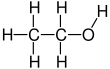
Ethanol
| |||
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈɛθənɒl/ | ||
| Systematic IUPAC name
ethanol[1] | |||
| Other names
Absolute alcohol
alcohol cologne spirit drinking alcohol ethylic alcohol EtOH ethyl alcohol ethyl hydrate ethyl hydroxide ethylol grain alcohol hydroxyethane methylcarbinol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Beilstein Reference | 1718733 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.526 | ||
| Gmelin Reference | 787 | ||
PubChem CID
|
|||
| UNII | |||
| UN number | UN 1170 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.07 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.7893 g/cm3 (at 20 °C)[2] | ||
| Melting point | −114.14 ± 0.03[2] °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.24 ± 0.09[2] °C (172.83 ± 0.16 °F; 351.39 ± 0.09 K) | ||
| miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO)[3][4] | ||
| −33.60·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3611[2] | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C)[5] | ||
| 1.69 D[6] | |||
| Hazards | |||
| NFPA 704 |
| ||
| Flash point | 14 °C (Absolute) | ||
| U.S. Permissible exposure limit (PEL) |
TWA 1000 ppm (1900 mg/m3) [7] | ||
| Related compounds | |||
| Related compounds | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

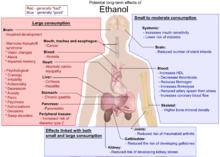
Ethanol, also known as ethyl alcohol, grain alcohol or just alcohol, is a flammable, colorless chemical compound whose chemical formula is C2H5OH, also written as C2H6O. It is the active part of alcoholic drinks, which are drunk in most cultures worldwide. Ethanol is also used as a solvent because it can dissolve many other chemicals and is not very toxic. Yeast makes most of the ethanol that people use.
Ethanol fuel
[change | change source]Ethanol fuel can be used instead of gasoline in cars and other engines. Engines can use pure ethanol or ethanol mixed with gasoline.
In Brazil, ethanol fuel made from sugar cane provides 18% of the fuel for cars. Therefore, the country does not have to buy petroleum from other countries.[8]To do so, much of the rainforest has been cut down to grow more sugarcane, which is then fermented into ethanol.
Most cars in the United States can run on fuels that have of up to 10% ethanol in them. Carmakers like Ford, DaimlerChrysler, and GM also make vehicles specifically designed to run on higher ethanol blends. Some of their engines can run on up to 85% ethanol (E85). By mid-2006, there were about six million E85-compatible vehicles on the country's roads, which can run on fuel wth 85% ethanol.[9]
References
[change | change source]- ↑ "Ethanol – Compound Summary". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ 2.0 2.1 2.2 2.3 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.246. ISBN 1439855110.
- ↑ Ballinger P, Long FA (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds1,2". Journal of the American Chemical Society. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ↑ Arnett EM, Venkatasubramaniam KG (1983). "Thermochemical acidities in three superbase systems". J. Org. Chem. 48 (10): 1569–1578. doi:10.1021/jo00158a001.
- ↑ Lide DR, ed. (2012). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL.: CRC Press/Taylor and Francis. pp. 6–232. ISBN 9781439855126.
- ↑ Lide DR, ed. (2008). CRC Handbook of Chemistry and Physics (89th ed.). Boca Raton: CRC Press. pp. 9–55. ISBN 9781420066791.
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0262". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "America and Brazil Intersect on Ethanol". Archived from the original on 2007-09-26. Retrieved 2007-06-05.
- ↑ American energy: The renewable path to energy security
Other websites
[change | change source]- US E85 refueling map Archived 2007-09-21 at the Wayback Machine
- International Chemical Safety Card 0044
- Molview from bluerhinos.co.uk Archived 2008-09-25 at the Wayback Machine See Ethanol in 3D (may take over a minute to load using a dial-up connection)