WO2024086194A1 - Combination therapy for treatment of cancer - Google Patents
Combination therapy for treatment of cancer Download PDFInfo
- Publication number
- WO2024086194A1 WO2024086194A1 PCT/US2023/035367 US2023035367W WO2024086194A1 WO 2024086194 A1 WO2024086194 A1 WO 2024086194A1 US 2023035367 W US2023035367 W US 2023035367W WO 2024086194 A1 WO2024086194 A1 WO 2024086194A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- substituted
- unsubstituted
- nhnr
- heterocycloalkyl
- heteroaryl
- Prior art date
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 78
- 201000011510 cancer Diseases 0.000 title claims abstract description 44
- 238000002648 combination therapy Methods 0.000 title abstract description 100
- 238000011282 treatment Methods 0.000 title description 42
- 150000001875 compounds Chemical class 0.000 claims abstract description 371
- 238000000034 method Methods 0.000 claims abstract description 146
- 239000002246 antineoplastic agent Substances 0.000 claims abstract description 135
- 230000010741 sumoylation Effects 0.000 claims abstract description 36
- 230000037361 pathway Effects 0.000 claims abstract description 9
- 230000006870 function Effects 0.000 claims abstract description 7
- -1 -CONH2 Chemical group 0.000 claims description 691
- 125000001072 heteroaryl group Chemical group 0.000 claims description 406
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 401
- 125000001424 substituent group Chemical group 0.000 claims description 254
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 246
- 125000003118 aryl group Chemical group 0.000 claims description 230
- 125000004404 heteroalkyl group Chemical group 0.000 claims description 212
- 125000000217 alkyl group Chemical group 0.000 claims description 209
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 196
- 229910052739 hydrogen Inorganic materials 0.000 claims description 189
- 239000001257 hydrogen Substances 0.000 claims description 189
- 229910052736 halogen Inorganic materials 0.000 claims description 179
- 150000002367 halogens Chemical group 0.000 claims description 177
- 229910052757 nitrogen Inorganic materials 0.000 claims description 170
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 162
- 239000000203 mixture Substances 0.000 claims description 80
- 239000003795 chemical substances by application Substances 0.000 claims description 64
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 54
- 229910052760 oxygen Inorganic materials 0.000 claims description 49
- 150000003839 salts Chemical class 0.000 claims description 43
- 125000000623 heterocyclic group Chemical group 0.000 claims description 40
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 27
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 claims description 24
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 22
- 229960003603 decitabine Drugs 0.000 claims description 22
- 229910052717 sulfur Inorganic materials 0.000 claims description 22
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 21
- 125000006583 (C1-C3) haloalkyl group Chemical group 0.000 claims description 19
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 19
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 18
- 102000004190 Enzymes Human genes 0.000 claims description 17
- 108090000790 Enzymes Proteins 0.000 claims description 17
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 15
- 229960004641 rituximab Drugs 0.000 claims description 14
- 206010009944 Colon cancer Diseases 0.000 claims description 13
- 125000004457 alkyl amino carbonyl group Chemical group 0.000 claims description 13
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 12
- 229940075628 hypomethylating agent Drugs 0.000 claims description 12
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 10
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 claims description 10
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 claims description 10
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 claims description 10
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 10
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 10
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 claims description 10
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 9
- 125000006599 (C1-C3) alkylaminocarbonylamino group Chemical group 0.000 claims description 9
- 125000006597 (C1-C3) alkylcarbonylamino group Chemical group 0.000 claims description 9
- 125000006602 (C1-C3) alkylsulfonylamino group Chemical group 0.000 claims description 9
- 208000029742 colonic neoplasm Diseases 0.000 claims description 9
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 9
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 9
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 8
- 230000002401 inhibitory effect Effects 0.000 claims description 8
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 claims description 7
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 claims description 7
- 101100256089 Caenorhabditis elegans uba-2 gene Proteins 0.000 claims description 7
- 229910006074 SO2NH2 Inorganic materials 0.000 claims description 7
- 229910006069 SO3H Inorganic materials 0.000 claims description 7
- 230000003213 activating effect Effects 0.000 claims description 7
- 125000004183 alkoxy alkyl group Chemical group 0.000 claims description 7
- 125000000717 hydrazino group Chemical group [H]N([*])N([H])[H] 0.000 claims description 7
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims description 6
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 claims description 6
- 125000001624 naphthyl group Chemical group 0.000 claims description 6
- 229960002621 pembrolizumab Drugs 0.000 claims description 6
- 229920006395 saturated elastomer Polymers 0.000 claims description 6
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 5
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 claims description 5
- 125000006642 (C1-C6) cyanoalkyl group Chemical group 0.000 claims description 5
- 125000006577 C1-C6 hydroxyalkyl group Chemical group 0.000 claims description 5
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 5
- 208000003950 B-cell lymphoma Diseases 0.000 claims description 4
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 4
- 125000004739 (C1-C6) alkylsulfonyl group Chemical group 0.000 claims description 3
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 3
- 206010005003 Bladder cancer Diseases 0.000 claims description 3
- 201000009030 Carcinoma Diseases 0.000 claims description 3
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 3
- 206010027406 Mesothelioma Diseases 0.000 claims description 3
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 3
- 206010041067 Small cell lung cancer Diseases 0.000 claims description 3
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 3
- 201000010881 cervical cancer Diseases 0.000 claims description 3
- 229910052711 selenium Inorganic materials 0.000 claims description 3
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 3
- 208000034578 Multiple myelomas Diseases 0.000 claims description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 2
- 208000034254 Squamous cell carcinoma of the cervix uteri Diseases 0.000 claims description 2
- 201000001531 bladder carcinoma Diseases 0.000 claims description 2
- 201000006612 cervical squamous cell carcinoma Diseases 0.000 claims description 2
- 208000030381 cutaneous melanoma Diseases 0.000 claims description 2
- 201000005243 lung squamous cell carcinoma Diseases 0.000 claims description 2
- 201000007426 ovarian cystadenocarcinoma Diseases 0.000 claims description 2
- 201000003708 skin melanoma Diseases 0.000 claims description 2
- 210000002784 stomach Anatomy 0.000 claims description 2
- 208000010570 urinary bladder carcinoma Diseases 0.000 claims description 2
- 150000002431 hydrogen Chemical group 0.000 claims 58
- 125000001475 halogen functional group Chemical group 0.000 claims 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 127
- 210000004027 cell Anatomy 0.000 description 45
- 229940126062 Compound A Drugs 0.000 description 44
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 44
- 125000005842 heteroatom Chemical group 0.000 description 37
- 102000002669 Small Ubiquitin-Related Modifier Proteins Human genes 0.000 description 33
- 108010043401 Small Ubiquitin-Related Modifier Proteins Proteins 0.000 description 33
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 31
- 239000008194 pharmaceutical composition Substances 0.000 description 29
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 25
- 239000003112 inhibitor Substances 0.000 description 23
- 238000011284 combination treatment Methods 0.000 description 22
- 230000004083 survival effect Effects 0.000 description 22
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 22
- 239000003814 drug Substances 0.000 description 21
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 20
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 19
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 17
- 125000001153 fluoro group Chemical group F* 0.000 description 16
- 125000003545 alkoxy group Chemical group 0.000 description 15
- 125000000732 arylene group Chemical group 0.000 description 15
- 125000005843 halogen group Chemical group 0.000 description 15
- 125000005549 heteroarylene group Chemical group 0.000 description 15
- 125000006588 heterocycloalkylene group Chemical group 0.000 description 15
- 239000001301 oxygen Substances 0.000 description 15
- 125000002947 alkylene group Chemical group 0.000 description 14
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 14
- 125000004474 heteroalkylene group Chemical group 0.000 description 14
- HXNOEHIMWZDRTK-UHFFFAOYSA-N 4,5,6,7-tetrahydrothieno[2,3-c]pyridine Chemical group C1NCCC2=C1SC=C2 HXNOEHIMWZDRTK-UHFFFAOYSA-N 0.000 description 13
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 13
- 239000003981 vehicle Substances 0.000 description 13
- 125000003282 alkyl amino group Chemical group 0.000 description 12
- 239000003886 aromatase inhibitor Substances 0.000 description 12
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 12
- 239000002552 dosage form Substances 0.000 description 12
- 238000009472 formulation Methods 0.000 description 12
- 150000003254 radicals Chemical class 0.000 description 12
- 125000004429 atom Chemical group 0.000 description 11
- 229940079593 drug Drugs 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- 239000000725 suspension Substances 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- 241000699670 Mus sp. Species 0.000 description 10
- 125000004122 cyclic group Chemical group 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 10
- 230000005764 inhibitory process Effects 0.000 description 10
- 229940124597 therapeutic agent Drugs 0.000 description 10
- 230000001225 therapeutic effect Effects 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- VWUXBMIQPBEWFH-WCCTWKNTSA-N Fulvestrant Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC2=C1 VWUXBMIQPBEWFH-WCCTWKNTSA-N 0.000 description 9
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 9
- 239000002502 liposome Substances 0.000 description 9
- 238000002560 therapeutic procedure Methods 0.000 description 9
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 description 8
- 125000000041 C6-C10 aryl group Chemical group 0.000 description 8
- 239000002253 acid Substances 0.000 description 8
- 125000002993 cycloalkylene group Chemical group 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 125000004458 methylaminocarbonyl group Chemical group [H]N(C(*)=O)C([H])([H])[H] 0.000 description 8
- 239000000546 pharmaceutical excipient Substances 0.000 description 8
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 8
- 239000003755 preservative agent Substances 0.000 description 8
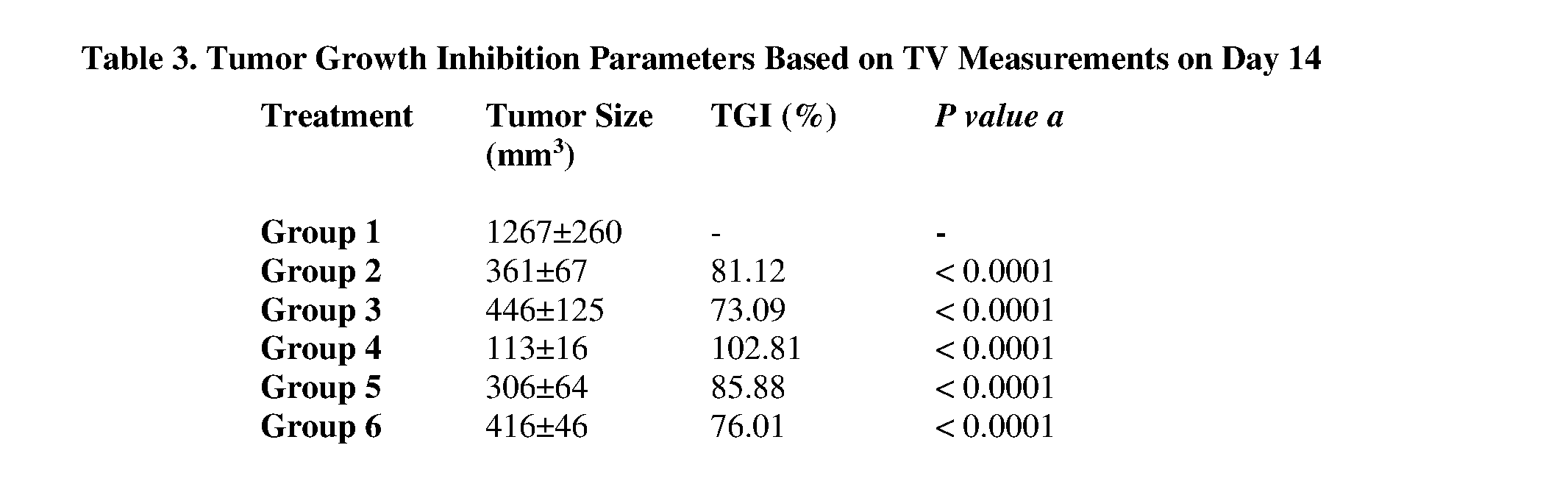
- 230000004614 tumor growth Effects 0.000 description 8
- 229940122815 Aromatase inhibitor Drugs 0.000 description 7
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 description 7
- 229940124647 MEK inhibitor Drugs 0.000 description 7
- 229940123751 PD-L1 antagonist Drugs 0.000 description 7
- 239000002256 antimetabolite Substances 0.000 description 7
- 239000002585 base Substances 0.000 description 7
- 229910052799 carbon Inorganic materials 0.000 description 7
- 230000003833 cell viability Effects 0.000 description 7
- 125000004663 dialkyl amino group Chemical group 0.000 description 7
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 7
- 231100000673 dose–response relationship Toxicity 0.000 description 7
- 229960002258 fulvestrant Drugs 0.000 description 7
- 229910052751 metal Inorganic materials 0.000 description 7
- 239000002184 metal Substances 0.000 description 7
- 238000007920 subcutaneous administration Methods 0.000 description 7
- 125000000547 substituted alkyl group Chemical group 0.000 description 7
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 6
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 6
- 108010074708 B7-H1 Antigen Proteins 0.000 description 6
- 102000008096 B7-H1 Antigen Human genes 0.000 description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 6
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 6
- 108010025468 Cyclin-Dependent Kinase 6 Proteins 0.000 description 6
- 102100036252 Cyclin-dependent kinase 4 Human genes 0.000 description 6
- 102100026804 Cyclin-dependent kinase 6 Human genes 0.000 description 6
- 241000196324 Embryophyta Species 0.000 description 6
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 6
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 6
- 206010025323 Lymphomas Diseases 0.000 description 6
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- 239000013543 active substance Substances 0.000 description 6
- MDFFNEOEWAXZRQ-UHFFFAOYSA-N aminyl Chemical compound [NH2] MDFFNEOEWAXZRQ-UHFFFAOYSA-N 0.000 description 6
- 230000000340 anti-metabolite Effects 0.000 description 6
- 229940100197 antimetabolite Drugs 0.000 description 6
- 238000013270 controlled release Methods 0.000 description 6
- 230000003111 delayed effect Effects 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 229940088597 hormone Drugs 0.000 description 6
- 239000005556 hormone Substances 0.000 description 6
- 125000002883 imidazolyl group Chemical group 0.000 description 6
- 210000002865 immune cell Anatomy 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 125000001786 isothiazolyl group Chemical group 0.000 description 6
- 125000000842 isoxazolyl group Chemical group 0.000 description 6
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 6
- 125000003226 pyrazolyl group Chemical group 0.000 description 6
- 125000004076 pyridyl group Chemical group 0.000 description 6
- 125000000168 pyrrolyl group Chemical group 0.000 description 6
- 150000003384 small molecules Chemical class 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 125000003107 substituted aryl group Chemical group 0.000 description 6
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 6
- 125000005717 substituted cycloalkylene group Chemical group 0.000 description 6
- 230000002195 synergetic effect Effects 0.000 description 6
- 125000000335 thiazolyl group Chemical group 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 125000001425 triazolyl group Chemical group 0.000 description 6
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 5
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 5
- 229940125431 BRAF inhibitor Drugs 0.000 description 5
- 229940124785 KRAS inhibitor Drugs 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 230000000996 additive effect Effects 0.000 description 5
- 125000003342 alkenyl group Chemical group 0.000 description 5
- 230000000259 anti-tumor effect Effects 0.000 description 5
- 229940046844 aromatase inhibitors Drugs 0.000 description 5
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 5
- 230000037396 body weight Effects 0.000 description 5
- 230000022131 cell cycle Effects 0.000 description 5
- 230000010261 cell growth Effects 0.000 description 5
- 229940124302 mTOR inhibitor Drugs 0.000 description 5
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 5
- 229960003301 nivolumab Drugs 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- 239000003381 stabilizer Substances 0.000 description 5
- 125000004434 sulfur atom Chemical group 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 230000003442 weekly effect Effects 0.000 description 5
- 125000003837 (C1-C20) alkyl group Chemical group 0.000 description 4
- 125000006716 (C1-C6) heteroalkyl group Chemical group 0.000 description 4
- 125000004066 1-hydroxyethyl group Chemical group [H]OC([H])([*])C([H])([H])[H] 0.000 description 4
- 125000004486 1-methylpiperidin-3-yl group Chemical group CN1CC(CCC1)* 0.000 description 4
- 125000004484 1-methylpiperidin-4-yl group Chemical group CN1CCC(CC1)* 0.000 description 4
- RTQWWZBSTRGEAV-PKHIMPSTSA-N 2-[[(2s)-2-[bis(carboxymethyl)amino]-3-[4-(methylcarbamoylamino)phenyl]propyl]-[2-[bis(carboxymethyl)amino]propyl]amino]acetic acid Chemical compound CNC(=O)NC1=CC=C(C[C@@H](CN(CC(C)N(CC(O)=O)CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O)C=C1 RTQWWZBSTRGEAV-PKHIMPSTSA-N 0.000 description 4
- 125000001731 2-cyanoethyl group Chemical group [H]C([H])(*)C([H])([H])C#N 0.000 description 4
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 4
- JTAYXEIPUKLUOX-UHFFFAOYSA-N 4-(dimethylamino)but-2-enal Chemical compound CN(C)CC=CC=O JTAYXEIPUKLUOX-UHFFFAOYSA-N 0.000 description 4
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 4
- 238000011725 BALB/c mouse Methods 0.000 description 4
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 4
- 108010025461 Cyclin-Dependent Kinase 9 Proteins 0.000 description 4
- 108010009392 Cyclin-Dependent Kinase Inhibitor p16 Proteins 0.000 description 4
- 102100024457 Cyclin-dependent kinase 9 Human genes 0.000 description 4
- 102100024458 Cyclin-dependent kinase inhibitor 2A Human genes 0.000 description 4
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 4
- 102100023266 Dual specificity mitogen-activated protein kinase kinase 2 Human genes 0.000 description 4
- 101710146529 Dual specificity mitogen-activated protein kinase kinase 2 Proteins 0.000 description 4
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 4
- 102000014150 Interferons Human genes 0.000 description 4
- 108010050904 Interferons Proteins 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 4
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 4
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 4
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- 102100033810 RAC-alpha serine/threonine-protein kinase Human genes 0.000 description 4
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 4
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical group CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 4
- 102000006275 Ubiquitin-Protein Ligases Human genes 0.000 description 4
- 108010083111 Ubiquitin-Protein Ligases Proteins 0.000 description 4
- 229930013930 alkaloid Natural products 0.000 description 4
- 125000000304 alkynyl group Chemical group 0.000 description 4
- 230000005975 antitumor immune response Effects 0.000 description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 4
- 125000002619 bicyclic group Chemical group 0.000 description 4
- AOJDZKCUAATBGE-UHFFFAOYSA-N bromomethane Chemical compound Br[CH2] AOJDZKCUAATBGE-UHFFFAOYSA-N 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 125000004432 carbon atom Chemical group C* 0.000 description 4
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 description 4
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 229960005167 everolimus Drugs 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 125000002541 furyl group Chemical group 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 239000003276 histone deacetylase inhibitor Substances 0.000 description 4
- 229960001001 ibritumomab tiuxetan Drugs 0.000 description 4
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 4
- 230000028993 immune response Effects 0.000 description 4
- 229940079322 interferon Drugs 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 229960003347 obinutuzumab Drugs 0.000 description 4
- 125000002971 oxazolyl group Chemical group 0.000 description 4
- 229910052697 platinum Inorganic materials 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 230000002335 preservative effect Effects 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 125000003373 pyrazinyl group Chemical group 0.000 description 4
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 4
- 125000000714 pyrimidinyl group Chemical group 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 125000006413 ring segment Chemical group 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 239000000829 suppository Substances 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 239000012730 sustained-release form Substances 0.000 description 4
- 125000001544 thienyl group Chemical group 0.000 description 4
- 150000007970 thio esters Chemical class 0.000 description 4
- DKVSUQWCZQBWCP-QAGGRKNESA-N (8R,9S,10R,13S,14S)-10,13-dimethyl-9,10,11,12,13,14,15,16-octahydro-3H-cyclopenta[alpha]phenanthrene-3,17(8H)-dione Natural products O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3C=CC2=C1 DKVSUQWCZQBWCP-QAGGRKNESA-N 0.000 description 3
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 3
- PAMIQIKDUOTOBW-UHFFFAOYSA-N 1-methylpiperidine Chemical compound CN1CCCCC1 PAMIQIKDUOTOBW-UHFFFAOYSA-N 0.000 description 3
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 3
- RHXHGRAEPCAFML-UHFFFAOYSA-N 7-cyclopentyl-n,n-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo[2,3-d]pyrimidine-6-carboxamide Chemical compound N1=C2N(C3CCCC3)C(C(=O)N(C)C)=CC2=CN=C1NC(N=C1)=CC=C1N1CCNCC1 RHXHGRAEPCAFML-UHFFFAOYSA-N 0.000 description 3
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 229940124291 BTK inhibitor Drugs 0.000 description 3
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 3
- 102100031480 Dual specificity mitogen-activated protein kinase kinase 1 Human genes 0.000 description 3
- 101710146526 Dual specificity mitogen-activated protein kinase kinase 1 Proteins 0.000 description 3
- 229940102550 Estrogen receptor antagonist Drugs 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 108700012941 GNRH1 Proteins 0.000 description 3
- 206010018338 Glioma Diseases 0.000 description 3
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 3
- 102000003964 Histone deacetylase Human genes 0.000 description 3
- 108090000353 Histone deacetylase Proteins 0.000 description 3
- 102000008135 Mechanistic Target of Rapamycin Complex 1 Human genes 0.000 description 3
- 108010035196 Mechanistic Target of Rapamycin Complex 1 Proteins 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- 108091008611 Protein Kinase B Proteins 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 206010039491 Sarcoma Diseases 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 description 3
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 150000003863 ammonium salts Chemical group 0.000 description 3
- 229940046836 anti-estrogen Drugs 0.000 description 3
- 230000001833 anti-estrogenic effect Effects 0.000 description 3
- 238000009166 antihormone therapy Methods 0.000 description 3
- 229940041181 antineoplastic drug Drugs 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 229960002756 azacitidine Drugs 0.000 description 3
- XSCHRSMBECNVNS-UHFFFAOYSA-N benzopyrazine Natural products N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 3
- 229910052796 boron Inorganic materials 0.000 description 3
- 229960004117 capecitabine Drugs 0.000 description 3
- 125000002837 carbocyclic group Chemical group 0.000 description 3
- 229960004562 carboplatin Drugs 0.000 description 3
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 3
- 230000006369 cell cycle progression Effects 0.000 description 3
- 230000003915 cell function Effects 0.000 description 3
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 229960000684 cytarabine Drugs 0.000 description 3
- 229940127089 cytotoxic agent Drugs 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 3
- 229960003668 docetaxel Drugs 0.000 description 3
- 229950009791 durvalumab Drugs 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 239000000328 estrogen antagonist Substances 0.000 description 3
- 229960000255 exemestane Drugs 0.000 description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 3
- 229960005277 gemcitabine Drugs 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 150000007529 inorganic bases Chemical class 0.000 description 3
- TWBYWOBDOCUKOW-UHFFFAOYSA-N isonicotinic acid Chemical compound OC(=O)C1=CC=NC=C1 TWBYWOBDOCUKOW-UHFFFAOYSA-N 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- RGLRXNKKBLIBQS-XNHQSDQCSA-N leuprolide acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 RGLRXNKKBLIBQS-XNHQSDQCSA-N 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 125000002950 monocyclic group Chemical group 0.000 description 3
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 3
- 125000004430 oxygen atom Chemical group O* 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- AHJRHEGDXFFMBM-UHFFFAOYSA-N palbociclib Chemical compound N1=C2N(C3CCCC3)C(=O)C(C(=O)C)=C(C)C2=CN=C1NC(N=C1)=CC=C1N1CCNCC1 AHJRHEGDXFFMBM-UHFFFAOYSA-N 0.000 description 3
- FPOHNWQLNRZRFC-ZHACJKMWSA-N panobinostat Chemical compound CC=1NC2=CC=CC=C2C=1CCNCC1=CC=C(\C=C\C(=O)NO)C=C1 FPOHNWQLNRZRFC-ZHACJKMWSA-N 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 229950003687 ribociclib Drugs 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 239000008247 solid mixture Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- FQZYTYWMLGAPFJ-OQKDUQJOSA-N tamoxifen citrate Chemical compound [H+].[H+].[H+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 FQZYTYWMLGAPFJ-OQKDUQJOSA-N 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- LIRYPHYGHXZJBZ-UHFFFAOYSA-N trametinib Chemical compound CC(=O)NC1=CC=CC(N2C(N(C3CC3)C(=O)C3=C(NC=4C(=CC(I)=CC=4)F)N(C)C(=O)C(C)=C32)=O)=C1 LIRYPHYGHXZJBZ-UHFFFAOYSA-N 0.000 description 3
- UELYDGOOJPRWGF-SRQXXRKNSA-N (2r,3r)-3-[2-[4-(cyclopropylsulfonimidoyl)anilino]-5-(trifluoromethyl)pyrimidin-4-yl]oxybutan-2-ol Chemical compound C1=C(C(F)(F)F)C(O[C@H](C)[C@H](O)C)=NC(NC=2C=CC(=CC=2)[S@](=N)(=O)C2CC2)=N1 UELYDGOOJPRWGF-SRQXXRKNSA-N 0.000 description 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 2
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 2
- 125000006002 1,1-difluoroethyl group Chemical group 0.000 description 2
- 125000004509 1,3,4-oxadiazol-2-yl group Chemical group O1C(=NN=C1)* 0.000 description 2
- ILROLYQPRYHHFG-UHFFFAOYSA-N 1-$l^{1}-oxidanylprop-2-en-1-one Chemical group [O]C(=O)C=C ILROLYQPRYHHFG-UHFFFAOYSA-N 0.000 description 2
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 2
- IHPYMWDTONKSCO-UHFFFAOYSA-N 2,2'-piperazine-1,4-diylbisethanesulfonic acid Chemical compound OS(=O)(=O)CCN1CCN(CCS(O)(=O)=O)CC1 IHPYMWDTONKSCO-UHFFFAOYSA-N 0.000 description 2
- 125000004778 2,2-difluoroethyl group Chemical group [H]C([H])(*)C([H])(F)F 0.000 description 2
- 125000004463 2,4-dimethyl-thiazol-5-yl group Chemical group CC=1SC(=C(N1)C)* 0.000 description 2
- WXTMDXOMEHJXQO-UHFFFAOYSA-N 2,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1O WXTMDXOMEHJXQO-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- PIMQWRZWLQKKBJ-SFHVURJKSA-N 2-[(2S)-1-[3-ethyl-7-[(1-oxido-3-pyridin-1-iumyl)methylamino]-5-pyrazolo[1,5-a]pyrimidinyl]-2-piperidinyl]ethanol Chemical compound C=1C(N2[C@@H](CCCC2)CCO)=NC2=C(CC)C=NN2C=1NCC1=CC=C[N+]([O-])=C1 PIMQWRZWLQKKBJ-SFHVURJKSA-N 0.000 description 2
- PEMUGDMSUDYLHU-ZEQRLZLVSA-N 2-[(2S)-4-[7-(8-chloronaphthalen-1-yl)-2-[[(2S)-1-methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile Chemical compound ClC=1C=CC=C2C=CC=C(C=12)N1CC=2N=C(N=C(C=2CC1)N1C[C@@H](N(CC1)C(C(=C)F)=O)CC#N)OC[C@H]1N(CCC1)C PEMUGDMSUDYLHU-ZEQRLZLVSA-N 0.000 description 2
- MRPGRAKIAJJGMM-OCCSQVGLSA-N 2-[2-chloro-4-(trifluoromethyl)phenyl]-5,7-dihydroxy-8-[(2r,3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl]chromen-4-one Chemical compound OC[C@@H]1N(C)CC[C@H]1C1=C(O)C=C(O)C2=C1OC(C=1C(=CC(=CC=1)C(F)(F)F)Cl)=CC2=O MRPGRAKIAJJGMM-OCCSQVGLSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- 125000004777 2-fluoroethyl group Chemical group [H]C([H])(F)C([H])([H])* 0.000 description 2
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 2
- DVLFYONBTKHTER-UHFFFAOYSA-N 3-(N-morpholino)propanesulfonic acid Chemical compound OS(=O)(=O)CCCN1CCOCC1 DVLFYONBTKHTER-UHFFFAOYSA-N 0.000 description 2
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 2
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 2
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 2
- CWDWFSXUQODZGW-UHFFFAOYSA-N 5-thiazolyl Chemical group [C]1=CN=CS1 CWDWFSXUQODZGW-UHFFFAOYSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- 108010029445 Agammaglobulinaemia Tyrosine Kinase Proteins 0.000 description 2
- 102000001714 Agammaglobulinaemia Tyrosine Kinase Human genes 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 206010003571 Astrocytoma Diseases 0.000 description 2
- 206010060971 Astrocytoma malignant Diseases 0.000 description 2
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 2
- 108010037003 Buserelin Proteins 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 2
- 108010024986 Cyclin-Dependent Kinase 2 Proteins 0.000 description 2
- 102100036239 Cyclin-dependent kinase 2 Human genes 0.000 description 2
- 102100026810 Cyclin-dependent kinase 7 Human genes 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- BWLUMTFWVZZZND-UHFFFAOYSA-N Dibenzylamine Chemical compound C=1C=CC=CC=1CNCC1=CC=CC=C1 BWLUMTFWVZZZND-UHFFFAOYSA-N 0.000 description 2
- 241000792859 Enema Species 0.000 description 2
- 206010014967 Ependymoma Diseases 0.000 description 2
- 102100030708 GTPase KRas Human genes 0.000 description 2
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 2
- 108010069236 Goserelin Proteins 0.000 description 2
- 101000911952 Homo sapiens Cyclin-dependent kinase 7 Proteins 0.000 description 2
- 101000584612 Homo sapiens GTPase KRas Proteins 0.000 description 2
- 101000693367 Homo sapiens SUMO-activating enzyme subunit 1 Proteins 0.000 description 2
- 101001094146 Homo sapiens SUMO-activating enzyme subunit 2 Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 2
- 108010000817 Leuprolide Proteins 0.000 description 2
- 206010025557 Malignant fibrous histiocytoma of bone Diseases 0.000 description 2
- 208000000172 Medulloblastoma Diseases 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 2
- 108091007960 PI3Ks Proteins 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- 102100032543 Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN Human genes 0.000 description 2
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 2
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 238000011579 SCID mouse model Methods 0.000 description 2
- 102100025809 SUMO-activating enzyme subunit 1 Human genes 0.000 description 2
- 102100035250 SUMO-activating enzyme subunit 2 Human genes 0.000 description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 2
- 108700042805 TRU-015 Proteins 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 229940123237 Taxane Drugs 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- 101150058395 US22 gene Proteins 0.000 description 2
- 102000003431 Ubiquitin-Conjugating Enzyme Human genes 0.000 description 2
- 108060008747 Ubiquitin-Conjugating Enzyme Proteins 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 2
- LXENKEWVEVKKGV-BQYQJAHWSA-N [2-methoxy-5-[[(e)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonylmethyl]phenyl] dihydrogen phosphate Chemical compound COC1=CC(OC)=CC(OC)=C1\C=C\S(=O)(=O)CC1=CC=C(OC)C(OP(O)(O)=O)=C1 LXENKEWVEVKKGV-BQYQJAHWSA-N 0.000 description 2
- KPCZJLGGXRGYIE-UHFFFAOYSA-N [C]1=CC=CN=C1 Chemical group [C]1=CC=CN=C1 KPCZJLGGXRGYIE-UHFFFAOYSA-N 0.000 description 2
- SZPWXAOBLNYOHY-UHFFFAOYSA-N [C]1=CC=NC2=CC=CC=C12 Chemical group [C]1=CC=NC2=CC=CC=C12 SZPWXAOBLNYOHY-UHFFFAOYSA-N 0.000 description 2
- 229950001573 abemaciclib Drugs 0.000 description 2
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 229940124988 adagrasib Drugs 0.000 description 2
- 150000003797 alkaloid derivatives Chemical class 0.000 description 2
- 125000003806 alkyl carbonyl amino group Chemical group 0.000 description 2
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 2
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 description 2
- 230000003281 allosteric effect Effects 0.000 description 2
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- BSJGASKRWFKGMV-UHFFFAOYSA-L ammonia dichloroplatinum(2+) Chemical compound N.N.Cl[Pt+2]Cl BSJGASKRWFKGMV-UHFFFAOYSA-L 0.000 description 2
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 230000000181 anti-adherent effect Effects 0.000 description 2
- 230000002280 anti-androgenic effect Effects 0.000 description 2
- 230000001093 anti-cancer Effects 0.000 description 2
- 229940121363 anti-inflammatory agent Drugs 0.000 description 2
- 239000002260 anti-inflammatory agent Substances 0.000 description 2
- 230000000118 anti-neoplastic effect Effects 0.000 description 2
- 239000003911 antiadherent Substances 0.000 description 2
- 239000000051 antiandrogen Substances 0.000 description 2
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 239000002216 antistatic agent Substances 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 229910052785 arsenic Inorganic materials 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 229960003852 atezolizumab Drugs 0.000 description 2
- 229950002916 avelumab Drugs 0.000 description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 125000002618 bicyclic heterocycle group Chemical group 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 229960000455 brentuximab vedotin Drugs 0.000 description 2
- 229950006429 briciclib Drugs 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical compound BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 239000007853 buffer solution Substances 0.000 description 2
- CUWODFFVMXJOKD-UVLQAERKSA-N buserelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](COC(C)(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 CUWODFFVMXJOKD-UVLQAERKSA-N 0.000 description 2
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 230000009702 cancer cell proliferation Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- 229960000590 celecoxib Drugs 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000019522 cellular metabolic process Effects 0.000 description 2
- 229940121420 cemiplimab Drugs 0.000 description 2
- 201000007335 cerebellar astrocytoma Diseases 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- 229950009221 chidamide Drugs 0.000 description 2
- SZMJVTADHFNAIS-BJMVGYQFSA-N chidamide Chemical compound NC1=CC(F)=CC=C1NC(=O)C(C=C1)=CC=C1CNC(=O)\C=C\C1=CC=CN=C1 SZMJVTADHFNAIS-BJMVGYQFSA-N 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- 229960002271 cobimetinib Drugs 0.000 description 2
- RESIMIUSNACMNW-BXRWSSRYSA-N cobimetinib fumarate Chemical compound OC(=O)\C=C\C(O)=O.C1C(O)([C@H]2NCCCC2)CN1C(=O)C1=CC=C(F)C(F)=C1NC1=CC=C(I)C=C1F.C1C(O)([C@H]2NCCCC2)CN1C(=O)C1=CC=C(F)C(F)=C1NC1=CC=C(I)C=C1F RESIMIUSNACMNW-BXRWSSRYSA-N 0.000 description 2
- 229940110456 cocoa butter Drugs 0.000 description 2
- 235000019868 cocoa butter Nutrition 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 229940111134 coxibs Drugs 0.000 description 2
- 229940043378 cyclin-dependent kinase inhibitor Drugs 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 2
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- UWFYSQMTEOIJJG-FDTZYFLXSA-N cyproterone acetate Chemical compound C1=C(Cl)C2=CC(=O)[C@@H]3C[C@@H]3[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 UWFYSQMTEOIJJG-FDTZYFLXSA-N 0.000 description 2
- BFSMGDJOXZAERB-UHFFFAOYSA-N dabrafenib Chemical compound S1C(C(C)(C)C)=NC(C=2C(=C(NS(=O)(=O)C=3C(=CC=CC=3F)F)C=CC=2)F)=C1C1=CC=NC(N)=N1 BFSMGDJOXZAERB-UHFFFAOYSA-N 0.000 description 2
- 229940059359 dacogen Drugs 0.000 description 2
- 230000001335 demethylating effect Effects 0.000 description 2
- 108010017271 denileukin diftitox Proteins 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 229910003460 diamond Inorganic materials 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 125000004655 dihydropyridinyl group Chemical group N1(CC=CC=C1)* 0.000 description 2
- 229950009859 dinaciclib Drugs 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000007920 enema Substances 0.000 description 2
- 229940079360 enema for constipation Drugs 0.000 description 2
- 238000013265 extended release Methods 0.000 description 2
- 229940087861 faslodex Drugs 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 229960004421 formestane Drugs 0.000 description 2
- OSVMTWJCGUFAOD-KZQROQTASA-N formestane Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1O OSVMTWJCGUFAOD-KZQROQTASA-N 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 229940121372 histone deacetylase inhibitor Drugs 0.000 description 2
- 239000003667 hormone antagonist Substances 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 125000002632 imidazolidinyl group Chemical group 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 2
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 2
- 238000011081 inoculation Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 2
- 125000004498 isoxazol-4-yl group Chemical group O1N=CC(=C1)* 0.000 description 2
- 229940043355 kinase inhibitor Drugs 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 2
- 229960004338 leuprorelin Drugs 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 206010024627 liposarcoma Diseases 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- GRVDJDISBSALJP-UHFFFAOYSA-N methyloxidanyl Chemical group [O]C GRVDJDISBSALJP-UHFFFAOYSA-N 0.000 description 2
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 2
- 229950009655 milciclib Drugs 0.000 description 2
- 239000002829 mitogen activated protein kinase inhibitor Substances 0.000 description 2
- RXZMYLDMFYNEIM-UHFFFAOYSA-N n,1,4,4-tetramethyl-8-[4-(4-methylpiperazin-1-yl)anilino]-5h-pyrazolo[4,3-h]quinazoline-3-carboxamide Chemical compound CNC(=O)C1=NN(C)C(C2=N3)=C1C(C)(C)CC2=CN=C3NC(C=C1)=CC=C1N1CCN(C)CC1 RXZMYLDMFYNEIM-UHFFFAOYSA-N 0.000 description 2
- UZWDCWONPYILKI-UHFFFAOYSA-N n-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine Chemical compound C1CN(CC)CCN1CC(C=N1)=CC=C1NC1=NC=C(F)C(C=2C=C3N(C(C)C)C(C)=NC3=C(F)C=2)=N1 UZWDCWONPYILKI-UHFFFAOYSA-N 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 239000006070 nanosuspension Substances 0.000 description 2
- 208000018795 nasal cavity and paranasal sinus carcinoma Diseases 0.000 description 2
- 229960002653 nilutamide Drugs 0.000 description 2
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 2
- 150000002825 nitriles Chemical group 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 239000002777 nucleoside Substances 0.000 description 2
- 229950009090 ocaratuzumab Drugs 0.000 description 2
- 229950005751 ocrelizumab Drugs 0.000 description 2
- 229960002450 ofatumumab Drugs 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 229940127084 other anti-cancer agent Drugs 0.000 description 2
- 125000004043 oxo group Chemical group O=* 0.000 description 2
- 229960004390 palbociclib Drugs 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 201000002530 pancreatic endocrine carcinoma Diseases 0.000 description 2
- 229960005184 panobinostat Drugs 0.000 description 2
- 239000002831 pharmacologic agent Substances 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- RDOWQLZANAYVLL-UHFFFAOYSA-N phenanthridine Chemical compound C1=CC=C2C3=CC=CC=C3C=NC2=C1 RDOWQLZANAYVLL-UHFFFAOYSA-N 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 239000008057 potassium phosphate buffer Substances 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 2
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 2
- 239000003197 protein kinase B inhibitor Substances 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 125000002098 pyridazinyl group Chemical group 0.000 description 2
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 description 2
- 125000001422 pyrrolinyl group Chemical group 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- FECGNJPYVFEKOD-VMPITWQZSA-N resminostat Chemical compound C1=CC(CN(C)C)=CC=C1S(=O)(=O)N1C=C(\C=C\C(=O)NO)C=C1 FECGNJPYVFEKOD-VMPITWQZSA-N 0.000 description 2
- OHRURASPPZQGQM-GCCNXGTGSA-N romidepsin Chemical compound O1C(=O)[C@H](C(C)C)NC(=O)C(=C/C)/NC(=O)[C@H]2CSSCC\C=C\[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N2 OHRURASPPZQGQM-GCCNXGTGSA-N 0.000 description 2
- OHRURASPPZQGQM-UHFFFAOYSA-N romidepsin Natural products O1C(=O)C(C(C)C)NC(=O)C(=CC)NC(=O)C2CSSCCC=CC1CC(=O)NC(C(C)C)C(=O)N2 OHRURASPPZQGQM-UHFFFAOYSA-N 0.000 description 2
- 108010091666 romidepsin Proteins 0.000 description 2
- 229950002433 roniciclib Drugs 0.000 description 2
- 102200006538 rs121913530 Human genes 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 229940125944 selective estrogen receptor degrader Drugs 0.000 description 2
- BTIHMVBBUGXLCJ-OAHLLOKOSA-N seliciclib Chemical compound C=12N=CN(C(C)C)C2=NC(N[C@@H](CO)CC)=NC=1NCC1=CC=CC=C1 BTIHMVBBUGXLCJ-OAHLLOKOSA-N 0.000 description 2
- 229950000055 seliciclib Drugs 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- NXQKSXLFSAEQCZ-SFHVURJKSA-N sotorasib Chemical group FC1=CC2=C(N(C(N=C2N2[C@H](CN(CC2)C(C=C)=O)C)=O)C=2C(=NC=CC=2C)C(C)C)N=C1C1=C(C=CC=C1O)F NXQKSXLFSAEQCZ-SFHVURJKSA-N 0.000 description 2
- 238000005563 spheronization Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- SUVMJBTUFCVSAD-UHFFFAOYSA-N sulforaphane Chemical compound CS(=O)CCCCN=C=S SUVMJBTUFCVSAD-UHFFFAOYSA-N 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- 229960005353 testolactone Drugs 0.000 description 2
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 2
- 125000000147 tetrahydroquinolinyl group Chemical group N1(CCCC2=CC=CC=C12)* 0.000 description 2
- 150000003536 tetrazoles Chemical class 0.000 description 2
- 238000011287 therapeutic dose Methods 0.000 description 2
- 229930192474 thiophene Natural products 0.000 description 2
- 208000008732 thymoma Diseases 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 239000012443 tonicity enhancing agent Substances 0.000 description 2
- 229960005267 tositumomab Drugs 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 229960004066 trametinib Drugs 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 229960001612 trastuzumab emtansine Drugs 0.000 description 2
- 125000004306 triazinyl group Chemical group 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 208000018417 undifferentiated high grade pleomorphic sarcoma of bone Diseases 0.000 description 2
- 229950000815 veltuzumab Drugs 0.000 description 2
- GPXBXXGIAQBQNI-UHFFFAOYSA-N vemurafenib Chemical compound CCCS(=O)(=O)NC1=CC=C(F)C(C(=O)C=2C3=CC(=CN=C3NC=2)C=2C=CC(Cl)=CC=2)=C1F GPXBXXGIAQBQNI-UHFFFAOYSA-N 0.000 description 2
- 229940065658 vidaza Drugs 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 229960001771 vorozole Drugs 0.000 description 2
- XLMPPFTZALNBFS-INIZCTEOSA-N vorozole Chemical compound C1([C@@H](C2=CC=C3N=NN(C3=C2)C)N2N=CN=C2)=CC=C(Cl)C=C1 XLMPPFTZALNBFS-INIZCTEOSA-N 0.000 description 2
- 229950003294 voruciclib Drugs 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- QVCAATSEPLQVBX-FPOVZHCZSA-N (3r,4s)-3,4-bis(4-hydroxyphenyl)-8-methyl-3,4-dihydro-2h-chromen-7-ol Chemical compound C1([C@H]2[C@H](C=3C=CC(O)=C(C=3OC2)C)C=2C=CC(O)=CC=2)=CC=C(O)C=C1 QVCAATSEPLQVBX-FPOVZHCZSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 1
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 description 1
- 125000006554 (C4-C8) cycloalkenyl group Chemical group 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- LAMIXXKAWNLXOC-INIZCTEOSA-N (S)-HDAC-42 Chemical compound O=C([C@@H](C(C)C)C=1C=CC=CC=1)NC1=CC=C(C(=O)NO)C=C1 LAMIXXKAWNLXOC-INIZCTEOSA-N 0.000 description 1
- PRXXYMVLYKJITB-IZZDOVSWSA-N (e)-n-(2-aminophenyl)-3-[1-[4-(1-methylpyrazol-4-yl)phenyl]sulfonylpyrrol-3-yl]prop-2-enamide Chemical compound C1=NN(C)C=C1C1=CC=C(S(=O)(=O)N2C=C(\C=C\C(=O)NC=3C(=CC=CC=3)N)C=C2)C=C1 PRXXYMVLYKJITB-IZZDOVSWSA-N 0.000 description 1
- AUGCSOFQTDKPSO-RGVLZGJSSA-N (e)-n-[3-(dimethylamino)propyl]-n'-hydroxy-2-(naphthalen-1-yloxymethyl)oct-2-enediamide Chemical compound C1=CC=C2C(OC/C(C(=O)NCCCN(C)C)=C\CCCCC(=O)NO)=CC=CC2=C1 AUGCSOFQTDKPSO-RGVLZGJSSA-N 0.000 description 1
- DKYBVKMIZODYKL-UHFFFAOYSA-N 1,3-diazinane Chemical compound C1CNCNC1 DKYBVKMIZODYKL-UHFFFAOYSA-N 0.000 description 1
- VBXZSFNZVNDOPB-UHFFFAOYSA-N 1,4,5,6-tetrahydropyrimidine Chemical compound C1CNC=NC1 VBXZSFNZVNDOPB-UHFFFAOYSA-N 0.000 description 1
- FLBAYUMRQUHISI-UHFFFAOYSA-N 1,8-naphthyridine Chemical compound N1=CC=CC2=CC=CN=C21 FLBAYUMRQUHISI-UHFFFAOYSA-N 0.000 description 1
- KSJVAYBCXSURMQ-UHFFFAOYSA-N 1-(3-chloro-4-methylphenyl)-3-[2,6-dinitro-4-(trifluoromethyl)anilino]thiourea Chemical compound C1=C(Cl)C(C)=CC=C1NC(=S)NNC1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O KSJVAYBCXSURMQ-UHFFFAOYSA-N 0.000 description 1
- JNKDVJKGFYTHJZ-UHFFFAOYSA-N 1-benzofuran;2h-benzotriazole Chemical compound C1=CC=C2OC=CC2=C1.C1=CC=C2NN=NC2=C1 JNKDVJKGFYTHJZ-UHFFFAOYSA-N 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- 125000004214 1-pyrrolidinyl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 1
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 1
- MFJCPDOGFAYSTF-UHFFFAOYSA-N 1H-isochromene Chemical compound C1=CC=C2COC=CC2=C1 MFJCPDOGFAYSTF-UHFFFAOYSA-N 0.000 description 1
- AAQTWLBJPNLKHT-UHFFFAOYSA-N 1H-perimidine Chemical compound N1C=NC2=CC=CC3=CC=CC1=C32 AAQTWLBJPNLKHT-UHFFFAOYSA-N 0.000 description 1
- ODMMNALOCMNQJZ-UHFFFAOYSA-N 1H-pyrrolizine Chemical compound C1=CC=C2CC=CN21 ODMMNALOCMNQJZ-UHFFFAOYSA-N 0.000 description 1
- VEPOHXYIFQMVHW-XOZOLZJESA-N 2,3-dihydroxybutanedioic acid (2S,3S)-3,4-dimethyl-2-phenylmorpholine Chemical compound OC(C(O)C(O)=O)C(O)=O.C[C@H]1[C@@H](OCCN1C)c1ccccc1 VEPOHXYIFQMVHW-XOZOLZJESA-N 0.000 description 1
- QLUYMIVVAYRECT-OCCSQVGLSA-N 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(2r,3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl]chromen-4-one Chemical compound OC[C@@H]1N(C)CC[C@H]1C1=C(O)C=C(O)C2=C1OC(C=1C(=CC=CC=1)Cl)=CC2=O QLUYMIVVAYRECT-OCCSQVGLSA-N 0.000 description 1
- NOIXNOMHHWGUTG-UHFFFAOYSA-N 2-[[4-[4-pyridin-4-yl-1-(2,2,2-trifluoroethyl)pyrazol-3-yl]phenoxy]methyl]quinoline Chemical group C=1C=C(OCC=2N=C3C=CC=CC3=CC=2)C=CC=1C1=NN(CC(F)(F)F)C=C1C1=CC=NC=C1 NOIXNOMHHWGUTG-UHFFFAOYSA-N 0.000 description 1
- PDGKHKMBHVFCMG-UHFFFAOYSA-N 2-[[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino]spiro[7,8-dihydropyrazino[5,6]pyrrolo[1,2-d]pyrimidine-9,1'-cyclohexane]-6-one Chemical compound C1CN(C)CCN1C(C=N1)=CC=C1NC1=NC=C(C=C2N3C4(CCCCC4)CNC2=O)C3=N1 PDGKHKMBHVFCMG-UHFFFAOYSA-N 0.000 description 1
- UXGVMFHEKMGWMA-UHFFFAOYSA-N 2-benzofuran Chemical compound C1=CC=CC2=COC=C21 UXGVMFHEKMGWMA-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- VHMICKWLTGFITH-UHFFFAOYSA-N 2H-isoindole Chemical compound C1=CC=CC2=CNC=C21 VHMICKWLTGFITH-UHFFFAOYSA-N 0.000 description 1
- RCLQNICOARASSR-SECBINFHSA-N 3-[(2r)-2,3-dihydroxypropyl]-6-fluoro-5-(2-fluoro-4-iodoanilino)-8-methylpyrido[2,3-d]pyrimidine-4,7-dione Chemical compound FC=1C(=O)N(C)C=2N=CN(C[C@@H](O)CO)C(=O)C=2C=1NC1=CC=C(I)C=C1F RCLQNICOARASSR-SECBINFHSA-N 0.000 description 1
- MAUCONCHVWBMHK-UHFFFAOYSA-N 3-[(dimethylamino)methyl]-N-[2-[4-[(hydroxyamino)-oxomethyl]phenoxy]ethyl]-2-benzofurancarboxamide Chemical compound O1C2=CC=CC=C2C(CN(C)C)=C1C(=O)NCCOC1=CC=C(C(=O)NO)C=C1 MAUCONCHVWBMHK-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- PRDJGNVQBVXXEO-UHFFFAOYSA-N 3-cyanopropyl carbamimidothioate Chemical compound NC(=N)SCCCC#N PRDJGNVQBVXXEO-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- ACWKGTGIJRCOOM-HHHXNRCGSA-N 4-(4-fluoro-2-methoxyphenyl)-N-[3-[(methylsulfonimidoyl)methyl]phenyl]-1,3,5-triazin-2-amine Chemical compound COc1cc(F)ccc1c2ncnc(Nc3cccc(C[S@](=N)(=O)C)c3)n2 ACWKGTGIJRCOOM-HHHXNRCGSA-N 0.000 description 1
- CLPFFLWZZBQMAO-UHFFFAOYSA-N 4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-yl)benzonitrile Chemical compound C1=CC(C#N)=CC=C1C1N2C=NC=C2CCC1 CLPFFLWZZBQMAO-UHFFFAOYSA-N 0.000 description 1
- SUVMJBTUFCVSAD-JTQLQIEISA-N 4-Methylsulfinylbutyl isothiocyanate Natural products C[S@](=O)CCCCN=C=S SUVMJBTUFCVSAD-JTQLQIEISA-N 0.000 description 1
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical group N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- PJMNEPMSGCRSRC-IEVKOWOJSA-N 4-androstene-3,6,17-trione Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=O)C2=C1 PJMNEPMSGCRSRC-IEVKOWOJSA-N 0.000 description 1
- GDRVFDDBLLKWRI-UHFFFAOYSA-N 4H-quinolizine Chemical compound C1=CC=CN2CC=CC=C21 GDRVFDDBLLKWRI-UHFFFAOYSA-N 0.000 description 1
- UPTYCYWTFGTCCG-UHFFFAOYSA-N 5-(1-piperazinylsulfonyl)isoquinoline Chemical compound C=1C=CC2=CN=CC=C2C=1S(=O)(=O)N1CCNCC1 UPTYCYWTFGTCCG-UHFFFAOYSA-N 0.000 description 1
- PLIVFNIUGLLCEK-UHFFFAOYSA-N 7-[4-(3-ethynylanilino)-7-methoxyquinazolin-6-yl]oxy-n-hydroxyheptanamide Chemical compound C=12C=C(OCCCCCCC(=O)NO)C(OC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 PLIVFNIUGLLCEK-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 150000008067 9-membered heterocyclic compounds Chemical class 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 208000002008 AIDS-Related Lymphoma Diseases 0.000 description 1
- BUROJSBIWGDYCN-GAUTUEMISA-N AP 23573 Chemical compound C1C[C@@H](OP(C)(C)=O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 BUROJSBIWGDYCN-GAUTUEMISA-N 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 229940080778 Adenosine deaminase inhibitor Drugs 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- 206010073360 Appendix cancer Diseases 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- 208000011691 Burkitt lymphomas Diseases 0.000 description 1
- 125000004406 C3-C8 cycloalkylene group Chemical group 0.000 description 1
- YVJKPZWMUQOLFJ-HKMNZKMDSA-N CCN1N=C(C(F)(F)F)C(C2=C([C@H](CN(C3)C(/C=C/CN(C)C)=O)C4=C3SC(C#N)=C4C)C=CC=C2F)=C1 Chemical compound CCN1N=C(C(F)(F)F)C(C2=C([C@H](CN(C3)C(/C=C/CN(C)C)=O)C4=C3SC(C#N)=C4C)C=CC=C2F)=C1 YVJKPZWMUQOLFJ-HKMNZKMDSA-N 0.000 description 1
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 1
- 229940124297 CDK 4/6 inhibitor Drugs 0.000 description 1
- 108091007914 CDKs Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 206010007279 Carcinoid tumour of the gastrointestinal tract Diseases 0.000 description 1
- 238000003734 CellTiter-Glo Luminescent Cell Viability Assay Methods 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- 150000000703 Cerium Chemical class 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 102000003903 Cyclin-dependent kinases Human genes 0.000 description 1
- 108090000266 Cyclin-dependent kinases Proteins 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical class OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 230000007067 DNA methylation Effects 0.000 description 1
- 208000008743 Desmoplastic Small Round Cell Tumor Diseases 0.000 description 1
- 206010064581 Desmoplastic small round cell tumour Diseases 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 102000056372 ErbB-3 Receptor Human genes 0.000 description 1
- 102000044591 ErbB-4 Receptor Human genes 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 208000006168 Ewing Sarcoma Diseases 0.000 description 1
- UKCVAQGKEOJTSR-UHFFFAOYSA-N Fadrozole hydrochloride Chemical compound Cl.C1=CC(C#N)=CC=C1C1N2C=NC=C2CCC1 UKCVAQGKEOJTSR-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 230000010190 G1 phase Effects 0.000 description 1
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 1
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical class Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 206010021042 Hypopharyngeal cancer Diseases 0.000 description 1
- 206010056305 Hypopharyngeal neoplasm Diseases 0.000 description 1
- 206010062767 Hypophysitis Diseases 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- WRYCSMQKUKOKBP-UHFFFAOYSA-N Imidazolidine Chemical compound C1CNCN1 WRYCSMQKUKOKBP-UHFFFAOYSA-N 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 206010061252 Intraocular melanoma Diseases 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 239000002177 L01XE27 - Ibrutinib Substances 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 206010061523 Lip and/or oral cavity cancer Diseases 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 206010025312 Lymphoma AIDS related Diseases 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 239000012820 MEK1 Inhibitor Substances 0.000 description 1
- 239000007993 MOPS buffer Substances 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000030070 Malignant epithelial tumor of ovary Diseases 0.000 description 1
- 206010073059 Malignant neoplasm of unknown primary site Diseases 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 1
- 102000009308 Mechanistic Target of Rapamycin Complex 2 Human genes 0.000 description 1
- 108010034057 Mechanistic Target of Rapamycin Complex 2 Proteins 0.000 description 1
- 244000062730 Melissa officinalis Species 0.000 description 1
- 235000010654 Melissa officinalis Nutrition 0.000 description 1
- 102000016397 Methyltransferase Human genes 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 description 1
- HRNLUBSXIHFDHP-UHFFFAOYSA-N N-(2-aminophenyl)-4-[[[4-(3-pyridinyl)-2-pyrimidinyl]amino]methyl]benzamide Chemical compound NC1=CC=CC=C1NC(=O)C(C=C1)=CC=C1CNC1=NC=CC(C=2C=NC=CC=2)=N1 HRNLUBSXIHFDHP-UHFFFAOYSA-N 0.000 description 1
- LKJPYSCBVHEWIU-UHFFFAOYSA-N N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide Chemical compound C=1C=C(C#N)C(C(F)(F)F)=CC=1NC(=O)C(O)(C)CS(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-UHFFFAOYSA-N 0.000 description 1
- QGZYDVAGYRLSKP-UHFFFAOYSA-N N-[7-(hydroxyamino)-7-oxoheptyl]-2-(N-phenylanilino)-5-pyrimidinecarboxamide Chemical compound N1=CC(C(=O)NCCCCCCC(=O)NO)=CN=C1N(C=1C=CC=CC=1)C1=CC=CC=C1 QGZYDVAGYRLSKP-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- 150000008522 N-ethylpiperidines Chemical class 0.000 description 1
- PAWIYAYFNXQGAP-UHFFFAOYSA-N N-hydroxy-2-[4-[[(1-methyl-3-indolyl)methylamino]methyl]-1-piperidinyl]-5-pyrimidinecarboxamide Chemical compound C12=CC=CC=C2N(C)C=C1CNCC(CC1)CCN1C1=NC=C(C(=O)NO)C=N1 PAWIYAYFNXQGAP-UHFFFAOYSA-N 0.000 description 1
- NCNRHFGMJRPRSK-UHFFFAOYSA-N N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]-2-propenamide Chemical compound ONC(=O)C=CC1=CC=CC(S(=O)(=O)NC=2C=CC=CC=2)=C1 NCNRHFGMJRPRSK-UHFFFAOYSA-N 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical compound ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 229910003849 O-Si Inorganic materials 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 208000035327 Oestrogen receptor positive breast cancer Diseases 0.000 description 1
- 206010031096 Oropharyngeal cancer Diseases 0.000 description 1
- 206010057444 Oropharyngeal neoplasm Diseases 0.000 description 1
- 208000007571 Ovarian Epithelial Carcinoma Diseases 0.000 description 1
- 206010061328 Ovarian epithelial cancer Diseases 0.000 description 1
- 229910003872 O—Si Inorganic materials 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108010011536 PTEN Phosphohydrolase Proteins 0.000 description 1
- 208000000821 Parathyroid Neoplasms Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 102100027913 Peptidyl-prolyl cis-trans isomerase FKBP1A Human genes 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- 101710132081 Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN Proteins 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 206010035052 Pineal germinoma Diseases 0.000 description 1
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 1
- 201000005746 Pituitary adenoma Diseases 0.000 description 1
- 206010061538 Pituitary tumour benign Diseases 0.000 description 1
- 201000008199 Pleuropulmonary blastoma Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 1
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 1
- 101710100963 Receptor tyrosine-protein kinase erbB-4 Proteins 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 230000018199 S phase Effects 0.000 description 1
- WINXNKPZLFISPD-UHFFFAOYSA-M Saccharin sodium Chemical compound [Na+].C1=CC=C2C(=O)[N-]S(=O)(=O)C2=C1 WINXNKPZLFISPD-UHFFFAOYSA-M 0.000 description 1
- 208000004337 Salivary Gland Neoplasms Diseases 0.000 description 1
- 206010061934 Salivary gland cancer Diseases 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 description 1
- 101710183263 Serine/threonine-protein kinase B-raf Proteins 0.000 description 1
- 229910007161 Si(CH3)3 Inorganic materials 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 1
- 206010042971 T-cell lymphoma Diseases 0.000 description 1
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 108010006877 Tacrolimus Binding Protein 1A Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 108010009978 Tec protein-tyrosine kinase Proteins 0.000 description 1
- CBPNZQVSJQDFBE-FUXHJELOSA-N Temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-FUXHJELOSA-N 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 206010043515 Throat cancer Diseases 0.000 description 1
- 201000009365 Thymic carcinoma Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- IWEQQRMGNVVKQW-OQKDUQJOSA-N Toremifene citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 IWEQQRMGNVVKQW-OQKDUQJOSA-N 0.000 description 1
- RTKIYFITIVXBLE-UHFFFAOYSA-N Trichostatin A Natural products ONC(=O)C=CC(C)=CC(C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046458 Urethral neoplasms Diseases 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 201000005969 Uveal melanoma Diseases 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- LYRLHTRLCKSXIV-ZRFIDHNTSA-N [[(2r,3s,4r,5r)-5-(2-amino-6-oxo-3h-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] 2-[(2-chloroacetyl)amino]ethyl hydrogen phosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O LYRLHTRLCKSXIV-ZRFIDHNTSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 229940028652 abraxane Drugs 0.000 description 1
- 229950009821 acalabrutinib Drugs 0.000 description 1
- WDENQIQQYWYTPO-IBGZPJMESA-N acalabrutinib Chemical compound CC#CC(=O)N1CCC[C@H]1C1=NC(C=2C=CC(=CC=2)C(=O)NC=2N=CC=CC=2)=C2N1C=CN=C2N WDENQIQQYWYTPO-IBGZPJMESA-N 0.000 description 1
- 159000000021 acetate salts Chemical class 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002487 adenosine deaminase inhibitor Substances 0.000 description 1
- 208000020990 adrenal cortex carcinoma Diseases 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 208000007128 adrenocortical carcinoma Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 229940042992 afinitor Drugs 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000005090 alkenylcarbonyl group Chemical group 0.000 description 1
- 125000005078 alkoxycarbonylalkyl group Chemical group 0.000 description 1
- 125000000278 alkyl amino alkyl group Chemical group 0.000 description 1
- 125000004689 alkyl amino carbonyl alkyl group Chemical group 0.000 description 1
- 125000004949 alkyl amino carbonyl amino group Chemical group 0.000 description 1
- 125000005094 alkyl carbonyl amino alkyl group Chemical group 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 125000005097 aminocarbonylalkyl group Chemical group 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 239000004037 angiogenesis inhibitor Substances 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 229940065524 anticholinergics inhalants for obstructive airway diseases Drugs 0.000 description 1
- 239000003472 antidiabetic agent Substances 0.000 description 1
- 229940125708 antidiabetic agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000002220 antihypertensive agent Substances 0.000 description 1
- 229940030600 antihypertensive agent Drugs 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- 208000021780 appendiceal neoplasm Diseases 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 229940078010 arimidex Drugs 0.000 description 1
- 229940087620 aromasin Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 125000003289 ascorbyl group Chemical class [H]O[C@@]([H])(C([H])([H])O*)[C@@]1([H])OC(=O)C(O*)=C1O* 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 229950010908 atuveciclib Drugs 0.000 description 1
- KLNFSAOEKUDMFA-UHFFFAOYSA-N azanide;2-hydroxyacetic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OCC(O)=O KLNFSAOEKUDMFA-UHFFFAOYSA-N 0.000 description 1
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 229960003094 belinostat Drugs 0.000 description 1
- NCNRHFGMJRPRSK-MDZDMXLPSA-N belinostat Chemical compound ONC(=O)\C=C\C1=CC=CC(S(=O)(=O)NC=2C=CC=CC=2)=C1 NCNRHFGMJRPRSK-MDZDMXLPSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- MYONAGGJKCJOBT-UHFFFAOYSA-N benzimidazol-2-one Chemical compound C1=CC=CC2=NC(=O)N=C21 MYONAGGJKCJOBT-UHFFFAOYSA-N 0.000 description 1
- 125000005605 benzo group Chemical group 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- ACWZRVQXLIRSDF-UHFFFAOYSA-N binimetinib Chemical compound OCCONC(=O)C=1C=C2N(C)C=NC2=C(F)C=1NC1=CC=C(Br)C=C1F ACWZRVQXLIRSDF-UHFFFAOYSA-N 0.000 description 1
- 229950003054 binimetinib Drugs 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000002715 bioenergetic effect Effects 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 229960003008 blinatumomab Drugs 0.000 description 1
- 229940101815 blincyto Drugs 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 201000008873 bone osteosarcoma Diseases 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 201000002143 bronchus adenoma Diseases 0.000 description 1
- 229960002719 buserelin Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 150000004648 butanoic acid derivatives Chemical class 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- 150000001661 cadmium Chemical class 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- 229910052792 caesium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 229940112129 campath Drugs 0.000 description 1
- 239000012830 cancer therapeutic Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 1
- 150000001722 carbon compounds Chemical class 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 125000004181 carboxyalkyl group Chemical group 0.000 description 1
- 239000002327 cardiovascular agent Substances 0.000 description 1
- 229940125692 cardiovascular agent Drugs 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 229940097647 casodex Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229940047495 celebrex Drugs 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 238000012054 celltiter-glo Methods 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000003576 central nervous system agent Substances 0.000 description 1
- 229940125693 central nervous system agent Drugs 0.000 description 1
- 208000030239 cerebral astrocytoma Diseases 0.000 description 1
- ZMIGMASIKSOYAM-UHFFFAOYSA-N cerium Chemical compound [Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce] ZMIGMASIKSOYAM-UHFFFAOYSA-N 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000013626 chemical specie Substances 0.000 description 1
- 230000002113 chemopreventative effect Effects 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 208000011654 childhood malignant neoplasm Diseases 0.000 description 1
- 239000012069 chiral reagent Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 239000000812 cholinergic antagonist Substances 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- WCZVZNOTHYJIEI-UHFFFAOYSA-N cinnoline Chemical compound N1=NC=CC2=CC=CC=C21 WCZVZNOTHYJIEI-UHFFFAOYSA-N 0.000 description 1
- 150000001860 citric acid derivatives Chemical class 0.000 description 1
- 229960002436 cladribine Drugs 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 150000001868 cobalt Chemical class 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229920001577 copolymer Chemical class 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 150000001879 copper Chemical class 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 1
- 125000004966 cyanoalkyl group Chemical group 0.000 description 1
- 239000002875 cyclin dependent kinase inhibitor Substances 0.000 description 1
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 description 1
- GLNWREBYRLDPQP-MHZLTWQESA-N cyclopentyl (2s)-2-[[4-[[8-(hydroxyamino)-8-oxooctanoyl]amino]phenyl]methylamino]-2-phenylacetate Chemical compound C1=CC(NC(=O)CCCCCCC(=O)NO)=CC=C1CN[C@@H](C=1C=CC=CC=1)C(=O)OC1CCCC1 GLNWREBYRLDPQP-MHZLTWQESA-N 0.000 description 1
- 229960003843 cyproterone Drugs 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 229960002465 dabrafenib Drugs 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 229960002923 denileukin diftitox Drugs 0.000 description 1
- 230000002074 deregulated effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- NIJJYAXOARWZEE-UHFFFAOYSA-N di-n-propyl-acetic acid Natural products CCCC(C(O)=O)CCC NIJJYAXOARWZEE-UHFFFAOYSA-N 0.000 description 1
- 229960001259 diclofenac Drugs 0.000 description 1
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 1
- 235000013325 dietary fiber Nutrition 0.000 description 1
- 229940043237 diethanolamine Drugs 0.000 description 1
- FKGKZBBDJSKCIS-UHFFFAOYSA-N diethyl-[[6-[[4-(hydroxycarbamoyl)phenyl]carbamoyloxymethyl]naphthalen-2-yl]methyl]azanium;chloride;hydrate Chemical compound O.[Cl-].C1=CC2=CC(C[NH+](CC)CC)=CC=C2C=C1COC(=O)NC1=CC=C(C(=O)NO)C=C1 FKGKZBBDJSKCIS-UHFFFAOYSA-N 0.000 description 1
- 125000001664 diethylamino group Chemical group [H]C([H])([H])C([H])([H])N(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 230000007783 downstream signaling Effects 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- INVTYAOGFAGBOE-UHFFFAOYSA-N entinostat Chemical compound NC1=CC=CC=C1NC(=O)C(C=C1)=CC=C1CNC(=O)OCC1=CC=CN=C1 INVTYAOGFAGBOE-UHFFFAOYSA-N 0.000 description 1
- 150000002118 epoxides Chemical class 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- 201000007281 estrogen-receptor positive breast cancer Diseases 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-N ethanesulfonic acid Chemical class CCS(O)(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-N 0.000 description 1
- 229940031098 ethanolamine Drugs 0.000 description 1
- 150000002169 ethanolamines Chemical class 0.000 description 1
- 125000005745 ethoxymethyl group Chemical group [H]C([H])([H])C([H])([H])OC([H])([H])* 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960004945 etoricoxib Drugs 0.000 description 1
- MNJVRJDLRVPLFE-UHFFFAOYSA-N etoricoxib Chemical compound C1=NC(C)=CC=C1C1=NC=C(Cl)C=C1C1=CC=C(S(C)(=O)=O)C=C1 MNJVRJDLRVPLFE-UHFFFAOYSA-N 0.000 description 1
- 229940085363 evista Drugs 0.000 description 1
- 229950011548 fadrozole Drugs 0.000 description 1
- 229940043168 fareston Drugs 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 239000010685 fatty oil Substances 0.000 description 1
- 229940087476 femara Drugs 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 239000004052 folic acid antagonist Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 150000004675 formic acid derivatives Chemical class 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 229940020967 gemzar Drugs 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 229960005219 gentisic acid Drugs 0.000 description 1
- 230000000762 glandular Effects 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 229950006191 gluconic acid Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 150000002306 glutamic acid derivatives Chemical class 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 230000034659 glycolysis Effects 0.000 description 1
- 229960002913 goserelin Drugs 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 201000000079 gynecomastia Diseases 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 125000004438 haloalkoxy group Chemical group 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 108010084091 heregulin beta1 Proteins 0.000 description 1
- 125000004446 heteroarylalkyl group Chemical group 0.000 description 1
- 125000004366 heterocycloalkenyl group Chemical group 0.000 description 1
- 125000004415 heterocyclylalkyl group Chemical group 0.000 description 1
- 208000029824 high grade glioma Diseases 0.000 description 1
- 229940125697 hormonal agent Drugs 0.000 description 1
- 108091008039 hormone receptors Proteins 0.000 description 1
- 125000001183 hydrocarbyl group Chemical group 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 230000037417 hyperactivation Effects 0.000 description 1
- 201000006866 hypopharynx cancer Diseases 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- 229940061301 ibrance Drugs 0.000 description 1
- 229960001507 ibrutinib Drugs 0.000 description 1
- XYFPWWZEPKGCCK-GOSISDBHSA-N ibrutinib Chemical compound C1=2C(N)=NC=NC=2N([C@H]2CN(CCC2)C(=O)C=C)N=C1C(C=C1)=CC=C1OC1=CC=CC=C1 XYFPWWZEPKGCCK-GOSISDBHSA-N 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 150000002460 imidazoles Chemical class 0.000 description 1
- 238000011493 immune profiling Methods 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 239000000677 immunologic agent Substances 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- LPAGFVYQRIESJQ-UHFFFAOYSA-N indoline Chemical compound C1=CC=C2NCCC2=C1 LPAGFVYQRIESJQ-UHFFFAOYSA-N 0.000 description 1
- HOBCFUWDNJPFHB-UHFFFAOYSA-N indolizine Chemical compound C1=CC=CN2C=CC=C21 HOBCFUWDNJPFHB-UHFFFAOYSA-N 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 150000002505 iron Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 229940011083 istodax Drugs 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 229960004752 ketorolac Drugs 0.000 description 1
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 150000003893 lactate salts Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229960000448 lactic acid Drugs 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- CMJCXYNUCSMDBY-ZDUSSCGKSA-N lgx818 Chemical compound COC(=O)N[C@@H](C)CNC1=NC=CC(C=2C(=NN(C=2)C(C)C)C=2C(=C(NS(C)(=O)=O)C=C(Cl)C=2)F)=N1 CMJCXYNUCSMDBY-ZDUSSCGKSA-N 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 150000002632 lipids Chemical group 0.000 description 1
- 239000008297 liquid dosage form Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 229910003002 lithium salt Inorganic materials 0.000 description 1
- 159000000002 lithium salts Chemical group 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 108010078259 luprolide acetate gel depot Proteins 0.000 description 1
- 229940087857 lupron Drugs 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 208000030883 malignant astrocytoma Diseases 0.000 description 1
- 201000011614 malignant glioma Diseases 0.000 description 1
- 208000026045 malignant tumor of parathyroid gland Diseases 0.000 description 1
- 150000002696 manganese Chemical class 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229940083118 mekinist Drugs 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 210000000716 merkel cell Anatomy 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 208000037970 metastatic squamous neck cancer Diseases 0.000 description 1
- GXHMMDRXHUIUMN-UHFFFAOYSA-N methanesulfonic acid Chemical compound CS(O)(=O)=O.CS(O)(=O)=O GXHMMDRXHUIUMN-UHFFFAOYSA-N 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 108091074487 miR-34 stem-loop Proteins 0.000 description 1
- 108091092493 miR-34-1 stem-loop Proteins 0.000 description 1
- 108091059780 miR-34-2 stem-loop Proteins 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 125000006682 monohaloalkyl group Chemical group 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- 229940113083 morpholine Drugs 0.000 description 1
- 150000002780 morpholines Chemical class 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 206010051747 multiple endocrine neoplasia Diseases 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 201000005962 mycosis fungoides Diseases 0.000 description 1
- 208000025113 myeloid leukemia Diseases 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- QRGHOAATPOLDPF-VQFNDLOPSA-N nanatinostat Chemical compound N1=CC(C(=O)NO)=CN=C1N1C[C@@H]([C@@H]2NCC=3N=C4C=CC(F)=CC4=CC=3)[C@@H]2C1 QRGHOAATPOLDPF-VQFNDLOPSA-N 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 229940042880 natural phospholipid Drugs 0.000 description 1
- 229950007221 nedaplatin Drugs 0.000 description 1
- 229960000801 nelarabine Drugs 0.000 description 1
- IXOXBSCIXZEQEQ-UHTZMRCNSA-N nelarabine Chemical compound C1=NC=2C(OC)=NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O IXOXBSCIXZEQEQ-UHTZMRCNSA-N 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- 201000008026 nephroblastoma Diseases 0.000 description 1
- 229940080607 nexavar Drugs 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 150000002826 nitrites Chemical class 0.000 description 1
- 125000006574 non-aromatic ring group Chemical group 0.000 description 1
- 102000037979 non-receptor tyrosine kinases Human genes 0.000 description 1
- 108091008046 non-receptor tyrosine kinases Proteins 0.000 description 1
- 231100001160 nonlethal Toxicity 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 125000003835 nucleoside group Chemical group 0.000 description 1
- 201000002575 ocular melanoma Diseases 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 229940100027 ontak Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 201000006958 oropharynx cancer Diseases 0.000 description 1
- MMMNTDFSPSQXJP-UHFFFAOYSA-N orphenadrine citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=C(C)C=1C(OCCN(C)C)C1=CC=CC=C1 MMMNTDFSPSQXJP-UHFFFAOYSA-N 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 208000021284 ovarian germ cell tumor Diseases 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 229940055726 pantothenic acid Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 208000028591 pheochromocytoma Diseases 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical compound C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- IIMIOEBMYPRQGU-UHFFFAOYSA-L picoplatin Chemical compound N.[Cl-].[Cl-].[Pt+2].CC1=CC=CC=N1 IIMIOEBMYPRQGU-UHFFFAOYSA-L 0.000 description 1
- 229950005566 picoplatin Drugs 0.000 description 1
- 201000007315 pineal gland astrocytoma Diseases 0.000 description 1
- 201000004838 pineal region germinoma Diseases 0.000 description 1
- 150000004885 piperazines Chemical class 0.000 description 1
- 150000003053 piperidines Chemical class 0.000 description 1
- 210000003635 pituitary gland Anatomy 0.000 description 1
- 208000021310 pituitary gland adenoma Diseases 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229940063179 platinol Drugs 0.000 description 1
- 125000006684 polyhaloalkyl group Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 1
- JHDKZFFAIZKUCU-ZRDIBKRKSA-N pracinostat Chemical compound ONC(=O)/C=C/C1=CC=C2N(CCN(CC)CC)C(CCCC)=NC2=C1 JHDKZFFAIZKUCU-ZRDIBKRKSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- 230000009822 protein phosphorylation Effects 0.000 description 1
- 230000004063 proteosomal degradation Effects 0.000 description 1
- CPNGPNLZQNNVQM-UHFFFAOYSA-N pteridine Chemical compound N1=CN=CC2=NC=CN=C21 CPNGPNLZQNNVQM-UHFFFAOYSA-N 0.000 description 1
- 239000000649 purine antagonist Substances 0.000 description 1
- 150000003216 pyrazines Chemical class 0.000 description 1
- USPWKWBDZOARPV-UHFFFAOYSA-N pyrazolidine Chemical compound C1CNNC1 USPWKWBDZOARPV-UHFFFAOYSA-N 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- UBQKCCHYAOITMY-UHFFFAOYSA-N pyridin-2-ol Chemical compound OC1=CC=CC=N1 UBQKCCHYAOITMY-UHFFFAOYSA-N 0.000 description 1
- 150000003222 pyridines Chemical class 0.000 description 1
- 239000003790 pyrimidine antagonist Substances 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- SBYHFKPVCBCYGV-UHFFFAOYSA-N quinuclidine Chemical compound C1CC2CCN1CC2 SBYHFKPVCBCYGV-UHFFFAOYSA-N 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 208000030859 renal pelvis/ureter urothelial carcinoma Diseases 0.000 description 1
- 229950002821 resminostat Drugs 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229960001302 ridaforolimus Drugs 0.000 description 1
- 229950006546 riviciclib Drugs 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 1
- 229960003452 romidepsin Drugs 0.000 description 1
- 102200006539 rs121913529 Human genes 0.000 description 1
- 150000003873 salicylate salts Chemical class 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 229960005399 satraplatin Drugs 0.000 description 1
- 190014017285 satraplatin Chemical compound 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- CYOHGALHFOKKQC-UHFFFAOYSA-N selumetinib Chemical compound OCCONC(=O)C=1C=C2N(C)C=NC2=C(F)C=1NC1=CC=C(Br)C=C1Cl CYOHGALHFOKKQC-UHFFFAOYSA-N 0.000 description 1
- 239000008299 semisolid dosage form Substances 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 201000008261 skin carcinoma Diseases 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 229940034810 soltamox Drugs 0.000 description 1
- 229960003787 sorafenib Drugs 0.000 description 1
- 229940073531 sotorasib Drugs 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 1
- 159000000008 strontium salts Chemical class 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 229960005559 sulforaphane Drugs 0.000 description 1
- 235000015487 sulforaphane Nutrition 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 230000003319 supportive effect Effects 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 201000008205 supratentorial primitive neuroectodermal tumor Diseases 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229940081616 tafinlar Drugs 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 229960003454 tamoxifen citrate Drugs 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 229960001367 tartaric acid Drugs 0.000 description 1
- 150000003892 tartrate salts Chemical class 0.000 description 1
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 description 1
- RCINICONZNJXQF-XAZOAEDWSA-N taxol® Chemical compound O([C@@H]1[C@@]2(CC(C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3(C21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-XAZOAEDWSA-N 0.000 description 1
- 229940063683 taxotere Drugs 0.000 description 1
- 229960000235 temsirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-UHFFFAOYSA-N temsirolimus Natural products C1CC(O)C(OC)CC1CC(C)C1OC(=O)C2CCCCN2C(=O)C(=O)C(O)(O2)C(C)CCC2CC(OC)C(C)=CC=CC=CC(C)CC(C)C(=O)C(OC)C(O)C(C)=CC(C)C(=O)C1 QFJCIRLUMZQUOT-UHFFFAOYSA-N 0.000 description 1
- IMCGHZIGRANKHV-AJNGGQMLSA-N tert-butyl (3s,5s)-2-oxo-5-[(2s,4s)-5-oxo-4-propan-2-yloxolan-2-yl]-3-propan-2-ylpyrrolidine-1-carboxylate Chemical compound O1C(=O)[C@H](C(C)C)C[C@H]1[C@H]1N(C(=O)OC(C)(C)C)C(=O)[C@H](C(C)C)C1 IMCGHZIGRANKHV-AJNGGQMLSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000005309 thioalkoxy group Chemical group 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 150000003608 titanium Chemical class 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- RTKIYFITIVXBLE-QEQCGCAPSA-N trichostatin A Chemical compound ONC(=O)/C=C/C(/C)=C/[C@@H](C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-QEQCGCAPSA-N 0.000 description 1
- 229940086542 triethylamine Drugs 0.000 description 1
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 1
- 229950007127 trilaciclib Drugs 0.000 description 1
- 190014017283 triplatin tetranitrate Chemical compound 0.000 description 1
- 229950002860 triplatin tetranitrate Drugs 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 229960004418 trolamine Drugs 0.000 description 1
- 208000029387 trophoblastic neoplasm Diseases 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 1
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 208000037965 uterine sarcoma Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- MSRILKIQRXUYCT-UHFFFAOYSA-M valproate semisodium Chemical compound [Na+].CCCC(C(O)=O)CCC.CCCC(C([O-])=O)CCC MSRILKIQRXUYCT-UHFFFAOYSA-M 0.000 description 1
- 229960000604 valproic acid Drugs 0.000 description 1
- 229960003862 vemurafenib Drugs 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 210000000239 visual pathway Anatomy 0.000 description 1
- 230000004400 visual pathway Effects 0.000 description 1
- WAEXFXRVDQXREF-UHFFFAOYSA-N vorinostat Chemical compound ONC(=O)CCCCCCC(=O)NC1=CC=CC=C1 WAEXFXRVDQXREF-UHFFFAOYSA-N 0.000 description 1
- 229960000237 vorinostat Drugs 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940053867 xeloda Drugs 0.000 description 1
- 229940034727 zelboraf Drugs 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229940033942 zoladex Drugs 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/12—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with radicals, substituted by hetero atoms, attached to carbon atoms of the nitrogen-containing ring
- C07D217/14—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with radicals, substituted by hetero atoms, attached to carbon atoms of the nitrogen-containing ring other than aralkyl radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4365—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system having sulfur as a ring hetero atom, e.g. ticlopidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2887—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against CD20
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/02—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with only hydrogen atoms or radicals containing only carbon and hydrogen atoms, directly attached to carbon atoms of the nitrogen-containing ring; Alkylene-bis-isoquinolines
- C07D217/06—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with only hydrogen atoms or radicals containing only carbon and hydrogen atoms, directly attached to carbon atoms of the nitrogen-containing ring; Alkylene-bis-isoquinolines with the ring nitrogen atom acylated by carboxylic or carbonic acids, or with sulfur or nitrogen analogues thereof, e.g. carbamates
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/12—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with radicals, substituted by hetero atoms, attached to carbon atoms of the nitrogen-containing ring
- C07D217/14—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with radicals, substituted by hetero atoms, attached to carbon atoms of the nitrogen-containing ring other than aralkyl radicals
- C07D217/16—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with radicals, substituted by hetero atoms, attached to carbon atoms of the nitrogen-containing ring other than aralkyl radicals substituted by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D223/00—Heterocyclic compounds containing seven-membered rings having one nitrogen atom as the only ring hetero atom
- C07D223/14—Heterocyclic compounds containing seven-membered rings having one nitrogen atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D223/16—Benzazepines; Hydrogenated benzazepines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/04—Ortho-condensed systems
- C07D491/044—Ortho-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring
- C07D491/048—Ortho-condensed systems with only one oxygen atom as ring hetero atom in the oxygen-containing ring the oxygen-containing ring being five-membered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
Definitions
- SUMOylation is a reversible post-translational modification by small ubiquitin-like modifier (SUMO). SUMOylation controls many cellular functions and plays a critical role in the regulation of genome integrity, cell cycle progression, and the immune response.
- SUMO E1 or SAE1/UBA2 small ubiquitin-like modifier
- UBC9 ubiquitin-conjugating enzyme 9
- the process of SUMOylation starts when SUMO E1 activates SUMO through ATP hydrolysis and forms a thioester conjugate with SUMO. Next, SUMO is transferred and forms a new thioester conjugate with E2. Finally, SUMO is attached to target proteins, a step catalyzed by an E3 ligase.
- E3 ligase Specific small molecule inhibitors of the SUMO E1 enzyme have recently been developed that allow for the exploration of pharmacological SUMOylation inhibitors as novel anticancer therapeutics. Inhibition of SUMOylation by small molecules impairs cancer cell proliferation and triggers antitumor immune responses by stimulating the interferon (IFN) response.
- IFN interferon
- SUMMARY Provided herein is a method of treating cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation.
- a compound of the disclosure comprises a substituted 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation.
- the compound binds to Uba2.
- the compound has an inhibitory effect on activating enzymes (E1) of SUMO.
- Group 1 [small circle] Vehicle control p.o, (BIWx2weeks)
- Group 2 [square] Compound A 50 mg/kg, p.o. (BIWx2weeks)
- Group 3 [triangle] rituximab 20 mg/kg, i.p. (BIWx2weeks)
- Group 4 [diamond] Compound A, 50 mg/kg, p.o.+ Rituximab, 20 mg/kg,i.p.
- Group 1 [small circle] Vehicle control p.o, (BIWx2weeks)
- Group 2 [square] Compound A 50 mg/kg, p.o.
- FIG.3 Effect upon combination treatment with Compound A and decitabine in AML cell lines.
- GDM-1 and MV4-11 cells were treated with indicated doses of Compound A and decitabine for 72-hrs, and cell viability was measured to generate dose response curves.
- IC50 values were calculated from treatment with Compound A alone, and in combination with decitabine, and these values were used to measure ZIP scores, see Table 4.
- Dose response graphs and IC50 calculations were generated using GraphPad Prism 9 (ver.9.4.0). ZIP scores were calculated using SynergyFinder 3.0 (https://synergyfinder.fim m.fi/synergy/20220701215205193938/).
- FIG.4 Combination of Compound A with anti-PD-1 therapy Inhibits TumorGrowth in a CT26 model of syngeneic colon cancer in BALB/c mice. Data points represent tumor volume curves for four treatment groups.
- Group 1 [small circle] Vehicle control.
- Group 2 [triangle] Compound A 50 mg/kg
- Group 3 [square] antiPD-12.5 mg/kg
- Group 4 [triangle] Compound A, 50 mg/kg, + antiPD-12.5 mg/kg
- the y-axis reflects the tumor volumes in mm 2 for each group of animals per length of experiment - 15 days [x-axis].
- FIG 5 Combination of Compound A with anti-PD-1 therapy Improves Survival in a CT26 model of syngeneic colon cancer in BALB/c mice. Data points represent survival for four treatment groups. Group 1: [small circle] Vehicle control. Group 2: [triangle] Compound A 50 mg/kg, Group 3: [square] antiPD-12.5 mg/kg, Group 4: [triangle] Compound A, 50 mg/kg, + antiPD-12.5 mg/kg The y-axis reflects the number of mice surviving as a percentage of the total in each group of animals per length of experiment - 80 days [x-axis]. [010] FIG 6. Compound A Induces CD8 + T Cell Infiltration in a CT26 model of syngeneic colon cancer in BALB/c mice.
- the bar graph represents the percent of CD8+ as a percent of CD45/CD3 for the treatment groups -
- Column 1 Vehicle control.
- Column 2 Compound A at 50 mg/kg.
- the present disclosure provides compounds and methods for blocking SUMOylation.
- the combinations of the present disclosure include SUMO inhibitors and at least one other anti-cancer agent that functions through a pathway other than SUMOylation.
- the SUMO inhibitors of the present disclosure bind to Uba2.
- the SUMO inhibitors of the present disclosure have an inhibitory effect on activating enzymes (E1) of SUMO.
- a method comprising: a) administering a therapeutically-effective amount of a compound to a subject in need thereof, wherein the compound blocks SUMOylation in the subject; and b) after the administering, observing that the administering increases an immune response in the subject against a cancer.
- the method comprises after the administering, obtaining a population of an immune cell in a biological sample obtained from the subject.
- the biological sample is obtained from a tumor.
- the administering increases a population of tumor infiltrating lymphocytes.
- the administering increases a population of CD8+ cells.
- the population of the immune cell is determined using a multiplexed immunofluorescence. In some embodiments, the population of the immune cell is determined using flow cytometry. In some embodiments, the population of the immune cell is determined using fluorescence activated cell sorting [FACS]. In some embodiments, the population of the immune cell is determined using antibody staining. [013] The disclosure further provides methods of treatment of a cancerous lesion or a tumor harboring a SUMOylation. [014] Cancer is a collection of related diseases characterized by uncontrolled proliferation of cells with the potential to metastasize throughout the body.
- Cancer can be classified into five broad categories including, for example: carcinomas, which can arise from cells that cover internal and external parts of the body such as the lung, breast, and colon; sarcomas, which can arise from cells that are located in bone, cartilage, fat, connective tissue, muscle, and other supportive tissues; lymphomas, which can arise in the lymph nodes and immune system tissues; leukemia, which can arise in the bone marrow and accumulate in the bloodstream; and adenomas, which can arise in the thyroid, the pituitary gland, the adrenal gland, and other glandular tissues. [015] Although different cancers can develop in virtually any of the body's tissues, and contain unique features, the basic processes that cause cancer can be similar in all forms of the disease.
- SUMOylation is a reversible post-translational modification by small ubiquitin-like modifier (SUMO). SUMOylation controls many cellular functions and plays a critical role in the regulation of genome integrity, cell cycle progression, and the immune response (1).
- SUMO E1 or SAE1/UBA2 small ubiquitin-like modifier
- UBC9 ubiquitin-conjugating enzyme 9
- the process of SUMOylation starts when SUMO E1 activates SUMO through ATP hydrolysis and forms a thioester conjugate with SUMO. Next, SUMO is transferred and forms a new thioester conjugate with E2. Finally, SUMO is attached to target proteins, a step catalyzed by an E3 ligase.
- E3 ligase Specific small molecule inhibitors of the SUMO E1 enzyme have recently been developed that allow for the exploration of pharmacological SUMOylation inhibitors as novel anticancer therapeutics. Inhibition of SUMOylation by small molecules impairs cancer cell proliferation and triggers antitumor immune responses by stimulating the interferon (IFN) response (2).
- One embodiment of the disclosure includes compounds that block SUMOylation when used in combination with other treatments for cancer. Mechanism of compounds disclosed herein.
- assays can be employed to detect, for example, binding of a compound to Uba2, or an inhibitory effect on activating enzymes (E1) of SUMO. Such assays are described in PCT application PCT/US22/73985.
- a compound of the disclosure can increase the inhibit SUMOylation by at least or up to about 0.1%, at least or up to about 0.2%, at least or up to about 0.3%, at least or up to about 0.4%, at least or up to about 0.5%, at least or up to about 0.6%, at least or up to about 0.7%, at least or up to about 0.8%, at least or up to about 0.9%, at least or up to about 1%, at least or up to about 2%, at least or up to about 3%, at least or up to about 4%, at least or up to about 5%, at least or up to about 6%, at least or up to about 7%, at least or up to about 8%, at least or up to about 9%, at least or up to about 10%, at least or up to about 11%, at least or up to about 12%, at least or up to about 13%, at least or up to about 14%, at least or up to about 15%, at least or up to about 16%, at least or up to about 17%, at least or up
- a compound described herein can bind SUMO E1 enzyme that is, for example, by at least or up to about 0.1%, at least or up to about 0.2%, at least or up to about 0.3%, at least or up to about 0.4%, at least or up to about 0.5%, at least or up to about 0.6%, at least or up to about 0.7%, at least or up to about 0.8%, at least or up to about 0.9%, at least or up to about 1%, at least or up to about 2%, at least or up to about 3%, at least or up to about 4%, at least or up to about 5%, at least or up to about 6%, at least or up to about 7%, at least or up to about 8%, at least or up to about 9%, at least or up to about 10%, at least or up to about 11%, at least or up to about 12%, at least or up to about 13%, at least or up to about 14%, at least or up to about 15%, at least or up to about 16%, at least or up to about 1
- a compound of the disclosure can be used, for example, to reduce cell proliferation or trigger antitumor immune response in a subject.
- Compounds of the disclosure [022]
- a compound of the disclosure comprises a substituted 4,5,6,7- tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation.
- the compound binds to Uba2.
- the compound has an inhibitory effect on activating enzymes (E1) of SUMO.
- the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has a nitrile substituent at a 2-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group.
- the nitrile substituent is alternatively described as a cyano group.
- the compound has plasma protein binding of less than about 99.5% or less than that of an analogous compound that lacks the cyano substituent.
- the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an electrophilic moiety at a 6-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group.
- the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an unsubstituted or substituted C2-6 alkenylcarbonyl or an unsubstituted or substituted C2-6 alkynylcarbonyl substituent at a 6-position of the 4,5,6,7- tetrahydrothieno[2,3-c]pyridine group.
- the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an unsubstituted or substituted aminopropenylcarbonyl or an unsubstituted or substituted aminobutenylcarbonyl substituent at a 6-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group.
- the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group comprises a substituted phenyl substituent at a 4-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group.
- R 1 is selected from hydrogen, halogen, -C(X 1 )3, -CHX 1 2, -CH2X 1 , -OCX 1 3, -OCH2X 1 , -OCHX 1 2, -CN, -SOn1R 1A , -SOv1NR 1A R 1B , -NHC(O)NR 1A R 1B , -N(O)m1, -NR 1A R 1B , -NHNR 1A R 1B , -C(O)R 1A , -C(O)-OR 1A , -C(O)NR 1A R 1B , -C(O)NHNR 1A R 1B , -OR 1A , -NR 1A SO2R 1B , -NR 1A C(O)R 1B , -NR 1A C(O)OR 1B , -NR 1A OR 1B , -N3, substituted or unsubstituted alky
- R 3 and R 3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
- R 5 is selected from hydrogen, halogen, -CX 5 3 , -CHX 5 2 , -CH 2 X 5 , -OCX 5 3 , -OCH 2 X 5 , -OCHX 5 2 , -CN, -SO n5 R 5A , -SO v5 NR 5A R 5B , -NHC(O)NR 5A R 5B , -N(O) m5 , -NR 5A R 5B , -NHNR 5A R 5B , -C(O)R 5A , -C(O)-OR 5A , -C(O)NR 5A R 5B , -C(O)NHNR 5A R 5B , -OR 5A , -NR 5A SO 2 R 5B ,
- Z is N.
- ring A is selected from 5-membered heteroaryl, wherein ring A is optionally substituted with one or more substituent groups.
- R 2 is selected from substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl.
- ring A is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein ring A is optionally substituted with one or more substituent groups. [032] In some embodiments, ring A is selected from
- R 2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cycloalkyl and substituted or unsubstituted 5- or 6-membered heteroaryl. [034] In some embodiments, R 2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cyclopentyl, substituted or unsubstituted cyclohexyl and substituted or unsubstituted pyridyl.
- M is CR 3 R 4 .
- Y is CR 6 R 7 .
- R 1 is selected from hydrogen, halogen, -CX 1 3, -CHX 1 2, -CH2X 1 , -OCX 1 3, -OCH2X 1 , -OCHX 1 2, -CN, -SOn1R 1A , -SOv1NR 1A R 1B , -NHC(O)NR 1A R 1B , -N(O)m1, -NR 1A R 1B , -NHNR 1A R 1B , -C(O)R 1A , -C(O)-OR 1A , -C(O)NR 1A R 1B , -C(O)NHNR 1A R 1B , -OR 1A , -NR 1A SO2R 1B , -NR 1A C(O)R 1B , -NR 1A C(O)OR 1B , -NR 1A OR 1B , -N3, substituted or unsubstituted alkyl, substituted or
- ring B is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein Ring B is optionally substituted with one or more substituents.
- ring B is selected from phenyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, and triazinyl, wherein ring B is optionally substituted with one or more substituent groups.
- ring B is selected from [044]
- W is S.
- R 10 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C 1-6 alkyl, substituted or unsubstituted C 1-6 heteroalkyl, substituted or unsubstituted C 3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl; and R 11 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C 1-6 alkyl, substituted or unsubstituted C 1-6 heteroalkyl, substituted or unsubstituted C 3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl; and R 11 is selected
- Non-limiting examples of compounds of the disclosure include compounds of the following formula wherein R 36 is H, halo, C 1 -C 4 alkyl, C 2 -C 4 alkenyl or C 3 -C 6 cycloalkyl; R 37 is substituted or unsubstituted C 2 -C 4 alkenyl or C 2 -C 4 alkynyl; and R 38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl, substituted or unsubstituted nitrogen containing 5- or 6- membered partially unsaturated heterocyclyl and substituted or unsubstituted nitrogen containing 6-10 membered heteroaryl; provided R 38 is not 4-pyridyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof.
- R 38 is selected from substituted or unsubstituted nitrogen containing 5- membered heteroaryl selected from pyrazolyl, isoxazolyl, isothiazolyl, pyrrolyl, thiazolyl, triazolyl and imidazolyl; substituted or unsubstituted nitrogen containing 6- membered heteroaryl selected from pyridinyl, pyrimidinyl and pyrazinyl; substituted or unsubstituted nitrogen containing 5-membered partially unsaturated heterocyclyl selected from pyrrolinyl, and imidazolidinyl; and substituted or unsubstituted dihydropyridinyl.
- R 36 is selected from chloro, methyl, ethyl, isopropyl, allylyl, and cyclopropyl;.
- R 38 is selected from substituted 5-pyrazolyl, substituted 4-pyrazolyl, substituted 1-pyrazolyl, substituted or unsubstituted 4-isoxazolyl, substituted or unsubstituted 4- isothiazolyl, substituted or unsubstituted 3-pyrrolyl, substituted or unsubstituted 5-thiazolyl, substituted or unsubstituted 5-imidazolyl, substituted or unsubstituted 1-imidazolyl, substituted or unsubstituted [1,2,4]triazol-5-yl, substituted or unsubstituted 3-pyridyl, and substituted or unsubstituted 5-pyrimidinyl.
- R 38 is selected from 3-trifluoromethyl-pyrazol-4-yl, 1-isopropyl-3- trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-trifluoromethyl-pyrazol-4-yl, 1-methyl-3-difluoromethyl-pyrazol- 4-yl, 1-butyl-3-trifluoromethyl-pyrazol-4-yl, 1-methoxymethyl-3-trifluoromethyl-pyrazol-4-yl, 1-propyl- 3-trifluoromethyl-pyrazol-4-yl, 1,3-dimethyl-pyrazol-4-yl, 1,3,5-trimethyl-pyrazol-4-yl, 1-methyl-3- cyclopropyl-pyrazol-4-yl, 1-methyl-3-trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-amino-pyrazol-4-yl, 1-ethyl- 3-methoxy-pyrazol-4-y
- R 38 is selected from 5-pyrimidinyl, 2-methyl-5-pyrimidinyl, 4-methyl-5- pyrimidinyl, 4,6-dimethoxy-5-pyrimidinyl, 4,6-dimethyl-5-pyrimidinyl, 4-triflouromethyl-5-pyrimidinyl, 4-pyrimidinyl, 2-methyl-4-pyrimidinyl, 4-methyl-6-pyrimidinyl, 2,4-dimethyl-6-pyrimidinyl, 3-pyridinyl, 2-pyridinyl, 4-methyl-2-pyridinyl, 2-triflouromethyl-3-pyridinyl, 4-triflouromethyl-3-pyridinyl, 2-methyl- 3-pyridinyl, 2,5-dimethyl-3-pyridinyl, 2,6-dimethyl-3-pyridinyl, 2,4-dimethyl-3-pyridinyl, 2-ethyl-3- pyridinyl, 5-methyl-3-pyridinyl, 5-methyl-3-pyri
- R 38 is selected from substituted or unsubstituted tetrahydroquinolinyl, substituted or unsubstituted 1-pyrrolin-3-yl, substituted or unsubstituted 1-imidazolidinyl, and substituted or unsubstituted dihydropyridin-3-yl.
- R 38 is selected from 4-isoquinolinyl, 1-methoxy-4-isoquinolinyl, 1-chloro- 4-isoquinolinyl, 6-methyl-4-isoquinolinyl, 1-oxo-2-methyl-4-isoquinolinyl, 3-quinolinyl, 4-quinolinyl, 5- pyrrolopyridinyl, [1,3,3a]-triazainden-5-yl, 1-ethyl-3-pyrrolopyridinyl, and 1-methyl-5-pyrrolopyridinyl.
- R 37 is selected from ethenyl, fluoroethenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl and nitrogen-containing heterocyclyl- propenyl.
- Non-limiting examples of compounds of the disclosure include compounds of the following formula wherein R 36 is selected from H, halo, C1-C4 alkyl, C2-C4 alkenyl and C3-C6 cycloalkyl; R 37 is substituted or unsubstituted C2-C4 alkenyl, or C2-C4 alkynyl; and R 41 is selected from H, C1-6 alkyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1-6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy- C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1- 6 alkyl, aryl-C1-6 alkyl, substituted
- R 41 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, D 3 -methyl, 1- hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2- methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, methoxycarbonylmethyl, dimethylaminocarbonylmethyl, dimethylaminocarbonylethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, aminocarbonyl-1,1-dimethyl, methylcarbonylaminoethyl, 1-methylcarbonylamino-2.2- dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl, cyanoethyl,
- R 36 is H, chloro, methyl, ethyl, isopropyl, propenyl, trifluoromethyl, 2-furyl, 2-pyridinyl, or cyclopropyl.
- R 37 is selected from ethenyl, fluoroethenyl, fluoropropenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethenyl and substituted or unsubstituted 3-7 membered nitrogen- containing heterocyclyl-propenyl.
- R 37 is azetidin-1-ylpropenyl, 1-methylpyrrolidin-2-ylethenyl, dimethylaminopropenyl, methylaminopropenyl, piperidin-1-ylpropenyl, 4-hydroxy-piperidin-1-ylpropenyl, pyrrolidin-1-ylpropenyl, 3-hydroxypyrrolidin-1-ylpropenyl, morpholin-1-ylpropenyl, or aminopropenyl.
- R 42 is selected from H, trifluoromethyl, difluoromethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy.
- R 43 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl.
- R 44 is one or more substituents selected from H, hydroxy, fluoro and methoxyl.
- Non-limiting examples of compounds of the disclosure include compounds of any one of the following formulae wherein R 1 is substituted or unsubstituted C 2 -C 6 alkenyl, or substituted or unsubstituted C 2 -C 6 alkynyl; R 6 is selected from H, C 1-6 alkyl, C 2-4 alkynyl, C 1-6 hydroxyalkyl, C 1-6 alkoxyalkyl, C 1-6 alkylamino-C 1- 6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C1-6 haloalkyl, C1-6 alkylsulfonyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl, aryl-
- R 1 is selected from C2-C6 alkenyl, halo-substituted C2-C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl, alkylamino substituted C2-C6 alkenyl, amino substituted C2-C6 alkenyl, hydroxy substituted amino-C2-C6 alkenyl, phenyl substituted amino-C2- C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C 2 -C 6 alkynyl, alkoxy substituted C 2 -C 6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C 2 -C 6 alkynyl, substituted or unsubstituted 3-7 membered cycloalkyl- substitute
- R 1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3- trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, alkoxypropenyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, 3-amino-4-hydroxy-butenyl, 3-amino-4-phenyl-butenyl, dialkylaminobutenyl, alkylaminobutenyl, aminobutenyl, dialkylaminopentenyl, alkylaminopentenyl, aminopentenyl, aminopropynyl, dialkylaminopropynyl, alkylaminopropynyl, methoxypropynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propynyl, substituted or unsubstituted 3-7 membered nitrogen
- R 1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3- trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, methoxypropenyl, ethoxypropenyl, aminopropenyl, 3- amino-butenyl, 3-methylamino-butenyl, 3-amino-4-hydroxy-butenyl, 3-methylamino-4-methoxy-butenyl, 3-amino-4-phenyl-butenyl, 3-amino-pentenyl, aminopropynyl, methoxypropynyl, dimethylaminopropenyl, di(d1,d2,d3-methyl)aminopropenyl, diethylaminopropenyl, 3-(N,N-dimethylamino)-3-phenyl-propenyl, 3- (N,N-dimethylamino)-3-pheny
- R 6 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, propynyl, 1- hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2- methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, carboxypropyl, methoxycarbonylmethyl, ethoxycarbonylmethyl, ethoxycarbonylethyl, ethoxycarbonylpropyl, dimethylaminocarbonylmethyl, dimethylaminocarbonyl-1-ethyl, methylaminocarbonyl-1-ethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, 1-aminocarbonylethyl
- R 7 is selected from H, trifluoromethyl, difluoromethyl, 1,1-difluoroethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy. methylaminocarbonyl, dimethylaminocarbonyl, and cyclopropyl.
- R 8 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl.
- R 9 is selected from H, fluoro, chloro, methyl, and cyano.
- R 10 is selected from H, fluoro, methylcarbonylamino, chloro, amino, hydroxy, methyl, difluoromethyl, methylsulfonylamino, and methylaminocarbonylamino.
- R 11 is H or fluoro.
- R 6 is ethyl; R 7 is trifluoromethyl; and R 8 is H.
- R 6 is methyl; R 7 is trifluoromethyl; and R 8 is H.
- R 6 is H; R 7 is trifluoromethyl; and R 8 is H.
- R 6 is 3,5-difluoropyridin-4-yl; R 7 is trifluoromethyl; and R 8 is H.
- R 1 is substituted ethenyl.
- R 1 is dimethylaminopropenyl.
- R 1 is methylaminopropenyl.
- R 1 is 3,3-difluoropropenyl.
- R 1 is 2-(azetin-2-yl)ethenyl.
- R 1 is 2-(2-methyl-pyrrolidiny-5-yl)ethenyl. [084] In some embodiments, R 1 is 3-(methylamino)butenyl. [085] In some embodiments, R 1 is ethylaminopropenyl. [086] In some embodiments, R 1 is 2-(3-methoxypyrrolidiny-5-yl)ethenyl. [087] In some embodiments, R 1 is 2-(3-fluoropyrrolidiny-5-yl)ethenyl. [088] In some embodiments, R 1 is 2-(pyrrolidiny-2-yl)ethenyl.
- R 1 is aminopropenyl.
- R 9 is fluoro;
- R 10 is H; and
- R 11 is H.
- Non-limiting examples of compounds of the current disclosure include the following compounds identified in Table A: TABLE A F F F F N N N S N O NH
- alkyl by itself or as part of another substituent, means, unless otherwise stated, a straight (i.e., unbranched) or branched carbon chain (or carbon), or combination thereof, which may be fully saturated, mono- or polyunsaturated and can include mono-, di- and multivalent radicals, having the number of carbon atoms designated (i.e., C1-C10 means one to ten carbons).
- Alkyl is an uncyclized chain.
- Preferred alkyl substituents are C1-C6 alkyl.
- saturated hydrocarbon radicals include, but are not limited to, groups such as methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, homologs and isomers of, for example, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like.
- alkylene by itself or as part of another substituent, means, unless otherwise stated, a divalent radical derived from an alkyl, as exemplified, but not limited by, -CH2CH2CH2CH2-.
- An unsaturated alkyl group is one having one or more double bonds or triple bonds referred to as “alkenyl” or “alkynyl” groups, respectively.
- Preferred alkenyl substituents are C2-C6 alkenyl and preferred alkynyl substituents are C2-C6 alkynyl.
- alkenyl or alkynyl groups include, but are not limited to, ethenyl, vinyl, 2-propenyl, butenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3-(1,4- pentadienyl), ethynyl, 1-propynyl, 3-propynyl, and 3-butynyl.
- heteroalkyl by itself or in combination with another term, means, unless otherwise stated, a stable straight or branched chain, or combinations thereof, including at least one carbon atom and at least one heteroatom (e.g., O, N, P, S, B, As, or Si), and wherein the nitrogen and sulfur atoms may optionally be oxidized, and the nitrogen heteroatom may optionally be quaternized.
- the heteroatom(s) e.g., O, N, P, S, B, As, or Si
- Heteroalkyl is an uncyclized chain.
- Heteroalkyl also includes terms such as alkoxy, and alkylamino.
- An alkoxy is an alkyl attached to the remainder of the molecule via an oxygen linker (-O-).
- Preferred alkoxy substituents include C1-4 alkoxy. Examples of alkoxy groups include, but are not limited to methoxy, ethoxy and propoxy.
- Preferred alkylamino substituents include mono substituted C1-4 alkylamino and disubstituted alkylamino. Examples of alkylamino groups include, but are not limited to methylamino, dimethylamino and diethylamino.
- heteroalkyl includes “alkoxyalkyl” where an alkyl group is substituted with an alkoxy group, as defined above.
- Preferred alkoxyalkyl substituents include C 1-4 alkoxy- C 1-4 alkyl.
- alkoxyalkyl groups include, but are not limited to methoxymethyl, ethoxymethyl and methoxyethyl.
- heteroalkyl groups include those groups that are attached to the remainder of the molecule through a heteroatom, such as -C(O)R', -C(O)NR', -NR'R'', -OR', -SR', and/or -SO 2 R'.
- cycloalkyl by itself or in combination with other terms, mean, unless otherwise stated, cyclic versions of “alkyl”. Cycloalkyl are not fully aromatic rings. Preferred cycloalkyl substituents include C 3 -C 6 cycloalkyl. A “cycloalkylene” alone or as part of another substituent, means a divalent radical derived from a cycloalkyl.
- a cycloalkyl group having 3 to 8 ring members may be referred to as a (C 3 -C 8 )cycloalkyl
- a cycloalkyl group having 3 to 7 ring members may be referred to as a (C 3 -C 7 )cycloalkyl
- a cycloalkyl group having 4 to 7 ring members may be referred to as a (C 4 - C 7 )cycloalkyl.
- the cycloalkyl group can be a (C 3 -C 10 )cycloalkyl, a (C 3 - C 8 )cycloalkyl, a (C 3 -C 7 )cycloalkyl, a (C 3 -C 6 )cycloalkyl, or a (C 4 -C 7 )cycloalkyl group and these may be referred to as C 3 -C 10 cycloalkyl, C 3 -C 8 cycloalkyl, C 3 -C 7 cycloalkyl, C 3 -C 6 cycloalkyl, or C 4 -C 7 cycloalkyl groups.
- cycloalkenyl by itself or in combination with other terms, mean, unless otherwise stated, cyclic versions of “alkenyl”. Cycloalkenyl are not fully aromatic rings. Preferred cycloalkenyl substituents include C4-C6 cycloalkenyl.
- a cycloalkenyl group having 4 to 8 ring members may be referred to as a (C4-C8)cycloalkenyl
- a cycloalkenyl group having 3 to 7 ring members may be referred to as a (C3-C7)cycloalkenyl
- a cycloalkenyl group having 4 to 6 ring members may be referred to as a (C4-C6)cycloalkenyl.
- Heterocycloalkyl is also referred by the term heterocyclyl.
- Preferred heterocyclyl substituents include C3-C7 oxygen or nitrogen containing rings, or both nitrogen and oxygen atms. Additionally, for heterocycloalkyl, a heteroatom can occupy the position at which the heterocycle is attached to the remainder of the molecule.
- a “heterocycloalkylene,” alone or as part of another substituent, means a divalent radical derived from heterocycloalkyl.
- “Heterocyclyl” refers to a cyclic group that includes at least one saturated, partially unsaturated, but non-aromatic, cyclic ring. Heterocyclyl groups include at least one heteroatom as a ring member.
- Typical heteroatoms include, O, S and N and are independently chosen.
- Heterocyclyl groups include monocyclic ring systems and bicyclic ring systems.
- Bicyclic heterocyclyl groups include at least one non-aromatic ring with at least one heteroatom ring member that may be fused to a cycloalkyl ring or may be fused to an aromatic ring where the aromatic ring may be carbocyclic or may include one or more heteroatoms.
- the point of attachment of a bicyclic heterocyclyl group may be at the non-aromatic cyclic ring that includes at least one heteroatom or at another ring of the heterocyclyl group.
- a heterocyclyl group derived by removal of a hydrogen atom from one of the 9 membered heterocyclic compounds shown below may be attached to the rest of the molecule at the 5-membered ring or at the 6- membered ring.
- a heterocyclyl group includes 5 to 10 ring members of which 1, 2, 3 or 4 or 1, 2, or 3 are heteroatoms independently selected from O, S, or N.
- a heterocyclyl group includes 3 to 7 ring members of which 1, 2, or 3 heteroatom are independently selected from O, S, or N.
- a heterocyclyl group includes 3 or 4 ring members of which 1 is a heteroatom selected from O, S, or N. In other embodiments, a heterocyclyl group includes 5 to 7 ring members of which 1, 2, or 3 are heteroatoms independently selected from O, S, or N.
- Typical heterocyclyl groups include, but are not limited to, groups derived from epoxides, aziridine, azetidine, imidazolidine, morpholine, piperazine, piperidine, hexahydropyrimidine, 1,4,5,6-tetrahydropyrimidine, pyrazolidine, pyrrolidine, quinuclidine, tetrahydrofuran, tetrahydropyran, benzimidazolone, pyridinone, and the like.
- Heterocyclyl groups may be fully saturated, but may also include one or more double bonds.
- heterocyclyl groups include, but are not limited to, 1,2,3,6-tetrahydropyridinyl, 3,6-dihydro-2H- pyranyl, 3,4-dihydro-2H-pyranyl, 2,5-dihydro-1H-pyrolyl, 2,3-dihydro-1H-pyrolyl, 1H-azirinyl, 1,2- dihydroazetenyl, and the like.
- halo or “halogen,” by themselves or as part of another substituent, mean, unless otherwise stated, a fluorine, chlorine, bromine, or iodine atom.
- haloalkyl is meant to include monohaloalkyl and polyhaloalkyl.
- C1-C3 -haloalkyl includes, but is not limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2,2,2- trifluoroethyl, 3-bromopropyl, and the like.
- acyl means, unless otherwise stated, -C(O)R where R is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl.
- aryl means, unless otherwise stated, a polyunsaturated, aromatic, hydrocarbon substituent, which can be a single ring or multiple rings (preferably from 1 to 3 rings) that are fused together (i.e., a fused ring aryl) or linked covalently.
- a fused ring aryl refers to multiple rings fused together wherein at least one of the fused rings is an aryl ring.
- heteroaryl refers to aryl groups (or rings) that contain at least one heteroatom such as N, O, or S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized.
- heteroaryl includes fused ring heteroaryl groups (i.e., multiple rings fused together wherein at least one of the fused rings is a heteroaromatic ring).
- a heteroaryl group can be attached to the remainder of the molecule through a carbon or heteroatom.
- Heteroaryl refers to a monovalent heteroaromatic group derived by the removal of one hydrogen atom from a single atom of a parent heteroaromatic ring system.
- Heteroaryl groups typically include 5- to 14-membered, but more typically include 5- to 10-membered aromatic, monocyclic, bicyclic, and tricyclic rings containing one or more, for example, 1, 2, 3, or 4, or in certain embodiments, 1, 2, or 3, heteroatoms chosen from O, S, or N, with the remaining ring atoms being carbon.
- monocyclic heteroaryl groups the single ring is aromatic and includes at least one heteroatom.
- a monocyclic heteroaryl group may include 5 or 6 ring members and may include 1, 2, 3, or 4 heteroatoms, 1, 2, or 3 heteroatoms, 1 or 2 heteroatoms, or 1 heteroatom where the heteroatom(s) are independently selected from O, S, or N.
- both rings are aromatic.
- bicyclic heteroaryl groups at least one of the rings must include a heteroatom, but it is not necessary that both rings include a heteroatom although it is permitted for them to do so.
- heteroaryl includes a 5- to 7-membered heteroaromatic ring fused to a carbocyclic aromatic ring or fused to another heteroaromatic ring.
- tricyclic aromatic rings all three of the rings are aromatic and at least one of the rings includes at least one heteroatom.
- the point of attachment may be at the ring including at least one heteroatom or at a carbocyclic ring.
- the total number of S and O atoms in the heteroaryl group exceeds 1, those heteroatoms are not adjacent to one another.
- the total number of S and O atoms in the heteroaryl group is not more than 2.
- the total number of S and O atoms in the aromatic heterocycle is not more than 1.
- Heteroaryl does not encompass or overlap with aryl as defined above.
- heteroaryl groups include, but are not limited to, groups derived from acridine, carbazole, cinnoline, furan, imidazole, indazole, indole, indolizine, isobenzofuran, isochromene, isoindole, isoquinoline, isothiazole, 2H-benzo[d][1,2,3]triazole, isoxazole, naphthyridine, oxadiazole, oxazole, perimidine, phenanthridine, phenanthroline, phenazine, phthalazine, pteridine, purine, pyrazine, pyrazole, pyridazine, pyridine, pyrimidine, pyrrole, pyrrolizine, quinazoline, quinoline, quinolizine, quinoxaline, tetrazole, thiadiazole, thiazole, thiophene, triazo
- the heteroaryl group can be between 5 to 20 membered heteroaryl, such as, for example, a 5 to 14 membered or 5 to 10 membered heteroaryl.
- heteroaryl groups can be those derived from thiophene, pyrrole, benzothiophene, 2H-benzo[d][1,2,3]triazole benzofuran, indole, pyridine, quinoline, imidazole, benzimidazole, oxazole, tetrazole, and pyrazine.
- cyano refers to the radical -CN.
- amino referes to the radical -NH2.
- aminocarbonyl refers to the radical -CO-NH2.
- Aminocarbonyl radicals may be substituted with one or two alkyl groups to form “alkylaminocarbonyl” groups.
- alkylcarbonyl refers to the radical alkyl-CO-.
- hydroxyl and “hydroxy” refers to the radical -OH.
- oxo means an oxygen that is double bonded to a carbon atom.
- R, R', R'', R'', and R''' each preferably independently refer to hydrogen, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl (e.g., aryl substituted with 1-3 halogens), substituted or unsubstituted heteroaryl, substituted or unsubstituted alkyl, alkoxy, or thioalkoxy groups, or arylalkyl groups.
- aryl e.g., aryl substituted with 1-3 halogens
- substituted or unsubstituted heteroaryl substituted or unsubstituted alkyl, alkoxy, or thioalkoxy groups, or arylalkyl groups.
- each of the R groups is independently selected as are each R', R'', R''', and R''' group when more than one of these groups is present.
- R' and R'' are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 4-, 5-, 6-, or 7-membered ring.
- -NR'R'' includes, but is not limited to, 1-pyrrolidinyl and 4- morpholinyl.
- alkyl is meant to include groups including carbon atoms bound to groups other than hydrogen groups, such as haloalkyl (e.g., -CF3 and -CH2CF3) and acyl (e.g., -C(O)CH3, -C(O)CF3, -C(O)CH2OCH3, and the like).
- haloalkyl e.g., -CF3 and -CH2CF3
- acyl e.g., -C(O)CH3, -C(O)CF3, -C(O)CH2OCH3, and the like.
- Such substituted alkyl groups include hydroxyalkyl; carboxyalkyl, alkoxycarbonylalkyl, cyanoalkyl, aminocarbonylalkyl, alkylaminocarbonylalkyl, alkylaminoalkyl, alkylcarbonylaminoalkyl, cycloalkylalkyl, aralkyl, heteroarylalkyl and heterocyclylalkyl; wherein the heterocyclyl, heteroaryl, aryl, cycloalkyl, hydroxyl; carboxyl, alkoxy, cyano, aminocarbonyl, alkylaminocarbonyl, alkylamino, and alkylcarbonylamino groups are defined above.
- alkenyl and alkynyl groups can be specifically substituted to form halo-substituted C2- C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl, alkylamino substituted C2-C6 alkenyl, amino substituted C2-C6 alkenyl, hydroxy substituted amino-C2-C6 alkenyl, phenyl substituted amino-C2-C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C2-C6 alkynyl, alkoxy substituted C2-C6 alkynyl, heterocyclyl- substituted C2-C6 alkynyl, cycloalkyl- substituted C2-C6 alkenyl, oxygen-containing heterocyclyl- substituted C2-C6 alkenyl, oxygen-containing heterocyclyl
- alkylsufonyl refers to the radical alkyl-SO 2 -; where alkyl is defined elseqhere.
- Substituted amino groups include “alkylcarbonylamino,” “alkylsulfonylamino,” “alkylaminocarbonylamino” and “alkylsulfonylamino”; wherein the amino radical is substituted, preferably with one substituent selected from alkylcarbonyl, alkylsulfonyl, alkylaminocarbonyl, defined elsewhere.
- each of the R groups is independently selected as are each R', R'', R'', and R''' groups when more than one of these groups is present.
- Substituents for rings e.g. cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene
- substituents on the ring may be depicted as substituents on the ring rather than on a specific atom of a ring (commonly referred to as a floating substituent).
- the substituent may be attached to any of the ring atoms (obeying the rules of chemical valency) and in the case of fused rings or spirocyclic rings, a substituent depicted as associated with one member of the fused rings or spirocyclic rings (a floating substituent on a single ring), may be a substituent on any of the fused rings or spirocyclic rings (a floating substituent on multiple rings).
- the multiple substituents may be on the same atom, same ring, different atoms, different fused rings, different spirocyclic rings, and each substituent may optionally be different.
- a point of attachment of a ring to the remainder of a molecule is not limited to a single atom (a floating substituent)
- the attachment point may be any atom of the ring and in the case of a fused ring or spirocyclic ring, any atom of any of the fused rings or spirocyclic rings while obeying the rules of chemical valency.
- a ring, fused rings, or spirocyclic rings contain one or more ring heteroatoms and the ring, fused rings, or spirocyclic rings are shown with one more floating substituents (including, but not limited to, points of attachment to the remainder of the molecule), the floating substituents may be bonded to the heteroatoms.
- the ring heteroatoms are shown bound to one or more hydrogens (e.g. a ring nitrogen with two bonds to ring atoms and a third bond to a hydrogen) in the structure or formula with the floating substituent, when the heteroatom is bonded to the floating substituent, the substituent will be understood to replace the hydrogen, while obeying the rules of chemical valency.
- Two or more substituents may optionally be joined to form aryl, heteroaryl, cycloalkyl, or heterocycloalkyl groups.
- Such so-called ring-forming substituents are typically, though not necessarily, found attached to a cyclic base structure.
- the ring-forming substituents are attached to adjacent members of the base structure.
- two ring-forming substituents attached to adjacent members of a cyclic base structure create a fused ring structure.
- the ring-forming substituents are attached to a single member of the base structure.
- two ring-forming substituents attached to a single member of a cyclic base structure create a spirocyclic structure.
- the ring-forming substituents are attached to non-adjacent members of the base structure.
- heteroatom or “ring heteroatom” are meant to include oxygen (O), nitrogen (N), sulfur (S), phosphorus (P), Boron (B), and silicon (Si).
- a “substituent group,” as used herein, means a group selected from the following moieties: (A) halogen, oxo, cyano, -CCl 3 , -CBr 3 , -CF 3 , -CI 3 , -CHCl 2 , -CHBr 2 , -CHF 2 , -CHI 2 , -CH 2 Cl, -CH 2 Br, -CH 2 F, -CH 2 I, -OH, -NH 2 , -COOH, -CONH 2 , -NO 2 , -SH, -SO 3 H, -SO 4 H, -SO 2 NH 2 , -NHNH 2 , -ONH 2 , -NHOH, -NHC(O)NHNH 2 , -NHC(O)NH 2 , -NHSO 2 H, -NHC(O)H, -NHC(O)OH, -OCCl 3 ,
- a “size-limited substituent” or “ size-limited substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C20 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl, and each substituted or unsubstituted heteroaryl is a group selected
- a “lower substituent” or “ lower substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C 1 -C 8 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C 3 -C 7 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C 6 -C 10 aryl, and each substituted or unsubstituted heteroaryl is
- each substituted group described in the compounds herein is substituted with at least one substituent group. More specifically, in some embodiments, each substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene described in the compounds herein are substituted with at least one substituent group. In other embodiments, at least one or all of these groups are substituted with at least one size-limited substituent group.
- each substituted or unsubstituted alkyl may be a substituted or unsubstituted C1-C20 alkyl
- each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl
- each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8 cycloalkyl
- each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl
- each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl
- each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5
- each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C20 alkylene
- each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 20 membered heteroalkylene
- each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C3-C8 cycloalkylene
- each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 8 membered heterocycloalkylene
- each substituted or unsubstituted arylene is a substituted or unsubstituted C6-C10 arylene
- each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 10 membered heteroarylene.
- each substituted or unsubstituted alkyl is a substituted or unsubstituted C1- C8 alkyl
- each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl
- each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C7 cycloalkyl
- each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl
- each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl
- each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 9 membered heteroaryl.
- each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C8 alkylene
- each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 8 membered heteroalkylene
- each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C 3 -C 7 cycloalkylene
- each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 7 membered heterocycloalkylene
- each substituted or unsubstituted arylene is a substituted or unsubstituted C 6 -C 10 arylene
- each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 9 membered heteroarylene.
- the compound is a chemical species set forth in the Examples section, figures, or tables below.
- a substituted or unsubstituted moiety e.g., substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, and/or substituted or unsubstituted heteroarylene) is unsubstituted (e.g., is an unsubstituted alkyl, unsubstituted cycloalkyl, substituted
- a substituted or unsubstituted moiety e.g., substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, and/or substituted or unsubstituted heteroarylene) is substituted (e.g., is a substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alky
- a substituted moiety e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene
- is substituted with at least one substituent group wherein if the substituted moiety is substituted with a plurality of substituent groups, each substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of substituent groups, each substituent group is different.
- a substituted moiety e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene
- is substituted with at least one size-limited substituent group wherein if the substituted moiety is substituted with a plurality of size-limited substituent groups, each size-limited substituent group may optionally be different.
- each size-limited substituent group is different.
- a substituted moiety e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene
- each lower substituent group is different.
- a substituted moiety e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene
- each substituent group, size-limited substituent group, and/or lower substituent group is different.
- Certain compounds of the present invention possess asymmetric carbon atoms (optical or chiral centers) or double bonds; the enantiomers, racemates, diastereomers, tautomers, geometric isomers, stereoisometric forms that may be defined, in terms of absolute stereochemistry, as (R)-or (S)- or, as (D)- or (L)- for amino acids, and individual isomers are encompassed within the scope of the present invention.
- the compounds of the present invention do not include those that are known in art to be too unstable to synthesize and/or isolate.
- the present invention is meant to include compounds in racemic and optically pure forms.
- Optically active (R)- and (S)-, or (D)- and (L)-isomers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques.
- R Optically active
- S S
- D dibenzyl- and (L)-isomers
- the term “isomers” refers to compounds having the same number and kind of atoms, and hence the same molecular weight, but differing in respect to the structural arrangement or configuration of the atoms.
- the term “tautomer,” as used herein, refers to one of two or more structural isomers which exist in equilibrium and which are readily converted from one isomeric form to another. [0140] It will be apparent to one skilled in the art that certain compounds of this invention may exist in tautomeric forms, all such tautomeric forms of the compounds being within the scope of the invention.
- stereoisomer or “stereomerically pure” means one stereoisomer of a compound that is substantially free of other stereoisomers of that compound.
- a stereomerically pure compound having one chiral center will be substantially free of the mirror image enantiomer of the compound.
- a stereomerically pure compound having two chiral centers will be substantially free of other diastereomers of the compound.
- a typical stereomerically pure compound comprises greater than about 80% by weight of one stereoisomer of the compound and less than about 20% by weight of other stereoisomers of the compound, more preferably greater than about 90% by weight of one stereoisomer of the compound and less than about 10% by weight of the other stereoisomers of the compound, even more preferably greater than about 95% by weight of one stereoisomer of the compound and less than about 5% by weight of the other stereoisomers of the compound, and most preferably greater than about 97% by weight of one stereoisomer of the compound and less than about 3% by weight of the other stereoisomers of the compound.
- stereochemistry of a structure or a portion of a structure is not indicated with, for example, bold or dashed lines, the structure or portion of the structure is to be interpreted as encompassing all stereoisomers of it.
- a bond drawn with a wavy line indicates that both stereoisomers are encompassed. This is not to be confused with a wavy line drawn perpendicular to a bond which indicates the point of attachment of a group to the rest of the molecule.
- this invention encompasses the use of stereomerically pure forms of such compounds, as well as the use of mixtures of those forms. For example, mixtures comprising equal or unequal amounts of the enantiomers of a particular compound of the invention may be used in methods and compositions of the invention.
- isomers may be asymmetrically synthesized or resolved using standard techniques such as chiral columns or chiral resolving agents. See, e.g., Jacques, J., et al., Enantiomers, Racemates and Resolutions (Wiley- Interscience, New York, 1981); Wilen, S. H., et al. (1997) Tetrahedron 33:2725; Eliel, E. L., Stereochemistry of Carbon Compounds (McGraw-Hill, NY, 1962); and Wilen, S. H., Tables of Resolving Agents and Optical Resolutions p.268 (E.L. Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, IN, 1972).
- structures depicted herein are also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms.
- compounds having the present structures except for the replacement of a hydrogen by a deuterium [D] or tritium, or the replacement of a carbon by 13 C- or 14 C-enriched carbon are within the scope of this is selected from invention.
- D deuterium
- carbon replacement of a carbon by 13 C- or 14 C-enriched carbon
- a or “an,” as used in herein means one or more.
- substituted with a[n] means the specified group may be substituted with one or more of any or all of the named substituents.
- a group such as an alkyl or heteroaryl group
- the group may contain one or more unsubstituted C 1 -C 20 alkyls, and/or one or more unsubstituted 2 to 20 membered heteroalkyls.
- R-substituted where a moiety is substituted with an R substituent, the group may be referred to as “R-substituted.” Where a moiety is R-substituted, the moiety is substituted with at least one R substituent and each R substituent is optionally different. Where a particular R group is present in the description of a chemical genus (such as Formula (XV)), a Roman alphabetic symbol may be used to distinguish each appearance of that particular R group. [0146] Any compound herein can be purified.
- a compound herein can be least 1% pure, at least 2% pure, at least 3% pure, at least 4% pure, at least 5% pure, at least 6% pure, at least 7% pure, at least 8% pure, at least 9% pure, at least 10% pure, at least 11% pure, at least 12% pure, at least 13% pure, at least 14% pure, at least 15% pure, at least 16% pure, at least 17% pure, at least 18% pure, at least 19% pure, at least 20% pure, at least 21% pure, at least 22% pure, at least 23% pure, at least 24% pure, at least 25% pure, at least 26% pure, at least 27% pure, at least 28% pure, at least 29% pure, at least 30% pure, at least 31% pure, at least 32% pure, at least 33% pure, at least 34% pure, at least 35% pure, at least 36% pure, at least 37% pure, at least 38% pure, at least 39% pure, at least 40% pure, at least 41% pure, at least 42% pure, at least 4
- compounds as disclosed herein can be used to treat cancer in a subject.
- a compound as disclosed herein can, for example, slow the proliferation of cancer cell lines, or kill cancer cells.
- cancer that can be treated by a compound as disclosed herein include: acute lymphoblastic leukemia, acute myeloid leukemia, adrenocortical carcinoma, AIDS-related cancers, AIDS-related lymphoma, anal cancer, appendix cancer, astrocytomas, basal cell carcinoma, bile duct cancer, bladder cancer, bone cancers, brain tumors, such as cerebellar astrocytoma, cerebral astrocytoma/malignant glioma, ependymoma, medulloblastoma, supratentorial primitive neuroectodermal tumors, visual pathway and hypothalamic glioma, breast cancer, bronchial adenomas, Burkitt lymphoma, carcinoma of unknown primary origin, central nervous system lympho
- the compounds of the disclosure show non-lethal toxicity.
- Pharmaceutically-acceptable salts include, for example, acid-addition salts and base- addition salts.
- the acid that is added to the compound to form an acid-addition salt can be an organic acid or an inorganic acid.
- a base that is added to the compound to form a base-addition salt can be an organic base or an inorganic base.
- a pharmaceutically-acceptable salt is a metal salt.
- a pharmaceutically-acceptable salt is an ammonium salt.
- Metal salts can arise from the addition of an inorganic base to a compound of the invention.
- the inorganic base consists of a metal cation paired with a basic counterion, such as, for example, hydroxide, carbonate, bicarbonate, or phosphate.
- the metal can be an alkali metal, alkaline earth metal, transition metal, or main group metal.
- the metal is lithium, sodium, potassium, cesium, cerium, magnesium, manganese, iron, calcium, strontium, cobalt, titanium, aluminum, copper, cadmium, or zinc.
- a metal salt is a lithium salt, a sodium salt, a potassium salt, a cesium salt, a cerium salt, a magnesium salt, a manganese salt, an iron salt, a calcium salt, a strontium salt, a cobalt salt, a titanium salt, an aluminum salt, a copper salt, a cadmium salt, or a zinc salt.
- Ammonium salts can arise from the addition of ammonia or an organic amine to a compound of the invention.
- the organic amine is triethyl amine, diisopropyl amine, ethanol amine, diethanol amine, triethanol amine, morpholine, N-methylmorpholine, piperidine, N- methylpiperidine, N-ethylpiperidine, dibenzylamine, piperazine, pyridine, pyrrazole, pipyrrazole, imidazole, pyrazine, or pipyrazine.
- an ammonium salt is a triethyl amine salt, a diisopropyl amine salt, an ethanol amine salt, a diethanol amine salt, a triethanol amine salt, a morpholine salt, an N- methylmorpholine salt, a piperidine salt, an N-methylpiperidine salt, an N-ethylpiperidine salt, a dibenzylamine salt, a piperazine salt, a pyridine salt, a pyrrazole salt, a pipyrrazole salt, an imidazole salt, a pyrazine salt, or a pipyrazine salt.
- Acid addition salts can arise from the addition of an acid to a compound of the invention.
- the acid is organic.
- the acid is inorganic.
- the acid is hydrochloric acid, hydrobromic acid, hydroiodic acid, nitric acid, nitrous acid, sulfuric acid, sulfurous acid, a phosphoric acid, isonicotinic acid, lactic acid, salicylic acid, tartaric acid, ascorbic acid, gentisinic acid, gluconic acid, glucaronic acid, saccaric acid, formic acid, benzoic acid, glutamic acid, pantothenic acid, acetic acid, propionic acid, butyric acid, fumaric acid, succinic acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, citric acid, oxalic acid, or maleic acid.
- the salt is a hydrochloride salt, a hydrobromide salt, a hydroiodide salt, a nitrate salt, a nitrite salt, a sulfate salt, a sulfite salt, a phosphate salt, isonicotinate salt, a lactate salt, a salicylate salt, a tartrate salt, an ascorbate salt, a gentisinate salt, a gluconate salt, a glucaronate salt, a saccarate salt, a formate salt, a benzoate salt, a glutamate salt, a pantothenate salt, an acetate salt, a propionate salt, a butyrate salt, a fumarate salt, a succinate salt, a methanesulfonate (mesylate) salt, an ethanesulfonate salt, a benzenesulfonate salt, a p-tolu
- compositions of the disclosure can be used, for example, before, during, or after treatment of a subject with, for example, another pharmaceutical agent.
- Subjects can be, for example, elderly adults, adults, adolescents, pre-adolescents, children, toddlers, infants, neonates, and non-human animals.
- a subject is a patient.
- a pharmaceutical composition of the disclosure can be a combination of any pharmaceutical compounds described herein with other chemical components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, and/or excipients. The pharmaceutical composition facilitates administration of the compound to an organism.
- compositions can be administered in therapeutically-effective amounts as pharmaceutical compositions by various forms and routes including, for example, intravenous, subcutaneous, intramuscular, oral, parenteral, ophthalmic, subcutaneous, transdermal, nasal, vaginal, and topical administration.
- a pharmaceutical composition can be administered in a local manner, for example, via injection of the compound directly into an organ, optionally in a depot or sustained release formulation or implant.
- Pharmaceutical compositions can be provided in the form of a rapid release formulation, in the form of an extended release formulation, or in the form of an intermediate release formulation.
- a rapid release form can provide an immediate release.
- An extended release formulation can provide a controlled release or a sustained delayed release.
- compositions can be formulated by combining the active compounds with pharmaceutically-acceptable carriers or excipients.
- Such carriers can be used to formulate liquids, gels, syrups, elixirs, slurries, or suspensions, for oral ingestion by a subject.
- Non- limiting examples of solvents used in an oral dissolvable formulation can include water, ethanol, isopropanol, saline, physiological saline, DMSO, dimethylformamide, potassium phosphate buffer, phosphate buffer saline (PBS), sodium phosphate buffer, 4-2-hydroxyethyl-1-piperazineethanesulfonic acid buffer (HEPES), 3-(N-morpholino)propanesulfonic acid buffer (MOPS), piperazine-N,N′-bis(2- ethanesulfonic acid) buffer (PIPES), and saline sodium citrate buffer (SSC).
- PBS phosphate buffer saline
- MOPS 4-2-hydroxyethyl-1-piperazineethanesulfonic acid buffer
- MOPS 3-(N-morpholino)propanesulfonic acid buffer
- PES piperazine-N,N′-bis(2- ethanesulfonic acid) buffer
- SSC saline sodium
- Non-limiting examples of co- solvents used in an oral dissolvable formulation can include sucrose, urea, cremaphor, DMSO, and potassium phosphate buffer.
- Pharmaceutical compositions can be formulated for intravenous administration.
- the pharmaceutical compositions can be in a form suitable for parenteral injection as a sterile suspension, solution or emulsion in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- Pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water-soluble form. Suspensions of the active compounds can be prepared as oily injection suspensions.
- Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes.
- the suspension can also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions.
- the active ingredient can be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- the active compounds can be administered topically and can be formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, medicated sticks, balms, creams, and ointments.
- Such pharmaceutical compositions can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives.
- the compounds of the disclosure can be applied topically to the skin, or a body cavity, for example, oral, vaginal, bladder, cranial, spinal, thoracic, or pelvic cavity of a subject.
- the compounds of the disclosure can be applied to an accessible body cavity.
- the compounds can also be formulated in rectal compositions such as enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly suppositories, or retention enemas, containing conventional suppository bases such as cocoa butter or other glycerides, as well as synthetic polymers such as polyvinylpyrrolidone, and PEG.
- rectal compositions such as enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly suppositories, or retention enemas
- conventional suppository bases such as cocoa butter or other glycerides
- synthetic polymers such as polyvinylpyrrolidone, and PEG.
- a low-melting wax such as a mixture of fatty acid glycerides, optionally in combination with cocoa butter, can be melted.
- therapeutically-effective amounts of the compounds described herein are administered in pharmaceutical compositions to a subject having a disease or condition to be treated.
- the subject is a mammal such as a human.
- a therapeutically-effective amount can vary widely depending on the severity of the disease, the age and relative health of the subject, the potency of the compounds used, and other factors.
- the compounds can be used singly or in combination with one or more therapeutic agents as components of mixtures.
- Pharmaceutical compositions can be formulated using one or more physiologically-acceptable carriers comprising excipients and auxiliaries, which facilitate processing of the active compounds into preparations that can be used pharmaceutically. Compositions can be modified depending upon the route of administration chosen.
- compositions comprising a compound described herein can be manufactured, for example, by mixing, dissolving, emulsifying, encapsulating, entrapping, or compression processes.
- the pharmaceutical compositions can include at least one pharmaceutically-acceptable carrier, diluent, or excipient and compounds described herein as free-base or pharmaceutically-acceptable salt form.
- Pharmaceutical compositions can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives.
- Methods for the preparation of compositions comprising the compounds described herein include formulating the compounds with one or more inert, pharmaceutically-acceptable excipients or carriers to form a solid, semi-solid, or liquid composition.
- Solid compositions include, for example, powders, tablets, dispersible granules, capsules, and cachets.
- Liquid compositions include, for example, solutions in which a compound is dissolved, emulsions comprising a compound, or a solution containing liposomes, micelles, or nanoparticles comprising a compound as disclosed herein.
- Semi-solid compositions include, for example, gels, suspensions and creams. The compositions can be in liquid solutions or suspensions, solid forms suitable for solution or suspension in a liquid prior to use, or as emulsions. These compositions can also contain minor amounts of nontoxic, auxiliary substances, such as wetting or emulsifying agents, pH buffering agents, and other pharmaceutically-acceptable additives.
- Non-limiting examples of dosage forms suitable for use in the disclosure include liquid, powder, gel, nanosuspension, nanoparticle, microgel, aqueous or oily suspensions, emulsion, and any combination thereof.
- Non-limiting examples of pharmaceutically-acceptable excipients suitable for use in the disclosure include binding agents, disintegrating agents, anti-adherents, anti-static agents, surfactants, anti-oxidants, coating agents, coloring agents, plasticizers, preservatives, suspending agents, emulsifying agents, anti-microbial agents, spheronization agents, and any combination thereof.
- a composition of the disclosure can be, for example, an immediate release form or a controlled release composition.
- An immediate release composition can be formulated to allow the compounds to act rapidly.
- immediate release compositions include readily dissolvable formulations.
- a controlled release compositions can be a pharmaceutical composition that has been adapted such that release rates and release profiles of the active agent can be matched to physiological and chronotherapeutic requirements or, alternatively, has been formulated to effect release of an active agent at a programmed rate.
- Non-limiting examples of controlled release compositions include granules, delayed release granules, hydrogels (e.g., of synthetic or natural origin), other gelling agents (e.g., gel- forming dietary fibers), matrix-based compositions (e.g., compositions comprising a polymeric material having at least one active ingredient dispersed through), granules within a matrix, polymeric mixtures, and granular masses.
- a controlled release composition is a delayed release form.
- a delayed release form can be formulated to delay a compound’s action for an extended period of time.
- a delayed release form can be formulated to delay the release of an effective dose of one or more compounds, for example, for about 4, about 8, about 12, about 16, or about 24 hours.
- a controlled release composition can be a sustained release form.
- a sustained release form can be formulated to sustain, for example, the compound’s action over an extended period of time.
- a sustained release form can be formulated to provide an effective dose of any compound described herein (e.g., provide a physiologically-effective blood profile) over about 4, about 8, about 12, about 16 or about 24 hours.
- Non-limiting examples of pharmaceutically-acceptable excipients can be found, for example, in Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover, John E., Remington’s Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania 1975; Liberman, H.A.
- Multiple therapeutic agents can be administered in any order or simultaneously.
- a compound of the disclosure is administered in combination with, before, or after treatment with another therapeutic agent.
- the multiple therapeutic agents can be provided in a single, unified form, or in multiple forms, for example, as multiple separate pills.
- the agents can be packed together or separately, in a single package or in a plurality of packages.
- One or all of the therapeutic agents can be given in multiple doses.
- compositions described herein can be administered before, during, or after the occurrence of a disease or condition, and the timing of administering the composition containing a therapeutic agent can vary.
- the compositions can be used as a prophylactic and can be administered continuously to subjects with a propensity to conditions or diseases in order to lessen a likelihood of the occurrence of the disease or condition.
- the compositions can be administered to a subject during or as soon as possible after the onset of the symptoms.
- the administration of the therapeutic agents can be initiated within the first 48 hours of the onset of the symptoms, within the first 24 hours of the onset of the symptoms, within the first 6 hours of the onset of the symptoms, or within 3 hours of the onset of the symptoms.
- the initial administration can be via any route practical, such as by any route described herein using any formulation described herein.
- a compound can be administered as soon as is practical after the onset of a disease or condition is detected or suspected, and for a length of time necessary for the treatment of the disease, such as, for example, from about 1 month to about 3 months.
- the length of time a compound can be administered can be about 1 day, about 2 days, about 3 days, about 4 days, about 5 days, about 6 days, about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 1 month, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 2 months, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 3 months, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 4 months, about 17 weeks, about 18 weeks, about 19 weeks, about 20 weeks, about 5 months, about 21 weeks, about 22 weeks, about 23 weeks, about 24 weeks, about 6 months, about 7 months, about 8 months, about 9 months, about 10 months, about 11 months, about 1 year, about 13 months, about 14 months, about 15 months, about 16 months, about 17 months, about 18 months, about 19 months, about 20 months, about 21 months, about 22 months about 23 months, about 2 years, about 2.5 years, about 3 years, about 3.5 years, about 4 years, about 4.5 years, about
- compositions described herein can be in unit dosage forms suitable for single administration of precise dosages.
- the formulation is divided into unit doses containing appropriate quantities of one or more compounds.
- the unit dosage can be in the form of a package containing discrete quantities of the formulation.
- Non-limiting examples are packaged injectables, vials, or ampoules.
- Aqueous suspension compositions can be packaged in single-dose non- reclosable containers. Multiple-dose reclosable containers can be used, for example, in combination with or without a preservative.
- Formulations for injection can be presented in unit dosage form, for example, in ampoules, or in multi-dose containers with a preservative.
- compositions provided herein can be administered in conjunction with other therapies, for example, chemotherapy, radiation, surgery, anti-inflammatory agents, and selected vitamins.
- the other agents can be administered prior to, after, or concomitantly with the pharmaceutical compositions.
- the pharmaceutical compositions can be in the form of solid, semi-solid or liquid dosage forms, such as, for example, tablets, suppositories, pills, capsules, powders, liquids, suspensions, lotions, creams, or gels, for example, in unit dosage form suitable for single administration of a precise dosage.
- nontoxic solid carriers include, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, talc, cellulose, glucose, sucrose, and magnesium carbonate.
- pharmaceutically active agents suitable for combination with compositions of the disclosure include anti-infectives, i.e., aminoglycosides, antiviral agents, antimicrobials, anticholinergics/antispasmotics, antidiabetic agents, antihypertensive agents, antineoplastics, cardiovascular agents, central nervous system agents, coagulation modifiers, hormones, immunologic agents, immunosuppressive agents, and ophthalmic preparations.
- Liposomes are composed of natural phospholipids, and can contain mixed lipid chains with surfactant properties (e.g., egg phosphatidylethanolamine).
- a liposome design can employ surface ligands for attaching to unhealthy tissue.
- Non-limiting examples of liposomes include the multilamellar vesicle (MLV), the small unilamellar vesicle (SUV), and the large unilamellar vesicle (LUV).
- Liposomal physicochemical properties can be modulated to optimize penetration through biological barriers and retention at the site of administration, and to reduce a likelihood of developing premature degradation and toxicity to non-target tissues.
- Optimal liposomal properties depend on the administration route: large-sized liposomes show good retention upon local injection, small-sized liposomes are better suited to achieve passive targeting.
- PEGylation reduces the uptake of the liposomes by the liver and spleen, and increases the circulation time, resulting in increased localization at the inflamed site due to the enhanced permeability and retention (EPR) effect.
- liposomal surfaces can be modified to achieve selective delivery of the encapsulated drug to specific target cells.
- Non-limiting examples of targeting ligands include monoclonal antibodies, vitamins, peptides, and polysaccharides specific for receptors concentrated on the surface of cells associated with the disease.
- Non-limiting examples of dosage forms suitable for use in the disclosure include liquid, elixir, nanosuspension, aqueous or oily suspensions, drops, syrups, and any combination thereof.
- compositions of the disclosure can be packaged as a kit.
- a kit includes written instructions on the administration/use of the composition.
- the written material can be, for example, a label.
- the written material can suggest conditions methods of administration.
- the instructions provide the subject and the supervising physician with the best guidance for achieving the optimal clinical outcome from the administration of the therapy.
- the written material can be a label.
- the label can be approved by a regulatory agency, for example the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or other regulatory agencies.
- Dosing can be in unit dosage forms suitable for single administration of precise dosages. In unit dosage form, the composition is divided into unit doses containing appropriate quantities of one or more compounds. The unit dosage can be in the form of a package containing discrete quantities of the composition. Non-limiting examples are liquids in vials or ampoules. Aqueous suspension compositions can be packaged in single-dose non-reclosable containers.
- compositions for parenteral injection can be presented in unit dosage form, for example, in ampoules, or in multi-dose containers with a preservative.
- a compound described herein can be present in a composition in a range of from about 1 mg to about 2000 mg; from about 10 mg to about 1000 mg; from about 100 mg to about 750 mg; from about 250 mg to about 500 mg, from about 100 mg to about 500 mg, from about 200 mg to about 500 mg, from about 250 mg to about450 mg, from about 100 mg to about 200 mg, from about 1 mg to about 50 mg, from about 50 mg to about 100 mg, from about 100 mg to about 150 mg, from about 150 mg to about 200 mg, from about 200 mg to about 250 mg, from about 250 mg to about 300 mg, from about 300 mg to about 350 mg, from about 350 mg to about 400 mg, from about 400 mg to about 450 mg, from about 450 mg to about 500 mg, from about 500 mg to about 550 mg
- a compound described herein can be present in a composition in an amount of about 1 mg, about 2 mg, about 3 mg, about 4 mg, about 5 mg, about 10 mg, about 15 mg, about 20 mg, about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 125 mg, about 150 mg, about 175 mg, about 200 mg, about 250 mg, about 300 mg, about 350 mg, about 400 mg, about 450 mg, about 500 mg, about 550 mg, about 600 mg, about 650 mg, about 700 mg, about 750 mg, about 800 mg, about 850 mg, about 900 mg, about 950 mg, or about 1000 mg.
- a dose can be expressed in terms of an amount of the drug divided by the mass of the subject, for example, milligrams of drug per kilograms of subject body mass.
- a compound is administered in an amount ranging from about 1 mg/kg to about 20 mg/kg, or about 5 mg/kg to about 10 mg/kg.
- a compound is administered in an amount of about 1 mg/kg, 2 mg/kg, 3 mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg, about 7 mg/kg, 8 mg/kg, about 9 mg/kg, or about 10 mg/kg.
- Combination Treatment with an anti-cancer agent can be used to treat a condition.
- the combination therapy can produce a significantly better therapeutic result than the additive effects achieved by each individual constituent when administered alone at a therapeutic dose.
- the dosage of the compound or anti-cancer agent described herein, in combination therapy can be reduced as compared to monotherapy with each agent, while still achieving an overall therapeutic effect.
- a compound and an anti-cancer agent described herein can exhibit a synergistic effect.
- the synergistic effect of a compound and anti-cancer agent described herein can be used to reduce the total amount drugs administered to a subject, which decrease side effects experienced by the subject.
- the compounds of the disclosure can be used in combination with at least one anti-cancer agent described herein.
- the at least one anti-cancer agent described herein can modulate the same or a different target as the compounds of the disclosure.
- the at least one anti-cancer agent described herein can modulate the same target as the compounds of the disclosure, or other components of the same pathway, or overlapping sets of target enzymes.
- the at least one anti-cancer agent described herein can modulate a different target from the compounds of the disclosure.
- the present disclosure provides a method for treating cancer, the method comprising administering to a subject in need thereof (a) an effective amount of a compound of the disclosure and (b) an effective amount of at least one anti-cancer agent described herein, to provide a combination therapy.
- the combination therapy may have an enhanced therapeutic effect compared to the effect of the compound and the at least one anti-cancer agent each administered alone.
- the combination therapy has a synergistic therapeutic effect.
- the combination therapy produces a significantly better therapeutic result (e.g., anti-cancer, cell growth arrest, apoptosis, induction of differentiation, cell death, etc.) than the additive effects achieved by each individual constituent when administered alone at a therapeutic dose.
- Combination therapy includes but is not limited to the combination of compounds of the disclosure with anti-cancer agents such as chemotherapeutic agents, therapeutic antibodies, or radiation treatment, to provide a synergistic therapeutic effect.
- the compounds of the disclosure are used in combination with one or more anti-cancer (antineoplastic or cytotoxic) chemotherapy drug.
- Suitable chemotherapeutic agents for use in the combinations of the present disclosure include, but are not limited to, alkylating agents, antibiotic agents, antimetabolic agents, hormonal agents, plant-derived agents, anti-angiogenic agents, differentiation inducing agents, cell growth arrest inducing agents, apoptosis inducing agents, cytotoxic agents, agents affecting cell bioenergetics, biologic agents, e.g., monoclonal antibodies, kinase inhibitors and inhibitors of growth factors and their receptors, gene therapy agents, cell therapy, or any combination thereof.
- a compound of the disclosure is used in combination with an estrogen receptor antagonist.
- a compound of the disclosure is used in combination with toremifene (Fareston®), fulvestrant (Faslodex®), or tamoxifen citrate (Soltamox®).
- Fulvestrant is a selective estrogen receptor degrader (SERD) and is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy.
- Fulvestrant is a complete estrogen receptor antagonist with little to no agonist effects and accelerates the proteasomal degradation of the estrogen receptor. Fulvestrant has poor oral bioavailability and is administered via intramuscular injection.
- Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes estrogen receptor-positive breast cancer cells to heregulin beta1.
- a compound of the disclosure is used in combination with fulvestrant.
- a compound of the disclosure is used in combination with an aromatase inhibitor.
- Aromatase inhibitors are used in the treatment of breast cancer in post-menopausal women and gynecomastia in men. Aromatase inhibitors can be used off-label to reduce estrogen conversion when using external testosterone. Aromatase inhibitors can also be used for chemoprevention in high-risk women.
- a compound of the disclosure is used in combination with a non-selective aromatase inhibitor.
- a compound of the disclosure is used in combination with a non-selective aromatase inhibitor, such as aminoglutethimide or testolactone (Teslac®).
- a compound of the disclosure is used in combination with a selective aromatase inhibitor.
- a compound of the disclosure is used in combination with a selective aromatase inhibitor, such as anastrozole (Arimidex®), letrozole (Femara®), exemestane (Aromasin®), vorozole (Rivizor®), formestane (Lentaron®), or fadrozole (Afema®).
- a compound of the disclosure is used in combination with exemestane.
- a compound of the disclosure is used in combination with an aromatase inhibitor such as 1,4,6-androstatrien-3,17-dione (ATD) or 4- androstene-3,6,17-trione.
- a compound of the disclosure is used in combination with an mTOR inhibitor.
- mTOR inhibitors are drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K)-related kinases (PIKKs).
- mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through the protein complexes mTORC1 and mTORC2.
- a compound of the disclosure is used in combination with an mTOR inhibitor, such as rapamycin, temsirolimus (CCI-779), everolimus (RAD001), ridaforolimus (AP-23573).
- an mTOR inhibitor such as rapamycin, temsirolimus (CCI-779), everolimus (RAD001), ridaforolimus (AP-23573).
- a compound of the disclosure is used in combination with everolimus (Afinitor®).
- Everolimus affects the mTORC1 protein complex and can lead to hyper-activation of the kinase AKT, which can lead to longer survival in some cell types.
- Everolimus binds to FKBP12, a protein receptor which directly interacts with mTORC1 and inhibits downstream signaling.
- a compound of the disclosure is used in combination with a mTOR inhibitor and an aromatase inhibitor.
- a compound of the disclosure is used in combination with everolimus and exemestane.
- Combination treatment with antimetabolites [0201] Antimetabolites are chemotherapy treatments that are similar to normal substances within the cell. When cells incorporate the antimetabolites into the cellular metabolism, the cells are unable to divide. Antimetabolites are cell-cycle specific and attack cells at specific phases in the cell cycle.
- a compound of the disclosure is used in combination with one or more antimetabolites, such as a folic acid antagonist, pyrimidine antagonist, purine antagonist, or an adenosine deaminase inhibitor.
- a compound of the disclosure is used in combination with an antimetabolite, such as methotrexate, 5-fluorouracil, foxuridine, cytarabine, capecitabine, gemcitabine, 6- mercaptopurine, 6-thioguanine, cladribine, fludarabine, nelarabine, or pentostatin.
- a compound of the disclosure is used in combination with capecitabine (Xeloda®), gemcitabine (Gemzar®), or cytarabine (Cytosar-U®).
- capecitabine Xeloda®
- gemcitabine gemcitabine
- Cytosar-U® cytarabine
- a compound of the disclosure is used in combination with a plant alkaloid.
- a plant alkaloid such as vinca alkaloids, taxanes, podophyllotoxins, or camptothecan analogues.
- a compound of the disclosure is used in combination with plant alkaloids, such as vincristine, vinblastine, vinorelbine, paclitaxel, docetaxel, etoposide, tenisopide, irinotecan, or topotecan.
- plant alkaloids such as vincristine, vinblastine, vinorelbine, paclitaxel, docetaxel, etoposide, tenisopide, irinotecan, or topotecan.
- a compound of the disclosure is used in combination with a taxane, such as paclitaxel (Abraxane® or Taxol®) and docetaxel (Taxotere®).
- paclitaxel Abraxane® or Taxol®
- docetaxel such as paclitaxel (Abraxane® or Taxol®)
- paclitaxel such as paclitaxel (Abraxane® or Taxol®) and docetaxel (
- a compound of the disclosure is used in combination with therapeutic antibodies.
- a compound of the disclosure is used in combination with monoclonal antibodies, such as alemtuzumab (Campath®) or trastuzumab (Herceptin®).
- a compound of the disclosure is used in combination with conjugated monoclonal antibodies, such as radiolabeled antibodies or chemolabeled antibodies.
- a compound of the disclosure is used in combination with conjugated monoclonal antibodies, such as ibritumomab tiuxetan (Zevalin®), brentuximab vedotin (Adcetris®), ado-trastuzumab emtansine (Kadcyla®), or denileukin diftitox (Ontak®).
- conjugated monoclonal antibodies such as ibritumomab tiuxetan (Zevalin®), brentuximab vedotin (Adcetris®), ado-trastuzumab emtansine (Kadcyla®), or denileukin diftitox (Ontak®).
- a compound of the disclosure is used in combination with bispecific monoclonal antibodies, such as blinatumomab (Blincyto®).
- a compound of the disclosure is used in combination with an anti-CD20 antibody, such as rituximab (Mabthera®/ Rituxan®), obinutuzumab (Gazyva®), ibritumomab tiuxetan, tositumomab, ofatumumab (Genmab®), ocaratuzumab, ocrelizumab, TRU-015, or veltuzumab.
- an anti-CD20 antibody such as rituximab (Mabthera®/ Rituxan®), obinutuzumab (Gazyva®), ibritumomab tiuxetan, tositumomab, ofatumumab (Genmab®), ocaratuzumab, ocrelizumab, TRU-015, or veltuzumab.
- the PD-1 pathway comprises the immune cell co-receptor Programmed Death-1 (PD-1) and the PD-1 ligands PD-L1 and PD-L2.
- the PD-1 pathway mediates local immunosuppression in the tumor microenvironment.
- PD-1 and PD-L1 antagonists suppress the immune system.
- a PD-1 or PD-L1 antagonist is a monoclonal antibody or antigen binding fragment thereof that specifically binds to, blocks, or downregulates PD-1 or PD-L1, respectively.
- a PD-1 or PD-L1 antagonist is a compound or biological molecule that specifically binds to, blocks, or downregulates PD-1 or PD-L1, respectively.
- the compounds of the disclosure are used in combination with a PD-1 or PD-L1 antagonist.
- the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist, for example, MK-3475, nivolumab (Opdivo®), pembrolizumab (Keytruda®), humanized antibodies (i.e., h409Al l, h409A16 and h409A17), AMP-514, BMS-936559, MEDI0680, MEDI4736, MPDL3280A, MSB0010718C, MDX-1105, MDX-1106, or pidilzumab.
- a PD-1/PD-L1 antagonist for example, MK-3475, nivolumab (Opdivo®), pembrolizumab (Keytruda®), humanized antibodies (i.e., h409Al l, h409A16 and h409A17), AMP-514, BMS-936559, MEDI0680, MEDI4736, MPDL3280A,
- the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist that is an immunoadhesion molecule, such as AMP-224. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist to treat cancer cells or a tumor that overexpresses PD-1 or PD-L1. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist to treat cancer cells or a tumor that overexpresses miR-34. h. Combination treatment with anti-hormone therapy [0209] Anti-hormone therapy uses an agent to suppress selected hormones or the effects.
- Anti-hormone therapy is achieved by antagonizing the function of hormones with a hormone antagonist and/or by preventing the production of hormones. In some embodiments, the suppression of hormones can be beneficial to subjects with certain cancers that grow in response to the presence of specific hormones.
- a compound of the disclosure is used in combination with a hormone antagonist. [0210] In some embodiments, a compound of the disclosure is used in combination with anti-androgens, anti-estrogens, aromatase inhibitors, or luteinizing hormone-releasing hormone (LHRH) agonists.
- a compound of the disclosure is used in combination with anti-androgens, such as bicalutamide (Casodex®), cyproterone (Androcur®), flutamide (Euflex®), or nilutamide (Anandron®).
- anti-estrogens such as fulvestrant (Faslodex®), raloxifene (Evista®), or tamoxifen (Novaladex®, Tamofen®).
- a compound of the disclosure is used in combination with LHRH agonists, such as buserelin (Suprefact®), goserelin (Zoladex®), or leuprolide (Lupron®, Lupron Depot®, Eligard®).
- LHRH agonists such as buserelin (Suprefact®), goserelin (Zoladex®), or leuprolide (Lupron®, Lupron Depot®, Eligard®).
- HMAs hypomethylating (demethylating) agents
- Treatment of these and other types of cancer may be improved by examining combinations that increase anti-tumor activities and may allow for similar activity at reduced doses compared to single agent treatment, leading to improved patient outcomes, and limiting adverse events.
- Hypomethylating (demethylating) agents inhibit DNA methylation, which affects cellular function through successive generations of cells without changing the underlying DNA sequence.
- Hypomethylating agents can block the activity of DNA methyltransferase.
- a compound of the disclosure is used in combination with hypomethylating agents, such as azacitidine (Vidaza®, Azadine®) or decitabine (Dacogen®).
- azacitidine such as azacitidine (Vidaza®, Azadine®) or decitabine (Dacogen®).
- a compound of the disclosure is used in combination with nonsteroidal anti-inflammatory drugs (NSAIDs), specific COX-2 inhibitors, or corticosteroids.
- NSAIDs nonsteroidal anti-inflammatory drugs
- a compound of the disclosure is used in combination with NSAIDs, such as aspirin, ibuprofen, naproxen, celecoxib, ketorolac, or diclofenac.
- NSAIDs such as aspirin, ibuprofen, naproxen, celecoxib, ketorolac, or diclofenac.
- a compound of the disclosure is used in combination with specific COX-2 inhibitors, such as celecoxib (Celebrex®), rofecoxib, or etoricoxib.
- a compound of the disclosure is used in combination with corticosteroids, such as dexamethasone or glucosteroids (e.g., hydrocortisone and prednisone). k.
- Histone deacetylase (HDAC) inhibitors are chemical compounds that inhibit histone deacetylase. HDAC inhibitors can induce p21 expression, a regulator of p53 activity. In some embodiments, a compound of the disclosure is used in combination with an HDAC inhibitor.
- a compound of the disclosure is used in combination with an HDAC inhibitor, such as vorinostat, romidepsin (Istodax®), chidamide, panobinostat (Farydak®), belinostat (PDX101), panobinostat (LBH589), valproic acid, mocetinostat (MGCD0103), abexinostat (PCI-24781), entinostat (MS-275), SB939, resminostat (4SC-201), givinostat (ITF2357), quisinostat (JNJ-26481585), HBI-8000, kevetrin, CUDC-101, AR-42, CHR-2845, CHR-3996, 4SC-202, CG200745, ACY-1215, ME-344, sulforaphane, or trichostatin A.
- HDAC inhibitor such as vorinostat, romidepsin (Istodax®), chid
- Platinum-based antineoplastic drugs are coordinated complex of platinum.
- a compound of the disclosure is used in combination with a platinum-based antineoplastic drug, such as cisplatin, oxaliplatin, carboplatin, nedaplatin, triplatin tetranitrate, phenanthriplatin, picoplatin, or satraplatin.
- a compound of the disclosure is used in combination with cisplatin or carboplatin.
- a compound of the disclosure is used in combination with cisplatinum, platamin, neoplatin, cismaplat, cis-diamminedichloroplatinum(II), or CDDP; Platinol®) and carboplatin (also known as cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II); tradenames Paraplatin® and Paraplatin-AQ®).
- Combination treatment with kinase inhibitors [0215] Abnormal activation of protein phosphorylation is frequently either a driver of direct consequence of cancer.
- MEK inhibitors are drugs that inhibit the mitogen-activated protein kinase enzymes MEK1 and/or MEK2.
- a compound of the disclosure is used in combination with a MEK1 inhibitor.
- a compound of the disclosure is used in combination with a MEK2 inhibitor.
- a compound of the disclosure is used in combination with an agent that can inhibit MEK1 and MEK2.
- a compound of the disclosure is used in combination with a MEK1/MEK2 inhibitor, such as trametinib (Mekinist®), cobimetinib, binimetinib, selumetinib (AZD6244), pimasertibe (AS-703026), PD-325901, CI-1040, PD035901, or TAK-733.
- a compound of the disclosure is used in combination with trametinib.
- a compound of the disclosure is used in combination with cobimetinib.
- BRAF inhibitors are drugs that inhibit the serine/threonine-protein kinase B- raf (BRAF) protein.
- BRAF serine/threonine-protein kinase B- raf
- a compound of the disclosure is used in combination with a BRAF inhibitor.
- a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit wild type BRAF.
- a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit mutated BRAF.
- a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit V600E mutated BRAF.
- a compound of the disclosure is used in combination with a BRAF inhibitor, such as vemurafenib (Zelboraf®), dabrafenib (Tafinlar®), C-1, NVP-LGX818, or sorafenib (Nexavar®).
- KRAS inhibitors KRAS is a gene that acts as an on/off switch in cell signaling.
- a compound of the disclosure is used in combination with a KRAS inhibitor.
- a compound of the disclosure is used in combination with a wild type KRAS inhibitor.
- a compound of the disclosure is used in combination with a mutated KRAS inhibitor (e.g., a G12C mutated KRAS).
- the KRAS inhibitor is sotorasib (AMG-510), COTI-219, ARS-3248, WDB-178, BI-3406, BI-1701963, SML-8-73-1 (G12C), Compound 3144 (G12D), Kobe0065, Kobe/2602, (Ras GTP), RT11, or adagrasib (MRTX-849).
- BTK inhibitors Bruton’s tyrosine kinase (BTK) is a non-receptor tyrosine kinase of the Tec kinase family that is involved in B-cell receptor signaling.
- a compound of the disclosure is used in combination with a BTK inhibitor.
- a compound of the disclosure is used in combination with a BTK inhibitor, such as ibrutinib or acalabrutinib.
- CDK inhibitors: CDK4 and CDK6 are cyclin-dependent kinases that control the transition between the G1 and S phases of the cell cycle. CDK4/CDK6 activity is deregulated and overactive in cancer cells.
- CDK4/CDK6 inhibitors can block cell-cycle progression in the mid-G1 phase of the cell cycle, causing arrest and preventing the proliferation of cancer cells.
- a compound of the disclosure is used in combination with a CDK4/CDK6 inhibitor.
- a compound of the disclosure is used in combination with a CDK4/CDK6 inhibitor, such as palbociclib (Ibrance®), ribociclib, trilaciclib, seliciclib, dinaciclib, milciclib, roniciclib, atuveciclib, briciclib, riviciclib, voruciclib, or abemaciclib.
- a compound of the disclosure is used in combination with palbociclib.
- a compound of the disclosure is used in combination with ribociclib. In some embodiments, a compound of the disclosure is used in combination with abemaciclib. [0221] In some examples, a compound of the disclosure is used in combination with an inhibitor of CDK4 and/or CDK6 and with an agent that reinforces the cytostatic activity of CDK4/6 inhibitors and/or with an agent that converts reversible cytostasis into irreversible growth arrest or cell death. Examples of cancer subtypes include NSCLC, melanoma, neuroblastoma, glioblastoma, liposarcoma, and mantle cell lymphoma.
- a compound of the disclosure is used in combination with at least one additional pharmaceutically active agent that alleviates CDKN2A (cyclin-dependent kinase inhibitor 2A) deletion.
- a compound of the disclosure is used in combination with at least one additional pharmaceutically active agent that alleviates CDK9 (cyclin-dependent kinase 9) abnormality.
- a compound of the disclosure is used in combination with a CDK2, CDK7, and/or CDK9 inhibitor.
- a compound of the disclosure is used in combination with a CDK2, CDK7, or CDK9 inhibitor, such as seliciclib, voruciclib, or milciclib.
- a compound of the disclosure is used in combination with a CDK inhibitor, such as dinaciclib, roniciclib (Kisqali®), or briciclib.
- a compound of the disclosure is used in combination with at least one additional pharmaceutically-active agent that alleviates CDKN2A (cyclin-dependent kinase inhibitor 2A) deletion.
- CDKN2A cyclin-dependent kinase inhibitor 2A
- ATM regulators A compound of the disclosure can be used in combination with one or more anti-cancer agent that regulates the ATM (upregulate or downregulate).
- the compounds described herein can synergize with one or more ATM regulators.
- one or more of the compounds described herein can synergize with all ATM regulators.
- AKT inhibitors In some embodiments, a compound of the disclosure is used in combination with one or more anti-cancer agent that inhibits the AKT (protein kinase B (PKB)). In some embodiments the compounds described herein can synergize with one or more AKT inhibitors. n. Combination treatment with other anti-cancer agents [0225] In some examples, a compound of the disclosure is used in combination with at least one anti- cancer agent that alleviates PTEN (phosphatase and tensin homolog) deletion. In some examples, a compound of the disclosure is used in combination with at least one anti-cancer agent that alleviates Wip- 1Alpha over expression.
- PTEN protein kinase B
- a compound of the disclosure is used in combination with at least one anti-cancer agent that is a Nucleoside metabolic inhibitor.
- nucleoside metabolic inhibitors include capecitabine, gemcitabine and cytarabine (Arac).
- Arac cytarabine
- a compound of the disclosure or a pharmaceutical composition comprising a compound of the disclosure and at least one anti-cancer agent as disclosed herein can be administered simultaneously (i.e., simultaneous administration) or sequentially (i.e., sequential administration).
- a compound of the disclosure and the at least one anti-cancer agent described herein are administered simultaneously, either in the same composition or in separate compositions.
- the compound and the at least one anti- cancer agent described herein may be contained in the same composition (e.g., a composition comprising both the compound and the at least one anti-cancer agent) or in separate compositions (e.g., the compound is contained in one composition and the at least one anti-cancer agent is contained in another composition).
- the compound and the at least one anti-cancer agent are administered sequentially, i.e., the compound is administered either prior to or after the administration of the anti- cancer agent.
- the compound is administered before the anti-cancer agent.
- the anti-cancer agent described herein is administered before the compound.
- the compound and the anti-cancer agent described herein are contained in separate compositions, which may be contained in the same or different packages.
- the administration of the compound and the anti-cancer agent described herein are concurrent, i.e., the administration period of the compounds and that of the anti-cancer agent overlap with each other.
- the administration of the compounds and the anti-cancer agent described herein are non-concurrent.
- the administration of the compound is terminated before the anti-cancer agent described herein is administered.
- the administration of the anti-cancer agent described herein is terminated before the compound is administered.
- the time period between these two non-concurrent administrations can range from being hours apart to being days apart to being weeks apart.
- the dosing frequency of the compound and the at least one anti-cancer agent described herein may be adjusted over the course of the treatment, based on the judgment of the administering physician.
- the compound and the at least one anti-cancer agent described herein can be administered at different dosing frequency or intervals.
- the compound can be administered weekly, while the at least one anti-cancer agent described herein can be administered more or less frequently.
- the compound can be administered twice weekly, while the at least one anti-cancer agent described herein can be administered more or less frequently.
- the compound and the at least one anti-cancer agent described herein can be administered using the same route of administration or using different routes of administration.
- a therapeutically-effective amount of a compound and/or the anti-cancer agent described herein for use in therapy can vary with the nature of the condition being treated, the length of treatment time desired, the age and the condition of the patient, and can be determined by the attending physician.
- the dosage of the compound when a compound of the disclosure is administered in combination with at least one anti-cancer agent described herein, can be given a lower dosage than when the compound is administered alone.
- the dosage of a compound of the disclosure in combination therapy can be from about 1 mg/kg to about 20 mg/kg. In some embodiments, the dosage of a compound of the disclosure in combination therapy can be from about 5 mg/kg to about 10 mg/kg..
- the dosage of a compound of the disclosure in combination therapy can be in an amount of about 0.5 mg/kg; about 1 mg/kg; about 2 mg/kg; about 3 mg/kg; about 4 mg/kg; about 5 mg/kg; about 6 mg/kg; about 7 mg/kg; about 8 mg/kg; about 9 mg/kg; or about 10 mg/kg.
- the dosage of the compound of the disclosure in combination therapy with at least one additional therapeutic agent can be at least 5% less than the dose of the compound of the disclosure administered alone, or at least 10% less, at least 15% less, at least 20% less, at least 25% less, at least 30% less, at least 35% less, at least 40% less, at least 45% less, at least 50% less, at least 55% less, at least 60% less, at least 65% less, at least 70% less, or at least 75% less than the dose of the compound of the disclosure administered alone.
- the dosage of the at least one additional anti-cancer agent can be given a lower dosage than when the anti-cancer agent is administered alone.
- the dosage of the at least one anti-cancer agent in combination therapy can be from about 50 ⁇ g to about 100 ⁇ g; from about 100 ⁇ g to about 150 ⁇ g; from about 150 ⁇ g to about 200 ⁇ g; from about 200 ⁇ g to about 250 ⁇ g; from about 250 ⁇ g to about 300 ⁇ g; from about 300 ⁇ g to about 350 ⁇ g; from about 350 ⁇ g to about 400 ⁇ g; from about 400 ⁇ g to about 450 ⁇ g; from about 450 ⁇ g to about 500 ⁇ g; from about 500 ⁇ g to about 600 ⁇ g; from about 600 ⁇ g to about 700 ⁇ g; from about 700 ⁇ g to about 800 ⁇ g; from about 800 ⁇ g to about 900 ⁇ g; or from about 900 ⁇ g to about 1000 ⁇ g.
- the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 ⁇ g to about 150 ⁇ g. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 150 ⁇ g to about 200 ⁇ g. In some embodiments, the dosage of the at least one additional anti-cancer agent in combination therapy can be from about 200 ⁇ g to about 250 ⁇ g.
- the dosage of the at least one anti-cancer agent in combination therapy can be in an amount of about 50 ⁇ g; about 100 ⁇ g; about 150 ⁇ g; about 200 ⁇ g; about 250 ⁇ g; about 300 ⁇ g; about 350 ⁇ g; about 400 ⁇ g; about 450 ⁇ g; or about 500 ⁇ g. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be in an amount of about 200 ⁇ g.
- the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 5 mg/kg; from about 5 mg/kg to about 25 mg/kg; from about 25 mg/kg to about 50 mg/kg; from about 50 mg/kg to about 75 mg/kg; from about 75 mg/kg to about 100 mg/kg; from about 100 mg/kg to about 150 mg/kg; from about 150 mg/kg to about 200 mg/kg; from about 200 mg/kg to about 250 mg/kg; from about 250 mg/kg to about 300 mg/kg; from about 300 mg/kg to about 350 mg/kg; from about 350 mg/kg to about 400 mg/kg; from about 400 mg/kg to about 450 mg/kg; from about 450 mg/kg to about 500 mg/kg; from about 500 mg/kg to about 600 mg/kg; from about 600 mg/kg to about 700 mg/kg; from about 700 mg/kg to about 800 mg/kg; from about 800 mg/kg to about 900 mg/kg; or from about 900
- the dosage of the at least one anti- cancer agent in combination therapy can be from about 1 mg/kg to about 5 mg/kg. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 mg/kg to about 150 mg/kg. In some embodiments, the dosage of the at least one additional therapeutic agent in combination therapy can be from about 200 mg/kg to about 250 mg/kg.
- the dosage of the at least one anti-cancer agent in combination therapy can be at least 5% less than the dose of the at least one anti-cancer agent administered alone, or at least 10% less, at least 15% less, at least 20% less, at least 25% less, at least 30% less, at least 35% less, at least 40% less, at least 45% less, at least 50% less, at least 55% less, at least 60% less, at least 65% less, at least 70% less, or at least 75% less than the dose of the at least one anti-cancer agent administered alone.
- the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 500 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 250 mg/kg. [0241] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 1 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg.
- the dosage of the compound of the disclosure administered in combination therapy can be about 2 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 4 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 6 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 8 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg.
- the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 ⁇ g to about 500 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 ⁇ g to about 500 ⁇ g. [0243] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 1 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg; and the dosage of the at least one anti- cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of anti-PD-1 agent as an anti- cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g. [0245] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 1 mg/kg or about 100 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg or about 150 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg or about 300 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg or about 400 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g..
- the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg or about 500 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of anti-CD20 agent as an anti- cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy is about 1 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg; and the dosage of anti- CD20 agent as an anti- cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g.
- the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 ⁇ g [0248]
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day. In some embodiments, a pharmaceutically- acceptable amount of a compound of the disclosure can be administered to a subject once a day.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject three times a day. [0249] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day once every 3 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day once every 3 days.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 7 days. In some embodiments, a pharmaceutically- acceptable amount of a compound of the disclosure can be administered to a subject once a day once every 7 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day once every 7 days. [0250] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for 1 to 50 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 5 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 10 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 15 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 20 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 25 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 30 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 35 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 7 days for about 5 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 7 days for about 10 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 15 doses.
- a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 3 days for about 20 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 3 days for about 35 doses. [0252] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 5 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 15 doses.
- compositions for combination treatment are administered within a single pharmaceutical composition.
- the compounds of the disclosure and the anti-cancer agent described herein can be provided in a single unit dosage form for being taken together.
- the pharmaceutical composition further comprises a pharmaceutically-acceptable diluent or carrier.
- the compounds of the disclosure and the anti-cancer agent described herein are administered within different pharmaceutical compositions.
- the compounds of the disclosure and the anti-cancer agent described herein can be provided in a single unit dosage as separate entities (e.g., in separate containers) to be administered simultaneously or with a certain time difference.
- the compounds of the disclosure and the anti-cancer agent described herein can be administered via the same route of administration. In some embodiments, the compounds of the disclosure and the anti-cancer agent described herein can be administered via the different route of administration. In some embodiments, a compound of the disclosure and the anti-cancer agent are administered orally. In some embodiments, a compound of the disclosure is administered orally, and the anti-cancer agent is not administered orally. In some embodiments, a compound of the disclosure is not administered orally, and the anti-cancer agent is administered orally.
- Treatment of a condition by administering a compound of the disclosure in combination with an anti-cancer agent can increase a median survival time of a subject compared to subjects who do not receive the combination therapy.
- a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that does not receive any cancer therapy.
- a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that receives therapy with a compound of the disclosure alone.
- a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that receives therapy with the anti- cancer agent alone.
- a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy (e.g., no therapy, a compound of the disclosure alone, or the anti-cancer agent alone) by at least about 50%, about 60%, about 70%, about 80%, about 90%, about 100%, about 110%, about 120%, about 130%, about 140%, about 150%, about 160%, about 170%, about 180%, about 190%, about 200%, about 210%, about 220%, about 230%, about 240%, about 250%, about 260%, about 270%, about 280%, about 290%, about 300%, about 310%, about 320%, about 330%, about 340%, about 350%, about 360%, about 370%, about 380%, about 390%, about 400%, about 410%, about 420%, about 430%, about 440%, about 450%, about 460%, about 470%, about 480%, about 490%, or about 500%.
- a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 50%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 100%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 150%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 200%.
- Methods of treatment Provided herein is a method of treating cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation.
- a compound of the disclosure comprises a substituted 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation.
- the compound binds to Uba2.
- the compound has an inhibitory effect on activating enzymes (E1) of SUMO.
- the cancer is ovarian cancer. In some embodiments, the cancer is breast cancer. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is AML. In some embodiments, the cancer is colon cancer. In some embodiments, the cancer is a lymphoma. In some embodiments, the subject is human. [0259] In some embodiments, the administering of the compound is oral. In some embodiments, the administering of the compound is subcutaneous. In some embodiments, the administering of the compound is topical. [0260] In some embodiments, the therapeutically-effective amount of the compound is from about 1 mg/kg to about 20 mg/kg. In some embodiments, the therapeutically-effective amount of the compound is from about 5 mg to about 10 mg..
- the therapeutically-effective amount of the compound is about 50 mg, about 5100 mg, about 150 mg, about 200 mg, about 250 mg, about 300 mg, about 350 mg, about 400 mg, about 450 mg, or about 500 mg. In some embodiments, the therapeutically- effective amount of the compound is about 150 mg. In some embodiments, the therapeutically-effective amount of the compound is about 200 mg. In some embodiments, the therapeutically-effective amount of the compound is about 300 mg. In some embodiments, the therapeutically-effective amount of the compound is about 400 mg. [0261] In some embodiments, the anti-cancer agent is a small molecule. In some embodiments, the anti- cancer agent is an antibody. [0262] In some embodiments, the anti-cancer agent is an immune checkpoint inhibitor.
- the immune checkpoint inhibitor is an anti-PD-1 agent.
- the anti-PD- 1 agent is nivolumab.
- the anti-PD-1 agent is pembrolizumab.
- the anti-PD-1 agent is cemiplimab.
- the immune checkpoint inhibitor is an anti-PD-L1 agent.
- the anti-PD-L1 agent is atezolizumab.
- the anti-PD-L1 agent is avelumab.
- the anti-PD-L1 agent is durvalumab.
- the administering of the anti-cancer agent is oral.
- the administering of the anti-cancer agent is subcutaneous. In some embodiments, the administering of the anti-cancer agent is topical. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is from about 5 mg/kg to about 500 mg/kg. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is from about 10 ⁇ g to about 500 ⁇ g. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is about 200 ⁇ g. [0263] In some embodiments, the anti-cancer agent is an anti-CD20 antibody.
- anti-CD-20 antibodies include rituximab (Mabthera®/ Rituxan®), obinutuzumab (Gazyva®), ibritumomab tiuxetan, tositumomab, ofatumumab (Genmab®), ocaratuzumab, ocrelizumab, TRU-015, and veltuzumab.
- the anti-cancer agent is rituximab.
- the therapeutically-effective amount of the anti-CD20 antibody is from about 5 mg/kg to about 500 mg/kg.
- the therapeutically-effective amount of the anti-CD20 antibody is from about 10 ⁇ g to about 500 ⁇ g.
- the anti-cancer agent is a hypomethylating agent (HMA).
- HMA hypomethylating agent
- a compound of the disclosure is used in combination with a HMA, such as azacitidine (Vidaza®, Azadine®) or decitabine (Dacogen®).
- the therapeutically-effective amount of the HMA is from about 5 mg/kg to about 500 mg/kg.
- the therapeutically-effective amount of the HMA is from about 0.005 ⁇ M to about 0.500 ⁇ M.
- Compound A is (S,E)-6-(4-(dimethylamino)but-2-enoyl)-4-(2-(1-ethyl-3-(trifluoromethyl)-1H- pyrazol-4-yl)-3-fluorophenyl)-3-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-2-carbonitrile.
- EXAMPLE 2 Combination of Compound A and anti-CD-20 agents [rituximab] in subcutaneous mouse lymphoma xenograft model [Raji human B cell] [0267]
- the anti-tumor efficacy of Compound A in combination with rituximab was evaluated in the subcutaneous Raji human lymphoma xenograft model in female CB17 SCID mice.
- the overall dosing regimen is included in Table 1.
- the Raji tumor cells (ATCC, Cat# CCL-86) were maintained in vitro as a suspension culture in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, 100 U/mL penicillin and 100 ⁇ g/mL streptomycin at 37oC in an atmosphere of 5% CO2 in air.
- the tumor cells were routinely subcultured twice weekly. The cells growing to a confluency around 70% - 80% were harvested and counted for tumor inoculation.
- 75 CB17 SCID mice [female, 6-8 weeks old, 18-20g weight] were inoculated subcutaneously at the right flanks with Raji cells for tumor development.
- mice were kept in individual ventilation cages at constant temperature and humidity with 4 animals in each cage [temperature 20-26 C and 40-70% humidity].
- 48 mice with tumor sizes ranging from 65-268 mm 3 (average tumor size 147.16 mm3) were selected and assigned into 6 groups using stratified randomization with 8 mice in each group based upon their tumor volumes.
- mice were treated as follows: Group 1: Vehicle control, p.o., (BIW on D1/4/8/11/14); Group 2: Compound A, 50 mg/kg, p.o., (BIW on D1/4/8/11/14/17); Group 3: Rituximab, 20 mg/kg, i.p., (BIW on D1/4/8/11/14); Group 4: Compound A, 50 mg/kg, p.o.,+ Rituximab, 20 mg/kg, i.p., (BIW on D1/4/8/11/14/17); Group 5: Compound A, 50 mg/kg, p.o., (QD x 17 days); and Group 6: SUMO comparator, 15 mg/kg, i.v., (BIW on D1/4/8/11/14/17).
- the tumor sizes were used for the calculations of both tumor growth inhibition (TGI) values.
- TVTreatment_DayN is the average tumor volume of a treatment group on a given day
- TVTreatment_Day0 is the average tumor volume of the treatment group on the first day of treatment
- TVVehicle_DayN is the average tumor volume of the vehicle control group on a given day
- TVVehicle_Day0 is the average tumor volume of the vehicle group on the first day of treatment.
- Mean tumor volumes over time are shown in Table 2 and tumor growth curves are shown in Figure 1.
- EXAMPLE 3 Combination of Compound A and a hypomethylating agent (HMA) in AML cell line model [GDM-1 cells and MV4-11]
- HMA hypomethylating agent
- Assay Principle This cellular assay measures the viability of leukemia cell lines upon treatment with a SUMO E1 inhibitor, Compound A, and a hypomethylating agent (HMA), decitabine, as single agents, as well as in combination. Cell viability was measured using the Promega CellTiter-Glo Luminescent Cell Viability Assay, which determines the number of viable cells based on the quantitation of ATP (as an indicator of metabolically active cells).
- AML acute myeloid leukemia
- GDM-1 Three acute myeloid leukemia (AML) cell lines, including GDM-1, and MV4-11, were first treated with Compound A or decitabine as single treatments to establish single agent IC50s to describe the quantity of compound needed to inhibit 50% of cell viability.
- cells were treated with both Compound A and decitabine, with 4 to 5 decreasing doses of each compound, to generate a matrix of 25 combinations.
- Dose response curves and IC50 values were generated, and Zero interaction potency (ZIP) synergy scores were calculated using SynergyFinder 3.0 (https://synergyfinder.fimm.fi/synergy/20220701215205193938/).
- the ZIP model determines whether combination treatments result in synergistic or additive effects by comparing the potency at specific dose levels between single and combination treatments.
- the ZIP model determines whether combination treatments result in synergistic or additive effects by comparing the potency at specific dose levels between single and combination treatments.
- the cell viability of three different AML cells lines were evaluated during single and combination treatment with Compound A and decitabine to determine the efficacy of combination therapy.
- GDM-1 and MV4-11 cells were plated overnight in white-walled 96-well plates (10,000 cells/well in 100ul total volume). The next day, compounds were diluted in cell culture media and cells were treated with 4 to 5 different doses of Compound A and decitabine (either as single treatment or in combination). Cells were then incubated for 72 hrs.
- FIG. 1 shows cell viability dose response curves of Compound A alone, along with the combination of escalating doses with decitabine in GDM-1 and MV-411 cells. The IC50 values from each curve, along with the calculated ZIP score are found in Table 4.
- mice Tumor-immune profiling of CT-26 and Colon 26 syngeneic mouse models reveals mechanism of anti-PD-1 response.
- BALB/c mice are injected subcutaneously with 1 x 10 5 CT26 cells on Day 0. Beginning day 5, mice are treated with Compound A [50 mpk]. and 250 pg of anti-PD-1 (BioXcell, Clone RMP1-14) or isotype control (BioXcell, Clone 2A3). Subsequently, all mice were continued on study the assigned combination therapy on days 12, 15, and 19.
- a method of treating a cancer in a subject in need thereof comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation.
- Embodiment 2. The method of embodiment 1, wherein the compound binds to Uba2.
- Embodiment 3 The method of embodiment 1, wherein the compound has an inhibitory effect on activating enzymes (E1) of SUMO.
- the cancer is selected from acute myeloid leukemia, large B-cell lymphoma, lung squamous cell carcinoma, pancreatic adenocarcoma, esophegeal carcinoma, cervical squamous cell carcinoma, endocervical adenocarcoma, stomach adenocarcinomathymoma, renal cell carcinoma, head and neck squamous cell carcinoma, bladder carcinoma, ovarian cystadenocarcinoma, multiple myeloma, non-Hodgkin’s lymphoma (NHL), and mesothelioma.
- Embodiment 5 Embodiment 5.
- Embodiment 6 The method of embodiment 1, wherein the cancer is selected from head and neck squamous cell carcinoma (HNSCC), non-squamous non-small cell lung cancer (NSCLC), cervical cancer, colorectal cancer (CRC), cutaneous melanoma, squamous NSCLC, and small cell lung cancer.
- HNSCC head and neck squamous cell carcinoma
- NSCLC non-squamous non-small cell lung cancer
- CRC colorectal cancer
- cutaneous melanoma squamous NSCLC
- small cell lung cancer small cell lung cancer.
- Embodiment 6 The method of embodiment 1, wherein the cancer is B Cell Lymphoma.
- Embodiment 7. The method of embodiment 1, wherein the cancer is acute myeloid leukemia.
- Embodiment 8 The method of embodiment 1, wherein the cancer is colon cancer.
- Embodiment 9. The method of embodiment 1, wherein the administering of the compound is oral.
- Embodiment 11 The method of embodiment 1, wherein the subject is human [0289] Embodiment 11. The method of embodiment 1, wherein the therapeutically-effective amount of the compound is from about 100 mg to about 500 mg. [0290] Embodiment 12. The method of any one of embodiments 1-12, wherein the therapeutically- effective amount of the compound is from about 200 mg to about 400 mg. [0291] Embodiment 13. The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 300 mg. [0292] Embodiment 14. The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 200 mg. [0293] Embodiment 15.
- Embodiment 18 The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 400 mg.
- Embodiment 18 The method of any one of embodiments 1-17, wherein the anti-cancer agent is a small molecule.
- Embodiment 19 The method of any one of embodiments 1-17, wherein the anti-cancer agent is an antibody.
- Embodiment 20 The method of any one of embodiments 1-17, wherein the anti-cancer agent is an immune checkpoint inhibitor.
- Embodiment 21 The method of any one of embodiments 1-17 or 20, wherein the immune checkpoint inhibitor is an anti-PD-1 agent.
- Embodiment 22 The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 400 mg.
- Embodiment 18 The method of any one of embodiments 1-17, wherein the anti-cancer agent is a small molecule.
- Embodiment 19 The method of any one of embodiments 1-17, wherein the anti-cancer
- Embodiment 23 The method of any one of embodiments 1-17, 20, or 21, wherein the anti-PD-1 agent is pembrolizumab.
- Embodiment 24 The method of any one of embodiments 1-17, 20, or 21, wherein the anti-PD-1 agent is cemiplimab.
- Embodiment 25 The method of any one of embodiments 1-17 or 20, wherein the immune checkpoint inhibitor is an anti-PD-L1 agent.
- Embodiment 26 Embodiment 26.
- Embodiment 27 The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is atezolizumab.
- Embodiment 28 The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is avelumab.
- Embodiment 28 The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is durvalumab.
- Embodiment 29 The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is oral.
- Embodiment 30 The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is subcutaneous.
- Embodiment 31 The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is topical.
- Embodiment 32 The method of any one of embodiments 1-31, wherein the therapeutically- effective amount of the anti-cancer agent is from about 1 mg/kg to about 20 mg/kg.
- Embodiment 33 The method of any one of embodiments 1-31, wherein the therapeutically- effective amount of the anti-cancer agent is from about 10 ⁇ g to about 500 ⁇ g.
- Embodiment 34 The method of any one of embodiments 1-31 or 33, wherein the therapeutically- effective amount of the anti-cancer agent is about 200 ⁇ g. [0311] Embodiment 35.
- R 1 is selected from hydrogen, halogen, -C(X 1 )3, -CHX 1 2, -CH2X 1 , -OCX 1 3, -OCH2X 1 , -OCHX 1 2, -CN, -SOn1R 1A , -SOv1NR 1A R 1B , -NHC(O)NR 1A R 1B , -N(O)m1, -NR 1A R 1B , -NHNR 1A R 1B , -C(O)R 1A , -C(O)-OR 1A , -C(O)NR 1A R 1B , -C(O)NHNR 1A R 1B , -OR 1A , -NR 1A SO2R 1B , -NR 1A C(O)R 1B , -NR 1A C(O)OR 1B , -NR 1A OR 1B , -N3, substituted or unsubstituted alky
- R 3 and R 3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
- R 5 is selected from hydrogen, halogen, -CX 5 3, -CHX 5 2, -CH2X 5 , -OCX 5 3, -OCH2X 5 , -OCHX 5 2, -CN, -SOn5R 5A , -SOv5NR 5A R 5B , -NHC(O)NR 5A R 5B , -N(O)m5, -NR 5A R 5B , -NHNR 5A R 5B , -C(O)R 5A , -C(O)-OR 5A , -C(O)NR 5A R 5B , -C(O)NHNR 5A R 5B , -OR 5A , -NR 5A SO2R 5B , -NR 5A C(O)R 5B , -NR
- Embodiment 36 The method of embodiment 35 wherein l is 1; m is 0; and n is 1.
- Embodiment 37 The method of embodiment 35 wherein Z is N.
- Embodiment 38 The method of embodiment 35 wherein ring A is selected from 5-membered heteroaryl, wherein ring A is optionally substituted with one or more substituent groups.
- Embodiment 39 The method of embodiment 35 R 2 is selected from substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl.
- Embodiment 40 Embodiment 40.
- ring A is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein ring A is optionally substituted with one or more substituent groups [0317] Embodiment 41.
- Embodiment 42 The method of embodiment 39 wherein R 2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cycloalkyl and substituted or unsubstituted 5- or 6- membered heteroaryl [0319]
- Embodiment 43 The method of embodiment 42, wherein R 2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cyclopentyl, substituted or unsubstituted cyclohexyl and substituted or unsubstituted pyridyl [0320]
- Embodiment 44 Embodiment 44.
- Embodiment 45 The method of embodiment 35 wherein M is CR 3 R 4 .
- Embodiment 46 The method of embodiment 35 wherein , .
- Embodiment 47 The method of embodiment 35 wherein Y is CR 6 R 7 .
- Embodiment 48 Embodiment 48.
- R 1 is selected from hydrogen, halogen, -CX 1 3, -CHX 1 2, -CH2X 1 , -OCX 1 3, -OCH2X 1 , -OCHX 1 2, -CN, -SOn1R 1A , -SOv1NR 1A R 1B , -NHC(O)NR 1A R 1B , -N(O)m1, -NR 1A R 1B , -NHNR 1A R 1B , -C(O)R 1A , -C(O)-OR 1A , -C(O)NR 1A R 1B , -C(O)NHNR 1A R 1B , -OR 1A , -NR 1A SO 2 R 1B , -NR 1A C(O)R 1B , -NR 1A C(O)OR 1B , -NR 1A OR 1B , -N3, substituted or unsubstituted alkyl, substituted or
- Embodiment 49 The method of embodiment 48 wherein ring B is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein Ring B is optionally substituted with one or more substituents.
- Embodiment 50 The method of embodiment 48 wherein ring B is selected from phenyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, and triazinyl, wherein ring B is optionally substituted with one or more substituent groups.
- Embodiment 51 The method of embodiment 50 wherein ring B is selected from [0328] Embodiment 52.
- Embodiment 53 The method of embodiment 48 wherein W is S.
- R 10 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl; and R 11 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl.
- Embodiment 55 The method of any one of embodiments 1-34, wherein the compound is of the formula: wherein R 36 is H, halo, C1-C4 alkyl, C2-C4 alkenyl or C3-C6 cycloalkyl; R 37 is substituted or unsubstituted C2-C4 alkenyl or C2-C4 alkynyl; and R 38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl, substituted or unsubstituted nitrogen containing 5- or 6- membered partially unsaturated heterocyclyl and substituted or unsubstituted nitrogen containing 6-10 membered heteroaryl; provided R 38 is not 4-pyridyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof.
- Embodiment 56 The method of embodiment 55 wherein R 38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl selected from pyrazolyl, isoxazolyl, isothiazolyl, pyrrolyl, thiazolyl, triazolyl and imidazolyl; substituted or unsubstituted nitrogen containing 6- membered heteroaryl selected from pyridinyl, pyrimidinyl and pyrazinyl; substituted or unsubstituted nitrogen containing 5-membered partially unsaturated heterocyclyl selected from pyrrolinyl, and imidazolidinyl; and substituted or unsubstituted dihydropyridinyl;.
- Embodiment 57 The method of embodiment 55 wherein R 36 is selected from chloro, methyl, ethyl, isopropyl, allylyl, and cyclopropyl;.
- Embodiment 58 Embodiment 58.
- R 38 is selected from substituted 5- pyrazolyl, substituted 4-pyrazolyl, substituted 1-pyrazolyl, substituted or unsubstituted 4-isoxazolyl, substituted or unsubstituted 4-isothiazolyl, substituted or unsubstituted 3-pyrrolyl, substituted or unsubstituted 5-thiazolyl, substituted or unsubstituted 5-imidazolyl, substituted or unsubstituted 1- imidazolyl, substituted or unsubstituted [1,2,4]triazol-5-yl, substituted or unsubstituted 3-pyridyl, and substituted or unsubstituted 5-pyrimidinyl;.
- Embodiment 59 The method of embodiment 58 wherein R 38 is selected from 3-trifluoromethyl- pyrazol-4-yl, 1-isopropyl-3-trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-trifluoromethyl-pyrazol-4-yl, 1- methyl-3-difluoromethyl-pyrazol-4-yl, 1-butyl-3-trifluoromethyl-pyrazol-4-yl, 1-methoxymethyl-3- trifluoromethyl-pyrazol-4-yl, 1-propyl-3-trifluoromethyl-pyrazol-4-yl, 1,3-dimethyl-pyrazol-4-yl, 1,3,5- trimethyl-pyrazol-4-yl, 1-methyl-3-cyclopropyl-pyrazol-4-yl, 1-methyl-3-trifluoromethyl-pyrazol-4-yl, 1- ethyl-3-amino-pyrazol-4-yl, 1-ethyl
- Embodiment 60 The method of embodiment 59 wherein R 38 is selected from 5-pyrimidinyl, 2- methyl-5-pyrimidinyl, 4-methyl-5-pyrimidinyl, 4,6-dimethoxy-5-pyrimidinyl, 4,6-dimethyl-5-pyrimidinyl, 4-triflouromethyl-5-pyrimidinyl, 4-pyrimidinyl, 2-methyl-4-pyrimidinyl, 4-methyl-6-pyrimidinyl, 2,4- dimethyl-6-pyrimidinyl, 3-pyridinyl, 2-pyridinyl, 4-methyl-2-pyridinyl, 2-triflouromethyl-3-pyridinyl, 4- triflouromethyl-3-pyridinyl, 2-methyl-3-pyridinyl, 2,5-dimethyl-3-pyridinyl, 2,6-dimethyl-3-pyridinyl, 2,4-dimethyl-3-pyridinyl, 2-ethyl-3-pyridinyl, 5-
- Embodiment 61 The method of embodiment 55 wherein R 38 is selected from substituted or unsubstituted tetrahydroquinolinyl, substituted or unsubstituted 1-pyrrolin-3-yl, substituted or unsubstituted 1-imidazolidinyl, and substituted or unsubstituted dihydropyridin-3-yl;.
- Embodiment 62 The method of embodiment 55 wherein R 38 is selected from substituted or unsubstituted tetrahydroquinolinyl, substituted or unsubstituted 1-pyrrolin-3-yl, substituted or unsubstituted 1-imidazolidinyl, and substituted or unsubstituted dihydropyridin-3-yl;.
- R 38 is selected from 4-isoquinolinyl, 1- methoxy-4-isoquinolinyl, 1-chloro-4-isoquinolinyl, 6-methyl-4-isoquinolinyl, 1-oxo-2-methyl-4- isoquinolinyl, 3-quinolinyl, 4-quinolinyl, 5-pyrrolopyridinyl, [1,3,3a]-triazainden-5-yl, 1-ethyl-3- pyrrolopyridinyl, and 1-methyl-5-pyrrolopyridinyl; [0339] Embodiment 63.
- R 37 is selected from ethenyl, fluoroethenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl and nitrogen- containing heterocyclyl-propenyl.
- R 36 is selected from H, halo, C 1 -C 4 alkyl, C 2 -C 4 alkenyl and C 3 -C 6 cycloalkyl
- R 37 is substituted or unsubstituted C 2 -C 4 alkenyl, or C 2 -C 4 alkynyl
- R 41 is selected from H, C 1-6 alkyl, C 1-6 hydroxyalkyl, C 1-6 alkoxyalkyl, C 1-6 alkylamino-C 1-6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl- C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl,
- Embodiment 65 The method of embodiment 64, wherein R 41 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, D3-methyl, 1-hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2-methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, methoxycarbonylmethyl, dimethylaminocarbonylmethyl, dimethylaminocarbonylethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, aminocarbonyl-1,1-dimethyl, methylcarbonylaminoethyl, 1-methylcarbonylamino-2.2-dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl,
- Embodiment 66 The method of embodiment 64, wherein R 36 is H, chloro, methyl, ethyl, isopropyl, propenyl, trifluoromethyl, 2-furyl, 2-pyridinyl, or cyclopropyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 67 Embodiment 67.
- R 37 is selected from ethenyl, fluoroethenyl, fluoropropenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethenyl and substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 68 Embodiment 68.
- R 37 is azetidin-1-ylpropenyl, 1- methylpyrrolidin-2-ylethenyl, dimethylaminopropenyl, methylaminopropenyl, piperidin-1-ylpropenyl, 4- hydroxy-piperidin-1-ylpropenyl, pyrrolidin-1-ylpropenyl, 3-hydroxypyrrolidin-1-ylpropenyl, morpholin- 1-ylpropenyl, or aminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 69 Embodiment 69.
- Embodiment 70 The method of embodiment 64, wherein R 43 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl; or an isomer or a pharmaceutically acceptable salt thereof.
- Embodiment 71 Embodiment 71.
- Embodiment 72 The method of any one of embodiments 1-34 wherein the compound is of the formula: wherein R 1 is substituted or unsubstituted C2-C6 alkenyl, or substituted or unsubstituted C2-C6 alkynyl; R 6 is selected from H, C 1-6 alkyl, C 2-4 alkynyl, C 1-6 hydroxyalkyl, C 1-6 alkoxyalkyl, C 1-6 alkylamino-C 1- 6 alkyl, C 1-6 alkylaminocarbonyl-C 1-6 alkyl, aminocarbonyl-C 1-6 alkyl, C 1-6 alkoxycarbonyl-C 1-6 alkyl, carboxy-C 1-6 alkyl, C 1-6 alkylcarbonylamino-C 1-6 alkyl, C
- Embodiment 73 The method of embodiment 72, wherein R 1 is selected from C2-C6 alkenyl, halo- substituted C2-C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl, alkylamino substituted C 2 -C 6 alkenyl, amino substituted C 2 -C 6 alkenyl, hydroxy substituted amino-C 2 -C 6 alkenyl, phenyl substituted amino-C2-C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C2-C6 alkynyl, alkoxy substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered
- Embodiment 74 The method of embodiment 73, wherein R 1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3-trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, alkoxypropenyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, 3-amino-4-hydroxy-butenyl, 3-amino-4- phenyl-butenyl, dialkylaminobutenyl, alkylaminobutenyl, aminobutenyl, dialkylaminopentenyl, alkylaminopentenyl, aminopentenyl, aminopropynyl, dialkylaminopropynyl, alkylaminopropynyl, methoxypropynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl
- R 1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3-trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, methoxypropenyl, ethoxypropenyl, aminopropenyl, 3-amino-butenyl, 3-methylamino-butenyl, 3-amino-4- hydroxy-butenyl, 3-methylamino-4-methoxy-butenyl, 3-amino-4-phenyl-butenyl, 3-amino-pentenyl, aminopropynyl, methoxypropynyl, dimethylaminopropenyl, di(d1,d2,d3-methyl)aminopropenyl, diethylaminopropenyl, 3-(N,N-dimethylamino)-3-phenyl-propenyl, 3-(N,N-
- R 6 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, propynyl, 1-hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2- hydroxypropyl, 2-hydroxy-2-methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, carboxypropyl, methoxycarbonylmethyl, ethoxycarbonylmethyl, ethoxycarbonylethyl, ethoxycarbonylpropyl, dimethylaminocarbonylmethyl, dimethylaminocarbonyl-1- ethyl, methylaminocarbonyl-1-ethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, 1-aminocarbonylethyl,
- Embodiment 80 The method of embodiment 72, wherein R 10 is selected from H, fluoro, methylcarbonylamino, chloro, amino, hydroxy, methyl, difluoromethyl, methylsulfonylamino, and methylaminocarbonylamino; [0357] Embodiment 81.
- R 11 is H or fluoro;
- Embodiment 82 The method of embodiment 72, wherein R 6 is ethyl; R 7 is trifluoromethyl; and R 8 is H; [0359] Embodiment 83.
- Embodiment 72 wherein R 6 is methyl; R 7 is trifluoromethyl; and R 8 is H; [0360] Embodiment 84.
- R 1 is substituted ethenyl;.
- Embodiment 87 The method of embodiment 72, wherein R 1 is dimethylaminopropenyl;.
- Embodiment 88 The method of embodiment 72, wherein R 1 is methylaminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 89 The method of embodiment 72, wherein R 1 is 3,3-difluoropropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 90 Embodiment 90.
- Embodiment 91 The method of embodiment 72, wherein R 1 is 2-(2-methyl-pyrrolidiny-5- yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 92 Embodiment 92.
- Embodiment 93 The method of embodiment 72, wherein R 1 is ethylaminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 94 Embodiment 94.
- Embodiment 95 The method of embodiment 72, wherein R 1 is 2-(3-fluoropyrrolidiny-5- yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 96 Embodiment 96.
- Embodiment 97 The method of embodiment 72, wherein R 1 is aminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 98 The method of embodiment 72, wherein R 9 is fluoro; R 10 is H; and R 11 is H; an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
- Embodiment 99 The method of any one of embodiments 1-34 wherein the compound is a compound of Table A.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present disclosure describes methods of treating a subject suffering from cancer using a combination therapy comprising (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation.
Description
COMBINATION THERAPY FOR TREATMENT OF CANCER BACKGROUND [001] SUMOylation is a reversible post-translational modification by small ubiquitin-like modifier (SUMO). SUMOylation controls many cellular functions and plays a critical role in the regulation of genome integrity, cell cycle progression, and the immune response. Three different enzyme components are involved in the SUMOylation cascade including a SUMO-activating enzyme E1 (SUMO E1 or SAE1/UBA2), an E2 enzyme (ubiquitin-conjugating enzyme 9 (UBC9)), and a limited set of E3 ligases. The process of SUMOylation starts when SUMO E1 activates SUMO through ATP hydrolysis and forms a thioester conjugate with SUMO. Next, SUMO is transferred and forms a new thioester conjugate with E2. Finally, SUMO is attached to target proteins, a step catalyzed by an E3 ligase. Specific small molecule inhibitors of the SUMO E1 enzyme have recently been developed that allow for the exploration of pharmacological SUMOylation inhibitors as novel anticancer therapeutics. Inhibition of SUMOylation by small molecules impairs cancer cell proliferation and triggers antitumor immune responses by stimulating the interferon (IFN) response. An allosteric covalent mechanism to inhibit SUMO E1 has been characterized, lending rationale towards the development of other novel pharmacological agents to block SUMOylation. [002] While many cancer therapeutics have shown efficacy as single agent treatments, combination therapies offer potentially superior outcomes due to synergistic effects. SUMMARY [003] Provided herein is a method of treating cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation. In some embodiments, a compound of the disclosure comprises a substituted 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation. In some embodiments, the compound binds to Uba2. In some embodiments, the compound has an inhibitory effect on activating enzymes (E1) of SUMO. [004] Disclosed herein is a method comprising: a) administering a therapeutically-effective amount of a compound to a subject in need thereof, wherein the compound blocks SUMOylation in the subject; and b) after the administering, observing that the administering increases an immune response in the subject against a cancer. BRIEF DESCRIPTION OF THE FIGURES [005] FIG.1 Tumor Growth Curve in the Mice in Different Groups. Data points represent means of each group, and error bars represent standard errors of the mean (SEM, n=8). Group 1: [small circle] Vehicle control p.o, (BIWx2weeks) Group 2: [square] Compound A 50 mg/kg, p.o. (BIWx2weeks) Group 3: [triangle] rituximab 20 mg/kg, i.p. (BIWx2weeks) Group 4: [diamond] Compound A, 50 mg/kg, p.o.+ Rituximab, 20 mg/kg,i.p. (BIWx2weeks) Group 5: [large circle] Compound A, 50 mg/kg, p.o. (QDx2weeks). The y-axis measures tumor volume (mm3) for each group of animals per length of the
experiment in days. [006] FIG.2 The Relative Change of Body Weights of the Mice in Different Groups (D1~D17). Data points represent mean body weights of each group. Error bars represent standard errors of the mean (SEM, n=8). Group 1: [small circle] Vehicle control p.o, (BIWx2weeks) Group 2: [square] Compound A 50 mg/kg, p.o. (BIWx2weeks) Group 3: [triangle] rituximab 20 mg/kg, i.p. (BIWx2weeks) Group 4: [diamond] Compound A, 50 mg/kg, p.o.+ rituximab, 20 mg/kg,i.p. (BIWx2weeks) Group 5: [large circle] Compound A, 50 mg/kg, p.o. (QDx2weeks). The y-axis reflects the RCBW for each group of animals in percent change versus vehicle control per length of experiment in days. [007] FIG.3 Effect upon combination treatment with Compound A and decitabine in AML cell lines. GDM-1 and MV4-11 cells were treated with indicated doses of Compound A and decitabine for 72-hrs, and cell viability was measured to generate dose response curves. IC50 values were calculated from treatment with Compound A alone, and in combination with decitabine, and these values were used to measure ZIP scores, see Table 4. Dose response graphs and IC50 calculations were generated using GraphPad Prism 9 (ver.9.4.0). ZIP scores were calculated using SynergyFinder 3.0 (https://synergyfinder.fim m.fi/synergy/20220701215205193938/). [008] FIG.4 Combination of Compound A with anti-PD-1 therapy Inhibits TumorGrowth in a CT26 model of syngeneic colon cancer in BALB/c mice. Data points represent tumor volume curves for four treatment groups. Group 1: [small circle] Vehicle control. Group 2: [triangle] Compound A 50 mg/kg, Group 3: [square] antiPD-12.5 mg/kg, Group 4: [triangle] Compound A, 50 mg/kg, + antiPD-12.5 mg/kg The y-axis reflects the tumor volumes in mm2 for each group of animals per length of experiment - 15 days [x-axis]. [009] FIG 5 Combination of Compound A with anti-PD-1 therapy Improves Survival in a CT26 model of syngeneic colon cancer in BALB/c mice. Data points represent survival for four treatment groups. Group 1: [small circle] Vehicle control. Group 2: [triangle] Compound A 50 mg/kg, Group 3: [square] antiPD-12.5 mg/kg, Group 4: [triangle] Compound A, 50 mg/kg, + antiPD-12.5 mg/kg The y-axis reflects the number of mice surviving as a percentage of the total in each group of animals per length of experiment - 80 days [x-axis]. [010] FIG 6. Compound A Induces CD8+ T Cell Infiltration in a CT26 model of syngeneic colon cancer in BALB/c mice. The bar graph represents the percent of CD8+ as a percent of CD45/CD3 for the treatment groups - Column 1: Vehicle control. Column 2: Compound A at 50 mg/kg. DETAILED DESCRIPTION [011] The present disclosure provides compounds and methods for blocking SUMOylation. The combinations of the present disclosure include SUMO inhibitors and at least one other anti-cancer agent that functions through a pathway other than SUMOylation. The SUMO inhibitors of the present disclosure bind to Uba2. The SUMO inhibitors of the present disclosure have an inhibitory effect on activating enzymes (E1) of SUMO. [012] Disclosed here in is a method comprising: a) administering a therapeutically-effective amount of a compound to a subject in need thereof, wherein the compound blocks SUMOylation in the subject;
and b) after the administering, observing that the administering increases an immune response in the subject against a cancer. In some embodiments, the method comprises after the administering, obtaining a population of an immune cell in a biological sample obtained from the subject. In some embodiments, the biological sample is obtained from a tumor. In some embodiments, the administering increases a population of tumor infiltrating lymphocytes. In some embodiments, the administering increases a population of CD8+ cells. In some embodiments, the population of the immune cell is determined using a multiplexed immunofluorescence. In some embodiments, the population of the immune cell is determined using flow cytometry. In some embodiments, the population of the immune cell is determined using fluorescence activated cell sorting [FACS]. In some embodiments, the population of the immune cell is determined using antibody staining. [013] The disclosure further provides methods of treatment of a cancerous lesion or a tumor harboring a SUMOylation. [014] Cancer is a collection of related diseases characterized by uncontrolled proliferation of cells with the potential to metastasize throughout the body. Cancer can be classified into five broad categories including, for example: carcinomas, which can arise from cells that cover internal and external parts of the body such as the lung, breast, and colon; sarcomas, which can arise from cells that are located in bone, cartilage, fat, connective tissue, muscle, and other supportive tissues; lymphomas, which can arise in the lymph nodes and immune system tissues; leukemia, which can arise in the bone marrow and accumulate in the bloodstream; and adenomas, which can arise in the thyroid, the pituitary gland, the adrenal gland, and other glandular tissues. [015] Although different cancers can develop in virtually any of the body's tissues, and contain unique features, the basic processes that cause cancer can be similar in all forms of the disease. Cancer begins when a cell breaks free from the normal restraints on cell division and begins to grow and divide out of control. MECHANISM. [016] SUMOylation is a reversible post-translational modification by small ubiquitin-like modifier (SUMO). SUMOylation controls many cellular functions and plays a critical role in the regulation of genome integrity, cell cycle progression, and the immune response (1). Three different enzyme components are involved in the SUMOylation cascade including a SUMO-activating enzyme E1 (SUMO E1 or SAE1/UBA2), an E2 enzyme (ubiquitin-conjugating enzyme 9 (UBC9)), and a limited set of E3 ligases. The process of SUMOylation starts when SUMO E1 activates SUMO through ATP hydrolysis and forms a thioester conjugate with SUMO. Next, SUMO is transferred and forms a new thioester conjugate with E2. Finally, SUMO is attached to target proteins, a step catalyzed by an E3 ligase. Specific small molecule inhibitors of the SUMO E1 enzyme have recently been developed that allow for the exploration of pharmacological SUMOylation inhibitors as novel anticancer therapeutics. Inhibition of SUMOylation by small molecules impairs cancer cell proliferation and triggers antitumor immune responses by stimulating the interferon (IFN) response (2). An allosteric covalent mechanism to inhibit
SUMO E1 was recently characterized (3), lending rationale towards the development of other novel pharmacological agents to block SUMOylation. [017] One embodiment of the disclosure includes compounds that block SUMOylation when used in combination with other treatments for cancer. Mechanism of compounds disclosed herein. [018] To determine the ability of a compound of the disclosure to blocks SUMOylation, assays can be employed to detect, for example, binding of a compound to Uba2, or an inhibitory effect on activating enzymes (E1) of SUMO. Such assays are described in PCT application PCT/US22/73985. [019] A compound of the disclosure can increase the inhibit SUMOylation by at least or up to about 0.1%, at least or up to about 0.2%, at least or up to about 0.3%, at least or up to about 0.4%, at least or up to about 0.5%, at least or up to about 0.6%, at least or up to about 0.7%, at least or up to about 0.8%, at least or up to about 0.9%, at least or up to about 1%, at least or up to about 2%, at least or up to about 3%, at least or up to about 4%, at least or up to about 5%, at least or up to about 6%, at least or up to about 7%, at least or up to about 8%, at least or up to about 9%, at least or up to about 10%, at least or up to about 11%, at least or up to about 12%, at least or up to about 13%, at least or up to about 14%, at least or up to about 15%, at least or up to about 16%, at least or up to about 17%, at least or up to about 18%, at least or up to about 19%, at least or up to about 20%, at least or up to about 21%, at least or up to about 22%, at least or up to about 23%, at least or up to about 24%, at least or up to about 25%, at least or up to about 26%, at least or up to about 27%, at least or up to about 28%, at least or up to about 29%, at least or up to about 30%, at least or up to about 31%, at least or up to about 32%, at least or up to about 33%, at least or up to about 34%, at least or up to about 35%, at least or up to about 36%, at least or up to about 37%, at least or up to about 38%, at least or up to about 39%, at least or up to about 40%, at least or up to about 41%, at least or up to about 42%, at least or up to about 43%, at least or up to about 44%, at least or up to about 45%, at least or up to about 46%, at least or up to about 47%, at least or up to about 48%, at least or up to about 49%, at least or up to about 50%, at least or up to about 51%, at least or up to about 52%, at least or up to about 53%, at least or up to about 54%, at least or up to about 55%, at least or up to about 56%, at least or up to about 57%, at least or up to about 58%, at least or up to about 59%, at least or up to about 60%, at least or up to about 61%, at least or up to about 62%, at least or up to about 63%, at least or up to about 64%, at least or up to about 65%, at least or up to about 66%, at least or up to about 67%, at least or up to about 68%, at least or up to about 69%, at least or up to about 70%, at least or up to about 71%, at least or up to about 72%, at least or up to about 73%, at least or up to about 74%, at least or up to about 75%, at least or up to about 76%, at least or up to about 77%, at least or up to about 78%, at least or up to about 79%, at least or up to about 80%, at least or up to about 81%, at least or up to about 82%, at least or up to about 83%, at least or up to about 84%, at least or up to about 85%, at least or up to about 86%, at least or up to about 87%, at least or up to about 88%, at least or up to about 89%, at least or up to about 90%, at least or up to about 91%, at least or up to about 92%, at least or up to about 93%, at least or up to about 94%, at least or up to about 95%, at least or up to about 96%, at least or up to about 97%, at
least or up to about 98%, at least or up to about 99%, at least or up to about 100%, at least or up to about 125%, at least or up to about 150%, at least or up to about 175%, at least or up to about 200%, at least or up to about 225%, or at least or up to about 250% as compared to the amount of SUMOylation in the absence of a compound of the disclosure. [020] A compound described herein can bind SUMO E1 enzyme that is, for example, by at least or up to about 0.1%, at least or up to about 0.2%, at least or up to about 0.3%, at least or up to about 0.4%, at least or up to about 0.5%, at least or up to about 0.6%, at least or up to about 0.7%, at least or up to about 0.8%, at least or up to about 0.9%, at least or up to about 1%, at least or up to about 2%, at least or up to about 3%, at least or up to about 4%, at least or up to about 5%, at least or up to about 6%, at least or up to about 7%, at least or up to about 8%, at least or up to about 9%, at least or up to about 10%, at least or up to about 11%, at least or up to about 12%, at least or up to about 13%, at least or up to about 14%, at least or up to about 15%, at least or up to about 16%, at least or up to about 17%, at least or up to about 18%, at least or up to about 19%, at least or up to about 20%, at least or up to about 21%, at least or up to about 22%, at least or up to about 23%, at least or up to about 24%, at least or up to about 25%, at least or up to about 26%, at least or up to about 27%, at least or up to about 28%, at least or up to about 29%, at least or up to about 30%, at least or up to about 31%, at least or up to about 32%, at least or up to about 33%, at least or up to about 34%, at least or up to about 35%, at least or up to about 36%, at least or up to about 37%, at least or up to about 38%, at least or up to about 39%, at least or up to about 40%, at least or up to about 41%, at least or up to about 42%, at least or up to about 43%, at least or up to about 44%, at least or up to about 45%, at least or up to about 46%, at least or up to about 47%, at least or up to about 48%, at least or up to about 49%, at least or up to about 50%, at least or up to about 51%, at least or up to about 52%, at least or up to about 53%, at least or up to about 54%, at least or up to about 55%, at least or up to about 56%, at least or up to about 57%, at least or up to about 58%, at least or up to about 59%, at least or up to about 60%, at least or up to about 61%, at least or up to about 62%, at least or up to about 63%, at least or up to about 64%, at least or up to about 65%, at least or up to about 66%, at least or up to about 67%, at least or up to about 68%, at least or up to about 69%, at least or up to about 70%, at least or up to about 71%, at least or up to about 72%, at least or up to about 73%, at least or up to about 74%, at least or up to about 75%, at least or up to about 76%, at least or up to about 77%, at least or up to about 78%, at least or up to about 79%, at least or up to about 80%, at least or up to about 81%, at least or up to about 82%, at least or up to about 83%, at least or up to about 84%, at least or up to about 85%, at least or up to about 86%, at least or up to about 87%, at least or up to about 88%, at least or up to about 89%, at least or up to about 90%, at least or up to about 91%, at least or up to about 92%, at least or up to about 93%, at least or up to about 94%, at least or up to about 95%, at least or up to about 96%, at least or up to about 97%, at least or up to about 98%, at least or up to about 99%, at least or up to about 100%. [021] A compound of the disclosure can be used, for example, to reduce cell proliferation or trigger antitumor immune response in a subject. Compounds of the disclosure. [022] In some embodiments, a compound of the disclosure comprises a substituted 4,5,6,7-
tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation. In some embodiments, the compound binds to Uba2. In some embodiments, the compound has an inhibitory effect on activating enzymes (E1) of SUMO. [023] In some embodiments, the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has a nitrile substituent at a 2-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group. The nitrile substituent is alternatively described as a cyano group. In some embodiments, the compound has plasma protein binding of less than about 99.5% or less than that of an analogous compound that lacks the cyano substituent. [024] In some embodiments, the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an electrophilic moiety at a 6-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group. In some embodiments, the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an unsubstituted or substituted C2-6 alkenylcarbonyl or an unsubstituted or substituted C2-6 alkynylcarbonyl substituent at a 6-position of the 4,5,6,7- tetrahydrothieno[2,3-c]pyridine group. In some embodiments, the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group has an unsubstituted or substituted aminopropenylcarbonyl or an unsubstituted or substituted aminobutenylcarbonyl substituent at a 6-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group. [025] In some embodiments, the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group comprises a substituted phenyl substituent at a 4-position of the 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group. In some embodiments, a substituted pyrazole group is attached to the phenyl substituent. [026] Non-limiting examples of compounds of the disclosure include compounds of the following formula:
wherein is a single bond or double bond; wherein l, m, n are each independently an integer from 0 to 2; wherein M is selected from CR3R4, NR5, C=O, O, S=O, O=S=O, and S; wherein Y is selected from CR6R7, NR8, C=O, O, S=O, O=S=O, and S; wherein Z is CR9, or N; wherein ring A is selected from a) 5- or 6-membered partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; wherein E is an electrophilic moiety, selected from:
; Wherein R1 is selected from hydrogen, halogen, -C(X1)3, -CHX12, -CH2X1, -OCX13, -OCH2X1, -OCHX12, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
Wherein R2 is selected from hydrogen, halogen, -CX2 3, -CHX2 2, -CH2X2, -OCX2 3, -OCH2X2, -OCHX22, -CN, -SOn2R2A, -SOv2NR2AR2B, -NHC(O)NR2AR2B, -N(O)m2, -NR2AR2B, -NHNR2AR2B, -C(O)R2A, -C(O)-OR2A, -C(O)NR2AR2B, -C(O)NHNR2AR2B, -OR2A, -NR2ASO2R2B, -NR2AC(O)R2B, -NR2AC(O)OR2B, -NR2AOR2B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R1 and R2 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; Wherein R3 is selected from hydrogen, halogen, -CX33, -CHX32, -CH2X3, -OCX33, -OCH2X3, -OCHX32, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; Wherein R5 is selected from hydrogen, halogen, -CX5 3, -CHX5 2, -CH2X5, -OCX5 3, -OCH2X5, -OCHX5 2, -CN, -SOn5R5A, -SOv5NR5AR5B, -NHC(O)NR5AR5B, -N(O)m5, -NR5AR5B, -NHNR5AR5B, -C(O)R5A, -C(O)-OR5A, -C(O)NR5AR5B, -C(O)NHNR5AR5B, -OR5A, -NR5ASO2R5B, -NR5AC(O)R5B, -NR5AC(O)OR5B, -NR5AOR5B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R6 is selected from hydrogen, halogen, -CX6 3, -CHX6 2, -CH2X6, -OCX6 3, -OCH2X6, -OCHX6 2, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B, -NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R7 is selected from hydrogen, halogen, -CX7 3, -CHX7 2, -CH2X7, -OCX7 3, -OCH2X7, -OCHX7 2, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7,
-NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R8 is selected from hydrogen, halogen, -CX83, -CHX82, -CH2X8, -OCX83, -OCH2X8, -OCHX82, -CN, -SOn8R8A, -SOv8NR8AR8B, -NHC(O)NR8AR8B, -N(O)m8, -NR8A87B, -NHNR8AR8B, -C(O)R8A, -C(O)-OR8A, -C(O)NR8AR8B, -C(O)NHNR8AR8B, -OR8A, -NR8ASO2R8B, -NR8AC(O)R8B, -NR8AC(O)OR8B, -NR8AOR8B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R10 is selected from hydrogen, halogen, -CX103, -CHX102, -CH2X10, -OCX103, -OCH2X10, -OCHX102, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R11 is selected from hydrogen, halogen, -CX11 3, -CHX11 2, -CH2X11, -OCX11 3, -OCH2X11, -OCHX11 2, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12, -NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B, -C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R13 is selected from hydrogen, halogen, -CX133, -CHX132, -CH2X13, -OCX133, -OCH2X13, -OCHX132, -CN, -SOn13R13A, -SOv13NR13AR13B, -NHC(O)NR13AR13B, -N(O)m13, -NR13AR13B, -NHNR13AR13B, -C(O)R13A, -C(O)-OR13A, -C(O)NR13AR13B, -C(O)NHNR13AR13B, -OR13A, -NR13ASO2R13B, -NR13AC(O)R13B, -NR13AC(O)OR13B, -NR13AOR13B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R14 is selected from hydrogen, halogen, -CX143, -CHX142, -CH2X14, -OCX143, -OCH2X14, -OCHX142, -CN, -SOn14R14A, -SOv14NR14AR14B, -NHC(O)NR14AR14B, -N(O)m14, -NR14AR14B, -NHNR14AR14B, -C(O)R14A, -C(O)-OR14A, -C(O)NR14AR14B, -C(O)NHNR14AR14B, -OR14A, -NR14ASO2R14B, -NR14AC(O)R14B, -NR14AC(O)OR14B, -NR14AOR14B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R15 is selected from hydrogen, halogen, -CX153, -CHX152, -CH2X15, -OCX153, -OCH2X15, -OCHX152, -CN, -SOn15R15A, -SOv15NR15AR15B, -NHC(O)NR15AR15B, -N(O)m15, -NR15AR15B, -NHNR15AR15B, -C(O)R15A, -C(O)-OR15A, -C(O)NR15AR15B, -C(O)NHNR15AR15B, -OR15A, -NR15ASO2R15B, -NR15AC(O)R15B, -NR15AC(O)OR15B, -NR15AOR15B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 is selected from hydrogen, halogen, -CX16 3, -CHX16 2, -CH2X16, -OCX16 3, -OCH2X16, -OCHX16 2, -CN, -SOn16R16A, -SOv16NR16AR16B, -NHC(O)NR16AR16B, -N(O)m16, -NR16AR16B, -NHNR16AR16B, -C(O)R16A, -C(O)-OR16A, -C(O)NR16AR16B, -C(O)NHNR16AR16B, -OR16A, -NR16ASO2R16B, -NR16AC(O)R16B, -NR16AC(O)OR16B, -NR16AOR16B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 is selected from hydrogen, halogen, -CX17 3, -CHX17 2, -CH2X17, -OCX17 3, -OCH2X17, -OCHX17 2, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R18 is selected from hydrogen, halogen, -CX18 3, -CHX18 2, -CH2X18, -OCX18 3, -OCH2X18, -OCHX182, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R19 is selected from hydrogen, halogen, -CX193, -CHX192, -CH2X19, -OCX193, -OCH2X19, -OCHX192, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 is selected from hydrogen, halogen, -CX203, -CHX202, -CH2X20, -OCX203, -OCH2X20, -OCHX202, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R20 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R21 is selected from hydrogen, halogen, -CX21 3, -CHX21 2, -CH2X21, -OCX21 3, -OCH2X21, -OCHX21 2, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R22 is selected from hydrogen, halogen, -CX22 3, -CHX22 2, -CH2X22, -OCX22 3, -OCH2X22, -OCHX22 2, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B, -NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 and R22 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
wherein R23 is selected from hydrogen, halogen, -CX23 3, -CHX23 2, -CH2X23, -OCX23 3, -OCH2X23, -OCHX232, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R24 is selected from hydrogen, halogen, -CX243, -CHX242, -CH2X24, -OCX243, -OCH2X24, -OCHX242, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R25 is selected from hydrogen, halogen, -CX253, -CHX252, -CH2X25, -OCX253, -OCH2X25, -OCHX252, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B, -C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R26 is selected from hydrogen, halogen, -CX26 3, -CHX26 2, -CH2X26, -OCX26 3, -OCH2X26, -OCHX26 2, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R27 is selected from hydrogen, halogen, -CX27 3, -CHX27 2, -CH2X27, -OCX27 3, -OCH2X27, -OCHX27 2, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B, -C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R28 is selected from hydrogen, halogen, -CX28 3, -CHX28 2, -CH2X28, -OCX28 3, -OCH2X28, -OCHX28 2, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B,
-C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R29 is selected from hydrogen, halogen, -CX293, -CHX292, -CH2X29, -OCX293, -OCH2X29, -OCHX292, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B, -C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R30 is selected from hydrogen, halogen, -CX303, -CHX302, -CH2X30, -OCX303, -OCH2X30, -OCHX302, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR30B, - N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R2A, R2B, R3A, R3B, R4A, R4B, R5A, R5B, R6A, R6B, R7A, R7B, R8A, R8B, R9A, R9B, R10A, R10B, R11A, R11B, R12A, R12B, R13A, R13B, R14A, R14B, R15A, R15B, R16A, R16B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B, R28A, R28B, R29A, R29B, R30A, R30B is independently selected from hydrogen, -CX3, -CHX2, - CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, ^NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; wherein R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5A and R5B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the
same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R8A and R8B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R10A and R10B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R11A and R11B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R12A and R12B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to
the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; Wherein each m1, m2, m3, m4, m5, m6, m7, m8, m9, m10, m11, m12, m13, m14, m15, m16, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, and m30 are independently 1 or 2; wherein each v1, v2, v3, v4, v5, v6, v7, v8, v9, v10, v11, v12, v13, v14, v15, v16, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29 and v30 are independently 1 or 2; wherein each n1, n2, n3, n4, n5, n6, n7, n8, n9, n10, n11, n12, n13, n14, n15, n16, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29 and n30 are independently an integer from 0 to 2; and wherein each X, X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12, X13, X14, X15, X16, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29 and X30 are independently -Cl, -Br, -I or –F. [027] In some embodiments, l is 1; m is 0; and n is 1. [028] In some embodiments, Z is N. [029] In some embodiments, ring A is selected from 5-membered heteroaryl, wherein ring A is optionally substituted with one or more substituent groups. [030] In some embodiments, R2 is selected from substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. [031] In some embodiments, ring A is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein ring A is optionally substituted with one or more substituent groups. [032] In some embodiments, ring A is selected from
. [033] In some embodiments, R2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cycloalkyl and substituted or unsubstituted 5- or 6-membered heteroaryl. [034] In some embodiments, R2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cyclopentyl, substituted or unsubstituted cyclohexyl and substituted or unsubstituted pyridyl. [035] In some embodiments,
[036] In some embodiments, E is selected from -C(=O)CH=CH2, -C(=O)-ethynyl, -C(=O)CH=CHCF3, -C(=O)CH=CHCHF2, -C(=O)CH=CHCH2F, 4-(dimethylamino)but-2-en-1-one and -C(=O)CH2Br. [037] In some embodiments, M is CR3R4. [038] In some embodiments, Y is CR6R7. [039] Non-limiting examples of compounds of the disclosure include compounds of the following formula
wherein Z is N, or CR9; W is selected from NR12, O, S, S=O, O=S=O, and Se; ring B is selected from a) 5- or 6-membered cycloalkyl, saturated or partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; E is selected from an electrophilic moiety, selected from
; R1 is selected from hydrogen, halogen, -CX13, -CHX12, -CH2X1, -OCX13, -OCH2X1, -OCHX12, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, rand substituted or unsubstituted heteroaryl; R3 is selected from hydrogen, halogen, -CX33, -CHX32, -CH2X3, -OCX33, -OCH2X3, -OCHX32, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R6 is selected from hydrogen, halogen, -CX63, -CHX62, -CH2X6, -OCX63, -OCH2X6, -OCHX62, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B, -NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl,
substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R7 is selected from hydrogen, halogen, -CX73, -CHX72, -CH2X7, -OCX73, -OCH2X7, -OCHX72, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7, -NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R10 is selected from hydrogen, halogen, -CX103, -CHX102, -CH2X10, -OCX103, -OCH2X10, -OCHX102, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R11 is selected from hydrogen, halogen, -CX11 3, -CHX11 2, -CH2X11, -OCX11 3, -OCH2X11, -OCHX11 2, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12, -NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B, -C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R17 is selected from hydrogen, halogen, -CX17 3, -CHX17 2, -CH2X17, -OCX17 3, -OCH2X17, -OCHX172, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R18 is selected from hydrogen, halogen, -CX183, -CHX182, -CH2X18, -OCX183, -OCH2X18, -OCHX182, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R19 is selected from hydrogen, halogen, -CX193, -CHX192, -CH2X19, -OCX193, -OCH2X19, -OCHX192, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R19 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R20 is selected from hydrogen, halogen, -CX20 3, -CHX20 2, -CH2X20, -OCX20 3, -OCH2X20, -OCHX20 2, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 is selected from hydrogen, halogen, -CX21 3, -CHX21 2, -CH2X21, -OCX21 3, -OCH2X21, -OCHX21 2, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 and R21 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
R22 is selected from hydrogen, halogen, -CX22 3, -CHX22 2, -CH2X22, -OCX22 3, -OCH2X22, -OCHX222, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B, -NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R23 is selected from hydrogen, halogen, -CX233, -CHX232, -CH2X23, -OCX233, -OCH2X23, -OCHX232, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R24 is selected from hydrogen, halogen, -CX243, -CHX242, -CH2X24, -OCX243, -OCH2X24, -OCHX242, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R25 is selected from hydrogen, halogen, -CX25 3, -CHX25 2, -CH2X25, -OCX25 3, -OCH2X25, -OCHX25 2, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B, -C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R26 is selected from hydrogen, halogen, -CX26 3, -CHX26 2, -CH2X26, -OCX26 3, -OCH2X26, -OCHX26 2, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R27 is selected from hydrogen, halogen, -CX27 3, -CHX27 2, -CH2X27, -OCX27 3, -OCH2X27, -OCHX27 2, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B,
-C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R28 is selected from hydrogen, halogen, -CX283, -CHX282, -CH2X28, -OCX283, -OCH2X28, -OCHX282, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B, -C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R29 is selected from hydrogen, halogen, -CX293, -CHX292, -CH2X29, -OCX293, -OCH2X29, -OCHX292, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B, -C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R30 is selected from hydrogen, halogen, -CX303, -CHX302, -CH2X30, -OCX303, -OCH2X30, -OCHX302, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR30B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R31 is selected from hydrogen, halogen, -CX31 3, -CHX31 2, -CH2X31, -OCX31 3, -OCH2X31, -OCHX31 2, -CN, -SOn31R31A, -SOv31NR31AR31B, -NHC(O)NR31AR31B, -N(O)m31, -NR31AR31B, -NHNR31AR31B, -C(O)R31A, -C(O)-OR31A, -C(O)NR31AR31B, -C(O)NHNR31AR31B, -OR31A, -NR31ASO2R31B, -NR31AC(O)R31B, -NR31AC(O)OR31B, -NR31AOR31B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R32 is selected from hydrogen, halogen, -CX32 3, -CHX32 2, -CH2X32, -OCX32 3, -OCH2X32, -OCHX32 2, -CN, -SOn32R32A, -SOv32NR32AR32B, -NHC(O)NR32AR32B, -N(O)m32, -NR32AR32B, -NHNR32AR32B, -C(O)R32A, -C(O)-OR32A, -C(O)NR32AR32B, -C(O)NHNR32AR32B, -OR32A, -NR32ASO2R32B, -NR32AC(O)R32B, -NR32AC(O)OR32B, -NR32AOR32B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted
or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R33 is selected from hydrogen, halogen, -CX333, -CHX332, -CH2X33, -OCX333, -OCH2X33, -OCHX332, -CN, -SOn33R33A, -SOv33NR33AR33B, -NHC(O)NR33AR33B, -N(O)m33, -NR33AR33B, -NHNR33AR33B, -C(O)R33A, -C(O)-OR33A, -C(O)NR33AR33B, -C(O)NHNR33AR33B, -OR33A, -NR33ASO2R33B, -NR33AC(O)R33B, -NR33AC(O)OR33B, -NR33AOR33B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R34 is selected from hydrogen, halogen, -CX343, -CHX342, -CH2X34, -OCX343, -OCH2X34, -OCHX342, -CN, -SOn34R34A, -SOv34NR34AR34B, -NHC(O)NR34AR34B, -N(O)m34, -NR34AR34B, -NHNR34AR34B, -C(O)R34A, -C(O)-OR34A, -C(O)NR34AR34B, -C(O)NHNR34AR34B, -OR34A, -NR34ASO2R34B, -NR34AC(O)R34B, -NR34AC(O)OR34B, -NR34AOR34B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R35 is selected from hydrogen, halogen, -CX353, -CHX352, -CH2X35, -OCX353, -OCH2X35, -OCHX352, -CN, -SOn35R35A, -SOv35NR35AR35B, -NHC(O)NR35AR35B, -N(O)m35, -NR35AR35B, -NHNR35AR35B, -C(O)R35A, -C(O)-OR35A, -C(O)NR35AR35B, -C(O)NHNR35AR35B, -OR35A, -NR35ASO2R35B, -NR35AC(O)R35B, -NR35AC(O)OR35B, -NR35AOR35B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R3A, R3B, R4A, R4B, R6A, R6B, R7A, R7B, R9A, R9B, R10A, R10B, R11A, R11B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B, R28A, R28B, R29A, R29B, R30A, R30B, R31A, R31B, R32A, R32B, R33A, R33B, R34A, R34B, R35A, R35B, is independently selected from hydrogen, -CX3, -CHX2, -CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, -NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may
optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl;
Each m1, m3, m4, m6, m7, m9, m10, m11, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, m30, m31, m32, m33, m34 and m35 is independently 1 or 2; Each v1, v3, v4, v6, v7, v9, v10, v11, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29, v30, v31, v32, v33, v34 and v35 is independently 1 or 2; Each n1, n3, n4, n6, n7, n9, n10, n11, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29, n30, n31, n32, n33, n34 and n35 is independently an integer from 0 to 2; and Each X1, X3, X4, X6, X7, X9, X10, X11, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29, X30, X31, X32, X33, X34, and X35 is independently -Cl, -Br, -I or –F. [040] In some embodiments, ring B is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein Ring B is optionally substituted with one or more substituents. [041] In some embodiments, ring B is selected from phenyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, and triazinyl, wherein ring B is optionally substituted with one or more substituent groups. [042] In some embodiments, ring B is selected from
[044] In some embodiments, E is selected from -C(=O)CH=CH2, -C(=O)-ethynyl, -C(=O)CH=CHCF3, -C(=O)CH=CHCHF2, -C(=O)CH=CHCH2F, 4-(dimethylamino)but-2-en-1-one and -C(=O)CH2Br. [045] In some embodiments, W is S. [046] In some embodiments, R10 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl; and R11 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl. [047] Non-limiting examples of compounds of the disclosure include compounds of the following
formula
wherein R36 is H, halo, C1-C4 alkyl, C2-C4 alkenyl or C3-C6 cycloalkyl; R37 is substituted or unsubstituted C2-C4 alkenyl or C2-C4 alkynyl; and R38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl, substituted or unsubstituted nitrogen containing 5- or 6- membered partially unsaturated heterocyclyl and substituted or unsubstituted nitrogen containing 6-10 membered heteroaryl; provided R38 is not 4-pyridyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof. [048] In some embodiments, R38 is selected from substituted or unsubstituted nitrogen containing 5- membered heteroaryl selected from pyrazolyl, isoxazolyl, isothiazolyl, pyrrolyl, thiazolyl, triazolyl and imidazolyl; substituted or unsubstituted nitrogen containing 6- membered heteroaryl selected from pyridinyl, pyrimidinyl and pyrazinyl; substituted or unsubstituted nitrogen containing 5-membered partially unsaturated heterocyclyl selected from pyrrolinyl, and imidazolidinyl; and substituted or unsubstituted dihydropyridinyl. [049] In some embodiments, R36 is selected from chloro, methyl, ethyl, isopropyl, allylyl, and cyclopropyl;. [050] In some embodiments, R38 is selected from substituted 5-pyrazolyl, substituted 4-pyrazolyl, substituted 1-pyrazolyl, substituted or unsubstituted 4-isoxazolyl, substituted or unsubstituted 4- isothiazolyl, substituted or unsubstituted 3-pyrrolyl, substituted or unsubstituted 5-thiazolyl, substituted or unsubstituted 5-imidazolyl, substituted or unsubstituted 1-imidazolyl, substituted or unsubstituted [1,2,4]triazol-5-yl, substituted or unsubstituted 3-pyridyl, and substituted or unsubstituted 5-pyrimidinyl. [051] In some embodiments, R38 is selected from 3-trifluoromethyl-pyrazol-4-yl, 1-isopropyl-3- trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-trifluoromethyl-pyrazol-4-yl, 1-methyl-3-difluoromethyl-pyrazol- 4-yl, 1-butyl-3-trifluoromethyl-pyrazol-4-yl, 1-methoxymethyl-3-trifluoromethyl-pyrazol-4-yl, 1-propyl- 3-trifluoromethyl-pyrazol-4-yl, 1,3-dimethyl-pyrazol-4-yl, 1,3,5-trimethyl-pyrazol-4-yl, 1-methyl-3- cyclopropyl-pyrazol-4-yl, 1-methyl-3-trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-amino-pyrazol-4-yl, 1-ethyl- 3-methoxy-pyrazol-4-yl, 1-hydroxyethyl-3-trifluoromethyl-pyrazol-4-yl, 1-[2-hydroxypropyl]-3- trifluoromethyl-pyrazol-4-yl, 1-[2-hydroxyisobutyl]-3-trifluoromethyl-pyrazol-4-yl, 1-methoxyethyl-3- trifluoromethyl-pyrazol-4-yl, 1-[N,N-dimethylaminoethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-[N- methylaminocarbonylmethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-[N-methylaminocarbonylethyl]-3-
trifluoromethyl-pyrazol-4-yl, 1-[N,N-dimethylaminocarbonylmethyl]-3-trifluoromethyl-pyrazol-4-yl, 1- aminocarbonylmethyl-3-trifluoromethyl-pyrazol-4-yl, 1-methylcarbonylaminoethyl-3-trifluoromethyl- pyrazol-4-yl, 1-methylcarbonylaminobutyl-3-trifluoromethyl-pyrazol-4-yl, 1-aminocarbonylethyl-3- trifluoromethyl-pyrazol-4-yl, 1-aminocarbonylpropyl-3-trifluoromethyl-pyrazol-4-yl, 1- aminocarbonylisopropyl-3-trifluoromethyl-pyrazol-4-yl, 1-cyanopropyl-3-trifluoromethyl-pyrazol-4-yl, 1- [N,N-dimethylaminocarbonylethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-carboxyethyl]-3-trifluoromethyl- pyrazol-4-yl, 1-carboxymethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-methoxycarbonylmethyl]-3- trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-carboxy-pyrazol-4-yl, 1-ethyl-5-carboxy-pyrazol-4-yl, 1-ethyl-3- methylaminocarbonyl-pyrazol-4-yl, 1-ethyl-3-[N,N-dimethylaminocarbonyl]-pyrazol-4-yl, 1-ethyl-5- methylaminocarbonyl-pyrazol-4-yl, 1-ethyl-5-[N,N-dimethylaminocarbonyl]-pyrazol-4-yl, 1-benzyl-3- methyl-pyrazol-4-yl, 1-(cyclopropylmethyl)-3-trifluoromethyl-pyrazol-4-yl, 1-cyclopropyl-3- trifluoromethyl-pyrazol-4-yl, 1-[1-methylazetidin-3-yl]-3-trifluoromethyl-pyrazol-4-yl, 1-[1- methylpyrrolidin-3-yl]-3-trifluoromethyl-pyrazol-4-yl, 1-[1-methylpiperidin-3-yl]-3-trifluoromethyl- pyrazol-4-yl, 1-[1-methylpiperidin-4-yl]-3-trifluoromethyl-pyrazol-4-yl, 1-(3-pyridinylmethyl)-3-methyl- pyrazol-4-yl, 1-(2-pyridinylmethyl)-3-methyl-pyrazol-4-yl, 1-[2-pyridyl]-3-methyl-pyrazol-4-yl, 1- methyl-5-pyrazolyl, 1-ethyl-5-trifluoromethylpyrazol-4-yl, 1,3-dimethyl-5-pyrazolyl, 1-methyl-3- cyclopropyl-pyrazol-5-yl, 1-methyl-4-pyrazolyl, 1-ethyl-3-methylpyrazol-4-yl, 1,5-dimethyl-4-pyrazolyl, 1,3,5-trimethyl-4-pyrazolyl, 1-methyl-3-pyrazolyl, 4-methyl-3-pyrazolyl, 1-methyl-[1,2,4]triazol-3-yl, 4- bromo-2-methyl-[1,2,4]triazol-5-yl, 4-bromo-2-ethyl-[1,2,4]triazol-5-yl, 1-methyl-[1,2,4]triazol-5-yl, 4- isothiazolyl, 4-methyl-2-oxazolyl, isoxazol-4-yl, 2,4-dimethylthiazol-5-yl, 3,5-dimethylisoxazol-4-yl, 2- methyl-5-thiazolyl and 4-methyl-5-thiazolyl. [052] In some embodiments, R38 is selected from 5-pyrimidinyl, 2-methyl-5-pyrimidinyl, 4-methyl-5- pyrimidinyl, 4,6-dimethoxy-5-pyrimidinyl, 4,6-dimethyl-5-pyrimidinyl, 4-triflouromethyl-5-pyrimidinyl, 4-pyrimidinyl, 2-methyl-4-pyrimidinyl, 4-methyl-6-pyrimidinyl, 2,4-dimethyl-6-pyrimidinyl, 3-pyridinyl, 2-pyridinyl, 4-methyl-2-pyridinyl, 2-triflouromethyl-3-pyridinyl, 4-triflouromethyl-3-pyridinyl, 2-methyl- 3-pyridinyl, 2,5-dimethyl-3-pyridinyl, 2,6-dimethyl-3-pyridinyl, 2,4-dimethyl-3-pyridinyl, 2-ethyl-3- pyridinyl, 5-methyl-3-pyridinyl, 2-ethoxy-3-pyridinyl, 2-ethoxy-5-methyl-3-pyridinyl, 2-methoxy-3- pyridinyl, 2-methoxy-6-methyl-3-pyridinyl, 2-ethoxy-6-methyl-3-pyridinyl, 2-isopropoxy-3-pyridinyl, 2- (3-pentoxy)-3-pyridinyl, 2-methoxyethoxy-3-pyridinyl, 2-cyclopropoxy-3-pyridinyl, 2-cyclopentyloxy-3- pyridinyl, 2-cyclohexloxy-3-pyridinyl, 2-fluoro-3-pyridinyl, 2-chloro-3-pyridinyl, 2-phenyl-3-pyridinyl, 2-flouro-3-methyl-5-pyridinyl, 2-flouro-3-chloro-5-pyridinyl, 3-fluoro-5-pyridinyl, 3-chloro-5-pyridinyl, 2-chloro-4-methyl-5-pyridinyl, 2-methoxy-5-pyridinyl, 3-methoxy-5-pyridinyl, 2-ethoxy-5-pyridinyl, 3- ethoxy-5-pyridinyl, 3-triflouromethyl-5-pyridinyl, 3-ethyl-5-pyridinyl, 2,3-dimethyl-5-pyridinyl, 2-(2- hydroxymethylpyrrolidin-1-yl)-5-pyridinyl, 2-(morpholin-1-yl)-3-chloro-5-pyridinyl, 2- (dimethylaminoethoxy)-5-pyridinyl, 2-(2-dimethylaminomethylpyrrolidin-1-yl)-5-pyridinyl, 2-phenyl-5- pyridinyl, 2-methyl-6-pyridinyl, 2,4-dimethyl-6-pyridinyl and 2-ethyl-6-pyridinyl.
[053] In some embodiments, R38 is selected from substituted or unsubstituted tetrahydroquinolinyl, substituted or unsubstituted 1-pyrrolin-3-yl, substituted or unsubstituted 1-imidazolidinyl, and substituted or unsubstituted dihydropyridin-3-yl. [054] In some embodiments, R38 is selected from 4-isoquinolinyl, 1-methoxy-4-isoquinolinyl, 1-chloro- 4-isoquinolinyl, 6-methyl-4-isoquinolinyl, 1-oxo-2-methyl-4-isoquinolinyl, 3-quinolinyl, 4-quinolinyl, 5- pyrrolopyridinyl, [1,3,3a]-triazainden-5-yl, 1-ethyl-3-pyrrolopyridinyl, and 1-methyl-5-pyrrolopyridinyl. [055] In some embodiments, R37 is selected from ethenyl, fluoroethenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl and nitrogen-containing heterocyclyl- propenyl. [056] Non-limiting examples of compounds of the disclosure include compounds of the following formula
wherein R36 is selected from H, halo, C1-C4 alkyl, C2-C4 alkenyl and C3-C6 cycloalkyl; R37 is substituted or unsubstituted C2-C4 alkenyl, or C2-C4 alkynyl; and R41 is selected from H, C1-6 alkyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1-6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy- C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1- 6 alkyl, aryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heteroaryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heterocyclyl and substituted or unsubstituted 5 or 6 membered heteroaryl; R42 is selected from H, carboxy, amino, C1-6 alkyl, C1-3 haloalkyl, C1-6 alkoxy, C1-6 alkylamino, C1-6 alkylaminocarbonyl, and C3-6 cycloalkyl; R43 is selected from H, carboxy, C1-4 alkyl, C1-3 haloalkyl, and C1-6 alkylaminocarbonyl; or an isomer or stereoisomer of any of the foregoing, and R44 is one or more substituents selected from H, halo, alkoxy and hydroxy; or a mixture thereof or a pharmaceutically acceptable salt thereof. [057] In one embodiment, R41 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, D3-methyl, 1- hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2-
methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, methoxycarbonylmethyl, dimethylaminocarbonylmethyl, dimethylaminocarbonylethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, aminocarbonyl-1,1-dimethylmethyl, methylcarbonylaminoethyl, 1-methylcarbonylamino-2.2- dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl, cyanoethyl, cyclopropyl, cyclopropylmethyl, benzyl, 3-pyridinylmethyl, 2-pyridinylmethyl, 4-oxazolylmethyl, oxadiazol-5-ylmethyl, 1-methylazetidin-3-yl, 1- methylpyrrolidin-3-yl, 1-methylpiperidin-4-yl, 1-methylpiperidin-3-yl, 3,5-difluoro-4-pyridyl, 3-chloro-4- pyridyl, 4-pyridyl and 2-pyridyl. [058] In one embodiment, R36 is H, chloro, methyl, ethyl, isopropyl, propenyl, trifluoromethyl, 2-furyl, 2-pyridinyl, or cyclopropyl. [059] In one embodiment, R37 is selected from ethenyl, fluoroethenyl, fluoropropenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethenyl and substituted or unsubstituted 3-7 membered nitrogen- containing heterocyclyl-propenyl. [060] In one embodiment, R37 is azetidin-1-ylpropenyl, 1-methylpyrrolidin-2-ylethenyl, dimethylaminopropenyl, methylaminopropenyl, piperidin-1-ylpropenyl, 4-hydroxy-piperidin-1-ylpropenyl, pyrrolidin-1-ylpropenyl, 3-hydroxypyrrolidin-1-ylpropenyl, morpholin-1-ylpropenyl, or aminopropenyl. [061] In one embodiment, R42 is selected from H, trifluoromethyl, difluoromethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy. methylaminocarbonyl, dimethylaminocarbonyl, and cyclopropyl. [062] In one embodiment, R43 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl. [063] In one embodiment, R44 is one or more substituents selected from H, hydroxy, fluoro and methoxyl. [064] Non-limiting examples of compounds of the disclosure include compounds of any one of the following formulae
wherein R1 is substituted or unsubstituted C2-C6 alkenyl, or substituted or unsubstituted C2-C6 alkynyl;
R6 is selected from H, C1-6 alkyl, C2-4 alkynyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1- 6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C1-6 haloalkyl, C1-6 alkylsulfonyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl, aryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heteroaryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heterocyclyl and substituted or unsubstituted 5 or 6 membered heteroaryl; R7 is selected from H, carboxy, amino, C1-6 alkyl, C1-3 haloalkyl, C1-6 alkoxy, C1-6 alkylamino, C1-6 alkylaminocarbonyl, and C3-6 cycloalkyl; R8 is selected from H, carboxy, cyano, C1-4 alkyl, C1-3 haloalkyl, and C1-6 alkylaminocarbonyl; R9 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; R10 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; and R11 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [065] In some embodiments, R1 is selected from C2-C6 alkenyl, halo-substituted C2-C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl, alkylamino substituted C2-C6 alkenyl, amino substituted C2-C6 alkenyl, hydroxy substituted amino-C2-C6 alkenyl, phenyl substituted amino-C2- C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C2-C6 alkynyl, alkoxy substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered cycloalkyl- substituted C2-C6 alkenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl- substituted C2-C6 alkenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl- substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C2-C6 alkenyl, and substituted or unsubstituted 3-7 membered cycloalkyl- substituted C2-C6 alkynyl . [066] In some embodiments, R1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3- trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, alkoxypropenyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, 3-amino-4-hydroxy-butenyl, 3-amino-4-phenyl-butenyl, dialkylaminobutenyl, alkylaminobutenyl, aminobutenyl, dialkylaminopentenyl, alkylaminopentenyl, aminopentenyl, aminopropynyl, dialkylaminopropynyl, alkylaminopropynyl, methoxypropynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propynyl, substituted or unsubstituted 3-7 membered cycloalkyl-ethenyl, substituted or unsubstituted 3-7 membered cycloalkyl-propenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propynyl, substituted or unsubstituted 3-7 membered oxygen-
containing heterocyclyl-ethenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl-propenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- ethenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propenyl, substituted or unsubstituted 3-7 membered cycloalkyl-ethynyl, substituted or unsubstituted 3-7 membered cycloalkyl- propynyl, substituted or unsubstituted 3-7 membered cycloalkyl-ethynyl, and substituted or unsubstituted 3-7 membered cycloalkyl-propynyl. [067] In some embodiments, R1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3- trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, methoxypropenyl, ethoxypropenyl, aminopropenyl, 3- amino-butenyl, 3-methylamino-butenyl, 3-amino-4-hydroxy-butenyl, 3-methylamino-4-methoxy-butenyl, 3-amino-4-phenyl-butenyl, 3-amino-pentenyl, aminopropynyl, methoxypropynyl, dimethylaminopropenyl, di(d1,d2,d3-methyl)aminopropenyl, diethylaminopropenyl, 3-(N,N-dimethylamino)-3-phenyl-propenyl, 3- (N,N-dimethylamino)-3-cyclopropyl-propenyl, (cyclopropylamino)propenyl, bicyclo[1.1.1]pent-1- ylamino, (1-methylcyclopropylamino)propenyl, (3-methyloxetan-3-yl)aminopropenyl, (3- methyltetrahydrofur-3-yl)aminopropenyl, (4-methyl-tetrahydropyran-4-yl)aminopropenyl, methylaminopropenyl, N-benzyl-N-methylaminopropenyl, N-(tert-butyl)aminopropenyl, N-sec- butylaminopropenyl, N-butylaminopropenyl, N-(isopropyl)aminopropenyl, N-(d2- isopropyl)aminopropenyl, ethylaminopropenyl, N-[3,3-difluorocyclobutyl]aminopropenyl, 1- hydroxymethyl-1-methyl-ethylaminopropenyl, 3-dimethylamino-butenyl, 3-(N-methylamino)-butenyl, methylaminobutenyl, N,N-dimethylaminobutenyl, piperidin-2-ylpropenyl, pyrrolidin-1-ylpropenyl, 3- methyl-oxetan-3-ylpropenyl, 4-methyl-tetrahydropyran-4-ylpropenyl, piperidin-2-ylethenyl, pyrrolidin-2- ylethenyl, azetidin-2-ylethenyl, morpholin-3-ylethenyl, 1-methylpyrrolidin-2-ylethenyl, 3- methylpyrrolidin-5-ylethenyl, 3-ethylpyrrolidin-5-ylethenyl, 2-methylpyrrolidin-5-ylethenyl, 2,2- dimethylpyrrolidin-5-ylethenyl, 3-methoxypyrrolidin-5-ylethenyl, 3-fluoropyrrolidin-5-ylethenyl, 3,3- difluoropyrrolidin-5-ylethenyl, 5-azaspiro[2.4]heptan-6-ylethenyl, 2-azabicyclo[3.1.0]hexan-3-ylethenyl, 3,3-dimethylpyrrolidin-5-ylethenyl, 3-methylpyrrolidin-1-ylpropenyl, 2-methylpyrrolidin-1-ylpropenyl, 1- methylcarbonylpyrrolidin-3-ylethenyl, 2-carboxypyrrolidin-1-ylpropenyl, 3-carboxypyrrolidin-1- ylpropenyl, tetrahydrofur-3-ylpropenyl, dimethylaminopropynyl, methylaminopropynyl, 2-amino-2- methylbutynyl, 2-(1-amino-cyclopropyl)-ethynyl, 2-(1-amino-cyclobutyl)-ethynyl, 2-(1-amino- cyclopentyl)-ethynyl, azetidin-2-ylethynyl, pyrrolidin-2-ylethynyl, pyrrolidin-3-ylethynyl, 2-methyl- pyrrolidin-2-ylethynyl, 4-methyl-piperazin-1-ylpropynyl, and piperidin-3-ylethynyl. [068] In some embodiments, R6 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, propynyl, 1- hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2- methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, carboxypropyl, methoxycarbonylmethyl, ethoxycarbonylmethyl, ethoxycarbonylethyl, ethoxycarbonylpropyl, dimethylaminocarbonylmethyl, dimethylaminocarbonyl-1-ethyl, methylaminocarbonyl-1-ethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, 1-aminocarbonylethyl, aminocarbonyl-1,1-dimethylmethyl,
methylcarbonylaminoethyl, 1-methylcarbonylamino-2,2-dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl, 2-cyanoethyl, 1-cyanoethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, methylsulfonyl, cyclopropyl, cyclopropylmethyl, benzyl, 4-pyridinylethyl, 2-pyridinylethyl, 3- pyridinylmethyl, 2-pyridinylmethyl, 4-oxazolylmethyl, 1,3,4-oxadiazol-2-yl]methyl, 1,2,4-oxadiazol-2- yl]methyl 1-methylazetidin-3-yl, 1-methylpyrrolidin-3-yl, 1-methylpiperidin-4-yl, 1-methylpiperidin-3-yl, 5-methoxypyrimidin-4-yl, 2-amino-4-pyridyl, 3-chloro-5-fluoro-4-pyridyl, 3,5-difluoro-4-pyridyl, 3- fluoro-2-pyridyl, 3-methoxy-2-pyridyl, 3-fluoro-4-pyridyl, 3-chloro-4-pyridyl, 4-pyridyl and 2-pyridyl. [069] In some embodiments, R7 is selected from H, trifluoromethyl, difluoromethyl, 1,1-difluoroethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy. methylaminocarbonyl, dimethylaminocarbonyl, and cyclopropyl. [070] In some embodiments, R8 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl. [071] In some embodiments, R9 is selected from H, fluoro, chloro, methyl, and cyano. [072] In some embodiments, R10 is selected from H, fluoro, methylcarbonylamino, chloro, amino, hydroxy, methyl, difluoromethyl, methylsulfonylamino, and methylaminocarbonylamino. [073] In some embodiments, R11 is H or fluoro. [074] In some embodiments, R6 is ethyl; R7 is trifluoromethyl; and R8 is H. [075] In some embodiments, R6 is methyl; R7 is trifluoromethyl; and R8 is H. [076] In some embodiments, R6 is H; R7 is trifluoromethyl; and R8 is H. [077] In some embodiments, R6 is 3,5-difluoropyridin-4-yl; R7 is trifluoromethyl; and R8 is H. [078] In some embodiments, R1 is substituted ethenyl. [079] In some embodiments, R1 is dimethylaminopropenyl. [080] In some embodiments, R1 is methylaminopropenyl. [081] In some embodiments, R1 is 3,3-difluoropropenyl. [082] In some embodiments, R1 is 2-(azetin-2-yl)ethenyl. [083] In some embodiments, R1 is 2-(2-methyl-pyrrolidiny-5-yl)ethenyl. [084] In some embodiments, R1 is 3-(methylamino)butenyl. [085] In some embodiments, R1 is ethylaminopropenyl. [086] In some embodiments, R1 is 2-(3-methoxypyrrolidiny-5-yl)ethenyl. [087] In some embodiments, R1 is 2-(3-fluoropyrrolidiny-5-yl)ethenyl. [088] In some embodiments, R1 is 2-(pyrrolidiny-2-yl)ethenyl.
[089] In some embodiments, R1 is aminopropenyl. [090] In some embodiments, R9 is fluoro; R10 is H; and R11 is H. [091] Non-limiting examples of compounds of the current disclosure include the following compounds identified in Table A: TABLE A
FF F F N N N S N O NH
Definitions [092] The abbreviations used herein have their conventional meaning within the chemical and biological arts. The chemical structures and formulae set forth herein are constructed according to the standard rules of chemical valency known in the chemical arts. [093] The term “alkyl,” by itself or as part of another substituent, means, unless otherwise stated, a straight (i.e., unbranched) or branched carbon chain (or carbon), or combination thereof, which may be fully saturated, mono- or polyunsaturated and can include mono-, di- and multivalent radicals, having the number of carbon atoms designated (i.e., C1-C10 means one to ten carbons). Alkyl is an uncyclized chain. Preferred alkyl substituents are C1-C6 alkyl. Examples of saturated hydrocarbon radicals include, but are not limited to, groups such as methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, homologs and isomers of, for example, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like. The term “alkylene,” by itself or as part of another substituent, means, unless otherwise stated, a divalent radical derived from an alkyl, as exemplified, but not limited by, -CH2CH2CH2CH2-.
[094] An unsaturated alkyl group is one having one or more double bonds or triple bonds referred to as “alkenyl” or “alkynyl” groups, respectively. Preferred alkenyl substituents are C2-C6 alkenyl and preferred alkynyl substituents are C2-C6 alkynyl. Examples of alkenyl or alkynyl groups include, but are not limited to, ethenyl, vinyl, 2-propenyl, butenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3-(1,4- pentadienyl), ethynyl, 1-propynyl, 3-propynyl, and 3-butynyl. [095] The term “heteroalkyl,” by itself or in combination with another term, means, unless otherwise stated, a stable straight or branched chain, or combinations thereof, including at least one carbon atom and at least one heteroatom (e.g., O, N, P, S, B, As, or Si), and wherein the nitrogen and sulfur atoms may optionally be oxidized, and the nitrogen heteroatom may optionally be quaternized. The heteroatom(s) (e.g., O, N, P, S, B, As, or Si) may be placed at any interior position of the heteroalkyl group or at the position at which the alkyl group is attached to the remainder of the molecule. Heteroalkyl is an uncyclized chain. Examples include, but are not limited to: -CH2-CH2-O-CH3, -CH2-CH2-NH-CH3, -CH2- CH2-N(CH3)-CH3, -CH2-S-CH2-CH3, -CH2-CH2, -S(O)-CH3, -CH2-CH2-S(O)2-CH3, -CH=CH-O-CH3, - Si(CH3)3, -CH2-CH=N-OCH3, -CH=CH-N(CH3)-CH3, -O-CH3, -O-CH2-CH3, and -CN. Up to two or three heteroatoms may be consecutive, such as, for example, -CH2-NH-OCH3 and -CH2-O-Si(CH3)3. Heteroalkyl also includes terms such as alkoxy, and alkylamino. [096] An alkoxy is an alkyl attached to the remainder of the molecule via an oxygen linker (-O-). Preferred alkoxy substituents include C1-4 alkoxy. Examples of alkoxy groups include, but are not limited to methoxy, ethoxy and propoxy. Preferred alkylamino substituents include mono substituted C1-4 alkylamino and disubstituted alkylamino. Examples of alkylamino groups include, but are not limited to methylamino, dimethylamino and diethylamino. [097] Another subgroup of heteroalkyl includes “alkoxyalkyl” where an alkyl group is substituted with an alkoxy group, as defined above. Preferred alkoxyalkyl substituents include C1-4 alkoxy- C1-4 alkyl. Examples of alkoxyalkyl groups include, but are not limited to methoxymethyl, ethoxymethyl and methoxyethyl. [098] As described above, heteroalkyl groups, as used herein, include those groups that are attached to the remainder of the molecule through a heteroatom, such as -C(O)R', -C(O)NR', -NR'R'', -OR', -SR', and/or -SO2R'. [099] The term “cycloalkyl” by itself or in combination with other terms, mean, unless otherwise stated, cyclic versions of “alkyl”. Cycloalkyl are not fully aromatic rings. Preferred cycloalkyl substituents include C3-C6 cycloalkyl. A “cycloalkylene” alone or as part of another substituent, means a divalent radical derived from a cycloalkyl. For example, a cycloalkyl group having 3 to 8 ring members may be referred to as a (C3-C8)cycloalkyl, a cycloalkyl group having 3 to 7 ring members may be referred to as a (C3-C7)cycloalkyl and a cycloalkyl group having 4 to 7 ring members may be referred to as a (C4- C7)cycloalkyl. In certain embodiments, the cycloalkyl group can be a (C3-C10)cycloalkyl, a (C3- C8)cycloalkyl, a (C3-C7)cycloalkyl, a (C3-C6)cycloalkyl, or a (C4-C7)cycloalkyl group and these may be referred to as C3-C10 cycloalkyl, C3-C8 cycloalkyl, C3-C7 cycloalkyl, C3-C6 cycloalkyl, or C4-C7 cycloalkyl groups.
[0100] The term “cycloalkenyl” by itself or in combination with other terms, mean, unless otherwise stated, cyclic versions of “alkenyl”. Cycloalkenyl are not fully aromatic rings. Preferred cycloalkenyl substituents include C4-C6 cycloalkenyl. For example, a cycloalkenyl group having 4 to 8 ring members may be referred to as a (C4-C8)cycloalkenyl, a cycloalkenyl group having 3 to 7 ring members may be referred to as a (C3-C7)cycloalkenyl and a cycloalkenyl group having 4 to 6 ring members may be referred to as a (C4-C6)cycloalkenyl. [0101] The term “heterocycloalkyl” by itself or in combination with other terms, mean, unless otherwise stated, cyclic versions of “heteroalkyl”. Heterocycloalkyl rings are not fully aromatic. Heterocycloalkyl is also referred by the term heterocyclyl. Preferred heterocyclyl substituents include C3-C7 oxygen or nitrogen containing rings, or both nitrogen and oxygen atms. Additionally, for heterocycloalkyl, a heteroatom can occupy the position at which the heterocycle is attached to the remainder of the molecule. A “heterocycloalkylene,” alone or as part of another substituent, means a divalent radical derived from heterocycloalkyl. “Heterocyclyl” refers to a cyclic group that includes at least one saturated, partially unsaturated, but non-aromatic, cyclic ring. Heterocyclyl groups include at least one heteroatom as a ring member. Typical heteroatoms include, O, S and N and are independently chosen. Heterocyclyl groups include monocyclic ring systems and bicyclic ring systems. Bicyclic heterocyclyl groups include at least one non-aromatic ring with at least one heteroatom ring member that may be fused to a cycloalkyl ring or may be fused to an aromatic ring where the aromatic ring may be carbocyclic or may include one or more heteroatoms. The point of attachment of a bicyclic heterocyclyl group may be at the non-aromatic cyclic ring that includes at least one heteroatom or at another ring of the heterocyclyl group. For example, a heterocyclyl group derived by removal of a hydrogen atom from one of the 9 membered heterocyclic compounds shown below may be attached to the rest of the molecule at the 5-membered ring or at the 6- membered ring. In some embodiments, a heterocyclyl group includes 5 to 10 ring members of which 1, 2, 3 or 4 or 1, 2, or 3 are heteroatoms independently selected from O, S, or N. In other embodiments, a heterocyclyl group includes 3 to 7 ring members of which 1, 2, or 3 heteroatom are independently selected from O, S, or N. In such 3-7 membered heterocyclyl groups, only 1 of the ring atoms is a heteroatom when the ring includes only 3 members and includes 1 or 2 heteroatoms when the ring includes 4 members. In some embodiments, a heterocyclyl group includes 3 or 4 ring members of which 1 is a heteroatom selected from O, S, or N. In other embodiments, a heterocyclyl group includes 5 to 7 ring members of which 1, 2, or 3 are heteroatoms independently selected from O, S, or N. Typical heterocyclyl groups include, but are not limited to, groups derived from epoxides, aziridine, azetidine, imidazolidine, morpholine, piperazine, piperidine, hexahydropyrimidine, 1,4,5,6-tetrahydropyrimidine, pyrazolidine, pyrrolidine, quinuclidine, tetrahydrofuran, tetrahydropyran, benzimidazolone, pyridinone, and the like. Heterocyclyl groups may be fully saturated, but may also include one or more double bonds. Examples of such heterocyclyl groups include, but are not limited to, 1,2,3,6-tetrahydropyridinyl, 3,6-dihydro-2H- pyranyl, 3,4-dihydro-2H-pyranyl, 2,5-dihydro-1H-pyrolyl, 2,3-dihydro-1H-pyrolyl, 1H-azirinyl, 1,2- dihydroazetenyl, and the like. Substituted heterocyclyl also includes ring systems substituted with one or more oxo (=O) or oxide (-O-) substituents, such as piperidinyl N-oxide, morpholinyl-N-oxide, 1-oxo-1-
thiomorpholinyl, pyridinonyl, benzimidazolonyl, benzo[d]oxazol-2(3H)-only, 3,4-dihydroisoquinolin- 1(2H)-only, indolin-only, 1H-imidazo[4,5-c]pyridin-2(3H)-only, 7H-purin-8(9H)-only, imidazolidin-2- only, 1H-imidazol-2(3H)-only, 1,1-dioxo-1-thiomorpholinyl, and the like. [0102] The terms “halo” or “halogen,” by themselves or as part of another substituent, mean, unless otherwise stated, a fluorine, chlorine, bromine, or iodine atom. [0103] The term “haloalkyl” is meant to include monohaloalkyl and polyhaloalkyl. For example, the term “C1-C3 -haloalkyl” includes, but is not limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2,2,2- trifluoroethyl, 3-bromopropyl, and the like. [0104] The term “acyl” means, unless otherwise stated, -C(O)R where R is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. [0105] The term “aryl” means, unless otherwise stated, a polyunsaturated, aromatic, hydrocarbon substituent, which can be a single ring or multiple rings (preferably from 1 to 3 rings) that are fused together (i.e., a fused ring aryl) or linked covalently. A fused ring aryl refers to multiple rings fused together wherein at least one of the fused rings is an aryl ring. [0106] The term “heteroaryl” refers to aryl groups (or rings) that contain at least one heteroatom such as N, O, or S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized. Thus, the term “heteroaryl” includes fused ring heteroaryl groups (i.e., multiple rings fused together wherein at least one of the fused rings is a heteroaromatic ring). A heteroaryl group can be attached to the remainder of the molecule through a carbon or heteroatom. “Heteroaryl” refers to a monovalent heteroaromatic group derived by the removal of one hydrogen atom from a single atom of a parent heteroaromatic ring system. Heteroaryl groups typically include 5- to 14-membered, but more typically include 5- to 10-membered aromatic, monocyclic, bicyclic, and tricyclic rings containing one or more, for example, 1, 2, 3, or 4, or in certain embodiments, 1, 2, or 3, heteroatoms chosen from O, S, or N, with the remaining ring atoms being carbon. In monocyclic heteroaryl groups, the single ring is aromatic and includes at least one heteroatom. In some embodiments, a monocyclic heteroaryl group may include 5 or 6 ring members and may include 1, 2, 3, or 4 heteroatoms, 1, 2, or 3 heteroatoms, 1 or 2 heteroatoms, or 1 heteroatom where the heteroatom(s) are independently selected from O, S, or N. In bicyclic aromatic rings, both rings are aromatic. In bicyclic heteroaryl groups, at least one of the rings must include a heteroatom, but it is not necessary that both rings include a heteroatom although it is permitted for them to do so. For example, the term “heteroaryl” includes a 5- to 7-membered heteroaromatic ring fused to a carbocyclic aromatic ring or fused to another heteroaromatic ring. In tricyclic aromatic rings, all three of the rings are aromatic and at least one of the rings includes at least one heteroatom. For fused, bicyclic and tricyclic heteroaryl ring systems where only one of the rings contains one or more heteroatoms, the point of attachment may be at the ring including at least one heteroatom or at a carbocyclic ring. When the total number of S and O atoms in the heteroaryl group exceeds 1, those heteroatoms are not adjacent to one another. In certain embodiments, the total number of S and O atoms in
the heteroaryl group is not more than 2. In certain embodiments, the total number of S and O atoms in the aromatic heterocycle is not more than 1. Heteroaryl does not encompass or overlap with aryl as defined above. Examples of heteroaryl groups include, but are not limited to, groups derived from acridine, carbazole, cinnoline, furan, imidazole, indazole, indole, indolizine, isobenzofuran, isochromene, isoindole, isoquinoline, isothiazole, 2H-benzo[d][1,2,3]triazole, isoxazole, naphthyridine, oxadiazole, oxazole, perimidine, phenanthridine, phenanthroline, phenazine, phthalazine, pteridine, purine, pyrazine, pyrazole, pyridazine, pyridine, pyrimidine, pyrrole, pyrrolizine, quinazoline, quinoline, quinolizine, quinoxaline, tetrazole, thiadiazole, thiazole, thiophene, triazole, and the like. In certain embodiments, the heteroaryl group can be between 5 to 20 membered heteroaryl, such as, for example, a 5 to 14 membered or 5 to 10 membered heteroaryl. In certain embodiments, heteroaryl groups can be those derived from thiophene, pyrrole, benzothiophene, 2H-benzo[d][1,2,3]triazole benzofuran, indole, pyridine, quinoline, imidazole, benzimidazole, oxazole, tetrazole, and pyrazine. [0107] An “arylene” and a “heteroarylene,” alone or as part of another substituent, mean a divalent radical derived from an aryl and heteroaryl, respectively. [0108] The term “carbonyl” refers to the radical–C(O) which may also be referred to as –C(=O) group. [0109] The term “carboxy” refers to the radical–C(O)OH which may also be referred to as–C(=O)OH. [0110] The term “cyano” refers to the radical -CN. [0111] The term “amino” referes to the radical -NH2. [0112] The term “aminocarbonyl” refers to the radical -CO-NH2. Aminocarbonyl radicals may be substituted with one or two alkyl groups to form “alkylaminocarbonyl” groups. [0113] The term “alkylcarbonyl” refers to the radical alkyl-CO-. [0114] The terms “hydroxyl” and “hydroxy” refers to the radical -OH. [0115] The term “oxo,” as used herein, means an oxygen that is double bonded to a carbon atom. [0116] Each of the above terms (e.g., “alkyl,” “heteroalkyl,” “cycloalkyl,” “heterocycloalkyl,” “aryl,” and “heteroaryl”) includes both substituted and unsubstituted forms of the indicated radical. [0117] Substituents for the alkyl and heteroalkyl radicals (including those groups often referred to as alkylene, alkenyl, heteroalkylene, heteroalkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl, and heterocycloalkenyl) can be one or more of a variety of groups selected from, but not limited to, -OR', =O, =NR', =N-OR', -NR'R'', -SR', -halogen, -SiR'R''R''', -OC(O)R', -C(O)R', -CO2R', -CONR'R'', -OC(O)NR'R'', -NR''C(O)R', -NR'-C(O)NR''R''', -NR''C(O)2R', -NR-C(NR'R''R''')=NR'''', -NR-C(NR'R'')=NR''', -S(O)R', -S(O)2R', -S(O)2NR'R'', -NRSO2R', -NR'NR''R''', -ONR'R'', -NR'C(O)NR''NR'''R'''', -CN, -NO2, -NR'SO2R'', -NR'C(O)R'', -NR'C(O)-OR'', -NR'OR'', in a number ranging from zero to (2m'+1), where m' is the total number of carbon atoms in such radical. R, R', R'', R''', and R'''' each preferably independently refer to hydrogen, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl (e.g., aryl substituted with 1-3 halogens), substituted or unsubstituted heteroaryl, substituted or unsubstituted alkyl, alkoxy, or thioalkoxy groups, or arylalkyl groups. When a compound described herein includes more than one R group, for example, each of the R groups is independently
selected as are each R', R'', R''', and R'''' group when more than one of these groups is present. When R' and R'' are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 4-, 5-, 6-, or 7-membered ring. For example, -NR'R'' includes, but is not limited to, 1-pyrrolidinyl and 4- morpholinyl. From the above discussion of substituents, one of skill in the art will understand that the term “alkyl” is meant to include groups including carbon atoms bound to groups other than hydrogen groups, such as haloalkyl (e.g., -CF3 and -CH2CF3) and acyl (e.g., -C(O)CH3, -C(O)CF3, -C(O)CH2OCH3, and the like). [0118] Such substituted alkyl groups include hydroxyalkyl; carboxyalkyl, alkoxycarbonylalkyl, cyanoalkyl, aminocarbonylalkyl, alkylaminocarbonylalkyl, alkylaminoalkyl, alkylcarbonylaminoalkyl, cycloalkylalkyl, aralkyl, heteroarylalkyl and heterocyclylalkyl; wherein the heterocyclyl, heteroaryl, aryl, cycloalkyl, hydroxyl; carboxyl, alkoxy, cyano, aminocarbonyl, alkylaminocarbonyl, alkylamino, and alkylcarbonylamino groups are defined above. [0119] Similarly, alkenyl and alkynyl groups can be specifically substituted to form halo-substituted C2- C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl, alkylamino substituted C2-C6 alkenyl, amino substituted C2-C6 alkenyl, hydroxy substituted amino-C2-C6 alkenyl, phenyl substituted amino-C2-C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C2-C6 alkynyl, alkoxy substituted C2-C6 alkynyl, heterocyclyl- substituted C2-C6 alkynyl, cycloalkyl- substituted C2-C6 alkenyl, oxygen-containing heterocyclyl- substituted C2-C6 alkenyl, oxygen-containing heterocyclyl- substituted C2-C6 alkynyl, nitrogen-containing heterocyclyl- substituted C2-C6 alkenyl, and cycloalkyl- substituted C2-C6 alkynyl groups; where the amino, halo, dialkylamino, alkylamino, alkoxy, cycloalkyl, oxygen-containing heterocyclyl, and nitrogen-containing heterocyclyl radicals are defined elswhere. [0120] The term “alkylsufonyl” refers to the radical alkyl-SO2-; where alkyl is defined elseqhere. [0121] Substituted amino groups include “alkylcarbonylamino,” “alkylsulfonylamino,” “alkylaminocarbonylamino” and “alkylsulfonylamino”; wherein the amino radical is substituted, preferably with one substituent selected from alkylcarbonyl, alkylsulfonyl, alkylaminocarbonyl, defined elsewhere. [0122] Similar to the substituents described for the alkyl radical, substituents for the aryl and heteroaryl groups are varied and are selected from, for example: -OR', -NR'R'', -SR', -halogen, -SiR'R''R''', -OC(O)R', -C(O)R', -CO2R', -CONR'R'', -OC(O)NR'R'', -NR''C(O)R', -NR'-C(O)NR''R''', - NR''C(O)2R', -NR-C(NR'R''R''')=NR'''', -NR-C(NR'R'')=NR''', -S(O)R', -S(O)2R', -S(O)2NR'R'', -NRSO2R', -NR'NR''R''', -ONR'R'', -NR'C(O)NR''NR'''R'''', -CN, -NO2, -R', -N3, -CH(Ph)2, fluoro(C1- C4)alkoxy, fluoro(C1-C4)alkyl, -NR'SO2R'', -NR'C(O)R'', -NR'C(O)-OR'', -NR'OR'', in a number ranging from zero to the total number of open valences on the aromatic ring system; and where R', R'', R''', and R'''' are preferably independently selected from hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. When a compound described herein includes more than one R group, for example, each of the R groups is
independently selected as are each R', R'', R''', and R'''' groups when more than one of these groups is present. [0123] Substituents for rings (e.g. cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene) may be depicted as substituents on the ring rather than on a specific atom of a ring (commonly referred to as a floating substituent). In such a case, the substituent may be attached to any of the ring atoms (obeying the rules of chemical valency) and in the case of fused rings or spirocyclic rings, a substituent depicted as associated with one member of the fused rings or spirocyclic rings (a floating substituent on a single ring), may be a substituent on any of the fused rings or spirocyclic rings (a floating substituent on multiple rings). When a substituent is attached to a ring, but not a specific atom (a floating substituent), and a subscript for the substituent is an integer greater than one, the multiple substituents may be on the same atom, same ring, different atoms, different fused rings, different spirocyclic rings, and each substituent may optionally be different. Where a point of attachment of a ring to the remainder of a molecule is not limited to a single atom (a floating substituent), the attachment point may be any atom of the ring and in the case of a fused ring or spirocyclic ring, any atom of any of the fused rings or spirocyclic rings while obeying the rules of chemical valency. Where a ring, fused rings, or spirocyclic rings contain one or more ring heteroatoms and the ring, fused rings, or spirocyclic rings are shown with one more floating substituents (including, but not limited to, points of attachment to the remainder of the molecule), the floating substituents may be bonded to the heteroatoms. Where the ring heteroatoms are shown bound to one or more hydrogens (e.g. a ring nitrogen with two bonds to ring atoms and a third bond to a hydrogen) in the structure or formula with the floating substituent, when the heteroatom is bonded to the floating substituent, the substituent will be understood to replace the hydrogen, while obeying the rules of chemical valency. [0124] Two or more substituents may optionally be joined to form aryl, heteroaryl, cycloalkyl, or heterocycloalkyl groups. Such so-called ring-forming substituents are typically, though not necessarily, found attached to a cyclic base structure. In one embodiment, the ring-forming substituents are attached to adjacent members of the base structure. For example, two ring-forming substituents attached to adjacent members of a cyclic base structure create a fused ring structure. In another embodiment, the ring-forming substituents are attached to a single member of the base structure. For example, two ring-forming substituents attached to a single member of a cyclic base structure create a spirocyclic structure. In yet another embodiment, the ring-forming substituents are attached to non-adjacent members of the base structure. [0125] As used herein, the terms “heteroatom” or “ring heteroatom” are meant to include oxygen (O), nitrogen (N), sulfur (S), phosphorus (P), Boron (B), and silicon (Si). [0126] A “substituent group,” as used herein, means a group selected from the following moieties: (A) halogen, oxo, cyano, -CCl3, -CBr3, -CF3, -CI3, -CHCl2, -CHBr2, -CHF2, -CHI2, -CH2Cl, -CH2Br, -CH2F, -CH2I, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHOH, -NHC(O)NHNH2, -NHC(O)NH2, -NHSO2H, -NHC(O)H, -NHC(O)OH, -OCCl3, -OCF3, -OCBr3, -OCI3, -OCHCl2, -OCHBr2, -OCHI2, -OCHF2, -OCH2Cl, -OCH2Br, -OCH2F, -OCH2I, -N3, unsubstituted alkyl
(e.g., C1-C20, C1-C12, C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 20 membered, 2 to 12 membered, 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C10, C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 10 membered, 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered), and (B) alkyl (e.g., C1-C20, C1-C12, C1-C8, C1-C6, C1-C4, or C1-C2), heteroalkyl (e.g., 2 to 20 membered, 2 to 12 membered, 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), cycloalkyl (e.g., C3-C10, C3-C8, C3-C6, C4-C6, or C5-C6), heterocycloalkyl (e.g., 3 to 10 membered, 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), aryl (e.g., C6-C12, C6-C10, or phenyl), or heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered), substituted with at least one substituent selected from: oxo, halo, haloalkyl cyano, hydroxyl, amino, carboxyl, amnocarbonyl, nitro, aminosulfonyl, haloalkoxy, cycloalkyl, heterocyclyl, aryl, or heteroaryl. [0127] A “size-limited substituent” or “ size-limited substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C20 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl, and each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 10 membered heteroaryl. [0128] A “lower substituent” or “ lower substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C8 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C7 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl, and each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 9 membered heteroaryl. [0129] In some embodiments, each substituted group described in the compounds herein is substituted with at least one substituent group. More specifically, in some embodiments, each substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene described in the compounds herein are substituted with at least one substituent group. In other embodiments, at least one or all of these groups are substituted with at least one size-limited substituent group. In other embodiments, at least one or all of these groups are substituted with at least one lower substituent group.
[0130] In other embodiments of the compounds herein, each substituted or unsubstituted alkyl may be a substituted or unsubstituted C1-C20 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl, and/or each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 10 membered heteroaryl. In some embodiments of the compounds herein, each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C20 alkylene, each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 20 membered heteroalkylene, each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C3-C8 cycloalkylene, each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 8 membered heterocycloalkylene, each substituted or unsubstituted arylene is a substituted or unsubstituted C6-C10 arylene, and/or each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 10 membered heteroarylene. [0131] In some embodiments, each substituted or unsubstituted alkyl is a substituted or unsubstituted C1- C8 alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C7 cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10 aryl, and/or each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 9 membered heteroaryl. In some embodiments, each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C8 alkylene, each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 8 membered heteroalkylene, each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C3-C7 cycloalkylene, each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 7 membered heterocycloalkylene, each substituted or unsubstituted arylene is a substituted or unsubstituted C6-C10 arylene, and/or each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 9 membered heteroarylene. In some embodiments, the compound is a chemical species set forth in the Examples section, figures, or tables below. [0132] In embodiments, a substituted or unsubstituted moiety (e.g., substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, and/or substituted or unsubstituted heteroarylene) is unsubstituted (e.g., is an unsubstituted alkyl, unsubstituted heteroalkyl, unsubstituted cycloalkyl, unsubstituted heterocycloalkyl, unsubstituted aryl, unsubstituted heteroaryl, unsubstituted alkylene, unsubstituted heteroalkylene, unsubstituted cycloalkylene, unsubstituted heterocycloalkylene, unsubstituted arylene, and/or unsubstituted heteroarylene, respectively). In embodiments, a substituted or unsubstituted moiety (e.g., substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, and/or substituted or unsubstituted heteroarylene) is substituted (e.g., is a substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene, respectively). [0133] In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one substituent group, wherein if the substituted moiety is substituted with a plurality of substituent groups, each substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of substituent groups, each substituent group is different. [0134] In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one size-limited substituent group, wherein if the substituted moiety is substituted with a plurality of size-limited substituent groups, each size-limited substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of size-limited substituent groups, each size-limited substituent group is different. [0135] In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one lower substituent group, wherein if the substituted moiety is substituted with a plurality of lower substituent groups, each lower substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of lower substituent groups, each lower substituent group is different. [0136] In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one substituent group, size-limited substituent group, or lower substituent group; wherein if the substituted moiety is substituted with a plurality of groups selected from substituent groups, size-limited substituent groups, and lower substituent groups; each substituent group, size-limited substituent group, and/or lower substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of groups selected from
substituent groups, size-limited substituent groups, and lower substituent groups; each substituent group, size-limited substituent group, and/or lower substituent group is different. [0137] Certain compounds of the present invention possess asymmetric carbon atoms (optical or chiral centers) or double bonds; the enantiomers, racemates, diastereomers, tautomers, geometric isomers, stereoisometric forms that may be defined, in terms of absolute stereochemistry, as (R)-or (S)- or, as (D)- or (L)- for amino acids, and individual isomers are encompassed within the scope of the present invention. The compounds of the present invention do not include those that are known in art to be too unstable to synthesize and/or isolate. The present invention is meant to include compounds in racemic and optically pure forms. Optically active (R)- and (S)-, or (D)- and (L)-isomers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. When the compounds described herein contain olefinic bonds or other centers of geometric asymmetry, and unless specified otherwise, it is intended that the compounds include both E and Z geometric isomers. [0138] As used herein, the term “isomers” refers to compounds having the same number and kind of atoms, and hence the same molecular weight, but differing in respect to the structural arrangement or configuration of the atoms. [0139] The term “tautomer,” as used herein, refers to one of two or more structural isomers which exist in equilibrium and which are readily converted from one isomeric form to another. [0140] It will be apparent to one skilled in the art that certain compounds of this invention may exist in tautomeric forms, all such tautomeric forms of the compounds being within the scope of the invention. [0141] Unless otherwise stated, structures depicted herein are also meant to include all stereochemical forms of the structure; i.e., the R and S configurations for each asymmetric center. Therefore, single stereochemical isomers as well as enantiomeric and diastereomeric mixtures of the present compounds are within the scope of the invention. As used herein and unless otherwise indicated, the term “stereoisomer” or “stereomerically pure” means one stereoisomer of a compound that is substantially free of other stereoisomers of that compound. For example, a stereomerically pure compound having one chiral center will be substantially free of the mirror image enantiomer of the compound. A stereomerically pure compound having two chiral centers will be substantially free of other diastereomers of the compound. A typical stereomerically pure compound comprises greater than about 80% by weight of one stereoisomer of the compound and less than about 20% by weight of other stereoisomers of the compound, more preferably greater than about 90% by weight of one stereoisomer of the compound and less than about 10% by weight of the other stereoisomers of the compound, even more preferably greater than about 95% by weight of one stereoisomer of the compound and less than about 5% by weight of the other stereoisomers of the compound, and most preferably greater than about 97% by weight of one stereoisomer of the compound and less than about 3% by weight of the other stereoisomers of the compound. If the stereochemistry of a structure or a portion of a structure is not indicated with, for example, bold or dashed lines, the structure or portion of the structure is to be interpreted as encompassing all stereoisomers of it. A bond drawn with a wavy line indicates that both stereoisomers are encompassed. This is not to be confused with a wavy line drawn perpendicular to a bond which indicates the point of
attachment of a group to the rest of the molecule. As described above, this invention encompasses the use of stereomerically pure forms of such compounds, as well as the use of mixtures of those forms. For example, mixtures comprising equal or unequal amounts of the enantiomers of a particular compound of the invention may be used in methods and compositions of the invention. These isomers may be asymmetrically synthesized or resolved using standard techniques such as chiral columns or chiral resolving agents. See, e.g., Jacques, J., et al., Enantiomers, Racemates and Resolutions (Wiley- Interscience, New York, 1981); Wilen, S. H., et al. (1997) Tetrahedron 33:2725; Eliel, E. L., Stereochemistry of Carbon Compounds (McGraw-Hill, NY, 1962); and Wilen, S. H., Tables of Resolving Agents and Optical Resolutions p.268 (E.L. Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, IN, 1972). [0142] Unless otherwise stated, structures depicted herein are also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium [D] or tritium, or the replacement of a carbon by 13C- or 14C-enriched carbon are within the scope of this is selected from invention. [0143] It should be noted that throughout the application that alternatives are written in Markush groups, for example, each amino acid position that contains more than one possible amino acid. It is specifically contemplated that each member of the Markush group should be considered separately, thereby comprising another embodiment, and the Markush group is not to be read as a single unit. [0144] The terms "a" or "an," as used in herein means one or more. In addition, the phrase "substituted with a[n]," as used herein, means the specified group may be substituted with one or more of any or all of the named substituents. For example, where a group, such as an alkyl or heteroaryl group, is "substituted with an unsubstituted C1-C20 alkyl, or unsubstituted 2 to 20 membered heteroalkyl," the group may contain one or more unsubstituted C1-C20 alkyls, and/or one or more unsubstituted 2 to 20 membered heteroalkyls. [0145] Moreover, where a moiety is substituted with an R substituent, the group may be referred to as “R-substituted.” Where a moiety is R-substituted, the moiety is substituted with at least one R substituent and each R substituent is optionally different. Where a particular R group is present in the description of a chemical genus (such as Formula (XV)), a Roman alphabetic symbol may be used to distinguish each appearance of that particular R group. [0146] Any compound herein can be purified. A compound herein can be least 1% pure, at least 2% pure, at least 3% pure, at least 4% pure, at least 5% pure, at least 6% pure, at least 7% pure, at least 8% pure, at least 9% pure, at least 10% pure, at least 11% pure, at least 12% pure, at least 13% pure, at least 14% pure, at least 15% pure, at least 16% pure, at least 17% pure, at least 18% pure, at least 19% pure, at least 20% pure, at least 21% pure, at least 22% pure, at least 23% pure, at least 24% pure, at least 25% pure, at least 26% pure, at least 27% pure, at least 28% pure, at least 29% pure, at least 30% pure, at least 31% pure, at least 32% pure, at least 33% pure, at least 34% pure, at least 35% pure, at least 36% pure, at least 37% pure, at least 38% pure, at least 39% pure, at least 40% pure, at least 41% pure, at least 42% pure, at least
43% pure, at least 44% pure, at least 45% pure, at least 46% pure, at least 47% pure, at least 48% pure, at least 49% pure, at least 50% pure, at least 51% pure, at least 52% pure, at least 53% pure, at least 54% pure, at least 55% pure, at least 56% pure, at least 57% pure, at least 58% pure, at least 59% pure, at least 60% pure, at least 61% pure, at least 62% pure, at least 63% pure, at least 64% pure, at least 65% pure, at least 66% pure, at least 67% pure, at least 68% pure, at least 69% pure, at least 70% pure, at least 71% pure, at least 72% pure, at least 73% pure, at least 74% pure, at least 75% pure, at least 76% pure, at least 77% pure, at least 78% pure, at least 79% pure, at least 80% pure, at least 81% pure, at least 82% pure, at least 83% pure, at least 84% pure, at least 85% pure, at least 86% pure, at least 87% pure, at least 88% pure, at least 89% pure, at least 90% pure, at least 91% pure, at least 92% pure, at least 93% pure, at least 94% pure, at least 95% pure, at least 96% pure, at least 97% pure, at least 98% pure, at least 99% pure, at least 99.1% pure, at least 99.2% pure, at least 99.3% pure, at least 99.4% pure, at least 99.5% pure, at least 99.6% pure, at least 99.7% pure, at least 99.8% pure, or at least 99.9% pure. [0147] In some embodiments, compounds as disclosed herein can be used to treat cancer in a subject. A compound as disclosed herein can, for example, slow the proliferation of cancer cell lines, or kill cancer cells. Non-limiting examples of cancer that can be treated by a compound as disclosed herein include: acute lymphoblastic leukemia, acute myeloid leukemia, adrenocortical carcinoma, AIDS-related cancers, AIDS-related lymphoma, anal cancer, appendix cancer, astrocytomas, basal cell carcinoma, bile duct cancer, bladder cancer, bone cancers, brain tumors, such as cerebellar astrocytoma, cerebral astrocytoma/malignant glioma, ependymoma, medulloblastoma, supratentorial primitive neuroectodermal tumors, visual pathway and hypothalamic glioma, breast cancer, bronchial adenomas, Burkitt lymphoma, carcinoma of unknown primary origin, central nervous system lymphoma, cerebellar astrocytoma, cervical cancer, childhood cancers, chronic lymphocytic leukemia, chronic myelogenous leukemia, chronic myeloproliferative disorders, colon cancer, cutaneous T-cell lymphoma, desmoplastic small round cell tumor, endometrial cancer, ependymoma, esophageal cancer, Ewing's sarcoma, germ cell tumors, gallbladder cancer, gastric cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumor, gliomas, hairy cell leukemia, head and neck cancer, heart cancer, hepatocellular (liver) cancer, Hodgkin lymphoma, Hypopharyngeal cancer, intraocular melanoma, islet cell carcinoma, Kaposi sarcoma, kidney cancer, laryngeal cancer, lip and oral cavity cancer, liposarcoma, liver cancer, lung cancers, such as non- small cell and small cell lung cancer, lymphomas, leukemias, macroglobulinemia, malignant fibrous histiocytoma of bone/osteosarcoma, medulloblastoma, melanomas, mesothelioma, metastatic squamous neck cancer with occult primary, mouth cancer, multiple endocrine neoplasia syndrome, myelodysplastic syndromes, myeloid leukemia, nasal cavity and paranasal sinus cancer, nasopharyngeal carcinoma, neuroblastoma, non-Hodgkin lymphoma, non-small cell lung cancer, oral cancer, oropharyngeal cancer, osteosarcoma/malignant fibrous histiocytoma of bone, ovarian cancer, ovarian epithelial cancer, ovarian germ cell tumor, pancreatic cancer, pancreatic cancer islet cell, paranasal sinus and nasal cavity cancer, parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma, pineal astrocytoma, pineal germinoma, pituitary adenoma, pleuropulmonary blastoma, plasma cell neoplasia, primary central nervous system lymphoma, prostate cancer, rectal cancer, renal cell carcinoma, renal pelvis and ureter transitional
cell cancer, retinoblastoma, rhabdomyosarcoma, salivary gland cancer, sarcomas, skin cancers, skin carcinoma merkel cell, small intestine cancer, soft tissue sarcoma, squamous cell carcinoma, stomach cancer, T-cell lymphoma, throat cancer, thymoma, thymic carcinoma, thyroid cancer, trophoblastic tumor (gestational), cancers of unknown primary site, urethral cancer, uterine sarcoma, vaginal cancer, vulvar cancer, Waldenström macroglobulinemia, and Wilms tumor. [0148] In some embodiments, the compounds of the disclosure show non-lethal toxicity. Pharmaceutically-acceptable salts. [0149] The disclosure provides the use of pharmaceutically-acceptable salts of any therapeutic compound described herein. Pharmaceutically-acceptable salts include, for example, acid-addition salts and base- addition salts. The acid that is added to the compound to form an acid-addition salt can be an organic acid or an inorganic acid. A base that is added to the compound to form a base-addition salt can be an organic base or an inorganic base. In some embodiments, a pharmaceutically-acceptable salt is a metal salt. In some embodiments, a pharmaceutically-acceptable salt is an ammonium salt. [0150] Metal salts can arise from the addition of an inorganic base to a compound of the invention. The inorganic base consists of a metal cation paired with a basic counterion, such as, for example, hydroxide, carbonate, bicarbonate, or phosphate. The metal can be an alkali metal, alkaline earth metal, transition metal, or main group metal. In some embodiments, the metal is lithium, sodium, potassium, cesium, cerium, magnesium, manganese, iron, calcium, strontium, cobalt, titanium, aluminum, copper, cadmium, or zinc. [0151] In some embodiments, a metal salt is a lithium salt, a sodium salt, a potassium salt, a cesium salt, a cerium salt, a magnesium salt, a manganese salt, an iron salt, a calcium salt, a strontium salt, a cobalt salt, a titanium salt, an aluminum salt, a copper salt, a cadmium salt, or a zinc salt. [0152] Ammonium salts can arise from the addition of ammonia or an organic amine to a compound of the invention. In some embodiments, the organic amine is triethyl amine, diisopropyl amine, ethanol amine, diethanol amine, triethanol amine, morpholine, N-methylmorpholine, piperidine, N- methylpiperidine, N-ethylpiperidine, dibenzylamine, piperazine, pyridine, pyrrazole, pipyrrazole, imidazole, pyrazine, or pipyrazine. [0153] In some embodiments, an ammonium salt is a triethyl amine salt, a diisopropyl amine salt, an ethanol amine salt, a diethanol amine salt, a triethanol amine salt, a morpholine salt, an N- methylmorpholine salt, a piperidine salt, an N-methylpiperidine salt, an N-ethylpiperidine salt, a dibenzylamine salt, a piperazine salt, a pyridine salt, a pyrrazole salt, a pipyrrazole salt, an imidazole salt, a pyrazine salt, or a pipyrazine salt. [0154] Acid addition salts can arise from the addition of an acid to a compound of the invention. In some embodiments, the acid is organic. In some embodiments, the acid is inorganic. In some embodiments, the acid is hydrochloric acid, hydrobromic acid, hydroiodic acid, nitric acid, nitrous acid, sulfuric acid, sulfurous acid, a phosphoric acid, isonicotinic acid, lactic acid, salicylic acid, tartaric acid, ascorbic acid, gentisinic acid, gluconic acid, glucaronic acid, saccaric acid, formic acid, benzoic acid, glutamic acid, pantothenic acid, acetic acid, propionic acid, butyric acid, fumaric acid, succinic acid, methanesulfonic
acid, ethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, citric acid, oxalic acid, or maleic acid. [0155] In some embodiments, the salt is a hydrochloride salt, a hydrobromide salt, a hydroiodide salt, a nitrate salt, a nitrite salt, a sulfate salt, a sulfite salt, a phosphate salt, isonicotinate salt, a lactate salt, a salicylate salt, a tartrate salt, an ascorbate salt, a gentisinate salt, a gluconate salt, a glucaronate salt, a saccarate salt, a formate salt, a benzoate salt, a glutamate salt, a pantothenate salt, an acetate salt, a propionate salt, a butyrate salt, a fumarate salt, a succinate salt, a methanesulfonate (mesylate) salt, an ethanesulfonate salt, a benzenesulfonate salt, a p-toluenesulfonate salt, a citrate salt, an oxalate salt , or a maleate salt. Pharmaceutical Compositions of the disclosure. [0156] A pharmaceutical composition of the disclosure can be used, for example, before, during, or after treatment of a subject with, for example, another pharmaceutical agent. [0157] Subjects can be, for example, elderly adults, adults, adolescents, pre-adolescents, children, toddlers, infants, neonates, and non-human animals. In some embodiments, a subject is a patient. [0158] A pharmaceutical composition of the disclosure can be a combination of any pharmaceutical compounds described herein with other chemical components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, and/or excipients. The pharmaceutical composition facilitates administration of the compound to an organism. Pharmaceutical compositions can be administered in therapeutically-effective amounts as pharmaceutical compositions by various forms and routes including, for example, intravenous, subcutaneous, intramuscular, oral, parenteral, ophthalmic, subcutaneous, transdermal, nasal, vaginal, and topical administration. [0159] A pharmaceutical composition can be administered in a local manner, for example, via injection of the compound directly into an organ, optionally in a depot or sustained release formulation or implant. Pharmaceutical compositions can be provided in the form of a rapid release formulation, in the form of an extended release formulation, or in the form of an intermediate release formulation. A rapid release form can provide an immediate release. An extended release formulation can provide a controlled release or a sustained delayed release. [0160] For oral administration, pharmaceutical compositions can be formulated by combining the active compounds with pharmaceutically-acceptable carriers or excipients. Such carriers can be used to formulate liquids, gels, syrups, elixirs, slurries, or suspensions, for oral ingestion by a subject. Non- limiting examples of solvents used in an oral dissolvable formulation can include water, ethanol, isopropanol, saline, physiological saline, DMSO, dimethylformamide, potassium phosphate buffer, phosphate buffer saline (PBS), sodium phosphate buffer, 4-2-hydroxyethyl-1-piperazineethanesulfonic acid buffer (HEPES), 3-(N-morpholino)propanesulfonic acid buffer (MOPS), piperazine-N,N′-bis(2- ethanesulfonic acid) buffer (PIPES), and saline sodium citrate buffer (SSC). Non-limiting examples of co- solvents used in an oral dissolvable formulation can include sucrose, urea, cremaphor, DMSO, and potassium phosphate buffer.
[0161] Pharmaceutical compositions can be formulated for intravenous administration. The pharmaceutical compositions can be in a form suitable for parenteral injection as a sterile suspension, solution or emulsion in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing and/or dispersing agents. Pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water-soluble form. Suspensions of the active compounds can be prepared as oily injection suspensions. Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes. The suspension can also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions. Alternatively, the active ingredient can be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use. [0162] The active compounds can be administered topically and can be formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, medicated sticks, balms, creams, and ointments. Such pharmaceutical compositions can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives. [0163] The compounds of the disclosure can be applied topically to the skin, or a body cavity, for example, oral, vaginal, bladder, cranial, spinal, thoracic, or pelvic cavity of a subject. The compounds of the disclosure can be applied to an accessible body cavity. [0164] The compounds can also be formulated in rectal compositions such as enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly suppositories, or retention enemas, containing conventional suppository bases such as cocoa butter or other glycerides, as well as synthetic polymers such as polyvinylpyrrolidone, and PEG. In suppository forms of the compositions, a low-melting wax such as a mixture of fatty acid glycerides, optionally in combination with cocoa butter, can be melted. [0165] In practicing the methods of treatment or use provided herein, therapeutically-effective amounts of the compounds described herein are administered in pharmaceutical compositions to a subject having a disease or condition to be treated. In some embodiments, the subject is a mammal such as a human. A therapeutically-effective amount can vary widely depending on the severity of the disease, the age and relative health of the subject, the potency of the compounds used, and other factors. The compounds can be used singly or in combination with one or more therapeutic agents as components of mixtures. [0166] Pharmaceutical compositions can be formulated using one or more physiologically-acceptable carriers comprising excipients and auxiliaries, which facilitate processing of the active compounds into preparations that can be used pharmaceutically. Compositions can be modified depending upon the route of administration chosen. Pharmaceutical compositions comprising a compound described herein can be manufactured, for example, by mixing, dissolving, emulsifying, encapsulating, entrapping, or compression processes. [0167] The pharmaceutical compositions can include at least one pharmaceutically-acceptable carrier, diluent, or excipient and compounds described herein as free-base or pharmaceutically-acceptable salt form. Pharmaceutical compositions can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives.
[0168] Methods for the preparation of compositions comprising the compounds described herein include formulating the compounds with one or more inert, pharmaceutically-acceptable excipients or carriers to form a solid, semi-solid, or liquid composition. Solid compositions include, for example, powders, tablets, dispersible granules, capsules, and cachets. Liquid compositions include, for example, solutions in which a compound is dissolved, emulsions comprising a compound, or a solution containing liposomes, micelles, or nanoparticles comprising a compound as disclosed herein. Semi-solid compositions include, for example, gels, suspensions and creams. The compositions can be in liquid solutions or suspensions, solid forms suitable for solution or suspension in a liquid prior to use, or as emulsions. These compositions can also contain minor amounts of nontoxic, auxiliary substances, such as wetting or emulsifying agents, pH buffering agents, and other pharmaceutically-acceptable additives. [0169] Non-limiting examples of dosage forms suitable for use in the disclosure include liquid, powder, gel, nanosuspension, nanoparticle, microgel, aqueous or oily suspensions, emulsion, and any combination thereof. [0170] Non-limiting examples of pharmaceutically-acceptable excipients suitable for use in the disclosure include binding agents, disintegrating agents, anti-adherents, anti-static agents, surfactants, anti-oxidants, coating agents, coloring agents, plasticizers, preservatives, suspending agents, emulsifying agents, anti-microbial agents, spheronization agents, and any combination thereof. [0171] A composition of the disclosure can be, for example, an immediate release form or a controlled release composition. An immediate release composition can be formulated to allow the compounds to act rapidly. Non-limiting examples of immediate release compositions include readily dissolvable formulations. A controlled release compositions can be a pharmaceutical composition that has been adapted such that release rates and release profiles of the active agent can be matched to physiological and chronotherapeutic requirements or, alternatively, has been formulated to effect release of an active agent at a programmed rate. Non-limiting examples of controlled release compositions include granules, delayed release granules, hydrogels (e.g., of synthetic or natural origin), other gelling agents (e.g., gel- forming dietary fibers), matrix-based compositions (e.g., compositions comprising a polymeric material having at least one active ingredient dispersed through), granules within a matrix, polymeric mixtures, and granular masses. [0172] In some, a controlled release composition is a delayed release form. A delayed release form can be formulated to delay a compound’s action for an extended period of time. A delayed release form can be formulated to delay the release of an effective dose of one or more compounds, for example, for about 4, about 8, about 12, about 16, or about 24 hours. [0173] A controlled release composition can be a sustained release form. A sustained release form can be formulated to sustain, for example, the compound’s action over an extended period of time. A sustained release form can be formulated to provide an effective dose of any compound described herein (e.g., provide a physiologically-effective blood profile) over about 4, about 8, about 12, about 16 or about 24 hours. [0174] Non-limiting examples of pharmaceutically-acceptable excipients can be found, for example, in
Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover, John E., Remington’s Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania 1975; Liberman, H.A. and Lachman, L., Eds., Pharmaceutical Dosage Forms, Marcel Decker, New York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug Delivery Systems, Seventh Ed. (Lippincott Williams & Wilkins1999), each of which is incorporated by reference in its entirety. [0175] Multiple therapeutic agents can be administered in any order or simultaneously. In some embodiments, a compound of the disclosure is administered in combination with, before, or after treatment with another therapeutic agent. If simultaneously, the multiple therapeutic agents can be provided in a single, unified form, or in multiple forms, for example, as multiple separate pills. The agents can be packed together or separately, in a single package or in a plurality of packages. One or all of the therapeutic agents can be given in multiple doses. If not simultaneous, the timing between the multiple doses can vary to as much as about a month. [0176] Therapeutic agents described herein can be administered before, during, or after the occurrence of a disease or condition, and the timing of administering the composition containing a therapeutic agent can vary. For example, the compositions can be used as a prophylactic and can be administered continuously to subjects with a propensity to conditions or diseases in order to lessen a likelihood of the occurrence of the disease or condition. The compositions can be administered to a subject during or as soon as possible after the onset of the symptoms. The administration of the therapeutic agents can be initiated within the first 48 hours of the onset of the symptoms, within the first 24 hours of the onset of the symptoms, within the first 6 hours of the onset of the symptoms, or within 3 hours of the onset of the symptoms. The initial administration can be via any route practical, such as by any route described herein using any formulation described herein. [0177] A compound can be administered as soon as is practical after the onset of a disease or condition is detected or suspected, and for a length of time necessary for the treatment of the disease, such as, for example, from about 1 month to about 3 months. In some embodiments, the length of time a compound can be administered can be about 1 day, about 2 days, about 3 days, about 4 days, about 5 days, about 6 days, about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 1 month, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 2 months, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 3 months, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 4 months, about 17 weeks, about 18 weeks, about 19 weeks, about 20 weeks, about 5 months, about 21 weeks, about 22 weeks, about 23 weeks, about 24 weeks, about 6 months, about 7 months, about 8 months, about 9 months, about 10 months, about 11 months, about 1 year, about 13 months, about 14 months, about 15 months, about 16 months, about 17 months, about 18 months, about 19 months, about 20 months, about 21 months, about 22 months about 23 months, about 2 years, about 2.5 years, about 3 years, about 3.5 years, about 4 years, about 4.5 years, about 5 years, about 6 years, about 7 years, about 8 years, about 9 years, or about 10 years. The length of treatment can vary for each subject. [0178] Pharmaceutical compositions described herein can be in unit dosage forms suitable for single administration of precise dosages. In unit dosage form, the formulation is divided into unit doses
containing appropriate quantities of one or more compounds. The unit dosage can be in the form of a package containing discrete quantities of the formulation. Non-limiting examples are packaged injectables, vials, or ampoules. Aqueous suspension compositions can be packaged in single-dose non- reclosable containers. Multiple-dose reclosable containers can be used, for example, in combination with or without a preservative. Formulations for injection can be presented in unit dosage form, for example, in ampoules, or in multi-dose containers with a preservative. [0179] Pharmaceutical compositions provided herein, can be administered in conjunction with other therapies, for example, chemotherapy, radiation, surgery, anti-inflammatory agents, and selected vitamins. The other agents can be administered prior to, after, or concomitantly with the pharmaceutical compositions. [0180] Depending on the intended mode of administration, the pharmaceutical compositions can be in the form of solid, semi-solid or liquid dosage forms, such as, for example, tablets, suppositories, pills, capsules, powders, liquids, suspensions, lotions, creams, or gels, for example, in unit dosage form suitable for single administration of a precise dosage. [0181] For solid compositions, nontoxic solid carriers include, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, talc, cellulose, glucose, sucrose, and magnesium carbonate. [0182] Non-limiting examples of pharmaceutically active agents suitable for combination with compositions of the disclosure include anti-infectives, i.e., aminoglycosides, antiviral agents, antimicrobials, anticholinergics/antispasmotics, antidiabetic agents, antihypertensive agents, antineoplastics, cardiovascular agents, central nervous system agents, coagulation modifiers, hormones, immunologic agents, immunosuppressive agents, and ophthalmic preparations. [0183] Compounds can be delivered via liposomal technology. The use of liposomes as drug carriers can increase the therapeutic index of the compounds. Liposomes are composed of natural phospholipids, and can contain mixed lipid chains with surfactant properties (e.g., egg phosphatidylethanolamine). A liposome design can employ surface ligands for attaching to unhealthy tissue. Non-limiting examples of liposomes include the multilamellar vesicle (MLV), the small unilamellar vesicle (SUV), and the large unilamellar vesicle (LUV). Liposomal physicochemical properties can be modulated to optimize penetration through biological barriers and retention at the site of administration, and to reduce a likelihood of developing premature degradation and toxicity to non-target tissues. Optimal liposomal properties depend on the administration route: large-sized liposomes show good retention upon local injection, small-sized liposomes are better suited to achieve passive targeting. PEGylation reduces the uptake of the liposomes by the liver and spleen, and increases the circulation time, resulting in increased localization at the inflamed site due to the enhanced permeability and retention (EPR) effect. Additionally, liposomal surfaces can be modified to achieve selective delivery of the encapsulated drug to specific target cells. Non-limiting examples of targeting ligands include monoclonal antibodies, vitamins, peptides, and polysaccharides specific for receptors concentrated on the surface of cells associated with the disease. [0184] Non-limiting examples of dosage forms suitable for use in the disclosure include liquid, elixir,
nanosuspension, aqueous or oily suspensions, drops, syrups, and any combination thereof. Non-limiting examples of pharmaceutically-acceptable excipients suitable for use in the disclosure include granulating agents, binding agents, lubricating agents, disintegrating agents, sweetening agents, glidants, anti- adherents, anti-static agents, surfactants, anti-oxidants, gums, coating agents, coloring agents, flavoring agents, coating agents, plasticizers, preservatives, suspending agents, emulsifying agents, plant cellulosic material and spheronization agents, and any combination thereof. [0185] Compositions of the disclosure can be packaged as a kit. In some embodiments, a kit includes written instructions on the administration/use of the composition. The written material can be, for example, a label. The written material can suggest conditions methods of administration. The instructions provide the subject and the supervising physician with the best guidance for achieving the optimal clinical outcome from the administration of the therapy. The written material can be a label. In some embodiments, the label can be approved by a regulatory agency, for example the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or other regulatory agencies. Dosing. [0186] Pharmaceutical compositions described herein can be in unit dosage forms suitable for single administration of precise dosages. In unit dosage form, the composition is divided into unit doses containing appropriate quantities of one or more compounds. The unit dosage can be in the form of a package containing discrete quantities of the composition. Non-limiting examples are liquids in vials or ampoules. Aqueous suspension compositions can be packaged in single-dose non-reclosable containers. Multiple-dose reclosable containers can be used, for example, in combination with a preservative. Compositions for parenteral injection can be presented in unit dosage form, for example, in ampoules, or in multi-dose containers with a preservative. [0187] A compound described herein can be present in a composition in a range of from about 1 mg to about 2000 mg; from about 10 mg to about 1000 mg; from about 100 mg to about 750 mg; from about 250 mg to about 500 mg, from about 100 mg to about 500 mg, from about 200 mg to about 500 mg, from about 250 mg to about450 mg, from about 100 mg to about 200 mg, from about 1 mg to about 50 mg, from about 50 mg to about 100 mg, from about 100 mg to about 150 mg, from about 150 mg to about 200 mg, from about 200 mg to about 250 mg, from about 250 mg to about 300 mg, from about 300 mg to about 350 mg, from about 350 mg to about 400 mg, from about 400 mg to about 450 mg, from about 450 mg to about 500 mg, from about 500 mg to about 550 mg, from about 550 mg to about 600 mg, from about 600 mg to about 650 mg, from about 650 mg to about 700 mg, from about 700 mg to about 750 mg, from about 750 mg to about 800 mg, from about 800 mg to about 850 mg, from about 850 mg to about 900 mg, from about 900 mg to about 950 mg, or from about 950 mg to about 1000 mg. [0188] A compound described herein can be present in a composition in an amount of about 1 mg, about 2 mg, about 3 mg, about 4 mg, about 5 mg, about 10 mg, about 15 mg, about 20 mg, about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about
125 mg, about 150 mg, about 175 mg, about 200 mg, about 250 mg, about 300 mg, about 350 mg, about 400 mg, about 450 mg, about 500 mg, about 550 mg, about 600 mg, about 650 mg, about 700 mg, about 750 mg, about 800 mg, about 850 mg, about 900 mg, about 950 mg, or about 1000 mg. [0189] In some embodiments, a dose can be expressed in terms of an amount of the drug divided by the mass of the subject, for example, milligrams of drug per kilograms of subject body mass. In some embodiments, a compound is administered in an amount ranging from about 1 mg/kg to about 20 mg/kg, or about 5 mg/kg to about 10 mg/kg. In some embodiments, a compound is administered in an amount of about 1 mg/kg, 2 mg/kg, 3 mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg, about 7 mg/kg, 8 mg/kg, about 9 mg/kg, or about 10 mg/kg. Combination Treatment with an anti-cancer agent [0190] Combination therapy with a compound of the disclosure and an anti-cancer agent described herein can be used to treat a condition. In some embodiments, the combination therapy can produce a significantly better therapeutic result than the additive effects achieved by each individual constituent when administered alone at a therapeutic dose. In some embodiments, the dosage of the compound or anti-cancer agent described herein, in combination therapy can be reduced as compared to monotherapy with each agent, while still achieving an overall therapeutic effect. In some embodiments, a compound and an anti-cancer agent described herein, can exhibit a synergistic effect. In some embodiments, the synergistic effect of a compound and anti-cancer agent described herein, can be used to reduce the total amount drugs administered to a subject, which decrease side effects experienced by the subject. [0191] The compounds of the disclosure can be used in combination with at least one anti-cancer agent described herein. In some embodiments, the at least one anti-cancer agent described herein, can modulate the same or a different target as the compounds of the disclosure. In some embodiments, the at least one anti-cancer agent described herein, can modulate the same target as the compounds of the disclosure, or other components of the same pathway, or overlapping sets of target enzymes. In some embodiments, the at least one anti-cancer agent described herein, can modulate a different target from the compounds of the disclosure. [0192] Accordingly, in one aspect, the present disclosure provides a method for treating cancer, the method comprising administering to a subject in need thereof (a) an effective amount of a compound of the disclosure and (b) an effective amount of at least one anti-cancer agent described herein, to provide a combination therapy. In some embodiments, the combination therapy may have an enhanced therapeutic effect compared to the effect of the compound and the at least one anti-cancer agent each administered alone. According to certain exemplary embodiments, the combination therapy has a synergistic therapeutic effect. According to this embodiment, the combination therapy produces a significantly better therapeutic result (e.g., anti-cancer, cell growth arrest, apoptosis, induction of differentiation, cell death, etc.) than the additive effects achieved by each individual constituent when administered alone at a therapeutic dose. [0193] Combination therapy includes but is not limited to the combination of compounds of the disclosure with anti-cancer agents such as chemotherapeutic agents, therapeutic antibodies, or radiation
treatment, to provide a synergistic therapeutic effect. In some embodiments, the compounds of the disclosure are used in combination with one or more anti-cancer (antineoplastic or cytotoxic) chemotherapy drug. Suitable chemotherapeutic agents for use in the combinations of the present disclosure include, but are not limited to, alkylating agents, antibiotic agents, antimetabolic agents, hormonal agents, plant-derived agents, anti-angiogenic agents, differentiation inducing agents, cell growth arrest inducing agents, apoptosis inducing agents, cytotoxic agents, agents affecting cell bioenergetics, biologic agents, e.g., monoclonal antibodies, kinase inhibitors and inhibitors of growth factors and their receptors, gene therapy agents, cell therapy, or any combination thereof. a. Combination treatment with estrogen receptor antagonists [0194] In some embodiments, a compound of the disclosure is used in combination with an estrogen receptor antagonist. In some embodiments, a compound of the disclosure is used in combination with toremifene (Fareston®), fulvestrant (Faslodex®), or tamoxifen citrate (Soltamox®). [0195] Fulvestrant is a selective estrogen receptor degrader (SERD) and is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. Fulvestrant is a complete estrogen receptor antagonist with little to no agonist effects and accelerates the proteasomal degradation of the estrogen receptor. Fulvestrant has poor oral bioavailability and is administered via intramuscular injection. Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes estrogen receptor-positive breast cancer cells to heregulin beta1. In some embodiments, a compound of the disclosure is used in combination with fulvestrant. b. Combination treatment with aromatase inhibitors [0196] In some embodiments, a compound of the disclosure is used in combination with an aromatase inhibitor. Aromatase inhibitors are used in the treatment of breast cancer in post-menopausal women and gynecomastia in men. Aromatase inhibitors can be used off-label to reduce estrogen conversion when using external testosterone. Aromatase inhibitors can also be used for chemoprevention in high-risk women. [0197] In some embodiments, a compound of the disclosure is used in combination with a non-selective aromatase inhibitor. In some embodiments, a compound of the disclosure is used in combination with a non-selective aromatase inhibitor, such as aminoglutethimide or testolactone (Teslac®). In some embodiments, a compound of the disclosure is used in combination with a selective aromatase inhibitor. In some embodiments, a compound of the disclosure is used in combination with a selective aromatase inhibitor, such as anastrozole (Arimidex®), letrozole (Femara®), exemestane (Aromasin®), vorozole (Rivizor®), formestane (Lentaron®), or fadrozole (Afema®). In some embodiments, a compound of the disclosure is used in combination with exemestane. In some embodiments, a compound of the disclosure is used in combination with an aromatase inhibitor such as 1,4,6-androstatrien-3,17-dione (ATD) or 4- androstene-3,6,17-trione. c. Combination treatment with mTOR inhibitors [0198] In some embodiments, a compound of the disclosure is used in combination with an mTOR inhibitor. mTOR inhibitors are drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a
serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K)-related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through the protein complexes mTORC1 and mTORC2. [0199] In some embodiments, a compound of the disclosure is used in combination with an mTOR inhibitor, such as rapamycin, temsirolimus (CCI-779), everolimus (RAD001), ridaforolimus (AP-23573). In some embodiments, a compound of the disclosure is used in combination with everolimus (Afinitor®). Everolimus affects the mTORC1 protein complex and can lead to hyper-activation of the kinase AKT, which can lead to longer survival in some cell types. Everolimus binds to FKBP12, a protein receptor which directly interacts with mTORC1 and inhibits downstream signaling. mRNAs that codify proteins implicated in the cell cycle and in the glycolysis process are impaired or altered as a result, inhibiting tumor growth and proliferation. [0200] In some embodiments, a compound of the disclosure is used in combination with a mTOR inhibitor and an aromatase inhibitor. For example, a compound of the disclosure is used in combination with everolimus and exemestane. d. Combination treatment with antimetabolites [0201] Antimetabolites are chemotherapy treatments that are similar to normal substances within the cell. When cells incorporate the antimetabolites into the cellular metabolism, the cells are unable to divide. Antimetabolites are cell-cycle specific and attack cells at specific phases in the cell cycle. [0202] In some examples, a compound of the disclosure is used in combination with one or more antimetabolites, such as a folic acid antagonist, pyrimidine antagonist, purine antagonist, or an adenosine deaminase inhibitor. In some embodiments, a compound of the disclosure is used in combination with an antimetabolite, such as methotrexate, 5-fluorouracil, foxuridine, cytarabine, capecitabine, gemcitabine, 6- mercaptopurine, 6-thioguanine, cladribine, fludarabine, nelarabine, or pentostatin. In some embodiments, a compound of the disclosure is used in combination with capecitabine (Xeloda®), gemcitabine (Gemzar®), or cytarabine (Cytosar-U®). e. Combination treatment with plant alkaloids [0203] In some embodiments, a compound of the disclosure is used in combination with a plant alkaloid. In some embodiments, a compound of the disclosure is used in combination with a plant alkaloid, such as vinca alkaloids, taxanes, podophyllotoxins, or camptothecan analogues. In some embodiments, a compound of the disclosure is used in combination with plant alkaloids, such as vincristine, vinblastine, vinorelbine, paclitaxel, docetaxel, etoposide, tenisopide, irinotecan, or topotecan. [0204] In some embodiments, a compound of the disclosure is used in combination with a taxane, such as paclitaxel (Abraxane® or Taxol®) and docetaxel (Taxotere®). In some embodiments, a compound of the disclosure is used in combination with paclitaxel. In some embodiments, a compound of the disclosure is used in combination with docetaxel. f. Combination treatment with therapeutic antibodies [0205] In some embodiments, a compound of the disclosure is used in combination with therapeutic antibodies. In some embodiments, a compound of the disclosure is used in combination with monoclonal
antibodies, such as alemtuzumab (Campath®) or trastuzumab (Herceptin®). In some embodiments, a compound of the disclosure is used in combination with conjugated monoclonal antibodies, such as radiolabeled antibodies or chemolabeled antibodies. In some embodiments, a compound of the disclosure is used in combination with conjugated monoclonal antibodies, such as ibritumomab tiuxetan (Zevalin®), brentuximab vedotin (Adcetris®), ado-trastuzumab emtansine (Kadcyla®), or denileukin diftitox (Ontak®). In some embodiments, a compound of the disclosure is used in combination with bispecific monoclonal antibodies, such as blinatumomab (Blincyto®). [0206] In some embodiments, a compound of the disclosure is used in combination with an anti-CD20 antibody, such as rituximab (Mabthera®/ Rituxan®), obinutuzumab (Gazyva®), ibritumomab tiuxetan, tositumomab, ofatumumab (Genmab®), ocaratuzumab, ocrelizumab, TRU-015, or veltuzumab. g. Combination treatment with PD-L1 and/or PD-1 antagonists [0207] The PD-1 pathway comprises the immune cell co-receptor Programmed Death-1 (PD-1) and the PD-1 ligands PD-L1 and PD-L2. The PD-1 pathway mediates local immunosuppression in the tumor microenvironment. PD-1 and PD-L1 antagonists suppress the immune system. In some embodiments, a PD-1 or PD-L1 antagonist is a monoclonal antibody or antigen binding fragment thereof that specifically binds to, blocks, or downregulates PD-1 or PD-L1, respectively. In some embodiments, a PD-1 or PD-L1 antagonist is a compound or biological molecule that specifically binds to, blocks, or downregulates PD-1 or PD-L1, respectively. [0208] In some embodiments, the compounds of the disclosure are used in combination with a PD-1 or PD-L1 antagonist. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist, for example, MK-3475, nivolumab (Opdivo®), pembrolizumab (Keytruda®), humanized antibodies (i.e., h409Al l, h409A16 and h409A17), AMP-514, BMS-936559, MEDI0680, MEDI4736, MPDL3280A, MSB0010718C, MDX-1105, MDX-1106, or pidilzumab. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist that is an immunoadhesion molecule, such as AMP-224. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist to treat cancer cells or a tumor that overexpresses PD-1 or PD-L1. In some embodiments, the compounds of the disclosure are used in combination with a PD-1/PD-L1 antagonist to treat cancer cells or a tumor that overexpresses miR-34. h. Combination treatment with anti-hormone therapy [0209] Anti-hormone therapy uses an agent to suppress selected hormones or the effects. Anti-hormone therapy is achieved by antagonizing the function of hormones with a hormone antagonist and/or by preventing the production of hormones. In some embodiments, the suppression of hormones can be beneficial to subjects with certain cancers that grow in response to the presence of specific hormones. In some embodiments, a compound of the disclosure is used in combination with a hormone antagonist. [0210] In some embodiments, a compound of the disclosure is used in combination with anti-androgens, anti-estrogens, aromatase inhibitors, or luteinizing hormone-releasing hormone (LHRH) agonists. In some embodiments, a compound of the disclosure is used in combination with anti-androgens, such as bicalutamide (Casodex®), cyproterone (Androcur®), flutamide (Euflex®), or nilutamide (Anandron®). In
some embodiments, a compound of the disclosure is used in combination with anti-estrogens, such as fulvestrant (Faslodex®), raloxifene (Evista®), or tamoxifen (Novaladex®, Tamofen®). In some embodiments, a compound of the disclosure is used in combination with LHRH agonists, such as buserelin (Suprefact®), goserelin (Zoladex®), or leuprolide (Lupron®, Lupron Depot®, Eligard®). i. Combination treatment with hypomethylating (demethylating) agents (HMAs) [0211] Treatment of these and other types of cancer may be improved by examining combinations that increase anti-tumor activities and may allow for similar activity at reduced doses compared to single agent treatment, leading to improved patient outcomes, and limiting adverse events. Hypomethylating (demethylating) agents inhibit DNA methylation, which affects cellular function through successive generations of cells without changing the underlying DNA sequence. Hypomethylating agents can block the activity of DNA methyltransferase. Two examples of HMAs for treatment of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) patients are decitabine and azacitidine. In some embodiments, a compound of the disclosure is used in combination with hypomethylating agents, such as azacitidine (Vidaza®, Azadine®) or decitabine (Dacogen®). j. Combination treatment with anti-inflammatory agents [0212] In some embodiments, a compound of the disclosure is used in combination with nonsteroidal anti-inflammatory drugs (NSAIDs), specific COX-2 inhibitors, or corticosteroids. In some embodiments, a compound of the disclosure is used in combination with NSAIDs, such as aspirin, ibuprofen, naproxen, celecoxib, ketorolac, or diclofenac. In some embodiments, a compound of the disclosure is used in combination with specific COX-2 inhibitors, such as celecoxib (Celebrex®), rofecoxib, or etoricoxib. In some embodiments, a compound of the disclosure is used in combination with corticosteroids, such as dexamethasone or glucosteroids (e.g., hydrocortisone and prednisone). k. Combination treatment with HDAC inhibitors [0213] Histone deacetylase (HDAC) inhibitors are chemical compounds that inhibit histone deacetylase. HDAC inhibitors can induce p21 expression, a regulator of p53 activity. In some embodiments, a compound of the disclosure is used in combination with an HDAC inhibitor. In some embodiments, a compound of the disclosure is used in combination with an HDAC inhibitor, such as vorinostat, romidepsin (Istodax®), chidamide, panobinostat (Farydak®), belinostat (PDX101), panobinostat (LBH589), valproic acid, mocetinostat (MGCD0103), abexinostat (PCI-24781), entinostat (MS-275), SB939, resminostat (4SC-201), givinostat (ITF2357), quisinostat (JNJ-26481585), HBI-8000, kevetrin, CUDC-101, AR-42, CHR-2845, CHR-3996, 4SC-202, CG200745, ACY-1215, ME-344, sulforaphane, or trichostatin A. l. Combination treatment with platinum-based antineoplastic drugs [0214] Platinum-based antineoplastic drugs are coordinated complex of platinum. In some embodiments, a compound of the disclosure is used in combination with a platinum-based antineoplastic drug, such as cisplatin, oxaliplatin, carboplatin, nedaplatin, triplatin tetranitrate, phenanthriplatin, picoplatin, or satraplatin. In some embodiments, a compound of the disclosure is used in combination with cisplatin or carboplatin. In some embodiments, a compound of the disclosure is used in combination with cisplatinum,
platamin, neoplatin, cismaplat, cis-diamminedichloroplatinum(II), or CDDP; Platinol®) and carboplatin (also known as cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II); tradenames Paraplatin® and Paraplatin-AQ®). m. Combination treatment with kinase inhibitors [0215] Abnormal activation of protein phosphorylation is frequently either a driver of direct consequence of cancer. Kinase signaling pathways are involved in the phenotypes of tumor biology, including proliferation, survival, motility, metabolism, angiogenesis, and evasion of antitumor immune responses. [0216] MEK inhibitors: MEK inhibitors are drugs that inhibit the mitogen-activated protein kinase enzymes MEK1 and/or MEK2. In some embodiments, a compound of the disclosure is used in combination with a MEK1 inhibitor. In some embodiments, a compound of the disclosure is used in combination with a MEK2 inhibitor. In some embodiments, a compound of the disclosure is used in combination with an agent that can inhibit MEK1 and MEK2. In some embodiments, a compound of the disclosure is used in combination with a MEK1/MEK2 inhibitor, such as trametinib (Mekinist®), cobimetinib, binimetinib, selumetinib (AZD6244), pimasertibe (AS-703026), PD-325901, CI-1040, PD035901, or TAK-733. In some embodiments, a compound of the disclosure is used in combination with trametinib. In some embodiments, a compound of the disclosure is used in combination with cobimetinib. [0217] BRAF inhibitors: BRAF inhibitors are drugs that inhibit the serine/threonine-protein kinase B- raf (BRAF) protein. In some embodiments, a compound of the disclosure is used in combination with a BRAF inhibitor. In some embodiments, a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit wild type BRAF. In some embodiments, a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit mutated BRAF. In some embodiments, a compound of the disclosure is used in combination with a BRAF inhibitor that can inhibit V600E mutated BRAF. In some embodiments, a compound of the disclosure is used in combination with a BRAF inhibitor, such as vemurafenib (Zelboraf®), dabrafenib (Tafinlar®), C-1, NVP-LGX818, or sorafenib (Nexavar®). [0218] KRAS inhibitors: KRAS is a gene that acts as an on/off switch in cell signaling. In some embodiments, a compound of the disclosure is used in combination with a KRAS inhibitor. In some embodiments, a compound of the disclosure is used in combination with a wild type KRAS inhibitor. In some embodiments, a compound of the disclosure is used in combination with a mutated KRAS inhibitor (e.g., a G12C mutated KRAS). In some embodiments, the KRAS inhibitor is sotorasib (AMG-510), COTI-219, ARS-3248, WDB-178, BI-3406, BI-1701963, SML-8-73-1 (G12C), Compound 3144 (G12D), Kobe0065, Kobe/2602, (Ras GTP), RT11, or adagrasib (MRTX-849). [0219] BTK inhibitors: Bruton’s tyrosine kinase (BTK) is a non-receptor tyrosine kinase of the Tec kinase family that is involved in B-cell receptor signaling. In some embodiments, a compound of the disclosure is used in combination with a BTK inhibitor. In some embodiments, a compound of the disclosure is used in combination with a BTK inhibitor, such as ibrutinib or acalabrutinib. [0220] CDK inhibitors: CDK4 and CDK6 are cyclin-dependent kinases that control the transition between the G1 and S phases of the cell cycle. CDK4/CDK6 activity is deregulated and overactive in
cancer cells. Selective CDK4/CDK6 inhibitors can block cell-cycle progression in the mid-G1 phase of the cell cycle, causing arrest and preventing the proliferation of cancer cells. In some embodiments, a compound of the disclosure is used in combination with a CDK4/CDK6 inhibitor. In some embodiments, a compound of the disclosure is used in combination with a CDK4/CDK6 inhibitor, such as palbociclib (Ibrance®), ribociclib, trilaciclib, seliciclib, dinaciclib, milciclib, roniciclib, atuveciclib, briciclib, riviciclib, voruciclib, or abemaciclib. In some embodiments, a compound of the disclosure is used in combination with palbociclib. In some embodiments, a compound of the disclosure is used in combination with ribociclib. In some embodiments, a compound of the disclosure is used in combination with abemaciclib. [0221] In some examples, a compound of the disclosure is used in combination with an inhibitor of CDK4 and/or CDK6 and with an agent that reinforces the cytostatic activity of CDK4/6 inhibitors and/or with an agent that converts reversible cytostasis into irreversible growth arrest or cell death. Examples of cancer subtypes include NSCLC, melanoma, neuroblastoma, glioblastoma, liposarcoma, and mantle cell lymphoma. In some examples, a compound of the disclosure is used in combination with at least one additional pharmaceutically active agent that alleviates CDKN2A (cyclin-dependent kinase inhibitor 2A) deletion. In some examples, a compound of the disclosure is used in combination with at least one additional pharmaceutically active agent that alleviates CDK9 (cyclin-dependent kinase 9) abnormality. [0222] In some embodiments, a compound of the disclosure is used in combination with a CDK2, CDK7, and/or CDK9 inhibitor. In some embodiments, a compound of the disclosure is used in combination with a CDK2, CDK7, or CDK9 inhibitor, such as seliciclib, voruciclib, or milciclib. In some embodiments, a compound of the disclosure is used in combination with a CDK inhibitor, such as dinaciclib, roniciclib (Kisqali®), or briciclib. In some examples, a compound of the disclosure is used in combination with at least one additional pharmaceutically-active agent that alleviates CDKN2A (cyclin-dependent kinase inhibitor 2A) deletion. [0223] ATM regulators: A compound of the disclosure can be used in combination with one or more anti-cancer agent that regulates the ATM (upregulate or downregulate). In some embodiments the compounds described herein can synergize with one or more ATM regulators. In some embodiments one or more of the compounds described herein can synergize with all ATM regulators. [0224] AKT inhibitors: In some embodiments, a compound of the disclosure is used in combination with one or more anti-cancer agent that inhibits the AKT (protein kinase B (PKB)). In some embodiments the compounds described herein can synergize with one or more AKT inhibitors. n. Combination treatment with other anti-cancer agents [0225] In some examples, a compound of the disclosure is used in combination with at least one anti- cancer agent that alleviates PTEN (phosphatase and tensin homolog) deletion. In some examples, a compound of the disclosure is used in combination with at least one anti-cancer agent that alleviates Wip- 1Alpha over expression. In some examples, a compound of the disclosure is used in combination with at least one anti-cancer agent that is a Nucleoside metabolic inhibitor. Examples of nucleoside metabolic inhibitors include capecitabine, gemcitabine and cytarabine (Arac).
[0226] The table below lists suitable anti-cancer agents for use with the methods described herein.
Administration of Combination Treatment of Compound of Disclosure and Anti-Cancer Agent [0227] A compound of the disclosure or a pharmaceutical composition comprising a compound of the disclosure and at least one anti-cancer agent as disclosed herein can be administered simultaneously (i.e., simultaneous administration) or sequentially (i.e., sequential administration). [0228] In some embodiments, a compound of the disclosure and the at least one anti-cancer agent described herein are administered simultaneously, either in the same composition or in separate compositions. When the drugs are administered simultaneously, the compound and the at least one anti- cancer agent described herein, may be contained in the same composition (e.g., a composition comprising both the compound and the at least one anti-cancer agent) or in separate compositions (e.g., the compound is contained in one composition and the at least one anti-cancer agent is contained in another composition). [0229] In some embodiments, the compound and the at least one anti-cancer agent are administered sequentially, i.e., the compound is administered either prior to or after the administration of the anti- cancer agent. In some embodiments, the compound is administered before the anti-cancer agent. In some embodiments, the anti-cancer agent described herein is administered before the compound. The compound and the anti-cancer agent described herein are contained in separate compositions, which may be contained in the same or different packages. [0230] In some embodiments, the administration of the compound and the anti-cancer agent described herein are concurrent, i.e., the administration period of the compounds and that of the anti-cancer agent overlap with each other. In some embodiments, the administration of the compounds and the anti-cancer agent described herein are non-concurrent. For example, in some embodiments, the administration of the compound is terminated before the anti-cancer agent described herein is administered. In some embodiments, the administration of the anti-cancer agent described herein is terminated before the compound is administered. The time period between these two non-concurrent administrations can range from being hours apart to being days apart to being weeks apart. [0231] The dosing frequency of the compound and the at least one anti-cancer agent described herein may be adjusted over the course of the treatment, based on the judgment of the administering physician. When administered separately, the compound and the at least one anti-cancer agent described herein can be administered at different dosing frequency or intervals. For example, the compound can be
administered weekly, while the at least one anti-cancer agent described herein can be administered more or less frequently. Or, the compound can be administered twice weekly, while the at least one anti-cancer agent described herein can be administered more or less frequently. In addition, the compound and the at least one anti-cancer agent described herein can be administered using the same route of administration or using different routes of administration. [0232] A therapeutically-effective amount of a compound and/or the anti-cancer agent described herein for use in therapy can vary with the nature of the condition being treated, the length of treatment time desired, the age and the condition of the patient, and can be determined by the attending physician. [0233] In some embodiments, when a compound of the disclosure is administered in combination with at least one anti-cancer agent described herein, the dosage of the compound can be given a lower dosage than when the compound is administered alone.. In some embodiments, the dosage of a compound of the disclosure in combination therapy can be from about 1 mg/kg to about 20 mg/kg. In some embodiments, the dosage of a compound of the disclosure in combination therapy can be from about 5 mg/kg to about 10 mg/kg.. [0234] In some embodiments, the dosage of a compound of the disclosure in combination therapy can be in an amount of about 0.5 mg/kg; about 1 mg/kg; about 2 mg/kg; about 3 mg/kg; about 4 mg/kg; about 5 mg/kg; about 6 mg/kg; about 7 mg/kg; about 8 mg/kg; about 9 mg/kg; or about 10 mg/kg.. [0235] In some embodiments, the dosage of the compound of the disclosure in combination therapy with at least one additional therapeutic agent can be at least 5% less than the dose of the compound of the disclosure administered alone, or at least 10% less, at least 15% less, at least 20% less, at least 25% less, at least 30% less, at least 35% less, at least 40% less, at least 45% less, at least 50% less, at least 55% less, at least 60% less, at least 65% less, at least 70% less, or at least 75% less than the dose of the compound of the disclosure administered alone. [0236] In some embodiments, when a compound of the disclosure is administered in combination with at least one anti-cancer agent described herein, the dosage of the at least one additional anti-cancer agent can be given a lower dosage than when the anti-cancer agent is administered alone. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 50 μg to about 100 μg; from about 100 μg to about 150 μg; from about 150 μg to about 200 μg; from about 200 μg to about 250 μg; from about 250 μg to about 300 μg; from about 300 μg to about 350 μg; from about 350 μg to about 400 μg; from about 400 μg to about 450 μg; from about 450 μg to about 500 μg; from about 500 μg to about 600 μg; from about 600 μg to about 700 μg; from about 700 μg to about 800 μg; from about 800 μg to about 900 μg; or from about 900 μg to about 1000 μg. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 μg to about 150 μg. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 150 μg to about 200 μg. In some embodiments, the dosage of the at least one additional anti-cancer agent in combination therapy can be from about 200 μg to about 250 μg. [0237] In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be in an amount of about 50 μg; about 100 μg; about 150 μg; about 200 μg; about 250 μg; about 300 μg;
about 350 μg; about 400 μg; about 450 μg; or about 500 μg. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be in an amount of about 200 μg. [0238] In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 5 mg/kg; from about 5 mg/kg to about 25 mg/kg; from about 25 mg/kg to about 50 mg/kg; from about 50 mg/kg to about 75 mg/kg; from about 75 mg/kg to about 100 mg/kg; from about 100 mg/kg to about 150 mg/kg; from about 150 mg/kg to about 200 mg/kg; from about 200 mg/kg to about 250 mg/kg; from about 250 mg/kg to about 300 mg/kg; from about 300 mg/kg to about 350 mg/kg; from about 350 mg/kg to about 400 mg/kg; from about 400 mg/kg to about 450 mg/kg; from about 450 mg/kg to about 500 mg/kg; from about 500 mg/kg to about 600 mg/kg; from about 600 mg/kg to about 700 mg/kg; from about 700 mg/kg to about 800 mg/kg; from about 800 mg/kg to about 900 mg/kg; or from about 900 mg/kg to about 1000 mg/kg. In some embodiments, the dosage of the at least one anti- cancer agent in combination therapy can be from about 1 mg/kg to about 5 mg/kg. In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 mg/kg to about 150 mg/kg. In some embodiments, the dosage of the at least one additional therapeutic agent in combination therapy can be from about 200 mg/kg to about 250 mg/kg. [0239] In some embodiments, the dosage of the at least one anti-cancer agent in combination therapy can be at least 5% less than the dose of the at least one anti-cancer agent administered alone, or at least 10% less, at least 15% less, at least 20% less, at least 25% less, at least 30% less, at least 35% less, at least 40% less, at least 45% less, at least 50% less, at least 55% less, at least 60% less, at least 65% less, at least 70% less, or at least 75% less than the dose of the at least one anti-cancer agent administered alone. [0240] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 500 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 1 mg/kg to about 250 mg/kg. [0241] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 1 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 2 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 4 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 6 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 8 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can about 5 mg/kg.
[0242] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 μg to about 500 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy can be from about 100 μg to about 500 μg. [0243] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be about 1 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg; and the dosage of the at least one anti- cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg; and the dosage of the at least one anti-cancer agent in combination therapy is about 200 μg. [0244] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of anti-PD-1 agent as an anti- cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg. [0245] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 1 mg/kg or about 100 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg or about 150 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg or about 300 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg or about 400 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg.. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg or about 500 mg; and the dosage of anti-PD-1 agent as an anti-cancer agent in combination therapy is about 200 μg. [0246] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy can be from about 1 mg/kg to about 20 mg/kg; and the dosage of anti-CD20 agent as an anti- cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound
of the disclosure administered in combination therapy can be from about 5 mg/kg to about 10 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 μg. [0247] In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 1 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 2 mg/kg; and the dosage of anti- CD20 agent as an anti- cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 4 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 6 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 μg. In some embodiments, the dosage of the compound of the disclosure administered in combination therapy is about 8 mg/kg; and the dosage of anti- CD20 agent as an anti-cancer agent in combination therapy is about 200 μg [0248] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day. In some embodiments, a pharmaceutically- acceptable amount of a compound of the disclosure can be administered to a subject once a day. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject three times a day. [0249] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day once every 3 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day once every 3 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 7 days. In some embodiments, a pharmaceutically- acceptable amount of a compound of the disclosure can be administered to a subject once a day once every 7 days. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day once every 7 days. [0250] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for 1 to 50 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 5 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 10 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 15 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can
be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 20 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 25 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 30 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject 1, 2, 3, 4, or 5 times a day once every 1, 2, 3, 4, 5, 6, or 7 days for about 35 doses. [0251] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 7 days for about 5 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 7 days for about 10 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 15 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 3 days for about 20 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject once a day every 3 days for about 35 doses. [0252] In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 5 doses. In some embodiments, a pharmaceutically-acceptable amount of a compound of the disclosure can be administered to a subject twice a day every 7 days for about 15 doses. Pharmaceutical compositions for combination treatment [0253] According to certain embodiments, the compounds and the anti-cancer agent described herein are administered within a single pharmaceutical composition. In some embodiments, the compounds of the disclosure and the anti-cancer agent described herein can be provided in a single unit dosage form for being taken together. According to some embodiments, the pharmaceutical composition further comprises a pharmaceutically-acceptable diluent or carrier. According to certain embodiments, the compounds of the disclosure and the anti-cancer agent described herein are administered within different pharmaceutical compositions. In some embodiments, the compounds of the disclosure and the anti-cancer agent described herein can be provided in a single unit dosage as separate entities (e.g., in separate containers) to be administered simultaneously or with a certain time difference. [0254] In some embodiments, the compounds of the disclosure and the anti-cancer agent described herein can be administered via the same route of administration. In some embodiments, the compounds of the disclosure and the anti-cancer agent described herein can be administered via the different route of administration. In some embodiments, a compound of the disclosure and the anti-cancer agent are administered orally. In some embodiments, a compound of the disclosure is administered orally, and the anti-cancer agent is not administered orally. In some embodiments, a compound of the disclosure is not administered orally, and the anti-cancer agent is administered orally.
[0255] Treatment of a condition by administering a compound of the disclosure in combination with an anti-cancer agent can increase a median survival time of a subject compared to subjects who do not receive the combination therapy. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that does not receive any cancer therapy. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that receives therapy with a compound of the disclosure alone. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population that receives therapy with the anti- cancer agent alone. [0256] In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy (e.g., no therapy, a compound of the disclosure alone, or the anti-cancer agent alone) by at least about 50%, about 60%, about 70%, about 80%, about 90%, about 100%, about 110%, about 120%, about 130%, about 140%, about 150%, about 160%, about 170%, about 180%, about 190%, about 200%, about 210%, about 220%, about 230%, about 240%, about 250%, about 260%, about 270%, about 280%, about 290%, about 300%, about 310%, about 320%, about 330%, about 340%, about 350%, about 360%, about 370%, about 380%, about 390%, about 400%, about 410%, about 420%, about 430%, about 440%, about 450%, about 460%, about 470%, about 480%, about 490%, or about 500%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 50%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 100%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 150%. In some embodiments, a median survival time of a first patient population receiving the combination therapy can be greater than a median survival time of a second patient population not receiving the combination therapy by at least about 200%. Methods of treatment [0257] Provided herein is a method of treating cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation. In some embodiments, a compound of the disclosure comprises a substituted 4,5,6,7-tetrahydrothieno[2,3-c]pyridine group, wherein the compound blocks SUMOylation. In some embodiments, the compound binds to Uba2. In some embodiments, the compound has an inhibitory effect on activating enzymes (E1) of SUMO. [0258] In some embodiments, the cancer is ovarian cancer. In some embodiments, the cancer is breast
cancer. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is AML. In some embodiments, the cancer is colon cancer. In some embodiments, the cancer is a lymphoma. In some embodiments, the subject is human. [0259] In some embodiments, the administering of the compound is oral. In some embodiments, the administering of the compound is subcutaneous. In some embodiments, the administering of the compound is topical. [0260] In some embodiments, the therapeutically-effective amount of the compound is from about 1 mg/kg to about 20 mg/kg. In some embodiments, the therapeutically-effective amount of the compound is from about 5 mg to about 10 mg.. In some embodiments, the therapeutically-effective amount of the compound is about 50 mg, about 5100 mg, about 150 mg, about 200 mg, about 250 mg, about 300 mg, about 350 mg, about 400 mg, about 450 mg, or about 500 mg. In some embodiments, the therapeutically- effective amount of the compound is about 150 mg. In some embodiments, the therapeutically-effective amount of the compound is about 200 mg. In some embodiments, the therapeutically-effective amount of the compound is about 300 mg. In some embodiments, the therapeutically-effective amount of the compound is about 400 mg. [0261] In some embodiments, the anti-cancer agent is a small molecule. In some embodiments, the anti- cancer agent is an antibody. [0262] In some embodiments, the anti-cancer agent is an immune checkpoint inhibitor. In some embodiments, the immune checkpoint inhibitor is an anti-PD-1 agent. In some embodiments, the anti-PD- 1 agent is nivolumab. In some embodiments, the anti-PD-1 agent is pembrolizumab. In some embodiments, the anti-PD-1 agent is cemiplimab. In some embodiments, the immune checkpoint inhibitor is an anti-PD-L1 agent. In some embodiments, the anti-PD-L1 agent is atezolizumab. In some embodiments, the anti-PD-L1 agent is avelumab. In some embodiments, the anti-PD-L1 agent is durvalumab. In some embodiments, the administering of the anti-cancer agent is oral. In some embodiments, the administering of the anti-cancer agent is subcutaneous. In some embodiments, the administering of the anti-cancer agent is topical. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is from about 5 mg/kg to about 500 mg/kg. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is from about 10 μg to about 500 μg. In some embodiments, the therapeutically-effective amount of the anti-cancer agent is about 200 μg. [0263] In some embodiments, the anti-cancer agent is an anti-CD20 antibody. Such anti-CD-20 antibodies include rituximab (Mabthera®/ Rituxan®), obinutuzumab (Gazyva®), ibritumomab tiuxetan, tositumomab, ofatumumab (Genmab®), ocaratuzumab, ocrelizumab, TRU-015, and veltuzumab. In some cases, the anti-cancer agent is rituximab. In some embodiments, the therapeutically-effective amount of the anti-CD20 antibody is from about 5 mg/kg to about 500 mg/kg. In some embodiments, the therapeutically-effective amount of the anti-CD20 antibody is from about 10 μg to about 500 μg. [0264] In some embodiments, the anti-cancer agent is a hypomethylating agent (HMA). In some embodiments, a compound of the disclosure is used in combination with a HMA, such as azacitidine (Vidaza®, Azadine®) or decitabine (Dacogen®). In some embodiments, the therapeutically-effective
amount of the HMA is from about 5 mg/kg to about 500 mg/kg. In some embodiments, the therapeutically-effective amount of the HMA is from about 0.005 μM to about 0.500 μM. [0265] Each patent, publication, and non-patent literature cited in this disclosure is hereby incorporated by reference in its entirety as if each was incorporated by reference individually. EXAMPLES EXAMPLE 1: Compounds of the disclosure [0266] SUMO inhibitors were prepared as described in PCT publication WO2020191151, WO2021150918 and PCT application PCT/US22/73985 and are shown in TABLE A. For the examples below, Compound A is (S,E)-6-(4-(dimethylamino)but-2-enoyl)-4-(2-(1-ethyl-3-(trifluoromethyl)-1H- pyrazol-4-yl)-3-fluorophenyl)-3-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-2-carbonitrile. EXAMPLE 2: Combination of Compound A and anti-CD-20 agents [rituximab] in subcutaneous mouse lymphoma xenograft model [Raji human B cell] [0267] The anti-tumor efficacy of Compound A in combination with rituximab was evaluated in the subcutaneous Raji human lymphoma xenograft model in female CB17 SCID mice. The overall dosing regimen is included in Table 1. The Raji tumor cells (ATCC, Cat# CCL-86) were maintained in vitro as a suspension culture in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37ºC in an atmosphere of 5% CO2 in air. The tumor cells were routinely subcultured twice weekly. The cells growing to a confluency around 70% - 80% were harvested and counted for tumor inoculation. 75 CB17 SCID mice [female, 6-8 weeks old, 18-20g weight] were inoculated subcutaneously at the right flanks with Raji cells for tumor development. The mice were kept in individual ventilation cages at constant temperature and humidity with 4 animals in each cage [temperature 20-26 C and 40-70% humidity]. Fourteen days after tumor inoculation, 48 mice with tumor sizes ranging from 65-268 mm3 (average tumor size 147.16 mm3) were selected and assigned into 6 groups using stratified randomization with 8 mice in each group based upon their tumor volumes. The treatments were started from the day of randomization (defined as PG-D1) and mice were treated as follows: Group 1: Vehicle control, p.o., (BIW on D1/4/8/11/14); Group 2: Compound A, 50 mg/kg, p.o., (BIW on D1/4/8/11/14/17); Group 3: Rituximab, 20 mg/kg, i.p., (BIW on D1/4/8/11/14); Group 4: Compound A, 50 mg/kg, p.o.,+ Rituximab, 20 mg/kg, i.p., (BIW on D1/4/8/11/14/17); Group 5: Compound A, 50 mg/kg, p.o., (QD x 17 days); and Group 6: SUMO comparator, 15 mg/kg, i.v., (BIW on D1/4/8/11/14/17). The entire study was terminated on day 17 and plasma samples were collected for analysis. Dosing was QD: daily, Q2D: once every 2 days, QW: once weekly. BIW: twice weekly. BID: twice daily. All the treatments groups showed significant statistically difference on tumor growth inhibition (p<0.0001 vs. vehicle control via Two-way RM ANOVA). P values represent statistical significance compared to the vehicle control group. COMPOUND A inhibits the growth of lymphoma tumors in vivo.
[0268] Body weights were measured at days 1, 4, 8, 11 and 14 after the start of treatment. No obvious body weight loss was observed during the dosing period, all the treatments were tolerated well. Relative change of body Weight (RCBW) of each mouse was calculated according to the following formula: RCBW (%) = (BWTreatment_DayN - BWTreatment_Day0)/ BWTreatment_Day0×100%. Weight change of less than 5% was observed. See Figure 2. [0269] Tumor Measurements and Endpoints - The major endpoint is to see if the tumor growth can be delayed or mice can be cured. Tumor sizes were measured two times a week in two dimensions using a caliper, and the volume was expressed in mm3 using the formula: V = 0.5 a x b2 where a and b are the long and short diameters of the tumor, respectively. The tumor sizes were used for the calculations of both tumor growth inhibition (TGI) values. TGI is calculated for each group using the formula: TGI (%) = [1- (TVTreatment_DayN- TVTreatment_Day0)/ (TVVehicle_DayN - TVVehicle_Day0)] ×100%; TVTreatment_DayN is the average tumor volume of a treatment group on a given day, TVTreatment_Day0 is the average tumor volume of the treatment group on the first day of treatment, TVVehicle_DayN is the average tumor volume of the vehicle control group on a given day, and TVVehicle_Day0 is the average tumor volume of the vehicle group on the first day of treatment. Mean tumor volumes over time are shown in Table 2 and tumor growth curves are shown in Figure 1. Tumor growth inhibition values are shown in Table 3.
EXAMPLE 3: Combination of Compound A and a hypomethylating agent (HMA) in AML cell line model [GDM-1 cells and MV4-11] [0270] Assay Principle - This cellular assay measures the viability of leukemia cell lines upon treatment with a SUMO E1 inhibitor, Compound A, and a hypomethylating agent (HMA), decitabine, as single agents, as well as in combination. Cell viability was measured using the Promega CellTiter-Glo Luminescent Cell Viability Assay, which determines the number of viable cells based on the quantitation of ATP (as an indicator of metabolically active cells). Three acute myeloid leukemia (AML) cell lines, including GDM-1, and MV4-11, were first treated with Compound A or decitabine as single treatments to establish single agent IC50s to describe the quantity of compound needed to inhibit 50% of cell viability. Next, cells were treated with both Compound A and decitabine, with 4 to 5 decreasing doses of each compound, to generate a matrix of 25 combinations. Dose response curves and IC50 values were generated, and Zero interaction potency (ZIP) synergy scores were calculated using SynergyFinder 3.0 (https://synergyfinder.fimm.fi/synergy/20220701215205193938/). The ZIP model determines whether combination treatments result in synergistic or additive effects by comparing the potency at specific dose levels between single and combination treatments. Overall, the cell viability of three different AML cells lines were evaluated during single and combination treatment with Compound A and decitabine to determine the efficacy of combination therapy. [0271] GDM-1 and MV4-11 cells were plated overnight in white-walled 96-well plates (10,000 cells/well in 100ul total volume). The next day, compounds were diluted in cell culture media and cells were treated with 4 to 5 different doses of Compound A and decitabine (either as single treatment or in combination). Cells were then incubated for 72 hrs. At 72 hrs post-treatment, cells were lysed with the CellTiter-Glo 2.0 reagent, and luminescence signal was read using the Glomax Discover Promega plate reader (program CellTiter-Glo). The raw data were analyzed in Excel to calculate percent inhibition compared to a DMSO control and the obtained values were plotted using GraphPad Prism 9 (ver.9.4.0) to derive dose response curves and IC50 values. Zero interaction potency (ZIP) synergy scores were calculated using SynergyFinder 3.0 (https://synergyfinder.fimm.fi/synergy/20220701215205193938/). [0272] Two different AML cell lines were tested to determine whether the efficacy of Compound A could be enhanced by combining with the chemotherapeutic compound decitabine. Cells were treated with 4 to 5 different doses of Compound A in combination with 4 to 5 doses of decitabine, and cell viability was measured at 72 hrs post-treatment. Figure 3A shows cell viability dose response curves of Compound
A alone, along with the combination of escalating doses with decitabine in GDM-1 and MV-411 cells. The IC50 values from each curve, along with the calculated ZIP score are found in Table 4. For all three cell lines, the combination of Compound A and decitabine impacted cell viability, as seen in the shift in the dose response curves (Figure 3A) and in the reduction in the IC50 values compared to treatment with Compound A alone (Table 4). In addition, all three cell lines exhibited an additive effect when the two drugs were combined, as shown by calculated ZIP values (between -10 and 10) using SynergyFinder 3.0 (Table 4). This additive effect can also be seen in Figure 3B, where combination treatment led to a greater % inhibition when compared to single treatment with Compound A or decitabine. As seen in Figure 3B, single treatment of MV-411 cells with the indicated doses of Compound A or decitabine resulted in between 40-80% inhibition, but that the combination of both compounds resulted in 100% inhibition. Overall, these data indicate that the efficacy of Compound A and was increased upon combination with decitabine. [0273] Compound A alone inhibited GDM-1 cell growth with an IC50 of 0.209 µM, and addition of decitabine caused left-shifted IC50 curves (left graph), and decreasing IC50 values for Compound A (right table) indicating enhanced anti-tumor activity. [0274] Compound A (0.167µM) or decitabine (0.167µM) as single agents inhibited MV4-11 cell growth, but the combination of the two agents resulted in greater inhibition of tumor cell growth (left graph). In dose response format, Compound A inhibited the growth of MV4-11 cells with an IC50 of 0.16 µM, and this decreased with increasing concentrations of decitabine (right table).
EXAMPLE 4: Combination of Compound A and anti-PD-1 agent in a mouse colon cancer model [0275] The combinations of the present invention are evaluated in CT26 model of syngeneic colon cancer. See Sato, Y., Fu, Y., Liu, H. et al. Tumor-immune profiling of CT-26 and Colon 26 syngeneic mouse models reveals mechanism of anti-PD-1 response. BMC Cancer 21, 1222 (2021). For
the CT26 tumor study, BALB/c mice are injected subcutaneously with 1 x 105 CT26 cells on Day 0. Beginning day 5, mice are treated with Compound A [50 mpk]. and 250 pg of anti-PD-1 (BioXcell, Clone RMP1-14) or isotype control (BioXcell, Clone 2A3). Subsequently, all mice were continued on study the assigned combination therapy on days 12, 15, and 19. [0276] Tumor volume is assessed using the formula TV = 0.5 x (L x W2) where length (L) is the longest dimension of the tumor and width (W) is the longest dimension perpendicular to the length. Statistical significance of treatment is assessed using Prism V8.0 software (GraphPad Software, San Diego, CA). [0277] Administration of Compound A results in dose responsive anti-tumor effect with tumor grow delay. Combination therapy with anti-PD-1 significantly improves the anti-tumor effect demonstrating regression, cures, and a significant increase in median survival time at the highest dose level. EMBODIMENTS [0278] The following non-limiting embodiments provide illustrative examples of the invention, but do not limit the scope of the invention. [0279] Embodiment 1. A method of treating a cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation. [0280] Embodiment 2. The method of embodiment 1, wherein the compound binds to Uba2. [0281] Embodiment 3. The method of embodiment 1, wherein the compound has an inhibitory effect on activating enzymes (E1) of SUMO. [0282] Embodiment 4. The method of embodiment 1, wherein the cancer is selected from acute myeloid leukemia, large B-cell lymphoma, lung squamous cell carcinoma, pancreatic adenocarcoma, esophegeal carcinoma, cervical squamous cell carcinoma, endocervical adenocarcoma, stomach adenocarcinomathymoma, renal cell carcinoma, head and neck squamous cell carcinoma, bladder carcinoma, ovarian cystadenocarcinoma, multiple myeloma, non-Hodgkin’s lymphoma (NHL), and mesothelioma. [0283] Embodiment 5. The method of embodiment 1, wherein the cancer is selected from head and neck squamous cell carcinoma (HNSCC), non-squamous non-small cell lung cancer (NSCLC), cervical cancer, colorectal cancer (CRC), cutaneous melanoma, squamous NSCLC, and small cell lung cancer. [0284] Embodiment 6. The method of embodiment 1, wherein the cancer is B Cell Lymphoma. [0285] Embodiment 7. The method of embodiment 1, wherein the cancer is acute myeloid leukemia. [0286] Embodiment 8. The method of embodiment 1, wherein the cancer is colon cancer. [0287] Embodiment 9. The method of embodiment 1, wherein the administering of the compound is oral. [0288] Embodiment 10. The method of embodiment 1, wherein the subject is human [0289] Embodiment 11. The method of embodiment 1, wherein the therapeutically-effective amount of the compound is from about 100 mg to about 500 mg. [0290] Embodiment 12. The method of any one of embodiments 1-12, wherein the therapeutically-
effective amount of the compound is from about 200 mg to about 400 mg. [0291] Embodiment 13. The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 300 mg. [0292] Embodiment 14. The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 200 mg. [0293] Embodiment 15. The method of any one of embodiments 1-13, wherein the therapeutically- effective amount of the compound is about 400 mg. [0294] Embodiment 18. The method of any one of embodiments 1-17, wherein the anti-cancer agent is a small molecule. [0295] Embodiment 19. The method of any one of embodiments 1-17, wherein the anti-cancer agent is an antibody. [0296] Embodiment 20. The method of any one of embodiments 1-17, wherein the anti-cancer agent is an immune checkpoint inhibitor. [0297] Embodiment 21. The method of any one of embodiments 1-17 or 20, wherein the immune checkpoint inhibitor is an anti-PD-1 agent. [0298] Embodiment 22. The method of any one of embodiments 1-17, 20, or 21, wherein the anti-PD-1 agent is nivolumab. [0299] Embodiment 23. The method of any one of embodiments 1-17, 20, or 21, wherein the anti-PD-1 agent is pembrolizumab. [0300] Embodiment 24. The method of any one of embodiments 1-17, 20, or 21, wherein the anti-PD-1 agent is cemiplimab. [0301] Embodiment 25. The method of any one of embodiments 1-17 or 20, wherein the immune checkpoint inhibitor is an anti-PD-L1 agent. [0302] Embodiment 26. The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is atezolizumab. [0303] Embodiment 27. The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is avelumab. [0304] Embodiment 28. The method of any one of embodiments 1-17, 20, or 25, wherein the anti-PD-L1 agent is durvalumab. [0305] Embodiment 29. The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is oral. [0306] Embodiment 30. The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is subcutaneous. [0307] Embodiment 31. The method of any one of embodiments 1-28, wherein the administering of the anti-cancer agent is topical. [0308] Embodiment 32. The method of any one of embodiments 1-31, wherein the therapeutically- effective amount of the anti-cancer agent is from about 1 mg/kg to about 20 mg/kg. [0309] Embodiment 33. The method of any one of embodiments 1-31, wherein the therapeutically-
effective amount of the anti-cancer agent is from about 10 μg to about 500 μg. [0310] Embodiment 34. The method of any one of embodiments 1-31 or 33, wherein the therapeutically- effective amount of the anti-cancer agent is about 200 μg. [0311] Embodiment 35. The method of any one of embodiments 1-34, wherein the compound is of the formula:
wherein is a single bond or double bond; wherein l, m, n are each independently an integer from 0 to 2; wherein M is selected from CR3R4, NR5, C=O, O, S=O, O=S=O, and S; wherein Y is selected from CR6R7, NR8, C=O, O, S=O, O=S=O, and S; wherein Z is CR9, or N; wherein ring A is selected from a) 5- or 6-membered partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; wherein E is an electrophilic moiety, selected from:
; Wherein R1 is selected from hydrogen, halogen, -C(X1)3, -CHX12, -CH2X1, -OCX13, -OCH2X1, -OCHX12, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R2 is selected from hydrogen, halogen, -CX23, -CHX22, -CH2X2, -OCX23, -OCH2X2, -OCHX22, -CN, -SOn2R2A, -SOv2NR2AR2B, -NHC(O)NR2AR2B, -N(O)m2, -NR2AR2B, -NHNR2AR2B, -C(O)R2A, -C(O)-OR2A, -C(O)NR2AR2B, -C(O)NHNR2AR2B, -OR2A, -NR2ASO2R2B, -NR2AC(O)R2B, -NR2AC(O)OR2B, -NR2AOR2B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R1 and R2 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
Wherein R3 is selected from hydrogen, halogen, -CX3 3, -CHX3 2, -CH2X3, -OCX3 3, -OCH2X3, -OCHX32, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; Wherein R5 is selected from hydrogen, halogen, -CX53, -CHX52, -CH2X5, -OCX53, -OCH2X5, -OCHX52, -CN, -SOn5R5A, -SOv5NR5AR5B, -NHC(O)NR5AR5B, -N(O)m5, -NR5AR5B, -NHNR5AR5B, -C(O)R5A, -C(O)-OR5A, -C(O)NR5AR5B, -C(O)NHNR5AR5B, -OR5A, -NR5ASO2R5B, -NR5AC(O)R5B, -NR5AC(O)OR5B, -NR5AOR5B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R6 is selected from hydrogen, halogen, -CX6 3, -CHX6 2, -CH2X6, -OCX6 3, -OCH2X6, -OCHX6 2, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B, -NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R7 is selected from hydrogen, halogen, -CX7 3, -CHX7 2, -CH2X7, -OCX7 3, -OCH2X7, -OCHX7 2, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7, -NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R8 is selected from hydrogen, halogen, -CX8 3, -CHX8 2, -CH2X8, -OCX8 3, -OCH2X8, -OCHX8 2, -CN, -SOn8R8A, -SOv8NR8AR8B, -NHC(O)NR8AR8B, -N(O)m8, -NR8A87B, -NHNR8AR8B, -C(O)R8A, -C(O)-OR8A, -C(O)NR8AR8B, -C(O)NHNR8AR8B,
-OR8A, -NR8ASO2R8B, -NR8AC(O)R8B, -NR8AC(O)OR8B, -NR8AOR8B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R10 is selected from hydrogen, halogen, -CX103, -CHX102, -CH2X10, -OCX103, -OCH2X10, -OCHX102, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R11 is selected from hydrogen, halogen, -CX113, -CHX112, -CH2X11, -OCX113, -OCH2X11, -OCHX112, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12, -NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B, -C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R13 is selected from hydrogen, halogen, -CX13 3, -CHX13 2, -CH2X13, -OCX13 3, -OCH2X13, -OCHX13 2, -CN, -SOn13R13A, -SOv13NR13AR13B, -NHC(O)NR13AR13B, -N(O)m13, -NR13AR13B, -NHNR13AR13B, -C(O)R13A, -C(O)-OR13A, -C(O)NR13AR13B, -C(O)NHNR13AR13B, -OR13A, -NR13ASO2R13B, -NR13AC(O)R13B, -NR13AC(O)OR13B, -NR13AOR13B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R14 is selected from hydrogen, halogen, -CX143, -CHX142, -CH2X14, -OCX143, -OCH2X14, -OCHX142, -CN, -SOn14R14A, -SOv14NR14AR14B, -NHC(O)NR14AR14B, -N(O)m14, -NR14AR14B, -NHNR14AR14B, -C(O)R14A, -C(O)-OR14A, -C(O)NR14AR14B, -C(O)NHNR14AR14B, -OR14A, -NR14ASO2R14B, -NR14AC(O)R14B, -NR14AC(O)OR14B, -NR14AOR14B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R15 is selected from hydrogen, halogen, -CX153, -CHX152, -CH2X15, -OCX153, -OCH2X15, -OCHX152, -CN, -SOn15R15A, -SOv15NR15AR15B, -NHC(O)NR15AR15B, -N(O)m15, -NR15AR15B, -NHNR15AR15B, -C(O)R15A, -C(O)-OR15A, -C(O)NR15AR15B, -C(O)NHNR15AR15B, -OR15A, -NR15ASO2R15B, -NR15AC(O)R15B, -NR15AC(O)OR15B, -NR15AOR15B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 is selected from hydrogen, halogen, -CX163, -CHX162, -CH2X16, -OCX163, -OCH2X16, -OCHX162, -CN, -SOn16R16A, -SOv16NR16AR16B, -NHC(O)NR16AR16B, -N(O)m16, -NR16AR16B, -NHNR16AR16B, -C(O)R16A, -C(O)-OR16A, -C(O)NR16AR16B, -C(O)NHNR16AR16B, -OR16A, -NR16ASO2R16B, -NR16AC(O)R16B, -NR16AC(O)OR16B, -NR16AOR16B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 is selected from hydrogen, halogen, -CX17 3, -CHX17 2, -CH2X17, -OCX17 3, -OCH2X17, -OCHX17 2, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R18 is selected from hydrogen, halogen, -CX18 3, -CHX18 2, -CH2X18, -OCX18 3, -OCH2X18, -OCHX18 2, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl;
R19 is selected from hydrogen, halogen, -CX19 3, -CHX19 2, -CH2X19, -OCX19 3, -OCH2X19, -OCHX192, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 is selected from hydrogen, halogen, -CX203, -CHX202, -CH2X20, -OCX203, -OCH2X20, -OCHX202, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R20 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R21 is selected from hydrogen, halogen, -CX213, -CHX212, -CH2X21, -OCX213, -OCH2X21, -OCHX212, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R22 is selected from hydrogen, halogen, -CX22 3, -CHX22 2, -CH2X22, -OCX22 3, -OCH2X22, -OCHX22 2, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B, -NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 and R22 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; wherein R23 is selected from hydrogen, halogen, -CX23 3, -CHX23 2, -CH2X23, -OCX23 3, -OCH2X23, -OCHX23 2, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
wherein R24 is selected from hydrogen, halogen, -CX24 3, -CHX24 2, -CH2X24, -OCX24 3, -OCH2X24, -OCHX242, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R25 is selected from hydrogen, halogen, -CX253, -CHX252, -CH2X25, -OCX253, -OCH2X25, -OCHX252, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B, -C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R26 is selected from hydrogen, halogen, -CX263, -CHX262, -CH2X26, -OCX263, -OCH2X26, -OCHX262, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R27 is selected from hydrogen, halogen, -CX27 3, -CHX27 2, -CH2X27, -OCX27 3, -OCH2X27, -OCHX27 2, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B, -C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R28 is selected from hydrogen, halogen, -CX28 3, -CHX28 2, -CH2X28, -OCX28 3, -OCH2X28, -OCHX28 2, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B, -C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R29 is selected from hydrogen, halogen, -CX29 3, -CHX29 2, -CH2X29, -OCX29 3, -OCH2X29, -OCHX29 2, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B,
-C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R30 is selected from hydrogen, halogen, -CX303, -CHX302, -CH2X30, -OCX303, -OCH2X30, -OCHX302, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR30B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R2A, R2B, R3A, R3B, R4A, R4B, R5A, R5B, R6A, R6B, R7A, R7B, R8A, R8B, R9A, R9B, R10A, R10B, R11A, R11B, R12A, R12B, R13A, R13B, R14A, R14B, R15A, R15B, R16A, R16B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B, R28A, R28B, R29A, R29B, R30A, R30B is independently selected from hydrogen, -CX3, -CHX2, -CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, -NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; wherein R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5A and R5B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R8A and R8B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R10A and R10B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R11A and R11B substituents bonded to
the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R12A and R12B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to
the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; Wherein each m1, m2, m3, m4, m5, m6, m7, m8, m9, m10, m11, m12, m13, m14, m15, m16, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, and m30 are independently 1 or 2; wherein each v1, v2, v3, v4, v5, v6, v7, v8, v9, v10, v11, v12, v13, v14, v15, v16, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29 and v30 are independently 1 or 2; wherein each n1, n2, n3, n4, n5, n6, n7, n8, n9, n10, n11, n12, n13, n14, n15, n16, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29 and n30 are independently an integer from 0 to 2; and wherein each X, X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12, X13, X14, X15, X16, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29 and X30 are independently -Cl, -Br, -I or –F. [0312] Embodiment 36. The method of embodiment 35 wherein l is 1; m is 0; and n is 1. [0313] Embodiment 37. The method of embodiment 35 wherein Z is N. [0314] Embodiment 38. The method of embodiment 35 wherein ring A is selected from 5-membered heteroaryl, wherein ring A is optionally substituted with one or more substituent groups. [0315] Embodiment 39. The method of embodiment 35 R2 is selected from substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. [0316] Embodiment 40. The method of embodiment 38 ring A is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein ring A is optionally substituted with one or more substituent groups [0317] Embodiment 41. The method of embodiment 40 wherein ring A is selected from
. [0318] Embodiment 42. The method of embodiment 39 wherein R2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cycloalkyl and substituted or unsubstituted 5- or 6- membered heteroaryl [0319] Embodiment 43. The method of embodiment 42, wherein R2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted cyclopentyl, substituted or unsubstituted cyclohexyl and substituted or unsubstituted pyridyl [0320] Embodiment 44. The method of embodiment 35 wherein E is selected from -C(=O)CH=CH2, - C(=O)-ethynyl, -C(=O)CH=CHCF3, -C(=O)CH=CHCHF2, -C(=O)CH=CHCH2F, 4-(dimethylamino)but- 2-en-1-one and -C(=O)CH2Br. [0321] Embodiment 45. The method of embodiment 35 wherein M is CR3R4. [0322] Embodiment 46. The method of embodiment 35 wherein
,
. [0323] Embodiment 47. The method of embodiment 35 wherein Y is CR6R7. [0324] Embodiment 48. The method of any one of embodiments 1-34, wherein the compound is of the formula:
wherein Z is N, or CR9; W is selected from NR12, O, S, S=O, O=S=O, and Se; ring B is selected from a) 5- or 6-membered cycloalkyl, saturated or partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; E is selected from an electrophilic moiety, selected from
; R1 is selected from hydrogen, halogen, -CX13, -CHX12, -CH2X1, -OCX13, -OCH2X1, -OCHX12, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, rand substituted or unsubstituted heteroaryl; R3 is selected from hydrogen, halogen, -CX33, -CHX32, -CH2X3, -OCX33, -OCH2X3, -OCHX32, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R6 is selected from hydrogen, halogen, -CX63, -CHX62, -CH2X6, -OCX63, -OCH2X6, -OCHX62, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B, -NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R7 is selected from hydrogen, halogen, -CX7 3, -CHX7 2, -CH2X7, -OCX7 3, -OCH2X7, -OCHX7 2, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7, -NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R10 is selected from hydrogen, halogen, -CX103, -CHX102, -CH2X10, -OCX103, -OCH2X10, -OCHX102, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R11 is selected from hydrogen, halogen, -CX113, -CHX112, -CH2X11, -OCX113, -OCH2X11, -OCHX112, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12, -NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B, -C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 is selected from hydrogen, halogen, -CX17 3, -CHX17 2, -CH2X17, -OCX17 3, -OCH2X17, -OCHX17 2, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R18 is selected from hydrogen, halogen, -CX18 3, -CHX18 2, -CH2X18, -OCX18 3, -OCH2X18, -OCHX18 2, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R19 is selected from hydrogen, halogen, -CX193, -CHX192, -CH2X19, -OCX193, -OCH2X19, -OCHX192, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R19 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R20 is selected from hydrogen, halogen, -CX203, -CHX202, -CH2X20, -OCX203, -OCH2X20, -OCHX202, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 is selected from hydrogen, halogen, -CX21 3, -CHX21 2, -CH2X21, -OCX21 3, -OCH2X21, -OCHX21 2, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 and R21 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R22 is selected from hydrogen, halogen, -CX22 3, -CHX22 2, -CH2X22, -OCX22 3, -OCH2X22, -OCHX22 2, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B, -NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R23 is selected from hydrogen, halogen, -CX23 3, -CHX23 2, -CH2X23, -OCX23 3, -OCH2X23, -OCHX23 2, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R24 is selected from hydrogen, halogen, -CX243, -CHX242, -CH2X24, -OCX243, -OCH2X24, -OCHX242, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R25 is selected from hydrogen, halogen, -CX253, -CHX252, -CH2X25, -OCX253, -OCH2X25, -OCHX252, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B, -C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R26 is selected from hydrogen, halogen, -CX263, -CHX262, -CH2X26, -OCX263, -OCH2X26, -OCHX262, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R27 is selected from hydrogen, halogen, -CX273, -CHX272, -CH2X27, -OCX273, -OCH2X27, -OCHX272, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B, -C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R28 is selected from hydrogen, halogen, -CX28 3, -CHX28 2, -CH2X28, -OCX28 3, -OCH2X28, -OCHX28 2, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B, -C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R29 is selected from hydrogen, halogen, -CX29 3, -CHX29 2, -CH2X29, -OCX29 3, -OCH2X29, -OCHX29 2, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B, -C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R30 is selected from hydrogen, halogen, -CX30 3, -CHX30 2, -CH2X30, -OCX30 3, -OCH2X30, -OCHX30 2, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR30B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R31 is selected from hydrogen, halogen, -CX313, -CHX312, -CH2X31, -OCX313, -OCH2X31, -OCHX312, -CN, -SOn31R31A, -SOv31NR31AR31B, -NHC(O)NR31AR31B, -N(O)m31, -NR31AR31B, -NHNR31AR31B, -C(O)R31A, -C(O)-OR31A, -C(O)NR31AR31B, -C(O)NHNR31AR31B, -OR31A, -NR31ASO2R31B, -NR31AC(O)R31B, -NR31AC(O)OR31B, -NR31AOR31B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R32 is selected from hydrogen, halogen, -CX323, -CHX322, -CH2X32, -OCX323, -OCH2X32, -OCHX322, -CN, -SOn32R32A, -SOv32NR32AR32B, -NHC(O)NR32AR32B, -N(O)m32, -NR32AR32B, -NHNR32AR32B, -C(O)R32A, -C(O)-OR32A, -C(O)NR32AR32B, -C(O)NHNR32AR32B, -OR32A, -NR32ASO2R32B, -NR32AC(O)R32B, -NR32AC(O)OR32B, -NR32AOR32B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R33 is selected from hydrogen, halogen, -CX333, -CHX332, -CH2X33, -OCX333, -OCH2X33, -OCHX332, -CN, -SOn33R33A, -SOv33NR33AR33B, -NHC(O)NR33AR33B, -N(O)m33, -NR33AR33B, -NHNR33AR33B, -C(O)R33A, -C(O)-OR33A, -C(O)NR33AR33B, -C(O)NHNR33AR33B, -OR33A, -NR33ASO2R33B, -NR33AC(O)R33B, -NR33AC(O)OR33B, -NR33AOR33B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R34 is selected from hydrogen, halogen, -CX34 3, -CHX34 2, -CH2X34, -OCX34 3, -OCH2X34, -OCHX34 2, -CN, -SOn34R34A, -SOv34NR34AR34B, -NHC(O)NR34AR34B, -N(O)m34, -NR34AR34B, -NHNR34AR34B, -C(O)R34A, -C(O)-OR34A, -C(O)NR34AR34B, -C(O)NHNR34AR34B, -OR34A, -NR34ASO2R34B, -NR34AC(O)R34B, -NR34AC(O)OR34B, -NR34AOR34B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R35 is selected from hydrogen, halogen, -CX35 3, -CHX35 2, -CH2X35, -OCX35 3, -OCH2X35, -OCHX35 2, -CN, -SOn35R35A, -SOv35NR35AR35B, -NHC(O)NR35AR35B, -N(O)m35, -NR35AR35B, -NHNR35AR35B, -C(O)R35A, -C(O)-OR35A, -C(O)NR35AR35B, -C(O)NHNR35AR35B, -OR35A, -NR35ASO2R35B, -NR35AC(O)R35B, -NR35AC(O)OR35B, -NR35AOR35B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R3A, R3B, R4A, R4B, R6A, R6B, R7A, R7B, R9A, R9B, R10A, R10B, R11A, R11B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B,
R28A, R28B, R29A, R29B, R30A, R30B, R31A, R31B, R32A, R32B, R33A, R33B, R34A, R34B, R35A, R35B, is independently selected from hydrogen, -CX3, -CHX2, -CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, -NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same
nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; Each m1, m3, m4, m6, m7, m9, m10, m11, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, m30, m31, m32, m33, m34 and m35 is independently 1 or 2; Each v1, v3, v4, v6, v7, v9, v10, v11, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29, v30, v31, v32, v33, v34 and v35 is independently 1 or 2; Each n1, n3, n4, n6, n7, n9, n10, n11, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29, n30, n31, n32, n33, n34 and n35 is independently an integer from 0 to 2; and Each X1, X3, X4, X6, X7, X9, X10, X11, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29, X30, X31, X32, X33, X34, and X35 is independently -Cl, -Br, -I or –F. [0325] Embodiment 49. The method of embodiment 48 wherein ring B is selected from thienyl, furanyl, pyrrolyl, thiazolyl, oxazolyl, imidazolyl, pyrazolyl, isoxazolyl, triazolyl and isothiazolyl, wherein Ring B is optionally substituted with one or more substituents. [0326] Embodiment 50. The method of embodiment 48 wherein ring B is selected from phenyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, and triazinyl, wherein ring B is optionally substituted with one or more substituent groups. [0327] Embodiment 51. The method of embodiment 50 wherein ring B is selected from
[0328] Embodiment 52. The method of embodiment 48 wherein E is selected from -C(=O)CH=CH2, - C(=O)-ethynyl, -C(=O)CH=CHCF3, -C(=O)CH=CHCHF2, -C(=O)CH=CHCH2F, 4-(dimethylamino)but-
2-en-1-one and -C(=O)CH2Br. [0329] Embodiment 53. The method of embodiment 48 wherein W is S. [0330] Embodiment 54. The method of embodiment 48 wherein R10 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl; and R11 is selected from hydrogen, fluoro, chloro, substituted or unsubstituted C1-6 alkyl, substituted or unsubstituted C1-6 heteroalkyl, substituted or unsubstituted C3-6 cycloalkyl, substituted or unsubstituted 3-6 membered heterocycloalkyl, substituted or unsubstituted 6-10 membered aryl, and substituted or unsubstituted 5-10 membered heteroaryl. [0331] Embodiment 55. The method of any one of embodiments 1-34, wherein the compound is of the formula:
wherein R36 is H, halo, C1-C4 alkyl, C2-C4 alkenyl or C3-C6 cycloalkyl; R37 is substituted or unsubstituted C2-C4 alkenyl or C2-C4 alkynyl; and R38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl, substituted or unsubstituted nitrogen containing 5- or 6- membered partially unsaturated heterocyclyl and substituted or unsubstituted nitrogen containing 6-10 membered heteroaryl; provided R38 is not 4-pyridyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof. [0332] Embodiment 56. The method of embodiment 55 wherein R38 is selected from substituted or unsubstituted nitrogen containing 5-membered heteroaryl selected from pyrazolyl, isoxazolyl, isothiazolyl, pyrrolyl, thiazolyl, triazolyl and imidazolyl; substituted or unsubstituted nitrogen containing 6- membered heteroaryl selected from pyridinyl, pyrimidinyl and pyrazinyl; substituted or unsubstituted nitrogen containing 5-membered partially unsaturated heterocyclyl selected from pyrrolinyl, and imidazolidinyl; and substituted or unsubstituted dihydropyridinyl;. [0333] Embodiment 57. The method of embodiment 55 wherein R36 is selected from chloro, methyl, ethyl, isopropyl, allylyl, and cyclopropyl;. [0334] Embodiment 58. The method of embodiment 55 wherein R38 is selected from substituted 5- pyrazolyl, substituted 4-pyrazolyl, substituted 1-pyrazolyl, substituted or unsubstituted 4-isoxazolyl, substituted or unsubstituted 4-isothiazolyl, substituted or unsubstituted 3-pyrrolyl, substituted or
unsubstituted 5-thiazolyl, substituted or unsubstituted 5-imidazolyl, substituted or unsubstituted 1- imidazolyl, substituted or unsubstituted [1,2,4]triazol-5-yl, substituted or unsubstituted 3-pyridyl, and substituted or unsubstituted 5-pyrimidinyl;. [0335] Embodiment 59. The method of embodiment 58 wherein R38 is selected from 3-trifluoromethyl- pyrazol-4-yl, 1-isopropyl-3-trifluoromethyl-pyrazol-4-yl, 1-ethyl-3-trifluoromethyl-pyrazol-4-yl, 1- methyl-3-difluoromethyl-pyrazol-4-yl, 1-butyl-3-trifluoromethyl-pyrazol-4-yl, 1-methoxymethyl-3- trifluoromethyl-pyrazol-4-yl, 1-propyl-3-trifluoromethyl-pyrazol-4-yl, 1,3-dimethyl-pyrazol-4-yl, 1,3,5- trimethyl-pyrazol-4-yl, 1-methyl-3-cyclopropyl-pyrazol-4-yl, 1-methyl-3-trifluoromethyl-pyrazol-4-yl, 1- ethyl-3-amino-pyrazol-4-yl, 1-ethyl-3-methoxy-pyrazol-4-yl, 1-hydroxyethyl-3-trifluoromethyl-pyrazol-4- yl, 1-[2-hydroxypropyl]-3-trifluoromethyl-pyrazol-4-yl, 1-[2-hydroxyisobutyl]-3-trifluoromethyl-pyrazol- 4-yl, 1-methoxyethyl-3-trifluoromethyl-pyrazol-4-yl, 1-[N,N-dimethylaminoethyl]-3-trifluoromethyl- pyrazol-4-yl, 1-[N-methylaminocarbonylmethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-[N- methylaminocarbonylethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-[N,N-dimethylaminocarbonylmethyl]-3- trifluoromethyl-pyrazol-4-yl, 1-aminocarbonylmethyl-3-trifluoromethyl-pyrazol-4-yl, 1- methylcarbonylaminoethyl-3-trifluoromethyl-pyrazol-4-yl, 1-methylcarbonylaminobutyl-3- trifluoromethyl-pyrazol-4-yl, 1-aminocarbonylethyl-3-trifluoromethyl-pyrazol-4-yl, 1- aminocarbonylpropyl-3-trifluoromethyl-pyrazol-4-yl, 1-aminocarbonylisopropyl-3-trifluoromethyl- pyrazol-4-yl, 1-cyanopropyl-3-trifluoromethyl-pyrazol-4-yl, 1-[N,N-dimethylaminocarbonylethyl]-3- trifluoromethyl-pyrazol-4-yl, 1-carboxyethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-carboxymethyl]-3- trifluoromethyl-pyrazol-4-yl, 1-methoxycarbonylmethyl]-3-trifluoromethyl-pyrazol-4-yl, 1-ethyl-3- carboxy-pyrazol-4-yl, 1-ethyl-5-carboxy-pyrazol-4-yl, 1-ethyl-3-methylaminocarbonyl-pyrazol-4-yl, 1- ethyl-3-[N,N-dimethylaminocarbonyl]-pyrazol-4-yl, 1-ethyl-5-methylaminocarbonyl-pyrazol-4-yl, 1- ethyl-5-[N,N-dimethylaminocarbonyl]-pyrazol-4-yl, 1-benzyl-3-methyl-pyrazol-4-yl, 1- (cyclopropylmethyl)-3-trifluoromethyl-pyrazol-4-yl, 1-cyclopropyl-3-trifluoromethyl-pyrazol-4-yl, 1-[1- methylazetidin-3-yl]-3-trifluoromethyl-pyrazol-4-yl, 1-[1-methylpyrrolidin-3-yl]-3-trifluoromethyl- pyrazol-4-yl, 1-[1-methylpiperidin-3-yl]-3-trifluoromethyl-pyrazol-4-yl, 1-[1-methylpiperidin-4-yl]-3- trifluoromethyl-pyrazol-4-yl, 1-(3-pyridinylmethyl)-3-methyl-pyrazol-4-yl, 1-(2-pyridinylmethyl)-3- methyl-pyrazol-4-yl, 1-[2-pyridyl]-3-methyl-pyrazol-4-yl, 1-methyl-5-pyrazolyl, 1-ethyl-5- trifluoromethylpyrazol-4-yl, 1,3-dimethyl-5-pyrazolyl, 1-methyl-3-cyclopropyl-pyrazol-5-yl, 1-methyl-4- pyrazolyl, 1-ethyl-3-methylpyrazol-4-yl, 1,5-dimethyl-4-pyrazolyl, 1,3,5-trimethyl-4-pyrazolyl, 1-methyl- 3-pyrazolyl, 4-methyl-3-pyrazolyl, 1-methyl-[1,2,4]triazol-3-yl, 4-bromo-2-methyl-[1,2,4]triazol-5-yl, 4- bromo-2-ethyl-[1,2,4]triazol-5-yl, 1-methyl-[1,2,4]triazol-5-yl, 4-isothiazolyl, 4-methyl-2-oxazolyl, isoxazol-4-yl, 2,4-dimethylthiazol-5-yl, 3,5-dimethylisoxazol-4-yl, 2-methyl-5-thiazolyl and 4-methyl-5- thiazolyl. [0336] Embodiment 60. The method of embodiment 59 wherein R38 is selected from 5-pyrimidinyl, 2- methyl-5-pyrimidinyl, 4-methyl-5-pyrimidinyl, 4,6-dimethoxy-5-pyrimidinyl, 4,6-dimethyl-5-pyrimidinyl, 4-triflouromethyl-5-pyrimidinyl, 4-pyrimidinyl, 2-methyl-4-pyrimidinyl, 4-methyl-6-pyrimidinyl, 2,4-
dimethyl-6-pyrimidinyl, 3-pyridinyl, 2-pyridinyl, 4-methyl-2-pyridinyl, 2-triflouromethyl-3-pyridinyl, 4- triflouromethyl-3-pyridinyl, 2-methyl-3-pyridinyl, 2,5-dimethyl-3-pyridinyl, 2,6-dimethyl-3-pyridinyl, 2,4-dimethyl-3-pyridinyl, 2-ethyl-3-pyridinyl, 5-methyl-3-pyridinyl, 2-ethoxy-3-pyridinyl, 2-ethoxy-5- methyl-3-pyridinyl, 2-methoxy-3-pyridinyl, 2-methoxy-6-methyl-3-pyridinyl, 2-ethoxy-6-methyl-3- pyridinyl, 2-isopropoxy-3-pyridinyl, 2-(3-pentoxy)-3-pyridinyl, 2-methoxyethoxy-3-pyridinyl, 2- cyclopropoxy-3-pyridinyl, 2-cyclopentyloxy-3-pyridinyl, 2-cyclohexloxy-3-pyridinyl, 2-fluoro-3- pyridinyl, 2-chloro-3-pyridinyl, 2-phenyl-3-pyridinyl, 2-flouro-3-methyl-5-pyridinyl, 2-flouro-3-chloro-5- pyridinyl, 3-fluoro-5-pyridinyl, 3-chloro-5-pyridinyl, 2-chloro-4-methyl-5-pyridinyl, 2-methoxy-5- pyridinyl, 3-methoxy-5-pyridinyl, 2-ethoxy-5-pyridinyl, 3-ethoxy-5-pyridinyl, 3-triflouromethyl-5- pyridinyl, 3-ethyl-5-pyridinyl, 2,3-dimethyl-5-pyridinyl, 2-(2-hydroxymethylpyrrolidin-1-yl)-5-pyridinyl, 2-(morpholin-1-yl)-3-chloro-5-pyridinyl, 2-(dimethylaminoethoxy)-5-pyridinyl, 2-(2- dimethylaminomethylpyrrolidin-1-yl)-5-pyridinyl, 2-phenyl-5-pyridinyl, 2-methyl-6-pyridinyl, 2,4- dimethyl-6-pyridinyl and 2-ethyl-6-pyridinyl. [0337] Embodiment 61. The method of embodiment 55 wherein R38 is selected from substituted or unsubstituted tetrahydroquinolinyl, substituted or unsubstituted 1-pyrrolin-3-yl, substituted or unsubstituted 1-imidazolidinyl, and substituted or unsubstituted dihydropyridin-3-yl;. [0338] Embodiment 62. The method of embodiment 61 wherein R38 is selected from 4-isoquinolinyl, 1- methoxy-4-isoquinolinyl, 1-chloro-4-isoquinolinyl, 6-methyl-4-isoquinolinyl, 1-oxo-2-methyl-4- isoquinolinyl, 3-quinolinyl, 4-quinolinyl, 5-pyrrolopyridinyl, [1,3,3a]-triazainden-5-yl, 1-ethyl-3- pyrrolopyridinyl, and 1-methyl-5-pyrrolopyridinyl; [0339] Embodiment 63. The method of embodiment 55 wherein R37 is selected from ethenyl, fluoroethenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl and nitrogen- containing heterocyclyl-propenyl. [0340] Embodiment 64. The method of any one of embodiments 1-34 wherein the compound is of the formula:
wherein R36 is selected from H, halo, C1-C4 alkyl, C2-C4 alkenyl and C3-C6 cycloalkyl; R37 is substituted or unsubstituted C2-C4 alkenyl, or C2-C4 alkynyl; and
R41 is selected from H, C1-6 alkyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1-6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl- C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl, aryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heteroaryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heterocyclyl and substituted or unsubstituted 5 or 6 membered heteroaryl; R42 is selected from H, carboxy, amino, C1-6 alkyl, C1-3 haloalkyl, C1-6 alkoxy, C1-6 alkylamino, C1-6 alkylaminocarbonyl, and C3-6 cycloalkyl; R43 is selected from H, carboxy, C1-4 alkyl, C1-3 haloalkyl, and C1-6 alkylaminocarbonyl; or an isomer or stereoisomer of any of the foregoing, and R44 is one or more substituents selected from H, halo, alkoxy and hydroxy; or a mixture thereof or a pharmaceutically acceptable salt thereof. [0341] Embodiment 65. The method of embodiment 64, wherein R41 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, D3-methyl, 1-hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2-hydroxypropyl, 2-hydroxy-2-methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, methoxycarbonylmethyl, dimethylaminocarbonylmethyl, dimethylaminocarbonylethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, aminocarbonyl-1,1-dimethylmethyl, methylcarbonylaminoethyl, 1-methylcarbonylamino-2.2-dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl, cyanoethyl, cyclopropyl, cyclopropylmethyl, benzyl, 3-pyridinylmethyl, 2-pyridinylmethyl, 4-oxazolylmethyl, oxadiazol-5-ylmethyl, 1-methylazetidin-3-yl, 1-methylpyrrolidin-3-yl, 1- methylpiperidin-4-yl, 1-methylpiperidin-3-yl, 3,5-difluoro-4-pyridyl, 3-chloro-4-pyridyl, 4-pyridyl and 2- pyridyl; or an isomer or a pharmaceutically acceptable salt thereof. [0342] Embodiment 66. The method of embodiment 64, wherein R36 is H, chloro, methyl, ethyl, isopropyl, propenyl, trifluoromethyl, 2-furyl, 2-pyridinyl, or cyclopropyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0343] Embodiment 67. The method of embodiment 64, wherein R37 is selected from ethenyl, fluoroethenyl, fluoropropenyl, propynyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethenyl and substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0344] Embodiment 68. The method of embodiment 64, wherein, R37 is azetidin-1-ylpropenyl, 1- methylpyrrolidin-2-ylethenyl, dimethylaminopropenyl, methylaminopropenyl, piperidin-1-ylpropenyl, 4- hydroxy-piperidin-1-ylpropenyl, pyrrolidin-1-ylpropenyl, 3-hydroxypyrrolidin-1-ylpropenyl, morpholin- 1-ylpropenyl, or aminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
[0345] Embodiment 69. The method of embodiment 64, wherein R42 is selected from H, trifluoromethyl, difluoromethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy. methylaminocarbonyl, dimethylaminocarbonyl, and cyclopropyl; or an isomer or a pharmaceutically acceptable salt thereof. [0346] Embodiment 70. The method of embodiment 64, wherein R43 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl; or an isomer or a pharmaceutically acceptable salt thereof. [0347] Embodiment 71. The method of embodiment 64, wherein R44 is one or more substituents selected from H, hydroxy, fluoro and methoxyl; or an isomer or a pharmaceutically acceptable salt thereof. [0348] Embodiment 72. The method of any one of embodiments 1-34 wherein the compound is of the formula:
wherein R1 is substituted or unsubstituted C2-C6 alkenyl, or substituted or unsubstituted C2-C6 alkynyl; R6 is selected from H, C1-6 alkyl, C2-4 alkynyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1- 6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C1-6 haloalkyl, C1-6 alkylsulfonyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl, aryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heteroaryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heterocyclyl and substituted or unsubstituted 5 or 6 membered heteroaryl; R7 is selected from H, carboxy, amino, C1-6 alkyl, C1-3 haloalkyl, C1-6 alkoxy, C1-6 alkylamino, C1-6 alkylaminocarbonyl, and C3-6 cycloalkyl; R8 is selected from H, carboxy, cyano, C1-4 alkyl, C1-3 haloalkyl, and C1-6 alkylaminocarbonyl; R9 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; R10 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; and R11 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0349] Embodiment 73. The method of embodiment 72, wherein R1 is selected from C2-C6 alkenyl, halo- substituted C2-C6 alkenyl; alkoxy substituted C2-C6 alkenyl; dialkylamino substituted C2-C6 alkenyl,
alkylamino substituted C2-C6 alkenyl, amino substituted C2-C6 alkenyl, hydroxy substituted amino-C2-C6 alkenyl, phenyl substituted amino-C2-C6 alkenyl, amino substituted C2-C6 alkynyl, dialkylamino substituted C2-C6 alkynyl, alkylamino substituted C2-C6 alkynyl, alkoxy substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered cycloalkyl- substituted C2-C6 alkenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl- substituted C2-C6 alkenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl- substituted C2-C6 alkynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl- substituted C2-C6 alkenyl, and substituted or unsubstituted 3-7 membered cycloalkyl- substituted C2-C6 alkynyl; . [0350] . Embodiment 74. The method of embodiment 73, wherein R1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3-trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, alkoxypropenyl, dialkylaminopropenyl, alkylaminopropenyl, aminopropenyl, 3-amino-4-hydroxy-butenyl, 3-amino-4- phenyl-butenyl, dialkylaminobutenyl, alkylaminobutenyl, aminobutenyl, dialkylaminopentenyl, alkylaminopentenyl, aminopentenyl, aminopropynyl, dialkylaminopropynyl, alkylaminopropynyl, methoxypropynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propynyl, substituted or unsubstituted 3-7 membered cycloalkyl-ethenyl, substituted or unsubstituted 3-7 membered cycloalkyl-propenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-ethynyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propynyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl-ethenyl, substituted or unsubstituted 3-7 membered oxygen-containing heterocyclyl-propenyl, substituted or unsubstituted 3-7 membered nitrogen- containing heterocyclyl-ethenyl, substituted or unsubstituted 3-7 membered nitrogen-containing heterocyclyl-propenyl, substituted or unsubstituted 3-7 membered cycloalkyl-ethynyl, substituted or unsubstituted 3-7 membered cycloalkyl-propynyl, substituted or unsubstituted 3-7 membered cycloalkyl- ethynyl, and substituted or unsubstituted 3-7 membered cycloalkyl-propynyl; [0351] Embodiment 75. The method of embodiment 74, wherein R1 is selected from ethenyl, fluoropropenyl, 3,3-difluoropropenyl, 3,3,3-trifluoropropenyl, 3,3,3-trifluoroprop-1-enyl, methoxypropenyl, ethoxypropenyl, aminopropenyl, 3-amino-butenyl, 3-methylamino-butenyl, 3-amino-4- hydroxy-butenyl, 3-methylamino-4-methoxy-butenyl, 3-amino-4-phenyl-butenyl, 3-amino-pentenyl, aminopropynyl, methoxypropynyl, dimethylaminopropenyl, di(d1,d2,d3-methyl)aminopropenyl, diethylaminopropenyl, 3-(N,N-dimethylamino)-3-phenyl-propenyl, 3-(N,N-dimethylamino)-3- cyclopropyl-propenyl, (cyclopropylamino)propenyl, bicyclo[1.1.1]pent-1-ylamino, (1- methylcyclopropylamino)propenyl, (3-methyloxetan-3-yl)aminopropenyl, (3-methyltetrahydrofur-3- yl)aminopropenyl, (4-methyl-tetrahydropyran-4-yl)aminopropenyl, methylaminopropenyl, N-benzyl-N- methylaminopropenyl, N-(tert-butyl)aminopropenyl, N-sec-butylaminopropenyl, N-butylaminopropenyl, N-(isopropyl)aminopropenyl, N-(d2-isopropyl)aminopropenyl, ethylaminopropenyl, N-[3,3- difluorocyclobutyl]aminopropenyl, 1-hydroxymethyl-1-methyl-ethylaminopropenyl, 3-dimethylamino- butenyl, 3-(N-methylamino)-butenyl, methylaminobutenyl, N,N-dimethylaminobutenyl, piperidin-2-
ylpropenyl, pyrrolidin-1-ylpropenyl, 3-methyl-oxetan-3-ylpropenyl, 4-methyl-tetrahydropyran-4- ylpropenyl, piperidin-2-ylethenyl, pyrrolidin-2-ylethenyl, azetidin-2-ylethenyl, morpholin-3-ylethenyl, 1- methylpyrrolidin-2-ylethenyl, 3-methylpyrrolidin-5-ylethenyl, 3-ethylpyrrolidin-5-ylethenyl, 2- methylpyrrolidin-5-ylethenyl, 2,2-dimethylpyrrolidin-5-ylethenyl, 3-methoxypyrrolidin-5-ylethenyl, 3- fluoropyrrolidin-5-ylethenyl, 3,3-difluoropyrrolidin-5-ylethenyl, 5-azaspiro[2.4]heptan-6-ylethenyl, 2- azabicyclo[3.1.0]hexan-3-ylethenyl, 3,3-dimethylpyrrolidin-5-ylethenyl, 3-methylpyrrolidin-1-ylpropenyl, 2-methylpyrrolidin-1-ylpropenyl, 1-methylcarbonylpyrrolidin-3-ylethenyl, 2-carboxypyrrolidin-1- ylpropenyl, 3-carboxypyrrolidin-1-ylpropenyl, tetrahydrofur-3-ylpropenyl, dimethylaminopropynyl, methylaminopropynyl, 2-amino-2-methylbutynyl, 2-(1-amino-cyclopropyl)-ethynyl, 2-(1-amino- cyclobutyl)-ethynyl, 2-(1-amino-cyclopentyl)-ethynyl, azetidin-2-ylethynyl, pyrrolidin-2-ylethynyl, pyrrolidin-3-ylethynyl, 2-methyl-pyrrolidin-2-ylethynyl, 4-methyl-piperazin-1-ylpropynyl, and piperidin- 3-ylethynyl; [0352] Embodiment 76. The method of embodiment 72, wherein R6 is selected from H, ethyl, isopropyl, butyl, propyl, methyl, propynyl, 1-hydroxyethyl, 2-hydroxymethylethyl, 1-hydroxy-2,2-dimethylethyl, 2- hydroxypropyl, 2-hydroxy-2-methylpropyl, methoxymethyl, methoxyethyl, dimethylaminoethyl, carboxymethyl, carboxyethyl, carboxypropyl, methoxycarbonylmethyl, ethoxycarbonylmethyl, ethoxycarbonylethyl, ethoxycarbonylpropyl, dimethylaminocarbonylmethyl, dimethylaminocarbonyl-1- ethyl, methylaminocarbonyl-1-ethyl, methylaminocarbonylethyl, methylaminocarbonylmethyl, aminocarbonylmethyl, aminocarbonylethyl, 1-aminocarbonylethyl, aminocarbonyl-1,1-dimethylmethyl, methylcarbonylaminoethyl, 1-methylcarbonylamino-2,2-dimethylethyl, 2-cyano-2-methylethyl, cyanomethyl, 2-cyanoethyl, 1-cyanoethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, methylsulfonyl, cyclopropyl, cyclopropylmethyl, benzyl, 4-pyridinylethyl, 2-pyridinylethyl, 3- pyridinylmethyl, 2-pyridinylmethyl, 4-oxazolylmethyl, 1,3,4-oxadiazol-2-yl]methyl, 1,2,4-oxadiazol-2- yl]methyl 1-methylazetidin-3-yl, 1-methylpyrrolidin-3-yl, 1-methylpiperidin-4-yl, 1-methylpiperidin-3-yl, 5-methoxypyrimidin-4-yl, 2-amino-4-pyridyl, 3-chloro-5-fluoro-4-pyridyl, 3,5-difluoro-4-pyridyl, 3- fluoro-2-pyridyl, 3-methoxy-2-pyridyl, 3-fluoro-4-pyridyl, 3-chloro-4-pyridyl, 4-pyridyl and 2-pyridyl; [0353] Embodiment 77. The method of embodiment 72, wherein R7 is selected from H, trifluoromethyl, difluoromethyl, 1,1-difluoroethyl, methyl, ethyl, methoxy, amino, dimethylamino, carboxy. methylaminocarbonyl, dimethylaminocarbonyl, and cyclopropyl; [0354] Embodiment 78. The method of embodiment 72, wherein R8 is selected from H, trifluoromethyl, methyl, ethyl, carboxy, cyano and methylaminocarbonyl; [0355] Embodiment 79. The method of embodiment 72, wherein R9 is selected from H, fluoro, chloro, methyl, and cyano; [0356] Embodiment 80. The method of embodiment 72, wherein R10 is selected from H, fluoro, methylcarbonylamino, chloro, amino, hydroxy, methyl, difluoromethyl, methylsulfonylamino, and methylaminocarbonylamino;
[0357] Embodiment 81. The method of embodiment 72, wherein R11 is H or fluoro; [0358] Embodiment 82. The method of embodiment 72, wherein R6 is ethyl; R7 is trifluoromethyl; and R8 is H; [0359] Embodiment 83. The method of embodiment 72, wherein R6 is methyl; R7 is trifluoromethyl; and R8 is H; [0360] Embodiment 84. The method of embodiment 72, wherein R6 is H; R7 is trifluoromethyl; and R8 is H; [0361] Embodiment 85. The method of embodiment 72, wherein R6 is 3,5-difluoropyridin-4-yl; R7 is trifluoromethyl; and R8 is H; [0362] Embodiment 86. The method of embodiment 72, wherein R1 is substituted ethenyl;. [0363] Embodiment 87. The method of embodiment 72, wherein R1 is dimethylaminopropenyl;. [0364] Embodiment 88. The method of embodiment 72, wherein R1 is methylaminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0365] Embodiment 89. The method of embodiment 72, wherein R1 is 3,3-difluoropropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0366] Embodiment 90. The method of embodiment 72, wherein R1 is 2-(azetin-2-yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0367] Embodiment 91. The method of embodiment 72, wherein R1 is 2-(2-methyl-pyrrolidiny-5- yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0368] Embodiment 92. The method of embodiment 72, wherein R1 is 3-(methylamino)butenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0369] Embodiment 93. The method of embodiment 72, wherein R1 is ethylaminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0370] Embodiment 94. The method of embodiment 72, wherein R1 is 2-(3-methoxypyrrolidiny-5- yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0371] Embodiment 95. The method of embodiment 72, wherein R1 is 2-(3-fluoropyrrolidiny-5- yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
[0372] Embodiment 96. The method of embodiment 72, wherein R1 is 2-(pyrrolidiny-2-yl)ethenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0373] Embodiment 97. The method of embodiment 72, wherein R1 is aminopropenyl; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0374] Embodiment 98. The method of embodiment 72, wherein R9 is fluoro; R10 is H; and R11 is H; an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof. [0375] Embodiment 99. The method of any one of embodiments 1-34 wherein the compound is a compound of Table A.
Claims
CLAIMS WHAT IS CLAIMED IS: 1. A method of treating a cancer in a subject in need thereof, the method comprising: (i) administering to the subject a therapeutically-effective amount of a compound that blocks SUMOylation in the subject; and (ii) administering to the subject a therapeutically-effective amount of an anti-cancer agent that functions through a pathway other than SUMOylation.
2. The method of claim 1, wherein the compound binds to Uba2.
3. The method of claim 1 or 2, wherein the compound has an inhibitory effect on activating enzymes (E1) of SUMO.
4. The method of claim 1, 2, or 3, wherein the cancer is selected from acute myeloid leukemia, large B- cell lymphoma, lung squamous cell carcinoma, pancreatic adenocarcoma, esophegeal carcinoma, cervical squamous cell carcinoma, endocervical adenocarcoma, stomach adenocarcinomathymoma, renal cell carcinoma, head and neck squamous cell carcinoma, bladder carcinoma, ovarian cystadenocarcinoma, multiple myeloma, non-Hodgkin’s lymphoma (NHL), and mesothelioma.
5. The method of claim 1, 2, or 3, wherein the cancer is selected from head and neck squamous cell carcinoma (HNSCC), non-squamous non-small cell lung cancer (NSCLC), cervical cancer, colorectal cancer (CRC), cutaneous melanoma, squamous NSCLC, and small cell lung cancer.
6. The method of claim 1, 2, or 3, wherein the cancer is B Cell Lymphoma.
7. The method of claim 1, 2, or 3, wherein the cancer is acute myeloid leukemia.
8. The method of claim 1, 2, or 3, wherein the cancer is colon cancer.
9. The method of anyone of claims 1-8, wherein the administering of the compound is oral.
10. The method of any one of claims 1-9, wherein the therapeutically-effective amount of the compound is from about 100 mg to about 500 mg.
11. The method of any one of claims 1-10, wherein the subject is human.
12. The method of any one of claims 1-11, wherein the anti-cancer agent is a hypomethylating agent.
13. The method of any one of claims 1-12, wherein the anti-cancer agent is an anti-CD20 antibody.
14. The method of any one of claims 1-12, wherein the anti-cancer agent is an immune checkpoint inhibitor.
15. The method of claim 14, wherein the immune checkpoint inhibitor is an anti-PD-1 agent.
16. The method of claim 14, wherein the immune checkpoint inhibitor is an anti-PD-L1 agent.
17. The method of any one of claims 1-16, wherein the therapeutically-effective amount of the anti-cancer agent is from about 5 mg/kg to about 500 mg/kg.
18. The method of any one of claims 1-16, wherein the therapeutically-effective amount of the anti-cancer agent is from about 10 μg to about 500 μg.
19. The method of any one of claims 1-18, wherein the compound is of the formula:
wherein is a single bond or double bond; wherein l, m, n are each independently an integer from 0 to 2; wherein M is selected from CR3R4, NR5, C=O, O, S=O, O=S=O, and S; wherein Y is selected from CR6R7, NR8, C=O, O, S=O, O=S=O, and S; wherein Z is CR9, or N; wherein ring A is selected from a) 5- or 6-membered partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; wherein E is an electrophilic moiety, selected from:
; Wherein R1 is selected from hydrogen, halogen, -C(X1)3, -CHX12, -CH2X1, -OCX13, -OCH2X1, -OCHX12, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
Wherein R2 is selected from hydrogen, halogen, -CX2 3, -CHX2 2, -CH2X2, -OCX2 3, -OCH2X2, -OCHX22, -CN, -SOn2R2A, -SOv2NR2AR2B, -NHC(O)NR2AR2B, -N(O)m2, -NR2AR2B, -NHNR2AR2B, -C(O)R2A, -C(O)-OR2A, -C(O)NR2AR2B, -C(O)NHNR2AR2B, -OR2A, -NR2ASO2R2B, -NR2AC(O)R2B, -NR2AC(O)OR2B, -NR2AOR2B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R1 and R2 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; Wherein R3 is selected from hydrogen, halogen, -CX33, -CHX32, -CH2X3, -OCX33, -OCH2X3, -OCHX32, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; Wherein R5 is selected from hydrogen, halogen, -CX5 3, -CHX5 2, -CH2X5, -OCX5 3, -OCH2X5, -OCHX5 2, -CN, -SOn5R5A, -SOv5NR5AR5B, -NHC(O)NR5AR5B, -N(O)m5, -NR5AR5B, -NHNR5AR5B, -C(O)R5A, -C(O)-OR5A, -C(O)NR5AR5B, -C(O)NHNR5AR5B, -OR5A, -NR5ASO2R5B, -NR5AC(O)R5B, -NR5AC(O)OR5B, -NR5AOR5B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R6 is selected from hydrogen, halogen, -CX6 3, -CHX6 2, -CH2X6, -OCX6 3, -OCH2X6, -OCHX6 2, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B, -NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
Wherein R7 is selected from hydrogen, halogen, -CX7 3, -CHX7 2, -CH2X7, -OCX7 3, -OCH2X7, -OCHX72, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7, -NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R8 is selected from hydrogen, halogen, -CX83, -CHX82, -CH2X8, -OCX83, -OCH2X8, -OCHX82, -CN, -SOn8R8A, -SOv8NR8AR8B, -NHC(O)NR8AR8B, -N(O)m8, -NR8A87B, -NHNR8AR8B, -C(O)R8A, -C(O)-OR8A, -C(O)NR8AR8B, -C(O)NHNR8AR8B, -OR8A, -NR8ASO2R8B, -NR8AC(O)R8B, -NR8AC(O)OR8B, -NR8AOR8B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R10 is selected from hydrogen, halogen, -CX10 3, -CHX10 2, -CH2X10, -OCX10 3, -OCH2X10, -OCHX10 2, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R11 is selected from hydrogen, halogen, -CX11 3, -CHX11 2, -CH2X11, -OCX11 3, -OCH2X11, -OCHX11 2, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12,
-NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B, -C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R13 is selected from hydrogen, halogen, -CX133, -CHX132, -CH2X13, -OCX133, -OCH2X13, -OCHX132, -CN, -SOn13R13A, -SOv13NR13AR13B, -NHC(O)NR13AR13B, -N(O)m13, -NR13AR13B, -NHNR13AR13B, -C(O)R13A, -C(O)-OR13A, -C(O)NR13AR13B, -C(O)NHNR13AR13B, -OR13A, -NR13ASO2R13B, -NR13AC(O)R13B, -NR13AC(O)OR13B, -NR13AOR13B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R14 is selected from hydrogen, halogen, -CX143, -CHX142, -CH2X14, -OCX143, -OCH2X14, -OCHX142, -CN, -SOn14R14A, -SOv14NR14AR14B, -NHC(O)NR14AR14B, -N(O)m14, -NR14AR14B, -NHNR14AR14B, -C(O)R14A, -C(O)-OR14A, -C(O)NR14AR14B, -C(O)NHNR14AR14B, -OR14A, -NR14ASO2R14B, -NR14AC(O)R14B, -NR14AC(O)OR14B, -NR14AOR14B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R15 is selected from hydrogen, halogen, -CX153, -CHX152, -CH2X15, -OCX153, -OCH2X15, -OCHX152, -CN, -SOn15R15A, -SOv15NR15AR15B, -NHC(O)NR15AR15B, -N(O)m15, -NR15AR15B, -NHNR15AR15B, -C(O)R15A, -C(O)-OR15A, -C(O)NR15AR15B, -C(O)NHNR15AR15B, -OR15A, -NR15ASO2R15B, -NR15AC(O)R15B, -NR15AC(O)OR15B, -NR15AOR15B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 is selected from hydrogen, halogen, -CX16 3, -CHX16 2, -CH2X16, -OCX16 3, -OCH2X16, -OCHX16 2, -CN, -SOn16R16A, -SOv16NR16AR16B, -NHC(O)NR16AR16B, -N(O)m16, -NR16AR16B, -NHNR16AR16B, -C(O)R16A, -C(O)-OR16A, -C(O)NR16AR16B, -C(O)NHNR16AR16B, -OR16A, -NR16ASO2R16B, -NR16AC(O)R16B, -NR16AC(O)OR16B, -NR16AOR16B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 is selected from hydrogen, halogen, -CX17 3, -CHX17 2, -CH2X17, -OCX17 3, -OCH2X17, -OCHX17 2, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R18 is selected from hydrogen, halogen, -CX183, -CHX182, -CH2X18, -OCX183, -OCH2X18, -OCHX182, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R19 is selected from hydrogen, halogen, -CX193, -CHX192, -CH2X19, -OCX193, -OCH2X19, -OCHX192, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 is selected from hydrogen, halogen, -CX203, -CHX202, -CH2X20, -OCX203, -OCH2X20, -OCHX202, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 and R20 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R21 is selected from hydrogen, halogen, -CX21 3, -CHX21 2, -CH2X21, -OCX21 3, -OCH2X21, -OCHX21 2, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R22 is selected from hydrogen, halogen, -CX22 3, -CHX22 2, -CH2X22, -OCX22 3, -OCH2X22, -OCHX22 2, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B,
-NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 and R22 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; wherein R23 is selected from hydrogen, halogen, -CX233, -CHX232, -CH2X23, -OCX233, -OCH2X23, -OCHX232, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R24 is selected from hydrogen, halogen, -CX243, -CHX242, -CH2X24, -OCX243, -OCH2X24, -OCHX242, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R25 is selected from hydrogen, halogen, -CX25 3, -CHX25 2, -CH2X25, -OCX25 3, -OCH2X25, -OCHX25 2, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B, -C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; wherein R26 is selected from hydrogen, halogen, -CX26 3, -CHX26 2, -CH2X26, -OCX26 3, -OCH2X26, -OCHX26 2, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
wherein R27 is selected from hydrogen, halogen, -CX27 3, -CHX27 2, -CH2X27, -OCX27 3, -OCH2X27, -OCHX272, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B, -C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R28 is selected from hydrogen, halogen, -CX283, -CHX282, -CH2X28, -OCX283, -OCH2X28, -OCHX282, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B, -C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Wherein R29 is selected from hydrogen, halogen, -CX293, -CHX292, -CH2X29, -OCX293, -OCH2X29, -OCHX292, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B, -C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R30 is selected from hydrogen, halogen, -CX30 3, -CHX30 2, -CH2X30, -OCX30 3, -OCH2X30, -OCHX30 2, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR 30B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R2A, R2B, R3A, R3B, R4A, R4B, R5A, R5B, R6A, R6B, R7A, R7B, R8A, R8B, R9A, R9B, R10A, R10B, R11A, R11B, R12A, R12B, R13A, R13B, R14A, R14B, R15A, R15B, R16A, R16B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B, R28A, R28B, R29A, R29B, R30A, R30B is independently selected from hydrogen, -CX3, -CHX2, -CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, -NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; wherein R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5A and R5B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R8A and R8B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R10A and R10B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R11A and R11B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R12A and R12B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A
and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; Wherein each m1, m2, m3, m4, m5, m6, m7, m8, m9, m10, m11, m12, m13, m14, m15, m16, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, and m30 are independently 1 or 2; wherein each v1, v2, v3, v4, v5, v6, v7, v8, v9, v10, v11, v12, v13, v14, v15, v16, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29 and v30 are independently 1 or 2; wherein each n1, n2, n3, n4, n5, n6, n7, n8, n9, n10, n11, n12, n13, n14, n15, n16, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29 and n30 are independently an integer from 0 to 2; and wherein each X, X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12, X13, X14, X15, X16, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29 and X30 are independently -Cl, -Br, -I or –F.
20. The method of any one of claims 1-18, wherein the compound is of the formula:
wherein Z is N, or CR9; W is selected from NR12, O, S, S=O, O=S=O, and Se; ring B is selected from a) 5- or 6-membered cycloalkyl, saturated or partially saturated heterocyclyl, b) 5- or 6-membered aryl or heteroaryl, c) 9-, 10- or 11-membered fused partially saturated heterocyclyl, d) 9- or 10-membered fused heteroaryl, e) naphthyl, and f) 4-, 5- or 6-membered cycloalkenyl; E is selected from an electrophilic moiety, selected from
; R1 is selected from hydrogen, halogen, -CX1 3, -CHX1 2, -CH2X1, -OCX1 3, -OCH2X1, -OCHX1 2, -CN, -SOn1R1A, -SOv1NR1AR1B, -NHC(O)NR1AR1B, -N(O)m1, -NR1AR1B, -NHNR1AR1B, -C(O)R1A, -C(O)-OR1A, -C(O)NR1AR1B, -C(O)NHNR1AR1B, -OR1A, -NR1ASO2R1B, -NR1AC(O)R1B, -NR1AC(O)OR1B, -NR1AOR1B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, rand substituted or unsubstituted heteroaryl; R3 is selected from hydrogen, halogen, -CX3 3, -CHX3 2, -CH2X3, -OCX3 3, -OCH2X3, -OCHX3 2, -CN, -SOn3R3A, -SOv3NR3AR3B, -NHC(O)NR3AR3B, -N(O)m3, -NR3AR3B, -NHNR3AR3B, -C(O)R3A, -C(O)-OR3A, -C(O)NR3AR3B, -OR3A, -NR3ASO2R3B, -NR3AC(O)R3B, -NR3AC(O)OR3B, -NR3AOR3B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R4 is selected from hydrogen, halogen, -CX43, -CHX42, -CH2X4, -OCX43, -OCH2X4, -OCHX42, -CN, -SOn4R4A, -SOv4NR4AR4B, -NHC(O)NR4AR4B, -N(O)m4, -NR4AR4B, -NHNR4AR4B, -C(O)R4A, -C(O)-OR4A, -C(O)NR4AR4B, -C(O)NHNR4AR4B, -OR4A, -NR4ASO2R4B, -NR4AC(O)R4B, -NR4AC(O)OR4B, -NR4AOR4B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R3 and R3 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R6 is selected from hydrogen, halogen, -CX63, -CHX62, -CH2X6, -OCX63, -OCH2X6, -OCHX62, -CN, -SOn6R6A, -SOv6NR6AR6B, -NHC(O)NR6AR6B, -N(O)m6, -NR6AR6B,
-NHNR6AR6B, -C(O)R6A, -C(O)-OR6A, -C(O)NR6AR6B, -C(O)NHNR6AR6B, -OR6A, -NR6ASO2R6B, -NR6AC(O)R6B, -NR6AC(O)OR6B, -NR6AOR6B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R7 is selected from hydrogen, halogen, -CX73, -CHX72, -CH2X7, -OCX73, -OCH2X7, -OCHX72, -CN, -SOn7R7A, -SOv7NR7AR7B, -NHC(O)NR7AR7B, -N(O)m7, -NR7AR7B, -NHNR7AR7B, -C(O)R7A, -C(O)-OR7A, -C(O)NR7AR7B, -C(O)NHNR7AR7B, -OR7A, -NR7ASO2R7B, -NR7AC(O)R7B, -NR7AC(O)OR7B, -NR7AOR7B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R9 is selected from hydrogen, halogen, -CX93, -CHX92, -CH2X9, -OCX93, -OCH2X9, -OCHX92, -CN, -SOn9R9A, -SOv9NR9AR9B, -NHC(O)NR9AR9B, -N(O)m9, -NR9AR9B, -NHNR9AR9B, -C(O)R9A, -C(O)-OR9A, -C(O)NR9AR9B, -C(O)NHNR9AR9B, -OR9A, -NR9ASO2R9B, -NR9AC(O)R9B, -NR9AC(O)OR9B, -NR9AOR9B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R10 is selected from hydrogen, halogen, -CX103, -CHX102, -CH2X10, -OCX103, -OCH2X10
, -OCHX102, -CN, -SOn10R10A, -SOv10NR10AR10B, -NHC(O)NR10AR10B, -N(O)m10, -NR10AR10B, -NHNR10AR10B, -C(O)R10A, -C(O)-OR10A, -C(O)NR10AR10B, -C(O)NHNR10AR10B, -OR10A, -NR10ASO2R10B, -NR10AC(O)R10B, -NR10AC(O)OR10B, -NR10AOR10B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R11 is selected from hydrogen, halogen, -CX11 3, -CHX11 2, -CH2X11, -OCX11 3, -OCH2X11, -OCHX11 2, -CN, -SOn11R11A, -SOv11NR11AR11B, -NHC(O)NR11AR11B, -N(O)m11, -NR11AR11B, -NHNR11AR11B, -C(O)R11A, -C(O)-OR11A, -C(O)NR11AR11B, -C(O)NHNR11AR11B, -OR11A, -NR11ASO2R11B, -NR11AC(O)R11B, -NR11AC(O)OR11B, -NR11AOR11B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R12 is selected from hydrogen, halogen, -CX12 3, -CHX12 2, -CH2X12, -OCX12 3, -OCH2X12, -OCHX12 2, -CN, -SOn12R12A, -SOv12NR12AR12B, -NHC(O)NR12AR12B, -N(O)m12, -NR12AR12B, -NHNR12AR12B, -C(O)R12A, -C(O)-OR12A, -C(O)NR12AR12B,
-C(O)NHNR12AR12B, -OR12A, -NR12ASO2R12B, -NR12AC(O)R12B, -NR12AC(O)OR12B, -NR12AOR12B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R17 is selected from hydrogen, halogen, -CX173, -CHX172, -CH2X17, -OCX173, -OCH2X17, -OCHX172, -CN, -SOn17R17A, -SOv17NR17AR17B, -NHC(O)NR17AR17B, -N(O)m17, -NR17AR17B, -NHNR17AR17B, -C(O)R17A, -C(O)-OR17A, -C(O)NR17AR17B, -C(O)NHNR17AR17B, -OR17A, -NR17ASO2R17B, -NR17AC(O)R17B, -NR17AC(O)OR17B, -NR17AOR17B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R18 is selected from hydrogen, halogen, -CX183, -CHX182, -CH2X18, -OCX183, -OCH2X18, -OCHX182, -CN, -SOn18R18A, -SOv18NR18AR18B, -NHC(O)NR18AR18B, -N(O)m18, -NR18AR18B, -NHNR18AR18B, -C(O)R18A, -C(O)-OR18A, -C(O)NR18AR18B, -C(O)NHNR18AR18B, -OR18A, -NR18ASO2R18B, -NR18AC(O)R18B, -NR18AC(O)OR18B, -NR18AOR18B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R18 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R19 is selected from hydrogen, halogen, -CX19 3, -CHX19 2, -CH2X19, -OCX19 3, -OCH2X19, -OCHX19 2, -CN, -SOn19R19A, -SOv19NR19AR19B, -NHC(O)NR19AR19B, -N(O)m19, -NR19AR19B, -NHNR19AR19B, -C(O)R19A, -C(O)-OR19A, -C(O)NR19AR19B, -C(O)NHNR19AR19B, -OR19A, -NR19ASO2R19B, -NR19AC(O)R19B, -NR19AC(O)OR19B, -NR19AOR19B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R16 and R19 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R20 is selected from hydrogen, halogen, -CX20 3, -CHX20 2, -CH2X20, -OCX20 3, -OCH2X20, -OCHX20 2, -CN, -SOn20R20A, -SOv20NR20AR20B, -NHC(O)NR20AR20B, -N(O)m20, -NR20AR20B, -NHNR20AR20B, -C(O)R20A, -C(O)-OR20A, -C(O)NR20AR20B, -C(O)NHNR20AR20B, -OR20A, -NR20ASO2R20B, -NR20AC(O)R20B, -NR20AC(O)OR20B, -NR20AOR20B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted
heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R21 is selected from hydrogen, halogen, -CX213, -CHX212, -CH2X21, -OCX213, -OCH2X21, -OCHX212, -CN, -SOn21R21A, -SOv21NR21AR21B, -NHC(O)NR21AR21B, -N(O)m21, -NR21AR21B, -NHNR21AR21B, -C(O)R21A, -C(O)-OR21A, -C(O)NR21AR21B, -C(O)NHNR21AR21B, -OR21A, -NR21ASO2R21B, -NR21AC(O)R21B, -NR21AC(O)OR21B, -NR21AOR21B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R20 and R21 substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl or heterocycloalkyl; R22 is selected from hydrogen, halogen, -CX223, -CHX222, -CH2X22, -OCX223, -OCH2X22, -OCHX222, -CN, -SOn22R22A, -SOv22NR22AR22B, -NHC(O)NR22AR22B, -N(O)m22, -NR22AR22B, -NHNR22AR22B, -C(O)R22A, -C(O)-OR22A, -C(O)NR22AR22B, -C(O)NHNR22AR22B, -OR22A, -NR22ASO2R22B, -NR22AC(O)R22B, -NR22AC(O)OR22B, -NR22AOR22B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R23 is selected from hydrogen, halogen, -CX233, -CHX232, -CH2X23, -OCX233, -OCH2X23, -OCHX232, -CN, -SOn23R23A, -SOv23NR23AR23B, -NHC(O)NR23AR23B, -N(O)m23, -NR23AR23B, -NHNR23AR23B, -C(O)R23A, -C(O)-OR23A, -C(O)NR23AR23B, -C(O)NHNR23AR23B, -OR23A, -NR23ASO2R23B, -NR23AC(O)R23B, -NR23AC(O)OR23B, -NR23AOR23B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R24 is selected from hydrogen, halogen, -CX24 3, -CHX24 2, -CH2X24, -OCX24 3, -OCH2X24, -OCHX24 2, -CN, -SOn24R24A, -SOv24NR24AR24B, -NHC(O)NR24AR24B, -N(O)m24, -NR24AR24B, -NHNR24AR24B, -C(O)R24A, -C(O)-OR24A, -C(O)NR24AR24B, -C(O)NHNR24AR24B, -OR24A, -NR24ASO2R24B, -NR24AC(O)R24B, -NR24AC(O)OR24B, -NR24AOR24B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R25 is selected from hydrogen, halogen, -CX25 3, -CHX25 2, -CH2X25, -OCX25 3, -OCH2X25, -OCHX25 2, -CN, -SOn25R25A, -SOv25NR25AR25B, -NHC(O)NR25AR25B, -N(O)m25, -NR25AR25B, -NHNR25AR25B, -C(O)R25A, -C(O)-OR25A, -C(O)NR25AR25B,
-C(O)NHNR25AR25B, -OR25A, -NR25ASO2R25B, -NR25AC(O)R25B, -NR25AC(O)OR25B, -NR25AOR25B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R26 is selected from hydrogen, halogen, -CX263, -CHX262, -CH2X26, -OCX263, -OCH2X26, -OCHX262, -CN, -SOn26R26A, -SOv26NR26AR26B, -NHC(O)NR26AR26B, -N(O)m26, -NR26AR26B, -NHNR26AR26B, -C(O)R26A, -C(O)-OR26A, -C(O)NR26AR26B, -C(O)NHNR26AR26B, -OR26A, -NR26ASO2R26B, -NR26AC(O)R26B, -NR26AC(O)OR26B, -NR26AOR26B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R27 is selected from hydrogen, halogen, -CX273, -CHX272, -CH2X27, -OCX273, -OCH2X27, -OCHX272, -CN, -SOn27R27A, -SOv27NR27AR27B, -NHC(O)NR27AR27B, -N(O)m27, -NR27AR27B, -NHNR27AR27B, -C(O)R27A, -C(O)-OR27A, -C(O)NR27AR27B, -C(O)NHNR27AR27B, -OR27A, -NR27ASO2R27B, -NR27AC(O)R27B, -NR27AC(O)OR27B, -NR27AOR27B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R28 is selected from hydrogen, halogen, -CX283, -CHX282, -CH2X28, -OCX283, -OCH2X28, -OCHX28 2, -CN, -SOn28R28A, -SOv28NR28AR28B, -NHC(O)NR28AR28B, -N(O)m28, -NR28AR28B, -NHNR28AR28B, -C(O)R28A, -C(O)-OR28A, -C(O)NR28AR28B, -C(O)NHNR28AR28B, -OR28A, -NR28ASO2R28B, -NR28AC(O)R28B, -NR28AC(O)OR28B, -NR28AOR28B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R29 is selected from hydrogen, halogen, -CX29 3, -CHX29 2, -CH2X29, -OCX29 3, -OCH2X29, -OCHX29 2, -CN, -SOn29R29A, -SOv29NR29AR29B, -NHC(O)NR29AR29B, -N(O)m29, -NR29AR29B, -NHNR29AR29B, -C(O)R29A, -C(O)-OR29A, -C(O)NR29AR29B, -C(O)NHNR29AR29B, -OR29A, -NR29ASO2R29B, -NR29AC(O)R29B, -NR29AC(O)OR29B, -NR29AOR29B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl;
R30 is selected from hydrogen, halogen, -CX30 3, -CHX30 2, -CH2X30, -OCX30 3, -OCH2X30, -OCHX302, -CN, -SOn30R30A, -SOv30NR30AR30B, -NHC(O)NR30AR30B, -N(O)m30, -NR30AR30B, -NHNR30AR30B, -C(O)R30A, -C(O)-OR30A, -C(O)NR30AR30B, -C(O)NHNR30AR30B, -OR30A, -NR30ASO2R30B, -NR30AC(O)R30B, -NR30AC(O)OR30B, -NR30AOR30B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R31 is selected from hydrogen, halogen, -CX313, -CHX312, -CH2X31, -OCX313, -OCH2X31, -OCHX312, -CN, -SOn31R31A, -SOv31NR31AR31B, -NHC(O)NR31AR31B, -N(O)m31, -NR31AR31B, -NHNR31AR31B, -C(O)R31A, -C(O)-OR31A, -C(O)NR31AR31B, -C(O)NHNR31AR31B, -OR31A, -NR31ASO2R31B, -NR31AC(O)R31B, -NR31AC(O)OR31B, -NR31AOR31B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R32 is selected from hydrogen, halogen, -CX323, -CHX322, -CH2X32, -OCX323, -OCH2X32, -OCHX322, -CN, -SOn32R32A, -SOv32NR32AR32B, -NHC(O)NR32AR32B, -N(O)m32, -NR32AR32B, -NHNR32AR32B, -C(O)R32A, -C(O)-OR32A, -C(O)NR32AR32B, -C(O)NHNR32AR32B, -OR32A, -NR32ASO2R32B, -NR32AC(O)R32B, -NR32AC(O)OR32B, -NR32AOR32B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted and unsubstituted heteroaryl; R33 is selected from hydrogen, halogen, -CX33 3, -CHX33 2, -CH2X33, -OCX33 3, -OCH2X33, -OCHX33 2, -CN, -SOn33R33A, -SOv33NR33AR33B, -NHC(O)NR33AR33B, -N(O)m33, -NR33AR33B, -NHNR33AR33B, -C(O)R33A, -C(O)-OR33A, -C(O)NR33AR33B, -C(O)NHNR33AR33B, -OR33A, -NR33ASO2R33B, -NR33AC(O)R33B, -NR33AC(O)OR33B, -NR33AOR33B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R34 is selected from hydrogen, halogen, -CX34 3, -CHX34 2, -CH2X34, -OCX34 3, -OCH2X34, -OCHX34 2, -CN, -SOn34R34A, -SOv34NR34AR34B, -NHC(O)NR34AR34B, -N(O)m34, -NR34AR34B, -NHNR34AR34B, -C(O)R34A, -C(O)-OR34A, -C(O)NR34AR34B, -C(O)NHNR34AR34B, -OR34A, -NR34ASO2R34B, -NR34AC(O)R34B, -NR34AC(O)OR34B, -NR34AOR34B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted
heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; R35 is selected from hydrogen, halogen, -CX353, -CHX352, -CH2X35, -OCX353, -OCH2X35, -OCHX352, -CN, -SOn35R35A, -SOv35NR35AR35B, -NHC(O)NR35AR35B, -N(O)m35, -NR35AR35B, -NHNR35AR35B, -C(O)R35A, -C(O)-OR35A, -C(O)NR35AR35B, -C(O)NHNR35AR35B, -OR35A, -NR35ASO2R35B, -NR35AC(O)R35B, -NR35AC(O)OR35B, -NR35AOR35B, -N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; Each R1A, R1B, R3A, R3B, R4A, R4B, R6A, R6B, R7A, R7B, R9A, R9B, R10A, R10B, R11A, R11B, R17A, R17B, R18A, R18B, R19A, R19B, R20A, R20B, R21A, R21B, R22A, R22B, R23A, R23B, R24A, R24B, R25A, R25B, R26A, R26B, R27A, R27B, R28A, R28B, R29A, R29B, R30A, R30B, R31A, R31B, R32A, R32B, R33A, R33B, R34A, R34B, R35A, R35B, is independently selected from hydrogen, -CX3, -CHX2, -CH2X, -C(O)OH, -C(O)NH2, -CN, -OH, -NH2, -COOH, -CONH2, -NO2, -SH, -SO3H, -SO4H, -SO2NH2, -NHNH2, -ONH2, -NHC=(O)NHNH2, -NHC=(O)NH2, -NHSO2H, -NHC=(O)H, -NHC(O)OH, -NHOH, -OCX3, -OCHX2, -OCH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1A and R1B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2A and R2B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4A and R4B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R6A and R6B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R7A and R7B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R9A and R9B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R13A and R13B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R14A and R14B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R15A and R15B substituents bonded to the same nitrogen atom may optionally be joined to
form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16A and R16B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17A and R17B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18A and R18B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R19A and R19B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R20A and R20B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R21A and R21B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R22A and R22B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R23A and R23B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R24A and R24B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R25A and R25B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R26A and R26B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R27A and R27B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R28A and R28B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R29A and R29B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R30A and R30B substituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; Each m1, m3, m4, m6, m7, m9, m10, m11, m17, m18, m19, m20, m21, m22, m23, m24, m25, m26, m27, m28, m29, m30, m31, m32, m33, m34 and m35 is independently 1 or 2; Each v1, v3, v4, v6, v7, v9, v10, v11, v17, v18, v19, v20, v21, v22, v23, v24, v25, v26, v27, v28, v29, v30, v31, v32, v33, v34 and v35 is independently 1 or 2;
Each n1, n3, n4, n6, n7, n9, n10, n11, n17, n18, n19, n20, n21, n22, n23, n24, n25, n26, n27, n28, n29, n30, n31, n32, n33, n34 and n35 is independently an integer from 0 to 2; and Each X1, X3, X4, X6, X7, X9, X10, X11, X17, X18, X19, X20, X21, X22, X23, X24, X25, X26, X27, X28, X29, X30, X31, X32, X33, X34, and X35 is independently -Cl, -Br, -I or –F.
21. The method of any one of claims 1-18, wherein the compound is of the formula:
wherein R1 is substituted or unsubstituted C2-C6 alkenyl, or substituted or unsubstituted C2-C6 alkynyl; R6 is selected from H, C1-6 alkyl, C2-4 alkynyl, C1-6 hydroxyalkyl, C1-6 alkoxyalkyl, C1-6 alkylamino-C1- 6 alkyl, C1-6 alkylaminocarbonyl-C1-6 alkyl, aminocarbonyl-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkylcarbonylamino-C1-6 alkyl, C1-6 cyanoalkyl, C1-6 haloalkyl, C1-6 alkylsulfonyl, C3-6 cycloalkyl, C3-6 cycloalkyl-C1-6 alkyl, aryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heteroaryl-C1-6 alkyl, substituted or unsubstituted 5 or 6 membered heterocyclyl and substituted or unsubstituted 5 or 6 membered heteroaryl; R7 is selected from H, carboxy, amino, C1-6 alkyl, C1-3 haloalkyl, C1-6 alkoxy, C1-6 alkylamino, C1-6 alkylaminocarbonyl, and C3-6 cycloalkyl; R8 is selected from H, carboxy, cyano, C1-4 alkyl, C1-3 haloalkyl, and C1-6 alkylaminocarbonyl; R9 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; R10 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; and R11 is selected from H, halo, hydroxy, C1-3 alkyl, C1-3 haloalkyl, cyano, amino, C1-3 alkylcarbonylamino, C1-3 alkylsulfonylamino, and C1-3 alkylaminocarbonylamino; or an isomer or stereoisomer of any of the foregoing, or a mixture thereof or a pharmaceutically acceptable salt thereof.
22. The method of any one of claims 1-19, wherein the compound is selected from a compound listed in Table A.
23. The method of claim 13, wherein the anti-CD20 antibody is rituximab.
24. The method of claim 14, wherein the immune checkpoint inhibitor is pembrolizumab.
25. The method of claim 12, wherein the HMA is decitabine.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202263417102P | 2022-10-18 | 2022-10-18 | |
| US63/417,102 | 2022-10-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024086194A1 true WO2024086194A1 (en) | 2024-04-25 |
Family
ID=90738299
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2023/035367 WO2024086194A1 (en) | 2022-10-18 | 2023-10-18 | Combination therapy for treatment of cancer |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2024086194A1 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021150918A1 (en) * | 2020-01-22 | 2021-07-29 | Oncovalent Therapeutics Inc. | 4,5,6,7-tetrahydrothieno[2,3-c]pyridine sumo inhibitors and uses thereof |
| US20220177488A1 (en) * | 2019-03-19 | 2022-06-09 | Suvalent Therapeutics, Inc. | Sumo inhibitor compounds and uses thereof |
| US20220298171A1 (en) * | 2018-07-31 | 2022-09-22 | City Of Hope | Sumo inhibitor compounds and uses thereof |
-
2023
- 2023-10-18 WO PCT/US2023/035367 patent/WO2024086194A1/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20220298171A1 (en) * | 2018-07-31 | 2022-09-22 | City Of Hope | Sumo inhibitor compounds and uses thereof |
| US20220177488A1 (en) * | 2019-03-19 | 2022-06-09 | Suvalent Therapeutics, Inc. | Sumo inhibitor compounds and uses thereof |
| WO2021150918A1 (en) * | 2020-01-22 | 2021-07-29 | Oncovalent Therapeutics Inc. | 4,5,6,7-tetrahydrothieno[2,3-c]pyridine sumo inhibitors and uses thereof |
Non-Patent Citations (1)
| Title |
|---|
| CANON JUDE R., CALLINAN ASHLEY, BRADLEY SARAH, WANG LAURIE, KUI MACKENZIE, WEIN ARIEL, SULLI GABRIELE, BUCHOWIECKI PETER, HONG FAN: "Abstract LB318: SB-4826, a first-in-class oral, covalent inhibitor of SUMO E1 that induces IFN signaling and inhibits tumor growth as monotherapy and in combination with immune checkpoint blockade", CANCER RESEARCH, AMERICAN ASSOCIATION FOR CANCER RESEARCH, vol. 83, no. 8_Suppl., 14 April 2023 (2023-04-14), pages LB318, XP009554941, ISSN: 0008-5472, DOI: 10.1158/1538-7445.AM2023-LB318 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2762140B1 (en) | Treatment of solid brain tumours with a rapamycin derivative | |
| US11938124B2 (en) | Combination therapy for treatment of cancer | |
| TWI654979B (en) | Method of treating cancer using TOR kinase inhibitor combination therapy | |
| US20170143725A1 (en) | Combination comprising an atp analog and an adenosine receptor antagonist or a nucleobase nucleoside analog for the treatment of cancer | |
| AU2020222296A1 (en) | Pharmaceutical combination comprising tno155 and ribociclib | |
| US20190060275A1 (en) | Administration of hypoxia activated prodrugs and antiangiogenic agents for the treatment of cancer | |
| US20160287605A1 (en) | Combination therapy | |
| KR20140025434A (en) | Combinations of akt inhibitor compounds and chemotherapeutic agents, and methods of use | |
| JP2024526145A (en) | Combination medicines containing KRAS G12C inhibitors and their use for the treatment of cancer - Patents.com | |
| CN112294965B (en) | Pharmaceutical compositions of MDM2 inhibitors and their use in the prevention and/or treatment of diseases | |
| Konar et al. | Synthesis and clinical development of Palbociclib: an overview | |
| NZ550174A (en) | Combinations comprising a vasculostatic compound such as vatalanib and epothilones, and pharmaceutical uses thereof | |
| AU2021267213B2 (en) | Pharmaceutical combination comprising TNO155 and nazartinib | |
| JP2019516728A (en) | Anti-cancer combination treatment | |
| WO2024086194A1 (en) | Combination therapy for treatment of cancer | |
| JP7571024B2 (en) | Therapeutic agent for anti-CCR4 antibody-resistant cancer | |
| CN117177752A (en) | Compounds and compositions for the treatment of MPNST | |
| CA3210632A1 (en) | Methods of treating cancer using a combination of serd dosing regimens | |
| JP2014034531A (en) | Combination of hsp90 inhibitor and gemcitabine | |
| WO2023111810A1 (en) | Combination therapies and uses for treating cancer | |
| WO2024130344A1 (en) | Transient receptor potential vanilloid 6 inhibitors | |
| AU2021271808A1 (en) | Administration of SUMO-activating enzyme inhibitor and anti-CD38 antibodies | |
| CN117529314A (en) | Pharmaceutical combinations comprising KRAS G12C inhibitors and their use for the treatment of cancer | |
| CA3141072A1 (en) | Methods and uses for treating cancer | |
| JP2014034534A (en) | COMBINATION OF HSP90 INHIBITOR AND mTOR INHIBITOR |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 23880520 Country of ref document: EP Kind code of ref document: A1 |