Nanoemulsão antifungica
- 1. Mycopathologia 155: 195–201, 2001. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. 195 The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi Andrzej Myc, Thomas Vanhecke, Jeffrey J. Landers, Tarek Hamouda1 & James R. Baker, Jr. Department of Internal Medicine, Division of Allergy and Center for Biologic Nanotechnology, University of Michigan, Ann Arbor, MI, USA; 1NanoBio Corporation, Ann Arbor, MI, USA Received 8 November 2001; acceptd in revised form 22 June 2002 Abstract Surfactant nanoemulsions are water in oil preparations that proved to have a broad spectrum biocidal activity against a variety of microorganisms including Gram-positive and Gram-negative bacteria, spores and enveloped viruses. These preparations are non-toxic to the skin, mucous membrane and gastrointestinal tissues at biocidal concentrations. In this study, 0.1% of the nanoemulsion designated X8W60PC has shown fungicidal activity against yeast including Candida albicans and C. tropicalis in 15 minutes. C. tropicalis was more sensitive than C. albicans, which required a longer time or a higher concentration of the nanoemulsion to achieve killing. Neutral to slightly alkaline pH was more effective in killing the yeast cells than acidic pH. Using the minimum inhibitory concen- tration assay, 0.08% of the nanoemulsion was inhibitory to C. albicans, and parapsilosis and filamentous fungi including Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, Aspergillus fumigatus and Fusarium oxysporum. None of the individual ingredients was as effective a fungicidal as the nanoemulsion at equiv- alent concentration. This shows that the nanoemulsion structure is an important factor in the anti-fungal activity. The X8W60PC has great potential as a topical anti-fungal agent and further investigation into the mechanism of fungicidal action is warranted. Key words: nanoemulsion, fungicidal, topical treatment, yeast, filamentous fungi, disinfectant. Introduction There are over 50,000 species of fungi. Fewer than 300 have been implicated in human diseases, and fewer than a dozen cause about 90% of all fungus infections. However, infections caused by unusual fungi are often difficult to identify and manage. Since fungi are eu- karyotes, as are human cells, they are more difficult to treat. They grow in the form of unicellular yeast or multicellular filamentous molds. Fungi are involved in the production of different forms of diseases, includ- ing allergies to fungal antigens, productions of toxins or direct invasion of hosts [1]. The search for effective anti-fungal topical agents has always been challenging due to drug side effects and acquired resistance. Many of the presently avail- able anti-fungal drugs used in topical treatment are inhibitors of fungus metabolic pathways. While they * Published in 2002. do not produce adverse and side effects at prescribed concentrations, they are not especially effective [2, 3]. Some antifungal drugs such as azoles are responsible for emergence of resistant strains [4–7]. While many disinfectants kill fungi, they are not suitable for topical treatment [8, 9]. For example, al- dehydes and phenols are used as surface disinfectants rather than topical antiseptics, because of their adverse effects on epithelial cells [10]. Quaternary ammonium compounds have limited application due to their low efficacy against fungi [11]. Given this, any non-toxic agent that has a rapid, broad-spectrum antifungal activity could be of great value. There is a continuing need to develop more ef- fective, safe, topical antifungal treatments that will not induce resistance. Nanoemulsions are micellar lipid nanoparticles in a uniform population of droplets ran- ging in diameter from 400–800 nm. They possess en- capsulating properties and can be tailored for a variety of uses [12–15]. Nanoemulsions can entrap and de-
- 2. 196 liver a wide variety of substances, including alcohols, non-polar solvents, detergents, solid particles, and aqueous materials. We have recently shown that the nanoemulsion stucture itself has extensive bactericidal and sporicidal activity [12, 16–18]. These components effectively inactivate enveloped viruses, including in- fluenza types A and B, sendai, sindbis, vaccinia, and Herpes simplex [18–20]. Soybean oil nanodroplets, stabilized by detergent and solvent, selectively fuse with bacterial membrane or viral envelope, destabil- izing lipids and initiating disruption of the pathogens [12]. Nanoemulsions are non-toxic to skin, mucous membranes, and gastrointestinal tissue at biocidal con- centrations [17]. Primary dermal irritation tests were conducted in rabbits and no skin irritation or other clinical signs of toxicity were seen during the study (unpublished data). In the present paper we report that a novel nanoemulsion, X8W60PC, has an effective anti-fungal activity at very low concentrations. X8W60PC con- sists of of oil, three non-ionic detergents, solvent, and water. Materials and methods Organisms and media Candida albicans (ATCC #90028), Candida trop- icalis (clinical isolates), Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, and Aspergillus fumigatus were provided by Carl Young (Clinical Pathology Laboratory, University of Michigan, Ann Arbor, MI). Candida parapsilosis (ATCC #90875) and Fusarium oxysporum (ATCC #26225) were purchased from ATCC. Yeast was grown on BHI agar or BHI broth supplemented with 5% sucrose (BHI-suc) at 37 ◦C. In some experiments, we used BHI broth with different pH. The medium was prepared by adding either 1N sodium hydroxide or hy- drochloric acid to obtain the desired pH. M. gypseum, T. spp., and A. fumigatus were grown on BHI agar at RT. F. oxysporum was grown on PDA agar at RT. Fungal suspensions Twenty-four hours prior to susceptibility testing, Can- dida spp. cultures were established from single colon- ies and incubated in BHI-suc at 37 ◦C on a shaker. The cells were counted using a hemocytometer and diluted to approximately 2 × 107 CFU per ml in BHI- suc for use in susceptibility testing as described below. Filamentous fungi were cultured on solid media until extensive fungal growth occurred. The mycelial mats were covered with 5 ml per plate of physiological sa- line. The fungi from the two plates were scraped and resuspended in 30 ml of physiological saline in a flask containing glass beads. The crude suspension was agitated vigorously for 2 to 3 min to dislodge spores and hyphal cells from any aggregates. After agitation, any remaining fungal clumps were removed by fil- tration through a 70 µm nylon cell strainer (Becton Dickinson, Franklin Lakes, NJ). The turbid suspen- sions were then concentrated by centrifugation and resuspended in a final volume of 8 ml with sterile saline. Viability counts of each suspension were per- formed on the day of their preparation. CFU were determined by plating 10 µl samples of 10-fold serial dilutions on agar plates. All suspensions were stored at 4 ◦C until used in susceptibility testing. X8W60PC and its compounds The X8W60PC surfactant nanoemulsion was prepared in a two-step procedure. An oil phase was prepared by blending the following ingredients: 64% oil, 8% solvent, 8% detergent 1 (D1), 1% detergent 2 (D2), 0.7% detergent 3 (D3). The mixture was heated at 70 ◦C for 30 minutes [12]. The nanoemulsion was then formed by mixing with water (18.3%) using the Silverson L4RT Mixer (fine Emulsor Screen) for 3 minutes at 10,000 rpm. All detergents were purchased from Sigma Chemicals (St. Louis, MO). Solvent was obtained from Aldrich (Milwaukee, WI) and oil was obtained from Croda Inc. (Mill Hill, PA). X8W60PC susceptibility testing of yeast One milliliter of C. albicans suspension (2 × 107 CFU per ml) was mixed with 1 ml of nanoemulsion or BHI medium (control) and incubated at 37 ◦C for different time intervals. After treatment, the yeast suspensions were centrifuged, supernatants were as- pirated and cells were diluted in fresh BHI medium. Serial ten-fold dilutions were prepared and plated on BHI plates. Plates were incubated at 37 ◦C for 48 h before the colony forming units were counted. Minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) assays Serial two-fold dilution of X8W60PC, each of its in- gredients or Nystatin (100 µl per well) in BHI-suc medium was prepared on a 96-well flat bottom plate.
- 3. 197 One hundred microliters inoculum of C. albicans and C. parapsilosis (2 × 104 CFU/well), M. gypseum (420 CFU per well), T. mentagrophytes (100 CFU per well), T. rubrum (100 CFU per well), A. fumigatus (100 CFU per well) or F. oxysrporum (100 CFU per well) were added to the wells. The plates were incubated either at 37 ◦C (C. albicans), or at RT (C. parapsilosis and filamentous fungi) until confluent growth appeared at the control wells. Fungal growth was examined both microscopically and by reading turbidity at 595 nm or 750 nm for C. parapsilosis and F. oxysporum on an ELISA reader. To assess MFC, fungal cells treated as described above were harvested at different time periods, washed three times in sterile water, resus- pended in 100 µl of BHI and plated on BHI or PDA (F. oxysporum) plates. Plates were incubated either at 37 ◦C (C. albicans) or RT (C. parapsilosis, and filamentous fungi) until colonial growth appeared. Electron microscopy photographs C. albicans at 2 × 104 CFU/100 µl were treated for 90 min with 100 µl of 2% nanoemulsion or incub- ated in BHI (control). Thermanox cover slips were pretreated with poly-L-lysine and 20 µl of each sus- pension was placed on the cover slips for one minute before draining the excess. These cover slips were then placed in fixative for 20 minutes (2.5% gluteralde- hyde in phosphate buffer) and dehydrated in a grated series of ethanol. The cover slips were dried using the HMDS (hexamethyldisilazane) technique [21]. The cover slips were then mounted, sputter coated with gold, and examined using an AMARY 1000-B SEM (Bedford, Massachusetts). Images were recorded on Polaroid film. Results To determine whether surfactant nanoemulsions have anti-fungal properties, we examined the effect of X8W60PC on two clinically important yeast species: C. albicans and C. tropicalis. As shown in Figure 1, X8W60PC had strong fungicidal effect on both spe- cies. One percent X8W60PC reduced C. albicans CFU more than four logs and C. tropicalis CFU approx- imately six logs in 15 minutes. Since C. albicans appeared to be less susceptible to nanoemulsion treat- ment, we chose it to investigate the fungicidal kinetics of X8W60PC. The organism was treated with two con- centrations of X8W60PC for 30, 60 and 120 minutes. Figure 1. Susceptibility of Candida spp to X8W60PC nanoemul- sion. Cells were either incubated in BHI medium (control) or treated with different concentrations of nanoemulsion or 6% bleach for 15 minutes at 37 ◦C. After treatment, cells were washed and plated on BHI plates to assess the number of CFU. The bars represent standard error. Figure 2. Kinetics of fungicidal activity of X8W60PC nanoemul- sion on Candida albicans. Cells were either incubated in BHI medium (control) or treated with different concentrations of the nanoemulsion for variable periods of time at 37 ◦C. After treatment, cells were washed and plated on BHI plates to assess the number of CFU. The bars represent standard error. Ten percent X8W60PC for 30 min or 1% X8W60PC treatment for 120 min resulted in six log reduction of C. albicans CFU (Figure 2). In order to depict the cell damage, scanning elec- tron microphotographs were taken. Candida albicans was treated for 90 min with 100 µl of 2% nanoemul- sion, fixed and prepared for electron microscopy scan- ning. As shown on Figure 3B–D, yeast cells treated with nanoemulsion lost their round shape and integrity due to coalescence with droplets nanoemulsion sur- rounded the cells. Figure 3 A shows control yeast cells. Untreated cells do not reveal any changes in shape and integrity and undergo budding proliferation process. To examine the pH variation on nanoemulsion anti-fungal activity, C. albicans was treated with
- 4. 198 Figure 3. Electron microphotographs of Candida albicans treated with naoemulsion. Yeasts were either untreated or incubated with 1% X8W60PC for 90 minutes at room temperature and were subjected to electron microscopy staining procedures. A – untreated yeasts, B, C, and D – depict different phases of cell damage caused by nanoemulsion. Arrows indicate the droplets of nanoemulsion. Magnification = 12,000×. 1% nanoemulsion at different pH (range 3–9). The nanoemulsion showed the highest fungicidal activity at pH range 7–9 (Figure 4). At pH lower than 7, the nanoemulsion was only slightly effective. pH higher than 9 was itself detrimental to the yeast (data not shown). Since some ingredients of X8W60PC have anti- microbial activity, we investigated the susceptibility of C. albicans to the individual ingredients at con- centrations equivalent to their concentrations in the nanoemulsion. Solvent, D1 and D3 were completely ineffective against C. albicans in the concentrations found in 0.1% of nanoemulsion. Only D2 partially reduced yeast growth (Figure 5). The efficacy of X8W60PC against yeast prompted us to determine the minimal inhibitory and fungicidal Figure 4. Effect of pH on susceptibility of Candida albicans to X8W60PC nanoemulsion. Cells were treated either with BHI me- dium or 1% nanoemulsion at different pH for 15 minutes at 37 ◦C. After treatment, cells were washed and plated on BHI plates to assess the number of CFU. The bars represent standard error.
- 5. 199 Figure 5. The susceptibility of Candida albicans to X8W60PC nanoemulsion or its individual active ingredients. Cells were treated with either 0.1% X8W60PC nanoemulsion or with individual in- gredients at the equivalent concentrations for 15 minutes at 37 ◦C. After treatment, cells were washed and plated on BHI plates to as- sess the number of CFU. The bars represent standard error. ∗ – D1, D2, and D3 depict three detergents used to prepare X8W60PC. Table 1. Minimal fungicidal concentration (MFC) of X8W60PC nanoemulsion and Nystatin on yeast and filamentous fungi. Microorganism X8W60PC Nystatin (%) (units/ml) Candida albicans 0.063 31.3 Candida parapsilosis 0.016 31.3 Microsporum gypseum 0.040 n/t∗ Trichophyton rubrum 0.032 n/t Trichophyton mentagrophytes 0.016 n/t Aspergillus fumigatus 0.010 n/t Fusarium oxysporum 0.032 62.5 ∗ Not tested. concentrations of the nanoemulsion to C. albicans and parapsilosis, T. spp., M. gypseum, A. fumigatus, and F. oxysporum (Figure 6 and Table 1). As a ref- erence anti-fungal agent, Nystatin has been used in some experiments in parallel to nanoemuslion. As- pergillus fumigatus was the most susceptible, <0.01% nanoemulsion resulted in complete killing and C. al- bicans was the most resistant to the nanoemulsion fungicidal activity (Figure 6 and Table 1). Overall, all tested fungi were susceptible to the nanoemulsion at a concentration below 0.1%. Discussion There is an ongoing need for new anti-fungal agents to combat the continuous development of resistant yeast or filamentous fungi species due to inappropriate use of anti-fungal drugs in humans and animals [22– 24]. Surfactant nanoemulsions are a treatment option. Nanoemulsions are novel water-in-oil formulations that are stabilized by the addition of small amounts of surfactant and solvent [12]. The water-immiscible, liquid phase is mixed into an aqueous phase by high stress mechanical extrusion, yielding a uniform popu- lation of droplets ranging in diameter from 400–800 nm. Due to their intrinsic features, nanoemulsions can be further diluted in aqueous solutions and stored at a broad range of temperature. Recently, surfactant nanoemulsions have been reported to have extens- ive bactericidal, sporicidal and virucidal effects [12, 16–20]. In this study, we showed that the surfactant nanoemulsion designated X8W60PC has fungicidal activity. At 1% concentration, X8W60PC reduced the number of C. albicans CFU by more than four logs within 15 minutes of treatment and two-hour treatment reduced the number of C. albicans CFU by six logs. Of note, Nystatin, which is presently used for topical treatment, needs to be applied for approximately four weeks to treat cutaneous candidiasis with a dose as high as 100, 000 units gram−1 [25]. Moreover, the nanoemulsion is most effective at physiological pH, another good feature in its potential application as an anti-fungal drug for topical treatment. Since some ingredients of X8W60PC are biocidal, we tested whether these ingredients alone, at concen- trations equivalent to those in the nanoemulsion, have anti-fungal activity. D1, D3 and solvent were inef- fective against yeast at equivalent concentrations. Ten to 1000 times higher concentrations of the individual ingredients were required to obtain a comparable fun- gicidal activity (data not shown). D2, at a concentra- tion equivalent to that of the nanoemulsion, had some fungicidal activity; it reduced the number of CFU by approximately two logs (Figure 5). These data suggest that the fungicidal activity of X8W60PC is not due to its ingredients, but to the nanoemulsion structure. Minimal Inhibitory Concentration assays further confirmed the anti-fungal activity of the nanoemul- sion. There was a slight variation in susceptibility among the tested fungi. C. albicans appeared to be the least susceptible (Figure 6A) and Candida parap- silosis (Figure 6B) and Trichophyton spp. (Figure
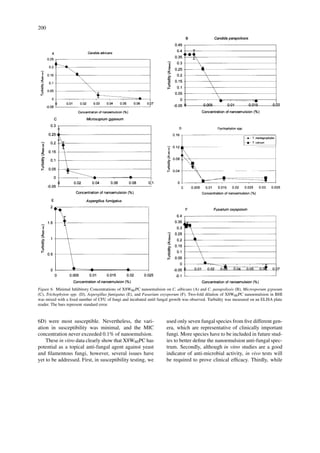
- 6. 200 Figure 6. Minimal Inhibitory Concentrations of X8W60PC nanoemulsion on C. albicans (A) and C. parapsilosis (B), Microsporum gypseum (C), Trichophyton spp. (D), Aspergillus fumigatus (E), and Fusarium oxysporum (F). Two-fold dilution of X8W60PC nanoemulsion in BHI was mixed with a fixed number of CFU of fungi and incubated until fungal growth was observed. Turbidity was measured on an ELISA plate reader. The bars represent standard error. 6D) were most susceptible. Nevertheless, the vari- ation in susceptibility was minimal, and the MIC concentration never exceeded 0.1% of nanoemulsion. These in vitro data clearly show that X8W60PC has potential as a topical anti-fungal agent against yeast and filamentous fungi, however, several issues have yet to be addressed. First, in susceptibility testing, we used only seven fungal species from five different gen- era, which are representative of clinically important fungi. More species have to be included in future stud- ies to better define the nanoemulsion anti-fungal spec- trum. Secondly, although in vitro studies are a good indicator of anti-microbial activity, in vivo tests will be required to prove clinical efficacy. Thirdly, while
- 7. 201 the surfactant nanoemulsion at biocidal concentrations is non-toxic in short term application to skin (unpub- lished data), mucous membranes, and gastrointestinal tract [17], its long term toxicity has never been tested. Therefore, chronic toxicity tests are essential. Finally, due to the rapid action of the nanoemulsion, it is un- likely that therapeutic concentrations could result in development of resistant strains, however, this can- not be excluded at this time. Currently, we are in the process of investigating all these issues. In conclusion, since X8W60PC exhibits fungicidal activity on yeast and filamentous fungi, this nanoemul- sion has potential as a topical treatment for a variety of mycoses. Acknowledgments This work was supported with DARPA (Defense Ad- vanced Research Project Agency) contract #MDA 972-1-007 of the Unconventional Pathogen Counter- measures Program. The authors wish to thank Dr. Nicholas Beeson for revision of the manuscript and critical review, and Chris Edwards for assistance with SEM microscopy. References 1. Mitchell TG. Medical Mycology. In: Joklik WK, Willett HP, Amos DB, Wilfert CM, eds. Zinsser Mirobiology 20th edtion. Appleton and Lange, Connecticut, 1992: 1071–1081. 2. Kruger W, Stockschlader M, Russmann, B., et al. Experi- ence with liposome Amphotericin-B in 60 patients undergoing high-dose therapy and bone marrow or peripheral blood stem cell transplantation. Br J Haematol. 1995; 91, 684–690. 3. Stevens DA, Diaz M, Negroni R, et al. Safety evaluation of chronic fluconazole therapy. Fluconazole Pan-American Study Group. Chemotherapy. 1997; 43: 371–377. 4. Yamaguchi H. Molecular and biochemical mechanisms of drug resistance in fungi. Nippon Ishinkin Gakkai Zasshi. 1999; 40: 199–208. 5. Wirsching S, Michel S, Kohler G, Morschhauser J. Activation of the multiple drug resistance gene MDR1 in fluconazole- resistant, clinical Candida albicans strains is caused by muta- tions in a trans-regulatory factor. Journal of Bacteriology. 2000; 182: 400–404. 6. Xu J, Ramos AR, Vilgalys R, Mitchell TG. Clonal and spon- taneous origins of fluconazole resistance in Candida albicans. Journal of Clinical Microbiology 2000; 38: 1214–1220. 7. Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson JB, Kohn LM. Evolution of drug resistance in experimental populations of Candida albicans. Journal of Bacteriology 2000; 182: 1515–1522. 8. Lineaweaver W, Howard R, Soucy D, et al. Topical antimicro- bial toxicity. Arch. Surg. 1985; 120: 267–270. 9. Rutala WA, Weber DJ. Uses of inogranic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997; 10: 597–610. 10. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999; 12: 147–179. 11. Bundgaard-Nielsen K, Nielsen PV. Fungicidal effect of 15 dis- infectants against 25 fungal contaminants commonly found in bread and cheese manufacturing. J. Food. Prot. 1996; 59: 268–275. 12. Baker JR, Jr, Wright DC, Hayes MM, Hamouda T, Brisker JM. Methods of inactivating bacteria including bacterial spores. U.S. Patent # 6015832; 2000. 13. Gregoriadis G. ed. Liposome Technology: Liposome Pre- parations and Related Techniques. Boca Raton, Fla.: CRC Press, 1993 (Non-ionic surfactant vesicles: preparation and characterization. 2nd ed. vol. 1). 14. Gregoriadis G, Florence AT. Liposomes in drug delivery. Clin- ical, diagnostic and ophthalmic potential. Drugs. 1993; 45: 15–28. 15. Wasan KM, Lopez-Berestein G. The past, present, and future uses of liposomes in treating infectious diseases. Immunophar- macol. Immunotoxicol. 1995; 17: 1–15. 16. Hamouda T, Wright DC, Brisker JM, Baker JR, Jr. Microbi- ocidal effects of liposome-like microemulsions on pathogenic Gram negative bacteria. American Society for Microbiology, 98th General Meeting, Atlanta, Georgia, 1998: 47. 17. Hamouda T, Hayes MM, Cao Z, et al. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus spores. J. Infect. Dis. 1999; 180: 1939–1949. 18. Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR, Jr. A novel surfactant nanoemulsion with a unique non- irritant topical antimicrobial activity against bacteria, envel- oped viruses and fungi. International Microbiol. Res. 2000; 156: 1–7. 19. Donovan BW, Reuter JD, Cao Z., Myc A, Johnson K, Baker JR, Jr. Prevention of murine influenza A virus pneumonitis by surfactant nano-emulsions Antivir. Chem. Chemother. 2000; 11: 41–49. 20. Myc A, Anderson, MJ, Wright DC, Brisker J, Baker JR, Jr. In- hibitory effect of non-phospholipid liposomes and nanoemul- sions on influenza A virus infectivity. 38th Interscience Con- ference on Antimicrobial Agents and Chemotherapy, San Diego, California, 1998: 336. 21. Bray, D.F., et al. (1993) Micros.Res. and Technique. 26: 489– 495. A comparison of HMDS, Peldri II, and critical drying methods for SEM of biological specimens. 22. Morse SS. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995; 1: 7–15. 23. Riley LW. Drug-resistant tuberculosis. Clin. Infect. Dis. 1993; 17, Suppl. 2, S442–446. 24. Tenover FC, Hughes JM. The challenges of emerging infec- tious diseases. Development and spread of multiply-resistant bacterial pathogens. JAMA 1996; 275: 300–304. 25. Clayton YM, Conner BL. Comparison of clotrimazole cream, Whitfield’s ointment and nystatin ointment for the topical treatment of ringworm infections, pityriasis versicolor, eryth- rasma and candidiasis. Br. J. Dermatol. 1973; 89: 297–303. Address for correspondence: James R. Baker, Jr., Room 9220 MSRB III, 1150 West Medical Center Drive, Ann Arbor, MI 48109- 0648, U.S.A. Phone (734) 647-2777; Fax: (734) 936-2990; E-mail: [email protected]

![Mycopathologia 155: 195–201, 2001.
© 2002 Kluwer Academic Publishers. Printed in the Netherlands.
195
The fungicidal activity of novel nanoemulsion (X8W60PC) against
clinically important yeast and filamentous fungi
Andrzej Myc, Thomas Vanhecke, Jeffrey J. Landers, Tarek Hamouda1 & James R. Baker, Jr.
Department of Internal Medicine, Division of Allergy and Center for Biologic Nanotechnology, University of
Michigan, Ann Arbor, MI, USA; 1NanoBio Corporation, Ann Arbor, MI, USA
Received 8 November 2001; acceptd in revised form 22 June 2002
Abstract
Surfactant nanoemulsions are water in oil preparations that proved to have a broad spectrum biocidal activity
against a variety of microorganisms including Gram-positive and Gram-negative bacteria, spores and enveloped
viruses. These preparations are non-toxic to the skin, mucous membrane and gastrointestinal tissues at biocidal
concentrations. In this study, 0.1% of the nanoemulsion designated X8W60PC has shown fungicidal activity against
yeast including Candida albicans and C. tropicalis in 15 minutes. C. tropicalis was more sensitive than C. albicans,
which required a longer time or a higher concentration of the nanoemulsion to achieve killing. Neutral to slightly
alkaline pH was more effective in killing the yeast cells than acidic pH. Using the minimum inhibitory concen-
tration assay, 0.08% of the nanoemulsion was inhibitory to C. albicans, and parapsilosis and filamentous fungi
including Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, Aspergillus fumigatus and
Fusarium oxysporum. None of the individual ingredients was as effective a fungicidal as the nanoemulsion at equiv-
alent concentration. This shows that the nanoemulsion structure is an important factor in the anti-fungal activity.
The X8W60PC has great potential as a topical anti-fungal agent and further investigation into the mechanism of
fungicidal action is warranted.
Key words: nanoemulsion, fungicidal, topical treatment, yeast, filamentous fungi, disinfectant.
Introduction
There are over 50,000 species of fungi. Fewer than 300
have been implicated in human diseases, and fewer
than a dozen cause about 90% of all fungus infections.
However, infections caused by unusual fungi are often
difficult to identify and manage. Since fungi are eu-
karyotes, as are human cells, they are more difficult
to treat. They grow in the form of unicellular yeast or
multicellular filamentous molds. Fungi are involved in
the production of different forms of diseases, includ-
ing allergies to fungal antigens, productions of toxins
or direct invasion of hosts [1].
The search for effective anti-fungal topical agents
has always been challenging due to drug side effects
and acquired resistance. Many of the presently avail-
able anti-fungal drugs used in topical treatment are
inhibitors of fungus metabolic pathways. While they
* Published in 2002.
do not produce adverse and side effects at prescribed
concentrations, they are not especially effective [2, 3].
Some antifungal drugs such as azoles are responsible
for emergence of resistant strains [4–7].
While many disinfectants kill fungi, they are not
suitable for topical treatment [8, 9]. For example, al-
dehydes and phenols are used as surface disinfectants
rather than topical antiseptics, because of their adverse
effects on epithelial cells [10]. Quaternary ammonium
compounds have limited application due to their low
efficacy against fungi [11].
Given this, any non-toxic agent that has a rapid,
broad-spectrum antifungal activity could be of great
value. There is a continuing need to develop more ef-
fective, safe, topical antifungal treatments that will not
induce resistance. Nanoemulsions are micellar lipid
nanoparticles in a uniform population of droplets ran-
ging in diameter from 400–800 nm. They possess en-
capsulating properties and can be tailored for a variety
of uses [12–15]. Nanoemulsions can entrap and de-](https://image.slidesharecdn.com/nanoemulsoantifungica-161026181933/85/Nanoemulsao-antifungica-1-320.jpg)
![196
liver a wide variety of substances, including alcohols,
non-polar solvents, detergents, solid particles, and
aqueous materials. We have recently shown that the
nanoemulsion stucture itself has extensive bactericidal
and sporicidal activity [12, 16–18]. These components
effectively inactivate enveloped viruses, including in-
fluenza types A and B, sendai, sindbis, vaccinia, and
Herpes simplex [18–20]. Soybean oil nanodroplets,
stabilized by detergent and solvent, selectively fuse
with bacterial membrane or viral envelope, destabil-
izing lipids and initiating disruption of the pathogens
[12]. Nanoemulsions are non-toxic to skin, mucous
membranes, and gastrointestinal tissue at biocidal con-
centrations [17]. Primary dermal irritation tests were
conducted in rabbits and no skin irritation or other
clinical signs of toxicity were seen during the study
(unpublished data).
In the present paper we report that a novel
nanoemulsion, X8W60PC, has an effective anti-fungal
activity at very low concentrations. X8W60PC con-
sists of of oil, three non-ionic detergents, solvent, and
water.
Materials and methods
Organisms and media
Candida albicans (ATCC #90028), Candida trop-
icalis (clinical isolates), Microsporum gypseum,
Trichophyton mentagrophytes, Trichophyton rubrum,
and Aspergillus fumigatus were provided by Carl
Young (Clinical Pathology Laboratory, University of
Michigan, Ann Arbor, MI). Candida parapsilosis
(ATCC #90875) and Fusarium oxysporum (ATCC
#26225) were purchased from ATCC. Yeast was
grown on BHI agar or BHI broth supplemented with
5% sucrose (BHI-suc) at 37 ◦C. In some experiments,
we used BHI broth with different pH. The medium was
prepared by adding either 1N sodium hydroxide or hy-
drochloric acid to obtain the desired pH. M. gypseum,
T. spp., and A. fumigatus were grown on BHI agar at
RT. F. oxysporum was grown on PDA agar at RT.
Fungal suspensions
Twenty-four hours prior to susceptibility testing, Can-
dida spp. cultures were established from single colon-
ies and incubated in BHI-suc at 37 ◦C on a shaker.
The cells were counted using a hemocytometer and
diluted to approximately 2 × 107 CFU per ml in BHI-
suc for use in susceptibility testing as described below.
Filamentous fungi were cultured on solid media until
extensive fungal growth occurred. The mycelial mats
were covered with 5 ml per plate of physiological sa-
line. The fungi from the two plates were scraped and
resuspended in 30 ml of physiological saline in a flask
containing glass beads. The crude suspension was
agitated vigorously for 2 to 3 min to dislodge spores
and hyphal cells from any aggregates. After agitation,
any remaining fungal clumps were removed by fil-
tration through a 70 µm nylon cell strainer (Becton
Dickinson, Franklin Lakes, NJ). The turbid suspen-
sions were then concentrated by centrifugation and
resuspended in a final volume of 8 ml with sterile
saline. Viability counts of each suspension were per-
formed on the day of their preparation. CFU were
determined by plating 10 µl samples of 10-fold serial
dilutions on agar plates. All suspensions were stored
at 4 ◦C until used in susceptibility testing.
X8W60PC and its compounds
The X8W60PC surfactant nanoemulsion was prepared
in a two-step procedure. An oil phase was prepared
by blending the following ingredients: 64% oil, 8%
solvent, 8% detergent 1 (D1), 1% detergent 2 (D2),
0.7% detergent 3 (D3). The mixture was heated at
70 ◦C for 30 minutes [12]. The nanoemulsion was
then formed by mixing with water (18.3%) using the
Silverson L4RT Mixer (fine Emulsor Screen) for 3
minutes at 10,000 rpm. All detergents were purchased
from Sigma Chemicals (St. Louis, MO). Solvent was
obtained from Aldrich (Milwaukee, WI) and oil was
obtained from Croda Inc. (Mill Hill, PA).
X8W60PC susceptibility testing of yeast
One milliliter of C. albicans suspension (2 × 107 CFU
per ml) was mixed with 1 ml of nanoemulsion or
BHI medium (control) and incubated at 37 ◦C for
different time intervals. After treatment, the yeast
suspensions were centrifuged, supernatants were as-
pirated and cells were diluted in fresh BHI medium.
Serial ten-fold dilutions were prepared and plated on
BHI plates. Plates were incubated at 37 ◦C for 48 h
before the colony forming units were counted.
Minimal inhibitory concentration (MIC) and minimal
fungicidal concentration (MFC) assays
Serial two-fold dilution of X8W60PC, each of its in-
gredients or Nystatin (100 µl per well) in BHI-suc
medium was prepared on a 96-well flat bottom plate.](https://image.slidesharecdn.com/nanoemulsoantifungica-161026181933/85/Nanoemulsao-antifungica-2-320.jpg)
![197
One hundred microliters inoculum of C. albicans and
C. parapsilosis (2 × 104 CFU/well), M. gypseum (420
CFU per well), T. mentagrophytes (100 CFU per well),
T. rubrum (100 CFU per well), A. fumigatus (100 CFU
per well) or F. oxysrporum (100 CFU per well) were
added to the wells. The plates were incubated either
at 37 ◦C (C. albicans), or at RT (C. parapsilosis and
filamentous fungi) until confluent growth appeared at
the control wells. Fungal growth was examined both
microscopically and by reading turbidity at 595 nm
or 750 nm for C. parapsilosis and F. oxysporum on
an ELISA reader. To assess MFC, fungal cells treated
as described above were harvested at different time
periods, washed three times in sterile water, resus-
pended in 100 µl of BHI and plated on BHI or PDA
(F. oxysporum) plates. Plates were incubated either
at 37 ◦C (C. albicans) or RT (C. parapsilosis, and
filamentous fungi) until colonial growth appeared.
Electron microscopy photographs
C. albicans at 2 × 104 CFU/100 µl were treated for
90 min with 100 µl of 2% nanoemulsion or incub-
ated in BHI (control). Thermanox cover slips were
pretreated with poly-L-lysine and 20 µl of each sus-
pension was placed on the cover slips for one minute
before draining the excess. These cover slips were then
placed in fixative for 20 minutes (2.5% gluteralde-
hyde in phosphate buffer) and dehydrated in a grated
series of ethanol. The cover slips were dried using the
HMDS (hexamethyldisilazane) technique [21]. The
cover slips were then mounted, sputter coated with
gold, and examined using an AMARY 1000-B SEM
(Bedford, Massachusetts). Images were recorded on
Polaroid film.
Results
To determine whether surfactant nanoemulsions have
anti-fungal properties, we examined the effect of
X8W60PC on two clinically important yeast species:
C. albicans and C. tropicalis. As shown in Figure 1,
X8W60PC had strong fungicidal effect on both spe-
cies. One percent X8W60PC reduced C. albicans CFU
more than four logs and C. tropicalis CFU approx-
imately six logs in 15 minutes. Since C. albicans
appeared to be less susceptible to nanoemulsion treat-
ment, we chose it to investigate the fungicidal kinetics
of X8W60PC. The organism was treated with two con-
centrations of X8W60PC for 30, 60 and 120 minutes.
Figure 1. Susceptibility of Candida spp to X8W60PC nanoemul-
sion. Cells were either incubated in BHI medium (control) or treated
with different concentrations of nanoemulsion or 6% bleach for 15
minutes at 37 ◦C. After treatment, cells were washed and plated on
BHI plates to assess the number of CFU. The bars represent standard
error.
Figure 2. Kinetics of fungicidal activity of X8W60PC nanoemul-
sion on Candida albicans. Cells were either incubated in BHI
medium (control) or treated with different concentrations of the
nanoemulsion for variable periods of time at 37 ◦C. After treatment,
cells were washed and plated on BHI plates to assess the number of
CFU. The bars represent standard error.
Ten percent X8W60PC for 30 min or 1% X8W60PC
treatment for 120 min resulted in six log reduction of
C. albicans CFU (Figure 2).
In order to depict the cell damage, scanning elec-
tron microphotographs were taken. Candida albicans
was treated for 90 min with 100 µl of 2% nanoemul-
sion, fixed and prepared for electron microscopy scan-
ning. As shown on Figure 3B–D, yeast cells treated
with nanoemulsion lost their round shape and integrity
due to coalescence with droplets nanoemulsion sur-
rounded the cells. Figure 3 A shows control yeast cells.
Untreated cells do not reveal any changes in shape and
integrity and undergo budding proliferation process.
To examine the pH variation on nanoemulsion
anti-fungal activity, C. albicans was treated with](https://image.slidesharecdn.com/nanoemulsoantifungica-161026181933/85/Nanoemulsao-antifungica-3-320.jpg)

![199
Figure 5. The susceptibility of Candida albicans to X8W60PC
nanoemulsion or its individual active ingredients. Cells were treated
with either 0.1% X8W60PC nanoemulsion or with individual in-
gredients at the equivalent concentrations for 15 minutes at 37 ◦C.
After treatment, cells were washed and plated on BHI plates to as-
sess the number of CFU. The bars represent standard error. ∗ – D1,
D2, and D3 depict three detergents used to prepare X8W60PC.
Table 1. Minimal fungicidal concentration (MFC) of
X8W60PC nanoemulsion and Nystatin on yeast and
filamentous fungi.
Microorganism X8W60PC Nystatin
(%) (units/ml)
Candida albicans 0.063 31.3
Candida parapsilosis 0.016 31.3
Microsporum gypseum 0.040 n/t∗
Trichophyton rubrum 0.032 n/t
Trichophyton mentagrophytes 0.016 n/t
Aspergillus fumigatus 0.010 n/t
Fusarium oxysporum 0.032 62.5
∗ Not tested.
concentrations of the nanoemulsion to C. albicans
and parapsilosis, T. spp., M. gypseum, A. fumigatus,
and F. oxysporum (Figure 6 and Table 1). As a ref-
erence anti-fungal agent, Nystatin has been used in
some experiments in parallel to nanoemuslion. As-
pergillus fumigatus was the most susceptible, <0.01%
nanoemulsion resulted in complete killing and C. al-
bicans was the most resistant to the nanoemulsion
fungicidal activity (Figure 6 and Table 1). Overall, all
tested fungi were susceptible to the nanoemulsion at a
concentration below 0.1%.
Discussion
There is an ongoing need for new anti-fungal agents
to combat the continuous development of resistant
yeast or filamentous fungi species due to inappropriate
use of anti-fungal drugs in humans and animals [22–
24]. Surfactant nanoemulsions are a treatment option.
Nanoemulsions are novel water-in-oil formulations
that are stabilized by the addition of small amounts
of surfactant and solvent [12]. The water-immiscible,
liquid phase is mixed into an aqueous phase by high
stress mechanical extrusion, yielding a uniform popu-
lation of droplets ranging in diameter from 400–800
nm. Due to their intrinsic features, nanoemulsions
can be further diluted in aqueous solutions and stored
at a broad range of temperature. Recently, surfactant
nanoemulsions have been reported to have extens-
ive bactericidal, sporicidal and virucidal effects [12,
16–20].
In this study, we showed that the surfactant
nanoemulsion designated X8W60PC has fungicidal
activity. At 1% concentration, X8W60PC reduced the
number of C. albicans CFU by more than four logs
within 15 minutes of treatment and two-hour treatment
reduced the number of C. albicans CFU by six logs.
Of note, Nystatin, which is presently used for topical
treatment, needs to be applied for approximately four
weeks to treat cutaneous candidiasis with a dose as
high as 100, 000 units gram−1 [25]. Moreover, the
nanoemulsion is most effective at physiological pH,
another good feature in its potential application as an
anti-fungal drug for topical treatment.
Since some ingredients of X8W60PC are biocidal,
we tested whether these ingredients alone, at concen-
trations equivalent to those in the nanoemulsion, have
anti-fungal activity. D1, D3 and solvent were inef-
fective against yeast at equivalent concentrations. Ten
to 1000 times higher concentrations of the individual
ingredients were required to obtain a comparable fun-
gicidal activity (data not shown). D2, at a concentra-
tion equivalent to that of the nanoemulsion, had some
fungicidal activity; it reduced the number of CFU by
approximately two logs (Figure 5). These data suggest
that the fungicidal activity of X8W60PC is not due to
its ingredients, but to the nanoemulsion structure.
Minimal Inhibitory Concentration assays further
confirmed the anti-fungal activity of the nanoemul-
sion. There was a slight variation in susceptibility
among the tested fungi. C. albicans appeared to be
the least susceptible (Figure 6A) and Candida parap-
silosis (Figure 6B) and Trichophyton spp. (Figure](https://image.slidesharecdn.com/nanoemulsoantifungica-161026181933/85/Nanoemulsao-antifungica-5-320.jpg)
![201
the surfactant nanoemulsion at biocidal concentrations
is non-toxic in short term application to skin (unpub-
lished data), mucous membranes, and gastrointestinal
tract [17], its long term toxicity has never been tested.
Therefore, chronic toxicity tests are essential. Finally,
due to the rapid action of the nanoemulsion, it is un-
likely that therapeutic concentrations could result in
development of resistant strains, however, this can-
not be excluded at this time. Currently, we are in the
process of investigating all these issues.
In conclusion, since X8W60PC exhibits fungicidal
activity on yeast and filamentous fungi, this nanoemul-
sion has potential as a topical treatment for a variety of
mycoses.
Acknowledgments
This work was supported with DARPA (Defense Ad-
vanced Research Project Agency) contract #MDA
972-1-007 of the Unconventional Pathogen Counter-
measures Program. The authors wish to thank Dr.
Nicholas Beeson for revision of the manuscript and
critical review, and Chris Edwards for assistance with
SEM microscopy.
References
1. Mitchell TG. Medical Mycology. In: Joklik WK, Willett HP,
Amos DB, Wilfert CM, eds. Zinsser Mirobiology 20th edtion.
Appleton and Lange, Connecticut, 1992: 1071–1081.
2. Kruger W, Stockschlader M, Russmann, B., et al. Experi-
ence with liposome Amphotericin-B in 60 patients undergoing
high-dose therapy and bone marrow or peripheral blood stem
cell transplantation. Br J Haematol. 1995; 91, 684–690.
3. Stevens DA, Diaz M, Negroni R, et al. Safety evaluation of
chronic fluconazole therapy. Fluconazole Pan-American Study
Group. Chemotherapy. 1997; 43: 371–377.
4. Yamaguchi H. Molecular and biochemical mechanisms of
drug resistance in fungi. Nippon Ishinkin Gakkai Zasshi. 1999;
40: 199–208.
5. Wirsching S, Michel S, Kohler G, Morschhauser J. Activation
of the multiple drug resistance gene MDR1 in fluconazole-
resistant, clinical Candida albicans strains is caused by muta-
tions in a trans-regulatory factor. Journal of Bacteriology.
2000; 182: 400–404.
6. Xu J, Ramos AR, Vilgalys R, Mitchell TG. Clonal and spon-
taneous origins of fluconazole resistance in Candida albicans.
Journal of Clinical Microbiology 2000; 38: 1214–1220.
7. Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson
JB, Kohn LM. Evolution of drug resistance in experimental
populations of Candida albicans. Journal of Bacteriology
2000; 182: 1515–1522.
8. Lineaweaver W, Howard R, Soucy D, et al. Topical antimicro-
bial toxicity. Arch. Surg. 1985; 120: 267–270.
9. Rutala WA, Weber DJ. Uses of inogranic hypochlorite
(bleach) in health-care facilities. Clin. Microbiol. Rev. 1997;
10: 597–610.
10. McDonnell G, Russell AD. Antiseptics and disinfectants:
activity, action, and resistance. Clin. Microbiol. Rev. 1999; 12:
147–179.
11. Bundgaard-Nielsen K, Nielsen PV. Fungicidal effect of 15 dis-
infectants against 25 fungal contaminants commonly found
in bread and cheese manufacturing. J. Food. Prot. 1996; 59:
268–275.
12. Baker JR, Jr, Wright DC, Hayes MM, Hamouda T, Brisker JM.
Methods of inactivating bacteria including bacterial spores.
U.S. Patent # 6015832; 2000.
13. Gregoriadis G. ed. Liposome Technology: Liposome Pre-
parations and Related Techniques. Boca Raton, Fla.: CRC
Press, 1993 (Non-ionic surfactant vesicles: preparation and
characterization. 2nd ed. vol. 1).
14. Gregoriadis G, Florence AT. Liposomes in drug delivery. Clin-
ical, diagnostic and ophthalmic potential. Drugs. 1993; 45:
15–28.
15. Wasan KM, Lopez-Berestein G. The past, present, and future
uses of liposomes in treating infectious diseases. Immunophar-
macol. Immunotoxicol. 1995; 17: 1–15.
16. Hamouda T, Wright DC, Brisker JM, Baker JR, Jr. Microbi-
ocidal effects of liposome-like microemulsions on pathogenic
Gram negative bacteria. American Society for Microbiology,
98th General Meeting, Atlanta, Georgia, 1998: 47.
17. Hamouda T, Hayes MM, Cao Z, et al. A novel surfactant
nanoemulsion with broad-spectrum sporicidal activity against
Bacillus spores. J. Infect. Dis. 1999; 180: 1939–1949.
18. Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker
JR, Jr. A novel surfactant nanoemulsion with a unique non-
irritant topical antimicrobial activity against bacteria, envel-
oped viruses and fungi. International Microbiol. Res. 2000;
156: 1–7.
19. Donovan BW, Reuter JD, Cao Z., Myc A, Johnson K, Baker
JR, Jr. Prevention of murine influenza A virus pneumonitis by
surfactant nano-emulsions Antivir. Chem. Chemother. 2000;
11: 41–49.
20. Myc A, Anderson, MJ, Wright DC, Brisker J, Baker JR, Jr. In-
hibitory effect of non-phospholipid liposomes and nanoemul-
sions on influenza A virus infectivity. 38th Interscience Con-
ference on Antimicrobial Agents and Chemotherapy, San
Diego, California, 1998: 336.
21. Bray, D.F., et al. (1993) Micros.Res. and Technique. 26: 489–
495. A comparison of HMDS, Peldri II, and critical drying
methods for SEM of biological specimens.
22. Morse SS. Factors in the emergence of infectious diseases.
Emerg. Infect. Dis. 1995; 1: 7–15.
23. Riley LW. Drug-resistant tuberculosis. Clin. Infect. Dis. 1993;
17, Suppl. 2, S442–446.
24. Tenover FC, Hughes JM. The challenges of emerging infec-
tious diseases. Development and spread of multiply-resistant
bacterial pathogens. JAMA 1996; 275: 300–304.
25. Clayton YM, Conner BL. Comparison of clotrimazole cream,
Whitfield’s ointment and nystatin ointment for the topical
treatment of ringworm infections, pityriasis versicolor, eryth-
rasma and candidiasis. Br. J. Dermatol. 1973; 89: 297–303.
Address for correspondence: James R. Baker, Jr., Room 9220
MSRB III, 1150 West Medical Center Drive, Ann Arbor, MI 48109-
0648, U.S.A.
Phone (734) 647-2777; Fax: (734) 936-2990;
E-mail: jbakerjr@umich.edu](https://image.slidesharecdn.com/nanoemulsoantifungica-161026181933/85/Nanoemulsao-antifungica-7-320.jpg)
