WO2022232650A1 - Methods for reducing agt expression - Google Patents
Methods for reducing agt expression Download PDFInfo
- Publication number
- WO2022232650A1 WO2022232650A1 PCT/US2022/027138 US2022027138W WO2022232650A1 WO 2022232650 A1 WO2022232650 A1 WO 2022232650A1 US 2022027138 W US2022027138 W US 2022027138W WO 2022232650 A1 WO2022232650 A1 WO 2022232650A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- less
- heart failure
- effective amount
- therapeutically effective
- oligomeric compound
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 187
- 206010020772 Hypertension Diseases 0.000 claims abstract description 121
- 208000015658 resistant hypertension Diseases 0.000 claims abstract description 92
- 206010019280 Heart failures Diseases 0.000 claims abstract description 83
- 208000038002 heart failure with reduced ejection fraction Diseases 0.000 claims abstract description 57
- 241000282414 Homo sapiens Species 0.000 claims abstract description 53
- 208000038003 heart failure with preserved ejection fraction Diseases 0.000 claims abstract description 52
- 208000007342 Diabetic Nephropathies Diseases 0.000 claims abstract description 48
- 208000033679 diabetic kidney disease Diseases 0.000 claims abstract description 48
- 206010007558 Cardiac failure chronic Diseases 0.000 claims abstract description 37
- 208000001826 Marfan syndrome Diseases 0.000 claims abstract description 32
- 208000020832 chronic kidney disease Diseases 0.000 claims abstract description 29
- 208000002251 Dissecting Aneurysm Diseases 0.000 claims abstract description 24
- 206010019708 Hepatic steatosis Diseases 0.000 claims abstract description 24
- 208000031953 Hereditary hemorrhagic telangiectasia Diseases 0.000 claims abstract description 24
- 201000008982 Thoracic Aortic Aneurysm Diseases 0.000 claims abstract description 24
- 208000007474 aortic aneurysm Diseases 0.000 claims abstract description 24
- 206010002895 aortic dissection Diseases 0.000 claims abstract description 24
- 238000002224 dissection Methods 0.000 claims abstract description 24
- 208000003457 familial thoracic 1 aortic aneurysm Diseases 0.000 claims abstract description 24
- 230000001717 pathogenic effect Effects 0.000 claims abstract description 24
- 230000011664 signaling Effects 0.000 claims abstract description 24
- 201000001320 Atherosclerosis Diseases 0.000 claims abstract description 23
- 201000005978 Loeys-Dietz syndrome Diseases 0.000 claims abstract description 22
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 13
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 11
- 150000001875 compounds Chemical class 0.000 claims description 103
- 230000035488 systolic blood pressure Effects 0.000 claims description 31
- 230000036772 blood pressure Effects 0.000 claims description 28
- 230000035487 diastolic blood pressure Effects 0.000 claims description 25
- 208000024891 symptom Diseases 0.000 claims description 25
- 150000003839 salts Chemical class 0.000 claims description 19
- 108091034117 Oligonucleotide Proteins 0.000 claims description 15
- 230000000747 cardiac effect Effects 0.000 claims description 14
- 230000002526 effect on cardiovascular system Effects 0.000 claims description 12
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 claims description 12
- 239000000126 substance Substances 0.000 claims description 12
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 claims description 12
- 101800000407 Brain natriuretic peptide 32 Proteins 0.000 claims description 11
- 230000002861 ventricular Effects 0.000 claims description 11
- YIMATHOGWXZHFX-WCTZXXKLSA-N (2r,3r,4r,5r)-5-(hydroxymethyl)-3-(2-methoxyethoxy)oxolane-2,4-diol Chemical compound COCCO[C@H]1[C@H](O)O[C@H](CO)[C@H]1O YIMATHOGWXZHFX-WCTZXXKLSA-N 0.000 claims description 9
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 claims description 8
- 206010013975 Dyspnoeas Diseases 0.000 claims description 7
- 102000004987 Troponin T Human genes 0.000 claims description 7
- 108090001108 Troponin T Proteins 0.000 claims description 7
- 229930024421 Adenine Natural products 0.000 claims description 6
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 claims description 6
- 229960000643 adenine Drugs 0.000 claims description 6
- 159000000000 sodium salts Chemical class 0.000 claims description 6
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 claims description 6
- 229940113082 thymine Drugs 0.000 claims description 6
- 239000008280 blood Substances 0.000 claims description 5
- 210000004369 blood Anatomy 0.000 claims description 5
- 239000012530 fluid Substances 0.000 claims description 5
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 claims description 5
- 238000010254 subcutaneous injection Methods 0.000 claims description 5
- 239000007929 subcutaneous injection Substances 0.000 claims description 5
- 238000012360 testing method Methods 0.000 claims description 5
- 206010003445 Ascites Diseases 0.000 claims description 4
- 208000000059 Dyspnea Diseases 0.000 claims description 4
- 208000037221 Hepatic congestion Diseases 0.000 claims description 4
- 206010030113 Oedema Diseases 0.000 claims description 4
- 206010031123 Orthopnoea Diseases 0.000 claims description 4
- 230000003205 diastolic effect Effects 0.000 claims description 4
- 230000003292 diminished effect Effects 0.000 claims description 4
- 208000002173 dizziness Diseases 0.000 claims description 4
- 230000003203 everyday effect Effects 0.000 claims description 4
- 210000005240 left ventricle Anatomy 0.000 claims description 4
- 208000012144 orthopnea Diseases 0.000 claims description 4
- 206010008479 Chest Pain Diseases 0.000 claims description 3
- 206010019233 Headaches Diseases 0.000 claims description 3
- 230000010343 cardiac dilation Effects 0.000 claims description 3
- 230000009787 cardiac fibrosis Effects 0.000 claims description 3
- 230000010339 dilation Effects 0.000 claims description 3
- 208000001780 epistaxis Diseases 0.000 claims description 3
- 206010016256 fatigue Diseases 0.000 claims description 3
- 231100000869 headache Toxicity 0.000 claims description 3
- 230000004217 heart function Effects 0.000 claims description 3
- 230000001788 irregular Effects 0.000 claims description 3
- 238000001356 surgical procedure Methods 0.000 claims description 3
- 210000002700 urine Anatomy 0.000 claims description 3
- ABEXEQSGABRUHS-UHFFFAOYSA-N 16-methylheptadecyl 16-methylheptadecanoate Chemical compound CC(C)CCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCCC(C)C ABEXEQSGABRUHS-UHFFFAOYSA-N 0.000 abstract description 175
- 241000764238 Isis Species 0.000 abstract description 175
- 238000005417 image-selected in vivo spectroscopy Methods 0.000 abstract description 175
- 238000012739 integrated shape imaging system Methods 0.000 abstract description 175
- 102000004887 Transforming Growth Factor beta Human genes 0.000 abstract 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 abstract 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 abstract 1
- 102000004881 Angiotensinogen Human genes 0.000 description 46
- 108090001067 Angiotensinogen Proteins 0.000 description 46
- 239000000902 placebo Substances 0.000 description 24
- 229940068196 placebo Drugs 0.000 description 24
- 229940127088 antihypertensive drug Drugs 0.000 description 23
- 238000012423 maintenance Methods 0.000 description 23
- 230000000694 effects Effects 0.000 description 19
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 16
- 239000008177 pharmaceutical agent Substances 0.000 description 16
- 230000009467 reduction Effects 0.000 description 15
- 239000002777 nucleoside Substances 0.000 description 14
- 239000008194 pharmaceutical composition Substances 0.000 description 10
- 239000005541 ACE inhibitor Substances 0.000 description 9
- 101710129690 Angiotensin-converting enzyme inhibitor Proteins 0.000 description 9
- 101710086378 Bradykinin-potentiating and C-type natriuretic peptides Proteins 0.000 description 9
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 9
- 229940079593 drug Drugs 0.000 description 8
- 239000003814 drug Substances 0.000 description 8
- 238000012216 screening Methods 0.000 description 8
- 239000002253 acid Substances 0.000 description 7
- 230000001684 chronic effect Effects 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 238000002483 medication Methods 0.000 description 7
- 150000003833 nucleoside derivatives Chemical class 0.000 description 7
- 230000036454 renin-angiotensin system Effects 0.000 description 7
- 230000003442 weekly effect Effects 0.000 description 7
- 229940127291 Calcium channel antagonist Drugs 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 239000002876 beta blocker Substances 0.000 description 6
- 229940097320 beta blocking agent Drugs 0.000 description 6
- 239000000480 calcium channel blocker Substances 0.000 description 6
- 230000001631 hypertensive effect Effects 0.000 description 6
- 125000003835 nucleoside group Chemical group 0.000 description 6
- 239000007864 aqueous solution Substances 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 150000001768 cations Chemical class 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical group O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- -1 2’-MOE nucleoside Chemical class 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 4
- 102000053602 DNA Human genes 0.000 description 4
- RPTUSVTUFVMDQK-UHFFFAOYSA-N Hidralazin Chemical compound C1=CC=C2C(NN)=NN=CC2=C1 RPTUSVTUFVMDQK-UHFFFAOYSA-N 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical group C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 4
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical class NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 4
- 239000005549 deoxyribonucleoside Substances 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- PQSUYGKTWSAVDQ-ZVIOFETBSA-N Aldosterone Chemical compound C([C@@]1([C@@H](C(=O)CO)CC[C@H]1[C@@H]1CC2)C=O)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 PQSUYGKTWSAVDQ-ZVIOFETBSA-N 0.000 description 3
- PQSUYGKTWSAVDQ-UHFFFAOYSA-N Aldosterone Natural products C1CC2C3CCC(C(=O)CO)C3(C=O)CC(O)C2C2(C)C1=CC(=O)CC2 PQSUYGKTWSAVDQ-UHFFFAOYSA-N 0.000 description 3
- 125000000824 D-ribofuranosyl group Chemical group [H]OC([H])([H])[C@@]1([H])OC([H])(*)[C@]([H])(O[H])[C@]1([H])O[H] 0.000 description 3
- 229940097420 Diuretic Drugs 0.000 description 3
- 208000001953 Hypotension Diseases 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical group [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 3
- 229940122767 Potassium sparing diuretic Drugs 0.000 description 3
- 102100028255 Renin Human genes 0.000 description 3
- 108090000783 Renin Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 229960002478 aldosterone Drugs 0.000 description 3
- 230000001174 ascending effect Effects 0.000 description 3
- 206010012601 diabetes mellitus Diseases 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000002934 diuretic Substances 0.000 description 3
- 230000001882 diuretic effect Effects 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 230000000004 hemodynamic effect Effects 0.000 description 3
- 208000021822 hypotensive Diseases 0.000 description 3
- 230000001077 hypotensive effect Effects 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000003286 potassium sparing diuretic agent Substances 0.000 description 3
- 229940097241 potassium-sparing diuretic Drugs 0.000 description 3
- 238000009097 single-agent therapy Methods 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000008223 sterile water Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 102000008873 Angiotensin II receptor Human genes 0.000 description 2
- 108050000824 Angiotensin II receptor Proteins 0.000 description 2
- 101800000734 Angiotensin-1 Proteins 0.000 description 2
- 102400000344 Angiotensin-1 Human genes 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 206010016807 Fluid retention Diseases 0.000 description 2
- 101000732617 Homo sapiens Angiotensinogen Proteins 0.000 description 2
- 208000002682 Hyperkalemia Diseases 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- 229940123518 Sodium/glucose cotransporter 2 inhibitor Drugs 0.000 description 2
- 239000000219 Sympatholytic Substances 0.000 description 2
- 206010047139 Vasoconstriction Diseases 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 239000000951 adrenergic alpha-1 receptor antagonist Substances 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- ORWYRWWVDCYOMK-HBZPZAIKSA-N angiotensin I Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C1=CC=C(O)C=C1 ORWYRWWVDCYOMK-HBZPZAIKSA-N 0.000 description 2
- 229940125364 angiotensin receptor blocker Drugs 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 229940104302 cytosine Drugs 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 229960002474 hydralazine Drugs 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 230000003907 kidney function Effects 0.000 description 2
- 230000002644 neurohormonal effect Effects 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 150000004713 phosphodiesters Chemical class 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 239000003087 receptor blocking agent Substances 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 229960004249 sodium acetate Drugs 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- 229910001415 sodium ion Inorganic materials 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 230000000948 sympatholitic effect Effects 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- RMMXLENWKUUMAY-UHFFFAOYSA-N telmisartan Chemical compound CCCC1=NC2=C(C)C=C(C=3N(C4=CC=CC=C4N=3)C)C=C2N1CC(C=C1)=CC=C1C1=CC=CC=C1C(O)=O RMMXLENWKUUMAY-UHFFFAOYSA-N 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 229940035893 uracil Drugs 0.000 description 2
- 230000025033 vasoconstriction Effects 0.000 description 2
- 229940124549 vasodilator Drugs 0.000 description 2
- 239000003071 vasodilator agent Substances 0.000 description 2
- BIDNLKIUORFRQP-XYGFDPSESA-N (2s,4s)-4-cyclohexyl-1-[2-[[(1s)-2-methyl-1-propanoyloxypropoxy]-(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylic acid Chemical compound C([P@@](=O)(O[C@H](OC(=O)CC)C(C)C)CC(=O)N1[C@@H](C[C@H](C1)C1CCCCC1)C(O)=O)CCCC1=CC=CC=C1 BIDNLKIUORFRQP-XYGFDPSESA-N 0.000 description 1
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 101800000733 Angiotensin-2 Proteins 0.000 description 1
- 102400000345 Angiotensin-2 Human genes 0.000 description 1
- 102000002723 Atrial Natriuretic Factor Human genes 0.000 description 1
- 101800001288 Atrial natriuretic factor Proteins 0.000 description 1
- 101800001890 Atrial natriuretic peptide Proteins 0.000 description 1
- 239000005485 Azilsartan Substances 0.000 description 1
- XPCFTKFZXHTYIP-PMACEKPBSA-N Benazepril Chemical compound C([C@@H](C(=O)OCC)N[C@@H]1C(N(CC(O)=O)C2=CC=CC=C2CC1)=O)CC1=CC=CC=C1 XPCFTKFZXHTYIP-PMACEKPBSA-N 0.000 description 1
- 102400000667 Brain natriuretic peptide 32 Human genes 0.000 description 1
- 101800002247 Brain natriuretic peptide 45 Proteins 0.000 description 1
- 239000002083 C09CA01 - Losartan Substances 0.000 description 1
- 239000002080 C09CA02 - Eprosartan Substances 0.000 description 1
- 239000004072 C09CA03 - Valsartan Substances 0.000 description 1
- 239000002947 C09CA04 - Irbesartan Substances 0.000 description 1
- 239000002053 C09CA06 - Candesartan Substances 0.000 description 1
- 239000005537 C09CA07 - Telmisartan Substances 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 206010007572 Cardiac hypertrophy Diseases 0.000 description 1
- 208000006029 Cardiomegaly Diseases 0.000 description 1
- JVHXJTBJCFBINQ-ADAARDCZSA-N Dapagliflozin Chemical compound C1=CC(OCC)=CC=C1CC1=CC([C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)=CC=C1Cl JVHXJTBJCFBINQ-ADAARDCZSA-N 0.000 description 1
- 108010061435 Enalapril Proteins 0.000 description 1
- 108010066671 Enalaprilat Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 101100108871 Homo sapiens AGT gene Proteins 0.000 description 1
- 101000629622 Homo sapiens Serine-pyruvate aminotransferase Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 208000029422 Hypernatremia Diseases 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 1
- 108010007859 Lisinopril Proteins 0.000 description 1
- 108091027974 Mature messenger RNA Proteins 0.000 description 1
- UWWDHYUMIORJTA-HSQYWUDLSA-N Moexipril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CC2=CC(OC)=C(OC)C=C2C1)C(O)=O)CC1=CC=CC=C1 UWWDHYUMIORJTA-HSQYWUDLSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 239000005480 Olmesartan Substances 0.000 description 1
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 1
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 1
- 206010037211 Psychomotor hyperactivity Diseases 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- VXFJYXUZANRPDJ-WTNASJBWSA-N Trandopril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](C[C@H]2CCCC[C@@H]21)C(O)=O)CC1=CC=CC=C1 VXFJYXUZANRPDJ-WTNASJBWSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229950006323 angiotensin ii Drugs 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 229940030600 antihypertensive agent Drugs 0.000 description 1
- 239000002220 antihypertensive agent Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 229960002731 azilsartan Drugs 0.000 description 1
- KGSXMPPBFPAXLY-UHFFFAOYSA-N azilsartan Chemical compound CCOC1=NC2=CC=CC(C(O)=O)=C2N1CC(C=C1)=CC=C1C1=CC=CC=C1C1=NOC(=O)N1 KGSXMPPBFPAXLY-UHFFFAOYSA-N 0.000 description 1
- 229960004530 benazepril Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 238000009530 blood pressure measurement Methods 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 229960000932 candesartan Drugs 0.000 description 1
- SGZAIDDFHDDFJU-UHFFFAOYSA-N candesartan Chemical compound CCOC1=NC2=CC=CC(C(O)=O)=C2N1CC(C=C1)=CC=C1C1=CC=CC=C1C1=NN=N[N]1 SGZAIDDFHDDFJU-UHFFFAOYSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229960000830 captopril Drugs 0.000 description 1
- FAKRSMQSSFJEIM-RQJHMYQMSA-N captopril Chemical compound SC[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O FAKRSMQSSFJEIM-RQJHMYQMSA-N 0.000 description 1
- 230000007211 cardiovascular event Effects 0.000 description 1
- NSQLIUXCMFBZME-MPVJKSABSA-N carperitide Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)=O)[C@@H](C)CC)C1=CC=CC=C1 NSQLIUXCMFBZME-MPVJKSABSA-N 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 208000035850 clinical syndrome Diseases 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000002354 daily effect Effects 0.000 description 1
- 229960003834 dapagliflozin Drugs 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 229960000873 enalapril Drugs 0.000 description 1
- GBXSMTUPTTWBMN-XIRDDKMYSA-N enalapril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)CC1=CC=CC=C1 GBXSMTUPTTWBMN-XIRDDKMYSA-N 0.000 description 1
- 229960002680 enalaprilat Drugs 0.000 description 1
- LZFZMUMEGBBDTC-QEJZJMRPSA-N enalaprilat (anhydrous) Chemical compound C([C@H](N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 LZFZMUMEGBBDTC-QEJZJMRPSA-N 0.000 description 1
- 239000002792 enkephalinase inhibitor Substances 0.000 description 1
- 229960004563 eprosartan Drugs 0.000 description 1
- OROAFUQRIXKEMV-LDADJPATSA-N eprosartan Chemical compound C=1C=C(C(O)=O)C=CC=1CN1C(CCCC)=NC=C1\C=C(C(O)=O)/CC1=CC=CS1 OROAFUQRIXKEMV-LDADJPATSA-N 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229960002490 fosinopril Drugs 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 125000003843 furanosyl group Chemical group 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 102000049538 human AGT Human genes 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 229960002198 irbesartan Drugs 0.000 description 1
- YCPOHTHPUREGFM-UHFFFAOYSA-N irbesartan Chemical compound O=C1N(CC=2C=CC(=CC=2)C=2C(=CC=CC=2)C=2[N]N=NN=2)C(CCCC)=NC21CCCC2 YCPOHTHPUREGFM-UHFFFAOYSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229960002394 lisinopril Drugs 0.000 description 1
- RLAWWYSOJDYHDC-BZSNNMDCSA-N lisinopril Chemical compound C([C@H](N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 RLAWWYSOJDYHDC-BZSNNMDCSA-N 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 229960004773 losartan Drugs 0.000 description 1
- KJJZZJSZUJXYEA-UHFFFAOYSA-N losartan Chemical compound CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C=2[N]N=NN=2)C=C1 KJJZZJSZUJXYEA-UHFFFAOYSA-N 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 229960005170 moexipril Drugs 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- HPNRHPKXQZSDFX-OAQDCNSJSA-N nesiritide Chemical compound C([C@H]1C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)CNC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CO)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1N=CNC=1)C(O)=O)=O)[C@@H](C)CC)C1=CC=CC=C1 HPNRHPKXQZSDFX-OAQDCNSJSA-N 0.000 description 1
- 230000002182 neurohumoral effect Effects 0.000 description 1
- 231100001079 no serious adverse effect Toxicity 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 229960005117 olmesartan Drugs 0.000 description 1
- VTRAEEWXHOVJFV-UHFFFAOYSA-N olmesartan Chemical compound CCCC1=NC(C(C)(C)O)=C(C(O)=O)N1CC1=CC=C(C=2C(=CC=CC=2)C=2NN=NN=2)C=C1 VTRAEEWXHOVJFV-UHFFFAOYSA-N 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 229960002582 perindopril Drugs 0.000 description 1
- IPVQLZZIHOAWMC-QXKUPLGCSA-N perindopril Chemical compound C1CCC[C@H]2C[C@@H](C(O)=O)N(C(=O)[C@H](C)N[C@@H](CCC)C(=O)OCC)[C@H]21 IPVQLZZIHOAWMC-QXKUPLGCSA-N 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 201000001474 proteinuria Diseases 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 229960001455 quinapril Drugs 0.000 description 1
- JSDRRTOADPPCHY-HSQYWUDLSA-N quinapril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CC2=CC=CC=C2C1)C(O)=O)CC1=CC=CC=C1 JSDRRTOADPPCHY-HSQYWUDLSA-N 0.000 description 1
- 229960003401 ramipril Drugs 0.000 description 1
- HDACQVRGBOVJII-JBDAPHQKSA-N ramipril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](C[C@@H]2CCC[C@@H]21)C(O)=O)CC1=CC=CC=C1 HDACQVRGBOVJII-JBDAPHQKSA-N 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- PYNXFZCZUAOOQC-UTKZUKDTSA-N sacubitril Chemical compound C1=CC(C[C@H](C[C@@H](C)C(=O)OCC)NC(=O)CCC(O)=O)=CC=C1C1=CC=CC=C1 PYNXFZCZUAOOQC-UTKZUKDTSA-N 0.000 description 1
- 229960003953 sacubitril Drugs 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 210000002820 sympathetic nervous system Anatomy 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960005187 telmisartan Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000759 toxicological effect Toxicity 0.000 description 1
- 229960002051 trandolapril Drugs 0.000 description 1
- 229960004699 valsartan Drugs 0.000 description 1
- SJSNUMAYCRRIOM-QFIPXVFZSA-N valsartan Chemical compound C1=CC(CN(C(=O)CCCC)[C@@H](C(C)C)C(O)=O)=CC=C1C1=CC=CC=C1C1=NN=N[N]1 SJSNUMAYCRRIOM-QFIPXVFZSA-N 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 230000002227 vasoactive effect Effects 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7125—Nucleic acids or oligonucleotides having modified internucleoside linkage, i.e. other than 3'-5' phosphodiesters
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/322—2'-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/334—Modified C
- C12N2310/3341—5-Methylcytosine
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/341—Gapmers, i.e. of the type ===---===
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
- C12N2310/3515—Lipophilic moiety, e.g. cholesterol
Definitions
- ISIS 757456 for treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject.
- AGT angiotensinogen
- renin-angiotensin-aldosterone system is an integral neuro-hormonal mechanism involved in the regulation of vascular tone and fluid homeostasis.
- the systemic RAAS cascade begins with renin- mediated cleavage of angiotensinogen (AGT), whose plasma levels are primarily liver derived, to generate angiotensin I.
- AGT angiotensinogen
- Angiotensin converting enzyme converts angiotensin I to angiotensin II (Ang II), a potent vasoactive peptide involved in vasoconstriction and aldosterone release.
- the RAAS acts as a key regulator of acute hemodynamic changes, but persistent activation results in excessive vasoconstriction, salt and water retention, cardiac hypertrophy, and fibrosis.
- Chronic overactivity of the RAAS pathway is considered a major contributor to the pathogenesis of cardiovascular disorders, including hypertension, chronic kidney disease and heart failure.
- Heart failure is a global public health issue characterized by significant mortality, frequent hospitalization, and poor QOL, with an overall prevalence that is steadily increasing across the globe. Heart failure afflicts approximately 6.5 million patients in the United States and 26 million worldwide (Savarese and Lund, Cardiac Failure Review 2017; 3: 7-l l). As the population ages, heart failure incidence is increasing, and >550,000 patients are diagnosed with new heart failure each year. Heart Failure is responsible for more hospitalizations than all forms of cancer combined and is the most common diagnosis in hospital patients age 65 years and older. Every year over 1 million patients are hospitalized for heart failure in the US and Europe, accounting for 6.5 million hospital days (Ambrosy et ak, Curr Heart Fail Rep 2014; 11: 416-427). High rates of hospitalizations with frequent readmission (almost 25% of patients with HF are readmitted within 30 days) along with other direct and indirect costs, also place an enormous economic burden on healthcare systems Summary
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject.
- angiotensinogen AGT
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
- Heart failure with reduced ejection fraction is a complex clinical syndrome characterized by the heart's reduced capacity to pump blood (Yancy et ah, Am Coll Cardiol 2013; 62: el47-239). Signs and symptoms of heart failure include those due to fluid volume excess (dyspnea, orthopnea, edema, hepatic congestion, ascites) and those due to a diminished cardiac output (fatigue, dizziness, weakness). Fluid retention in heart failure is initiated by the fall in cardiac output, leading to alterations in renal function, and subsequent compensatory activation of the sodium-retaining renin-angiotensinaldosterone system (RAAS) and sympathetic nervous systems. The net effect of these neurohumoral responses is to produce hemodynamic changes in heart, kidneys and vasculature to increase blood volume and then ventricular fdling. However, chronic activation of neurohormonal response results in hemodynamic stress and progression of HF.
- RAAS sodium-retaining renin-angiotensinaldosterone system
- HF with preserved EF is characterized by a normal cardiac output but abnormal diastolic function, often with left ventricular (LV) concentric remodeling or hypertrophy.
- LV left ventricular
- RAAS suppression may benefit HFpEF as well.
- HTN Hypertension
- BP blood pressure
- CVD cardiovascular disease
- Inadequate BP control can lead to increase cardiovascular risk.
- Some patients have pseudo-resistance HTN as they are not compliant with their medications or they have white coat HTN.
- CV cardiovascular
- CHD chronic heart disease
- stroke heart failure
- renal failure all-cause mortality
- SBP Night-time systolic blood pressure
- a 10 mmHg increase in night-time SBP can increase risk of total CV events, stroke and cardiac mortality in diabetic patients (Draman et ak, JHypertens 2015; 33: 1373-1377).
- Resistant hypertension is characterized as failure to achieve blood pressure (BP) goal in patients on 3 or more antihypertensive medications, which typically belong to one or more of the following classes of medications: a diuretic, a long -acting calcium channel blocker, a beta blocker, or a renin-angiotensin pathway blocker such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.
- BP blood pressure
- antihypertensive medications typically belong to one or more of the following classes of medications: a diuretic, a long -acting calcium channel blocker, a beta blocker, or a renin-angiotensin pathway blocker such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.
- methods comprise administering a therapeutically effective amount of a modified oligonucleotide.
- the modified oligonucleotide is ISIS 757456.
- the therapeutically effective amount is within the range of about 5 mg to about 200 mg of ISIS 757456.
- the therapeutically effective amount is or is about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, about 80 mg, about 100 mg, or about 120 mg of ISIS 757456.
- the therapeutically effective amount is or is about 80 mg of ISIS 757456.
- the therapeutically effective amount is or is about 120 mg of ISIS 757456.
- the therapeutically effective amount is administered once every day. In certain embodiments, the therapeutically effective amount is administered once every other day. In certain embodiments, the therapeutically effective amount is administered once every 3 days. In certain embodiments, the therapeutically effective amount is administered once every 4 days. In certain embodiments, the therapeutically effective amount is administered once every 5 days. In certain embodiments, the therapeutically effective amount is administered once every 6 days. In certain embodiments, the therapeutically effective amount is administered once every week. In certain embodiments, the therapeutically effective amount is administered once every 2 weeks. In certain embodiments, the therapeutically effective amount is administered once every 3 weeks. In certain embodiments, the therapeutically effective amount is administered once every 4 weeks. In certain embodiments, the therapeutically effective amount is administered twice every week.
- the therapeutically effective amount is administered three times every week. In certain embodiments, the therapeutically effective amount is administered four times every week. In certain embodiments, the therapeutically effective amount is administered five times every week. In certain embodiments, the therapeutically effective amount is administered six times every week. In any of the foregoing embodiments, ISIS 757456 can be administered by a syringe.
- 2’-deoxyribonucleoside means a nucleoside comprising a 2’-H(H) deoxyribosyl sugar moiety.
- a 2’-deoxyribonucleoside is a 2 -b- ⁇ deoxyribonucleoside and comprises a 2’ ⁇ -D-deoxyribosyl sugar moiety, which has the b-D configuration as found in naturally occurring deoxyribonucleic acids (DNA).
- a 2 ’-deoxyribonucleoside may comprise a modified nucleobase or may comprise an RNA nucleobase (uracil).
- 2’-MOE means a 2’-0CH 2 CH 2 0CH 3 group in place of the 2 ’-OH group of a ribosyl sugar moiety.
- a “2’-MOE sugar moiety” is a sugar moiety with a 2’-0CH 2 CH 2 0CH 3 group in place of the 2’- OH group of a ribosyl sugar moiety. Unless otherwise indicated, a 2’-MOE sugar moiety is in the b-D configuration. “MOE” means O-methoxyethyl.
- 2’-MOE nucleoside means a nucleoside comprising a 2’-MOE sugar moiety.
- 5-methyl cytosine means a cytosine modified with a methyl group attached to the 5 position.
- a 5-methyl cytosine is a modified nucleobase.
- “about” means plus or minus 7% of the provided value.
- administering means providing a pharmaceutical agent to a human subject.
- dose means a quantity of a pharmaceutical agent administered.
- AGT RNA is the RNA expression product of the human gene, angiotensinogen.
- AGT protein is the protein expression product of AGT RNA.
- intemucleoside linkage means the covalent linkage between contiguous nucleosides in an oligonucleotide.
- modified intemucleoside linkage means any intemucleoside linkage other than a phosphodiester intemucleoside linkage.
- Phosphorothioate intemucleoside linkage is a modified intemucleoside linkage in which one of the non-bridging oxygen atoms of a phosphodiester intemucleoside linkage is replaced with a sulfur atom.
- loading dose means a therapeutically effective amount of a pharmaceutical agent administered during an initial dosing phase during which steady state concentration of the pharmaceutical agent is achieved.
- Initial loading dose means the first loading dose administered.
- Last loading dose means the loading dose administered most recently prior to administering a first maintenance dose.
- maintenance dose means a therapeutically effective amount of a pharmaceutical agent administered during a dosing phase after steady state concentration of the pharmaceutical agent has been achieved.
- nucleobase means an unmodified nucleobase or modified nucleobase.
- An “unmodified nucleobase” is adenine (A), thymine (T), cytosine (C), uracil (U), or guanine (G).
- a “modified nucleobase” is group of atoms other than unmodified A, T, C, U, or G capable of pairing with at least one unmodified nucleobase.
- a “5-methyl cytosine” is a modified nucleobase.
- nucleobase sequence means the order of contiguous nucleobases in a target nucleic acid or oligonucleotide independent of any sugar or intemucleoside linkage modification.
- nucleoside means a compound comprising a nucleobase and a sugar moiety.
- the nucleobase and sugar moiety are each, independently, unmodified or modified.
- modified nucleoside means a nucleoside comprising a modified nucleobase and/or a modified sugar moiety.
- Linked nucleosides are nucleosides that are connected in a contiguous sequence (i.e., no additional nucleosides are presented between those that are linked).
- oligonucleotide means a strand of linked nucleosides connected via intemucleoside linkages, wherein each nucleoside and intemucleoside linkage may be modified or unmodified. Unless otherwise indicated, oligonucleotides consist of 8-50 linked nucleosides.
- modified oligonucleotide means an oligonucleotide, wherein at least one nucleoside or intemucleoside linkage is modified.
- pharmaceutically acceptable carrier or diluent means any substance suitable for use in administering to a human subject. Certain such carriers enable pharmaceutical compositions to be formulated as, for example, tablets, pills, dragees, capsules, liquids, gels, symps, slurries, suspension, and lozenges for the oral ingestion by a human subject.
- a pharmaceutically acceptable carrier or diluent is sterile water, sterile saline, or sterile buffer solution.
- pharmaceutically acceptable salts means physiologically and pharmaceutically acceptable salts of compounds. Pharmaceutically acceptable salts retain the desired biological activity of the parent compound and do not impart undesired toxicological effects thereto.
- potassium salt means a salt of a modified oligonucleotide, wherein the cation of the salt is potassium.
- RNA means an RNA transcript and includes pre-mRNA and mature mRNA unless otherwise specified.
- sodium salt means a salt of a modified oligonucleotide, wherein the cation of the salt is sodium.
- subject means a human or non-human animal. In certain embodiments, the subject is a human subject. A “subject in need thereof,” is a subject who would benefit from administration of a modified oligonucleotide disclosed herein.
- the subject in need thereof has hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary He
- sugar moiety means an unmodified sugar moiety or a modified sugar moiety.
- Unmodified sugar moiety means a 2’-OH(H) b-D ribosyl moiety, as found in RNA (an “unmodified RNA sugar moiety”), or a 2’-H(H) b-D deoxyribosyl moiety, as found in DNA (an “unmodified DNA sugar moiety”).
- Unmodified sugar moieties have one hydrogen at each of the G, 3’, and 4’ positions, an oxygen at the 3’ position, and two hydrogens at the 5’ position.
- Modified sugar moiety or “modified sugar” means a modified furanosyl sugar moiety or a sugar surrogate.
- symptom means any physical feature or test result that indicates the existence or extent of a disease or disorder. In certain embodiments, a symptom is apparent to a subject or to a medical professional examining or testing the subject.
- treating refers to administering a compound or pharmaceutical composition to a subject in order to effect an alteration or improvement of a disease, disorder, or condition in the subject. In certain embodiments, treating includes amelioration.
- terapéuticaally effective amount means an amount of a pharmaceutical agent that provides a therapeutic benefit to a human subject. For example, a therapeutically effective amount improves a symptom of a disease.
- week means 7 days.
- Embodiment 1 A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure: Embodiment 2. The method of embodiment 1, wherein the oligomeric compound is the sodium salt or the potassium salt.
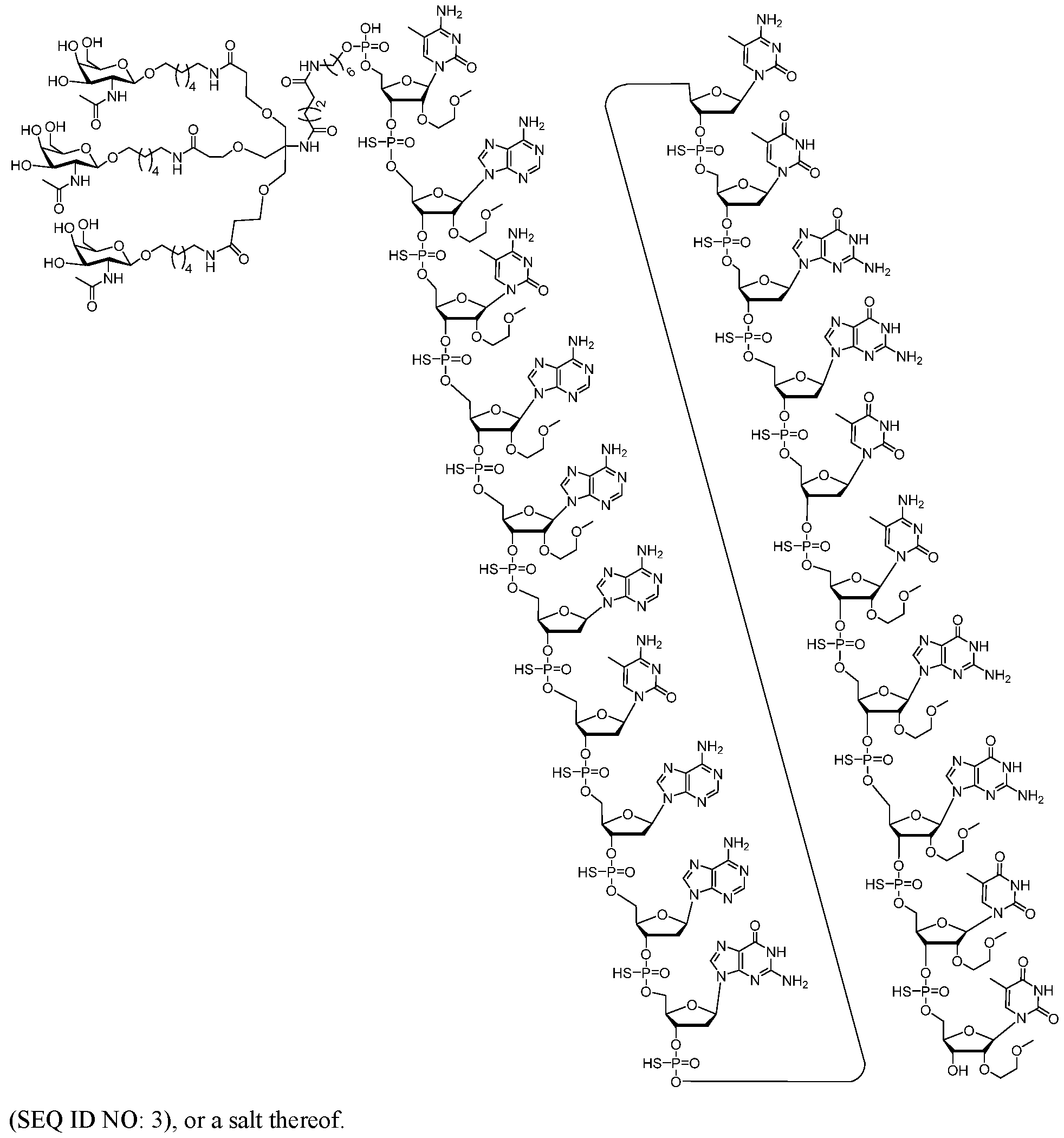
- Embodiment 3 A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure: (SEQ ID NO: 3).
- Embodiment 4 A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosisin a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according to the following formula: GalNAc 3 -7 a-0 ⁇ mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Td
- A an adenine nucleobase
- mC a 5-methyl cytosine nucleobase
- G a guanine nucleobase
- T a thymine nucleobase
- e a 2’-MOE sugar moiety
- d a 2’ ⁇ -D-deoxyribosyl sugar moiety
- s a phosphorothioate intemucleoside linkage
- Embodiment 5 A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Foeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
- Embodiment 6 The method of embodiment 5, wherein the oligomeric compound is the sodium salt or the potassium salt.
- Embodiment 7 A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Foeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
- Embodiment 8 A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according to the following formula: GalNAc 3 -7 a-0 ⁇ mCes Aes mCes Aes Aes Ads mCds Ads Ads Gd
- G a guanine nucleobase
- T a thymine nucleobase
- e a 2’-MOE sugar moiety
- d a 2’- -D-deoxyribosyl sugar moiety
- s a phosphorothioate intemucleoside linkage
- Embodiment 9 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 25 mg.
- Embodiment 10 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 40 mg.
- Embodiment 11 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 60 mg.
- Embodiment 12 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 80 mg.
- Embodiment 13 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 120 mg.
- Embodiment 14 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 25 mg.
- Embodiment 15 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 40 mg.
- Embodiment 16 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 60 mg.
- Embodiment 17 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 80 mg.
- Embodiment 18 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 120 mg.
- Embodiment 19 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg, 175 mg, 180 mg, 185 mg, 190 mg, 195 mg, or 200 mg.
- the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg
- Embodiment 20 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 105 mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg, about 135 mg, about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about 165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about 195 mg, or about 200 mg.
- the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about
- Embodiment 21 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is within the range of any of 25 mg to 200 mg, 40 mg to 200 mg, 60 mg to 200 mg, 80 mg to 200 mg, 100 mg to 200 mg, 120 mg to 200 mg, 150 mg to 200 mg, 25 mg to 120 mg, 30 mg to 120 mg, 40 mg to 120 mg, 60 mg to 120 mg, 80 mg to 120 mg, 40 mg to 80 mg, or 60 mg to 80 mg.
- Embodiment 22 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than 170 mg, less than 165 mg, less than 160 mg, less than 150 mg, less than 145 mg, less than 140 mg, less than 135 mg, less than 130 mg, less than 125 mg, less than 120 mg, less than 115 mg, less than 110 mg, less than 105 mg, less than 100 mg, less than 95 mg, less than 90 mg, less than 85 mg, less than 80 mg, less than 75 mg, less than 70 mg, less than 65 mg, less than 60 mg, less than 55 mg, less than 50 mg, less than 45 mg, less than 40 mg, less than 35 mg, and less than 30 mg.
- the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than
- Embodiment 23 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of less than about 200 mg, less than about 195 mg, less than about 190 mg, less than about 185 mg, less than about 180 mg, less than about 175 mg, less than about 170 mg, less than about 165 mg, less than about 160 mg, less than about 150 mg, less than about 145 mg, less than about 140 mg, less than about 135 mg, less than about 130 mg, less than about 125 mg, less than about 120 mg, less than about 115 mg, less than about 110 mg, less than about 105 mg, less than about 100 mg, less than about 95 mg, less than about 90 mg, less than about 85 mg, less than about 80 mg, less than about 75 mg, less than about 70 mg, less than about 65 mg, less than about 60 mg, less than about 55 mg, less than about 50 mg, less than about 45 mg, less than about 40 mg, less than about 35 mg, and less than about 30 mg.
- the therapeutically effective amount is any of less than
- Embodiment 24 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of at least at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 mg, at least 60 mg, at least 65 mg, at least 70 mg, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 mg, at least 95 mg, at least about 100 mg, at least 105 mg, at least 115 mg, at least 120 mg, at least 125 mg, at least 130 mg, at least 135 mg, at least 140 mg, at least 145 mg, at least 150 mg, at least 155 mg, at least 160 mg, at least 165 mg, at least 170 mg, at least 175 mg, at least 180 mg, at least 185, at least 190 mg, at least 195 mg, and at least 200 mg.
- the therapeutically effective amount is any of at least at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at
- Embodiment 25 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, at least about 70 mg, at least about 75 mg, at least about 80 mg, at least about 85 mg, at least about 90 mg, at least about 95 mg, at least about 100 mg, at least about 105 mg, at least about 115 mg, at least about 120 mg, at least about 125 mg, at least about 130 mg, at least about 135 mg, at least about 140 mg, at least about 145 mg, or at least about 150 mg, at least about 155 mg, at least about 160 mg, at least about 165 mg, at least about 170 mg, at least about 175 mg, at least about 180 mg, at least about 185, at least about 190 mg, at least about 195 mg, and at least about 200 mg.
- Embodiment 26 The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 80 mg to about 120 mg.
- Embodiment 27 The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every week.
- Embodiment 28 The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 2 weeks.
- Embodiment 29 The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 3 weeks.
- Embodiment 30 The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 4 weeks.
- Embodiment 31 The method of any one of embodiments 1-26, comprising administering the oligomeric compound twice every week.
- Embodiment 32 The method of any one of embodiments 1-26, comprising administering the oligomeric compound three times every week.
- Embodiment 33 The method of any one of embodiments 1-26, comprising administering the oligomeric compound four times every week.
- Embodiment 34 The method of any one of embodiments 1-26, comprising administering the oligomeric compound five times every week.
- Embodiment 35 The method of any one of embodiments 1-26, comprising administering the oligomeric compound six times every week.
- Embodiment 36 The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every day, once every other day, once every 3 days, once every 4 days, once every 5 days, or once every 6 days.
- Embodiment 37 The method of any one of embodiments 1-36, wherein at least one symptom of hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis is reduced or improved.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced
- Embodiment 38 The method of any one of embodiments 1-36, wherein the human subject has hypertension or resistant hypertension and at least one symptom of hypertension or resistant hypertension is reduced or improved.
- Embodiment 39 The method of embodiment 38, wherein at least one symptom is headaches, nosebleeds, fatigue, confusion, vision problems, chest pain, difficulty breathing, irregular heartbeat, blood in urine, or a combination thereof.
- Embodiment 40 The method of embodiment 38 or 39, wherein administering the oligomeric compound reduces blood pressure (BP), reduces systolic blood pressure (SBP), reduces diastolic blood pressure (DBP), achieves ⁇ 140/90 mmHg BP, achieves ⁇ 130/80 mmHg BP, improves quality of life as assessed by patient reported outcomes, or any combination thereof.
- BP blood pressure
- SBP systolic blood pressure
- DBP diastolic blood pressure
- Embodiment 41 The method of any one of embodiments 1-36, wherein the human subject has heart failure and at least one symptom of heart failure is reduced or improved.
- Embodiment 42 The method of embodiment 41, wherein at least one symptom is a symptom due to fluid volume excess including dyspnea, orthopnea, edema, hepatic congestion, and ascites; a symptom due to a diminished cardiac output including fatigue, dizziness, and weakness; or a combination thereof.
- Embodiment 43 The method of embodiment 41 or 42, wherein administering the oligomeric compound reduces rates of cardiovascular (CV) mortality, reduces heart failure hospitalization and urgent visits, reduces N-terminal prohormone B-type natriuretic peptide (NT-proBNP) levels, reduces B-type natriuretic peptide (BNP) levels, reduces cardiac troponin T (cTnT) levels, reduces high-sensitive cardiac troponin T (hs-cTnT) levels, improves cardiac function, reduces cardiac dilation, reduces cardiac fibrosis, increases or improves LVEF (left ventricular ejection fraction), reduces or improves LVESV (left ventricular end systolic volume), reduces or improves LVEDV (left ventricular end diastolic volume), increases or improves left ventricle (LV) strain, improves 6 minute walk test, improves quality of life, or any combination thereof.
- CV cardiovascular
- NT-proBNP N-terminal prohormon
- Embodiment 44 The method of any one of embodiments 1-36, wherein the human subject has Marfan syndrome and at least one symptom of Marfan syndrome is reduced or improved.
- Embodiment 45 The method of embodiment 44, wherein administering the oligomeric compound reduces or improves aortic root dilation, mortality, aortic root surgery, or any combination thereof.
- Embodiment 46 The method of any one of embodiments 1-45, wherein the oligomeric compound is administered by subcutaneous injection.
- Embodiment 47 The method of embodiment 46, wherein the oligomeric compound is administered by a syringe.
- AGT RNA is encoded by the human angiotensinogen (AGT) gene.
- AGT protein is the protein expression product of AGT RNA.
- a representative nucleobase sequence for a human AGT gene is the complement of the nucleotides of GenBank Accession No. NT_167186.1 truncated from nucleotides 24354000 to 24370100 (designated herein as SEQ ID NO: 1).
- a representative nucleobase sequence for a human AGT RNA is GENBANK Accession No. NM_000029.3 (designated herein as SEQ ID NO: 2).
- a method comprises administering oligomeric compound, ISIS 757456, to a subject in need thereof.
- ISIS 757456 is represented by the following chemical notation (5’ to 3’): GalNAc 3 -7 a-0 mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
- A an adenine nucleobase
- mC a 5-methyl cytosine nucleobase
- G a guanine nucleobase
- T a thymine nucleobase
- e a 2’-MOE sugar moiety
- d a 2’- -D-deoxyribosyl sugar moiety
- s a phosphorothioate intemucleoside linkage
- ISIS 757456 is represented by the following chemical structure:
- the sodium salt of ISIS 757456 is represented by the following chemical 5 structure:
- the pharmaceutical composition comprises a pharmaceutically acceptable diluent or carrier.
- the pharmaceutical composition comprises or consists essentially of a sterile saline solution and the oligomeric compound ISIS 757456.
- the sterile saline is pharmaceutical grade saline.
- the pharmaceutical composition comprises or consists essentially of sterile water and the oligomeric compound ISIS 757456.
- the sterile water is pharmaceutical grade water.
- the pharmaceutical composition comprises or consists essentially of the oligomeric compound ISIS 757456 in 2mM phosphate buffered isotonic saline, pH 7.4.
- compositions comprising the oligomeric compound ISIS 757456 encompass any pharmaceutically acceptable salt of the oligomeric compound ISIS 757456, esters of the oligomeric compound ISIS 757456, or salts of such esters.
- pharmaceutical compositions comprising the oligomeric compound ISIS 757456 are capable of providing (directly or indirectly) the biologically active metabolite or residue thereof upon administration to a human subject. Accordingly, for example, the disclosure is also drawn to pharmaceutically acceptable salts of the oligomeric compound ISIS 757456, prodrugs of the oligomeric compound ISIS 757456, pharmaceutically acceptable salts of such prodrugs, and other bioequivalents.
- Suitable pharmaceutically acceptable salts include, but are not limited to, sodium and potassium salts.
- the oligomeric compound ISIS 757456 acts as an acid.
- ISIS 757456 may be drawn or described in protonated (free acid) form, or ionized and in association with a cation (salt) form, aqueous solutions of ISIS 757456 exist in equilibrium among such forms.
- a phosphate linkage of ISIS 757456 in aqueous solution exists in equilibrium among free acid, anion, and salt forms.
- the term, “ISIS 757456,” is intended to include all such forms.
- ISIS 757456 has several such linkages, each of which is in equilibrium.
- ISIS 757456 exists in solution in an ensemble of forms at multiple positions all at equilibrium.
- ISIS 757456 is intended to include all such forms. Drawn structures necessarily depict a single form. Nevertheless, unless otherwise indicated, such drawings are likewise intended to include corresponding forms.
- a structure depicting the free acid of ISIS 757456 followed by the term “or a salt thereof’ expressly includes all such forms that may be fully or partially protonated/de-protonated/in association with a cation. In certain instances, one or more specific cation is identified.
- ISIS 757456 is in aqueous solution with sodium. In certain embodiments, ISIS 757456 is in aqueous solution with potassium. In certain embodiments, ISIS 757456 is in PBS. In certain embodiments, ISIS 757456 is in water. In certain such embodiments, the pH of the solution is adjusted with NaOH and/or HC1 to achieve a desired pH.
- ISIS 757456 in milligrams indicates the mass of the free acid form of ISIS 757456.
- the free acid in aqueous solution, the free acid is in equilibrium with anionic and salt forms.
- ISIS 757456 exists as a solvent-free, sodium-acetate free, anhydrous, free acid.
- ISIS 757456 may be partially or fully de-protonated and in association with Na+ ions.
- ISIS 757456 is administered by subcutaneous injection. In certain embodiments, ISIS 757456 is administered by a syringe.
- a method comprises administering to a subject a therapeutically effective amount of the oligomeric compound ISIS 757456.
- the therapeutically effective amount is 80 mg or about 80 mg.
- the therapeutically effective amount is 120 mg or about 120 mg.
- the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is 75 mg to 85 mg. In certain embodiments, the therapeutically effective amount is 110 mg to 130 mg or 115 mg to 125 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 37.5 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg, 175 mg, 180 mg, 185 mg, 190 mg, 195 mg, and 200 mg.
- the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 37.5 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 105 mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg, about 135 mg, about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about 165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about 195 mg, and about 200 mg.
- the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of 25 mg to 200 mg, 25 mg to 80 mg, 40 mg to 80 mg, 40 mg to 120 mg, or 80 mg to 120 mg. In certain embodiments, the therapeutically effective amount is any of about 40 mg to about 80 mg, about 40 mg to about 120 mg, or about 80 mg to about 120 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of 40 mg to 200 mg, 40 mg to 190 mg, 40 mg to 180 mg, 40 mg to 170 mg, from 40 mg to 160 mg, 40 mg to 150 mg, 40 mg to 140 mg, 40 mg to 120 mg, 40 mg to 110 mg, 40 mg to 100 mg, 40 mg to 80 mg, 40 mg to 70 mg, 40 mg to 60 mg, 40 mg to 50 mg, 50 mg to 200 mg, 50 mg to 190 mg, 50 mg to 180 mg, 50 mg to 170 mg, 50 mg to 160 mg, 50 mg to 150 mg, 50 mg to 140 mg, 50 mg to 120 mg, 50 mg to 110 mg, 50 mg to 100 mg, 50 mg to 80 mg, 50 mg to 70 mg, 50 mg to 60 mg, 60 mg to 200 mg, 60 mg to 190 mg, 60 mg to 180 mg, 60 mg to 170 mg, 60 mg to 160 mg, 60 mg to 150 mg, 60 mg to 140 mg, 60 mg to 120 mg, 60 mg to 110 mg, 60 mg to 100 mg, 60 mg to 80 mg, 60 mg to 70 mg, 60 mg to 60 mg
- the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than 170 mg, less than 165 mg, less than 160 mg, less than 150 mg, less than 145 mg, less than 140 mg, less than 135 mg, less than 130 mg, less than 125 mg, less than 120 mg, less than 115 mg, less than 110 mg, less than 105 mg, less than 100 mg, less than 95 mg, less than 90 mg, less than 85 mg, less than 80 mg, less than 75 mg, less than 70 mg, less than 65 mg, less than 60 mg, less than 55 mg, less than 50 mg, less than 45 mg, less than 40 mg, less than 35 mg and less than 30 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of less than about 200 mg, less than about 195 mg, less than about 190 mg, less than about 185 mg, less than about 180 mg, less than about 175 mg, less than about 170 mg, less than about 165 mg, less than about 160 mg, less than about 150 mg, less than about 145 mg, less than about 140 mg, less than about 135 mg, less than about 130 mg, less than about 125 mg, less than about 120 mg, less than about 115 mg, less than about 110 mg, less than about 105 mg, less than about 100 mg, less than about 95 mg, less than about 90 mg, less than about 85 mg, less than about 80 mg, less than about 75 mg, less than about 70 mg, less than about 65 mg, less than about 60 mg, less than about 55 mg, less than about 50 mg, less than about 45 mg, less than about 40 mg, less than about 35 mg, and less than about 30 mg.
- the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 mg, at least 60 mg, at least 65 mg, at least 70 mg, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 mg, at least 95 mg, at least 100 mg, at least 105 mg, at least 115 mg, at least 120 mg, at least 125 mg, at least 130 mg, at least 135 mg, at least 140 mg, at least 145 mg, at least 150 mg, at least 155 mg, at least 160 mg, at least 165 mg, at least 170 mg, at least 175 mg, at least 180 mg, at least 185, at least 190 mg, at least 195 mg, and at least 200 mg.
- the therapeutically effective amount is administered once every week.
- the therapeutically effective amount is any of at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, at least about 70 mg, at least about 75 mg, at least about 80 mg, at least about 85 mg, at least about 90 mg, at least about 95 mg, at least about 100 mg, at least about 105 mg, at least about 115 mg, at least about 120 mg, at least about 125 mg, at least about 130 mg, at least about 135 mg, at least about 140 mg, at least about 145 mg, or at least about 150 mg, at least about 155 mg, at least about 160 mg, at least about 165 mg, at least about 170 mg, at least about 175 mg, at least about 180 mg, at least about 185, at least about 190 mg, at least about 195 mg, and at least about 200 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
- a method comprises administering to a subject about 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method comprises administering to a subject 80 mg of the oligomeric compound ISIS 757456 administered subcutaneously once every week. In certain embodiments a method comprises administering to a subject about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method comprises administering to a subject 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- methods of administering to a subject a therapeutically effective amount of the oligomeric compound ISIS 757456 one or more times. In certain embodiments, methods comprise administering the therapeutically effective amount at least 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 times. In certain embodiments, methods comprise administering the therapeutically effective amount once every week. In certain embodiments, methods comprise administering the therapeutically effective amount once every other week.
- methods comprise administering the therapeutically effective amount once every week, once every 2 weeks, once every 3 weeks, once every 4 weeks, once every 5 weeks, once every 6 weeks, once every 7 weeks, or once every 8 weeks.
- methods comprise administering the therapeutically effective amount once every week, twice every week, three times every week, four times every week, five times every week, or six times every week.
- methods comprise administering the therapeutically effective amount once every day, once every 2 days, once every 3 days, once every 4 days, once every 5 days, or once every 6 days.
- methods comprise administering the therapeutically effective amount for at least about 1 month, at least about 2 months, at least about 3 months, at least about 4 months, at least about 5 months, at least about 6 months, at least about 7 months, at least about 8 months, at least about 9 months, at least about 10 months, at least about 11 months, or at least about 12 months.
- methods comprise administering the therapeutically effective amount for as long as the subject needs treatment for hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loe
- the therapeutically effective amount is administered as a loading dose and/or a maintenance dose.
- methods comprise administering a loading dose or doses and subsequently administering a maintenance dose or doses.
- methods comprise administering a loading dose once about every 4 weeks, and subsequently administering a maintenance dose once about every 8 weeks.
- methods comprise administering a loading dose once about every 4 weeks, and subsequently administering a maintenance dose once about every 16 weeks.
- methods comprise administering at least 2 loading doses, at least 3 loading doses, at least 4 loading doses, at least 5 loading doses, or at least 6 loading doses. In certain embodiments, methods comprise administering 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 loading doses. In certain embodiments, methods comprise administering a loading dose or doses about every 1 week, about every 2 weeks, about every 3 weeks, about every 4 weeks, about every 5 weeks, about every 6 weeks, about every 7 weeks, about every 8 weeks, about every 9 weeks, about every 10 weeks, about every 11 weeks, or about every 12 weeks.
- methods comprise administering an initial loading dose and administering a second loading dose about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, or about 12 weeks after administering the initial loading dose.
- methods comprise administering at least 2 maintenance doses, at least 3 maintenance doses, at least 4 maintenance doses, at least 5 maintenance doses, or at least 6 maintenance doses. In certain embodiments, methods comprise administering 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 maintenance doses. In some instances, methods comprise administering a maintenance dose or doses about every 4 weeks, about every 5 weeks, about every 6 weeks, about every 7 weeks, about every 8 weeks, about every 9 weeks, about every 10 weeks, about every 11 weeks, about every 12 weeks, about every 13 weeks, about every 14 weeks, about every 15 weeks, about every 16 weeks, about every 17 weeks, about every 18 weeks, about every 19 weeks, or about every 20 weeks.
- methods comprise administering a first maintenance dose and administering a second maintenance dose about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 17 weeks, about 18 weeks, about 19 weeks, or about 20 weeks after administering the first maintenance dose.
- methods comprise administering a first maintenance dose or doses about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 17 weeks, about 18 weeks, about 19 weeks, or about 20 weeks after administering the last loading dose.
- methods comprise administering a loading dose of about 5 mg to about 120 mg of ISIS 757456 in the same week as administering a maintenance dose of about 5 mg to about 120 mg of ISIS 757456.
- the loading dose is about 5, 10, 20, 40, 60, 80 or 120 mg of ISIS 757456.
- the maintenance dose is about 5, 10, 20, 40, 60, 80 or 120 mg of ISIS 757456.
- the loading dose is about 80 mg of ISIS 757456.
- the maintenance dose is about 80 mg of ISIS 757456.
- the loading dose is about 120 mg of ISIS 757456.
- the maintenance dose is about 120 mg of ISIS 757456.
- the loading dose is administered for 1 week and the maintenance dose is administered for as long as the human subject needs treatment.
- Certain embodiments are drawn to administering a first dose of ISIS 757456 for a first period of time followed by a second dose of ISIS 757456 for a second period of time, wherein the second dose is smaller than the first dose.
- the first period of time and the second period of time do not overlap.
- a method comprises administering to a subject about 120 mg of ISIS 757456 for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 for a second period of time.
- a method comprises administering to a subject about 120 mg of ISIS 757456 once every week for a first period of time followed by administering to the subject about mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 once every week for a second period of time.
- the first period of time and the second period of time do not overlap.
- a method comprises administering to a subject about 80 mg of ISIS 757456 for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, or about 60 mg of ISIS 757456 for a second period of time.
- a method comprises administering to a subject about 80 mg of ISIS 757456 once every week for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 once every week for a second period of time.
- the first period of time and the second period of time do not overlap.
- methods comprise co-administering ISIS 757456 with at least one other pharmaceutical agent.
- the at least one other pharmaceutical agent treats hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis, or a symptom thereof.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- ISIS 757456 is co-administered with the at least one other pharmaceutical agent to produce a combinational effect. In certain embodiments, ISIS 757456 is co-administered with the at least one other pharmaceutical agent to produce a synergistic effect.
- ISIS 757456 and the at least one other pharmaceutical agent are administered at the same time. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are administered at different times. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are prepared together in a single formulation. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are administered are prepared separately.
- pharmaceutical agents that may be co-administered with ISIS 757456 include angiotensin-converting enzyme inhibitor (ACEi, e.g., lisinopril, ramipril, perindopril, enalapril, benazepril, quinapril, captopril, fosinopril, trandolapril, moexipril, enalaprilat), angiotensin II receptor blocker (ARB, e.g., losartan, olmesartan, valsartan, candesartan, irbesartan, telmisartan, azilsartan, eprosartan), beta blocker, calcium channel blocker, non-potassium sparing diuretic, alpha- 1 blocker, centrally acting sympatholytic agent, SGLT2 inhibitor (SGLT2i, e.g., empaglifozin, canaglifozin, dapag
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
- methods, doses, or dosing regimens described herein can be used for treating RHTN in a subject who has failed to achieve a blood pressure ⁇ 140/90 mmHg despite taking or having taken 3 or more medications for hypertension. In certain embodiments, methods, doses, or dosing regimens described herein can be used for treating RHTN in a subject who has failed to achieve a blood pressure ⁇ 130/80 mmHg despite taking or having taken 3 or more medications for hypertension.
- the 3 or more medications for hypertension belong to one or more of the following classes of medications: a diuretic, a long -acting calcium channel blocker, a beta blocker, or a renin-angiotensin pathway blocker such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.
- administering ISIS 757456 in any of the foregoing doses or dosing regimens can improve or reduce at least one symptom in a subject have any of the aforementioned diseases.
- methods described herein are sufficiently effective to treat hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Die
- methods described herein improves symptoms of a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
- HTN hypertension
- RHTN resistant hypertension
- HFrEF heart failure with reduced ejection fraction
- HFpEF heart failure with preserved ejection fraction
- DN diabetic nephropathy
- aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Diet
- a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension, thereby treating hypertension or resistant hypertension in the subject.
- administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension reduces blood pressure (BP), reduces systolic blood pressure (SBP), reduces diastolic blood pressure (DBP), achieves ⁇ 140/90 mmHg BP, achieves ⁇ 130/80 mmHg BP, improves quality of life as assessed by patient reported outcomes, or any combination thereof, in the subject.
- BP blood pressure
- SBP systolic blood pressure
- DBP diastolic blood pressure
- administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension reduces or improves headaches, nosebleeds, fatigue, confusion, vision problems, chest pain, difficulty breathing, irregular heartbeat, blood in urine, or any combination thereof, in the subject.
- a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 once a week to a subject having hypertension or resistant hypertension, thereby treating hypertension or resistant hypertension in the subject.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 to a subject having uncontrolled hypertension on two or three or more antihypertensive medications, thereby treating hypertension or resistant hypertension in the subject.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 120 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure, thereby treating heart failure in the subject.
- administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure reduces rates of cardiovascular (CV) mortality, reduces heart failure hospitalization and urgent heart failure visits, reduces N-terminal prohormone B-type natriuretic peptide (NT-proBNP) levels, reduces B- type natriuretic peptide (BNP) levels, reduces cardiac troponin T (cTnT) levels, reduces high-sensitive cardiac troponin T (hs-cTnT) levels, improves cardiac function, reduces cardiac dilation, reduces cardiac fibrosis, increases or improves LVEF (left ventricular ejection fraction), reduces or improves LVESV (left ventricular end systolic volume), reduces or improves LVEDV (left ventricular end diastolic volume), increases
- CV cardiovascular
- a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 once a week to a subject having heart failure, thereby treating heart failure in the subject.
- administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure reduces or improves symptoms due to fluid volume excess including dyspnea, orthopnea, edema, hepatic congestion, and ascites; reduces or improves symptoms due to a diminished cardiac output including fatigue, dizziness, and weakness; or any combination thereof, in the subject.
- a method of treating heart failure comprises administering to a subject having heart failure about 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating heart failure comprises administering to a subject having heart failure 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating heart failure comprises administering to a subject having heart failure 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 to a subject having chronic heart failure with reduced ejection fraction, thereby treating chronic heart failure in the subject.
- a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 80 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 120 mg of the oligomeric compound ISIS 757456 once every week.
- a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction failure 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
- a method of treating Marfan syndrome comprises administering a therapeutically effective amount of ISIS 757456 to a subject having Marfan syndrome, thereby treating Marfan syndrome in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having Marfan syndrome reduces or improves aortic root dilation, mortality, aortic root surgery, or any combination thereof, in the subject.
- the phase 1 trial in healthy volunteers was a randomized, double-blind, placebo-controlled study assessing safety and tolerability of ISIS 757456 in 5 single-ascending dose cohorts (5-80 mg) and 2 multiple- ascending dose cohorts dosed weekly for 6-weeks (40 to 80 mg in an 8:2 randomization ratio).
- Subjects with a screening plasma AGT level ⁇ 20 pg/mL were not eligible for the multiple-dose cohorts.
- the primary objective was to assess safety and tolerability. Plasma AGT levels over time were also measured.
- phase 1 Healthy Volunteer Study single ascending doses (29 subjects treated with ISIS 757456 and 12 with placebo) and multiple ascending doses (16 subjects treated with ISIS 757456 and 4 with placebo), were well tolerated with no drug related SAEs, no clinically meaningful changes in laboratory assessments, no hypotensive events, acute renal changes or hyperkalemia.
- Plasma AGT levels returned to baseline levels 10 weeks after the last dose. There was no significant effect on blood pressure, Ang II, brain natriuretic peptide, atrial natriuretic peptide or renin mass and activity.
- ISIS 757456 results in significant AGT reductions with favorable safety and tolerability profile.
- SBP systolic blood pressure
- the phase 2 monotherapy study was a randomized, double-blind, placebo-controlled trial evaluating ISIS 757456.
- systolic blood pressure SBP
- All patients received weekly, in clinic, subcutaneous injections for 6 weeks with a loading dose administered on Day 3, then followed for 12 weeks in the post treatment period.
- the primary efficacy endpoint was the comparison of percent change in plasma AGT from baseline to study week 7 (Day 43) between 80 mg ISIS 757456 and placebo.
- Exploratory endpoints included post-baseline changes in SBP, diastolic blood pressure (DBP), percentage of patients reaching the goals of in-clinic SBP ⁇ 140 mmHg, DBP ⁇ 90 mmHg, and both over time. Blood pressure was measured by study personnel at every in-clinic visit in a quiet room after 5 minutes of resting in a chair with feet on the floor. Three consecutive blood pressure measurements were averaged to obtain an average blood pressure.
- the AGT levels tended to decline following Wash-Out of anti-hypertensive medications.
- ISIS 757456 was well tolerated with no hypotensive events, hyperkalemia or renal abnormalities (Supplemental Table 3). After 6 weeks of dosing at Day 43, a significant mean absolute reduction in AGT levels was noted in the ISIS 757456 group compared to placebo (-11.2 pg/mL versus 2.0, p ⁇ 0.001). Similarly, the mean percent reduction in AGT levels was significantly lower with ISIS 757456 compared to placebo (- 54% versus 12.6%, p ⁇ 0.001). In the ISIS 757456 group, 6 treated patients had AGT levels below the lower level of detection of the assay and were assigned values of 4.7 pg/ml. The reductions were sustained and demonstrated reversibility in the post treatment period.
- ISIS 757456 results in significant AGT reductions with favorable trends in SBP and DBP reduction in subjects with uncontrolled HTN with concomitant RAAS blockade. No on-target effects were observed.
- a dose-ranging, Phase 2B study is underway in subjects with resistant HTN on 3 medications, as well a Phase 2 study in patients with heart failure with reduced ejection fraction.
- Example 3 Phase 2 Human Clinical Trial with ISIS 757456 in Patients with Uncontrolled Hypertension on Two or Three Antihypertensive Medications
- Baseline mean AGT levels were 25.5 and 25.2 pg/mL in the placebo and ISIS 757456 groups, respectively. Approximately two thirds of patients were taking 2 anti-hypertensive medications and one third 3 antihypertensive medications.
- Baseline mean SBP was 152 mmHg and DBP 87 in the placebo group and SBP 154 and DBP 89 mmHg in the ISIS 757456 group.
- ISIS 757456 was well tolerated with no serious adverse events, hypotensive events or renal abnormalities. After 8 weeks of dosing at Day 57, a significant absolute reduction in mean AGT levels was noted in the ISIS 757456 group compared to placebo (-17.0 versus -1.1 pg/mL pO.OOl). Similarly, the mean percent reduction in AGT levels was significantly lower in ISIS 757456 compared to placebo (67% versus 3.4%, p ⁇ 0.001). In the ISIS 757456 group, 2 treated patients had AGT levels below the lower level of detection and were assigned values of 4.7 pg/ml.
- Example 4 Phase 2 Human Clinical Trial to Assess the Safety, Tolerability and Efficacy of ISIS 757456 Administered to Hypertensive Patients with Uncontrolled Blood Pressure
- a Phase 2 study to evaluate the effect of ISIS 757456 compared to placebo on seated automated office systolic blood pressure (SBP) from Baseline in uncontrolled hypertensive patients on 3 or more antihypertensive medications in the following categories is conducted: angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB), beta blocker, calcium channel blocker, non-potassium sparing diuretic, alpha- 1 blocker, centrally acting sympatholytic agent, or direct acting vasodilators (e.g. hydralazine).
- ACEi angiotensin-converting enzyme inhibitor
- ARB angiotensin II receptor blocker
- beta blocker calcium channel blocker
- non-potassium sparing diuretic e.g. hydralazine
- 80 mg or 120 mg of ISIS 757456 is administered to the patients once every week. The following are evaluated:
- ISIS 757456 plasma angiotensinogen (AGT) at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications.
- AGT plasma angiotensinogen
- ISIS 757456 The effect of ISIS 757456 on seated automated office SBP at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications.
- ISIS 757456 on seated automated office diastolic blood pressure (DBP) at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications.
- DBP diastolic blood pressure
- Example 5 Phase 2 Human Clinical Trial Assessing the Safety, Tolerability and Efficacy of ISIS 757456 in Patients with Chronic Heart Failure with Reduced Ejection Fraction
- ISIS 757456 weekly subcutaneous (SC) injection on plasma angiotensinogen (AGT) concentration from Baseline to Study Day 85 (W eek 13) in patients with chronic heart failure (HF) with reduced ejection fraction (HFrEF) is evaluated. 40 mg or 80 mg or 120 mg of ISIS 757456 is administered to the patients once weekly. The following are evaluated:
- ISIS 757456 weekly SC injection on plasma angiotensinogen (AGT) concentration at each scheduled visit in patients with chronic HFrEF.
- AGT angiotensinogen
- NT-proBNP N-terminal prohormone of B-type natriuretic peptide
- ISIS 757456 high sensitivity cardiac troponin T (hs-cTnT) at each scheduled visit in patients with chronic HFrEF.
- ISIS 757456 The effect of ISIS 757456 on functional capacity, patient-reported outcomes and disease scores related to the quality of life (QOL) at each scheduled visit in patients with chronic HFrEF.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biochemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Physics & Mathematics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Provided herein are methods of administering ISIS 757456 for treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-β signaling including Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis. Also provide herein are methods of reducing AGT RNA or protein in a human subject in need thereof.
Description
METHODS FOR REDUCING AGT EXPRESSION
Sequence Listing
The present application is being filed along with a Sequence Listing in electronic format. The Sequence Listing is provided as a file entitled BIOL0412WOSEQ_ST25.txt, created on April 25, 2022, which is 25 KB in size. The information in the electronic format of the sequence listing is incorporated herein by reference in its entirety.
Field
Provided herein are methods of administering ISIS 757456 for treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject. Also provided herein are methods of administering ISIS 757456 for reducing angiotensinogen (AGT) RNA or protein in a human subject in need thereof.
Background
The renin-angiotensin-aldosterone system (RAAS) is an integral neuro-hormonal mechanism involved in the regulation of vascular tone and fluid homeostasis. The systemic RAAS cascade begins with renin- mediated cleavage of angiotensinogen (AGT), whose plasma levels are primarily liver derived, to generate angiotensin I. Angiotensin converting enzyme converts angiotensin I to angiotensin II (Ang II), a potent vasoactive peptide involved in vasoconstriction and aldosterone release. The RAAS acts as a key regulator of acute hemodynamic changes, but persistent activation results in excessive vasoconstriction, salt and water retention, cardiac hypertrophy, and fibrosis. Chronic overactivity of the RAAS pathway is considered a major contributor to the pathogenesis of cardiovascular disorders, including hypertension, chronic kidney disease and heart failure.
Heart failure (HF) is a global public health issue characterized by significant mortality, frequent hospitalization, and poor QOL, with an overall prevalence that is steadily increasing across the globe. Heart failure afflicts approximately 6.5 million patients in the United States and 26 million worldwide (Savarese and Lund, Cardiac Failure Review 2017; 3: 7-l l). As the population ages, heart failure incidence is increasing, and >550,000 patients are diagnosed with new heart failure each year. Heart Failure is responsible for more hospitalizations than all forms of cancer combined and is the most common diagnosis in hospital patients age 65 years and older. Every year over 1 million patients are hospitalized for heart failure in the US and Europe, accounting for 6.5 million hospital days (Ambrosy et ak, Curr Heart Fail Rep 2014; 11: 416-427). High rates of hospitalizations with frequent readmission (almost 25% of patients with HF are readmitted within 30 days) along with other direct and indirect costs, also place an enormous economic burden on healthcare systems
Summary
Provided herein are methods for treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject. Also provided herein are methods of reducing angiotensinogen (AGT) RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
Heart failure with reduced ejection fraction (HFrEF) is a complex clinical syndrome characterized by the heart's reduced capacity to pump blood (Yancy et ah, Am Coll Cardiol 2013; 62: el47-239). Signs and symptoms of heart failure include those due to fluid volume excess (dyspnea, orthopnea, edema, hepatic congestion, ascites) and those due to a diminished cardiac output (fatigue, dizziness, weakness). Fluid retention in heart failure is initiated by the fall in cardiac output, leading to alterations in renal function, and subsequent compensatory activation of the sodium-retaining renin-angiotensinaldosterone system (RAAS) and sympathetic nervous systems. The net effect of these neurohumoral responses is to produce hemodynamic changes in heart, kidneys and vasculature to increase blood volume and then ventricular fdling. However, chronic activation of neurohormonal response results in hemodynamic stress and progression of HF.
HF with preserved EF (HFpEF) is characterized by a normal cardiac output but abnormal diastolic function, often with left ventricular (LV) concentric remodeling or hypertrophy. Currently, no clinical trials have shown benefits in the subset of subjects with HFpEF, which represents approximately 50% of HF cases. Comorbidities such as hypertension, diabetes mellitus, and CKDs are highly prevalent and are implicated in development and progression of HFpEF. Thus, it seems reasonable that RAAS suppression may benefit HFpEF as well.
Hypertension (HTN) is characterized as failure to achieve blood pressure (BP) goal < 140/90 mmHg. HTN is a major contributor to cardiovascular disease (CVD) morbidity and mortality and chronic kidney disease. Approximately 1.5 M people in the US have myocardial infarction or stroke annually, with -50% of these major adverse cardiovascular events attributed to HTN (Lawes et ah, Lancet 2008; 371: 1513-1518; Korsnes et ah, J Manag Care Spec Pharm 2015; 21: 443-450). Inadequate BP control can lead to increase cardiovascular risk. Some patients have pseudo-resistance HTN as they are not compliant with their
medications or they have white coat HTN. Lowering blood pressure reduces cardiovascular (CV) risk including major CV events, chronic heart disease (CHD), stroke, heart failure, renal failure, all-cause mortality (Ettehad et ak, The Lancet 2016; 387: 957-967). Providing sustained and controlled blood pressure with weekly or less administration could benefit patients inadequately controlled with daily administered therapies. Night-time systolic blood pressure (SBP) has consistently been shown to be an important predictor of CV risk. A 10 mmHg increase in night-time SBP can increase risk of total CV events, stroke and cardiac mortality in diabetic patients (Draman et ak, JHypertens 2015; 33: 1373-1377).
Resistant hypertension (RHTN) is characterized as failure to achieve blood pressure (BP) goal in patients on 3 or more antihypertensive medications, which typically belong to one or more of the following classes of medications: a diuretic, a long -acting calcium channel blocker, a beta blocker, or a renin-angiotensin pathway blocker such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. (Carey RM et ak, Hypertension 2018 Nov; 72(5):e53-e90; Judd and Calhoun, J Hum Hypertens 2014; 28: 463-468; Sigmund et ak, Hypertension 2020; 75: 902-917). In US alone, 70 million adults have HTN, of which 12-15% have RHTN. Among these patients 33% of them have uncontrolled RHTN (Judd and Calhoun 2014). In an analysis of National Health and Nutrition Examination Survey database, these patients are more likely to be black, with diabetes, with chronic kidney disease (CKD) Stage 3, with proteinuria and congestive heart failure compared to patients with HTN and without resistant HTN.
In certain embodiments, methods comprise administering a therapeutically effective amount of a modified oligonucleotide. In certain embodiments, the modified oligonucleotide is ISIS 757456. In certain embodiments, the therapeutically effective amount is within the range of about 5 mg to about 200 mg of ISIS 757456. In certain embodiments, the therapeutically effective amount is or is about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, about 80 mg, about 100 mg, or about 120 mg of ISIS 757456. In certain embodiments, the therapeutically effective amount is or is about 80 mg of ISIS 757456. In certain embodiments, the therapeutically effective amount is or is about 120 mg of ISIS 757456. In certain embodiments, the therapeutically effective amount is administered once every day. In certain embodiments, the therapeutically effective amount is administered once every other day. In certain embodiments, the therapeutically effective amount is administered once every 3 days. In certain embodiments, the therapeutically effective amount is administered once every 4 days. In certain embodiments, the therapeutically effective amount is administered once every 5 days. In certain embodiments, the therapeutically effective amount is administered once every 6 days. In certain embodiments, the therapeutically effective amount is administered once every week. In certain embodiments, the therapeutically effective amount is administered once every 2 weeks. In certain embodiments, the therapeutically effective amount is administered once every 3 weeks. In certain embodiments, the therapeutically effective amount is administered once every 4 weeks. In certain embodiments, the therapeutically effective amount is administered twice every week. In certain embodiments, the therapeutically effective amount is administered three times every week. In certain embodiments, the therapeutically effective amount is administered four times every week. In certain embodiments, the
therapeutically effective amount is administered five times every week. In certain embodiments, the therapeutically effective amount is administered six times every week. In any of the foregoing embodiments, ISIS 757456 can be administered by a syringe.
Detailed Description
It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive. Herein, the use of the singular includes the plural unless specifically stated otherwise. As used herein, the use of “or” means “and/or” unless stated otherwise. Furthermore, the use of the term “including” as well as other forms, such as “includes” and “included”, is not limiting. Also, terms such as “element” or “component” encompass both elements and components comprising one unit and elements and components that comprise more than one subunit, unless specifically stated otherwise.
The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. All documents, or portions of documents, cited in this application, including, but not limited to, patents, patent applications, articles, books, and treatises, are hereby expressly incorporated-by-reference for the portions of the document discussed herein, as well as in their entirety.
DEFINITIONS
Unless specific definitions are provided, the nomenclature used in connection with, and the procedures and techniques of, analytical chemistry, synthetic organic chemistry, and medicinal and pharmaceutical chemistry described herein are those well-known and commonly used in the art. Where permitted, all patents, applications, published applications and other publications and other data referred to throughout in the disclosure are incorporated by reference herein in their entirety.
Unless otherwise indicated, the following terms have the following meanings:
As used herein, “2’-deoxyribonucleoside” means a nucleoside comprising a 2’-H(H) deoxyribosyl sugar moiety. In certain embodiments, a 2’-deoxyribonucleoside is a 2 -b-ϋ deoxyribonucleoside and comprises a 2’^-D-deoxyribosyl sugar moiety, which has the b-D configuration as found in naturally occurring deoxyribonucleic acids (DNA). In certain embodiments, a 2 ’-deoxyribonucleoside may comprise a modified nucleobase or may comprise an RNA nucleobase (uracil).
As used herein, “2’-MOE” means a 2’-0CH2CH20CH3 group in place of the 2 ’-OH group of a ribosyl sugar moiety. A “2’-MOE sugar moiety” is a sugar moiety with a 2’-0CH2CH20CH3 group in place of the 2’- OH group of a ribosyl sugar moiety. Unless otherwise indicated, a 2’-MOE sugar moiety is in the b-D configuration. “MOE” means O-methoxyethyl.
As used herein, “2’-MOE nucleoside” means a nucleoside comprising a 2’-MOE sugar moiety.
As used herein, “5-methyl cytosine” means a cytosine modified with a methyl group attached to the 5 position. A 5-methyl cytosine is a modified nucleobase.
As used herein, “about” means plus or minus 7% of the provided value.
As used herein, “administering” means providing a pharmaceutical agent to a human subject.
As used herein, “dose” means a quantity of a pharmaceutical agent administered.
As used herein, “AGT RNA” is the RNA expression product of the human gene, angiotensinogen.
As used herein, “AGT protein” is the protein expression product of AGT RNA.
As used herein, the term “intemucleoside linkage” means the covalent linkage between contiguous nucleosides in an oligonucleotide. As used herein “modified intemucleoside linkage” means any intemucleoside linkage other than a phosphodiester intemucleoside linkage. “Phosphorothioate intemucleoside linkage” is a modified intemucleoside linkage in which one of the non-bridging oxygen atoms of a phosphodiester intemucleoside linkage is replaced with a sulfur atom.
As used herein, “loading dose” means a therapeutically effective amount of a pharmaceutical agent administered during an initial dosing phase during which steady state concentration of the pharmaceutical agent is achieved. “Initial loading dose” means the first loading dose administered. “Last loading dose” means the loading dose administered most recently prior to administering a first maintenance dose.
As used herein, “maintenance dose” means a therapeutically effective amount of a pharmaceutical agent administered during a dosing phase after steady state concentration of the pharmaceutical agent has been achieved.
As used herein, “nucleobase” means an unmodified nucleobase or modified nucleobase. An “unmodified nucleobase” is adenine (A), thymine (T), cytosine (C), uracil (U), or guanine (G). A “modified nucleobase” is group of atoms other than unmodified A, T, C, U, or G capable of pairing with at least one unmodified nucleobase. A “5-methyl cytosine” is a modified nucleobase. As used herein, “nucleobase sequence” means the order of contiguous nucleobases in a target nucleic acid or oligonucleotide independent of any sugar or intemucleoside linkage modification.
As used herein, “nucleoside” means a compound comprising a nucleobase and a sugar moiety. The nucleobase and sugar moiety are each, independently, unmodified or modified. As used herein, “modified nucleoside” means a nucleoside comprising a modified nucleobase and/or a modified sugar moiety. “Linked nucleosides” are nucleosides that are connected in a contiguous sequence (i.e., no additional nucleosides are presented between those that are linked). As used herein, “oligonucleotide” means a strand of linked nucleosides connected via intemucleoside linkages, wherein each nucleoside and intemucleoside linkage may be modified or unmodified. Unless otherwise indicated, oligonucleotides consist of 8-50 linked nucleosides. As used herein, “modified oligonucleotide” means an oligonucleotide, wherein at least one nucleoside or intemucleoside linkage is modified.
As used herein, “pharmaceutically acceptable carrier or diluent” means any substance suitable for use in administering to a human subject. Certain such carriers enable pharmaceutical compositions to be formulated as, for example, tablets, pills, dragees, capsules, liquids, gels, symps, slurries, suspension, and
lozenges for the oral ingestion by a human subject. In certain embodiments, a pharmaceutically acceptable carrier or diluent is sterile water, sterile saline, or sterile buffer solution.
As used herein, “pharmaceutically acceptable salts” means physiologically and pharmaceutically acceptable salts of compounds. Pharmaceutically acceptable salts retain the desired biological activity of the parent compound and do not impart undesired toxicological effects thereto.
As used herein, “potassium salt” means a salt of a modified oligonucleotide, wherein the cation of the salt is potassium.
As used herein, “RNA” means an RNA transcript and includes pre-mRNA and mature mRNA unless otherwise specified.
As used herein, “sodium salt” means a salt of a modified oligonucleotide, wherein the cation of the salt is sodium.
As used herein, “subject” means a human or non-human animal. In certain embodiments, the subject is a human subject. A “subject in need thereof,” is a subject who would benefit from administration of a modified oligonucleotide disclosed herein. In certain embodiments, the subject in need thereof has hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
As used herein, “sugar moiety” means an unmodified sugar moiety or a modified sugar moiety. “Unmodified sugar moiety” means a 2’-OH(H) b-D ribosyl moiety, as found in RNA (an “unmodified RNA sugar moiety”), or a 2’-H(H) b-D deoxyribosyl moiety, as found in DNA (an “unmodified DNA sugar moiety”). Unmodified sugar moieties have one hydrogen at each of the G, 3’, and 4’ positions, an oxygen at the 3’ position, and two hydrogens at the 5’ position. “Modified sugar moiety” or “modified sugar” means a modified furanosyl sugar moiety or a sugar surrogate.
As used herein, “symptom” means any physical feature or test result that indicates the existence or extent of a disease or disorder. In certain embodiments, a symptom is apparent to a subject or to a medical professional examining or testing the subject.
As used herein, “treating” refers to administering a compound or pharmaceutical composition to a subject in order to effect an alteration or improvement of a disease, disorder, or condition in the subject. In certain embodiments, treating includes amelioration.
As used herein, “therapeutically effective amount” means an amount of a pharmaceutical agent that provides a therapeutic benefit to a human subject. For example, a therapeutically effective amount improves a symptom of a disease.
As used herein, “week” means 7 days.
CERTAIN EMBODIMENTS
Embodiment 1. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
Embodiment 2. The method of embodiment 1, wherein the oligomeric compound is the sodium salt or the potassium salt.
Embodiment 3. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
(SEQ ID NO: 3).
Embodiment 4. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosisin a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according to the following formula: GalNAc3-7a-0· mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
A = an adenine nucleobase, mC = a 5-methyl cytosine nucleobase,
G = a guanine nucleobase,
T = a thymine nucleobase, e = a 2’-MOE sugar moiety, d = a 2’^-D-deoxyribosyl sugar moiety, s = a phosphorothioate intemucleoside linkage, o’ = 5’-P(0H)(=0)-0-3’, and
Embodiment 5. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Foeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
(SEQ ID NO: 3), or a salt thereof.
Embodiment 6. The method of embodiment 5, wherein the oligomeric compound is the sodium salt or the potassium salt.
Embodiment 7. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Foeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
(SEQ ID NO: 3).
Embodiment 8. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according to the following formula: GalNAc3-7a-0· mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
A = an adenine nucleobase, mC = a 5-methyl cytosine nucleobase,
G = a guanine nucleobase,
T = a thymine nucleobase, e = a 2’-MOE sugar moiety, d = a 2’- -D-deoxyribosyl sugar moiety, s = a phosphorothioate intemucleoside linkage, o’ = 5’-P(0H)(=0)-0-3’, and
Embodiment 9. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 25 mg.
Embodiment 10. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 40 mg.
Embodiment 11. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 60 mg.
Embodiment 12. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 80 mg.
Embodiment 13. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is 120 mg.
Embodiment 14. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 25 mg.
Embodiment 15. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 40 mg.
Embodiment 16. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 60 mg.
Embodiment 17. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 80 mg.
Embodiment 18. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 120 mg.
Embodiment 19. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg, 175 mg, 180 mg, 185 mg, 190 mg, 195 mg, or 200 mg.
Embodiment 20. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 105 mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg, about 135 mg, about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about 165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about 195 mg, or about 200 mg.
Embodiment 21. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is within the range of any of 25 mg to 200 mg, 40 mg to 200 mg, 60 mg to 200 mg, 80 mg to 200 mg, 100 mg to 200 mg, 120 mg to 200 mg, 150 mg to 200 mg, 25 mg to 120 mg, 30 mg to 120 mg, 40 mg to 120 mg, 60 mg to 120 mg, 80 mg to 120 mg, 40 mg to 80 mg, or 60 mg to 80 mg.
Embodiment 22. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than 170 mg, less than 165 mg, less than 160 mg, less than 150 mg, less than 145 mg, less than 140 mg, less than 135 mg, less than 130 mg, less than 125 mg, less than 120 mg, less than 115 mg, less than 110 mg, less than 105 mg, less than 100 mg, less than 95 mg, less than 90 mg, less than 85 mg, less than 80 mg, less than 75 mg, less than 70 mg, less than 65 mg, less than 60 mg, less than 55 mg, less than 50 mg, less than 45 mg, less than 40 mg, less than 35 mg, and less than 30 mg.
Embodiment 23. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of less than about 200 mg, less than about 195 mg, less than about 190 mg, less than about 185 mg, less than about 180 mg, less than about 175 mg, less than about 170 mg, less than about 165 mg, less than about 160 mg, less than about 150 mg, less than about 145 mg, less than about 140 mg, less than about 135 mg, less than about 130 mg, less than about 125 mg, less than about 120 mg, less than about 115 mg, less than about 110 mg, less than about 105 mg, less than about 100 mg, less than about 95 mg, less than about 90 mg, less than about 85 mg, less than about 80 mg, less than about 75 mg, less than about 70 mg, less than about 65 mg, less than about 60 mg, less than about 55 mg, less than about 50 mg, less than about 45 mg, less than about 40 mg, less than about 35 mg, and less than about 30 mg.
Embodiment 24. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of at least at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 mg, at least 60 mg, at least 65 mg, at least 70 mg, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 mg, at least 95 mg, at least about 100 mg, at least 105 mg, at least 115 mg, at least 120 mg, at least
125 mg, at least 130 mg, at least 135 mg, at least 140 mg, at least 145 mg, at least 150 mg, at least 155 mg, at least 160 mg, at least 165 mg, at least 170 mg, at least 175 mg, at least 180 mg, at least 185, at least 190 mg, at least 195 mg, and at least 200 mg.
Embodiment 25. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is any of at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, at least about 70 mg, at least about 75 mg, at least about 80 mg, at least about 85 mg, at least about 90 mg, at least about 95 mg, at least about 100 mg, at least about 105 mg, at least about 115 mg, at least about 120 mg, at least about 125 mg, at least about 130 mg, at least about 135 mg, at least about 140 mg, at least about 145 mg, or at least about 150 mg, at least about 155 mg, at least about 160 mg, at least about 165 mg, at least about 170 mg, at least about 175 mg, at least about 180 mg, at least about 185, at least about 190 mg, at least about 195 mg, and at least about 200 mg.
Embodiment 26. The method of any one of embodiments 1-8, wherein the therapeutically effective amount is about 80 mg to about 120 mg.
Embodiment 27. The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every week.
Embodiment 28. The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 2 weeks.
Embodiment 29. The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 3 weeks.
Embodiment 30. The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every 4 weeks.
Embodiment 31. The method of any one of embodiments 1-26, comprising administering the oligomeric compound twice every week.
Embodiment 32. The method of any one of embodiments 1-26, comprising administering the oligomeric compound three times every week.
Embodiment 33. The method of any one of embodiments 1-26, comprising administering the oligomeric compound four times every week.
Embodiment 34. The method of any one of embodiments 1-26, comprising administering the oligomeric compound five times every week.
Embodiment 35. The method of any one of embodiments 1-26, comprising administering the oligomeric compound six times every week.
Embodiment 36. The method of any one of embodiments 1-26, comprising administering the oligomeric compound once every day, once every other day, once every 3 days, once every 4 days, once every 5 days, or once every 6 days.
Embodiment 37. The method of any one of embodiments 1-36, wherein at least one symptom of hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis is reduced or improved.
Embodiment 38. The method of any one of embodiments 1-36, wherein the human subject has hypertension or resistant hypertension and at least one symptom of hypertension or resistant hypertension is reduced or improved.
Embodiment 39. The method of embodiment 38, wherein at least one symptom is headaches, nosebleeds, fatigue, confusion, vision problems, chest pain, difficulty breathing, irregular heartbeat, blood in urine, or a combination thereof.
Embodiment 40. The method of embodiment 38 or 39, wherein administering the oligomeric compound reduces blood pressure (BP), reduces systolic blood pressure (SBP), reduces diastolic blood pressure (DBP), achieves <140/90 mmHg BP, achieves <130/80 mmHg BP, improves quality of life as assessed by patient reported outcomes, or any combination thereof.
Embodiment 41. The method of any one of embodiments 1-36, wherein the human subject has heart failure and at least one symptom of heart failure is reduced or improved.
Embodiment 42. The method of embodiment 41, wherein at least one symptom is a symptom due to fluid volume excess including dyspnea, orthopnea, edema, hepatic congestion, and ascites; a symptom due to a diminished cardiac output including fatigue, dizziness, and weakness; or a combination thereof.
Embodiment 43. The method of embodiment 41 or 42, wherein administering the oligomeric compound reduces rates of cardiovascular (CV) mortality, reduces heart failure hospitalization and urgent visits, reduces N-terminal prohormone B-type natriuretic peptide (NT-proBNP) levels, reduces B-type natriuretic peptide (BNP) levels, reduces cardiac troponin T (cTnT) levels, reduces high-sensitive cardiac troponin T (hs-cTnT) levels, improves cardiac function, reduces cardiac dilation, reduces cardiac fibrosis, increases or improves LVEF (left ventricular ejection fraction), reduces or improves LVESV (left ventricular end systolic volume), reduces or improves LVEDV (left ventricular end diastolic volume), increases or improves left ventricle (LV) strain, improves 6 minute walk test, improves quality of life, or any combination thereof.
Embodiment 44. The method of any one of embodiments 1-36, wherein the human subject has Marfan syndrome and at least one symptom of Marfan syndrome is reduced or improved.
Embodiment 45. The method of embodiment 44, wherein administering the oligomeric compound reduces or improves aortic root dilation, mortality, aortic root surgery, or any combination thereof.
Embodiment 46. The method of any one of embodiments 1-45, wherein the oligomeric compound is administered by subcutaneous injection.
Embodiment 47. The method of embodiment 46, wherein the oligomeric compound is administered by a syringe.
I. AGT
In certain embodiments, described herein are methods of reducing AGT RNA and/or AGT protein in a cell or a biological fluid of a subject. AGT RNA is encoded by the human angiotensinogen (AGT) gene. AGT protein is the protein expression product of AGT RNA. A representative nucleobase sequence for a human AGT gene is the complement of the nucleotides of GenBank Accession No. NT_167186.1 truncated from nucleotides 24354000 to 24370100 (designated herein as SEQ ID NO: 1). A representative nucleobase sequence for a human AGT RNA is GENBANK Accession No. NM_000029.3 (designated herein as SEQ ID NO: 2).
II. ISIS 757456
In certain embodiments, a method comprises administering oligomeric compound, ISIS 757456, to a subject in need thereof.
In certain embodiments, ISIS 757456 is represented by the following chemical notation (5’ to 3’): GalNAc3-7a-0 mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
A = an adenine nucleobase, mC = a 5-methyl cytosine nucleobase,
G = a guanine nucleobase,
T = a thymine nucleobase, e = a 2’-MOE sugar moiety, d = a 2’- -D-deoxyribosyl sugar moiety, s = a phosphorothioate intemucleoside linkage, o’ = 5’-P(0H)(=0)-0-3’, and
Structure 1. ISIS 757456
In certain embodiments, ISIS 757456 is represented by the following chemical structure:
In certain embodiments, the sodium salt of ISIS 757456 is represented by the following chemical 5 structure:
(SEQ ID NO: 3).
III. Certain Pharmaceutical Compositions
In certain embodiments, described herein are methods of administering to a subject a pharmaceutical composition comprising the oligomeric compound ISIS 757456. In certain embodiments, the pharmaceutical composition comprises a pharmaceutically acceptable diluent or carrier. In certain embodiments, the pharmaceutical composition comprises or consists essentially of a sterile saline solution and the oligomeric compound ISIS 757456. In certain embodiments, the sterile saline is pharmaceutical grade saline. In certain embodiments, the pharmaceutical composition comprises or consists essentially of sterile water and the oligomeric compound ISIS 757456. In certain embodiments, the sterile water is pharmaceutical grade water.
In certain embodiments, the pharmaceutical composition comprises or consists essentially of the oligomeric compound ISIS 757456 in 2mM phosphate buffered isotonic saline, pH 7.4.
In certain embodiments, pharmaceutical compositions comprising the oligomeric compound ISIS 757456 encompass any pharmaceutically acceptable salt of the oligomeric compound ISIS 757456, esters of the oligomeric compound ISIS 757456, or salts of such esters. In certain embodiments, pharmaceutical compositions comprising the oligomeric compound ISIS 757456 are capable of providing (directly or indirectly) the biologically active metabolite or residue thereof upon administration to a human subject. Accordingly, for example, the disclosure is also drawn to pharmaceutically acceptable salts of the oligomeric compound ISIS 757456, prodrugs of the oligomeric compound ISIS 757456, pharmaceutically acceptable salts of such prodrugs, and other bioequivalents. Suitable pharmaceutically acceptable salts include, but are not limited to, sodium and potassium salts.
Under certain conditions, the oligomeric compound ISIS 757456 acts as an acid. Although ISIS 757456 may be drawn or described in protonated (free acid) form, or ionized and in association with a cation (salt) form, aqueous solutions of ISIS 757456 exist in equilibrium among such forms. For example, a phosphate linkage of ISIS 757456 in aqueous solution exists in equilibrium among free acid, anion, and salt forms. Unless otherwise indicated, the term, “ISIS 757456,” is intended to include all such forms. Moreover, ISIS 757456 has several such linkages, each of which is in equilibrium. Thus, ISIS 757456 exists in solution in an ensemble of forms at multiple positions all at equilibrium. The term “ISIS 757456” is intended to include all such forms. Drawn structures necessarily depict a single form. Nevertheless, unless otherwise indicated, such drawings are likewise intended to include corresponding forms. Herein, a structure depicting the free acid of ISIS 757456 followed by the term “or a salt thereof’ expressly includes all such forms that may be fully or partially protonated/de-protonated/in association with a cation. In certain instances, one or more specific cation is identified.
In certain embodiments, ISIS 757456 is in aqueous solution with sodium. In certain embodiments, ISIS 757456 is in aqueous solution with potassium. In certain embodiments, ISIS 757456 is in PBS. In certain embodiments, ISIS 757456 is in water. In certain such embodiments, the pH of the solution is adjusted with NaOH and/or HC1 to achieve a desired pH.
Herein, certain specific doses are described. For clarity, a dose of ISIS 757456 in milligrams indicates the mass of the free acid form of ISIS 757456. As described above, in aqueous solution, the free acid is in equilibrium with anionic and salt forms. However, for the purpose of calculating dose, it is assumed that ISIS 757456 exists as a solvent-free, sodium-acetate free, anhydrous, free acid. For example, where ISIS 757456 is in solution comprising sodium (e.g., saline), ISIS 757456 may be partially or fully de-protonated and in association with Na+ ions. However, the mass of the protons is nevertheless counted toward the weight of the dose, and the mass of the Na+ ions are not counted toward the weight of the dose. Thus, for example, a dose of 80 mg of ISIS 757456 equals the number of fully protonated molecules that weighs 80 mg. This would be equivalent to 83.8 mg of solvent-free, sodium-acetate free, anhydrous sodiated ISIS 757456.
In certain embodiments, ISIS 757456 is administered by subcutaneous injection. In certain embodiments, ISIS 757456 is administered by a syringe.
Certain Dosage Amounts
In certain embodiments, a method comprises administering to a subject a therapeutically effective amount of the oligomeric compound ISIS 757456. In certain embodiments, the therapeutically effective amount is 80 mg or about 80 mg. In certain embodiments, the therapeutically effective amount is 120 mg or about 120 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is 75 mg to 85 mg. In certain embodiments, the therapeutically effective amount is 110 mg to 130 mg or 115 mg to 125 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 37.5 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg, 175 mg, 180 mg, 185 mg, 190 mg, 195 mg, and 200 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 37.5 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 105 mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg, about 135 mg, about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about 165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about 195 mg, and about 200 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of 25 mg to 200 mg, 25 mg to 80 mg, 40 mg to 80 mg, 40 mg to 120 mg, or 80 mg to 120 mg. In certain embodiments, the therapeutically effective amount is any of about 40 mg to about 80 mg, about 40 mg to about 120 mg, or about 80 mg to about 120 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of 40 mg to 200 mg, 40 mg to 190 mg, 40 mg to 180 mg, 40 mg to 170 mg, from 40 mg to 160 mg, 40 mg to 150 mg, 40 mg to 140 mg, 40 mg to 120 mg, 40 mg to 110 mg, 40 mg to 100 mg, 40 mg to 80 mg, 40 mg to 70 mg, 40 mg to 60 mg, 40 mg to 50 mg, 50 mg to 200 mg, 50 mg to 190 mg, 50 mg to 180 mg, 50 mg to 170 mg, 50 mg to 160 mg, 50 mg to 150 mg, 50 mg to 140 mg, 50 mg to 120 mg, 50 mg to 110 mg, 50 mg to 100 mg, 50 mg to 80 mg, 50 mg to 70 mg, 50 mg to 60 mg, 60 mg to 200 mg, 60 mg to 190 mg, 60 mg to 180 mg, 60 mg to 170 mg, 60 mg to 160 mg, 60 mg to 150 mg, 60 mg to 140 mg, 60 mg to 120 mg, 60 mg to 110 mg, 60 mg to 100 mg, 60 mg to 80 mg, 60 mg to 70 mg, 70 mg to 200 mg, 70 mg to 190 mg, 70 mg to 180 mg, 70 mg to 170 mg, 70 mg to 160 mg, 70 mg to 150 mg, 70 mg to 140 mg, 70 mg to 120 mg, 70 mg to 110 mg, 70 mg to 100 mg, 70 mg to 80
mg, 80 mg to 200 mg, 80 mg to 190 mg, 80 mg to 180 mg, 80 mg to 170 mg, 80 mg to 160 mg, 80 mg to 150 mg, 80 mg to 140 mg, 80 mg to 120 mg, 80 mg to 110 mg, 80 mg to 100 mg, 80 mg to 90 mg, 90 mg to 200 mg, 90 mg to 190 mg, 90 mg to 180 mg, 90 mg to 170 mg, 90 mg to 160 mg, 90 mg to 150 mg, 90 mg to 140 mg, 90 mg to 120 mg, 90 mg to 110 mg, 90 mg to 100 mg, 100 mg to 200 mg, 100 mg to 190 mg, 100 mg to
180 mg, 100 mg to 170 mg, 100 mg to 160 mg, 100 mg to 150 mg, 100 mg to 140 mg, 100 mg to 120 mg, 100 mg to 110 mg, 110 mg to 200 mg, 110 mg to 190 mg, 110 mg to 180 mg, 110 mg to 170 mg, 110 mg to 160 mg, 110 mg to 150 mg, 110 mg to 140 mg, 110 mg to 130 mg, 110 mg to 120 mg, 120 mg to 200 mg, 120 mg to 190 mg, 120 mg to 180 mg, 120 mg to 170 mg, 120 mg to 160 mg, 120 mg to 150 mg, 120 mg to 140 mg, 120 mg to 130 mg, 130 mg to 200 mg, 130 mg to 190 mg, 130 mg to 180 mg, 130 mg to 170 mg, 130 mg to 160 mg, 130 mg to 150 mg, 130 mg to 140 mg, 140 mg to 200 mg, 140 mg to 190 mg, 140 mg to 180 mg, 140 mg to 170 mg, 140 mg to 160 mg, 140 mg to 150 mg, 150 mg to 200 mg, 150 mg to 190 mg, 150 mg to 180 mg, 150 mg to 170 mg, 150 mg to 160 mg, 160 mg to 200 mg, 160 mg to 190 mg, 160 mg to 180 mg, 160 mg to 170 mg, 180 mg to 200 mg, 180 mg to 190 mg, 190 mg to 200 mg, 105 mg to 135 mg, 105 mg to 130 mg, 105 mg to 125 mg 105 mg to 120 mg, 110 mg to 135 mg, 110 mg to 130 mg, 110 mg to 125 mg, 110 mg to 120 mg, 115 mg to 135 mg, 115 mg to 130 mg, 115 mg to 125 mg, 115 mg to 120 mg, 115 mg to 125 mg, 115 mg to 120 mg, 120 mg to 135 mg, 120 mg to 125 mg, 125 mg to 140 mg, 125 mg to 130 mg, 130 mg to 135 mg, or 135 mg to 140 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than 170 mg, less than 165 mg, less than 160 mg, less than 150 mg, less than 145 mg, less than 140 mg, less than 135 mg, less than 130 mg, less than 125 mg, less than 120 mg, less than 115 mg, less than 110 mg, less than 105 mg, less than 100 mg, less than 95 mg, less than 90 mg, less than 85 mg, less than 80 mg, less than 75 mg, less than 70 mg, less than 65 mg, less than 60 mg, less than 55 mg, less than 50 mg, less than 45 mg, less than 40 mg, less than 35 mg and less than 30 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of less than about 200 mg, less than about 195 mg, less than about 190 mg, less than about 185 mg, less than about 180 mg, less than about 175 mg, less than about 170 mg, less than about 165 mg, less than about 160 mg, less than about 150 mg, less than about 145 mg, less than about 140 mg, less than about 135 mg, less than about 130 mg, less than about 125 mg, less than about 120 mg, less than about 115 mg, less than about 110 mg, less than about 105 mg, less than about 100 mg, less than about 95 mg, less than about 90 mg, less than about 85 mg, less than about 80 mg, less than about 75 mg, less than about 70 mg, less than about 65 mg, less than about 60 mg, less than about 55 mg, less than about 50 mg, less than about 45 mg, less than about 40 mg, less than about 35 mg, and less than about 30 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 mg, at least 60 mg, at least 65 mg, at least 70 mg, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 mg, at least 95 mg, at least 100 mg, at least 105 mg, at least 115 mg, at least 120 mg, at least 125 mg, at least 130 mg, at least 135 mg, at least 140 mg, at least 145 mg, at least 150 mg, at least 155 mg, at least 160 mg, at least 165 mg, at least 170 mg, at least 175 mg, at least 180 mg, at least 185, at least 190 mg, at least 195 mg, and at least 200 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments, the therapeutically effective amount is any of at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, at least about 70 mg, at least about 75 mg, at least about 80 mg, at least about 85 mg, at least about 90 mg, at least about 95 mg, at least about 100 mg, at least about 105 mg, at least about 115 mg, at least about 120 mg, at least about 125 mg, at least about 130 mg, at least about 135 mg, at least about 140 mg, at least about 145 mg, or at least about 150 mg, at least about 155 mg, at least about 160 mg, at least about 165 mg, at least about 170 mg, at least about 175 mg, at least about 180 mg, at least about 185, at least about 190 mg, at least about 195 mg, and at least about 200 mg. In certain embodiments, the therapeutically effective amount is administered once every week.
In certain embodiments a method comprises administering to a subject about 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method comprises administering to a subject about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method comprises administering to a subject 80 mg of the oligomeric compound ISIS 757456 administered subcutaneously once every week. In certain embodiments a method comprises administering to a subject about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method comprises administering to a subject 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
IV. Certain Dosing Regimens
In certain embodiments, described herein are methods of administering to a subject a therapeutically effective amount of the oligomeric compound ISIS 757456 one or more times. In certain embodiments, methods comprise administering the therapeutically effective amount at least 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 times. In certain embodiments, methods comprise administering the therapeutically effective amount once every week. In certain embodiments, methods comprise administering the therapeutically effective amount once every other week.
In certain embodiments, methods comprise administering the therapeutically effective amount once
every week, once every 2 weeks, once every 3 weeks, once every 4 weeks, once every 5 weeks, once every 6 weeks, once every 7 weeks, or once every 8 weeks.
In certain embodiments, methods comprise administering the therapeutically effective amount once every week, twice every week, three times every week, four times every week, five times every week, or six times every week.
In certain embodiments, methods comprise administering the therapeutically effective amount once every day, once every 2 days, once every 3 days, once every 4 days, once every 5 days, or once every 6 days.
In certain embodiments, methods comprise administering the therapeutically effective amount for at least about 1 month, at least about 2 months, at least about 3 months, at least about 4 months, at least about 5 months, at least about 6 months, at least about 7 months, at least about 8 months, at least about 9 months, at least about 10 months, at least about 11 months, or at least about 12 months.
In certain embodiments, methods comprise administering the therapeutically effective amount for as long as the subject needs treatment for hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
Loading and Maintenance Doses
In certain embodiment, the therapeutically effective amount is administered as a loading dose and/or a maintenance dose. In certain embodiments, methods comprise administering a loading dose or doses and subsequently administering a maintenance dose or doses. In certain embodiments, methods comprise administering a loading dose once about every 4 weeks, and subsequently administering a maintenance dose once about every 8 weeks. In certain embodiments, methods comprise administering a loading dose once about every 4 weeks, and subsequently administering a maintenance dose once about every 16 weeks.
In certain embodiments, methods comprise administering at least 2 loading doses, at least 3 loading doses, at least 4 loading doses, at least 5 loading doses, or at least 6 loading doses. In certain embodiments, methods comprise administering 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 loading doses. In certain embodiments, methods comprise administering a loading dose or doses about every 1 week, about every 2 weeks, about every 3 weeks, about every 4 weeks, about every 5 weeks, about every 6 weeks, about every 7 weeks, about every 8 weeks, about every 9 weeks, about every 10 weeks, about every 11 weeks, or about every 12 weeks. In certain embodiments, methods comprise administering an initial loading dose and administering a second loading dose about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, or about 12 weeks after
administering the initial loading dose.
In certain embodiments, methods comprise administering at least 2 maintenance doses, at least 3 maintenance doses, at least 4 maintenance doses, at least 5 maintenance doses, or at least 6 maintenance doses. In certain embodiments, methods comprise administering 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 maintenance doses. In some instances, methods comprise administering a maintenance dose or doses about every 4 weeks, about every 5 weeks, about every 6 weeks, about every 7 weeks, about every 8 weeks, about every 9 weeks, about every 10 weeks, about every 11 weeks, about every 12 weeks, about every 13 weeks, about every 14 weeks, about every 15 weeks, about every 16 weeks, about every 17 weeks, about every 18 weeks, about every 19 weeks, or about every 20 weeks. In certain embodiments, methods comprise administering a first maintenance dose and administering a second maintenance dose about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 17 weeks, about 18 weeks, about 19 weeks, or about 20 weeks after administering the first maintenance dose.
In certain embodiments, methods comprise administering a first maintenance dose or doses about 1 week, about 2 weeks, about 3 weeks, about 4 weeks, about 5 weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 13 weeks, about 14 weeks, about 15 weeks, about 16 weeks, about 17 weeks, about 18 weeks, about 19 weeks, or about 20 weeks after administering the last loading dose.
In certain embodiments, methods comprise administering a loading dose of about 5 mg to about 120 mg of ISIS 757456 in the same week as administering a maintenance dose of about 5 mg to about 120 mg of ISIS 757456. In certain embodiments, the loading dose is about 5, 10, 20, 40, 60, 80 or 120 mg of ISIS 757456. In certain embodiments, the maintenance dose is about 5, 10, 20, 40, 60, 80 or 120 mg of ISIS 757456. In certain embodiments, the loading dose is about 80 mg of ISIS 757456. In certain embodiments, the maintenance dose is about 80 mg of ISIS 757456. In certain embodiments, the loading dose is about 120 mg of ISIS 757456. In certain embodiments, the maintenance dose is about 120 mg of ISIS 757456. In certain embodiments, the loading dose is administered for 1 week and the maintenance dose is administered for as long as the human subject needs treatment.
Dose Titration
Certain embodiments are drawn to administering a first dose of ISIS 757456 for a first period of time followed by a second dose of ISIS 757456 for a second period of time, wherein the second dose is smaller than the first dose. In certain embodiments, the first period of time and the second period of time do not overlap.
In certain embodiments, a method comprises administering to a subject about 120 mg of ISIS 757456 for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 for a second period of time. In certain embodiments, a
method comprises administering to a subject about 120 mg of ISIS 757456 once every week for a first period of time followed by administering to the subject about mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 once every week for a second period of time. In certain embodiments, the first period of time and the second period of time do not overlap.
In certain embodiments, a method comprises administering to a subject about 80 mg of ISIS 757456 for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, or about 60 mg of ISIS 757456 for a second period of time. In certain embodiments, a method comprises administering to a subject about 80 mg of ISIS 757456 once every week for a first period of time followed by administering to the subject about 5 mg, about 10 mg, about 20 mg, about 40 mg, about 60 mg, or about 80 mg of ISIS 757456 once every week for a second period of time. In certain embodiments, the first period of time and the second period of time do not overlap.
V. Certain Combination Therapies
In certain embodiments, methods comprise co-administering ISIS 757456 with at least one other pharmaceutical agent. In certain embodiments, the at least one other pharmaceutical agent treats hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis, or a symptom thereof. In certain embodiments, ISIS 757456 is co-administered with the at least one other pharmaceutical agent to produce a combinational effect. In certain embodiments, ISIS 757456 is co-administered with the at least one other pharmaceutical agent to produce a synergistic effect.
In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are administered at the same time. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are administered at different times. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are prepared together in a single formulation. In certain embodiments, ISIS 757456 and the at least one other pharmaceutical agent are administered are prepared separately.
In certain embodiments, pharmaceutical agents that may be co-administered with ISIS 757456 include angiotensin-converting enzyme inhibitor (ACEi, e.g., lisinopril, ramipril, perindopril, enalapril, benazepril, quinapril, captopril, fosinopril, trandolapril, moexipril, enalaprilat), angiotensin II receptor blocker (ARB, e.g., losartan, olmesartan, valsartan, candesartan, irbesartan, telmisartan, azilsartan, eprosartan), beta blocker, calcium channel blocker, non-potassium sparing diuretic, alpha- 1 blocker, centrally acting sympatholytic agent, SGLT2 inhibitor (SGLT2i, e.g., empaglifozin, canaglifozin, dapaglifozin, ertuglifozin), or direct acting vasodilators (e.g. hydralazine), neprilysin inhibitor (e.g., sacubitril).
VI. Certain Indications
Any of the foregoing methods, doses, or dosing regimens can be used for treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis. In certain embodiments, methods, doses, or dosing regimens described herein can be used for treating RHTN in a subject who has failed to achieve a blood pressure <140/90 mmHg despite taking or having taken 3 or more medications for hypertension. In certain embodiments, methods, doses, or dosing regimens described herein can be used for treating RHTN in a subject who has failed to achieve a blood pressure <130/80 mmHg despite taking or having taken 3 or more medications for hypertension. In certain embodiments, the 3 or more medications for hypertension belong to one or more of the following classes of medications: a diuretic, a long -acting calcium channel blocker, a beta blocker, or a renin-angiotensin pathway blocker such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. In certain embodiments, administering ISIS 757456 in any of the foregoing doses or dosing regimens can improve or reduce at least one symptom in a subject have any of the aforementioned diseases.
VII. Efficacy
Assessing Efficacy of ISIS 757456
In certain embodiments, methods described herein are sufficiently effective to treat hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject.
In certain embodiments, methods described herein improves symptoms of a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling including, e.g., Marfan Syndrome, Loeys-Dietz Syndrome, Hereditary Hemorrhagic Telangiectasia, and Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis.
In certain embodiments, a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension, thereby treating hypertension or resistant hypertension in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension reduces blood pressure (BP), reduces systolic blood pressure (SBP), reduces diastolic blood pressure (DBP), achieves <140/90 mmHg BP, achieves <130/80 mmHg BP, improves quality of life as assessed
by patient reported outcomes, or any combination thereof, in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having hypertension or resistant hypertension reduces or improves headaches, nosebleeds, fatigue, confusion, vision problems, chest pain, difficulty breathing, irregular heartbeat, blood in urine, or any combination thereof, in the subject.
In certain embodiments, a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 once a week to a subject having hypertension or resistant hypertension, thereby treating hypertension or resistant hypertension in the subject. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having hypertension or resistant hypertension 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
In certain embodiments, a method of treating hypertension or resistant hypertension comprises administering a therapeutically effective amount of ISIS 757456 to a subject having uncontrolled hypertension on two or three or more antihypertensive medications, thereby treating hypertension or resistant hypertension in the subject. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 120
mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating hypertension or resistant hypertension comprises administering to a subject having uncontrolled hypertension on two or three antihypertensive medications 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
In certain embodiments, a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure, thereby treating heart failure in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure reduces rates of cardiovascular (CV) mortality, reduces heart failure hospitalization and urgent heart failure visits, reduces N-terminal prohormone B-type natriuretic peptide (NT-proBNP) levels, reduces B- type natriuretic peptide (BNP) levels, reduces cardiac troponin T (cTnT) levels, reduces high-sensitive cardiac troponin T (hs-cTnT) levels, improves cardiac function, reduces cardiac dilation, reduces cardiac fibrosis, increases or improves LVEF (left ventricular ejection fraction), reduces or improves LVESV (left ventricular end systolic volume), reduces or improves LVEDV (left ventricular end diastolic volume), increases or improves left ventricle (LV) strain, improves 6 minute walk test, improves quality of life, or any combination thereof, in the subject.
In certain embodiments, a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 once a week to a subject having heart failure, thereby treating heart failure in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having heart failure reduces or improves symptoms due to fluid volume excess including dyspnea, orthopnea, edema, hepatic congestion, and ascites; reduces or improves symptoms due to a diminished cardiac output including fatigue, dizziness, and weakness; or any combination thereof, in the subject. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of
treating heart failure comprises administering to a subject having heart failure about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having heart failure 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
In certain embodiments, a method of treating heart failure comprises administering a therapeutically effective amount of ISIS 757456 to a subject having chronic heart failure with reduced ejection fraction, thereby treating chronic heart failure in the subject. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 80 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 120 mg of the oligomeric compound ISIS 757456 once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction 80 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction about 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week. In certain embodiments a method of treating heart failure comprises administering to a subject having chronic heart failure with reduced ejection fraction failure 120 mg of the oligomeric compound ISIS 757456 subcutaneously once every week.
In certain embodiments, a method of treating Marfan syndrome comprises administering a therapeutically effective amount of ISIS 757456 to a subject having Marfan syndrome, thereby treating Marfan syndrome in the subject. In certain embodiments, administering a therapeutically effective amount of ISIS 757456 to a subject having Marfan syndrome reduces or improves aortic root dilation, mortality, aortic root surgery, or any combination thereof, in the subject.
EXAMPLES
The following examples illustrate certain embodiments of the present disclosure and are not limiting.
Moreover, where specific embodiments are provided, the inventors have contemplated generic application of those specific embodiments.
Example 1: Phase 1 Human Clinical Trial with ISIS 757456 in Healthy Volunteers
The phase 1 trial in healthy volunteers was a randomized, double-blind, placebo-controlled study assessing safety and tolerability of ISIS 757456 in 5 single-ascending dose cohorts (5-80 mg) and 2 multiple- ascending dose cohorts dosed weekly for 6-weeks (40 to 80 mg in an 8:2 randomization ratio). Subjects with a screening plasma AGT level < 20 pg/mL were not eligible for the multiple-dose cohorts. The primary objective was to assess safety and tolerability. Plasma AGT levels over time were also measured.
In the phase 1 Healthy Volunteer Study, single ascending doses (29 subjects treated with ISIS 757456 and 12 with placebo) and multiple ascending doses (16 subjects treated with ISIS 757456 and 4 with placebo), were well tolerated with no drug related SAEs, no clinically meaningful changes in laboratory assessments, no hypotensive events, acute renal changes or hyperkalemia. Six weeks of treatment resulted in significant and dose-dependent reductions in AGT (46%, p=0.004 and 60%, p=0.006 mean reductions in the 40 mg and 80 mg groups respectively). Plasma AGT levels returned to baseline levels 10 weeks after the last dose. There was no significant effect on blood pressure, Ang II, brain natriuretic peptide, atrial natriuretic peptide or renin mass and activity.
ISIS 757456 results in significant AGT reductions with favorable safety and tolerability profile. As monotherapy, proof of principle was demonstrated as evidenced by reduction in systolic blood pressure (SBP) thereby providing support to proceed to larger phase 2 trials in patients with resistant hypertension and heart failure.
Example 2: Phase 2 Human Clinical Trial with ISIS 757456 Monotherapy in Patients with Hypertension
The phase 2 monotherapy study was a randomized, double-blind, placebo-controlled trial evaluating ISIS 757456. Patients aged 18-72 years, inclusive, with controlled hypertension on 2 antihypertensive medications , one of which was an ACEi or an ARB and the other was either a beta blocker, calcium channel blocker, or diuretic, were enrolled. Patients with a screening plasma AGT level <20 pg/mL or with K+ >4.85 and UPCR >=0.3 mg/mg were excluded. All anti-hypertensive medications were stopped for 14 days (Wash-Out). Patients who met the inclusion criteria of a systolic blood pressure (SBP) >140 - <165 mmHg after Wash-Out were randomized 2: 1 to 80 mg ISIS 757456 or placebo. Patients were also stratified by screening plasma AGT level (<30 pg/mL vs. >30 pg/mL). All patients received weekly, in clinic, subcutaneous injections for 6 weeks with a loading dose administered on Day 3, then followed for 12 weeks in the post treatment period. The primary efficacy endpoint was the comparison of percent change in plasma AGT from baseline to study week 7 (Day 43) between 80 mg ISIS 757456 and placebo. Exploratory endpoints included post-baseline changes in SBP, diastolic blood pressure (DBP), percentage of patients reaching the goals of in-clinic SBP < 140 mmHg, DBP < 90 mmHg, and both over time. Blood pressure was
measured by study personnel at every in-clinic visit in a quiet room after 5 minutes of resting in a chair with feet on the floor. Three consecutive blood pressure measurements were averaged to obtain an average blood pressure.
At screening the mean (SD) AGT levels were 27.4 (13.1) pg/mL in the placebo group and 23.5 (3.7) pg/ml in the ISIS 757456 group (p=0.80). All patients had confirmed controlled SBP/DBP on HTN therapy at Screening. The AGT levels tended to decline following Wash-Out of anti-hypertensive medications. An expected, a rise in blood pressure occurred after stopping the anti-hypertensive medications for 14 days, reaching mean SBP 149 mmHg and 146 mmHg and DBP 88 and 86 mmHg for the placebo and ISIS 757456 groups, respectively. Renal function and potassium levels were normal at baseline.
ISIS 757456 was well tolerated with no hypotensive events, hyperkalemia or renal abnormalities (Supplemental Table 3). After 6 weeks of dosing at Day 43, a significant mean absolute reduction in AGT levels was noted in the ISIS 757456 group compared to placebo (-11.2 pg/mL versus 2.0, p<0.001). Similarly, the mean percent reduction in AGT levels was significantly lower with ISIS 757456 compared to placebo (- 54% versus 12.6%, p<0.001). In the ISIS 757456 group, 6 treated patients had AGT levels below the lower level of detection of the assay and were assigned values of 4.7 pg/ml. The reductions were sustained and demonstrated reversibility in the post treatment period.
There was a numerically larger reduction in in SBP (-8 mmHg) or DBP (- 1 mmHg) observed with ISIS 757456 compared to placebo but these did not reach statistical significance. A higher percentage of subjects treated with ISIS 757456 compared to placebo achieved >5, >10 and >15 mmHg reductions in SBP and DBP. Similarly, a higher percentage of patients treated with ISIS 757456 compared to placebo reached SBP <140 mmHg or DBP <90 mmHg. There were no significant changes in ANGII, aldosterone, or renin mass or activity.
ISIS 757456 results in significant AGT reductions with favorable trends in SBP and DBP reduction in subjects with uncontrolled HTN with concomitant RAAS blockade. No on-target effects were observed. A dose-ranging, Phase 2B study is underway in subjects with resistant HTN on 3 medications, as well a Phase 2 study in patients with heart failure with reduced ejection fraction.
Example 3: Phase 2 Human Clinical Trial with ISIS 757456 in Patients with Uncontrolled Hypertension on Two or Three Antihypertensive Medications
A phase 2 randomized, double-blind, placebo-controlled add-on trial evaluating ISIS 757456 was conducted. Patients aged 18-75 years, inclusive, on a stable regimen of 2 to 3 antihypertensive medications, including an ACEi or ARB and 1 or 2 additional antihypertensives in the beta blocker, calcium channel blocker, or non-potassium sparing diuretic classes were eligible. The inclusion criteria also required that patients have an average SBP within >140 and <170 mmHg and DBP >80 mmHg at screening and pre-dose Day 1. Subjects with a screening AGT level <20 pg/mL or with K+ >4.9 and UPCR >=0.3 mg/mg were excluded. Patients were stratified by screening ACEi or ARB dose then randomized 2: 1 to 80 mg ISIS 757456 or placebo, respectively.
All patients received weekly in-clinic subcutaneous injections for 8 weeks with a loading dose administered on Day 3, then followed for 12 weeks in the post treatment period. The primary efficacy endpoint was the comparison of percent change from baseline to study week 9 (Day 57) in plasma AGT between 80 mg ISIS 757456 and placebo. Exploratory endpoints included post-baseline changes in SBP, DBP and percentage of patients reaching goals of SPB <140 mmHg, DBP <80 mmHg and both during the study. Blood pressure was measured as in the Example 2.
Baseline mean AGT levels were 25.5 and 25.2 pg/mL in the placebo and ISIS 757456 groups, respectively. Approximately two thirds of patients were taking 2 anti-hypertensive medications and one third 3 antihypertensive medications. Baseline mean SBP was 152 mmHg and DBP 87 in the placebo group and SBP 154 and DBP 89 mmHg in the ISIS 757456 group.
ISIS 757456 was well tolerated with no serious adverse events, hypotensive events or renal abnormalities. After 8 weeks of dosing at Day 57, a significant absolute reduction in mean AGT levels was noted in the ISIS 757456 group compared to placebo (-17.0 versus -1.1 pg/mL pO.OOl). Similarly, the mean percent reduction in AGT levels was significantly lower in ISIS 757456 compared to placebo (67% versus 3.4%, p<0.001). In the ISIS 757456 group, 2 treated patients had AGT levels below the lower level of detection and were assigned values of 4.7 pg/ml.
There was a numerically larger reduction in SBP (-12 mmHg) and DBP (-6 mmHg) observed with ISIS 757456 compared to placebo but these did not reach statistical significance. A higher percentage of subjects treated with ISIS 757456 compared to placebo achieved >5, >10 and >15 mmHg reductions in SBP and DBP. Similarly, a higher percentage of patients treated with ISIS 757456 compared to placebo reached SBP <140 mmHg or DBP <90 mmHg. There was no significant difference based on whether patients were on 2 or 3 anti hypertensive medications. There were no significant changes in ANGII, aldosterone or renin mass or activity.
Example 4: Phase 2 Human Clinical Trial to Assess the Safety, Tolerability and Efficacy of ISIS 757456 Administered to Hypertensive Patients with Uncontrolled Blood Pressure
A Phase 2 study to evaluate the effect of ISIS 757456 compared to placebo on seated automated office systolic blood pressure (SBP) from Baseline in uncontrolled hypertensive patients on 3 or more antihypertensive medications in the following categories is conducted: angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB), beta blocker, calcium channel blocker, non-potassium sparing diuretic, alpha- 1 blocker, centrally acting sympatholytic agent, or direct acting vasodilators (e.g. hydralazine). 80 mg or 120 mg of ISIS 757456 is administered to the patients once every week. The following are evaluated:
The effect of ISIS 757456 on plasma angiotensinogen (AGT) at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications.
The effect of ISIS 757456 on 24-hour ambulatory blood pressure at Study Day 85 in uncontrolled hypertensive patients on > 3 antihypertensive medications.
The effect of ISIS 757456 on seated automated office SBP at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications. The effect of ISIS 757456 on seated automated office diastolic blood pressure (DBP) at each scheduled visit in uncontrolled hypertensive patients on > 3 antihypertensive medications
Example 5: Phase 2 Human Clinical Trial Assessing the Safety, Tolerability and Efficacy of ISIS 757456 in Patients with Chronic Heart Failure with Reduced Ejection Fraction
The effect of ISIS 757456, weekly subcutaneous (SC) injection on plasma angiotensinogen (AGT) concentration from Baseline to Study Day 85 (W eek 13) in patients with chronic heart failure (HF) with reduced ejection fraction (HFrEF) is evaluated. 40 mg or 80 mg or 120 mg of ISIS 757456 is administered to the patients once weekly. The following are evaluated:
The effect of ISIS 757456 weekly SC injection on plasma angiotensinogen (AGT) concentration at each scheduled visit in patients with chronic HFrEF. The effect of ISIS 757456 on N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) at each scheduled visit in patients with chronic HFrEF.
The effect of ISIS 757456 on high sensitivity cardiac troponin T (hs-cTnT) at each scheduled visit in patients with chronic HFrEF.
The effect of ISIS 757456 on functional capacity, patient-reported outcomes and disease scores related to the quality of life (QOL) at each scheduled visit in patients with chronic HFrEF.
The effect of ISIS 757456 on clinical outcomes in patients with HFrEF.
Claims
1. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
2. The method of claim 1, wherein the oligomeric compound is the sodium salt or the potassium salt.
3. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
4. A method of treating hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis in a human subject comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according to the following formula: GalNAc3- 7a-o’ mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
A = an adenine nucleobase, mC = a 5-methyl cytosine nucleobase,
G = a guanine nucleobase,
T = a thymine nucleobase, e = a 2’-MOE sugar moiety, d = a 2’^-D-deoxyribosyl sugar moiety, s = a phosphorothioate intemucleoside linkage, o’ = 5’-P(0H)(=0)-0-3’, and
5. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
(SEQ ID NO: 3), or a salt thereof.
6. The method of claim 5, wherein the oligomeric compound is the sodium salt or the potassium salt.
7. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease;
hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound according to the following chemical structure:
(SEQ ID NO: 3).
8. A method of reducing AGT RNA or protein in a human subject having hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction (HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis comprising administering to the human subject a therapeutically effective amount of an oligomeric compound comprising a modified oligonucleotide and a conjugate group according
to the following formula: GalNAc3-7a-0· mCes Aes mCes Aes Aes Ads mCds Ads Ads Gds mCds Tds Gds Gds Tds mCes Ges Ges Tes Te (SEQ ID NO: 3); wherein,
A = an adenine nucleobase, mC = a 5-methyl cytosine nucleobase,
G = a guanine nucleobase,
T = a thymine nucleobase, e = a 2’-MOE sugar moiety, d = a 2’- -D-deoxyribosyl sugar moiety, s = a phosphorothioate intemucleoside linkage, o’ = 5’-P(0H)(=0)-0-3’, and
9. The method of any one of claims 1-8, wherein the therapeutically effective amount is 25 mg.
10 The method of any one of claims 1-8, wherein the therapeutically effective amount is 40 mg.
11 The method of any one of claims 1-8, wherein the therapeutically effective amount is 60 mg.
12 The method of any one of claims 1-8, wherein the therapeutically effective amount is 80 mg.
13. The method of any one of claims 1-8, wherein the therapeutically effective amount is 120 mg.
14. The method of any one of claims 1-8, wherein the therapeutically effective amount is about 25 mg.
15. The method of any one of claims 1-8, wherein the therapeutically effective amount is about 40 mg.
16. The method of any one of claims 1-8, wherein the therapeutically effective amount is about 60 mg.
17. The method of any one of claims 1-8, wherein the therapeutically effective amount is about 80 mg.
18. The method of any one of claims 1-8, wherein the therapeutically effective amount is about
120 mg.
19. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of 25 mg, 30 mg, 35 mg, 40 mg, 45 mg, 50 mg, 55 mg, 60 mg, 65 mg, 70 mg, 75 mg, 80 mg, 85 mg, 90 mg, 95 mg, 100 mg, 105 mg, 110 mg, 115 mg, 120 mg, 125 mg, 130 mg, 135 mg, 140 mg, 145 mg, 150 mg, 155 mg, 160 mg, 165 mg, 170 mg, 175 mg, 180 mg, 185 mg, 190 mg, 195 mg, or 200 mg.
20. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg, about 105 mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg, about 135 mg, about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about 165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about 195 mg, or about 200 mg.
21. The method of any one of claims 1-8, wherein the therapeutically effective amount is within the range of any of 25 mg to 200 mg, 30 mg to 200 mg, 40 mg to 200 mg, 60 mg to 200 mg, 80 mg to 200 mg, 100 mg to 200 mg, 120 mg to 200 mg, 150 mg to 200 mg, 25 mg to 120 mg, 30 mg to 120 mg, 40 mg to 120 mg, 60 mg to 120 mg, 80 mg to 120 mg, 40 mg to 80 mg, or 60 mg to 80 mg.
22. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of less than 200 mg, less than 195 mg, less than 190 mg, less than 185 mg, less than 180 mg, less than 175 mg, less than 170 mg, less than 165 mg, less than 160 mg, less than 150 mg, less than 145 mg, less than 140 mg, less than 135 mg, less than 130 mg, less than 125 mg, less than 120 mg, less than 115 mg, less than 110 mg, less than 105 mg, less than 100 mg, less than 95 mg, less than 90 mg, less than 85 mg, less than 80 mg, less than 75 mg, less than 70 mg, less than 65 mg, less than 60 mg, less than 55 mg, less than 50 mg, less than 45 mg, less than 40 mg, less than 35 mg, or less than 30 mg.
23. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of less than about 200 mg, less than about 195 mg, less than about 190 mg, less than about 185 mg, less than about 180 mg, less than about 175 mg, less than about 170 mg, less than about 165 mg, less than about 160 mg, less than about 150 mg, less than about 145 mg, less than about 140 mg, less than about 135 mg, less than about 130 mg, less than about 125 mg, less than about 120 mg, less than about 115 mg, less than about 110 mg, less than about 105 mg, less than about 100 mg, less than about 95 mg, less than about 90 mg, less than about 85 mg, less than about 80 mg, less than about 75 mg, less than about 70 mg, less than about 65 mg, less than about 60 mg, less than about 55 mg, less than about 50 mg, less than about 45 mg, less than about 40 mg, less than about 35 mg, or less than about 30 mg.
24. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of at least 25 mg, at least 30 mg, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 mg, at least 60 mg, at least 65 mg, at least 70 mg, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 mg, at least 95 mg, at least about 100 mg, at least 105 mg, at least 115 mg, at least 120 mg, at least 125 mg, at least 130 mg, at least 135 mg, at least 140 mg, at least 145 mg, at least 150 mg, at least 155 mg, at least 160 mg, at least
165 mg, at least 170 mg, at least 175 mg, at least 180 mg, at least 185, at least 190 mg, at least 195 mg, and at least 200 mg.
25. The method of any one of claims 1-8, wherein the therapeutically effective amount is any of at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, at least about 70 mg, at least about 75 mg, at least about 80 mg, at least about 85 mg, at least about 90 mg, at least about 95 mg, at least about 100 mg, at least about 105 mg, at least about 115 mg, at least about 120 mg, at least about 125 mg, at least about 130 mg, at least about 135 mg, at least about 140 mg, at least about 145 mg, or at least about 150 mg, at least about 155 mg, at least about 160 mg, at least about 165 mg, at least about 170 mg, at least about 175 mg, at least about 180 mg, at least about 185, at least about 190 mg, at least about 195 mg, and at least about 200 mg.
26. The method of any one of claims 1-8, wherein the therapeutically effective amount is about 80 mg to about 120 mg.
27. The method of any one of claims 1-26, comprising administering the oligomeric compound once every week.
28. The method of any one of claims 1-26, comprising administering the oligomeric compound once every 2 weeks.
29. The method of any one of claims 1-26, comprising administering the oligomeric compound once every 3 weeks.
30. The method of any one of claims 1-26, comprising administering the oligomeric compound once every 4 weeks.
31. The method of any one of claims 1-26, comprising administering the oligomeric compound twice every week.
32. The method of any one of claims 1-26, comprising administering the oligomeric compound three times every week.
33. The method of any one of claims 1-26, comprising administering the oligomeric compound four times every week.
34. The method of any one of claims 1-26, comprising administering the oligomeric compound five times every week.
35. The method of any one of claims 1-26, comprising administering the oligomeric compound six times every week.
36. The method of any one of claims 1-26, comprising administering the oligomeric compound once every day, once every other day, once every 3 days, once every 4 days, once every 5 days, or once every 6 days.
37. The method of any one of claims 1-36, wherein at least one symptom of hypertension (HTN); resistant hypertension (RHTN); heart failure; chronic heart failure; heart failure with reduced ejection fraction
(HFrEF); heart failure with preserved ejection fraction (HFpEF); diabetic nephropathy (DN); aortopathies associated with pathogenic TGF-b signaling; Marfan Syndrome; Loeys-Dietz Syndrome; Hereditary Hemorrhagic Telangiectasia; Familial Thoracic Aortic Aneurysm and Dissection; chronic kidney disease; hepatic steatosis; or atherosclerosis is reduced or improved.
38. The method of any one of claims 1-36, wherein the human subject has hypertension or resistant hypertension and at least one symptom of hypertension or resistant hypertension is reduced or improved.
39. The method of claim 38, wherein at least one symptom is headaches, nosebleeds, fatigue, confusion, vision problems, chest pain, difficulty breathing, irregular heartbeat, blood in urine, or a combination thereof.
40. The method of claim 38 or 39, wherein administering the oligomeric compound reduces blood pressure (BP), reduces systolic blood pressure (SBP), reduces diastolic blood pressure (DBP), achieves <140/90 mmHg BP, achieves <130/80 mmHg BP, improves quality of life as assessed by patient reported outcomes, or any combination thereof.
41. The method of any one of claims 1-36, wherein the human subject has heart failure and at least one symptom of heart failure is reduced or improved.
42. The method of claim 41, wherein at least one symptom is a symptom due to fluid volume excess including dyspnea, orthopnea, edema, hepatic congestion, and ascites; a symptom due to a diminished cardiac output including fatigue, dizziness, and weakness; or a combination thereof.
43. The method of claim 41 or 42, wherein administering the oligomeric compound reduces rates of cardiovascular (CV) mortality, reduces heart failure hospitalization and urgent visits, reduces N-terminal prohormone B-type natriuretic peptide (NT-proBNP) levels, reduces B-type natriuretic peptide (BNP) levels, reduces cardiac troponin T (cTnT) levels, reduces high-sensitive cardiac troponin T (hs-cTnT) levels, improves cardiac function, reduces cardiac dilation, reduces cardiac fibrosis, increases or improves LVEF (left ventricular ejection fraction), reduces or improves LVESV (left ventricular end systolic volume), reduces or improves LVEDV (left ventricular end diastolic volume), increases or improves left ventricle (LV) strain, improves 6 minute walk test, improves quality of life, or any combination thereof.
44. The method of any one of claims 1-36, wherein the human subject has Marfan syndrome and at least one symptom of Marfan syndrome is reduced or improved.
45. The method of claim 44, wherein administering the oligomeric compound reduces or improves aortic root dilation, mortality, aortic root surgery, or any combination thereof.
46. The method of any one of claims 1-45, wherein the oligomeric compound is administered by subcutaneous injection.
47. The method of claim 46, wherein the oligomeric compound is administered by a syringe.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202163182518P | 2021-04-30 | 2021-04-30 | |
| US63/182,518 | 2021-04-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022232650A1 true WO2022232650A1 (en) | 2022-11-03 |
Family
ID=83847359
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2022/027138 WO2022232650A1 (en) | 2021-04-30 | 2022-04-29 | Methods for reducing agt expression |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2022232650A1 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014179629A2 (en) * | 2013-05-01 | 2014-11-06 | Isis Pharmaceuticals, Inc. | Compositions and methods |
| WO2017062816A2 (en) * | 2015-10-08 | 2017-04-13 | Ionis Pharmaceuiticals, Inc. | Compounds and methods for modulating angiotensinogen expression |
| US20180251764A1 (en) * | 2012-11-15 | 2018-09-06 | Roche Innovation Center Copenhagen A/S | Oligonucleotide Conjugates |
-
2022
- 2022-04-29 WO PCT/US2022/027138 patent/WO2022232650A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180251764A1 (en) * | 2012-11-15 | 2018-09-06 | Roche Innovation Center Copenhagen A/S | Oligonucleotide Conjugates |
| WO2014179629A2 (en) * | 2013-05-01 | 2014-11-06 | Isis Pharmaceuticals, Inc. | Compositions and methods |
| US20180273952A1 (en) * | 2013-05-01 | 2018-09-27 | Ionis Pharmaceuticals, Inc. | Compositions and methods for modulating apolipoprotein c-iii expression |
| WO2017062816A2 (en) * | 2015-10-08 | 2017-04-13 | Ionis Pharmaceuiticals, Inc. | Compounds and methods for modulating angiotensinogen expression |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Al Ghorani et al. | Arterial hypertension–Clinical trials update 2021 | |
| Kusumoto et al. | Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin II type 1 receptor blocker, azilsartan medoxomil, in rat and dog models | |
| Karlsson et al. | Beta1‐adrenergic receptor gene polymorphisms and response to beta1‐adrenergic receptor blockade in patients with essential hypertension | |
| Nussberger et al. | Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension | |
| AU731656B2 (en) | Method of treating heart failure | |
| Griffin et al. | Transthyretin cardiac amyloidosis: a treatable form of heart failure with a preserved ejection fraction | |
| Arruda-Junior et al. | Unraveling the interplay between dipeptidyl peptidase 4 and the renin-angiotensin system in heart failure | |
| US20070293518A1 (en) | Prolonged improvement of renal function comprising infrequent administration of an aa1ra | |
| Enzan et al. | The use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers is associated with the recovered ejection fraction in patients with dilated cardiomyopathy | |
| Zambelli et al. | Efficacy of aminaftone in a rat model of monocrotaline-induced pulmonary hypertension | |
| Borghi et al. | THE USE OF LERCANIDIPINE CAN IMPROVE THE INDIVIDUAL TOLERABILITY TO DIHYDROPIRIDINE CALCIUM BLOCKERS IN HYPERTENSIVE PATIENTS: P3. 18 | |
| Liang et al. | Serum uric acid level and left ventricular hypertrophy in elderly male patients with nonvalvular atrial fibrillation | |
| WO2022232650A1 (en) | Methods for reducing agt expression | |
| Feldman et al. | Low-dose oral enoximone enhances the ability to wean patients with ultra-advanced heart failure from intravenous inotropic support: results of the oral enoximone in intravenous inotrope-dependent subjects trial | |
| Cavallari et al. | Association of aldosterone concentration and mineralocorticoid receptor genotype with potassium response to spironolactone in patients with heart failure | |
| Buckley et al. | Recent advances in the treatment of chronic heart failure | |
| TW202103709A (en) | Methods for treating subjects with chronic kidney disease | |
| Lin et al. | The effect of angiotensin-converting enzyme gene polymorphisms on the clinical efficacy of perindopril prescribed for acute myocardial infarction in Chinese Han patients | |
| KR20220152569A (en) | Compositions and methods for treating and preventing prekallikrein-associated conditions | |
| RU2811365C2 (en) | Treatment of heart failure in humans | |
| Saito et al. | Cost-effectiveness analysis: controlled-release nifedipine and valsartan combination therapy in patients with essential hypertension: the adalat CR and valsartan cost-effectiveness combination (ADVANCE-Combi) study | |
| Haddad et al. | Usual ACE inhibitor therapy in CKD patients is associated with lower plasma aldosterone levels than usual angiotensin receptor blocker therapy | |
| JP2005531492A (en) | Method for reducing type II diabetes in high-risk patients | |
| Ogihara et al. | Clinical efficacy of a new angiotensin II type 1 receptor blocker, pratosartan, in hypertensive patients | |
| TW202413393A (en) | Methods of using neuregulin-4 compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22796896 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 22796896 Country of ref document: EP Kind code of ref document: A1 |