WO2024142830A1 - Aluminum alloy forging material, aluminum alloy forged product, and method for manufacturing same - Google Patents
Aluminum alloy forging material, aluminum alloy forged product, and method for manufacturing same Download PDFInfo
- Publication number
- WO2024142830A1 WO2024142830A1 PCT/JP2023/043832 JP2023043832W WO2024142830A1 WO 2024142830 A1 WO2024142830 A1 WO 2024142830A1 JP 2023043832 W JP2023043832 W JP 2023043832W WO 2024142830 A1 WO2024142830 A1 WO 2024142830A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mass
- less
- range
- aluminum alloy
- content
- Prior art date
Links
- 229910000838 Al alloy Inorganic materials 0.000 title claims abstract description 221
- 238000005242 forging Methods 0.000 title claims description 99
- 238000000034 method Methods 0.000 title claims description 41
- 239000000463 material Substances 0.000 title claims description 38
- 238000004519 manufacturing process Methods 0.000 title claims description 20
- 239000000047 product Substances 0.000 claims abstract description 65
- 239000013078 crystal Substances 0.000 claims abstract description 28
- 239000002244 precipitate Substances 0.000 claims abstract description 23
- 239000012535 impurity Substances 0.000 claims abstract description 19
- 238000005266 casting Methods 0.000 claims description 46
- 229910052751 metal Inorganic materials 0.000 claims description 35
- 239000002184 metal Substances 0.000 claims description 35
- 239000000956 alloy Substances 0.000 claims description 28
- 229910045601 alloy Inorganic materials 0.000 claims description 22
- 238000010438 heat treatment Methods 0.000 claims description 21
- 239000000203 mixture Substances 0.000 claims description 21
- 238000010791 quenching Methods 0.000 claims description 14
- 230000000171 quenching effect Effects 0.000 claims description 14
- 230000032683 aging Effects 0.000 claims description 12
- 238000000265 homogenisation Methods 0.000 claims description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 11
- 230000008018 melting Effects 0.000 claims description 9
- 238000002844 melting Methods 0.000 claims description 9
- 229910052802 copper Inorganic materials 0.000 claims description 7
- 239000002245 particle Substances 0.000 claims description 7
- 238000012545 processing Methods 0.000 claims description 5
- 239000004033 plastic Substances 0.000 claims description 4
- 239000000498 cooling water Substances 0.000 description 49
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 42
- 238000001816 cooling Methods 0.000 description 26
- 150000001875 compounds Chemical class 0.000 description 20
- 230000000694 effects Effects 0.000 description 19
- 238000009749 continuous casting Methods 0.000 description 15
- 239000010949 copper Substances 0.000 description 14
- 238000001953 recrystallisation Methods 0.000 description 14
- 229910052782 aluminium Inorganic materials 0.000 description 13
- 239000012530 fluid Substances 0.000 description 13
- 239000000243 solution Substances 0.000 description 13
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical group [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 11
- 238000011156 evaluation Methods 0.000 description 11
- 230000004907 flux Effects 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 230000001050 lubricating effect Effects 0.000 description 10
- 239000010687 lubricating oil Substances 0.000 description 10
- 230000002093 peripheral effect Effects 0.000 description 9
- 238000007670 refining Methods 0.000 description 9
- 229910052710 silicon Inorganic materials 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- 230000007797 corrosion Effects 0.000 description 8
- 238000005260 corrosion Methods 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 229910052742 iron Inorganic materials 0.000 description 8
- 229910052804 chromium Inorganic materials 0.000 description 7
- 229910052748 manganese Inorganic materials 0.000 description 7
- 239000012071 phase Substances 0.000 description 6
- 239000006104 solid solution Substances 0.000 description 6
- 229910052725 zinc Inorganic materials 0.000 description 6
- 229910017082 Fe-Si Inorganic materials 0.000 description 5
- 229910017133 Fe—Si Inorganic materials 0.000 description 5
- 230000000052 comparative effect Effects 0.000 description 5
- 230000007547 defect Effects 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 229910019018 Mg 2 Si Inorganic materials 0.000 description 4
- 229910019064 Mg-Si Inorganic materials 0.000 description 4
- 229910019406 Mg—Si Inorganic materials 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 4
- 239000000314 lubricant Substances 0.000 description 4
- 239000002994 raw material Substances 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 229910052726 zirconium Inorganic materials 0.000 description 4
- 229910000831 Steel Inorganic materials 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 229910000765 intermetallic Inorganic materials 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 238000001556 precipitation Methods 0.000 description 3
- 238000005204 segregation Methods 0.000 description 3
- 238000007711 solidification Methods 0.000 description 3
- 230000008023 solidification Effects 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- 238000005728 strengthening Methods 0.000 description 3
- 229910021365 Al-Mg-Si alloy Inorganic materials 0.000 description 2
- 229910018464 Al—Mg—Si Inorganic materials 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 229910018565 CuAl Inorganic materials 0.000 description 2
- 229910019752 Mg2Si Inorganic materials 0.000 description 2
- 238000007550 Rockwell hardness test Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000005868 electrolysis reaction Methods 0.000 description 2
- 238000001887 electron backscatter diffraction Methods 0.000 description 2
- 230000005496 eutectics Effects 0.000 description 2
- 238000000445 field-emission scanning electron microscopy Methods 0.000 description 2
- -1 for example Substances 0.000 description 2
- 229910002804 graphite Inorganic materials 0.000 description 2
- 239000010439 graphite Substances 0.000 description 2
- 238000009863 impact test Methods 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 150000001247 metal acetylides Chemical class 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 230000001376 precipitating effect Effects 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 230000001360 synchronised effect Effects 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 229910018191 Al—Fe—Si Inorganic materials 0.000 description 1
- 229910000521 B alloy Inorganic materials 0.000 description 1
- 229910017827 Cu—Fe Inorganic materials 0.000 description 1
- 229910017060 Fe Cr Inorganic materials 0.000 description 1
- 229910002544 Fe-Cr Inorganic materials 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 240000008415 Lactuca sativa Species 0.000 description 1
- 229910017708 MgZn2 Inorganic materials 0.000 description 1
- 238000006124 Pilkington process Methods 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229910052790 beryllium Inorganic materials 0.000 description 1
- 238000009529 body temperature measurement Methods 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000011362 coarse particle Substances 0.000 description 1
- 239000002826 coolant Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000005461 lubrication Methods 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000011819 refractory material Substances 0.000 description 1
- 239000003507 refrigerant Substances 0.000 description 1
- 235000012045 salad Nutrition 0.000 description 1
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 1
- 229910010271 silicon carbide Inorganic materials 0.000 description 1
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 230000008016 vaporization Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/02—Alloys based on aluminium with silicon as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/06—Alloys based on aluminium with magnesium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
- C22F1/05—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon of alloys of the Al-Si-Mg type, i.e. containing silicon and magnesium in approximately equal proportions
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
Definitions
- the present invention relates to an aluminum alloy material for forging, an aluminum alloy forging, and a method for producing the same.
- iron-based materials were used exclusively for automobile parts, particularly suspension parts.
- aluminum or aluminum alloy materials primarily for the purpose of reducing weight.
- Zr is effective in preventing recrystallization, it has the following problems.
- Zr forms compounds with Al-Ti-B based alloys. These compounds accumulate at the bottom of the furnace in which the molten alloy is stored, contaminating the furnace. In addition, these compounds also crystallize out as coarse particles in the produced ingot, reducing its strength.
- Aspect 1 of the present invention is a composition in which Cu is in the range of 0.25 mass% or more and 0.55 mass% or less, Mg is in the range of 0.60 mass% or more and 1.25 mass% or less, Si is in the range of 0.90 mass% or more and 1.4 mass% or less, Mn is in the range of 0.35 mass% or more and 0.60 mass% or less, Fe is in the range of 0.15 mass% or more and 0.30 mass% or less, Zn is in the range of 0.25 mass% or less, Cr is in the range of 0.050 mass% or more and 0.30 mass% or less, Ti is in the range of 0.01 mass% or more and 0.1 mass% or less, and B is in the range of 0.0010 mass% or less.
- the aluminum alloy forging material is composed of an aluminum alloy having an alloy composition containing 0.030% by mass or more, 0.0010% by mass or more and 0.050% by mass or less of Zr, a ratio of the Fe content to the Mn content Fe/Mn of 0.3 to 1.2 by mass, and the balance being Al and unavoidable impurities, and has a post-cast electrical conductivity of 25% IACS to 35% IACS and a Rockwell hardness HRF of 62 to 82.
- Aspect 3 of the present invention is a steel sheet having a Cu content in the range of 0.25 mass% or more and 0.55 mass% or less, a Mg content in the range of 0.60 mass% or more and 1.25 mass% or less, a Si content in the range of 0.90 mass% or more and 1.4 mass% or less, a Mn content in the range of 0.35 mass% or more and 0.60 mass% or less, a Fe content in the range of 0.15 mass% or more and 0.30 mass% or less, a Zn content in the range of 0.25 mass% or less, a Cr content in the range of 0.050 mass% or more and 0.30 mass% or less, a Ti content in the range of 0.01 mass% or more and 0.1 % or less by mass, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less
- FIG. 1 is a perspective view showing an example of an aluminum alloy forging according to an embodiment of the present invention.
- FIG. FIG. 2 is a plan view showing another example of an aluminum alloy forged product according to an embodiment of the present invention.
- FIG. 2 is a perspective view showing yet another example of an aluminum alloy forged product according to an embodiment of the present invention.
- 1 is a cross-sectional view showing an example of the vicinity of a mold of a horizontal continuous casting apparatus for producing an aluminum alloy forging according to an embodiment of the present invention.
- 5 is an enlarged cross-sectional view of a main portion near a cooling water cavity of the horizontal continuous casting machine shown in FIG. 4.
- FIG. 2 is an explanatory diagram illustrating a heat flux in a cooling wall portion of a horizontal continuous casting apparatus.
- FIG. 2 is a plan view showing the position at which the central portion was sampled from the aluminum alloy forging obtained in this example for preparing a test piece for evaluating mechanical properties.
- FIG. 2 is a plan view showing a test
- an aluminum alloy forging material contains Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, Ti in the range of 0.01 mass% or more and 0.02 mass% or less, and Zn in the range of 0.03 mass% or more and 0.04 mass% or less.
- An aluminum alloy forging material has Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, and the range below, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content Fe/Mn being 0.3 to 1.2 in mass ratio, with the balance
- the electrical conductivity of the aluminum alloy forging material of the above embodiment is 25% IACS or more and 35% IACS or less. This electrical conductivity is the electrical conductivity at room temperature, which is about 20°C ⁇ 15°C. If the electrical conductivity is less than 25% IACS, the material will be too hard and its workability will decrease, whereas if it exceeds 35% IACS, the material will be too soft, resulting in poor machinability (ability to break away chips), and the guaranteed strength of the final product may not be satisfied.
- the Rockwell hardness HRF of the aluminum alloy forging material of the above embodiment is 62 or more and 82 or less.
- the Rockwell hardness HRF is a value measured in accordance with JIS Z2245:2016 "Rockwell hardness test - Test method". This is because the workability is good if the Rockwell hardness HRF is within this range. That is, if the Rockwell hardness HRF is less than 62, the material is too soft, resulting in poor machinability (chip breakability) and inability to satisfy the guaranteed strength of the final product, while if it exceeds 82, the material is too hard, resulting in poor workability.
- the aluminum alloy forged product of this embodiment has an impact value of 10 J/cm 2 or more at room temperature.
- the size of precipitates containing Mn within 2.0 ⁇ m, including grain boundaries is 0.5 ⁇ m or less.
- the aluminum alloy forgings of this embodiment correspond to 6000 series aluminum alloy forgings in that they contain Mg and Si.
- Cu has the effect of finely dispersing Mg-Si compounds in the aluminum alloy and the effect of improving the tensile strength of the aluminum alloy by precipitating as Al-Cu-Mg-Si compounds including the Q phase.
- the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
- Mg has the effect of improving the tensile strength of the aluminum alloy.
- Mg contributes to strengthening the aluminum alloy by dissolving in the aluminum parent phase or precipitating as Mg-Si compounds (Mg 2 Si) such as the ⁇ ′′ phase, or Al-Cu-Mg-Si compounds (AlCuMgSi) such as the Q phase.
- Mg 2 Si also has the effect of suppressing the formation of CuAl 2 phase in the aluminum alloy. By suppressing the formation of CuAl 2 phase, the corrosion resistance of the aluminum alloy forging 1a is improved. By keeping the Mg content within the above range, it is possible to improve the corrosion resistance as well as the mechanical properties at room temperature of the aluminum alloy forging 1a.
- Si 0.90 mass% or more, 1.4 mass% or less
- Si has the effect of improving the mechanical properties and corrosion resistance of the aluminum alloy forging 1a at room temperature.
- coarse primary crystal Si grains may crystallize, which may reduce the tensile strength of the aluminum alloy.
- Mn 0.35 mass% or more, 0.60 mass% or less
- Mn has the effect of improving the tensile strength of the aluminum alloy by forming fine granular precipitates containing intermetallic compounds such as Al-Mn-Fe-Si and Al-Mn-Cr-Fe-Si in the aluminum alloy.
- the Mn content is within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
- FIG. 3 is a plan view of yet another example of an aluminum alloy forged product according to an embodiment of the present invention.
- the aluminum alloy forging 1c shown in Fig. 3 has three connecting portions 4f, 4g, and 4h.
- the connecting portions 4f and 4g are connected to each other by the long portion 2, and the connecting portions 4f and 4h are connected to each other by the long portion 2.
- a through hole is provided in the connecting portion 4f.
- This aluminum alloy forging 1b can be used as, for example, an A-type suspension arm.
- Cu is in the range of 0.25 mass% or more and 0.55 mass% or less
- Mg is in the range of 0.60 mass% or more and 1.25 mass% or less
- Si is in the range of 0.90 mass% or more and 1.4 mass% or less
- Mn is in the range of 0.35 mass% or more and 0.60 mass% or less
- Fe is in the range of 0.15 mass% or more and 0.30 mass% or less
- Zn is in the range of 0.25 mass% or less
- Cr is in the range of 0.050 mass% or more and 0.30 mass% or less
- Ti is 0.01 mass% and 0.1% by mass or less
- B in the range of 0.0010% by mass or more and 0.030% by mass or less
- Zr in the range of 0.0010% by mass or more and 0.050% by mass or less
- a ratio of the Fe content to the Mn content Fe/Mn is 0.3 to 1.2 in mass ratio
- the balance is Al and unavoidable impurities.
- the molten metal receiving section 11 is composed of a molten metal inlet section 11a that receives the molten aluminum alloy M obtained in the above-mentioned molten metal forming process, a molten metal holding section 11b, and an outlet section 11c into the hollow section 21 of the mold 12.
- the molten aluminum alloy M held in the molten metal holding portion 11b in the molten metal receiving portion 11 is poured into the hollow portion 21 of the mold 12 through the pouring passage 13a provided in the refractory plate body 13.
- the molten aluminum alloy M supplied into the hollow portion 21 is then cooled and solidified by the cooling device 23 described below, and is drawn out from the other end side 12b of the mold 12 as an aluminum alloy rod B, which is a solidified ingot.
- the elevation angle is less than 0°, when the aluminum alloy rod B is pulled out of the mold 12, it encounters resistance at the other end 12b, which is the mold outlet, and casting may become difficult.
- the elevation angle exceeds 3°, the contact of the inner peripheral surface 21a with the molten aluminum alloy M may become insufficient, and the effect of removing heat from the molten aluminum alloy M and its solidified shell to the mold 12 may decrease, resulting in insufficient solidification.
- a remelted skin may appear on the surface of the aluminum alloy rod B, or unsolidified molten aluminum alloy M may erupt from the end of the aluminum alloy rod B, which is not preferable, as this may lead to casting problems.
- the inner surface 21a of the mold 12 is cooled by the cooling water W contained in the cooling water cavity 24, which removes heat from the molten aluminum alloy M filling the hollow portion 21 of the mold 12 from the surface in contact with the inner surface 21a of the mold 12, forming a solidified shell on the surface of the molten aluminum alloy M.
- the mold 12 is formed so that the thickness t of the cooling wall 27 of the mold 12, i.e., the distance between the inner bottom surface 24a of the cooling water cavity 24 and the inner peripheral surface 21a of the hollow portion 21 of the mold 12, is within a range of, for example, 0.5 mm to 3.0 mm, and preferably 0.5 mm to 2.5 mm.
- the material for forming the mold 12 is selected so that the thermal conductivity of at least the cooling wall 27 of the mold 12 is within a range of 100 W/m ⁇ K to 400 W/m ⁇ K.
- the aluminum alloy rod B is pulled out at a constant speed by a pull-out drive device (not shown) installed near the other end 12b of the mold 12, so that it is cast continuously to form a long aluminum alloy rod B.
- the pulled aluminum alloy rod B is then cut to the desired length, for example, by a synchronous cut-off machine (not shown).
- composition ratio of the cast aluminum alloy rod B can be confirmed, for example, by a method using a photoelectric emission spectrophotometric analyzer (example: Shimadzu PDA-5500, manufactured by Japan) as described in "JIS H 1305.”
- the lubricating oil supply rate is preferably 0.05 mL/min to 5 mL/min (more preferably 0.1 mL/min to 1 mL/min). If the supply rate is too low, the molten aluminum alloy M of the aluminum alloy rod B may not solidify and may leak from the mold 12 due to insufficient lubrication. If the amount of supply is excessive, the excess may be mixed into the aluminum alloy bar B and cause internal defects.
- the casting speed which is the speed at which the aluminum alloy rod B is pulled out of the mold 12, is preferably 200 mm/min or more and 1500 mm/min or less (more preferably 400 mm/min or more and 1000 mm/min or less). This is because, at a casting speed within this range, the network structure of the crystals formed by casting becomes uniform and fine, which increases the resistance of the aluminum matrix to deformation at high temperatures and improves the high-temperature mechanical strength.
- the amount of cooling water sprayed from the shower opening 25a of the cooling water spray passage 25 is preferably 10 L/min or more and 50 L/min or less (more preferably 25 L/min or more and 40 L/min or less) per mold. If the amount of cooling water is less than this, the molten aluminum alloy M may not solidify and may leak from the mold 12. In addition, the surface of the cast aluminum alloy bar B may remelt, forming an uneven structure that may remain as an internal defect. On the other hand, if the amount of cooling water is more than this range, the mold 12 may lose too much heat, causing it to solidify midway.
- the average temperature of the molten aluminum alloy M flowing from the molten metal receiving portion 11 into the mold 12 is, for example, preferably 650°C or higher and 750°C or lower (more preferably 680°C or higher and 720°C or lower). If the temperature of the molten aluminum alloy M is too low, there is a risk that coarse crystals will form in the mold 12 or in front of it and will be incorporated into the aluminum alloy bar B as internal defects. On the other hand, if the temperature of the molten aluminum alloy M is too high, a large amount of hydrogen gas will be easily incorporated into the molten aluminum alloy M, which will be incorporated into the aluminum alloy bar B as porosity and may cause internal cavities.
- the cooling wall portion 27 of the mold 12 is configured so that the heat flux value per unit area is 10 ⁇ 10 5 W/m 2 or more, thereby making it possible to prevent seizure of the cast aluminum alloy bar B. In addition, it is preferable that the heat flux value per unit area is 50 ⁇ 10 5 W/m 2 or less.
- the aluminum alloy rod B thus obtained is cooled and solidified under conditions where the heat flux value per unit area in the cooling wall 27 is 10 ⁇ 10 5 W/m 2 or more, thereby suppressing adhesion of reaction products, such as carbides, caused by contact between the lubricating oil gas and the molten aluminum alloy M. This makes it unnecessary to cut and remove carbides, etc., on the surface of the aluminum alloy rod B, and allows the aluminum alloy rod B to be produced with a high yield.
- Molds are generally made of metal components with good thermal conductivity, and have a hollow structure to allow the introduction of a coolant inside.
- the mold used in this embodiment is appropriately selected from metals such as copper and aluminum, or graphite, from the viewpoint of heat transfer performance and durability at the contact point with the molten metal.
- the header is generally made of a refractory material and is installed on the upper side of the mold. There are no particular restrictions on the material and size of the header, and it can be appropriately selected depending on the composition range of the alloy to be cast and the dimensions of the cast product.
- the casting method described above makes it possible to obtain a uniform metal structure even in medium to large castings.
- the forging process is a process in which the aluminum alloy casting after casting is cut to a predetermined size, the obtained forging material is heated to a predetermined temperature, and then pressure is applied by a press machine to mold it into a die.
- the forging process is performed without performing the homogenization process that was conventionally performed after casting to remove segregation. Therefore, since it is necessary to perform the segregation removal performed by the homogenization process by heating the material during forging, it is necessary to perform the heating at a temperature of 500°C or higher and below the melting point. Then, the forging process is performed to obtain a forged product (for example, a suspension arm part of an automobile).
- the material heating temperature during forging is less than 500°C, compounds such as AlFeSi and Mg 2 Si in the alloy structure remain in a segregated state, the deformation resistance increases, making it impossible to perform sufficient processing, and cracks occur. Furthermore, if the temperature exceeds the melting point, defects such as eutectic melting are likely to occur.
- the forged product is subjected to solution treatment by holding it at a treatment temperature of 530°C or more and 560°C or less for 0.3 or more and 3 hours or less.
- the heating rate from room temperature to the above-mentioned treatment temperature is preferably 5.0°C/min or more. If the treatment temperature is less than 530°C, the solute elements may not be dissolved in solid solution. On the other hand, if the treatment temperature exceeds 560°C, the solute elements may be dissolved in solid solution more, but eutectic melting and recrystallization may occur easily. In addition, if the heating rate is less than 5.0°C/min, Mg 2 Si may precipitate coarsely. On the other hand, if the treatment temperature is less than 530°C, the solution treatment may not proceed, making it difficult to achieve high strength by aging precipitation.
- the quenching process is a process in which the forged product in the solid-solution state obtained in the solution treatment process is rapidly cooled to form a supersaturated solid solution.
- the forged product is placed in a water tank that stores water (quenching water) and quenched by submerging the forged product.
- the temperature of the water in the tank is preferably 20°C or higher and 60°C or lower.
- the forged product is preferably placed in the water tank for 5 seconds or higher and 60 seconds or lower after solution treatment so that all surfaces of the forged product are in contact with water.
- the submersion time of the forged product varies depending on the size of the casting, but is, for example, between more than 1 minute and 30 minutes.
- the aging treatment process is a process in which the forged product is heated and held at a relatively low temperature to precipitate the elements that are supersaturated in solid solution, thereby imparting an appropriate hardness.
- a method for producing an aluminum alloy forged product further includes, between the casting step and the forging step, a homogenization heat treatment step in which the aluminum alloy cast product is subjected to homogenization heat treatment by holding the aluminum alloy cast product at a temperature range of 370° C. or higher and 560° C. or lower for 2 hours or higher and 10 hours or lower.
- the method for producing an aluminum alloy forged product of this embodiment differs from the method for producing an aluminum alloy forged product of the above-described embodiment in that it includes a homogenization heat treatment step.
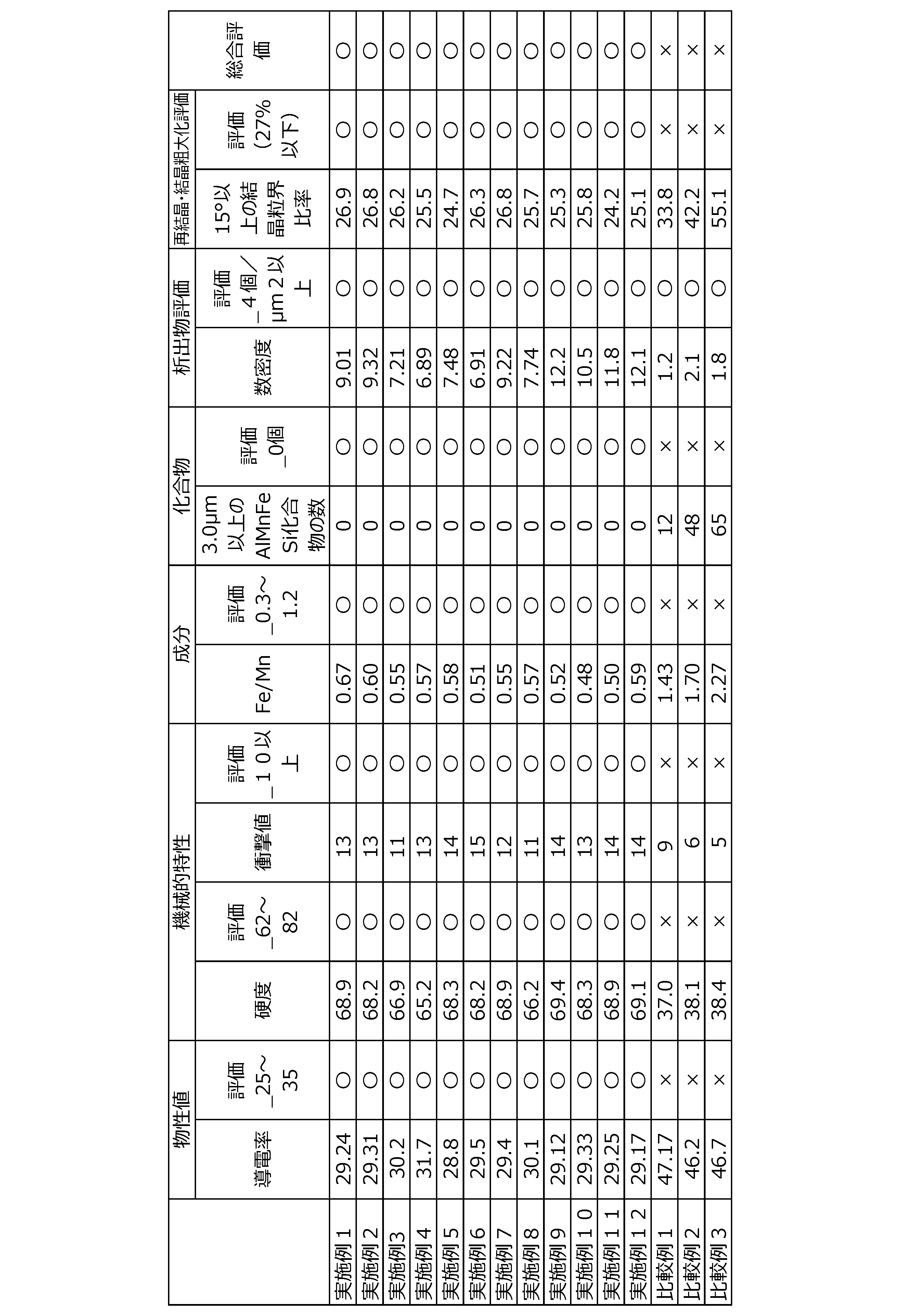
- Examples 1 to 8 and Comparative Examples 1 to 3 (Production of continuous cast products) First, an aluminum alloy having the alloy composition (the balance being aluminum) shown in the following Table 1 was prepared. A continuous cast product having a circular cross section and a diameter of 82 mm was produced from the prepared aluminum alloy.
- ⁇ Conductivity (%IACS)> The conductivity was measured at room temperature. (Judgment criteria) "Good”: 25% IACS or more and 35% IACS or less. “X”: Less than 25% IACS or more than 35% IACS.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Forging (AREA)
Abstract
Provided is an aluminum alloy forged product containing: 0.25-0.55 mass% of Cu; 0.60-1.25 mass% of Mg; 0.95-1.4 mass% of Si; 0.35-0.60 mass% of Mn; 0.15-0.30 mass% of Fe; at most 0.25 mass% of Zn; 0.050-0.30 mass% of Cr; 0.01-0.1 mass% of Ti; 0.0010-0.030 mass% of B; and 0.0010-0.050 mass% of Zr, wherein the Fe/Mn ratio is less than 1.4, the remainder consists of Al and unavoidable impurities, the number density of Mn-containing precipitates within 2.0 μm including grain boundaries is 4/μm2 or more, and when the fraction of high-angle grain boundaries having a crystal misorientation of 15° or more is 27% or less, the impact value at room temperature is 10 J/cm2 or more.
Description
本発明は、アルミニウム合金鍛造用素材、アルミニウム合金鍛造品及びその製造方法に関する。
本願は、2022年12月27日に、日本に出願された特願2022-210255号に基づき優先権を主張し、その内容をここに援用する。 The present invention relates to an aluminum alloy material for forging, an aluminum alloy forging, and a method for producing the same.
This application claims priority based on Japanese Patent Application No. 2022-210255, filed on December 27, 2022, the contents of which are incorporated herein by reference.
本願は、2022年12月27日に、日本に出願された特願2022-210255号に基づき優先権を主張し、その内容をここに援用する。 The present invention relates to an aluminum alloy material for forging, an aluminum alloy forging, and a method for producing the same.
This application claims priority based on Japanese Patent Application No. 2022-210255, filed on December 27, 2022, the contents of which are incorporated herein by reference.
近年、アルミニウム合金は、軽量性を生かして各種製品の構造部材としての用途が拡大しつつある。例えば、自動車の足廻りやバンパー部品では、今まで高張力鋼が用いられてきた。一方、近年は高強度アルミニウム合金材が用いられるようになっている。
In recent years, aluminum alloys have been increasingly used as structural components for various products, taking advantage of their light weight. For example, high-tensile steel has traditionally been used for automobile suspension and bumper parts. However, in recent years, high-strength aluminum alloy materials have come to be used.
また、自動車部品、その中でも、例えばサスペンション部品には、専ら鉄系材料が使用されていた。一方、近年は軽量化を主目的として、アルミニウム材料又はアルミニウム合金材料に置き換えられることが多くなってきた。
Furthermore, in the past, iron-based materials were used exclusively for automobile parts, particularly suspension parts. However, in recent years, they have increasingly been replaced with aluminum or aluminum alloy materials, primarily for the purpose of reducing weight.
これらの自動車部品では、優れた耐食性、高強度及び優れた加工性が要求されることから、アルミニウム合金材料としてAl-Mg-Si系合金、特にA6061が多用されている。そして、このような自動車部品は、強度の向上を図るため、アルミニウム合金材料を加工用素材として塑性加工の1つである鍛造加工を行って製造される。
Since these automotive parts require excellent corrosion resistance, high strength and excellent workability, Al-Mg-Si alloys, especially A6061, are often used as the aluminum alloy material. In order to improve the strength of these automotive parts, they are manufactured by forging, a type of plastic processing, using the aluminum alloy material as the processing material.
また、最近では、コストダウンを図る必要があるため、押出をせずに鋳造部材をそのまま素材として鍛造した後、溶体化処理と人工時効処理を行う処理(T6処理)して得たサスペンション部品が実用化され始めており、さらなる軽量化を目的として、従来のA6061に代わる高強度合金の開発が進められている(例えば、特許文献1~3を参照。)。
In addition, recently, due to the need to reduce costs, suspension parts have begun to be put to practical use in which the cast components are used as they are without extrusion, and then subjected to a solution treatment and artificial aging treatment (T6 treatment). In order to further reduce weight, development of high-strength alloys to replace the conventional A6061 is underway (see, for example, Patent Documents 1 to 3).
しかしながら、上述したAl-Mg-Si系の高強度合金は、鍛造及び熱処理工程において加工組織が再結晶し、粗大結晶粒が発生することにより、十分な高強度を得ることができないという問題があった。そのため、粗大再結晶粒生成防止のため、Zr(ジルコニウム)を添加して再結晶を防止しているものがある(例えば、上記特許文献1,2を参照。)。
However, the above-mentioned high-strength Al-Mg-Si alloys have the problem that the processed structure recrystallizes during the forging and heat treatment processes, resulting in the generation of coarse crystal grains, making it impossible to obtain sufficiently high strength. For this reason, some alloys have been designed to prevent recrystallization by adding Zr (zirconium) to prevent the generation of coarse recrystallized grains (see, for example, Patent Documents 1 and 2 above).
しかしながら、Zrを添加することは、再結晶防止に効果があるものの、次のような問題点があった。
(1)Zrの添加により、Al-Ti-B系合金の結晶粒微細化効果が弱められ、鋳塊自体の結晶粒が粗くなり、塑性加工後の加工品(鍛造品)の強度低下を招く。
(2)鋳塊自体の結晶粒微細化効果が弱められるため、鋳塊割れが発生し易くなり、内部欠陥が増加し、歩留まりが悪化する。
(3)Zrは、Al-Ti-B系合金と化合物を形成し、合金溶湯を貯留する炉の底に化合物が堆積し、炉を汚染すると共に、製造した鋳塊においてもこれら化合物が鋳塊中に粗大に晶出し、強度を低下させる。 Although the addition of Zr is effective in preventing recrystallization, it has the following problems.
(1) The addition of Zr weakens the effect of refining the crystal grains of the Al-Ti-B alloy, making the crystal grains of the ingot itself coarse, which leads to a decrease in the strength of the processed product (forged product) after plastic working.
(2) The effect of refining the crystal grains of the ingot itself is weakened, so that the ingot is more likely to crack, internal defects increase, and the yield decreases.
(3) Zr forms compounds with Al-Ti-B based alloys. These compounds accumulate at the bottom of the furnace in which the molten alloy is stored, contaminating the furnace. In addition, these compounds also crystallize out as coarse particles in the produced ingot, reducing its strength.
(1)Zrの添加により、Al-Ti-B系合金の結晶粒微細化効果が弱められ、鋳塊自体の結晶粒が粗くなり、塑性加工後の加工品(鍛造品)の強度低下を招く。
(2)鋳塊自体の結晶粒微細化効果が弱められるため、鋳塊割れが発生し易くなり、内部欠陥が増加し、歩留まりが悪化する。
(3)Zrは、Al-Ti-B系合金と化合物を形成し、合金溶湯を貯留する炉の底に化合物が堆積し、炉を汚染すると共に、製造した鋳塊においてもこれら化合物が鋳塊中に粗大に晶出し、強度を低下させる。 Although the addition of Zr is effective in preventing recrystallization, it has the following problems.
(1) The addition of Zr weakens the effect of refining the crystal grains of the Al-Ti-B alloy, making the crystal grains of the ingot itself coarse, which leads to a decrease in the strength of the processed product (forged product) after plastic working.
(2) The effect of refining the crystal grains of the ingot itself is weakened, so that the ingot is more likely to crack, internal defects increase, and the yield decreases.
(3) Zr forms compounds with Al-Ti-B based alloys. These compounds accumulate at the bottom of the furnace in which the molten alloy is stored, contaminating the furnace. In addition, these compounds also crystallize out as coarse particles in the produced ingot, reducing its strength.
このように、Zrの添加は、再結晶防止に効果があるものの、強度の安定性を維持するのが困難であった。
Thus, although the addition of Zr was effective in preventing recrystallization, it was difficult to maintain strength stability.
本発明は、かかる技術的背景に鑑みてなされたものであって、常温における機械的特性に優れたアルミニウム合金鍛造用素材、アルミニウム合金鍛造品及びその製造方法を提供することを目的とする。
The present invention was made in consideration of this technical background, and aims to provide an aluminum alloy forging material that has excellent mechanical properties at room temperature, an aluminum alloy forging product, and a manufacturing method thereof.
本発明は、上記課題を解決するため、以下の手段を提供する。
The present invention provides the following means to solve the above problems.
本発明の態様1は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造用素材であって、鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である、アルミニウム合金鍛造用素材である。
Aspect 1 of the present invention is a composition in which Cu is in the range of 0.25 mass% or more and 0.55 mass% or less, Mg is in the range of 0.60 mass% or more and 1.25 mass% or less, Si is in the range of 0.90 mass% or more and 1.4 mass% or less, Mn is in the range of 0.35 mass% or more and 0.60 mass% or less, Fe is in the range of 0.15 mass% or more and 0.30 mass% or less, Zn is in the range of 0.25 mass% or less, Cr is in the range of 0.050 mass% or more and 0.30 mass% or less, Ti is in the range of 0.01 mass% or more and 0.1 mass% or less, and B is in the range of 0.0010 mass% or less. This aluminum alloy forging material is composed of an aluminum alloy having an alloy composition containing 0.010% to 0.030% by mass, 0.0010% to 0.050% by mass of Zr, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 by mass, and the balance consisting of Al and unavoidable impurities, and has a post-cast electrical conductivity of 25% IACS to 35% IACS and a Rockwell hardness HRF of 62 to 82.
本発明の態様2は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造用素材であって、鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である、アルミニウム合金鍛造用素材である。
Aspect 2 of the present invention is a composition comprising Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, Ti in the range of 0.01 mass% or more and 0.1 mass% or less, and B in the range of 0.0010 mass% or less. The aluminum alloy forging material is composed of an aluminum alloy having an alloy composition containing 0.030% by mass or more, 0.0010% by mass or more and 0.050% by mass or less of Zr, a ratio of the Fe content to the Mn content Fe/Mn of 0.3 to 1.2 by mass, and the balance being Al and unavoidable impurities, and has a post-cast electrical conductivity of 25% IACS to 35% IACS and a Rockwell hardness HRF of 62 to 82.
本発明の態様3は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下であり、かつ、常温における衝撃値が10J/cm2以上である、アルミニウム合金鍛造品である。
Aspect 3 of the present invention is a steel sheet having a Cu content in the range of 0.25 mass% or more and 0.55 mass% or less, a Mg content in the range of 0.60 mass% or more and 1.25 mass% or less, a Si content in the range of 0.90 mass% or more and 1.4 mass% or less, a Mn content in the range of 0.35 mass% or more and 0.60 mass% or less, a Fe content in the range of 0.15 mass% or more and 0.30 mass% or less, a Zn content in the range of 0.25 mass% or less, a Cr content in the range of 0.050 mass% or more and 0.30 mass% or less, a Ti content in the range of 0.01 mass% or more and 0.1 % or less by mass, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 in terms of mass ratio, and the balance being Al and unavoidable impurities, wherein the number density of precipitates containing Mn within 2.0 μm including grain boundaries is 4 precipitates/μm2 or more , the ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more is 27% or less, and the impact value at room temperature is 10 J/ cm2 or more.
本発明の態様4は、態様3のアルミニウム合金鍛造品において、前記析出物のサイズが0.5μm以下である。
In aspect 4 of the present invention, in the aluminum alloy forged product of aspect 3, the size of the precipitates is 0.5 μm or less.
本発明の態様5は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下であり、かつ、常温における衝撃値が10J/cm2以上である、アルミニウム合金鍛造品である。
Aspect 5 of the present invention is a steel sheet having a Cu content in the range of 0.25 mass% or more and 0.55 mass% or less, an Mg content in the range of 0.60 mass% or more and 1.25 mass% or less, an Si content in the range of 0.90 mass% or more and 1.4 mass% or less, an Mn content in the range of 0.35 mass% or more and 0.60 mass% or less, an Fe content in the range of 0.15 mass% or more and 0.30 mass% or less, an Zn content in the range of 0.25 mass% or less, an Cr content in the range of 0.050 mass% or more and 0.30 mass% or less, an Ti content in the range of 0.01 mass% or more and 0.1 mass% or less, and an Zn content in the range of 0.02 mass% or more and 0.05 mass% or less. % or less, B in the range of 0.0010 mass % or more and 0.030 mass % or less, Zr in the range of 0.0010 mass % or more and 0.050 mass % or less, a ratio of the Fe content to the Mn content Fe/Mn is 0.3 to 1.2 in mass ratio, and the balance is an alloy composition consisting of Al and unavoidable impurities, wherein the number density of precipitates containing Mn within 2.0 μm including grain boundaries is 4 precipitates/μm2 or more , the ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more is 27% or less, and the aluminum alloy forging has an impact value of 10 J/cm2 or more at room temperature.
本発明の態様6は、態様5のアルミニウム合金鍛造品において、前記析出物のサイズが0.5μm以下である。
Aspect 6 of the present invention is an aluminum alloy forged product according to aspect 5, in which the size of the precipitates is 0.5 μm or less.
本発明の態様7は、態様3から態様6のいずれか一つのアルミニウム合金鍛造品の製造方法であって、前記アルミニウム合金の溶湯を得る溶湯形成工程と、前記得られた溶湯を鋳造加工することによって鋳造品を得る鋳造工程と、前記鋳造品を500℃~融点以下の温度で素材加熱し塑性加工を施して鍛造品を得る鍛造工程と、前記得られた鍛造品に20℃~500℃までの昇温速度が5.0℃/min以上で昇温し、530~560℃で0.3~3時間以内で保持する溶体化処理を行う溶体化処理工程と、前記溶体化処理後5~60秒以内に前記鍛造品の全ての表面が焼き入れ水に接触し、1分を超え、40分以内水槽内で焼き入れする焼き入れ工程と、前記焼き入れ処理工程を経た鍛造品に180℃~220℃の温度で0.5時間~8時間加熱して時効処理を行う時効処理工程と、を有する、アルミニウム合金鍛造品の製造方法である。
Aspect 7 of the present invention is a method for producing an aluminum alloy forging according to any one of aspects 3 to 6, comprising the steps of: forming a molten metal of the aluminum alloy; casting the molten metal to obtain a casting; heating the casting at a temperature between 500°C and the melting point and performing plastic processing to obtain a forging; solution treatment of the forging at a temperature of 20°C to 500°C at a heating rate of 5.0°C/min or more and maintaining the temperature at 530 to 560°C for 0.3 to 3 hours; quenching the entire surface of the forging in contact with quenching water within 5 to 60 seconds after the solution treatment and quenching in a water tank for more than 1 minute and not exceeding 40 minutes; and aging treatment of the forging after the quenching treatment at a temperature of 180°C to 220°C for 0.5 to 8 hours to perform aging treatment.
本発明の態様8は、態様4のアルミニウム合金鍛造品の製造方法において、前記鋳造工程と前記鍛造工程との間に、前記アルミニウム合金鋳造品を、370℃以上560℃以下の温度範囲で2時間以上10時間以下保持して均質化熱処理を行う均質化熱処理工程を更に有する。
Aspect 8 of the present invention is a method for manufacturing an aluminum alloy forged product according to aspect 4, which further includes a homogenization heat treatment step between the casting step and the forging step, in which the aluminum alloy casting product is held at a temperature range of 370°C to 560°C for 2 hours to 10 hours to perform homogenization heat treatment.
本発明によれば、常温における機械的特性に優れたアルミニウム合金鍛造用素材を提供できる。
本発明によれば、常温における機械的特性に優れたアルミニウム合金鍛造品を提供できる。
また、本発明によれば、これまでアルミ合金溶湯を鋳造したのちに、偏析除去するために施していた均質化処理工程を削減している為、低コスト・省エネなアルミニウム合金鍛造品の製造方法を提供できる。 According to the present invention, it is possible to provide an aluminum alloy material for forging that has excellent mechanical properties at room temperature.
According to the present invention, it is possible to provide an aluminum alloy forging having excellent mechanical properties at room temperature.
Furthermore, according to the present invention, the homogenization treatment step that has been conventionally performed after casting of the molten aluminum alloy to remove segregation is eliminated, thereby providing a low-cost, energy-saving method for producing aluminum alloy forged products.
本発明によれば、常温における機械的特性に優れたアルミニウム合金鍛造品を提供できる。
また、本発明によれば、これまでアルミ合金溶湯を鋳造したのちに、偏析除去するために施していた均質化処理工程を削減している為、低コスト・省エネなアルミニウム合金鍛造品の製造方法を提供できる。 According to the present invention, it is possible to provide an aluminum alloy material for forging that has excellent mechanical properties at room temperature.
According to the present invention, it is possible to provide an aluminum alloy forging having excellent mechanical properties at room temperature.
Furthermore, according to the present invention, the homogenization treatment step that has been conventionally performed after casting of the molten aluminum alloy to remove segregation is eliminated, thereby providing a low-cost, energy-saving method for producing aluminum alloy forged products.
以下、本発明の実施形態について、図面を参照して詳細に説明する。
なお、以下の説明で用いる図面は、特徴をわかりやすくするために、便宜上特徴となる部分を拡大して示している場合があり、各構成要素の寸法比率などが実際と同じであるとは限らない。また、以下の説明において例示される材料、寸法等は一例であって、本発明はそれらに必ずしも限定されるものではなく、その効果を変更しない範囲で適宜変更して実施することが可能である。 Hereinafter, an embodiment of the present invention will be described in detail with reference to the drawings.
In addition, the drawings used in the following description may show characteristic parts in an enlarged scale for the sake of convenience in order to make the characteristics easier to understand, and the dimensional ratios of each component may not necessarily be the same as in reality. Furthermore, the materials, dimensions, etc. exemplified in the following description are merely examples, and the present invention is not necessarily limited to them, and may be appropriately modified and implemented within a range that does not change the effects of the present invention.
なお、以下の説明で用いる図面は、特徴をわかりやすくするために、便宜上特徴となる部分を拡大して示している場合があり、各構成要素の寸法比率などが実際と同じであるとは限らない。また、以下の説明において例示される材料、寸法等は一例であって、本発明はそれらに必ずしも限定されるものではなく、その効果を変更しない範囲で適宜変更して実施することが可能である。 Hereinafter, an embodiment of the present invention will be described in detail with reference to the drawings.
In addition, the drawings used in the following description may show characteristic parts in an enlarged scale for the sake of convenience in order to make the characteristics easier to understand, and the dimensional ratios of each component may not necessarily be the same as in reality. Furthermore, the materials, dimensions, etc. exemplified in the following description are merely examples, and the present invention is not necessarily limited to them, and may be appropriately modified and implemented within a range that does not change the effects of the present invention.
[アルミニウム合金鍛造用素材]
先ず、本発明の一実施形態に係るアルミニウム合金鍛造用素材である。
本実施形態のアルミニウム合金鍛造用素材は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金鍛造用素材であって、鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である。 [Aluminum alloy forging materials]
First, an aluminum alloy forging material according to one embodiment of the present invention.
The aluminum alloy forging material of this embodiment contains Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, Ti in the range of 0.01 mass% or more and 0.02 mass% or less, and Zn in the range of 0.03 mass% or more and 0.04 mass% or less. 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 by mass, with the remainder being Al and unavoidable impurities, and the aluminum alloy forging material has an electrical conductivity after casting of 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less.
先ず、本発明の一実施形態に係るアルミニウム合金鍛造用素材である。
本実施形態のアルミニウム合金鍛造用素材は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金鍛造用素材であって、鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である。 [Aluminum alloy forging materials]
First, an aluminum alloy forging material according to one embodiment of the present invention.
The aluminum alloy forging material of this embodiment contains Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, Ti in the range of 0.01 mass% or more and 0.02 mass% or less, and Zn in the range of 0.03 mass% or more and 0.04 mass% or less. 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 by mass, with the remainder being Al and unavoidable impurities, and the aluminum alloy forging material has an electrical conductivity after casting of 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less.
本発明の別の実施形態に係るアルミニウム合金鍛造用素材は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造用素材であって、鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である。
Fe/Mnは、質量比で0.3以上1.0以下とすることができ、また、0.4以上0.8以下とすることができ、また、0.4以上0.7以下とすることができる。 An aluminum alloy forging material according to another embodiment of the present invention has Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, and the range below, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content Fe/Mn being 0.3 to 1.2 in mass ratio, with the balance being Al and unavoidable impurities, and the aluminum alloy forging material is composed of an aluminum alloy having an alloy composition, the electrical conductivity after casting being 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less.
The Fe/Mn mass ratio can be set to 0.3 or more and 1.0 or less, can be set to 0.4 or more and 0.8 or less, or can be set to 0.4 or more and 0.7 or less.
Fe/Mnは、質量比で0.3以上1.0以下とすることができ、また、0.4以上0.8以下とすることができ、また、0.4以上0.7以下とすることができる。 An aluminum alloy forging material according to another embodiment of the present invention has Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, and the range below, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content Fe/Mn being 0.3 to 1.2 in mass ratio, with the balance being Al and unavoidable impurities, and the aluminum alloy forging material is composed of an aluminum alloy having an alloy composition, the electrical conductivity after casting being 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less.
The Fe/Mn mass ratio can be set to 0.3 or more and 1.0 or less, can be set to 0.4 or more and 0.8 or less, or can be set to 0.4 or more and 0.7 or less.
上記実施形態のアルミニウム合金鍛造用素材は、MgとSiを含む点で6000系アルミニウム合金に相当する。
The aluminum alloy forging material in the above embodiment corresponds to the 6000 series aluminum alloy in that it contains Mg and Si.
(導電率:25%IACS以上35%IACS以下)
上記実施形態のアルミニウム合金鍛造用素材の導電率は、25%IACS以上35%IACS以下である。この導電率は室温における導電率であるとし、この室温とは20℃±15℃程度とする。
導電率が25%IACS未満の場合は硬すぎて加工性が低下する、35%IACSを超える場合は柔らかいことで切削性(切粉分断性)の悪化、及び最終製品の保証強度を満足できないことがある。 (Conductivity: 25% IACS or more and 35% IACS or less)
The electrical conductivity of the aluminum alloy forging material of the above embodiment is 25% IACS or more and 35% IACS or less. This electrical conductivity is the electrical conductivity at room temperature, which is about 20°C ± 15°C.
If the electrical conductivity is less than 25% IACS, the material will be too hard and its workability will decrease, whereas if it exceeds 35% IACS, the material will be too soft, resulting in poor machinability (ability to break away chips), and the guaranteed strength of the final product may not be satisfied.
上記実施形態のアルミニウム合金鍛造用素材の導電率は、25%IACS以上35%IACS以下である。この導電率は室温における導電率であるとし、この室温とは20℃±15℃程度とする。
導電率が25%IACS未満の場合は硬すぎて加工性が低下する、35%IACSを超える場合は柔らかいことで切削性(切粉分断性)の悪化、及び最終製品の保証強度を満足できないことがある。 (Conductivity: 25% IACS or more and 35% IACS or less)
The electrical conductivity of the aluminum alloy forging material of the above embodiment is 25% IACS or more and 35% IACS or less. This electrical conductivity is the electrical conductivity at room temperature, which is about 20°C ± 15°C.
If the electrical conductivity is less than 25% IACS, the material will be too hard and its workability will decrease, whereas if it exceeds 35% IACS, the material will be too soft, resulting in poor machinability (ability to break away chips), and the guaranteed strength of the final product may not be satisfied.
(ロックウェル硬さHRF:62以上82以下)
上記実施形態のアルミニウム合金鍛造用素材のロックウェル硬さHRFは、62以上82以下である。ここで、ロックウェル硬さHRFはJISZ2245:2016の「ロックウェル硬さ試験-試験方法」に準拠して測定された値である。
ロックウェル硬さHRFがこの範囲であれば、加工性が良好だからである。すなわち、ロックウェル硬さHRFが62未満の場合は柔らかいことで切削性(切粉分断性)の悪化、及び最終製品の保証強度を満足できないことがあり、82を超える場合は硬すぎて加工性が低下する。 (Rockwell hardness HRF: 62 or more and 82 or less)
The Rockwell hardness HRF of the aluminum alloy forging material of the above embodiment is 62 or more and 82 or less. Here, the Rockwell hardness HRF is a value measured in accordance with JIS Z2245:2016 "Rockwell hardness test - Test method".
This is because the workability is good if the Rockwell hardness HRF is within this range. That is, if the Rockwell hardness HRF is less than 62, the material is too soft, resulting in poor machinability (chip breakability) and inability to satisfy the guaranteed strength of the final product, while if it exceeds 82, the material is too hard, resulting in poor workability.
上記実施形態のアルミニウム合金鍛造用素材のロックウェル硬さHRFは、62以上82以下である。ここで、ロックウェル硬さHRFはJISZ2245:2016の「ロックウェル硬さ試験-試験方法」に準拠して測定された値である。
ロックウェル硬さHRFがこの範囲であれば、加工性が良好だからである。すなわち、ロックウェル硬さHRFが62未満の場合は柔らかいことで切削性(切粉分断性)の悪化、及び最終製品の保証強度を満足できないことがあり、82を超える場合は硬すぎて加工性が低下する。 (Rockwell hardness HRF: 62 or more and 82 or less)
The Rockwell hardness HRF of the aluminum alloy forging material of the above embodiment is 62 or more and 82 or less. Here, the Rockwell hardness HRF is a value measured in accordance with JIS Z2245:2016 "Rockwell hardness test - Test method".
This is because the workability is good if the Rockwell hardness HRF is within this range. That is, if the Rockwell hardness HRF is less than 62, the material is too soft, resulting in poor machinability (chip breakability) and inability to satisfy the guaranteed strength of the final product, while if it exceeds 82, the material is too hard, resulting in poor workability.
[アルミニウム合金鍛造品]
本発明の一実施形態に係るアルミニウム合金鍛造品について説明する。
図1は、本発明の一実施形態に係るアルミニウム合金鍛造品の斜視図である。
図1に示すように、アルミニウム合金鍛造品1aは、長尺部2と、長尺部2の長手方向の両端にそれぞれ接続された連結部4a,4bとを有する。長尺部は断面が4角形とされている。これら2つの連結部4には、それぞれ貫通孔が設けられていればよい。この形状のアルミニウム合金鍛造品1aは、例えば、I型サスペンションアームとして用いることができる。 [Aluminum alloy forgings]
An aluminum alloy forging according to one embodiment of the present invention will be described.
FIG. 1 is a perspective view of an aluminum alloy forging according to one embodiment of the present invention.
As shown in Fig. 1, an aluminum alloy forging 1a has along portion 2 and connecting portions 4a, 4b connected to both ends of the long portion 2 in the longitudinal direction. The long portion has a rectangular cross section. Each of the two connecting portions 4 may have a through hole. The aluminum alloy forging 1a having this shape can be used as, for example, an I-type suspension arm.
本発明の一実施形態に係るアルミニウム合金鍛造品について説明する。
図1は、本発明の一実施形態に係るアルミニウム合金鍛造品の斜視図である。
図1に示すように、アルミニウム合金鍛造品1aは、長尺部2と、長尺部2の長手方向の両端にそれぞれ接続された連結部4a,4bとを有する。長尺部は断面が4角形とされている。これら2つの連結部4には、それぞれ貫通孔が設けられていればよい。この形状のアルミニウム合金鍛造品1aは、例えば、I型サスペンションアームとして用いることができる。 [Aluminum alloy forgings]
An aluminum alloy forging according to one embodiment of the present invention will be described.
FIG. 1 is a perspective view of an aluminum alloy forging according to one embodiment of the present invention.
As shown in Fig. 1, an aluminum alloy forging 1a has a
本実施形態のアルミニウム合金鍛造品は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下である。
また、本実施形態のアルミニウム合金鍛造品は、常温における衝撃値において、10J/cm2以上とされている。 The aluminum alloy forging of this embodiment contains Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, and Ti in the range of 0.01 mass% or less. and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 in terms of mass ratio, and the balance being Al and unavoidable impurities.
Moreover, the aluminum alloy forged product of this embodiment has an impact value of 10 J/cm 2 or more at room temperature.
また、本実施形態のアルミニウム合金鍛造品は、常温における衝撃値において、10J/cm2以上とされている。 The aluminum alloy forging of this embodiment contains Cu in the range of 0.25 mass% or more and 0.55 mass% or less, Mg in the range of 0.60 mass% or more and 1.25 mass% or less, Si in the range of 0.90 mass% or more and 1.4 mass% or less, Mn in the range of 0.35 mass% or more and 0.60 mass% or less, Fe in the range of 0.15 mass% or more and 0.30 mass% or less, Zn in the range of 0.25 mass% or less, Cr in the range of 0.050 mass% or more and 0.30 mass% or less, and Ti in the range of 0.01 mass% or less. and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) of less than 1.4 in terms of mass ratio, and the balance being Al and unavoidable impurities.
Moreover, the aluminum alloy forged product of this embodiment has an impact value of 10 J/cm 2 or more at room temperature.
粒界を含む2.0μm以内に含まれるMnを含む析出物のサイズを例示すると、0.5μm以下である。
For example, the size of precipitates containing Mn within 2.0 μm, including grain boundaries, is 0.5 μm or less.
本実施形態のアルミニウム合金鍛造品は、MgとSiを含む点で6000系アルミニウム合金の鍛造品に相当する。
The aluminum alloy forgings of this embodiment correspond to 6000 series aluminum alloy forgings in that they contain Mg and Si.
(Cu:0.25質量%以上、0.55質量%以下)
Cuは、アルミニウム合金中でMg-Si系化合物を微細に分散させる作用や、Q相を始めとするAl-Cu-Mg-Si系化合物として析出することでアルミニウム合金の引張強さを向上させる作用を有する。Cuの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Cu: 0.25% by mass or more, 0.55% by mass or less)
Cu has the effect of finely dispersing Mg-Si compounds in the aluminum alloy and the effect of improving the tensile strength of the aluminum alloy by precipitating as Al-Cu-Mg-Si compounds including the Q phase. By keeping the Cu content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
Cuは、アルミニウム合金中でMg-Si系化合物を微細に分散させる作用や、Q相を始めとするAl-Cu-Mg-Si系化合物として析出することでアルミニウム合金の引張強さを向上させる作用を有する。Cuの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Cu: 0.25% by mass or more, 0.55% by mass or less)
Cu has the effect of finely dispersing Mg-Si compounds in the aluminum alloy and the effect of improving the tensile strength of the aluminum alloy by precipitating as Al-Cu-Mg-Si compounds including the Q phase. By keeping the Cu content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
(Mg:0.60質量%以上、1.25質量%以下)
Mgは、アルミニウム合金の引張強さを向上させる作用を有する。アルミニウム母相へMgが固溶する、あるいは、β”相などのMg-Si系化合物(Mg2Si)、またはQ相を始めとするAl-Cu-Mg-Si系化合物(AlCuMgSi)として析出することで、アルミニウム合金の強化に寄与する。また、Mg2Siは、アルミニウム合金中でのCuAl2相の生成を抑制する作用がある。CuAl2相の生成が抑制されることによって、アルミニウム合金鍛造品1aの耐食性が向上する。Mgの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性とともに耐食性を向上させることができる。 (Mg: 0.60% by mass or more, 1.25% by mass or less)
Mg has the effect of improving the tensile strength of the aluminum alloy. Mg contributes to strengthening the aluminum alloy by dissolving in the aluminum parent phase or precipitating as Mg-Si compounds (Mg 2 Si) such as the β″ phase, or Al-Cu-Mg-Si compounds (AlCuMgSi) such as the Q phase. Mg 2 Si also has the effect of suppressing the formation of CuAl 2 phase in the aluminum alloy. By suppressing the formation of CuAl 2 phase, the corrosion resistance of the aluminum alloy forging 1a is improved. By keeping the Mg content within the above range, it is possible to improve the corrosion resistance as well as the mechanical properties at room temperature of the aluminum alloy forging 1a.
Mgは、アルミニウム合金の引張強さを向上させる作用を有する。アルミニウム母相へMgが固溶する、あるいは、β”相などのMg-Si系化合物(Mg2Si)、またはQ相を始めとするAl-Cu-Mg-Si系化合物(AlCuMgSi)として析出することで、アルミニウム合金の強化に寄与する。また、Mg2Siは、アルミニウム合金中でのCuAl2相の生成を抑制する作用がある。CuAl2相の生成が抑制されることによって、アルミニウム合金鍛造品1aの耐食性が向上する。Mgの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性とともに耐食性を向上させることができる。 (Mg: 0.60% by mass or more, 1.25% by mass or less)
Mg has the effect of improving the tensile strength of the aluminum alloy. Mg contributes to strengthening the aluminum alloy by dissolving in the aluminum parent phase or precipitating as Mg-Si compounds (Mg 2 Si) such as the β″ phase, or Al-Cu-Mg-Si compounds (AlCuMgSi) such as the Q phase. Mg 2 Si also has the effect of suppressing the formation of CuAl 2 phase in the aluminum alloy. By suppressing the formation of CuAl 2 phase, the corrosion resistance of the aluminum alloy forging 1a is improved. By keeping the Mg content within the above range, it is possible to improve the corrosion resistance as well as the mechanical properties at room temperature of the aluminum alloy forging 1a.
(Si:0.90質量%以上、1.4質量%以下)
Siは、Mgと同様にアルミニウム合金鍛造品1aの常温における機械的特性と共に耐食性を向上させる作用を有する。但し、アルミニウム合金にSiを過剰に添加すると、粗大な初晶Si粒が晶出することにより、アルミニウム合金の引張強さが低下するおそれがある。Siの含有率が上記の範囲内にあることによって、初晶Siの晶出を抑えつつ、アルミニウム合金鍛造品1aの常温における機械的特性と共に耐食性を向上させることができる。 (Si: 0.90 mass% or more, 1.4 mass% or less)
Like Mg, Si has the effect of improving the mechanical properties and corrosion resistance of the aluminum alloy forging 1a at room temperature. However, if excessive Si is added to the aluminum alloy, coarse primary crystal Si grains may crystallize, which may reduce the tensile strength of the aluminum alloy. By keeping the Si content within the above range, it is possible to improve the mechanical properties and corrosion resistance of the aluminum alloy forging 1a at room temperature while suppressing the crystallization of primary crystal Si.
Siは、Mgと同様にアルミニウム合金鍛造品1aの常温における機械的特性と共に耐食性を向上させる作用を有する。但し、アルミニウム合金にSiを過剰に添加すると、粗大な初晶Si粒が晶出することにより、アルミニウム合金の引張強さが低下するおそれがある。Siの含有率が上記の範囲内にあることによって、初晶Siの晶出を抑えつつ、アルミニウム合金鍛造品1aの常温における機械的特性と共に耐食性を向上させることができる。 (Si: 0.90 mass% or more, 1.4 mass% or less)
Like Mg, Si has the effect of improving the mechanical properties and corrosion resistance of the aluminum alloy forging 1a at room temperature. However, if excessive Si is added to the aluminum alloy, coarse primary crystal Si grains may crystallize, which may reduce the tensile strength of the aluminum alloy. By keeping the Si content within the above range, it is possible to improve the mechanical properties and corrosion resistance of the aluminum alloy forging 1a at room temperature while suppressing the crystallization of primary crystal Si.
(Mn:0.35質量%以上、0.60質量%以下)
Mnは、アルミニウム合金中でAl-Mn-Fe-SiやAl-Mn-Cr-Fe-Siなどの金属間化合物を含む微細な粒状の晶出物を形成することで、アルミニウム合金の引張強さを向上させる作用を有する。Mnの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Mn: 0.35 mass% or more, 0.60 mass% or less)
Mn has the effect of improving the tensile strength of the aluminum alloy by forming fine granular precipitates containing intermetallic compounds such as Al-Mn-Fe-Si and Al-Mn-Cr-Fe-Si in the aluminum alloy. When the Mn content is within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
Mnは、アルミニウム合金中でAl-Mn-Fe-SiやAl-Mn-Cr-Fe-Siなどの金属間化合物を含む微細な粒状の晶出物を形成することで、アルミニウム合金の引張強さを向上させる作用を有する。Mnの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Mn: 0.35 mass% or more, 0.60 mass% or less)
Mn has the effect of improving the tensile strength of the aluminum alloy by forming fine granular precipitates containing intermetallic compounds such as Al-Mn-Fe-Si and Al-Mn-Cr-Fe-Si in the aluminum alloy. When the Mn content is within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
(Fe:0.15質量%以上、0.30質量%以下)
Feは、アルミニウム合金中でAl-Mn-Fe-Si、Al-Mn-Cr-Fe-Si、Al-Fe-Si、Al-Cu-Fe、Al-Mn-Feなどの金属間化合物を含む微細な晶出物として晶出することで、アルミニウム合金の引張強さを向上させる作用がある。Feの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。
なお、Fe/Mnの関係は1.4未満である。Fe/Mnの関係が1.4未満であることによって、3.0μm以上のAlFeSi系化合物の晶出を抑制することができ、且つ結晶粒内にAlMn系化合物の数密度を向上させることができる。 (Fe: 0.15 mass% or more, 0.30 mass% or less)
Fe has the effect of improving the tensile strength of the aluminum alloy by crystallizing in the aluminum alloy as fine crystallized products including intermetallic compounds such as Al-Mn-Fe-Si, Al-Mn-Cr-Fe-Si, Al-Fe-Si, Al-Cu-Fe, Al-Mn-Fe, etc. By keeping the Fe content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
The Fe/Mn relationship is less than 1.4. By making the Fe/Mn relationship less than 1.4, it is possible to suppress crystallization of AlFeSi-based compounds having a size of 3.0 μm or more, and to improve the number density of AlMn-based compounds in crystal grains.
Feは、アルミニウム合金中でAl-Mn-Fe-Si、Al-Mn-Cr-Fe-Si、Al-Fe-Si、Al-Cu-Fe、Al-Mn-Feなどの金属間化合物を含む微細な晶出物として晶出することで、アルミニウム合金の引張強さを向上させる作用がある。Feの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。
なお、Fe/Mnの関係は1.4未満である。Fe/Mnの関係が1.4未満であることによって、3.0μm以上のAlFeSi系化合物の晶出を抑制することができ、且つ結晶粒内にAlMn系化合物の数密度を向上させることができる。 (Fe: 0.15 mass% or more, 0.30 mass% or less)
Fe has the effect of improving the tensile strength of the aluminum alloy by crystallizing in the aluminum alloy as fine crystallized products including intermetallic compounds such as Al-Mn-Fe-Si, Al-Mn-Cr-Fe-Si, Al-Fe-Si, Al-Cu-Fe, Al-Mn-Fe, etc. By keeping the Fe content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
The Fe/Mn relationship is less than 1.4. By making the Fe/Mn relationship less than 1.4, it is possible to suppress crystallization of AlFeSi-based compounds having a size of 3.0 μm or more, and to improve the number density of AlMn-based compounds in crystal grains.
(Cr:0.050質量%以上、0.30質量%以下)
Crは、アルミニウム合金中でAl-Mn-Cr-Fe-SiやAl-Fe-Crなどの金属間化合物を含む微細な粒状の晶出物を形成することで、アルミニウム合金の引張強さを向上させる作用を有する。Crの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Cr: 0.050 mass% or more, 0.30 mass% or less)
Cr has the effect of improving the tensile strength of the aluminum alloy by forming fine granular crystallized products including intermetallic compounds such as Al-Mn-Cr-Fe-Si and Al-Fe-Cr in the aluminum alloy. By having the Cr content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
Crは、アルミニウム合金中でAl-Mn-Cr-Fe-SiやAl-Fe-Crなどの金属間化合物を含む微細な粒状の晶出物を形成することで、アルミニウム合金の引張強さを向上させる作用を有する。Crの含有率が上記の範囲内にあることによって、アルミニウム合金鍛造品1aの常温における機械的特性を向上させることができる。 (Cr: 0.050 mass% or more, 0.30 mass% or less)
Cr has the effect of improving the tensile strength of the aluminum alloy by forming fine granular crystallized products including intermetallic compounds such as Al-Mn-Cr-Fe-Si and Al-Fe-Cr in the aluminum alloy. By having the Cr content within the above range, the mechanical properties of the aluminum alloy forging 1a at room temperature can be improved.
(Ti:0.01質量%以上、0.1質量%以下)
Tiは、アルミニウム合金の結晶粒を微細化し、展伸加工性を向上させる作用を有する。Ti含有率が0.01質量%未満の場合、結晶粒の微細化効果が十分に得られないおそれがある。一方、Ti含有率が0.1質量%を超えると、粗大な晶出物を形成し、展伸加工性が低下するおそれがある。また、アルミニウム合金鍛造品1aにTiを含む粗大な晶出物が多量に混入すると靭性が低下する場合がある。したがって、Tiの含有率は0.012質量%以上、0.035質量%以下とする。Tiの含有率は、好ましくは0.015質量%以上、0.050質量%以下である。 (Ti: 0.01 mass% or more, 0.1 mass% or less)
Ti has the effect of refining the crystal grains of the aluminum alloy and improving the wrought workability. If the Ti content is less than 0.01% by mass, the effect of refining the crystal grains may not be sufficiently obtained. On the other hand, if the Ti content exceeds 0.1% by mass, coarse crystallized products may be formed, and the wrought workability may be reduced. In addition, if a large amount of coarse crystallized products containing Ti are mixed into the aluminum alloy forgedproduct 1a, the toughness may be reduced. Therefore, the Ti content is set to 0.012% by mass or more and 0.035% by mass or less. The Ti content is preferably 0.015% by mass or more and 0.050% by mass or less.
Tiは、アルミニウム合金の結晶粒を微細化し、展伸加工性を向上させる作用を有する。Ti含有率が0.01質量%未満の場合、結晶粒の微細化効果が十分に得られないおそれがある。一方、Ti含有率が0.1質量%を超えると、粗大な晶出物を形成し、展伸加工性が低下するおそれがある。また、アルミニウム合金鍛造品1aにTiを含む粗大な晶出物が多量に混入すると靭性が低下する場合がある。したがって、Tiの含有率は0.012質量%以上、0.035質量%以下とする。Tiの含有率は、好ましくは0.015質量%以上、0.050質量%以下である。 (Ti: 0.01 mass% or more, 0.1 mass% or less)
Ti has the effect of refining the crystal grains of the aluminum alloy and improving the wrought workability. If the Ti content is less than 0.01% by mass, the effect of refining the crystal grains may not be sufficiently obtained. On the other hand, if the Ti content exceeds 0.1% by mass, coarse crystallized products may be formed, and the wrought workability may be reduced. In addition, if a large amount of coarse crystallized products containing Ti are mixed into the aluminum alloy forged
(B:0.001質量%以上、0.03質量%以下)
Bは、アルミニウム合金の結晶粒を微細化し、展伸加工性を向上させる作用を有する。上述したTiと共にBをアルミニウム合金に添加することによって、結晶粒の微細化効果が向上する。Bの含有率が0.0010質量%未満では、結晶粒の微細化効果が十分に得られないおそれがある。一方、Bの含有率が0.030質量%を超えると、粗大な晶出物を形成し、介在物としてアルミニウム合金鍛造品1aに混入するおそれがある。また、アルミニウム合金の最終製品にBを含む粗大な晶出物が多量に混入すると靭性が低下する場合がある。したがって、Bの含有率は0.0010質量%以上、0.030質量%とする。Bの含有率は、好ましくは0.0050質量%以上、0.025質量%である。 (B: 0.001% by mass or more, 0.03% by mass or less)
B has the effect of refining the crystal grains of the aluminum alloy and improving the wrought workability. By adding B to the aluminum alloy together with the above-mentioned Ti, the effect of refining the crystal grains is improved. If the content of B is less than 0.0010% by mass, the effect of refining the crystal grains may not be sufficiently obtained. On the other hand, if the content of B exceeds 0.030% by mass, coarse crystallized products may be formed and may be mixed into the aluminum alloy forgedproduct 1a as inclusions. In addition, if a large amount of coarse crystallized products containing B are mixed into the final product of the aluminum alloy, the toughness may decrease. Therefore, the content of B is set to 0.0010% by mass or more and 0.030% by mass. The content of B is preferably 0.0050% by mass or more and 0.025% by mass.
Bは、アルミニウム合金の結晶粒を微細化し、展伸加工性を向上させる作用を有する。上述したTiと共にBをアルミニウム合金に添加することによって、結晶粒の微細化効果が向上する。Bの含有率が0.0010質量%未満では、結晶粒の微細化効果が十分に得られないおそれがある。一方、Bの含有率が0.030質量%を超えると、粗大な晶出物を形成し、介在物としてアルミニウム合金鍛造品1aに混入するおそれがある。また、アルミニウム合金の最終製品にBを含む粗大な晶出物が多量に混入すると靭性が低下する場合がある。したがって、Bの含有率は0.0010質量%以上、0.030質量%とする。Bの含有率は、好ましくは0.0050質量%以上、0.025質量%である。 (B: 0.001% by mass or more, 0.03% by mass or less)
B has the effect of refining the crystal grains of the aluminum alloy and improving the wrought workability. By adding B to the aluminum alloy together with the above-mentioned Ti, the effect of refining the crystal grains is improved. If the content of B is less than 0.0010% by mass, the effect of refining the crystal grains may not be sufficiently obtained. On the other hand, if the content of B exceeds 0.030% by mass, coarse crystallized products may be formed and may be mixed into the aluminum alloy forged
(Zr:0.0010質量%以上、0.05質量%以下)
Zrは、0.05質量%以下であれば、Al3ZrおよびAl-(Ti,Zr)という形で析出することで、再結晶抑制効果や析出強化によりアルミニウム合金鍛造品1aの強度の向上に寄与する。Zrの含有率が0.050質量%を超えると粗大なZr化合物として晶出することによって、アルミニウム合金鍛造品1aの耐食性の低下につながるおそれがある。このため、Zrの含有率は、0.050質量%以下とする。また、上記の再結晶抑制効果や析出強化による鍛造品の強度の向上の効果を得るためにはZrの含有率は、0.0010質量%以上であることが好ましい。 (Zr: 0.0010 mass% or more, 0.05 mass% or less)
If Zr is 0.05% by mass or less, it precipitates in the form of Al 3 Zr and Al-(Ti, Zr), which contributes to improving the strength of the aluminum alloy forging 1a by suppressing recrystallization and precipitation strengthening. If the Zr content exceeds 0.050% by mass, it may crystallize as coarse Zr compounds, which may lead to a decrease in the corrosion resistance of the aluminum alloy forging 1a. For this reason, the Zr content is set to 0.050% by mass or less. In order to obtain the above-mentioned recrystallization suppression effect and the effect of improving the strength of the forging by precipitation strengthening, the Zr content is preferably 0.0010% by mass or more.
Zrは、0.05質量%以下であれば、Al3ZrおよびAl-(Ti,Zr)という形で析出することで、再結晶抑制効果や析出強化によりアルミニウム合金鍛造品1aの強度の向上に寄与する。Zrの含有率が0.050質量%を超えると粗大なZr化合物として晶出することによって、アルミニウム合金鍛造品1aの耐食性の低下につながるおそれがある。このため、Zrの含有率は、0.050質量%以下とする。また、上記の再結晶抑制効果や析出強化による鍛造品の強度の向上の効果を得るためにはZrの含有率は、0.0010質量%以上であることが好ましい。 (Zr: 0.0010 mass% or more, 0.05 mass% or less)
If Zr is 0.05% by mass or less, it precipitates in the form of Al 3 Zr and Al-(Ti, Zr), which contributes to improving the strength of the aluminum alloy forging 1a by suppressing recrystallization and precipitation strengthening. If the Zr content exceeds 0.050% by mass, it may crystallize as coarse Zr compounds, which may lead to a decrease in the corrosion resistance of the aluminum alloy forging 1a. For this reason, the Zr content is set to 0.050% by mass or less. In order to obtain the above-mentioned recrystallization suppression effect and the effect of improving the strength of the forging by precipitation strengthening, the Zr content is preferably 0.0010% by mass or more.
(Zn:0.250質量%以下)
Znは0.250質量%以下であればよい。Znの含有率が0.250質量%を超えるとMgZn2が生成し、Al母相から粒界に析出することで粒界腐食を起こし、アルミニウム合金鍛造品の耐食性の低下につながる。このため、Znの含有率は、0.250質量%以下、あるいは全く含まないことが好ましい。 (Zn: 0.250 mass% or less)
Zn may be 0.250% by mass or less. If the Zn content exceeds 0.250% by mass, MgZn2 is generated and precipitates from the Al matrix to the grain boundaries, causing intergranular corrosion and leading to a decrease in the corrosion resistance of the aluminum alloy forged product. For this reason, it is preferable that the Zn content is 0.250% by mass or less, or that it is not contained at all.
Znは0.250質量%以下であればよい。Znの含有率が0.250質量%を超えるとMgZn2が生成し、Al母相から粒界に析出することで粒界腐食を起こし、アルミニウム合金鍛造品の耐食性の低下につながる。このため、Znの含有率は、0.250質量%以下、あるいは全く含まないことが好ましい。 (Zn: 0.250 mass% or less)
Zn may be 0.250% by mass or less. If the Zn content exceeds 0.250% by mass, MgZn2 is generated and precipitates from the Al matrix to the grain boundaries, causing intergranular corrosion and leading to a decrease in the corrosion resistance of the aluminum alloy forged product. For this reason, it is preferable that the Zn content is 0.250% by mass or less, or that it is not contained at all.
(不可避不純物)
不可避不純物は、原料又は製造工程から不可避的にアルミニウム合金に混入する不純物である。不可避不純物の例としては、Ni、Sn、Beなどを挙げることができる。これらの不可避不純物の含有率は0.1質量%を超えないことが好ましい。 (Inevitable impurities)
Inevitable impurities are impurities that are inevitably mixed into the aluminum alloy from the raw materials or manufacturing process. Examples of inevitable impurities include Ni, Sn, Be, etc. The content of these inevitable impurities is preferably not more than 0.1 mass%.
不可避不純物は、原料又は製造工程から不可避的にアルミニウム合金に混入する不純物である。不可避不純物の例としては、Ni、Sn、Beなどを挙げることができる。これらの不可避不純物の含有率は0.1質量%を超えないことが好ましい。 (Inevitable impurities)
Inevitable impurities are impurities that are inevitably mixed into the aluminum alloy from the raw materials or manufacturing process. Examples of inevitable impurities include Ni, Sn, Be, etc. The content of these inevitable impurities is preferably not more than 0.1 mass%.
本実施形態のアルミニウム合金鍛造品1aの長尺部2の長手方向の中央部2aは、例えば、アルミニウム合金鍛造品1aを車両のサスペンションアームとして使用した場合に、最大主応力がかかる部分である。中央部2aは、例えば、長尺部2の長手方向の中心を含む長尺部2の全体に対して1%以上80%以下の範囲内の領域である。長尺部2のアスペクト比(長手方向の長さ/長手方向に垂直な方向の短辺の長さ)は、例えば、2以上100以下の範囲内にある。長尺部2の中央部2aの断面は、鍛造加工によってアルミニウム合金鍛造品1aを製造する際に、圧力を加えた方向に沿った方向の断面(以下、中央部断面と称することがある)である。
The longitudinal center portion 2a of the long portion 2 of the aluminum alloy forging 1a of this embodiment is the portion to which the maximum principal stress is applied when the aluminum alloy forging 1a is used, for example, as a suspension arm of a vehicle. The central portion 2a is, for example, a region in the range of 1% to 80% of the entire long portion 2 including the longitudinal center of the long portion 2. The aspect ratio of the long portion 2 (longitudinal length/length of the short side perpendicular to the longitudinal direction) is, for example, in the range of 2 to 100. The cross section of the central portion 2a of the long portion 2 is a cross section in the direction along which pressure is applied when manufacturing the aluminum alloy forging 1a by forging (hereinafter, sometimes referred to as the central cross section).
(平均粒子径が3.0μm以上のAIFeSi(Mn)系化合物を含まない)
アルミニウム合金鍛造品1aの中央部断面における合金組織には、平均粒子径が3.0μm以上のAIFeSi(Mn)系化合物を含まないようにする。平均粒子径が3.0μm以上のAIFeSi(Mn)系化合物が存在すると、機械的特性(引張特性/疲労特性等)が低下するおそれがある。 (Does not contain AIFeSi(Mn) based compounds with an average particle size of 3.0 μm or more)
The alloy structure in the central cross section of the aluminum alloy forging 1a is not to contain AIFeSi(Mn)-based compounds having an average particle size of 3.0 μm or more. If AIFeSi(Mn)-based compounds having an average particle size of 3.0 μm or more are present, there is a risk of deterioration of mechanical properties (tensile properties/fatigue properties, etc.).
アルミニウム合金鍛造品1aの中央部断面における合金組織には、平均粒子径が3.0μm以上のAIFeSi(Mn)系化合物を含まないようにする。平均粒子径が3.0μm以上のAIFeSi(Mn)系化合物が存在すると、機械的特性(引張特性/疲労特性等)が低下するおそれがある。 (Does not contain AIFeSi(Mn) based compounds with an average particle size of 3.0 μm or more)
The alloy structure in the central cross section of the aluminum alloy forging 1a is not to contain AIFeSi(Mn)-based compounds having an average particle size of 3.0 μm or more. If AIFeSi(Mn)-based compounds having an average particle size of 3.0 μm or more are present, there is a risk of deterioration of mechanical properties (tensile properties/fatigue properties, etc.).
(衝撃値が10J/cm2以上)
アルミニウム合金製鍛造品1aの中央部断面は、常温(20℃)での衝撃値が10J/cm2以上の機械的特性を有する。この衝撃値が10J/cm2未満では、部品の耐久性が低下する虞がある。
本明細書において、「衝撃値」はJIS Z2242-2005の「金属材料のシャルピー衝撃試験方法」の規定に準拠して測定して得られたシャルピー衝撃強度を意味するものである。試験片としては、円柱状の試験片を使用して測定するものとする。 (Impact value is 10 J/cm2 or more )
The central cross section of the aluminum alloy forgedproduct 1a has mechanical properties such that the impact value at room temperature (20° C.) is 10 J/cm 2 or more. If this impact value is less than 10 J/cm 2 , there is a risk that the durability of the part will decrease.
In this specification, the term "impact value" refers to the Charpy impact strength measured in accordance with the provisions of JIS Z2242-2005 "Charpy impact test method for metallic materials." The test specimen used for the measurement is a cylindrical test specimen.
アルミニウム合金製鍛造品1aの中央部断面は、常温(20℃)での衝撃値が10J/cm2以上の機械的特性を有する。この衝撃値が10J/cm2未満では、部品の耐久性が低下する虞がある。
本明細書において、「衝撃値」はJIS Z2242-2005の「金属材料のシャルピー衝撃試験方法」の規定に準拠して測定して得られたシャルピー衝撃強度を意味するものである。試験片としては、円柱状の試験片を使用して測定するものとする。 (Impact value is 10 J/cm2 or more )
The central cross section of the aluminum alloy forged
In this specification, the term "impact value" refers to the Charpy impact strength measured in accordance with the provisions of JIS Z2242-2005 "Charpy impact test method for metallic materials." The test specimen used for the measurement is a cylindrical test specimen.
(結晶方位差15°以上の大角粒界の比率27%以下)
アルミニウム合金鍛造品1aの中央部断面において、結晶方位差15°以上の結晶粒界(大角粒界)は、アルミニウム合金鍛造品1aの長尺部2の再結晶化の進行程度の指標となる。この大角粒界の比率が27%以下であることは、再結晶化が十分に抑制されていることを表す。再結晶化が十分に抑制されていることによって、長尺部2の機械的特性が向上する。大角粒界の比率は、EBSD像から得ることができる。 (The ratio of high-angle grain boundaries with a crystal orientation difference of 15° or more is 27% or less)
In the cross section of the central portion of the aluminum alloy forging 1a, grain boundaries (high-angle grain boundaries) with a crystal orientation difference of 15° or more are indicators of the degree of progress of recrystallization in thelong portion 2 of the aluminum alloy forging 1a. The ratio of these high-angle grain boundaries being 27% or less indicates that recrystallization is sufficiently suppressed. The mechanical properties of the long portion 2 are improved by sufficiently suppressing recrystallization. The ratio of high-angle grain boundaries can be obtained from an EBSD image.
アルミニウム合金鍛造品1aの中央部断面において、結晶方位差15°以上の結晶粒界(大角粒界)は、アルミニウム合金鍛造品1aの長尺部2の再結晶化の進行程度の指標となる。この大角粒界の比率が27%以下であることは、再結晶化が十分に抑制されていることを表す。再結晶化が十分に抑制されていることによって、長尺部2の機械的特性が向上する。大角粒界の比率は、EBSD像から得ることができる。 (The ratio of high-angle grain boundaries with a crystal orientation difference of 15° or more is 27% or less)
In the cross section of the central portion of the aluminum alloy forging 1a, grain boundaries (high-angle grain boundaries) with a crystal orientation difference of 15° or more are indicators of the degree of progress of recrystallization in the
以上のような構成とされた本実施形態のアルミニウム合金鍛造品1aは、その材料であるアルミニウム合金が上記の合金組成とされているので、鍛造品を製造する際に再結晶化が起こりにくい。このため、過度に粗大な結晶粒が生成しにくい。また、本実施形態のアルミニウム合金製鍛造品1aの長尺部2の中央部2aの断面は、粒界を含む2.0μm以内にサイズが0.5μm以下のMnを含有する析出物の数密度が4個/μm2以上含まれており、且つ結晶方位差15°以上の大角粒界の比率27%以下の合金組織を有する。
このため、長尺部2の中央部2aは、引張特性や疲労特性が高く、靭性に優れ耐衝撃性が向上する。 The aluminum alloy forging 1a of the present embodiment configured as described above is made of the above-mentioned alloy composition, so that recrystallization is unlikely to occur when the forging is manufactured. Therefore, excessively coarse crystal grains are unlikely to be generated. In addition, the cross section of thecentral portion 2a of the long portion 2 of the aluminum alloy forging 1a of the present embodiment has an alloy structure in which the number density of precipitates containing Mn and having a size of 0.5 μm or less within 2.0 μm including the grain boundaries is 4 pieces/μm2 or more , and the ratio of high-angle grain boundaries with a crystal orientation difference of 15° or more is 27% or less.
Therefore, thecentral portion 2a of the long portion 2 has high tensile properties and fatigue properties, excellent toughness, and improved impact resistance.
このため、長尺部2の中央部2aは、引張特性や疲労特性が高く、靭性に優れ耐衝撃性が向上する。 The aluminum alloy forging 1a of the present embodiment configured as described above is made of the above-mentioned alloy composition, so that recrystallization is unlikely to occur when the forging is manufactured. Therefore, excessively coarse crystal grains are unlikely to be generated. In addition, the cross section of the
Therefore, the
本実施形態のアルミニウム合金鍛造品1aは、長尺部2の中央部2aの強度や耐久性が高く、かつ軽量であるため、自動車などの車両のサスペンションアーム用として有利に使用することができる。
The aluminum alloy forged product 1a of this embodiment has high strength and durability in the central portion 2a of the long portion 2, and is also lightweight, so it can be advantageously used for suspension arms of vehicles such as automobiles.
図1に示す本実施形態のアルミニウム合金鍛造品1aにおいては、一方の連結部4aは相対的に直径が小さい円柱状で、一方の連結部4bは相対的に直径が大きい円柱状とされ、長尺部2は、一方の連結部4a側の端辺から他方の連結部4b側の端辺に向けて幅が広くなった形状とされているが、アルミニウム合金鍛造品1aの形状は、これに限定されるものでない。例えば、アルミニウム合金鍛造品1aの一方の連結部4aと他方の連結部4bは同じ形状であってもよい。長尺部2の幅は一定であってもよい。また、長尺部2は湾曲した形状であってもよい。連結部4は3個以上形成されていてもよい。
In the aluminum alloy forging 1a of this embodiment shown in FIG. 1, one connecting portion 4a is cylindrical with a relatively small diameter, and one connecting portion 4b is cylindrical with a relatively large diameter, and the long portion 2 has a shape in which the width increases from the end edge on the side of one connecting portion 4a toward the end edge on the side of the other connecting portion 4b, but the shape of the aluminum alloy forging 1a is not limited to this. For example, one connecting portion 4a and the other connecting portion 4b of the aluminum alloy forging 1a may have the same shape. The width of the long portion 2 may be constant. Also, the long portion 2 may have a curved shape. Three or more connecting portions 4 may be formed.
図2は、本発明の一実施形態に係るアルミニウム合金鍛造品の別の一例の平面図である。
図2に示すアルミニウム合金鍛造品1bは、3つの連結部4c,4d,4eを有する。連結部4cと連結部4dは長尺部2で接続され、連結部4dと連結部4eとは、長尺部2よりも相対的に長さが短く短尺部5で接続されている。連結部4cには、貫通孔が設けられている。このアルミニウム合金鍛造品1bは、例えば、L型サスペンションアームとして用いることができる。 FIG. 2 is a plan view of another example of an aluminum alloy forged product according to an embodiment of the present invention.
The aluminum alloy forging 1b shown in Fig. 2 has three connecting portions 4c, 4d, and 4e. The connecting portions 4c and 4d are connected by a long portion 2, and the connecting portions 4d and 4e are connected by a short portion 5 that is relatively shorter than the long portion 2. A through hole is provided in the connecting portion 4c. This aluminum alloy forging 1b can be used, for example, as an L-shaped suspension arm.
図2に示すアルミニウム合金鍛造品1bは、3つの連結部4c,4d,4eを有する。連結部4cと連結部4dは長尺部2で接続され、連結部4dと連結部4eとは、長尺部2よりも相対的に長さが短く短尺部5で接続されている。連結部4cには、貫通孔が設けられている。このアルミニウム合金鍛造品1bは、例えば、L型サスペンションアームとして用いることができる。 FIG. 2 is a plan view of another example of an aluminum alloy forged product according to an embodiment of the present invention.
The aluminum alloy forging 1b shown in Fig. 2 has three connecting
図3は、本発明の一実施形態に係るアルミニウム合金鍛造品のさらに別の一例の平面図である。
図3に示すアルミニウム合金鍛造品1cは、3つの連結部4f,4g,4hを有する。連結部4fと連結部4g及び連結部4fと連結部4hはそれぞれ長尺部2で接続されている。連結部4fには、貫通孔が設けられている。このアルミニウム合金鍛造品1bは、例えば、A型サスペンションアームとして用いることができる。 FIG. 3 is a plan view of yet another example of an aluminum alloy forged product according to an embodiment of the present invention.
The aluminum alloy forging 1c shown in Fig. 3 has three connecting portions 4f, 4g, and 4h. The connecting portions 4f and 4g are connected to each other by the long portion 2, and the connecting portions 4f and 4h are connected to each other by the long portion 2. A through hole is provided in the connecting portion 4f. This aluminum alloy forging 1b can be used as, for example, an A-type suspension arm.
図3に示すアルミニウム合金鍛造品1cは、3つの連結部4f,4g,4hを有する。連結部4fと連結部4g及び連結部4fと連結部4hはそれぞれ長尺部2で接続されている。連結部4fには、貫通孔が設けられている。このアルミニウム合金鍛造品1bは、例えば、A型サスペンションアームとして用いることができる。 FIG. 3 is a plan view of yet another example of an aluminum alloy forged product according to an embodiment of the present invention.
The aluminum alloy forging 1c shown in Fig. 3 has three connecting
本発明の別の実施形態のアルミニウム合金鍛造品は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下である。
また、本実施形態のアルミニウム合金鍛造品は、常温における衝撃値において、10J/cm2以上とされている。
Fe/Mnは、質量比で0.3以上1.0以下とすることができ、また、0.4以上0.8以下とすることができ、また、0.4以上0.7以下とすることができる。 In another embodiment of the aluminum alloy forging of the present invention, Cu is in the range of 0.25 mass% or more and 0.55 mass% or less, Mg is in the range of 0.60 mass% or more and 1.25 mass% or less, Si is in the range of 0.90 mass% or more and 1.4 mass% or less, Mn is in the range of 0.35 mass% or more and 0.60 mass% or less, Fe is in the range of 0.15 mass% or more and 0.30 mass% or less, Zn is in the range of 0.25 mass% or less, Cr is in the range of 0.050 mass% or more and 0.30 mass% or less, Ti is 0.01 mass% and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content Fe/Mn is 0.3 to 1.2 in mass ratio, and the balance is Al and unavoidable impurities.
Moreover, the aluminum alloy forged product of this embodiment has an impact value of 10 J/cm 2 or more at room temperature.
The Fe/Mn mass ratio can be set to 0.3 or more and 1.0 or less, can be set to 0.4 or more and 0.8 or less, or can be set to 0.4 or more and 0.7 or less.
また、本実施形態のアルミニウム合金鍛造品は、常温における衝撃値において、10J/cm2以上とされている。
Fe/Mnは、質量比で0.3以上1.0以下とすることができ、また、0.4以上0.8以下とすることができ、また、0.4以上0.7以下とすることができる。 In another embodiment of the aluminum alloy forging of the present invention, Cu is in the range of 0.25 mass% or more and 0.55 mass% or less, Mg is in the range of 0.60 mass% or more and 1.25 mass% or less, Si is in the range of 0.90 mass% or more and 1.4 mass% or less, Mn is in the range of 0.35 mass% or more and 0.60 mass% or less, Fe is in the range of 0.15 mass% or more and 0.30 mass% or less, Zn is in the range of 0.25 mass% or less, Cr is in the range of 0.050 mass% or more and 0.30 mass% or less, Ti is 0.01 mass% and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content Fe/Mn is 0.3 to 1.2 in mass ratio, and the balance is Al and unavoidable impurities.
Moreover, the aluminum alloy forged product of this embodiment has an impact value of 10 J/cm 2 or more at room temperature.
The Fe/Mn mass ratio can be set to 0.3 or more and 1.0 or less, can be set to 0.4 or more and 0.8 or less, or can be set to 0.4 or more and 0.7 or less.
粒界を含む2.0μm以内に含まれるMnを含む析出物のサイズを例示すると、0.5μm以下である。
For example, the size of precipitates containing Mn within 2.0 μm, including grain boundaries, is 0.5 μm or less.
[アルミニウム合金鍛造品の製造方法]
次に、本実施形態のアルミニウム合金鍛造品の製造方法について説明する。
本実施形態のアルミニウム合金鍛造品の製造方法は、例えば、溶湯形成工程と、鋳造工程と、鍛造工程と、溶体化処理工程と、焼き入れ処理工程と、時効処理工程とを含む。 [Method of manufacturing aluminum alloy forged products]
Next, a method for producing an aluminum alloy forged product according to this embodiment will be described.
The method for producing an aluminum alloy forged product of this embodiment includes, for example, a molten metal forming step, a casting step, a forging step, a solution treatment step, a quenching treatment step, and an aging treatment step.
次に、本実施形態のアルミニウム合金鍛造品の製造方法について説明する。
本実施形態のアルミニウム合金鍛造品の製造方法は、例えば、溶湯形成工程と、鋳造工程と、鍛造工程と、溶体化処理工程と、焼き入れ処理工程と、時効処理工程とを含む。 [Method of manufacturing aluminum alloy forged products]
Next, a method for producing an aluminum alloy forged product according to this embodiment will be described.
The method for producing an aluminum alloy forged product of this embodiment includes, for example, a molten metal forming step, a casting step, a forging step, a solution treatment step, a quenching treatment step, and an aging treatment step.
(溶湯形成工程)
溶湯形成工程は、原料を溶解して組成を調製したアルミニウム合金溶湯を得る工程である。アルミニウム合金溶湯の組成は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成になるように調整して6000系アルミニウム合金の溶湯を得る。
Fe/Mnが質量比で0.3以上1.2以下となるように調整してもよい。 (Molten metal forming process)
The molten metal forming step is a step of preparing a molten aluminum alloy by melting raw materials and adjusting the composition thereof. The molten aluminum alloy has a composition of Cu in the range of 0.25 mass% to 0.55 mass%, Mg in the range of 0.60 mass% to 1.25 mass%, Si in the range of 0.90 mass% to 1.4 mass%, Mn in the range of 0.35 mass% to 0.60 mass%, Fe in the range of 0.15 mass% to 0.30 mass%, Zn in the range of 0.25 mass%, Cr in the range of 0.050 mass% to 0.30 mass%, and Cr in the range of 0.050 mass% to 0.30 mass%. The molten aluminum alloy of 6000 series is obtained by adjusting the content of the alloy so as to have an alloy composition in which the content of Fe to the content of Mn is less than 1.4 in terms of mass ratio, the content of Ti being within the range of 0.01% by mass or more and 0.1% by mass or less, the content of B being within the range of 0.0010% by mass or more and 0.030% by mass or less, and the content of Zr being within the range of 0.0010% by mass or more and 0.050% by mass or less, and the ratio of the content of Fe to the content of Mn, Fe/Mn, is less than 1.4 in terms of mass ratio, with the balance being Al and unavoidable impurities.
The Fe/Mn mass ratio may be adjusted to be 0.3 or more and 1.2 or less.
溶湯形成工程は、原料を溶解して組成を調製したアルミニウム合金溶湯を得る工程である。アルミニウム合金溶湯の組成は、Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成になるように調整して6000系アルミニウム合金の溶湯を得る。
Fe/Mnが質量比で0.3以上1.2以下となるように調整してもよい。 (Molten metal forming process)
The molten metal forming step is a step of preparing a molten aluminum alloy by melting raw materials and adjusting the composition thereof. The molten aluminum alloy has a composition of Cu in the range of 0.25 mass% to 0.55 mass%, Mg in the range of 0.60 mass% to 1.25 mass%, Si in the range of 0.90 mass% to 1.4 mass%, Mn in the range of 0.35 mass% to 0.60 mass%, Fe in the range of 0.15 mass% to 0.30 mass%, Zn in the range of 0.25 mass%, Cr in the range of 0.050 mass% to 0.30 mass%, and Cr in the range of 0.050 mass% to 0.30 mass%. The molten aluminum alloy of 6000 series is obtained by adjusting the content of the alloy so as to have an alloy composition in which the content of Fe to the content of Mn is less than 1.4 in terms of mass ratio, the content of Ti being within the range of 0.01% by mass or more and 0.1% by mass or less, the content of B being within the range of 0.0010% by mass or more and 0.030% by mass or less, and the content of Zr being within the range of 0.0010% by mass or more and 0.050% by mass or less, and the ratio of the content of Fe to the content of Mn, Fe/Mn, is less than 1.4 in terms of mass ratio, with the balance being Al and unavoidable impurities.
The Fe/Mn mass ratio may be adjusted to be 0.3 or more and 1.2 or less.
上記組成のアルミニウム合金溶湯を用いてこれ以降の工程を行うことで、再結晶発生がしにくく、常温における機械的特性に優れたAl-Mg-Si系のアルミニウム合金鍛造品を得ることができる。なお、アルミニウム新塊とは、鉱物から製造されたアルミナに、電解精錬と呼ばれる電気分解を行うことで得られる濃度99%以上のアルミニウムである。
By carrying out the subsequent steps using molten aluminum alloy with the above composition, it is possible to obtain Al-Mg-Si aluminum alloy forgings that are less prone to recrystallization and have excellent mechanical properties at room temperature. Note that virgin aluminum ingot is aluminum with a concentration of 99% or more, obtained by subjecting alumina produced from minerals to electrolysis, a process known as electrolytic refining.
アルミニウム合金溶湯は、アルミニウム合金を加熱して溶融させることによって得ることができる。また、アルミニウム合金の原料となる元素の単体若しくは元素を2種以上含む化合物を、目的のアルミニウム合金を生成する割合で含む混合物を溶融させることによって成形してもよい。例えば、鋳造工程で生成させるアルミニウム合金の結晶粒径を制御する目的で、TiやBをAl-Ti-Bロッドなどの結晶粒微細化材として混合してもよい。
A molten aluminum alloy can be obtained by heating and melting an aluminum alloy. Alternatively, the aluminum alloy may be formed by melting a mixture containing the elemental elements or compounds containing two or more elements that are the raw materials for the aluminum alloy, in a ratio that produces the desired aluminum alloy. For example, Ti and B may be mixed as grain refiners, such as Al-Ti-B rods, for the purpose of controlling the grain size of the aluminum alloy produced in the casting process.
また、アルミニウム合金溶湯の原料として1000系、2000系、3000系、4000系、5000系、6000系、7000系のアルミニウム合金のスクラップ材を10%以上使用し、残部がアルミニウム新塊、上記の添加元素であるものを用い、これらを溶解して組成を調製したアルミニウム合金溶湯を得てもよい。この場合、再結晶発生がしにくく常温における機械的特性に優れたAl-Mg-Si系アルミニウム合金鍛造品を得ることができる。なお、アルミニウム新塊とは、鉱物から製造されたアルミナに、電解精錬と称される電気分解を行うことで得られる、例えば純度が99%以上のアルミニウムである。
Also, 10% or more of scrap material of 1000 series, 2000 series, 3000 series, 4000 series, 5000 series, 6000 series, or 7000 series aluminum alloys may be used as raw materials for the molten aluminum alloy, with the remainder being new aluminum ingots and the above-mentioned additive elements, and these may be melted to obtain a molten aluminum alloy having a composition adjusted. In this case, it is possible to obtain an Al-Mg-Si-based aluminum alloy forging that is less prone to recrystallization and has excellent mechanical properties at room temperature. Note that new aluminum ingots are aluminum with a purity of, for example, 99% or more, which is obtained by subjecting alumina produced from minerals to electrolysis, a process known as electrolytic refining.
(鋳造工程)
鋳造工程では、アルミニウム合金の溶湯(液相)を冷却して固体(固相)に凝固させて、アルミニウム合金鋳造品を得る。鋳造工程は、例えば、水平連続鋳造法を用いることができる。 (Casting process)
In the casting process, the molten aluminum alloy (liquid phase) is cooled and solidified into a solid (solid phase) to obtain an aluminum alloy cast product. The casting process can be, for example, a horizontal continuous casting method.
鋳造工程では、アルミニウム合金の溶湯(液相)を冷却して固体(固相)に凝固させて、アルミニウム合金鋳造品を得る。鋳造工程は、例えば、水平連続鋳造法を用いることができる。 (Casting process)
In the casting process, the molten aluminum alloy (liquid phase) is cooled and solidified into a solid (solid phase) to obtain an aluminum alloy cast product. The casting process can be, for example, a horizontal continuous casting method.
ここで、本実施形態のアルミニウム合金鋳造品の製造に用いることができる水平連続鋳造装置を図4及び図5に示す。
なお、図4は、水平連続鋳造装置10の鋳型12付近の一例を示す断面図である。図5は、水平連続鋳造装置10の冷却水キャビティ24付近の要部を拡大した断面図である。 A horizontal continuous casting apparatus that can be used to manufacture the aluminum alloy casting of this embodiment is shown in FIGS.
4 is a cross-sectional view showing an example of the vicinity of themold 12 of the horizontal continuous casting apparatus 10. FIG 5 is an enlarged cross-sectional view showing a main portion of the horizontal continuous casting apparatus 10 near the cooling water cavity 24.
なお、図4は、水平連続鋳造装置10の鋳型12付近の一例を示す断面図である。図5は、水平連続鋳造装置10の冷却水キャビティ24付近の要部を拡大した断面図である。 A horizontal continuous casting apparatus that can be used to manufacture the aluminum alloy casting of this embodiment is shown in FIGS.
4 is a cross-sectional view showing an example of the vicinity of the
図4及び図5に示す水平連続鋳造装置10は、溶湯受部(タンディッシュ)11と、中空円筒状の鋳型12と、この鋳型12の一端側12aと溶湯受部11との間に配される耐火物製板状体(断熱部材)13とを有している。
The horizontal continuous casting device 10 shown in Figures 4 and 5 has a molten metal receiving portion (tundish) 11, a hollow cylindrical mold 12, and a refractory plate-like body (insulating member) 13 arranged between one end side 12a of the mold 12 and the molten metal receiving portion 11.
溶湯受部11は、上記の溶湯形成工程で得られたアルミニウム合金溶湯Mを受ける溶湯流入部11a、溶湯保持部11b、鋳型12の中空部21への流出部11cから構成されている。
The molten metal receiving section 11 is composed of a molten metal inlet section 11a that receives the molten aluminum alloy M obtained in the above-mentioned molten metal forming process, a molten metal holding section 11b, and an outlet section 11c into the hollow section 21 of the mold 12.
溶湯受部11は、アルミニウム合金溶湯Mの上液面のレベルを鋳型12の中空部21の上面よりも高い位置に維持し、且つ、多連鋳造の場合には、それぞれの鋳型12にアルミニウム合金溶湯Mを安定的に分配するものである。
The molten metal receiving portion 11 maintains the upper liquid level of the molten aluminum alloy M at a position higher than the upper surface of the hollow portion 21 of the mold 12, and in the case of multiple casting, distributes the molten aluminum alloy M stably to each mold 12.
溶湯受部11内の溶湯保持部11bに保持されたアルミニウム合金溶湯Mは、耐火物製板状体13に設けられた注湯用通路13aから鋳型12の中空部21内に注湯される。そして、中空部21内に供給されたアルミニウム合金溶湯Mは、後述する冷却装置23によって冷却されて固化し、凝固鋳塊であるアルミニウム合金棒Bとして、鋳型12の他端側12bから引き出される。
The molten aluminum alloy M held in the molten metal holding portion 11b in the molten metal receiving portion 11 is poured into the hollow portion 21 of the mold 12 through the pouring passage 13a provided in the refractory plate body 13. The molten aluminum alloy M supplied into the hollow portion 21 is then cooled and solidified by the cooling device 23 described below, and is drawn out from the other end side 12b of the mold 12 as an aluminum alloy rod B, which is a solidified ingot.
鋳型12の他端側12bには、鋳造されたアルミニウム合金棒Bを一定速度で引き出す引出駆動装置(図示略)が設置されていればよい。また、連続して引き出されたアルミニウム合金棒Bを任意の長さに切断する同調切断機(図示略)が設置されていることも好ましい。
A pull-out drive device (not shown) that pulls out the cast aluminum alloy rod B at a constant speed may be installed on the other end 12b of the mold 12. It is also preferable to install a synchronized cutter (not shown) that cuts the continuously pulled aluminum alloy rod B to the desired length.
耐火物製板状体13は、溶湯受部11と鋳型12との間の熱移動を遮断する部材であり、例えば、ケイ酸カルシウム、アルミナ、シリカ、アルミナとシリカの混合物、窒化珪素、炭化珪素、グラファイト等の材料で構成されていてもよい。こうした耐火物製板状体13は、互いに構成材料の異なる複数の層から構成することもできる。
The refractory plate 13 is a member that blocks heat transfer between the molten metal receiving portion 11 and the mold 12, and may be made of materials such as calcium silicate, alumina, silica, a mixture of alumina and silica, silicon nitride, silicon carbide, graphite, etc. Such a refractory plate 13 may also be made up of multiple layers made of different materials.
鋳型12は、本実施形態では中空円筒状の部材であり、例えば、アルミニウム、銅、若しくはそれらの合金から選ばれる1種又は2種以上の組み合わせた材料から形成されている。こうした鋳型12の材料は、熱伝導性、耐熱性、機械強度の点から最適な組み合わせを選択すればよい。
In this embodiment, the mold 12 is a hollow cylindrical member, and is made of, for example, one or a combination of two or more materials selected from aluminum, copper, or alloys thereof. The materials for the mold 12 can be selected from an optimal combination in terms of thermal conductivity, heat resistance, and mechanical strength.
鋳型12の中空部21は、鋳造するアルミニウム合金棒Bを円筒棒状にするために断面円形に形成されており、この中空部21の中心を通る鋳型中心軸(中心軸)Cがほぼ水平方向に沿うように鋳型12が保持されている。
The hollow portion 21 of the mold 12 is formed with a circular cross section to cast the aluminum alloy rod B into a cylindrical rod shape, and the mold 12 is held so that the mold central axis (center axis) C, which passes through the center of this hollow portion 21, is aligned approximately horizontally.
鋳型12の中空部21の内周面21aは、アルミニウム合金棒Bの鋳造方向(図1を参照)に向けて鋳型中心軸Cに対して0°~3°(より好ましくは0°~1°)の仰角で形成されている。すなわち、内周面21aは、鋳造方向に向かってコーン状に開いたテーパー状に構成されている。そしてそのテーパーのなす角度が仰角である。
The inner peripheral surface 21a of the hollow portion 21 of the mold 12 is formed at an elevation angle of 0° to 3° (more preferably 0° to 1°) with respect to the central axis C of the mold toward the casting direction of the aluminum alloy bar B (see FIG. 1). In other words, the inner peripheral surface 21a is configured in a tapered shape that opens out like a cone toward the casting direction. The angle of this taper is the elevation angle.
仰角が0°未満では、アルミニウム合金棒Bが鋳型12から引き出される際に、鋳型出口である他端側12bで抵抗を受けるために鋳造が困難になるおそれがある。一方、仰角が3°を越えると、内周面21aのアルミニウム合金溶湯Mへの接触が不十分になり、アルミニウム合金溶湯Mやこれが冷却固化した凝固殻から鋳型12への抜熱効果が低下することによって凝固が不十分になるおそれがある。その結果、アルミニウム合金棒Bの表面に再溶融肌が生じ、又は、アルミニウム合金棒Bの端部から未凝固のアルミニウム合金溶湯Mが噴出するなどの鋳造トラブルにつながるおそれがあるので好ましくない。
If the elevation angle is less than 0°, when the aluminum alloy rod B is pulled out of the mold 12, it encounters resistance at the other end 12b, which is the mold outlet, and casting may become difficult. On the other hand, if the elevation angle exceeds 3°, the contact of the inner peripheral surface 21a with the molten aluminum alloy M may become insufficient, and the effect of removing heat from the molten aluminum alloy M and its solidified shell to the mold 12 may decrease, resulting in insufficient solidification. As a result, a remelted skin may appear on the surface of the aluminum alloy rod B, or unsolidified molten aluminum alloy M may erupt from the end of the aluminum alloy rod B, which is not preferable, as this may lead to casting problems.
なお、鋳型12の中空部21の断面形状(鋳型12の中空部21を他端側21bから見たときの平面形状)は、本実施形態の円形以外にも、例えば、三角形や矩形断面形状、多角形、半円、楕円若しくは対称軸や対称面を持たない異形断面形状を有した形状など、鋳造するアルミニウム合金棒の形状に合わせて選択されればよい。
The cross-sectional shape of the hollow portion 21 of the mold 12 (the planar shape of the hollow portion 21 of the mold 12 when viewed from the other end side 21b) may be selected to match the shape of the aluminum alloy rod to be cast, such as a triangular or rectangular cross-sectional shape, a polygon, a semicircle, an ellipse, or an irregular cross-sectional shape that does not have an axis or plane of symmetry, other than the circular shape of this embodiment.
鋳型12の一端側12aには、鋳型12の中空部21内に潤滑流体を供給する流体供給管22が配置されている。流体供給管22から供給される潤滑流体としては、気体潤滑材、液体潤滑材から選ばれる何れか1種又は2種以上の潤滑流体とすることができる。気体潤滑材と液体潤滑材を両方供給する場合には、それぞれ流体供給管を別々に設けることが好ましい。流体供給管22から加圧供給された潤滑流体は、環状の潤滑材供給口22aを通って鋳型12の中空部21内に供給される。
A fluid supply pipe 22 is disposed on one end side 12a of the mold 12 to supply lubricating fluid into the hollow portion 21 of the mold 12. The lubricating fluid supplied from the fluid supply pipe 22 can be one or more types of lubricating fluid selected from a gas lubricant and a liquid lubricant. When supplying both a gas lubricant and a liquid lubricant, it is preferable to provide separate fluid supply pipes for each. The lubricating fluid supplied under pressure from the fluid supply pipe 22 is supplied into the hollow portion 21 of the mold 12 through the annular lubricant supply port 22a.
本実施形態では、圧送された潤滑流体が潤滑材供給口22aから鋳型12の内周面21aに供給される。なお、液体潤滑材は加熱されて分解気体となって、鋳型12の内周面21aに供給される構成であってもよい。また、潤滑材供給口22aに多孔質材料を配して、この多孔質材料を介して潤滑流体を鋳型12の内周面21aに滲出させる構成であってもよい。
In this embodiment, the pressurized lubricating fluid is supplied from the lubricating material supply port 22a to the inner circumferential surface 21a of the mold 12. The liquid lubricating material may be heated to decompose into a gas and supplied to the inner circumferential surface 21a of the mold 12. Alternatively, a porous material may be disposed in the lubricating material supply port 22a, and the lubricating fluid may be allowed to seep out onto the inner circumferential surface 21a of the mold 12 through the porous material.
鋳型12の内部には、アルミニウム合金溶湯Mを冷却、固化させる冷却手段である冷却装置23が形成されている。本実施形態の冷却装置23は、鋳型12の中空部21の内周面21aを冷却するための冷却水Wを収容する冷却水キャビティ24と、この冷却水キャビティ24と鋳型12の中空部21とを連通させる冷却水噴射通路25とを有している。
A cooling device 23 is formed inside the mold 12 as a cooling means for cooling and solidifying the molten aluminum alloy M. The cooling device 23 in this embodiment has a cooling water cavity 24 that contains cooling water W for cooling the inner circumferential surface 21a of the hollow portion 21 of the mold 12, and a cooling water injection passage 25 that connects the cooling water cavity 24 to the hollow portion 21 of the mold 12.
冷却水キャビティ24は、鋳型12の内部で中空部21の内周面21aよりも外側に、中空部21を取り巻くように環状に形成され、冷却水供給管26を介して冷却水Wが供給される。
The cooling water cavity 24 is formed in the mold 12 outside the inner circumferential surface 21a of the hollow portion 21, in a ring shape surrounding the hollow portion 21, and cooling water W is supplied through a cooling water supply pipe 26.
鋳型12は、冷却水キャビティ24に収容される冷却水Wによって内周面21aが冷却されることにより、鋳型12の中空部21内に充満したアルミニウム合金溶湯Mの熱を鋳型12の内周面21aに接触する面から奪って、アルミニウム合金溶湯Mの表面に凝固殻を形成させる。
The inner surface 21a of the mold 12 is cooled by the cooling water W contained in the cooling water cavity 24, which removes heat from the molten aluminum alloy M filling the hollow portion 21 of the mold 12 from the surface in contact with the inner surface 21a of the mold 12, forming a solidified shell on the surface of the molten aluminum alloy M.
また、冷却水噴射通路25は、中空部21に臨むシャワー開口25aから、鋳型12の他端側12bにおいてアルミニウム合金棒Bに向けて直接、冷却水Wを当ててアルミニウム合金棒Bを冷却する。こうした冷却水噴射通路25の縦断面形状は、本実施形態の円状以外にも、例えば、半円、洋ナシ形状、馬蹄形状であってもよい。
The cooling water injection passage 25 also sprays cooling water W from a shower opening 25a facing the hollow portion 21 directly toward the aluminum alloy rod B at the other end side 12b of the mold 12 to cool the aluminum alloy rod B. The vertical cross-sectional shape of the cooling water injection passage 25 may be, for example, semicircular, pear-shaped, or horseshoe-shaped, other than the circular shape of this embodiment.
なお、本実施形態では、冷却水供給管26を介して供給される冷却水Wを、先ず冷却水キャビティ24に収容して鋳型12の中空部21の内周面21aの冷却を行い、更に冷却水キャビティ24の冷却水Wを冷却水噴射通路25からアルミニウム合金棒Bに向けて噴射しているが、これらをそれぞれ別系統の冷却水供給管によって供給する構成にすることもできる。
In this embodiment, the cooling water W supplied through the cooling water supply pipe 26 is first stored in the cooling water cavity 24 to cool the inner surface 21a of the hollow portion 21 of the mold 12, and then the cooling water W in the cooling water cavity 24 is sprayed toward the aluminum alloy bar B from the cooling water spray passage 25. However, it is also possible to configure the configuration so that each of these is supplied through a separate cooling water supply pipe.
冷却水噴射通路25のシャワー開口25aの中心軸の延長線が、鋳造されたアルミニウム合金棒Bの表面に当る位置から、鋳型12と耐火物製板状体13との接触面までの長さを有効モールド長Lと称し、この有効モールド長Lは、例えば、10mm以上40mm以下であるのが好ましい。この有効モールド長Lが、10mm未満では、良好な皮膜が形成されない等から鋳造不可となり、40mmを超えると、強制冷却の効果が低くなり、鋳型壁による凝固が支配的になって、鋳型12とアルミニウム合金溶湯M又はアルミニウム合金棒Bとの接触抵抗が大きくなって、鋳肌に割れが生じたり、鋳型内部で千切れたりする等、鋳造が不安定になるおそれがあるので好ましくない。
The length from the position where the extension line of the central axis of the shower opening 25a of the cooling water injection passage 25 hits the surface of the cast aluminum alloy bar B to the contact surface between the mold 12 and the refractory plate-like body 13 is called the effective mold length L, and this effective mold length L is preferably, for example, 10 mm or more and 40 mm or less. If this effective mold length L is less than 10 mm, casting is not possible because a good film is not formed, and if it exceeds 40 mm, the effect of forced cooling is reduced, solidification by the mold wall becomes dominant, and the contact resistance between the mold 12 and the aluminum alloy molten metal M or aluminum alloy bar B increases, which may cause cracks on the casting surface or tearing inside the mold, making the casting unstable, and is not preferable.
これら冷却水キャビティ24への冷却水Wの供給や、冷却水噴射通路25のシャワー開口25aからの冷却水Wの噴射は、制御装置(図示略)からの制御信号によってそれぞれ動作を制御できることが好ましい。
It is preferable that the supply of cooling water W to the cooling water cavity 24 and the spraying of cooling water W from the shower opening 25a of the cooling water spray passage 25 can each be controlled by a control signal from a control device (not shown).
冷却水キャビティ24は、鋳型12の中空部21寄りの内底面24aが、鋳型12の中空部21の内周面21aに対して、互いに平行面になるように形成されている。
The cooling water cavity 24 is formed so that the inner bottom surface 24a near the hollow portion 21 of the mold 12 is parallel to the inner peripheral surface 21a of the hollow portion 21 of the mold 12.
なお、ここでいう平行とは、冷却水キャビティ24の内底面24aに対して、鋳型12の中空部21の内周面21aが0°~3°の仰角で形成されている場合、すなわち、内底面24aが内周面21aに対して0°を超えて3°まで傾斜している場合も含む。
Note that "parallel" in this context also includes cases where the inner peripheral surface 21a of the hollow portion 21 of the mold 12 is formed at an elevation angle of 0° to 3° relative to the inner bottom surface 24a of the cooling water cavity 24, i.e., where the inner bottom surface 24a is inclined from 0° to 3° relative to the inner peripheral surface 21a.
図4に示すように、こうした冷却水キャビティ24の内底面24aと鋳型12の中空部21の内周面21aとが対向する部分である鋳型12の冷却壁部27は、中空部21のアルミニウム合金溶湯Mから冷却水キャビティ24の冷却水Wに向かう単位面積当たりの熱流束値が10×105W/m2以上、50×105W/m2以下の範囲内になるように形成されている。
As shown in Figure 4, the cooling wall portion 27 of the mold 12, which is the portion where the inner bottom surface 24a of the cooling water cavity 24 faces the inner surface 21a of the hollow portion 21 of the mold 12, is formed so that the heat flux value per unit area from the molten aluminum alloy M in the hollow portion 21 to the cooling water W in the cooling water cavity 24 is in the range of 10 x 105 W/m2 or more and 50 x 105 W/ m2 or less.
こうした鋳型12の冷却壁部27の厚みt、即ち冷却水キャビティ24の内底面24aと鋳型12の中空部21の内周面21aとの間隔が、例えば、0.5mm以上3.0mm以下、好ましくは0.5mm以上2.5mm以下の範囲内になるように鋳型12が形成されていればよい。また、鋳型12の少なくとも冷却壁部27の熱伝導率が100W/m・K以上400W/m・K以下の範囲内になるように、鋳型12の形成材料が選択されればよい。
The mold 12 is formed so that the thickness t of the cooling wall 27 of the mold 12, i.e., the distance between the inner bottom surface 24a of the cooling water cavity 24 and the inner peripheral surface 21a of the hollow portion 21 of the mold 12, is within a range of, for example, 0.5 mm to 3.0 mm, and preferably 0.5 mm to 2.5 mm. In addition, the material for forming the mold 12 is selected so that the thermal conductivity of at least the cooling wall 27 of the mold 12 is within a range of 100 W/m·K to 400 W/m·K.
図4において、溶湯受部11中のアルミニウム合金溶湯Mは、耐火物製板状体13を経て鋳型中心軸Cがほぼ水平になるように保持された鋳型12の一端側12aから供給され、鋳型12の他端側12bで強制冷却されてアルミニウム合金棒Bとなる。
In FIG. 4, the molten aluminum alloy M in the molten metal receiving section 11 is supplied to one end 12a of the mold 12, which is held so that the central axis C of the mold is nearly horizontal, via a refractory plate 13, and is forcibly cooled at the other end 12b of the mold 12 to become an aluminum alloy rod B.
アルミニウム合金棒Bは、鋳型12の他端側12b近くに設置された引出駆動装置(図示略)によって一定速度で引き出されるため、連続的に鋳造されて長尺のアルミニウム合金棒Bが形成される。引き出されたアルミニウム合金棒Bは、例えば、同調切断機(図示略)によって所望の長さに切断される。
The aluminum alloy rod B is pulled out at a constant speed by a pull-out drive device (not shown) installed near the other end 12b of the mold 12, so that it is cast continuously to form a long aluminum alloy rod B. The pulled aluminum alloy rod B is then cut to the desired length, for example, by a synchronous cut-off machine (not shown).
なお、鋳造されたアルミニウム合金棒Bの組成比は、例えば、「JIS H 1305」に記載されているような光電測光式発光分光分析装置(装置例:日本島津製作所製PDA-5500)による方法で確認できる。
The composition ratio of the cast aluminum alloy rod B can be confirmed, for example, by a method using a photoelectric emission spectrophotometric analyzer (example: Shimadzu PDA-5500, manufactured by Japan) as described in "JIS H 1305."
溶湯受部11内に貯留されたアルミニウム合金溶湯Mの液面レベルの高さと、鋳型12の上側の内周面21aとの高さの差は、0mm~250mm(より好ましくは50mm~170mm。)とするのが好ましい。こうした範囲にすることで、鋳型12内に供給されるアルミニウム合金溶湯Mの圧力と潤滑油及び潤滑油が気化したガスとが好適にバランスするために鋳造性が安定する。
The difference in height between the liquid level of the molten aluminum alloy M stored in the molten metal receiving portion 11 and the upper inner peripheral surface 21a of the mold 12 is preferably 0 mm to 250 mm (more preferably 50 mm to 170 mm). By setting it in this range, the pressure of the molten aluminum alloy M supplied to the mold 12 and the lubricating oil and the gas produced by vaporizing the lubricating oil are appropriately balanced, resulting in stable castability.
液体潤滑材は、潤滑油である植物油を用いることができる。例えば、菜種油、ひまし油、サラダ油を挙げることができる。これらは環境への悪影響が小さいので好ましい。
For the liquid lubricant, vegetable oils, which are lubricating oils, can be used. Examples include rapeseed oil, castor oil, and salad oil. These are preferred because they have a small negative impact on the environment.
潤滑油供給量は0.05mL/分~5mL/分(より好ましくは0.1mL/分以上、1mL/分以下。)であるのが好ましい。供給量が過少だと、潤滑不足によってアルミニウム合金棒Bのアルミニウム合金溶湯Mが固まらずに鋳型12から漏れるおそれがある。
供給量が過多であると、余剰分がアルミニウム合金棒B中に混入して内部欠陥となるおそれがある。 The lubricating oil supply rate is preferably 0.05 mL/min to 5 mL/min (more preferably 0.1 mL/min to 1 mL/min). If the supply rate is too low, the molten aluminum alloy M of the aluminum alloy rod B may not solidify and may leak from themold 12 due to insufficient lubrication.
If the amount of supply is excessive, the excess may be mixed into the aluminum alloy bar B and cause internal defects.
供給量が過多であると、余剰分がアルミニウム合金棒B中に混入して内部欠陥となるおそれがある。 The lubricating oil supply rate is preferably 0.05 mL/min to 5 mL/min (more preferably 0.1 mL/min to 1 mL/min). If the supply rate is too low, the molten aluminum alloy M of the aluminum alloy rod B may not solidify and may leak from the
If the amount of supply is excessive, the excess may be mixed into the aluminum alloy bar B and cause internal defects.
鋳型12からアルミニウム合金棒Bを引き抜く速度である鋳造速度は、200mm/分以上、1500mm/分以下(より好ましくは400mm/分以上、1000mm/分以下。)であるのが好ましい。それは、この範囲内の鋳造速度であれば、鋳造で形成される晶出物のネットワーク組織が均一微細となり、高温下でのアルミニウム生地の変形に対する抵抗が増し、高温機械的強度が向上するためである。
The casting speed, which is the speed at which the aluminum alloy rod B is pulled out of the mold 12, is preferably 200 mm/min or more and 1500 mm/min or less (more preferably 400 mm/min or more and 1000 mm/min or less). This is because, at a casting speed within this range, the network structure of the crystals formed by casting becomes uniform and fine, which increases the resistance of the aluminum matrix to deformation at high temperatures and improves the high-temperature mechanical strength.
冷却水噴射通路25のシャワー開口25aから噴射される冷却水量は、鋳型当り10L/分以上、50L/分以下(より好ましくは25L/分以上、40L/分以下。)であるのが好ましい。冷却水量がこれよりも少ないと、アルミニウム合金溶湯Mが固まらずに鋳型12から漏れるおそれがある。また、鋳造したアルミニウム合金棒Bの表面が再溶融して不均一な組織が形成され、内部欠陥として残存するおそれがある。一方、冷却水量がこの範囲よりも多い場合、鋳型12の抜熱が大き過ぎて途中で凝固してしまうおそれがある。
The amount of cooling water sprayed from the shower opening 25a of the cooling water spray passage 25 is preferably 10 L/min or more and 50 L/min or less (more preferably 25 L/min or more and 40 L/min or less) per mold. If the amount of cooling water is less than this, the molten aluminum alloy M may not solidify and may leak from the mold 12. In addition, the surface of the cast aluminum alloy bar B may remelt, forming an uneven structure that may remain as an internal defect. On the other hand, if the amount of cooling water is more than this range, the mold 12 may lose too much heat, causing it to solidify midway.
溶湯受部11内から鋳型12へ流入するアルミニウム合金溶湯Mの平均温度は、例えば、650℃以上、750℃以下(より好ましくは680℃以上、720℃以下。)であるのが好ましい。アルミニウム合金溶湯Mの温度が低すぎると、鋳型12及びその手前で粗大な晶出物を形成してアルミニウム合金棒Bの内部に内部欠陥として取り込まれるおそれがある。一方、アルミニウム合金溶湯Mの温度が高すぎると、アルミニウム合金溶湯M中に大量の水素ガスが取り込まれやすく、アルミニウム合金棒B中にポロシティーとして取り込まれ、内部の空洞となるおそれがある。
The average temperature of the molten aluminum alloy M flowing from the molten metal receiving portion 11 into the mold 12 is, for example, preferably 650°C or higher and 750°C or lower (more preferably 680°C or higher and 720°C or lower). If the temperature of the molten aluminum alloy M is too low, there is a risk that coarse crystals will form in the mold 12 or in front of it and will be incorporated into the aluminum alloy bar B as internal defects. On the other hand, if the temperature of the molten aluminum alloy M is too high, a large amount of hydrogen gas will be easily incorporated into the molten aluminum alloy M, which will be incorporated into the aluminum alloy bar B as porosity and may cause internal cavities.
そして、鋳型12の冷却壁部27において、中空部21のアルミニウム合金溶湯Mから冷却水キャビティ24の冷却水Wに向かう単位面積当たりの熱流束値は、10×105W/m2以上50×105W/m2以下の範囲内にすることによって、アルミニウム合金棒Bの焼き付きが発生することを防止できる。
Furthermore, by setting the heat flux value per unit area from the molten aluminum alloy M in the hollow portion 21 to the cooling water W in the cooling water cavity 24 in the cooling wall portion 27 of the mold 12 to a range of 10 x 10 5 W/m 2 or more and 50 x 10 5 W/m 2 or less, the occurrence of seizure of the aluminum alloy rod B can be prevented.
鋳型12の冷却壁部27は、アルミニウム合金溶湯Mからの抜熱によって熱を受け、この熱を冷却水キャビティ24に収容される冷却水Wで冷却することで熱交換を行っているが、この熱交換の状態について、図6に示す説明図のように、単位面積あたりの熱流束に着目した。単位面積あたりの熱流束は、フーリエの法則にて以下の式(1)で表される。
Q=-k×(T1-T2)/L・・・(1)
Q:熱流束
k:熱を通過する箇所(本実施形態では鋳型12の冷却壁部27)の熱伝導率(W/m・K)
T1:熱が通過する箇所の低温側温度(本実施形態では冷却水キャビティ24の内底面24a)
T2:熱が通過する箇所の高温側温度(本実施形態では鋳型12の中空部21の内周面21a)
L:熱が通過する箇所の区間長さ(mm)(本実施形態では鋳型12の冷却壁部27の厚みt) The coolingwall 27 of the mold 12 receives heat from the molten aluminum alloy M and exchanges the heat by cooling it with the cooling water W contained in the cooling water cavity 24. Regarding the state of this heat exchange, attention is focused on the heat flux per unit area as shown in the explanatory diagram in Fig. 6. The heat flux per unit area is expressed by the following formula (1) according to Fourier's law.
Q = -k × (T1 - T2) / L (1)
Q: heat flux k: thermal conductivity (W/m·K) of the portion through which heat passes (the coolingwall portion 27 of the mold 12 in this embodiment)
T1: Low temperature side temperature of the portion through which heat passes (in this embodiment, theinner bottom surface 24a of the cooling water cavity 24)
T2: High temperature side temperature of the portion through which heat passes (in this embodiment, the innercircumferential surface 21a of the hollow portion 21 of the mold 12)
L: Length (mm) of the section where heat passes through (in this embodiment, the thickness t of thecooling wall portion 27 of the mold 12)
Q=-k×(T1-T2)/L・・・(1)
Q:熱流束
k:熱を通過する箇所(本実施形態では鋳型12の冷却壁部27)の熱伝導率(W/m・K)
T1:熱が通過する箇所の低温側温度(本実施形態では冷却水キャビティ24の内底面24a)
T2:熱が通過する箇所の高温側温度(本実施形態では鋳型12の中空部21の内周面21a)
L:熱が通過する箇所の区間長さ(mm)(本実施形態では鋳型12の冷却壁部27の厚みt) The cooling
Q = -k × (T1 - T2) / L (1)
Q: heat flux k: thermal conductivity (W/m·K) of the portion through which heat passes (the cooling
T1: Low temperature side temperature of the portion through which heat passes (in this embodiment, the
T2: High temperature side temperature of the portion through which heat passes (in this embodiment, the inner
L: Length (mm) of the section where heat passes through (in this embodiment, the thickness t of the
鋳造時に潤滑油量を減らしても良好な結果が得られた鋳型材質、厚み、測温データに基づいて、単位面積当たりの熱流束値が10×105W/m2以上になるように鋳型12の冷却壁部27を構成することで、鋳造したアルミニウム合金棒Bの焼き付きを防止することができる。また、単位面積当たりの熱流束値が50×105W/m2以下にすることが好ましい。
Based on the mold material, thickness, and temperature measurement data that show good results even when the amount of lubricating oil is reduced during casting, the cooling wall portion 27 of the mold 12 is configured so that the heat flux value per unit area is 10×10 5 W/m 2 or more, thereby making it possible to prevent seizure of the cast aluminum alloy bar B. In addition, it is preferable that the heat flux value per unit area is 50×10 5 W/m 2 or less.
鋳型12の冷却壁部27をこうした熱流束値の範囲にするために、鋳型12の冷却壁部27の厚みtを例えば、0.5mm以上、3.0mm以下の範囲になるように鋳型12を形成すればよい。また、鋳型12の少なくとも冷却壁部27の熱伝導率を100W/m・K以上、400W/m・K以下の範囲にすればよい。
In order to set the cooling wall portion 27 of the mold 12 within this range of heat flux values, the mold 12 may be formed so that the thickness t of the cooling wall portion 27 of the mold 12 is, for example, in the range of 0.5 mm or more and 3.0 mm or less. In addition, the thermal conductivity of at least the cooling wall portion 27 of the mold 12 may be set in the range of 100 W/m·K or more and 400 W/m·K or less.
本実施形態のアルミニウム合金棒Bを製造する際には、上述した水平連続鋳造装置10を用いて、溶湯受部11内に貯留されたアルミニウム合金溶湯Mを、鋳型12の一端側12aから中空部21内に連続して供給する。また、冷却水キャビティ24に冷却水Wを供給すると共に、流体供給管22から潤滑流体、例えば潤滑油を供給する。
When manufacturing the aluminum alloy rod B of this embodiment, the above-mentioned horizontal continuous casting device 10 is used to continuously supply the molten aluminum alloy M stored in the molten metal receiving portion 11 into the hollow portion 21 from one end side 12a of the mold 12. In addition, cooling water W is supplied to the cooling water cavity 24, and a lubricating fluid, for example, lubricating oil, is supplied from the fluid supply pipe 22.
そして、中空部21内に供給されたアルミニウム合金溶湯Mを、冷却壁部27における単位面積当たりの熱流束値が10×105W/m2以上の条件で冷却、凝固させてアルミニウム合金棒Bを鋳造する。また、アルミニウム合金棒Bを鋳造時において、冷却水Wによって冷却される鋳型12の冷却壁部27の壁面温度を100℃以下にすることが好ましい。
The molten aluminum alloy M supplied into the hollow portion 21 is then cooled and solidified under conditions where the heat flux value per unit area in the cooling wall portion 27 is 10× 105 W/ m2 or more, to cast the aluminum alloy bar B. In addition, when casting the aluminum alloy bar B, it is preferable to set the wall surface temperature of the cooling wall portion 27 of the mold 12, which is cooled by cooling water W, to 100° C. or less.
こうして得られるアルミニウム合金棒Bは、冷却壁部27における単位面積当たりの熱流束値が10×105W/m2以上の条件で冷却、凝固させることによって、潤滑油のガスとアルミニウム合金溶湯Mとの接触による反応生成物、例えば炭化物の固着が抑制される。これにより、アルミニウム合金棒Bの表面の炭化物等を切削除去する必要がなく、高収率でアルミニウム合金棒Bを製造することができる。
The aluminum alloy rod B thus obtained is cooled and solidified under conditions where the heat flux value per unit area in the cooling wall 27 is 10×10 5 W/m 2 or more, thereby suppressing adhesion of reaction products, such as carbides, caused by contact between the lubricating oil gas and the molten aluminum alloy M. This makes it unnecessary to cut and remove carbides, etc., on the surface of the aluminum alloy rod B, and allows the aluminum alloy rod B to be produced with a high yield.
アルミニウム合金溶湯Mから鋳造品を得る鋳造工程は、上述の水平連続鋳造法に限定されるものではなく、垂直連続鋳造法など公知の連続鋳造法を用いることができる。垂直連続鋳造法は、アルミニウム合金溶湯Mのモールド(鋳型12)への供給方式によってフロート法やホットトップ法に分類されるが、以下では、ホットトップ法を用いる場合について簡単に説明する。
The casting process for obtaining a cast product from the molten aluminum alloy M is not limited to the horizontal continuous casting method described above, and any known continuous casting method such as vertical continuous casting can be used. Vertical continuous casting methods are classified into the float method and the hot top method depending on the method of supplying the molten aluminum alloy M to the mold (casting mold 12), but the following will briefly explain the case where the hot top method is used.
ホットトップ法に用いられる鋳造装置は、モールド、溶湯受容器(ヘッダー)等を備えている。溶湯受部へ供給された溶湯は、出湯口を通り、ヘッダーを通ることで流速を調整され、ほぼ水平に設置された筒状鋳型内に入り、ここで強制冷却されて溶湯の外表面に凝固殻が形成される。
The casting equipment used in the hot top method is equipped with a mold, a molten metal receiving vessel (header), etc. The molten metal supplied to the molten metal receiving vessel passes through a spout and through the header, where the flow rate is adjusted, and enters a cylindrical mold that is installed almost horizontally, where it is forcibly cooled and a solidified shell is formed on the outer surface of the molten metal.
さらに、鋳型から引き出された鋳造品に冷却水が直接放射され、鋳造品内部まで金属の凝固が進行しつつ鋳造品が連続的に引き出される。一般的にモールドは熱伝導性の良い金属部材が用いられ、内部に冷媒を導入するための中空構造を有している。
In addition, cooling water is sprayed directly onto the casting as it is pulled out of the mold, and the solidification of the metal progresses all the way to the inside of the casting as it is continuously pulled out. Molds are generally made of metal components with good thermal conductivity, and have a hollow structure to allow the introduction of a coolant inside.
使用する冷媒は、工業的に利用可能なものから適宜選べばよいが、利用しやすさの観点から水が推奨される。
The refrigerant to be used can be selected from those that are commercially available, but water is recommended from the standpoint of ease of use.
本実施形態で使用するモールドは、溶湯との接触部における伝熱性能及び耐久性の観点から銅やアルミニウムなどの金属、若しくはグラファイトから適宜選択する。ヘッダーは、一般に耐火物製であり、モールドの上側に設置されている。ヘッダーの材料やサイズは鋳造する合金の成分範囲や鋳造品の寸法によって適宜選択すればよく、特に制約されるものではない。
The mold used in this embodiment is appropriately selected from metals such as copper and aluminum, or graphite, from the viewpoint of heat transfer performance and durability at the contact point with the molten metal. The header is generally made of a refractory material and is installed on the upper side of the mold. There are no particular restrictions on the material and size of the header, and it can be appropriately selected depending on the composition range of the alloy to be cast and the dimensions of the cast product.
鋳造時の平均冷却速度は、例えば10~300℃/秒などの一般的に推奨される範囲から適宜選定すればよい。鋳造速度は水平連続鋳造において一般的な範囲から適宜選択すればよく、例えば200~600mm/分の範囲から適宜選定すればよい。
The average cooling rate during casting may be appropriately selected from a generally recommended range, such as 10 to 300°C/sec. The casting speed may be appropriately selected from a range that is typical for horizontal continuous casting, such as 200 to 600 mm/min.
以上に記載した鋳造方法によって、中型~大型の鋳造品であっても、均一な金属組織が得られるようになる。対象とする鋳造品の直径は特に制限されるものでなく、直径30~100mmの棒材に対して好適に用いられる。
The casting method described above makes it possible to obtain a uniform metal structure even in medium to large castings. There are no particular restrictions on the diameter of the castings, and the method is suitable for use with rods with diameters of 30 to 100 mm.
(鍛造工程)
鍛造工程は、鋳造後のアルミニウム合金鋳造品を所定のサイズに切断して、得られた鍛造用素材を所定の温度に加熱し、その後プレス機で圧力をかけて金型成型する工程である。本実施形態では、従来、鋳造後に偏析除去のために実施していた均質化処理を施さずに鍛造加工を実施する。そのため均質化処理でおこなっていた偏析除去を鍛造時素材加熱で実施する必要があるため、加熱は500℃以上、融点以下の温度で素材加熱を実施する必要がある。その後鍛造加工を行って鍛造品(例えば自動車のサスペンションアーム部品等)を得る。鍛造時素材加熱温度が500℃未満になると合金組織中のAlFeSi系、Mg2Si系等の化合物が偏析した状態で残存し、変形抵抗が高くなって十分な加工ができなくなる、及び割れが発生する。また融点温度を超えると共晶融解等の欠陥が発生し易くなる。 (Forging process)
The forging process is a process in which the aluminum alloy casting after casting is cut to a predetermined size, the obtained forging material is heated to a predetermined temperature, and then pressure is applied by a press machine to mold it into a die. In this embodiment, the forging process is performed without performing the homogenization process that was conventionally performed after casting to remove segregation. Therefore, since it is necessary to perform the segregation removal performed by the homogenization process by heating the material during forging, it is necessary to perform the heating at a temperature of 500°C or higher and below the melting point. Then, the forging process is performed to obtain a forged product (for example, a suspension arm part of an automobile). If the material heating temperature during forging is less than 500°C, compounds such as AlFeSi and Mg 2 Si in the alloy structure remain in a segregated state, the deformation resistance increases, making it impossible to perform sufficient processing, and cracks occur. Furthermore, if the temperature exceeds the melting point, defects such as eutectic melting are likely to occur.
鍛造工程は、鋳造後のアルミニウム合金鋳造品を所定のサイズに切断して、得られた鍛造用素材を所定の温度に加熱し、その後プレス機で圧力をかけて金型成型する工程である。本実施形態では、従来、鋳造後に偏析除去のために実施していた均質化処理を施さずに鍛造加工を実施する。そのため均質化処理でおこなっていた偏析除去を鍛造時素材加熱で実施する必要があるため、加熱は500℃以上、融点以下の温度で素材加熱を実施する必要がある。その後鍛造加工を行って鍛造品(例えば自動車のサスペンションアーム部品等)を得る。鍛造時素材加熱温度が500℃未満になると合金組織中のAlFeSi系、Mg2Si系等の化合物が偏析した状態で残存し、変形抵抗が高くなって十分な加工ができなくなる、及び割れが発生する。また融点温度を超えると共晶融解等の欠陥が発生し易くなる。 (Forging process)
The forging process is a process in which the aluminum alloy casting after casting is cut to a predetermined size, the obtained forging material is heated to a predetermined temperature, and then pressure is applied by a press machine to mold it into a die. In this embodiment, the forging process is performed without performing the homogenization process that was conventionally performed after casting to remove segregation. Therefore, since it is necessary to perform the segregation removal performed by the homogenization process by heating the material during forging, it is necessary to perform the heating at a temperature of 500°C or higher and below the melting point. Then, the forging process is performed to obtain a forged product (for example, a suspension arm part of an automobile). If the material heating temperature during forging is less than 500°C, compounds such as AlFeSi and Mg 2 Si in the alloy structure remain in a segregated state, the deformation resistance increases, making it impossible to perform sufficient processing, and cracks occur. Furthermore, if the temperature exceeds the melting point, defects such as eutectic melting are likely to occur.
(溶体化処理工程)
溶体化処理工程は、鍛造工程で得られた鍛造品を加熱して溶体化させることにより、鍛造工程で導入された歪みを緩和し、溶質元素の固溶を行う工程である。 (Solution treatment process)
The solution treatment process is a process in which the forged product obtained in the forging process is heated to bring about a solution, thereby relieving the distortion introduced in the forging process and causing the solute elements to dissolve in solid solution.
溶体化処理工程は、鍛造工程で得られた鍛造品を加熱して溶体化させることにより、鍛造工程で導入された歪みを緩和し、溶質元素の固溶を行う工程である。 (Solution treatment process)
The solution treatment process is a process in which the forged product obtained in the forging process is heated to bring about a solution, thereby relieving the distortion introduced in the forging process and causing the solute elements to dissolve in solid solution.
本実施形態では、鍛造品を530℃以上、560℃以下の処理温度で0.3以上、3時間以下で保持することにより溶体化処理を行う。室温から上述した処理温度までの昇温速度は、5.0℃/分以上であることが好ましい。処理温度が530℃未満であると、溶質元素の固溶が不十分となるおそれがある。一方、560℃を超えると、溶質元素の固溶がより促進されるものの、共晶融解や再結晶が生じ易くなるおそれがある。また、昇温速度が5.0℃/分未満である場合は、Mg2Siが粗大析出するおそれがある。一方、処理温度が530℃未満である場合は、溶体化が進まず時効析出による高強度化を実現しにくくなるおそれがある。
In this embodiment, the forged product is subjected to solution treatment by holding it at a treatment temperature of 530°C or more and 560°C or less for 0.3 or more and 3 hours or less. The heating rate from room temperature to the above-mentioned treatment temperature is preferably 5.0°C/min or more. If the treatment temperature is less than 530°C, the solute elements may not be dissolved in solid solution. On the other hand, if the treatment temperature exceeds 560°C, the solute elements may be dissolved in solid solution more, but eutectic melting and recrystallization may occur easily. In addition, if the heating rate is less than 5.0°C/min, Mg 2 Si may precipitate coarsely. On the other hand, if the treatment temperature is less than 530°C, the solution treatment may not proceed, making it difficult to achieve high strength by aging precipitation.
(焼き入れ処理工程)
焼き入れ処理工程は、溶体化処理工程によって得られた固溶状態の鍛造品を急速に冷却せしめて、過飽和固溶体を形成する工程である。 (Quenching process)
The quenching process is a process in which the forged product in the solid-solution state obtained in the solution treatment process is rapidly cooled to form a supersaturated solid solution.
焼き入れ処理工程は、溶体化処理工程によって得られた固溶状態の鍛造品を急速に冷却せしめて、過飽和固溶体を形成する工程である。 (Quenching process)
The quenching process is a process in which the forged product in the solid-solution state obtained in the solution treatment process is rapidly cooled to form a supersaturated solid solution.
本実施形態では、水(焼き入れ水)が貯留された水槽に鍛造品を投入して、鍛造品を水没させることによって焼き入れ処理を行う。水槽内の水温は、20℃以上、60℃以下であることが好ましい。鍛造品の水槽への投入は、溶体化処理後に5秒以上、60秒以下で鍛造品の全ての表面が水に接触するように行うことが好ましい。鍛造品の水没時間は、鋳造品のサイズによっても異なるが、例えば、1分を超え30分以内の間である。
In this embodiment, the forged product is placed in a water tank that stores water (quenching water) and quenched by submerging the forged product. The temperature of the water in the tank is preferably 20°C or higher and 60°C or lower. The forged product is preferably placed in the water tank for 5 seconds or higher and 60 seconds or lower after solution treatment so that all surfaces of the forged product are in contact with water. The submersion time of the forged product varies depending on the size of the casting, but is, for example, between more than 1 minute and 30 minutes.
(時効処理工程)
時効処理工程は、鍛造品を比較的低温で加熱保持し過飽和に固溶した元素を析出させて、適度な硬さを付与する工程である。 (Aging treatment process)
The aging treatment process is a process in which the forged product is heated and held at a relatively low temperature to precipitate the elements that are supersaturated in solid solution, thereby imparting an appropriate hardness.
時効処理工程は、鍛造品を比較的低温で加熱保持し過飽和に固溶した元素を析出させて、適度な硬さを付与する工程である。 (Aging treatment process)
The aging treatment process is a process in which the forged product is heated and held at a relatively low temperature to precipitate the elements that are supersaturated in solid solution, thereby imparting an appropriate hardness.
本実施形態では、焼き入れ処理工程後の鍛造品に170℃以上、210℃以下の温度に加熱し、その温度で0.5時間以上、7時間以下で保持することにより時効処理を行う。処理温度が170℃未満、若しくは保持時間が0.5時間未満では、引張強度を向上させるMg2Si系析出物が十分に成長できなくなるおそれがある。一方、処理温度が190℃を超える場合、若しくは保持時間が7時間を超える場合、Mg2Si系析出物が粗大になり過ぎて引張強度を十分に向上させることができなくなるおそれがある。
In this embodiment, the forged product after the quenching process is heated to a temperature of 170°C or more and 210°C or less, and is held at that temperature for 0.5 hours or more and 7 hours or less to perform aging treatment. If the treatment temperature is less than 170°C or the holding time is less than 0.5 hours, the Mg2Si -based precipitates that improve the tensile strength may not grow sufficiently. On the other hand, if the treatment temperature exceeds 190°C or the holding time exceeds 7 hours, the Mg2Si -based precipitates may become too coarse to sufficiently improve the tensile strength.
本発明の別の実施形態のアルミニウム合金鍛造品の製造方法は、上記鋳造工程と上記鍛造工程との間に、アルミニウム合金鋳造品を、370℃以上560℃以下の温度範囲で2時間以上10時間以下保持して均質化熱処理を行う均質化熱処理工程を更に有する。
この実施形態のアルミニウム合金鍛造品の製造方法は、上記実施形態のアルミニウム合金鍛造品の製造方法とは、均質化熱処理工程を有する点が異なる。 In another embodiment of the present invention, a method for producing an aluminum alloy forged product further includes, between the casting step and the forging step, a homogenization heat treatment step in which the aluminum alloy cast product is subjected to homogenization heat treatment by holding the aluminum alloy cast product at a temperature range of 370° C. or higher and 560° C. or lower for 2 hours or higher and 10 hours or lower.
The method for producing an aluminum alloy forged product of this embodiment differs from the method for producing an aluminum alloy forged product of the above-described embodiment in that it includes a homogenization heat treatment step.
この実施形態のアルミニウム合金鍛造品の製造方法は、上記実施形態のアルミニウム合金鍛造品の製造方法とは、均質化熱処理工程を有する点が異なる。 In another embodiment of the present invention, a method for producing an aluminum alloy forged product further includes, between the casting step and the forging step, a homogenization heat treatment step in which the aluminum alloy cast product is subjected to homogenization heat treatment by holding the aluminum alloy cast product at a temperature range of 370° C. or higher and 560° C. or lower for 2 hours or higher and 10 hours or lower.
The method for producing an aluminum alloy forged product of this embodiment differs from the method for producing an aluminum alloy forged product of the above-described embodiment in that it includes a homogenization heat treatment step.
次に、本発明の具体的実施例について説明するが、本発明はこれら実施例のものに特に限定されるものではない。
Next, specific examples of the present invention will be described, but the present invention is not particularly limited to these examples.
[実施例1~8及び比較例1~3]
(連続鋳造品の作製)
先ず、下記の表1に示す合金組成(残部はアルミニウム)のアルミニウム合金を用意した。用意したアルミニウム合金を用いて、直径82mmの断面円形の連続鋳造品を作製した。 [Examples 1 to 8 and Comparative Examples 1 to 3]
(Production of continuous cast products)
First, an aluminum alloy having the alloy composition (the balance being aluminum) shown in the following Table 1 was prepared. A continuous cast product having a circular cross section and a diameter of 82 mm was produced from the prepared aluminum alloy.
(連続鋳造品の作製)
先ず、下記の表1に示す合金組成(残部はアルミニウム)のアルミニウム合金を用意した。用意したアルミニウム合金を用いて、直径82mmの断面円形の連続鋳造品を作製した。 [Examples 1 to 8 and Comparative Examples 1 to 3]
(Production of continuous cast products)
First, an aluminum alloy having the alloy composition (the balance being aluminum) shown in the following Table 1 was prepared. A continuous cast product having a circular cross section and a diameter of 82 mm was produced from the prepared aluminum alloy.
(アルミニウム合金鍛造品の製造)
次に、得られた連続鋳造品に対して、均質化熱処理工程(比較例1~3のみ)、鍛造加工工程、溶体化処理工程、焼き入れ処理工程、人工時効処理工程をこの順で行って、図1に示す形状のアルミニウム合金鍛造品1aを得た。均質化熱処理工程(比較例1~3のみ)、鍛造加工工程、溶体化処理工程、焼き入れ処理工程、人工時効処理工程の条件を下記の表2に示す。 (Manufacturing of aluminum alloy forgings)
Next, the obtained continuous cast product was subjected to a homogenization heat treatment step (only Comparative Examples 1 to 3), a forging step, a solution treatment step, a quenching treatment step, and an artificial aging treatment step in this order to obtain an aluminum alloy forgedproduct 1a having the shape shown in Figure 1. The conditions of the homogenization heat treatment step (only Comparative Examples 1 to 3), the forging step, the solution treatment step, the quenching treatment step, and the artificial aging treatment step are shown in Table 2 below.
次に、得られた連続鋳造品に対して、均質化熱処理工程(比較例1~3のみ)、鍛造加工工程、溶体化処理工程、焼き入れ処理工程、人工時効処理工程をこの順で行って、図1に示す形状のアルミニウム合金鍛造品1aを得た。均質化熱処理工程(比較例1~3のみ)、鍛造加工工程、溶体化処理工程、焼き入れ処理工程、人工時効処理工程の条件を下記の表2に示す。 (Manufacturing of aluminum alloy forgings)
Next, the obtained continuous cast product was subjected to a homogenization heat treatment step (only Comparative Examples 1 to 3), a forging step, a solution treatment step, a quenching treatment step, and an artificial aging treatment step in this order to obtain an aluminum alloy forged
[評価]
以上のようにして得られた実施例1~8及び比較例1~3のアルミニウム合金鍛造品1aにおける、長尺部2の長さ方向の中央部2aについて、下記の評価を行った。長尺部2の中央部2aの評価結果を下記の表3に示す。 [evaluation]
The following evaluations were performed on thecentral portion 2a in the longitudinal direction of the long portion 2 in the aluminum alloy forgings 1a of Examples 1 to 8 and Comparative Examples 1 to 3 obtained as described above. The evaluation results of the central portion 2a of the long portion 2 are shown in Table 3 below.
以上のようにして得られた実施例1~8及び比較例1~3のアルミニウム合金鍛造品1aにおける、長尺部2の長さ方向の中央部2aについて、下記の評価を行った。長尺部2の中央部2aの評価結果を下記の表3に示す。 [evaluation]
The following evaluations were performed on the
<Fe/Mn>
Fe/Mnは実施例が0.3以上1.2以下、比較例が1.2超えとなるように調整した。
「〇」・・・0.3以上1.2以下である。
「×」・・・1.2超えである。 <Fe/Mn>
The Fe/Mn ratio was adjusted to 0.3 or more and 1.2 or less in the examples, and to more than 1.2 in the comparative examples.
"Good": 0.3 or more and 1.2 or less.
"X": Over 1.2.
Fe/Mnは実施例が0.3以上1.2以下、比較例が1.2超えとなるように調整した。
「〇」・・・0.3以上1.2以下である。
「×」・・・1.2超えである。 <Fe/Mn>
The Fe/Mn ratio was adjusted to 0.3 or more and 1.2 or less in the examples, and to more than 1.2 in the comparative examples.
"Good": 0.3 or more and 1.2 or less.
"X": Over 1.2.
<導電率(%IACS)>
導電率は、室温にて測定した。
(判定基準)
「〇」・・・25%IACS以上35%IACS以下である。
「×」・・・25%IACS未満、または、35%IACS超えである。 <Conductivity (%IACS)>
The conductivity was measured at room temperature.
(Judgment criteria)
"Good": 25% IACS or more and 35% IACS or less.
"X": Less than 25% IACS or more than 35% IACS.
導電率は、室温にて測定した。
(判定基準)
「〇」・・・25%IACS以上35%IACS以下である。
「×」・・・25%IACS未満、または、35%IACS超えである。 <Conductivity (%IACS)>
The conductivity was measured at room temperature.
(Judgment criteria)
"Good": 25% IACS or more and 35% IACS or less.
"X": Less than 25% IACS or more than 35% IACS.
<ロックウェル硬さ(HRF)>
ロックウェル硬さ(HRF)は、JISZ2245:2016の「ロックウェル硬さ試験-試験方法」に準拠して測定した。
(判定基準)
「〇」・・・62以上82以下である。
「×」・・・62未満、または、82超えである。 <Rockwell hardness (HRF)>
The Rockwell hardness (HRF) was measured in accordance with JIS Z2245:2016 "Rockwell hardness test - test method".
(Judgment criteria)
“O”: Between 62 and 82.
"X": Less than 62 or more than 82.
ロックウェル硬さ(HRF)は、JISZ2245:2016の「ロックウェル硬さ試験-試験方法」に準拠して測定した。
(判定基準)
「〇」・・・62以上82以下である。
「×」・・・62未満、または、82超えである。 <Rockwell hardness (HRF)>
The Rockwell hardness (HRF) was measured in accordance with JIS Z2245:2016 "Rockwell hardness test - test method".
(Judgment criteria)
“O”: Between 62 and 82.
"X": Less than 62 or more than 82.
<機械的特性(衝撃特性)評価>
アルミニウム合金製鍛造品1aの長尺部2の中央部2aを、図7に示すように切断して、機械的特性(衝撃特性)評価用試験片の作製用の角柱体を採取した。得られた角柱体を加工して、図8に示す円柱状の機械的特性評価用試験片を作製した。機械的特性評価用試験片の平行部直径Aは8.0mm、標点間距離Gは30.0mmとした。機械的特性評価用試験片について、常温(25℃)でJIS Z2242に準拠してシャルピー衝撃試験を行うことによって、衝撃値を測定した。得られた衝撃値を、下記の判定基準に基づいて評価した。
(判定基準)
「〇」・・・常温で衝撃値が10J/cm2以上である。
「×」・・・常温で衝撃値が10J/cm2未満である。 <Mechanical property (impact property) evaluation>
Thecentral portion 2a of the long portion 2 of the aluminum alloy forged product 1a was cut as shown in FIG. 7 to obtain a rectangular column for preparing a test piece for evaluating mechanical properties (impact properties). The obtained rectangular column was processed to prepare a cylindrical test piece for evaluating mechanical properties as shown in FIG. 8. The parallel portion diameter A of the test piece for evaluating mechanical properties was 8.0 mm, and the gauge length G was 30.0 mm. The test piece for evaluating mechanical properties was subjected to a Charpy impact test at room temperature (25° C.) in accordance with JIS Z2242 to measure the impact value. The obtained impact value was evaluated based on the following criteria.
(Judgment criteria)
"Good": Impact value is 10 J/ cm2 or more at room temperature.
"X": The impact value at room temperature is less than 10 J/ cm2 .
アルミニウム合金製鍛造品1aの長尺部2の中央部2aを、図7に示すように切断して、機械的特性(衝撃特性)評価用試験片の作製用の角柱体を採取した。得られた角柱体を加工して、図8に示す円柱状の機械的特性評価用試験片を作製した。機械的特性評価用試験片の平行部直径Aは8.0mm、標点間距離Gは30.0mmとした。機械的特性評価用試験片について、常温(25℃)でJIS Z2242に準拠してシャルピー衝撃試験を行うことによって、衝撃値を測定した。得られた衝撃値を、下記の判定基準に基づいて評価した。
(判定基準)
「〇」・・・常温で衝撃値が10J/cm2以上である。
「×」・・・常温で衝撃値が10J/cm2未満である。 <Mechanical property (impact property) evaluation>
The
(Judgment criteria)
"Good": Impact value is 10 J/ cm2 or more at room temperature.
"X": The impact value at room temperature is less than 10 J/ cm2 .
<微細化評価、再結晶・結晶粗大化評価>
アルミニウム合金鍛造品1aの長尺部2の中央部2aを、中央部2aの表面に対して垂直方向に切断して、微細化評価用試験片の作製用の板状体(厚さ2mm)を採取した。得られた板状体を7mm角に切断して、7mm×7mm×厚さ2mmの微細化評価用の試験片とした。採取した微細化評価用の試験片の表面(中央部2aの断面)について、SEM-EBSD(走査型電子顕微鏡-電子線後方散乱回折装置)を用いて、3.0μm以上のAlFeSi系化合物の数、及び、結晶方位差15゜以上の大角粒界の比率を測定した。得られた3.0μm以上のAlFeSi系化合物の数、及び、大角粒界の比率をそれぞれ下記の基準に基づいて判定して、結晶粒子の微細化及び再結晶・結晶粗大化を評価した。なお、SEM-EBSDの測定条件は、加速電圧を15kV、測定ピッチを0.5μm/px、解析領域を500×500μm2、粒界定義角を15゜とした。
(判定基準)
(3.0μm以上のAlFeSi系化合物の数の判定基準)
「O」・・・無い場合(0個)。
「×」・・・有る場合。
(大角粒界の比率の判定基準)
「O」・・・27%以下である。
「×」・・・27%超えである。 <Evaluation of refinement, recrystallization and coarsening of crystals>
Thecentral portion 2a of the long portion 2 of the aluminum alloy forging 1a was cut in a direction perpendicular to the surface of the central portion 2a to obtain a plate-shaped body (thickness 2 mm) for preparing a test piece for refinement evaluation. The obtained plate-shaped body was cut into a 7 mm square to obtain a test piece for refinement evaluation of 7 mm x 7 mm x 2 mm thick. The number of AlFeSi-based compounds of 3.0 μm or more and the ratio of high-angle grain boundaries with a crystal orientation difference of 15 ° or more were measured using SEM-EBSD (scanning electron microscope-electron backscatter diffraction device) for the surface (cross section of the central portion 2a) of the obtained test piece for refinement evaluation. The number of AlFeSi-based compounds of 3.0 μm or more and the ratio of high-angle grain boundaries obtained were judged based on the following criteria, respectively, to evaluate the refinement of crystal grains and the recrystallization and coarsening of crystal grains. The measurement conditions for SEM-EBSD were an acceleration voltage of 15 kV, a measurement pitch of 0.5 μm/px, an analysis area of 500×500 μm 2 , and a grain boundary definition angle of 15°.
(Judgment criteria)
(Criteria for the number of AlFeSi compounds of 3.0 μm or more)
"O": None (0).
"×": if available.
(Criteria for determining the ratio of high angle grain boundaries)
"O": Less than 27%.
"X": Over 27%.
アルミニウム合金鍛造品1aの長尺部2の中央部2aを、中央部2aの表面に対して垂直方向に切断して、微細化評価用試験片の作製用の板状体(厚さ2mm)を採取した。得られた板状体を7mm角に切断して、7mm×7mm×厚さ2mmの微細化評価用の試験片とした。採取した微細化評価用の試験片の表面(中央部2aの断面)について、SEM-EBSD(走査型電子顕微鏡-電子線後方散乱回折装置)を用いて、3.0μm以上のAlFeSi系化合物の数、及び、結晶方位差15゜以上の大角粒界の比率を測定した。得られた3.0μm以上のAlFeSi系化合物の数、及び、大角粒界の比率をそれぞれ下記の基準に基づいて判定して、結晶粒子の微細化及び再結晶・結晶粗大化を評価した。なお、SEM-EBSDの測定条件は、加速電圧を15kV、測定ピッチを0.5μm/px、解析領域を500×500μm2、粒界定義角を15゜とした。
(判定基準)
(3.0μm以上のAlFeSi系化合物の数の判定基準)
「O」・・・無い場合(0個)。
「×」・・・有る場合。
(大角粒界の比率の判定基準)
「O」・・・27%以下である。
「×」・・・27%超えである。 <Evaluation of refinement, recrystallization and coarsening of crystals>
The
(Judgment criteria)
(Criteria for the number of AlFeSi compounds of 3.0 μm or more)
"O": None (0).
"×": if available.
(Criteria for determining the ratio of high angle grain boundaries)
"O": Less than 27%.
"X": Over 27%.
<アルミニウム合金鍛造品におけるMn系を含む析出物の数密度測定法>
また、各アルミニウム合金鍛造品についてFE―SEM装置(電界放出型走査電子顕微鏡装置)を用いて、粒界を含む2.0μm以内にMnを含む析出物の数密度測定を行った。なお、アルミニウム合金鍛造品1aの長尺部2の中央部2aより約10mm×横10mm×厚さ10mmの大きさの組織観察用サンプル片を切り出し、このサンプル片を断面試料作製装置(Cross section polisher)を用いて研磨した。そして、この研磨後のサンプル片のFE-SEM写真(電界放出型走査電子顕微鏡写真)を撮影し、このSEM写真における視野面積1.5815mm2の範囲において、粒界を含む2.0μm以内にMnを含む析出物の数密度を求め、以下の判定基準で数密度を評価した。
(数密度の判定基準)
「O」・・・4個/μm2以上である。
「×」・・・4個/μm2以下である。 <Method for measuring the number density of precipitates containing Mn in aluminum alloy forgings>
In addition, for each aluminum alloy forging, a FE-SEM device (field emission scanning electron microscope device) was used to measure the number density of precipitates containing Mn within 2.0 μm including grain boundaries. A sample piece for observing the structure having a size of about 10 mm x 10 mm x 10 mm was cut out from thecentral part 2a of the long part 2 of the aluminum alloy forging 1a, and this sample piece was polished using a cross section sample preparation device (Cross section polisher). Then, an FE-SEM photograph (field emission scanning electron microscope photograph) of the sample piece after this polishing was taken, and the number density of precipitates containing Mn within 2.0 μm including grain boundaries was obtained in the range of a field area of 1.5815 mm 2 in this SEM photograph, and the number density was evaluated according to the following criteria.
(Number density criteria)
"O": 4 particles/ μm2 or more.
"X": 4 particles/ μm2 or less.
また、各アルミニウム合金鍛造品についてFE―SEM装置(電界放出型走査電子顕微鏡装置)を用いて、粒界を含む2.0μm以内にMnを含む析出物の数密度測定を行った。なお、アルミニウム合金鍛造品1aの長尺部2の中央部2aより約10mm×横10mm×厚さ10mmの大きさの組織観察用サンプル片を切り出し、このサンプル片を断面試料作製装置(Cross section polisher)を用いて研磨した。そして、この研磨後のサンプル片のFE-SEM写真(電界放出型走査電子顕微鏡写真)を撮影し、このSEM写真における視野面積1.5815mm2の範囲において、粒界を含む2.0μm以内にMnを含む析出物の数密度を求め、以下の判定基準で数密度を評価した。
(数密度の判定基準)
「O」・・・4個/μm2以上である。
「×」・・・4個/μm2以下である。 <Method for measuring the number density of precipitates containing Mn in aluminum alloy forgings>
In addition, for each aluminum alloy forging, a FE-SEM device (field emission scanning electron microscope device) was used to measure the number density of precipitates containing Mn within 2.0 μm including grain boundaries. A sample piece for observing the structure having a size of about 10 mm x 10 mm x 10 mm was cut out from the
(Number density criteria)
"O": 4 particles/ μm2 or more.
"X": 4 particles/ μm2 or less.
<総合評価>
Fe/Mn比、導電率、ロックウェル硬さ、衝撃特性、3.0μm以上のAlFeSi系化合物の数、AlMn系化合物の数密度、結晶方位差15°以上の大角粒界の比率の7つの評価結果を、下記の判定基準に基づいて評価した。
(判定基準)
「O」・・・7つの評価の全てが「O」である。
「×」・・・7つの評価のうち1つ以上が「×」である。 <Overall evaluation>
The seven evaluation results, namely, Fe/Mn ratio, electrical conductivity, Rockwell hardness, impact properties, the number of AlFeSi-based compounds having a size of 3.0 μm or more, the number density of AlMn-based compounds, and the ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more, were evaluated based on the following criteria.
(Judgment criteria)
"O": All seven ratings are "O".
"X": One or more of the seven evaluations is "X".
Fe/Mn比、導電率、ロックウェル硬さ、衝撃特性、3.0μm以上のAlFeSi系化合物の数、AlMn系化合物の数密度、結晶方位差15°以上の大角粒界の比率の7つの評価結果を、下記の判定基準に基づいて評価した。
(判定基準)
「O」・・・7つの評価の全てが「O」である。
「×」・・・7つの評価のうち1つ以上が「×」である。 <Overall evaluation>
The seven evaluation results, namely, Fe/Mn ratio, electrical conductivity, Rockwell hardness, impact properties, the number of AlFeSi-based compounds having a size of 3.0 μm or more, the number density of AlMn-based compounds, and the ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more, were evaluated based on the following criteria.
(Judgment criteria)
"O": All seven ratings are "O".
"X": One or more of the seven evaluations is "X".
10…水平連続鋳造装置
11…溶湯受部(タンディッシュ)
11a…溶湯流入部
11b…溶湯保持部
11c…流出部
12…鋳型
12a…一端側
12b…他端側
13…耐火物製板状体(断熱部材)
13a…注湯用通路
21…中空部
21a…内周面
21b…他端側
22…流体供給管
22a…潤滑材供給口
23…冷却装置
24…冷却水キャビティ
24a…内底面
25…冷却水噴射通路
25a…シャワー開口
26…冷却水供給管
27…冷却壁部
B…アルミニウム合金棒
M…合金溶湯
W…冷却水
100…アルミニウム合金鍛造品 10...Horizontalcontinuous casting device 11...Molten metal receiving portion (tundish)
11a: Moltenmetal inlet portion 11b: Molten metal holding portion 11c: Outlet portion 12: Mold 12a: One end side 12b: Other end side 13: Refractory plate-like body (thermal insulation member)
Reference Signs List 13a: Passage for pouring molten metal 21: Hollow portion 21a: Inner peripheral surface 21b: Other end side 22: Fluid supply pipe 22a: Lubricant supply port 23: Cooling device 24: Cooling water cavity 24a: Inner bottom surface 25: Cooling water injection passage 25a: Shower opening 26: Cooling water supply pipe 27: Cooling wall portion B: Aluminum alloy rod M: Molten alloy W: Cooling water 100: Aluminum alloy forging
11…溶湯受部(タンディッシュ)
11a…溶湯流入部
11b…溶湯保持部
11c…流出部
12…鋳型
12a…一端側
12b…他端側
13…耐火物製板状体(断熱部材)
13a…注湯用通路
21…中空部
21a…内周面
21b…他端側
22…流体供給管
22a…潤滑材供給口
23…冷却装置
24…冷却水キャビティ
24a…内底面
25…冷却水噴射通路
25a…シャワー開口
26…冷却水供給管
27…冷却壁部
B…アルミニウム合金棒
M…合金溶湯
W…冷却水
100…アルミニウム合金鍛造品 10...Horizontal
11a: Molten
Claims (8)
- Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金鍛造用素材であって、
鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である、アルミニウム合金鍛造用素材。 1. An aluminum alloy forging material having an alloy composition comprising Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, and Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) being less than 1.4 in terms of mass ratio, with the remainder being Al and unavoidable impurities,
An aluminum alloy forging material having a post-cast electrical conductivity of 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less. - Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金鍛造用素材であって、
鋳造後の導電率が25%IACS以上35%IACS以下、かつ、ロックウェル硬さHRFが62以上82以下である、アルミニウム合金鍛造用素材。 1. An aluminum alloy forging material having an alloy composition comprising Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, and Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) being 0.3 to 1.2 in mass ratio, with the balance being Al and unavoidable impurities,
An aluminum alloy forging material having a post-cast electrical conductivity of 25% IACS or more and 35% IACS or less, and a Rockwell hardness HRF of 62 or more and 82 or less. - Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で1.4未満であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、
粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下であり、かつ、常温における衝撃値が10J/cm2以上である、アルミニウム合金鍛造品。 1. An aluminum alloy forging comprising an aluminum alloy having an alloy composition comprising Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, and Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, wherein a ratio of the Fe content to the Mn content (Fe/Mn) is less than 1.4 in mass ratio, and the balance is composed of Al and unavoidable impurities,
An aluminum alloy forging having a number density of Mn-containing precipitates of 4 particles/ μm2 or more within 2.0 μm including grain boundaries, a ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more of 27% or less, and an impact value of 10 J/ cm2 or more at room temperature. - 前記析出物のサイズが0.5μm以下である、請求項3に記載のアルミニウム合金鍛造品。 The aluminum alloy forged product according to claim 3, wherein the precipitates have a size of 0.5 μm or less.
- Cuを0.25質量%以上0.55質量%以下の範囲内、Mgを0.60質量%以上1.25質量%以下の範囲内、Siを0.90質量%以上1.4質量%以下の範囲内、Mnを0.35質量%以上0.60質量%以下の範囲内、Feを0.15質量%以上0.30質量%以下の範囲内、Znを0.25質量%以下の範囲内、Crを0.050質量%以上0.30質量%以下の範囲内、Tiを0.01質量%以上0.1質量%以下の範囲内、Bを0.0010質量%以上0.030質量%以下の範囲内、Zrを0.0010質量%以上0.050質量%以下の範囲内で含有し、Mnの含有量に対するFeの含有量の比Fe/Mnが質量比で0.3以上1.2以下であり、残部がAl及び不可避不純物からなる合金組成を有するアルミニウム合金から構成されるアルミニウム合金鍛造品であって、
粒界を含む2.0μm以内にMnを含有する析出物の数密度が4個/μm2以上含まれており、結晶方位差15゜以上の大角粒界の比率が27%以下であり、かつ、常温における衝撃値が10J/cm2以上である、アルミニウム合金鍛造品。 1. An aluminum alloy forging comprising an aluminum alloy having an alloy composition comprising Cu in the range of 0.25% by mass or more and 0.55% by mass or less, Mg in the range of 0.60% by mass or more and 1.25% by mass or less, Si in the range of 0.90% by mass or more and 1.4% by mass or less, Mn in the range of 0.35% by mass or more and 0.60% by mass or less, Fe in the range of 0.15% by mass or more and 0.30% by mass or less, Zn in the range of 0.25% by mass or less, Cr in the range of 0.050% by mass or more and 0.30% by mass or less, Ti in the range of 0.01% by mass or more and 0.1% by mass or less, B in the range of 0.0010% by mass or more and 0.030% by mass or less, and Zr in the range of 0.0010% by mass or more and 0.050% by mass or less, a ratio of the Fe content to the Mn content (Fe/Mn) being 0.3 to 1.2 in mass ratio, with the remainder being Al and unavoidable impurities;
An aluminum alloy forging having a number density of Mn-containing precipitates of 4 particles/ μm2 or more within 2.0 μm including grain boundaries, a ratio of high-angle grain boundaries having a crystal orientation difference of 15° or more of 27% or less, and an impact value of 10 J/ cm2 or more at room temperature. - 前記析出物のサイズが0.5μm以下である、請求項5に記載のアルミニウム合金鍛造品。 The aluminum alloy forged product according to claim 5, wherein the size of the precipitates is 0.5 μm or less.
- 請求項3~6のいずれか一項に記載のアルミニウム合金鍛造品の製造方法であって、
アルミニウム合金の溶湯を得る溶湯形成工程と、
前記得られた溶湯を鋳造加工することによって鋳造品を得る鋳造工程と、
前記鋳造品を500℃~融点以下の温度で素材加熱し塑性加工を施して鍛造品を得る鍛造工程と、
前記得られた鍛造品に20℃~500℃までの昇温速度が5.0℃/min以上で昇温し、530~560℃で0.3~3時間以内で保持する溶体化処理を行う溶体化処理工程と、
前記溶体化処理の後5~60秒以内に前記鍛造品の全ての表面が焼き入れ水に接触し、1分を超え、40分以内水槽内で焼き入れする焼き入れ工程と、
前記焼き入れ処理工程を経た鍛造品に180℃~220℃の温度で0.5時間~8時間加熱して時効処理を行う時効処理工程と、を有する、アルミニウム合金鍛造品の製造方法。 A method for producing an aluminum alloy forged product according to any one of claims 3 to 6,
a molten metal forming step for obtaining a molten aluminum alloy;
a casting step of obtaining a casting by casting the obtained molten metal;
a forging process for heating the cast product at a temperature of 500° C. to the melting point and subjecting it to plastic processing to obtain a forged product;
A solution treatment process in which the obtained forged product is heated to 20°C to 500°C at a heating rate of 5.0°C/min or more and then held at 530°C to 560°C for 0.3 to 3 hours;
a quenching step in which all surfaces of the forged product are contacted with quenching water within 5 to 60 seconds after the solution treatment, and the forged product is quenched in a water tank for more than 1 minute and less than 40 minutes;
and an aging treatment step of heating the forged product that has been subjected to the quenching treatment step at a temperature of 180°C to 220°C for 0.5 hours to 8 hours to perform aging treatment. - 前記鋳造工程と前記鍛造工程との間に、前記アルミニウム合金の鋳造品を、370℃以上560℃以下の温度範囲で2時間以上10時間以下保持して均質化熱処理を行う均質化熱処理工程を更に有する、請求項7に記載のアルミニウム合金鍛造品の製造方法。
8. The method for producing an aluminum alloy forged product according to claim 7, further comprising a homogenization heat treatment step between the casting step and the forging step, in which the aluminum alloy cast product is held at a temperature range of 370°C or higher and 560°C or lower for 2 hours or longer and 10 hours or shorter to perform homogenization heat treatment.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022-210255 | 2022-12-27 | ||
| JP2022210255 | 2022-12-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024142830A1 true WO2024142830A1 (en) | 2024-07-04 |
Family
ID=91717272
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2023/043832 WO2024142830A1 (en) | 2022-12-27 | 2023-12-07 | Aluminum alloy forging material, aluminum alloy forged product, and method for manufacturing same |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024142830A1 (en) |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006274415A (en) * | 2005-03-30 | 2006-10-12 | Kobe Steel Ltd | Aluminum alloy forging for high strength structural member |
| WO2007114078A1 (en) * | 2006-03-31 | 2007-10-11 | Kabushiki Kaisha Kobe Seiko Sho | Aluminum alloy forging member and process for producing the same |
| WO2013114928A1 (en) * | 2012-02-02 | 2013-08-08 | 株式会社神戸製鋼所 | Forged aluminum alloy material and method for producing same |
| WO2015146654A1 (en) * | 2014-03-27 | 2015-10-01 | 株式会社神戸製鋼所 | Forged aluminum alloy material and method for producing same |
| JP2021143374A (en) * | 2020-03-11 | 2021-09-24 | 昭和電工株式会社 | Aluminum alloy forged article and method for producing aluminum alloy forged article |
| JP2022093990A (en) * | 2020-12-14 | 2022-06-24 | 昭和電工株式会社 | Aluminum alloy forging and method for producing the same |
| WO2023139960A1 (en) * | 2022-01-18 | 2023-07-27 | 株式会社レゾナック | Aluminum alloy forged product, and manufacturing method thereof |
| JP2023161784A (en) * | 2022-04-26 | 2023-11-08 | 株式会社レゾナック | Aluminum alloy forging and method for manufacturing the same |
-
2023
- 2023-12-07 WO PCT/JP2023/043832 patent/WO2024142830A1/en unknown
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006274415A (en) * | 2005-03-30 | 2006-10-12 | Kobe Steel Ltd | Aluminum alloy forging for high strength structural member |
| WO2007114078A1 (en) * | 2006-03-31 | 2007-10-11 | Kabushiki Kaisha Kobe Seiko Sho | Aluminum alloy forging member and process for producing the same |
| WO2013114928A1 (en) * | 2012-02-02 | 2013-08-08 | 株式会社神戸製鋼所 | Forged aluminum alloy material and method for producing same |
| WO2015146654A1 (en) * | 2014-03-27 | 2015-10-01 | 株式会社神戸製鋼所 | Forged aluminum alloy material and method for producing same |
| JP2021143374A (en) * | 2020-03-11 | 2021-09-24 | 昭和電工株式会社 | Aluminum alloy forged article and method for producing aluminum alloy forged article |
| JP2022093990A (en) * | 2020-12-14 | 2022-06-24 | 昭和電工株式会社 | Aluminum alloy forging and method for producing the same |
| WO2023139960A1 (en) * | 2022-01-18 | 2023-07-27 | 株式会社レゾナック | Aluminum alloy forged product, and manufacturing method thereof |
| JP2023161784A (en) * | 2022-04-26 | 2023-11-08 | 株式会社レゾナック | Aluminum alloy forging and method for manufacturing the same |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5027844B2 (en) | Method for producing aluminum alloy molded product | |
| JP4768925B2 (en) | Method for manufacturing aluminum alloy ingot for plastic working, method for manufacturing aluminum alloy plastic processed product, and aluminum alloy plastic processed product | |
| JP4359231B2 (en) | Method for producing aluminum alloy molded product, and aluminum alloy molded product | |
| JP3552577B2 (en) | Aluminum alloy piston excellent in high temperature fatigue strength and wear resistance and method of manufacturing the same | |
| WO2023139960A1 (en) | Aluminum alloy forged product, and manufacturing method thereof | |
| JP2023161784A (en) | Aluminum alloy forging and method for manufacturing the same | |
| WO2024142830A1 (en) | Aluminum alloy forging material, aluminum alloy forged product, and method for manufacturing same | |
| JP7533743B2 (en) | Aluminum alloy forging material, aluminum alloy forging product and its manufacturing method | |
| JP7533746B2 (en) | Aluminum alloy forging material, aluminum alloy forging product and manufacturing method thereof | |
| JP7533745B2 (en) | Aluminum alloy forging material, aluminum alloy forging product and manufacturing method thereof | |
| JP5081791B2 (en) | Manufacturing method of automobile parts | |
| JP2024109944A (en) | Aluminum alloy casting, aluminum alloy forging, and method for manufacturing the same | |
| JP2024093687A (en) | Aluminum alloy forged product and method for manufacturing the same | |
| JP2024085797A (en) | Aluminum alloy forged product and method for producing the same | |
| JP2024085798A (en) | Aluminum alloy forged product and method for producing the same | |
| JP2024093725A (en) | Aluminum alloy forged product and method for manufacturing the same | |
| JP2024086593A (en) | Material for aluminum alloy forging, aluminum alloy forged product and method for producing the same | |
| JP2024085792A (en) | Aluminum alloy forged product and method for producing the same | |
| JP2024085793A (en) | Aluminum alloy forged product and method for producing the same | |
| JP2024086591A (en) | Material for aluminum alloy forging, aluminum alloy forged product and method for producing the same | |
| JP5435371B2 (en) | Aluminum alloy ingot for plastic working | |
| US11840748B2 (en) | Aluminum alloy forging | |
| WO2023084867A1 (en) | Aluminum alloy ingot, aluminum alloy material, and method for manufacturing aluminum alloy material | |
| WO2023084864A1 (en) | Aluminum alloy ingot, aluminum alloy material, and method for manufacturing aluminum alloy material | |
| US20240209479A1 (en) | Aluminum alloy forging material, aluminum alloy forged product and method of producing same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 23911611 Country of ref document: EP Kind code of ref document: A1 |