-
Notifications
You must be signed in to change notification settings - Fork 3
TransPlot 0.0.3 documentation
here I introduced bedVis and trackVis functions to visualize peak files and bigwig files which work better with trancriptVis. You can use these functions to make a complex track plot with more ajustable parameters to control you graph produced.
Note:
all the test files can be downloaded from:
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE211475
# install.packages("devtools")
devtools::install_github("junjunlab/transPlotR")bedVis function can be used to visualize peaks data (bed format).
bed files:
file <- list.files(pattern = '.bed')
file
# [1] "H524-NeuroD1_peaks.bed" "H524-Promoter_peaks.bed" "H82-NeuroD1_peaks.bed"
# [4] "H82-Promoter_peaks.bed"choose the region and chromesome to be plotted:
# plot
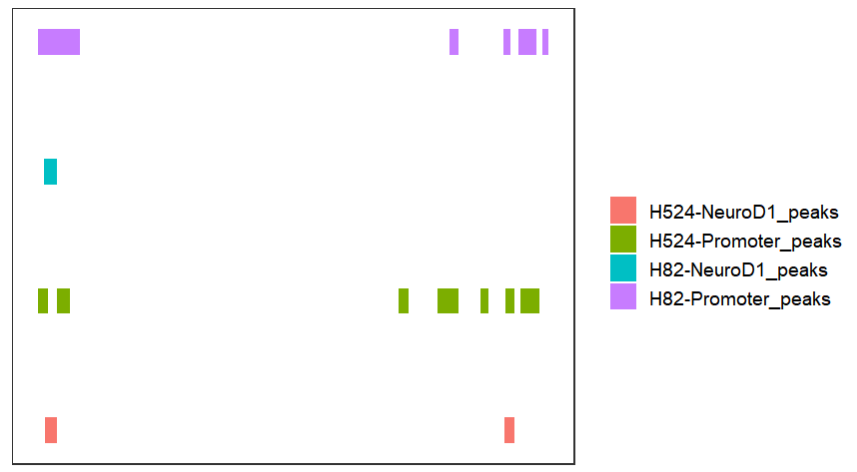
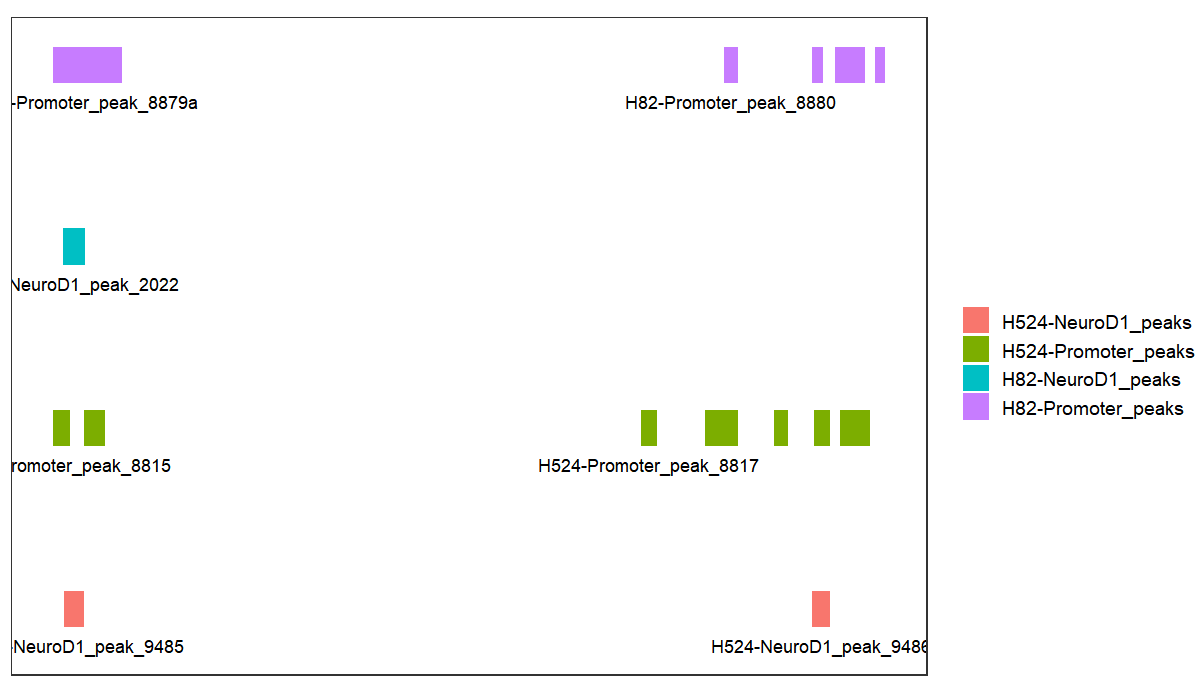
bedVis(bdFile = file,
chr = "chr19",
region.min = 39875973,
region.max = 39919857)collapse the tracks:
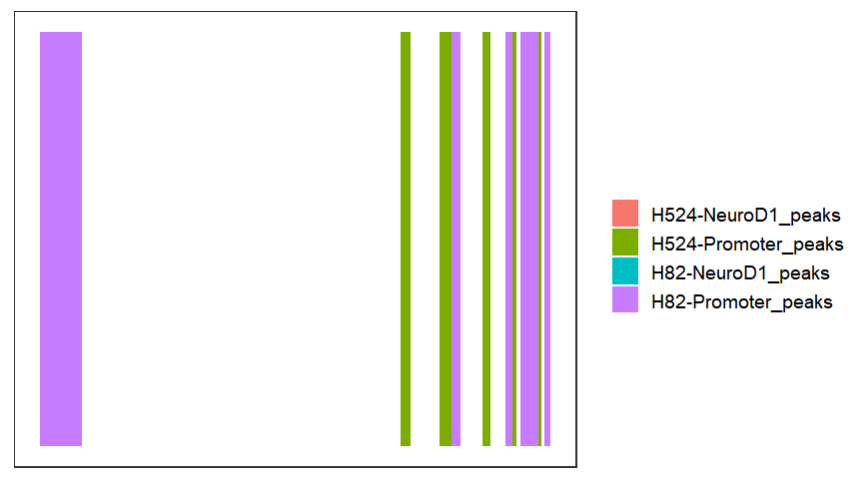
# collapse tracks
bedVis(bdFile = file,
chr = "chr19",
region.min = 39875973,
region.max = 39919857,
collapse = TRUE)change color:
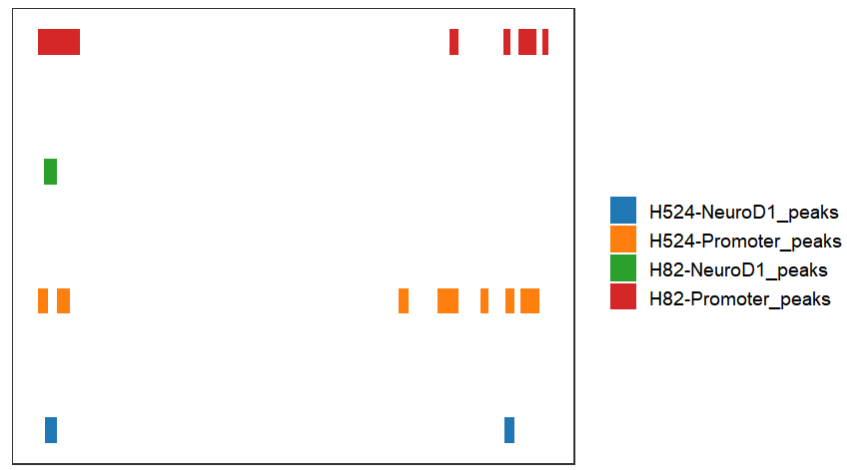
# change colors
bedVis(bdFile = file,
chr = "chr19",
region.min = 39875973,
region.max = 39919857,
fill = ggsci::pal_d3()(4))remove legend:
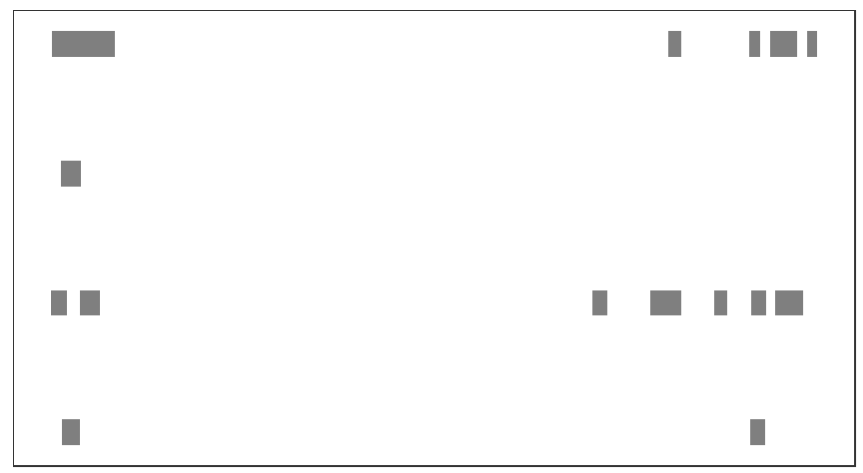
# change to grey50 and turn off legend
bedVis(bdFile = file,
chr = "chr19",
region.min = 39875973,
region.max = 39919857,
fill = rep('grey50',4),
show.legend = FALSE)add peak name:
# add label
bd <-
bedVis(bdFile = file,
chr = "chr19",
region.min = 39875973,
region.max = 39919857,
add.label = TRUE,label.column = 'name')
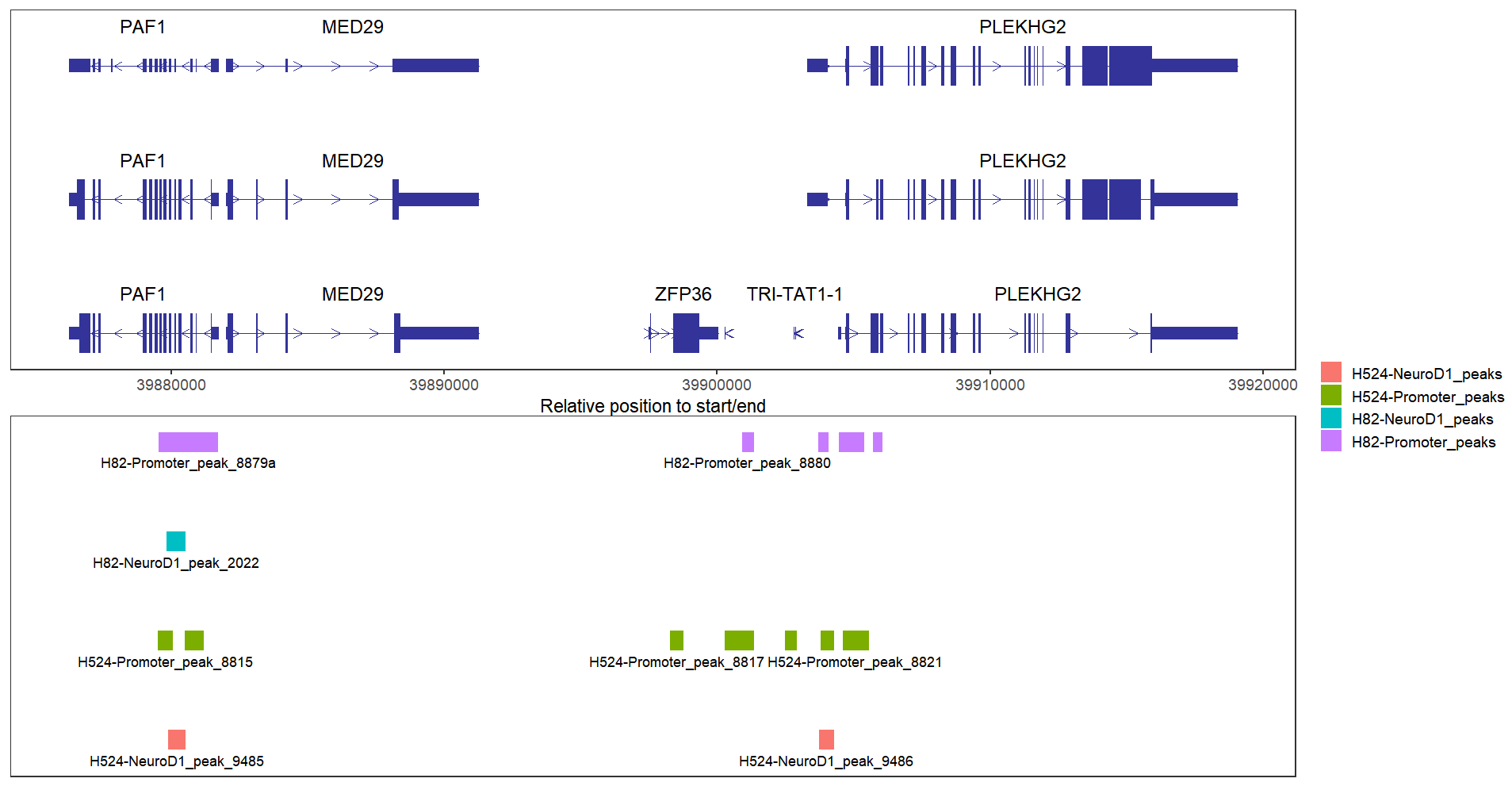
bdyou can combine with trancriptVis function:
# combine
gtf <- import('hg19.ncbiRefSeq.gtf',format = "gtf") %>%
data.frame()
p <-
trancriptVis(gtfFile = gtf,
Chr = "chr19",
posStart = 39875973,
posEnd = 39919857,
textLabel = "gene_name")
# combine
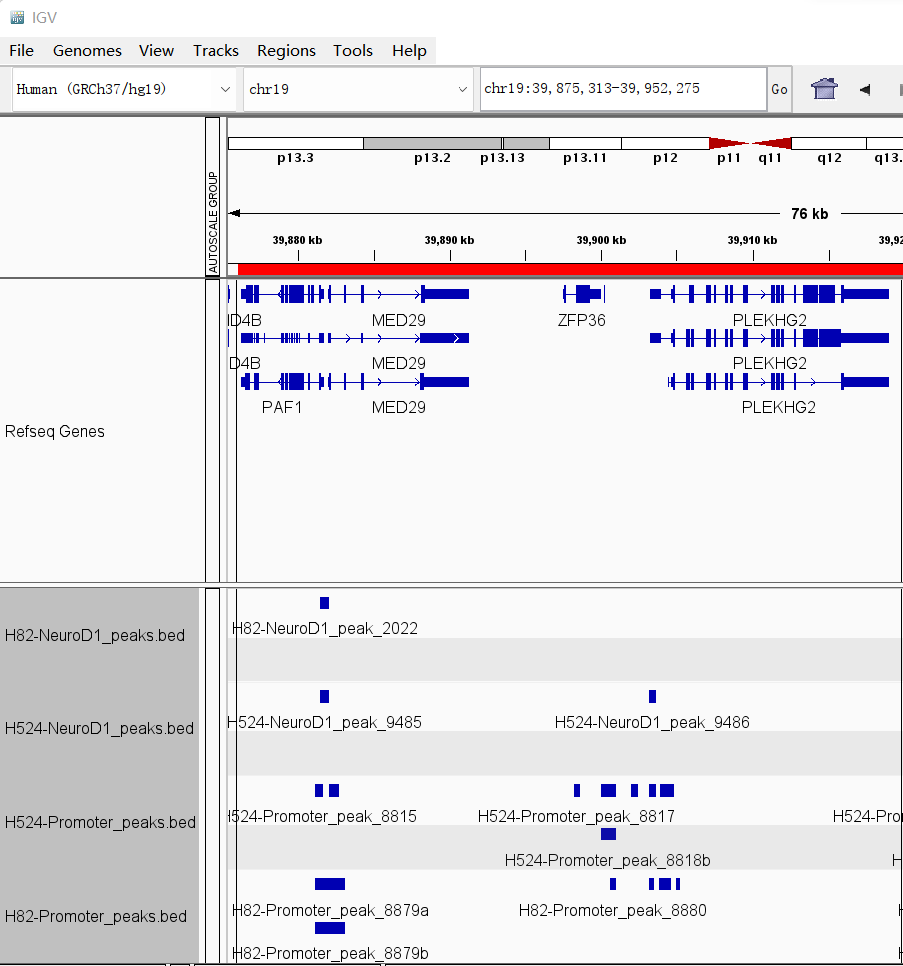
p %>% insert_bottom(bd)here we can show the results in IGV:
loadBigWig function will load bigwig files and transform them into data.frame format.
# test
file <- list.files(pattern = '.bw')
# [1] "H524-Input.bw" "H524-NeuroD1.bw" "H524-Promoter.bw" "H82-Input.bw" "H82-NeuroD1.bw"
# [6] "H82-Promoter.bw"
# read file
mybw <- loadBigWig(file)
# check
head(mybw,3)
# seqnames start end width strand score fileName
# 1 chr19 74845 74945 101 * 1 H524-Input
# 2 chr19 75000 75100 101 * 1 H524-Input
# 3 chr19 80776 80876 101 * 1 H524-InputtrackVis function can be used to visualize bigwig files in an elegant way. The trackVis will extend 3000bps upstream and downstream by defalut. You can set the extend.up/extend.dn to ajust a suitable value.
plot a region:
# load data
load('bWData.Rda')
mybw <- bWData
# plot with specific region
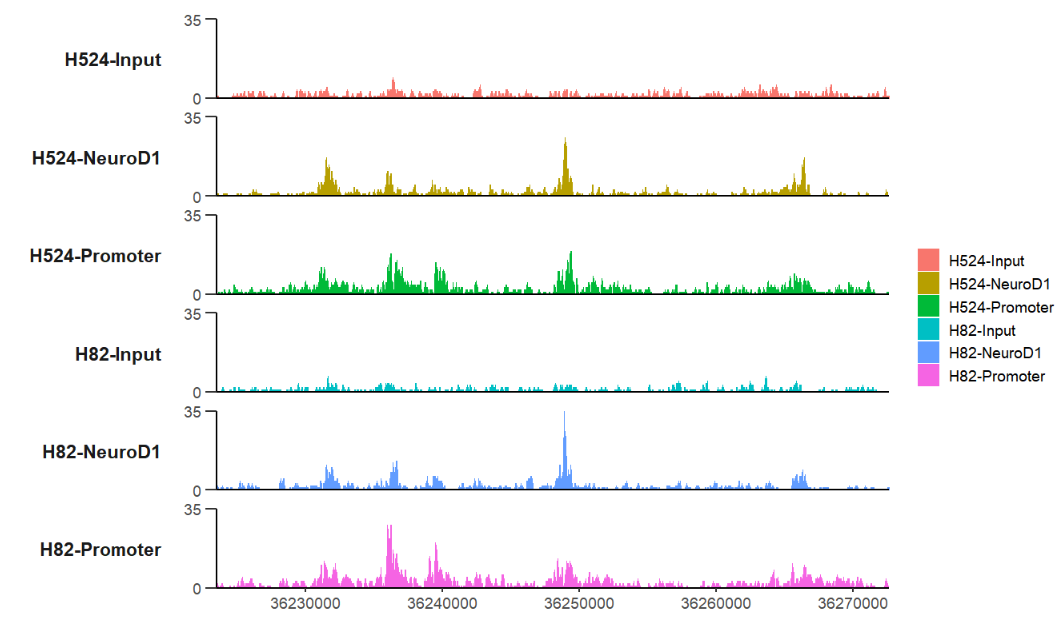
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
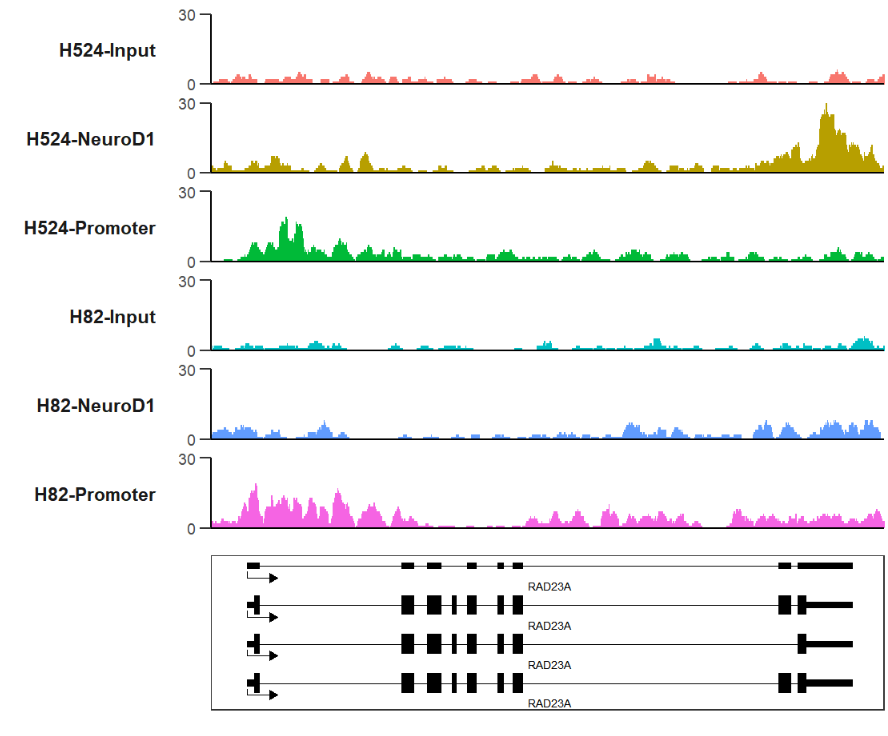
region.max = 36269673)plot a specific gene:
# load gtf
gtf <- import('hg19.ncbiRefSeq.gtf',format = "gtf") %>%
data.frame()
# plot with specific gene
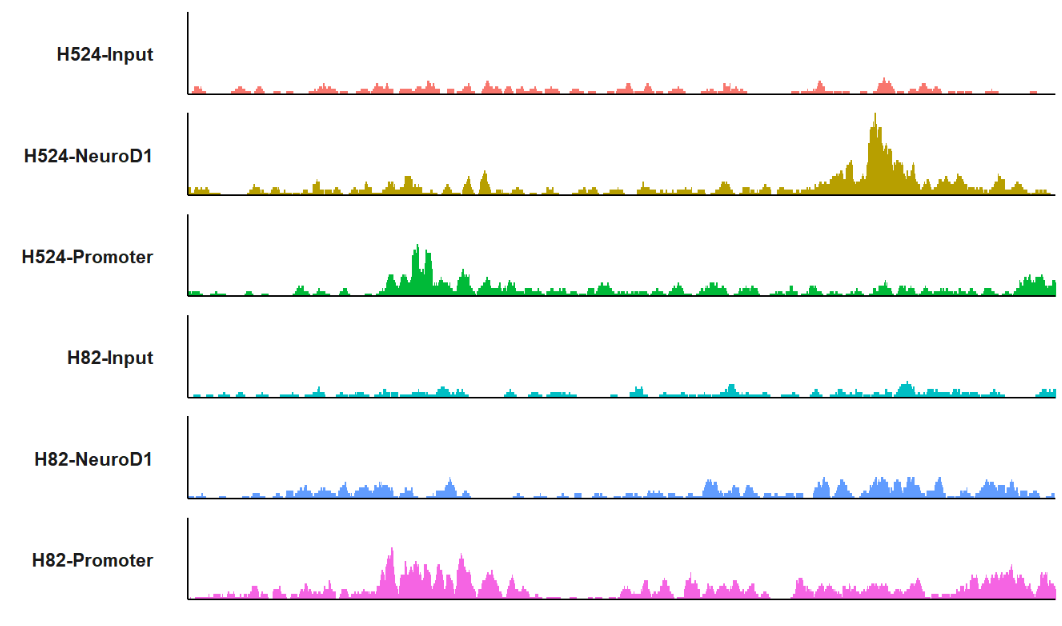
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A")here we show the same results in IGV:
show the color legend:
# show legend
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
show.legend = TRUE)remove Y axis information:
# remove axis info
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
xAxis.info = FALSE,
yAxis.info = FALSE)change a theme:
# change theme
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
yAxis.info = FALSE,
theme = "bw")change scales and layout:
# free scales and draw two columns
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
scales = "free",
ncol = 2,
label.angle = 90)change track colors:
# change color
c1 <- trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
color = ggsci::pal_npg()(6))
c2 <- trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
color = rep('grey20',6))
c3 <- trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
color = jjAnno::useMyCol(platte = "stallion",n = 6))
# combine
cowplot::plot_grid(plotlist = list(c1,c2,c3),
align = 'hv',ncol = 3)sometimes we want to highlight some regions like peak site or modification site et. trackVis can also achive this. You just need to supply a list object include
startandendcoordinates.
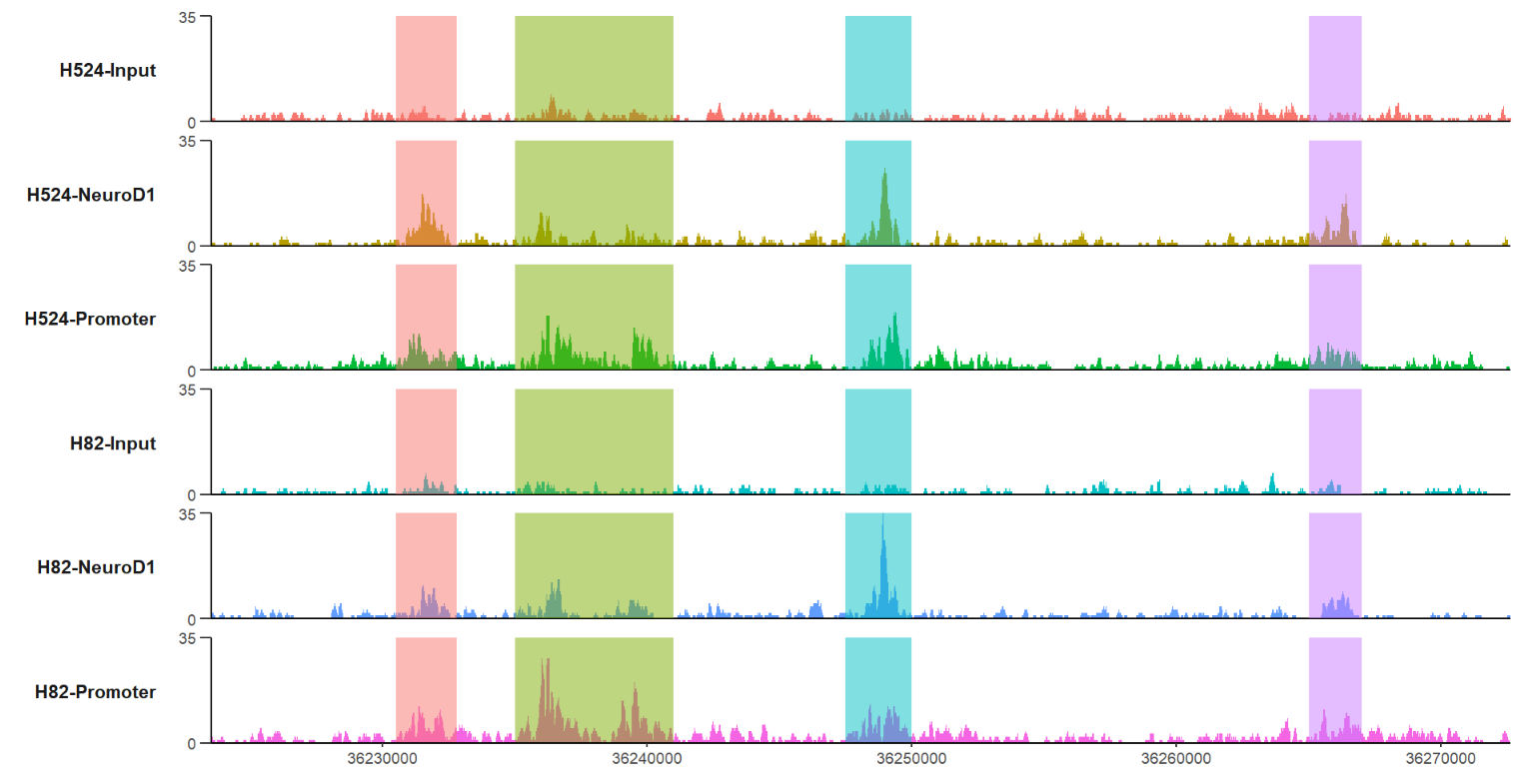
mark three regions:
# mark some regions
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
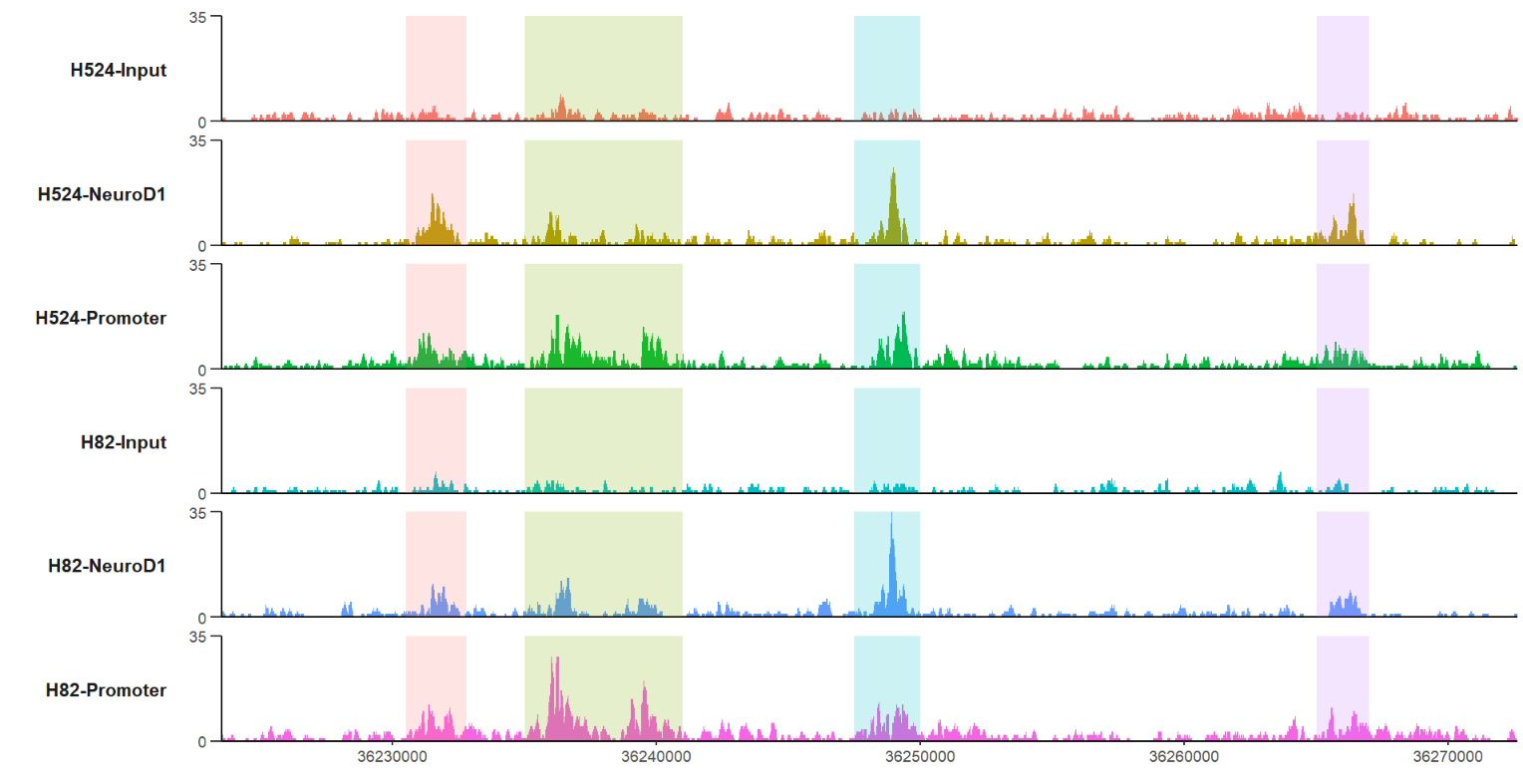
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)))ajust the color alpha:
# change alpha
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)),
mark.alpha = 0.2)changing the marked regions color also is acceptable:
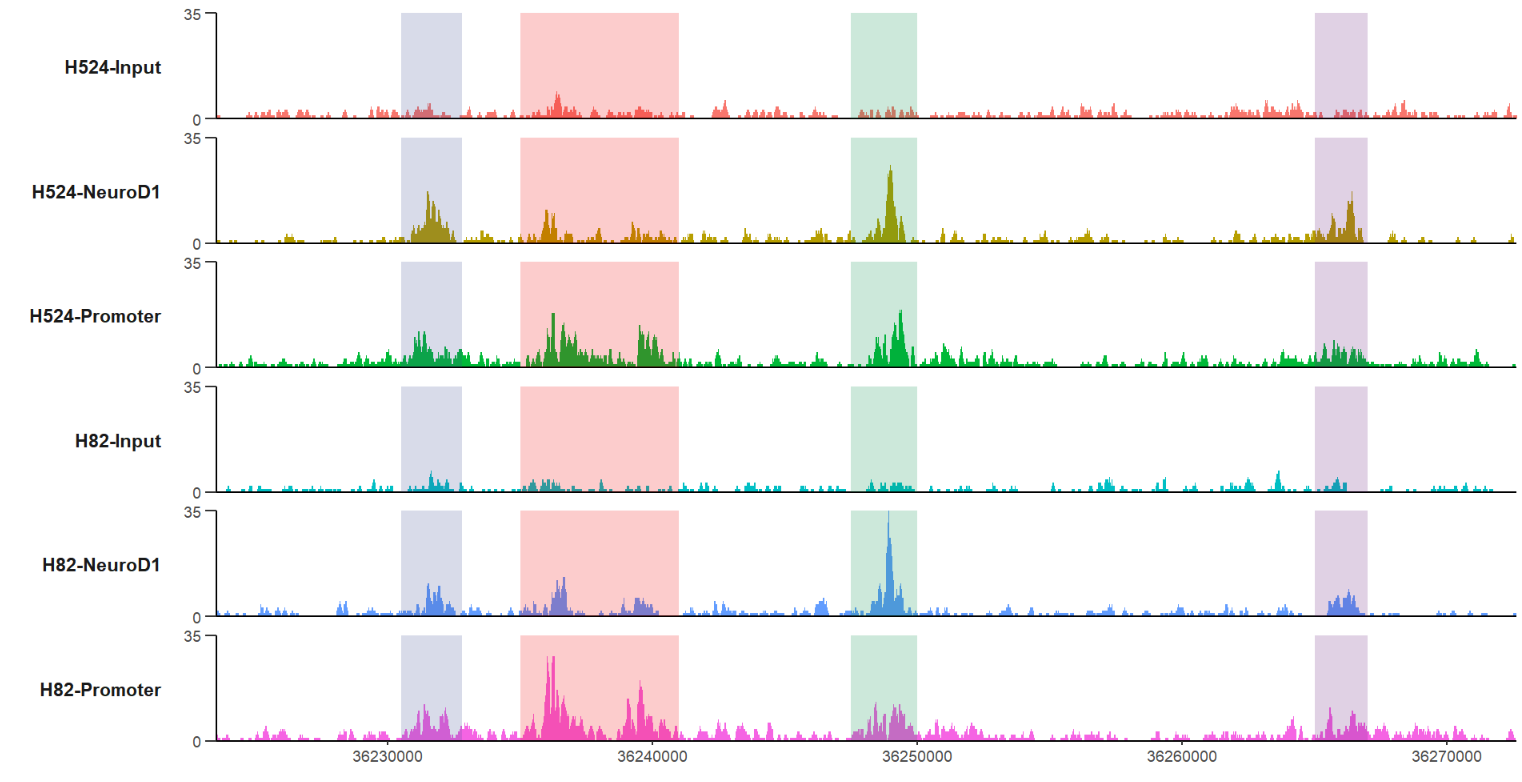
# change color
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)),
mark.alpha = 0.2,
mark.col = ggsci::pal_aaas()(4))change a theme:
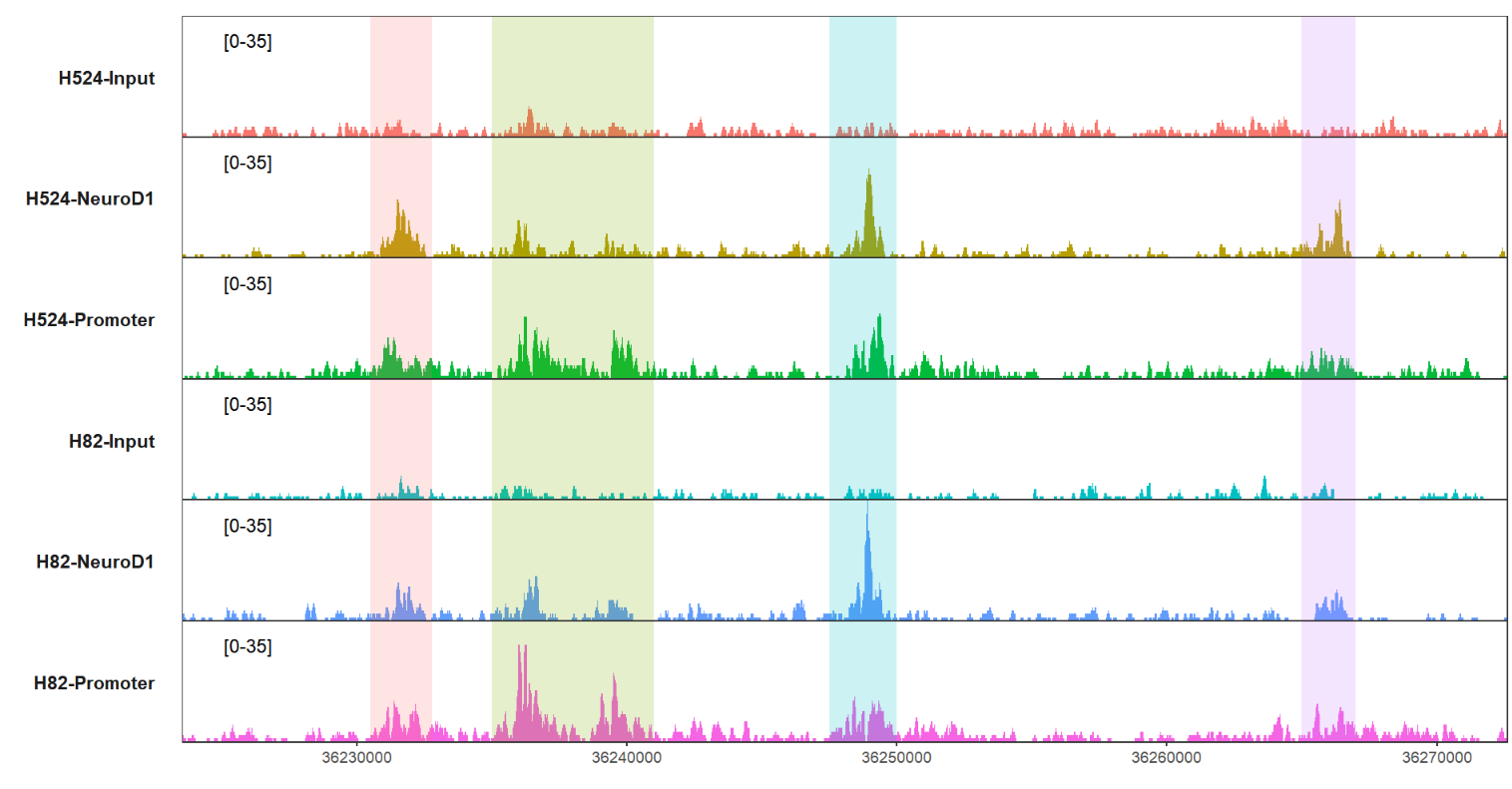
# change theme
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)),
mark.alpha = 0.2,
theme = "bw",yAxis.info = FALSE)giving a gene name to plot with marked regions:
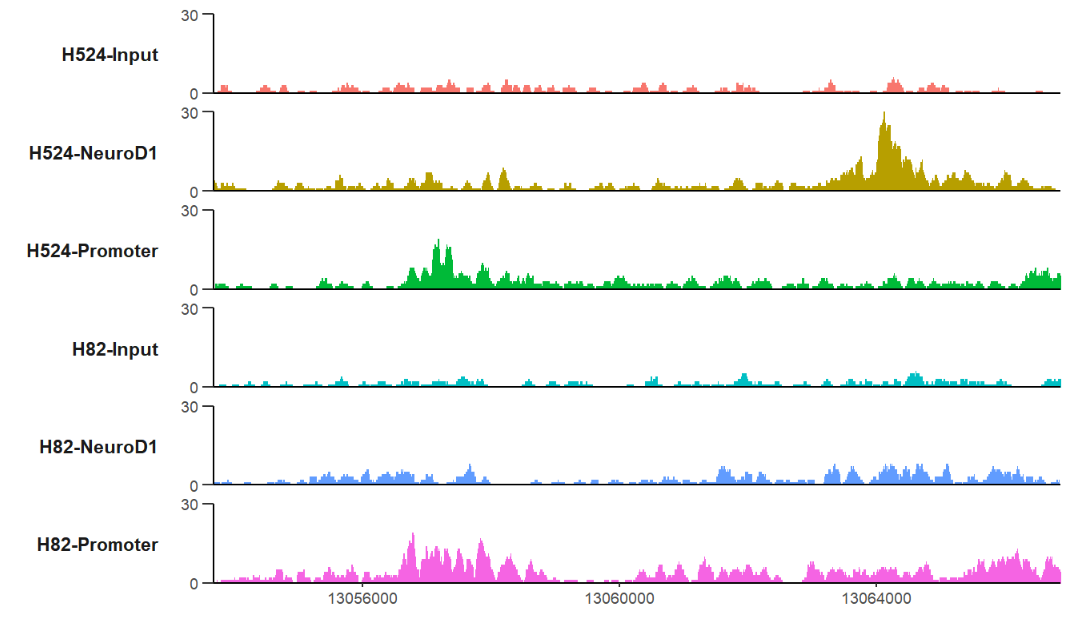
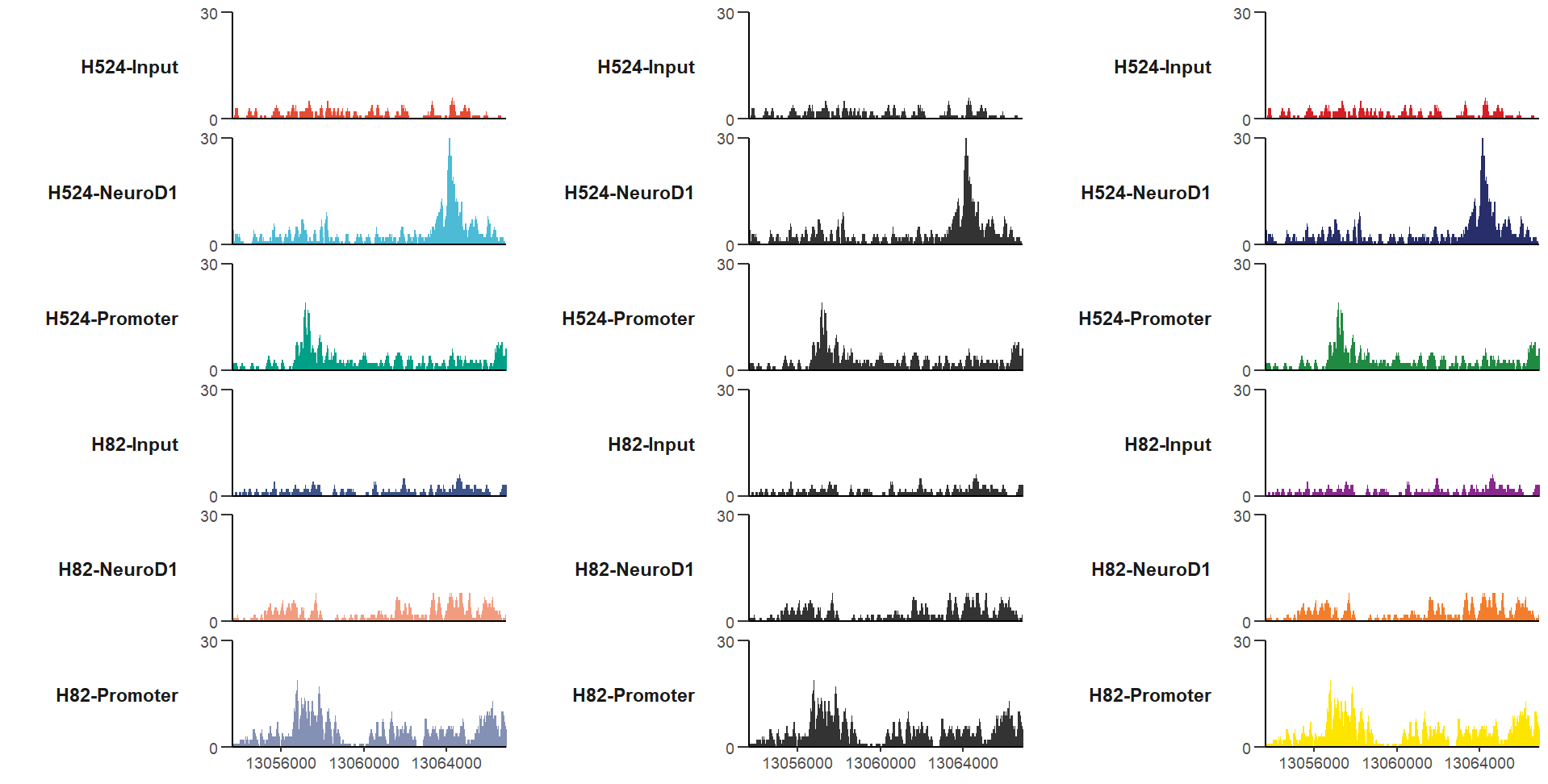
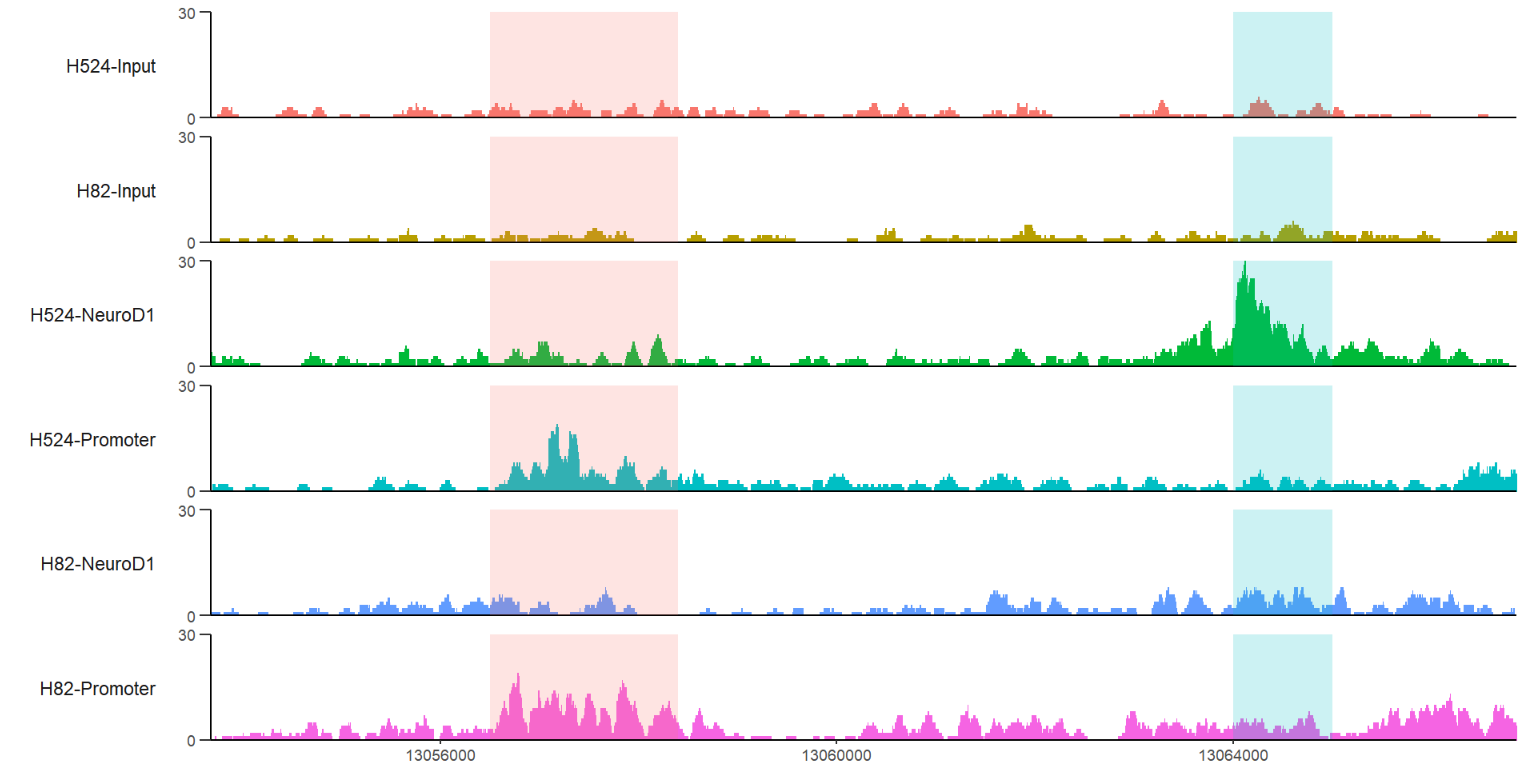
# define gene name with mark region
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
mark.region = list(c(13056500,13064000),
c(13058400,13065000)),
mark.alpha = 0.2,
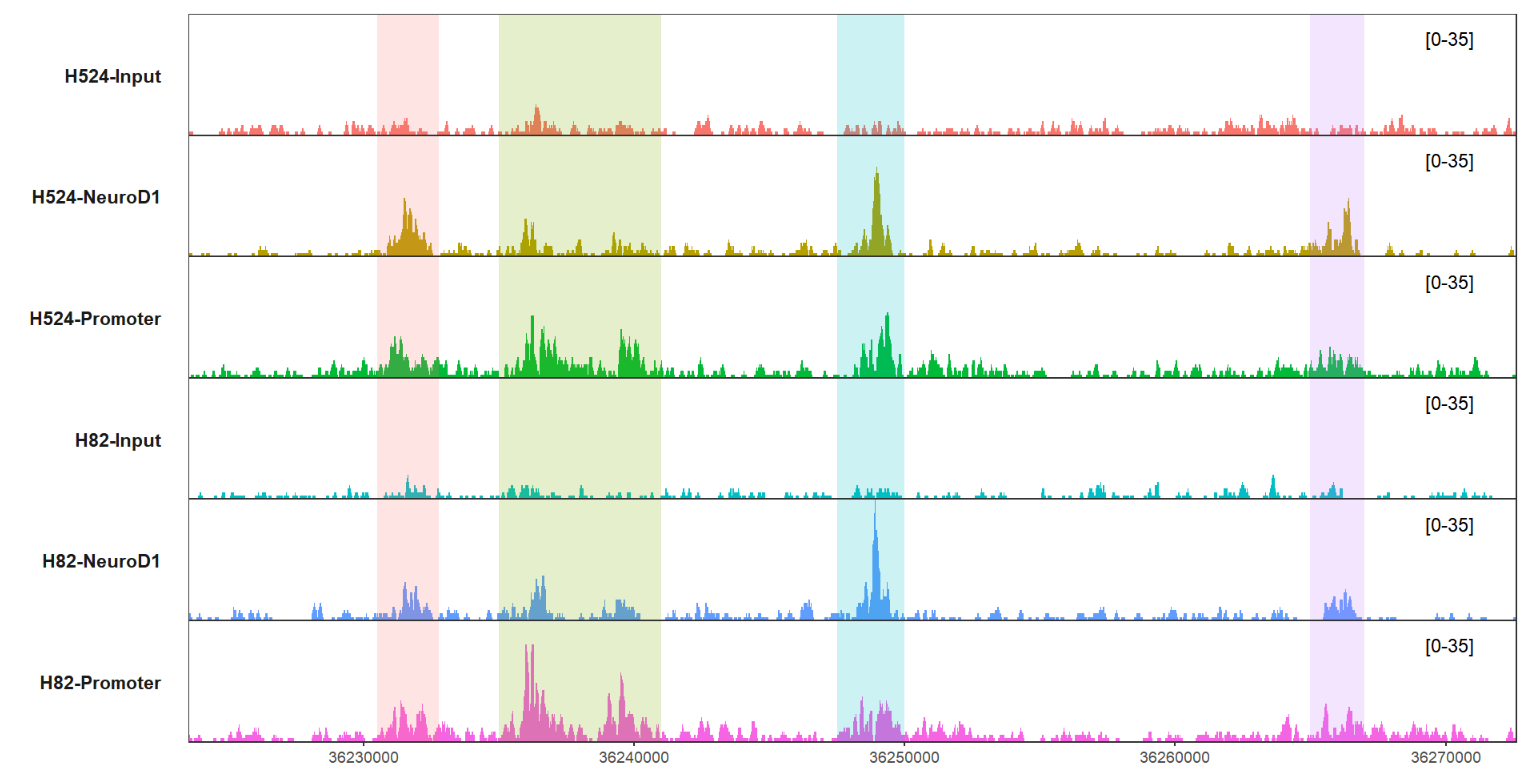
label.face = 'plain')here I provide a new style Y axis which often ocurrs in papers, you can try this style.
add a new style Y axis:
# add new y range
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)),
mark.alpha = 0.2,
theme = "bw",yAxis.info = FALSE,
new.yaxis = TRUE)you can ajust the pos.ration arg to palace the label:
# ajust position
trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673,
mark.region = list(c(36230500,36235000,36247500,36265000),
c(36232800,36241000,36250000,36267000)),
mark.alpha = 0.2,
theme = "bw",yAxis.info = FALSE,
new.yaxis = TRUE,
pos.ratio = c(0.95,0.8))you can also change the plot orders through sample.order arg.
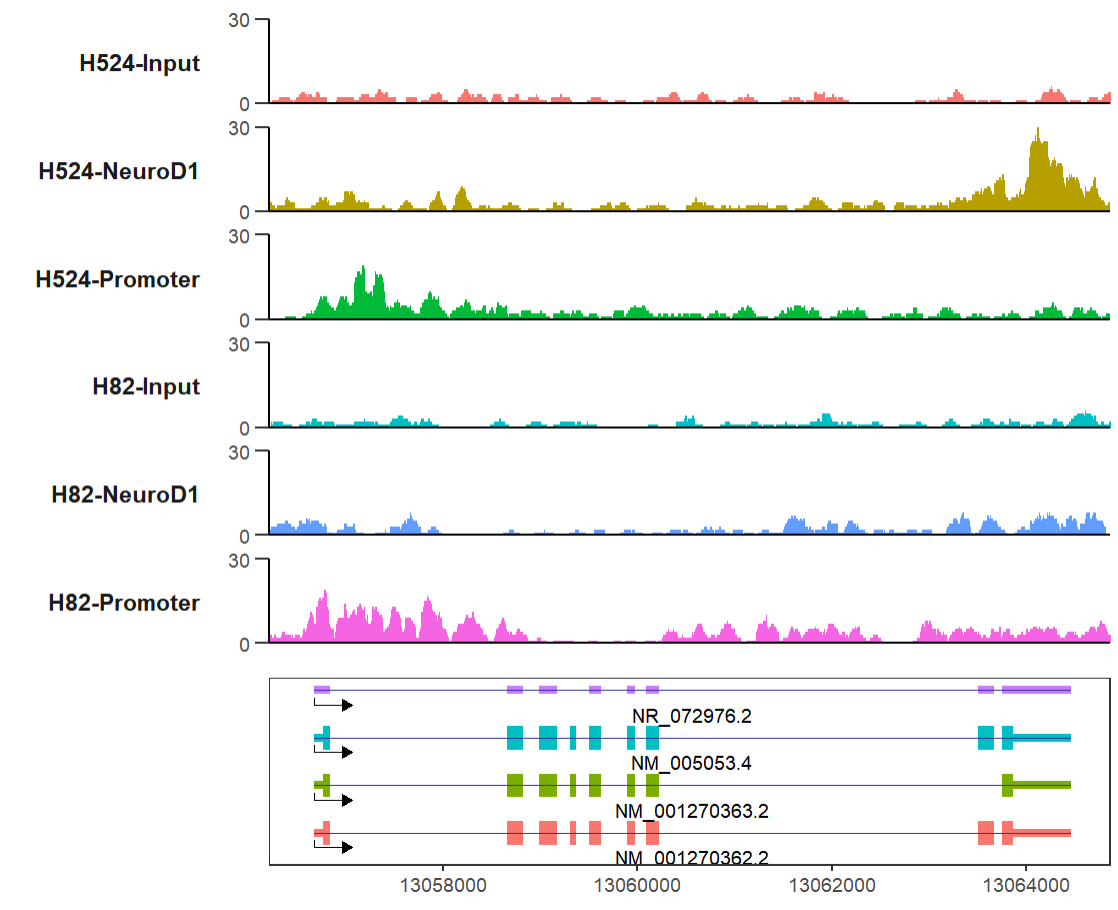
change the orders:
# ajust order
order <- c("H524-Input","H82-Input","H524-NeuroD1","H524-Promoter","H82-NeuroD1","H82-Promoter")
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
mark.region = list(c(13056500,13064000),
c(13058400,13065000)),
mark.alpha = 0.2,
label.face = 'plain',
sample.order = order)let's see some cases working with other functions.
ptrack <- trackVis(bWData = mybw,
chr = "chr19",
region.min = 36226490,
region.max = 36269673)
trans <-
trancriptVis(gtfFile = gtf,
Chr = "chr19",
posStart = 36226490 - 3000,
posEnd = 36269673 + 3000,
addNormalArrow = FALSE,
newStyleArrow = T,
absSpecArrowLen = T,
speArrowRelLen = 0.2,
textLabel = "gene_name",
textLabelSize = 3,
relTextDist = 0.5,
exonWidth = 0.9)
# combine
ptrack %>% insert_bottom(trans,height = 0.75)IGV:
collapse the gene structures:
trans <-
trancriptVis(gtfFile = gtf,
Chr = "chr19",
posStart = 36226490 - 3000,
posEnd = 36269673 + 3000,
absSpecArrowLen = T,
speArrowRelLen = 0.2,
textLabel = "gene_name",
textLabelSize = 4,
relTextDist = 0.2,
exonWidth = 0.5,
collapse = T)
# combine
ptrack %>% insert_bottom(trans,height = 0.1)supply with gene name:
RAD23A <-
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
extend.up = 500,
extend.dn = 1000,
xAxis.info = F)
p <-
trancriptVis(gtfFile = gtf,
gene = "RAD23A",
relTextDist = -0.5,
exonWidth = 0.5,
exonColorBy = 'transcript_id',
textLabelSize = 4,
addNormalArrow = FALSE,
newStyleArrow = TRUE) +
xlab('')
# combine
RAD23A %>% insert_bottom(p,height = 0.3)IGV shows:
change transcript colors:
# change transcript color
p <-
trancriptVis(gtfFile = gtf,
gene = "RAD23A",
relTextDist = -0.5,
exonWidth = 0.5,
exonFill = 'black',
arrowCol = 'black',
textLabelSize = 3,
addNormalArrow = FALSE,
newStyleArrow = TRUE,
xAxis.info = FALSE,
textLabel = 'gene_name') +
xlab('')
# combine
RAD23A %>% insert_bottom(p,height = 0.3)let's combine track, gene and peak:
# track + gene + peak
RAD23A <-
trackVis(bWData = mybw,
gtf.file = gtf,
gene.name = "RAD23A",
extend.up = 500,
extend.dn = 1000,
xAxis.info = F,
theme = "bw",
yAxis.info = F,new.yaxis = T,
pos.ratio = c(0.06,0.8),
color = jjAnno::useMyCol(platte = "stallion",n = 6))
# peak
bd <- bedVis(bdFile = file,
chr = "chr19",
track.width = 0.3,
show.legend = T)
# combine
RAD23A %>% insert_bottom(p,height = 0.3) %>%
insert_bottom(bd,height = 0.15)linkVis can be used to visualize chromtin accessbility , peak correlation or modification site relation on the chromosome coordinate.
# load data
link <- read.table('link-data.txt',header = T)
# check
head(link,3)
# chr start end grou val
# 1 chr1 714173 714486 group1 5.38023
# 2 chr1 714173 968410 group1 10.56281
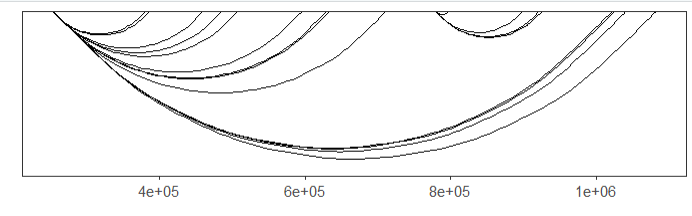
# 3 chr1 714173 995186 group1 4.04307default plot:
# no facet
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "black",
facet = FALSE)ajust the curvature:
# change curvature
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "black",
facet = FALSE,
curvature = 0.2)mapping color:
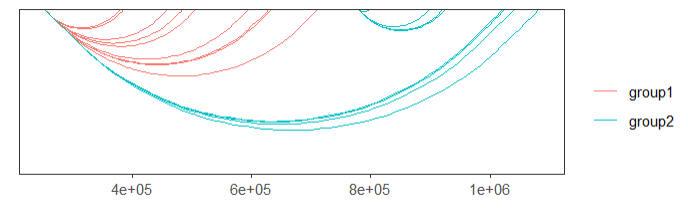
# mapping color
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "grou",
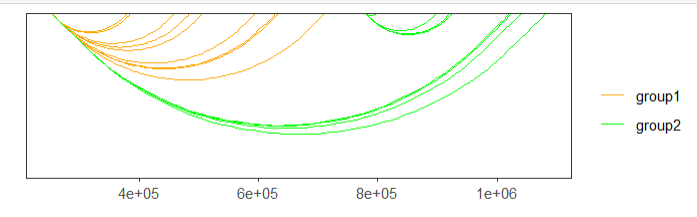
facet = FALSE)you can also change the color what you like:
# change color
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "grou",
facet = FALSE,
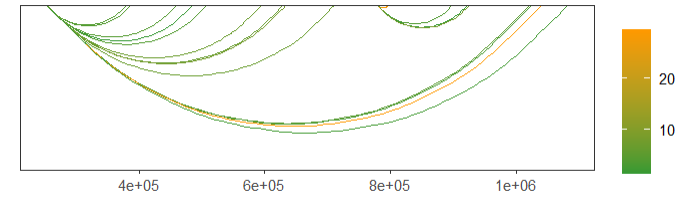
link.color = c('orange','green'))mapping with continues values:
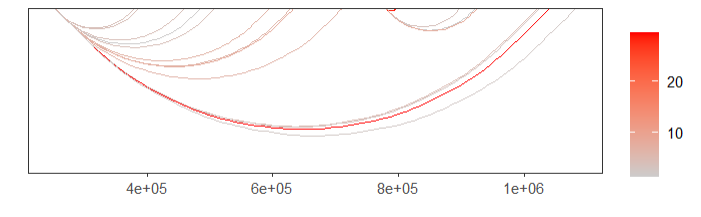
# mapping continues value
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = FALSE)change color:
# change color
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = FALSE,
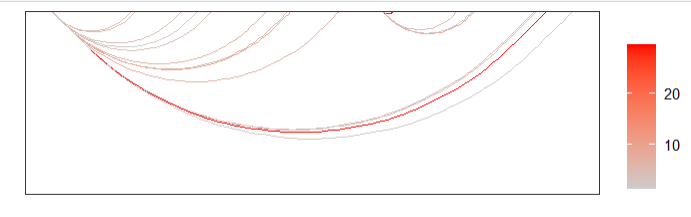
link.color = c('grey80','red'))remove X axis information:
# remove x Axis info
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = FALSE,
link.color = c('grey80','red'),
xAixs.info = FALSE)reverse direction:
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = TRUE,
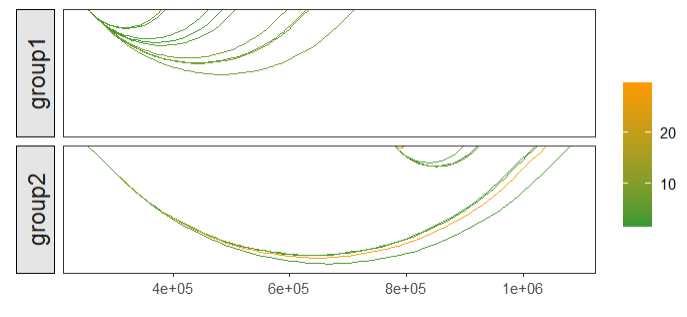
group = "grou",curvature = -0.5,yshift = -0.1)if you have multiple groups with links, you can plot them with facet plot.
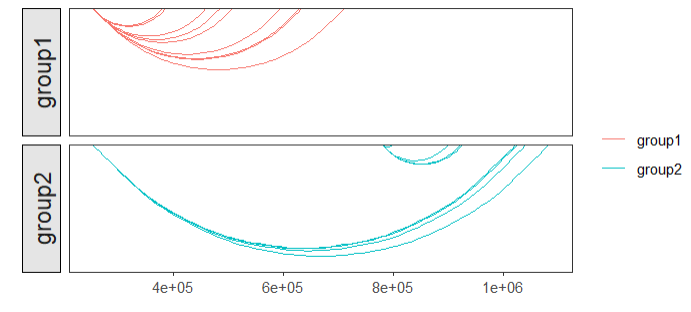
facet:
# facet link
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "grou",
facet = TRUE,
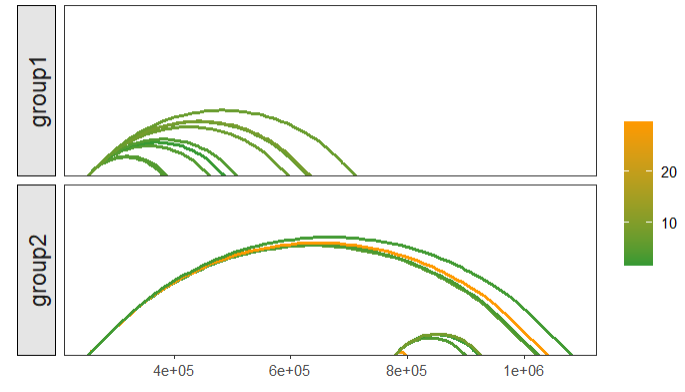
group = "grou")mapping with continues values:
# mapping continues value
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = TRUE,
group = "grou")some facet settings:
# ajust facet settings
linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = TRUE,
group = "grou",
facet.placement = "inside",
facet.fill = "white",
facet.text.angle = 0)let's combine with gene structure and bigwig track:
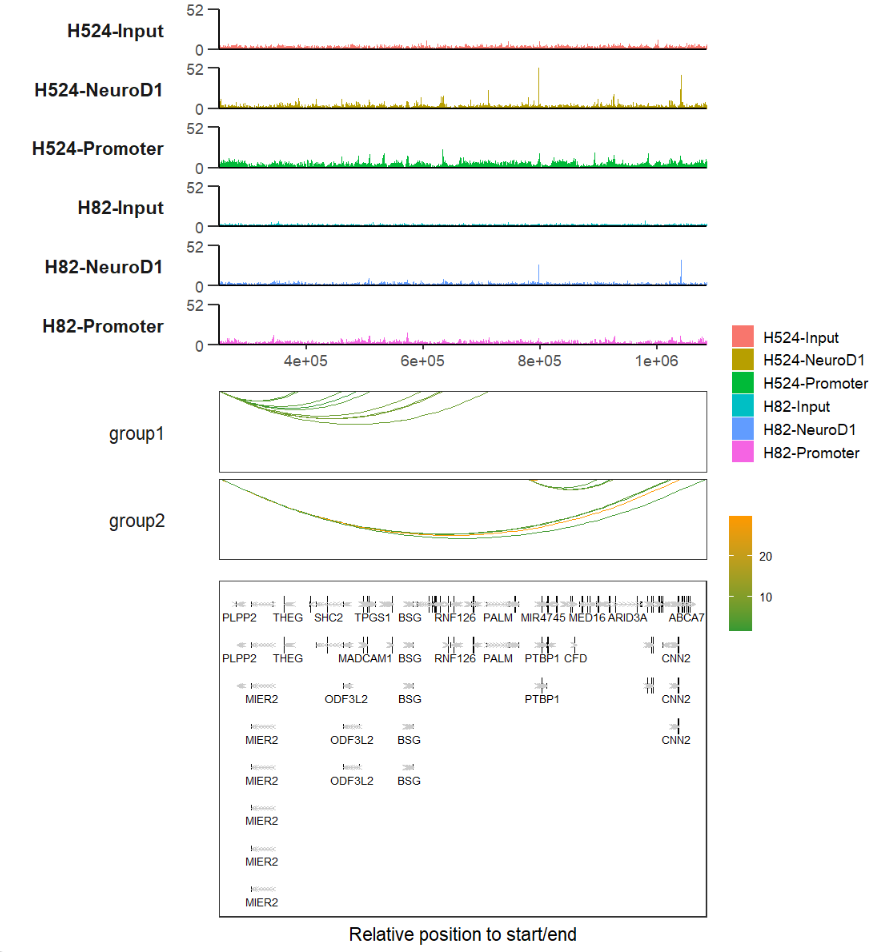
load('bWData.Rda')
# combine track and link
track <- trackVis(bWData = bWData,
chr = "chr19",
region.min = min(link$start),
region.max = max(link$end),
show.legend = T)
# load gtf
library(rtracklayer)
gtf <- import('hg19.ncbiRefSeq.gtf',format = "gtf") %>%
data.frame()
gene <-
trancriptVis(gtf,
Chr = "chr19",
posStart = min(link$start),
posEnd = max(link$end),
arrowLength = 0.05,
textLabel = 'gene_name',
textLabelSize = 3,
exonFill = 'black',
arrowCol = 'grey80',
exonWidth = 0.4,intronSize = 0.5,
reverse.y = T,
xAxis.info = F)
linkp <- linkVis(linkData = link,
start = "start",
end = "end",
link.aescolor = "val",
facet = TRUE,
group = "grou",
facet.fill = 'white',
facet.color = 'white',
facet.text.angle = 0,
curvature = 0.25,
line.size = 0.5,
xAixs.info = F)
# combine
track %>% insert_bottom(linkp,height = 0.5) %>%
insert_bottom(gene)if you have any advice or douts please leave words on: