WO2024190050A1 - Secondary battery electrolyte and secondary battery - Google Patents
Secondary battery electrolyte and secondary battery Download PDFInfo
- Publication number
- WO2024190050A1 WO2024190050A1 PCT/JP2024/000259 JP2024000259W WO2024190050A1 WO 2024190050 A1 WO2024190050 A1 WO 2024190050A1 JP 2024000259 W JP2024000259 W JP 2024000259W WO 2024190050 A1 WO2024190050 A1 WO 2024190050A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- electrolyte
- secondary battery
- lithium
- imide
- Prior art date
Links
- 239000003792 electrolyte Substances 0.000 title claims abstract description 233
- -1 imide anions Chemical class 0.000 claims abstract description 180
- 150000003839 salts Chemical class 0.000 claims abstract description 105
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims abstract description 52
- 239000008151 electrolyte solution Substances 0.000 claims description 69
- 229910001416 lithium ion Inorganic materials 0.000 claims description 37
- 150000001768 cations Chemical class 0.000 claims description 36
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 claims description 35
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 32
- 125000002947 alkylene group Chemical group 0.000 claims description 30
- 229910052744 lithium Inorganic materials 0.000 claims description 30
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims description 29
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 claims description 28
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims description 28
- 125000000217 alkyl group Chemical group 0.000 claims description 25
- 125000001153 fluoro group Chemical group F* 0.000 claims description 23
- 150000005676 cyclic carbonates Chemical class 0.000 claims description 22
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 claims description 17
- VDVLPSWVDYJFRW-UHFFFAOYSA-N lithium;bis(fluorosulfonyl)azanide Chemical compound [Li+].FS(=O)(=O)[N-]S(F)(=O)=O VDVLPSWVDYJFRW-UHFFFAOYSA-N 0.000 claims description 16
- 229910021645 metal ion Inorganic materials 0.000 claims description 12
- 239000012948 isocyanate Substances 0.000 claims description 8
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 claims description 7
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 6
- 229910001496 lithium tetrafluoroborate Inorganic materials 0.000 claims description 6
- IGILRSKEFZLPKG-UHFFFAOYSA-M lithium;difluorophosphinate Chemical compound [Li+].[O-]P(F)(F)=O IGILRSKEFZLPKG-UHFFFAOYSA-M 0.000 claims description 6
- 150000008065 acid anhydrides Chemical class 0.000 claims description 5
- 150000003459 sulfonic acid esters Chemical class 0.000 claims description 5
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 3
- 239000000460 chlorine Substances 0.000 abstract description 4
- 229910052801 chlorine Inorganic materials 0.000 abstract description 3
- 239000010410 layer Substances 0.000 description 57
- 230000014759 maintenance of location Effects 0.000 description 45
- 239000002904 solvent Substances 0.000 description 39
- 238000003860 storage Methods 0.000 description 28
- 150000001875 compounds Chemical class 0.000 description 26
- 230000000694 effects Effects 0.000 description 25
- 239000000203 mixture Substances 0.000 description 22
- 238000000034 method Methods 0.000 description 20
- 230000005012 migration Effects 0.000 description 18
- 238000013508 migration Methods 0.000 description 18
- 239000007774 positive electrode material Substances 0.000 description 18
- 238000007789 sealing Methods 0.000 description 17
- 238000005516 engineering process Methods 0.000 description 16
- 239000007773 negative electrode material Substances 0.000 description 16
- 229920000642 polymer Polymers 0.000 description 16
- 150000001450 anions Chemical class 0.000 description 14
- 239000004020 conductor Substances 0.000 description 13
- 238000000354 decomposition reaction Methods 0.000 description 13
- 229910052751 metal Inorganic materials 0.000 description 12
- 238000001514 detection method Methods 0.000 description 11
- 238000000576 coating method Methods 0.000 description 10
- 239000007769 metal material Substances 0.000 description 10
- 239000002002 slurry Substances 0.000 description 10
- 230000006641 stabilisation Effects 0.000 description 10
- 238000011105 stabilization Methods 0.000 description 10
- 229910052782 aluminium Inorganic materials 0.000 description 9
- 239000011883 electrode binding agent Substances 0.000 description 9
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 8
- 239000011248 coating agent Substances 0.000 description 8
- 238000007599 discharging Methods 0.000 description 8
- 239000011808 electrode reactant Substances 0.000 description 8
- 238000004519 manufacturing process Methods 0.000 description 8
- 239000002184 metal Substances 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 150000002148 esters Chemical group 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 239000003960 organic solvent Substances 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 6
- 239000002033 PVDF binder Substances 0.000 description 6
- 239000000470 constituent Substances 0.000 description 6
- 125000004122 cyclic group Chemical group 0.000 description 6
- 230000004927 fusion Effects 0.000 description 6
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 239000004743 Polypropylene Substances 0.000 description 5
- 239000012790 adhesive layer Substances 0.000 description 5
- 239000003575 carbonaceous material Substances 0.000 description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 5
- 150000002500 ions Chemical class 0.000 description 5
- 239000005001 laminate film Substances 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- 239000002243 precursor Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- ZPFAVCIQZKRBGF-UHFFFAOYSA-N 1,3,2-dioxathiolane 2,2-dioxide Chemical group O=S1(=O)OCCO1 ZPFAVCIQZKRBGF-UHFFFAOYSA-N 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 229910013872 LiPF Inorganic materials 0.000 description 4
- 229910013870 LiPF 6 Inorganic materials 0.000 description 4
- 101150058243 Lipf gene Proteins 0.000 description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 239000003125 aqueous solvent Substances 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 239000006258 conductive agent Substances 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- FSSPGSAQUIYDCN-UHFFFAOYSA-N 1,3-Propane sultone Chemical compound O=S1(=O)CCCO1 FSSPGSAQUIYDCN-UHFFFAOYSA-N 0.000 description 3
- VAYTZRYEBVHVLE-UHFFFAOYSA-N 1,3-dioxol-2-one Chemical compound O=C1OC=CO1 VAYTZRYEBVHVLE-UHFFFAOYSA-N 0.000 description 3
- RYIRMSRYCSMGJA-UHFFFAOYSA-N 1,5,2,4-dioxadithiepane 2,2,4,4-tetraoxide Chemical compound O=S1(=O)CS(=O)(=O)OCCO1 RYIRMSRYCSMGJA-UHFFFAOYSA-N 0.000 description 3
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 3
- DSMUTQTWFHVVGQ-UHFFFAOYSA-N 4,5-difluoro-1,3-dioxolan-2-one Chemical compound FC1OC(=O)OC1F DSMUTQTWFHVVGQ-UHFFFAOYSA-N 0.000 description 3
- BJWMSGRKJIOCNR-UHFFFAOYSA-N 4-ethenyl-1,3-dioxolan-2-one Chemical compound C=CC1COC(=O)O1 BJWMSGRKJIOCNR-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 3
- 239000005057 Hexamethylene diisocyanate Substances 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 229910052783 alkali metal Inorganic materials 0.000 description 3
- 150000001340 alkali metals Chemical class 0.000 description 3
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 3
- 150000001342 alkaline earth metals Chemical class 0.000 description 3
- 150000008064 anhydrides Chemical class 0.000 description 3
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzonitrile Chemical compound N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 239000011888 foil Substances 0.000 description 3
- 229910002804 graphite Inorganic materials 0.000 description 3
- 239000010439 graphite Substances 0.000 description 3
- RRAMGCGOFNQTLD-UHFFFAOYSA-N hexamethylene diisocyanate Chemical compound O=C=NCCCCCCN=C=O RRAMGCGOFNQTLD-UHFFFAOYSA-N 0.000 description 3
- 150000002596 lactones Chemical class 0.000 description 3
- 229910003002 lithium salt Inorganic materials 0.000 description 3
- 159000000002 lithium salts Chemical class 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 229940014800 succinic anhydride Drugs 0.000 description 3
- IAHFWCOBPZCAEA-UHFFFAOYSA-N succinonitrile Chemical compound N#CCCC#N IAHFWCOBPZCAEA-UHFFFAOYSA-N 0.000 description 3
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 3
- 238000004804 winding Methods 0.000 description 3
- AVPYLKIIPLFMHQ-UHFFFAOYSA-N 1,2,6-oxadithiane 2,2,6,6-tetraoxide Chemical compound O=S1(=O)CCCS(=O)(=O)O1 AVPYLKIIPLFMHQ-UHFFFAOYSA-N 0.000 description 2
- SBLRHMKNNHXPHG-UHFFFAOYSA-N 4-fluoro-1,3-dioxolan-2-one Chemical compound FC1COC(=O)O1 SBLRHMKNNHXPHG-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 229910013063 LiBF 4 Inorganic materials 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910001413 alkali metal ion Inorganic materials 0.000 description 2
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 229910021383 artificial graphite Inorganic materials 0.000 description 2
- 238000001636 atomic emission spectroscopy Methods 0.000 description 2
- 229910052790 beryllium Inorganic materials 0.000 description 2
- ATBAMAFKBVZNFJ-UHFFFAOYSA-N beryllium atom Chemical compound [Be] ATBAMAFKBVZNFJ-UHFFFAOYSA-N 0.000 description 2
- 238000009529 body temperature measurement Methods 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 150000005678 chain carbonates Chemical class 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 239000011889 copper foil Substances 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- GAEKPEKOJKCEMS-UHFFFAOYSA-N gamma-valerolactone Chemical compound CC1CCC(=O)O1 GAEKPEKOJKCEMS-UHFFFAOYSA-N 0.000 description 2
- 238000009616 inductively coupled plasma Methods 0.000 description 2
- 229910010272 inorganic material Inorganic materials 0.000 description 2
- 239000011147 inorganic material Substances 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 239000011244 liquid electrolyte Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 229910052814 silicon oxide Inorganic materials 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 150000003871 sulfonates Chemical class 0.000 description 2
- 230000008961 swelling Effects 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 229920003051 synthetic elastomer Polymers 0.000 description 2
- 239000005061 synthetic rubber Substances 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- YFHICDDUDORKJB-UHFFFAOYSA-N trimethylene carbonate Chemical compound O=C1OCCCO1 YFHICDDUDORKJB-UHFFFAOYSA-N 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- KYPOHTVBFVELTG-OWOJBTEDSA-N (e)-but-2-enedinitrile Chemical compound N#C\C=C\C#N KYPOHTVBFVELTG-OWOJBTEDSA-N 0.000 description 1
- OQHXCCQBSGTCGM-UHFFFAOYSA-N 1,2,5-oxadithiolane 2,2,5,5-tetraoxide Chemical compound O=S1(=O)CCS(=O)(=O)O1 OQHXCCQBSGTCGM-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- HNAGHMKIPMKKBB-UHFFFAOYSA-N 1-benzylpyrrolidine-3-carboxamide Chemical compound C1C(C(=O)N)CCN1CC1=CC=CC=C1 HNAGHMKIPMKKBB-UHFFFAOYSA-N 0.000 description 1
- UHOPWFKONJYLCF-UHFFFAOYSA-N 2-(2-sulfanylethyl)isoindole-1,3-dione Chemical compound C1=CC=C2C(=O)N(CCS)C(=O)C2=C1 UHOPWFKONJYLCF-UHFFFAOYSA-N 0.000 description 1
- HBZYYOYCJQHAEL-UHFFFAOYSA-N 2-[3-(dicyanomethylidene)inden-1-ylidene]propanedinitrile Chemical compound C1=CC=C2C(=C(C#N)C#N)CC(=C(C#N)C#N)C2=C1 HBZYYOYCJQHAEL-UHFFFAOYSA-N 0.000 description 1
- BCGCCTGNWPKXJL-UHFFFAOYSA-N 3-(2-cyanoethoxy)propanenitrile Chemical compound N#CCCOCCC#N BCGCCTGNWPKXJL-UHFFFAOYSA-N 0.000 description 1
- AWVNJBFNHGQUQU-UHFFFAOYSA-N 3-butoxypropanenitrile Chemical compound CCCCOCCC#N AWVNJBFNHGQUQU-UHFFFAOYSA-N 0.000 description 1
- VWEYDBUEGDKEHC-UHFFFAOYSA-N 3-methyloxathiolane 2,2-dioxide Chemical compound CC1CCOS1(=O)=O VWEYDBUEGDKEHC-UHFFFAOYSA-N 0.000 description 1
- KLLQVNFCMHPYGL-UHFFFAOYSA-N 5h-oxathiole 2,2-dioxide Chemical compound O=S1(=O)OCC=C1 KLLQVNFCMHPYGL-UHFFFAOYSA-N 0.000 description 1
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229920000178 Acrylic resin Polymers 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- OIFBSDVPJOWBCH-UHFFFAOYSA-N Diethyl carbonate Chemical compound CCOC(=O)OCC OIFBSDVPJOWBCH-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- JGFBQFKZKSSODQ-UHFFFAOYSA-N Isothiocyanatocyclopropane Chemical compound S=C=NC1CC1 JGFBQFKZKSSODQ-UHFFFAOYSA-N 0.000 description 1
- 229910004190 Li1.15(Mn0.65Ni0.22Co0.13)O2 Inorganic materials 0.000 description 1
- 229910008744 Li1.2Mn0.52Co0.175Ni0.1O2 Inorganic materials 0.000 description 1
- 229910013075 LiBF Inorganic materials 0.000 description 1
- 229910012278 LiCo0.98Al0.01Mg0.01O2 Inorganic materials 0.000 description 1
- 229910032387 LiCoO2 Inorganic materials 0.000 description 1
- 229910011857 LiFe0.3Mn0.7PO4 Inorganic materials 0.000 description 1
- 229910011990 LiFe0.5Mn0.5PO4 Inorganic materials 0.000 description 1
- 229910052493 LiFePO4 Inorganic materials 0.000 description 1
- 229910000668 LiMnPO4 Inorganic materials 0.000 description 1
- 229910013716 LiNi Inorganic materials 0.000 description 1
- 229910013825 LiNi0.33Co0.33Mn0.33O2 Inorganic materials 0.000 description 1
- 229910002991 LiNi0.5Co0.2Mn0.3O2 Inorganic materials 0.000 description 1
- 229910002995 LiNi0.8Co0.15Al0.05O2 Inorganic materials 0.000 description 1
- 229910003005 LiNiO2 Inorganic materials 0.000 description 1
- 229910002097 Lithium manganese(III,IV) oxide Inorganic materials 0.000 description 1
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 1
- RJUFJBKOKNCXHH-UHFFFAOYSA-N Methyl propionate Chemical compound CCC(=O)OC RJUFJBKOKNCXHH-UHFFFAOYSA-N 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 229910008479 TiSi2 Inorganic materials 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 239000006230 acetylene black Substances 0.000 description 1
- BTGRAWJCKBQKAO-UHFFFAOYSA-N adiponitrile Chemical compound N#CCCCCC#N BTGRAWJCKBQKAO-UHFFFAOYSA-N 0.000 description 1
- 229910001423 beryllium ion Inorganic materials 0.000 description 1
- DFJQEGUNXWZVAH-UHFFFAOYSA-N bis($l^{2}-silanylidene)titanium Chemical compound [Si]=[Ti]=[Si] DFJQEGUNXWZVAH-UHFFFAOYSA-N 0.000 description 1
- 229910001593 boehmite Inorganic materials 0.000 description 1
- 150000001642 boronic acid derivatives Chemical class 0.000 description 1
- OBNCKNCVKJNDBV-UHFFFAOYSA-N butanoic acid ethyl ester Natural products CCCC(=O)OCC OBNCKNCVKJNDBV-UHFFFAOYSA-N 0.000 description 1
- PWLNAUNEAKQYLH-UHFFFAOYSA-N butyric acid octyl ester Natural products CCCCCCCCOC(=O)CCC PWLNAUNEAKQYLH-UHFFFAOYSA-N 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 239000003990 capacitor Substances 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000000748 compression moulding Methods 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- VMUOSHREZKXCIV-UHFFFAOYSA-N cyclohexane-1,3,5-tricarbonitrile Chemical compound N#CC1CC(C#N)CC(C#N)C1 VMUOSHREZKXCIV-UHFFFAOYSA-N 0.000 description 1
- SVPZJHKVRMRREG-UHFFFAOYSA-N cyclopentanecarbonitrile Chemical compound N#CC1CCCC1 SVPZJHKVRMRREG-UHFFFAOYSA-N 0.000 description 1
- DFJYZCUIKPGCSG-UHFFFAOYSA-N decanedinitrile Chemical compound N#CCCCCCCCCC#N DFJYZCUIKPGCSG-UHFFFAOYSA-N 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- SXWUDUINABFBMK-UHFFFAOYSA-L dilithium;fluoro-dioxido-oxo-$l^{5}-phosphane Chemical compound [Li+].[Li+].[O-]P([O-])(F)=O SXWUDUINABFBMK-UHFFFAOYSA-L 0.000 description 1
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- PYMZYVXDCJXPAM-UHFFFAOYSA-N ethane-1,2-diol;propanenitrile Chemical compound CCC#N.CCC#N.OCCO PYMZYVXDCJXPAM-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- HHEIMYAXCOIQCJ-UHFFFAOYSA-N ethyl 2,2-dimethylpropanoate Chemical compound CCOC(=O)C(C)(C)C HHEIMYAXCOIQCJ-UHFFFAOYSA-N 0.000 description 1
- JBTWLSYIZRCDFO-UHFFFAOYSA-N ethyl methyl carbonate Chemical compound CCOC(=O)OC JBTWLSYIZRCDFO-UHFFFAOYSA-N 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005669 field effect Effects 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229920001973 fluoroelastomer Polymers 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 1
- 239000011245 gel electrolyte Substances 0.000 description 1
- VANNPISTIUFMLH-UHFFFAOYSA-N glutaric anhydride Chemical compound O=C1CCCC(=O)O1 VANNPISTIUFMLH-UHFFFAOYSA-N 0.000 description 1
- ZTOMUSMDRMJOTH-UHFFFAOYSA-N glutaronitrile Chemical compound N#CCCCC#N ZTOMUSMDRMJOTH-UHFFFAOYSA-N 0.000 description 1
- 229910021469 graphitizable carbon Inorganic materials 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- LNLFLMCWDHZINJ-UHFFFAOYSA-N hexane-1,3,6-tricarbonitrile Chemical compound N#CCCCC(C#N)CCC#N LNLFLMCWDHZINJ-UHFFFAOYSA-N 0.000 description 1
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 1
- 239000003273 ketjen black Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 150000002641 lithium Chemical class 0.000 description 1
- 229910003473 lithium bis(trifluoromethanesulfonyl)imide Inorganic materials 0.000 description 1
- QSZMZKBZAYQGRS-UHFFFAOYSA-N lithium;bis(trifluoromethylsulfonyl)azanide Chemical compound [Li+].FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F QSZMZKBZAYQGRS-UHFFFAOYSA-N 0.000 description 1
- QVXQYMZVJNYDNG-UHFFFAOYSA-N lithium;bis(trifluoromethylsulfonyl)methylsulfonyl-trifluoromethane Chemical compound [Li+].FC(F)(F)S(=O)(=O)[C-](S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F QVXQYMZVJNYDNG-UHFFFAOYSA-N 0.000 description 1
- MCVFFRWZNYZUIJ-UHFFFAOYSA-M lithium;trifluoromethanesulfonate Chemical compound [Li+].[O-]S(=O)(=O)C(F)(F)F MCVFFRWZNYZUIJ-UHFFFAOYSA-M 0.000 description 1
- 229910001425 magnesium ion Inorganic materials 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 229940017219 methyl propionate Drugs 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- UUIQMZJEGPQKFD-UHFFFAOYSA-N n-butyric acid methyl ester Natural products CCCC(=O)OC UUIQMZJEGPQKFD-UHFFFAOYSA-N 0.000 description 1
- KTQDYGVEEFGIIL-UHFFFAOYSA-N n-fluorosulfonylsulfamoyl fluoride Chemical compound FS(=O)(=O)NS(F)(=O)=O KTQDYGVEEFGIIL-UHFFFAOYSA-N 0.000 description 1
- 229910021382 natural graphite Inorganic materials 0.000 description 1
- 229910021470 non-graphitizable carbon Inorganic materials 0.000 description 1
- 239000011255 nonaqueous electrolyte Substances 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229920006284 nylon film Polymers 0.000 description 1
- YSIMAPNUZAVQER-UHFFFAOYSA-N octanenitrile Chemical compound CCCCCCCC#N YSIMAPNUZAVQER-UHFFFAOYSA-N 0.000 description 1
- 125000001979 organolithium group Chemical group 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 238000007500 overflow downdraw method Methods 0.000 description 1
- MHYFEEDKONKGEB-UHFFFAOYSA-N oxathiane 2,2-dioxide Chemical compound O=S1(=O)CCCCO1 MHYFEEDKONKGEB-UHFFFAOYSA-N 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 125000005004 perfluoroethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- XQZYPMVTSDWCCE-UHFFFAOYSA-N phthalonitrile Chemical compound N#CC1=CC=CC=C1C#N XQZYPMVTSDWCCE-UHFFFAOYSA-N 0.000 description 1
- 229920006391 phthalonitrile polymer Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229910001414 potassium ion Inorganic materials 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- OWAHJGWVERXJMI-UHFFFAOYSA-N prop-2-ynyl methanesulfonate Chemical compound CS(=O)(=O)OCC#C OWAHJGWVERXJMI-UHFFFAOYSA-N 0.000 description 1
- RAFBXJGDOLMWDJ-UHFFFAOYSA-N propane-1,2,2,3-tetracarbonitrile Chemical compound N#CCC(C#N)(C#N)CC#N RAFBXJGDOLMWDJ-UHFFFAOYSA-N 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 238000005245 sintering Methods 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- PCCVSPMFGIFTHU-UHFFFAOYSA-N tetracyanoquinodimethane Chemical compound N#CC(C#N)=C1C=CC(=C(C#N)C#N)C=C1 PCCVSPMFGIFTHU-UHFFFAOYSA-N 0.000 description 1
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 238000007751 thermal spraying Methods 0.000 description 1
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0568—Liquid materials characterised by the solutes
Definitions
- This technology relates to electrolytes for secondary batteries and secondary batteries.
- secondary batteries are being developed as a power source that is small, lightweight, and has a high energy density.
- These secondary batteries contain a positive electrode, a negative electrode, and an electrolyte (secondary battery electrolyte), and various studies are being conducted on the configuration of these secondary batteries.
- Faiz Ahmed et al. “Novel divalent organo-lithium salts with high electrochemical and thermal stability for aqueous rechargeable Li-Ion batteries”, Electrochimica Acta, 298, 2019, 709-716 Faiz Ahmed et al., “Highly conductive divalent fluorosulfonyl imide based electrolytes improving Li-ion battery performance: Additive potentiating”, Journal of Power Sources, 455, 2020, 227980
- the electrolyte for a secondary battery contains an electrolyte salt and chloride ions.
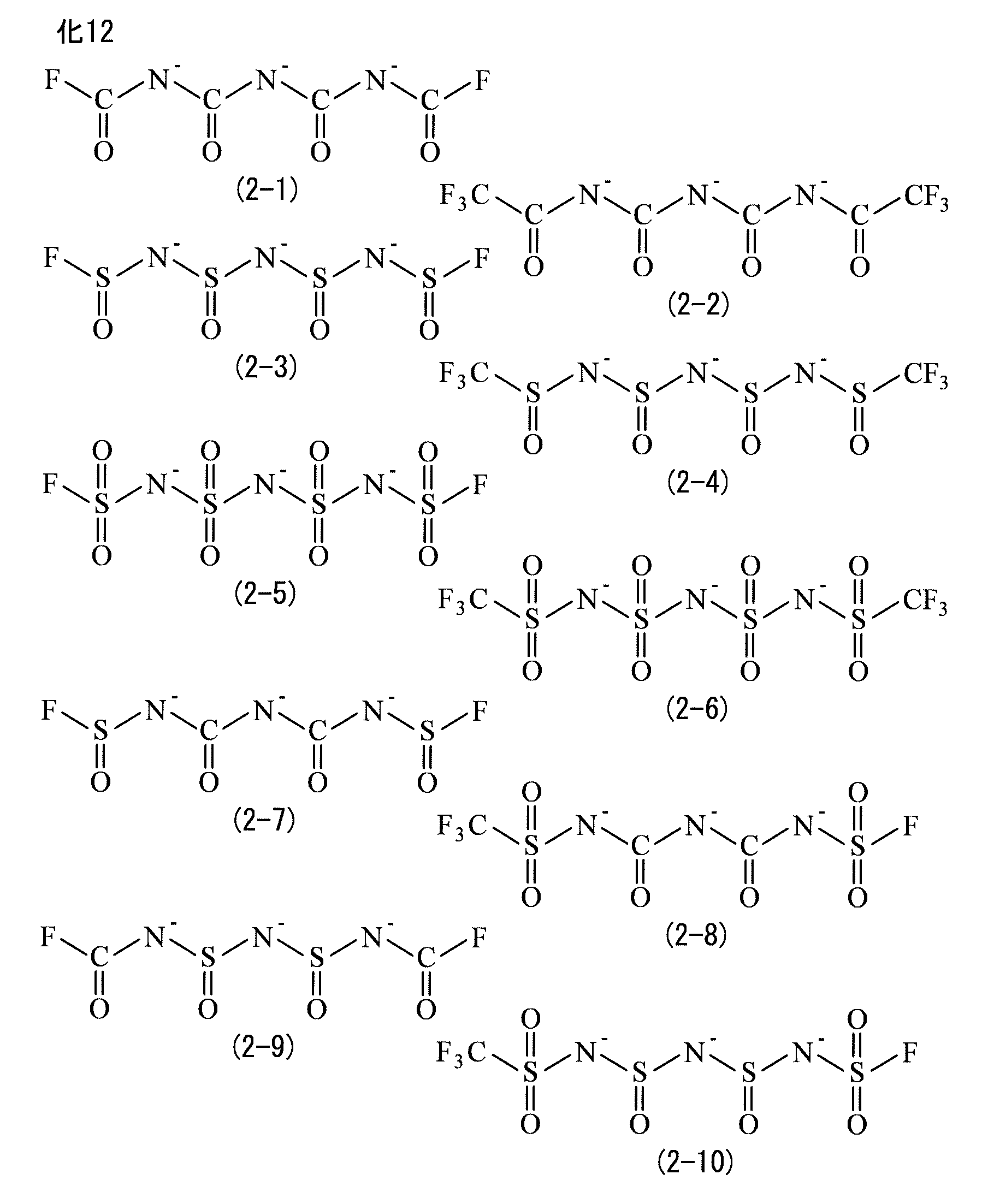
- the electrolyte salt contains imide anions, and the imide anions include at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4).
- the chloride ion content is 5,000 ppm by weight or less.
- Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group.
- Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group.
- Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.
- R5 is a fluorinated alkylene group.
- Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.
- Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group.
- R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group.
- Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- the secondary battery of one embodiment of the present technology includes a positive electrode, a negative electrode, and an electrolyte, and the electrolyte has a configuration similar to that of the electrolyte for a secondary battery of one embodiment of the present technology described above.
- the secondary battery electrolyte contains an electrolyte salt and chloride ions
- the electrolyte salt contains imide anions
- the imide anions contain one or more of the first imide anion, the second imide anion, the third imide anion, and the fourth imide anion
- the chloride ion content is 5,000 ppm by weight or less, so that excellent battery characteristics can be obtained.
- FIG. 1 is a perspective view illustrating a configuration of a secondary battery according to an embodiment of the present technology.
- FIG. 2 is a cross-sectional view showing the configuration of the battery element shown in FIG.
- FIG. 3 is a block diagram showing a configuration of an application example of a secondary battery.
- Electrolyte for secondary batteries First, an electrolyte for a secondary battery (hereinafter simply referred to as an "electrolyte”) according to an embodiment of the present technology will be described.
- the electrolytic solution is a liquid electrolyte used in a secondary battery, which is an electrochemical device.
- the electrolytic solution may be used in other electrochemical devices.
- the type of the other electrochemical device is not particularly limited, but specifically, it is a capacitor or the like.
- the electrolyte contains an electrolyte salt and chloride ions (Cl ⁇ ). More specifically, the electrolyte further contains a solvent that disperses (ionizes) the electrolyte salt.
- the electrolyte salt is a compound that ionizes in a solvent and contains an anion and a cation.
- the anion includes an imide anion.
- the imide anion includes one or more of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4). That is, the electrolyte salt includes one imide anion as an anion.
- the type of the first imide anion may be one type or two or more types. The same applies to each of the second imide anion, the third imide anion, and the fourth imide anion.
- Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group.
- Each of W1, W2, and W3 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group.
- Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.
- R5 is a fluorinated alkylene group.
- Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.
- Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group.
- R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group.
- Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- the anion contains an imide anion is as follows. First, when a secondary battery using an electrolyte is charged and discharged, a good quality coating derived from the electrolyte salt is formed on the surface of each of the positive and negative electrodes, suppressing the decomposition reaction of the electrolyte on the surface of each of the positive and negative electrodes. In this case, the decomposition reaction of the solvent is particularly suppressed. Second, the above-mentioned coating is used to improve the migration speed of cations near the surfaces of each of the positive and negative electrodes. Third, the migration speed of cations is improved even in the electrolyte liquid.
- the first imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms (N) and three functional groups (W1 to W3).
- R1 and R2 are not particularly limited as long as they are either a fluorine group (-F) or a fluorinated alkyl group. Thus, R1 and R2 are not, for example, a hydrogen group (-H) or an alkyl group. R1 and R2 may be the same group or different groups.
- a fluorinated alkyl group is an alkyl group in which one or more hydrogen groups (-H) have been replaced with a fluorine group.
- the fluorinated alkyl group may be linear or branched with one or more side chains.
- the number of carbon atoms in the fluorinated alkyl group is not particularly limited, but is specifically 1 to 10. This is because the solubility and ionization properties of the electrolyte salt containing the first imide anion are improved.
- fluorinated alkyl groups include a perfluoromethyl group (--CF 3 ) and a perfluoroethyl group (--C 2 F 5 ).
- W1 to W3 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- W1 to W3 may be the same group or different groups.
- any two of W1 to W3 may be the same group.
- the second imide anion is a chain anion (trivalent negative ion) containing three nitrogen atoms and four functional groups (X1 to X4).
- R3 and R4 are similar to those for R1 and R2.
- X1 to X4 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- X1 to X4 may be the same group or different groups.
- any two of X1 to X4 may be the same group, or any three of X1 to X4 may be the same group.
- the third imide anion is a cyclic anion (divalent negative ion) containing two nitrogen atoms, three functional groups (Y1 to Y3), and one connecting group (R5).
- the fluorinated alkylene group R5 is an alkylene group in which one or more hydrogen groups have been replaced with fluorine groups.
- the fluorinated alkylene group may be linear or branched with one or more side chains.
- the number of carbon atoms in the fluorinated alkylene group is not particularly limited, but is specifically 1 to 10. This is because it improves the solubility and ionization properties of the electrolyte salt containing the third imide anion.
- fluorinated alkylene groups include a perfluoromethylene group (--CF 2 --) and a perfluoroethylene group (--C 2 F 4 --).
- Y1 to Y3 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- Y1 to Y3 may be the same group or different groups.
- any two of Y1 to Y3 may be the same group.
- the quaternary imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms, four functional groups (Z1 to Z4), and one connecting group (R8).
- R6 and R7 are similar to those for R1 and R2.
- R8 is not particularly limited as long as it is any one of an alkylene group, a phenylene group, a fluorinated alkylene group, and a fluorinated phenylene group.
- the alkylene group may be linear or branched having one or more side chains.
- the number of carbon atoms in the alkylene group is not particularly limited, but specifically, it is 1 to 10. This is because the solubility and ionization property of the electrolyte salt containing the quaternary imide anion are improved.
- Specific examples of the alkylene group include a methylene group ( -CH2- ), an ethylene group (-C2H4- ) , and a propylene group ( -C3H6- ).
- fluorinated alkylene group R8 Details regarding the fluorinated alkylene group R8 are the same as those regarding the fluorinated alkylene group R5.
- a fluorinated phenylene group is a phenylene group in which one or more hydrogen groups have been substituted with fluorine groups.
- a specific example of a fluorinated phenylene group is a monofluorophenylene group (-C 6 H 3 F-).
- Z1 to Z4 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group. In other words, Z1 to Z4 may be the same group or different groups. Of course, any two of Z1 to Z4 may be the same group, or any three of Z1 to Z4 may be the same group.
- Specific examples of the first imide anion include anions represented by each of formulas (1-1) to (1-30).
- second imide anion examples include the anions represented by formulas (2-1) to (2-22).
- third imide anion examples include the anions represented by formulas (3-1) to (3-15).
- fourth imide anion examples include the anions represented by formulas (4-1) to (4-65).
- the type of cation is not particularly limited. Specifically, the cation contains one or more types of light metal ions. That is, the electrolyte salt contains light metal ions as cations. This is because a high voltage can be obtained.
- the type of light metal ion is not particularly limited, but specific examples include alkali metal ions and alkaline earth metal ions. Specific examples of alkali metal ions include lithium ions, sodium ions, and potassium ions. Specific examples of alkaline earth metal ions include beryllium ions, magnesium ions, and calcium ions. In addition, the light metal ion may be an aluminum ion, etc.
- the light metal ions contain lithium ions, because this allows a sufficiently high voltage to be obtained.
- the content of the electrolyte salt in the electrolytic solution is not particularly limited and can be set arbitrarily.
- the content of the electrolyte salt in the electrolytic solution is preferably 0.2 mol/kg to 2 mol/kg. This is because high ionic conductivity can be obtained.
- the "content of the electrolyte salt” described here refers to the content of the electrolyte salt relative to the solvent.
- the electrolyte is analyzed using one or more of the following analytical methods: inductively coupled plasma (ICP) atomic emission spectroscopy, nuclear magnetic resonance spectroscopy (NMR), and gas chromatography-mass spectrometry (GC-MS).
- ICP inductively coupled plasma
- NMR nuclear magnetic resonance spectroscopy
- GC-MS gas chromatography-mass spectrometry
- Chloride ion Chloride ions are included in the electrolyte primarily for reasons that will be explained below.
- chloride ions are generated during the synthesis of electrolyte salts containing imide anions, and the chloride ions are mixed into the electrolyte solution.
- chloride ions derived from thionyl chloride (SOCl 2 ) are generated.
- electrolyte salts containing imide anions originally contain chloride ions, and these chloride ions are mixed into the electrolyte solution.
- chloride ions are generated because electrolyte salts containing imide anions contain chlorine as a constituent element.
- electrolyte salts containing imide anions contain chlorine as a constituent element, so chloride ions are generated, and chloride ions are also generated because they are mixed into the electrolyte.
- chloride ions may be present in the electrolyte for reasons other than those mentioned above.
- the content of chloride ions in the electrolyte is 5,000 ppm by weight or less, more specifically, 0 ppm by weight to 5,000 ppm by weight. This is because the chemical stability of the electrolyte is improved, and the decomposition reaction of the electrolyte is suppressed.
- chloride ions affect the chemical stability of the electrolyte.
- the chloride ion content is more than 5,000 ppm by weight, the chemical stability of the electrolyte decreases, making the electrolyte more susceptible to decomposition when a secondary battery using that electrolyte is charged and discharged.
- the chloride ion content is 5,000 ppm by weight or less, the chemical stability of the electrolyte improves, making the electrolyte less susceptible to decomposition when a secondary battery using that electrolyte is charged and discharged.
- the content of chloride ions in the electrolyte is 100 ppm by weight or less, more specifically 0 ppm by weight to 100 ppm by weight, even more preferably 50 ppm by weight or less, more specifically 0 ppm by weight to 50 ppm by weight, and particularly preferably 30 ppm by weight or less, more specifically 0 ppm by weight to 30 ppm by weight. This is because the chemical stability of the electrolyte is further improved.
- the electrolyte is analyzed using an analytical method such as ion chromatography. This separates the chloride ions and measures their amount.
- the solvent contains one or more of non-aqueous solvents (organic solvents), and the electrolyte solution containing the non-aqueous solvent is a so-called non-aqueous electrolyte solution.
- the non-aqueous solvent is an ester, an ether, or the like, more specifically, a carbonate ester compound, a carboxylate ester compound, a lactone compound, or the like.
- Carbonate compounds include cyclic carbonates and chain carbonates.
- cyclic carbonates are ethylene carbonate and propylene carbonate.
- chain carbonates are dimethyl carbonate, diethyl carbonate, and ethyl methyl carbonate.
- Carboxylic acid ester compounds include chain carboxylates.
- chain carboxylates include methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, propyl propionate, ethyl trimethylacetate, methyl butyrate, and ethyl butyrate.
- Lactone compounds include lactones. Specific examples of lactones include gamma-butyrolactone and gamma-valerolactone.
- the ethers may be 1,2-dimethoxyethane, tetrahydrofuran, 1,3-dioxolane, 1,4-dioxane, etc.
- the electrolyte may further contain one or more of the other electrolyte salts. This is because the cation migration speed is improved in the vicinity of the surfaces of the positive electrode and the negative electrode, and the cation migration speed is also improved in the electrolyte.
- the content of the other electrolyte salt in the electrolyte is not particularly limited and can be set arbitrarily.
- the type of other electrolyte salt is not particularly limited, but specifically, it is a light metal salt such as a lithium salt. However, the above electrolyte salts are excluded from the lithium salts described here.
- lithium salts include lithium hexafluorophosphate (LiPF 6 ), lithium tetrafluoroborate (LiBF 4 ), lithium trifluoromethanesulfonate (LiCF 3 SO 3 ), lithium bis(fluorosulfonyl)imide (LiN(FSO 2 ) 2 ), lithium bis(trifluoromethanesulfonyl)imide (LiN(CF 3 SO 2 ) 2 ), lithium tris(trifluoromethanesulfonyl)methide (LiC(CF 3 SO 2 ) 3 ), lithium bis(oxalato)borate (LiB(C 2 O 4 ) 2 ), lithium difluorooxalatoborate (LiBF 2 (C 2 O 4 )), and lithium difluorodi(oxalato)borate (LiPF 2 (C 2 O 4 ) 2 ) . ), lithium tetrafluorooxalatophosphate (Li
- the other electrolyte salt contains one or more of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate.
- the cation migration speed is sufficiently improved near the surfaces of the positive electrode and negative electrode, and the cation migration speed is also sufficiently improved in the electrolyte solution.
- the electrolyte may further contain one or more of the other solvents.
- a coating derived from the other solvent is formed on the surface of each of the positive and negative electrodes, so that the decomposition reaction of the electrolyte is suppressed.
- the content of the other solvent in the electrolyte is not particularly limited and can be set arbitrarily.
- the types of other solvents are not particularly limited, but specific examples include unsaturated cyclic carbonates, fluorinated cyclic carbonates, sulfonic acid esters, dicarboxylic acid anhydrides, disulfonic acid anhydrides, sulfates, nitrile compounds, and isocyanate compounds.
- the unsaturated cyclic carbonate is a cyclic carbonate containing an unsaturated carbon bond (carbon-carbon double bond).
- the number of unsaturated carbon bonds is not particularly limited, and may be one or more.
- Specific examples of the unsaturated cyclic carbonate include vinylene carbonate, vinylethylene carbonate, and methyleneethylene carbonate.
- a fluorinated cyclic carbonate is a cyclic carbonate containing fluorine as a constituent element. That is, a fluorinated cyclic carbonate is a compound in which one or more hydrogen groups of a cyclic carbonate are substituted with fluorine groups. Specific examples of the fluorinated cyclic carbonate include monofluoroethylene carbonate and difluoroethylene carbonate.
- the sulfonate esters include cyclic monosulfonate esters, cyclic disulfonate esters, chain monosulfonate esters, chain disulfonate esters, etc.
- cyclic monosulfonate esters include 1,3-propane sultone, 1-propene-1,3-sultone, 1,4-butane sultone, 2,4-butane sultone, methanesulfonic acid propargyl ester, etc.
- Specific examples of cyclic disulfonate esters include cyclodisone, etc.
- dicarboxylic acid anhydride Specific examples of dicarboxylic acid anhydrides include succinic anhydride, glutaric anhydride, and maleic anhydride.
- disulfonic anhydride Specific examples of disulfonic anhydrides include ethanedisulfonic anhydride and propanedisulfonic anhydride.
- sulfate ester is ethylene sulfate (1,3,2-dioxathiolane 2,2-dioxide).
- the nitrile compound is a compound having one or more cyano groups (-CN).
- Specific examples of the nitrile compound include octanenitrile, benzonitrile, phthalonitrile, succinonitrile, glutaronitrile, adiponitrile, sebaconitrile, 1,3,6-hexanetricarbonitrile, 3,3'-oxydipropionitrile, 3-butoxypropionitrile, ethylene glycol bispropionitrile ether, 1,2,2,3-tetracyanopropane, tetracyanopropane, fumaronitrile, 7,7,8,8-tetracyanoquinodimethane, cyclopentanecarbonitrile, 1,3,5-cyclohexanetricarbonitrile, and 1,3-bis(dicyanomethylidene)indane.
- the isocyanate compound is a compound having one or more isocyanate groups (-NCO).
- a specific example of the isocyanate compound is hexamethylene diisocyanate.
- an electrolyte salt is added to a solvent.
- another electrolyte salt may be added to the solvent, or an additive may be added to the solvent.
- the electrolyte salt and the like are dispersed or dissolved in the solvent by the above-mentioned process, and thus an electrolyte solution is prepared.
- This electrolyte solution contains chloride ions for the above-mentioned reasons.
- the electrolyte contains an electrolyte salt and chloride ions
- the electrolyte salt contains imide anions
- the content of chloride ions in the electrolyte is 5000 ppm by weight or less.
- the electrolyte salt contains imide anions, as described above, when a secondary battery using an electrolyte is charged and discharged, the decomposition reaction of the electrolyte is suppressed by utilizing the high-quality coating derived from the electrolyte salt, and the migration rate of cations is improved.
- the content of chloride ions in the electrolyte is 5,000 ppm by weight or less, the chemical stability of the electrolyte is improved, as described above. This further suppresses the decomposition reaction of the electrolyte when a secondary battery using the electrolyte is charged and discharged.
- the electrolyte salt contains light metal ions as cations, a higher voltage can be obtained, and therefore a greater effect can be achieved.
- the light metal ions contain lithium ions, a higher voltage can be obtained, and therefore an even greater effect can be achieved.
- the content of electrolyte salt in the electrolyte solution is 0.2 mol/kg to 2 mol/kg, high ionic conductivity can be obtained, resulting in even greater effects.
- the electrolyte further contains one or more of the following electrolyte salts as other electrolyte salts: lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate, the cation migration rate is further improved, and thus a greater effect can be obtained.
- the electrolyte further contains one or more of the following solvents as other solvents: unsaturated cyclic carbonates, fluorinated cyclic carbonates, sulfonic acid esters, dicarboxylic acid anhydrides, disulfonic acid anhydrides, sulfate esters, nitrile compounds, and isocyanate compounds, the decomposition reaction of the electrolyte is suppressed, and thus a greater effect can be obtained.
- the secondary battery described here is a secondary battery that obtains battery capacity by utilizing the absorption and release of electrode reactants, and is equipped with a positive electrode, a negative electrode, and an electrolyte.
- the charge capacity of the negative electrode is preferably greater than the discharge capacity of the positive electrode.
- the electrochemical capacity per unit area of the negative electrode is preferably greater than the electrochemical capacity per unit area of the positive electrode. This is to prevent electrode reactants from depositing on the surface of the negative electrode during charging.
- the type of electrode reactant is not particularly limited, but specifically includes light metals such as alkali metals and alkaline earth metals.
- alkali metals include lithium, sodium, and potassium
- alkaline earth metals include beryllium, magnesium, and calcium.
- the electrode reactant is lithium.
- a secondary battery that obtains battery capacity by utilizing the absorption and release of lithium is known as a lithium-ion secondary battery.
- lithium-ion secondary battery lithium is absorbed and released in an ionic state.
- Fig. 1 shows a perspective view of a secondary battery
- Fig. 2 shows a cross-sectional view of a battery element 20 shown in Fig. 1.
- FIG. 1 the exterior film 10 and the battery element 20 are shown in a state where they are separated from each other, and a cross section of the battery element 20 along the XZ plane is shown by a dashed line. In FIG. 2, only a part of the battery element 20 is shown.
- this secondary battery includes an exterior film 10, a battery element 20, a positive electrode lead 31, a negative electrode lead 32, and sealing films 41 and 42.
- the secondary battery described here uses a flexible or pliable exterior film 10 as an exterior member for housing the battery element 20 inside. Therefore, the secondary battery shown in Figures 1 and 2 is a so-called laminate film type secondary battery.
- the exterior film 10 has a bag-like structure that is sealed with the battery element 20 housed therein. As a result, the exterior film 10 houses a positive electrode 21, a negative electrode 22, and a separator 23, which will be described later.
- the exterior film 10 is a single film-like member that is folded in the folding direction F.
- This exterior film 10 is provided with a recessed portion 10U (a so-called deep drawn portion) for accommodating the battery element 20.
- the exterior film 10 is a three-layer laminate film in which a fusion layer, a metal layer, and a surface protection layer are laminated in this order from the inside, and when the exterior film 10 is folded, the outer peripheral edges of the opposing fusion layers are fused to each other.
- the fusion layer contains a polymer compound such as polypropylene.
- the metal layer contains a metallic material such as aluminum.
- the surface protection layer contains a polymer compound such as nylon.
- the configuration (number of layers) of the exterior film 10 is not particularly limited, so it may be one or two layers, or four or more layers.
- the battery element 20 is housed inside the exterior film 10.
- the battery element 20 is a so-called power generating element, and includes a positive electrode 21, a negative electrode 22, a separator 23, and an electrolyte (not shown), as shown in Figures 1 and 2 .
- the battery element 20 is a so-called wound electrode body, so that the positive electrode 21 and the negative electrode 22 are wound around the winding axis P while facing each other via the separator 23.
- This winding axis P is a virtual axis extending in the Y-axis direction, as shown in FIG. 1.
- the three-dimensional shape of the battery element 20 is not particularly limited.
- the battery element 20 has a flat three-dimensional shape, so that the shape of the cross section (cross section along the XZ plane) of the battery element 20 intersecting the winding axis P is a flat shape defined by the major axis J1 and the minor axis J2.
- the long axis J1 is an imaginary axis extending in the X-axis direction and has a length greater than that of the short axis J2.
- the short axis J2 is an imaginary axis extending in the Z-axis direction intersecting the X-axis direction and has a length less than that of the long axis J1.
- the three-dimensional shape of the battery element 20 is a flattened cylinder, and therefore the cross-sectional shape of the battery element 20 is a flattened, approximately elliptical shape.
- the positive electrode 21 includes a positive electrode current collector 21A and a positive electrode active material layer 21B.
- the positive electrode collector 21A has a pair of surfaces on which the positive electrode active material layer 21B is provided.
- This positive electrode collector 21A contains a conductive material such as a metal material, and a specific example of the conductive material is aluminum.
- the positive electrode active material layer 21B contains one or more types of positive electrode active materials that absorb and release lithium. However, the positive electrode active material layer 21B may further contain one or more types of other materials such as a positive electrode binder and a positive electrode conductor.
- the method of forming the positive electrode active material layer 21B is not particularly limited, but specifically includes a coating method.
- the positive electrode active material layer 21B is provided on both sides of the positive electrode collector 21A.
- the positive electrode active material layer 21B may be provided on only one side of the positive electrode collector 21A on the side where the positive electrode 21 faces the negative electrode 22.
- the type of positive electrode active material is not particularly limited, but specifically includes lithium-containing compounds.
- This lithium-containing compound is a compound that contains one or more transition metal elements as constituent elements along with lithium, and may further contain one or more other elements as constituent elements.
- the type of other element is not particularly limited, so long as it is an element other than lithium and transition metal elements, but specifically includes elements belonging to groups 2 to 15 of the long period periodic table.
- the type of lithium-containing compound is not particularly limited, but specifically includes oxides, phosphate compounds, silicate compounds, and borate compounds.
- oxides include LiNiO2 , LiCoO2 , LiCo0.98Al0.01Mg0.01O2 , LiNi0.5Co0.2Mn0.3O2 , LiNi0.8Co0.15Al0.05O2 , LiNi0.33Co0.33Mn0.33O2 , Li1.2Mn0.52Co0.175Ni0.1O2 , Li1.15 ( Mn0.65Ni0.22Co0.13 ) O2 , and LiMn2O4 .
- phosphate compounds include LiFePO4 , LiMnPO4 , LiFe0.5Mn0.5PO4 , and LiFe0.3Mn0.7PO4 .
- the positive electrode binder contains one or more of the following materials: synthetic rubber and polymeric compounds.
- synthetic rubber include styrene-butadiene rubber, fluororubber, and ethylene-propylene-diene.
- polymeric compounds include polyvinylidene fluoride, polyimide, and carboxymethyl cellulose.
- the positive electrode conductive agent contains one or more conductive materials such as carbon materials, metal materials, and conductive polymer compounds.
- conductive materials such as carbon materials, metal materials, and conductive polymer compounds.
- Specific examples of carbon materials include graphite, carbon black, acetylene black, and ketjen black.
- the negative electrode 22 includes a negative electrode current collector 22A and a negative electrode active material layer 22B.
- the negative electrode current collector 22A has a pair of surfaces on which the negative electrode active material layer 22B is provided.
- This negative electrode current collector 22A contains a conductive material such as a metal material, and a specific example of the conductive material is copper.
- the negative electrode active material layer 22B contains one or more types of negative electrode active materials that absorb and release lithium. However, the negative electrode active material layer 22B may further contain one or more types of other materials such as a negative electrode binder and a negative electrode conductor.
- the method of forming the negative electrode active material layer 22B is not particularly limited, but specifically includes one or more types of a coating method, a gas phase method, a liquid phase method, a thermal spraying method, and a baking method (sintering method).
- the negative electrode active material layer 22B is provided on both sides of the negative electrode collector 22A.
- the negative electrode active material layer 22B may be provided on only one side of the negative electrode collector 22A on the side where the negative electrode 22 faces the positive electrode 21.
- the type of negative electrode active material is not particularly limited, but specific examples include carbon materials and metal-based materials, because they provide high energy density.
- carbon materials include graphitizable carbon, non-graphitizable carbon, and graphite.
- the graphite may be natural graphite or artificial graphite.
- Metallic materials are a general term for materials that contain one or more of metallic elements and semi-metallic elements that can form an alloy with lithium as constituent elements, and specific examples of the metallic elements and semi-metallic elements include silicon and tin.
- the metallic materials may be a single element, an alloy, a compound, a mixture of two or more of these, or a material that contains two or more of these phases.
- Specific examples of metallic materials include TiSi2 and SiOx (0 ⁇ x ⁇ 2 or 0.2 ⁇ x ⁇ 1.4).
- the separator 23 is an insulating porous film interposed between the positive electrode 21 and the negative electrode 22, and allows lithium to pass through in an ion state while preventing the occurrence of a short circuit due to contact between the positive electrode 21 and the negative electrode 22.
- This separator 23 contains a polymer compound such as polyethylene.
- the electrolyte is impregnated into each of the positive electrode 21, the negative electrode 22, and the separator 23, and has the above-mentioned configuration. That is, the electrolyte contains an electrolyte salt and chloride ions, the electrolyte salt contains imide anions, and the content of chloride ions in the electrolyte is within the above-mentioned range.
- the positive electrode lead 31 is a positive electrode wiring connected to the positive electrode current collector 21A of the positive electrode 21, and is led out of the exterior film 10.
- the positive electrode lead 31 contains a conductive material such as a metal material, and a specific example of the conductive material is aluminum.
- the shape of the positive electrode lead 31 is either a thin plate shape or a mesh shape.
- the negative electrode lead 32 is a negative electrode wiring connected to the negative electrode current collector 22A of the negative electrode 22, and is led out of the exterior film 10.
- the lead-out direction of the negative electrode lead 32 is the same as the lead-out direction of the positive electrode lead 31.
- This negative electrode lead 32 contains a conductive material such as a metal material, and a specific example of the conductive material is copper.
- the details of the shape of the negative electrode lead 32 are the same as the details of the shape of the positive electrode lead 31.
- the sealing film 41 is inserted between the exterior film 10 and the positive electrode lead 31, and the sealing film 42 is inserted between the exterior film 10 and the negative electrode lead 32.
- the sealing films 41 and 42 may be omitted.
- the sealing film 41 is a sealing member that prevents outside air and the like from entering the inside of the exterior film 10.
- This sealing film 41 contains a polymer compound such as polyolefin that has adhesion to the positive electrode lead 31, and a specific example of the polymer compound is polypropylene.
- the configuration of the sealing film 42 is the same as that of the sealing film 41, except that the sealing film 42 is a sealing member that has adhesion to the negative electrode lead 32.
- the sealing film 42 contains a polymer compound such as polyolefin that has adhesion to the negative electrode lead 32.
- This secondary battery operates in the battery element 20 as follows.
- lithium When charging, lithium is released from the positive electrode 21 and is absorbed into the negative electrode 22 via the electrolyte.
- discharging lithium is released from the negative electrode 22 and is absorbed into the positive electrode 21 via the electrolyte.
- discharging and charging lithium is absorbed and released in an ionic state.
- the positive electrode 21 and the negative electrode 22 are each produced and an electrolyte solution is prepared according to the procedure described below. Then, the positive electrode 21, the negative electrode 22, and the electrolyte solution are used to manufacture the secondary battery. A secondary battery is assembled and a stabilization process is performed on the secondary battery after assembly.
- a positive electrode active material, a positive electrode binder, and a positive electrode conductive agent are mixed together to prepare a positive electrode mixture. Then, the positive electrode mixture is poured into a solvent to prepare a paste-like positive electrode mixture slurry.
- the solvent may be an aqueous solvent or an organic solvent.
- the positive electrode active material layer 21B is formed by applying the positive electrode mixture slurry to both sides of the positive electrode current collector 21A.
- the positive electrode active material layer 21B is compression molded using a roll press or the like. In this case, the positive electrode active material layer 21B may be heated, or the compression molding of the positive electrode active material layer 21B may be repeated multiple times. In this way, the positive electrode active material layer 21B is formed on both sides of the positive electrode current collector 21A, and the positive electrode 21 is produced.
- the negative electrode 22 is formed by the same procedure as the procedure for producing the positive electrode 21 described above. Specifically, first, a mixture (negative electrode mixture) in which the negative electrode active material, the negative electrode binder, and the negative electrode conductive agent are mixed together is put into a solvent to prepare a paste-like negative electrode mixture slurry. Details regarding the solvent are as described above. Next, the negative electrode mixture slurry is applied to both sides of the negative electrode current collector 22A to form the negative electrode active material layer 22B. Finally, the negative electrode active material layer 22B is compression molded. As a result, the negative electrode active material layer 22B is formed on both sides of the negative electrode current collector 22A, and the negative electrode 22 is produced.
- the positive electrode lead 31 is connected to the positive electrode collector 21A of the positive electrode 21 using a joining method such as welding, and the negative electrode lead 32 is connected to the negative electrode collector 22A of the negative electrode 22 using a joining method such as welding.
- the positive electrode 21 and the negative electrode 22 are stacked on top of each other with the separator 23 interposed therebetween to form a laminate (not shown).
- the laminate is then wound to produce a wound body (not shown), which is then pressed using a press or the like to form the wound body into a flat shape.
- the wound body after this formation has a configuration similar to that of the battery element 20, except that it is not impregnated with the electrolyte.
- the exterior film 10 adheresive layer/metal layer/surface protection layer
- the exterior film 10 is folded so that the exterior films 10 face each other.
- the outer edges of two of the opposing adhesive layers are joined to each other using an adhesive method such as heat fusion, thereby placing the roll inside the bag-shaped exterior film 10.
- the outer peripheral edges of the remaining sides of the opposing fusion layers are joined to each other by using an adhesive method such as a heat fusion method.
- a sealing film 41 is inserted between the exterior film 10 and the positive electrode lead 31, and a sealing film 42 is inserted between the exterior film 10 and the negative electrode lead 32.
- the wound body is impregnated with the electrolyte, producing the battery element 20.
- the battery element 20 is then enclosed inside the bag-shaped exterior film 10, and a secondary battery is assembled.
- Stabilization treatment of secondary battery after assembly The assembled secondary battery is charged and discharged. Stabilization conditions such as the environmental temperature, the number of charge/discharge cycles (number of cycles), and charge/discharge conditions can be set arbitrarily. As a result, a coating is formed on the surface of each of the positive electrode 21 and the negative electrode 22, and the state of the battery element 20 is electrochemically stabilized. Thus, the secondary battery is completed.
- the secondary battery includes an electrolyte, and the electrolyte has the above-mentioned configuration, and therefore, for the above-mentioned reasons, the decomposition reaction of the electrolyte is significantly suppressed while the lithium ion migration speed is guaranteed, and therefore excellent battery characteristics can be obtained.
- the secondary battery is a lithium-ion secondary battery
- sufficient battery capacity can be stably obtained by utilizing the absorption and release of lithium, resulting in even greater effects.
- the electrolyte solution may contain other electrolyte salts in addition to the electrolyte salt containing the imide anion.
- the electrolyte solution contains lithium hexafluorophosphate as another electrolyte salt, and that the content of the electrolyte salt in the electrolyte solution is optimized in relation to the content of the other electrolyte salt in the electrolyte solution.
- the electrolyte salt contains a cation and an imide anion.
- the hexafluorophosphate ion contains a lithium ion and a hexafluorophosphate ion.
- the sum T (mol/kg) of the cation content C1 in the electrolyte and the lithium ion content C2 in the electrolyte is preferably 0.7 mol/kg to 2.2 mol/kg.
- the ratio R (mol%) of the number of moles M2 of hexafluorophosphate ions in the electrolyte to the number of moles M1 of imide anions in the electrolyte is preferably 13 mol% to 6000 mol%. This is because the migration speeds of the cations and lithium ions in the vicinity of the surfaces of the positive electrode 21 and the negative electrode 22 are sufficiently improved, and the migration speeds of the cations and lithium ions in the electrolyte are also sufficiently improved.
- the “content of cations in the electrolyte” described here is the content of electrolyte salt of cations relative to the solvent, and the “content of lithium ions in the electrolyte” is the content of lithium ions relative to the solvent.
- the secondary battery When calculating the sum T and the ratio R, the secondary battery is disassembled to recover the electrolyte, and the electrolyte is then analyzed using ICP atomic emission spectrometry. This allows the contents C1, C2 and the mole numbers M1, M2 to be determined, and the sum T and the ratio R to be calculated.
- the electrolyte solution contains electrolyte salt, and therefore the same effect can be obtained.
- the electrolyte salt is used in combination with another electrolyte salt (lithium hexafluorophosphate)
- the total amount of both (sum T) is optimized, and the mixing ratio (ratio R) of both is also optimized. This further improves the migration speed of cations and lithium ions near the surfaces of the positive electrode 21 and the negative electrode 22, and also further improves the migration speed of cations and lithium ions in the electrolyte solution. Therefore, a greater effect can be obtained.
- the electrolyte solution contains lithium hexafluorophosphate as another electrolyte salt.
- the electrolyte solution may contain lithium bis(fluorosulfonyl)imide as another electrolyte salt instead of lithium hexafluorophosphate. Even in this case, it is preferable that the content of the electrolyte salt in the electrolyte solution is optimized in relation to the content of the other electrolyte salt in the electrolyte solution.
- the bis(fluorosulfonyl)imide lithium contains lithium ions and bis(fluorosulfonyl)imide ions.
- the sum T (mol/kg) of the cation content C1 in the electrolyte and the lithium ion content C2 in the electrolyte is preferably 0.7 mol/kg to 2.2 mol/kg.
- the ratio R (mol%) of the number of moles M3 of bis(fluorosulfonyl)imide ions in the electrolyte to the number of moles M1 of imide anions in the electrolyte is preferably 13 mol% to 6000 mol%. This is because the migration speeds of the cations and lithium ions are sufficiently improved near the surfaces of the positive electrode 21 and the negative electrode 22, and the migration speeds of the cations and lithium ions are also sufficiently improved in the electrolyte.
- the procedure for calculating the ratio R is as described above, except that the number of moles M3 is specified instead of the number of moles M2.
- the electrolyte solution contains an electrolyte salt, and therefore the same effect can be obtained.
- the electrolyte salt is used in combination with another electrolyte salt (lithium bis(fluorosulfonyl)imide)
- the total amount (sum T) of the two is optimized, and the mixing ratio (ratio R) of the two is also optimized. This further improves the migration speeds of the cations and lithium ions near the surfaces of the positive electrode 21 and the negative electrode 22, and also further improves the migration speeds of the cations and lithium ions in the electrolyte solution. Therefore, a greater effect can be obtained.
- a porous membrane separator 23 was used. However, although not specifically shown here, a laminated separator including a polymer compound layer may be used instead of the porous membrane separator 23.
- the laminated separator includes a porous membrane having a pair of surfaces, and a polymer compound layer provided on one or both surfaces of the porous membrane.
- the polymer compound layer includes polyvinylidene fluoride, etc. This is because polyvinylidene fluoride has excellent physical strength and is electrochemically stable.
- one or both of the porous film and the polymer compound layer may contain one or more types of insulating particles. This is because the insulating particles dissipate heat when the secondary battery generates heat, improving the safety (heat resistance) of the secondary battery.
- the insulating particles contain one or more types of insulating materials such as inorganic materials and resin materials. Specific examples of inorganic materials include aluminum oxide, aluminum nitride, boehmite, silicon oxide, titanium oxide, magnesium oxide, and zirconium oxide. Specific examples of resin materials include acrylic resin and styrene resin.
- a precursor solution containing a polymer compound and an organic solvent is prepared, and then the precursor solution is applied to one or both sides of a porous film.
- the precursor solution may contain multiple insulating particles.
- the lithium can move in an ionic state between the positive electrode 21 and the negative electrode 22, so the same effect can be obtained.
- swelling of the secondary battery is particularly suppressed, so a greater effect can be obtained.
- a positive electrode 21 and a negative electrode 22 are wound facing each other with a separator 23 and an electrolyte layer interposed between them.
- the electrolyte layer is interposed between the positive electrode 21 and the separator 23, and also between the negative electrode 22 and the separator 23.
- the electrolyte layer contains a polymer compound together with an electrolyte solution, and the electrolyte solution is held by the polymer compound. This is because leakage of the electrolyte solution is prevented.
- the composition of the electrolyte solution is as described above.
- the polymer compound contains polyvinylidene fluoride and the like.
- the lithium ions can move between the positive electrode 21 and the negative electrode 22 via the electrolyte layer, so the same effect can be obtained.
- leakage of the electrolyte is particularly prevented as described above, so a greater effect can be obtained.
- a secondary battery used as a power source may be a main power source or an auxiliary power source in electronic devices and electric vehicles.
- a main power source is a power source that is used preferentially regardless of the presence or absence of other power sources.
- An auxiliary power source may be a power source used in place of the main power source or a power source that can be switched from the main power source.
- Secondary batteries are as follows: Electronic devices such as video cameras, digital still cameras, mobile phones, notebook computers, headphone stereos, portable radios, and portable information terminals. Storage devices such as backup power sources and memory cards. Power tools such as electric drills and power saws. Battery packs installed in electronic devices. Medical electronic devices such as pacemakers and hearing aids. Electric vehicles such as electric cars (including hybrid cars). Power storage systems such as home or industrial battery systems that store power in preparation for emergencies. In these applications, one secondary battery may be used, or multiple secondary batteries may be used.
- the battery pack may include a single cell or a battery pack.
- the electric vehicle is a vehicle that runs on a secondary battery as a driving power source, and may be a hybrid vehicle that also includes a driving source other than the secondary battery.
- a home power storage system it is possible to use home electrical appliances and the like by utilizing the power stored in the secondary battery, which is a power storage source.
- FIG. 3 shows the block diagram of a battery pack, which is an example of an application of a secondary battery.
- the battery pack described here is a battery pack (a so-called soft pack) that uses one secondary battery, and is installed in electronic devices such as smartphones.
- this battery pack includes a power source 51 and a circuit board 52.
- This circuit board 52 is connected to the power source 51 and includes a positive terminal 53, a negative terminal 54, and a temperature detection terminal 55.
- the power source 51 includes one secondary battery.
- the positive electrode lead is connected to the positive electrode terminal 53
- the negative electrode lead is connected to the negative electrode terminal 54.
- This power source 51 is connected to the outside via the positive electrode terminal 53 and the negative electrode terminal 54, and is therefore capable of charging and discharging.
- the circuit board 52 includes a control unit 56, a switch 57, a PTC element 58 which is a thermosensitive resistor, and a temperature detection unit 59. However, the PTC element 58 may be omitted.
- the control unit 56 includes a central processing unit (CPU) and memory, and controls the operation of the entire battery pack. This control unit 56 detects and controls the usage status of the power source 51.
- CPU central processing unit
- the control unit 56 turns off the switch 57 to prevent charging current from flowing through the current path of the power source 51.
- the overcharge detection voltage is not particularly limited, but is specifically 4.20V ⁇ 0.05V, and the overdischarge detection voltage is not particularly limited, but is specifically 2.40V ⁇ 0.10V.
- Switch 57 includes a charge control switch, a discharge control switch, a charge diode, and a discharge diode, and switches between the presence and absence of a connection between power source 51 and an external device in response to an instruction from control unit 56.
- Switch 57 includes a field effect transistor (MOSFET) that uses a metal oxide semiconductor, and the charge current and discharge current are each detected based on the ON resistance of switch 57.
- MOSFET field effect transistor
- the temperature detection unit 59 includes a temperature detection element such as a thermistor. This temperature detection unit 59 measures the temperature of the power supply 51 using the temperature detection terminal 55, and outputs the temperature measurement result to the control unit 56. The temperature measurement result measured by the temperature detection unit 59 is used when the control unit 56 performs charge/discharge control in the event of abnormal heat generation, and when the control unit 56 performs correction processing when calculating the remaining capacity.
- a positive electrode active material LiNi 0.82 Co 0.14 Al 0.04 O 2 which is a lithium-containing compound (oxide)
- 3 parts by mass of a positive electrode binder polyvinylidene fluoride
- 6 parts by mass of a positive electrode conductive agent carbon black
- the positive electrode mixture was put into a solvent (N-methyl-2-pyrrolidone which is an organic solvent), and the organic solvent was stirred to prepare a paste-like positive electrode mixture slurry.
- the positive electrode mixture slurry was applied to both sides of a positive electrode current collector 21A (a strip-shaped aluminum foil having a thickness of 12 ⁇ m) using a coating device, and then the positive electrode mixture slurry was dried to form a positive electrode active material layer 21B. Finally, the positive electrode active material layer 21B was compression-molded using a roll press machine. As a result, the positive electrode 21 was produced.
- a positive electrode current collector 21A a strip-shaped aluminum foil having a thickness of 12 ⁇ m
- the solvent used was a mixture of ethylene carbonate, a cyclic carbonate ester, and gamma-butyrolactone, a lactone.
- Lithium ions (Li + ) were used as the cations of the electrolyte salt.
- the anions (imide anions) of the electrolyte salt the first imide anions shown in formulas (1-5), (1-6), (1-21) and (1-22), the second imide anion shown in formula (2-5), the third imide anion shown in formula (3-5) and the fourth imide anion shown in formula (4-37) were used.
- the contents (mol/kg) of the electrolyte salts in the electrolytic solution were as shown in Tables 1 and 2.
- This electrolyte contains chloride ions, and the chloride ion content (ppm by weight) in the electrolyte is as shown in Tables 1 and 2.
- Tables 1 and 2 To change the chloride ion content in the electrolyte, the number of recrystallizations during purification of the electrolyte salt containing imide anions was changed.
- an electrolyte solution was prepared in the same manner, except that an electrolyte salt not containing an imide anion (lithium hexafluorophosphate (LiPF 6 )) was used instead of the electrolyte salt containing the imide anion.
- an electrolyte salt not containing an imide anion lithium hexafluorophosphate (LiPF 6 )
- the positive electrode lead 31 (aluminum foil) was welded to the positive electrode current collector 21 A of the positive electrode 21
- the negative electrode lead 32 (copper foil) was welded to the negative electrode current collector 22 A of the negative electrode 22 .
- the positive electrode 21 and the negative electrode 22 were laminated on each other with a separator 23 (a microporous polyethylene film having a thickness of 25 ⁇ m) interposed therebetween to produce a laminate.
- the laminate was then wound to produce a wound body, which was then pressed using a press machine to form the wound body into a flat shape.
- the exterior film 10 (adhesive layer/metal layer/surface protection layer) was folded so as to sandwich the roll housed in the recess 10U, and the outer peripheral edges of two sides of the adhesive layer were heat-sealed to each other to house the roll inside the bag-shaped exterior film 10.
- the exterior film 10 used was an aluminum laminate film in which the adhesive layer (polypropylene film with a thickness of 30 ⁇ m), the metal layer (aluminum foil with a thickness of 40 ⁇ m), and the surface protection layer (nylon film with a thickness of 25 ⁇ m) were laminated in this order from the inside.
- the battery element 20 is enclosed inside the exterior film 10, and a secondary battery is assembled.
- the battery was charged at a constant current of 0.1 C until the voltage reached 4.2 V, and then charged at a constant voltage of 4.2 V until the current reached 0.05 C.
- the battery was discharged at a constant current of 0.1 C until the voltage reached 2.5 V.
- 0.1 C is the current value at which the battery capacity (theoretical capacity) is fully discharged in 10 hours
- 0.05 C is the current value at which the battery capacity is fully discharged in 20 hours.
- the secondary battery was repeatedly charged and discharged until the total number of cycles reached 100, and the discharge capacity (discharge capacity at the 100th cycle) was measured.
- the charge and discharge conditions were the same as those during the stabilization treatment described above.
- cycle retention rate (%) (discharge capacity at 100th cycle/discharge capacity at 1st cycle) x 100.
- the charge and discharge conditions were the same as those during the stabilization treatment described above.
- storage retention rate (%) (discharge capacity after storage/discharge capacity before storage) x 100.
- the charge and discharge conditions were the same as those during the stabilization treatment described above.
- the charge and discharge conditions were the same as those during the stabilization process described above, except that the discharge current was changed to 1C.
- 1C is the current value at which the battery capacity is fully discharged in 1 hour.
- Load retention rate (%) (discharge capacity at 100th cycle/discharge capacity at 1st cycle) x 100.
- the cycle retention rate, storage retention rate, and load retention rate were all sufficiently high, regardless of the type of imide anion.
- the content of chloride ions in the electrolyte was 50 ppm by weight or less, the cycle retention rate, storage retention rate, and load retention rate all increased more.
- the electrolyte salt contained light metal ions (lithium ions) as cations, the cycle retention rate, storage retention rate, and load retention rate all increased sufficiently.
- the content of electrolyte salt in the electrolyte was 0.2 mol/kg to 2 mol/kg, the cycle retention rate, storage retention rate, and load retention rate all increased more.
- Examples 19 to 36 Secondary batteries were produced and their battery characteristics were evaluated in the same manner as in Example 3, except that other solvents or other electrolyte salts were added to the electrolyte solution as shown in Tables 3 and 4. In this case, the other solvents or other electrolyte salts were added to the electrolyte solution, and then the electrolyte solution was stirred.
- LiPF 6 lithium hexafluorophosphate
- LiBF 4 lithium tetrafluoroborate
- LiFSI lithium bis(fluorosulfonyl)imide
- LiBOB lithium bis(oxalato)borate
- LiPF 2 O 2 lithium difluorophosphate
- Examples 37 to 112 As shown in Tables 5 to 10, secondary batteries were prepared in a manner similar to that of Example 3, except that the electrolyte solution contained lithium hexafluorophosphate (LiPF 6 ) or lithium bis(fluorosulfonyl)imide (LiFSI) as another electrolyte salt, and the battery characteristics were then evaluated.
- LiPF 6 lithium hexafluorophosphate
- LiFSI lithium bis(fluorosulfonyl)imide
- the battery structure of the secondary battery has been described as being of a laminate film type.
- the battery structure of the secondary battery is not particularly limited, and may be of a cylindrical type, a square type, a coin type, a button type, etc.
- the battery element has been described as having a wound structure.
- the structure of the battery element is not particularly limited, and may be a stacked type or a zigzag type.
- the positive and negative electrodes are alternately stacked with a separator between them, while in the zigzag type, the positive and negative electrodes are folded in a zigzag pattern while facing each other with the separator between them.
- the electrode reactant is lithium in the above description, the electrode reactant is not particularly limited. Specifically, as described above, the electrode reactant may be other alkali metals such as sodium and potassium, or alkaline earth metals such as beryllium, magnesium, and calcium. In addition, the electrode reactant may be other light metals such as aluminum.
- the present technology can also be configured as follows. ⁇ 1> A positive electrode and A negative electrode; an electrolyte solution containing an electrolyte salt and chloride ions;
- the electrolyte salt contains an imide anion,
- the imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
- the content of the chloride ions in the electrolytic solution is 5000 ppm by weight or less.
- Secondary battery Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group.
- Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group.
- Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.
- R5 is a fluorinated alkylene group.
- Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.)
- Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group.
- R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group.
- Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.
- the secondary battery according to ⁇ 1>. ⁇ 3> The electrolyte contains light metal ions as cations. The secondary battery according to ⁇ 1> or ⁇ 2>. ⁇ 4> The light metal ions include lithium ions. The secondary battery according to ⁇ 3>. ⁇ 5> The content of the electrolyte salt in the electrolytic solution is 0.2 mol/kg or more and 2 mol/kg or less. ⁇ 4> The secondary battery according to any one of ⁇ 1> to ⁇ 4>.
- the electrolyte solution further contains at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate;
- the secondary battery according to ⁇ 5> contains at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate;
- the electrolyte solution further contains lithium hexafluorophosphate or lithium bis(fluorosulfonyl)imide, the electrolyte salt comprises a cation and the imide anion,
- the lithium hexafluorophosphate contains lithium ions and hexafluorophosphate ions
- the lithium bis(fluorosulfonyl)imide contains a lithium ion and a bis(fluorosulfonyl)imide ion, the sum of the content of the cation in the electrolyte solution and the content of the lithium ion in the electrolyte solution is 0.7 mol/kg or more and 2.2 mol/kg or less; a ratio of the number of moles of the hexafluorophosphate ion or the bis(fluorosulfonyl)imide ion in the electrolyte solution to the number of moles of the imide anion in the electrolyte solution is 13 mol% or more and
- the electrolytic solution further contains at least one of an unsaturated cyclic carbonate, a fluorinated cyclic carbonate, a sulfonic acid ester, a dicarboxylic acid anhydride, a disulfonic acid anhydride, a sulfuric acid ester, a nitrile compound, and an isocyanate compound.
- ⁇ 9> It is a lithium-ion secondary battery.
- the electrolyte salt contains an imide anion
- the imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
- the content of the chloride ions is 5000 ppm by weight or less.
- Electrolyte for secondary batteries (Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group.
- Each of W1, W2, and W3 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.)
- Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group.
- Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.
- R5 is a fluorinated alkylene group.
- Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.)
- Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group.
- R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group.
- Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Inorganic Chemistry (AREA)
- Secondary Cells (AREA)
Abstract
Provided is a secondary battery with which it is possible to attain excellent battery characteristics. This secondary battery comprises: a positive electrode; a negative electrode; and an electrolyte including an electrolytic salt and chloride ions. The electrolyte salt contains imide anions. The imide anions include at least one of first imide anions represented by formula (1), second imide anions represented by formula (2), third imide anions represented by formula (3), or fourth imide anions represented by formula (4). The content of chlorine ions in the electrolyte is 5000 ppm by weight or less.
Description
本技術は、二次電池用電解液および二次電池に関する。
This technology relates to electrolytes for secondary batteries and secondary batteries.
携帯電話機などの多様な電子機器が普及しているため、小型かつ軽量であると共に高エネルギー密度が得られる電源として二次電池の開発が進められている。この二次電池は、正極および負極と共に電解液(二次電池用電解液)を備えており、その二次電池の構成に関しては、様々な検討がなされている。
With the widespread use of a wide variety of electronic devices such as mobile phones, secondary batteries are being developed as a power source that is small, lightweight, and has a high energy density. These secondary batteries contain a positive electrode, a negative electrode, and an electrolyte (secondary battery electrolyte), and various studies are being conducted on the configuration of these secondary batteries.
具体的には、電解液がRF
1-S(=O)2 -NH-S(=O)2 -NH-S(=O)2 -RF
2で表されるイミド化合物を含んでいる(例えば、特許文献1参照。)。また、電解液の電解質塩がF-S(=O)2 -N- -C(=O)-N- -S(=O)2 -FまたはF-S(=O)2 -N- -S(=O)2 -C6 H4 -S(=O)2 -N- -S(=O)2 -Fで表されるイミドアニオンを含んでいる(例えば、非特許文献1,2参照。)。
Specifically, the electrolyte contains an imide compound represented by R F 1 -S(=O) 2 -NH-S(=O) 2 -NH-S(=O) 2 -R F 2 (see, for example, Patent Document 1). Also, the electrolyte salt of the electrolyte contains an imide anion represented by F-S(=O) 2 -N - -C(=O)-N - -S(=O) 2 -F or F-S(=O) 2 -N - -S(=O) 2 -C 6 H 4 -S(=O) 2 -N - -S(=O) 2 -F (see, for example, Non-Patent Documents 1 and 2).
二次電池の構成に関する様々な検討がなされているが、その二次電池の電池特性は未だ十分でないため、改善の余地がある。
Various studies have been conducted on the configuration of secondary batteries, but the battery characteristics of these batteries are still insufficient, leaving room for improvement.
優れた電池特性を得ることが可能である二次電池用電解液および二次電池が望まれている。
There is a demand for secondary battery electrolytes and secondary batteries that can provide excellent battery characteristics.
本技術の一実施形態の二次電池用電解液は、電解質塩および塩素イオンを含むものである。電解質塩は、イミドアニオンを含み、そのイミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含む。塩素イオンの含有量は、5000重量ppm以下である。
The electrolyte for a secondary battery according to one embodiment of the present technology contains an electrolyte salt and chloride ions. The electrolyte salt contains imide anions, and the imide anions include at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4). The chloride ion content is 5,000 ppm by weight or less.
本技術の一実施形態の二次電池は、正極と、負極と、電解液とを備え、その電解液が上記した本技術の一実施形態の二次電池用電解液の構成と同様の構成を有するものである。
The secondary battery of one embodiment of the present technology includes a positive electrode, a negative electrode, and an electrolyte, and the electrolyte has a configuration similar to that of the electrolyte for a secondary battery of one embodiment of the present technology described above.
本技術の一実施形態の二次電池用電解液または二次電池によれば、その二次電池用電解液が電解質塩および塩素イオンを含んでおり、その電解質塩がイミドアニオンを含んでおり、そのイミドアニオンが第1イミドアニオン、第2イミドアニオン、第3イミドアニオンおよび第4イミドアニオンのうちのいずれか1種類または2種類以上を含んでおり、その塩素イオンの含有量が5000重量ppm以下であるので、優れた電池特性を得ることができる。
According to one embodiment of the secondary battery electrolyte or secondary battery of the present technology, the secondary battery electrolyte contains an electrolyte salt and chloride ions, the electrolyte salt contains imide anions, and the imide anions contain one or more of the first imide anion, the second imide anion, the third imide anion, and the fourth imide anion, and the chloride ion content is 5,000 ppm by weight or less, so that excellent battery characteristics can be obtained.
なお、本技術の効果は、必ずしもここで説明された効果に限定されるわけではなく、後述する本技術に関連する一連の効果のうちのいずれの効果でもよい。
Note that the effects of this technology are not necessarily limited to the effects described here, but may be any of a series of effects related to this technology described below.
以下、本技術の一実施形態に関して、図面を参照しながら詳細に説明する。なお、説明する順序は、下記の通りである。
1.二次電池用電解液
1-1.構成
1-2.製造方法
1-3.作用および効果
2.二次電池
2-1.構成
2-2.動作
2-3.製造方法
2-4.作用および効果
3.変形例
4.二次電池の用途
Hereinafter, an embodiment of the present technology will be described in detail with reference to the drawings. The description will be given in the following order.
1. Electrolyte for secondary battery 1-1. Configuration 1-2. Manufacturing method 1-3. Action and effect 2. Secondary battery 2-1. Configuration 2-2. Operation 2-3. Manufacturing method 2-4. Action and effect 3. Modification 4. Use of secondary battery
1.二次電池用電解液
1-1.構成
1-2.製造方法
1-3.作用および効果
2.二次電池
2-1.構成
2-2.動作
2-3.製造方法
2-4.作用および効果
3.変形例
4.二次電池の用途
Hereinafter, an embodiment of the present technology will be described in detail with reference to the drawings. The description will be given in the following order.
1. Electrolyte for secondary battery 1-1. Configuration 1-2. Manufacturing method 1-3. Action and effect 2. Secondary battery 2-1. Configuration 2-2. Operation 2-3. Manufacturing method 2-4. Action and effect 3. Modification 4. Use of secondary battery
<1.二次電池用電解液>
まず、本技術の一実施形態の二次電池用電解液(以下、単に「電解液」と呼称する。)に関して説明する。 <1. Electrolyte for secondary batteries>
First, an electrolyte for a secondary battery (hereinafter simply referred to as an "electrolyte") according to an embodiment of the present technology will be described.
まず、本技術の一実施形態の二次電池用電解液(以下、単に「電解液」と呼称する。)に関して説明する。 <1. Electrolyte for secondary batteries>
First, an electrolyte for a secondary battery (hereinafter simply referred to as an "electrolyte") according to an embodiment of the present technology will be described.
<1-1.構成>
電解液は、電気化学デバイスである二次電池に用いられる液状の電解質である。ただし、電解液は、他の電気化学デバイスに用いられてもよい。他の電気化学デバイスの種類は、特に限定されないが、具体的には、キャパシタなどである。 <1-1. Configuration>
The electrolytic solution is a liquid electrolyte used in a secondary battery, which is an electrochemical device. However, the electrolytic solution may be used in other electrochemical devices. The type of the other electrochemical device is not particularly limited, but specifically, it is a capacitor or the like.
電解液は、電気化学デバイスである二次電池に用いられる液状の電解質である。ただし、電解液は、他の電気化学デバイスに用いられてもよい。他の電気化学デバイスの種類は、特に限定されないが、具体的には、キャパシタなどである。 <1-1. Configuration>
The electrolytic solution is a liquid electrolyte used in a secondary battery, which is an electrochemical device. However, the electrolytic solution may be used in other electrochemical devices. The type of the other electrochemical device is not particularly limited, but specifically, it is a capacitor or the like.
この電解液は、電解質塩および塩素イオン(Cl- )を含んでいる。より具体的には、電解液は、さらに、電解質塩を分散(電離)させる溶媒を含んでいる。
The electrolyte contains an electrolyte salt and chloride ions (Cl − ). More specifically, the electrolyte further contains a solvent that disperses (ionizes) the electrolyte salt.
[電解質塩]
電解質塩は、溶媒中において電離する化合物であり、アニオンおよびカチオンを含んでいる。 [Electrolyte salt]
The electrolyte salt is a compound that ionizes in a solvent and contains an anion and a cation.
電解質塩は、溶媒中において電離する化合物であり、アニオンおよびカチオンを含んでいる。 [Electrolyte salt]
The electrolyte salt is a compound that ionizes in a solvent and contains an anion and a cation.
(アニオン)
アニオンは、イミドアニオンを含んでいる。具体的には、イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちのいずれか1種類または2種類以上を含んでいる。すなわち、電解質塩は、アニオンとして1イミドアニオンを含んでいる。 (anion)
The anion includes an imide anion. Specifically, the imide anion includes one or more of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4). That is, the electrolyte salt includes one imide anion as an anion.
アニオンは、イミドアニオンを含んでいる。具体的には、イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちのいずれか1種類または2種類以上を含んでいる。すなわち、電解質塩は、アニオンとして1イミドアニオンを含んでいる。 (anion)
The anion includes an imide anion. Specifically, the imide anion includes one or more of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4). That is, the electrolyte salt includes one imide anion as an anion.
ただし、第1イミドアニオンの種類は、1種類だけでもよいし、2種類以上でもよい。このように種類が1種類でも2種類以上でもよいことは、第2イミドアニオン、第3イミドアニオンおよび第4イミドアニオンのそれぞれに関しても同様である。
However, the type of the first imide anion may be one type or two or more types. The same applies to each of the second imide anion, the third imide anion, and the fourth imide anion.
アニオンがイミドアニオンを含んでいる理由は、以下で説明する通りである。第1に、電解液を用いた二次電池の充放電時において、電解質塩に由来する良質な被膜が正極および負極のそれぞれの表面に形成されるため、その正極および負極のそれぞれの表面における電解液の分解反応が抑制される。この場合には、特に、溶媒の分解反応が抑制される。第2に、上記した被膜を利用して、正極および負極のそれぞれの表面近傍においてカチオンの移動速度が向上する。第3に、電解液の液中においてもカチオンの移動速度が向上する。
The reason why the anion contains an imide anion is as follows. First, when a secondary battery using an electrolyte is charged and discharged, a good quality coating derived from the electrolyte salt is formed on the surface of each of the positive and negative electrodes, suppressing the decomposition reaction of the electrolyte on the surface of each of the positive and negative electrodes. In this case, the decomposition reaction of the solvent is particularly suppressed. Second, the above-mentioned coating is used to improve the migration speed of cations near the surfaces of each of the positive and negative electrodes. Third, the migration speed of cations is improved even in the electrolyte liquid.
(第1イミドアニオン)
第1イミドアニオンは、式(1)に示したように、2個の窒素原子(N)および3個の官能基(W1~W3)を含む鎖状のアニオン(2価のマイナスイオン)である。 (First imide anion)
As shown in formula (1), the first imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms (N) and three functional groups (W1 to W3).
第1イミドアニオンは、式(1)に示したように、2個の窒素原子(N)および3個の官能基(W1~W3)を含む鎖状のアニオン(2価のマイナスイオン)である。 (First imide anion)
As shown in formula (1), the first imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms (N) and three functional groups (W1 to W3).
R1およびR2のそれぞれは、フッ素基(-F)およびフッ素化アルキル基のうちのいずれかであれば、特に限定されない。これにより、R1およびR2のそれぞれは、水素基(-H)およびアルキル基などではない。なお、R1およびR2のそれぞれは、互いに同じ基でもよいし、互いに異なる基でもよい。
R1 and R2 are not particularly limited as long as they are either a fluorine group (-F) or a fluorinated alkyl group. Thus, R1 and R2 are not, for example, a hydrogen group (-H) or an alkyl group. R1 and R2 may be the same group or different groups.
フッ素化アルキル基は、アルキル基のうちの1個または2個以上の水素基(-H)がフッ素基により置換された基である。ただし、フッ素化アルキル基は、直鎖状でもよいし、1本または2本以上の側鎖を有する分岐状でもよい。
A fluorinated alkyl group is an alkyl group in which one or more hydrogen groups (-H) have been replaced with a fluorine group. However, the fluorinated alkyl group may be linear or branched with one or more side chains.
フッ素化アルキル基の炭素数は、特に限定されないが、具体的には、1~10である。第1イミドアニオンを含む電解質塩の溶解性および電離性が向上するからである。
The number of carbon atoms in the fluorinated alkyl group is not particularly limited, but is specifically 1 to 10. This is because the solubility and ionization properties of the electrolyte salt containing the first imide anion are improved.
フッ素化アルキル基の具体例は、パーフルオロメチル基(-CF3 )およびパーフルオロエチル基(-C2 F5 )などである。
Specific examples of fluorinated alkyl groups include a perfluoromethyl group (--CF 3 ) and a perfluoroethyl group (--C 2 F 5 ).
W1~W3のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかであれば、特に限定されない。すなわち、W1~W3のそれぞれは、互いに同じ基でもよいし、互いに異なる基でもよい。もちろん、W1~W3のうちの任意の2つだけが互いに同じ基でもよい。
W1 to W3 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group. In other words, W1 to W3 may be the same group or different groups. Of course, any two of W1 to W3 may be the same group.
(第2イミドアニオン)
第2イミドアニオンは、式(2)に示したように、3個の窒素原子および4個の官能基(X1~X4)を含む鎖状のアニオン(3価のマイナスイオン)である。 (Second Imide Anion)
As shown in formula (2), the second imide anion is a chain anion (trivalent negative ion) containing three nitrogen atoms and four functional groups (X1 to X4).
第2イミドアニオンは、式(2)に示したように、3個の窒素原子および4個の官能基(X1~X4)を含む鎖状のアニオン(3価のマイナスイオン)である。 (Second Imide Anion)
As shown in formula (2), the second imide anion is a chain anion (trivalent negative ion) containing three nitrogen atoms and four functional groups (X1 to X4).
R3およびR4のそれぞれに関する詳細は、R1およびR2のそれぞれに関する詳細と同様である。
The details for R3 and R4 are similar to those for R1 and R2.
X1~X4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかであれば、特に限定されない。すなわち、X1~X4のそれぞれは、互いに同じ基でもよいし、互いに異なる基でもよい。もちろん、X1~X4のうちの任意の2つだけが互いに同じ基でもよいし、X1~X4のうちの任意の3つだけが互いに同じ基でもよい。
X1 to X4 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group. In other words, X1 to X4 may be the same group or different groups. Of course, any two of X1 to X4 may be the same group, or any three of X1 to X4 may be the same group.
(第3イミドアニオン)
第3イミドアニオンは、式(3)に示したように、2個の窒素原子、3個の官能基(Y1~Y3)および1個の接続基(R5)を含む環状のアニオン(2価のマイナスイオン)である。 (Tertiary imide anion)
As shown in formula (3), the third imide anion is a cyclic anion (divalent negative ion) containing two nitrogen atoms, three functional groups (Y1 to Y3), and one connecting group (R5).
第3イミドアニオンは、式(3)に示したように、2個の窒素原子、3個の官能基(Y1~Y3)および1個の接続基(R5)を含む環状のアニオン(2価のマイナスイオン)である。 (Tertiary imide anion)
As shown in formula (3), the third imide anion is a cyclic anion (divalent negative ion) containing two nitrogen atoms, three functional groups (Y1 to Y3), and one connecting group (R5).
R5であるフッ素化アルキレン基は、アルキレン基のうちの1個または2個以上の水素基がフッ素基により置換された基である。ただし、フッ素化アルキレン基は、直鎖状でもよいし、1本または2本以上の側鎖を有する分岐状でもよい。
The fluorinated alkylene group R5 is an alkylene group in which one or more hydrogen groups have been replaced with fluorine groups. However, the fluorinated alkylene group may be linear or branched with one or more side chains.
フッ素化アルキレン基の炭素数は、特に限定されないが、具体的には、1~10である。第3イミドアニオンを含む電解質塩の溶解性および電離性が向上するからである。
The number of carbon atoms in the fluorinated alkylene group is not particularly limited, but is specifically 1 to 10. This is because it improves the solubility and ionization properties of the electrolyte salt containing the third imide anion.
フッ素化アルキレン基の具体例は、パーフルオロメチレン基(-CF2 -)およびパーフルオロエチレン基(-C2 F4 -)などである。
Specific examples of fluorinated alkylene groups include a perfluoromethylene group (--CF 2 --) and a perfluoroethylene group (--C 2 F 4 --).
Y1~Y3のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかであれば、特に限定されない。すなわち、Y1~Y3のそれぞれは、互いに同じ基でもよいし、互いに異なる基でもよい。もちろん、Y1~Y3のうちの任意の2つだけが互いに同じ基でもよい。
Y1 to Y3 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group. In other words, Y1 to Y3 may be the same group or different groups. Of course, any two of Y1 to Y3 may be the same group.
(第4イミドアニオン)
第4イミドアニオンは、式(4)に示したように、2個の窒素原子、4個の官能基(Z1~Z4)および1個の接続基(R8)を含む鎖状のアニオン(2価のマイナスイオン)である。 (Quaternary imide anion)
As shown in formula (4), the quaternary imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms, four functional groups (Z1 to Z4), and one connecting group (R8).
第4イミドアニオンは、式(4)に示したように、2個の窒素原子、4個の官能基(Z1~Z4)および1個の接続基(R8)を含む鎖状のアニオン(2価のマイナスイオン)である。 (Quaternary imide anion)
As shown in formula (4), the quaternary imide anion is a chain anion (divalent negative ion) containing two nitrogen atoms, four functional groups (Z1 to Z4), and one connecting group (R8).
R6およびR7のそれぞれに関する詳細は、R1およびR2のそれぞれに関する詳細と同様である。
The details for R6 and R7 are similar to those for R1 and R2.
R8は、アルキレン基、フェニレン基、フッ素化アルキレン基およびフッ素化フェニレン基のうちのいずれかであれば、特に限定されない。
R8 is not particularly limited as long as it is any one of an alkylene group, a phenylene group, a fluorinated alkylene group, and a fluorinated phenylene group.
アルキレン基は、直鎖状でもよいし、1本または2本以上の側鎖を有する分岐状でもよい。アルキレン基の炭素数は、特に限定されないが、具体的には、1~10である。第4イミドアニオンを含む電解質塩の溶解性および電離性が向上するからである。アルキレン基の具体例は、メチレン基(-CH2 -)、エチレン基(-C2 H4 -)およびプロピレン基(-C3 H6 -)などである。
The alkylene group may be linear or branched having one or more side chains. The number of carbon atoms in the alkylene group is not particularly limited, but specifically, it is 1 to 10. This is because the solubility and ionization property of the electrolyte salt containing the quaternary imide anion are improved. Specific examples of the alkylene group include a methylene group ( -CH2- ), an ethylene group (-C2H4- ) , and a propylene group ( -C3H6- ).
R8であるフッ素化アルキレン基に関する詳細は、R5であるフッ素化アルキレン基に関する詳細と同様である。
Details regarding the fluorinated alkylene group R8 are the same as those regarding the fluorinated alkylene group R5.
フッ素化フェニレン基は、フェニレン基のうちの1個または2個以上の水素基がフッ素基により置換された基である。フッ素化フェニレン基の具体例は、モノフルオロフェニレン基(-C6 H3 F-)などである。
A fluorinated phenylene group is a phenylene group in which one or more hydrogen groups have been substituted with fluorine groups. A specific example of a fluorinated phenylene group is a monofluorophenylene group (-C 6 H 3 F-).
Z1~Z4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかであれば、特に限定されない。すなわち、Z1~Z4のそれぞれは、互いに同じ基でもよいし、互いに異なる基でもよい。もちろん、Z1~Z4のうちの任意の2つだけが互いに同じ基でもよいし、Z1~Z4のうちの任意の3つだけが互いに同じ基でもよい。
Z1 to Z4 are not particularly limited as long as they are either a carbonyl group, a sulfinyl group, or a sulfonyl group. In other words, Z1 to Z4 may be the same group or different groups. Of course, any two of Z1 to Z4 may be the same group, or any three of Z1 to Z4 may be the same group.
(アニオンの具体例)
第1イミドアニオンの具体例は、式(1-1)~式(1-30)のそれぞれで表されるアニオンなどである。 (Specific examples of anions)
Specific examples of the first imide anion include anions represented by each of formulas (1-1) to (1-30).
第1イミドアニオンの具体例は、式(1-1)~式(1-30)のそれぞれで表されるアニオンなどである。 (Specific examples of anions)
Specific examples of the first imide anion include anions represented by each of formulas (1-1) to (1-30).
第2イミドアニオンの具体例は、式(2-1)~式(2-22)のそれぞれで表されるアニオンなどである。
Specific examples of the second imide anion include the anions represented by formulas (2-1) to (2-22).
第3イミドアニオンの具体例は、式(3-1)~式(3-15)のそれぞれで表されるアニオンなどである。
Specific examples of the third imide anion include the anions represented by formulas (3-1) to (3-15).
第4イミドアニオンの具体例は、式(4-1)~式(4-65)のそれぞれで表されるアニオンなどである。
Specific examples of the fourth imide anion include the anions represented by formulas (4-1) to (4-65).
(カチオン)
カチオンの種類は、特に限定されない。具体的には、カチオンは、軽金属イオンのうちのいずれか1種類または2種類以上を含んでいる。すなわち、電解質塩は、カチオンとして軽金属イオンを含んでいる。高い電圧が得られるからである。 (Cation)
The type of cation is not particularly limited. Specifically, the cation contains one or more types of light metal ions. That is, the electrolyte salt contains light metal ions as cations. This is because a high voltage can be obtained.
カチオンの種類は、特に限定されない。具体的には、カチオンは、軽金属イオンのうちのいずれか1種類または2種類以上を含んでいる。すなわち、電解質塩は、カチオンとして軽金属イオンを含んでいる。高い電圧が得られるからである。 (Cation)
The type of cation is not particularly limited. Specifically, the cation contains one or more types of light metal ions. That is, the electrolyte salt contains light metal ions as cations. This is because a high voltage can be obtained.
軽金属イオンの種類は、特に限定されないが、具体的には、アルカリ金属イオンおよびアルカリ土類金属イオンなどである。アルカリ金属イオンの具体例は、リチウムイオン、ナトリウムイオンおよびカリウムイオンなどである。アルカリ土類金属イオンの具体例は、ベリリウムイオン、マグネシウムイオンおよびカルシウムイオンなどである。この他、軽金属イオンは、アルミニウムイオンなどでもよい。
The type of light metal ion is not particularly limited, but specific examples include alkali metal ions and alkaline earth metal ions. Specific examples of alkali metal ions include lithium ions, sodium ions, and potassium ions. Specific examples of alkaline earth metal ions include beryllium ions, magnesium ions, and calcium ions. In addition, the light metal ion may be an aluminum ion, etc.
中でも、軽金属イオンは、リチウムイオンを含んでいることが好ましい。十分に高い電圧が得られるからである。
Among them, it is preferable that the light metal ions contain lithium ions, because this allows a sufficiently high voltage to be obtained.
(含有量)
電解液における電解質塩の含有量は、特に限定されないため、任意に設定可能である。中でも、電解液における電解質塩の含有量は、0.2mol/kg~2mol/kgであることが好ましい。高いイオン伝導性が得られるからである。ここで説明する「電解質塩の含有量」とは、溶媒に対する電解質塩の含有量である。 (Content)
The content of the electrolyte salt in the electrolytic solution is not particularly limited and can be set arbitrarily. In particular, the content of the electrolyte salt in the electrolytic solution is preferably 0.2 mol/kg to 2 mol/kg. This is because high ionic conductivity can be obtained. The "content of the electrolyte salt" described here refers to the content of the electrolyte salt relative to the solvent.
電解液における電解質塩の含有量は、特に限定されないため、任意に設定可能である。中でも、電解液における電解質塩の含有量は、0.2mol/kg~2mol/kgであることが好ましい。高いイオン伝導性が得られるからである。ここで説明する「電解質塩の含有量」とは、溶媒に対する電解質塩の含有量である。 (Content)
The content of the electrolyte salt in the electrolytic solution is not particularly limited and can be set arbitrarily. In particular, the content of the electrolyte salt in the electrolytic solution is preferably 0.2 mol/kg to 2 mol/kg. This is because high ionic conductivity can be obtained. The "content of the electrolyte salt" described here refers to the content of the electrolyte salt relative to the solvent.
電解液における電解質塩の含有量を測定する場合には、高周波誘導結合プラズマ(ICP)発光分光分析法、核磁気共鳴分光法(NMR)およびガスクロマトグラフ質量分析法(GC-MS)などの分析方法のうちのいずれか1種類または2種類以上を用いて電解液を分析する。これにより、溶媒の重量および電解質塩の重量のそれぞれが特定されるため、その電解質塩の含有量が算出される。
When measuring the content of electrolyte salt in an electrolyte, the electrolyte is analyzed using one or more of the following analytical methods: inductively coupled plasma (ICP) atomic emission spectroscopy, nuclear magnetic resonance spectroscopy (NMR), and gas chromatography-mass spectrometry (GC-MS). This allows the weight of the solvent and the weight of the electrolyte salt to be determined, and the content of the electrolyte salt can be calculated.
[塩素イオン]
塩素イオンは、主に、以下で説明する理由により、電解液に含まれている。 [Chloride ion]
Chloride ions are included in the electrolyte primarily for reasons that will be explained below.
塩素イオンは、主に、以下で説明する理由により、電解液に含まれている。 [Chloride ion]
Chloride ions are included in the electrolyte primarily for reasons that will be explained below.
第1に、イミドアニオンを含んでいる電解質塩などの合成過程において塩素イオンが発生するため、その塩素イオンが電解液に混入する。一例を挙げると、イミドアニオンを含んでいる電解質塩の合成過程において脱水縮合反応が進行した場合には、塩化チオニル(SOCl2 )などに由来する塩素イオンが発生する。
First, chloride ions are generated during the synthesis of electrolyte salts containing imide anions, and the chloride ions are mixed into the electrolyte solution. For example, when a dehydration condensation reaction occurs during the synthesis of electrolyte salts containing imide anions, chloride ions derived from thionyl chloride (SOCl 2 ) are generated.
第2に、イミドアニオンを含んでいる電解質塩などに塩素イオンが元々含まれているため、その塩素イオンが電解液に混入する。この場合には、イミドアニオンを含んでいる電解質塩などが塩素を構成元素として含んでいるため、塩素イオンが発生する。
Secondly, electrolyte salts containing imide anions originally contain chloride ions, and these chloride ions are mixed into the electrolyte solution. In this case, chloride ions are generated because electrolyte salts containing imide anions contain chlorine as a constituent element.
第3に、電解液を用いた二次電池の充放電時において、その電解液の分解反応などに起因して塩素イオンが発生するため、その塩素イオンが電解液に混入する。この場合には、上記したように、イミドアニオンを含んでいる電解質塩などが塩素を構成元素として含んでいるため、塩素イオンが発生すると共に、電解液に塩素イオンが混入しているため、その塩素イオンが発生する。
Thirdly, when a secondary battery using an electrolyte is charged and discharged, chloride ions are generated due to the decomposition reaction of the electrolyte, etc., and these chloride ions are mixed into the electrolyte. In this case, as described above, electrolyte salts containing imide anions contain chlorine as a constituent element, so chloride ions are generated, and chloride ions are also generated because they are mixed into the electrolyte.
ただし、塩素イオンは、上記以外の理由により、電解液に含まれていてもよい。
However, chloride ions may be present in the electrolyte for reasons other than those mentioned above.
ここで、電解液における塩素イオンの含有量は、5000重量ppm以下であり、より具体的には、0重量ppm~5000重量ppmである。電解液の化学的安定性が向上するため、その電解液の分解反応が抑制されるからである。
Here, the content of chloride ions in the electrolyte is 5,000 ppm by weight or less, more specifically, 0 ppm by weight to 5,000 ppm by weight. This is because the chemical stability of the electrolyte is improved, and the decomposition reaction of the electrolyte is suppressed.
詳細には、塩素イオンは、電解液の化学的安定性に影響を及ぼす。この場合には、塩素イオンの含有量が5000重量ppmよりも多いと、電解液の化学的安定性が低下するため、その電解液を用いた二次電池の充放電時において電解液が分解されやすくなる。これに対して、塩素イオンの含有量が5000重量ppm以下であると、電解液の化学的安定性が向上するため、その電解液を用いた二次電池の充放電時において電解液が分解されにくくなる。
In more detail, chloride ions affect the chemical stability of the electrolyte. In this case, if the chloride ion content is more than 5,000 ppm by weight, the chemical stability of the electrolyte decreases, making the electrolyte more susceptible to decomposition when a secondary battery using that electrolyte is charged and discharged. In contrast, if the chloride ion content is 5,000 ppm by weight or less, the chemical stability of the electrolyte improves, making the electrolyte less susceptible to decomposition when a secondary battery using that electrolyte is charged and discharged.
中でも、電解液における塩素イオンの含有量は、100重量ppm以下、より具体的には0重量ppm~100重量ppmであることがより好ましく、50重量ppm以下、より具体的には0重量ppm~50重量ppmであることがさらに好ましく、30重量ppm以下、より具体的には0重量ppm~30重量ppmであることが特に好ましい。電解液の化学的安定性がより向上するからである。
Among these, it is more preferable that the content of chloride ions in the electrolyte is 100 ppm by weight or less, more specifically 0 ppm by weight to 100 ppm by weight, even more preferably 50 ppm by weight or less, more specifically 0 ppm by weight to 50 ppm by weight, and particularly preferably 30 ppm by weight or less, more specifically 0 ppm by weight to 30 ppm by weight. This is because the chemical stability of the electrolyte is further improved.
電解液における塩素イオンの含有量を測定する場合には、イオンクロマトグラフィ法などの分析方法を用いて電解液を分析する。これにより、塩素イオンが分離されると共に、その塩素イオンの含有量が測定される。
When measuring the amount of chloride ions in an electrolyte, the electrolyte is analyzed using an analytical method such as ion chromatography. This separates the chloride ions and measures their amount.
[溶媒]
溶媒は、非水溶媒(有機溶剤)のうちのいずれか1種類または2種類以上を含んでおり、その非水溶媒を含んでいる電解液は、いわゆる非水電解液である。非水溶媒は、エステル類およびエーテル類などであり、より具体的には、炭酸エステル系化合物、カルボン酸エステル系化合物およびラクトン系化合物などである。 [solvent]
The solvent contains one or more of non-aqueous solvents (organic solvents), and the electrolyte solution containing the non-aqueous solvent is a so-called non-aqueous electrolyte solution. The non-aqueous solvent is an ester, an ether, or the like, more specifically, a carbonate ester compound, a carboxylate ester compound, a lactone compound, or the like.
溶媒は、非水溶媒(有機溶剤)のうちのいずれか1種類または2種類以上を含んでおり、その非水溶媒を含んでいる電解液は、いわゆる非水電解液である。非水溶媒は、エステル類およびエーテル類などであり、より具体的には、炭酸エステル系化合物、カルボン酸エステル系化合物およびラクトン系化合物などである。 [solvent]
The solvent contains one or more of non-aqueous solvents (organic solvents), and the electrolyte solution containing the non-aqueous solvent is a so-called non-aqueous electrolyte solution. The non-aqueous solvent is an ester, an ether, or the like, more specifically, a carbonate ester compound, a carboxylate ester compound, a lactone compound, or the like.
炭酸エステル系化合物は、環状炭酸エステルおよび鎖状炭酸エステルなどである。環状炭酸エステルの具体例は、炭酸エチレンおよび炭酸プロピレンなどである。鎖状炭酸エステルの具体例は、炭酸ジメチル、炭酸ジエチルおよび炭酸エチルメチルなどである。
Carbonate compounds include cyclic carbonates and chain carbonates. Examples of cyclic carbonates are ethylene carbonate and propylene carbonate. Examples of chain carbonates are dimethyl carbonate, diethyl carbonate, and ethyl methyl carbonate.
カルボン酸エステル系化合物は、鎖状カルボン酸エステルなどである。鎖状カルボン酸エステルの具体例は、酢酸メチル、酢酸エチル、プロピオン酸メチル、プロピオン酸エチル、プロピオン酸プロピル、トリメチル酢酸エチル、酪酸メチルおよび酪酸エチルなどである。
Carboxylic acid ester compounds include chain carboxylates. Specific examples of chain carboxylates include methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, propyl propionate, ethyl trimethylacetate, methyl butyrate, and ethyl butyrate.
ラクトン系化合物は、ラクトンなどである。ラクトンの具体例は、γ-ブチロラクトンおよびγ-バレロラクトンなどである。
Lactone compounds include lactones. Specific examples of lactones include gamma-butyrolactone and gamma-valerolactone.
なお、エーテル類は、1,2-ジメトキシエタン、テトラヒドロフラン、1,3-ジオキソランおよび1,4-ジオキサンなどでもよい。
The ethers may be 1,2-dimethoxyethane, tetrahydrofuran, 1,3-dioxolane, 1,4-dioxane, etc.
[他の電解質塩]
なお、電解液は、さらに、他の電解質塩のうちのいずれか1種類または2種類以上を含んでいてもよい。正極および負極のそれぞれの表面近傍においてカチオンの移動速度がより向上すると共に、電解液の液中においてもカチオンの移動速度がより向上するからである。電解液における他の電解質塩の含有量は、特に限定されないため、任意に設定可能である。 [Other electrolyte salts]
The electrolyte may further contain one or more of the other electrolyte salts. This is because the cation migration speed is improved in the vicinity of the surfaces of the positive electrode and the negative electrode, and the cation migration speed is also improved in the electrolyte. The content of the other electrolyte salt in the electrolyte is not particularly limited and can be set arbitrarily.
なお、電解液は、さらに、他の電解質塩のうちのいずれか1種類または2種類以上を含んでいてもよい。正極および負極のそれぞれの表面近傍においてカチオンの移動速度がより向上すると共に、電解液の液中においてもカチオンの移動速度がより向上するからである。電解液における他の電解質塩の含有量は、特に限定されないため、任意に設定可能である。 [Other electrolyte salts]
The electrolyte may further contain one or more of the other electrolyte salts. This is because the cation migration speed is improved in the vicinity of the surfaces of the positive electrode and the negative electrode, and the cation migration speed is also improved in the electrolyte. The content of the other electrolyte salt in the electrolyte is not particularly limited and can be set arbitrarily.
他の電解質塩の種類は、特に限定されないが、具体的には、リチウム塩などの軽金属塩である。ただし、上記した電解質塩は、ここで説明するリチウム塩から除かれる。
The type of other electrolyte salt is not particularly limited, but specifically, it is a light metal salt such as a lithium salt. However, the above electrolyte salts are excluded from the lithium salts described here.
リチウム塩の具体例は、六フッ化リン酸リチウム(LiPF6 )、四フッ化ホウ酸リチウム(LiBF4 )、トリフルオロメタンスルホン酸リチウム(LiCF3 SO3 )、ビス(フルオロスルホニル)イミドリチウム(LiN(FSO2 )2 )、ビス(トリフルオロメタンスルホニル)イミドリチウム(LiN(CF3 SO2 )2 )、リチウムトリス(トリフルオロメタンスルホニル)メチド(LiC(CF3 SO2 )3 )、ビス(オキサラト)ホウ酸リチウム(LiB(C2 O4 )2 )、ジフルオロオキサラトホウ酸リチウム(LiBF2 (C2 O4 ))、ジフルオロジ(オキサラト)ホウ酸リチウム(LiPF2 (C2 O4 )2 )、テトラフルオロオキサラトリン酸リチウム(LiPF4 (C2 O4 ))、モノフルオロリン酸リチウム(Li2 PFO3 )およびジフルオロリン酸リチウム(LiPF2 O2 )などである。
Specific examples of lithium salts include lithium hexafluorophosphate (LiPF 6 ), lithium tetrafluoroborate (LiBF 4 ), lithium trifluoromethanesulfonate (LiCF 3 SO 3 ), lithium bis(fluorosulfonyl)imide (LiN(FSO 2 ) 2 ), lithium bis(trifluoromethanesulfonyl)imide (LiN(CF 3 SO 2 ) 2 ), lithium tris(trifluoromethanesulfonyl)methide (LiC(CF 3 SO 2 ) 3 ), lithium bis(oxalato)borate (LiB(C 2 O 4 ) 2 ), lithium difluorooxalatoborate (LiBF 2 (C 2 O 4 )), and lithium difluorodi(oxalato)borate (LiPF 2 (C 2 O 4 ) 2 ) . ), lithium tetrafluorooxalatophosphate (LiPF 4 (C 2 O 4 )), lithium monofluorophosphate (Li 2 PFO 3 ), and lithium difluorophosphate (LiPF 2 O 2 ).
中でも、他の電解質塩は、六フッ化リン酸リチウム、四フッ化ホウ酸リチウム、ビス(フルオロスルホニル)イミドリチウム、ビス(オキサラト)ホウ酸リチウムおよびジフルオロリン酸リチウムのうちのいずれか1種類または2種類以上を含んでいることが好ましい。正極および負極のそれぞれの表面近傍においてカチオンの移動速度が十分に向上すると共に、電解液の液中においてもカチオンの移動速度が十分に向上するからである。
Among them, it is preferable that the other electrolyte salt contains one or more of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate. This is because the cation migration speed is sufficiently improved near the surfaces of the positive electrode and negative electrode, and the cation migration speed is also sufficiently improved in the electrolyte solution.
[他の溶媒]
また、電解液は、さらに、他の溶媒のうちのいずれか1種類または2種類以上を含んでいてもよい。電解液を用いた二次電池の充放電時において、他の溶媒に由来する被膜が正極および負極のそれぞれの表面に形成されるため、その電解液の分解反応が抑制されるからである。なお、電解液における他の溶媒の含有量は、特に限定されないため、任意に設定可能である。 [Other Solvents]
The electrolyte may further contain one or more of the other solvents. During charging and discharging of the secondary battery using the electrolyte, a coating derived from the other solvent is formed on the surface of each of the positive and negative electrodes, so that the decomposition reaction of the electrolyte is suppressed. The content of the other solvent in the electrolyte is not particularly limited and can be set arbitrarily.
また、電解液は、さらに、他の溶媒のうちのいずれか1種類または2種類以上を含んでいてもよい。電解液を用いた二次電池の充放電時において、他の溶媒に由来する被膜が正極および負極のそれぞれの表面に形成されるため、その電解液の分解反応が抑制されるからである。なお、電解液における他の溶媒の含有量は、特に限定されないため、任意に設定可能である。 [Other Solvents]
The electrolyte may further contain one or more of the other solvents. During charging and discharging of the secondary battery using the electrolyte, a coating derived from the other solvent is formed on the surface of each of the positive and negative electrodes, so that the decomposition reaction of the electrolyte is suppressed. The content of the other solvent in the electrolyte is not particularly limited and can be set arbitrarily.
他の溶媒の種類は、特に限定されないが、具体的には、不飽和環状炭酸エステル、フッ素化環状炭酸エステル、スルホン酸エステル、ジカルボン酸無水物、ジスルホン酸無水物、硫酸エステル、ニトリル化合物およびイソシアネート化合物などである。
The types of other solvents are not particularly limited, but specific examples include unsaturated cyclic carbonates, fluorinated cyclic carbonates, sulfonic acid esters, dicarboxylic acid anhydrides, disulfonic acid anhydrides, sulfates, nitrile compounds, and isocyanate compounds.
(不飽和環状炭酸エステル)
不飽和環状炭酸エステルは、不飽和炭素結合(炭素間二重結合)を含む環状炭酸エステルである。不飽和炭素結合の数は、特に限定されないため、1個だけでもよいし、2個以上でもよい。不飽和環状炭酸エステルの具体例は、炭酸ビニレン、炭酸ビニルエチレンおよび炭酸メチレンエチレンなどである。 (Unsaturated cyclic carbonate)
The unsaturated cyclic carbonate is a cyclic carbonate containing an unsaturated carbon bond (carbon-carbon double bond). The number of unsaturated carbon bonds is not particularly limited, and may be one or more. Specific examples of the unsaturated cyclic carbonate include vinylene carbonate, vinylethylene carbonate, and methyleneethylene carbonate.
不飽和環状炭酸エステルは、不飽和炭素結合(炭素間二重結合)を含む環状炭酸エステルである。不飽和炭素結合の数は、特に限定されないため、1個だけでもよいし、2個以上でもよい。不飽和環状炭酸エステルの具体例は、炭酸ビニレン、炭酸ビニルエチレンおよび炭酸メチレンエチレンなどである。 (Unsaturated cyclic carbonate)
The unsaturated cyclic carbonate is a cyclic carbonate containing an unsaturated carbon bond (carbon-carbon double bond). The number of unsaturated carbon bonds is not particularly limited, and may be one or more. Specific examples of the unsaturated cyclic carbonate include vinylene carbonate, vinylethylene carbonate, and methyleneethylene carbonate.
(フッ素化環状炭酸エステル)
フッ素化環状炭酸エステルは、フッ素を構成元素として含む環状炭酸エステルである。すなわち、フッ素化環状炭酸エステルは、環状炭酸エステルのうちの1個または2個以上の水素基がフッ素基により置換された化合物である。フッ素化環状炭酸エステルの具体例は、モノフルオロ炭酸エチレンおよびジフルオロ炭酸エチレンなどである。 (Fluorinated cyclic carbonate)
A fluorinated cyclic carbonate is a cyclic carbonate containing fluorine as a constituent element. That is, a fluorinated cyclic carbonate is a compound in which one or more hydrogen groups of a cyclic carbonate are substituted with fluorine groups. Specific examples of the fluorinated cyclic carbonate include monofluoroethylene carbonate and difluoroethylene carbonate.
フッ素化環状炭酸エステルは、フッ素を構成元素として含む環状炭酸エステルである。すなわち、フッ素化環状炭酸エステルは、環状炭酸エステルのうちの1個または2個以上の水素基がフッ素基により置換された化合物である。フッ素化環状炭酸エステルの具体例は、モノフルオロ炭酸エチレンおよびジフルオロ炭酸エチレンなどである。 (Fluorinated cyclic carbonate)
A fluorinated cyclic carbonate is a cyclic carbonate containing fluorine as a constituent element. That is, a fluorinated cyclic carbonate is a compound in which one or more hydrogen groups of a cyclic carbonate are substituted with fluorine groups. Specific examples of the fluorinated cyclic carbonate include monofluoroethylene carbonate and difluoroethylene carbonate.
(スルホン酸エステル)
スルホン酸エステルは、環状モノスルホン酸エステル、環状ジスルホン酸エステル、鎖状モノスルホン酸エステルおよび鎖状ジスルホン酸エステルなどである。環状モノスルホン酸エステルの具体例は、1,3-プロパンスルトン、1-プロペン-1,3-スルトン、1,4-ブタンスルトン、2,4-ブタンスルトンおよびメタンスルホン酸プロパルギルエステルなどである。環状ジスルホン酸エステルの具体例は、シクロジソンなどである。 (Sulfonic acid ester)
The sulfonate esters include cyclic monosulfonate esters, cyclic disulfonate esters, chain monosulfonate esters, chain disulfonate esters, etc. Specific examples of cyclic monosulfonate esters include 1,3-propane sultone, 1-propene-1,3-sultone, 1,4-butane sultone, 2,4-butane sultone, methanesulfonic acid propargyl ester, etc. Specific examples of cyclic disulfonate esters include cyclodisone, etc.
スルホン酸エステルは、環状モノスルホン酸エステル、環状ジスルホン酸エステル、鎖状モノスルホン酸エステルおよび鎖状ジスルホン酸エステルなどである。環状モノスルホン酸エステルの具体例は、1,3-プロパンスルトン、1-プロペン-1,3-スルトン、1,4-ブタンスルトン、2,4-ブタンスルトンおよびメタンスルホン酸プロパルギルエステルなどである。環状ジスルホン酸エステルの具体例は、シクロジソンなどである。 (Sulfonic acid ester)
The sulfonate esters include cyclic monosulfonate esters, cyclic disulfonate esters, chain monosulfonate esters, chain disulfonate esters, etc. Specific examples of cyclic monosulfonate esters include 1,3-propane sultone, 1-propene-1,3-sultone, 1,4-butane sultone, 2,4-butane sultone, methanesulfonic acid propargyl ester, etc. Specific examples of cyclic disulfonate esters include cyclodisone, etc.
(ジカルボン酸無水物)
ジカルボン酸無水物の具体例は、無水コハク酸、無水グルタル酸および無水マレイン酸などである。 (Dicarboxylic acid anhydride)
Specific examples of dicarboxylic acid anhydrides include succinic anhydride, glutaric anhydride, and maleic anhydride.
ジカルボン酸無水物の具体例は、無水コハク酸、無水グルタル酸および無水マレイン酸などである。 (Dicarboxylic acid anhydride)
Specific examples of dicarboxylic acid anhydrides include succinic anhydride, glutaric anhydride, and maleic anhydride.
(ジスルホン酸無水物)
ジスルホン酸無水物の具体例は、無水エタンジスルホン酸および無水プロパンジスルホン酸などである。 (Disulfonic acid anhydride)
Specific examples of disulfonic anhydrides include ethanedisulfonic anhydride and propanedisulfonic anhydride.
ジスルホン酸無水物の具体例は、無水エタンジスルホン酸および無水プロパンジスルホン酸などである。 (Disulfonic acid anhydride)
Specific examples of disulfonic anhydrides include ethanedisulfonic anhydride and propanedisulfonic anhydride.
(硫酸エステル)
硫酸エステルの具体例は、エチレンスルファート(1,3,2-ジオキサチオラン 2,2-ジオキシド)などである。 (Sulfuric acid ester)
A specific example of a sulfate ester is ethylene sulfate (1,3,2-dioxathiolane 2,2-dioxide).
硫酸エステルの具体例は、エチレンスルファート(1,3,2-ジオキサチオラン 2,2-ジオキシド)などである。 (Sulfuric acid ester)
A specific example of a sulfate ester is ethylene sulfate (1,3,2-dioxathiolane 2,2-dioxide).
(ニトリル化合物)
ニトリル化合物は、1個または2個以上のシアノ基(-CN)を有する化合物である。ニトリル化合物の具体例は、オクタンニトリル、ベンゾニトリル、フタロニトリル、スクシノニトリル、グルタロニトリル、アジポニトリル、セバコニトリル、1,3,6-ヘキサントリカルボニトリル、3,3’-オキシジプロピオニトリル、3-ブトキシプロピオニトリル、エチレングリコールビスプロピオニトリルエーテル、1,2,2,3-テトラシアノプロパン、テトラシアノプロパン、フマロニトリル、7,7,8,8-テトラシアノキノジメタン、シクロペンタンカルボニトリル、1,3,5-シクロヘキサントリカルボニトリルおよび1,3-ビス(ジシアノメチリデン)インダンなどである。 (Nitrile compounds)
The nitrile compound is a compound having one or more cyano groups (-CN). Specific examples of the nitrile compound include octanenitrile, benzonitrile, phthalonitrile, succinonitrile, glutaronitrile, adiponitrile, sebaconitrile, 1,3,6-hexanetricarbonitrile, 3,3'-oxydipropionitrile, 3-butoxypropionitrile, ethylene glycol bispropionitrile ether, 1,2,2,3-tetracyanopropane, tetracyanopropane, fumaronitrile, 7,7,8,8-tetracyanoquinodimethane, cyclopentanecarbonitrile, 1,3,5-cyclohexanetricarbonitrile, and 1,3-bis(dicyanomethylidene)indane.
ニトリル化合物は、1個または2個以上のシアノ基(-CN)を有する化合物である。ニトリル化合物の具体例は、オクタンニトリル、ベンゾニトリル、フタロニトリル、スクシノニトリル、グルタロニトリル、アジポニトリル、セバコニトリル、1,3,6-ヘキサントリカルボニトリル、3,3’-オキシジプロピオニトリル、3-ブトキシプロピオニトリル、エチレングリコールビスプロピオニトリルエーテル、1,2,2,3-テトラシアノプロパン、テトラシアノプロパン、フマロニトリル、7,7,8,8-テトラシアノキノジメタン、シクロペンタンカルボニトリル、1,3,5-シクロヘキサントリカルボニトリルおよび1,3-ビス(ジシアノメチリデン)インダンなどである。 (Nitrile compounds)
The nitrile compound is a compound having one or more cyano groups (-CN). Specific examples of the nitrile compound include octanenitrile, benzonitrile, phthalonitrile, succinonitrile, glutaronitrile, adiponitrile, sebaconitrile, 1,3,6-hexanetricarbonitrile, 3,3'-oxydipropionitrile, 3-butoxypropionitrile, ethylene glycol bispropionitrile ether, 1,2,2,3-tetracyanopropane, tetracyanopropane, fumaronitrile, 7,7,8,8-tetracyanoquinodimethane, cyclopentanecarbonitrile, 1,3,5-cyclohexanetricarbonitrile, and 1,3-bis(dicyanomethylidene)indane.
(イソシアネート化合物)
イソシアネート化合物は、1個または2個以上のイソシアネート基(-NCO)を有する化合物である。イソシアネート化合物の具体例は、ヘキサメチレンジイソシアネートなどである。 (Isocyanate Compounds)
The isocyanate compound is a compound having one or more isocyanate groups (-NCO). A specific example of the isocyanate compound is hexamethylene diisocyanate.
イソシアネート化合物は、1個または2個以上のイソシアネート基(-NCO)を有する化合物である。イソシアネート化合物の具体例は、ヘキサメチレンジイソシアネートなどである。 (Isocyanate Compounds)
The isocyanate compound is a compound having one or more isocyanate groups (-NCO). A specific example of the isocyanate compound is hexamethylene diisocyanate.
<1-2.製造方法>
電解液を製造する場合には、溶媒に電解質塩を投入する。この場合には、溶媒にさらに他の電解質塩を添加してもよいし、溶媒にさらに添加剤を添加してもよい。これにより、溶媒中において電解質塩などが分散または溶解されるため、電解液が調製される。この電解液は、上記した理由により、塩素イオンを含んでいる。 <1-2. Manufacturing method>
When producing an electrolytic solution, an electrolyte salt is added to a solvent. In this case, another electrolyte salt may be added to the solvent, or an additive may be added to the solvent. The electrolyte salt and the like are dispersed or dissolved in the solvent by the above-mentioned process, and thus an electrolyte solution is prepared. This electrolyte solution contains chloride ions for the above-mentioned reasons.
電解液を製造する場合には、溶媒に電解質塩を投入する。この場合には、溶媒にさらに他の電解質塩を添加してもよいし、溶媒にさらに添加剤を添加してもよい。これにより、溶媒中において電解質塩などが分散または溶解されるため、電解液が調製される。この電解液は、上記した理由により、塩素イオンを含んでいる。 <1-2. Manufacturing method>
When producing an electrolytic solution, an electrolyte salt is added to a solvent. In this case, another electrolyte salt may be added to the solvent, or an additive may be added to the solvent. The electrolyte salt and the like are dispersed or dissolved in the solvent by the above-mentioned process, and thus an electrolyte solution is prepared. This electrolyte solution contains chloride ions for the above-mentioned reasons.
<1-3.作用および効果>
この電解液によれば、その電解液が電解質塩および塩素イオンを含んでおり、その電解質塩がイミドアニオンを含んでおり、その電解液における塩素イオンの含有量が5000重量ppm以下である。 <1-3. Actions and Effects>
According to this electrolyte, the electrolyte contains an electrolyte salt and chloride ions, the electrolyte salt contains imide anions, and the content of chloride ions in the electrolyte is 5000 ppm by weight or less.
この電解液によれば、その電解液が電解質塩および塩素イオンを含んでおり、その電解質塩がイミドアニオンを含んでおり、その電解液における塩素イオンの含有量が5000重量ppm以下である。 <1-3. Actions and Effects>
According to this electrolyte, the electrolyte contains an electrolyte salt and chloride ions, the electrolyte salt contains imide anions, and the content of chloride ions in the electrolyte is 5000 ppm by weight or less.
この場合には、電解質塩がイミドアニオンを含んでいるため、上記したように、電解液を用いた二次電池の充放電時において、その電解質塩に由来する良質な被膜を利用して電解液の分解反応が抑制されると共に、カチオンの移動速度が向上する。
In this case, since the electrolyte salt contains imide anions, as described above, when a secondary battery using an electrolyte is charged and discharged, the decomposition reaction of the electrolyte is suppressed by utilizing the high-quality coating derived from the electrolyte salt, and the migration rate of cations is improved.
しかも、電解液における塩素イオンの含有量が5000重量ppm以下であるため、上記したように、その電解液の化学的安定性が向上する。これにより、電解液を用いた二次電池の充放電時において、その電解液の分解反応がより抑制される。
In addition, because the content of chloride ions in the electrolyte is 5,000 ppm by weight or less, the chemical stability of the electrolyte is improved, as described above. This further suppresses the decomposition reaction of the electrolyte when a secondary battery using the electrolyte is charged and discharged.
これらのことから、電解液を用いた二次電池の充放電時において、カチオンの移動速度が担保されながら、電解液の分解反応が著しく抑制される。よって、電解液を用いて、優れた電池特性を有する二次電池を実現することができる。
As a result, when a secondary battery using an electrolyte is charged and discharged, the decomposition reaction of the electrolyte is significantly suppressed while the cation migration rate is guaranteed. Therefore, a secondary battery with excellent battery characteristics can be realized by using an electrolyte.
特に、電解質塩がカチオンとして軽金属イオンを含んでいれば、高い電圧が得られるため、より高い効果を得ることができる。この場合には、軽金属イオンがリチウムイオンを含んでいれば、より高い電圧が得られるため、さらに高い効果を得ることができる。
In particular, if the electrolyte salt contains light metal ions as cations, a higher voltage can be obtained, and therefore a greater effect can be achieved. In this case, if the light metal ions contain lithium ions, a higher voltage can be obtained, and therefore an even greater effect can be achieved.
また、電解液における電解質塩の含有量が0.2mol/kg~2mol/kgであれば、高いイオン伝導性が得られるため、より高い効果を得ることができる。
In addition, if the content of electrolyte salt in the electrolyte solution is 0.2 mol/kg to 2 mol/kg, high ionic conductivity can be obtained, resulting in even greater effects.
また、電解液がさらに他の電解質塩として六フッ化リン酸リチウム、四フッ化ホウ酸リチウム、ビス(フルオロスルホニル)イミドリチウム、ビス(オキサラト)ホウ酸リチウムおよびジフルオロリン酸リチウムのうちのいずれか1種類または2種類以上を含んでいれば、カチオンの移動速度がより向上するため、より高い効果を得ることができる。
In addition, if the electrolyte further contains one or more of the following electrolyte salts as other electrolyte salts: lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate, the cation migration rate is further improved, and thus a greater effect can be obtained.
また、電解液がさらに他の溶媒として不飽和環状炭酸エステル、フッ素化環状炭酸エステル、スルホン酸エステル、ジカルボン酸無水物、ジスルホン酸無水物、硫酸エステル、ニトリル化合物およびイソシアネート化合物のうちのいずれか1種類または2種類以上を含んでいれば、その電解液の分解反応が抑制されるため、より高い効果を得ることができる。
In addition, if the electrolyte further contains one or more of the following solvents as other solvents: unsaturated cyclic carbonates, fluorinated cyclic carbonates, sulfonic acid esters, dicarboxylic acid anhydrides, disulfonic acid anhydrides, sulfate esters, nitrile compounds, and isocyanate compounds, the decomposition reaction of the electrolyte is suppressed, and thus a greater effect can be obtained.
<2.二次電池>
次に、上記した電解液を用いた二次電池に関して説明する。 2. Secondary battery
Next, a secondary battery using the above-mentioned electrolyte will be described.
次に、上記した電解液を用いた二次電池に関して説明する。 2. Secondary battery
Next, a secondary battery using the above-mentioned electrolyte will be described.
ここで説明する二次電池は、電極反応物質の吸蔵放出を利用して電池容量が得られる二次電池であり、正極および負極と共に電解液を備えている。
The secondary battery described here is a secondary battery that obtains battery capacity by utilizing the absorption and release of electrode reactants, and is equipped with a positive electrode, a negative electrode, and an electrolyte.
負極の充電容量は、正極の放電容量よりも大きいことが好ましい。すなわち、負極の単位面積当たりの電気化学容量は、正極の単位面積当たりの電気化学容量よりも大きいことが好ましい。充電途中において負極の表面に電極反応物質が析出することを抑制するためである。
The charge capacity of the negative electrode is preferably greater than the discharge capacity of the positive electrode. In other words, the electrochemical capacity per unit area of the negative electrode is preferably greater than the electrochemical capacity per unit area of the positive electrode. This is to prevent electrode reactants from depositing on the surface of the negative electrode during charging.
電極反応物質の種類は、特に限定されないが、具体的には、アルカリ金属およびアルカリ土類金属などの軽金属である。アルカリ金属の具体例は、リチウム、ナトリウムおよびカリウムなどであると共に、アルカリ土類金属の具体例は、ベリリウム、マグネシウムおよびカルシウムなどである。
The type of electrode reactant is not particularly limited, but specifically includes light metals such as alkali metals and alkaline earth metals. Specific examples of alkali metals include lithium, sodium, and potassium, while specific examples of alkaline earth metals include beryllium, magnesium, and calcium.
以下では、電極反応物質がリチウムである場合を例に挙げる。リチウムの吸蔵放出を利用して電池容量が得られる二次電池は、いわゆるリチウムイオン二次電池である。このリチウムイオン二次電池では、リチウムがイオン状態で吸蔵放出される。
Below, we will use an example where the electrode reactant is lithium. A secondary battery that obtains battery capacity by utilizing the absorption and release of lithium is known as a lithium-ion secondary battery. In this lithium-ion secondary battery, lithium is absorbed and released in an ionic state.
<2-1.構成>
図1は、二次電池の斜視構成を表している。図2は、図1に示した電池素子20の断面構成を表している。 <2-1. Configuration>
Fig. 1 shows a perspective view of a secondary battery, and Fig. 2 shows a cross-sectional view of abattery element 20 shown in Fig. 1.
図1は、二次電池の斜視構成を表している。図2は、図1に示した電池素子20の断面構成を表している。 <2-1. Configuration>
Fig. 1 shows a perspective view of a secondary battery, and Fig. 2 shows a cross-sectional view of a
ただし、図1では、外装フィルム10と電池素子20とが互いに分離された状態を示していると共に、XZ面に沿った電池素子20の断面を破線で示している。図2では、電池素子20の一部だけを示している。
However, in FIG. 1, the exterior film 10 and the battery element 20 are shown in a state where they are separated from each other, and a cross section of the battery element 20 along the XZ plane is shown by a dashed line. In FIG. 2, only a part of the battery element 20 is shown.
この二次電池は、図1および図2に示したように、外装フィルム10と、電池素子20と、正極リード31と、負極リード32と、封止フィルム41,42とを備えている。
As shown in Figures 1 and 2, this secondary battery includes an exterior film 10, a battery element 20, a positive electrode lead 31, a negative electrode lead 32, and sealing films 41 and 42.
ここで説明する二次電池は、上記したように、電池素子20を内部に収納するための外装部材として、可撓性または柔軟性を有する外装フィルム10を用いている。よって、図1および図2に示した二次電池は、いわゆるラミネートフィルム型の二次電池である。
As described above, the secondary battery described here uses a flexible or pliable exterior film 10 as an exterior member for housing the battery element 20 inside. Therefore, the secondary battery shown in Figures 1 and 2 is a so-called laminate film type secondary battery.
[外装フィルム]
外装フィルム10は、図1に示したように、電池素子20が内部に収納された状態において封止された袋状の構造を有している。これにより、外装フィルム10は、後述する正極21、負極22およびセパレータ23を収納している。 [Exterior film]
1, theexterior film 10 has a bag-like structure that is sealed with the battery element 20 housed therein. As a result, the exterior film 10 houses a positive electrode 21, a negative electrode 22, and a separator 23, which will be described later.
外装フィルム10は、図1に示したように、電池素子20が内部に収納された状態において封止された袋状の構造を有している。これにより、外装フィルム10は、後述する正極21、負極22およびセパレータ23を収納している。 [Exterior film]
1, the
ここでは、外装フィルム10は、1枚のフィルム状の部材であり、折り畳み方向Fに折り畳まれている。この外装フィルム10には、電池素子20を収容するための窪み部10U(いわゆる深絞り部)が設けられている。
Here, the exterior film 10 is a single film-like member that is folded in the folding direction F. This exterior film 10 is provided with a recessed portion 10U (a so-called deep drawn portion) for accommodating the battery element 20.
具体的には、外装フィルム10は、融着層、金属層および表面保護層が内側からこの順に積層された3層のラミネートフィルムであり、その外装フィルム10が折り畳まれた状態において、互いに対向する融着層のうちの外周縁部同士が互いに融着されている。融着層は、ポリプロピレンなどの高分子化合物を含んでいる。金属層は、アルミニウムなどの金属材料を含んでいる。表面保護層は、ナイロンなどの高分子化合物を含んでいる。
Specifically, the exterior film 10 is a three-layer laminate film in which a fusion layer, a metal layer, and a surface protection layer are laminated in this order from the inside, and when the exterior film 10 is folded, the outer peripheral edges of the opposing fusion layers are fused to each other. The fusion layer contains a polymer compound such as polypropylene. The metal layer contains a metallic material such as aluminum. The surface protection layer contains a polymer compound such as nylon.
ただし、外装フィルム10の構成(層数)は、特に、限定されないため、1層または2層でもよいし、4層以上でもよい。
However, the configuration (number of layers) of the exterior film 10 is not particularly limited, so it may be one or two layers, or four or more layers.
[電池素子]
電池素子20は、外装フィルム10の内部に収納されている。この電池素子20は、いわゆる発電素子であり、図1および図2に示したように、正極21、負極22、セパレータ23および電解液(図示せず)を含んでいる。 [Battery element]
Thebattery element 20 is housed inside the exterior film 10. The battery element 20 is a so-called power generating element, and includes a positive electrode 21, a negative electrode 22, a separator 23, and an electrolyte (not shown), as shown in Figures 1 and 2 .
電池素子20は、外装フィルム10の内部に収納されている。この電池素子20は、いわゆる発電素子であり、図1および図2に示したように、正極21、負極22、セパレータ23および電解液(図示せず)を含んでいる。 [Battery element]
The
ここでは、電池素子20は、いわゆる巻回電極体であるため、正極21および負極22は、セパレータ23を介して互いに対向しながら巻回軸Pを中心として巻回されている。この巻回軸Pは、図1に示したように、Y軸方向に延在する仮想軸である。
Here, the battery element 20 is a so-called wound electrode body, so that the positive electrode 21 and the negative electrode 22 are wound around the winding axis P while facing each other via the separator 23. This winding axis P is a virtual axis extending in the Y-axis direction, as shown in FIG. 1.
電池素子20の立体的形状は、特に限定されない。ここでは、電池素子20は、扁平状の立体的形状を有しているため、巻回軸Pと交差する電池素子20の断面(XZ面に沿った断面)の形状は、長軸J1および短軸J2により規定される扁平形状である。
The three-dimensional shape of the battery element 20 is not particularly limited. Here, the battery element 20 has a flat three-dimensional shape, so that the shape of the cross section (cross section along the XZ plane) of the battery element 20 intersecting the winding axis P is a flat shape defined by the major axis J1 and the minor axis J2.
長軸J1は、X軸方向に延在する仮想軸であり、短軸J2の長さよりも大きい長さを有している。短軸J2は、X軸方向と交差するZ軸方向に延在する仮想軸であり、長軸J1の長さよりも小さい長さを有している。ここでは、電池素子20の立体的形状は、扁平な円筒状であるため、その電池素子20の断面の形状は、扁平な略楕円形状である。
The long axis J1 is an imaginary axis extending in the X-axis direction and has a length greater than that of the short axis J2. The short axis J2 is an imaginary axis extending in the Z-axis direction intersecting the X-axis direction and has a length less than that of the long axis J1. Here, the three-dimensional shape of the battery element 20 is a flattened cylinder, and therefore the cross-sectional shape of the battery element 20 is a flattened, approximately elliptical shape.
(正極)
正極21は、図2に示したように、正極集電体21Aおよび正極活物質層21Bを含んでいる。 (Positive electrode)
As shown in FIG. 2, thepositive electrode 21 includes a positive electrode current collector 21A and a positive electrode active material layer 21B.
正極21は、図2に示したように、正極集電体21Aおよび正極活物質層21Bを含んでいる。 (Positive electrode)
As shown in FIG. 2, the
正極集電体21Aは、正極活物質層21Bが設けられる一対の面を有している。この正極集電体21Aは、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、アルミニウムなどである。
The positive electrode collector 21A has a pair of surfaces on which the positive electrode active material layer 21B is provided. This positive electrode collector 21A contains a conductive material such as a metal material, and a specific example of the conductive material is aluminum.
正極活物質層21Bは、リチウムを吸蔵放出する正極活物質のうちのいずれか1種類または2種類以上を含んでいる。ただし、正極活物質層21Bは、さらに、正極結着剤および正極導電剤などの他の材料のうちのいずれか1種類または2種類以上を含んでいてもよい。正極活物質層21Bの形成方法は、特に限定されないが、具体的には、塗布法などである。
The positive electrode active material layer 21B contains one or more types of positive electrode active materials that absorb and release lithium. However, the positive electrode active material layer 21B may further contain one or more types of other materials such as a positive electrode binder and a positive electrode conductor. The method of forming the positive electrode active material layer 21B is not particularly limited, but specifically includes a coating method.
ここでは、正極活物質層21Bは、正極集電体21Aの両面に設けられている。ただし、正極活物質層21Bは、正極21が負極22に対向する側において正極集電体21Aの片面だけに設けられていてもよい。
Here, the positive electrode active material layer 21B is provided on both sides of the positive electrode collector 21A. However, the positive electrode active material layer 21B may be provided on only one side of the positive electrode collector 21A on the side where the positive electrode 21 faces the negative electrode 22.
正極活物質の種類は、特に限定されないが、具体的には、リチウム含有化合物などである。このリチウム含有化合物は、リチウムと共に1種類または2種類以上の遷移金属元素を構成元素として含む化合物であり、さらに、1種類または2種類以上の他元素を構成元素として含んでいてもよい。他元素の種類は、リチウムおよび遷移金属元素のそれぞれ以外の元素であれば、特に限定されないが、具体的には、長周期型周期表中の2族~15族に属する元素である。リチウム含有化合物の種類は、特に限定されないが、具体的には、リチウム含有化合物は、酸化物、リン酸化合物、ケイ酸化合物およびホウ酸化合物などである。
The type of positive electrode active material is not particularly limited, but specifically includes lithium-containing compounds. This lithium-containing compound is a compound that contains one or more transition metal elements as constituent elements along with lithium, and may further contain one or more other elements as constituent elements. The type of other element is not particularly limited, so long as it is an element other than lithium and transition metal elements, but specifically includes elements belonging to groups 2 to 15 of the long period periodic table. The type of lithium-containing compound is not particularly limited, but specifically includes oxides, phosphate compounds, silicate compounds, and borate compounds.
酸化物の具体例は、LiNiO2 、LiCoO2 、LiCo0.98Al0.01Mg0.01O2 、LiNi0.5 Co0.2 Mn0.3 O2 、LiNi0.8 Co0.15Al0.05O2 、LiNi0.33Co0.33Mn0.33O2 、Li1.2 Mn0.52Co0.175 Ni0.1 O2 、Li1.15(Mn0.65Ni0.22Co0.13)O2 およびLiMn2 O4 などである。リン酸化合物の具体例は、LiFePO4 、LiMnPO4 、LiFe0.5 Mn0.5 PO4 およびLiFe0.3 Mn0.7 PO4 などである。
Specific examples of oxides include LiNiO2 , LiCoO2 , LiCo0.98Al0.01Mg0.01O2 , LiNi0.5Co0.2Mn0.3O2 , LiNi0.8Co0.15Al0.05O2 , LiNi0.33Co0.33Mn0.33O2 , Li1.2Mn0.52Co0.175Ni0.1O2 , Li1.15 ( Mn0.65Ni0.22Co0.13 ) O2 , and LiMn2O4 . Specific examples of phosphate compounds include LiFePO4 , LiMnPO4 , LiFe0.5Mn0.5PO4 , and LiFe0.3Mn0.7PO4 .
正極結着剤は、合成ゴムおよび高分子化合物などの材料のうちのいずれか1種類または2種類以上を含んでいる。合成ゴムの具体例は、スチレンブタジエン系ゴム、フッ素系ゴムおよびエチレンプロピレンジエンなどである。高分子化合物の具体例は、ポリフッ化ビニリデン、ポリイミドおよびカルボキシメチルセルロースなどである。
The positive electrode binder contains one or more of the following materials: synthetic rubber and polymeric compounds. Specific examples of synthetic rubber include styrene-butadiene rubber, fluororubber, and ethylene-propylene-diene. Specific examples of polymeric compounds include polyvinylidene fluoride, polyimide, and carboxymethyl cellulose.
正極導電剤は、炭素材料、金属材料および導電性高分子化合物などの導電性材料のうちのいずれか1種類または2種類以上を含んでおり、その炭素材料の具体例は、黒鉛、カーボンブラック、アセチレンブラックおよびケッチェンブラックなどである。
The positive electrode conductive agent contains one or more conductive materials such as carbon materials, metal materials, and conductive polymer compounds. Specific examples of carbon materials include graphite, carbon black, acetylene black, and ketjen black.
(負極)
負極22は、図2に示したように、負極集電体22Aおよび負極活物質層22Bを含んでいる。 (Negative electrode)
As shown in FIG. 2, thenegative electrode 22 includes a negative electrode current collector 22A and a negative electrode active material layer 22B.
負極22は、図2に示したように、負極集電体22Aおよび負極活物質層22Bを含んでいる。 (Negative electrode)
As shown in FIG. 2, the
負極集電体22Aは、負極活物質層22Bが設けられる一対の面を有している。この負極集電体22Aは、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、銅などである。
The negative electrode current collector 22A has a pair of surfaces on which the negative electrode active material layer 22B is provided. This negative electrode current collector 22A contains a conductive material such as a metal material, and a specific example of the conductive material is copper.
負極活物質層22Bは、リチウムを吸蔵放出する負極活物質のうちのいずれか1種類または2種類以上を含んでいる。ただし、負極活物質層22Bは、さらに、負極結着剤および負極導電剤などの他の材料のうちのいずれか1種類または2種類以上を含んでいてもよい。負極活物質層22Bの形成方法は、特に限定されないが、具体的には、塗布法、気相法、液相法、溶射法および焼成法(焼結法)などのうちのいずれか1種類または2種類以上である。
The negative electrode active material layer 22B contains one or more types of negative electrode active materials that absorb and release lithium. However, the negative electrode active material layer 22B may further contain one or more types of other materials such as a negative electrode binder and a negative electrode conductor. The method of forming the negative electrode active material layer 22B is not particularly limited, but specifically includes one or more types of a coating method, a gas phase method, a liquid phase method, a thermal spraying method, and a baking method (sintering method).
ここでは、負極活物質層22Bは、負極集電体22Aの両面に設けられている。ただし、負極活物質層22Bは、負極22が正極21に対向する側において負極集電体22Aの片面だけに設けられていてもよい。
Here, the negative electrode active material layer 22B is provided on both sides of the negative electrode collector 22A. However, the negative electrode active material layer 22B may be provided on only one side of the negative electrode collector 22A on the side where the negative electrode 22 faces the positive electrode 21.
負極活物質の種類は、特に限定されないが、具体的には、炭素材料および金属系材料などである。高いエネルギー密度が得られるからである。
The type of negative electrode active material is not particularly limited, but specific examples include carbon materials and metal-based materials, because they provide high energy density.
炭素材料の具体例は、易黒鉛化性炭素、難黒鉛化性炭素および黒鉛などである。この黒鉛は、天然黒鉛でもよいし、人造黒鉛でもよい。
Specific examples of carbon materials include graphitizable carbon, non-graphitizable carbon, and graphite. The graphite may be natural graphite or artificial graphite.
金属系材料は、リチウムと合金を形成可能である金属元素および半金属元素のうちのいずれか1種類または2種類以上を構成元素として含む材料の総称であり、その金属元素および半金属元素の具体例は、ケイ素およびスズなどである。この金属系材料は、単体でもよいし、合金でもよいし、化合物でもよいし、それらの2種類以上の混合物でもよいし、それらの2種類以上の相を含む材料でもよい。金属系材料の具体例は、TiSi2 およびSiOx (0<x≦2または0.2<x<1.4)などである。
Metallic materials are a general term for materials that contain one or more of metallic elements and semi-metallic elements that can form an alloy with lithium as constituent elements, and specific examples of the metallic elements and semi-metallic elements include silicon and tin. The metallic materials may be a single element, an alloy, a compound, a mixture of two or more of these, or a material that contains two or more of these phases. Specific examples of metallic materials include TiSi2 and SiOx (0<x≦2 or 0.2<x<1.4).
負極結着剤に関する詳細は、正極結着剤に関する詳細と同様であると共に、負極導電剤に関する詳細は、正極導電剤に関する詳細と同様である。
Details regarding the negative electrode binder are the same as those regarding the positive electrode binder, and details regarding the negative electrode conductor are the same as those regarding the positive electrode conductor.
(セパレータ)
セパレータ23は、図2に示したように、正極21と負極22との間に介在している絶縁性の多孔質膜であり、その正極21と負極22との接触に起因する短絡の発生を防止しながらリチウムをイオン状態で通過させる。このセパレータ23は、ポリエチレンなどの高分子化合物を含んでいる。 (Separator)
2, theseparator 23 is an insulating porous film interposed between the positive electrode 21 and the negative electrode 22, and allows lithium to pass through in an ion state while preventing the occurrence of a short circuit due to contact between the positive electrode 21 and the negative electrode 22. This separator 23 contains a polymer compound such as polyethylene.
セパレータ23は、図2に示したように、正極21と負極22との間に介在している絶縁性の多孔質膜であり、その正極21と負極22との接触に起因する短絡の発生を防止しながらリチウムをイオン状態で通過させる。このセパレータ23は、ポリエチレンなどの高分子化合物を含んでいる。 (Separator)
2, the
(電解液)
電解液は、正極21、負極22およびセパレータ23のそれぞれに含浸されており、上記した構成を有している。すなわち、電解液は、電解質塩および塩素イオンを含んでおり、その電解質塩は、イミドアニオンを含んでおり、その電解液における塩素イオンの含有量は、上記した範囲である。 (Electrolyte)
The electrolyte is impregnated into each of thepositive electrode 21, the negative electrode 22, and the separator 23, and has the above-mentioned configuration. That is, the electrolyte contains an electrolyte salt and chloride ions, the electrolyte salt contains imide anions, and the content of chloride ions in the electrolyte is within the above-mentioned range.
電解液は、正極21、負極22およびセパレータ23のそれぞれに含浸されており、上記した構成を有している。すなわち、電解液は、電解質塩および塩素イオンを含んでおり、その電解質塩は、イミドアニオンを含んでおり、その電解液における塩素イオンの含有量は、上記した範囲である。 (Electrolyte)
The electrolyte is impregnated into each of the
[正極リード]
正極リード31は、図1および図2に示したように、正極21の正極集電体21Aに接続されている正極配線であり、外装フィルム10の外部に導出されている。この正極リード31は、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、アルミニウムなどである。なお、正極リード31の形状は、薄板状および網目状などのうちのいずれかである。 [Positive lead]
1 and 2, thepositive electrode lead 31 is a positive electrode wiring connected to the positive electrode current collector 21A of the positive electrode 21, and is led out of the exterior film 10. The positive electrode lead 31 contains a conductive material such as a metal material, and a specific example of the conductive material is aluminum. The shape of the positive electrode lead 31 is either a thin plate shape or a mesh shape.
正極リード31は、図1および図2に示したように、正極21の正極集電体21Aに接続されている正極配線であり、外装フィルム10の外部に導出されている。この正極リード31は、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、アルミニウムなどである。なお、正極リード31の形状は、薄板状および網目状などのうちのいずれかである。 [Positive lead]
1 and 2, the
[負極リード]
負極リード32は、図1および図2に示したように、負極22の負極集電体22Aに接続されている負極配線であり、外装フィルム10の外部に導出されている。ここでは、負極リード32の導出方向は、正極リード31の導出方向と同様の方向である。この負極リード32は、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、銅などである。なお、負極リード32の形状に関する詳細は、正極リード31の形状に関する詳細と同様である。 [Negative lead]
As shown in Fig. 1 and Fig. 2, thenegative electrode lead 32 is a negative electrode wiring connected to the negative electrode current collector 22A of the negative electrode 22, and is led out of the exterior film 10. Here, the lead-out direction of the negative electrode lead 32 is the same as the lead-out direction of the positive electrode lead 31. This negative electrode lead 32 contains a conductive material such as a metal material, and a specific example of the conductive material is copper. The details of the shape of the negative electrode lead 32 are the same as the details of the shape of the positive electrode lead 31.
負極リード32は、図1および図2に示したように、負極22の負極集電体22Aに接続されている負極配線であり、外装フィルム10の外部に導出されている。ここでは、負極リード32の導出方向は、正極リード31の導出方向と同様の方向である。この負極リード32は、金属材料などの導電性材料を含んでおり、その導電性材料の具体例は、銅などである。なお、負極リード32の形状に関する詳細は、正極リード31の形状に関する詳細と同様である。 [Negative lead]
As shown in Fig. 1 and Fig. 2, the
[封止フィルム]
封止フィルム41は、外装フィルム10と正極リード31との間に挿入されていると共に、封止フィルム42は、外装フィルム10と負極リード32との間に挿入されている。ただし、封止フィルム41,42のうちの一方または双方は、省略されてもよい。 [Sealing film]
The sealingfilm 41 is inserted between the exterior film 10 and the positive electrode lead 31, and the sealing film 42 is inserted between the exterior film 10 and the negative electrode lead 32. However, one or both of the sealing films 41 and 42 may be omitted.
封止フィルム41は、外装フィルム10と正極リード31との間に挿入されていると共に、封止フィルム42は、外装フィルム10と負極リード32との間に挿入されている。ただし、封止フィルム41,42のうちの一方または双方は、省略されてもよい。 [Sealing film]
The sealing
封止フィルム41は、外装フィルム10の内部に外気などが侵入することを防止する封止部材である。この封止フィルム41は、正極リード31に対して密着性を有するポリオレフィンなどの高分子化合物を含んでおり、その高分子化合物の具体例は、ポリプロピレンなどである。
The sealing film 41 is a sealing member that prevents outside air and the like from entering the inside of the exterior film 10. This sealing film 41 contains a polymer compound such as polyolefin that has adhesion to the positive electrode lead 31, and a specific example of the polymer compound is polypropylene.
封止フィルム42の構成は、負極リード32に対して密着性を有する封止部材であることを除いて、封止フィルム41の構成と同様である。すなわち、封止フィルム42は、負極リード32に対して密着性を有するポリオレフィンなどの高分子化合物を含んでいる。
The configuration of the sealing film 42 is the same as that of the sealing film 41, except that the sealing film 42 is a sealing member that has adhesion to the negative electrode lead 32. In other words, the sealing film 42 contains a polymer compound such as polyolefin that has adhesion to the negative electrode lead 32.
<2-2.動作>
この二次電池は、電池素子20において、以下のように動作する。 <2-2. Operation>
This secondary battery operates in thebattery element 20 as follows.
この二次電池は、電池素子20において、以下のように動作する。 <2-2. Operation>
This secondary battery operates in the
充電時には、正極21からリチウムが放出されると共に、そのリチウムが電解液を介して負極22に吸蔵される。一方、放電時には、負極22からリチウムが放出されると共に、そのリチウムが電解液を介して正極21に吸蔵される。放電時および充電時のそれぞれでは、リチウムがイオン状態で吸蔵放出される。
When charging, lithium is released from the positive electrode 21 and is absorbed into the negative electrode 22 via the electrolyte. When discharging, lithium is released from the negative electrode 22 and is absorbed into the positive electrode 21 via the electrolyte. When discharging and charging, lithium is absorbed and released in an ionic state.
<2-3.製造方法>
二次電池を製造する場合には、以下で説明する一例の手順により、正極21および負極22のそれぞれを作製すると共に、電解液を調製したのち、その正極21、負極22および電解液を用いて二次電池を組み立てると共に、その組み立て後の二次電池の安定化処理を行う。 <2-3. Manufacturing method>
In the case of manufacturing a secondary battery, thepositive electrode 21 and the negative electrode 22 are each produced and an electrolyte solution is prepared according to the procedure described below. Then, the positive electrode 21, the negative electrode 22, and the electrolyte solution are used to manufacture the secondary battery. A secondary battery is assembled and a stabilization process is performed on the secondary battery after assembly.
二次電池を製造する場合には、以下で説明する一例の手順により、正極21および負極22のそれぞれを作製すると共に、電解液を調製したのち、その正極21、負極22および電解液を用いて二次電池を組み立てると共に、その組み立て後の二次電池の安定化処理を行う。 <2-3. Manufacturing method>
In the case of manufacturing a secondary battery, the
なお、電解液の製造方法に関しては既に説明したため、以下では、その電解液の製造方法に関する説明を省略する。
Because the method for producing the electrolyte has already been explained, the explanation of the method for producing the electrolyte will be omitted below.
[正極の作製]
最初に、正極活物質、正極結着剤および正極導電剤を互いに混合させることにより、正極合剤とする。続いて、溶媒に正極合剤を投入することにより、ペースト状の正極合剤スラリーを調製する。この溶媒は、水性溶媒でもよいし、有機溶剤でもよい。 [Preparation of Positive Electrode]
First, a positive electrode active material, a positive electrode binder, and a positive electrode conductive agent are mixed together to prepare a positive electrode mixture. Then, the positive electrode mixture is poured into a solvent to prepare a paste-like positive electrode mixture slurry. The solvent may be an aqueous solvent or an organic solvent.
最初に、正極活物質、正極結着剤および正極導電剤を互いに混合させることにより、正極合剤とする。続いて、溶媒に正極合剤を投入することにより、ペースト状の正極合剤スラリーを調製する。この溶媒は、水性溶媒でもよいし、有機溶剤でもよい。 [Preparation of Positive Electrode]
First, a positive electrode active material, a positive electrode binder, and a positive electrode conductive agent are mixed together to prepare a positive electrode mixture. Then, the positive electrode mixture is poured into a solvent to prepare a paste-like positive electrode mixture slurry. The solvent may be an aqueous solvent or an organic solvent.
続いて、正極集電体21Aの両面に正極合剤スラリーを塗布することにより、正極活物質層21Bを形成する。最後に、ロールプレス機などを用いて正極活物質層21Bを圧縮成形する。この場合には、正極活物質層21Bを加熱してもよいし、正極活物質層21Bの圧縮成形を複数回繰り返してもよい。これにより、正極集電体21Aの両面に正極活物質層21Bが形成されるため、正極21が作製される。
Then, the positive electrode active material layer 21B is formed by applying the positive electrode mixture slurry to both sides of the positive electrode current collector 21A. Finally, the positive electrode active material layer 21B is compression molded using a roll press or the like. In this case, the positive electrode active material layer 21B may be heated, or the compression molding of the positive electrode active material layer 21B may be repeated multiple times. In this way, the positive electrode active material layer 21B is formed on both sides of the positive electrode current collector 21A, and the positive electrode 21 is produced.
[負極の作製]
上記した正極21の作製手順と同様の手順により、負極22を形成する。具体的には、最初に、負極活物質、負極結着剤および負極導電剤が互いに混合された混合物(負極合剤)を溶媒に投入することにより、ペースト状の負極合剤スラリーを調製する。溶媒に関する詳細は、上記した通りである。続いて、負極集電体22Aの両面に負極合剤スラリーを塗布することにより、負極活物質層22Bを形成する。最後に、負極活物質層22Bを圧縮成形する。これにより、負極集電体22Aの両面に負極活物質層22Bが形成されるため、負極22が作製される。 [Preparation of negative electrode]
Thenegative electrode 22 is formed by the same procedure as the procedure for producing the positive electrode 21 described above. Specifically, first, a mixture (negative electrode mixture) in which the negative electrode active material, the negative electrode binder, and the negative electrode conductive agent are mixed together is put into a solvent to prepare a paste-like negative electrode mixture slurry. Details regarding the solvent are as described above. Next, the negative electrode mixture slurry is applied to both sides of the negative electrode current collector 22A to form the negative electrode active material layer 22B. Finally, the negative electrode active material layer 22B is compression molded. As a result, the negative electrode active material layer 22B is formed on both sides of the negative electrode current collector 22A, and the negative electrode 22 is produced.
上記した正極21の作製手順と同様の手順により、負極22を形成する。具体的には、最初に、負極活物質、負極結着剤および負極導電剤が互いに混合された混合物(負極合剤)を溶媒に投入することにより、ペースト状の負極合剤スラリーを調製する。溶媒に関する詳細は、上記した通りである。続いて、負極集電体22Aの両面に負極合剤スラリーを塗布することにより、負極活物質層22Bを形成する。最後に、負極活物質層22Bを圧縮成形する。これにより、負極集電体22Aの両面に負極活物質層22Bが形成されるため、負極22が作製される。 [Preparation of negative electrode]
The
[二次電池の組み立て]
最初に、溶接法などの接合法を用いて、正極21の正極集電体21Aに正極リード31を接続させると共に、溶接法などの接合法を用いて、負極22の負極集電体22Aに負極リード32を接続させる。 [Assembly of secondary battery]
First, thepositive electrode lead 31 is connected to the positive electrode collector 21A of the positive electrode 21 using a joining method such as welding, and the negative electrode lead 32 is connected to the negative electrode collector 22A of the negative electrode 22 using a joining method such as welding.
最初に、溶接法などの接合法を用いて、正極21の正極集電体21Aに正極リード31を接続させると共に、溶接法などの接合法を用いて、負極22の負極集電体22Aに負極リード32を接続させる。 [Assembly of secondary battery]
First, the
続いて、セパレータ23を介して正極21および負極22を互いに積層させることにより、積層体(図示せず)を形成する。続いて、積層体を巻回させることにより、巻回体(図示せず)を作製したのち、プレス機などを用いて巻回体を押圧することにより、扁平形状となるように巻回体を成形する。この成形後の巻回体は、電解液が含浸されていないことを除いて、電池素子20の構成と同様の構成を有している。
Then, the positive electrode 21 and the negative electrode 22 are stacked on top of each other with the separator 23 interposed therebetween to form a laminate (not shown). The laminate is then wound to produce a wound body (not shown), which is then pressed using a press or the like to form the wound body into a flat shape. The wound body after this formation has a configuration similar to that of the battery element 20, except that it is not impregnated with the electrolyte.
続いて、窪み部10Uの内部に巻回体を収容したのち、外装フィルム10(融着層/金属層/表面保護層)を折り畳むことにより、その外装フィルム10同士を互いに対向させる。続いて、熱融着法などの接着法を用いて、互いに対向する融着層のうちの2辺の外周縁部同士を互いに接合させることにより、袋状の外装フィルム10の内部に巻回体を収納する。
Then, after the roll is placed inside the recess 10U, the exterior film 10 (adhesive layer/metal layer/surface protection layer) is folded so that the exterior films 10 face each other. Next, the outer edges of two of the opposing adhesive layers are joined to each other using an adhesive method such as heat fusion, thereby placing the roll inside the bag-shaped exterior film 10.
最後に、袋状の外装フィルム10に電解液を注入したのち、熱融着法などの接着法を用いて、互いに対向する融着層のうちの残りの1辺の外周縁部同士を互いに接合させる。この場合には、外装フィルム10と正極リード31との間に封止フィルム41を挿入すると
共に、外装フィルム10と負極リード32との間に封止フィルム42を挿入する。 Finally, after injecting an electrolyte solution into the bag-shapedexterior film 10, the outer peripheral edges of the remaining sides of the opposing fusion layers are joined to each other by using an adhesive method such as a heat fusion method. In this case, a sealing film 41 is inserted between the exterior film 10 and the positive electrode lead 31, and a sealing film 42 is inserted between the exterior film 10 and the negative electrode lead 32.
共に、外装フィルム10と負極リード32との間に封止フィルム42を挿入する。 Finally, after injecting an electrolyte solution into the bag-shaped
これにより、巻回体に電解液が含浸されるため、電池素子20が作製される。よって、袋状の外装フィルム10の内部に電池素子20が封入されるため、二次電池が組み立てられる。
As a result, the wound body is impregnated with the electrolyte, producing the battery element 20. The battery element 20 is then enclosed inside the bag-shaped exterior film 10, and a secondary battery is assembled.
[組み立て後の二次電池の安定化処理]
組み立て後の二次電池を充放電させる。環境温度、充放電回数(サイクル数)および充放電条件などの安定化条件は、任意に設定可能である。これにより、正極21および負極22のそれぞれの表面に被膜が形成されるため、電池素子20の状態が電気化学的に安定化する。よって、二次電池が完成する。 [Stabilization treatment of secondary battery after assembly]
The assembled secondary battery is charged and discharged. Stabilization conditions such as the environmental temperature, the number of charge/discharge cycles (number of cycles), and charge/discharge conditions can be set arbitrarily. As a result, a coating is formed on the surface of each of thepositive electrode 21 and the negative electrode 22, and the state of the battery element 20 is electrochemically stabilized. Thus, the secondary battery is completed.
組み立て後の二次電池を充放電させる。環境温度、充放電回数(サイクル数)および充放電条件などの安定化条件は、任意に設定可能である。これにより、正極21および負極22のそれぞれの表面に被膜が形成されるため、電池素子20の状態が電気化学的に安定化する。よって、二次電池が完成する。 [Stabilization treatment of secondary battery after assembly]
The assembled secondary battery is charged and discharged. Stabilization conditions such as the environmental temperature, the number of charge/discharge cycles (number of cycles), and charge/discharge conditions can be set arbitrarily. As a result, a coating is formed on the surface of each of the
<2-4.作用および効果>
この二次電池によれば、その二次電池が電解液を備えており、その電解液が上記した構成を有している。よって、上記した理由により、リチウムイオンの移動速度が担保されながら電解液の分解反応が著しく抑制されるため、優れた電池特性を得ることができる。 <2-4. Actions and Effects>
According to this secondary battery, the secondary battery includes an electrolyte, and the electrolyte has the above-mentioned configuration, and therefore, for the above-mentioned reasons, the decomposition reaction of the electrolyte is significantly suppressed while the lithium ion migration speed is guaranteed, and therefore excellent battery characteristics can be obtained.
この二次電池によれば、その二次電池が電解液を備えており、その電解液が上記した構成を有している。よって、上記した理由により、リチウムイオンの移動速度が担保されながら電解液の分解反応が著しく抑制されるため、優れた電池特性を得ることができる。 <2-4. Actions and Effects>
According to this secondary battery, the secondary battery includes an electrolyte, and the electrolyte has the above-mentioned configuration, and therefore, for the above-mentioned reasons, the decomposition reaction of the electrolyte is significantly suppressed while the lithium ion migration speed is guaranteed, and therefore excellent battery characteristics can be obtained.
特に、二次電池がリチウムイオン二次電池であれば、リチウムの吸蔵放出を利用して十分な電池容量が安定に得られるため、より高い効果を得ることができる。
In particular, if the secondary battery is a lithium-ion secondary battery, sufficient battery capacity can be stably obtained by utilizing the absorption and release of lithium, resulting in even greater effects.
この二次電池に関する他の作用および効果は、上記した電解液に関する他の作用および効果と同様である。
Other functions and effects of this secondary battery are similar to those of the electrolyte described above.
<3.変形例>
二次電池の構成は、以下で説明するように、適宜、変更可能である。ただし、以下で説明する一連の変形例は、互いに組み合わされてもよい。 3. Modifications
The configuration of the secondary battery can be modified as appropriate, as described below, although the series of modifications described below may be combined with each other.
二次電池の構成は、以下で説明するように、適宜、変更可能である。ただし、以下で説明する一連の変形例は、互いに組み合わされてもよい。 3. Modifications
The configuration of the secondary battery can be modified as appropriate, as described below, although the series of modifications described below may be combined with each other.
[変形例1]
上記したように、電解液は、イミドアニオンを含む電解質塩と共に、他の電解質塩を含んでいてもよい。 [Modification 1]
As described above, the electrolyte solution may contain other electrolyte salts in addition to the electrolyte salt containing the imide anion.
上記したように、電解液は、イミドアニオンを含む電解質塩と共に、他の電解質塩を含んでいてもよい。 [Modification 1]
As described above, the electrolyte solution may contain other electrolyte salts in addition to the electrolyte salt containing the imide anion.
中でも、電解液は、他の電解質塩として六フッ化リン酸リチウムを含んでいると共に、その電解液における電解質塩の含有量は、その電解液における他の電解質塩の含有量との関係において適正化されていることが好ましい。
In particular, it is preferable that the electrolyte solution contains lithium hexafluorophosphate as another electrolyte salt, and that the content of the electrolyte salt in the electrolyte solution is optimized in relation to the content of the other electrolyte salt in the electrolyte solution.
具体的には、電解質塩は、カチオンおよびイミドアニオンを含んでいる。また、六フッ化リン酸イオンは、リチウムイオンおよび六フッ化リン酸イオンを含んでいる。
Specifically, the electrolyte salt contains a cation and an imide anion. Also, the hexafluorophosphate ion contains a lithium ion and a hexafluorophosphate ion.
この場合において、電解液におけるカチオンの含有量C1と、その電解液におけるリチウムイオンの含有量C2との和T(mol/kg)は、0.7mol/kg~2.2mol/kgであることが好ましい。また、電解液におけるイミドアニオンのモル数M1に対する、その電解液における六フッ化リン酸イオンのモル数M2の割合R(mol%)は、13mol%~6000mol%であることが好ましい。正極21および負極22のそれぞれの表面近傍においてカチオンおよびリチウムイオンのそれぞれの移動速度が十分に向上すると共に、電解液の液中においてもカチオンおよびリチウムイオンのそれぞれの移動速度が十分に向上するからである。
In this case, the sum T (mol/kg) of the cation content C1 in the electrolyte and the lithium ion content C2 in the electrolyte is preferably 0.7 mol/kg to 2.2 mol/kg. In addition, the ratio R (mol%) of the number of moles M2 of hexafluorophosphate ions in the electrolyte to the number of moles M1 of imide anions in the electrolyte is preferably 13 mol% to 6000 mol%. This is because the migration speeds of the cations and lithium ions in the vicinity of the surfaces of the positive electrode 21 and the negative electrode 22 are sufficiently improved, and the migration speeds of the cations and lithium ions in the electrolyte are also sufficiently improved.
ここで説明した「電解液におけるカチオンの含有量」は、溶媒に対するカチオンの電解質塩の含有量であると共に、「電解液におけるリチウムイオンの含有量」は、溶媒に対するリチウムイオンの含有量である。なお、和Tは、T=C1+C2という計算式に基づいて算出されると共に、割合Rは、R=(M2/M1)×100という計算式に基づいて算出される。
The "content of cations in the electrolyte" described here is the content of electrolyte salt of cations relative to the solvent, and the "content of lithium ions in the electrolyte" is the content of lithium ions relative to the solvent. The sum T is calculated based on the formula T = C1 + C2, and the ratio R is calculated based on the formula R = (M2/M1) x 100.
和Tおよび割合Rのそれぞれを算出する場合には、二次電池を解体することにより、電解液を回収したのち、ICP発光分光分析法を用いて電解液を分析する。これにより、含有量C1,C2およびモル数M1,M2のそれぞれが特定されるため、和Tおよび割合Rのそれぞれが算出される。
When calculating the sum T and the ratio R, the secondary battery is disassembled to recover the electrolyte, and the electrolyte is then analyzed using ICP atomic emission spectrometry. This allows the contents C1, C2 and the mole numbers M1, M2 to be determined, and the sum T and the ratio R to be calculated.
この場合においても、電解液が電解質塩を含んでいるため、同様の効果を得ることができる。この場合には、特に、電解質塩と他の電解質塩(六フッ化リン酸リチウム)とを併用した場合において、両者の総量(和T)が適正化されると共に、両者の混合比(割合R)も適正化される。これにより、正極21および負極22のそれぞれの表面近傍においてカチオンおよびリチウムイオンのそれぞれの移動速度がさらに向上すると共に、電解液の液中においてもカチオンおよびリチウムイオンのそれぞれの移動速度がさらに向上する。よって、より高い効果を得ることができる。
In this case, the electrolyte solution contains electrolyte salt, and therefore the same effect can be obtained. In this case, particularly when the electrolyte salt is used in combination with another electrolyte salt (lithium hexafluorophosphate), the total amount of both (sum T) is optimized, and the mixing ratio (ratio R) of both is also optimized. This further improves the migration speed of cations and lithium ions near the surfaces of the positive electrode 21 and the negative electrode 22, and also further improves the migration speed of cations and lithium ions in the electrolyte solution. Therefore, a greater effect can be obtained.
[変形例2]
変形例1において、電解液は、他の電解質塩として六フッ化リン酸リチウムを含んでいる。しかしながら、電解液は、他の電解質塩として六フッ化リン酸リチウムの代わりにビス(フルオロスルホニル)イミドリチウムを含んでいてもよい。この場合においても、電解液における電解質塩の含有量は、その電解液における他の電解質塩の含有量との関係において適正化されていることが好ましい。 [Modification 2]
In the first modification, the electrolyte solution contains lithium hexafluorophosphate as another electrolyte salt. However, the electrolyte solution may contain lithium bis(fluorosulfonyl)imide as another electrolyte salt instead of lithium hexafluorophosphate. Even in this case, it is preferable that the content of the electrolyte salt in the electrolyte solution is optimized in relation to the content of the other electrolyte salt in the electrolyte solution.
変形例1において、電解液は、他の電解質塩として六フッ化リン酸リチウムを含んでいる。しかしながら、電解液は、他の電解質塩として六フッ化リン酸リチウムの代わりにビス(フルオロスルホニル)イミドリチウムを含んでいてもよい。この場合においても、電解液における電解質塩の含有量は、その電解液における他の電解質塩の含有量との関係において適正化されていることが好ましい。 [Modification 2]
In the first modification, the electrolyte solution contains lithium hexafluorophosphate as another electrolyte salt. However, the electrolyte solution may contain lithium bis(fluorosulfonyl)imide as another electrolyte salt instead of lithium hexafluorophosphate. Even in this case, it is preferable that the content of the electrolyte salt in the electrolyte solution is optimized in relation to the content of the other electrolyte salt in the electrolyte solution.
具体的には、ビス(フルオロスルホニル)イミドリチウムは、リチウムイオンおよびビス(フルオロスルホニル)イミドイオンを含んでいる。この場合において、電解液におけるカチオンの含有量C1と、その電解液におけるリチウムイオンの含有量C2との和T(mol/kg)は、0.7mol/kg~2.2mol/kgであることが好ましい。また、電解液におけるイミドアニオンのモル数M1に対する、その電解液におけるビス(フルオロスルホニル)イミドイオンのモル数M3の割合R(mol%)は、13mol%~6000mol%であることが好ましい。正極21および負極22のそれぞれの表面近傍においてカチオンおよびリチウムイオンのそれぞれの移動速度が十分に向上すると共に、電解液の液中においてもカチオンおよびリチウムイオンのそれぞれの移動速度が十分に向上するからである。
Specifically, the bis(fluorosulfonyl)imide lithium contains lithium ions and bis(fluorosulfonyl)imide ions. In this case, the sum T (mol/kg) of the cation content C1 in the electrolyte and the lithium ion content C2 in the electrolyte is preferably 0.7 mol/kg to 2.2 mol/kg. In addition, the ratio R (mol%) of the number of moles M3 of bis(fluorosulfonyl)imide ions in the electrolyte to the number of moles M1 of imide anions in the electrolyte is preferably 13 mol% to 6000 mol%. This is because the migration speeds of the cations and lithium ions are sufficiently improved near the surfaces of the positive electrode 21 and the negative electrode 22, and the migration speeds of the cations and lithium ions are also sufficiently improved in the electrolyte.
なお、割合Rは、R=(M3/M1)×100という計算式に基づいて算出される。割合Rを算出する手順は、モル数M2の代わりにモル数M3を特定することを除いて、上記した通りである。
The ratio R is calculated based on the formula R = (M3/M1) x 100. The procedure for calculating the ratio R is as described above, except that the number of moles M3 is specified instead of the number of moles M2.
この場合においても、電解液が電解質塩を含んでいるため、同様の効果を得ることができる。この場合には、特に、電解質塩と他の電解質塩(ビス(フルオロスルホニル)イミドリチウム)とを併用した場合において、両者の総量(和T)が適正化されると共に、両者の混合比(割合R)も適正化される。これにより、正極21および負極22のそれぞれの表面近傍においてカチオンおよびリチウムイオンのそれぞれの移動速度がさらに向上すると共に、電解液の液中においてもカチオンおよびリチウムイオンのそれぞれの移動速度がさらに向上する。よって、より高い効果を得ることができる。
In this case, the electrolyte solution contains an electrolyte salt, and therefore the same effect can be obtained. In this case, particularly when the electrolyte salt is used in combination with another electrolyte salt (lithium bis(fluorosulfonyl)imide), the total amount (sum T) of the two is optimized, and the mixing ratio (ratio R) of the two is also optimized. This further improves the migration speeds of the cations and lithium ions near the surfaces of the positive electrode 21 and the negative electrode 22, and also further improves the migration speeds of the cations and lithium ions in the electrolyte solution. Therefore, a greater effect can be obtained.
[変形例3]
多孔質膜であるセパレータ23を用いた。しかしながら、ここでは具体的に図示しないが、多孔質膜であるセパレータ23の代わりに、高分子化合物層を含む積層型のセパレータを用いてもよい。 [Modification 3]
Aporous membrane separator 23 was used. However, although not specifically shown here, a laminated separator including a polymer compound layer may be used instead of the porous membrane separator 23.
多孔質膜であるセパレータ23を用いた。しかしながら、ここでは具体的に図示しないが、多孔質膜であるセパレータ23の代わりに、高分子化合物層を含む積層型のセパレータを用いてもよい。 [Modification 3]
A
具体的には、積層型のセパレータは、一対の面を有する多孔質膜と、その多孔質膜の片面または両面に設けられた高分子化合物層とを含んでいる。正極21および負極22のそれぞれに対するセパレータの密着性が向上するため、電池素子20の位置ずれが抑制されるからである。これにより、正極21、負極22およびセパレータのそれぞれの巻きずれが抑制されるため、電解液の分解反応が発生しても二次電池の膨れが抑制される。高分子化合物層は、ポリフッ化ビニリデンなどを含んでいる。ポリフッ化ビニリデンは、物理的強度に優れていると共に、電気化学的に安定だからである。
Specifically, the laminated separator includes a porous membrane having a pair of surfaces, and a polymer compound layer provided on one or both surfaces of the porous membrane. This is because the adhesion of the separator to each of the positive electrode 21 and the negative electrode 22 is improved, thereby suppressing misalignment of the battery element 20. This suppresses miswinding of the positive electrode 21, the negative electrode 22, and the separator, thereby suppressing swelling of the secondary battery even if a decomposition reaction of the electrolyte occurs. The polymer compound layer includes polyvinylidene fluoride, etc. This is because polyvinylidene fluoride has excellent physical strength and is electrochemically stable.
なお、多孔質膜および高分子化合物層のうちの一方または双方は、複数の絶縁性粒子のうちのいずれか1種類または2種類以上を含んでいてもよい。二次電池の発熱時において複数の絶縁性粒子が放熱するため、その二次電池の安全性(耐熱性)が向上するからである。複数の絶縁性粒子は、無機材料および樹脂材料などの絶縁性材料のうちのいずれか1種類または2種類以上を含んでいる。無機材料の具体例は、酸化アルミニウム、窒化アルミニウム、ベーマイト、酸化ケイ素、酸化チタン、酸化マグネシウムおよび酸化ジルコニウムなどである。樹脂材料の具体例は、アクリル樹脂およびスチレン樹脂などである。
In addition, one or both of the porous film and the polymer compound layer may contain one or more types of insulating particles. This is because the insulating particles dissipate heat when the secondary battery generates heat, improving the safety (heat resistance) of the secondary battery. The insulating particles contain one or more types of insulating materials such as inorganic materials and resin materials. Specific examples of inorganic materials include aluminum oxide, aluminum nitride, boehmite, silicon oxide, titanium oxide, magnesium oxide, and zirconium oxide. Specific examples of resin materials include acrylic resin and styrene resin.
積層型のセパレータを作製する場合には、高分子化合物および有機溶剤などを含む前駆溶液を調製したのち、多孔質膜の片面または両面に前駆溶液を塗布する。この場合には、前駆溶液中に複数の絶縁性粒子を含有させてもよい。
When making a laminated separator, a precursor solution containing a polymer compound and an organic solvent is prepared, and then the precursor solution is applied to one or both sides of a porous film. In this case, the precursor solution may contain multiple insulating particles.
この積層型のセパレータを用いた場合においても、正極21と負極22との間においてリチウムがイオン状態で移動可能になるため、同様の効果を得ることができる。この場合には、特に、上記したように、二次電池の膨れが抑制されるため、より高い効果を得ることができる。
Even when this laminated separator is used, the lithium can move in an ionic state between the positive electrode 21 and the negative electrode 22, so the same effect can be obtained. In this case, as described above, swelling of the secondary battery is particularly suppressed, so a greater effect can be obtained.
[変形例4]
液状の電解質である電解液を用いた。しかしながら、ここでは具体的に図示しないが、ゲル状の電解質である電解質層を用いてもよい。 [Modification 4]
An electrolyte solution that is a liquid electrolyte is used, but an electrolyte layer that is a gel electrolyte may also be used, although this is not specifically shown.
液状の電解質である電解液を用いた。しかしながら、ここでは具体的に図示しないが、ゲル状の電解質である電解質層を用いてもよい。 [Modification 4]
An electrolyte solution that is a liquid electrolyte is used, but an electrolyte layer that is a gel electrolyte may also be used, although this is not specifically shown.
電解質層を用いた電池素子20では、正極21および負極22がセパレータ23および電解質層を介して互いに対向しながら巻回されている。この電解質層は、正極21とセパレータ23との間に介在していると共に、負極22とセパレータ23との間に介在している。
In a battery element 20 using an electrolyte layer, a positive electrode 21 and a negative electrode 22 are wound facing each other with a separator 23 and an electrolyte layer interposed between them. The electrolyte layer is interposed between the positive electrode 21 and the separator 23, and also between the negative electrode 22 and the separator 23.
具体的には、電解質層は、電解液と共に高分子化合物を含んでおり、その電解液は、高分子化合物により保持されている。電解液の漏液が防止されるからである。電解液の構成は、上記した通りである。高分子化合物は、ポリフッ化ビニリデンなどを含んでいる。電解質層を形成する場合には、電解液、高分子化合物および溶媒などを含む前駆溶液を調製したのち、正極21および負極22のそれぞれの片面または両面に前駆溶液を塗布する。
Specifically, the electrolyte layer contains a polymer compound together with an electrolyte solution, and the electrolyte solution is held by the polymer compound. This is because leakage of the electrolyte solution is prevented. The composition of the electrolyte solution is as described above. The polymer compound contains polyvinylidene fluoride and the like. When forming the electrolyte layer, a precursor solution containing an electrolyte solution, a polymer compound, a solvent, and the like is prepared, and then the precursor solution is applied to one or both sides of each of the positive electrode 21 and the negative electrode 22.
この電解質層を用いた場合においても、正極21と負極22との間において電解質層を介してリチウムイオンが移動可能になるため、同様の効果を得ることができる。この場合には、特に、上記したように、電解液の漏液が防止されるため、より高い効果を得ることができる。
Even when this electrolyte layer is used, the lithium ions can move between the positive electrode 21 and the negative electrode 22 via the electrolyte layer, so the same effect can be obtained. In this case, leakage of the electrolyte is particularly prevented as described above, so a greater effect can be obtained.
<4.二次電池の用途>
最後に、二次電池の用途(適用例)に関して説明する。 <4. Uses of secondary batteries>
Finally, uses (application examples) of the secondary battery will be described.
最後に、二次電池の用途(適用例)に関して説明する。 <4. Uses of secondary batteries>
Finally, uses (application examples) of the secondary battery will be described.
二次電池の用途は、特に限定されない。電源として用いられる二次電池は、電子機器および電動車両などにおいて、主電源でもよいし、補助電源でもよい。主電源とは、他の電源の有無に関係なく、優先的に用いられる電源である。補助電源は、主電源の代わりに用いられる電源でもよいし、主電源から切り替えられる電源でもよい。
The use of the secondary battery is not particularly limited. A secondary battery used as a power source may be a main power source or an auxiliary power source in electronic devices and electric vehicles. A main power source is a power source that is used preferentially regardless of the presence or absence of other power sources. An auxiliary power source may be a power source used in place of the main power source or a power source that can be switched from the main power source.
二次電池の用途の具体例は、以下で説明する通りである。ビデオカメラ、デジタルスチルカメラ、携帯電話機、ノート型パソコン、ヘッドホンステレオ、携帯用ラジオおよび携帯用情報端末などの電子機器である。バックアップ電源およびメモリーカードなどの記憶用装置である。電動ドリルおよび電動鋸などの電動工具である。電子機器などに搭載される電池パックである。ペースメーカおよび補聴器などの医療用電子機器である。電気自動車(ハイブリッド自動車を含む。)などの電動車両である。非常時などに備えて電力を蓄積しておく家庭用または産業用のバッテリシステムなどの電力貯蔵システムである。これらの用途では、1個の二次電池が用いられてもよいし、複数個の二次電池が用いられてもよい。
Specific examples of uses for secondary batteries are as follows: Electronic devices such as video cameras, digital still cameras, mobile phones, notebook computers, headphone stereos, portable radios, and portable information terminals. Storage devices such as backup power sources and memory cards. Power tools such as electric drills and power saws. Battery packs installed in electronic devices. Medical electronic devices such as pacemakers and hearing aids. Electric vehicles such as electric cars (including hybrid cars). Power storage systems such as home or industrial battery systems that store power in preparation for emergencies. In these applications, one secondary battery may be used, or multiple secondary batteries may be used.
電池パックは、単電池を備えていてもよいし、組電池を備えていてもよい。電動車両は、駆動用電源として二次電池を用いて走行する車両であり、その二次電池以外の駆動源を併せて備えたハイブリッド自動車でもよい。家庭用の電力貯蔵システムでは、電力貯蔵源である二次電池に蓄積された電力を利用して家庭用の電気製品などを使用可能である。
The battery pack may include a single cell or a battery pack. The electric vehicle is a vehicle that runs on a secondary battery as a driving power source, and may be a hybrid vehicle that also includes a driving source other than the secondary battery. In a home power storage system, it is possible to use home electrical appliances and the like by utilizing the power stored in the secondary battery, which is a power storage source.
ここで、二次電池の用途の一例に関して具体的に説明する。以下で説明する構成は、あくまで一例であるため、適宜、変更可能である。
Here, we will specifically explain an example of a use of a secondary battery. The configuration described below is merely an example and can be modified as appropriate.
図3は、二次電池の適用例である電池パックのブロック構成を表している。ここで説明する電池パックは、1個の二次電池を用いた電池パック(いわゆるソフトパック)であり、スマートフォンに代表される電子機器などに搭載される。
Figure 3 shows the block diagram of a battery pack, which is an example of an application of a secondary battery. The battery pack described here is a battery pack (a so-called soft pack) that uses one secondary battery, and is installed in electronic devices such as smartphones.
この電池パックは、図3に示したように、電源51と、回路基板52とを備えている。この回路基板52は、電源51に接続されていると共に、正極端子53、負極端子54および温度検出端子55を含んでいる。
As shown in FIG. 3, this battery pack includes a power source 51 and a circuit board 52. This circuit board 52 is connected to the power source 51 and includes a positive terminal 53, a negative terminal 54, and a temperature detection terminal 55.
電源51は、1個の二次電池を含んでいる。この二次電池では、正極リードが正極端子53に接続されていると共に、負極リードが負極端子54に接続されている。この電源51は、正極端子53および負極端子54を介して外部と接続されるため、充放電可能である。回路基板52は、制御部56と、スイッチ57と、熱感抵抗素子であるPTC素子58と、温度検出部59とを含んでいる。ただし、PTC素子58は省略されてもよい。
The power source 51 includes one secondary battery. In this secondary battery, the positive electrode lead is connected to the positive electrode terminal 53, and the negative electrode lead is connected to the negative electrode terminal 54. This power source 51 is connected to the outside via the positive electrode terminal 53 and the negative electrode terminal 54, and is therefore capable of charging and discharging. The circuit board 52 includes a control unit 56, a switch 57, a PTC element 58 which is a thermosensitive resistor, and a temperature detection unit 59. However, the PTC element 58 may be omitted.
制御部56は、中央演算処理装置(CPU)およびメモリなどを含んでおり、電池パック全体の動作を制御する。この制御部56は、電源51の使用状態に関する検出および制御などを行う。
The control unit 56 includes a central processing unit (CPU) and memory, and controls the operation of the entire battery pack. This control unit 56 detects and controls the usage status of the power source 51.
なお、制御部56は、電源51(二次電池)の電圧が過充電検出電圧または過放電検出電圧に到達すると、スイッチ57を切断することにより、その電源51の電流経路に充電電流が流れないようにする。過充電検出電圧は、特に限定されないが、具体的には、4.20V±0.05Vであると共に、過放電検出電圧は、特に限定されないが、具体的には、2.40V±0.10Vである。
When the voltage of the power source 51 (secondary battery) reaches the overcharge detection voltage or overdischarge detection voltage, the control unit 56 turns off the switch 57 to prevent charging current from flowing through the current path of the power source 51. The overcharge detection voltage is not particularly limited, but is specifically 4.20V±0.05V, and the overdischarge detection voltage is not particularly limited, but is specifically 2.40V±0.10V.
スイッチ57は、充電制御スイッチ、放電制御スイッチ、充電用ダイオードおよび放電用ダイオードなどを含んでおり、制御部56の指示に応じて電源51と外部機器との接続の有無を切り換える。このスイッチ57は、金属酸化物半導体を用いた電界効果トランジスタ(MOSFET)などを含んでおり、充電電流および放電電流のそれぞれは、スイッチ57のON抵抗に基づいて検出される。
Switch 57 includes a charge control switch, a discharge control switch, a charge diode, and a discharge diode, and switches between the presence and absence of a connection between power source 51 and an external device in response to an instruction from control unit 56. Switch 57 includes a field effect transistor (MOSFET) that uses a metal oxide semiconductor, and the charge current and discharge current are each detected based on the ON resistance of switch 57.
温度検出部59は、サーミスタなどの温度検出素子を含んでいる。この温度検出部59は、温度検出端子55を用いて電源51の温度を測定すると共に、その温度の測定結果を制御部56に出力する。温度検出部59により測定された温度の測定結果は、異常発熱時において制御部56が充放電制御を行う場合および残容量の算出時において制御部56が補正処理を行う場合などに用いられる。
The temperature detection unit 59 includes a temperature detection element such as a thermistor. This temperature detection unit 59 measures the temperature of the power supply 51 using the temperature detection terminal 55, and outputs the temperature measurement result to the control unit 56. The temperature measurement result measured by the temperature detection unit 59 is used when the control unit 56 performs charge/discharge control in the event of abnormal heat generation, and when the control unit 56 performs correction processing when calculating the remaining capacity.
本技術の実施例に関して説明する。
We will explain an example of this technology.
<実施例1~18および比較例1~3>
以下で説明するように、二次電池を製造したのち、その二次電池の電池特性を評価した。 <Examples 1 to 18 and Comparative Examples 1 to 3>
As described below, after the secondary batteries were manufactured, the battery characteristics of the secondary batteries were evaluated.
以下で説明するように、二次電池を製造したのち、その二次電池の電池特性を評価した。 <Examples 1 to 18 and Comparative Examples 1 to 3>
As described below, after the secondary batteries were manufactured, the battery characteristics of the secondary batteries were evaluated.
[二次電池の作製]
以下の手順により、図1および図2に示したラミネートフィルム型の二次電池(リチウムイオン二次電池)を作製した。 [Preparation of secondary battery]
A laminate film type secondary battery (lithium ion secondary battery) shown in FIGS. 1 and 2 was fabricated by the following procedure.
以下の手順により、図1および図2に示したラミネートフィルム型の二次電池(リチウムイオン二次電池)を作製した。 [Preparation of secondary battery]
A laminate film type secondary battery (lithium ion secondary battery) shown in FIGS. 1 and 2 was fabricated by the following procedure.
(正極の作製)
最初に、正極活物質(リチウム含有化合物(酸化物)であるLiNi0.82Co0.14Al0.04O2 )91質量部と、正極結着剤(ポリフッ化ビニリデン)3質量部と、正極導電剤(カーボンブラック)6質量部とを互いに混合させることにより、正極合剤とした。続いて、溶媒(有機溶剤であるN-メチル-2-ピロリドン)に正極合剤を投入したのち、その有機溶剤を撹拌することにより、ペースト状の正極合剤スラリーを調製した。続いて、コーティング装置を用いて正極集電体21A(厚さ=12μmである帯状のアルミニウム箔)の両面に正極合剤スラリーを塗布したのち、その正極合剤スラリーを乾燥させることにより、正極活物質層21Bを形成した。最後に、ロールプレス機を用いて正極活物質層21Bを圧縮成形した。これにより、正極21が作製された。 (Preparation of Positive Electrode)
First, 91 parts by mass of a positive electrode active material (LiNi 0.82 Co 0.14 Al 0.04 O 2 which is a lithium-containing compound (oxide), 3 parts by mass of a positive electrode binder (polyvinylidene fluoride), and 6 parts by mass of a positive electrode conductive agent (carbon black) were mixed together to prepare a positive electrode mixture. Next, the positive electrode mixture was put into a solvent (N-methyl-2-pyrrolidone which is an organic solvent), and the organic solvent was stirred to prepare a paste-like positive electrode mixture slurry. Next, the positive electrode mixture slurry was applied to both sides of a positive electrodecurrent collector 21A (a strip-shaped aluminum foil having a thickness of 12 μm) using a coating device, and then the positive electrode mixture slurry was dried to form a positive electrode active material layer 21B. Finally, the positive electrode active material layer 21B was compression-molded using a roll press machine. As a result, the positive electrode 21 was produced.
最初に、正極活物質(リチウム含有化合物(酸化物)であるLiNi0.82Co0.14Al0.04O2 )91質量部と、正極結着剤(ポリフッ化ビニリデン)3質量部と、正極導電剤(カーボンブラック)6質量部とを互いに混合させることにより、正極合剤とした。続いて、溶媒(有機溶剤であるN-メチル-2-ピロリドン)に正極合剤を投入したのち、その有機溶剤を撹拌することにより、ペースト状の正極合剤スラリーを調製した。続いて、コーティング装置を用いて正極集電体21A(厚さ=12μmである帯状のアルミニウム箔)の両面に正極合剤スラリーを塗布したのち、その正極合剤スラリーを乾燥させることにより、正極活物質層21Bを形成した。最後に、ロールプレス機を用いて正極活物質層21Bを圧縮成形した。これにより、正極21が作製された。 (Preparation of Positive Electrode)
First, 91 parts by mass of a positive electrode active material (LiNi 0.82 Co 0.14 Al 0.04 O 2 which is a lithium-containing compound (oxide), 3 parts by mass of a positive electrode binder (polyvinylidene fluoride), and 6 parts by mass of a positive electrode conductive agent (carbon black) were mixed together to prepare a positive electrode mixture. Next, the positive electrode mixture was put into a solvent (N-methyl-2-pyrrolidone which is an organic solvent), and the organic solvent was stirred to prepare a paste-like positive electrode mixture slurry. Next, the positive electrode mixture slurry was applied to both sides of a positive electrode
(負極の作製)
最初に、負極活物質(炭素材料である人造黒鉛)93質量部と、負極結着剤(ポリフッ化ビニリデン)7質量部とを互いに混合させることにより、負極合剤とした。続いて、溶媒(有機溶剤であるN-メチル-2-ピロリドン)に負極合剤を投入したのち、その有機溶剤を撹拌することにより、ペースト状の負極合剤スラリーを調製した。続いて、コーティング装置を用いて負極集電体22A(厚さ=15μmである帯状の銅箔)の両面に負極合剤スラリーを塗布したのち、その負極合剤スラリーを乾燥させることにより、負極活物質層22Bを形成した。最後に、ロールプレス機を用いて負極活物質層22Bを圧縮成形した。これにより、負極22が作製された。 (Preparation of negative electrode)
First, 93 parts by mass of the negative electrode active material (artificial graphite, which is a carbon material) and 7 parts by mass of the negative electrode binder (polyvinylidene fluoride) were mixed together to prepare a negative electrode mixture. Next, the negative electrode mixture was added to a solvent (N-methyl-2-pyrrolidone, which is an organic solvent), and the organic solvent was stirred to prepare a paste-like negative electrode mixture slurry. Next, the negative electrode mixture slurry was applied to both sides of the negative electrodecurrent collector 22A (a strip-shaped copper foil having a thickness of 15 μm) using a coating device, and then the negative electrode mixture slurry was dried to form the negative electrode active material layer 22B. Finally, the negative electrode active material layer 22B was compression-molded using a roll press machine. This resulted in the negative electrode 22 being produced.
最初に、負極活物質(炭素材料である人造黒鉛)93質量部と、負極結着剤(ポリフッ化ビニリデン)7質量部とを互いに混合させることにより、負極合剤とした。続いて、溶媒(有機溶剤であるN-メチル-2-ピロリドン)に負極合剤を投入したのち、その有機溶剤を撹拌することにより、ペースト状の負極合剤スラリーを調製した。続いて、コーティング装置を用いて負極集電体22A(厚さ=15μmである帯状の銅箔)の両面に負極合剤スラリーを塗布したのち、その負極合剤スラリーを乾燥させることにより、負極活物質層22Bを形成した。最後に、ロールプレス機を用いて負極活物質層22Bを圧縮成形した。これにより、負極22が作製された。 (Preparation of negative electrode)
First, 93 parts by mass of the negative electrode active material (artificial graphite, which is a carbon material) and 7 parts by mass of the negative electrode binder (polyvinylidene fluoride) were mixed together to prepare a negative electrode mixture. Next, the negative electrode mixture was added to a solvent (N-methyl-2-pyrrolidone, which is an organic solvent), and the organic solvent was stirred to prepare a paste-like negative electrode mixture slurry. Next, the negative electrode mixture slurry was applied to both sides of the negative electrode
(電解液の調製)
イミドアニオンを含んでいる電解質塩を溶媒に投入したのち、その溶媒を攪拌した。これにより、電解液が調製された。 (Preparation of Electrolyte)
The electrolyte salt containing the imide anion was added to the solvent, and the solvent was stirred to prepare the electrolyte solution.
イミドアニオンを含んでいる電解質塩を溶媒に投入したのち、その溶媒を攪拌した。これにより、電解液が調製された。 (Preparation of Electrolyte)
The electrolyte salt containing the imide anion was added to the solvent, and the solvent was stirred to prepare the electrolyte solution.
溶媒としては、環状炭酸エステルである炭酸エチレンと、ラクトンであるγ-ブチロラクトンとの混合物を用いた。この場合には、溶媒の混合比(重量比)を炭酸エチレン:γ-ブチロラクトン=30:70とした。
The solvent used was a mixture of ethylene carbonate, a cyclic carbonate ester, and gamma-butyrolactone, a lactone. In this case, the solvent mixing ratio (weight ratio) was ethylene carbonate:gamma-butyrolactone = 30:70.
電解質塩のカチオンとしては、リチウムイオン(Li+ )を用いた。電解質塩のアニオン(イミドアニオン)としては、式(1-5)、式(1-6)、式(1-21)および式(1-22)のそれぞれに示した第1イミドアニオンと、式(2-5)に示した第2イミドアニオンと、式(3-5)に示した第3イミドアニオンと、式(4-37)に示した第4イミドアニオンとを用いた。電解液における電解質塩の含有量(mol/kg)は、表1および表2に示した通りであった。
Lithium ions (Li + ) were used as the cations of the electrolyte salt. As the anions (imide anions) of the electrolyte salt, the first imide anions shown in formulas (1-5), (1-6), (1-21) and (1-22), the second imide anion shown in formula (2-5), the third imide anion shown in formula (3-5) and the fourth imide anion shown in formula (4-37) were used. The contents (mol/kg) of the electrolyte salts in the electrolytic solution were as shown in Tables 1 and 2.
この電解液は、塩素イオンを含んでおり、その電解液における塩素イオンの含有量(重量ppm)は、表1および表2に示した通りであった。電解液における塩素イオンの含有量を変化させる場合には、イミドアニオンを含んでいる電解質塩の精製時における再結晶の回数を変更した。
This electrolyte contains chloride ions, and the chloride ion content (ppm by weight) in the electrolyte is as shown in Tables 1 and 2. To change the chloride ion content in the electrolyte, the number of recrystallizations during purification of the electrolyte salt containing imide anions was changed.
なお、比較のために、イミドアニオンを含んでいる電解質塩の代わりに、そのイミドアニオンを含んでいない電解質塩(六フッ化リン酸リチウム(LiPF6 ))を用いたことを除いて同様の手順により、電解液を調製した。
For comparison, an electrolyte solution was prepared in the same manner, except that an electrolyte salt not containing an imide anion (lithium hexafluorophosphate (LiPF 6 )) was used instead of the electrolyte salt containing the imide anion.
(二次電池の組み立て)
最初に、正極21の正極集電体21Aに正極リード31(アルミニウム箔)を溶接したと共に、負極22の負極集電体22Aに負極リード32(銅箔)を溶接した。 (Assembly of secondary batteries)
First, the positive electrode lead 31 (aluminum foil) was welded to the positive electrodecurrent collector 21 A of the positive electrode 21 , and the negative electrode lead 32 (copper foil) was welded to the negative electrode current collector 22 A of the negative electrode 22 .
最初に、正極21の正極集電体21Aに正極リード31(アルミニウム箔)を溶接したと共に、負極22の負極集電体22Aに負極リード32(銅箔)を溶接した。 (Assembly of secondary batteries)
First, the positive electrode lead 31 (aluminum foil) was welded to the positive electrode
続いて、セパレータ23(厚さ=25μmである微多孔性ポリエチレンフィルム)を介して正極21および負極22を互いに積層させることにより、積層体を作製した。続いて、積層体を巻回させることにより、巻回体を作製したのち、プレス機を用いて巻回体をプレスすることにより、扁平形状となるように巻回体を成形した。
Then, the positive electrode 21 and the negative electrode 22 were laminated on each other with a separator 23 (a microporous polyethylene film having a thickness of 25 μm) interposed therebetween to produce a laminate. The laminate was then wound to produce a wound body, which was then pressed using a press machine to form the wound body into a flat shape.
続いて、窪み部10Uに収容された巻回体を挟むように外装フィルム10(融着層/金属層/表面保護層)を折り畳んだのち、その融着層のうちの2辺の外周縁部同士を互いに熱融着させることにより、袋状の外装フィルム10の内部に巻回体を収納した。外装フィルム10としては、融着層(厚さ=30μmであるポリプロピレンフィルム)と、金属層(厚さ=40μmであるアルミニウム箔)と、表面保護層(厚さ=25μmであるナイロンフィルム)とが内側からこの順に積層されたアルミラミネートフィルムを用いた。
Then, the exterior film 10 (adhesive layer/metal layer/surface protection layer) was folded so as to sandwich the roll housed in the recess 10U, and the outer peripheral edges of two sides of the adhesive layer were heat-sealed to each other to house the roll inside the bag-shaped exterior film 10. The exterior film 10 used was an aluminum laminate film in which the adhesive layer (polypropylene film with a thickness of 30 μm), the metal layer (aluminum foil with a thickness of 40 μm), and the surface protection layer (nylon film with a thickness of 25 μm) were laminated in this order from the inside.
最後に、袋状の外装フィルム10の内部に電解液を注入したのち、減圧環境中において融着層のうちの残りの1辺の外周縁部同士を互いに熱融着させた。この場合には、外装フィルム10と正極リード31との間に封止フィルム41(厚さ=5μmであるポリプロピレンフィルム)を挿入したと共に、外装フィルム10と負極リード32との間に封止フィルム42(厚さ=5μmであるポリプロピレンフィルム)を挿入した。これにより、巻回体に電解液が含浸されたため、電池素子20が作製された。
Finally, after injecting electrolyte into the bag-shaped exterior film 10, the outer edges of the remaining side of the fusion layer were heat-sealed to each other in a reduced pressure environment. In this case, a sealing film 41 (a polypropylene film with a thickness of 5 μm) was inserted between the exterior film 10 and the positive electrode lead 31, and a sealing film 42 (a polypropylene film with a thickness of 5 μm) was inserted between the exterior film 10 and the negative electrode lead 32. As a result, the electrolyte was impregnated into the wound body, and the battery element 20 was produced.
よって、外装フィルム10の内部に電池素子20が封入されたため、二次電池が組み立てられた。
As a result, the battery element 20 is enclosed inside the exterior film 10, and a secondary battery is assembled.
(組み立て後の二次電池の安定化処理)
常温環境中(温度=23℃)において組み立て後の二次電池を1サイクル充放電させた。充電時には、0.1Cの電流で電圧が4.2Vに到達するまで定電流充電したのち、その4.2Vの電圧で電流が0.05Cに到達するまで定電圧充電した。放電時には、0.1Cの電流で電圧が2.5Vに到達するまで定電流放電した。0.1Cとは、電池容量(理論容量)を10時間で放電しきる電流値であると共に、0.05Cとは、電池容量を20時間で放電しきる電流値である。 (Stabilization of secondary batteries after assembly)
The assembled secondary battery was charged and discharged for one cycle in a room temperature environment (temperature = 23 ° C.). During charging, the battery was charged at a constant current of 0.1 C until the voltage reached 4.2 V, and then charged at a constant voltage of 4.2 V until the current reached 0.05 C. During discharging, the battery was discharged at a constant current of 0.1 C until the voltage reached 2.5 V. 0.1 C is the current value at which the battery capacity (theoretical capacity) is fully discharged in 10 hours, and 0.05 C is the current value at which the battery capacity is fully discharged in 20 hours.
常温環境中(温度=23℃)において組み立て後の二次電池を1サイクル充放電させた。充電時には、0.1Cの電流で電圧が4.2Vに到達するまで定電流充電したのち、その4.2Vの電圧で電流が0.05Cに到達するまで定電圧充電した。放電時には、0.1Cの電流で電圧が2.5Vに到達するまで定電流放電した。0.1Cとは、電池容量(理論容量)を10時間で放電しきる電流値であると共に、0.05Cとは、電池容量を20時間で放電しきる電流値である。 (Stabilization of secondary batteries after assembly)
The assembled secondary battery was charged and discharged for one cycle in a room temperature environment (temperature = 23 ° C.). During charging, the battery was charged at a constant current of 0.1 C until the voltage reached 4.2 V, and then charged at a constant voltage of 4.2 V until the current reached 0.05 C. During discharging, the battery was discharged at a constant current of 0.1 C until the voltage reached 2.5 V. 0.1 C is the current value at which the battery capacity (theoretical capacity) is fully discharged in 10 hours, and 0.05 C is the current value at which the battery capacity is fully discharged in 20 hours.
これにより、正極21および負極22のそれぞれの表面に被膜が形成されたため、電池素子20の状態が電気化学的に安定化した。よって、二次電池が完成した。
As a result, a coating was formed on the surface of each of the positive electrode 21 and the negative electrode 22, electrochemically stabilizing the state of the battery element 20. Thus, the secondary battery was completed.
[電池特性の評価]
以下で説明する手順により、電池特性(サイクル特性、保存特性および負荷特性)を評価したところ、表1および表2に示した結果が得られた。 [Evaluation of Battery Characteristics]
The battery characteristics (cycle characteristics, storage characteristics, and load characteristics) were evaluated according to the procedures described below, and the results shown in Tables 1 and 2 were obtained.
以下で説明する手順により、電池特性(サイクル特性、保存特性および負荷特性)を評価したところ、表1および表2に示した結果が得られた。 [Evaluation of Battery Characteristics]
The battery characteristics (cycle characteristics, storage characteristics, and load characteristics) were evaluated according to the procedures described below, and the results shown in Tables 1 and 2 were obtained.
(サイクル特性)
最初に、高温環境中(温度=60℃)において二次電池を充放電させることにより、放電容量(1サイクル目の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Cycle characteristics)
First, the discharge capacity (discharge capacity at the first cycle) was measured by charging and discharging the secondary battery in a high-temperature environment (temperature = 60 ° C.) The charge and discharge conditions were the same as those during the stabilization treatment described above.
最初に、高温環境中(温度=60℃)において二次電池を充放電させることにより、放電容量(1サイクル目の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Cycle characteristics)
First, the discharge capacity (discharge capacity at the first cycle) was measured by charging and discharging the secondary battery in a high-temperature environment (temperature = 60 ° C.) The charge and discharge conditions were the same as those during the stabilization treatment described above.
続いて、同環境中においてサイクル数の総数が100サイクルに到達するまで二次電池を繰り返して充放電させることにより、放電容量(100サイクル目の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。
Then, in the same environment, the secondary battery was repeatedly charged and discharged until the total number of cycles reached 100, and the discharge capacity (discharge capacity at the 100th cycle) was measured. The charge and discharge conditions were the same as those during the stabilization treatment described above.
最後に、サイクル維持率(%)=(100サイクル目の放電容量/1サイクル目の放電容量)×100という計算式に基づいて、サイクル特性を評価するための指標であるサイクル維持率を算出した。
Finally, the cycle retention rate, which is an index for evaluating cycle characteristics, was calculated based on the formula: cycle retention rate (%) = (discharge capacity at 100th cycle/discharge capacity at 1st cycle) x 100.
(保存特性)
最初に、常温環境中(温度=23℃)において二次電池を1サイクル充放電させることにより、放電容量(保存前の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Storage characteristics)
First, the discharge capacity (discharge capacity before storage) was measured by charging and discharging the secondary battery one cycle in a room temperature environment (temperature = 23 ° C.) The charge and discharge conditions were the same as the charge and discharge conditions during the stabilization treatment described above.
最初に、常温環境中(温度=23℃)において二次電池を1サイクル充放電させることにより、放電容量(保存前の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Storage characteristics)
First, the discharge capacity (discharge capacity before storage) was measured by charging and discharging the secondary battery one cycle in a room temperature environment (temperature = 23 ° C.) The charge and discharge conditions were the same as the charge and discharge conditions during the stabilization treatment described above.
続いて、同環境中において二次電池を充電させることにより、高温環境中(温度=80℃)において充電状態の二次電池を保存(保存時間=10日間)したのち、常温環境中において二次電池を放電させることにより、放電容量(保存後の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。
Subsequently, the secondary battery was charged in the same environment, and the charged secondary battery was stored (storage time = 10 days) in a high-temperature environment (temperature = 80°C), and then the secondary battery was discharged in a room-temperature environment to measure the discharge capacity (discharge capacity after storage). The charge and discharge conditions were the same as those during the stabilization treatment described above.
最後に、保存維持率(%)=(保存後の放電容量/保存前の放電容量)×100という計算式に基づいて、保存特性を評価するための指標である保存維持率を算出した。
Finally, the storage retention rate, which is an index for evaluating storage characteristics, was calculated based on the formula: storage retention rate (%) = (discharge capacity after storage/discharge capacity before storage) x 100.
(負荷特性)
最初に、常温環境中(温度=23℃)において二次電池を1サイクル充放電させることにより、放電容量(1サイクル目の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Load characteristics)
First, the discharge capacity (discharge capacity at the first cycle) was measured by charging and discharging the secondary battery one cycle in a room temperature environment (temperature = 23 ° C.) The charge and discharge conditions were the same as those during the stabilization treatment described above.
最初に、常温環境中(温度=23℃)において二次電池を1サイクル充放電させることにより、放電容量(1サイクル目の放電容量)を測定した。充放電条件は、上記した安定化処理時の充放電条件と同様にした。 (Load characteristics)
First, the discharge capacity (discharge capacity at the first cycle) was measured by charging and discharging the secondary battery one cycle in a room temperature environment (temperature = 23 ° C.) The charge and discharge conditions were the same as those during the stabilization treatment described above.
続いて、低温環境中(温度=-10℃)においてサイクル数の総数が100サイクルに到達するまで二次電池を繰り返して充放電させることにより、放電容量(100サイクル目の放電容量)を測定した。充放電条件は、放電時の電流を1Cに変更したことを除いて、上記した安定化処理時の充放電条件と同様にした。1Cとは、電池容量を1時間で放電しきる電流値である。
Then, the secondary battery was repeatedly charged and discharged in a low-temperature environment (temperature = -10°C) until the total number of cycles reached 100, and the discharge capacity (discharge capacity at the 100th cycle) was measured. The charge and discharge conditions were the same as those during the stabilization process described above, except that the discharge current was changed to 1C. 1C is the current value at which the battery capacity is fully discharged in 1 hour.
最後に、負荷維持率(%)=(100サイクル目の放電容量/1サイクル目の放電容量)×100という計算式に基づいて、負荷特性を評価するための指標である負荷維持率を算出した。
Finally, the load retention rate, which is an index for evaluating load characteristics, was calculated based on the formula: Load retention rate (%) = (discharge capacity at 100th cycle/discharge capacity at 1st cycle) x 100.
[考察]
表1および表2に示したように、サイクル維持率、保存維持率および負荷維持率のそれぞれは、電解液の構成に応じて大きく変動した。 [Discussion]
As shown in Tables 1 and 2, the cycle retention rate, storage retention rate, and load retention rate each varied greatly depending on the composition of the electrolyte.
表1および表2に示したように、サイクル維持率、保存維持率および負荷維持率のそれぞれは、電解液の構成に応じて大きく変動した。 [Discussion]
As shown in Tables 1 and 2, the cycle retention rate, storage retention rate, and load retention rate each varied greatly depending on the composition of the electrolyte.
具体的には、電解質塩がイミドアニオンを含んでいないと共に、電解液における塩素イオンの含有量が5000重量ppmよりも大きい場合(比較例1)には、サイクル維持率、保存維持率および負荷維持率がいずれも減少した。
Specifically, when the electrolyte salt did not contain imide anions and the content of chloride ions in the electrolyte was greater than 5,000 ppm by weight (Comparative Example 1), the cycle retention rate, storage retention rate, and load retention rate all decreased.
また、電解質塩はイミドアニオンを含んでいるが、電解液における塩素イオンの含有量が5000重量ppmよりも大きい場合(比較例2)においても同様に、サイクル維持率、保存維持率および負荷維持率がいずれも減少した。
Furthermore, even though the electrolyte salt contains imide anions, when the content of chloride ions in the electrolyte solution was greater than 5,000 ppm by weight (Comparative Example 2), the cycle retention rate, storage retention rate, and load retention rate all decreased.
さらに、電解液における塩素イオンの含有量は5000重量ppm以下であるが、電解質塩はイミドアニオンを含んでいない場合(比較例3)においても同様に、サイクル維持率、保存維持率および負荷維持率がいずれも減少した。
Furthermore, even when the content of chloride ions in the electrolyte was 5,000 ppm by weight or less, but the electrolyte salt did not contain imide anions (Comparative Example 3), the cycle retention rate, storage retention rate, and load retention rate all decreased.
これに対して、電解質塩がイミドアニオンを含んでいると共に、電解液における塩素イオンの含有量が5000重量ppm以下である場合(実施例1~18)には、サイクル維持率、保存維持率および負荷維持率がいずれも増加した。
In contrast, when the electrolyte salt contained imide anions and the content of chloride ions in the electrolyte was 5,000 ppm by weight or less (Examples 1 to 18), the cycle retention rate, storage retention rate, and load retention rate all increased.
この場合(実施例1~18)には、特に、以下で説明する傾向が得られた。
In this case (Examples 1 to 18), the following trends were observed.
第1に、イミドアニオンの種類に依存せずに、サイクル維持率、保存維持率および負荷維持率のそれぞれが十分に高くなった。第2に、電解液における塩素イオンの含有量が50重量ppm以下であると、サイクル維持率、保存維持率および負荷維持率のそれぞれがより増加した。第3に、電解質塩がカチオンとして軽金属イオン(リチウムイオン)を含んでいると、サイクル維持率、保存維持率および負荷維持率のそれぞれが十分に高くなった。第4に、電解液における電解質塩の含有量が0.2mol/kg~2mol/kgであると、サイクル維持率、保存維持率および負荷維持率のそれぞれがより増加した。
First, the cycle retention rate, storage retention rate, and load retention rate were all sufficiently high, regardless of the type of imide anion. Second, when the content of chloride ions in the electrolyte was 50 ppm by weight or less, the cycle retention rate, storage retention rate, and load retention rate all increased more. Third, when the electrolyte salt contained light metal ions (lithium ions) as cations, the cycle retention rate, storage retention rate, and load retention rate all increased sufficiently. Fourth, when the content of electrolyte salt in the electrolyte was 0.2 mol/kg to 2 mol/kg, the cycle retention rate, storage retention rate, and load retention rate all increased more.
<実施例19~36>
表3および表4に示したように、電解液に他の溶媒または他の電解質塩を含有させたことを除いて実施例3と同様の手順により、二次電池を作製したのち、電池特性を評価した。この場合には、電解液に他の溶媒または他の電解質塩を添加したのち、その電解液を攪拌した。 <Examples 19 to 36>
Secondary batteries were produced and their battery characteristics were evaluated in the same manner as in Example 3, except that other solvents or other electrolyte salts were added to the electrolyte solution as shown in Tables 3 and 4. In this case, the other solvents or other electrolyte salts were added to the electrolyte solution, and then the electrolyte solution was stirred.
表3および表4に示したように、電解液に他の溶媒または他の電解質塩を含有させたことを除いて実施例3と同様の手順により、二次電池を作製したのち、電池特性を評価した。この場合には、電解液に他の溶媒または他の電解質塩を添加したのち、その電解液を攪拌した。 <Examples 19 to 36>
Secondary batteries were produced and their battery characteristics were evaluated in the same manner as in Example 3, except that other solvents or other electrolyte salts were added to the electrolyte solution as shown in Tables 3 and 4. In this case, the other solvents or other electrolyte salts were added to the electrolyte solution, and then the electrolyte solution was stirred.
他の溶媒に関する詳細は、以下で説明する通りである。不飽和環状炭酸エステルとしては、炭酸ビニレン(VC)、炭酸ビニルエチレン(VEC)および炭酸メチレンエチレン(MEC)を用いた。フッ素化環状炭酸エステルとしては、モノフルオロ炭酸エチレン(FEC)およびジフルオロ炭酸エチレン(DFEC)を用いた。スルホン酸エステルとしては、環状モノスルホン酸エステルであるプロパンスルトン(PS)およびプロペンスルトン(PRS)と、環状ジスルホン酸エステルであるシクロジソン(CD)とを用いた。ジカルボン酸無水物としては、無水コハク酸(SA)を用いた。ジスルホン酸無水物としては、無水プロパンジスルホン酸(PSAH)を用いた。硫酸エステルとしては、エチレンスルファート(DTD)を用いた。ニトリル化合物としては、スクシノニトリル(SN)を用いた。イソシアネート化合物としては、ヘキサメチレンジイソシアネート(HMI)を用いた。
Details of other solvents are as follows. As unsaturated cyclic carbonates, vinylene carbonate (VC), vinylethylene carbonate (VEC), and methyleneethylene carbonate (MEC) were used. As fluorinated cyclic carbonates, monofluoroethylene carbonate (FEC) and difluoroethylene carbonate (DFEC) were used. As sulfonates, cyclic monosulfonates, propane sultone (PS) and propene sultone (PRS), and cyclic disulfonates, cyclodisone (CD), were used. As dicarboxylic anhydrides, succinic anhydride (SA) was used. As disulfonic anhydrides, propane disulfonic anhydride (PSAH) was used. As sulfates, ethylene sulfate (DTD) was used. As nitrile compounds, succinonitrile (SN) was used. As isocyanate compounds, hexamethylene diisocyanate (HMI) was used.
他の電解質塩としては、六フッ化リン酸リチウム(LiPF6 )、四フッ化ホウ酸リチウム(LiBF4 )、ビス(フルオロスルホニル)イミドリチウム(LiFSI)、ビス(オキサラト)ホウ酸リチウム(LiBOB)およびジフルオロリン酸リチウム(LiPF2 O2 )を用いた。
Other electrolyte salts used were lithium hexafluorophosphate (LiPF 6 ), lithium tetrafluoroborate (LiBF 4 ), lithium bis(fluorosulfonyl)imide (LiFSI), lithium bis(oxalato)borate (LiBOB) and lithium difluorophosphate (LiPF 2 O 2 ).
電解液における他の溶媒の含有量(重量%)と、電解液における他の電解質塩の含有量(重量%)とは、表3および表4に示した通りであった。
The contents (wt%) of other solvents in the electrolyte and the contents (wt%) of other electrolyte salts in the electrolyte are as shown in Tables 3 and 4.
表3に示したように、電解液が他の溶媒を含んでいる場合(実施例19~31)には、電解液が他の溶媒を含んでいない場合(実施例3)と比較して、サイクル維持率、保存維持率および負荷維持率のうちの2つ以上がより増加した。
As shown in Table 3, when the electrolyte contained other solvents (Examples 19 to 31), two or more of the cycle retention rate, storage retention rate, and load retention rate were increased more than when the electrolyte did not contain other solvents (Example 3).
また、表4に示したように、電解液が他の電解質塩を含んでいる場合(実施例32~36)には、電解液が他の電解質塩を含んでいない場合(実施例3)と比較して、サイクル維持率、保存維持率および負荷維持率ののうちの2つ以上がより増加した。
Also, as shown in Table 4, when the electrolyte solution contained other electrolyte salts (Examples 32 to 36), two or more of the cycle retention rate, storage retention rate, and load retention rate were increased more than when the electrolyte solution did not contain other electrolyte salts (Example 3).
<実施例37~112>
表5~表10に示したように、電解液に他の電解質塩として六フッ化リン酸リチウム(LiPF6 )またはビス(フルオロスルホニル)イミドリチウム(LiFSI)を含有させたことを除いて実施例3とほぼ同様の手順により、二次電池を作製したのち、電池特性を評価した。 <Examples 37 to 112>
As shown in Tables 5 to 10, secondary batteries were prepared in a manner similar to that of Example 3, except that the electrolyte solution contained lithium hexafluorophosphate (LiPF 6 ) or lithium bis(fluorosulfonyl)imide (LiFSI) as another electrolyte salt, and the battery characteristics were then evaluated.
表5~表10に示したように、電解液に他の電解質塩として六フッ化リン酸リチウム(LiPF6 )またはビス(フルオロスルホニル)イミドリチウム(LiFSI)を含有させたことを除いて実施例3とほぼ同様の手順により、二次電池を作製したのち、電池特性を評価した。 <Examples 37 to 112>
As shown in Tables 5 to 10, secondary batteries were prepared in a manner similar to that of Example 3, except that the electrolyte solution contained lithium hexafluorophosphate (LiPF 6 ) or lithium bis(fluorosulfonyl)imide (LiFSI) as another electrolyte salt, and the battery characteristics were then evaluated.
この場合には、溶媒にさらに他の電解質塩を添加したのち、その溶媒を攪拌した。電解液における電解質塩の含有量(mol/kg)と、電解液における他の電解質塩の含有量(mol/kg)と、和T(mol/kg)と、割合R(mol%)とは、表5~表10に示した通りであった。
In this case, other electrolyte salts were added to the solvent, and the solvent was then stirred. The electrolyte salt content (mol/kg) in the electrolyte solution, the content (mol/kg) of other electrolyte salts in the electrolyte solution, the sum T (mol/kg), and the ratio R (mol%) were as shown in Tables 5 to 10.
他の電解質塩として六フッ化リン酸リチウムを用いた場合には、表5~表7に示した結果が得られた。すなわち、和Tが0.7mol/kg~2.2mol/kgであると共に割合Rが13mol%~6000mol%という2種類の条件が満たされている場合(実施例41,42,46~54,56~60,62~65,69~74)には、その2種類の条件が満たされていない場合(実施例37~40,43~45,55,61,66~68)と比較して、サイクル維持率、保存維持率および負荷維持率のうちの2つ以上がより増加した。
When lithium hexafluorophosphate was used as another electrolyte salt, the results shown in Tables 5 to 7 were obtained. That is, when the two conditions of the sum T being 0.7 mol/kg to 2.2 mol/kg and the ratio R being 13 mol% to 6000 mol% were met (Examples 41, 42, 46-54, 56-60, 62-65, 69-74), two or more of the cycle retention rate, storage retention rate, and load retention rate increased more than when these two conditions were not met (Examples 37-40, 43-45, 55, 61, 66-68).
ここで説明した優劣に関する傾向は、表8~表10に示したように、他の電解質塩としてビス(フルオロスルホニル)イミドリチウムを用いた場合(実施例75~112)においても同様に得られた。
The trends regarding superiority and inferiority described here were also observed when lithium bis(fluorosulfonyl)imide was used as another electrolyte salt (Examples 75 to 112), as shown in Tables 8 to 10.
[まとめ]
表1~表10に示した結果から、電解液が電解質塩および塩素イオンを含んでおり、その電解質塩がイミドアニオンを含んでおり、その電解液における塩素イオンの含有量が5000重量ppm以下であると、高いサイクル維持率、高い保存維持率および高い負荷維持率が得られた。よって、サイクル特性、保存特性および負荷特性のそれぞれが改善されたため、二次電池において優れた電池特性が得られた。 [summary]
From the results shown in Tables 1 to 10, when the electrolyte solution contains an electrolyte salt and a chloride ion, the electrolyte salt contains an imide anion, and the content of the chloride ion in the electrolyte solution is 5000 ppm by weight or less, a high cycle retention rate, a high storage retention rate, and a high load retention rate were obtained. Therefore, since each of the cycle characteristics, storage characteristics, and load characteristics was improved, excellent battery characteristics were obtained in the secondary battery.
表1~表10に示した結果から、電解液が電解質塩および塩素イオンを含んでおり、その電解質塩がイミドアニオンを含んでおり、その電解液における塩素イオンの含有量が5000重量ppm以下であると、高いサイクル維持率、高い保存維持率および高い負荷維持率が得られた。よって、サイクル特性、保存特性および負荷特性のそれぞれが改善されたため、二次電池において優れた電池特性が得られた。 [summary]
From the results shown in Tables 1 to 10, when the electrolyte solution contains an electrolyte salt and a chloride ion, the electrolyte salt contains an imide anion, and the content of the chloride ion in the electrolyte solution is 5000 ppm by weight or less, a high cycle retention rate, a high storage retention rate, and a high load retention rate were obtained. Therefore, since each of the cycle characteristics, storage characteristics, and load characteristics was improved, excellent battery characteristics were obtained in the secondary battery.
以上、一実施形態および実施例を挙げながら本技術に関して説明したが、その本技術の構成は、一実施形態および実施例において説明された構成に限定されないため、種々に変形可能である。
The present technology has been described above with reference to one embodiment and examples, but the configuration of the present technology is not limited to the configuration described in the embodiment and examples, and can be modified in various ways.
具体的には、二次電池の電池構造がラミネートフィルム型である場合に関して説明した。しかしながら、二次電池の電池構造は、特に限定されないため、円筒型、角型、コイン型およびボタン型などでもよい。
Specifically, the battery structure of the secondary battery has been described as being of a laminate film type. However, the battery structure of the secondary battery is not particularly limited, and may be of a cylindrical type, a square type, a coin type, a button type, etc.
また、電池素子の素子構造が巻回型である場合に関して説明した。しかしながら、電池素子の素子構造は、特に限定されないため、積層型および九十九折り型などでもよい。積層型では、正極および負極がセパレータを介して交互に積層されていると共に、九十九折り型では、正極および負極がセパレータを介して互いに対向しながらジグザグに折り畳まれている。
Also, the battery element has been described as having a wound structure. However, the structure of the battery element is not particularly limited, and may be a stacked type or a zigzag type. In the stacked type, the positive and negative electrodes are alternately stacked with a separator between them, while in the zigzag type, the positive and negative electrodes are folded in a zigzag pattern while facing each other with the separator between them.
また、電極反応物質がリチウムである場合に関して説明したが、その電極反応物質は、特に限定されない。具体的には、電極反応物質は、上記したように、ナトリウムおよびカリウムなどの他のアルカリ金属でもよいし、ベリリウム、マグネシウムおよびカルシウムなどのアルカリ土類金属でもよい。この他、電極反応物質は、アルミニウムなどの他の軽金属でもよい。
Although the electrode reactant is lithium in the above description, the electrode reactant is not particularly limited. Specifically, as described above, the electrode reactant may be other alkali metals such as sodium and potassium, or alkaline earth metals such as beryllium, magnesium, and calcium. In addition, the electrode reactant may be other light metals such as aluminum.
本明細書中に記載された効果は、あくまで例示であるため、本技術の効果は、本明細書中に記載された効果に限定されない。よって、本技術に関して、他の効果が得られてもよい。
The effects described in this specification are merely examples, and the effects of this technology are not limited to the effects described in this specification. Therefore, other effects may be obtained with respect to this technology.
なお、本技術は、以下のような構成を取ることもできる。
<1>
正極と、
負極と、
電解質塩および塩素イオンを含む電解液と
を備え、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記電解液における前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池。
(R1およびR2のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。W1、W2およびW3のそれぞれは、カルボニル基(>C=O)、スルフィニル基(>S=O)およびスルホニル基(>S(=O)2 )のうちのいずれかである。)
(R3およびR4のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。X1、X2、X3およびX4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
(R5は、フッ素化アルキレン基である。Y1、Y2およびY3のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
(R6およびR7のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。R8は、アルキレン基、フェニレン基、フッ素化アルキレン基およびフッ素化フェニレン基のうちのいずれかである。Z1、Z2、Z3およびZ4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
<2>
前記電解液における前記塩素イオンの含有量は、50重量ppm以下である、
<1>に記載の二次電池。
<3>
前記電解液は、カチオンとして軽金属イオンを含む、
<1>または<2>に記載の二次電池。
<4>
前記軽金属イオンは、リチウムイオンを含む、
<3>に記載の二次電池。
<5>
前記電解液における前記電解質塩の含有量は、0.2mol/kg以上2mol/kg以下である、
<1>ないし<4>のいずれか1つに記載の二次電池。
<6>
前記電解液は、さらに、六フッ化リン酸リチウム、四フッ化ホウ酸リチウム、ビス(フルオロスルホニル)イミドリチウム、ビス(オキサラト)ホウ酸リチウムおよびジフルオロリン酸リチウムのうちの少なくとも1種を含む、
<5>に記載の二次電池。
<7>
前記電解液は、さらに、六フッ化リン酸リチウムまたはビス(フルオロスルホニル)イミドリチウムを含み、
前記電解質塩は、カチオンおよび前記イミドアニオンを含み、
前記六フッ化リン酸リチウムは、リチウムイオンおよび六フッ化リン酸イオンを含み、
前記ビス(フルオロスルホニル)イミドリチウムは、リチウムイオンおよびビス(フルオロスルホニル)イミドイオンを含み、
前記電解液における前記カチオンの含有量と、前記電解液における前記リチウムイオンの含有量との和は、0.7mol/kg以上2.2mol/kg以下であり、
前記電解液における前記イミドアニオンのモル数に対する、前記電解液における前記六フッ化リン酸イオンまたは前記ビス(フルオロスルホニル)イミドイオンのモル数の割合は、13mol%以上6000mol%以下である、
<1>ないし<4>のいずれか1つに記載の二次電池。
<8>
前記電解液は、さらに、不飽和環状炭酸エステル、フッ素化環状炭酸エステル、スルホン酸エステル、ジカルボン酸無水物、ジスルホン酸無水物、硫酸エステル、ニトリル化合物およびイソシアネート化合物のうちの少なくとも1種を含む、
<1>ないし<7>のいずれか1つに記載の二次電池。
<9>
リチウムイオン二次電池である、
<1>ないし<8>のいずれか1つに記載の二次電池。
<10>
電解質塩および塩素イオンを含み、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池用電解液。
(R1およびR2のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。W1、W2およびW3のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
(R3およびR4のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。X1、X2、X3およびX4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
(R5は、フッ素化アルキレン基である。Y1、Y2およびY3のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
(R6およびR7のそれぞれは、フッ素基およびフッ素化アルキル基のうちのいずれかである。R8は、アルキレン基、フェニレン基、フッ素化アルキレン基およびフッ素化フェニレン基のうちのいずれかである。Z1、Z2、Z3およびZ4のそれぞれは、カルボニル基、スルフィニル基およびスルホニル基のうちのいずれかである。)
The present technology can also be configured as follows.
<1>
A positive electrode and
A negative electrode;
an electrolyte solution containing an electrolyte salt and chloride ions;
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions in the electrolytic solution is 5000 ppm by weight or less.
Secondary battery.
(Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group. Each of W1, W2 and W3 is either a carbonyl group (>C=O), a sulfinyl group (>S=O) or a sulfonyl group (>S(=O) 2 ).)
(Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group. Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.)
(R5 is a fluorinated alkylene group. Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.)
(Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group. R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group. Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.)
<2>
The content of the chloride ions in the electrolytic solution is 50 ppm by weight or less.
The secondary battery according to <1>.
<3>
The electrolyte contains light metal ions as cations.
The secondary battery according to <1> or <2>.
<4>
The light metal ions include lithium ions.
The secondary battery according to <3>.
<5>
The content of the electrolyte salt in the electrolytic solution is 0.2 mol/kg or more and 2 mol/kg or less.
<4> The secondary battery according to any one of <1> to <4>.
<6>
The electrolyte solution further contains at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate;
The secondary battery according to <5>.
<7>
The electrolyte solution further contains lithium hexafluorophosphate or lithium bis(fluorosulfonyl)imide,
the electrolyte salt comprises a cation and the imide anion,
The lithium hexafluorophosphate contains lithium ions and hexafluorophosphate ions,
The lithium bis(fluorosulfonyl)imide contains a lithium ion and a bis(fluorosulfonyl)imide ion,
the sum of the content of the cation in the electrolyte solution and the content of the lithium ion in the electrolyte solution is 0.7 mol/kg or more and 2.2 mol/kg or less;
a ratio of the number of moles of the hexafluorophosphate ion or the bis(fluorosulfonyl)imide ion in the electrolyte solution to the number of moles of the imide anion in the electrolyte solution is 13 mol% or more and 6000 mol% or less;
<4> The secondary battery according to any one of <1> to <4>.
<8>
The electrolytic solution further contains at least one of an unsaturated cyclic carbonate, a fluorinated cyclic carbonate, a sulfonic acid ester, a dicarboxylic acid anhydride, a disulfonic acid anhydride, a sulfuric acid ester, a nitrile compound, and an isocyanate compound.
<7> The secondary battery according to any one of <1> to <7>.
<9>
It is a lithium-ion secondary battery.
<8> The secondary battery according to any one of <1> to <8>.
<10>
Contains an electrolyte salt and chloride ions,
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions is 5000 ppm by weight or less.
Electrolyte for secondary batteries.
(Each of R1 and R2 is either a fluorine group or a fluorinated alkyl group. Each of W1, W2, and W3 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.)
(Each of R3 and R4 is either a fluorine group or a fluorinated alkyl group. Each of X1, X2, X3 and X4 is either a carbonyl group, a sulfinyl group or a sulfonyl group.)
(R5 is a fluorinated alkylene group. Each of Y1, Y2, and Y3 is any one of a carbonyl group, a sulfinyl group, and a sulfonyl group.)
(Each of R6 and R7 is either a fluorine group or a fluorinated alkyl group. R8 is either an alkylene group, a phenylene group, a fluorinated alkylene group, or a fluorinated phenylene group. Each of Z1, Z2, Z3, and Z4 is either a carbonyl group, a sulfinyl group, or a sulfonyl group.)
<1>
正極と、
負極と、
電解質塩および塩素イオンを含む電解液と
を備え、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記電解液における前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池。
<2>
前記電解液における前記塩素イオンの含有量は、50重量ppm以下である、
<1>に記載の二次電池。
<3>
前記電解液は、カチオンとして軽金属イオンを含む、
<1>または<2>に記載の二次電池。
<4>
前記軽金属イオンは、リチウムイオンを含む、
<3>に記載の二次電池。
<5>
前記電解液における前記電解質塩の含有量は、0.2mol/kg以上2mol/kg以下である、
<1>ないし<4>のいずれか1つに記載の二次電池。
<6>
前記電解液は、さらに、六フッ化リン酸リチウム、四フッ化ホウ酸リチウム、ビス(フルオロスルホニル)イミドリチウム、ビス(オキサラト)ホウ酸リチウムおよびジフルオロリン酸リチウムのうちの少なくとも1種を含む、
<5>に記載の二次電池。
<7>
前記電解液は、さらに、六フッ化リン酸リチウムまたはビス(フルオロスルホニル)イミドリチウムを含み、
前記電解質塩は、カチオンおよび前記イミドアニオンを含み、
前記六フッ化リン酸リチウムは、リチウムイオンおよび六フッ化リン酸イオンを含み、
前記ビス(フルオロスルホニル)イミドリチウムは、リチウムイオンおよびビス(フルオロスルホニル)イミドイオンを含み、
前記電解液における前記カチオンの含有量と、前記電解液における前記リチウムイオンの含有量との和は、0.7mol/kg以上2.2mol/kg以下であり、
前記電解液における前記イミドアニオンのモル数に対する、前記電解液における前記六フッ化リン酸イオンまたは前記ビス(フルオロスルホニル)イミドイオンのモル数の割合は、13mol%以上6000mol%以下である、
<1>ないし<4>のいずれか1つに記載の二次電池。
<8>
前記電解液は、さらに、不飽和環状炭酸エステル、フッ素化環状炭酸エステル、スルホン酸エステル、ジカルボン酸無水物、ジスルホン酸無水物、硫酸エステル、ニトリル化合物およびイソシアネート化合物のうちの少なくとも1種を含む、
<1>ないし<7>のいずれか1つに記載の二次電池。
<9>
リチウムイオン二次電池である、
<1>ないし<8>のいずれか1つに記載の二次電池。
<10>
電解質塩および塩素イオンを含み、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池用電解液。
<1>
A positive electrode and
A negative electrode;
an electrolyte solution containing an electrolyte salt and chloride ions;
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions in the electrolytic solution is 5000 ppm by weight or less.
Secondary battery.
<2>
The content of the chloride ions in the electrolytic solution is 50 ppm by weight or less.
The secondary battery according to <1>.
<3>
The electrolyte contains light metal ions as cations.
The secondary battery according to <1> or <2>.
<4>
The light metal ions include lithium ions.
The secondary battery according to <3>.
<5>
The content of the electrolyte salt in the electrolytic solution is 0.2 mol/kg or more and 2 mol/kg or less.
<4> The secondary battery according to any one of <1> to <4>.
<6>
The electrolyte solution further contains at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate;
The secondary battery according to <5>.
<7>
The electrolyte solution further contains lithium hexafluorophosphate or lithium bis(fluorosulfonyl)imide,
the electrolyte salt comprises a cation and the imide anion,
The lithium hexafluorophosphate contains lithium ions and hexafluorophosphate ions,
The lithium bis(fluorosulfonyl)imide contains a lithium ion and a bis(fluorosulfonyl)imide ion,
the sum of the content of the cation in the electrolyte solution and the content of the lithium ion in the electrolyte solution is 0.7 mol/kg or more and 2.2 mol/kg or less;
a ratio of the number of moles of the hexafluorophosphate ion or the bis(fluorosulfonyl)imide ion in the electrolyte solution to the number of moles of the imide anion in the electrolyte solution is 13 mol% or more and 6000 mol% or less;
<4> The secondary battery according to any one of <1> to <4>.
<8>
The electrolytic solution further contains at least one of an unsaturated cyclic carbonate, a fluorinated cyclic carbonate, a sulfonic acid ester, a dicarboxylic acid anhydride, a disulfonic acid anhydride, a sulfuric acid ester, a nitrile compound, and an isocyanate compound.
<7> The secondary battery according to any one of <1> to <7>.
<9>
It is a lithium-ion secondary battery.
<8> The secondary battery according to any one of <1> to <8>.
<10>
Contains an electrolyte salt and chloride ions,
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions is 5000 ppm by weight or less.
Electrolyte for secondary batteries.
Claims (10)
- 正極と、
負極と、
電解質塩および塩素イオンを含む電解液と
を備え、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記電解液における前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池。
A negative electrode;
an electrolyte solution containing an electrolyte salt and chloride ions;
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions in the electrolytic solution is 5000 ppm by weight or less.
Secondary battery.
- 前記電解液における前記塩素イオンの含有量は、50重量ppm以下である、
請求項1に記載の二次電池。 The content of the chloride ions in the electrolytic solution is 50 ppm by weight or less.
The secondary battery according to claim 1 . - 前記電解液は、カチオンとして軽金属イオンを含む、
請求項1または請求項2に記載の二次電池。 The electrolyte contains light metal ions as cations.
The secondary battery according to claim 1 or 2. - 前記軽金属イオンは、リチウムイオンを含む、
請求項3に記載の二次電池。 The light metal ions include lithium ions.
The secondary battery according to claim 3 . - 前記電解液における前記電解質塩の含有量は、0.2mol/kg以上2mol/kg以下である、
請求項1ないし請求項4のいずれか1項に記載の二次電池。 The content of the electrolyte salt in the electrolytic solution is 0.2 mol/kg or more and 2 mol/kg or less.
The secondary battery according to claim 1 . - 前記電解液は、さらに、六フッ化リン酸リチウム、四フッ化ホウ酸リチウム、ビス(フルオロスルホニル)イミドリチウム、ビス(オキサラト)ホウ酸リチウムおよびジフルオロリン酸リチウムのうちの少なくとも1種を含む、
請求項5に記載の二次電池。 The electrolyte solution further contains at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(fluorosulfonyl)imide, lithium bis(oxalato)borate, and lithium difluorophosphate;
The secondary battery according to claim 5 . - 前記電解液は、さらに、六フッ化リン酸リチウムまたはビス(フルオロスルホニル)イミドリチウムを含み、
前記電解質塩は、カチオンおよび前記イミドアニオンを含み、
前記六フッ化リン酸リチウムは、リチウムイオンおよび六フッ化リン酸イオンを含み、
前記ビス(フルオロスルホニル)イミドリチウムは、リチウムイオンおよびビス(フルオロスルホニル)イミドイオンを含み、
前記電解液における前記カチオンの含有量と、前記電解液における前記リチウムイオンの含有量との和は、0.7mol/kg以上2.2mol/kg以下であり、
前記電解液における前記イミドアニオンのモル数に対する、前記電解液における前記六フッ化リン酸イオンまたは前記ビス(フルオロスルホニル)イミドイオンのモル数の割合は、13mol%以上6000mol%以下である、
請求項1ないし請求項4のいずれか1項に記載の二次電池。 The electrolyte solution further contains lithium hexafluorophosphate or lithium bis(fluorosulfonyl)imide,
the electrolyte salt comprises a cation and the imide anion,
The lithium hexafluorophosphate contains lithium ions and hexafluorophosphate ions,
The lithium bis(fluorosulfonyl)imide contains a lithium ion and a bis(fluorosulfonyl)imide ion,
the sum of the content of the cation in the electrolyte solution and the content of the lithium ion in the electrolyte solution is 0.7 mol/kg or more and 2.2 mol/kg or less;
a ratio of the number of moles of the hexafluorophosphate ion or the bis(fluorosulfonyl)imide ion in the electrolyte solution to the number of moles of the imide anion in the electrolyte solution is 13 mol% or more and 6000 mol% or less;
The secondary battery according to claim 1 . - 前記電解液は、さらに、不飽和環状炭酸エステル、フッ素化環状炭酸エステル、スルホン酸エステル、ジカルボン酸無水物、ジスルホン酸無水物、硫酸エステル、ニトリル化合物およびイソシアネート化合物のうちの少なくとも1種を含む、
請求項1ないし請求項7のいずれか1項に記載の二次電池。 The electrolytic solution further contains at least one of an unsaturated cyclic carbonate, a fluorinated cyclic carbonate, a sulfonic acid ester, a dicarboxylic acid anhydride, a disulfonic acid anhydride, a sulfuric acid ester, a nitrile compound, and an isocyanate compound.
The secondary battery according to claim 1 . - リチウムイオン二次電池である、
請求項1ないし請求項8のいずれか1項に記載の二次電池。 It is a lithium-ion secondary battery.
The secondary battery according to claim 1 . - 電解質塩および塩素イオンを含み、
前記電解質塩は、イミドアニオンを含み、
前記イミドアニオンは、式(1)により表される第1イミドアニオン、式(2)により表される第2イミドアニオン、式(3)により表される第3イミドアニオンおよび式(4)により表される第4イミドアニオンのうちの少なくとも1種を含み、
前記塩素イオンの含有量は、5000重量ppm以下である、
二次電池用電解液。
The electrolyte salt contains an imide anion,
The imide anion includes at least one of a first imide anion represented by formula (1), a second imide anion represented by formula (2), a third imide anion represented by formula (3), and a fourth imide anion represented by formula (4),
The content of the chloride ions is 5000 ppm by weight or less.
Electrolyte for secondary batteries.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2023042221 | 2023-03-16 | ||
| JP2023-042221 | 2023-03-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024190050A1 true WO2024190050A1 (en) | 2024-09-19 |
Family
ID=92754680
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2024/000259 WO2024190050A1 (en) | 2023-03-16 | 2024-01-10 | Secondary battery electrolyte and secondary battery |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024190050A1 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012054156A (en) * | 2010-09-02 | 2012-03-15 | Sony Corp | Electrolyte for secondary battery, secondary battery, electric tool, electric vehicle, and power storage system |
| WO2021015264A1 (en) * | 2019-07-24 | 2021-01-28 | セントラル硝子株式会社 | Nonaqueous electrolyte solution, nonaqueous electrolyte battery and compound |
| WO2022208978A1 (en) * | 2021-03-30 | 2022-10-06 | パナソニックIpマネジメント株式会社 | Lithium ion secondary battery |
-
2024
- 2024-01-10 WO PCT/JP2024/000259 patent/WO2024190050A1/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012054156A (en) * | 2010-09-02 | 2012-03-15 | Sony Corp | Electrolyte for secondary battery, secondary battery, electric tool, electric vehicle, and power storage system |
| WO2021015264A1 (en) * | 2019-07-24 | 2021-01-28 | セントラル硝子株式会社 | Nonaqueous electrolyte solution, nonaqueous electrolyte battery and compound |
| WO2022208978A1 (en) * | 2021-03-30 | 2022-10-06 | パナソニックIpマネジメント株式会社 | Lithium ion secondary battery |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2022196266A1 (en) | Secondary battery | |
| JP7459960B2 (en) | Electrolyte for secondary battery and secondary battery | |
| WO2024190050A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2023119948A1 (en) | Secondary battery | |
| WO2023119945A1 (en) | Secondary battery electrolyte solution and secondary battery | |
| WO2024195306A1 (en) | Secondary battery | |
| WO2023119946A1 (en) | Secondary battery electrolyte solution and secondary battery | |
| WO2023162429A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2023162430A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2024195305A1 (en) | Secondary battery | |
| WO2023162432A1 (en) | Secondary battery | |
| WO2023162431A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2024195303A1 (en) | Secondary battery | |
| WO2023162852A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2023119949A1 (en) | Secondary battery | |
| WO2022196238A1 (en) | Electrolyte solution for secondary battery, and secondary battery | |
| WO2024195302A1 (en) | Secondary battery | |
| WO2023120687A1 (en) | Secondary battery | |
| WO2024142877A1 (en) | Secondary battery | |
| WO2023162428A1 (en) | Secondary battery | |
| WO2023120688A1 (en) | Secondary battery | |
| WO2024070821A1 (en) | Secondary battery electrolytic solution and secondary battery | |
| WO2024150541A1 (en) | Electrolyte solution for lithium ion secondary batteries, and lithium ion secondary battery | |
| WO2023188949A1 (en) | Secondary battery electrolyte and secondary battery | |
| WO2022163138A1 (en) | Electrolyte for secondary battery, and secondary battery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 24770172 Country of ref document: EP Kind code of ref document: A1 |