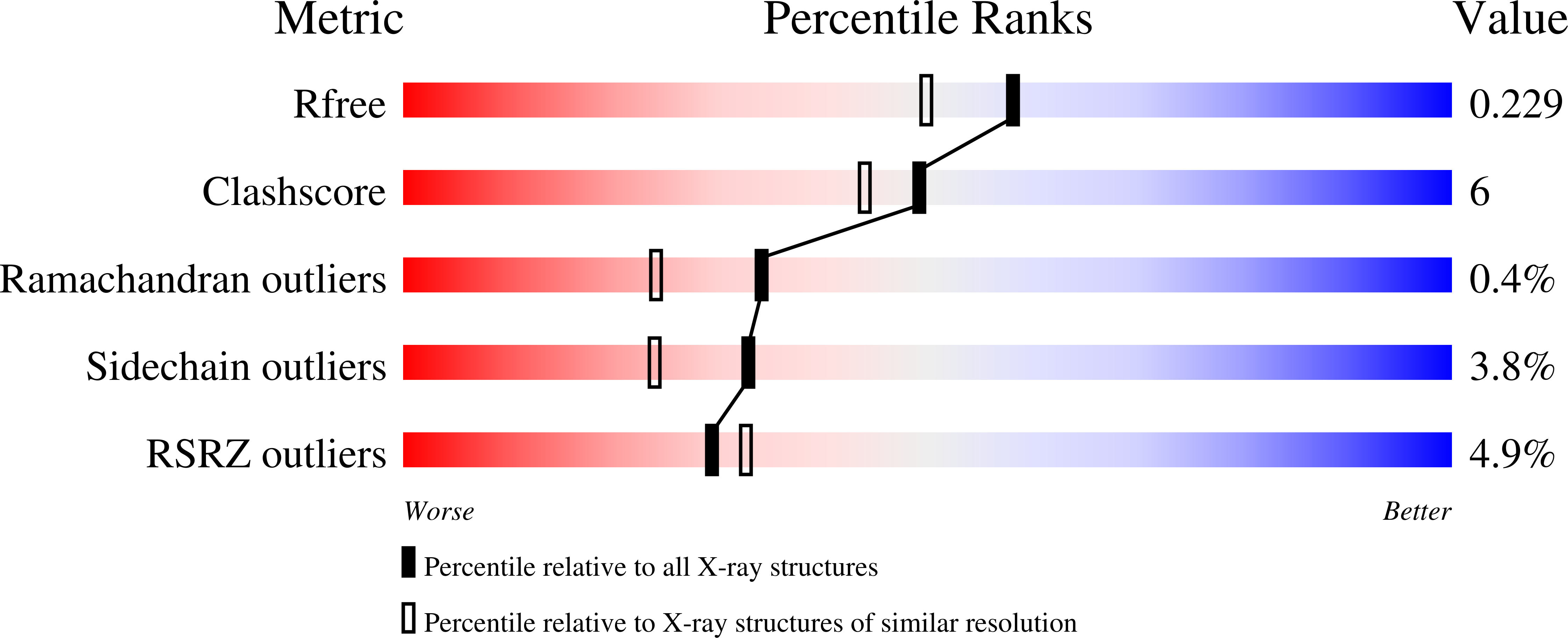
Conformational plasticity at the IgE-binding site of the B-cell receptor CD23.
Dhaliwal, B., Pang, M.O., Yuan, D., Yahya, N., Fabiane, S.M., McDonnell, J.M., Gould, H.J., Beavil, A.J., Sutton, B.J.(2013) Mol Immunol 56: 693-697
- PubMed: 23933509
- DOI: https://doi.org/10.1016/j.molimm.2013.07.005
- Primary Citation of Related Structures:
4J6J, 4J6K, 4J6L, 4J6M, 4J6N, 4J6P, 4J6Q - PubMed Abstract:
IgE antibodies play a central role in allergic disease. They recognize allergens via their Fab regions, whilst their effector functions are controlled through interactions of the Fc region with two principal cell surface receptors, FcɛRI and CD23. Crosslinking of FcɛRI-bound IgE on mast cells and basophils by allergen initiates an immediate inflammatory response, while the interaction of IgE with CD23 on B-cells regulates IgE production. We have determined the structures of the C-type lectin "head" domain of CD23 from seven crystal forms. The thirty-five independent structures reveal extensive conformational plasticity in two loops that are critical for IgE binding.
Organizational Affiliation:
King's College London, Randall Division of Cell and Molecular Biophysics, New Hunt's House, Guy's Campus, London SE1 1UL, UK; MRC & Asthma UK Centre in Allergic Mechanisms of Asthma, London, UK. Electronic address: [email protected].