CN109897029B - Terpyridyl derivative, and preparation method, application and device thereof - Google Patents
Terpyridyl derivative, and preparation method, application and device thereof Download PDFInfo
- Publication number
- CN109897029B CN109897029B CN201711293130.9A CN201711293130A CN109897029B CN 109897029 B CN109897029 B CN 109897029B CN 201711293130 A CN201711293130 A CN 201711293130A CN 109897029 B CN109897029 B CN 109897029B
- Authority
- CN
- China
- Prior art keywords
- terpyridyl
- carbazole
- added
- layer
- terpyridyl derivative
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Landscapes
- Indole Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
The invention belongs to the technical field of organic photoelectric material application, and particularly relates to a terpyridyl derivative, and a preparation method, application and device thereof. The electronic material is mainly based on a terpyridine core, the periphery of the terpyridine core is regulated and controlled by carbazole derivatives and the like, different bridging groups are changed through D-A structural design, and the distribution of a proper lowest occupied orbital (HOMO) and highest unoccupied orbital (LUMO) is regulated and controlled, so that the electronic material is mainly applied to TADF guest materials, host materials and carrier transport materials.
Description
Technical Field
The invention belongs to the technical field of organic photoelectric material application, and particularly relates to a terpyridyl derivative, and a preparation method, application and device thereof.
Background
Organic Light Emitting Diodes (OLEDs) are light, thin, self-emissive, high contrast, easy to manufacture in large areas, and can be used in flexible and transparent display and illumination applications, and are known as "illusion display" and next generation solid state illumination technologies. In 1963, Pope teaches that a bias of several hundred volts is applied to a crystal of anthracene to emit weak blue light, but this is not appreciated due to excessive voltage and poor luminous efficiency. Since 1987, the multilayer OLED device manufactured by dune green cloud doctor (c.w. tang) and Steve VanSlyke through vacuum evaporation greatly reduces the driving voltage of the device and improves the light emitting efficiency, so that the OLED has attracted wide attention in the fields of illumination and display. The development of OLEDs has generally undergone three stages of development, fluorescence → phosphorescence → thermally-induced delayed fluorescence. The development of the first generation of pure fluorescence materials and the second generation of phosphorescence materials tends to be perfect, and the materials are more materials used in the industry at present. In order to realize the three primary colors of red, green and blue, the industry mainly adopts blue light fluorescent materials; green and red phosphorescent materials. However, the quantum efficiency of blue fluorescence is only 25% in the theory of the electrogenerated condition, and the coupled light-out constant is about 20% under the influence of the optical waveguide, so the theoretical maximum external quantum yield is about 5%. In the second generation, the phosphorescence blue light material can realize the internal quantum yield of 100% theoretically through the external heavy atom effect, but the cost is increased due to the containing of noble metal, and the luminescent material of heavy metal is easy to degrade in the electroluminescent process of the device, so that the stability of the device is poor. For the third generation of thermally induced delayed fluorescence (TADF), which is a potential material to realize high efficiency, the efficiency roll-off problem is very serious, and the phenomenon is particularly obvious on blue light. Therefore, the problems of high efficiency and efficiency roll-off are urgently solved for the blue light material of the OLED.
In the D-A structural design for realizing the blue light TADF, the reasonable collocation of the electron-withdrawing group A and the electron-donating group D and the bridging group between D, A are used, so that the molecule realizes a weak intramolecular charge transfer state, and the method is an important way for realizing the blue light. Triazine, benzophenone, diphenyl sulfide sulfone, phosphorus and the like, and due to the strong electron withdrawing ability and the matching of weak electron groups, most of blue light realized belongs to sky blue light, and deep blue light high-efficiency luminescent materials are in short supply. Terpyridine is widely applied to metal complexes, but has limited application in organic light-emitting small molecules due to low quantum yield of the terpyridine.
Disclosure of Invention
The invention mainly bases on terpyridine core, uses carbazole derivatives and the like at the periphery to regulate and control proper electron-withdrawing ability, changes different bridging groups through D-A structure design, regulates and controls the distribution of proper lowest occupied orbital (HOMO) and highest unoccupied orbital (LUMO), and is mainly applied to TADF guest materials, host materials and carrier transport materials. The purpose is to synthesize the terpyridyl derivative by adopting a simple and feasible synthesis scheme and apply the terpyridyl derivative to an electroluminescent material, thereby solving the technical problems of poor stability and low efficiency of the blue light material in the prior art.
The technical scheme provided by the invention is as follows:
the terpyridyl derivative is characterized by having the following structural general formula:
wherein the bridging group L is selected from any one of the following four:
r is an alkyl group having 6 to 60 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 6 to 60 carbon atoms in total and 6 to 60 hetero atoms, or a substituted or unsubstituted aromatic cyclic group having 6 to 60 carbon atoms in total.
Preferably, R is a carbazole-based derivative group.
Preferably, the carbazole-based derivative group has the formula: and A is crossed with B, wherein A is carbazolyl, B is nitrogen fluorenyl, oxygen fluorenyl, sulfur fluorenyl, 1-dimethyl fluorenyl, 1-diphenyl fluorenyl or spirofluorenyl, and A and B are crossed on a benzene ring.
Specifically, the carbazole derivative group is selected from any one of the following groups:
specifically, the terpyridyl derivative is selected from any one of the following:
the invention also provides a preparation method of the terpyridyl derivative, which comprises the following steps: R-H and Br-L-terpyridine are coupled to obtain terpyridyl derivativeWherein:
the bridging group L is selected from any one of the following four:
r is an alkyl group having 6 to 60 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 6 to 60 carbon atoms in total and 6 to 60 hetero atoms, or a substituted or unsubstituted aromatic cyclic group having 6 to 60 carbon atoms in total.
Preferably, R is a carbazole-based derivative group.
The carbazole-based derivative group and the obtained terpyridyl derivative are as described above.
The invention also provides application of the terpyridyl derivative provided by the invention as an electroluminescent layer material in an organic electroluminescent device, wherein the electroluminescent layer material is a fluorescent or thermally-induced delayed fluorescent material, a phosphorescent main body material or a fluorescent main body material.
The invention also provides an electroluminescent device which at least comprises a pair of electrodes and an organic luminescent medium arranged between the pair of electrodes, wherein the organic luminescent medium at least contains one terpyridyl derivative provided by the invention.
The terpyridyl derivative has weaker electron-withdrawing capability, and can realize light color regulation from deep blue to sky blue by matching with different power supply groups. In an OLED device, the hole mobility of an organic material is generally one to two orders of magnitude higher than that of electrons, and the terpyridyl derivative can improve the electron mobility by utilizing the intramolecular hydrogen bonding action, so that the carrier of a light-emitting region is more balanced, the efficiency is reduced, and the roll off is small. The terpyridyl derivative is simple to prepare, has good thermal stability, and is suitable to be used as an organic light-emitting functional layer material through thermal evaporation.
Drawings
FIG. 1 is a graph of the spectra of compounds 13, 14 and 15 provided by the present invention under electroluminescent conditions.
Fig. 2 is a life decay curve under light induced conditions for compounds 13, 14 and 15 provided by the present invention.
Fig. 3 is a structural diagram of an electro-device provided by the present invention.
Detailed Description
The principles and features of this invention are described below in conjunction with examples which are set forth to illustrate, but are not to be construed to limit the scope of the invention.
Example 1
Preparation of (5- (4- ([2,2 ': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -12, 12-dimethyl-5, 12-dihydroindeno [1,2-c ] carbo-) by:
(1) in a dry 500mL two-necked flask, 3-carbazole boronic acid pinacol ester (15g,51mmol), o-dibromobenzene (14.5g,61.2mmol), toluene (120mL), ethanol (60mL) and 2mol/L potassium carbonate solution (60mL) were added, sonicated for 5-10 minutes followed by a flash nitrogen sparge for 5 minutes, the catalyst tetrakis (triphenylphosphine) palladium (1.8g,1.53mmol) was added rapidly and nitrogen sparged for a large amount of 10 minutes. Heated to 100 ℃ and stirred and refluxed for 12 h. During treatment, the white solid product 3- (2-bromophenyl) -9H-carbazole can be obtained through extraction, spin-drying and chromatography by petroleum ether and dichloromethane, and the yield is 93%.
(2) 3- (2-bromophenyl) -9H-carbazole (20.73g,64.4mmol), di-tert-butyl dicarbonate (19.67g,90.13mmol) and 4-dimethylaminopyridine (0.787g,6.44mmol) were placed in a 500mL single-neck flask, dissolved by adding 250mL of tetrahydrofuran, and stirred at room temperature for 4 hours. Spin-dry, with ethyl acetate: column chromatography of the eluent with petroleum ether at a ratio of 1:50 gave the white intermediate, tert-butyl 3- (2-bromophenyl) -9H-carbazole-9-carboxylate, in 95% yield.
(3) A dried two-necked 250mL round-bottomed flask, magnetons and a constant pressure dropping funnel were prepared, then 3- (2-bromophenyl) -9H-carbazole-9-carboxylic acid tert-butyl ester (8g,19mmol) obtained in step (2) was added, the round-bottomed flask was evacuated and purged with nitrogen, redistilled tetrahydrofuran (80mL) was immediately added, after dissolution by stirring, nitrogen was added and cooled, and n-butyllithium (8.36mL, 20.9mmol) was added dropwise after 10 minutes and the addition was completed within 30 minutes. After 1h of reaction, dry acetone (1.2g,20.9mmol) was added dropwise, and after 2h of reaction the cold pot was removed and allowed to warm to room temperature. After 12h of reaction, quench with a small amount of water. Spin-dry to remove water and THF. It was a viscous solid by spin-drying in a 100mL single-necked flask. Acetic acid (100mL) was added as a solvent and hydrochloric acid (25mL) was used to close the ring. Heated to 120 ℃ and stirred under reflux for 12 h. After the reaction, water is added to separate out solid, and the solid is filtered. White product 12, 12-dimethyl-5, 12-dihydroindeno [1,2-c ] carbo is obtained by petroleum ether and ethyl acetate column chromatography, and the yield is 63 percent.
(4) Into a 50mL single-necked flask was charged 12, 12-dimethyl-5, 12-dihydroindeno [1,2-c ] synthesized according to steps (1) to (3) of example 1]3-carbohydramine (2g, 7.06mmol), 4'- (4-bromophenyl) -2, 2': 6', 2 "-terpyridine (2.73g,7.06mmol), cuprous iodide (0.8g, 4.2mmol), K2CO3(5.83g,42.0mmol) and 18-crown-6 (1.08g, 4.2mmol) were dissolved in a 5mL of the MPU solution. Under the protection of N2, the temperature is raised to 175 ℃ and the reaction is carried out for 36 h. After the reaction is finished, cooling to room temperature, extracting, spin-drying and carrying out column chromatography. Column chromatography on petroleum ether and dichloromethane afforded the product as a pale yellow solid in 78% yield.13C-NMR(CDCl 3100 MHz). delta. (ppm)155.9,155.3,152.0,149.2,147.8,145.4,141.0,139.1,137.3,137.2,134.0,133.9,130.9,129.7,129.6,128.0,126.7,126.6,123.6,123.2,121.6,121.4,119.8,118.0,117.3,114.1,109.5,109.1,104.4,42.9,31.2. high resolution Mass Spectrometry: 590.568.
the results of the nuclear magnetic resonance and the mass spectrum revealed that the pale yellow solid product had a correct structure and was a compound represented by formula 13 (5- (4- ([2,2 ': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -12, 12-dimethyl-5, 12-dihydroindeno [1,2-c ] carbo).
Example 2
Preparation of (5- (4- ([2,2 ': 6', 2 '-terpyridyl ] -4' -yl) phenyl) -12, 12-diphenyl-5, 12-dihydroindeno [1,2-c ] carbo)
(1) In a dry 500mL two-necked flask, 3-carbazole boronic acid pinacol ester (15g,51mmol), o-dibromobenzene (14.5g,61.2mmol), toluene (120mL), ethanol (60mL) and 2mol/L potassium carbonate solution (60mL) were added, sonicated for 5-10 minutes followed by a flash nitrogen sparge for 5 minutes, the catalyst tetrakis (triphenylphosphine) palladium (1.8g,1.53mmol) was added rapidly and nitrogen sparged for a large amount of 10 minutes. Heated to 100 ℃ and stirred and refluxed for 12 h. During treatment, the white solid product 3- (2-bromophenyl) -9H-carbazole can be obtained through extraction, spin-drying and chromatography by petroleum ether and dichloromethane, and the yield is 93%.
(2) 3- (2-bromophenyl) -9H-carbazole (20.73g,64.4mmol), di-tert-butyl dicarbonate (19.67g,90.13mmol) and 4-dimethylaminopyridine (0.787g,6.44mmol) were placed in a 500mL single-neck flask, dissolved by adding 250mL of tetrahydrofuran, and stirred at room temperature for 4 hours. Spin-dry, with ethyl acetate: column chromatography of the eluent with petroleum ether at a ratio of 1:50 gave the white intermediate, tert-butyl 3- (2-bromophenyl) -9H-carbazole-9-carboxylate, in 95% yield.
(3) A dried two-necked 250mL round-bottomed flask, magnetons and a constant pressure dropping funnel were prepared, then 3- (2-bromophenyl) -9H-carbazole-9-carboxylic acid tert-butyl ester (8g,19mmol) obtained in step (2) was added, the round-bottomed flask was evacuated and purged with nitrogen, redistilled tetrahydrofuran (80mL) was immediately added, after dissolution by stirring, nitrogen was added and cooled, and n-butyllithium (8.36mL, 20.9mmol) was added dropwise after 10 minutes and the addition was completed within 30 minutes. After the addition reaction was completed for 1h, benzophenone (3.8g,20.9mmol) was added dropwise, and after 2h the cold pot was removed and allowed to warm to room temperature naturally. After 12h of reaction, quench with a small amount of water. Spin-dry to remove water and THF. It was a viscous solid by spin-drying in a 100mL single-necked flask. Acetic acid (100mL) was added as a solvent and hydrochloric acid (25mL) was used to close the ring. Heated to 120 ℃ and stirred under reflux for 12 h. After the reaction, water is added to separate out solid, and the solid is filtered. White product 12, 12-diphenyl-5, 12-dihydroindeno [1,2-c ] carbo is obtained by petroleum ether and ethyl acetate column chromatography, and the yield is 70%.
(4) A50 mL single-necked flask was charged with 12, 12-diphenyl-5, 12-dihydroindeno [1,2-c ] synthesized according to steps (1) to (3) of example 2]3-carbohydramine (2g, 4.9mmol), 4'- (4-bromophenyl) -2, 2': 6', 2 "-terpyridine (1.9g,4.9mmol), cuprous iodide (0.55g, 2.91mmol), K2CO3(4.03g,29.1mmol) and 18-crown-6 (0.75g, 2.91mmol) were dissolved in a 5mL of the MPU solution. Under the protection of N2, the temperature is raised to 175 ℃ and the reaction is carried out for 36 h. After the reaction is finished, cooling to room temperature, extracting, spin-drying and carrying out column chromatography. Column chromatography with petroleum ether and dichloromethane afforded the product as a pale yellow solid in 80% yield.13C-NMR(CDCl3,100MHz):δ(ppm)155.9,155.3,152.0,149.2,147.7,145.4,141.9,141.0,139.1,137.3,137.2,134.0,133.9,131.9,130.9,129.7,129.2,128.7,128.1,128.0,126.7,126.6,126.2,123.6,121.4,119.8,118.0,117.3,114.1,109.5,109.1,104.4,
60.3. High resolution mass spectrometry: 714.893.
the results of the nuclear magnetic resonance and the mass spectrum revealed that the pale yellow solid product had a correct structure and was a compound represented by formula 14 (5- (4- ([2,2 ': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -12, 12-dimethyl-5, 12-dihydroindeno [1,2-c ] carbo).
Example 3
Preparation of (5'- (4- ([2, 2': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -5 'H-spiro [ fluorene 9,12' indeno [1,2-c ] carbazole ])
(1) In a dry 500mL two-necked flask, 3-carbazole boronic acid pinacol ester (15g,51mmol), o-dibromobenzene (14.5g,61.2mmol), toluene (120mL), ethanol (60mL) and 2mol/L potassium carbonate solution (60mL) were added, sonicated for 5-10 minutes followed by a flash nitrogen sparge for 5 minutes, the catalyst tetrakis (triphenylphosphine) palladium (1.8g,1.53mmol) was added rapidly and nitrogen sparged for a large amount of 10 minutes. Heated to 100 ℃ and stirred and refluxed for 12 h. During treatment, the white solid product 3- (2-bromophenyl) -9H-carbazole can be obtained through extraction, spin-drying and chromatography by petroleum ether and dichloromethane, and the yield is 93%.
(2) 3- (2-bromophenyl) -9H-carbazole (20.73g,64.4mmol), di-tert-butyl dicarbonate (19.67g,90.13mmol) and 4-dimethylaminopyridine (0.787g,6.44mmol) were placed in a 500mL single-neck flask, dissolved by adding 250mL of tetrahydrofuran, and stirred at room temperature for 4 hours. Spin-dry, with ethyl acetate: column chromatography of the eluent with petroleum ether at a ratio of 1:50 gave the white intermediate, tert-butyl 3- (2-bromophenyl) -9H-carbazole-9-carboxylate, in 95% yield.
(3) A dried two-necked 250mL round-bottomed flask, magnetons and a constant pressure dropping funnel were prepared, then 3- (2-bromophenyl) -9H-carbazole-9-carboxylic acid tert-butyl ester (8g,19mmol) obtained in step (2) was added, the round-bottomed flask was evacuated and purged with nitrogen, redistilled tetrahydrofuran (80mL) was immediately added, after dissolution by stirring, nitrogen was added and cooled, and n-butyllithium (8.36mL, 20.9mmol) was added dropwise after 10 minutes and the addition was completed within 30 minutes. After 1h of reaction, 9-fluorenone (3.7g,20.9mmol) was added dropwise, and after 2h of reaction, the cold pot was removed and the temperature was naturally raised to room temperature. After 12h of reaction, quench with a small amount of water. Spin-dry to remove water and THF. It was a viscous solid by spin-drying in a 100mL single-necked flask. Acetic acid (100mL) was added as a solvent and hydrochloric acid (25mL) was used to close the ring. Heated to 120 ℃ and stirred under reflux for 12 h. After the reaction, water is added to separate out solid, and the solid is filtered. The white product 5' H-spirofluorene-9, 8-indene carbazole is obtained by petroleum ether and ethyl acetate column chromatography, and the yield is 75%.
(4) To a 50mL single-necked flask were added 5' H-spirofluorene-9, 8-indene carbazole (1.98g, 4.9mmol) synthesized in steps (1) to (3) of example 3, 4' - (4-bromophenyl) -2,2 ': 6', 2' -terpyridine (1.89g,4.9mmol), cuprous iodide (0.55g, 2.91mmol), K2CO3(4.03g,29.1mmol) and 18-crown-6 (0.75g, 2.91mmol) were dissolved in a 5mL of the MPU solution. Under the protection of N2, the temperature is raised to 175 ℃ and the reaction is carried out for 36 h. After the reaction is finished, cooling to room temperature, extracting, spin-drying and carrying out column chromatography. Column chromatography with petroleum ether and dichloromethane afforded the product as a pale yellow solid in 80% yield.13C-NMR(CDCl 3100 MHz). delta. (ppm)155.9,155.3,152.0,149.2,145.4, 141.9, 141.0,139.1,137.3,137.2,134.0,133.9,130.9,129.7,128.7,128.1,128.0,126.7,126.6,126.2,123.6,121.4,119.8,118.0,117.3,114.1,109.5,109.1,104.4,60.3. high resolution Mass Spectrometry: 712.725.
from the nuclear magnetic resonance results and mass spectrometry, the pale yellow solid product had a correct structure and was a compound represented by formula 15 (5'- (4- ([2, 2': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -5 'H-spiro [ fluorene 9,12' indeno [1,2-c ] carbazole).
Example 4
Preparation of (5- (4- ([2,2 ': 6', 2 '-terpyridyl ] -4' -yl) phenyl) -5H-benzofuro [3,2-c ] carbo)
(1)50ml of 4-bromodibenzofuran (7.4g, 30mmol, 1eq) in a three-necked flask, adding a dry tetrahydrofuran solution, cooling to-78 deg.C, and removing N2Three times, guarantee bodyIn an oxygen-free environment, n-butyllithium (2.5M, 13.2ml, 33mmol, 1.1eq) is added dropwise, the mixture is kept at-78 ℃ for reaction for 1.5h, triisopropyl borate (rho is 0.815g/ml, 7.6ml, 33mmol, 1.1eq) is added dropwise, the mixture is heated to room temperature after the dropwise addition is finished, diluted hydrochloric acid is added dropwise for quenching reaction after 8h, tetrahydrofuran is distilled out under reduced pressure, dichloromethane is added for dissolution, anhydrous magnesium sulfate is used for washing, drying and filtering are carried out, and the filtrate is recrystallized to obtain 6g of a white solid product, namely 4-dibenzofuran borate (yield is 94%).
(2) In a 500ml three-necked flask, 6g of 4-boronic acid dibenzofuran (28mmol, 1.1eq) and 5.05g of o-bromonitrobenzene (25mmol), 6.9g of potassium carbonate (50mmol, 2eq) were dissolved in tetrahydrofuran: water 3: 1 (volume ratio) is 200ml in total, and N is pumped out2Three times, the oxygen-free environment of the system is ensured, and the content of N2Adding 0.72g (0.62mmol, 2.5% eq) of palladium tetratriphenylphosphine catalyst under the protection of (1), reacting for 16h under reflux, distilling under reduced pressure to remove tetrahydrofuran after the reaction is finished, dissolving with dichloromethane, washing organic phase, extracting aqueous phase three times, mixing and passing through a column, and passing through a column with petroleum ether and dichloromethane mixed solvent to obtain bright yellow gel 4- (2-nitrophenyl) dibenzo [ b, d ]]Furan 7.0g (96% yield).
(3) Preparation of 5-hydro-benzofuran [3,2-c ] carbazole:
7.0g of 4- (2-nitrophenyl) dibenzo [ b, d ]]Furan (24mmol, 1eq), 15.7g of triphenylphosphine (60mmol, 2.5eq) were added to a 500ml three-necked flask, 220ml of o-dichlorobenzene was added, and N was pumped off2Thirdly, ensuring the anaerobic environment of the system, refluxing for 48h, distilling off the o-DCB under reduced pressure, dissolving the dichloromethane, washing the organic phase for three times, drying and filtering by anhydrous magnesium sulfate, mixing the sample, and passing through a column to obtain the off-white solid 5-hydro-benzofuran [3,2-c ]]Carbazole 3.9g (yield 63%).
(4) 5-hydro-benzofuran [3,2-c ] synthesized in steps (1) to (3) of example 4 was charged into a 50mL single-necked flask]Carbazole (1.26g, 4.9mmol), 4'- (4-bromophenyl) -2, 2': 6', 2' -terpyridine (1.89g,4.9mmol), cuprous iodide (0.55g, 2.91mmol), K2CO3(4.03g,29.1mmol) and 18-crown-6 (0.75g, 2.91mmol) were dissolved in a 5mL of the MPU solution. Under the protection of N2, the temperature is raised to 175 ℃ and the reaction is carried out for 36 h. To be treatedAfter the reaction is finished, cooling to room temperature, extracting, spin-drying and carrying out column chromatography. Performing column chromatography with petroleum ether and dichloromethane to obtain pale yellow solid product 5- (4- ([2,2 ': 6', 2' -terpyridine)]-4' -yl) phenyl) -5H-benzofuro [3,2-c]Carbazide, yield 78%.13C-NMR(CDCl 3100 MHz). delta (ppm)156.5,155.9,155.3,152.0,149.2,145.4,141.2,139.1,137.3,137.2,134.0,130.9,128.0,126.6,124.7,124.6,123.6,123.3,123.2,121.4,120.9,119.8,118.0,114.1,111.8,111.5,109.5,106.4 high resolution Mass Spectrometry: 564.812.
the results of the nuclear magnetic resonance and the mass spectrum revealed that the pale yellow solid product had a correct structure and was a compound represented by formula 19 (5- (4- ([2,2 ': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -5H-benzofuro [3,2-c ] carbo).
Example 5
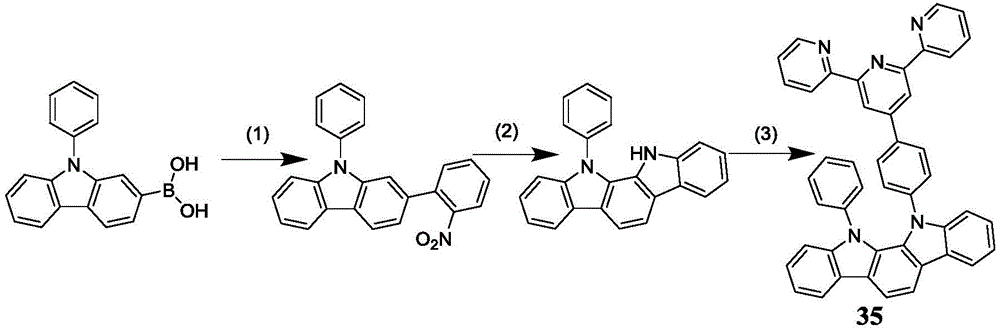
Preparation of 11- (4- ([2,2 ': 6', 2 '-terpyridyl ] -4' -yl) phenyl) -12-phenyl-11, 12-indolino [2,3-a ] carbazole.
(1) (9-phenyl-9H-carbazol-2-yl) boronic acid (10g,34.84mmol), o-bromonitrobenzene (7.74g,38.32mmol), potassium carbonate (13.8g,100mmol) and Pd (PPh)3)4(0.4g,0.34mmol) was added to a 500mL three-necked flask, 100mL of toluene was added, 50mL of ethanol and 50mL of water were added sequentially, the flask was evacuated for 5min, and the mixture was heated to 100 ℃ under reflux for 12 hours with stirring under the protection of N2. Performing column chromatography with dichloromethane and petroleum ether to obtain white solid 2- (2-nitrophenyl) -9-phenyl-9H-carbazole. The yield was 92%.
(2) 2- (2-nitrophenyl) -9-phenyl-9H-carbazole (5g,14.75mmol) and triphenylphosphine (9.67g,36.87mmol) were dissolved in 150mL of o-dichlorobenzene, heated to 180 ℃ under protection of N2, and refluxed for 24 hours. And (3) performing reaction treatment, namely performing reduced pressure distillation on o-dichlorobenzene to remove the o-dichlorobenzene, and then performing column chromatography purification on dichloromethane and petroleum ether to obtain white solid 11-phenyl-11, 12-indolino [2,3-a ] carbazole. The yield was 55%.
(3) A50 mL single-necked flask was charged with the solution obtained in the same manner as in example 5 step (1)11-phenyl-11, 12-indolino [2,3-a ] obtained by synthesis]Carbazole (1.62g, 4.9mmol), 4'- (4-bromophenyl) -2, 2': 6', 2' -terpyridine (1.89g,4.9mmol), cuprous iodide (0.55g, 2.91mmol), K2CO3(4.03g,29.1mmol) and 18-crown-6 (0.75g, 2.91mmol) were dissolved in a 5mL of the MPU solution. Under the protection of N2, the temperature is raised to 175 ℃ and the reaction is carried out for 36 h. After the reaction is finished, cooling to room temperature, extracting, spin-drying and carrying out column chromatography. Performing column chromatography with petroleum ether and dichloromethane to obtain light yellow solid product 11- (4- ([2,2 ': 6', 2' -terpyridine)]-4' -yl) phenyl) -12-phenyl-11, 12-indolino [2,3-a]Carbazole in 78% yield.13C-NMR(CDCl 3100 MHz). delta (ppm)155.9,155.3,152.0,149.2,145.4,139.1,137.3,137.2,136.8,135.5,129.3,128.0,126.6,125.5,125.1,123.6,121.4,120.5,120.0,119.8,118.8,118.0,114.1,111.0,109.5,105.4 high resolution Mass Spectrometry: 639.584.
the results of the nuclear magnetic resonance and the mass spectrum revealed that the pale yellow solid product had a correct structure and was 11- (4- ([2,2 ': 6', 2 "-terpyridyl ] -4' -yl) phenyl) -12-phenyl-11, 12-indolino [2,3-a ] carbazole, which is a compound represented by formula 35.
Example 6
A device was prepared using the terpyridyl derivative of formula 13 prepared in example 1 as a light emitting guest. This example shows 13 a performance verification of an electroluminescent device prepared as guest light emitting material. The ITO (indium tin oxide) glass was successively cleaned with ultrasound in a detergent and deionized water for 30 minutes. Then drying for 2 hours in vacuum (105 ℃), putting the ITO glass into a plasma reactor for oxygen plasma treatment for 5 minutes, transferring the ITO glass into a vacuum chamber to prepare an organic film and a metal electrode, preparing a layer of 10nm hole injection material molybdenum trioxide by a vacuum evaporation method, evaporating a 60nm thick hole transport material 4,4' -bis [ N- (1-naphthyl) -N-phenylamino ] biphenyl (NPB), and forming an electron blocking layer of 10nm thick 1, 3-di-9-carbazolyl benzene (mCP); then a layer of luminescent layer 30nm bis [2- (diphenylphosphino) phenyl ] ether oxide (DPEPO) is evaporated, wherein the proportion is 10 percent and 13 percent; then a layer of DPEPO with the thickness of 10nm is evaporated to be used as a hole blocking layer, then 4, 7-diphenyl-1, 10-phenanthroline (Bphen) with the thickness of 40nm is evaporated to be a layer of LiF with the thickness of 1nm and Al with the thickness of 100 nm.
Aluminum is used as a cathode of the device, a positive electrode of direct current is added to an ITO (indium tin oxide) layer, a negative electrode is added to a metal layer, and bright and uniform light emitted from the ITO (indium tin oxide) layer can be obtained, the CIE color coordinate is (0.155,0.108), the starting voltage is 4.9V, the maximum external quantum efficiency is 15.4%, and the maximum current efficiency is 12.4 cd/A. The experimental device has the structure that: ITO (indium tin oxide)/MoO3(10nm)/NPB(60nm)/mCP(10nm)/DPEPO:10wt%13(30nm)/Bphen(40nm)/LiF(1nm)/Al(100nm)。
Example 7
The terpyridyl derivative of formula 14 prepared in example 2 was used as a light-emitting object to prepare a device. This example demonstrates 14 performance verification of an electroluminescent device prepared as a guest light emitting material. The ITO (indium tin oxide) glass was successively cleaned with ultrasound in a detergent and deionized water for 30 minutes. Then drying for 2 hours in vacuum (105 ℃), putting the ITO glass into a plasma reactor for oxygen plasma treatment for 5 minutes, transferring the ITO glass into a vacuum chamber to prepare an organic film and a metal electrode, preparing a layer of 10nm hole injection material molybdenum trioxide by a vacuum evaporation method, evaporating a 60nm thick hole transport material 4,4' -bis [ N- (1-naphthyl) -N-phenylamino ] biphenyl (NPB), and forming an electron blocking layer of 10nm thick 1, 3-di-9-carbazolyl benzene (mCP); then a layer of luminescent layer 30nm bis [2- (diphenylphosphino) phenyl ] ether oxide (DPEPO) is evaporated, wherein the proportion is 10 percent to 14 percent; then a layer of DPEPO with the thickness of 10nm is evaporated to be used as a hole blocking layer, then 4, 7-diphenyl-1, 10-phenanthroline (Bphen) with the thickness of 40nm is evaporated to be a layer of LiF with the thickness of 1nm and Al with the thickness of 100 nm.
Aluminum is used as a cathode of the device, a positive electrode of direct current is added to an ITO (indium tin oxide) layer, a negative electrode is added to a metal layer, and bright and uniform light emitted from the ITO (indium tin oxide) layer can be obtained, the CIE color coordinate is (0.154,0.109), the starting voltage is 4.8V, the maximum external quantum efficiency is 17.8%, and the maximum current efficiency is 14.9 cd/A. The experimental device has the structure that: ITO (indium tin oxide)/MoO3(10nm)/NPB(60nm)/mCP(10nm)/DPEPO:10wt%14(30nm)/Bphen(40nm)/LiF(1nm)/Al(100nm)。
Example 8
The terpyridyl derivative shown in formula 15 prepared in example 3 was used as a light-emitting object to prepare a device. This example demonstrates 15 performance verification of an electroluminescent device prepared as a guest light emitting material. The ITO (indium tin oxide) glass was successively cleaned with ultrasound in a detergent and deionized water for 30 minutes. Then drying for 2 hours in vacuum (105 ℃), putting the ITO glass into a plasma reactor for oxygen plasma treatment for 5 minutes, transferring the ITO glass into a vacuum chamber to prepare an organic film and a metal electrode, preparing a layer of 10nm hole injection material molybdenum trioxide by a vacuum evaporation method, evaporating a 60nm thick hole transport material 4,4' -bis [ N- (1-naphthyl) -N-phenylamino ] biphenyl (NPB), and forming an electron blocking layer of 10nm thick 1, 3-di-9-carbazolyl benzene (mCP); then a layer of luminescent layer 30nm bis [2- (diphenylphosphino) phenyl ] ether oxide (DPEPO) is evaporated, wherein the proportion is 10 percent to 15 percent; then a layer of DPEPO with the thickness of 10nm is evaporated to be used as a hole blocking layer, then 4, 7-diphenyl-1, 10-phenanthroline (Bphen) with the thickness of 40nm is evaporated to be a layer of LiF with the thickness of 1nm and Al with the thickness of 100 nm.
Aluminum is used as a cathode of the device, a positive electrode of direct current is added to an ITO (indium tin oxide) layer, a negative electrode is added to a metal layer, and bright and uniform light emitted from the ITO (indium tin oxide) layer can be obtained, the CIE color coordinate is (0.158 and 0.157), the starting voltage is 4.7V, the maximum external quantum efficiency is 8.35 percent, and the maximum current efficiency is 10.3 cd/A. The experimental device has the structure that: ITO (indium tin oxide)/MoO3(10nm)/NPB(60nm)/mCP(10nm)/DPEPO:10wt%15(30nm)/Bphen(40nm)/LiF(1nm)/Al(100nm)。
The invention takes the correlation maps of the compounds 13, 14 and 15 as examples to illustrate the relevant properties of the compound materials provided by the invention:
as can be seen from fig. 1, the emission of the three materials in the electroluminescent device in the figure is at 448nm,448nm and 456nm, respectively, which all achieve deep blue emission.
As shown in FIG. 2, the transient delay lives of the three materials are very obvious and all reach hundreds of microseconds, which indicates that all the three materials have the phenomenon of thermally-induced delayed fluorescence.
As shown in fig. 3, in the electroluminescent device structure of such a deep blue TADF material, since the triplet plays a key role in the light emitting process, the Host (Host) needs to select a high triplet state, such as DPEPO, and in order to confine excitons in the light emitting layer, an Electron Blocking Layer (EBL) and a Hole Blocking Layer (HBL) are used. The additional Electron Transport Layer (ETL) and Hole Transport Layer (HTL) need to be energy level matched with other functional layers, and the transport rates of electrons and holes are comparable.
The device performance of the compounds in other implementations is shown in the following table, all with good performance:
the above description is only for the purpose of illustrating the preferred embodiments of the present invention and is not to be construed as limiting the invention, and any modifications, equivalents, improvements and the like that fall within the spirit and principle of the present invention are intended to be included therein.
Claims (5)
4. Use of a terpyridyl derivative according to any one of claims 1 or 2, wherein: the electroluminescent layer material is a thermal-induced delayed fluorescent material as an electroluminescent layer material in an organic electroluminescent device.
5. An electroluminescent device comprising at least a pair of electrodes and an organic light-emitting layer disposed between the pair of electrodes, characterized in that: the organic light-emitting layer contains at least the terpyridyl derivative according to any one of claims 1 or 2.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711293130.9A CN109897029B (en) | 2017-12-08 | 2017-12-08 | Terpyridyl derivative, and preparation method, application and device thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711293130.9A CN109897029B (en) | 2017-12-08 | 2017-12-08 | Terpyridyl derivative, and preparation method, application and device thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109897029A CN109897029A (en) | 2019-06-18 |
| CN109897029B true CN109897029B (en) | 2021-03-30 |
Family
ID=66940170
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201711293130.9A Active CN109897029B (en) | 2017-12-08 | 2017-12-08 | Terpyridyl derivative, and preparation method, application and device thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN109897029B (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110452227A (en) * | 2019-08-05 | 2019-11-15 | 北京大学深圳研究生院 | A kind of organic blue-light fluorescent material and blue-light device based on indole derivatives |
| CN113278018B (en) * | 2020-02-20 | 2022-12-16 | 苏州大学 | Pyridine-based thermally activated delayed fluorescence material and application thereof |
| CN111689946A (en) * | 2020-06-17 | 2020-09-22 | 深圳大学 | Carbazole aromatic ring thermal activation delayed fluorescent material and organic electroluminescent device thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011108901A2 (en) * | 2010-03-05 | 2011-09-09 | 덕산하이메탈(주) | Spiro-carbazole compound comprising a spiro skeleton, and an organic electronic element using the same and a terminal thereof |
| CN106935712A (en) * | 2015-12-29 | 2017-07-07 | 三星显示有限公司 | Organic luminescent device |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101718887B1 (en) * | 2013-07-01 | 2017-04-05 | 삼성디스플레이 주식회사 | Organic light emitting diodes |
| US20170170407A1 (en) * | 2014-06-12 | 2017-06-15 | Duk San Neolux Co., Ltd. | Compound for organic electronic element, organic electronic element using same, and electronic device thereof |
| CN107586299A (en) * | 2017-09-29 | 2018-01-16 | 江苏三月光电科技有限公司 | A kind of organic compound and its application using pyridine as core |
-
2017
- 2017-12-08 CN CN201711293130.9A patent/CN109897029B/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011108901A2 (en) * | 2010-03-05 | 2011-09-09 | 덕산하이메탈(주) | Spiro-carbazole compound comprising a spiro skeleton, and an organic electronic element using the same and a terminal thereof |
| CN106935712A (en) * | 2015-12-29 | 2017-07-07 | 三星显示有限公司 | Organic luminescent device |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109897029A (en) | 2019-06-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101427605B1 (en) | Novel organic electroluminescent compounds and organic electroluminescent device using the same | |
| JP6182145B2 (en) | Spirobifluorene compounds for light emitting devices | |
| CN108586188B (en) | chrysene derivative, material containing chrysene derivative and organic electroluminescent device | |
| TWI454469B (en) | Compound for organic electroluminescent device and organic electroluminescent devices using the same | |
| JP7119204B2 (en) | Organic light-emitting compound and organic electroluminescent device using the same | |
| KR20110132721A (en) | Novel organic electroluminescent compounds and organic electroluminescent device using the same | |
| CN110078755B (en) | Compound, display panel and display device | |
| CN111777633B (en) | Boron-containing compound and organic electroluminescent device containing same | |
| CN113336782A (en) | Green light narrow spectrum three-coordination boron luminescent compound containing carbazole skeleton, preparation method and application thereof | |
| KR20190049525A (en) | Hetero-cyclic compound and organic light emitting device comprising same | |
| CN109053696B (en) | Bipyrazine derivative and application thereof in organic photoelectric device | |
| CN109897029B (en) | Terpyridyl derivative, and preparation method, application and device thereof | |
| KR20130121516A (en) | Using new alylamine as hole transporting mateial and organic electroluminescent device using the same | |
| CN113563871A (en) | Host material, organic photoelectric device and display or lighting device | |
| CN113980040B (en) | Boron-containing asymmetric spiro compound and application thereof in organic electroluminescent device | |
| CN113528123B (en) | Host material and organic electroluminescent device comprising same | |
| KR101815653B1 (en) | Azaboradibenzochrysene derivatives organic electroluminescent compound, ink composition and organic electroluminescent device | |
| KR20130120855A (en) | Using thiophen derivative as hole transporting mateial and organic electroluminescent device using the same | |
| CN111574505B (en) | Compound with benzo [ c ] cinnoline as receptor and application thereof | |
| KR20120128386A (en) | Organic compounds for organic electro luminescente device and organic electro luminescent device using same | |
| CN107344923B (en) | Organic compound for electric field light-emitting device | |
| KR101327301B1 (en) | Amine derivative as hole transporting material and organic electroluminescent device using the same | |
| KR20110119282A (en) | Novel organic electroluminescent compounds and organic electroluminescent device using the same | |
| CN113004262B (en) | Organic material and application thereof | |
| CN111635420B (en) | Novel polyheterocyclic compound and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |