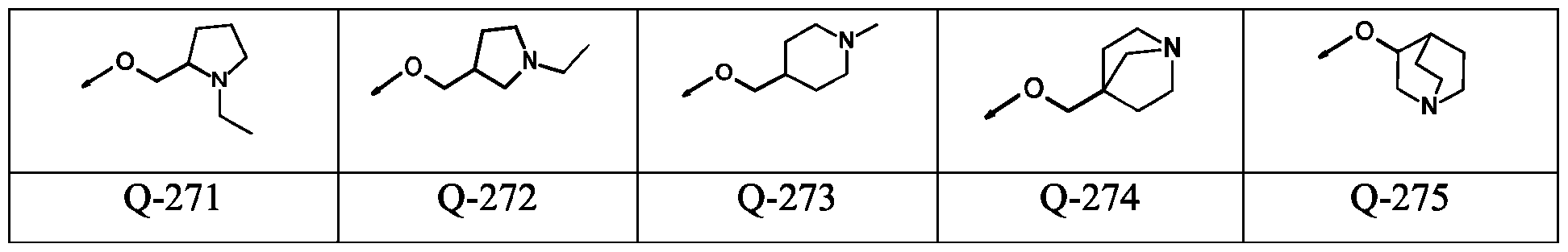
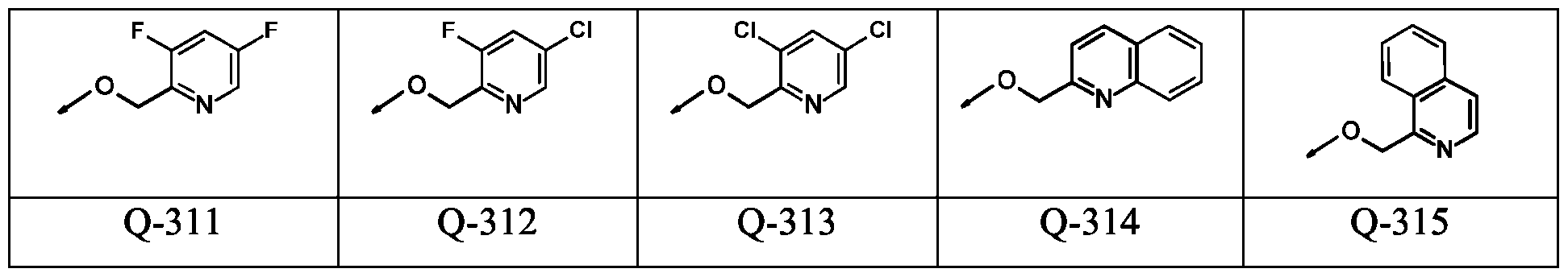
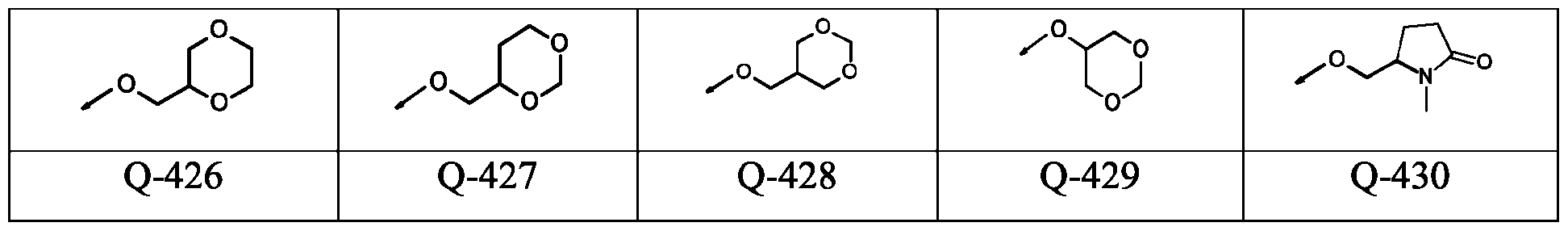
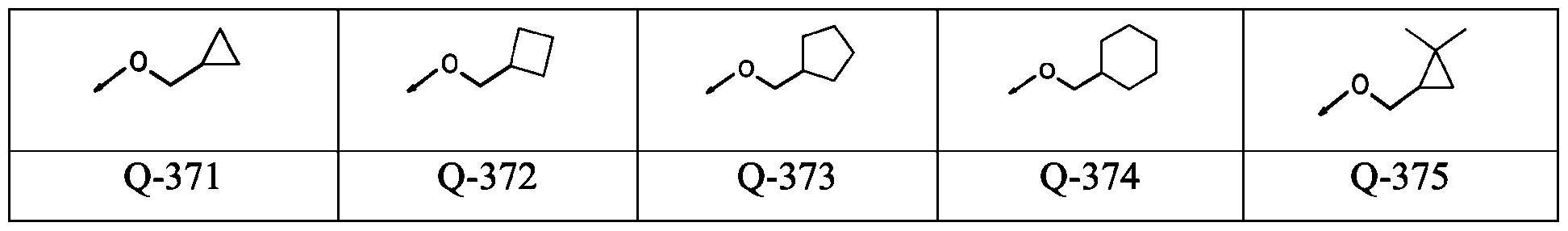
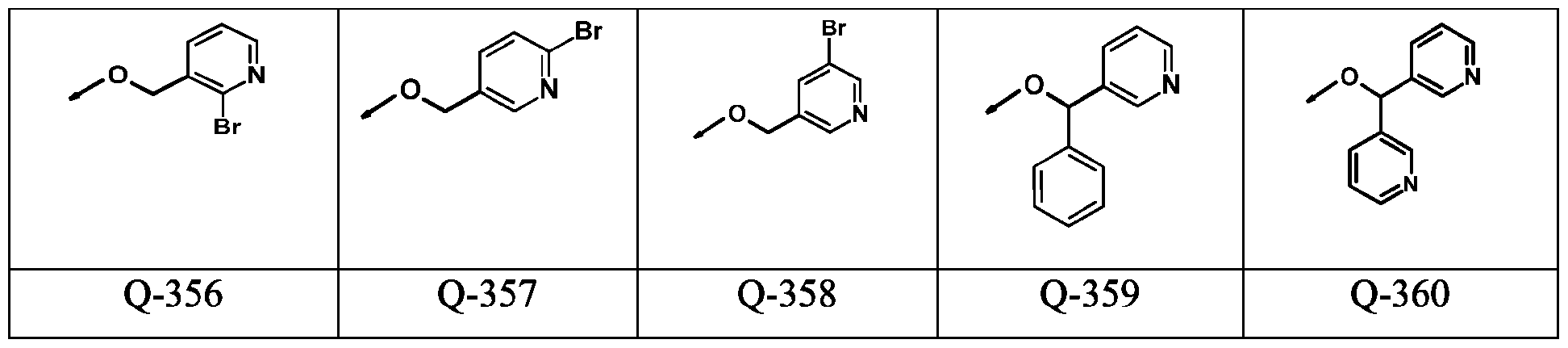
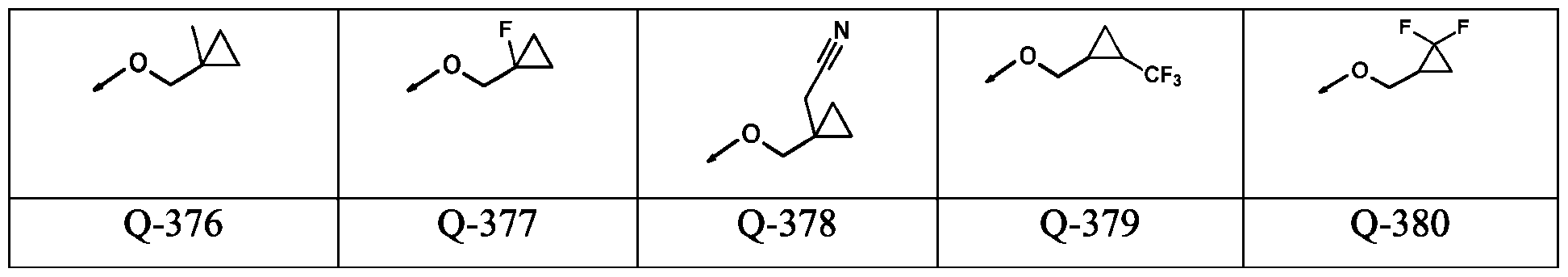
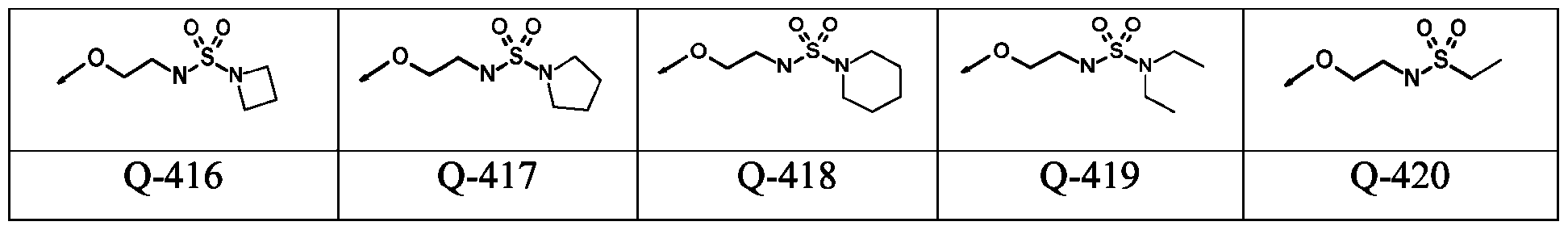
WO2024178190A2 - Methods and compositions for ppo herbicide tolerance - Google Patents
Methods and compositions for ppo herbicide tolerance Download PDFInfo
- Publication number
- WO2024178190A2 WO2024178190A2 PCT/US2024/016844 US2024016844W WO2024178190A2 WO 2024178190 A2 WO2024178190 A2 WO 2024178190A2 US 2024016844 W US2024016844 W US 2024016844W WO 2024178190 A2 WO2024178190 A2 WO 2024178190A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- cas
- methyl
- aryl
- alkoxy
- Prior art date
Links
- 239000004009 herbicide Substances 0.000 title claims abstract description 140
- 230000002363 herbicidal effect Effects 0.000 title claims abstract description 131
- 238000000034 method Methods 0.000 title claims abstract description 73
- 239000000203 mixture Substances 0.000 title description 33
- 241000196324 Embryophyta Species 0.000 claims abstract description 229
- 150000003839 salts Chemical class 0.000 claims abstract description 48
- 108020004511 Recombinant DNA Proteins 0.000 claims abstract description 40
- 230000012010 growth Effects 0.000 claims abstract description 23
- 230000008635 plant growth Effects 0.000 claims abstract description 16
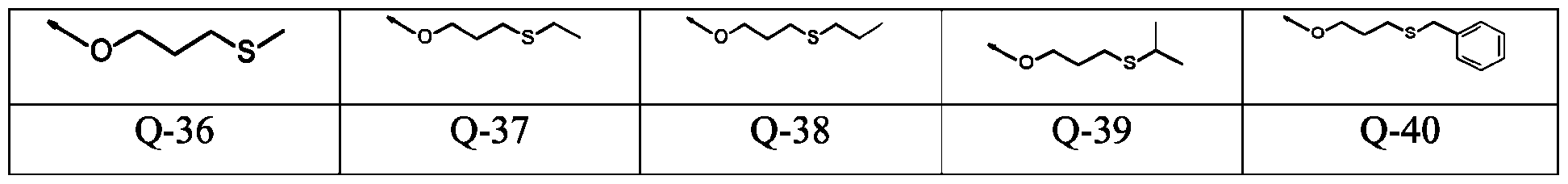
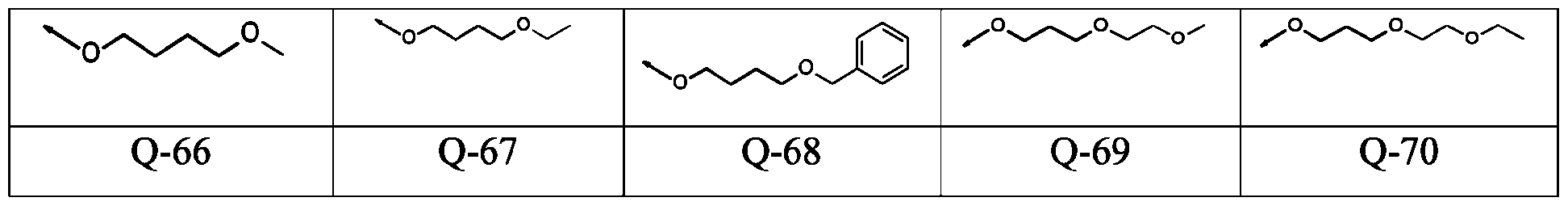
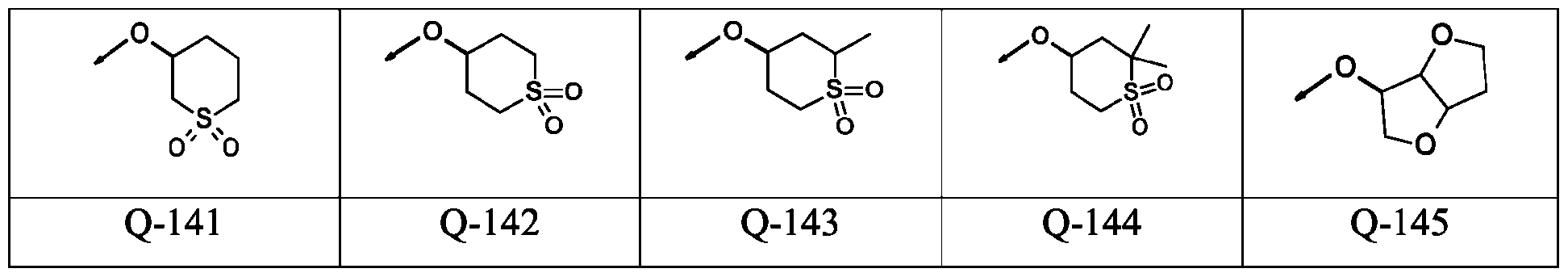
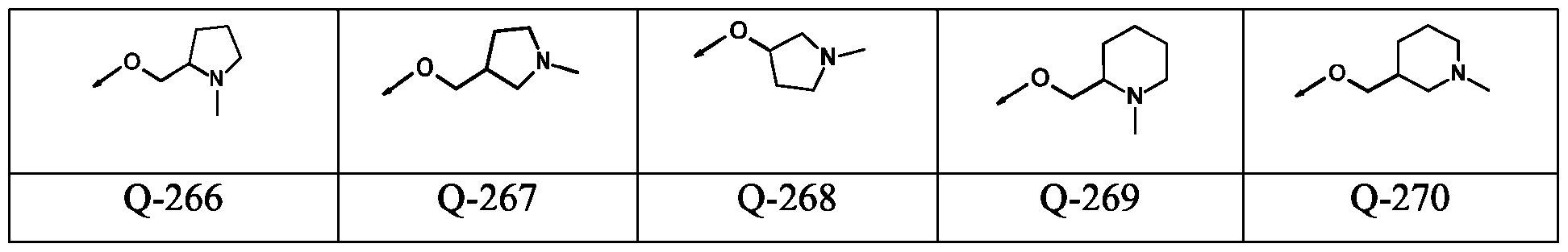
- -1 aryl-(C1-C8)-alkyl Chemical group 0.000 claims description 346
- 150000001875 compounds Chemical class 0.000 claims description 131
- 108020001991 Protoporphyrinogen Oxidase Proteins 0.000 claims description 113
- 102000005135 Protoporphyrinogen oxidase Human genes 0.000 claims description 111
- 108090000623 proteins and genes Proteins 0.000 claims description 100
- 102000004169 proteins and genes Human genes 0.000 claims description 99
- 239000003112 inhibitor Substances 0.000 claims description 86
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 claims description 76
- 102000053602 DNA Human genes 0.000 claims description 69
- 229910052739 hydrogen Inorganic materials 0.000 claims description 69
- 239000001257 hydrogen Substances 0.000 claims description 69
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 47
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 47
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 46
- 150000001413 amino acids Chemical group 0.000 claims description 44
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 43
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 42
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 42
- 125000003118 aryl group Chemical group 0.000 claims description 38
- 125000000623 heterocyclic group Chemical group 0.000 claims description 36
- 230000006378 damage Effects 0.000 claims description 31
- 229910052736 halogen Inorganic materials 0.000 claims description 31
- 150000002367 halogens Chemical group 0.000 claims description 31
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 30
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 30
- 125000001072 heteroaryl group Chemical group 0.000 claims description 26
- 108010031100 chloroplast transit peptides Proteins 0.000 claims description 24
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 24
- 229910052757 nitrogen Inorganic materials 0.000 claims description 22
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 21
- 229910052760 oxygen Inorganic materials 0.000 claims description 19
- 125000005842 heteroatom Chemical group 0.000 claims description 18
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 17
- 229920006395 saturated elastomer Polymers 0.000 claims description 17
- 229910052717 sulfur Inorganic materials 0.000 claims description 16
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 claims description 15
- 239000005562 Glyphosate Substances 0.000 claims description 15
- 229910052731 fluorine Chemical group 0.000 claims description 15
- 238000006467 substitution reaction Methods 0.000 claims description 15
- IAJOBQBIJHVGMQ-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid Chemical group CP(O)(=O)CCC(N)C(O)=O IAJOBQBIJHVGMQ-UHFFFAOYSA-N 0.000 claims description 14
- 229910052799 carbon Inorganic materials 0.000 claims description 14
- 230000000694 effects Effects 0.000 claims description 14
- 239000011737 fluorine Chemical group 0.000 claims description 14
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 claims description 13
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 claims description 13
- XDDAORKBJWWYJS-UHFFFAOYSA-N glyphosate Chemical group OC(=O)CNCP(O)(O)=O XDDAORKBJWWYJS-UHFFFAOYSA-N 0.000 claims description 13
- 150000001721 carbon Chemical group 0.000 claims description 12
- 229940097068 glyphosate Drugs 0.000 claims description 12
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 12
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 claims description 12
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 12
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 12
- 239000005561 Glufosinate Substances 0.000 claims description 11
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 11
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical group C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 claims description 11
- 239000001301 oxygen Substances 0.000 claims description 11
- SEOVTRFCIGRIMH-UHFFFAOYSA-N indole-3-acetic acid Chemical compound C1=CC=C2C(CC(=O)O)=CNC2=C1 SEOVTRFCIGRIMH-UHFFFAOYSA-N 0.000 claims description 10
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 claims description 9
- 108010020183 3-phosphoshikimate 1-carboxyvinyltransferase Proteins 0.000 claims description 9
- 230000015572 biosynthetic process Effects 0.000 claims description 9
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims description 8
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 8
- MFUPLJQNEXUUDW-UHFFFAOYSA-N 2-phenylisoindole-1,3-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1=CC=CC=C1 MFUPLJQNEXUUDW-UHFFFAOYSA-N 0.000 claims description 7
- 229930192334 Auxin Natural products 0.000 claims description 7
- 239000002363 auxin Substances 0.000 claims description 7
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical group BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 7
- 229910052794 bromium Inorganic materials 0.000 claims description 7
- 239000000460 chlorine Substances 0.000 claims description 7
- 229910052801 chlorine Inorganic materials 0.000 claims description 7
- 230000029553 photosynthesis Effects 0.000 claims description 7
- 238000010672 photosynthesis Methods 0.000 claims description 7
- 238000003786 synthesis reaction Methods 0.000 claims description 7
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 claims description 6
- CAAMSDWKXXPUJR-UHFFFAOYSA-N 3,5-dihydro-4H-imidazol-4-one Chemical compound O=C1CNC=N1 CAAMSDWKXXPUJR-UHFFFAOYSA-N 0.000 claims description 6
- 239000005964 Acibenzolar-S-methyl Substances 0.000 claims description 6
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical group O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 claims description 6
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 6
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 6
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 6
- 125000002619 bicyclic group Chemical group 0.000 claims description 6
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 6
- 150000004668 long chain fatty acids Chemical class 0.000 claims description 6
- 125000002950 monocyclic group Chemical group 0.000 claims description 6
- 150000002825 nitriles Chemical class 0.000 claims description 6
- 239000011593 sulfur Substances 0.000 claims description 6
- FNQJDLTXOVEEFB-UHFFFAOYSA-N 1,2,3-benzothiadiazole Chemical compound C1=CC=C2SN=NC2=C1 FNQJDLTXOVEEFB-UHFFFAOYSA-N 0.000 claims description 5
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 5
- 229940123561 Fatty acid inhibitor Drugs 0.000 claims description 5
- 229940100389 Sulfonylurea Drugs 0.000 claims description 5
- 235000010233 benzoic acid Nutrition 0.000 claims description 5
- OILAIQUEIWYQPH-UHFFFAOYSA-N cyclohexane-1,2-dione Chemical compound O=C1CCCCC1=O OILAIQUEIWYQPH-UHFFFAOYSA-N 0.000 claims description 5
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 claims description 5
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 5
- 229920001184 polypeptide Polymers 0.000 claims description 5
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical compound O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 claims description 5
- 150000007659 semicarbazones Chemical class 0.000 claims description 5
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 claims description 5
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims description 4
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical group FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 4
- VXIVSQZSERGHQP-UHFFFAOYSA-N chloroacetamide Chemical group NC(=O)CCl VXIVSQZSERGHQP-UHFFFAOYSA-N 0.000 claims description 4
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 4
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 claims description 3
- QMNWYGTWTXOQTP-UHFFFAOYSA-N 1h-triazin-6-one Chemical compound O=C1C=CN=NN1 QMNWYGTWTXOQTP-UHFFFAOYSA-N 0.000 claims description 3
- 239000005711 Benzoic acid Substances 0.000 claims description 3
- WCHBDQIRELTLEK-UHFFFAOYSA-N CCOC(COC1=NC=CC=C1OC(C=C(C(F)=C1)N(C(C=C(C(C)(F)F)N2C)=O)C2=O)=C1[N+]([O-])=O)=O Chemical compound CCOC(COC1=NC=CC=C1OC(C=C(C(F)=C1)N(C(C=C(C(C)(F)F)N2C)=O)C2=O)=C1[N+]([O-])=O)=O WCHBDQIRELTLEK-UHFFFAOYSA-N 0.000 claims description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 claims description 3
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 claims description 3
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 claims description 3
- 125000002947 alkylene group Chemical group 0.000 claims description 3
- 239000004202 carbamide Substances 0.000 claims description 3
- 150000001732 carboxylic acid derivatives Chemical class 0.000 claims description 3
- 229960005437 etoperidone Drugs 0.000 claims description 3
- 239000013459 phenoxy herbicide Substances 0.000 claims description 3
- YROXIXLRRCOBKF-UHFFFAOYSA-N sulfonylurea Chemical class OC(=N)N=S(=O)=O YROXIXLRRCOBKF-UHFFFAOYSA-N 0.000 claims description 3
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 2
- 125000001153 fluoro group Chemical group F* 0.000 claims description 2
- 125000000842 isoxazolyl group Chemical group 0.000 claims description 2
- 102100029028 Protoporphyrinogen oxidase Human genes 0.000 claims 2
- 101100339555 Zymoseptoria tritici HPPD gene Proteins 0.000 claims 2
- IZBNNCFOBMGTQX-UHFFFAOYSA-N etoperidone Chemical compound O=C1N(CC)C(CC)=NN1CCCN1CCN(C=2C=C(Cl)C=CC=2)CC1 IZBNNCFOBMGTQX-UHFFFAOYSA-N 0.000 claims 1
- 230000009261 transgenic effect Effects 0.000 abstract description 66
- 230000002401 inhibitory effect Effects 0.000 abstract description 24
- 244000038559 crop plants Species 0.000 abstract description 22
- 108020004414 DNA Proteins 0.000 description 93
- 235000018102 proteins Nutrition 0.000 description 90
- 210000004027 cell Anatomy 0.000 description 38
- 239000004480 active ingredient Substances 0.000 description 37
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 27
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 26
- 150000003254 radicals Chemical class 0.000 description 26
- 230000014509 gene expression Effects 0.000 description 24
- 125000004432 carbon atom Chemical group C* 0.000 description 23
- 102000004190 Enzymes Human genes 0.000 description 19
- 108090000790 Enzymes Proteins 0.000 description 19
- 244000068988 Glycine max Species 0.000 description 19
- 229940088598 enzyme Drugs 0.000 description 19
- 230000004048 modification Effects 0.000 description 18
- 238000012986 modification Methods 0.000 description 18
- 235000010469 Glycine max Nutrition 0.000 description 17
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 16
- 125000003342 alkenyl group Chemical group 0.000 description 14
- 125000000217 alkyl group Chemical group 0.000 description 14
- 238000009472 formulation Methods 0.000 description 14
- 239000011734 sodium Substances 0.000 description 14
- 229910052708 sodium Inorganic materials 0.000 description 14
- 230000009466 transformation Effects 0.000 description 14
- 108700019146 Transgenes Proteins 0.000 description 12
- 208000027418 Wounds and injury Diseases 0.000 description 12
- 235000011054 acetic acid Nutrition 0.000 description 12
- 230000001276 controlling effect Effects 0.000 description 12
- 239000004615 ingredient Substances 0.000 description 12
- 208000014674 injury Diseases 0.000 description 12
- 125000001424 substituent group Chemical group 0.000 description 12
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 12
- 108010000700 Acetolactate synthase Proteins 0.000 description 11
- 244000062793 Sorghum vulgare Species 0.000 description 11
- IWEDIXLBFLAXBO-UHFFFAOYSA-N dicamba Chemical compound COC1=C(Cl)C=CC(Cl)=C1C(O)=O IWEDIXLBFLAXBO-UHFFFAOYSA-N 0.000 description 11
- 239000002773 nucleotide Substances 0.000 description 11
- 125000003729 nucleotide group Chemical group 0.000 description 11
- 239000013598 vector Substances 0.000 description 11
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 10
- 241000219146 Gossypium Species 0.000 description 10
- 101710163270 Nuclease Proteins 0.000 description 10
- 241000209094 Oryza Species 0.000 description 10
- 125000004430 oxygen atom Chemical group O* 0.000 description 10
- 239000005631 2,4-Dichlorophenoxyacetic acid Substances 0.000 description 9
- WVQBLGZPHOPPFO-UHFFFAOYSA-N 2-chloro-N-(2-ethyl-6-methylphenyl)-N-(1-methoxypropan-2-yl)acetamide Chemical compound CCC1=CC=CC(C)=C1N(C(C)COC)C(=O)CCl WVQBLGZPHOPPFO-UHFFFAOYSA-N 0.000 description 9
- 229920000742 Cotton Polymers 0.000 description 9
- 235000004341 Gossypium herbaceum Nutrition 0.000 description 9
- 240000002024 Gossypium herbaceum Species 0.000 description 9
- 235000007164 Oryza sativa Nutrition 0.000 description 9
- 240000008042 Zea mays Species 0.000 description 9
- 230000005782 double-strand break Effects 0.000 description 9
- 150000002148 esters Chemical class 0.000 description 9
- BGZZWXTVIYUUEY-UHFFFAOYSA-N fomesafen Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)C)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 BGZZWXTVIYUUEY-UHFFFAOYSA-N 0.000 description 9
- 238000010362 genome editing Methods 0.000 description 9
- 238000003780 insertion Methods 0.000 description 9
- 230000037431 insertion Effects 0.000 description 9
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 9
- 235000009566 rice Nutrition 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 8
- 108010068327 4-hydroxyphenylpyruvate dioxygenase Proteins 0.000 description 8
- 102100028626 4-hydroxyphenylpyruvate dioxygenase Human genes 0.000 description 8
- 102000000452 Acetyl-CoA carboxylase Human genes 0.000 description 8
- 108010016219 Acetyl-CoA carboxylase Proteins 0.000 description 8
- 108010018763 Biotin carboxylase Proteins 0.000 description 8
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 8
- 239000002253 acid Substances 0.000 description 8
- 125000003545 alkoxy group Chemical group 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 125000005843 halogen group Chemical group 0.000 description 8
- 125000004269 oxiran-2-yl group Chemical group [H]C1([H])OC1([H])* 0.000 description 8
- FIKAKWIAUPDISJ-UHFFFAOYSA-L paraquat dichloride Chemical compound [Cl-].[Cl-].C1=C[N+](C)=CC=C1C1=CC=[N+](C)C=C1 FIKAKWIAUPDISJ-UHFFFAOYSA-L 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- GQQIAHNFBAFBCS-UHFFFAOYSA-N 2-[2-chloro-5-(1,3-dioxo-4,5,6,7-tetrahydroisoindol-2-yl)-4-fluorophenoxy]acetic acid Chemical compound C1=C(Cl)C(OCC(=O)O)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1F GQQIAHNFBAFBCS-UHFFFAOYSA-N 0.000 description 7
- XTDZGXBTXBEZDN-UHFFFAOYSA-N 3-(difluoromethyl)-N-(9-isopropyl-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl)-1-methylpyrazole-4-carboxamide Chemical compound CC(C)C1C2CCC1C1=C2C=CC=C1NC(=O)C1=CN(C)N=C1C(F)F XTDZGXBTXBEZDN-UHFFFAOYSA-N 0.000 description 7
- 108020004705 Codon Proteins 0.000 description 7
- 239000005504 Dicamba Substances 0.000 description 7
- 239000005799 Isopyrazam Substances 0.000 description 7
- CHNUNORXWHYHNE-UHFFFAOYSA-N Oxadiazon Chemical compound C1=C(Cl)C(OC(C)C)=CC(N2C(OC(=N2)C(C)(C)C)=O)=C1Cl CHNUNORXWHYHNE-UHFFFAOYSA-N 0.000 description 7
- OQMBBFQZGJFLBU-UHFFFAOYSA-N Oxyfluorfen Chemical compound C1=C([N+]([O-])=O)C(OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 OQMBBFQZGJFLBU-UHFFFAOYSA-N 0.000 description 7
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 7
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 7
- 150000001450 anions Chemical class 0.000 description 7
- JEDYYFXHPAIBGR-UHFFFAOYSA-N butafenacil Chemical compound O=C1N(C)C(C(F)(F)F)=CC(=O)N1C1=CC=C(Cl)C(C(=O)OC(C)(C)C(=O)OCC=C)=C1 JEDYYFXHPAIBGR-UHFFFAOYSA-N 0.000 description 7
- QMTNOLKHSWIQBE-KSZLIROESA-N exo-(-)-cinmethylin Chemical compound O([C@@H]1[C@@]2(C)CC[C@](O2)(C1)C(C)C)CC1=CC=CC=C1C QMTNOLKHSWIQBE-KSZLIROESA-N 0.000 description 7
- XFZUQTKDBCOXPP-UHFFFAOYSA-N florpyrauxifen Chemical compound COC1=C(Cl)C=CC(C=2C(=C(N)C(Cl)=C(C(O)=O)N=2)F)=C1F XFZUQTKDBCOXPP-UHFFFAOYSA-N 0.000 description 7
- YFONKFDEZLYQDH-BOURZNODSA-N indaziflam Chemical compound CC(F)C1=NC(N)=NC(N[C@H]2C3=CC(C)=CC=C3C[C@@H]2C)=N1 YFONKFDEZLYQDH-BOURZNODSA-N 0.000 description 7
- ZNJFBWYDHIGLCU-HWKXXFMVSA-N jasmonic acid Chemical compound CC\C=C/C[C@@H]1[C@@H](CC(O)=O)CCC1=O ZNJFBWYDHIGLCU-HWKXXFMVSA-N 0.000 description 7
- 150000007523 nucleic acids Chemical class 0.000 description 7
- 239000011591 potassium Substances 0.000 description 7
- 229910052700 potassium Inorganic materials 0.000 description 7
- 241000894007 species Species 0.000 description 7
- OORLZFUTLGXMEF-UHFFFAOYSA-N sulfentrazone Chemical compound O=C1N(C(F)F)C(C)=NN1C1=CC(NS(C)(=O)=O)=C(Cl)C=C1Cl OORLZFUTLGXMEF-UHFFFAOYSA-N 0.000 description 7
- AZHZOGYUMMIAOF-UHFFFAOYSA-N trifludimoxazin Chemical compound O=C1N(C)C(=S)N(C)C(=O)N1C(C(=C1)F)=CC2=C1OC(F)(F)C(=O)N2CC#C AZHZOGYUMMIAOF-UHFFFAOYSA-N 0.000 description 7
- AIAYSXFWIUNXRC-PHIMTYICSA-N (1r,5s)-3-[hydroxy-[2-(2-methoxyethoxymethyl)-6-(trifluoromethyl)pyridin-3-yl]methylidene]bicyclo[3.2.1]octane-2,4-dione Chemical compound COCCOCC1=NC(C(F)(F)F)=CC=C1C(O)=C1C(=O)[C@@H](C2)CC[C@@H]2C1=O AIAYSXFWIUNXRC-PHIMTYICSA-N 0.000 description 6
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 6
- WVQBLGZPHOPPFO-LBPRGKRZSA-N (S)-metolachlor Chemical compound CCC1=CC=CC(C)=C1N([C@@H](C)COC)C(=O)CCl WVQBLGZPHOPPFO-LBPRGKRZSA-N 0.000 description 6
- YIVXMZJTEQBPQO-UHFFFAOYSA-N 2,4-DB Chemical compound OC(=O)CCCOC1=CC=C(Cl)C=C1Cl YIVXMZJTEQBPQO-UHFFFAOYSA-N 0.000 description 6
- NUPJIGQFXCQJBK-UHFFFAOYSA-N 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)-5-(methoxymethyl)nicotinic acid Chemical compound OC(=O)C1=CC(COC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 NUPJIGQFXCQJBK-UHFFFAOYSA-N 0.000 description 6
- YHKBGVDUSSWOAB-UHFFFAOYSA-N 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]-4-fluorophenyl}propanoic acid Chemical compound O=C1N(C(F)F)C(C)=NN1C1=CC(CC(Cl)C(O)=O)=C(Cl)C=C1F YHKBGVDUSSWOAB-UHFFFAOYSA-N 0.000 description 6
- JLYFCTQDENRSOL-UHFFFAOYSA-N 2-chloro-N-(2,4-dimethylthiophen-3-yl)-N-(1-methoxypropan-2-yl)acetamide Chemical compound COCC(C)N(C(=O)CCl)C=1C(C)=CSC=1C JLYFCTQDENRSOL-UHFFFAOYSA-N 0.000 description 6
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 6
- CASLETQIYIQFTQ-UHFFFAOYSA-N 3-[[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)pyrazol-4-yl]methylsulfonyl]-5,5-dimethyl-4h-1,2-oxazole Chemical compound CN1N=C(C(F)(F)F)C(CS(=O)(=O)C=2CC(C)(C)ON=2)=C1OC(F)F CASLETQIYIQFTQ-UHFFFAOYSA-N 0.000 description 6
- VTNQPKFIQCLBDU-UHFFFAOYSA-N Acetochlor Chemical compound CCOCN(C(=O)CCl)C1=C(C)C=CC=C1CC VTNQPKFIQCLBDU-UHFFFAOYSA-N 0.000 description 6
- KXDAEFPNCMNJSK-UHFFFAOYSA-N Benzamide Chemical compound NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 6
- BXEHUCNTIZGSOJ-UHFFFAOYSA-N Esprocarb Chemical compound CC(C)C(C)N(CC)C(=O)SCC1=CC=CC=C1 BXEHUCNTIZGSOJ-UHFFFAOYSA-N 0.000 description 6
- HWATZEJQIXKWQS-UHFFFAOYSA-N Flazasulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)C(F)(F)F)=N1 HWATZEJQIXKWQS-UHFFFAOYSA-N 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- XVOKUMIPKHGGTN-UHFFFAOYSA-N Imazethapyr Chemical compound OC(=O)C1=CC(CC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 XVOKUMIPKHGGTN-UHFFFAOYSA-N 0.000 description 6
- 239000005588 Oxadiazon Substances 0.000 description 6
- FCOHEOSCARXMMS-UHFFFAOYSA-N Oxaziclomefone Chemical compound C1OC(C)=C(C=2C=CC=CC=2)C(=O)N1C(C)(C)C1=CC(Cl)=CC(Cl)=C1 FCOHEOSCARXMMS-UHFFFAOYSA-N 0.000 description 6
- 239000005590 Oxyfluorfen Substances 0.000 description 6
- 108010091086 Recombinases Proteins 0.000 description 6
- 102000018120 Recombinases Human genes 0.000 description 6
- MWBPRDONLNQCFV-UHFFFAOYSA-N Tri-allate Chemical compound CC(C)N(C(C)C)C(=O)SCC(Cl)=C(Cl)Cl MWBPRDONLNQCFV-UHFFFAOYSA-N 0.000 description 6
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 6
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 6
- NUFNQYOELLVIPL-UHFFFAOYSA-N acifluorfen Chemical compound C1=C([N+]([O-])=O)C(C(=O)O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NUFNQYOELLVIPL-UHFFFAOYSA-N 0.000 description 6
- DDBMQDADIHOWIC-UHFFFAOYSA-N aclonifen Chemical compound C1=C([N+]([O-])=O)C(N)=C(Cl)C(OC=2C=CC=CC=2)=C1 DDBMQDADIHOWIC-UHFFFAOYSA-N 0.000 description 6
- 125000004414 alkyl thio group Chemical group 0.000 description 6
- 125000000304 alkynyl group Chemical group 0.000 description 6
- MXWJVTOOROXGIU-UHFFFAOYSA-N atrazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)C)=N1 MXWJVTOOROXGIU-UHFFFAOYSA-N 0.000 description 6
- 239000002585 base Substances 0.000 description 6
- ZOMSMJKLGFBRBS-UHFFFAOYSA-N bentazone Chemical compound C1=CC=C2NS(=O)(=O)N(C(C)C)C(=O)C2=C1 ZOMSMJKLGFBRBS-UHFFFAOYSA-N 0.000 description 6
- SILSDTWXNBZOGF-KUZBFYBWSA-N chembl111058 Chemical compound CCSC(C)CC1CC(O)=C(\C(CC)=N\OC\C=C\Cl)C(=O)C1 SILSDTWXNBZOGF-KUZBFYBWSA-N 0.000 description 6
- BXIGJZDQFDFASM-UHFFFAOYSA-N cyclopyrimorate Chemical compound N=1N=C(Cl)C=C(OC(=O)N2CCOCC2)C=1OC=1C(C)=CC=CC=1C1CC1 BXIGJZDQFDFASM-UHFFFAOYSA-N 0.000 description 6
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 6
- WCGGWVOVFQNRRS-UHFFFAOYSA-N dichloroacetamide Chemical compound NC(=O)C(Cl)Cl WCGGWVOVFQNRRS-UHFFFAOYSA-N 0.000 description 6
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 6
- SYJFEGQWDCRVNX-UHFFFAOYSA-N diquat Chemical compound C1=CC=[N+]2CC[N+]3=CC=CC=C3C2=C1 SYJFEGQWDCRVNX-UHFFFAOYSA-N 0.000 description 6
- 241001233957 eudicotyledons Species 0.000 description 6
- IANUJLZYFUDJIH-UHFFFAOYSA-N flufenacet Chemical compound C=1C=C(F)C=CC=1N(C(C)C)C(=O)COC1=NN=C(C(F)(F)F)S1 IANUJLZYFUDJIH-UHFFFAOYSA-N 0.000 description 6
- FOUWCSDKDDHKQP-UHFFFAOYSA-N flumioxazin Chemical compound FC1=CC=2OCC(=O)N(CC#C)C=2C=C1N(C1=O)C(=O)C2=C1CCCC2 FOUWCSDKDDHKQP-UHFFFAOYSA-N 0.000 description 6
- 102000005396 glutamine synthetase Human genes 0.000 description 6
- 108020002326 glutamine synthetase Proteins 0.000 description 6
- 239000008187 granular material Substances 0.000 description 6
- 125000001188 haloalkyl group Chemical group 0.000 description 6
- OYIKARCXOQLFHF-UHFFFAOYSA-N isoxaflutole Chemical compound CS(=O)(=O)C1=CC(C(F)(F)F)=CC=C1C(=O)C1=C(C2CC2)ON=C1 OYIKARCXOQLFHF-UHFFFAOYSA-N 0.000 description 6
- CONWAEURSVPLRM-UHFFFAOYSA-N lactofen Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC(C)C(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 CONWAEURSVPLRM-UHFFFAOYSA-N 0.000 description 6
- 235000009973 maize Nutrition 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- KPUREKXXPHOJQT-UHFFFAOYSA-N mesotrione Chemical compound [O-][N+](=O)C1=CC(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O KPUREKXXPHOJQT-UHFFFAOYSA-N 0.000 description 6
- QPTPZPIXUPELRM-UHFFFAOYSA-N methyl 3-[2-[2-chloro-4-fluoro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]phenyl]sulfanylpropanoylamino]propanoate Chemical compound C1=C(Cl)C(SC(C)C(=O)NCCC(=O)OC)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1F QPTPZPIXUPELRM-UHFFFAOYSA-N 0.000 description 6
- 230000002438 mitochondrial effect Effects 0.000 description 6
- FBUKVWPVBMHYJY-UHFFFAOYSA-N nonanoic acid Chemical compound CCCCCCCCC(O)=O FBUKVWPVBMHYJY-UHFFFAOYSA-N 0.000 description 6
- CHIFOSRWCNZCFN-UHFFFAOYSA-N pendimethalin Chemical compound CCC(CC)NC1=C([N+]([O-])=O)C=C(C)C(C)=C1[N+]([O-])=O CHIFOSRWCNZCFN-UHFFFAOYSA-N 0.000 description 6
- SIOXPEMLGUPBBT-UHFFFAOYSA-M picolinate Chemical compound [O-]C(=O)C1=CC=CC=N1 SIOXPEMLGUPBBT-UHFFFAOYSA-M 0.000 description 6
- YXIIPOGUBVYZIW-UHFFFAOYSA-N pyraflufen Chemical compound ClC1=C(OC(F)F)N(C)N=C1C1=CC(OCC(O)=O)=C(Cl)C=C1F YXIIPOGUBVYZIW-UHFFFAOYSA-N 0.000 description 6
- GNHDVXLWBQYPJE-UHFFFAOYSA-N saflufenacil Chemical compound C1=C(Cl)C(C(=O)NS(=O)(=O)N(C)C(C)C)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1F GNHDVXLWBQYPJE-UHFFFAOYSA-N 0.000 description 6
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 6
- IUQAXCIUEPFPSF-UHFFFAOYSA-N tembotrione Chemical compound ClC1=C(COCC(F)(F)F)C(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O IUQAXCIUEPFPSF-UHFFFAOYSA-N 0.000 description 6
- FZXISNSWEXTPMF-UHFFFAOYSA-N terbutylazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)(C)C)=N1 FZXISNSWEXTPMF-UHFFFAOYSA-N 0.000 description 6
- GLDAZAQRGCSFNP-UHFFFAOYSA-N thiencarbazone Chemical compound O=C1N(C)C(OC)=NN1C(=O)NS(=O)(=O)C1=C(C)SC=C1C(O)=O GLDAZAQRGCSFNP-UHFFFAOYSA-N 0.000 description 6
- JLIDBLDQVAYHNE-YKALOCIXSA-N (+)-Abscisic acid Chemical compound OC(=O)/C=C(/C)\C=C\[C@@]1(O)C(C)=CC(=O)CC1(C)C JLIDBLDQVAYHNE-YKALOCIXSA-N 0.000 description 5
- 125000004767 (C1-C4) haloalkoxy group Chemical group 0.000 description 5
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 description 5
- GHLCSCRDVVEUQD-UHFFFAOYSA-N 1-({1-ethyl-4-[3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoyl]-1H-pyrazol-5-yl}oxy)ethyl methyl carbonate Chemical compound CCN1N=CC(C(=O)C=2C(=C(OCCOC)C(=CC=2)S(C)(=O)=O)C)=C1OC(C)OC(=O)OC GHLCSCRDVVEUQD-UHFFFAOYSA-N 0.000 description 5
- 239000002794 2,4-DB Substances 0.000 description 5
- MZHCENGPTKEIGP-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1Cl MZHCENGPTKEIGP-UHFFFAOYSA-N 0.000 description 5
- WNTGYJSOUMFZEP-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1C WNTGYJSOUMFZEP-UHFFFAOYSA-N 0.000 description 5
- CLQMBPJKHLGMQK-UHFFFAOYSA-N 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)nicotinic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC=CC=C1C(O)=O CLQMBPJKHLGMQK-UHFFFAOYSA-N 0.000 description 5
- MAYMYMXYWIVVOK-UHFFFAOYSA-N 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-4-(methanesulfonamidomethyl)benzoic acid Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=C(CNS(C)(=O)=O)C=2)C(O)=O)=N1 MAYMYMXYWIVVOK-UHFFFAOYSA-N 0.000 description 5
- IRJQWZWMQCVOLA-DNTJNYDQSA-N 2-[(e)-n-[(3,5-difluorophenyl)carbamoylamino]-c-methylcarbonimidoyl]pyridine-3-carboxylic acid Chemical compound N=1C=CC=C(C(O)=O)C=1C(/C)=N/NC(=O)NC1=CC(F)=CC(F)=C1 IRJQWZWMQCVOLA-DNTJNYDQSA-N 0.000 description 5
- UPMXNNIRAGDFEH-UHFFFAOYSA-N 3,5-dibromo-4-hydroxybenzonitrile Chemical compound OC1=C(Br)C=C(C#N)C=C1Br UPMXNNIRAGDFEH-UHFFFAOYSA-N 0.000 description 5
- WGAMTMFKUSYNHH-UHFFFAOYSA-N 4,5-dihydro-1,2-oxazole-5-carboxamide Chemical compound NC(=O)C1CC=NO1 WGAMTMFKUSYNHH-UHFFFAOYSA-N 0.000 description 5
- MBFHUWCOCCICOK-UHFFFAOYSA-N 4-iodo-2-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]benzoic acid Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=C(I)C=2)C(O)=O)=N1 MBFHUWCOCCICOK-UHFFFAOYSA-N 0.000 description 5
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 5
- 239000002890 Aclonifen Substances 0.000 description 5
- 102000008682 Argonaute Proteins Human genes 0.000 description 5
- 108010088141 Argonaute Proteins Proteins 0.000 description 5
- 239000005476 Bentazone Substances 0.000 description 5
- 239000005497 Clethodim Substances 0.000 description 5
- 108091026890 Coding region Proteins 0.000 description 5
- NNYRZQHKCHEXSD-UHFFFAOYSA-N Daimuron Chemical compound C1=CC(C)=CC=C1NC(=O)NC(C)(C)C1=CC=CC=C1 NNYRZQHKCHEXSD-UHFFFAOYSA-N 0.000 description 5
- 239000005510 Diuron Substances 0.000 description 5
- 241000588724 Escherichia coli Species 0.000 description 5
- 239000005514 Flazasulfuron Substances 0.000 description 5
- 239000005531 Flufenacet Substances 0.000 description 5
- DHAHEVIQIYRFRG-UHFFFAOYSA-N Fluoroglycofen Chemical compound C1=C([N+]([O-])=O)C(C(=O)OCC(=O)O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 DHAHEVIQIYRFRG-UHFFFAOYSA-N 0.000 description 5
- 239000005566 Imazamox Substances 0.000 description 5
- 239000005568 Iodosulfuron Substances 0.000 description 5
- 239000005571 Isoxaflutole Substances 0.000 description 5
- 239000005577 Mesosulfuron Substances 0.000 description 5
- 239000005578 Mesotrione Substances 0.000 description 5
- RRVIAQKBTUQODI-UHFFFAOYSA-N Methabenzthiazuron Chemical compound C1=CC=C2SC(N(C)C(=O)NC)=NC2=C1 RRVIAQKBTUQODI-UHFFFAOYSA-N 0.000 description 5
- 239000005583 Metribuzin Substances 0.000 description 5
- 108050006807 Nicotinic acetylcholine receptors Proteins 0.000 description 5
- 239000005591 Pendimethalin Substances 0.000 description 5
- 239000005617 S-Metolachlor Substances 0.000 description 5
- 239000005620 Tembotrione Substances 0.000 description 5
- 239000005621 Terbuthylazine Substances 0.000 description 5
- 239000005622 Thiencarbazone Substances 0.000 description 5
- WHKUVVPPKQRRBV-UHFFFAOYSA-N Trasan Chemical compound CC1=CC(Cl)=CC=C1OCC(O)=O WHKUVVPPKQRRBV-UHFFFAOYSA-N 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- CNBGNNVCVSKAQZ-UHFFFAOYSA-N benzidamine Natural products C12=CC=CC=C2C(OCCCN(C)C)=NN1CC1=CC=CC=C1 CNBGNNVCVSKAQZ-UHFFFAOYSA-N 0.000 description 5
- GINJFDRNADDBIN-FXQIFTODSA-N bilanafos Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCP(C)(O)=O GINJFDRNADDBIN-FXQIFTODSA-N 0.000 description 5
- FHUKASKVKWSLCY-UHFFFAOYSA-N bixlozone Chemical compound O=C1C(C)(C)CON1CC1=CC=C(Cl)C=C1Cl FHUKASKVKWSLCY-UHFFFAOYSA-N 0.000 description 5
- 239000011575 calcium Substances 0.000 description 5
- 229910052791 calcium Inorganic materials 0.000 description 5
- 235000013877 carbamide Nutrition 0.000 description 5
- 125000002837 carbocyclic group Chemical group 0.000 description 5
- 125000003636 chemical group Chemical group 0.000 description 5
- KIEDNEWSYUYDSN-UHFFFAOYSA-N clomazone Chemical compound O=C1C(C)(C)CON1CC1=CC=CC=C1Cl KIEDNEWSYUYDSN-UHFFFAOYSA-N 0.000 description 5
- MZZBPDKVEFVLFF-UHFFFAOYSA-N cyanazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)(C)C#N)=N1 MZZBPDKVEFVLFF-UHFFFAOYSA-N 0.000 description 5
- 125000000753 cycloalkyl group Chemical group 0.000 description 5
- WYEHFWKAOXOVJD-UHFFFAOYSA-N diflufenican Chemical compound FC1=CC(F)=CC=C1NC(=O)C1=CC=CN=C1OC1=CC=CC(C(F)(F)F)=C1 WYEHFWKAOXOVJD-UHFFFAOYSA-N 0.000 description 5
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 5
- 239000000839 emulsion Substances 0.000 description 5
- ZCNQYNHDVRPZIH-UHFFFAOYSA-N fluthiacet-methyl Chemical group C1=C(Cl)C(SCC(=O)OC)=CC(N=C2N3CCCCN3C(=O)S2)=C1F ZCNQYNHDVRPZIH-UHFFFAOYSA-N 0.000 description 5
- PXDNXJSDGQBLKS-UHFFFAOYSA-N foramsulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=C(NC=O)C=2)C(=O)N(C)C)=N1 PXDNXJSDGQBLKS-UHFFFAOYSA-N 0.000 description 5
- KKLBEFSLWYDQFI-UHFFFAOYSA-N halauxifen Chemical compound COC1=C(Cl)C=CC(C=2N=C(C(Cl)=C(N)C=2)C(O)=O)=C1F KKLBEFSLWYDQFI-UHFFFAOYSA-N 0.000 description 5
- 125000004995 haloalkylthio group Chemical group 0.000 description 5
- RUCAXVJJQQJZGU-UHFFFAOYSA-M hydron;2-(phosphonatomethylamino)acetate;trimethylsulfanium Chemical compound C[S+](C)C.OP(O)(=O)CNCC([O-])=O RUCAXVJJQQJZGU-UHFFFAOYSA-M 0.000 description 5
- 230000001939 inductive effect Effects 0.000 description 5
- PUIYMUZLKQOUOZ-UHFFFAOYSA-N isoproturon Chemical compound CC(C)C1=CC=C(NC(=O)N(C)C)C=C1 PUIYMUZLKQOUOZ-UHFFFAOYSA-N 0.000 description 5
- MWKVXOJATACCCH-UHFFFAOYSA-N isoxadifen-ethyl Chemical compound C1C(C(=O)OCC)=NOC1(C=1C=CC=CC=1)C1=CC=CC=C1 MWKVXOJATACCCH-UHFFFAOYSA-N 0.000 description 5
- 229940088649 isoxaflutole Drugs 0.000 description 5
- FOXFZRUHNHCZPX-UHFFFAOYSA-N metribuzin Chemical compound CSC1=NN=C(C(C)(C)C)C(=O)N1N FOXFZRUHNHCZPX-UHFFFAOYSA-N 0.000 description 5
- 230000000813 microbial effect Effects 0.000 description 5
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 125000004043 oxo group Chemical group O=* 0.000 description 5
- MGOHCFMYLBAPRN-UHFFFAOYSA-N pinoxaden Chemical compound CCC1=CC(C)=CC(CC)=C1C(C1=O)=C(OC(=O)C(C)(C)C)N2N1CCOCC2 MGOHCFMYLBAPRN-UHFFFAOYSA-N 0.000 description 5
- 239000005648 plant growth regulator Substances 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- DWSPRBSLSXQIEJ-UHFFFAOYSA-N pyrasulfotole Chemical compound CC1=NN(C)C(O)=C1C(=O)C1=CC=C(C(F)(F)F)C=C1S(C)(=O)=O DWSPRBSLSXQIEJ-UHFFFAOYSA-N 0.000 description 5
- 239000004094 surface-active agent Substances 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- NYHLMHAKWBUZDY-QMMMGPOBSA-N (2s)-2-[2-chloro-5-[2-chloro-4-(trifluoromethyl)phenoxy]benzoyl]oxypropanoic acid Chemical compound C1=C(Cl)C(C(=O)O[C@@H](C)C(O)=O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NYHLMHAKWBUZDY-QMMMGPOBSA-N 0.000 description 4
- RBSXHDIPCIWOMG-UHFFFAOYSA-N 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethylsulfonylimidazo[1,2-a]pyridin-3-yl)sulfonylurea Chemical compound CCS(=O)(=O)C=1N=C2C=CC=CN2C=1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 RBSXHDIPCIWOMG-UHFFFAOYSA-N 0.000 description 4
- SNTWKPAKVQFCCF-UHFFFAOYSA-N 2,3-dihydro-1h-triazole Chemical compound N1NC=CN1 SNTWKPAKVQFCCF-UHFFFAOYSA-N 0.000 description 4
- HCNBYBFTNHEQQJ-UHFFFAOYSA-N 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-6-(trifluoromethyl)pyridine-3-carboxylic acid Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=C(N=2)C(F)(F)F)C(O)=O)=N1 HCNBYBFTNHEQQJ-UHFFFAOYSA-N 0.000 description 4
- UWHURBUBIHUHSU-UHFFFAOYSA-N 2-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]benzoic acid Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 UWHURBUBIHUHSU-UHFFFAOYSA-N 0.000 description 4
- OOLBCHYXZDXLDS-UHFFFAOYSA-N 2-[4-(2,4-dichlorophenoxy)phenoxy]propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CC=C(Cl)C=C1Cl OOLBCHYXZDXLDS-UHFFFAOYSA-N 0.000 description 4
- MPPOHAUSNPTFAJ-UHFFFAOYSA-N 2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 MPPOHAUSNPTFAJ-UHFFFAOYSA-N 0.000 description 4
- CABMTIJINOIHOD-UHFFFAOYSA-N 2-[4-methyl-5-oxo-4-(propan-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline-3-carboxylic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC2=CC=CC=C2C=C1C(O)=O CABMTIJINOIHOD-UHFFFAOYSA-N 0.000 description 4
- LLWADFLAOKUBDR-UHFFFAOYSA-N 2-methyl-4-chlorophenoxybutyric acid Chemical compound CC1=CC(Cl)=CC=C1OCCCC(O)=O LLWADFLAOKUBDR-UHFFFAOYSA-N 0.000 description 4
- ABOOPXYCKNFDNJ-UHFFFAOYSA-N 2-{4-[(6-chloroquinoxalin-2-yl)oxy]phenoxy}propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 ABOOPXYCKNFDNJ-UHFFFAOYSA-N 0.000 description 4
- BPPVUXSMLBXYGG-UHFFFAOYSA-N 4-[3-(4,5-dihydro-1,2-oxazol-3-yl)-2-methyl-4-methylsulfonylbenzoyl]-2-methyl-1h-pyrazol-3-one Chemical compound CC1=C(C(=O)C=2C(N(C)NC=2)=O)C=CC(S(C)(=O)=O)=C1C1=NOCC1 BPPVUXSMLBXYGG-UHFFFAOYSA-N 0.000 description 4
- NYRMIJKDBAQCHC-UHFFFAOYSA-N 5-(methylamino)-2-phenyl-4-[3-(trifluoromethyl)phenyl]furan-3(2H)-one Chemical compound O1C(NC)=C(C=2C=C(C=CC=2)C(F)(F)F)C(=O)C1C1=CC=CC=C1 NYRMIJKDBAQCHC-UHFFFAOYSA-N 0.000 description 4
- QQOGZMUZAZWLJH-UHFFFAOYSA-N 5-[2-chloro-6-fluoro-4-(trifluoromethyl)phenoxy]-n-ethylsulfonyl-2-nitrobenzamide Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)CC)=CC(OC=2C(=CC(=CC=2F)C(F)(F)F)Cl)=C1 QQOGZMUZAZWLJH-UHFFFAOYSA-N 0.000 description 4
- PVSGXWMWNRGTKE-UHFFFAOYSA-N 5-methyl-2-[4-methyl-5-oxo-4-(propan-2-yl)-4,5-dihydro-1H-imidazol-2-yl]pyridine-3-carboxylic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC=C(C)C=C1C(O)=O PVSGXWMWNRGTKE-UHFFFAOYSA-N 0.000 description 4
- DVOODWOZJVJKQR-UHFFFAOYSA-N 5-tert-butyl-3-(2,4-dichloro-5-prop-2-ynoxyphenyl)-1,3,4-oxadiazol-2-one Chemical group O=C1OC(C(C)(C)C)=NN1C1=CC(OCC#C)=C(Cl)C=C1Cl DVOODWOZJVJKQR-UHFFFAOYSA-N 0.000 description 4
- HZKBYBNLTLVSPX-UHFFFAOYSA-N 6-[(6,6-dimethyl-5,7-dihydropyrrolo[2,1-c][1,2,4]thiadiazol-3-ylidene)amino]-7-fluoro-4-prop-2-ynyl-1,4-benzoxazin-3-one Chemical compound C#CCN1C(=O)COC(C=C2F)=C1C=C2N=C1SN=C2CC(C)(C)CN21 HZKBYBNLTLVSPX-UHFFFAOYSA-N 0.000 description 4
- XKJMBINCVNINCA-UHFFFAOYSA-N Alfalone Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XKJMBINCVNINCA-UHFFFAOYSA-N 0.000 description 4
- KLSJWNVTNUYHDU-UHFFFAOYSA-N Amitrole Chemical compound NC1=NC=NN1 KLSJWNVTNUYHDU-UHFFFAOYSA-N 0.000 description 4
- 241000193388 Bacillus thuringiensis Species 0.000 description 4
- 239000005489 Bromoxynil Substances 0.000 description 4
- 108091033409 CRISPR Proteins 0.000 description 4
- DXXVCXKMSWHGTF-UHFFFAOYSA-N Chlomethoxyfen Chemical compound C1=C([N+]([O-])=O)C(OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 DXXVCXKMSWHGTF-UHFFFAOYSA-N 0.000 description 4
- NLYNUTMZTCLNOO-UHFFFAOYSA-N Chlorbromuron Chemical compound CON(C)C(=O)NC1=CC=C(Br)C(Cl)=C1 NLYNUTMZTCLNOO-UHFFFAOYSA-N 0.000 description 4
- 239000005499 Clomazone Substances 0.000 description 4
- VYNOULHXXDFBLU-UHFFFAOYSA-N Cumyluron Chemical compound C=1C=CC=CC=1C(C)(C)NC(=O)NCC1=CC=CC=C1Cl VYNOULHXXDFBLU-UHFFFAOYSA-N 0.000 description 4
- QNXAVFXEJCPCJO-UHFFFAOYSA-N Diclosulam Chemical compound N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1Cl QNXAVFXEJCPCJO-UHFFFAOYSA-N 0.000 description 4
- 239000005507 Diflufenican Substances 0.000 description 4
- 108010028143 Dioxygenases Proteins 0.000 description 4
- 102000016680 Dioxygenases Human genes 0.000 description 4
- 239000005630 Diquat Substances 0.000 description 4
- 108010042407 Endonucleases Proteins 0.000 description 4
- 102000004533 Endonucleases Human genes 0.000 description 4
- UWVKRNOCDUPIDM-UHFFFAOYSA-N Ethoxysulfuron Chemical compound CCOC1=CC=CC=C1OS(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 UWVKRNOCDUPIDM-UHFFFAOYSA-N 0.000 description 4
- YQVMVCCFZCMYQB-UHFFFAOYSA-N Flamprop Chemical compound C=1C=C(F)C(Cl)=CC=1N(C(C)C(O)=O)C(=O)C1=CC=CC=C1 YQVMVCCFZCMYQB-UHFFFAOYSA-N 0.000 description 4
- QZXATCCPQKOEIH-UHFFFAOYSA-N Florasulam Chemical compound N=1N2C(OC)=NC=C(F)C2=NC=1S(=O)(=O)NC1=C(F)C=CC=C1F QZXATCCPQKOEIH-UHFFFAOYSA-N 0.000 description 4
- RXCPQSJAVKGONC-UHFFFAOYSA-N Flumetsulam Chemical compound N1=C2N=C(C)C=CN2N=C1S(=O)(=O)NC1=C(F)C=CC=C1F RXCPQSJAVKGONC-UHFFFAOYSA-N 0.000 description 4
- YWBVHLJPRPCRSD-UHFFFAOYSA-N Fluridone Chemical compound O=C1C(C=2C=C(C=CC=2)C(F)(F)F)=CN(C)C=C1C1=CC=CC=C1 YWBVHLJPRPCRSD-UHFFFAOYSA-N 0.000 description 4
- 239000005560 Foramsulfuron Substances 0.000 description 4
- 241000238631 Hexapoda Species 0.000 description 4
- CAWXEEYDBZRFPE-UHFFFAOYSA-N Hexazinone Chemical compound O=C1N(C)C(N(C)C)=NC(=O)N1C1CCCCC1 CAWXEEYDBZRFPE-UHFFFAOYSA-N 0.000 description 4
- NAGRVUXEKKZNHT-UHFFFAOYSA-N Imazosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N3C=CC=CC3=NC=2Cl)=N1 NAGRVUXEKKZNHT-UHFFFAOYSA-N 0.000 description 4
- GEWDNTWNSAZUDX-UHFFFAOYSA-N Jasmonic Acid Methyl Ester Chemical compound CCC=CCC1C(CC(=O)OC)CCC1=O GEWDNTWNSAZUDX-UHFFFAOYSA-N 0.000 description 4
- 241000209510 Liliopsida Species 0.000 description 4
- SUSRORUBZHMPCO-UHFFFAOYSA-N MC-4379 Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 SUSRORUBZHMPCO-UHFFFAOYSA-N 0.000 description 4
- 239000005574 MCPA Substances 0.000 description 4
- LGDSHSYDSCRFAB-UHFFFAOYSA-N Methyl isothiocyanate Chemical compound CN=C=S LGDSHSYDSCRFAB-UHFFFAOYSA-N 0.000 description 4
- WLFDQEVORAMCIM-UHFFFAOYSA-N Metobromuron Chemical compound CON(C)C(=O)NC1=CC=C(Br)C=C1 WLFDQEVORAMCIM-UHFFFAOYSA-N 0.000 description 4
- VGHPMIFEKOFHHQ-UHFFFAOYSA-N Metosulam Chemical compound N1=C2N=C(OC)C=C(OC)N2N=C1S(=O)(=O)NC1=C(Cl)C=CC(C)=C1Cl VGHPMIFEKOFHHQ-UHFFFAOYSA-N 0.000 description 4
- LKJPSUCKSLORMF-UHFFFAOYSA-N Monolinuron Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C=C1 LKJPSUCKSLORMF-UHFFFAOYSA-N 0.000 description 4
- FFQPZWRNXKPNPX-UHFFFAOYSA-N N-benzyl-2-[4-fluoro-3-(trifluoromethyl)phenoxy]butanamide Chemical compound C=1C=CC=CC=1CNC(=O)C(CC)OC1=CC=C(F)C(C(F)(F)F)=C1 FFQPZWRNXKPNPX-UHFFFAOYSA-N 0.000 description 4
- 102000019315 Nicotinic acetylcholine receptors Human genes 0.000 description 4
- 239000005643 Pelargonic acid Substances 0.000 description 4
- SYJGKVOENHZYMQ-UHFFFAOYSA-N Penoxsulam Chemical compound N1=C2C(OC)=CN=C(OC)N2N=C1NS(=O)(=O)C1=C(OCC(F)F)C=CC=C1C(F)(F)F SYJGKVOENHZYMQ-UHFFFAOYSA-N 0.000 description 4
- 239000005597 Pinoxaden Substances 0.000 description 4
- YLPGTOIOYRQOHV-UHFFFAOYSA-N Pretilachlor Chemical compound CCCOCCN(C(=O)CCl)C1=C(CC)C=CC=C1CC YLPGTOIOYRQOHV-UHFFFAOYSA-N 0.000 description 4
- LTUNNEGNEKBSEH-UHFFFAOYSA-N Prosulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)CCC(F)(F)F)=N1 LTUNNEGNEKBSEH-UHFFFAOYSA-N 0.000 description 4
- IHHMUBRVTJMLQO-UHFFFAOYSA-N Pyraclonil Chemical compound C#CCN(C)C1=C(C#N)C=NN1C1=NN(CCCC2)C2=C1Cl IHHMUBRVTJMLQO-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- NBQCNZYJJMBDKY-UHFFFAOYSA-N Terbacil Chemical compound CC=1NC(=O)N(C(C)(C)C)C(=O)C=1Cl NBQCNZYJJMBDKY-UHFFFAOYSA-N 0.000 description 4
- 239000005625 Tri-allate Substances 0.000 description 4
- 241000209140 Triticum Species 0.000 description 4
- 108010017070 Zinc Finger Nucleases Proteins 0.000 description 4
- 238000007792 addition Methods 0.000 description 4
- XCSGPAVHZFQHGE-UHFFFAOYSA-N alachlor Chemical compound CCC1=CC=CC(CC)=C1N(COC)C(=O)CCl XCSGPAVHZFQHGE-UHFFFAOYSA-N 0.000 description 4
- 125000003282 alkyl amino group Chemical group 0.000 description 4
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 4
- RQVYBGPQFYCBGX-UHFFFAOYSA-N ametryn Chemical compound CCNC1=NC(NC(C)C)=NC(SC)=N1 RQVYBGPQFYCBGX-UHFFFAOYSA-N 0.000 description 4
- KWAIHLIXESXTJL-UHFFFAOYSA-N aminocyclopyrachlor Chemical compound OC(=O)C1=C(Cl)C(N)=NC(C2CC2)=N1 KWAIHLIXESXTJL-UHFFFAOYSA-N 0.000 description 4
- NIXXQNOQHKNPEJ-UHFFFAOYSA-N aminopyralid Chemical compound NC1=CC(Cl)=NC(C(O)=O)=C1Cl NIXXQNOQHKNPEJ-UHFFFAOYSA-N 0.000 description 4
- XOEMATDHVZOBSG-UHFFFAOYSA-N azafenidin Chemical compound C1=C(OCC#C)C(Cl)=CC(Cl)=C1N1C(=O)N2CCCCC2=N1 XOEMATDHVZOBSG-UHFFFAOYSA-N 0.000 description 4
- 229940097012 bacillus thuringiensis Drugs 0.000 description 4
- LVKBXDHACCFCTA-UHFFFAOYSA-N bencarbazone Chemical compound C1=C(C(N)=S)C(NS(=O)(=O)CC)=CC(N2C(N(C)C(=N2)C(F)(F)F)=O)=C1F LVKBXDHACCFCTA-UHFFFAOYSA-N 0.000 description 4
- 150000001735 carboxylic acids Chemical class 0.000 description 4
- PNCNFDRSHBFIDM-WOJGMQOQSA-N chembl111617 Chemical compound C=CCO\N=C(/CCC)C1=C(O)C(C(=O)OC)C(C)(C)CC1=O PNCNFDRSHBFIDM-WOJGMQOQSA-N 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- WYKYKTKDBLFHCY-UHFFFAOYSA-N chloridazon Chemical compound O=C1C(Cl)=C(N)C=NN1C1=CC=CC=C1 WYKYKTKDBLFHCY-UHFFFAOYSA-N 0.000 description 4
- RIUXZHMCCFLRBI-UHFFFAOYSA-N chlorimuron Chemical compound COC1=CC(Cl)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 RIUXZHMCCFLRBI-UHFFFAOYSA-N 0.000 description 4
- JXCGFZXSOMJFOA-UHFFFAOYSA-N chlorotoluron Chemical compound CN(C)C(=O)NC1=CC=C(C)C(Cl)=C1 JXCGFZXSOMJFOA-UHFFFAOYSA-N 0.000 description 4
- NNKKTZOEKDFTBU-YBEGLDIGSA-N cinidon ethyl Chemical group C1=C(Cl)C(/C=C(\Cl)C(=O)OCC)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1 NNKKTZOEKDFTBU-YBEGLDIGSA-N 0.000 description 4
- HUBANNPOLNYSAD-UHFFFAOYSA-N clopyralid Chemical compound OC(=O)C1=NC(Cl)=CC=C1Cl HUBANNPOLNYSAD-UHFFFAOYSA-N 0.000 description 4
- YIANBKOBVRMNPR-UHFFFAOYSA-N cloransulam Chemical compound N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1C(O)=O YIANBKOBVRMNPR-UHFFFAOYSA-N 0.000 description 4
- 125000000392 cycloalkenyl group Chemical group 0.000 description 4
- OAWUUPVZMNKZRY-UHFFFAOYSA-N cyprosulfamide Chemical compound COC1=CC=CC=C1C(=O)NS(=O)(=O)C1=CC=C(C(=O)NC2CC2)C=C1 OAWUUPVZMNKZRY-UHFFFAOYSA-N 0.000 description 4
- 238000012217 deletion Methods 0.000 description 4
- 230000037430 deletion Effects 0.000 description 4
- 125000004663 dialkyl amino group Chemical group 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 239000003995 emulsifying agent Substances 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- GINFBXXYGUODAT-UHFFFAOYSA-N flucarbazone Chemical compound O=C1N(C)C(OC)=NN1C(=O)NS(=O)(=O)C1=CC=CC=C1OC(F)(F)F GINFBXXYGUODAT-UHFFFAOYSA-N 0.000 description 4
- OQZCSNDVOWYALR-UHFFFAOYSA-N flurochloridone Chemical compound FC(F)(F)C1=CC=CC(N2C(C(Cl)C(CCl)C2)=O)=C1 OQZCSNDVOWYALR-UHFFFAOYSA-N 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 125000004438 haloalkoxy group Chemical group 0.000 description 4
- COYBRKAVBMYYSF-UHFFFAOYSA-N heptan-2-yl [(5-chloroquinolin-8-yl)oxy]acetate Chemical compound C1=CN=C2C(OCC(=O)OC(C)CCCCC)=CC=C(Cl)C2=C1 COYBRKAVBMYYSF-UHFFFAOYSA-N 0.000 description 4
- NRXQIUSYPAHGNM-UHFFFAOYSA-N ioxynil Chemical compound OC1=C(I)C=C(C#N)C=C1I NRXQIUSYPAHGNM-UHFFFAOYSA-N 0.000 description 4
- PMHURSZHKKJGBM-UHFFFAOYSA-N isoxaben Chemical compound O1N=C(C(C)(CC)CC)C=C1NC(=O)C1=C(OC)C=CC=C1OC PMHURSZHKKJGBM-UHFFFAOYSA-N 0.000 description 4
- ZNJFBWYDHIGLCU-UHFFFAOYSA-N jasmonic acid Natural products CCC=CCC1C(CC(O)=O)CCC1=O ZNJFBWYDHIGLCU-UHFFFAOYSA-N 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- JTHMVYBOQLDDIY-UHFFFAOYSA-N methyl 2-[(4-methyl-5-oxo-3-propoxy-1,2,4-triazole-1-carbonyl)sulfamoyl]benzoate Chemical compound O=C1N(C)C(OCCC)=NN1C(=O)NS(=O)(=O)C1=CC=CC=C1C(=O)OC JTHMVYBOQLDDIY-UHFFFAOYSA-N 0.000 description 4
- LYPWWQLKWQNQKV-UHFFFAOYSA-N methyl 2-[5-ethyl-2-[[4-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]phenoxy]methyl]phenoxy]propanoate Chemical compound COC(=O)C(C)OC1=CC(CC)=CC=C1COC1=CC=C(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)C=C1 LYPWWQLKWQNQKV-UHFFFAOYSA-N 0.000 description 4
- DSRNRYQBBJQVCW-UHFFFAOYSA-N metoxuron Chemical compound COC1=CC=C(NC(=O)N(C)C)C=C1Cl DSRNRYQBBJQVCW-UHFFFAOYSA-N 0.000 description 4
- 235000019713 millet Nutrition 0.000 description 4
- GLBLPMUBLHYFCW-UHFFFAOYSA-N n-(5,7-dimethoxy-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)-2-methoxy-4-(trifluoromethyl)pyridine-3-sulfonamide Chemical compound N1=C2N=C(OC)C=C(OC)N2N=C1NS(=O)(=O)C1=C(OC)N=CC=C1C(F)(F)F GLBLPMUBLHYFCW-UHFFFAOYSA-N 0.000 description 4
- GBHVIWKSEHWFDD-UHFFFAOYSA-N n-[2-(4,6-dimethoxy-1,3,5-triazine-2-carbonyl)-6-fluorophenyl]-1,1-difluoro-n-methylmethanesulfonamide Chemical compound COC1=NC(OC)=NC(C(=O)C=2C(=C(F)C=CC=2)N(C)S(=O)(=O)C(F)F)=N1 GBHVIWKSEHWFDD-UHFFFAOYSA-N 0.000 description 4
- CHEDHKBPPDKBQF-UPONEAKYSA-N n-[5-[(6s,7ar)-6-fluoro-1,3-dioxo-5,6,7,7a-tetrahydropyrrolo[1,2-c]imidazol-2-yl]-2-chloro-4-fluorophenyl]-1-chloromethanesulfonamide Chemical compound N1([C@@H](C2=O)C[C@@H](C1)F)C(=O)N2C1=CC(NS(=O)(=O)CCl)=C(Cl)C=C1F CHEDHKBPPDKBQF-UPONEAKYSA-N 0.000 description 4
- RTCOGUMHFFWOJV-UHFFFAOYSA-N nicosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)C(=O)N(C)C)=N1 RTCOGUMHFFWOJV-UHFFFAOYSA-N 0.000 description 4
- NVGOPFQZYCNLDU-UHFFFAOYSA-N norflurazon Chemical compound O=C1C(Cl)=C(NC)C=NN1C1=CC=CC(C(F)(F)F)=C1 NVGOPFQZYCNLDU-UHFFFAOYSA-N 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- IOXAXYHXMLCCJJ-UHFFFAOYSA-N oxetan-3-yl 2-[(4,6-dimethylpyrimidin-2-yl)carbamoylsulfamoyl]benzoate Chemical compound CC1=CC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(=O)OC2COC2)=N1 IOXAXYHXMLCCJJ-UHFFFAOYSA-N 0.000 description 4
- IZUPBVBPLAPZRR-UHFFFAOYSA-N pentachlorophenol Chemical compound OC1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1Cl IZUPBVBPLAPZRR-UHFFFAOYSA-N 0.000 description 4
- JZPKLLLUDLHCEL-UHFFFAOYSA-N pentoxazone Chemical compound O=C1C(=C(C)C)OC(=O)N1C1=CC(OC2CCCC2)=C(Cl)C=C1F JZPKLLLUDLHCEL-UHFFFAOYSA-N 0.000 description 4
- NQQVFXUMIDALNH-UHFFFAOYSA-N picloram Chemical compound NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl NQQVFXUMIDALNH-UHFFFAOYSA-N 0.000 description 4
- CWKFPEBMTGKLKX-UHFFFAOYSA-N picolinafen Chemical compound C1=CC(F)=CC=C1NC(=O)C1=CC=CC(OC=2C=C(C=CC=2)C(F)(F)F)=N1 CWKFPEBMTGKLKX-UHFFFAOYSA-N 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- FKLQIONHGSFYJY-UHFFFAOYSA-N propan-2-yl 5-[4-bromo-1-methyl-5-(trifluoromethyl)pyrazol-3-yl]-2-chloro-4-fluorobenzoate Chemical compound C1=C(Cl)C(C(=O)OC(C)C)=CC(C=2C(=C(N(C)N=2)C(F)(F)F)Br)=C1F FKLQIONHGSFYJY-UHFFFAOYSA-N 0.000 description 4
- NQLVQOSNDJXLKG-UHFFFAOYSA-N prosulfocarb Chemical compound CCCN(CCC)C(=O)SCC1=CC=CC=C1 NQLVQOSNDJXLKG-UHFFFAOYSA-N 0.000 description 4
- APTZNLHMIGJTEW-UHFFFAOYSA-N pyraflufen-ethyl Chemical group C1=C(Cl)C(OCC(=O)OCC)=CC(C=2C(=C(OC(F)F)N(C)N=2)Cl)=C1F APTZNLHMIGJTEW-UHFFFAOYSA-N 0.000 description 4
- ASRAWSBMDXVNLX-UHFFFAOYSA-N pyrazolynate Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OS(=O)(=O)C1=CC=C(C)C=C1 ASRAWSBMDXVNLX-UHFFFAOYSA-N 0.000 description 4
- 150000008512 pyrimidinediones Chemical class 0.000 description 4
- ABOOPXYCKNFDNJ-SNVBAGLBSA-N quizalofop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 ABOOPXYCKNFDNJ-SNVBAGLBSA-N 0.000 description 4
- MEFOUWRMVYJCQC-UHFFFAOYSA-N rimsulfuron Chemical compound CCS(=O)(=O)C1=CC=CN=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 MEFOUWRMVYJCQC-UHFFFAOYSA-N 0.000 description 4
- ODCWYMIRDDJXKW-UHFFFAOYSA-N simazine Chemical compound CCNC1=NC(Cl)=NC(NCC)=N1 ODCWYMIRDDJXKW-UHFFFAOYSA-N 0.000 description 4
- DEWVPZYHFVYXMZ-QCILGFJPSA-M sodium;(3ar,4as,8ar,8bs)-2,2,7,7-tetramethyl-4a,5,8a,8b-tetrahydro-[1,3]dioxolo[3,4]furo[1,3-d][1,3]dioxine-3a-carboxylate Chemical compound [Na+].O([C@H]12)C(C)(C)OC[C@@H]1O[C@]1(C([O-])=O)[C@H]2OC(C)(C)O1 DEWVPZYHFVYXMZ-QCILGFJPSA-M 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 4
- PQTBTIFWAXVEPB-UHFFFAOYSA-N sulcotrione Chemical compound ClC1=CC(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O PQTBTIFWAXVEPB-UHFFFAOYSA-N 0.000 description 4
- FZMKKCQHDROFNI-UHFFFAOYSA-N sulfometuron Chemical compound CC1=CC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 FZMKKCQHDROFNI-UHFFFAOYSA-N 0.000 description 4
- 230000008685 targeting Effects 0.000 description 4
- XLNZEKHULJKQBA-UHFFFAOYSA-N terbufos Chemical compound CCOP(=S)(OCC)SCSC(C)(C)C XLNZEKHULJKQBA-UHFFFAOYSA-N 0.000 description 4
- IROINLKCQGIITA-UHFFFAOYSA-N terbutryn Chemical compound CCNC1=NC(NC(C)(C)C)=NC(SC)=N1 IROINLKCQGIITA-UHFFFAOYSA-N 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- LOQQVLXUKHKNIA-UHFFFAOYSA-N thifensulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C2=C(SC=C2)C(O)=O)=N1 LOQQVLXUKHKNIA-UHFFFAOYSA-N 0.000 description 4
- 230000014616 translation Effects 0.000 description 4
- REZQBEBOWJAQKS-UHFFFAOYSA-N triacontan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCO REZQBEBOWJAQKS-UHFFFAOYSA-N 0.000 description 4
- BQZXUHDXIARLEO-UHFFFAOYSA-N tribenuron Chemical compound COC1=NC(C)=NC(N(C)C(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 BQZXUHDXIARLEO-UHFFFAOYSA-N 0.000 description 4
- ZSDSQXJSNMTJDA-UHFFFAOYSA-N trifluralin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O ZSDSQXJSNMTJDA-UHFFFAOYSA-N 0.000 description 4
- ZCVAOQKBXKSDMS-PVAVHDDUSA-N (+)-trans-(S)-allethrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)O[C@@H]1C(C)=C(CC=C)C(=O)C1 ZCVAOQKBXKSDMS-PVAVHDDUSA-N 0.000 description 3
- GXEKYRXVRROBEV-FBXFSONDSA-N (1r,2s,3r,4s)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid Chemical compound C1C[C@@H]2[C@@H](C(O)=O)[C@@H](C(=O)O)[C@H]1O2 GXEKYRXVRROBEV-FBXFSONDSA-N 0.000 description 3
- ROBSGBGTWRRYSK-SNVBAGLBSA-N (2r)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propanoic acid Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CC=C(C#N)C=C1F ROBSGBGTWRRYSK-SNVBAGLBSA-N 0.000 description 3
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 description 3
- ADDQHLREJDZPMT-AWEZNQCLSA-N (S)-metamifop Chemical compound O=C([C@@H](OC=1C=CC(OC=2OC3=CC(Cl)=CC=C3N=2)=CC=1)C)N(C)C1=CC=CC=C1F ADDQHLREJDZPMT-AWEZNQCLSA-N 0.000 description 3
- ZFHGXWPMULPQSE-UKSCLKOJSA-N (Z)-(1R)-cis-tefluthrin Chemical compound FC1=C(F)C(C)=C(F)C(F)=C1COC(=O)[C@H]1C(C)(C)[C@H]1\C=C(/Cl)C(F)(F)F ZFHGXWPMULPQSE-UKSCLKOJSA-N 0.000 description 3
- OVXMBIVWNJDDSM-UHFFFAOYSA-N (benzhydrylideneamino) 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate Chemical compound COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C(=O)ON=C(C=2C=CC=CC=2)C=2C=CC=CC=2)=N1 OVXMBIVWNJDDSM-UHFFFAOYSA-N 0.000 description 3
- MHULQDZDXMHODA-UHFFFAOYSA-N 1-(2,2-dichloroacetyl)-3,3,8a-trimethyl-2,4,7,8-tetrahydropyrrolo[1,2-a]pyrimidin-6-one Chemical compound C1C(C)(C)CN(C(=O)C(Cl)Cl)C2(C)N1C(=O)CC2 MHULQDZDXMHODA-UHFFFAOYSA-N 0.000 description 3
- DHYXNIKICPUXJI-UHFFFAOYSA-N 1-(2,4-dichlorophenyl)-n-(2,4-difluorophenyl)-5-oxo-n-propan-2-yl-1,2,4-triazole-4-carboxamide Chemical compound C=1C=C(F)C=C(F)C=1N(C(C)C)C(=O)N(C1=O)C=NN1C1=CC=C(Cl)C=C1Cl DHYXNIKICPUXJI-UHFFFAOYSA-N 0.000 description 3
- IXWKBUKANTXHJH-UHFFFAOYSA-N 1-[5-chloro-2-methyl-4-(5-methyl-5,6-dihydro-1,4,2-dioxazin-3-yl)pyrazol-3-yl]sulfonyl-3-(4,6-dimethoxypyrimidin-2-yl)urea Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=C(Cl)C=2C=2OC(C)CON=2)C)=N1 IXWKBUKANTXHJH-UHFFFAOYSA-N 0.000 description 3
- MCNOFYBITGAAGM-UHFFFAOYSA-N 2,2-dichloro-1-[5-(furan-2-yl)-2,2-dimethyl-1,3-oxazolidin-3-yl]ethanone Chemical compound C1N(C(=O)C(Cl)Cl)C(C)(C)OC1C1=CC=CO1 MCNOFYBITGAAGM-UHFFFAOYSA-N 0.000 description 3
- YOYAIZYFCNQIRF-UHFFFAOYSA-N 2,6-dichlorobenzonitrile Chemical compound ClC1=CC=CC(Cl)=C1C#N YOYAIZYFCNQIRF-UHFFFAOYSA-N 0.000 description 3
- BDQWWOHKFDSADC-UHFFFAOYSA-N 2-(2,4-dichloro-3-methylphenoxy)-n-phenylpropanamide Chemical compound C=1C=CC=CC=1NC(=O)C(C)OC1=CC=C(Cl)C(C)=C1Cl BDQWWOHKFDSADC-UHFFFAOYSA-N 0.000 description 3
- GOCUAJYOYBLQRH-UHFFFAOYSA-N 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl GOCUAJYOYBLQRH-UHFFFAOYSA-N 0.000 description 3
- YUVKUEAFAVKILW-UHFFFAOYSA-N 2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 YUVKUEAFAVKILW-UHFFFAOYSA-N 0.000 description 3
- KRQUFUKTQHISJB-YYADALCUSA-N 2-[(E)-N-[2-(4-chlorophenoxy)propoxy]-C-propylcarbonimidoyl]-3-hydroxy-5-(thian-3-yl)cyclohex-2-en-1-one Chemical compound CCC\C(=N/OCC(C)OC1=CC=C(Cl)C=C1)C1=C(O)CC(CC1=O)C1CCCSC1 KRQUFUKTQHISJB-YYADALCUSA-N 0.000 description 3
- KPSTXQYTZBZXMM-UHFFFAOYSA-N 2-[8-chloro-4-(4-methoxyphenyl)-3-oxoquinoxaline-2-carbonyl]cyclohexane-1,3-dione Chemical compound C1=CC(OC)=CC=C1N1C(=O)C(C(=O)C2C(CCCC2=O)=O)=NC2=C(Cl)C=CC=C21 KPSTXQYTZBZXMM-UHFFFAOYSA-N 0.000 description 3
- KZNDFYDURHAESM-UHFFFAOYSA-N 2-chloro-n-(2-ethyl-6-methylphenyl)-n-(propan-2-yloxymethyl)acetamide Chemical compound CCC1=CC=CC(C)=C1N(COC(C)C)C(=O)CCl KZNDFYDURHAESM-UHFFFAOYSA-N 0.000 description 3
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 3
- MVQVNTPHUGQQHK-UHFFFAOYSA-N 3-pyridinemethanol Chemical compound OCC1=CC=CN=C1 MVQVNTPHUGQQHK-UHFFFAOYSA-N 0.000 description 3
- CIPJEXPBJDLNIP-UHFFFAOYSA-N 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1h-indol-6-yl)pyridine-2-carboxylic acid Chemical compound OC(=O)C1=C(Cl)C(N)=C(F)C(C=2C(=C3NC=CC3=CC=2)F)=N1 CIPJEXPBJDLNIP-UHFFFAOYSA-N 0.000 description 3
- ZMYKITJYWFYRFJ-UHFFFAOYSA-N 4-oxo-4-(2-phenylethylamino)butanoic acid Chemical compound OC(=O)CCC(=O)NCCC1=CC=CC=C1 ZMYKITJYWFYRFJ-UHFFFAOYSA-N 0.000 description 3
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 3
- ZOGDSYNXUXQGHF-UHFFFAOYSA-N 5-(3-butanoyl-2,4,6-trimethylphenyl)-2-[(Z)-N-ethoxy-C-ethylcarbonimidoyl]-3-hydroxycyclohex-2-en-1-one Chemical compound C(C)ON=C(CC)/C=1C(CC(CC1O)C1=C(C(=C(C=C1C)C)C(CCC)=O)C)=O ZOGDSYNXUXQGHF-UHFFFAOYSA-N 0.000 description 3
- CTSLUCNDVMMDHG-UHFFFAOYSA-N 5-bromo-3-(butan-2-yl)-6-methylpyrimidine-2,4(1H,3H)-dione Chemical compound CCC(C)N1C(=O)NC(C)=C(Br)C1=O CTSLUCNDVMMDHG-UHFFFAOYSA-N 0.000 description 3
- SKYNPRKUXHXZFJ-UHFFFAOYSA-L 6,7-dihydrodipyrido[1,2-b:1',2'-e]pyrazine-5,8-diium;dichloride Chemical compound [Cl-].[Cl-].C1=CC=[N+]2CC[N+]3=CC=CC=C3C2=C1 SKYNPRKUXHXZFJ-UHFFFAOYSA-L 0.000 description 3
- ZUSHSDOEVHPTCU-UHFFFAOYSA-N 6-chloro-3-phenyl-1h-pyridazin-4-one Chemical compound N1C(Cl)=CC(=O)C(C=2C=CC=CC=2)=N1 ZUSHSDOEVHPTCU-UHFFFAOYSA-N 0.000 description 3
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 3
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- CTTHWASMBLQOFR-UHFFFAOYSA-N Amidosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)N(C)S(C)(=O)=O)=N1 CTTHWASMBLQOFR-UHFFFAOYSA-N 0.000 description 3
- 239000005468 Aminopyralid Substances 0.000 description 3
- NXQDBZGWYSEGFL-UHFFFAOYSA-N Anilofos Chemical compound COP(=S)(OC)SCC(=O)N(C(C)C)C1=CC=C(Cl)C=C1 NXQDBZGWYSEGFL-UHFFFAOYSA-N 0.000 description 3
- 244000075850 Avena orientalis Species 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 239000005470 Beflubutamid Substances 0.000 description 3
- JDWQITFHZOBBFE-UHFFFAOYSA-N Benzofenap Chemical compound C=1C=C(Cl)C(C)=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=C(C)C=C1 JDWQITFHZOBBFE-UHFFFAOYSA-N 0.000 description 3
- 239000005484 Bifenox Substances 0.000 description 3
- XTFNPKDYCLFGPV-OMCISZLKSA-N Bromofenoxim Chemical compound C1=C(Br)C(O)=C(Br)C=C1\C=N\OC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O XTFNPKDYCLFGPV-OMCISZLKSA-N 0.000 description 3
- OEYOMNZEMCPTKN-UHFFFAOYSA-N Butamifos Chemical compound CCC(C)NP(=S)(OCC)OC1=CC(C)=CC=C1[N+]([O-])=O OEYOMNZEMCPTKN-UHFFFAOYSA-N 0.000 description 3
- SPNQRCTZKIBOAX-UHFFFAOYSA-N Butralin Chemical compound CCC(C)NC1=C([N+]([O-])=O)C=C(C(C)(C)C)C=C1[N+]([O-])=O SPNQRCTZKIBOAX-UHFFFAOYSA-N 0.000 description 3
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 3
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 description 3
- KSFOVUSSGSKXFI-GAQDCDSVSA-N CC1=C/2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(C=C)=C5C)C(C=C)=C4C)C(C)=C3CCC(O)=O)=C1CCC(O)=O Chemical compound CC1=C/2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(C=C)=C5C)C(C=C)=C4C)C(C)=C3CCC(O)=O)=C1CCC(O)=O KSFOVUSSGSKXFI-GAQDCDSVSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- 229920002101 Chitin Polymers 0.000 description 3
- 229920001661 Chitosan Polymers 0.000 description 3
- HSSBORCLYSCBJR-UHFFFAOYSA-N Chloramben Chemical compound NC1=CC(Cl)=CC(C(O)=O)=C1Cl HSSBORCLYSCBJR-UHFFFAOYSA-N 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- 239000005494 Chlorotoluron Substances 0.000 description 3
- KZCBXHSWMMIEQU-UHFFFAOYSA-N Chlorthal Chemical compound OC(=O)C1=C(Cl)C(Cl)=C(C(O)=O)C(Cl)=C1Cl KZCBXHSWMMIEQU-UHFFFAOYSA-N 0.000 description 3
- WMLPCIHUFDKWJU-UHFFFAOYSA-N Cinosulfuron Chemical compound COCCOC1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC)=NC(OC)=N1 WMLPCIHUFDKWJU-UHFFFAOYSA-N 0.000 description 3
- 239000005500 Clopyralid Substances 0.000 description 3
- OFSLKOLYLQSJPB-UHFFFAOYSA-N Cyclosulfamuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)NC=2C(=CC=CC=2)C(=O)C2CC2)=N1 OFSLKOLYLQSJPB-UHFFFAOYSA-N 0.000 description 3
- 230000004568 DNA-binding Effects 0.000 description 3
- 239000005644 Dazomet Substances 0.000 description 3
- 239000005506 Diclofop Substances 0.000 description 3
- DHWRNDJOGMTCPB-UHFFFAOYSA-N Dimefuron Chemical compound ClC1=CC(NC(=O)N(C)C)=CC=C1N1C(=O)OC(C(C)(C)C)=N1 DHWRNDJOGMTCPB-UHFFFAOYSA-N 0.000 description 3
- IKYICRRUVNIHPP-UHFFFAOYSA-N Dimethametryn Chemical compound CCNC1=NC(NC(C)C(C)C)=NC(SC)=N1 IKYICRRUVNIHPP-UHFFFAOYSA-N 0.000 description 3
- OFDYMSKSGFSLLM-UHFFFAOYSA-N Dinitramine Chemical compound CCN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C(N)=C1[N+]([O-])=O OFDYMSKSGFSLLM-UHFFFAOYSA-N 0.000 description 3
- YUBJPYNSGLJZPQ-UHFFFAOYSA-N Dithiopyr Chemical compound CSC(=O)C1=C(C(F)F)N=C(C(F)(F)F)C(C(=O)SC)=C1CC(C)C YUBJPYNSGLJZPQ-UHFFFAOYSA-N 0.000 description 3
- PTFJIKYUEPWBMS-UHFFFAOYSA-N Ethalfluralin Chemical compound CC(=C)CN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O PTFJIKYUEPWBMS-UHFFFAOYSA-N 0.000 description 3
- NRFQZTCQAYEXEE-UHFFFAOYSA-N Fenclorim Chemical compound ClC1=CC(Cl)=NC(C=2C=CC=CC=2)=N1 NRFQZTCQAYEXEE-UHFFFAOYSA-N 0.000 description 3
- LLQPHQFNMLZJMP-UHFFFAOYSA-N Fentrazamide Chemical compound N1=NN(C=2C(=CC=CC=2)Cl)C(=O)N1C(=O)N(CC)C1CCCCC1 LLQPHQFNMLZJMP-UHFFFAOYSA-N 0.000 description 3
- 239000005529 Florasulam Substances 0.000 description 3
- FICWGWVVIRLNRB-UHFFFAOYSA-N Flucetosulfuron Chemical compound COCC(=O)OC(C(C)F)C1=NC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 FICWGWVVIRLNRB-UHFFFAOYSA-N 0.000 description 3
- IRECWLYBCAZIJM-UHFFFAOYSA-N Flumiclorac pentyl Chemical group C1=C(Cl)C(OCC(=O)OCCCCC)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1F IRECWLYBCAZIJM-UHFFFAOYSA-N 0.000 description 3
- AOQMRUTZEYVDIL-UHFFFAOYSA-N Flupoxam Chemical compound C=1C=C(Cl)C(COCC(F)(F)C(F)(F)F)=CC=1N1N=C(C(=O)N)N=C1C1=CC=CC=C1 AOQMRUTZEYVDIL-UHFFFAOYSA-N 0.000 description 3
- GXAMYUGOODKVRM-UHFFFAOYSA-N Flurecol Chemical compound C1=CC=C2C(C(=O)O)(O)C3=CC=CC=C3C2=C1 GXAMYUGOODKVRM-UHFFFAOYSA-N 0.000 description 3
- 239000005535 Flurochloridone Substances 0.000 description 3
- 239000005559 Flurtamone Substances 0.000 description 3
- 239000005981 Imazaquin Substances 0.000 description 3
- 239000005567 Imazosulfuron Substances 0.000 description 3
- PMAAYIYCDXGUAP-UHFFFAOYSA-N Indanofan Chemical compound O=C1C2=CC=CC=C2C(=O)C1(CC)CC1(C=2C=C(Cl)C=CC=2)CO1 PMAAYIYCDXGUAP-UHFFFAOYSA-N 0.000 description 3
- 102100034343 Integrase Human genes 0.000 description 3
- 108010061833 Integrases Proteins 0.000 description 3
- 241000207783 Ipomoea Species 0.000 description 3
- 239000005867 Iprodione Substances 0.000 description 3
- JLLJHQLUZAKJFH-UHFFFAOYSA-N Isouron Chemical compound CN(C)C(=O)NC=1C=C(C(C)(C)C)ON=1 JLLJHQLUZAKJFH-UHFFFAOYSA-N 0.000 description 3
- 239000005570 Isoxaben Substances 0.000 description 3
- 239000005573 Linuron Substances 0.000 description 3
- 239000005575 MCPB Substances 0.000 description 3
- BWPYBAJTDILQPY-UHFFFAOYSA-N Methoxyphenone Chemical compound C1=C(C)C(OC)=CC=C1C(=O)C1=CC=CC(C)=C1 BWPYBAJTDILQPY-UHFFFAOYSA-N 0.000 description 3
- 239000005581 Metobromuron Substances 0.000 description 3
- 239000005582 Metosulam Substances 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- IUFUITYPUYMIHI-UHFFFAOYSA-N N-[1-(3,5-dimethylphenoxy)propan-2-yl]-6-(2-fluoropropan-2-yl)-1,3,5-triazine-2,4-diamine Chemical compound N=1C(N)=NC(C(C)(C)F)=NC=1NC(C)COC1=CC(C)=CC(C)=C1 IUFUITYPUYMIHI-UHFFFAOYSA-N 0.000 description 3
- OVRNDRQMDRJTHS-RTRLPJTCSA-N N-acetyl-D-glucosamine Chemical group CC(=O)N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-RTRLPJTCSA-N 0.000 description 3
- NTBVTCXMRYKRTB-UHFFFAOYSA-N N-{2-[(4,6-dimethoxypyrimidin-2-yl)(hydroxy)methyl]-6-(methoxymethyl)phenyl}-1,1-difluoromethanesulfonamide Chemical compound COCC1=CC=CC(C(O)C=2N=C(OC)C=C(OC)N=2)=C1NS(=O)(=O)C(F)F NTBVTCXMRYKRTB-UHFFFAOYSA-N 0.000 description 3
- CCGPUGMWYLICGL-UHFFFAOYSA-N Neburon Chemical compound CCCCN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 CCGPUGMWYLICGL-UHFFFAOYSA-N 0.000 description 3
- 239000005586 Nicosulfuron Substances 0.000 description 3
- 239000005642 Oleic acid Substances 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 239000005592 Penoxsulam Substances 0.000 description 3
- 239000005595 Picloram Substances 0.000 description 3
- 239000005596 Picolinafen Substances 0.000 description 3
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 3
- UNLYSVIDNRIVFJ-UHFFFAOYSA-N Piperophos Chemical compound CCCOP(=S)(OCCC)SCC(=O)N1CCCCC1C UNLYSVIDNRIVFJ-UHFFFAOYSA-N 0.000 description 3
- 241000219843 Pisum Species 0.000 description 3
- GPGLBXMQFQQXDV-UHFFFAOYSA-N Primisulfuron Chemical compound OC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC(F)F)=CC(OC(F)F)=N1 GPGLBXMQFQQXDV-UHFFFAOYSA-N 0.000 description 3
- 239000005601 Propoxycarbazone Substances 0.000 description 3
- 239000005603 Prosulfocarb Substances 0.000 description 3
- 239000005604 Prosulfuron Substances 0.000 description 3
- 239000005605 Pyraflufen-ethyl Substances 0.000 description 3
- VXMNDQDDWDDKOQ-UHFFFAOYSA-N Pyrazosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=CC=2C(O)=O)C)=N1 VXMNDQDDWDDKOQ-UHFFFAOYSA-N 0.000 description 3
- JTZCTMAVMHRNTR-UHFFFAOYSA-N Pyridate Chemical compound CCCCCCCCSC(=O)OC1=CC(Cl)=NN=C1C1=CC=CC=C1 JTZCTMAVMHRNTR-UHFFFAOYSA-N 0.000 description 3
- RRKHIAYNPVQKEF-UHFFFAOYSA-N Pyriftalid Chemical compound COC1=CC(OC)=NC(SC=2C=3C(=O)OC(C)C=3C=CC=2)=N1 RRKHIAYNPVQKEF-UHFFFAOYSA-N 0.000 description 3
- CNILNQMBAHKMFS-UHFFFAOYSA-M Pyrithiobac-sodium Chemical compound [Na+].COC1=CC(OC)=NC(SC=2C(=C(Cl)C=CC=2)C([O-])=O)=N1 CNILNQMBAHKMFS-UHFFFAOYSA-M 0.000 description 3
- 239000005607 Pyroxsulam Substances 0.000 description 3
- 239000005609 Quizalofop-P Substances 0.000 description 3
- 239000005616 Rimsulfuron Substances 0.000 description 3
- CSPPKDPQLUUTND-NBVRZTHBSA-N Sethoxydim Chemical compound CCO\N=C(/CCC)C1=C(O)CC(CC(C)SCC)CC1=O CSPPKDPQLUUTND-NBVRZTHBSA-N 0.000 description 3
- JXVIIQLNUPXOII-UHFFFAOYSA-N Siduron Chemical compound CC1CCCCC1NC(=O)NC1=CC=CC=C1 JXVIIQLNUPXOII-UHFFFAOYSA-N 0.000 description 3
- 239000005618 Sulcotrione Substances 0.000 description 3
- 239000005619 Sulfosulfuron Substances 0.000 description 3
- HBPDKDSFLXWOAE-UHFFFAOYSA-N Tebuthiuron Chemical compound CNC(=O)N(C)C1=NN=C(C(C)(C)C)S1 HBPDKDSFLXWOAE-UHFFFAOYSA-N 0.000 description 3
- IOYNQIMAUDJVEI-BMVIKAAMSA-N Tepraloxydim Chemical compound C1C(=O)C(C(=N/OC\C=C\Cl)/CC)=C(O)CC1C1CCOCC1 IOYNQIMAUDJVEI-BMVIKAAMSA-N 0.000 description 3
- KDWQYMVPYJGPHS-UHFFFAOYSA-N Thenylchlor Chemical compound C1=CSC(CN(C(=O)CCl)C=2C(=CC=CC=2C)C)=C1OC KDWQYMVPYJGPHS-UHFFFAOYSA-N 0.000 description 3
- YIJZJEYQBAAWRJ-UHFFFAOYSA-N Thiazopyr Chemical compound N1=C(C(F)F)C(C(=O)OC)=C(CC(C)C)C(C=2SCCN=2)=C1C(F)(F)F YIJZJEYQBAAWRJ-UHFFFAOYSA-N 0.000 description 3
- HFCYZXMHUIHAQI-UHFFFAOYSA-N Thidiazuron Chemical compound C=1C=CC=CC=1NC(=O)NC1=CN=NS1 HFCYZXMHUIHAQI-UHFFFAOYSA-N 0.000 description 3
- 239000005624 Tralkoxydim Substances 0.000 description 3
- 239000005626 Tribenuron Substances 0.000 description 3
- HFBWPRKWDIRYNX-UHFFFAOYSA-N Trietazine Chemical compound CCNC1=NC(Cl)=NC(N(CC)CC)=N1 HFBWPRKWDIRYNX-UHFFFAOYSA-N 0.000 description 3
- 235000021307 Triticum Nutrition 0.000 description 3
- LALGZGDVRILFNW-UHFFFAOYSA-N [2,5-dimethyl-4-[2-methylsulfonyl-4-(trifluoromethyl)benzoyl]pyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound CN1N=C(C(=C1)C(=O)OC1=C(C(=NN1C)C)C(C1=C(C=C(C=C1)C(F)(F)F)S(=O)(=O)C)=O)C LALGZGDVRILFNW-UHFFFAOYSA-N 0.000 description 3
- 230000000895 acaricidal effect Effects 0.000 description 3
- 239000000642 acaricide Substances 0.000 description 3
- UELITFHSCLAHKR-UHFFFAOYSA-N acibenzolar-S-methyl Chemical group CSC(=O)C1=CC=CC2=C1SN=N2 UELITFHSCLAHKR-UHFFFAOYSA-N 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 125000004457 alkyl amino carbonyl group Chemical group 0.000 description 3
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 3
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 3
- ORFPWVRKFLOQHK-UHFFFAOYSA-N amicarbazone Chemical compound CC(C)C1=NN(C(=O)NC(C)(C)C)C(=O)N1N ORFPWVRKFLOQHK-UHFFFAOYSA-N 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 125000003710 aryl alkyl group Chemical group 0.000 description 3
- VGPYEHKOIGNJKV-UHFFFAOYSA-N asulam Chemical compound COC(=O)NS(=O)(=O)C1=CC=C(N)C=C1 VGPYEHKOIGNJKV-UHFFFAOYSA-N 0.000 description 3
- MAHPNPYYQAIOJN-UHFFFAOYSA-N azimsulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=CC=2C2=NN(C)N=N2)C)=N1 MAHPNPYYQAIOJN-UHFFFAOYSA-N 0.000 description 3
- HYJSGOXICXYZGS-UHFFFAOYSA-N benazolin Chemical compound C1=CC=C2SC(=O)N(CC(=O)O)C2=C1Cl HYJSGOXICXYZGS-UHFFFAOYSA-N 0.000 description 3
- WQRCEBAZAUAUQC-UHFFFAOYSA-N benazolin-ethyl Chemical group C1=CC=C2SC(=O)N(CC(=O)OCC)C2=C1Cl WQRCEBAZAUAUQC-UHFFFAOYSA-N 0.000 description 3
- SMDHCQAYESWHAE-UHFFFAOYSA-N benfluralin Chemical compound CCCCN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O SMDHCQAYESWHAE-UHFFFAOYSA-N 0.000 description 3
- XMQFTWRPUQYINF-UHFFFAOYSA-N bensulfuron-methyl Chemical group COC(=O)C1=CC=CC=C1CS(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 XMQFTWRPUQYINF-UHFFFAOYSA-N 0.000 description 3
- 238000009395 breeding Methods 0.000 description 3
- PSGPXWYGJGGEEG-UHFFFAOYSA-N butyl 9-hydroxyfluorene-9-carboxylate Chemical group C1=CC=C2C(C(=O)OCCCC)(O)C3=CC=CC=C3C2=C1 PSGPXWYGJGGEEG-UHFFFAOYSA-N 0.000 description 3
- HFEJHAAIJZXXRE-UHFFFAOYSA-N cafenstrole Chemical compound CCN(CC)C(=O)N1C=NC(S(=O)(=O)C=2C(=CC(C)=CC=2C)C)=N1 HFEJHAAIJZXXRE-UHFFFAOYSA-N 0.000 description 3
- GGWHBJGBERXSLL-NBVRZTHBSA-N chembl113137 Chemical compound C1C(=O)C(C(=N/OCC)/CCC)=C(O)CC1C1CSCCC1 GGWHBJGBERXSLL-NBVRZTHBSA-N 0.000 description 3
- VJYIFXVZLXQVHO-UHFFFAOYSA-N chlorsulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)Cl)=N1 VJYIFXVZLXQVHO-UHFFFAOYSA-N 0.000 description 3
- 229960001231 choline Drugs 0.000 description 3
- YUIKUTLBPMDDNQ-MRVPVSSYSA-N clodinafop Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC=C(Cl)C=C1F YUIKUTLBPMDDNQ-MRVPVSSYSA-N 0.000 description 3
- JBDHZKLJNAIJNC-LLVKDONJSA-N clodinafop-propargyl Chemical group C1=CC(O[C@H](C)C(=O)OCC#C)=CC=C1OC1=NC=C(Cl)C=C1F JBDHZKLJNAIJNC-LLVKDONJSA-N 0.000 description 3
- ICJSJAJWTWPSBD-UHFFFAOYSA-N cloquintocet Chemical compound C1=CN=C2C(OCC(=O)O)=CC=C(Cl)C2=C1 ICJSJAJWTWPSBD-UHFFFAOYSA-N 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 125000000000 cycloalkoxy group Chemical group 0.000 description 3
- 125000005167 cycloalkylaminocarbonyl group Chemical group 0.000 description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 3
- QAYICIQNSGETAS-UHFFFAOYSA-N dazomet Chemical compound CN1CSC(=S)N(C)C1 QAYICIQNSGETAS-UHFFFAOYSA-N 0.000 description 3
- WZJZMXBKUWKXTQ-UHFFFAOYSA-N desmedipham Chemical compound CCOC(=O)NC1=CC=CC(OC(=O)NC=2C=CC=CC=2)=C1 WZJZMXBKUWKXTQ-UHFFFAOYSA-N 0.000 description 3
- FCRACOPGPMPSHN-UHFFFAOYSA-N desoxyabscisic acid Natural products OC(=O)C=C(C)C=CC1C(C)=CC(=O)CC1(C)C FCRACOPGPMPSHN-UHFFFAOYSA-N 0.000 description 3
- PYJWLFDSOCOIHD-UHFFFAOYSA-N dicamba-olamine Chemical compound NCCO.COC1=C(Cl)C=CC(Cl)=C1C(O)=O PYJWLFDSOCOIHD-UHFFFAOYSA-N 0.000 description 3
- OPGCOAPTHCZZIW-UHFFFAOYSA-N diethyl 1-(2,4-dichlorophenyl)-5-methyl-4h-pyrazole-3,5-dicarboxylate Chemical group CCOC(=O)C1(C)CC(C(=O)OCC)=NN1C1=CC=C(Cl)C=C1Cl OPGCOAPTHCZZIW-UHFFFAOYSA-N 0.000 description 3
- BWUPSGJXXPATLU-UHFFFAOYSA-N dimepiperate Chemical compound C=1C=CC=CC=1C(C)(C)SC(=O)N1CCCCC1 BWUPSGJXXPATLU-UHFFFAOYSA-N 0.000 description 3
- SCCDDNKJYDZXMM-UHFFFAOYSA-N dimethachlor Chemical compound COCCN(C(=O)CCl)C1=C(C)C=CC=C1C SCCDDNKJYDZXMM-UHFFFAOYSA-N 0.000 description 3
- ZPZKADHMBHMAES-PXYBLNDHSA-L dipotassium;(1s,2r,3s,4r)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate Chemical compound [K+].[K+].C1C[C@@H]2[C@@H](C([O-])=O)[C@@H](C(=O)[O-])[C@H]1O2 ZPZKADHMBHMAES-PXYBLNDHSA-L 0.000 description 3
- XRHVZWWRFMCBAZ-PXYBLNDHSA-L disodium;(1s,2r,3s,4r)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate Chemical compound [Na+].[Na+].C1C[C@@H]2[C@@H](C([O-])=O)[C@@H](C(=O)[O-])[C@H]1O2 XRHVZWWRFMCBAZ-PXYBLNDHSA-L 0.000 description 3
- 239000004495 emulsifiable concentrate Substances 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- IRLGCAJYYKDTCG-UHFFFAOYSA-N ethametsulfuron Chemical compound CCOC1=NC(NC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 IRLGCAJYYKDTCG-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 150000002191 fatty alcohols Chemical class 0.000 description 3
- NYPJDWWKZLNGGM-UHFFFAOYSA-N fenvalerate Aalpha Natural products C=1C=C(Cl)C=CC=1C(C(C)C)C(=O)OC(C#N)C(C=1)=CC=CC=1OC1=CC=CC=C1 NYPJDWWKZLNGGM-UHFFFAOYSA-N 0.000 description 3
- WFZSZAXUALBVNX-UHFFFAOYSA-N flufenpyr Chemical compound O=C1C(C)=C(C(F)(F)F)C=NN1C1=CC(OCC(O)=O)=C(Cl)C=C1F WFZSZAXUALBVNX-UHFFFAOYSA-N 0.000 description 3
- DNUAYCRATWAJQE-UHFFFAOYSA-N flufenpyr-ethyl Chemical group C1=C(Cl)C(OCC(=O)OCC)=CC(N2C(C(C)=C(C=N2)C(F)(F)F)=O)=C1F DNUAYCRATWAJQE-UHFFFAOYSA-N 0.000 description 3
- RZILCCPWPBTYDO-UHFFFAOYSA-N fluometuron Chemical compound CN(C)C(=O)NC1=CC=CC(C(F)(F)F)=C1 RZILCCPWPBTYDO-UHFFFAOYSA-N 0.000 description 3
- MEFQWPUMEMWTJP-UHFFFAOYSA-N fluroxypyr Chemical compound NC1=C(Cl)C(F)=NC(OCC(O)=O)=C1Cl MEFQWPUMEMWTJP-UHFFFAOYSA-N 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 238000010353 genetic engineering Methods 0.000 description 3
- 125000005347 halocycloalkyl group Chemical group 0.000 description 3
- 101150102931 hemG gene Proteins 0.000 description 3
- 229930195733 hydrocarbon Natural products 0.000 description 3
- ORTFAQDWJHRMNX-UHFFFAOYSA-N hydroxidooxidocarbon(.) Chemical group O[C]=O ORTFAQDWJHRMNX-UHFFFAOYSA-N 0.000 description 3
- 239000002917 insecticide Substances 0.000 description 3
- 230000010354 integration Effects 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- ONUFESLQCSAYKA-UHFFFAOYSA-N iprodione Chemical compound O=C1N(C(=O)NC(C)C)CC(=O)N1C1=CC(Cl)=CC(Cl)=C1 ONUFESLQCSAYKA-UHFFFAOYSA-N 0.000 description 3
- ZTMKADLOSYKWCA-UHFFFAOYSA-N lenacil Chemical compound O=C1NC=2CCCC=2C(=O)N1C1CCCCC1 ZTMKADLOSYKWCA-UHFFFAOYSA-N 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- XIGAUIHYSDTJHW-UHFFFAOYSA-N mefenacet Chemical compound N=1C2=CC=CC=C2SC=1OCC(=O)N(C)C1=CC=CC=C1 XIGAUIHYSDTJHW-UHFFFAOYSA-N 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- VHCNQEUWZYOAEV-UHFFFAOYSA-N metamitron Chemical compound O=C1N(N)C(C)=NN=C1C1=CC=CC=C1 VHCNQEUWZYOAEV-UHFFFAOYSA-N 0.000 description 3
- STEPQTYSZVCJPV-UHFFFAOYSA-N metazachlor Chemical compound CC1=CC=CC(C)=C1N(C(=O)CCl)CN1N=CC=C1 STEPQTYSZVCJPV-UHFFFAOYSA-N 0.000 description 3
- LYEFBCLHLUGNES-SJKOYZFVSA-N methyl (3R)-3-[[(5R)-3-(3,5-difluorophenyl)-5-methyl-4H-1,2-oxazole-5-carbonyl]amino]-2,3-dihydrofuran-5-carboxylate Chemical compound C[C@@]1(CC(=NO1)C2=CC(=CC(=C2)F)F)C(=O)N[C@H]3COC(=C3)C(=O)OC LYEFBCLHLUGNES-SJKOYZFVSA-N 0.000 description 3
- VWGAYSCWLXQJBQ-UHFFFAOYSA-N methyl 4-iodo-2-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]benzoate Chemical group COC(=O)C1=CC=C(I)C=C1S(=O)(=O)NC(=O)NC1=NC(C)=NC(OC)=N1 VWGAYSCWLXQJBQ-UHFFFAOYSA-N 0.000 description 3
- WXUNXXKSYBUHMK-UHFFFAOYSA-N methyl 4-methyl-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)benzoate;methyl 5-methyl-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)benzoate Chemical compound COC(=O)C1=CC=C(C)C=C1C1=NC(C)(C(C)C)C(=O)N1.COC(=O)C1=CC(C)=CC=C1C1=NC(C)(C(C)C)C(=O)N1 WXUNXXKSYBUHMK-UHFFFAOYSA-N 0.000 description 3
- BUTXJHNFXHLCIM-UHFFFAOYSA-N n-(3-chloro-1-pyridin-3-ylpyrazol-4-yl)-n-ethyl-3-(3,3,3-trifluoropropylsulfinyl)propanamide Chemical compound N1=C(Cl)C(N(C(=O)CCS(=O)CCC(F)(F)F)CC)=CN1C1=CC=CN=C1 BUTXJHNFXHLCIM-UHFFFAOYSA-N 0.000 description 3
- AIMMSOZBPYFASU-UHFFFAOYSA-N n-(4,6-dimethoxypyrimidin-2-yl)-n'-[3-(2,2,2-trifluoroethoxy)pyridin-1-ium-2-yl]sulfonylcarbamimidate Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)OCC(F)(F)F)=N1 AIMMSOZBPYFASU-UHFFFAOYSA-N 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- UCDPMNSCCRBWIC-UHFFFAOYSA-N orthosulfamuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)NC=2C(=CC=CC=2)C(=O)N(C)C)=N1 UCDPMNSCCRBWIC-UHFFFAOYSA-N 0.000 description 3
- UNAHYJYOSSSJHH-UHFFFAOYSA-N oryzalin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(S(N)(=O)=O)C=C1[N+]([O-])=O UNAHYJYOSSSJHH-UHFFFAOYSA-N 0.000 description 3
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 3
- CSWIKHNSBZVWNQ-UHFFFAOYSA-N pethoxamide Chemical compound CCOCCN(C(=O)CCl)C(=C(C)C)C1=CC=CC=C1 CSWIKHNSBZVWNQ-UHFFFAOYSA-N 0.000 description 3
- IDOWTHOLJBTAFI-UHFFFAOYSA-N phenmedipham Chemical compound COC(=O)NC1=CC=CC(OC(=O)NC=2C=C(C)C=CC=2)=C1 IDOWTHOLJBTAFI-UHFFFAOYSA-N 0.000 description 3
- 108010001545 phytoene dehydrogenase Proteins 0.000 description 3
- 229920000151 polyglycol Polymers 0.000 description 3
- 239000010695 polyglycol Substances 0.000 description 3
- 238000003752 polymerase chain reaction Methods 0.000 description 3
- WHHIPMZEDGBUCC-UHFFFAOYSA-N probenazole Chemical compound C1=CC=C2C(OCC=C)=NS(=O)(=O)C2=C1 WHHIPMZEDGBUCC-UHFFFAOYSA-N 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- ISEUFVQQFVOBCY-UHFFFAOYSA-N prometon Chemical compound COC1=NC(NC(C)C)=NC(NC(C)C)=N1 ISEUFVQQFVOBCY-UHFFFAOYSA-N 0.000 description 3
- AAEVYOVXGOFMJO-UHFFFAOYSA-N prometryn Chemical compound CSC1=NC(NC(C)C)=NC(NC(C)C)=N1 AAEVYOVXGOFMJO-UHFFFAOYSA-N 0.000 description 3
- MFOUDYKPLGXPGO-UHFFFAOYSA-N propachlor Chemical compound ClCC(=O)N(C(C)C)C1=CC=CC=C1 MFOUDYKPLGXPGO-UHFFFAOYSA-N 0.000 description 3
- LFULEKSKNZEWOE-UHFFFAOYSA-N propanil Chemical compound CCC(=O)NC1=CC=C(Cl)C(Cl)=C1 LFULEKSKNZEWOE-UHFFFAOYSA-N 0.000 description 3
- FROBCXTULYFHEJ-OAHLLOKOSA-N propaquizafop Chemical compound C1=CC(O[C@H](C)C(=O)OCCON=C(C)C)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 FROBCXTULYFHEJ-OAHLLOKOSA-N 0.000 description 3
- WJNRPILHGGKWCK-UHFFFAOYSA-N propazine Chemical compound CC(C)NC1=NC(Cl)=NC(NC(C)C)=N1 WJNRPILHGGKWCK-UHFFFAOYSA-N 0.000 description 3
- VXPLXMJHHKHSOA-UHFFFAOYSA-N propham Chemical compound CC(C)OC(=O)NC1=CC=CC=C1 VXPLXMJHHKHSOA-UHFFFAOYSA-N 0.000 description 3
- PHNUZKMIPFFYSO-UHFFFAOYSA-N propyzamide Chemical compound C#CC(C)(C)NC(=O)C1=CC(Cl)=CC(Cl)=C1 PHNUZKMIPFFYSO-UHFFFAOYSA-N 0.000 description 3
- 229950003776 protoporphyrin Drugs 0.000 description 3
- UHSGPDMIQQYNAX-UHFFFAOYSA-N protoporphyrinogen Chemical compound C1C(=C(C=2C=C)C)NC=2CC(=C(C=2CCC(O)=O)C)NC=2CC(N2)=C(CCC(O)=O)C(C)=C2CC2=C(C)C(C=C)=C1N2 UHSGPDMIQQYNAX-UHFFFAOYSA-N 0.000 description 3
- FKERUJTUOYLBKB-UHFFFAOYSA-N pyrazoxyfen Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=CC=C1 FKERUJTUOYLBKB-UHFFFAOYSA-N 0.000 description 3
- DEIKMOQTJBGGAX-DJKKODMXSA-N pyriminobac Chemical compound CO\N=C(/C)C1=CC=CC(OC=2N=C(OC)C=C(OC)N=2)=C1C(O)=O DEIKMOQTJBGGAX-DJKKODMXSA-N 0.000 description 3
- FFSSWMQPCJRCRV-UHFFFAOYSA-N quinclorac Chemical compound ClC1=CN=C2C(C(=O)O)=C(Cl)C=CC2=C1 FFSSWMQPCJRCRV-UHFFFAOYSA-N 0.000 description 3
- ALZOLUNSQWINIR-UHFFFAOYSA-N quinmerac Chemical compound OC(=O)C1=C(Cl)C=CC2=CC(C)=CN=C21 ALZOLUNSQWINIR-UHFFFAOYSA-N 0.000 description 3
- XSCHRSMBECNVNS-UHFFFAOYSA-N quinoxaline Chemical compound N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 229960004889 salicylic acid Drugs 0.000 description 3
- MGLWZSOBALDPEK-UHFFFAOYSA-N simetryn Chemical compound CCNC1=NC(NCC)=NC(SC)=N1 MGLWZSOBALDPEK-UHFFFAOYSA-N 0.000 description 3
- XWAFIZUTHLRWBE-UHFFFAOYSA-M sodium;2-(2,4-dichlorophenoxy)propanoate Chemical compound [Na+].[O-]C(=O)C(C)OC1=CC=C(Cl)C=C1Cl XWAFIZUTHLRWBE-UHFFFAOYSA-M 0.000 description 3
- YPMAQKXGDCKXPE-WCCKRBBISA-M sodium;[(3s)-3-amino-3-carboxypropyl]-methylphosphinate Chemical compound [Na+].CP([O-])(=O)CC[C@H](N)C(O)=O YPMAQKXGDCKXPE-WCCKRBBISA-M 0.000 description 3
- 238000001228 spectrum Methods 0.000 description 3
- 239000004546 suspension concentrate Substances 0.000 description 3
- BCQMBFHBDZVHKU-UHFFFAOYSA-N terbumeton Chemical compound CCNC1=NC(NC(C)(C)C)=NC(OC)=N1 BCQMBFHBDZVHKU-UHFFFAOYSA-N 0.000 description 3
- QQDYOLJZDUADHV-CJNGLKHVSA-N tetflupyrolimet Chemical compound FC1=C(C=CC=C1)NC(=O)[C@H]1C(N(C[C@@H]1C1=CC(=CC=C1)C(F)(F)F)C)=O QQDYOLJZDUADHV-CJNGLKHVSA-N 0.000 description 3
- DQFPEYARZIQXRM-LTGZKZEYSA-N tralkoxydim Chemical compound C1C(=O)C(C(/CC)=N/OCC)=C(O)CC1C1=C(C)C=C(C)C=C1C DQFPEYARZIQXRM-LTGZKZEYSA-N 0.000 description 3
- XOPFESVZMSQIKC-UHFFFAOYSA-N triasulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)OCCCl)=N1 XOPFESVZMSQIKC-UHFFFAOYSA-N 0.000 description 3
- 150000003918 triazines Chemical class 0.000 description 3
- REEQLXCGVXDJSQ-UHFFFAOYSA-N trichlopyr Chemical compound OC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl REEQLXCGVXDJSQ-UHFFFAOYSA-N 0.000 description 3
- 229960004418 trolamine Drugs 0.000 description 3
- 150000003672 ureas Chemical class 0.000 description 3
- CXBMCYHAMVGWJQ-CABCVRRESA-N (1,3-dioxo-4,5,6,7-tetrahydroisoindol-2-yl)methyl (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OCN1C(=O)C(CCCC2)=C2C1=O CXBMCYHAMVGWJQ-CABCVRRESA-N 0.000 description 2
- YNWVFADWVLCOPU-MDWZMJQESA-N (1E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol Chemical compound C1=NC=NN1/C(C(O)C(C)(C)C)=C/C1=CC=C(Cl)C=C1 YNWVFADWVLCOPU-MDWZMJQESA-N 0.000 description 2
- KAATUXNTWXVJKI-NSHGMRRFSA-N (1R)-cis-(alphaS)-cypermethrin Chemical compound CC1(C)[C@@H](C=C(Cl)Cl)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 KAATUXNTWXVJKI-NSHGMRRFSA-N 0.000 description 2
- VIXCLRUCUMWJFF-KGLIPLIRSA-N (1R,5S)-benzobicyclon Chemical compound CS(=O)(=O)c1ccc(C(=O)C2=C(Sc3ccccc3)[C@H]3CC[C@H](C3)C2=O)c(Cl)c1 VIXCLRUCUMWJFF-KGLIPLIRSA-N 0.000 description 2
- PGMZYNZXIYOOHJ-UHFFFAOYSA-N (2,6-dibromo-4-cyanophenyl) butanoate Chemical compound CCCC(=O)OC1=C(Br)C=C(C#N)C=C1Br PGMZYNZXIYOOHJ-UHFFFAOYSA-N 0.000 description 2
- BHZWBQPHPLFZSV-UHFFFAOYSA-N (2,6-dibromo-4-cyanophenyl) heptanoate Chemical compound CCCCCCC(=O)OC1=C(Br)C=C(C#N)C=C1Br BHZWBQPHPLFZSV-UHFFFAOYSA-N 0.000 description 2
- DQKWXTIYGWPGOO-UHFFFAOYSA-N (2,6-dibromo-4-cyanophenyl) octanoate Chemical compound CCCCCCCC(=O)OC1=C(Br)C=C(C#N)C=C1Br DQKWXTIYGWPGOO-UHFFFAOYSA-N 0.000 description 2
- UDPGUMQDCGORJQ-UHFFFAOYSA-N (2-chloroethyl)phosphonic acid Chemical compound OP(O)(=O)CCCl UDPGUMQDCGORJQ-UHFFFAOYSA-N 0.000 description 2
- IPPAUTOBDWNELX-UHFFFAOYSA-N (2-ethoxy-2-oxoethyl) 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical group C1=C([N+]([O-])=O)C(C(=O)OCC(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 IPPAUTOBDWNELX-UHFFFAOYSA-N 0.000 description 2
- RYAUSSKQMZRMAI-ALOPSCKCSA-N (2S,6R)-4-[3-(4-tert-butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine Chemical compound C=1C=C(C(C)(C)C)C=CC=1CC(C)CN1C[C@H](C)O[C@H](C)C1 RYAUSSKQMZRMAI-ALOPSCKCSA-N 0.000 description 2
- MPPOHAUSNPTFAJ-SECBINFHSA-N (2r)-2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoic acid Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 MPPOHAUSNPTFAJ-SECBINFHSA-N 0.000 description 2
- XHSDUVBUZOUAOQ-WJQMYRPNSA-N (3e,3ar,8bs)-3-[[(2r)-4-methyl-5-oxo-2h-furan-2-yl]oxymethylidene]-4,8b-dihydro-3ah-indeno[1,2-b]furan-2-one Chemical compound O1C(=O)C(C)=C[C@@H]1O\C=C/1C(=O)O[C@@H]2C3=CC=CC=C3C[C@@H]2\1 XHSDUVBUZOUAOQ-WJQMYRPNSA-N 0.000 description 2
- LNGRZPZKVUBWQV-UHFFFAOYSA-N (4-chloro-2-methylsulfonylphenyl)-(5-cyclopropyl-1,2-oxazol-4-yl)methanone Chemical compound CS(=O)(=O)C1=CC(Cl)=CC=C1C(=O)C1=C(C2CC2)ON=C1 LNGRZPZKVUBWQV-UHFFFAOYSA-N 0.000 description 2
- WMMQJAQJAPXWDO-UHFFFAOYSA-N (4-chlorophenyl) n-methylcarbamate Chemical compound CNC(=O)OC1=CC=C(Cl)C=C1 WMMQJAQJAPXWDO-UHFFFAOYSA-N 0.000 description 2
- 125000004737 (C1-C6) haloalkoxy group Chemical group 0.000 description 2
- 125000006643 (C2-C6) haloalkenyl group Chemical group 0.000 description 2
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- WCXDHFDTOYPNIE-RIYZIHGNSA-N (E)-acetamiprid Chemical compound N#C/N=C(\C)N(C)CC1=CC=C(Cl)N=C1 WCXDHFDTOYPNIE-RIYZIHGNSA-N 0.000 description 2
- WNTGYJSOUMFZEP-SSDOTTSWSA-N (R)-mecoprop Chemical compound OC(=O)[C@@H](C)OC1=CC=C(Cl)C=C1C WNTGYJSOUMFZEP-SSDOTTSWSA-N 0.000 description 2
- FFQPZWRNXKPNPX-INIZCTEOSA-N (S)-beflubutamid Chemical compound O([C@@H](CC)C(=O)NCC=1C=CC=CC=1)C1=CC=C(F)C(C(F)(F)F)=C1 FFQPZWRNXKPNPX-INIZCTEOSA-N 0.000 description 2
- DARPYRSDRJYGIF-PTNGSMBKSA-N (Z)-3-ethoxy-2-naphthalen-2-ylsulfonylprop-2-enenitrile Chemical compound C1=CC=CC2=CC(S(=O)(=O)C(\C#N)=C/OCC)=CC=C21 DARPYRSDRJYGIF-PTNGSMBKSA-N 0.000 description 2
- HOKKPVIRMVDYPB-UVTDQMKNSA-N (Z)-thiacloprid Chemical compound C1=NC(Cl)=CC=C1CN1C(=N/C#N)/SCC1 HOKKPVIRMVDYPB-UVTDQMKNSA-N 0.000 description 2
- PYKLUAIDKVVEOS-JLHYYAGUSA-N (z)-n-(cyanomethoxy)benzenecarboximidoyl cyanide Chemical compound N#CCO\N=C(/C#N)C1=CC=CC=C1 PYKLUAIDKVVEOS-JLHYYAGUSA-N 0.000 description 2
- WNWOTMKHOLCHRJ-UHFFFAOYSA-N 1,4-dihydrotriazol-5-one Chemical compound O=C1CN=NN1 WNWOTMKHOLCHRJ-UHFFFAOYSA-N 0.000 description 2
- MVHWKYHDYCGNQN-UHFFFAOYSA-N 1,5-dichloro-3-fluoro-2-(4-nitrophenoxy)benzene Chemical compound C1=CC([N+](=O)[O-])=CC=C1OC1=C(F)C=C(Cl)C=C1Cl MVHWKYHDYCGNQN-UHFFFAOYSA-N 0.000 description 2
- PYCINWWWERDNKE-UHFFFAOYSA-N 1-(2-chloro-6-propylimidazo[1,2-b]pyridazin-3-yl)sulfonyl-3-(4,6-dimethoxypyrimidin-2-yl)urea Chemical compound N12N=C(CCC)C=CC2=NC(Cl)=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 PYCINWWWERDNKE-UHFFFAOYSA-N 0.000 description 2
- RMOGWMIKYWRTKW-UHFFFAOYSA-N 1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)CC1=CC=C(Cl)C=C1 RMOGWMIKYWRTKW-UHFFFAOYSA-N 0.000 description 2
- PXMNMQRDXWABCY-UHFFFAOYSA-N 1-(4-chlorophenyl)-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol Chemical compound C1=NC=NN1CC(O)(C(C)(C)C)CCC1=CC=C(Cl)C=C1 PXMNMQRDXWABCY-UHFFFAOYSA-N 0.000 description 2
- LPVPNRSVMLNXGQ-UHFFFAOYSA-N 1-(azepan-1-yl)-2,2-dichloroethanone Chemical compound ClC(Cl)C(=O)N1CCCCCC1 LPVPNRSVMLNXGQ-UHFFFAOYSA-N 0.000 description 2
- 239000005969 1-Methyl-cyclopropene Substances 0.000 description 2
- JZWGZVIBYWRECK-RUMWWMSVSA-N 1-[(e)-[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidene]amino]-3-[4-(difluoromethoxy)phenyl]urea Chemical compound C1=CC(OC(F)F)=CC=C1NC(=O)N\N=C(C=1C=C(C=CC=1)C(F)(F)F)/CC1=CC=C(C#N)C=C1 JZWGZVIBYWRECK-RUMWWMSVSA-N 0.000 description 2
- AGLQRPBHLYHVLV-UHFFFAOYSA-N 1-[4-(trifluoromethyl)pyridin-2-yl]imidazolidin-2-one Chemical compound FC(F)(F)c1ccnc(c1)N1CCNC1=O AGLQRPBHLYHVLV-UHFFFAOYSA-N 0.000 description 2
- UUHXXNQVWVFJLW-UHFFFAOYSA-N 1-dimethoxyphosphorylethyl 2-(2,4-dichlorophenoxy)acetate Chemical compound COP(=O)(OC)C(C)OC(=O)COC1=CC=C(Cl)C=C1Cl UUHXXNQVWVFJLW-UHFFFAOYSA-N 0.000 description 2
- WXJYKXOIFICRPR-UHFFFAOYSA-L 1-methyl-4-(1-methylpyridin-1-ium-4-yl)pyridin-1-ium;methyl sulfate Chemical compound COS([O-])(=O)=O.COS([O-])(=O)=O.C1=C[N+](C)=CC=C1C1=CC=[N+](C)C=C1 WXJYKXOIFICRPR-UHFFFAOYSA-L 0.000 description 2
- SHDPRTQPPWIEJG-UHFFFAOYSA-N 1-methylcyclopropene Chemical compound CC1=CC1 SHDPRTQPPWIEJG-UHFFFAOYSA-N 0.000 description 2
- PRPINYUDVPFIRX-UHFFFAOYSA-N 1-naphthaleneacetic acid Chemical compound C1=CC=C2C(CC(=O)O)=CC=CC2=C1 PRPINYUDVPFIRX-UHFFFAOYSA-N 0.000 description 2
- 239000005971 1-naphthylacetic acid Substances 0.000 description 2
- 125000006023 1-pentenyl group Chemical group 0.000 description 2
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 2
- QWWHRELOCZEQNZ-UHFFFAOYSA-N 2,2-dichloro-1-(1-oxa-4-azaspiro[4.5]decan-4-yl)ethanone Chemical compound ClC(Cl)C(=O)N1CCOC11CCCCC1 QWWHRELOCZEQNZ-UHFFFAOYSA-N 0.000 description 2
- XZIDTOHMJBOSOX-UHFFFAOYSA-N 2,3,6-TBA Chemical compound OC(=O)C1=C(Cl)C=CC(Cl)=C1Cl XZIDTOHMJBOSOX-UHFFFAOYSA-N 0.000 description 2
- OVSKIKFHRZPJSS-UHFFFAOYSA-N 2,4-D Chemical compound OC(=O)COC1=CC=C(Cl)C=C1Cl OVSKIKFHRZPJSS-UHFFFAOYSA-N 0.000 description 2
- UIAFKZKHHVMJGS-UHFFFAOYSA-N 2,4-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1O UIAFKZKHHVMJGS-UHFFFAOYSA-N 0.000 description 2
- KGKGSIUWJCAFPX-UHFFFAOYSA-N 2,6-dichlorothiobenzamide Chemical compound NC(=S)C1=C(Cl)C=CC=C1Cl KGKGSIUWJCAFPX-UHFFFAOYSA-N 0.000 description 2
- WRXSEWUFHVTFEX-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)propanoate;dimethylazanium Chemical compound CNC.OC(=O)C(C)OC1=CC=C(Cl)C=C1Cl WRXSEWUFHVTFEX-UHFFFAOYSA-N 0.000 description 2
- QURLONWWPWCPIC-UHFFFAOYSA-N 2-(2-aminoethoxy)ethanol;3,6-dichloro-2-methoxybenzoic acid Chemical compound NCCOCCO.COC1=C(Cl)C=CC(Cl)=C1C(O)=O QURLONWWPWCPIC-UHFFFAOYSA-N 0.000 description 2
- ROKVVMOXSZIDEG-UHFFFAOYSA-N 2-(3,5,6-trichloropyridin-2-yl)oxyacetate;triethylazanium Chemical compound CCN(CC)CC.OC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl ROKVVMOXSZIDEG-UHFFFAOYSA-N 0.000 description 2
- YNTJKQDWYXUTLZ-UHFFFAOYSA-N 2-(3-chlorophenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=CC(Cl)=C1 YNTJKQDWYXUTLZ-UHFFFAOYSA-N 0.000 description 2
- CDRNTSYQTIKJNX-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)propanoate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.OC(=O)C(C)OC1=CC=C(Cl)C=C1C CDRNTSYQTIKJNX-UHFFFAOYSA-N 0.000 description 2
- UFNOUKDBUJZYDE-UHFFFAOYSA-N 2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1CC(O)(C=1C=CC(Cl)=CC=1)C(C)C1CC1 UFNOUKDBUJZYDE-UHFFFAOYSA-N 0.000 description 2
- KCTKQZUYHSKJLP-UHFFFAOYSA-N 2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)pyridine-3-carboxylate;propan-2-ylazanium Chemical compound CC(C)[NH3+].N1C(=O)C(C(C)C)(C)N=C1C1=NC=CC=C1C([O-])=O KCTKQZUYHSKJLP-UHFFFAOYSA-N 0.000 description 2
- DSUPUOGOCIFZBG-UHFFFAOYSA-N 2-(phenylcarbamoyl)benzoic acid Chemical compound OC(=O)C1=CC=CC=C1C(=O)NC1=CC=CC=C1 DSUPUOGOCIFZBG-UHFFFAOYSA-N 0.000 description 2
- MNHVNIJQQRJYDH-UHFFFAOYSA-N 2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]-1,2-dihydro-1,2,4-triazole-3-thione Chemical compound N1=CNC(=S)N1CC(C1(Cl)CC1)(O)CC1=CC=CC=C1Cl MNHVNIJQQRJYDH-UHFFFAOYSA-N 0.000 description 2
- NQQBTWVFKDDVIB-UHFFFAOYSA-N 2-aminoethanol;3,6-dichloropyridine-2-carboxylic acid Chemical compound NCCO.OC(=O)C1=NC(Cl)=CC=C1Cl NQQBTWVFKDDVIB-UHFFFAOYSA-N 0.000 description 2
- KWGQOJWITMQEOT-UHFFFAOYSA-N 2-benzhydryloxyacetic acid Chemical class C=1C=CC=CC=1C(OCC(=O)O)C1=CC=CC=C1 KWGQOJWITMQEOT-UHFFFAOYSA-N 0.000 description 2
- NCMJQZVNCFTQPG-UHFFFAOYSA-N 2-butoxyethyl 2-(2,4-dichlorophenoxy)propanoate Chemical group CCCCOCCOC(=O)C(C)OC1=CC=C(Cl)C=C1Cl NCMJQZVNCFTQPG-UHFFFAOYSA-N 0.000 description 2
- IVDRCZNHVGQBHZ-UHFFFAOYSA-N 2-butoxyethyl 2-(3,5,6-trichloropyridin-2-yl)oxyacetate Chemical group CCCCOCCOC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl IVDRCZNHVGQBHZ-UHFFFAOYSA-N 0.000 description 2
- WKGKFWXGAHXMCE-UHFFFAOYSA-N 2-butoxyethyl 2-(4-chloro-2-methylphenoxy)acetate Chemical group CCCCOCCOC(=O)COC1=CC=C(Cl)C=C1C WKGKFWXGAHXMCE-UHFFFAOYSA-N 0.000 description 2
- QEGVVEOAVNHRAA-UHFFFAOYSA-N 2-chloro-6-(4,6-dimethoxypyrimidin-2-yl)sulfanylbenzoic acid Chemical compound COC1=CC(OC)=NC(SC=2C(=C(Cl)C=CC=2)C(O)=O)=N1 QEGVVEOAVNHRAA-UHFFFAOYSA-N 0.000 description 2
- IRCMYGHHKLLGHV-UHFFFAOYSA-N 2-ethoxy-3,3-dimethyl-2,3-dihydro-1-benzofuran-5-yl methanesulfonate Chemical compound C1=C(OS(C)(=O)=O)C=C2C(C)(C)C(OCC)OC2=C1 IRCMYGHHKLLGHV-UHFFFAOYSA-N 0.000 description 2
- CCRUKTGQASMHHB-UHFFFAOYSA-M 2-hydroxyethyl(trimethyl)azanium;2-(3,5,6-trichloropyridin-2-yl)oxyacetate Chemical compound C[N+](C)(C)CCO.[O-]C(=O)COC1=NC(Cl)=C(Cl)C=C1Cl CCRUKTGQASMHHB-UHFFFAOYSA-M 0.000 description 2
- RZCJYMOBWVJQGV-UHFFFAOYSA-N 2-naphthyloxyacetic acid Chemical compound C1=CC=CC2=CC(OCC(=O)O)=CC=C21 RZCJYMOBWVJQGV-UHFFFAOYSA-N 0.000 description 2
- LOHXCLHVVJGTLU-UHFFFAOYSA-N 2-oxopropyl 2-(5-chloroquinolin-8-yl)oxyacetate Chemical compound C1=CN=C2C(OCC(=O)OCC(=O)C)=CC=C(Cl)C2=C1 LOHXCLHVVJGTLU-UHFFFAOYSA-N 0.000 description 2
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- UFAPVJDEYHLLBG-UHFFFAOYSA-N 2-{2-chloro-4-(methylsulfonyl)-3-[(tetrahydrofuran-2-ylmethoxy)methyl]benzoyl}cyclohexane-1,3-dione Chemical compound ClC1=C(COCC2OCCC2)C(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O UFAPVJDEYHLLBG-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- UYEMGAFJOZZIFP-UHFFFAOYSA-N 3,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC(O)=C1 UYEMGAFJOZZIFP-UHFFFAOYSA-N 0.000 description 2
- JDRFUUBRGGDEIZ-UHFFFAOYSA-N 3,6-dichloro-2-methoxybenzoate;dimethylazanium Chemical compound CNC.COC1=C(Cl)C=CC(Cl)=C1C(O)=O JDRFUUBRGGDEIZ-UHFFFAOYSA-N 0.000 description 2
- DLUOWQZURZVAEN-UHFFFAOYSA-N 3,6-dichloro-2-methoxybenzoic acid;propan-2-amine Chemical compound CC(C)N.COC1=C(Cl)C=CC(Cl)=C1C(O)=O DLUOWQZURZVAEN-UHFFFAOYSA-N 0.000 description 2
- PIJRTJAKUMDAKQ-UHFFFAOYSA-N 3-(cyclopropen-1-yl)propanoic acid Chemical compound OC(=O)CCC1=CC1 PIJRTJAKUMDAKQ-UHFFFAOYSA-N 0.000 description 2
- RSSKZIYCSDAOJD-UHFFFAOYSA-N 3-amino-2,5-dichlorobenzoic acid;azane Chemical compound [NH4+].NC1=CC(Cl)=CC(C([O-])=O)=C1Cl RSSKZIYCSDAOJD-UHFFFAOYSA-N 0.000 description 2
- SGSBXGMWMFNPKQ-UHFFFAOYSA-N 3-amino-2,5-dichlorobenzoic acid;methanamine Chemical compound NC.NC1=CC(Cl)=CC(C(O)=O)=C1Cl SGSBXGMWMFNPKQ-UHFFFAOYSA-N 0.000 description 2
- ZXVONLUNISGICL-UHFFFAOYSA-N 4,6-dinitro-o-cresol Chemical compound CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1O ZXVONLUNISGICL-UHFFFAOYSA-N 0.000 description 2
- UTNPLPXWDUEIJI-UHFFFAOYSA-N 4-[3-[2,6-dichloro-4-(3,3-dichloroprop-2-enoxy)phenoxy]propoxy]-2-methoxy-6-(trifluoromethyl)pyrimidine Chemical compound FC(F)(F)C1=NC(OC)=NC(OCCCOC=2C(=CC(OCC=C(Cl)Cl)=CC=2Cl)Cl)=C1 UTNPLPXWDUEIJI-UHFFFAOYSA-N 0.000 description 2
- WFMULRZXIUQYFV-UHFFFAOYSA-N 4-amino-3,5,6-trichloropyridine-2-carboxylic acid;n,n-diethylethanamine Chemical compound CCN(CC)CC.NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl WFMULRZXIUQYFV-UHFFFAOYSA-N 0.000 description 2
- SLYAVQCIBHSIQW-UHFFFAOYSA-N 4-amino-3,6-dichloropyridine-2-carboxylic acid;1-[bis(2-hydroxypropyl)amino]propan-2-ol Chemical compound NC1=CC(Cl)=NC(C(O)=O)=C1Cl.CC(O)CN(CC(C)O)CC(C)O SLYAVQCIBHSIQW-UHFFFAOYSA-N 0.000 description 2
- 125000004487 4-tetrahydropyranyl group Chemical group [H]C1([H])OC([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- PWVXXGRKLHYWKM-UHFFFAOYSA-N 5-[2-(benzenesulfonyl)ethyl]-3-[(1-methylpyrrolidin-2-yl)methyl]-1h-indole Chemical compound CN1CCCC1CC(C1=C2)=CNC1=CC=C2CCS(=O)(=O)C1=CC=CC=C1 PWVXXGRKLHYWKM-UHFFFAOYSA-N 0.000 description 2
- ZOCSXAVNDGMNBV-UHFFFAOYSA-N 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile Chemical compound NC1=C(S(=O)C(F)(F)F)C(C#N)=NN1C1=C(Cl)C=C(C(F)(F)F)C=C1Cl ZOCSXAVNDGMNBV-UHFFFAOYSA-N 0.000 description 2
- ODPOAESBSUKMHD-UHFFFAOYSA-L 6,7-dihydrodipyrido[1,2-b:1',2'-e]pyrazine-5,8-diium;dibromide Chemical compound [Br-].[Br-].C1=CC=[N+]2CC[N+]3=CC=CC=C3C2=C1 ODPOAESBSUKMHD-UHFFFAOYSA-L 0.000 description 2
- NGZWZIAGFHOSDS-UHFFFAOYSA-N 6-methylheptyl 4-amino-3,5,6-trichloropyridine-2-carboxylate Chemical group CC(C)CCCCCOC(=O)C1=NC(Cl)=C(Cl)C(N)=C1Cl NGZWZIAGFHOSDS-UHFFFAOYSA-N 0.000 description 2
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 239000005660 Abamectin Substances 0.000 description 2
- 239000005875 Acetamiprid Substances 0.000 description 2
- 102000012440 Acetylcholinesterase Human genes 0.000 description 2
- 108010022752 Acetylcholinesterase Proteins 0.000 description 2
- 239000005652 Acrinathrin Substances 0.000 description 2
- 241000589158 Agrobacterium Species 0.000 description 2
- 241000234282 Allium Species 0.000 description 2
- 241000219318 Amaranthus Species 0.000 description 2
- 239000003666 Amidosulfuron Substances 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- 241000219195 Arabidopsis thaliana Species 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- 235000005781 Avena Nutrition 0.000 description 2
- 239000005469 Azimsulfuron Substances 0.000 description 2
- 101100113088 Bacillus subtilis (strain 168) cgoX gene Proteins 0.000 description 2
- 239000005734 Benalaxyl Substances 0.000 description 2
- 239000005471 Benfluralin Substances 0.000 description 2
- NCLRGQCNIDLTDW-UHFFFAOYSA-M Bentazone-sodium Chemical compound [Na+].C1=CC=C2[N-]S(=O)(=O)N(C(C)C)C(=O)C2=C1 NCLRGQCNIDLTDW-UHFFFAOYSA-M 0.000 description 2
- 241000219198 Brassica Species 0.000 description 2
- 235000014698 Brassica juncea var multisecta Nutrition 0.000 description 2
- 240000002791 Brassica napus Species 0.000 description 2
- 235000006008 Brassica napus var napus Nutrition 0.000 description 2
- 240000000385 Brassica napus var. napus Species 0.000 description 2
- 235000011299 Brassica oleracea var botrytis Nutrition 0.000 description 2
- 240000003259 Brassica oleracea var. botrytis Species 0.000 description 2
- 235000006618 Brassica rapa subsp oleifera Nutrition 0.000 description 2
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 2
- IXVMHGVQKLDRKH-VRESXRICSA-N Brassinolide Natural products O=C1OC[C@@H]2[C@@H]3[C@@](C)([C@H]([C@@H]([C@@H](O)[C@H](O)[C@H](C(C)C)C)C)CC3)CC[C@@H]2[C@]2(C)[C@@H]1C[C@H](O)[C@H](O)C2 IXVMHGVQKLDRKH-VRESXRICSA-N 0.000 description 2
- 125000003320 C2-C6 alkenyloxy group Chemical group 0.000 description 2
- SVRNNESSZBFZIJ-LQNPISAESA-N CCOC([C@@H](C1)C=C[C@@H]1NC(C(C1)(OC)ON=C1C1=CC(F)=CC(F)=C1)=O)=O Chemical compound CCOC([C@@H](C1)C=C[C@@H]1NC(C(C1)(OC)ON=C1C1=CC(F)=CC(F)=C1)=O)=O SVRNNESSZBFZIJ-LQNPISAESA-N 0.000 description 2
- CRJNVKRUGKSRPD-IJPZITHUSA-N C[C@@](C1)(C(N[C@H](C2)C=C[C@H]2C(O)=O)=O)ON=C1C1=CC(F)=CC(F)=C1 Chemical compound C[C@@](C1)(C(N[C@H](C2)C=C[C@H]2C(O)=O)=O)ON=C1C1=CC(F)=CC(F)=C1 CRJNVKRUGKSRPD-IJPZITHUSA-N 0.000 description 2
- TWFZGCMQGLPBSX-UHFFFAOYSA-N Carbendazim Natural products C1=CC=C2NC(NC(=O)OC)=NC2=C1 TWFZGCMQGLPBSX-UHFFFAOYSA-N 0.000 description 2
- 239000005490 Carbetamide Substances 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 239000005493 Chloridazon (aka pyrazone) Substances 0.000 description 2
- 239000005496 Chlorsulfuron Substances 0.000 description 2
- 239000005498 Clodinafop Substances 0.000 description 2
- 108010051219 Cre recombinase Proteins 0.000 description 2
- 241000219112 Cucumis Species 0.000 description 2
- 235000009854 Cucurbita moschata Nutrition 0.000 description 2
- XZMCDFZZKTWFGF-UHFFFAOYSA-N Cyanamide Chemical compound NC#N XZMCDFZZKTWFGF-UHFFFAOYSA-N 0.000 description 2
- 239000005754 Cyazofamid Substances 0.000 description 2
- 239000005501 Cycloxydim Substances 0.000 description 2
- TYIYMOAHACZAMQ-CQSZACIVSA-N Cyhalofop-butyl Chemical group C1=CC(O[C@H](C)C(=O)OCCCC)=CC=C1OC1=CC=C(C#N)C=C1F TYIYMOAHACZAMQ-CQSZACIVSA-N 0.000 description 2
- 239000005946 Cypermethrin Substances 0.000 description 2
- 239000005757 Cyproconazole Substances 0.000 description 2
- NPOJQCVWMSKXDN-UHFFFAOYSA-N Dacthal Chemical group COC(=O)C1=C(Cl)C(Cl)=C(C(=O)OC)C(Cl)=C1Cl NPOJQCVWMSKXDN-UHFFFAOYSA-N 0.000 description 2
- NOQGZXFMHARMLW-UHFFFAOYSA-N Daminozide Chemical compound CN(C)NC(=O)CCC(O)=O NOQGZXFMHARMLW-UHFFFAOYSA-N 0.000 description 2
- 239000005975 Daminozide Substances 0.000 description 2
- 239000005892 Deltamethrin Substances 0.000 description 2
- 239000005503 Desmedipham Substances 0.000 description 2
- HCRWJJJUKUVORR-UHFFFAOYSA-N Desmetryn Chemical compound CNC1=NC(NC(C)C)=NC(SC)=N1 HCRWJJJUKUVORR-UHFFFAOYSA-N 0.000 description 2
- YRMLFORXOOIJDR-UHFFFAOYSA-N Dichlormid Chemical compound ClC(Cl)C(=O)N(CC=C)CC=C YRMLFORXOOIJDR-UHFFFAOYSA-N 0.000 description 2
- 101100409079 Dictyostelium discoideum ppox gene Proteins 0.000 description 2
- 239000005759 Diethofencarb Substances 0.000 description 2
- 239000005508 Dimethachlor Substances 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- 108010024882 Electron Transport Complex III Proteins 0.000 description 2
- 102000015782 Electron Transport Complex III Human genes 0.000 description 2
- 239000005976 Ethephon Substances 0.000 description 2
- KCOCSOWTADCKOL-UHFFFAOYSA-N Ethidimuron Chemical compound CCS(=O)(=O)C1=NN=C(N(C)C(=O)NC)S1 KCOCSOWTADCKOL-UHFFFAOYSA-N 0.000 description 2
- 239000005961 Ethoprophos Substances 0.000 description 2
- 239000005958 Fenamiphos (aka phenamiphos) Substances 0.000 description 2
- 239000005778 Fenpropimorph Substances 0.000 description 2
- PNVJTZOFSHSLTO-UHFFFAOYSA-N Fenthion Chemical compound COP(=S)(OC)OC1=CC=C(SC)C(C)=C1 PNVJTZOFSHSLTO-UHFFFAOYSA-N 0.000 description 2
- 239000005899 Fipronil Substances 0.000 description 2
- 239000005978 Flumetralin Substances 0.000 description 2
- PWNAWOCHVWERAR-UHFFFAOYSA-N Flumetralin Chemical compound [O-][N+](=O)C=1C=C(C(F)(F)F)C=C([N+]([O-])=O)C=1N(CC)CC1=C(F)C=CC=C1Cl PWNAWOCHVWERAR-UHFFFAOYSA-N 0.000 description 2
- 239000005533 Fluometuron Substances 0.000 description 2
- 239000005783 Fluopyram Substances 0.000 description 2
- 239000005558 Fluroxypyr Substances 0.000 description 2
- VEVZCONIUDBCDC-UHFFFAOYSA-N Flurprimidol Chemical compound C=1N=CN=CC=1C(O)(C(C)C)C1=CC=C(OC(F)(F)F)C=C1 VEVZCONIUDBCDC-UHFFFAOYSA-N 0.000 description 2
- UKSLKNUCVPZQCQ-UHFFFAOYSA-N Fluxofenim Chemical compound C=1C=C(Cl)C=CC=1C(C(F)(F)F)=NOCC1OCCO1 UKSLKNUCVPZQCQ-UHFFFAOYSA-N 0.000 description 2
- 239000005789 Folpet Substances 0.000 description 2
- 239000005979 Forchlorfenuron Substances 0.000 description 2
- 239000005980 Gibberellic acid Substances 0.000 description 2
- 108010014458 Gin recombinase Proteins 0.000 description 2
- 108050006905 Glutamate-Gated Chloride Channel Proteins 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 108030006517 Glyphosate oxidoreductases Proteins 0.000 description 2
- PDYXIVPKOMYDOK-UHFFFAOYSA-N Glyphosate-monoammonium Chemical compound [NH4+].OC(=O)CNCP(O)([O-])=O PDYXIVPKOMYDOK-UHFFFAOYSA-N 0.000 description 2
- LXKOADMMGWXPJQ-UHFFFAOYSA-N Halosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=C(Cl)C=2C(O)=O)C)=N1 LXKOADMMGWXPJQ-UHFFFAOYSA-N 0.000 description 2
- FMGZEUWROYGLAY-UHFFFAOYSA-N Halosulfuron-methyl Chemical group ClC1=NN(C)C(S(=O)(=O)NC(=O)NC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OC FMGZEUWROYGLAY-UHFFFAOYSA-N 0.000 description 2
- 239000005906 Imidacloprid Substances 0.000 description 2
- 240000007171 Imperata cylindrica Species 0.000 description 2
- PFDCOZXELJAUTR-UHFFFAOYSA-N Inabenfide Chemical compound C=1C(Cl)=CC=C(NC(=O)C=2C=CN=CC=2)C=1C(O)C1=CC=CC=C1 PFDCOZXELJAUTR-UHFFFAOYSA-N 0.000 description 2
- JUJFQMPKBJPSFZ-UHFFFAOYSA-M Iodosulfuron-methyl-sodium Chemical compound [Na+].COC(=O)C1=CC=C(I)C=C1S(=O)(=O)[N-]C(=O)NC1=NC(C)=NC(OC)=N1 JUJFQMPKBJPSFZ-UHFFFAOYSA-M 0.000 description 2
- 235000021506 Ipomoea Nutrition 0.000 description 2
- 239000005797 Iprovalicarb Substances 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- NWUWYYSKZYIQAE-ZBFHGGJFSA-N L-(R)-iprovalicarb Chemical compound CC(C)OC(=O)N[C@@H](C(C)C)C(=O)N[C@H](C)C1=CC=C(C)C=C1 NWUWYYSKZYIQAE-ZBFHGGJFSA-N 0.000 description 2
- FQPGMQABJNQLLF-VKHMYHEASA-N L-canaline Chemical compound NOCC[C@H](N)C(O)=O FQPGMQABJNQLLF-VKHMYHEASA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- 239000005572 Lenacil Substances 0.000 description 2
- 241000227653 Lycopersicon Species 0.000 description 2
- 101150039283 MCPB gene Proteins 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 239000005983 Maleic hydrazide Substances 0.000 description 2
- BGRDGMRNKXEXQD-UHFFFAOYSA-N Maleic hydrazide Chemical compound OC1=CC=C(O)N=N1 BGRDGMRNKXEXQD-UHFFFAOYSA-N 0.000 description 2
- 239000002169 Metam Substances 0.000 description 2
- 239000005579 Metamitron Substances 0.000 description 2
- 239000005580 Metazachlor Substances 0.000 description 2
- 239000005951 Methiocarb Substances 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- NWBJYWHLCVSVIJ-UHFFFAOYSA-N N-benzyladenine Chemical compound N=1C=NC=2NC=NC=2C=1NCC1=CC=CC=C1 NWBJYWHLCVSVIJ-UHFFFAOYSA-N 0.000 description 2
- GRSMWKLPSNHDHA-UHFFFAOYSA-N Naphthalic anhydride Chemical compound C1=CC(C(=O)OC2=O)=C3C2=CC=CC3=C1 GRSMWKLPSNHDHA-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 239000005587 Oryzalin Substances 0.000 description 2
- 239000005589 Oxasulfuron Substances 0.000 description 2
- 241000209117 Panicum Species 0.000 description 2
- 235000006443 Panicum miliaceum subsp. miliaceum Nutrition 0.000 description 2
- 235000009037 Panicum miliaceum subsp. ruderale Nutrition 0.000 description 2
- 239000005814 Pencycuron Substances 0.000 description 2
- WGVWLKXZBUVUAM-UHFFFAOYSA-N Pentanochlor Chemical compound CCCC(C)C(=O)NC1=CC=C(C)C(Cl)=C1 WGVWLKXZBUVUAM-UHFFFAOYSA-N 0.000 description 2
- 239000005594 Phenmedipham Substances 0.000 description 2
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 description 2
- 108030002884 Phosphinothricin acetyltransferases Proteins 0.000 description 2
- 108010081996 Photosystem I Protein Complex Proteins 0.000 description 2
- 108010060806 Photosystem II Protein Complex Proteins 0.000 description 2
- 235000010582 Pisum sativum Nutrition 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 239000005820 Prochloraz Substances 0.000 description 2
- 239000005599 Profoxydim Substances 0.000 description 2
- 239000005986 Prohexadione Substances 0.000 description 2
- 239000005821 Propamocarb Substances 0.000 description 2
- 239000005600 Propaquizafop Substances 0.000 description 2
- 239000005823 Propineb Substances 0.000 description 2
- 239000005602 Propyzamide Substances 0.000 description 2
- 239000005825 Prothioconazole Substances 0.000 description 2
- BGNQYGRXEXDAIQ-UHFFFAOYSA-N Pyrazosulfuron-ethyl Chemical group C1=NN(C)C(S(=O)(=O)NC(=O)NC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OCC BGNQYGRXEXDAIQ-UHFFFAOYSA-N 0.000 description 2
- 239000005606 Pyridate Substances 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 239000005828 Pyrimethanil Substances 0.000 description 2
- 239000005608 Quinmerac Substances 0.000 description 2
- 241000220259 Raphanus Species 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 235000002634 Solanum Nutrition 0.000 description 2
- 241000207763 Solanum Species 0.000 description 2
- 235000017967 Sphenoclea zeylanica Nutrition 0.000 description 2
- 244000273618 Sphenoclea zeylanica Species 0.000 description 2
- 239000005837 Spiroxamine Substances 0.000 description 2
- 108091081024 Start codon Proteins 0.000 description 2
- 101000951943 Stenotrophomonas maltophilia Dicamba O-demethylase, oxygenase component Proteins 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 238000010459 TALEN Methods 0.000 description 2
- 239000005839 Tebuconazole Substances 0.000 description 2
- 239000005939 Tefluthrin Substances 0.000 description 2
- PNRAZZZISDRWMV-UHFFFAOYSA-N Terbucarb Chemical compound CNC(=O)OC1=C(C(C)(C)C)C=C(C)C=C1C(C)(C)C PNRAZZZISDRWMV-UHFFFAOYSA-N 0.000 description 2
- 239000005940 Thiacloprid Substances 0.000 description 2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 2
- 108010043645 Transcription Activator-Like Effector Nucleases Proteins 0.000 description 2
- 108010020764 Transposases Proteins 0.000 description 2
- 102000008579 Transposases Human genes 0.000 description 2
- APEOYHMMWLHUAL-UHFFFAOYSA-N Triacetyl-gallussaeure-aethylester Natural products CCOC(=O)C1=CC(OC(C)=O)=C(OC(C)=O)C(OC(C)=O)=C1 APEOYHMMWLHUAL-UHFFFAOYSA-N 0.000 description 2
- 239000005846 Triadimenol Substances 0.000 description 2
- 239000005627 Triclopyr Substances 0.000 description 2
- 239000005857 Trifloxystrobin Substances 0.000 description 2
- 239000005994 Trinexapac Substances 0.000 description 2
- AMRQXHFXNZFDCH-SECBINFHSA-N [(2r)-1-(ethylamino)-1-oxopropan-2-yl] n-phenylcarbamate Chemical compound CCNC(=O)[C@@H](C)OC(=O)NC1=CC=CC=C1 AMRQXHFXNZFDCH-SECBINFHSA-N 0.000 description 2
- WXQMIXCKKFECIU-UHFFFAOYSA-N [4-[2-chloro-3-[(3,5-dimethylpyrazol-1-yl)methyl]-4-methylsulfonylbenzoyl]-2,5-dimethylpyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound CN1N=C(C(=C1)C(=O)OC1=C(C(=NN1C)C)C(C1=C(C(=C(C=C1)S(=O)(=O)C)CN1N=C(C=C1C)C)Cl)=O)C WXQMIXCKKFECIU-UHFFFAOYSA-N 0.000 description 2
- NWBFHIJKEYFVND-UHFFFAOYSA-N [4-[2-chloro-4-methylsulfonyl-3-(2,2,2-trifluoroethoxymethyl)benzoyl]-2-ethylpyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound N1(C=C(C(=O)OC=2N(CC)N=CC=2C(=O)C2=C(C(=C(S(=O)(=O)C)C=C2)COCC(F)(F)F)Cl)C(C)=N1)C NWBFHIJKEYFVND-UHFFFAOYSA-N 0.000 description 2
- MNLVCBKWEVXHGE-UHFFFAOYSA-N [CH]1[CH]CC[CH]1 Chemical group [CH]1[CH]CC[CH]1 MNLVCBKWEVXHGE-UHFFFAOYSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 2
- 229940022698 acetylcholinesterase Drugs 0.000 description 2
- CGIHPACLZJDCBQ-UHFFFAOYSA-N acibenzolar Chemical compound SC(=O)C1=CC=CC2=C1SN=N2 CGIHPACLZJDCBQ-UHFFFAOYSA-N 0.000 description 2
- YLFSVIMMRPNPFK-WEQBUNFVSA-N acrinathrin Chemical compound CC1(C)[C@@H](\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 YLFSVIMMRPNPFK-WEQBUNFVSA-N 0.000 description 2
- QGLZXHRNAYXIBU-WEVVVXLNSA-N aldicarb Chemical compound CNC(=O)O\N=C\C(C)(C)SC QGLZXHRNAYXIBU-WEVVVXLNSA-N 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 125000003302 alkenyloxy group Chemical group 0.000 description 2
- 125000005092 alkenyloxycarbonyl group Chemical group 0.000 description 2
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 2
- 125000005083 alkoxyalkoxy group Chemical group 0.000 description 2
- 125000005078 alkoxycarbonylalkyl group Chemical group 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 150000001350 alkyl halides Chemical class 0.000 description 2
- 125000004644 alkyl sulfinyl group Chemical group 0.000 description 2
- 125000001118 alkylidene group Chemical group 0.000 description 2
- 125000005225 alkynyloxycarbonyl group Chemical group 0.000 description 2
- 239000011717 all-trans-retinol Substances 0.000 description 2
- 235000019169 all-trans-retinol Nutrition 0.000 description 2
- POJWUDADGALRAB-UHFFFAOYSA-N allantoin Chemical compound NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 description 2
- 125000005282 allenyl group Chemical group 0.000 description 2
- 230000003281 allosteric effect Effects 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- MDWRNPOBHVLALB-UHFFFAOYSA-N aminocyclopyrachlor-methyl Chemical group NC1=C(Cl)C(C(=O)OC)=NC(C2CC2)=N1 MDWRNPOBHVLALB-UHFFFAOYSA-N 0.000 description 2
- BYXPJQZAPGGUGH-UHFFFAOYSA-M aminocyclopyrachlor-potassium Chemical compound [K+].[O-]C(=O)C1=C(Cl)C(N)=NC(C2CC2)=N1 BYXPJQZAPGGUGH-UHFFFAOYSA-M 0.000 description 2
- 229960002749 aminolevulinic acid Drugs 0.000 description 2
- MDFFNEOEWAXZRQ-UHFFFAOYSA-N aminyl Chemical compound [NH2] MDFFNEOEWAXZRQ-UHFFFAOYSA-N 0.000 description 2
- 229960002587 amitraz Drugs 0.000 description 2
- QXAITBQSYVNQDR-ZIOPAAQOSA-N amitraz Chemical compound C=1C=C(C)C=C(C)C=1/N=C/N(C)\C=N\C1=CC=C(C)C=C1C QXAITBQSYVNQDR-ZIOPAAQOSA-N 0.000 description 2
- 229940051880 analgesics and antipyretics pyrazolones Drugs 0.000 description 2
- HUTDUHSNJYTCAR-UHFFFAOYSA-N ancymidol Chemical compound C1=CC(OC)=CC=C1C(O)(C=1C=NC=NC=1)C1CC1 HUTDUHSNJYTCAR-UHFFFAOYSA-N 0.000 description 2
- 125000005098 aryl alkoxy carbonyl group Chemical group 0.000 description 2
- 125000002102 aryl alkyloxo group Chemical group 0.000 description 2
- 125000004104 aryloxy group Chemical group 0.000 description 2
- PEXLHWBDBQUUOG-UHFFFAOYSA-M asulam-sodium Chemical compound [Na+].COC(=O)[N-]S(=O)(=O)C1=CC=C(N)C=C1 PEXLHWBDBQUUOG-UHFFFAOYSA-M 0.000 description 2
- FTNJWQUOZFUQQJ-NDAWSKJSSA-N azadirachtin A Chemical compound C([C@@H]([C@]1(C=CO[C@H]1O1)O)[C@]2(C)O3)[C@H]1[C@]23[C@]1(C)[C@H](O)[C@H](OC[C@@]2([C@@H](C[C@@H]3OC(=O)C(\C)=C\C)OC(C)=O)C(=O)OC)[C@@H]2[C@]32CO[C@@](C(=O)OC)(O)[C@@H]12 FTNJWQUOZFUQQJ-NDAWSKJSSA-N 0.000 description 2
- GNZZHGJSMCDMBU-UHFFFAOYSA-N azane;5-(methoxymethyl)-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)pyridine-3-carboxylic acid Chemical compound [NH4+].[O-]C(=O)C1=CC(COC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 GNZZHGJSMCDMBU-UHFFFAOYSA-N 0.000 description 2
- FBJUTZMAUXJMMH-UHFFFAOYSA-N azane;5-methyl-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)pyridine-3-carboxylic acid Chemical compound [NH4+].N1C(=O)C(C(C)C)(C)N=C1C1=NC=C(C)C=C1C([O-])=O FBJUTZMAUXJMMH-UHFFFAOYSA-N 0.000 description 2
- OTSAMNSACVKIOJ-UHFFFAOYSA-N azane;carbamoyl(ethoxy)phosphinic acid Chemical compound [NH4+].CCOP([O-])(=O)C(N)=O OTSAMNSACVKIOJ-UHFFFAOYSA-N 0.000 description 2
- 238000002869 basic local alignment search tool Methods 0.000 description 2
- CJPQIRJHIZUAQP-MRXNPFEDSA-N benalaxyl-M Chemical compound CC=1C=CC=C(C)C=1N([C@H](C)C(=O)OC)C(=O)CC1=CC=CC=C1 CJPQIRJHIZUAQP-MRXNPFEDSA-N 0.000 description 2
- KKTYYONKWVCNJI-UHFFFAOYSA-N benazolin-dimethylammonium Chemical compound CNC.C1=CC=C2SC(=O)N(CC(=O)O)C2=C1Cl KKTYYONKWVCNJI-UHFFFAOYSA-N 0.000 description 2
- BVAQOPQXUNGRCM-UHFFFAOYSA-M benazolin-potassium Chemical compound [K+].C1=CC=C2SC(=O)N(CC(=O)[O-])C2=C1Cl BVAQOPQXUNGRCM-UHFFFAOYSA-M 0.000 description 2
- PPWBRCCBKOWDNB-UHFFFAOYSA-N bensulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)CC=2C(=CC=CC=2)C(O)=O)=N1 PPWBRCCBKOWDNB-UHFFFAOYSA-N 0.000 description 2
- 150000001559 benzoic acids Chemical class 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- OMFRMAHOUUJSGP-IRHGGOMRSA-N bifenthrin Chemical compound C1=CC=C(C=2C=CC=CC=2)C(C)=C1COC(=O)[C@@H]1[C@H](\C=C(/Cl)C(F)(F)F)C1(C)C OMFRMAHOUUJSGP-IRHGGOMRSA-N 0.000 description 2
- VEMKTZHHVJILDY-UXHICEINSA-N bioresmethrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OCC1=COC(CC=2C=CC=CC=2)=C1 VEMKTZHHVJILDY-UXHICEINSA-N 0.000 description 2
- LLEMOWNGBBNAJR-UHFFFAOYSA-N biphenyl-2-ol Chemical compound OC1=CC=CC=C1C1=CC=CC=C1 LLEMOWNGBBNAJR-UHFFFAOYSA-N 0.000 description 2
- CGFQAAGKJZMVNF-UHFFFAOYSA-N bis(2-hydroxyethyl)azanium;2-(4-chloro-2-methylphenoxy)propanoate Chemical compound OCCNCCO.OC(=O)C(C)OC1=CC=C(Cl)C=C1C CGFQAAGKJZMVNF-UHFFFAOYSA-N 0.000 description 2
- FUHMZYWBSHTEDZ-UHFFFAOYSA-M bispyribac-sodium Chemical compound [Na+].COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C([O-])=O)=N1 FUHMZYWBSHTEDZ-UHFFFAOYSA-M 0.000 description 2
- IXVMHGVQKLDRKH-KNBKMWSGSA-N brassinolide Chemical compound C1OC(=O)[C@H]2C[C@H](O)[C@H](O)C[C@]2(C)[C@H]2CC[C@]3(C)[C@@H]([C@H](C)[C@@H](O)[C@H](O)[C@@H](C)C(C)C)CC[C@H]3[C@@H]21 IXVMHGVQKLDRKH-KNBKMWSGSA-N 0.000 description 2
- GZUXJHMPEANEGY-UHFFFAOYSA-N bromomethane Chemical compound BrC GZUXJHMPEANEGY-UHFFFAOYSA-N 0.000 description 2
- HKPHPIREJKHECO-UHFFFAOYSA-N butachlor Chemical compound CCCCOCN(C(=O)CCl)C1=C(CC)C=CC=C1CC HKPHPIREJKHECO-UHFFFAOYSA-N 0.000 description 2
- NLKUPINTOLSSLD-UHFFFAOYSA-L calcium;4-(1-oxidopropylidene)-3,5-dioxocyclohexane-1-carboxylate Chemical compound [Ca+2].CCC([O-])=C1C(=O)CC(C([O-])=O)CC1=O NLKUPINTOLSSLD-UHFFFAOYSA-L 0.000 description 2
- JHRWWRDRBPCWTF-OLQVQODUSA-N captafol Chemical compound C1C=CC[C@H]2C(=O)N(SC(Cl)(Cl)C(Cl)Cl)C(=O)[C@H]21 JHRWWRDRBPCWTF-OLQVQODUSA-N 0.000 description 2
- 229960005286 carbaryl Drugs 0.000 description 2
- CVXBEEMKQHEXEN-UHFFFAOYSA-N carbaryl Chemical compound C1=CC=C2C(OC(=O)NC)=CC=CC2=C1 CVXBEEMKQHEXEN-UHFFFAOYSA-N 0.000 description 2
- JNPZQRQPIHJYNM-UHFFFAOYSA-N carbendazim Chemical compound C1=C[CH]C2=NC(NC(=O)OC)=NC2=C1 JNPZQRQPIHJYNM-UHFFFAOYSA-N 0.000 description 2
- 239000006013 carbendazim Substances 0.000 description 2
- DUEPRVBVGDRKAG-UHFFFAOYSA-N carbofuran Chemical compound CNC(=O)OC1=CC=CC2=C1OC(C)(C)C2 DUEPRVBVGDRKAG-UHFFFAOYSA-N 0.000 description 2
- ULDHMXUKGWMISQ-UHFFFAOYSA-N carvone Chemical compound CC(=C)C1CC=C(C)C(=O)C1 ULDHMXUKGWMISQ-UHFFFAOYSA-N 0.000 description 2
- 235000005487 catechin Nutrition 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- NSWAMPCUPHPTTC-UHFFFAOYSA-N chlorimuron-ethyl Chemical group CCOC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(Cl)=CC(OC)=N1 NSWAMPCUPHPTTC-UHFFFAOYSA-N 0.000 description 2
- 210000003763 chloroplast Anatomy 0.000 description 2
- IVUXTESCPZUGJC-UHFFFAOYSA-N chloroxuron Chemical compound C1=CC(NC(=O)N(C)C)=CC=C1OC1=CC=C(Cl)C=C1 IVUXTESCPZUGJC-UHFFFAOYSA-N 0.000 description 2
- CWJSHJJYOPWUGX-UHFFFAOYSA-N chlorpropham Chemical compound CC(C)OC(=O)NC1=CC=CC(Cl)=C1 CWJSHJJYOPWUGX-UHFFFAOYSA-N 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 239000004927 clay Substances 0.000 description 2
- BIKACRYIQSLICJ-UHFFFAOYSA-N cloransulam-methyl Chemical group N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1C(=O)OC BIKACRYIQSLICJ-UHFFFAOYSA-N 0.000 description 2
- 239000000084 colloidal system Substances 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- YXKMMRDKEKCERS-UHFFFAOYSA-N cyazofamid Chemical compound CN(C)S(=O)(=O)N1C(C#N)=NC(Cl)=C1C1=CC=C(C)C=C1 YXKMMRDKEKCERS-UHFFFAOYSA-N 0.000 description 2
- GLWWLNJJJCTFMZ-UHFFFAOYSA-N cyclanilide Chemical compound C=1C=C(Cl)C=C(Cl)C=1NC(=O)C1(C(=O)O)CC1 GLWWLNJJJCTFMZ-UHFFFAOYSA-N 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 229960001591 cyfluthrin Drugs 0.000 description 2
- QQODLKZGRKWIFG-QSFXBCCZSA-N cyfluthrin Chemical compound CC1(C)[C@@H](C=C(Cl)Cl)[C@H]1C(=O)O[C@@H](C#N)C1=CC=C(F)C(OC=2C=CC=CC=2)=C1 QQODLKZGRKWIFG-QSFXBCCZSA-N 0.000 description 2
- KAATUXNTWXVJKI-UHFFFAOYSA-N cypermethrin Chemical compound CC1(C)C(C=C(Cl)Cl)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 KAATUXNTWXVJKI-UHFFFAOYSA-N 0.000 description 2
- 229960005424 cypermethrin Drugs 0.000 description 2
- NDYULEPTCXJCJM-UHFFFAOYSA-N dazomet, sodium salt Chemical compound [Na+].CN1CN(C)C(=S)S[CH-]1 NDYULEPTCXJCJM-UHFFFAOYSA-N 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 229960002483 decamethrin Drugs 0.000 description 2
- 238000006356 dehydrogenation reaction Methods 0.000 description 2
- OWZREIFADZCYQD-NSHGMRRFSA-N deltamethrin Chemical compound CC1(C)[C@@H](C=C(Br)Br)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 OWZREIFADZCYQD-NSHGMRRFSA-N 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- CPHCYTUHSKEDOI-UHFFFAOYSA-N diazanium;2-(phosphonatomethylamino)acetic acid Chemical compound [NH4+].[NH4+].OC(=O)CNCP([O-])([O-])=O CPHCYTUHSKEDOI-UHFFFAOYSA-N 0.000 description 2
- XNYCXKRWVHWIBE-UHFFFAOYSA-N dicamba-biproamine Chemical compound NCCCN(C)CCCN.COC1=C(Cl)C=CC(Cl)=C1C(O)=O XNYCXKRWVHWIBE-UHFFFAOYSA-N 0.000 description 2
- AWSBKDYHGOOSML-UHFFFAOYSA-N dicamba-methyl Chemical group COC(=O)C1=C(Cl)C=CC(Cl)=C1OC AWSBKDYHGOOSML-UHFFFAOYSA-N 0.000 description 2
- RVJMEWSAFHIEJX-UHFFFAOYSA-M dicamba-potassium Chemical compound [K+].COC1=C(Cl)C=CC(Cl)=C1C([O-])=O RVJMEWSAFHIEJX-UHFFFAOYSA-M 0.000 description 2
- HLZCHRAMVPCKDU-UHFFFAOYSA-M dicamba-sodium Chemical compound [Na+].COC1=C(Cl)C=CC(Cl)=C1C([O-])=O HLZCHRAMVPCKDU-UHFFFAOYSA-M 0.000 description 2
- OEBRKCOSUFCWJD-UHFFFAOYSA-N dichlorvos Chemical compound COP(=O)(OC)OC=C(Cl)Cl OEBRKCOSUFCWJD-UHFFFAOYSA-N 0.000 description 2
- LNJNFVJKDJYTEU-UHFFFAOYSA-N diethofencarb Chemical compound CCOC1=CC=C(NC(=O)OC(C)C)C=C1OCC LNJNFVJKDJYTEU-UHFFFAOYSA-N 0.000 description 2
- XXWNKVBJDWSYBN-UHFFFAOYSA-N diethoxy-phenoxy-sulfanylidene-$l^{5}-phosphane Chemical compound CCOP(=S)(OCC)OC1=CC=CC=C1 XXWNKVBJDWSYBN-UHFFFAOYSA-N 0.000 description 2
- FWCBATIDXGJRMF-UHFFFAOYSA-N dikegulac Natural products C12OC(C)(C)OCC2OC2(C(O)=O)C1OC(C)(C)O2 FWCBATIDXGJRMF-UHFFFAOYSA-N 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- JLYFCTQDENRSOL-VIFPVBQESA-N dimethenamid-P Chemical compound COC[C@H](C)N(C(=O)CCl)C=1C(C)=CSC=1C JLYFCTQDENRSOL-VIFPVBQESA-N 0.000 description 2
- 229950010286 diolamine Drugs 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- DOFZAZXDOSGAJZ-UHFFFAOYSA-N disulfoton Chemical compound CCOP(=S)(OCC)SCCSCC DOFZAZXDOSGAJZ-UHFFFAOYSA-N 0.000 description 2
- 238000010410 dusting Methods 0.000 description 2
- RDYMFSUJUZBWLH-SVWSLYAFSA-N endosulfan Chemical compound C([C@@H]12)OS(=O)OC[C@@H]1[C@]1(Cl)C(Cl)=C(Cl)[C@@]2(Cl)C1(Cl)Cl RDYMFSUJUZBWLH-SVWSLYAFSA-N 0.000 description 2
- 229960002125 enilconazole Drugs 0.000 description 2
- 230000036253 epinasty Effects 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- VJYFKVYYMZPMAB-UHFFFAOYSA-N ethoprophos Chemical compound CCCSP(=O)(OCC)SCCC VJYFKVYYMZPMAB-UHFFFAOYSA-N 0.000 description 2
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 2
- PQKBPHSEKWERTG-LLVKDONJSA-N ethyl (2r)-2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoate Chemical group C1=CC(O[C@H](C)C(=O)OCC)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 PQKBPHSEKWERTG-LLVKDONJSA-N 0.000 description 2
- KXAVVWXJUDQGDA-UHFFFAOYSA-N ethyl 2-(3,5,6-trichloropyridin-2-yl)oxyacetate Chemical group CCOC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl KXAVVWXJUDQGDA-UHFFFAOYSA-N 0.000 description 2
- MLKCGVHIFJBRCD-UHFFFAOYSA-N ethyl 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]-4-fluorophenyl}propanoate Chemical group C1=C(Cl)C(CC(Cl)C(=O)OCC)=CC(N2C(N(C(F)F)C(C)=N2)=O)=C1F MLKCGVHIFJBRCD-UHFFFAOYSA-N 0.000 description 2
- OSUHJPCHFDQAIT-UHFFFAOYSA-N ethyl 2-{4-[(6-chloroquinoxalin-2-yl)oxy]phenoxy}propanoate Chemical group C1=CC(OC(C)C(=O)OCC)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 OSUHJPCHFDQAIT-UHFFFAOYSA-N 0.000 description 2
- ZKDUJUNNBWZZCO-UHFFFAOYSA-N ethyl 5-[(2,4-dichlorophenyl)methyl]-4,5-dihydro-1,2-oxazole-3-carboxylate Chemical compound C1C(C(=O)OCC)=NOC1CC1=CC=C(Cl)C=C1Cl ZKDUJUNNBWZZCO-UHFFFAOYSA-N 0.000 description 2
- YNZGZAJXHXGDBF-UHFFFAOYSA-N ethyl 5-phenyl-4,5-dihydro-1,2-oxazole-3-carboxylate Chemical compound C1C(C(=O)OCC)=NOC1C1=CC=CC=C1 YNZGZAJXHXGDBF-UHFFFAOYSA-N 0.000 description 2
- 125000000219 ethylidene group Chemical group [H]C(=[*])C([H])([H])[H] 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 150000002190 fatty acyls Chemical group 0.000 description 2
- ZCJPOPBZHLUFHF-UHFFFAOYSA-N fenamiphos Chemical compound CCOP(=O)(NC(C)C)OC1=CC=C(SC)C(C)=C1 ZCJPOPBZHLUFHF-UHFFFAOYSA-N 0.000 description 2
- XXOYNJXVWVNOOJ-UHFFFAOYSA-N fenuron Chemical compound CN(C)C(=O)NC1=CC=CC=C1 XXOYNJXVWVNOOJ-UHFFFAOYSA-N 0.000 description 2
- RWTNPBWLLIMQHL-UHFFFAOYSA-N fexofenadine Chemical compound C1=CC(C(C)(C(O)=O)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 RWTNPBWLLIMQHL-UHFFFAOYSA-N 0.000 description 2
- 229940013764 fipronil Drugs 0.000 description 2
- WNZCDFOXYNRBRB-UHFFFAOYSA-N florpyrauxifen-benzyl Chemical group COC1=C(Cl)C=CC(C=2C(=C(N)C(Cl)=C(C(=O)OCC=3C=CC=CC=3)N=2)F)=C1F WNZCDFOXYNRBRB-UHFFFAOYSA-N 0.000 description 2
- YUVKUEAFAVKILW-SECBINFHSA-N fluazifop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 YUVKUEAFAVKILW-SECBINFHSA-N 0.000 description 2
- VAIZTNZGPYBOGF-CYBMUJFWSA-N fluazifop-P-butyl Chemical group C1=CC(O[C@H](C)C(=O)OCCCC)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 VAIZTNZGPYBOGF-CYBMUJFWSA-N 0.000 description 2
- UOUXAYAIONPXDH-UHFFFAOYSA-M flucarbazone-sodium Chemical compound [Na+].O=C1N(C)C(OC)=NN1C(=O)[N-]S(=O)(=O)C1=CC=CC=C1OC(F)(F)F UOUXAYAIONPXDH-UHFFFAOYSA-M 0.000 description 2
- MBHXIQDIVCJZTD-RVDMUPIBSA-N flufenoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC=C(C(F)(F)F)C=C1Cl MBHXIQDIVCJZTD-RVDMUPIBSA-N 0.000 description 2
- KVDJTXBXMWJJEF-UHFFFAOYSA-N fluopyram Chemical compound ClC1=CC(C(F)(F)F)=CN=C1CCNC(=O)C1=CC=CC=C1C(F)(F)F KVDJTXBXMWJJEF-UHFFFAOYSA-N 0.000 description 2
- HKIOYBQGHSTUDB-UHFFFAOYSA-N folpet Chemical compound C1=CC=C2C(=O)N(SC(Cl)(Cl)Cl)C(=O)C2=C1 HKIOYBQGHSTUDB-UHFFFAOYSA-N 0.000 description 2
- CRHGSCXKJPJNAB-UHFFFAOYSA-M fomesafen-sodium Chemical compound [Na+].C1=C([N+]([O-])=O)C(C(/[O-])=N/S(=O)(=O)C)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 CRHGSCXKJPJNAB-UHFFFAOYSA-M 0.000 description 2
- GPXLRLUVLMHHIK-UHFFFAOYSA-N forchlorfenuron Chemical compound C1=NC(Cl)=CC(NC(=O)NC=2C=CC=CC=2)=C1 GPXLRLUVLMHHIK-UHFFFAOYSA-N 0.000 description 2
- 230000005714 functional activity Effects 0.000 description 2
- 239000000417 fungicide Substances 0.000 description 2
- IXORZMNAPKEEDV-UHFFFAOYSA-N gibberellic acid GA3 Natural products OC(=O)C1C2(C3)CC(=C)C3(O)CCC2C2(C=CC3O)C1C3(C)C(=O)O2 IXORZMNAPKEEDV-UHFFFAOYSA-N 0.000 description 2
- IXORZMNAPKEEDV-OBDJNFEBSA-N gibberellin A3 Chemical compound C([C@@]1(O)C(=C)C[C@@]2(C1)[C@H]1C(O)=O)C[C@H]2[C@]2(C=C[C@@H]3O)[C@H]1[C@]3(C)C(=O)O2 IXORZMNAPKEEDV-OBDJNFEBSA-N 0.000 description 2
- 108010039239 glyphosate N-acetyltransferase Proteins 0.000 description 2
- ZEKANFGSDXODPD-UHFFFAOYSA-N glyphosate-isopropylammonium Chemical compound CC(C)N.OC(=O)CNCP(O)(O)=O ZEKANFGSDXODPD-UHFFFAOYSA-N 0.000 description 2
- 150000003278 haem Chemical class 0.000 description 2
- KDHKOPYYWOHESS-UHFFFAOYSA-N halauxifen-methyl Chemical group NC1=C(Cl)C(C(=O)OC)=NC(C=2C(=C(OC)C(Cl)=CC=2)F)=C1 KDHKOPYYWOHESS-UHFFFAOYSA-N 0.000 description 2
- GOCUAJYOYBLQRH-MRVPVSSYSA-N haloxyfop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl GOCUAJYOYBLQRH-MRVPVSSYSA-N 0.000 description 2
- MFSWTRQUCLNFOM-SECBINFHSA-N haloxyfop-P-methyl Chemical group C1=CC(O[C@H](C)C(=O)OC)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl MFSWTRQUCLNFOM-SECBINFHSA-N 0.000 description 2
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 150000002390 heteroarenes Chemical class 0.000 description 2
- 125000005114 heteroarylalkoxy group Chemical group 0.000 description 2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 2
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 2
- 208000006278 hypochromic anemia Diseases 0.000 description 2
- 229940056881 imidacloprid Drugs 0.000 description 2
- YWTYJOPNNQFBPC-UHFFFAOYSA-N imidacloprid Chemical compound [O-][N+](=O)\N=C1/NCCN1CC1=CC=C(Cl)N=C1 YWTYJOPNNQFBPC-UHFFFAOYSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- JTEDVYBZBROSJT-UHFFFAOYSA-N indole-3-butyric acid Chemical compound C1=CC=C2C(CCCC(=O)O)=CNC2=C1 JTEDVYBZBROSJT-UHFFFAOYSA-N 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- UFHLMYOGRXOCSL-UHFFFAOYSA-N isoprothiolane Chemical compound CC(C)OC(=O)C(C(=O)OC(C)C)=C1SCCS1 UFHLMYOGRXOCSL-UHFFFAOYSA-N 0.000 description 2
- ITGSCCPVERXFGN-UHFFFAOYSA-N isoxadifen Chemical compound C1C(C(=O)O)=NOC1(C=1C=CC=CC=1)C1=CC=CC=C1 ITGSCCPVERXFGN-UHFFFAOYSA-N 0.000 description 2
- 150000002545 isoxazoles Chemical class 0.000 description 2
- 229930014550 juvenile hormone Natural products 0.000 description 2
- 239000002949 juvenile hormone Substances 0.000 description 2
- 150000003633 juvenile hormone derivatives Chemical class 0.000 description 2
- PVTHJAPFENJVNC-MHRBZPPQSA-N kasugamycin Chemical compound N[C@H]1C[C@H](NC(=N)C(O)=O)[C@@H](C)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O PVTHJAPFENJVNC-MHRBZPPQSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 150000002596 lactones Chemical class 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- WMMYHMKUTCMAIJ-UHFFFAOYSA-M lithium;4-cyano-2,6-diiodophenolate Chemical compound [Li+].[O-]C1=C(I)C=C(C#N)C=C1I WMMYHMKUTCMAIJ-UHFFFAOYSA-M 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 230000036244 malformation Effects 0.000 description 2
- YKSNLCVSTHTHJA-UHFFFAOYSA-L maneb Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S YKSNLCVSTHTHJA-UHFFFAOYSA-L 0.000 description 2
- 229920000940 maneb Polymers 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- ZQEIXNIJLIKNTD-GFCCVEGCSA-N metalaxyl-M Chemical compound COCC(=O)N([C@H](C)C(=O)OC)C1=C(C)C=CC=C1C ZQEIXNIJLIKNTD-GFCCVEGCSA-N 0.000 description 2
- HYVVJDQGXFXBRZ-UHFFFAOYSA-N metam Chemical compound CNC(S)=S HYVVJDQGXFXBRZ-UHFFFAOYSA-N 0.000 description 2
- NNKVPIKMPCQWCG-UHFFFAOYSA-N methamidophos Chemical compound COP(N)(=O)SC NNKVPIKMPCQWCG-UHFFFAOYSA-N 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- CKJNUZNMWOVDFN-UHFFFAOYSA-N methanone Chemical compound O=[CH-] CKJNUZNMWOVDFN-UHFFFAOYSA-N 0.000 description 2
- YFBPRJGDJKVWAH-UHFFFAOYSA-N methiocarb Chemical compound CNC(=O)OC1=CC(C)=C(SC)C(C)=C1 YFBPRJGDJKVWAH-UHFFFAOYSA-N 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- RBNIGDFIUWJJEV-LLVKDONJSA-N methyl (2r)-2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N([C@H](C)C(=O)OC)C(=O)C1=CC=CC=C1 RBNIGDFIUWJJEV-LLVKDONJSA-N 0.000 description 2
- HRWAENGVBFKIFS-FZKQIMNGSA-N methyl (3R)-3-[[(5S)-3-(3,5-difluorophenyl)-5-ethenyl-4H-1,2-oxazole-5-carbonyl]amino]-2,3-dihydrofuran-5-carboxylate Chemical compound COC(=O)C1=C[C@H](CO1)NC(=O)[C@]2(CC(=NO2)C3=CC(=CC(=C3)F)F)C=C HRWAENGVBFKIFS-FZKQIMNGSA-N 0.000 description 2
- SCHCPDWDIOTCMJ-UHFFFAOYSA-N methyl 2-(2,4-dichlorophenoxy)propanoate Chemical group COC(=O)C(C)OC1=CC=C(Cl)C=C1Cl SCHCPDWDIOTCMJ-UHFFFAOYSA-N 0.000 description 2
- YWGAULPFWIQKRB-UHFFFAOYSA-N methyl 2-(4-chloro-2-methylphenoxy)propanoate Chemical group COC(=O)C(C)OC1=CC=C(Cl)C=C1C YWGAULPFWIQKRB-UHFFFAOYSA-N 0.000 description 2
- NIFKBBMCXCMCAO-UHFFFAOYSA-N methyl 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-4-(methanesulfonamidomethyl)benzoate Chemical group COC(=O)C1=CC=C(CNS(C)(=O)=O)C=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 NIFKBBMCXCMCAO-UHFFFAOYSA-N 0.000 description 2
- BACHBFVBHLGWSL-UHFFFAOYSA-N methyl 2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-UHFFFAOYSA-N 0.000 description 2
- LQPRNRFJJUSONA-UHFFFAOYSA-N methyl 2-benzhydryloxyacetate Chemical compound C=1C=CC=CC=1C(OCC(=O)OC)C1=CC=CC=C1 LQPRNRFJJUSONA-UHFFFAOYSA-N 0.000 description 2
- HQTUEAOWLVWJLF-UHFFFAOYSA-N methyl 3,6-dichloropyridine-2-carboxylate Chemical group COC(=O)C1=NC(Cl)=CC=C1Cl HQTUEAOWLVWJLF-UHFFFAOYSA-N 0.000 description 2
- AHGMXAFUHVRQAD-UHFFFAOYSA-N methyl 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical group C1=C([N+]([O-])=O)C(C(=O)OC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 AHGMXAFUHVRQAD-UHFFFAOYSA-N 0.000 description 2
- CJPQIRJHIZUAQP-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-(phenylacetyl)alaninate Chemical compound CC=1C=CC=C(C)C=1N(C(C)C(=O)OC)C(=O)CC1=CC=CC=C1 CJPQIRJHIZUAQP-UHFFFAOYSA-N 0.000 description 2
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 2
- 229960002939 metizoline Drugs 0.000 description 2
- VOEYXMAFNDNNED-UHFFFAOYSA-N metolcarb Chemical compound CNC(=O)OC1=CC=CC(C)=C1 VOEYXMAFNDNNED-UHFFFAOYSA-N 0.000 description 2
- RSMUVYRMZCOLBH-UHFFFAOYSA-N metsulfuron methyl Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(C)=NC(OC)=N1 RSMUVYRMZCOLBH-UHFFFAOYSA-N 0.000 description 2
- ZLBGSRMUSVULIE-GSMJGMFJSA-N milbemycin A3 Chemical class O1[C@H](C)[C@@H](C)CC[C@@]11O[C@H](C\C=C(C)\C[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 ZLBGSRMUSVULIE-GSMJGMFJSA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- DEDOPGXGGQYYMW-UHFFFAOYSA-N molinate Chemical compound CCSC(=O)N1CCCCCC1 DEDOPGXGGQYYMW-UHFFFAOYSA-N 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- QBHMPGXYSYRJEW-UHFFFAOYSA-N n-[(2,4-dimethyl-6-nitrophenoxy)-ethoxyphosphinothioyl]propan-2-amine Chemical compound CCOP(=S)(NC(C)C)OC1=C(C)C=C(C)C=C1[N+]([O-])=O QBHMPGXYSYRJEW-UHFFFAOYSA-N 0.000 description 2
- UWKQSUQMFIRFMM-UHFFFAOYSA-N n-[2-(tert-butylcarbamoyl)-4-chloro-6-methylphenyl]-2-(3-chloropyridin-2-yl)-5-(fluoromethoxy)pyrazole-3-carboxamide Chemical compound CC1=CC(Cl)=CC(C(=O)NC(C)(C)C)=C1NC(=O)C1=CC(OCF)=NN1C1=NC=CC=C1Cl UWKQSUQMFIRFMM-UHFFFAOYSA-N 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- VDCHTAFVUAPYGO-UHFFFAOYSA-N n-methylmethanamine;2-(phosphonomethylamino)acetic acid Chemical compound CNC.OC(=O)CNCP(O)(O)=O VDCHTAFVUAPYGO-UHFFFAOYSA-N 0.000 description 2
- 230000017074 necrotic cell death Effects 0.000 description 2
- 230000006780 non-homologous end joining Effects 0.000 description 2
- OLZQTUCTGLHFTQ-UHFFFAOYSA-N octan-2-yl 2-(4-amino-3,5-dichloro-6-fluoropyridin-2-yl)oxyacetate Chemical group CCCCCCC(C)OC(=O)COC1=NC(F)=C(Cl)C(N)=C1Cl OLZQTUCTGLHFTQ-UHFFFAOYSA-N 0.000 description 2
- 229950004864 olamine Drugs 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- PMCVMORKVPSKHZ-UHFFFAOYSA-N oxydemeton-methyl Chemical compound CCS(=O)CCSP(=O)(OC)OC PMCVMORKVPSKHZ-UHFFFAOYSA-N 0.000 description 2
- OGYFATSSENRIKG-UHFFFAOYSA-N pencycuron Chemical compound C1=CC(Cl)=CC=C1CN(C(=O)NC=1C=CC=CC=1)C1CCCC1 OGYFATSSENRIKG-UHFFFAOYSA-N 0.000 description 2
- JGCSKOVQDXEQHI-UHFFFAOYSA-N phenazine-1-carboxylic acid Chemical compound C1=CC=C2N=C3C(C(=O)O)=CC=CC3=NC2=C1 JGCSKOVQDXEQHI-UHFFFAOYSA-N 0.000 description 2
- 229950009215 phenylbutanoic acid Drugs 0.000 description 2
- 150000008048 phenylpyrazoles Chemical class 0.000 description 2
- BULVZWIRKLYCBC-UHFFFAOYSA-N phorate Chemical compound CCOP(=S)(OCC)SCSCC BULVZWIRKLYCBC-UHFFFAOYSA-N 0.000 description 2
- RJQUHEYNLDNJLN-UHFFFAOYSA-N picloram-methyl Chemical group COC(=O)C1=NC(Cl)=C(Cl)C(N)=C1Cl RJQUHEYNLDNJLN-UHFFFAOYSA-N 0.000 description 2
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 2
- 125000003367 polycyclic group Chemical group 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- HKSBGIRAPYUOPP-UHFFFAOYSA-M potassium;2,6-dibromo-4-cyanophenolate Chemical compound [K+].[O-]C1=C(Br)C=C(C#N)C=C1Br HKSBGIRAPYUOPP-UHFFFAOYSA-M 0.000 description 2
- SIVJKMBJGCUUNS-UHFFFAOYSA-M potassium;2-(2,4-dichlorophenoxy)propanoate Chemical compound [K+].[O-]C(=O)C(C)OC1=CC=C(Cl)C=C1Cl SIVJKMBJGCUUNS-UHFFFAOYSA-M 0.000 description 2
- OFCQYQOZASISIU-UHFFFAOYSA-M potassium;2-(4-chloro-2-methylphenoxy)propanoate Chemical compound [K+].[O-]C(=O)C(C)OC1=CC=C(Cl)C=C1C OFCQYQOZASISIU-UHFFFAOYSA-M 0.000 description 2
- LIOPHZNMBKHGAV-UHFFFAOYSA-M potassium;2-(phosphonomethylamino)acetate Chemical compound [K+].OC(=O)CNCP(O)([O-])=O LIOPHZNMBKHGAV-UHFFFAOYSA-M 0.000 description 2
- RLQCYSVYYHHLIL-UHFFFAOYSA-M potassium;3,6-dichloropyridine-2-carboxylate Chemical compound [K+].[O-]C(=O)C1=NC(Cl)=CC=C1Cl RLQCYSVYYHHLIL-UHFFFAOYSA-M 0.000 description 2
- ZRHANBBTXQZFSP-UHFFFAOYSA-M potassium;4-amino-3,5,6-trichloropyridine-2-carboxylate Chemical compound [K+].NC1=C(Cl)C(Cl)=NC(C([O-])=O)=C1Cl ZRHANBBTXQZFSP-UHFFFAOYSA-M 0.000 description 2
- WKJOGZIRDGAEFO-UHFFFAOYSA-N potassium;methyl n-(4-aminophenyl)sulfonylcarbamate Chemical compound [K+].COC(=O)NS(=O)(=O)C1=CC=C(N)C=C1 WKJOGZIRDGAEFO-UHFFFAOYSA-N 0.000 description 2
- TVLSRXXIMLFWEO-UHFFFAOYSA-N prochloraz Chemical compound C1=CN=CN1C(=O)N(CCC)CCOC1=C(Cl)C=C(Cl)C=C1Cl TVLSRXXIMLFWEO-UHFFFAOYSA-N 0.000 description 2
- BUCOQPHDYUOJSI-UHFFFAOYSA-N prohexadione Chemical compound CCC(=O)C1C(=O)CC(C(O)=O)CC1=O BUCOQPHDYUOJSI-UHFFFAOYSA-N 0.000 description 2
- WZZLDXDUQPOXNW-UHFFFAOYSA-N propamocarb Chemical compound CCCOC(=O)NCCCN(C)C WZZLDXDUQPOXNW-UHFFFAOYSA-N 0.000 description 2
- IKVXBIIHQGXQRQ-CYBMUJFWSA-N propan-2-yl (2r)-2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N([C@H](C)C(=O)OC(C)C)C(=O)C1=CC=CC=C1 IKVXBIIHQGXQRQ-CYBMUJFWSA-N 0.000 description 2
- OYJMHAFVOZPIOY-UHFFFAOYSA-N propan-2-yl 2-chloro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]benzoate Chemical compound C1=C(Cl)C(C(=O)OC(C)C)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1 OYJMHAFVOZPIOY-UHFFFAOYSA-N 0.000 description 2
- KKMLIVYBGSAJPM-UHFFFAOYSA-L propineb Chemical compound [Zn+2].[S-]C(=S)NC(C)CNC([S-])=S KKMLIVYBGSAJPM-UHFFFAOYSA-L 0.000 description 2
- DVBARFVOVUTLAH-UHFFFAOYSA-N propyl 5,5-diphenyl-4h-1,2-oxazole-3-carboxylate Chemical compound C1C(C(=O)OCCC)=NOC1(C=1C=CC=CC=1)C1=CC=CC=C1 DVBARFVOVUTLAH-UHFFFAOYSA-N 0.000 description 2
- FITIWKDOCAUBQD-UHFFFAOYSA-N prothiofos Chemical compound CCCSP(=S)(OCC)OC1=CC=C(Cl)C=C1Cl FITIWKDOCAUBQD-UHFFFAOYSA-N 0.000 description 2
- KIDHWZJUCRJVML-UHFFFAOYSA-N putrescine Chemical compound NCCCCN KIDHWZJUCRJVML-UHFFFAOYSA-N 0.000 description 2
- 150000003217 pyrazoles Chemical class 0.000 description 2
- MIOBBYRMXGNORL-UHFFFAOYSA-N pyrifluquinazon Chemical compound C1C2=CC(C(F)(C(F)(F)F)C(F)(F)F)=CC=C2N(C(=O)C)C(=O)N1NCC1=CC=CN=C1 MIOBBYRMXGNORL-UHFFFAOYSA-N 0.000 description 2
- ZLIBICFPKPWGIZ-UHFFFAOYSA-N pyrimethanil Chemical compound CC1=CC(C)=NC(NC=2C=CC=CC=2)=N1 ZLIBICFPKPWGIZ-UHFFFAOYSA-N 0.000 description 2
- USSIUIGPBLPCDF-KEBDBYFISA-N pyriminobac-methyl Chemical group CO\N=C(/C)C1=CC=CC(OC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OC USSIUIGPBLPCDF-KEBDBYFISA-N 0.000 description 2
- PJPDUXCVLFAQBM-UHFFFAOYSA-N quinclorac-dimethylammonium Chemical compound CNC.ClC1=CN=C2C(C(=O)O)=C(Cl)C=CC2=C1 PJPDUXCVLFAQBM-UHFFFAOYSA-N 0.000 description 2
- LNKVWJBNESETCJ-UHFFFAOYSA-N quinclorac-methyl Chemical group ClC1=CN=C2C(C(=O)OC)=C(Cl)C=CC2=C1 LNKVWJBNESETCJ-UHFFFAOYSA-N 0.000 description 2
- FBQQHUGEACOBDN-UHFFFAOYSA-N quinomethionate Chemical compound N1=C2SC(=O)SC2=NC2=CC(C)=CC=C21 FBQQHUGEACOBDN-UHFFFAOYSA-N 0.000 description 2
- OSUHJPCHFDQAIT-GFCCVEGCSA-N quizalofop-P-ethyl Chemical group C1=CC(O[C@H](C)C(=O)OCC)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 OSUHJPCHFDQAIT-GFCCVEGCSA-N 0.000 description 2
- BBKDWPHJZANJGB-IKJXHCRLSA-N quizalofop-P-tefuryl Chemical group O=C([C@H](OC=1C=CC(OC=2N=C3C=CC(Cl)=CC3=NC=2)=CC=1)C)OCC1CCCO1 BBKDWPHJZANJGB-IKJXHCRLSA-N 0.000 description 2
- 239000000018 receptor agonist Substances 0.000 description 2
- 229940044601 receptor agonist Drugs 0.000 description 2
- 230000035806 respiratory chain Effects 0.000 description 2
- JUVIOZPCNVVQFO-UHFFFAOYSA-N rotenone Natural products O1C2=C3CC(C(C)=C)OC3=CC=C2C(=O)C2C1COC1=C2C=C(OC)C(OC)=C1 JUVIOZPCNVVQFO-UHFFFAOYSA-N 0.000 description 2
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- HPYNBECUCCGGPA-UHFFFAOYSA-N silafluofen Chemical compound C1=CC(OCC)=CC=C1[Si](C)(C)CCCC1=CC=C(F)C(OC=2C=CC=CC=2)=C1 HPYNBECUCCGGPA-UHFFFAOYSA-N 0.000 description 2
- RTWIRLHWLMNVCC-WQYNNSOESA-M sodium (2S)-2-[[(2S)-2-[[(2S)-2-amino-4-[hydroxy(methyl)phosphoryl]butanoyl]amino]propanoyl]amino]propanoate Chemical compound [Na+].[O-]C(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCP(C)(O)=O RTWIRLHWLMNVCC-WQYNNSOESA-M 0.000 description 2
- DHJHUXNTNSRSEX-UHFFFAOYSA-M sodium;2-(4-chloro-2-methylphenoxy)propanoate Chemical compound [Na+].[O-]C(=O)C(C)OC1=CC=C(Cl)C=C1C DHJHUXNTNSRSEX-UHFFFAOYSA-M 0.000 description 2
- JUNDBKFAZXZKRA-MAFYXNADSA-M sodium;2-[(e)-n-[(3,5-difluorophenyl)carbamoylamino]-c-methylcarbonimidoyl]pyridine-3-carboxylate Chemical compound [Na+].N=1C=CC=C(C([O-])=O)C=1C(/C)=N/NC(=O)NC1=CC(F)=CC(F)=C1 JUNDBKFAZXZKRA-MAFYXNADSA-M 0.000 description 2
- WMWBBXKLYSGKDY-UHFFFAOYSA-M sodium;3-amino-2,5-dichlorobenzoate Chemical compound [Na+].NC1=CC(Cl)=CC(C([O-])=O)=C1Cl WMWBBXKLYSGKDY-UHFFFAOYSA-M 0.000 description 2
- JRQGDDUXDKCWRF-UHFFFAOYSA-M sodium;n-(2-methoxycarbonylphenyl)sulfonyl-4-methyl-5-oxo-3-propoxy-1,2,4-triazole-1-carboximidate Chemical compound [Na+].O=C1N(C)C(OCCC)=NN1C(=O)[N-]S(=O)(=O)C1=CC=CC=C1C(=O)OC JRQGDDUXDKCWRF-UHFFFAOYSA-M 0.000 description 2
- 239000002689 soil Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 description 2
- PFNFFQXMRSDOHW-UHFFFAOYSA-N spermine Chemical compound NCCCNCCCCNCCCN PFNFFQXMRSDOHW-UHFFFAOYSA-N 0.000 description 2
- WOPFPAIGRGHWAQ-UHFFFAOYSA-N spiropidion Chemical compound CCOC(=O)OC1=C(C=2C(=CC(Cl)=CC=2C)C)C(=O)N(C)C11CCN(OC)CC1 WOPFPAIGRGHWAQ-UHFFFAOYSA-N 0.000 description 2
- PUYXTUJWRLOUCW-UHFFFAOYSA-N spiroxamine Chemical compound O1C(CN(CC)CCC)COC11CCC(C(C)(C)C)CC1 PUYXTUJWRLOUCW-UHFFFAOYSA-N 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 2
- ZDXMLEQEMNLCQG-UHFFFAOYSA-N sulfometuron methyl Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(C)=CC(C)=N1 ZDXMLEQEMNLCQG-UHFFFAOYSA-N 0.000 description 2
- 150000003460 sulfonic acids Chemical class 0.000 description 2
- RWSOTUBLDIXVET-UHFFFAOYSA-O sulfonium Chemical compound [SH3+] RWSOTUBLDIXVET-UHFFFAOYSA-O 0.000 description 2
- JMSVCTWVEWCHDZ-UHFFFAOYSA-N syringic acid Chemical compound COC1=CC(C(O)=O)=CC(OC)=C1O JMSVCTWVEWCHDZ-UHFFFAOYSA-N 0.000 description 2
- RJKCKKDSSSRYCB-UHFFFAOYSA-N tebutam Chemical compound CC(C)(C)C(=O)N(C(C)C)CC1=CC=CC=C1 RJKCKKDSSSRYCB-UHFFFAOYSA-N 0.000 description 2
- XQTLDIFVVHJORV-UHFFFAOYSA-N tecnazene Chemical compound [O-][N+](=O)C1=C(Cl)C(Cl)=CC(Cl)=C1Cl XQTLDIFVVHJORV-UHFFFAOYSA-N 0.000 description 2
- 229960005199 tetramethrin Drugs 0.000 description 2
- XSKZXGDFSCCXQX-UHFFFAOYSA-N thiencarbazone-methyl Chemical compound COC(=O)C1=CSC(C)=C1S(=O)(=O)NC(=O)N1C(=O)N(C)C(OC)=N1 XSKZXGDFSCCXQX-UHFFFAOYSA-N 0.000 description 2
- BAKXBZPQTXCKRR-UHFFFAOYSA-N thiodicarb Chemical compound CSC(C)=NOC(=O)NSNC(=O)ON=C(C)SC BAKXBZPQTXCKRR-UHFFFAOYSA-N 0.000 description 2
- 125000003396 thiol group Chemical group [H]S* 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- YWSCPYYRJXKUDB-KAKFPZCNSA-N tralomethrin Chemical compound CC1(C)[C@@H](C(Br)C(Br)(Br)Br)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 YWSCPYYRJXKUDB-KAKFPZCNSA-N 0.000 description 2
- 238000011426 transformation method Methods 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- BAZVSMNPJJMILC-UHFFFAOYSA-N triadimenol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC1=CC=C(Cl)C=C1 BAZVSMNPJJMILC-UHFFFAOYSA-N 0.000 description 2
- AMFGTOFWMRQMEM-UHFFFAOYSA-N triazophos Chemical compound N1=C(OP(=S)(OCC)OCC)N=CN1C1=CC=CC=C1 AMFGTOFWMRQMEM-UHFFFAOYSA-N 0.000 description 2
- NFACJZMKEDPNKN-UHFFFAOYSA-N trichlorfon Chemical compound COP(=O)(OC)C(O)C(Cl)(Cl)Cl NFACJZMKEDPNKN-UHFFFAOYSA-N 0.000 description 2
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 2
- ONCZDRURRATYFI-TVJDWZFNSA-N trifloxystrobin Chemical compound CO\N=C(\C(=O)OC)C1=CC=CC=C1CO\N=C(/C)C1=CC=CC(C(F)(F)F)=C1 ONCZDRURRATYFI-TVJDWZFNSA-N 0.000 description 2
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 2
- AKTQJCBOGPBERP-UHFFFAOYSA-N triflusulfuron Chemical compound FC(F)(F)COC1=NC(N(C)C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2C)C(O)=O)=N1 AKTQJCBOGPBERP-UHFFFAOYSA-N 0.000 description 2
- IMEVJVISCHQJRM-UHFFFAOYSA-N triflusulfuron-methyl Chemical group COC(=O)C1=CC=CC(C)=C1S(=O)(=O)NC(=O)NC1=NC(OCC(F)(F)F)=NC(N(C)C)=N1 IMEVJVISCHQJRM-UHFFFAOYSA-N 0.000 description 2
- DFFWZNDCNBOKDI-UHFFFAOYSA-N trinexapac Chemical compound O=C1CC(C(=O)O)CC(=O)C1=C(O)C1CC1 DFFWZNDCNBOKDI-UHFFFAOYSA-N 0.000 description 2
- RVKCCVTVZORVGD-UHFFFAOYSA-N trinexapac-ethyl Chemical group O=C1CC(C(=O)OCC)CC(=O)C1=C(O)C1CC1 RVKCCVTVZORVGD-UHFFFAOYSA-N 0.000 description 2
- ICESBGJHRQHALQ-UHFFFAOYSA-K trisodium;(carboxymethylamino)methyl-hydroxyphosphinate;2-[[hydroxy(oxido)phosphoryl]methylamino]acetate Chemical compound [Na+].[Na+].[Na+].OC(=O)CNCP(O)([O-])=O.OC(=O)CNCP([O-])([O-])=O ICESBGJHRQHALQ-UHFFFAOYSA-K 0.000 description 2
- DBHVHTPMRCXCIY-UHFFFAOYSA-N tyclopyrazoflor Chemical compound N1=C(Cl)C(N(C(=O)CCSCCC(F)(F)F)CC)=CN1C1=CC=CN=C1 DBHVHTPMRCXCIY-UHFFFAOYSA-N 0.000 description 2
- YNWVFADWVLCOPU-MAUPQMMJSA-N uniconazole P Chemical compound C1=NC=NN1/C([C@@H](O)C(C)(C)C)=C/C1=CC=C(Cl)C=C1 YNWVFADWVLCOPU-MAUPQMMJSA-N 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 239000004562 water dispersible granule Substances 0.000 description 2
- PFTAWBLQPZVEMU-DZGCQCFKSA-N (+)-catechin Chemical compound C1([C@H]2OC3=CC(O)=CC(O)=C3C[C@@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-DZGCQCFKSA-N 0.000 description 1
- ZCVAOQKBXKSDMS-AQYZNVCMSA-N (+)-trans-allethrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OC1C(C)=C(CC=C)C(=O)C1 ZCVAOQKBXKSDMS-AQYZNVCMSA-N 0.000 description 1
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 1
- PFTAWBLQPZVEMU-HIFRSBDPSA-N (-)-catechin Chemical compound C1([C@@H]2OC3=CC(O)=CC(O)=C3C[C@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-HIFRSBDPSA-N 0.000 description 1
- SJWOCKTZFQIKHZ-UHFFFAOYSA-N (1-ethoxy-1-oxopropan-2-yl) 3,6-dichloro-2-methoxybenzoate Chemical compound CCOC(=O)C(C)OC(=O)C1=C(Cl)C=CC(Cl)=C1OC SJWOCKTZFQIKHZ-UHFFFAOYSA-N 0.000 description 1
- ZXCBTCBNCGLDCD-UHFFFAOYSA-N (1-ethoxy-3-methyl-1-oxobut-3-en-2-yl) 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC(C(=O)OCC)C(C)=C)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 ZXCBTCBNCGLDCD-UHFFFAOYSA-N 0.000 description 1
- KAATUXNTWXVJKI-GGPKGHCWSA-N (1R)-trans-(alphaS)-cypermethrin Chemical compound CC1(C)[C@H](C=C(Cl)Cl)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 KAATUXNTWXVJKI-GGPKGHCWSA-N 0.000 description 1
- FJDPATXIBIBRIM-QFMSAKRMSA-N (1R)-trans-cyphenothrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 FJDPATXIBIBRIM-QFMSAKRMSA-N 0.000 description 1
- SBNFWQZLDJGRLK-RTWAWAEBSA-N (1R)-trans-phenothrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OCC1=CC=CC(OC=2C=CC=CC=2)=C1 SBNFWQZLDJGRLK-RTWAWAEBSA-N 0.000 description 1
- ZXQYGBMAQZUVMI-RDDWSQKMSA-N (1S)-cis-(alphaR)-cyhalothrin Chemical compound CC1(C)[C@H](\C=C(/Cl)C(F)(F)F)[C@@H]1C(=O)O[C@@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-RDDWSQKMSA-N 0.000 description 1
- XERJKGMBORTKEO-VZUCSPMQSA-N (1e)-2-(ethylcarbamoylamino)-n-methoxy-2-oxoethanimidoyl cyanide Chemical compound CCNC(=O)NC(=O)C(\C#N)=N\OC XERJKGMBORTKEO-VZUCSPMQSA-N 0.000 description 1
- SPXBEYPYQKZKGX-PVAVHDDUSA-N (1s,2r,5r)-2-(chloromethyl)-5-[(4-chlorophenyl)methyl]-2-methyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol Chemical compound C([C@H]1CC[C@]([C@]1(O)CN1N=CN=C1)(CCl)C)C1=CC=C(Cl)C=C1 SPXBEYPYQKZKGX-PVAVHDDUSA-N 0.000 description 1
- HTBQOINACZOEPC-UHFFFAOYSA-N (2,4-dichlorophenyl)-(1-methyl-5-phenylmethoxypyrazol-4-yl)methanone Chemical compound C=1C=CC=CC=1COC=1N(C)N=CC=1C(=O)C1=CC=C(Cl)C=C1Cl HTBQOINACZOEPC-UHFFFAOYSA-N 0.000 description 1
- FWYMRCLEYOTUEU-UHFFFAOYSA-N (2-hexoxy-2-oxoethyl) 2-aminooxy-3-methylbut-2-enoate Chemical compound C(CCCCC)OC(COC(C(ON)=C(C)C)=O)=O FWYMRCLEYOTUEU-UHFFFAOYSA-N 0.000 description 1
- RPGLJGQKRRFSFG-UHFFFAOYSA-N (2-methoxy-2-oxoethyl) 2-aminooxy-3-methylbut-2-enoate Chemical compound COC(=O)COC(=O)C(ON)=C(C)C RPGLJGQKRRFSFG-UHFFFAOYSA-N 0.000 description 1
- BMJYKXALMDAIEG-UHFFFAOYSA-N (2-tert-butyl-4,6-dinitrophenyl) acetate Chemical compound CC(=O)OC1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1C(C)(C)C BMJYKXALMDAIEG-UHFFFAOYSA-N 0.000 description 1
- ZMYFCFLJBGAQRS-IRXDYDNUSA-N (2R,3S)-epoxiconazole Chemical compound C1=CC(F)=CC=C1[C@@]1(CN2N=CN=C2)[C@H](C=2C(=CC=CC=2)Cl)O1 ZMYFCFLJBGAQRS-IRXDYDNUSA-N 0.000 description 1
- HPMOLIHDZUCPIZ-KOLCDFICSA-N (2S)-2-(1-chlorocyclopropyl)-4-[(1R)-2,2-dichlorocyclopropyl]-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound O[C@@](CC[C@@H]1CC1(Cl)Cl)(Cn1cncn1)C1(Cl)CC1 HPMOLIHDZUCPIZ-KOLCDFICSA-N 0.000 description 1
- HPMOLIHDZUCPIZ-ONGXEEELSA-N (2S)-2-(1-chlorocyclopropyl)-4-[(1S)-2,2-dichlorocyclopropyl]-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound O[C@@](CC[C@H]1CC1(Cl)Cl)(Cn1cncn1)C1(Cl)CC1 HPMOLIHDZUCPIZ-ONGXEEELSA-N 0.000 description 1
- WRXSEWUFHVTFEX-NUBCRITNSA-N (2r)-2-(2,4-dichlorophenoxy)propanoic acid;n-methylmethanamine Chemical compound CNC.OC(=O)[C@@H](C)OC1=CC=C(Cl)C=C1Cl WRXSEWUFHVTFEX-NUBCRITNSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- MUNXLKSMKBJOIQ-VEQVDCDKSA-N (2z,4e)-5-(6-ethynyl-1-hydroxy-2,6-dimethyl-4-oxocyclohex-2-en-1-yl)-3-methylpenta-2,4-dienoic acid Chemical compound OC(=O)/C=C(/C)\C=C\C1(O)C(C)=CC(=O)CC1(C)C#C MUNXLKSMKBJOIQ-VEQVDCDKSA-N 0.000 description 1
- LDVVMCZRFWMZSG-OLQVQODUSA-N (3ar,7as)-2-(trichloromethylsulfanyl)-3a,4,7,7a-tetrahydroisoindole-1,3-dione Chemical compound C1C=CC[C@H]2C(=O)N(SC(Cl)(Cl)Cl)C(=O)[C@H]21 LDVVMCZRFWMZSG-OLQVQODUSA-N 0.000 description 1
- KAEYISLSIRRSJI-YRNVUSSQSA-N (3e)-3-[1-[(6-chloropyridin-3-yl)methyl]pyridin-2-ylidene]-1,1,1-trifluoropropan-2-one Chemical compound FC(F)(F)C(=O)\C=C1/C=CC=CN1CC1=CC=C(Cl)N=C1 KAEYISLSIRRSJI-YRNVUSSQSA-N 0.000 description 1
- NJSUAAJHYPEVBZ-SHUUEZRQSA-N (3s,4r,5r,6r)-1,3,4,5,6-pentahydroxyheptan-2-one Chemical compound C[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(=O)CO NJSUAAJHYPEVBZ-SHUUEZRQSA-N 0.000 description 1
- SODPIMGUZLOIPE-UHFFFAOYSA-N (4-chlorophenoxy)acetic acid Chemical compound OC(=O)COC1=CC=C(Cl)C=C1 SODPIMGUZLOIPE-UHFFFAOYSA-N 0.000 description 1
- XUNYDVLIZWUPAW-UHFFFAOYSA-N (4-chlorophenyl) n-(4-methylphenyl)sulfonylcarbamate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)NC(=O)OC1=CC=C(Cl)C=C1 XUNYDVLIZWUPAW-UHFFFAOYSA-N 0.000 description 1
- WLSQDEYDCAGPIR-GCJKJVERSA-N (4R,5S)-isocycloseram Chemical compound ClC=1C=C(C=C(C=1F)Cl)[C@@]1(CC(=NO1)C1=CC(=C(C(=O)N[C@H]2C(N(OC2)CC)=O)C=C1)C)C(F)(F)F WLSQDEYDCAGPIR-GCJKJVERSA-N 0.000 description 1
- PPDBOQMNKNNODG-NTEUORMPSA-N (5E)-5-(4-chlorobenzylidene)-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentanol Chemical compound C1=NC=NN1CC1(O)C(C)(C)CC\C1=C/C1=CC=C(Cl)C=C1 PPDBOQMNKNNODG-NTEUORMPSA-N 0.000 description 1
- RFZZKBWDDKMWNM-GTBMBKLPSA-N (5s,7r,8s,9r)-8,9-dihydroxy-7-(hydroxymethyl)-6-oxa-1,3-diazaspiro[4.4]nonane-2,4-dione Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@]11C(=O)NC(=O)N1 RFZZKBWDDKMWNM-GTBMBKLPSA-N 0.000 description 1
- 125000006274 (C1-C3)alkoxy group Chemical group 0.000 description 1
- 125000004768 (C1-C4) alkylsulfinyl group Chemical group 0.000 description 1
- 125000004738 (C1-C6) alkyl sulfinyl group Chemical group 0.000 description 1
- 125000004739 (C1-C6) alkylsulfonyl group Chemical group 0.000 description 1
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 description 1
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 description 1
- 125000006652 (C3-C12) cycloalkyl group Chemical group 0.000 description 1
- PGOOBECODWQEAB-UHFFFAOYSA-N (E)-clothianidin Chemical compound [O-][N+](=O)\N=C(/NC)NCC1=CN=C(Cl)S1 PGOOBECODWQEAB-UHFFFAOYSA-N 0.000 description 1
- BKBSMMUEEAWFRX-NBVRZTHBSA-N (E)-flumorph Chemical compound C1=C(OC)C(OC)=CC=C1C(\C=1C=CC(F)=CC=1)=C\C(=O)N1CCOCC1 BKBSMMUEEAWFRX-NBVRZTHBSA-N 0.000 description 1
- CFRPSFYHXJZSBI-DHZHZOJOSA-N (E)-nitenpyram Chemical compound [O-][N+](=O)/C=C(\NC)N(CC)CC1=CC=C(Cl)N=C1 CFRPSFYHXJZSBI-DHZHZOJOSA-N 0.000 description 1
- SDVVLIIVFBKBMG-ONEGZZNKSA-N (E)-penta-2,4-dienoic acid Chemical compound OC(=O)\C=C\C=C SDVVLIIVFBKBMG-ONEGZZNKSA-N 0.000 description 1
- FZRBKIRIBLNOAM-UHFFFAOYSA-N (E,E)-2-propynyl 3,7,11-trimethyl-2,4-dodecadienoate Chemical compound CC(C)CCCC(C)CC=CC(C)=CC(=O)OCC#C FZRBKIRIBLNOAM-UHFFFAOYSA-N 0.000 description 1
- IQVNEKKDSLOHHK-FNCQTZNRSA-N (E,E)-hydramethylnon Chemical compound N1CC(C)(C)CNC1=NN=C(/C=C/C=1C=CC(=CC=1)C(F)(F)F)\C=C\C1=CC=C(C(F)(F)F)C=C1 IQVNEKKDSLOHHK-FNCQTZNRSA-N 0.000 description 1
- MZHCENGPTKEIGP-RXMQYKEDSA-N (R)-dichlorprop Chemical compound OC(=O)[C@@H](C)OC1=CC=C(Cl)C=C1Cl MZHCENGPTKEIGP-RXMQYKEDSA-N 0.000 description 1
- BACHBFVBHLGWSL-SNVBAGLBSA-N (R)-diclofop-methyl Chemical group C1=CC(O[C@H](C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-SNVBAGLBSA-N 0.000 description 1
- ZEXXEODAXHSRDJ-HXUWFJFHSA-N (R)-fluoxapiprolin Chemical compound CS(=O)(=O)Oc1cccc(Cl)c1[C@H]1CC(=NO1)c1csc(n1)C1CCN(CC1)C(=O)Cn1nc(cc1C(F)F)C(F)F ZEXXEODAXHSRDJ-HXUWFJFHSA-N 0.000 description 1
- PDPWCKVFIFAQIQ-GOSISDBHSA-N (R)-mandestrobin Chemical compound CNC(=O)[C@H](OC)C1=CC=CC=C1COC1=CC(C)=CC=C1C PDPWCKVFIFAQIQ-GOSISDBHSA-N 0.000 description 1
- XCGBHLLWJZOLEM-VIFPVBQESA-N (S)-fluindapyr Chemical compound C([C@@H]1C)C(C)(C)C(C(=CC=2)F)=C1C=2NC(=O)C1=CN(C)N=C1C(F)F XCGBHLLWJZOLEM-VIFPVBQESA-N 0.000 description 1
- PDPWCKVFIFAQIQ-SFHVURJKSA-N (S)-mandestrobin Chemical compound CNC(=O)[C@@H](OC)C1=CC=CC=C1COC1=CC(C)=CC=C1C PDPWCKVFIFAQIQ-SFHVURJKSA-N 0.000 description 1
- JERZEQUMJNCPRJ-QGZVFWFLSA-N (S)-mefentrifluconazole Chemical compound C([C@](O)(C)C=1C(=CC(OC=2C=CC(Cl)=CC=2)=CC=1)C(F)(F)F)N1C=NC=N1 JERZEQUMJNCPRJ-QGZVFWFLSA-N 0.000 description 1
- XGWIJUOSCAQSSV-XHDPSFHLSA-N (S,S)-hexythiazox Chemical compound S([C@H]([C@@H]1C)C=2C=CC(Cl)=CC=2)C(=O)N1C(=O)NC1CCCCC1 XGWIJUOSCAQSSV-XHDPSFHLSA-N 0.000 description 1
- RMOGWMIKYWRTKW-UONOGXRCSA-N (S,S)-paclobutrazol Chemical compound C([C@@H]([C@@H](O)C(C)(C)C)N1N=CN=C1)C1=CC=C(Cl)C=C1 RMOGWMIKYWRTKW-UONOGXRCSA-N 0.000 description 1
- QNBTYORWCCMPQP-JXAWBTAJSA-N (Z)-dimethomorph Chemical compound C1=C(OC)C(OC)=CC=C1C(\C=1C=CC(Cl)=CC=1)=C/C(=O)N1CCOCC1 QNBTYORWCCMPQP-JXAWBTAJSA-N 0.000 description 1
- PCKNFPQPGUWFHO-SXBRIOAWSA-N (Z)-flucycloxuron Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC(C=C1)=CC=C1CO\N=C(C=1C=CC(Cl)=CC=1)\C1CC1 PCKNFPQPGUWFHO-SXBRIOAWSA-N 0.000 description 1
- NQRKYASMKDDGHT-UHFFFAOYSA-N (aminooxy)acetic acid Chemical compound NOCC(O)=O NQRKYASMKDDGHT-UHFFFAOYSA-N 0.000 description 1
- QEUOHPLVFSQWME-XDJHFCHBSA-N (e)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4-yl)-1-morpholin-4-ylprop-2-en-1-one Chemical compound C1=CC(C(C)(C)C)=CC=C1C(\C=1C=C(Cl)N=CC=1)=C/C(=O)N1CCOCC1 QEUOHPLVFSQWME-XDJHFCHBSA-N 0.000 description 1
- QEUOHPLVFSQWME-CYVLTUHYSA-N (z)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4-yl)-1-morpholin-4-ylprop-2-en-1-one Chemical compound C1=CC(C(C)(C)C)=CC=C1C(\C=1C=C(Cl)N=CC=1)=C\C(=O)N1CCOCC1 QEUOHPLVFSQWME-CYVLTUHYSA-N 0.000 description 1
- 125000006112 1, 1-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- IAKOZHOLGAGEJT-UHFFFAOYSA-N 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)-Ethane Chemical compound C1=CC(OC)=CC=C1C(C(Cl)(Cl)Cl)C1=CC=C(OC)C=C1 IAKOZHOLGAGEJT-UHFFFAOYSA-N 0.000 description 1
- FIARMZDBEGVMLV-UHFFFAOYSA-N 1,1,2,2,2-pentafluoroethanolate Chemical group [O-]C(F)(F)C(F)(F)F FIARMZDBEGVMLV-UHFFFAOYSA-N 0.000 description 1
- 125000006120 1,1,2-trimethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006150 1,1,2-trimethylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000006142 1,1-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- 125000006098 1,1-dimethylethyl sulfinyl group Chemical group 0.000 description 1
- ZQMAFHIMPLLGDW-UHFFFAOYSA-N 1,1-dimethylpiperidin-1-ium (7-oxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonan-3-yl)oxy-oxoborane Chemical compound C[N+]1(C)CCCCC1.[O-]B1OB2OB(OB=O)OB(O1)O2 ZQMAFHIMPLLGDW-UHFFFAOYSA-N 0.000 description 1
- 125000006103 1,1-dimethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006133 1,1-dimethylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000006121 1,2,2-trimethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006151 1,2,2-trimethylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000001607 1,2,3-triazol-1-yl group Chemical group [*]N1N=NC([H])=C1[H] 0.000 description 1
- 125000006061 1,2-dimethyl-1-butenyl group Chemical group 0.000 description 1
- 125000006062 1,2-dimethyl-2-butenyl group Chemical group 0.000 description 1
- XQEMNBNCQVQXMO-UHFFFAOYSA-M 1,2-dimethyl-3,5-diphenylpyrazol-1-ium;methyl sulfate Chemical compound COS([O-])(=O)=O.C[N+]=1N(C)C(C=2C=CC=CC=2)=CC=1C1=CC=CC=C1 XQEMNBNCQVQXMO-UHFFFAOYSA-M 0.000 description 1
- 125000006113 1,2-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006143 1,2-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- 125000006104 1,2-dimethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006134 1,2-dimethylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000004509 1,3,4-oxadiazol-2-yl group Chemical group O1C(=NN=C1)* 0.000 description 1
- TUBQDCKAWGHZPF-UHFFFAOYSA-N 1,3-benzothiazol-2-ylsulfanylmethyl thiocyanate Chemical compound C1=CC=C2SC(SCSC#N)=NC2=C1 TUBQDCKAWGHZPF-UHFFFAOYSA-N 0.000 description 1
- 125000004227 1,3-benzoxazol-2-yl group Chemical group [H]C1=C([H])C([H])=C2N=C(*)OC2=C1[H] 0.000 description 1
- 125000006064 1,3-dimethyl-1-butenyl group Chemical group 0.000 description 1
- QYPFLRIVEVOAGA-LLVKDONJSA-N 1,3-dimethyl-n-[(3r)-1,1,3-trimethyl-2,3-dihydroinden-4-yl]pyrazole-4-carboxamide Chemical compound C([C@H](C=12)C)C(C)(C)C2=CC=CC=1NC(=O)C1=CN(C)N=C1C QYPFLRIVEVOAGA-LLVKDONJSA-N 0.000 description 1
- QYPFLRIVEVOAGA-NSHDSACASA-N 1,3-dimethyl-n-[(3s)-1,1,3-trimethyl-2,3-dihydroinden-4-yl]pyrazole-4-carboxamide Chemical compound C([C@@H](C=12)C)C(C)(C)C2=CC=CC=1NC(=O)C1=CN(C)N=C1C QYPFLRIVEVOAGA-NSHDSACASA-N 0.000 description 1
- 125000006114 1,3-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006144 1,3-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- 125000000196 1,4-pentadienyl group Chemical group [H]C([*])=C([H])C([H])([H])C([H])=C([H])[H] 0.000 description 1
- VMLKTERJLVWEJJ-UHFFFAOYSA-N 1,5-naphthyridine Chemical compound C1=CC=NC2=CC=CN=C21 VMLKTERJLVWEJJ-UHFFFAOYSA-N 0.000 description 1
- VSOSXKMEQPYESP-UHFFFAOYSA-N 1,6-naphthyridine Chemical compound C1=CN=CC2=CC=CN=C21 VSOSXKMEQPYESP-UHFFFAOYSA-N 0.000 description 1
- MXBVNILGVJVVMH-UHFFFAOYSA-N 1,7-naphthyridine Chemical compound C1=NC=CC2=CC=CN=C21 MXBVNILGVJVVMH-UHFFFAOYSA-N 0.000 description 1
- BAPDWOBBGGGGOC-UHFFFAOYSA-N 1,8-naphthyridine;2,6-naphthyridine Chemical compound N1=CC=CC2=CC=CN=C21.N1=CC=C2C=NC=CC2=C1 BAPDWOBBGGGGOC-UHFFFAOYSA-N 0.000 description 1
- HKELWJONQIFBPO-UHFFFAOYSA-N 1-(2,4-dichlorophenyl)-5-(trichloromethyl)-1,2,4-triazole-3-carboxylic acid Chemical compound N1=C(C(=O)O)N=C(C(Cl)(Cl)Cl)N1C1=CC=C(Cl)C=C1Cl HKELWJONQIFBPO-UHFFFAOYSA-N 0.000 description 1
- CJIUSUMMIWIYMH-UHFFFAOYSA-N 1-(2-aminoethyl)-3-thiophen-2-ylquinoxalin-2-one;hydrochloride Chemical compound Cl.O=C1N(CCN)C2=CC=CC=C2N=C1C1=CC=CS1 CJIUSUMMIWIYMH-UHFFFAOYSA-N 0.000 description 1
- DAGDLSRRQJATCV-UHFFFAOYSA-N 1-(2-bromoethoxy)-2-propan-2-ylbenzene Chemical compound CC(C)C1=CC=CC=C1OCCBr DAGDLSRRQJATCV-UHFFFAOYSA-N 0.000 description 1
- JWUCHKBSVLQQCO-UHFFFAOYSA-N 1-(2-fluorophenyl)-1-(4-fluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanol Chemical compound C=1C=C(F)C=CC=1C(C=1C(=CC=CC=1)F)(O)CN1C=NC=N1 JWUCHKBSVLQQCO-UHFFFAOYSA-N 0.000 description 1
- QNGFJGCALLNSDE-UHFFFAOYSA-N 1-(3-chloro-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridin-2-yl)-5-(cyclopropylmethylamino)pyrazole-4-carbonitrile Chemical compound ClC=1C(=NN2C=1CCCC2)N1N=CC(=C1NCC1CC1)C#N QNGFJGCALLNSDE-UHFFFAOYSA-N 0.000 description 1
- IHGSAQHSAGRWNI-UHFFFAOYSA-N 1-(4-bromophenyl)-2,2,2-trifluoroethanone Chemical compound FC(F)(F)C(=O)C1=CC=C(Br)C=C1 IHGSAQHSAGRWNI-UHFFFAOYSA-N 0.000 description 1
- ULERLVZDMYNWNA-UHFFFAOYSA-N 1-(4-chloro-3-fluorophenyl)-n-[(2-methyl-3-phenylphenyl)methoxy]-2-methylsulfanylethanimine Chemical compound C=1C=C(Cl)C(F)=CC=1C(CSC)=NOCC(C=1C)=CC=CC=1C1=CC=CC=C1 ULERLVZDMYNWNA-UHFFFAOYSA-N 0.000 description 1
- WURBVZBTWMNKQT-UHFFFAOYSA-N 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one Chemical compound C1=NC=NN1C(C(=O)C(C)(C)C)OC1=CC=C(Cl)C=C1 WURBVZBTWMNKQT-UHFFFAOYSA-N 0.000 description 1
- IAQLCKZJGNTRDO-UHFFFAOYSA-N 1-(4-{4-[5-(2,6-difluorophenyl)-4,5-dihydro-1,2-oxazol-3-yl]-1,3-thiazol-2-yl}piperidin-1-yl)-2-[5-methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl]ethanone Chemical compound CC1=CC(C(F)(F)F)=NN1CC(=O)N1CCC(C=2SC=C(N=2)C=2CC(ON=2)C=2C(=CC=CC=2F)F)CC1 IAQLCKZJGNTRDO-UHFFFAOYSA-N 0.000 description 1
- VGPIBGGRCVEHQZ-UHFFFAOYSA-N 1-(biphenyl-4-yloxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C1=NC=NN1C(C(O)C(C)(C)C)OC(C=C1)=CC=C1C1=CC=CC=C1 VGPIBGGRCVEHQZ-UHFFFAOYSA-N 0.000 description 1
- HVQHXBNMBZJPLK-UHFFFAOYSA-N 1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-5-[(2-methylprop-2-en-1-yl)amino]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile Chemical compound CC(=C)CNC1=C([S+]([O-])C(F)(F)F)C(C#N)=NN1C1=C(Cl)C=C(C(F)(F)F)C=C1Cl HVQHXBNMBZJPLK-UHFFFAOYSA-N 0.000 description 1
- XVTXMTOYQVRHSK-UHFFFAOYSA-N 1-[2-(2,4-dichlorophenyl)-2-prop-2-enoxyethyl]imidazole;sulfuric acid Chemical compound OS(O)(=O)=O.ClC1=CC(Cl)=CC=C1C(OCC=C)CN1C=NC=C1 XVTXMTOYQVRHSK-UHFFFAOYSA-N 0.000 description 1
- LQDARGUHUSPFNL-UHFFFAOYSA-N 1-[2-(2,4-dichlorophenyl)-3-(1,1,2,2-tetrafluoroethoxy)propyl]1,2,4-triazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(COC(F)(F)C(F)F)CN1C=NC=N1 LQDARGUHUSPFNL-UHFFFAOYSA-N 0.000 description 1
- PZBPKYOVPCNPJY-UHFFFAOYSA-N 1-[2-(allyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole Chemical compound ClC1=CC(Cl)=CC=C1C(OCC=C)CN1C=NC=C1 PZBPKYOVPCNPJY-UHFFFAOYSA-N 0.000 description 1
- MGNFYQILYYYUBS-UHFFFAOYSA-N 1-[3-(4-tert-butylphenyl)-2-methylpropyl]piperidine Chemical compound C=1C=C(C(C)(C)C)C=CC=1CC(C)CN1CCCCC1 MGNFYQILYYYUBS-UHFFFAOYSA-N 0.000 description 1
- XAGZJIQIVXSURR-UHFFFAOYSA-N 1-[4-(trifluoromethyl)phenyl]piperidin-2-one Chemical compound C1=CC(C(F)(F)F)=CC=C1N1C(=O)CCCC1 XAGZJIQIVXSURR-UHFFFAOYSA-N 0.000 description 1
- XZXSSWDGVSHUNZ-UHFFFAOYSA-N 1-[[2-(4-cyano-3-cyclopropylphenyl)acetyl]amino]cyclohexane-1-carboxylic acid Chemical compound C1C(C2=C(C#N)C=CC(CC(=O)NC3(CCCCC3)C(=O)O)=C2)C1 XZXSSWDGVSHUNZ-UHFFFAOYSA-N 0.000 description 1
- SRPJGPPDQIFOGY-UHFFFAOYSA-N 1-[bis(2-hydroxypropyl)amino]propan-2-ol;2-(2,4-dichlorophenoxy)acetic acid Chemical compound CC(O)CN(CC(C)O)CC(C)O.OC(=O)COC1=CC=C(Cl)C=C1Cl SRPJGPPDQIFOGY-UHFFFAOYSA-N 0.000 description 1
- JSJFUVBHOQIFNC-UHFFFAOYSA-N 1-[bis(2-hydroxypropyl)amino]propan-2-ol;3,6-dichloropyridine-2-carboxylic acid Chemical compound OC(=O)C1=NC(Cl)=CC=C1Cl.CC(O)CN(CC(C)O)CC(C)O JSJFUVBHOQIFNC-UHFFFAOYSA-N 0.000 description 1
- PAJPWUMXBYXFCZ-UHFFFAOYSA-N 1-aminocyclopropanecarboxylic acid Chemical compound OC(=O)C1(N)CC1 PAJPWUMXBYXFCZ-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- ZKFARSBUEBZZJT-UHFFFAOYSA-N 1-butoxypropan-2-yl 2-(4-amino-3,5-dichloro-6-fluoropyridin-2-yl)oxyacetate Chemical group CCCCOCC(C)OC(=O)COC1=NC(F)=C(Cl)C(N)=C1Cl ZKFARSBUEBZZJT-UHFFFAOYSA-N 0.000 description 1
- 125000004972 1-butynyl group Chemical group [H]C([H])([H])C([H])([H])C#C* 0.000 description 1
- YIKWKLYQRFRGPM-UHFFFAOYSA-N 1-dodecylguanidine acetate Chemical compound CC(O)=O.CCCCCCCCCCCCN=C(N)N YIKWKLYQRFRGPM-UHFFFAOYSA-N 0.000 description 1
- 125000006073 1-ethyl-1-butenyl group Chemical group 0.000 description 1
- 125000006122 1-ethyl-1-methylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006152 1-ethyl-1-methylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000006036 1-ethyl-1-propenyl group Chemical group 0.000 description 1
- 125000006123 1-ethyl-2-methylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000004736 1-ethyl-2-methylpropylthio group Chemical group C(C)C(C(C)C)S* 0.000 description 1
- 125000006118 1-ethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006148 1-ethylbutyl sulfonyl group Chemical group 0.000 description 1
- QHFUTZLUZYEBIN-UHFFFAOYSA-N 1-ethylcyclopropene Chemical compound CCC1=CC1 QHFUTZLUZYEBIN-UHFFFAOYSA-N 0.000 description 1
- 125000006106 1-ethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006136 1-ethylpropyl sulfonyl group Chemical group 0.000 description 1
- 125000006039 1-hexenyl group Chemical group 0.000 description 1
- 125000006025 1-methyl-1-butenyl group Chemical group 0.000 description 1
- 125000006019 1-methyl-1-propenyl group Chemical group 0.000 description 1
- 125000006028 1-methyl-2-butenyl group Chemical group 0.000 description 1
- DUWKZHIPEWZXNC-UHFFFAOYSA-N 1-methyl-3-(trifluoromethyl)-n-[2-[2-(trifluoromethyl)phenyl]phenyl]pyrazole-4-carboxamide Chemical compound FC(F)(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1C1=CC=CC=C1C(F)(F)F DUWKZHIPEWZXNC-UHFFFAOYSA-N 0.000 description 1
- IPBFEHWNKZHUSY-UHFFFAOYSA-N 1-methyl-3-thiophen-2-ylquinoxalin-2-one Chemical compound O=C1N(C)C2=CC=CC=C2N=C1C1=CC=CS1 IPBFEHWNKZHUSY-UHFFFAOYSA-N 0.000 description 1
- VOWPJWPJWXZTID-UHFFFAOYSA-N 1-methyl-3-thiophen-2-ylquinoxaline-2-thione Chemical compound S=C1N(C)C2=CC=CC=C2N=C1C1=CC=CS1 VOWPJWPJWXZTID-UHFFFAOYSA-N 0.000 description 1
- PFFIDZXUXFLSSR-UHFFFAOYSA-N 1-methyl-N-[2-(4-methylpentan-2-yl)-3-thienyl]-3-(trifluoromethyl)pyrazole-4-carboxamide Chemical compound S1C=CC(NC(=O)C=2C(=NN(C)C=2)C(F)(F)F)=C1C(C)CC(C)C PFFIDZXUXFLSSR-UHFFFAOYSA-N 0.000 description 1
- 125000006018 1-methyl-ethenyl group Chemical group 0.000 description 1
- 125000006100 1-methylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006130 1-methylbutyl sulfonyl group Chemical group 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- 125000006094 1-methylethyl sulfinyl group Chemical group 0.000 description 1
- 125000006108 1-methylpentyl sulfinyl group Chemical group 0.000 description 1
- 125000006138 1-methylpentyl sulfonyl group Chemical group 0.000 description 1
- 125000006096 1-methylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006127 1-methylpropyl sulfonyl group Chemical group 0.000 description 1
- XFNJVKMNNVCYEK-UHFFFAOYSA-N 1-naphthaleneacetamide Chemical compound C1=CC=C2C(CC(=O)N)=CC=CC2=C1 XFNJVKMNNVCYEK-UHFFFAOYSA-N 0.000 description 1
- VCSWZXSTJNUEPD-UHFFFAOYSA-N 1-o-ethyl 3-o-methyl 2-(5-chloroquinolin-8-yl)oxypropanedioate Chemical compound C1=CN=C2C(OC(C(=O)OCC)C(=O)OC)=CC=C(Cl)C2=C1 VCSWZXSTJNUEPD-UHFFFAOYSA-N 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- WTNKNGDMXNJRPB-UHFFFAOYSA-N 1-propylcyclopropene Chemical compound CCCC1=CC1 WTNKNGDMXNJRPB-UHFFFAOYSA-N 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- FMTFEIJHMMQUJI-NJAFHUGGSA-N 102130-98-3 Natural products CC=CCC1=C(C)[C@H](CC1=O)OC(=O)[C@@H]1[C@@H](C=C(C)C)C1(C)C FMTFEIJHMMQUJI-NJAFHUGGSA-N 0.000 description 1
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Natural products C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 1
- 125000004793 2,2,2-trifluoroethoxy group Chemical group FC(CO*)(F)F 0.000 description 1
- QQNIGXQDICUWGT-UHFFFAOYSA-N 2,2-dichloro-1-(1-oxa-3-azaspiro[4.5]decan-3-yl)ethanone Chemical compound C1N(C(=O)C(Cl)Cl)COC21CCCCC2 QQNIGXQDICUWGT-UHFFFAOYSA-N 0.000 description 1
- ZAWPDPLWGKAAHU-UHFFFAOYSA-N 2,2-dichloro-n-[2-oxo-2-(prop-2-enylamino)ethyl]-n-prop-2-enylacetamide Chemical compound ClC(Cl)C(=O)N(CC=C)CC(=O)NCC=C ZAWPDPLWGKAAHU-UHFFFAOYSA-N 0.000 description 1
- 125000006067 2,2-dimethyl-3-butenyl group Chemical group 0.000 description 1
- KNGWEAQJZJKFLI-UHFFFAOYSA-N 2,2-dimethyl-4h-1,3-benzodioxine-6-carbaldehyde Chemical compound O=CC1=CC=C2OC(C)(C)OCC2=C1 KNGWEAQJZJKFLI-UHFFFAOYSA-N 0.000 description 1
- 125000006115 2,2-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006145 2,2-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- 125000006105 2,2-dimethylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006135 2,2-dimethylpropyl sulfonyl group Chemical group 0.000 description 1
- SXINVWXSZUQKSW-UHFFFAOYSA-N 2,3,5,6-tetrachloro-4-methoxycarbonylbenzoic acid Chemical compound COC(=O)C1=C(Cl)C(Cl)=C(C(O)=O)C(Cl)=C1Cl SXINVWXSZUQKSW-UHFFFAOYSA-N 0.000 description 1
- 125000006068 2,3-dimethyl-1-butenyl group Chemical group 0.000 description 1
- 125000006069 2,3-dimethyl-2-butenyl group Chemical group 0.000 description 1
- 125000006070 2,3-dimethyl-3-butenyl group Chemical group 0.000 description 1
- 125000006116 2,3-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006146 2,3-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- OXJISOJFVQITNG-UHFFFAOYSA-M 2,4-D choline Chemical compound C[N+](C)(C)CCO.[O-]C(=O)COC1=CC=C(Cl)C=C1Cl OXJISOJFVQITNG-UHFFFAOYSA-M 0.000 description 1
- 108010053957 2,4-dichlorophenoxyacetate-alpha-ketoglutarate dioxygenase Proteins 0.000 description 1
- NIOPZPCMRQGZCE-WEVVVXLNSA-N 2,4-dinitro-6-(octan-2-yl)phenyl (E)-but-2-enoate Chemical compound CCCCCCC(C)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1OC(=O)\C=C\C NIOPZPCMRQGZCE-WEVVVXLNSA-N 0.000 description 1
- YTOPFCCWCSOHFV-UHFFFAOYSA-N 2,6-dimethyl-4-tridecylmorpholine Chemical compound CCCCCCCCCCCCCN1CC(C)OC(C)C1 YTOPFCCWCSOHFV-UHFFFAOYSA-N 0.000 description 1
- VQRSXYVRBBWVSQ-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetate;diethylazanium Chemical compound CCNCC.OC(=O)COC1=CC=C(Cl)C=C1Cl VQRSXYVRBBWVSQ-UHFFFAOYSA-N 0.000 description 1
- IUQJDHJVPLLKFL-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetate;dimethylazanium Chemical compound CNC.OC(=O)COC1=CC=C(Cl)C=C1Cl IUQJDHJVPLLKFL-UHFFFAOYSA-N 0.000 description 1
- DLDBIAPNKRBPRS-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetate;tetradecylazanium Chemical compound OC(=O)COC1=CC=C(Cl)C=C1Cl.CCCCCCCCCCCCCCN DLDBIAPNKRBPRS-UHFFFAOYSA-N 0.000 description 1
- OLHMQEQGSVBLTJ-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.OC(=O)COC1=CC=C(Cl)C=C1Cl OLHMQEQGSVBLTJ-UHFFFAOYSA-N 0.000 description 1
- FDMDZIBZKGXZPT-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetic acid;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.OC(=O)COC1=CC=C(Cl)C=C1Cl FDMDZIBZKGXZPT-UHFFFAOYSA-N 0.000 description 1
- GDOSCZDDZQGBAJ-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetic acid;dodecan-1-amine Chemical compound CCCCCCCCCCCCN.OC(=O)COC1=CC=C(Cl)C=C1Cl GDOSCZDDZQGBAJ-UHFFFAOYSA-N 0.000 description 1
- UTILIQPPRUUSFN-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetic acid;heptan-1-amine Chemical compound CCCCCCCN.OC(=O)COC1=CC=C(Cl)C=C1Cl UTILIQPPRUUSFN-UHFFFAOYSA-N 0.000 description 1
- FEGJYGNUIXVOOQ-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetic acid;n,n-diethylethanamine Chemical compound CCN(CC)CC.OC(=O)COC1=CC=C(Cl)C=C1Cl FEGJYGNUIXVOOQ-UHFFFAOYSA-N 0.000 description 1
- HXMBBZAHRWCZOX-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)acetic acid;propan-2-amine Chemical compound CC(C)[NH3+].[O-]C(=O)COC1=CC=C(Cl)C=C1Cl HXMBBZAHRWCZOX-UHFFFAOYSA-N 0.000 description 1
- UJVZXCWQMZWYKI-UHFFFAOYSA-N 2-(2-methoxyethenylamino)acetic acid Chemical compound COC=CNCC(O)=O UJVZXCWQMZWYKI-UHFFFAOYSA-N 0.000 description 1
- PTQBEFQWTBZMED-UHFFFAOYSA-N 2-(3-ethylsulfonylpyridin-2-yl)-5-(trifluoromethylsulfonyl)-1,3-benzoxazole Chemical group CCS(=O)(=O)C1=CC=CN=C1C1=NC2=CC(S(=O)(=O)C(F)(F)F)=CC=C2O1 PTQBEFQWTBZMED-UHFFFAOYSA-N 0.000 description 1
- LWZVSTQWRNKGPB-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)acetate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.CC1=CC(Cl)=CC=C1OCC(O)=O LWZVSTQWRNKGPB-UHFFFAOYSA-N 0.000 description 1
- XQAVWNJMMDWIKG-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)acetic acid;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.CC1=CC(Cl)=CC=C1OCC(O)=O XQAVWNJMMDWIKG-UHFFFAOYSA-N 0.000 description 1
- PKAUICCNAWQPAU-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)acetic acid;n-methylmethanamine Chemical compound CNC.CC1=CC(Cl)=CC=C1OCC(O)=O PKAUICCNAWQPAU-UHFFFAOYSA-N 0.000 description 1
- ROGDGDPDLIVQFZ-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)propanoate;dimethylazanium Chemical compound CNC.OC(=O)C(C)OC1=CC=C(Cl)C=C1C ROGDGDPDLIVQFZ-UHFFFAOYSA-N 0.000 description 1
- HZJKXKUJVSEEFU-UHFFFAOYSA-N 2-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile Chemical compound C=1C=C(Cl)C=CC=1C(CCCC)(C#N)CN1C=NC=N1 HZJKXKUJVSEEFU-UHFFFAOYSA-N 0.000 description 1
- KWLVWJPJKJMCSH-UHFFFAOYSA-N 2-(4-chlorophenyl)-N-{2-[3-methoxy-4-(prop-2-yn-1-yloxy)phenyl]ethyl}-2-(prop-2-yn-1-yloxy)acetamide Chemical compound C1=C(OCC#C)C(OC)=CC(CCNC(=O)C(OCC#C)C=2C=CC(Cl)=CC=2)=C1 KWLVWJPJKJMCSH-UHFFFAOYSA-N 0.000 description 1
- NGLCOYIAJMJYQI-UHFFFAOYSA-N 2-(4-methoxyiminocyclohexyl)-2-(3,3,3-trifluoropropylsulfonyl)acetonitrile Chemical compound CON=C1CCC(C(C#N)S(=O)(=O)CCC(F)(F)F)CC1 NGLCOYIAJMJYQI-UHFFFAOYSA-N 0.000 description 1
- WBBVOIKLKLBBAZ-UHFFFAOYSA-N 2-(4-pyridazin-3-ylpyridazin-1-ium-1-yl)ethanesulfonate Chemical class N1=NC(=CC=C1)C1=CN=[N+](C=C1)CCS(=O)(=O)[O-] WBBVOIKLKLBBAZ-UHFFFAOYSA-N 0.000 description 1
- MFTUAFIIUACGJW-UHFFFAOYSA-N 2-(4-pyrimidin-2-ylpyridazin-1-ium-1-yl)ethanesulfonate Chemical class N1=C(N=CC=C1)C1=CN=[N+](C=C1)CCS(=O)(=O)[O-] MFTUAFIIUACGJW-UHFFFAOYSA-N 0.000 description 1
- AXHCGXRBOHBEQA-UHFFFAOYSA-N 2-(5-chloro-2-methylphenoxy)acetic acid Chemical compound CC1=CC=C(Cl)C=C1OCC(O)=O AXHCGXRBOHBEQA-UHFFFAOYSA-N 0.000 description 1
- JAXUXISKXAOIDD-UHFFFAOYSA-N 2-(5-chloroquinolin-8-yl)oxypropanedioic acid Chemical compound C1=CN=C2C(OC(C(=O)O)C(O)=O)=CC=C(Cl)C2=C1 JAXUXISKXAOIDD-UHFFFAOYSA-N 0.000 description 1
- AGVACZYQCJRDQE-UHFFFAOYSA-N 2-(6-benzylpyridin-2-yl)quinazoline Chemical compound C=1C=CC(C=2N=C3C=CC=CC3=CN=2)=NC=1CC1=CC=CC=C1 AGVACZYQCJRDQE-UHFFFAOYSA-N 0.000 description 1
- SPXBEYPYQKZKGX-UHFFFAOYSA-N 2-(chloromethyl)-5-[(4-chlorophenyl)methyl]-2-methyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol Chemical compound C1=NC=NN1CC1(O)C(C)(CCl)CCC1CC1=CC=C(Cl)C=C1 SPXBEYPYQKZKGX-UHFFFAOYSA-N 0.000 description 1
- OHXLAOJLJWLEIP-UHFFFAOYSA-N 2-(dichloromethyl)-2-methyl-1,3-dioxolane Chemical compound ClC(Cl)C1(C)OCCO1 OHXLAOJLJWLEIP-UHFFFAOYSA-N 0.000 description 1
- ZTMOLOVAQWCURR-UHFFFAOYSA-N 2-[(2,5-dichlorophenyl)methyl]-4,4-dimethyl-1,2-oxazolidin-3-one Chemical compound O=C1C(C)(C)CON1CC1=CC(Cl)=CC=C1Cl ZTMOLOVAQWCURR-UHFFFAOYSA-N 0.000 description 1
- IYRCSBXNZXXLHZ-LYRGGWFBSA-N 2-[(2r,4r,5r)-1-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-1h-1,2,4-triazole-3-thione Chemical compound N1([C@@H]([C@H](O)C(C)(C)C)C[C@H](C)CC=2C(=CC(Cl)=CC=2)Cl)NC=NC1=S IYRCSBXNZXXLHZ-LYRGGWFBSA-N 0.000 description 1
- IYRCSBXNZXXLHZ-RLCCDNCMSA-N 2-[(2r,4s,5r)-1-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-1h-1,2,4-triazole-3-thione Chemical compound N1([C@H]([C@H](O)C(C)(C)C)C[C@H](C)CC=2C(=CC(Cl)=CC=2)Cl)NC=NC1=S IYRCSBXNZXXLHZ-RLCCDNCMSA-N 0.000 description 1
- QKJJCZYFXJCKRX-HZHKWBLPSA-N 2-[(2s,3s,6r)-6-[4-amino-5-(hydroxymethyl)-2-oxopyrimidin-1-yl]-3-[[(2s)-2-amino-3-hydroxypropanoyl]amino]-3,6-dihydro-2h-pyran-2-yl]-5-(diaminomethylideneamino)-2,4-dihydroxypentanoic acid Chemical compound O1[C@H](C(O)(CC(O)CN=C(N)N)C(O)=O)[C@@H](NC(=O)[C@H](CO)N)C=C[C@@H]1N1C(=O)N=C(N)C(CO)=C1 QKJJCZYFXJCKRX-HZHKWBLPSA-N 0.000 description 1
- ZEXXEODAXHSRDJ-UHFFFAOYSA-N 2-[(ethanesulfonyl)amino]-5-fluoro-4-[4-methyl-5-oxo-3-(trifluoromethyl)-4,5-dihydro-1H-1,2,4-triazol-1-yl]benzene-1-carbothioamide Chemical compound CS(=O)(=O)OC1=CC=CC(Cl)=C1C1ON=C(C=2N=C(SC=2)C2CCN(CC2)C(=O)CN2C(=CC(=N2)C(F)F)C(F)F)C1 ZEXXEODAXHSRDJ-UHFFFAOYSA-N 0.000 description 1
- YEKNJVQWXXDDQO-UHFFFAOYSA-N 2-[2-(2-aminoethoxy)ethenylamino]acetic acid Chemical compound NCCOC=CNCC(O)=O YEKNJVQWXXDDQO-UHFFFAOYSA-N 0.000 description 1
- KWQQMZDAELQQGX-UHFFFAOYSA-N 2-[2-(2-methoxyethoxymethyl)-6-methylpyridine-3-carbonyl]cyclohexane-1,3-dione Chemical compound COCCOCC1=NC(C)=CC=C1C(=O)C1C(=O)CCCC1=O KWQQMZDAELQQGX-UHFFFAOYSA-N 0.000 description 1
- GSSLIXUCWSWMKG-UHFFFAOYSA-N 2-[2-chloro-4-(4-chlorophenoxy)phenyl]-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C=1C=C(OC=2C=CC(Cl)=CC=2)C=C(Cl)C=1C(O)(CC)CN1C=NC=N1 GSSLIXUCWSWMKG-UHFFFAOYSA-N 0.000 description 1
- GYATVWZBWGYBFH-UHFFFAOYSA-N 2-[2-fluoro-6-(8-fluoro-2-methylquinolin-3-yl)oxyphenyl]propan-2-ol Chemical compound CC1=NC2=C(F)C=CC=C2C=C1OC1=CC=CC(F)=C1C(C)(C)O GYATVWZBWGYBFH-UHFFFAOYSA-N 0.000 description 1
- BOTNFCTYKJBUMU-UHFFFAOYSA-N 2-[4-(2-methylpropyl)piperazin-4-ium-1-yl]-2-oxoacetate Chemical compound CC(C)C[NH+]1CCN(C(=O)C([O-])=O)CC1 BOTNFCTYKJBUMU-UHFFFAOYSA-N 0.000 description 1
- HSUIUGJIZSLSPE-UHFFFAOYSA-N 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]-1-(1,2,4-triazol-1-yl)butan-2-ol Chemical compound C=1C=C(OC=2C=CC(Cl)=CC=2)C=C(C(F)(F)F)C=1C(O)(CC)CN1C=NC=N1 HSUIUGJIZSLSPE-UHFFFAOYSA-N 0.000 description 1
- JERZEQUMJNCPRJ-UHFFFAOYSA-N 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]-1-(1H-1,2,4-triazol-1-yl)propan-2-ol Chemical compound C=1C=C(OC=2C=CC(Cl)=CC=2)C=C(C(F)(F)F)C=1C(O)(C)CN1C=NC=N1 JERZEQUMJNCPRJ-UHFFFAOYSA-N 0.000 description 1
- YANWOMFJWXJQEF-UHFFFAOYSA-N 2-[6-(3-fluoro-4-methoxyphenyl)-5-methylpyridin-2-yl]quinazoline Chemical compound C1=C(F)C(OC)=CC=C1C1=NC(C=2N=C3C=CC=CC3=CN=2)=CC=C1C YANWOMFJWXJQEF-UHFFFAOYSA-N 0.000 description 1
- VQRYVKJGEDNMNC-UHFFFAOYSA-N 2-[[2-chloro-3-[2-(1,3-dioxolan-2-yl)ethoxy]-4-methylsulfonylphenyl]-hydroxymethylidene]cyclohexane-1,3-dione Chemical compound ClC1=C(OCCC2OCCO2)C(S(=O)(=O)C)=CC=C1C(O)=C1C(=O)CCCC1=O VQRYVKJGEDNMNC-UHFFFAOYSA-N 0.000 description 1
- ZMLZTMPXQOOCNI-UHFFFAOYSA-N 2-[bis(2-hydroxyethyl)amino]ethanol;3,6-dichloro-2-methoxybenzoic acid Chemical compound OCCN(CCO)CCO.COC1=C(Cl)C=CC(Cl)=C1C(O)=O ZMLZTMPXQOOCNI-UHFFFAOYSA-N 0.000 description 1
- RCOHXZITQFQFRZ-UHFFFAOYSA-N 2-[n-[2-(3,5-dichloro-2-methoxybenzoyl)oxyethyl]anilino]ethyl 3,6-dichloro-2-methoxybenzoate Chemical compound COC1=C(Cl)C=C(Cl)C=C1C(=O)OCCN(C=1C=CC=CC=1)CCOC(=O)C1=C(Cl)C=CC(Cl)=C1OC RCOHXZITQFQFRZ-UHFFFAOYSA-N 0.000 description 1
- PVTHJAPFENJVNC-UHFFFAOYSA-N 2-amino-2-[5-amino-2-methyl-6-(2,3,4,5,6-pentahydroxycyclohexyl)oxyoxan-3-yl]iminoacetic acid Chemical compound NC1CC(N=C(N)C(O)=O)C(C)OC1OC1C(O)C(O)C(O)C(O)C1O PVTHJAPFENJVNC-UHFFFAOYSA-N 0.000 description 1
- BOBATFKNMFWLFG-UHFFFAOYSA-N 2-amino-2-cyano-n-methylacetamide Chemical compound CNC(=O)C(N)C#N BOBATFKNMFWLFG-UHFFFAOYSA-N 0.000 description 1
- IWMQIGXEXKJFPR-UHFFFAOYSA-N 2-aminoethanol;2-(4-chloro-2-methylphenoxy)acetic acid Chemical compound NCCO.CC1=CC(Cl)=CC=C1OCC(O)=O IWMQIGXEXKJFPR-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- ZMWGIGHRZQTQRE-UHFFFAOYSA-N 2-butoxyethyl 2-(2,4-dichlorophenoxy)acetate Chemical compound CCCCOCCOC(=O)COC1=CC=C(Cl)C=C1Cl ZMWGIGHRZQTQRE-UHFFFAOYSA-N 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- QCTALYCTVMUWPT-UHFFFAOYSA-N 2-chloro-3-(4-chlorophenyl)propanoic acid Chemical compound OC(=O)C(Cl)CC1=CC=C(Cl)C=C1 QCTALYCTVMUWPT-UHFFFAOYSA-N 0.000 description 1
- SVOAUHHKPGKPQK-UHFFFAOYSA-N 2-chloro-9-hydroxyfluorene-9-carboxylic acid Chemical compound C1=C(Cl)C=C2C(C(=O)O)(O)C3=CC=CC=C3C2=C1 SVOAUHHKPGKPQK-UHFFFAOYSA-N 0.000 description 1
- TVAJZOVLQZNNCJ-UHFFFAOYSA-N 2-chloro-n-(1-cyanocyclopropyl)-5-[1-[2-methyl-5-(1,1,2,2,2-pentafluoroethyl)-4-(trifluoromethyl)pyrazol-3-yl]pyrazol-4-yl]benzamide Chemical compound CN1N=C(C(F)(F)C(F)(F)F)C(C(F)(F)F)=C1N1N=CC(C=2C=C(C(Cl)=CC=2)C(=O)NC2(CC2)C#N)=C1 TVAJZOVLQZNNCJ-UHFFFAOYSA-N 0.000 description 1
- 125000004182 2-chlorophenyl group Chemical group [H]C1=C([H])C(Cl)=C(*)C([H])=C1[H] 0.000 description 1
- MIJLZGZLQLAQCM-UHFFFAOYSA-N 2-ethoxyethyl 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OCCOCC)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl MIJLZGZLQLAQCM-UHFFFAOYSA-N 0.000 description 1
- 125000006076 2-ethyl-1-butenyl group Chemical group 0.000 description 1
- 125000006077 2-ethyl-2-butenyl group Chemical group 0.000 description 1
- 125000006078 2-ethyl-3-butenyl group Chemical group 0.000 description 1
- 125000006119 2-ethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006149 2-ethylbutyl sulfonyl group Chemical group 0.000 description 1
- QZSFJRIWRPJUOH-UHFFFAOYSA-N 2-ethylhexyl 2-(2,4-dichlorophenoxy)acetate Chemical compound CCCCC(CC)COC(=O)COC1=CC=C(Cl)C=C1Cl QZSFJRIWRPJUOH-UHFFFAOYSA-N 0.000 description 1
- IDGRPSMONFWWEK-UHFFFAOYSA-N 2-ethylhexyl 2-(4-chloro-2-methylphenoxy)acetate Chemical compound CCCCC(CC)COC(=O)COC1=CC=C(Cl)C=C1C IDGRPSMONFWWEK-UHFFFAOYSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000006040 2-hexenyl group Chemical group 0.000 description 1
- AWSZRJQNBMEZOI-UHFFFAOYSA-N 2-methoxyethyl 2-(4-tert-butylphenyl)-2-cyano-3-oxo-3-[2-(trifluoromethyl)phenyl]propanoate Chemical compound C=1C=C(C(C)(C)C)C=CC=1C(C#N)(C(=O)OCCOC)C(=O)C1=CC=CC=C1C(F)(F)F AWSZRJQNBMEZOI-UHFFFAOYSA-N 0.000 description 1
- 125000006026 2-methyl-1-butenyl group Chemical group 0.000 description 1
- 125000006045 2-methyl-1-pentenyl group Chemical group 0.000 description 1
- 125000006029 2-methyl-2-butenyl group Chemical group 0.000 description 1
- 125000006049 2-methyl-2-pentenyl group Chemical group 0.000 description 1
- 125000006022 2-methyl-2-propenyl group Chemical group 0.000 description 1
- 125000006031 2-methyl-3-butenyl group Chemical group 0.000 description 1
- 125000006053 2-methyl-3-pentenyl group Chemical group 0.000 description 1
- 125000006056 2-methyl-4-pentenyl group Chemical group 0.000 description 1
- 125000006101 2-methylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006131 2-methylbutyl sulfonyl group Chemical group 0.000 description 1
- 125000006109 2-methylpentyl sulfinyl group Chemical group 0.000 description 1
- 125000006139 2-methylpentyl sulfonyl group Chemical group 0.000 description 1
- 125000006097 2-methylpropyl sulfinyl group Chemical group 0.000 description 1
- 125000006128 2-methylpropyl sulfonyl group Chemical group 0.000 description 1
- IQUPABOKLQSFBK-UHFFFAOYSA-N 2-nitrophenol Chemical compound OC1=CC=CC=C1[N+]([O-])=O IQUPABOKLQSFBK-UHFFFAOYSA-N 0.000 description 1
- HNUKTDKISXPDPA-UHFFFAOYSA-N 2-oxopropyl Chemical group [CH2]C(C)=O HNUKTDKISXPDPA-UHFFFAOYSA-N 0.000 description 1
- JYVLIDXNZAXMDK-UHFFFAOYSA-N 2-pentanol Substances CCCC(C)O JYVLIDXNZAXMDK-UHFFFAOYSA-N 0.000 description 1
- 125000006024 2-pentenyl group Chemical group 0.000 description 1
- 229940061334 2-phenylphenol Drugs 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- 125000004485 2-pyrrolidinyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])C1([H])* 0.000 description 1
- PNKYIZBUGAZUHQ-UHFFFAOYSA-N 2-quinolin-8-yloxyacetic acid Chemical compound C1=CN=C2C(OCC(=O)O)=CC=CC2=C1 PNKYIZBUGAZUHQ-UHFFFAOYSA-N 0.000 description 1
- NBNQOWVYEXFQJC-UHFFFAOYSA-N 2-sulfanyl-3h-thiadiazole Chemical compound SN1NC=CS1 NBNQOWVYEXFQJC-UHFFFAOYSA-N 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- PDPWCKVFIFAQIQ-UHFFFAOYSA-N 2-{2-[(2,5-dimethylphenoxy)methyl]phenyl}-2-methoxy-N-methylacetamide Chemical compound CNC(=O)C(OC)C1=CC=CC=C1COC1=CC(C)=CC=C1C PDPWCKVFIFAQIQ-UHFFFAOYSA-N 0.000 description 1
- HJIKODJJEORHMZ-NNPZUXBVSA-N 28-Homobrassinolide Chemical group C1OC(=O)[C@H]2C[C@H](O)[C@H](O)C[C@]2(C)[C@H]2CC[C@]3(C)[C@@H]([C@H](C)[C@@H](O)[C@H](O)[C@H](C(C)C)CC)CC[C@H]3[C@@H]21 HJIKODJJEORHMZ-NNPZUXBVSA-N 0.000 description 1
- GTODOEDLCNTSLG-UHFFFAOYSA-N 2h-triazole-4-carboxylic acid Chemical compound OC(=O)C1=CNN=N1 GTODOEDLCNTSLG-UHFFFAOYSA-N 0.000 description 1
- 108020005345 3' Untranslated Regions Proteins 0.000 description 1
- 125000006071 3,3-dimethyl-1-butenyl group Chemical group 0.000 description 1
- 125000006072 3,3-dimethyl-2-butenyl group Chemical group 0.000 description 1
- 125000006117 3,3-dimethylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006147 3,3-dimethylbutyl sulfonyl group Chemical group 0.000 description 1
- AUQAUAIUNJIIEP-UHFFFAOYSA-N 3,4,5-trimethylphenyl methylcarbamate Chemical compound CNC(=O)OC1=CC(C)=C(C)C(C)=C1 AUQAUAIUNJIIEP-UHFFFAOYSA-N 0.000 description 1
- XCSKMKLNIBUVPP-UHFFFAOYSA-N 3,4-dihydroisothiochromen-1-one Chemical class C1=CC=C2C(=O)SCCC2=C1 XCSKMKLNIBUVPP-UHFFFAOYSA-N 0.000 description 1
- SOUGWDPPRBKJEX-UHFFFAOYSA-N 3,5-dichloro-N-(1-chloro-3-methyl-2-oxopentan-3-yl)-4-methylbenzamide Chemical compound ClCC(=O)C(C)(CC)NC(=O)C1=CC(Cl)=C(C)C(Cl)=C1 SOUGWDPPRBKJEX-UHFFFAOYSA-N 0.000 description 1
- 125000004211 3,5-difluorophenyl group Chemical group [H]C1=C(F)C([H])=C(*)C([H])=C1F 0.000 description 1
- RAKSGYQDDJHPCC-UHFFFAOYSA-N 3,6-dichloro-2-methoxybenzoic acid;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.COC1=C(Cl)C=CC(Cl)=C1C(O)=O RAKSGYQDDJHPCC-UHFFFAOYSA-N 0.000 description 1
- NRAYWXLNSHEHQO-UHFFFAOYSA-N 3-(1-benzothiophen-2-yl)-5,6-dihydro-1,4,2-oxathiazine 4-oxide Chemical compound O=S1CCON=C1C1=CC2=CC=CC=C2S1 NRAYWXLNSHEHQO-UHFFFAOYSA-N 0.000 description 1
- MEBWABJHRAYGFW-UHFFFAOYSA-N 3-(2,4-dichlorophenyl)prop-2-enoic acid Chemical compound OC(=O)C=CC1=CC=C(Cl)C=C1Cl MEBWABJHRAYGFW-UHFFFAOYSA-N 0.000 description 1
- SWBHWUYHHJCADA-UHFFFAOYSA-N 3-(2-chlorophenyl)-6-(2,6-difluorophenyl)-1,2,4,5-tetrazine Chemical compound FC1=CC=CC(F)=C1C1=NN=C(C=2C(=CC=CC=2)Cl)N=N1 SWBHWUYHHJCADA-UHFFFAOYSA-N 0.000 description 1
- BBPCQVYYILTZAN-UHFFFAOYSA-N 3-(2h-tetrazole-5-carbonyl)-1h-quinolin-2-one Chemical class C=1C2=CC=CC=C2NC(=O)C=1C(=O)C1=NN=NN1 BBPCQVYYILTZAN-UHFFFAOYSA-N 0.000 description 1
- FSCWZHGZWWDELK-UHFFFAOYSA-N 3-(3,5-dichlorophenyl)-5-ethenyl-5-methyl-2,4-oxazolidinedione Chemical compound O=C1C(C)(C=C)OC(=O)N1C1=CC(Cl)=CC(Cl)=C1 FSCWZHGZWWDELK-UHFFFAOYSA-N 0.000 description 1
- MSBFKZLNCGASNW-OIHKITLTSA-N 3-(3,5-difluorophenyl)-N-[(1R,4S)-4-(oxazinane-2-carbonyl)cyclopent-2-en-1-yl]-5-(trifluoromethyl)-4H-1,2-oxazole-5-carboxamide Chemical compound O=C([C@@H](C1)C=C[C@@H]1NC(C(C1)(C(F)(F)F)ON=C1C1=CC(F)=CC(F)=C1)=O)N1OCCCC1 MSBFKZLNCGASNW-OIHKITLTSA-N 0.000 description 1
- OIJVCMGWZZTWJT-UHFFFAOYSA-N 3-(4-chloro-2,6-dimethylphenyl)-4-hydroxy-8-methoxy-1,8-diazaspiro[4.5]dec-3-en-2-one Chemical compound ClC1=CC(=C(C(=C1)C)C=1C(NC2(C=1O)CCN(CC2)OC)=O)C OIJVCMGWZZTWJT-UHFFFAOYSA-N 0.000 description 1
- REIWAEMSRXWEDS-UHFFFAOYSA-N 3-(4-chloro-2,6-dimethylphenyl)-8-methoxy-1-methyl-1,8-diazaspiro[4.5]decane-2,4-dione Chemical compound C1CN(OC)CCC21C(=O)C(C=1C(=CC(Cl)=CC=1C)C)C(=O)N2C REIWAEMSRXWEDS-UHFFFAOYSA-N 0.000 description 1
- SCFGLMLXZAQXGC-UHFFFAOYSA-N 3-(5-tert-butyl-1,2-oxazol-3-yl)-4-hydroxy-1-methylimidazolidin-2-one Chemical compound O=C1N(C)CC(O)N1C1=NOC(C(C)(C)C)=C1 SCFGLMLXZAQXGC-UHFFFAOYSA-N 0.000 description 1
- YTCIYOXHHQLDEI-UHFFFAOYSA-N 3-(difluoromethyl)-1-methyl-n-(1,1,3-trimethyl-2,3-dihydroinden-4-yl)pyrazole-4-carboxamide Chemical compound C=12C(C)CC(C)(C)C2=CC=CC=1NC(=O)C1=CN(C)N=C1C(F)F YTCIYOXHHQLDEI-UHFFFAOYSA-N 0.000 description 1
- YTCIYOXHHQLDEI-JTQLQIEISA-N 3-(difluoromethyl)-1-methyl-n-[(3s)-1,1,3-trimethyl-2,3-dihydroinden-4-yl]pyrazole-4-carboxamide Chemical compound C([C@@H](C=12)C)C(C)(C)C2=CC=CC=1NC(=O)C1=CN(C)N=C1C(F)F YTCIYOXHHQLDEI-JTQLQIEISA-N 0.000 description 1
- AGJBBAXNZINQHC-UHFFFAOYSA-N 3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(N)=O)=C1F AGJBBAXNZINQHC-UHFFFAOYSA-N 0.000 description 1
- XCGBHLLWJZOLEM-UHFFFAOYSA-N 3-(difluoromethyl)-N-(7-fluoro-1,1,3-trimethyl-2,3-dihydro-1H-inden-4-yl)-1-methyl-1H-pyrazole-4-carboxamide Chemical compound CC1CC(C)(C)C(C(=CC=2)F)=C1C=2NC(=O)C1=CN(C)N=C1C(F)F XCGBHLLWJZOLEM-UHFFFAOYSA-N 0.000 description 1
- DGOAXBPOVUPPEB-UHFFFAOYSA-N 3-(difluoromethyl)-N-methoxy-1-methyl-N-[1-(2,4,6-trichlorophenyl)propan-2-yl]pyrazole-4-carboxamide Chemical compound C=1N(C)N=C(C(F)F)C=1C(=O)N(OC)C(C)CC1=C(Cl)C=C(Cl)C=C1Cl DGOAXBPOVUPPEB-UHFFFAOYSA-N 0.000 description 1
- MIHUUQQKVLCKAT-UHFFFAOYSA-N 3-(difluoromethyl)-n-[(2-ethyl-5-fluorophenyl)methyl]-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CCC1=CC=C(F)C=C1CNC(=O)C1=C(F)N(C)N=C1C(F)F MIHUUQQKVLCKAT-UHFFFAOYSA-N 0.000 description 1
- VIXCLRUCUMWJFF-UHFFFAOYSA-N 3-[2-chloro-4-(methylsulfonyl)benzoyl]-4-(phenylthio)bicyclo[3.2.1]oct-3-en-2-one Chemical compound ClC1=CC(S(=O)(=O)C)=CC=C1C(=O)C(C(C1CCC2C1)=O)=C2SC1=CC=CC=C1 VIXCLRUCUMWJFF-UHFFFAOYSA-N 0.000 description 1
- WYJOEQHHWHAJRB-UHFFFAOYSA-N 3-[5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrophenoxy]oxolane Chemical compound C1=C(OC2COCC2)C([N+](=O)[O-])=CC=C1OC1=CC=C(C(F)(F)F)C=C1Cl WYJOEQHHWHAJRB-UHFFFAOYSA-N 0.000 description 1
- NXZLJFLADUDSQR-UHFFFAOYSA-N 3-amino-2,5-dichlorobenzoic acid;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.NC1=CC(Cl)=CC(C(O)=O)=C1Cl NXZLJFLADUDSQR-UHFFFAOYSA-N 0.000 description 1
- CJQNJRRDTPULTL-UHFFFAOYSA-N 3-azabicyclo[3.2.1]octane Chemical compound C1C2CCC1CNC2 CJQNJRRDTPULTL-UHFFFAOYSA-N 0.000 description 1
- RAMUASXTSSXCMB-UHFFFAOYSA-N 3-bromo-N-{2-bromo-4-chloro-6-[(1-cyclopropylethyl)carbamoyl]phenyl}-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamide Chemical compound C1CC1C(C)NC(=O)C1=CC(Cl)=CC(Br)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl RAMUASXTSSXCMB-UHFFFAOYSA-N 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- INDMHHREARZNOU-UHFFFAOYSA-N 3-chloro-5-(6-chloropyridin-3-yl)-6-methyl-4-(2,4,6-trifluorophenyl)pyridazine Chemical compound C=1C=C(Cl)N=CC=1C=1C(C)=NN=C(Cl)C=1C1=C(F)C=C(F)C=C1F INDMHHREARZNOU-UHFFFAOYSA-N 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000006041 3-hexenyl group Chemical group 0.000 description 1
- RUXHWBMJNBBYNL-UHFFFAOYSA-N 3-hydroxy-1,2-dihydropyrrol-5-one Chemical class OC1=CC(=O)NC1 RUXHWBMJNBBYNL-UHFFFAOYSA-N 0.000 description 1
- 125000006027 3-methyl-1-butenyl group Chemical group 0.000 description 1
- 125000006046 3-methyl-1-pentenyl group Chemical group 0.000 description 1
- 125000006050 3-methyl-2-pentenyl group Chemical group 0.000 description 1
- 125000006032 3-methyl-3-butenyl group Chemical group 0.000 description 1
- 125000006054 3-methyl-3-pentenyl group Chemical group 0.000 description 1
- 125000006057 3-methyl-4-pentenyl group Chemical group 0.000 description 1
- HDKWFBCPLKNOCK-SFHVURJKSA-N 3-methyl-n-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]-5-[(5s)-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4h-1,2-oxazol-3-yl]thiophene-2-carboxamide Chemical compound S1C(C(=O)NCC(=O)NCC(F)(F)F)=C(C)C=C1C1=NO[C@](C(F)(F)F)(C=2C=C(Cl)C(Cl)=C(Cl)C=2)C1 HDKWFBCPLKNOCK-SFHVURJKSA-N 0.000 description 1
- 125000006102 3-methylbutyl sulfinyl group Chemical group 0.000 description 1
- 125000006132 3-methylbutyl sulfonyl group Chemical group 0.000 description 1
- FAPGNCCCFGCZKP-UHFFFAOYSA-N 3-methylcyclopropene Chemical compound CC1C=C1 FAPGNCCCFGCZKP-UHFFFAOYSA-N 0.000 description 1
- 125000006110 3-methylpentyl sulfinyl group Chemical group 0.000 description 1
- 125000006140 3-methylpentyl sulfonyl group Chemical group 0.000 description 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000004575 3-pyrrolidinyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- SWTPIYGGSMJRTB-UHFFFAOYSA-N 4,4-difluoro-3,3-dimethyl-1-quinolin-3-ylisoquinoline Chemical compound C12=CC=CC=C2C(F)(F)C(C)(C)N=C1C1=CN=C(C=CC=C2)C2=C1 SWTPIYGGSMJRTB-UHFFFAOYSA-N 0.000 description 1
- WVQFVFHYUXCSBD-UHFFFAOYSA-N 4-(2,3-dichlorophenyl)-1h-pyrazole-5-carboxylic acid Chemical class N1N=CC(C=2C(=C(Cl)C=CC=2)Cl)=C1C(=O)O WVQFVFHYUXCSBD-UHFFFAOYSA-N 0.000 description 1
- KEIXJOCOXNOKHI-UHFFFAOYSA-N 4-(2,4-dichlorophenoxy)butanoate;dimethylazanium Chemical compound CNC.OC(=O)CCCOC1=CC=C(Cl)C=C1Cl KEIXJOCOXNOKHI-UHFFFAOYSA-N 0.000 description 1
- CMQKKWSPWPFZCL-UHFFFAOYSA-N 4-(2-bromo-4-fluorophenyl)-n-(2-bromo-6-fluorophenyl)-2,5-dimethylpyrazol-3-amine Chemical compound C=1C=C(F)C=C(Br)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=CC=C1Br CMQKKWSPWPFZCL-UHFFFAOYSA-N 0.000 description 1
- LBGMYUQCXJJLIR-UHFFFAOYSA-N 4-(2-bromo-4-fluorophenyl)-n-(2-chloro-6-fluorophenyl)-2,5-dimethylpyrazol-3-amine Chemical compound C=1C=C(F)C=C(Br)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=CC=C1Cl LBGMYUQCXJJLIR-UHFFFAOYSA-N 0.000 description 1
- AQSDPFVDUFPUSI-UHFFFAOYSA-N 4-(2-chloro-4-fluorophenyl)-n-(2-chloro-6-fluorophenyl)-2,5-dimethylpyrazol-3-amine Chemical compound C=1C=C(F)C=C(Cl)C=1C=1C(C)=NN(C)C=1NC1=C(F)C=CC=C1Cl AQSDPFVDUFPUSI-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- SIYAHZSHQIPQLY-UHFFFAOYSA-N 4-(4-chlorophenoxy)butanoic acid Chemical compound OC(=O)CCCOC1=CC=C(Cl)C=C1 SIYAHZSHQIPQLY-UHFFFAOYSA-N 0.000 description 1
- FAOFKIOARCDPBY-UHFFFAOYSA-N 4-(benzoylsulfamoyl)benzamide Chemical compound C1=CC(C(=O)N)=CC=C1S(=O)(=O)NC(=O)C1=CC=CC=C1 FAOFKIOARCDPBY-UHFFFAOYSA-N 0.000 description 1
- ZAYKVYVNMLEAMI-UHFFFAOYSA-N 4-(carboxymethyl)-2,3-dihydrochromene-4-carboxylic acid Chemical compound C1=CC=C2C(CC(=O)O)(C(O)=O)CCOC2=C1 ZAYKVYVNMLEAMI-UHFFFAOYSA-N 0.000 description 1
- XFYYQDHEDOXWGA-UHFFFAOYSA-N 4-[(5-bromopyridin-2-yl)amino]-4-oxobutanoic acid Chemical compound OC(=O)CCC(=O)NC1=CC=C(Br)C=N1 XFYYQDHEDOXWGA-UHFFFAOYSA-N 0.000 description 1
- BPFUIWLQXNPZHI-UHFFFAOYSA-N 4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]-N-[(methoxyamino)methylidene]-2-methylbenzamide Chemical compound C1=C(C)C(C(=O)N\C=N/OC)=CC=C1C1=NOC(C(F)(F)F)(C=2C=C(Cl)C=C(Cl)C=2)C1 BPFUIWLQXNPZHI-UHFFFAOYSA-N 0.000 description 1
- OXDDDHGGRFRLEE-UHFFFAOYSA-N 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-4h-1,2-oxazol-3-yl]-n-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]naphthalene-1-carboxamide Chemical compound C12=CC=CC=C2C(C(=O)NCC(=O)NCC(F)(F)F)=CC=C1C(C1)=NOC1(C(F)(F)F)C1=CC(Cl)=CC(C(F)(F)F)=C1 OXDDDHGGRFRLEE-UHFFFAOYSA-N 0.000 description 1
- KLSSIPFIAURKSX-UHFFFAOYSA-N 4-amino-3,5,6-trichloropyridine-2-carboxylic acid 2-[bis(2-hydroxyethyl)amino]ethanol Chemical compound OCCN(CCO)CCO.NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl KLSSIPFIAURKSX-UHFFFAOYSA-N 0.000 description 1
- NPLPNFIMSLUIMN-UHFFFAOYSA-N 4-amino-3,5,6-trichloropyridine-2-carboxylic acid N-methylmethanamine Chemical compound CNC.NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl NPLPNFIMSLUIMN-UHFFFAOYSA-N 0.000 description 1
- XQVYHLBQRMKACP-UHFFFAOYSA-N 4-amino-3,5,6-trichloropyridine-2-carboxylic acid;1-[bis(2-hydroxypropyl)amino]propan-2-ol Chemical compound CC(O)CN(CC(C)O)CC(C)O.NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl XQVYHLBQRMKACP-UHFFFAOYSA-N 0.000 description 1
- HYOWIYLMXADQFD-UHFFFAOYSA-N 4-amino-3,6-dichloropyridine-2-carboxylic acid;n-methylmethanamine Chemical compound CNC.NC1=CC(Cl)=NC(C(O)=O)=C1Cl HYOWIYLMXADQFD-UHFFFAOYSA-N 0.000 description 1
- ADZSGNDOZREKJK-UHFFFAOYSA-N 4-amino-6-tert-butyl-3-ethylsulfanyl-1,2,4-triazin-5-one Chemical compound CCSC1=NN=C(C(C)(C)C)C(=O)N1N ADZSGNDOZREKJK-UHFFFAOYSA-N 0.000 description 1
- PGYDGBCATBINCB-UHFFFAOYSA-N 4-diethoxyphosphoryl-n,n-dimethylaniline Chemical compound CCOP(=O)(OCC)C1=CC=C(N(C)C)C=C1 PGYDGBCATBINCB-UHFFFAOYSA-N 0.000 description 1
- TTZOLDXHOCCNMF-UHFFFAOYSA-N 4-fluoro-2-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(F)C=C1O TTZOLDXHOCCNMF-UHFFFAOYSA-N 0.000 description 1
- 125000006042 4-hexenyl group Chemical group 0.000 description 1
- DXPNWQHGOPWNTO-UHFFFAOYSA-N 4-hydroxy-1-methoxy-5-methyl-3-[4-(trifluoromethyl)pyridin-2-yl]imidazolidin-2-one Chemical compound CON1C(C)C(O)N(C1=O)c1cc(ccn1)C(F)(F)F DXPNWQHGOPWNTO-UHFFFAOYSA-N 0.000 description 1
- ZOQWKQZKRNTDHF-UHFFFAOYSA-N 4-hydroxy-1-methyl-3-(2h-tetrazole-5-carbonyl)quinolin-2-one Chemical compound O=C1N(C)C2=CC=CC=C2C(O)=C1C(=O)C1=NN=NN1 ZOQWKQZKRNTDHF-UHFFFAOYSA-N 0.000 description 1
- VCSXIASJLLCLHT-UHFFFAOYSA-N 4-hydroxy-1-methyl-3-[4-(trifluoromethyl)pyridin-2-yl]imidazolidin-2-one Chemical compound CN1CC(O)N(C1=O)c1cc(ccn1)C(F)(F)F VCSXIASJLLCLHT-UHFFFAOYSA-N 0.000 description 1
- 125000006051 4-methyl-2-pentenyl group Chemical group 0.000 description 1
- 125000003119 4-methyl-3-pentenyl group Chemical group [H]\C(=C(/C([H])([H])[H])C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000006058 4-methyl-4-pentenyl group Chemical group 0.000 description 1
- QRAOZQGIUIDZQZ-UHFFFAOYSA-N 4-methyl-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,3-dihydro-1,4-benzoxazine Chemical compound C=1C=C2N(C)CCOC2=CC=1B1OC(C)(C)C(C)(C)O1 QRAOZQGIUIDZQZ-UHFFFAOYSA-N 0.000 description 1
- QMNWXSUXINMZIM-UHFFFAOYSA-N 4-methylpentan-2-yl 2-(5-chloroquinolin-8-yl)oxyacetate Chemical compound C1=CN=C2C(OCC(=O)OC(C)CC(C)C)=CC=C(Cl)C2=C1 QMNWXSUXINMZIM-UHFFFAOYSA-N 0.000 description 1
- 125000006111 4-methylpentyl sulfinyl group Chemical group 0.000 description 1
- 125000006141 4-methylpentyl sulfonyl group Chemical group 0.000 description 1
- JWIIZZMRHCJZEJ-UHFFFAOYSA-N 4-n-(benzenesulfonyl)benzene-1,4-dicarboxamide Chemical compound C1=CC(C(=O)N)=CC=C1C(=O)NS(=O)(=O)C1=CC=CC=C1 JWIIZZMRHCJZEJ-UHFFFAOYSA-N 0.000 description 1
- LHZOTJOOBRODLL-UHFFFAOYSA-N 4-oxo-1-(pyrimidin-5-ylmethyl)-3-[3-(trifluoromethyl)phenyl]pyrido[1,2-a]pyrimidin-5-ium-2-olate Chemical compound O=C1[N+]2=CC=CC=C2N(CC=2C=NC=NC=2)C([O-])=C1C1=CC=CC(C(F)(F)F)=C1 LHZOTJOOBRODLL-UHFFFAOYSA-N 0.000 description 1
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- HQQTZCPKNZVLFF-UHFFFAOYSA-N 4h-1,2-benzoxazin-3-one Chemical class C1=CC=C2ONC(=O)CC2=C1 HQQTZCPKNZVLFF-UHFFFAOYSA-N 0.000 description 1
- 108020003589 5' Untranslated Regions Proteins 0.000 description 1
- QGHKONZVJQXPBQ-UHFFFAOYSA-N 5,8-difluoro-n-[2-[2-fluoro-4-[4-(trifluoromethyl)pyridin-2-yl]oxyphenyl]ethyl]quinazolin-4-amine Chemical compound C=1C=C(CCNC=2C3=C(F)C=CC(F)=C3N=CN=2)C(F)=CC=1OC1=CC(C(F)(F)F)=CC=N1 QGHKONZVJQXPBQ-UHFFFAOYSA-N 0.000 description 1
- OPEJGICLTMWFNQ-UHFFFAOYSA-N 5-[(2,6-difluorophenyl)methoxymethyl]-5-methyl-3-(3-methylthiophen-2-yl)-4h-1,2-oxazole Chemical compound C1=CSC(C=2CC(C)(COCC=3C(=CC=CC=3F)F)ON=2)=C1C OPEJGICLTMWFNQ-UHFFFAOYSA-N 0.000 description 1
- IVJKCSCRNVMPNG-OWOJBTEDSA-N 5-[[(E)-3-chloroprop-2-enyl]amino]-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethylsulfinyl)pyrazole-3-carbonitrile Chemical compound Cl/C=C/CNC1=C(C(=NN1C1=C(C=C(C=C1Cl)C(F)(F)F)Cl)C#N)S(=O)C(F)(F)F IVJKCSCRNVMPNG-OWOJBTEDSA-N 0.000 description 1
- UUPFHBMPCZWWAC-UHFFFAOYSA-N 5-bromo-4-chloro-N-[4-chloro-2-methyl-6-(methylcarbamoyl)phenyl]-2-(3-chloropyridin-2-yl)pyrazole-3-carboxamide Chemical compound BrC=1C(=C(N(N=1)C1=NC=CC=C1Cl)C(=O)NC1=C(C=C(C=C1C(NC)=O)Cl)C)Cl UUPFHBMPCZWWAC-UHFFFAOYSA-N 0.000 description 1
- WXPLKPHFZNDLBX-UHFFFAOYSA-N 5-bromo-N-[4-chloro-2-methyl-6-(methylcarbamothioyl)phenyl]-2-(3-chloropyridin-2-yl)pyrazole-3-carboxamide Chemical compound BrC1=NN(C(=C1)C(=O)NC1=C(C=C(C=C1C(=S)NC)Cl)C)C1=NC=CC=C1Cl WXPLKPHFZNDLBX-UHFFFAOYSA-N 0.000 description 1
- ONILAONOGQYBHW-UHFFFAOYSA-N 5-bromo-n-[2,4-dichloro-6-(methylcarbamoyl)phenyl]-2-(3,5-dichloropyridin-2-yl)pyrazole-3-carboxamide Chemical compound CNC(=O)C1=CC(Cl)=CC(Cl)=C1NC(=O)C1=CC(Br)=NN1C1=NC=C(Cl)C=C1Cl ONILAONOGQYBHW-UHFFFAOYSA-N 0.000 description 1
- RXVUMQMPWMCDNE-UHFFFAOYSA-N 5-bromo-n-[4-carbamothioyl-2-methyl-6-(methylcarbamoyl)phenyl]-2-(3-chloropyridin-2-yl)pyrazole-3-carboxamide Chemical compound CNC(=O)C1=CC(C(N)=S)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl RXVUMQMPWMCDNE-UHFFFAOYSA-N 0.000 description 1
- XJFIKRXIJXAJGH-UHFFFAOYSA-N 5-chloro-1,3-dihydroimidazo[4,5-b]pyridin-2-one Chemical group ClC1=CC=C2NC(=O)NC2=N1 XJFIKRXIJXAJGH-UHFFFAOYSA-N 0.000 description 1
- NRTLIYOWLVMQBO-UHFFFAOYSA-N 5-chloro-1,3-dimethyl-N-(1,1,3-trimethyl-1,3-dihydro-2-benzofuran-4-yl)pyrazole-4-carboxamide Chemical compound C=12C(C)OC(C)(C)C2=CC=CC=1NC(=O)C=1C(C)=NN(C)C=1Cl NRTLIYOWLVMQBO-UHFFFAOYSA-N 0.000 description 1
- QKRORLUXLQYKAA-UHFFFAOYSA-N 5-chloro-n'-phenyl-n'-prop-2-ynylthiophene-2-sulfonohydrazide Chemical compound S1C(Cl)=CC=C1S(=O)(=O)NN(CC#C)C1=CC=CC=C1 QKRORLUXLQYKAA-UHFFFAOYSA-N 0.000 description 1
- GOFJDXZZHFNFLV-UHFFFAOYSA-N 5-fluoro-1,3-dimethyl-N-[2-(4-methylpentan-2-yl)phenyl]pyrazole-4-carboxamide Chemical compound CC(C)CC(C)C1=CC=CC=C1NC(=O)C1=C(F)N(C)N=C1C GOFJDXZZHFNFLV-UHFFFAOYSA-N 0.000 description 1
- CGRCPZUQMBYVPZ-UHFFFAOYSA-N 5-fluoro-2-[(4-methylphenyl)methoxy]pyrimidin-4-amine Chemical compound C1=CC(C)=CC=C1COC1=NC=C(F)C(N)=N1 CGRCPZUQMBYVPZ-UHFFFAOYSA-N 0.000 description 1
- 125000006043 5-hexenyl group Chemical group 0.000 description 1
- PCCSBWNGDMYFCW-UHFFFAOYSA-N 5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-1,3-oxazolidine-2,4-dione Chemical compound O=C1C(C)(C=2C=CC(OC=3C=CC=CC=3)=CC=2)OC(=O)N1NC1=CC=CC=C1 PCCSBWNGDMYFCW-UHFFFAOYSA-N 0.000 description 1
- GABNAHQQEVWYNS-UHFFFAOYSA-N 5-phenyl-2,3-dihydro-1,4-dithiine 1,1,4,4-tetraoxide Chemical compound O=S1(=O)CCS(=O)(=O)C(C=2C=CC=CC=2)=C1 GABNAHQQEVWYNS-UHFFFAOYSA-N 0.000 description 1
- VRODIKIAQUNGJI-UHFFFAOYSA-N 5-phenyl-4,5-dihydro-1,2-oxazole-3-carboxylic acid Chemical compound C1C(C(=O)O)=NOC1C1=CC=CC=C1 VRODIKIAQUNGJI-UHFFFAOYSA-N 0.000 description 1
- IBSREHMXUMOFBB-JFUDTMANSA-N 5u8924t11h Chemical compound O1[C@@H](C)[C@H](O)[C@@H](OC)C[C@@H]1O[C@@H]1[C@@H](OC)C[C@H](O[C@@H]2C(=C/C[C@@H]3C[C@@H](C[C@@]4(O3)C=C[C@H](C)[C@@H](C(C)C)O4)OC(=O)[C@@H]3C=C(C)[C@@H](O)[C@H]4OC\C([C@@]34O)=C/C=C/[C@@H]2C)/C)O[C@H]1C.C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 IBSREHMXUMOFBB-JFUDTMANSA-N 0.000 description 1
- QSERAAUJOZXWGA-UHFFFAOYSA-N 6-methylheptyl 2-(4-chloro-2-methylphenoxy)propanoate Chemical group CC(C)CCCCCOC(=O)C(C)OC1=CC=C(Cl)C=C1C QSERAAUJOZXWGA-UHFFFAOYSA-N 0.000 description 1
- VSVKOUBCDZYAQY-UHFFFAOYSA-N 7-chloro-1,2-benzothiazole Chemical compound ClC1=CC=CC2=C1SN=C2 VSVKOUBCDZYAQY-UHFFFAOYSA-N 0.000 description 1
- 230000002407 ATP formation Effects 0.000 description 1
- 241000219144 Abutilon Species 0.000 description 1
- 239000005651 Acequinocyl Substances 0.000 description 1
- 108010052875 Adenine deaminase Proteins 0.000 description 1
- 241000209758 Aegilops Species 0.000 description 1
- 241000589155 Agrobacterium tumefaciens Species 0.000 description 1
- 241000209136 Agropyron Species 0.000 description 1
- 241000743339 Agrostis Species 0.000 description 1
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Natural products NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 description 1
- MDBGGTQNNUOQRC-UHFFFAOYSA-N Allidochlor Chemical compound ClCC(=O)N(CC=C)CC=C MDBGGTQNNUOQRC-UHFFFAOYSA-N 0.000 description 1
- 235000005254 Allium ampeloprasum Nutrition 0.000 description 1
- 240000006108 Allium ampeloprasum Species 0.000 description 1
- 235000002732 Allium cepa var. cepa Nutrition 0.000 description 1
- 241000743985 Alopecurus Species 0.000 description 1
- 239000005877 Alpha-Cypermethrin Substances 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000482638 Amaranthus tuberculatus Species 0.000 description 1
- 239000005726 Ametoctradin Substances 0.000 description 1
- 239000005727 Amisulbrom Substances 0.000 description 1
- GEHMBYLTCISYNY-UHFFFAOYSA-N Ammonium sulfamate Chemical compound [NH4+].NS([O-])(=O)=O GEHMBYLTCISYNY-UHFFFAOYSA-N 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- 241001547866 Anoda Species 0.000 description 1
- 241000404028 Anthemis Species 0.000 description 1
- 241001666377 Apera Species 0.000 description 1
- 241000581616 Aphanes Species 0.000 description 1
- 240000007087 Apium graveolens Species 0.000 description 1
- 235000015849 Apium graveolens Dulce Group Nutrition 0.000 description 1
- 235000010591 Appio Nutrition 0.000 description 1
- 101100071444 Arabidopsis thaliana HSP90-5 gene Proteins 0.000 description 1
- 235000003911 Arachis Nutrition 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 235000003826 Artemisia Nutrition 0.000 description 1
- 235000003261 Artemisia vulgaris Nutrition 0.000 description 1
- 240000006891 Artemisia vulgaris Species 0.000 description 1
- 235000005340 Asparagus officinalis Nutrition 0.000 description 1
- 241000219305 Atriplex Species 0.000 description 1
- 235000007319 Avena orientalis Nutrition 0.000 description 1
- 235000007558 Avena sp Nutrition 0.000 description 1
- 235000000832 Ayote Nutrition 0.000 description 1
- 239000005878 Azadirachtin Substances 0.000 description 1
- 239000005730 Azoxystrobin Substances 0.000 description 1
- 241000193747 Bacillus firmus Species 0.000 description 1
- 241000132028 Bellis Species 0.000 description 1
- 239000005735 Benalaxyl-M Substances 0.000 description 1
- QGQSRQPXXMTJCM-UHFFFAOYSA-N Benfuresate Chemical compound CCS(=O)(=O)OC1=CC=C2OCC(C)(C)C2=C1 QGQSRQPXXMTJCM-UHFFFAOYSA-N 0.000 description 1
- PFJJMJDEVDLPNE-UHFFFAOYSA-N Benoxacor Chemical compound C1=CC=C2N(C(=O)C(Cl)Cl)C(C)COC2=C1 PFJJMJDEVDLPNE-UHFFFAOYSA-N 0.000 description 1
- 239000005472 Bensulfuron methyl Substances 0.000 description 1
- RRNIZKPFKNDSRS-UHFFFAOYSA-N Bensulide Chemical compound CC(C)OP(=S)(OC(C)C)SCCNS(=O)(=O)C1=CC=CC=C1 RRNIZKPFKNDSRS-UHFFFAOYSA-N 0.000 description 1
- 239000005736 Benthiavalicarb Substances 0.000 description 1
- 239000005737 Benzovindiflupyr Substances 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- 241000335053 Beta vulgaris Species 0.000 description 1
- 241000219310 Beta vulgaris subsp. vulgaris Species 0.000 description 1
- 239000005884 Beta-Cyfluthrin Substances 0.000 description 1
- 241000143476 Bidens Species 0.000 description 1
- 239000005653 Bifenazate Substances 0.000 description 1
- 239000005874 Bifenthrin Substances 0.000 description 1
- 239000005488 Bispyribac Substances 0.000 description 1
- 239000005738 Bixafen Substances 0.000 description 1
- 239000005739 Bordeaux mixture Substances 0.000 description 1
- 239000005740 Boscalid Substances 0.000 description 1
- 241000339490 Brachyachne Species 0.000 description 1
- 235000011331 Brassica Nutrition 0.000 description 1
- 235000011293 Brassica napus Nutrition 0.000 description 1
- 240000007124 Brassica oleracea Species 0.000 description 1
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 1
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 1
- 235000017647 Brassica oleracea var italica Nutrition 0.000 description 1
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 1
- 235000010149 Brassica rapa subsp chinensis Nutrition 0.000 description 1
- 235000000536 Brassica rapa subsp pekinensis Nutrition 0.000 description 1
- 241000499436 Brassica rapa subsp. pekinensis Species 0.000 description 1
- 239000005741 Bromuconazole Substances 0.000 description 1
- 241000209200 Bromus Species 0.000 description 1
- 239000005885 Buprofezin Substances 0.000 description 1
- BMTAFVWTTFSTOG-UHFFFAOYSA-N Butylate Chemical compound CCSC(=O)N(CC(C)C)CC(C)C BMTAFVWTTFSTOG-UHFFFAOYSA-N 0.000 description 1
- DZGJYZRGHITTMM-UHFFFAOYSA-N C(C)(=O)OC1=CC1.[Na] Chemical compound C(C)(=O)OC1=CC1.[Na] DZGJYZRGHITTMM-UHFFFAOYSA-N 0.000 description 1
- WLHSDSOCJVGOGT-UHFFFAOYSA-N C(C)(=O)OC1C=C1.[Na] Chemical compound C(C)(=O)OC1C=C1.[Na] WLHSDSOCJVGOGT-UHFFFAOYSA-N 0.000 description 1
- ZJNACCUTXGXPEE-HNNXBMFYSA-N C(C)(C)(C)C=1C=CC(=NC=1)OC=1C=C(O[C@H](C(=O)OCC#C)C)C=CC=1 Chemical compound C(C)(C)(C)C=1C=CC(=NC=1)OC=1C=C(O[C@H](C(=O)OCC#C)C)C=CC=1 ZJNACCUTXGXPEE-HNNXBMFYSA-N 0.000 description 1
- DTHLKBCRDIUBAF-UHFFFAOYSA-N C(C)N1C(C=2SC=3C(=NSC=3C#N)SC=2C1=O)=O Chemical compound C(C)N1C(C=2SC=3C(=NSC=3C#N)SC=2C1=O)=O DTHLKBCRDIUBAF-UHFFFAOYSA-N 0.000 description 1
- 125000004399 C1-C4 alkenyl group Chemical group 0.000 description 1
- 125000006577 C1-C6 hydroxyalkyl group Chemical group 0.000 description 1
- 125000004650 C1-C8 alkynyl group Chemical group 0.000 description 1
- 229910014585 C2-Ce Inorganic materials 0.000 description 1
- YAPDTLVTAQLZHQ-UHFFFAOYSA-N CC(C=C1C(C(C(CCC2)=O)=C2O)=O)=NN(C(C=C2)=CC(OC)=C2OC)C1=O Chemical compound CC(C=C1C(C(C(CCC2)=O)=C2O)=O)=NN(C(C=C2)=CC(OC)=C2OC)C1=O YAPDTLVTAQLZHQ-UHFFFAOYSA-N 0.000 description 1
- XFGBYNJAFZOFSQ-UHFFFAOYSA-N CC1=C(C=C(C(=C1)OC1=CC(=CC=C1)SC(C(F)(F)F)(F)F)C)N=CN(C)CC Chemical compound CC1=C(C=C(C(=C1)OC1=CC(=CC=C1)SC(C(F)(F)F)(F)F)C)N=CN(C)CC XFGBYNJAFZOFSQ-UHFFFAOYSA-N 0.000 description 1
- NUUXJNFQORMREK-UHFFFAOYSA-N CC1=C(C=C(C(=C1)SC1=CC(=CC=C1)OC(C(F)(F)F)(F)F)C)N=CN(C)CC Chemical compound CC1=C(C=C(C(=C1)SC1=CC(=CC=C1)OC(C(F)(F)F)(F)F)C)N=CN(C)CC NUUXJNFQORMREK-UHFFFAOYSA-N 0.000 description 1
- KKZFOFOWAVOUSF-UHFFFAOYSA-N CC1=CC(C)=NN1CC(C(Cl)=C(C=C1)C(C2=C(C3=C(C(O)=O)C(C)=NN3C)N(C)N=C2)=O)=C1S(C)(=O)=O Chemical compound CC1=CC(C)=NN1CC(C(Cl)=C(C=C1)C(C2=C(C3=C(C(O)=O)C(C)=NN3C)N(C)N=C2)=O)=C1S(C)(=O)=O KKZFOFOWAVOUSF-UHFFFAOYSA-N 0.000 description 1
- SSUFDOMYCBCHML-UHFFFAOYSA-N CCCCC[S](=O)=O Chemical group CCCCC[S](=O)=O SSUFDOMYCBCHML-UHFFFAOYSA-N 0.000 description 1
- XMSYZKCBUKZYIU-WKILWMFISA-N CCN(C)C=Nc1cc(Br)c(O[C@H]2CC[C@@H](CC2)C(C)C)nc1C Chemical compound CCN(C)C=Nc1cc(Br)c(O[C@H]2CC[C@@H](CC2)C(C)C)nc1C XMSYZKCBUKZYIU-WKILWMFISA-N 0.000 description 1
- XMSYZKCBUKZYIU-IYBDPMFKSA-N CCN(C)C=Nc1cc(Br)c(O[C@H]2CC[C@H](CC2)C(C)C)nc1C Chemical compound CCN(C)C=Nc1cc(Br)c(O[C@H]2CC[C@H](CC2)C(C)C)nc1C XMSYZKCBUKZYIU-IYBDPMFKSA-N 0.000 description 1
- QZAUYGXRMCIGIE-UHFFFAOYSA-N CCN(C)C=Nc1cc(C)c(Oc2cccc(SCC(F)(F)C(F)F)c2)cc1C Chemical compound CCN(C)C=Nc1cc(C)c(Oc2cccc(SCC(F)(F)C(F)F)c2)cc1C QZAUYGXRMCIGIE-UHFFFAOYSA-N 0.000 description 1
- GCSQWUIZJXIGNA-UHFFFAOYSA-N CCN(C)C=Nc1cc(C)c(Oc2cccc(SCC(F)(F)F)c2)cc1C Chemical compound CCN(C)C=Nc1cc(C)c(Oc2cccc(SCC(F)(F)F)c2)cc1C GCSQWUIZJXIGNA-UHFFFAOYSA-N 0.000 description 1
- MMINILZEVQHMLJ-UHFFFAOYSA-N CCN(C)C=Nc1cc(C)c(Sc2cccc(OCC(F)(F)C(F)F)c2)cc1C Chemical compound CCN(C)C=Nc1cc(C)c(Sc2cccc(OCC(F)(F)C(F)F)c2)cc1C MMINILZEVQHMLJ-UHFFFAOYSA-N 0.000 description 1
- JFLRKDZMHNBDQS-UCQUSYKYSA-N CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C(=C[C@H]3[C@@H]2CC(=O)O1)C)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C.CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C=C[C@H]3C2CC(=O)O1)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C Chemical compound CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C(=C[C@H]3[C@@H]2CC(=O)O1)C)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C.CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C=C[C@H]3C2CC(=O)O1)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C JFLRKDZMHNBDQS-UCQUSYKYSA-N 0.000 description 1
- LJSVEVMXBDRYAW-XGHSRDQLSA-N CC\C(/C=C/C(C(C)(C)C1)(C(C)=CC1=O)O)=C\C(O)=O Chemical compound CC\C(/C=C/C(C(C)(C)C1)(C(C)=CC1=O)O)=C\C(O)=O LJSVEVMXBDRYAW-XGHSRDQLSA-N 0.000 description 1
- RFRRFALLZDAMGS-UHFFFAOYSA-N COC(=O)C1=NC(=C(F)C(N)=C1Cl)C1=CC=C2C=CN(C(C)=O)C2=C1F Chemical compound COC(=O)C1=NC(=C(F)C(N)=C1Cl)C1=CC=C2C=CN(C(C)=O)C2=C1F RFRRFALLZDAMGS-UHFFFAOYSA-N 0.000 description 1
- VGAVFMUCIIYQFJ-UHFFFAOYSA-N CON=C(C(=O)NC)C(=CC)C Chemical compound CON=C(C(=O)NC)C(=CC)C VGAVFMUCIIYQFJ-UHFFFAOYSA-N 0.000 description 1
- 101150018129 CSF2 gene Proteins 0.000 description 1
- 101150069031 CSN2 gene Proteins 0.000 description 1
- 239000006009 Calcium phosphide Substances 0.000 description 1
- 241000220244 Capsella <angiosperm> Species 0.000 description 1
- 235000002566 Capsicum Nutrition 0.000 description 1
- 239000005745 Captan Substances 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 239000005746 Carboxin Substances 0.000 description 1
- 241000320316 Carduus Species 0.000 description 1
- 239000005492 Carfentrazone-ethyl Substances 0.000 description 1
- 239000005973 Carvone Substances 0.000 description 1
- 241000132570 Centaurea Species 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- 241000219312 Chenopodium Species 0.000 description 1
- 235000000509 Chenopodium ambrosioides Nutrition 0.000 description 1
- 244000098897 Chenopodium botrys Species 0.000 description 1
- 235000005490 Chenopodium botrys Nutrition 0.000 description 1
- 239000005886 Chlorantraniliprole Substances 0.000 description 1
- YJKIALIXRCSISK-UHFFFAOYSA-N Chlorfenprop-methyl Chemical group COC(=O)C(Cl)CC1=CC=C(Cl)C=C1 YJKIALIXRCSISK-UHFFFAOYSA-N 0.000 description 1
- 108010062745 Chloride Channels Proteins 0.000 description 1
- 102000011045 Chloride Channels Human genes 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 239000005647 Chlorpropham Substances 0.000 description 1
- 239000005945 Chlorpyrifos-methyl Substances 0.000 description 1
- 239000005887 Chromafenozide Substances 0.000 description 1
- 244000192528 Chrysanthemum parthenium Species 0.000 description 1
- 244000037364 Cinnamomum aromaticum Species 0.000 description 1
- 235000014489 Cinnamomum aromaticum Nutrition 0.000 description 1
- 241000132536 Cirsium Species 0.000 description 1
- 108091062157 Cis-regulatory element Proteins 0.000 description 1
- 244000241235 Citrullus lanatus Species 0.000 description 1
- 235000012828 Citrullus lanatus var citroides Nutrition 0.000 description 1
- LWOLGHMOQLJMIH-UHFFFAOYSA-N ClC1(C=C(NN1C1=CC=CC=C1)C(=O)O)Cl Chemical compound ClC1(C=C(NN1C1=CC=CC=C1)C(=O)O)Cl LWOLGHMOQLJMIH-UHFFFAOYSA-N 0.000 description 1
- PZOVUAFSUXEQII-UHFFFAOYSA-N ClC1=C(C(=O)C=2C(CCCC=2O)=O)C=CC(=C1CN1CCOCC1)S(=O)(=O)C Chemical compound ClC1=C(C(=O)C=2C(CCCC=2O)=O)C=CC(=C1CN1CCOCC1)S(=O)(=O)C PZOVUAFSUXEQII-UHFFFAOYSA-N 0.000 description 1
- 239000005654 Clofentezine Substances 0.000 description 1
- 239000005888 Clothianidin Substances 0.000 description 1
- 241000254173 Coleoptera Species 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 241000207892 Convolvulus Species 0.000 description 1
- JJLJMEJHUUYSSY-UHFFFAOYSA-L Copper hydroxide Chemical compound [OH-].[OH-].[Cu+2] JJLJMEJHUUYSSY-UHFFFAOYSA-L 0.000 description 1
- 239000005750 Copper hydroxide Substances 0.000 description 1
- QPLDLSVMHZLSFG-UHFFFAOYSA-N Copper oxide Chemical compound [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 description 1
- 239000005751 Copper oxide Substances 0.000 description 1
- 239000005752 Copper oxychloride Substances 0.000 description 1
- 235000015510 Cucumis melo subsp melo Nutrition 0.000 description 1
- 235000010071 Cucumis prophetarum Nutrition 0.000 description 1
- 240000008067 Cucumis sativus Species 0.000 description 1
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 1
- 241000219122 Cucurbita Species 0.000 description 1
- 240000004244 Cucurbita moschata Species 0.000 description 1
- 240000001980 Cucurbita pepo Species 0.000 description 1
- 235000009852 Cucurbita pepo Nutrition 0.000 description 1
- 235000009804 Cucurbita pepo subsp pepo Nutrition 0.000 description 1
- 239000005889 Cyantraniliprole Substances 0.000 description 1
- DFCAFRGABIXSDS-UHFFFAOYSA-N Cycloate Chemical compound CCSC(=O)N(CC)C1CCCCC1 DFCAFRGABIXSDS-UHFFFAOYSA-N 0.000 description 1
- 239000005655 Cyflumetofen Substances 0.000 description 1
- 239000005502 Cyhalofop-butyl Substances 0.000 description 1
- 239000005756 Cymoxanil Substances 0.000 description 1
- PYKLUAIDKVVEOS-UHFFFAOYSA-N Cyometrinil Chemical compound N#CCON=C(C#N)C1=CC=CC=C1 PYKLUAIDKVVEOS-UHFFFAOYSA-N 0.000 description 1
- 241000234653 Cyperus Species 0.000 description 1
- 239000005758 Cyprodinil Substances 0.000 description 1
- 239000005891 Cyromazine Substances 0.000 description 1
- 102100026846 Cytidine deaminase Human genes 0.000 description 1
- 108010031325 Cytidine deaminase Proteins 0.000 description 1
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 1
- YVGGHNCTFXOJCH-UHFFFAOYSA-N DDT Chemical compound C1=CC(Cl)=CC=C1C(C(Cl)(Cl)Cl)C1=CC=C(Cl)C=C1 YVGGHNCTFXOJCH-UHFFFAOYSA-N 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 241000320605 Dactyloctenium Species 0.000 description 1
- NDUPDOJHUQKPAG-UHFFFAOYSA-N Dalapon Chemical compound CC(Cl)(Cl)C(O)=O NDUPDOJHUQKPAG-UHFFFAOYSA-N 0.000 description 1
- 241000208296 Datura Species 0.000 description 1
- 241000208175 Daucus Species 0.000 description 1
- 235000002767 Daucus carota Nutrition 0.000 description 1
- 244000000626 Daucus carota Species 0.000 description 1
- 208000006558 Dental Calculus Diseases 0.000 description 1
- 244000147058 Derris elliptica Species 0.000 description 1
- 241000522190 Desmodium Species 0.000 description 1
- 239000005505 Dichlorprop-P Substances 0.000 description 1
- 239000005760 Difenoconazole Substances 0.000 description 1
- LBGPXIPGGRQBJW-UHFFFAOYSA-N Difenzoquat Chemical compound C[N+]=1N(C)C(C=2C=CC=CC=2)=CC=1C1=CC=CC=C1 LBGPXIPGGRQBJW-UHFFFAOYSA-N 0.000 description 1
- 239000005893 Diflubenzuron Substances 0.000 description 1
- 235000017896 Digitaria Nutrition 0.000 description 1
- 241001303487 Digitaria <clam> Species 0.000 description 1
- 239000005509 Dimethenamid-P Substances 0.000 description 1
- 239000005947 Dimethoate Substances 0.000 description 1
- 239000005761 Dimethomorph Substances 0.000 description 1
- 239000005762 Dimoxystrobin Substances 0.000 description 1
- IIPZYDQGBIWLBU-UHFFFAOYSA-N Dinoterb Chemical compound CC(C)(C)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1O IIPZYDQGBIWLBU-UHFFFAOYSA-N 0.000 description 1
- QAHFOPIILNICLA-UHFFFAOYSA-N Diphenamid Chemical compound C=1C=CC=CC=1C(C(=O)N(C)C)C1=CC=CC=C1 QAHFOPIILNICLA-UHFFFAOYSA-N 0.000 description 1
- 239000005764 Dithianon Substances 0.000 description 1
- 239000005766 Dodine Substances 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- AIGRXSNSLVJMEA-UHFFFAOYSA-N EPN Chemical compound C=1C=CC=CC=1P(=S)(OCC)OC1=CC=C([N+]([O-])=O)C=C1 AIGRXSNSLVJMEA-UHFFFAOYSA-N 0.000 description 1
- GUVLYNGULCJVDO-UHFFFAOYSA-N EPTC Chemical compound CCCN(CCC)C(=O)SCC GUVLYNGULCJVDO-UHFFFAOYSA-N 0.000 description 1
- 241000192043 Echinochloa Species 0.000 description 1
- 241000202829 Eleocharis Species 0.000 description 1
- 241000209215 Eleusine Species 0.000 description 1
- 235000007351 Eleusine Nutrition 0.000 description 1
- 235000006369 Emex spinosa Nutrition 0.000 description 1
- 244000294661 Emex spinosa Species 0.000 description 1
- YUGWDVYLFSETPE-JLHYYAGUSA-N Empenthrin Chemical compound CC\C=C(/C)C(C#C)OC(=O)C1C(C=C(C)C)C1(C)C YUGWDVYLFSETPE-JLHYYAGUSA-N 0.000 description 1
- 241000588697 Enterobacter cloacae Species 0.000 description 1
- 239000005767 Epoxiconazole Substances 0.000 description 1
- 241001518935 Eragrostis Species 0.000 description 1
- 241000044408 Eriochloa Species 0.000 description 1
- 241001071608 Erodium Species 0.000 description 1
- 241000919496 Erysimum Species 0.000 description 1
- 239000005895 Esfenvalerate Substances 0.000 description 1
- FNELVJVBIYMIMC-UHFFFAOYSA-N Ethiprole Chemical compound N1=C(C#N)C(S(=O)CC)=C(N)N1C1=C(Cl)C=C(C(F)(F)F)C=C1Cl FNELVJVBIYMIMC-UHFFFAOYSA-N 0.000 description 1
- 239000005512 Ethofumesate Substances 0.000 description 1
- ICWUMLXQKFTJMH-UHFFFAOYSA-N Etobenzanid Chemical compound C1=CC(OCOCC)=CC=C1C(=O)NC1=CC=CC(Cl)=C1Cl ICWUMLXQKFTJMH-UHFFFAOYSA-N 0.000 description 1
- 239000005896 Etofenprox Substances 0.000 description 1
- 239000005897 Etoxazole Substances 0.000 description 1
- 241001473317 Eupatorium cannabinum Species 0.000 description 1
- 241000221079 Euphorbia <genus> Species 0.000 description 1
- 108010046276 FLP recombinase Proteins 0.000 description 1
- 239000005656 Fenazaquin Substances 0.000 description 1
- GMBRUAIJEFRHFQ-UHFFFAOYSA-N Fenchlorazole-ethyl Chemical group N1=C(C(=O)OCC)N=C(C(Cl)(Cl)Cl)N1C1=CC=C(Cl)C=C1Cl GMBRUAIJEFRHFQ-UHFFFAOYSA-N 0.000 description 1
- 239000005776 Fenhexamid Substances 0.000 description 1
- PQKBPHSEKWERTG-UHFFFAOYSA-N Fenoxaprop ethyl Chemical group C1=CC(OC(C)C(=O)OCC)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 PQKBPHSEKWERTG-UHFFFAOYSA-N 0.000 description 1
- 239000005513 Fenoxaprop-P Substances 0.000 description 1
- 239000005898 Fenoxycarb Substances 0.000 description 1
- 239000005777 Fenpropidin Substances 0.000 description 1
- 239000005779 Fenpyrazamine Substances 0.000 description 1
- 239000005657 Fenpyroximate Substances 0.000 description 1
- 241000234642 Festuca Species 0.000 description 1
- 241001290564 Fimbristylis Species 0.000 description 1
- YQVMVCCFZCMYQB-SNVBAGLBSA-N Flamprop-M Chemical compound C=1C=C(F)C(Cl)=CC=1N([C@H](C)C(O)=O)C(=O)C1=CC=CC=C1 YQVMVCCFZCMYQB-SNVBAGLBSA-N 0.000 description 1
- 239000005900 Flonicamid Substances 0.000 description 1
- 239000005530 Fluazifop-P Substances 0.000 description 1
- 239000005780 Fluazinam Substances 0.000 description 1
- 239000005901 Flubendiamide Substances 0.000 description 1
- MNFMIVVPXOGUMX-UHFFFAOYSA-N Fluchloralin Chemical compound CCCN(CCCl)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O MNFMIVVPXOGUMX-UHFFFAOYSA-N 0.000 description 1
- 239000005781 Fludioxonil Substances 0.000 description 1
- 239000005784 Fluoxastrobin Substances 0.000 description 1
- PXRROZVNOOEPPZ-UHFFFAOYSA-N Flupropanate Chemical compound OC(=O)C(F)(F)C(F)F PXRROZVNOOEPPZ-UHFFFAOYSA-N 0.000 description 1
- 239000005902 Flupyradifurone Substances 0.000 description 1
- 239000005785 Fluquinconazole Substances 0.000 description 1
- AJKQZRAAQMBNKM-UHFFFAOYSA-N Flurenol methyl ester Chemical group C1=CC=C2C(C(=O)OC)(O)C3=CC=CC=C3C2=C1 AJKQZRAAQMBNKM-UHFFFAOYSA-N 0.000 description 1
- 239000005786 Flutolanil Substances 0.000 description 1
- 239000005787 Flutriafol Substances 0.000 description 1
- 239000005788 Fluxapyroxad Substances 0.000 description 1
- 239000005948 Formetanate Substances 0.000 description 1
- 239000005790 Fosetyl Substances 0.000 description 1
- 239000005959 Fosthiazate Substances 0.000 description 1
- 241000816457 Galeopsis Species 0.000 description 1
- 241000748465 Galinsoga Species 0.000 description 1
- 241001101998 Galium Species 0.000 description 1
- 239000005903 Gamma-cyhalothrin Substances 0.000 description 1
- 241000208152 Geranium Species 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 235000009438 Gossypium Nutrition 0.000 description 1
- 108020005004 Guide RNA Proteins 0.000 description 1
- 239000005563 Halauxifen-methyl Substances 0.000 description 1
- 239000005564 Halosulfuron methyl Substances 0.000 description 1
- 239000005565 Haloxyfop-P Substances 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- 241000208818 Helianthus Species 0.000 description 1
- 244000020551 Helianthus annuus Species 0.000 description 1
- 235000003222 Helianthus annuus Nutrition 0.000 description 1
- 241000258937 Hemiptera Species 0.000 description 1
- 239000005661 Hexythiazox Substances 0.000 description 1
- 235000005206 Hibiscus Nutrition 0.000 description 1
- 235000007185 Hibiscus lunariifolius Nutrition 0.000 description 1
- 244000284380 Hibiscus rosa sinensis Species 0.000 description 1
- 241000209219 Hordeum Species 0.000 description 1
- 240000005979 Hordeum vulgare Species 0.000 description 1
- 235000007340 Hordeum vulgare Nutrition 0.000 description 1
- RFZZKBWDDKMWNM-UHFFFAOYSA-N Hydantocidin Natural products OC1C(O)C(CO)OC11C(=O)NC(=O)N1 RFZZKBWDDKMWNM-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 241000169108 Hydrothrix Species 0.000 description 1
- 241000257303 Hymenoptera Species 0.000 description 1
- 239000005795 Imazalil Substances 0.000 description 1
- PPCUNNLZTNMXFO-ACCUITESSA-N Imicyafos Chemical compound CCCSP(=O)(OCC)N1CCN(CC)\C1=N/C#N PPCUNNLZTNMXFO-ACCUITESSA-N 0.000 description 1
- 239000005907 Indoxacarb Substances 0.000 description 1
- 239000005796 Ipconazole Substances 0.000 description 1
- 239000005798 Isofetamid Substances 0.000 description 1
- UQVYUTAMNICZNI-UHFFFAOYSA-N Karbutilate Chemical compound CN(C)C(=O)NC1=CC=CC(NC(=O)OC(C)(C)C)=C1 UQVYUTAMNICZNI-UHFFFAOYSA-N 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241000110847 Kochia Species 0.000 description 1
- 239000005800 Kresoxim-methyl Substances 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 241000208822 Lactuca Species 0.000 description 1
- 235000003228 Lactuca sativa Nutrition 0.000 description 1
- 240000008415 Lactuca sativa Species 0.000 description 1
- 241000520028 Lamium Species 0.000 description 1
- 241000801118 Lepidium Species 0.000 description 1
- 241000255777 Lepidoptera Species 0.000 description 1
- 241000320639 Leptochloa Species 0.000 description 1
- 241000064140 Lindernia Species 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-N Linoleic acid Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(O)=O OYHQOLUKZRVURQ-HZJYTTRNSA-N 0.000 description 1
- 241000208204 Linum Species 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 241000209082 Lolium Species 0.000 description 1
- 239000005912 Lufenuron Substances 0.000 description 1
- 235000002262 Lycopersicon Nutrition 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 241000193386 Lysinibacillus sphaericus Species 0.000 description 1
- 239000005949 Malathion Substances 0.000 description 1
- 239000005802 Mancozeb Substances 0.000 description 1
- 239000005804 Mandipropamid Substances 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000017945 Matricaria Nutrition 0.000 description 1
- 235000007232 Matricaria chamomilla Nutrition 0.000 description 1
- 239000005576 Mecoprop-P Substances 0.000 description 1
- 240000004658 Medicago sativa Species 0.000 description 1
- 235000017587 Medicago sativa ssp. sativa Nutrition 0.000 description 1
- OKIBNKKYNPBDRS-UHFFFAOYSA-N Mefluidide Chemical compound CC(=O)NC1=CC(NS(=O)(=O)C(F)(F)F)=C(C)C=C1C OKIBNKKYNPBDRS-UHFFFAOYSA-N 0.000 description 1
- 235000014435 Mentha Nutrition 0.000 description 1
- 241001072983 Mentha Species 0.000 description 1
- 239000005806 Meptyldinocap Substances 0.000 description 1
- 241000221024 Mercurialis Species 0.000 description 1
- 239000005914 Metaflumizone Substances 0.000 description 1
- MIFOMMKAVSCNKQ-HWIUFGAZSA-N Metaflumizone Chemical compound C1=CC(OC(F)(F)F)=CC=C1NC(=O)N\N=C(C=1C=C(C=CC=1)C(F)(F)F)\CC1=CC=C(C#N)C=C1 MIFOMMKAVSCNKQ-HWIUFGAZSA-N 0.000 description 1
- 239000005807 Metalaxyl Substances 0.000 description 1
- 239000005808 Metalaxyl-M Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 239000005868 Metconazole Substances 0.000 description 1
- 239000005916 Methomyl Substances 0.000 description 1
- 239000005917 Methoxyfenozide Substances 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 239000005809 Metiram Substances 0.000 description 1
- 239000005584 Metsulfuron-methyl Substances 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- 239000005918 Milbemectin Substances 0.000 description 1
- 102000013379 Mitochondrial Proton-Translocating ATPases Human genes 0.000 description 1
- 108010026155 Mitochondrial Proton-Translocating ATPases Proteins 0.000 description 1
- SOWBFZRMHSNYGE-UHFFFAOYSA-N Monoamide-Oxalic acid Natural products NC(=O)C(O)=O SOWBFZRMHSNYGE-UHFFFAOYSA-N 0.000 description 1
- 235000003990 Monochoria hastata Nutrition 0.000 description 1
- 240000000178 Monochoria vaginalis Species 0.000 description 1
- 101100494762 Mus musculus Nedd9 gene Proteins 0.000 description 1
- 239000005811 Myclobutanil Substances 0.000 description 1
- 241001442129 Myosotis Species 0.000 description 1
- WXZVAROIGSFCFJ-UHFFFAOYSA-N N,N-diethyl-2-(naphthalen-1-yloxy)propanamide Chemical compound C1=CC=C2C(OC(C)C(=O)N(CC)CC)=CC=CC2=C1 WXZVAROIGSFCFJ-UHFFFAOYSA-N 0.000 description 1
- YJHRKNSAKJZXBO-UHFFFAOYSA-N N-[2-(3-thiophen-2-yl-2H-quinoxalin-1-yl)ethyl]methanesulfonamide Chemical compound CS(=O)(=O)NCCN1CC(=NC2=CC=CC=C12)C=1SC=CC=1 YJHRKNSAKJZXBO-UHFFFAOYSA-N 0.000 description 1
- CCCGEKHKTPTUHJ-UHFFFAOYSA-N N-[9-(dichloromethylene)-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-1-methylpyrazole-4-carboxamide Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC2=C1C1CCC2C1=C(Cl)Cl CCCGEKHKTPTUHJ-UHFFFAOYSA-N 0.000 description 1
- FHKZNLSPTAEYSH-UHFFFAOYSA-N N-[[2-(4,6-dimethoxypyrimidin-2-yl)oxyphenyl]methyl]aniline Chemical compound COC1=NC(=NC(=C1)OC)OC1=C(CNC2=CC=CC=C2)C=CC=C1 FHKZNLSPTAEYSH-UHFFFAOYSA-N 0.000 description 1
- NQRFDNJEBWAUBL-UHFFFAOYSA-N N-[cyano(2-thienyl)methyl]-4-ethyl-2-(ethylamino)-1,3-thiazole-5-carboxamide Chemical compound S1C(NCC)=NC(CC)=C1C(=O)NC(C#N)C1=CC=CS1 NQRFDNJEBWAUBL-UHFFFAOYSA-N 0.000 description 1
- BFFLCMLOXQNHJA-LJQANCHMSA-N N-[ethoxy-(4-methyl-2-nitrophenoxy)phosphinothioyl]propan-2-amine Chemical compound CCO[P@](=S)(NC(C)C)Oc1ccc(C)cc1[N+]([O-])=O BFFLCMLOXQNHJA-LJQANCHMSA-N 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- XQJQCBDIXRIYRP-UHFFFAOYSA-N N-{2-[1,1'-bi(cyclopropyl)-2-yl]phenyl}-3-(difluoromethyl)-1-methyl-1pyrazole-4-carboxamide Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1C1C(C2CC2)C1 XQJQCBDIXRIYRP-UHFFFAOYSA-N 0.000 description 1
- 101710198130 NADPH-cytochrome P450 reductase Proteins 0.000 description 1
- YZZZUTGJDODTAJ-UHFFFAOYSA-N NC1=C(C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC)Cl Chemical compound NC1=C(C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC)Cl YZZZUTGJDODTAJ-UHFFFAOYSA-N 0.000 description 1
- WNWPZIPVRMSQIH-UHFFFAOYSA-N NC1=C(Cl)C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC1=CC=CC=C1 Chemical compound NC1=C(Cl)C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC1=CC=CC=C1 WNWPZIPVRMSQIH-UHFFFAOYSA-N 0.000 description 1
- 239000005585 Napropamide Substances 0.000 description 1
- 241000169176 Natronobacterium gregoryi Species 0.000 description 1
- 101100385413 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) csm-3 gene Proteins 0.000 description 1
- 241000208125 Nicotiana Species 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- VJAWBEFMCIINFU-UHFFFAOYSA-N Nitrothal-isopropyl Chemical group CC(C)OC(=O)C1=CC(C(=O)OC(C)C)=CC([N+]([O-])=O)=C1 VJAWBEFMCIINFU-UHFFFAOYSA-N 0.000 description 1
- 238000000636 Northern blotting Methods 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 241000238814 Orthoptera Species 0.000 description 1
- WFVUIONFJOAYPK-KAMYIIQDSA-N Oxabetrinil Chemical compound C=1C=CC=CC=1C(/C#N)=N\OCC1OCCO1 WFVUIONFJOAYPK-KAMYIIQDSA-N 0.000 description 1
- 239000005950 Oxamyl Substances 0.000 description 1
- 239000005812 Oxathiapiprolin Substances 0.000 description 1
- YXLXNENXOJSQEI-UHFFFAOYSA-L Oxine-copper Chemical compound [Cu+2].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 YXLXNENXOJSQEI-UHFFFAOYSA-L 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 239000005985 Paclobutrazol Substances 0.000 description 1
- 235000011096 Papaver Nutrition 0.000 description 1
- 240000001090 Papaver somniferum Species 0.000 description 1
- 241001268782 Paspalum dilatatum Species 0.000 description 1
- SGEJQUSYQTVSIU-UHFFFAOYSA-N Pebulate Chemical compound CCCCN(CC)C(=O)SCCC SGEJQUSYQTVSIU-UHFFFAOYSA-N 0.000 description 1
- 239000005815 Penflufen Substances 0.000 description 1
- 239000005816 Penthiopyrad Substances 0.000 description 1
- 239000006002 Pepper Substances 0.000 description 1
- 239000005593 Pethoxamid Substances 0.000 description 1
- 240000007377 Petunia x hybrida Species 0.000 description 1
- 241000745991 Phalaris Species 0.000 description 1
- 241000219833 Phaseolus Species 0.000 description 1
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 1
- 244000046052 Phaseolus vulgaris Species 0.000 description 1
- 241000746981 Phleum Species 0.000 description 1
- 239000005921 Phosmet Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 239000005818 Picoxystrobin Substances 0.000 description 1
- 235000016761 Piper aduncum Nutrition 0.000 description 1
- 240000003889 Piper guineense Species 0.000 description 1
- 235000017804 Piper guineense Nutrition 0.000 description 1
- 235000008184 Piper nigrum Nutrition 0.000 description 1
- 239000005923 Pirimicarb Substances 0.000 description 1
- 239000005924 Pirimiphos-methyl Substances 0.000 description 1
- 108010064851 Plant Proteins Proteins 0.000 description 1
- 241001127637 Plantago Species 0.000 description 1
- 241000209048 Poa Species 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 241000205407 Polygonum Species 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- 241000219295 Portulaca Species 0.000 description 1
- RSVPPPHXAASNOL-UHFFFAOYSA-N Prodiamine Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C(N)=C1[N+]([O-])=O RSVPPPHXAASNOL-UHFFFAOYSA-N 0.000 description 1
- IPDFPNNPBMREIF-CHWSQXEVSA-N Prohydrojasmon Chemical compound CCCCC[C@@H]1[C@@H](CC(=O)OCCC)CCC1=O IPDFPNNPBMREIF-CHWSQXEVSA-N 0.000 description 1
- ZGUGWUXLJSTTMA-UHFFFAOYSA-N Promazinum Chemical compound C1=CC=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZGUGWUXLJSTTMA-UHFFFAOYSA-N 0.000 description 1
- MKIMSXGUTQTKJU-UHFFFAOYSA-N Propamocarb hydrochloride Chemical compound [Cl-].CCCOC(=O)NCCC[NH+](C)C MKIMSXGUTQTKJU-UHFFFAOYSA-N 0.000 description 1
- 239000005822 Propiconazole Substances 0.000 description 1
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 1
- 239000005824 Proquinazid Substances 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 239000005700 Putrescine Substances 0.000 description 1
- 239000005925 Pymetrozine Substances 0.000 description 1
- 239000005869 Pyraclostrobin Substances 0.000 description 1
- 239000005663 Pyridaben Substances 0.000 description 1
- 239000005926 Pyridalyl Substances 0.000 description 1
- 239000005829 Pyriofenone Substances 0.000 description 1
- 239000005927 Pyriproxyfen Substances 0.000 description 1
- 241000205156 Pyrococcus furiosus Species 0.000 description 1
- OBLNWSCLAYSJJR-UHFFFAOYSA-N Quinoclamin Chemical compound C1=CC=C2C(=O)C(N)=C(Cl)C(=O)C2=C1 OBLNWSCLAYSJJR-UHFFFAOYSA-N 0.000 description 1
- 239000002167 Quinoclamine Substances 0.000 description 1
- 239000005831 Quinoxyfen Substances 0.000 description 1
- 239000005614 Quizalofop-P-ethyl Substances 0.000 description 1
- 239000005615 Quizalofop-P-tefuryl Substances 0.000 description 1
- 241000218206 Ranunculus Species 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- 235000006140 Raphanus sativus var sativus Nutrition 0.000 description 1
- ISRUGXGCCGIOQO-UHFFFAOYSA-N Rhoden Chemical compound CNC(=O)OC1=CC=CC=C1OC(C)C ISRUGXGCCGIOQO-UHFFFAOYSA-N 0.000 description 1
- 241000490453 Rorippa Species 0.000 description 1
- 241000341978 Rotala Species 0.000 description 1
- 241000857233 Rottboellia Species 0.000 description 1
- 241000219053 Rumex Species 0.000 description 1
- 102000001424 Ryanodine receptors Human genes 0.000 description 1
- ZJUKTBDSGOFHSH-WFMPWKQPSA-N S-Adenosylhomocysteine Chemical compound O[C@@H]1[C@H](O)[C@@H](CSCC[C@H](N)C(O)=O)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZJUKTBDSGOFHSH-WFMPWKQPSA-N 0.000 description 1
- OKUGPJPKMAEJOE-UHFFFAOYSA-N S-propyl dipropylcarbamothioate Chemical compound CCCSC(=O)N(CCC)CCC OKUGPJPKMAEJOE-UHFFFAOYSA-N 0.000 description 1
- 241000209051 Saccharum Species 0.000 description 1
- 240000000111 Saccharum officinarum Species 0.000 description 1
- 235000007201 Saccharum officinarum Nutrition 0.000 description 1
- 240000009132 Sagittaria sagittifolia Species 0.000 description 1
- 241001632050 Salsola Species 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 241000202758 Scirpus Species 0.000 description 1
- BTANRVKWQNVYAZ-UHFFFAOYSA-N Sec-butyl alcohol Natural products CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 1
- 241000209056 Secale Species 0.000 description 1
- 241000228160 Secale cereale x Triticum aestivum Species 0.000 description 1
- 239000005834 Sedaxane Substances 0.000 description 1
- 241000780602 Senecio Species 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 244000275012 Sesbania cannabina Species 0.000 description 1
- 235000005775 Setaria Nutrition 0.000 description 1
- 241000232088 Setaria <nematode> Species 0.000 description 1
- 239000005835 Silthiofam Substances 0.000 description 1
- 241000220261 Sinapis Species 0.000 description 1
- 108010052164 Sodium Channels Proteins 0.000 description 1
- 102000018674 Sodium Channels Human genes 0.000 description 1
- 235000002597 Solanum melongena Nutrition 0.000 description 1
- 244000061458 Solanum melongena Species 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 241000488874 Sonchus Species 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- 235000009337 Spinacia oleracea Nutrition 0.000 description 1
- 244000300264 Spinacia oleracea Species 0.000 description 1
- 239000005929 Spinetoram Substances 0.000 description 1
- GOENIMGKWNZVDA-OAMCMWGQSA-N Spinetoram Chemical compound CO[C@@H]1[C@H](OCC)[C@@H](OC)[C@H](C)O[C@H]1OC1C[C@H]2[C@@H]3C=C4C(=O)[C@H](C)[C@@H](O[C@@H]5O[C@H](C)[C@H](CC5)N(C)C)CCC[C@H](CC)OC(=O)CC4[C@@H]3CC[C@@H]2C1 GOENIMGKWNZVDA-OAMCMWGQSA-N 0.000 description 1
- 239000005930 Spinosad Substances 0.000 description 1
- 239000005664 Spirodiclofen Substances 0.000 description 1
- 239000005665 Spiromesifen Substances 0.000 description 1
- 239000005931 Spirotetramat Substances 0.000 description 1
- 240000006694 Stellaria media Species 0.000 description 1
- 235000021536 Sugar beet Nutrition 0.000 description 1
- 239000005934 Sulfoxaflor Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 239000005935 Sulfuryl fluoride Substances 0.000 description 1
- SAQSTQBVENFSKT-UHFFFAOYSA-M TCA-sodium Chemical compound [Na+].[O-]C(=O)C(Cl)(Cl)Cl SAQSTQBVENFSKT-UHFFFAOYSA-M 0.000 description 1
- 102000003563 TRPV Human genes 0.000 description 1
- 108060008564 TRPV Proteins 0.000 description 1
- 240000004460 Tanacetum coccineum Species 0.000 description 1
- 241000245665 Taraxacum Species 0.000 description 1
- 239000005937 Tebufenozide Substances 0.000 description 1
- 239000005658 Tebufenpyrad Substances 0.000 description 1
- 239000005938 Teflubenzuron Substances 0.000 description 1
- 239000005840 Tetraconazole Substances 0.000 description 1
- 229920002359 Tetronic® Polymers 0.000 description 1
- 239000005941 Thiamethoxam Substances 0.000 description 1
- QHTQREMOGMZHJV-UHFFFAOYSA-N Thiobencarb Chemical compound CCN(CC)C(=O)SCC1=CC=C(Cl)C=C1 QHTQREMOGMZHJV-UHFFFAOYSA-N 0.000 description 1
- GNVMUORYQLCPJZ-UHFFFAOYSA-M Thiocarbamate Chemical compound NC([S-])=O GNVMUORYQLCPJZ-UHFFFAOYSA-M 0.000 description 1
- 241001061127 Thione Species 0.000 description 1
- 239000005842 Thiophanate-methyl Substances 0.000 description 1
- 239000005843 Thiram Substances 0.000 description 1
- 241000722118 Thlaspi Species 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 241001414989 Thysanoptera Species 0.000 description 1
- 239000005845 Tolclofos-methyl Substances 0.000 description 1
- 239000005942 Triflumuron Substances 0.000 description 1
- 239000005628 Triflusulfuron Substances 0.000 description 1
- 241000219793 Trifolium Species 0.000 description 1
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 1
- 235000019714 Triticale Nutrition 0.000 description 1
- 239000005859 Triticonazole Substances 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 108091023045 Untranslated Region Proteins 0.000 description 1
- ICMGLRUYEQNHPF-UHFFFAOYSA-N Uraprene Chemical compound COC1=CC=CC=C1N1CCN(CCCNC=2N(C(=O)N(C)C(=O)C=2)C)CC1 ICMGLRUYEQNHPF-UHFFFAOYSA-N 0.000 description 1
- 241000219422 Urtica Species 0.000 description 1
- 239000005860 Valifenalate Substances 0.000 description 1
- 241000219873 Vicia Species 0.000 description 1
- CVQODEWAPZVVBU-UHFFFAOYSA-N XMC Chemical compound CNC(=O)OC1=CC(C)=CC(C)=C1 CVQODEWAPZVVBU-UHFFFAOYSA-N 0.000 description 1
- 241001506766 Xanthium Species 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 241000209149 Zea Species 0.000 description 1
- 235000007244 Zea mays Nutrition 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 239000006011 Zinc phosphide Substances 0.000 description 1
- 239000005870 Ziram Substances 0.000 description 1
- 239000005863 Zoxamide Substances 0.000 description 1
- GBAWQJNHVWMTLU-RQJHMYQMSA-N [(1R,5S)-7-chloro-6-bicyclo[3.2.0]hepta-2,6-dienyl] dimethyl phosphate Chemical compound C1=CC[C@@H]2C(OP(=O)(OC)OC)=C(Cl)[C@@H]21 GBAWQJNHVWMTLU-RQJHMYQMSA-N 0.000 description 1
- VXSIXFKKSNGRRO-MXOVTSAMSA-N [(1s)-2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate;[(1s)-2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-3-[(e)-3-methoxy-2-methyl-3-oxoprop-1-enyl Chemical class CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1.CC1(C)[C@H](/C=C(\C)C(=O)OC)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1 VXSIXFKKSNGRRO-MXOVTSAMSA-N 0.000 description 1
- LUZZPGJQJKMMDM-JTQLQIEISA-N [(2s)-1-ethoxy-1-oxopropan-2-yl] 2-chloro-5-[2-chloro-4-(trifluoromethyl)phenoxy]benzoate Chemical group C1=C(Cl)C(C(=O)O[C@@H](C)C(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 LUZZPGJQJKMMDM-JTQLQIEISA-N 0.000 description 1
- QQODLKZGRKWIFG-RUTXASTPSA-N [(R)-cyano-(4-fluoro-3-phenoxyphenyl)methyl] (1S)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate Chemical compound CC1(C)C(C=C(Cl)Cl)[C@@H]1C(=O)O[C@@H](C#N)C1=CC=C(F)C(OC=2C=CC=CC=2)=C1 QQODLKZGRKWIFG-RUTXASTPSA-N 0.000 description 1
- FZSVSABTBYGOQH-XFFZJAGNSA-N [(e)-(3,3-dimethyl-1-methylsulfanylbutan-2-ylidene)amino] n-methylcarbamate Chemical compound CNC(=O)O\N=C(C(C)(C)C)\CSC FZSVSABTBYGOQH-XFFZJAGNSA-N 0.000 description 1
- CTJBHIROCMPUKL-WEVVVXLNSA-N [(e)-3-methylsulfonylbutan-2-ylideneamino] n-methylcarbamate Chemical compound CNC(=O)O\N=C(/C)C(C)S(C)(=O)=O CTJBHIROCMPUKL-WEVVVXLNSA-N 0.000 description 1
- BZMIHNKNQJJVRO-LVZFUZTISA-N [(e)-c-(3-chloro-2,6-dimethoxyphenyl)-n-ethoxycarbonimidoyl] benzoate Chemical compound COC=1C=CC(Cl)=C(OC)C=1C(=N/OCC)\OC(=O)C1=CC=CC=C1 BZMIHNKNQJJVRO-LVZFUZTISA-N 0.000 description 1
- KAATUXNTWXVJKI-QPIRBTGLSA-N [(s)-cyano-(3-phenoxyphenyl)methyl] 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate Chemical compound CC1(C)C(C=C(Cl)Cl)C1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 KAATUXNTWXVJKI-QPIRBTGLSA-N 0.000 description 1
- FSAVDKDHPDSCTO-WQLSENKSSA-N [(z)-2-chloro-1-(2,4-dichlorophenyl)ethenyl] diethyl phosphate Chemical compound CCOP(=O)(OCC)O\C(=C/Cl)C1=CC=C(Cl)C=C1Cl FSAVDKDHPDSCTO-WQLSENKSSA-N 0.000 description 1
- QSGNQELHULIMSJ-POHAHGRESA-N [(z)-2-chloro-1-(2,4-dichlorophenyl)ethenyl] dimethyl phosphate Chemical compound COP(=O)(OC)O\C(=C/Cl)C1=CC=C(Cl)C=C1Cl QSGNQELHULIMSJ-POHAHGRESA-N 0.000 description 1
- AMRQXHFXNZFDCH-UHFFFAOYSA-N [1-(ethylamino)-1-oxopropan-2-yl] n-phenylcarbamate Chemical compound CCNC(=O)C(C)OC(=O)NC1=CC=CC=C1 AMRQXHFXNZFDCH-UHFFFAOYSA-N 0.000 description 1
- DPJITPZADZSLBP-PIPQINALSA-N [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl (1r,3r)-3-[(e)-2-cyanoprop-1-enyl]-2,2-dimethylcyclopropane-1-carboxylate Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)[C@H]1C(C)(C)[C@@H]1\C=C(/C)C#N DPJITPZADZSLBP-PIPQINALSA-N 0.000 description 1
- MWFQAAWRPDRKDG-KOLCDFICSA-N [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl (1r,3s)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)[C@H]1C(C)(C)[C@@H]1C=C(Cl)Cl MWFQAAWRPDRKDG-KOLCDFICSA-N 0.000 description 1
- APEPLROGLDYWBS-UHFFFAOYSA-N [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl 2,2,3,3-tetramethylcyclopropane-1-carboxylate Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)C1C(C)(C)C1(C)C APEPLROGLDYWBS-UHFFFAOYSA-N 0.000 description 1
- FMPFURNXXAKYNE-UHFFFAOYSA-N [2-ethyl-3,7-dimethyl-6-[4-(trifluoromethoxy)phenoxy]quinolin-4-yl] methyl carbonate Chemical compound C1=C2C(OC(=O)OC)=C(C)C(CC)=NC2=CC(C)=C1OC1=CC=C(OC(F)(F)F)C=C1 FMPFURNXXAKYNE-UHFFFAOYSA-N 0.000 description 1
- ROVGZAWFACYCSP-MQBLHHJJSA-N [2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OC1C(C)=C(C\C=C/C=C)C(=O)C1 ROVGZAWFACYCSP-MQBLHHJJSA-N 0.000 description 1
- MVEFZZKZBYQFPP-UHFFFAOYSA-N [3-(ethoxycarbonylamino)phenyl] n-(3-methylphenyl)carbamate Chemical group CCOC(=O)NC1=CC=CC(OC(=O)NC=2C=C(C)C=CC=2)=C1 MVEFZZKZBYQFPP-UHFFFAOYSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- FJJCIZWZNKZHII-UHFFFAOYSA-N [4,6-bis(cyanoamino)-1,3,5-triazin-2-yl]cyanamide Chemical compound N#CNC1=NC(NC#N)=NC(NC#N)=N1 FJJCIZWZNKZHII-UHFFFAOYSA-N 0.000 description 1
- QSSIDEQRZPKZHQ-UHFFFAOYSA-N [4-(1H-benzimidazol-2-yl)-5-(2,2-dichloroethenyl)-6,6-dimethylcyclohexa-2,4-dien-1-yl] cyclopropanecarboxylate Chemical compound ClC(=CC=1C(C(C=CC=1C1=NC2=C(N1)C=CC=C2)OC(=O)C1CC1)(C)C)Cl QSSIDEQRZPKZHQ-UHFFFAOYSA-N 0.000 description 1
- DANONAHLRKWMTD-UHFFFAOYSA-N [6,6,6-trifluoro-3-methyl-1-[(4-methylbenzoyl)amino]hexan-2-yl] carbamate Chemical compound C(N)(OC(CNC(C1=CC=C(C=C1)C)=O)C(CCC(F)(F)F)C)=O DANONAHLRKWMTD-UHFFFAOYSA-N 0.000 description 1
- DNMKNKDKNHDRTL-UHFFFAOYSA-M [K+].NC1=C(Cl)C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C([O-])=O Chemical compound [K+].NC1=C(Cl)C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C([O-])=O DNMKNKDKNHDRTL-UHFFFAOYSA-M 0.000 description 1
- SSBRSHIQIANGKS-UHFFFAOYSA-N [amino(hydroxy)methylidene]azanium;hydrogen sulfate Chemical compound NC(N)=O.OS(O)(=O)=O SSBRSHIQIANGKS-UHFFFAOYSA-N 0.000 description 1
- CWVZGJORVTZXFW-UHFFFAOYSA-N [benzyl(dimethyl)silyl]methyl carbamate Chemical compound NC(=O)OC[Si](C)(C)CC1=CC=CC=C1 CWVZGJORVTZXFW-UHFFFAOYSA-N 0.000 description 1
- YXWCBRDRVXHABN-JCMHNJIXSA-N [cyano-(4-fluoro-3-phenoxyphenyl)methyl] 3-[(z)-2-chloro-2-(4-chlorophenyl)ethenyl]-2,2-dimethylcyclopropane-1-carboxylate Chemical compound C=1C=C(F)C(OC=2C=CC=CC=2)=CC=1C(C#N)OC(=O)C1C(C)(C)C1\C=C(/Cl)C1=CC=C(Cl)C=C1 YXWCBRDRVXHABN-JCMHNJIXSA-N 0.000 description 1
- ZVQOOHYFBIDMTQ-UHFFFAOYSA-N [methyl(oxido){1-[6-(trifluoromethyl)pyridin-3-yl]ethyl}-lambda(6)-sulfanylidene]cyanamide Chemical compound N#CN=S(C)(=O)C(C)C1=CC=C(C(F)(F)F)N=C1 ZVQOOHYFBIDMTQ-UHFFFAOYSA-N 0.000 description 1
- 229950008167 abamectin Drugs 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- YASYVMFAVPKPKE-UHFFFAOYSA-N acephate Chemical compound COP(=O)(SC)NC(C)=O YASYVMFAVPKPKE-UHFFFAOYSA-N 0.000 description 1
- QDRXWCAVUNHOGA-UHFFFAOYSA-N acequinocyl Chemical group C1=CC=C2C(=O)C(CCCCCCCCCCCC)=C(OC(C)=O)C(=O)C2=C1 QDRXWCAVUNHOGA-UHFFFAOYSA-N 0.000 description 1
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 description 1
- 125000003670 adamantan-2-yl group Chemical group [H]C1([H])C(C2([H])[H])([H])C([H])([H])C3([H])C([*])([H])C1([H])C([H])([H])C2([H])C3([H])[H] 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- LRZWFURXIMFONG-HRSIRGMGSA-N afidopyropen Chemical compound C([C@@]1(C)[C@H]2[C@]([C@H]3[C@@H](O)C=4C(=O)OC(=CC=4O[C@]3(C)[C@@H](O)C2)C=2C=NC=CC=2)(C)CC[C@@H]1OC(=O)C1CC1)OC(=O)C1CC1 LRZWFURXIMFONG-HRSIRGMGSA-N 0.000 description 1
- 229960000982 afoxolaner Drugs 0.000 description 1
- 244000193174 agave Species 0.000 description 1
- GMAUQNJOSOMMHI-JXAWBTAJSA-N alanycarb Chemical compound CSC(\C)=N/OC(=O)N(C)SN(CCC(=O)OCC)CC1=CC=CC=C1 GMAUQNJOSOMMHI-JXAWBTAJSA-N 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical group 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000005090 alkenylcarbonyl group Chemical group 0.000 description 1
- 125000004466 alkoxycarbonylamino group Chemical group 0.000 description 1
- 125000003806 alkyl carbonyl amino group Chemical group 0.000 description 1
- 125000005103 alkyl silyl group Chemical group 0.000 description 1
- 125000005038 alkynylalkyl group Chemical group 0.000 description 1
- 125000005087 alkynylcarbonyl group Chemical group 0.000 description 1
- 125000005133 alkynyloxy group Chemical group 0.000 description 1
- 229960000458 allantoin Drugs 0.000 description 1
- ZCVAOQKBXKSDMS-UHFFFAOYSA-N allethrin Chemical compound CC1(C)C(C=C(C)C)C1C(=O)OC1C(C)=C(CC=C)C(=O)C1 ZCVAOQKBXKSDMS-UHFFFAOYSA-N 0.000 description 1
- 229940024113 allethrin Drugs 0.000 description 1
- 125000005336 allyloxy group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- GGKQIOFASHYUJZ-UHFFFAOYSA-N ametoctradin Chemical compound NC1=C(CCCCCCCC)C(CC)=NC2=NC=NN21 GGKQIOFASHYUJZ-UHFFFAOYSA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- BREATYVWRHIPIY-UHFFFAOYSA-N amisulbrom Chemical compound CN(C)S(=O)(=O)N1C=NC(S(=O)(=O)N2C3=CC(F)=CC=C3C(Br)=C2C)=N1 BREATYVWRHIPIY-UHFFFAOYSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 125000005428 anthryl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C(*)=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 1
- 229940111121 antirheumatic drug quinolines Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 1
- 235000009052 artemisia Nutrition 0.000 description 1
- 125000005018 aryl alkenyl group Chemical group 0.000 description 1
- 125000005015 aryl alkynyl group Chemical group 0.000 description 1
- 125000004658 aryl carbonyl amino group Chemical group 0.000 description 1
- 125000005129 aryl carbonyl group Chemical group 0.000 description 1
- 125000005104 aryl silyl group Chemical group 0.000 description 1
- VEHPJKVTJQSSKL-UHFFFAOYSA-N azadirachtin Natural products O1C2(C)C(C3(C=COC3O3)O)CC3C21C1(C)C(O)C(OCC2(OC(C)=O)C(CC3OC(=O)C(C)=CC)OC(C)=O)C2C32COC(C(=O)OC)(O)C12 VEHPJKVTJQSSKL-UHFFFAOYSA-N 0.000 description 1
- FTNJWQUOZFUQQJ-IRYYUVNJSA-N azadirachtin A Natural products C([C@@H]([C@]1(C=CO[C@H]1O1)O)[C@]2(C)O3)[C@H]1[C@]23[C@]1(C)[C@H](O)[C@H](OC[C@@]2([C@@H](C[C@@H]3OC(=O)C(\C)=C/C)OC(C)=O)C(=O)OC)[C@@H]2[C@]32CO[C@@](C(=O)OC)(O)[C@@H]12 FTNJWQUOZFUQQJ-IRYYUVNJSA-N 0.000 description 1
- VNKBTWQZTQIWDV-UHFFFAOYSA-N azamethiphos Chemical compound C1=C(Cl)C=C2OC(=O)N(CSP(=O)(OC)OC)C2=N1 VNKBTWQZTQIWDV-UHFFFAOYSA-N 0.000 description 1
- AQKNQRMIGNOQQF-UHFFFAOYSA-N azane;2-(2,4-dichlorophenoxy)acetic acid Chemical compound [NH4+].[O-]C(=O)COC1=CC=C(Cl)C=C1Cl AQKNQRMIGNOQQF-UHFFFAOYSA-N 0.000 description 1
- QOHVLZBCVSJGFV-UHFFFAOYSA-N azane;2-methyl-4,6-dinitrophenol Chemical compound [NH4+].CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1[O-] QOHVLZBCVSJGFV-UHFFFAOYSA-N 0.000 description 1
- QRSHQJLLXXEYPS-UHFFFAOYSA-N azane;5-ethyl-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)pyridine-3-carboxylic acid Chemical compound [NH4+].[O-]C(=O)C1=CC(CC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 QRSHQJLLXXEYPS-UHFFFAOYSA-N 0.000 description 1
- VJBCNMFKFZIXHC-UHFFFAOYSA-N azanium;2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)quinoline-3-carboxylate Chemical compound N.N1C(=O)C(C(C)C)(C)N=C1C1=NC2=CC=CC=C2C=C1C(O)=O VJBCNMFKFZIXHC-UHFFFAOYSA-N 0.000 description 1
- 125000002785 azepinyl group Chemical group 0.000 description 1
- RQVGAIADHNPSME-UHFFFAOYSA-N azinphos-ethyl Chemical group C1=CC=C2C(=O)N(CSP(=S)(OCC)OCC)N=NC2=C1 RQVGAIADHNPSME-UHFFFAOYSA-N 0.000 description 1
- CJJOSEISRRTUQB-UHFFFAOYSA-N azinphos-methyl Chemical group C1=CC=C2C(=O)N(CSP(=S)(OC)OC)N=NC2=C1 CJJOSEISRRTUQB-UHFFFAOYSA-N 0.000 description 1
- ONHBDDJJTDTLIR-UHFFFAOYSA-N azocyclotin Chemical compound C1CCCCC1[Sn](N1N=CN=C1)(C1CCCCC1)C1CCCCC1 ONHBDDJJTDTLIR-UHFFFAOYSA-N 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- WFDXOXNFNRHQEC-GHRIWEEISA-N azoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1OC1=CC(OC=2C(=CC=CC=2)C#N)=NC=N1 WFDXOXNFNRHQEC-GHRIWEEISA-N 0.000 description 1
- 229940005348 bacillus firmus Drugs 0.000 description 1
- 230000010310 bacterial transformation Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- XEGGRYVFLWGFHI-UHFFFAOYSA-N bendiocarb Chemical compound CNC(=O)OC1=CC=CC2=C1OC(C)(C)O2 XEGGRYVFLWGFHI-UHFFFAOYSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- FYZBOYWSHKHDMT-UHFFFAOYSA-N benfuracarb Chemical compound CCOC(=O)CCN(C(C)C)SN(C)C(=O)OC1=CC=CC2=C1OC(C)(C)C2 FYZBOYWSHKHDMT-UHFFFAOYSA-N 0.000 description 1
- YFXPPSKYMBTNAV-UHFFFAOYSA-N bensultap Chemical compound C=1C=CC=CC=1S(=O)(=O)SCC(N(C)C)CSS(=O)(=O)C1=CC=CC=C1 YFXPPSKYMBTNAV-UHFFFAOYSA-N 0.000 description 1
- VVSLYIKSEBPRSN-PELKAZGASA-N benthiavalicarb Chemical compound C1=C(F)C=C2SC([C@@H](C)NC(=O)[C@@H](NC(O)=O)C(C)C)=NC2=C1 VVSLYIKSEBPRSN-PELKAZGASA-N 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- ZYXYTGQFPZEUFX-UHFFFAOYSA-N benzpyrimoxan Chemical compound O1C(OCCC1)C=1C(=NC=NC=1)OCC1=CC=C(C=C1)C(F)(F)F ZYXYTGQFPZEUFX-UHFFFAOYSA-N 0.000 description 1
- MKQSWTQPLLCSOB-UHFFFAOYSA-N benzyl 2-chloro-4-(trifluoromethyl)-1,3-thiazole-5-carboxylate Chemical compound N1=C(Cl)SC(C(=O)OCC=2C=CC=CC=2)=C1C(F)(F)F MKQSWTQPLLCSOB-UHFFFAOYSA-N 0.000 description 1
- VHLKTXFWDRXILV-UHFFFAOYSA-N bifenazate Chemical compound C1=C(NNC(=O)OC(C)C)C(OC)=CC=C1C1=CC=CC=C1 VHLKTXFWDRXILV-UHFFFAOYSA-N 0.000 description 1
- 229960001901 bioallethrin Drugs 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229950002373 bioresmethrin Drugs 0.000 description 1
- OIPMQULDKWSNGX-UHFFFAOYSA-N bis[[ethoxy(oxo)phosphaniumyl]oxy]alumanyloxy-ethoxy-oxophosphanium Chemical compound [Al+3].CCO[P+]([O-])=O.CCO[P+]([O-])=O.CCO[P+]([O-])=O OIPMQULDKWSNGX-UHFFFAOYSA-N 0.000 description 1
- RYVIXQCRCQLFCM-UHFFFAOYSA-N bispyribac Chemical compound COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C(O)=O)=N1 RYVIXQCRCQLFCM-UHFFFAOYSA-N 0.000 description 1
- LDLMOOXUCMHBMZ-UHFFFAOYSA-N bixafen Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=C(F)C=C1C1=CC=C(Cl)C(Cl)=C1 LDLMOOXUCMHBMZ-UHFFFAOYSA-N 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- 229940118790 boscalid Drugs 0.000 description 1
- WYEMLYFITZORAB-UHFFFAOYSA-N boscalid Chemical compound C1=CC(Cl)=CC=C1C1=CC=CC=C1NC(=O)C1=CC=CN=C1Cl WYEMLYFITZORAB-UHFFFAOYSA-N 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- QSLZKWPYTWEWHC-UHFFFAOYSA-N broflanilide Chemical compound C=1C=CC(C(=O)NC=2C(=CC(=CC=2Br)C(F)(C(F)(F)F)C(F)(F)F)C(F)(F)F)=C(F)C=1N(C)C(=O)C1=CC=CC=C1 QSLZKWPYTWEWHC-UHFFFAOYSA-N 0.000 description 1
- WZDDLAZXUYIVMU-UHFFFAOYSA-N bromobutide Chemical compound CC(C)(C)C(Br)C(=O)NC(C)(C)C1=CC=CC=C1 WZDDLAZXUYIVMU-UHFFFAOYSA-N 0.000 description 1
- FOANIXZHAMJWOI-UHFFFAOYSA-N bromopropylate Chemical compound C=1C=C(Br)C=CC=1C(O)(C(=O)OC(C)C)C1=CC=C(Br)C=C1 FOANIXZHAMJWOI-UHFFFAOYSA-N 0.000 description 1
- HJJVPARKXDDIQD-UHFFFAOYSA-N bromuconazole Chemical compound ClC1=CC(Cl)=CC=C1C1(CN2N=CN=C2)OCC(Br)C1 HJJVPARKXDDIQD-UHFFFAOYSA-N 0.000 description 1
- PRLVTUNWOQKEAI-VKAVYKQESA-N buprofezin Chemical compound O=C1N(C(C)C)\C(=N\C(C)(C)C)SCN1C1=CC=CC=C1 PRLVTUNWOQKEAI-VKAVYKQESA-N 0.000 description 1
- RHDGNLCLDBVESU-UHFFFAOYSA-N but-3-en-4-olide Chemical compound O=C1CC=CO1 RHDGNLCLDBVESU-UHFFFAOYSA-N 0.000 description 1
- YXXPNGIZHZHBTQ-UHFFFAOYSA-N butan-2-ol Chemical compound CC[C](C)O YXXPNGIZHZHBTQ-UHFFFAOYSA-N 0.000 description 1
- SFNPDDSJBGRXLW-UITAMQMPSA-N butocarboxim Chemical compound CNC(=O)O\N=C(\C)C(C)SC SFNPDDSJBGRXLW-UITAMQMPSA-N 0.000 description 1
- VAIZTNZGPYBOGF-UHFFFAOYSA-N butyl 2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OCCCC)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 VAIZTNZGPYBOGF-UHFFFAOYSA-N 0.000 description 1
- IXXKVXJYFVAQBI-UHFFFAOYSA-N butyl 4-(2,4-dichlorophenoxy)butanoate Chemical group CCCCOC(=O)CCCOC1=CC=C(Cl)C=C1Cl IXXKVXJYFVAQBI-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- KXRPCFINVWWFHQ-UHFFFAOYSA-N cadusafos Chemical compound CCC(C)SP(=O)(OCC)SC(C)CC KXRPCFINVWWFHQ-UHFFFAOYSA-N 0.000 description 1
- PAFYVDNYOJAWDX-UHFFFAOYSA-L calcium;2,2,2-trichloroacetate Chemical compound [Ca+2].[O-]C(=O)C(Cl)(Cl)Cl.[O-]C(=O)C(Cl)(Cl)Cl PAFYVDNYOJAWDX-UHFFFAOYSA-L 0.000 description 1
- JBGIVEALFOAXPA-UHFFFAOYSA-L calcium;2,2-dichloropropanoate Chemical compound [Ca+2].CC(Cl)(Cl)C([O-])=O.CC(Cl)(Cl)C([O-])=O JBGIVEALFOAXPA-UHFFFAOYSA-L 0.000 description 1
- OOCMUZJPDXYRFD-UHFFFAOYSA-L calcium;2-dodecylbenzenesulfonate Chemical compound [Ca+2].CCCCCCCCCCCCC1=CC=CC=C1S([O-])(=O)=O.CCCCCCCCCCCCC1=CC=CC=C1S([O-])(=O)=O OOCMUZJPDXYRFD-UHFFFAOYSA-L 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229940117949 captan Drugs 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- JLQUFIHWVLZVTJ-UHFFFAOYSA-N carbosulfan Chemical compound CCCCN(CCCC)SN(C)C(=O)OC1=CC=CC2=C1OC(C)(C)C2 JLQUFIHWVLZVTJ-UHFFFAOYSA-N 0.000 description 1
- GYSSRZJIHXQEHQ-UHFFFAOYSA-N carboxin Chemical compound S1CCOC(C)=C1C(=O)NC1=CC=CC=C1 GYSSRZJIHXQEHQ-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- RXDMAYSSBPYBFW-UHFFFAOYSA-N carpropamid Chemical compound C=1C=C(Cl)C=CC=1C(C)NC(=O)C1(CC)C(C)C1(Cl)Cl RXDMAYSSBPYBFW-UHFFFAOYSA-N 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- ADRVNXBAWSRFAJ-UHFFFAOYSA-N catechin Natural products OC1Cc2cc(O)cc(O)c2OC1c3ccc(O)c(O)c3 ADRVNXBAWSRFAJ-UHFFFAOYSA-N 0.000 description 1
- 125000004403 catechin group Chemical group 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000010307 cell transformation Effects 0.000 description 1
- 230000008166 cellulose biosynthesis Effects 0.000 description 1
- 108010040093 cellulose synthase Proteins 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- KXZJHVJKXJLBKO-UHFFFAOYSA-N chembl1408157 Chemical compound N=1C2=CC=CC=C2C(C(=O)O)=CC=1C1=CC=C(O)C=C1 KXZJHVJKXJLBKO-UHFFFAOYSA-N 0.000 description 1
- NDHXMRFNYMNBKO-PWSUYJOCSA-N chembl2227757 Chemical compound [O-][N+](=O)C([C@H]1CC[C@H](O1)N1CC2)=C1N2CC1=CC=C(Cl)N=C1 NDHXMRFNYMNBKO-PWSUYJOCSA-N 0.000 description 1
- DQFPEYARZIQXRM-UHFFFAOYSA-N chembl2355904 Chemical compound C1C(=O)C(C(CC)=NOCC)=C(O)CC1C1=C(C)C=C(C)C=C1C DQFPEYARZIQXRM-UHFFFAOYSA-N 0.000 description 1
- CSPPKDPQLUUTND-UHFFFAOYSA-N chembl3186232 Chemical compound CCON=C(CCC)C1=C(O)CC(CC(C)SCC)CC1=O CSPPKDPQLUUTND-UHFFFAOYSA-N 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- BIWJNBZANLAXMG-YQELWRJZSA-N chloordaan Chemical compound ClC1=C(Cl)[C@@]2(Cl)C3CC(Cl)C(Cl)C3[C@]1(Cl)C2(Cl)Cl BIWJNBZANLAXMG-YQELWRJZSA-N 0.000 description 1
- PSOVNZZNOMJUBI-UHFFFAOYSA-N chlorantraniliprole Chemical compound CNC(=O)C1=CC(Cl)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl PSOVNZZNOMJUBI-UHFFFAOYSA-N 0.000 description 1
- XFDJMIHUAHSGKG-UHFFFAOYSA-N chlorethoxyfos Chemical compound CCOP(=S)(OCC)OC(Cl)C(Cl)(Cl)Cl XFDJMIHUAHSGKG-UHFFFAOYSA-N 0.000 description 1
- QZXCCPZJCKEPSA-UHFFFAOYSA-N chlorfenac Chemical compound OC(=O)CC1=C(Cl)C=CC(Cl)=C1Cl QZXCCPZJCKEPSA-UHFFFAOYSA-N 0.000 description 1
- DZRJBVFMFCMALE-UHFFFAOYSA-N chlorfenac-ammonium Chemical compound N.OC(=O)CC1=C(Cl)C=CC(Cl)=C1Cl DZRJBVFMFCMALE-UHFFFAOYSA-N 0.000 description 1
- YPWJVPXHSDMPQB-UHFFFAOYSA-M chlorfenac-sodium Chemical compound [Na+].[O-]C(=O)CC1=C(Cl)C=CC(Cl)=C1Cl YPWJVPXHSDMPQB-UHFFFAOYSA-M 0.000 description 1
- CWFOCCVIPCEQCK-UHFFFAOYSA-N chlorfenapyr Chemical compound BrC1=C(C(F)(F)F)N(COCC)C(C=2C=CC(Cl)=CC=2)=C1C#N CWFOCCVIPCEQCK-UHFFFAOYSA-N 0.000 description 1
- UISUNVFOGSJSKD-UHFFFAOYSA-N chlorfluazuron Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC(C=C1Cl)=CC(Cl)=C1OC1=NC=C(C(F)(F)F)C=C1Cl UISUNVFOGSJSKD-UHFFFAOYSA-N 0.000 description 1
- QGTYWWGEWOBMAK-UHFFFAOYSA-N chlormephos Chemical compound CCOP(=S)(OCC)SCCl QGTYWWGEWOBMAK-UHFFFAOYSA-N 0.000 description 1
- JUZXDNPBRPUIOR-UHFFFAOYSA-N chlormequat Chemical compound C[N+](C)(C)CCCl JUZXDNPBRPUIOR-UHFFFAOYSA-N 0.000 description 1
- HKMOPYJWSFRURD-UHFFFAOYSA-N chloro hypochlorite;copper Chemical compound [Cu].ClOCl HKMOPYJWSFRURD-UHFFFAOYSA-N 0.000 description 1
- 229930002875 chlorophyll Natural products 0.000 description 1
- 235000019804 chlorophyll Nutrition 0.000 description 1
- ATNHDLDRLWWWCB-AENOIHSZSA-M chlorophyll a Chemical compound C1([C@@H](C(=O)OC)C(=O)C2=C3C)=C2N2C3=CC(C(CC)=C3C)=[N+]4C3=CC3=C(C=C)C(C)=C5N3[Mg-2]42[N+]2=C1[C@@H](CCC(=O)OC\C=C(/C)CCC[C@H](C)CCC[C@H](C)CCCC(C)C)[C@H](C)C2=C5 ATNHDLDRLWWWCB-AENOIHSZSA-M 0.000 description 1
- LFHISGNCFUNFFM-UHFFFAOYSA-N chloropicrin Chemical compound [O-][N+](=O)C(Cl)(Cl)Cl LFHISGNCFUNFFM-UHFFFAOYSA-N 0.000 description 1
- RQNZCZHWQZMZER-ZLDLUXBVSA-N chloroprallethrin Chemical compound CC1=C(CC#C)C(=O)C[C@@H]1OC(=O)[C@H]1C(C)(C)[C@@H]1C=C(Cl)Cl RQNZCZHWQZMZER-ZLDLUXBVSA-N 0.000 description 1
- CRQQGFGUEAVUIL-UHFFFAOYSA-N chlorothalonil Chemical compound ClC1=C(Cl)C(C#N)=C(Cl)C(C#N)=C1Cl CRQQGFGUEAVUIL-UHFFFAOYSA-N 0.000 description 1
- SBPBAQFWLVIOKP-UHFFFAOYSA-N chlorpyrifos Chemical compound CCOP(=S)(OCC)OC1=NC(Cl)=C(Cl)C=C1Cl SBPBAQFWLVIOKP-UHFFFAOYSA-N 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- HPNSNYBUADCFDR-UHFFFAOYSA-N chromafenozide Chemical compound CC1=CC(C)=CC(C(=O)N(NC(=O)C=2C(=C3CCCOC3=CC=2)C)C(C)(C)C)=C1 HPNSNYBUADCFDR-UHFFFAOYSA-N 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 229950001002 cianidanol Drugs 0.000 description 1
- WCZVZNOTHYJIEI-UHFFFAOYSA-N cinnoline Chemical compound N1=NC=CC2=CC=CC=C21 WCZVZNOTHYJIEI-UHFFFAOYSA-N 0.000 description 1
- UXADOQPNKNTIHB-UHFFFAOYSA-N clofentezine Chemical compound ClC1=CC=CC=C1C1=NN=C(C=2C(=CC=CC=2)Cl)N=N1 UXADOQPNKNTIHB-UHFFFAOYSA-N 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000007859 condensation product Substances 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 229910001956 copper hydroxide Inorganic materials 0.000 description 1
- 229940120693 copper naphthenate Drugs 0.000 description 1
- 229910000431 copper oxide Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- SEVNKWFHTNVOLD-UHFFFAOYSA-L copper;3-(4-ethylcyclohexyl)propanoate;3-(3-ethylcyclopentyl)propanoate Chemical compound [Cu+2].CCC1CCC(CCC([O-])=O)C1.CCC1CCC(CCC([O-])=O)CC1 SEVNKWFHTNVOLD-UHFFFAOYSA-L 0.000 description 1
- 101150055601 cops2 gene Proteins 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 235000011655 cotton Nutrition 0.000 description 1
- BXNANOICGRISHX-UHFFFAOYSA-N coumaphos Chemical compound CC1=C(Cl)C(=O)OC2=CC(OP(=S)(OCC)OCC)=CC=C21 BXNANOICGRISHX-UHFFFAOYSA-N 0.000 description 1
- CWVRPJSBNHNJSI-XQNSMLJCSA-N coumoxystrobin Chemical compound C1=C2OC(=O)C(CCCC)=C(C)C2=CC=C1OCC1=CC=CC=C1\C(=C/OC)C(=O)OC CWVRPJSBNHNJSI-XQNSMLJCSA-N 0.000 description 1
- 238000012272 crop production Methods 0.000 description 1
- 229910001610 cryolite Inorganic materials 0.000 description 1
- 230000001955 cumulated effect Effects 0.000 description 1
- SCKHCCSZFPSHGR-UHFFFAOYSA-N cyanophos Chemical compound COP(=S)(OC)OC1=CC=C(C#N)C=C1 SCKHCCSZFPSHGR-UHFFFAOYSA-N 0.000 description 1
- DVBUIBGJRQBEDP-UHFFFAOYSA-N cyantraniliprole Chemical compound CNC(=O)C1=CC(C#N)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl DVBUIBGJRQBEDP-UHFFFAOYSA-N 0.000 description 1
- 125000004465 cycloalkenyloxy group Chemical group 0.000 description 1
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 description 1
- 125000006310 cycloalkyl amino group Chemical group 0.000 description 1
- 125000006254 cycloalkyl carbonyl group Chemical group 0.000 description 1
- 125000005112 cycloalkylalkoxy group Chemical group 0.000 description 1
- 125000005357 cycloalkylalkynyl group Chemical group 0.000 description 1
- 125000005169 cycloalkylcarbonylamino group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- PYRZPBDTPRQYKG-UHFFFAOYSA-M cyclopentene-1-carboxylate Chemical compound [O-]C(=O)C1=CCCC1 PYRZPBDTPRQYKG-UHFFFAOYSA-M 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- CXKXKAHHMPBRPT-UHFFFAOYSA-N cyclopropen-1-ylmethanol Chemical compound OCC1=CC1 CXKXKAHHMPBRPT-UHFFFAOYSA-N 0.000 description 1
- LSFUGNKKPMBOMG-UHFFFAOYSA-N cycloprothrin Chemical compound ClC1(Cl)CC1(C=1C=CC=CC=1)C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 LSFUGNKKPMBOMG-UHFFFAOYSA-N 0.000 description 1
- APJLTUBHYCOZJI-VZCXRCSSSA-N cyenopyrafen Chemical compound CC1=NN(C)C(\C(OC(=O)C(C)(C)C)=C(/C#N)C=2C=CC(=CC=2)C(C)(C)C)=C1C APJLTUBHYCOZJI-VZCXRCSSSA-N 0.000 description 1
- NNRSYETYEADPBW-UHFFFAOYSA-N cyhalodiamide Chemical compound CC1=CC(C(F)(C(F)(F)F)C(F)(F)F)=CC=C1NC(=O)C1=CC=CC(Cl)=C1C(=O)NC(C)(C)C#N NNRSYETYEADPBW-UHFFFAOYSA-N 0.000 description 1
- ZXQYGBMAQZUVMI-UNOMPAQXSA-N cyhalothrin Chemical compound CC1(C)C(\C=C(/Cl)C(F)(F)F)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-UNOMPAQXSA-N 0.000 description 1
- HAORKNGNJCEJBX-UHFFFAOYSA-N cyprodinil Chemical compound N=1C(C)=CC(C2CC2)=NC=1NC1=CC=CC=C1 HAORKNGNJCEJBX-UHFFFAOYSA-N 0.000 description 1
- LVQDKIWDGQRHTE-UHFFFAOYSA-N cyromazine Chemical compound NC1=NC(N)=NC(NC2CC2)=N1 LVQDKIWDGQRHTE-UHFFFAOYSA-N 0.000 description 1
- 229950000775 cyromazine Drugs 0.000 description 1
- 229940008203 d-transallethrin Drugs 0.000 description 1
- 238000005034 decoration Methods 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- WEBQKRLKWNIYKK-UHFFFAOYSA-N demeton-S-methyl Chemical group CCSCCSP(=O)(OC)OC WEBQKRLKWNIYKK-UHFFFAOYSA-N 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- WOWBFOBYOAGEEA-UHFFFAOYSA-N diafenthiuron Chemical compound CC(C)C1=C(NC(=S)NC(C)(C)C)C(C(C)C)=CC(OC=2C=CC=CC=2)=C1 WOWBFOBYOAGEEA-UHFFFAOYSA-N 0.000 description 1
- 125000004984 dialkylaminoalkoxy group Chemical group 0.000 description 1
- 125000004473 dialkylaminocarbonyl group Chemical group 0.000 description 1
- 150000001470 diamides Chemical class 0.000 description 1
- NJPDFPLPWOPSKC-PXYBLNDHSA-N diazanium;(1s,2r,3s,4r)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate Chemical compound [NH4+].[NH4+].C1C[C@@H]2[C@@H](C([O-])=O)[C@@H](C(=O)[O-])[C@H]1O2 NJPDFPLPWOPSKC-PXYBLNDHSA-N 0.000 description 1
- FHIVAFMUCKRCQO-UHFFFAOYSA-N diazinon Chemical compound CCOP(=S)(OCC)OC1=CC(C)=NC(C(C)C)=N1 FHIVAFMUCKRCQO-UHFFFAOYSA-N 0.000 description 1
- PFBUKDPBVNJDEW-UHFFFAOYSA-N dichlorocarbene Chemical group Cl[C]Cl PFBUKDPBVNJDEW-UHFFFAOYSA-N 0.000 description 1
- 229950001327 dichlorvos Drugs 0.000 description 1
- PVDQXPBKBSCNJZ-UHFFFAOYSA-N dicloromezotiaz Chemical compound CC1=CC=C[N+](C(C(C=2C=C(Cl)C=C(Cl)C=2)=C2[O-])=O)=C1N2CC1=CN=C(Cl)S1 PVDQXPBKBSCNJZ-UHFFFAOYSA-N 0.000 description 1
- UOAMTSKGCBMZTC-UHFFFAOYSA-N dicofol Chemical compound C=1C=C(Cl)C=CC=1C(C(Cl)(Cl)Cl)(O)C1=CC=C(Cl)C=C1 UOAMTSKGCBMZTC-UHFFFAOYSA-N 0.000 description 1
- VEENJGZXVHKXNB-VOTSOKGWSA-N dicrotophos Chemical compound COP(=O)(OC)O\C(C)=C\C(=O)N(C)C VEENJGZXVHKXNB-VOTSOKGWSA-N 0.000 description 1
- LPVVTHQFIVXMEZ-UHFFFAOYSA-N diethyl 2-(5-chloroquinolin-8-yl)oxypropanedioate Chemical compound C1=CN=C2C(OC(C(=O)OCC)C(=O)OCC)=CC=C(Cl)C2=C1 LPVVTHQFIVXMEZ-UHFFFAOYSA-N 0.000 description 1
- JXSJBGJIGXNWCI-UHFFFAOYSA-N diethyl 2-[(dimethoxyphosphorothioyl)thio]succinate Chemical compound CCOC(=O)CC(SP(=S)(OC)OC)C(=O)OCC JXSJBGJIGXNWCI-UHFFFAOYSA-N 0.000 description 1
- BQYJATMQXGBDHF-UHFFFAOYSA-N difenoconazole Chemical compound O1C(C)COC1(C=1C(=CC(OC=2C=CC(Cl)=CC=2)=CC=1)Cl)CN1N=CN=C1 BQYJATMQXGBDHF-UHFFFAOYSA-N 0.000 description 1
- 229940019503 diflubenzuron Drugs 0.000 description 1
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 description 1
- MCWXGJITAZMZEV-UHFFFAOYSA-N dimethoate Chemical compound CNC(=O)CSP(=S)(OC)OC MCWXGJITAZMZEV-UHFFFAOYSA-N 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- WXUZAHCNPWONDH-DYTRJAOYSA-N dimoxystrobin Chemical compound CNC(=O)C(=N\OC)\C1=CC=CC=C1COC1=CC(C)=CC=C1C WXUZAHCNPWONDH-DYTRJAOYSA-N 0.000 description 1
- YKBZOVFACRVRJN-UHFFFAOYSA-N dinotefuran Chemical compound [O-][N+](=O)\N=C(/NC)NCC1CCOC1 YKBZOVFACRVRJN-UHFFFAOYSA-N 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- PYZSVQVRHDXQSL-UHFFFAOYSA-N dithianon Chemical compound S1C(C#N)=C(C#N)SC2=C1C(=O)C1=CC=CC=C1C2=O PYZSVQVRHDXQSL-UHFFFAOYSA-N 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 108010057988 ecdysone receptor Proteins 0.000 description 1
- 101150036810 eco gene Proteins 0.000 description 1
- AWZOLILCOUMRDG-UHFFFAOYSA-N edifenphos Chemical compound C=1C=CC=CC=1SP(=O)(OCC)SC1=CC=CC=C1 AWZOLILCOUMRDG-UHFFFAOYSA-N 0.000 description 1
- CXEGAUYXQAKHKJ-NSBHKLITSA-N emamectin B1a Chemical compound C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](NC)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 CXEGAUYXQAKHKJ-NSBHKLITSA-N 0.000 description 1
- 239000002895 emetic Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 150000002085 enols Chemical group 0.000 description 1
- VMNULHCTRPXWFJ-UJSVPXBISA-N enoxastrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1CO\N=C(/C)\C=C\C1=CC=C(Cl)C=C1 VMNULHCTRPXWFJ-UJSVPXBISA-N 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000007824 enzymatic assay Methods 0.000 description 1
- 230000009088 enzymatic function Effects 0.000 description 1
- KVIZNNVXXNFLMU-DUVUQDDDSA-N epsilon-metofluthrin Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)[C@H]1C(C)(C)[C@@H]1\C=C/C KVIZNNVXXNFLMU-DUVUQDDDSA-N 0.000 description 1
- 230000008686 ergosterol biosynthesis Effects 0.000 description 1
- NYPJDWWKZLNGGM-RPWUZVMVSA-N esfenvalerate Chemical compound C=1C([C@@H](C#N)OC(=O)[C@@H](C(C)C)C=2C=CC(Cl)=CC=2)=CC=CC=1OC1=CC=CC=C1 NYPJDWWKZLNGGM-RPWUZVMVSA-N 0.000 description 1
- ZINJLDJMHCUBIP-UHFFFAOYSA-N ethametsulfuron-methyl Chemical compound CCOC1=NC(NC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(=O)OC)=N1 ZINJLDJMHCUBIP-UHFFFAOYSA-N 0.000 description 1
- ZCRZCMUDOWDGOB-UHFFFAOYSA-N ethanesulfonimidic acid Chemical compound CCS(N)(=O)=O ZCRZCMUDOWDGOB-UHFFFAOYSA-N 0.000 description 1
- HEZNVIYQEUHLNI-UHFFFAOYSA-N ethiofencarb Chemical compound CCSCC1=CC=CC=C1OC(=O)NC HEZNVIYQEUHLNI-UHFFFAOYSA-N 0.000 description 1
- RIZMRRKBZQXFOY-UHFFFAOYSA-N ethion Chemical compound CCOP(=S)(OCC)SCSP(=S)(OCC)OCC RIZMRRKBZQXFOY-UHFFFAOYSA-N 0.000 description 1
- YKRQBWKLHCEKQH-KHPPLWFESA-N ethyl (z)-3-amino-2-cyano-3-phenylprop-2-enoate Chemical compound CCOC(=O)C(\C#N)=C(/N)C1=CC=CC=C1 YKRQBWKLHCEKQH-KHPPLWFESA-N 0.000 description 1
- FPIQZBQZKBKLEI-UHFFFAOYSA-N ethyl 1-[[2-chloroethyl(nitroso)carbamoyl]amino]cyclohexane-1-carboxylate Chemical compound ClCCN(N=O)C(=O)NC1(C(=O)OCC)CCCCC1 FPIQZBQZKBKLEI-UHFFFAOYSA-N 0.000 description 1
- JEMXUSHXYOXNFL-UHFFFAOYSA-N ethyl 2-(5-chloroquinolin-8-yl)oxyacetate Chemical compound C1=CN=C2C(OCC(=O)OCC)=CC=C(Cl)C2=C1 JEMXUSHXYOXNFL-UHFFFAOYSA-N 0.000 description 1
- VZZVOKHLRXJIEP-UHFFFAOYSA-N ethyl 2-benzhydryloxyacetate Chemical compound C=1C=CC=CC=1C(OCC(=O)OCC)C1=CC=CC=C1 VZZVOKHLRXJIEP-UHFFFAOYSA-N 0.000 description 1
- VBXKZVDAIHPRLJ-UHFFFAOYSA-N ethyl 3-(4-chloro-2,6-dimethylphenyl)-8-methoxy-1-methyl-2-oxo-1,8-diazaspiro[4.5]dec-3-ene-4-carboxylate Chemical compound C(C)OC(=O)C1=C(C(N(C11CCN(CC1)OC)C)=O)C1=C(C=C(C=C1C)Cl)C VBXKZVDAIHPRLJ-UHFFFAOYSA-N 0.000 description 1
- SQFPMCDRPGJOQH-UHFFFAOYSA-N ethyl 5-(4-fluorophenyl)-5-phenyl-4h-1,2-oxazole-3-carboxylate Chemical compound C1C(C(=O)OCC)=NOC1(C=1C=CC(F)=CC=1)C1=CC=CC=C1 SQFPMCDRPGJOQH-UHFFFAOYSA-N 0.000 description 1
- 125000006125 ethylsulfonyl group Chemical group 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- YREQHYQNNWYQCJ-UHFFFAOYSA-N etofenprox Chemical compound C1=CC(OCC)=CC=C1C(C)(C)COCC1=CC=CC(OC=2C=CC=CC=2)=C1 YREQHYQNNWYQCJ-UHFFFAOYSA-N 0.000 description 1
- 229950005085 etofenprox Drugs 0.000 description 1
- IXSZQYVWNJNRAL-UHFFFAOYSA-N etoxazole Chemical compound CCOC1=CC(C(C)(C)C)=CC=C1C1N=C(C=2C(=CC=CC=2F)F)OC1 IXSZQYVWNJNRAL-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- QMTNOLKHSWIQBE-FGTMMUONSA-N exo-(+)-cinmethylin Chemical compound O([C@H]1[C@]2(C)CC[C@@](O2)(C1)C(C)C)CC1=CC=CC=C1C QMTNOLKHSWIQBE-FGTMMUONSA-N 0.000 description 1
- JISACBWYRJHSMG-UHFFFAOYSA-N famphur Chemical compound COP(=S)(OC)OC1=CC=C(S(=O)(=O)N(C)C)C=C1 JISACBWYRJHSMG-UHFFFAOYSA-N 0.000 description 1
- DMYHGDXADUDKCQ-UHFFFAOYSA-N fenazaquin Chemical compound C1=CC(C(C)(C)C)=CC=C1CCOC1=NC=NC2=CC=CC=C12 DMYHGDXADUDKCQ-UHFFFAOYSA-N 0.000 description 1
- VDLGAVXLJYLFDH-UHFFFAOYSA-N fenhexamid Chemical compound C=1C=C(O)C(Cl)=C(Cl)C=1NC(=O)C1(C)CCCCC1 VDLGAVXLJYLFDH-UHFFFAOYSA-N 0.000 description 1
- ZNOLGFHPUIJIMJ-UHFFFAOYSA-N fenitrothion Chemical compound COP(=S)(OC)OC1=CC=C([N+]([O-])=O)C(C)=C1 ZNOLGFHPUIJIMJ-UHFFFAOYSA-N 0.000 description 1
- DIRFUJHNVNOBMY-UHFFFAOYSA-N fenobucarb Chemical compound CCC(C)C1=CC=CC=C1OC(=O)NC DIRFUJHNVNOBMY-UHFFFAOYSA-N 0.000 description 1
- ACDZDIIWZVQMIX-UHFFFAOYSA-N fenoxasulfone Chemical compound C1=C(Cl)C(OCC)=CC(Cl)=C1CS(=O)(=O)C1=NOC(C)(C)C1 ACDZDIIWZVQMIX-UHFFFAOYSA-N 0.000 description 1
- HJUFTIJOISQSKQ-UHFFFAOYSA-N fenoxycarb Chemical compound C1=CC(OCCNC(=O)OCC)=CC=C1OC1=CC=CC=C1 HJUFTIJOISQSKQ-UHFFFAOYSA-N 0.000 description 1
- XQUXKZZNEFRCAW-UHFFFAOYSA-N fenpropathrin Chemical compound CC1(C)C(C)(C)C1C(=O)OC(C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 XQUXKZZNEFRCAW-UHFFFAOYSA-N 0.000 description 1
- UTOHZQYBSYOOGC-UHFFFAOYSA-N fenpyrazamine Chemical compound O=C1N(C(C)C)N(C(=O)SCC=C)C(N)=C1C1=CC=CC=C1C UTOHZQYBSYOOGC-UHFFFAOYSA-N 0.000 description 1
- YYJNOYZRYGDPNH-MFKUBSTISA-N fenpyroximate Chemical compound C=1C=C(C(=O)OC(C)(C)C)C=CC=1CO/N=C/C=1C(C)=NN(C)C=1OC1=CC=CC=C1 YYJNOYZRYGDPNH-MFKUBSTISA-N 0.000 description 1
- WDQNIWFZKXZFAY-UHFFFAOYSA-M fentin acetate Chemical compound CC([O-])=O.C1=CC=CC=C1[Sn+](C=1C=CC=CC=1)C1=CC=CC=C1 WDQNIWFZKXZFAY-UHFFFAOYSA-M 0.000 description 1
- RLQJEEJISHYWON-UHFFFAOYSA-N flonicamid Chemical compound FC(F)(F)C1=CC=NC=C1C(=O)NCC#N RLQJEEJISHYWON-UHFFFAOYSA-N 0.000 description 1
- MXWAGQASUDSFBG-RVDMUPIBSA-N fluacrypyrim Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC(C(F)(F)F)=NC(OC(C)C)=N1 MXWAGQASUDSFBG-RVDMUPIBSA-N 0.000 description 1
- PHCCDUCBMCYSNQ-UHFFFAOYSA-N fluazaindolizine Chemical compound COC1=CC=C(Cl)C(S(=O)(=O)NC(=O)C=2N=C3C(Cl)=CC(=CN3C=2)C(F)(F)F)=C1 PHCCDUCBMCYSNQ-UHFFFAOYSA-N 0.000 description 1
- UZCGKGPEKUCDTF-UHFFFAOYSA-N fluazinam Chemical compound [O-][N+](=O)C1=CC(C(F)(F)F)=C(Cl)C([N+]([O-])=O)=C1NC1=NC=C(C(F)(F)F)C=C1Cl UZCGKGPEKUCDTF-UHFFFAOYSA-N 0.000 description 1
- ZGNITFSDLCMLGI-UHFFFAOYSA-N flubendiamide Chemical compound CC1=CC(C(F)(C(F)(F)F)C(F)(F)F)=CC=C1NC(=O)C1=CC=CC(I)=C1C(=O)NC(C)(C)CS(C)(=O)=O ZGNITFSDLCMLGI-UHFFFAOYSA-N 0.000 description 1
- GBIHOLCMZGAKNG-CGAIIQECSA-N flucythrinate Chemical compound O=C([C@@H](C(C)C)C=1C=CC(OC(F)F)=CC=1)OC(C#N)C(C=1)=CC=CC=1OC1=CC=CC=C1 GBIHOLCMZGAKNG-CGAIIQECSA-N 0.000 description 1
- MUJOIMFVNIBMKC-UHFFFAOYSA-N fludioxonil Chemical compound C=12OC(F)(F)OC2=CC=CC=1C1=CNC=C1C#N MUJOIMFVNIBMKC-UHFFFAOYSA-N 0.000 description 1
- XSNMWAPKHUGZGQ-UHFFFAOYSA-N fluensulfone Chemical compound FC(F)=C(F)CCS(=O)(=O)C1=NC=C(Cl)S1 XSNMWAPKHUGZGQ-UHFFFAOYSA-N 0.000 description 1
- GJEREQYJIQASAW-UHFFFAOYSA-N flufenerim Chemical compound CC(F)C1=NC=NC(NCCC=2C=CC(OC(F)(F)F)=CC=2)=C1Cl GJEREQYJIQASAW-UHFFFAOYSA-N 0.000 description 1
- RYLHNOVXKPXDIP-UHFFFAOYSA-N flufenoxuron Chemical compound C=1C=C(NC(=O)NC(=O)C=2C(=CC=CC=2F)F)C(F)=CC=1OC1=CC=C(C(F)(F)F)C=C1Cl RYLHNOVXKPXDIP-UHFFFAOYSA-N 0.000 description 1
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- 125000003709 fluoroalkyl group Chemical group 0.000 description 1
- IPENDKRRWFURRE-UHFFFAOYSA-N fluoroimide Chemical compound C1=CC(F)=CC=C1N1C(=O)C(Cl)=C(Cl)C1=O IPENDKRRWFURRE-UHFFFAOYSA-N 0.000 description 1
- VUWZPRWSIVNGKG-UHFFFAOYSA-N fluoromethane Chemical compound F[CH2] VUWZPRWSIVNGKG-UHFFFAOYSA-N 0.000 description 1
- 125000004785 fluoromethoxy group Chemical group [H]C([H])(F)O* 0.000 description 1
- UFEODZBUAFNAEU-NLRVBDNBSA-N fluoxastrobin Chemical compound C=1C=CC=C(OC=2C(=C(OC=3C(=CC=CC=3)Cl)N=CN=2)F)C=1C(=N/OC)\C1=NOCCO1 UFEODZBUAFNAEU-NLRVBDNBSA-N 0.000 description 1
- QOIYTRGFOFZNKF-UHFFFAOYSA-N flupyradifurone Chemical compound C=1C(=O)OCC=1N(CC(F)F)CC1=CC=C(Cl)N=C1 QOIYTRGFOFZNKF-UHFFFAOYSA-N 0.000 description 1
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 description 1
- MLBZKOGAMRTSKP-UHFFFAOYSA-N fluralaner Chemical compound C1=C(C(=O)NCC(=O)NCC(F)(F)F)C(C)=CC(C=2CC(ON=2)(C=2C=C(Cl)C=C(Cl)C=2)C(F)(F)F)=C1 MLBZKOGAMRTSKP-UHFFFAOYSA-N 0.000 description 1
- 229960004498 fluralaner Drugs 0.000 description 1
- XWROTTLWMHCFEC-UHFFFAOYSA-N fluthiacet Chemical compound C1=C(Cl)C(SCC(=O)O)=CC(N=C2N3CCCCN3C(=O)S2)=C1F XWROTTLWMHCFEC-UHFFFAOYSA-N 0.000 description 1
- XWROTTLWMHCFEC-LGMDPLHJSA-N fluthiacet Chemical compound C1=C(Cl)C(SCC(=O)O)=CC(\N=C/2N3CCCCN3C(=O)S\2)=C1F XWROTTLWMHCFEC-LGMDPLHJSA-N 0.000 description 1
- KGXUEPOHGFWQKF-ZCXUNETKSA-N flutianil Chemical compound COC1=CC=CC=C1N(CCS\1)C/1=C(C#N)/SC1=CC(C(F)(F)F)=CC=C1F KGXUEPOHGFWQKF-ZCXUNETKSA-N 0.000 description 1
- PTCGDEVVHUXTMP-UHFFFAOYSA-N flutolanil Chemical compound CC(C)OC1=CC=CC(NC(=O)C=2C(=CC=CC=2)C(F)(F)F)=C1 PTCGDEVVHUXTMP-UHFFFAOYSA-N 0.000 description 1
- SXSGXWCSHSVPGB-UHFFFAOYSA-N fluxapyroxad Chemical compound FC(F)C1=NN(C)C=C1C(=O)NC1=CC=CC=C1C1=CC(F)=C(F)C(F)=C1 SXSGXWCSHSVPGB-UHFFFAOYSA-N 0.000 description 1
- KVGLBTYUCJYMND-UHFFFAOYSA-N fonofos Chemical compound CCOP(=S)(CC)SC1=CC=CC=C1 KVGLBTYUCJYMND-UHFFFAOYSA-N 0.000 description 1
- RMFNNCGOSPBBAD-MDWZMJQESA-N formetanate Chemical compound CNC(=O)OC1=CC=CC(\N=C\N(C)C)=C1 RMFNNCGOSPBBAD-MDWZMJQESA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- VUERQRKTYBIULR-UHFFFAOYSA-N fosetyl Chemical compound CCOP(O)=O VUERQRKTYBIULR-UHFFFAOYSA-N 0.000 description 1
- DUFVKSUJRWYZQP-UHFFFAOYSA-N fosthiazate Chemical compound CCC(C)SP(=O)(OCC)N1CCSC1=O DUFVKSUJRWYZQP-UHFFFAOYSA-N 0.000 description 1
- HAWJXYBZNNRMNO-UHFFFAOYSA-N furathiocarb Chemical compound CCCCOC(=O)N(C)SN(C)C(=O)OC1=CC=CC2=C1OC(C)(C)C2 HAWJXYBZNNRMNO-UHFFFAOYSA-N 0.000 description 1
- ZXQYGBMAQZUVMI-GCMPRSNUSA-N gamma-cyhalothrin Chemical compound CC1(C)[C@@H](\C=C(/Cl)C(F)(F)F)[C@H]1C(=O)O[C@H](C#N)C1=CC=CC(OC=2C=CC=CC=2)=C1 ZXQYGBMAQZUVMI-GCMPRSNUSA-N 0.000 description 1
- 238000012215 gene cloning Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000037442 genomic alteration Effects 0.000 description 1
- IAJOBQBIJHVGMQ-BYPYZUCNSA-N glufosinate-P Chemical compound CP(O)(=O)CC[C@H](N)C(O)=O IAJOBQBIJHVGMQ-BYPYZUCNSA-N 0.000 description 1
- ZBMRKNMTMPPMMK-WCCKRBBISA-N glufosinate-P-ammonium Chemical compound N.CP(O)(=O)CC[C@H](N)C(O)=O ZBMRKNMTMPPMMK-WCCKRBBISA-N 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 239000003966 growth inhibitor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- WIFXJBMOTMKRMM-UHFFFAOYSA-N halfenprox Chemical compound C=1C=C(OC(F)(F)Br)C=CC=1C(C)(C)COCC(C=1)=CC=CC=1OC1=CC=CC=C1 WIFXJBMOTMKRMM-UHFFFAOYSA-N 0.000 description 1
- 125000005291 haloalkenyloxy group Chemical group 0.000 description 1
- 125000005292 haloalkynyloxy group Chemical group 0.000 description 1
- 125000006277 halobenzyl group Chemical group 0.000 description 1
- CNKHSLKYRMDDNQ-UHFFFAOYSA-N halofenozide Chemical compound C=1C=CC=CC=1C(=O)N(C(C)(C)C)NC(=O)C1=CC=C(Cl)C=C1 CNKHSLKYRMDDNQ-UHFFFAOYSA-N 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- BKACAEJQMLLGAV-PLNGDYQASA-N heptafluthrin Chemical compound FC1=C(F)C(COC)=C(F)C(F)=C1COC(=O)C1C(C)(C)C1\C=C/C(F)(F)F BKACAEJQMLLGAV-PLNGDYQASA-N 0.000 description 1
- 125000004446 heteroarylalkyl group Chemical group 0.000 description 1
- 125000005312 heteroarylalkynyl group Chemical group 0.000 description 1
- 125000005223 heteroarylcarbonyl group Chemical group 0.000 description 1
- 125000005553 heteroaryloxy group Chemical group 0.000 description 1
- 125000004415 heterocyclylalkyl group Chemical group 0.000 description 1
- 125000005844 heterocyclyloxy group Chemical group 0.000 description 1
- 125000004468 heterocyclylthio group Chemical group 0.000 description 1
- RGNPBRKPHBKNKX-UHFFFAOYSA-N hexaflumuron Chemical compound C1=C(Cl)C(OC(F)(F)C(F)F)=C(Cl)C=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F RGNPBRKPHBKNKX-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000003898 horticulture Methods 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- FYQGBXGJFWXIPP-UHFFFAOYSA-N hydroprene Chemical compound CCOC(=O)C=C(C)C=CCC(C)CCCC(C)C FYQGBXGJFWXIPP-UHFFFAOYSA-N 0.000 description 1
- 229930000073 hydroprene Natural products 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- HICUREFSAIZXFQ-JOWPUVSESA-N i9z29i000j Chemical compound C1C[C@H](C)[C@@H](CC)O[C@@]21O[C@H](C\C=C(C)\[C@H](OC(=O)C(=N/OC)\C=1C=CC=CC=1)[C@@H](C)\C=C\C=C/1[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\1)O)C[C@H]4C2 HICUREFSAIZXFQ-JOWPUVSESA-N 0.000 description 1
- RONFGUROBZGJKP-UHFFFAOYSA-N iminoctadine Chemical compound NC(N)=NCCCCCCCCNCCCCCCCCN=C(N)N RONFGUROBZGJKP-UHFFFAOYSA-N 0.000 description 1
- VPRAQYXPZIFIOH-UHFFFAOYSA-N imiprothrin Chemical compound CC1(C)C(C=C(C)C)C1C(=O)OCN1C(=O)N(CC#C)CC1=O VPRAQYXPZIFIOH-UHFFFAOYSA-N 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 1
- 125000003454 indenyl group Chemical group C1(C=CC2=CC=CC=C12)* 0.000 description 1
- 239000003617 indole-3-acetic acid Substances 0.000 description 1
- VBCVPMMZEGZULK-NRFANRHFSA-N indoxacarb Chemical compound C([C@@]1(OC2)C(=O)OC)C3=CC(Cl)=CC=C3C1=NN2C(=O)N(C(=O)OC)C1=CC=C(OC(F)(F)F)C=C1 VBCVPMMZEGZULK-NRFANRHFSA-N 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- YTCIYOXHHQLDEI-SNVBAGLBSA-N inpyrfluxam Chemical compound C([C@H](C=12)C)C(C)(C)C2=CC=CC=1NC(=O)C1=CN(C)N=C1C(F)F YTCIYOXHHQLDEI-SNVBAGLBSA-N 0.000 description 1
- 230000000749 insecticidal effect Effects 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- QTYCMDBMOLSEAM-UHFFFAOYSA-N ipconazole Chemical compound C1=NC=NN1CC1(O)C(C(C)C)CCC1CC1=CC=C(Cl)C=C1 QTYCMDBMOLSEAM-UHFFFAOYSA-N 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- YFVOXLJXJBQDEF-UHFFFAOYSA-N isocarbophos Chemical compound COP(N)(=S)OC1=CC=CC=C1C(=O)OC(C)C YFVOXLJXJBQDEF-UHFFFAOYSA-N 0.000 description 1
- HOQADATXFBOEGG-UHFFFAOYSA-N isofenphos Chemical compound CCOP(=S)(NC(C)C)OC1=CC=CC=C1C(=O)OC(C)C HOQADATXFBOEGG-UHFFFAOYSA-N 0.000 description 1
- WMKZDPFZIZQROT-UHFFFAOYSA-N isofetamid Chemical compound CC1=CC(OC(C)C)=CC=C1C(=O)C(C)(C)NC(=O)C1=C(C)C=CS1 WMKZDPFZIZQROT-UHFFFAOYSA-N 0.000 description 1
- VFQXVTODMYMSMJ-UHFFFAOYSA-N isonicotinamide Chemical compound NC(=O)C1=CC=NC=C1 VFQXVTODMYMSMJ-UHFFFAOYSA-N 0.000 description 1
- QBSJMKIUCUGGNG-UHFFFAOYSA-N isoprocarb Chemical compound CNC(=O)OC1=CC=CC=C1C(C)C QBSJMKIUCUGGNG-UHFFFAOYSA-N 0.000 description 1
- 125000004254 isoquinolin-1-yl group Chemical group [H]C1=C([H])C2=C([H])C([H])=C([H])C([H])=C2C(*)=N1 0.000 description 1
- 125000004551 isoquinolin-3-yl group Chemical group C1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 125000004552 isoquinolin-4-yl group Chemical group C1=NC=C(C2=CC=CC=C12)* 0.000 description 1
- 150000002537 isoquinolines Chemical class 0.000 description 1
- 125000001793 isothiazol-3-yl group Chemical group [H]C1=C([H])C(*)=NS1 0.000 description 1
- 125000004500 isothiazol-4-yl group Chemical group S1N=CC(=C1)* 0.000 description 1
- 125000004501 isothiazol-5-yl group Chemical group S1N=CC=C1* 0.000 description 1
- WLPCAERCXQSYLQ-UHFFFAOYSA-N isotianil Chemical compound ClC1=NSC(C(=O)NC=2C(=CC=CC=2)C#N)=C1Cl WLPCAERCXQSYLQ-UHFFFAOYSA-N 0.000 description 1
- SDMSCIWHRZJSRN-UHFFFAOYSA-N isoxathion Chemical compound O1N=C(OP(=S)(OCC)OCC)C=C1C1=CC=CC=C1 SDMSCIWHRZJSRN-UHFFFAOYSA-N 0.000 description 1
- 125000004284 isoxazol-3-yl group Chemical group [H]C1=C([H])C(*)=NO1 0.000 description 1
- 125000004498 isoxazol-4-yl group Chemical group O1N=CC(=C1)* 0.000 description 1
- 125000004499 isoxazol-5-yl group Chemical group O1N=CC=C1* 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- UGWALRUNBSBTGI-ZKMZRDRYSA-N kadethrin Chemical compound C(/[C@@H]1C([C@@H]1C(=O)OCC=1C=C(CC=2C=CC=CC=2)OC=1)(C)C)=C1/CCSC1=O UGWALRUNBSBTGI-ZKMZRDRYSA-N 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- LKAHTZMLAOKUJF-UHFFFAOYSA-N ketospiradox-potassium Chemical compound [K+].CC=1C(C2(OCCO2)CCS2(=O)=O)=C2C(C)=CC=1C(=O)[C-]1C(=O)CCCC1=O LKAHTZMLAOKUJF-UHFFFAOYSA-N 0.000 description 1
- 229930001540 kinoprene Natural products 0.000 description 1
- ZOTBXTZVPHCKPN-HTXNQAPBSA-N kresoxim-methyl Chemical compound CO\N=C(\C(=O)OC)C1=CC=CC=C1COC1=CC=CC=C1C ZOTBXTZVPHCKPN-HTXNQAPBSA-N 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 239000005910 lambda-Cyhalothrin Substances 0.000 description 1
- 125000005647 linker group Chemical group 0.000 description 1
- 235000020778 linoleic acid Nutrition 0.000 description 1
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- JLUHDLXQXJRHKA-UHFFFAOYSA-M lithium;2,3,6-trichlorobenzoate Chemical compound [Li+].[O-]C(=O)C1=C(Cl)C=CC(Cl)=C1Cl JLUHDLXQXJRHKA-UHFFFAOYSA-M 0.000 description 1
- WBGFKUKWGNKHTN-UHFFFAOYSA-M lithium;2-(2,4-dichlorophenoxy)acetate Chemical compound [Li+].[O-]C(=O)COC1=CC=C(Cl)C=C1Cl WBGFKUKWGNKHTN-UHFFFAOYSA-M 0.000 description 1
- VIMSQXDDXJYTLW-UHFFFAOYSA-M lithium;5-bromo-3-butan-2-yl-6-methylpyrimidin-1-ide-2,4-dione Chemical compound [Li+].CCC(C)N1C(=O)[N-]C(C)=C(Br)C1=O VIMSQXDDXJYTLW-UHFFFAOYSA-M 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 229950002303 lotilaner Drugs 0.000 description 1
- 229960000521 lufenuron Drugs 0.000 description 1
- PWPJGUXAGUPAHP-UHFFFAOYSA-N lufenuron Chemical compound C1=C(Cl)C(OC(F)(F)C(C(F)(F)F)F)=CC(Cl)=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F PWPJGUXAGUPAHP-UHFFFAOYSA-N 0.000 description 1
- YMNRJYYHNXITFZ-UHFFFAOYSA-L magnesium;2,2,2-trichloroacetate Chemical compound [Mg+2].[O-]C(=O)C(Cl)(Cl)Cl.[O-]C(=O)C(Cl)(Cl)Cl YMNRJYYHNXITFZ-UHFFFAOYSA-L 0.000 description 1
- QPPXJFBMSFYKMM-UHFFFAOYSA-L magnesium;2,2-dichloropropanoate Chemical compound [Mg+2].CC(Cl)(Cl)C([O-])=O.CC(Cl)(Cl)C([O-])=O QPPXJFBMSFYKMM-UHFFFAOYSA-L 0.000 description 1
- 229960000453 malathion Drugs 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- KLGMSAOQDHLCOS-UHFFFAOYSA-N mecarbam Chemical compound CCOC(=O)N(C)C(=O)CSP(=S)(OCC)OCC KLGMSAOQDHLCOS-UHFFFAOYSA-N 0.000 description 1
- 230000008099 melanin synthesis Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- JCHMGYRXQDASJE-UHFFFAOYSA-N metcamifen Chemical compound C1=CC(NC(=O)NC)=CC=C1S(=O)(=O)NC(=O)C1=CC=CC=C1OC JCHMGYRXQDASJE-UHFFFAOYSA-N 0.000 description 1
- XWPZUHJBOLQNMN-UHFFFAOYSA-N metconazole Chemical compound C1=NC=NN1CC1(O)C(C)(C)CCC1CC1=CC=C(Cl)C=C1 XWPZUHJBOLQNMN-UHFFFAOYSA-N 0.000 description 1
- HNQIVZYLYMDVSB-NJFSPNSNSA-N methanesulfonamide Chemical compound [14CH3]S(N)(=O)=O HNQIVZYLYMDVSB-NJFSPNSNSA-N 0.000 description 1
- MEBQXILRKZHVCX-UHFFFAOYSA-N methidathion Chemical compound COC1=NN(CSP(=S)(OC)OC)C(=O)S1 MEBQXILRKZHVCX-UHFFFAOYSA-N 0.000 description 1
- UHXUZOCRWCRNSJ-QPJJXVBHSA-N methomyl Chemical compound CNC(=O)O\N=C(/C)SC UHXUZOCRWCRNSJ-QPJJXVBHSA-N 0.000 description 1
- 229930002897 methoprene Natural products 0.000 description 1
- 229950003442 methoprene Drugs 0.000 description 1
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 1
- QCAWEPFNJXQPAN-UHFFFAOYSA-N methoxyfenozide Chemical compound COC1=CC=CC(C(=O)NN(C(=O)C=2C=C(C)C=C(C)C=2)C(C)(C)C)=C1C QCAWEPFNJXQPAN-UHFFFAOYSA-N 0.000 description 1
- AIBVECHZVPKHDS-GHYOLMRSSA-N methyl (2z,4e)-5-(6-ethynyl-1-hydroxy-2,6-dimethyl-4-oxocyclohex-2-en-1-yl)-3-methylpenta-2,4-dienoate Chemical compound COC(=O)\C=C(\C)/C=C/C1(O)C(C)=CC(=O)CC1(C)C#C AIBVECHZVPKHDS-GHYOLMRSSA-N 0.000 description 1
- GEPDYQSQVLXLEU-AATRIKPKSA-N methyl (e)-3-dimethoxyphosphoryloxybut-2-enoate Chemical compound COC(=O)\C=C(/C)OP(=O)(OC)OC GEPDYQSQVLXLEU-AATRIKPKSA-N 0.000 description 1
- HWIGZMADSFQMOI-UHFFFAOYSA-N methyl 2-(2,4-dichlorophenoxy)acetate Chemical compound COC(=O)COC1=CC=C(Cl)C=C1Cl HWIGZMADSFQMOI-UHFFFAOYSA-N 0.000 description 1
- FFCCBBNQPIMUJI-UHFFFAOYSA-N methyl 2-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)-para-toluate Chemical compound COC(=O)C1=CC=C(C)C=C1C1=NC(C)(C(C)C)C(=O)N1 FFCCBBNQPIMUJI-UHFFFAOYSA-N 0.000 description 1
- PMJRLYSMEOLBIU-UHFFFAOYSA-N methyl 2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)quinoline-3-carboxylate Chemical compound COC(=O)C1=CC2=CC=CC=C2N=C1C1=NC(C)(C(C)C)C(=O)N1 PMJRLYSMEOLBIU-UHFFFAOYSA-N 0.000 description 1
- MFSWTRQUCLNFOM-UHFFFAOYSA-N methyl 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl MFSWTRQUCLNFOM-UHFFFAOYSA-N 0.000 description 1
- NDQZMBNEHYSVPL-UHFFFAOYSA-N methyl 2-(5-chloroquinolin-8-yl)oxyacetate Chemical compound C1=CN=C2C(OCC(=O)OC)=CC=C(Cl)C2=C1 NDQZMBNEHYSVPL-UHFFFAOYSA-N 0.000 description 1
- RBNIGDFIUWJJEV-UHFFFAOYSA-N methyl 2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N(C(C)C(=O)OC)C(=O)C1=CC=CC=C1 RBNIGDFIUWJJEV-UHFFFAOYSA-N 0.000 description 1
- BOIUPYHMTRFIJN-UHFFFAOYSA-N methyl 2-[(3-oxo-1h-isothiochromen-4-ylidene)methoxy]acetate Chemical compound C1=CC=C2C(=COCC(=O)OC)C(=O)SCC2=C1 BOIUPYHMTRFIJN-UHFFFAOYSA-N 0.000 description 1
- DTVOKYWXACGVGO-UHFFFAOYSA-N methyl 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-6-(trifluoromethyl)pyridine-3-carboxylate Chemical compound COC(=O)C1=CC=C(C(F)(F)F)N=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 DTVOKYWXACGVGO-UHFFFAOYSA-N 0.000 description 1
- OUGVRUOROTYZSS-UHFFFAOYSA-N methyl 2-[(4-methoxy-6-methylsulfanylpyrimidin-2-yl)carbamoylsulfamoyl]benzoate Chemical compound COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(SC)=N1 OUGVRUOROTYZSS-UHFFFAOYSA-N 0.000 description 1
- VGBNSONMEGTIDX-UHFFFAOYSA-N methyl 2-[(4-methylpyrimidin-2-yl)carbamoylsulfamoyl]benzoate Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC=CC(C)=N1 VGBNSONMEGTIDX-UHFFFAOYSA-N 0.000 description 1
- IAUMNRCGDHLAMJ-UHFFFAOYSA-N methyl 2-[4-[5-(trifluoromethyl)pyridin-2-yl]oxyphenoxy]propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 IAUMNRCGDHLAMJ-UHFFFAOYSA-N 0.000 description 1
- ZTYVMAQSHCZXLF-UHFFFAOYSA-N methyl 2-[[4,6-bis(difluoromethoxy)pyrimidin-2-yl]carbamoylsulfamoyl]benzoate Chemical compound COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC(F)F)=CC(OC(F)F)=N1 ZTYVMAQSHCZXLF-UHFFFAOYSA-N 0.000 description 1
- LINPVWIEWJTEEJ-UHFFFAOYSA-N methyl 2-chloro-9-hydroxyfluorene-9-carboxylate Chemical group C1=C(Cl)C=C2C(C(=O)OC)(O)C3=CC=CC=C3C2=C1 LINPVWIEWJTEEJ-UHFFFAOYSA-N 0.000 description 1
- RTAXDHMFSZODHZ-UHFFFAOYSA-N methyl 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1h-indol-6-yl)pyridine-2-carboxylate Chemical compound NC1=C(Cl)C(C(=O)OC)=NC(C=2C(=C3NC=CC3=CC=2)F)=C1F RTAXDHMFSZODHZ-UHFFFAOYSA-N 0.000 description 1
- ZQEIXNIJLIKNTD-UHFFFAOYSA-N methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alaninate Chemical compound COCC(=O)N(C(C)C(=O)OC)C1=C(C)C=CC=C1C ZQEIXNIJLIKNTD-UHFFFAOYSA-N 0.000 description 1
- 229940102396 methyl bromide Drugs 0.000 description 1
- HAMGRBXTJNITHG-UHFFFAOYSA-N methyl isocyanate Chemical compound CN=C=O HAMGRBXTJNITHG-UHFFFAOYSA-N 0.000 description 1
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 229920000257 metiram Polymers 0.000 description 1
- HIIRDDUVRXCDBN-OBGWFSINSA-N metominostrobin Chemical compound CNC(=O)C(=N\OC)\C1=CC=CC=C1OC1=CC=CC=C1 HIIRDDUVRXCDBN-OBGWFSINSA-N 0.000 description 1
- 229960001952 metrifonate Drugs 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- FXWHFKOXMBTCMP-WMEDONTMSA-N milbemycin Natural products COC1C2OCC3=C/C=C/C(C)CC(=CCC4CC(CC5(O4)OC(C)C(C)C(OC(=O)C(C)CC(C)C)C5O)OC(=O)C(C=C1C)C23O)C FXWHFKOXMBTCMP-WMEDONTMSA-N 0.000 description 1
- KCIRYJNISRMYFI-UHFFFAOYSA-N mildiomycin Natural products NC(CO)C(=O)NC1C=CC(OC1C(O)(CC(O)CNC(=N)N)C(=O)O)N2CN=C(N)C(=C2)CO KCIRYJNISRMYFI-UHFFFAOYSA-N 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000003129 miticidal effect Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- KRTSDMXIXPKRQR-AATRIKPKSA-N monocrotophos Chemical compound CNC(=O)\C=C(/C)OP(=O)(OC)OC KRTSDMXIXPKRQR-AATRIKPKSA-N 0.000 description 1
- 125000006682 monohaloalkyl group Chemical group 0.000 description 1
- 238000002887 multiple sequence alignment Methods 0.000 description 1
- 239000003471 mutagenic agent Substances 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- GIPNVQZZSSKOQF-UHFFFAOYSA-N n'-(2,5-dimethyl-4-phenoxyphenyl)-n-ethyl-n-methylmethanimidamide Chemical compound C1=C(C)C(N=CN(C)CC)=CC(C)=C1OC1=CC=CC=C1 GIPNVQZZSSKOQF-UHFFFAOYSA-N 0.000 description 1
- CLDHVFBXNWTWKV-UHFFFAOYSA-N n'-[2,5-dimethyl-4-[3-(1,1,2,2-tetrafluoroethoxy)phenyl]sulfanylphenyl]-n-ethyl-n-methylmethanimidamide Chemical compound C1=C(C)C(N=CN(C)CC)=CC(C)=C1SC1=CC=CC(OC(F)(F)C(F)F)=C1 CLDHVFBXNWTWKV-UHFFFAOYSA-N 0.000 description 1
- FXLMOAQSXRPYGT-UHFFFAOYSA-N n'-[4-[3-(difluoromethoxy)phenyl]sulfanyl-2,5-dimethylphenyl]-n-ethyl-n-methylmethanimidamide Chemical compound C1=C(C)C(N=CN(C)CC)=CC(C)=C1SC1=CC=CC(OC(F)F)=C1 FXLMOAQSXRPYGT-UHFFFAOYSA-N 0.000 description 1
- QPWDBFQQYNCENC-UHFFFAOYSA-N n'-[5-bromo-6-(2,3-dihydro-1h-inden-2-yloxy)-2-methylpyridin-3-yl]-n-ethyl-n-methylmethanimidamide Chemical compound N1=C(C)C(N=CN(C)CC)=CC(Br)=C1OC1CC2=CC=CC=C2C1 QPWDBFQQYNCENC-UHFFFAOYSA-N 0.000 description 1
- QUBHBTSVQMARKX-GFCCVEGCSA-N n'-[5-bromo-6-[(1r)-1-(3,5-difluorophenyl)ethoxy]-2-methylpyridin-3-yl]-n-ethyl-n-methylmethanimidamide Chemical compound N1=C(C)C(N=CN(C)CC)=CC(Br)=C1O[C@H](C)C1=CC(F)=CC(F)=C1 QUBHBTSVQMARKX-GFCCVEGCSA-N 0.000 description 1
- NVFPHKSEIQNWCE-UHFFFAOYSA-N n'-methylmethanimidamide Chemical compound CNC=N NVFPHKSEIQNWCE-UHFFFAOYSA-N 0.000 description 1
- TUCSJFNYZJYLHE-UHFFFAOYSA-N n'-tert-butyl-n'-(3,5-dimethylbenzoyl)-2,7-dimethyl-2,3-dihydro-1-benzofuran-6-carbohydrazide Chemical compound CC1=C2OC(C)CC2=CC=C1C(=O)NN(C(C)(C)C)C(=O)C1=CC(C)=CC(C)=C1 TUCSJFNYZJYLHE-UHFFFAOYSA-N 0.000 description 1
- BLCKKNLGFULNRC-UHFFFAOYSA-L n,n-dimethylcarbamodithioate;nickel(2+) Chemical compound [Ni+2].CN(C)C([S-])=S.CN(C)C([S-])=S BLCKKNLGFULNRC-UHFFFAOYSA-L 0.000 description 1
- XESCSVABNVAQQS-UHFFFAOYSA-N n-(3-chloro-4-propan-2-ylphenyl)-2-methylpentanamide Chemical compound CCCC(C)C(=O)NC1=CC=C(C(C)C)C(Cl)=C1 XESCSVABNVAQQS-UHFFFAOYSA-N 0.000 description 1
- MEWYBGBLQURRNP-UHFFFAOYSA-N n-(3-ethyl-3,5,5-trimethylcyclohexyl)-3-formamido-2-hydroxybenzamide Chemical compound C1C(C)(C)CC(CC)(C)CC1NC(=O)C1=CC=CC(NC=O)=C1O MEWYBGBLQURRNP-UHFFFAOYSA-N 0.000 description 1
- QTGVGIVRLSGTJJ-UHFFFAOYSA-N n-(acetamidomethyl)-2-chloro-n-(2,6-diethylphenyl)acetamide Chemical compound CCC1=CC=CC(CC)=C1N(CNC(C)=O)C(=O)CCl QTGVGIVRLSGTJJ-UHFFFAOYSA-N 0.000 description 1
- YGGNKXSVYCKXCA-UHFFFAOYSA-N n-[(2-cyclopentyl-5-fluorophenyl)methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=C(F)C=2)C2CCCC2)C2CC2)=C1F YGGNKXSVYCKXCA-UHFFFAOYSA-N 0.000 description 1
- ACDCNNFPJPHJCQ-UHFFFAOYSA-N n-[(2-tert-butyl-5-methylphenyl)methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CC1=CC=C(C(C)(C)C)C(CN(C2CC2)C(=O)C=2C(=NN(C)C=2F)C(F)F)=C1 ACDCNNFPJPHJCQ-UHFFFAOYSA-N 0.000 description 1
- PZSVWPHTFIJDAX-UHFFFAOYSA-N n-[(2-tert-butylphenyl)methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=CC=2)C(C)(C)C)C2CC2)=C1F PZSVWPHTFIJDAX-UHFFFAOYSA-N 0.000 description 1
- ZNPUHPIMSDMYGC-UHFFFAOYSA-N n-[(5-chloro-2-ethylphenyl)methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CCC1=CC=C(Cl)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 ZNPUHPIMSDMYGC-UHFFFAOYSA-N 0.000 description 1
- JEFUQUGZXLEHLD-UHFFFAOYSA-N n-[(5-chloro-2-propan-2-ylphenyl)methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CC(C)C1=CC=C(Cl)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 JEFUQUGZXLEHLD-UHFFFAOYSA-N 0.000 description 1
- CAGKXPHIFFSYLL-UHFFFAOYSA-N n-[1-(2,4-dichlorophenyl)-1-methoxypropan-2-yl]-3-(difluoromethyl)-1-methylpyrazole-4-carboxamide Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(OC)C(C)NC(=O)C1=CN(C)N=C1C(F)F CAGKXPHIFFSYLL-UHFFFAOYSA-N 0.000 description 1
- DHQKLWKZSFCKTA-UHFFFAOYSA-N n-[1-[(6-chloropyridin-3-yl)methyl]pyridin-2-ylidene]-2,2,2-trifluoroacetamide Chemical compound FC(F)(F)C(=O)N=C1C=CC=CN1CC1=CC=C(Cl)N=C1 DHQKLWKZSFCKTA-UHFFFAOYSA-N 0.000 description 1
- JDMXBYDZNLFFCJ-UHFFFAOYSA-N n-[2,4-dimethyl-5-(trifluoromethylsulfonylamino)phenyl]acetamide;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.CC(=O)NC1=CC(NS(=O)(=O)C(F)(F)F)=C(C)C=C1C JDMXBYDZNLFFCJ-UHFFFAOYSA-N 0.000 description 1
- FITSYTCYOITKJL-UHFFFAOYSA-N n-[2-[(3,3-dimethyl-2-oxoazetidin-1-yl)methyl]phenyl]-1,1,1-trifluoromethanesulfonamide Chemical compound O=C1C(C)(C)CN1CC1=CC=CC=C1NS(=O)(=O)C(F)(F)F FITSYTCYOITKJL-UHFFFAOYSA-N 0.000 description 1
- DPLHJUQFGXECAS-UHFFFAOYSA-N n-[3-(benzylcarbamoyl)-4-chlorophenyl]-2-methyl-5-(1,1,2,2,2-pentafluoroethyl)-4-(trifluoromethyl)pyrazole-3-carboxamide Chemical compound CN1N=C(C(F)(F)C(F)(F)F)C(C(F)(F)F)=C1C(=O)NC1=CC=C(Cl)C(C(=O)NCC=2C=CC=CC=2)=C1 DPLHJUQFGXECAS-UHFFFAOYSA-N 0.000 description 1
- NYDKNJDNBUFWRJ-UHFFFAOYSA-N n-[4-(dimethylcarbamoylamino)phenyl]sulfonyl-2-methoxybenzamide Chemical compound COC1=CC=CC=C1C(=O)NS(=O)(=O)C1=CC=C(NC(=O)N(C)C)C=C1 NYDKNJDNBUFWRJ-UHFFFAOYSA-N 0.000 description 1
- HZDIJTXDRLNTIS-DAXSKMNVSA-N n-[[(z)-but-2-enoxy]methyl]-2-chloro-n-(2,6-diethylphenyl)acetamide Chemical compound CCC1=CC=CC(CC)=C1N(COC\C=C/C)C(=O)CCl HZDIJTXDRLNTIS-DAXSKMNVSA-N 0.000 description 1
- YNKFZRGTXAPYFD-UHFFFAOYSA-N n-[[2-chloro-3,5-bis(trifluoromethyl)phenyl]carbamoyl]-2,6-difluorobenzamide Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1Cl YNKFZRGTXAPYFD-UHFFFAOYSA-N 0.000 description 1
- UTXLNSPIBIMGQJ-UHFFFAOYSA-N n-[[2-chloro-6-(trifluoromethyl)phenyl]methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=CC=2Cl)C(F)(F)F)C2CC2)=C1F UTXLNSPIBIMGQJ-UHFFFAOYSA-N 0.000 description 1
- POIXHZKAAMCWOT-UHFFFAOYSA-N n-[[3-chloro-2-fluoro-6-(trifluoromethyl)phenyl]methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=C(Cl)C=2F)C(F)(F)F)C2CC2)=C1F POIXHZKAAMCWOT-UHFFFAOYSA-N 0.000 description 1
- HEMPTJWLSOMNER-UHFFFAOYSA-N n-[[5-chloro-2-(trifluoromethyl)phenyl]methyl]-n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=C(Cl)C=2)C(F)(F)F)C2CC2)=C1F HEMPTJWLSOMNER-UHFFFAOYSA-N 0.000 description 1
- BFFLCMLOXQNHJA-UHFFFAOYSA-N n-[ethoxy-(4-methyl-2-nitrophenoxy)phosphinothioyl]propan-2-amine Chemical compound CCOP(=S)(NC(C)C)OC1=CC=C(C)C=C1[N+]([O-])=O BFFLCMLOXQNHJA-UHFFFAOYSA-N 0.000 description 1
- VHEWQRWLIDWRMR-UHFFFAOYSA-N n-[methoxy-(4-methyl-2-nitrophenoxy)phosphinothioyl]propan-2-amine Chemical compound CC(C)NP(=S)(OC)OC1=CC=C(C)C=C1[N+]([O-])=O VHEWQRWLIDWRMR-UHFFFAOYSA-N 0.000 description 1
- YSVBFKLFADDJEI-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methyl-n-[(2-propan-2-ylphenyl)methyl]pyrazole-4-carbothioamide Chemical compound CC(C)C1=CC=CC=C1CN(C(=S)C=1C(=NN(C)C=1F)C(F)F)C1CC1 YSVBFKLFADDJEI-UHFFFAOYSA-N 0.000 description 1
- YBQARPUVLHEOSY-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methyl-n-[(2-propan-2-ylphenyl)methyl]pyrazole-4-carboxamide Chemical compound CC(C)C1=CC=CC=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 YBQARPUVLHEOSY-UHFFFAOYSA-N 0.000 description 1
- MRCJJCNYJGXHAU-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methyl-n-[(5-methyl-2-propan-2-ylphenyl)methyl]pyrazole-4-carboxamide Chemical compound CC(C)C1=CC=C(C)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 MRCJJCNYJGXHAU-UHFFFAOYSA-N 0.000 description 1
- GZSDSDAVLHAHBW-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-1-methyl-n-[[5-methyl-2-(trifluoromethyl)phenyl]methyl]pyrazole-4-carboxamide Chemical compound CC1=CC=C(C(F)(F)F)C(CN(C2CC2)C(=O)C=2C(=NN(C)C=2F)C(F)F)=C1 GZSDSDAVLHAHBW-UHFFFAOYSA-N 0.000 description 1
- YBQVYCCAGISJDK-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-n-[(2-fluoro-6-propan-2-ylphenyl)methyl]-1-methylpyrazole-4-carboxamide Chemical compound CC(C)C1=CC=CC(F)=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 YBQVYCCAGISJDK-UHFFFAOYSA-N 0.000 description 1
- KBWODRLEGDCUHW-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-5-fluoro-n-[(5-fluoro-2-propan-2-ylphenyl)methyl]-1-methylpyrazole-4-carboxamide Chemical compound CC(C)C1=CC=C(F)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 KBWODRLEGDCUHW-UHFFFAOYSA-N 0.000 description 1
- FLQNESNIAKFCNJ-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-n-[(2-ethyl-4,5-dimethylphenyl)methyl]-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CCC1=CC(C)=C(C)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 FLQNESNIAKFCNJ-UHFFFAOYSA-N 0.000 description 1
- GVRURMVTCDBWMT-UHFFFAOYSA-N n-cyclopropyl-3-(difluoromethyl)-n-[(2-ethyl-5-methylphenyl)methyl]-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CCC1=CC=C(C)C=C1CN(C(=O)C=1C(=NN(C)C=1F)C(F)F)C1CC1 GVRURMVTCDBWMT-UHFFFAOYSA-N 0.000 description 1
- PBBIVBOBRLKLGM-UHFFFAOYSA-N n-cyclopropyl-n-[(2-cyclopropyl-5-fluorophenyl)methyl]-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound CN1N=C(C(F)F)C(C(=O)N(CC=2C(=CC=C(F)C=2)C2CC2)C2CC2)=C1F PBBIVBOBRLKLGM-UHFFFAOYSA-N 0.000 description 1
- ZDVWJXRWGQXPIA-UHFFFAOYSA-N n-cyclopropyl-n-[(2-cyclopropyl-5-methylphenyl)methyl]-3-(difluoromethyl)-5-fluoro-1-methylpyrazole-4-carboxamide Chemical compound C1CC1N(C(=O)C=1C(=NN(C)C=1F)C(F)F)CC1=CC(C)=CC=C1C1CC1 ZDVWJXRWGQXPIA-UHFFFAOYSA-N 0.000 description 1
- PEQJBOMPGWYIRO-UHFFFAOYSA-N n-ethyl-3,4-dimethoxyaniline Chemical compound CCNC1=CC=C(OC)C(OC)=C1 PEQJBOMPGWYIRO-UHFFFAOYSA-N 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- QSFRJEUITMJQCX-UHFFFAOYSA-N n-methylmethanamine;2,3,6-trichlorobenzoic acid Chemical compound C[NH2+]C.[O-]C(=O)C1=C(Cl)C=CC(Cl)=C1Cl QSFRJEUITMJQCX-UHFFFAOYSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- UQJQVUOTMVCFHX-UHFFFAOYSA-L nabam Chemical compound [Na+].[Na+].[S-]C(=S)NCCNC([S-])=S UQJQVUOTMVCFHX-UHFFFAOYSA-L 0.000 description 1
- BUYMVQAILCEWRR-UHFFFAOYSA-N naled Chemical compound COP(=O)(OC)OC(Br)C(Cl)(Cl)Br BUYMVQAILCEWRR-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 235000010298 natamycin Nutrition 0.000 description 1
- 239000004311 natamycin Substances 0.000 description 1
- NCXMLFZGDNKEPB-FFPOYIOWSA-N natamycin Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C[C@@H](C)OC(=O)/C=C/[C@H]2O[C@@H]2C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 NCXMLFZGDNKEPB-FFPOYIOWSA-N 0.000 description 1
- 229960003255 natamycin Drugs 0.000 description 1
- 230000001069 nematicidal effect Effects 0.000 description 1
- 239000005645 nematicide Substances 0.000 description 1
- RJMUSRYZPJIFPJ-UHFFFAOYSA-N niclosamide Chemical compound OC1=CC=C(Cl)C=C1C(=O)NC1=CC=C([N+]([O-])=O)C=C1Cl RJMUSRYZPJIFPJ-UHFFFAOYSA-N 0.000 description 1
- 229960001920 niclosamide Drugs 0.000 description 1
- 229960002715 nicotine Drugs 0.000 description 1
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 1
- 229940079888 nitenpyram Drugs 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- JVJQPDTXIALXOG-UHFFFAOYSA-N nitryl fluoride Chemical compound [O-][N+](F)=O JVJQPDTXIALXOG-UHFFFAOYSA-N 0.000 description 1
- 239000003284 nod factor Substances 0.000 description 1
- 230000024121 nodulation Effects 0.000 description 1
- BMQNWLUEXNQIGL-UHFFFAOYSA-N nonanoic acid Chemical compound CCCCCCCCC(O)=O.CCCCCCCCC(O)=O BMQNWLUEXNQIGL-UHFFFAOYSA-N 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 108010003516 norsynephrine receptor Proteins 0.000 description 1
- NJPPVKZQTLUDBO-UHFFFAOYSA-N novaluron Chemical compound C1=C(Cl)C(OC(F)(F)C(OC(F)(F)F)F)=CC=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F NJPPVKZQTLUDBO-UHFFFAOYSA-N 0.000 description 1
- YTYGAJLZOJPJGH-UHFFFAOYSA-N noviflumuron Chemical compound FC1=C(Cl)C(OC(F)(F)C(C(F)(F)F)F)=C(Cl)C=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F YTYGAJLZOJPJGH-UHFFFAOYSA-N 0.000 description 1
- 239000003865 nucleic acid synthesis inhibitor Substances 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- PMOWTIHVNWZYFI-UHFFFAOYSA-N o-Coumaric acid Natural products OC(=O)C=CC1=CC=CC=C1O PMOWTIHVNWZYFI-UHFFFAOYSA-N 0.000 description 1
- ZAPVNKQEZNTYQV-UHFFFAOYSA-N octan-3-yl 4-(2,4-dichlorophenoxy)butanoate Chemical compound CCCCCC(CC)OC(=O)CCCOC1=CC=C(Cl)C=C1Cl ZAPVNKQEZNTYQV-UHFFFAOYSA-N 0.000 description 1
- 150000002482 oligosaccharides Polymers 0.000 description 1
- PZXOQEXFMJCDPG-UHFFFAOYSA-N omethoate Chemical compound CNC(=O)CSP(=O)(OC)OC PZXOQEXFMJCDPG-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- LLLFASISUZUJEQ-UHFFFAOYSA-N orbencarb Chemical compound CCN(CC)C(=O)SCC1=CC=CC=C1Cl LLLFASISUZUJEQ-UHFFFAOYSA-N 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000005416 organic matter Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 150000002903 organophosphorus compounds Chemical class 0.000 description 1
- 235000010292 orthophenyl phenol Nutrition 0.000 description 1
- 238000009401 outcrossing Methods 0.000 description 1
- 150000004866 oxadiazoles Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- KZAUOCCYDRDERY-UHFFFAOYSA-N oxamyl Chemical compound CNC(=O)ON=C(SC)C(=O)N(C)C KZAUOCCYDRDERY-UHFFFAOYSA-N 0.000 description 1
- 150000001475 oxazolidinediones Chemical class 0.000 description 1
- ATYBXHSAIOKLMG-UHFFFAOYSA-N oxepin Chemical compound O1C=CC=CC=C1 ATYBXHSAIOKLMG-UHFFFAOYSA-N 0.000 description 1
- 125000003585 oxepinyl group Chemical group 0.000 description 1
- 230000010627 oxidative phosphorylation Effects 0.000 description 1
- 125000000466 oxiranyl group Chemical group 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- INFDPOAKFNIJBF-UHFFFAOYSA-N paraquat Chemical compound C1=C[N+](C)=CC=C1C1=CC=[N+](C)C=C1 INFDPOAKFNIJBF-UHFFFAOYSA-N 0.000 description 1
- RLBIQVVOMOPOHC-UHFFFAOYSA-N parathion-methyl Chemical group COP(=S)(OC)OC1=CC=C([N+]([O-])=O)C=C1 RLBIQVVOMOPOHC-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- LKPLKUMXSAEKID-UHFFFAOYSA-N pentachloronitrobenzene Chemical compound [O-][N+](=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1Cl LKPLKUMXSAEKID-UHFFFAOYSA-N 0.000 description 1
- WKOKIMCPHGHWIF-UHFFFAOYSA-N pentan-2-ol Chemical compound C[CH]CC(C)O WKOKIMCPHGHWIF-UHFFFAOYSA-N 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000005010 perfluoroalkyl group Chemical group 0.000 description 1
- 229960000490 permethrin Drugs 0.000 description 1
- RLLPVAHGXHCWKJ-UHFFFAOYSA-N permethrin Chemical compound CC1(C)C(C=C(Cl)Cl)C1C(=O)OCC1=CC=CC(OC=2C=CC=CC=2)=C1 RLLPVAHGXHCWKJ-UHFFFAOYSA-N 0.000 description 1
- 230000000361 pesticidal effect Effects 0.000 description 1
- 239000000575 pesticide Substances 0.000 description 1
- 239000003090 pesticide formulation Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 description 1
- 229960003536 phenothrin Drugs 0.000 description 1
- XAMUDJHXFNRLCY-UHFFFAOYSA-N phenthoate Chemical compound CCOC(=O)C(SP(=S)(OC)OC)C1=CC=CC=C1 XAMUDJHXFNRLCY-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- IOUNQDKNJZEDEP-UHFFFAOYSA-N phosalone Chemical compound C1=C(Cl)C=C2OC(=O)N(CSP(=S)(OCC)OCC)C2=C1 IOUNQDKNJZEDEP-UHFFFAOYSA-N 0.000 description 1
- LMNZTLDVJIUSHT-UHFFFAOYSA-N phosmet Chemical compound C1=CC=C2C(=O)N(CSP(=S)(OC)OC)C(=O)C2=C1 LMNZTLDVJIUSHT-UHFFFAOYSA-N 0.000 description 1
- RGCLLPNLLBQHPF-HJWRWDBZSA-N phosphamidon Chemical compound CCN(CC)C(=O)C(\Cl)=C(/C)OP(=O)(OC)OC RGCLLPNLLBQHPF-HJWRWDBZSA-N 0.000 description 1
- HOKBIQDJCNTWST-UHFFFAOYSA-N phosphanylidenezinc;zinc Chemical compound [Zn].[Zn]=P.[Zn]=P HOKBIQDJCNTWST-UHFFFAOYSA-N 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003003 phosphines Chemical class 0.000 description 1
- 150000004714 phosphonium salts Chemical class 0.000 description 1
- 235000011007 phosphoric acid Nutrition 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 1
- ATROHALUCMTWTB-OWBHPGMISA-N phoxim Chemical compound CCOP(=S)(OCC)O\N=C(\C#N)C1=CC=CC=C1 ATROHALUCMTWTB-OWBHPGMISA-N 0.000 description 1
- 229950001664 phoxim Drugs 0.000 description 1
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical compound C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 1
- IBSNKSODLGJUMQ-SDNWHVSQSA-N picoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC=CC(C(F)(F)F)=N1 IBSNKSODLGJUMQ-SDNWHVSQSA-N 0.000 description 1
- 125000004483 piperidin-3-yl group Chemical group N1CC(CCC1)* 0.000 description 1
- YFGYUFNIOHWBOB-UHFFFAOYSA-N pirimicarb Chemical compound CN(C)C(=O)OC1=NC(N(C)C)=NC(C)=C1C YFGYUFNIOHWBOB-UHFFFAOYSA-N 0.000 description 1
- QHOQHJPRIBSPCY-UHFFFAOYSA-N pirimiphos-methyl Chemical group CCN(CC)C1=NC(C)=CC(OP(=S)(OC)OC)=N1 QHOQHJPRIBSPCY-UHFFFAOYSA-N 0.000 description 1
- 239000004069 plant analysis Substances 0.000 description 1
- 210000000745 plant chromosome Anatomy 0.000 description 1
- 230000037039 plant physiology Effects 0.000 description 1
- 235000021118 plant-derived protein Nutrition 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 210000002706 plastid Anatomy 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001522 polyglycol ester Polymers 0.000 description 1
- 125000006684 polyhaloalkyl group Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000005077 polysulfide Substances 0.000 description 1
- 229920001021 polysulfide Polymers 0.000 description 1
- 150000008117 polysulfides Polymers 0.000 description 1
- NNFCIKHAZHQZJG-UHFFFAOYSA-N potassium cyanide Chemical compound [K+].N#[C-] NNFCIKHAZHQZJG-UHFFFAOYSA-N 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- SIVJKMBJGCUUNS-NUBCRITNSA-M potassium;(2r)-2-(2,4-dichlorophenoxy)propanoate Chemical compound [K+].[O-]C(=O)[C@@H](C)OC1=CC=C(Cl)C=C1Cl SIVJKMBJGCUUNS-NUBCRITNSA-M 0.000 description 1
- QTWDVSMYLOZQBZ-UHFFFAOYSA-N potassium;(5-acetamido-2,4-dimethylphenyl)-(trifluoromethylsulfonyl)azanide Chemical compound [K+].CC(=O)NC1=CC([N-]S(=O)(=O)C(F)(F)F)=C(C)C=C1C QTWDVSMYLOZQBZ-UHFFFAOYSA-N 0.000 description 1
- KJMADHVXBWVFBX-UHFFFAOYSA-M potassium;2,3,6-trichlorobenzoate Chemical compound [K+].[O-]C(=O)C1=C(Cl)C=CC(Cl)=C1Cl KJMADHVXBWVFBX-UHFFFAOYSA-M 0.000 description 1
- ZSUHWKOUWKJIOR-UHFFFAOYSA-M potassium;2-methyl-4,6-dinitrophenolate Chemical compound [K+].CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1[O-] ZSUHWKOUWKJIOR-UHFFFAOYSA-M 0.000 description 1
- WRBJKRRJBCSNLE-UHFFFAOYSA-M potassium;4-(2,4-dichlorophenoxy)butanoate Chemical compound [K+].[O-]C(=O)CCCOC1=CC=C(Cl)C=C1Cl WRBJKRRJBCSNLE-UHFFFAOYSA-M 0.000 description 1
- XUYNZTABHAFFAO-UHFFFAOYSA-M potassium;4-amino-3,6-dichloropyridine-2-carboxylate Chemical compound [K+].NC1=CC(Cl)=NC(C([O-])=O)=C1Cl XUYNZTABHAFFAO-UHFFFAOYSA-M 0.000 description 1
- PWMWGKRAALHLEH-UHFFFAOYSA-M potassium;4-phenylbutanoate Chemical compound [K+].[O-]C(=O)CCCC1=CC=CC=C1 PWMWGKRAALHLEH-UHFFFAOYSA-M 0.000 description 1
- SMKRKQBMYOFFMU-UHFFFAOYSA-N prallethrin Chemical compound CC1(C)C(C=C(C)C)C1C(=O)OC1C(C)=C(CC#C)C(=O)C1 SMKRKQBMYOFFMU-UHFFFAOYSA-N 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 125000001844 prenyl group Chemical group [H]C([*])([H])C([H])=C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- QXJKBPAVAHBARF-BETUJISGSA-N procymidone Chemical compound O=C([C@]1(C)C[C@@]1(C1=O)C)N1C1=CC(Cl)=CC(Cl)=C1 QXJKBPAVAHBARF-BETUJISGSA-N 0.000 description 1
- QYMMJNLHFKGANY-UHFFFAOYSA-N profenofos Chemical compound CCCSP(=O)(OCC)OC1=CC=C(Br)C=C1Cl QYMMJNLHFKGANY-UHFFFAOYSA-N 0.000 description 1
- 229960003598 promazine Drugs 0.000 description 1
- LLBYUPOTPHGXIL-UHFFFAOYSA-N prop-2-enyl 1-oxa-4-azaspiro[4.5]decane-4-carbodithioate Chemical compound C=CCSC(=S)N1CCOC11CCCCC1 LLBYUPOTPHGXIL-UHFFFAOYSA-N 0.000 description 1
- REJMLNWDJIPPSE-UHFFFAOYSA-N propan-2-yl 4-[[2-(4,6-dimethoxypyrimidin-2-yl)oxyphenyl]methylamino]benzoate Chemical group COC1=CC(OC)=NC(OC=2C(=CC=CC=2)CNC=2C=CC(=CC=2)C(=O)OC(C)C)=N1 REJMLNWDJIPPSE-UHFFFAOYSA-N 0.000 description 1
- ZYHMJXZULPZUED-UHFFFAOYSA-N propargite Chemical compound C1=CC(C(C)(C)C)=CC=C1OC1C(OS(=O)OCC#C)CCCC1 ZYHMJXZULPZUED-UHFFFAOYSA-N 0.000 description 1
- BZNDWPRGXNILMS-VQHVLOKHSA-N propetamphos Chemical compound CCNP(=S)(OC)O\C(C)=C\C(=O)OC(C)C BZNDWPRGXNILMS-VQHVLOKHSA-N 0.000 description 1
- STJLVHWMYQXCPB-UHFFFAOYSA-N propiconazole Chemical compound O1C(CCC)COC1(C=1C(=CC(Cl)=CC=1)Cl)CN1N=CN=C1 STJLVHWMYQXCPB-UHFFFAOYSA-N 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- ZHYPDEKPSXOZKN-UHFFFAOYSA-N propyl 4-[[2-(4,6-dimethoxypyrimidin-2-yl)oxyphenyl]methylamino]benzoate Chemical group C1=CC(C(=O)OCCC)=CC=C1NCC1=CC=CC=C1OC1=NC(OC)=CC(OC)=N1 ZHYPDEKPSXOZKN-UHFFFAOYSA-N 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- FLVBXVXXXMLMOX-UHFFFAOYSA-N proquinazid Chemical compound C1=C(I)C=C2C(=O)N(CCC)C(OCCC)=NC2=C1 FLVBXVXXXMLMOX-UHFFFAOYSA-N 0.000 description 1
- 230000026447 protein localization Effects 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- ROSDSFDQCJNGOL-UHFFFAOYSA-N protonated dimethyl amine Natural products CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 1
- 150000003195 pteridines Chemical class 0.000 description 1
- 235000015136 pumpkin Nutrition 0.000 description 1
- DZVWKNFPXMUIFA-UHFFFAOYSA-N pyflubumide Chemical compound C1=C(CC(C)C)C(C(OC)(C(F)(F)F)C(F)(F)F)=CC=C1N(C(=O)C(C)C)C(=O)C1=C(C)N(C)N=C1C DZVWKNFPXMUIFA-UHFFFAOYSA-N 0.000 description 1
- QHMTXANCGGJZRX-WUXMJOGZSA-N pymetrozine Chemical compound C1C(C)=NNC(=O)N1\N=C\C1=CC=CN=C1 QHMTXANCGGJZRX-WUXMJOGZSA-N 0.000 description 1
- QHGVXILFMXYDRS-UHFFFAOYSA-N pyraclofos Chemical compound C1=C(OP(=O)(OCC)SCCC)C=NN1C1=CC=C(Cl)C=C1 QHGVXILFMXYDRS-UHFFFAOYSA-N 0.000 description 1
- HZRSNVGNWUDEFX-UHFFFAOYSA-N pyraclostrobin Chemical compound COC(=O)N(OC)C1=CC=CC=C1COC1=NN(C=2C=CC(Cl)=CC=2)C=C1 HZRSNVGNWUDEFX-UHFFFAOYSA-N 0.000 description 1
- DWTVBEZBWMDXIY-UHFFFAOYSA-N pyrametostrobin Chemical compound COC(=O)N(OC)C1=CC=CC=C1COC1=C(C)C(C=2C=CC=CC=2)=NN1C DWTVBEZBWMDXIY-UHFFFAOYSA-N 0.000 description 1
- URXNNPCNKVAQRA-XMHGGMMESA-N pyraoxystrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC(C=2C=CC(Cl)=CC=2)=NN1C URXNNPCNKVAQRA-XMHGGMMESA-N 0.000 description 1
- KKEJMLAPZVXPOF-UHFFFAOYSA-N pyraziflumid Chemical compound C1=C(F)C(F)=CC=C1C1=CC=CC=C1NC(=O)C1=NC=CN=C1C(F)(F)F KKEJMLAPZVXPOF-UHFFFAOYSA-N 0.000 description 1
- 125000004307 pyrazin-2-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 1
- 125000004944 pyrazin-3-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 1
- HYJYGLGUBUDSLJ-UHFFFAOYSA-N pyrethrin Natural products CCC(=O)OC1CC(=C)C2CC3OC3(C)C2C2OC(=O)C(=C)C12 HYJYGLGUBUDSLJ-UHFFFAOYSA-N 0.000 description 1
- 229940070846 pyrethrins Drugs 0.000 description 1
- 239000002728 pyrethroid Substances 0.000 description 1
- 229940015367 pyrethrum Drugs 0.000 description 1
- VTRWMTJQBQJKQH-UHFFFAOYSA-N pyributicarb Chemical compound COC1=CC=CC(N(C)C(=S)OC=2C=C(C=CC=2)C(C)(C)C)=N1 VTRWMTJQBQJKQH-UHFFFAOYSA-N 0.000 description 1
- DWFZBUWUXWZWKD-UHFFFAOYSA-N pyridaben Chemical compound C1=CC(C(C)(C)C)=CC=C1CSC1=C(Cl)C(=O)N(C(C)(C)C)N=C1 DWFZBUWUXWZWKD-UHFFFAOYSA-N 0.000 description 1
- AEHJMNVBLRLZKK-UHFFFAOYSA-N pyridalyl Chemical group N1=CC(C(F)(F)F)=CC=C1OCCCOC1=C(Cl)C=C(OCC=C(Cl)Cl)C=C1Cl AEHJMNVBLRLZKK-UHFFFAOYSA-N 0.000 description 1
- CXJSOEPQXUCJSA-UHFFFAOYSA-N pyridaphenthion Chemical compound N1=C(OP(=S)(OCC)OCC)C=CC(=O)N1C1=CC=CC=C1 CXJSOEPQXUCJSA-UHFFFAOYSA-N 0.000 description 1
- 125000002206 pyridazin-3-yl group Chemical group [H]C1=C([H])C([H])=C(*)N=N1 0.000 description 1
- 125000004940 pyridazin-4-yl group Chemical group N1=NC=C(C=C1)* 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- YEYHFKBVNARCNE-UHFFFAOYSA-N pyrido[2,3-b]pyrazine Chemical class N1=CC=NC2=CC=CN=C21 YEYHFKBVNARCNE-UHFFFAOYSA-N 0.000 description 1
- OHZYAOYVLLHTGW-UHFFFAOYSA-N pyrido[3,2-c]pyridazine Chemical class C1=CN=NC2=CC=CN=C21 OHZYAOYVLLHTGW-UHFFFAOYSA-N 0.000 description 1
- 150000008518 pyridopyrimidines Chemical class 0.000 description 1
- ITKAIUGKVKDENI-UHFFFAOYSA-N pyrimidifen Chemical compound CC1=C(C)C(CCOCC)=CC=C1OCCNC1=NC=NC(CC)=C1Cl ITKAIUGKVKDENI-UHFFFAOYSA-N 0.000 description 1
- 125000000246 pyrimidin-2-yl group Chemical group [H]C1=NC(*)=NC([H])=C1[H] 0.000 description 1
- OYRRZWATULMEPF-UHFFFAOYSA-N pyrimidin-4-amine Chemical compound NC1=CC=NC=N1 OYRRZWATULMEPF-UHFFFAOYSA-N 0.000 description 1
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- JOZPEVMCAKXSEY-UHFFFAOYSA-N pyrimido[5,4-d]pyrimidine Chemical class N1=CN=CC2=NC=NC=C21 JOZPEVMCAKXSEY-UHFFFAOYSA-N 0.000 description 1
- YYXSCUSVVALMNW-FOWTUZBSSA-N pyriminostrobin Chemical compound CO\C=C(\C(=O)OC)C1=CC=CC=C1COC1=CC(C(F)(F)F)=NC(NC=2C(=CC(Cl)=CC=2)Cl)=N1 YYXSCUSVVALMNW-FOWTUZBSSA-N 0.000 description 1
- BAUQXSYUDSNRHL-UHFFFAOYSA-N pyrimorph Chemical compound C1=CC(C(C)(C)C)=CC=C1C(C=1C=NC(Cl)=CC=1)=CC(=O)N1CCOCC1 BAUQXSYUDSNRHL-UHFFFAOYSA-N 0.000 description 1
- NMVCBWZLCXANER-UHFFFAOYSA-N pyriofenone Chemical compound COC1=C(OC)C(OC)=CC(C)=C1C(=O)C1=C(C)C(Cl)=CN=C1OC NMVCBWZLCXANER-UHFFFAOYSA-N 0.000 description 1
- NHDHVHZZCFYRSB-UHFFFAOYSA-N pyriproxyfen Chemical compound C=1C=CC=NC=1OC(C)COC(C=C1)=CC=C1OC1=CC=CC=C1 NHDHVHZZCFYRSB-UHFFFAOYSA-N 0.000 description 1
- DHTJFQWHCVTNRY-OEMAIJDKSA-N pyrisoxazole Chemical compound C1([C@@]2(C)CC(ON2C)C=2C=CC(Cl)=CC=2)=CC=CN=C1 DHTJFQWHCVTNRY-OEMAIJDKSA-N 0.000 description 1
- 229910052903 pyrophyllite Inorganic materials 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- JYQUHIFYBATCCY-UHFFFAOYSA-N quinalphos Chemical compound C1=CC=CC2=NC(OP(=S)(OCC)OCC)=CN=C21 JYQUHIFYBATCCY-UHFFFAOYSA-N 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 125000004159 quinolin-2-yl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C([H])C(*)=NC2=C1[H] 0.000 description 1
- 125000004548 quinolin-3-yl group Chemical group N1=CC(=CC2=CC=CC=C12)* 0.000 description 1
- 125000004549 quinolin-4-yl group Chemical group N1=CC=C(C2=CC=CC=C12)* 0.000 description 1
- 125000004550 quinolin-6-yl group Chemical group N1=CC=CC2=CC(=CC=C12)* 0.000 description 1
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical compound C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 1
- MRUMAIRJPMUAPZ-UHFFFAOYSA-N quinolin-8-ol;sulfuric acid Chemical compound OS(O)(=O)=O.C1=CN=C2C(O)=CC=CC2=C1 MRUMAIRJPMUAPZ-UHFFFAOYSA-N 0.000 description 1
- 150000003248 quinolines Chemical class 0.000 description 1
- WRPIRSINYZBGPK-UHFFFAOYSA-N quinoxyfen Chemical compound C1=CC(F)=CC=C1OC1=CC=NC2=CC(Cl)=CC(Cl)=C12 WRPIRSINYZBGPK-UHFFFAOYSA-N 0.000 description 1
- BACHBFVBHLGWSL-JTQLQIEISA-N rac-diclofop methyl Natural products C1=CC(O[C@@H](C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-JTQLQIEISA-N 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 230000006950 reactive oxygen species formation Effects 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 229940108410 resmethrin Drugs 0.000 description 1
- VEMKTZHHVJILDY-FIWHBWSRSA-N resmethrin Chemical compound CC1(C)[C@H](C=C(C)C)C1C(=O)OCC1=COC(CC=2C=CC=CC=2)=C1 VEMKTZHHVJILDY-FIWHBWSRSA-N 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- SLUXPOIDTZWGCG-UHFFFAOYSA-N rhizobitoxine Natural products OCC(N)COC=CC(N)C(O)=O SLUXPOIDTZWGCG-UHFFFAOYSA-N 0.000 description 1
- 229940080817 rotenone Drugs 0.000 description 1
- JUVIOZPCNVVQFO-HBGVWJBISA-N rotenone Chemical compound O([C@H](CC1=C2O3)C(C)=C)C1=CC=C2C(=O)[C@@H]1[C@H]3COC2=C1C=C(OC)C(OC)=C2 JUVIOZPCNVVQFO-HBGVWJBISA-N 0.000 description 1
- 108091052345 ryanodine receptor (TC 1.A.3.1) family Proteins 0.000 description 1
- MSHXTAQSSIEBQS-UHFFFAOYSA-N s-[3-carbamoylsulfanyl-2-(dimethylamino)propyl] carbamothioate;hydron;chloride Chemical compound [Cl-].NC(=O)SCC([NH+](C)C)CSC(N)=O MSHXTAQSSIEBQS-UHFFFAOYSA-N 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 229940043230 sarcosine Drugs 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- QYOJSKGCWNAKGW-HCWXCVPCSA-N shikimate-3-phosphate Chemical compound O[C@H]1CC(C(O)=O)=C[C@H](OP(O)(O)=O)[C@@H]1O QYOJSKGCWNAKGW-HCWXCVPCSA-N 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- MXMXHPPIGKYTAR-UHFFFAOYSA-N silthiofam Chemical compound CC=1SC([Si](C)(C)C)=C(C(=O)NCC=C)C=1C MXMXHPPIGKYTAR-UHFFFAOYSA-N 0.000 description 1
- 230000005783 single-strand break Effects 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000003195 sodium channel blocking agent Substances 0.000 description 1
- 229920005552 sodium lignosulfonate Polymers 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000004328 sodium tetraborate Substances 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- JKPSVOHVUGMYGH-UHFFFAOYSA-M sodium;(4,6-dimethoxypyrimidin-2-yl)-[[3-methoxycarbonyl-6-(trifluoromethyl)pyridin-2-yl]sulfonylcarbamoyl]azanide Chemical compound [Na+].COC(=O)C1=CC=C(C(F)(F)F)N=C1S(=O)(=O)NC(=O)[N-]C1=NC(OC)=CC(OC)=N1 JKPSVOHVUGMYGH-UHFFFAOYSA-M 0.000 description 1
- UFEIWEXHHOXPGP-UHFFFAOYSA-M sodium;(4,6-dimethoxypyrimidin-2-yl)carbamoyl-[3-(2,2,2-trifluoroethoxy)pyridin-2-yl]sulfonylazanide Chemical compound [Na+].COC1=CC(OC)=NC(NC(=O)[N-]S(=O)(=O)C=2C(=CC=CN=2)OCC(F)(F)F)=N1 UFEIWEXHHOXPGP-UHFFFAOYSA-M 0.000 description 1
- UEFBGOFTQWGYCJ-BNSHTTSQSA-M sodium;(6z)-2-methoxycarbonyl-3,3-dimethyl-5-oxo-6-[1-(prop-2-enoxyamino)butylidene]cyclohexen-1-olate Chemical compound [Na+].C=CCONC(/CCC)=C1\C(=O)CC(C)(C)C(C(=O)OC)=C1[O-] UEFBGOFTQWGYCJ-BNSHTTSQSA-M 0.000 description 1
- SGUSXTMVKMPQKI-UHFFFAOYSA-M sodium;2,2,3,3-tetrafluoropropanoate Chemical compound [Na+].[O-]C(=O)C(F)(F)C(F)F SGUSXTMVKMPQKI-UHFFFAOYSA-M 0.000 description 1
- PDEFQWNXOUGDJR-UHFFFAOYSA-M sodium;2,2-dichloropropanoate Chemical compound [Na+].CC(Cl)(Cl)C([O-])=O PDEFQWNXOUGDJR-UHFFFAOYSA-M 0.000 description 1
- DPMABEYFRYKAHD-UHFFFAOYSA-M sodium;2,3,6-trichlorobenzoate Chemical compound [Na+].[O-]C(=O)C1=C(Cl)C=CC(Cl)=C1Cl DPMABEYFRYKAHD-UHFFFAOYSA-M 0.000 description 1
- KZOJQMWTKJDSQJ-UHFFFAOYSA-M sodium;2,3-dibutylnaphthalene-1-sulfonate Chemical compound [Na+].C1=CC=C2C(S([O-])(=O)=O)=C(CCCC)C(CCCC)=CC2=C1 KZOJQMWTKJDSQJ-UHFFFAOYSA-M 0.000 description 1
- RFOHRSIAXQACDB-UHFFFAOYSA-M sodium;2-(2,4-dichlorophenoxy)acetate Chemical compound [Na+].[O-]C(=O)COC1=CC=C(Cl)C=C1Cl RFOHRSIAXQACDB-UHFFFAOYSA-M 0.000 description 1
- STAPBGVGYWCRTF-UHFFFAOYSA-M sodium;2-(4-chloro-2-methylphenoxy)acetate Chemical compound [Na+].CC1=CC(Cl)=CC=C1OCC([O-])=O STAPBGVGYWCRTF-UHFFFAOYSA-M 0.000 description 1
- NAGWPMWBTLVULC-UHFFFAOYSA-M sodium;2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)quinoline-3-carboxylate Chemical compound [Na+].N1C(=O)C(C(C)C)(C)N=C1C1=NC2=CC=CC=C2C=C1C([O-])=O NAGWPMWBTLVULC-UHFFFAOYSA-M 0.000 description 1
- YWICANUUQPYHOW-UHFFFAOYSA-M sodium;2-(phosphonomethylamino)acetate Chemical compound [Na+].OP(O)(=O)CNCC([O-])=O YWICANUUQPYHOW-UHFFFAOYSA-M 0.000 description 1
- DUZVBQWEFKXWJM-UHFFFAOYSA-M sodium;2-[4-[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxyphenoxy]propanoate Chemical compound [Na+].C1=CC(OC(C)C([O-])=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl DUZVBQWEFKXWJM-UHFFFAOYSA-M 0.000 description 1
- JQYJSVBNPUHHKB-UHFFFAOYSA-M sodium;2-methyl-4,6-dinitrophenolate Chemical compound [Na+].CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1[O-] JQYJSVBNPUHHKB-UHFFFAOYSA-M 0.000 description 1
- KBRBTPNRBSCEJD-UHFFFAOYSA-M sodium;3-cycloprop-2-en-1-ylpropanoate Chemical compound [Na+].[O-]C(=O)CCC1C=C1 KBRBTPNRBSCEJD-UHFFFAOYSA-M 0.000 description 1
- PPKIJAQKBAYNNL-UHFFFAOYSA-M sodium;4-(2,4-dichlorophenoxy)butanoate Chemical compound [Na+].[O-]C(=O)CCCOC1=CC=C(Cl)C=C1Cl PPKIJAQKBAYNNL-UHFFFAOYSA-M 0.000 description 1
- GABUSZPTCJGKGB-UHFFFAOYSA-M sodium;4-(4-chloro-2-methylphenoxy)butanoate Chemical compound [Na+].CC1=CC(Cl)=CC=C1OCCCC([O-])=O GABUSZPTCJGKGB-UHFFFAOYSA-M 0.000 description 1
- RVULBHWZFCBODE-UHFFFAOYSA-M sodium;5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical compound [Na+].C1=C([N+]([O-])=O)C(C(=O)[O-])=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 RVULBHWZFCBODE-UHFFFAOYSA-M 0.000 description 1
- JXIAOZNHSODSNJ-UHFFFAOYSA-M sodium;5-bromo-3-butan-2-yl-6-methylpyrimidin-1-ide-2,4-dione Chemical compound [Na+].CCC(C)N1C(=O)[N-]C(C)=C(Br)C1=O JXIAOZNHSODSNJ-UHFFFAOYSA-M 0.000 description 1
- IYYIUOWKEMQYNV-UHFFFAOYSA-N sodium;ethoxy-oxido-oxophosphanium Chemical compound [Na+].CCO[P+]([O-])=O IYYIUOWKEMQYNV-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000004550 soluble concentrate Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 229940063673 spermidine Drugs 0.000 description 1
- 229940063675 spermine Drugs 0.000 description 1
- 229940014213 spinosad Drugs 0.000 description 1
- 229930185156 spinosyn Natural products 0.000 description 1
- HEOYPZMAGQITRO-UHFFFAOYSA-N spirobudifen Chemical compound C(OCCCC)(OC1=C(C(OC12CCCCC2)=O)C2=C(C=C(C=C2)Cl)Cl)=O HEOYPZMAGQITRO-UHFFFAOYSA-N 0.000 description 1
- DTDSAWVUFPGDMX-UHFFFAOYSA-N spirodiclofen Chemical compound CCC(C)(C)C(=O)OC1=C(C=2C(=CC(Cl)=CC=2)Cl)C(=O)OC11CCCCC1 DTDSAWVUFPGDMX-UHFFFAOYSA-N 0.000 description 1
- GOLXNESZZPUPJE-UHFFFAOYSA-N spiromesifen Chemical compound CC1=CC(C)=CC(C)=C1C(C(O1)=O)=C(OC(=O)CC(C)(C)C)C11CCCC1 GOLXNESZZPUPJE-UHFFFAOYSA-N 0.000 description 1
- CLSVJBIHYWPGQY-GGYDESQDSA-N spirotetramat Chemical compound CCOC(=O)OC1=C(C=2C(=CC=C(C)C=2)C)C(=O)N[C@@]11CC[C@H](OC)CC1 CLSVJBIHYWPGQY-GGYDESQDSA-N 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 235000020354 squash Nutrition 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 230000000707 stereoselective effect Effects 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- CCEKAJIANROZEO-UHFFFAOYSA-N sulfluramid Chemical compound CCNS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F CCEKAJIANROZEO-UHFFFAOYSA-N 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- XIUROWKZWPIAIB-UHFFFAOYSA-N sulfotep Chemical compound CCOP(=S)(OCC)OP(=S)(OCC)OCC XIUROWKZWPIAIB-UHFFFAOYSA-N 0.000 description 1
- 125000005555 sulfoximide group Chemical group 0.000 description 1
- 125000005537 sulfoxonium group Chemical group 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- OBTWBSRJZRCYQV-UHFFFAOYSA-N sulfuryl difluoride Chemical compound FS(F)(=O)=O OBTWBSRJZRCYQV-UHFFFAOYSA-N 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000005936 tau-Fluvalinate Substances 0.000 description 1
- INISTDXBRIBGOC-XMMISQBUSA-N tau-fluvalinate Chemical compound N([C@H](C(C)C)C(=O)OC(C#N)C=1C=C(OC=2C=CC=CC=2)C=CC=1)C1=CC=C(C(F)(F)F)C=C1Cl INISTDXBRIBGOC-XMMISQBUSA-N 0.000 description 1
- QYPNKSZPJQQLRK-UHFFFAOYSA-N tebufenozide Chemical compound C1=CC(CC)=CC=C1C(=O)NN(C(C)(C)C)C(=O)C1=CC(C)=CC(C)=C1 QYPNKSZPJQQLRK-UHFFFAOYSA-N 0.000 description 1
- ZZYSLNWGKKDOML-UHFFFAOYSA-N tebufenpyrad Chemical compound CCC1=NN(C)C(C(=O)NCC=2C=CC(=CC=2)C(C)(C)C)=C1Cl ZZYSLNWGKKDOML-UHFFFAOYSA-N 0.000 description 1
- LWLJEQHTPVPKSJ-UHFFFAOYSA-N tebufloquin Chemical compound C1=C(C(C)(C)C)C=C2C(OC(=O)C)=C(C)C(C)=NC2=C1F LWLJEQHTPVPKSJ-UHFFFAOYSA-N 0.000 description 1
- AWYOMXWDGWUJHS-UHFFFAOYSA-N tebupirimfos Chemical compound CCOP(=S)(OC(C)C)OC1=CN=C(C(C)(C)C)N=C1 AWYOMXWDGWUJHS-UHFFFAOYSA-N 0.000 description 1
- ROZUQUDEWZIBHV-UHFFFAOYSA-N tecloftalam Chemical compound OC(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C(=O)NC1=CC=CC(Cl)=C1Cl ROZUQUDEWZIBHV-UHFFFAOYSA-N 0.000 description 1
- CJDWRQLODFKPEL-UHFFFAOYSA-N teflubenzuron Chemical compound FC1=CC=CC(F)=C1C(=O)NC(=O)NC1=CC(Cl)=C(F)C(Cl)=C1F CJDWRQLODFKPEL-UHFFFAOYSA-N 0.000 description 1
- WWJZWCUNLNYYAU-UHFFFAOYSA-N temephos Chemical compound C1=CC(OP(=S)(OC)OC)=CC=C1SC1=CC=C(OP(=S)(OC)OC)C=C1 WWJZWCUNLNYYAU-UHFFFAOYSA-N 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- UBCKGWBNUIFUST-YHYXMXQVSA-N tetrachlorvinphos Chemical compound COP(=O)(OC)O\C(=C/Cl)C1=CC(Cl)=C(Cl)C=C1Cl UBCKGWBNUIFUST-YHYXMXQVSA-N 0.000 description 1
- MLGCXEBRWGEOQX-UHFFFAOYSA-N tetradifon Chemical compound C1=CC(Cl)=CC=C1S(=O)(=O)C1=CC(Cl)=C(Cl)C=C1Cl MLGCXEBRWGEOQX-UHFFFAOYSA-N 0.000 description 1
- 125000001712 tetrahydronaphthyl group Chemical group C1(CCCC2=CC=CC=C12)* 0.000 description 1
- KNDVJPKNBVIKML-UHFFFAOYSA-N tetraniliprole Chemical compound CNC(=O)C1=CC(C#N)=CC(C)=C1NC(=O)C1=CC(CN2N=C(N=N2)C(F)(F)F)=NN1C1=NC=CC=C1Cl KNDVJPKNBVIKML-UHFFFAOYSA-N 0.000 description 1
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 239000004308 thiabendazole Substances 0.000 description 1
- 235000010296 thiabendazole Nutrition 0.000 description 1
- WJCNZQLZVWNLKY-UHFFFAOYSA-N thiabendazole Chemical compound S1C=NC(C=2NC3=CC=CC=C3N=2)=C1 WJCNZQLZVWNLKY-UHFFFAOYSA-N 0.000 description 1
- 229960004546 thiabendazole Drugs 0.000 description 1
- 150000004867 thiadiazoles Chemical class 0.000 description 1
- NWWZPOKUUAIXIW-FLIBITNWSA-N thiamethoxam Chemical compound [O-][N+](=O)\N=C/1N(C)COCN\1CC1=CN=C(Cl)S1 NWWZPOKUUAIXIW-FLIBITNWSA-N 0.000 description 1
- 125000003777 thiepinyl group Chemical group 0.000 description 1
- AHTPATJNIAFOLR-UHFFFAOYSA-N thifensulfuron-methyl Chemical compound S1C=CC(S(=O)(=O)NC(=O)NC=2N=C(OC)N=C(C)N=2)=C1C(=O)OC AHTPATJNIAFOLR-UHFFFAOYSA-N 0.000 description 1
- 125000001730 thiiranyl group Chemical group 0.000 description 1
- DNVLJEWNNDHELH-UHFFFAOYSA-N thiocyclam Chemical compound CN(C)C1CSSSC1 DNVLJEWNNDHELH-UHFFFAOYSA-N 0.000 description 1
- OPASCBHCTNRLRM-UHFFFAOYSA-N thiometon Chemical compound CCSCCSP(=S)(OC)OC OPASCBHCTNRLRM-UHFFFAOYSA-N 0.000 description 1
- QGHREAKMXXNCOA-UHFFFAOYSA-N thiophanate-methyl Chemical compound COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC QGHREAKMXXNCOA-UHFFFAOYSA-N 0.000 description 1
- QSOHVSNIQHGFJU-UHFFFAOYSA-L thiosultap disodium Chemical compound [Na+].[Na+].[O-]S(=O)(=O)SCC(N(C)C)CSS([O-])(=O)=O QSOHVSNIQHGFJU-UHFFFAOYSA-L 0.000 description 1
- 125000000464 thioxo group Chemical group S=* 0.000 description 1
- 229960002447 thiram Drugs 0.000 description 1
- KUAZQDVKQLNFPE-UHFFFAOYSA-N thiram Chemical compound CN(C)C(=S)SSC(=S)N(C)C KUAZQDVKQLNFPE-UHFFFAOYSA-N 0.000 description 1
- VJQYLJSMBWXGDV-UHFFFAOYSA-N tiadinil Chemical compound N1=NSC(C(=O)NC=2C=C(Cl)C(C)=CC=2)=C1C VJQYLJSMBWXGDV-UHFFFAOYSA-N 0.000 description 1
- 229950007146 tigolaner Drugs 0.000 description 1
- IHNSIFFSNUQGQN-UHFFFAOYSA-N tioxazafen Chemical compound C1=CSC(C=2ON=C(N=2)C=2C=CC=CC=2)=C1 IHNSIFFSNUQGQN-UHFFFAOYSA-N 0.000 description 1
- OBZIQQJJIKNWNO-UHFFFAOYSA-N tolclofos-methyl Chemical compound COP(=S)(OC)OC1=C(Cl)C=C(C)C=C1Cl OBZIQQJJIKNWNO-UHFFFAOYSA-N 0.000 description 1
- WPALTCMYPARVNV-UHFFFAOYSA-N tolfenpyrad Chemical compound CCC1=NN(C)C(C(=O)NCC=2C=CC(OC=3C=CC(C)=CC=3)=CC=2)=C1Cl WPALTCMYPARVNV-UHFFFAOYSA-N 0.000 description 1
- HYVWIQDYBVKITD-UHFFFAOYSA-N tolylfluanid Chemical compound CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)C1=CC=C(C)C=C1 HYVWIQDYBVKITD-UHFFFAOYSA-N 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- DDVNRFNDOPPVQJ-HQJQHLMTSA-N transfluthrin Chemical compound CC1(C)[C@H](C=C(Cl)Cl)[C@H]1C(=O)OCC1=C(F)C(F)=CC(F)=C1F DDVNRFNDOPPVQJ-HQJQHLMTSA-N 0.000 description 1
- 125000005270 trialkylamine group Chemical group 0.000 description 1
- 125000004665 trialkylsilyl group Chemical group 0.000 description 1
- VLCQZHSMCYCDJL-UHFFFAOYSA-N tribenuron methyl Chemical compound COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)N(C)C1=NC(C)=NC(OC)=N1 VLCQZHSMCYCDJL-UHFFFAOYSA-N 0.000 description 1
- YMXOXAPKZDWXLY-QWRGUYRKSA-N tribenuron methyl Chemical group COC(=O)[C@H]1CCCC[C@@H]1S(=O)(=O)NC(=O)N(C)C1=NC(C)=NC(OC)=N1 YMXOXAPKZDWXLY-QWRGUYRKSA-N 0.000 description 1
- DQJCHOQLCLEDLL-UHFFFAOYSA-N tricyclazole Chemical compound CC1=CC=CC2=C1N1C=NN=C1S2 DQJCHOQLCLEDLL-UHFFFAOYSA-N 0.000 description 1
- UGCNRZFAUBJVPT-UHFFFAOYSA-N tricyclohexyltin;hydrate Chemical compound O.C1CCCCC1[Sn](C1CCCCC1)C1CCCCC1 UGCNRZFAUBJVPT-UHFFFAOYSA-N 0.000 description 1
- XAIPTRIXGHTTNT-UHFFFAOYSA-N triflumuron Chemical compound C1=CC(OC(F)(F)F)=CC=C1NC(=O)NC(=O)C1=CC=CC=C1Cl XAIPTRIXGHTTNT-UHFFFAOYSA-N 0.000 description 1
- 125000005034 trifluormethylthio group Chemical group FC(S*)(F)F 0.000 description 1
- AZHWSDHSXZKYQT-UHFFFAOYSA-N trifluoromethyl penta-2,4-dienoate Chemical compound C(C=CC=C)(=O)OC(F)(F)F AZHWSDHSXZKYQT-UHFFFAOYSA-N 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- KVEQCVKVIFQSGC-UHFFFAOYSA-N tritosulfuron Chemical compound FC(F)(F)C1=NC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(F)(F)F)=N1 KVEQCVKVIFQSGC-UHFFFAOYSA-N 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- DBXFMOWZRXXBRN-LWKPJOBUSA-N valifenalate Chemical compound CC(C)OC(=O)N[C@@H](C(C)C)C(=O)NC(CC(=O)OC)C1=CC=C(Cl)C=C1 DBXFMOWZRXXBRN-LWKPJOBUSA-N 0.000 description 1
- LESVOLZBIFDZGS-UHFFFAOYSA-N vamidothion Chemical compound CNC(=O)C(C)SCCSP(=O)(OC)OC LESVOLZBIFDZGS-UHFFFAOYSA-N 0.000 description 1
- 150000004669 very long chain fatty acids Chemical class 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000001238 wet grinding Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- WCJYTPVNMWIZCG-UHFFFAOYSA-N xylylcarb Chemical compound CNC(=O)OC1=CC=C(C)C(C)=C1 WCJYTPVNMWIZCG-UHFFFAOYSA-N 0.000 description 1
- 239000005943 zeta-Cypermethrin Substances 0.000 description 1
- 229940048462 zinc phosphide Drugs 0.000 description 1
- AMHNZOICSMBGDH-UHFFFAOYSA-L zineb Chemical compound [Zn+2].[S-]C(=S)NCCNC([S-])=S AMHNZOICSMBGDH-UHFFFAOYSA-L 0.000 description 1
- DUBNHZYBDBBJHD-UHFFFAOYSA-L ziram Chemical compound [Zn+2].CN(C)C([S-])=S.CN(C)C([S-])=S DUBNHZYBDBBJHD-UHFFFAOYSA-L 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8241—Phenotypically and genetically modified plants via recombinant DNA technology
- C12N15/8261—Phenotypically and genetically modified plants via recombinant DNA technology with agronomic (input) traits, e.g. crop yield
- C12N15/8271—Phenotypically and genetically modified plants via recombinant DNA technology with agronomic (input) traits, e.g. crop yield for stress resistance, e.g. heavy metal resistance
- C12N15/8274—Phenotypically and genetically modified plants via recombinant DNA technology with agronomic (input) traits, e.g. crop yield for stress resistance, e.g. heavy metal resistance for herbicide resistance
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/82—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with three ring hetero atoms
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/0004—Oxidoreductases (1.)
- C12N9/001—Oxidoreductases (1.) acting on the CH-CH group of donors (1.3)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y103/00—Oxidoreductases acting on the CH-CH group of donors (1.3)
- C12Y103/03—Oxidoreductases acting on the CH-CH group of donors (1.3) with oxygen as acceptor (1.3.3)
- C12Y103/03004—Protoporphyrinogen oxidase (1.3.3.4)
Definitions
- the present disclosure relates to the fields of agriculture, plant biotechnology, and molecular biology. More specifically, the disclosure relates to the use of substituted phenyluracils, or agrochemically acceptable salts thereof, for controlling or preventing weed growth in plant growth areas of transgenic crop plants that are tolerant to protoporphyrinogen oxidase (PPO) inhibiting herbicides.
- PPO protoporphyrinogen oxidase
- herbicides are often used to control the growth and spread of weeds or other plants that are unwanted in a particular environment. These chemicals are active at one or more target sites within a plant where they interrupt normal plant functions. Herbicides vary in their modes of action, in their effects on weeds and crop plants, and how they are used. While herbicides are very effective in controlling growth of undesirable vegetation, their use may also cause incidental damage to desired plants located in the same vicinity, such as crop plants. In order to minimize crop damage, extensive research has been directed toward the development of herbicide tolerant plants, especially through use of transgenic traits. Examples of transgenic herbicide tolerance traits include glyphosate tolerance, glufosinate tolerance, and dicamba tolerance.
- Herbicides of particular interest include herbicides that inhibit protoporphyrinogen oxidase (PPO, EC 1.3.3.4), referred to as PPO inhibitor herbicides.
- PPO inhibitor herbicides provide control of a spectrum of herbicide-resistant weeds, thus making a trait conferring tolerance to these herbicides particularly useful in a cropping system combined with one or more other herbicide-tolerance trait(s).
- a method for controlling or preventing weed growth in a plant growth area comprises the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the general formula (I) or an agrochemically acceptable salt thereof
- R 8 represents hydrogen, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, aryl, aryl-(C 1 - C8)-alkyl, heteroaryl, (C 2 -C 8 )-alkynyl, (C 2 -C 8 )-alkenyl, C(O)R 13 , C(O)OR 13 , or (C 1 -C 8 )-alkoxy- (C 1 -C 8 )-alkyl; “R 9 ” represents hydrogen or (C 1 -C 8 )-alkyl; “R 10 ” represents hydrogen, halogen, cyano, NO2, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (C 3 -C 8 )-cycloalkyl, (C 3 -C 8 )-cycloalkyl-(C 1 -C 8 )- alky
- Also provided herein is a method for controlling or preventing weed growth in a plant growth area comprising the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the of the general formula (I), or an agrochemically acceptable salt thereof, that is further characterized in that “W” represents a group W-l to W-2; and wherein “A” represents nitrogen or CH; “R 1 ” represents hydrogen; “R 2 ” represents hydrogen or fluorine; “R 3 ” represents fluorine; “R 4 ” represents chlorine, bromine, or cyano; “R 5 ” represents hydrogen; “R 6 ” represents hydrogen or fluorine; “R 7 ” represents hydrogen; “G” represents methylene, (methyl)methylene or (methoxy
- Also provided herein is a method for controlling or preventing weed growth in a plant growth area comprising the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the of the general formula (I), or an agrochemically acceptable salt thereof, wherein said compound corresponds to:
- the heterologous HemG protein has herbicide-insensitive protoporphyrinogen oxidase activity.
- the heterologous HemG protein has at least 85% sequence identity to a polypeptide sequence selected from the group consisting of SEQ ID NOs:l-20 and 65-193.
- the DNA sequence encoding the heterologous HemG protein is selected from the group consisting of SEQ ID NOs:22-64 and 194-322.
- the heterologous HemG protein comprises an amino acid sequence selected from the group consisting of SEQ ID NOs:l-20 and 65-193.
- the DNA sequence encoding a heterologous HemG protein is operably linked to a DNA sequence encoding a chloroplast transit peptide (CTP).
- CTP comprises an amino acid sequence with at least 97% sequence identity to a sequence selected from the group consisting of SEQ ID NOs:323-328, 340, and 342-407.
- the DNA sequence encoding the CTP comprises at least 97% identity to a sequence selected from the group consisting of SEQ ID NOs:329-339, 341, and 408-483.
- said recombinant DNA molecule further comprises a heterologous promoter operably linked to the DNA sequence encoding said HemG protein.
- the plant comprising the recombinant DNA molecule is a monocotyledonous plant. In other embodiments, the plant comprising the recombinant DNA molecule is a dicotyledonous plant.
- the herbicidally active compound is applied to the area at a rate of about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, about 0.25 g a.i./ha to about 300 g a.i./ha, about 0.02 g a.i./ha to about 250 g a.i./ha, about 0.05 g a.i./ha to about 150 g a.i./ha, or about 0.25 g a.i./ha to about 120 g a.i./ha.
- the method is further defined as comprising applying said compound to said area at least twice.
- the herbicidally active compound is applied in an amount that does not damage said plant comprising the recombinant DNA molecule.
- said applying of the compound is carried out pre-emergence.
- said applying of the compound is carried out post-emergence.
- said applying of the compound comprises contacting said plant with the compound.
- said applying of the compound comprises an over the top application of said compound.
- said applying of the compound results in an increase in the growth or yield of said plant relative to a plant of the same genotype cultivated in a growth area in which said compound has not been applied.
- the method further comprises applying to said area an effective amount of at least a second herbicide.
- the second herbicide is selected from the group consisting of: an ACCase inhibitor, an ALS inhibitor, an EPSPS inhibitor, a synthetic auxin, a photosynthesis inhibitor, a glutamine synthesis inhibitor, a HPPD inhibitor, a PPO inhibitor, and a long-chain fatty acid inhibitor.
- the ACCase inhibitor is an aryloxyphenoxy propionate or a cyclohexanedione;
- the ALS inhibitor is a sulfonylurea, imidazolinone, triazoloyrimidine, or a triazolinone;
- the EPSPS inhibitor is glyphosate;
- the synthetic auxin is a phenoxy herbicide, a benzoic acid, a carboxylic acid, or a semicarbazone;
- the photosynthesis inhibitor is a triazine, a triazinone, a nitrile, a benzothiadiazole, or a urea;
- the glutamine synthesis inhibitor is glufosinate;
- the HPPD inhibitor is an isoxazole, a pyrazolone, or a triketone;
- the PPO inhibitor is a diphenylether, a N- phenylphthalimide, an aryl triazinone, or a
- SEQ ID NO: 1 is the amino acid sequence of the HemG PPO H_N90.
- SEQ ID NO:2 is the amino acid sequence of the HemG PPO H_N20.
- SEQ ID NO:3 is the amino acid sequence of the HemG PPO H_N60.
- SEQ ID NO:4 is the amino acid sequence of H_N10, which is the E. coli wild-type HemG protoporphyrinogen oxidase (NCBI GenBank Accession No. WP 021498199).
- SEQ ID NO:5 is the amino acid sequence of the HemG PPG H N30.
- SEQ ID NO:6 is the amino acid sequence of the HemG PPO H_N40.
- SEQ ID NO:7 is the amino acid sequence of the HemG PPO H_N50.
- SEQ ID NO:8 is the amino acid sequence of the HemG PPO H_N70.
- SEQ ID NO:9 is the amino acid sequence of the HemG PPO H N100.
- SEQ ID NO: 10 is the amino acid sequence of the HemG PPO H_N 110.
- SEQ ID NOs: 11-17 are amino acid sequences lacking the start methionine and corresponding to SEQ ID NOs:l, 2, 4, 5, 6, 7, and 9, respectively.
- SEQ ID Nos: 18-19 are amino acid sequences of two variants of SEQ ID NO:11.
- SEQ ID NO:20 is the amino acid sequence of a variant of SEQ ID NO: 17.
- SEQ ID NO:21 is the amino acid sequence of the wild-type PPO from Amaranthus tuberculatus (waterhemp).
- SEQ ID NOs:22-31 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for E. coli expression.
- SEQ ID NOs:32-41 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for dicot expression.
- SEQ ID NOs:42-48 are nucleotide sequences encoding SEQ ID NOs: 11-17, respectively, codon optimized for dicot expression.
- SEQ ID NOs:49-50 are recombinant nucleotide sequences encoding SEQ ID NO: 11.
- SEQ ID NO:51 is a recombinant nucleotide sequence encoding SEQ ID NO: 12.
- SEQ ID NOs:52-54 are recombinant nucleotide sequences encoding SEQ ID NOs: 18-
- SEQ ID NOs:55-64 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for monocot expression.
- SEQ ID Nos: 65-77 are amino acid sequences of HemG PPOs from different species with variations in the long chain insert loop.
- SEQ ID NOs:78-193 are amino acid sequences of recombinant HemG PPO variants, each incorporating a mutation to the long chain insert loop.
- SEQ ID NOs: 194-206 are nucleotide sequences encoding the HemG PPOs of SEQ ID NOs: 65-77.
- SEQ ID NOs: 207-322 are nucleotide sequences encoding the recombinant HemG PPO variants of SEQ ID NOs:78-193.
- SEQ ID NO:323 is the amino acid sequence of the Arabidopsis thaliana albino and pale green (APG6) chloroplast transit peptide (CTP).
- SEQ ID NO:324 is the amino acid sequence of an amino-terminal optimized variant of the APG6 CTP.
- SEQ ID NO:325 is the amino acid sequence of the Arabidopsis thaliana 90 kDa heat shock protein (CR88) CTP.
- SEQ ID NO:326 is the amino acid sequence of the petunia ShkG-EPSPS CTP.
- SEQ ID NO:327 is the amino acid sequence of the pea rbcS-3C CTP.
- SEQ ID NO:328 is the amino acid sequence of the rice Waxy CTP.
- SEQ ID NOs:329-333 are the nucleotide sequences encoding APG6 CTP of SEQ ID NOs:329-333 are the nucleotide sequences encoding APG6 CTP of SEQ ID NOs:329-333.
- SEQ ID NO:334 is the nucleotide sequence encoding APG6 CTP of SEQ ID NO:324.
- SEQ ID NOs:335-336 are nucleotide sequences encoding AtCR88 CTP optimized for dicot and monocot expression, respectively.
- SEQ ID NOs:337-339 are nucleotide sequences encoding SEQ ID NOs:326-328.
- SEQ ID NO:340 is the amino acid sequence of the cotton 12G088600TP CTP.
- SEQ ID NO:341 is the nucleotide sequence encoding the cotton 12G088600TP CTP, optimized for dicot expression.
- SEQ ID NOs:342-407 are amino acid sequences of transit peptides from different species.
- SEQ ID NOs:408-483 are nucleotide sequences encoding SEQ ID NOs:342-407.
- PPO is an essential enzyme in plants that catalyzes the dehydrogenation of protoporphyrinogen IX to form protoporphyrin IX, which is the precursor to heme and chlorophyll.
- PPO inhibition in plant cells causes accumulation of intermediate tetrapyrroles and the formation of reactive oxygen species, resulting in membrane disruption and ultimately cell death.
- PPO inhibitors such as diphenyl ethers, aryl triazolinones, pyrimidinediones, and N- phenylphthalimides.
- C 6 rtain compounds of the structure class of substituted phenyluracils have been identified as having herbicidal activity and can be used for controlling monocotyledonous and dicotyledonous weeds. These compounds are effective against a broad spectrum of harmful plants when applied both preemergence and postemergence, with the possibility of non-selective use for control of unwanted plant growth or selective use in plant crops.
- the present application shows that these specific substituted phenyluracils or salts thereof can be applied to transgenic crop plants that comprise one or more genes conferring tolerance to PPO inhibitor herbicides.
- the herbicide tolerance trait described herein provides tolerance to one of more of the herbicidally active compound of the general formula (I) or agrochemically acceptable salts thereof.
- A represents nitrogen or CH
- R 1 represents hydrogen or methyl
- R 2 represents hydrogen or fluorine
- R 3 represents hydrogen, halogen, or (C 1 -C 8 )-alkoxy
- R 4 represents halogen, cyano, NO2, C(O)NH 2 , C(S)NH 2 , (C 1 -C 8 )-haloalkyl, or (C 1 -C 8 )- alkynyl;
- R 5 , R 6 , and R 7 independently of one another represent hydrogen, halogen, cyano, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (C 1 -C 8 )-alkoxy, or (C 1 -C 8 )-haloalkoxy;
- G represents a branched or unbranched alkylene group optionally substituted with an (Ci -C3)- alkoxy group
- Q represents hydroxy or a group Q-l , Q-2;
- R 8 represents hydrogen, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, aryl, aryl-(C 1 -C 8 )-alkyl, heteroaryl, (C 2 -C 8 )-alkynyl, (C 2 -C 8 )-alkenyl, C(O)R 13 , C(O)OR 13 , or (C 1 -C 8 )-alkoxy-(C 1 -C 8 )-alkyl;
- R 9 represents hydrogen or (C 1 -C 8 )-alkyl
- R 10 represents hydrogen, halogen, cyano, NO2, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (Cs-Cg)- cycloalkyl, (C3-Cg)-cycloalkyl-(C 1 -C 8 )-alkyl, (C3-Cg)-halocycloalkyl, (Cs-Cg)- halocycloalkyl-(C 1 -C 8 )-alkyl, (C2-Cg)-alkenyl, (C2-Cg)-alkynyl, aryl, aryl-(C 1 -C 8 )-alkyl, heteroaryl, heteroaryl-(C 1 -C 8 )-alkyl, heterocyclyl, heterocyclyl-(C 1 -C 8 )-alkyl, R 11 R 12 N-
- R 8 and R 10 together with the carbon atom to which they are attached form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having further substitution;
- R 11 and R 12 are identical or different and independently of one another represent hydrogen, (C 1 - Cg)-alkyl, (C2-Cg)-alkenyl, (C2-Cg)-alkynyl, (C 1 -C 8 )-cyanoalkyl, (C 1 -C 10 )-haloalkyl, (C2- Cg)-haloalkenyl, (C3-Cg)-haloalkynyl, (C3-C 10 )-cycloalkyl, (C3-C 10 )-halocycloalkyl, (C 4 - C 10 )-cycloalkenyl, (C 4 -C 10 )-halocycloalkenyl, (C 1 -C 8 )-alkoxy-(C 1 -C 8 )-alkyl, (C 1 -C 8 )- haloalkoxy-(C 1 -C 8 )-alkyl, (C 1 -C 8 )-
- R 13 represents hydrogen, (C 1 -C 8 )-alkyl, (C 2 -C 8 )-alkenyl, (C 2 -C 8 )-alkynyl, (C 1 -C 8 )-cyanoalkyl, (C 1 -C 10 )-haloalkyl, (C 2 -C 8 )-haloalkenyl, (C 3 -C 8 )-haloalkynyl, (C3-C 10 )-cycloalkyl, (C3- C 10 )-halocycloalkyl, (C 4 -C 10 )-cycloalkenyl, (C 4 -C 10 )-halocycloalkenyl, (C 1 -C 8 )-alkoxy- (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkoxy-(C 1 -C 8 )-alkyl, (C 1 -C 8 )-al
- R 14 represents hydrogen, (C 1 -C 8 )-alkyl, (C 2 -C 8 )-alkenyl, (C2-C 8 )-alkynyl, (C 1 -C 8 )-cyanoalkyl, (C 1 -C 10 )-haloalkyl, (C2-C 8 )-haloalkenyl, (C3-C 8 )-haloalkynyl, (C3-C 10 )-cycloalkyl, (C3- C 10 )-halocycloalkyl, (C 4 -C 10 )-cycloalkenyl, (C 4 -C 10 )-halocycloalkenyl, (C 1 -C 8 )-alkoxy- (C 1 -C 8 )-alkyl, (C 1 -C 8 )-alkoxy-(C 1 -C 8 )-haloalkyl, aryl, aryl-(C 1 -C 8
- R 15 and R 16 together, with the carbon atom to which they are attached, form an unsubstituted (C3- C?)-cycloalkyl
- X and Y independently of one another, represent oxygen or sulfur
- Z represents nitrogen or CH.
- the compound of the general formula (I) or an agrochemically acceptable salt thereof is further characterized in that
- W represents a group W-l to W-2
- A represents nitrogen or CH
- R 1 represents hydrogen
- R 2 represents hydrogen or fluorine
- R 3 represents fluorine
- R 4 represents chlorine, bromine, or cyano
- R 5 represents hydrogen
- R 6 represents hydrogen or fluorine
- R 7 represents hydrogen
- G represents methylene, (methyl)methylene, or (methoxy)methylene
- X represents oxygen
- Z represents nitrogen or CH; and Q represents one of the following moieties Q-l to Q-500:
- plants comprise a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein that has herbicide-insensitive PPO activity.
- the compound of the general formula (I) or an agrochemically acceptable salt thereof corresponds to one of the compounds denoted (a)-(n) below, with their corresponding IUPAC names: (a) ethyl [(3- ⁇ 2-chloro-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy ⁇ pyridin-2-yl)oxy]acetate;
- radicals listed above in general terms or within areas of preference apply to the end products of the general formula (I) and as well as to the starting materials or intermediates required for preparation in each case. These radical definitions can be combined with one another as desired, i.e. including combinations between the given preferred ranges.
- compounds of the general formula (I) described herein or their salts and their use according to the present disclosure are of particular interest in which individual radicals have one of the preferred meanings already specified or specified below, or in particular those in which one or more of the preferred meanings already specified or specified below occur in combination.
- the terms used above and further below will be elucidated. These are familiar to the person skilled in the art and especially have the definitions elucidated hereinafter.
- names of chemical groups are generally to be understood such that attachment to the skeleton or the remainder of the molecule is via the structural element mentioned last, i.e. for example in the case of (C2-Cg)-alkenyloxy via the oxygen atom and in the case of (C 1 -C 8 )-alkoxy-(C 1 -C4)-alkyl or (C 1 -C 8 )-alkoxycarbonyl-(C 1 -C 8 )-alkyl, in each case via the carbon atom of the alkyl group.
- alkylsulfonyl refers to straight-chain or branched alkylsulfonyl, preferably having 1 to 6 or 1 to 8 carbon atoms, of such alkylsulfonyls include, but are but not limited to, (C 1 -C6)-alkylsulfonyl such as methylsulfonyl, ethylsulfonyl, propylsulfonyl, 1 -methylethylsulfonyl, butylsulfonyl, 1- methylpropylsulfonyl, 2-methylpropylsulfonyl, 1,1 -dimethylethylsulfonyl, pentylsulfonyl, 1- methylbutylsulfonyl, 2-methylbutylsulfonyl, 3 -methylbutylsulfonyl
- alkylthio refers to a straight-chain or branched S-alkyl, preferably having 1 to 6 or 1 to 8 carbon atoms, such as (C 1 -C 10 )-, (C 1 -C 6 )- or (C 1 -C4)-alkylthio.
- alkylthios examples include, but are but not limited to, (C 1 -C6)-alkylthio such as methylthio, ethylthio, propylthio, 1 -methylethylthio, butylthio, 1 -methylpropylthio, 2-methylpropylthio, 1,1 -dimethylethylthio, pentylthio, 1- methylbutylthio, 2-methylbutylthio, 3 -methylbutylthio, 1,1 -dimethylpropylthio, 1,2- dimethylpropylthio, 2,2-dimethylpropylthio, 1 -ethylpropylthio, hexylthio, 1 -methylpentylthio, 2- methylpentylthio, 3 -methylpentylthio, 4-methylpentylthio, 1,1 -dimethylbutylthio, 1,2- dimethylbutylthio, 1,2-
- alkylsulfinyls include, but are but not limited to, (C 1 -C 8 )- alkylsulfinyl such as methylsulfinyl, ethylsulfinyl, propylsulfinyl, 1-methylethylsulfinyl, butylsulfinyl, 1 -methylpropylsulfinyl, 2-methylpropylsulfinyl, 1,1 -dimethylethylsulfinyl, pentylsulfinyl, 1 -methylbutylsulfinyl, 2-methylbutylsulfinyl, 3 -methylbutylsulfinyl, 1,1- dimethylpropylsulfinyl, 1,2-dimethylpropylsulfinyl, 2,2-dimethylpropylsulfinyl, 1- ethylpropylsulfinyl, he
- alkoxy refers to an alkyl radical bonded via an oxygen atom.
- alkoxys include, but are but not limited to, (C 1 -C 6 )-alkoxy such as methoxy, ethoxy, propoxy, 1 -methylethoxy, butoxy, 1 -methylpropoxy, 2-methylpropoxy, 1,1- dimethylethoxy, pentoxy, 1 -methylbutoxy, 2-methylbutoxy, 3 -methylbutoxy, 1,1- dimethylpropoxy, 1,2-dimethylpropoxy, 2,2-dimethylpropoxy, 1 -ethylpropoxy, hexoxy, 1- methylpentoxy, 2-methylpentoxy, 3 -methylpentoxy, 4-methylpentoxy, 1,1 -dimethylbutoxy, 1,2- dimethylbutoxy, 1,3 -dimethylbutoxy, 2,2-dimethylbutoxy, 2,3 -dimethylbutoxy, 3,3- dimethylbutoxy, 1 -ethylbut
- haloalkoxy is, for example, OCF 3 , OCHF 2 , OCH 2 F, OCF 2 CF 3 , OCH 2 CF 3 , and OCH 2 CH 2 C1; this applies correspondingly to haloalkenyloxy, haloalkynyloxy, and other halogen-substituted radicals.
- C 1 -C 8 -haloalkoxy as mentioned herein by way of example thus refers to a C 1 -C 6 -alkoxy group, as defined above, in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different.
- C 1 -C 6 - haloalkoxy groups include, but are not limited to, chloromethoxy, bromomethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 1 -chloroethoxy, 1- bromoethoxy, 1 -fluoroethoxy, 2-fluoroethoxy, 2,2-difluoroethoxy, 2,2,2-trifluoroethoxy, 2- chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy, 2,2-dichloro-2-fluoroethoxy, 2,2,2- trichloroethoxy, pentafluoroethoxy, and l,l,l-trifluoroprop-2-oxy.
- alkenyloxy refers to an alkenyl radical attached via an oxygen atom
- alkynyloxy refers to an alkynyl radical attached via an oxygen atom, such as (C 2 -C 10 )-, (C 2 -C6)-, or (C 2 -C4)-alkenyloxy, and (C 3 -C 10 )-, (C 3 -C 6 )-, or (C 3 -C4)-alkynyloxy.
- C 2 -C6-alkenyloxy refers to a formula (C 2 -C 6 -alkenyl)-O-, in which the term “C 2 -C6-alkenyl” group is which the as defined herein.
- C 2 -C 6 -alkenyloxy examples include but are not limited to ethenyloxy (or "vinyloxy”), prop-2 - en-l-yloxy (or “allyloxy”), prop-l-en-l-yloxy, prop-l-en-2-yloxy (or “isopropenyloxy”), but-3- enyloxy, but-2-enyloxy, but-l-enyloxy, 2-methylprop-2-enyloxy, l-methylprop-2-enyloxy, 2- methylprop-l-enyloxy, and 1-methylprop-l-enyloxy.
- cycloalkoxy refers to a cycloalkyl radical attached via an oxygen atom
- cycloalkenyloxy refers to a cycloalkenyl radical attached via an oxygen atom.
- the number of the carbon atoms refers to the alkyl radical in the alkylcarbonyl group.
- the number of the carbon atoms refers to the alkenyl or alkynyl radical in the alkenyl or alkynyl group.
- the number of the carbon atoms refers to the alkyl radical in the alkoxycarbonyl group.
- alkenyloxycarbonyl and “alkynyloxycarbonyl,” unless defined differently elsewhere, respectively represent alkenyl and alkynyl radicals attached to the skeleton via -O-C( O)-, such as (C2-C10)-, (C2-C6)-, or (C2-C4)-alkenyloxycarbonyl and (C3-C10)-, (C3-C6)- , or (C3-C4)-alkynyloxycarbonyl.
- the number of the carbon atoms refers to the alkenyl or alkynyl radical in the alkenyloxycarbonyl or alkynyloxycarbonyl group.
- aryl denotes an optionally substituted mono-, bi-, or polycyclic aromatic system having preferably 6 to 14, especially 6 to 10, ring carbon atoms, for example phenyl, naphthyl, anthryl, phenanthrenyl and the like, preferably phenyl.
- optionally substituted aryl also includes polycyclic systems, such as tetrahydronaphthyl, indenyl, indanyl, fluorenyl, biphenylyl, where the bonding site is on the aromatic system.
- aryl is generally also encompassed by the term “optionally substituted phenyl.”
- Preferred aryl substituents here are, for example, hydrogen, halogen, alkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, halocycloalkyl, alkenyl, alkynyl, aryl, arylalkyl, arylalkenyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, alkoxyalkyl, alkylthio, haloalkylthio, haloalkyl, alkoxy, haloalkoxy, cycloalkoxy, cycloalkylalkoxy, aryloxy, heteroraryloxy, alkoxyalkoxy, alkynylalkoxy, alkenyloxy, dialkylamino-alkoxy, tris-[alkyl]silyl, di-[alkyl
- optionally substituted heterocyclyl polycyclic systems are also included, for example 8- azabicyclo[3.2.1]octanyl, 8-azabicyclo[2.2.2]octanyl or l-azabicyclo[2.2.1]heptyl.
- Optionally substituted heterocyclyl also includes spirocyclic systems, such as, for example, l-oxa-5-aza- spiro[2.3]hexyl.
- the heterocyclic ring preferably contains 3 to 9 ring atoms, in particular 3 to 6 ring atoms, and one or more, preferably 1 to 4, in particular 1, 2 or 3 heteroatoms in the heterocyclic ring, preferably from the group N, O, and S, where, however, two oxygen atoms must not be directly adjacent to one another, for example having one heteroatom from the group consisting of N, O, and S 1- or 2- or 3-pyrrolidinyl, 3,4-dihydro-2H-pyrrol-2- or - 3-yl, 2,3 -dihydro- IH-pyrrol-l- or -2- or -3- or -4- or -5-yl; 2,5-dihydro-lH-pyrrol-l- or -2- or -3- yl, 1 - or 2- or 3- or 4-piperidinyl; 2,3,4,5-tetrahydropyridin-2- or -3- or -4- or -5-yl or -6-yl
- 3 -membered and 4-membered heterocycles are, for example, 1- or 2-aziridinyl, oxiranyl, thiiranyl, 1- or 2- or 3-azetidinyl, 2- or 3-oxetanyl, 2- or 3-thietanyl, l,3-dioxetan-2-yl.
- heterocyclyl are a partially or fully hydrogenated heterocyclic radical having two heteroatoms from the group of N, O and S, for example 1- or 2- or 3- or 4-pyrazolidinyl; 4,5-dihydro-3H- pyrazol-3- or -4- or -5-yl; 4,5-dihydro-lH-pyrazol-l- or -3- or -4- or -5-yl; 2,3 -dihydro- 1H- pyrazol-1- or -2- or -3- or -4- or -5-yl; 1- or -2- or -3- or -4-imidazolidinyl; 2,3 -dihydro- 1H- imidazol-1- or -2- or -3- or -4-yl; 2,5-dihydro-lH-imidazol-l- or -2- or -4- or -5-yl; 4,5-dihydro- IH-imidazol-l-l-
- heterocyclyl are a partially or fully hydrogenated heterocyclic radical having 3 heteroatoms from the group of N, O and S, for example 1,4,2- dioxazolidin-2- or -3- or -5-yl; l,4,2-dioxazol-3- or -5-yl; l,4,2-dioxazinan-2- or -3- or -5- or -6- yl; 5,6-dihydro-l,4,2-dioxazin-3- or -5- or -6-yl; l,4,2-dioxazin-3- or -5- or -6-yl; 1,4,2- dioxazepan-2- or -3- or -5- or -6- or -7-yl; 6,7-dihydro-5H-l,4,2-dioxazepin-3- or -5- or -6- or -7- yl; 2,3-dihydro
- heterocycles listed above are preferably substituted by, for example, hydrogen, halogen, alkyl, haloalkyl, hydroxyl, alkoxy, cycloalkoxy, aryloxy, alkoxyalkyl, alkoxyalkoxy, cycloalkyl, halocycloalkyl, aryl, arylalkyl, heteroaryl, heterocyclyl, alkenyl, alkylcarbonyl, cycloalkylcarbonyl, arylcarbonyl, heteroarylcarbonyl, alkoxycarbonyl, hydroxycarbonyl, cycloalkoxycarbonyl, cycloalkylalkoxycarbonyl, alkoxycarbonylalkyl, arylalkoxycarbonyl, arylalkoxycarbonylalkyl, alkynyl, alkynylalkyl, alkylalkynyl, trisalkylsilylalkynyl, nitro, amino,
- substituents for a substituted heterocyclic radical are the substituents specified further down, and additionally also oxo and thioxo.
- the oxo group as a substituent on a ring carbon atom is then, for example, a carbonyl group in the heterocyclic ring.
- lactones and lactams are preferably also included.
- the oxo group may also occur on the ring heteroatoms, which may exist in different oxidation states, for example in the case of N and S, and in that case form, for example, the divalent -N(O)-, -S(O)- (also SO for short) and - S(O) 2 - (also SO 2 for short) groups in the heterocyclic ring.
- -N(O)- and -S(O)- groups both enantiomers in each case are included.
- heteroaryl refers to heteroaromatic compounds, i.e. fully unsaturated aromatic heterocyclic compounds, preferably 5- to 7-membered rings having 1 to 4, preferably 1 or 2, identical or different heteroatoms, preferably O, S, or N.
- Inventive heteroaryls are, for example, IH-pyrrol-l-yl; lH-pyrrol-2-yl; lH-pyrrol-3-yl; furan-2-yl; furan-3-yl; thien-2-yl; thien- 3-yl, IH-imidazol-l-yl; lH-imidazol-2-yl; lH-imidazol-4-yl; lH-imidazol-5-yl; lH-pyrazol-l-yl; lH-pyrazol-3-yl; lH-pyrazol-4-yl; lH-pyrazol-5-yl, 1 H- 1,2, 3 -triazol- 1-yl, lH-l,2,3-triazol-4-yl, lH-l,2,3-triazol-5-yl, 2H-l,2,3-triazol-2-yl, 2H-l,2,3-triazol-4
- heteroaryl groups described herein may also be substituted by one or more identical or different radicals. If two adjacent carbon atoms are part of a further aromatic ring, the systems are fused heteroaromatic systems, such as benzofiised or polyannealed heteroaromatics. Preferred examples are quinolines (e.g.
- quinolin-2-yl quinolin-3-yl, quinolin-4- yl, quinolin-5-yl, quinolin-6-yl, quinolin-7-yl, quinolin-8-yl
- isoquinolines e.g.
- heteroaryl are also 5- or 6-membered benzofused rings from the group of IH-indol- 1-yl, lH-indol-2-yl, lH-indol-3-yl, lH-indol-4-yl, lH-indol-5-yl, lH-indol-6-yl, lH-indol-7-yl, 1- benzofuran-2-yl, l-benzofuran-3-yl, l-benzofuran-4-yl, l-benzofuran-5-yl, l-benzofuran-6-yl, 1- benzofuran-7-yl, l-benzothiophen-2-yl, l-benzothiophen-3-yl, l-benzothiophen-4-yl, 1- benzothiophen-5-yl, l-benzothiophen-6-yl, l-benzothiophen-7-yl,
- halogen denotes, for example, fluorine, chlorine, bromine or iodine. If the term is used for a radical, "halogen" denotes, for example, a fluorine, chlorine, bromine or iodine atom.
- alkyl refers to a straight-chain or branched open-chain, saturated hydrocarbon radical that is optionally mono- or polysubstituted, and in the latter case is referred to as "substituted alkyl.”
- Preferred substituents are halogen atoms, alkoxy, haloalkoxy, cyano, alkylthio, haloalkylthio, amino, or nitro groups, particular preference being given to methoxy, methyl, fluoroalkyl, cyano, nitro, fluorine, chlorine, bromine, or iodine.
- the prefix “di” includes the combination of equal or different alkyl radicals, e.g. dimethyl or methyl(ethyl) or ethyl(methyl).
- haloalkyl denote an alkyl, alkenyl, and alkynyl, respectively, that is partially or fiilly substituted by identical or different halogen atoms, for example monohaloalkyl, such as CH 2 CH 2 C1, CH 2 CH 2 Br, CHC1CH 3 , CH 2 C1, and CH 2 F; perhaloalkyl or perfluoroalkyl, such as CC1 3 , CC1F 2 , CFC1 2 ,CF 2 CC1F 2 , and CF 2 CC1FCF 3 ; polyhaloalkyl, such as CH 2 CHFC1, CF 2 CC1FH, CF 2 CBrFH, and CH 2 CF 3 .
- C 2 -C6-haloalkenyl refers to a C 2 - C 6 -alkenyl group as defined above in which one or more hydrogen atoms are replaced with one or more halogen atoms that may be the same or different.
- C 2 -C6-haloalkenyl comprises up to 9 halogen atoms that can be the same or different.
- C 2 -C6-haloalkynyl refers to a C 2 - C 6 -alkynyl group as defined above in which one or more hydrogen atoms are replaced with one or more halogen atoms that may be the same or different.
- C 2 -C6-haloalkynyl comprises up to 9 halogen atoms that can be the same or different.
- (C 1 -C4)-alkyl mentioned here by way of example is a brief notation for straight-chain or branched alkyl having one to 4 carbon atoms according to the range stated for carbon atoms, i.e. encompasses the methyl, ethyl, 1 -propyl, 2-propyl, 1 -butyl, 2-butyl, 2- methylpropyl, or tert-butyl radicals.
- General alkyl radicals with a larger specified range of carbon atoms e.g. "(C 1 -C6)-alkyl” also encompass straight-chain or branched alkyl radicals with a greater number of carbon atoms, i.e.
- alkyl radicals having 5 and 6 carbon atoms.
- Alkyl radicals including in composite radicals such as alkoxy, haloalkyl, etc., are, for example, methyl, ethyl, n- propyl or i-propyl, n-, i-, t- or 2-butyl, pentyls; hexyls, such as n-hexyl, i-hexyl, and 1,3- dimethylbutyl; heptyls, such as n-heptyl, 1 -methylhexyl, and 1,4-dimethylpentyl.
- Alkenyl and alkynyl radicals are defined as the possible unsaturated radicals corresponding to the alkyl radicals, where at least one double bond or triple bond is present. Preference is given to radicals having one double bond or triple bond.
- alkenyl also includes, in particular, straight-chain or branched open-chain hydrocarbon radicals having more than one double bond, such as 1,3- butadienyl and 1,4-pentadienyl, but also allenyl or cumulenyl radicals having one or more cumulated double bonds, for example allenyl (1,2-propadienyl), 1,2-butadienyl and 1,2,3- pentatrienyl.
- Alkenyl denotes, for example, vinyl which may optionally be substituted by further alkyl radicals, for example (but not limited thereto) (C2-C 6 )-alkenyl such as ethenyl, 1 -propenyl, 2-propenyl, 1 -methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1 -methyl- 1 -propenyl, 2-methyl-l- propenyl, l-methyl-2-propenyl, 2-methyl-2 -propenyl, 1 -pentenyl, 2-pentenyl, 3 -pentenyl, 4- pentenyl, 1 -methyl- 1-butenyl, 2-methyl- 1-butenyl, 3 -methyl- 1-butenyl, 1 -methyl-2-butenyl, 2- methyl-2-butenyl, 3-methyl-2-butenyl, l-methyl-3-butenyl, 2-methyl-3-butenyl, 3-methyl
- alkynyl also includes, in particular, straight-chain or branched open-chain hydrocarbon radicals having more than one triple bond, or else having one or more triple bonds and one or more double bonds, for example 1 ,3-butatrienyl or 3-penten-l -yn- 1-yl.
- C2-C6)-alkynyl denotes, for example, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl,
- cycloalkyl denotes a carbocyclic saturated ring system having preferably 3-8 ring carbon atoms, for example cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, which optionally has further substitution, preferably by hydrogen, alkyl, alkoxy, cyano, nitro, alkylthio, haloalkylthio, halogen, alkenyl, alkynyl, haloalkyl, amino, alkylamino, dialkylamino, alkoxycarbonyl, hydroxycarbonyl, arylalkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl, and cycloalkylaminocarbonyl.
- cyclic systems with substituents are included, also including substituents with a double bond on the cycloalkyl radical, for example an alkylidene group such as methylidene.
- polycyclic aliphatic systems are also included, for example bicyclo[l .1 ,0]butan-l -yl, bicyclo[ 1.1.0]butan-2-yl, bicyclo[2.1 ,0]pentan- 1 -yl, bicyclo[l .1.1 ]pentan- 1 -yl, bicyclo[2.1 ,0]pentan-2-yl, bicyclo[2.1 ,0]pentan-5-yl, bicyclo[2.1.1 ]hexyl, bicyclo[2.2.1 ]hept-2-yl, bicyclo[2.2.2]octan-2-yl, bicyclo[3.2.1 ]oct
- (C3-C7)-cycloalkyl is a brief notation for cycloalkyl having three to 7 carbon atoms, corresponding to the range specified for carbon atoms.
- substituted cycloalkyl spirocyclic aliphatic systems are also included, for example spiro[2.2]pent-l-yl, spiro[2.3]hex-l-yl, spiro[2.3]hex-4-yl, 3-spiro[2.3]hex- 5-yl, spiro[3.3]hept-l-yl, and spiro[3.3]hept-2-yl.
- Cycloalkenyl denotes a carbocyclic, nonaromatic, partially unsaturated ring system having preferably 4-8 carbon atoms, e.g. 1-cyclobutenyl, 2-cyclobutenyl, 1 -cyclopentenyl, 2- cyclopentenyl, 3 -cyclopentenyl, or 1 -cyclohexenyl, 2-cyclohexenyl, 3 -cyclohexenyl, 1,3- cyclohexadienyl or 1,4-cyclohexadienyl, also including substituents with a double bond on the cycloalkenyl radical, for example an alkylidene group such as methylidene.
- the elucidations for substituted cycloalkyl apply correspondingly.
- haloalkylthio on its own or as constituent part of a chemical group - represents straight-chain or branched S-haloalkyl, preferably having 1 to 8, or having 1 to 6 carbon atoms, such as (C 1 -C 8 )-, (C 1 -C 6 )-, or (C 1 -C4)-haloalkylthio, for example (but not limited thereto) trifluoromethylthio, pentafluoroethylthio, difluoromethyl, 2,2-difluoroeth-l-ylthio, 2,2,2- difluoroeth-l-ylthio, and 3,3,3-prop-l-ylthio.
- Halocycloalkyl and halocycloalkenyl denote cycloalkyl and cycloalkenyl, respectively, which are partially or fully substituted by identical or different halogen atoms, such as F, Cl and Br, or by haloalkyl, such as trifluoromethyl or difluoromethyl, for example 1- fluorocycloprop-l-yl, 2-fhiorocycloprop-l-yl, 2,2-difluorocycloprop-l-yl, 1-fluorocyclobut-l-yl, 1-trifluoromethylcycloprop-l-yl, 2-trifluoromethylcycloprop-l-yl, 1 -chlorocycloprop- 1-yl, 2- chlorocycloprop-l-yl, 2,2-dichlorocycloprop-l-yl, and 3, 3 -difluorocyclobutyl.
- “trialkylsilyl” used alone or as constituent part of a chemical group represents a straight-chain or branched Si-alkyl, preferably having 1 to 8, or having 1 to 6 carbon atoms, such as tri[(C 1 -C 8 )-, (C 1 -C 6 )- or (C 1 -C4)-alkyl]silyl, for example (but not limited thereto) trimethyl silyl, triethylsilyl, tri(n-propyl)silyl, tri(isopropyl)silyl, tri(n-butyl)silyl, tri(l- methylprop-l-yl)silyl, tri(2-methylprop-l-yl)silyl, tri(l,l-dimethyleth-l-yl)silyl, and tri(2,2- dimethyleth- 1 -yl)silyl.
- oxo refers to an oxygen atom that is bonded to a carbon atom or sulfur atom by a double bond.
- methylidene refers to a CH2 group connected to a carbon atom by a double bond.
- halomethylidene refers to a CX2 group connected to a carbon atom by a double bond, where X is halogen.
- the compounds can form, through a hydrogen shift, tautomers whose structure is not formally covered by the general formula (I), these tautomers are nevertheless covered by the definition of the inventive compounds of the general formula (I), unless a particular tautomer is under consideration.
- many carbonyl compounds may be present both in the keto form and in the enol form, both forms being encompassed by the definition of the compound of the general formula (I).
- the compounds of the formula (I) have acidic properties and can form salts, and if appropriate also internal salts or adducts with inorganic or organic bases or with metal ions. If the compounds of the formula (I) bear hydroxyl, carboxyl or other groups which induce acidic properties, these compounds can be reacted with bases to give salts.
- Suitable bases are, for example, hydroxides, carbonates, hydrogen carbonates of the alkali metals and alkaline earth metals, especially those of sodium, potassium, magnesium, and calcium, and also ammonia, primary, secondary, and tertiary amines having (C 1 - C4)-alkyl groups, mono-, di-, and trialkanolamines of (C 1 -C4)-alkanols, choline and chlorocholine, and organic amines, such as trialkylamines, morpholine, piperidine, or pyridine.
- salts are compounds in which the acidic hydrogen is replaced by an agriculturally suitable cation, for example metal salts, especially alkali metal salts or alkaline earth metal salts, especially sodium and potassium salts, or else ammonium salts, salts with organic amines or quaternary ammonium salts, for example with cations of the formula [NRR'R''R'”] + in which R to R'" are each independently an organic radical, especially alkyl, aryl, aralkyl, or alkylaryl.
- an agriculturally suitable cation for example metal salts, especially alkali metal salts or alkaline earth metal salts, especially sodium and potassium salts, or else ammonium salts, salts with organic amines or quaternary ammonium salts, for example with cations of the formula [NRR'R''R'”] + in which R to R'" are each independently an organic radical, especially alkyl, aryl, aralkyl, or alkylaryl.
- alkyl sulfonium and alkyl sulfoxonium salts such as (C 1 -C4)-trialkyl sulfonium and (C1-C4)- trialkyl sulfoxonium salts.
- the compounds of the formula (I) can form salts by addition of a suitable inorganic or organic acid, for example mineral acids, for example HO, HBr, H2SO4, H3PO4 or HNO3, or organic acids, for example carboxylic acids such as formic acid, acetic acid, propionic acid, oxalic acid, lactic acid or salicylic acid or sulfonic acids, for example p-Toluenesulfonic acid, onto a basic group, for example amino, alkylamino, dialkylamino, piperidino, morpholino, or pyridino.
- these salts comprise the conjugate base of the acid as the anion.
- Suitable substituents present in deprotonated form may form internal salts with groups which for their part can be protonated, such as amino groups.
- the compounds of the general formula (I) may be present as stereoisomers.
- the possible stereoisomers defined by the specific three-dimensional form thereof, such as enantiomers, diastereomers, Z and E isomers, are all encompassed by the general formula (I). If, for example, one or more alkenyl groups are present, diastereomers (Z and E isomers) may occur. If, for example, one or more asymmetric carbon atoms are present, enantiomers and diastereomers may occur.
- Stereoisomers can be obtained from the mixtures obtained in the preparation by customary separation methods.
- the chromatographic separation can be effected either on the analytical scale to find the enantiomeric excess or the diastereomeric excess, or else on the preparative scale to produce test specimens for biological testing. It is likewise possible to selectively prepare stereoisomers by using stereoselective reactions with use of optically active starting materials and/or auxiliaries.
- the present disclosure thus also relates to all stereoisomers which are embraced by the general formula (I) but are not shown in their specific stereomeric form, and to mixtures thereof.
- a “herbicide” is any molecule that is used to control, prevent, or interfere with the growth of one or more plants.
- Illustrative herbicides include acetyl-CoA carboxylase (ACCase) inhibitors (for example, aryloxyphenoxy propionates and cyclohexanediones); acetolactate synthase (ALS) inhibitors (for example, sulfonylureas, imidazolinones, triazolopyrimidines, and triazolinones); 5 -enolpyruvylshikimate-3 -phosphate synthase (EPSPS) inhibitors (for example, glyphosate), synthetic auxins (for example, phenoxys, benzoic acids, carboxylic acids, and semicarbazones), photosynthesis (photosystem II) inhibitors (for example, triazines, triazinones, nitriles, benzothiadiazoles, and
- PPO inhibiting herbicides are known in the art and commercially available.
- PPO inhibiting herbicides include, but are not limited to, diphenylethers (such as acifluorfen, its salts and esters, bifenox, its salts and esters, ethoxyfen, its salts and esters, fluoronitrofen, furyloxyfen, halosafen, chlomethoxyfen, fluoroglycofen, its salts and esters, lactofen, its salts and esters, oxyfluorfen, and fomesafen, its salts and esters); thiadiazoles (such as fluthiacet-methyl and thidiazimin); pyrimidinediones or phenyluracils (such as benzfendizone, butafenacil, ethyl [3 -2-chloro-4-fluoro-5-( 1 -methyl-6-trifhioromethyl-2,
- herbicide-tolerant or “herbicide-tolerance” means the ability to be wholly or partially unaffected by the presence or application of one of more herbicide(s), for example to resist the toxic effects of an herbicide when applied.
- a cell or organism is “herbicide- tolerant” if it is able to maintain at least some normal growth or phenotype in the presence of one or more herbicide(s).
- a trait is an herbicide-tolerance trait if its presence can confer improved tolerance to an herbicide upon a cell, plant, or seed as compared to the wild-type or control cell, plant, or seed. Crops comprising a herbicide-tolerance trait can continue to grow and are minimally affected by the presence of the herbicide.
- a target enzyme is “herbicide-tolerant” if it exhibits improved enzyme activity relative to a wild-type or control enzyme in the presence of the herbicide.
- Herbicide-tolerance may be complete or partial insensitivity to a particular herbicide, and may be expressed as a percent (%) tolerance or insensitivity to a particular herbicide.
- Contemplated plants which might be produced with an herbicide tolerance trait of the present disclosure could include, for instance, any plant susceptible to a PPO inhibitor herbicide, including crop plants such as soybean (Glycine max), maize (Zea mays), cotton (Gossypium sp.), Brassica plants, alfalfa, barley, beans, beet, broccoli, cabbage, carrot, canola, cauliflower, celery, Chinese cabbage, cucumber, eggplant, leek, lettuce, melon, oat, onion, pea, pepper, peanut, potato, pumpkin, radish, rice, sweet com, sorghum, spinach, squash, sugar beet, sugar cane, sunflower, tomato, watermelon, and wheat, among others.
- crop plants such as soybean (Glycine max), maize (Zea mays), cotton (Gossypium sp.), Brassica plants, alfalfa, barley, beans, beet, broccoli, cabbage, carrot, canola, cauliflower, celery, Chinese cabbage, cucumber,
- Herbicides may be applied to a plant growth area comprising the plants and seeds provided by the disclosure as a method for controlling weeds.
- Plants and seeds provided by the disclosure comprise an herbicide tolerance trait and as such are tolerant to the application of one or more PPO inhibiting herbicides.
- the herbicide application may be the recommended commercial rate (IX) or any fraction or multiple thereof, such as twice the recommended commercial rate (2X).
- Herbicide rates may be expressed as acid equivalent per pound per acre (lb ae/acre) or acid equivalent per gram per hectare (g ae/ha) or as pounds active ingredient per acre (lb ai/acre) or grams active ingredient per hectare (g ai/ha), depending on the herbicide and the formulation.
- the herbicide application comprises at least one PPO inhibiting herbicide.
- the plant growth area may or may not comprise weed plants at the time of herbicide application.
- a herbicidally-effective dose of PPO inhibiting herbicide(s) for use in an area for controlling weeds may consist of a range from about 0. IX to about 30X label rate(s) over a growing season.
- One (1) hectare is equivalent to 2.47105 acres and one (1) pound is equivalent to 453.592 grams.
- the desired application rate of the compounds of the general formula (I) and/or their salts is generally impacted to a certain extent by external conditions such as temperature, humidity, etc.
- the application rate may therefore vary within wide limits, and can be determined empirically by one of skill in the art in view of the present disclosure.
- the total amount of compounds of the general formula (I) and their salts is often desirably in the range from about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, or about 0.25 g a.i./ha to about 300 g a.i./ha, but preferably from about 0.02 g a.i./ha to about 250 g a.i./ha, especially from about 0.05 g a.i./ha to about 150 g a.i./ha, and most preferably from about 0.25 g a.i./ha to about 120 g a.i./ha. This applies both to the pre-emergence or the post-emergence application.
- Herbicide applications may be sequentially or tank mixed with one, two, or a combination of several PPO inhibiting herbicides or any other compatible herbicide. Multiple applications of one herbicide or of two or more herbicides, in combination or alone, may be used over a growing season to areas comprising transgenic plants of the disclosure for the control of a broad spectrum of dicot weeds, monocot weeds, or both, for example, two applications (such as a pre-planting application and a post-emergence application or a pre-emergence application and a post-emergence application) or three applications (such as a pre-planting application, a preemergence application, and a post-emergence application or a pre-emergence application and two post-emergence applications).
- two applications such as a pre-planting application and a post-emergence application or a pre-emergence application and a post-emergence application
- three applications such as a pre-planting application, a preemergence application, and a post-emergence application or a pre-emergence application and two post-emergence applications.
- Substituted phenyluracils to be used according to the disclosure and its salts have excellent herbicidal efficacy against a broad spectrum of economically important monocotyledonous and dicotyledonous annual harmful plants.
- the present disclosure therefore provides methods for controlling weeds, in areas of transgenic crop plants being tolerant to PPO inhibitor herbicides wherein the plants comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein the protein confers tolerance to such herbicides, comprising the application of the compounds of the general formula (I) and/or salts as defined above, to the plants (for example harmful plants such as monocotyledonous or dicotyledonous weeds or undesired crop plants), to the seed (for example grains, seeds or vegetative propagules such as tubers or shoot parts with buds) or to the area on which the plants grow (for example the area under cultivation), including any possible combinations thereof.
- the spectrum of action extends to species such as, for example, Abutilon, Amaranthus, Ambrosia, Anoda, Anthemis, Aphanes, Artemisia, Atriplex, Bellis, Bidens, Capsella, Carduus, Cassia, Centaurea, Chenopodium, Cirsium, Convolvulus, Datura, Desmodium, Emex, Erodium, Erysimum, Euphorbia, Galeopsis, Galinsoga, Galium, Geranium, Hibiscus, Ipomoea, Kochia, Lamium, Lepidium, Lindernia, Matricaria, Mentha, Mercurialis, Mullugo, Myosotis, Papaver, Pharbitis, Plantago, Polygonum, Portulaca, Ranunculus, Raphanus, Rorippa, Rotala, Rumex, Salsola, Senecio, Sesbania, Sida, Sin
- Economically important crops here are, for example, dicotyledonous crops from the genera of Arachis, Beta, Brassica, Cucumis, Cucurbita, Helianthus, Daucus, Glycine, Gossypium, Ipomoea, Lactuca, Linum, Lycopersicon, Nicotiana, Phaseolus, Pisum, Solanum, and Vicia, or monocotyledonous crops from the genera of Allium, Ananas, Asparagus, Avena, Hordeum, Oryza, Panicum, Saccharum, Secale, Sorghum, Triticale, Triticum, and Zea.
- the compounds of the general formula (I) and/or salts thereof can be formulated in various ways according to which biological and/or physicochemical parameters are required.
- general formulation options are: wettable powders (WP), water-soluble powders (SP), emulsifiable concentrates (EC), water-soluble concentrates, aqueous solutions (SL), emulsions (EW), such as oil-in-water and water-in-oil emulsions, sprayable solutions or emulsions, dispersions based on oil or water, oil dispersions (OD), suspoemulsions (SE), suspension concentrates (SC), oil-miscible solutions, capsule suspensions (CS), dusting products (DP), dressings, granules for soil application or scattering, granules (GR) in the form of microgranules, spray granules, absorption and adsorption granules, water-dispersible granules (WG), water- soluble gran
- Wettable powders are preparations which can be dispersed uniformly in water and, in addition to the active ingredient, apart from a diluent or inert substance, also comprise surfactants of the ionic and/or nonionic type (wetting agents, dispersants), for example polyoxyethylated alkylphenols, polyoxyethylated fatty alcohols, polyoxyethylated fatty amines, fatty alcohol polyglycol ether sulfates, alkanesulfonates, alkylbenzene sulfonates, sodium lignosulfonate, sodium 2, 2'-dinaphthylmethane-6, 6' -disulfonate, sodium dibutylnaphthalenesulfonate, or sodium oleoyl methyltaurate.
- the active herbicidal ingredients are finely ground, for example, in customary apparatuses such as hammer mills, blower
- Emulsifiable concentrates are produced by dissolving the active ingredient in an organic solvent, for example butanol, cyclohexanone, dimethylformamide, xylene, or else relatively high-boiling aromatics or hydrocarbons or mixtures of the organic solvents, with addition of one or more ionic and/or nonionic surfactants (emulsifiers).
- organic solvent for example butanol, cyclohexanone, dimethylformamide, xylene, or else relatively high-boiling aromatics or hydrocarbons or mixtures of the organic solvents.
- emulsifiers which may be used are: calcium alkyl aryl sulfonate salts, such as calcium dodecylbenzenesulfonate, or nonionic emulsifiers such as fatty acid polyglycol esters, alkyl aryl polyglycol ethers, fatty alcohol polyglycol ethers, propylene oxide-ethylene oxide condensation products, alkyl polyethers, sorbitan esters, for example sorbitan fatty acid esters, or for example polyoxyethylene sorbitan fatty acid esters.
- calcium alkyl aryl sulfonate salts such as calcium dodecylbenzenesulfonate
- nonionic emulsifiers such as fatty acid polyglycol esters, alkyl aryl polyglycol ethers, fatty alcohol polyglycol ethers, propylene oxide-ethylene oxide condensation products, alkyl polyethers, sorbitan esters, for example sorbit
- Dusting products are obtained by grinding the active ingredient with finely distributed solids, for example talc, natural clays, such as kaolin, bentonite and pyrophyllite, or diatomaceous earth.
- finely distributed solids for example talc, natural clays, such as kaolin, bentonite and pyrophyllite, or diatomaceous earth.
- Suspension concentrates may be water- or oil-based. They may be produced, for example, by wet-grinding by means of commercial bead mills and optional addition of surfactants as already listed above, for example, for the other formulation types.
- Emulsions for example oil-in-water emulsions (EW)
- EW oil-in-water emulsions
- Active compounds that can be employed in combination with the compounds of the general formula (I) described herein in compositions described herein are, for example, known active compounds which are based on inhibition of, for example, acetolactate synthase, acetyl-CoA carboxylase, cellulose synthase, enolpyruvylshikimate-3 -phosphate synthase, glutamine synthetase, p-hydroxyphenylpyruvate dioxygenase, phytoene desaturase, photosystem I, photosystem II, or protoporphyrinogen oxidase, as are described in, for example, “Glossary of Common Names and Abbreviations of Herbicides,” Weed Research 26:441-445, 1986 or MacBean, "The Pesticide Manual,” 16 th ed., British Crop Protection Council, Alton, UK, 2012, and the literature cited therein.
- Known herbicides or plant growth regulators which can be combined with the compounds of the described herein are, for example, the following, where said active compounds are designated either with their "common name” in accordance with the International Organization for Standardization (ISO) or with the chemical name or with the code number. They always encompass all the use forms, for example acids, salts, esters and also all isomeric forms such as stereoisomers and optical isomers, even if they are not mentioned explicitly.
- ISO International Organization for Standardization
- herbicidal mixing partners include one or more of the following: acetochlor, acifluorfen, acifluorfen-methyl, acifluorfen-sodium, aclonifen, alachlor, allidochlor, alloxydim, alloxydim-sodium, ametryn, amicarbazone, amidochlor, amidosulfuron, 4-amino-3- chloro-6-(4-chloro-2-fhioro-3-methylphenyl)-5-fluoropyridine-2-carboxylic acid, aminocyclopyrachlor, aminocyclopyrachlor-potassium, aminocyclopyrachlor-methyl, aminopyralid, aminopyralid-dimethylammonium, aminopyralid-tripromine, amitrole, ammonium sulfamate, anilofos, asulam, asulam-potassium, asulam sodium, atrazine, azafenidin, azims
- a combination described herein may be a composition containing herbicidally active compounds (A) and (B), where (A) is one or more compounds of the general formula (I) or their agrochemically compatible salts [component (A)] and (B) is one or more herbicides [component (B)] selected from the group consisting of the herbicidally active compounds (Bl) to (Bl l), wherein:
- (Bl) represents active herbicidal ingredients from the group of the following 1,3 -diketo compounds:
- (B2) represents active herbicidal ingredients from the group of the following (sulfon)amides:
- (B3) represents active herbicidal ingredients from the group of the following aryl nitriles:
- (B4) represents active herbicidal ingredients from the group of the following azoles:
- (B5) represents further active herbicidal ingredients specified below:
- (B6) represents active herbicidal ingredients from the group of the following (het)arylcarboxylic acids:
- (B7) represents active herbicidal ingredients from the group of the following organic phosphorus compounds:
- (B8) represents active herbicidal ingredients from the group of the following phenyl ethers:
- (B9) represents active herbicidal ingredients from the group of the following pyrimidines:
- (B10) represents active herbicidal ingredients from the group of the following (thio)ureas:
- (Bl 1) represents active herbicidal ingredients from the group of the following triazines:
- herbicides (B) are believed to be particularly good combination partners.
- the preferred, particularly preferred, and most preferred herbicides (B) are listed below as further embodiments of the present invention.
- Preferred components from B 1 are the herbicidal active ingredients are bicyclopyrone, clethodim, mesotrione, pinoxaden, sethoxydim, sulcotrione, tembotrione, and tralkoxydim.
- Particularly preferred components from Bl are the herbicidal active ingredients bicyclopyrone, clethodim, mesotrione, pinoxaden, and tembotrione.
- Most preferred from group Bl are bicyclopyrone, clethodim, mesotrione, and tembotrione.
- Preferred components from B2 are the herbicidal active ingredients acetochlor, asularn, beflubutamid, chlorimuron, chlorsulfuron, cloransulam, diclosulam, diflufenican, dimethenamid, ethoxysulfuron, flazasulfuron, florasulam, flucarbazone, flufenacet, flumetsulam, flupyrsulfuron, foramsulfuron, iodosulfuron, mesosulfuron, metolachlor, metosulam, metsulfuron, nicosulfuron, penoxsulam, picolinafen, propoxycarbazone, prosulfocarb, prosulfuron, pyroxsulam, rimsulfiiron, S-metolachlor, sulfometuron, sulfosulfuron, thiencarbazone, thifens
- Particularly preferred components from B2 are the herbicidal active ingredients acetochlor, diflufenican, dimethenamid, flazasulfuron, flufenacet, foramsulfuron, iodosulfuron, mesosulfuron, metolachlor, nicosulfuron, rimsulfuron, S- metolachlor, thiencarbazone, methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5-methoxy-4H- isoxazole-5-carbonyl]amino]tetrahydrofuran-2-carboxylate, methyl rel-(2R,4R)-4-[[3-(3,5- dichlorophenyl)-5-vinyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2 -carboxylate, methyl rel-(2R,4R)-4-
- Preferred components from group B3 are the herbicidal active ingredients bromoxynil and ioxynil.
- the most preferred component from group B3 is bromoxynil.
- Preferred components from group B4 are the herbicidal active ingredients amitrole, carfentrazone, imazamethabenz, imazamox, imazapic, imazapyr, imazethapyr, isoxaben, isoxaflutole, oxadiazon, pyraflufen, pyrasulfotole, pyroxasulfone, sulfentrazone, tolpyralate, topramezone, flupoxam.
- Particularly preferred components from group B4 are carfentrazone, imazamox, imazapyr, imazethapyr, isoxaflutole, oxadiazon, pyraflufen, pyrasulfotole, pyroxasulfone, sulfentrazone, and tolpyralate.
- the most preferred components from group B4 are imazamox, imazethapyr, isoxaflutole, oxadiazon, pyraflufen-ethyl, pyroxasulfone, and sulfentrazone.
- Preferred components from group B5 are the herbicidal active ingredients aminocyclopyrachlor, aminopyralid, benazolin, bentazone, bixlozone, cinidon, cinmethylin, clomazone, ethofiimesate, flamprop, florpyrauxifen, flumiclorac, flumioxazin, fluridone, flurochloridone, flurtamone, fluthiacet-methyl, halauxifen, indanofan, paraquat, pelargonic acid, pendimethalin, triafamone, trifluralin, 4-amino-3-chloro-5-fluoro-6-(7-fluoro-lH-indol-6- yl)pyridine-2-carboxylic acid, cyclopyrimorate, diquat, oxaziclomefone, and 4-hydroxy-l -methyl -
- Particularly preferred components from group B5 are the herbicidal active ingredients bentazone, florpyrauxifen, flumiclorac, flumioxazin, fluthiacet-methyl, halauxifen, paraquat, pelargonic acid, and pendimethalin.
- the most preferred components from group B5 are bentazone, clomazone, florpyrauxifen, flumiclorac, flumioxazin, and paraquat.
- Preferred components from group B6 are the herbicidal active ingredients clopyralid, dicamba, fhiroxypyr, and picloram. Particularly preferred components from group B6 are the herbicidal active ingredient dicamba and dicamba salts. A most preferred component from group B6 is dicamba.
- Preferred components from group B7 are the herbicidal active ingredients bialaphos, glufosinate, glyphosate, and sulfosate. Particularly preferred components from group B7 are the herbicidal active ingredients glufosinate, glyphosate, and sulfosate. The most preferred components from group B7 are glufosinate and glyphosate.
- Preferred components from group B8 are the herbicidal active ingredients 2,4-D, 2,4- DB, 2,4-DP, acifluorfen, aclonifen, clodinafop, diclofop, fenoxaprop, fomesafen, lactofen, MCPA, mecoprop, oxyfluorfen, quizalofop, and quizalofop-P.
- Particularly preferred components from group B8 are the herbicidal active ingredients 2,4-D, 2,4-DB, 2,4-DP, acifluorfen, aclonifen, fomesafen, lactofen, and oxyfluorfen.
- the most preferred components from group B8 are 2,4-D, aclonifen, fomesafen, and oxyfluorfen.
- Preferred components from group B9 are the herbicidal active ingredients butafenacil, safhifenacil, terbacil, tiafenacil, trifludimoxazin, and ethyl [3-[2-chloro-4-fluoro-5-(l-methyl-6- trifhioromethyl-2,4-dioxo-l,2,3,4-tetrahydropyrimidin-3-yl)phenoxy]-2-pyridyloxy]acetate.
- Particularly preferred components from group B9 are the herbicidal active ingredients butafenacil, safhifenacil, tiafenacil, trifludimoxazin, and ethyl[3-[2-chloro-4-fluoro-5-(l-methyl-6-trifluoromethyl- 2,4-dioxo-l,2,3,4-tetrahydropyrimidin-3-yl)phenoxy]-2-pyridyl-oxy]acetate.
- the most preferred components from group B9 are butafenacil, saflufenacil, and trifludimoxazin.
- Preferred components from group B10 are the herbicidal active ingredients chlorobromuron, chlorotoluron, diuron, isoproturon, linuron, methabenzthiazuron, metobromuron, metoxuron, and monolinuron.
- Particularly preferred components from group B10 are the herbicidal active ingredients diuron and isoproturon. The most preferred component from group B10 is diuron.
- Preferred components from group Bl 1 are the herbicidal active ingredients ametryne, atrazine, hexazinone, indaziflam, metribuzin, simazine, terbuthylazine, and terbutryne.
- Particularly preferred components from group Bl 1 are the herbicidal active ingredients atrazine, indaziflam, metribuzin, and terbuthylazine.
- the most preferred components from group Bl 1 are atrazine, indaziflam, metribuzin, and terbuthylazine.
- the herbicide combinations according to the invention may contain other components, e.g. plant growth regulators.
- plant growth regulators as possible mixing partners are: abscisic acid and related analogs [e.g. (2Z,4E)-5-[6-Ethynyl-l -hydroxy-2, 6-dimethyl-4- oxocyclohex-2-en- 1 -yl]-3-methylpenta-2,4-dienoic acid, methyl-(2Z,4E)-5-[6-ethynyl- 1 - hydroxy-2, 6-dimethyl-4-oxocyclohex-2-en- 1 -yl] -3 -methylpenta-2,4-dienoate, (2Z,4E)-3 -ethyl-5- (1 -hydroxy-2, 6, 6-trimethyl-4-oxocyclohex-2-en- 1 -yl)penta-2,4-dienoic acid, (2E,4E)-5-( 1 -
- COs sometimes referred to as N-acetylchitooligosaccharides, are also composed of GlcNAc residues but have side chain decorations that make them different from chitin molecules [(CsHi3NO5)n, CAS No. 1398-61-4] and chitosan molecules [(CsHnNO ⁇ n, CAS No.
- chitinous compounds chlormequat chloride, cloprop, cyclanilide, 3-(Cycloprop-l-enyl)propionic acid, l-[2-(4-cyano-3,5- dicyclopropylphenyl)acetamido]cyclohexanecarboxylic acid, 1 -[2-(4-cyano-3- cyclopropylphenyl)acetamido] cyclohexanecarboxylic acid, daminozide, dazomet, dazomet- sodium, n-decanol, dikegulac, dikegulac-sodium, endothal, endothal-dipotassium, -disodium, and mono(N,N-dimethylalkylammonium), ethephon, flumetralin, flurenol, flurenol-butyl, flurenol- methyl, flurprimidol, forchlorfen
- LCO lipo-chitooligosaccharides
- Nod symbiotic nodulation
- Myc factors consist of an oligosaccharide backbone of 0-1,4-linked JV-acetyl-D-glucosamine (“GlcNAc”) residues with an N-linked fatty acyl chain condensed at the non-reducing end.
- LCOs differ in the number of GlcNAc residues in the backbone, in the length and degree of saturation of the fatty acyl chain and in the substitutions of reducing and non-reducing sugar residues), linoleic acid or derivatives thereof, linolenic acid or derivatives thereof, maleic hydrazide, mepiquat chloride, mepiquat pentaborate, 1 -methylcyclopropene, 3- methylcyclopropene, 1 -ethylcyclopropene, 1-n-propylcyclopropene, 1 -cyclopropenylmethanol, methoxyvinylglycin (MVG), 3’-methyl abscisic acid, l-(4-methylphenyl)-N-(2-oxo-l-propyl- 1 ,2,3,4-tetrahydroquinolin-6-yl)methanesulfonamide and related substituted tetrahydroquinolin
- Active compounds which can be employed in combination with the compounds of the general formula (I) according to the present disclosure in compositions according to the present disclosure are, for example, the following safeners: SI) Compounds from the group of heterocyclic carboxylic acid derivatives of formula SI : wherein symbols and indices are defined as follows: nA is an integer value in the range of 0 to 5, preferably 0 to 3;
- RA 1 is halogen, (C 1 -C4)-alkyl, (C 1 -C4)-alkoxy, nitro or (C 1 -C4)-haloalkyl;
- WA is an unsubstituted or substituted divalent heterocyclic moiety selected from the group of partially unsaturated or aromatic five-membered heterocycles carrying 1 to 3 hetero ring atoms selected from the group of nitrogen (N) und oxygen (O), and carrying at least one N-atom and not more than one O-atom in the ring, preferably a five-membered heterocyclic moiety selected from the group (WA 1 ) to (WA 5 ); mA is 0 or 1 ;
- RA 2 is ORA 3 , SRA 3 or NRA 3 RA 4 or a saturated or unsaturated 3- to 7-membered heterocycle containing at least one N-atom and up to 3 heteroatoms, preferably combined with other heteroatoms from the group of O (oxygen) and S (sulfur), and which is linked to the carbonyl group in (SI) via a nitrogen atom, and which is unsubstituted or substituted by moieties selected from the group of (C 1 -C4)-alkyl, (C 1 -C4)-alkoxy or possibly substituted phenyl, preferably ORA 3 , NHRA 4 , or N(CH3)2, particularly ORA 3
- RA 3 is hydrogen or an unsubstituted or substituted aliphatic hydrocarbon moiety, preferably containing 1 to 18 C-atoms;
- RA 4 is hydrogen, (C 1 -C6)-alkyl, (C 1 -C6)-alkoxy or substituted or unsubstituted phenyl;
- RA 5 is hydrogen, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (C 1 -C4)-alkoxy-(C 1 -C 8 )-alkyl, cyano or COORA 9 , wherein RA 9 is hydrogen, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (C1-C4)- alkoxy-(C 1 -C4)-alkyl, (C 1 -C6)-hydroxyalkyl, (C3-Ci2)-cycloalkyl, or tris-(C 1 -
- RA 6 , RA 7 , RA 8 are independently hydrogen, (C 1 -C 8 )-alkyl, (C 1 -C 8 )-haloalkyl, (C3-C12)- cycloalkyl or substituted or unsubstituted phenyl;
- Sl a Compounds of the dichlorophenylpyrazoline-3 -carboxylic acid type (Sl a ), preferably compounds such as l-(2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline-3- carboxylic acid, ethyl l-(2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline- 3 -carboxylate (SI -1) ("mefenpyr-diethyl”), and related compounds, as described in WO- A-91/07874;
- Sl b Derivatives of dichlorophenylpyrazolecarboxylic acid (Sl b ), preferably compounds such as ethyl l-(2,4-dichlorophenyl)-5-methylpyrazole-3-carboxylate (Sl-2), ethyl l-(2,4- dichlorophenyl)-5-isopropylpyrazole-3-carboxylate (Sl-3), ethyl 1 -(2,4-dichlorophenyl)- 5-(l,l-dimethylethyl)pyrazole-3-carboxylate (Sl-4) and related compounds as described in EP-A-333131 and EP-A-269806;
- Sl c Derivatives of 1, 5 -diphenylpyrazole-3 -carboxy lie acid (Sl c ), preferably compounds such as ethyl l-(2,4-dichlorophenyl)-5-phenylpyrazole-3-carboxylate (Sl-5), methyl l-(2- chlorophenyl)-5-phenylpyrazole-3 -carboxylate (SI -6) and related compounds as described, for example, in EP-A-268554;
- Sl d Compounds of the triazolecarboxylic acid type (Sl d ), preferably compounds such as fenchlorazole(-ethyl ester), i.e. ethyl l-(2,4-dichlorophenyl)-5-trichloromethyl-lH-l,2,4- triazole-3-carboxylate (SI -7), and related compounds, as described in EP-A- 174562 and EP-A-346620;
- Sl f Compounds of the triazolyloxy acetic acid type (Sl f ), preferably compounds such as methyl- ⁇ [ 1 ,5-bis(4-chloro-2-fhiorophenyl)- 1 H- 1 ,2,4-triazol-3 -yl]oxy ⁇ acetate (S 1 - 14) or ⁇ [l,5-Bis(4-chloro-2-fhiorophenyl)-lH-l,2,4-triazol-3-yl]oxy ⁇ acetic acid (Sl-15) or methyl- ⁇ [5-(4-chloro-2-fluorophenyl)- 1 -(2,4-difluorophenyl)- 1 H- 1 ,2,4-triazol-3 - yl]oxy ⁇ acetate (Sl-16) or ⁇ [5-(4-chloro-2-fluorphenyl)-l-(2,4-difluorophenyl)
- S2 a Compounds of the 8-quinolinoxyacetic acid type (S2 a ), preferably 1 -methylhexyl (5- chloro-8-quinolinoxy)acetate ("cloquintocet-mexyl") (S2-1), 1,3-dimethylbut-l-yl (5- chloro-8-quinolinoxy)acetate (S2-2), 4-allyloxybutyl (5-chloro-8-quinolinoxy)acetate (S2- 3), 1 -allyloxyprop-2 -yl (5-chloro-8-quinolinoxy)acetate (S2-4), ethyl (5-chloro-8- quinolinoxy)acetate (S2-5), methyl (5-chloro-8-quinolinoxy)acetate (S2-6), allyl (5- chloro-8-quinolinoxy)acetate (S2-7), 2-(2-propylideneiminoxy)-l -ethyl (5-chloro-8- quinolinoxy)acetate (S2-7)
- S2 b Compounds of the (5-chloro-8-quinolinoxy)malonic acid type (S2 b ), preferably compounds such as diethyl (5-chloro-8-quinolinoxy)malonate, diallyl (5-chloro-8- quinolinoxy)malonate, methyl ethyl (5-chloro-8-quinolinoxy)malonate and related compounds, as described in EP-A-0 582 198. 53) Active ingredients of the dichloroacetamide type (S3), which are frequently used as preemergence safeners (soil-acting safeners), for example
- AD-67 or "MON 4660” (3-dichloroacetyl-l-oxa-3-azaspiro[4.5]decane) from Nitrokemia or Monsanto (S3 -7),
- TI-35 (1-dichloroacetylazepane) from TRI-Chemical RT (S3-8), "diclonon” (dicyclonon) or "BAS145138” or “LAB145138” (S3-9) ((RS)- 1 -dichloroacetyl-3 ,3 , 8a-trimethylperhydropyrrolo[ 1 ,2-a]pyrimidin-6-one) from TRI-Chemical RT (S3-8), "diclonon” (dicyclonon) or "BAS145138” or “LAB145138” (S3-9) ((RS)- 1 -dichloroacetyl-3 ,3 , 8a-trimethylperhydropyrrolo[ 1 ,2-a]pyrimidin-6-one) from TRI-Chemical RT (S3-8), "diclonon” (dicyclonon) or "BAS145138” or “LAB145138” (S3-9) ((RS)- 1
- RA 1 is (C 1 -C6)-alkyl, (C3-C6)-cycloalkyl, where the 2 latter radicals are substituted by VA substituents from the group of halogen, (C 1 -C4)-alkoxy, (C 1 -C6)-haloalkoxy and (C 1 -C4)-alkylthio and, in the case of cyclic radicals, also by (C 1 -C4)-alkyl and (C 1 - C4)-haloalkyl;
- RA 2 is halogen, (C 1 -C4)-alkyl, (C 1 -C4)-alkoxy, CF3; mA is 1 or 2;
- VA is 0, 1, 2 or 3;
- RB 1 , RB 2 are independently hydrogen, (C 1 -C6)-alkyl, (C3-C6)-cycloalkyl, (C3-C6)-alkenyl, (C3-C6)-alkynyl;
- RB 3 is halogen, (C 1 -C 4 )-alkyl, (C 1 -C4)-haloalkyl or (C 1 -C4)-alkoxy; and mB is 1 or 2, e.g. those in which
- RB 1 cyclopropyl
- RB 2 hydrogen
- (RB 3 ) 2-OMe
- RB 1 cyclopropyl
- RB 2 hydrogen
- (RB 3 ) 5-Cl-2-OMe (S4-2)
- RB 1 isopropyl
- RB 2 hydrogen
- (RB 3 ) 5-Cl-2-OMe (S4-4) and
- RB 1 isopropyl
- RB 2 hydrogen
- (RB 3 ) 2-OMe (S4-5);
- Rc 1 Rc 2 are independently hydrogen, (C 1 -C 8 )-alkyl, (C 3 -C 8 )-cycloalkyl, (C3-C6)- alkenyl, (C3-C6)-alkynyl,
- Rc 3 is halogen, (C 1 -C4)-alkyl, (C 1 -C4)-alkoxy, CF3 and me is 1 or 2; for example 1-[4-(N-2-methoxybenzoylsulfamoyl)phenyl]-3-methylurea,
- RD 4 is halogen, (C 1 -C4)-alkyl, (C 1 -C4)-alkoxy, CF3; mD is 1 or 2;
- RD 5 is hydrogen, (C 1 -C 6 )-alkyl, (C3-C6)-cycloalkyl, (C 2 -C 8 )-alkenyl, (C2-C 6 )-alkynyl, (C5-C6)-cycloalkenyl.
- Active ingredients from the class of the hydroxyaromatics and the aromatic-aliphatic carboxylic acid derivatives (S5) for example ethyl 3, 4, 5 -triacetoxybenzoate, 3,5-dimethoxy-4-hydroxybenzoic acid, 3,5- dihydroxybenzoic acid, 4-hydroxysalicylic acid, 4-fluorosalicylic acid, 2-hydroxycinnamic acid, 2,4-dichlorocinnamic acid, as described in WO-A-2004/084631, WO-A- 2005/015994, WO-A-2005/016001.
- RD 1 is halogen, (C 1 -C4)-alkyl, (C 1 -C4)-haloalkyl, (C 1 -C4)-alkoxy, (C 1 -C4)-haloalkoxy,
- RD 2 is hydrogen or (C 1 -C4)-alkyl
- RD 3 is hydrogen, (C 1 -C 8 )-alkyl, (C2-C4)-alkenyl, (C2-C4)-alkynyl or aryl, where each of the aforementioned carbon-containing radicals is unsubstituted or substituted by one or more, preferably up to three, identical or different radicals from the group consisting of halogen and alkoxy; or salts thereof, no is an integer from 0 to 2.
- Active ingredients from the class of the 3-(5-tetrazolylcarbonyl)-2-quinolones for example l,2-dihydro-4-hydroxy-l-ethyl-3-(5-tetrazolylcarbonyl)-2-quinolone (CAS Reg. No.: 219479- 18-2), 1 ,2-dihydro-4-hydroxy- 1 -methyl-3 -(5-tetrazolylcarbonyl)-2-quinolone
- RE 1 is halogen, (C 1 -C4)-alkyl, methoxy, nitro, cyano, CF3, OCF3,
- YE, ZE are independently O or S, nE is an integer from 0 to 4,
- RE 2 is (C 1 -Ci6)-alkyl, (C2-C 6 )-alkenyl, (C3-C6)-cycloalkyl, aryl; benzyl, halobenzyl, RE 3 is hydrogen or (Ci-C6)-alkyl.
- CGA-43089 (Z)-cyanomethoxyimino(phenyl)acetonitrile) (SI 1-3), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage.
- naphthalic anhydride (1,8-naphthalenedicarboxylic anhydride) (SI 3-1), which is known as a seed-dressing safener for maize against thiocarbamate herbicide damage,
- flurazole (benzyl 2-chloro-4-trifluoromethyl-l,3-thiazole-5-carboxylate) (S13-3), which is known as a seed-dressing safener for millet/sorghum against alachlor and metolachlor damage,
- JC-940 3-(2-chlorophenylmethyl)-l-(l-methyl-l-phenylethyl)urea, see JP-A-60087254), which is known as a safener for rice against damage by some herbicides
- methoxyphenone or "NK 049” (3,3'-dimethyl-4-methoxybenzophenone), which is known as a safener for rice against damage by some herbicides
- RH 1 is a (C 1 -C6)-haloalkyl radical
- RH 2 is hydrogen or halogen
- RH 3 , RH 4 are independently hydrogen, (C 1 -Ci6)-alkyl, (C2-Ci6)-alkenyl or (C2-C16)- alkynyl, where each of the 3 latter radicals is unsubstituted or substituted by one or more radicals from the group of halogen, hydroxyl, cyano, (C 1 -C4)-alkoxy, (C1-C4)- haloalkoxy, (C 1 -C4)-alkylthio, (C 1 -C4)-alkylamino, di[(C 1 -C4)-alkyl]amino, [(C 1 - C4)-alkoxy]carbonyl, [(C 1 -C4)-haloalkoxy]carbonyl, (C3-C6)-cycloalkyl which is unsubstituted or substituted, phenyl which is unsubstituted or substituted, and heterocyclyl which is unsubstituted or substituted
- RH 3 is (Ci-C4)-alkoxy, (C2-C4)-alkenyloxy, (C2-Ce)-alkynyloxy or (C2-C4)-haloalkoxy and
- RH 4 is hydrogen or (Ci-C4)-alkyl or
- RH 3 and RH 4 together with the directly attached nitrogen atom represent a four- to eightmembered heterocyclic ring which, as well as the nitrogen atom, may also contain further ring heteroatoms, preferably up to two further ring heteroatoms from the group of N, O, and S, and which is unsubstituted or substituted by one or more radicals from the group of halogen, cyano, nitro, (Ci-C4)-alkyl, (Ci-C4)-haloalkyl, (Ci-C4)-alkoxy, (C1-C4)- haloalkoxy and (Ci-C4)-alkylthio.
- Active ingredients which are used primarily as herbicides but also have safener action on crop plants, for example (2,4-dichlorophenoxy)acetic acid (2,4-D), (4-chlorophenoxy)acetic acid, (R,S)-2-(4-chloro-o-tolyloxy)propionic acid (mecoprop), 4-(2,4-dichlorophenoxy)butyric acid (2,4-DB), (4-chloro-o-tolyloxy)acetic acid (MCPA), 4-(4-chloro-o-tolyloxy)butyric acid, 4-(4-chlorophenoxy)butyric acid,
- Safeners that may be used in combination with the herbicidal compounds described herein include, but are not limited to, cloquintocet-mexyl, cyprosulfamide, fenchlorazole ethyl ester, isoxadifen-ethyl, mefenpyr-diethyl, fenclorim, cumyluron, S4-1, and S4-5.
- Preferred safeners include cloquintocet-mexyl, cyprosulfamide, isoxadifen-ethyl, and mefenpyr-diethyl.
- the herbicide combinations described herein may comprise further components, for example plant growth regulators or compounds that prevent or eliminate unwanted species.
- Such compounds include, but are not limited to herbicides, fungicides, insecticides, acaricides, nematicides, miticides, and related substances.
- plant growth regulators examples include, but are not limited to, acibenzolar, acibenzolar-S-methyl, 5-aminolevulinic acid, ancymidol, 6-benzylaminopurine, brassinolide, catechol, chlormequat chloride, cloprop, cyclanilide, 3 -(cycloprop- l-enyl)propionic acid, daminozide, dazomet, n-decanol, dikegulac, dikegulac-sodium, endothal, endothal- dipotassium, -disodium, and mono(N,N-dimethylalkylammonium), ethephon, flumetralin, flurenol, flurenol -butyl, flurprimidol, forchlorfenuron, gibberellic acid, inabenfide, indole-3 -acetic acid (IAA), 4-indol-3-ylbut
- Active compounds that may be used in combination with the compounds of the general formula (I) described herein (in, for example, mixed formulations or a tank mix) are, for example, fungicidally active compounds.
- the preferred fungicidally active compounds comprise at least one standard commercial active ingredient, and include, but are not limited to:
- Ergosterol biosynthesis inhibitors for example (1.001) cyproconazole, (1.002) difenoconazole, (1.003) epoxiconazole, (1.004) fenhexamid, (1.005) fenpropidin, (1.006) fenpropimorph, (1.007) fenpyrazamine, (1.008) fhiquinconazole, (1.009) flutriafol, (1.010) imazalil, (1.011) imazalil sulfate, (1.012) ipconazole, (1.013) metconazole, (1.014) myclobutanil, (1.015) paclobutrazole, (1.016) prochloraz, (1.017) propiconazole, (1.018) prothioconazole, (1.019) pyrisoxazole, (1.020) spiroxamine, (1.021) tebuconazole, (1.022) tetraconazole, (1.023) t
- N-methylimidoformamide (1.065) N'-(2,5-dimethyl-4- ⁇ [3-(2, 2,3,3- tetrafluoropropoxy)phenyl]sulfanyl ⁇ phenyl)-N-ethyl-N-methylimidoformamide, (1.066) N'-(2,5-dimethyl-4- ⁇ [3-(pentafluoroethoxy)phenyl]sulfanyl ⁇ phenyl)-N-ethyl-N- methylimidoformamide, (1.067) N'-(2,5-dimethyl-4- ⁇ 3-[(l,l,2,2- tetrafluoroethyl)sulfanyl]phenoxy ⁇ phenyl)-N-ethyl-N-methylimidoformamide, (1.068) N'-(2,5-dimethyl-4- ⁇ 3-[(2,2,2-trifluoroethyl)sulfanyl]phenoxy ⁇ phenyl)-
- Inhibitors of the respiratory chain in complex I or II for example (2.001) benzovindiflupyr, (2.002) bixafen, (2.003) boscalid, (2.004) carboxin, (2.005) fluopyram, (2.006) flutolanil, (2.007) fluxapyroxad, (2.008) furametpyr, (2.009) isofetamid, (2.010) isopyrazam (anti-epimeric enantiomer 1R,4S,9S), (2.011) isopyrazam (anti-epimeric enantiomer 1S,4R,9R), (2.012) isopyrazam (anti-epimeric racemate 1RS,4SR,9SR), (2.013) isopyrazam (mixture of the syn-epimeric racemate 1RS,4SR,9RS and the anti- epimeric racemate 1RS,4SR,9SR), (2.014) isopyrazam (syn-epimeric enantiomer 1R,4S,
- Amino acid and/or protein biosynthesis inhibitors for example (7.001) cyprodinil, (7.002) kasugamycin, (7.003) kasugamycin hydrochloride hydrate, (7.004) oxytetracycline, (7.005) pyrimethanil, (7.006) 3-(5-fhioro-3,3,4,4-tetramethyl-3,4- dihydroisoquinolin- 1 -yl)quinoline.
- ATP production inhibitors for example (8.001) silthiofam.
- C 6 ll wall synthesis inhibitors for example (9.001) benthiavalicarb, (9.002) dimethomorph, (9.003) flumorph, (9.004) iprovalicarb, (9.005) mandipropamid, (9.006) pyrimorph, (9.007) valifenalate, (9.008) (2E)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4- yl)- 1 -(morpholin-4-yl)prop-2-en- 1 -one, (9.009) (2Z)-3 -(4-tert-butylphenyl)-3 -(2- chloropyridin-4-yl)- 1 -(morpholin-4-yl)prop-2-en- 1 -one.
- Lipid and membrane synthesis inhibitors for example (10.001) propamocarb, (10.002) propamocarb hydrochloride, (10.003) tolclofos-methyl.
- Nucleic acid synthesis inhibitors for example (12.001) benalaxyl, (12.002) benalaxyl-M (kiralaxyl), (12.003) metalaxyl, (12.004) metalaxyl-M (mefenoxam).
- Signal transduction inhibitors for example (13.001) fludioxonil, (13.002) iprodione, (13.003) procymidone, (13.004) proquinazid, (13.005) quinoxyfen, (13.006) vinclozolin.
- Compounds that can act as decouplers for example (14.001) fluazinam, (14.002) meptyldinocap.
- Preferred fungicides are selected from the group consisting of benalaxyl, bitertanol, bromuconazole, captafol, carbendazim, carpropamid, cyazofamid, cyproconazole, diethofencarb, edifenphos, fenpropimorph, fentin acetate, fluquinconazole, fosetyl, fluoroimide, folpet, iminoctadine, iprodione, iprovalicarb, kasugamycin, maneb, nabam, pencycuron, prochloraz, propamocarb, propineb, prothioconazole, pyrimethanil, spiroxamine, quintozene, tebuconazole, tolylfluanid, triadimefon, triadimenol, trifloxystrobin, and zineb.
- Active compounds which can be employed in combination with the compounds of the general formula (I) according to the present disclosure in compositions described herein are, for example, insecticidal, acaricidal, nematicidal, miticidal and related active ingredients are, for example (the compounds are, if possible, referred to by their common names): (1) Acetylcholinesterase (AChE) inhibitors, preferably carbamates selected from alanycarb, aldicarb, bendiocarb, benfuracarb, butocarboxim, butoxycarboxim, carbaryl, carbofuran, carbosulfan, ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, metolcarb, oxamyl, pirimicarb, propoxur, thiodicarb, thiofa
- AChE Acetylcholinesterase
- GABA-gated chloride channel blockers preferably cyclodiene-organochlorines selected from chlordane and endosulfan, or phenylpyrazoles (fiproles) selected from ethiprole and fipronil.
- Sodium channel modulators preferably pyrethroids selected from acrinathrin, allethrin, d-cis-trans allethrin, d-trans allethrin, bifenthrin, bioallethrin, bioallethrin S- cyclopentenyl isomer, bioresmethrin, cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda-cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, cyphenothrin [(IR)-trans isomer], deltamethrin, empenthrin [(EZ)-(IR) isomer], esfenvalerate,
- Nicotinic acetylcholine receptor (nAChR) competitive modulators preferably neonicotinoids selected from acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, and thiamethoxam, or nicotine, or sulfoximines such as sulfoxaflor, or butenolides such as flupyradifurone.
- Nicotinic acetylcholine receptor (nAChR) allosteric modulators preferably spinosyns selected from spinetoram and spinosad.
- Glutamate-gated chloride channel (GluCl) allosteric modulators preferably avermectins/milbemycins selected from abamectin, emamectin benzoate, lepimectin, and milbemectin.
- Juvenile hormone mimics preferably juvenile hormone analogues selected from hydroprene, kinoprene and methoprene, or fenoxycarb or pyriproxyfen.
- Miscellaneous non-specific (multi-site) inhibitors preferably alkyl halides selected from methyl bromide and other alkyl halides; or chloropicrin or sulfuryl fluoride or borax or tartar emetic or methyl isocyanate generators selected from diazomet and metam.
- Mite growth inhibitors selected from clofentezine, hexythiazox, diflovidazin, and etoxazole.
- Microbial disruptors of insect midgut membranes selected from Bacillus thuringiensis subspecies israelensis, Bacillus sphaericus, Bacillus thuringiensis subspecies aizawai, Bacillus thuringiensis subspecies kurstaki, Bacillus thuringiensis subspecies tenebrionis, and B.t. plant proteins selected from CrylAb, CrylAc, CrylFa, CrylA.105, Cry2Ab, VIP3A, mCry3A, Cry3Ab, Cry3Bb, and Cry34Abl/35Abl.
- Inhibitors of mitochondrial ATP synthase preferably ATP disruptors selected from diafenthiuron, or organotin compounds selected from azocyclotin, cyhexatin and fenbutatin oxide, or propargite or tetradifon.
- Nicotinic acetylcholine receptor channel blockers selected from bensultap, cartap hydrochloride, thiocyclam, and thiosultap-sodium.
- Molting disruptors (especially in the case of Diptera) such as cyromazine.
- Ecdysone receptor agonists selected from chromafenozide, halofenozide, methoxyfenozide, and tebufenozide.
- Octopamine receptor agonists such as amitraz.
- Mitochondrial complex III electron transport inhibitors selected from hydramethylnon, acequinocyl, and fluacrypyrim.
- Mitochondrial complex I electron transport inhibitors preferably METI acaricides selected from fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad and tolfenpyrad, or rotenone (Derris).
- Inhibitors of acetyl-CoA carboxylase preferably tetronic and tetramic acid derivatives selected from spirodiclofen, spiromesifen, and spirotetramat.
- Mitochondrial complex IV electron transport inhibitors preferably phosphines selected from aluminum phosphide, calcium phosphide, phosphine, and zinc phosphide, or cyanides selected from calcium cyanide, potassium cyanide, and sodium cyanide.
- Mitochondrial complex II electron transport inhibitors preferably beta-keto nitrile derivatives selected from cyenopyrafen and cyflumetofen, or carboxanilides such as pyflubumide.
- Ryanodine receptor modulators preferably diamides selected from chlorantraniliprole, cyantraniliprole, and flubendiamide.
- Insecticides that can preferably be used together with the herbicides are, for example, as follows: acetamiprid, acrinathrin, aldicarb, amitraz, acinphos-methyl, cyfluthrin, carbaryl, cypermethrin, deltamethrin, endosulfan, ethoprophos, fenamiphos, fenthion, fipronil, imidacloprid, methamidophos, methiocarb, niclosamide, oxydemeton-methyl, prothiophos, silafluofen, thiacloprid, thiodicarb, tralomethrin, triazophos, trichlorfon, triflumuron, terbufos, fonofos, phorate, chlorpyriphos, carbofuran, and tefluthrin.
- the disclosure relates, in certain embodiments, to recombinant DNA molecules that encode herbicide-insensitive protoporphyrinogen oxidases (PPOs) and the proteins encoded thereby.
- PPOs herbicide-insensitive protoporphyrinogen oxidases
- engineered refers to a non-natural DNA, protein, cell, or organism that would not normally be found in nature and was created by human intervention.
- an “engineered protein,” “engineered enzyme,” or “engineered PPO,” refers to a protein, enzyme, or PPO whose amino acid sequence was conceived of and created in the laboratory using one or more of the techniques of biotechnology, protein design, or protein engineering, such as molecular biology, protein biochemistry, bacterial transformation, plant transformation, site-directed mutagenesis, directed evolution using random mutagenesis, genome editing, gene editing, gene cloning, DNA ligation, DNA synthesis, protein synthesis, and DNA shuffling.
- an engineered protein may have one or more deletions, insertions, or substitutions relative to the coding sequence of the wild-type protein and each deletion, insertion, or substitution may consist of one or more amino acids.
- Genetic engineering can be used to create a DNA molecule encoding an engineered protein, such as an engineered PPO that is herbicide tolerant and comprises at least a first amino acid substitution relative to a wild-type PPO protein as described herein.
- proteins provided herein have herbicide-tolerant protoporphyrinogen oxidase activity.
- herbicide-tolerant protoporphyrinogen oxidase means the ability of a protoporphyrinogen oxidase to maintain at least some of its protoporphyrinogen oxidase activity in the presence of one or more PPO inhibiting herbicide(s).
- protoporphyrinogen oxidase activity means the ability to catalyze the six-electron oxidation (removal of electrons) of protoporphyrinogen IX to form protoporphyrin IX, that is, to catalyze the dehydrogenation of protoporphyrinogen to form protoporphyrin.
- Enzymatic activity of a protoporphyrinogen oxidase can be measured by any means known in the art, for example, by an enzymatic assay in which the production of the product of protoporphyrinogen oxidase or the consumption of the substrate of protoporphyrinogen oxidase in the presence of one or more PPO inhibiting herbicide(s) is measured via fluorescence, high performance liquid chromatography (HPLC), or mass spectrometry (MS).
- HPLC high performance liquid chromatography
- MS mass spectrometry
- an assay for measuring enzymatic activity of a protoporphyrinogen oxidase is a bacterial assay, such as the assays described herein, whereby a recombinant protoporphyrinogen oxidase is expressed in a bacterial cell otherwise lacking PPO activity and the ability of the recombinant protoporphyrinogen oxidase to complement this knockout phenotype is measured.
- a "hemG knockout strain” means an organism or cell of an organism, such as E.
- a hemG knockout strain of, for instance, E. coli may be prepared in view of knowledge in the art, for instance in view of the E. coli HemG PPO sequence (Ecogene Accession No. EG11485; Sasarman et al., "Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity ofE. coll K12" Can. J. Microbiol. 39:1155-1161, 1993).
- the term “recombinant” refers to a non-naturally occurring DNA, protein, cell, seed, or organism that is the result of genetic engineering and was created by human intervention.
- a “recombinant DNA molecule” is a DNA molecule comprising a DNA sequence that does not naturally occur and as such is the result of human intervention, such as a DNA molecule comprising at least two DNA molecules heterologous to each other.
- An example of a recombinant DNA molecule is a DNA molecule provided herein encoding an herbicide-tolerant protoporphyrinogen oxidase operably linked to a heterologous promoter.
- a “recombinant protein” is a protein comprising an amino acid sequence that does not naturally occur and as such is the result of human intervention, such as an engineered protein.
- a recombinant cell, seed, or organism is a cell, seed, or organism comprising transgenic or heterologous DNA or protein, for example a transgenic plant cell, seed, or plant comprising a DNA construct or engineered protein described herein.
- wild-type means a naturally occurring.
- a “wild-type DNA molecule,” “wild-type protein” is a naturally occurring version of a DNA molecule or protein, that is, a version of a DNA molecule or protein pre-existing in nature.
- a wild-type version of a DNA molecule or protein may be useful for comparison with a recombinant or engineered DNA molecule or protein.
- An example of a wild-type protein useful for comparison with the engineered proteins provided by the present disclosure is the PPO enzyme from E. cloacae (H_N90) provided as SEQ ID NO:l.
- a “wild-type plant” is a naturally occurring plant. Such wild-type plants may also be useful for comparison with a plant comprising a recombinant or engineered DNA molecule or protein.
- An example of a wild-type plant useful for comparison with plants comprising a recombinant or engineered DNA molecule or protein may be a plant of the same type as the plant comprising the engineered DNA molecule or protein, such as a protein conferring an herbicide tolerance trait, and as such is genetically distinct from the plant comprising the herbicide tolerance trait.
- control plants means an experimental control designed for comparison purposes.
- a control plant in a transgenic plant analysis is a plant of the same type as the experimental plant (that is, the plant to be tested) but does not contain the transgenic insert, recombinant DNA molecule, or DNA construct of the experimental plant.
- Examples of control plants useful for comparison with transgenic plants include: for maize plants, non-transgenic LH244 maize (U.S. Patent No. 6,252,148) or non-transgenic 01DKD2 maize (U.S. Patent No.
- DNA refers to a double-stranded DNA molecule of genomic or synthetic origin (that is, a polymer of deoxyribonucleotide bases or a polynucleotide molecule) read from the 5' (upstream) end to the 3' (downstream) end.
- DNA sequence refers to the nucleotide sequence of a DNA molecule. The nomenclature used herein corresponds to that of by Title 37 of the United States Code of Federal Regulations ⁇ 1.822, and set forth in the tables in WIPO Standard ST.25 (1998), Appendix 2, Tables 1 and 3.
- protein-coding DNA molecule refers to a DNA molecule comprising a DNA sequence that encodes a protein.
- protein refers to a chain of amino acids linked by peptide (amide) bonds and includes both polypeptide chains that are folded or arranged in a biologically functional way and polypeptide chains that are not.
- a “protein-coding sequence” means a DNA sequence that encodes a protein.
- a “sequence” means a sequential arrangement of nucleotides or amino acids.
- a “DNA sequence” may refer to a sequence of nucleotides or to the DNA molecule comprising of a sequence of nucleotides; a “protein sequence” may refer to a sequence of amino acids or to the protein comprising a sequence of amino acids.
- the boundaries of a protein-coding sequence are usually determined by a translation start codon at the 5'-terminus and a translation stop codon at the 3'-terminus.
- the term “isolated” refers to at least partially separating a molecule from other molecules typically associated with it in its natural state.
- the term “isolated” refers to a DNA molecule that is separated from the nucleic acids that normally flank the DNA molecule in its natural state.
- a DNA molecule encoding a protein that is naturally present in a bacterium would be an isolated DNA molecule if it was not within the DNA of the bacterium from which the DNA molecule encoding the protein is naturally found.
- DNA molecule fused to or operably linked to one or more other DNA molecule(s) with which it would not be associated in nature is considered isolated herein.
- Such molecules are considered isolated even when integrated into the chromosome of a host cell or present in a nucleic acid solution with other DNA molecules.
- DNA molecules, or fragment thereof can also be obtained by other techniques, such as by directly synthesizing the fragment by chemical means, as is commonly practiced by using an automated oligonucleotide synthesizer.
- DNA sequences can encode proteins, such as the altered or engineered proteins disclosed herein. It is well within the capability of one of skill in the art to create alternative DNA sequences encoding the same, or essentially the same, altered or engineered proteins as described herein. These variant or alternative DNA sequences are within the scope of the embodiments described herein. As used herein, references to “essentially the same” sequence refers to sequences which encode amino acid substitutions, deletions, additions, or insertions that do not materially alter the functional activity of the protein encoded by the DNA molecule of the embodiments described herein.
- nucleotide sequences encoding a wild-type or engineered protein are also encompassed within the scope of the embodiments described herein.
- Substitution of amino acids other than those specifically exemplified or naturally present in a wild-type or engineered PPO enzyme are also contemplated within the scope of the embodiments described herein, so long as the PPO enzyme having the substitution still retains substantially the same functional activity described herein.
- Recombinant DNA molecules of the present disclosure may be synthesized and modified by methods known in the art, either completely or in part, where it is desirable to provide sequences useful for DNA manipulation (such as restriction enzyme recognition sites or recombination-based cloning sites), plant-preferred sequences (such as plant-codon usage or Kozak consensus sequences), or sequences useful for DNA construct design (such as spacer or linker sequences).
- sequences useful for DNA manipulation such as restriction enzyme recognition sites or recombination-based cloning sites
- plant-preferred sequences such as plant-codon usage or Kozak consensus sequences
- sequences useful for DNA construct design such as spacer or linker sequences.
- the present disclosure includes recombinant DNA molecules and engineered proteins having at least 50% sequence identity, at least 60% sequence identity, at least 70% sequence identity, at least 80% sequence identity, at least 85% sequence identity, at least 90% sequence identity, at least 91% sequence identity, at least 92% sequence identity, at least 93% sequence identity, at least 94% sequence identity, at least 95% sequence identity, at least 96% sequence identity, at least 97% sequence identity, at least 98% sequence identity, and at least 99% sequence identity to any of the recombinant DNA molecule or amino acid sequences provided herein, and having herbicide-tolerant protoporphyrinogen oxidase activity.
- percent sequence identity refers to the percentage of identical nucleotides or amino acids in a linear polynucleotide or amino acid sequence of a reference (“query”) sequence (or its complementary strand) as compared to a test (“subject”) sequence (or its complementary strand) when the two sequences are optimally aligned (with appropriate nucleotide or amino acid insertions, deletions, or gaps totaling less than 20 percent of the reference sequence over the window of comparison).
- Optimal alignment of sequences for aligning a comparison window are well known to those skilled in the art and may be conducted by tools such as the local homology algorithm of Smith and Waterman, the homology alignment algorithm of Needleman and Wunsch, the search for similarity method of Pearson and Lipman, and by computerized implementations of these algorithms such as GAP, BESTFIT, FASTA, and TFASTA available as part of the Sequence Analysis software package of the GCG® Wisconsin Package® (Accelrys Inc., San Diego, CA), MEGAlign (DNAStar Inc., 1228 S.
- tools such as the local homology algorithm of Smith and Waterman, the homology alignment algorithm of Needleman and Wunsch, the search for similarity method of Pearson and Lipman, and by computerized implementations of these algorithms such as GAP, BESTFIT, FASTA, and TFASTA available as part of the Sequence Analysis software package of the GCG® Wisconsin Package® (Accelrys Inc., San Diego, CA), MEGAlign (DNAStar Inc., 1228 S.
- An “identity fraction” for aligned segments of a test sequence and a reference sequence is the number of identical components that are shared by the two aligned sequences divided by the total number of components in the portion of the reference sequence segment being aligned, that is, the entire reference sequence or a smaller defined part of the reference sequence. Percent sequence identity is represented as the identity fraction multiplied by 100. The comparison of one or more sequences may be to a full-length sequence or a portion thereof, or to a longer sequence.
- a “DNA construct” is a recombinant DNA molecule comprising two or more heterologous DNA sequences.
- DNA constructs are useful for transgene expression and may be comprised in vectors and plasmids.
- DNA constructs may be used in vectors for transformation (that is, the introduction of heterologous DNA into a host cell) to produce recombinant bacteria or transgenic plants and cells (and as such may also be contained in the plastid DNA or genomic DNA of a transgenic plant, seed, cell, or plant part).
- a “vector” means any recombinant DNA molecule that may be used for bacterial or plant transformation.
- DNA molecules provided by the present disclosure can, for example, be inserted into a vector as part of a DNA construct having the DNA molecule operably linked to a heterologous gene expression element that functions in a plant to affect expression of the engineered protein encoded by the DNA molecule.
- Methods for making and using DNA constructs and vectors are well known in the art and described in detail in, for example, handbooks and laboratory manuals including Green and Sambrook, “Molecular Cloning: A Laboratory Manual” Vol. 1, 4 th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2012.
- the components for a DNA construct, or a vector comprising a DNA construct include one or more gene expression elements operably linked to a transcribable nucleic acid sequence, such as the following: a promoter for the expression of an operably linked DNA, an operably linked protein-coding DNA molecule, and an operably linked 3’ untranslated region (UTR).
- Gene expression elements useful in practicing the present disclosure include, but are not limited to, one or more of the following type of elements: promoter, 5’ UTR, enhancer, leader, cis-acting element, intron, transit sequence, 3’ UTR, and one or more selectable marker transgenes.
- transgenic refers to a DNA molecule artificially incorporated into the genome of an organism as a result of human intervention, such as by plant transformation methods.
- transgenic means comprising a transgene, for example a “transgenic plant” refers to a plant comprising a transgene in its genome and a “transgenic trait” refers to a characteristic or phenotype conveyed or conferred by the presence of a transgene incorporated into the plant genome.
- a transgenic plant refers to a plant comprising a transgene in its genome
- a “transgenic trait” refers to a characteristic or phenotype conveyed or conferred by the presence of a transgene incorporated into the plant genome.
- Transgenic plants of the present disclosure comprise the recombinant DNA molecules and proteins described herein.
- heterologous refers to the relationship between two or more things not normally associated in nature, for instance that are derived from different sources or not normally found in nature together in any other manner.
- a DNA molecule or protein may be heterologous with respect to another DNA molecule, protein, cell, plant, seed, or organism if not normally found in nature together or in the same context.
- a first DNA molecule is heterologous to a second DNA molecule if the two DNA molecules are not normally found in nature together in the same context.
- a protein-coding recombinant DNA molecule is heterologous with respect to an operably linked promoter if such a combination is not normally found in nature.
- a protein is heterologous with respect to a second operably linked protein, such as a transit peptide, if such combination is not normally found in nature.
- a recombinant DNA molecule encoding a PPO enzyme is heterologous with respect to an operably linked promoter that is functional in a plant cell if such combination is not normally found in nature.
- a recombinant DNA molecule also may be heterologous with respect to a cell, seed, or organism into which it is inserted when it would not naturally occur in that cell, seed, or organism.
- heterologous protein is a protein present in a plant, seed, cell, tissue, or organism in which it does not naturally occur or operably linked to a protein with which it is not naturally linked.
- heterologous proteins are the PPO enzymes described herein that is expressed in any plant, seed, cell, tissue, or organism.
- Another example is a protein operably linked to a second protein, such as a transit peptide or herbicide-tolerant protein, with which it is not naturally linked, or a protein introduced into a plant cell in which it does not naturally occur using the techniques of genetic engineering.
- operably linked means two or more DNA molecules or two or more proteins linked in manner so that one may affect the function of the other.
- Operably linked DNA molecules or operably linked proteins may be part of a single contiguous molecule and may or may not be adjacent.
- a promoter is operably linked with a protein-coding DNA molecule in a DNA construct where the two DNA molecules are so arranged that the promoter may affect the expression of the transgene.
- the DNA constructs described herein may include a promoter operably linked to a protein-coding DNA molecule provided herein, whereby the promoter drives expression of the protein.
- Useful promoters include those that function in a cell for expression of an operably linked DNA molecule, such as a bacterial or plant promoter. Plant promoters are varied and well known in the art and include, for instance, those that are inducible, viral, synthetic, constitutive, temporally regulated, spatially regulated, or spatio-temporally regulated.
- a DNA construct provided herein includes a DNA sequence encoding a transit sequence that is operably linked to a heterologous DNA sequence encoding a PPO enzyme, whereby the transit sequence facilitates localizing the protein molecule within the cell.
- Transit sequences are known in the art as signal sequences, targeting peptides, targeting sequences, localization sequences, and transit peptides.
- An example of a transit sequence is a chloroplast transit peptide (CTP), a mitochondrial transit sequence (MTS), or a dual chloroplast and mitochondrial transit peptide.
- the transit sequence may increase the accumulation of recombinant protein, protect the protein from proteolytic degradation, or enhance the level of herbicide tolerance, and thereby reduce levels of injury in the cell, seed, or organism after herbicide application.
- CTPs and other targeting molecules that may be used in connection with the present disclosure are well known in the art.
- transgene expression means the production of a protein through the process of transcribing a DNA molecule into messenger RNA (mRNA) and translating the mRNA into polypeptide chains, which are ultimately folded into proteins.
- mRNA messenger RNA
- a protein-coding DNA molecule may be operably linked to a heterologous promoter in a DNA construct for use in expressing the protein in a cell transformed with the recombinant DNA molecule.
- cells, tissues, plants, and seeds that comprising the recombinant DNA molecules or proteins are provided herein. These cells, tissues, plants, and seeds comprising the recombinant DNA molecules or proteins exhibit tolerance to one or more PPO inhibiting herbicide(s).
- PPO inhibiting herbicide(s) In the commercial production of crops, it is desirable to eliminate under reliable pesticidal management unwanted plants (z.e., "weeds") from a field of crop plants. An ideal treatment would be one which could be applied to an entire field but which would eliminate only the unwanted plants while leaving the crop plants unaffected.
- One such treatment system would involve the use of crop plants which are tolerant to an herbicide so that when the herbicide is sprayed on a field of herbicide-tolerant crop plants, the crop plants would continue to thrive while non-herbicide-tolerant weeds are killed or severely damaged.
- Such treatment systems would take advantage of varying herbicide properties so that weed control could provide the best possible combination of flexibility and economy. For example, individual herbicides have different longevities in the field, and some herbicides persist and are effective for a relatively long time after they are applied to a field while other herbicides are quickly broken down into other and/or nonactive compounds.
- An ideal treatment system would allow the use of different herbicides so that growers could tailor the choice of herbicides for a particular situation.
- One method of producing such cells, tissues, plants, and seeds is through plant transformation.
- Suitable methods for transformation of host plant cells for use with the current disclosure include any method by which DNA can be introduced into a cell (for example, where a recombinant DNA construct is stably integrated into a plant chromosome) and are well known in the art.
- Two effective, and widely utilized, methods for cell transformation are Agrobacterium- mediated transformation and microprojectile bombardment-mediated transformation. Microprojectile bombardment methods are illustrated, for example, in US Patent Nos. 5,550,318; 5,538,880; 6,160,208; and 6,399,861.
- Agrobacterium-mediated transformation methods are described, for example in US Patent No. 5,591,616.
- a cell with a recombinant DNA molecule or protein of the present disclosure may be selected for the presence of the recombinant DNA molecule or protein, for instance through its encoded enzymatic activity, before or after regenerating such a cell into a plant.
- Another method of producing the cells, plants, and seeds of the present disclosure is through genome modification using site-specific integration or genome editing.
- Targeted modification of plant genomes through the use of genome editing methods can be used to create improved plant lines through modification of plant genomic DNA.
- site-directed integration refers to genome editing methods the enable targeted insertion of one or more nucleic acids of interest into a plant genome. Suitable methods for altering a wild-type DNA sequence or a preexisting transgenic sequence or for inserting DNA into a plant genome at a pre-determined chromosomal site include any method known in the art.
- Exemplary methods include the use of sequence specific nucleases, such as zinc-finger nucleases, engineered or native meganucleases, TALE-endonucleases, or an RNA-guided endonucleases (for example, a Clustered Regularly Interspersed Short Palindromic Repeat (CRISPR)/Cas9 system, a CRISPR/Cpfl system, a CRISPR/CasX system, a CRISPR/CasY system, a CRISPR/Cascade system).
- sequence specific nucleases such as zinc-finger nucleases, engineered or native meganucleases, TALE-endonucleases, or an RNA-guided endonucleases (for example, a Clustered Regularly Interspersed Short Palindromic Repeat (CRISPR)/Cas9 system, a CRISPR/Cpfl system, a CRISPR/CasX system, a CRISPR/CasY
- the present disclosure provides modification or replacement of an existing coding sequence, such as a PPO coding sequence or another existing transgenic insert, within a plant genome with a sequence encoding a protein, such as a PPO coding sequence of the present disclosure, or an expression cassette comprising such a protein.
- an existing coding sequence such as a PPO coding sequence or another existing transgenic insert
- RNA-guided endonucleases for example, a Clustered Regularly Interspersed Short Palindromic Repeat (CRISPR)/Cas9 system, a CRISPR/Cpfl system, a CRISPR/CasX system, a CRISPR/CasY system, a CRISPR/Cascade system.
- CRISPR Clustered Regularly Interspersed Short Palindromic Repeat
- RNA construct comprising an expression cassette(s) encoding a site-specific nuclease and, optionally, any associated protein(s) to carry out genome modification.
- These nuclease-expressing cassette(s) may be present in the same molecule or vector as a donor template for templated editing or an expression cassette comprising nucleic acid sequence encoding a PPO protein as described herein (in cis) or on a separate molecule or vector (in trans).
- DSB double strand break
- nick a desired genomic site or locus.
- the donor template DNA, transgene, or expression cassette may become integrated into the genome at the site of the DSB or nick.
- the presence of the homology arm(s) in the DNA to be integrated may promote the adoption and targeting of the insertion sequence into the plant genome during the repair process through homologous recombination, although an insertion event may occur through non-homologous end joining (NHEJ).
- NHEJ non-homologous end joining
- double-strand break inducing agent refers to any agent that can induce a double-strand break (DSB) in a DNA molecule.
- the doublestrand break inducing agent is a site-specific genome modification enzyme.
- site-specific genome modification enzyme refers to any enzyme that can modify a nucleotide sequence in a sequence-specific manner.
- a site-specific genome modification enzyme modifies the genome by inducing a single-strand break.
- a site-specific genome modification enzyme modifies the genome by inducing a double-strand break.
- a site-specific genome modification enzyme comprises a cytidine deaminase.
- a site-specific genome modification enzyme comprises an adenine deaminase.
- sitespecific genome modification enzymes include endonucleases, recombinases, transposases, deaminases, helicases and any combination thereof.
- the site-specific genome modification enzyme is a sequence-specific nuclease.
- the endonuclease is selected from a meganuclease, a zinc-finger nuclease (ZFN), a transcription activator-like effector nucleases (TALEN), an Argonaute (non-limiting examples of Argonaute proteins include Thermits thermophilus Argonaute (TtAgo), Pyrococcus furiosus Argonaute (PfAgo), Natronobacterium gregoryi Argonaute (NgAgo), an RNA-guided nuclease, such as a CRISPR associated nuclease (non-limiting examples of CRISPR associated nucleases include Casl, CaslB, Cas2, Cas3, Cas4, Cas5, Cas6, Cas7, Cas8, Cas9 (also known as Csnl and Csxl2), CaslO, Csyl, Csy2, Csy3, Csel, Cse2, Cscl,
- the site-specific genome modification enzyme is a recombinase.
- recombinases include a tyrosine recombinase attached to a DNA recognition motif and is selected from the group consisting of a Cre recombinase, a Gin recombinase, a Flp recombinase, and a Tnpl recombinase.
- a Cre recombinase or a Gin recombinase provided herein is tethered to a zinc-finger DNA-binding domain, or a TALE DNA-binding domain, or a Cas9 nuclease.
- a serine recombinase attached to a DNA recognition motif is selected from the group consisting of a PhiC31 integrase, an R4 integrase, and a TP-901 integrase.
- a DNA transposase attached to a DNA binding domain provided herein is selected from the group consisting of a TALE-piggyBac and TALE- Mutator.
- a “weed” is any undesired plant.
- a plant may be considered generally undesirable for agriculture or horticulture purposes (for example, Amaranthus species) or may be considered undesirable in a particular situation (for example, a crop plant of one species in a field of a different species, also known as a volunteer plant).
- transgenic plants, progeny, seeds, plant cells, and plant parts described herein may also contain one or more additional traits. Additional traits may be introduced by crossing a plant containing a transgene comprising the recombinant DNA molecules provided herein with another plant containing one or more additional trait(s). As used herein, “crossing” means breeding two individual plants to produce a progeny plant. Two plants may thus be crossed to produce progeny that contain the desirable traits from each parent. As used herein “progeny” means the offspring of any generation of a parent plant, and transgenic progeny comprise a DNA construct provided herein and inherited from at least one parent plant.
- Additional trait(s) also may be introduced by co-transforming a DNA construct for that additional transgenic trait(s) with a DNA construct comprising the recombinant DNA molecules provided herein (for example, with all the DNA constructs present as part of the same vector used for plant transformation) or by inserting the additional trait(s) into a transgenic plant comprising a DNA construct provided by the herein or vice versa (for example, by using any of the methods of plant transformation or genome editing on a transgenic plant or plant cell).
- additional traits include, but are not limited to, increased insect resistance, increased water use efficiency, increased yield performance, increased drought resistance, increased seed quality, improved nutritional quality, hybrid seed production, and herbicide-tolerance, in which the trait is measured with respect to a wild-type plant.
- Illustrative additional herbicide-tolerance traits may include transgenic or non-transgenic tolerance to one or more herbicides such as ACCase inhibitors (for example aryloxyphenoxy propionates and cyclohexanediones), ALS inhibitors (for example sulfonylureas, imidazolinones, triazolopyrimidines, and triazolinones) EPSPS inhibitors (for example glyphosate), synthetic auxins (for example phenoxys, benzoic acids, carboxylic acids, semicarbazones), photosynthesis inhibitors (for example triazines, triazinones, nitriles, benzothiadiazoles, and ureas), glutamine synthesis inhibitors (for example glufosinate), HPPD inhibitors (for example isoxazoles, pyrazolones, and triketones), PPO inhibitors (for example diphenylethers, N-phenylphthalimide, aryl
- herbicide-tolerance proteins useful for producing additional herbicidetolerance traits are well known in the art and include, but are not limited to, glyphosate-tolerant 5- enolypyruvyl shikimate 3-phosphate synthases (e.g., CP4 EPSPS, 2mEPSPS), glyphosate oxidoreductases (GOX), glyphosate N-acetyltransferases (GAT), herbicide-tolerant acetolactate synthases (ALS) / acetohydroxyacid synthases (AHAS), herbicide-tolerant 4- hydroxyphenylpyruvate dioxygenases (HPPD), dicamba monooxygenases (DMO), phosphinothricin acetyl transferases (PAT), herbicide-tolerant glutamine synthetases (GS), 2,4- dichlorophenoxyproprionate dioxygenases (TfdA), R-2,4-dichlorophenoxypropionate dioxygenases (R
- Exemplary insect resistance traits may include resistance to one or more insect members within one or more of the orders of Lepidoptera, Coleoptera, Hemiptera, Thysanoptera, Diptera, Hymenoptera, and Orthoptera, among others.
- additional traits are well known to one of skill in the art; for example, and a list of such transgenic traits is provided by the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS).
- Transgenic plants and progeny that are tolerant to PPO inhibiting herbicides may be used with any breeding methods that are known in the art.
- the traits may be independently segregating, linked, or a combination of both in plant lines comprising three or more transgenic traits.
- Backcrossing to a parental plant and out-crossing with a non-transgenic plant are also contemplated, as is vegetative propagation. Descriptions of breeding methods that are commonly used for different traits and crops are well known to those of skill in the art.
- Such assays include, for example, molecular biology assays, such as Southern and Northern blotting, PCR, and DNA sequencing; biochemical assays, such as detecting the presence of a protein product, for example, by immunological means (ELISAs and western blots) or by enzymatic function; plant part assays, such as leaf or root assays; and also, by analyzing the phenotype of the whole plant.
- molecular biology assays such as Southern and Northern blotting, PCR, and DNA sequencing
- biochemical assays such as detecting the presence of a protein product, for example, by immunological means (ELISAs and western blots) or by enzymatic function
- plant part assays such as leaf or root assays
- analyzing the phenotype of the whole plant include, for example, molecular biology assays, such as Southern and Northern blotting, PCR, and DNA sequencing; biochemical assays, such as detecting the presence of a protein product, for example,
- Introgression of a transgenic trait into a plant genotype is achieved as the result of the process of backcross conversion.
- a plant genotype into which a transgenic trait has been introgressed may be referred to as a backcross converted genotype, line, inbred, or hybrid.
- a plant genotype lacking the desired transgenic trait may be referred to as an unconverted genotype, line, inbred, or hybrid.
- Novel microbial HemG protoporphyrinogen oxidases that are tolerant to PPO inhibitor herbicides were previously identified from microbial sequence databases using bioinformatic methods and a herbicide bacterial screening system and are provided as SEQ ID NOs:l-20 and recombinant variants of these microbial HemG protoporphyrinogen oxidases are provided as SEQ ID NOs:65-193.
- DNA sequences encoding microbial HemG protoporphyrinogen oxidases and their variants, along with DNA sequences that are optimized for expression in a monocot or dicot can optionally be synthesized and are provided as SEQ ID NOs:22-64 and 194-322.
- a codon for a methionine commonly known as a start codon
- this codon (and optionally a few amino-terminal amino acids, for example 2 to 7), can be eliminated to facilitate operable linkage of a transit peptide sequence to the 5 ’ end of the coding sequence.
- Novel transit peptides were previously identified by using bioinformatic methods and tools, such as hidden Markov models (HMM), the Pfam database, and basic local alignment search tool (BLAST), to identify thousands of EST and genomic sequences predicted to encode proteins known to be localized to the chloroplast and mitochondria in plant cells and are provided herein as SEQ ID NOs:323-328, 340, and 342-407, along with their corresponding nucleotide sequences, provided herein as SEQ ID NOs:329-339, 341, and 408-483.
- HMM hidden Markov models
- BLAST basic local alignment search tool
- Protoporphyrinogen oxidases operably linked to transit peptides were tested in transgenic soybean, com, and cotton plants for tolerance to PPO inhibiting herbicides and for weed control in the field.
- Plant transformation vectors were constructed for expressing a chloroplast transit peptide operably linked to the PPO H_N90 (SEQ ID NO:1) in transgenic soybean, inbred com, hybrid com, and cotton plants, and introduced into seed-derived explants of soybean, inbred and hybrid com, and cotton, respectively, through Agrobacterium tumefaciens-ms ⁇ iatQ ⁇ transformation using standard methods known in the art.
- the regenerated Ro plants were analyzed to select for events with a single copy insertion for advancement to Ri nursery for Ri seed production.
- Each herbicide treatment was applied at a rate of 100 ai/ha or 200 g ai/ha.
- Methylated rapeseed oil (Mero) was used as an adjuvant and added to the spray mix for each herbicide at 0.5% v/v.
- Each treatment consisted of four replicates (pots).
- H_N90 conferred to transgenic soybean, com, and cotton plants 100% complete tolerance to the herbicidal compounds (a), (b), and (o), 28 days after application for both application rates.
- Protoporphyrinogen oxidases operably linked to transit peptides are tested in transgenic soybean, com, and cotton plants for tolerance to PPO inhibiting herbicides and for weed control in the field.
- Plant transformation vectors are constructed for expressing a chloroplast transit peptide operably linked to a HemG PPO enzyme, such as H_N90 (SEQ ID NO:1) in transgenic soybean, com, and cotton plants, and introduced into seed-derived explants of soybean, com, and cotton, respectively, through Agrobacterium tumefaciens-mediated transformation using standard methods known in the art.
- the regenerated Ro plants are analyzed to select for events with a single copy insertion for advancement to Ri nursery for Ri seed production. Homozygous Ri or later generation events are tested under field conditions to confirm their tolerance to PPO inhibitor herbicides.
- herbicidal compound(s) of the general formula (I) described herein and a commercial PPO inhibitor herbicide such as saflufenacil
- a commercial PPO inhibitor herbicide such as saflufenacil
- Plants are visually assessed for herbicide injury 14 days after treatment for VE, and 7 days after treatment for V3 and RI stages.
- Unsprayed transgenic plants are used for phenotypic comparison with unsprayed wild-type plants.
- herbicidal compound(s) of the general formula (I) described herein and a commercial PPO inhibitor herbicide such as fomesafen
- VE emergence
- V2 V6, and VT developmental stages at one of two rates. Plants are visually assessed for herbicide injury 10-14 days after herbicide treatment. Unsprayed transgenic plants are used for phenotypic comparison with unsprayed wild-type plants.
- the injury rating is determined as the percentage of leaf area of a plant exhibiting damage such as necrosis (brown or dead tissue), chlorosis (yellow tissue or yellow spotting), and malformation (misshapen leaves or plant structures, epinasty or twisting of stem, cupping of leaves) caused by herbicide application and is measured on a scale of 0-100, with zero being no injury and 100 being complete crop death.
- Transgenic crop seeds conferring PPO inhibiting herbicide tolerance prepared as described above are planted in a field or crop growing area.
- PPO inhibiting herbicide formulations such as the herbicidal compound(s) of the general formula (I) described herein, are applied to the field or crop growing area before or/and after planting the seeds to control weed growth.
- the herbicide application comprises an effective amount of at least one PPO inhibiting herbicide that prevents or controls the growth of weeds, but does not damage or injure the transgenic soybean, com, or cotton crop plants comprising a recombinant DNA molecule encoding a chloroplast- targeted heterologous HemG protein.
- the PPO inhibiting herbicides can be applied, once or more than once, at about IX application rate.
- the rate may be adjusted or varied depending on environmental conditions (such as temperature and humidity) and the type of weeds being controlled, as is known in the art. Therefore, the application rate may vary within wide limits, and may consist of a range from about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, about 0.25 g a.i./ha to about 300 g a.i./ha, about 0.02 g a.i./ha to about 250 g a.i./ha, about 0.05 g a.i./ha to about 150 g a.i./ha, or about 0.25 g a.i./ha to about 120 g a.i./ha.
- the PPO inhibiting herbicides may be applied pre-emergence and/or post emergence. In the case of post emergence application, the PPO inhibiting herbicides may be applied over the top of the crop growing area.
- an effective amount of at least a second herbicide can be applied for weed control.
- a second herbicide include, but are not limited to, an ACCase inhibitor (such as an aryloxyphenoxy propionate or a cyclohexanedione), an ALS inhibitor (such as sulfonylurea, imidazolinone, triazoloyrimidine, or a triazolinone), an EPSPS inhibitor (such as glyphosate), a synthetic auxin (such as a phenoxy herbicide, a benzoic acid, a carboxylic acid, or a semicarbazone), a photosynthesis inhibitor (such as a triazine, a triazinone, a nitrile, a benzothiadiazole, or a urea), a glutamine synthetase inhibitor (such as glufosinate), a HPPD inhibitor (such as an iso
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Dentistry (AREA)
- Environmental Sciences (AREA)
- Cell Biology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
The present disclosure relates to the use of substituted phenyluracils or agrochemically acceptable salts thereof, for controlling or preventing weed growth in plant growth areas of transgenic crop plants that are tolerant to PPO inhibiting herbicides. The disclosure also provides herbicide tolerant transgenic plants, seeds, cells, and plant parts containing the recombinant DNA molecules, as well as methods of using the same.
Description
TITLE OF THE INVENTION METHODS AND COMPOSITIONS FOR PPO HERBICIDE TOLERANCE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the priority of U.S. Provisional Appl. Ser. No. 63/486,768, filed
February 24, 2023, which is incorporated herein by reference in its entirety.
FIELD OF THE INVENTION
[0002] The present disclosure relates to the fields of agriculture, plant biotechnology, and molecular biology. More specifically, the disclosure relates to the use of substituted phenyluracils, or agrochemically acceptable salts thereof, for controlling or preventing weed growth in plant growth areas of transgenic crop plants that are tolerant to protoporphyrinogen oxidase (PPO) inhibiting herbicides.
INCORPORATION OF SEQUENCE LISTING
[0003] A computer readable form of a sequence listing is filed with this application by electronic submission and is incorporated into this application by reference in its entirety. The sequence listing is contained in the file named “MONS553WO_ST26.xml”, which is 626 kilobytes in size (measured in operating system MS Windows) and was created on February 6, 2024.
BACKGROUND OF THE INVENTION
[0004] Chemical herbicides are often used to control the growth and spread of weeds or other plants that are unwanted in a particular environment. These chemicals are active at one or more target sites within a plant where they interrupt normal plant functions. Herbicides vary in their modes of action, in their effects on weeds and crop plants, and how they are used. While herbicides are very effective in controlling growth of undesirable vegetation, their use may also cause incidental damage to desired plants located in the same vicinity, such as crop plants. In order to minimize crop damage, extensive research has been directed toward the development of herbicide tolerant plants, especially through use of transgenic traits. Examples of transgenic herbicide tolerance traits include glyphosate tolerance, glufosinate tolerance, and dicamba tolerance. With the increase of weed species resistant to the commonly used herbicides, especially glyphosate,
growers have turned to use of herbicides having different modes of action and thus new herbicide tolerance traits are needed in the field. Herbicides of particular interest include herbicides that inhibit protoporphyrinogen oxidase (PPO, EC 1.3.3.4), referred to as PPO inhibitor herbicides. PPO inhibitor herbicides provide control of a spectrum of herbicide-resistant weeds, thus making a trait conferring tolerance to these herbicides particularly useful in a cropping system combined with one or more other herbicide-tolerance trait(s).
SUMMARY OF THE INVENTION
[0005] Provided herein is a method for controlling or preventing weed growth in a plant growth area, wherein the method comprises the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the general formula (I) or an agrochemically acceptable salt thereof
And in which: “A” represents nitrogen or CH; “R1” represents hydrogen or methyl; “R2” represents hydrogen or fluorine; “R3” represents hydrogen, halogen, or (C1-C8)-alkoxy; “R4”
represents halogen, cyano, NO2, C(O)NH2, C(S)NH2, (C1-C8)-haloalkyl, or (C2-C8)-alkynyl; “R5,” “R6,” and “R7,” independently of each other, represent hydrogen, halogen, cyano, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C1-C8)-alkoxy, or (C1-C8)-haloalkoxy; “G” represents a branched or unbranched alkylene group, optionally substituted with an (C1-C3)-alkoxy group; and “Q” represents hydroxy or a group Q-l, Q-2;
In which: “R8” represents hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, aryl, aryl-(C1- C8)-alkyl, heteroaryl, (C2-C8)-alkynyl, (C2-C8)-alkenyl, C(O)R13, C(O)OR13, or (C1-C8)-alkoxy- (C1-C8)-alkyl; “R9” represents hydrogen or (C1-C8)-alkyl; “R10” represents hydrogen, halogen, cyano, NO2, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C3-C8)-cycloalkyl, (C3-C8)-cycloalkyl-(C1-C8)- alkyl, (C3-C8)-halocycloalkyl, (C3-C8)-halocycloalkyl-(C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)- alkynyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl, heterocyclyl- (C1-C8)-alkyl, R11R12N-(C1-C8)-alkyl, R13O-(C1-C8)-alkyl, cyano-(C1-C8)-alkyl, (C1-C8)- alkylcarbonyloxy-(C1-C8)-alkyl, (C3-C8)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy- (C1-C8)-alkyl, heteroarylcarbonyloxy-(C1-C8)-alkyl, heterocyclylcarbonyloxy-(C1-C8)-alkyl, OR13, NR11R12, SR14, S(O)R14, SO2R14, R14S-(C1-C8)-alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(CI- C8)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl](aryl)silyl(C1-C8)-alkyl, [(C1- C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, tris- [(C1-C8)-alkyl] silyl, bis-hydroxyboryl-(C1-C8)-alkyl, bis-[(C1-C8)-alkoxy]boryl-(C1-C8)-alkyl, tetramethyl-l,3,2-dioxaborolan-2-yl, tetramethyl- 1,3,2- dioxaborolan-2-yl-(C1-C8)-alkyl, nitro-(C1-C8)-alkyl, C(O)OR13, C(O)R13, C(O)N R11R12, R13O(O)C-(C1-C8)-alkyl, R11R12N(O)C-(C1-C8)-alkyl, bis-(C1-C8)-alkoxy-(C1-C8)-alkyl, or “R8” and “R10” together, with the carbon atom to which they are attached, form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having a further substitution; “R11” and “R12” are either identical or different and, independently of one another, represent hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-
haloalkynyl, (C3-C10)-cycloalkyl, (C3-C10)-halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)- halocycloalkenyl, (C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)-haloalkoxy-(C1-C8)-alkyl, (C1-C8)- alkylthio-(C1-C8)-alkyl, (C1-C8)-haloalkylthio-(C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, COR13, SO2R14, heterocyclyl, (C1-C8)-alkoxycarbonyl, bis- [(C1-C8)-alkyl]aminocarbonyl-(C1-C8)-alkyl, (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl- (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxycarbonyl, heteroaryl-(C1-C8)- alkoxycarbonyl, (C2-C8)-alkenyloxycarbonyl, (C2-C8)-alkynyloxycarbonyl, or heterocyclyl-(C1- C8)-alkyl, or “R11” and “R12” together, with the nitrogen atom to which they are bonded, form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having a further substitution; “R13” represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)- haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3-C10)-halocycloalkyl, (C4-C10)- cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)-haloalkoxy-(C1- C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, (C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl, (C1- C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1- C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl, aryl, aryl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxy-(C1-C8)- alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)- cycloalkenyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl]aminocarbonyl-(C1-C8)-alkyl, (C1-C8)-alkyl- aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, bis-[(C1-C8)- alkyl]amino-(C2-C6)-alkyl, (C1-C8)-alkyl amino-(C2-C6)-alkyl, aryl-(C1-C8)-alkyl-amino-(C2-C6)- alkyl, R14S-(C1-C8)-alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(C1-C8)-alkyl, hydroxycarbonyl-(C1- C8)-alkyl, heterocyclyl, heterocyclyl-(C1-C8)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis- [(C1-C8)-alkyl](aryl)silyl(C1-C8)-alkyl, [(C1-C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, (C1-C8)- alkylcarbonyloxy-(C1-C8)-alkyl, (C3-C8)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy- (C1-C8)-alkyl, heteroarylcarbonyloxy-(C1-C8)-alkyl, heterocyclylcarbonyloxy-(C1-C8)-alkyl, aryloxy-(C1-C8)-alkyl, heteroaryloxy-(C1-C8)-alkyl, or (C1-C8)-alkoxycarbonyl; “R14” represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)- haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3-C10)- halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy-(C1-C8)-alkyl,
(C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl-(C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)- alkyl, bis-[(C1-C8)-alkyl]amino, (C1-C8)-alkyl-amino, aryl-(C1-C8)-amino, aryl-(C1-C6)-alkyl- amino, aryl-[(C1-C8)-alkyl]amino, (C3-C8)-cycloalkyl-amino, (C3-C8)-cycloalkyl-[(C1-C8)- alkyl]amino; N-azetidinyl, N-pyrrolidinyl, N-piperidinyl, or N-morpholinyl; “R15” and “R16” independently of each other represent (C1-C8)-alkyl, (C3-C8)-cycloalkyl, aryl, heteroaryl, or heterocyclyl or “R15” and “R16” together, with the carbon atom to which they are attached, form an unsubstituted (C3-C7)-cycloalkyl; “X” and “Y,” independently of one another, represent oxygen or sulfur; and “Z” represents nitrogen or CH; and (b) applying to said area an amount of said compound effective to control or prevent weed growth in the area.
[0006] Also provided herein is a method for controlling or preventing weed growth in a plant growth area, wherein the method comprises the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the of the general formula (I), or an agrochemically acceptable salt thereof, that is further characterized in that “W” represents a group W-l to W-2;
and wherein “A” represents nitrogen or CH; “R1” represents hydrogen; “R2” represents hydrogen or fluorine; “R3” represents fluorine; “R4” represents chlorine, bromine, or cyano; “R5” represents hydrogen; “R6” represents hydrogen or fluorine; “R7” represents hydrogen; “G” represents methylene, (methyl)methylene or (methoxy)methylene; “X” represents oxygen; “Y” represents oxygen; “Z” represents nitrogen or CH; and “Q” represents one of the following moieties Q-l to Q-500:
and (b) applying to said area an amount of said compound effective to control or prevent weed growth in the area.
[0007] Also provided herein is a method for controlling or preventing weed growth in a plant growth area, wherein the method comprises the steps of: (a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said
protein confers tolerance in said plant to an herbicidally active compound of the of the general formula (I), or an agrochemically acceptable salt thereof, wherein said compound corresponds to:
(a) ethyl [(3- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(b) [(3- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l (2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(c) ethyl (2- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)acetate;
(d) (2- {2-chloro-5- [4-( 1 , 1 -difhioroethyl)-3 -methyl -2, 6-dioxo-3 ,6-dihydropyrimidin- 1 (2H)- yl] -4-fluorophenoxy } phenoxy)acetic acid;
(e) ethyl (2- {2-chloro-4-fluoro-5- [4-( 1 -fluoroethyl)-3 -methyl-2,6-dioxo-3 ,6- dihydropyrimidin-l(2H)-yl]phenoxy}phenoxy)acetate;
(f) 2-methoxyethyl [(3-{2-chloro-5-[4-(l,l-difhioroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(g) tetrahydrofuran-2-ylmethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(h) cyanomethyl [(3-{2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(i) methyl (2- {2-chloro-5- [4-( 1 , 1 -difluoroethyl)-3 -methyl-2,6-dioxo-3 ,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(j) methyl (2-{2-bromo-5-[4-(l,l-difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(k) [(3-{2-bromo-5-[4-(l,l-difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l(2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(l) ethyl [(3- {2-bromo-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fhiorophenoxy}pyridin-2-yl)oxy]acetate;
(m) 2-methoxyethyl [(3 - {2-bromo-5 -[4-( 1 , 1 -difluoroethyl)-3 -methyl -2, 6-dioxo-3 ,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy } pyridin-2-yl)oxy] acetate;
(n) tetrahydrofuran-2-ylmethyl [(3-{2-bromo-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate; or
(o) ethyl 2-[[3-[5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-pyrimidin-l -yl]-4-fluoro-2- nitro-phenoxy]- 2-pyridyl]oxy]acetate; and (b) applying to said area an amount of said compound effective to control or prevent weed growth in the area.
[0008] In some embodiments of the methods provided herein, the heterologous HemG protein has herbicide-insensitive protoporphyrinogen oxidase activity. In some embodiments, the heterologous HemG protein has at least 85% sequence identity to a polypeptide sequence selected from the group consisting of SEQ ID NOs:l-20 and 65-193. In some embodiments, the DNA sequence encoding the heterologous HemG protein is selected from the group consisting of SEQ ID NOs:22-64 and 194-322. In further embodiments, the heterologous HemG protein comprises an amino acid sequence selected from the group consisting of SEQ ID NOs:l-20 and 65-193. In some embodiments, the DNA sequence encoding a heterologous HemG protein is operably linked to a DNA sequence encoding a chloroplast transit peptide (CTP). In some embodiments, the CTP comprises an amino acid sequence with at least 97% sequence identity to a sequence selected from the group consisting of SEQ ID NOs:323-328, 340, and 342-407. In further embodiments, the DNA sequence encoding the CTP comprises at least 97% identity to a sequence selected from the group consisting of SEQ ID NOs:329-339, 341, and 408-483. In additional embodiments, said recombinant DNA molecule further comprises a heterologous promoter operably linked to the DNA sequence encoding said HemG protein. In some embodiments, the plant comprising the recombinant DNA molecule is a monocotyledonous plant. In other embodiments, the plant comprising the recombinant DNA molecule is a dicotyledonous plant.
[0009] In some embodiments of the methods provided herein, the herbicidally active compound is applied to the area at a rate of about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, about 0.25 g a.i./ha to about 300 g a.i./ha, about 0.02 g a.i./ha to
about 250 g a.i./ha, about 0.05 g a.i./ha to about 150 g a.i./ha, or about 0.25 g a.i./ha to about 120 g a.i./ha.
[0010] In some embodiments, the method is further defined as comprising applying said compound to said area at least twice. In some embodiments, the herbicidally active compound is applied in an amount that does not damage said plant comprising the recombinant DNA molecule. In some embodiments, said applying of the compound is carried out pre-emergence. In some embodiments, said applying of the compound is carried out post-emergence. In some embodiments, said applying of the compound comprises contacting said plant with the compound. In further embodiments, said applying of the compound comprises an over the top application of said compound. In additional embodiments, said applying of the compound results in an increase in the growth or yield of said plant relative to a plant of the same genotype cultivated in a growth area in which said compound has not been applied.
[0011] In some embodiments of the methods provided herein, the method further comprises applying to said area an effective amount of at least a second herbicide. In further embodiments, the second herbicide is selected from the group consisting of: an ACCase inhibitor, an ALS inhibitor, an EPSPS inhibitor, a synthetic auxin, a photosynthesis inhibitor, a glutamine synthesis inhibitor, a HPPD inhibitor, a PPO inhibitor, and a long-chain fatty acid inhibitor. In even further embodiments, the ACCase inhibitor is an aryloxyphenoxy propionate or a cyclohexanedione; the ALS inhibitor is a sulfonylurea, imidazolinone, triazoloyrimidine, or a triazolinone; the EPSPS inhibitor is glyphosate; the synthetic auxin is a phenoxy herbicide, a benzoic acid, a carboxylic acid, or a semicarbazone; the photosynthesis inhibitor is a triazine, a triazinone, a nitrile, a benzothiadiazole, or a urea; the glutamine synthesis inhibitor is glufosinate; the HPPD inhibitor is an isoxazole, a pyrazolone, or a triketone; the PPO inhibitor is a diphenylether, a N- phenylphthalimide, an aryl triazinone, or a pyrimidinedione; or the long-chain fatty acid inhibitor is a chloroacetamide, an oxyacetamide, or a pyrazole.
BRIEF DESCRIPTION OF THE SEQUENCES
[0012] SEQ ID NO: 1 is the amino acid sequence of the HemG PPO H_N90.
[0013] SEQ ID NO:2 is the amino acid sequence of the HemG PPO H_N20.
[0014] SEQ ID NO:3 is the amino acid sequence of the HemG PPO H_N60.
[0015] SEQ ID NO:4 is the amino acid sequence of H_N10, which is the E. coli wild-type HemG protoporphyrinogen oxidase (NCBI GenBank Accession No. WP 021498199).
[0016] SEQ ID NO:5 is the amino acid sequence of the HemG PPG H N30.
[0017] SEQ ID NO:6 is the amino acid sequence of the HemG PPO H_N40.
[0018] SEQ ID NO:7 is the amino acid sequence of the HemG PPO H_N50.
[0019] SEQ ID NO:8 is the amino acid sequence of the HemG PPO H_N70.
[0020] SEQ ID NO:9 is the amino acid sequence of the HemG PPO H N100.
[0021] SEQ ID NO: 10 is the amino acid sequence of the HemG PPO H_N 110.
[0022] SEQ ID NOs: 11-17 are amino acid sequences lacking the start methionine and corresponding to SEQ ID NOs:l, 2, 4, 5, 6, 7, and 9, respectively.
[0023] SEQ ID NOs: 18-19 are amino acid sequences of two variants of SEQ ID NO:11.
[0024] SEQ ID NO:20 is the amino acid sequence of a variant of SEQ ID NO: 17.
[0025] SEQ ID NO:21 is the amino acid sequence of the wild-type PPO from Amaranthus tuberculatus (waterhemp).
[0026] SEQ ID NOs:22-31 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for E. coli expression.
[0027] SEQ ID NOs:32-41 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for dicot expression.
[0028] SEQ ID NOs:42-48 are nucleotide sequences encoding SEQ ID NOs: 11-17, respectively, codon optimized for dicot expression.
[0029] SEQ ID NOs:49-50 are recombinant nucleotide sequences encoding SEQ ID NO: 11.
[0030] SEQ ID NO:51 is a recombinant nucleotide sequence encoding SEQ ID NO: 12.
[0031] SEQ ID NOs:52-54 are recombinant nucleotide sequences encoding SEQ ID NOs: 18-
20.
[0032] SEQ ID NOs:55-64 are nucleotide sequences encoding SEQ ID NOs:l-10, respectively, codon optimized for monocot expression.
[0033] SEQ ID NOs: 65-77 are amino acid sequences of HemG PPOs from different species with variations in the long chain insert loop.
[0034] SEQ ID NOs:78-193 are amino acid sequences of recombinant HemG PPO variants, each incorporating a mutation to the long chain insert loop.
[0035] SEQ ID NOs: 194-206 are nucleotide sequences encoding the HemG PPOs of SEQ ID NOs: 65-77.
[0036] SEQ ID NOs: 207-322 are nucleotide sequences encoding the recombinant HemG PPO variants of SEQ ID NOs:78-193.
[0037] SEQ ID NO:323 is the amino acid sequence of the Arabidopsis thaliana albino and pale green (APG6) chloroplast transit peptide (CTP).
[0038] SEQ ID NO:324 is the amino acid sequence of an amino-terminal optimized variant of the APG6 CTP.
[0039] SEQ ID NO:325 is the amino acid sequence of the Arabidopsis thaliana 90 kDa heat shock protein (CR88) CTP.
[0040] SEQ ID NO:326 is the amino acid sequence of the petunia ShkG-EPSPS CTP.
[0041] SEQ ID NO:327 is the amino acid sequence of the pea rbcS-3C CTP.
[0042] SEQ ID NO:328 is the amino acid sequence of the rice Waxy CTP.
[0043] SEQ ID NOs:329-333 are the nucleotide sequences encoding APG6 CTP of SEQ ID
NO:323, optimized for plant expression.
[0044] SEQ ID NO:334 is the nucleotide sequence encoding APG6 CTP of SEQ ID NO:324. [0045] SEQ ID NOs:335-336 are nucleotide sequences encoding AtCR88 CTP optimized for dicot and monocot expression, respectively.
[0046] SEQ ID NOs:337-339 are nucleotide sequences encoding SEQ ID NOs:326-328.
[0047] SEQ ID NO:340 is the amino acid sequence of the cotton 12G088600TP CTP.
[0048] SEQ ID NO:341 is the nucleotide sequence encoding the cotton 12G088600TP CTP, optimized for dicot expression.
[0049] SEQ ID NOs:342-407 are amino acid sequences of transit peptides from different species.
[0050] SEQ ID NOs:408-483 are nucleotide sequences encoding SEQ ID NOs:342-407.
DETAILED DESCRIPTION
[0051] The following descriptions and definitions are provided to better define the invention and to guide those of ordinary skill in the art in the practice of the invention. Unless otherwise noted, terms are to be understood according to conventional usage by those of ordinary skill in the relevant art.
[0052] The present disclosure provides for the use of substituted phenyluracils, or agrochemically acceptable salts thereof, in controlling or preventing weed growth in plant growth areas of transgenic crop plants that express herbicide insensitive PPOs (protoporphyrinogen oxidases), and that are therefore tolerant to PPO inhibiting herbicides. PPO is an essential enzyme in plants that catalyzes the dehydrogenation of protoporphyrinogen IX to form protoporphyrin IX, which is the precursor to heme and chlorophyll. PPO inhibition in plant cells causes accumulation of intermediate tetrapyrroles and the formation of reactive oxygen species, resulting in membrane disruption and ultimately cell death. There are several herbicide families that are classified as PPO inhibitors, such as diphenyl ethers, aryl triazolinones, pyrimidinediones, and N- phenylphthalimides.
[0053] C6rtain compounds of the structure class of substituted phenyluracils have been identified as having herbicidal activity and can be used for controlling monocotyledonous and dicotyledonous weeds. These compounds are effective against a broad spectrum of harmful plants when applied both preemergence and postemergence, with the possibility of non-selective use for control of unwanted plant growth or selective use in plant crops. The present application shows that these specific substituted phenyluracils or salts thereof can be applied to transgenic crop plants that comprise one or more genes conferring tolerance to PPO inhibitor herbicides. The herbicide tolerance trait described herein provides tolerance to one of more of the herbicidally active compound of the general formula (I) or agrochemically acceptable salts thereof.
I. Herbicides and Herbicidally Active Compounds
[0054] Provided herein are uses for specific substituted phenyluracils of the general formula (I):
in which
w represents a group W-l to W-2
A represents nitrogen or CH;
R1 represents hydrogen or methyl;
R2 represents hydrogen or fluorine;
R3 represents hydrogen, halogen, or (C1-C8)-alkoxy;
R4 represents halogen, cyano, NO2, C(O)NH2, C(S)NH2, (C1-C8)-haloalkyl, or (C1-C8)- alkynyl;
R5, R6, and R7 independently of one another represent hydrogen, halogen, cyano, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C1-C8)-alkoxy, or (C1-C8)-haloalkoxy;
G represents a branched or unbranched alkylene group optionally substituted with an (Ci -C3)- alkoxy group;
R8 represents hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, (C2-C8)-alkynyl, (C2-C8)-alkenyl, C(O)R13, C(O)OR13, or (C1-C8)-alkoxy-(C1-C8)-alkyl;
R9 represents hydrogen or (C1-C8)-alkyl;
R10 represents hydrogen, halogen, cyano, NO2, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (Cs-Cg)- cycloalkyl, (C3-Cg)-cycloalkyl-(C1-C8)-alkyl, (C3-Cg)-halocycloalkyl, (Cs-Cg)- halocycloalkyl-(C1-C8)-alkyl, (C2-Cg)-alkenyl, (C2-Cg)-alkynyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl, heterocyclyl-(C1-C8)-alkyl, R11R12N- (C1-C8)-alkyl, R13O-(C1-C8)-alkyl, cyano-(C1-C8)-alkyl, (C1-C8)-alkylcarbonyloxy-(C1- Cg)-alkyl, (C3-Cg)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy-(C1-C8)-alkyl, heteroarylcarbonyloxy-(Ci -Cg)-alkyl, heterocyclylcarbonyloxy-(Ci -Cg)-alkyl, OR13,
NR11R12, SR14, S(O)R14, SO2R14, R14S-(C1-C8)-alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(CI- Cg)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl](aryl)silyl(C1-C8)- alkyl, [(C1-C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, tris-[(C1-C8)-alkyl]silyl, bis- hydroxyboryl-(C1-C8)-alkyl, bis-[(C1-C8)-alkoxy]boryl-(C1-C8)-alkyl, tetramethyl- 1,3, 2- dioxaborolan-2-yl, tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl-(Ci -Cg)-alkyl, nitro-(Ci -Cg)- alkyl, C(O)OR13, C(O)R13, C(O)NR11R12, R13O(O)C-(C1-C8)-alkyl, R11R12N(O)C-(C1- Cg)-alkyl, orbis-(C1-C8)-alkoxy-(C1-C8)-alkyl, or
R8 and R10 together with the carbon atom to which they are attached form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having further substitution;
R11 and R12 are identical or different and independently of one another represent hydrogen, (C1- Cg)-alkyl, (C2-Cg)-alkenyl, (C2-Cg)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2- Cg)-haloalkenyl, (C3-Cg)-haloalkynyl, (C3-C10)-cycloalkyl, (C3-C10)-halocycloalkyl, (C4- C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)- haloalkoxy-(C1-C8)-alkyl, (C1-C8)-alkylthio-(C1-C8)-alkyl, (C1-C8)-haloalkylthio-(C1-C8)- alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl- (C1-C8)-alkyl, (C3-Cg)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, COR13, SO2R14, heterocyclyl, (C1-C8)-alkoxycarbonyl, bis-[(C1-C8)-alkyl]aminocarbonyl- (C1-C8)-alkyl, (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkyl- aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxycarbonyl, heteroaryl-(C1-C8)- alkoxycarbonyl, (C2-Cg)-alkenyloxycarbonyl, (C2-Cg)-alkynyloxycarbonyl, or heterocyclyl-(C1-C8)-alkyl, or
R11 and R12 together with the nitrogen atom to which they are attached form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having further substitution;
R13 represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3- C10)-halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-haloalkoxy-(C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, (C1- C8)-alkoxy-(Ci -C8)-alkoxy-(Ci -C8)-alkyl, (Ci -C8)-alkoxy-(Ci -C8)-alkoxy-(Ci -C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)- alkyl, aryl, aryl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxy-(C1-C8)-alkyl, heteroaryl, heteroaryl- (C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, bis- [(C1-C8)-alkyl]aminocarbonyl-(C1-C8)-alkyl, (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl]amino-(C2-C6)-alkyl, (C1-C8)-alkyl amino-(C2-C6)-alkyl, aryl-(C1-C8)-alkyl-amino-(C2-C6)-alkyl, R14S-(CI-CS)- alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(C1-C8)-alkyl, hydroxycarbonyl-(C1-C8)-alkyl, heterocyclyl, heterocyclyl-(C1-C8)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis-[(C1- C8)-alkyl](aryl)silyl(C1-C8)-alkyl, [(C1-C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, (C1-C8)- alkylcarbonyloxy-(C1-C8)-alkyl, (C3-C8)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy-(C1-C8)-alkyl, heteroarylcarbonyloxy-(C1-C8)-alkyl, heterocyclylcarbonyloxy-(C1-C8)-alkyl, aryloxy-(C1-C8)-alkyl, heteroaryloxy-(Ci -C8)- alkyl, or (C1-C8)-alkoxycarbonyl;
R14 represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3- C10)-halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl-(Ci -C8)-alkyl, (C3 -C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl]amino, (C1-C8)-alkyl-amino, aryl- (C1-C8)-amino, aryl-(C1-C6)-alkyl-amino, aryl-[(C1-C8)-alkyl]amino, (C3-C8)-cycloalkyl- amino, (C3-C8)-cycloalkyl-[(C1-C8)-alkyl]amino; N-azetidinyl, N-pyrrolidinyl, N- piperidinyl, or N-morpholinyl;
R15 and R16 independently of each other represent (C1-C8)-alkyl, (C3-Cg)-cycloalkyl, aryl, heteroaryl, or heterocyclyl or
R15 and R16 together, with the carbon atom to which they are attached, form an unsubstituted (C3- C?)-cycloalkyl; and
X and Y, independently of one another, represent oxygen or sulfur; and
Z represents nitrogen or CH.
[0055] In certain embodiments, the compound of the general formula (I) or an agrochemically acceptable salt thereof is further characterized in that
A represents nitrogen or CH;
R1 represents hydrogen;
R2 represents hydrogen or fluorine;
R3 represents fluorine;
R4 represents chlorine, bromine, or cyano;
R5 represents hydrogen;
R6 represents hydrogen or fluorine;
R7 represents hydrogen;,
G represents methylene, (methyl)methylene, or (methoxy)methylene;
X represents oxygen;
Y represents oxygen;
or agrochemically acceptable salts thereof for controlling or preventing weed growth in plant growth areas of transgenic crop plants that are tolerant to PPO inhibiting herbicides wherein the plants comprise a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein that has herbicide-insensitive PPO activity.
[0056] In preferred embodiments, the compound of the general formula (I) or an agrochemically acceptable salt thereof, corresponds to one of the compounds denoted (a)-(n) below, with their corresponding IUPAC names:
(a) ethyl [(3- {2-chloro-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(b) [(3- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l (2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(c) ethyl (2- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)acetate;
(d) (2- {2-chloro-5- [4-( 1 , 1 -difluoroethyl)-3 -methyl -2, 6-dioxo-3 ,6-dihydropyrimidin- 1 (2H)- yl] -4-fluorophenoxy } phenoxy)acetic acid;
(e) ethyl (2- {2-chloro-4-fluoro-5- [4-( 1 -fluoroethyl)-3 -methyl-2,6-dioxo-3 ,6- dihydropyrimidin-l(2H)-yl]phenoxy}phenoxy)acetate;
(f) 2-methoxyethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(g) tetrahydrofuran-2-ylmethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(h) cyanomethyl [(3-{2-chloro-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(i) methyl (2- {2-chloro-5- [4-(l , 1 -difluoroethyl)-3 -methyl-2,6-dioxo-3 ,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(j) methyl (2-{2-bromo-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(k) [(3- {2-bromo-5-[4-(l, 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l (2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(l) ethyl [(3- {2-bromo-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(m) 2-methoxyethyl [(3 - {2-bromo-5 -[4-( 1 , 1 -difluoroethyl)-3 -methyl -2, 6-dioxo-3 ,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(n) tetrahydrofura -2 -ylrn ethyl [(3-{2-bromo-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate; or
(o) ethyl 2-[ [3 - [5 -[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-pyrimidin-l -yl]-4-fluoro-2- nitro-phenoxy]- 2-pyridyl]oxy]acetate.
[0057] The definitions of radicals listed above in general terms or within areas of preference apply to the end products of the general formula (I) and as well as to the starting materials or intermediates required for preparation in each case. These radical definitions can be combined with one another as desired, i.e. including combinations between the given preferred ranges. Primarily for reasons of higher herbicidal activity, better selectivity and/or better producibility, compounds of the general formula (I) described herein or their salts and their use according to the present disclosure are of particular interest in which individual radicals have one of the preferred meanings already specified or specified below, or in particular those in which one or more of the preferred meanings already specified or specified below occur in combination. With regard to the compounds according to the present disclosure, the terms used above and further below will be elucidated. These are familiar to the person skilled in the art and especially have the definitions elucidated hereinafter.
[0058] Unless defined differently, names of chemical groups are generally to be understood such that attachment to the skeleton or the remainder of the molecule is via the structural element mentioned last, i.e. for example in the case of (C2-Cg)-alkenyloxy via the oxygen atom and in the case of (C1-C8)-alkoxy-(C1-C4)-alkyl or (C1-C8)-alkoxycarbonyl-(C1-C8)-alkyl, in each case via the carbon atom of the alkyl group. In the case of specified groups, such as OR13, NR11R12, S(O)mR14, C(=O)R13, C(=O)OR13, or C(=O)NR11R12 the attachment to the skeleton is via the structural element mentioned first, i.e. for example in the case of OR13 via the oxygen atom and in the case of C(=O)OR13 via the carbonyl atom.
[0059] As used herein and when described alone or as part of a chemical group, "alkylsulfonyl" refers to straight-chain or branched alkylsulfonyl, preferably having 1 to 6 or 1 to 8 carbon atoms, of such alkylsulfonyls include, but are but not limited to, (C1-C6)-alkylsulfonyl such as methylsulfonyl, ethylsulfonyl, propylsulfonyl, 1 -methylethylsulfonyl, butylsulfonyl, 1- methylpropylsulfonyl, 2-methylpropylsulfonyl, 1,1 -dimethylethylsulfonyl, pentylsulfonyl, 1- methylbutylsulfonyl, 2-methylbutylsulfonyl, 3 -methylbutylsulfonyl, 1,1 -dimethylpropylsulfonyl,
1,2-dimethylpropylsulfonyl, 2,2-dimethylpropylsulfonyl, 1 -ethylpropylsulfonyl, hexylsulfonyl, 1- methylpentylsulfonyl, 2-methylpentylsulfonyl, 3 -methylpentylsulfonyl, 4-methylpentylsulfonyl, 1,1 -dimethylbutylsulfonyl, 1,2-dimethylbutylsulfonyl, 1,3 -dimethylbutylsulfonyl, 2,2- dimethylbutylsulfonyl, 2,3 -dimethylbutylsulfonyl, 3, 3 -dimethylbutylsulfonyl, 1- ethylbutylsulfonyl, 2-ethylbutylsulfonyl, 1,1,2-trimethylpropylsulfonyl, 1,2,2- trimethylpropylsulfonyl, 1 -ethyl- 1 -methylpropylsulfonyl and l-ethyl-2-methylpropylsulfonyl. [0060] As used herein and when described alone or as part of a chemical group, "alkylthio refers to a straight-chain or branched S-alkyl, preferably having 1 to 6 or 1 to 8 carbon atoms, such as (C1-C10)-, (C1-C6)- or (C1-C4)-alkylthio. Examples of such alkylthios include, but are but not limited to, (C1-C6)-alkylthio such as methylthio, ethylthio, propylthio, 1 -methylethylthio, butylthio, 1 -methylpropylthio, 2-methylpropylthio, 1,1 -dimethylethylthio, pentylthio, 1- methylbutylthio, 2-methylbutylthio, 3 -methylbutylthio, 1,1 -dimethylpropylthio, 1,2- dimethylpropylthio, 2,2-dimethylpropylthio, 1 -ethylpropylthio, hexylthio, 1 -methylpentylthio, 2- methylpentylthio, 3 -methylpentylthio, 4-methylpentylthio, 1,1 -dimethylbutylthio, 1,2- dimethylbutylthio, 1,3 -dimethylbutylthio, 2,2-dimethylbutylthio, 2,3-dimethylbutylthio, 3,3- dimethylbutylthio, 1 -ethylbutylthio, 2-ethylbutylthio, 1,1,2-trimethylpropylthio, 1,2,2- trimethylpropylthio, 1 -ethyl- 1 -methylpropylthio, and 1 -ethyl-2-methylpropylthio.
[0061] As used herein and unless defined differently elsewhere, “alkylsulfinyl (alkyl-S(=O)),” refers to alkyl radicals that are attached to the skeleton via -S(=O)-, such as (C1-C10)-, (C1-C6)-, or (C1-C4)-alkylsulfinyl. Examples of such alkylsulfinyls include, but are but not limited to, (C1-C8)- alkylsulfinyl such as methylsulfinyl, ethylsulfinyl, propylsulfinyl, 1-methylethylsulfinyl, butylsulfinyl, 1 -methylpropylsulfinyl, 2-methylpropylsulfinyl, 1,1 -dimethylethylsulfinyl, pentylsulfinyl, 1 -methylbutylsulfinyl, 2-methylbutylsulfinyl, 3 -methylbutylsulfinyl, 1,1- dimethylpropylsulfinyl, 1,2-dimethylpropylsulfinyl, 2,2-dimethylpropylsulfinyl, 1- ethylpropylsulfinyl, hexylsulfinyl, 1 -methylpentylsulfinyl, 2-methylpentylsulfinyl, 3- methylpentylsulfinyl, 4-methylpentylsulfinyl, 1,1 -dimethylbutylsulfinyl, 1,2- dimethylbutylsulfinyl, 1,3 -dimethylbutylsulfinyl, 2,2-dimethylbutylsulfinyl, 2,3- dimethylbutylsulfinyl, 3,3-dimethylbutylsulfinyl, 1 -ethylbutylsulfinyl, 2-ethylbutylsulfinyl, 1,1,2- trimethylpropylsulfinyl, 1,2,2-trimethylpropylsulfinyl, 1 -ethyl- 1 -methylpropylsulfinyl, and 1- ethyl-2 -methylpropylsulfinyl.
[0062] As used herein, “alkoxy” refers to an alkyl radical bonded via an oxygen atom. Examples of such alkoxys include, but are but not limited to, (C1-C6)-alkoxy such as methoxy, ethoxy, propoxy, 1 -methylethoxy, butoxy, 1 -methylpropoxy, 2-methylpropoxy, 1,1- dimethylethoxy, pentoxy, 1 -methylbutoxy, 2-methylbutoxy, 3 -methylbutoxy, 1,1- dimethylpropoxy, 1,2-dimethylpropoxy, 2,2-dimethylpropoxy, 1 -ethylpropoxy, hexoxy, 1- methylpentoxy, 2-methylpentoxy, 3 -methylpentoxy, 4-methylpentoxy, 1,1 -dimethylbutoxy, 1,2- dimethylbutoxy, 1,3 -dimethylbutoxy, 2,2-dimethylbutoxy, 2,3 -dimethylbutoxy, 3,3- dimethylbutoxy, 1 -ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy, 1 -ethyl- 1 -methylpropoxy, and l-ethyl-2-methylpropoxy.
[0063] As used herein, “haloalkoxy” is, for example, OCF3, OCHF2, OCH2F, OCF2CF3, OCH2CF3, and OCH2CH2C1; this applies correspondingly to haloalkenyloxy, haloalkynyloxy, and other halogen-substituted radicals. The term “C1-C8-haloalkoxy” as mentioned herein by way of example thus refers to a C1-C6-alkoxy group, as defined above, in which one or more hydrogen atoms are replaced with halogen atoms that may be the same or different. Examples of C1-C6- haloalkoxy groups include, but are not limited to, chloromethoxy, bromomethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 1 -chloroethoxy, 1- bromoethoxy, 1 -fluoroethoxy, 2-fluoroethoxy, 2,2-difluoroethoxy, 2,2,2-trifluoroethoxy, 2- chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy, 2,2-dichloro-2-fluoroethoxy, 2,2,2- trichloroethoxy, pentafluoroethoxy, and l,l,l-trifluoroprop-2-oxy.
[0064] Likewise, “alkenyloxy” refers to an alkenyl radical attached via an oxygen atom, and alkynyloxy refers to an alkynyl radical attached via an oxygen atom, such as (C2-C10)-, (C2-C6)-, or (C2-C4)-alkenyloxy, and (C3-C10)-, (C3-C6)-, or (C3-C4)-alkynyloxy.
[0065] The term “C2-C6-alkenyloxy” mentioned here by way of example refers to a formula (C2-C6-alkenyl)-O-, in which the term "C2-C6-alkenyl” group is which the as defined herein. Examples of C2-C6-alkenyloxy include but are not limited to ethenyloxy (or "vinyloxy"), prop-2 - en-l-yloxy (or "allyloxy"), prop-l-en-l-yloxy, prop-l-en-2-yloxy (or "isopropenyloxy"), but-3- enyloxy, but-2-enyloxy, but-l-enyloxy, 2-methylprop-2-enyloxy, l-methylprop-2-enyloxy, 2- methylprop-l-enyloxy, and 1-methylprop-l-enyloxy.
[0066] As used herein, “cycloalkoxy” refers to a cycloalkyl radical attached via an oxygen atom and cycloalkenyloxy refers to a cycloalkenyl radical attached via an oxygen atom.
[0067] As used herein, “alkylcarbonyl” (alkyl-C(=O)-), unless defined differently elsewhere, represents alkyl radicals attached to the skeleton via -C(=O)-, such as (C1-C10)-, (C1-C8)-, or (C1- C4)-alkylcarbonyl. Here, the number of the carbon atoms refers to the alkyl radical in the alkylcarbonyl group.
[0068] Analogously, unless defined differently elsewhere, “alkenylcarbonyl” and “alkynylcarbonyl” represent alkenyl and alkynyl radicals, respectively, attached to the skeleton via -C(=O)-, such as (C2-C10)-, (C2-C6)-> or (C2-C4)-alkenylcarbonyl and (C2-C10)-, (C2-C6)-, or (C2- C4)-alkynylcarbonyl. Here, the number of the carbon atoms refers to the alkenyl or alkynyl radical in the alkenyl or alkynyl group.
[0069] “Alkoxycarbonyl (alkyl-O-C(=O)-),” unless defined differently elsewhere refers to alkyl radicals attached to the skeleton via -O-C(=O)-, such as (C1-C10)-, (C1-C8)-, or (C1-C4)- alkoxycarbonyl. Here, the number of the carbon atoms refers to the alkyl radical in the alkoxycarbonyl group.
[0070] Analogously, “alkenyloxycarbonyl” and “alkynyloxycarbonyl,” unless defined differently elsewhere, respectively represent alkenyl and alkynyl radicals attached to the skeleton via -O-C(=O)-, such as (C2-C10)-, (C2-C6)-, or (C2-C4)-alkenyloxycarbonyl and (C3-C10)-, (C3-C6)- , or (C3-C4)-alkynyloxycarbonyl. Here, the number of the carbon atoms refers to the alkenyl or alkynyl radical in the alkenyloxycarbonyl or alkynyloxycarbonyl group.
[0071] The term "aryl" denotes an optionally substituted mono-, bi-, or polycyclic aromatic system having preferably 6 to 14, especially 6 to 10, ring carbon atoms, for example phenyl, naphthyl, anthryl, phenanthrenyl and the like, preferably phenyl.
[0072] The term "optionally substituted aryl" also includes polycyclic systems, such as tetrahydronaphthyl, indenyl, indanyl, fluorenyl, biphenylyl, where the bonding site is on the aromatic system. In systematic terms, "aryl" is generally also encompassed by the term "optionally substituted phenyl." Preferred aryl substituents here are, for example, hydrogen, halogen, alkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, halocycloalkyl, alkenyl, alkynyl, aryl, arylalkyl, arylalkenyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, alkoxyalkyl, alkylthio, haloalkylthio, haloalkyl, alkoxy, haloalkoxy, cycloalkoxy, cycloalkylalkoxy, aryloxy,
heteroraryloxy, alkoxyalkoxy, alkynylalkoxy, alkenyloxy, dialkylamino-alkoxy, tris-[alkyl]silyl, di-[alkyl]arylsilyl, di-[alkyl]alkylsilyl, tris-[alkyl]silylalkynyl, arylalkynyl, heteroarylalkynyl, alkylalkynyl, cycloalkylalkynyl, haloalkylalkynyl, heterocyclyl-N-alkoxy, nitro, cyano, amino, alkylamino, dialkylamino, alkylcarbonylamino, cycloalkylcarbonylamino, arylcarbonylamino, alkoxycarbonylamino, alkoxycarbonylalkylamino, arylalkoxycarbonylalkylamino, hydroxycarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl, cycloalkylaminocarbonyl, di-alkylaminocarbonyl, heteroarylalkoxy, and arylalkoxy.
[0073] A heterocyclic radical (heterocyclyl) contains at least one heterocyclic ring (=carbocyclic ring in which at least one carbon atom has been replaced by a heteroatom, preferably by a heteroatom from the group of N, O, S, and P) which is saturated, unsaturated, partially saturated, or heteroaromatic and may be unsubstituted or substituted, in which case the bonding site is localized on a ring atom. If the heterocyclyl radical or the heterocyclic ring is optionally substituted, it may be fused to other carbocyclic or heterocyclic rings. In the case of optionally substituted heterocyclyl, polycyclic systems are also included, for example 8- azabicyclo[3.2.1]octanyl, 8-azabicyclo[2.2.2]octanyl or l-azabicyclo[2.2.1]heptyl. Optionally substituted heterocyclyl also includes spirocyclic systems, such as, for example, l-oxa-5-aza- spiro[2.3]hexyl. Unless defined otherwise, the heterocyclic ring preferably contains 3 to 9 ring atoms, in particular 3 to 6 ring atoms, and one or more, preferably 1 to 4, in particular 1, 2 or 3 heteroatoms in the heterocyclic ring, preferably from the group N, O, and S, where, however, two oxygen atoms must not be directly adjacent to one another, for example having one heteroatom from the group consisting of N, O, and S 1- or 2- or 3-pyrrolidinyl, 3,4-dihydro-2H-pyrrol-2- or - 3-yl, 2,3 -dihydro- IH-pyrrol-l- or -2- or -3- or -4- or -5-yl; 2,5-dihydro-lH-pyrrol-l- or -2- or -3- yl, 1 - or 2- or 3- or 4-piperidinyl; 2,3,4,5-tetrahydropyridin-2- or -3- or -4- or -5-yl or -6-yl; 1 ,2,3,6- tetrahydropyridin-1- or -2- or -3- or -4- or -5- or -6-yl; 1,2,3,4-tetrahydropyridin-l- or -2- or -3- or -4- or -5- or -6-yl; 1,4-dihydropyridin-l- or -2- or -3- or -4-yl; 2,3-dihydropyridin-2- or -3- or -4- or -5- or -6-yl; 2,5-dihydropyridin-2- or -3- or -4- or -5- or -6-yl, 1- or 2- or 3- or 4-azepanyl; 2,3,4,5-tetrahydro-lH-azepin-l- or -2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,4,7-tetrahydro-lH- azepin-1- or -2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,6,7-tetrahydro-lH-azepin-l- or -2- or -3- or -4-yl; 3,4,5,6-tetrahydro-2H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4,5-dihydro-lH-azepin- 1- or -2- or -3- or -4-yl; 2,5-dihydro-lH-azepin-l- or -2- or -3- or -4- or -5- or -6- or -7-yl; 2,7-
dihydro- IH-azepin-l- or -2- or -3- or -4-yl; 2,3-dihydro-lH-azepin-l- or -2- or -3- or -4- or -5- or -6- or -7-yl; 3,4-dihydro-2H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; 3,6-dihydro-2H-azepin- 2- or -3- or -4- or -5- or -6- or -7-yl; 5,6-dihydro-2H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4,5-dihydro-3H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; IH-azepin-l- or -2- or -3- or -4- or - 5- or -6- or -7-yl; 2H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; 3H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4H-azepin-2- or -3- or -4- or -5- or -6- or -7-yl, 2- or 3-oxolanyl (= 2- or 3- tetrahydrofuranyl); 2,3-dihydrofuran-2- or -3- or -4- or -5-yl; 2,5-dihydrofuran-2- or -3-yl, 2- or 3- or 4-oxanyl (= 2- or 3- or 4-tetrahydropyranyl); 3,4-dihydro-2H-pyran-2- or -3- or -4- or -5- or -6- yl; 3,6-dihydro-2H-pyran-2- or -3-or -4- or -5- or -6-yl; 2H-pyran-2- or -3- or -4- or -5- or -6-yl; 4H-pyran-2- or -3- or -4-yl, 2- or -3- or -4-oxepanyl; 2,3,4,5-tetrahydrooxepin-2- or -3- or -4- or - 5- or -6- or -7-yl; 2,3,4,7-tetrahydrooxepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2, 3,6,7- tetrahydrooxepin-2- or -3- or -4-yl; 2,3-dihydrooxepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4,5- dihydrooxepin-2- or -3- or -4-yl; 2,5-dihydrooxepin-2- or -3- or -4- or -5- or -6- or -7-yl; oxepin-
2- or -3- or -4- or -5- or -6- or -7-yl; 2- or 3 -tetrahydrothiophenyl; 2,3-dihydrothiophen-2- or -3- or -4- or -5-yl; 2,5-dihydrothiophen-2- or -3-yl; tetrahydro-2H-thiopyran-2- or -3- or -4-yl; 3,4- dihydro-2H-thiopyran-2- or -3- or -4- or -5- or -6-yl; 3,6-dihydro-2H-thiopyran-2- or -3- or -4- or -5- or -6-yl; 2H-thiopyran-2- or -3- or -4- or -5- or -6-yl; 4H-thiopyran-2- or -3- or -4-yl. Preferred
3 -membered and 4-membered heterocycles are, for example, 1- or 2-aziridinyl, oxiranyl, thiiranyl, 1- or 2- or 3-azetidinyl, 2- or 3-oxetanyl, 2- or 3-thietanyl, l,3-dioxetan-2-yl. Further examples of "heterocyclyl" are a partially or fully hydrogenated heterocyclic radical having two heteroatoms from the group of N, O and S, for example 1- or 2- or 3- or 4-pyrazolidinyl; 4,5-dihydro-3H- pyrazol-3- or -4- or -5-yl; 4,5-dihydro-lH-pyrazol-l- or -3- or -4- or -5-yl; 2,3 -dihydro- 1H- pyrazol-1- or -2- or -3- or -4- or -5-yl; 1- or -2- or -3- or -4-imidazolidinyl; 2,3 -dihydro- 1H- imidazol-1- or -2- or -3- or -4-yl; 2,5-dihydro-lH-imidazol-l- or -2- or -4- or -5-yl; 4,5-dihydro- IH-imidazol-l- or -2- or -4- or -5-yl; hexahydropyridazin-1- or -2- or -3- or -4-yl; 1,2,3 ,4- tetrahydropyridazin-1- or -2- or -3- or -4- or -5- or -6-yl; 1,2,3,6-tetrahydropyridazin-l- or -2- or - 3- or -4- or -5- or -6-yl; 1,4,5,6-tetrahydropyridazin-l- or -3- or -4- or -5- or -6-yl; 3, 4,5,6- tetrahydropyridazin-3- or -4- or -5-yl; 4,5-dihydropyridazin-3- or -4-yl; 3,4-dihydropyridazin-3- or -4- or -5- or -6-yl; 3,6-dihydropyridazin-3- or -4-yl; 1,6-dihydropyridazin-l- or -3- or -4- or -5- or -6-yl; hexahydropyrimidin-1- or -2- or -3- or -4-yl; 1,4,5,6-tetrahydropyrimidin-l- or -2- or -4-
or -5- or -6-yl; 1,2,5,6-tetrahydropyrimidin-l- or -2- or -4- or -5- or -6-yl; 1 ,2,3,4- tetrahydropyrimidin-1- or -2- or -3- or -4- or -5- or -6-yl; 1,6-dihydropyrimidin-l- or -2- or -4- or -5- or -6-yl; 1,2-dihydropyrimidin-l- or -2- or -4- or -5- or -6-yl; 2,5-dihydropyrimidin-2- or -4- or -5-yl; 4,5-dihydropyrimidin- 4- or -5- or -6-yl; 1,4-dihydropyrimidin-l- or -2- or -4- or -5- or - 6-yl; 1- or -2- or -3-piperazinyl; 1,2,3,6-tetrahydropyrazin-l- or -2- or -3- or -5- or -6-yl; 1 ,2,3,4- tetrahydropyrazin-1- or -2- or -3- or -4- or -5- or -6-yl; 1,2-dihydropyrazin-l- or -2- or -3- or -5- or -6-yl; 1,4-dihydropyrazin-l- or -2- or -3-yl; 2,3-dihydropyrazin-2- or -3- or -5- or -6-yl; 2,5- dihydropyrazin-2- or -3-yl; l,3-dioxolan-2- or -4- or -5-yl; l,3-dioxol-2- or -4-yl; l,3-dioxan-2- or -4- or -5-yl; 4H- 1,3 -dioxin-2- or -4- or -5- or -6-yl; l,4-dioxan-2- or -3- or -5- or -6-yl; 2,3-dihydro- l,4-dioxin-2- or -3- or -5- or -6-yl; l,4-dioxin-2- or -3-yl; l,2-dithiolan-3- or -4-yl; 3H-l,2-dithiol-
3- or -4- or -5-yl; l,3-dithiolan-2- or -4-yl; l,3-dithiol-2- or -4-yl; l,2-dithian-3- or -4-yl; 3,4- dihydro-l,2-dithiin-3- or -4- or -5- or -6-yl; 3,6-dihydro-l,2-dithiin-3- or -4-yl; l,2-dithiin-3- or -
4-yl; l,3-dithian-2- or -4- or -5-yl; 4H-l,3-dithiin-2- or -4- or -5- or -6-yl; isoxazolidin-2- or -3- or -4- or -5-yl; 2,3-dihydroisoxazol-2- or -3- or -4- or -5-yl; 2,5-dihydroisoxazol-2- or -3- or -4- or -
5-yl; 4,5-dihydroisoxazol-3- or -4- or -5-yl; l,3-oxazolidin-2- or -3- or -4- or -5-yl; 2,3-dihydro- l,3-oxazol-2- or -3- or -4- or -5-yl; 2,5-dihydro-l,3-oxazol-2- or -4- or -5-yl; 4,5-dihydro-l,3- oxazol-2- or -4- or -5-yl; l,2-oxazinan-2- or -3- or -4- or -5- or -6-yl; 3,4-dihydro-2H-l,2-oxazin- 2- or -3- or -4- or -5- or -6-yl; 3,6-dihydro-2H-l,2-oxazin-2- or -3- or -4- or -5- or -6-yl; 5,6- dihydro-2H-l,2-oxazin-2- or -3- or -4- or -5- or -6-yl; 5,6-dihydro-4H-l,2-oxazin-3- or -4- or -5- or -6-yl; 2H- 1,2 -oxazin-2- or -3- or -4- or -5- or -6-yl; 6H-l,2-oxazin-3- or -4- or -5- or -6-yl; 4H-
1.2-oxazin-3- or -4- or -5- or -6-yl; l,3-oxazinan-2- or -3- or -4- or -5- or -6-yl; 3,4-dihydro-2H-
1.3-oxazin-2- or -3- or -4- or -5- or -6-yl; 3,6-dihydro-2H-l,3-oxazin-2- or -3- or -4- or -5- or -6- yl; 5,6-dihydro-2H-l,3-oxazin-2- or -4- or -5- or -6-yl; 5,6-dihydro-4H-l,3-oxazin-2- or -4- or -5- or -6-yl; 2H- 1,3 -oxazin-2- or -4- or -5- or -6-yl; 6H-l,3-oxazin-2- or -4- or -5- or -6-yl; 4H-1,3- oxazin-2- or -4- or -5- or -6-yl; morpholin-2- or -3- or -4-yl; 3,4-dihydro-2H-l,4-oxazin-2- or -3- or -4- or -5- or -6-yl; 3,6-dihydro-2H-l,4-oxazin-2- or -3- or -5- or -6-yl; 2H-l,4-oxazin-2- or -3- or -5- or -6-yl; 4H-l,4-oxazin-2- or -3-yl; l,2-oxazepan-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,4,5-tetrahydro-l,2-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,4,7-tetrahydro-l,2- oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,6,7-tetrahydro-l,2-oxazepin-2- or -3- or -4- or - 5- or -6- or -7-yl; 2,5,6,7-tetrahydro-l,2-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4, 5, 6, 7-
tetrahydro-1, 2-oxazepin-3- or -4- or -5- or -6- or -7-yl; 2,3-dihydro-l,2-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,5-dihydro-l,2-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,7-dihydro-
1.2-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4,5-dihydro-l,2-oxazepin-3- or -4- or -5- or -6- or -7-yl; 4,7-dihydro-l,2-oxazepin-3- or -4- or -5- or -6- or -7-yl; 6,7-dihydro-l,2-oxazepin-3- or -4- or -5- or -6- or -7-yl; l,2-oxazepin-3- or -4- or -5- or -6- or -7-yl; l,3-oxazepan-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,4,5-tetrahydro-l,3-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2, 3,4,7- tetrahydro-l,3-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,6,7-tetrahydro-l,3-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,5,6,7-tetrahydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; 4,5,6,7-tetrahydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; 2,3-dihydro-l,3-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,5-dihydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; 2,7-dihydro-
1.3-oxazepin-2- or -4- or -5- or -6- or -7-yl; 4,5-dihydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7- yl; 4,7-dihydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; 6,7-dihydro-l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; l,3-oxazepin-2- or -4- or -5- or -6- or -7-yl; l,4-oxazepan-2- or -3- or -5- or - 6- or -7-yl; 2,3,4,5-tetrahydro-l,4-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,4,7-tetrahydro-
1.4-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3,6,7-tetrahydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; 2,5,6,7-tetrahydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; 4,5,6,7-tetrahydro-
1.4-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 2,3-dihydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; 2,5-dihydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; 2,7-dihydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; 4,5-dihydro-l,4-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 4,7- dihydro-l,4-oxazepin-2- or -3- or -4- or -5- or -6- or -7-yl; 6,7-dihydro-l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; l,4-oxazepin-2- or -3- or -5- or -6- or -7-yl; isothiazolidin-2- or -3- or -4- or -5- yl; 2,3-dihydroisothiazol-2- or -3- or -4- or -5-yl; 2,5-dihydroisothiazol-2- or -3- or -4- or -5-yl;
4.5-dihydroisothiazol-3- or -4- or -5-yl; l,3-thiazolidin-2- or -3- or -4- or -5-yl; 2,3-dihydro-l,3- thiazol-2- or -3- or -4- or -5-yl; 2,5-dihydro-l,3-thiazol-2- or -4- or -5-yl; 4,5-dihydro-l,3-thiazol-
2- or -4- or -5-yl; l,3-thiazinan-2- or -3- or -4- or -5- or -6-yl; 3,4-dihydro-2H-l,3-thiazin-2- or -
3- or -4- or -5- or -6-yl; 3,6-dihydro-2H-l,3-thiazin-2- or -3- or -4- or -5- or -6-yl; 5,6-dihydro- 2H-l,3-thiazin-2- or -4- or -5- or -6-yl; 5,6-dihydro-4H-l,3-thiazin-2- or -4- or -5- or -6-yl; 2H- l,3-thiazin-2- or -4- or -5- or -6-yl; 6H-l,3-thiazin-2- or -4- or -5- or -6-yl; 4H-l,3-thiazin-2- or -
4- or -5- or -6-yl. Further examples of "heterocyclyl" are a partially or fully hydrogenated heterocyclic radical having 3 heteroatoms from the group of N, O and S, for example 1,4,2-
dioxazolidin-2- or -3- or -5-yl; l,4,2-dioxazol-3- or -5-yl; l,4,2-dioxazinan-2- or -3- or -5- or -6- yl; 5,6-dihydro-l,4,2-dioxazin-3- or -5- or -6-yl; l,4,2-dioxazin-3- or -5- or -6-yl; 1,4,2- dioxazepan-2- or -3- or -5- or -6- or -7-yl; 6,7-dihydro-5H-l,4,2-dioxazepin-3- or -5- or -6- or -7- yl; 2,3-dihydro-7H-l,4,2-dioxazepin-2- or -3- or -5- or -6- or -7-yl; 2,3-dihydro-5H-l,4,2- dioxazepin-2- or -3- or -5- or -6- or -7-yl; 5H-l,4,2-dioxazepin-3- or -5- or -6- or -7-yl; 7H-1,4,2- dioxazepin-3- or -5- or -6- or -7-yl. Structural examples of heterocycles that are optionally substituted further are also listed below:
[0074] The heterocycles listed above are preferably substituted by, for example, hydrogen, halogen, alkyl, haloalkyl, hydroxyl, alkoxy, cycloalkoxy, aryloxy, alkoxyalkyl, alkoxyalkoxy, cycloalkyl, halocycloalkyl, aryl, arylalkyl, heteroaryl, heterocyclyl, alkenyl, alkylcarbonyl, cycloalkylcarbonyl, arylcarbonyl, heteroarylcarbonyl, alkoxycarbonyl, hydroxycarbonyl, cycloalkoxycarbonyl, cycloalkylalkoxycarbonyl, alkoxycarbonylalkyl, arylalkoxycarbonyl, arylalkoxycarbonylalkyl, alkynyl, alkynylalkyl, alkylalkynyl, trisalkylsilylalkynyl, nitro, amino, cyano, haloalkoxy, haloalkylthio, alkylthio, hydrothio, hydroxyalkyl, oxo, heteroarylalkoxy, arylalkoxy, heterocyclylalkoxy, heterocyclylalkylthio, heterocyclyloxy, heterocyclylthio, heteroaryloxy, dialkylamino, alkylamino, cycloalkylamino, hydroxycarbonylalkylamino, alkoxycarbonylalkylamino, arylalkoxycarbonylalkylamino, alkoxycarbonylalkyl(alkyl)amino, aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, cycloalkylaminocarbonyl,
hydroxycarbonylalkylaminocarbonyl, alkoxycarbonylalkylaminocarbonyl, and arylalkoxycarbonylalkylaminocarbonyl.
[0075] When a base structure is substituted "by one or more substituents" from a list of radicals (= group) or a generically defined group of radicals, this in each case includes simultaneous substitution by a plurality of identical and/or structurally different radicals. In the case of a partially or fully saturated nitrogen heterocycle, this may be joined to the remainder of the molecule either via carbon or via the nitrogen. Suitable substituents for a substituted heterocyclic radical are the substituents specified further down, and additionally also oxo and thioxo. The oxo group as a substituent on a ring carbon atom is then, for example, a carbonyl group in the heterocyclic ring. As a result, lactones and lactams are preferably also included. The oxo group may also occur on the ring heteroatoms, which may exist in different oxidation states, for example in the case of N and S, and in that case form, for example, the divalent -N(O)-, -S(O)- (also SO for short) and - S(O)2- (also SO2 for short) groups in the heterocyclic ring. In the case of -N(O)- and -S(O)- groups, both enantiomers in each case are included.
[0076] As used herein, "heteroaryl" refers to heteroaromatic compounds, i.e. fully unsaturated aromatic heterocyclic compounds, preferably 5- to 7-membered rings having 1 to 4, preferably 1 or 2, identical or different heteroatoms, preferably O, S, or N. Inventive heteroaryls are, for example, IH-pyrrol-l-yl; lH-pyrrol-2-yl; lH-pyrrol-3-yl; furan-2-yl; furan-3-yl; thien-2-yl; thien- 3-yl, IH-imidazol-l-yl; lH-imidazol-2-yl; lH-imidazol-4-yl; lH-imidazol-5-yl; lH-pyrazol-l-yl; lH-pyrazol-3-yl; lH-pyrazol-4-yl; lH-pyrazol-5-yl, 1 H- 1,2, 3 -triazol- 1-yl, lH-l,2,3-triazol-4-yl, lH-l,2,3-triazol-5-yl, 2H-l,2,3-triazol-2-yl, 2H-l,2,3-triazol-4-yl, lH-l,2,4-triazol-l-yl, 1H-
1.2.4-triazol-3-yl, 4H-l,2,4-triazol-4-yl, l,2,4-oxadiazol-3-yl, l,2,4-oxadiazol-5-yl, 1,3,4- oxadiazol-2-yl, l,2,3-oxadiazol-4-yl, l,2,3-oxadiazol-5-yl, l,2,5-oxadiazol-3-yl, azepinyl, pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyrazin-2-yl, pyrazin-3-yl, pyrimidin-2-yl, pyrimidineyl, pyrimidin-5-yl, pyridazin-3-yl, pyridazin-4-yl, l,3,5-triazin-2-yl, l,2,4-triazin-3-yl, 1,2,4- triazin-5-yl, l,2,4-triazin-6-yl, l,2,3-triazin-4-yl, l,2,3-triazin-5-yl, 1,2,4-, 1,3,2-, 1,3,6- and 1,2,6- oxazinyl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl, l,3-oxazol-2-yl, l,3-oxazol-4-yl, 1,3-oxazol- 5-yl, isothiazol-3-yl, isothiazol-4-yl, isothiazol-5-yl, l,3-thiazol-2-yl, 1,3-thiazoM-yl, 1,3-thiazol- 5-yl, oxepinyl, thiepinyl, 1,2,4-triazolonyl and 1,2,4-diazepinyl, 2H-l,2,3,4-tetrazol-5-yl, 1H-
1.2.3.4-tetrazol-5-yl, l,2,3,4-oxatriazol-5-yl, l,2,3,4-thiatriazol-5-yl, l,2,3,5-oxatriazol-4-yl,
l,2,3,5-thiatriazol-4-yl. The heteroaryl groups described herein may also be substituted by one or more identical or different radicals. If two adjacent carbon atoms are part of a further aromatic ring, the systems are fused heteroaromatic systems, such as benzofiised or polyannealed heteroaromatics. Preferred examples are quinolines (e.g. quinolin-2-yl, quinolin-3-yl, quinolin-4- yl, quinolin-5-yl, quinolin-6-yl, quinolin-7-yl, quinolin-8-yl); isoquinolines e.g. isoquinolin- 1-yl, isoquinolin-3-yl, isoquinolin-4-yl, isoquinolin-5-yl, isoquinolin-6-yl, isoquinolin-7-yl, isoquinolin-8-yl); quinoxaline; quinazoline; cinnoline; 1,5-naphthyridine; 1,6-naphthyridine; 1,7- naphthyridine; 1,8-naphthyridine; 2,6-naphthyridine; 2,7-naphthyridine; phthalazine; pyridopyrazines; pyridopyrimidines; pyridopyridazines; pteridines; pyrimidopyrimidines. Examples of heteroaryl are also 5- or 6-membered benzofused rings from the group of IH-indol- 1-yl, lH-indol-2-yl, lH-indol-3-yl, lH-indol-4-yl, lH-indol-5-yl, lH-indol-6-yl, lH-indol-7-yl, 1- benzofuran-2-yl, l-benzofuran-3-yl, l-benzofuran-4-yl, l-benzofuran-5-yl, l-benzofuran-6-yl, 1- benzofuran-7-yl, l-benzothiophen-2-yl, l-benzothiophen-3-yl, l-benzothiophen-4-yl, 1- benzothiophen-5-yl, l-benzothiophen-6-yl, l-benzothiophen-7-yl, IH-indazol-l-yl, IH-indazol- 3-yl, lH-indazol-4-yl, lH-indazol-5-yl, lH-indazol-6-yl, lH-indazol-7-yl, 2H-indazol-2-yl, 2H- indazol-3-yl, 2H-indazol-4-yl, 2H-indazol-5-yl, 2H-indazol-6-yl, 2H-indazol-7-yl, 2H-isoindol-2- yl, 2H-isoindol-l-yl, 2H-isoindol-3-yl, 2H-isoindol-4-yl, 2H-isoindol-5-yl, 2H-isoindol-6-yl; 2H- isoindol-7-yl, 1 H-benzimidazol- 1 -yl, 1 H-benzimidazol-2-yl, 1 H-benzimidazol-4-yl, 1H- benzimidazol-5 -yl, 1 H-benzimidazol-6-yl, , lH-benzimidazol-7-yl, , 1 ,3 -benzoxazol-2-yl, 1,3- benzoxazol-4-yl, 1 ,3-benzoxazol-5-yl, 1 ,3-benzoxazol-6-yl, 1 ,3-benzoxazol-7-yl, 1,3- benzothiazol-2 -yl, 1 ,3 -benzothiazol-4-yl, 1 , 3 -benzothiazol-5 -yl, 1 ,3 -benzothiazol-6-yl, 1,3- benzothiazol-7 -yl, 1.2-benzisoxazol-3 -yl, 1 ,2-benzisoxazol-4-yl, 1.2-benzisoxazol-5-yl, 1,2- benzisoxazol-6-yl, 1.2-benzisoxazol-7-yl, 11 ,2-benzisothiazol-3 -yl, 1.2-benzisothiazol-4-yl, 1,2- benzisothiazol-5-yl, 1 ,2-benzisothiazol-6-yl, 1 ,2-benzisothiazol-7-yl.
[0077] The term "halogen" denotes, for example, fluorine, chlorine, bromine or iodine. If the term is used for a radical, "halogen" denotes, for example, a fluorine, chlorine, bromine or iodine atom.
[0078] As used herein, "alkyl" refers to a straight-chain or branched open-chain, saturated hydrocarbon radical that is optionally mono- or polysubstituted, and in the latter case is referred to as "substituted alkyl." Preferred substituents are halogen atoms, alkoxy, haloalkoxy, cyano,
alkylthio, haloalkylthio, amino, or nitro groups, particular preference being given to methoxy, methyl, fluoroalkyl, cyano, nitro, fluorine, chlorine, bromine, or iodine.
[0079] The prefix “di” includes the combination of equal or different alkyl radicals, e.g. dimethyl or methyl(ethyl) or ethyl(methyl).
[0080] As used herein, "haloalkyl," "-alkenyl," and "-alkynyl" denote an alkyl, alkenyl, and alkynyl, respectively, that is partially or fiilly substituted by identical or different halogen atoms, for example monohaloalkyl, such as CH2CH2C1, CH2CH2Br, CHC1CH3, CH2C1, and CH2F; perhaloalkyl or perfluoroalkyl, such as CC13, CC1F2, CFC12,CF2CC1F2, and CF2CC1FCF3; polyhaloalkyl, such as CH2CHFC1, CF2CC1FH, CF2CBrFH, and CH2CF3.
[0081] The term “C2-C6-haloalkenyl” as mentioned herein by way of example refers to a C2- C6-alkenyl group as defined above in which one or more hydrogen atoms are replaced with one or more halogen atoms that may be the same or different. Typically, C2-C6-haloalkenyl comprises up to 9 halogen atoms that can be the same or different.
[0082] The term “C2-C6-haloalkynyl” as mentioned herein by way of example refers to a C2- C6-alkynyl group as defined above in which one or more hydrogen atoms are replaced with one or more halogen atoms that may be the same or different. Typically, C2-C6-haloalkynyl comprises up to 9 halogen atoms that can be the same or different.
[0083] As used herein, "(C1-C4)-alkyl" mentioned here by way of example is a brief notation for straight-chain or branched alkyl having one to 4 carbon atoms according to the range stated for carbon atoms, i.e. encompasses the methyl, ethyl, 1 -propyl, 2-propyl, 1 -butyl, 2-butyl, 2- methylpropyl, or tert-butyl radicals. General alkyl radicals with a larger specified range of carbon atoms, e.g. "(C1-C6)-alkyl," also encompass straight-chain or branched alkyl radicals with a greater number of carbon atoms, i.e. alkyl radicals having 5 and 6 carbon atoms. Unless stated specifically, preference is given to the lower carbon skeletons, for example, having from 1 to 6 carbon atoms, or having from 2 to 6 carbon atoms in the case of unsaturated groups, in the case of the hydrocarbyl radicals such as alkyl, alkenyl and alkynyl radicals, including in composite radicals. Alkyl radicals, including in composite radicals such as alkoxy, haloalkyl, etc., are, for example, methyl, ethyl, n- propyl or i-propyl, n-, i-, t- or 2-butyl, pentyls; hexyls, such as n-hexyl, i-hexyl, and 1,3- dimethylbutyl; heptyls, such as n-heptyl, 1 -methylhexyl, and 1,4-dimethylpentyl.
[0084] Alkenyl and alkynyl radicals are defined as the possible unsaturated radicals corresponding to the alkyl radicals, where at least one double bond or triple bond is present. Preference is given to radicals having one double bond or triple bond.
[0085] As used herein, the term "alkenyl" also includes, in particular, straight-chain or branched open-chain hydrocarbon radicals having more than one double bond, such as 1,3- butadienyl and 1,4-pentadienyl, but also allenyl or cumulenyl radicals having one or more cumulated double bonds, for example allenyl (1,2-propadienyl), 1,2-butadienyl and 1,2,3- pentatrienyl. Alkenyl denotes, for example, vinyl which may optionally be substituted by further alkyl radicals, for example (but not limited thereto) (C2-C6)-alkenyl such as ethenyl, 1 -propenyl, 2-propenyl, 1 -methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1 -methyl- 1 -propenyl, 2-methyl-l- propenyl, l-methyl-2-propenyl, 2-methyl-2 -propenyl, 1 -pentenyl, 2-pentenyl, 3 -pentenyl, 4- pentenyl, 1 -methyl- 1-butenyl, 2-methyl- 1-butenyl, 3 -methyl- 1-butenyl, 1 -methyl-2-butenyl, 2- methyl-2-butenyl, 3-methyl-2-butenyl, l-methyl-3-butenyl, 2-methyl-3-butenyl, 3-methyl-3- butenyl, l,l-dimethyl-2-propenyl, 1,2-dimethyl-l -propenyl, l,2-dimethyl-2-propenyl, 1 -ethyl- 1- propenyl, l-ethyl-2-propenyl, 1 -hexenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-
1 -pentenyl, 2-methyl- 1 -pentenyl, 3 -methyl- 1 -pentenyl, 4-methyl-l -pentenyl, l-methyl-2- pentenyl, 2-methyl-2 -pentenyl, 3-methyl-2-pentenyl, 4-methyl-2-pentenyl, l-methyl-3 -pentenyl,
2-methyl-3 -pentenyl, 3 -methyl-3 -pentenyl, 4-methyl-3 -pentenyl, l-methyl-4-pentenyl, 2-methyl- 4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl, l,l-dimethyl-2-butenyl, l,l-dimethyl-3- butenyl, 1,2-dimethyl- 1-butenyl, 1,2-dimethyl -2-butenyl, l,2-dimethyl-3-butenyl, 1,3 -dimethyl- 1- butenyl, l,3-dimethyl-2-butenyl, l,3-dimethyl-3-butenyl, 2,2-dimethyl-3-butenyl, 2,3 -dimethyl- 1- butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl, 3, 3 -dimethyl- 1-butenyl, 3,3-dimethyl-2- butenyl, 1 -ethyl- 1-butenyl, l-ethyl-2-butenyl, l-ethyl-3-butenyl, 2 -ethyl- 1-butenyl, 2-ethyl-2- butenyl, 2-ethyl-3-butenyl, l,l,2-trimethyl-2 -propenyl, 1 -ethyl- l-methyl-2-propenyl, l-ethyl-2- methyl-1 -propenyl, and l-ethyl-2-methyl-2 -propenyl.
[0086] As used herein, the term "alkynyl" also includes, in particular, straight-chain or branched open-chain hydrocarbon radicals having more than one triple bond, or else having one or more triple bonds and one or more double bonds, for example 1 ,3-butatrienyl or 3-penten-l -yn- 1-yl. (C2-C6)-alkynyl denotes, for example, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl,
3-butynyl, l-methyl-2-propynyl, 1 -pentynyl, 2-pentynyl, 3 -pentynyl, 4-pentynyl, l-methyl-2-
butynyl, l-methyl-3-butynyl, 2 -methyl-3 -butynyl, 3 -methyl- 1-butynyl, l,l-dimethyl-2-propynyl, l-ethyl-2-propynyl, 1 -hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, l-methyl-2- pentynyl, l-methyl-3 -pentynyl, l-methyl-4-pentynyl, 2-methyl-3 -pentynyl, 2-methyl-4-pentynyl, 3 -methyl- 1 -pentynyl, 3-methyl-4-pentynyl, 4-methyl-l -pentynyl, 4-methyl-2 -pentynyl, 1,1- dimethyl-2-butynyl, l,l-dimethyl-3 -butynyl, l,2-dimethyl-3-butynyl, 2, 2-dimethyl-3 -butynyl, 3, 3 -dimethyl- 1-butynyl, l-ethyl-2-butynyl, l-ethyl-3-butynyl, 2-ethyl-3 -butynyl, and 1-ethyl-l- methyl-2-propynyl.
[0087] As used herein, the term "cycloalkyl" denotes a carbocyclic saturated ring system having preferably 3-8 ring carbon atoms, for example cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, which optionally has further substitution, preferably by hydrogen, alkyl, alkoxy, cyano, nitro, alkylthio, haloalkylthio, halogen, alkenyl, alkynyl, haloalkyl, amino, alkylamino, dialkylamino, alkoxycarbonyl, hydroxycarbonyl, arylalkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl, and cycloalkylaminocarbonyl. In the case of optionally substituted cycloalkyl, cyclic systems with substituents are included, also including substituents with a double bond on the cycloalkyl radical, for example an alkylidene group such as methylidene. In the case of optionally substituted cycloalkyl, polycyclic aliphatic systems are also included, for example bicyclo[l .1 ,0]butan-l -yl, bicyclo[ 1.1.0]butan-2-yl, bicyclo[2.1 ,0]pentan- 1 -yl, bicyclo[l .1.1 ]pentan- 1 -yl, bicyclo[2.1 ,0]pentan-2-yl, bicyclo[2.1 ,0]pentan-5-yl, bicyclo[2.1.1 ]hexyl, bicyclo[2.2.1 ]hept-2-yl, bicyclo[2.2.2]octan-2-yl, bicyclo[3.2.1 ]octan-2-yl, bicyclo[3.2.2]nonan-2-yl, adamantan-l-yl, and adamantan-2-yl, but also systems such as 1,1’- bi(cyclopropyl)-l-yl, l,l'-bi(cyclopropyl)-2-yl, for example. The term "(C3-C7)-cycloalkyl" is a brief notation for cycloalkyl having three to 7 carbon atoms, corresponding to the range specified for carbon atoms. In the case of substituted cycloalkyl, spirocyclic aliphatic systems are also included, for example spiro[2.2]pent-l-yl, spiro[2.3]hex-l-yl, spiro[2.3]hex-4-yl, 3-spiro[2.3]hex- 5-yl, spiro[3.3]hept-l-yl, and spiro[3.3]hept-2-yl.
[0088] "Cycloalkenyl" denotes a carbocyclic, nonaromatic, partially unsaturated ring system having preferably 4-8 carbon atoms, e.g. 1-cyclobutenyl, 2-cyclobutenyl, 1 -cyclopentenyl, 2- cyclopentenyl, 3 -cyclopentenyl, or 1 -cyclohexenyl, 2-cyclohexenyl, 3 -cyclohexenyl, 1,3- cyclohexadienyl or 1,4-cyclohexadienyl, also including substituents with a double bond on the cycloalkenyl radical, for example an alkylidene group such as methylidene. In the case of
optionally substituted cycloalkenyl, the elucidations for substituted cycloalkyl apply correspondingly.
[0089] As used herein, “haloalkylthio” - on its own or as constituent part of a chemical group - represents straight-chain or branched S-haloalkyl, preferably having 1 to 8, or having 1 to 6 carbon atoms, such as (C1-C8)-, (C1-C6)-, or (C1-C4)-haloalkylthio, for example (but not limited thereto) trifluoromethylthio, pentafluoroethylthio, difluoromethyl, 2,2-difluoroeth-l-ylthio, 2,2,2- difluoroeth-l-ylthio, and 3,3,3-prop-l-ylthio.
[0090] “Halocycloalkyl” and “halocycloalkenyl” denote cycloalkyl and cycloalkenyl, respectively, which are partially or fully substituted by identical or different halogen atoms, such as F, Cl and Br, or by haloalkyl, such as trifluoromethyl or difluoromethyl, for example 1- fluorocycloprop-l-yl, 2-fhiorocycloprop-l-yl, 2,2-difluorocycloprop-l-yl, 1-fluorocyclobut-l-yl, 1-trifluoromethylcycloprop-l-yl, 2-trifluoromethylcycloprop-l-yl, 1 -chlorocycloprop- 1-yl, 2- chlorocycloprop-l-yl, 2,2-dichlorocycloprop-l-yl, and 3, 3 -difluorocyclobutyl.
[0091] As used herein, “trialkylsilyl” used alone or as constituent part of a chemical group represents a straight-chain or branched Si-alkyl, preferably having 1 to 8, or having 1 to 6 carbon atoms, such as tri[(C1-C8)-, (C1-C6)- or (C1-C4)-alkyl]silyl, for example (but not limited thereto) trimethyl silyl, triethylsilyl, tri(n-propyl)silyl, tri(isopropyl)silyl, tri(n-butyl)silyl, tri(l- methylprop-l-yl)silyl, tri(2-methylprop-l-yl)silyl, tri(l,l-dimethyleth-l-yl)silyl, and tri(2,2- dimethyleth- 1 -yl)silyl.
[0092] As used herein, “oxo” refers to an oxygen atom that is bonded to a carbon atom or sulfur atom by a double bond.
[0093] As used herein, “methylidene” refers to a CH2 group connected to a carbon atom by a double bond.
[0094] As used herein, “halomethylidene” refers to a CX2 group connected to a carbon atom by a double bond, where X is halogen.
[0095] If the compounds can form, through a hydrogen shift, tautomers whose structure is not formally covered by the general formula (I), these tautomers are nevertheless covered by the definition of the inventive compounds of the general formula (I), unless a particular tautomer is under consideration. For example, many carbonyl compounds may be present both in the keto form
and in the enol form, both forms being encompassed by the definition of the compound of the general formula (I).
[0096] According to the nature of the substituents defined above, the compounds of the formula (I) have acidic properties and can form salts, and if appropriate also internal salts or adducts with inorganic or organic bases or with metal ions. If the compounds of the formula (I) bear hydroxyl, carboxyl or other groups which induce acidic properties, these compounds can be reacted with bases to give salts. Suitable bases are, for example, hydroxides, carbonates, hydrogen carbonates of the alkali metals and alkaline earth metals, especially those of sodium, potassium, magnesium, and calcium, and also ammonia, primary, secondary, and tertiary amines having (C1- C4)-alkyl groups, mono-, di-, and trialkanolamines of (C1-C4)-alkanols, choline and chlorocholine, and organic amines, such as trialkylamines, morpholine, piperidine, or pyridine. These salts are compounds in which the acidic hydrogen is replaced by an agriculturally suitable cation, for example metal salts, especially alkali metal salts or alkaline earth metal salts, especially sodium and potassium salts, or else ammonium salts, salts with organic amines or quaternary ammonium salts, for example with cations of the formula [NRR'R''R'”]+ in which R to R'" are each independently an organic radical, especially alkyl, aryl, aralkyl, or alkylaryl. Also suitable are alkyl sulfonium and alkyl sulfoxonium salts, such as (C1-C4)-trialkyl sulfonium and (C1-C4)- trialkyl sulfoxonium salts.
[0097] The compounds of the formula (I) can form salts by addition of a suitable inorganic or organic acid, for example mineral acids, for example HO, HBr, H2SO4, H3PO4 or HNO3, or organic acids, for example carboxylic acids such as formic acid, acetic acid, propionic acid, oxalic acid, lactic acid or salicylic acid or sulfonic acids, for example p-Toluenesulfonic acid, onto a basic group, for example amino, alkylamino, dialkylamino, piperidino, morpholino, or pyridino. In this case, these salts comprise the conjugate base of the acid as the anion.
[0098] Suitable substituents present in deprotonated form, for example sulfonic acids or carboxylic acids, may form internal salts with groups which for their part can be protonated, such as amino groups.
[0099] If a group is polysubstituted by radicals, this means that this group is substituted by one or more identical or different radicals from those mentioned.
[00100] The present disclosure also relates to all stereoisomers and mixtures thereof which are encompassed by the formula (I) but not defined specifically. For the sake of simplicity, however, reference will always be made hereinafter to compounds of the formula (I), even though this means not only the pure compounds but also, if appropriate, mixtures with different proportions of isomeric compounds.
[00101] Depending on the nature of the substituents and the manner in which they are attached, the compounds of the general formula (I) may be present as stereoisomers. The possible stereoisomers defined by the specific three-dimensional form thereof, such as enantiomers, diastereomers, Z and E isomers, are all encompassed by the general formula (I). If, for example, one or more alkenyl groups are present, diastereomers (Z and E isomers) may occur. If, for example, one or more asymmetric carbon atoms are present, enantiomers and diastereomers may occur. Stereoisomers can be obtained from the mixtures obtained in the preparation by customary separation methods. The chromatographic separation can be effected either on the analytical scale to find the enantiomeric excess or the diastereomeric excess, or else on the preparative scale to produce test specimens for biological testing. It is likewise possible to selectively prepare stereoisomers by using stereoselective reactions with use of optically active starting materials and/or auxiliaries. The present disclosure thus also relates to all stereoisomers which are embraced by the general formula (I) but are not shown in their specific stereomeric form, and to mixtures thereof.
[00102] As used herein, a “herbicide” is any molecule that is used to control, prevent, or interfere with the growth of one or more plants. Illustrative herbicides include acetyl-CoA carboxylase (ACCase) inhibitors (for example, aryloxyphenoxy propionates and cyclohexanediones); acetolactate synthase (ALS) inhibitors (for example, sulfonylureas, imidazolinones, triazolopyrimidines, and triazolinones); 5 -enolpyruvylshikimate-3 -phosphate synthase (EPSPS) inhibitors (for example, glyphosate), synthetic auxins (for example, phenoxys, benzoic acids, carboxylic acids, and semicarbazones), photosynthesis (photosystem II) inhibitors (for example, triazines, triazinones, nitriles, benzothiadiazoles, and ureas), glutamine synthetase (GS) inhibitors (for example, glufosinate and bialaphos), 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors (for example, isoxazoles, pyrazolones, and triketones), protoporphyrinogen oxidase (PPO) inhibitors (for example, diphenylethers, N-phenylphthalimide, aryl triazinones, and
pyrimidinediones), very long-chain fatty acid inhibitors (for example, chloroacetamides, oxyacetamides, and pyrazoles), cellulose biosynthesis inhibitors (for example, indaziflam), photosystem I inhibitors (for example, paraquat), microtubule assembly inhibitors (for example, pendimethalin), and phytoene desaturase (PDS) inhibitors (for example, norflurazone), among others.
[00103] Specific PPO inhibiting herbicides are known in the art and commercially available. Examples of PPO inhibiting herbicides include, but are not limited to, diphenylethers (such as acifluorfen, its salts and esters, bifenox, its salts and esters, ethoxyfen, its salts and esters, fluoronitrofen, furyloxyfen, halosafen, chlomethoxyfen, fluoroglycofen, its salts and esters, lactofen, its salts and esters, oxyfluorfen, and fomesafen, its salts and esters); thiadiazoles (such as fluthiacet-methyl and thidiazimin); pyrimidinediones or phenyluracils (such as benzfendizone, butafenacil, ethyl [3 -2-chloro-4-fluoro-5-( 1 -methyl-6-trifhioromethyl-2,4-dioxo- 1 ,2,3,4- tetrahydropyrimidin-3-yl)phenoxy]-2-pyridyloxy]acetate (CAS Registry Number 353292-31-6 and referred to herein as S-3100), flupropacil, saflufenacil, and tiafenacil); phenylpyrazoles (such as fluazolate, pyraflufen, and pyraflufen-ethyl); oxadiazoles (such as oxadiargyl and oxadiazon); triazolinones (such as azafenidin, bencarbazone, carfentrazone, its salts and esters, and sulfentrazone); oxazolidinediones (such as pentoxazone); N-phenylphthalimides (such as cinidon- ethyl, flumiclorac, flumiclorac-pentyl, and flumioxazin); benzoxazinone derivatives (such as 1,5- dimethyl-6-thioxo-3-(2,2,7-trifluoro-3,4-dihydro-3-oxo-4-prop-2-ynyl-2H-l,4-benzoxazin-6-yl)- 1, 3, 5-triazinane-2, 4-dione (trifludimoxazin)); flufenpyr and flufenpyr-ethyl; pyraclonil; and profluazol.
[00104] As used herein, “herbicide-tolerant” or “herbicide-tolerance” means the ability to be wholly or partially unaffected by the presence or application of one of more herbicide(s), for example to resist the toxic effects of an herbicide when applied. A cell or organism is “herbicide- tolerant” if it is able to maintain at least some normal growth or phenotype in the presence of one or more herbicide(s). A trait is an herbicide-tolerance trait if its presence can confer improved tolerance to an herbicide upon a cell, plant, or seed as compared to the wild-type or control cell, plant, or seed. Crops comprising a herbicide-tolerance trait can continue to grow and are minimally affected by the presence of the herbicide. A target enzyme is “herbicide-tolerant” if it exhibits improved enzyme activity relative to a wild-type or control enzyme in the presence of the
herbicide. Herbicide-tolerance may be complete or partial insensitivity to a particular herbicide, and may be expressed as a percent (%) tolerance or insensitivity to a particular herbicide.
[00105] Contemplated plants which might be produced with an herbicide tolerance trait of the present disclosure could include, for instance, any plant susceptible to a PPO inhibitor herbicide, including crop plants such as soybean (Glycine max), maize (Zea mays), cotton (Gossypium sp.), Brassica plants, alfalfa, barley, beans, beet, broccoli, cabbage, carrot, canola, cauliflower, celery, Chinese cabbage, cucumber, eggplant, leek, lettuce, melon, oat, onion, pea, pepper, peanut, potato, pumpkin, radish, rice, sweet com, sorghum, spinach, squash, sugar beet, sugar cane, sunflower, tomato, watermelon, and wheat, among others.
[00106] Herbicides may be applied to a plant growth area comprising the plants and seeds provided by the disclosure as a method for controlling weeds. Plants and seeds provided by the disclosure comprise an herbicide tolerance trait and as such are tolerant to the application of one or more PPO inhibiting herbicides. The herbicide application may be the recommended commercial rate (IX) or any fraction or multiple thereof, such as twice the recommended commercial rate (2X). Herbicide rates may be expressed as acid equivalent per pound per acre (lb ae/acre) or acid equivalent per gram per hectare (g ae/ha) or as pounds active ingredient per acre (lb ai/acre) or grams active ingredient per hectare (g ai/ha), depending on the herbicide and the formulation. The herbicide application comprises at least one PPO inhibiting herbicide. The plant growth area may or may not comprise weed plants at the time of herbicide application. A herbicidally-effective dose of PPO inhibiting herbicide(s) for use in an area for controlling weeds may consist of a range from about 0. IX to about 30X label rate(s) over a growing season. One (1) hectare is equivalent to 2.47105 acres and one (1) pound is equivalent to 453.592 grams. Herbicide rates can be converted between English and metric as: (lb ai/ac) multiplied by 1.12 = (kg ai/ha) and (kg ai/ha) multiplied by 0.89 = (lb ai/ac).
[00107] The desired application rate of the compounds of the general formula (I) and/or their salts is generally impacted to a certain extent by external conditions such as temperature, humidity, etc. The application rate may therefore vary within wide limits, and can be determined empirically by one of skill in the art in view of the present disclosure. For the application as a herbicide for controlling weeds or other undesirable plants, the total amount of compounds of the general formula (I) and their salts is often desirably in the range from about 0.02 g a.i./ha to about 750 g
a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, or about 0.25 g a.i./ha to about 300 g a.i./ha, but preferably from about 0.02 g a.i./ha to about 250 g a.i./ha, especially from about 0.05 g a.i./ha to about 150 g a.i./ha, and most preferably from about 0.25 g a.i./ha to about 120 g a.i./ha. This applies both to the pre-emergence or the post-emergence application.
[00108] Herbicide applications may be sequentially or tank mixed with one, two, or a combination of several PPO inhibiting herbicides or any other compatible herbicide. Multiple applications of one herbicide or of two or more herbicides, in combination or alone, may be used over a growing season to areas comprising transgenic plants of the disclosure for the control of a broad spectrum of dicot weeds, monocot weeds, or both, for example, two applications (such as a pre-planting application and a post-emergence application or a pre-emergence application and a post-emergence application) or three applications (such as a pre-planting application, a preemergence application, and a post-emergence application or a pre-emergence application and two post-emergence applications).
[00109] Substituted phenyluracils to be used according to the disclosure and its salts, have excellent herbicidal efficacy against a broad spectrum of economically important monocotyledonous and dicotyledonous annual harmful plants. The present disclosure therefore provides methods for controlling weeds, in areas of transgenic crop plants being tolerant to PPO inhibitor herbicides wherein the plants comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein the protein confers tolerance to such herbicides, comprising the application of the compounds of the general formula (I) and/or salts as defined above, to the plants (for example harmful plants such as monocotyledonous or dicotyledonous weeds or undesired crop plants), to the seed (for example grains, seeds or vegetative propagules such as tubers or shoot parts with buds) or to the area on which the plants grow (for example the area under cultivation), including any possible combinations thereof. Specific examples may be mentioned of some representatives of the monocotyledonous and dicotyledonous weed flora which can be controlled by the compounds and methods as described herein, without the enumeration being restricted to certain species. Among the monocotyledonous weed species, for example, Aegilops, Agropyron, Agrostis, Alopecurus, Apera, Avena, Brachicaria, Bromus, Cynodon, Dactyloctenium, Digitaria, Echinochloa, Eleocharis, Eleusine, Eragrostis, Eriochloa, Festuca, Fimbristylis, Imperata, Ischaemurn, Heteranthera, Imperata,
Leptochloa, Lolium, Monochoria, Panicum, Paspalum, Phalaris, Phleum, Poa, Rottboellia, Sagittaria, Scirpus, Setaria, Sorghum, Sphenoclea, and Cyperus species are covered by the annual group. In the case of dicotyledonous weed species, the spectrum of action extends to species such as, for example, Abutilon, Amaranthus, Ambrosia, Anoda, Anthemis, Aphanes, Artemisia, Atriplex, Bellis, Bidens, Capsella, Carduus, Cassia, Centaurea, Chenopodium, Cirsium, Convolvulus, Datura, Desmodium, Emex, Erodium, Erysimum, Euphorbia, Galeopsis, Galinsoga, Galium, Geranium, Hibiscus, Ipomoea, Kochia, Lamium, Lepidium, Lindernia, Matricaria, Mentha, Mercurialis, Mullugo, Myosotis, Papaver, Pharbitis, Plantago, Polygonum, Portulaca, Ranunculus, Raphanus, Rorippa, Rotala, Rumex, Salsola, Senecio, Sesbania, Sida, Sinapis, Solanum, Sonchus, Sphenoclea, Stellaria, Taraxacum, Thlaspi, Trifolium, Urtica, Veronica, Viola, and Xanthium.
[00110] Although the compounds described herein display outstanding herbicidal activity against monocotyledonous and dicotyledonous weeds, many economically important crop plants, depending on the structure of the respective active ingredients and the application rate thereof, are damaged only insignificantly, if at all. Economically important crops here are, for example, dicotyledonous crops from the genera of Arachis, Beta, Brassica, Cucumis, Cucurbita, Helianthus, Daucus, Glycine, Gossypium, Ipomoea, Lactuca, Linum, Lycopersicon, Nicotiana, Phaseolus, Pisum, Solanum, and Vicia, or monocotyledonous crops from the genera of Allium, Ananas, Asparagus, Avena, Hordeum, Oryza, Panicum, Saccharum, Secale, Sorghum, Triticale, Triticum, and Zea.
[00111] The compounds of the general formula (I) and/or salts thereof can be formulated in various ways according to which biological and/or physicochemical parameters are required. Examples of general formulation options are: wettable powders (WP), water-soluble powders (SP), emulsifiable concentrates (EC), water-soluble concentrates, aqueous solutions (SL), emulsions (EW), such as oil-in-water and water-in-oil emulsions, sprayable solutions or emulsions, dispersions based on oil or water, oil dispersions (OD), suspoemulsions (SE), suspension concentrates (SC), oil-miscible solutions, capsule suspensions (CS), dusting products (DP), dressings, granules for soil application or scattering, granules (GR) in the form of microgranules, spray granules, absorption and adsorption granules, water-dispersible granules (WG), water- soluble granules (SG), ULV formulations, microcapsules, or waxes.
[00112] The individual formulation types are known in principle and are described in, for example, Winnacker-Kuchler, "Chemische Technologic,” Vol. 7, 4th ed., Carl Hanser Verlag, Munich, 1986; Van Valkenburg, "Pesticide Formulations," Marcel Dekker Inc., New York, NY, 1973; and Masters, "Spray Drying Handbook," 3rd ed., George Goodwin Ltd. London, 1979. The necessary formulation auxiliaries such as inert materials, surfactants, solvents and further additives are likewise known and are described in, for example, Watkins, "Handbook of Insecticide Dust Diluents and Carriers," 2nd ed., Dorland Books, Caldwell, NJ, 1955; Van Olphen, "An Introduction to Clay Colloid Chemistry," 2nd ed., J. Wiley & Sons, New York, NY, 1974; Marsden, "Solvents Guide," 2nd ed., Interscience Publishers Inc., New York, NY, 1963; McCutcheon's "Detergents and Emulsifiers Annual," MC Publishing Corp., Ridgewood, NJ, 1998; Sisley and Wood, “Encyclopedia of Surface- Active Agents,” Chemical Publishing Company, New York, NY, 1964; Schonfeldt, "Grenzflachenaktive Athylenoxid-Addukte," Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, 1976; and Winnacker-Kuchler, "Chemische Technologic,” Vol. 7, 4th ed., Carl Hanser Verlag, Munich, 1986.
[00113] Wettable powders are preparations which can be dispersed uniformly in water and, in addition to the active ingredient, apart from a diluent or inert substance, also comprise surfactants of the ionic and/or nonionic type (wetting agents, dispersants), for example polyoxyethylated alkylphenols, polyoxyethylated fatty alcohols, polyoxyethylated fatty amines, fatty alcohol polyglycol ether sulfates, alkanesulfonates, alkylbenzene sulfonates, sodium lignosulfonate, sodium 2, 2'-dinaphthylmethane-6, 6' -disulfonate, sodium dibutylnaphthalenesulfonate, or sodium oleoyl methyltaurate. To produce the wettable powders, the active herbicidal ingredients are finely ground, for example, in customary apparatuses such as hammer mills, blower mills, and air-jet mills, and simultaneously or subsequently mixed with the formulation auxiliaries.
[00114] Emulsifiable concentrates are produced by dissolving the active ingredient in an organic solvent, for example butanol, cyclohexanone, dimethylformamide, xylene, or else relatively high-boiling aromatics or hydrocarbons or mixtures of the organic solvents, with addition of one or more ionic and/or nonionic surfactants (emulsifiers). Examples of emulsifiers which may be used are: calcium alkyl aryl sulfonate salts, such as calcium dodecylbenzenesulfonate, or nonionic emulsifiers such as fatty acid polyglycol esters, alkyl aryl polyglycol ethers, fatty alcohol polyglycol ethers, propylene oxide-ethylene oxide condensation
products, alkyl polyethers, sorbitan esters, for example sorbitan fatty acid esters, or for example polyoxyethylene sorbitan fatty acid esters.
[00115] Dusting products are obtained by grinding the active ingredient with finely distributed solids, for example talc, natural clays, such as kaolin, bentonite and pyrophyllite, or diatomaceous earth.
[00116] Suspension concentrates may be water- or oil-based. They may be produced, for example, by wet-grinding by means of commercial bead mills and optional addition of surfactants as already listed above, for example, for the other formulation types.
[00117] Emulsions, for example oil-in-water emulsions (EW), can be produced, for example, by means of stirrers, colloid mills and/or static mixers using aqueous organic solvents and optionally surfactants as already listed above, for example, for the other formulation types.
[00118] Active compounds that can be employed in combination with the compounds of the general formula (I) described herein in compositions described herein (for example in mixed formulations or in the tank mix) are, for example, known active compounds which are based on inhibition of, for example, acetolactate synthase, acetyl-CoA carboxylase, cellulose synthase, enolpyruvylshikimate-3 -phosphate synthase, glutamine synthetase, p-hydroxyphenylpyruvate dioxygenase, phytoene desaturase, photosystem I, photosystem II, or protoporphyrinogen oxidase, as are described in, for example, “Glossary of Common Names and Abbreviations of Herbicides,” Weed Research 26:441-445, 1986 or MacBean, "The Pesticide Manual,” 16th ed., British Crop Protection Council, Alton, UK, 2012, and the literature cited therein. Known herbicides or plant growth regulators which can be combined with the compounds of the described herein are, for example, the following, where said active compounds are designated either with their "common name" in accordance with the International Organization for Standardization (ISO) or with the chemical name or with the code number. They always encompass all the use forms, for example acids, salts, esters and also all isomeric forms such as stereoisomers and optical isomers, even if they are not mentioned explicitly.
[00119] Examples of such herbicidal mixing partners include one or more of the following: acetochlor, acifluorfen, acifluorfen-methyl, acifluorfen-sodium, aclonifen, alachlor, allidochlor, alloxydim, alloxydim-sodium, ametryn, amicarbazone, amidochlor, amidosulfuron, 4-amino-3- chloro-6-(4-chloro-2-fhioro-3-methylphenyl)-5-fluoropyridine-2-carboxylic acid,
aminocyclopyrachlor, aminocyclopyrachlor-potassium, aminocyclopyrachlor-methyl, aminopyralid, aminopyralid-dimethylammonium, aminopyralid-tripromine, amitrole, ammonium sulfamate, anilofos, asulam, asulam-potassium, asulam sodium, atrazine, azafenidin, azimsulfuron, beflubutamid, (S)-(-)-beflubutamid, beflubutamid-M, benazolin, benazolin-ethyl, benazolin-dimethylammonium, benazolin-potassium, bencarbazone, benfluralin, benfuresate, bensulfuron, bensulfuron-methyl, bensulide, bentazone, bentazone-sodium, benzfendizone, benzobicyclon, benzofenap, bicyclopyrone, bifenox, bilanafos, bilanafos-sodium, bipyrazone, bispyribac, bispyribac-sodium, bixlozone, bromacil, bromacil-lithium, bromacil-sodium, bromobutide, bromofenoxim, bromoxynil, bromoxynil-butyrate, -potassium, -heptanoate and - octanoate, busoxinone, butachlor, butafenacil, butamifos, butenachlor, butralin, butroxydim, butylate, cafenstrole, cambendichlor, carbetamide, carfentrazone, carfentrazone-ethyl, chlomethoxyfen, chloramben, chloramben-ammonium, chloramben-diolamine, chlroamben- methyl, chloramben-methylammonium, chloramben-sodium, chlorbromuron, chlorfenac, chlorfenac-ammonium, chlorfenac-sodium, chlorfenprop, chlorfenprop-methyl, chlorflurenol, chlorflurenol-methyl, chloridazon, chlorimuron, chlorimuron-ethyl, chlorophthalim, chlorotoluron, chlorsulfuron, chlorthal, chlorthal-dimethyl, cinidon, cinidon-ethyl, cinmethylin, exo-(+)-cinmethylin ((lR,2S,4S)-4-isopropyl-l-methyl-2-[(2-methylbenzyl)oxy]-7- oxabicyclo[2.2.1 ]heptane), exo-(-)-cinmethylin ((lR,2S,4S)-4-isopropyl- 1 -methyl-2-[(2- methylbenzyl)oxy]-7-oxabicyclo[2.2.1]heptane), cinosulfuron, clacyfos, clethodim, clodinafop, clodinafop-ethyl, clodinafop-propargyl, clomazone, clomeprop, clopyralid, clopyralid-methyl, clopyralid-olamine, clopyralid-potassium, clopyralid-tripomine, cloransulam, cloransulam- methyl, cumyluron, cyanamide, cyanazine, cycloate, cyclopyranil, cyclopyrimorate, cyclosulfamuron, cycloxydim, cyhalofop, cyhalofop-butyl, cy'prazine, 2,4-D, 2,4-D salts (including the -ammonium, -butotyl, -butyl, -choline, -diethylammonium, -dimethylammonium, - diolamine, -doboxyl, -dodecylammonium, -etexyl, -ethyl, 2-ethylhexyl, -heptylammonium, - isobutyl, -isooctyl, -isopropyl, -isopropylammonium, -lithium, -meptyl, -methyl, -potassium, - tetradecylammonium, -triethylammonium, -triisopropanolammonium, -tripromine, and -trolamine salts of 2,4-D), 2,4-DB, 2,4-DB-butyl, -dimethylammonium, isooctyl, -potassium and -sodium, daimuron (dymron), dalapon, dalapon-calcium, dalapon-magnesium, dalapon-sodium, dazomet, dazomet-sodium, n-decanol, 7-deoxy-D-sedoheptulose, desmedipham, detosyl-pyrazolate (DTP),
dicamba, dicamba salts (including dicamba-biproamine, dicamba-N,N-Bis(3- aminopropyl)methylamine, dicamba-butotyl, dicamba-choline, dicamba-diglycolamine, dicamba- dimethylammonium, dicamba-diethanolamine ammonium, dicamba-diethylammonium, dicamba- isopropylammonium, dicamba-methyl, dicamba-monoethanolamine, dicamba-olamine, dicamba- potassium, dicamba-sodium, dicamba-triethanolamine), dichlobenil, 2-(2,4-dichlorobenzyl)-4,4- dimethyl- 1 ,2-oxazolidin-3 -one, 2-(2,5 -dichlorobenzyl)-4,4-dimethyl- 1 ,2-oxazolidin-3 -one, dichlorprop, dichlorprop-butotyl, dichlorprop-dimethylammonium, dichlorprop-etexyl, dichlorprop-ethylammonium, dichlorprop-isoctyl, dichlorprop-methyl, dichlorprop-potassium, dichlorprop-sodium, dichlorprop-P, dichlorprop-P-dimethylammonium, dichlorprop-P-etexyl, dichlorprop-P-potassium, dichlorprop-sodium, diclofop, diclofop-methyl, diclofop-P, diclofop-P- methyl, diclosulam, difenzoquat, difenzoquat-metilsulfate, diflufenican, diflufenzopyr, diflufenzopyr-sodium, dimefuron, dimepiperate, dimesulfazet, dimethachlor, dimethametryn, dimethenamid, dimethenamid-P, dimetrasulfuron, dinitramine, dinoterb, dinoterb-acetate, diphenamid, diquat, diquat-dibromid, diquat-dichloride, dithiopyr, diuron, DNOC, DNOC- ammonium, DNOC-potassium, DNOC-sodium, endothal, endothal-diammonium, endothal- dipotassium, endothal-disodium, EPTC, esprocarb, ethalfluralin, ethametsulfuron, ethametsuliuron-methyl, ethiozin, ethofiimesate, ethoxyfen, ethoxyfen-ethyl, ethoxysulfuron, ethyl [3 -2-chloro-4-fluoro-5-( 1 -methyl-6-trifluoromethyl-2,4-dioxo- 1 ,2,3 ,4-tetrahydropyrimidin- 3-yl)phenoxy]-2-pyridyloxy]acetate (CAS Registry Number 353292-31-6 and referred to herein as S-3100), etobenzanid, F-5231(N-[2-chloro-4-fhioro-5-[4-(3-fhioropropyl)-4,5-dihydro-5-oxo- lH-tetrazol-l-yl]phenyl] ethanesulfonamide) F-7967 (3-[7-chloro-5-fluoro-2-(trifluoromethyl)- lH-benzimidazol-4-yl]-l-methyl-6-(trifluoromethyl)pyrimidine-2,4(lH,3H)-dione), fenoxaprop, fenoxaprop-P, fenoxaprop-ethyl, fenoxaprop-P-ethyl, fenoxasulfone, fenpyrazone, fenquinotrione, fentrazamide, flamprop, flamprop-isoproyl, flamprop-methyl, flamprop-M- isopropyl, flamprop-M-methyl, flazasulfuron, florasulam, florpyrauxifen, florpyrauxifen-benzyl, fluazifop, fluazifop-butyl, fluazifop-methyl, fluazifop-P, fluazifop-P-butyl, fluazolate, flucarbazone, flucarbazone-sodium, flucetosulfuron, fluchloralin, flufenacet, fhifenpyr, flufenpyr- ethyl, flumetsulam, flumiclorac, flumiclorac-pentyl, fhimioxazin, fluometuron, fluoroglycofen, fluoronitrofen, flurenol, flurenol-butyl, -dimethylammonium and -methyl, fluoroglycofen, fluoroglycofen-ethyl, flupropacil, flupropanate, flupropanate-sodium, flupyrsulfuron,
flupyrsulturon-methyl, flupyrsulfiiron-methyl-sodium, fluridone, flurochloridone, fluroxypyr, fluroxypyr-butometyl, fluroxypyr-meptyl, flurtamone, fluthiacet, fluthiacet-methyl, fomesafen, fomesafen-sodium, foramsulfuron, foramsulfiiron sodium salt, fosamine, fosamine-ammonium, fiiryloxyfen, glufosinate, glufosinate-ammonium, glufosinate-sodium, L-glufosinate-ammonium, L-glufosiante-sodium, glufosinate-P-sodium, glufosinate-P-ammonium, glufosinate-P-sodium, glyphosate, glyphosate-ammonium, -isopropylammonium, -diammonium, -dimethylammonium, - potassium, -sodium, -sesquisodium and -trimesium, H-9201 (O-(2,4-dimethyl-6-nitrophenyl) O- ethyl isopropylphosphoramidothioate, halauxifen, halauxifen-methyl, halosafen, halosulfuron, halosulfuron-methyl, haloxyfop, haloxyfop-P, haloxyfop-ethoxyethyl, haloxyfop-P-ethoxyethyl, haloxyfop-methyl, haloxyfop-P-methyl, haloxyfop-sodium, hexazinone, HNPC-A8169 (prop-2 - yn-l-yl (2S)-2-{3-[(5-tert-butylpyridin-2-yl)oxy]phenoxy}propanoate), HW-02 (1-
(dimethoxyphosphoryl)ethyl (2,4-dichlorophenoxy)acetate), hydantocidin, imazamethabenz, imazamethabenz-methyl, imazamox, imazamox-ammonium, imazapic, imazapic-ammonium, imazapyr, imazapyr-isopropylammonium, imazaquin, imazaquin-ammonium, imazaquin.methyl, imazethapyr, imazethapyr-immonium, imazosulfuron, indanofan, indaziflam, iodosulfuron, iodosulfuron-methyl, iodosulfuron-methyl-sodium, ioxynil, ioxynil-lithium, -octanoate, - potassium and sodium, ipfencarbazone, isoproturon, isouron, isoxaben, isoxaflutole, karbutilate, KUH-043 (3 -( { [5-(difluoromethyl)- 1 -methyl-3 -(trifluoromethyl)- 1 H-pyrazol-4- yl]methyl} sulfonyl)-5,5-dimethyl-4,5-dihydro-l ,2-oxazole), ketospiradox, ketospiradox- potassium, lactofen, lancotrione, lenacil, linuron, MCPA, MCPA-butotyl, -butyl, - dimethylammonium, -diolamine, -2-ethylhexyl, -ethyl, -isobutyl, isoctyl, -isopropyl, - isopropylammonium, -methyl, -olamine, -potassium, -sodium, and -trolamine, MCPB, MCPB- methyl, -ethyl and -sodium, mecoprop, mecoprop-butotyl, mecoprop-demethylammonium, mecoprop-diolamine, mecoprop-etexyl, mecoprop-ethadyl, mecoprop-isoctyl, mecoprop-methyl, mecoprop-potassium, mecoprop-sodium, mecoprop-trolamine mecoprop-P, mecoprop-P-butotyl, -dimethylammonium, -2-ethylhexyl and -potassium, mefenacet, mefluidide, mefluidide- diolamine, mefluidide-potassium, mesosulfuron, mesosulfuron-methyl, mesosulfuron sodium salt, mesotrione, methabenzthiazuron, metam, metamifop, metamitron, metazachlor, metazosulfuron, methabenzthiazuron, methiopyrsulfuron, methiozolin, methyl isothiocyanate, metobromuron, metolachlor, S-metolachlor, metosulam, metoxuron, metribuzin, metsulfuron, metsulfuron-
methyl, molinate, monolinuron, monosulfuron, monosulfuron-methyl, MT-5950 (N-[3-chloro-4- (l-methylethyl)phenyl]-2-methylpentanamide), NGGC-011, napropamide, NC-310 (4-(2,4- dichlorobenzoyl)-l -methyl-5-benzyloxypyrazole), NC-656, (3-[(isopropylsulfonyl)methyl]-N-(5- methyl-l,3,4-oxadiazol-2-yl)-5-(trifluoromethyl)[l,2,4]triazolo[4,3-a]pyridine-8-carboxamide), neburon, nicosulfuron, nonanoic acid (pelargonic acid), norflurazon, oleic acid (fatty acids), orbencarb, orthosulfamuron, oryzalin, oxadiargyl, oxadiazon, oxasulfuron, oxaziclomefone, oxyfluorfen, paraquat, paraquat-dichloride, paraquat-dimethylsulfate, pebulate, pendimethalin, penoxsulam, pentachlorophenol, pentoxazone, pethoxamid, petroleum oils, phenmedipham, phenmedipham-ethyl, picloram, picloram-dimethylammonium, picloram-etexyl, picloram-isoctyl, picloram-methyl, picloram-olamine, picloram-potassium, picloram-triethylammonium, picloram- tripromine, picloram-trolamine, picolinafen, pinoxaden, piperophos, pretilachlor, primisulfuron, primisulfiiron-methyl, prodiamine, profluazol, profoxydim, prometon, prometryn, propachlor, propanil, propaquizafop, propazine, propham, propisochlor, propoxycarbazone, propoxycarbazone-sodium, propyrisulfuron, propyzamide, prosulfocarb, prosulfuron, pyraclonil, pyraflufen, pyraflufen-ethyl, pyrasulfotole, pyrazolynate (pyrazolate), pyrazosulfuron, pyrazosulfuron-ethyl, pyrazoxyfen, pyribambenz, pyribambenz-isopropyl, pyribambenz-propyl, pyribenzoxim, pyributicarb, pyridafol, pyridate, pyriftalid, pyriminobac, pyriminobac-methyl, pyrimisulfan, pyrithiobac, pyrithiobac-sodium, pyroxasulfone, pyroxsulam, quinclorac, quinclorac-dimethylammonium, quinclorac-methyl, quinmerac, quinoclamine, quizalofop, quizalofop-ethyl, quizalofop-P, quizalofop-P-ethyl, quizalofop-P-tefuryl, QYM201 (l-{2-chloro- 3 - [(3 -cyclopropyl-5 -hydroxy- 1 -methyl- 1 H-pyrazol-4-yl)carbonyl] -6-(trifluoromethyl)phenyl } piperidin-2-one), rimsulfiiron, saflufenacil, sethoxydim, siduron, simazine, simetryn, SL-261, sulcotrione, sulfentrazone, sulfometuron, sulfometuron-methyl, sulfosulfuron, SYP-249 (1- ethoxy-3 -methyl- l-oxobut-3-en-2-yl 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate), SYP-300 (l-[7-fluoro-3-oxo-4-(prop-2-yn-l-yl)-3,4-dihydro-2H-l,4-benzoxazin-6-yl]-3-propyl- 2-thioxoimidazolidine-4, 5-dione), 2,3,6-TBA, TCA (trichloroacetic acid) and its salts (e.g., TCA- ammonium, TCA-calcium, TCA-ethyl, TCA-magnesium, and TCA-sodium), tebuthiuron, teiuryltrione, tembotrione, tepraloxydim, terbacil, terbucarb, terbumeton, terbuthylazine, terbutryn, tetflupyrolimet, thaxtomin, thenylchlor, thiazopyr, thidiazimin, thiencarbazone, thiencarbazone-methyl, thifensulfuron, thifensulfiiron-methyl, thiobencarb, tiafenacil, tolpyralate,
topramezone, tralkoxydim, triafamone, tri-allate, triasulfuron, triaziflam, tribenuron, tribenuron- methyl, triclopyr, triclopyr-butotyl, triclopyr-choline, triclopyr-ethyl, triclopyr-triethylammonium, trietazine, trifloxysulfuron, trifloxysulfiiron-sodium, trifludimoxazin, trifluralin, trifhisulfuron, triflusulfuron-methyl, tritosulfiiron, urea sulfate, vernolate, XDE-848, ZJ-0862 (3,4-dichloro-N-
{2-[(4,6-dimethoxypyrimidin-2-yl)oxy]benzyl} aniline), 3-chloro-2-[3-
(difluoromethyl)isoxazolyl-5-yl]phenyl-5-chloropyrimidin-2-yl ether, 2-(3,4-dimethoxyphenyl)- 4-[(2 -hydroxy -6-oxocyclohex- 1 -en-1 -yl)carbonyl]-6-methylpyridazine-3(2H)-one, 2-( {2-[(2- methoxyethoxy)methyl] -6-methylpyridin-3 -yl } carbonyl)cyclohexane- 1 , 3 -dione, (5 -hydroxy- 1 - methyl-lH-pyrazol-4-yl)(3,3,4-trimethyl-l,l-dioxido-2,3-dihydro-l-benzothiophen-5- yl)methanone, 1 -methyl-4-[(3,3,4-trimethyl-l , 1 -dioxido-2,3-dihydro-l -benzothiophen-5- yl)carbonyl] - 1 H-pyrazol-5-yl propane- 1 -sulfonate, 4- {2-chloro-3 -[(3 ,5-dimethyl- 1 H-pyrazol- 1 - yl)methyl] -4-(methylsulfonyl)benzoyl } - 1 -methyl- 1 H-pyrazol-5-yl- 1 ,3 -dimethyl- 1 H-pyrazole-4- carboxylate; cyanomethyl 4-amino-3-chloro-5-fhioro-6-(7-fluoro-lH-indol-6-yl)pyridine-2- carboxylate, prop-2-yn-l-yl 4-amino-3-chloro-5-fhioro-6-(7-fluoro-lH-indol-6-yl)pyridine-2- carboxylate, methyl 4-amino-3-chloro-5-fluoro-6-(7-fluoro-lH-indol-6-yl)pyridine-2- carboxylate, 4-amino-3 -chloro-5-fluoro-6-(7-fluoro- lH-indol-6-yl)pyridine-2-carboxylic acid, benzyl 4-amino-3-chloro-5-fluoro-6-(7-fluoro-lH-indol-6-yl)pyridine-2-carboxylate, ethyl 4- amino-3 -chloro-5-fluoro-6-(7-fluoro- 1 H-indol-6-yl)pyridine-2-carboxylate, methyl 4-amino-3 - chloro-5-fhioro-6-(7-fluoro-l-isobutyryl-lH-indol-6-yl)pyridine-2 -carboxylate, methyl 6-(l- acetyl-7-fluoro-lH-indol-6-yl)-4-amino-3-chloro-5-fluoropyridine-2-carboxylate, methyl 4- amino-3-chloro-6-[l-(2,2-dimethylpropanoyl)-7-fluoro-lH-indol-6-yl]-5-fluoropyridine-2- carboxylate, methyl 4-amino-3-chloro-5-fhioro-6-[7-fhioro-l-(methoxyacetyl)-lH-indol-6- yl]pyridine-2-carboxylate, potassium 4-amino-3-chloro-5-fluoro-6-(7-fluoro-lH-indol-6- yl)pyridine-2-carboxylate, sodium 4-amino-3-chloro-5-fluoro-6-(7-fhioro-lH-indol-6- yl)pyridine-2-carboxylate, butyl 4-amino-3-chloro-5-fhioro-6-(7 -fluoro- lH-indol-6-yl)pyridine- 2-carboxylate, 4-hydroxy-l-methyl-3-[4-(trifluoromethyl)pyridin-2-yl]imidazolidin-2-one, 3-(5- tert-butyl-l,2-oxazol-3-yl)-4-hydroxy-l-methylimidazolidin-2-one, 3-[5-chloro-4-
(trifhiormethyl)pyridin-2-yl]-4-hydroxy-l-methylimidazolidin-2-one, 4-hydroxy-l-methoxy-5- methyl-3-[4-(trifluormethyl)pyridin-2-yl]imidazolidin-2-one, 6-[(2-hydroxy-6-oxocyclohex-l- en-l-yl)carbonyl]-l,5-dimethyl-3-(2-methylphenyl)quinazolin-2,4(lH,3H)-dione, 3-(2,6-
dimethylphenyl)-6- [(2-hydroxy-6-oxocyclohex- 1 -en- 1 -yl)carbonyl] - 1 -methylquinazolin-
2,4( 1 H,3H)-dione, 2- [2-chloro-4-(methylsulfonyl)-3 -(morpholin-4-ylmethyl)benzoyl]-3 - hydroxycyclohex-2-en-l-one, l-(2-carboxyethyl)-4-(pyrimidin-2-yl)pyridazin-l-ium salt (with anions such as chloride, acetate or trifluoroacetate), l-(2-carboxyethyl)-4-(pyridazin-3- yl)pyridazin-l-ium salt (with anions such as chloride, acetate or trifluoroacetate), 4-(pyrimidin-2- yl)-l-(2-sulfoethyl)pyridazin-l-ium salt (with anions such as chloride, acetate or trifluoroacetate), 4-(pyridazin-3-yl)-l-(2-sulfoethyl)pyridazin-l-ium salt (with anions such as chloride, acetate or trifluoroacetate), l-(2-Carboxyethyl)-4-(l,3-thiazol-2-yl)pyridazin-l-ium salt (with anions such as chloride, acetate or trifluoroacetate), l-(2-Carboxyethyl)-4-(l,3-thiazol-2-yl)pyridazin-l-ium salt (with anions such as chloride, acetate, or trifluoroacetate).
[00120] A combination described herein may be a composition containing herbicidally active compounds (A) and (B), where (A) is one or more compounds of the general formula (I) or their agrochemically compatible salts [component (A)] and (B) is one or more herbicides [component (B)] selected from the group consisting of the herbicidally active compounds (Bl) to (Bl l), wherein:
(Bl) represents active herbicidal ingredients from the group of the following 1,3 -diketo compounds:
(Bl.l) alloxydim CAS 55634-91-8; CAS 55635-13-7
(Bl.2) bicyclopyrone CAS 352010-68-5
(B1.3) butroxydim CAS 138164-12-2
(B1.4) clethodim CAS 99129-21-2
(Bl.5) cycloxydim CAS 101205-02-1
(Bl.6) fenquinotrione CAS 1342891-70-6
(Bl.7) mesotrione CAS 104206-82-8
(Bl.8) pinoxaden CAS 243973-20-8
(Bl.9) profoxydim CAS 139001-49-3
(Bl.10) sethoxydim CAS 74051-80-2
(Bl.ll) sulcotrione CAS 99105-77-8
(Bl.12) SYP-9121 CAS 1976053-87-8
(Bl.13) tefuryltrione CAS 473278-76-1
(Bl.14) tembotrione CAS 335104-84-2
(Bl.15) tepraloxydim CAS 149979-41-9
(Bl.16) tralkoxydim CAS 87820-88-0
(Bl.17) Y13161 CAS 1639426-14-4
(Bl.18) Y13287 CAS 1639426-42-8
(B2) represents active herbicidal ingredients from the group of the following (sulfon)amides:
(B2.1) acetochlor CAS 34256-82-1
(B2.2) alachlor CAS 15972-60-8
(B2.3) amidosulfuron CAS 120923-37-7
(B2.4) asulam CAS 3337-71-1; CAS 14089-43-1; CAS 2302-17-2
(B2.5) azimsulfuron CAS 120162-55-2
(B2.6) beflubutamid CAS 113614-08-7, CAS 113614-09-8
(B2.7) bensulfuron CAS 83055-99-6; CAS 83055-99-6
(B2.8) butachlor CAS 23184-66-99
(B2.9) carbetamide CAS 16118-49-3
(B2.10) chlorimuron CAS 99283-00-8; CAS 90982-32-4
(B2.l l) chlorpropham CAS 101-21-3
(B2.12) chlorsulfiiron CAS 64902-72-3
(B2.13) cinosulfuron CAS 94593-91-6
(B2.14) cloransulam CAS 159518-97-5; CAS 147150-35-4
(B2.15) cyclosulfamuron CAS 136849-15-5
(B2.16) desmedipham CAS 13684-56-5
(B2.17) diclosulam CAS 145701-21-9
(B2.18) diflufemcan CAS 83164-33-4
(B2.19) dimethachlor CAS 50563-36-5
(B2.20) dimethenamid CAS 87674-68-8; CAS 163515-14-8
(B2.21) esprocarb CAS 85785-20-2
(B2.22) ethametsulfuron CAS 111353-84-5; CAS 97780-06-8
(B2.23) ethoxysulfuron CAS 126801-58-9
(B2.24) flazasulfuron CAS 104040-78-0
(B2.25) florasulam CAS 145701-23-1
(B2.26) flucarbazone CAS 145026-88-6; CAS 181274-17-9
(B2.27) flucetosulfuron CAS 412928-75-7
(B2.28) flufenacet CAS 142459-58-3
(B2.29) flumetsulam CAS 98967-40-9
(B2.30) flupyrsulfuron CAS 150315-10-9; CAS 144740-53-4; CAS 144740-54-5
(B2.31) foramsulfuron CAS 173159-57-4
(B2.32) halosulfiiron CAS 135397-30-7; CAS 100784-20-1
(B2.33) imazosulfuron CAS 122548-33-8
(B2.34) iodosulfuron CAS 185119-76-0; CAS 144550-06-1; CAS 144550-36-7
(B2.35) ipfencarbazone CAS 212201-70-2
(B2.36) mefenacet CAS 73250-68-7
(B2.37) mesosulfiiron CAS 400852-66-6; CAS 208465-21-8
(B2.38) metazachlor CAS 67129-08-2
(B2.39) metazosulfuron CAS 868680-84-6
(B2.40) metolachlor CAS 51218-45-2
(B2.41) metosulam CAS 139528-85-1
(B2.42) metsulfuron CAS 79510-48-8; CAS 74223-64-6
(B2.43) nicosulfiiron CAS 111991-09-4
(B2.44) orthosulfamuron CAS 213464-77-8
(B2.45) oxasulfuron CAS 144651-06-9
(B2.46) penoxsulam CAS 219714-96-2
(B2.47) pethoxamide CAS 106700-29-2
(B2.48) phenmedipham CAS 13684-63-4
(B2.49) picolinafen CAS 137641-05-5
(B2.50) pretilachlor CAS 51218-49-6
(B2.51 ) primisulfuron CAS 113036-87-6; CAS 86209-51-0
(B2.52) propachlor CAS 1918-16-7
(B2.53) propanil CAS 709-98-8
(B2.54) propham CAS 122-42-9
(B2.55) propisochlor CAS 86763-47-5
(B2.56) propoxycarbazone CAS 145026-81-9; CAS 181274-15-7
(B2.57) propyrisulfiiron CAS 570415-88-2
(B2.58) propyzamide CAS 23950-58-5
(B2.59) prosulfocarb CAS 52888-80-9
(B2.60) prosulfuron CAS 94125-34-5
(B2.61) pyrazosulfuron CAS 98389-04-9; CAS 93697-74-6
(B2.62) pyroxsulam CAS 422556-08-9
(B2.63) rimsulfuron CAS 122931-48-0
(B2.64) S-metolachlor CAS 87392-12-9
(B2.65) sulfometuron CAS 74223-56-6; CAS 74222-97-2; CAS 144651-06-9
(B2.66) sulfosulfuron CAS 141776-32-1
(B2.67) thenylchlor CAS 96491-05-3
(B2.68) thiencarbazone CAS 936331-72-5; CAS 317815-83-1
(B2.69) thifensulfuron CAS 79277-67-1; CAS 79277-27-3
(B2.70) tri-allate CAS 2303-17-5
(B2.71) triasulfuron CAS 82097-50-5
(B2.72) tribenuron CAS 106040-48-6; CAS 101200-48-0
(B2.73) trifloxysulfuron CAS 145099-21-4; CAS 199119-58-9
(B2.74) triflusulfuron CAS 135990-29-3; CAS 126535-15-7
(B2.75) tritosulfiiron CAS 142469-14-5
(B2.76) esprocarb CAS 85785-20-2
(B2.77) profluazol CAS 190314-43-3
(B2.78) tri-allate CAS 2303-17-5
(B2.79) methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5-methoxy-4H-isoxazole-5- carbonyl]amino]tetrahydrofiiran-2-carboxylate,
(B2.80) methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5-vinyl-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2-carboxylate
(B2.81 ) methyl rel-(2R,4R)-4-[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2-carboxylate
(B2.82) isopropyl rel-(2R,4R)-4-[[3-(3-fluorophenyl)-5-methyl-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2-carboxylate
(B2.83) methyl (3R)-3-[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-isoxazole-5- carbonyl]amino]-2,3-dihydrofiiran-5-carboxylate
(B2.84) methyl (3R)-3-[[(5R)-3-(3,5-difluorophenyl)-5-methyl-4H-isoxazole-5- carbonyl]amino]-2,3-dihydrofuran-5-carboxylate
(B2.85) methyl (lS,4R)-4-[[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-l,2-oxazol-5- yl]carbonyl]amino]cyclopent-2-ene-l-carboxylate
(B2.86) ethyl ( 1 S,4R)-4-[[[3-(3,5-difluorophenyl)-5-methoxy-4H- 1 ,2-oxazol-5- yl]carbonyl]amino]cyclopent-2-ene-l-carboxylate
(B2.87) 2-methoxyethyl(lS,4R)-4-[[[(5R)-3-(3-cyano-5-fluorophenyl)-5-
(trifluoromethyl)-4H- 1 ,2-oxazol-5-yl]carbonyl]amino]cyclopent-2-ene- 1 - carboxylate
(B2.88) methyl (4S)-4-[[[3-(3,5-difluorophenyl)-5-methyl-4H-l,2-oxazol-5- yl]carbonyl]amino]cyclopentene-l-carboxylate
(B2.89) methyl (3S)-3-[[[(5R)-3-(3,5-difluorophenyl)-5-methyl-4H-l ,2-oxazol-5- yl] carbonyl] amino] cyclopentene- 1 -carboxylate
(B2.90) 3-(3,5-difluorophenyl)-N-[(lR,4S)-4-(oxazinan-2-ylcarbonyl)cyclopent-2-en-l- yl]-5-(trifluoromethyl)-4H- 1 ,2-oxazole-5-carboxamide
(B2.91) 3-(3,5-difluorophenyl)-N-[(lR,4S)-4-[(propylsulfonylamino)carbonyl]cyclopent-
2-en-l-yl]-5-(trifluoromethyl)-4H-l,2-oxazole-5-carboxamide
(B2.92) (lS,4R)-4-[[(5R)-3-(3,5-difluorophenyl)-5-methyl-4H-isoxazole-5- carbonyl] amino] cyclopent-2-ene- 1 -carboxylic acid,
(B3) represents active herbicidal ingredients from the group of the following aryl nitriles:
(B3.1) bromoxynil CAS 1689-84-5; CAS 3861-41-4; CAS 56634-95-8; CAS
1689-99-2; CAS 2961-68-4
(B3.2) chlorthiamid CAS 1918-13-4
(B3.3) dichlobenil CAS 1194-65-6
(B3.4) ioxynil CAS 1689-83-4; CAS 2961-61-7; CAS 3861-47-0; CAS
2961-62-8
(B3.5) pyraclonil CAS 158353-15-2
(B4) represents active herbicidal ingredients from the group of the following azoles:
(B4.1) amicarbazone CAS 129909-90-6
(B4.2) amitrole CAS 61-82-5
(B4.3) azafenidin CAS 68049-83-2
(B4.4) benzofenap CAS 82692-44-2
(B4.5) benzuofiicaotong CAS 1992017-55-6
(B4.6) biscarfentrazone CAS 1622908-18-2
(B4.7) cafenstrole CAS 125306-83-4
(B4.8) carfentrazone CAS 128621-72-7; CAS 128639-02-1
(B4.9) fentrazamide CAS 158237-07-1
(B4.10) imazamethabenz CAS 100728-84-5; CAS 81405-85-8
(B4.11) imazamox CAS 114311-32-9; CAS 247057-22-3
(B4.12) imazapic CAS 104098-48-8; CAS 115136-53-3
(B4.13) imazapyr CAS 81334-34-1; CAS 81510-83-0
(B4.14) imazaquin CAS 81335-37-7; CAS 81335-47-9; CAS 81335-43-5; CAS
81335-46-8
(B4.15) imazethapyr CAS 81335-77-5; CAS 101917-66-2
(B4.16) isouron CAS 55861-78-4
(B4.17) isoxaben CAS 82558-50-7
(B4.18) isoxaflutole CAS 141112-29-0
(B4.19) oxadiargyl CAS 39807-15-3
(B4.20) oxadiazon CAS 19666-30-9
(B4.21) pyraflufen CAS 129630-17-7; CAS 129630-19-9
(B4.22) pyrasulfotole CAS 365400-11-9
(B4.23) pyrazolynate CAS 58011-68-0
(B4.24) pyrazoxyfen CAS 71561-11-0
(B4.25) pyroxasulfone CAS 447399-55-5
(B4.26) sulfentrazone CAS 122836-35-5
(B4.27) tolpyralate CAS 1101132-67-5
(B4.28) topramezone CAS 210631-68-8
(B4.29) triazole sulcotrione CAS 1911613-97-2
(B4.30) QYM201 CAS 1855925-45-1
(B4.31) bencarbazone CAS 173980-17-1
(B4.32) fluazolate CAS 174514-07-9
(B4.33) flupoxam CAS 119126-15-7
(B4.34) isoxachlortole CAS 141112-06-3
(B5) represents further active herbicidal ingredients specified below:
(B5.1) aminocyclopyrachlor CAS 858956-08-8; CAS 858954-83-3; CAS 858956-35-1
(B5.2) aminopyralid CAS 150114-71-9; CAS 566191-87-5; CAS 566191-89-7
(B5.3) benazolin-ethyl CAS 3813-05-6; CAS 38561-76-1; CAS 25059-80-7;
CAS 67338-65-2
(B5.4) benfluralin CAS 1861-40-1
(B5.5) bentazone CAS 25057-89-0; CAS 50723-80-3
(B5.6) benzobicyclon CAS 156963-66-5
(B5.7) bixlozone CAS 81777-95-9
(B5.8) bromofenoxim CAS 13181-17-4
(B5.9) butralin CAS 33629-47-9
(B5.10) chloridazon/pyrazon CAS 1698-60-8
(B5.l l) chlorthal CAS 2136-79-0; CAS 1861-32-1; CAS 887-54-7
(B5.12) cinidon-ethyl CAS 142891-20-1
(B5.13) cinmethylin CAS 87818-31-3
(B5.14) clomazone CAS 81777-89-1
(B5.15) cyclopyrimorate CAS 499231-24-2
(B5.16) dinitramine CAS 29091-05-2
(B5.17) diquat CAS 2764-72-9; CAS 85-00-7; CAS 4032-26-2
(B5.18) dithiopyr CAS 97886-45-8
(B5.19) acetic acid CAS 64-19-7
(B5.20) ethalfluralin CAS 55283-68-6
(B5.21) ethofumesate CAS 26225-79-6
(B5.22) flamprop CAS 58667-63-3; CAS 90134-59-1; CAS 63782-90-1;
CAS 63729-98-6
(B5.23) florpyrauxifen CAS 943832-81-3; CAS 1390661-72-9
(B5.24) flufenpyr CAS 188490-07-5; CAS 188489-07-8
(B5.25) flumiclorac CAS 87547-04-4; CAS 87546-18-7
(B5.26) flumioxazin CAS 103361-09-7
(B5.27) fluridone CAS 59756-60-4
(B5.28) flurochloridone CAS 61213-25-0
(B5.29) flurtamone CAS 96525-23-4
(B5.30) fluthiacet-methyl CAS 149253-65-6
(B5.31) halauxifen CAS 943832-60-8; CAS 943831-98-9
(B5.32) indanofan CAS 13320-30-1
(B5.33) norflurazon CAS 27314-13-2
(B5.34) oleic acid CAS 112-80-1
(B5.35) oryzalin CAS 19044-88-3
(B5.36) oxaziclomefone CAS 153197-14-9
(B5.37) paraquat CAS 4685-14-7; CAS 1910-42-5; CAS 2074-50-2
(B5.38) pelargonic acid CAS 112-05-0
(B5.39) pendimethalin CAS 40487-42-1
(B5.40) pentoxazone CAS 110956-75-7
(B5.41) pyridafol CAS 40020-01-7
(B5.42) pyridate CAS 55512-33-9
(B5.43) tetflupyrolimet CAS 2053901-33-8
(B5.44) thiazopyr CAS 117718-60-2
(B5.45) triafamone CAS 874195-61-6
(B5.46) trifluralin CAS 1582-09-8
(B5.47) 4-amino-3-chloro-5- fluoro-6-(7-fluoro-lH- indol-6-yl)pyridine-2- carboxylic acid
(B5.48) cyclopyrimorate CAS 499231-24-2
(B5.49) diquat CAS 2764-72-9; CAS 85-00-7; CAS 4032-26-2
(B5.50) oxaziclomefone CAS 153197-14-9
(B5.51) pentanochlor CAS 2307-68-8
(B5.52) tebutam CAS 35256-85-0
(B5.53) thidiazimin CAS 123249-43-4
(B5.54) 4-hydroxy-l-methyl-3- CAS 1708087-22-2
[4-(trifluoromethyl)-2- pyridinyl]-2- imidazolidinone
(B6) represents active herbicidal ingredients from the group of the following (het)arylcarboxylic acids:
(B6.1 ) chloramben CAS 133-90-4; CAS 1076-46-6; CAS 53404- 16-7286;
CAS 25182-03-0; CAS 1954-81-0
(B6.2) clopyralid CAS 1702-17-6; CAS 1532-24-7; CAS 57754-85-5; CAS
58509-83-4; CAS 73455-09-1
(B6.3) dicamba CAS 1918-00-9; CAS 1286239-22-2; CAS 104040-79-1;
CAS 2300-66-5; CAS 25059-78-3; CAS 55871-02-8;
CAS 6597-78-0; CAS 53404-28-7; CAS 10007-85-9;
CAS 1982-69-0; CAS 53404-29-8; CAS 56141-00-5
(B6.4) fluroxypyr CAS 69377-81-7; CAS -27-8; CAS 81406-37-3
(B6.5) picloram CAS 1918-02-1; CAS 55870-98-9; CAS 36374-99-9;
CAS 26952-20-5; CAS 14143-55-6; CAS 55871-00-6;
CAS 2545-60-0; CAS 35832-11-2; CAS 6753-47-5; CAS 82683-78-1
(B6.6) quinclorac CAS 84087-01-4; CAS 84087-48-9; CAS 84087-33-2
(B6.7) quinmerac CAS 90717-03-6
(B6.8) TBA CAS 50-31-7; CAS 3426-62-8; CAS 71750-37-3; CAS
4559-30-2; CAS 2078-42-4
(B6.9) Triclopyr CAS 55335-06-3; CAS 64700-56-7; CAS 1048373-85-8;
CAS 60825-27-6; CAS 57213-69-1
(B7) represents active herbicidal ingredients from the group of the following organic phosphorus compounds:
(B7.1) anilofos CAS 64249-01-0
(B7.2) bialaphos CAS 35597-43-4; CAS 71048-99-2
(B7.3) butamifos CAS 36335-67-8
(B7.4) glufosinate CAS 51276-47-2; CAS 35597-44-5; CAS 77182-82-2;
CAS 70033-13-5
(B7.5) glyphosate CAS 1071-83-6; CAS 69254-40-6; CAS 34494-04-7;
CAS 38641-94-0; CAS 40465-66-5; CAS 39600-42-5;
CAS 70393-85-0; CAS 81591-81-3
(B7.6) piperophos CAS 24151-93-7
(B7.7) sulfosate CAS 1591-81-3
(B7.8) Amiprofos CAS 33857-23-7; CAS 36001-88-4
(B8) represents active herbicidal ingredients from the group of the following phenyl ethers:
(B8.1) 2,4-D CAS 94-75-7; CAS 2307-55-3; CAS 1929-73-3; CAS
1320-18-9; CAS 1928-45-6; CAS 94-80-4; CAS
1048373-72-3; CAS 20940-37-8; CAS 2008-39-1; CAS 5742-19-8; CAS 2212-54-6; CAS 533-23-3; CAS 1928- 43-4; CAS 37102-63-9; CAS 713-15-1; CAS 25168-26- 7; CAS 94-11-1; CAS 5742-17-6; CAS 3766-27-6; CAS 1917-97-1; CAS 1928-38-7; CAS 1928-44-5; CAS 1917-92-6; CAS 1928-61-6; CAS 2702-72-9; CAS 15146-99-3; CAS 28685-18-9; CAS 2646-78-8; CAS 18584-79-7; CAS 2569-01-9; CAS 215655-76-8
(B8.2) 2,4-DB CAS 94-82-6; CAS 2758-42-1; CAS 1320-15-6; CAS 19480-40-1; CAS 10433-59-7
(B8.3) 2,4-DP CAS 120-36-5; CAS 53404-31-2; CAS 53404-32-3; CAS 79270-78-3; CAS 28631-35-8; CAS 57153-17-0; CAS 5746-17-8; CAS 39104-30-8
(B8.4) acifluorfen CAS 50594-66-6; CAS 50594-67-7; CAS 62476-59-9 (B8.5) aclonifen CAS 74070-46-5 (B8.6) bifenox CAS 42576-02-3 (B8.7) chlomethoxyfen, CAS 32861-85-1 (B8.8) clodinafop-propargyl CAS 114420-56-3; CAS 105512-06-9 (B8.9) clomeprop CAS 84496-56-0 (B8.10) cyhalofop CAS 122008-78-0; CAS 122008-85-9; (B8.ll) diclofop CAS 40843-25-2; CAS 51338-27-3; (B8.12) ethoxyfen CAS 188634-90-4; CAS 131086-42-5; (B8.13) fenoxaprop CAS 95617-09-7; CAS 113158-40-0; CAS 71283-80-2 (B8.14) fluazifop CAS 69335-91-7; CAS 83066-88-0; CAS 79241-46-6
(B8.15) fluoroglycofen CAS 77501-60-1; CAS 77501-90-7 (B8.16) fomesafen CAS 72178-02-0; CAS 108731-70-0 (B8.17) halosafen CAS 77227-69-1 (B8.18) haloxyfop CAS 69806-34-4; CAS 95977-29-0; CAS 72619-32-0 (B8.19) lactofen CAS 77501-63-4 (B8.20) MCPA CAS 94-74-6; CAS 19480-43-4; CAS 1713-12-8; CAS 2039-46-5; CAS 20405-19-0; CAS 2698-38-6; CAS 29450-45-1; CAS 1713-11-7; CAS 26544-20-7; CAS
2698-40-0; CAS 2436-73-9; CAS 6365-62-4; CAS 5221 - 16-9; CAS 3653-48-3; CAS 42459-68-7
(B8.21) MCPB CAS 94-81-5; CAS 10443-70-6; CAS 57153-18-1; CAS 6062-26-6
(B8.22) mecoprop CAS 93-65-2; CAS 32351-70-5; CAS 1432-14-0; CAS 71526-69-7; CAS 28473-03-2; CAS 2786-19-8; CAS 1929-86-8; CAS 19095-88-6; CAS 53404-61-8; CAS 16484-77-8
(B8.23) metamifop CAS 256412-89-2
(B8.24) oxyfluorfen CAS 42874-03-3
(B8.25) propaquizafop CAS 111479-05-1
(B8.26) quizalofop CAS 76578-12-6; CAS 76578-14-8
(B8.27) quizalofop-P CAS 94051-08-8; CAS 100646-51-3; CAS 200509-41-7
(B8.28) benzfendizone CAS 158755-95-4
(B9) represents active herbicidal ingredients from the group of the following pyrimidines:
(B9.1) bispyrac-sodium CAS 125401-92-5
(B9.2) bromacil CAS 314-40-9; CAS 53404- 19-6; CAS 69484- 12-4
(B9.3) butafenacil CAS 134605-64-4
(B9.4) lenacil CAS 2164-08-1
(B9.5) pyribenzoxim CAS 168088-61-7
(B9.6) pyriftalid CAS 135186-78-6
(B9.7) pyriminobac CAS 136191-56-5; CAS 136191-64-5
(B9.8) pyrimisulfan CAS 221205-90-9
(B9.9) pyrithiobac-sodium CAS 123342-93-8; CAS 123343-16-8
(B9.10) saflufenacil CAS 372137-35-4
(B9.ll) terbacil CAS 5902-51-2
(B9.12) tiafenacil CAS 1220411-29-9
(B9.13) trifludimoxazin CAS 1258836-72-4
(B9.14) ethyl [3-[2-chloro-4-fluoro- CAS 353292-31-6
5-(l-methyl-6- trifluoromethyl-2,4-dioxo-
1, 2,3,4-
tetrahydropyrimidin-3- yl)phenoxy]-2- pyridyloxy]acetate
(B10) represents active herbicidal ingredients from the group of the following (thio)ureas:
(Bl 0.1) chlorobromuron CAS 13360-45-7
(Bl 0.2) chlorotoluron CAS 15545-48-9
(Bl 0.3) daimuron CAS 42609-52-9
(Bl 0.4) dimefuron CAS 34205-21-5
(Bl 0.5) diuron CAS 330-54-1
(Bl 0.6) diflufenzopyr CAS 1957168-02-3
(Bl 0.7) fluometuron CAS 2164-17-2
(Bl 0.8) isoproturon CAS 34123-59-6
(Bl 0.9) linuron CAS 330-55-2
(Bl 0.10) methabenzthiazuron CAS 18691-97-9
(B10.l l) metobromuron CAS 3060-89-7
(Bl 0.12) metoxuron CAS 19937-59-8
(Bl 0.13) monolinuron CAS 1746-81-2
(B10.14) neburon CAS 555-37-3
(Bl 0.15) siduron CAS 1982-49-6
(Bl 0.16) tebuthiuron CAS 34014-18-1
(Bl 0.17) fenuron CAS 101-42-8
(Bl 0.18) chloroxuron CAS 1982-47-4
(Bl 0.1 ) diflufenzopyr CAS 1957168-02-3; CAS 109293-98-3
(Bl 0.20) ethidimuron CAS 30043-49-3
(Bl 1) represents active herbicidal ingredients from the group of the following triazines:
(Bl 1.1) ametryne CAS 834-12-8
(Bl 1.2) atrazine CAS 1912-24-9
(Bl 1.3) cyanazine CAS 21725-46-2
(B 11.4) dimethametryn CAS 22936-75-0
(B 11.5) hexazinone CAS 51235-04-2
(B 11.6) indaziflam CAS 950782-86-2
(B 11.7) metamitron CAS 41394-05-2
(B 11.8) metribuzin CAS 1087-64-9
(B 11.9) prometon CAS 1610-18-0
(B 11.10) prometryne CAS 7287-19-6
(B 11.11 ) propazine CAS 139-40-2
(Bl 1.12) simazine CAS 122-34-9
(Bl 1.13) simetryne CAS 1014-70-6
(B 11.14) terbumeton CAS 33693-04-8
(B 11.15) terbuthylazine CAS 5915-41-3
(B 11.16) terbutryne CAS 886-50-0
(Bl 1.17) triaziflam CAS 131475-57-5
(Bl 1.18) trietazine CAS 1912-26-1
(B 11.19) desmetryne CAS 1014-69-3
[00121] Some herbicides (B) are believed to be particularly good combination partners. The preferred, particularly preferred, and most preferred herbicides (B) are listed below as further embodiments of the present invention.
[00122] Preferred components from B 1 are the herbicidal active ingredients are bicyclopyrone, clethodim, mesotrione, pinoxaden, sethoxydim, sulcotrione, tembotrione, and tralkoxydim. Particularly preferred components from Bl are the herbicidal active ingredients bicyclopyrone, clethodim, mesotrione, pinoxaden, and tembotrione. Most preferred from group Bl are bicyclopyrone, clethodim, mesotrione, and tembotrione.
[00123] Preferred components from B2 are the herbicidal active ingredients acetochlor, asularn, beflubutamid, chlorimuron, chlorsulfuron, cloransulam, diclosulam, diflufenican, dimethenamid, ethoxysulfuron, flazasulfuron, florasulam, flucarbazone, flufenacet, flumetsulam, flupyrsulfuron, foramsulfuron, iodosulfuron, mesosulfuron, metolachlor, metosulam, metsulfuron, nicosulfuron, penoxsulam, picolinafen, propoxycarbazone, prosulfocarb, prosulfuron, pyroxsulam, rimsulfiiron, S-metolachlor, sulfometuron, sulfosulfuron, thiencarbazone, thifensulfuron, tribenuron, esprocarb, tri-allate, methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5-methoxy-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2 -carboxylate, methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5- vinyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2-carboxylate, methyl rel-(2R,4R)-4- [[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2-
carboxylate, isopropyl rel-(2R,4R)-4-[[3-(3-fluorophenyl)-5-methyl-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2 -carboxylate, methyl (3R)-3-[[(5S)-3-(3,5-difluorophenyl)-5- vinyl-4H-isoxazole-5-carbonyl]amino]-2,3-dihydrofuran-5-carboxylate, methyl (3R)-3-[[(5R)-3- (3 , 5 -difluorophenyl)-5 -methyl-4H-isoxazole-5 -carbonyl]amino] -2, 3 -dihydrofuran-5 -carboxylate, methyl (lS,4R)-4-[[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-l,2-oxazol-5- yl]carbonyl]amino]cyclopent-2-ene-l -carboxylate, ethyl (lS,4R)-4-[[[3-(3,5-difluorophenyl)-5- methoxy-4H-l,2-oxazol-5-yl]carbonyl]amino]cyclopent-2-ene-l -carboxylate, 2-methoxyethyl (lS,4R)-4-[[[(5R)-3-(3-cyano-5-fluorophenyl)-5-(trifluoromethyl)-4H-l,2-oxazol-5- yl]carbonyl]amino]cyclopent-2-ene-l -carboxylate, methyl (4S)-4-[[[3-(3,5-difluorophenyl)-5- methyl-4H-l,2-oxazol-5-yl]carbonyl]amino]cyclopentene-l -carboxylate, methyl (3S)-3-[[[(5R)- 3-(3,5-difluorophenyl)-5-methyl-4H-l,2-oxazol-5-yl]carbonyl]amino]cyclopentene-l- carboxylate, 3 -(3 ,5-difluorophenyl)-N-[(l R,4S)-4-(oxazinan-2-ylcarbonyl)cyclopent-2-en- 1 -yl] - 5-(trifluoromethyl)-4H-l,2-oxazole-5-carboxamide, 3-(3,5-difluorophenyl)-N-[(lR,4S)-4- [(propylsulfonylamino)carbonyl]cyclopent-2-en-l-yl]-5-(trifluoromethyl)-4H-l,2-oxazole-5- carboxamide, and (1 S,4R)-4-[[(5R)-3-(3,5-difluorophenyl)-5-methyl-4H-isoxazole-5- carbonyl]amino]cyclopent-2-ene-l -carboxylic acid. Particularly preferred components from B2 are the herbicidal active ingredients acetochlor, diflufenican, dimethenamid, flazasulfuron, flufenacet, foramsulfuron, iodosulfuron, mesosulfuron, metolachlor, nicosulfuron, rimsulfuron, S- metolachlor, thiencarbazone, methyl rel-(2R,4R)-4-[[3-(3,5-dichlorophenyl)-5-methoxy-4H- isoxazole-5-carbonyl]amino]tetrahydrofuran-2-carboxylate, methyl rel-(2R,4R)-4-[[3-(3,5- dichlorophenyl)-5-vinyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2 -carboxylate, methyl rel-(2R,4R)-4-[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-isoxazole-5- carbonyl]amino]tetrahydrofuran-2 -carboxylate, isopropyl rel-(2R,4R)-4-[[3-(3-fluorophenyl)-5- methyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2-carboxylate, methyl (3R)-3-[[(5S)-3- (3 , 5 -difluorophenyl)-5 -vinyl-4H-isoxazole-5 -carbonyl]amino] -2, 3 -dihydrofuran-5 -carboxylate, methyl (3R)-3 - [ [(5R)-3-(3 ,5 -difluorophenyl)-5-methyl-4H-isoxazole-5-carbonyl]amino]-2,3 - dihydrofuran-5-carboxylate, methyl (lS,4R)-4-[[[(5S)-3-(3,5-difluorophenyl)-5-vinyl-4H-l,2- oxazol-5-yl]carbonyl]amino]cyclopent-2-ene-l -carboxylate, ethyl (lS,4R)-4-[[[3-(3,5- difluorophenyl)-5-methoxy-4H- 1 ,2-oxazol-5-yl]carbonyl]amino]cyclopent-2-ene- 1 -carboxylate, 2-methoxyethyl (lS,4R)-4-[[[(5R)-3-(3-cyano-5-fhiorophenyl)-5-(trifluoromethyl)-4H-l,2-
oxazol-5 -y 1] carbonyl] amino]cyclopent-2-ene- 1 -carboxylate, methyl (4S)-4- [ [ [3 -(3 , 5 - difluorophenyl)-5-methyl-4H-l,2-oxazol-5-yl]carbonyl]amino]cyclopentene-l-carboxylate, methyl (3S)-3-[[[(5R)-3-(3,5-difhiorophenyl)-5-methyl-4H-l,2-oxazol-5- yl]carbonyl]amino]cyclopentene-l -carboxylate, 3-(3,5-difluorophenyl)-N-[(lR,4S)-4-(oxazinan-
2-ylcarbonyl)cyclopent-2-en-l-yl]-5-(trifluoromethyl)-4H-l,2-oxazole-5-carboxamide, and 3- (3 , 5 -difluorophenyl)-N - [( 1 R,4S)-4- [(propylsulfonylamino)carbonyl] cyclopent-2-en- 1 -yl] -5 - (trifluoromethyl) 4H-l,2-oxazole-5-carboxamide. The most preferred components from B2 acetochlor, diflufenican, dimethenamid, flazasulfuron, flufenacet, foramsulfiiron, iodosulfuron, mesosulfuron, rimsulfuron, S-metolachlor, thiencarbazone, and methyl rel-(2R,4R)-4-[[(5S)-3- (3, 5-difluorophenyl)-5-vinyl-4H-isoxazole-5-carbonyl]amino]tetrahydrofuran-2 -carboxylate.
[00124] Preferred components from group B3 are the herbicidal active ingredients bromoxynil and ioxynil. The most preferred component from group B3 is bromoxynil.
[00125] Preferred components from group B4 are the herbicidal active ingredients amitrole, carfentrazone, imazamethabenz, imazamox, imazapic, imazapyr, imazethapyr, isoxaben, isoxaflutole, oxadiazon, pyraflufen, pyrasulfotole, pyroxasulfone, sulfentrazone, tolpyralate, topramezone, flupoxam. Particularly preferred components from group B4 are carfentrazone, imazamox, imazapyr, imazethapyr, isoxaflutole, oxadiazon, pyraflufen, pyrasulfotole, pyroxasulfone, sulfentrazone, and tolpyralate. The most preferred components from group B4 are imazamox, imazethapyr, isoxaflutole, oxadiazon, pyraflufen-ethyl, pyroxasulfone, and sulfentrazone.
[00126] Preferred components from group B5 are the herbicidal active ingredients aminocyclopyrachlor, aminopyralid, benazolin, bentazone, bixlozone, cinidon, cinmethylin, clomazone, ethofiimesate, flamprop, florpyrauxifen, flumiclorac, flumioxazin, fluridone, flurochloridone, flurtamone, fluthiacet-methyl, halauxifen, indanofan, paraquat, pelargonic acid, pendimethalin, triafamone, trifluralin, 4-amino-3-chloro-5-fluoro-6-(7-fluoro-lH-indol-6- yl)pyridine-2-carboxylic acid, cyclopyrimorate, diquat, oxaziclomefone, and 4-hydroxy-l -methyl -
3-[4-(trifluoromethyl)-2-pyridinyl]-2-imidazolidinone. Particularly preferred components from group B5 are the herbicidal active ingredients bentazone, florpyrauxifen, flumiclorac, flumioxazin, fluthiacet-methyl, halauxifen, paraquat, pelargonic acid, and pendimethalin. The most preferred
components from group B5 are bentazone, clomazone, florpyrauxifen, flumiclorac, flumioxazin, and paraquat.
[00127] Preferred components from group B6 are the herbicidal active ingredients clopyralid, dicamba, fhiroxypyr, and picloram. Particularly preferred components from group B6 are the herbicidal active ingredient dicamba and dicamba salts. A most preferred component from group B6 is dicamba.
[00128] Preferred components from group B7 are the herbicidal active ingredients bialaphos, glufosinate, glyphosate, and sulfosate. Particularly preferred components from group B7 are the herbicidal active ingredients glufosinate, glyphosate, and sulfosate. The most preferred components from group B7 are glufosinate and glyphosate.
[00129] Preferred components from group B8 are the herbicidal active ingredients 2,4-D, 2,4- DB, 2,4-DP, acifluorfen, aclonifen, clodinafop, diclofop, fenoxaprop, fomesafen, lactofen, MCPA, mecoprop, oxyfluorfen, quizalofop, and quizalofop-P. Particularly preferred components from group B8 are the herbicidal active ingredients 2,4-D, 2,4-DB, 2,4-DP, acifluorfen, aclonifen, fomesafen, lactofen, and oxyfluorfen. The most preferred components from group B8 are 2,4-D, aclonifen, fomesafen, and oxyfluorfen.
[00130] Preferred components from group B9 are the herbicidal active ingredients butafenacil, safhifenacil, terbacil, tiafenacil, trifludimoxazin, and ethyl [3-[2-chloro-4-fluoro-5-(l-methyl-6- trifhioromethyl-2,4-dioxo-l,2,3,4-tetrahydropyrimidin-3-yl)phenoxy]-2-pyridyloxy]acetate.
Particularly preferred components from group B9 are the herbicidal active ingredients butafenacil, safhifenacil, tiafenacil, trifludimoxazin, and ethyl[3-[2-chloro-4-fluoro-5-(l-methyl-6-trifluoromethyl- 2,4-dioxo-l,2,3,4-tetrahydropyrimidin-3-yl)phenoxy]-2-pyridyl-oxy]acetate. The most preferred components from group B9 are butafenacil, saflufenacil, and trifludimoxazin.
[00131] Preferred components from group B10 are the herbicidal active ingredients chlorobromuron, chlorotoluron, diuron, isoproturon, linuron, methabenzthiazuron, metobromuron, metoxuron, and monolinuron. Particularly preferred components from group B10 are the herbicidal active ingredients diuron and isoproturon. The most preferred component from group B10 is diuron.
[00132] Preferred components from group Bl 1 are the herbicidal active ingredients ametryne, atrazine, hexazinone, indaziflam, metribuzin, simazine, terbuthylazine, and terbutryne.
Particularly preferred components from group Bl 1 are the herbicidal active ingredients atrazine, indaziflam, metribuzin, and terbuthylazine. The most preferred components from group Bl 1 are atrazine, indaziflam, metribuzin, and terbuthylazine.
[00133] The herbicide combinations according to the invention may contain other components, e.g. plant growth regulators. Examples of plant growth regulators as possible mixing partners are: abscisic acid and related analogs [e.g. (2Z,4E)-5-[6-Ethynyl-l -hydroxy-2, 6-dimethyl-4- oxocyclohex-2-en- 1 -yl]-3-methylpenta-2,4-dienoic acid, methyl-(2Z,4E)-5-[6-ethynyl- 1 - hydroxy-2, 6-dimethyl-4-oxocyclohex-2-en- 1 -yl] -3 -methylpenta-2,4-dienoate, (2Z,4E)-3 -ethyl-5- (1 -hydroxy-2, 6, 6-trimethyl-4-oxocyclohex-2-en- 1 -yl)penta-2,4-dienoic acid, (2E,4E)-5-( 1 - hydroxy-2, 6, 6-frimethyl-4-oxocyclohex-2-en-l-yl)-3-(trifluoromethyl)penta-2,4-dienoic acid, methyl (2E,4E)-5-(l -hydroxy-2, 6, 6-trimethyl-4-oxocyclohex-2-en- 1 -yl)-3 -
(trifluoromethyl)penta-2,4-dienoate, (2Z,4E)-5-(2 -hydroxy-1, 3-dimethyl-5- oxobicyclo[4.1.0]hept-3-en-2-yl)-3-methylpenta-2,4-dienoic acid], acibenzolar, acibenzolar-S- methyl, S-adenosylhomocysteine, allantoin, 2-Aminoethoxyvinylglycine (AVG), aminooxyacetic acid and related esters [e.g. (Isopropylidene)-aminooxyacetic acid-2-(methoxy)-2-oxoethylester, (Isopropylidene)-aminooxyacetic acid-2-(hexyloxy)-2-oxoethylester, (Cyclohexylidene)- aminooxyacetic acid-2-(isopropyloxy)-2-oxoethylester], 1 -aminocycloprop- 1-yl carboxylic acid and derivatives thereof, e.g. disclosed in DE3335514, EP30287, DE2906507 or US5123951, 5- aminolevulinic acid, ancymidol, 6-benzylaminopurine, bikinin, brassinolide, brassinolide-ethyl, L-canaline, catechin and catechines (e.g. (2S,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H- chromen-3,5,7-triol), chitooligosaccharides (CO; COs differ from LCOs in that they lack the pendant fatty acid chain that is characteristic of LCOs. COs, sometimes referred to as N-acetylchitooligosaccharides, are also composed of GlcNAc residues but have side chain decorations that make them different from chitin molecules [(CsHi3NO5)n, CAS No. 1398-61-4] and chitosan molecules [(CsHnNO^n, CAS No. 9012-76-4]), chitinous compounds, chlormequat chloride, cloprop, cyclanilide, 3-(Cycloprop-l-enyl)propionic acid, l-[2-(4-cyano-3,5- dicyclopropylphenyl)acetamido]cyclohexanecarboxylic acid, 1 -[2-(4-cyano-3- cyclopropylphenyl)acetamido] cyclohexanecarboxylic acid, daminozide, dazomet, dazomet- sodium, n-decanol, dikegulac, dikegulac-sodium, endothal, endothal-dipotassium, -disodium, and mono(N,N-dimethylalkylammonium), ethephon, flumetralin, flurenol, flurenol-butyl, flurenol-
methyl, flurprimidol, forchlorfenuron, gibberellic acid, inabenfide, indol-3 -acetic acid (IAA), 4- indol-3-ylbutyric acid, isoprothiolane, probenazole, jasmonic acid, Jasmonic acid or derivatives thereof (e.g. jasmonic acid methyl ester, jasmonic acid ethyl ester), lipo-chitooligosaccharides (LCO, sometimes referred to as symbiotic nodulation (Nod) signals (or Nod factors) or as Myc factors, consist of an oligosaccharide backbone of 0-1,4-linked JV-acetyl-D-glucosamine (“GlcNAc”) residues with an N-linked fatty acyl chain condensed at the non-reducing end. As understood in the art, LCOs differ in the number of GlcNAc residues in the backbone, in the length and degree of saturation of the fatty acyl chain and in the substitutions of reducing and non-reducing sugar residues), linoleic acid or derivatives thereof, linolenic acid or derivatives thereof, maleic hydrazide, mepiquat chloride, mepiquat pentaborate, 1 -methylcyclopropene, 3- methylcyclopropene, 1 -ethylcyclopropene, 1-n-propylcyclopropene, 1 -cyclopropenylmethanol, methoxyvinylglycin (MVG), 3’-methyl abscisic acid, l-(4-methylphenyl)-N-(2-oxo-l-propyl- 1 ,2,3,4-tetrahydroquinolin-6-yl)methanesulfonamide and related substituted tetrahydroquinolin-6- yl)methanesulfonamides, (3E,3aR,8bS)-3-({[(2R)-4-Methyl-5-oxo-2,5-dihydrofuran-2- yl]oxy}methylen)-3,3a,4,8b-tetrahydro-2H-indeno[l,2-b]furan-2-one and related lactones as outlined in EP2248421, 2-(l-naphthyl)acetamide, 1 -naphthylacetic acid, 2- naphthyloxyacetic acid, nitrophenolate-mixture, 4-oxo-4[(2-phenylethyl)amino]butyric acid, paclobutrazol, 4- phenylbutyric acid and its related salts (e.g., sodium-4-phenylbutanoate, potassium-4- phenylbutanoate), phenylalanine, N-phenylphthalamic acid, prohexadione, prohexadione- calcium, putrescine, prohydrojasmon, rhizobitoxin, salicylic acid, salicylic acid methyl ester, sarcosine, sodium cycloprop- 1-en-l-yl acetate, sodium cycloprop-2-en-l-yl acetate, sodium-3- (cycloprop-2-en-l-yl)propanoate, sodium-3 -(cycloprop- 1-en-l-yl) propanoate, sidefimgin, spermidine, spermine, strigolactone, tecnazene, thidiazuron, triacontanol, trinexapac, trinexapac- ethyl, tryptophan, tsitodef, uniconazole, uniconazole-P, and 2-fluoro-N-(3-methoxyphenyl)-9/7- purin-6-amine.
[00134] Active compounds which can be employed in combination with the compounds of the general formula (I) according to the present disclosure in compositions according to the present disclosure (for example in mixed formulations or in the tank mix) are, for example, the following safeners:
SI) Compounds from the group of heterocyclic carboxylic acid derivatives of formula SI :
wherein symbols and indices are defined as follows: nA is an integer value in the range of 0 to 5, preferably 0 to 3;
WA is an unsubstituted or substituted divalent heterocyclic moiety selected from the group of partially unsaturated or aromatic five-membered heterocycles carrying 1 to 3 hetero ring atoms selected from the group of nitrogen (N) und oxygen (O), and carrying at least one N-atom and not more than one O-atom in the ring, preferably a five-membered heterocyclic moiety selected from the group (WA1) to (WA5); mA is 0 or 1 ;
RA2 is ORA3, SRA3 or NRA3RA4 or a saturated or unsaturated 3- to 7-membered heterocycle containing at least one N-atom and up to 3 heteroatoms, preferably combined with other heteroatoms from the group of O (oxygen) and S (sulfur), and which is linked to the carbonyl group in (SI) via a nitrogen atom, and which is unsubstituted or substituted by moieties selected from the group of (C1-C4)-alkyl, (C1-C4)-alkoxy or possibly substituted phenyl, preferably ORA3, NHRA4, or N(CH3)2, particularly ORA3
RA3 is hydrogen or an unsubstituted or substituted aliphatic hydrocarbon moiety, preferably containing 1 to 18 C-atoms;
RA4 is hydrogen, (C1-C6)-alkyl, (C1-C6)-alkoxy or substituted or unsubstituted phenyl; RA5 is hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C1-C4)-alkoxy-(C1-C8)-alkyl, cyano or COORA9, wherein RA9 is hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C1-C4)- alkoxy-(C1-C4)-alkyl, (C1-C6)-hydroxyalkyl, (C3-Ci2)-cycloalkyl, or tris-(C1-C4)- alkylsilyl;
RA6, RA7, RA8 are independently hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C3-C12)- cycloalkyl or substituted or unsubstituted phenyl;
Sla) Compounds of the dichlorophenylpyrazoline-3 -carboxylic acid type (Sla), preferably compounds such as l-(2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline-3- carboxylic acid, ethyl l-(2,4-dichlorophenyl)-5-(ethoxycarbonyl)-5-methyl-2-pyrazoline- 3 -carboxylate (SI -1) ("mefenpyr-diethyl"), and related compounds, as described in WO- A-91/07874;
Slb) Derivatives of dichlorophenylpyrazolecarboxylic acid (Slb), preferably compounds such as ethyl l-(2,4-dichlorophenyl)-5-methylpyrazole-3-carboxylate (Sl-2), ethyl l-(2,4- dichlorophenyl)-5-isopropylpyrazole-3-carboxylate (Sl-3), ethyl 1 -(2,4-dichlorophenyl)- 5-(l,l-dimethylethyl)pyrazole-3-carboxylate (Sl-4) and related compounds as described in EP-A-333131 and EP-A-269806;
Slc) Derivatives of 1, 5 -diphenylpyrazole-3 -carboxy lie acid (Slc), preferably compounds such as ethyl l-(2,4-dichlorophenyl)-5-phenylpyrazole-3-carboxylate (Sl-5), methyl l-(2- chlorophenyl)-5-phenylpyrazole-3 -carboxylate (SI -6) and related compounds as described, for example, in EP-A-268554;
Sld) Compounds of the triazolecarboxylic acid type (Sld), preferably compounds such as fenchlorazole(-ethyl ester), i.e. ethyl l-(2,4-dichlorophenyl)-5-trichloromethyl-lH-l,2,4- triazole-3-carboxylate (SI -7), and related compounds, as described in EP-A- 174562 and EP-A-346620;
Sle) Compounds of the 5-benzyl- or 5-phenyl-2-isoxazoline-3-carboxylic acid or of the 5,5- diphenyl-2-isoxazoline-3 -carboxylic acid type (Sle), preferably compounds such as ethyl 5-(2,4-dichlorobenzyl)-2-isoxazoline-3-carboxylate (Sl-8) or ethyl 5-phenyl-2- isoxazoline-3 -carboxylate (Sl-9) and related compounds as described in WO-A-91/08202, or 5,5-diphenyl-2-isoxazolinecarboxylic acid (SI -10) or ethyl 5,5-diphenyl-2-isoxazoline-
3-carboxylate (Sl-11) ("isoxadifen-ethyl") or n-propyl 5,5-diphenyl-2-isoxazoline-3- carboxylate (Sl-12) or ethyl 5-(4-fluorophenyl)-5-phenyl-2-isoxazoline-3-carboxylate (S 1 - 13) as described in patent application WO-A-95/07897;
Slf) Compounds of the triazolyloxy acetic acid type (Slf), preferably compounds such as methyl- { [ 1 ,5-bis(4-chloro-2-fhiorophenyl)- 1 H- 1 ,2,4-triazol-3 -yl]oxy } acetate (S 1 - 14) or {[l,5-Bis(4-chloro-2-fhiorophenyl)-lH-l,2,4-triazol-3-yl]oxy}acetic acid (Sl-15) or methyl- { [5-(4-chloro-2-fluorophenyl)- 1 -(2,4-difluorophenyl)- 1 H- 1 ,2,4-triazol-3 - yl]oxy} acetate (Sl-16) or {[5-(4-chloro-2-fluorphenyl)-l-(2,4-difluorophenyl)-lH-l,2,4- triazol-3-yl]oxy}acetic acid (Sl-17) or methyl-{[l-(4-chloro-2-fluorophenyl)-5-(2,4- difhiorophenyl)-lH-l,2,4-triazol-3-yl]oxy}acetate (Sl-18) or {[l-(4-chloro-2- fhiorophenyl)-5-(2,4-difluorophenyl)-lH-l,2,4-triazol-3-yl]oxy}acetic acid (Sl-19), as described in patent application WO 2021105101.
S2) Compounds from the group of the 8-quinolinoxy derivatives (S2):
S2a) Compounds of the 8-quinolinoxyacetic acid type (S2a), preferably 1 -methylhexyl (5- chloro-8-quinolinoxy)acetate ("cloquintocet-mexyl") (S2-1), 1,3-dimethylbut-l-yl (5- chloro-8-quinolinoxy)acetate (S2-2), 4-allyloxybutyl (5-chloro-8-quinolinoxy)acetate (S2- 3), 1 -allyloxyprop-2 -yl (5-chloro-8-quinolinoxy)acetate (S2-4), ethyl (5-chloro-8- quinolinoxy)acetate (S2-5), methyl (5-chloro-8-quinolinoxy)acetate (S2-6), allyl (5- chloro-8-quinolinoxy)acetate (S2-7), 2-(2-propylideneiminoxy)-l -ethyl (5-chloro-8- quinolinoxy)acetate (S2-8), 2-oxoprop-l-yl (5-chloro-8-quinolinoxy)acetate (S2-9) and related compounds, as described in EP-A-86750, EP-A-94349 and EP-A-191736 or EP-A- 0492 366, and also (5-chloro-8-quinolinoxy)acetic acid (S2-10), hydrates and salts thereof, for example the lithium, sodium, potassium, calcium, magnesium, aluminium, iron, ammonium, quaternary ammonium, sulfonium or phosphonium salts thereof, as described in WO-A-2002/34048;
S2b) Compounds of the (5-chloro-8-quinolinoxy)malonic acid type (S2b), preferably compounds such as diethyl (5-chloro-8-quinolinoxy)malonate, diallyl (5-chloro-8- quinolinoxy)malonate, methyl ethyl (5-chloro-8-quinolinoxy)malonate and related compounds, as described in EP-A-0 582 198.
53) Active ingredients of the dichloroacetamide type (S3), which are frequently used as preemergence safeners (soil-acting safeners), for example
"dichlormid" (N,N-diallyl-2,2-dichloroacetamide) (S3-1), "R-29148" (3-dichloroacetyl-2,2,5-trimethyl-l,3-oxazolidine) from Stauffer (S3-2), "R-28725" (3-dichloroacetyl-2,2-dimethyl-l,3-oxazolidine) from Stauffer (S3-3), "benoxacor" (4-dichloroacetyl-3,4-dihydro-3-methyl-2H-l ,4-benzoxazine) (S3-4), "PPG-1292" (N-allyl-N-[(l,3-dioxolan-2-yl)methyl]dichloroacetamide) from PPG
Industries (S3 -5),
"DKA-24" (N-allyl-N-[(allylaminocarbonyl)methyl]dichloroacetamide) from Sagro- Chem (S3-6),
"AD-67" or "MON 4660" (3-dichloroacetyl-l-oxa-3-azaspiro[4.5]decane) from Nitrokemia or Monsanto (S3 -7),
"TI-35" (1-dichloroacetylazepane) from TRI-Chemical RT (S3-8), "diclonon" (dicyclonon) or "BAS145138" or "LAB145138" (S3-9) ((RS)- 1 -dichloroacetyl-3 ,3 , 8a-trimethylperhydropyrrolo[ 1 ,2-a]pyrimidin-6-one) from
BASF, "furilazole" or "MON 13900" ((RS)-3-dichloroacetyl-5-(2-furyl)-2,2- dimethyloxazolidine) (S3-10), and the (R) isomer thereof (S3-11).
54) Compounds from the class of the acyl sulfonamides (S4):
S4a) N-acylsulfonamides of the formula (S4a) and salts thereof, as described in WO-A- 97/45016,
in which
RA1 is (C1-C6)-alkyl, (C3-C6)-cycloalkyl, where the 2 latter radicals are substituted by VA substituents from the group of halogen, (C1-C4)-alkoxy, (C1-C6)-haloalkoxy and (C1-C4)-alkylthio and, in the case of cyclic radicals, also by (C1-C4)-alkyl and (C1- C4)-haloalkyl;
RA2 is halogen, (C1-C4)-alkyl, (C1-C4)-alkoxy, CF3;
mA is 1 or 2;
VA is 0, 1, 2 or 3;
S4b) Compounds of the 4-(benzoylsulfamoyl)benzamide type of the formula (S4b) and salts thereof, as described in WO-A-99/16744,
in which
RB1, RB2are independently hydrogen, (C1-C6)-alkyl, (C3-C6)-cycloalkyl, (C3-C6)-alkenyl, (C3-C6)-alkynyl;
RB3 is halogen, (C1-C4)-alkyl, (C1-C4)-haloalkyl or (C1-C4)-alkoxy; and mB is 1 or 2, e.g. those in which
RB1 = cyclopropyl, RB2 = hydrogen, and (RB3) = 2-OMe ("cyprosulfamide," S4-1),
RB1 = cyclopropyl, RB2 = hydrogen, and (RB3) = 5-Cl-2-OMe (S4-2),
RB1 = ethyl, RB2 = hydrogen, and (RB3) = 2-OMe (S4-3),
RB1 = isopropyl, RB2 = hydrogen, and (RB3) = 5-Cl-2-OMe (S4-4) and
RB1 = isopropyl, RB2 = hydrogen, and (RB3) = 2-OMe (S4-5);
S4C) Compounds from the class of the benzoylsulfamoylphenylureas of the formula (S4C), as described in EP- A-365484,
in which
Rc1 Rc2 are independently hydrogen, (C1-C8)-alkyl, (C3-C8)-cycloalkyl, (C3-C6)- alkenyl, (C3-C6)-alkynyl,
Rc3 is halogen, (C1-C4)-alkyl, (C1-C4)-alkoxy, CF3 and me is 1 or 2; for example
1-[4-(N-2-methoxybenzoylsulfamoyl)phenyl]-3-methylurea,
1 - [4-(N-2-methoxybenzoylsulfamoyl)phenyl] -3,3 -dimethylurea,
1 - [4-(N-4, 5 -dimethylbenzoylsulfamoyl)phenyl] -3 -methylurea;
S4d) Compounds of the N-phenylsulfonylterephthalamide type of the formula (S4d) and salts thereof, which are known, for example, from CN 101838227,
in which
RD4 is halogen, (C1-C4)-alkyl, (C1-C4)-alkoxy, CF3; mD is 1 or 2;
RD5 is hydrogen, (C1-C6)-alkyl, (C3-C6)-cycloalkyl, (C2-C8)-alkenyl, (C2-C6)-alkynyl, (C5-C6)-cycloalkenyl.
55) Active ingredients from the class of the hydroxyaromatics and the aromatic-aliphatic carboxylic acid derivatives (S5), for example ethyl 3, 4, 5 -triacetoxybenzoate, 3,5-dimethoxy-4-hydroxybenzoic acid, 3,5- dihydroxybenzoic acid, 4-hydroxysalicylic acid, 4-fluorosalicylic acid, 2-hydroxycinnamic acid, 2,4-dichlorocinnamic acid, as described in WO-A-2004/084631, WO-A- 2005/015994, WO-A-2005/016001.
56) Active ingredients from the class of the l,2-dihydroquinoxalin-2-ones (S6), for example
1 -methyl-3 -(2 -thienyl)- 1 ,2-dihydroquinoxalin-2-one, 1 -methyl-3 -(2 -thienyl)- 1,2- dihydroquinoxaline-2-thione, 1 -(2-aminoethyl)-3 -(2 -thienyl)- 1 ,2-dihydroquinoxalin-2- one hydrochloride, 1 -(2-methylsulfonylaminoethyl)-3 -(2 -thienyl)- 1 ,2-dihydroquinoxalin-
2-one, as described in WO-A-2005/112630.
57) Compounds from the class of the diphenylmethoxy acetic acid derivatives (S7), e.g. methyl diphenylmethoxyacetate (CAS Reg. No. 41858-19-9) (S7-1), ethyl diphenylmethoxyacetate or diphenylmethoxyacetic acid, as described in WO-A-98/38856.
58) Compounds of the formula (S8), as described in WO-A-98/27049,
in which the symbols and indices are defined as follows:
RD1 is halogen, (C1-C4)-alkyl, (C1-C4)-haloalkyl, (C1-C4)-alkoxy, (C1-C4)-haloalkoxy,
RD2 is hydrogen or (C1-C4)-alkyl,
RD3 is hydrogen, (C1-C8)-alkyl, (C2-C4)-alkenyl, (C2-C4)-alkynyl or aryl, where each of the aforementioned carbon-containing radicals is unsubstituted or substituted by one or more, preferably up to three, identical or different radicals from the group consisting of halogen and alkoxy; or salts thereof, no is an integer from 0 to 2.
S9) Active ingredients from the class of the 3-(5-tetrazolylcarbonyl)-2-quinolones (S9), for example l,2-dihydro-4-hydroxy-l-ethyl-3-(5-tetrazolylcarbonyl)-2-quinolone (CAS Reg. No.: 219479- 18-2), 1 ,2-dihydro-4-hydroxy- 1 -methyl-3 -(5-tetrazolylcarbonyl)-2-quinolone
(CAS Reg. No. 95855-00-8), as described in WO-A- 1999/000020.
S 10) Compounds of the formulae (S 10a) or (S 10b) as described in WO-A-2007/023719 and WO-A-2007/023764
in which
RE1 is halogen, (C1-C4)-alkyl, methoxy, nitro, cyano, CF3, OCF3,
YE, ZE are independently O or S, nE is an integer from 0 to 4,
RE2 is (C1-Ci6)-alkyl, (C2-C6)-alkenyl, (C3-C6)-cycloalkyl, aryl; benzyl, halobenzyl,
RE3 is hydrogen or (Ci-C6)-alkyl.
S11) Active ingredients of the oxyimino compounds type (Si l), which are known as seeddressing agents, for example
"oxabetrinil" ((Z)-l,3-dioxolan-2-ylmethoxyimino(phenyl)acetonitrile) (SI 1-1), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage, "fluxofenim" (1 -(4-chlorophenyl)-2, 2, 2 -trifluoro- 1 -ethanone O-(l ,3-dioxolan-2- ylmethyl)oxime) (SI 1-2), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage, and
"cyometrinil" or "CGA-43089" ((Z)-cyanomethoxyimino(phenyl)acetonitrile) (SI 1-3), which is known as a seed-dressing safener for millet/sorghum against metolachlor damage.
S12) Active ingredients from the class of the isothiochromanones (SI 2), for example methyl [(3-oxo-lH-2-benzothiopyran-4(3H)-ylidene)methoxy]acetate (CAS Reg. No. 205121-04- 6) (S12-1) and related compounds from WO-A-1998/13361.
S13) One or more compounds from group (S13):
"naphthalic anhydride" (1,8-naphthalenedicarboxylic anhydride) (SI 3-1), which is known as a seed-dressing safener for maize against thiocarbamate herbicide damage,
"fenclorim" (4,6-dichloro-2-phenylpyrimidine) (SI 3 -2), which is known as a safener for pretilachlor in sown rice,
"flurazole" (benzyl 2-chloro-4-trifluoromethyl-l,3-thiazole-5-carboxylate) (S13-3), which is known as a seed-dressing safener for millet/sorghum against alachlor and metolachlor damage,
"CL 304415" (CAS Reg. No. 31541-57-8)
(4-carboxy-3,4-dihydro-2H-l-benzopyran-4-acetic acid) (SI 3 -4) from American
Cyanamid, which is known as a safener for maize against damage by imidazolinones, "MG 191" (CAS Reg. No. 96420-72-3) (2-dichloromethyl-2-methyl- 1,3 -dioxolane) (S13- 5) from Nitrokemia, which is known as a safener for maize, "MG 838" (CAS Reg. No. 133993-74-5)
(2 -propenyl l-oxa-4-azaspiro[4.5]decane-4-carbodithioate) (SI 3 -6) from Nitrokemia "disulfoton" (O,O-diethyl S-2-ethylthioethyl phosphorodithioate) (SI 3-7), "dietholate" (O,O-diethyl O-phenyl phosphorothioate) (SI 3-8),
"mephenate" (4-chlorophenyl methylcarbamate) (S13-9). ) Active ingredients which, in addition to herbicidal action against harmful plants, also have safener action on crop plants such as rice, for example
"dimepiperate" or "MY-93" (S-l -methyl 1 -phenylethylpiperidine- 1 -carbothioate), which is known as a safener for rice against damage by the herbicide molinate,
"daimuron" or "SK 23" (l-(l-methyl-l-phenylethyl)-3-p-tolylurea), which is known as a safener for rice against damage by the herbicide imazosulfuron,
"cumyluron" or "JC-940" (3-(2-chlorophenylmethyl)-l-(l-methyl-l-phenylethyl)urea, see JP-A-60087254), which is known as a safener for rice against damage by some herbicides, "methoxyphenone" or "NK 049" (3,3'-dimethyl-4-methoxybenzophenone), which is known as a safener for rice against damage by some herbicides,
“CSB" (l-bromo-4-(chloromethylsulfonyl)benzene) from Kumiai, (CAS Reg. No. 54091- 06-4), which is known as a safener against damage by some herbicides in rice. ) Compounds of the formula (S 15) or tautomers thereof
as described in WO-A-2008/131861 and WO-A-2008/131860 in which
RH1 is a (C1-C6)-haloalkyl radical and
RH2 is hydrogen or halogen and
RH3, RH4 are independently hydrogen, (C1-Ci6)-alkyl, (C2-Ci6)-alkenyl or (C2-C16)- alkynyl, where each of the 3 latter radicals is unsubstituted or substituted by one or more radicals from the group of halogen, hydroxyl, cyano, (C1-C4)-alkoxy, (C1-C4)- haloalkoxy, (C1-C4)-alkylthio, (C1-C4)-alkylamino, di[(C1-C4)-alkyl]amino, [(C1- C4)-alkoxy]carbonyl, [(C1-C4)-haloalkoxy]carbonyl, (C3-C6)-cycloalkyl which is unsubstituted or substituted, phenyl which is unsubstituted or substituted, and heterocyclyl which is unsubstituted or substituted,
or
(C3-C6)-cycloalkyl, (C4-C6)-cycloalkenyl, (C3-C6)-cycloalkyl fused on one side of the ring to a 4 to 6-membered saturated or unsaturated carbocyclic ring, or (C4-C6)- cycloalkenyl fused on one side of the ring to a 4 to 6-membered saturated or unsaturated carbocyclic ring, and where each of the 4 latter radicals is unsubstituted or substituted by one or more radicals from the group of halogen, hydroxyl, cyano, (Ci-C4)-alkyl, (C1-C4)- haloalkyl, (Ci-C4)-alkoxy, (Ci-C4)-haloalkoxy, (Ci-C4)-alkylthio, (C1-C4)- alkylamino, di[(Ci-C4)-alkyl]amino, [(Ci-C4)-alkoxy]carbonyl, [(C1-C4)- haloalkoxy] carbonyl, (C3-C6)-cycloalkyl which is unsubstituted or substituted, phenyl which is unsubstituted or substituted, and heterocyclyl which is unsubstituted or substituted, or
RH3 is (Ci-C4)-alkoxy, (C2-C4)-alkenyloxy, (C2-Ce)-alkynyloxy or (C2-C4)-haloalkoxy and
RH4 is hydrogen or (Ci-C4)-alkyl or
RH3 and RH4 together with the directly attached nitrogen atom represent a four- to eightmembered heterocyclic ring which, as well as the nitrogen atom, may also contain further ring heteroatoms, preferably up to two further ring heteroatoms from the group of N, O, and S, and which is unsubstituted or substituted by one or more radicals from the group of halogen, cyano, nitro, (Ci-C4)-alkyl, (Ci-C4)-haloalkyl, (Ci-C4)-alkoxy, (C1-C4)- haloalkoxy and (Ci-C4)-alkylthio.
SI 6) Active ingredients which are used primarily as herbicides but also have safener action on crop plants, for example (2,4-dichlorophenoxy)acetic acid (2,4-D), (4-chlorophenoxy)acetic acid, (R,S)-2-(4-chloro-o-tolyloxy)propionic acid (mecoprop), 4-(2,4-dichlorophenoxy)butyric acid (2,4-DB), (4-chloro-o-tolyloxy)acetic acid (MCPA), 4-(4-chloro-o-tolyloxy)butyric acid,
4-(4-chlorophenoxy)butyric acid,
3,6-dichloro-2-methoxybenzoic acid (dicamba),
1 -(ethoxycarbonyl)ethyl 3,6-dichloro-2-methoxybenzoate (lactidichlor-ethyl).
[00135] Safeners that may be used in combination with the herbicidal compounds described herein include, but are not limited to, cloquintocet-mexyl, cyprosulfamide, fenchlorazole ethyl ester, isoxadifen-ethyl, mefenpyr-diethyl, fenclorim, cumyluron, S4-1, and S4-5. Preferred safeners include cloquintocet-mexyl, cyprosulfamide, isoxadifen-ethyl, and mefenpyr-diethyl.
[00136] The herbicide combinations described herein may comprise further components, for example plant growth regulators or compounds that prevent or eliminate unwanted species. Such compounds include, but are not limited to herbicides, fungicides, insecticides, acaricides, nematicides, miticides, and related substances.
[00137] Examples of plant growth regulators that may be used include, but are not limited to, acibenzolar, acibenzolar-S-methyl, 5-aminolevulinic acid, ancymidol, 6-benzylaminopurine, brassinolide, catechol, chlormequat chloride, cloprop, cyclanilide, 3 -(cycloprop- l-enyl)propionic acid, daminozide, dazomet, n-decanol, dikegulac, dikegulac-sodium, endothal, endothal- dipotassium, -disodium, and mono(N,N-dimethylalkylammonium), ethephon, flumetralin, flurenol, flurenol -butyl, flurprimidol, forchlorfenuron, gibberellic acid, inabenfide, indole-3 -acetic acid (IAA), 4-indol-3-ylbutyric acid, isoprothiolane, probenazole, jasmonic acid, jasmonic acid methyl ester, maleic hydrazide, mepiquat chloride, 1 -methylcyclopropene, 2-(l- naphthyljacetamide, 1 -naphthylacetic acid, 2-naphthyloxyacetic acid, nitrophenolate mixture, 4- oxo-4[(2-phenylethyl)amino]butyric acid, paclobutrazole, N-phenylphthalamic acid, prohexadione, prohexadione-calcium, prohydrojasmone, salicylic acid, strigolactone, tecnazene, thidiazuron, triacontanol, trinexapac, trinexapac-ethyl, tsitodef, uniconazole, and uniconazole-P.
[00138] Active compounds that may be used in combination with the compounds of the general formula (I) described herein (in, for example, mixed formulations or a tank mix) are, for example, fungicidally active compounds. The preferred fungicidally active compounds comprise at least one standard commercial active ingredient, and include, but are not limited to:
1) Ergosterol biosynthesis inhibitors, for example (1.001) cyproconazole, (1.002) difenoconazole, (1.003) epoxiconazole, (1.004) fenhexamid, (1.005) fenpropidin, (1.006) fenpropimorph, (1.007) fenpyrazamine, (1.008) fhiquinconazole, (1.009) flutriafol, (1.010)
imazalil, (1.011) imazalil sulfate, (1.012) ipconazole, (1.013) metconazole, (1.014) myclobutanil, (1.015) paclobutrazole, (1.016) prochloraz, (1.017) propiconazole, (1.018) prothioconazole, (1.019) pyrisoxazole, (1.020) spiroxamine, (1.021) tebuconazole, (1.022) tetraconazole, (1.023) triadimenol, (1.024) tridemorph, (1.025) triticonazole, (1.026) (1 R,2S,5 S)-5-(4-chlorobenzyl)-2-(chloromethyl)-2 -methyl- 1 -(1 H- 1 ,2,4-triazol- 1 - ylmethyl)cyclopentanol, (1.027) (1 S,2R,5R)-5-(4-chlorobenzyl)-2-(chloromethyl)-2- methyl-1 -(1H-1 ,2,4-triazol-l -ylmethyl)cyclopentanol, (1.028) (2R)-2-(l - chlorocyclopropyl)-4-[( 1 R)-2,2-dichlorocyclopropyl] - 1 -( 1 H- 1 ,2,4-triazol- 1 -yl)butan-2-ol, (1.029) (2R)-2-(l-chlorocyclopropyl)-4-[(lS)-2,2-dichlorocyclopropyl]-l-(lH-l,2,4- triazol-l-yl)butan-2-ol, (1.030) (2R)-2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]- l-(lH-l,2,4-triazol-l-yl)propan-2-ol, (1.031) (2S)-2-(l-chlorocyclopropyl)-4-[(lR)-2,2- dichlorocyclopropyl]-l -(1H-1 ,2,4-triazol-l -yl)butan-2-ol, (1.032) (2S)-2-(l - chlorocyclopropyl)-4-[( 1 S)-2,2-dichlorocyclopropyl]- 1 -(1 H- 1 ,2,4-triazol- 1 -yl)butan-2-ol, (1.033) (2S)-2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]- 1 -(1H-1 ,2,4-triazol- 1 - yl)propan-2-ol, (1.034) (R)-[3-(4-chloro-2-fluorophenyl)-5-(2,4-difluorophenyl)-l ,2- oxazol-4-yl](pyridin-3-yl)methanol, (1.035) (S)-[3-(4-chloro-2-fluorophenyl)-5-(2,4- difluorophenyl)-l ,2-oxazol-4-yl](pyridin-3-yl)methanol, (1.036) [3-(4-chloro-2- fluorophenyl)-5-(2,4-difluorophenyl)-l,2-oxazol-4-yl](pyridin-3-yl)methanol, (1.037) 1- ({(2R,4S)-2-[2-chloro-4-(4-chlorophenoxy)phenyl]-4-methyl-l,3-dioxolan-2-yl}methyl)- lH-l,2,4-triazole, (1.038) l-({(2S,4S)-2-[2-chloro-4-(4-chlorophenoxy)phenyl]-4-methyl- l,3-dioxolan-2-yl}methyl)-lH-l,2,4-triazole, (1.039) l-{[3-(2-chlorophenyl)-2-(2,4- difluorophenyl)oxiran-2-yl]methyl}-lH-l,2,4-triazol-5-yl thiocyanate, (1.040) 1-
{ [rel(2R,3R)-3-(2-chlorophenyl)-2-(2,4-difluorophenyl)oxiran-2-yl]methyl} - 1 H-l ,2,4- triazol-5-yl thiocyanate, (1.041) l-{[rel(2R,3S)-3-(2-chlorophenyl)-2-(2,4- difluorophenyl)oxiran-2-yl]methyl}-lH-l,2,4-triazol-5-yl thiocyanate, (1.042) 2-
[(2R,4R,5R)-l-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-2,4-dihydro- 3H-1 ,2,4-triazole-3-thione, (1.043) 2-[(2R,4R,5S)-l -(2,4-dichlorophenyl)-5-hydroxy-
2,6,6-trimethylheptan-4-yl] -2,4-dihydro-3H- 1 ,2,4-triazole-3 -thione, ( 1.044) 2-
[(2R,4S,5R)-l-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-2,4-dihydro- 3H-1, 2, 4-triazole-3 -thione, (1.045) 2-[(2R,4S,5S)-l-(2,4-dichlorophenyl)-5-hydroxy-
2,6,6-trimethylheptan-4-yl]-2,4-dihydro-3H-l,2,4-triazole-3-thione, (1.046) 2-
[(2S,4R,5R)-l-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-2,4-dihydro- 3H-l,2,4-triazole-3-thione, (1.047) 2-[(2S,4R,5S)-l-(2,4-dichlorophenyl)-5-hydroxy-
2.6.6-trimethylheptan-4-yl]-2,4-dihydro-3H-l,2,4-triazole-3-thione, (1.048) 2-
[(2S,4S,5R)-l-(2,4-dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-2,4-dihydro- 3H-l,2,4-triazole-3-thione, (1.049) 2-[(2S,4S,5S)-l-(2,4-dichlorophenyl)-5-hydroxy-
2.6.6-trimethylheptan-4-yl] -2,4-dihydro-3H- 1 ,2,4-triazole-3 -thione, (1.050) 2-[l-(2,4- dichlorophenyl)-5-hydroxy-2,6,6-trimethylheptan-4-yl]-2,4-dihydro-3H-l,2,4-triazole-3- thione, (1.051) 2-[2-chloro-4-(2,4-dichlorophenoxy)phenyl]-l-(lH-l,2,4-triazol-l- yl)propan-2-ol, ( 1.052) 2-[2-chloro-4-(4-chlorophenoxy)phenyl] - 1 -( 1 H- 1 ,2,4-triazol- 1 - yl)butan-2-ol, ( 1.053) 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl] - 1 -( 1 H- 1 ,2,4- triazol- 1 -yl)butan-2-ol, (1.054) 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]- 1 - (lH-l,2,4-triazol-l-yl)pentan-2-ol, (1.055) 2-[4-(4-chlorophenoxy)-2-
(trifluoromethyl)phenyl] - 1 -( 1 H- 1 ,2,4-triazol- 1 -yl)propan-2-ol, (1.056) 2-{[3-(2- chlorophenyl)-2-(2,4-difluorophenyl)oxiran-2-yl]methyl}-2,4-dihydro-3H-l,2,4-triazole- 3 -thione, ( 1.057) 2- { [rel(2R,3R)-3-(2-chlorophenyl)-2-(2,4-difluorophenyl)oxiran-2- yl]methyl}-2,4-dihydro-3H-l,2,4-triazole-3-thione, (1.058) 2-{[rel(2R,3S)-3-(2- chlorophenyl)-2-(2,4-difluorophenyl)oxiran-2-yl]methyl}-2,4-dihydro-3H-l,2,4-triazole- 3 -thione, ( 1.059) 5-(4-chlorobenzyl)-2-(chloromethyl)-2-methyl- 1 -( 1 H- 1 ,2,4-triazol- 1 - ylmethyl)cyclopentanol, (1.060) 5-(allylsulfanyl)-l - { [3 -(2-chlorophenyl)-2-(2,4- difluorophenyl)oxiran-2-yl]methyl} -1 H- 1 ,2,4-triazole, ( 1.061 ) 5 -(allylsulfanyl)- 1 -
{[rel(2R,3R)-3-(2-chlorophenyl)-2-(2,4-difluorophenyl)oxiran-2-yl]methyl}-lH-l,2,4- triazole, (1.062) 5-(allylsulfanyl)-l-{[rel(2R,3S)-3-(2-chlorophenyl)-2-(2,4- difluorophenyl)oxiran-2-yl]methyl}-lH-l,2,4-triazole, (1.063) N'-(2,5-dimethyl-4-{[3- (1,1 ,2,2-tetrafluoroethoxy)phenyl]sulfanyl}phenyl)-N-ethyl-N-methylimidoformamide, (1.064) N'-(2,5-dimethyl-4-{[3-(2,2,2-trifluoroethoxy)phenyl]sulfanyl}phenyl)-N-ethyl-
N-methylimidoformamide, (1.065) N'-(2,5-dimethyl-4-{[3-(2, 2,3,3- tetrafluoropropoxy)phenyl]sulfanyl}phenyl)-N-ethyl-N-methylimidoformamide, (1.066) N'-(2,5-dimethyl-4-{[3-(pentafluoroethoxy)phenyl]sulfanyl}phenyl)-N-ethyl-N- methylimidoformamide, (1.067) N'-(2,5-dimethyl-4-{3-[(l,l,2,2-
tetrafluoroethyl)sulfanyl]phenoxy}phenyl)-N-ethyl-N-methylimidoformamide, (1.068) N'-(2,5-dimethyl-4-{3-[(2,2,2-trifluoroethyl)sulfanyl]phenoxy}phenyl)-N-ethyl-N- methylimidoformamide, (1.069) N'-(2,5-dimethyl-4- {3-[(2, 2,3,3- tetrafluoropropyl)sulfanyl]phenoxy}phenyl)-N-ethyl-N-methylimidoformamide, (1.070) N'-(2,5-dimethyl-4-{3-[(pentafluoroethyl)sulfanyl]phenoxy}phenyl)-N-ethyl-N- methylimidoformamide, (1.071) N'-(2,5-dimethyl-4-phenoxyphenyl)-N-ethyl-N- methylimidoformamide, (1.072) N'-(4-{[3-(difluoromethoxy)phenyl]sulfanyl}-2,5- dimethylphenyl)-N-ethyl-N-methylimidoformamide, (1.073) N'-(4-{3-
[(difluoromethyl)sulfanyl]phenoxy}-2,5-dimethylphenyl)-N-ethyl-N- methylimidoformamide, (1.074) N'-[5-bromo-6-(2,3-dihydro-lH-inden-2-yloxy)-2- methylpyridin-3-yl]-N-ethyl-N-methylimidoformamide, (1.075) N'-{4-[(4,5-dichloro-l,3- thiazol-2-yl)oxy]-2,5-dimethylphenyl}-N-ethyl-N-methylimidoformamide, (1.076) N'-{5- bromo-6-[(lR)-l-(3,5-difluorophenyl)ethoxy]-2-methylpyridin-3-yl}-N-ethyl-N- methylimidoformamide, (1.077) N'-{5-bromo-6-[(l S)-l -(3,5-difluorophenyl)ethoxy]-2- methylpyridin-3 -yl } -N -ethyl-N-methylimidoformamide, (1.078) N'- { 5 -bromo-6- [(cis-4- isopropylcyclohexyl)oxy]-2-methylpyridin-3-yl}-N-ethyl-N-methylimidoformamide, ( 1.079) N'- { 5 -bromo-6- [(trans-4-isopropylcy clohexyl)oxy] -2-methylpyridin-3 -yl } -N - ethyl-N-methylimidoformamide, (1.080) N'-{5-bromo-6-[l-(3,5-difluorophenyl)ethoxy]- 2-methylpyridin-3 -yl } -N -ethyl-N -methylimidoformamide, (1.081) mefentrifluconazole, (1.082) ipfentrifhiconazole.
2) Inhibitors of the respiratory chain in complex I or II, for example (2.001) benzovindiflupyr, (2.002) bixafen, (2.003) boscalid, (2.004) carboxin, (2.005) fluopyram, (2.006) flutolanil, (2.007) fluxapyroxad, (2.008) furametpyr, (2.009) isofetamid, (2.010) isopyrazam (anti-epimeric enantiomer 1R,4S,9S), (2.011) isopyrazam (anti-epimeric enantiomer 1S,4R,9R), (2.012) isopyrazam (anti-epimeric racemate 1RS,4SR,9SR), (2.013) isopyrazam (mixture of the syn-epimeric racemate 1RS,4SR,9RS and the anti- epimeric racemate 1RS,4SR,9SR), (2.014) isopyrazam (syn-epimeric enantiomer 1R,4S,9R), (2.015) isopyrazam (syn-epimeric enantiomer 1S,4R,9S), (2.016) isopyrazam (syn-epimeric racemate 1RS,4SR,9RS), (2.017) penflufen, (2.018) penthiopyrad, (2.019) pydiflumetofen, (2.020) pyraziflumid, (2.021) sedaxane, (2.022) l,3-dimethyl-N-(l,l,3-
trimethyl-2, 3-dihydro-lH-inden-4-yl)-lH-pyrazole-4-carboxamide, (2.023) 1,3-dimethyl- N- [(3R)- 1 , 1 , 3 -trimethyl-2, 3 -dihydro- 1 H-inden-4-yl] - 1 H-pyrazole-4-carboxamide, (2.024) 1 ,3 -dimethyl -N - [(3 S)- 1 , 1 ,3 -trimethyl-2, 3 -dihydro- 1 H-inden-4-yl] - 1 H-pyrazole-4- carboxamide, (2.025) 1 -methyl-3-(trifluoromethyl)-N-[2'-(trifluoromethyl)biphenyl-2-yl]- lH-pyrazole-4-carboxamide, (2.026) 2-fluoro-6-(trifluoromethyl)-N-(l,l,3-trimethyl-2,3- dihydro- 1 H-inden-4-yl)benzamide, (2.027) 3 -(difluoromethyl)- 1 -methyl-N-( 1,1,3- trimethyl -2, 3 -dihydro- 1 H-inden-4-yl)- 1 H-pyrazole-4-carboxamide, (2.028) 3 -
(difluoromethyl)- 1 -methyl-N-[(3R)- 1 , 1 ,3 -trimethyl-2, 3 -dihydro- 1 H-inden-4-yl]- 1 H- pyrazole-4-carboxamide, (2.029) 3 -(difluoromethyl)- 1 -methyl-N-[(3 S)- 1 , 1 ,3-trimethyl- 2,3 -dihydro- 1 H-inden-4-yl]- 1 H-pyrazole-4-carboxamide, (2.030) 3 -(difluoromethyl)-N- (7-fluoro- 1 , 1 ,3 -trimethyl-2, 3 -dihydro- 1 H-inden-4-yl)- 1 -methyl- 1 H-pyrazole-4- carboxamide, (2.031) 3-(difluoromethyl)-N-[(3R)-7-fluoro-l,l,3-trimethyl-2,3-dihydro- 1 H-inden-4-yl] - 1 -methyl- 1 H-pyrazole-4-carboxamide, (2.032) 3 -(difluoromethyl)-N - [(3 S)-7-fluoro- 1 , 1 ,3 -trimethyl-2, 3 -dihydro- 1 H-inden-4-yl]- 1 -methyl- 1 H-pyrazole-4- carboxamide, (2.033) 5,8-difluoro-N-[2-(2-fluoro-4-{[4-(trifluoromethyl)pyridin-2- yl]oxy}phenyl)ethyl]quinazolin-4-amine, (2.034) N-(2-cyclopentyl-5-fluorobenzyl)-N- cyclopropyl-3-(difluoromethyl)-5-fluoro-l-methyl-lH-pyrazole-4-carboxamide, (2.035) N-(2-tert-butyl-5-methylbenzyl)-N-cyclopropyl-3-(difluoromethyl)-5-fluoro-l-methyl- lH-pyrazole-4-carboxamide, (2.036) N-(2-tert-butylbenzyl)-N-cyclopropyl-3- (difluoromethyl)-5 -fluoro- 1 -methyl- lH-pyrazole-4-carboxamide, (2.037) N-(5-chloro-2- ethylbenzyl)-N-cyclopropyl-3 -(difluoromethyl)-5-fluoro- 1 -methyl- 1 H-pyrazole-4- carboxamide, (2.038) N-(5-chloro-2-isopropylbenzyl)-N-cyclopropyl-3 -(difluoromethyl)- 5-fluoro-l -methyl- lH-pyrazole-4-carboxamide, (2.039) N-[(lR,4S)-9-
(dichloromethylene)-l,2,3,4-tetrahydro-l,4-methanonaphthalen-5-yl]-3-(difluoromethyl)- 1 -methyl- lH-pyrazole-4-carboxamide, (2.040) N-[(lS,4R)-9-(dichloromethylene)- l,2,3,4-tetrahydro-l,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-l-methyl-lH- pyrazole-4-carboxamide, (2.041 ) N- [ 1 -(2,4-dichlorophenyl)- 1 -methoxypropan-2-yl] -3 - (difluoromethyl)- 1 -methyl- 1 H-pyrazole-4-carboxamide, (2.042) N-[2-chloro-6- (trifluoromethyl)benzyl] -N -cyclopropyl-3 -(difluoromethyl)-5 -fluoro- 1 -methyl- 1 H- pyrazole-4-carboxamide, (2.043) N-[3-chloro-2-fluoro-6-(trifluoromethyl)benzyl]-N-
cyclopropyl-3-(difluoromethyl)-5-fluoro-l -methyl- lH-pyrazole-4-carboxamide, (2.044) N-[5-chloro-2-(trifluoromethyl)benzyl]-N-cyclopropyl-3-(difluoromethyl)-5-fluoro-l- methyl-lH-pyrazole-4-carboxamide, (2.045) N-cyclopropyl-3-(difluoromethyl)-5-fluoro- l-methyl-N-[5-methyl-2-(trifluoromethyl)benzyl]-lH-pyrazole-4-carboxamide, (2.046) N-cyclopropyl-3-(difluoromethyl)-5-fluoro-N-(2-fluoro-6-isopropylbenzyl)-l-methyl- lH-pyrazole-4-carboxamide, (2.047) N-cyclopropyl-3-(difluoromethyl)-5-fluoro-N-(2- isopropyl-5-methylbenzyl)-l -methyl- lH-pyrazole-4-carboxamide, (2.048) N- cyclopropyl-3-(difluoromethyl)-5-fluoro-N-(2-isopropylbenzyl)-l-methyl-lH-pyrazole-4- carbothioamide, (2.049) N-cyclopropyl-3-(difluoromethyl)-5-fluoro-N-(2- isopropylbenzyl)-l -methyl- lH-pyrazole-4-carboxamide, (2.050) N-cyclopropyl-3- (difluoromethyl)-5-fluoro-N-(5-fluoro-2-isopropylbenzyl)-l-methyl-lH-pyrazole-4- carboxamide, (2.051 ) N-cyclopropyl-3-(difluoromethyl)-N-(2-ethyl-4,5-dimethylbenzyl)- 5 -fluoro- 1 -methyl- lH-pyrazole-4-carboxamide, (2.052) N-cyclopropyl-3-
(difluoromethyl)-N-(2-ethyl-5-fluorobenzyl)-5-fluoro-l-methyl-lH-pyrazole-4- carboxamide, (2.053) N-cyclopropyl-3-(difluoromethyl)-N-(2-ethyl-5-methylbenzyl)-5- fluoro-1 -methyl- lH-pyrazole-4-carboxamide, (2.054) N-cyclopropyl-N-(2-cyclopropyl-5- fluorobenzyl)-3-(difluoromethyl)-5-fluoro-l-methyl-lH-pyrazole-4-carboxamide, (2.055) N-cyclopropyl-N-(2-cyclopropyl-5-methylbenzyl)-3-(difluoromethyl)-5-fluoro-l-methyl- 1 H-pyrazole-4-carboxamide, (2.056) N -cyclopropyl-N -(2-cyclopropylbenzyl)-3 -
(difluoromethyl)-5 -fluoro- 1 -methyl- 1 H-pyrazole-4-carboxamide.
3) Respiratory chain inhibitors acting on complex III, for example (3.001) ametoctradin, (3.002) amisulbrom, (3.003) azoxystrobin, (3.004) coumethoxystrobin, (3.005) coumoxystrobin, (3.006) cyazofamid, (3.007) dimoxystrobin, (3.008) enoxastrobin, (3.009) famoxadon, (3.010) fenamidon, (3.011) flufenoxystrobin, (3.012) fluoxastrobin, (3.013) kresoxim-methyl, (3.014) metominostrobin, (3.015) oiysastrobin, (3.016) picoxystrobin, (3.017) pyraclostrobin, (3.018) pyrametostrobin, (3.019) pyraoxystrobin, (3.020) trifloxystrobin, (3.021) (2E)-2-{2-[({[(lE)-l-(3-{[(E)-l-fluoro-2- phenylvinyl]oxy } phenyl)ethylidene] amino } oxy)methyl]phenyl } -2-(methoxyimino)-N - methylacetamide, (3.022) (2E,3Z)-5- { [ 1 -(4-chlorophenyl)- 1 H-pyrazol-3 -yl]oxy } -2-
(methoxyimino)-N,3-dimethylpent-3-enamide, (3.023) (2R)-2-{2-[(2,5-
dimethylphenoxy)methyl]phenyl} -2-methoxy-N-methylacetamide, (3.024) (2S)-2- {2- [(2,5-dimethylphenoxy)methyl]phenyl} -2-methoxy-N-methylacetamide, (3.025)
(3S,6S,7R,8R)-8-benzyl-3-[({3-[(isobutyryloxy)methoxy]-4-methoxypyridin-2- yl}carbonyl)amino]-6-methyl-4,9-dioxo-l,5-dioxonan-7-yl 2-methylpropanoate, (3.026) 2-{2-[(2,5-dimethylphenoxy)methyl]phenyl}-2-methoxy-N-methylacetamide, (3.027) N- (3-ethyl-3,5,5-trimethylcyclohexyl)-3-formamido-2-hydroxybenzamide, (3.028) (2E,3Z)- 5-{[l-(4-chloro-2-fluorophenyl)-lH-pyrazol-3-yl]oxy}-2-(methoxyimino)-N,3- dimethylpent-3-enamide, (3.029) methyl {5-[3-(2,4-dimethylphenyl)-lH-pyrazol-l-yl]-2- methylbenzyl} carbamate.
4) Mitosis and cell division inhibitors, for example (4.001) carbendazim, (4.002) diethofencarb, (4.003) ethaboxam, (4.004) fluopicolid, (4.005) pencycuron, (4.006) thiabendazole, (4.007) thiophanate-methyl, (4.008) zoxamide, (4.009) 3-chloro-4-(2,6- difhiorophenyl)-6-methyl-5-phenylpyridazine, (4.010) 3-chloro-5-(4-chlorophenyl)-4- (2,6-difhiorophenyl)-6-methylpyridazine, (4.011) 3-chloro-5-(6-chloropyridin-3-yl)-6- methyl-4-(2,4,6-trifluorophenyl)pyridazine, (4.012) 4-(2-bromo-4-fluorophenyl)-N-(2,6- difluorophenyl)-l ,3-dimethyl-lH-pyrazol-5-amine, (4.013) 4-(2-bromo-4-fluorophenyl)- N-(2-bromo-6-fluorophenyl)- 1 ,3 -dimethyl- 1 H-pyrazol-5-amine, (4.014) 4-(2-bromo-4- fhiorophenyl)-N-(2-bromophenyl)-l,3-dimethyl-lH-pyrazol-5-amine, (4.015) 4-(2- bromo-4-fluorophenyl)-N-(2-chloro-6-fluorophenyl)- 1 ,3 -dimethyl- 1 H-pyrazol-5-amine, (4.016) 4-(2-bromo-4-fluorophenyl)-N-(2-chlorophenyl)-l,3-dimethyl-lH-pyrazol-5- amine, (4.017) 4-(2-bromo-4-fhiorophenyl)-N-(2-fluorophenyl)- 1 ,3 -dimethyl- 1 H- pyrazol-5-amine, (4.018) 4-(2-chloro-4-fluorophenyl)-N-(2,6-difluorophenyl)-l,3- dimethyl-lH-pyrazol-5-amine, (4.019) 4-(2-chloro-4-fluorophenyl)-N-(2-chloro-6- fluorophenyl)- 1 ,3 -dimethyl- lH-pyrazol-5 -amine, (4.020) 4-(2-chloro-4-fhiorophenyl)-N- (2-chlorophenyl)-l,3-dimethyl-lH-pyrazol-5-amine, (4.021) 4-(2-chloro-4-fluorophenyl)- N-(2-fhiorophenyl)-l,3-dimethyl-lH-pyrazol-5-amine, (4.022) 4-(4-chlorophenyl)-5-(2,6- difhiorophenyl)-3,6-dimethylpyridazine, (4.023) N-(2-bromo-6-fluorophenyl)-4-(2- chloro-4-fluorophenyl)-l,3-dimethyl-lH-pyrazol-5-amine, (4.024) N-(2-bromophenyl)-4- (2-chloro-4-fluorophenyl)-l ,3 -dimethyl- lH-pyrazol-5 -amine, (4.025) N-(4-chloro-2,6- difhiorophenyl)-4-(2-chloro-4-fhiorophenyl)-l,3-dimethyl-lH-pyrazol-5-amine.
5) Compounds with multisite activity, for example (5.001) Bordeaux mixture, (5.002) captafol, (5.003) captan, (5.004) chlorthalonil, (5.005) copper hydroxide, (5.006) copper naphthenate, (5.007) copper oxide, (5.008) copper oxychloride, (5.009) copper(2+) sulfate, (5.010) dithianon, (5.011) dodine, (5.012) folpet, (5.013) mancozeb, (5.014) maneb, (5.015) metiram, (5.016) zinc metiram, (5.017) copper oxine, (5.018) propineb, (5.019) sulfur and sulfur preparations including calcium polysulfide, (5.020) thiram, (5.021) zineb, (5.022) ziram, (5.023) 6-ethyl-5,7-dioxo-6,7-dihydro-5H- pyrrolo[3',4' : 5,6] [ 1 ,4]dithiino[2, 3 -c] [ 1 ,2]thiazole-3 -carbonitrile.
6) Compounds capable of triggering host defense, for example (6.001) acibenzolar-S- methyl, (6.002) isotianil, (6.003) probenazole, (6.004) tiadinil.
7) Amino acid and/or protein biosynthesis inhibitors, for example (7.001) cyprodinil, (7.002) kasugamycin, (7.003) kasugamycin hydrochloride hydrate, (7.004) oxytetracycline, (7.005) pyrimethanil, (7.006) 3-(5-fhioro-3,3,4,4-tetramethyl-3,4- dihydroisoquinolin- 1 -yl)quinoline.
8) ATP production inhibitors, for example (8.001) silthiofam.
9) C6ll wall synthesis inhibitors, for example (9.001) benthiavalicarb, (9.002) dimethomorph, (9.003) flumorph, (9.004) iprovalicarb, (9.005) mandipropamid, (9.006) pyrimorph, (9.007) valifenalate, (9.008) (2E)-3-(4-tert-butylphenyl)-3-(2-chloropyridin-4- yl)- 1 -(morpholin-4-yl)prop-2-en- 1 -one, (9.009) (2Z)-3 -(4-tert-butylphenyl)-3 -(2- chloropyridin-4-yl)- 1 -(morpholin-4-yl)prop-2-en- 1 -one.
10) Lipid and membrane synthesis inhibitors, for example (10.001) propamocarb, (10.002) propamocarb hydrochloride, (10.003) tolclofos-methyl.
11) Melanin biosynthesis inhibitors, for example (11.001) tricyclazole, (11.002) 2,2,2- trifluoroethyl- {3-methyl- 1 -[(4-methylbenzoyl)amino]butan-2-yl} carbamate.
12) Nucleic acid synthesis inhibitors, for example (12.001) benalaxyl, (12.002) benalaxyl-M (kiralaxyl), (12.003) metalaxyl, (12.004) metalaxyl-M (mefenoxam).
13) Signal transduction inhibitors, for example (13.001) fludioxonil, (13.002) iprodione, (13.003) procymidone, (13.004) proquinazid, (13.005) quinoxyfen, (13.006) vinclozolin.
14) Compounds that can act as decouplers, for example (14.001) fluazinam, (14.002) meptyldinocap.
15) Further compounds, for example (15.001) abscisic acid, (15.002) benthiazole,
(15.003) bethoxazin, (15.004) capsimycin, (15.005) carvone, (15.006) chinomethionat, (15.007) cufraneb, (15.008) cyfhifenamid, (15.009) cymoxanil, (15.010) cyprosulfamide, (15.011) flutianil, (15.012) fosetyl-aluminium, (15.013) fosetyl-calcium, (15.014) fosetyl- sodium, (15.015) methyl isothiocyanate, (15.016) metrafenon, (15.017) mildiomycin, (15.018) natamycin, (15.019) nickel dimethyldithiocarbamate, (15.020) nitrothal- isopropyl, (15.021) oxamocarb, (15.022) oxathiapiprolin, (15.023) oxyfenthiin, (15.024) pentachlorophenol and salts, (15.025) phosphonic acid and salts thereof, (15.026) propamocarb-fosetylate, (15.027) pyriofenone (chlazafenone), (15.028) tebufloquin, (15.029) tecloftalam, (15.030) tolnifanide, (15.031) l-(4-{4-[(5R)-5-(2,6-difhiorophenyl)- 4,5-dihydro-l,2-oxazol-3-yl]-l,3-thiazol-2-yl}piperidin-l-yl)-2-[5-methyl-3- (trifluoromethyl)- 1 H-pyrazol- 1 -yl]ethanone, ( 15.032) 1 -(4- {4- [(5 S)-5-(2,6- difhiorophenyl)-4,5-dihydro-l ,2-oxazol-3-yl]-l ,3-thiazol-2-yl}piperidin-l -yl)-2-[5- methyl-3 -(trifluoromethyl)- 1 H-pyrazol- 1 -yl]ethanone, (15.033) 2-(6-benzylpyridin-2- yl)quinazoline, (15.034) 2,6-dimethyl-lH,5H-[l,4]dithiino[2,3-c:5,6-c']dipyrrole- l,3,5,7(2H,6H)-tetrone, (15.035) 2-[3,5-bis(difhioromethyl)-lH-pyrazol-l-yl]-l-[4-(4-{5- [2-(prop-2-yn-l-yloxy)phenyl]-4,5-dihydro-l,2-oxazol-3-yl}-l,3-thiazol-2-yl)piperidin- 1-yl] ethanone, (15.036) 2-[3,5-bis(difhioromethyl)-lH-pyrazol-l-yl]-l-[4-(4-{5-[2- chloro-6-(prop-2-yn- 1 -yloxy)phenyl]-4,5-dihydro-l ,2-oxazol-3 -yl} - 1 ,3-thiazol-2- yl)piperidin-l -yl]ethanone, (15.037) 2-[3,5-bis(difluoromethyl)-lH-pyrazol- 1 -yl]-l-[4-(4- {5-[2-fluoro-6-(prop-2-yn-l-yloxy)phenyl]-4,5-dihydro-l,2-oxazol-3-yl}-l,3-thiazol-2- yl)piperidin- 1 -yl]ethanone, ( 15.038) 2- [6-(3 -fluoro-4-methoxyphenyl)-5 -methylpyridin-2- yl]quinazoline, (15.039) 2- {(5R)-3 - [2-( 1 - { [3 ,5-bis(difluoromethyl)- 1 H-pyrazol- 1 - yl]acetyl } piperidin-4-yl)- 1 , 3 -thiazol-4-yl] -4,5 -dihydro- 1 ,2-oxazol-5 -yl } -3 -chlorophenyl methanesulfonate, (15.040) 2-{(5S)-3-[2-(l -{ [3,5-bis(difluoromethyl)-lH-pyrazol-l - yl]acetyl}piperidin-4-yl)-l,3-thiazol-4-yl]-4,5-dihydro-l,2-oxazol-5-yl}-3-chlorophenyl methanesulfonate, (15.041) 2- {2-[(7, 8-difhioro-2-methylquinolin-3 -yl)oxy] -6- fhiorophenyl}propan-2-ol, (15.042) 2- {2-fluoro-6-[(8-fluoro-2-methylquinolin-3-
yl)oxy]phenyl}propan-2-ol, (15.043) 2-{3-[2-(l-{[3,5-bis(difluoromethyl)-lH-pyrazol-l- yl]acetyl } piperidin-4-yl)- 1 , 3 -thiazol-4-yl] -4,5 -dihydro- 1 ,2-oxazol-5 -yl } -3 -chlorophenyl methanesulfonate, (15.044) 2-{3-[2-(l-{[3,5-bis(difluoromethyl)-lH-pyrazol-l- yl]acetyl}piperidin-4-yl)-l,3-thiazol-4-yl]-4,5-dihydro-l,2-oxazol-5-yl}phenyl methanesulfonate, (15.045) 2-phenylphenol and salts thereof, (15.046) 3-(4,4,5-trifluoro-
3.3-dimethyl-3,4-dihydroisoquinolin-l-yl)quinoline, (15.047) 3-(4,4-difluoro-3,3- dimethyl-3,4-dihydroisoquinolin-l-yl)quinoline, (15.048) 4-amino-5-fhioropyrimidin-2- ol (tautomeric form: 4-amino-5-fhioropyrimidin-2(lH)-one), (15.049) 4-oxo-4-[(2- phenylethyl)amino]butyric acid, (15.050) 5-amino-l,3,4-thiadiazole-2-thiol, (15.051) 5- chloro-N'-phenyl-N'-(prop-2-yn-l-yl)thiophene 2-sulfonohydrazide, (15.052) 5-fluoro-2- [(4-fhiorobenzyl)oxy]pyrimidin-4-amine, (15.053) 5-fluoro-2-[(4- methylbenzyl)oxy]pyrimidin-4-amine, (15.054) 9-fluoro-2,2-dimethyl-5-(quinolin-3-yl)-
2.3-dihydro-l,4-benzoxazepine, (15.055) but-3-yn-l-yl {6-[({[(Z)-(l-methyl-lH-tetrazol-
5-yl)(phenyl)methylene]amino}oxy)methyl]pyridin-2-yl}carbamate, (15.056) ethyl (2Z)- 3 -amino-2-cyano-3 -phenylacrylate, (15.057) phenazine- 1 -carboxylic acid, (15.058) propyl 3, 4, 5 -trihydroxybenzoate, (15.059) quinolin-8-ol, (15.060) quinolin-8-ol sulfate (2:1), (15.061) tert-butyl {6-[({[(l-methyl-lH-tetrazol-5- yl)(phenyl)methylene]amino } oxy)methyl]pyridin-2-yl } carbamate, ( 15.062) 5 -fluoro-4- imino-3 -methyl- 1 )sulfonyl]-3 ,4-dihydropyrimidin-2( 1 H)-one.
[00139] Preferred fungicides are selected from the group consisting of benalaxyl, bitertanol, bromuconazole, captafol, carbendazim, carpropamid, cyazofamid, cyproconazole, diethofencarb, edifenphos, fenpropimorph, fentin acetate, fluquinconazole, fosetyl, fluoroimide, folpet, iminoctadine, iprodione, iprovalicarb, kasugamycin, maneb, nabam, pencycuron, prochloraz, propamocarb, propineb, prothioconazole, pyrimethanil, spiroxamine, quintozene, tebuconazole, tolylfluanid, triadimefon, triadimenol, trifloxystrobin, and zineb.
[00140] Active compounds which can be employed in combination with the compounds of the general formula (I) according to the present disclosure in compositions described herein (for example in mixed formulations or in the tank mix) are, for example, insecticidal, acaricidal, nematicidal, miticidal and related active ingredients are, for example (the compounds are, if possible, referred to by their common names):
(1) Acetylcholinesterase (AChE) inhibitors, preferably carbamates selected from alanycarb, aldicarb, bendiocarb, benfuracarb, butocarboxim, butoxycarboxim, carbaryl, carbofuran, carbosulfan, ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, metolcarb, oxamyl, pirimicarb, propoxur, thiodicarb, thiofanox, triazemate, trimethacarb, XMC, and xylylcarb; or organophosphates selected from acephate, azamethiphos, azinphos-ethyl, azinphos-methyl, cadusafos, chlorethoxyfos, chlorfenvinphos, chlormephos, chlorpyrifos-methyl, coumaphos, cyanophos, demeton-S- methyl, diazinon, dichlorvos/DDVP, dicrotophos, dimethoate, dimethylvinphos, disulfoton, EPN, ethion, ethoprophos, famphur, fenamiphos, fenitrothion, fenthion, fosthiazate, heptenophos, imicyafos, isofenphos, isopropyl O- (methoxyaminothiophosphoryl) salicylate, isoxathion, malathion, mecarbam, methamidophos, methidathion, mevinphos, monocrotophos, naled, omethoate, oxydemeton-methyl, parathion-methyl, phenthoate, phorate, phosalone, phosmet, phosphamidon, phoxim, pirimiphos-methyl, profenofos, propetamphos, prothiofos, pyraclofos, pyridaphenthion, quinalphos, sulfotep, tebupirimfos, temephos, terbufos, tetrachlorvinphos, thiometon, triazophos, triclorfon, and vamidothion.
(2) GABA-gated chloride channel blockers, preferably cyclodiene-organochlorines selected from chlordane and endosulfan, or phenylpyrazoles (fiproles) selected from ethiprole and fipronil.
(3) Sodium channel modulators, preferably pyrethroids selected from acrinathrin, allethrin, d-cis-trans allethrin, d-trans allethrin, bifenthrin, bioallethrin, bioallethrin S- cyclopentenyl isomer, bioresmethrin, cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda-cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, cyphenothrin [(IR)-trans isomer], deltamethrin, empenthrin [(EZ)-(IR) isomer], esfenvalerate, etofenprox, fenpropathrin, fenvalerate, flucythrinate, flumethrin, tau-fluvalinate, halfenprox, imiprothrin, kadethrin, momfluorothrin, permethrin, phenothrin [(lR)-trans isomer], prallethrin, pyrethrins (pyrethrum), resmethrin, silafluofen, tefluthrin, tetramethrin, tetramethrin [(1R) isomer], tralomethrin and transfluthrin or DDT or methoxychlor.
(4) Nicotinic acetylcholine receptor (nAChR) competitive modulators, preferably neonicotinoids selected from acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, and thiamethoxam, or nicotine, or sulfoximines such as sulfoxaflor, or butenolides such as flupyradifurone.
(5) Nicotinic acetylcholine receptor (nAChR) allosteric modulators, preferably spinosyns selected from spinetoram and spinosad.
(6) Glutamate-gated chloride channel (GluCl) allosteric modulators, preferably avermectins/milbemycins selected from abamectin, emamectin benzoate, lepimectin, and milbemectin.
(7) Juvenile hormone mimics, preferably juvenile hormone analogues selected from hydroprene, kinoprene and methoprene, or fenoxycarb or pyriproxyfen.
(8) Miscellaneous non-specific (multi-site) inhibitors, preferably alkyl halides selected from methyl bromide and other alkyl halides; or chloropicrin or sulfuryl fluoride or borax or tartar emetic or methyl isocyanate generators selected from diazomet and metam.
(9) Chordotonal organ TRPV channel modulators selected from pymetrozine and pyrifluquinazon.
(10) Mite growth inhibitors selected from clofentezine, hexythiazox, diflovidazin, and etoxazole.
(11) Microbial disruptors of insect midgut membranes selected from Bacillus thuringiensis subspecies israelensis, Bacillus sphaericus, Bacillus thuringiensis subspecies aizawai, Bacillus thuringiensis subspecies kurstaki, Bacillus thuringiensis subspecies tenebrionis, and B.t. plant proteins selected from CrylAb, CrylAc, CrylFa, CrylA.105, Cry2Ab, VIP3A, mCry3A, Cry3Ab, Cry3Bb, and Cry34Abl/35Abl.
(12) Inhibitors of mitochondrial ATP synthase, preferably ATP disruptors selected from diafenthiuron, or organotin compounds selected from azocyclotin, cyhexatin and fenbutatin oxide, or propargite or tetradifon.
(13) Uncouplers of oxidative phosphorylation via disruption of the proton gradient selected from chlorfenapyr, DNOC, and sulfluramid.
(14) Nicotinic acetylcholine receptor channel blockers selected from bensultap, cartap hydrochloride, thiocyclam, and thiosultap-sodium.
(15) Inhibitors of chitin biosynthesis, type 0, selected from bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novaluron, noviflumuron, teflubenzuron, and trifhimuron.
(16) Inhibitors of chitin biosynthesis, type 1, such as buprofezin.
(17) Molting disruptors (especially in the case of Diptera) such as cyromazine.
(18) Ecdysone receptor agonists selected from chromafenozide, halofenozide, methoxyfenozide, and tebufenozide.
(19) Octopamine receptor agonists such as amitraz.
(20) Mitochondrial complex III electron transport inhibitors selected from hydramethylnon, acequinocyl, and fluacrypyrim.
(21) Mitochondrial complex I electron transport inhibitors, preferably METI acaricides selected from fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad and tolfenpyrad, or rotenone (Derris).
(22) Voltage-dependent sodium channel blockers selected from indoxacarb and metaflumizone.
(23) Inhibitors of acetyl-CoA carboxylase, preferably tetronic and tetramic acid derivatives selected from spirodiclofen, spiromesifen, and spirotetramat.
(24) Mitochondrial complex IV electron transport inhibitors, preferably phosphines selected from aluminum phosphide, calcium phosphide, phosphine, and zinc phosphide, or cyanides selected from calcium cyanide, potassium cyanide, and sodium cyanide.
(25) Mitochondrial complex II electron transport inhibitors, preferably beta-keto nitrile derivatives selected from cyenopyrafen and cyflumetofen, or carboxanilides such as pyflubumide.
(26) Ryanodine receptor modulators, preferably diamides selected from chlorantraniliprole, cyantraniliprole, and flubendiamide.
(27) Chordotonal organ modulators (with undefined target structure) such as flonicamid.
(28) Further active ingredients selected from acynonapyr, afidopyropen, afoxolaner, azadirachtin, benclothiaz, benzoximate, benzpyrimoxan, bifenazate, broflanilide, bromopropylate, chinomethionat, chloroprallethrin, cryolite, cyclaniliprole, cycloxaprid,
cyhalodiamide, dicloromezotiaz, dicofol, epsilon metofluthrin, epsilon momfluthrin, flometoquin, fluazaindolizine, fluensulfone, flufenerim, flufenoxystrobin, flufiprole, fluhexafon, fluopyram, flupyrimin, fluralaner, fluxametamide, fufenozide, guadipyr, heptafluthrin, imidaclothiz, iprodione, kappa bifenthrin, kappa tefluthrin, lotilaner, meperfluthrin, oxazosulfyl, paichongding, pyridalyl, pyrifluquinazon, pyriminostrobin, spirobudiclofen, spiropidion, tetramethylfluthrin, tetraniliprole, tetrachlorantraniliprole, tigolaner, tioxazafen, thiofluoximate, triflumezopyrim, and iodomethane; additionally preparations based on Bacillus firmus (1-1582, BioNeem, Votivo), and the following compounds: l-{2-fluoro-4-methyl-5-[(2,2,2-trifluoroethyl)sulfinyl]phenyl}-3-
(trifluoromethyl)-lH-l,2,4-triazole-5-amine (known from WO 2006/043635) (CAS 885026-50-6), {l'-[(2E)-3-(4-chlorophenyl)prop-2-en-l-yl]-5-fluorospiro[indole-3,4'- piperidine]-l(2H)-yl}(2-chloropyridin-4-yl)methanone (known from WO 2003/106457) (CAS 637360-23-7), 2-chloro-N-[2-{l-[(2E)-3-(4-chlorophenyl)prop-2-en-l-yl]piperidin- 4-yl}-4-(trifluoromethyl) phenyl]isonicotinamide (known from WO 2006/003494) (CAS 872999-66- 1 ), 3 -(4-chloro-2,6-dimethylphenyl)-4-hydroxy-8-methoxy- 1,8- diazaspiro[4.5]dec-3-en-2-one (known from WO 2010052161) (CAS 1225292-17-0), 3- (4-chloro-2,6-dimethylphenyl)-8-methoxy-2 -oxo-1, 8-diazaspiro[4.5]dec-3-en-4-yl ethylcarbonate (known from EP 2647626) (CAS- 1440516-42-6), 4-(but-2-yn-l-yloxy)-6- (3,5-dimethylpiperidin-l-yl)-5-fhioropyrimidine (known from WO 2004/099160) (CAS 792914-58-0), PF1364 (known from JP2010/018586) (CAS Reg. No. 1204776-60-2), (3E)-3 - [ 1 - [(6-chloro-3 -pyridyl)methyl] -2-pyridylidene]- 1 ,1,1 -trifluoropropan-2-one (known from WO 2013/144213) (CAS 1461743-15-6), N-[3-(benzylcarbamoyl)-4- chlorophenyl] - 1 -methyl-3-(pentafluoroethyl)-4-(frifluoromethyl)- 1 H-pyrazole-5- carboxamide (known from WO 2010/051926) (CAS 1226889-14-0), 5-bromo-4-chloro-N- [4-chloro-2-methyl-6-(methylcarbamoyl)phenyl] -2-(3 -chloro-2-pyridyl)pyrazole-3 - carboxamide (known from CN 103232431) (CAS 1449220-44-3), 4-[5-(3,5- dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-2-methyl-N-(cis-l-oxido- 3-thietanyl)benzamide, 4-[5-(3,5-dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3- isoxazolyl]-2-methyl-N-(trans-l-oxido-3-thietanyl)benzamide and 4-[(5S)-5-(3,5- dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-2-methyl-N-(cis-l-oxido-
3-thietanyl)benzamide (known from WO 2013/050317 Al) (CAS 1332628-83-7), N-[3- chloro- 1 -(3 -pyridinyl)- 1 H-pyrazol-4-yl] -N -ethyl-3 -[(3,3,3- trifluoropropyl)sulfinyl]propanamide, (+)-N-[3-chloro-l-(3-pyridinyl)-lH-pyrazol-4-yl]- N-ethyl-3-[(3,3,3-trifluoropropyl)sulfinyl]propanamide and (-)-N-[3-chloro-l -(3- pyridinyl)- 1 H-pyrazol-4-yl] -N -ethyl-3 - [(3 , 3 ,3 -trifluoropropyl)sulfinyl]propanamide (known from WO 2013/162715 A2, WO 2013/162716 A2, US 2014/0213448 Al) (CAS 1477923-37-7), 5-[[(2E)-3-chloro-2-propen-l-yl]amino]-l-[2,6-dichloro-4-
(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-lH-pyrazole-3-carbonitrile (known from CN 101337937 A) (CAS 1105672-77-2), 3-bromo-N-[4-chloro-2-methyl-6- [(methylamino)thioxomethyl]phenyl]-l-(3-chloro-2-pyridinyl)-lH-pyrazole-5- carboxamide (Liudaibenjiaxuanan, known from CN 103109816 A) (CAS 1232543-85-9); N-[4-chloro-2-[[(l , 1 -dimethylethyl)amino] carbonyl] -6-methylphenyl]-l -(3-chloro-2- pyridinyl)-3-(fluoromethoxy)-lH-pyrazole-5-carboxamide (known from WO 2012/034403 Al) (CAS 1268277-22-0), N-[2-(5-amino-l,3,4-thiadiazol-2-yl)-4-chloro-6- methylphenyl] -3 -bromo- 1 -(3 -chloro-2-pyridinyl)- 1 H-pyrazole-5 -carboxamide (known from WO 2011/085575 Al) (CAS 1233882-22-8), 4-[3-[2,6-dichloro-4-[(3,3-dichloro-2- propen- 1 -yl)oxy]phenoxy]propoxy]-2-methoxy-6-(trifluoromethyl)pyrimidine (known from CN 101337940 A) (CAS 1108184-52-6); (2E)- and 2(Z)-2-[2-(4-cyanophenyl)-l-[3- (trifluoromethyl)phenyl] ethylidene] -N- [4- (difluoromethoxy)phenyl]hydrazinecarboxamide (known from CN 101715774 A) (CAS 1232543-85-9); cyclopropanecarboxylic acid 3-(2,2-dichloroethenyl)-2,2-dimethyl-4-(lH- benzimidazol-2-yl)phenyl ester (known from CN 103524422 A) (CAS 1542271-46-4); (4aS)-7 -chloro-2, 5 -dihydro-2- [ [(methoxycarbonyl) [4- [(trifluoromethyl)thio]phenyl]amino]carbonyl]indeno[l,2-e][l,3,4]oxadiazine-4a(3H)- carboxylic acid methyl ester (known from CN 102391261 A) (CAS 1370358-69-2); 6- deoxy-3-O-ethyl-2,4-di-O-methyl-l -[N-[4-[l -[4-(l , 1 ,2,2,2-pentafluoroethoxy)phenyl]- lH-l,2,4-triazol-3-yl]phenyl]carbamate]-a-L-mannopyranose (known from US 2014/0275503 Al) (CAS 1181213-14-8); 8-(2-cyclopropylmethoxy-4- trifluoromethylphenoxy)-3 -(6-trifluoromethylpyridazin-3 -y 1) -3 -azabicyclo[3.2.1 ]octane (CAS 1253850-56-4), (8-anti)-8-(2-cyclopropyhnethoxy-4-trifhioromethylphenoxy)-3-(6-
trifluoromethylpyridazin-3-yl)-3-azabicyclo[3.2.1]octane (CAS 933798-27-7), (8-syn)-8- (2-cyclopropylmethoxy-4-trifhioromethylphenoxy)-3-(6-trifluoromethylpyridazin-3-yl)-
3-azabicyclo[3.2.1]octane (known from WO 2007040280 Al, WO 2007040282 Al) (CAS
934001-66-8), N-[3-chloro-l-(3-pyridinyl)-lH-pyrazol-4-yl]-N-ethyl-3-[(3,3,3- trifluoropropyl)thio]propanamide (known from WO 2015/058021 Al, WO 2015/058028 Al) (CAS 1477919-27-9) and N-[4-(aminothioxomethyl)-2-methyl-6- [(methylamino)carbonyl]phenyl] -3-bromo- 1 -(3 -chloro-2-pyridinyl)- 1 H-pyrazole-5 - carboxamide (known from CN 103265527 A) (CAS 1452877-50-7), 5-(l,3-dioxan-2-yl)-
4-[[4-(trifluoromethyl)phenyl]methoxy]pyrimidine (known from WO 2013/115391 Al)
(CAS 1449021 -97-9), 3-(4-chloro-2,6-dimethylphenyl)-8-methoxy- 1 -methyl- 1 ,8- diazaspiro[4.5]decane-2, 4-dione (known from WO 2014/187846 Al) (CAS 1638765-58- 8), ethyl 3-(4-chloro-2,6-dimethylphenyl)-8-methoxy- 1 -methyl-2-oxo- 1 ,8- diazaspiro[4.5]dec-3-en-4-ylcarboxylate (known from WO 2010/066780 Al, WO 2011151146 Al) (CAS 1229023-00-0), 4-[(5S)-5-(3,5-dichloro-4-fluorophenyl)-4,5- dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[(4R)-2-ethyl-3-oxo-4-isoxazolidinyl]-2- methylbenzamide (known from WO 2011/067272, WO2013/050302) (CAS 1309959-62- 3).
[00141] Insecticides that can preferably be used together with the herbicides are, for example, as follows: acetamiprid, acrinathrin, aldicarb, amitraz, acinphos-methyl, cyfluthrin, carbaryl, cypermethrin, deltamethrin, endosulfan, ethoprophos, fenamiphos, fenthion, fipronil, imidacloprid, methamidophos, methiocarb, niclosamide, oxydemeton-methyl, prothiophos, silafluofen, thiacloprid, thiodicarb, tralomethrin, triazophos, trichlorfon, triflumuron, terbufos, fonofos, phorate, chlorpyriphos, carbofuran, and tefluthrin.
II. Recombinant DNA Molecules
[00142] The disclosure relates, in certain embodiments, to recombinant DNA molecules that encode herbicide-insensitive protoporphyrinogen oxidases (PPOs) and the proteins encoded thereby. As used herein, the term “engineered” refers to a non-natural DNA, protein, cell, or organism that would not normally be found in nature and was created by human intervention. An “engineered protein,” “engineered enzyme,” or “engineered PPO,” refers to a protein, enzyme, or PPO whose amino acid sequence was conceived of and created in the laboratory using one or more
of the techniques of biotechnology, protein design, or protein engineering, such as molecular biology, protein biochemistry, bacterial transformation, plant transformation, site-directed mutagenesis, directed evolution using random mutagenesis, genome editing, gene editing, gene cloning, DNA ligation, DNA synthesis, protein synthesis, and DNA shuffling. For example, an engineered protein may have one or more deletions, insertions, or substitutions relative to the coding sequence of the wild-type protein and each deletion, insertion, or substitution may consist of one or more amino acids. Genetic engineering can be used to create a DNA molecule encoding an engineered protein, such as an engineered PPO that is herbicide tolerant and comprises at least a first amino acid substitution relative to a wild-type PPO protein as described herein.
[00143] In one embodiment, proteins provided herein have herbicide-tolerant protoporphyrinogen oxidase activity. As used herein, “herbicide-tolerant protoporphyrinogen oxidase” means the ability of a protoporphyrinogen oxidase to maintain at least some of its protoporphyrinogen oxidase activity in the presence of one or more PPO inhibiting herbicide(s). The term “protoporphyrinogen oxidase activity” means the ability to catalyze the six-electron oxidation (removal of electrons) of protoporphyrinogen IX to form protoporphyrin IX, that is, to catalyze the dehydrogenation of protoporphyrinogen to form protoporphyrin. Enzymatic activity of a protoporphyrinogen oxidase can be measured by any means known in the art, for example, by an enzymatic assay in which the production of the product of protoporphyrinogen oxidase or the consumption of the substrate of protoporphyrinogen oxidase in the presence of one or more PPO inhibiting herbicide(s) is measured via fluorescence, high performance liquid chromatography (HPLC), or mass spectrometry (MS). Another example of an assay for measuring enzymatic activity of a protoporphyrinogen oxidase is a bacterial assay, such as the assays described herein, whereby a recombinant protoporphyrinogen oxidase is expressed in a bacterial cell otherwise lacking PPO activity and the ability of the recombinant protoporphyrinogen oxidase to complement this knockout phenotype is measured. As used herein, a "hemG knockout strain" means an organism or cell of an organism, such as E. coli, that lacks HemG activity to the extent that it is unable to grow on heme-free growth medium, or such that its growth is detectably impaired in the absence of heme relative to an otherwise isogenic strain comprising a functional HemG. A hemG knockout strain of, for instance, E. coli may be prepared in view of knowledge in the art, for instance in view of the E. coli HemG PPO sequence (Ecogene Accession No. EG11485;
Sasarman et al., "Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity ofE. coll K12" Can. J. Microbiol. 39:1155-1161, 1993).
[00144] As used herein, the term “recombinant” refers to a non-naturally occurring DNA, protein, cell, seed, or organism that is the result of genetic engineering and was created by human intervention. A “recombinant DNA molecule” is a DNA molecule comprising a DNA sequence that does not naturally occur and as such is the result of human intervention, such as a DNA molecule comprising at least two DNA molecules heterologous to each other. An example of a recombinant DNA molecule is a DNA molecule provided herein encoding an herbicide-tolerant protoporphyrinogen oxidase operably linked to a heterologous promoter. A “recombinant protein” is a protein comprising an amino acid sequence that does not naturally occur and as such is the result of human intervention, such as an engineered protein. A recombinant cell, seed, or organism is a cell, seed, or organism comprising transgenic or heterologous DNA or protein, for example a transgenic plant cell, seed, or plant comprising a DNA construct or engineered protein described herein.
[00145] As used herein, “wild-type” means a naturally occurring. A “wild-type DNA molecule,” “wild-type protein” is a naturally occurring version of a DNA molecule or protein, that is, a version of a DNA molecule or protein pre-existing in nature. A wild-type version of a DNA molecule or protein may be useful for comparison with a recombinant or engineered DNA molecule or protein. An example of a wild-type protein useful for comparison with the engineered proteins provided by the present disclosure is the PPO enzyme from E. cloacae (H_N90) provided as SEQ ID NO:l.
[00146] A “wild-type plant” is a naturally occurring plant. Such wild-type plants may also be useful for comparison with a plant comprising a recombinant or engineered DNA molecule or protein. An example of a wild-type plant useful for comparison with plants comprising a recombinant or engineered DNA molecule or protein may be a plant of the same type as the plant comprising the engineered DNA molecule or protein, such as a protein conferring an herbicide tolerance trait, and as such is genetically distinct from the plant comprising the herbicide tolerance trait.
[00147] In certain embodiments, wild-type plants may also be used or referred to as "control plants." As used herein, “control” means an experimental control designed for comparison
purposes. For example, a control plant in a transgenic plant analysis is a plant of the same type as the experimental plant (that is, the plant to be tested) but does not contain the transgenic insert, recombinant DNA molecule, or DNA construct of the experimental plant. Examples of control plants useful for comparison with transgenic plants include: for maize plants, non-transgenic LH244 maize (U.S. Patent No. 6,252,148) or non-transgenic 01DKD2 maize (U.S. Patent No. 7,166,779); for comparison with soybean plants, non-transgenic A3555 soybean (ATCC deposit number PTA-10207); for comparison with cotton plants, non-transgenic DP393 (U.S. Patent No. 6,930,228, PVP 200400266); for comparison with canola or Brassica napus plants, non-transgenic Brassica napus variety 65037 Restorer line (Canada Plant Breeders' Rights Application 06-5517); for comparison with wheat plants, non-transgenic wheat variety Samson germplasm (PVP 1994). [00148] As used herein, the term “DNA” or “DNA molecule” refers to a double-stranded DNA molecule of genomic or synthetic origin (that is, a polymer of deoxyribonucleotide bases or a polynucleotide molecule) read from the 5' (upstream) end to the 3' (downstream) end. As used herein, the term “DNA sequence” refers to the nucleotide sequence of a DNA molecule. The nomenclature used herein corresponds to that of by Title 37 of the United States Code of Federal Regulations § 1.822, and set forth in the tables in WIPO Standard ST.25 (1998), Appendix 2, Tables 1 and 3.
[00149] As used herein, the term “protein-coding DNA molecule” refers to a DNA molecule comprising a DNA sequence that encodes a protein. As used herein, the term “protein” refers to a chain of amino acids linked by peptide (amide) bonds and includes both polypeptide chains that are folded or arranged in a biologically functional way and polypeptide chains that are not. As used herein, a “protein-coding sequence” means a DNA sequence that encodes a protein. As used herein, a “sequence” means a sequential arrangement of nucleotides or amino acids. A “DNA sequence” may refer to a sequence of nucleotides or to the DNA molecule comprising of a sequence of nucleotides; a “protein sequence” may refer to a sequence of amino acids or to the protein comprising a sequence of amino acids. The boundaries of a protein-coding sequence are usually determined by a translation start codon at the 5'-terminus and a translation stop codon at the 3'-terminus.
[00150] As used herein, the term “isolated” refers to at least partially separating a molecule from other molecules typically associated with it in its natural state. In one embodiment, the term
“isolated” refers to a DNA molecule that is separated from the nucleic acids that normally flank the DNA molecule in its natural state. For example, a DNA molecule encoding a protein that is naturally present in a bacterium would be an isolated DNA molecule if it was not within the DNA of the bacterium from which the DNA molecule encoding the protein is naturally found. Thus, a DNA molecule fused to or operably linked to one or more other DNA molecule(s) with which it would not be associated in nature, for example as the result of recombinant DNA or plant transformation techniques, is considered isolated herein. Such molecules are considered isolated even when integrated into the chromosome of a host cell or present in a nucleic acid solution with other DNA molecules.
[00151] Any number of methods well known to those skilled in the art can be used to isolate and manipulate a DNA molecule, or fragment thereof, as disclosed herein. For example, polymerase chain reaction (PCR) technology can be used to amplify a particular starting DNA molecule or to produce variants of the original molecule. DNA molecules, or fragment thereof, can also be obtained by other techniques, such as by directly synthesizing the fragment by chemical means, as is commonly practiced by using an automated oligonucleotide synthesizer.
[00152] Because of the degeneracy of the genetic code, a variety of different DNA sequences can encode proteins, such as the altered or engineered proteins disclosed herein. It is well within the capability of one of skill in the art to create alternative DNA sequences encoding the same, or essentially the same, altered or engineered proteins as described herein. These variant or alternative DNA sequences are within the scope of the embodiments described herein. As used herein, references to “essentially the same” sequence refers to sequences which encode amino acid substitutions, deletions, additions, or insertions that do not materially alter the functional activity of the protein encoded by the DNA molecule of the embodiments described herein. Allelic variants of the nucleotide sequences encoding a wild-type or engineered protein are also encompassed within the scope of the embodiments described herein. Substitution of amino acids other than those specifically exemplified or naturally present in a wild-type or engineered PPO enzyme are also contemplated within the scope of the embodiments described herein, so long as the PPO enzyme having the substitution still retains substantially the same functional activity described herein.
[00153] Recombinant DNA molecules of the present disclosure may be synthesized and modified by methods known in the art, either completely or in part, where it is desirable to provide
sequences useful for DNA manipulation (such as restriction enzyme recognition sites or recombination-based cloning sites), plant-preferred sequences (such as plant-codon usage or Kozak consensus sequences), or sequences useful for DNA construct design (such as spacer or linker sequences). The present disclosure includes recombinant DNA molecules and engineered proteins having at least 50% sequence identity, at least 60% sequence identity, at least 70% sequence identity, at least 80% sequence identity, at least 85% sequence identity, at least 90% sequence identity, at least 91% sequence identity, at least 92% sequence identity, at least 93% sequence identity, at least 94% sequence identity, at least 95% sequence identity, at least 96% sequence identity, at least 97% sequence identity, at least 98% sequence identity, and at least 99% sequence identity to any of the recombinant DNA molecule or amino acid sequences provided herein, and having herbicide-tolerant protoporphyrinogen oxidase activity. As used herein, the term “percent sequence identity” or “% sequence identity” refers to the percentage of identical nucleotides or amino acids in a linear polynucleotide or amino acid sequence of a reference (“query”) sequence (or its complementary strand) as compared to a test (“subject”) sequence (or its complementary strand) when the two sequences are optimally aligned (with appropriate nucleotide or amino acid insertions, deletions, or gaps totaling less than 20 percent of the reference sequence over the window of comparison). Optimal alignment of sequences for aligning a comparison window are well known to those skilled in the art and may be conducted by tools such as the local homology algorithm of Smith and Waterman, the homology alignment algorithm of Needleman and Wunsch, the search for similarity method of Pearson and Lipman, and by computerized implementations of these algorithms such as GAP, BESTFIT, FASTA, and TFASTA available as part of the Sequence Analysis software package of the GCG® Wisconsin Package® (Accelrys Inc., San Diego, CA), MEGAlign (DNAStar Inc., 1228 S. Park St., Madison, WI 53715), and MUSCLE (version 3.6) (RC Edgar, “MUSCLE: multiple sequence alignment with high accuracy and high throughput” Nucleic Acids Research 32(5): 1792-7 (2004)) for instance with default parameters. An “identity fraction” for aligned segments of a test sequence and a reference sequence is the number of identical components that are shared by the two aligned sequences divided by the total number of components in the portion of the reference sequence segment being aligned, that is, the entire reference sequence or a smaller defined part of the reference sequence. Percent sequence identity is represented as the identity fraction multiplied by
100. The comparison of one or more sequences may be to a full-length sequence or a portion thereof, or to a longer sequence.
III. Expression Constructs
[00154] As used herein, a “DNA construct” is a recombinant DNA molecule comprising two or more heterologous DNA sequences. DNA constructs are useful for transgene expression and may be comprised in vectors and plasmids. DNA constructs may be used in vectors for transformation (that is, the introduction of heterologous DNA into a host cell) to produce recombinant bacteria or transgenic plants and cells (and as such may also be contained in the plastid DNA or genomic DNA of a transgenic plant, seed, cell, or plant part). As used herein, a “vector” means any recombinant DNA molecule that may be used for bacterial or plant transformation. DNA molecules provided by the present disclosure can, for example, be inserted into a vector as part of a DNA construct having the DNA molecule operably linked to a heterologous gene expression element that functions in a plant to affect expression of the engineered protein encoded by the DNA molecule. Methods for making and using DNA constructs and vectors are well known in the art and described in detail in, for example, handbooks and laboratory manuals including Green and Sambrook, “Molecular Cloning: A Laboratory Manual” Vol. 1, 4th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2012. The components for a DNA construct, or a vector comprising a DNA construct, include one or more gene expression elements operably linked to a transcribable nucleic acid sequence, such as the following: a promoter for the expression of an operably linked DNA, an operably linked protein-coding DNA molecule, and an operably linked 3’ untranslated region (UTR). Gene expression elements useful in practicing the present disclosure include, but are not limited to, one or more of the following type of elements: promoter, 5’ UTR, enhancer, leader, cis-acting element, intron, transit sequence, 3’ UTR, and one or more selectable marker transgenes.
[00155] The term “transgene” refers to a DNA molecule artificially incorporated into the genome of an organism as a result of human intervention, such as by plant transformation methods. As used herein, the term “transgenic” means comprising a transgene, for example a “transgenic plant” refers to a plant comprising a transgene in its genome and a “transgenic trait” refers to a characteristic or phenotype conveyed or conferred by the presence of a transgene incorporated into the plant genome. As a result of such genomic alteration, the transgenic plant is something
distinctly different from the related wild-type plant and the transgenic trait is a trait not naturally found in the wild-type plant. Transgenic plants of the present disclosure comprise the recombinant DNA molecules and proteins described herein.
[00156] As used herein, the term “heterologous” refers to the relationship between two or more things not normally associated in nature, for instance that are derived from different sources or not normally found in nature together in any other manner. For example, a DNA molecule or protein may be heterologous with respect to another DNA molecule, protein, cell, plant, seed, or organism if not normally found in nature together or in the same context. In certain embodiments, a first DNA molecule is heterologous to a second DNA molecule if the two DNA molecules are not normally found in nature together in the same context. For instance, a protein-coding recombinant DNA molecule is heterologous with respect to an operably linked promoter if such a combination is not normally found in nature. Similarly, a protein is heterologous with respect to a second operably linked protein, such as a transit peptide, if such combination is not normally found in nature. In another embodiment, a recombinant DNA molecule encoding a PPO enzyme is heterologous with respect to an operably linked promoter that is functional in a plant cell if such combination is not normally found in nature. A recombinant DNA molecule also may be heterologous with respect to a cell, seed, or organism into which it is inserted when it would not naturally occur in that cell, seed, or organism.
[00157] A “heterologous protein” is a protein present in a plant, seed, cell, tissue, or organism in which it does not naturally occur or operably linked to a protein with which it is not naturally linked. Examples of heterologous proteins are the PPO enzymes described herein that is expressed in any plant, seed, cell, tissue, or organism. Another example is a protein operably linked to a second protein, such as a transit peptide or herbicide-tolerant protein, with which it is not naturally linked, or a protein introduced into a plant cell in which it does not naturally occur using the techniques of genetic engineering.
[00158] As used herein, “operably linked” means two or more DNA molecules or two or more proteins linked in manner so that one may affect the function of the other. Operably linked DNA molecules or operably linked proteins may be part of a single contiguous molecule and may or may not be adjacent. For example, a promoter is operably linked with a protein-coding DNA
molecule in a DNA construct where the two DNA molecules are so arranged that the promoter may affect the expression of the transgene.
[00159] The DNA constructs described herein may include a promoter operably linked to a protein-coding DNA molecule provided herein, whereby the promoter drives expression of the protein. Useful promoters include those that function in a cell for expression of an operably linked DNA molecule, such as a bacterial or plant promoter. Plant promoters are varied and well known in the art and include, for instance, those that are inducible, viral, synthetic, constitutive, temporally regulated, spatially regulated, or spatio-temporally regulated.
[00160] In one embodiment, a DNA construct provided herein includes a DNA sequence encoding a transit sequence that is operably linked to a heterologous DNA sequence encoding a PPO enzyme, whereby the transit sequence facilitates localizing the protein molecule within the cell. Transit sequences are known in the art as signal sequences, targeting peptides, targeting sequences, localization sequences, and transit peptides. An example of a transit sequence is a chloroplast transit peptide (CTP), a mitochondrial transit sequence (MTS), or a dual chloroplast and mitochondrial transit peptide. By facilitating protein localization within the cell, the transit sequence may increase the accumulation of recombinant protein, protect the protein from proteolytic degradation, or enhance the level of herbicide tolerance, and thereby reduce levels of injury in the cell, seed, or organism after herbicide application. CTPs and other targeting molecules that may be used in connection with the present disclosure are well known in the art.
[00161] As used herein, “transgene expression,” “expressing a transgene,” “protein expression,” and “expressing a protein,” mean the production of a protein through the process of transcribing a DNA molecule into messenger RNA (mRNA) and translating the mRNA into polypeptide chains, which are ultimately folded into proteins. A protein-coding DNA molecule may be operably linked to a heterologous promoter in a DNA construct for use in expressing the protein in a cell transformed with the recombinant DNA molecule.
IV. Transgenic Plants
[00162] In one aspect, cells, tissues, plants, and seeds that comprising the recombinant DNA molecules or proteins are provided herein. These cells, tissues, plants, and seeds comprising the recombinant DNA molecules or proteins exhibit tolerance to one or more PPO inhibiting herbicide(s).
[00163] In the commercial production of crops, it is desirable to eliminate under reliable pesticidal management unwanted plants (z.e., "weeds") from a field of crop plants. An ideal treatment would be one which could be applied to an entire field but which would eliminate only the unwanted plants while leaving the crop plants unaffected. One such treatment system would involve the use of crop plants which are tolerant to an herbicide so that when the herbicide is sprayed on a field of herbicide-tolerant crop plants, the crop plants would continue to thrive while non-herbicide-tolerant weeds are killed or severely damaged. Ideally, such treatment systems would take advantage of varying herbicide properties so that weed control could provide the best possible combination of flexibility and economy. For example, individual herbicides have different longevities in the field, and some herbicides persist and are effective for a relatively long time after they are applied to a field while other herbicides are quickly broken down into other and/or nonactive compounds. An ideal treatment system would allow the use of different herbicides so that growers could tailor the choice of herbicides for a particular situation.
[00164] While a number of herbicide-tolerant crop plants are presently commercially available, one issue that has arisen for many commercial herbicides and herbicide/crop combinations is that individual herbicides typically have incomplete spectrum of activity against common weed species. For most individual herbicides which have been in use for some time, populations of herbicide resistant weed species and biotypes have become more prevalent (see, e.g., Tranel and Wright, Weed Science 50:700-712, 2002; Owen and Zelaya, Pest Manag. Sci. 61:301-311, 2005). Transgenic plants which are resistant to more than one herbicide have been described (see, e.g., WO 2005/012515). However, improvements in every aspect of crop production, weed control options, extension of residual weed control, and improvement in crop yield are continuously in demand.
[00165] One method of producing such cells, tissues, plants, and seeds is through plant transformation. Suitable methods for transformation of host plant cells for use with the current disclosure include any method by which DNA can be introduced into a cell (for example, where a recombinant DNA construct is stably integrated into a plant chromosome) and are well known in the art. Two effective, and widely utilized, methods for cell transformation are Agrobacterium- mediated transformation and microprojectile bombardment-mediated transformation. Microprojectile bombardment methods are illustrated, for example, in US Patent Nos. 5,550,318;
5,538,880; 6,160,208; and 6,399,861. Agrobacterium-mediated transformation methods are described, for example in US Patent No. 5,591,616. A cell with a recombinant DNA molecule or protein of the present disclosure may be selected for the presence of the recombinant DNA molecule or protein, for instance through its encoded enzymatic activity, before or after regenerating such a cell into a plant.
[00166] Another method of producing the cells, plants, and seeds of the present disclosure is through genome modification using site-specific integration or genome editing. Targeted modification of plant genomes through the use of genome editing methods can be used to create improved plant lines through modification of plant genomic DNA. As used herein “site-directed integration” refers to genome editing methods the enable targeted insertion of one or more nucleic acids of interest into a plant genome. Suitable methods for altering a wild-type DNA sequence or a preexisting transgenic sequence or for inserting DNA into a plant genome at a pre-determined chromosomal site include any method known in the art. Exemplary methods include the use of sequence specific nucleases, such as zinc-finger nucleases, engineered or native meganucleases, TALE-endonucleases, or an RNA-guided endonucleases (for example, a Clustered Regularly Interspersed Short Palindromic Repeat (CRISPR)/Cas9 system, a CRISPR/Cpfl system, a CRISPR/CasX system, a CRISPR/CasY system, a CRISPR/Cascade system). Several embodiments relate to methods of genome editing by using single-stranded oligonucleotides to introduce precise base pair modifications in a plant genome, as described by Sauer et al., Plant Physiology 170(4): 1917-1928, 2016. Methods of genome editing to modify, delete, or insert nucleic acid sequences into genomic DNA are known in the art.
[00167] In certain embodiments, the present disclosure provides modification or replacement of an existing coding sequence, such as a PPO coding sequence or another existing transgenic insert, within a plant genome with a sequence encoding a protein, such as a PPO coding sequence of the present disclosure, or an expression cassette comprising such a protein. Several embodiments relate to the use of a known genome editing methods, such as zinc-finger nucleases, engineered or native meganucleases, TALE-endonucleases, or an RNA-guided endonucleases (for example, a Clustered Regularly Interspersed Short Palindromic Repeat (CRISPR)/Cas9 system, a CRISPR/Cpfl system, a CRISPR/CasX system, a CRISPR/CasY system, a CRISPR/Cascade system).
[00168] Several embodiments may therefore relate to a recombinant DNA construct comprising an expression cassette(s) encoding a site-specific nuclease and, optionally, any associated protein(s) to carry out genome modification. These nuclease-expressing cassette(s) may be present in the same molecule or vector as a donor template for templated editing or an expression cassette comprising nucleic acid sequence encoding a PPO protein as described herein (in cis) or on a separate molecule or vector (in trans). Several methods for site-directed integration are known in the art involving different sequence-specific nucleases (or complexes of proteins or guide RNA or both) that cut the genomic DNA to produce a double strand break (DSB) or nick at a desired genomic site or locus. As understood in the art, during the process of repairing the DSB or nick introduced by the nuclease enzyme, the donor template DNA, transgene, or expression cassette may become integrated into the genome at the site of the DSB or nick. The presence of the homology arm(s) in the DNA to be integrated may promote the adoption and targeting of the insertion sequence into the plant genome during the repair process through homologous recombination, although an insertion event may occur through non-homologous end joining (NHEJ).
[00169] As used herein, the term “double-strand break inducing agent” refers to any agent that can induce a double-strand break (DSB) in a DNA molecule. In some embodiments, the doublestrand break inducing agent is a site-specific genome modification enzyme.
[00170] As used herein, the term “site-specific genome modification enzyme” refers to any enzyme that can modify a nucleotide sequence in a sequence-specific manner. In some embodiments, a site-specific genome modification enzyme modifies the genome by inducing a single-strand break. In some embodiments, a site-specific genome modification enzyme modifies the genome by inducing a double-strand break. In some embodiments, a site-specific genome modification enzyme comprises a cytidine deaminase. In some embodiments, a site-specific genome modification enzyme comprises an adenine deaminase. In the present disclosure, sitespecific genome modification enzymes include endonucleases, recombinases, transposases, deaminases, helicases and any combination thereof. In some embodiments, the site-specific genome modification enzyme is a sequence-specific nuclease.
[00171] In one aspect, the endonuclease is selected from a meganuclease, a zinc-finger nuclease (ZFN), a transcription activator-like effector nucleases (TALEN), an Argonaute (non-limiting
examples of Argonaute proteins include Thermits thermophilus Argonaute (TtAgo), Pyrococcus furiosus Argonaute (PfAgo), Natronobacterium gregoryi Argonaute (NgAgo), an RNA-guided nuclease, such as a CRISPR associated nuclease (non-limiting examples of CRISPR associated nucleases include Casl, CaslB, Cas2, Cas3, Cas4, Cas5, Cas6, Cas7, Cas8, Cas9 (also known as Csnl and Csxl2), CaslO, Csyl, Csy2, Csy3, Csel, Cse2, Cscl, Csc2, Csa5, Csn2, Csm2, Csm3, Csm4, Csm5, Csm6, Cmrl, Cmr3, Cmr4, Cmr5, Cmr6, Csbl, Csb2, Csb3, Csxl7, Csxl4, CsxlO, Csxl6, CsaX, Csx3, Csxl, Csxl5, Csfl, Csf2, Csf3, Csf4, Cpfl, CasX, CasY, homologs thereof, or modified versions thereof).
[00172] In some embodiments, the site-specific genome modification enzyme is a recombinase. Non-limiting examples of recombinases include a tyrosine recombinase attached to a DNA recognition motif and is selected from the group consisting of a Cre recombinase, a Gin recombinase, a Flp recombinase, and a Tnpl recombinase. In an aspect, a Cre recombinase or a Gin recombinase provided herein is tethered to a zinc-finger DNA-binding domain, or a TALE DNA-binding domain, or a Cas9 nuclease. In another aspect, a serine recombinase attached to a DNA recognition motif is selected from the group consisting of a PhiC31 integrase, an R4 integrase, and a TP-901 integrase. In another aspect, a DNA transposase attached to a DNA binding domain provided herein is selected from the group consisting of a TALE-piggyBac and TALE- Mutator.
[00173] Any of the DNA of interest provided herein can be integrated into a target site of a chromosome sequence by introducing the DNA of interest and the provided site-specific genome modification enzymes. Any method provided herein can utilize any site-specific genome modification enzyme provided herein.
[00174] As used herein, a “weed” is any undesired plant. A plant may be considered generally undesirable for agriculture or horticulture purposes (for example, Amaranthus species) or may be considered undesirable in a particular situation (for example, a crop plant of one species in a field of a different species, also known as a volunteer plant).
[00175] The transgenic plants, progeny, seeds, plant cells, and plant parts described herein may also contain one or more additional traits. Additional traits may be introduced by crossing a plant containing a transgene comprising the recombinant DNA molecules provided herein with another plant containing one or more additional trait(s). As used herein, “crossing” means breeding two
individual plants to produce a progeny plant. Two plants may thus be crossed to produce progeny that contain the desirable traits from each parent. As used herein “progeny” means the offspring of any generation of a parent plant, and transgenic progeny comprise a DNA construct provided herein and inherited from at least one parent plant.
[00176] Additional trait(s) also may be introduced by co-transforming a DNA construct for that additional transgenic trait(s) with a DNA construct comprising the recombinant DNA molecules provided herein (for example, with all the DNA constructs present as part of the same vector used for plant transformation) or by inserting the additional trait(s) into a transgenic plant comprising a DNA construct provided by the herein or vice versa (for example, by using any of the methods of plant transformation or genome editing on a transgenic plant or plant cell). Such additional traits include, but are not limited to, increased insect resistance, increased water use efficiency, increased yield performance, increased drought resistance, increased seed quality, improved nutritional quality, hybrid seed production, and herbicide-tolerance, in which the trait is measured with respect to a wild-type plant. Illustrative additional herbicide-tolerance traits may include transgenic or non-transgenic tolerance to one or more herbicides such as ACCase inhibitors (for example aryloxyphenoxy propionates and cyclohexanediones), ALS inhibitors (for example sulfonylureas, imidazolinones, triazolopyrimidines, and triazolinones) EPSPS inhibitors (for example glyphosate), synthetic auxins (for example phenoxys, benzoic acids, carboxylic acids, semicarbazones), photosynthesis inhibitors (for example triazines, triazinones, nitriles, benzothiadiazoles, and ureas), glutamine synthesis inhibitors (for example glufosinate), HPPD inhibitors (for example isoxazoles, pyrazolones, and triketones), PPO inhibitors (for example diphenylethers, N-phenylphthalimide, aryl triazinones, and pyrimidinediones), and long-chain fatty acid inhibitors (for example chloroacetamindes, oxyacetamides, and pyrazoles), among others. Examples of herbicide-tolerance proteins useful for producing additional herbicidetolerance traits are well known in the art and include, but are not limited to, glyphosate-tolerant 5- enolypyruvyl shikimate 3-phosphate synthases (e.g., CP4 EPSPS, 2mEPSPS), glyphosate oxidoreductases (GOX), glyphosate N-acetyltransferases (GAT), herbicide-tolerant acetolactate synthases (ALS) / acetohydroxyacid synthases (AHAS), herbicide-tolerant 4- hydroxyphenylpyruvate dioxygenases (HPPD), dicamba monooxygenases (DMO), phosphinothricin acetyl transferases (PAT), herbicide-tolerant glutamine synthetases (GS), 2,4-
dichlorophenoxyproprionate dioxygenases (TfdA), R-2,4-dichlorophenoxypropionate dioxygenases (RdpA), S-2,4-dichlorophenoxypropionate dioxygenases (SdpA), herbicide-tolerant protoporphyrinogen oxidases (PPO), and cytochrome P450 monooxygenases. Exemplary insect resistance traits may include resistance to one or more insect members within one or more of the orders of Lepidoptera, Coleoptera, Hemiptera, Thysanoptera, Diptera, Hymenoptera, and Orthoptera, among others. Such additional traits are well known to one of skill in the art; for example, and a list of such transgenic traits is provided by the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS).
[00177] Transgenic plants and progeny that are tolerant to PPO inhibiting herbicides may be used with any breeding methods that are known in the art. In plant lines comprising two or more traits, the traits may be independently segregating, linked, or a combination of both in plant lines comprising three or more transgenic traits. Backcrossing to a parental plant and out-crossing with a non-transgenic plant are also contemplated, as is vegetative propagation. Descriptions of breeding methods that are commonly used for different traits and crops are well known to those of skill in the art. To confirm the presence of the transgene(s) in a particular plant or seed, a variety of assays may be performed. Such assays include, for example, molecular biology assays, such as Southern and Northern blotting, PCR, and DNA sequencing; biochemical assays, such as detecting the presence of a protein product, for example, by immunological means (ELISAs and western blots) or by enzymatic function; plant part assays, such as leaf or root assays; and also, by analyzing the phenotype of the whole plant.
[00178] Introgression of a transgenic trait into a plant genotype is achieved as the result of the process of backcross conversion. A plant genotype into which a transgenic trait has been introgressed may be referred to as a backcross converted genotype, line, inbred, or hybrid. Similarly, a plant genotype lacking the desired transgenic trait may be referred to as an unconverted genotype, line, inbred, or hybrid.
[00179] As used herein, the term “comprising” means “including but not limited to.”
[00180] Having described the invention in detail, it will be apparent that modifications, variations, and equivalent embodiments are possible without departing the scope of the invention defined in the appended claims. Furthermore, it should be appreciated that the examples in the present disclosure are provided as non-limiting examples.
EXAMPLES
Example 1: HemG Protoporphyrinogen Oxidases and Transit Peptides
[00181] Novel microbial HemG protoporphyrinogen oxidases that are tolerant to PPO inhibitor herbicides were previously identified from microbial sequence databases using bioinformatic methods and a herbicide bacterial screening system and are provided as SEQ ID NOs:l-20 and recombinant variants of these microbial HemG protoporphyrinogen oxidases are provided as SEQ ID NOs:65-193. DNA sequences encoding microbial HemG protoporphyrinogen oxidases and their variants, along with DNA sequences that are optimized for expression in a monocot or dicot can optionally be synthesized and are provided as SEQ ID NOs:22-64 and 194-322.
[00182] At the 5’ end of the DNA sequence encoding a protoporphyrinogen oxidase, a codon for a methionine, commonly known as a start codon, may be present. Alternatively, this codon (and optionally a few amino-terminal amino acids, for example 2 to 7), can be eliminated to facilitate operable linkage of a transit peptide sequence to the 5 ’ end of the coding sequence. Novel transit peptides were previously identified by using bioinformatic methods and tools, such as hidden Markov models (HMM), the Pfam database, and basic local alignment search tool (BLAST), to identify thousands of EST and genomic sequences predicted to encode proteins known to be localized to the chloroplast and mitochondria in plant cells and are provided herein as SEQ ID NOs:323-328, 340, and 342-407, along with their corresponding nucleotide sequences, provided herein as SEQ ID NOs:329-339, 341, and 408-483.
Example 2: Herbicide Tolerance Evaluation of Crop Plants Expressing HemG PPOs
[00183] Protoporphyrinogen oxidases operably linked to transit peptides were tested in transgenic soybean, com, and cotton plants for tolerance to PPO inhibiting herbicides and for weed control in the field.
[00184] Plant transformation vectors were constructed for expressing a chloroplast transit peptide operably linked to the PPO H_N90 (SEQ ID NO:1) in transgenic soybean, inbred com, hybrid com, and cotton plants, and introduced into seed-derived explants of soybean, inbred and hybrid com, and cotton, respectively, through Agrobacterium tumefaciens-ms^iatQ^ transformation using standard methods known in the art. The regenerated Ro plants were analyzed
to select for events with a single copy insertion for advancement to Ri nursery for Ri seed production.
[00185] Seeds of the regenerated transgenic plants described above were sown in 12-cm tall plastic pots containing standard soil (14.7% sand, 19.9% clay, 65.4% silt, and 1.8% organic matter). Transgenic cotton and com seed were sown at a density of one seed per pot, whereas soybean was sown at a density of three seeds per pot. Plants were grown in a greenhouse with 60% relative humidity in a light cycle of 13 -hour day and 11 -hour night. The temperature was kept at 23°C during the day and 12°C at night.
[00186] To confirm herbicide tolerance of transgenic soybean, com, and cotton plants, the herbicidal formulations of ethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy }pyridin-2-yl)oxy]acetate, [(3 - {2-chloro-5 -[4-( 1,1- difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2- yl)oxy]acetic acid, and ethyl 2-[[3-[5-[4-(l,l-difhioroethyl)-3-methyl-2,6-dioxo-pyrimidin-l-yl]- 4-fluoro-2-nitro-phenoxy]-2-pyridyl]oxy] acetate, designated below as compound (a), compound (b), and compound (o), respectively, were applied to plants at the 2-4 leaf stage (corresponding to BBCH 12-14). Each herbicide treatment was applied at a rate of 100 ai/ha or 200 g ai/ha. Methylated rapeseed oil (Mero) was used as an adjuvant and added to the spray mix for each herbicide at 0.5% v/v. Each treatment consisted of four replicates (pots).
[00187] Plants were visually assessed for herbicide injury at the following time points: 8 days, 15 days, 22 days, and 28 days after application. Unsprayed transgenic plants were used for phenotypic comparison with unsprayed wild-type plants. Injury rating was determined as the percentage of leaf area of a plant exhibiting damage such as necrosis (brown or dead tissue), chlorosis (yellow tissue or yellow spotting), and malformation (misshapen leaves or plant structures, epinasty or twisting of stem, cupping of leaves) caused by herbicide application and was measured on a scale of 0-100, with zero being no injury and 100 being complete crop death. The data obtained at 28 days after application of compounds (a), (b), and (o) are shown in Table 1 below.
Table 1. Injury ratings after 28 days after application of PPO inhibiting herbicides in soybean, corn, and cotton.
[00188] H_N90 conferred to transgenic soybean, com, and cotton plants 100% complete tolerance to the herbicidal compounds (a), (b), and (o), 28 days after application for both application rates. Conventional (wild-type) soybean, com, and cotton plants had injury ratings of nearly 100 for each treatment, with the exception of treatment with compound (o), which caused 92% crop damage to wild-type soybean. Similar results were observed for both conventional and transgenic plants at 8 days, 15 days, and 22 days after application for both application rates (data not shown).
Example 3: Weed Control in Plant Growth Area Containing Plants Expressing HemG PPOs
[00189] Protoporphyrinogen oxidases operably linked to transit peptides are tested in transgenic soybean, com, and cotton plants for tolerance to PPO inhibiting herbicides and for weed control in the field.
[00190] Plant transformation vectors are constructed for expressing a chloroplast transit peptide operably linked to a HemG PPO enzyme, such as H_N90 (SEQ ID NO:1) in transgenic soybean, com, and cotton plants, and introduced into seed-derived explants of soybean, com, and cotton, respectively, through Agrobacterium tumefaciens-mediated transformation using standard methods known in the art. The regenerated Ro plants are analyzed to select for events with a single copy insertion for advancement to Ri nursery for Ri seed production. Homozygous Ri or later generation events are tested under field conditions to confirm their tolerance to PPO inhibitor herbicides.
[00191] To confirm herbicide tolerance of transgenic soybean plants under field conditions, herbicidal compound(s) of the general formula (I) described herein and a commercial PPO inhibitor herbicide (such as saflufenacil) are applied at emergence (VE), V3, and RI developmental stages at one of two rates. Plants are visually assessed for herbicide injury 14 days after treatment for VE, and 7 days after treatment for V3 and RI stages. Unsprayed transgenic plants are used for phenotypic comparison with unsprayed wild-type plants.
[00192] To confirm herbicide tolerance of transgenic com plants under field conditions, herbicidal compound(s) of the general formula (I) described herein and a commercial PPO inhibitor herbicide (such as fomesafen) are applied to transgenic inbred or hybrid plants at emergence (VE), V2, V6, and VT developmental stages at one of two rates. Plants are visually
assessed for herbicide injury 10-14 days after herbicide treatment. Unsprayed transgenic plants are used for phenotypic comparison with unsprayed wild-type plants.
[00193] To confirm herbicide tolerance of transgenic cotton plants under field conditions, herbicidal compound(s) of the general formula (I) described herein and a commercial PPO inhibitor herbicide (such as fomesafen) are applied at pre-emergence (PRE), V4, and V8 developmental stages at one of two rates. Plants are visually assessed for herbicide injury 10-14 days after herbicide treatment. Unsprayed transgenic plants are used for phenotypic comparison with unsprayed wild-type plants.
[00194] For soybean, com, and cotton, the injury rating is determined as the percentage of leaf area of a plant exhibiting damage such as necrosis (brown or dead tissue), chlorosis (yellow tissue or yellow spotting), and malformation (misshapen leaves or plant structures, epinasty or twisting of stem, cupping of leaves) caused by herbicide application and is measured on a scale of 0-100, with zero being no injury and 100 being complete crop death.
[00195] Transgenic crop seeds conferring PPO inhibiting herbicide tolerance prepared as described above are planted in a field or crop growing area. PPO inhibiting herbicide formulations, such as the herbicidal compound(s) of the general formula (I) described herein, are applied to the field or crop growing area before or/and after planting the seeds to control weed growth. The herbicide application comprises an effective amount of at least one PPO inhibiting herbicide that prevents or controls the growth of weeds, but does not damage or injure the transgenic soybean, com, or cotton crop plants comprising a recombinant DNA molecule encoding a chloroplast- targeted heterologous HemG protein. The PPO inhibiting herbicides can be applied, once or more than once, at about IX application rate. However, the rate may be adjusted or varied depending on environmental conditions (such as temperature and humidity) and the type of weeds being controlled, as is known in the art. Therefore, the application rate may vary within wide limits, and may consist of a range from about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, about 0.25 g a.i./ha to about 300 g a.i./ha, about 0.02 g a.i./ha to about 250 g a.i./ha, about 0.05 g a.i./ha to about 150 g a.i./ha, or about 0.25 g a.i./ha to about 120 g a.i./ha.
[00196] A desired application rate in any particular environment or in the context of a particular weed can be determined empirically by one of skill in the art in view of the present disclosure. The herbicide rate is carefiilly selected to avoid 1) over use than what is needed, which could lead to
injury to the herbicide tolerant crop(s); 2) under use, resulting in poor weed control, which could lead to development of herbicide tolerant weeds.
[00197] The PPO inhibiting herbicides may be applied pre-emergence and/or post emergence. In the case of post emergence application, the PPO inhibiting herbicides may be applied over the top of the crop growing area.
[00198] In addition to a PPO inhibiting herbicide, an effective amount of at least a second herbicide can be applied for weed control. Examples of such a second herbicide include, but are not limited to, an ACCase inhibitor (such as an aryloxyphenoxy propionate or a cyclohexanedione), an ALS inhibitor (such as sulfonylurea, imidazolinone, triazoloyrimidine, or a triazolinone), an EPSPS inhibitor (such as glyphosate), a synthetic auxin (such as a phenoxy herbicide, a benzoic acid, a carboxylic acid, or a semicarbazone), a photosynthesis inhibitor (such as a triazine, a triazinone, a nitrile, a benzothiadiazole, or a urea), a glutamine synthetase inhibitor (such as glufosinate), a HPPD inhibitor (such as an isoxazole, a pyrazolone, or a triketone), a PPO inhibitor (such as a diphenylether, a N-phenylphthalimide, an aryl triazinone, or a pyrimidinedione), and a long-chain fatty acid inhibitor (such as a chloroacetamide, an oxyacetamide, or a pyrazole)
Claims
1. A method for controlling or preventing weed growth in a plant growth area, wherein the method comprises the steps of:
(a) providing in said plant growth area a plant or a seed that when grown produces said plant, wherein the plant comprises a recombinant DNA molecule comprising a DNA sequence encoding a heterologous HemG protein, wherein said protein confers tolerance in said plant to an herbicidally active compound of the general formula (I) or an agrochemically acceptable salt thereof
in which:
A represents nitrogen or CH;
R1 represents hydrogen or methyl;
R2 represents hydrogen or fluorine;
R3 represents hydrogen, halogen, or (C1-C8)-alkoxy;
R4 represents halogen, cyano, NO2, C(O)NH2, C(S)NH2, (C1-C8)-haloalkyl, or (Cz-Cs)- alkynyl;
R5, R6, and R7 independently of one another represent hydrogen, halogen, cyano, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (C1-C8)-alkoxy, or (C1-C8)-haloalkoxy;
G represents a branched or unbranched alkylene group optionally substituted with an (Ci -C3)- alkoxy group;
R8 represents hydrogen, (C1-C8)-alkyl, (C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, (C2-C8)-alkynyl, (C2-C8)-alkenyl, C(O)R13, C(O)OR13, or (C1-C8)-alkoxy-(C1-C8)-alkyl;
R9 represents hydrogen or (C1-C8)-alkyl;
R10 represents hydrogen, halogen, cyano, NO2, (C1-C8)-alkyl, (C1-C8)-haloalkyl, (Cs-Cs)- cycloalkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C3-C8)-halocycloalkyl, (Cs-Cs)- halocycloalkyl-(C1-C8)-alkyl, (C2-Cs)-alkenyl, (C2-C8)-alkynyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl, heterocyclyl-(C1-C8)-alkyl, R11R12N- (C1-C8)-alkyl, R13O-(C1-C8)-alkyl, cyano-(C1-C8)-alkyl, (C1-C8)-alkylcarbonyloxy-(C1- C8)-alkyl, (C3-C8)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy-(C1-C8)-alkyl, heteroarylcarbonyloxy-(C1-C8)-alkyl, heterocyclylcarbonyloxy-(C1-C8)-alkyl, OR13, NR11R12, SR14, S(O)R14, SO2R14, R14S-(C1-C8)-alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(CI- C8)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl](aryl)silyl(C1-C8)- alkyl, [(C1-C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, tris-[(C1-C8)-alkyl]silyl, bis- hydroxyboryl-(C1-C8)-alkyl, bis-[(C1-C8)-alkoxy]boryl-(C1-C8)-alkyl, tetramethyl- 1,3,2- dioxaborolan-2-yl, tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl-(C1-C8)-alkyl, nitro-(C1-C8)- alkyl, C(O)OR13, C(O)R13, C(O)NR11R12, R13O(O)C-(C1-C8)-alkyl, R11R12N(O)C-(C1- C8)-alkyl, orbis-(C1-C8)-alkoxy-(C1-C8)-alkyl, or
R8 and R10 together with the carbon atom to which they are attached form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having further substitution;
R11 and R12 are identical or different and independently of one another represent hydrogen, (C1- Cs)-alkyl, (C2-C8)-alkenyl, (C2-Cg)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2- C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3-C10)-halocycloalkyl, (C4- C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)- haloalkoxy-(C1-C8)-alkyl, (C1-C8)-alkylthio-(C1-C8)-alkyl, (C1-C8)-haloalkylthio-(C1-C8)- alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl- (C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, COR13, SO2R14, heterocyclyl, (C1-C8)-alkoxycarbonyl, bis-[(C1-C8)-alkyl]aminocarbonyl- (C1-C8)-alkyl, (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkyl- aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxycarbonyl, heteroaryl-(C1-C8)- alkoxycarbonyl, (C2-C8)-alkenyloxycarbonyl, (C2-C8)-alkynyloxycarbonyl, or heterocyclyl-(C1-C8)-alkyl, or
R11 and R12 together with the nitrogen atom to which they are attached form a fully saturated or partially saturated 3- to 10-membered monocyclic or bicyclic ring optionally interrupted by heteroatoms and optionally having further substitution;
R13 represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3- C10)-halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-haloalkoxy-(C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, (C1- C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy-(C1-C8)- alkyl, aryl, aryl-(C1-C8)-alkyl, aryl-(C1-C8)-alkoxy-(C1-C8)-alkyl, heteroaryl, heteroaryl- (C1-C8)-alkyl, (C3-C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, bis- [(C1-C8)-alkyl]aminocarbonyl-(C1-C8)-alkyl, (C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, aryl-(C1-C8)-alkyl-aminocarbonyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl]amino-(C2-C6)-alkyl, (C1-C8)-alkyl amino-(C2-C6)-alkyl, aryl-(C1-C8)-alkyl-amino-(C2-C6)-alkyl, R14S-(CI-C8)- alkyl, R14(O)S-(C1-C8)-alkyl, R14O2S-(C1-C8)-alkyl, hydroxycarbonyl-(C1-C8)-alkyl,
heterocyclyl, heterocyclyl-(C1-C8)-alkyl, tris-[(C1-C8)-alkyl]silyl-(C1-C8)-alkyl, bis-[(C1- C8)-alkyl](aryl)silyl(C1-C8)-alkyl, [(C1-C8)-alkyl]-bis-(aryl)silyl-(C1-C8)-alkyl, (C1-C8)- alkylcarbonyloxy-(C1-C8)-alkyl, (C3-C8)-cycloalkylcarbonyloxy-(C1-C8)-alkyl, arylcarbonyloxy-(C1-C8)-alkyl, heteroarylcarbonyloxy-(C1-C8)-alkyl, heterocyclylcarbonyloxy-(C1-C8)-alkyl, aryloxy-(C1-C8)-alkyl, heteroaryloxy-(C1-C8)- alkyl, or (C1-C8)-alkoxycarbonyl;
R14 represents hydrogen, (C1-C8)-alkyl, (C2-C8)-alkenyl, (C2-C8)-alkynyl, (C1-C8)-cyanoalkyl, (C1-C10)-haloalkyl, (C2-C8)-haloalkenyl, (C3-C8)-haloalkynyl, (C3-C10)-cycloalkyl, (C3- C10)-halocycloalkyl, (C4-C10)-cycloalkenyl, (C4-C10)-halocycloalkenyl, (C1-C8)-alkoxy- (C1-C8)-alkyl, (C1-C8)-alkoxy-(C1-C8)-haloalkyl, aryl, aryl-(C1-C8)-alkyl, heteroaryl, heteroaryl-(C1-C8)-alkyl, heterocyclyl-(C1-C8)-alkyl, (C3 -C8)-cycloalkyl-(C1-C8)-alkyl, (C4-C10)-cycloalkenyl-(C1-C8)-alkyl, bis-[(C1-C8)-alkyl]amino, (C1-C8)-alkyl-amino, aryl- (C1-C8)-amino, aryl-(C1-C6)-alkyl-amino, aryl-[(C1-C8)-alkyl]amino, (C3-C8)-cycloalkyl- amino, (C3-C8)-cycloalkyl-[(C1-C8)-alkyl]amino; N-azetidinyl, N-pyrrolidinyl, N- piperidinyl, or N-morpholinyl;
R15 and R16 independently of each other represent (C1-C8)-alkyl, (C3-C8)-cycloalkyl, aryl, heteroaryl, or heterocyclyl or
R15 and R16 together, with the carbon atom to which they are attached, form an unsubstituted (C3- C?)-cycloalkyl;
X and Y, independently of one another, represent oxygen or sulfur; and
Z represents nitrogen or CH; and
(b) applying to said area an amount of said compound effective to control or prevent weed growth in the area.
2. The method of claim 1, wherein said compound of the general formula (I) or an agrochemically acceptable salt thereof is further characterized in that
A represents nitrogen or CH;
R1 represents hydrogen;
R2 represents hydrogen or fluorine;
R3 represents fluorine;
R4 represents chlorine, bromine, or cyano;
R5 represents hydrogen;
R6 represents hydrogen or fluorine;
R7 represents hydrogen;,
G represents methylene, (methyl)methylene, or (methoxy)methylene;
X represents oxygen;
Y represents oxygen;
Z represents nitrogen or CH; and
3. The method of claim 1, wherein said compound corresponds to:
(a) ethyl [(3- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(b) [(3- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l (2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(c) ethyl (2- {2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fhiorophenoxy}phenoxy)acetate;
(d) (2- {2-chloro-5- [4-( 1 , 1 -difhioroethyl)-3 -methyl-2,6-dioxo-3 ,6-dihydropyrimidin- 1 (2H)- yl] -4-fluorophenoxy } phenoxy)acetic acid;
(e) ethyl (2- {2-chloro-4-fluoro-5- [4-( 1 -fluoroethyl)-3 -methyl-2,6-dioxo-3 ,6- dihydropyrimidin-l(2H)-yl]phenoxy}phenoxy)acetate;
(f) 2-methoxyethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(g) tetrahydrofuran-2-ylmethyl [(3-{2-chloro-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(h) cyanomethyl [(3-{2-chloro-5-[4-(l , 1 -difhioroethyl)-3-methyl-2,6-dioxo-3,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy} pyridin-2-yl)oxy] acetate;
(i) methyl (2- {2-chloro-5- [4-(l , 1 -difluoroethyl)-3 -methyl-2,6-dioxo-3 ,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(j) methyl (2-{2-bromo-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}phenoxy)(methoxy)acetate;
(k) [(3- {2-bromo-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin-l (2H)- yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetic acid;
(l) ethyl [(3- {2-bromo-5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-3,6-dihydropyrimidin- l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate;
(m) 2-methoxyethyl [(3 - {2-bromo-5 -[4-( 1 , 1 -difluoroethyl)-3 -methyl-2,6-dioxo-3 ,6- dihydropyrimidin- 1 (2H)-yl] -4-fluorophenoxy } pyridin-2-yl)oxy] acetate;
(n) tefrahydrofuran-2-ylmethyl [(3-{2-bromo-5-[4-(l,l-difluoroethyl)-3-methyl-2,6-dioxo- 3,6-dihydropyrimidin-l(2H)-yl]-4-fluorophenoxy}pyridin-2-yl)oxy]acetate; or
(o) ethyl 2-[[3-[5-[4-(l , 1 -difluoroethyl)-3-methyl-2,6-dioxo-pyrimidin-l -yl]-4-fluoro-2- nitro-phenoxy]- 2-pyridyl]oxy]acetate.
4. The method of any of claims 1 -3, wherein the heterologous HemG protein has herbicideinsensitive protoporphyrinogen oxidase activity.
5. The method of any of claims 1 -4, wherein the heterologous HemG protein has at least 85% sequence identity to a polypeptide sequence selected from the group consisting of SEQ ID NOs:l- 20 and 65-193.
6. The method of any of claims 1-5, wherein the DNA sequence encoding the heterologous HemG protein is selected from the group consisting of SEQ ID NOs:22-64 and 194-322.
7. The method of any of claims 1-6, wherein the heterologous HemG protein comprises an amino acid sequence selected from the group consisting of SEQ ID NOs:l-20 and 65-193.
8. The method of any of claims 1-7, wherein the DNA sequence encoding a heterologous HemG protein is operably linked to a DNA sequence encoding a chloroplast transit peptide (CTP).
9. The method of claim 8, wherein the CTP comprises an amino acid sequence with at least 97% sequence identity to a sequence selected from the group consisting of SEQ ID NOs:323-328, 340, and 342-407.
10. The method of claim 8, wherein the DNA sequence encoding the CTP comprises at least 97% identity to a sequence selected from the group consisting of SEQ ID NOs:329-339, 341, and 408-483.
11. The method of any of claims 1-10, wherein said recombinant DNA molecule further comprises a heterologous promoter operably linked to the DNA sequence encoding said HemG protein.
12. The method of any of claims 1-11, wherein said herbicidally active compound is applied to the area at a rate of about 0.02 g a.i./ha to about 750 g a.i./ha, about 0.05 g a.i./ha to about 400 g a.i./ha, about 0.25 g a.i./ha to about 300 g a.i./ha, about 0.02 g a.i./ha to about 250 g a.i./ha, about 0.05 g a.i./ha to about 150 g a.i./ha, or about 0.25 g a.i./ha to about 120 g a.i./ha.
13. The method of any of claims 1-12, further defined as comprising applying said compound to said area at least twice.
14. The method of any of claims 1-13, wherein the herbicidally active compound is applied in an amount that does not damage said plant comprising the recombinant DNA molecule.
15. The method of any of claims 1-14, wherein the plant comprising the recombinant DNA molecule is a monocotyledonous plant.
16. The method of any of claims 1-14, wherein the plant comprising the recombinant DNA molecule is a dicotyledonous plant.
17. The method of any of claims 1-16, wherein the method further comprises applying to said area an effective amount of at least a second herbicide.
18. The method of claim 17, wherein the second herbicide is selected from the group consisting of: an ACCase inhibitor, an ALS inhibitor, an EPSPS inhibitor, a synthetic auxin, a photosynthesis inhibitor, a glutamine synthesis inhibitor, a HPPD inhibitor, a PPO inhibitor, and a long-chain fatty acid inhibitor.
19. The method of claim 18, wherein the ACCase inhibitor is an aryloxyphenoxy propionate or a cyclohexanedione; the ALS inhibitor is a sulfonylurea, imidazolinone, triazoloyrimidine, or a triazolinone; the EPSPS inhibitor is glyphosate; the synthetic auxin is a phenoxy herbicide, a benzoic acid, a carboxylic acid, or a semicarbazone; the photosynthesis inhibitor is a triazine, a triazinone, a nitrile, a benzothiadiazole, or a urea; the glutamine synthesis inhibitor is glufosinate;
the HPPD inhibitor is an isoxazole, a pyrazolone, or a triketone; the PPO inhibitor is a diphenylether, a N-phenylphthalimide, an aryl triazinone, or a pyrimidinedione; or the long-chain fatty acid inhibitor is a chloroacetamide, an oxyacetamide, or a pyrazole.
20. The method of any of claims 1-19, wherein said applying of the compound is carried out pre-emergence.
21. The method of any of claims 1-19, wherein said applying of the compound is carried out post-emergence.
22. The method of any of claims 1-21, wherein said applying of the compound comprises contacting said plant with the compound.
23. The method of any of claims 1-22, wherein said applying of the compound comprises an over the top application of said compound.
24. The method of any of claims 1-23, wherein said applying of the compound results in an increase in the growth or yield of said plant relative to a plant of the same genotype cultivated in a growth area in which said compound has not been applied.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202363486768P | 2023-02-24 | 2023-02-24 | |
| US63/486,768 | 2023-02-24 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2024178190A2 true WO2024178190A2 (en) | 2024-08-29 |
| WO2024178190A3 WO2024178190A3 (en) | 2024-10-17 |
Family
ID=92501792
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2024/016844 WO2024178190A2 (en) | 2023-02-24 | 2024-02-22 | Methods and compositions for ppo herbicide tolerance |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024178190A2 (en) |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EA202091289A1 (en) * | 2017-11-29 | 2020-10-12 | Басф Се | PLANTS WITH INCREASED HERBICIDE RESISTANCE |
| CA3026528A1 (en) * | 2017-12-15 | 2019-06-15 | Monsanto Technology Llc | Methods and compositions for ppo herbicide tolerance |
| BR112021015898A2 (en) * | 2019-02-15 | 2021-10-05 | Syngenta Crop Protection Ag | HERBICIDAL COMPOSITIONS |
| CA3225358A1 (en) * | 2021-07-16 | 2023-01-19 | Tobias SEISER | Herbicidal phenyluracils |
| WO2024015948A2 (en) * | 2022-07-14 | 2024-01-18 | Monsanto Technology Llc | Methods and compositions for ppo herbicide tolerance |
| WO2024015949A2 (en) * | 2022-07-14 | 2024-01-18 | Monsanto Technology Llc | Methods and compositions for ppo herbicide tolerance |
-
2024
- 2024-02-22 WO PCT/US2024/016844 patent/WO2024178190A2/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| WO2024178190A3 (en) | 2024-10-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2941285T3 (en) | herbicidal compositions | |
| ES2942215T3 (en) | herbicidal compositions | |
| WO2024015950A1 (en) | Methods and compositions for ppo herbicide tolerance | |
| JP2024037963A (en) | Controlled release formulation for agricultural chemical | |
| US20210147371A1 (en) | Oxadiazoline derivatives | |
| US20220386605A1 (en) | Herbicidal compositions | |
| WO2022152728A1 (en) | Herbicidal compositions | |
| CN111225565B (en) | Aqueous suspension concentrates | |
| WO2024015949A2 (en) | Methods and compositions for ppo herbicide tolerance | |
| WO2024013015A1 (en) | Herbicidal compositions | |
| WO2024015948A2 (en) | Methods and compositions for ppo herbicide tolerance | |
| US20240251788A1 (en) | Herbicide/safener combination based on safeners from the class of substituted [(1,5-diphenyl-1h-1,2,4-triazol-3-yl)oxy]acetic acids and their salts | |
| WO2024178190A2 (en) | Methods and compositions for ppo herbicide tolerance | |
| WO2023036707A1 (en) | Substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines, salts thereof and their use as herbicidally active substances | |
| EP4097087A1 (en) | [(1,4,5-trisubstituted-1h-pyrazol-3-yl)sulfanyl]acetic acid derivatives, salts thereof, and use thereof as herbicidal ingredients | |
| WO2024013016A1 (en) | Herbicidal compositions | |
| WO2024020316A2 (en) | Methods and compositions for ppo herbicide tolerance | |
| WO2024167793A1 (en) | Methods and compositions for ppo herbicide tolerance | |
| EP4353082A1 (en) | Herbicidal compositions | |
| WO2024170472A1 (en) | Herbicidal mixtures | |
| US20230416770A1 (en) | Methods and Compositions for Generating Dominant Brachytic Alleles Using Genome Editing | |
| WO2018178010A1 (en) | N-cyclopropyl-2-oxo-4-phenyl-piperidine-3-carboxamide derivatives and related compounds as herbicidal plant protection agents | |
| OA20567A (en) | Herbicidal compositions. | |
| JP2024542693A (en) | (1,4,5-trisubstituted-1H-pyrazol-3-yl)oxy-2-alkoxythioalkyl acids and derivatives thereof, their salts and their use as herbicidal active agents | |
| KR20240108800A (en) | (1,4,5-trisubstituted-1H-pyrazol-3-yl)oxy-2-alkoxythio alkyl acids and their derivatives, salts thereof, and their use as herbicides as active herbicidal ingredients. |