WO2024219896A1 - Non-aqueous electrolyte and lithium secondary battery comprising same - Google Patents
Non-aqueous electrolyte and lithium secondary battery comprising same Download PDFInfo
- Publication number
- WO2024219896A1 WO2024219896A1 PCT/KR2024/005354 KR2024005354W WO2024219896A1 WO 2024219896 A1 WO2024219896 A1 WO 2024219896A1 KR 2024005354 W KR2024005354 W KR 2024005354W WO 2024219896 A1 WO2024219896 A1 WO 2024219896A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- chemical formula
- aqueous electrolyte
- carbon atoms
- organic solvent
- Prior art date
Links
- 239000011255 nonaqueous electrolyte Substances 0.000 title claims abstract description 63
- 229910052744 lithium Inorganic materials 0.000 title claims description 43
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 title claims description 39
- 239000000126 substance Substances 0.000 claims abstract description 122
- 239000003960 organic solvent Substances 0.000 claims abstract description 48
- -1 cyclic siloxane compound Chemical class 0.000 claims abstract description 30
- 239000000654 additive Substances 0.000 claims abstract description 20
- 230000000996 additive effect Effects 0.000 claims abstract description 19
- 229910003002 lithium salt Inorganic materials 0.000 claims abstract description 16
- 159000000002 lithium salts Chemical class 0.000 claims abstract description 16
- 125000004432 carbon atom Chemical group C* 0.000 claims description 60
- 150000001875 compounds Chemical class 0.000 claims description 40
- 239000007773 negative electrode material Substances 0.000 claims description 26
- 125000001424 substituent group Chemical group 0.000 claims description 21
- 125000003545 alkoxy group Chemical group 0.000 claims description 18
- 239000002409 silicon-based active material Substances 0.000 claims description 18
- 125000001174 sulfone group Chemical group 0.000 claims description 12
- 125000000217 alkyl group Chemical group 0.000 claims description 11
- 125000004185 ester group Chemical group 0.000 claims description 11
- 229910052731 fluorine Inorganic materials 0.000 claims description 11
- QAOWNCQODCNURD-UHFFFAOYSA-L sulfate group Chemical group S(=O)(=O)([O-])[O-] QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 11
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 claims description 11
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 claims description 10
- 125000002947 alkylene group Chemical group 0.000 claims description 8
- 150000005676 cyclic carbonates Chemical class 0.000 claims description 8
- 125000003342 alkenyl group Chemical group 0.000 claims description 6
- 125000000304 alkynyl group Chemical group 0.000 claims description 6
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical group [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 5
- 229910013870 LiPF 6 Inorganic materials 0.000 claims description 5
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical group OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 claims description 5
- 229910052794 bromium Inorganic materials 0.000 claims description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 5
- 229910052801 chlorine Inorganic materials 0.000 claims description 5
- 150000002148 esters Chemical class 0.000 claims description 5
- 125000001033 ether group Chemical group 0.000 claims description 5
- 229910052740 iodine Inorganic materials 0.000 claims description 5
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 claims description 5
- ZBKFYXZXZJPWNQ-UHFFFAOYSA-N isothiocyanate group Chemical group [N-]=C=S ZBKFYXZXZJPWNQ-UHFFFAOYSA-N 0.000 claims description 5
- 125000000468 ketone group Chemical group 0.000 claims description 5
- 125000002560 nitrile group Chemical group 0.000 claims description 5
- 125000005373 siloxane group Chemical group [SiH2](O*)* 0.000 claims description 5
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 claims description 5
- AMXOYNBUYSYVKV-UHFFFAOYSA-M lithium bromide Chemical compound [Li+].[Br-] AMXOYNBUYSYVKV-UHFFFAOYSA-M 0.000 claims description 4
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 claims description 4
- 229910013063 LiBF 4 Inorganic materials 0.000 claims description 3
- 229910013684 LiClO 4 Inorganic materials 0.000 claims description 3
- 229910010941 LiFSI Inorganic materials 0.000 claims description 3
- 229910013398 LiN(SO2CF2CF3)2 Inorganic materials 0.000 claims description 3
- ACFSQHQYDZIPRL-UHFFFAOYSA-N lithium;bis(1,1,2,2,2-pentafluoroethylsulfonyl)azanide Chemical compound [Li+].FC(F)(F)C(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)C(F)(F)F ACFSQHQYDZIPRL-UHFFFAOYSA-N 0.000 claims description 3
- VDVLPSWVDYJFRW-UHFFFAOYSA-N lithium;bis(fluorosulfonyl)azanide Chemical compound [Li+].FS(=O)(=O)[N-]S(F)(=O)=O VDVLPSWVDYJFRW-UHFFFAOYSA-N 0.000 claims description 3
- 229910010238 LiAlCl 4 Inorganic materials 0.000 claims description 2
- 229910010090 LiAlO 4 Inorganic materials 0.000 claims description 2
- 229910015015 LiAsF 6 Inorganic materials 0.000 claims description 2
- 229910015044 LiB Inorganic materials 0.000 claims description 2
- 229910012513 LiSbF 6 Inorganic materials 0.000 claims description 2
- HSZCZNFXUDYRKD-UHFFFAOYSA-M lithium iodide Inorganic materials [Li+].[I-] HSZCZNFXUDYRKD-UHFFFAOYSA-M 0.000 claims description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims 1
- 229910013188 LiBOB Inorganic materials 0.000 claims 1
- 239000011737 fluorine Substances 0.000 claims 1
- 239000006259 organic additive Substances 0.000 claims 1
- 239000010408 film Substances 0.000 description 26
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 23
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 23
- 239000010410 layer Substances 0.000 description 22
- 239000007774 positive electrode material Substances 0.000 description 17
- 229910052723 transition metal Inorganic materials 0.000 description 16
- 239000011230 binding agent Substances 0.000 description 13
- 239000004020 conductor Substances 0.000 description 12
- 238000003860 storage Methods 0.000 description 11
- 239000002905 metal composite material Substances 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- SBLRHMKNNHXPHG-UHFFFAOYSA-N 4-fluoro-1,3-dioxolan-2-one Chemical compound FC1COC(=O)O1 SBLRHMKNNHXPHG-UHFFFAOYSA-N 0.000 description 9
- 239000000203 mixture Substances 0.000 description 9
- OIFBSDVPJOWBCH-UHFFFAOYSA-N Diethyl carbonate Chemical compound CCOC(=O)OCC OIFBSDVPJOWBCH-UHFFFAOYSA-N 0.000 description 8
- 230000000052 comparative effect Effects 0.000 description 8
- 239000011572 manganese Substances 0.000 description 8
- 229910052759 nickel Inorganic materials 0.000 description 8
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 7
- 229910052782 aluminium Inorganic materials 0.000 description 7
- IGILRSKEFZLPKG-UHFFFAOYSA-M lithium;difluorophosphinate Chemical compound [Li+].[O-]P(F)(F)=O IGILRSKEFZLPKG-UHFFFAOYSA-M 0.000 description 7
- 239000002388 carbon-based active material Substances 0.000 description 6
- 239000003792 electrolyte Substances 0.000 description 6
- 238000005755 formation reaction Methods 0.000 description 6
- 229910002804 graphite Inorganic materials 0.000 description 6
- 239000010439 graphite Substances 0.000 description 6
- 239000002002 slurry Substances 0.000 description 6
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 5
- 239000006182 cathode active material Substances 0.000 description 5
- 230000008602 contraction Effects 0.000 description 5
- 239000011267 electrode slurry Substances 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 125000001153 fluoro group Chemical group F* 0.000 description 5
- 230000014759 maintenance of location Effects 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 150000003624 transition metals Chemical class 0.000 description 5
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 4
- 239000005977 Ethylene Substances 0.000 description 4
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 4
- 239000004372 Polyvinyl alcohol Substances 0.000 description 4
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 239000006229 carbon black Substances 0.000 description 4
- 239000002041 carbon nanotube Substances 0.000 description 4
- 229910021393 carbon nanotube Inorganic materials 0.000 description 4
- 239000010949 copper Substances 0.000 description 4
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- JBTWLSYIZRCDFO-UHFFFAOYSA-N ethyl methyl carbonate Chemical compound CCOC(=O)OC JBTWLSYIZRCDFO-UHFFFAOYSA-N 0.000 description 4
- 239000004745 nonwoven fabric Substances 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 239000010936 titanium Substances 0.000 description 4
- 229910052719 titanium Inorganic materials 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 229910014689 LiMnO Inorganic materials 0.000 description 3
- 229910013290 LiNiO 2 Inorganic materials 0.000 description 3
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 229920000459 Nitrile rubber Polymers 0.000 description 3
- 239000002033 PVDF binder Substances 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- 229910021383 artificial graphite Inorganic materials 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 229910017052 cobalt Inorganic materials 0.000 description 3
- 239000010941 cobalt Substances 0.000 description 3
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 3
- 239000006258 conductive agent Substances 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 229910052802 copper Inorganic materials 0.000 description 3
- 239000012153 distilled water Substances 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 229910001416 lithium ion Inorganic materials 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910021382 natural graphite Inorganic materials 0.000 description 3
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 238000007086 side reaction Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- DHKHKXVYLBGOIT-UHFFFAOYSA-N 1,1-Diethoxyethane Chemical compound CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 2
- VAYTZRYEBVHVLE-UHFFFAOYSA-N 1,3-dioxol-2-one Chemical compound O=C1OC=CO1 VAYTZRYEBVHVLE-UHFFFAOYSA-N 0.000 description 2
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 2
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 2
- 229920000049 Carbon (fiber) Polymers 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 101150065749 Churc1 gene Proteins 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 229920002943 EPDM rubber Polymers 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 229910003936 Li(Ni0.5Mn0.3Co0.2)O2 Inorganic materials 0.000 description 2
- 229910004406 Li(Ni0.6Mn0.2CO0.2)O2 Inorganic materials 0.000 description 2
- 229910004427 Li(Ni0.7Mn0.15Co0.15)O2 Inorganic materials 0.000 description 2
- 229910004437 Li(Ni0.8Mn0.1Co0.1)O2 Inorganic materials 0.000 description 2
- 229910012851 LiCoO 2 Inorganic materials 0.000 description 2
- 229910015643 LiMn 2 O 4 Inorganic materials 0.000 description 2
- 229910013716 LiNi Inorganic materials 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 229920000265 Polyparaphenylene Polymers 0.000 description 2
- 102100038239 Protein Churchill Human genes 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 239000006230 acetylene black Substances 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical compound [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 239000004917 carbon fiber Substances 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000006231 channel black Substances 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- NJLLQSBAHIKGKF-UHFFFAOYSA-N dipotassium dioxido(oxo)titanium Chemical compound [K+].[K+].[O-][Ti]([O-])=O NJLLQSBAHIKGKF-UHFFFAOYSA-N 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 2
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 229920001973 fluoroelastomer Polymers 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- JBFHTYHTHYHCDJ-UHFFFAOYSA-N gamma-caprolactone Chemical compound CCC1CCC(=O)O1 JBFHTYHTHYHCDJ-UHFFFAOYSA-N 0.000 description 2
- GAEKPEKOJKCEMS-UHFFFAOYSA-N gamma-valerolactone Chemical compound CC1CCC(=O)O1 GAEKPEKOJKCEMS-UHFFFAOYSA-N 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000003273 ketjen black Substances 0.000 description 2
- 239000006233 lamp black Substances 0.000 description 2
- VGYDTVNNDKLMHX-UHFFFAOYSA-N lithium;manganese;nickel;oxocobalt Chemical class [Li].[Mn].[Ni].[Co]=O VGYDTVNNDKLMHX-UHFFFAOYSA-N 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 229910052750 molybdenum Inorganic materials 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- SUSQOBVLVYHIEX-UHFFFAOYSA-N phenylacetonitrile Chemical compound N#CCC1=CC=CC=C1 SUSQOBVLVYHIEX-UHFFFAOYSA-N 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 229920002239 polyacrylonitrile Polymers 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920006254 polymer film Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 2
- 239000004627 regenerated cellulose Substances 0.000 description 2
- 238000005096 rolling process Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 229920003048 styrene butadiene rubber Polymers 0.000 description 2
- 239000006234 thermal black Substances 0.000 description 2
- 239000010409 thin film Substances 0.000 description 2
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 2
- 229910000314 transition metal oxide Inorganic materials 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- 239000011787 zinc oxide Substances 0.000 description 2
- ZZXUZKXVROWEIF-UHFFFAOYSA-N 1,2-butylene carbonate Chemical compound CCC1COC(=O)O1 ZZXUZKXVROWEIF-UHFFFAOYSA-N 0.000 description 1
- ZPFAVCIQZKRBGF-UHFFFAOYSA-N 1,3,2-dioxathiolane 2,2-dioxide Chemical compound O=S1(=O)OCCO1 ZPFAVCIQZKRBGF-UHFFFAOYSA-N 0.000 description 1
- FSSPGSAQUIYDCN-UHFFFAOYSA-N 1,3-Propane sultone Chemical compound O=S1(=O)CCCO1 FSSPGSAQUIYDCN-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- NVJUHMXYKCUMQA-UHFFFAOYSA-N 1-ethoxypropane Chemical compound CCCOCC NVJUHMXYKCUMQA-UHFFFAOYSA-N 0.000 description 1
- HFZLSTDPRQSZCQ-UHFFFAOYSA-N 1-pyrrolidin-3-ylpyrrolidine Chemical compound C1CCCN1C1CNCC1 HFZLSTDPRQSZCQ-UHFFFAOYSA-N 0.000 description 1
- PQNNUQKDERZMPQ-UHFFFAOYSA-N 2,2-bis(trifluoromethyl)-1,3-dioxolane Chemical compound FC(F)(F)C1(C(F)(F)F)OCCO1 PQNNUQKDERZMPQ-UHFFFAOYSA-N 0.000 description 1
- KTPHYLJFAZNALV-UHFFFAOYSA-N 2,3,4-trifluorobenzonitrile Chemical compound FC1=CC=C(C#N)C(F)=C1F KTPHYLJFAZNALV-UHFFFAOYSA-N 0.000 description 1
- GKPHNZYMLJPYJJ-UHFFFAOYSA-N 2,3-difluorobenzonitrile Chemical compound FC1=CC=CC(C#N)=C1F GKPHNZYMLJPYJJ-UHFFFAOYSA-N 0.000 description 1
- DAVJMKMVLKOQQC-UHFFFAOYSA-N 2-(2-fluorophenyl)acetonitrile Chemical compound FC1=CC=CC=C1CC#N DAVJMKMVLKOQQC-UHFFFAOYSA-N 0.000 description 1
- UHOPWFKONJYLCF-UHFFFAOYSA-N 2-(2-sulfanylethyl)isoindole-1,3-dione Chemical compound C1=CC=C2C(=O)N(CCS)C(=O)C2=C1 UHOPWFKONJYLCF-UHFFFAOYSA-N 0.000 description 1
- JHQBLYITVCBGTO-UHFFFAOYSA-N 2-(4-fluorophenyl)acetonitrile Chemical compound FC1=CC=C(CC#N)C=C1 JHQBLYITVCBGTO-UHFFFAOYSA-N 0.000 description 1
- GDHXJNRAJRCGMX-UHFFFAOYSA-N 2-fluorobenzonitrile Chemical compound FC1=CC=CC=C1C#N GDHXJNRAJRCGMX-UHFFFAOYSA-N 0.000 description 1
- LWLOKSXSAUHTJO-UHFFFAOYSA-N 4,5-dimethyl-1,3-dioxolan-2-one Chemical compound CC1OC(=O)OC1C LWLOKSXSAUHTJO-UHFFFAOYSA-N 0.000 description 1
- BJWMSGRKJIOCNR-UHFFFAOYSA-N 4-ethenyl-1,3-dioxolan-2-one Chemical compound C=CC1COC(=O)O1 BJWMSGRKJIOCNR-UHFFFAOYSA-N 0.000 description 1
- LSUWCXHZPFTZSF-UHFFFAOYSA-N 4-ethyl-5-methyl-1,3-dioxolan-2-one Chemical compound CCC1OC(=O)OC1C LSUWCXHZPFTZSF-UHFFFAOYSA-N 0.000 description 1
- AEKVBBNGWBBYLL-UHFFFAOYSA-N 4-fluorobenzonitrile Chemical compound FC1=CC=C(C#N)C=C1 AEKVBBNGWBBYLL-UHFFFAOYSA-N 0.000 description 1
- AUXJVUDWWLIGRU-UHFFFAOYSA-N 4-propyl-1,3-dioxolan-2-one Chemical compound CCCC1COC(=O)O1 AUXJVUDWWLIGRU-UHFFFAOYSA-N 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 229910000925 Cd alloy Inorganic materials 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- 229910004424 Li(Ni0.8Co0.15Al0.05)O2 Inorganic materials 0.000 description 1
- 229910013733 LiCo Inorganic materials 0.000 description 1
- 229910015645 LiMn Inorganic materials 0.000 description 1
- 229910015644 LiMn 2 - z Ni Inorganic materials 0.000 description 1
- 229910013528 LiN(SO2 CF3)2 Inorganic materials 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- XOBKSJJDNFUZPF-UHFFFAOYSA-N Methoxyethane Chemical compound CCOC XOBKSJJDNFUZPF-UHFFFAOYSA-N 0.000 description 1
- RJUFJBKOKNCXHH-UHFFFAOYSA-N Methyl propionate Chemical compound CCC(=O)OC RJUFJBKOKNCXHH-UHFFFAOYSA-N 0.000 description 1
- RFFFKMOABOFIDF-UHFFFAOYSA-N Pentanenitrile Chemical compound CCCCC#N RFFFKMOABOFIDF-UHFFFAOYSA-N 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 229910052772 Samarium Inorganic materials 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- VIEVWNYBKMKQIH-UHFFFAOYSA-N [Co]=O.[Mn].[Li] Chemical class [Co]=O.[Mn].[Li] VIEVWNYBKMKQIH-UHFFFAOYSA-N 0.000 description 1
- QTHKJEYUQSLYTH-UHFFFAOYSA-N [Co]=O.[Ni].[Li] Chemical class [Co]=O.[Ni].[Li] QTHKJEYUQSLYTH-UHFFFAOYSA-N 0.000 description 1
- SOXUFMZTHZXOGC-UHFFFAOYSA-N [Li].[Mn].[Co].[Ni] Chemical compound [Li].[Mn].[Co].[Ni] SOXUFMZTHZXOGC-UHFFFAOYSA-N 0.000 description 1
- IDSMHEZTLOUMLM-UHFFFAOYSA-N [Li].[O].[Co] Chemical class [Li].[O].[Co] IDSMHEZTLOUMLM-UHFFFAOYSA-N 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 229920000800 acrylic rubber Polymers 0.000 description 1
- BTGRAWJCKBQKAO-UHFFFAOYSA-N adiponitrile Chemical compound N#CCCCCC#N BTGRAWJCKBQKAO-UHFFFAOYSA-N 0.000 description 1
- NDPGDHBNXZOBJS-UHFFFAOYSA-N aluminum lithium cobalt(2+) nickel(2+) oxygen(2-) Chemical compound [Li+].[O--].[O--].[O--].[O--].[Al+3].[Co++].[Ni++] NDPGDHBNXZOBJS-UHFFFAOYSA-N 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- IAQRGUVFOMOMEM-UHFFFAOYSA-N butene Natural products CC=CC IAQRGUVFOMOMEM-UHFFFAOYSA-N 0.000 description 1
- 229920005549 butyl rubber Polymers 0.000 description 1
- KVNRLNFWIYMESJ-UHFFFAOYSA-N butyronitrile Chemical compound CCCC#N KVNRLNFWIYMESJ-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 229910000428 cobalt oxide Inorganic materials 0.000 description 1
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(ii) oxide Chemical compound [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- VBWIZSYFQSOUFQ-UHFFFAOYSA-N cyclohexanecarbonitrile Chemical compound N#CC1CCCCC1 VBWIZSYFQSOUFQ-UHFFFAOYSA-N 0.000 description 1
- SVPZJHKVRMRREG-UHFFFAOYSA-N cyclopentanecarbonitrile Chemical compound N#CC1CCCC1 SVPZJHKVRMRREG-UHFFFAOYSA-N 0.000 description 1
- 238000009831 deintercalation Methods 0.000 description 1
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 1
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 1
- VUPKGFBOKBGHFZ-UHFFFAOYSA-N dipropyl carbonate Chemical compound CCCOC(=O)OCCC VUPKGFBOKBGHFZ-UHFFFAOYSA-N 0.000 description 1
- POLCUAVZOMRGSN-UHFFFAOYSA-N dipropyl ether Chemical compound CCCOCCC POLCUAVZOMRGSN-UHFFFAOYSA-N 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000011883 electrode binding agent Substances 0.000 description 1
- 229940093499 ethyl acetate Drugs 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- QKBJDEGZZJWPJA-UHFFFAOYSA-N ethyl propyl carbonate Chemical compound [CH2]COC(=O)OCCC QKBJDEGZZJWPJA-UHFFFAOYSA-N 0.000 description 1
- 125000003709 fluoroalkyl group Chemical group 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 229910021389 graphene Inorganic materials 0.000 description 1
- 125000003106 haloaryl group Chemical group 0.000 description 1
- 229910021385 hard carbon Inorganic materials 0.000 description 1
- SDAXRHHPNYTELL-UHFFFAOYSA-N heptanenitrile Chemical compound CCCCCCC#N SDAXRHHPNYTELL-UHFFFAOYSA-N 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 125000004366 heterocycloalkenyl group Chemical group 0.000 description 1
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 230000002687 intercalation Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 229910003473 lithium bis(trifluoromethanesulfonyl)imide Inorganic materials 0.000 description 1
- 229910000625 lithium cobalt oxide Inorganic materials 0.000 description 1
- 229910002102 lithium manganese oxide Inorganic materials 0.000 description 1
- FRMOHNDAXZZWQI-UHFFFAOYSA-N lithium manganese(2+) nickel(2+) oxygen(2-) Chemical class [O-2].[Mn+2].[Ni+2].[Li+] FRMOHNDAXZZWQI-UHFFFAOYSA-N 0.000 description 1
- QEXMICRJPVUPSN-UHFFFAOYSA-N lithium manganese(2+) oxygen(2-) Chemical class [O-2].[Mn+2].[Li+] QEXMICRJPVUPSN-UHFFFAOYSA-N 0.000 description 1
- QSZMZKBZAYQGRS-UHFFFAOYSA-N lithium;bis(trifluoromethylsulfonyl)azanide Chemical compound [Li+].FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F QSZMZKBZAYQGRS-UHFFFAOYSA-N 0.000 description 1
- URIIGZKXFBNRAU-UHFFFAOYSA-N lithium;oxonickel Chemical class [Li].[Ni]=O URIIGZKXFBNRAU-UHFFFAOYSA-N 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- VNKYTQGIUYNRMY-UHFFFAOYSA-N methoxypropane Chemical compound CCCOC VNKYTQGIUYNRMY-UHFFFAOYSA-N 0.000 description 1
- 229940017219 methyl propionate Drugs 0.000 description 1
- KKQAVHGECIBFRQ-UHFFFAOYSA-N methyl propyl carbonate Chemical compound CCCOC(=O)OC KKQAVHGECIBFRQ-UHFFFAOYSA-N 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- YKYONYBAUNKHLG-UHFFFAOYSA-N n-Propyl acetate Natural products CCCOC(C)=O YKYONYBAUNKHLG-UHFFFAOYSA-N 0.000 description 1
- 229910000480 nickel oxide Inorganic materials 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- YSIMAPNUZAVQER-UHFFFAOYSA-N octanenitrile Chemical compound CCCCCCCC#N YSIMAPNUZAVQER-UHFFFAOYSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- GNRSAWUEBMWBQH-UHFFFAOYSA-N oxonickel Chemical compound [Ni]=O GNRSAWUEBMWBQH-UHFFFAOYSA-N 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- FVSKHRXBFJPNKK-UHFFFAOYSA-N propionitrile Chemical compound CCC#N FVSKHRXBFJPNKK-UHFFFAOYSA-N 0.000 description 1
- 229940090181 propyl acetate Drugs 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- 229920001384 propylene homopolymer Polymers 0.000 description 1
- 230000001603 reducing effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 229910021384 soft carbon Inorganic materials 0.000 description 1
- 239000007784 solid electrolyte Substances 0.000 description 1
- 229910052596 spinel Inorganic materials 0.000 description 1
- 239000011029 spinel Substances 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- IAHFWCOBPZCAEA-UHFFFAOYSA-N succinonitrile Chemical compound N#CCCC#N IAHFWCOBPZCAEA-UHFFFAOYSA-N 0.000 description 1
- 229920005608 sulfonated EPDM Polymers 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- ZUHZGEOKBKGPSW-UHFFFAOYSA-N tetraglyme Chemical compound COCCOCCOCCOCCOC ZUHZGEOKBKGPSW-UHFFFAOYSA-N 0.000 description 1
- YFNKIDBQEZZDLK-UHFFFAOYSA-N triglyme Chemical compound COCCOCCOCCOC YFNKIDBQEZZDLK-UHFFFAOYSA-N 0.000 description 1
- QJMMCGKXBZVAEI-UHFFFAOYSA-N tris(trimethylsilyl) phosphate Chemical compound C[Si](C)(C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C QJMMCGKXBZVAEI-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
- PAPBSGBWRJIAAV-UHFFFAOYSA-N ε-Caprolactone Chemical compound O=C1CCCCCO1 PAPBSGBWRJIAAV-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to a non-aqueous electrolyte and a lithium secondary battery including the same.
- secondary batteries are the most suitable for various applications, and among these secondary batteries, lithium secondary batteries are attracting attention as they can be miniaturized to the point where they can be applied to personal IT devices and have the highest energy density.
- lithium secondary batteries are manufactured by injecting or impregnating a non-aqueous electrolyte into an electrode assembly consisting of a cathode, an anode, and a porous separator.
- the positive active materials of these lithium secondary batteries are being considered to include lithium-containing cobalt oxide, LiMnO 2 with a layered crystal structure, LiMn 2 O 4 with a spinel crystal structure, lithium-containing nickel oxide (LiNiO 2 ), and lithium nickel-cobalt-manganese transition metal oxides.
- silicon-based active materials such as graphite have been used as negative active materials, but recently, silicon-based active materials are also being considered because they have higher capacity than carbon-based active materials.
- the silicon-based active material has the advantage of having a high capacity, but has the problem of very large volume expansion/contraction during the charge/discharge process. This large volume expansion/contraction greatly reduces the conductivity of the negative electrode, which causes a decrease in life performance.
- a solid electrolyte interface layer hereinafter referred to as SEI film
- SEI film solid electrolyte interface layer
- silicon-based active materials have a large volume expansion, which causes problems such as SEI film breakage and continuous generation of new negative electrode surfaces. Accordingly, SEI film formation reactions continuously occur, which accelerates electrolyte side reactions, and the thickness of the SEI film increases, which increases resistance.
- One object of the present invention is to solve the above problems, and to provide a non-aqueous electrolyte capable of forming an SEI film having excellent resilience and improved durability on a negative electrode, thereby implementing a lithium secondary battery having improved life performance and storage performance.
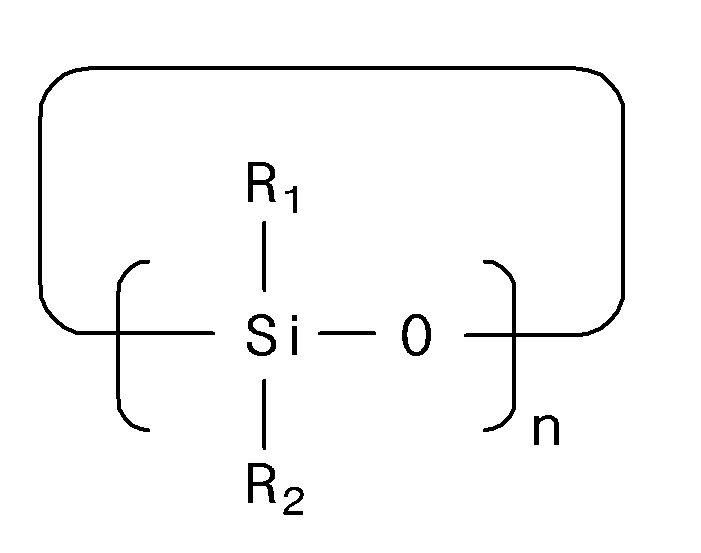
- the present invention provides a non-aqueous electrolyte comprising a lithium salt, an organic solvent and an additive, wherein the additive comprises a compound represented by the following chemical formula 1.
- R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises
- L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms
- R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom
- * is a bonding site.
- the present invention provides a lithium secondary battery including a cathode; a cathode opposite to the cathode; a separator interposed between the cathode and the cathode; and the non-aqueous electrolyte described above.
- the non-aqueous electrolyte of the present invention is characterized by including a cyclic siloxane compound having a specific structure including an alkoxy group in which at least one F is substituted as an additive.
- the compound is capable of forming a polymer-type siloxane SEI film upon reduction at an anode, and such a polymer-type siloxane SEI film has a high shear modulus and can contribute to the formation of an SEI film having excellent thermal stability and chemical and electrochemical safety.
- the alkoxy group in which at least one F is substituted included in the cyclic siloxane compound enables the formation of an inorganic SEI film such as LiF upon reduction at an anode, thereby improving the durability of the SEI film, and in particular, since the alkoxy group is a good leaving group, it can strongly induce an SEI film formation reaction. Accordingly, a lithium secondary battery including the non-aqueous electrolyte according to the present invention can have improved cycle performance and storage performance, particularly improved cycle performance and storage performance at high temperatures.
- an alkyl group having 1 to 5 carbon atoms means an alkyl group including 1 to 5 carbon atoms, that is, CH 3 -, CH 3 CH 2 -, CH 3 CH 2 CH 2 -, (CH 3 ) 2 CH-, CH 3 CH 2 CH 2 CH 2 -, (CH 3 ) 2 CHCH 2 -, CH 3 CH 2 CH 2 CH 2 -, (CH 3 ) 2 CHCH 2 CH 2 -, etc.
- both the alkyl group and the aryl group may be substituted or unsubstituted.
- substitution means, unless otherwise defined, that at least one hydrogen bonded to carbon is replaced with an element other than hydrogen, for example, an alkyl group having 1 to 20 carbon atoms, an alkenyl group having 2 to 20 carbon atoms, an alkynyl group having 2 to 20 carbon atoms, an alkoxy group having 1 to 20 carbon atoms, a cycloalkyl group having 3 to 12 carbon atoms, a cycloalkenyl group having 3 to 12 carbon atoms, a cycloalkynyl group having 3 to 12 carbon atoms, a heterocycloalkyl group having 3 to 12 carbon atoms, a heterocycloalkenyl group having 3 to 12 carbon atoms, a heterocycloalkynyl group having 2 to 12 carbon atoms, an aryloxy group having 6 to 12 carbon atoms, a
- the present invention relates to a non-aqueous electrolyte.
- the non-aqueous electrolyte according to the present invention comprises a lithium salt, an organic solvent and an additive, and is characterized in that the additive comprises a compound represented by the following chemical formula 1.
- R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises
- L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms
- R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom
- * is a bonding site.
- the lithium salt used in the present invention various lithium salts commonly used in non-aqueous electrolytes for lithium secondary batteries can be used without limitation.
- the lithium salt contains Li + as a cation and F- , Cl- , Br- , I- , NO3- , N(CN) 2- , BF4- , ClO4- , AlO4- , AlCl4- , PF6- , SbF6- , AsF6-, B10Cl10-, BF2C2O4- , BC4O8- , PF4C2O4- , PF2C4O8- , ( CF3 ) 2PF4- , ( CF3 ) 3PF3- , ( CF3 ) 4PF2- , ( CF3 ) 5PF- , ( CF3 ) 6P- , CF3SO3- , C It may include at least one selected from the group consisting of 4 F 9 SO 3 - ,
- the lithium salt may include at least one selected from the group consisting of LiCl, LiBr, LiI, LiBF 4 , LiClO 4 , LiAlO 4 , LiAlCl 4 , LiPF 6 , LiSbF 6 , LiAsF 6 , LiB 10 Cl 10 , LiBOB (LiB(C 2 O 4 ) 2 ), LiCF 3 SO 3 , LiFSI (LiN(SO 2 F) 2 ), LiCH 3 SO 3 , LiCF 3 CO 2 , LiCH 3 CO 2 and LiBETI (LiN(SO 2 CF 2 CF 3 ) 2 ).
- the lithium salt may include at least one selected from the group consisting of LiBF 4 , LiClO 4 , LiPF 6 , LiBOB (LiB(C 2 O 4 ) 2 ), LiCF 3 SO 3 , LiTFSI (LiN(SO 2 CF 3 ) 2 ), LiFSI ((LiN(SO 2 F) 2 ) and LiBETI (LiN(SO 2 CF 2 CF 3 ) 2 ).
- the above lithium salt may be included in the non-aqueous electrolyte at a concentration of 0.5 M to 5 M, specifically at a concentration of 0.8 M to 4 M, and more specifically at a concentration of 0.8 M to 2.0 M.
- concentration of the lithium salt satisfies the above range, the lithium ion yield (Li + transference number) and the degree of dissociation of lithium ions may be improved, thereby improving the output characteristics of the battery.
- the above organic solvent is a non-aqueous solvent commonly used in lithium secondary batteries, and is not particularly limited as long as decomposition due to oxidation reactions, etc. during the charge/discharge process of the secondary battery can be minimized.
- the organic solvent may include at least one selected from the group consisting of a cyclic carbonate-based organic solvent, a linear carbonate-based organic solvent, a linear ester-based organic solvent, and a cyclic ester-based organic solvent.
- the organic solvent may include a cyclic carbonate-based organic solvent, a linear carbonate-based organic solvent, or a mixture thereof.
- the above cyclic carbonate-based organic solvent is a high-viscosity organic solvent having a high dielectric constant and capable of dissociating a lithium salt in the electrolyte well, and specifically, may include at least one organic solvent selected from the group consisting of ethylene carbonate (EC), fluoroethylene carbonate (FEC), propylene carbonate (PC), 1,2-butylene carbonate, 2,3-butylene carbonate, 1,2-pentylene carbonate, 2,3-pentylene carbonate, and vinylene carbonate, more specifically, may include at least one selected from the group consisting of ethylene carbonate (EC) and fluoroethylene carbonate (FEC), and even more specifically, may include fluoroethylene carbonate (FEC) in terms of contributing to the formation of an inorganic (LiF)-containing SEI film.
- EC ethylene carbonate
- FEC fluoroethylene carbonate
- PC propylene carbonate
- 1,2-butylene carbonate 2,3-butylene carbonate
- the linear carbonate-based organic solvent is an organic solvent having low viscosity and low dielectric constant, and specifically may include at least one selected from the group consisting of dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate, ethylmethyl carbonate (EMC), methylpropyl carbonate, and ethylpropyl carbonate, and more specifically may include at least one selected from the group consisting of ethylmethyl carbonate (EMC) and diethyl carbonate (DEC), and more specifically may include diethyl carbonate (DEC) in that it can further improve the oxidation stability of the non-aqueous electrolyte.
- DMC dimethyl carbonate
- DEC diethyl carbonate
- EMC ethylmethyl carbonate
- EMC ethylmethyl carbonate
- DEC diethyl carbonate
- DEC diethyl carbonate
- DEC diethyl carbonate
- DEC diethyl carbonate
- the above organic solvent may be a mixture of a cyclic carbonate-based organic solvent and a linear carbonate-based organic solvent.
- the cyclic carbonate-based organic solvent and the linear carbonate-based organic solvent may be mixed in a volume ratio of 5:95 to 40:60, specifically, a volume ratio of 7:93 to 25:75.
- the organic solvent may further include at least one carbonate organic solvent selected from the group consisting of the cyclic carbonate organic solvent and the linear carbonate organic solvent, and at least one ester organic solvent selected from the group consisting of the linear ester organic solvent and the cyclic ester organic solvent, in order to produce an electrolyte having high ionic conductivity.
- the above linear ester organic solvent may specifically include at least one selected from the group consisting of methyl acetate, ethyl acetate, propyl acetate, methyl propionate, ethyl propionate, propyl propionate, and butyl propionate.
- the cyclic ester organic solvent may specifically include at least one selected from the group consisting of ⁇ -butyrolactone, ⁇ -valerolactone, ⁇ -caprolactone, ⁇ -valerolactone, and ⁇ -caprolactone.
- the organic solvent may be used without limitation by adding an organic solvent commonly used in a non-aqueous electrolyte as needed.
- an organic solvent commonly used in a non-aqueous electrolyte
- at least one organic solvent from among an ether-based organic solvent, a glyme-based solvent, and a nitrile-based organic solvent may be additionally included.
- any one selected from the group consisting of dimethyl ether, diethyl ether, dipropyl ether, methyl ethyl ether, methyl propyl ether, ethyl propyl ether, 1,3-dioxolane (DOL), and 2,2-bis(trifluoromethyl)-1,3-dioxolane (TFDOL) or a mixture of two or more thereof may be used, but is not limited thereto.
- the above-mentioned glyme solvent has a high dielectric constant and low surface tension compared to linear carbonate-based organic solvents, and is a solvent with low reactivity with metals, and may include at least one selected from the group consisting of dimethoxyethane (glyme, DME), diethoxyethane, diglyme, tri-glyme, and tetra-glyme (TEGDME), but is not limited thereto.
- the above nitrile solvent may be at least one selected from the group consisting of acetonitrile, propionitrile, butyronitrile, valeronitrile, caprylonitrile, heptanenitrile, cyclopentane carbonitrile, cyclohexane carbonitrile, 2-fluorobenzonitrile, 4-fluorobenzonitrile, difluorobenzonitrile, trifluorobenzonitrile, phenylacetonitrile, 2-fluorophenylacetonitrile, and 4-fluorophenylacetonitrile, but is not limited thereto.
- the above non-aqueous electrolyte contains an additive.
- the above additive comprises a compound represented by the following chemical formula 1.
- R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises
- L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms
- R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom
- * is a bonding site.
- R 1 and/or R 2 substituted on Si is an alkoxy group having 1 to 10 carbon atoms substituted with at least one F, specifically a cyclic siloxane compound including a substituent represented by the above chemical formula 2.
- the cyclic siloxane structure is opened upon reduction at the cathode to form a polymer-type siloxane SEI film.
- This polymer-type siloxane SEI film not only has excellent flexibility and resilience, but also has a high shear modulus and excellent thermal stability, chemical stability, and electrochemical stability.
- the compound represented by the chemical formula 1 includes a substituent represented by the chemical formula 2, and the substituent is reduced at the cathode to form an inorganic SEI film containing an inorganic substance such as LiF.
- This inorganic SEI film can significantly improve the durability of the SEI film.
- the compound of the chemical formula 1 according to the present invention forms the above-described polymer type/inorganic composite SEI film, the durability, flexibility, and stability of the SEI film can be improved simultaneously.
- the substituent (R 1 and/or R 2 ) included in the compound represented by the above chemical formula 1 is characterized by including a fluorine-substituted alkoxy group, specifically a substituent represented by the above chemical formula 2, and the fluorine-substituted alkoxy group is a rather weak electron withdrawn group and functions as a good leaving group. This can induce and promote the formation of an inorganic SEI film such as LiF.
- the non-aqueous electrolyte according to the present invention enables improvement of the life performance and storage performance of a lithium secondary battery, particularly improvement of the life performance and storage performance at high temperatures.
- the compound represented by the chemical formula 1 can be more preferably applied to an anode using a silicon-based active material. Since Li of lithiated Si formed by activation of an anode including a silicon-based active material and F derived from the compound represented by the chemical formula 1 can have a strong interaction with each other (Glue effect), it can be more advantageous in forming a durable and resilient SEI film on a silicon-based active material which is extremely subject to volume expansion during charge and discharge.
- R 1 and R 2 may independently include F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof.
- at least one of the R 1 and R 2 may independently
- L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms
- R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom
- * is a bonding site.
- either one of R 1 and R 2 may include a substituent represented by the chemical formula 2.
- R 1 It includes a substituent represented by the above chemical formula 2, and the above R 2 may not include a substituent represented by the above chemical formula 2.
- R 2 may be an alkyl group having 1 to 5 carbon atoms, more specifically an ethyl group or a methyl group, and even more specifically a methyl group.
- L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms
- R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom
- * is a bonding site.
- L 1 can be specifically an alkylene group having 1 to 10 carbon atoms, a sulfone group or a combination thereof, more specifically an alkylene group having 1 to 5 carbon atoms, and even more specifically a methylene group or an ethylene group in terms of easily accepting electrons during cathodic reduction, thereby further improving the reducing property, and even more specifically an ethylene group.
- R 3 may be an alkoxy group having 1 to 10 carbon atoms substituted with at least one F, specifically an alkoxy group having 1 to 5 carbon atoms substituted with at least one F, more specifically one selected from the group consisting of -OCF 3 , -OCF 2 CF 3 and -OCF 2 CF 2 CF 3 , and even more specifically -OCF 3 in terms of preventing a decrease in reactivity due to steric hindrance.
- n may be an integer of 3 to 8, specifically 3 or 4, and more specifically 3.
- R 1 and/or R 2 in each repeating unit may be the same or different.
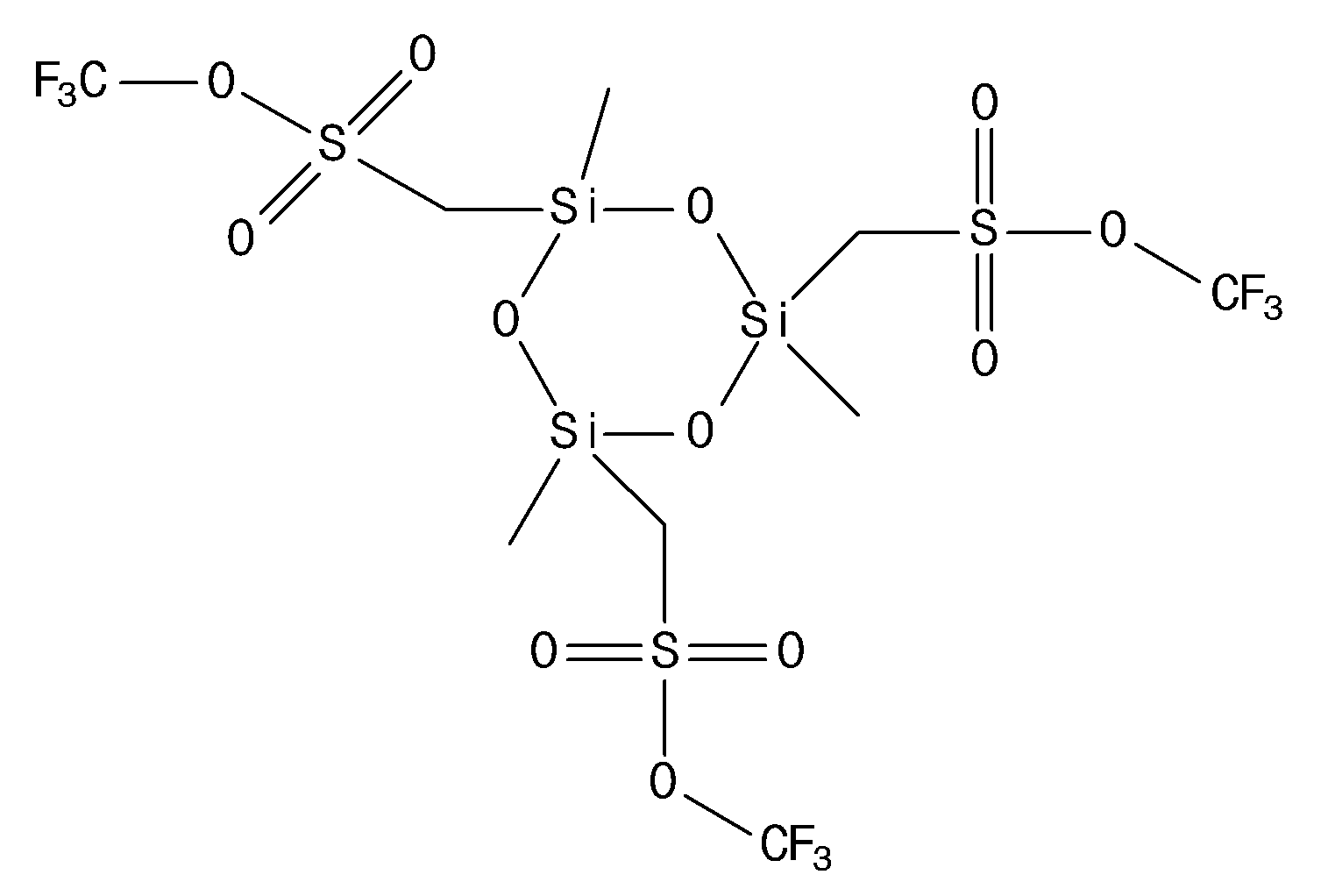
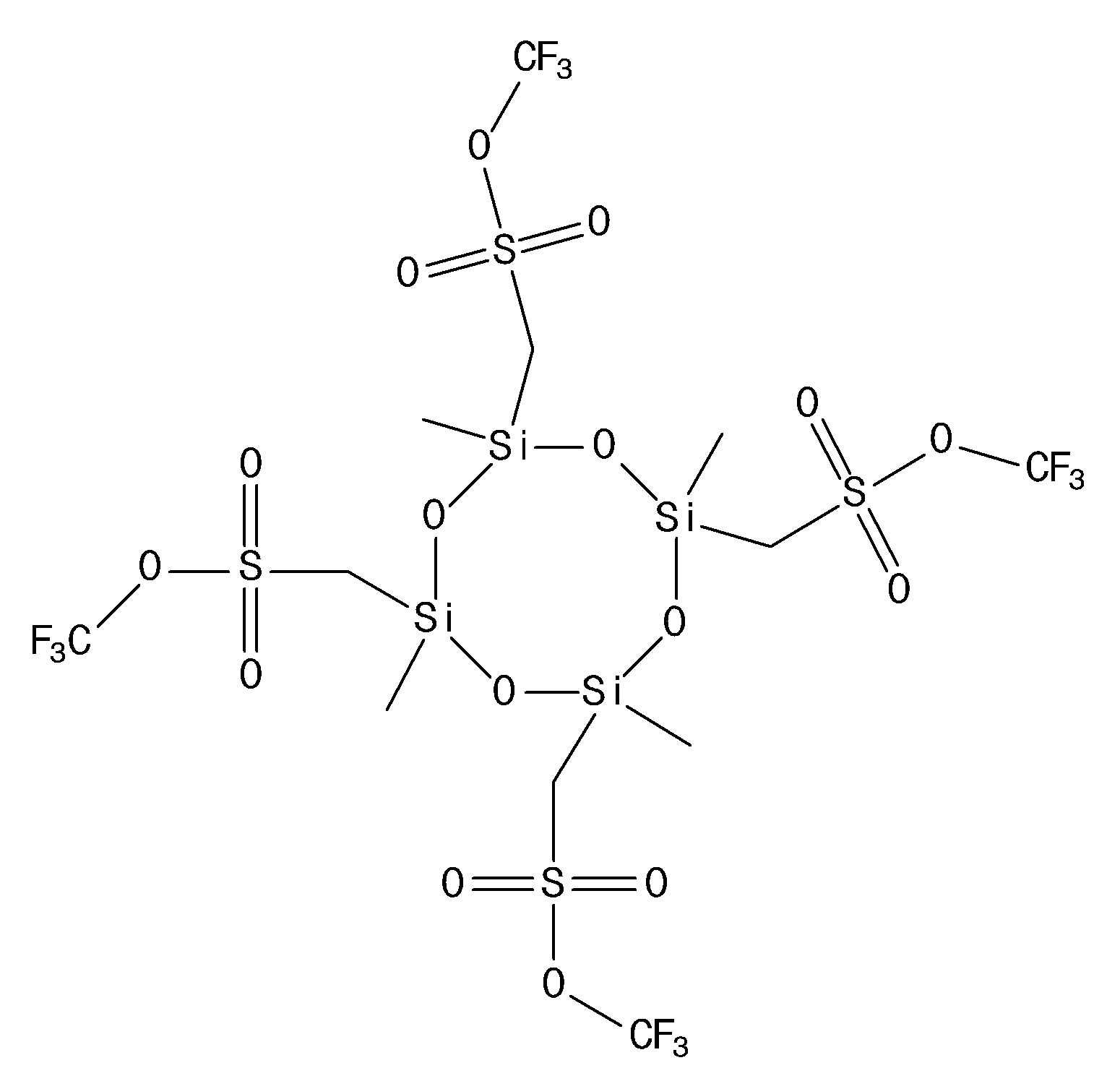
- the compound represented by the chemical formula 1 may include at least one compound selected from the group consisting of chemical formulas 1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-11, and 1-12, more specifically, may include at least one compound selected from the group consisting of chemical formulas 1-1, 1-3, 1-9, and 1-10, and even more specifically, may include at least one compound selected from the group consisting of chemical formulas 1-1 and 1-3, and even more specifically, may include a compound represented by chemical formula 1-1.
- the compound represented by the above chemical formula 1 may be included in an amount of 0.01 wt% to 10 wt%, specifically 0.3 wt% to 7 wt%, more specifically 0.5 wt% to 5 wt%, and even more specifically 1 wt% to 3 wt%, based on the weight of the non-aqueous electrolyte.
- a flexible and durable SEI film can be formed on the negative electrode, while preventing an increase in resistance when added in excessive amounts.
- the above additive may further include an additional additive together with the compound represented by Chemical Formula 1.
- the above additional additive may be included in the non-aqueous electrolyte to prevent decomposition of the non-aqueous electrolyte in a high-power environment, causing cathode collapse, or to provide low-temperature high-rate discharge characteristics, high-temperature stability, overcharge prevention, and suppression of battery expansion at high temperatures.
- the additional additive may include at least one selected from the group consisting of lithium difluorophosphate (LiDFP), vinylene carbonate, vinyl ethylene carbonate, fluoroethylene carbonate, propane sultone, propene sultone, succinonitrile, adiponitrile, ethylene sulfate, lithium bis-(oxalato)borate (LiBOB), 3-trimethoxysilanyl-propyl-N-aniline (TMSPa), and tris(trimethylsilyl) phosphate (TMSPi), and specifically, lithium difluorophosphate (LiDFP).
- LiDFP lithium difluorophosphate
- vinylene carbonate vinyl ethylene carbonate
- fluoroethylene carbonate propane sultone, propene sultone
- succinonitrile propane sultone
- adiponitrile ethylene sulfate
- LiBOB lithium bis-(oxalato)borate
- TMSPa 3-tri
- the above additional additive may be included in the non-aqueous electrolyte in an amount of 0.1 wt% to 15 wt%, more specifically 0.3 wt% to 3 wt%.
- the weight ratio of the compound represented by the chemical formula 1 and the additional additive may be 45:55 to 99:1, specifically 50:50 to 95:5, and more specifically 70:30 to 85:15, and when within the above range, the life performance and high-temperature storage performance may be improved to a more desirable level.
- the present invention provides a lithium secondary battery including the above-described non-aqueous electrolyte.
- a lithium secondary battery according to the present invention includes a negative electrode; a positive electrode opposite to the negative electrode; a separator interposed between the negative electrode and the positive electrode; and the non-aqueous electrolyte described above.
- the above lithium secondary battery can be manufactured by housing an electrode assembly including the negative electrode; a positive electrode opposing the negative electrode; and a separator interposed between the negative electrode and the positive electrode in a battery case, and then injecting the above-described non-aqueous electrolyte.
- the above negative electrode includes a negative electrode active material.
- the above negative active material may be any material used as a negative active material in the relevant field without limitation.
- the above negative active material may specifically include at least one selected from a silicon-based active material and a carbon-based active material, and more specifically may include a silicon-based active material.
- the above silicon-based active material exhibits higher capacity than the carbon-based active material, but has a problem in that the degree of volume expansion/contraction due to charge/discharge is large.
- a flexible, resilient, and durable SEI film can be formed on the negative electrode, thereby preventing electrolyte side reactions and enabling the implementation of a lithium secondary battery having high life performance and storage performance.
- the above silicon-based active material may include a compound represented by the following chemical formula A.
- the silicon-based active material may be Si.
- the average particle diameter (D 50 ) of the above silicon-based active material may be 1 ⁇ m to 20 ⁇ m.
- the above carbon-based active material may include at least one selected from the group consisting of graphite, hard carbon, soft carbon, carbon black, graphene, and fibrous carbon, and preferably may include graphite.
- the graphite may include at least one selected from the group consisting of artificial graphite and natural graphite.
- the average particle diameter (D 50 ) of the above carbon-based active material may be 10 ⁇ m to 30 ⁇ m, preferably 15 ⁇ m to 25 ⁇ m, in order to ensure structural stability during charge and discharge and reduce side reactions with the electrolyte.
- the above negative electrode may include a negative electrode current collector; and a negative electrode active material layer disposed on at least one surface of the negative electrode current collector.
- the negative electrode active material may be included in the negative electrode active material layer.
- the above negative electrode current collector is not particularly limited as long as it has high conductivity without causing chemical changes in the battery.
- the negative electrode current collector may be made of copper, stainless steel, aluminum, nickel, titanium, calcined carbon, copper or stainless steel surface-treated with carbon, nickel, titanium, silver, etc., an aluminum-cadmium alloy, etc.
- the above negative electrode collector may typically have a thickness of 3 to 500 ⁇ m.
- the above negative electrode current collector may form fine irregularities on the surface to strengthen the bonding strength of the negative electrode active material.
- the above negative electrode current collector may be used in various forms such as a film, a sheet, a foil, a net, a porous body, a foam, a non-woven fabric, etc.
- the negative electrode active material layer is disposed on at least one surface of the negative electrode current collector. Specifically, the negative electrode active material layer may be disposed on one surface or both surfaces of the negative electrode current collector.
- the above negative active material may be included in the negative active material layer at 60 wt% to 99 wt% in order to sufficiently express the capacity in the secondary battery while minimizing the effect of volume expansion/contraction on the battery.
- the above negative active material layer may further include a conductive material and/or a binder together with the silicon-based active material.
- the above binder can be used to improve the adhesion between the negative electrode active material layer and the negative electrode current collector described later, or to improve the bonding strength between silicon-based active materials.
- the binder comprises at least one selected from the group consisting of styrene butadiene rubber (SBR), nitrile butadiene rubber (NBR), acrylonitrile butadiene rubber, acrylic rubber, butyl rubber, fluoro rubber, polyvinyl alcohol, carboxymethyl cellulose (CMC), starch, hydroxypropyl cellulose, regenerated cellulose, polyvinyl alcohol (PVA), polyacrylic acid (PAA), polyethylene glycol (PEG), polyacrylonitrile (PAN), and polyacryl amide (PAM), in that it can further improve electrode adhesion and provide sufficient resistance to volume expansion/contraction of the silicon-based active material. Can be.
- SBR styrene butadiene rubber
- NBR nitrile butadiene rubber
- acrylonitrile butadiene rubber acrylic rubber
- butyl rubber fluoro rubber
- polyvinyl alcohol carboxymethyl cellulose (CMC), starch, hydroxypropyl cellulose, regenerated
- the above binder may be included in the negative electrode active material layer at 1 wt% to 30 wt%, and when present in the above range, the negative electrode active material may be better bound, thereby minimizing the problem of volume expansion of the active material, while at the same time facilitating dispersion of the binder during the preparation of a slurry for forming the negative electrode active material layer, thereby improving the coatability and phase stability of the slurry.
- the conductive material may be used to assist and improve conductivity in a secondary battery, and is not particularly limited as long as it has conductivity without causing a chemical change.
- the conductive material may include at least one selected from the group consisting of graphite such as natural graphite or artificial graphite; carbon black such as carbon black, acetylene black, Ketjen black, channel black, paneth black, lamp black, and thermal black; conductive fibers such as carbon fibers or metal fibers; conductive tubes such as carbon nanotubes; metal powders such as fluorocarbon, aluminum, and nickel powder; conductive whiskers such as zinc oxide and potassium titanate; conductive metal oxides such as titanium oxide; and polyphenylene derivatives.
- graphite such as natural graphite or artificial graphite
- carbon black such as carbon black, acetylene black, Ketjen black, channel black, paneth black, lamp black, and thermal black
- conductive fibers such as carbon fibers or metal fibers
- conductive tubes such as carbon nano
- the above-mentioned conductive agent may be included in the negative electrode active material layer at 1 wt% to 20 wt%, and when within the above range, it is preferable in that it can form an excellent conductive network while alleviating the increase in resistance due to the binder.
- the thickness of the above negative active material layer may be 5 ⁇ m to 500 ⁇ m, preferably 5 ⁇ m to 100 ⁇ m.
- the above negative electrode can be manufactured by coating a negative electrode slurry including a negative electrode active material and optionally a binder, a conductive material, and a solvent for forming a negative electrode slurry on the negative electrode current collector, and then drying and rolling.
- the solvent for forming the negative electrode slurry may include at least one selected from the group consisting of distilled water, ethanol, methanol and isopropyl alcohol, preferably distilled water, in order to facilitate dispersion of the negative electrode active material, binder and/or conductive agent.
- the above positive electrode contains a positive electrode active material.
- the above positive electrode active material is a compound capable of reversible intercalation and deintercalation of lithium, and specifically, may include a lithium-transition metal composite oxide including lithium and at least one transition metal selected from nickel, cobalt, manganese, and aluminum, preferably a lithium-transition metal composite oxide including lithium and a transition metal selected from nickel, cobalt, and manganese.
- the lithium transition metal composite oxides include lithium-manganese oxides (e.g., LiMnO 2 , LiMn 2 O 4 , etc.), lithium-cobalt oxides (e.g., LiCoO 2 , etc.), lithium-nickel oxides (e.g., LiNiO 2 , etc.), lithium-nickel-manganese oxides (e.g., LiNi 1-Y Mn Y O 2 (wherein, 0 ⁇ Y ⁇ 1), LiMn 2-z Ni z O 4 (wherein, 0 ⁇ Z ⁇ 2)), lithium-nickel-cobalt oxides (e.g., LiNi 1-Y1 Co Y1 O 2 (wherein, 0 ⁇ Y1 ⁇ 1)), lithium-manganese-cobalt oxides (e.g., LiCo 1-Y2 Mn Y2 O 2 (wherein, 0 ⁇ Y2 ⁇ 1), LiMn 2-z1 Co z1 O 4 (wherein, (0 ⁇ Z1 ⁇ 2) etc
- the lithium transition metal composite oxide may be LiCoO 2 , LiMnO 2 , LiNiO 2 , lithium nickel-manganese-cobalt oxide (for example, Li(Ni 0.6 Mn 0.2 Co 0.2 )O 2 , Li(Ni 0.5 Mn 0.3 Co 0.2 )O 2 , Li(Ni 0.7 Mn 0.15 Co 0.15 )O 2 or Li(Ni 0.8 Mn 0.1 Co 0.1 )O 2 , or lithium nickel cobalt aluminum oxide (for example, Li(Ni 0.8 Co 0.15 Al 0.05 )O 2 , etc.), and considering the prominence of the improvement effect according to the control of the type and content ratio of the constituent elements forming the lithium transition metal composite oxide, the lithium transition metal composite oxide may be The oxide may be Li ( Ni0.6Mn0.2Co0.2 ) O2 , Li(Ni0.5Mn0.3Co0.2)O2, Li(Ni0.7Mn0.15Co0.15)O2 or Li(Ni0.8Mn0.1Co0.1 )
- the cathode active material may be a lithium-transition metal composite oxide, and may contain nickel in an amount of 60 mol% or more based on the total mole number of transition metals included in the lithium-transition metal composite oxide.
- the cathode active material may be a lithium-transition metal composite oxide, wherein the transition metal includes nickel; and at least one selected from manganese, cobalt, and aluminum, and may contain nickel in an amount of 60 mol% or more, specifically 60 to 90 mol%, based on the total mole number of transition metals.
- the positive electrode active material may include a lithium composite transition metal oxide represented by the following chemical formula B.
- a, b, c and d may be 0.70 ⁇ a ⁇ 0.95, 0.025 ⁇ b ⁇ 0.20, 0.025 ⁇ c ⁇ 0.20, 0 ⁇ d ⁇ 0.05, respectively.
- a, b, c, and d may be 0.80 ⁇ a ⁇ 0.95, 0.025 ⁇ b ⁇ 0.15, 0.025 ⁇ c ⁇ 0.15, and 0 ⁇ d ⁇ 0.05, respectively.
- a, b, c, and d may be 0.85 ⁇ a ⁇ 0.90, 0.05 ⁇ b ⁇ 0.10, 0.05 ⁇ c ⁇ 0.10, and 0 ⁇ d ⁇ 0.03, respectively.
- the above positive electrode may include a positive electrode current collector; and a positive electrode active material layer disposed on at least one surface of the positive electrode current collector.
- the positive electrode active material layer may include the above-described positive electrode active material.
- the thickness of the above positive electrode collector can typically be 3 to 500 ⁇ m.
- the above-mentioned positive electrode current collector may form fine irregularities on the surface to strengthen the bonding strength of the negative electrode active material.
- the above-mentioned positive electrode current collector may be used in various forms such as a film, a sheet, a foil, a net, a porous body, a foam, a non-woven fabric, etc.
- the positive electrode active material layer is disposed on at least one surface of the positive electrode current collector. Specifically, the positive electrode active material layer may be disposed on one surface or both surfaces of the positive electrode current collector.
- the above cathode active material may be included in the cathode active material layer at 80 to 99 wt%, taking into account sufficient capacity of the cathode active material.
- the above-described positive electrode active material layer may further include a binder and/or a conductive material together with the above-described positive electrode active material.
- the above binder is a component that assists in the binding of the active material and the conductive material and the binding to the current collector, and specifically, may include at least one selected from the group consisting of polyvinylidene fluoride, polyvinyl alcohol, carboxymethyl cellulose (CMC), starch, hydroxypropyl cellulose, regenerated cellulose, polyvinyl pyrrolidone, polytetrafluoroethylene, polyethylene, polypropylene, ethylene-propylene-diene terpolymer (EPDM), sulfonated EPDM, styrene-butadiene rubber, and fluororubber, preferably polyvinylidene fluoride.
- CMC carboxymethyl cellulose
- EPDM ethylene-propylene-diene terpolymer
- EPDM ethylene-propylene-diene terpolymer
- EPDM ethylene-propylene-diene terpolymer
- EPDM ethylene-propylene-diene terpoly
- the above binder may be included in the positive electrode active material layer at 1 to 20 wt%, preferably 1.2 to 10 wt%, in order to sufficiently secure binding force between components such as the positive electrode active material.
- the conductive material may be used to assist and improve conductivity in a secondary battery, and is not particularly limited as long as it has conductivity without causing a chemical change.
- the positive electrode conductive material may include at least one selected from the group consisting of graphite such as natural graphite or artificial graphite; carbon black such as carbon black, acetylene black, Ketjen black, channel black, paneth black, lamp black, thermal black, etc.; conductive fibers such as carbon fibers or metal fibers; conductive tubes such as carbon nanotubes; fluorocarbons; metal powders such as aluminum or nickel powder; conductive whiskers such as zinc oxide or potassium titanate; conductive metal oxides such as titanium oxide; and polyphenylene derivatives, and preferably, the positive electrode conductive material may include carbon nanotubes in terms of improving conductivity.
- the above-mentioned conductive material may be included in the positive electrode active material layer at 1 wt% to 20 wt%, preferably 1.2 wt% to 10 wt%, in order to sufficiently secure electrical conductivity.
- the thickness of the above positive electrode active material layer may be 5 ⁇ m to 500 ⁇ m, preferably 20 ⁇ m to 200 ⁇ m.
- the above positive electrode can be manufactured by coating a positive electrode slurry including a positive electrode active material and optionally a binder, a conductive material, and a solvent for forming a positive electrode slurry on the positive electrode current collector, and then drying and rolling.
- the above separator may be interposed between the anode and the cathode.
- a conventional porous polymer film used as a conventional separator for example, a porous polymer film made of a polyolefin polymer such as an ethylene homopolymer, a propylene homopolymer, an ethylene/butene copolymer, an ethylene/hexene copolymer, and an ethylene/methacrylate copolymer, can be used alone or in a laminated manner, or a conventional porous nonwoven fabric, for example, a nonwoven fabric made of high-melting-point glass fiber, polyethylene terephthalate fiber, etc., can be used, but is not limited thereto.
- a coated separator containing a ceramic component or a polymer material to secure heat resistance or mechanical strength can be used, and can optionally be used in a single-layer or multi-layer structure.
- the external shape of the lithium secondary battery of the present invention may be in the shape of a cylinder, a square, a pouch, or a coin using a can.
- FEC fluoroethylene carbonate
- DEC diethyl carbonate
- a non-aqueous electrolyte was prepared by adding LiPF 6 as a lithium salt, a compound represented by the chemical formula 1-1 as an additive, and lithium difluorophosphate (LiDFP) to the organic solvent.
- LiPF 6 lithium difluorophosphate
- the above LiPF 6 was included in the non-aqueous electrolyte at a molar concentration of 1.5 M.
- the compound represented by the following chemical formula 1-1 was included in the non-aqueous electrolyte at 2 wt%, and lithium difluorophosphate was included in the non-aqueous electrolyte at 0.5 wt%.
- a positive electrode mixture slurry (solid content 75.5 wt%) was prepared by adding positive electrode active material (Li[Ni 0.85 Co 0.05 Mn 0.07 Al 0.03 ]O 2 ): conductive material (carbon nanotube): binder (polyvinylidene fluoride) in a weight ratio of 97.74:0.70:1.56 to N-methyl-2-pyrrolidone (NMP) as a solvent.
- the positive electrode mixture slurry was applied to one surface of a positive electrode current collector (Al thin film) having a thickness of 12 ⁇ m, and drying and roll pressing were performed to prepare a positive electrode.
- a negative electrode mixture slurry (solid content 26 wt%) was prepared by adding negative active material (silicon-based active material, Si): conductive agent (carbon black): binder (styrene-stadiene rubber) to distilled water as a solvent in a weight ratio of 70.0:20.3:9.7.
- the negative electrode mixture slurry was applied to one surface of a 15 ⁇ m thick negative electrode current collector (Cu thin film), and drying and roll pressing were performed to prepare a negative electrode.
- a polyethylene porous film separator was interposed between the positive and negative electrodes manufactured above in a dry room, and then the non-aqueous electrolyte manufactured above was injected to manufacture a secondary battery.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was added to the non-aqueous electrolyte in an amount of 0.5 wt% instead of 2 wt%.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was added to the non-aqueous electrolyte in an amount of 5 wt% instead of 2 wt%.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula 1-9 was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was not added.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula X was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula Y was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
- a non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula Z was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
- the lithium secondary batteries manufactured in Examples 1 to 4 and Comparative Examples 1 to 4 above were charged to 4.2 V, 0.05 C using an electrochemical charger/discharger under CC/CV, 1 C conditions at 45°C, and then discharged to 3.0 V under CC, 0.5 C conditions, which constituted one cycle, and 200 charge/discharge cycles were performed.
- the capacity retention rate is calculated using the formula below, and the results are shown in Table 1 below.
- Capacity retention rate (%) ⁇ (discharge capacity after 200 cycles/discharge capacity after 1 cycle) ⁇ ⁇ 100
- the lithium secondary batteries manufactured in Examples 1 to 4 and Comparative Examples 1 to 4 above were charged to 4.2 V, 0.05 C under CC/CV, 0.33 C conditions at 25°C and discharged to 2.5 V at 0.33 C to perform initial charge/discharge, and then charged to 4.2 V, 0.05 C under CC/CV, 0.33 C conditions at 25°C and stored at 60°C for 8 weeks.
- the lithium secondary battery was charged to 4.2 V, 0.05 C at 25°C under 0.33 C conditions and discharged to 3.0 V at 0.33 C to measure the capacity during discharge.
- Capacity retention rate (%) (discharge capacity after 8 weeks of storage/initial discharge capacity) ⁇ 100
- the lithium secondary batteries of Examples 1 to 4 using the non-aqueous electrolyte according to the present invention exhibit significantly superior capacity retention rates during high-temperature cycle charge/discharge and high-temperature storage compared to Comparative Examples 1 to 4 that do not use the non-aqueous electrolyte.
Landscapes
- Secondary Cells (AREA)
Abstract
The present invention relates to a non-aqueous electrolyte comprising lithium salt, an organic solvent, and an additive, wherein the additive comprises a cyclic siloxane compound represented by a specific chemical formula.
Description
관련출원과의 상호인용Cross-citation with related applications
본 출원은 2023년 4월 21일 자 한국 특허 출원 제10-2023-0052976호에 기초한 우선권의 이익을 주장하며, 해당 한국 특허 출원의 문헌에 개시된 모든 내용은 본 명세서의 일부로서 포함된다.This application claims the benefit of priority to Korean Patent Application No. 10-2023-0052976, filed April 21, 2023, the entire contents of which are incorporated herein by reference.
기술분야Technical field
본 발명은 비수 전해질 및 이를 포함하는 리튬 이차전지에 관한 것이다.The present invention relates to a non-aqueous electrolyte and a lithium secondary battery including the same.
정보사회의 발달로 인한 개인 IT 디바이스와 전산망이 발달되고 이에 수반하여 전반적인 사회의 전기 에너지에 대한 의존도가 높아지면서, 전기 에너지를 효율적으로 저장하고 활용하기 위한 기술 개발이 요구되고 있다.As personal IT devices and computer networks have developed due to the development of the information society, and as a result, the overall dependence of society on electric energy has increased, there is a demand for technology development to efficiently store and utilize electric energy.
이차전지는 개발된 기술 중 여러 용도에 가장 적합한 기술로서, 이러한 이차전지 중에서도 개인 IT 디바이스 등에 적용될 수 있을 정도로 소형화가 가능할 뿐만 아니라, 에너지 밀도가 가장 높은 리튬 이차전지에 대한 관심이 대두되고 있다.Among the developed technologies, secondary batteries are the most suitable for various applications, and among these secondary batteries, lithium secondary batteries are attracting attention as they can be miniaturized to the point where they can be applied to personal IT devices and have the highest energy density.
일반적으로 리튬 이차전지는 양극, 음극 및 다공성 분리막으로 이루어진 전극 조립체에 비수 전해질이 주입 또는 함침되어 제조된다.Typically, lithium secondary batteries are manufactured by injecting or impregnating a non-aqueous electrolyte into an electrode assembly consisting of a cathode, an anode, and a porous separator.
이러한 리튬 이차전지의 양극 활물질로는 리튬 함유 코발트 산화물, 층상 결정 구조의 LiMnO2, 스피넬 결정 구조의 LiMn2O4, 리튬 함유 니켈 산화물(LiNiO2) 리튬 니켈-코발트-망간 전이금속 산화물 등의 사용이 고려되고 있다.The positive active materials of these lithium secondary batteries are being considered to include lithium-containing cobalt oxide, LiMnO 2 with a layered crystal structure, LiMn 2 O 4 with a spinel crystal structure, lithium-containing nickel oxide (LiNiO 2 ), and lithium nickel-cobalt-manganese transition metal oxides.
한편, 음극 활물질로는 흑연 등의 탄소계 활물질이 사용되어 왔으나, 최근 탄소계 활물질에 비해 높은 용량을 갖는다는 측면에서 실리콘계 활물질의 사용도 고려되고 있다. 상기 실리콘계 활물질은 높은 용량을 갖는다는 점에서 장점이 있지만, 충방전 과정에서 부피 팽창/수축이 매우 크다는 문제가 있다. 이러한 큰 부피 팽창/수축 정도는 음극의 도전성을 크게 저하시켜 수명 성능을 저하시키는 원인이 된다. 또한, 초기 활성화 시 음극 표면에는 고체 전해질 계면막(Solid Electrolyte Interface layer, 이하 SEI 막)이 형성되는데, 실리콘계 활물질은 부피 팽창 정도가 커 SEI 막 깨짐, 새로운 음극 표면의 계속적인 발생이 문제되며, 이에 따라 SEI 막 형성 반응이 계속적으로 발생하는 등으로 전해질 부반응 가속화의 문제가 있고, SEI 막의 두께가 두꺼워져 저항이 증가하는 문제가 있다.Meanwhile, carbon-based active materials such as graphite have been used as negative active materials, but recently, silicon-based active materials are also being considered because they have higher capacity than carbon-based active materials. The silicon-based active material has the advantage of having a high capacity, but has the problem of very large volume expansion/contraction during the charge/discharge process. This large volume expansion/contraction greatly reduces the conductivity of the negative electrode, which causes a decrease in life performance. In addition, a solid electrolyte interface layer (hereinafter referred to as SEI film) is formed on the negative electrode surface during initial activation, but silicon-based active materials have a large volume expansion, which causes problems such as SEI film breakage and continuous generation of new negative electrode surfaces. Accordingly, SEI film formation reactions continuously occur, which accelerates electrolyte side reactions, and the thickness of the SEI film increases, which increases resistance.
본 발명의 일 과제는 상기와 같은 문제점을 해결하기 위한 것으로, 음극에 회복성이 우수하면서도 내구성이 향상된 SEI 피막을 형성하여, 수명 성능 및 저장 성능이 향상된 리튬 이차전지를 구현할 수 있는 비수 전해질을 제공하는 것이다.One object of the present invention is to solve the above problems, and to provide a non-aqueous electrolyte capable of forming an SEI film having excellent resilience and improved durability on a negative electrode, thereby implementing a lithium secondary battery having improved life performance and storage performance.
본 발명은 리튬 염, 유기 용매 및 첨가제를 포함하고, 상기 첨가제는 하기 화학식 1로 표시되는 화합물을 포함하는 비수 전해질을 제공한다.The present invention provides a non-aqueous electrolyte comprising a lithium salt, an organic solvent and an additive, wherein the additive comprises a compound represented by the following chemical formula 1.
[화학식 1][Chemical Formula 1]
상기 화학식 1에서, R1 및 R2는 서로 독립적으로 F, Br, Cl, I, 나이트릴기, 에스터기, 에테르기, 케톤기, 카르복시기, 치환 또는 비치환된 탄소수 1 내지 10의 알킬기, 치환 또는 비치환된 탄소수 1 내지 10의 알케닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알키닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알콕시기, 보론기, 보레이트기, 이소시아네이트기, 이소티오시아네이트기, 실릴기, 실록산기, 설폰기, 설포네이트기, 설페이트기, 하기 화학식 2로 표시되는 치환기 또는 이들의 2 이상의 조합을 포함하고, 상기 R1 및 R2 중 적어도 하나는 하기 화학식 2로 표시되는 치환기를 포함하고, n은 3 내지 8의 정수이다.In the chemical formula 1, R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises a substituent represented by the following chemical formula 2, and n is an integer of 3 to 8.
[화학식 2][Chemical formula 2]
상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고, R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고, *는 결합 부위이다.In the above chemical formula 2, L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms, R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom, and * is a bonding site.
또한, 본 발명은 음극; 상기 음극에 대향하는 양극; 상기 음극 및 상기 양극 사이에 개재되는 분리막; 및 전술한 비수 전해질;을 포함하는 리튬 이차전지를 제공한다.In addition, the present invention provides a lithium secondary battery including a cathode; a cathode opposite to the cathode; a separator interposed between the cathode and the cathode; and the non-aqueous electrolyte described above.
본 발명의 비수 전해질은 첨가제로서 F가 하나 이상 치환된 알콕시기를 포함하는 특정 구조의 고리형 실록산 화합물을 포함하는 것을 특징으로 한다. 상기 화합물은 음극에서 환원 시 폴리머형의 실록산 SEI 피막의 형성이 가능하며, 이러한 폴리머형 실록산 SEI 피막은 전단 계수(shear modulus)가 높고, 열 안전성 및 화학적, 전기화학적 안전성이 우수한 SEI 피막 형성에 기여할 수 있다. 또한, 상기 고리형 실록산 화합물에 포함된 F가 하나 이상 치환된 알콕시기는 음극에서 환원 시 LiF 등의 무기형 SEI 피막의 형성을 가능케 하여 SEI 피막의 내구성을 향상시킬 수 있으며, 특히 상기 알콕시기는 좋은 이탈기(good leaving group)이므로 SEI 피막 형성 반응을 강하게 유도할 수 있다. 이에 따라, 본 발명에 따른 비수 전해질을 포함하는 리튬 이차전지는 수명 성능 및 저장 성능의 향상, 특히 고온에서의 수명 성능 및 저장 성능의 향상이 가능할 수 있다.The non-aqueous electrolyte of the present invention is characterized by including a cyclic siloxane compound having a specific structure including an alkoxy group in which at least one F is substituted as an additive. The compound is capable of forming a polymer-type siloxane SEI film upon reduction at an anode, and such a polymer-type siloxane SEI film has a high shear modulus and can contribute to the formation of an SEI film having excellent thermal stability and chemical and electrochemical safety. In addition, the alkoxy group in which at least one F is substituted included in the cyclic siloxane compound enables the formation of an inorganic SEI film such as LiF upon reduction at an anode, thereby improving the durability of the SEI film, and in particular, since the alkoxy group is a good leaving group, it can strongly induce an SEI film formation reaction. Accordingly, a lithium secondary battery including the non-aqueous electrolyte according to the present invention can have improved cycle performance and storage performance, particularly improved cycle performance and storage performance at high temperatures.
본 명세서 및 청구범위에 사용된 용어나 단어는 통상적이거나 사전적인 의미로 한정해서 해석되어서는 아니 되며, 발명자는 그 자신의 발명을 가장 최선의 방법으로 설명하기 위해 용어의 개념을 적절하게 정의할 수 있다는 원칙에 입각하여 본 발명의 기술적 사상에 부합하는 의미와 개념으로 해석되어야 한다.The terms or words used in this specification and claims should not be interpreted as limited to their usual or dictionary meanings, but should be interpreted as having meanings and concepts that conform to the technical idea of the present invention, based on the principle that the inventor can appropriately define the concept of the term in order to explain his or her own invention in the best manner.
본 명세서에서 "포함하다", "구비하다" 또는 "가지다" 등의 용어는 실시된 특징, 숫자, 단계, 구성 요소 또는 이들을 조합한 것이 존재함을 지정하려는 것이지, 하나 또는 그 이상의 다른 특징들이나 숫자, 단계, 구성 요소, 또는 이들을 조합한 것들의 존재 또는 부가 가능성을 미리 배제하지 않는 것으로 이해되어야 한다.It should be understood that the terms “comprise,” “include,” or “have,” as used herein, are intended to specify the presence of a feature, number, step, component, or combination thereof, but do not exclude in advance the possibility of the presence or addition of one or more other features, numbers, steps, components, or combinations thereof.
한편, 본 발명을 설명하기에 앞서, 본 발명에서 특별한 언급이 없는 한 "* "는 동일하거나, 상이한 원자 또는 화학식의 말단부 간의 연결된 부분(결합 부위)을 의미한다.Meanwhile, before explaining the present invention, unless otherwise specifically stated, "*" in the present invention means a connected portion (bonding site) between terminals of identical or different atoms or chemical formulas.
또한, 본 명세서 내에서 "탄소수 a 내지 b"의 기재에 있어서, "a" 및 "b"는 구체적인 작용기에 포함되는 탄소 원자의 개수를 의미한다. 즉, 상기 작용기는 "a" 내지 "b" 개의 탄소원자를 포함할 수 있다. 예를 들어, "탄소수 1 내지 5의 알킬기"는 탄소수 1 내지 5의 탄소 원자를 포함하는 알킬기, 즉 CH3-, CH3CH2-, CH3CH2CH2-, (CH3)2CH-, CH3CH2CH2CH2-, (CH3)2CHCH2-, CH3CH2CH2CH2CH2-, (CH3)2CHCH2CH2- 등을 의미한다.In addition, in the description of "carbon atoms a to b" in the present specification, "a" and "b" mean the number of carbon atoms included in a specific functional group. That is, the functional group may include "a" to "b" carbon atoms. For example, "an alkyl group having 1 to 5 carbon atoms" means an alkyl group including 1 to 5 carbon atoms, that is, CH 3 -, CH 3 CH 2 -, CH 3 CH 2 CH 2 -, (CH 3 ) 2 CH-, CH 3 CH 2 CH 2 CH 2 -, (CH 3 ) 2 CHCH 2 -, CH 3 CH 2 CH 2 CH 2 CH 2 -, (CH 3 ) 2 CHCH 2 CH 2 -, etc.
또한, 본 명세서에서 알킬기, 또는 아릴기는 모두 치환 또는 비치환될 수 있다. 상기 "치환"이란 별도의 정의가 없는 한, 탄소에 결합된 적어도 하나 이상의 수소가 수소 이외의 원소로 치환된 것을 의미하는 것으로, 예를 들면, 탄소수 1 내지 20의 알킬기, 탄소수 2 내지 20의 알케닐기, 탄소수 2 내지 20의 알키닐기, 탄소수 1 내지 20의 알콕시기, 탄소수 3 내지 12의 사이클로알킬기, 탄소수 3 내지 12의 사이클로알케닐기, 탄소수 3 내지 12의 사이클로알키닐기, 탄소수 3 내지 12의 헤테로사이클로알킬기, 탄소수 3 내지 12의 헤테로사이클로알케닐기, 탄소수 2 내지 12의 헤테로사이클로알키닐기, 탄소수 6 내지 12의 아릴옥시기, 할로겐 원자, 탄소수 1 내지 20의 플루오로알킬기, 니트로기, 탄소수 6 내지 20의 아릴기, 탄소수 2 내지 20의 헤테로아릴기, 탄소수 6 내지 20의 할로아릴기 등으로 치환된 것을 의미한다.Additionally, in the present specification, both the alkyl group and the aryl group may be substituted or unsubstituted. The above "substitution" means, unless otherwise defined, that at least one hydrogen bonded to carbon is replaced with an element other than hydrogen, for example, an alkyl group having 1 to 20 carbon atoms, an alkenyl group having 2 to 20 carbon atoms, an alkynyl group having 2 to 20 carbon atoms, an alkoxy group having 1 to 20 carbon atoms, a cycloalkyl group having 3 to 12 carbon atoms, a cycloalkenyl group having 3 to 12 carbon atoms, a cycloalkynyl group having 3 to 12 carbon atoms, a heterocycloalkyl group having 3 to 12 carbon atoms, a heterocycloalkenyl group having 3 to 12 carbon atoms, a heterocycloalkynyl group having 2 to 12 carbon atoms, an aryloxy group having 6 to 12 carbon atoms, a halogen atom, a fluoroalkyl group having 1 to 20 carbon atoms, a nitro group, an aryl group having 6 to 20 carbon atoms, a halogen atom, an aryl group having 2 to 20 carbon atoms, a cycloalkyl ... It means substituted with a heteroaryl group having 20 carbon atoms, a haloaryl group having 6 to 20 carbon atoms, etc.
이하, 본 발명을 더욱 상세하게 설명한다.Hereinafter, the present invention will be described in more detail.
비수 전해질Electrolyte of the dagger
본 발명은 비수 전해질에 관한 것이다.The present invention relates to a non-aqueous electrolyte.
구체적으로, 본 발명에 따른 비수 전해질은 리튬 염, 유기 용매 및 첨가제를 포함하고, 상기 첨가제는 하기 화학식 1로 표시되는 화합물을 포함하는 것을 특징으로 한다.Specifically, the non-aqueous electrolyte according to the present invention comprises a lithium salt, an organic solvent and an additive, and is characterized in that the additive comprises a compound represented by the following chemical formula 1.
[화학식 1][Chemical Formula 1]
상기 화학식 1에서, R1 및 R2는 서로 독립적으로 F, Br, Cl, I, 나이트릴기, 에스터기, 에테르기, 케톤기, 카르복시기, 치환 또는 비치환된 탄소수 1 내지 10의 알킬기, 치환 또는 비치환된 탄소수 1 내지 10의 알케닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알키닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알콕시기, 보론기, 보레이트기, 이소시아네이트기, 이소티오시아네이트기, 실릴기, 실록산기, 설폰기, 설포네이트기, 설페이트기, 하기 화학식 2로 표시되는 치환기 또는 이들의 2 이상의 조합을 포함하고, 상기 R1 및 R2 중 적어도 하나는 하기 화학식 2로 표시되는 치환기를 포함하고, n은 3 내지 8의 정수이다.In the chemical formula 1, R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises a substituent represented by the following chemical formula 2, and n is an integer of 3 to 8.
[화학식 2][Chemical formula 2]
상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고, R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고, *는 결합 부위이다.In the above chemical formula 2, L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms, R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom, and * is a bonding site.
(1) 리튬 염(1) Lithium salt
본 발명에서 사용되는 리튬 염으로는, 리튬 이차전지용 비수 전해질에 통상적으로 사용되는 다양한 리튬 염들이 제한 없이 사용될 수 있다. 예를 들어, 상기 리튬 염은, 양이온으로 Li+를 포함하고, 음이온으로는 F-, Cl-, Br-, I-, NO3
-, N(CN)2
-, BF4
-, ClO4
-, AlO4
-, AlCl4
-, PF6
-, SbF6
-, AsF6
-, B10Cl10
-, BF2C2O4
-, BC4O8
-, PF4C2O4
-, PF2C4O8
-, (CF3)2PF4
-, (CF3)3PF3
-, (CF3)4PF2
-, (CF3)5PF-, (CF3)6P-, CF3SO3
-, C4F9SO3
-, CF3CF2SO3
-, (FSO2)2N-, CF3CF2(CF3)2CO-, (CF3SO2)2CH-, CH3SO3
-, CF3(CF2)7SO3
-, CF3CO2
-, CH3CO2
-, SCN- 및 (CF3CF2SO2)2N-로 이루어진 군으로부터 선택된 적어도 어느 하나를 포함하는 것일 수 있다.As the lithium salt used in the present invention, various lithium salts commonly used in non-aqueous electrolytes for lithium secondary batteries can be used without limitation. For example , the lithium salt contains Li + as a cation and F- , Cl- , Br- , I- , NO3- , N(CN) 2- , BF4- , ClO4- , AlO4- , AlCl4- , PF6- , SbF6- , AsF6-, B10Cl10-, BF2C2O4- , BC4O8- , PF4C2O4- , PF2C4O8- , ( CF3 ) 2PF4- , ( CF3 ) 3PF3- , ( CF3 ) 4PF2- , ( CF3 ) 5PF- , ( CF3 ) 6P- , CF3SO3- , C It may include at least one selected from the group consisting of 4 F 9 SO 3 - , CF 3 CF 2 SO 3 - , (FSO 2 ) 2 N - , CF 3 CF 2 (CF 3 ) 2 CO - , (CF 3 SO 2 ) 2 CH - , CH 3 SO 3 - , CF 3 (CF 2 ) 7 SO 3 - , CF 3 CO 2 - , CH 3 CO 2 - , SCN - and (CF 3 CF 2 SO 2 ) 2 N - .
구체적으로, 상기 리튬 염은 LiCl, LiBr, LiI, LiBF4, LiClO4, LiAlO4, LiAlCl4, LiPF6, LiSbF6, LiAsF6, LiB10Cl10, LiBOB (LiB(C2O4)2), LiCF3SO3, LiFSI (LiN(SO2F)2), LiCH3SO3, LiCF3CO2, LiCH3CO2 및 LiBETI (LiN(SO2CF2CF3)2)로 이루어진 군으로부터 선택된 적어도 1종을 포함할 수 있다. 구체적으로 상기 리튬 염은 LiBF4, LiClO4, LiPF6, LiBOB (LiB(C2O4)2), LiCF3SO3, LiTFSI (LiN(SO2CF3)2), LiFSI ((LiN(SO2F)2) 및 LiBETI (LiN(SO2CF2CF3)2)로 이루어진 군으로부터 선택된 적어도 1종을 포함할 수 있다.Specifically, the lithium salt may include at least one selected from the group consisting of LiCl, LiBr, LiI, LiBF 4 , LiClO 4 , LiAlO 4 , LiAlCl 4 , LiPF 6 , LiSbF 6 , LiAsF 6 , LiB 10 Cl 10 , LiBOB (LiB(C 2 O 4 ) 2 ), LiCF 3 SO 3 , LiFSI (LiN(SO 2 F) 2 ), LiCH 3 SO 3 , LiCF 3 CO 2 , LiCH 3 CO 2 and LiBETI (LiN(SO 2 CF 2 CF 3 ) 2 ). Specifically, the lithium salt may include at least one selected from the group consisting of LiBF 4 , LiClO 4 , LiPF 6 , LiBOB (LiB(C 2 O 4 ) 2 ), LiCF 3 SO 3 , LiTFSI (LiN(SO 2 CF 3 ) 2 ), LiFSI ((LiN(SO 2 F) 2 ) and LiBETI (LiN(SO 2 CF 2 CF 3 ) 2 ).
상기 리튬 염은 상기 비수 전해질에 0.5M 내지 5M의 농도, 구체적으로 0.8M 내지 4M의 농도, 보다 구체적으로 0.8M 내지 2.0M의 농도로 포함될 수 있다. 상기 리튬 염의 농도가 상기 범위를 만족할 때, 리튬 이온 수율(Li+ transference number) 및 리튬 이온의 해리도가 향상되어 전지의 출력 특성이 향상될 수 있다.The above lithium salt may be included in the non-aqueous electrolyte at a concentration of 0.5 M to 5 M, specifically at a concentration of 0.8 M to 4 M, and more specifically at a concentration of 0.8 M to 2.0 M. When the concentration of the lithium salt satisfies the above range, the lithium ion yield (Li + transference number) and the degree of dissociation of lithium ions may be improved, thereby improving the output characteristics of the battery.
(2) 유기 용매(2) Organic solvent
상기 유기 용매로는 리튬 이차전지에 통상적으로 사용되는 비수계 용매로서, 이차전지의 충방전 과정에서 산화 반응 등에 의한 분해가 최소화될 수 있는 것이라면 특별히 제한되지 않는다.The above organic solvent is a non-aqueous solvent commonly used in lithium secondary batteries, and is not particularly limited as long as decomposition due to oxidation reactions, etc. during the charge/discharge process of the secondary battery can be minimized.
구체적으로, 상기 유기 용매는 환형 카보네이트계 유기 용매, 선형 카보네이트계 유기 용매, 선형 에스터계 유기 용매 및 환형 에스터계 유기 용매로 이루어진 군으로부터 선택된 적어도 1종을 포함할 수 있다.Specifically, the organic solvent may include at least one selected from the group consisting of a cyclic carbonate-based organic solvent, a linear carbonate-based organic solvent, a linear ester-based organic solvent, and a cyclic ester-based organic solvent.
구체적으로, 상기 유기 용매는 환형 카보네이트계 유기 용매, 선형 카보네이트계 유기 용매 또는 이들의 혼합물을 포함할 수 있다. Specifically, the organic solvent may include a cyclic carbonate-based organic solvent, a linear carbonate-based organic solvent, or a mixture thereof.
상기 환형 카보네이트계 유기 용매는 고점도의 유기 용매로서 유전율이 높아 전해질 내의 리튬염을 잘 해리시킬 수 있는 유기 용매로서, 구체적으로 에틸렌 카보네이트(EC), 플루오로에틸렌 카보네이트(FEC), 프로필렌 카보네이트(PC), 1,2-부틸렌 카보네이트, 2,3-부틸렌 카보네이트, 1,2-펜틸렌 카보네이트, 2,3-펜틸렌 카보네이트 및 비닐렌 카보네이트로 이루어진 군으로부터 선택되는 적어도 1종의 유기 용매를 포함할 수 있으며, 보다 구체적으로 에틸렌 카보네이트(EC) 및 플루오로에틸렌 카보네이트(FEC)로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있고, 보다 더 구체적으로 무기물(LiF) 함유 SEI 피막 형성에 기여하는 측면에서 플루오로에틸렌 카보네이트(FEC)를 포함할 수 있다.The above cyclic carbonate-based organic solvent is a high-viscosity organic solvent having a high dielectric constant and capable of dissociating a lithium salt in the electrolyte well, and specifically, may include at least one organic solvent selected from the group consisting of ethylene carbonate (EC), fluoroethylene carbonate (FEC), propylene carbonate (PC), 1,2-butylene carbonate, 2,3-butylene carbonate, 1,2-pentylene carbonate, 2,3-pentylene carbonate, and vinylene carbonate, more specifically, may include at least one selected from the group consisting of ethylene carbonate (EC) and fluoroethylene carbonate (FEC), and even more specifically, may include fluoroethylene carbonate (FEC) in terms of contributing to the formation of an inorganic (LiF)-containing SEI film.
또한, 상기 선형 카보네이트계 유기 용매는 저점도 및 저유전율을 가지는 유기 용매로서, 구체적으로 디메틸 카보네이트(dimethyl carbonate, DMC), 디에틸 카보네이트(diethyl carbonate, DEC), 디프로필 카보네이트, 에틸메틸 카보네이트(EMC), 메틸프로필 카보네이트 및 에틸프로필 카보네이트로 이루어진 군으로부터 선택되는 적어도 1종을 포함할 수 있으며, 보다 구체적으로 에틸메틸 카보네이트(EMC) 및 디에틸 카보네이트(DEC)로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있고, 보다 구체적으로 비수 전해질의 산화 안정성을 더 향상시킬 수 있다는 측면에서 디에틸 카보네이트(DEC)를 포함할 수 있다.In addition, the linear carbonate-based organic solvent is an organic solvent having low viscosity and low dielectric constant, and specifically may include at least one selected from the group consisting of dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate, ethylmethyl carbonate (EMC), methylpropyl carbonate, and ethylpropyl carbonate, and more specifically may include at least one selected from the group consisting of ethylmethyl carbonate (EMC) and diethyl carbonate (DEC), and more specifically may include diethyl carbonate (DEC) in that it can further improve the oxidation stability of the non-aqueous electrolyte.
상기 유기 용매는 환형 카보네이트계 유기 용매와 선형 카보네이트계 유기 용매의 혼합물일 수 있다. 이때, 상기 환형 카보네이트계 유기 용매와 선형 카보네이트계 유기 용매는 5:95 내지 40:60의 부피비, 구체적으로 7:93 내지 25:75의 부피비로 혼합될 수 있다. 환형 카보네이트계 유기 용매와 선형 카보네이트계 유기 용매의 혼합비가 상기 범위를 만족할 경우, 고유전율과 저점도 특성을 동시에 만족하며, 우수한 이온 전도도 특성을 구현할 수 있다.The above organic solvent may be a mixture of a cyclic carbonate-based organic solvent and a linear carbonate-based organic solvent. At this time, the cyclic carbonate-based organic solvent and the linear carbonate-based organic solvent may be mixed in a volume ratio of 5:95 to 40:60, specifically, a volume ratio of 7:93 to 25:75. When the mixing ratio of the cyclic carbonate-based organic solvent and the linear carbonate-based organic solvent satisfies the above range, high dielectric constant and low viscosity characteristics can be simultaneously satisfied, and excellent ion conductivity characteristics can be implemented.
또한, 상기 유기 용매는 높은 이온 전도율을 갖는 전해질을 제조하기 위하여, 상기 환형 카보네이트계 유기 용매 및 선형 카보네이트계 유기 용매로 이루어진 군으로부터 선택된 적어도 1종의 카보네이트계 유기 용매에 선형 에스터계 유기 용매 및 환형 에스터계 유기 용매로 이루어진 군으로부터 선택된 적어도 1종의 에스터계 유기 용매를 추가로 포함할 수 있다.In addition, the organic solvent may further include at least one carbonate organic solvent selected from the group consisting of the cyclic carbonate organic solvent and the linear carbonate organic solvent, and at least one ester organic solvent selected from the group consisting of the linear ester organic solvent and the cyclic ester organic solvent, in order to produce an electrolyte having high ionic conductivity.
상기 선형 에스터계 유기 용매는 구체적으로 메틸 아세테이트, 에틸 아세테이트, 프로필 아세테이트, 메틸 프로피오네이트, 에틸 프로피오네이트, 프로필 프로피오네이트 및 부틸 프로피오네이트로 이루어진 군으로부터 선택되는 적어도 1종을 포함할 수 있다.The above linear ester organic solvent may specifically include at least one selected from the group consisting of methyl acetate, ethyl acetate, propyl acetate, methyl propionate, ethyl propionate, propyl propionate, and butyl propionate.
또한, 상기 환형 에스터계 유기 용매는 구체적으로 γ-부티로락톤, γ-발레로락톤, γ-카프로락톤, σ-발레로락톤 및 ε-카프로락톤으로 이루어진 군으로부터 선택되는 적어도 1종을 포함할 수 있다.In addition, the cyclic ester organic solvent may specifically include at least one selected from the group consisting of γ-butyrolactone, γ-valerolactone, γ-caprolactone, σ-valerolactone, and ε-caprolactone.
한편, 상기 유기 용매는 필요에 따라 비수 전해질에 통상적으로 사용되는 유기 용매를 제한 없이 추가하여 사용할 수 있다. 예를 들면, 에테르계 유기 용매, 글라임계 용매 및 니트릴계 유기 용매 중 적어도 하나 이상의 유기 용매를 추가로 포함할 수도 있다.Meanwhile, the organic solvent may be used without limitation by adding an organic solvent commonly used in a non-aqueous electrolyte as needed. For example, at least one organic solvent from among an ether-based organic solvent, a glyme-based solvent, and a nitrile-based organic solvent may be additionally included.
상기 에테르계 용매로는 디메틸에테르, 디에틸에테르, 디프로필 에테르, 메틸에틸에테르, 메틸프로필 에테르, 에틸프로필 에테르, 1,3-디옥소란(DOL) 및 2,2-비스(트리플루오로메틸)-1,3-디옥소란(TFDOL)으로 이루어진 군으로부터 선택되는 어느 하나 또는 이들 중 2종 이상의 혼합물을 사용할 수 있으나, 이에 한정되는 것은 아니다.As the above ether solvent, any one selected from the group consisting of dimethyl ether, diethyl ether, dipropyl ether, methyl ethyl ether, methyl propyl ether, ethyl propyl ether, 1,3-dioxolane (DOL), and 2,2-bis(trifluoromethyl)-1,3-dioxolane (TFDOL) or a mixture of two or more thereof may be used, but is not limited thereto.
상기 글라임계 용매는 선형 카보네이트계 유기 용매에 비해 높은 유전율 및 낮은 표면 장력을 가지며, 메탈과의 반응성이 적은 용매로서, 디메톡시에탄 (글라임, DME), 디에톡시에탄, 디글라임 (digylme), 트리-글라임(Triglyme), 및 테트라-글라임 (TEGDME)으로 이루어진 군으로부터 선택된 적어도 하나 이상을 포함할 수 있으나 이에 한정되는 것은 아니다. The above-mentioned glyme solvent has a high dielectric constant and low surface tension compared to linear carbonate-based organic solvents, and is a solvent with low reactivity with metals, and may include at least one selected from the group consisting of dimethoxyethane (glyme, DME), diethoxyethane, diglyme, tri-glyme, and tetra-glyme (TEGDME), but is not limited thereto.
상기 니트릴계 용매는 아세토니트릴, 프로피오니트릴, 부티로니트릴, 발레로니트릴, 카프릴로니트릴, 헵탄니트릴, 싸이클로펜탄 카보니트릴, 싸이클로헥산 카보니트릴, 2-플루오로벤조니트릴, 4-플루오로벤조니트릴, 다이플루오로벤조니트릴, 트리플루오로벤조니트릴, 페닐아세토니트릴, 2-플루오로페닐아세토니트릴, 4-플루오로페닐아세토니트릴로 이루어진 군에서 선택되는 1종 이상인 것일 수 있으나 이에 한정되는 것은 아니다.The above nitrile solvent may be at least one selected from the group consisting of acetonitrile, propionitrile, butyronitrile, valeronitrile, caprylonitrile, heptanenitrile, cyclopentane carbonitrile, cyclohexane carbonitrile, 2-fluorobenzonitrile, 4-fluorobenzonitrile, difluorobenzonitrile, trifluorobenzonitrile, phenylacetonitrile, 2-fluorophenylacetonitrile, and 4-fluorophenylacetonitrile, but is not limited thereto.
(3) 첨가제(3) Additives
상기 비수 전해질은 첨가제를 포함한다. The above non-aqueous electrolyte contains an additive.
상기 첨가제는 하기 화학식 1로 표시되는 화합물을 포함한다.The above additive comprises a compound represented by the following chemical formula 1.
[화학식 1][Chemical Formula 1]
상기 화학식 1에서, R1 및 R2는 서로 독립적으로 F, Br, Cl, I, 나이트릴기, 에스터기, 에테르기, 케톤기, 카르복시기, 치환 또는 비치환된 탄소수 1 내지 10의 알킬기, 치환 또는 비치환된 탄소수 1 내지 10의 알케닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알키닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알콕시기, 보론기, 보레이트기, 이소시아네이트기, 이소티오시아네이트기, 실릴기, 실록산기, 설폰기, 설포네이트기, 설페이트기, 하기 화학식 2로 표시되는 치환기 또는 이들의 2 이상의 조합을 포함하고, 상기 R1 및 R2 중 적어도 하나는 하기 화학식 2로 표시되는 치환기를 포함하고, n은 3 내지 8의 정수이다.In the chemical formula 1, R 1 and R 2 independently represent F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof, at least one of the R 1 and R 2 comprises a substituent represented by the following chemical formula 2, and n is an integer of 3 to 8.
[화학식 2][Chemical formula 2]
상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고, R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고, *는 결합 부위이다.In the above chemical formula 2, L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms, R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom, and * is a bonding site.
상기 화학식 1로 표시되는 화합물은 Si에 치환된 R1 및/또는 R2가 F가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기, 구체적으로 상기 화학식 2로 표시되는 치환기를 포함하는 고리형 실록산 화합물인 것을 특징으로 한다.The compound represented by the above chemical formula 1 is characterized in that R 1 and/or R 2 substituted on Si is an alkoxy group having 1 to 10 carbon atoms substituted with at least one F, specifically a cyclic siloxane compound including a substituent represented by the above chemical formula 2.
상기 화학식 1로 표시되는 화합물이 비수 전해질 첨가제로 사용될 경우, 음극에서 환원 시 고리형 실록산 구조가 개환되어 폴리머형의 실록산 SEI 피막을 형성한다. 이러한 폴리머형 실록산 SEI 피막은 유연성과 회복성이 우수할 뿐 아니라, 전단 계수(Shear modulus)가 높고, 열 안정성, 화학적 안정성 및 전기화학적 안정성이 우수하다.When the compound represented by the above chemical formula 1 is used as a non-aqueous electrolyte additive, the cyclic siloxane structure is opened upon reduction at the cathode to form a polymer-type siloxane SEI film. This polymer-type siloxane SEI film not only has excellent flexibility and resilience, but also has a high shear modulus and excellent thermal stability, chemical stability, and electrochemical stability.
또한, 상기 화학식 1로 표시되는 화합물은 상기 화학식 2로 표시되는 치환기 를 포함하는 것으로서, 상기 치환기는 음극에서 환원되어 LiF 등의 무기물이 함유된 무기형 SEI 피막을 형성케 한다. 이러한 무기형 SEI 피막은 SEI 피막의 내구성을 현저한 수준으로 향상시킬 수 있다. 특히, 본 발명에 따른 화학식 1 화합물은 상술한 폴리머형/무기형 복합 SEI 피막을 형성하므로, SEI 피막의 내구성, 유연성 및 안정성이 동시에 향상시킬 수 있다.In addition, the compound represented by the chemical formula 1 includes a substituent represented by the chemical formula 2, and the substituent is reduced at the cathode to form an inorganic SEI film containing an inorganic substance such as LiF. This inorganic SEI film can significantly improve the durability of the SEI film. In particular, since the compound of the chemical formula 1 according to the present invention forms the above-described polymer type/inorganic composite SEI film, the durability, flexibility, and stability of the SEI film can be improved simultaneously.
또한, 상기 화학식 1로 표시되는 화합물에 포함되는 치환기(R1 및/또는 R2)는 불소가 치환된 알콕시기, 구체적으로 상기 화학식 2로 표시되는 치환기를 포함하는 것을 특징으로 하며, 상기 불소가 치환된 알콕시기는 다소 약한 전자 끄는 기(electron withdrawn group)이며 좋은 이탈기(good leaving group)으로 기능한다. 이는 LiF 등의 무기형 SEI 피막의 형성을 유도, 촉진시킬 수 있다.In addition, the substituent (R 1 and/or R 2 ) included in the compound represented by the above chemical formula 1 is characterized by including a fluorine-substituted alkoxy group, specifically a substituent represented by the above chemical formula 2, and the fluorine-substituted alkoxy group is a rather weak electron withdrawn group and functions as a good leaving group. This can induce and promote the formation of an inorganic SEI film such as LiF.
상술한 효과에 따라, 본 발명에 따른 비수 전해질은 리튬 이차전지의 수명 성능 및 저장 성능의 향상, 특히 고온에서의 수명 성능 및 저장 성능의 향상을 가능케 한다. 특히, 상기 화학식 1로 표시되는 화합물은 실리콘계 활물질을 사용하는 음극에 더욱 바람직하게 적용될 수 있다. 실리콘계 활물질을 포함하는 음극의 활성화로 인해 형성되는 리튬화 Si(lithiated Si)의 Li와 상기 화학식 1로 표시되는 화합물로부터 유래된 F는 서로 강한 interation을 가질 수 있으므로(Glue effect), 충방전 시 부피 팽창이 극심한 실리콘계 활물질에 내구성, 회복성이 강한 SEI 피막 형성에 더욱 유리할 수 있다.According to the effects described above, the non-aqueous electrolyte according to the present invention enables improvement of the life performance and storage performance of a lithium secondary battery, particularly improvement of the life performance and storage performance at high temperatures. In particular, the compound represented by the chemical formula 1 can be more preferably applied to an anode using a silicon-based active material. Since Li of lithiated Si formed by activation of an anode including a silicon-based active material and F derived from the compound represented by the chemical formula 1 can have a strong interaction with each other (Glue effect), it can be more advantageous in forming a durable and resilient SEI film on a silicon-based active material which is extremely subject to volume expansion during charge and discharge.
상기 화학식 1에서, R1 및 R2는 서로 독립적으로 F, Br, Cl, I, 나이트릴기, 에스터기, 에테르기, 케톤기, 카르복시기, 치환 또는 비치환된 탄소수 1 내지 10의 알킬기, 치환 또는 비치환된 탄소수 1 내지 10의 알케닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알키닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알콕시기, 보론기, 보레이트기, 이소시아네이트기, 이소티오시아네이트기, 실릴기, 실록산기, 설폰기, 설포네이트기, 설페이트기, 하기 화학식 2로 표시되는 치환기 또는 이들의 2 이상의 조합을 포함할 수 있다. 이때, 상기 R1 및 R2 중 적어도 하나는 하기 화학식 2로 표시되는 치환기를 포함한다.In the chemical formula 1, R 1 and R 2 may independently include F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof. At this time, at least one of the R 1 and R 2 includes a substituent represented by the following chemical formula 2.
[화학식 2][Chemical formula 2]
상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고, R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고, *는 결합 부위이다.In the above chemical formula 2, L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms, R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom, and * is a bonding site.
구체적으로, 입체 장애(Steric hindrance)에 따른 반응성 저하를 방지하는 측면에서 상기 R1 및 R2 중 어느 하나가 상기 화학식 2로 표시되는 치환기를 포함할 수 있다. 예를 들어, 화학식 1에서 상기 R1이 상기 화학식 2로 표시되는 치환기를 포함하고, 상기 R2는 상기 화학식 2로 표시되는 치환기를 포함하지 않을 수 있다. 구체적으로 화학식 1에서 상기 R1이 상기 화학식 2로 표시되는 치환기를 포함할 경우, 상기 R2는 탄소수 1 내지 5의 알킬기, 보다 구체적으로 에틸기 또는 메틸기, 보다 더 구체적으로 메틸기일 수 있다.Specifically, in terms of preventing a decrease in reactivity due to steric hindrance, either one of R 1 and R 2 may include a substituent represented by the chemical formula 2. For example, in the chemical formula 1, R 1 It includes a substituent represented by the above chemical formula 2, and the above R 2 may not include a substituent represented by the above chemical formula 2. Specifically, in the chemical formula 1, the above R 1 When including a substituent represented by the above chemical formula 2, R 2 may be an alkyl group having 1 to 5 carbon atoms, more specifically an ethyl group or a methyl group, and even more specifically a methyl group.
상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고, R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고, *는 결합 부위이다.In the above chemical formula 2, L 1 is an alkylene group, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof having 1 to 10 carbon atoms, R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine atom, and * is a bonding site.
상기 화학식 2에서, L1은 구체적으로 탄소수 1 내지 10의 알킬렌기, 설폰기 또는 이들의 조합일 수 있고, 보다 구체적으로 탄소수 1 내지 5의 알킬렌기일 수 있고, 보다 더 구체적으로 음극 환원 시 전자 받음이 용이하여 환원성을 더욱 향상시킬 수 있다는 측면에서 메틸렌기 또는 에틸렌기일 수 있고, 보다 더 구체적으로 에틸렌기일 수 있다.In the above chemical formula 2, L 1 can be specifically an alkylene group having 1 to 10 carbon atoms, a sulfone group or a combination thereof, more specifically an alkylene group having 1 to 5 carbon atoms, and even more specifically a methylene group or an ethylene group in terms of easily accepting electrons during cathodic reduction, thereby further improving the reducing property, and even more specifically an ethylene group.
상기 화학식 2에서, R3는 F가 하나 이상 치환된 탄소수 1 내지 10의 알콕시기, 구체적으로 F가 하나 이상 치환된 탄소수 1 내지 5의 알콕시기, 보다 구체적으로 -OCF3, -OCF2CF3 및 -OCF2CF2CF3로 이루어진 군에서 선택된 1종, 보다 더 구체적으로 입체 장애에 따른 반응성 저하를 방지하는 측면에서 -OCF3일 수 있다.In the above chemical formula 2, R 3 may be an alkoxy group having 1 to 10 carbon atoms substituted with at least one F, specifically an alkoxy group having 1 to 5 carbon atoms substituted with at least one F, more specifically one selected from the group consisting of -OCF 3 , -OCF 2 CF 3 and -OCF 2 CF 2 CF 3 , and even more specifically -OCF 3 in terms of preventing a decrease in reactivity due to steric hindrance.
상기 화학식 1에서, n은 3 내지 8의 정수, 구체적으로 3 또는 4일 수 있고, 보다 구체적으로 3일 수 있다. 상기 n이 3 내지 8일 경우, 각각의 반복 단위 내의 R1 및/또는 R2는 서로 동일할 수도 있고 상이할 수도 있다.In the above chemical formula 1, n may be an integer of 3 to 8, specifically 3 or 4, and more specifically 3. When n is 3 to 8, R 1 and/or R 2 in each repeating unit may be the same or different.
구체적으로, 상기 화학식 1로 표시되는 화합물은 하기 화학식 1-1, 화학식 1-2, 화학식 1-3, 화학식 1-4, 화학식 1-5, 화학식 1-6, 화학식 1-7, 화학식 1-8, 화학식 1-9, 화학식 1-10, 화학식 1-11 및 화학식 1-12로 이루어진 군에서 선택된 적어도 1종의 화합물을 포함할 수 있고, 보다 구체적으로 화학식 1-1, 화학식 1-3, 화학식 1-9 및 화학식 1-10으로 이루어진 군에서 선택된 적어도 1종의 화합물을 포함할 수 있고, 보다 더 구체적으로 화학식 1-1 및 화학식 1-3으로 이루어진 군에서 선택된 적어도 1종의 화합물을 포함할 수 있고, 보다 더 구체적으로 화학식 1-1로 표시되는 화합물을 포함할 수 있다.Specifically, the compound represented by the chemical formula 1 may include at least one compound selected from the group consisting of chemical formulas 1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-11, and 1-12, more specifically, may include at least one compound selected from the group consisting of chemical formulas 1-1, 1-3, 1-9, and 1-10, and even more specifically, may include at least one compound selected from the group consisting of chemical formulas 1-1 and 1-3, and even more specifically, may include a compound represented by chemical formula 1-1.
[화학식 1-1][Chemical Formula 1-1]
[화학식 1-2][Chemical Formula 1-2]
[화학식 1-3][Chemical Formula 1-3]
[화학식 1-4][Chemical Formula 1-4]
[화학식 1-5][Chemical Formula 1-5]
[화학식 1-6][Chemical Formula 1-6]
[화학식 1-7][Chemical Formula 1-7]
[화학식 1-8][Chemical Formula 1-8]
[화학식 1-9][Chemical Formula 1-9]
[화학식 1-10][Chemical Formula 1-10]
[화학식 1-11][Chemical Formula 1-11]
[화학식 1-12][Chemical Formula 1-12]
상기 화학식 1로 표시되는 화합물은 상기 비수 전해질의 중량을 기준으로 0.01중량% 내지 10중량%, 구체적으로 0.3중량% 내지 7중량%, 보다 구체적으로 0.5중량% 내지 5중량%, 보다 더 구체적으로 1중량% 내지 3중량%로 포함될 수 있다. 상기 화학식 1로 표시되는 화합물이 상술한 함량 범위로 사용될 때, 유연하면서도 내구성이 우수한 SEI 피막을 음극에 형성할 수 있으면서도 과량 첨가 시의 저항 증가가 방지될 수 있다.The compound represented by the above chemical formula 1 may be included in an amount of 0.01 wt% to 10 wt%, specifically 0.3 wt% to 7 wt%, more specifically 0.5 wt% to 5 wt%, and even more specifically 1 wt% to 3 wt%, based on the weight of the non-aqueous electrolyte. When the compound represented by the above chemical formula 1 is used in the above-described content range, a flexible and durable SEI film can be formed on the negative electrode, while preventing an increase in resistance when added in excessive amounts.
상기 첨가제는 화학식 1로 표시되는 화합물과 함께 추가 첨가제를 더 포함할 수 있다. 상기 추가 첨가제는 고출력의 환경에서 비수 전해질이 분해되어 음극 붕괴가 유발되는 것을 방지하거나, 저온 고율방전 특성, 고온 안정성, 과충전 방지, 고온에서의 전지 팽창 억제 효과 등을 위해 비수 전해질에 포함될 수 있다.The above additive may further include an additional additive together with the compound represented by Chemical Formula 1. The above additional additive may be included in the non-aqueous electrolyte to prevent decomposition of the non-aqueous electrolyte in a high-power environment, causing cathode collapse, or to provide low-temperature high-rate discharge characteristics, high-temperature stability, overcharge prevention, and suppression of battery expansion at high temperatures.
구체적으로 상기 추가 첨가제는 리튬 디플루오로포스페이트(LiDFP, lithium difluorophosphate), 비닐렌 카보네이트(Vinylene Carbonate), 비닐에틸렌 카보네이트(vinyl ethylene carbonate), 플루오로에틸렌 카본네이트(fluoroethylene carbonate), 프로판 설톤(Propane sultone), 프로펜설톤(Propene Sultone), 숙시노니트릴(succinonitrile), 아디포니트릴(Adiponitrile), 에틸렌 설페이트(ethylene sulfate), LiBOB(Lithium bis-(oxalato)borate), TMSPa(3-trimethoxysilanyl-propyl-N-aniline), 및 TMSPi(Tris(trimethylsilyl) Phosphite)로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있으며, 구체적으로 리튬 디플루오로포스페이트(LiDFP, lithium difluorophosphate)를 포함할 수 있다. Specifically, the additional additive may include at least one selected from the group consisting of lithium difluorophosphate (LiDFP), vinylene carbonate, vinyl ethylene carbonate, fluoroethylene carbonate, propane sultone, propene sultone, succinonitrile, adiponitrile, ethylene sulfate, lithium bis-(oxalato)borate (LiBOB), 3-trimethoxysilanyl-propyl-N-aniline (TMSPa), and tris(trimethylsilyl) phosphate (TMSPi), and specifically, lithium difluorophosphate (LiDFP).
상기 추가 첨가제는 상기 비수 전해질에 0.1중량% 내지 15중량%, 보다 구체적으로 0.3중량% 내지 3중량%로 포함될 수 있다.The above additional additive may be included in the non-aqueous electrolyte in an amount of 0.1 wt% to 15 wt%, more specifically 0.3 wt% to 3 wt%.
상기 비수 전해질이 상기 추가 첨가제를 더 포함할 경우, 상기 화학식 1로 표시되는 화합물과 추가 첨가제의 중량비는 45:55 내지 99:1, 구체적으로 50:50 내지 95:5, 보다 더 구체적으로 70:30 내지 85:15일 수 있으며, 상기 범위에 있을 때 수명 성능 및 고온 저장 성능이 보다 바람직한 수준으로 향상될 수 있다.When the non-aqueous electrolyte further includes the additional additive, the weight ratio of the compound represented by the chemical formula 1 and the additional additive may be 45:55 to 99:1, specifically 50:50 to 95:5, and more specifically 70:30 to 85:15, and when within the above range, the life performance and high-temperature storage performance may be improved to a more desirable level.
리튬 이차전지Lithium secondary battery
또한, 본 발명은 전술한 비수 전해질을 포함하는 리튬 이차전지를 제공한다.In addition, the present invention provides a lithium secondary battery including the above-described non-aqueous electrolyte.
구체적으로, 본 발명에 따른 리튬 이차전지는 음극; 상기 음극에 대향하는 양극; 상기 음극 및 상기 양극 사이에 개재되는 분리막; 및 전술한 비수 전해질을 포함한다.Specifically, a lithium secondary battery according to the present invention includes a negative electrode; a positive electrode opposite to the negative electrode; a separator interposed between the negative electrode and the positive electrode; and the non-aqueous electrolyte described above.
상기 리튬 이차전지는 상기 음극; 상기 음극에 대향하는 양극; 및 상기 음극 및 상기 양극 사이에 개재되는 분리막;을 포함하는 전극 조립체를 전지 케이스에 수납한 후, 전술한 비수 전해질을 주입하여 제조될 수 있다.The above lithium secondary battery can be manufactured by housing an electrode assembly including the negative electrode; a positive electrode opposing the negative electrode; and a separator interposed between the negative electrode and the positive electrode in a battery case, and then injecting the above-described non-aqueous electrolyte.
비수 전해질에 대한 설명은 전술하였으므로, 이하 음극, 양극 및 분리막에 대해 설명한다.Since the description of the non-aqueous electrolyte has been provided above, the cathode, anode, and separator will be described below.
(1) 음극(1) Cathode
상기 음극은 음극 활물질을 포함한다.The above negative electrode includes a negative electrode active material.
상기 음극 활물질은 당분야에서 음극 활물질로 사용되는 물질을 제한 없이 사용할 수 있다. 상기 음극 활물질은 구체적으로 실리콘계 활물질 및 탄소계 활물질 중에서 선택된 적어도 1종을 포함할 수 있고, 보다 구체적으로 실리콘계 활물질을 포함할 수 있다.The above negative active material may be any material used as a negative active material in the relevant field without limitation. The above negative active material may specifically include at least one selected from a silicon-based active material and a carbon-based active material, and more specifically may include a silicon-based active material.
상기 실리콘계 활물질은 탄소계 활물질에 비해 높은 용량을 발휘하지만, 충방전에 따른 부피 팽창/수축 정도가 크다는 문제가 있다. 그러나, 상기 실리콘계 활물질과 전술한 비수 전해질을 함께 사용할 경우, 유연하고 회복성이 강하면서도 내구성이 우수한 SEI 피막을 음극에 형성할 수 있으므로, 전해질 부반응이 방지되고 높은 수명 성능 및 저장 성능을 갖는 리튬 이차전지의 구현이 가능하다.The above silicon-based active material exhibits higher capacity than the carbon-based active material, but has a problem in that the degree of volume expansion/contraction due to charge/discharge is large. However, when the above silicon-based active material and the above-mentioned non-aqueous electrolyte are used together, a flexible, resilient, and durable SEI film can be formed on the negative electrode, thereby preventing electrolyte side reactions and enabling the implementation of a lithium secondary battery having high life performance and storage performance.
상기 실리콘계 활물질은 하기 화학식 A로 표시되는 화합물을 포함할 수 있다.The above silicon-based active material may include a compound represented by the following chemical formula A.
[화학식 A][Chemical Formula A]
SiOx(0 ≤ x < 2)SiO x (0 ≤ x < 2)
상기 화학식 A에서, SiO2의 경우 리튬 이온과 반응하지 않아 리튬을 저장할 수 없으므로, x는 상기 범위 내인 것이 바람직하다. 보다 구체적으로 상기 실리콘계 활물질은 Si일 수 있다.In the above chemical formula A, since SiO 2 does not react with lithium ions and thus cannot store lithium, it is preferable that x is within the above range. More specifically, the silicon-based active material may be Si.
상기 실리콘계 활물질의 평균 입경(D50)은 1㎛ 내지 20㎛일 수 있다.The average particle diameter (D 50 ) of the above silicon-based active material may be 1 μm to 20 μm.
상기 탄소계 활물질은 흑연, 하드카본, 소프트카본, 카본 블랙, 그래핀 및 섬유상 탄소로 이루어진 군으로부터 선택되는 적어도 1종을 포함할 수 있으며, 바람직하게는 흑연을 포함할 수 있다. 상기 흑연은 인조흑연 및 천연흑연으로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있다.The above carbon-based active material may include at least one selected from the group consisting of graphite, hard carbon, soft carbon, carbon black, graphene, and fibrous carbon, and preferably may include graphite. The graphite may include at least one selected from the group consisting of artificial graphite and natural graphite.
상기 탄소계 활물질의 평균 입경(D50)은 충방전 시에 구조적 안정성을 기하고 전해액과의 부반응을 줄이는 측면에서 10㎛ 내지 30㎛, 바람직하게는 15㎛ 내지 25㎛일 수 있다.The average particle diameter (D 50 ) of the above carbon-based active material may be 10 ㎛ to 30 ㎛, preferably 15 ㎛ to 25 ㎛, in order to ensure structural stability during charge and discharge and reduce side reactions with the electrolyte.
상기 음극은 음극 집전체; 및 상기 음극 집전체의 적어도 일면에 배치된 음극 활물질층;을 포함할 수 있다. 이때, 상기 음극 활물질은 상기 음극 활물질층에 포함될 수 있다.The above negative electrode may include a negative electrode current collector; and a negative electrode active material layer disposed on at least one surface of the negative electrode current collector. In this case, the negative electrode active material may be included in the negative electrode active material layer.
상기 음극 집전체는 전지에 화학적 변화를 유발하지 않으면서 높은 도전성을 가지는 것이라면 특별히 제한되지 않는다. 구체적으로 상기 음극 집전체는 구리, 스테인레스 스틸, 알루미늄, 니켈, 티탄, 소성 탄소, 구리나 스테인레스 스틸의 표면에 탄소, 니켈, 티탄, 은 등으로 표면 처리한 것, 알루미늄-카드뮴 합금 등이 사용될 수 있다.The above negative electrode current collector is not particularly limited as long as it has high conductivity without causing chemical changes in the battery. Specifically, the negative electrode current collector may be made of copper, stainless steel, aluminum, nickel, titanium, calcined carbon, copper or stainless steel surface-treated with carbon, nickel, titanium, silver, etc., an aluminum-cadmium alloy, etc.
상기 음극 집전체는 통상적으로 3 내지 500㎛의 두께를 가질 수 있다.The above negative electrode collector may typically have a thickness of 3 to 500 μm.
상기 음극 집전체는 표면에 미세한 요철을 형성하여 음극 활물질의 결합력을 강화시킬 수도 있다. 예를 들어, 상기 음극 집전체는 필름, 시트, 호일, 네트, 다공질체, 발포체, 부직포체 등 다양한 형태로 사용될 수 있다.The above negative electrode current collector may form fine irregularities on the surface to strengthen the bonding strength of the negative electrode active material. For example, the above negative electrode current collector may be used in various forms such as a film, a sheet, a foil, a net, a porous body, a foam, a non-woven fabric, etc.
상기 음극 활물질층은 상기 음극 집전체의 적어도 일면에 배치된다. 구체적으로, 상기 음극 활물질층은 상기 음극 집전체의 일면 또는 양면에 배치될 수 있다.The negative electrode active material layer is disposed on at least one surface of the negative electrode current collector. Specifically, the negative electrode active material layer may be disposed on one surface or both surfaces of the negative electrode current collector.
상기 음극 활물질은 부피 팽창/수축이 전지에 미치는 영향을 최소화하면서, 용량을 이차전지에 충분히 발현하기 위한 측면에서 상기 음극 활물질층 내에 60중량% 내지 99중량%으로 포함될 수 있다.The above negative active material may be included in the negative active material layer at 60 wt% to 99 wt% in order to sufficiently express the capacity in the secondary battery while minimizing the effect of volume expansion/contraction on the battery.
상기 음극 활물질층은 상기 실리콘계 활물질과 함께 도전재 및/또는 바인더를 더 포함할 수 있다.The above negative active material layer may further include a conductive material and/or a binder together with the silicon-based active material.
상기 바인더는 상기 음극 활물질층과 후술할 음극 집전체와의 접착력을 향상시키거나, 실리콘계 활물질 간의 결착력을 향상시키기 위해 사용될 수 있다.The above binder can be used to improve the adhesion between the negative electrode active material layer and the negative electrode current collector described later, or to improve the bonding strength between silicon-based active materials.
구체적으로, 상기 바인더는 전극 접착력을 더욱 향상시키고 실리콘계 활물질의 부피 팽창/수축에 충분한 저항력을 부여할 수 있다는 측면에서, 스티렌부타디엔 고무(SBR: styrene butadiene rubber), 니트릴부타디엔 고무(NBR: nitrile butadiene rubber), 아크릴로니트릴부타디엔 고무(acrylonitrile butadiene rubber), 아크릴 고무(acrylic rubber), 부틸 고무(butyl rubber), 플루오르 고무(fluoro rubber), 폴리비닐알코올, 카르복시메틸셀룰로오스(CMC), 전분, 히드록시프로필셀룰로오스, 재생 셀룰로오스, 폴리비닐알코올(PVA: polyvinyl alcohol), 폴리아크릴산(PAA: polyacrylic acid), 폴리에틸렌 글리콜(PEG: polyethylene glycol), 폴리아크릴로니트릴(PAN: polyacrylonitrile) 및 폴리아크릴 아미드(PAM: polyacryl amide)로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있다.Specifically, the binder comprises at least one selected from the group consisting of styrene butadiene rubber (SBR), nitrile butadiene rubber (NBR), acrylonitrile butadiene rubber, acrylic rubber, butyl rubber, fluoro rubber, polyvinyl alcohol, carboxymethyl cellulose (CMC), starch, hydroxypropyl cellulose, regenerated cellulose, polyvinyl alcohol (PVA), polyacrylic acid (PAA), polyethylene glycol (PEG), polyacrylonitrile (PAN), and polyacryl amide (PAM), in that it can further improve electrode adhesion and provide sufficient resistance to volume expansion/contraction of the silicon-based active material. Can be.
상기 바인더는 상기 음극 활물질층 내에 1중량% 내지 30중량%로 포함될 수 있으며, 상기 범위에 있을 때 음극 활물질을 보다 잘 결착시켜 활물질의 부피 팽창 문제를 최소화할 수 있음과 동시에 음극 활물질층 형성을 위한 슬러리 제조 시에 바인더의 분산이 용이하도록 하고 코팅성 및 슬러리의 상 안정성을 향상시킬 수 있다.The above binder may be included in the negative electrode active material layer at 1 wt% to 30 wt%, and when present in the above range, the negative electrode active material may be better bound, thereby minimizing the problem of volume expansion of the active material, while at the same time facilitating dispersion of the binder during the preparation of a slurry for forming the negative electrode active material layer, thereby improving the coatability and phase stability of the slurry.
상기 도전재는 이차전지에 도전성을 보조 및 향상시키기 위해 사용될 수 있고, 화학적 변화를 유발하지 않으면서 도전성을 가진 것이라면 특별히 제한되는 것은 아니다. 구체적으로 상기 도전재는 천연 흑연이나 인조 흑연 등의 흑연; 카본블랙, 아세틸렌 블랙, 케첸 블랙, 채널 블랙, 파네스 블랙, 램프 블랙, 서멀 블랙 등의 카본블랙; 탄소 섬유나 금속 섬유 등의 도전성 섬유; 탄소 나노 튜브 등의 도전성 튜브; 플루오로카본, 알루미늄, 니켈 분말 등의 금속 분말; 산화아연, 티탄산 칼륨 등의 도전성 위스커; 산화 티탄 등의 도전성 금속 산화물; 및 폴리페닐렌 유도체로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있다.The conductive material may be used to assist and improve conductivity in a secondary battery, and is not particularly limited as long as it has conductivity without causing a chemical change. Specifically, the conductive material may include at least one selected from the group consisting of graphite such as natural graphite or artificial graphite; carbon black such as carbon black, acetylene black, Ketjen black, channel black, paneth black, lamp black, and thermal black; conductive fibers such as carbon fibers or metal fibers; conductive tubes such as carbon nanotubes; metal powders such as fluorocarbon, aluminum, and nickel powder; conductive whiskers such as zinc oxide and potassium titanate; conductive metal oxides such as titanium oxide; and polyphenylene derivatives.
상기 도전재는 상기 음극 활물질층 내에 1중량% 내지 20중량%로 포함될 수 있으며, 상기 범위일 때 바인더로 인한 저항 증가를 완화시키면서도 우수한 도전성 네트워크를 형성할 수 있다는 측면에서 바람직하다.The above-mentioned conductive agent may be included in the negative electrode active material layer at 1 wt% to 20 wt%, and when within the above range, it is preferable in that it can form an excellent conductive network while alleviating the increase in resistance due to the binder.
상기 음극 활물질층의 두께는 5㎛ 내지 500㎛, 바람직하게는 5㎛ 내지 100㎛일 수 있다.The thickness of the above negative active material layer may be 5 ㎛ to 500 ㎛, preferably 5 ㎛ to 100 ㎛.
상기 음극은 상기 음극 집전체 상에 음극 활물질 및 선택적으로 바인더, 도전재 및 음극 슬러리 형성용 용매를 포함하는 음극 슬러리를 코팅한 다음, 건조 및 압연하여 제조될 수 있다.The above negative electrode can be manufactured by coating a negative electrode slurry including a negative electrode active material and optionally a binder, a conductive material, and a solvent for forming a negative electrode slurry on the negative electrode current collector, and then drying and rolling.
상기 음극 슬러리 형성용 용매는 예를 들어 음극 활물질, 바인더 및/또는 도전재의 분산을 용이하게 하는 측면에서, 증류수, 에탄올, 메탄올 및 이소프로필 알코올로 이루어진 군에서 선택된 적어도 1종, 바람직하게는 증류수를 포함할 수 있다.The solvent for forming the negative electrode slurry may include at least one selected from the group consisting of distilled water, ethanol, methanol and isopropyl alcohol, preferably distilled water, in order to facilitate dispersion of the negative electrode active material, binder and/or conductive agent.
(2) 양극(2) Bipolar
상기 양극은 양극 활물질을 포함한다.The above positive electrode contains a positive electrode active material.
상기 양극 활물질은 리튬의 가역적인 인터칼레이션 및 디인터칼레이션이 가능한 화합물로서, 구체적으로는 니켈, 코발트, 망간 및 알루미늄으로 이루어진 적어도 1종의 전이금속과 리튬을 포함하는 리튬 전이금속 복합 산화물, 바람직하게는 니켈, 코발트 및 망간을 포함하는 전이금속과 리튬을 포함하는 리튬 전이금속 복합 산화물을 포함할 수 있다.The above positive electrode active material is a compound capable of reversible intercalation and deintercalation of lithium, and specifically, may include a lithium-transition metal composite oxide including lithium and at least one transition metal selected from nickel, cobalt, manganese, and aluminum, preferably a lithium-transition metal composite oxide including lithium and a transition metal selected from nickel, cobalt, and manganese.
예를 들어, 상기 리튬 전이금속 복합 산화물로는 리튬-망간계 산화물(예를 들면, LiMnO2, LiMn2O4 등), 리튬-코발트계 산화물(예를 들면, LiCoO2 등), 리튬-니켈계 산화물(예를 들면, LiNiO2 등), 리튬-니켈-망간계 산화물(예를 들면, LiNi1-YMnYO2(여기에서, 0<Y<1), LiMn2-zNizO4(여기에서, 0<Z<2) 등), 리튬-니켈-코발트계 산화물(예를 들면, LiNi1-Y1CoY1O2(여기에서, 0<Y1<1) 등), 리튬-망간-코발트계 산화물(예를 들면, LiCo1-Y2MnY2O2(여기에서, 0<Y2<1), LiMn2-z1Coz1O4(여기에서, 0<Z1<2) 등), 리튬-니켈-망간-코발트계 산화물(예를 들면, Li(NipCoqMnr1)O2(여기에서, 0<p<1, 0<q<1, 0<r1<1, p+q+r1=1) 또는 Li(Nip1Coq1Mnr2)O4(여기에서, 0<p1<2, 0<q1<2, 0<r2<2, p1+q1+r2=2) 등), 또는 리튬-니켈-코발트-전이금속(M) 산화물 (예를 들면, Li(Nip2Coq2Mnr3MS2)O2(여기에서, M은 Al, Fe, V, Cr, Ti, Ta, Mg 및 Mo로 이루어지는 군으로부터 선택되고, p2, q2, r3 및 s2는 각각 독립적인 원소들의 원자분율로서, 0<p2<1, 0<q2<1, 0<r3<1, 0<s2<1, p2+q2+r3+s2=1이다) 등) 등을 들 수 있으며, 이들 중 어느 하나 또는 둘 이상의 화합물이 포함될 수 있다. 이중에서도 전지의 용량 특성 및 안정성을 높일 수 있다는 점에서 상기 리튬 전이금속 복합 산화물은 LiCoO2, LiMnO2, LiNiO2, 리튬 니켈-망간-코발트 산화물(예를 들면, Li(Ni0.6Mn0.2Co0.2)O2, Li(Ni0.5Mn0.3Co0.2)O2, Li(Ni0.7Mn0.15Co0.15)O2 또는 Li(Ni0.8Mn0.1Co0.1)O2 등), 또는 리튬 니켈코발트알루미늄 산화물(예를 들면, Li(Ni0.8Co0.15Al0.05)O2 등) 등일 수 있으며, 리튬 전이금속 복합 산화물을 형성하는 구성원소의 종류 및 함량비 제어에 따른 개선 효과의 현저함을 고려할 때 상기 리튬 전이금속 복합 산화물은 Li(Ni0.6Mn0.2Co0.2)O2, Li(Ni0.5Mn0.3Co0.2)O2, Li(Ni0.7Mn0.15Co0.15)O2 또는 Li(Ni0.8Mn0.1Co0.1)O2 등일 수 있으며, 이들 중 어느 하나 또는 둘 이상의 혼합물이 사용될 수 있다.For example, the lithium transition metal composite oxides include lithium-manganese oxides (e.g., LiMnO 2 , LiMn 2 O 4 , etc.), lithium-cobalt oxides (e.g., LiCoO 2 , etc.), lithium-nickel oxides (e.g., LiNiO 2 , etc.), lithium-nickel-manganese oxides (e.g., LiNi 1-Y Mn Y O 2 (wherein, 0<Y<1), LiMn 2-z Ni z O 4 (wherein, 0<Z<2)), lithium-nickel-cobalt oxides (e.g., LiNi 1-Y1 Co Y1 O 2 (wherein, 0<Y1<1)), lithium-manganese-cobalt oxides (e.g., LiCo 1-Y2 Mn Y2 O 2 (wherein, 0<Y2<1), LiMn 2-z1 Co z1 O 4 (wherein, (0<Z1<2) etc.), lithium-nickel-manganese-cobalt oxides (e.g., Li(Ni p Co q Mn r1 )O 2 (wherein, 0<p<1, 0<q<1, 0<r1<1, p+q+r1=1) or Li(Ni p1 Co q1 Mn r2 )O 4 (wherein, 0<p1<2, 0<q1<2, 0<r2<2, p1+q1+r2=2) etc.), or lithium-nickel-cobalt-transition metal (M) oxides (e.g., Li(Ni p2 Co q2 Mn r3 M S2 )O 2 (wherein, M is selected from the group consisting of Al, Fe, V, Cr, Ti, Ta, Mg and Mo, and p2, q2, r3 and s2 are atomic fractions of independent elements, 0<p2<1, 0<q2<1, 0<r3<1, 0<s2<1, p2+q2+r3+s2=1), etc.), and one or more compounds among these may be included. Among these, the lithium transition metal composite oxide may be LiCoO 2 , LiMnO 2 , LiNiO 2 , lithium nickel-manganese-cobalt oxide (for example, Li(Ni 0.6 Mn 0.2 Co 0.2 )O 2 , Li(Ni 0.5 Mn 0.3 Co 0.2 )O 2 , Li(Ni 0.7 Mn 0.15 Co 0.15 )O 2 or Li(Ni 0.8 Mn 0.1 Co 0.1 )O 2 , or lithium nickel cobalt aluminum oxide (for example, Li(Ni 0.8 Co 0.15 Al 0.05 )O 2 , etc.), and considering the prominence of the improvement effect according to the control of the type and content ratio of the constituent elements forming the lithium transition metal composite oxide, the lithium transition metal composite oxide may be The oxide may be Li ( Ni0.6Mn0.2Co0.2 ) O2 , Li(Ni0.5Mn0.3Co0.2)O2, Li(Ni0.7Mn0.15Co0.15)O2 or Li(Ni0.8Mn0.1Co0.1 ) O2 , and any one of these or a mixture of two or more thereof may be used .
보다 구체적으로, 상기 양극 활물질은 리튬 전이금속 복합 산화물로서, 상기 리튬 전이금속 복합 산화물에 포함된 전이금속의 전체 몰수를 기준으로 니켈을 60몰% 이상 포함하는 것일 수 있다. 구체적으로, 상기 양극 활물질은 리튬 전이금속 복합 산화물로서, 상기 전이금속은 니켈; 및 망간, 코발트 및 알루미늄 중에서 선택된 적어도 1종을 포함하고, 상기 니켈을 상기 전이금속의 전체 몰수를 기준으로 60몰% 이상, 구체적으로 60몰% 내지 90몰%로 포함하는 것일 수 있다. 이러한 니켈을 고함량으로 사용하는 리튬 전이금속 복합 산화물을 전술한 비수 전해액을 함께 사용할 때, 구조 붕괴에 의해 발생되는 가스 상에 부산물을 감소시켜 줄 수 있다는 측면에서 바람직하다.More specifically, the cathode active material may be a lithium-transition metal composite oxide, and may contain nickel in an amount of 60 mol% or more based on the total mole number of transition metals included in the lithium-transition metal composite oxide. Specifically, the cathode active material may be a lithium-transition metal composite oxide, wherein the transition metal includes nickel; and at least one selected from manganese, cobalt, and aluminum, and may contain nickel in an amount of 60 mol% or more, specifically 60 to 90 mol%, based on the total mole number of transition metals. When such a lithium-transition metal composite oxide using a high nickel content is used together with the above-described non-aqueous electrolyte, it is preferable in that by-products in a gas phase generated by structural collapse can be reduced.
또한, 상기 양극 활물질은 하기 화학식 B로 표시되는 리튬 복합 전이금속 산화물을 포함할 수 있다.In addition, the positive electrode active material may include a lithium composite transition metal oxide represented by the following chemical formula B.
[화학식 B][Chemical Formula B]
Li1+x(NiaCobMncMd)O2
Li 1+x (Ni a Co b Mn c M d )O 2
상기 화학식 B에서, M은 W, Cu, Fe, V, Cr, Ti, Zr, Zn, Al, In, Ta, Y, La, Sr, Ga, Sc, Gd, Sm, Ca, Ce, Nb, Mg, B 및 Mo 중 선택된 1종 이상이고, 1+x, a, b, c 및 d는 각각 독립적인 원소들의 원자분율로서, 0≤x≤0.2, 0.50≤a<1, 0<b≤0.25, 0<c≤0.25, 0≤d≤0.1, a+b+c+d=1이다.In the chemical formula B, M is at least one selected from W, Cu, Fe, V, Cr, Ti, Zr, Zn, Al, In, Ta, Y, La, Sr, Ga, Sc, Gd, Sm, Ca, Ce, Nb, Mg, B, and Mo, and 1+x, a, b, c, and d are atomic fractions of independent elements, respectively, 0≤x≤0.2, 0.50≤a<1, 0<b≤0.25, 0<c≤0.25, 0≤d≤0.1, a+b+c+d=1.
바람직하게는, 상기 a, b, c 및 d는 각각 0.70≤a≤0.95, 0.025≤b≤0.20, 0.025≤c≤0.20, 0≤d≤0.05일 수 있다.Preferably, a, b, c and d may be 0.70≤a≤0.95, 0.025≤b≤0.20, 0.025≤c≤0.20, 0≤d≤0.05, respectively.
또한, 상기 a, b, c 및 d는 각각 0.80≤a≤0.95, 0.025≤b≤0.15, 0.025≤c≤0.15, 0≤d≤0.05일 수 있다.Additionally, a, b, c, and d may be 0.80≤a≤0.95, 0.025≤b≤0.15, 0.025≤c≤0.15, and 0≤d≤0.05, respectively.
또한, 상기 a, b, c 및 d는 각각 0.85≤a≤0.90, 0.05≤b≤0.10, 0.05≤c≤0.10, 0≤d≤0.03일 수 있다.Additionally, a, b, c, and d may be 0.85≤a≤0.90, 0.05≤b≤0.10, 0.05≤c≤0.10, and 0≤d≤0.03, respectively.
상기 양극은 양극 집전체; 및 상기 양극 집전체의 적어도 일면에 배치된 양극 활물질층;을 포함할 수 있다. 이때, 상기 양극 활물질층은 전술한 양극 활물질을 포함할 수 있다.The above positive electrode may include a positive electrode current collector; and a positive electrode active material layer disposed on at least one surface of the positive electrode current collector. In this case, the positive electrode active material layer may include the above-described positive electrode active material.
상기 양극 집전체의 두께는 통상적으로 3 내지 500㎛의 두께를 가질 수 있다.The thickness of the above positive electrode collector can typically be 3 to 500 μm.
상기 양극 집전체는 표면에 미세한 요철을 형성하여 음극 활물질의 결합력을 강화시킬 수도 있다. 예를 들어, 상기 양극 집전체는 필름, 시트, 호일, 네트, 다공질체, 발포체, 부직포체 등 다양한 형태로 사용될 수 있다.The above-mentioned positive electrode current collector may form fine irregularities on the surface to strengthen the bonding strength of the negative electrode active material. For example, the above-mentioned positive electrode current collector may be used in various forms such as a film, a sheet, a foil, a net, a porous body, a foam, a non-woven fabric, etc.
상기 양극 활물질층은 상기 양극 집전체의 적어도 일면에 배치된다. 구체적으로, 상기 양극 활물질층은 상기 양극 집전체의 일면 또는 양면에 배치될 수 있다.The positive electrode active material layer is disposed on at least one surface of the positive electrode current collector. Specifically, the positive electrode active material layer may be disposed on one surface or both surfaces of the positive electrode current collector.
상기 양극 활물질은 양극 활물질의 충분한 용량 발휘 등을 고려하여 양극 활물질층에 80중량% 내지 99중량%로 포함될 수 있다.The above cathode active material may be included in the cathode active material layer at 80 to 99 wt%, taking into account sufficient capacity of the cathode active material.
상기 양극 활물질층은 전술한 양극 활물질과 함께 바인더 및/또는 도전재를 더 포함할 수 있다.The above-described positive electrode active material layer may further include a binder and/or a conductive material together with the above-described positive electrode active material.
상기 바인더는 활물질과 도전재 등의 결착과 집전체에 대한 결착에 조력하는 성분이며, 구체적으로 폴리비닐리덴플루오라이드, 폴리비닐알코올, 카르복시메틸셀룰로우즈(CMC), 전분, 히드록시프로필셀룰로우즈, 재생 셀룰로우즈, 폴리비닐피롤리돈, 폴리테트라플루오로에틸렌, 폴리에틸렌, 폴리프로필렌, 에틸렌-프로필렌-디엔 테르 폴리머(EPDM), 술폰화 EPDM, 스티렌-부타디엔 고무 및 불소 고무로 이루어진 군에서 선택된 적어도 1종, 바람직하게는 폴리비닐리덴플루오라이드를 포함할 수 있다.The above binder is a component that assists in the binding of the active material and the conductive material and the binding to the current collector, and specifically, may include at least one selected from the group consisting of polyvinylidene fluoride, polyvinyl alcohol, carboxymethyl cellulose (CMC), starch, hydroxypropyl cellulose, regenerated cellulose, polyvinyl pyrrolidone, polytetrafluoroethylene, polyethylene, polypropylene, ethylene-propylene-diene terpolymer (EPDM), sulfonated EPDM, styrene-butadiene rubber, and fluororubber, preferably polyvinylidene fluoride.
상기 바인더는 양극 활물질 등 성분 간 결착력을 충분히 확보하는 측면에서 상기 양극 활물질층에 1중량% 내지 20중량%, 바람직하게는 1.2중량% 내지 10중량%로 포함될 수 있다.The above binder may be included in the positive electrode active material layer at 1 to 20 wt%, preferably 1.2 to 10 wt%, in order to sufficiently secure binding force between components such as the positive electrode active material.
상기 도전재는 이차전지에 도전성을 보조 및 향상시키기 위해 사용될 수 있고, 화학적 변화를 유발하지 않으면서 도전성을 가진 것이라면 특별히 제한되는 것은 아니다. 구체적으로 상기 양극 도전재는 천연 흑연이나 인조 흑연 등의 흑연; 카본블랙, 아세틸렌 블랙, 케첸 블랙, 채널 블랙, 파네스 블랙, 램프 블랙, 서멀 블랙 등의 카본블랙; 탄소 섬유나 금속 섬유 등의 도전성 섬유; 탄소 나노 튜브 등의 도전성 튜브; 플루오로카본; 알루미늄, 니켈 분말 등의 금속 분말; 산화아연, 티탄산 칼륨 등의 도전성 위스커; 산화 티탄 등의 도전성 금속 산화물; 및 폴리페닐렌 유도체로 이루어진 군에서 선택된 적어도 1종을 포함할 수 있으며, 바람직하게는 도전성 향상 측면에서 탄소 나노 튜브를 포함할 수 있다.The conductive material may be used to assist and improve conductivity in a secondary battery, and is not particularly limited as long as it has conductivity without causing a chemical change. Specifically, the positive electrode conductive material may include at least one selected from the group consisting of graphite such as natural graphite or artificial graphite; carbon black such as carbon black, acetylene black, Ketjen black, channel black, paneth black, lamp black, thermal black, etc.; conductive fibers such as carbon fibers or metal fibers; conductive tubes such as carbon nanotubes; fluorocarbons; metal powders such as aluminum or nickel powder; conductive whiskers such as zinc oxide or potassium titanate; conductive metal oxides such as titanium oxide; and polyphenylene derivatives, and preferably, the positive electrode conductive material may include carbon nanotubes in terms of improving conductivity.
상기 도전재는 전기 전도성을 충분히 확보하는 측면에서 상기 양극 활물질층 내에 1중량% 내지 20중량%, 바람직하게는 1.2중량% 내지 10중량%로 포함될 수 있다.The above-mentioned conductive material may be included in the positive electrode active material layer at 1 wt% to 20 wt%, preferably 1.2 wt% to 10 wt%, in order to sufficiently secure electrical conductivity.
상기 양극 활물질층의 두께는 5㎛ 내지 500㎛, 바람직하게는 20㎛ 내지 200㎛일 수 있다.The thickness of the above positive electrode active material layer may be 5 µm to 500 µm, preferably 20 µm to 200 µm.
상기 양극은 상기 양극 집전체 상에 양극 활물질 및 선택적으로 바인더, 도전재 및 양극 슬러리 형성용 용매를 포함하는 양극 슬러리를 코팅한 다음, 건조 및 압연하여 제조될 수 있다.The above positive electrode can be manufactured by coating a positive electrode slurry including a positive electrode active material and optionally a binder, a conductive material, and a solvent for forming a positive electrode slurry on the positive electrode current collector, and then drying and rolling.
(3) 분리막(3) Membrane
상기 분리막은 상기 양극 및 상기 음극 사이에 개재될 수 있다.The above separator may be interposed between the anode and the cathode.
상기 분리막으로는 종래에 분리막으로 사용된 통상적인 다공성 고분자 필름, 예를 들어 에틸렌 단독공중합체, 프로필렌 단독공중합체, 에틸렌/부텐 공중합체, 에틸렌/헥센 공중합체 및 에틸렌/메타크릴레이트 공중합체 등과 같은 폴리올레핀계 고분자로 제조한 다공성 고분자 필름을 단독으로 또는 이들을 적층하여 사용할 수 있으며, 또는 통상적인 다공성 부직포, 예를 들어 고융점의 유리 섬유, 폴리에틸렌테레프탈레이트 섬유 등으로 된 부직포를 사용할 수 있으나, 이에 한정되는 것은 아니다. 또한, 내열성 또는 기계적 강도 확보를 위해 세라믹 성분 또는 고분자 물질이 포함된 코팅된 분리막이 사용될 수도 있으며, 선택적으로 단층 또는 다층 구조로 사용될 수 있다.As the above-mentioned separator, a conventional porous polymer film used as a conventional separator, for example, a porous polymer film made of a polyolefin polymer such as an ethylene homopolymer, a propylene homopolymer, an ethylene/butene copolymer, an ethylene/hexene copolymer, and an ethylene/methacrylate copolymer, can be used alone or in a laminated manner, or a conventional porous nonwoven fabric, for example, a nonwoven fabric made of high-melting-point glass fiber, polyethylene terephthalate fiber, etc., can be used, but is not limited thereto. In addition, a coated separator containing a ceramic component or a polymer material to secure heat resistance or mechanical strength can be used, and can optionally be used in a single-layer or multi-layer structure.
본 발명의 리튬 이차전지의 외형은 특별한 제한이 없으나, 캔을 사용한 원통형, 각형, 파우치(pouch)형 또는 코인(coin)형 등이 될 수 있다.There is no particular limitation on the external shape of the lithium secondary battery of the present invention, but it may be in the shape of a cylinder, a square, a pouch, or a coin using a can.
이하, 구체적인 실시예를 통해 본 발명을 보다 구체적으로 설명한다. 다만, 하기 실시예는 본 발명의 이해를 돕기 위한 예시일 뿐, 본 발명의 범위를 한정하는 것은 아니다. 본 기재의 범주 및 기술사상 범위 내에서 다양한 변경 및 수정이 가능함은 당업자에게 있어서 명백한 것이며, 이러한 변형 및 수정이 첨부된 특허청구범위에 속하는 것은 당연한 것이다.Hereinafter, the present invention will be described in more detail through specific examples. However, the following examples are merely examples to help understand the present invention and do not limit the scope of the present invention. It will be obvious to those skilled in the art that various changes and modifications are possible within the scope and technical idea of the present description, and it is natural that such changes and modifications fall within the scope of the appended patent claims.
실시예 및 비교예Examples and Comparative Examples
실시예 1Example 1
(비수 전해질의 제조)(Manufacture of non-aqueous electrolyte)
유기 용매로서 플루오로에틸렌 카보네이트(FEC) 및 디에틸카보네이트(DEC)를 10:90의 부피비로 혼합한 것을 사용하였다.A mixture of fluoroethylene carbonate (FEC) and diethyl carbonate (DEC) in a volume ratio of 10:90 was used as an organic solvent.
상기 유기 용매에 리튬 염으로서 LiPF6, 첨가제로서 상기 화학식 1-1로 표시되는 화합물 및 리튬 디플루오로포스페이트(LiDFP)를 첨가하여 비수 전해질을 제조하였다.A non-aqueous electrolyte was prepared by adding LiPF 6 as a lithium salt, a compound represented by the chemical formula 1-1 as an additive, and lithium difluorophosphate (LiDFP) to the organic solvent.
상기 LiPF6은 비수 전해질에 1.5M의 몰 농도로 포함되었다.The above LiPF 6 was included in the non-aqueous electrolyte at a molar concentration of 1.5 M.
하기 화학식 1-1로 표시되는 화합물은 상기 비수 전해질에 2중량%로 포함되었고, 리튬 디플루오로포스페이트는 상기 비수 전해질에 0.5중량%로 포함되었다.The compound represented by the following chemical formula 1-1 was included in the non-aqueous electrolyte at 2 wt%, and lithium difluorophosphate was included in the non-aqueous electrolyte at 0.5 wt%.
(리튬 이차전지 제조)(Lithium secondary battery manufacturing)
양극 활물질(Li[Ni0.85Co0.05Mn0.07Al0.03]O2) : 도전재(탄소나노튜브): 바인더 (폴리비닐리덴플루오라이드)를 97.74:0.70:1.56 중량비로 용제인 N-메틸-2-피롤리돈(NMP) 에 첨가하여 양극 합제 슬러리(고형분 75.5 중량%)를 제조하였다. 상기 양극 합제 슬러리를 두께가 12 ㎛인 양극 집전체(Al 박막) 일면에 도포하고, 건조 및 롤 프레스(roll press)를 실시하여 양극을 제조하였다. A positive electrode mixture slurry (solid content 75.5 wt%) was prepared by adding positive electrode active material (Li[Ni 0.85 Co 0.05 Mn 0.07 Al 0.03 ]O 2 ): conductive material (carbon nanotube): binder (polyvinylidene fluoride) in a weight ratio of 97.74:0.70:1.56 to N-methyl-2-pyrrolidone (NMP) as a solvent. The positive electrode mixture slurry was applied to one surface of a positive electrode current collector (Al thin film) having a thickness of 12 ㎛, and drying and roll pressing were performed to prepare a positive electrode.
음극 활물질(실리콘계 활물질, Si) : 도전재(카본블랙) : 바인더(스티렌-스타디엔 고무)를 70.0:20.3:9.7의 중량비로 용제인 증류수에 첨가하여 음극 합제 슬러리(고형분 26 중량%)를 제조하였다. 상기 음극 합제 슬러리를 두께가 15㎛인 음극 집전체(Cu 박막) 일면에 도포하고, 건조 및 롤 프레스(roll press)를 실시하여 음극을 제조하였다. A negative electrode mixture slurry (solid content 26 wt%) was prepared by adding negative active material (silicon-based active material, Si): conductive agent (carbon black): binder (styrene-stadiene rubber) to distilled water as a solvent in a weight ratio of 70.0:20.3:9.7. The negative electrode mixture slurry was applied to one surface of a 15 ㎛ thick negative electrode current collector (Cu thin film), and drying and roll pressing were performed to prepare a negative electrode.
드라이 룸에서 상기 제조된 양극과 음극 사이에 폴리에틸렌 다공성 필름 세퍼레이터를 개재한 다음, 상기 제조된 비수 전해질을 주액하여 이차 전지를 제조하였다.A polyethylene porous film separator was interposed between the positive and negative electrodes manufactured above in a dry room, and then the non-aqueous electrolyte manufactured above was injected to manufacture a secondary battery.
실시예 2Example 2
상기 화학식 1-1로 표시되는 화합물을 2중량% 대신 0.5중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was added to the non-aqueous electrolyte in an amount of 0.5 wt% instead of 2 wt%.
실시예 3Example 3
상기 화학식 1-1로 표시되는 화합물을 2중량% 대신 5중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was added to the non-aqueous electrolyte in an amount of 5 wt% instead of 2 wt%.
실시예 4Example 4
상기 화학식 1-1로 표시되는 화합물 대신 상기 화학식 1-9로 표시되는 화합물을 2중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula 1-9 was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
비교예 1Comparative Example 1
상기 화학식 1-1로 표시되는 화합물을 첨가하지 않은 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that the compound represented by the chemical formula 1-1 was not added.
비교예 2Comparative Example 2
상기 화학식 1-1로 표시되는 화합물 대신 화학식 X로 표시되는 화합물을 2중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula X was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
[화학식 X][chemical formula X]
비교예 3Comparative Example 3
상기 화학식 1-1로 표시되는 화합물 대신 화학식 Y로 표시되는 화합물을 2중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula Y was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
[화학식 Y][chemical formula Y]
비교예 4Comparative Example 4
상기 화학식 1-1로 표시되는 화합물 대신 화학식 Z로 표시되는 화합물을 2중량%의 함량으로 비수 전해질에 첨가한 것을 제외하고는 실시예 1과 동일한 방법으로 비수 전해질, 리튬 이차전지를 제조하였다.A non-aqueous electrolyte and a lithium secondary battery were manufactured in the same manner as in Example 1, except that a compound represented by the chemical formula Z was added to the non-aqueous electrolyte in an amount of 2 wt% instead of the compound represented by the chemical formula 1-1.
[화학식 Z][chemical formula Z]
실험예Experimental example
실험예 1: 고온 사이클 성능 평가Experimental Example 1: Evaluation of High Temperature Cycle Performance
상기에서 제조된 실시예 1~4, 비교예 1~4의 리튬 이차전지를 전기화학 충방전기를 사용하여 45℃에서 CC/CV, 1C 조건으로 4.2V, 0.05C까지 충전한 다음, CC, 0.5C 조건으로 3.0V까지 방전하는 것을 1 사이클로 하여 200 사이클 충방전을 실시하였다.The lithium secondary batteries manufactured in Examples 1 to 4 and Comparative Examples 1 to 4 above were charged to 4.2 V, 0.05 C using an electrochemical charger/discharger under CC/CV, 1 C conditions at 45°C, and then discharged to 3.0 V under CC, 0.5 C conditions, which constituted one cycle, and 200 charge/discharge cycles were performed.
용량 유지율을 아래 식으로 계산하고, 그 결과를 하기 표 1에 나타내었다.The capacity retention rate is calculated using the formula below, and the results are shown in Table 1 below.
용량 유지율(%) = {(200사이클 후의 방전 용량/1 사이클 후의 방전 용량)} × 100Capacity retention rate (%) = {(discharge capacity after 200 cycles/discharge capacity after 1 cycle)} × 100
실험예 2: 고온 저장 성능 평가Experimental Example 2: Evaluation of High Temperature Storage Performance
상기에서 제조된 실시예 1~4, 비교예 1~4의 리튬 이차전지를 25℃에서 CC/CV, 0.33C 조건으로 4.2V, 0.05C까지 충전하고 0.33C로 2.5V까지 방전하여 초기 충방전을 수행하였고, 이후 25℃에서 CC/CV, 0.33C 조건으로 4.2V, 0.05C까지 충전한 후, 60℃에서 8주 저장하였다. The lithium secondary batteries manufactured in Examples 1 to 4 and Comparative Examples 1 to 4 above were charged to 4.2 V, 0.05 C under CC/CV, 0.33 C conditions at 25°C and discharged to 2.5 V at 0.33 C to perform initial charge/discharge, and then charged to 4.2 V, 0.05 C under CC/CV, 0.33 C conditions at 25°C and stored at 60°C for 8 weeks.
8주 저장 후, 상기 리튬 이차전지를 25℃에서 0.33C 조건으로 4.2V, 0.05C까지 충전하고 0.33C로 3.0V까지 방전하여 방전 시의 용량을 측정하였다.After 8 weeks of storage, the lithium secondary battery was charged to 4.2 V, 0.05 C at 25°C under 0.33 C conditions and discharged to 3.0 V at 0.33 C to measure the capacity during discharge.
하기 식에 따라 용량 유지율을 평가하고, 그 결과를 하기 표 1에 나타내었다.The capacity retention rate was evaluated according to the following formula, and the results are shown in Table 1 below.
용량 유지율(%) = (8주 저장 후 방전 용량/초기 방전 용량) × 100Capacity retention rate (%) = (discharge capacity after 8 weeks of storage/initial discharge capacity) × 100
상기 표 1을 참조하면, 본 발명에 따른 비수 전해질을 사용한 실시예 1 내지 4의 리튬 이차전지는 그렇지 않은 비교예 1 내지 4에 비해 고온 사이클 충방전 및 고온 저장 시 현저하게 우수한 수준의 용량 유지율을 보이는 것을 확인할 수 있다.Referring to Table 1 above, it can be confirmed that the lithium secondary batteries of Examples 1 to 4 using the non-aqueous electrolyte according to the present invention exhibit significantly superior capacity retention rates during high-temperature cycle charge/discharge and high-temperature storage compared to Comparative Examples 1 to 4 that do not use the non-aqueous electrolyte.
Claims (12)
- 리튬 염, 유기 용매 및 첨가제를 포함하고,Containing lithium salt, organic solvent and additives,상기 첨가제는 하기 화학식 1로 표시되는 화합물을 포함하는 비수 전해질:The above additive is a non-aqueous electrolyte comprising a compound represented by the following chemical formula 1:[화학식 1][Chemical Formula 1]상기 화학식 1에서, R1 및 R2는 서로 독립적으로 F, Br, Cl, I, 나이트릴기, 에스터기, 에테르기, 케톤기, 카르복시기, 치환 또는 비치환된 탄소수 1 내지 10의 알킬기, 치환 또는 비치환된 탄소수 1 내지 10의 알케닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알키닐기, 치환 또는 비치환된 탄소수 1 내지 10의 알콕시기, 보론기, 보레이트기, 이소시아네이트기, 이소티오시아네이트기, 실릴기, 실록산기, 설폰기, 설포네이트기, 설페이트기, 하기 화학식 2로 표시되는 치환기 또는 이들의 2 이상의 조합을 포함하고,In the chemical formula 1, R 1 and R 2 independently comprise F, Br, Cl, I, a nitrile group, an ester group, an ether group, a ketone group, a carboxyl group, a substituted or unsubstituted alkyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkenyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkynyl group having 1 to 10 carbon atoms, a substituted or unsubstituted alkoxy group having 1 to 10 carbon atoms, a boron group, a borate group, an isocyanate group, an isothiocyanate group, a silyl group, a siloxane group, a sulfone group, a sulfonate group, a sulfate group, a substituent represented by the following chemical formula 2, or a combination of two or more thereof,상기 R1 및 R2 중 적어도 하나는 하기 화학식 2로 표시되는 치환기를 포함하고,At least one of the above R 1 and R 2 comprises a substituent represented by the following chemical formula 2,n은 3 내지 8의 정수이고,n is an integer from 3 to 8,[화학식 2][Chemical formula 2]상기 화학식 2에서, L1은 탄소수 1 내지 10의 알킬렌기, 에스터기, 설폰기, 설포네이트기, 설페이트기 또는 이들의 2 이상의 조합이고,In the above chemical formula 2, L 1 is an alkylene group having 1 to 10 carbon atoms, an ester group, a sulfone group, a sulfonate group, a sulfate group, or a combination of two or more thereof,R3은 불소가 적어도 하나 치환된 탄소수 1 내지 10의 알콕시기이고,R 3 is an alkoxy group having 1 to 10 carbon atoms substituted with at least one fluorine,*는 결합 부위이다.* is the binding site.
- 청구항 1에 있어서,In claim 1,상기 화학식 1에서, n은 3 또는 4인 비수 전해질.A non-aqueous electrolyte in the above chemical formula 1, wherein n is 3 or 4.
- 청구항 1에 있어서,In claim 1,상기 R3은 -OCF3, -OCF2CF3 및 -OCF2CF2CF3로 이루어진 군에서 선택된 1종을 포함하는 비수 전해질.A non-aqueous electrolyte wherein the above R 3 comprises one selected from the group consisting of -OCF 3 , -OCF 2 CF 3 , and -OCF 2 CF 2 CF 3 .
- 청구항 1에 있어서,In claim 1,상기 R1이 상기 화학식 2로 표시되는 치환기를 포함하고, 상기 R2는 상기 화학식 2로 표시되는 치환기를 포함하지 않는 비수 전해질.The above R 1 A non-aqueous electrolyte comprising a substituent represented by the above chemical formula 2, wherein R 2 does not comprise a substituent represented by the above chemical formula 2.
- 청구항 4에 있어서,In claim 4,상기 R2는 탄소수 1 내지 5의 알킬기인 비수 전해질.A non-aqueous electrolyte wherein R 2 is an alkyl group having 1 to 5 carbon atoms.
- 청구항 1에 있어서,In claim 1,상기 화학식 1로 표시되는 화합물은 하기 화학식 1-1, 화학식 1-2, 화학식 1-3, 화학식 1-4, 화학식 1-5, 화학식 1-6, 화학식 1-7, 화학식 1-8, 화학식 1-9, 화학식 1-10, 화학식 1-11 및 화학식 1-12로 이루어진 군에서 선택된 적어도 1종의 화합물을 포함하는 비수 전해질:The compound represented by the above chemical formula 1 is a non-aqueous electrolyte including at least one compound selected from the group consisting of the following chemical formulas 1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-11 and 1-12:[화학식 1-1][Chemical Formula 1-1][화학식 1-2][Chemical Formula 1-2][화학식 1-3][Chemical Formula 1-3][화학식 1-4][Chemical Formula 1-4][화학식 1-5][Chemical Formula 1-5][화학식 1-6][Chemical Formula 1-6][화학식 1-7][Chemical Formula 1-7][화학식 1-8][Chemical Formula 1-8][화학식 1-9][Chemical Formula 1-9][화학식 1-10][Chemical Formula 1-10][화학식 1-11][Chemical Formula 1-11][화학식 1-12][Chemical Formula 1-12]
- 청구항 1에 있어서,In claim 1,상기 화학식 1로 표시되는 화합물은 상기 비수 전해질의 중량을 기준으로 0.01중량% 내지 10중량%로 포함되는 비수 전해질.A non-aqueous electrolyte in which the compound represented by the chemical formula 1 is contained in an amount of 0.01 wt% to 10 wt% based on the weight of the non-aqueous electrolyte.
- 청구항 1에 있어서,In claim 1,상기 리튬 염은 LiCl, LiBr, LiI, LiBF4, LiClO4, LiAlO4, LiAlCl4, LiPF6, LiSbF6, LiAsF6, LiB10Cl10, LiBOB(LiB(C2O4)2), LiCF3SO3, LiFSI(LiN(SO2F)2), LiCH3SO3, LiCF3CO2, LiCH3CO2 및 LiBETI(LiN(SO2CF2CF3)2)로 이루어진 군으로부터 선택된 적어도 1종을 포함하는 비수 전해질.A non-aqueous electrolyte comprising at least one lithium salt selected from the group consisting of LiCl, LiBr, LiI, LiBF 4 , LiClO 4 , LiAlO 4 , LiAlCl 4 , LiPF 6 , LiSbF 6 , LiAsF 6 , LiB 10 Cl 10 , LiBOB (LiB(C 2 O 4 ) 2 ), LiCF 3 SO 3 , LiFSI (LiN(SO 2 F) 2 ), LiCH 3 SO 3 , LiCF 3 CO 2 , LiCH 3 CO 2 and LiBETI (LiN(SO 2 CF 2 CF 3 ) 2 ).
- 청구항 1에 있어서,In claim 1,상기 리튬 염은 상기 비수 전해질에 0.5 M 내지 5.0 M의 몰 농도로 포함되는 것인 비수 전해질.A non-aqueous electrolyte wherein the lithium salt is contained in the non-aqueous electrolyte at a molar concentration of 0.5 M to 5.0 M.
- 청구항 1에 있어서,In claim 1,상기 유기 용매는 환형 카보네이트계 유기 용매, 선형 카보네이트계 유기 용매, 선형 에스터계 유기 용매 및 환형 에스터계 유기 용매로 이루어진 군으로부터 선택된 적어도 하나를 포함하는 비수 전해질.A non-aqueous electrolyte wherein the organic solvent comprises at least one selected from the group consisting of a cyclic carbonate-based organic solvent, a linear carbonate-based organic solvent, a linear ester-based organic solvent, and a cyclic ester-based organic solvent.
- 음극;cathode;상기 음극에 대향하는 양극;An anode opposite to the cathode;상기 음극 및 상기 양극 사이에 개재되는 분리막; 및A separator interposed between the cathode and the anode; and청구항 1에 따른 비수 전해질;을 포함하는 리튬 이차전지.A lithium secondary battery comprising a non-aqueous electrolyte according to claim 1.
- 청구항 11에 있어서,In claim 11,상기 음극은 음극 활물질을 포함하며,The above negative electrode contains a negative electrode active material,상기 음극 활물질은 실리콘계 활물질을 포함하는 리튬 이차전지.A lithium secondary battery wherein the negative active material includes a silicon-based active material.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20230052976 | 2023-04-21 | ||
| KR10-2023-0052976 | 2023-04-21 | ||
| KR10-2024-0052747 | 2024-04-19 | ||
| KR1020240052747A KR20240156323A (en) | 2023-04-21 | 2024-04-19 | Non-aqueous electrolyte and lithium secondary battery comprising the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024219896A1 true WO2024219896A1 (en) | 2024-10-24 |
Family
ID=93153078
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2024/005354 WO2024219896A1 (en) | 2023-04-21 | 2024-04-19 | Non-aqueous electrolyte and lithium secondary battery comprising same |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024219896A1 (en) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20150049301A (en) * | 2013-10-30 | 2015-05-08 | 솔브레인 주식회사 | Electrolyte and lithium secondary battery with the same |
| US20150171475A1 (en) * | 2012-06-13 | 2015-06-18 | Central Glass Company, Limited | Electrolyte for Non-Aqueous Electrolyte Battery, and Non-Aqueous Electrolyte Battery Using Same |
| JP6032504B2 (en) * | 2012-07-17 | 2016-11-30 | トヨタ自動車株式会社 | Lithium secondary battery and manufacturing method thereof |
| EP3273519A1 (en) * | 2015-03-17 | 2018-01-24 | Adeka Corporation | Non-aqueous electrolyte, and non-aqueous electrolyte secondary cell |
| US20200203764A1 (en) * | 2018-12-21 | 2020-06-25 | Enevate Corporation | Silicon-based energy storage devices with cyclic organosilicon containing electrolyte additives |
-
2024
- 2024-04-19 WO PCT/KR2024/005354 patent/WO2024219896A1/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150171475A1 (en) * | 2012-06-13 | 2015-06-18 | Central Glass Company, Limited | Electrolyte for Non-Aqueous Electrolyte Battery, and Non-Aqueous Electrolyte Battery Using Same |
| JP6032504B2 (en) * | 2012-07-17 | 2016-11-30 | トヨタ自動車株式会社 | Lithium secondary battery and manufacturing method thereof |
| KR20150049301A (en) * | 2013-10-30 | 2015-05-08 | 솔브레인 주식회사 | Electrolyte and lithium secondary battery with the same |
| EP3273519A1 (en) * | 2015-03-17 | 2018-01-24 | Adeka Corporation | Non-aqueous electrolyte, and non-aqueous electrolyte secondary cell |
| US20200203764A1 (en) * | 2018-12-21 | 2020-06-25 | Enevate Corporation | Silicon-based energy storage devices with cyclic organosilicon containing electrolyte additives |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2018135889A1 (en) | Non-aqueous electrolyte for lithium secondary battery, and lithium secondary battery comprising same | |
| WO2020149678A1 (en) | Non-aqueous electrolyte for lithium secondary battery and lithium secondary battery comprising same | |
| WO2019151725A1 (en) | Lithium secondary battery having improved high-temperature storage characteristics | |
| WO2021033987A1 (en) | Non-aqueous electrolyte solution for lithium secondary battery and lithium secondary battery comprising same | |
| WO2021040388A1 (en) | Non-aqueous electrolytic solution, and lithium secondary battery comprising same | |
| WO2023085843A1 (en) | Non-aqueous electrolyte containing non-aqueous electrolyte additive, and lithium secondary battery including same | |
| WO2020153791A1 (en) | Electrolyte for lithium secondary battery and lithium secondary battery comprising same | |
| WO2020096411A1 (en) | Non-aqueous electrolyte for lithium secondary battery, and lithium secondary battering including same | |
| WO2023075379A1 (en) | Additive for non-aqueous electrolyte, non-aqueous electrolyte comprising same, and lithium secondary battery | |
| WO2023063648A1 (en) | Non-aqueous electrolyte for lithium secondary battery and lithium secondary battery comprising same | |
| WO2023153813A1 (en) | Non-aqueous electrolyte and lithium secondary battery comprising same | |
| WO2023014174A1 (en) | Non-aqueous electrolyte containing additive for non-aqueous electrolyte and lithium secondary battery comprising same | |
| WO2023121028A1 (en) | Non-aqueous electrolyte comprising additive for non-aqueous electrolyte, and lithium secondary battery comprising same | |
| WO2020222469A1 (en) | Non-aqueous electrolyte for lithium secondary battery, and lithium secondary battery comprising same | |
| WO2024219896A1 (en) | Non-aqueous electrolyte and lithium secondary battery comprising same | |
| WO2020190076A1 (en) | Non-aqueous electrolyte for lithium secondary battery and lithium secondary battery comprising same | |
| WO2019009595A1 (en) | Electrolyte additive and nonaqueous electrolyte solution for lithium secondary battery containing same | |
| WO2024117826A1 (en) | Non-aqueous electrolyte and lithium secondary battery comprising same | |
| WO2024071830A1 (en) | Nonaqueous electrolyte and lithium secondary battery comprising same | |
| WO2024035169A1 (en) | Nonaqueous electrolyte and lithium secondary battery comprising same | |
| WO2024039232A1 (en) | Lithium secondary battery | |
| WO2024210609A1 (en) | Nonaqueous electrolyte and secondary lithium battery comprising same | |
| WO2024071832A1 (en) | Nonaqueous electrolyte and lithium secondary battery comprising same | |
| WO2024210729A1 (en) | Nonaqueous electrolyte and lithium secondary battery comprising same | |
| WO2024035167A1 (en) | Non-aqueous electrolyte and lithium secondary battery comprising same |