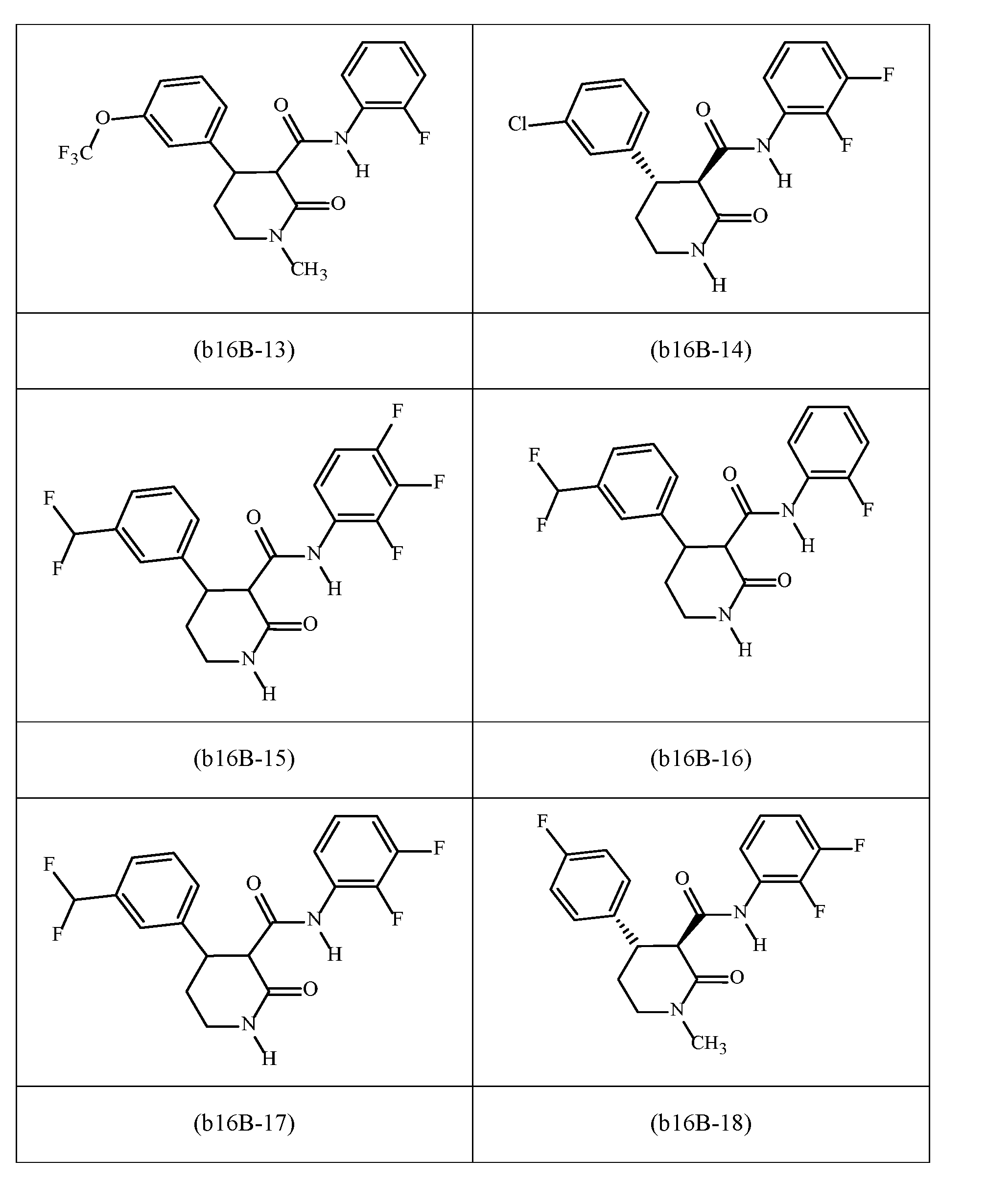
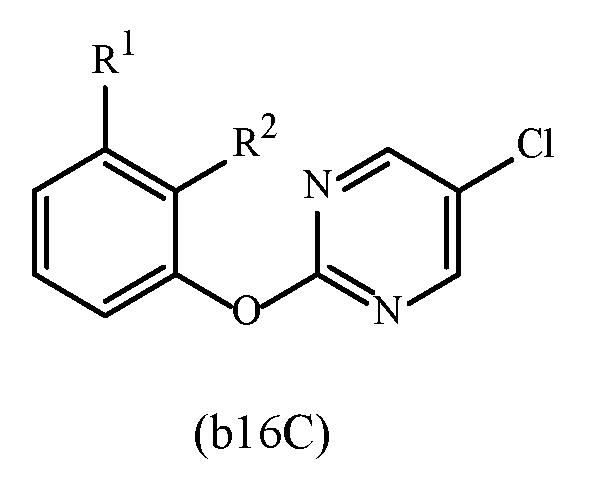
WO2023129493A1 - Substituted cyclopropylpyrimidne herbicides - Google Patents
Substituted cyclopropylpyrimidne herbicides Download PDFInfo
- Publication number
- WO2023129493A1 WO2023129493A1 PCT/US2022/053915 US2022053915W WO2023129493A1 WO 2023129493 A1 WO2023129493 A1 WO 2023129493A1 US 2022053915 W US2022053915 W US 2022053915W WO 2023129493 A1 WO2023129493 A1 WO 2023129493A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- inhibitors
- methyl
- trifluoromethyl
- formula
- Prior art date
Links
- 239000004009 herbicide Substances 0.000 title claims abstract description 52
- 150000001875 compounds Chemical class 0.000 claims abstract description 251
- 239000000203 mixture Substances 0.000 claims abstract description 95
- 150000003839 salts Chemical class 0.000 claims abstract description 31
- 150000001204 N-oxides Chemical class 0.000 claims abstract description 15
- -1 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine Chemical compound 0.000 claims description 102
- 239000003112 inhibitor Substances 0.000 claims description 56
- 229910052736 halogen Inorganic materials 0.000 claims description 50
- 230000002363 herbicidal effect Effects 0.000 claims description 37
- 150000002367 halogens Chemical group 0.000 claims description 29
- 239000004480 active ingredient Substances 0.000 claims description 27
- 239000003085 diluting agent Substances 0.000 claims description 26
- 238000000034 method Methods 0.000 claims description 26
- 239000007788 liquid Substances 0.000 claims description 25
- 125000001188 haloalkyl group Chemical group 0.000 claims description 24
- 239000004094 surface-active agent Substances 0.000 claims description 21
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 16
- 125000003545 alkoxy group Chemical group 0.000 claims description 15
- 229910052739 hydrogen Inorganic materials 0.000 claims description 15
- 239000007787 solid Substances 0.000 claims description 15
- 229910052801 chlorine Inorganic materials 0.000 claims description 14
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 14
- 125000004414 alkyl thio group Chemical group 0.000 claims description 12
- 229910052799 carbon Inorganic materials 0.000 claims description 12
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 12
- 230000012010 growth Effects 0.000 claims description 11
- SEOVTRFCIGRIMH-UHFFFAOYSA-N indole-3-acetic acid Chemical class C1=CC=C2C(CC(=O)O)=CNC2=C1 SEOVTRFCIGRIMH-UHFFFAOYSA-N 0.000 claims description 11
- SNOOUWRIMMFWNE-UHFFFAOYSA-M sodium;6-[(3,4,5-trimethoxybenzoyl)amino]hexanoate Chemical compound [Na+].COC1=CC(C(=O)NCCCCCC([O-])=O)=CC(OC)=C1OC SNOOUWRIMMFWNE-UHFFFAOYSA-M 0.000 claims description 10
- 102000000452 Acetyl-CoA carboxylase Human genes 0.000 claims description 8
- 108010016219 Acetyl-CoA carboxylase Proteins 0.000 claims description 8
- 229930192334 Auxin Natural products 0.000 claims description 8
- 108010018763 Biotin carboxylase Proteins 0.000 claims description 8
- 108030006708 Homogentisate solanesyltransferases Proteins 0.000 claims description 8
- 239000002363 auxin Substances 0.000 claims description 8
- 229910052731 fluorine Inorganic materials 0.000 claims description 8
- FBUKVWPVBMHYJY-UHFFFAOYSA-N nonanoic acid Chemical compound CCCCCCCCC(O)=O FBUKVWPVBMHYJY-UHFFFAOYSA-N 0.000 claims description 8
- 108010068327 4-hydroxyphenylpyruvate dioxygenase Proteins 0.000 claims description 7
- 102100028626 4-hydroxyphenylpyruvate dioxygenase Human genes 0.000 claims description 7
- VYNOULHXXDFBLU-UHFFFAOYSA-N Cumyluron Chemical compound C=1C=CC=CC=1C(C)(C)NC(=O)NCC1=CC=CC=C1Cl VYNOULHXXDFBLU-UHFFFAOYSA-N 0.000 claims description 7
- 108020001991 Protoporphyrinogen Oxidase Proteins 0.000 claims description 7
- 102000005135 Protoporphyrinogen oxidase Human genes 0.000 claims description 7
- OTSAMNSACVKIOJ-UHFFFAOYSA-N azane;carbamoyl(ethoxy)phosphinic acid Chemical compound [NH4+].CCOP([O-])(=O)C(N)=O OTSAMNSACVKIOJ-UHFFFAOYSA-N 0.000 claims description 7
- 229940125904 compound 1 Drugs 0.000 claims description 7
- 102000005396 glutamine synthetase Human genes 0.000 claims description 7
- 108020002326 glutamine synthetase Proteins 0.000 claims description 7
- 108010001545 phytoene dehydrogenase Proteins 0.000 claims description 7
- 125000004767 (C1-C4) haloalkoxy group Chemical group 0.000 claims description 6
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 6
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 claims description 6
- 150000004669 very long chain fatty acids Chemical class 0.000 claims description 6
- QUTYKIXIUDQOLK-PRJMDXOYSA-N 5-O-(1-carboxyvinyl)-3-phosphoshikimic acid Chemical compound O[C@H]1[C@H](OC(=C)C(O)=O)CC(C(O)=O)=C[C@H]1OP(O)(O)=O QUTYKIXIUDQOLK-PRJMDXOYSA-N 0.000 claims description 5
- 108010000700 Acetolactate synthase Proteins 0.000 claims description 5
- 108010060806 Photosystem II Protein Complex Proteins 0.000 claims description 5
- 230000008166 cellulose biosynthesis Effects 0.000 claims description 5
- 125000004995 haloalkylthio group Chemical group 0.000 claims description 5
- 239000005644 Dazomet Substances 0.000 claims description 4
- ICWUMLXQKFTJMH-UHFFFAOYSA-N Etobenzanid Chemical compound C1=CC(OCOCC)=CC=C1C(=O)NC1=CC=CC(Cl)=C1Cl ICWUMLXQKFTJMH-UHFFFAOYSA-N 0.000 claims description 4
- GXAMYUGOODKVRM-UHFFFAOYSA-N Flurecol Chemical compound C1=CC=C2C(C(=O)O)(O)C3=CC=CC=C3C2=C1 GXAMYUGOODKVRM-UHFFFAOYSA-N 0.000 claims description 4
- 239000002169 Metam Substances 0.000 claims description 4
- FMINYZXVCTYSNY-UHFFFAOYSA-N Methyldymron Chemical compound C=1C=CC=CC=1N(C)C(=O)NC(C)(C)C1=CC=CC=C1 FMINYZXVCTYSNY-UHFFFAOYSA-N 0.000 claims description 4
- FCOHEOSCARXMMS-UHFFFAOYSA-N Oxaziclomefone Chemical compound C1OC(C)=C(C=2C=CC=CC=2)C(=O)N1C(C)(C)C1=CC(Cl)=CC(Cl)=C1 FCOHEOSCARXMMS-UHFFFAOYSA-N 0.000 claims description 4
- 239000005643 Pelargonic acid Substances 0.000 claims description 4
- 108010081996 Photosystem I Protein Complex Proteins 0.000 claims description 4
- VGPYEHKOIGNJKV-UHFFFAOYSA-N asulam Chemical compound COC(=O)NS(=O)(=O)C1=CC=C(N)C=C1 VGPYEHKOIGNJKV-UHFFFAOYSA-N 0.000 claims description 4
- WZDDLAZXUYIVMU-UHFFFAOYSA-N bromobutide Chemical compound CC(C)(C)C(Br)C(=O)NC(C)(C)C1=CC=CC=C1 WZDDLAZXUYIVMU-UHFFFAOYSA-N 0.000 claims description 4
- QAYICIQNSGETAS-UHFFFAOYSA-N dazomet Chemical compound CN1CSC(=S)N(C)C1 QAYICIQNSGETAS-UHFFFAOYSA-N 0.000 claims description 4
- QMTNOLKHSWIQBE-FGTMMUONSA-N exo-(+)-cinmethylin Chemical compound O([C@H]1[C@]2(C)CC[C@@](O2)(C1)C(C)C)CC1=CC=CC=C1C QMTNOLKHSWIQBE-FGTMMUONSA-N 0.000 claims description 4
- 125000000262 haloalkenyl group Chemical group 0.000 claims description 4
- 125000000232 haloalkynyl group Chemical group 0.000 claims description 4
- 229910052760 oxygen Inorganic materials 0.000 claims description 4
- VTRWMTJQBQJKQH-UHFFFAOYSA-N pyributicarb Chemical compound COC1=CC=CC(N(C)C(=S)OC=2C=C(C=CC=2)C(C)(C)C)=N1 VTRWMTJQBQJKQH-UHFFFAOYSA-N 0.000 claims description 4
- RFZZKBWDDKMWNM-GTBMBKLPSA-N (5s,7r,8s,9r)-8,9-dihydroxy-7-(hydroxymethyl)-6-oxa-1,3-diazaspiro[4.4]nonane-2,4-dione Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@]11C(=O)NC(=O)N1 RFZZKBWDDKMWNM-GTBMBKLPSA-N 0.000 claims description 3
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims description 3
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 claims description 3
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 claims description 3
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 claims description 3
- LBGPXIPGGRQBJW-UHFFFAOYSA-N Difenzoquat Chemical compound C[N+]=1N(C)C(C=2C=CC=CC=2)=CC=1C1=CC=CC=C1 LBGPXIPGGRQBJW-UHFFFAOYSA-N 0.000 claims description 3
- RFZZKBWDDKMWNM-UHFFFAOYSA-N Hydantocidin Natural products OC1C(O)C(CO)OC11C(=O)NC(=O)N1 RFZZKBWDDKMWNM-UHFFFAOYSA-N 0.000 claims description 3
- 239000005642 Oleic acid Substances 0.000 claims description 3
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 claims description 3
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 claims description 3
- HYVVJDQGXFXBRZ-UHFFFAOYSA-N metam Chemical compound CNC(S)=S HYVVJDQGXFXBRZ-UHFFFAOYSA-N 0.000 claims description 3
- 230000000394 mitotic effect Effects 0.000 claims description 3
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 claims description 3
- 229910052717 sulfur Inorganic materials 0.000 claims description 3
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 claims description 2
- 229940125773 compound 10 Drugs 0.000 claims description 2
- 229940125898 compound 5 Drugs 0.000 claims description 2
- 125000005291 haloalkenyloxy group Chemical group 0.000 claims description 2
- 125000005292 haloalkynyloxy group Chemical group 0.000 claims description 2
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 claims description 2
- 241000196324 Embryophyta Species 0.000 description 67
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 36
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 30
- 239000002904 solvent Substances 0.000 description 30
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 27
- 238000009472 formulation Methods 0.000 description 27
- 125000005843 halogen group Chemical group 0.000 description 24
- 125000000217 alkyl group Chemical group 0.000 description 22
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 20
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 19
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 18
- 238000006243 chemical reaction Methods 0.000 description 16
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 15
- 230000015572 biosynthetic process Effects 0.000 description 15
- 239000000460 chlorine Substances 0.000 description 15
- 239000000543 intermediate Substances 0.000 description 15
- 230000000694 effects Effects 0.000 description 14
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 14
- 235000010469 Glycine max Nutrition 0.000 description 13
- 244000068988 Glycine max Species 0.000 description 13
- 240000008042 Zea mays Species 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 12
- ZHXTWWCDMUWMDI-UHFFFAOYSA-N dihydroxyboron Chemical compound O[B]O ZHXTWWCDMUWMDI-UHFFFAOYSA-N 0.000 description 12
- 125000001424 substituent group Chemical group 0.000 description 12
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 11
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- 238000003786 synthesis reaction Methods 0.000 description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 11
- 244000036975 Ambrosia artemisiifolia Species 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- 240000005702 Galium aparine Species 0.000 description 10
- 235000014820 Galium aparine Nutrition 0.000 description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 10
- 241000209140 Triticum Species 0.000 description 10
- 235000021307 Triticum Nutrition 0.000 description 10
- 235000014113 dietary fatty acids Nutrition 0.000 description 10
- 239000000194 fatty acid Substances 0.000 description 10
- 229930195729 fatty acid Natural products 0.000 description 10
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 239000000047 product Substances 0.000 description 10
- 238000001228 spectrum Methods 0.000 description 10
- 241001621841 Alopecurus myosuroides Species 0.000 description 9
- 244000237956 Amaranthus retroflexus Species 0.000 description 9
- 235000013479 Amaranthus retroflexus Nutrition 0.000 description 9
- 235000003129 Ambrosia artemisiifolia var elatior Nutrition 0.000 description 9
- 235000014716 Eleusine indica Nutrition 0.000 description 9
- 235000003484 annual ragweed Nutrition 0.000 description 9
- 235000003488 common ragweed Nutrition 0.000 description 9
- 150000002148 esters Chemical group 0.000 description 9
- 125000000623 heterocyclic group Chemical group 0.000 description 9
- 239000002689 soil Substances 0.000 description 9
- 235000004135 Amaranthus viridis Nutrition 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 8
- 235000007319 Avena orientalis Nutrition 0.000 description 8
- 235000009344 Chenopodium album Nutrition 0.000 description 8
- 235000005484 Chenopodium berlandieri Nutrition 0.000 description 8
- 235000009332 Chenopodium rubrum Nutrition 0.000 description 8
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- 235000005822 corn Nutrition 0.000 description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 8
- 159000000000 sodium salts Chemical class 0.000 description 8
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 8
- 238000010792 warming Methods 0.000 description 8
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 7
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 description 7
- 244000075850 Avena orientalis Species 0.000 description 7
- 241000110847 Kochia Species 0.000 description 7
- 241000209082 Lolium Species 0.000 description 7
- 230000009471 action Effects 0.000 description 7
- 239000000654 additive Substances 0.000 description 7
- 150000001412 amines Chemical class 0.000 description 7
- 235000006263 bur ragweed Nutrition 0.000 description 7
- 125000004122 cyclic group Chemical group 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- 229910000027 potassium carbonate Inorganic materials 0.000 description 7
- 235000009736 ragweed Nutrition 0.000 description 7
- 150000003871 sulfonates Chemical class 0.000 description 7
- KAESVJOAVNADME-UHFFFAOYSA-N 1H-pyrrole Natural products C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 6
- 240000001592 Amaranthus caudatus Species 0.000 description 6
- 235000009328 Amaranthus caudatus Nutrition 0.000 description 6
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 6
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 6
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 6
- 238000009835 boiling Methods 0.000 description 6
- 229910052794 bromium Inorganic materials 0.000 description 6
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 125000001309 chloro group Chemical group Cl* 0.000 description 6
- 125000001153 fluoro group Chemical group F* 0.000 description 6
- 125000000524 functional group Chemical group 0.000 description 6
- 239000011591 potassium Substances 0.000 description 6
- 229910052700 potassium Inorganic materials 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 241000894007 species Species 0.000 description 6
- 238000011282 treatment Methods 0.000 description 6
- 239000011701 zinc Substances 0.000 description 6
- WVQBLGZPHOPPFO-LBPRGKRZSA-N (S)-metolachlor Chemical compound CCC1=CC=CC(C)=C1N([C@@H](C)COC)C(=O)CCl WVQBLGZPHOPPFO-LBPRGKRZSA-N 0.000 description 5
- 239000005631 2,4-Dichlorophenoxyacetic acid Substances 0.000 description 5
- KPSTXQYTZBZXMM-UHFFFAOYSA-N 2-[8-chloro-4-(4-methoxyphenyl)-3-oxoquinoxaline-2-carbonyl]cyclohexane-1,3-dione Chemical compound C1=CC(OC)=CC=C1N1C(=O)C(C(=O)C2C(CCCC2=O)=O)=NC2=C(Cl)C=CC=C21 KPSTXQYTZBZXMM-UHFFFAOYSA-N 0.000 description 5
- CASLETQIYIQFTQ-UHFFFAOYSA-N 3-[[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)pyrazol-4-yl]methylsulfonyl]-5,5-dimethyl-4h-1,2-oxazole Chemical compound CN1N=C(C(F)(F)F)C(CS(=O)(=O)C=2CC(C)(C)ON=2)=C1OC(F)F CASLETQIYIQFTQ-UHFFFAOYSA-N 0.000 description 5
- 244000074881 Conyza canadensis Species 0.000 description 5
- 235000004385 Conyza canadensis Nutrition 0.000 description 5
- NNYRZQHKCHEXSD-UHFFFAOYSA-N Daimuron Chemical compound C1=CC(C)=CC=C1NC(=O)NC(C)(C)C1=CC=CC=C1 NNYRZQHKCHEXSD-UHFFFAOYSA-N 0.000 description 5
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 5
- 239000005534 Flupyrsulfuron-methyl Substances 0.000 description 5
- 239000005562 Glyphosate Substances 0.000 description 5
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 5
- 239000005578 Mesotrione Substances 0.000 description 5
- 240000007594 Oryza sativa Species 0.000 description 5
- 235000007164 Oryza sativa Nutrition 0.000 description 5
- 239000005617 S-Metolachlor Substances 0.000 description 5
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 5
- 239000005623 Thifensulfuron-methyl Substances 0.000 description 5
- 150000001298 alcohols Chemical class 0.000 description 5
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 5
- MXWJVTOOROXGIU-UHFFFAOYSA-N atrazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)C)=N1 MXWJVTOOROXGIU-UHFFFAOYSA-N 0.000 description 5
- FHUKASKVKWSLCY-UHFFFAOYSA-N bixlozone Chemical compound O=C1C(C)(C)CON1CC1=CC=C(Cl)C=C1Cl FHUKASKVKWSLCY-UHFFFAOYSA-N 0.000 description 5
- 210000000170 cell membrane Anatomy 0.000 description 5
- NSWAMPCUPHPTTC-UHFFFAOYSA-N chlorimuron-ethyl Chemical group CCOC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(Cl)=CC(OC)=N1 NSWAMPCUPHPTTC-UHFFFAOYSA-N 0.000 description 5
- 238000006073 displacement reaction Methods 0.000 description 5
- 239000004495 emulsifiable concentrate Substances 0.000 description 5
- 150000004665 fatty acids Chemical class 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- KPUREKXXPHOJQT-UHFFFAOYSA-N mesotrione Chemical compound [O-][N+](=O)C1=CC(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O KPUREKXXPHOJQT-UHFFFAOYSA-N 0.000 description 5
- DTVOKYWXACGVGO-UHFFFAOYSA-N methyl 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-6-(trifluoromethyl)pyridine-3-carboxylate Chemical group COC(=O)C1=CC=C(C(F)(F)F)N=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 DTVOKYWXACGVGO-UHFFFAOYSA-N 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 239000000575 pesticide Substances 0.000 description 5
- 230000008635 plant growth Effects 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 150000003254 radicals Chemical class 0.000 description 5
- AHTPATJNIAFOLR-UHFFFAOYSA-N thifensulfuron-methyl Chemical group S1C=CC(S(=O)(=O)NC(=O)NC=2N=C(OC)N=C(C)N=2)=C1C(=O)OC AHTPATJNIAFOLR-UHFFFAOYSA-N 0.000 description 5
- 125000004737 (C1-C6) haloalkoxy group Chemical group 0.000 description 4
- IAJOBQBIJHVGMQ-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid Chemical compound CP(O)(=O)CCC(N)C(O)=O IAJOBQBIJHVGMQ-UHFFFAOYSA-N 0.000 description 4
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 4
- 235000007320 Avena fatua Nutrition 0.000 description 4
- 241000209764 Avena fatua Species 0.000 description 4
- 239000005469 Azimsulfuron Substances 0.000 description 4
- 239000005492 Carfentrazone-ethyl Substances 0.000 description 4
- 239000005499 Clomazone Substances 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- RXCPQSJAVKGONC-UHFFFAOYSA-N Flumetsulam Chemical compound N1=C2N=C(C)C=CN2N=C1S(=O)(=O)NC1=C(F)C=CC=C1F RXCPQSJAVKGONC-UHFFFAOYSA-N 0.000 description 4
- XVOKUMIPKHGGTN-UHFFFAOYSA-N Imazethapyr Chemical compound OC(=O)C1=CC(CC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 XVOKUMIPKHGGTN-UHFFFAOYSA-N 0.000 description 4
- 239000005572 Lenacil Substances 0.000 description 4
- 239000005583 Metribuzin Substances 0.000 description 4
- 239000005584 Metsulfuron-methyl Substances 0.000 description 4
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 4
- 239000005593 Pethoxamid Substances 0.000 description 4
- 239000005595 Picloram Substances 0.000 description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 4
- 239000005616 Rimsulfuron Substances 0.000 description 4
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 4
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 4
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 4
- 150000001408 amides Chemical class 0.000 description 4
- 229910021529 ammonia Inorganic materials 0.000 description 4
- MAHPNPYYQAIOJN-UHFFFAOYSA-N azimsulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=CC=2C2=NN(C)N=N2)C)=N1 MAHPNPYYQAIOJN-UHFFFAOYSA-N 0.000 description 4
- 239000003054 catalyst Substances 0.000 description 4
- KIEDNEWSYUYDSN-UHFFFAOYSA-N clomazone Chemical compound O=C1C(C)(C)CON1CC1=CC=CC=C1Cl KIEDNEWSYUYDSN-UHFFFAOYSA-N 0.000 description 4
- BIKACRYIQSLICJ-UHFFFAOYSA-N cloransulam-methyl Chemical group N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1C(=O)OC BIKACRYIQSLICJ-UHFFFAOYSA-N 0.000 description 4
- MZZBPDKVEFVLFF-UHFFFAOYSA-N cyanazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)(C)C#N)=N1 MZZBPDKVEFVLFF-UHFFFAOYSA-N 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- SWXVUIWOUIDPGS-UHFFFAOYSA-N diacetone alcohol Chemical compound CC(=O)CC(C)(C)O SWXVUIWOUIDPGS-UHFFFAOYSA-N 0.000 description 4
- ZINJLDJMHCUBIP-UHFFFAOYSA-N ethametsulfuron-methyl Chemical group CCOC1=NC(NC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(=O)OC)=N1 ZINJLDJMHCUBIP-UHFFFAOYSA-N 0.000 description 4
- MLKCGVHIFJBRCD-UHFFFAOYSA-N ethyl 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]-4-fluorophenyl}propanoate Chemical group C1=C(Cl)C(CC(Cl)C(=O)OCC)=CC(N2C(N(C(F)F)C(C)=N2)=O)=C1F MLKCGVHIFJBRCD-UHFFFAOYSA-N 0.000 description 4
- ZCNQYNHDVRPZIH-UHFFFAOYSA-N fluthiacet-methyl Chemical group C1=C(Cl)C(SCC(=O)OC)=CC(N=C2N3CCCCN3C(=O)S2)=C1F ZCNQYNHDVRPZIH-UHFFFAOYSA-N 0.000 description 4
- BGZZWXTVIYUUEY-UHFFFAOYSA-N fomesafen Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)C)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 BGZZWXTVIYUUEY-UHFFFAOYSA-N 0.000 description 4
- IAJOBQBIJHVGMQ-BYPYZUCNSA-N glufosinate-P Chemical class CP(O)(=O)CC[C@H](N)C(O)=O IAJOBQBIJHVGMQ-BYPYZUCNSA-N 0.000 description 4
- 150000002314 glycerols Chemical class 0.000 description 4
- 150000004820 halides Chemical class 0.000 description 4
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 4
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 4
- ZTMKADLOSYKWCA-UHFFFAOYSA-N lenacil Chemical compound O=C1NC=2CCCC=2C(=O)N1C1CCCCC1 ZTMKADLOSYKWCA-UHFFFAOYSA-N 0.000 description 4
- 150000002632 lipids Chemical class 0.000 description 4
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 4
- 235000009973 maize Nutrition 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 150000004702 methyl esters Chemical class 0.000 description 4
- FOXFZRUHNHCZPX-UHFFFAOYSA-N metribuzin Chemical compound CSC1=NN=C(C(C)(C)C)C(=O)N1N FOXFZRUHNHCZPX-UHFFFAOYSA-N 0.000 description 4
- RSMUVYRMZCOLBH-UHFFFAOYSA-N metsulfuron methyl Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(C)=NC(OC)=N1 RSMUVYRMZCOLBH-UHFFFAOYSA-N 0.000 description 4
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 4
- JXTHEWSKYLZVJC-UHFFFAOYSA-N naptalam Chemical compound OC(=O)C1=CC=CC=C1C(=O)NC1=CC=CC2=CC=CC=C12 JXTHEWSKYLZVJC-UHFFFAOYSA-N 0.000 description 4
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 4
- 239000002736 nonionic surfactant Substances 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 238000007254 oxidation reaction Methods 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- CSWIKHNSBZVWNQ-UHFFFAOYSA-N pethoxamide Chemical compound CCOCCN(C(=O)CCl)C(=C(C)C)C1=CC=CC=C1 CSWIKHNSBZVWNQ-UHFFFAOYSA-N 0.000 description 4
- 150000002989 phenols Chemical class 0.000 description 4
- 150000003014 phosphoric acid esters Chemical class 0.000 description 4
- NQQVFXUMIDALNH-UHFFFAOYSA-N picloram Chemical compound NC1=C(Cl)C(Cl)=NC(C(O)=O)=C1Cl NQQVFXUMIDALNH-UHFFFAOYSA-N 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- FFSSWMQPCJRCRV-UHFFFAOYSA-N quinclorac Chemical compound ClC1=CN=C2C(C(=O)O)=C(Cl)C=CC2=C1 FFSSWMQPCJRCRV-UHFFFAOYSA-N 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 235000009566 rice Nutrition 0.000 description 4
- MEFOUWRMVYJCQC-UHFFFAOYSA-N rimsulfuron Chemical compound CCS(=O)(=O)C1=CC=CN=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 MEFOUWRMVYJCQC-UHFFFAOYSA-N 0.000 description 4
- 239000012312 sodium hydride Substances 0.000 description 4
- 229910000104 sodium hydride Inorganic materials 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- OORLZFUTLGXMEF-UHFFFAOYSA-N sulfentrazone Chemical compound O=C1N(C(F)F)C(C)=NN1C1=CC(NS(C)(=O)=O)=C(Cl)C=C1Cl OORLZFUTLGXMEF-UHFFFAOYSA-N 0.000 description 4
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 4
- YMXOXAPKZDWXLY-QWRGUYRKSA-N tribenuron methyl Chemical group COC(=O)[C@H]1CCCC[C@@H]1S(=O)(=O)NC(=O)N(C)C1=NC(C)=NC(OC)=N1 YMXOXAPKZDWXLY-QWRGUYRKSA-N 0.000 description 4
- IMEVJVISCHQJRM-UHFFFAOYSA-N triflusulfuron-methyl Chemical group COC(=O)C1=CC=CC(C)=C1S(=O)(=O)NC(=O)NC1=NC(OCC(F)(F)F)=NC(N(C)C)=N1 IMEVJVISCHQJRM-UHFFFAOYSA-N 0.000 description 4
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 4
- 229910052725 zinc Inorganic materials 0.000 description 4
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Polymers OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 3
- 125000006677 (C1-C3) haloalkoxy group Chemical group 0.000 description 3
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 description 3
- WXZVAROIGSFCFJ-CYBMUJFWSA-N (R)-napropamide Chemical compound C1=CC=C2C(O[C@H](C)C(=O)N(CC)CC)=CC=CC2=C1 WXZVAROIGSFCFJ-CYBMUJFWSA-N 0.000 description 3
- FFQPZWRNXKPNPX-INIZCTEOSA-N (S)-beflubutamid Chemical compound O([C@@H](CC)C(=O)NCC=1C=CC=CC=1)C1=CC=C(F)C(C(F)(F)F)=C1 FFQPZWRNXKPNPX-INIZCTEOSA-N 0.000 description 3
- DHYXNIKICPUXJI-UHFFFAOYSA-N 1-(2,4-dichlorophenyl)-n-(2,4-difluorophenyl)-5-oxo-n-propan-2-yl-1,2,4-triazole-4-carboxamide Chemical compound C=1C=C(F)C=C(F)C=1N(C(C)C)C(=O)N(C1=O)C=NN1C1=CC=C(Cl)C=C1Cl DHYXNIKICPUXJI-UHFFFAOYSA-N 0.000 description 3
- PYCINWWWERDNKE-UHFFFAOYSA-N 1-(2-chloro-6-propylimidazo[1,2-b]pyridazin-3-yl)sulfonyl-3-(4,6-dimethoxypyrimidin-2-yl)urea Chemical compound N12N=C(CCC)C=CC2=NC(Cl)=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 PYCINWWWERDNKE-UHFFFAOYSA-N 0.000 description 3
- VQHHIQJPQOLZGF-UHFFFAOYSA-N 1-(2-iodophenyl)sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)I)=N1 VQHHIQJPQOLZGF-UHFFFAOYSA-N 0.000 description 3
- GHLCSCRDVVEUQD-UHFFFAOYSA-N 1-({1-ethyl-4-[3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoyl]-1H-pyrazol-5-yl}oxy)ethyl methyl carbonate Chemical compound CCN1N=CC(C(=O)C=2C(=C(OCCOC)C(=CC=2)S(C)(=O)=O)C)=C1OC(C)OC(=O)OC GHLCSCRDVVEUQD-UHFFFAOYSA-N 0.000 description 3
- IXWKBUKANTXHJH-UHFFFAOYSA-N 1-[5-chloro-2-methyl-4-(5-methyl-5,6-dihydro-1,4,2-dioxazin-3-yl)pyrazol-3-yl]sulfonyl-3-(4,6-dimethoxypyrimidin-2-yl)urea Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=C(Cl)C=2C=2OC(C)CON=2)C)=N1 IXWKBUKANTXHJH-UHFFFAOYSA-N 0.000 description 3
- AHXUVTKVCMOKIX-UHFFFAOYSA-N 1-bromo-4-(chloromethylsulfonyl)benzene Chemical compound ClCS(=O)(=O)C1=CC=C(Br)C=C1 AHXUVTKVCMOKIX-UHFFFAOYSA-N 0.000 description 3
- 238000005160 1H NMR spectroscopy Methods 0.000 description 3
- KRQUFUKTQHISJB-YYADALCUSA-N 2-[(E)-N-[2-(4-chlorophenoxy)propoxy]-C-propylcarbonimidoyl]-3-hydroxy-5-(thian-3-yl)cyclohex-2-en-1-one Chemical compound CCC\C(=N/OCC(C)OC1=CC=C(Cl)C=C1)C1=C(O)CC(CC1=O)C1CCCSC1 KRQUFUKTQHISJB-YYADALCUSA-N 0.000 description 3
- WVQBLGZPHOPPFO-UHFFFAOYSA-N 2-chloro-N-(2-ethyl-6-methylphenyl)-N-(1-methoxypropan-2-yl)acetamide Chemical compound CCC1=CC=CC(C)=C1N(C(C)COC)C(=O)CCl WVQBLGZPHOPPFO-UHFFFAOYSA-N 0.000 description 3
- UPMXNNIRAGDFEH-UHFFFAOYSA-N 3,5-dibromo-4-hydroxybenzonitrile Chemical compound OC1=C(Br)C=C(C#N)C=C1Br UPMXNNIRAGDFEH-UHFFFAOYSA-N 0.000 description 3
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 3
- DWEXQMNCDCYZAG-UHFFFAOYSA-N 3-(4-pyrimidin-2-ylpyridazin-1-ium-1-yl)propanoate Chemical compound C1=CN=C(N=C1)C2=CN=[N+](C=C2)CCC(=O)[O-] DWEXQMNCDCYZAG-UHFFFAOYSA-N 0.000 description 3
- OYJGTWPEVWCBMZ-UHFFFAOYSA-N 4-(2,6-diethyl-4-methylphenyl)-5-hydroxy-2,6-dimethylpyridazin-3-one Chemical compound CCC1=CC(C)=CC(CC)=C1C1=C(O)C(C)=NN(C)C1=O OYJGTWPEVWCBMZ-UHFFFAOYSA-N 0.000 description 3
- CTSLUCNDVMMDHG-UHFFFAOYSA-N 5-bromo-3-(butan-2-yl)-6-methylpyrimidine-2,4(1H,3H)-dione Chemical compound CCC(C)N1C(=O)NC(C)=C(Br)C1=O CTSLUCNDVMMDHG-UHFFFAOYSA-N 0.000 description 3
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 3
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- 239000005470 Beflubutamid Substances 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 3
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 3
- 239000005489 Bromoxynil Substances 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- 239000005510 Diuron Substances 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 239000005533 Fluometuron Substances 0.000 description 3
- 241001101998 Galium Species 0.000 description 3
- 244000020551 Helianthus annuus Species 0.000 description 3
- 235000003222 Helianthus annuus Nutrition 0.000 description 3
- CAWXEEYDBZRFPE-UHFFFAOYSA-N Hexazinone Chemical compound O=C1N(C)C(N(C)C)=NC(=O)N1C1CCCCC1 CAWXEEYDBZRFPE-UHFFFAOYSA-N 0.000 description 3
- 235000007340 Hordeum vulgare Nutrition 0.000 description 3
- 240000005979 Hordeum vulgare Species 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 244000100545 Lolium multiflorum Species 0.000 description 3
- FFQPZWRNXKPNPX-UHFFFAOYSA-N N-benzyl-2-[4-fluoro-3-(trifluoromethyl)phenoxy]butanamide Chemical compound C=1C=CC=CC=1CNC(=O)C(CC)OC1=CC=C(F)C(C(F)(F)F)=C1 FFQPZWRNXKPNPX-UHFFFAOYSA-N 0.000 description 3
- GRSMWKLPSNHDHA-UHFFFAOYSA-N Naphthalic anhydride Chemical compound C1=CC(C(=O)OC2=O)=C3C2=CC=CC3=C1 GRSMWKLPSNHDHA-UHFFFAOYSA-N 0.000 description 3
- 239000005586 Nicosulfuron Substances 0.000 description 3
- 239000005597 Pinoxaden Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000005606 Pyridate Substances 0.000 description 3
- JTZCTMAVMHRNTR-UHFFFAOYSA-N Pyridate Chemical compound CCCCCCCCSC(=O)OC1=CC(Cl)=NN=C1C1=CC=CC=C1 JTZCTMAVMHRNTR-UHFFFAOYSA-N 0.000 description 3
- 235000017016 Setaria faberi Nutrition 0.000 description 3
- 241001355178 Setaria faberi Species 0.000 description 3
- 235000002595 Solanum tuberosum Nutrition 0.000 description 3
- 244000061456 Solanum tuberosum Species 0.000 description 3
- 239000005620 Tembotrione Substances 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 241000607479 Yersinia pestis Species 0.000 description 3
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 3
- 125000004644 alkyl sulfinyl group Chemical group 0.000 description 3
- 229940024606 amino acid Drugs 0.000 description 3
- KWAIHLIXESXTJL-UHFFFAOYSA-N aminocyclopyrachlor Chemical compound OC(=O)C1=C(Cl)C(N)=NC(C2CC2)=N1 KWAIHLIXESXTJL-UHFFFAOYSA-N 0.000 description 3
- 239000003945 anionic surfactant Substances 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- ZOMSMJKLGFBRBS-UHFFFAOYSA-N bentazone Chemical compound C1=CC=C2NS(=O)(=O)N(C(C)C)C(=O)C2=C1 ZOMSMJKLGFBRBS-UHFFFAOYSA-N 0.000 description 3
- HUYBEDCQLAEVPD-MNOVXSKESA-N bicyclopyrone Chemical compound COCCOCc1nc(ccc1C(=O)C1=C(O)[C@@H]2CC[C@@H](C2)C1=O)C(F)(F)F HUYBEDCQLAEVPD-MNOVXSKESA-N 0.000 description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 3
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 239000006227 byproduct Substances 0.000 description 3
- 239000003093 cationic surfactant Substances 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- GITFHTZGVMIBGS-UHFFFAOYSA-M chloropalladium(1+);ditert-butyl-[2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane;2-phenylethanamine Chemical compound [Pd+]Cl.NCCC1=CC=CC=[C-]1.CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C(C)(C)C)C(C)(C)C GITFHTZGVMIBGS-UHFFFAOYSA-M 0.000 description 3
- 210000003763 chloroplast Anatomy 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 229940125782 compound 2 Drugs 0.000 description 3
- 239000012141 concentrate Substances 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 239000013078 crystal Substances 0.000 description 3
- OVIZXYUPMXRUSD-UHFFFAOYSA-N cyanomethyl 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)pyridine-2-carboxylate Chemical compound NC1=C(C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC#N)Cl OVIZXYUPMXRUSD-UHFFFAOYSA-N 0.000 description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 3
- BXIGJZDQFDFASM-UHFFFAOYSA-N cyclopyrimorate Chemical compound N=1N=C(Cl)C=C(OC(=O)N2CCOCC2)C=1OC=1C(C)=CC=CC=1C1CC1 BXIGJZDQFDFASM-UHFFFAOYSA-N 0.000 description 3
- MXFYYFVVIIWKFE-UHFFFAOYSA-N dicyclohexyl-[2-[2,6-di(propan-2-yloxy)phenyl]phenyl]phosphane Chemical group CC(C)OC1=CC=CC(OC(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 MXFYYFVVIIWKFE-UHFFFAOYSA-N 0.000 description 3
- BWUPSGJXXPATLU-UHFFFAOYSA-N dimepiperate Chemical compound C=1C=CC=CC=1C(C)(C)SC(=O)N1CCCCC1 BWUPSGJXXPATLU-UHFFFAOYSA-N 0.000 description 3
- 235000013399 edible fruits Nutrition 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- ZFQQLFKHTMIGJX-UHFFFAOYSA-N ethyl 3-[2-chloro-4-fluoro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]phenyl]-5-methyl-4H-1,2-oxazole-5-carboxylate Chemical compound C(C)OC(=O)C1(CC(=NO1)C1=C(C=C(C(=C1)N1C(N(C(=CC1=O)C(F)(F)F)C)=O)F)Cl)C ZFQQLFKHTMIGJX-UHFFFAOYSA-N 0.000 description 3
- ACDZDIIWZVQMIX-UHFFFAOYSA-N fenoxasulfone Chemical compound C1=C(Cl)C(OCC)=CC(Cl)=C1CS(=O)(=O)C1=NOC(C)(C)C1 ACDZDIIWZVQMIX-UHFFFAOYSA-N 0.000 description 3
- RZILCCPWPBTYDO-UHFFFAOYSA-N fluometuron Chemical compound CN(C)C(=O)NC1=CC=CC(C(F)(F)F)=C1 RZILCCPWPBTYDO-UHFFFAOYSA-N 0.000 description 3
- XDDAORKBJWWYJS-UHFFFAOYSA-N glyphosate Chemical compound OC(=O)CNCP(O)(O)=O XDDAORKBJWWYJS-UHFFFAOYSA-N 0.000 description 3
- 229940097068 glyphosate Drugs 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- KKLBEFSLWYDQFI-UHFFFAOYSA-N halauxifen Chemical compound COC1=C(Cl)C=CC(C=2N=C(C(Cl)=C(N)C=2)C(O)=O)=C1F KKLBEFSLWYDQFI-UHFFFAOYSA-N 0.000 description 3
- KDHKOPYYWOHESS-UHFFFAOYSA-N halauxifen-methyl Chemical group NC1=C(Cl)C(C(=O)OC)=NC(C=2C(=C(OC)C(Cl)=CC=2)F)=C1 KDHKOPYYWOHESS-UHFFFAOYSA-N 0.000 description 3
- YFONKFDEZLYQDH-BOURZNODSA-N indaziflam Chemical compound CC(F)C1=NC(N)=NC(N[C@H]2C3=CC(C)=CC=C3C[C@@H]2C)=N1 YFONKFDEZLYQDH-BOURZNODSA-N 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 239000002917 insecticide Substances 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 239000011630 iodine Substances 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 230000000155 isotopic effect Effects 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- QPTPZPIXUPELRM-UHFFFAOYSA-N methyl 3-[2-[2-chloro-4-fluoro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]phenyl]sulfanylpropanoylamino]propanoate Chemical compound C1=C(Cl)C(SC(C)C(=O)NCCC(=O)OC)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1F QPTPZPIXUPELRM-UHFFFAOYSA-N 0.000 description 3
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 3
- GBHVIWKSEHWFDD-UHFFFAOYSA-N n-[2-(4,6-dimethoxy-1,3,5-triazine-2-carbonyl)-6-fluorophenyl]-1,1-difluoro-n-methylmethanesulfonamide Chemical compound COC1=NC(OC)=NC(C(=O)C=2C(=C(F)C=CC=2)N(C)S(=O)(=O)C(F)F)=N1 GBHVIWKSEHWFDD-UHFFFAOYSA-N 0.000 description 3
- RTCOGUMHFFWOJV-UHFFFAOYSA-N nicosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)C(=O)N(C)C)=N1 RTCOGUMHFFWOJV-UHFFFAOYSA-N 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- MGOHCFMYLBAPRN-UHFFFAOYSA-N pinoxaden Chemical compound CCC1=CC(C)=CC(CC)=C1C(C1=O)=C(OC(=O)C(C)(C)C)N2N1CCOCC2 MGOHCFMYLBAPRN-UHFFFAOYSA-N 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- TUWNZICUVDSWIN-UHFFFAOYSA-N prop-2-ynyl 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)pyridine-2-carboxylate Chemical compound NC1=C(C(=NC(=C1F)C1=CC=C2C=CNC2=C1F)C(=O)OCC#C)Cl TUWNZICUVDSWIN-UHFFFAOYSA-N 0.000 description 3
- 125000004076 pyridyl group Chemical group 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 239000011877 solvent mixture Substances 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000004546 suspension concentrate Substances 0.000 description 3
- IUQAXCIUEPFPSF-UHFFFAOYSA-N tembotrione Chemical compound ClC1=C(COCC(F)(F)F)C(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O IUQAXCIUEPFPSF-UHFFFAOYSA-N 0.000 description 3
- 150000003512 tertiary amines Chemical class 0.000 description 3
- QQDYOLJZDUADHV-CJNGLKHVSA-N tetflupyrolimet Chemical compound FC1=C(C=CC=C1)NC(=O)[C@H]1C(N(C[C@@H]1C1=CC(=CC=C1)C(F)(F)F)C)=O QQDYOLJZDUADHV-CJNGLKHVSA-N 0.000 description 3
- WYRSGXAIHNMKOL-UHFFFAOYSA-N $l^{1}-sulfanylethane Chemical compound CC[S] WYRSGXAIHNMKOL-UHFFFAOYSA-N 0.000 description 2
- QSLPNSWXUQHVLP-UHFFFAOYSA-N $l^{1}-sulfanylmethane Chemical compound [S]C QSLPNSWXUQHVLP-UHFFFAOYSA-N 0.000 description 2
- VIXCLRUCUMWJFF-KGLIPLIRSA-N (1R,5S)-benzobicyclon Chemical compound CS(=O)(=O)c1ccc(C(=O)C2=C(Sc3ccccc3)[C@H]3CC[C@H](C3)C2=O)c(Cl)c1 VIXCLRUCUMWJFF-KGLIPLIRSA-N 0.000 description 2
- IPPAUTOBDWNELX-UHFFFAOYSA-N (2-ethoxy-2-oxoethyl) 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical group C1=C([N+]([O-])=O)C(C(=O)OCC(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 IPPAUTOBDWNELX-UHFFFAOYSA-N 0.000 description 2
- QUJMAWONJHVEOF-UHFFFAOYSA-N (2-fluorophenyl)sulfonylurea Chemical compound NC(=O)NS(=O)(=O)C1=C(C=CC=C1)F QUJMAWONJHVEOF-UHFFFAOYSA-N 0.000 description 2
- FHTLRVQASCAGSZ-UHFFFAOYSA-N (2-methylphenyl)sulfonylurea Chemical compound CC1=CC=CC=C1S(=O)(=O)NC(N)=O FHTLRVQASCAGSZ-UHFFFAOYSA-N 0.000 description 2
- LNGRZPZKVUBWQV-UHFFFAOYSA-N (4-chloro-2-methylsulfonylphenyl)-(5-cyclopropyl-1,2-oxazol-4-yl)methanone Chemical compound CS(=O)(=O)C1=CC(Cl)=CC=C1C(=O)C1=C(C2CC2)ON=C1 LNGRZPZKVUBWQV-UHFFFAOYSA-N 0.000 description 2
- WMMQJAQJAPXWDO-UHFFFAOYSA-N (4-chlorophenyl) n-methylcarbamate Chemical compound CNC(=O)OC1=CC=C(Cl)C=C1 WMMQJAQJAPXWDO-UHFFFAOYSA-N 0.000 description 2
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 2
- 125000004738 (C1-C6) alkyl sulfinyl group Chemical group 0.000 description 2
- 125000004739 (C1-C6) alkylsulfonyl group Chemical group 0.000 description 2
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 description 2
- OVXMBIVWNJDDSM-UHFFFAOYSA-N (benzhydrylideneamino) 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate Chemical compound COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C(=O)ON=C(C=2C=CC=CC=2)C=2C=CC=CC=2)=N1 OVXMBIVWNJDDSM-UHFFFAOYSA-N 0.000 description 2
- PYKLUAIDKVVEOS-RAXLEYEMSA-N (e)-n-(cyanomethoxy)benzenecarboximidoyl cyanide Chemical compound N#CCO\N=C(\C#N)C1=CC=CC=C1 PYKLUAIDKVVEOS-RAXLEYEMSA-N 0.000 description 2
- MHULQDZDXMHODA-UHFFFAOYSA-N 1-(2,2-dichloroacetyl)-3,3,8a-trimethyl-2,4,7,8-tetrahydropyrrolo[1,2-a]pyrimidin-6-one Chemical compound C1C(C)(C)CN(C(=O)C(Cl)Cl)C2(C)N1C(=O)CC2 MHULQDZDXMHODA-UHFFFAOYSA-N 0.000 description 2
- RBSXHDIPCIWOMG-UHFFFAOYSA-N 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethylsulfonylimidazo[1,2-a]pyridin-3-yl)sulfonylurea Chemical compound CCS(=O)(=O)C=1N=C2C=CC=CN2C=1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 RBSXHDIPCIWOMG-UHFFFAOYSA-N 0.000 description 2
- UUHXXNQVWVFJLW-UHFFFAOYSA-N 1-dimethoxyphosphorylethyl 2-(2,4-dichlorophenoxy)acetate Chemical compound COP(=O)(OC)C(C)OC(=O)COC1=CC=C(Cl)C=C1Cl UUHXXNQVWVFJLW-UHFFFAOYSA-N 0.000 description 2
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical compound CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 2
- QWWHRELOCZEQNZ-UHFFFAOYSA-N 2,2-dichloro-1-(1-oxa-4-azaspiro[4.5]decan-4-yl)ethanone Chemical compound ClC(Cl)C(=O)N1CCOC11CCCCC1 QWWHRELOCZEQNZ-UHFFFAOYSA-N 0.000 description 2
- MCNOFYBITGAAGM-UHFFFAOYSA-N 2,2-dichloro-1-[5-(furan-2-yl)-2,2-dimethyl-1,3-oxazolidin-3-yl]ethanone Chemical compound C1N(C(=O)C(Cl)Cl)C(C)(C)OC1C1=CC=CO1 MCNOFYBITGAAGM-UHFFFAOYSA-N 0.000 description 2
- XZIDTOHMJBOSOX-UHFFFAOYSA-N 2,3,6-TBA Chemical compound OC(=O)C1=C(Cl)C=CC(Cl)=C1Cl XZIDTOHMJBOSOX-UHFFFAOYSA-N 0.000 description 2
- 239000002794 2,4-DB Substances 0.000 description 2
- YIVXMZJTEQBPQO-UHFFFAOYSA-N 2,4-DB Chemical compound OC(=O)CCCOC1=CC=C(Cl)C=C1Cl YIVXMZJTEQBPQO-UHFFFAOYSA-N 0.000 description 2
- YOYAIZYFCNQIRF-UHFFFAOYSA-N 2,6-dichlorobenzonitrile Chemical compound ClC1=CC=CC(Cl)=C1C#N YOYAIZYFCNQIRF-UHFFFAOYSA-N 0.000 description 2
- KGKGSIUWJCAFPX-UHFFFAOYSA-N 2,6-dichlorothiobenzamide Chemical compound NC(=S)C1=C(Cl)C=CC=C1Cl KGKGSIUWJCAFPX-UHFFFAOYSA-N 0.000 description 2
- BDQWWOHKFDSADC-UHFFFAOYSA-N 2-(2,4-dichloro-3-methylphenoxy)-n-phenylpropanamide Chemical compound C=1C=CC=CC=1NC(=O)C(C)OC1=CC=C(Cl)C(C)=C1Cl BDQWWOHKFDSADC-UHFFFAOYSA-N 0.000 description 2
- MZHCENGPTKEIGP-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1Cl MZHCENGPTKEIGP-UHFFFAOYSA-N 0.000 description 2
- WNTGYJSOUMFZEP-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1C WNTGYJSOUMFZEP-UHFFFAOYSA-N 0.000 description 2
- NUPJIGQFXCQJBK-UHFFFAOYSA-N 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)-5-(methoxymethyl)nicotinic acid Chemical compound OC(=O)C1=CC(COC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 NUPJIGQFXCQJBK-UHFFFAOYSA-N 0.000 description 2
- CLQMBPJKHLGMQK-UHFFFAOYSA-N 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)nicotinic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC=CC=C1C(O)=O CLQMBPJKHLGMQK-UHFFFAOYSA-N 0.000 description 2
- OHXLAOJLJWLEIP-UHFFFAOYSA-N 2-(dichloromethyl)-2-methyl-1,3-dioxolane Chemical compound ClC(Cl)C1(C)OCCO1 OHXLAOJLJWLEIP-UHFFFAOYSA-N 0.000 description 2
- ZTMOLOVAQWCURR-UHFFFAOYSA-N 2-[(2,5-dichlorophenyl)methyl]-4,4-dimethyl-1,2-oxazolidin-3-one Chemical compound O=C1C(C)(C)CON1CC1=CC(Cl)=CC=C1Cl ZTMOLOVAQWCURR-UHFFFAOYSA-N 0.000 description 2
- IRJQWZWMQCVOLA-ZBKNUEDVSA-N 2-[(z)-n-[(3,5-difluorophenyl)carbamoylamino]-c-methylcarbonimidoyl]pyridine-3-carboxylic acid Chemical compound N=1C=CC=C(C(O)=O)C=1C(/C)=N\NC(=O)NC1=CC(F)=CC(F)=C1 IRJQWZWMQCVOLA-ZBKNUEDVSA-N 0.000 description 2
- CABMTIJINOIHOD-UHFFFAOYSA-N 2-[4-methyl-5-oxo-4-(propan-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline-3-carboxylic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC2=CC=CC=C2C=C1C(O)=O CABMTIJINOIHOD-UHFFFAOYSA-N 0.000 description 2
- IOYNQIMAUDJVEI-ZFNPBRLTSA-N 2-[N-[(E)-3-chloroprop-2-enoxy]-C-ethylcarbonimidoyl]-3-hydroxy-5-(oxan-4-yl)cyclohex-2-en-1-one Chemical compound C1C(=O)C(C(=NOC\C=C\Cl)CC)=C(O)CC1C1CCOCC1 IOYNQIMAUDJVEI-ZFNPBRLTSA-N 0.000 description 2
- JLYFCTQDENRSOL-UHFFFAOYSA-N 2-chloro-N-(2,4-dimethylthiophen-3-yl)-N-(1-methoxypropan-2-yl)acetamide Chemical compound COCC(C)N(C(=O)CCl)C=1C(C)=CSC=1C JLYFCTQDENRSOL-UHFFFAOYSA-N 0.000 description 2
- KZNDFYDURHAESM-UHFFFAOYSA-N 2-chloro-n-(2-ethyl-6-methylphenyl)-n-(propan-2-yloxymethyl)acetamide Chemical compound CCC1=CC=CC(C)=C1N(COC(C)C)C(=O)CCl KZNDFYDURHAESM-UHFFFAOYSA-N 0.000 description 2
- CPCVDCYAKMUIKW-UHFFFAOYSA-N 2-cyclopropylpyrimidine Chemical class C1CC1C1=NC=CC=N1 CPCVDCYAKMUIKW-UHFFFAOYSA-N 0.000 description 2
- YIWUKEYIRIRTPP-UHFFFAOYSA-N 2-ethylhexan-1-ol Chemical compound CCCCC(CC)CO YIWUKEYIRIRTPP-UHFFFAOYSA-N 0.000 description 2
- KNJZLEGWAJKFKK-UHFFFAOYSA-N 2-methyl-3-methylsulfonyl-n-(1-methyltetrazol-5-yl)-4-(trifluoromethyl)benzamide Chemical compound C1=CC(C(F)(F)F)=C(S(C)(=O)=O)C(C)=C1C(=O)NC1=NN=NN1C KNJZLEGWAJKFKK-UHFFFAOYSA-N 0.000 description 2
- LLWADFLAOKUBDR-UHFFFAOYSA-N 2-methyl-4-chlorophenoxybutyric acid Chemical compound CC1=CC(Cl)=CC=C1OCCCC(O)=O LLWADFLAOKUBDR-UHFFFAOYSA-N 0.000 description 2
- HMDNIKJIOUZBLO-UHFFFAOYSA-N 2-methyl-n-(4-methyl-1,2,5-oxadiazol-3-yl)-3-methylsulfinyl-4-(trifluoromethyl)benzamide Chemical compound CC1=NON=C1NC(=O)C1=CC=C(C(F)(F)F)C(S(C)=O)=C1C HMDNIKJIOUZBLO-UHFFFAOYSA-N 0.000 description 2
- UFAPVJDEYHLLBG-UHFFFAOYSA-N 2-{2-chloro-4-(methylsulfonyl)-3-[(tetrahydrofuran-2-ylmethoxy)methyl]benzoyl}cyclohexane-1,3-dione Chemical compound ClC1=C(COCC2OCCC2)C(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O UFAPVJDEYHLLBG-UHFFFAOYSA-N 0.000 description 2
- VLLDNNSDBRDZAF-UHFFFAOYSA-N 3-(2-chloro-3,6-difluorophenyl)-4-hydroxy-1-methyl-1,5-naphthyridin-2-one Chemical compound O=C1N(C)C2=CC=CN=C2C(O)=C1C1=C(F)C=CC(F)=C1Cl VLLDNNSDBRDZAF-UHFFFAOYSA-N 0.000 description 2
- 108010020183 3-phosphoshikimate 1-carboxyvinyltransferase Proteins 0.000 description 2
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 2
- ZOGDSYNXUXQGHF-UHFFFAOYSA-N 5-(3-butanoyl-2,4,6-trimethylphenyl)-2-[(Z)-N-ethoxy-C-ethylcarbonimidoyl]-3-hydroxycyclohex-2-en-1-one Chemical compound C(C)ON=C(CC)/C=1C(CC(CC1O)C1=C(C(=C(C=C1C)C)C(CCC)=O)C)=O ZOGDSYNXUXQGHF-UHFFFAOYSA-N 0.000 description 2
- NYRMIJKDBAQCHC-UHFFFAOYSA-N 5-(methylamino)-2-phenyl-4-[3-(trifluoromethyl)phenyl]furan-3(2H)-one Chemical compound O1C(NC)=C(C=2C=C(C=CC=2)C(F)(F)F)C(=O)C1C1=CC=CC=C1 NYRMIJKDBAQCHC-UHFFFAOYSA-N 0.000 description 2
- OPEJGICLTMWFNQ-UHFFFAOYSA-N 5-[(2,6-difluorophenyl)methoxymethyl]-5-methyl-3-(3-methylthiophen-2-yl)-4h-1,2-oxazole Chemical compound C1=CSC(C=2CC(C)(COCC=3C(=CC=CC=3F)F)ON=2)=C1C OPEJGICLTMWFNQ-UHFFFAOYSA-N 0.000 description 2
- PVSGXWMWNRGTKE-UHFFFAOYSA-N 5-methyl-2-[4-methyl-5-oxo-4-(propan-2-yl)-4,5-dihydro-1H-imidazol-2-yl]pyridine-3-carboxylic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC=C(C)C=C1C(O)=O PVSGXWMWNRGTKE-UHFFFAOYSA-N 0.000 description 2
- DVOODWOZJVJKQR-UHFFFAOYSA-N 5-tert-butyl-3-(2,4-dichloro-5-prop-2-ynoxyphenyl)-1,3,4-oxadiazol-2-one Chemical group O=C1OC(C(C)(C)C)=NN1C1=CC(OCC#C)=C(Cl)C=C1Cl DVOODWOZJVJKQR-UHFFFAOYSA-N 0.000 description 2
- ZNORIBFUKCCYPJ-UHFFFAOYSA-N 7-(3,5-dichloropyridin-4-yl)-5-(2,2-difluoroethyl)-8-hydroxypyrido[2,3-b]pyrazin-6-one Chemical compound O=C1N(CC(F)F)C2=NC=CN=C2C(O)=C1C1=C(Cl)C=NC=C1Cl ZNORIBFUKCCYPJ-UHFFFAOYSA-N 0.000 description 2
- VTNQPKFIQCLBDU-UHFFFAOYSA-N Acetochlor Chemical compound CCOCN(C(=O)CCl)C1=C(C)C=CC=C1CC VTNQPKFIQCLBDU-UHFFFAOYSA-N 0.000 description 2
- 239000002890 Aclonifen Substances 0.000 description 2
- XKJMBINCVNINCA-UHFFFAOYSA-N Alfalone Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XKJMBINCVNINCA-UHFFFAOYSA-N 0.000 description 2
- 241001542006 Amaranthus palmeri Species 0.000 description 2
- 235000009051 Ambrosia paniculata var. peruviana Nutrition 0.000 description 2
- 239000003666 Amidosulfuron Substances 0.000 description 2
- CTTHWASMBLQOFR-UHFFFAOYSA-N Amidosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)N(C)S(C)(=O)=O)=N1 CTTHWASMBLQOFR-UHFFFAOYSA-N 0.000 description 2
- 239000005468 Aminopyralid Substances 0.000 description 2
- KLSJWNVTNUYHDU-UHFFFAOYSA-N Amitrole Chemical compound NC1=NC=NN1 KLSJWNVTNUYHDU-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- NXQDBZGWYSEGFL-UHFFFAOYSA-N Anilofos Chemical compound COP(=S)(OC)SCC(=O)N(C(C)C)C1=CC=C(Cl)C=C1 NXQDBZGWYSEGFL-UHFFFAOYSA-N 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 235000004535 Avena sterilis Nutrition 0.000 description 2
- PFJJMJDEVDLPNE-UHFFFAOYSA-N Benoxacor Chemical compound C1=CC=C2N(C(=O)C(Cl)Cl)C(C)COC2=C1 PFJJMJDEVDLPNE-UHFFFAOYSA-N 0.000 description 2
- 239000005472 Bensulfuron methyl Substances 0.000 description 2
- JDWQITFHZOBBFE-UHFFFAOYSA-N Benzofenap Chemical compound C=1C=C(Cl)C(C)=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=C(C)C=C1 JDWQITFHZOBBFE-UHFFFAOYSA-N 0.000 description 2
- 241000219310 Beta vulgaris subsp. vulgaris Species 0.000 description 2
- 239000005484 Bifenox Substances 0.000 description 2
- 244000024671 Brassica kaber Species 0.000 description 2
- 240000002791 Brassica napus Species 0.000 description 2
- XTFNPKDYCLFGPV-OMCISZLKSA-N Bromofenoxim Chemical compound C1=C(Br)C(O)=C(Br)C=C1\C=N\OC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O XTFNPKDYCLFGPV-OMCISZLKSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- DXXVCXKMSWHGTF-UHFFFAOYSA-N Chlomethoxyfen Chemical compound C1=C([N+]([O-])=O)C(OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 DXXVCXKMSWHGTF-UHFFFAOYSA-N 0.000 description 2
- HSSBORCLYSCBJR-UHFFFAOYSA-N Chloramben Chemical compound NC1=CC(Cl)=CC(C(O)=O)=C1Cl HSSBORCLYSCBJR-UHFFFAOYSA-N 0.000 description 2
- NLYNUTMZTCLNOO-UHFFFAOYSA-N Chlorbromuron Chemical compound CON(C)C(=O)NC1=CC=C(Br)C(Cl)=C1 NLYNUTMZTCLNOO-UHFFFAOYSA-N 0.000 description 2
- 239000005493 Chloridazon (aka pyrazone) Substances 0.000 description 2
- 239000005494 Chlorotoluron Substances 0.000 description 2
- 239000005496 Chlorsulfuron Substances 0.000 description 2
- WMLPCIHUFDKWJU-UHFFFAOYSA-N Cinosulfuron Chemical compound COCCOC1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC)=NC(OC)=N1 WMLPCIHUFDKWJU-UHFFFAOYSA-N 0.000 description 2
- 241000207199 Citrus Species 0.000 description 2
- 239000005497 Clethodim Substances 0.000 description 2
- 239000005500 Clopyralid Substances 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- OFSLKOLYLQSJPB-UHFFFAOYSA-N Cyclosulfamuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)NC=2C(=CC=CC=2)C(=O)C2CC2)=N1 OFSLKOLYLQSJPB-UHFFFAOYSA-N 0.000 description 2
- 239000005501 Cycloxydim Substances 0.000 description 2
- 239000005502 Cyhalofop-butyl Substances 0.000 description 2
- TYIYMOAHACZAMQ-CQSZACIVSA-N Cyhalofop-butyl Chemical group C1=CC(O[C@H](C)C(=O)OCCCC)=CC=C1OC1=CC=C(C#N)C=C1F TYIYMOAHACZAMQ-CQSZACIVSA-N 0.000 description 2
- 244000108484 Cyperus difformis Species 0.000 description 2
- 235000005853 Cyperus esculentus Nutrition 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 239000005503 Desmedipham Substances 0.000 description 2
- HCRWJJJUKUVORR-UHFFFAOYSA-N Desmetryn Chemical compound CNC1=NC(NC(C)C)=NC(SC)=N1 HCRWJJJUKUVORR-UHFFFAOYSA-N 0.000 description 2
- 239000005504 Dicamba Substances 0.000 description 2
- YRMLFORXOOIJDR-UHFFFAOYSA-N Dichlormid Chemical compound ClC(Cl)C(=O)N(CC=C)CC=C YRMLFORXOOIJDR-UHFFFAOYSA-N 0.000 description 2
- QNXAVFXEJCPCJO-UHFFFAOYSA-N Diclosulam Chemical compound N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1Cl QNXAVFXEJCPCJO-UHFFFAOYSA-N 0.000 description 2
- 239000005507 Diflufenican Substances 0.000 description 2
- DHWRNDJOGMTCPB-UHFFFAOYSA-N Dimefuron Chemical compound ClC1=CC(NC(=O)N(C)C)=CC=C1N1C(=O)OC(C(C)(C)C)=N1 DHWRNDJOGMTCPB-UHFFFAOYSA-N 0.000 description 2
- 239000005508 Dimethachlor Substances 0.000 description 2
- IKYICRRUVNIHPP-UHFFFAOYSA-N Dimethametryn Chemical compound CCNC1=NC(NC(C)C(C)C)=NC(SC)=N1 IKYICRRUVNIHPP-UHFFFAOYSA-N 0.000 description 2
- QAHFOPIILNICLA-UHFFFAOYSA-N Diphenamid Chemical compound C=1C=CC=CC=1C(C(=O)N(C)C)C1=CC=CC=C1 QAHFOPIILNICLA-UHFFFAOYSA-N 0.000 description 2
- 239000005630 Diquat Substances 0.000 description 2
- 244000058871 Echinochloa crus-galli Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- UWVKRNOCDUPIDM-UHFFFAOYSA-N Ethoxysulfuron Chemical compound CCOC1=CC=CC=C1OS(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 UWVKRNOCDUPIDM-UHFFFAOYSA-N 0.000 description 2
- NXCLHVLTKKEXCV-UHFFFAOYSA-N FC1=CC=C(C=C1)N1C(N(N=C(C1=O)C(=O)C1=C(CCCC1=O)O)C)=O Chemical compound FC1=CC=C(C=C1)N1C(N(N=C(C1=O)C(=O)C1=C(CCCC1=O)O)C)=O NXCLHVLTKKEXCV-UHFFFAOYSA-N 0.000 description 2
- GMBRUAIJEFRHFQ-UHFFFAOYSA-N Fenchlorazole-ethyl Chemical group N1=C(C(=O)OCC)N=C(C(Cl)(Cl)Cl)N1C1=CC=C(Cl)C=C1Cl GMBRUAIJEFRHFQ-UHFFFAOYSA-N 0.000 description 2
- NRFQZTCQAYEXEE-UHFFFAOYSA-N Fenclorim Chemical compound ClC1=CC(Cl)=NC(C=2C=CC=CC=2)=N1 NRFQZTCQAYEXEE-UHFFFAOYSA-N 0.000 description 2
- LLQPHQFNMLZJMP-UHFFFAOYSA-N Fentrazamide Chemical compound N1=NN(C=2C(=CC=CC=2)Cl)C(=O)N1C(=O)N(CC)C1CCCCC1 LLQPHQFNMLZJMP-UHFFFAOYSA-N 0.000 description 2
- 239000005514 Flazasulfuron Substances 0.000 description 2
- HWATZEJQIXKWQS-UHFFFAOYSA-N Flazasulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)C(F)(F)F)=N1 HWATZEJQIXKWQS-UHFFFAOYSA-N 0.000 description 2
- 239000005529 Florasulam Substances 0.000 description 2
- QZXATCCPQKOEIH-UHFFFAOYSA-N Florasulam Chemical compound N=1N2C(OC)=NC=C(F)C2=NC=1S(=O)(=O)NC1=C(F)C=CC=C1F QZXATCCPQKOEIH-UHFFFAOYSA-N 0.000 description 2
- 239000005531 Flufenacet Substances 0.000 description 2
- IRECWLYBCAZIJM-UHFFFAOYSA-N Flumiclorac pentyl Chemical group C1=C(Cl)C(OCC(=O)OCCCCC)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1F IRECWLYBCAZIJM-UHFFFAOYSA-N 0.000 description 2
- AOQMRUTZEYVDIL-UHFFFAOYSA-N Flupoxam Chemical compound C=1C=C(Cl)C(COCC(F)(F)C(F)(F)F)=CC=1N1N=C(C(=O)N)N=C1C1=CC=CC=C1 AOQMRUTZEYVDIL-UHFFFAOYSA-N 0.000 description 2
- YWBVHLJPRPCRSD-UHFFFAOYSA-N Fluridone Chemical compound O=C1C(C=2C=C(C=CC=2)C(F)(F)F)=CN(C)C=C1C1=CC=CC=C1 YWBVHLJPRPCRSD-UHFFFAOYSA-N 0.000 description 2
- 239000005535 Flurochloridone Substances 0.000 description 2
- 239000005558 Fluroxypyr Substances 0.000 description 2
- 239000005559 Flurtamone Substances 0.000 description 2
- UKSLKNUCVPZQCQ-UHFFFAOYSA-N Fluxofenim Chemical compound C=1C=C(Cl)C=CC=1C(C(F)(F)F)=NOCC1OCCO1 UKSLKNUCVPZQCQ-UHFFFAOYSA-N 0.000 description 2
- 239000005560 Foramsulfuron Substances 0.000 description 2
- 239000005561 Glufosinate Substances 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 244000299507 Gossypium hirsutum Species 0.000 description 2
- 239000005563 Halauxifen-methyl Substances 0.000 description 2
- 239000005564 Halosulfuron methyl Substances 0.000 description 2
- FMGZEUWROYGLAY-UHFFFAOYSA-N Halosulfuron-methyl Chemical group ClC1=NN(C)C(S(=O)(=O)NC(=O)NC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OC FMGZEUWROYGLAY-UHFFFAOYSA-N 0.000 description 2
- 241000169130 Heteranthera limosa Species 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 239000005566 Imazamox Substances 0.000 description 2
- 239000005981 Imazaquin Substances 0.000 description 2
- 239000005567 Imazosulfuron Substances 0.000 description 2
- NAGRVUXEKKZNHT-UHFFFAOYSA-N Imazosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N3C=CC=CC3=NC=2Cl)=N1 NAGRVUXEKKZNHT-UHFFFAOYSA-N 0.000 description 2
- PMAAYIYCDXGUAP-UHFFFAOYSA-N Indanofan Chemical compound O=C1C2=CC=CC=C2C(=O)C1(CC)CC1(C=2C=C(Cl)C=CC=2)CO1 PMAAYIYCDXGUAP-UHFFFAOYSA-N 0.000 description 2
- JLLJHQLUZAKJFH-UHFFFAOYSA-N Isouron Chemical compound CN(C)C(=O)NC=1C=C(C(C)(C)C)ON=1 JLLJHQLUZAKJFH-UHFFFAOYSA-N 0.000 description 2
- 239000005570 Isoxaben Substances 0.000 description 2
- 239000005571 Isoxaflutole Substances 0.000 description 2
- 239000005573 Linuron Substances 0.000 description 2
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 2
- SUSRORUBZHMPCO-UHFFFAOYSA-N MC-4379 Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 SUSRORUBZHMPCO-UHFFFAOYSA-N 0.000 description 2
- 239000005574 MCPA Substances 0.000 description 2
- 239000005575 MCPB Substances 0.000 description 2
- 101150039283 MCPB gene Proteins 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 239000005579 Metamitron Substances 0.000 description 2
- 239000005580 Metazachlor Substances 0.000 description 2
- RRVIAQKBTUQODI-UHFFFAOYSA-N Methabenzthiazuron Chemical compound C1=CC=C2SC(N(C)C(=O)NC)=NC2=C1 RRVIAQKBTUQODI-UHFFFAOYSA-N 0.000 description 2
- BWPYBAJTDILQPY-UHFFFAOYSA-N Methoxyphenone Chemical compound C1=C(C)C(OC)=CC=C1C(=O)C1=CC=CC(C)=C1 BWPYBAJTDILQPY-UHFFFAOYSA-N 0.000 description 2
- 239000005581 Metobromuron Substances 0.000 description 2
- WLFDQEVORAMCIM-UHFFFAOYSA-N Metobromuron Chemical compound CON(C)C(=O)NC1=CC=C(Br)C=C1 WLFDQEVORAMCIM-UHFFFAOYSA-N 0.000 description 2
- 239000005582 Metosulam Substances 0.000 description 2
- VGHPMIFEKOFHHQ-UHFFFAOYSA-N Metosulam Chemical compound N1=C2N=C(OC)C=C(OC)N2N=C1S(=O)(=O)NC1=C(Cl)C=CC(C)=C1Cl VGHPMIFEKOFHHQ-UHFFFAOYSA-N 0.000 description 2
- LKJPSUCKSLORMF-UHFFFAOYSA-N Monolinuron Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C=C1 LKJPSUCKSLORMF-UHFFFAOYSA-N 0.000 description 2
- 240000005561 Musa balbisiana Species 0.000 description 2
- WXZVAROIGSFCFJ-UHFFFAOYSA-N N,N-diethyl-2-(naphthalen-1-yloxy)propanamide Chemical compound C1=CC=C2C(OC(C)C(=O)N(CC)CC)=CC=CC2=C1 WXZVAROIGSFCFJ-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- IUFUITYPUYMIHI-UHFFFAOYSA-N N-[1-(3,5-dimethylphenoxy)propan-2-yl]-6-(2-fluoropropan-2-yl)-1,3,5-triazine-2,4-diamine Chemical compound N=1C(N)=NC(C(C)(C)F)=NC=1NC(C)COC1=CC(C)=CC(C)=C1 IUFUITYPUYMIHI-UHFFFAOYSA-N 0.000 description 2
- LVKTWOXHRYGDMM-UHFFFAOYSA-N Naproanilide Chemical compound C=1C=C2C=CC=CC2=CC=1OC(C)C(=O)NC1=CC=CC=C1 LVKTWOXHRYGDMM-UHFFFAOYSA-N 0.000 description 2
- 239000005585 Napropamide Substances 0.000 description 2
- CCGPUGMWYLICGL-UHFFFAOYSA-N Neburon Chemical compound CCCCN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 CCGPUGMWYLICGL-UHFFFAOYSA-N 0.000 description 2
- WFVUIONFJOAYPK-KAMYIIQDSA-N Oxabetrinil Chemical compound C=1C=CC=CC=1C(/C#N)=N\OCC1OCCO1 WFVUIONFJOAYPK-KAMYIIQDSA-N 0.000 description 2
- 239000005588 Oxadiazon Substances 0.000 description 2
- CHNUNORXWHYHNE-UHFFFAOYSA-N Oxadiazon Chemical compound C1=C(Cl)C(OC(C)C)=CC(N2C(OC(=N2)C(C)(C)C)=O)=C1Cl CHNUNORXWHYHNE-UHFFFAOYSA-N 0.000 description 2
- 239000005589 Oxasulfuron Substances 0.000 description 2
- 239000005590 Oxyfluorfen Substances 0.000 description 2
- OQMBBFQZGJFLBU-UHFFFAOYSA-N Oxyfluorfen Chemical compound C1=C([N+]([O-])=O)C(OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 OQMBBFQZGJFLBU-UHFFFAOYSA-N 0.000 description 2
- 239000005592 Penoxsulam Substances 0.000 description 2
- SYJGKVOENHZYMQ-UHFFFAOYSA-N Penoxsulam Chemical compound N1=C2C(OC)=CN=C(OC)N2N=C1NS(=O)(=O)C1=C(OCC(F)F)C=CC=C1C(F)(F)F SYJGKVOENHZYMQ-UHFFFAOYSA-N 0.000 description 2
- WGVWLKXZBUVUAM-UHFFFAOYSA-N Pentanochlor Chemical compound CCCC(C)C(=O)NC1=CC=C(C)C(Cl)=C1 WGVWLKXZBUVUAM-UHFFFAOYSA-N 0.000 description 2
- 239000005594 Phenmedipham Substances 0.000 description 2
- 239000005596 Picolinafen Substances 0.000 description 2
- UNLYSVIDNRIVFJ-UHFFFAOYSA-N Piperophos Chemical compound CCCOP(=S)(OCCC)SCC(=O)N1CCCCC1C UNLYSVIDNRIVFJ-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- YLPGTOIOYRQOHV-UHFFFAOYSA-N Pretilachlor Chemical compound CCCOCCN(C(=O)CCl)C1=C(CC)C=CC=C1CC YLPGTOIOYRQOHV-UHFFFAOYSA-N 0.000 description 2
- 239000005599 Profoxydim Substances 0.000 description 2
- 239000005600 Propaquizafop Substances 0.000 description 2
- 239000005604 Prosulfuron Substances 0.000 description 2
- LTUNNEGNEKBSEH-UHFFFAOYSA-N Prosulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)CCC(F)(F)F)=N1 LTUNNEGNEKBSEH-UHFFFAOYSA-N 0.000 description 2
- IHHMUBRVTJMLQO-UHFFFAOYSA-N Pyraclonil Chemical compound C#CCN(C)C1=C(C#N)C=NN1C1=NN(CCCC2)C2=C1Cl IHHMUBRVTJMLQO-UHFFFAOYSA-N 0.000 description 2
- 239000005605 Pyraflufen-ethyl Substances 0.000 description 2
- BGNQYGRXEXDAIQ-UHFFFAOYSA-N Pyrazosulfuron-ethyl Chemical group C1=NN(C)C(S(=O)(=O)NC(=O)NC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OCC BGNQYGRXEXDAIQ-UHFFFAOYSA-N 0.000 description 2
- RRKHIAYNPVQKEF-UHFFFAOYSA-N Pyriftalid Chemical compound COC1=CC(OC)=NC(SC=2C=3C(=O)OC(C)C=3C=CC=2)=N1 RRKHIAYNPVQKEF-UHFFFAOYSA-N 0.000 description 2
- CNILNQMBAHKMFS-UHFFFAOYSA-M Pyrithiobac-sodium Chemical compound [Na+].COC1=CC(OC)=NC(SC=2C(=C(Cl)C=CC=2)C([O-])=O)=N1 CNILNQMBAHKMFS-UHFFFAOYSA-M 0.000 description 2
- 239000005607 Pyroxsulam Substances 0.000 description 2
- 239000005608 Quinmerac Substances 0.000 description 2
- YNQSILKYZQZHFJ-UHFFFAOYSA-N R-29148 Chemical compound CC1CN(C(=O)C(Cl)Cl)C(C)(C)O1 YNQSILKYZQZHFJ-UHFFFAOYSA-N 0.000 description 2
- 235000004443 Ricinus communis Nutrition 0.000 description 2
- 240000000111 Saccharum officinarum Species 0.000 description 2
- 235000007201 Saccharum officinarum Nutrition 0.000 description 2
- CSPPKDPQLUUTND-NBVRZTHBSA-N Sethoxydim Chemical compound CCO\N=C(/CCC)C1=C(O)CC(CC(C)SCC)CC1=O CSPPKDPQLUUTND-NBVRZTHBSA-N 0.000 description 2
- JXVIIQLNUPXOII-UHFFFAOYSA-N Siduron Chemical compound CC1CCCCC1NC(=O)NC1=CC=CC=C1 JXVIIQLNUPXOII-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 2
- 240000003768 Solanum lycopersicum Species 0.000 description 2
- 240000006394 Sorghum bicolor Species 0.000 description 2
- 240000002439 Sorghum halepense Species 0.000 description 2
- 235000021536 Sugar beet Nutrition 0.000 description 2
- 239000005618 Sulcotrione Substances 0.000 description 2
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical class OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 description 2
- 239000005619 Sulfosulfuron Substances 0.000 description 2
- HBPDKDSFLXWOAE-UHFFFAOYSA-N Tebuthiuron Chemical compound CNC(=O)N(C)C1=NN=C(C(C)(C)C)S1 HBPDKDSFLXWOAE-UHFFFAOYSA-N 0.000 description 2
- NBQCNZYJJMBDKY-UHFFFAOYSA-N Terbacil Chemical compound CC=1NC(=O)N(C(C)(C)C)C(=O)C=1Cl NBQCNZYJJMBDKY-UHFFFAOYSA-N 0.000 description 2
- 239000005621 Terbuthylazine Substances 0.000 description 2
- KDWQYMVPYJGPHS-UHFFFAOYSA-N Thenylchlor Chemical compound C1=CSC(CN(C(=O)CCl)C=2C(=CC=CC=2C)C)=C1OC KDWQYMVPYJGPHS-UHFFFAOYSA-N 0.000 description 2
- 239000005622 Thiencarbazone Substances 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 239000005624 Tralkoxydim Substances 0.000 description 2
- WHKUVVPPKQRRBV-UHFFFAOYSA-N Trasan Chemical compound CC1=CC(Cl)=CC=C1OCC(O)=O WHKUVVPPKQRRBV-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 2
- 239000005627 Triclopyr Substances 0.000 description 2
- HFBWPRKWDIRYNX-UHFFFAOYSA-N Trietazine Chemical compound CCNC1=NC(Cl)=NC(N(CC)CC)=N1 HFBWPRKWDIRYNX-UHFFFAOYSA-N 0.000 description 2
- 239000005629 Tritosulfuron Substances 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 244000067505 Xanthium strumarium Species 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 230000000895 acaricidal effect Effects 0.000 description 2
- 239000000642 acaricide Substances 0.000 description 2
- DDBMQDADIHOWIC-UHFFFAOYSA-N aclonifen Chemical compound C1=C([N+]([O-])=O)C(N)=C(Cl)C(OC=2C=CC=CC=2)=C1 DDBMQDADIHOWIC-UHFFFAOYSA-N 0.000 description 2
- 238000005054 agglomeration Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- XCSGPAVHZFQHGE-UHFFFAOYSA-N alachlor Chemical compound CCC1=CC=CC(CC)=C1N(COC)C(=O)CCl XCSGPAVHZFQHGE-UHFFFAOYSA-N 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 125000003282 alkyl amino group Chemical group 0.000 description 2
- 150000004996 alkyl benzenes Chemical class 0.000 description 2
- 125000005093 alkyl carbonyl alkyl group Chemical group 0.000 description 2
- 125000005196 alkyl carbonyloxy group Chemical group 0.000 description 2
- 125000005119 alkyl cycloalkyl group Chemical group 0.000 description 2
- 125000006350 alkyl thio alkyl group Chemical group 0.000 description 2
- 230000002152 alkylating effect Effects 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- ORFLOUYIJLPLPL-WOJGMQOQSA-N alloxydim Chemical compound CCC\C(=N/OCC=C)C1=C(O)CC(C)(C)C(C(=O)OC)C1=O ORFLOUYIJLPLPL-WOJGMQOQSA-N 0.000 description 2
- RQVYBGPQFYCBGX-UHFFFAOYSA-N ametryn Chemical compound CCNC1=NC(NC(C)C)=NC(SC)=N1 RQVYBGPQFYCBGX-UHFFFAOYSA-N 0.000 description 2
- ORFPWVRKFLOQHK-UHFFFAOYSA-N amicarbazone Chemical compound CC(C)C1=NN(C(=O)NC(C)(C)C)C(=O)N1N ORFPWVRKFLOQHK-UHFFFAOYSA-N 0.000 description 2
- NIXXQNOQHKNPEJ-UHFFFAOYSA-N aminopyralid Chemical compound NC1=CC(Cl)=NC(C(O)=O)=C1Cl NIXXQNOQHKNPEJ-UHFFFAOYSA-N 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 150000001543 aryl boronic acids Chemical class 0.000 description 2
- 238000000065 atmospheric pressure chemical ionisation Methods 0.000 description 2
- WQRCEBAZAUAUQC-UHFFFAOYSA-N benazolin-ethyl Chemical group C1=CC=C2SC(=O)N(CC(=O)OCC)C2=C1Cl WQRCEBAZAUAUQC-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- XMQFTWRPUQYINF-UHFFFAOYSA-N bensulfuron-methyl Chemical group COC(=O)C1=CC=CC=C1CS(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 XMQFTWRPUQYINF-UHFFFAOYSA-N 0.000 description 2
- DMSMPAJRVJJAGA-UHFFFAOYSA-N benzo[d]isothiazol-3-one Chemical compound C1=CC=C2C(=O)NSC2=C1 DMSMPAJRVJJAGA-UHFFFAOYSA-N 0.000 description 2
- 125000002047 benzodioxolyl group Chemical group O1OC(C2=C1C=CC=C2)* 0.000 description 2
- MKQSWTQPLLCSOB-UHFFFAOYSA-N benzyl 2-chloro-4-(trifluoromethyl)-1,3-thiazole-5-carboxylate Chemical compound N1=C(Cl)SC(C(=O)OCC=2C=CC=CC=2)=C1C(F)(F)F MKQSWTQPLLCSOB-UHFFFAOYSA-N 0.000 description 2
- GINJFDRNADDBIN-FXQIFTODSA-N bilanafos Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCP(C)(O)=O GINJFDRNADDBIN-FXQIFTODSA-N 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000000853 biopesticidal effect Effects 0.000 description 2
- 125000001246 bromo group Chemical group Br* 0.000 description 2
- HKPHPIREJKHECO-UHFFFAOYSA-N butachlor Chemical compound CCCCOCN(C(=O)CCl)C1=C(CC)C=CC=C1CC HKPHPIREJKHECO-UHFFFAOYSA-N 0.000 description 2
- JEDYYFXHPAIBGR-UHFFFAOYSA-N butafenacil Chemical compound O=C1N(C)C(C(F)(F)F)=CC(=O)N1C1=CC=C(Cl)C(C(=O)OC(C)(C)C(=O)OCC=C)=C1 JEDYYFXHPAIBGR-UHFFFAOYSA-N 0.000 description 2
- HFEJHAAIJZXXRE-UHFFFAOYSA-N cafenstrole Chemical compound CCN(CC)C(=O)N1C=NC(S(=O)(=O)C=2C(=CC(C)=CC=2C)C)=N1 HFEJHAAIJZXXRE-UHFFFAOYSA-N 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N caprylic alcohol Natural products CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 235000021466 carotenoid Nutrition 0.000 description 2
- 150000001747 carotenoids Chemical class 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- GGWHBJGBERXSLL-NBVRZTHBSA-N chembl113137 Chemical compound C1C(=O)C(C(=N/OCC)/CCC)=C(O)CC1C1CSCCC1 GGWHBJGBERXSLL-NBVRZTHBSA-N 0.000 description 2
- WYKYKTKDBLFHCY-UHFFFAOYSA-N chloridazon Chemical compound O=C1C(Cl)=C(N)C=NN1C1=CC=CC=C1 WYKYKTKDBLFHCY-UHFFFAOYSA-N 0.000 description 2
- DZNFQIYYEXFFGV-UHFFFAOYSA-M chloropalladium(1+) 2-phenylaniline tritert-butylphosphane Chemical compound [Pd+]Cl.CC(C)(C)P(C(C)(C)C)C(C)(C)C.NC1=CC=CC=C1C1=CC=CC=[C-]1 DZNFQIYYEXFFGV-UHFFFAOYSA-M 0.000 description 2
- JXCGFZXSOMJFOA-UHFFFAOYSA-N chlorotoluron Chemical compound CN(C)C(=O)NC1=CC=C(C)C(Cl)=C1 JXCGFZXSOMJFOA-UHFFFAOYSA-N 0.000 description 2
- VJYIFXVZLXQVHO-UHFFFAOYSA-N chlorsulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)Cl)=N1 VJYIFXVZLXQVHO-UHFFFAOYSA-N 0.000 description 2
- NNKKTZOEKDFTBU-YBEGLDIGSA-N cinidon ethyl Chemical compound C1=C(Cl)C(/C=C(\Cl)C(=O)OCC)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1 NNKKTZOEKDFTBU-YBEGLDIGSA-N 0.000 description 2
- 235000020971 citrus fruits Nutrition 0.000 description 2
- SILSDTWXNBZOGF-JWGBMQLESA-N clethodim Chemical compound CCSC(C)CC1CC(O)=C(C(CC)=NOC\C=C\Cl)C(=O)C1 SILSDTWXNBZOGF-JWGBMQLESA-N 0.000 description 2
- JBDHZKLJNAIJNC-LLVKDONJSA-N clodinafop-propargyl Chemical group C1=CC(O[C@H](C)C(=O)OCC#C)=CC=C1OC1=NC=C(Cl)C=C1F JBDHZKLJNAIJNC-LLVKDONJSA-N 0.000 description 2
- HUBANNPOLNYSAD-UHFFFAOYSA-N clopyralid Chemical compound OC(=O)C1=NC(Cl)=CC=C1Cl HUBANNPOLNYSAD-UHFFFAOYSA-N 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000006880 cross-coupling reaction Methods 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- RWGFKTVRMDUZSP-UHFFFAOYSA-N cumene Chemical compound CC(C)C1=CC=CC=C1 RWGFKTVRMDUZSP-UHFFFAOYSA-N 0.000 description 2
- 125000004966 cyanoalkyl group Chemical group 0.000 description 2
- 125000001316 cycloalkyl alkyl group Chemical group 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 2
- WZJZMXBKUWKXTQ-UHFFFAOYSA-N desmedipham Chemical compound CCOC(=O)NC1=CC=CC(OC(=O)NC=2C=CC=CC=2)=C1 WZJZMXBKUWKXTQ-UHFFFAOYSA-N 0.000 description 2
- IWEDIXLBFLAXBO-UHFFFAOYSA-N dicamba Chemical compound COC1=C(Cl)C=CC(Cl)=C1C(O)=O IWEDIXLBFLAXBO-UHFFFAOYSA-N 0.000 description 2
- XXWNKVBJDWSYBN-UHFFFAOYSA-N diethoxy-phenoxy-sulfanylidene-$l^{5}-phosphane Chemical compound CCOP(=S)(OCC)OC1=CC=CC=C1 XXWNKVBJDWSYBN-UHFFFAOYSA-N 0.000 description 2
- OPGCOAPTHCZZIW-UHFFFAOYSA-N diethyl 1-(2,4-dichlorophenyl)-5-methyl-4h-pyrazole-3,5-dicarboxylate Chemical group CCOC(=O)C1(C)CC(C(=O)OCC)=NN1C1=CC=C(Cl)C=C1Cl OPGCOAPTHCZZIW-UHFFFAOYSA-N 0.000 description 2
- WYEHFWKAOXOVJD-UHFFFAOYSA-N diflufenican Chemical compound FC1=CC(F)=CC=C1NC(=O)C1=CC=CN=C1OC1=CC=CC(C(F)(F)F)=C1 WYEHFWKAOXOVJD-UHFFFAOYSA-N 0.000 description 2
- SCCDDNKJYDZXMM-UHFFFAOYSA-N dimethachlor Chemical compound COCCN(C(=O)CCl)C1=C(C)C=CC=C1C SCCDDNKJYDZXMM-UHFFFAOYSA-N 0.000 description 2
- OGGXGZAMXPVRFZ-UHFFFAOYSA-N dimethylarsinic acid Chemical compound C[As](C)(O)=O OGGXGZAMXPVRFZ-UHFFFAOYSA-N 0.000 description 2
- 230000003828 downregulation Effects 0.000 description 2
- 238000000132 electrospray ionisation Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- XPEVJXBWHXAUDR-UHFFFAOYSA-N epyrifenacil Chemical compound CCOC(=O)COC1=NC=CC=C1OC1=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C(F)C=C1Cl XPEVJXBWHXAUDR-UHFFFAOYSA-N 0.000 description 2
- PQKBPHSEKWERTG-LLVKDONJSA-N ethyl (2r)-2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoate Chemical group C1=CC(O[C@H](C)C(=O)OCC)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 PQKBPHSEKWERTG-LLVKDONJSA-N 0.000 description 2
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 241001233957 eudicotyledons Species 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- XXOYNJXVWVNOOJ-UHFFFAOYSA-N fenuron Chemical compound CN(C)C(=O)NC1=CC=CC=C1 XXOYNJXVWVNOOJ-UHFFFAOYSA-N 0.000 description 2
- 230000009969 flowable effect Effects 0.000 description 2
- IANUJLZYFUDJIH-UHFFFAOYSA-N flufenacet Chemical compound C=1C=C(F)C=CC=1N(C(C)C)C(=O)COC1=NN=C(C(F)(F)F)S1 IANUJLZYFUDJIH-UHFFFAOYSA-N 0.000 description 2
- DNUAYCRATWAJQE-UHFFFAOYSA-N flufenpyr-ethyl Chemical group C1=C(Cl)C(OCC(=O)OCC)=CC(N2C(C(C)=C(C=N2)C(F)(F)F)=O)=C1F DNUAYCRATWAJQE-UHFFFAOYSA-N 0.000 description 2
- FOUWCSDKDDHKQP-UHFFFAOYSA-N flumioxazin Chemical compound FC1=CC=2OCC(=O)N(CC#C)C=2C=C1N(C1=O)C(=O)C2=C1CCCC2 FOUWCSDKDDHKQP-UHFFFAOYSA-N 0.000 description 2
- 125000004428 fluoroalkoxy group Chemical group 0.000 description 2
- 125000003709 fluoroalkyl group Chemical group 0.000 description 2
- 125000004785 fluoromethoxy group Chemical group [H]C([H])(F)O* 0.000 description 2
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 2
- OQZCSNDVOWYALR-UHFFFAOYSA-N flurochloridone Chemical compound FC(F)(F)C1=CC=CC(N2C(C(Cl)C(CCl)C2)=O)=C1 OQZCSNDVOWYALR-UHFFFAOYSA-N 0.000 description 2
- MEFQWPUMEMWTJP-UHFFFAOYSA-N fluroxypyr Chemical compound NC1=C(Cl)C(F)=NC(OCC(O)=O)=C1Cl MEFQWPUMEMWTJP-UHFFFAOYSA-N 0.000 description 2
- PXDNXJSDGQBLKS-UHFFFAOYSA-N foramsulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=C(NC=O)C=2)C(=O)N(C)C)=N1 PXDNXJSDGQBLKS-UHFFFAOYSA-N 0.000 description 2
- 239000000417 fungicide Substances 0.000 description 2
- 230000030279 gene silencing Effects 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 125000004994 halo alkoxy alkyl group Chemical group 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- COYBRKAVBMYYSF-UHFFFAOYSA-N heptan-2-yl [(5-chloroquinolin-8-yl)oxy]acetate Chemical group C1=CN=C2C(OCC(=O)OC(C)CCCCC)=CC=C(Cl)C2=C1 COYBRKAVBMYYSF-UHFFFAOYSA-N 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 2
- AOGQPLXWSUTHQB-UHFFFAOYSA-N hexyl acetate Chemical compound CCCCCCOC(C)=O AOGQPLXWSUTHQB-UHFFFAOYSA-N 0.000 description 2
- IGMNYECMUMZDDF-UHFFFAOYSA-N homogentisic acid Chemical compound OC(=O)CC1=CC(O)=CC=C1O IGMNYECMUMZDDF-UHFFFAOYSA-N 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- RUCAXVJJQQJZGU-UHFFFAOYSA-M hydron;2-(phosphonatomethylamino)acetate;trimethylsulfanium Chemical compound C[S+](C)C.OP(O)(=O)CNCC([O-])=O RUCAXVJJQQJZGU-UHFFFAOYSA-M 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- NRXQIUSYPAHGNM-UHFFFAOYSA-N ioxynil Chemical compound OC1=C(I)C=C(C#N)C=C1I NRXQIUSYPAHGNM-UHFFFAOYSA-N 0.000 description 2
- 238000003973 irrigation Methods 0.000 description 2
- 230000002262 irrigation Effects 0.000 description 2
- MLFHJEHSLIIPHL-UHFFFAOYSA-N isoamyl acetate Chemical compound CC(C)CCOC(C)=O MLFHJEHSLIIPHL-UHFFFAOYSA-N 0.000 description 2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Chemical compound CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- HJOVHMDZYOCNQW-UHFFFAOYSA-N isophorone Chemical compound CC1=CC(=O)CC(C)(C)C1 HJOVHMDZYOCNQW-UHFFFAOYSA-N 0.000 description 2
- JJWLVOIRVHMVIS-UHFFFAOYSA-O isopropylaminium Chemical compound CC(C)[NH3+] JJWLVOIRVHMVIS-UHFFFAOYSA-O 0.000 description 2
- PUIYMUZLKQOUOZ-UHFFFAOYSA-N isoproturon Chemical compound CC(C)C1=CC=C(NC(=O)N(C)C)C=C1 PUIYMUZLKQOUOZ-UHFFFAOYSA-N 0.000 description 2
- PMHURSZHKKJGBM-UHFFFAOYSA-N isoxaben Chemical compound O1N=C(C(C)(CC)CC)C=C1NC(=O)C1=C(OC)C=CC=C1OC PMHURSZHKKJGBM-UHFFFAOYSA-N 0.000 description 2
- MWKVXOJATACCCH-UHFFFAOYSA-N isoxadifen-ethyl Chemical group C1C(C(=O)OCC)=NOC1(C=1C=CC=CC=1)C1=CC=CC=C1 MWKVXOJATACCCH-UHFFFAOYSA-N 0.000 description 2
- OYIKARCXOQLFHF-UHFFFAOYSA-N isoxaflutole Chemical compound CS(=O)(=O)C1=CC(C(F)(F)F)=CC=C1C(=O)C1=C(C2CC2)ON=C1 OYIKARCXOQLFHF-UHFFFAOYSA-N 0.000 description 2
- 229940088649 isoxaflutole Drugs 0.000 description 2
- CONWAEURSVPLRM-UHFFFAOYSA-N lactofen Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC(C)C(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 CONWAEURSVPLRM-UHFFFAOYSA-N 0.000 description 2
- 229920005610 lignin Chemical class 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- XIGAUIHYSDTJHW-UHFFFAOYSA-N mefenacet Chemical compound N=1C2=CC=CC=C2SC=1OCC(=O)N(C)C1=CC=CC=C1 XIGAUIHYSDTJHW-UHFFFAOYSA-N 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- VHCNQEUWZYOAEV-UHFFFAOYSA-N metamitron Chemical compound O=C1N(N)C(C)=NN=C1C1=CC=CC=C1 VHCNQEUWZYOAEV-UHFFFAOYSA-N 0.000 description 2
- STEPQTYSZVCJPV-UHFFFAOYSA-N metazachlor Chemical compound CC1=CC=CC(C)=C1N(C(=O)CCl)CN1N=CC=C1 STEPQTYSZVCJPV-UHFFFAOYSA-N 0.000 description 2
- JCHMGYRXQDASJE-UHFFFAOYSA-N metcamifen Chemical compound C1=CC(NC(=O)NC)=CC=C1S(=O)(=O)NC(=O)C1=CC=CC=C1OC JCHMGYRXQDASJE-UHFFFAOYSA-N 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- RBNIGDFIUWJJEV-LLVKDONJSA-N methyl (2r)-2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N([C@H](C)C(=O)OC)C(=O)C1=CC=CC=C1 RBNIGDFIUWJJEV-LLVKDONJSA-N 0.000 description 2
- NIFKBBMCXCMCAO-UHFFFAOYSA-N methyl 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-4-(methanesulfonamidomethyl)benzoate Chemical group COC(=O)C1=CC=C(CNS(C)(=O)=O)C=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 NIFKBBMCXCMCAO-UHFFFAOYSA-N 0.000 description 2
- BACHBFVBHLGWSL-UHFFFAOYSA-N methyl 2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-UHFFFAOYSA-N 0.000 description 2
- ZTYVMAQSHCZXLF-UHFFFAOYSA-N methyl 2-[[4,6-bis(difluoromethoxy)pyrimidin-2-yl]carbamoylsulfamoyl]benzoate Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC(F)F)=CC(OC(F)F)=N1 ZTYVMAQSHCZXLF-UHFFFAOYSA-N 0.000 description 2
- PZSVMKWRONODDG-UHFFFAOYSA-N methyl 4-amino-3-chloro-6-(4-chloro-2-fluoro-3-methoxyphenyl)-5-fluoropyridine-2-carboxylate Chemical compound NC1=C(Cl)C(C(=O)OC)=NC(C=2C(=C(OC)C(Cl)=CC=2)F)=C1F PZSVMKWRONODDG-UHFFFAOYSA-N 0.000 description 2
- VWGAYSCWLXQJBQ-UHFFFAOYSA-N methyl 4-iodo-2-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]benzoate Chemical group COC(=O)C1=CC=C(I)C=C1S(=O)(=O)NC(=O)NC1=NC(C)=NC(OC)=N1 VWGAYSCWLXQJBQ-UHFFFAOYSA-N 0.000 description 2
- QYPPRTNMGCREIM-UHFFFAOYSA-N methylarsonic acid Chemical compound C[As](O)(O)=O QYPPRTNMGCREIM-UHFFFAOYSA-N 0.000 description 2
- DSRNRYQBBJQVCW-UHFFFAOYSA-N metoxuron Chemical compound COC1=CC=C(NC(=O)N(C)C)C=C1Cl DSRNRYQBBJQVCW-UHFFFAOYSA-N 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- AIMMSOZBPYFASU-UHFFFAOYSA-N n-(4,6-dimethoxypyrimidin-2-yl)-n'-[3-(2,2,2-trifluoroethoxy)pyridin-1-ium-2-yl]sulfonylcarbamimidate Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)OCC(F)(F)F)=N1 AIMMSOZBPYFASU-UHFFFAOYSA-N 0.000 description 2
- GLBLPMUBLHYFCW-UHFFFAOYSA-N n-(5,7-dimethoxy-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)-2-methoxy-4-(trifluoromethyl)pyridine-3-sulfonamide Chemical compound N1=C2N=C(OC)C=C(OC)N2N=C1NS(=O)(=O)C1=C(OC)N=CC=C1C(F)(F)F GLBLPMUBLHYFCW-UHFFFAOYSA-N 0.000 description 2
- FITSYTCYOITKJL-UHFFFAOYSA-N n-[2-[(3,3-dimethyl-2-oxoazetidin-1-yl)methyl]phenyl]-1,1,1-trifluoromethanesulfonamide Chemical compound O=C1C(C)(C)CN1CC1=CC=CC=C1NS(=O)(=O)C(F)(F)F FITSYTCYOITKJL-UHFFFAOYSA-N 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 230000001069 nematicidal effect Effects 0.000 description 2
- 239000005645 nematicide Substances 0.000 description 2
- 125000004971 nitroalkyl group Chemical group 0.000 description 2
- GJQIMXVRFNLMTB-UHFFFAOYSA-N nonyl acetate Chemical compound CCCCCCCCCOC(C)=O GJQIMXVRFNLMTB-UHFFFAOYSA-N 0.000 description 2
- 230000000269 nucleophilic effect Effects 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 235000014571 nuts Nutrition 0.000 description 2
- YLYBTZIQSIBWLI-UHFFFAOYSA-N octyl acetate Chemical compound CCCCCCCCOC(C)=O YLYBTZIQSIBWLI-UHFFFAOYSA-N 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- IOXAXYHXMLCCJJ-UHFFFAOYSA-N oxetan-3-yl 2-[(4,6-dimethylpyrimidin-2-yl)carbamoylsulfamoyl]benzoate Chemical compound CC1=CC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(=O)OC2COC2)=N1 IOXAXYHXMLCCJJ-UHFFFAOYSA-N 0.000 description 2
- MUJIDPITZJWBSW-UHFFFAOYSA-N palladium(2+) Chemical compound [Pd+2] MUJIDPITZJWBSW-UHFFFAOYSA-N 0.000 description 2
- FIKAKWIAUPDISJ-UHFFFAOYSA-L paraquat dichloride Chemical compound [Cl-].[Cl-].C1=C[N+](C)=CC=C1C1=CC=[N+](C)C=C1 FIKAKWIAUPDISJ-UHFFFAOYSA-L 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 235000020232 peanut Nutrition 0.000 description 2
- JZPKLLLUDLHCEL-UHFFFAOYSA-N pentoxazone Chemical compound O=C1C(=C(C)C)OC(=O)N1C1=CC(OC2CCCC2)=C(Cl)C=C1F JZPKLLLUDLHCEL-UHFFFAOYSA-N 0.000 description 2
- IDOWTHOLJBTAFI-UHFFFAOYSA-N phenmedipham Chemical compound COC(=O)NC1=CC=CC(OC(=O)NC=2C=C(C)C=CC=2)=C1 IDOWTHOLJBTAFI-UHFFFAOYSA-N 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- CWKFPEBMTGKLKX-UHFFFAOYSA-N picolinafen Chemical compound C1=CC(F)=CC=C1NC(=O)C1=CC=CC(OC=2C=C(C=CC=2)C(F)(F)F)=N1 CWKFPEBMTGKLKX-UHFFFAOYSA-N 0.000 description 2
- 230000005080 plant death Effects 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 229920002689 polyvinyl acetate Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 2
- RLQCYSVYYHHLIL-UHFFFAOYSA-M potassium;3,6-dichloropyridine-2-carboxylate Chemical compound [K+].[O-]C(=O)C1=NC(Cl)=CC=C1Cl RLQCYSVYYHHLIL-UHFFFAOYSA-M 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- ISEUFVQQFVOBCY-UHFFFAOYSA-N prometon Chemical compound COC1=NC(NC(C)C)=NC(NC(C)C)=N1 ISEUFVQQFVOBCY-UHFFFAOYSA-N 0.000 description 2
- AAEVYOVXGOFMJO-UHFFFAOYSA-N prometryn Chemical compound CSC1=NC(NC(C)C)=NC(NC(C)C)=N1 AAEVYOVXGOFMJO-UHFFFAOYSA-N 0.000 description 2
- MFOUDYKPLGXPGO-UHFFFAOYSA-N propachlor Chemical compound ClCC(=O)N(C(C)C)C1=CC=CC=C1 MFOUDYKPLGXPGO-UHFFFAOYSA-N 0.000 description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 2
- IKVXBIIHQGXQRQ-CYBMUJFWSA-N propan-2-yl (2r)-2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N([C@H](C)C(=O)OC(C)C)C(=O)C1=CC=CC=C1 IKVXBIIHQGXQRQ-CYBMUJFWSA-N 0.000 description 2
- FKLQIONHGSFYJY-UHFFFAOYSA-N propan-2-yl 5-[4-bromo-1-methyl-5-(trifluoromethyl)pyrazol-3-yl]-2-chloro-4-fluorobenzoate Chemical compound C1=C(Cl)C(C(=O)OC(C)C)=CC(C=2C(=C(N(C)N=2)C(F)(F)F)Br)=C1F FKLQIONHGSFYJY-UHFFFAOYSA-N 0.000 description 2
- LFULEKSKNZEWOE-UHFFFAOYSA-N propanil Chemical compound CCC(=O)NC1=CC=C(Cl)C(Cl)=C1 LFULEKSKNZEWOE-UHFFFAOYSA-N 0.000 description 2
- FROBCXTULYFHEJ-OAHLLOKOSA-N propaquizafop Chemical compound C1=CC(O[C@H](C)C(=O)OCCON=C(C)C)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 FROBCXTULYFHEJ-OAHLLOKOSA-N 0.000 description 2
- WJNRPILHGGKWCK-UHFFFAOYSA-N propazine Chemical compound CC(C)NC1=NC(Cl)=NC(NC(C)C)=N1 WJNRPILHGGKWCK-UHFFFAOYSA-N 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- APTZNLHMIGJTEW-UHFFFAOYSA-N pyraflufen-ethyl Chemical group C1=C(Cl)C(OCC(=O)OCC)=CC(C=2C(=C(OC(F)F)N(C)N=2)Cl)=C1F APTZNLHMIGJTEW-UHFFFAOYSA-N 0.000 description 2
- DWSPRBSLSXQIEJ-UHFFFAOYSA-N pyrasulfotole Chemical compound CC1=NN(C)C(O)=C1C(=O)C1=CC=C(C(F)(F)F)C=C1S(C)(=O)=O DWSPRBSLSXQIEJ-UHFFFAOYSA-N 0.000 description 2
- 125000003226 pyrazolyl group Chemical group 0.000 description 2
- ASRAWSBMDXVNLX-UHFFFAOYSA-N pyrazolynate Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OS(=O)(=O)C1=CC=C(C)C=C1 ASRAWSBMDXVNLX-UHFFFAOYSA-N 0.000 description 2
- FKERUJTUOYLBKB-UHFFFAOYSA-N pyrazoxyfen Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=CC=C1 FKERUJTUOYLBKB-UHFFFAOYSA-N 0.000 description 2
- UMLDUMMLRZFROX-UHFFFAOYSA-N pyridin-2-ylboronic acid Chemical compound OB(O)C1=CC=CC=N1 UMLDUMMLRZFROX-UHFFFAOYSA-N 0.000 description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 2
- USSIUIGPBLPCDF-KEBDBYFISA-N pyriminobac-methyl Chemical group CO\N=C(/C)C1=CC=CC(OC=2N=C(OC)C=C(OC)N=2)=C1C(=O)OC USSIUIGPBLPCDF-KEBDBYFISA-N 0.000 description 2
- ALZOLUNSQWINIR-UHFFFAOYSA-N quinmerac Chemical compound OC(=O)C1=C(Cl)C=CC2=CC(C)=CN=C21 ALZOLUNSQWINIR-UHFFFAOYSA-N 0.000 description 2
- BACHBFVBHLGWSL-JTQLQIEISA-N rac-diclofop methyl Natural products C1=CC(O[C@@H](C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-JTQLQIEISA-N 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 238000006722 reduction reaction Methods 0.000 description 2
- GNHDVXLWBQYPJE-UHFFFAOYSA-N saflufenacil Chemical compound C1=C(Cl)C(C(=O)NS(=O)(=O)N(C)C(C)C)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1F GNHDVXLWBQYPJE-UHFFFAOYSA-N 0.000 description 2
- 239000004576 sand Substances 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- ODCWYMIRDDJXKW-UHFFFAOYSA-N simazine Chemical compound CCNC1=NC(Cl)=NC(NCC)=N1 ODCWYMIRDDJXKW-UHFFFAOYSA-N 0.000 description 2
- MGLWZSOBALDPEK-UHFFFAOYSA-N simetryn Chemical compound CCNC1=NC(NCC)=NC(SC)=N1 MGLWZSOBALDPEK-UHFFFAOYSA-N 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000000021 stimulant Substances 0.000 description 2
- PQTBTIFWAXVEPB-UHFFFAOYSA-N sulcotrione Chemical compound ClC1=CC(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O PQTBTIFWAXVEPB-UHFFFAOYSA-N 0.000 description 2
- ZDXMLEQEMNLCQG-UHFFFAOYSA-N sulfometuron methyl Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(C)=CC(C)=N1 ZDXMLEQEMNLCQG-UHFFFAOYSA-N 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 239000003760 tallow Substances 0.000 description 2
- BCQMBFHBDZVHKU-UHFFFAOYSA-N terbumeton Chemical compound CCNC1=NC(NC(C)(C)C)=NC(OC)=N1 BCQMBFHBDZVHKU-UHFFFAOYSA-N 0.000 description 2
- IROINLKCQGIITA-UHFFFAOYSA-N terbutryn Chemical compound CCNC1=NC(NC(C)(C)C)=NC(SC)=N1 IROINLKCQGIITA-UHFFFAOYSA-N 0.000 description 2
- FZXISNSWEXTPMF-UHFFFAOYSA-N terbutylazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)(C)C)=N1 FZXISNSWEXTPMF-UHFFFAOYSA-N 0.000 description 2
- GLDAZAQRGCSFNP-UHFFFAOYSA-N thiencarbazone Chemical compound O=C1N(C)C(OC)=NN1C(=O)NS(=O)(=O)C1=C(C)SC=C1C(O)=O GLDAZAQRGCSFNP-UHFFFAOYSA-N 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- ARYHTUPFQTUBBG-UHFFFAOYSA-N thiophen-2-ylboronic acid Chemical compound OB(O)C1=CC=CS1 ARYHTUPFQTUBBG-UHFFFAOYSA-N 0.000 description 2
- IYMLUHWAJFXAQP-UHFFFAOYSA-N topramezone Chemical compound CC1=C(C(=O)C2=C(N(C)N=C2)O)C=CC(S(C)(=O)=O)=C1C1=NOCC1 IYMLUHWAJFXAQP-UHFFFAOYSA-N 0.000 description 2
- DQFPEYARZIQXRM-LTGZKZEYSA-N tralkoxydim Chemical compound C1C(=O)C(C(/CC)=N/OCC)=C(O)CC1C1=C(C)C=C(C)C=C1C DQFPEYARZIQXRM-LTGZKZEYSA-N 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- XOPFESVZMSQIKC-UHFFFAOYSA-N triasulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)OCCCl)=N1 XOPFESVZMSQIKC-UHFFFAOYSA-N 0.000 description 2
- REEQLXCGVXDJSQ-UHFFFAOYSA-N trichlopyr Chemical compound OC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl REEQLXCGVXDJSQ-UHFFFAOYSA-N 0.000 description 2
- AZHZOGYUMMIAOF-UHFFFAOYSA-N trifludimoxazin Chemical compound O=C1N(C)C(=S)N(C)C(=O)N1C(C(=C1)F)=CC2=C1OC(F)(F)C(=O)N2CC#C AZHZOGYUMMIAOF-UHFFFAOYSA-N 0.000 description 2
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 2
- 235000019798 tripotassium phosphate Nutrition 0.000 description 2
- KVEQCVKVIFQSGC-UHFFFAOYSA-N tritosulfuron Chemical compound FC(F)(F)C1=NC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(F)(F)F)=N1 KVEQCVKVIFQSGC-UHFFFAOYSA-N 0.000 description 2
- 239000001993 wax Substances 0.000 description 2
- 239000011592 zinc chloride Substances 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- PFTAWBLQPZVEMU-DZGCQCFKSA-N (+)-catechin Chemical compound C1([C@H]2OC3=CC(O)=CC(O)=C3C[C@@H]2O)=CC=C(O)C(O)=C1 PFTAWBLQPZVEMU-DZGCQCFKSA-N 0.000 description 1
- GXEKYRXVRROBEV-FBXFSONDSA-N (1r,2s,3r,4s)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid Chemical compound C1C[C@@H]2[C@@H](C(O)=O)[C@@H](C(=O)O)[C@H]1O2 GXEKYRXVRROBEV-FBXFSONDSA-N 0.000 description 1
- DQKWXTIYGWPGOO-UHFFFAOYSA-N (2,6-dibromo-4-cyanophenyl) octanoate Chemical compound CCCCCCCC(=O)OC1=C(Br)C=C(C#N)C=C1Br DQKWXTIYGWPGOO-UHFFFAOYSA-N 0.000 description 1
- HCMJWOGOISXSDL-UHFFFAOYSA-N (2-isothiocyanato-1-phenylethyl)benzene Chemical group C=1C=CC=CC=1C(CN=C=S)C1=CC=CC=C1 HCMJWOGOISXSDL-UHFFFAOYSA-N 0.000 description 1
- ROBSGBGTWRRYSK-SNVBAGLBSA-N (2r)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propanoic acid Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CC=C(C#N)C=C1F ROBSGBGTWRRYSK-SNVBAGLBSA-N 0.000 description 1
- MPPOHAUSNPTFAJ-SECBINFHSA-N (2r)-2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoic acid Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 MPPOHAUSNPTFAJ-SECBINFHSA-N 0.000 description 1
- NYHLMHAKWBUZDY-QMMMGPOBSA-N (2s)-2-[2-chloro-5-[2-chloro-4-(trifluoromethyl)phenoxy]benzoyl]oxypropanoic acid Chemical compound C1=C(Cl)C(C(=O)O[C@@H](C)C(O)=O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NYHLMHAKWBUZDY-QMMMGPOBSA-N 0.000 description 1
- RINPOXNCRXGFAV-FNXAHOOKSA-N (2s,4s,5r,6r)-5-amino-2-[(2s,3r,4r,5s,6r)-5-[(2s,3r,4r,5r,6r)-3-amino-5-hydroxy-6-(hydroxymethyl)-4-[(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2-[(2r,3s,4r,5r,6r)-6-[(e)-2-amino-3-hydroxyicos-4-enoxy]-4,5-dihydroxy-2-(h Chemical compound O[C@@H]1[C@@H](O)[C@H](OCC(N)C(O)/C=C/CCCCCCCCCCCCCCC)O[C@H](CO)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@]2(O[C@H]([C@H](N)[C@@H](O)C2)C(O)C(O)CO)C(O)=O)[C@@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O3)O)[C@@H](O)[C@@H](CO)O2)N)[C@@H](CO)O1 RINPOXNCRXGFAV-FNXAHOOKSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- 125000006274 (C1-C3)alkoxy group Chemical group 0.000 description 1
- 125000004769 (C1-C4) alkylsulfonyl group Chemical group 0.000 description 1
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 description 1
- WNTGYJSOUMFZEP-SSDOTTSWSA-N (R)-mecoprop Chemical compound OC(=O)[C@@H](C)OC1=CC=C(Cl)C=C1C WNTGYJSOUMFZEP-SSDOTTSWSA-N 0.000 description 1
- ADDQHLREJDZPMT-AWEZNQCLSA-N (S)-metamifop Chemical compound O=C([C@@H](OC=1C=CC(OC=2OC3=CC(Cl)=CC=C3N=2)=CC=1)C)N(C)C1=CC=CC=C1F ADDQHLREJDZPMT-AWEZNQCLSA-N 0.000 description 1
- DARPYRSDRJYGIF-PTNGSMBKSA-N (Z)-3-ethoxy-2-naphthalen-2-ylsulfonylprop-2-enenitrile Chemical compound C1=CC=CC2=CC(S(=O)(=O)C(\C#N)=C/OCC)=CC=C21 DARPYRSDRJYGIF-PTNGSMBKSA-N 0.000 description 1
- USGUVNUTPWXWBA-JRIXXDKMSA-N (e,2s)-2-amino-4-(2-aminoethoxy)but-3-enoic acid Chemical compound NCCO\C=C\[C@H](N)C(O)=O USGUVNUTPWXWBA-JRIXXDKMSA-N 0.000 description 1
- MAUMSNABMVEOGP-UHFFFAOYSA-N (methyl-$l^{2}-azanyl)methane Chemical compound C[N]C MAUMSNABMVEOGP-UHFFFAOYSA-N 0.000 description 1
- COLOHWPRNRVWPI-UHFFFAOYSA-N 1,1,1-trifluoroethane Chemical compound [CH2]C(F)(F)F COLOHWPRNRVWPI-UHFFFAOYSA-N 0.000 description 1
- FNQJDLTXOVEEFB-UHFFFAOYSA-N 1,2,3-benzothiadiazole Chemical class C1=CC=C2SN=NC2=C1 FNQJDLTXOVEEFB-UHFFFAOYSA-N 0.000 description 1
- ZZXUZKXVROWEIF-UHFFFAOYSA-N 1,2-butylene carbonate Chemical compound CCC1COC(=O)O1 ZZXUZKXVROWEIF-UHFFFAOYSA-N 0.000 description 1
- XQEMNBNCQVQXMO-UHFFFAOYSA-M 1,2-dimethyl-3,5-diphenylpyrazol-1-ium;methyl sulfate Chemical compound COS([O-])(=O)=O.C[N+]=1N(C)C(C=2C=CC=CC=2)=CC=1C1=CC=CC=C1 XQEMNBNCQVQXMO-UHFFFAOYSA-M 0.000 description 1
- DAGDLSRRQJATCV-UHFFFAOYSA-N 1-(2-bromoethoxy)-2-propan-2-ylbenzene Chemical compound CC(C)C1=CC=CC=C1OCCBr DAGDLSRRQJATCV-UHFFFAOYSA-N 0.000 description 1
- XFRVVPUIAFSTFO-UHFFFAOYSA-N 1-Tridecanol Chemical compound CCCCCCCCCCCCCO XFRVVPUIAFSTFO-UHFFFAOYSA-N 0.000 description 1
- BXKKQFGRMSOANI-UHFFFAOYSA-N 1-methoxy-3-[4-[(2-methoxy-2,4,4-trimethyl-3h-chromen-7-yl)oxy]phenyl]-1-methylurea Chemical compound C1=CC(NC(=O)N(C)OC)=CC=C1OC1=CC=C2C(C)(C)CC(C)(OC)OC2=C1 BXKKQFGRMSOANI-UHFFFAOYSA-N 0.000 description 1
- UUFQTNFCRMXOAE-UHFFFAOYSA-N 1-methylmethylene Chemical compound C[CH] UUFQTNFCRMXOAE-UHFFFAOYSA-N 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- RAXXELZNTBOGNW-UHFFFAOYSA-N 1H-imidazole Chemical compound C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 1
- KNGWEAQJZJKFLI-UHFFFAOYSA-N 2,2-dimethyl-4h-1,3-benzodioxine-6-carbaldehyde Chemical compound O=CC1=CC=C2OC(C)(C)OCC2=C1 KNGWEAQJZJKFLI-UHFFFAOYSA-N 0.000 description 1
- TVFWYUWNQVRQRG-UHFFFAOYSA-N 2,3,4-tris(2-phenylethenyl)phenol Chemical class C=1C=CC=CC=1C=CC1=C(C=CC=2C=CC=CC=2)C(O)=CC=C1C=CC1=CC=CC=C1 TVFWYUWNQVRQRG-UHFFFAOYSA-N 0.000 description 1
- XTVIFVALDYTCLL-UHFFFAOYSA-N 2,3,5-trichloro-1h-pyridin-4-one Chemical compound ClC1=CNC(Cl)=C(Cl)C1=O XTVIFVALDYTCLL-UHFFFAOYSA-N 0.000 description 1
- JKTAIYGNOFSMCE-UHFFFAOYSA-N 2,3-di(nonyl)phenol Chemical compound CCCCCCCCCC1=CC=CC(O)=C1CCCCCCCCC JKTAIYGNOFSMCE-UHFFFAOYSA-N 0.000 description 1
- KKSKKVUKDFJQMS-UHFFFAOYSA-N 2,4-dichloro-5-cyclopropylpyrimidine Chemical compound ClC1=NC(Cl)=NC=C1C1CC1 KKSKKVUKDFJQMS-UHFFFAOYSA-N 0.000 description 1
- JNYAEWCLZODPBN-UHFFFAOYSA-N 2-(1,2-dihydroxyethyl)oxolane-3,4-diol Polymers OCC(O)C1OCC(O)C1O JNYAEWCLZODPBN-UHFFFAOYSA-N 0.000 description 1
- ROKVVMOXSZIDEG-UHFFFAOYSA-N 2-(3,5,6-trichloropyridin-2-yl)oxyacetate;triethylazanium Chemical compound CCN(CC)CC.OC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl ROKVVMOXSZIDEG-UHFFFAOYSA-N 0.000 description 1
- PKAUICCNAWQPAU-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)acetic acid;n-methylmethanamine Chemical compound CNC.CC1=CC(Cl)=CC=C1OCC(O)=O PKAUICCNAWQPAU-UHFFFAOYSA-N 0.000 description 1
- GOCUAJYOYBLQRH-UHFFFAOYSA-N 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl GOCUAJYOYBLQRH-UHFFFAOYSA-N 0.000 description 1
- YUVKUEAFAVKILW-UHFFFAOYSA-N 2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 YUVKUEAFAVKILW-UHFFFAOYSA-N 0.000 description 1
- ANQRDMDBJYHVII-UHFFFAOYSA-N 2-[2-(3,4-dimethoxyphenyl)-6-methyl-3-oxopyridazine-4-carbonyl]cyclohexane-1,3-dione Chemical compound COC=1C=C(C=CC=1OC)N1N=C(C=C(C1=O)C(=O)C1C(CCCC1=O)=O)C ANQRDMDBJYHVII-UHFFFAOYSA-N 0.000 description 1
- OOLBCHYXZDXLDS-UHFFFAOYSA-N 2-[4-(2,4-dichlorophenoxy)phenoxy]propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CC=C(Cl)C=C1Cl OOLBCHYXZDXLDS-UHFFFAOYSA-N 0.000 description 1
- MPPOHAUSNPTFAJ-UHFFFAOYSA-N 2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 MPPOHAUSNPTFAJ-UHFFFAOYSA-N 0.000 description 1
- NQQBTWVFKDDVIB-UHFFFAOYSA-N 2-aminoethanol;3,6-dichloropyridine-2-carboxylic acid Chemical compound NCCO.OC(=O)C1=NC(Cl)=CC=C1Cl NQQBTWVFKDDVIB-UHFFFAOYSA-N 0.000 description 1
- IVDRCZNHVGQBHZ-UHFFFAOYSA-N 2-butoxyethyl 2-(3,5,6-trichloropyridin-2-yl)oxyacetate Chemical group CCCCOCCOC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl IVDRCZNHVGQBHZ-UHFFFAOYSA-N 0.000 description 1
- WKGKFWXGAHXMCE-UHFFFAOYSA-N 2-butoxyethyl 2-(4-chloro-2-methylphenoxy)acetate Chemical group CCCCOCCOC(=O)COC1=CC=C(Cl)C=C1C WKGKFWXGAHXMCE-UHFFFAOYSA-N 0.000 description 1
- YHKBGVDUSSWOAB-UHFFFAOYSA-N 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]-4-fluorophenyl}propanoic acid Chemical compound O=C1N(C(F)F)C(C)=NN1C1=CC(CC(Cl)C(O)=O)=C(Cl)C=C1F YHKBGVDUSSWOAB-UHFFFAOYSA-N 0.000 description 1
- QEGVVEOAVNHRAA-UHFFFAOYSA-N 2-chloro-6-(4,6-dimethoxypyrimidin-2-yl)sulfanylbenzoic acid Chemical compound COC1=CC(OC)=NC(SC=2C(=C(Cl)C=CC=2)C(O)=O)=N1 QEGVVEOAVNHRAA-UHFFFAOYSA-N 0.000 description 1
- FOQGODXEDCGTKZ-UHFFFAOYSA-N 2-chloro-n-(1-methyltetrazol-5-yl)-6-(trifluoromethyl)pyridine-3-carboxamide Chemical compound CN1N=NN=C1NC(=O)C1=CC=C(C(F)(F)F)N=C1Cl FOQGODXEDCGTKZ-UHFFFAOYSA-N 0.000 description 1
- UNCQVRBWJWWJBF-UHFFFAOYSA-N 2-chloropyrimidine Chemical compound ClC1=NC=CC=N1 UNCQVRBWJWWJBF-UHFFFAOYSA-N 0.000 description 1
- CYEJMVLDXAUOPN-UHFFFAOYSA-N 2-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=CC=C1O CYEJMVLDXAUOPN-UHFFFAOYSA-N 0.000 description 1
- IRCMYGHHKLLGHV-UHFFFAOYSA-N 2-ethoxy-3,3-dimethyl-2,3-dihydro-1-benzofuran-5-yl methanesulfonate Chemical compound C1=C(OS(C)(=O)=O)C=C2C(C)(C)C(OCC)OC2=C1 IRCMYGHHKLLGHV-UHFFFAOYSA-N 0.000 description 1
- MIJLZGZLQLAQCM-UHFFFAOYSA-N 2-ethoxyethyl 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OCCOCC)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl MIJLZGZLQLAQCM-UHFFFAOYSA-N 0.000 description 1
- IDGRPSMONFWWEK-UHFFFAOYSA-N 2-ethylhexyl 2-(4-chloro-2-methylphenoxy)acetate Chemical group CCCCC(CC)COC(=O)COC1=CC=C(Cl)C=C1C IDGRPSMONFWWEK-UHFFFAOYSA-N 0.000 description 1
- SWKACZQJGXABCN-JSGWLJPKSA-N 2-methyl-6-all-trans-nonaprenyl-1,4-benzoquinone Chemical compound CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC1=CC(O)=CC(C)=C1O SWKACZQJGXABCN-JSGWLJPKSA-N 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- ABOOPXYCKNFDNJ-UHFFFAOYSA-N 2-{4-[(6-chloroquinoxalin-2-yl)oxy]phenoxy}propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 ABOOPXYCKNFDNJ-UHFFFAOYSA-N 0.000 description 1
- UGEJOEBBMPOJMT-UHFFFAOYSA-N 3-(trifluoromethyl)phenol Chemical compound OC1=CC=CC(C(F)(F)F)=C1 UGEJOEBBMPOJMT-UHFFFAOYSA-N 0.000 description 1
- XWSSUYOEOWLFEI-UHFFFAOYSA-N 3-phenylpyridazine Chemical class C1=CC=CC=C1C1=CC=CN=N1 XWSSUYOEOWLFEI-UHFFFAOYSA-N 0.000 description 1
- ZXVONLUNISGICL-UHFFFAOYSA-N 4,6-dinitro-o-cresol Chemical compound CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1O ZXVONLUNISGICL-UHFFFAOYSA-N 0.000 description 1
- BCFOOQRXUXKJCL-UHFFFAOYSA-N 4-amino-4-oxo-2-sulfobutanoic acid Chemical class NC(=O)CC(C(O)=O)S(O)(=O)=O BCFOOQRXUXKJCL-UHFFFAOYSA-N 0.000 description 1
- ADZSGNDOZREKJK-UHFFFAOYSA-N 4-amino-6-tert-butyl-3-ethylsulfanyl-1,2,4-triazin-5-one Chemical compound CCSC1=NN=C(C(C)(C)C)C(=O)N1N ADZSGNDOZREKJK-UHFFFAOYSA-N 0.000 description 1
- PYXNITNKYBLBMW-UHFFFAOYSA-N 5-(trifluoromethyl)-1h-pyrazole Chemical compound FC(F)(F)C1=CC=NN1 PYXNITNKYBLBMW-UHFFFAOYSA-N 0.000 description 1
- SOZRVJLJEGLPGH-UHFFFAOYSA-N 5-[2-chloro-6-(5-chloropyrimidin-2-yl)oxyphenyl]-3-(difluoromethyl)-1,2-oxazole Chemical compound ClC=1C=NC(=NC=1)OC1=C(C(=CC=C1)Cl)C1=CC(=NO1)C(F)F SOZRVJLJEGLPGH-UHFFFAOYSA-N 0.000 description 1
- QQOGZMUZAZWLJH-UHFFFAOYSA-N 5-[2-chloro-6-fluoro-4-(trifluoromethyl)phenoxy]-n-ethylsulfonyl-2-nitrobenzamide Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)CC)=CC(OC=2C(=CC(=CC=2F)C(F)(F)F)Cl)=C1 QQOGZMUZAZWLJH-UHFFFAOYSA-N 0.000 description 1
- WWJLCYHYLZZXBE-UHFFFAOYSA-N 5-chloro-1,3-dihydroindol-2-one Chemical compound ClC1=CC=C2NC(=O)CC2=C1 WWJLCYHYLZZXBE-UHFFFAOYSA-N 0.000 description 1
- HZKBYBNLTLVSPX-UHFFFAOYSA-N 6-[(6,6-dimethyl-5,7-dihydropyrrolo[2,1-c][1,2,4]thiadiazol-3-ylidene)amino]-7-fluoro-4-prop-2-ynyl-1,4-benzoxazin-3-one Chemical compound C#CCN1C(=O)COC(C=C2F)=C1C=C2N=C1SN=C2CC(C)(C)CN21 HZKBYBNLTLVSPX-UHFFFAOYSA-N 0.000 description 1
- ZUSHSDOEVHPTCU-UHFFFAOYSA-N 6-chloro-3-phenyl-1h-pyridazin-4-one Chemical compound N1C(Cl)=CC(=O)C(C=2C=CC=CC=2)=N1 ZUSHSDOEVHPTCU-UHFFFAOYSA-N 0.000 description 1
- WBFYVDCHGVNRBH-UHFFFAOYSA-N 7,8-dihydropteroic acid Chemical compound N=1C=2C(=O)NC(N)=NC=2NCC=1CNC1=CC=C(C(O)=O)C=C1 WBFYVDCHGVNRBH-UHFFFAOYSA-N 0.000 description 1
- PLLBRTOLHQQAQQ-UHFFFAOYSA-N 8-methylnonan-1-ol Chemical compound CC(C)CCCCCCCO PLLBRTOLHQQAQQ-UHFFFAOYSA-N 0.000 description 1
- 241001290610 Abildgaardia Species 0.000 description 1
- 241001133760 Acoelorraphe Species 0.000 description 1
- 241001103808 Albifimbria verrucaria Species 0.000 description 1
- MDBGGTQNNUOQRC-UHFFFAOYSA-N Allidochlor Chemical compound ClCC(=O)N(CC=C)CC=C MDBGGTQNNUOQRC-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000584608 Alternaria destruens Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 235000003133 Ambrosia artemisiifolia Nutrition 0.000 description 1
- GEHMBYLTCISYNY-UHFFFAOYSA-N Ammonium sulfamate Chemical compound [NH4+].NS([O-])(=O)=O GEHMBYLTCISYNY-UHFFFAOYSA-N 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 241001666377 Apera Species 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 241000209761 Avena Species 0.000 description 1
- 241000193755 Bacillus cereus Species 0.000 description 1
- 241000193388 Bacillus thuringiensis Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 241001645380 Bassia scoparia Species 0.000 description 1
- 239000005471 Benfluralin Substances 0.000 description 1
- QGQSRQPXXMTJCM-UHFFFAOYSA-N Benfuresate Chemical compound CCS(=O)(=O)OC1=CC=C2OCC(C)(C)C2=C1 QGQSRQPXXMTJCM-UHFFFAOYSA-N 0.000 description 1
- RRNIZKPFKNDSRS-UHFFFAOYSA-N Bensulide Chemical compound CC(C)OP(=S)(OC(C)C)SCCNS(=O)(=O)C1=CC=CC=C1 RRNIZKPFKNDSRS-UHFFFAOYSA-N 0.000 description 1
- 239000005476 Bentazone Substances 0.000 description 1
- 235000010662 Bidens pilosa Nutrition 0.000 description 1
- 244000104272 Bidens pilosa Species 0.000 description 1
- 239000005488 Bispyribac Substances 0.000 description 1
- 235000014750 Brassica kaber Nutrition 0.000 description 1
- 235000006008 Brassica napus var napus Nutrition 0.000 description 1
- 235000011292 Brassica rapa Nutrition 0.000 description 1
- OEYOMNZEMCPTKN-UHFFFAOYSA-N Butamifos Chemical compound CCC(C)NP(=S)(OCC)OC1=CC(C)=CC=C1[N+]([O-])=O OEYOMNZEMCPTKN-UHFFFAOYSA-N 0.000 description 1
- SPNQRCTZKIBOAX-UHFFFAOYSA-N Butralin Chemical compound CCC(C)NC1=C([N+]([O-])=O)C=C(C(C)(C)C)C=C1[N+]([O-])=O SPNQRCTZKIBOAX-UHFFFAOYSA-N 0.000 description 1
- BMTAFVWTTFSTOG-UHFFFAOYSA-N Butylate Chemical compound CCSC(=O)N(CC(C)C)CC(C)C BMTAFVWTTFSTOG-UHFFFAOYSA-N 0.000 description 1
- SSUFDOMYCBCHML-UHFFFAOYSA-N CCCCC[S](=O)=O Chemical class CCCCC[S](=O)=O SSUFDOMYCBCHML-UHFFFAOYSA-N 0.000 description 1
- OVPOQARSILRTKD-UHFFFAOYSA-N COCCCn1c(ncc(C(=O)C2=C(O)CCCC2=O)c1=O)-c1cccc(OC)c1 Chemical compound COCCCn1c(ncc(C(=O)C2=C(O)CCCC2=O)c1=O)-c1cccc(OC)c1 OVPOQARSILRTKD-UHFFFAOYSA-N 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000011305 Capsella bursa pastoris Nutrition 0.000 description 1
- 240000008867 Capsella bursa-pastoris Species 0.000 description 1
- 239000005490 Carbetamide Substances 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 235000003255 Carthamus tinctorius Nutrition 0.000 description 1
- 244000020518 Carthamus tinctorius Species 0.000 description 1
- 239000005647 Chlorpropham Substances 0.000 description 1
- 235000005918 Cirsium arvense Nutrition 0.000 description 1
- 240000001579 Cirsium arvense Species 0.000 description 1
- 239000005498 Clodinafop Substances 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 240000007154 Coffea arabica Species 0.000 description 1
- 241001133184 Colletotrichum agaves Species 0.000 description 1
- 241001529387 Colletotrichum gloeosporioides Species 0.000 description 1
- 241001478752 Commelina benghalensis Species 0.000 description 1
- 241000218631 Coniferophyta Species 0.000 description 1
- DFCAFRGABIXSDS-UHFFFAOYSA-N Cycloate Chemical compound CCSC(=O)N(CC)C1CCCCC1 DFCAFRGABIXSDS-UHFFFAOYSA-N 0.000 description 1
- 244000052363 Cynodon dactylon Species 0.000 description 1
- 244000285774 Cyperus esculentus Species 0.000 description 1
- 240000001505 Cyperus odoratus Species 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- NPOJQCVWMSKXDN-UHFFFAOYSA-N Dacthal Chemical group COC(=O)C1=C(Cl)C(Cl)=C(C(=O)OC)C(Cl)=C1Cl NPOJQCVWMSKXDN-UHFFFAOYSA-N 0.000 description 1
- NDUPDOJHUQKPAG-UHFFFAOYSA-N Dalapon Chemical compound CC(Cl)(Cl)C(O)=O NDUPDOJHUQKPAG-UHFFFAOYSA-N 0.000 description 1
- 240000008853 Datura stramonium Species 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000005506 Diclofop Substances 0.000 description 1
- 244000152970 Digitaria sanguinalis Species 0.000 description 1
- 235000010823 Digitaria sanguinalis Nutrition 0.000 description 1
- 108010052167 Dihydroorotate Dehydrogenase Proteins 0.000 description 1
- 102100032823 Dihydroorotate dehydrogenase (quinone), mitochondrial Human genes 0.000 description 1
- 239000005509 Dimethenamid-P Substances 0.000 description 1
- PHVNLLCAQHGNKU-UHFFFAOYSA-N Dimethipin Chemical compound CC1=C(C)S(=O)(=O)CCS1(=O)=O PHVNLLCAQHGNKU-UHFFFAOYSA-N 0.000 description 1
- OFDYMSKSGFSLLM-UHFFFAOYSA-N Dinitramine Chemical compound CCN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C(N)=C1[N+]([O-])=O OFDYMSKSGFSLLM-UHFFFAOYSA-N 0.000 description 1
- IIPZYDQGBIWLBU-UHFFFAOYSA-N Dinoterb Chemical compound CC(C)(C)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1O IIPZYDQGBIWLBU-UHFFFAOYSA-N 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- YUBJPYNSGLJZPQ-UHFFFAOYSA-N Dithiopyr Chemical compound CSC(=O)C1=C(C(F)F)N=C(C(F)(F)F)C(C(=O)SC)=C1CC(C)C YUBJPYNSGLJZPQ-UHFFFAOYSA-N 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- GUVLYNGULCJVDO-UHFFFAOYSA-N EPTC Chemical compound CCCN(CCC)C(=O)SCC GUVLYNGULCJVDO-UHFFFAOYSA-N 0.000 description 1
- 235000001950 Elaeis guineensis Nutrition 0.000 description 1
- 244000127993 Elaeis melanococca Species 0.000 description 1
- 244000025670 Eleusine indica Species 0.000 description 1
- 241000508725 Elymus repens Species 0.000 description 1
- BXEHUCNTIZGSOJ-UHFFFAOYSA-N Esprocarb Chemical compound CC(C)C(C)N(CC)C(=O)SCC1=CC=CC=C1 BXEHUCNTIZGSOJ-UHFFFAOYSA-N 0.000 description 1
- PTFJIKYUEPWBMS-UHFFFAOYSA-N Ethalfluralin Chemical compound CC(=C)CN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O PTFJIKYUEPWBMS-UHFFFAOYSA-N 0.000 description 1
- KCOCSOWTADCKOL-UHFFFAOYSA-N Ethidimuron Chemical compound CCS(=O)(=O)C1=NN=C(N(C)C(=O)NC)S1 KCOCSOWTADCKOL-UHFFFAOYSA-N 0.000 description 1
- 239000005512 Ethofumesate Substances 0.000 description 1
- 244000004281 Eucalyptus maculata Species 0.000 description 1
- 244000248416 Fagopyrum cymosum Species 0.000 description 1
- 241001289540 Fallopia convolvulus Species 0.000 description 1
- PQKBPHSEKWERTG-UHFFFAOYSA-N Fenoxaprop ethyl Chemical group C1=CC(OC(C)C(=O)OCC)=CC=C1OC1=NC2=CC=C(Cl)C=C2O1 PQKBPHSEKWERTG-UHFFFAOYSA-N 0.000 description 1
- 239000005513 Fenoxaprop-P Substances 0.000 description 1
- XDWSZOWLJIWERG-UHFFFAOYSA-N Fenuron-TCA Chemical compound [O-]C(=O)C(Cl)(Cl)Cl.C[NH+](C)C(=O)NC1=CC=CC=C1 XDWSZOWLJIWERG-UHFFFAOYSA-N 0.000 description 1
- 241000234642 Festuca Species 0.000 description 1
- 239000005530 Fluazifop-P Substances 0.000 description 1
- FICWGWVVIRLNRB-UHFFFAOYSA-N Flucetosulfuron Chemical compound COCC(=O)OC(C(C)F)C1=NC=CC=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 FICWGWVVIRLNRB-UHFFFAOYSA-N 0.000 description 1
- MNFMIVVPXOGUMX-UHFFFAOYSA-N Fluchloralin Chemical compound CCCN(CCCl)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O MNFMIVVPXOGUMX-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 239000005980 Gibberellic acid Substances 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- 239000005565 Haloxyfop-P Substances 0.000 description 1
- 235000008694 Humulus lupulus Nutrition 0.000 description 1
- 244000025221 Humulus lupulus Species 0.000 description 1
- QBEXFUOWUYCXNI-UHFFFAOYSA-N Ioxynil octanoate Chemical compound CCCCCCCC(=O)OC1=C(I)C=C(C#N)C=C1I QBEXFUOWUYCXNI-UHFFFAOYSA-N 0.000 description 1
- 240000001549 Ipomoea eriocarpa Species 0.000 description 1
- 235000005146 Ipomoea eriocarpa Nutrition 0.000 description 1
- 241000032989 Ipomoea lacunosa Species 0.000 description 1
- KGEKLUUHTZCSIP-UHFFFAOYSA-N Isobornyl acetate Natural products C1CC2(C)C(OC(=O)C)CC1C2(C)C KGEKLUUHTZCSIP-UHFFFAOYSA-N 0.000 description 1
- 239000004440 Isodecyl alcohol Substances 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 229920001732 Lignosulfonate Polymers 0.000 description 1
- 240000006240 Linum usitatissimum Species 0.000 description 1
- 235000004431 Linum usitatissimum Nutrition 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- AZFKQCNGMSSWDS-UHFFFAOYSA-N MCPA-thioethyl Chemical group CCSC(=O)COC1=CC=C(Cl)C=C1C AZFKQCNGMSSWDS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000218922 Magnoliophyta Species 0.000 description 1
- 239000005983 Maleic hydrazide Substances 0.000 description 1
- BGRDGMRNKXEXQD-UHFFFAOYSA-N Maleic hydrazide Chemical compound OC1=CC=C(O)N=N1 BGRDGMRNKXEXQD-UHFFFAOYSA-N 0.000 description 1
- 239000005576 Mecoprop-P Substances 0.000 description 1
- 235000010624 Medicago sativa Nutrition 0.000 description 1
- 240000004658 Medicago sativa Species 0.000 description 1
- 235000017587 Medicago sativa ssp. sativa Nutrition 0.000 description 1
- OKIBNKKYNPBDRS-UHFFFAOYSA-N Mefluidide Chemical compound CC(=O)NC1=CC(NS(=O)(=O)C(F)(F)F)=C(C)C=C1C OKIBNKKYNPBDRS-UHFFFAOYSA-N 0.000 description 1
- 240000007298 Megathyrsus maximus Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- LSDPWZHWYPCBBB-UHFFFAOYSA-N Methanethiol Chemical compound SC LSDPWZHWYPCBBB-UHFFFAOYSA-N 0.000 description 1
- 235000003805 Musa ABB Group Nutrition 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- NWBJYWHLCVSVIJ-UHFFFAOYSA-N N-benzyladenine Chemical compound N=1C=NC=2NC=NC=2C=1NCC1=CC=CC=C1 NWBJYWHLCVSVIJ-UHFFFAOYSA-N 0.000 description 1
- NTBVTCXMRYKRTB-UHFFFAOYSA-N N-{2-[(4,6-dimethoxypyrimidin-2-yl)(hydroxy)methyl]-6-(methoxymethyl)phenyl}-1,1-difluoromethanesulfonamide Chemical compound COCC1=CC=CC(C(O)C=2N=C(OC)C=C(OC)N=2)=C1NS(=O)(=O)C(F)F NTBVTCXMRYKRTB-UHFFFAOYSA-N 0.000 description 1
- 238000006411 Negishi coupling reaction Methods 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 239000005587 Oryzalin Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 235000011999 Panicum crusgalli Nutrition 0.000 description 1
- 240000004674 Papaver rhoeas Species 0.000 description 1
- 235000007846 Papaver rhoeas Nutrition 0.000 description 1
- SGEJQUSYQTVSIU-UHFFFAOYSA-N Pebulate Chemical compound CCCCN(CC)C(=O)SCCC SGEJQUSYQTVSIU-UHFFFAOYSA-N 0.000 description 1
- 239000005591 Pendimethalin Substances 0.000 description 1
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 1
- WHTBVLXUSXVMEV-UHFFFAOYSA-N Perfluidone Chemical compound C1=C(NS(=O)(=O)C(F)(F)F)C(C)=CC(S(=O)(=O)C=2C=CC=CC=2)=C1 WHTBVLXUSXVMEV-UHFFFAOYSA-N 0.000 description 1
- 241000978467 Persicaria pensylvanica Species 0.000 description 1
- 241000257649 Phalaris minor Species 0.000 description 1
- 241000233637 Phytophthora palmivora Species 0.000 description 1
- 235000008566 Pinus taeda Nutrition 0.000 description 1
- 241000218679 Pinus taeda Species 0.000 description 1
- 235000015266 Plantago major Nutrition 0.000 description 1
- 244000292693 Poa annua Species 0.000 description 1
- 241000209049 Poa pratensis Species 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000004349 Polyvinylpyrrolidone-vinyl acetate copolymer Substances 0.000 description 1
- RSVPPPHXAASNOL-UHFFFAOYSA-N Prodiamine Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C(N)=C1[N+]([O-])=O RSVPPPHXAASNOL-UHFFFAOYSA-N 0.000 description 1
- IPDFPNNPBMREIF-CHWSQXEVSA-N Prohydrojasmon Chemical compound CCCCC[C@@H]1[C@@H](CC(=O)OCCC)CCC1=O IPDFPNNPBMREIF-CHWSQXEVSA-N 0.000 description 1
- 239000005601 Propoxycarbazone Substances 0.000 description 1
- 239000005602 Propyzamide Substances 0.000 description 1
- 239000005603 Prosulfocarb Substances 0.000 description 1
- 241000578611 Puccinia thlaspeos Species 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- OBLNWSCLAYSJJR-UHFFFAOYSA-N Quinoclamin Chemical compound C1=CC=C2C(=O)C(N)=C(Cl)C(=O)C2=C1 OBLNWSCLAYSJJR-UHFFFAOYSA-N 0.000 description 1
- 239000002167 Quinoclamine Substances 0.000 description 1
- 239000005609 Quizalofop-P Substances 0.000 description 1
- 239000005614 Quizalofop-P-ethyl Substances 0.000 description 1
- 239000005615 Quizalofop-P-tefuryl Substances 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- OKUGPJPKMAEJOE-UHFFFAOYSA-N S-propyl dipropylcarbamothioate Chemical compound CCCSC(=O)N(CCC)CCC OKUGPJPKMAEJOE-UHFFFAOYSA-N 0.000 description 1
- 238000000297 Sandmeyer reaction Methods 0.000 description 1
- 235000003434 Sesamum indicum Nutrition 0.000 description 1
- 244000040738 Sesamum orientale Species 0.000 description 1
- 235000008515 Setaria glauca Nutrition 0.000 description 1
- 235000001155 Setaria leucopila Nutrition 0.000 description 1
- 244000010062 Setaria pumila Species 0.000 description 1
- 235000002248 Setaria viridis Nutrition 0.000 description 1
- 240000003461 Setaria viridis Species 0.000 description 1
- 235000010086 Setaria viridis var. viridis Nutrition 0.000 description 1
- 240000006410 Sida spinosa Species 0.000 description 1
- 239000004965 Silica aerogel Substances 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 235000002594 Solanum nigrum Nutrition 0.000 description 1
- 240000002307 Solanum ptychanthum Species 0.000 description 1
- 102100038803 Somatotropin Human genes 0.000 description 1
- FBPFZTCFMRRESA-NQAPHZHOSA-N Sorbitol Polymers OCC(O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-NQAPHZHOSA-N 0.000 description 1
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 1
- 235000006923 Sorghum x drummondii Nutrition 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 241000044578 Stenotaphrum secundatum Species 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- SAQSTQBVENFSKT-UHFFFAOYSA-M TCA-sodium Chemical compound [Na+].[O-]C(=O)C(Cl)(Cl)Cl SAQSTQBVENFSKT-UHFFFAOYSA-M 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- YIJZJEYQBAAWRJ-UHFFFAOYSA-N Thiazopyr Chemical compound N1=C(C(F)F)C(C(=O)OC)=C(CC(C)C)C(C=2SCCN=2)=C1C(F)(F)F YIJZJEYQBAAWRJ-UHFFFAOYSA-N 0.000 description 1
- QHTQREMOGMZHJV-UHFFFAOYSA-N Thiobencarb Chemical compound CCN(CC)C(=O)SCC1=CC=C(Cl)C=C1 QHTQREMOGMZHJV-UHFFFAOYSA-N 0.000 description 1
- PHSUVQBHRAWOQD-UHFFFAOYSA-N Tiocarbazil Chemical compound CCC(C)N(C(C)CC)C(=O)SCC1=CC=CC=C1 PHSUVQBHRAWOQD-UHFFFAOYSA-N 0.000 description 1
- 239000005625 Tri-allate Substances 0.000 description 1
- MWBPRDONLNQCFV-UHFFFAOYSA-N Tri-allate Chemical compound CC(C)N(C(C)C)C(=O)SCC(Cl)=C(Cl)Cl MWBPRDONLNQCFV-UHFFFAOYSA-N 0.000 description 1
- 239000005626 Tribenuron Substances 0.000 description 1
- IBZHOAONZVJLOB-UHFFFAOYSA-N Tridiphane Chemical compound ClC1=CC(Cl)=CC(C2(CC(Cl)(Cl)Cl)OC2)=C1 IBZHOAONZVJLOB-UHFFFAOYSA-N 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical class OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 241001141210 Urochloa platyphylla Species 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 241000394440 Viola arvensis Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 241000219094 Vitaceae Species 0.000 description 1
- 235000007244 Zea mays Nutrition 0.000 description 1
- 241001148683 Zostera marina Species 0.000 description 1
- 239000001940 [(1R,4S,6R)-1,7,7-trimethyl-6-bicyclo[2.2.1]heptanyl] acetate Substances 0.000 description 1
- FHOXQLTVOMNIOR-QZPAGEHASA-N [(1S,2R,4R,5S,7S,11S,12S,15R,16S)-2,16-dimethyl-15-[(1S)-1-[(2R,3R)-3-[(3S)-2-methylpentan-3-yl]oxiran-2-yl]ethyl]-8-oxo-4-propanoyloxy-9-oxatetracyclo[9.7.0.02,7.012,16]octadecan-5-yl] propanoate Chemical compound CC[C@@H](C(C)C)[C@H]1O[C@@H]1[C@@H](C)[C@@H]1[C@@]2(C)CC[C@@H]3[C@@]4(C)C[C@@H](OC(=O)CC)[C@@H](OC(=O)CC)C[C@@H]4C(=O)OC[C@H]3[C@@H]2CC1 FHOXQLTVOMNIOR-QZPAGEHASA-N 0.000 description 1
- 239000001089 [(2R)-oxolan-2-yl]methanol Substances 0.000 description 1
- AMRQXHFXNZFDCH-SECBINFHSA-N [(2r)-1-(ethylamino)-1-oxopropan-2-yl] n-phenylcarbamate Chemical compound CCNC(=O)[C@@H](C)OC(=O)NC1=CC=CC=C1 AMRQXHFXNZFDCH-SECBINFHSA-N 0.000 description 1
- LALGZGDVRILFNW-UHFFFAOYSA-N [2,5-dimethyl-4-[2-methylsulfonyl-4-(trifluoromethyl)benzoyl]pyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound CN1N=C(C(=C1)C(=O)OC1=C(C(=NN1C)C)C(C1=C(C=C(C=C1)C(F)(F)F)S(=O)(=O)C)=O)C LALGZGDVRILFNW-UHFFFAOYSA-N 0.000 description 1
- ALMFIOZYDASRRC-UHFFFAOYSA-N [4-(trifluoromethyl)phenyl]boronic acid Chemical compound OB(O)C1=CC=C(C(F)(F)F)C=C1 ALMFIOZYDASRRC-UHFFFAOYSA-N 0.000 description 1
- WXQMIXCKKFECIU-UHFFFAOYSA-N [4-[2-chloro-3-[(3,5-dimethylpyrazol-1-yl)methyl]-4-methylsulfonylbenzoyl]-2,5-dimethylpyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound CN1N=C(C(=C1)C(=O)OC1=C(C(=NN1C)C)C(C1=C(C(=C(C=C1)S(=O)(=O)C)CN1N=C(C=C1C)C)Cl)=O)C WXQMIXCKKFECIU-UHFFFAOYSA-N 0.000 description 1
- NWBFHIJKEYFVND-UHFFFAOYSA-N [4-[2-chloro-4-methylsulfonyl-3-(2,2,2-trifluoroethoxymethyl)benzoyl]-2-ethylpyrazol-3-yl] 1,3-dimethylpyrazole-4-carboxylate Chemical compound N1(C=C(C(=O)OC=2N(CC)N=CC=2C(=O)C2=C(C(=C(S(=O)(=O)C)C=C2)COCC(F)(F)F)Cl)C(C)=N1)C NWBFHIJKEYFVND-UHFFFAOYSA-N 0.000 description 1
- 230000036579 abiotic stress Effects 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- ZCZSIDMEHXZRLG-UHFFFAOYSA-N acetic acid heptyl ester Natural products CCCCCCCOC(C)=O ZCZSIDMEHXZRLG-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- NUFNQYOELLVIPL-UHFFFAOYSA-N acifluorfen Chemical compound C1=C([N+]([O-])=O)C(C(=O)O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NUFNQYOELLVIPL-UHFFFAOYSA-N 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 230000009418 agronomic effect Effects 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 125000005083 alkoxyalkoxy group Chemical group 0.000 description 1
- 125000005078 alkoxycarbonylalkyl group Chemical group 0.000 description 1
- 229920000180 alkyd Polymers 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001409 amidines Chemical class 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- KJBRXNXZODWCMC-UHFFFAOYSA-N anisiflupurin Chemical compound COC1=CC=CC(NC=2C=3NC=NC=3N=C(F)N=2)=C1 KJBRXNXZODWCMC-UHFFFAOYSA-N 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000002528 anti-freeze Effects 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 239000005667 attractant Substances 0.000 description 1
- 208000014347 autosomal dominant hyaline body myopathy Diseases 0.000 description 1
- XOEMATDHVZOBSG-UHFFFAOYSA-N azafenidin Chemical compound C1=C(OCC#C)C(Cl)=CC(Cl)=C1N1C(=O)N2CCCCC2=N1 XOEMATDHVZOBSG-UHFFFAOYSA-N 0.000 description 1
- QRSHQJLLXXEYPS-UHFFFAOYSA-N azane;5-ethyl-2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)pyridine-3-carboxylic acid Chemical compound [NH4+].[O-]C(=O)C1=CC(CC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 QRSHQJLLXXEYPS-UHFFFAOYSA-N 0.000 description 1
- 229940097012 bacillus thuringiensis Drugs 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- LVKBXDHACCFCTA-UHFFFAOYSA-N bencarbazone Chemical compound C1=C(C(N)=S)C(NS(=O)(=O)CC)=CC(N2C(N(C)C(=N2)C(F)(F)F)=O)=C1F LVKBXDHACCFCTA-UHFFFAOYSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- SMDHCQAYESWHAE-UHFFFAOYSA-N benfluralin Chemical compound CCCCN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O SMDHCQAYESWHAE-UHFFFAOYSA-N 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- CNBGNNVCVSKAQZ-UHFFFAOYSA-N benzidamine Natural products C12=CC=CC=C2C(OCCCN(C)C)=NN1CC1=CC=CC=C1 CNBGNNVCVSKAQZ-UHFFFAOYSA-N 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- 230000004790 biotic stress Effects 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- RYVIXQCRCQLFCM-UHFFFAOYSA-N bispyribac Chemical compound COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C(O)=O)=N1 RYVIXQCRCQLFCM-UHFFFAOYSA-N 0.000 description 1
- FUHMZYWBSHTEDZ-UHFFFAOYSA-M bispyribac-sodium Chemical compound [Na+].COC1=CC(OC)=NC(OC=2C(=C(OC=3N=C(OC)C=C(OC)N=3)C=CC=2)C([O-])=O)=N1 FUHMZYWBSHTEDZ-UHFFFAOYSA-M 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- VAIZTNZGPYBOGF-UHFFFAOYSA-N butyl 2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OCCCC)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 VAIZTNZGPYBOGF-UHFFFAOYSA-N 0.000 description 1
- PSGPXWYGJGGEEG-UHFFFAOYSA-N butyl 9-hydroxyfluorene-9-carboxylate Chemical group C1=CC=C2C(C(=O)OCCCC)(O)C3=CC=CC=C3C2=C1 PSGPXWYGJGGEEG-UHFFFAOYSA-N 0.000 description 1
- 125000004744 butyloxycarbonyl group Chemical group 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- 229950004243 cacodylic acid Drugs 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- NLKUPINTOLSSLD-UHFFFAOYSA-L calcium;4-(1-oxidopropylidene)-3,5-dioxocyclohexane-1-carboxylate Chemical compound [Ca+2].CCC([O-])=C1C(=O)CC(C([O-])=O)CC1=O NLKUPINTOLSSLD-UHFFFAOYSA-L 0.000 description 1
- 239000004490 capsule suspension Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- ADRVNXBAWSRFAJ-UHFFFAOYSA-N catechin Natural products OC1Cc2cc(O)cc(O)c2OC1c3ccc(O)c(O)c3 ADRVNXBAWSRFAJ-UHFFFAOYSA-N 0.000 description 1
- 235000005487 catechin Nutrition 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 150000001793 charged compounds Chemical class 0.000 description 1
- MXZACTZQSGYANA-UHFFFAOYSA-N chembl545463 Chemical compound Cl.C1=CC(OC)=CC=C1C(N=C1)=CN2C1=NC(C)=C2O MXZACTZQSGYANA-UHFFFAOYSA-N 0.000 description 1
- 238000003889 chemical engineering Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 230000003559 chemosterilizing effect Effects 0.000 description 1
- VXIVSQZSERGHQP-UHFFFAOYSA-N chloroacetamide Chemical class NC(=O)CCl VXIVSQZSERGHQP-UHFFFAOYSA-N 0.000 description 1
- 229930002875 chlorophyll Natural products 0.000 description 1
- 235000019804 chlorophyll Nutrition 0.000 description 1
- ATNHDLDRLWWWCB-AENOIHSZSA-M chlorophyll a Chemical compound C1([C@@H](C(=O)OC)C(=O)C2=C3C)=C2N2C3=CC(C(CC)=C3C)=[N+]4C3=CC3=C(C=C)C(C)=C5N3[Mg-2]42[N+]2=C1[C@@H](CCC(=O)OC\C=C(/C)CCC[C@H](C)CCC[C@H](C)CCCC(C)C)[C@H](C)C2=C5 ATNHDLDRLWWWCB-AENOIHSZSA-M 0.000 description 1
- 150000005698 chloropyrimidines Chemical class 0.000 description 1
- IVUXTESCPZUGJC-UHFFFAOYSA-N chloroxuron Chemical compound C1=CC(NC(=O)N(C)C)=CC=C1OC1=CC=C(Cl)C=C1 IVUXTESCPZUGJC-UHFFFAOYSA-N 0.000 description 1
- CWJSHJJYOPWUGX-UHFFFAOYSA-N chlorpropham Chemical compound CC(C)OC(=O)NC1=CC=CC(Cl)=C1 CWJSHJJYOPWUGX-UHFFFAOYSA-N 0.000 description 1
- 229950001002 cianidanol Drugs 0.000 description 1
- YUIKUTLBPMDDNQ-MRVPVSSYSA-N clodinafop Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC=C(Cl)C=C1F YUIKUTLBPMDDNQ-MRVPVSSYSA-N 0.000 description 1
- 235000012716 cod liver oil Nutrition 0.000 description 1
- 239000003026 cod liver oil Substances 0.000 description 1
- 235000016213 coffee Nutrition 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 230000002301 combined effect Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- 244000038559 crop plants Species 0.000 description 1
- 238000012272 crop production Methods 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 description 1
- OAWUUPVZMNKZRY-UHFFFAOYSA-N cyprosulfamide Chemical compound COC1=CC=CC=C1C(=O)NS(=O)(=O)C1=CC=C(C(=O)NC2CC2)C=C1 OAWUUPVZMNKZRY-UHFFFAOYSA-N 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- JLYFCTQDENRSOL-VIFPVBQESA-N dimethenamid-P Chemical compound COC[C@H](C)N(C(=O)CCl)C=1C(C)=CSC=1C JLYFCTQDENRSOL-VIFPVBQESA-N 0.000 description 1
- FFHWGQQFANVOHV-UHFFFAOYSA-N dimethyldioxirane Chemical compound CC1(C)OO1 FFHWGQQFANVOHV-UHFFFAOYSA-N 0.000 description 1
- 229950010286 diolamine Drugs 0.000 description 1
- 150000004844 dioxiranes Chemical class 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- SYJFEGQWDCRVNX-UHFFFAOYSA-N diquat Chemical compound C1=CC=[N+]2CC[N+]3=CC=CC=C3C2=C1 SYJFEGQWDCRVNX-UHFFFAOYSA-N 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- SDIXRDNYIMOKSG-UHFFFAOYSA-L disodium methyl arsenate Chemical compound [Na+].[Na+].C[As]([O-])([O-])=O SDIXRDNYIMOKSG-UHFFFAOYSA-L 0.000 description 1
- 239000004491 dispersible concentrate Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- SACNIGZYDTUHKB-UHFFFAOYSA-N ditert-butyl-[2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane Chemical group CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C(C)(C)C)C(C)(C)C SACNIGZYDTUHKB-UHFFFAOYSA-N 0.000 description 1
- LQZZUXJYWNFBMV-UHFFFAOYSA-N dodecan-1-ol Chemical compound CCCCCCCCCCCCO LQZZUXJYWNFBMV-UHFFFAOYSA-N 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000009837 dry grinding Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000007350 electrophilic reaction Methods 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 239000004497 emulsifiable granule Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000000967 entomopathogenic effect Effects 0.000 description 1
- LRMHFDNWKCSEQU-UHFFFAOYSA-N ethoxyethane;phenol Chemical compound CCOCC.OC1=CC=CC=C1 LRMHFDNWKCSEQU-UHFFFAOYSA-N 0.000 description 1
- GMRBKRLUOIKWFH-UHFFFAOYSA-N ethyl 1-(2-methoxyphenyl)-6-oxo-2-phenylpyrimidine-5-carboxylate Chemical compound CCOC(=O)c1cnc(-c2ccccc2)n(-c2ccccc2OC)c1=O GMRBKRLUOIKWFH-UHFFFAOYSA-N 0.000 description 1
- OSUHJPCHFDQAIT-UHFFFAOYSA-N ethyl 2-{4-[(6-chloroquinoxalin-2-yl)oxy]phenoxy}propanoate Chemical group C1=CC(OC(C)C(=O)OCC)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 OSUHJPCHFDQAIT-UHFFFAOYSA-N 0.000 description 1
- XNKARWLGLZGMGX-UHFFFAOYSA-N ethyl 4-(4-chloro-2-methylphenoxy)butanoate Chemical group CCOC(=O)CCCOC1=CC=C(Cl)C=C1C XNKARWLGLZGMGX-UHFFFAOYSA-N 0.000 description 1
- 125000006437 ethyl cyclopropyl group Chemical group 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 230000004136 fatty acid synthesis Effects 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 235000004426 flaxseed Nutrition 0.000 description 1
- YUVKUEAFAVKILW-SECBINFHSA-N fluazifop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 YUVKUEAFAVKILW-SECBINFHSA-N 0.000 description 1
- VAIZTNZGPYBOGF-CYBMUJFWSA-N fluazifop-P-butyl Chemical group C1=CC(O[C@H](C)C(=O)OCCCC)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 VAIZTNZGPYBOGF-CYBMUJFWSA-N 0.000 description 1
- GINFBXXYGUODAT-UHFFFAOYSA-N flucarbazone Chemical compound O=C1N(C)C(OC)=NN1C(=O)NS(=O)(=O)C1=CC=CC=C1OC(F)(F)F GINFBXXYGUODAT-UHFFFAOYSA-N 0.000 description 1
- UOUXAYAIONPXDH-UHFFFAOYSA-M flucarbazone-sodium Chemical compound [Na+].O=C1N(C)C(OC)=NN1C(=O)[N-]S(=O)(=O)C1=CC=CC=C1OC(F)(F)F UOUXAYAIONPXDH-UHFFFAOYSA-M 0.000 description 1
- LEIWLFHGOGNSAD-UWTATZPHSA-N fluchloraminopyr Chemical compound C[C@H](C(=O)O)OC1=NC(=C(C(=C1Cl)N)Cl)F LEIWLFHGOGNSAD-UWTATZPHSA-N 0.000 description 1
- WFZSZAXUALBVNX-UHFFFAOYSA-N flufenpyr Chemical compound O=C1C(C)=C(C(F)(F)F)C=NN1C1=CC(OCC(O)=O)=C(Cl)C=C1F WFZSZAXUALBVNX-UHFFFAOYSA-N 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- IXORZMNAPKEEDV-UHFFFAOYSA-N gibberellic acid GA3 Natural products OC(=O)C1C2(C3)CC(=C)C3(O)CCC2C2(C=CC3O)C1C3(C)C(=O)O2 IXORZMNAPKEEDV-UHFFFAOYSA-N 0.000 description 1
- IXORZMNAPKEEDV-OBDJNFEBSA-N gibberellin A3 Chemical compound C([C@@]1(O)C(=C)C[C@@]2(C1)[C@H]1C(O)=O)C[C@H]2[C@]2(C=C[C@@H]3O)[C@H]1[C@]3(C)C(=O)O2 IXORZMNAPKEEDV-OBDJNFEBSA-N 0.000 description 1
- RSQSQJNRHICNNH-NFMPGMCNSA-N gibberellin A4 Chemical compound C([C@@H]1C[C@]2(CC1=C)[C@H]1C(O)=O)C[C@H]2[C@@]2(OC3=O)[C@H]1[C@@]3(C)[C@@H](O)CC2 RSQSQJNRHICNNH-NFMPGMCNSA-N 0.000 description 1
- SEEGHKWOBVVBTQ-NFMPGMCNSA-N gibberellin A7 Chemical compound C([C@@H]1C[C@]2(CC1=C)[C@H]1C(O)=O)C[C@H]2[C@]2(C=C[C@@H]3O)[C@H]1[C@]3(C)C(=O)O2 SEEGHKWOBVVBTQ-NFMPGMCNSA-N 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 229940087559 grape seed Drugs 0.000 description 1
- 235000021021 grapes Nutrition 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 239000003630 growth substance Substances 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- MJAWMRVEIWPJRW-UHFFFAOYSA-N haloxydine Chemical compound FC=1NC(F)=C(Cl)C(=O)C=1Cl MJAWMRVEIWPJRW-UHFFFAOYSA-N 0.000 description 1
- GOCUAJYOYBLQRH-MRVPVSSYSA-N haloxyfop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl GOCUAJYOYBLQRH-MRVPVSSYSA-N 0.000 description 1
- JPXGPRBLTIYFQG-UHFFFAOYSA-N heptan-4-yl acetate Chemical compound CCCC(CCC)OC(C)=O JPXGPRBLTIYFQG-UHFFFAOYSA-N 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003707 hexyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000005980 hexynyl group Chemical group 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229920001600 hydrophobic polymer Polymers 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- VDEGQTCMQUFPFH-UHFFFAOYSA-N hydroxy-dimethyl-arsine Natural products C[As](C)O VDEGQTCMQUFPFH-UHFFFAOYSA-N 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229940117955 isoamyl acetate Drugs 0.000 description 1
- 229940035429 isobutyl alcohol Drugs 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 150000003893 lactate salts Chemical class 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 150000002689 maleic acids Chemical class 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000008204 material by function Substances 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- AFCCDDWKHLHPDF-UHFFFAOYSA-M metam-sodium Chemical compound [Na+].CNC([S-])=S AFCCDDWKHLHPDF-UHFFFAOYSA-M 0.000 description 1
- MFSWTRQUCLNFOM-UHFFFAOYSA-N methyl 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl MFSWTRQUCLNFOM-UHFFFAOYSA-N 0.000 description 1
- RBNIGDFIUWJJEV-UHFFFAOYSA-N methyl 2-(n-benzoyl-3-chloro-4-fluoroanilino)propanoate Chemical group C=1C=C(F)C(Cl)=CC=1N(C(C)C(=O)OC)C(=O)C1=CC=CC=C1 RBNIGDFIUWJJEV-UHFFFAOYSA-N 0.000 description 1
- JTHMVYBOQLDDIY-UHFFFAOYSA-N methyl 2-[(4-methyl-5-oxo-3-propoxy-1,2,4-triazole-1-carbonyl)sulfamoyl]benzoate Chemical compound O=C1N(C)C(OCCC)=NN1C(=O)NS(=O)(=O)C1=CC=CC=C1C(=O)OC JTHMVYBOQLDDIY-UHFFFAOYSA-N 0.000 description 1
- LYPWWQLKWQNQKV-UHFFFAOYSA-N methyl 2-[5-ethyl-2-[[4-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]phenoxy]methyl]phenoxy]propanoate Chemical compound COC(=O)C(C)OC1=CC(CC)=CC=C1COC1=CC=C(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)C=C1 LYPWWQLKWQNQKV-UHFFFAOYSA-N 0.000 description 1
- LINPVWIEWJTEEJ-UHFFFAOYSA-N methyl 2-chloro-9-hydroxyfluorene-9-carboxylate Chemical group C1=C(Cl)C=C2C(C(=O)OC)(O)C3=CC=CC=C3C2=C1 LINPVWIEWJTEEJ-UHFFFAOYSA-N 0.000 description 1
- 125000006431 methyl cyclopropyl group Chemical group 0.000 description 1
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 1
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 1
- NDNKHWUXXOFHTD-UHFFFAOYSA-N metizoline Chemical compound CC=1SC2=CC=CC=C2C=1CC1=NCCN1 NDNKHWUXXOFHTD-UHFFFAOYSA-N 0.000 description 1
- 229960002939 metizoline Drugs 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- DEDOPGXGGQYYMW-UHFFFAOYSA-N molinate Chemical compound CCSC(=O)N1CCCCCC1 DEDOPGXGGQYYMW-UHFFFAOYSA-N 0.000 description 1
- JITOKQVGRJSHHA-UHFFFAOYSA-M monosodium methyl arsenate Chemical compound [Na+].C[As](O)([O-])=O JITOKQVGRJSHHA-UHFFFAOYSA-M 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- UMCKXPWHWSKWJJ-UHFFFAOYSA-N n,n-dimethyl-2-oxo-6-(trifluoromethyl)-1h-pyridine-3-carboxamide Chemical compound CN(C)C(=O)C1=CC=C(C(F)(F)F)N=C1O UMCKXPWHWSKWJJ-UHFFFAOYSA-N 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N n-Octanol Natural products CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- CHEDHKBPPDKBQF-UPONEAKYSA-N n-[5-[(6s,7ar)-6-fluoro-1,3-dioxo-5,6,7,7a-tetrahydropyrrolo[1,2-c]imidazol-2-yl]-2-chloro-4-fluorophenyl]-1-chloromethanesulfonamide Chemical compound N1([C@@H](C2=O)C[C@@H](C1)F)C(=O)N2C1=CC(NS(=O)(=O)CCl)=C(Cl)C=C1F CHEDHKBPPDKBQF-UPONEAKYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 229920000847 nonoxynol Polymers 0.000 description 1
- 231100001184 nonphytotoxic Toxicity 0.000 description 1
- NVGOPFQZYCNLDU-UHFFFAOYSA-N norflurazon Chemical compound O=C1C(Cl)=C(NC)C=NN1C1=CC=CC(C(F)(F)F)=C1 NVGOPFQZYCNLDU-UHFFFAOYSA-N 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 239000012038 nucleophile Substances 0.000 description 1
- 238000007344 nucleophilic reaction Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 239000004533 oil dispersion Substances 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 229940055577 oleyl alcohol Drugs 0.000 description 1
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 1
- LLLFASISUZUJEQ-UHFFFAOYSA-N orbencarb Chemical compound CCN(CC)C(=O)SCC1=CC=CC=C1Cl LLLFASISUZUJEQ-UHFFFAOYSA-N 0.000 description 1
- 210000003463 organelle Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 238000010653 organometallic reaction Methods 0.000 description 1
- UCDPMNSCCRBWIC-UHFFFAOYSA-N orthosulfamuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)NC=2C(=CC=CC=2)C(=O)N(C)C)=N1 UCDPMNSCCRBWIC-UHFFFAOYSA-N 0.000 description 1
- UNAHYJYOSSSJHH-UHFFFAOYSA-N oryzalin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(S(N)(=O)=O)C=C1[N+]([O-])=O UNAHYJYOSSSJHH-UHFFFAOYSA-N 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- CHIFOSRWCNZCFN-UHFFFAOYSA-N pendimethalin Chemical compound CCC(CC)NC1=C([N+]([O-])=O)C=C(C)C(C)=C1[N+]([O-])=O CHIFOSRWCNZCFN-UHFFFAOYSA-N 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N pentanoic acid group Chemical class C(CCCC)(=O)O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000001148 pentyloxycarbonyl group Chemical group 0.000 description 1
- 125000005981 pentynyl group Chemical group 0.000 description 1
- 150000004965 peroxy acids Chemical class 0.000 description 1
- 235000020030 perry Nutrition 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 1
- 239000003016 pheromone Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 1
- 230000029553 photosynthesis Effects 0.000 description 1
- 238000010672 photosynthesis Methods 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000000885 phytotoxic effect Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 230000008654 plant damage Effects 0.000 description 1
- 239000005648 plant growth regulator Substances 0.000 description 1
- 239000003375 plant hormone Substances 0.000 description 1
- 150000004291 polyenes Chemical class 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 235000019448 polyvinylpyrrolidone-vinyl acetate copolymer Nutrition 0.000 description 1
- 235000015277 pork Nutrition 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- ORHJUFUQMQEFPQ-UHFFFAOYSA-M potassium;2-(4-chloro-2-methylphenoxy)acetate Chemical compound [K+].CC1=CC(Cl)=CC=C1OCC([O-])=O ORHJUFUQMQEFPQ-UHFFFAOYSA-M 0.000 description 1
- ZRHANBBTXQZFSP-UHFFFAOYSA-M potassium;4-amino-3,5,6-trichloropyridine-2-carboxylate Chemical compound [K+].NC1=C(Cl)C(Cl)=NC(C([O-])=O)=C1Cl ZRHANBBTXQZFSP-UHFFFAOYSA-M 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- ZKWPMZVVAJSYNI-UHFFFAOYSA-N prop-2-enal Chemical compound C=CC=O.C=CC=O ZKWPMZVVAJSYNI-UHFFFAOYSA-N 0.000 description 1
- VXPLXMJHHKHSOA-UHFFFAOYSA-N propham Chemical compound CC(C)OC(=O)NC1=CC=CC=C1 VXPLXMJHHKHSOA-UHFFFAOYSA-N 0.000 description 1
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 1
- PHNUZKMIPFFYSO-UHFFFAOYSA-N propyzamide Chemical compound C#CC(C)(C)NC(=O)C1=CC(Cl)=CC(Cl)=C1 PHNUZKMIPFFYSO-UHFFFAOYSA-N 0.000 description 1
- NQLVQOSNDJXLKG-UHFFFAOYSA-N prosulfocarb Chemical compound CCCN(CCC)C(=O)SCC1=CC=CC=C1 NQLVQOSNDJXLKG-UHFFFAOYSA-N 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- VTGOHKSTWXHQJK-UHFFFAOYSA-N pyrimidin-2-ol Chemical class OC1=NC=CC=N1 VTGOHKSTWXHQJK-UHFFFAOYSA-N 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- ABOOPXYCKNFDNJ-SNVBAGLBSA-N quizalofop-P Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 ABOOPXYCKNFDNJ-SNVBAGLBSA-N 0.000 description 1
- OSUHJPCHFDQAIT-GFCCVEGCSA-N quizalofop-P-ethyl Chemical group C1=CC(O[C@H](C)C(=O)OCC)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 OSUHJPCHFDQAIT-GFCCVEGCSA-N 0.000 description 1
- BBKDWPHJZANJGB-IKJXHCRLSA-N quizalofop-P-tefuryl Chemical group O=C([C@H](OC=1C=CC(OC=2N=C3C=CC(Cl)=CC3=NC=2)=CC=1)C)OCC1CCCO1 BBKDWPHJZANJGB-IKJXHCRLSA-N 0.000 description 1
- 238000007348 radical reaction Methods 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical class C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 239000003620 semiochemical Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000017550 sodium carbonate Nutrition 0.000 description 1
- RMBAVIFYHOYIFM-UHFFFAOYSA-M sodium methanethiolate Chemical compound [Na+].[S-]C RMBAVIFYHOYIFM-UHFFFAOYSA-M 0.000 description 1
- 229960001922 sodium perborate Drugs 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- PDEFQWNXOUGDJR-UHFFFAOYSA-M sodium;2,2-dichloropropanoate Chemical compound [Na+].CC(Cl)(Cl)C([O-])=O PDEFQWNXOUGDJR-UHFFFAOYSA-M 0.000 description 1
- STAPBGVGYWCRTF-UHFFFAOYSA-M sodium;2-(4-chloro-2-methylphenoxy)acetate Chemical compound [Na+].CC1=CC(Cl)=CC=C1OCC([O-])=O STAPBGVGYWCRTF-UHFFFAOYSA-M 0.000 description 1
- AXKBOWBNOCUNJL-UHFFFAOYSA-M sodium;2-nitrophenolate Chemical compound [Na+].[O-]C1=CC=CC=C1[N+]([O-])=O AXKBOWBNOCUNJL-UHFFFAOYSA-M 0.000 description 1
- GABUSZPTCJGKGB-UHFFFAOYSA-M sodium;4-(4-chloro-2-methylphenoxy)butanoate Chemical compound [Na+].CC1=CC(Cl)=CC=C1OCCCC([O-])=O GABUSZPTCJGKGB-UHFFFAOYSA-M 0.000 description 1
- QGKPUZOFTJQTHL-UHFFFAOYSA-M sodium;4-cyano-2,6-diiodophenolate Chemical compound [Na+].[O-]C1=C(I)C=C(C#N)C=C1I QGKPUZOFTJQTHL-UHFFFAOYSA-M 0.000 description 1
- JRQGDDUXDKCWRF-UHFFFAOYSA-M sodium;n-(2-methoxycarbonylphenyl)sulfonyl-4-methyl-5-oxo-3-propoxy-1,2,4-triazole-1-carboximidate Chemical compound [Na+].O=C1N(C)C(OCCC)=NN1C(=O)[N-]S(=O)(=O)C1=CC=CC=C1C(=O)OC JRQGDDUXDKCWRF-UHFFFAOYSA-M 0.000 description 1
- YKLJGMBLPUQQOI-UHFFFAOYSA-M sodium;oxidooxy(oxo)borane Chemical compound [Na+].[O-]OB=O YKLJGMBLPUQQOI-UHFFFAOYSA-M 0.000 description 1
- 239000004550 soluble concentrate Substances 0.000 description 1
- JNYAEWCLZODPBN-CTQIIAAMSA-N sorbitan Polymers OCC(O)C1OCC(O)[C@@H]1O JNYAEWCLZODPBN-CTQIIAAMSA-N 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- VNFWTIYUKDMAOP-UHFFFAOYSA-N sphos Chemical compound COC1=CC=CC(OC)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 VNFWTIYUKDMAOP-UHFFFAOYSA-N 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 235000011044 succinic acid Nutrition 0.000 description 1
- 150000003444 succinic acids Chemical class 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000003445 sucroses Chemical class 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- HHVIBTZHLRERCL-UHFFFAOYSA-N sulfonyldimethane Chemical group CS(C)(=O)=O HHVIBTZHLRERCL-UHFFFAOYSA-N 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000004548 suspo-emulsion Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 239000000271 synthetic detergent Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- RJKCKKDSSSRYCB-UHFFFAOYSA-N tebutam Chemical compound CC(C)(C)C(=O)N(C(C)C)CC1=CC=CC=C1 RJKCKKDSSSRYCB-UHFFFAOYSA-N 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 1
- BSYVTEYKTMYBMK-UHFFFAOYSA-N tetrahydrofurfuryl alcohol Chemical compound OCC1CCCO1 BSYVTEYKTMYBMK-UHFFFAOYSA-N 0.000 description 1
- CZDYPVPMEAXLPK-UHFFFAOYSA-N tetramethylsilane Chemical compound C[Si](C)(C)C CZDYPVPMEAXLPK-UHFFFAOYSA-N 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 230000009974 thixotropic effect Effects 0.000 description 1
- 210000002377 thylakoid Anatomy 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 150000003918 triazines Chemical class 0.000 description 1
- BQZXUHDXIARLEO-UHFFFAOYSA-N tribenuron Chemical compound COC1=NC(C)=NC(N(C)C(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 BQZXUHDXIARLEO-UHFFFAOYSA-N 0.000 description 1
- ZDRNMODJXFOYMN-UHFFFAOYSA-N tridecyl acetate Chemical compound CCCCCCCCCCCCCOC(C)=O ZDRNMODJXFOYMN-UHFFFAOYSA-N 0.000 description 1
- 229940087291 tridecyl alcohol Drugs 0.000 description 1
- MCVUKOYZUCWLQQ-UHFFFAOYSA-N tridecylbenzene Chemical class CCCCCCCCCCCCCC1=CC=CC=C1 MCVUKOYZUCWLQQ-UHFFFAOYSA-N 0.000 description 1
- DQWPFSLDHJDLRL-UHFFFAOYSA-N triethyl phosphate Chemical compound CCOP(=O)(OCC)OCC DQWPFSLDHJDLRL-UHFFFAOYSA-N 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
- ZSDSQXJSNMTJDA-UHFFFAOYSA-N trifluralin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O ZSDSQXJSNMTJDA-UHFFFAOYSA-N 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 1
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 239000004562 water dispersible granule Substances 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- UGOMMVLRQDMAQQ-UHFFFAOYSA-N xphos Chemical compound CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C1=CC=CC=C1P(C1CCCCC1)C1CCCCC1 UGOMMVLRQDMAQQ-UHFFFAOYSA-N 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 235000014692 zinc oxide Nutrition 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/54—1,3-Diazines; Hydrogenated 1,3-diazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/74—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,3
- A01N43/78—1,3-Thiazoles; Hydrogenated 1,3-thiazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01P—BIOCIDAL, PEST REPELLANT, PEST ATTRACTANT OR PLANT GROWTH REGULATORY ACTIVITY OF CHEMICAL COMPOUNDS OR PREPARATIONS
- A01P13/00—Herbicides; Algicides
- A01P13/02—Herbicides; Algicides selective
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/34—One oxygen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
Definitions
- This invention relates to certain substituted cyclopropylpyrimidine herbicides, their N-oxides, salts and compositions, and methods of their use for controlling undesirable vegetation.
- BACKGROUND OF THE INVENTION The control of undesired vegetation is extremely important in achieving high crop efficiency. Achievement of selective control of the growth of weeds especially in such useful crops as rice, soybean, sugar beet, maize, potato, wheat, barley, tomato and plantation crops, among others, is very desirable. Unchecked weed growth in such useful crops can cause significant reduction in productivity and thereby result in increased costs to the consumer.
- This invention is directed to compounds of Formula 1, all stereoisomers, N-oxides, and salts thereof, agricultural compositions containing them and their use as herbicides: wherein J is selected from the group consisting of , R 1 is halogen, C 1 –C 4 haloalkyl, C 1 –C 4 haloalkoxy, C 1 –C 4 alkoxy, C 1 –C 4 haloalkenyl, C 1 –C 4 haloalkynyl, C 1 –C 4 haloalkenyloxy, C 1 –C 4 haloalkynyloxy, C 1 –C 4 haloalkylthio, C 1 –C 4 alkylthio or -CN; R 2 is H or
- this invention pertains to a compound of Formula 1 (including all stereoisomers), an N-oxide or a salt thereof.
- This invention also relates to a herbicidal composition comprising a compound of the invention (i.e. in a herbicidally effective amount) and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents.
- This invention further relates to a method for controlling the growth of undesired vegetation comprising contacting the vegetation or its environment with a herbicidally effective amount of a compound of the invention (e.g., as a composition described herein).
- This invention also includes a herbicidal mixture comprising (a) a compound selected from Formula 1, N-oxides, and salts thereof, and (b) at least one additional active ingredient selected from (b1) through (b17), and salts of compounds of (b1) through (b17), as described below.
- a herbicidal mixture comprising (a) a compound selected from Formula 1, N-oxides, and salts thereof, and (b) at least one additional active ingredient selected from (b1) through (b17), and salts of compounds of (b1) through (b17), as described below.
- compositions, mixture, process or method that comprises a list of elements is not necessarily limited to only those elements but may include other elements not expressly listed or inherent to such composition, mixture, process or method.
- the transitional phrase “consisting of” excludes any element, step, or ingredient not specified. If in the claim, such would close the claim to the inclusion of materials other than those recited except for impurities ordinarily associated therewith.
- the phrase “consisting of” appears in a clause of the body of a claim, rather than immediately following the preamble, it limits only the element set forth in that clause; other elements are not excluded from the claim as a whole.
- transitional phrase “consisting essentially of” is used to define a composition or method that includes materials, steps, features, components, or elements, in addition to those literally disclosed, provided that these additional materials, steps, features, components, or elements do not materially affect the basic and novel characteristic(s) of the claimed invention.
- the term “consisting essentially of” occupies a middle ground between “comprising” and “consisting of”.
- the indefinite articles “a” and “an” preceding an element or component of the invention are intended to be nonrestrictive regarding the number of instances (i.e. occurrences) of the element or component. Therefore “a” or “an” should be read to include one or at least one, and the singular word form of the element or component also includes the plural unless the number is obviously meant to be singular.
- seedling used either alone or in a combination of words means a young plant developing from the embryo of a seed.
- the term “broadleaf” used either alone or in words such as “broadleaf weed” means dicot or dicotyledon, a term used to describe a group of angiosperms characterized by embryos having two cotyledons.
- the term “alkylating” refers reaction in which nucleophile displaces a leaving group such as halide or sulfonate from a carbon-containing radical. Unless otherwise indicated, the term “alkylating” does not limit the carbon-containing radical to alkyl.
- alkyl used either alone or in compound words such as “alkylthio” or “haloalkyl” includes straight-chain or branched alkyl, such as, methyl, ethyl, n-propyl, i-propyl, or the different butyl, pentyl or hexyl isomers.
- Alkenyl includes straight-chain or branched alkenes such as ethenyl, 1-propenyl, 2-propenyl, and the different butenyl, pentenyl and hexenyl isomers.
- Alkenyl also includes polyenes such as 1,2-propadienyl and 2,4-hexadienyl.
- Alkynyl includes straight-chain or branched alkynes such as ethynyl, 1-propynyl, 2-propynyl and the different butynyl, pentynyl and hexynyl isomers.
- Alkynyl can also include moieties comprised of multiple triple bonds such as 2,5-hexadiynyl.
- Alkoxy includes, for example, methoxy, ethoxy, n-propyloxy, isopropyloxy and the different butoxy, pentoxy and hexyloxy isomers.
- Alkoxyalkyl denotes alkoxy substitution on alkyl. Examples of “alkoxyalkyl” include CH 3 OCH 2 , CH 3 OCH 2 CH 2 , CH 3 CH 2 OCH 2 , CH 3 CH 2 CH 2 CH 2 OCH 2 and CH 3 CH 2 OCH 2 CH 2 .
- Alkoxyalkoxy denotes alkoxy substitution on alkoxy.
- Alkylthio includes branched or straight-chain alkylthio moieties such as methylthio, ethylthio, and the different propylthio, butylthio, pentylthio and hexylthio isomers.
- Alkylthioalkyl denotes alkylthio substitution on alkyl. Examples of “alkylthioalkyl” include CH 3 SCH 2 , CH 3 SCH 2 CH 2 , CH 3 CH 2 SCH 2 , CH 3 CH 2 CH 2 CH 2 SCH 2 and CH 3 CH 2 SCH 2 CH 2 .
- Alkylsulfinyl includes both enantiomers of an alkylsulfinyl group.
- alkylsulfinyl examples include CH 3 S(O)-, CH 3 CH 2 S(O)-, CH 3 CH 2 CH 2 S(O)-, (CH 3 ) 2 CHS(O)- and the different butylsulfinyl, pentylsulfinyl and hexylsulfinyl isomers.
- alkylsulfonyl examples include CH 3 S(O) 2 -, CH 3 CH 2 S(O) 2 -, CH 3 CH 2 CH 2 S(O) 2 -, (CH 3 ) 2 CHS(O) 2 -, and the different butylsulfonyl, pentylsulfonyl and hexylsulfonyl isomers.
- Cyanoalkyl denotes an alkyl group substituted with one cyano group. Examples of “cyanoalkyl” include NCCH 2 and NCCH 2 CH 2 (alternatively identified as CH 2 CH 2 CN).
- Niroalkyl denotes an alkyl group substituted with one nitro group.
- nitroalkyl examples include (NO 2 )CH 2 and (NO 2 )CH 2 CH 2 (alternatively identified as CH 2 CH 2 (NO 2 )).
- Alkylamino includes an NH radical substituted with straight-chain or branched alkyl. Examples of “alkylamino” include CH 3 CH 2 NH, CH 3 CH 2 CH 2 NH, and (CH 3 ) 2 CHCH 2 NH. Examples of “dialkylamino” include (CH 3 ) 2 N, (CH 3 CH 2 CH 2 ) 2 N and CH 3 CH 2 (CH 3 )N.
- Cycloalkyl includes, for example, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
- cycloalkylalkyl denotes cycloalkyl substitution on an alkyl moiety. Examples of “cycloalkylalkyl” include cyclopropylmethyl, cyclopentylethyl, and other cycloalkyl moieties bonded to straight-chain or branched alkyl groups.
- alkylcycloalkyl donotes an alkyl group bonded to a cycloalkyl moiety.
- halogen either alone or in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” includes fluorine, chlorine, bromine or iodine. Further, when used in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” said alkyl may be partially or fully substituted with halogen atoms which may be the same or different. Examples of “haloalkyl” or “alkyl substituted with halogen” include F 3 C, ClCH 2 , CF 3 CH 2 and CF 3 CCl 2 .
- haloalkoxy examples include CF 3 O- , CCl 3 CH 2 O-, HCF 2 CH 2 CH 2 O- and CF 3 CH 2 O-.
- haloalkoxyalkyl examples include CF 3 OCH 2 -, CCl 3 CH 2 OCH 2 -, HCF 2 CH 2 CH 2 OCH 2 - and CF 3 CH 2 OCH 2 -.
- haloalkylthio examples include CCl 3 S-, CF 3 S-, CCl 3 CH 2 S- and ClCH 2 CH 2 CH 2 S-.
- haloalkynyl examples include HC ⁇ CCHCl-, CF 3 C ⁇ C-, CCl 3 C ⁇ C- and FCH 2 C ⁇ CCH 2 -.
- alkoxycarbonylalkyl denotes a straight-chain or branched alkoxycarbonyl moiety bonded through an alkyl moiety.
- alkylcarbonylalkyl denotes a straight or branched alkylcarbonyl moiety bonded through an alkyl moiety.
- alkanediyl or alkenediyl refers to a linear or branched alkane or alkene linking chain respectively.
- alkanediyl include —CH 2 –, –CH 2 CH(CH 3 )– and –CH 2 CH 2 CH 2 –.
- the term “adjacent” in the context of locating a substituent means “next to” or “immediately next to”.
- the total number of carbon atoms in a substituent group is indicated by the “C i –C j ” prefix where i and j are numbers from 1 to 8.
- C 1 –C 4 alkylsulfonyl designates methylsulfonyl through butylsulfonyl
- C 3 –C 8 alkylcarbonylalkyl can be, for example, CH 3 COCH 2 -, CH 3 COCH 2 CH 2 - or CH 3 CH 2 CH 2 COCH 2 CH 2 CH 2 CH 2 -
- C 4 –C 7 alkylcycloalkyl can be, for example, methylcyclopropyl, methylcyclobutyl, ethylcyclopropyl, or propylcyclobutyl
- C 2 alkoxyalkyl designates CH 3 OCH 2 -
- C 3 alkoxyalkyl designates, for example, CH 3 CH(OCH 3 )-, CH 3 OCH 2 CH 2 - or CH 3 CH 2 OCH 2 -
- C 4 alkoxyalkyl designates the various isomers of an alkyl group substituted with an alkoxy group containing a total of four
- ring system denotes two or more fused rings.
- bicyclic ring system denotes a ring system consisting of two fused rings.
- Stereoisomers are isomers of identical constitution but differing in the arrangement of their atoms in space and include enantiomers, diastereomers, cis-trans isomers (also known as geometric isomers) and atropisomers. Atropisomers result from restricted rotation about single bonds where the rotational barrier is high enough to permit isolation of the isomeric species.
- one stereoisomer may be more active and/or may exhibit beneficial effects when enriched relative to the other stereoisomer(s) or when separated from the other stereoisomer(s).
- the compounds of the invention may be present as a mixture of stereoisomers, individual stereoisomers or as an optically active form.
- Compounds of Formula 1 typically exist in more than one form, and Formula 1 thus include all crystalline and non-crystalline forms of the compounds they represent.
- Non- crystalline forms include embodiments which are solids such as waxes and gums as well as embodiments which are liquids such as solutions and melts.
- Crystalline forms include embodiments which represent essentially a single crystal type and embodiments which represent a mixture of polymorphs (i.e. different crystalline types).
- polymorph refers to a particular crystalline form of a chemical compound that can crystallize in different crystalline forms, these forms having different arrangements and/or conformations of the molecules in the crystal lattice. Although polymorphs can have the same chemical composition, they can also differ in composition due to the presence or absence of co- crystallized water or other molecules, which can be weakly or strongly bound in the lattice. Polymorphs can differ in such chemical, physical and biological properties as crystal shape, density, hardness, color, chemical stability, melting point, hygroscopicity, suspensibility, dissolution rate and biological availability.
- a polymorph of a compound of Formula 1 can exhibit beneficial effects (e.g., suitability for preparation of useful formulations, improved biological performance) relative to another polymorph or a mixture of polymorphs of the same compound of Formula 1.
- Preparation and isolation of a particular polymorph of a compound of Formula 1 can be achieved by methods known to those skilled in the art including, for example, crystallization using selected solvents and temperatures.
- crystallization using selected solvents and temperatures.
- nitrogen-containing heterocycles can form N-oxides since the nitrogen requires an available lone pair for oxidation to the oxide; one skilled in the art will recognize those nitrogen-containing heterocycles which can form N-oxides.
- nitrogen-containing heterocycles which can form N-oxides.
- tertiary amines can form N-oxides.
- N-oxides of heterocycles and tertiary amines are very well known by one skilled in the art including the oxidation of heterocycles and tertiary amines with peroxy acids such as peracetic and m-chloroperbenzoic acid (MCPBA), hydrogen peroxide, alkyl hydroperoxides such as t-butyl hydroperoxide, sodium perborate, and dioxiranes such as dimethyldioxirane.
- MCPBA peroxy acids
- alkyl hydroperoxides such as t-butyl hydroperoxide
- sodium perborate sodium perborate
- dioxiranes such as dimethyldioxirane
- salts of chemical compounds are in equilibrium with their corresponding nonsalt forms, salts share the biological utility of the nonsalt forms.
- salts of a compound of Formula 1 are useful for control of undesired vegetation (i.e. are agriculturally suitable).
- the salts of a compound of Formula 1 include acid-addition salts with inorganic or organic acids such as hydrobromic, hydrochloric, nitric, phosphoric, sulfuric, acetic, butyric, fumaric, lactic, maleic, malonic, oxalic, propionic, salicylic, tartaric, 4-toluenesulfonic or valeric acids.
- salts also include those formed with organic or inorganic bases such as pyridine, triethylamine or ammonia, or amides, hydrides, hydroxides or carbonates of sodium, potassium, lithium, calcium, magnesium or barium.
- the present invention comprises compounds selected from Formula 1, N-oxides and agriculturally suitable salts thereof.
- a wide variety of synthetic methods are known in the art to enable preparation of aromatic and nonaromatic heterocyclic rings and ring systems; for extensive reviews see the eight volume set of Comprehensive Heterocyclic Chemistry, A. R. Katritzky and C. W. Rees editors-in-chief, Pergamon Press, Oxford, 1984 and the twelve volume set of Comprehensive Heterocyclic Chemistry II, A. R. Katritzky, C. W. Rees and E. F. V. Scriven editors-in-chief, Pergamon Press, Oxford, 1996.
- Embodiments of the present invention as described in the Summary of the Invention include those described below.
- Formula 1 includes stereoisomers, N-oxides and salts thereof, and reference to “a compound of Formula 1” includes the definitions of substituents specified in the Summary of the Invention unless further defined in the Embodiments.
- Embodiment 1. A compound of Formula 1, stereoisomers, N-oxides, and salts thereof, agricultural compositions containing them and their use as herbicides as described in the Summary of the Invention.
- Embodiment 2. A compound of Formula 1 or Embodiment 1 wherein J is J-1, J-2, J-3, J-5, J-6, J-8,J-9 or J-10.
- Embodiment 2a A compound of Formula 1 or Embodiment 2 wherein J is J-1, J-2 or J-3.
- Embodiment 2b A compound of Embodiment 2a wherein J is J-1.
- Embodiment 2c A compound of Embodiment 2a wherein J is J-2.
- Embodiment 2d A compound of Embodiment 2a wherein J is J-3.
- Embodiment 2e A compound of Formula 1 or Embodiment 2 wherein J is J-5, J-6 or J-8.
- Embodiment 2f A compound of Embodiment 2e wherein J is J-5.
- Embodiment 2g A compound of Embodiment 2e wherein J is J-6.
- Embodiment 2h A compound of Embodiment 2e wherein J is J-8.
- Embodiment 3a A compound of Embodiment 3 wherein G is G-1, G-2, G-3 or G-7.
- Embodiment 3aa A compound of Embodiment 3a wherein G is G-1, G-2 or G-3.
- Embodiment 3b A compound of Embodiment 3a where G is G-1 wherein W, X, and Y are independently N or CR 6 .
- Embodiment 3c A compound of Embodiment 3b wherein X is N, Y is CR 6 and W is CR 6 .
- Embodiment 3d A compound of Embodiment 3d.
- Embodiment 3b wherein X is CR 6 , Y is N and W is CR 6 .
- Embodiment 3dd A compound of Embodiment 3b wherein X is CR 6 , Y is CR 6 and W is N.
- Embodiment 3e A compound of Embodiment 3b wherein X is CR 6 , Y is CR 6 and W is CR 6 .
- Embodiment 3f A compound of Embodiment 3b wherein X is N, Y is N and W is CR 6 .
- Embodiment 3g A compound of Embodiment 3 wherein G is G-2.
- Embodiment 3h A compound of Embodiment 3 wherein G is G-3.
- Embodiment 3i A compound of Embodiment 3 wherein G is G-4.
- Embodiment 3j A compound of Embodiment 3 wherein G is G-7.
- Embodiment 4. A compound of Formula 1 or any one of the preceding Embodiments wherein R 1 is halogen, C 1 –C 4 haloalkyl, C 1 –C 4 haloalkoxy, C 1 –C 4 alkoxy or C 1 –C 4 haloalkylthio.
- Embodiment 4a A compound of Embodiment 4 wherein R 1 is C 1 –C 4 haloalkyl or C 1 – C 4 haloalkoxy.
- Embodiment 4b A compound of Embodiment 4 wherein R 1 is C 1 –C 4 haloalkyl or C 1 – C 4 haloalkoxy.
- Embodiment 4a wherein OCF 3 .
- Embodiment 4c A compound of Embodiment 4a wherein haloalkyl.
- Embodiment 4d A compound of Embodiment 4c wherein Embodiment 4e.
- Embodiment 4a wherein haloalkoxy.
- Embodiment 4f A compound of Embodiment 4e wherein Embodiment 5.
- a compound of Formula 1 or any one of the preceding Embodiments wherein R 2 is H.
- Embodiment 5a A compound of Formula 1 or any one of the preceding Embodiments wherein R 2 is halogen.
- Embodiment 6a A compound of Embodiment 6 wherein R3 is C 1 –C 5 alkyl, halogen, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C 1 –C 5 haloalkoxy.
- Embodiment 6b A compound of Formula 1 or any one of the preceding Embodiments wherein R 3 is C 1 –C 5 alkyl, halogen, C 3 –C 7 cycloalkyl, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C 1 –C 5 haloalkoxy.
- Embodiment 6a wherein R 3 is halogen, C 1 –C 5 haloalkyl or C 1 –C 5 haloalkoxy.
- Embodiment 6c A compound of Embodiment 6b wherein R 3 is halogen or C 1 –C 5 haloalkyl.
- Embodiment 6d A compound of Embodiment 6c wherein R 3 is halogen.
- Embodiment 6e A compound of Embodiment 6c wherein R 3 is C 1 –C 5 haloalkyl.
- Embodiment 6f A compound of Embodiment 6c wherein R 3 is F, Cl or CF 3 .
- Embodiment 6g A compound of Embodiment 6g.
- Embodiment 7. A compound of Formula 1 or any one of the preceding Embodiments w herein R4 is H, C 1 –C 5 alkyl, halogen, -CN, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C 1 –C 5 alkylthio.
- Embodiment 7b A compound of Embodiment 7a wherein R 4 is H, halogen, -CN or CF 3 .
- Embodiment 7c A compound of Embodiment 7b wherein R 4 is H.
- Embodiment 8a A compound of Embodiment 8 wherein Q is O or -CH 2 -.
- Embodiment 8b A compound of Embodiment 8a wherein Q is O.
- Embodiment 8c A compound of Embodiment 8a wherein Q is -CH 2 -.
- Embodiment 8d A compound of Embodiment 8a wherein Q is -CH 2 -.
- Embodiment 9 A compound of Formula 1 or any one of the preceding Embodiments w herein R5 is H, C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C 3 –C 7 cycloalkyl, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy, C 1 –C 5 haloalkoxy or C 1 –C 5 alkylthio.
- E mbodiment 9a A compound of Embodiment 9 wherein R5 is H, C 1 –C 5 alkyl or halogen.
- Embodiment 9b A compound of Embodiment 9 wherein R5 is H, C 1 –C 5 alkyl or halogen.
- Embodiment 9a wherein R 5 is H.
- E mbodiment 9c A compound of Embodiment 9a wherein R5 is C 1 –C 3 alkyl.
- Embodiment 9d A compound of Embodiment 9a wherein R 5 is halogen.
- Embodiments of this invention including Embodiments 1–9d above as well as any other embodiments described herein, can be combined in any manner, and the descriptions of variables in the embodiments pertain not only to the compounds of Formula 1 but also to the starting compounds and intermediate compounds useful for preparing the compounds of Formula 1.
- Embodiment X A compound of Formula 1 as described in the Summary of the Invention wherein J is J-1, J-2, J-3, J-5, J-6, J-8, J-9 or J-10; and G is G-1, G-2, G-3 or G-7.
- Embodiment XX A compound of Formula 1 as described in the Summary of the Invention wherein J is J-1, J-2, J-3, J-5, J-6, J-8, J-9 or J-10; and G is G-1, G-2, G-3 or G-7.
- Embodiment X wherein R 1 is C 1 –C 4 haloalkyl or C 1 –C 4 haloalkoxy; R 3 is halogen or C 1 –C 5 haloalkyl; R 4 is H; and Q is O or -CH 2 -.
- Embodiment XXX A compound of Embodiment XX wherein Q is O.
- Embodiment A1. A compound of Embodiment X wherein J is J-1, J-2 or J-3; R 1 is CF 3 or OCF 3 ; and R 3 is F, Cl or CF 3 .
- Embodiment A2. A compound of Embodiment A1 wherein J is J-1; and R 2 is H or halogen.
- Embodiment A3 A compound of Embodiment A2 wherein R 2 is H. Embodiment A4. A compound of Embodiment A1 wherein J is J-2; and R 1 is CF 3 . Embodiment A5. A compound of Embodiment A1 wherein J is J-3; and R 1 is CF 3 .
- Embodiment A6 A compound of Embodiment X wherein G is G-1, G-2 or G-3.
- Embodiment A7 A compound of Embodiment A6 wherein G is G-1; X is N; Y is CH; W is CH; and R 5 is CF 3 .
- Embodiment A8 A compound of Embodiment A6 wherein G is G-2; and R 3 is CF 3 .
- Embodiment A11 A compound of Embodiment A6 wherein G is G-3; and R 3 is CF 3 .
- Embodiment B1. A compound of Embodiment X wherein J is J-5, J-6 or J-8; R 1 is CF 3 or OCF 3 ; and R 3 is F, Cl, CF 3 .
- Embodiment B2. A compound of Embodiment B1 wherein J is J-5.
- Embodiment B4. A compound of Embodiment B1 wherein J is J-8.
- Embodiment C A compound of Summary of the Invention wherein Q is O..
- Specific embodiments include a compound of Formula 1 selected from the group consisting of: 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 1) 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[4-(trifluoromethyl)phenyl]pyrimidine; (Compound 5) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-pyrazol-1-yl]-4-[[2-(trifluoromethyl)-4- pyridinyl]oxy]pyrimidine; (Compound 9) 5-cyclopropyl-4-[3-(trifluoromethoxy)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 10) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-
- This invention also relates to a method for controlling undesired vegetation comprising applying to the locus of the vegetation herbicidally effective amounts of the compounds of the invention (e.g., as a composition described herein).
- the compounds of the invention e.g., as a composition described herein.
- embodiments relating to methods of use are those involving the compounds of embodiments described above.
- Compounds of the invention are particularly useful for selective control of weeds in crops such as wheat, barley, maize, soybean, sunflower, cotton, oilseed rape and rice, and specialty crops such as sugarcane, citrus, fruit and nut crops.
- herbicidal compositions of the present invention comprising the compounds of embodiments described above.
- This invention also includes a herbicidal mixture comprising (a) a compound selected from Formula 1, N-oxides, and salts thereof, and (b) at least one additional active ingredient selected from (b1) photosystem II inhibitors, (b2) acetohydroxy acid synthase (AHAS) inhibitors, (b3) acetyl-CoA carboxylase (ACCase) inhibitors, (b4) auxin mimics, (b5) 5-enol- pyruvylshikimate-3-phosphate (EPSP) synthase inhibitors, (b6) photosystem I electron diverters, (b7) protoporphyrinogen oxidase (PPO) inhibitors, (b8) glutamine synthetase (GS) inhibitors, (b9) very long chain fatty acid (VLCFA) elongase inhibitors, (b10) auxin transport inhibitors, (b11) phytoene desaturase (PDS) inhibitors, (b12) 4-hydroxyphenyl-pyruvate dioxygena
- Photosystem II inhibitors are chemical compounds that bind to the D-1 protein at the Q B -binding niche and thus block electron transport from Q A to Q B in the chloroplast thylakoid membranes. The electrons blocked from passing through photosystem II are transferred through a series of reactions to form toxic compounds that disrupt cell membranes and cause chloroplast swelling, membrane leakage, and ultimately cellular destruction.
- the Q B -binding niche has three different binding sites: binding site A binds the triazines such as atrazine, triazinones such as hexazinone, and uracils such as bromacil, binding site B binds the phenylureas such as diuron, and binding site C binds benzothiadiazoles such as bentazon, nitriles such as bromoxynil and phenyl-pyridazines such as pyridate.
- triazines such as atrazine
- triazinones such as hexazinone
- uracils such as bromacil
- binding site B binds the phenylureas such as diuron
- binding site C binds benzothiadiazoles such as bentazon, nitriles such as bromoxynil and phenyl-pyridazines such as pyridate.
- photosystem II inhibitors include ametryn, amicarbazone, atrazine, bentazon, bromacil, bromofenoxim, bromoxynil, chlorbromuron, chloridazon, chlorotoluron, chloroxuron, cumyluron, cyanazine, daimuron, desmedipham, desmetryn, dimefuron, dimethametryn, diuron, ethidimuron, fenuron, fluometuron, hexazinone, ioxynil, isoproturon, isouron, lenacil, linuron, metamitron, methabenzthiazuron, metobromuron, metoxuron, metribuzin, monolinuron, neburon, pentanochlor, phenmedipham, prometon, prometryn, propanil, propazine, pyridafol, pyridate, siduron, simazine, simetryn,
- AHAS inhibitors are chemical compounds that inhibit acetohydroxy acid synthase (AHAS), also known as acetolactate synthase (ALS), and thus kill plants by inhibiting the production of the branched-chain aliphatic amino acids such as valine, leucine and isoleucine, which are required for protein synthesis and cell growth.
- AHAS acetohydroxy acid synthase
- ALS acetolactate synthase
- AHAS inhibitors include amidosulfuron, azimsulfuron, bensulfuron-methyl, bispyribac-sodium, cloransulam-methyl, chlorimuron-ethyl, chlorsulfuron, cinosulfuron, cyclosulfamuron, diclosulam, ethametsulfuron-methyl, ethoxysulfuron, flazasulfuron, florasulam, flucarbazone-sodium, flumetsulam, flupyrsulfuron-methyl, flupyrsulfuron-sodium, foramsulfuron, halosulfuron-methyl, imazamethabenz-methyl, imazamox, imazapic, imazapyr, imazaquin, imazethapyr, imazosulfuron, iodosulfuron-methyl (including sodium salt), iofensulfuron (2-iodo-N-[[(4-methoxy
- ACCase inhibitors are chemical compounds that inhibit the acetyl-CoA carboxylase enzyme, which is responsible for catalyzing an early step in lipid and fatty acid synthesis in plants. Lipids are essential components of cell membranes, and without them, new cells cannot be produced. The inhibition of acetyl CoA carboxylase and the subsequent lack of lipid production leads to losses in cell membrane integrity, especially in regions of active growth such as meristems. Eventually shoot and rhizome growth ceases, and shoot meristems and rhizome buds begin to die back.
- ACCase inhibitors include alloxydim, butroxydim, clethodim, clodinafop, cycloxydim, cyhalofop, diclofop, fenoxaprop, fluazifop, haloxyfop, pinoxaden, profoxydim, propaquizafop, quizalofop, sethoxydim, tepraloxydim and tralkoxydim, including resolved forms such as fenoxaprop-P, fluazifop-P, haloxyfop-P and quizalofop-P and ester forms such as clodinafop-propargyl, cyhalofop-butyl, diclofop-methyl and fenoxaprop-P-ethyl.
- auxin is a plant hormone that regulates growth in many plant tissues.
- auxin mimics are chemical compounds mimicking the plant growth hormone auxin, thus causing uncontrolled and disorganized growth leading to plant death in susceptible species.
- auxin mimics include aminocyclopyrachlor (6-amino-5-chloro-2-cyclopropyl-4- pyrimidinecarboxylic acid) and its methyl and ethyl esters and its sodium and potassium salts, 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)-2-Pyridinecarboxylic 2-propyn-1-yl ester (CAS No.
- EPSP synthase inhibitors are chemical compounds that inhibit the enzyme, 5-enol-pyruvylshikimate-3-phosphate synthase, which is involved in the synthesis of aromatic amino acids such as tyrosine, tryptophan and phenylalanine.
- EPSP inhibitor herbicides are readily absorbed through plant foliage and translocated in the phloem to the growing points.
- Glyphosate is a relatively nonselective postemergence herbicide that belongs to this group. Glyphosate includes esters and salts such as ammonium, isopropylammonium, potassium, sodium (including sesquisodium) and trimesium (alternatively named sulfosate).
- Photosystem I electron diverters are chemical compounds that accept electrons from Photosystem I, and after several cycles, generate hydroxyl radicals. These radicals are extremely reactive and readily destroy unsaturated lipids, including membrane fatty acids and chlorophyll. This destroys cell membrane integrity, so that cells and organelles “leak”, leading to rapid leaf wilting and desiccation, and eventually to plant death. Examples of this second type of photosynthesis inhibitor include diquat, paraquat and 1-(2-carboxyethyl)-4-(2- pyrimidinyl)pyridazinium (CAS No.2285384-11-2).
- PPO inhibitors are chemical compounds that inhibit the enzyme protoporphyrinogen oxidase, quickly resulting in formation of highly reactive compounds in plants that rupture cell membranes, causing cell fluids to leak out.
- PPO inhibitors include acifluorfen-sodium, azafenidin, benzfendizone, bifenox, butafenacil, carfentrazone, carfentrazone-ethyl, chlomethoxyfen, 3-[2-chloro-5-[3,6-dihydro-3-methyl-2,6-dioxo-4- (trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluorophenyl]-4,5-dihydro-5-methyl-5- Isoxazolecarboxylic ethyl ester (CAS No.
- GS inhibitors are chemical compounds that inhibit the activity of the glutamine synthetase enzyme, which plants use to convert ammonia into glutamine. Consequently, ammonia accumulates and glutamine levels decrease. Plant damage probably occurs due to the combined effects of ammonia toxicity and deficiency of amino acids required for other metabolic processes.
- the GS inhibitors include glufosinate and its esters and salts such as glufosinate-ammonium and other phosphinothricin derivatives, glufosinate-P ((2S)-2-amino- 4-(hydroxymethylphosphinyl)butanoic acid) and bilanaphos.
- VLCFA elongase inhibitors are herbicides having a wide variety of chemical structures, which inhibit the elongase.
- Elongase is one of the enzymes located in or near chloroplasts which are involved in biosynthesis of VLCFAs.
- very-long-chain fatty acids are the main constituents of hydrophobic polymers that prevent desiccation at the leaf surface and provide stability to pollen grains.
- Such herbicides include acetochlor, alachlor, anilofos, butachlor, cafenstrole, dimethachlor, dimethenamid, diphenamid, fenoxasulfone (3- [[(2,5-dichloro-4-ethoxyphenyl)methyl]sulfonyl]-4,5-dihydro-5,5-dimethylisoxazole), fentrazamide, flufenacet, indanofan, mefenacet, metazachlor, metolachlor, naproanilide, napropamide, napropamide-M ((2R)-N,N-diethyl-2-(1-naphthalenyloxy)propanamide), pethoxamid, piperophos, pretilachlor, propachlor, propisochlor, pyroxasulfone, and thenylchlor, including resolved forms such as S-metolachlor and chloroacetamides and oxyace
- auxin transport inhibitors are chemical substances that inhibit auxin transport in plants, such as by binding with an auxin-carrier protein.
- auxin transport inhibitors include diflufenzopyr, naptalam (also known as N-(1-naphthyl)phthalamic acid and 2-[(1-naphthalenylamino)carbonyl]benzoic acid).
- PDS inhibitors are chemical compounds that inhibit carotenoid biosynthesis pathway at the phytoene desaturase step. Examples of PDS inhibitors include beflubutamid, diflufenican, fluridone, flurochloridone, flurtamone norflurzon and picolinafen.
- HPPD inhibitors are chemical substances that inhibit the biosynthesis of synthesis of 4-hydroxyphenyl-pyruvate dioxygenase.
- HPPD inhibitors include benzobicyclon, benzofenap, bicyclopyrone (4-hydroxy-3-[[2-[(2-methoxyethoxy)methyl]-6- (trifluoromethyl)-3-pyridinyl]carbonyl]bicyclo[3.2.1]oct-3-en-2-one), fenquinotrione (2-[[8- chloro-3,4-dihydro-4-(4-methoxyphenyl)-3-oxo-2-quinoxalinyl]carbonyl]-1,3- cyclohexanedione), flusulfinam, iptriazopyrid, isoxachlortole, isoxaflutole, mesotrione, pyrasulfotole, pyrazolynate, pyrazoxyfen, sulcotrion
- HST homogentisate solanesyltransferase inhibitors
- HST inhibitors include cyclopyrimorate (6-chloro-3-(2- cyclopropyl-6-methylphenoxy)-4-pyridazinyl 4-morpholinecarboxylate), haloxydine, pyriclor, 3-(2-chloro-3,6-difluorophenyl)-4-hydroxy-1-methyl-1,5-naphthyridin-2(1H)-one, 7-(3,5-dichloro-4-pyridinyl)-5-(2,2-difluoroethyl)-8-hydroxypyrido[2,3-b]pyrazin-6(5H)-one and 4-(2,6-diethyl-4-methylphenyl)-5-hydroxy-2,6-dimethyl-3(2H)-pyridazinone.
- cyclopyrimorate 6-chloro-3-(2- cyclopropyl-6-methylphenoxy)-4-pyridazinyl 4-morpholinecarboxylate
- Cellulose biosynthesis inhibitors inhibit the biosynthesis of cellulose in certain plants. They are most effective when applied preemergence or early postemergence on young or rapidly growing plants. Examples of cellulose biosynthesis inhibitors include chlorthiamid, dichlobenil, flupoxam, indaziflam (N 2 -[(1R,2S)-2,3-dihydro-2,6-dimethyl-1H-inden-1-yl]-6- (1-fluoroethyl)-1,3,5-triazine-2,4-diamine), isoxaben and triaziflam.
- DHODH (dihydroorotate dehydrogenase) inhibitors act through inhibiting catalysis of the fourth step of pyrimidine biosynthesis in plant systems. Inhibition of pyrimidine biosynthesis leads to the cessation of plant growth.
- DOHDH inhibitors examples include tetflupyrolimet ((3S,4S)-N-(2-fluorophenyl)-1-methyl-2-oxo-4-[3- (trifluoromethyl)phenyl]-3-pyrrolidinecarboxamide) and (3S,4R)-N-(2,3-difluorophenyl)-1- methyl-4-[1-methyl-5-(trifluoromethyl)-1H-pyrazol-3-yl]-2-oxo-3-pyrrolidinecaboxamide.
- “Other herbicides” include herbicides that act through a variety of different modes of action such as mitotic disruptors (e.g., flamprop-M-methyl and flamprop-M-isopropyl), organic arsenicals (e.g., DSMA, and MSMA), 7,8-dihydropteroate synthase inhibitors, chloroplast isoprenoid synthesis inhibitors and cell-wall biosynthesis inhibitors.
- Other herbicides include those herbicides having unknown modes of action or do not fall into a specific category listed in (b1) through (b14) or act through a combination of modes of action listed above.
- herbicides examples include aclonifen, asulam, amitrole, bixlozone, broclozone, bromobutide, cinmethylin, clomazone, cumyluron, daimuron, difenzoquat, dimesulfazet, epyrifenacil, etobenzanid, fluometuron, flurenol, fosamine, fosamine-ammonium, dazomet, dymron, ipfencarbazone (1-(2,4-dichlorophenyl)-N-(2,4- difluorophenyl)-1,5-dihydro-N-(1-methylethyl)-5-oxo-4H-1,2,4-triazole-4-carboxamide), metam, methyldymron, oleic acid, oxaziclomefone, pelargonic acid, pyributicarb, 2,5- anhydro-3,4-dide
- “Other herbicides” also include a compound of Formula (b16A) wherein R 12 is H, C 1 –C 6 alkyl, C 1 –C 6 haloalkyl or C 4 –C 8 cycloalkyl; R 13 is H, C 1 –C 6 alkyl or C 1 –C 6 alkoxy; Q 1 is an optionally substituted ring system selected from the group consisting of phenyl, thienyl, pyridinyl, benzodioxolyl, naphthyl, naphthalenyl, benzofuranyl, furanyl, benzothiophenyl and pyrazolyl, wherein when substituted said ring system is substituted by 1 to 3 R 14
- R 12 is H or C 1 –C 6 alkyl; more preferably R 12 is H or methyl.
- R 13 is H.
- Q 1 is either a phenyl ring or a pyridinyl ring, each ring substituted by 1 to 3 R 14 ; more preferably Q 1 is a phenyl ring substituted by 1 to 2 R 14 .
- Q 2 is a phenyl ring substituted by 1 to 3 R 15 ; more preferably Q 2 is a phenyl ring substituted by 1 to 2 R 15 .
- each R 14 is independently halogen, C 1 –C 4 alkyl, C 1 –C 3 haloalkyl, C 1 –C 3 alkoxy or C 1 –C 3 haloalkoxy; more preferably each R 14 is independently chloro, fluoro, bromo, C 1 –C 2 haloalkyl, C 1 –C 2 haloalkoxy or C 1 –C 2 alkoxy.
- each R 15 is independently halogen, C 1 –C 4 alkyl, C 1 –C 3 haloalkoxy; more preferably each R 15 is independently chloro, fluoro, bromo, C 1 –C 2 haloalkyl, C 1 –C 2 haloalkoxy or C 1 –C 2 alkoxy.
- other herbicides include any one of the following (b16A-1) through (b16A-15):
- “Other herbicides” also include a compound of Formula (b16B) wherein R 18 is H, C 1 –C 6 alkyl, C 1 –C 6 haloalkyl or C 4 –C 8 cycloalkyl; each R 19 is independently halogen, C 1 –C 6 haloalkyl or C 1 –C 6 haloalkoxy; p is an integer of 0, 1, 2 or 3; each R 20 is independently halogen, C 1 –C 6 haloalkyl or C 1 –C 6 haloalkoxy; and q is an integer of 0, 1, 2 or 3.
- R 18 is H, methyl, ethyl or propyl; more preferably R 18 is H or methyl; most preferably R 18 is H.
- each R 19 is independently chloro, fluoro, C 1 – C 3 haloalkyl or C 1 –C 3 haloalkoxy; more preferably each R 19 is independently chloro, fluoro, C 1 fluoroalkyl (i.e. fluoromethyl, difluoromethyl or trifluoromethyl) or C 1 fluoroalkoxy (i.e. trifluoromethoxy, difluoromethoxy or fluoromethoxy).
- each R 20 is independently chloro, fluoro, C 1 haloalkyl or C 1 haloalkoxy; more preferably each R 20 is independently chloro, fluoro, C 1 fluoroalkyl (i.e. fluoromethyl, difluorormethyl or trifluromethyl) or C 1 fluoroalkoxy (i.e. trifluoromethoxy, difluoromethoxy or fluoromethoxy).
- other herbicides include any one of the following (b15B-1) through (b16B-19):
- herbicide safeners are substances added to a herbicide formulation to eliminate or reduce phytotoxic effects of the herbicide to certain crops. These compounds protect crops from injury by herbicides but typically do not prevent the herbicide from controlling undesired vegetation.
- herbicide safeners include but are not limited to benoxacor, cloquintocet-mexyl, cumyluron, cyometrinil, cyprosulfamide, daimuron, dichlormid, dicyclonon, dietholate, dimepiperate, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr-diethyl, mephenate, methoxyphenone, naphthalic anhydride, oxabetrinil, N-(aminocarbonyl)-2-methylbenzenesulfonamide and N- (aminocarbonyl)-2-fluorobenzenesulfonamide, 1-bromo-4-[(chloromethyl)sulfonyl]benzene, 2-(dichloromethyl)-2-methyl-1,3-dioxolane (MG 19
- Preferred for better control of undesired vegetation e.g., lower use rate such as from greater-than-additive effects, broader spectrum of weeds controlled, or enhanced crop safety
- a herbicide selected from the group consisting of 4-amino-3-chloro-5-fluoro- 6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic 2-propyn-1-yl ester (CAS No.2251111-17- 6), 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic cyanomethyl ester (CAS No.2251111-18-7), 2,5-anhydro-3,4-dideoxy-4-[[[(5S)-3-(3,5-difluorophenyl)-5- ethenyl-4,5-dihydr
- Reacting a cyclopropyl dihalopyrimidine of Formula 2 (wherein X is a halogen as leaving group) with an appropriately substituted phenol or hydroxy heterocycle of Formula J-OH, in the presence of a suitable base and solvent, can provide intermediates of Formula 3.
- suitable bases for this reaction include but are not limited to potassium carbonate, sodium hydroxide, potassium t-butoxide, sodium hydride or potassium hydroxide, and compatible solvents include but not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide.
- Temperatures for this reaction generally range from 0° C to warming below or heating at the boiling point of the solvent.
- halogen X at the 4-position of a pyrimidine of Formula 2 is generally favored as the leaving group over the halogen X at the 2-position, one skilled in the art will recognize that some regioisomeric side-product of intermediate 3 can form from displacement of the halogen at the 2-position and side-adduct may require separation from 3 by chromatography or recrystallization.
- the halogen substituent X can be chlorine, bromine or in some cases iodine.
- Preferable palladium catalysts include but are not limited to the commercially available chloro[2-(di-tert-butylphosphino)- 2′,4′,6′-triisopropyl-1,1′-biphenyl][2-(2-aminoethyl)phenyl)] palladium(II) [t-BuXPhos palladium(II) phenethylamine chloride, tBuXPhos-Pd-G1], chloro[(tri-tert-butylphosphine)- 2-(2-aminobiphenyl)] palladium(II) [P(t-Bu)3 Pd G2], methanesulfonato(2-di-t- butylphosphino-2;methanesulfonato(2-di-t-butylphosphino-2',4',
- halogen X at the 4-position of a pyrimidine of Formula 2 is usually favored as the preferred leaving group over the halogen X at the 2-position
- some regioisomeric side-product of intermediate 4 can form from displacement of the halogen at the 2-position. Separation by chromatography or recrystallization may be needed to isolate the desired isomers.
- Reagents of Formula J-CH 2 -ZnX 1 are readily generated from commercially available benzyl and heteroarylmethyl halides by reacting with an appropriate zinc reagent (i.e.
- the halogen substituent X can be chlorine, bromine or in some cases iodine.
- compounds of Formula 1b can also be made without use of a palladium catalyst, instead by reacting an intermediate of Formula 4 with an azole of Formula (G-1)H in the presence of a base such as potassium carbonate, sodium hydride or sodium hydroxide in a compatible solvent for the particular base used.
- a base such as potassium carbonate, sodium hydride or sodium hydroxide
- solvents include but are not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide.
- Temperatures for this nucleophilic displacement reaction can range from 0° C to ambient temperature to warming below or heating at the boiling point of the solvent.
- a base preferably potassium carbonate, sodium hydride or sodium hydroxide
- a compatible solvent i.e. acetonitrile, toluene, dioxane or N,N-dimethylformamide
- the methyl sulfone substituent is now an activated leaving group, where upon reacting 7 with phenols and hydroxyheterocycles of Formula J-OH, in the presence of base and solvent (e.g. potassium carbonate in N,N-dimethylformamide) at ambient temperature to warming below the reflux temperature of the solvent, gives final products of Formula 1c.
- base and solvent e.g. potassium carbonate in N,N-dimethylformamide
- Heating the compound of Formula 10 in phosphorous oxychloride (and in some cases with thionyl chloride or oxalyl chloride, optionally with a cosolvent such as toluene or dichloromethane) provides chloropyrimidines of Formula 11.
- Reacting a compound of Formula 11 with an appropriately substituted phenol or hydroxy heterocycle of Formula J- OH, in the presence of a suitable base and solvent, can provide final products of Formula 1d.
- suitable bases for this reaction include but are not limited to potassium carbonate, sodium hydroxide, potassium t-butoxide, sodium hydride or potassium hydroxide; and compatible solvents include but not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide. Temperatures for this reaction generally range from 0° C to warming below or heating at the boiling point of the solvent.
- intermediates for the preparation of compounds of Formula 1 may contain aromatic nitro groups, which can be reduced to amino groups, and then be converted via reactions well known in the art such as the Sandmeyer reaction, to various halides, providing compounds of Formula 1.
- the above reactions can also in many cases be performed in alternate order. It is recognized that some reagents and reaction conditions described above for preparing compounds of Formula 1 may not be compatible with certain functionalities present in the intermediates. In these instances, the incorporation of protection/deprotection sequences or functional group interconversions into the synthesis will aid in obtaining the desired products.
- the use and choice of the protecting groups will be apparent to one skilled in chemical synthesis (see, for example, Greene, T. W.; Wuts, P. G. M.
- step A of Synthesis Example 1 0.25 g, 0.79 mmol
- 4-(trifluoromethyl)phenylboronic acid (0.18 g, 0.95 mmol
- potassium phosphate tribasic (0.20 g, 0.95 mmol
- Xphos Pd G2 0.063 g, 0.079 mmol
- Dioxane (3.97 mL) was added and the vial was purged twice more with N 2 and heated at 70 °C overnight.
- a compound of this invention will generally be used as a herbicidal active ingredient in a composition, i.e. formulation, with at least one additional component selected from the group consisting of surfactants, solid diluents and liquid diluents, which serves as a carrier.
- the formulation or composition ingredients are selected to be consistent with the physical properties of the active ingredient, mode of application and environmental factors such as soil type, moisture and temperature.
- Useful formulations include both liquid and solid compositions.
- Liquid compositions include solutions (including emulsifiable concentrates), suspensions, emulsions (including microemulsions, oil-in -water emulsions, flowable concentrates and/or suspoemulsions) and the like, which optionally can be thickened into gels.
- aqueous liquid compositions are soluble concentrate, suspension concentrate, capsule suspension, concentrated emulsion, microemulsion, oil-in-water emulsion, flowable concentrate and suspo-emulsion.
- the general types of nonaqueous liquid compositions are emulsifiable concentrate, microemulsifiable concentrate, dispersible concentrate and oil dispersion.
- the general types of solid compositions are dusts, powders, granules, pellets, prills, pastilles, tablets, filled films (including seed coatings) and the like, which can be water-dispersible (“wettable”) or water-soluble. Films and coatings formed from film- forming solutions or flowable suspensions are particularly useful for seed treatment.
- Active ingredient can be (micro)encapsulated and further formed into a suspension or solid formulation; alternatively, the entire formulation of active ingredient can be encapsulated (or “overcoated”). Encapsulation can control or delay release of the active ingredient.
- An emulsifiable granule combines the advantages of both an emulsifiable concentrate formulation and a dry granular formulation.
- High-strength compositions are primarily used as intermediates for further formulation.
- Sprayable formulations are typically extended in a suitable medium before spraying. Such liquid and solid formulations are formulated to be readily diluted in the spray medium, usually water, but occasionally another suitable medium like an aromatic or paraffinic hydrocarbon or vegetable oil. Spray volumes can range from about from about one to several thousand liters per hectare, but more typically are in the range from about ten to several hundred liters per hectare.
- Sprayable formulations can be tank mixed with water or another suitable medium for foliar treatment by aerial or ground application, or for application to the growing medium of the plant. Liquid and dry formulations can be metered directly into drip irrigation systems or metered into the furrow during planting. The formulations will typically contain effective amounts of active ingredient, diluent and surfactant within the following approximate ranges which add up to 100 percent by weight.
- Solid diluents include, for example, clays such as bentonite, montmorillonite, attapulgite and kaolin, gypsum, cellulose, titanium dioxide, zinc oxide, starch, dextrin, sugars (e.g., lactose, sucrose), silica, talc, mica, diatomaceous earth, urea, calcium carbonate, sodium carbonate and bicarbonate, and sodium sulfate.
- Typical solid diluents are described in Watkins et al., Handbook of Insecticide Dust Diluents and Carriers, 2nd Ed., Dorland Books, Caldwell, New Jersey.
- Liquid diluents include, for example, water, N,N-dimethylalkanamides (e.g., N,N-dimethylformamide), limonene, dimethyl sulfoxide, N-alkylpyrrolidones (e.g., N-methylpyrrolidinone), alkyl phosphates (e.g., triethyl phosphate), ethylene glycol, triethylene glycol, propylene glycol, dipropylene glycol, polypropylene glycol, propylene carbonate, butylene carbonate, paraffins (e.g., white mineral oils, normal paraffins, isoparaffins), alkylbenzenes, alkylnaphthalenes, glycerine, glycerol triacetate, sorbitol, aromatic hydrocarbons, dearomatized aliphatics, alkylbenzenes, alkylnaphthalenes, ketones such as cyclohexanone
- Liquid diluents also include glycerol esters of saturated and unsaturated fatty acids (typically C 6 –C 22 ), such as plant seed and fruit oils (e.g., oils of olive, castor, linseed, sesame, corn (maize), peanut, sunflower, grapeseed, safflower, cottonseed, soybean, rapeseed, coconut and palm kernel), animal-sourced fats (e.g., beef tallow, pork tallow, lard, cod liver oil, fish oil), and mixtures thereof.
- plant seed and fruit oils e.g., oils of olive, castor, linseed, sesame, corn (maize), peanut, sunflower, grapeseed, safflower, cottonseed, soybean, rapeseed, coconut and palm kernel
- animal-sourced fats e.g., beef tallow, pork tallow, lard, cod liver oil, fish oil
- Liquid diluents also include alkylated fatty acids (e.g., methylated, ethylated, butylated) wherein the fatty acids may be obtained by hydrolysis of glycerol esters from plant and animal sources, and can be purified by distillation. Typical liquid diluents are described in Marsden, Solvents Guide, 2nd Ed., Interscience, New York, 1950.
- the solid and liquid compositions of the present invention often include one or more surfactants. When added to a liquid, surfactants (also known as “surface-active agents”) generally modify, most often reduce, the surface tension of the liquid.
- surfactants can be useful as wetting agents, dispersants, emulsifiers or defoaming agents.
- surfactants can be classified as nonionic, anionic or cationic.
- Nonionic surfactants useful for the present compositions include, but are not limited to: alcohol alkoxylates such as alcohol alkoxylates based on natural and synthetic alcohols (which may be branched or linear) and prepared from the alcohols and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof; amine ethoxylates, alkanolamides and ethoxylated alkanolamides; alkoxylated triglycerides such as ethoxylated soybean, castor and rapeseed oils; alkylphenol alkoxylates such as octylphenol ethoxylates, nonylphenol ethoxylates, dinonyl phenol ethoxylates and dodecyl phenol ethoxylates (prepared from the phenols and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof); block polymers prepared from ethylene oxide or propylene oxide and reverse block polymers where the terminal blocks are prepared from propylene oxide
- Useful anionic surfactants include, but are not limited to: alkylaryl sulfonic acids and their salts; carboxylated alcohol or alkylphenol ethoxylates; diphenyl sulfonate derivatives; lignin and lignin derivatives such as lignosulfonates; maleic or succinic acids or their anhydrides; olefin sulfonates; phosphate esters such as phosphate esters of alcohol alkoxylates, phosphate esters of alkylphenol alkoxylates and phosphate esters of styryl phenol ethoxylates; protein-based surfactants; sarcosine derivatives; styryl phenol ether sulfate; sulfates and sulfonates of oils and fatty acids; sulfates and sulfonates of ethoxylated alkylphenols; sulfates of alcohols; sulfates of e
- Useful cationic surfactants include, but are not limited to: amides and ethoxylated amides; amines such as N-alkyl propanediamines, tripropylenetriamines and dipropylenetetramines, and ethoxylated amines, ethoxylated diamines and propoxylated amines (prepared from the amines and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof); amine salts such as amine acetates and diamine salts; quaternary ammonium salts such as quaternary salts, ethoxylated quaternary salts and diquaternary salts; and amine oxides such as alkyldimethylamine oxides and bis-(2-hydroxyethyl)-alkylamine oxides.
- amines such as N-alkyl propanediamines, tripropylenetriamines and dipropylenetetramines, and ethoxylated amine
- Nonionic, anionic and cationic surfactants and their recommended uses are disclosed in a variety of published references including McCutcheon’s Emulsifiers and Detergents, annual American and International Editions published by McCutcheon’s Division, The Manufacturing Confectioner Publishing Co.; Sisely and Wood, Encyclopedia of Surface Active Agents, Chemical Publ. Co., Inc., New York, 1964; and A. S. Davidson and B. Milwidsky, Synthetic Detergents, Seventh Edition, John Wiley and Sons, New York, 1987.
- compositions of this invention may also contain formulation auxiliaries and additives, known to those skilled in the art as formulation aids (some of which may be considered to also function as solid diluents, liquid diluents or surfactants).
- formulation auxiliaries and additives may control: pH (buffers), foaming during processing (antifoams such polyorganosiloxanes), sedimentation of active ingredients (suspending agents), viscosity (thixotropic thickeners), in-container microbial growth (antimicrobials), product freezing (antifreezes), color (dyes/pigment dispersions), wash-off (film formers or stickers), evaporation (evaporation retardants), and other formulation attributes.
- Film formers include, for example, polyvinyl acetates, polyvinyl acetate copolymers, polyvinylpyrrolidone-vinyl acetate copolymer, polyvinyl alcohols, polyvinyl alcohol copolymers and waxes.
- formulation auxiliaries and additives include those listed in McCutcheon’s Volume 2: Functional Materials, annual International and North American editions published by McCutcheon’s Division, The Manufacturing Confectioner Publishing Co.; and PCT Publication WO 03/024222.
- the compound of Formula 1 and any other active ingredients are typically incorporated into the present compositions by dissolving the active ingredient in a solvent or by grinding in a liquid or dry diluent.
- Solutions including emulsifiable concentrates, can be prepared by simply mixing the ingredients. If the solvent of a liquid composition intended for use as an emulsifiable concentrate is water-immiscible, an emulsifier is typically added to emulsify the active-containing solvent upon dilution with water. Active ingredient slurries, with particle diameters of up to 2,000 ⁇ m can be wet milled using media mills to obtain particles with average diameters below 3 ⁇ m. Aqueous slurries can be made into finished suspension concentrates (see, for example, U.S.3,060,084) or further processed by spray drying to form water-dispersible granules.
- Dusts and powders can be prepared by blending and usually grinding (such as with a hammer mill or fluid-energy mill).
- Granules and pellets can be prepared by spraying the active material upon preformed granular carriers or by agglomeration techniques. See Browning, “Agglomeration”, Chemical Engineering, December 4, 1967, pp 147–48, Perry’s Chemical Engineer’s Handbook, 4th Ed., McGraw-Hill, New York, 1963, pages 8–57 and following, and WO 91/13546.
- Pellets can be prepared as described in U.S.4,172,714.
- Water-dispersible and water-soluble granules can be prepared as taught in U.S. 4,144,050, U.S. 3,920,442 and DE 3,246,493. Tablets can be prepared as taught in U.S. 5,180,587, U.S. 5,232,701 and U.S. 5,208,030. Films can be prepared as taught in GB 2,095,558 and U.S.3,299,566.
- T. S. Woods “The Formulator’s Toolbox – Product Forms for Modern Agriculture” in Pesticide Chemistry and Bioscience, The Food–Environment Challenge, T. Brooks and T. R.
- the compounds of the inention generally show highest activity for postemergence weed control (i.e. applied after weed seedlings emerge from the soil) and preemergence weed control (i.e. applied before weed seedlings emerge from the soil). Many of them have utility for broad-spectrum pre- and/or postemergence weed control in areas where complete control of all vegetation is desired such as around fuel storage tanks, industrial storage areas, parking lots, drive-in theaters, air fields, river banks, irrigation and other waterways, around billboards and highway and railroad structures.
- Compounds of this invention may show tolerance to important agronomic crops including, but is not limited to, alfalfa, barley, cotton, wheat, rape, sugar beets, corn (maize), sorghum, soybeans, rice, oats, peanuts, vegetables, tomato, potato, perennial plantation crops including coffee, cocoa, oil palm, rubber, sugarcane, citrus, grapes, fruit trees, nut trees, banana, plantain, pineapple, hops, tea and forests such as eucalyptus and conifers (e.g., loblolly pine), and turf species (e.g., Kentucky bluegrass, St. Augustine grass, Kentucky fescue and Bermuda grass).
- important agronomic crops including, but is not limited to, alfalfa, barley, cotton, wheat, rape, sugar beets, corn (maize), sorghum, soybeans, rice, oats, peanuts, vegetables, tomato, potato, perennial plantation crops including coffee, cocoa
- Compounds of this invention can be used in crops genetically transformed or bred to incorporate resistance to herbicides, express proteins toxic to invertebrate pests (such as Bacillus thuringiensis toxin), and/or express other useful traits. Those skilled in the art will appreciate that not all compounds are equally effective against all weeds. Alternatively, the subject compounds are useful to modify plant growth.
- the compounds of the invention have both preemergent and postemergent herbicidal activity, to control undesired vegetation by killing or injuring the vegetation or reducing its growth
- the compounds can be usefully applied by a variety of methods involving contacting a herbicidally effective amount of a compound of the invention, or a composition comprising said compound and at least one of a surfactant, a solid diluent or a liquid diluent, to the foliage or other part of the undesired vegetation or to the environment of the undesired vegetation such as the soil or water in which the undesired vegetation is growing or which surrounds the seed or other propagule of the undesired vegetation.
- Undesired vegetation includes at least one selected from the group consisting of grass weeds and broadleaf weeds.
- Undesired vegetation is selected from the group consisting of annual bluegrass, Benghal dayflower, blackgrass, black nightshade, broadleaf signalgrass, Canada thistle, cheat, common cocklebur (Xanthium pensylvanicum), common ragweed, corn poppies, field violet, giant foxtail, goosegrass, green foxtail, guinea grass, hairy beggarticks, herbicide-resistant black grass, horseweed, Italian rye grass, jimsonweed, Johnson grass (Sorghum halepense), large crabgrass, little seed canary grass, morning glory, Pennsylvania smartweed, pitted morning glory, prickly sida, quackgrass, redroot pigweed, shattercane, shepherd's purse, silky windgrass, sunflower (as weed in potato), wild buckwheat (Polygonum convolvulus), wild mustard (Brass
- a herbicidally effective amount of the compounds of this invention is determined by a number of factors. These factors include: formulation selected, method of application, amount and type of vegetation present, growing conditions, etc. In general, a herbicidally effective amount of compounds of this invention is about 0.001 to 20 kg/ha with a preferred range of about 0.004 to 1 kg/ha. One skilled in the art can easily determine the herbicidally effective amount necessary for the desired level of weed control. In one common embodiment, a compound of the invention is applied, typically in a formulated composition, to a locus comprising desired vegetation (e.g., crops) and undesired vegetation (i.e.
- weeds both of which may be seeds, seedlings and/or larger plants, in contact with a growth medium (e.g., soil).
- a composition comprising a compound of the invention can be directly applied to a plant or a part thereof, particularly of the undesired vegetation, and/or to the growth medium in contact with the plant.
- compounds of the invention are used to control undesired vegetation
- contact of desired vegetation in the treated locus with compounds of the invention may result in super-additive or enhanced effects with genetic traits in the desired vegetation, including traits incorporated through genetic modification. For example, resistance to phytophagous insect pests or plant diseases, tolerance to biotic/abiotic stresses or storage stability may be greater than expected from the genetic traits in the desired vegetation.
- Compounds of this invention can also be mixed with one or more other biologically active compounds or agents including herbicides, herbicide safeners, fungicides, insecticides, nematocides, bactericides, acaricides, growth regulators such as insect molting inhibitors and rooting stimulants, chemosterilants, semiochemicals, repellents, attractants, pheromones, feeding stimulants, plant nutrients, other biologically active compounds or entomopathogenic bacteria, virus or fungi to form a multi-component pesticide giving an even broader spectrum of agricultural protection.
- Mixtures of the compounds of the invention with other herbicides can broaden the spectrum of activity against additional weed species, and suppress the proliferation of any resistant biotypes.
- the present invention also pertains to a composition
- a composition comprising a compound of Formula 1 (in a herbicidally effective amount) and at least one additional biologically active compound or agent (in a biologically effective amount) and can further comprise at least one of a surfactant, a solid diluent or a liquid diluent.
- the other biologically active compounds or agents can be formulated in compositions comprising at least one of a surfactant, solid or liquid diluent.
- one or more other biologically active compounds or agents can be formulated together with a compound of Formula 1, to form a premix, or one or more other biologically active compounds or agents can be formulated separately from the compound of Formula 1, and the formulations combined together before application (e.g., in a spray tank) or, alternatively, applied in succession.
- a mixture of one or more of the following herbicides with a compound of this invention may be particularly useful for weed control: acetochlor, acifluorfen and its sodium salt, aclonifen, acrolein (2-propenal), alachlor, alloxydim, ametryn, amicarbazone, amidosulfuron, aminocyclopyrachlor and its esters (e.g., methyl, ethyl) and salts (e.g., sodium, potassium), 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)-2- Pyridinecarboxylic 2-propyn-1-yl ester (CAS No.2251111-17-6), 4-amino-3-chloro-5- fluoro-6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic cyanomethyl ester (CAS No.
- herbicides also include bioherbicides such as Alternaria destruens Simmons, Colletotrichum gloeosporiodes (Penz.) Penz. & Sacc., Drechsiera monoceras (MTB-951), Myrothecium verrucaria (Albertini & Schweinitz) Ditmar: Fries, Phytophthora palmivora (Butl.) Butl. and Puccinia thlaspeos Schub.
- bioherbicides such as Alternaria destruens Simmons, Colletotrichum gloeosporiodes (Penz.) Penz. & Sacc., Drechsiera monoceras (MTB-951), Myrothecium verrucaria (Albertini & Schweinitz) Ditmar: Fries, Phytophthora palmivora (Butl.) Butl. and Puccinia thlaspeos Schub.
- Preferred for better control of undesired vegetation e.g., lower use rate such as from enhanced effects, broader spectrum of weeds controlled, or enhanced crop safety
- a herbicide selected from the group consisting of atrazine, azimsulfuron, S-beflubutamid, benzisothiazolinone, carfentrazone-ethyl, chlorimuron-ethyl, chlorsulfuron-methyl, clomazone, clopyralid potassium, cloransulam-methyl, 2-[(2,4-dichlorophenyl)methyl]- 4,4-dimethyl-3-isoxazolidinone, 2-[(2,5-dichlorophenyl)methyl]-4,4-dimethyl-3- isoxazolidinone, ethametsulfuron-methyl, flumetsulam, 4-(4-fluorophenyl)-6-[(2-hydroxy- 6-
- Plant growth regulators such as aviglycine, N-(phenylmethyl)-1H-purin-6-amine, epocholeone, gibberellic acid, gibberellin A 4 and A 7 , harpin protein, mepiquat chloride, prohexadione calcium, prohydrojasmon, sodium nitrophenolate and trinexapac-methyl, and plant growth modifying organisms such as Bacillus cereus strain BP01.
- plant growth regulators such as aviglycine, N-(phenylmethyl)-1H-purin-6-amine, epocholeone, gibberellic acid, gibberellin A 4 and A 7 , harpin protein, mepiquat chloride, prohexadione calcium, prohydrojasmon, sodium nitrophenolate and trinexapac-methyl
- plant growth modifying organisms such as Bacillus cereus strain BP01.
- General references for agricultural protectants i.e. herbicides, herbicide safeners, insecticides
- the mixing partners are typically used in the amounts similar to amounts customary when the mixture partners are used alone. More particularly in mixtures, active ingredients are often applied at an application rate between one-half and the full application rate specified on product labels for use of active ingredient alone. These amounts are listed in references such as The Pesticide Manual and The BioPesticide Manual.
- the weight ratio of these various mixing partners (in total) to the compound of Formula 1 is typically between about 1:3000 and about 3000:1.
- weight ratios between about 1:300 and about 300:1 for example ratios between about 1:30 and about 30:1.
- One skilled in the art can easily determine through simple experimentation the biologically effective amounts of active ingredients necessary for the desired spectrum of biological activity. It will be evident that including these additional components may expand the spectrum of weeds controlled beyond the spectrum controlled by the compound of Formula 1 alone.
- combinations of a compound of this invention with other biologically active (particularly herbicidal) compounds or agents (i.e. active ingredients) can result in a greater-than-additive (i.e. enhanced) effect on weeds and/or a less-than-additive effect (i.e. safening) on crops or other desirable plants.
- composition of the present invention can further comprise (in a herbicidally effective amount) at least one additional herbicidal active ingredient having a similar spectrum of control but a different site of action.
- herbicide safeners such as allidochlor, benoxacor, cloquintocet-mexyl, cumyluron, cyometrinil, cyprosulfonamide, daimuron, dichlormid, dicyclonon, dietholate, dimepiperate, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr- diethyl, mephenate, methoxyphenone naphthalic anhydride (1,8-naphthalic anhydride), oxabetrinil, N-(aminocarbonyl)-2-methylbenzenesulfonamide, N-(aminocarbonyl)- 2-fluorobenzenesulfonamide, 1-bromo-4-[(chloromethyl)sulfonyl]benzene (BC
- Antidotally effective amounts of the herbicide safeners can be applied at the same time as the compounds of this invention, or applied as seed treatments. Therefore an aspect of the present invention relates to a herbicidal mixture comprising a compound of this invention and an antidotally effective amount of a herbicide safener. Seed treatment is particularly useful for selective weed control, because it physically restricts antidoting to the crop plants. Therefore a particularly useful embodiment of the present invention is a method for selectively controlling the growth of undesired vegetation in a crop comprising contacting the locus of the crop with a herbicidally effective amount of a compound of this invention wherein seed from which the crop is grown is treated with an antidotally effective amount of safener.
- Antidotally effective amounts of safeners can be easily determined by one skilled in the art through simple experimentation.
- Compounds of the invention can also be mixed with: (1) polynucleotides including but not limited to DNA, RNA, and/or chemically modified nucleotides influencing the amount of a particular target through down regulation, interference, suppression or silencing of the genetically derived transcript that render a herbicidal effect; or (2) polynucleotides including but not limited to DNA, RNA, and/or chemically modified nucleotides influencing the amount of a particular target through down regulation, interference, suppression or silencing of the genetically derived transcript that render a safening effect.
- compositions comprising a compound of the invention (in a herbicidally effective amount), at least one additional active ingredient selected from the group consisting of other herbicides and herbicide safeners (in an effective amount), and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents.
- Table A1 lists specific combinations of a Component (a) with Component (b) illustrative of the mixtures, compositions and methods of the present invention.
- Compound No. (Compound Number) (i.e. Compound 1) in the Component (a) column is identified in Index Table A.
- the second column of Table A1 lists the specific Component (b) compound (e.g., “2,4-D” in the first line).
- the third, fourth and fifth columns of Table A1 lists ranges of weight ratios for rates at which the Component (a) compound is typically applied to a field-grown crop relative to Component (b) (i.e. (a):(b)).
- the first line of Table A1 specifically discloses the combination of Component (a) (i.e. Compound 1 in Index Table A) with 2,4-D is typically applied in a weight ratio between 1:384 – 6:1.
- the remaining lines of Table A1 are to be construed similarly.
- Table A2 is constructed the same as Table A1 above except that entries below the “Component (a)” column heading are replaced with the respective Component (a) Column Entry shown below. Compound No. in the Component (a) column is identified in Index Table A. Thus, for example, in Table A2 the entries below the “Component (a)” column heading all recite “Compound 2” (i.e. Compound 2 identified in Index Table A), and the first line below the column headings in Table A2 specifically discloses a mixture of Compound 2 with 2,4-D. Tables A3 through A60 are constructed similarly.
- Preferred for better control of undesired vegetation e.g., lower use rate such as from enhanced effects, broader spectrum of weeds controlled, or enhanced crop safety
- a herbicide selected from the group consisting of chlorimuron-ethyl, nicosulfuron, mesotrione, thifensulfuron-methyl, flupyrsulfuron-methyl, tribenuron, pyroxasulfone, pinoxaden, tembotrione, pyroxsulam, metolachlor and S-metolachlor.
- the following TESTS demonstrate the control efficacy of compounds of this invention on specific pathogens.
- the pathogen control protection afforded by the compounds is not limited, however, to these species. See Index Tables A and B below for compound descriptions.
- the abbreviation “Cmpd.” stands for “Compound”, and the abbreviation “Ex.” stands for “Example” and is followed by a number indicating in which example the compound is prepared.
- the numerical value reported in the column “MS” is the molecular weight of the highest isotopic abundance positively charged parent ion (M+1) formed by addition of H+ (molecular weight of 1) to the molecule having the highest isotopic abundance, or the highest isotopic abundance negatively charged ion (M–1) formed by loss of H+ (molecular weight of 1).
- “Pyr” means “Pyridine”.
- Przl means “Pyrazol”.
- Thzl means “Thiazole”.
- Imdzl mean “Imidazol”. The presence of molecular ions containing one or more higher atomic weight isotopes of lower abundance (e.g., 37Cl, 81Br) is not reported. The reported MS peaks were observed by mass spectrometry using electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI).
- ESI electrospray ionization
- APCI atmospheric pressure chemical ionization
- BIOLOGICAL EXAMPLES OF THE INVENTION TEST A Seeds of plant species selected from blackgrass (Alopecurus myosuroides), corn (Zea mays), foxtail, giant (giant foxtail, Setaria faberi), goosegrass (Eleusine indica), kochia (Bassia scoparia), oat, wild (wild oat, Avena fatua), pigweed, palmer (palmer amaranth, palmer pigweed, Amaranthus palmeri), ragweed (common ragweed, Ambrosia artemisiifolia), ryegrass, Italian (italian ryegrass, Lolium multiflorum), soybean (Glycine max) and wheat (Triticum aestivum) were planted into a blend of loam soil and sand and treated preemergence with a directed soil spray using test chemicals formulated in a non-phytotoxic solvent mixture which included a surfactant.
- plants selected from these crop and weed species and also galium (catchweed bedstraw, Galium aparine) and horseweed (Erigeron canadensis) were planted in pots containing the same blend of loam soil and sand and treated with postemergence applications of test chemicals formulated in the same manner. Plants ranged in height from 2 to 10 cm and were in the one- to two-leaf stage for the postemergence treatment. Treated plants and untreated controls were maintained in a greenhouse for 10 days, after which time all treated plants were compared to untreated controls and visually evaluated for injury. Plant response ratings, summarized in Table A, are based on a 0 to 100 scale where 0 is no effect and 100 is complete control. A dash (–) response means no test result.
- test pots were flooded to 3 cm above the soil surface, treated by application of test compounds directly to the paddy water, and then maintained at that water depth for the duration of the test.
- Treated plants and controls were maintained in a greenhouse for 13 days, after which time all species were compared to controls and visually evaluated.
- Plant response ratings, summarized in Table B, are based on a scale of 0 to 100 where 0 is no effect and 100 is complete control. A dash (–) response means no test result.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental Sciences (AREA)
- Wood Science & Technology (AREA)
- Plant Pathology (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Pest Control & Pesticides (AREA)
- General Health & Medical Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Disclosed are compounds of Formula I, including all stereoisomers, N-oxides, and salts thereof, agricultural compositions containing them and their use as herbicides, wherein J is selected from the group consisting of J-1 to J-10, G is selected from the group consisting of G-1 to G7, and R1, R2, R3, R4, R5, W, X, Y and Q are as defined in the disclosure.
Description
TITLE SUBSTITUTED CYCLOPROPYLPYRIMIDINE HERBICIDES FIELD OF THE INVENTION This invention relates to certain substituted cyclopropylpyrimidine herbicides, their N-oxides, salts and compositions, and methods of their use for controlling undesirable vegetation. BACKGROUND OF THE INVENTION The control of undesired vegetation is extremely important in achieving high crop efficiency. Achievement of selective control of the growth of weeds especially in such useful crops as rice, soybean, sugar beet, maize, potato, wheat, barley, tomato and plantation crops, among others, is very desirable. Unchecked weed growth in such useful crops can cause significant reduction in productivity and thereby result in increased costs to the consumer. The control of undesired vegetation in noncrop areas is also important. Many products are commercially available for these purposes, but the need continues for new compounds that are more effective, less costly, less toxic, environmentally safer or have different sites of action. SUMMARY OF THE INVENTION This invention is directed to compounds of Formula 1, all stereoisomers, N-oxides, and salts thereof, agricultural compositions containing them and their use as herbicides:
wherein J is selected from the group consisting of ,
R1 is halogen, C1–C4 haloalkyl, C1–C4 haloalkoxy, C1–C4 alkoxy, C1–C4 haloalkenyl, C1–C4 haloalkynyl, C1–C4 haloalkenyloxy, C1–C4 haloalkynyloxy, C1–C4 haloalkylthio, C1–C4 alkylthio or -CN; R2 is H or halogen; W, X, and Y are independently N or CR5; R3 is C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C 3 –C 7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy, C1–C5 haloalkoxy or C1–C5 alkylthio; R4 is H, C 1 –C 5 alkyl, halogen, -CN, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C 1 –C 5 alkylthio; Q is O, S, -CH2- or -(C=O)-; R5 is H, C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C 3 –C 7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy, C1–C5 haloalkoxy or C1–C5 alkylthio. More particularly, this invention pertains to a compound of Formula 1 (including all stereoisomers), an N-oxide or a salt thereof. This invention also relates to a herbicidal composition comprising a compound of the invention (i.e. in a herbicidally effective amount) and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents. This invention further relates to a method for controlling the growth of undesired vegetation comprising contacting the vegetation or its environment with a herbicidally effective amount of a compound of the invention (e.g., as a composition described herein). This invention also includes a herbicidal mixture comprising (a) a compound selected from Formula 1, N-oxides, and salts thereof, and (b) at least one additional active ingredient selected from (b1) through (b17), and salts of compounds of (b1) through (b17), as described below. DETAILS OF THE INVENTION As used herein, the terms “comprises,” “comprising,” “includes,” “including,” “has,” “having,” “contains”, “containing,” “characterized by” or any other variation thereof, are intended to cover a non-exclusive inclusion, subject to any limitation explicitly indicated. For example, a composition, mixture, process or method that comprises a list of elements is not necessarily limited to only those elements but may include other elements not expressly listed or inherent to such composition, mixture, process or method. The transitional phrase “consisting of” excludes any element, step, or ingredient not specified. If in the claim, such would close the claim to the inclusion of materials other than those recited except for impurities ordinarily associated therewith. When the phrase “consisting of” appears in a clause of the body of a claim, rather than immediately following the preamble, it limits only the element set forth in that clause; other elements are not excluded from the claim as a whole.
The transitional phrase “consisting essentially of” is used to define a composition or method that includes materials, steps, features, components, or elements, in addition to those literally disclosed, provided that these additional materials, steps, features, components, or elements do not materially affect the basic and novel characteristic(s) of the claimed invention. The term “consisting essentially of” occupies a middle ground between “comprising” and “consisting of”. Where applicants have defined an invention or a portion thereof with an open-ended term such as “comprising,” it should be readily understood that (unless otherwise stated) the description should be interpreted to also describe such an invention using the terms “consisting essentially of” or “consisting of.” Further, unless expressly stated to the contrary, “or” refers to an inclusive or and not to an exclusive or. For example, a condition A or B is satisfied by any one of the following: A is true (or present) and B is false (or not present), A is false (or not present) and B is true (or present), and both A and B are true (or present). Also, the indefinite articles “a” and “an” preceding an element or component of the invention are intended to be nonrestrictive regarding the number of instances (i.e. occurrences) of the element or component. Therefore “a” or “an” should be read to include one or at least one, and the singular word form of the element or component also includes the plural unless the number is obviously meant to be singular. As referred to herein, the term “seedling”, used either alone or in a combination of words means a young plant developing from the embryo of a seed. As referred to herein, the term “broadleaf” used either alone or in words such as “broadleaf weed” means dicot or dicotyledon, a term used to describe a group of angiosperms characterized by embryos having two cotyledons. As used herein, the term “alkylating” refers reaction in which nucleophile displaces a leaving group such as halide or sulfonate from a carbon-containing radical. Unless otherwise indicated, the term “alkylating” does not limit the carbon-containing radical to alkyl. In the above recitations, the term “alkyl”, used either alone or in compound words such as “alkylthio” or “haloalkyl” includes straight-chain or branched alkyl, such as, methyl, ethyl, n-propyl, i-propyl, or the different butyl, pentyl or hexyl isomers. “Alkenyl” includes straight-chain or branched alkenes such as ethenyl, 1-propenyl, 2-propenyl, and the different butenyl, pentenyl and hexenyl isomers. “Alkenyl” also includes polyenes such as 1,2-propadienyl and 2,4-hexadienyl. “Alkynyl” includes straight-chain or branched alkynes such as ethynyl, 1-propynyl, 2-propynyl and the different butynyl, pentynyl and hexynyl isomers. “Alkynyl” can also include moieties comprised of multiple triple bonds such as 2,5-hexadiynyl. “Alkoxy” includes, for example, methoxy, ethoxy, n-propyloxy, isopropyloxy and the different butoxy, pentoxy and hexyloxy isomers. “Alkoxyalkyl” denotes alkoxy substitution
on alkyl. Examples of “alkoxyalkyl” include CH3OCH2, CH3OCH2CH2, CH3CH2OCH2, CH3CH2CH2CH2OCH2 and CH3CH2OCH2CH2. “Alkoxyalkoxy” denotes alkoxy substitution on alkoxy. “Alkylthio” includes branched or straight-chain alkylthio moieties such as methylthio, ethylthio, and the different propylthio, butylthio, pentylthio and hexylthio isomers. “Alkylthioalkyl” denotes alkylthio substitution on alkyl. Examples of “alkylthioalkyl” include CH3SCH2, CH3SCH2CH2, CH3CH2SCH2, CH3CH2CH2CH2SCH2 and CH3CH2SCH2CH2. “Alkylsulfinyl” includes both enantiomers of an alkylsulfinyl group. Examples of “alkylsulfinyl” include CH3S(O)-, CH3CH2S(O)-, CH3CH2CH2S(O)-, (CH3)2CHS(O)- and the different butylsulfinyl, pentylsulfinyl and hexylsulfinyl isomers. Examples of “alkylsulfonyl” include CH3S(O)2-, CH3CH2S(O)2-, CH3CH2CH2S(O)2-, (CH3)2CHS(O)2-, and the different butylsulfonyl, pentylsulfonyl and hexylsulfonyl isomers. “Cyanoalkyl” denotes an alkyl group substituted with one cyano group. Examples of “cyanoalkyl” include NCCH2 and NCCH2CH2 (alternatively identified as CH2CH2CN). “Nitroalkyl” denotes an alkyl group substituted with one nitro group. Examples of “nitroalkyl” include (NO2)CH2 and (NO2)CH2CH2 (alternatively identified as CH2CH2(NO2)). “Cyano” means -CN, and “formyl” means HC(=O)-. “Alkylamino” includes an NH radical substituted with straight-chain or branched alkyl. Examples of “alkylamino” include CH3CH2NH, CH3CH2CH2NH, and (CH3)2CHCH2NH. Examples of “dialkylamino” include (CH3)2N, (CH3CH2CH2)2N and CH3CH2(CH3)N. “Cycloalkyl” includes, for example, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. The term “cycloalkylalkyl” denotes cycloalkyl substitution on an alkyl moiety. Examples of “cycloalkylalkyl” include cyclopropylmethyl, cyclopentylethyl, and other cycloalkyl moieties bonded to straight-chain or branched alkyl groups. The term “alkylcycloalkyl” donotes an alkyl group bonded to a cycloalkyl moiety. The term “halogen”, either alone or in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” includes fluorine, chlorine, bromine or iodine. Further, when used in compound words such as “haloalkyl”, or when used in descriptions such as “alkyl substituted with halogen” said alkyl may be partially or fully substituted with halogen atoms which may be the same or different. Examples of “haloalkyl” or “alkyl substituted with halogen” include F3C, ClCH2, CF3CH2 and CF3CCl2. The terms “haloalkoxy”, “haloalkoxyalkyl”, “haloalkylthio”, “haloalkenyl”, “haloalkynyl”, and the like, are as defined analogously to the term “haloalkyl”. Examples of “haloalkoxy” include CF3O- , CCl3CH2O-, HCF2CH2CH2O- and CF3CH2O-. Examples of “haloalkoxyalkyl” include CF3OCH2-, CCl3CH2OCH2-, HCF2CH2CH2OCH2- and CF3CH2OCH2-. Examples of “haloalkylthio” include CCl3S-, CF3S-, CCl3CH2S- and ClCH2CH2CH2S-. Examples of “haloalkenyl” include (Cl)2C=CHCH2- and CF3CH2CH=CHCH2-. Examples of “haloalkynyl” include HC≡CCHCl-, CF3C≡C-, CCl3C≡C- and FCH2C≡CCH2-.
“Alkylcarbonyl” denotes a straight-chain or branched alkyl moiety bonded to a C(=O) moiety. Examples of “alkylcarbonyl” include CH3C(=O)-, CH3CH2C(=O)-, CH3CH2CH2C(=O)- and (CH3)2CHC(=O)-. “Alkoxycarbonyl” denotes a straight-chain or branched alkoxy moieties bonded to a C(=O) moiety. Examples of “alkoxycarbonyl” include CH3OC(=O)-, CH3CH2OC(=O)-, CH3CH2CH2OC(=O)-, (CH3)2CHOC(=O)- and the different butoxy- or pentoxycarbonyl isomers. C(=O) or C(O) designates carbonyl. The term “alkoxycarbonylalkyl” denotes a straight-chain or branched alkoxycarbonyl moiety bonded through an alkyl moiety. The term “alkylcarbonylalkyl” denotes a straight or branched alkylcarbonyl moiety bonded through an alkyl moiety. The term “alkylcarbonyloxy” donates an alkylcarbony moiety bonded through oxygen. Examples of alkylcarbonyloxy include CH3C(=O)O-, CH3CH2C(=O)O-, CH3CH2CH2C(=O)O- and (CH3)2CHC(=O)-. The term alkanediyl or alkenediyl refers to a linear or branched alkane or alkene linking chain respectively. Examples of alkanediyl include –CH2–, –CH2CH(CH3)– and –CH2CH2CH2–. Examples of alkenediyl include –CH=CH–, –CH2C=CH– and –CH=C(CH3)–. The term “adjacent” in the context of locating a substituent means “next to” or “immediately next to”. The total number of carbon atoms in a substituent group is indicated by the “Ci–Cj” prefix where i and j are numbers from 1 to 8. For example, C1–C4 alkylsulfonyl designates methylsulfonyl through butylsulfonyl; C3–C8 alkylcarbonylalkyl can be, for example, CH3COCH2-, CH3COCH2CH2- or CH3CH2CH2COCH2CH2CH2CH2-; C4–C7 alkylcycloalkyl can be, for example, methylcyclopropyl, methylcyclobutyl, ethylcyclopropyl, or propylcyclobutyl; C2 alkoxyalkyl designates CH3OCH2-; C3 alkoxyalkyl designates, for example, CH3CH(OCH3)-, CH3OCH2CH2- or CH3CH2OCH2-; and C4 alkoxyalkyl designates the various isomers of an alkyl group substituted with an alkoxy group containing a total of four carbon atoms, examples including CH3CH2CH2OCH2- and CH3CH2OCH2CH2-. When a group contains a substituent which can be hydrogen, for example R2 or R5, then when this substituent is taken as hydrogen, it is recognized that this is equivalent to said group being unsubstituted. When one or more positions on a group are said to be “not substituted” or “unsubstituted”, then hydrogen atoms are attached to take up any free valency. Unless otherwise indicated as being optionally substituted, the term “phenyl” means unsubstituted phenyl. Unless otherwise indicated as being optionally substituted, the term “benzyl” means unsubstituted benzyl. When a compound is substituted with a substituent bearing a subscript that indicates the number of said substituents can exceed 1, said substituents (when they exceed 1) are independently selected from the group of defined substituents.When a functional group or a compound is shown to be optionally substituted with a substituent, the said functional group or compound may be unsubstituted or substituted. When one or more positions on a group are
said to be “not substituted” or “unsubstituted”, then hydrogen atoms are attached to take up any free valency. The term “ring system” denotes two or more fused rings. The term “bicyclic ring system” denotes a ring system consisting of two fused rings. Compounds of this invention can exist as one or more stereoisomers. The various stereoisomers include enantiomers, diastereomers, atropisomers and geometric isomers. Stereoisomers are isomers of identical constitution but differing in the arrangement of their atoms in space and include enantiomers, diastereomers, cis-trans isomers (also known as geometric isomers) and atropisomers. Atropisomers result from restricted rotation about single bonds where the rotational barrier is high enough to permit isolation of the isomeric species. One skilled in the art will appreciate that one stereoisomer may be more active and/or may exhibit beneficial effects when enriched relative to the other stereoisomer(s) or when separated from the other stereoisomer(s). Additionally, the skilled artisan knows how to separate, enrich, and/or to selectively prepare said stereoisomers. The compounds of the invention may be present as a mixture of stereoisomers, individual stereoisomers or as an optically active form. Compounds of Formula 1 typically exist in more than one form, and Formula 1 thus include all crystalline and non-crystalline forms of the compounds they represent. Non- crystalline forms include embodiments which are solids such as waxes and gums as well as embodiments which are liquids such as solutions and melts. Crystalline forms include embodiments which represent essentially a single crystal type and embodiments which represent a mixture of polymorphs (i.e. different crystalline types). The term “polymorph” refers to a particular crystalline form of a chemical compound that can crystallize in different crystalline forms, these forms having different arrangements and/or conformations of the molecules in the crystal lattice. Although polymorphs can have the same chemical composition, they can also differ in composition due to the presence or absence of co- crystallized water or other molecules, which can be weakly or strongly bound in the lattice. Polymorphs can differ in such chemical, physical and biological properties as crystal shape, density, hardness, color, chemical stability, melting point, hygroscopicity, suspensibility, dissolution rate and biological availability. One skilled in the art will appreciate that a polymorph of a compound of Formula 1 can exhibit beneficial effects (e.g., suitability for preparation of useful formulations, improved biological performance) relative to another polymorph or a mixture of polymorphs of the same compound of Formula 1. Preparation and isolation of a particular polymorph of a compound of Formula 1 can be achieved by methods known to those skilled in the art including, for example, crystallization using selected solvents and temperatures. For a comprehensive discussion of polymorphism see R. Hilfiker, Ed., Polymorphism in the Pharmaceutical Industry, Wiley-VCH, Weinheim, 2006.
One skilled in the art will appreciate that not all nitrogen-containing heterocycles can form N-oxides since the nitrogen requires an available lone pair for oxidation to the oxide; one skilled in the art will recognize those nitrogen-containing heterocycles which can form N-oxides. One skilled in the art will also recognize that tertiary amines can form N-oxides. Synthetic methods for the preparation of N-oxides of heterocycles and tertiary amines are very well known by one skilled in the art including the oxidation of heterocycles and tertiary amines with peroxy acids such as peracetic and m-chloroperbenzoic acid (MCPBA), hydrogen peroxide, alkyl hydroperoxides such as t-butyl hydroperoxide, sodium perborate, and dioxiranes such as dimethyldioxirane. These methods for the preparation of N-oxides have been extensively described and reviewed in the literature, see for example: T. L. Gilchrist in Comprehensive Organic Synthesis, vol. 7, pp 748–750, S. V. Ley, Ed., Pergamon Press; M. Tisler and B. Stanovnik in Comprehensive Heterocyclic Chemistry, vol. 3, pp 18–20, A. J. Boulton and A. McKillop, Eds., Pergamon Press; M. R. Grimmett and B. R. T. Keene in Advances in Heterocyclic Chemistry, vol. 43, pp 149–161, A. R. Katritzky, Ed., Academic Press; M. Tisler and B. Stanovnik in Advances in Heterocyclic Chemistry, vol.9, pp 285–291, A. R. Katritzky and A. J. Boulton, Eds., Academic Press; and G. W. H. Cheeseman and E. S. G. Werstiuk in Advances in Heterocyclic Chemistry, vol. 22, pp 390–392, A. R. Katritzky and A. J. Boulton, Eds., Academic Press. One skilled in the art recognizes that because in the environment and under physiological conditions salts of chemical compounds are in equilibrium with their corresponding nonsalt forms, salts share the biological utility of the nonsalt forms. Thus, a wide variety of salts of a compound of Formula 1 are useful for control of undesired vegetation (i.e. are agriculturally suitable). The salts of a compound of Formula 1 include acid-addition salts with inorganic or organic acids such as hydrobromic, hydrochloric, nitric, phosphoric, sulfuric, acetic, butyric, fumaric, lactic, maleic, malonic, oxalic, propionic, salicylic, tartaric, 4-toluenesulfonic or valeric acids. When a compound of Formula 1 contains an acidic moiety, salts also include those formed with organic or inorganic bases such as pyridine, triethylamine or ammonia, or amides, hydrides, hydroxides or carbonates of sodium, potassium, lithium, calcium, magnesium or barium. Accordingly, the present invention comprises compounds selected from Formula 1, N-oxides and agriculturally suitable salts thereof. A wide variety of synthetic methods are known in the art to enable preparation of aromatic and nonaromatic heterocyclic rings and ring systems; for extensive reviews see the eight volume set of Comprehensive Heterocyclic Chemistry, A. R. Katritzky and C. W. Rees editors-in-chief, Pergamon Press, Oxford, 1984 and the twelve volume set of Comprehensive Heterocyclic Chemistry II, A. R. Katritzky, C. W. Rees and E. F. V. Scriven editors-in-chief, Pergamon Press, Oxford, 1996.
Embodiments of the present invention as described in the Summary of the Invention include those described below. In the following Embodiments, Formula 1 includes stereoisomers, N-oxides and salts thereof, and reference to “a compound of Formula 1” includes the definitions of substituents specified in the Summary of the Invention unless further defined in the Embodiments. Embodiment 1. A compound of Formula 1, stereoisomers, N-oxides, and salts thereof, agricultural compositions containing them and their use as herbicides as described in the Summary of the Invention. Embodiment 2. A compound of Formula 1 or Embodiment 1 wherein J is J-1, J-2, J-3, J-5, J-6, J-8,J-9 or J-10. Embodiment 2a. A compound of Formula 1 or Embodiment 2 wherein J is J-1, J-2 or J-3. Embodiment 2b. A compound of Embodiment 2a wherein J is J-1. Embodiment 2c. A compound of Embodiment 2a wherein J is J-2. Embodiment 2d. A compound of Embodiment 2a wherein J is J-3. Embodiment 2e. A compound of Formula 1 or Embodiment 2 wherein J is J-5, J-6 or J-8. Embodiment 2f. A compound of Embodiment 2e wherein J is J-5. Embodiment 2g. A compound of Embodiment 2e wherein J is J-6. Embodiment 2h. A compound of Embodiment 2e wherein J is J-8. Embodiment 3. A compound of Formula 1 or any one of the preceding Embodiments wherein G is G-1, G-2, G-3, G-4 or G-7. Embodiment 3a. A compound of Embodiment 3 wherein G is G-1, G-2, G-3 or G-7. Embodiment 3aa. A compound of Embodiment 3a wherein G is G-1, G-2 or G-3. Embodiment 3b. A compound of Embodiment 3a where G is G-1 wherein W, X, and Y are independently N or CR6. Embodiment 3c. A compound of Embodiment 3b wherein X is N, Y is CR6 and W is CR6. Embodiment 3d. A compound of Embodiment 3b wherein X is CR6, Y is N and W is CR6. Embodiment 3dd. A compound of Embodiment 3b wherein X is CR6, Y is CR6 and W is N. Embodiment 3e. A compound of Embodiment 3b wherein X is CR6, Y is CR6 and W is CR6. Embodiment 3f. A compound of Embodiment 3b wherein X is N, Y is N and W is CR6. Embodiment 3g. A compound of Embodiment 3 wherein G is G-2.
Embodiment 3h. A compound of Embodiment 3 wherein G is G-3. Embodiment 3i. A compound of Embodiment 3 wherein G is G-4. Embodiment 3j. A compound of Embodiment 3 wherein G is G-7. Embodiment 4. A compound of Formula 1 or any one of the preceding Embodiments wherein R1 is halogen, C1–C4 haloalkyl, C1–C4 haloalkoxy, C1–C4 alkoxy or C1–C4 haloalkylthio. Embodiment 4a. A compound of Embodiment 4 wherein R1 is C1–C4 haloalkyl or C1– C4 haloalkoxy. Embodiment 4b. A compound of Embodiment 4a wherein OCF3. Embodiment 4c. A compound of Embodiment 4a wherein haloalkyl. Embodiment 4d. A compound of Embodiment 4c wherein Embodiment 4e. A compound of Embodiment 4a wherein haloalkoxy. Embodiment 4f. A compound of Embodiment 4e wherein
Embodiment 5. A compound of Formula 1 or any one of the preceding Embodiments wherein R2 is H. Embodiment 5a. A compound of Formula 1 or any one of the preceding Embodiments wherein R2 is halogen. Embodiment 6. A compound of Formula 1 or any one of the preceding Embodiments wherein R3 is C1–C5 alkyl, halogen, C3–C7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy or C1–C5 haloalkoxy. Embodiment 6a. A compound of Embodiment 6 wherein R3 is C 1 –C 5 alkyl, halogen, C1–C5 haloalkyl, C1–C5 alkoxy or C1–C5 haloalkoxy. Embodiment 6b. A compound of Embodiment 6a wherein R3 is halogen, C1–C5 haloalkyl or C1–C5 haloalkoxy. Embodiment 6c. A compound of Embodiment 6b wherein R3 is halogen or C1–C5 haloalkyl. Embodiment 6d. A compound of Embodiment 6c wherein R3 is halogen. Embodiment 6e. A compound of Embodiment 6c wherein R3 is C1–C5 haloalkyl. Embodiment 6f. A compound of Embodiment 6c wherein R3 is F, Cl or CF3. Embodiment 6g. A compound of Embodiment 6f wherein R3 is CF3. Embodiment 6h. A compound of Embodiment 6f wherein R3 is F. Embodiment 6i. A compound of Embodiment 6f wherein R3 is Cl. Embodiment 7. A compound of Formula 1 or any one of the preceding Embodiments wherein R4 is H, C 1 –C 5 alkyl, halogen, -CN, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C1–C5 alkylthio.
Embodiment 7a. A compound of Embodiment 7 wherein R4 is H, halogen, -CN or C1– C5 haloalkyl. Embodiment 7b. A compound of Embodiment 7a wherein R4 is H, halogen, -CN or CF3. Embodiment 7c. A compound of Embodiment 7b wherein R4 is H. Embodiment 8. A compound of Formula 1 or any one of the preceding Embodiments wherein Q is O, S, -CH2- or –(C=O)-. Embodiment 8a. A compound of Embodiment 8 wherein Q is O or -CH2-. Embodiment 8b. A compound of Embodiment 8a wherein Q is O. Embodiment 8c. A compound of Embodiment 8a wherein Q is -CH2-. Embodiment 8d. A compound of Embodiment 8 wherein Q is S. Embodiment 9. A compound of Formula 1 or any one of the preceding Embodiments wherein R5 is H, C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C3–C7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy, C1–C5 haloalkoxy or C1–C5 alkylthio. Embodiment 9a. A compound of Embodiment 9 wherein R5 is H, C 1 –C 5 alkyl or halogen. Embodiment 9b. A compound of Embodiment 9a wherein R5 is H. Embodiment 9c. A compound of Embodiment 9a wherein R5 is C 1 –C 3 alkyl. Embodiment 9d. A compound of Embodiment 9a wherein R5 is halogen. Embodiments of this invention, including Embodiments 1–9d above as well as any other embodiments described herein, can be combined in any manner, and the descriptions of variables in the embodiments pertain not only to the compounds of Formula 1 but also to the starting compounds and intermediate compounds useful for preparing the compounds of Formula 1. In addition, embodiments of this invention, including Embodiments 1–9d above as well as any other embodiments described herein, and any combination thereof, pertain to the compositions and methods of the present invention. Combinations of Embodiments 1–9d are illustrated by: Embodiment X. A compound of Formula 1 as described in the Summary of the Invention wherein J is J-1, J-2, J-3, J-5, J-6, J-8, J-9 or J-10; and G is G-1, G-2, G-3 or G-7. Embodiment XX. A compound of Embodiment X wherein R1 is C1–C4 haloalkyl or C1–C4 haloalkoxy;
R3 is halogen or C1–C5 haloalkyl; R4 is H; and Q is O or -CH2-. Embodiment XXX. A compound of Embodiment XX wherein Q is O. Embodiment A1. A compound of Embodiment X wherein J is J-1, J-2 or J-3; R1 is CF3 or OCF3; and R3 is F, Cl or CF3. Embodiment A2. A compound of Embodiment A1 wherein J is J-1; and R2 is H or halogen. Embodiment A3. A compound of Embodiment A2 wherein R2 is H. Embodiment A4. A compound of Embodiment A1 wherein J is J-2; and R1 is CF3. Embodiment A5. A compound of Embodiment A1 wherein J is J-3; and R1 is CF3. Embodiment A6. A compound of Embodiment X wherein G is G-1, G-2 or G-3. Embodiment A7. A compound of Embodiment A6 wherein G is G-1; X is N; Y is CH; W is CH; and R5 is CF3. Embodiment A8. A compound of Embodiment A6 wherein
G is G-2; and R3 is CF3. Embodiment A11. A compound of Embodiment A6 wherein G is G-3; and R3 is CF3. Embodiment B1. A compound of Embodiment X wherein J is J-5, J-6 or J-8; R1 is CF3 or OCF3; and R3 is F, Cl, CF3. Embodiment B2. A compound of Embodiment B1 wherein J is J-5. Embodiment B3. A compound of Embodiment B1 wherein J is J-6. Embodiment B4. A compound of Embodiment B1 wherein J is J-8. Embodiment C. A compound of Summary of the Invention wherein Q is O.. Specific embodiments include a compound of Formula 1 selected from the group consisting of: 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 1) 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[4-(trifluoromethyl)phenyl]pyrimidine; (Compound 5) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-pyrazol-1-yl]-4-[[2-(trifluoromethyl)-4- pyridinyl]oxy]pyrimidine; (Compound 9) 5-cyclopropyl-4-[3-(trifluoromethoxy)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 10) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-pyrazol-1-yl]-4-[[6-(trifluoromethyl)-2- pyridinyl]oxy]pyrimidine; (Compound 8) and 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[6-(trifluoromethyl)-3- pyridinyl]pyrimidine. (Compound 13)
This invention also relates to a method for controlling undesired vegetation comprising applying to the locus of the vegetation herbicidally effective amounts of the compounds of the invention (e.g., as a composition described herein). Of note as embodiments relating to methods of use are those involving the compounds of embodiments described above. Compounds of the invention are particularly useful for selective control of weeds in crops such as wheat, barley, maize, soybean, sunflower, cotton, oilseed rape and rice, and specialty crops such as sugarcane, citrus, fruit and nut crops. Also noteworthy as embodiments are herbicidal compositions of the present invention comprising the compounds of embodiments described above. This invention also includes a herbicidal mixture comprising (a) a compound selected from Formula 1, N-oxides, and salts thereof, and (b) at least one additional active ingredient selected from (b1) photosystem II inhibitors, (b2) acetohydroxy acid synthase (AHAS) inhibitors, (b3) acetyl-CoA carboxylase (ACCase) inhibitors, (b4) auxin mimics, (b5) 5-enol- pyruvylshikimate-3-phosphate (EPSP) synthase inhibitors, (b6) photosystem I electron diverters, (b7) protoporphyrinogen oxidase (PPO) inhibitors, (b8) glutamine synthetase (GS) inhibitors, (b9) very long chain fatty acid (VLCFA) elongase inhibitors, (b10) auxin transport inhibitors, (b11) phytoene desaturase (PDS) inhibitors, (b12) 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitors, (b13) homogentisate solanesyltransferase (HST) inhibitors, (b14) cellulose biosynthesis inhibitors, (b15) dehydrooritate dehydrogenase (DHODH) inhibitors, (b16) other herbicides including mitotic disruptors, organic arsenicals, asulam, bromobutide, cinmethylin, cumyluron, dazomet, difenzoquat, dymron, etobenzanid, flurenol, fosamine, fosamine-ammonium, hydantocidin, metam, methyldymron, oleic acid, oxaziclomefone, pelargonic acid and pyributicarb, (b17) herbicide safeners, and salts of compounds of (b1) through (b17). “Photosystem II inhibitors” (b1) are chemical compounds that bind to the D-1 protein at the QB-binding niche and thus block electron transport from QA to QB in the chloroplast thylakoid membranes. The electrons blocked from passing through photosystem II are transferred through a series of reactions to form toxic compounds that disrupt cell membranes and cause chloroplast swelling, membrane leakage, and ultimately cellular destruction. The QB-binding niche has three different binding sites: binding site A binds the triazines such as atrazine, triazinones such as hexazinone, and uracils such as bromacil, binding site B binds the phenylureas such as diuron, and binding site C binds benzothiadiazoles such as bentazon, nitriles such as bromoxynil and phenyl-pyridazines such as pyridate. Examples of photosystem II inhibitors include ametryn, amicarbazone, atrazine, bentazon, bromacil, bromofenoxim, bromoxynil, chlorbromuron, chloridazon, chlorotoluron, chloroxuron, cumyluron, cyanazine, daimuron, desmedipham, desmetryn, dimefuron, dimethametryn, diuron, ethidimuron, fenuron, fluometuron, hexazinone, ioxynil, isoproturon, isouron, lenacil, linuron, metamitron, methabenzthiazuron, metobromuron, metoxuron, metribuzin,
monolinuron, neburon, pentanochlor, phenmedipham, prometon, prometryn, propanil, propazine, pyridafol, pyridate, siduron, simazine, simetryn, tebuthiuron, terbacil, terbumeton, terbuthylazine, terbutryn and trietazine. “AHAS inhibitors” (b2) are chemical compounds that inhibit acetohydroxy acid synthase (AHAS), also known as acetolactate synthase (ALS), and thus kill plants by inhibiting the production of the branched-chain aliphatic amino acids such as valine, leucine and isoleucine, which are required for protein synthesis and cell growth. Examples of AHAS inhibitors include amidosulfuron, azimsulfuron, bensulfuron-methyl, bispyribac-sodium, cloransulam-methyl, chlorimuron-ethyl, chlorsulfuron, cinosulfuron, cyclosulfamuron, diclosulam, ethametsulfuron-methyl, ethoxysulfuron, flazasulfuron, florasulam, flucarbazone-sodium, flumetsulam, flupyrsulfuron-methyl, flupyrsulfuron-sodium, foramsulfuron, halosulfuron-methyl, imazamethabenz-methyl, imazamox, imazapic, imazapyr, imazaquin, imazethapyr, imazosulfuron, iodosulfuron-methyl (including sodium salt), iofensulfuron (2-iodo-N-[[(4-methoxy-6-methyl-1,3,5-triazin-2- yl)amino]carbonyl]benzenesulfonamide), mesosulfuron-methyl, metazosulfuron (3-chloro-4- (5,6-dihydro-5-methyl-1,4,2-dioxazin-3-yl)-N-[[(4,6-dimethoxy-2- pyrimidinyl)amino]carbonyl]-1-methyl-1H-pyrazole-5-sulfonamide), metosulam, metsulfuron-methyl, nicosulfuron, oxasulfuron, penoxsulam, primisulfuron-methyl, propoxycarbazone-sodium, propyrisulfuron (2-chloro-N-[[(4,6-dimethoxy-2- pyrimidinyl)amino]carbonyl]-6-propylimidazo[1,2-b]pyridazine-3-sulfonamide), prosulfuron, pyrazosulfuron-ethyl, pyribenzoxim, pyriftalid, pyriminobac-methyl, pyrithiobac-sodium, rimsulfuron, sulfometuron-methyl, sulfosulfuron, thiencarbazone, thifensulfuron-methyl, triafamone (N-[2-[(4,6-dimethoxy-1,3,5-triazin-2-yl)carbonyl]-6- fluorophenyl]-1,1-difluoro-N-methylmethanesulfonamide), triasulfuron, tribenuron-methyl, trifloxysulfuron (including sodium salt), triflusulfuron-methyl and tritosulfuron. “ACCase inhibitors” (b3) are chemical compounds that inhibit the acetyl-CoA carboxylase enzyme, which is responsible for catalyzing an early step in lipid and fatty acid synthesis in plants. Lipids are essential components of cell membranes, and without them, new cells cannot be produced. The inhibition of acetyl CoA carboxylase and the subsequent lack of lipid production leads to losses in cell membrane integrity, especially in regions of active growth such as meristems. Eventually shoot and rhizome growth ceases, and shoot meristems and rhizome buds begin to die back. Examples of ACCase inhibitors include alloxydim, butroxydim, clethodim, clodinafop, cycloxydim, cyhalofop, diclofop, fenoxaprop, fluazifop, haloxyfop, pinoxaden, profoxydim, propaquizafop, quizalofop, sethoxydim, tepraloxydim and tralkoxydim, including resolved forms such as fenoxaprop-P, fluazifop-P, haloxyfop-P and quizalofop-P and ester forms such as clodinafop-propargyl, cyhalofop-butyl, diclofop-methyl and fenoxaprop-P-ethyl.
Auxin is a plant hormone that regulates growth in many plant tissues. “Auxin mimics” (b4) are chemical compounds mimicking the plant growth hormone auxin, thus causing uncontrolled and disorganized growth leading to plant death in susceptible species. Examples of auxin mimics include aminocyclopyrachlor (6-amino-5-chloro-2-cyclopropyl-4- pyrimidinecarboxylic acid) and its methyl and ethyl esters and its sodium and potassium salts, 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)-2-Pyridinecarboxylic 2-propyn-1-yl ester (CAS No. 2251111-17-6), 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)-2- Pyridinecarboxylic cyanomethyl ester (CAS No. 2251111-18-7), aminopyralid, benazolin-ethyl, chloramben, clacyfos, clomeprop, clopyralid, dicamba, 2,4-D, 2,4-DB, dichlorprop, fluroxypyr, halauxifen (4-amino-3-chloro-6-(4-chloro-2-fluoro-3- methoxyphenyl)-2-pyridinecarboxylic acid), halauxifen-methyl (methyl 4-amino-3-chloro-6- (4-chloro-2-fluoro-3-methoxyphenyl)-2-pyridinecarboxylate), MCPA, MCPB, mecoprop, picloram, quinclorac, quinmerac, 2,3,6-TBA, triclopyr, and methyl 4-amino-3-chloro-6-(4- chloro-2-fluoro-3-methoxyphenyl)-5-fluoro-2-pyridinecarboxylate. “EPSP synthase inhibitors” (b5) are chemical compounds that inhibit the enzyme, 5-enol-pyruvylshikimate-3-phosphate synthase, which is involved in the synthesis of aromatic amino acids such as tyrosine, tryptophan and phenylalanine. EPSP inhibitor herbicides are readily absorbed through plant foliage and translocated in the phloem to the growing points. Glyphosate is a relatively nonselective postemergence herbicide that belongs to this group. Glyphosate includes esters and salts such as ammonium, isopropylammonium, potassium, sodium (including sesquisodium) and trimesium (alternatively named sulfosate). “Photosystem I electron diverters” (b6) are chemical compounds that accept electrons from Photosystem I, and after several cycles, generate hydroxyl radicals. These radicals are extremely reactive and readily destroy unsaturated lipids, including membrane fatty acids and chlorophyll. This destroys cell membrane integrity, so that cells and organelles “leak”, leading to rapid leaf wilting and desiccation, and eventually to plant death. Examples of this second type of photosynthesis inhibitor include diquat, paraquat and 1-(2-carboxyethyl)-4-(2- pyrimidinyl)pyridazinium (CAS No.2285384-11-2). “PPO inhibitors” (b7) are chemical compounds that inhibit the enzyme protoporphyrinogen oxidase, quickly resulting in formation of highly reactive compounds in plants that rupture cell membranes, causing cell fluids to leak out. Examples of PPO inhibitors include acifluorfen-sodium, azafenidin, benzfendizone, bifenox, butafenacil, carfentrazone, carfentrazone-ethyl, chlomethoxyfen, 3-[2-chloro-5-[3,6-dihydro-3-methyl-2,6-dioxo-4- (trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluorophenyl]-4,5-dihydro-5-methyl-5- Isoxazolecarboxylic ethyl ester (CAS No. 1949837-17-5), cinidon-ethyl, fluazolate, flufenoximacil, flufenpyr-ethyl, flumiclorac-pentyl, flumioxazin, fluoroglycofen-ethyl, fluthiacet-methyl, fomesafen, halosafen, lactofen, oxadiargyl, oxadiazon, oxyfluorfen, pentoxazone, profluazol, pyraclonil, pyraflufen-ethyl, saflufenacil, sulfentrazone, thidiazimin,
trifludimoxazin (dihydro-1,5-dimehyl-6-thioxo-3-[2,2,7-trifluoro-3,4-dihydro-3-oxo-4-(2- propyn-1-yl)-2H-1,4-benzoxazin-6-yl]-1,3,5-triazine-2,4(1H,3H)-dione) and tiafenacil (methyl N-[2-[[2-chloro-5-[3,6-dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)- pyrimidinyl]-4-fluorophenyl]thio]-1-oxopropyl]-β-alaninate). “GS inhibitors” (b8) are chemical compounds that inhibit the activity of the glutamine synthetase enzyme, which plants use to convert ammonia into glutamine. Consequently, ammonia accumulates and glutamine levels decrease. Plant damage probably occurs due to the combined effects of ammonia toxicity and deficiency of amino acids required for other metabolic processes. The GS inhibitors include glufosinate and its esters and salts such as glufosinate-ammonium and other phosphinothricin derivatives, glufosinate-P ((2S)-2-amino- 4-(hydroxymethylphosphinyl)butanoic acid) and bilanaphos. “VLCFA elongase inhibitors” (b9) are herbicides having a wide variety of chemical structures, which inhibit the elongase. Elongase is one of the enzymes located in or near chloroplasts which are involved in biosynthesis of VLCFAs. In plants, very-long-chain fatty acids are the main constituents of hydrophobic polymers that prevent desiccation at the leaf surface and provide stability to pollen grains. Such herbicides include acetochlor, alachlor, anilofos, butachlor, cafenstrole, dimethachlor, dimethenamid, diphenamid, fenoxasulfone (3- [[(2,5-dichloro-4-ethoxyphenyl)methyl]sulfonyl]-4,5-dihydro-5,5-dimethylisoxazole), fentrazamide, flufenacet, indanofan, mefenacet, metazachlor, metolachlor, naproanilide, napropamide, napropamide-M ((2R)-N,N-diethyl-2-(1-naphthalenyloxy)propanamide), pethoxamid, piperophos, pretilachlor, propachlor, propisochlor, pyroxasulfone, and thenylchlor, including resolved forms such as S-metolachlor and chloroacetamides and oxyacetamides. “Auxin transport inhibitors” (b10) are chemical substances that inhibit auxin transport in plants, such as by binding with an auxin-carrier protein. Examples of auxin transport inhibitors include diflufenzopyr, naptalam (also known as N-(1-naphthyl)phthalamic acid and 2-[(1-naphthalenylamino)carbonyl]benzoic acid). “PDS inhibitors” (b11) are chemical compounds that inhibit carotenoid biosynthesis pathway at the phytoene desaturase step. Examples of PDS inhibitors include beflubutamid, diflufenican, fluridone, flurochloridone, flurtamone norflurzon and picolinafen. “HPPD inhibitors” (b12) are chemical substances that inhibit the biosynthesis of synthesis of 4-hydroxyphenyl-pyruvate dioxygenase. Examples of HPPD inhibitors include benzobicyclon, benzofenap, bicyclopyrone (4-hydroxy-3-[[2-[(2-methoxyethoxy)methyl]-6- (trifluoromethyl)-3-pyridinyl]carbonyl]bicyclo[3.2.1]oct-3-en-2-one), fenquinotrione (2-[[8- chloro-3,4-dihydro-4-(4-methoxyphenyl)-3-oxo-2-quinoxalinyl]carbonyl]-1,3- cyclohexanedione), flusulfinam, iptriazopyrid, isoxachlortole, isoxaflutole, mesotrione, pyrasulfotole, pyrazolynate, pyrazoxyfen, sulcotrione, tefuryltrione, tembotrione, tolpyralate (1-[[1-ethyl-4-[3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoyl]-1H-pyrazol-5-
yl]oxy]ethyl methyl carbonate), topramezone, 5-chloro-3-[(2-hydroxy-6-oxo-1-cyclohexen-1- yl)carbonyl]-1-(4-methoxyphenyl)-2(1H)-quinoxalinone, 4-(2,6-diethyl-4-methylphenyl)-5- hydroxy-2,6-dimethyl-3(2H)-pyridazinone, 4-(4-fluorophenyl)-6-[(2-hydroxy-6-oxo-1- cyclohexen-1-yl)carbonyl]-2-methyl-1,2,4-triazine-3,5(2H,4H)-dione, 5-[(2-hydroxy-6-oxo- 1-cyclohexen-1-yl)carbonyl]-2-(3-methoxyphenyl)-3-(3-methoxypropyl)-4(3H)- pyrimidinone, 2-methyl-N-(4-methyl-1,2,5-oxadiazol-3-yl)-3-(methylsulfinyl)-4- (trifluoromethyl)benzamide and 2-methyl-3-(methylsulfonyl)-N-(1-methyl-1H-tetrazol-5-yl)- 4-(trifluoromethyl)benzamide. “HST (homogentisate solanesyltransferase) inhibitors” (b13) disrupt a plant’s ability to convert homogentisate to 2-methyl-6-solanyl-1,4-benzoquinone, thereby disrupting carotenoid biosynthesis. Examples of HST inhibitors include cyclopyrimorate (6-chloro-3-(2- cyclopropyl-6-methylphenoxy)-4-pyridazinyl 4-morpholinecarboxylate), haloxydine, pyriclor, 3-(2-chloro-3,6-difluorophenyl)-4-hydroxy-1-methyl-1,5-naphthyridin-2(1H)-one, 7-(3,5-dichloro-4-pyridinyl)-5-(2,2-difluoroethyl)-8-hydroxypyrido[2,3-b]pyrazin-6(5H)-one and 4-(2,6-diethyl-4-methylphenyl)-5-hydroxy-2,6-dimethyl-3(2H)-pyridazinone. HST inhibitors also include compounds of Formulae A and B.
wherein Rd1 is H, Cl or CF3; Rd2 is H, Cl or Br; Rd3 is H or Cl; Rd4 is H, Cl or CF3; Rd5 is CH3, CH2CH3 or CH2CHF2; and Rd6 is OH, or -OC(=O)-i-Pr; and Re1 is H, F, Cl, CH3 or CH2CH3; Re2 is H or CF3; Re3 is H, CH3 or CH2CH3; Re4 is H, F or Br; Re5 is Cl, CH3, CF3, OCF3 or CH2CH3; Re6 is H, CH3, CH2CHF2 or C≡CH; Re7 is OH, -OC(=O)Et, -OC(=O)-i-Pr or -OC(=O)-t-Bu; and Ae8 is N or CH. “Cellulose biosynthesis inhibitors” (b14) inhibit the biosynthesis of cellulose in certain plants. They are most effective when applied preemergence or early postemergence on young or rapidly growing plants. Examples of cellulose biosynthesis inhibitors include chlorthiamid, dichlobenil, flupoxam, indaziflam (N2-[(1R,2S)-2,3-dihydro-2,6-dimethyl-1H-inden-1-yl]-6- (1-fluoroethyl)-1,3,5-triazine-2,4-diamine), isoxaben and triaziflam. “DHODH (dihydroorotate dehydrogenase) inhibitors” (b15) act through inhibiting catalysis of the fourth step of pyrimidine biosynthesis in plant systems. Inhibition of pyrimidine biosynthesis leads to the cessation of plant growth. Examples of DOHDH
inhibitors include tetflupyrolimet ((3S,4S)-N-(2-fluorophenyl)-1-methyl-2-oxo-4-[3- (trifluoromethyl)phenyl]-3-pyrrolidinecarboxamide) and (3S,4R)-N-(2,3-difluorophenyl)-1- methyl-4-[1-methyl-5-(trifluoromethyl)-1H-pyrazol-3-yl]-2-oxo-3-pyrrolidinecaboxamide. “Other herbicides” (b16) include herbicides that act through a variety of different modes of action such as mitotic disruptors (e.g., flamprop-M-methyl and flamprop-M-isopropyl), organic arsenicals (e.g., DSMA, and MSMA), 7,8-dihydropteroate synthase inhibitors, chloroplast isoprenoid synthesis inhibitors and cell-wall biosynthesis inhibitors. Other herbicides include those herbicides having unknown modes of action or do not fall into a specific category listed in (b1) through (b14) or act through a combination of modes of action listed above. Examples of other herbicides include aclonifen, asulam, amitrole, bixlozone, broclozone, bromobutide, cinmethylin, clomazone, cumyluron, daimuron, difenzoquat, dimesulfazet, epyrifenacil, etobenzanid, fluometuron, flurenol, fosamine, fosamine-ammonium, dazomet, dymron, ipfencarbazone (1-(2,4-dichlorophenyl)-N-(2,4- difluorophenyl)-1,5-dihydro-N-(1-methylethyl)-5-oxo-4H-1,2,4-triazole-4-carboxamide), metam, methyldymron, oleic acid, oxaziclomefone, pelargonic acid, pyributicarb, 2,5- anhydro-3,4-dideoxy-4-[[[(5S)-3-(3,5-difluorophenyl)-5-ethenyl-4,5-dihydro-5- isoxazolyl]carbonyl]amino]-threo-Pentonic methyl ester (CAS No. 27499989-21-6) and 5- [[(2,6-difluorophenyl)methoxy]methyl]-4,5-dihydro-5-methyl-3-(3-methyl-2- thienyl)isoxazole. “Other herbicides” (b16) also include a compound of Formula (b16A)
wherein R12 is H, C1–C6 alkyl, C1–C6 haloalkyl or C4–C8 cycloalkyl; R13 is H, C1–C6 alkyl or C1–C6 alkoxy; Q1 is an optionally substituted ring system selected from the group consisting of phenyl, thienyl, pyridinyl, benzodioxolyl, naphthyl, naphthalenyl, benzofuranyl, furanyl, benzothiophenyl and pyrazolyl, wherein when substituted said ring system is substituted by 1 to 3 R14; Q2 is an optionally substituted ring system selected from the group consisting of phenyl, pyridinyl, benzodioxolyl, pyridinonyl, thiadiazolyl, thiazolyl, and oxazolyl, wherein when substituted said ring system is substituted by 1 to 3 R15;
each R14 is independently halogen, C1–C6 alkyl, C1–C6 haloalkyl, C1–C6 alkoxy, C1– C6 haloalkoxy, C3–C8 cyaloalkyl, cyano, C1–C6 alkylthio, C1–C6 alkylsulfinyl, C1–C6 alkylsulfonyl, SF5, NHR17; or phenyl optionally substituted by 1 to 3 R16; or pyrazolyl optionally substituted by 1 to 3 R16; each R15 is independently halogen, C1–C6 alkyl, C1–C6 haloalkyl, C1–C6 alkoxy, C1– C6 haloalkoxy, cyano, nitro, C1–C6 alkylthio, C1–C6 alkylsulfinyl, C1–C6 alkylsulfonyl; each R16 is independently halogen, C1–C6 alkyl or C1–C6 haloalkyl; R17 is C1–C4 alkoxycarbonyl. In one Embodiment wherein “other herbicides” (b16) also include a compound of Formula (b16A), it is preferred that R12 is H or C1–C6 alkyl; more preferably R12 is H or methyl. Preferrably R13 is H. Preferably Q1 is either a phenyl ring or a pyridinyl ring, each ring substituted by 1 to 3 R14; more preferably Q1 is a phenyl ring substituted by 1 to 2 R14. Preferably Q2 is a phenyl ring substituted by 1 to 3 R15; more preferably Q2 is a phenyl ring substituted by 1 to 2 R15. Preferably each R14 is independently halogen, C1–C4 alkyl, C1–C3 haloalkyl, C1–C3 alkoxy or C1–C3 haloalkoxy; more preferably each R14 is independently chloro, fluoro, bromo, C1–C2 haloalkyl, C1–C2 haloalkoxy or C1–C2 alkoxy. Preferrably each R15 is independently halogen, C1–C4 alkyl, C1–C3 haloalkoxy; more preferably each R15 is independently chloro, fluoro, bromo, C1–C2 haloalkyl, C1–C2 haloalkoxy or C1–C2 alkoxy. Specifically preferred as “other herbicides” (b16) include any one of the following (b16A-1) through (b16A-15):
“Other herbicides” (b16) also include a compound of Formula (b16B)
wherein R18 is H, C1–C6 alkyl, C1–C6 haloalkyl or C4–C8 cycloalkyl; each R19 is independently halogen, C1–C6 haloalkyl or C1–C6 haloalkoxy; p is an integer of 0, 1, 2 or 3; each R20 is independently halogen, C1–C6 haloalkyl or C1–C6 haloalkoxy; and q is an integer of 0, 1, 2 or 3. In one Embodiment wherein “other herbicides” (b16) also include a compound of Formula (b15B), it is preferred that R18 is H, methyl, ethyl or propyl; more preferably R18 is H or methyl; most preferably R18 is H. Preferrably each R19 is independently chloro, fluoro, C1– C3 haloalkyl or C1–C3 haloalkoxy; more preferably each R19 is independently chloro, fluoro, C1 fluoroalkyl (i.e. fluoromethyl, difluoromethyl or trifluoromethyl) or C1 fluoroalkoxy (i.e. trifluoromethoxy, difluoromethoxy or fluoromethoxy). Preferably each R20 is independently chloro, fluoro, C1 haloalkyl or C1 haloalkoxy; more preferably each R20 is independently chloro, fluoro, C1 fluoroalkyl (i.e. fluoromethyl, difluorormethyl or trifluromethyl) or C1 fluoroalkoxy (i.e. trifluoromethoxy, difluoromethoxy or fluoromethoxy). Specifically
preferred as “other herbicides” (b16) include any one of the following (b15B-1) through (b16B-19):
Another Embodiment wherein “other herbicides” (b16) also include a compound of Formula (b16C),
wherein R1 is Cl, Br or CN; and R2 is C(=O)CH2CH2CF3, CH2CH2CH2CH2CF3 or 3-CHF2-isoxazol-5-yl. “Herbicide safeners” (b17) are substances added to a herbicide formulation to eliminate or reduce phytotoxic effects of the herbicide to certain crops. These compounds protect crops from injury by herbicides but typically do not prevent the herbicide from controlling undesired vegetation. Examples of herbicide safeners include but are not limited to benoxacor, cloquintocet-mexyl, cumyluron, cyometrinil, cyprosulfamide, daimuron, dichlormid, dicyclonon, dietholate, dimepiperate, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr-diethyl, mephenate, methoxyphenone, naphthalic anhydride, oxabetrinil, N-(aminocarbonyl)-2-methylbenzenesulfonamide and N- (aminocarbonyl)-2-fluorobenzenesulfonamide, 1-bromo-4-[(chloromethyl)sulfonyl]benzene, 2-(dichloromethyl)-2-methyl-1,3-dioxolane (MG 191), 4-(dichloroacetyl)-1-oxa- 4-azospiro[4.5]decane (MON 4660), 2,2-dichloro-1-(2,2,5-trimethyl-3-oxazolidinyl)- ethanone and 2-methoxy-N-[[4-[[(methylamino)carbonyl]amino]phenyl]sulfonyl]- benzamide. Preferred for better control of undesired vegetation (e.g., lower use rate such as from greater-than-additive effects, broader spectrum of weeds controlled, or enhanced crop safety) or for preventing the development of resistant weeds are mixtures of a compound of this
invention with a herbicide selected from the group consisting of 4-amino-3-chloro-5-fluoro- 6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic 2-propyn-1-yl ester (CAS No.2251111-17- 6), 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic cyanomethyl ester (CAS No.2251111-18-7), 2,5-anhydro-3,4-dideoxy-4-[[[(5S)-3-(3,5-difluorophenyl)-5- ethenyl-4,5-dihydro-5-isoxazolyl]carbonyl]amino]-threo-Pentonic methyl ester (CAS No. 27499989-21-6), atrazine, azimsulfuron, beflubutamid, beflubutamid-M, bixlozone, broclozone, benzisothiazolinone, 1-(2-carboxyethyl)-4-(2-pyrimidinyl)pyridazinium (CAS No. 2285384-11-2) and salts thereof, carfentrazone-ethyl, chlorimuron-ethyl, 3-[2-chloro-5- [3,6-dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluorophenyl]- 4,5-dihydro-5-methyl-5-isoxazolecarboxylic ethyl ester (CAS No. 1949837-17-5), chlorsulfuron-methyl, clomazone, clopyralid potassium, cloransulam-methyl, 2-[(2,4- dichlorophenyl)methyl]-4,4-dimethyl-3-isoxazolidinone, 2-[(2,5- dichlorophenyl)methyl]-4,4-dimethyl-3-isoxazolidinone, ethametsulfuron-methyl, flumetsulam, 4-(4-fluorophenyl)-6-[(2-hydroxy-6-oxo-1-cyclohexen-1-yl)carbonyl]-2- methyl-1,2,4-triazine-3,5-(2H,4H)-dione, flupyrsulfuron-methyl, fluthiacet-methyl, fomesafen, imazethapyr, lenacil, mesotrione, metribuzin, metsulfuron-methyl, pethoxamid, picloram, pyroxasulfone, quinclorac, rimsulfuron, S-metolachlor, sulfentrazone, thifensulfuron-methyl, triflusulfuron-methyl and tribenuron-methyl. One or more of the following methods and variations as described in Schemes 1–5 can be used to prepare the compounds of Formula 1. The definitions of X, J and G in the compounds of Formulae 1–11 below are as defined above in the Summary of the Invention unless otherwise noted. Compounds of Formulae 1a, 1b, 1c, 1d and 1e are various subsets of the compounds of Formula 1. Compounds of Formula 1a (i.e. compounds of Formula 1 wherein Q is O) can be made by the general synthetic route outlined in Scheme 1. Reacting a cyclopropyl dihalopyrimidine of Formula 2 (wherein X is a halogen as leaving group) with an appropriately substituted phenol or hydroxy heterocycle of Formula J-OH, in the presence of a suitable base and solvent, can provide intermediates of Formula 3. Examples of suitable bases for this reaction include but are not limited to potassium carbonate, sodium hydroxide, potassium t-butoxide, sodium hydride or potassium hydroxide, and compatible solvents include but not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide. Temperatures for this reaction generally range from 0° C to warming below or heating at the boiling point of the solvent. Although the halogen X at the 4-position of a pyrimidine of Formula 2 is generally favored as the leaving group over the halogen X at the 2-position, one skilled in the art will recognize that some regioisomeric side-product of intermediate 3 can form from displacement of the halogen at the 2-position and side-adduct may require separation from 3 by chromatography or recrystallization.
Cross-coupling of intermediates of Formula 3 with an azole heterocycle of Formula (G- 1)H, an aryl boronic acid of Formula (G-2)B(OH)2, a pyridyl boronic acid of Formula (G- 3)B(OH)2, (G-4)B(OH)2 or (G-5)B(OH)2, a thienyl boronic acid of Formula (G-6) B(OH)2 or a thiazolyl boronic acid of Formula (G-7)B(OH)2, in the presence of a suitable palladium catalyst in a solvent such as tetrahydrofuran, acetonitrile, toluene, dioxane or N,N- dimethylformamide or dichloromethane, affords compounds of Formula 1a. The halogen substituent X can be chlorine, bromine or in some cases iodine. Preferable palladium catalysts include but are not limited to the commercially available chloro[2-(di-tert-butylphosphino)- 2′,4′,6′-triisopropyl-1,1′-biphenyl][2-(2-aminoethyl)phenyl)] palladium(II) [t-BuXPhos palladium(II) phenethylamine chloride, tBuXPhos-Pd-G1], chloro[(tri-tert-butylphosphine)- 2-(2-aminobiphenyl)] palladium(II) [P(t-Bu)3 Pd G2], methanesulfonato(2-di-t- butylphosphino-2;methanesulfonato(2-di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl) (2'-amino-1,1'-biphenyl-2-yl)palladium(II) [tBuXPhos-Pd-G3], 2-Dicyclohexylphosphino- 2′,6′-diisopropoxybiphenyl [RuPhos], (2-Dicyclohexylphosphino-2′,6′-diisopropoxy-1,1′- biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate [RuPhos-G3] or Pd2(dba)3 with SPhos. Temperatures for this coupling reaction generally range from 0° C to warming below or heating at the boiling point of the solvent.
Compounds of Formula 1b (i.e. compounds of Formula 1 where Q is CH2) can be made by the general synthetic route usually referred to as a “Negishi Coupling” as outlined in Scheme 2. Reacting a cyclopropyl dihalopyrimidine of Formula 2 (where X is a halogen as leaving group) with an appropriately substituted benzyl or pyridylmethyl zinc reagent of Formula J-CH2-ZnX1 (X1 is halogen) in a solvent such as tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide, provides an intermediate of Formula 4.
Temperatures for this reaction generally range from –78° C to warming below or heating at the boiling point of the solvent. Although the halogen X at the 4-position of a pyrimidine of Formula 2 is usually favored as the preferred leaving group over the halogen X at the 2-position, one skilled in the art will recognize that some regioisomeric side-product of intermediate 4 can form from displacement of the halogen at the 2-position. Separation by chromatography or recrystallization may be needed to isolate the desired isomers. Reagents of Formula J-CH2-ZnX1 are readily generated from commercially available benzyl and heteroarylmethyl halides by reacting with an appropriate zinc reagent (i.e. zinc dust, ZnCl2, TMPZnCl·LiCl, or TMP2Zn·2MgCl2·2LiCl], generally in the solvent used for the coupling. Cross-coupling of intermediates of Formula 4 with an azole heterocycle of Formula (G-1)H, an aryl boronic acid of Formula (G-2)B(OH)2, a pyridyl boronic acid of Formula (G-3)B(OH)2, (G-4)B(OH)2 or (G-5)B(OH)2, a thienyl boronic acid of Formula (G-6)B(OH)2 or a thiazolyl halide of Formula (G-7)B(OH)2, in the presence of a suitable palladium catalyst (as listed above for Scheme 1) in a solvent such as tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide or dichloromethane, affords compounds of Formula 1b. The halogen substituent X can be chlorine, bromine or in some cases iodine. In the case wherein G is G-1, compounds of Formula 1b can also be made without use of a palladium catalyst, instead by reacting an intermediate of Formula 4 with an azole of Formula (G-1)H in the presence of a base such as potassium carbonate, sodium hydride or sodium hydroxide in a compatible solvent for the particular base used. Examples of solvents include but are not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide. Temperatures for this nucleophilic displacement reaction can range from 0° C to ambient temperature to warming below or heating at the boiling point of the solvent.
An alternative method for accessing compounds of Formula 1c (i.e. compounds of Formula 1 where X is O and G is G-1) regioselectivity, is summarized in Scheme 3. Reacting a dihalopyrimidine or Formula 2 with sodium thiomethoxide in a solvent such as tetrahydrofuran or dioxane at a temperature ranging usually from 0° C to ambient temperature, affords intermediates of Formula 5 as the major product. This reaction can sometimes give rise to an isomeric side-product resulting from mercaptan displacement of the halogen at 2- position in various amounts where that isomeric impurity may require separation by chromatography or crystallization. Displacement of the halogen at 2- position on intermediates of Formula 5 with an azole of Formula G-1, in the presence of a base (preferably potassium carbonate, sodium hydride or sodium hydroxide) in a compatible solvent (i.e. acetonitrile, toluene, dioxane or N,N-dimethylformamide) at temperature ranging usually from ambient temperature to warming below the reflux temperature of the solvent, gives intermediates of Formula 6. Oxidation of the compound of Formula 6 with an oxidizing agent, generally meta-chloroperoxybenzoic acid (MCPBA) in dichloromethane (DCM), affords the corresponding sulfones 7. The methyl sulfone substituent is now an activated leaving group, where upon reacting 7 with phenols and hydroxyheterocycles of Formula J-OH, in the presence of base and solvent (e.g. potassium carbonate in N,N-dimethylformamide) at ambient temperature to warming below the reflux temperature of the solvent, gives final products of Formula 1c.
Another method for making pyrimidines of Formula 1d (i.e. compounds of Formula 1 where X is O and G is G-2 to G-7) is outlined in Scheme 4. Reaction of an amidine of Formula 8 (which is commercially available in many cases or readily prepared by standard methods know in the art) with ethyl α-formyl-cyclopropaneacetate of Formula 9, in the presence of an appropriate base such as sodium methoxide, potassium carbonate, or potassium t-butoxide in a solvent that includes but not limited to methanol, ethanol, dimethyl sulfoxide, N,N-dimethylformamide or acetonitrile, at temperatures ranging usually from ambient temperature to the reflux temperature of the solvent, affords hydroxypyrimidines of Formula 10. Heating the compound of Formula 10 in phosphorous oxychloride (and in some cases with thionyl chloride or oxalyl chloride, optionally with a cosolvent such as toluene or dichloromethane) provides chloropyrimidines of Formula 11. Reacting a compound of Formula 11 with an appropriately substituted phenol or hydroxy heterocycle of Formula J- OH, in the presence of a suitable base and solvent, can provide final products of Formula 1d. Examples of suitable bases for this reaction include but are not limited to potassium carbonate, sodium hydroxide, potassium t-butoxide, sodium hydride or potassium hydroxide; and compatible solvents include but not limited to tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide. Temperatures for this reaction generally range from 0° C to warming below or heating at the boiling point of the solvent.
s C Reaction of the chloropyrimidine of Formula 11 with an appropriately substituted benzyl or pyridylmethyl zinc reagent of Formula JCH2ZnX (X is halogen), in a solvent such as tetrahydrofuran, acetonitrile, toluene, dioxane or N,N-dimethylformamide, also provides end products of Formula 1e. Temperatures for this reaction generally range from –78° C to warming below or heating at the boiling point of the solvent. Reagents of Formula JCH2ZnBr are readily generated from commercially available benzyl and heteroarylmethyl halides by reacting with an appropriate zinc reagent (i.e. zinc dust, ZnCl2, TMPZnCl·LiCl, or TMP2Zn·2MgCl2·2LiCl], generally in the solvent used for the coupling.
It is recognized by one skilled in the art that various functional groups can be converted into others to provide different compounds of Formula 1. For a valuable resource that illustrates the interconversion of functional groups in a simple and straightforward fashion, see Larock, R. C., Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 2nd Ed., Wiley-VCH, New York, 1999. For example, intermediates for the preparation of compounds of Formula 1 may contain aromatic nitro groups, which can be reduced to amino groups, and then be converted via reactions well known in the art such as
the Sandmeyer reaction, to various halides, providing compounds of Formula 1. The above reactions can also in many cases be performed in alternate order. It is recognized that some reagents and reaction conditions described above for preparing compounds of Formula 1 may not be compatible with certain functionalities present in the intermediates. In these instances, the incorporation of protection/deprotection sequences or functional group interconversions into the synthesis will aid in obtaining the desired products. The use and choice of the protecting groups will be apparent to one skilled in chemical synthesis (see, for example, Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991). One skilled in the art will recognize that, in some cases, after the introduction of a given reagent as depicted in any individual scheme, it may be necessary to perform additional routine synthetic steps not described in detail to complete the synthesis of compounds of Formula 1. One skilled in the art will also recognize that it may be necessary to perform a combination of the steps illustrated in the above schemes in an order other than that implied by the partcular presented to prepare the compounds of Formula 1. One skilled in the art will also recognize that compounds of Formula 1 and the intermediates described herein can be subjected to various electrophilic, nucleophilic, radical, organometallic, oxidation, and reduction reactions to add substituents or modify existing substituents. Without further elaboration, it is believed that one skilled in the art using the preceding description can utilize the present invention to its fullest extent. The following non-limiting Examples are illustrative of the invention. Steps in the following Examples illustrate a procedure for each step in an overall synthetic transformation, and the starting material for each step may not have necessarily been prepared by a particular preparative run whose procedure is described in other Examples or Steps. Percentages are by weight except for chromatographic solvent mixtures or where otherwise indicated. Parts and percentages for chromatographic solvent mixtures are by volume unless otherwise indicated. All NMR spectra are reported in CDCl3 downfield from tetramethylsilane at 500 MHz unless otherwise indicated where s means singlet, brs means broad singlet, d means doublet, t means triplet and m means multiplet. SYNTHESIS EXAMPLE 1 Preparation of 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[3-(trifluoromethyl)- 1H-pyrazol-1-yl]pyrimidine (Compound 1) Step A: Preparation of 2-chloro-5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy] pyrimidine A vial was charged with 2,4-dichloro-5-cyclopropylpyrimidine (0.5 g, 2.65 mmol), potassium carbonate (0.40 g, 2.90 mmol), and m-trifluoromethylphenol (0.47 g, 2.90 mmol). N,N-Dimethylformamide 13.23 mL was added and the mixture was heated at 80 °C for 1 h.
The reaction was partitioned between diethyl ether and water. The organic phase was separated, dried over magnesium sulfate, filtered, and concentrated to give 0.80 g of the title compound. 1H NMR δ ppm 8.10 (s, 1H), 7.53–7.61 (m, 2H), 7.44–7.48 (m, 1H), 7.38–7.42 (m, 1H), 2.06 (m, 1H), 1.07–1.13 (m, 2H), 0.82–0.88 (m, 2H).
g y y rifluoromethyl)phenoxy]pyrimidine (i.e. the product of step A of Synthesis Example 1, 0.25 g, 0.79 mmol), 4-(trifluoromethyl)phenylboronic acid (0.18 g, 0.95 mmol), potassium phosphate tribasic (0.20 g, 0.95 mmol), and Xphos Pd G2 (0.063 g, 0.079 mmol) and purged with N2. Dioxane (3.97 mL) was added and the vial was purged twice more with N2 and heated at 70 °C overnight. The crude reaction was concentrated onto Celite® (diatomaceaous-earth filter aid) and purified via silica gel column chromatography (eluting with a hexanes/ethyl acetate gradient) to obtain 0.19 g of the title compound. 1H NMR (500 MHz) δ ppm 8.34 (s, 1H), 8.24 (d, 2H), 7.55–7.66 (m, 5H), 7.43–7.49 (m, 1H), 2.11–2.21 (m, 1H), 1.09–1.16 (m, 2H), 0.90–0.95 (m, 2H). SYNTHESIS EXAMPLE 3 Preparation of 5-cyclopropyl-4-[[3-(trifluoromethyl)phenyl]methyl]-2-[3-(trifluoromethyl)- 1H-pyrazol-1-yl]pyrimidine (Compound 7)
(trifluoromethyl)phenyl]methyl]pyrimidine (i.e. the product of Step A)(0.25 g, 0.79 mmol), 3- (trifluoromethyl)-1H-pyrazole (0.11 g, 0.794 mmol), potassium phosphate tribasic (0.20 g, 0.95 mmol), and tBuXphos Pd G3 (0.063 g, 0.079 mmol) and purged with N2. Dioxane 3.97 mL was added and the vial was purged 2x more with N2 and heated at 50 °C overnight. The crude reaction mixture was concentrated onto Celite® (diatomaceaous-earth filter aid) and purified via silica gel column chromatography (eluting with a hexanes/ethyl acetate gradient) to obtain 0.19 g of the title compound. 1H NMR (500 MHz) δ ppm 8.52–8.60 (m, 1H), 8.44 (s, 1H), 7.56–7.62 (m, 1H), 7.53 (d, 1H), 7.41–7.50 (m, 2H), 6.71 (d, 1H), 4.43 (s, 2H), 1.79–1.86 (m, 1H), 1.03–1.11 (m, 2H), 0.68– 0.76 (m, 2H). By the procedures described herein together with methods known in the art, the following compounds of Tables 1 to 18 can be prepared. The following abbreviations are used in the Tables which follow: t means tertiary, s means secondary, n means normal, i means iso, c means cyclo, Me means methyl, Et means ethyl, Pr means propyl, Bu means butyl, i-Pr means isopropyl, c-Pr means cyclopropyl, t-Bu means tertiary butyl, Ph means phenyl, OMe means methoxy, OEt means ethoxy, SMe means methylthio, -CN means cyano, -NO2 means nitro, TMS means trimethylsilyl, SOMe means methylsulfinyl, C2F5 means CF2CF3 and SO2Me means methylsulfonyl.
The present disclosure also includes Tables 2 through 18.
Table 2 Table 3
Table 8 Table 9
Table 14 Table 15
Formulation/Utility A compound of this invention will generally be used as a herbicidal active ingredient in a composition, i.e. formulation, with at least one additional component selected from the group consisting of surfactants, solid diluents and liquid diluents, which serves as a carrier. The formulation or composition ingredients are selected to be consistent with the physical
properties of the active ingredient, mode of application and environmental factors such as soil type, moisture and temperature. Useful formulations include both liquid and solid compositions. Liquid compositions include solutions (including emulsifiable concentrates), suspensions, emulsions (including microemulsions, oil-in -water emulsions, flowable concentrates and/or suspoemulsions) and the like, which optionally can be thickened into gels. The general types of aqueous liquid compositions are soluble concentrate, suspension concentrate, capsule suspension, concentrated emulsion, microemulsion, oil-in-water emulsion, flowable concentrate and suspo-emulsion. The general types of nonaqueous liquid compositions are emulsifiable concentrate, microemulsifiable concentrate, dispersible concentrate and oil dispersion. The general types of solid compositions are dusts, powders, granules, pellets, prills, pastilles, tablets, filled films (including seed coatings) and the like, which can be water-dispersible (“wettable”) or water-soluble. Films and coatings formed from film- forming solutions or flowable suspensions are particularly useful for seed treatment. Active ingredient can be (micro)encapsulated and further formed into a suspension or solid formulation; alternatively, the entire formulation of active ingredient can be encapsulated (or “overcoated”). Encapsulation can control or delay release of the active ingredient. An emulsifiable granule combines the advantages of both an emulsifiable concentrate formulation and a dry granular formulation. High-strength compositions are primarily used as intermediates for further formulation. Sprayable formulations are typically extended in a suitable medium before spraying. Such liquid and solid formulations are formulated to be readily diluted in the spray medium, usually water, but occasionally another suitable medium like an aromatic or paraffinic hydrocarbon or vegetable oil. Spray volumes can range from about from about one to several thousand liters per hectare, but more typically are in the range from about ten to several hundred liters per hectare. Sprayable formulations can be tank mixed with water or another suitable medium for foliar treatment by aerial or ground application, or for application to the growing medium of the plant. Liquid and dry formulations can be metered directly into drip irrigation systems or metered into the furrow during planting. The formulations will typically contain effective amounts of active ingredient, diluent and surfactant within the following approximate ranges which add up to 100 percent by weight.
Solid diluents include, for example, clays such as bentonite, montmorillonite, attapulgite and kaolin, gypsum, cellulose, titanium dioxide, zinc oxide, starch, dextrin, sugars (e.g., lactose, sucrose), silica, talc, mica, diatomaceous earth, urea, calcium carbonate, sodium carbonate and bicarbonate, and sodium sulfate. Typical solid diluents are described in Watkins et al., Handbook of Insecticide Dust Diluents and Carriers, 2nd Ed., Dorland Books, Caldwell, New Jersey. Liquid diluents include, for example, water, N,N-dimethylalkanamides (e.g., N,N-dimethylformamide), limonene, dimethyl sulfoxide, N-alkylpyrrolidones (e.g., N-methylpyrrolidinone), alkyl phosphates (e.g., triethyl phosphate), ethylene glycol, triethylene glycol, propylene glycol, dipropylene glycol, polypropylene glycol, propylene carbonate, butylene carbonate, paraffins (e.g., white mineral oils, normal paraffins, isoparaffins), alkylbenzenes, alkylnaphthalenes, glycerine, glycerol triacetate, sorbitol, aromatic hydrocarbons, dearomatized aliphatics, alkylbenzenes, alkylnaphthalenes, ketones such as cyclohexanone, 2-heptanone, isophorone and 4-hydroxy-4-methyl-2-pentanone, acetates such as isoamyl acetate, hexyl acetate, heptyl acetate, octyl acetate, nonyl acetate, tridecyl acetate and isobornyl acetate, other esters such as alkylated lactate esters, dibasic esters, alkyl and aryl benzoates and γ-butyrolactone, and alcohols, which can be linear, branched, saturated or unsaturated, such as methanol, ethanol, n-propanol, isopropyl alcohol, n-butanol, isobutyl alcohol, n-hexanol, 2-ethylhexanol, n-octanol, decanol, isodecyl alcohol, isooctadecanol, cetyl alcohol, lauryl alcohol, tridecyl alcohol, oleyl alcohol, cyclohexanol, tetrahydrofurfuryl alcohol, diacetone alcohol, cresol and benzyl alcohol. Liquid diluents also include glycerol esters of saturated and unsaturated fatty acids (typically C6–C22), such as plant seed and fruit oils (e.g., oils of olive, castor, linseed, sesame, corn (maize), peanut, sunflower, grapeseed, safflower, cottonseed, soybean, rapeseed, coconut and palm kernel), animal-sourced fats (e.g., beef tallow, pork tallow, lard, cod liver oil, fish oil), and mixtures thereof. Liquid diluents also include alkylated fatty acids (e.g., methylated,
ethylated, butylated) wherein the fatty acids may be obtained by hydrolysis of glycerol esters from plant and animal sources, and can be purified by distillation. Typical liquid diluents are described in Marsden, Solvents Guide, 2nd Ed., Interscience, New York, 1950. The solid and liquid compositions of the present invention often include one or more surfactants. When added to a liquid, surfactants (also known as “surface-active agents”) generally modify, most often reduce, the surface tension of the liquid. Depending on the nature of the hydrophilic and lipophilic groups in a surfactant molecule, surfactants can be useful as wetting agents, dispersants, emulsifiers or defoaming agents. Surfactants can be classified as nonionic, anionic or cationic. Nonionic surfactants useful for the present compositions include, but are not limited to: alcohol alkoxylates such as alcohol alkoxylates based on natural and synthetic alcohols (which may be branched or linear) and prepared from the alcohols and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof; amine ethoxylates, alkanolamides and ethoxylated alkanolamides; alkoxylated triglycerides such as ethoxylated soybean, castor and rapeseed oils; alkylphenol alkoxylates such as octylphenol ethoxylates, nonylphenol ethoxylates, dinonyl phenol ethoxylates and dodecyl phenol ethoxylates (prepared from the phenols and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof); block polymers prepared from ethylene oxide or propylene oxide and reverse block polymers where the terminal blocks are prepared from propylene oxide; ethoxylated fatty acids; ethoxylated fatty esters and oils; ethoxylated methyl esters; ethoxylated tristyrylphenol (including those prepared from ethylene oxide, propylene oxide, butylene oxide or mixtures thereof); fatty acid esters, glycerol esters, lanolin- based derivatives, polyethoxylate esters such as polyethoxylated sorbitan fatty acid esters, polyethoxylated sorbitol fatty acid esters and polyethoxylated glycerol fatty acid esters; other sorbitan derivatives such as sorbitan esters; polymeric surfactants such as random copolymers, block copolymers, alkyd peg (polyethylene glycol) resins, graft or comb polymers and star polymers; polyethylene glycols (pegs); polyethylene glycol fatty acid esters; silicone-based surfactants; and sugar-derivatives such as sucrose esters, alkyl polyglycosides and alkyl polysaccharides. Useful anionic surfactants include, but are not limited to: alkylaryl sulfonic acids and their salts; carboxylated alcohol or alkylphenol ethoxylates; diphenyl sulfonate derivatives; lignin and lignin derivatives such as lignosulfonates; maleic or succinic acids or their anhydrides; olefin sulfonates; phosphate esters such as phosphate esters of alcohol alkoxylates, phosphate esters of alkylphenol alkoxylates and phosphate esters of styryl phenol ethoxylates; protein-based surfactants; sarcosine derivatives; styryl phenol ether sulfate; sulfates and sulfonates of oils and fatty acids; sulfates and sulfonates of ethoxylated alkylphenols; sulfates of alcohols; sulfates of ethoxylated alcohols; sulfonates of amines and amides such as N,N- alkyltaurates; sulfonates of benzene, cumene, toluene, xylene, and dodecyl and tridecylbenzenes; sulfonates of condensed naphthalenes; sulfonates of naphthalene and alkyl
naphthalene; sulfonates of fractionated petroleum; sulfosuccinamates; and sulfosuccinates and their derivatives such as dialkyl sulfosuccinate salts. Useful cationic surfactants include, but are not limited to: amides and ethoxylated amides; amines such as N-alkyl propanediamines, tripropylenetriamines and dipropylenetetramines, and ethoxylated amines, ethoxylated diamines and propoxylated amines (prepared from the amines and ethylene oxide, propylene oxide, butylene oxide or mixtures thereof); amine salts such as amine acetates and diamine salts; quaternary ammonium salts such as quaternary salts, ethoxylated quaternary salts and diquaternary salts; and amine oxides such as alkyldimethylamine oxides and bis-(2-hydroxyethyl)-alkylamine oxides. Also useful for the present compositions are mixtures of nonionic and anionic surfactants or mixtures of nonionic and cationic surfactants. Nonionic, anionic and cationic surfactants and their recommended uses are disclosed in a variety of published references including McCutcheon’s Emulsifiers and Detergents, annual American and International Editions published by McCutcheon’s Division, The Manufacturing Confectioner Publishing Co.; Sisely and Wood, Encyclopedia of Surface Active Agents, Chemical Publ. Co., Inc., New York, 1964; and A. S. Davidson and B. Milwidsky, Synthetic Detergents, Seventh Edition, John Wiley and Sons, New York, 1987. Compositions of this invention may also contain formulation auxiliaries and additives, known to those skilled in the art as formulation aids (some of which may be considered to also function as solid diluents, liquid diluents or surfactants). Such formulation auxiliaries and additives may control: pH (buffers), foaming during processing (antifoams such polyorganosiloxanes), sedimentation of active ingredients (suspending agents), viscosity (thixotropic thickeners), in-container microbial growth (antimicrobials), product freezing (antifreezes), color (dyes/pigment dispersions), wash-off (film formers or stickers), evaporation (evaporation retardants), and other formulation attributes. Film formers include, for example, polyvinyl acetates, polyvinyl acetate copolymers, polyvinylpyrrolidone-vinyl acetate copolymer, polyvinyl alcohols, polyvinyl alcohol copolymers and waxes. Examples of formulation auxiliaries and additives include those listed in McCutcheon’s Volume 2: Functional Materials, annual International and North American editions published by McCutcheon’s Division, The Manufacturing Confectioner Publishing Co.; and PCT Publication WO 03/024222. The compound of Formula 1 and any other active ingredients are typically incorporated into the present compositions by dissolving the active ingredient in a solvent or by grinding in a liquid or dry diluent. Solutions, including emulsifiable concentrates, can be prepared by simply mixing the ingredients. If the solvent of a liquid composition intended for use as an emulsifiable concentrate is water-immiscible, an emulsifier is typically added to emulsify the active-containing solvent upon dilution with water. Active ingredient slurries, with particle diameters of up to 2,000 μm can be wet milled using media mills to obtain particles with
average diameters below 3 μm. Aqueous slurries can be made into finished suspension concentrates (see, for example, U.S.3,060,084) or further processed by spray drying to form water-dispersible granules. Dry formulations usually require dry milling processes, which produce average particle diameters in the 2 to 10 μm range. Dusts and powders can be prepared by blending and usually grinding (such as with a hammer mill or fluid-energy mill). Granules and pellets can be prepared by spraying the active material upon preformed granular carriers or by agglomeration techniques. See Browning, “Agglomeration”, Chemical Engineering, December 4, 1967, pp 147–48, Perry’s Chemical Engineer’s Handbook, 4th Ed., McGraw-Hill, New York, 1963, pages 8–57 and following, and WO 91/13546. Pellets can be prepared as described in U.S.4,172,714. Water-dispersible and water-soluble granules can be prepared as taught in U.S. 4,144,050, U.S. 3,920,442 and DE 3,246,493. Tablets can be prepared as taught in U.S. 5,180,587, U.S. 5,232,701 and U.S. 5,208,030. Films can be prepared as taught in GB 2,095,558 and U.S.3,299,566. For further information regarding the art of formulation, see T. S. Woods, “The Formulator’s Toolbox – Product Forms for Modern Agriculture” in Pesticide Chemistry and Bioscience, The Food–Environment Challenge, T. Brooks and T. R. Roberts, Eds., Proceedings of the 9th International Congress on Pesticide Chemistry, The Royal Society of Chemistry, Cambridge, 1999, pp.120–133. See also U.S.3,235,361, Col.6, line 16 through Col.7, line 19 and Examples 10–41; U.S.3,309,192, Col.5, line 43 through Col.7, line 62 and Examples 8, 12, 15, 39, 41, 52, 53, 58, 132, 138–140, 162–164, 166, 167 and 169–182; U.S.2,891,855, Col.3, line 66 through Col.5, line 17 and Examples 1–4; Klingman, Weed Control as a Science, John Wiley and Sons, Inc., New York, 1961, pp 81–96; Hance et al., Weed Control Handbook, 8th Ed., Blackwell Scientific Publications, Oxford, 1989; and Developments in formulation technology, PJB Publications, Richmond, UK, 2000. In the following Examples, all percentages are by weight and all formulations are prepared in conventional ways. Compound numbers refer to compounds in Index Table A. Without further elaboration, it is believed that one skilled in the art using the preceding description can utilize the present invention to its fullest extent. The following Examples are, therefore, to be construed as merely illustrative, and not limiting of the disclosure in any way whatsoever. Percentages are by weight except where otherwise indicated. Example A High Strength Concentrate Compound 1 98.5% silica aerogel 0.5% synthetic amorphous fine silica 1.0% Example B Wettable Powder Compound 1 65.0%
Water 53.7%
Test results indicate that the compounds of the present invention are highly active preemergent and/or postemergent herbicides and/or plant growth regulants. The compounds of the inention generally show highest activity for postemergence weed control (i.e. applied after weed seedlings emerge from the soil) and preemergence weed control (i.e. applied before weed seedlings emerge from the soil). Many of them have utility for broad-spectrum pre- and/or postemergence weed control in areas where complete control of all vegetation is desired such as around fuel storage tanks, industrial storage areas, parking lots, drive-in theaters, air fields, river banks, irrigation and other waterways, around billboards and highway and railroad structures. Many of the compounds of this invention, by virtue of selective metabolism in crops versus weeds, or by selective activity at the locus of physiological inhibition in crops and weeds, or by selective placement on or within the environment of a mixture of crops and weeds, are useful for the selective control of grass and broadleaf weeds within a crop/weed mixture. One skilled in the art will recognize that the preferred
combination of these selectivity factors within a compound or group of compounds can readily be determined by performing routine biological and/or biochemical assays. Compounds of this invention may show tolerance to important agronomic crops including, but is not limited to, alfalfa, barley, cotton, wheat, rape, sugar beets, corn (maize), sorghum, soybeans, rice, oats, peanuts, vegetables, tomato, potato, perennial plantation crops including coffee, cocoa, oil palm, rubber, sugarcane, citrus, grapes, fruit trees, nut trees, banana, plantain, pineapple, hops, tea and forests such as eucalyptus and conifers (e.g., loblolly pine), and turf species (e.g., Kentucky bluegrass, St. Augustine grass, Kentucky fescue and Bermuda grass). Compounds of this invention can be used in crops genetically transformed or bred to incorporate resistance to herbicides, express proteins toxic to invertebrate pests (such as Bacillus thuringiensis toxin), and/or express other useful traits. Those skilled in the art will appreciate that not all compounds are equally effective against all weeds. Alternatively, the subject compounds are useful to modify plant growth. As the compounds of the invention have both preemergent and postemergent herbicidal activity, to control undesired vegetation by killing or injuring the vegetation or reducing its growth, the compounds can be usefully applied by a variety of methods involving contacting a herbicidally effective amount of a compound of the invention, or a composition comprising said compound and at least one of a surfactant, a solid diluent or a liquid diluent, to the foliage or other part of the undesired vegetation or to the environment of the undesired vegetation such as the soil or water in which the undesired vegetation is growing or which surrounds the seed or other propagule of the undesired vegetation. Undesired vegetation includes at least one selected from the group consisting of grass weeds and broadleaf weeds. Undesired vegetation is selected from the group consisting of annual bluegrass, Benghal dayflower, blackgrass, black nightshade, broadleaf signalgrass, Canada thistle, cheat, common cocklebur (Xanthium pensylvanicum), common ragweed, corn poppies, field violet, giant foxtail, goosegrass, green foxtail, guinea grass, hairy beggarticks, herbicide-resistant black grass, horseweed, Italian rye grass, jimsonweed, Johnson grass (Sorghum halepense), large crabgrass, little seed canary grass, morning glory, Pennsylvania smartweed, pitted morning glory, prickly sida, quackgrass, redroot pigweed, shattercane, shepherd's purse, silky windgrass, sunflower (as weed in potato), wild buckwheat (Polygonum convolvulus), wild mustard (Brassica kaber), wild oat (Avena fatua), wild pointsettia, yellow foxtail, and yellow nutsedge (Cyperus esculentus). A herbicidally effective amount of the compounds of this invention is determined by a number of factors. These factors include: formulation selected, method of application, amount and type of vegetation present, growing conditions, etc. In general, a herbicidally effective amount of compounds of this invention is about 0.001 to 20 kg/ha with a preferred range of about 0.004 to 1 kg/ha. One skilled in the art can easily determine the herbicidally effective amount necessary for the desired level of weed control.
In one common embodiment, a compound of the invention is applied, typically in a formulated composition, to a locus comprising desired vegetation (e.g., crops) and undesired vegetation (i.e. weeds), both of which may be seeds, seedlings and/or larger plants, in contact with a growth medium (e.g., soil). In this locus, a composition comprising a compound of the invention can be directly applied to a plant or a part thereof, particularly of the undesired vegetation, and/or to the growth medium in contact with the plant. Although most typically, compounds of the invention are used to control undesired vegetation, contact of desired vegetation in the treated locus with compounds of the invention may result in super-additive or enhanced effects with genetic traits in the desired vegetation, including traits incorporated through genetic modification. For example, resistance to phytophagous insect pests or plant diseases, tolerance to biotic/abiotic stresses or storage stability may be greater than expected from the genetic traits in the desired vegetation. Compounds of this invention can also be mixed with one or more other biologically active compounds or agents including herbicides, herbicide safeners, fungicides, insecticides, nematocides, bactericides, acaricides, growth regulators such as insect molting inhibitors and rooting stimulants, chemosterilants, semiochemicals, repellents, attractants, pheromones, feeding stimulants, plant nutrients, other biologically active compounds or entomopathogenic bacteria, virus or fungi to form a multi-component pesticide giving an even broader spectrum of agricultural protection. Mixtures of the compounds of the invention with other herbicides can broaden the spectrum of activity against additional weed species, and suppress the proliferation of any resistant biotypes. Thus the present invention also pertains to a composition comprising a compound of Formula 1 (in a herbicidally effective amount) and at least one additional biologically active compound or agent (in a biologically effective amount) and can further comprise at least one of a surfactant, a solid diluent or a liquid diluent. The other biologically active compounds or agents can be formulated in compositions comprising at least one of a surfactant, solid or liquid diluent. For mixtures of the present invention, one or more other biologically active compounds or agents can be formulated together with a compound of Formula 1, to form a premix, or one or more other biologically active compounds or agents can be formulated separately from the compound of Formula 1, and the formulations combined together before application (e.g., in a spray tank) or, alternatively, applied in succession. A mixture of one or more of the following herbicides with a compound of this invention may be particularly useful for weed control: acetochlor, acifluorfen and its sodium salt, aclonifen, acrolein (2-propenal), alachlor, alloxydim, ametryn, amicarbazone, amidosulfuron, aminocyclopyrachlor and its esters (e.g., methyl, ethyl) and salts (e.g., sodium, potassium), 4-amino-3-chloro-5-fluoro-6-(7-fluoro-1H-indol-6-yl)-2- Pyridinecarboxylic 2-propyn-1-yl ester (CAS No.2251111-17-6), 4-amino-3-chloro-5- fluoro-6-(7-fluoro-1H-indol-6-yl)- 2-Pyridinecarboxylic cyanomethyl ester (CAS No.
2251111-18-7), aminopyralid, amitrole, ammonium sulfamate, 2,5-anhydro-3,4-dideoxy-4- [[[(5S)-3-(3,5-difluorophenyl)-5-ethenyl-4,5-dihydro-5-isoxazolyl]carbonyl]amino]-threo- Pentonic methyl ester (CAS No.27499989-21-6), anilofos, anisiflupurin, asulam, atrazine, azimsulfuron, bixlozone, beflubutamid, beflubutamid-M, benazolin, benazolin-ethyl, bencarbazone, benfluralin, benfuresate, benquitrione, bensulfuron-methyl, bensulide, bentazone, benzobicyclon, benzofenap, bicyclopyrone, bifenox, bilanafos, bispyribac and its sodium salt, bromacil, bromobutide, bromofenoxim, bromoxynil, bromoxynil octanoate, butachlor, butafenacil, butamifos, butralin, butroxydim, butylate, bipyrazone, cafenstrole, carbetamide, 1-(2-carboxyethyl)-4-(2-pyrimidinyl)pyridazinium (CAS No.2285384-11-2) and salts thereof, carfentrazone-ethyl, catechin, chlomethoxyfen, chloramben, chlorbromuron, chlorflurenol-methyl, chloridazon, chlorimuron-ethyl, 3-[2-chloro-5-[3,6- dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluorophenyl]-4,5- dihydro-5-methyl-5-Isoxazolecarboxylic ethyl ester (CAS No.1949837-17-5), chlorotoluron, chlorpropham, chlorsulfuron, chlorthal-dimethyl, chlorthiamid, cinidon-ethyl, cinmethylin, cinosulfuron, clacyfos, clefoxydim, clethodim, clodinafop-propargyl, clomazone, clomeprop, clopyralid, clopyralid-olamine, cloransulam-methyl, cumyluron, cyanazine, cycloate, cyclopyrimorate, cyclosulfamuron, cycloxydim, cyhalofop-butyl, 2,4-D and its butotyl, butyl, isoctyl and isopropyl esters and its dimethylammonium, diolamine and trolamine salts, cyprafluone, daimuron, dalapon, dalapon-sodium, dazomet, 2,4-DB and its dimethylammonium, potassium and sodium salts, desmedipham, desmetryn, dicamba and its diglycolammonium, dimethylammonium, potassium and sodium salts, dichlobenil, dichlorprop, diclofop-methyl, diclosulam, difenzoquat metilsulfate, diflufenican, diflufenzopyr, dimefuron, dimepiperate, dimesulfazet, dimethachlor, dimethametryn, dimethenamid, dimethenamid-P, dimethipin, dimethylarsinic acid and its sodium salt, dinitramine, dinoterb, dioxopyritrione, diphenamid, diquat dibromide, dithiopyr, diuron, DNOC, endothal, EPTC, epyrifenacil, esprocarb, ethalfluralin, ethametsulfuron-methyl, ethiozin, ethofumesate, ethoxyfen, ethoxysulfuron, etobenzanid, fenoxaprop-ethyl, fenoxaprop-P-ethyl, fenoxasulfone, fenpyrazone, fenquinotrione, fentrazamide, fenuron, fenuron-TCA, flamprop-methyl, flamprop-M-isopropyl, flamprop-M-methyl, flazasulfuron, florasulam, fluazifop-butyl, fluazifop-P-butyl, fluazolate, flucarbazone, flucetosulfuron, fluchloralin, fluchloraminopyr, flufenacet, flufenoximacil, flufenpyr, flufenpyr-ethyl, flumetsulam, flumiclorac-pentyl, flumioxazin, fluometuron, fluoroglycofen-ethyl, flupoxam, flupyrsulfuron-methyl and its sodium salt, flurenol, flurenol-butyl, fluridone, flurochloridone, fluroxypyr, flurtamone, flusulfinam, fluthiacet-methyl, fomesafen, foramsulfuron, fosamine-ammonium, glufosinate, glufosinate-ammonium, glufosinate-P, glyphosate and its salts such as ammonium, isopropylammonium, potassium, sodium (including sesquisodium) and trimesium (alternatively named sulfosate), halauxifen, halauxifen-methyl, halosulfuron-methyl, haloxyfop-etotyl, haloxyfop-methyl, hexazinone,
hydantocidin, imazamethabenz-methyl, imazamox, imazapic, imazapyr, imazaquin, imazaquin-ammonium, imazethapyr, imazethapyr-ammonium, imazosulfuron, indanofan, indaziflam, iofensulfuron, iodosulfuron-methyl, ioxynil, ioxynil octanoate, ioxynil-sodium, ipfencarbazone, isoproturon, isouron, isoxaben, isoxaflutole, isoxachlortole, lactofen, lenacil, linuron, maleic hydrazide, MCPA and its salts (e.g., MCPA-dimethylammonium, MCPA- potassium and MCPA-sodium, esters (e.g., MCPA-2-ethylhexyl, MCPA-butotyl) and thioesters (e.g., MCPA-thioethyl), MCPB and its salts (e.g., MCPB-sodium) and esters (e.g., MCPB-ethyl), mecoprop, mecoprop-P, mefenacet, mefluidide, mesosulfuron-methyl, mesotrione, metam-sodium, metamifop, metamitron, metazachlor, metazosulfuron, methabenzthiazuron, methylarsonic acid and its calcium, monoammonium, monosodium and disodium salts, methyldymron, metobenzuron, metobromuron, metolachlor, S-metolachlor, metosulam, metoxuron, metribuzin, metsulfuron-methyl, molinate, monolinuron, naproanilide, napropamide, napropamide-M, naptalam, neburon, nicosulfuron, norflurazon, orbencarb, orthosulfamuron, oryzalin, oxadiargyl, oxadiazon, oxasulfuron, oxaziclomefone, oxyfluorfen, paraquat dichloride, pebulate, pelargonic acid, pendimethalin, penoxsulam, pentanochlor, pentoxazone, perfluidone, pethoxamid, pethoxyamid, phenmedipham, picloram, picloram-potassium, picolinafen, pinoxaden, piperophos, pretilachlor, primisulfuron-methyl, prodiamine, profoxydim, prometon, prometryn, propachlor, propanil, propaquizafop, propazine, propham, propisochlor, propoxycarbazone, propyrisulfuron, propyzamide, prosulfocarb, prosulfuron, pyraclonil, pyraflufen-ethyl, pyrasulfotole, pyrazogyl, pyrazolynate, pyrazoxyfen, pyrazosulfuron-ethyl, pyribenzoxim, pyributicarb, pyridate, pyriflubenzoxim, pyriftalid, pyriminobac-methyl, pyrimisulfan, pyrithiobac, pyrithiobac-sodium, pyroxasulfone, pyroxsulam, quinclorac, quinmerac, quinoclamine, quizalofop-ethyl, quizalofop-P-ethyl, quizalofop-P-tefuryl, rimisoxafen, rimsulfuron, saflufenacil, sethoxydim, siduron, simazine, simetryn, sulcotrione, sulfentrazone, sulfometuron-methyl, sulfosulfuron, 2,3,6-TBA, TCA, TCA-sodium, tebutam, tebuthiuron, tefuryltrione, tembotrione, tepraloxydim, terbacil, terbumeton, terbuthylazine, terbutryn, tetflupyrolimet, thenylchlor, thiazopyr, thiencarbazone, thifensulfuron-methyl, thiobencarb, tiafenacil, tiocarbazil, tolpyralate, topramezone, tralkoxydim, tri-allate, triafamone, triasulfuron, triaziflam, tribenuron-methyl, triclopyr, triclopyr-butotyl, triclopyr- triethylammonium, tridiphane, trietazine, trifloxysulfuron, trifludimoxazin, trifluralin, triflusulfuron-methyl, tripyrasulfone, tritosulfuron, vernolate, 3-(2-chloro-3,6- difluorophenyl)-4-hydroxy-1-methyl-1,5-naphthyridin-2(1H)-one, 5-chloro-3-[(2-hydroxy-6- oxo-1-cyclohexen-1-yl)carbonyl]-1-(4-methoxyphenyl)-2(1H)-quinoxalinone, 2-chloro-N- (1-methyl-1H-tetrazol-5-yl)-6-(trifluoromethyl)-3-pyridinecarboxamide, 7-(3,5-dichloro-4- pyridinyl)-5-(2,2-difluoroethyl)-8-hydroxypyrido[2,3-b]pyrazin-6(5H)-one), 4-(2,6-diethyl- 4-methylphenyl)-5-hydroxy-2,6-dimethyl-3(2H)-pyridazinone), 5-[[(2,6- difluorophenyl)methoxy]methyl]-4,5-dihydro-5-methyl-3-(3-methyl-2-thienyl)isoxazole
(previously methioxolin), 4-(4-fluorophenyl)-6-[(2-hydroxy-6-oxo-1-cyclohexen-1- yl)carbonyl]-2-methyl-1,2,4-triazine-3,5(2H,4H)-dione, methyl 4-amino-3-chloro-6-(4- chloro-2-fluoro-3-methoxyphenyl)-5-fluoro-2-pyridinecarboxylate, 2-methyl-3- (methylsulfonyl)-N-(1-methyl-1H-tetrazol-5-yl)-4-(trifluoromethyl)benzamide and 2-methyl- N-(4-methyl-1,2,5-oxadiazol-3-yl)-3-(methylsulfinyl)-4-(trifluoromethyl)benzamide. Other herbicides also include bioherbicides such as Alternaria destruens Simmons, Colletotrichum gloeosporiodes (Penz.) Penz. & Sacc., Drechsiera monoceras (MTB-951), Myrothecium verrucaria (Albertini & Schweinitz) Ditmar: Fries, Phytophthora palmivora (Butl.) Butl. and Puccinia thlaspeos Schub. Preferred for better control of undesired vegetation (e.g., lower use rate such as from enhanced effects, broader spectrum of weeds controlled, or enhanced crop safety) or for preventing the development of resistant weeds are mixtures of a compound of this invention with a herbicide selected from the group consisting of atrazine, azimsulfuron, S-beflubutamid, benzisothiazolinone, carfentrazone-ethyl, chlorimuron-ethyl, chlorsulfuron-methyl, clomazone, clopyralid potassium, cloransulam-methyl, 2-[(2,4-dichlorophenyl)methyl]- 4,4-dimethyl-3-isoxazolidinone, 2-[(2,5-dichlorophenyl)methyl]-4,4-dimethyl-3- isoxazolidinone, ethametsulfuron-methyl, flumetsulam, 4-(4-fluorophenyl)-6-[(2-hydroxy- 6-oxo-1-cyclohexen-1-yl)carbonyl]-2-methyl-1,2,4-triazine-3,5-(2H,4H)-dione, flupyrsulfuron-methyl, fluthiacet-methyl, fomesafen, imazethapyr, lenacil, mesotrione, metribuzin, metsulfuron-methyl, pethoxamid, picloram, pyroxasulfone, quinclorac, rimsulfuron, S-metolachlor, sulfentrazone, thifensulfuron-methyl, triflusulfuron-methyl and tribenuron-methyl. Compounds of this invention can also be used in combination with plant growth regulators such as aviglycine, N-(phenylmethyl)-1H-purin-6-amine, epocholeone, gibberellic acid, gibberellin A4 and A7, harpin protein, mepiquat chloride, prohexadione calcium, prohydrojasmon, sodium nitrophenolate and trinexapac-methyl, and plant growth modifying organisms such as Bacillus cereus strain BP01. General references for agricultural protectants (i.e. herbicides, herbicide safeners, insecticides, fungicides, nematocides, acaricides and biological agents) include The Pesticide Manual, 13th Edition, C. D. S. Tomlin, Ed., British Crop Protection Council, Farnham, Surrey, U.K., 2003 and The BioPesticide Manual, 2nd Edition, L. G. Copping, Ed., British Crop Protection Council, Farnham, Surrey, U.K., 2001. For embodiments where one or more of these various mixing partners are used, the mixing partners are typically used in the amounts similar to amounts customary when the mixture partners are used alone. More particularly in mixtures, active ingredients are often applied at an application rate between one-half and the full application rate specified on product labels for use of active ingredient alone. These amounts are listed in references such as The Pesticide Manual and The BioPesticide Manual. The weight ratio of these various
mixing partners (in total) to the compound of Formula 1 is typically between about 1:3000 and about 3000:1. Of note are weight ratios between about 1:300 and about 300:1 (for example ratios between about 1:30 and about 30:1). One skilled in the art can easily determine through simple experimentation the biologically effective amounts of active ingredients necessary for the desired spectrum of biological activity. It will be evident that including these additional components may expand the spectrum of weeds controlled beyond the spectrum controlled by the compound of Formula 1 alone. In certain instances, combinations of a compound of this invention with other biologically active (particularly herbicidal) compounds or agents (i.e. active ingredients) can result in a greater-than-additive (i.e. enhanced) effect on weeds and/or a less-than-additive effect (i.e. safening) on crops or other desirable plants. Reducing the quantity of active ingredients released in the environment while ensuring effective pest control is always desirable. Ability to use greater amounts of active ingredients to provide more effective weed control without excessive crop injury is also desirable. When the enhanced effects of herbicidal mixtures of active ingredients occurs on weeds at application rates giving agronomically satisfactory levels of weed control, such combinations can be advantageous for reducing crop production cost and decreasing environmental load. When safening of herbicidal active ingredients occurs on crops, such combinations can be advantageous for increasing crop protection by reducing weed competition. Of note is a combination of a compound of the invention with at least one other herbicidal active ingredient. Of particular note is such a combination where the other herbicidal active ingredient has different site of action from the compound of the invention. In certain instances, a combination with at least one other herbicidal active ingredient having a similar spectrum of control but a different site of action will be particularly advantageous for resistance management. Thus, a composition of the present invention can further comprise (in a herbicidally effective amount) at least one additional herbicidal active ingredient having a similar spectrum of control but a different site of action. Compounds of this invention can also be used in combination with herbicide safeners such as allidochlor, benoxacor, cloquintocet-mexyl, cumyluron, cyometrinil, cyprosulfonamide, daimuron, dichlormid, dicyclonon, dietholate, dimepiperate, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr- diethyl, mephenate, methoxyphenone naphthalic anhydride (1,8-naphthalic anhydride), oxabetrinil, N-(aminocarbonyl)-2-methylbenzenesulfonamide, N-(aminocarbonyl)- 2-fluorobenzenesulfonamide, 1-bromo-4-[(chloromethyl)sulfonyl]benzene (BCS), 4- (dichloroacetyl)-1-oxa-4-azospiro[4.5]decane (MON 4660), 2-(dichloromethyl)-2-methyl- 1,3-dioxolane (MG 191), ethyl 1,6-dihydro-1-(2-methoxyphenyl)-6-oxo-2-phenyl-5- pyrimidinecarboxylate, 2-hydroxy-N,N-dimethyl-6-(trifluoromethyl)pyridine-3-carboxamide, and 3-oxo-1-cyclohexen-l-yl 1-(3,4-dimethylphenyl)-l,6-dihydro-6-oxo-2-phenyl-5-
pyrimidinecarboxylate, 2,2-dichloro-1-(2,2,5-trimethyl-3-oxazolidinyl)-ethanone and 2- methoxy-N-[[4-[[(methylamino)carbonyl]amino]phenyl]sulfonyl]-benzamide to increase safety to certain crops. Antidotally effective amounts of the herbicide safeners can be applied at the same time as the compounds of this invention, or applied as seed treatments. Therefore an aspect of the present invention relates to a herbicidal mixture comprising a compound of this invention and an antidotally effective amount of a herbicide safener. Seed treatment is particularly useful for selective weed control, because it physically restricts antidoting to the crop plants. Therefore a particularly useful embodiment of the present invention is a method for selectively controlling the growth of undesired vegetation in a crop comprising contacting the locus of the crop with a herbicidally effective amount of a compound of this invention wherein seed from which the crop is grown is treated with an antidotally effective amount of safener. Antidotally effective amounts of safeners can be easily determined by one skilled in the art through simple experimentation. Compounds of the invention can also be mixed with: (1) polynucleotides including but not limited to DNA, RNA, and/or chemically modified nucleotides influencing the amount of a particular target through down regulation, interference, suppression or silencing of the genetically derived transcript that render a herbicidal effect; or (2) polynucleotides including but not limited to DNA, RNA, and/or chemically modified nucleotides influencing the amount of a particular target through down regulation, interference, suppression or silencing of the genetically derived transcript that render a safening effect. Of note is a composition comprising a compound of the invention (in a herbicidally effective amount), at least one additional active ingredient selected from the group consisting of other herbicides and herbicide safeners (in an effective amount), and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents. Table A1 lists specific combinations of a Component (a) with Component (b) illustrative of the mixtures, compositions and methods of the present invention. Compound No. (Compound Number) (i.e. Compound 1) in the Component (a) column is identified in Index Table A. The second column of Table A1 lists the specific Component (b) compound (e.g., “2,4-D” in the first line). The third, fourth and fifth columns of Table A1 lists ranges of weight ratios for rates at which the Component (a) compound is typically applied to a field-grown crop relative to Component (b) (i.e. (a):(b)). Thus, for example, the first line of Table A1 specifically discloses the combination of Component (a) (i.e. Compound 1 in Index Table A) with 2,4-D is typically applied in a weight ratio between 1:384 – 6:1. The remaining lines of Table A1 are to be construed similarly. TABLE A1
Table A2 is constructed the same as Table A1 above except that entries below the “Component (a)” column heading are replaced with the respective Component (a) Column Entry shown below. Compound No. in the Component (a) column is identified in Index Table A. Thus, for example, in Table A2 the entries below the “Component (a)” column heading all recite “Compound 2” (i.e. Compound 2 identified in Index Table A), and the first line below the column headings in Table A2 specifically discloses a mixture of Compound 2 with 2,4-D. Tables A3 through A60 are constructed similarly.
Preferred for better control of undesired vegetation (e.g., lower use rate such as from enhanced effects, broader spectrum of weeds controlled, or enhanced crop safety) or for preventing the development of resistant weeds are mixtures of a compound of this invention with a herbicide selected from the group consisting of chlorimuron-ethyl, nicosulfuron, mesotrione, thifensulfuron-methyl, flupyrsulfuron-methyl, tribenuron, pyroxasulfone, pinoxaden, tembotrione, pyroxsulam, metolachlor and S-metolachlor. The following TESTS demonstrate the control efficacy of compounds of this invention on specific pathogens. The pathogen control protection afforded by the compounds is not limited, however, to these species. See Index Tables A and B below for compound descriptions. The abbreviation “Cmpd.” stands for “Compound”, and the abbreviation “Ex.”
stands for “Example” and is followed by a number indicating in which example the compound is prepared. The numerical value reported in the column “MS” is the molecular weight of the highest isotopic abundance positively charged parent ion (M+1) formed by addition of H+ (molecular weight of 1) to the molecule having the highest isotopic abundance, or the highest isotopic abundance negatively charged ion (M–1) formed by loss of H+ (molecular weight of 1). “Pyr” means “Pyridine”. “Przl” means “Pyrazol”. “Thzl” means “Thiazole”. “Imdzl” mean “Imidazol”. The presence of molecular ions containing one or more higher atomic weight isotopes of lower abundance (e.g., 37Cl, 81Br) is not reported. The reported MS peaks were observed by mass spectrometry using electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI).
BIOLOGICAL EXAMPLES OF THE INVENTION TEST A Seeds of plant species selected from blackgrass (Alopecurus myosuroides), corn (Zea mays), foxtail, giant (giant foxtail, Setaria faberi), goosegrass (Eleusine indica), kochia (Bassia scoparia), oat, wild (wild oat, Avena fatua), pigweed, palmer (palmer amaranth, palmer pigweed, Amaranthus palmeri), ragweed (common ragweed, Ambrosia artemisiifolia), ryegrass, Italian (italian ryegrass, Lolium multiflorum), soybean (Glycine max) and wheat (Triticum aestivum) were planted into a blend of loam soil and sand and treated preemergence with a directed soil spray using test chemicals formulated in a non-phytotoxic solvent mixture which included a surfactant. At the same time, plants selected from these crop and weed species and also galium (catchweed bedstraw, Galium aparine) and horseweed (Erigeron canadensis) were planted in pots containing the same blend of loam soil and sand and treated with postemergence applications of test chemicals formulated in the same manner. Plants ranged in height from 2 to 10 cm and were in the one- to two-leaf stage for the postemergence treatment. Treated plants and untreated controls were maintained in a greenhouse for 10 days, after which time all treated plants were compared to untreated controls and visually evaluated for injury. Plant response ratings, summarized in Table A, are based on a 0 to 100 scale where 0 is no effect and 100 is complete control. A dash (–) response means no test result. Table A Compounds 125 g ai/ha 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Preemergence Blackgrass 90 40 40 30 80 30 90 70 90 70 0 10 90 80 Corn 30 0 0 0 0 0 20 60 40 10 0 10 10 10 Foxtail, Giant 100100 100 90100 30100100100100 10 80100100 Goosegrass 90 90 90 90 90 50100100100100 0 70100 100 Kochia 100 60 30 40 70 10100100100100 0 50100 90 Oat, Wild 80 30 10 20 70 0 50 90 80 70 0 20 50 50 Pigweed, Palmer 100100 90 90100 80100100100100 80100100 100 Ragweed 90 60 10 10 80 0 40 90 60100 0 10 80 50 Ryegrass, Italian 100 70 60 90100 20 90100100100 0 50100 100 Soybean 30 10 0 0 10 0 50 50 0 50 0 0 20 30 Wheat 70 20 10 10 30 0 20 40 30 50 0 10 30 20 Table A Compounds 125 g ai/ha 15 16 Preemergence Blackgrass 70 90
Corn 40 80 Foxtail, Giant 100100 Goosegrass 100100 Kochia 80100 Oat, Wild 30 80 Pigweed, Palmer 100 0 Ragweed 100 70 Ryegrass, Italian 30 80 Soybean 10 60 Wheat 20 80 Table A Compounds 31 g ai/ha 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Preemergence Blackgrass 90 30 0 0 0 0 30 50 20 10 0 0 40 20 Corn 0 0 0 0 0 0 0 30 0 0 0 0 0 0 Foxtail, Giant 100 90 30 20 70 0100 100100 90 0 20 90 90 Goosegrass 90 80 40 30 90 10 80100 90100 0 10 90 80 Kochia 60 60 10 10 30 0 80100100 80 0 10 10 90 Oat, Wild 30 10 0 0 30 0 20 30 30 30 0 0 20 30 Pigweed, Palmer 100 90 80 60 90 40100100100100 10 50100100 Ragweed 30 20 10 0 20 0 20 10 10 40 0 0 40 10 Ryegrass, Italian 80 50 10 20 40 0 90 60 40100 0 0 80100 Soybean 10 0 0 0 0 0 0 10 0 30 0 0 10 10 Wheat 20 10 0 0 10 0 10 20 20 10 0 0 10 10 Table A Compounds 31 g ai/ha 15 16 Preemergence Blackgrass 20 20 Corn 0 30 Foxtail, Giant 90100 Goosegrass 90 90 Kochia 70 90 Oat, Wild 10 30 Pigweed, Palmer 60 0 Ragweed 50 0 Ryegrass, Italian 10 80 Soybean 0 10 Wheat 0 20
Galium 60 70 50 60 90 60 70 70 70 70 0 50 70 60 Goosegrass 80 60 30 40 70 30 40 40 30 90 0 20 50 40 Horseweed 40 30 20 30 60 40 30 40 30 60 0 10 70 20 Kochia 90 90 90 70 90 60 90 90 60 80 0 60 80 80 Oat, Wild 50 30 20 20 30 20 20 30 20 20 0 20 20 20 Pigweed, Palmer 100100 90100 90 90100100 90100 60 90100100 Ragweed 70 60 50 40 40 20 40 40 30 80 0 40 60 50 Ryegrass, Italian 60 50 20 30 80 20 50 50 40 80 0 30 30 50 Soybean 60 50 40 40 60 30 80 40 60 60 0 30 50 50 Wheat 30 20 10 10 20 0 10 30 20 20 0 20 20 20 Table A Compounds 31 g ai/ha 15 16 Postemergence Blackgrass 40 40 Corn 20 40 Foxtail, Giant 60 60 Galium 70 60 Goosegrass 30 30 Horseweed 40 10 Kochia 20 90 Oat, Wild 20 50 Pigweed, Palmer 50100 Ragweed 60 50 Ryegrass, Italian 30 50 Soybean 0 60 Wheat 20 20 TEST B Plant species in the flooded paddy test selected from barnyardgrass (Echinochloa crus- galli), ducksalad (Heteranthera limosa), rice (Oryza sativa), and sedge, umbrella (small- flower umbrella sedge, Cyperus difformis) were grown to the 2-leaf stage for testing. At time of treatment, test pots were flooded to 3 cm above the soil surface, treated by application of test compounds directly to the paddy water, and then maintained at that water depth for the duration of the test. Treated plants and controls were maintained in a greenhouse for 13 days, after which time all species were compared to controls and visually evaluated. Plant response ratings, summarized in Table B, are based on a scale of 0 to 100 where 0 is no effect and 100 is complete control. A dash (–) response means no test result.
Claims
61481 CLAIMS What is claimed is: 1. A compound of Formula 1, all stereoisomers, N-oxides, and salts thereof
wherein J is selected from the group consisting of
G is selected from the group consisting of
R1 is halogen, C1–C4 haloalkyl, C1–C4 haloalkoxy, C1–C4 alkoxy, C1–C4 haloalkenyl, C1–C4 haloalkynyl, C1–C4 haloalkenyloxy, C1–C4 haloalkynyloxy, C1–C4 haloalkylthio, C1–C4 alkylthio or -CN; R2 is H or halogen; W, X, and Y are independently N or CR5; R3 is C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C 3 –C 7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy, C1–C5 haloalkoxy or C1–C5 alkylthio; R4 is H, C 1 –C 5 alkyl, halogen, -CN, C 1 –C 5 haloalkyl, C 1 –C 5 alkoxy or C 1 –C 5 alkylthio; Q is O, S, -CH2- or –(C=O)-; and R5 is H, C 1 –C 5 alkyl, halogen, -CN, C 3 –C 5 alkenyl, C 3 –C 5 alkynyl, C 3 –C 7 cycloalkyl, C1–C5 haloalkyl, C1–C5 alkoxy, C1–C5 haloalkoxy or C1–C5 alkylthio.
2. The compound of Claim 1 wherein J is J-1, J-2, J-3, J-5, J-6, J-8, J-9 or J-10; and G is G-1, G-2, G-3 or G-7.
3. The compound of Claim 1 wherein R1 is C1–C4 haloalkyl or C1–C4 haloalkoxy; R3 is halogen or C1–C5 haloalkyl;
R4 is H; and Q is O or -CH2-.
4. The compound of Claim 3 wherein Q is O.
5. The compound of Claim 1 wherein J is J-1, J-2 or J-3; R1 is CF3 or OCF3; and R3 is F, Cl or CF3.
6. The compound of Claim 5 wherein J is J-1; and R2 is H or halogen.
7. The compound of Claim 6 wherein R2 is H.
8. The compound of Claim 5 wherein J is J-2; and R1 is CF3.
9. The compound of Claim 5 wherein J is J-3; and R1 is CF3.
10. The compound of Claim 1 wherein G is G-1, G-2 or G-3.
11. The compound of Claim 10 wherein G is G-1; X is N; Y is CH; W is CH; and R5 is CF3.
12. The compound of Claim 10 wherein G is G-2; and R3 is CF3.
13. The compound of Claim 10 wherein G is G-3; and R3 is CF3.
14. The compound of Claim 1 wherein J is J-5, J-6 or J-8; R1 is CF3 or OCF3; and R3 is F, Cl or CF3.
15. The compound of Claim 14 wherein J is J-5.
16. The compound of Claim 14 wherein J is J-6.
17. The compound of Claim 1 wherein Q is O.
18. The compound of Claim 1 selected from the group consisting of 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 1) 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[4-(trifluoromethyl)phenyl]pyrimidine; (Compound 5) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-pyrazol-1-yl]-4-[[2-(trifluoromethyl)-4- pyridinyl]oxy]pyrimidine; (Compound 9) 5-cyclopropyl-4-[3-(trifluoromethoxy)phenoxy]-2-[3-(trifluoromethyl)-1H-pyrazol-1- yl]pyrimidine; (Compound 10) 5-cyclopropyl-2-[3-(trifluoromethyl)-1H-pyrazol-1-yl]-4-[[6-(trifluoromethyl)-2- pyridinyl]oxy]pyrimidine; (Compound 8) and 5-cyclopropyl-4-[3-(trifluoromethyl)phenoxy]-2-[6-(trifluoromethyl)-3- pyridinyl]pyrimidine. (Compound 13) 19. A herbicidal composition comprising a compound of Claim 1 and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents. 20. A herbicidal composition comprising a compound of Claim 1, at least one additional active ingredient selected from the group consisting of other herbicides and herbicide safeners, and at least one component selected from the group consisting of surfactants, solid diluents and liquid diluents. 21. A herbicidal mixture comprising (a) a compound of Claim 1, and (b) at least one additional active ingredient selected from (b1) photosystem II inhibitors, (b2) acetohydroxy acid synthase (AHAS) inhibitors, (b3) acetyl-CoA carboxylase (ACCase) inhibitors, (b4) auxin mimics, (b5) 5-enol-pyruvylshikimate-3-phosphate (EPSP) synthase inhibitors, (b6) photosystem I electron diverters, (b7) protoporphyrinogen oxidase (PPO) inhibitors, (b8)
glutamine synthetase (GS) inhibitors, (b9) very long chain fatty acid (VLCFA) elongase inhibitors, (b10) auxin transport inhibitors, (b11) phytoene desaturase (PDS) inhibitors, (b12) 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitors, (b13) homogentisate solanesyltransferase (HST) inhibitors, (b14) cellulose biosynthesis inhibitors, (b15) other herbicides including mitotic disruptors, organic arsenicals, asulam, bromobutide, cinmethylin, cumyluron, dazomet, difenzoquat, dymron, etobenzanid, flurenol, fosamine, fosamine-ammonium, hydantocidin, metam, methyldymron, oleic acid, oxaziclomefone, pelargonic acid and pyributicarb, (b16) herbicide safeners, and salts of compounds of (b1) through (b16). 22. A method for controlling the growth of undesired vegetation comprising contacting the vegetation or its environment with a herbicidally effective amount of a compound of Claim 1.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202163294264P | 2021-12-28 | 2021-12-28 | |
| US63/294,264 | 2021-12-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023129493A1 true WO2023129493A1 (en) | 2023-07-06 |
Family
ID=85150281
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2022/053915 WO2023129493A1 (en) | 2021-12-28 | 2022-12-23 | Substituted cyclopropylpyrimidne herbicides |
Country Status (2)
| Country | Link |
|---|---|
| AR (1) | AR128122A1 (en) |
| WO (1) | WO2023129493A1 (en) |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2891855A (en) | 1954-08-16 | 1959-06-23 | Geigy Ag J R | Compositions and methods for influencing the growth of plants |
| US3060084A (en) | 1961-06-09 | 1962-10-23 | Du Pont | Improved homogeneous, readily dispersed, pesticidal concentrate |
| US3235361A (en) | 1962-10-29 | 1966-02-15 | Du Pont | Method for the control of undesirable vegetation |
| US3299566A (en) | 1964-06-01 | 1967-01-24 | Olin Mathieson | Water soluble film containing agricultural chemicals |
| US3309192A (en) | 1964-12-02 | 1967-03-14 | Du Pont | Method of controlling seedling weed grasses |
| US3920442A (en) | 1972-09-18 | 1975-11-18 | Du Pont | Water-dispersible pesticide aggregates |
| US4144050A (en) | 1969-02-05 | 1979-03-13 | Hoechst Aktiengesellschaft | Micro granules for pesticides and process for their manufacture |
| US4172714A (en) | 1976-12-20 | 1979-10-30 | E. I. Du Pont De Nemours And Company | Dry compactible, swellable herbicidal compositions and pellets produced therefrom |
| GB2095558A (en) | 1981-03-30 | 1982-10-06 | Avon Packers Ltd | Formulation of agricultural chemicals |
| DE3246493A1 (en) | 1982-12-16 | 1984-06-20 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING WATER-DISPERSIBLE GRANULES |
| WO1991013546A1 (en) | 1990-03-12 | 1991-09-19 | E.I. Du Pont De Nemours And Company | Water-dispersible or water-soluble pesticide granules from heat-activated binders |
| US5180587A (en) | 1988-06-28 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Tablet formulations of pesticides |
| US5208030A (en) | 1989-08-30 | 1993-05-04 | Imperial Chemical Industries Plc | Active ingredient dosage device |
| US5232701A (en) | 1990-10-11 | 1993-08-03 | Sumitomo Chemical Company, Limited | Boron carbonate and solid acid pesticidal composition |
| WO2003024222A1 (en) | 2001-09-21 | 2003-03-27 | E. I. Du Pont De Nemours And Company | Anthranilamide arthropodicide treatment |
| WO2016149315A1 (en) * | 2015-03-18 | 2016-09-22 | E. I. Du Pont De Nemours And Company | Substituted pyrimidinyloxy pyridine derivatives as herbicides |
| WO2020087694A1 (en) * | 2018-10-31 | 2020-05-07 | 青岛清原化合物有限公司 | Substituted nitrogen-containing heterocyclic formamide derivative, weeding composition and uses thereof |
-
2022
- 2022-12-23 WO PCT/US2022/053915 patent/WO2023129493A1/en unknown
- 2022-12-27 AR ARP220103604A patent/AR128122A1/en unknown
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2891855A (en) | 1954-08-16 | 1959-06-23 | Geigy Ag J R | Compositions and methods for influencing the growth of plants |
| US3060084A (en) | 1961-06-09 | 1962-10-23 | Du Pont | Improved homogeneous, readily dispersed, pesticidal concentrate |
| US3235361A (en) | 1962-10-29 | 1966-02-15 | Du Pont | Method for the control of undesirable vegetation |
| US3299566A (en) | 1964-06-01 | 1967-01-24 | Olin Mathieson | Water soluble film containing agricultural chemicals |
| US3309192A (en) | 1964-12-02 | 1967-03-14 | Du Pont | Method of controlling seedling weed grasses |
| US4144050A (en) | 1969-02-05 | 1979-03-13 | Hoechst Aktiengesellschaft | Micro granules for pesticides and process for their manufacture |
| US3920442A (en) | 1972-09-18 | 1975-11-18 | Du Pont | Water-dispersible pesticide aggregates |
| US4172714A (en) | 1976-12-20 | 1979-10-30 | E. I. Du Pont De Nemours And Company | Dry compactible, swellable herbicidal compositions and pellets produced therefrom |
| GB2095558A (en) | 1981-03-30 | 1982-10-06 | Avon Packers Ltd | Formulation of agricultural chemicals |
| DE3246493A1 (en) | 1982-12-16 | 1984-06-20 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING WATER-DISPERSIBLE GRANULES |
| US5180587A (en) | 1988-06-28 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Tablet formulations of pesticides |
| US5208030A (en) | 1989-08-30 | 1993-05-04 | Imperial Chemical Industries Plc | Active ingredient dosage device |
| WO1991013546A1 (en) | 1990-03-12 | 1991-09-19 | E.I. Du Pont De Nemours And Company | Water-dispersible or water-soluble pesticide granules from heat-activated binders |
| US5232701A (en) | 1990-10-11 | 1993-08-03 | Sumitomo Chemical Company, Limited | Boron carbonate and solid acid pesticidal composition |
| WO2003024222A1 (en) | 2001-09-21 | 2003-03-27 | E. I. Du Pont De Nemours And Company | Anthranilamide arthropodicide treatment |
| WO2016149315A1 (en) * | 2015-03-18 | 2016-09-22 | E. I. Du Pont De Nemours And Company | Substituted pyrimidinyloxy pyridine derivatives as herbicides |
| WO2020087694A1 (en) * | 2018-10-31 | 2020-05-07 | 青岛清原化合物有限公司 | Substituted nitrogen-containing heterocyclic formamide derivative, weeding composition and uses thereof |
Non-Patent Citations (18)
| Title |
|---|
| "Comprehensive Heterocyclic Chemistry II", 1996, PERGAMON PRESS |
| "Developments in formulation technology", 2000, PJB PUBLICATIONS |
| "Perry's Chemical Engineer's Handbook", 1963, MCGRAW-HILL, pages: 8 - 57 |
| "Polymorphism in the Pharmaceutical Industry", 2006, WILEY-VCH |
| "The BioPesticide Manual", 2001, BRITISH CROP PROTECTION COUNCIL |
| "The Pesticide Manual", 2003, BRITISH CROP PROTECTION COUNCIL |
| A. S. DAVIDSONB. MILWIDSKY: "Synthetic Detergents", 1987, JOHN WILEY AND SONS |
| BROWNING: "Agglomeration", CHEMICAL ENGINEERING, 4 December 1967 (1967-12-04), pages 147 - 48 |
| CAS , no. 2285384-11-2 |
| CAS, no. 27499989-21-6 |
| G. W. H. CHEESEMANE. S. G. WERSTIUK: "Comprehensive Heterocyclic Chemistry", vol. 22, 1984, PERGAMON PRESS, pages: 390 - 392 |
| GREENE, T. WWUTS, P. G. M: "Protective Groups in Organic Synthesis", 1991, WILEY |
| HANCE ET AL.: "Weed Control Handbook", 1989, BLACKWELL SCIENTIFIC PUBLICATIONS |
| KLINGMAN: "Weed Control as a Science", 1961, JOHN WILEY AND SONS, INC, pages: 81 - 96 |
| LAROCK, R. C: "Comprehensive Organic Transformations: A Guide to Functional Group Preparations", 1999, THE ROYAL SOCIETY OF CHEMISTRY, article "The Formulator's Toolbox - Product Forms for Modern Agriculture", pages: 120 - 133 |
| MCCUTCHEON'S: "annual International and North American", vol. 2, THE MANUFACTURING CONFECTIONER PUBLISHING CO, article "Functional Materials" |
| SISELYWOOD: "McCutcheon's Emulsifiers and Detergents", 1964, THE MANUFACTURING CONFECTIONER PUBLISHING CO |
| WATKINS ET AL.: "Handbook of Insecticide Dust Diluents and Carriers", 1950, DORLAND BOOKS |
Also Published As
| Publication number | Publication date |
|---|---|
| AR128122A1 (en) | 2024-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2016259529B2 (en) | Aryl substituted bicyclic compounds as herbicides | |
| AU2018277041B2 (en) | Herbicidal 3-substituted lactams | |
| US11292770B2 (en) | Pyridazinone herbicides | |
| WO2016196019A1 (en) | Substituted cyclic amides as herbicides | |
| AU2018277537B2 (en) | Herbicidal amides | |
| EP3645515B1 (en) | 4-(3,4-dihydronaphth-1-yl or 2h-chromen-4-yl)-5-hydroxy-2h-pyradizin-3-ones as herbicides | |
| EP3137456A1 (en) | Pyridazinone herbicides | |
| WO2016149315A1 (en) | Substituted pyrimidinyloxy pyridine derivatives as herbicides | |
| AU2018262478B2 (en) | Pyrimidinyloxy benzo-fused compounds as herbicides | |
| AU2015290153A1 (en) | Bis(aryl)catechol derivatives as herbicides | |
| US20220281848A1 (en) | Pyrazole-substituted pyrrolidinones as herbicides | |
| WO2016014814A1 (en) | Pyridones as herbicides | |
| WO2021183980A1 (en) | Substituted pyrimidines and triazines as herbicides | |
| WO2015191377A1 (en) | Herbicidal substituted 3-arylpyrazoles | |
| EP3185684A1 (en) | Herbicidal triazoles | |
| WO2023129493A1 (en) | Substituted cyclopropylpyrimidne herbicides | |
| WO2024072768A1 (en) | Substituted fluoropyridine as herbicides | |
| WO2022177892A1 (en) | Herbicidal cyclic amides n-substituted with a haloalkylsulfonylanilide group | |
| WO2022140309A1 (en) | Substituted pyridazinone herbicides | |
| EP4188093A1 (en) | Substituted haloalkyl sulfonanilide herbicides | |
| WO2024015425A1 (en) | Herbicidal benzoxazines | |
| IL269625A (en) | Novel pyridazinone herbicides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22850913 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |