,CDCl3)δ207.3,163.5(t,J=32.5Hz),161.6,142.9,138.3,134.6,132.9,128.9,128.7,128.1,127.7,118.8,115.6(t,J=253.6Hz),111.5,110.4,63.4,59.9(t,J=3.4Hz),57.3,38.9(t,J=21.9Hz),13.9.19FNMR(564MHz,CDCl3)δ-96.0(d,J=262.8Hz),-102.1(d,J=263.0Hz).HRMS(ESI)m/zcalcd.For C22H19BrClF2NNaO3[M+Na]+520.0097,found 520.0104.
实施例8
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(2-甲氧基苯基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-8):
以2-(1-(2-甲氧基苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=16h,步骤2中使用的反应溶剂为4mL三氯甲烷;催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=15/1–10/1),得到黄色油状的产物V-8,核磁收率74%,分离收率69%,>16/1dr。HPLC分析得92%ee(Chiralcel IA,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=11.6min,tr(minor)=9.7min;[α]D 20=–53.8(c=0.78,in CHCl3).1H NMR(500MHz,CDCl3)δ7.52–7.44(m,1H),7.15(t,J=6.4Hz,1H),7.08–6.97(m,3H),6.82–6.67(m,3H),6.50(t,J=6.5Hz,1H),6.45–6.40(m,1H),5.29(s,1H),4.62–4.18(m,3H),3.58(s,3H),3.30(s,1H),3.05(q,J=16.7Hz,1H),1.35(t,J=6.7Hz,3H).13C NMR(126MHz,CDCl3)δ208.8,163.6(t,J=32.2Hz),156.7,143.4,142.7,133.8,129.7,129.2,129.2,128.4,127.5,120.8,118.9,117.5,114.8(t,J=253.6Hz),112.1,111.1,63.3,61.4–61.1(m),55.6,54.7,40.0–39.3(m),13.9.19F NMR(564MHz,CDCl3)δ-99.1(d,J=264.4Hz),-100.2(d,J=264.2Hz).HRMS(ESI)m/zcalcd.For C23H22ClF2NNaO4[M+Na]+472.1098,found 472.1103.
实施例9
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(3-甲氧基苯基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-9):
以2-(1-(3-甲氧基苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=40h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/三氯甲烷=6/1–4/1),得到黄色油状的产物V-9,核磁收率49%,分离收率40%,7.2/1dr。HPLC分析得93%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=16.3min,tr(minor)=13.1min;[α]D 20=–110.6(c=0.91,in CHCl3).1H NMR(500MHz,CDCl3)δ7.61–7.58(m,1H),7.23–7.13(m,3H),6.91(d,J=8.1Hz,1H),6.81(d,J=8.3Hz,1H),6.68–6.57(m,3H),6.54(s,1H),5.43(d,J=10.5Hz,1H),4.31(q,J=7.1Hz,2H),3.92(d,J=10.5Hz,1H),3.65(s,3H),3.24–2.99(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.1,162.7(t,J=34.9Hz),161.7,159.7,142.1,139.6,134.5,129.8,129.5,128.0,119.8,119.8,118.2,115.7(t,J=249.2Hz),113.9,113.4,111.5,63.4,59.8(t,J=2.7Hz),57.4,55.2,39.0(t,J=21.8Hz),13.9.19F NMR(377MHz,CDCl3)δ-95.6(d,J=262.2Hz),-102.5(d,J=262.3Hz).HRMS(ESI)m/z calcd.For C23H22ClF2NNaO4[M+Na]+472.1098,found 472.1102.
实施例10
3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-(间甲基苯基)环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-10):
以2-(1-(间甲苯基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=20h,t2=3h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–6/1),得到黄色油状的产物V-10,核磁收率42%,分离收率39%,>20/1dr。HPLC分析得89%ee(FLM Chiral NS,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=6.1min,tr(minor)=8.0min;[α]D 20=–78.5(c=0.45,in CHCl3).1H NMR(500MHz,CDCl3)δ7.60–7.57(m,1H),7.22–7.13(m,3H),7.08(d,J=7.5Hz,1H),6.91(d,J=8.2Hz,1H),6.82(d,J=10.0Hz,2H),6.65(t,J=7.6Hz,1H),6.58(d,J=5.7Hz,1H),5.43(s,1H),4.30(q,J=7.1Hz,2H),3.87(br,1H),3.25–2.96(m,2H),2.23(s,3H),1.34(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.5,163.6(t,J=62.5Hz),161.7,142.1,138.5,138.1,134.5,129.5,128.8,128.7,128.5,128.0,124.6,119.8,118.2,115.6(t,J=253.6Hz),111.5,63.4,59.8(t,J=3.5Hz),57.4,39.1(t,J=21.9Hz),21.6,13.9.19F NMR(564MHz,CDCl3)δ-95.7(d,J=261.8Hz),-102.4(d,J=262.5Hz).HRMS(ESI)m/z calcd.For C23H22ClF2NNaO3[M+Na]+456.1148,found456.1153.
实施例11
3-((1R,2R)-2-((2-氯苯基)氨基)-1-(萘-2-基)-5-氧代环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-11):
以2-(1-(萘-2-基)乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=3h,步骤2中使用的反应温度为10℃,反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3,6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/三氯甲烷=10/1–8/1),得到黄色油状的产物V-11,核磁收率52%,分离收率50%,>20/1dr。HPLC分析得94%ee(FLMChiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=13.8min,tr(minor)=13.0min;[α]D 20=–49.6(c=0.81,in CHCl3).1H NMR(500MHz,CDCl3)δ7.78(d,J=7.6Hz,1H),7.74(d,J=8.7Hz,1H),7.69(d,J=7.6Hz,1H),7.64–7.60(m,1H),7.50–7.43(m,3H),7.22–7.15(m,2H),7.07(d,J=7.9Hz,1H),6.96(d,J=8.2Hz,1H),6.70–6.61(m,2H),5.52(d,J=10.0Hz,1H),4.32(q,J=7.2Hz,2H),3.87(d,J=10.6Hz,1H),3.39–3.12(m,2H),1.35(t,J=7.2Hz,3H).13C NMR(126MHz,CDCl3)δ207.2,163.6(t,J=34.4Hz),161.8,142.0,135.5,134.5,133.0,132.5,129.5,128.4,128.2,128.0,127.4,127.4,126.7,126.5,125.2,119.9,118.3,115.7(t,J=253.3Hz),111.6,63.5,60.0(t,J=3.2Hz),57.7,39.3(t,J=21.9Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.2–-96.3(m),-101.9–-102.8(m).HRMS(ESI)m/z calcd.For C26H22ClF2NNaO3[M+Na]+492.1148,found492.1173.
实施例12
3-((1R,2R)-2-((2-溴苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸酯(V-12):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-溴苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=10h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–6/1),得到黄色油状的产物V-12,核磁收率42%,分离收率39%,>20/1dr。HPLC分析得95%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=14.4min,tr(minor)=10.1min;[α]D 20=–131.6(c=0.8,in CHCl3).1H NMR(500MHz,CDCl3)δ7.63–7.58(m,1H),7.34–7.27(m,4H),7.21(t,J=7.5Hz,1H),7.11–7.04(m,2H),6.87(d,J=8.1Hz,1H),6.63–6.55(m,2H),5.42(d,J=10.2Hz,1H),4.31(q,J=6.6Hz,2H),3.86(d,J=10.1Hz,1H),3.27–3.00(m,2H),1.35(t,J=6.9Hz,3H).13C NMR(126MHz,CDCl3)δ207.3,163.5(t,J=32.4Hz),161.6,142.9,138.3,134.5,132.8,128.9,128.7,128.1,127.7,118.8,115.63(dd,J=255.8,250.4Hz),111.5,110.4,63.4,59.9(t,J=6.3Hz),57.3,38.9(t,J=21.9Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=262.6Hz),-102.3(d,J=262.5Hz).HRMS(ESI)m/z calcd.For C22H20BrF2NNaO3[M+Na]+486.0487,found 486.0493.
实施例13
3-((1R,2R)-2-((2-乙酰苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸酯(V-13):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、1-(2-氨基苯基)乙烷-1-酮(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=12h,t2=42h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–5/1),得到黄色油状的产物V-13,核磁收率94%,分离收率76%,4.8/1dr。HPLC分析得92%ee(FLMChiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=17.5min,tr(minor)=14.2min;[α]D 20=–410.1(c=0.89,in CHCl3).1H NMR(500MHz,CDCl3)δ8.38(d,J=9.3Hz,1H),7.62(d,J=7.6Hz,2H),7.40(t,J=7.8Hz,1H),7.21–7.21(m,3H),6.99(t,J=8.8Hz,3H),6.64(t,J=7.6Hz,1H),6.60–6.56(m,1H),5.57(d,J=8.4Hz,1H),4.31(q,J=7.1Hz,2H),3.26–3.05(m,2H),2.35(s,3H),1.34(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.7,200.3 163.6(t,J=32.4Hz),161.9,149.3,138.6,135.1,134.6,132.9,128.4,127.7,127.6,118.6,115.7(t,J=250.4Hz),115.4,111.9,63.4,60.0–59.9(m),57.6,39.3(t,J=21.9Hz),27.8,13.9.19F NMR(564MHz,CDCl3)δ-95.9(ddd,J=262.1,25.2,14.3Hz),-102.4(ddd,J=262.1,20.1,12.8Hz).HRMS(ESI)m/z calcd.ForC24H23F2NNaO4[M+Na]+450.1487,found 450.1508.
实施例14
3-((1R,2R)-2-((2-苯甲酰基苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸酯(V-14):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、(2-氨基苯基)(苯基)甲酮(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=10h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=5/1–4/1),得到黄色油状的产物V-14,核磁收率77%,分离收率73%,8.5/1dr。HPLC分析得90%ee(ChiralcelOD,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=12.6min,tr(minor)=16.5min;[α]D 20=–358.6(c=0.89,in CHCl3).1H NMR(500MHz,CDCl3)δ8.05(d,J=10.0Hz,1H),7.70–7.65(m,1H),7.50–7.44(m,1H),7.44–7.37(m,5H),7.33(t,J=7.7Hz,1H),7.15–7.03(m,4H),6.99(d,J=7.3Hz,2H),6.64–6.52(m,2H),5.65(d,J=10.0Hz,1H),4.32(q,J=6.8Hz,2H),3.34–3.09(m,2H),1.35(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.8,198.5,163.6(t,J=32.3Hz),161.8,149.9,139.9,138.6,135.6,135.0,134.9,130.9,129.0,128.4,127.9,127.6,127.6,118.2,115.8(t,J=253.5Hz),115.1,111.9,63.4,60.0,57.7,39.4(t,J=21.8Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=261.7Hz),-102.3(d,J=262.0Hz).HRMS(ESI)m/z calcd.For C29H25F2NNaO4[M+Na]+512.1644,found 512.1652.
实施例15
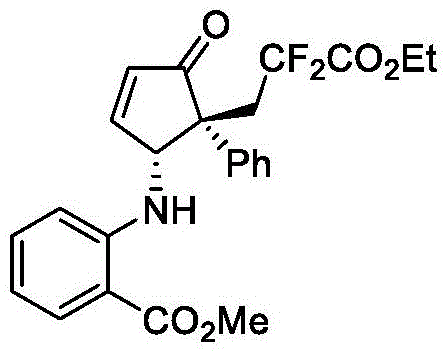
2-((1R,5R)-5-(3-乙氧基-2,2-二氟-3-氧丙基)-4-氧代-5-苯基-环戊-2-烯-1-基)氨基)苯甲酸甲酯(V-15):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=53h,t2=12h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色油状的产物V-15,核磁收率68%,分离收率50%,3/1dr。HPLC分析得92%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=14.4min,tr(minor)=11.8min;[α]D 20=–259.5(c=0.99,in CHCl3).1H NMR(500MHz,CDCl3)δ7.77(d,J=8.0Hz,1H),7.64–7.60(m,1H),7.40(t,J=7.8Hz,1H),7.23–7.08(m,4H),7.03–6.94(m,3H),6.64(t,J=7.6Hz,1H),6.59(d,J=5.7Hz,1H),5.58(s,1H),4.31(q,J=7.1Hz,2H),3.61(s,3H),3.31–2.95(m,2H),2.43(br,1H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.9,168.0,163.6,162.1,149.3,138.8,134.7,134.6,131.9,128.35,127.7,127.5,116.0,111.7,115.7(t,J=253.3Hz),111.4,63.4,60.2–60.0(m),57.8,51.4,39.4(t,J=21.4Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(ddd,J=262.1,25.3,14.2Hz),-102.4(ddd,J=262.1,20.4,13.1Hz).HRMS(ESI)m/z calcd.For C24H23F2NNaO5[M+Na]+466.1437,found 466.1457.
实施例16
3-((1R,2R)-2-((3-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-16):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、3-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=34h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=5/1–4/1),得到黄色固体的产物V-16,核磁收率61%,分离收率51%,5.6/1dr。HPLC分析得72%ee(Chiralcel AD-H,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=10.1min,tr(minor)=13.7min;[α]D 20=–21.6(c=0.88,in CHCl3);mp=89–91℃.1H NMR(500MHz,CDCl3)δ7.58–7.55(m,1H),7.31–7.27(m,3H),7.09–7.01(m,3H),6.70(d,J=7.9Hz,1H),6.58–6.52(m,1H),6.42(s,1H),6.37(d,J=8.2Hz,1H),5.35(d,J=10.6Hz,1H),4.30(q,J=7.2Hz,2H),3.25–3.00(m,3H),1.35(t,J=7.2Hz,3H).13C NMR(126MHz,CDCl3)δ206.1,162.4(t,J=32.4Hz),160.8,146.2,137.2,134.1,133.4,129.4,127.6,127.0,126.8,117.4,115.5(t,J=255.0Hz),112.4,110.5,62.4,59.4(d,J=4.3Hz),56.4,38.3(t,J=22.2Hz),12.8.19F NMR(564MHz,CDCl3)δ-96.0(d,J=263.4Hz),-102.1(d,J=263.0Hz).HRMS(ESI)m/zcalcd.For C22H20ClF2NNaO3[M+Na]+442.0992,found 442.0997.
实施例17
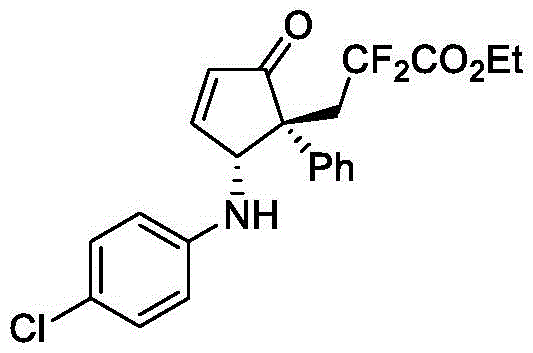
3-((1R,2R)-2-((4-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-17):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、4-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=20h,t2=120h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应温度为15℃,反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–6/1),得到黄色油状的产物V-17,核磁收率77%,分离收率63%,2.7/1dr。HPLC分析得67%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=15.9min,tr(minor)=17.2min;[α]D 20=–26.7(c=0.3,in CHCl3).1H NMR(500MHz,CDCl3)δ7.61–7.54(m,1H),7.31–7.25(m,3H),7.08(d,J=8.8Hz,2H),7.25–6.99(m,2H),6.59–6.52(m,1H),6.39(d,J=11.3Hz,2H),5.36(d,J=10.0Hz,1H),4.30(q,J=7.2Hz,2H),3.21–2.98(m,3H),1.35(t,J=7.2Hz,3H).13C NMR(126MHz,CDCl3)δ207.4,163.4(t,J=32.3Hz),162.1,144.7,138.4,134.5,129.3,128.7,128.0,127.9,123.2,115.6(dd,J=255.9,251,5Hz),114.7,63.5,60.8(t,J=2.2Hz),57.6,39.1(t,J=22.5Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=262.5Hz),-102.3(d,J=262.5Hz).HRMS(ESI)m/z calcd.For C22H20ClF2NNaO3[M+Na]+442.0992,found 442.0995.
实施例18
2,2-二氟-3-((1R,5R)-2-氧代-1-苯基-5-((4-(三氟甲基)苯基)氨基)环戊-3-烯-1-基)丙酸酯(V-18):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、4-(三氟甲基)苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=82h。步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=5/1–4/1),得到黄色油状的产物V-18,核磁收率65%,分离收率56%,9/1dr。HPLC分析得80%ee(Chiralcel AD-H,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=10.7min,tr(minor)=13.2min;[α]D 20=–115.1(c=0.2,in CHCl3).1H NMR(500MHz,CDCl3)δ7.59–7.54(m,1H),7.38(d,J=8.2Hz,2H),7.31–7.27(m,3H),7.07–6.99(m,2H),6.61–6.56(m,1H),6.51(d,J=8.2Hz,2H),5.46(d,J=10.5Hz,1H),4.31(q,J=7.4Hz,2H),3.38(d,J=10.8Hz,1H),3.29–2.99(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.1,163.3(t,J=32.4Hz),161.6,148.7,138.3,134.7,128.7,128.1,127.9,126.9(q,J=3.6Hz),122.5(q,J=270.8Hz),120.2(q,J=32.3Hz),115.6(t,J=253.0Hz),112.6,63.5,60.1(t,J=2.5Hz),57.5,39.2(t,J=22.4Hz),13.9.19F NMR(564MHz,CDCl3)δ-61.3(s),-95.9(ddd,J=262.4,25.1,13.9Hz),-102.3(ddd,J=259.6,17.1,9.0Hz).HRMS(ESI)m/z calcd.F ForC23H20F5NNaO3[M+Na]+476.1256,found 476.1274.
实施例19
2-(((1R,5R)-5-(3-乙氧基-2,2-二氟-3-氧丙基)-4-氧代-5-苯基环戊-2-烯-1-基)氨基)-4-甲氧基苯甲酸酯(V-19):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基-4-甲氧基苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=16h,t2=10h。步骤2中使用的反应温度为10℃,反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=5/1–4/1),得到黄色油状的产物V-19,核磁收率87%,分离收率67%,4/1dr。HPLC分析得92%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=12.9min,tr(minor)=14.7min;[α]D 20=–186.3(c=0.96,in CHCl3).1H NMR(500MHz,CDCl3)δ7.71(d,J=8.9Hz,1H),7.64–7.55(m,1H),7.39(d,J=10.3Hz,1H),7.20–7.14(m,3H),7.03–6.88(m,2H),6.65–6.56(m,1H),6.50(s,1H),6.21(d,J=8.9Hz,1H),5.57(d,J=10.3Hz,1H),4.31(q,J=7.0Hz,2H),3.86(s,3H),3.58(s,3H),3.31–3.05(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.8,167.7,164.9,163.5(t,J=32.5Hz),162.0,151.4,138.8,134.6,133.7,128.4,127.7,127.5,115.9(dd,J=256.8,253.2Hz),104.7,103.6,95.5,63.4,60.1–59.9(m),58.0,55.3,51.1,39.6(t,J=21.8Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.3(d,J=260.6Hz),-102.7(d,J=260.6Hz).HRMS(ESI)m/z calcd.For C25H25F2NNaO6[M+Na]+496.1542,found 496.1548.
实施例20
2-((1R,5R)-5-(3-乙氧基-2,2-二氟-3-氧丙基)-4-氧代-5-苯基环戊-2-烯-1-基)氨基)-4-甲基苯甲酸酯(V-20):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基-4-甲基苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=11h,t2=40h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=5/1–4/1),得到黄色油状的产物V-20,核磁收率70%,分离收率53%,3.5/1dr。HPLC分析得93%ee(FLMChiral NS,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=8.4min,tr(minor)=9.7min;[α]D 20=–241.9(c=0.81,in CHCl3).1H NMR(500MHz,CDCl3)δ7.66(d,J=8.1Hz,1H),7.63–7.57(m,1H),7.22–7.16(m,4H),7.01–6.94(m,2H),6.83(s,1H),6.60–6.56(m,1H),6.46(d,J=8.1Hz,1H),5.58(s,1H),4.31(q,J=7.1Hz,2H),3.58(s,3H),3.26–3.03(m,2H),2.35(s,3H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.9,168.0,163.6,162.2,149.3,145.5,138.8,134.4,131.8,128.4,127.7,127.5,117.3,115.7(t,J=251.1Hz),112.1,109.0,63.4,60.1–59.9(m),57.9,51.3,39.4(t,J=21.9Hz),22.2,13.9.19F NMR(564MHz,CDCl3)δ-95.7(ddd,J=262.0,25.1,14.4Hz),-102.6(ddd,J=262.7,20.1,12.9Hz).HRMS(ESI)m/z calcd.For C25H25F2NNaO5[M+Na]+480.1593,found480.1615.
实施例21
2-((1R,5R)-5-(3-乙氧基-2,2-二氟-3-氧丙基)-4-氧代-5-苯基环戊-2-烯-1-基)氨基-5-氟苯甲酸甲酯(V-21):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基-5-氟苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=87h,t2=40h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–4/1),得到黄色油状的产物V-21,核磁收率79%,分离收率70%,8.6/1dr。HPLC分析得91%ee(Chiralcel OD,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=9.0min,tr(minor)=9.9min;[α]D 20=–294.0(c=0.8,in CHCl3).1H NMR(500MHz,CDCl3)δ7.64–7.56(m,1H),7.49–7.43(m,1H),7.20–7.13(m,4H),7.06(d,J=10.3Hz,1H),6.94(t,J=11.6Hz,3H),6.62–6.58(m,1H),5.53(d,J=10.4Hz,1H),4.31(q,J=7.0Hz,2H),3.62(s,3H),3.28–2.93(m,2H),1.35(t,J=7.1Hz,3H).13C NMR(126MHz,CDCl3)δ207.7,167.0(d,J=2.6Hz),163.5(t,J=32.4Hz),161.8,153.6(d,J=235.4Hz),146.1,138.7,134.7,128.3,127.7,127.5,122.2(d,J=22.9Hz),117.3(d,J=23.4Hz),115.7(dd,J=255.7,250.2Hz),113.0(d,J=7.6Hz),111.6(d,J=6.4Hz),60.8–60.5(m),57.7,51.7,39.4(t,J=21.8Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=261.7Hz),-102.4(d,J=262.0Hz),-128.4(s).HRMS(ESI)m/z calcd.For C24H22F3NNaO5[M+Na]+484.1342,found 484.1348.
实施例22
5-氯-2-((1R,5R)-5-(3-乙氧基-2,2-二氟-3-氧丙基)-4-氧代-5-苯基环戊-2-烯-1-基)氨基)苯甲酸甲酯(V-22):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基-5-氯苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=48h,t2=18h,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–4/1),得到黄色固体的产物V-22,核磁收率76%,分离收率70%,14/1dr。HPLC分析得90%ee(FLM Chiral INB,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=13.6min,tr(minor)=12.4min;[α]D 20=–214.9(c=0.87,in CHCl3);mp=64–66℃.1H NMR(500MHz,CDCl3)δ7.74(s,1H),7.65–7.52(m,1H),7.34(d,J=8.9Hz,1H),7.24–7.13(m,4H),6.95(s,3H),6.64–6.57(m,1H),5.53(d,J=10.2Hz,1H),4.31(q,J=6.9Hz,2H),3.62(s,3H),3.26–3.01(m,2H),1.35(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.5,167.0,163.5(t,J=32.5Hz),161.5,147.9,138.6,134.8,134.5,131.2,128.4,127.7,127.6,120.6,115.7(t,J=256.4,253.4Hz),113.2,112.3,63.5,60.3–60.2(m),57.7,51.7,39.4(t,J=21.9Hz),13.9.19F NMR(564MHz,CDCl3)δ-95.8(d,J=261.9Hz),-102.4(d,J=262.0Hz).HRMS(ESI)m/z calcd.For C24H22ClF2NNaO5[M+Na]+500.1047,found500.1053.
实施例23
3-((1R,2R)-2-((4-氯苯基)(甲基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-23):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、N-甲基-4-(三氟甲基)苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(0.4mmol,2.0eq)为反应原料,反应时间为t1=87h,t2=40h,步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–5/1),得到黄色固体的产物V-23,核磁收率77%,分离收率75%,>20/1dr。HPLC分析得60%ee(FLMChiral INB,hexane/i-PrOH=95/5,flow rate=1.0mL/min,l=254nm),tr(major)=15.9min,tr(minor)=15.0min;[α]D 20=60.2(c=0.99,in CHCl3);mp=107–109℃.1H NMR(500MHz,CDCl3)δ7.53(d,J=7.8Hz,3H),7.24–7.14(m,3H),6.96(d,J=7.6Hz,2H),6.82(d,J=8.2Hz,2H),6.64–6.60(m,1H),5.89(s,1H),4.32(q,J=7.0Hz,2H),3.22–2.99(m,2H),1.84(s,3H),1.35(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.3,163.3(t,J=32.4Hz),161.2,151.0,138.3,134.8,128.5,128.2,127.7,127.0(q,J=271.1Hz),125.0(q,J=3.5Hz),118.7(q,J=32.8Hz),115.6(t,J=256.5,251.2Hz),111.0,66.0,60.4,58.1,41.9(t,J=21.7Hz),33.5,13.9.19F NMR(564MHz,CDCl3)δ-61.0(s),-95.6(ddd,J=261.9,25.6,15.0Hz),-102.1(ddd,J=262.0,19.7,12.9Hz).HRMS(ESI)m/z calcd.ForC24H22F5NNaO3[M+Na]+490.1412,found 490.1417.
实施例24
(4R,5R)-4-((2-氯苯基)氨基)-5-(2,2-二氟-3-氧代-3-苯基丙基)-5-苯基环戊-2-烯-1-酮(V-24):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氨基苯甲酸甲酯(0.2mmol,1.0eq)和2-溴-2,2-二氟-1-苯基乙烷-1-酮(0.4mmol,2.0eq)为反应原料,反应时间为t1=10h,t2=17h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1;其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=15/1–10/1),得到黄色油状的产物V-24,核磁收率62%,分离收率60%,4.7/1dr。HPLC分析得96%ee(Chiralcel OD,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=10.4min,tr(minor)=8.7min;[α]D 20=–265.9(c=0.96,in CHCl3).1H NMR(500MHz,CDCl3)δ8.08(d,J=7.6Hz,2H),7.77(d,J=8.0Hz,1H),7.69–7.64(m,1H),7.62(t,J=7.7Hz,1H),7.49(t,J=7.8Hz,2H),7.39(t,J=7.9Hz,1H),7.25(s,1H),7.18–7.14(m,3H),7.06–6.95(m,3H),6.65–6.61(m,2H),5.69(d,J=10.1Hz,1H),4.96(d,J=9.1Hz,0.22H),3.61(s,3H),3.44–3.32(m,1H),3.26–3.16(m,1H).13C NMR(500MHz,CDCl3)δ8.08(d,J=7.6Hz,2H),7.77(d,J=8.0Hz,1H),7.69–7.64(m,1H),7.62(t,J=7.7Hz,1H),7.49(t,J=7.8Hz,2H),7.39(t,J=7.9Hz,1H),7.25(s,1H),7.18–7.14(m,3H),7.06–6.95(m,3H),6.65–6.61(m,2H),5.69(d,J=10.1Hz,1H),4.96(d,J=9.1Hz,0.22H),3.61(s,3H),3.44–3.32(m,1H),3.26–3.16(m,1H).19F NMR(377MHz,CDCl3)δ-92.6(d,J=276.0Hz),-96.3(d,J=275.8Hz).HRMS(ESI)m/z calcd.For C28H23F2NNaO4[M+Na]+498.1487,found 498.1506.
实施例25
(4R,5R)-4-((2-氯苯基)氨基)-5-(2,2-二氟-3-(4-甲氧基苯基)-3-氧丙基)-5-苯基环戊-2-烯-1-酮(V-25):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟-1-(4-甲氧基苯基)乙烷-1-酮(0.4mmol,2.0eq)为反应原料,反应时间为t1=14h,t2=16h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色油状的产物V-25,核磁收率71%,分离收率68%,>20/1dr。HPLC分析得94%ee(FLM Chiral INB,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=19.4min,tr(minor)=17.0min;[α]D 20=–162.0(c=0.89,in CHCl3).1H NMR(500MHz,CDCl3)δ8.08(d,J=8.4Hz,2H),7.67–7.59(m,1H),7.25(s,3H),7.20–7.10(m,2H),7.09–7.04(m,2H),7.01–6.88(m,3H),6.72–6.56(m,2H),5.57(d,J=10.5Hz,1H),3.86(d,J=2.8Hz,4H),3.44–3.28(m,1H),3.25–3.11(m,1H).13C NMR(126MHz,CDCl3)δ208.1,186.9(t,J=29.5Hz),164.7,161.8,142.1,138.4,134.6,133.0,129.5,128.8,128.0,127.7,124.3,124.2,121.7,119.9,119.7(dd,J=258.4,252.8Hz),118.2,114.1,113.9,111.6,59.9(d,J=6.4Hz),57.6,55.6,38.5(t,J=21.1Hz).19F NMR(377MHz,CDCl3)δ-92.5(d,J=272.4Hz),-95.8(d,J=272.6Hz).HRMS(ESI)m/z calcd.ForC27H22ClF2NNaO3[M+Na]+504.1148,found 504.1162.
实施例26
N-苄基-3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酰胺(V-26):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和N-苄基-2-溴-2,2-二氟乙酰胺(0.4mmol,2.0eq)为反应原料,反应时间为t1=12h,t2=43h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1;其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–4/1),得到黄色油状的产物V-26,核磁收率52%,分离收率47%,>20/1dr。HPLC分析得93%ee(Chiralcel IA,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=11.6min,tr(minor)=10.2min;[α]D 20=–30.3(c=0.72,in CHCl3).1H NMR(500MHz,CDCl3)δ7.57–7.51(m,1H),7.36–7.24(m,8H),7.15(t,J=7.9Hz,1H),7.11(d,J=7.9Hz,1H),7.06–6.99(m,2H),6.92(d,J=8.2Hz,1H),6.82(br,1H),6.63(t,J=7.7Hz,1H),6.55–6.49(m,1H),5.41(d,J=10.5Hz,1H),4.45(dd,J=14.9,5.9Hz,1H),4.35(dd,J=14.9,5.5Hz,1H),3.83(d,J=10.5Hz,1H),3.35–3.19(m,1H),3.16–3.00(m,1H).13C NMR(126MHz,CDCl3)δ207.9,163.8(t,J=28.6Hz),161.7,142.0,138.1,136.7,134.5,129.5,128.9,128.8,128.1,128.0,127.9,127.7,119.8,118.3,117.4(dd,J=257.9,253.4Hz),111.7,60.3(d,J=4.8Hz),57.5,43.7,39.0(t,J=22.1Hz).19F NMR((377MHz,CDCl3)δ-97.1(d,J=254.5Hz),-101.4(d,J=254.6Hz).HRMS(ESI)m/z calcd.For C27H23ClF2N2NaO2[M+Na]+503.1308,found 503.1326.
实施例27
3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-N-环己基-2,2-二氟丙烷(27):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-N-环己基-2,2-二氟乙酰胺(0.4mmol,2.0eq)为反应原料,反应时间为t1=72h,t2=12h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1;其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–5/1),得到黄色油状的产物V-27,核磁收率36%,分离收率34%,>20/1dr。HPLC分析得84%ee(Chiralcel ID,hexane/i-PrOH=95/5,flow rate=1.0mL/min,l=254nm),tr(major)=40.1min,tr(minor)=29.6min;[α]D 20=–96.8(c=0.84,in CHCl3).1H NMR(500MHz,CDCl3)δ7.62–7.58(m,1H),7.29–7.25(m,3H),7.17(t,J=7.9Hz,1H),7.12(d,J=7.8Hz,1H),7.08–7.03(m,2H),6.94(d,J=8.2Hz,1H),6.64(t,J=7.7Hz,1H),6.61–6.55(m,1H),6.18(d,J=8.1Hz,1H),5.45(d,J=10.6Hz,1H),3.84(d,J=10.6Hz,1H),3.77–3.67(m,1H),3.32–3.18(m,1H),3.11–2.96(m,1H),1.94(d,J=12.5Hz,1H),1.88(d,J=12.4Hz,1H),1.75–1.58(m,4H),1.41–1.31(m,2H),1.22–1.16(m,2H).13C NMR(126MHz,CDCl3)δ208.0,163.0(t,J=26.7Hz),161.7,142.0,138.2,134.6,129.4,128.7,128.0,127.9,127.7,119.8,118.2,116.3(t,J=254.5Hz),111.7,60.2(d,J=4.6Hz),57.5,48.8,39.0(t,J=22.2Hz),32.6,32.5,25.3,24.6.19F NMR(377MHz,CDCl3)δ-97.6(d,J=253.1Hz),-101.3(d,J=253.2Hz).HRMS(ESI)m/zcalcd.For C26H27ClF2N2NaO2[M+Na]+495.1621,found 495.1636.
实施例28
3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟-N-苯基丙胺(V-28):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟-N-苯基乙酰胺(0.4mmol,2.0eq)为反应原料,反应时间为t1=24h,t2=40h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1;其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=3/1–2/1),得到白色固体的产物V-28,核磁收率51%,分离收率49%,>20/1dr。HPLC分析得91%ee(Chiralcel OD,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=14.0min,tr(minor)=12.4min;[α]D 20=–130.3(c=0.72,in CHCl3);mp=175–177℃.1H NMR(500MHz,DMSO)δ10.57(s,1H),7.88–7.82(m,1H),7.67(d,J=8.0Hz,2H),7.36(t,J=7.0Hz,2H),7.29–7.23(m,3H),7.19–7.08(m,5H),6.92(d,J=8.2Hz,1H),6.70–6.64(m,1H),6.59(t,J=7.7Hz,1H),5.31(d,J=9.5Hz,1H),4.14(d,J=9.4Hz,1H),3.50–3.37(m,1H),3.17–3.03(m,1H).13C NMR(126MHz,DMSO)δ207.2,162.6,161.6(t,J=29.6Hz),141.9,138.3,137.1,134.2,129.0,128.7,128.3,128.0,127.6,127.4,124.9,121.0,118.2,117.8,111.8,117.3(t,J=256.2Hz),60.1,56.6,37.8(t,J=21.9Hz).19F NMR(377MHz,DMSO)δ-95.9(d,J=255.6Hz),-99.2(d,J=255.6Hz).HRMS(ESI)m/z calcd.For C26H21ClF2N2NaO2[M+Na]+489.1152,found489.1173.
实施例29
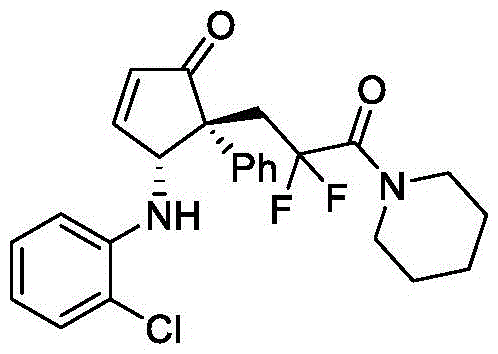
(4R,5R)-4-((2-氯苯基)氨基)-5-(2,2-二氟-3-氧代-3-(哌啶-1-基)丙基)-5-苯基环戊-2-烯-1-酮(V-29):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟-1-(哌啶-1-基)乙烷-1-酮(0.4mmol,2.0eq)为反应原料,反应时间为t1=12h,t2=4h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1;其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–5/1),得到黄色油状的产物V-29,核磁收率78%,分离收率75%,>20/1dr。HPLC分析得92%ee(FLM Chiral INB,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=11.7min,tr(minor)=9.4min;[α]D 20=–147.9(c=0.94,in CHCl3).1H NMR(500MHz,CDCl3)δ7.62–7.58(m,1H),7.27–7.24(m,3H),7.18–7.03(m,4H),6.91(d,J=8.1Hz,1H),6.62(t,J=7.8Hz,1H),6.59–6.55(m,1H),5.52(d,J=10.4Hz,1H),3.84(d,J=10.4Hz,1H),3.64–3.51(m,4H),3.35–3.13(m,2H),1.69–1.58(m,6H).13C NMR(126MHz,CDCl3)δ208.1,161.8,161.4(t,J=28.2Hz),142.1,138.6,134.5,129.4,128.7,128.0,127.9,127.7,119.8,119.0(dd,J=259.1,252.6Hz),118.1,111.7,59.7(d,J=6.4Hz),57.4,46.9(t,J=6.0Hz),44.7,39.3(t,J=21.4Hz),26.4,25.6,24.4.19F NMR(377MHz,CDCl3)δ-91.3(d,J=268.4Hz),-94.8(d,J=267.8Hz).HRMS(ESI)m/z calcd.For C25H25ClF2N2NaO2[M+Na]+481.1465,found481.1480.
实施例30
(4R,5R)-4-((2-氯苯基)氨基)-5-(2,2-二氟-3-吗啉-3-氧丙基)-5-苯基环戊-2-烯-1-酮(V-30):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-2,2-二氟-1-吗啉酮(0.4mmol,2.0eq)为反应原料,反应时间为t1=83h,t2=4h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱,2mL二氯甲烷作为溶剂;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=3/1–2/1),得到黄色油状的产物V-30,核磁收率38%,分离收率36%,>20/1dr。HPLC分析得89%ee(FLM Chiral NS,hexane/i-PrOH=70/30,flow rate=1.0mL/min,l=254nm),tr(major)=10.4min,tr(minor)=8.2min;[α]D 20=–142.7(c=0.71,in CHCl3).1H NMR(500MHz,CDCl3)δ7.63–7.58(m,1H),7.29–7.25(m,3H),7.19–7.11(m,2H),7.10–7.05(m,2H),6.90(d,J=8.1Hz,1H),6.64(t,J=7.7Hz,1H),6.61–6.56(m,1H),5.49(d,J=10.5Hz,1H),3.85(d,J=10.5Hz,1H),3.77–3.59(m,8H),3.33–3.13(m,2H).13C NMR(126MHz,CDCl3)δ208.0,161.9,161.5(t,J=29.5Hz),142.0,138.4,134.5,129.5,128.8,128.0,128.0,127.7,119.8,118.8(dd,J=258.8,251.8Hz),118.2,111.6,66.6,59.7(d,J=6.0Hz),57.4,46.6(t,J=5.6Hz),43.6,39.1(t,J=21.4Hz).19F NMR(377MHz,CDCl3)δ-91.0(d,J=270.3Hz),-95.0(d,J=270.3Hz).HRMS(ESI)m/z calcd.For C24H23ClF2N2NaO3[M+Na]+483.1257,found 483.1294.
实施例31
3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-N,N-二乙基-2,2-二氟丙烷(V-31):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和2-溴-N,N-二乙基-2,2-二氟乙酰胺(0.4mmol,2.0eq)为反应原料,反应时间为t1=11h,t2=4h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=10/1–8/1),得到黄色油状的产物V-32,核磁收率82%,分离收率80%,>20/1dr。HPLC分析得88%ee(FLM Chiral INB,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=9.0min,tr(minor)=6.9min;[α]D 20=–130.3(c=0.92,in CHCl3).1H NMR(500MHz,CDCl3)δ7.62–7.56(m,1H),7.27–7.23(m,3H),7.18–7.05(m,4H),6.92(d,J=8.2Hz,1H),6.62(t,J=7.7Hz,1H),6.59–6.59(m,1H),5.52(d,J=10.5Hz,1H),3.84(d,J=10.3Hz,1H),3.51–3.32(m,4H),3.29–3.19(m,2H),1.22–1.13(m,6H).13C(126MHz,CDCl3)δ208.1,162.5(t,J=28.4Hz),161.8,142.1,138.6,134.5,129.4,128.7,128.0,127.9,127.8,119.8,118.9(dd,J=258.9,252.5Hz),118.1,111.7,59.6(d,J=6.0Hz),57.4,42.1(t,J=5.1Hz),41.9,39.5(t,J=21.7Hz),14.3,12.3.19F NMR(377MHz,CDCl3)δ-91.8(d,J=267.7Hz),-95.4(d,J=267.7Hz).HRMS(ESI)m/z calcd.For C24H25ClF2N2NaO2[M+Na]+469.1465,found 469.1465.
实施例32
二乙基(2-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-1,1-二氟乙基)膦酸酯(V-32):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和二乙基(溴二氟甲基)膦酸盐(0.4mmol,2.0eq)为反应原料,反应时间为t1=96h,t2=15h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1经硅胶柱层析纯化(石油醚/乙酸乙酯=8/1–6/1),得到黄色油状的产物V-32,核磁收率55%,分离收率53%,>20/1dr。HPLC分析得87%ee(FLM Chiral INB,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=13.8min,tr(minor)=7.5min;[α]D 20=–87.9(c=0.79,in CHCl3).1H NMR(500MHz,CDCl3)δ7.60–7.56(m,1H),7.26(s,2H),7.19–7.10(m,2H),7.07–7.01(m,2H),6.93(d,J=8.3Hz,1H),6.68–6.56(m,2H),5.50(d,J=10.5Hz,1H),4.45–4.22(m,4H),3.82(d,J=10.6Hz,1H),3.29–3.01(m,2H),1.44–1.36(m,6H),1.35–1.23(m,1H).13C NMR(126MHz,CDCl3)δ207.7,161.3,142.1,138.2,134.4,129.5,128.8,125.1(dt,J=255.8,212.9Hz),119.8,118.2,111.6,65.0(dd,J=17.9,6.8Hz),59.9,59.8,57.4,57.3,38.1(q,J=18.6,17.8Hz),16.4,16.4.19F NMR(377MHz,CDCl3)δ-102.9(dd,J=295.5,110.3Hz),-107.6(dd,J=295.7,101.5Hz).HRMS(ESI)m/z calcd.ForC23H25ClF2NNaO4P[M+Na]+506.1070,found506.1101.
实施例33
(3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟烷基)-L-苯丙氨酸乙酯(V-33):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和(2-溴-2,2-二氟乙酰基)-L-苯丙酸乙酯(0.4mmol,1.0eq)为反应原料,反应时间为t1=34h,t2=16h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3、6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=6/1–5/1),得到黄色油状的产物V-33,核磁收率45%,分离收率43%,>20/1syn:anti。HPLC分析得89%de(Chiralcel IA,hexane/i-PrOH=80/20,flow rate=1.0mL/min,l=254nm),tr(major)=9.0min,tr(minor)=10.7min;[α]D 20=–66.7(c=0.91,in CHCl3).1H NMR(500MHz,CDCl3)δ7.59–7.55(m,1H),7.28–7.22(m,5H),7.20–7.14(m,2H),7.11(d,J=7.7Hz,3H),7.05–7.00(m,2H),6.89(d,J=8.3Hz,1H),6.83(d,J=8.1Hz,1H),6.63(t,J=7.7Hz,1H),6.60–6.56(m,1H),5.38(d,J=10.7Hz,1H),4.82(q,J=6.9Hz,1H),4.20(q,J=7.5Hz,2H),3.83(d,J=10.5Hz,1H),3.21–3.04(m,3H),3.02–2.90(m,1H),1.25(t,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.7,170.4,163.3(t,J=29.4Hz),161.5,142.0,138.2,135.1,134.6,129.4,129.4,128.7,128.0,128.0,127.7,127.4,119.8,118.2,117.2(t,J=253.9Hz),111.7,61.9,60.0(d,J=4.9Hz),57.3,53.2,38.8(t,J=21.7Hz),37.8,14.1.19F NMR(377MHz,CDCl3)δ-98.4(d,J=255.1Hz),-101.8(d,J=255.0Hz).HRMS(ESI)m/z calcd.For C31H29ClF2N2NaO4[M+Na]+589.1676,found 589.1697.
实施例34
(1S,2R,5S)-2-异丙基-5-甲基环己基3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸酯(V-34):
以2-(1-苯基乙烯基)呋喃(0.24mmol,1.2eq)、2-氯苯胺(0.2mmol,1.0eq)和(1S,2R,5S)-2-异丙基-5-甲基环己基2-溴-2,2-二氟乙酸(0.4mmol,2.0eq)为反应原料,反应时间为t1=20h,t2=24h,步骤1中使用的光催化剂为2mol%mer-Ir(ppy)3,NaHCO3(0.6mmol,3.0eq)作为碱;步骤2中使用的反应溶剂为4mL三氯甲烷,催化剂用量为2mol%Dy(OTf)3和6mol%A1,其余操作同实施例1。经硅胶柱层析纯化(石油醚/乙酸乙酯=20/1–15/1),得到黄色油状的产物V-34,核磁收率61%,分离收率59%,>20/1syn:anti。HPLC分析得94%de(Chiralcel ID,hexane/i-PrOH=90/10,flow rate=1.0mL/min,l=254nm),tr(major)=5.7min,tr(minor)=7.1min;[α]D 20=–110.8(c=0.97,in CHCl3).1H NMR(500MHz,CDCl3)δ7.62–7.54(m,1H),7.31–7.26(m,3H),7.20–7.11(m,2H),7.08–7.02(m,2H),6.90(d,J=8.1Hz,1H),6.64(t,J=7.8Hz,1H),6.60–6.56(m,1H),5.45(d,J=10.3Hz,1H),4.86–4.76(m,1H),3.82(d,J=10.5Hz,1H),3.26–3.01(m,2H),2.08(d,J=12.2Hz,1H),1.91–1.81(m,1H),1.72–1.65(m,2H),1.54–1.45(m,2H),1.14–1.01(m,2H),0.96–0.86(m,7H),0.75(d,J=7.0Hz,3H).13C NMR(126MHz,CDCl3)δ207.3,163.1(t,J=32.2Hz),161.5,142.0,138.4,134.6,129.5,128.8,128.0,127.7,119.9,118.2,115.8(dd,J=255.6Hz,255.6Hz),111.5,78.1,59.8(d,J=3.7Hz),57.3,46.8,40.2,38.8(t,J=21.6Hz),34.0,31.5,26.2,23.4,22.0,20.7,16.2.19F NMR(377MHz,CDCl3)δ-96.1(d,J=261.6Hz),-101.9(d,J=261.6Hz).HRMS(ESI)m/z calcd.For C30H34ClF2NNaO3[M+Na]+552.2087,found 552.2117.
本发明的扩大量实验
3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯(V-1)的合成路线:
步骤1:将光催化剂mer-Ir(ppy)3(19.5mg,0.03mmol)和K2CO3(829.5mg,6.0mmol,2.0eq)置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入30mL乙腈。然后依次加入2-(1-苯基乙烯基)呋喃(612mg,3.6mmol,1.2eq)、邻氯苯胺(762mg,3.0mmol,1.0eq)和2-溴-2,2-二氟乙酸乙酯(1.22g,6.0mmol,2.0eq)。将反应混合物用氩气脱气10分钟,然后在功率为3W的蓝色LED灯照射下室温反应,用TLC监测反应。当TLC显示芳胺类化合物完全转化时(t1=60h),将反应混合物经硅藻土过滤,用二氯甲烷洗涤并浓缩,得到的粗产品中间物IV直接用于下一步。
步骤2:将Dy(OTf)3(36mg,0.06mol)和布朗斯特酸A1(64.8mg,0.072mmol)。置于反应管中,放入合适大小磁力搅拌子。抽真空干燥,用氩气置换三次,氩气保护下加入三氯甲烷(5.0mL)并在室温下搅拌1分钟。随后,将步骤1所得中间物IV溶解在三氯甲烷(15.0mL)并加入反应的混合物中,室温下反应。待反应完成后(t2=48h),浓缩反应液,通过硅胶柱层析分离(石油醚/乙酸乙酯=20/1–15/1),得到0.9g的产物3-((1R,2R)-2-((2-氯苯基)氨基)-5-氧代-1-苯基环戊-3-烯-1-基)-2,2-二氟丙酸乙酯,分离收率70%。
附图说明
图1为本发明所采用的技术方案的化学式
图2为一系列含二氟烷基的手性多官能化环戊烯酮衍生物的结构式
以上各化合反应所用的原料来源列表如下:
| 试剂名称 | CAS号 | 纯度 | 规格 | 厂家 |
| 碳酸钾 | 584-08-7 | AR | 500g | 科隆 |
| 三氟甲磺酸镝 | 139177-62-1 | 98% | 25g | Adamas |
| 三氯甲烷 | 887144-97-0 | 98% | 5g | 毕得医药 |
| 乙腈 | 75-05-8 | AR | 500mL | 科隆 |
| 石油醚 | 8032-32-4 | AR | 15kg | 科隆 |
| 乙酸乙酯 | 141-78-6 | AR | 20kg | 科隆 |
| 二氯甲烷 | 75-09-2 | AR | 25kg | 科隆 |
Claims (8)
1.一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法,其特征在于,包括以下步骤:
步骤S1以呋喃烯烃、芳胺类化合物和二氟烷基卤化物为原料在可见光下进行的自由基介导的三组分二氟烷基化胺化反应,其反应式如下:
步骤S2取中间物IV,加入手性布朗斯特酸和非手性路易斯酸,在有机溶剂中进行不对称重排反应得到产物V,其反应式如下:
其中
R为苯基、2-萘基或者含有取代基的苯环,苯环上的取代基为甲基、甲氧基或卤素中的一种;
R1为H或者甲基;
Ar为连有吸电子或供电子的不同基团的苯环,所述不同基团为甲基、甲氧基、卤素、酯基、三氟甲基、苯甲酰基或者乙酰基中的一种或者两种;
RF为连有不同基团的二氟亚甲基,其原料二氟烷基卤化物III选自下列化合物中的一种:
步骤S1中所述的光催化剂为mer-Ir(ppy)3,碱为碳酸钾,反应温度为室温;
步骤S1中所述的可见光由功率为3W、波长为460-465nm的蓝色LED灯提供;
步骤S2中所述的非手性路易斯酸为三氟甲磺酸镝,反应温度为室温;
步骤S2中所述的手性布朗斯特酸结构式如下:
2.如权利要求1所述的方法,其特征在于:步骤S1反应中的呋喃烯烃、芳胺类化合物和二氟烷基卤化物的用量比为0.24mmol:0.20mmol:0.40mmol。
3.如权利要求1所述的方法,其特征在于:步骤S1反应中的mer-Ir(ppy)3的摩尔用量为芳胺类化合物的0.33%,碳酸钾和芳胺类化合物的用量比为0.4mmol:0.20mmol。
4.如权利要求1所述的方法,其特征在于:步骤S1反应时间t1为10-96h。
5.如权利要求1所述的方法,其特征在于:步骤S1反应中的有机溶剂为乙腈,芳胺类化合物的物质的量与乙腈的体积之比为0.1mmol:2mL,反应在氩气氛围下进行。
6.如权利要求1所述的方法,其特征在于:步骤S1反应中的可见光由功率为3W、波长为460-465nm的蓝色LED灯提供,所述蓝灯和反应物之间的距离为4cm。
7.如权利要求1所述的方法,其特征在于:步骤S2反应中的Dy(OTf)3、手性布朗斯特酸A1摩尔用量分别为芳胺类化合物的0.33%、1%。
8.如权利要求1所述的方法,其特征在于:步骤S2反应中的有机溶剂为三氯甲烷,溶剂用量为2mL,反应时间t2为3-120h。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110770331.3A CN113501766B (zh) | 2021-07-07 | 2021-07-07 | 一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110770331.3A CN113501766B (zh) | 2021-07-07 | 2021-07-07 | 一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113501766A CN113501766A (zh) | 2021-10-15 |
| CN113501766B true CN113501766B (zh) | 2023-05-23 |
Family
ID=78012064
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110770331.3A Active CN113501766B (zh) | 2021-07-07 | 2021-07-07 | 一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113501766B (zh) |
-
2021
- 2021-07-07 CN CN202110770331.3A patent/CN113501766B/zh active Active
Non-Patent Citations (4)
| Title |
|---|
| Guanglong Pan 等.Heterogeneous photocatalytic cyanomethylarylation of alkenes with acetonitrile: synthesis of diverse nitrogenous heterocyclic compounds .《Beilstein J. Org. Chem.》.2021,第17卷第 1171-1180 页. * |
| Huilin Li 等.Catalytic Enantioselective Aza-Piancatelli Rearrangement.《Angew. Chem. Int. Ed.》.2016,第55卷第 15125-15 128 页. * |
| Lei Xu 等.Ln(III)/Chiral Brønsted Acid catalyzed Asymmetric Cascade Ring Opening/Aza-Piancatelli Rearrangement of D-A Cyclopropanes.《Org. Lett.》.2020,第22卷第 9016-902 1 页. * |
| Yunfei Cai 等.Catalytic Asymmetric Piancatelli Rearrangement: Brønsted Acid Catalyzed 4p Electrocyclization for the Synthesis of Multisubstituted Cyclopentenones.《Angew. Chem. Int. Ed.》.2016,第55卷第 14126-14 130 页. * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113501766A (zh) | 2021-10-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4502293B2 (ja) | 軸不斉を有する光学活性な4級アンモニウム塩、その製法およびα−アミノ酸誘導体の不斉合成への応用 | |
| CN113501766B (zh) | 一种含二氟烷基的多官能化环戊烯酮衍生物的不对称合成方法 | |
| EP2914574A2 (en) | New process | |
| CN114901644B (zh) | 制备右美托咪定的方法 | |
| US6340753B1 (en) | Optically active quarternary ammonium salt with axial chirality, method for producing thereof, and application thereof for asymmetric synthesis of α-amino acid | |
| EP4151620A1 (en) | Method for preparing chiral 4-aryl-beta-amino acid derivative | |
| CN113501764B (zh) | 一种含三氟甲基的多官能化环戊烯酮衍生物的不对称合成方法 | |
| US6441231B1 (en) | Optically active quarternary ammonium salt with axial chirality, method for producing thereof, and application thereof for asymmetric synthesis of α-amino acid | |
| JP4943185B2 (ja) | 光学活性スルホニルイミン化合物の製造方法 | |
| CN109956893B (zh) | 一种多取代3-氨基吡咯化合物的制备方法 | |
| CN113072486B (zh) | 氨基醇-硼-联萘酚配合物及利用该配合物的光学活性氨基醇衍生物的制备方法 | |
| CN110028448B (zh) | 一种3-羟基-2,3-二氢异喹啉-1,4-二酮化合物的制备方法 | |
| KR101109942B1 (ko) | 방향족 불포화 화합물의 제조 방법 | |
| AU705387B2 (en) | Racemisation of quaternary chiral centers | |
| CN110684043B (zh) | 一种c-n轴手性芳胺化合物及其制备方法 | |
| Ahadi et al. | Diastereoselective synthesis of polysubstituted cyclopentanols and cyclopentenes containing stereogenic centers via domino Michael/cyclization reaction | |
| JP5344523B2 (ja) | 立体選択的にストレッカー反応を進行させ得る触媒、およびそれを用いたα−アミノニトリル誘導体を立体選択的に製造するための方法 | |
| KR20060136357A (ko) | 방향족 불포화 화합물의 제조 방법 | |
| WO2012108367A1 (ja) | 第4級アンモニウム塩 | |
| CN112441934B (zh) | 一种卤代氧杂烯丙基胺类化合物及其制备方法和应用 | |
| JP5106562B2 (ja) | 軸不斉を有する光学活性な4級アンモニウム塩、その製法およびα−アミノ酸誘導体の不斉合成への応用 | |
| CN109810020B (zh) | 一种氰基甲酰胺类化合物的合成方法 | |
| WO2013038427A1 (en) | ARYLATED β-DICARBOIMYL COMPOUNDS AND PROCESS FOR THE PREPARATION THEREOF | |
| CN113461690B (zh) | 一种手性4,6-二氧八氢吡咯并[3,4-c]吡咯-1-羧酸酯类化合物的合成方法 | |
| Mi et al. | A Facile and Practical Synthesis of (-)-tasimelteon |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |