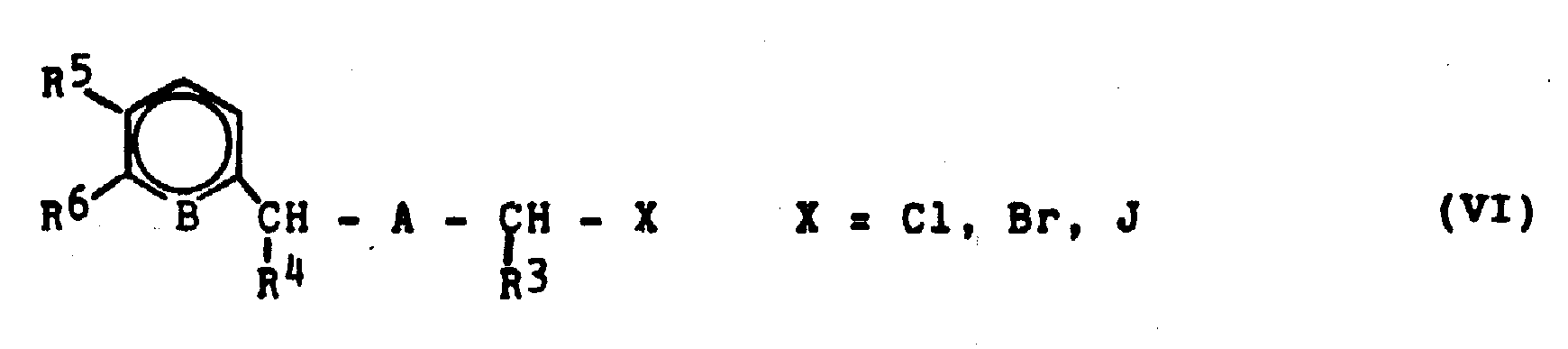CN1046156A - 杂芳基-芳基-丁烯衍生物和杂芳基-芳基-丁烷衍生物制备它们的方法含有它们的制剂以及它们作为农药的应用 - Google Patents
杂芳基-芳基-丁烯衍生物和杂芳基-芳基-丁烷衍生物制备它们的方法含有它们的制剂以及它们作为农药的应用 Download PDFInfo
- Publication number
- CN1046156A CN1046156A CN90101246A CN90101246A CN1046156A CN 1046156 A CN1046156 A CN 1046156A CN 90101246 A CN90101246 A CN 90101246A CN 90101246 A CN90101246 A CN 90101246A CN 1046156 A CN1046156 A CN 1046156A
- Authority
- CN
- China
- Prior art keywords
- formula
- compound
- carbon
- alkyl
- represent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/28—Radicals substituted by singly-bound oxygen or sulphur atoms
- C07D213/30—Oxygen atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/08—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/10—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with sulfur as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/50—1,3-Diazoles; Hydrogenated 1,3-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/54—1,3-Diazines; Hydrogenated 1,3-diazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/74—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/26—Radicals substituted by halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/64—One oxygen atom attached in position 2 or 6
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/26—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D277/22—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/38—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D307/40—Radicals substituted by oxygen atoms
- C07D307/42—Singly bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/12—Radicals substituted by halogen atoms or nitro or nitroso radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/14—Radicals substituted by singly bound hetero atoms other than halogen
- C07D333/16—Radicals substituted by singly bound hetero atoms other than halogen by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/30—Hetero atoms other than halogen
- C07D333/32—Oxygen atoms
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Environmental Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Dentistry (AREA)
- Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- Pest Control & Pesticides (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Tropical Medicine & Parasitology (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Plural Heterocyclic Compounds (AREA)
- Heterocyclic Compounds Containing Sulfur Atoms (AREA)
- Pyridine Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Thiazole And Isothizaole Compounds (AREA)
- Furan Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
本发明涉及的式I和II化合物,它们的立体异构体、以及这些立体异构体的混合物具有很好的杀虫和杀螨性能。
Description
已知某些1,4-二芳基丁烷和1,4-二芳基丁烯化合物具有杀虫或杀螨性能(见GB 2,187,452A或Chem.Abstr.Vol.109,73119Z)。但是,这些化合物的活性在一些情况下并不令人满意。
通过把杂芳基基团引入到芳基丁烯和芳基丁烷中,可以制备出新的芳基丁烯和芳基丁烷的衍生物,它们作为农药是优良的,尤其具有优良的杀虫和/或杀螨性能。
本发明涉及式Ⅰ和Ⅱ的化合物、涉及它们的立体异构体、以及这些立体异构体的混合物
其中,
R1代表吡啶基、嘧啶基、噻吩基、噻唑基、呋喃基、吡唑基、1,3-噁唑基或咪唑基,所有这些基团可以是取代了的;
R3代表H或(1-4)碳烷基;
R4代表H、(1-4)碳烷基、-CN或-C≡CH;
A代表CH2、O或S;
B代表CH、N或C(CH3);
R5代表H或卤素,
R6代表苯基、苯氧基或苄基,它们都可以被卤素或(1-3)碳卤代烷基取代1-5位。
式Ⅰ和Ⅱ化合物中优选的是以下这些,其中,
R1是式A、B、C、D、E或F基团之一:
其中R7是H、卤素、(1-4)碳烷基、(1-4)碳烷氧基、(1-4)碳卤代烷基、(1-4)碳卤代烷氧基,同时m是1或2;
R2是(1-3)碳烷基、环丙基或CF3;
R3是H或甲基;
B是CH;
R4是H、甲基、-CN或-C≡CH;
R6是苯氧基,在它的4-位可被Cl、Br或CF3取代。
式Ⅰ和Ⅱ中尤其优选的是这样的化合物,其中R7是Cl、Br、(1-3)碳烷氧基、-OCF3、-OCH2CF3或-OCF2CF2H。
取代基R1以式A、B或C代表的基团为好,尤其好的是A。
本发明包括了式Ⅰ和Ⅱ可能出现的所有立体异构体,尤其是关于式Ⅰ中烯官能团的Z-和E-非对映异构体以及它们的混合物,还有式Ⅰ和Ⅱ的那些单型对映体以及那些对映体对。烷基可以为直链的和支链的。
本发明还涉及式Ⅰ和Ⅱ化合物的制备方法,它包括
a)对式Ⅰ化合物而言,使一种式Ⅲ化合物与一种式Ⅳ化合物反应
其中R1和R2同式Ⅰ中的定义
其中R3、R4、R5、R6、A和B同式Ⅰ中的定义,同时R8是苯基、环己基或丁基,及
b)对式Ⅱ化合物而言,用氢或一种释放出氢的化合物来使式Ⅰ所对应的化合物上的烯烃官能团进行催化加氢。
a)所描述的方法是已知的Wittig反应。其过程和反应参数如文献所述(见H.J.Bestmann,Jopics in Current Chemistry 109(1983);Houben-Weyl,Methoden der Organ.Chemie〔Methods of Organic Chemistry〕,Vol.El and 5/lb)。化合物Ⅲ的羰基官能团可用化合物Ⅳ上的基团=P(R8)3交换,反过来亦可。
此外,基团R1可以借助一种有机金属化合物被转化成对应的羰基化合物。然后,通过使生成了的叔醇脱水,即产生出式Ⅰ化合物(Houben-Weyl Vol.6/1a/2)。
用作Wittig反应a)的起始物料的酮类Ⅲ是通过对含有足够电子的芳类化合物进行Friedel-Crafts酰化作用而得到的(Houben-Weyl,Vol.7/2a,39,83),或是采用下面方法来得到:用恰当的羧酸衍生物如羧酸卤化物、酐、酯或腈来与芳基金属化合物反应,从而合成其碳结构部分(Houben-Weyl,7/2a,548-621;X.Creary,J.Org.Chem.52,5026-30(1987))。
内鎓盐化合物Ⅳ是通过利用强碱的去质子化作用,或是在添加酮之前、或是在酮Ⅲ的存在下从对应的鏻盐或膦酸酯而形成的,其温度从-20至100℃。所说强碱如碱金属氢化物、有机锂化合物、碱金属氨化物和碱金属醇盐,这些强碱以醚或芳烃为溶剂(Houben-Weyl,Vol.El and 5/1b)。
在文献公开的反应条件下(Houben-Weyl,Vol.El,495-515,and 12/1,79-124),鏻盐V
是用式Ⅵ所示的芳基烷基卤化物
使三苯膦或三烷基膦进行烷基化而得到的。
当A=CH2时,烷基化剂Ⅵ从对应的3-芳基丙醇Ⅶ和卤化氢或无机酸卤化物如亚硫酰氯反应而得到;但当A=O或S时,它们是从对应的苄醇和甲醛及卤化氢而制得(Houben-Weyl,Vol 5/3 and 5/4,DE-A-3,125,338)。用铝氢化合物如铝氢化锂使肉桂酸和它们的酯Ⅷ还原,可制得3-芳基丙醇Ⅶ(Houben-Weyl,Vol.4/ld,190-216)。
在文献公开的反应条件(Houben-Weyl,Vol.4/1C)及钯、铂、镍或铑化合物的催化诱导下,通过用氢或一种释氢化合物如肼、环己烯或甲酸衍生物来使式Ⅰ所示的芳基丁烯上的烯烃官能团加氢,就合成得到了式Ⅱ的芳基丁烷化合物。
还可通过乙硼烷、甲硼烷加合物或烷基取代的甲硼烷与式Ⅰ中的碳碳双键的加成反应、随后用水或醇使硼/碳键水解来制备化合物Ⅱ(Houben-Weyl,Vol.4/1d,P.49-59)。
本发明的有效成分适宜于防治有害动物,尤其适用于昆虫、蛛形纲、蠕虫和软体动物,特别优先推荐用于防治昆虫、蛛形纲,这些有害动物在农业、畜牧业、林业、仓贮产品和原材料的保护以及在卫生领域中会遇到,这些有效成分对植物安全,且对温血动物低毒。它们能有效地防治敏感的和具有抗药性的有害动物种群,适用于全部或部分生育期。以上提到的害虫包括:
在蜱螨目中的例子有(如):粗脚粉螨、Agras Spp.、钝缘蜱属、鸡皮刺螨、茶藨瘿螨、柑枯锈螨、牛蜱属、扇头蜱属、花蜱属、璃眼蜱属、硬蜱属、瘙螨属、痒螨属、疥螨属、跗线螨属、苜蓿苔螨、全爪螨属、叶螨属、始叶螨属、小爪螨属、真叶螨属。在等足目中有如:oniscus asellus、鼠妇和粗皱皮鼠妇、在倍足亚纲中有(如):Blaniulus guttulatus。在唇足亚纲中有(如)食果地蜈蚣和蚰蜒属。在综合亚纲中有(如)一种么蚰。在缨尾目中有(如)西洋衣鱼。在弹尾目中有(如)一种棘跳虫。在直翅目中有(如)东方
蠊、美洲大蠊、马德拉
蠊、德国小蠊、家蟋蟀、蛄蝼属、热带飞蝗、长额负蝗和沙漠蝗。在革翅目中有(如)欧洲球
。在等翅目中有(如)散白蚁属。在
目中有(如)葡萄根瘤蚜、瘿绵蚜属、体虱、血虱属和顎虱属。在食毛目中有(如)兽鸟虱属和畜虱属。在缨翅目中有(如)温室条蓟马和棉蓟马。在异翅亚目中有(如)扁盾蝽属、介棉红蝽(Dysdercus intermedius)、甜菜拟网蝽、温带臭虫、长红猎蝽和锥猎蝽属。在同翅亚目中有(如)甘蓝粉虱、甘薯粉虱、温室粉虱、棉蚜、甘蓝蚜、茶藨隐瘤额蚜、Doralis fabae、Doralis pomi、苹果绵蚜、桃大尾蚜、麦长管蚜、瘤蚜属、忽布疣额蚜、禾谷缢管蚜、微叶蝉属、具二叶叶蝉、黑尾叶蝉、褐盔蜡蚧、乌盔蚧、灰稻虱、稻褐飞虱、红圆蚧、夹竹桃圆蚧、粉蚧属和木虱属。在鳞翅目中有(如)棉红铃虫、松尺蠖、冬尺蛾、苹细蛾、苹果巢蛾、小菜蛾、天幕毛虫、黄毒蛾、毒蛾属、棉叶穿孔潜蛾、桔叶潜蛾、地老虎属、切根虫属、夜蛾属、棉斑实蛾、实夜蛾属、甜菜夜蛾、甘蓝夜蛾、斜纹夜蛾、粘虫属、粉纹夜蛾、苹果蠹蛾、粉蝶属、螟属、玉米螟、地中海粉斑螟、大猎螟、荷兰石竹卷蛾、reticulana烟卷蛾、云杉卷叶蛾、葡萄果蠹蛾茶长卷蛾和栎绿卷叶蛾。在鞘翅目中有(如)家俱窃蠹、谷蠹、被甲豆象、菜豆象、家天牛、马铃薯甲虫、辣根猿叶甲、叶甲属、油菜金头跳甲、墨西哥豆瓢虫、隐食甲属、锯胸谷盔、花象属、谷象属、黑葡萄耳象甲、香蕉根颈象、甘蓝荚象甲、苜蓿叶象甲、皮蠹属、斑皮蠹属、圆皮蠹属、黑皮蠹属、粉蠹属、油菜花露尾甲、蛛甲属、金黄蛛甲、麦蛛甲、拟谷盗属、黄粉甲、叩甲属、金针虫属、西方五月鳃角金龟、六月金龟子和褐新西兰肋翅鳃角金龟。在膜翅目中有(如)锯角叶蜂属、(实)叶蜂属、黄蚁属、厨蚁和胡蜂属。在双翅目中有(如)伊蚁属、按蚊属、库蚊属、黄猩猩果蝇、家蝇属、厩蝇属、红头丽蝇、绿蝇属、金蝇属、疽蝇属、胃蝇属、虱蝇属、厩螫蝇属、狂蝇属、螫蝇属、皮蝇属、虻属、Tannia Spp、花园毛蚊、瑞典麦秆蝇、种蝇属、甜菜潜叶蝇、地中海实蝇、油橄榄实蝇和欧洲大蚊。在蚤目中有(如)东方鼠蚤和毛列蚤属。在蛛形纲中有(如)Scorpio maurus和黑寡妇球腹蛛。在蠕虫纲中有(如)血矛线虫属、毛圆线虫属、奥斯脱线虫属、古柏线虫属、夏柏线虫属、类圆线虫属、结节线虫属、下圆线虫属、鉤口线虫属、蛔虫属和异刺线虫属,还包括有片形吸虫属和植物病原线虫,如根结线虫属、异皮线虫属、双垫刃线虫属、滑刃线虫(属)、穿孔线虫属、球孢囊线虫属、短体线虫属、长针线虫属和剑线虫属。在腹足纲中有(如)灰蛞蝓属阿勇蛞蝓属、椎实螺属、土蜗螺属、琥珀螺属、Biophalaria spp.、泡螺属和钉螺属。在瓣鳃纲中有(如)饰贝属。
本发明也涉及农药制剂,这些制剂中含有式Ⅰ和Ⅱ所示化合物以及适宜的剂型助剂。
本发明的制剂通常含有1-95%(重量)式Ⅰ和Ⅱ所示的有效成分。
在满足生物学和/或化学物理学参数的先决条件下,可采用多种方法来配制这些制剂。可以配制成的剂型有:可湿性粉剂(WP)、乳油(EC)、水剂(SC)、浓乳剂、可直接喷洒剂、油基或水基悬浮剂(SC)、悬浮性乳油(SC)、粉剂(DP)、种衣剂,以下形式的粒剂:微粒剂、喷涂粒剂、包衣粒剂和吸附粒剂、可水分散粒剂(WG)、还有超低容量剂、微胶囊剂、蜡丸或毒饵。
这些各别的剂型在原则上是已知的,且可见以下文献叙述(如)Winnacker-Kü chler,“Chemische Technologie(化学工艺学)”,Volume 7,C.Hauser Verlag,Munich,4th Ed.,1986;Van Falkenberg,“pesticides Formulations”,Marcel Dekker N.Y.,2nd Ed.,1972-73;K.Martens,“Spray Drying Handbook”,3rd Ed.,1979 G.Goodwin Ltd.London.
所需的剂型助剂如惰性材料,表面活性剂、溶剂和其它添加剂,也同样是已知的且已见诸报导,如见以下文献:Watkins,“Handbook of Insecticide Dust Diluentsand Carriers”,2nd Ed.,Darland books,Caldwell N.J.;H.V.Olphen,“Introduction to Clay Colloid Chemistry”,2nd Ed.,J.Wiley & Sons,N.Y.;Marschen,“Solvents Guide”,2nd Ed.,Interscience,N.Y.1950;McCutcheon′s,“Detergents and Emulsifiers Annual”,Mc Publ.Corp.Ridgewood N.J.;Sisley and wood,“Encyclopedia of Surface Active Agents”,Chem.Publ.Co.Inc.,N.Y.1964;Schoenfeldt,“Grenzflaechenaktive Aethylenoxidaddukte(具有表面活性的环氧乙烷加成物)”,Wiss.Verlagsgesell.,Stuttgart 1976;Winnacker-Kü chler,“Chemische Technologie(化学工艺学)”,Volume 7,C.Hauser Verlag Munich,4th Ed.1986。
也可在这些制剂的基础上,加入其它的杀虫有效成分、肥料和/或生长调节剂而制成一些混合制剂,如事先混配或临时桶混制得。可湿性粉剂可均匀地分散在水中,除含有所说有效成分外,还含有湿润剂如聚氧乙烯基化的烷基酚、聚氧乙烯基化的脂肪醇、烷基磺酸酯或烷基酚基磺酸酯,还含有分散剂如木素磺酸钠、2,2′-二萘甲烷-6,6′-二磺酸钠、二丁基萘磺酸钠,或改用油酰甲基牛磺酸钠,此外还含有稀释剂或惰性物质。通过以下方法可制得乳油:把有效成分溶解在一种有机溶剂如丁醇、环己酮、二甲基甲酰胺、二甲苯和较高沸点的芳烃类化合物或烃类中,加入一种或多种乳化剂。可用的乳化剂的例子有:烷基芳基磺酸的钙盐如十二烷苯磺酸钙,或是非离子型乳化剂如脂肪酸聚乙二醇酯、烷基芳基聚乙二醇醚、脂肪醇聚乙二醇醚、环氧丙烷/环氧乙烷缩合物、烷基多醚、脱水山梨糖醇脂肪酸酯、聚氧乙烯脱氧山梨糖醇脂肪酸酯或聚氧乙烯山梨糖醇酯。
把有效成分与粉碎的固体材料一起研磨,可以制得粉剂。所说固体材料是(如)滑石和天然粘土,例如高岭土、膨润土、叶蜡石粉或者硅藻土。粒剂可经以下两法中任一种方法制得:或是把有效成分喷涂在具有吸附性的、已成粒的惰性材料上,或是通过使用粘结剂如聚乙烯醇、聚丙烯酸钠或改用矿物油,使有效成分的浓缩液涂敷于载体表面,载体可用(如)砂、高岭土或已成粒的惰性材料。如果需要与肥料混用,恰当的有效成分也可按照通常的肥料造粒的方法来造粒。
在可湿性粉剂中,有效成分的含量可在(如)10-90%(重量,以下同),其余部分由制剂的其他常用成分补足。对乳油而言,有效成分可占5-80%。粉剂中通常含有5-20%的有效成分,可直接喷洒剂中则占2-20%。在粒剂中,有效成分的含量要部分地取决于有效成分是液体还是固体,且部分地取决于采用了什么成粒助剂、填料等。
此外,如果需要的话,所提到的有效成分制剂中可含有粘合剂、湿润剂、分散剂、乳化剂、渗透剂、溶剂、填料或载体,它们都是常用材料。
在使用时,市售形式的浓剂要以通常的方式进行适当的稀释,例如对可湿性粉剂、乳油、悬浮剂、以及微粒等其它一些制剂,要用水稀释。粉剂或粒剂、还有可直接喷洒剂在使用前通常不需要用惰性材料再作稀释。
所需的用药量随外部条件而变化,(如)特别是随温度、湿度等而变。用药量可在一个宽限内变化,如0.005-10.0千克有效成分/公顷或更高,但推荐量为0.01-5千克/公顷。
本发明的有效成分可以其市售制剂形式存在,同时还可把这些制剂与其它有效成分制成一种混合制剂以供施用,所说其它有效成分有(如)杀虫剂、引诱剂、消毒剂、杀螨剂、杀线虫剂、杀(真)菌剂、生长调节剂或除草剂。
杀虫剂包括(如)磷酸酯、氨基甲酸酯、羧酸酯、甲脒、锡化合物、以及那些由微生物法制得的杀虫物质。
选用的混合成分有:
1.含磷化合物中有乙酰甲胺磷、甲基吡恶磷、益棉磷、保棉磷、溴硫磷、乙基溴硫磷、毒虫畏、氯甲硫磷、甲基氯蜱硫磷、内吸磷、甲基内吸磷、砜吸磷、氯亚胺硫磷、二嗪磷、敌敌畏、百治磷、O,O-1,2,2,2-四氯乙基-硫逐磷酸酯(SD-208,304)、乐果、乙拌磷、苯硫磷、乙硫磷、灭克磷、乙嘧硫磷、伐灭磷、苯胺磷、杀螟松、丰索磷、倍硫磷、地虫磷、安果、庚虫磷、异唑磷、叶蚜磷、异噁唑磷、马拉松、丁烯硫磷、甲胺磷、杀扑磷、蔬果磷、速灭磷、久效磷、二溴磷、氧乐果、亚砜吸磷、对硫磷、甲基对硫磷、稻丰散、甲拌磷、伏杀硫磷、硫环磷、亚胺硫磷、磷胺、辛硫磷、嘧啶磷、甲基嘧啶磷、溴丙磷、丙虫磷、胺丙畏、丙硫磷、Pyraclofos、哒嗪硫磷、喹硫磷、乙丙硫磷、双硫磷、特丁磷、杀虫畏、甲基乙拌磷、三唑磷、敌百虫、蚜灭多;
2.含氨基甲酸酯的化合物有:涕灭威、仲丁威、甲萘威、克百威、丁硫克百威、除线威、丙硫克百威、乙硫苯威、呋线威、异丙威灭多威、5-甲基-间-枯烯基-丁酰基(甲基)氨基甲酸酯、杀线威、抗蚜威、残杀威、双灭多威、久效威、4,6,9-三氮杂-4-苄基-6,10-二甲基-8-噁-7-氧代-5,11-二硫杂-9-十二烷酸乙酯(OK 135)、1-甲硫基-(亚乙基氨基)-N-甲基-N-(吗啉代硫基)氨基甲酸酯(UC 51717);
3.含羧酸酯的化合物有:
烯丙菊酯、顺式氯氰菊酯、(E)-(lR)顺-2,2-二甲基-3-(2-氧代硫羟烷-3-内鎓盐式甲基)环丙烷甲酸(5-苄基-3-呋喃甲基)酯(5-benzyl-3-furylmethyl(E)-(IR) Cis-2,2-dimethyl-3-(2-Oxothiolan-3-ylidene-methyl) cycloprop-anecarboxylate)、反式烯丙菊酯、反式烯丙菊酯((S)环戊基异构体)、反式苄呋菊酯、(lRS)-反-3-(4-叔丁苯基)-2,2-二甲基环丙烷甲酸(联苯基(RS)-1-氰基-1-(6-苯氧-2-吡啶基)甲基)酯(NCI 85193)〔biphenate (RS)-1-cyano-1-(6-phenoxy-2-pyridyl)methyl (lRS)-trans-3-(4-tert.butylphenyl)-2,2-dimethyl-cyclopropanecarboxylate(NCI 85193)〕、乙氰菊酯、氟氯氰菊酯、氯氟氰菊酯、氯氰菊酯、cyphenothrin、deltamethrin、烯炔菊酯、高氯戊菊酯、五氟苯菊酯、甲氰菊酯、氰戊菊酯、氟氰戊菊酯、氟氯苯菊酯、氟胺氰菊酯(D-异构体体)、氯菊酯、pheothrin((R)-异构体)、d-pralethrin、除虫菊酯(天然产品)、苄呋菊酯、七氟菊酯、胺菊酯、四溴菊酯;
4.脒类化合物有:双甲脒、杀虫脒;
5.含锡化合物有:三环锡、杀螨锡;
6.其它
aba mectin、苏云金杆菌、杀虫磺、乐杀螨、溴螨酯、噻嗪酮、毒杀芬、杀螟丹、乙酯杀螨醇、chlorfluazuron、2-((4-氯苯基)-4,5-二苯基噻吩(UBI-T 930)、四螨嗪、环丙烷甲酸(2-萘甲基)酯(RO12-0470)、灭蝇胺、N-(3,5-二氯代-4-(1,1,2,3,3,3-六氟代-1-丙氧基)苯基氨基甲酰基-2-氯苯并羰基亚氨酸乙酯(ethyl N-(3,5-dichloro-4-(1,1,2,3,3,3-hexafluoro-1-propyloxy)phenylcar-bamoyl-2-chlorobenzocarboximidate)、滴滴涕、三氯杀螨醇、氟铃脲、除虫脲、N-(2,3-二氢-3-甲基-1,3-噻唑-2-内鎓盐(式))-2,4-二甲代苯胺、消螨通、消螨普、硫丹、醚菊酯、(4-乙氧苯基)(二甲基)(3-(3-苯氧苯基)丙基)硅烷、(4-乙氧苯基)(3-(4-氟-3-苯氧苯基)丙基)二甲基硅烷、双氧威、2-氟-5-(4-(4-乙氧苯基)-4-甲基-1-戊基)联苯基醚(MTI 800)、颗粒体和核多角体病毒、苯硫威、氟螨噻、flucycloxuron、flufenoxuron、氟虫脲、林丹、噻螨酮、伏蚁腙、ivermectin、2-硝基甲基-4,5-二氢-6H-噻嗪(SD 52618)、2-硝基甲基-3,4-二氢噻唑(SD 35651)、噻虫醛、克螨特、teflubenzuron、三氯杀螨砜、杀螨硫醚、杀虫环、triflumuron。
从市售制剂制得的施用形态中,有效成分含量可从0.00000001-100%不等,推荐含量为0.00001-1%。
可采用一种通常的、适应药剂施用形态的方式施药。
本发明的有效成分也宜于对付体内寄生物和体外寄生物,优选用于兽药领域或畜牧业中。
本发明的有效成分以通常方式施用,如以片剂、胶囊、水剂或粒剂等方式口服,或通过(如)浸渍、喷洒、浇淋和点样以及喷粉等方式经表皮给药,也可通过如注射等非肠道方式给药。
依照本发明的式Ⅰ所示新化合物尤其适用于家畜饲养业(如牛、羊、猪和家禽如鸡、鹅等)。在本发明的一个推荐的实施方案中,以适宜的制剂形式(见上)存在的新化合物适当地与饮水或饲料一起,经口服向动物给药。因为药剂随动物粪便排泄,故以这种方式就非常完全地防止了昆虫在粪便中发育。适合于这种给药方式的剂量和剂型主要取决于受药动物的类型和发育阶段,也取决于昆虫的危害程度,这可用通常的方法方便地确定。对牛而言,新化合物可用的剂量为0.01-1毫克/千克体重。
以下实施例供进一步阐述本发明。
A.制剂实施例
a)用下法制得一种粉剂:将10份(重量,以下同)有效成分与90份作为惰性材料的滑石混合,并在一个锤磨机中将该混合物弄成粉末。
b)易于分散于水中的一种可湿性粉剂可如下得到:把25份有效成分、65份用作惰性材料的不含石英的高岭土、10份木素磺酸钾和1份油酰甲基牛磺酸钠(这两种材料用作湿润剂和分散剂)混合,在一个气流粉碎机中研磨该混合物。
c)易于分散于水中前一种悬浮剂可如下制得:将以下成分混合起来并在一个球磨机中研磨至粒度小于5微米,这些成分是40份有效成分、7份琥珀磺基单酯、2份木素磺酸钠和51份水。
d)用以下物质可制得乳油:15份有效成分、75份环己酮作为溶剂、10份乙氧基化的壬基酚(10 EO)作为乳化剂。
e)由2至15份有效成分和惰性粒状载体如阿泰土(attapulgite)、成粒了的浮石和/或石英砂可制成粒剂。
较为有利的作法是,用实施例b)中制得的可湿性粉剂配成一个悬浮液,使其固体含量为30%,把该悬浮液喷洒在阿泰土粒子表面上,使之干燥并充分混和。成品粒剂中,可湿性粉剂的比例约为5%,惰性载体则占约95%。
B.化学实施例
1,1,1-三氟-2-(2-乙氧基-5-吡啶基)-5-(3-苯氧基-苯基)-2-戊烯(式Ⅰ之实例,序号1)
在一种保护性气体的气氛中,把以下三种物质混合在50ml甲苯中,并在20℃下搅拌该混合物6小时,它们是:2.5g(11.4mmol)2-乙氧基-5-三氟乙酰吡啶、6.0g(10.8mmol)溴化3-(3-苯氧苯基)-丙基三苯基鏻和1.3g(12.0mmol)叔丁醇钾。然后用冰洗去溴化钾,对甲苯相进行浓缩,并用柱层析法分离该甲苯萃取物(洗脱剂:乙酸乙酯∶庚烷=2∶8;固定相为硅胶)。由此得到3.50g(75%)无色液态烯烃产物(l)。溶解性:易于溶于庚烷和甲苯中;不溶于水。1H-NMR和GC测试结果表明,产物为非对映体(Z和E异构体)的混合物,其比例为2.3∶1,沸点(b.P.1013)为386和395℃(GC测得)
1,1,1-三氟-2-(2-乙氧基-5-吡啶基)-5-(3-苯氧苯基)-戊烷(式Ⅱ之实例,序号61)
在氢气氛下,把1.0g载在活性炭上的铂快速加到100ml乙醇中,随后再加入5.0g(12.1mmol)烯烃(l)。在1013 mbar下保持4小时之后,用硅胶过滤该反应混合物,并进行浓缩,得到序号为61的化合物,为一种无色液体,产量4.9g(97%)。
以下所列出的式Ⅰ和式Ⅱ化合物皆可按类似上述过程制得。
缩写:Me=CH3,Et=C2H5,pH=C6H5,iPr=CH(CH3)2
表1
表1(续)
表1(续)
1H-NMR数据(洗脱剂:CDCl3,标准物:TMS,δ(H)[ppm])
4 E-烯烃:1,39,t(3H,OCH2CH3,7.3Hz);2,30,m(2H,CH2CH2CH);2,61,t(2H,ArCH2CH2,7.0Hz);4,36,q,(2H,OCH2CH3,7.3Hz);6,42tq(1H,C=CHCH2,1.7Hz,7.5Hz);6,68-7,35,m(10H;芳基-H);7,86,d(1H,6-吡啶基-H,2.5Hz).
Z-烯烃:1,38,t(3H,OCH2CH3,7.3Hz);2,73,m(4H,ArCH2CH2CH);4,36,q(2H,OCH2CH3,7.3Hz);5,95,tq(1H,C=CHCH2,6,9Hz);6,65,d(1H,3-吡啶基-H,8.8Hz);6,85-7,48,m(10H,芳基-H);7,99,d(1H,6-吡啶基-H,2.5Hz).
表2
表2(续)
表2(续)
表2(续)
实施例 R1R2R3A R4B R5R6物理数据
序 号 (b.p.℃,GC)
1013 mbar
105 ″ CH3H CH2H CH F OPh
106 ″ CH3H O H CH F OPh
1H-NMR数据(洗脱剂:CDCl3,标准物:TMS,δ(H)[ppm])
实施例序号75
1,48,m(2H,CH2);1,90,m(2H,CH2);2,56,t(ArCH2,2H,7.3Hz);3,19,m(1H,F3CCH);4,73,q(2H,OCH2CF3,8,6Hz);6,75-7,36,m(10H,芳基-H);7,51,dd(1H,4-吡啶基-H,8.8Hz,2.5Hz);7,98,d(1H,6-吡啶基-H,2.5Hz).
C.生物学实例
例1
把一个陪氏培养皿的盖子和底部的内侧均匀地涂上1ml待试验的制剂(已在水中乳化),待涂层干了后,把每批10个普通家蝇成虫放入皿中。把试验皿封口,放入室温下保持3小时,然后确定供试动物的死亡率。在250ppm的浓度下(指有效成分含量),制品1、2、6、7、11、15、16、19、20、51、52、61、64、65、75和79显示了很好的杀死普通家蝇的药效(100%死亡)。
例2
把数批(每批10只)德国小蠊的若虫放入经例1中处理过的陪氏培养皿中,封住这些皿,5天后确定这些供试动物的死亡率。在100ppm的浓度下(指有效成分含量),制品1、2、11、15、20、61、65、75和79显示了对德国小蠊很好的药效(100%死亡)。
例3
在每个陪氏培养皿底部内侧放入一层滤纸,其上再涂敷3-5ml的半合成饲料。把待试制剂用水配成递降浓度的药液,冷却后,喷洒在饲料及滤纸的表面上,待喷洒涂层已干了时,放入数批(每批10只)斜纹夜蛾幼虫(L3-L4)。把这些皿用盖子封口,在室温下保持7天,然后确定供试动物的死亡率。
在1000ppm下(指有效成分含量),制品1、2、6、15、16、19、61、64、65、75和79对斜纹夜蛾幼虫显示了很好的药效。
例4
在喷雾装置中用指定浓度的有效成分的药液处理墨西哥豆瓢虫。同时,把矮生菜豆叶浸到同样的有效成分药液中,待喷洒涂层干了后,把这些幼虫接到叶子上。3天后,确定这些幼虫的死亡率(用百分数表示),在1000ppm下(指有效成分浓度),制品第1、2、6、7、15、16、19、20、51、52、61、64、65、75和79号对墨西哥豆瓢虫显示了很好的药效。
例5
每个陪氏培养皿底部放入一张处理过的吸水纸,再把一些稻褐飞虱的老熟若虫放入这些皿中。在放入供试动物前,每张滤纸用2ml蒸馏水弄湿,随后喷洒上递降剂量的待试药剂,这些药剂已按600l/ha的用水量配成乳液。装入动物后,立即封住这些皿,并在实验条件下保持24小时。此后,评价药效(%死亡率)。当有效成分在喷洒液中的浓度为125ppm时,制品第1、6、51、64、65和79号对稻褐飞虱显示了100%的药效。
例6
对被豆蚜严重侵害的蚕豆喷以可湿性粉剂的水稀释液(有效成分含量为100ppm)直至达到滴流点。3天后检查蚜虫的死亡率,用实施例1、15、16、19、61、64、65、75和79的化合物制品可达到100%的死亡率。
Claims (8)
1、制备式I和Ⅱ化合物,它们的立体异构体、以及立体异构体混合物的方法
其中,
R1代表吡啶基、嘧啶基、噻吩基、噻唑基、呋喃基、吡唑基、1,3-噁唑基或咪唑基,所有这些基团可以是取代了的;
R2代表H、(1-4)碳烷基、(1-4)碳卤代烷基、(1-4)碳烷氧基、(3-6)碳环烷基、-CH=CH2或-C≡CH;
R3代表H或(1-4)碳烷基;
R4代表H、(1-4)碳烷基、-CN或-C≡CH;
A代表CH2、O或S;
B代表CH、N或C(CH3);
R5代表H或卤素,
R6代表苯基、苯氧基或苄基,它们都可以被卤素或(1-3)碳卤代烷基取代1-5位。
该方法包括:
a)对式I化合物而言,使式Ⅲ化合物
其中R1和R2同式I中的定义,与式Ⅳ的一种化合物反应
其中R3、R4、R5、R6、A和B同式I中的定义,R8为苯基、环己基或丁基,以及
b)对式Ⅱ化合物而言,用氢或一种释氢化合物来对对应的式I化合物上的烯烃官能团进行催化加氢。
3、如权利要求1或2要求的方法,其中,式Ⅰ和Ⅱ中的R7是Cl、Br、(1-3)碳烷氧基、-OCF3、-OCH2CF3或-OCF2H。
4、如权利要求1-3中任意一个所要求的方法,其中,式Ⅰ和Ⅱ中的R1是式A、B或C所示的一个基团。
5、如权利要求1-4中任意一个所要求的方法,其中,式Ⅰ和Ⅱ中的R1是式A所示基团。
6、一种杀虫剂或杀螨剂,其中含有有效量的、如权利要求1-5中任意一个所要求的一种式Ⅰ和/或Ⅱ化合物。
7、权利要求1-5中任一个所要求的一种式Ⅰ和/或式Ⅱ化合物的应用,以用于控制害虫或害螨。
8、控制害虫或害螨的一种方法,其作法包括:向这些害虫或害螨、或者是被它们侵害的植物、大田或底物上施用有效量的如权利要求1-5中任意一个所要求的式Ⅰ和/或式Ⅱ化合物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3907784A DE3907784A1 (de) | 1989-03-10 | 1989-03-10 | Heteroaryl-aryl-buten- und -butanderivate, verfahren zu ihrer herstellung, sie enthaltende mittel und ihre verwendung als schaedlingsbekaempfungsmittel |
| DEP3907784.5 | 1989-03-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1046156A true CN1046156A (zh) | 1990-10-17 |
Family
ID=6376018
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN90101246A Pending CN1046156A (zh) | 1989-03-10 | 1990-03-09 | 杂芳基-芳基-丁烯衍生物和杂芳基-芳基-丁烷衍生物制备它们的方法含有它们的制剂以及它们作为农药的应用 |
Country Status (12)
| Country | Link |
|---|---|
| EP (1) | EP0386715B1 (zh) |
| JP (1) | JPH0774203B2 (zh) |
| KR (1) | KR900014319A (zh) |
| CN (1) | CN1046156A (zh) |
| AT (1) | ATE118480T1 (zh) |
| AU (1) | AU622292B2 (zh) |
| CA (1) | CA2011832A1 (zh) |
| DE (2) | DE3907784A1 (zh) |
| ES (1) | ES2070200T3 (zh) |
| HU (1) | HUT53082A (zh) |
| IL (1) | IL93688A0 (zh) |
| ZA (1) | ZA901846B (zh) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103214413A (zh) * | 2013-03-22 | 2013-07-24 | 郑州泰基鸿诺药物科技有限公司 | 一种含杂环的三氟甲基酮化合物及其制备方法 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3810379A1 (de) * | 1988-03-26 | 1989-10-12 | Hoechst Ag | Azaneophyl- und silazaneophylsulfide, verfahren zu ihrer herstellung, sie enthaltende mittel und ihre verwendung als schaedlingsbekaempfungsmittel |
| NZ597375A (en) | 2009-07-08 | 2013-08-30 | Dermira Canada Inc | Tofa analogs useful in treating dermatological disorders or conditions |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8520027D0 (en) * | 1985-08-09 | 1985-09-18 | Ici Plc | Insecticidal ethers |
| GB2187452A (en) * | 1986-03-04 | 1987-09-09 | Ici Plc | Insecticidal fluoroalkyl benzene derivatives |
| US4840971A (en) * | 1986-04-07 | 1989-06-20 | Sumitomo Chemical Company, Limited | Novel ether compound, a process for manufacturing the same, a composition containing the same and a use thereof |
| DE3780291T2 (de) * | 1987-01-08 | 1993-01-07 | Ici Plc | Insektizide aether. |
| DE3712752A1 (de) * | 1987-04-15 | 1988-11-03 | Hoechst Ag | Heterocyclische neophananaloga, verfahren zu ihrer herstellung und ihre verwendung als schaedlingsbekaempfungsmittel |
| US4808762A (en) * | 1987-04-23 | 1989-02-28 | Fmc Corporation | Insecticidal cyclopropyl-substituted di(aryl) compounds |
| BR8807472A (pt) * | 1987-04-23 | 1990-03-27 | Fmc Corp | Composto,composicao inseticida,processo de controle de insetos e acarideos e processo para preparacao de um composto |
-
1989
- 1989-03-10 DE DE3907784A patent/DE3907784A1/de not_active Withdrawn
-
1990
- 1990-03-07 ES ES90104312T patent/ES2070200T3/es not_active Expired - Lifetime
- 1990-03-07 DE DE59008451T patent/DE59008451D1/de not_active Expired - Fee Related
- 1990-03-07 AT AT90104312T patent/ATE118480T1/de not_active IP Right Cessation
- 1990-03-07 EP EP90104312A patent/EP0386715B1/de not_active Expired - Lifetime
- 1990-03-08 IL IL93688A patent/IL93688A0/xx not_active IP Right Cessation
- 1990-03-09 HU HU901378A patent/HUT53082A/hu unknown
- 1990-03-09 KR KR1019900003116A patent/KR900014319A/ko not_active Application Discontinuation
- 1990-03-09 AU AU51180/90A patent/AU622292B2/en not_active Ceased
- 1990-03-09 CA CA002011832A patent/CA2011832A1/en not_active Abandoned
- 1990-03-09 JP JP2056770A patent/JPH0774203B2/ja not_active Expired - Lifetime
- 1990-03-09 CN CN90101246A patent/CN1046156A/zh active Pending
- 1990-03-09 ZA ZA901846A patent/ZA901846B/xx unknown
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103214413A (zh) * | 2013-03-22 | 2013-07-24 | 郑州泰基鸿诺药物科技有限公司 | 一种含杂环的三氟甲基酮化合物及其制备方法 |
| CN103214413B (zh) * | 2013-03-22 | 2015-05-13 | 郑州泰基鸿诺药物科技有限公司 | 一种含杂环的三氟甲基酮化合物及其制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU5118090A (en) | 1990-09-13 |
| HU901378D0 (en) | 1990-05-28 |
| EP0386715A2 (de) | 1990-09-12 |
| ATE118480T1 (de) | 1995-03-15 |
| AU622292B2 (en) | 1992-04-02 |
| JPH02289547A (ja) | 1990-11-29 |
| ZA901846B (en) | 1990-11-28 |
| DE59008451D1 (de) | 1995-03-23 |
| CA2011832A1 (en) | 1990-09-10 |
| EP0386715B1 (de) | 1995-02-15 |
| JPH0774203B2 (ja) | 1995-08-09 |
| DE3907784A1 (de) | 1990-09-13 |
| HUT53082A (en) | 1990-09-28 |
| ES2070200T3 (es) | 1995-06-01 |
| KR900014319A (ko) | 1990-10-23 |
| IL93688A0 (en) | 1990-12-23 |
| EP0386715A3 (de) | 1991-12-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2619606B2 (ja) | 置換ピリミジン類,それらの製造方法およびそれらの害虫防除剤および殺菌剤としての用途 | |
| JP2875630B2 (ja) | 縮合窒素含有複素環および殺虫剤、殺菌剤および抗眞菌剤としてのその用途 | |
| CN1082960C (zh) | 取代的吡啶基吡唑类化合物 | |
| CN1044661C (zh) | 含新型硅烷衍生物的农药,杀虫,杀螨组合物 | |
| CN1067069C (zh) | 作为杀虫剂的双环胺 | |
| JP3046354B2 (ja) | 置換されたピリジン、それらの製造方法、及びそれらを農薬及び殺菌剤として使用する方法 | |
| JP2931407B2 (ja) | 殺ダニ性,殺虫性および殺線虫性の置換された(ヘテロ)アリールアルキルケトンオキシムo−エーテル,それらの製造方法,それらを含む薬剤,およびそれらの有害生物防除剤としての使用 | |
| CS268533B2 (en) | Insecticide and acaricide and method of efficient substances production | |
| CN1148383A (zh) | 杂环基氨基-及杂环基氧基-环烷基衍生物,其制备方法和作为农药与杀真菌剂的应用 | |
| JPH09502966A (ja) | 置換ピリジンおよびピリミジン、それらの製造方法および殺害虫剤および殺菌剤としてのそれらの用途 | |
| KR20010033455A (ko) | 2,2-디메틸 3-(2-플루오로 비닐)사이클로프로판 카복실산유도체, 그의 제조방법 및 살충제로서의 그의 용도 | |
| CN1046156A (zh) | 杂芳基-芳基-丁烯衍生物和杂芳基-芳基-丁烷衍生物制备它们的方法含有它们的制剂以及它们作为农药的应用 | |
| CN1373636A (zh) | 防治稻类及其他农作物虫害的组合物和方法 | |
| JP3046355B2 (ja) | 置換ピリジン、その製造方法および殺害虫剤および殺菌剤としてのその用途 | |
| JPH021441A (ja) | N‐フエニルベンズアミドおよびn‐フエニルベンズアミドオキシム、それらの製造方法、それらを含有する剤およびそれらの農薬としての用途 | |
| JPS6377829A (ja) | フルオロ置換ベンジルアルコ−ル及びその殺虫性エステル及びその製造法 | |
| JPH02149535A (ja) | 殺虫、殺ダニ性化合物、その製造方法及びそれを含有する殺虫、殺ダニ組成物 | |
| AU612736B2 (en) | (Thio) Benzoylureas and functional derivatives thereof, processes for their preparation, agents containing them and their use as agents for combating pests | |
| CN1126200A (zh) | 磺酰胺衍生物 | |
| JP2002515007A (ja) | ヘテロシクリル−アミノ−およびヘテロシクリル−オキシ−シクロアルケニル誘導体、害虫防除剤および殺菌剤としてのそれらの使用 | |
| CN1130858A (zh) | 增效农药 | |
| KR820000772B1 (ko) | α-시아노-3-펜옥시벤질-S-(+)-2-(4-클로로페닐)이소발레르산의 제조방법 | |
| US20030008913A1 (en) | Derivatives of 2,2-dimethyl 3(2-fluoro vinyl) cyclopropane carboxylic acid, their preparation process and their use as pesticides | |
| DE19544100A1 (de) | Cyclohexylmethyl- und Cyclohexylidenmethyl-Pyridine, Verfahren zu ihrer Herstellung, diese enthaltende Mittel und ihre Verwendung als Schädlingsbekämpfungsmittel und Fungizide | |
| CN1189830A (zh) | 取代的1,3-二噁烷-5-基氨基-杂环化合物,其制备方法及其作为害虫防治组合物的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| AD01 | Patent right deemed abandoned | ||
| C20 | Patent right or utility model deemed to be abandoned or is abandoned |