CN112763590A - Determination of sodium azide in antibiotics by LC-MS derivatization method - Google Patents
Determination of sodium azide in antibiotics by LC-MS derivatization method Download PDFInfo
- Publication number
- CN112763590A CN112763590A CN202011466669.1A CN202011466669A CN112763590A CN 112763590 A CN112763590 A CN 112763590A CN 202011466669 A CN202011466669 A CN 202011466669A CN 112763590 A CN112763590 A CN 112763590A
- Authority
- CN
- China
- Prior art keywords
- solution
- sample
- diluting
- water
- stock solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/04—Preparation or injection of sample to be analysed
- G01N30/06—Preparation
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/72—Mass spectrometers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/04—Preparation or injection of sample to be analysed
- G01N30/06—Preparation
- G01N2030/067—Preparation by reaction, e.g. derivatising the sample
Landscapes
- Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Abstract
The invention provides a method for determining sodium azide in antibiotics by an LC-MS derivatization method, in particular to the technical field of drug detection, and phosphate buffer solution with the pH value of 5.0 is prepared; preparing 10mg/ml dansyl chloride solution as a derivatization agent; precisely taking 1.0ml of water, putting the water into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution with the pH value of 5.0, 3.0ml of acetonitrile and 1.0ml of a derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the mixture to a scale with the acetonitrile, and shaking the mixture uniformly to obtain a blank solution; preparing a reference substance solution; precisely weighing about 13.3mg of olmesartan medoxomil sample, placing the sample in a 50ml measuring flask, ultrasonically dissolving the sample with a proper amount of acetonitrile, diluting the sample to a scale, and shaking the sample uniformly to obtain a sample stock solution. Precisely measuring 1.0ml of a sample stock solution, placing the sample stock solution into a 10ml measuring flask, sequentially adding 1.0ml of water, 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 2.0ml of acetonitrile and 1.0ml of a derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the sample stock solution to a scale with the acetonitrile, and shaking up to obtain a sample solution. The invention can obviously improve the sensitivity of visual observation and detection, has higher specificity, strong anti-interference capability and detection efficiency, and saves the labor cost.
Description
Technical Field
The invention belongs to the technical field of drug detection, and particularly relates to a method for determining sodium azide in antibiotics by an LC-MS derivatization method.
Background
Sodium azide is a hexagonal crystal or white crystal, soluble in water and liquid ammonia, slightly soluble in ethanol, insoluble in diethyl ether, and can be prepared by reacting sodium amide with nitrous oxide. The tetrazole compound is a raw material for further synthesizing an antibiotic cephalosporin medicament, and is also a key material in the synthesis of other medicaments, for example, sodium azide and heptanoyl chloride are required to synthesize an intermediate ethyl isocyanate in the preparation of an anticancer medicament carmofur.
However, sodium azide is a highly toxic product, has similar toxicity to cyanide, has an inhibitory effect on cytochrome oxidase and other enzymes, can block the formation of oxygenated hemoglobin in vivo, and has a significant antihypertensive effect. At lower concentration levels, which may also cause direct DNA damage and induce DNA mutagenesis and thus cancer, azides should be controlled as mutagenic impurities according to the international harmonization society (ICH) -M7 guidelines for human drug registration, and thus the azide content of drugs and pharmaceutical intermediates must be strictly controlled during drug production.
At present, azide compounds are detected by a plurality of methods, and the azide analysis methods reported in documents comprise a capacity analysis method, an ultraviolet spectroscopy analysis method, a GC-MS method, an HPLC method and the like. Among them, the volumetric analysis and spectroscopic analysis are not accurate enough for measuring azide at low concentration and are rarely used. GC method and HPLC method are low in sensitivity and poor in selectivity, so that it is desired to develop a method for detecting azide in bulk drugs with high sensitivity and good selectivity.
Disclosure of Invention
The invention aims to provide a method for determining sodium azide in antibiotics by an LC-MS derivatization method, which has better selectivity, can be controlled to be completed within 10min, has higher sensitivity, and can greatly improve the detection efficiency.
The invention provides the following technical scheme:
the specific determination of sodium azide in antibiotics by LC-MS derivatization method is as follows:
s1, preparing a phosphate buffer solution with pH5.0;
s2, preparing 10mg/ml dansyl chloride solution as a derivatizing agent; s3, precisely weighing 1.0ml of water, placing the water into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution with the pH value of 5.0, 3.0ml of acetonitrile and 1.0ml of derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the solution to a scale with the acetonitrile, and shaking up the solution to obtain a blank solution;
s4, weighing 1.0g of sodium azide, placing the sodium azide in a 10ml measuring flask, dissolving the sodium azide in water, fixing the volume, and shaking up to obtain a 10% sodium azide solution;
precisely measuring 1.0ml of 10% sodium azide solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up the solution to serve as a grade-1 reference substance stock solution; precisely measuring 1.0ml of the grade-1 reference substance stock solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a grade-2 reference substance stock solution; precisely measuring 1.0ml of the 2-grade reference stock solution, placing the 2-grade reference stock solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a 3-grade reference stock solution; precisely measuring 5.0ml of 3-grade reference substance stock solution, placing in a 50ml measuring flask, diluting with water to scale, and shaking up to obtain 4-grade reference substance stock solution;
precisely measuring 1.0ml of 4-grade reference substance stock solution, placing the stock solution into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 3.0ml of acetonitrile and 1.0ml of derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the solution to a scale with the acetonitrile, and shaking up to obtain a reference substance solution;
s5, precisely weighing about 13.3mg of olmesartan medoxomil sample, placing the sample in a 50ml measuring flask, ultrasonically dissolving the sample with a proper amount of acetonitrile, diluting the sample to a scale, and shaking the sample uniformly to obtain a sample stock solution.
Precisely measuring 1.0ml of a stock solution of a test sample, putting the stock solution into a 10ml measuring flask, sequentially adding 1.0ml of water, 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 2.0ml of acetonitrile and 1.0ml of a derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the mixture to a scale with the acetonitrile, and shaking the mixture uniformly to obtain a test sample solution;
s5, confirming LC-MS parameters according to the spectrogram
The results of the peak areas of the control at different ultrasound times are as follows
| Time of ultrasound | Peak area of control |
| 5min | 2125167 |
| 10min | 2121965 |
| 30min | 2131146 |
Preferably, the chromatographic parameters are as follows:
preferably, in the step S1, 7.1g of anhydrous disodium hydrogen phosphate is weighed, diluted with water to 1000ml for dissolution, adjusted to pH5.0 with phosphoric acid, and mixed uniformly to obtain the disodium hydrogen phosphate.
Preferably, in the step S2, about 1g of dansyl chloride is weighed, placed in a 100ml measuring flask, ultrasonically dissolved by using a proper amount of water, diluted to a scale and uniformly mixed to obtain the dansyl chloride.
The invention has the beneficial effects that:
the invention uses dansyl chloride derivatization reagent to derivatize sodium azide; the method has the advantages that the retention behavior of sodium azide in reverse liquid chromatography is remarkably improved, and the detection sensitivity of sodium azide in mass spectrum is greatly improved. The method has the advantages of high sensitivity, good selectivity and simple and convenient operation, and can be used for quantitatively detecting the content of sodium azide in the antibiotic in a suspicious manner without carrying out fussy sample pretreatment.
Drawings
The accompanying drawings, which are included to provide a further understanding of the invention and are incorporated in and constitute a part of this specification, illustrate embodiments of the invention and together with the description serve to explain the principles of the invention and not to limit the invention. In the drawings:
FIG. 1 is an ion pair identification diagram;
FIG. 2 is a CE value confirmation diagram;
FIG. 3 shows the DP value confirmation;
FIG. 4 blank solution diagram;
FIG. 5 is a solution diagram of a control;
FIG. 6 sodium azide line graph.
Detailed Description
The principle of the derivatization method is as follows:
the invention discloses a method for determining sodium azide in antibiotics by a derivatization LC-MS (liquid chromatography-mass spectrometry), which comprises the following steps of using dansyl chloride derivatization reagent to perform derivatization on sodium azide; the method has the advantages that the retention behavior of sodium azide in reverse liquid chromatography is remarkably improved, and the detection sensitivity of sodium azide in mass spectrum is greatly improved. The method has the advantages of high sensitivity, good selectivity and simple and convenient operation, and can be used for quantitatively detecting the content of sodium azide in the antibiotic in a suspicious manner without carrying out fussy sample pretreatment.
Chromatographic parameters
Solution preparation
Phosphate buffer solution (pH5.0)
Weighing 7.1g of anhydrous disodium hydrogen phosphate, diluting with water to 1000ml, dissolving, adjusting pH to 5.0 with phosphoric acid, and mixing well to obtain the final product.
A derivatizing agent: dansyl chloride solution (10 mg/ml):
weighing dansyl chloride about 1g, placing into a 100ml measuring flask, ultrasonically dissolving with appropriate amount of water, diluting to scale, and mixing.
Blank solution
Precisely taking 1.0ml of water, placing the water into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 3.0ml of acetonitrile and 1.0ml of derivatizing agent, carrying out ultrasonic reaction at room temperature for 10min, diluting the mixture to a scale with the acetonitrile, and shaking up the mixture to obtain a blank solution.
Control solution
Weighing 1.0g of sodium azide, placing the sodium azide in a 10ml measuring flask, dissolving the sodium azide with water, fixing the volume and shaking up to obtain a 10% sodium azide solution.
Precisely measuring 1.0ml of 10% sodium azide solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up the solution to serve as a grade-1 reference substance stock solution; precisely measuring 1.0ml of the grade-1 reference substance stock solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a grade-2 reference substance stock solution; precisely measuring 1.0ml of the 2-grade reference stock solution, placing the 2-grade reference stock solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a 3-grade reference stock solution; precisely measuring 5.0ml of the 3-grade reference stock solution, placing the 3-grade reference stock solution in a 50ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain the 4-grade reference stock solution.
Precisely measuring 1.0ml of 4-grade reference substance stock solution, placing the stock solution into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 3.0ml of acetonitrile and 1.0ml of derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the mixture to a scale with the acetonitrile, and shaking up to obtain a reference substance solution.
Test solution
Precisely weighing about 13.3mg of olmesartan medoxomil sample, placing the sample in a 50ml measuring flask, ultrasonically dissolving the sample with a proper amount of acetonitrile, diluting the sample to a scale, and shaking the sample uniformly to obtain a sample stock solution.
Precisely measuring 1.0ml of a sample stock solution, placing the sample stock solution into a 10ml measuring flask, sequentially adding 1.0ml of water, 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 2.0ml of acetonitrile and 1.0ml of a derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the sample stock solution to a scale with the acetonitrile, and shaking up to obtain a sample solution.
Method validation
Confirmation of Mass Spectrometry conditions, the following LC-MS parameters were confirmed from FIGS. 1 to 3
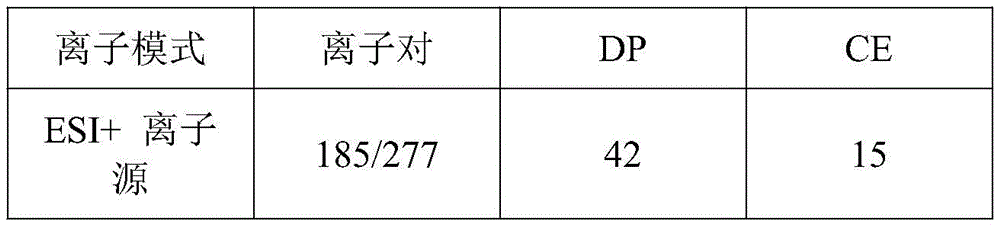
| Ion mode | Ion pair | DP | CE |
| ESI + ion source | 185/277 | 42 | 15 |
Confirmation of the conditions of derivation
Peak area results of control samples at different ultrasound times
| Time of ultrasound | Peak area of control |
| 5min | 2125167 |
| 10min | 2121965 |
| 30min | 2131146 |
The peak areas of the control samples were not different at different sonication times, indicating that the derivatization reaction was complete within 5 minutes.
Method verification
Specificity
Referring to FIGS. 4 to 5, the blank solution showed a weak response at the target peak, but less than < LOD, and no interference with quantitative detection of the target peak
Continuously injecting 6 samples of the control solution STD1, wherein the target peak area RSD is 0.55%, and the retention time RSD is 0.28%; meets the requirements. The results are detailed in the table below.
Result of sample introduction precision
Referring to fig. 6, the target peak is in the concentration range of 0.1987-2.9801 ng/ml, the linear relation is good, the linear equation is that y is 1,801,966.0679x +198,774.3138(y is the peak area, x is the target concentration), the correlation coefficient r is 0.9984
Linear result
Although the present invention has been described in detail with reference to the foregoing embodiments, those skilled in the art will understand that various changes, modifications and substitutions can be made without departing from the spirit and scope of the invention as defined by the appended claims. Any modification, equivalent replacement, or improvement made within the spirit and principle of the present invention should be included in the protection scope of the present invention.
Claims (4)
- The method for determining sodium azide in antibiotics by using an LC-MS derivatization method is characterized by comprising the following steps:s1, preparing a phosphate buffer solution with pH5.0;s2, preparing 10mg/ml dansyl chloride solution as a derivatizing agent; s3, precisely weighing 1.0ml of water, placing the water into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution with the pH value of 5.0, 3.0ml of acetonitrile and 1.0ml of derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the solution to a scale with the acetonitrile, and shaking up the solution to obtain a blank solution;s4, weighing 1.0g of sodium azide, placing the sodium azide in a 10ml measuring flask, dissolving the sodium azide in water, fixing the volume, and shaking up to obtain a 10% sodium azide solution;precisely measuring 1.0ml of 10% sodium azide solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up the solution to serve as a grade-1 reference substance stock solution; precisely measuring 1.0ml of the grade-1 reference substance stock solution, placing the solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a grade-2 reference substance stock solution; precisely measuring 1.0ml of the 2-grade reference stock solution, placing the 2-grade reference stock solution in a 100ml measuring flask, diluting the solution to a scale with water, and shaking up to obtain a 3-grade reference stock solution; precisely measuring 5.0ml of 3-grade reference substance stock solution, placing in a 50ml measuring flask, diluting with water to scale, and shaking up to obtain 4-grade reference substance stock solution;precisely measuring 1.0ml of 4-grade reference substance stock solution, placing the stock solution into a 10ml measuring flask, sequentially adding 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 3.0ml of acetonitrile and 1.0ml of derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the solution to a scale with the acetonitrile, and shaking up to obtain a reference substance solution;s5, precisely weighing about 13.3mg of olmesartan medoxomil sample, placing the sample in a 50ml measuring flask, ultrasonically dissolving the sample with a proper amount of acetonitrile, diluting the sample to a scale, and shaking the sample uniformly to obtain a sample stock solution.Precisely measuring 1.0ml of a stock solution of a test sample, putting the stock solution into a 10ml measuring flask, sequentially adding 1.0ml of water, 3.0ml of 0.05mol/L phosphate buffer solution (pH5.0), 2.0ml of acetonitrile and 1.0ml of a derivative, carrying out ultrasonic reaction at room temperature for 10min, diluting the mixture to a scale with the acetonitrile, and shaking the mixture uniformly to obtain a test sample solution;s5, confirming LC-MS parameters according to the spectrogramThe results of the peak areas of the control at different ultrasound times are as follows
Time of ultrasound Peak area of control 5min 2125167 10min 2121965 30min 2131146 - 3. the method for determining sodium azide in antibiotics by LC-MS derivatization according to claim 2, wherein the method comprises the following steps: s1, weighing 7.1g of anhydrous disodium hydrogen phosphate, diluting with water to 1000ml, dissolving, adjusting the pH value to 5.0 with phosphoric acid, and mixing uniformly to obtain the disodium hydrogen phosphate.
- 4. The LC-MS derivatization method for determining sodium azide in antibiotics according to claim 3, wherein the method comprises the following steps: s2, weighing 1g dansyl chloride, placing the dansyl chloride into a 100ml measuring flask, ultrasonically dissolving the dansyl chloride with a proper amount of water, diluting the dansyl chloride to a scale, and uniformly mixing the dansyl chloride and the water.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011466669.1A CN112763590A (en) | 2020-12-14 | 2020-12-14 | Determination of sodium azide in antibiotics by LC-MS derivatization method |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011466669.1A CN112763590A (en) | 2020-12-14 | 2020-12-14 | Determination of sodium azide in antibiotics by LC-MS derivatization method |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112763590A true CN112763590A (en) | 2021-05-07 |
Family
ID=75693670
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011466669.1A Pending CN112763590A (en) | 2020-12-14 | 2020-12-14 | Determination of sodium azide in antibiotics by LC-MS derivatization method |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112763590A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113866300A (en) * | 2021-09-26 | 2021-12-31 | 山东建筑大学 | Method for detecting sodium azide in medicine or intermediate thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0401657A1 (en) * | 1989-06-03 | 1990-12-12 | Forschungszentrum Jülich Gmbh | Process for modifying the C-terminal end of proteins |
| CN103154735A (en) * | 2009-05-11 | 2013-06-12 | 连接Dx股份有限公司 | Methods and compositions for analyte detection |
| CN104040332A (en) * | 2012-01-20 | 2014-09-10 | Dh科技发展私人贸易有限公司 | Analysis of estradiol and analytes with phenolic OH using labeling chemistry and LC-MSMS workflow |
| WO2019089846A1 (en) * | 2017-10-31 | 2019-05-09 | Encodia, Inc. | Methods and compositions for polypeptide analysis |
-
2020
- 2020-12-14 CN CN202011466669.1A patent/CN112763590A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0401657A1 (en) * | 1989-06-03 | 1990-12-12 | Forschungszentrum Jülich Gmbh | Process for modifying the C-terminal end of proteins |
| CN103154735A (en) * | 2009-05-11 | 2013-06-12 | 连接Dx股份有限公司 | Methods and compositions for analyte detection |
| CN104040332A (en) * | 2012-01-20 | 2014-09-10 | Dh科技发展私人贸易有限公司 | Analysis of estradiol and analytes with phenolic OH using labeling chemistry and LC-MSMS workflow |
| WO2019089846A1 (en) * | 2017-10-31 | 2019-05-09 | Encodia, Inc. | Methods and compositions for polypeptide analysis |
Non-Patent Citations (3)
| Title |
|---|
| LIEVE DILLEN等: "Quantitative LC-MS/MS analysis of azide and azidoalanine in in vitro samples following derivatisation with dansyl chloride", 《ANAL. METHODS》 * |
| 付林等: "2,6-dansyl azide快速测定血清中H_2S含量", 《分析试验室》 * |
| 张鹏等: "用于检测多硫化氢和亚硝酰氢的小分子荧光探针", 《影像科学与光化学》 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113866300A (en) * | 2021-09-26 | 2021-12-31 | 山东建筑大学 | Method for detecting sodium azide in medicine or intermediate thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8987233B2 (en) | Bruton's tyrosine kinase activity probe and method of using | |
| US7332343B2 (en) | Determining enantiomeric excess using indicator-displacement assays | |
| US10816552B2 (en) | Organotellurium compounds, compositions and methods of use thereof | |
| Mittelmaier et al. | Identification and quantification of the glucose degradation product glucosone in peritoneal dialysis fluids by HPLC/DAD/MSMS | |
| CN112763590A (en) | Determination of sodium azide in antibiotics by LC-MS derivatization method | |
| WO2020214336A2 (en) | Sulfur-heterocycle exchange chemistry and uses thereof | |
| US20130196325A1 (en) | In Situ Chemiluminescent Substrates and Assays | |
| Moeker et al. | Design and synthesis of thiourea compounds that inhibit transmembrane anchored carbonic anhydrases | |
| CN101880290A (en) | Preparation method of cefamandole nafate | |
| Musser et al. | Alkylation of DNA with aziridine produced during the hydrolysis of N, N', N''-triethylenethiophosphoramide | |
| Abbas et al. | Spectrophotometric method for the determination of metoclopramide in pharmaceutical forms | |
| CN111620908A (en) | Diastereoisomer of tenofovir alafenamide, preparation method and application thereof | |
| CN112098549B (en) | Method for determining impurity content in arbidol hydrochloride solution | |
| Huang et al. | High performance liquid chromatography for the determination of glucosamine sulfate in human plasma after derivatization with 9‐fluorenylmethyl chloroformate | |
| Stobaugh et al. | Aspects of the stability of isoindoles derived from the reaction of o-phthalaldehyde—Ethanethiol with primary amino compounds | |
| CN110407745A (en) | Hydroxychloroquine nitrogen oxidation derivative and application thereof | |
| Yao et al. | Development of an interference-free chemiluminescence method for monitoring acetylcholine and choline based on immobilized enzymes | |
| Attia et al. | Validated spectrofluorimetric method for the determination of cefoxitin sodium in its pure form and powder for injection via derivatization with 4-chloro-7-nitrobenzo-2-oxa-1, 3-diazole (NBD-CL) | |
| CN109134380A (en) | Chloro aminooimidazole class compound and preparation method thereof, purposes and detection method | |
| CN114354810A (en) | Method for detecting impurity N in clindamycin phosphate and method for separating impurity | |
| Rizk et al. | Fluorimetric determination of nalidixic acid in formulations and biological fluids through ternary complex formation | |
| Tsang et al. | The aminolysis of N-aroyl β-lactams occurs by a concerted mechanism | |
| Stanisz | The influence of relative humidity and temperature on stability of moexipril hydrochloride in solid phase | |
| CN113075331A (en) | Novel method for determining related substances in mecobalamin tablets | |
| CN116425858B (en) | Fluorescence-modified semaglutin derivative and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20210507 |
|
| RJ01 | Rejection of invention patent application after publication |