atomic mass unit
- Also called:
- dalton
- Related Topics:
- atom
- physical constant
- atomic mass
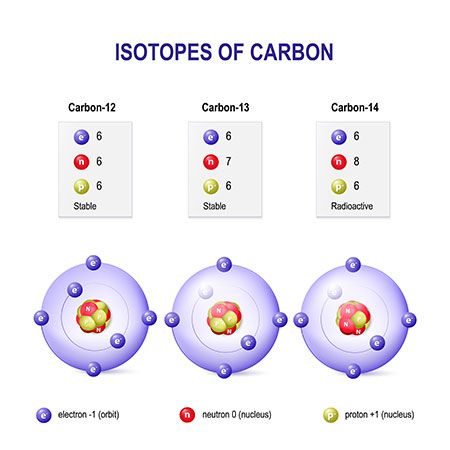
atomic mass unit (AMU), in physics and chemistry, a unit for expressing masses of atoms, molecules, or subatomic particles. An atomic mass unit is equal to 1/12 the mass of a single atom of carbon-12, the most abundant isotope of carbon, or 1.660538921 × 10 −24 gram. The mass of an atom consists of the mass of the nucleus plus that of the electrons, so the atomic mass unit is not exactly the same as the mass of the proton or neutron. Atomic mass units are also called daltons (Da), for chemist John Dalton.
Atomic mass units are often used to describe an element’s atomic weight, which is the weighted average of the atomic masses of an element’s naturally occurring isotopes. For example, while the atomic mass of helium-3 is 3.016029 AMU and that of helium-4 is 4.002603 AMU, the atomic weight of helium is 4.002602 AMU because the 3He isotope makes up less than 1 percent of all helium found naturally. In biochemistry, the molecular masses of nucleic acids and proteins are commonly expressed in kilodaltons (kDa) or megadaltons (MDa).