Metana
Praèn
| Metana | |
|---|---|
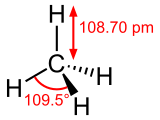
| |
 |
 |
Jeneng liya
| |
| Identifikasi | |
| Nomor CAS | [74-82-8] |
| PubChem | |
| Nomer EINECS | |
| KEGG | C01438 |
| MeSH | Methane |
| ChEBI | CHEBI:16183 |
| Nomer RTECS | PA1490000 |
| SMILES | C |
| Réferènsi Baeilstein | 1718732 |
| Réferènsi Gmelin | 59 |
| 3DMet | B01450 |
| Sifat | |
| Rumus kimia | CH4 |
| Massa molar | 16.04 g mol−1 |
| Panampilan | gas tanpa warna |
| Ambu | ora mambu |
| Densitas | 655.6 μg cm−3 |
| Titik leleh |
-187 °C, 86 K, -305 °F |
| Titik umob |
-162 °C, 111 K, -260 °F |
| Kelarutan dalam air | 35 mg dm−3 (at 17 °C) |
| log P | 1.09 |
| Termokimia | |
| Entalpi pambentukan standar (ΔfH |
−74.87 kJ mol−1 |
| Entalpi pangobongan standar ΔcH |
−891.1–−890.3 kJ mol−1 |
| Entropi molar standar S |
186.25 J K−1 mol−1 |
| Kapasitas panas, C | {{{HeactCapacity}}} |
| Bahaya | |
| Klasifikasi EU | |
| EU Index | 601-001-00-4 |
| NFPA 704 | |
| Frasa-R | Cithakan:R12 |
| Frasa-S | Cithakan:S2, Cithakan:S9, S16, Cithakan:S33 |
| Titik murub | −188 °C |
| Autoignition temperature |
537 °C |
| Explosive limits | 5–15% [3] |
| Senyawa kagandhèng | |
| alkana kagandhèng | Etana |
| Senyawa kagandhèng | Klorometana |
| Kejaba ditélakaké suwaliké, data ing ndhuwur kanggo ing suhu lan tekanan standar (25°C, 100 kPa) | |
Metana hidrokarbon paling prasaja sing wanguné gas kanthi rumus kimia CH4. Metana murni ora mambu, nanging yèn dipigunakaké kanggo kaperlon komersial, racaké ditambahaké sithik ambu welirang kanggo ndhétèksi kabocoran sing mungkin dumadi. Metana duwé sipat racun lan gampang kobong
Réferènsi
[besut | besut sumber]- ↑ a b "methane (CHEBI:16183)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 17 October 2009. Main. Dibukak ing 10 October 2011.
- ↑ a b Linstrom, P.J.; Mallard, W.G., èd. (2011). "Methane". NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology. Dibukak ing 4 December 2011.
- ↑ Matheson Tri-Gas (Dec 4, 2009). "Safety Data Sheet: Methane". Matheson Tri-Gas. Diarsip saka sing asli (PDF) ing 26 December 2018. Dibukak ing 4 December 2011.
Pranala njaba
[besut | besut sumber]| Wikimedia Commons duwé médhia ngenani Methane. |
- Gavin Schmidt, Methane: A Scientific Journey from Obscurity to Climate Super-Stardom Archived 2004-09-10 at the Wayback Machine., NASA Goddard, September 2004
- Methane thermodynamics
- International Chemical Safety Card 0291
- Methane Hydrates
- Safety data for methane Archived 2007-10-11 at the Wayback Machine.
- Methane-eating bug holds promise for cutting greenhouse gas Archived 2010-06-04 at the Wayback Machine.. Media Release, GNS Science, New Zealand]
- Catalytic conversion of methane to more useful chemicals and fuels Archived 2010-07-02 at the Wayback Machine.
- Methane as a Savior of the Dairy Industry Archived 2009-08-28 at the Wayback Machine.

