Difference between coenzyme vs cofactor
- 2. Cofactors: Inorganic substances like metal ions that are required to increase the rate of catalysis. Chemical compunds or elements associated with the enzyme to increase its efficiency. There are mainly two types- inorganic ions and organic compounds. Inorganic ions: Known as enzyme activators (Cl ion in salivary amylase). Organic compunds: Divided into two types- co-enzymes and prosthetic groups.
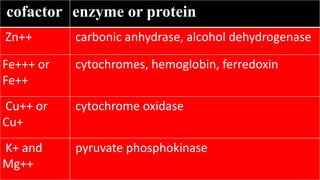
- 3. cofactor enzyme or protein Zn++ carbonic anhydrase, alcohol dehydrogenase Fe+++ or Fe++ cytochromes, hemoglobin, ferredoxin Cu++ or Cu+ cytochrome oxidase K+ and Mg++ pyruvate phosphokinase
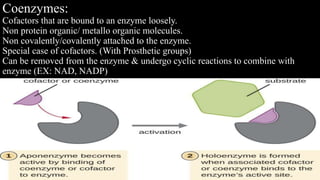
- 4. Coenzymes: Cofactors that are bound to an enzyme loosely. Non protein organic/ metallo organic molecules. Non covalently/covalently attached to the enzyme. Special case of cofactors. (With Prosthetic groups) Can be removed from the enzyme & undergo cyclic reactions to combine with enzyme (EX: NAD, NADP)
- 5. VITAMIN COENZYME RxN MEDIATED NIACIN nicotine adenine dinucleotide, nicotine adenine dinucelotide phosphate (NADH,NADHP-Partly composed of niacin) OXIDATION-REDUCTION RxN electron (hydrogen atom) transfer RIBOFLAVIN (VITAMIN B2) flavine adenine dinucleotide(FADH,FAD+ Partly composed of riboflavin - vit. B2) OXIDATION-REDUCTION RxN electron (hydrogen atom) transfer PANTOTHENATE (PANTOTHENIC ACID) coenzyme A Acyl groups activation & transfer Co-Q10 coenzymeQ10 OXIDATION-REDUCTION RxN electron (hydrogen atom) transfer THIAMINE (VITAMIN B1) thiamine pyrophosphate Activation & transfer of aldehydes (carbonyl groups) PYRIDOXINE (VITAMIN B6) pyridoxal phosphate Amino acids activation & amino groups transfer
- 6. VITAMIN COENZYME RxN MEDIATED COBALAMIN (VITAMIN B12) Carbamide coenzymes Alkyl groups / alkylation RxN (Isomerization & Methyl group transfer) BIOTIN biotin CARBOXYLATION RxN (carbon dioxide activation & transfer) NICOTINAMIDE nicotinamide OXIDATION-REDUCTION RxN electron (hydrogen atom) transfer FOLIC ACID tetrahydrofolate Activation & transfer of single carbon groups. LIPOIC ACID lipoamide Acylgroup activation & transfer OXIDATION-REDUCTION RxN
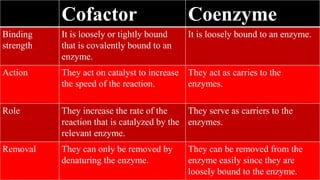
- 7. Cofactor Coenzyme Definition It is a non-protein chemical compounds that are bound tightly or loosely to an enzyme or other protein molecules. It is defined as small, organic, non-protein molecules, which carry chemical groups between enzymes. Molecule type These are organic as well as inorganic compounds / substances. These are organic molecules / substances. Function It assists in biological transformations. It aids or helps the function of an enzyme or the relative enzyme. Type They are chemical compounds. Two types of cofactors are: They are chemical molecules, include under type of cofactor.
- 8. Cofactor Coenzyme Binding strength It is loosely or tightly bound that is covalently bound to an enzyme. It is loosely bound to an enzyme. Action They act on catalyst to increase the speed of the reaction. They act as carries to the enzymes. Role They increase the rate of the reaction that is catalyzed by the relevant enzyme. They serve as carriers to the enzymes. Removal They can only be removed by denaturing the enzyme. They can be removed from the enzyme easily since they are loosely bound to the enzyme.
- 9. Cofactor Coenzyme Characteristic Its presence is important for the activity of an enzyme. They bind with an enzyme to catalyze a reaction. Example Metal Ions like Zn++, K+ and Mg++, etc. Vitamins, Biotin, Coenzyme A, etc
- 10. TERMINOLOGY PROSTHETIC GROUPS Co factors /co enzymes that are tightly bound to the enzyme molecules(EX : FAD). TURNOVER RATE The number of substrate molecules acted upon by an enzyme in one second under appropriate conditions. INTERNATIONAL UNIT Amount of enzyme causing Transformation of 1μmol of substrate/minute under optimal conditions. APOENZYME Protein component of enzyme. HOLOENZYME Apoenzyme + non protein component.
- 11. TERMINOLOGY ACTIVE SITE Special pocket or cleft in an enzyme molecule. Has amino acid side chains for substrate binding &catalysis. ALLOSTERIC SITES Enzymes have allosteric sites(other than active site) where effectors (chemical reagents acting as modifiers) bindthrough non covalent linkage. ACTIVITY Total units of enzymes in a given solution. SPECIFIC ACTIVITY Number of enzyme units/mg of total protein. KATAL Amount of an enzyme which catalyzes a reaction rate of 1 mole of substrate / second. SUBSTRATE The substance upon which an enzyme acts is called the substrate.
- 12. Reference: 1. Helmenstine, Ph.D. Anne Marie. “What Is a Coenzyme? Definition and Examples.” ThoughtCo. N.p., n.d. <https://www.thoughtco.com/definition-of- coenzyme-and-examples-604932>. 2. “Cofactor.” Encyclopædia Britannica. Encyclopædia Britannica, inc., n.d. Web. <https://www.britannica.com/science/cofactor>. 3. “Coenzymes and cofactors.” Coenzymes and cofactors. N.p., n.d. Web. <https://academic.brooklyn.cuny.edu/biology/bio4fv/page/coenzy_.htm>. 4. J.H. Freeland-Graves, C. Bavik, in Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003
- 13. Thank You