WO2022184604A1 - Liquid-crystal medium - Google Patents
Liquid-crystal medium Download PDFInfo
- Publication number
- WO2022184604A1 WO2022184604A1 PCT/EP2022/054906 EP2022054906W WO2022184604A1 WO 2022184604 A1 WO2022184604 A1 WO 2022184604A1 EP 2022054906 W EP2022054906 W EP 2022054906W WO 2022184604 A1 WO2022184604 A1 WO 2022184604A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- atoms
- compounds
- formula
- denotes
- alkyl
- Prior art date
Links
- 239000004973 liquid crystal related substance Substances 0.000 title abstract description 246
- 239000003381 stabilizer Substances 0.000 claims abstract description 19
- 238000000034 method Methods 0.000 claims abstract description 17
- 230000008569 process Effects 0.000 claims abstract description 7
- 150000001875 compounds Chemical class 0.000 claims description 226
- 125000004432 carbon atom Chemical group C* 0.000 claims description 106
- -1 alkenyl radical Chemical class 0.000 claims description 96
- 125000000217 alkyl group Chemical group 0.000 claims description 65
- 229910052731 fluorine Inorganic materials 0.000 claims description 47
- 229910052801 chlorine Inorganic materials 0.000 claims description 40
- 125000003342 alkenyl group Chemical group 0.000 claims description 37
- 125000003545 alkoxy group Chemical group 0.000 claims description 26
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 19
- 150000003254 radicals Chemical class 0.000 claims description 19
- 239000000758 substrate Substances 0.000 claims description 17
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 16
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 16
- 229910052739 hydrogen Inorganic materials 0.000 claims description 14
- 229910052736 halogen Inorganic materials 0.000 claims description 13
- 150000002367 halogens Chemical class 0.000 claims description 12
- JNCMHMUGTWEVOZ-UHFFFAOYSA-N F[CH]F Chemical compound F[CH]F JNCMHMUGTWEVOZ-UHFFFAOYSA-N 0.000 claims description 10
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 claims description 10
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 9
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 9
- 239000002019 doping agent Substances 0.000 claims description 8
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 7
- 125000006850 spacer group Chemical group 0.000 claims description 7
- 125000003302 alkenyloxy group Chemical group 0.000 claims description 6
- 125000004183 alkoxy alkyl group Chemical group 0.000 claims description 6
- 229910052757 nitrogen Inorganic materials 0.000 claims description 6
- 125000004434 sulfur atom Chemical group 0.000 claims description 6
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 claims description 6
- 238000002156 mixing Methods 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- 239000000654 additive Substances 0.000 claims description 4
- ORGHESHFQPYLAO-UHFFFAOYSA-N vinyl radical Chemical compound C=[CH] ORGHESHFQPYLAO-UHFFFAOYSA-N 0.000 claims description 4
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 229910052717 sulfur Inorganic materials 0.000 claims description 3
- KWKAKUADMBZCLK-UHFFFAOYSA-N 1-octene Chemical group CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 claims description 2
- VUWZPRWSIVNGKG-UHFFFAOYSA-N fluoromethane Chemical compound F[CH2] VUWZPRWSIVNGKG-UHFFFAOYSA-N 0.000 claims description 2
- 230000003287 optical effect Effects 0.000 abstract description 7
- 238000004519 manufacturing process Methods 0.000 abstract description 5
- 239000000203 mixture Substances 0.000 description 100
- 239000000460 chlorine Substances 0.000 description 30
- 239000000306 component Substances 0.000 description 18
- 230000000875 corresponding effect Effects 0.000 description 11
- 239000004642 Polyimide Substances 0.000 description 9
- 229920001721 polyimide Polymers 0.000 description 9
- 230000035882 stress Effects 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 239000004990 Smectic liquid crystal Substances 0.000 description 7
- 239000011159 matrix material Substances 0.000 description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 239000000470 constituent Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 230000005684 electric field Effects 0.000 description 4
- 229910052740 iodine Inorganic materials 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 4
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 125000002947 alkylene group Chemical group 0.000 description 3
- 230000005540 biological transmission Effects 0.000 description 3
- 235000010290 biphenyl Nutrition 0.000 description 3
- 150000004074 biphenyls Chemical class 0.000 description 3
- 229910052794 bromium Inorganic materials 0.000 description 3
- WVIIMZNLDWSIRH-UHFFFAOYSA-N cyclohexylcyclohexane Chemical group C1CCCCC1C1CCCCC1 WVIIMZNLDWSIRH-UHFFFAOYSA-N 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 3
- 125000001424 substituent group Chemical group 0.000 description 3
- 150000001911 terphenyls Chemical class 0.000 description 3
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 2
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 2
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 125000005194 alkoxycarbonyloxy group Chemical group 0.000 description 2
- IGARGHRYKHJQSM-UHFFFAOYSA-N cyclohexylbenzene Chemical class C1CCCCC1C1=CC=CC=C1 IGARGHRYKHJQSM-UHFFFAOYSA-N 0.000 description 2
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000004786 difluoromethoxy group Chemical group [H]C(F)(F)O* 0.000 description 2
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 230000032050 esterification Effects 0.000 description 2
- 238000005886 esterification reaction Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000005446 heptyloxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000002958 pentadecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 125000006413 ring segment Chemical group 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000010409 thin film Substances 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 125000002889 tridecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 description 1
- 125000006701 (C1-C7) alkyl group Chemical group 0.000 description 1
- PKORYTIUMAOPED-UHFFFAOYSA-N 1,2,3,4-tetrahydroquinazoline Chemical compound C1=CC=C2NCNCC2=C1 PKORYTIUMAOPED-UHFFFAOYSA-N 0.000 description 1
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 1
- VDFVNEFVBPFDSB-UHFFFAOYSA-N 1,3-dioxane Chemical group C1COCOC1 VDFVNEFVBPFDSB-UHFFFAOYSA-N 0.000 description 1
- 125000001140 1,4-phenylene group Chemical group [H]C1=C([H])C([*:2])=C([H])C([H])=C1[*:1] 0.000 description 1
- RAYZALBEMJMGEA-UHFFFAOYSA-N 1-cyclohexylnaphthalene Chemical class C1CCCCC1C1=CC=CC2=CC=CC=C12 RAYZALBEMJMGEA-UHFFFAOYSA-N 0.000 description 1
- KGFKYWUYESESLF-UHFFFAOYSA-N 2,2-difluoro-3,4-dihydrophenanthro[9,10-b]pyran Chemical class C12=CC=CC=C2C2=CC=CC=C2C2=C1OC(F)(F)CC2 KGFKYWUYESESLF-UHFFFAOYSA-N 0.000 description 1
- SZTBMYHIYNGYIA-UHFFFAOYSA-M 2-chloroacrylate Chemical compound [O-]C(=O)C(Cl)=C SZTBMYHIYNGYIA-UHFFFAOYSA-M 0.000 description 1
- SMHSPYVJAUGNOI-UHFFFAOYSA-N 2-cyclohexyl-1,4-dioxane Chemical class C1CCCCC1C1OCCOC1 SMHSPYVJAUGNOI-UHFFFAOYSA-N 0.000 description 1
- YJDDXMSIMBMMGY-UHFFFAOYSA-N 2-cyclohexylpyrimidine Chemical class C1CCCCC1C1=NC=CC=N1 YJDDXMSIMBMMGY-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- 239000004988 Nematic liquid crystal Substances 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- HFBMWMNUJJDEQZ-UHFFFAOYSA-N acryloyl chloride Chemical compound ClC(=O)C=C HFBMWMNUJJDEQZ-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 1
- 125000005196 alkyl carbonyloxy group Chemical group 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 150000001482 benzyl phenyl ethers Chemical class 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- XITRBUPOXXBIJN-UHFFFAOYSA-N bis(2,2,6,6-tetramethylpiperidin-4-yl) decanedioate Chemical group C1C(C)(C)NC(C)(C)CC1OC(=O)CCCCCCCCC(=O)OC1CC(C)(C)NC(C)(C)C1 XITRBUPOXXBIJN-UHFFFAOYSA-N 0.000 description 1
- 125000005569 butenylene group Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 229940114081 cinnamate Drugs 0.000 description 1
- 150000001851 cinnamic acid derivatives Chemical class 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 150000003983 crown ethers Chemical class 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004850 cyclobutylmethyl group Chemical group C1(CCC1)C* 0.000 description 1
- DQZKGSRJOUYVPL-UHFFFAOYSA-N cyclohexyl benzoate Chemical class C=1C=CC=CC=1C(=O)OC1CCCCC1 DQZKGSRJOUYVPL-UHFFFAOYSA-N 0.000 description 1
- 150000002666 cyclohexyl cyclohexanecarboxylates Chemical class 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000004851 cyclopentylmethyl group Chemical group C1(CCCC1)C* 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 125000004186 cyclopropylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C1([H])[H] 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- RBBNOVKRLWDEGC-UHFFFAOYSA-M dodecyl-ethyl-dimethylazanium;4-hexoxybenzoate Chemical compound CCCCCCOC1=CC=C(C([O-])=O)C=C1.CCCCCCCCCCCC[N+](C)(C)CC RBBNOVKRLWDEGC-UHFFFAOYSA-M 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000005678 ethenylene group Chemical group [H]C([*:1])=C([H])[*:2] 0.000 description 1
- 125000005745 ethoxymethyl group Chemical group [H]C([H])([H])C([H])([H])OC([H])([H])* 0.000 description 1
- QUPDWYMUPZLYJZ-UHFFFAOYSA-N ethyl Chemical compound C[CH2] QUPDWYMUPZLYJZ-UHFFFAOYSA-N 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- ZYMKZMDQUPCXRP-UHFFFAOYSA-N fluoro prop-2-enoate Chemical compound FOC(=O)C=C ZYMKZMDQUPCXRP-UHFFFAOYSA-N 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 125000003392 indanyl group Chemical class C1(CCC2=CC=CC=C12)* 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000004611 light stabiliser Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- UVEWQKMPXAHFST-UHFFFAOYSA-N n,1-diphenylmethanimine Chemical class C=1C=CC=CC=1C=NC1=CC=CC=C1 UVEWQKMPXAHFST-UHFFFAOYSA-N 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 150000002987 phenanthrenes Chemical class 0.000 description 1
- ARJOQCYCJMAIFR-UHFFFAOYSA-N prop-2-enoyl prop-2-enoate Chemical compound C=CC(=O)OC(=O)C=C ARJOQCYCJMAIFR-UHFFFAOYSA-N 0.000 description 1
- 125000006410 propenylene group Chemical group 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000001846 repelling effect Effects 0.000 description 1
- 239000011369 resultant mixture Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 150000001629 stilbenes Chemical class 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- LMBFAGIMSUYTBN-MPZNNTNKSA-N teixobactin Chemical compound C([C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H]1C(N[C@@H](C)C(=O)N[C@@H](C[C@@H]2NC(=N)NC2)C(=O)N[C@H](C(=O)O[C@H]1C)[C@@H](C)CC)=O)NC)C1=CC=CC=C1 LMBFAGIMSUYTBN-MPZNNTNKSA-N 0.000 description 1
- 125000001712 tetrahydronaphthyl group Chemical group C1(CCCC2=CC=CC=C12)* 0.000 description 1
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 1
- 230000008646 thermal stress Effects 0.000 description 1
- 125000004862 thiobutyl group Chemical group 0.000 description 1
- 125000004014 thioethyl group Chemical group [H]SC([H])([H])C([H])([H])* 0.000 description 1
- 125000004055 thiomethyl group Chemical group [H]SC([H])([H])* 0.000 description 1
- 125000004035 thiopropyl group Chemical group [H]SC([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000005407 trans-1,4-cyclohexylene group Chemical group [H]C1([H])C([H])([H])[C@]([H])([*:2])C([H])([H])C([H])([H])[C@@]1([H])[*:1] 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M trans-cinnamate Chemical compound [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 229940086542 triethylamine Drugs 0.000 description 1
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3491—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having sulfur as hetero atom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3098—Unsaturated non-aromatic rings, e.g. cyclohexene rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K2019/0444—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group
- C09K2019/0448—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit characterized by a linking chain between rings or ring systems, a bridging chain between extensive mesogenic moieties or an end chain group the end chain group being a polymerizable end group, e.g. -Sp-P or acrylate
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/10—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings
- C09K19/12—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings at least two benzene rings directly linked, e.g. biphenyls
- C09K2019/121—Compounds containing phenylene-1,4-diyl (-Ph-)
- C09K2019/122—Ph-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/10—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings
- C09K19/12—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing at least two benzene rings at least two benzene rings directly linked, e.g. biphenyls
- C09K2019/121—Compounds containing phenylene-1,4-diyl (-Ph-)
- C09K2019/123—Ph-Ph-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3003—Compounds containing at least two rings in which the different rings are directly linked (covalent bond)
- C09K2019/3004—Cy-Cy
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3003—Compounds containing at least two rings in which the different rings are directly linked (covalent bond)
- C09K2019/3009—Cy-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3003—Compounds containing at least two rings in which the different rings are directly linked (covalent bond)
- C09K2019/301—Cy-Cy-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3003—Compounds containing at least two rings in which the different rings are directly linked (covalent bond)
- C09K2019/3016—Cy-Ph-Ph
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/08—Non-steroidal liquid crystal compounds containing at least two non-condensed rings
- C09K19/30—Non-steroidal liquid crystal compounds containing at least two non-condensed rings containing saturated or unsaturated non-aromatic rings, e.g. cyclohexane rings
- C09K19/3001—Cyclohexane rings
- C09K19/3003—Compounds containing at least two rings in which the different rings are directly linked (covalent bond)
- C09K2019/3027—Compounds comprising 1,4-cyclohexylene and 2,3-difluoro-1,4-phenylene
Definitions
- the present invention relates to a liquid-crystal (LC) medium comprising a stabiliser, to its use for optical, electro-optical and electronic purposes, in particular in LC displays, especially in LC displays of the vertically aligned mode, to an LC display of the vertically aligned mode comprising the LC medium, and to a process of manufacturing the LC display.
- LC liquid-crystal
- the popularity of 8K and gaming monitors leads to an increased need for LC display (LCD) panels having higher refresh rates and thus for LC media having faster response times. Many of these LCD panels are using display modes wherein the LC molecules are aligned substantially perpendicular or slightly tilted relative to the electrode surface in the switched-off state.
- VA vertical aligned
- the LC cell of a VA display contains a layer of an LC medium between two transparent electrodes, where the LC medium usually has a negative value of the dielectric anisotropy ( ⁇ ).
- ⁇ dielectric anisotropy
- the molecules of the LC layer are aligned perpendicular to the electrode surfaces (homeotropically) or have a tilted homeotropic alignment.
- FFS field switching
- FFS displays have a low viewing-angle dependence of the contrast.
- FFS displays usually contain an LC medium with positive dielectric anisotropy, and an alignment layer, usually of polyimide, which provides planar alignment to the molecules of the LC medium.
- FFS displays can be operated as active-matrix or passive-matrix displays.
- IPS in-plane switching
- FFS displays have been disclosed (see S.H. Lee et al., Appl. Phys. Lett.73(20), 1998, 2882-2883 and S.H. Lee et al., Liquid Crystals 39(9), 2012, 1141-1148), which have similar electrode design and layer thickness as FFS displays, but comprise a layer of an LC medium with negative dielectric anisotropy instead of an LC medium with positive dielectric anisotropy.
- the LC medium with negative dielectric anisotropy shows a more favourable director orientation that has less tilt and more twist orientation compared to the LC medium with positive dielectric anisotropy, as a result of which these displays have a higher transmission.
- VA displays which use an alignment layer that is prepared by photoalignment, also known as UV 2 A mode (see e.g. Q. Tang et al., SID Symposium Digest of Technical Papers 2018, 414-417).
- These displays utilize an alignment layer prepared from crosslinkable and photoorientable monomers or prepolymers, e.g. cinnamate chromophores which are irradiated obliquely with linearly polarized UV light.
- crosslinked alignment layer is formed which induces uniaxial alignment with a pretilt angle in the LC molecules close to its surface.
- LC media with negative dielectric anisotropy have also several drawbacks. For example, they have a significantly lower reliability compared to LC media with positive dielectric anisotropy.
- the term "reliability” as used hereinafter means the quality of the performance of the display during time and with different stress loads, such as light load, temperature, humidity, or voltage which cause display defects such as image sticking (area and line image sticking), mura, yogore etc. and which are known to the skilled person in the field of LC displays.
- VHR voltage holding ration
- the reduced reliability of an LC medium with negative dielectric anisotropy in a VA or FFS display can be explained by an interaction of the LC molecules with the polyimide of the alignment layer, as a result of which ions are extracted from the polyimide alignment layer, and wherein LC molecules with negative dielectric anisotropy do more effectively extract such ions.
- the LC medium has to show a high reliability and a high VHR value after UV exposure.
- the UV component of daylight or the backlight can cause undesired decomposition reactions of the LC molecules therein and thus initiate the production of ionic or free-radical impurities. These may accumulate, in particular, at the electrodes or the alignment layers, where they may reduce the effective applied voltage.
- LC media for use in displays including but not limited to VA and FFS displays, do often exhibit high viscosities and, as a consequence, high switching times. In order to reduce the viscosity and switching time of the LC medium, it has been suggested in prior art to add LC compounds with an alkenyl group.
- LC media containing alkenyl compounds often show a decrease of the reliability and stability, and a decrease of the VHR especially after exposure to UV radiation but also to visible light from the backlight of a display, that usually does not emit UV light.
- the use of stabilisers was proposed, such as for example compounds of the HALS- (hindered amine light stabiliser) type.
- HALS- hindered amine light stabiliser
- Tinuvin 770 a compound of the formula Nevertheless, these LC mixtures can still exhibit insufficient reliability during the operation of a display, e.g. upon irradiation with the typical CCFL-(Cold Cathode Fluorescent Lamp) backlight.
- a different class of compound used for the stabilisation of liquid crystals are antioxidants derived from phenol, such as for example the compound as described in DE 19539141 A1.
- Such stabilisers can be used to stabilise LC mixtures against heat or the influence of oxygen but typically do not show advantages under light stress.
- the liquid crystal a complex mixture of many different types of compounds itself, interacts with different kinds of species, including the polyimide, it is a challenging task also for the skilled person to choose the right stabiliser in order to identify the best material combination.
- a further object of the invention is to provide FFS displays with good transmission, high reliability, a VHR value especially after backlight exposure, a high specific resistance, a large working-temperature range, short response times even at low tempera- tures, a low threshold voltage, a multiplicity of grey levels, high contrast and a broad viewing angle, and reduced image sticking. It was found that one or more of these objects could be achieved by providing an LC medium as disclosed and claimed hereinafter.
- an LC medium comprising a small amount of a stabiliser, which is a compound of formula I as described hereinafter, in a VA-, IPS or FFS display. It has also been found that when using such stabilisers in an LC medium for use in an FFS display, surprisingly the reliability and the VHR value after backlight load are higher, compared to an LC medium without a stabiliser according to the present invention. Also, the use of an LC medium comprising a stabiliser as described hereinafter allows to exploit the known advantages of alkenyl-containing LC media, like reduced viscosity and faster switching time, and at the same time leads to improved reliability and high VHR value especially after backlight exposure.
- a stabiliser which is a compound of formula I as described hereinafter
- the invention further relates to the use of the LC medium as described above and below in LC displays, preferably in LC displays of the VA, IPS, FFS, UB-FFS or UV 2 A mode.
- the LC medium has negative dielectric anisotropy.
- the invention furthermore relates to a process for preparing an LC medium as described above and below, comprising the steps of mixing one or more compounds of formula I with one or more compounds of formula II and optionally with further LC compounds and/or additives.
- the invention furthermore relates to an LC display comprising an LC medium according to the invention as described above and below, preferably an LC display of the VA, IPS, FFS, UB-FFS or UV 2 A mode.
- the invention furthermore relates to a process for manufacturing an LC display as described above and below, comprising the steps of filling or otherwise providing an LC medium as described above and below between the substrates of the display.
- the compounds of formula I although they carry potentially reactive groups P like acrylate or methacrylate, quite contrary to being harmful in terms of reliability of the LC, are able to stabilise LC mixtures under light stress. It was also found that, if the concentration of the compounds of formula I is kept low enough, an undesired generation of a pretilt angle in the LC medium, which is usually observed when using such compounds in VA mode displays and exposing them to UV irradiation, can be suppressed.
- the reliability and the VHR value after backlight load are higher, compared to an LC medium without a compound of formula I according to the present invention.
- the use of an LC medium comprising a compound of formula I as described hereinafter allows to exploit the known advantages of alkenyl- containing LC media, like reduced viscosity and faster switching time, and at the same time leads to improved reliability and high VHR value especially after backlight exposure.
- the compounds of formula I are preferably selected from achiral compounds.
- the terms “active layer” and “switchable layer” mean a layer in an electrooptical display, for example an LC display, that comprises one or more molecules having structural and optical anisotropy, like for example LC molecules, which change their orientation upon an external stimulus like an electric or magnetic field, resulting in a change of the transmission of the layer for polarized or unpolarized light.
- the terms “tilt” and “tilt angle” will be understood to mean a tilted alignment of the LC molecules of an LC medium relative to the surfaces of the cell in an LC display (here preferably a PSA display), and will be understood to be inclusive of “pretilt” and "pretilt angle”.
- the tilt angle here denotes the average angle ( ⁇ 90°) between the longitudinal molecular axes of the LC molecules (LC director) and the surface of the plane-parallel outer plates which form the LC cell.
- a low absolute value for the tilt angle i.e. a large deviation from the 90° angle
- a suitable method for measurement of the tilt angle is given in the examples. Unless indicated otherwise, tilt angle values disclosed above and below relate to this measurement method.
- mesogenic group as used herein is known to the person skilled in the art and described in the literature, and means a group which, due to the anisotropy of its attracting and repelling interactions, essentially contributes to causing a liquid-crystal (LC) phase in low-molecular-weight or polymeric substances.
- LC liquid-crystal
- Compounds containing mesogenic groups do not necessarily have to have an LC phase themselves. It is also possible for mesogenic compounds to exhibit LC phase behaviour only after mixing with other compounds and/or after polymerization. Typical mesogenic groups are, for example, rigid rod- or disc-shaped units.
- spacer group hereinafter also referred to as "Sp”, as used herein is known to the person skilled in the art and is described in the literature, see, for example, Pure Appl. Chem.2001, 73(5), 888 and C. Tschierske, G. Pelzl, S. Diele, Angew. Chem.2004, 116, 6340-6368.
- spacer group or "spacer” mean a flexible group, for example an alkylene group, which connects the mesogenic group and the polymerizable group(s) in a polymerizable mesogenic compound.
- alkylene group which connects the mesogenic group and the polymerizable group(s) in a polymerizable mesogenic compound.
- the single bond shown between the two ring atoms can be attached to any free position of the benzene ring.
- R 1-12 , R Q , R or L denotes an alkyl radical and/or an alkoxy radical, this may be straight- chain or branched.
- It is preferably straight-chain, has 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes ethyl, propyl, butyl, pentyl, hexyl, heptyl, ethoxy, propoxy, butoxy, pentoxy, hexyloxy or heptyloxy, furthermore methyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetra- decyl, pentadecyl, methoxy, octyloxy, nonyloxy, decyloxy, undecyloxy, dodecyloxy, tridecyloxy or tetradecyloxy.
- R 1-13 R 51 , R 52 , R Q , R, R 2A , R 2B , R IIIA , R 1N , R 2N , R B1 , R B2 , R CR1 , R CR2 , R or L denotes an alkyl radical and/or an alkoxy radical, this may be straight-chain or branched.
- It is preferably straight-chain, has 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes ethyl, propyl, butyl, pentyl, hexyl, heptyl, ethoxy, propoxy, butoxy, pentoxy, hexyloxy or heptyloxy, furthermore methyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl, methoxy, octyloxy, nonyloxy, decyloxy, undecyloxy, dodecyloxy, tridecyloxy or tetradecyloxy.
- R 1-13 R 51 , R 52 , R Q , R, R 2A , R 2B , R IIIA , R 1N , R 2N , R B1 , R B2 , R CR1 , R CR2 , R or L denotes an alkyl radical wherein one or more CH 2 groups are replaced by S, this may be straight-chain or branched. It is preferably straight-chain, has 1, 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes thiomethyl, thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl or thioheptyl.
- R 1-13 R 51 , R 52 , R Q , R, R 2A , R 2B , R IIIA , R 1N , R 2N , R B1 , R B2 , R CR1 , R CR2 , R or L denotes an alkoxy or oxaalkyl group it may also contain one or more additional oxygen atoms, provided that oxygen atoms are not linked directly to one another.
- one or more of R 1-13 , R 51 , R 52 , R Q , R, R 2A , R 2B , R IIIA , R 1N , R 2N , R B1 , R B2 , R CR1 , R CR2 , R or L are selected from the group consisting of -S 1 -F, -O-S 1 -F, -O-S1-O-S2, wherein S 1 is C 1-12 -alkylene or C 2-12 - alkenylene and S 2 is H, C 1-12 -alkyl or C 2-12 -alkenyl, and very preferably are selected from the group consisting of -OCH 2 OCH 3 , -O(CH 2 ) 2 OCH 3 , -O(CH 2 ) 3 OCH 3 , -O(CH 2 ) 4 OCH 3 , -O(CH 2 ) 2 F, - O(CH 2 ) 3 F and -O(CH 2
- R 1-13 R 51 , R 52 , R Q , R, R 2A , R 2B , R IIIA , R 1N , R 2N , R B1 , R B2 , R CR1 , R CR2 , R or L denotes an alkyl or alkenyl radical which is at least monosubstituted by halogen, this radical is preferably straight-chain, and halogen is preferably F or Cl. In the case of polysubstitution, halogen is preferably F.
- the resultant radicals also include perfluorinated radicals.
- the fluorine or chlorine substituent may be in any desired position, but is preferably in the ⁇ -position.
- Halogen is preferably F or Cl, very preferably F.
- substituents L are, for example, F, Cl, CN, NO 2 , CH 3 ,C 2 H 5 , OCH 3 , OC 2 H 5 , COCH 3 , COC 2 H 5 , COOCH 3 , COOC 2 H 5 ,CF 3 , OCF 3 , OCHF 2 , OC 2 F 5 , furthermore phenyl.
- L has one of the meanings indicated above.
- the group P is preferably selected from the group consisting of acrylate, methacrylate, fluoroacrylate and chloroacrylate, more preferably from acrylate and methacrylate, most preferably P denotes methacrylate.
- X" is preferably -O-, -S-, -CO-, -COO-, -OCO-, -O-COO-, -CO-NR 0 -, -NR 0 - CO-, -NR 0 -CO-NR 00 - or a single bond.
- Typical spacer groups Sp 1-3 and -Sp"-X"- are, for example, -(CH 2 ) p1 -, - (CH 2 ) p1 -O-, -(CH 2 ) p1 -O-CO-, -(CH 2 ) p1 -CO-O-, -(CH 2 ) p1 -O-CO-O-, - (CH 2 CH 2 O) q1 -CH 2 CH 2 -, -CH 2 CH 2 -S-CH 2 CH 2 -, -CH 2 CH 2 -NH-CH 2 CH 2 - or - (SiR 0 R 00 -O) p1 -, in which p1 is an integer from 1 to 12, q1 is an integer from 1 to 3, and R 0 and R 00 have the meanings indicated above.
- Particularly preferred groups Sp 1-3 and -Sp"-X"- are -(CH 2 ) p1 -, -(CH 2 ) p1 -O-, - (CH 2 ) p1 -O-CO-, -(CH 2 ) p1 -CO-O-, -(CH 2 ) p1 -O-CO-O-, in which p1 and q1 have the meanings indicated above.
- Particularly preferred groups Sp are, in each case straight-chain, ethylene, propylene, butylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, octadecylene, ethyleneoxyethylene, methyleneoxybutylene, ethylenethioethylene, ethylene-N-methyliminoethylene, 1-methylalkylene, ethenylene, propenylene and butenylene.
- Preferred compounds of formula I and its subformulae are those wherein at least one of the groups Sp 1-3 is a single bond.
- Further preferred compounds or formula I and its subformulae are those wherein at least one of the groups Sp 1-3 is different from a single bond.
- Very preferred are compounds of formula I and its subformulae, wherein Sp 1-3 are selected from the group consisting of a single bond, -(CH 2 ) p1 -, - O-(CH 2 ) p1 -, -O-CO-(CH 2 ) p1 and -CO-O-(CH 2 ) p1 , wherein p1 is 2, 3, 4, 5 or 6, and, if Sp is -O-(CH 2 ) p1 -, -O-CO-(CH 2 ) p1 or -CO-O-(CH 2 ) p1 the O-atom or CO-group, respectively, is linked to the benzene ring.
- - all groups P have the same meaning, and very preferably denote acrylate or methacrylate, most preferably methacrylate, - all of Sp 1-3 are a single bond - all of Sp 1-3 are different from a single bond, - one of Sp 1-3 is a single bond and the other two are different from a single bond, - one of Sp 1-3 is different from a single bond and the other two each denote a single bond, - Sp 1 is a single bond and one or both of Sp 2 and Sp 3 , preferably both of Sp 2 and Sp 3 , are different from a single bond, - Sp 1-3 , when being different from a single bond, are selected from the group consisting of -(CH 2 ) 2 -, -(CH 2 ) 3 -, -(CH 2 ) 4 -, -O-(CH 2 ) 2 -, -O
- Sp’ in formulae I1 to I8 is preferably selected from the group consisting of - (CH 2 ) 2 -, -(CH 2 ) 3 -, -(CH 2 ) 4 -, -O-(CH 2 ) 2 -, -O-(CH 2 ) 3 -, -O-CO-(CH 2 ) 2 and -CO- O-(CH) 2 -, wherein the O atom or the CO group is attached to the benzene ring.
- L in formulae I1 to I8 preferably denotes F, Cl, CN or OCH 3 , very preferably F or OCH 3 .
- Very preferred compounds of formula I are selected from the following subformulae:
- concentration of the compounds of formula I and its subformulae in the LC medium is preferably from 0.001 to 0.02%, very preferably from 0.002 to 0.015%, most preferably from 0.005 to 0.015%. In another rpreferred embodiment the concentration of the compounds of formula I and its subformulae in the LC medium is from 10 to 250ppm, preferably from 20 to 200ppm, most preferably from 50 to 150 ppm.
- the compounds of the formula I can be prepared analogously to processes known to the person skilled in the art and described in standard works of organic chemistry, such as, for example, in Houben-Weyl, Methoden der organischen Chemie [Methods of Organic Chemistry], Thieme-Verlag, Stuttgart.
- acrylic or methacrylic esters can be prepared by esterification of the corresponding alcohols with acid derivatives like, for example, (meth)acryloyl chloride or (meth)acrylic anhydride in the presence of a base like pyridine or triethyl amine, and 4-(N,N-dimethylamino)pyridine (DMAP).
- the esters can be prepared by esterification of the alcohols with (meth)acrylic acid in the presence of a dehydrating reagent, for example according to Steglich with dicyclohexylcarbodiimide (DCC), N-(3- dimethylaminopropyl)-N’-ethylcarbodiimide (EDC) or N-(3- dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride and DMAP.
- the LC media for use in the LC displays according to the invention comprise an LC mixture ("host mixture") comprising one or more, preferably two or more LC compounds, at least one of which is a compound of formula II. Particularly preferred embodiments of such an LC medium are shown below.
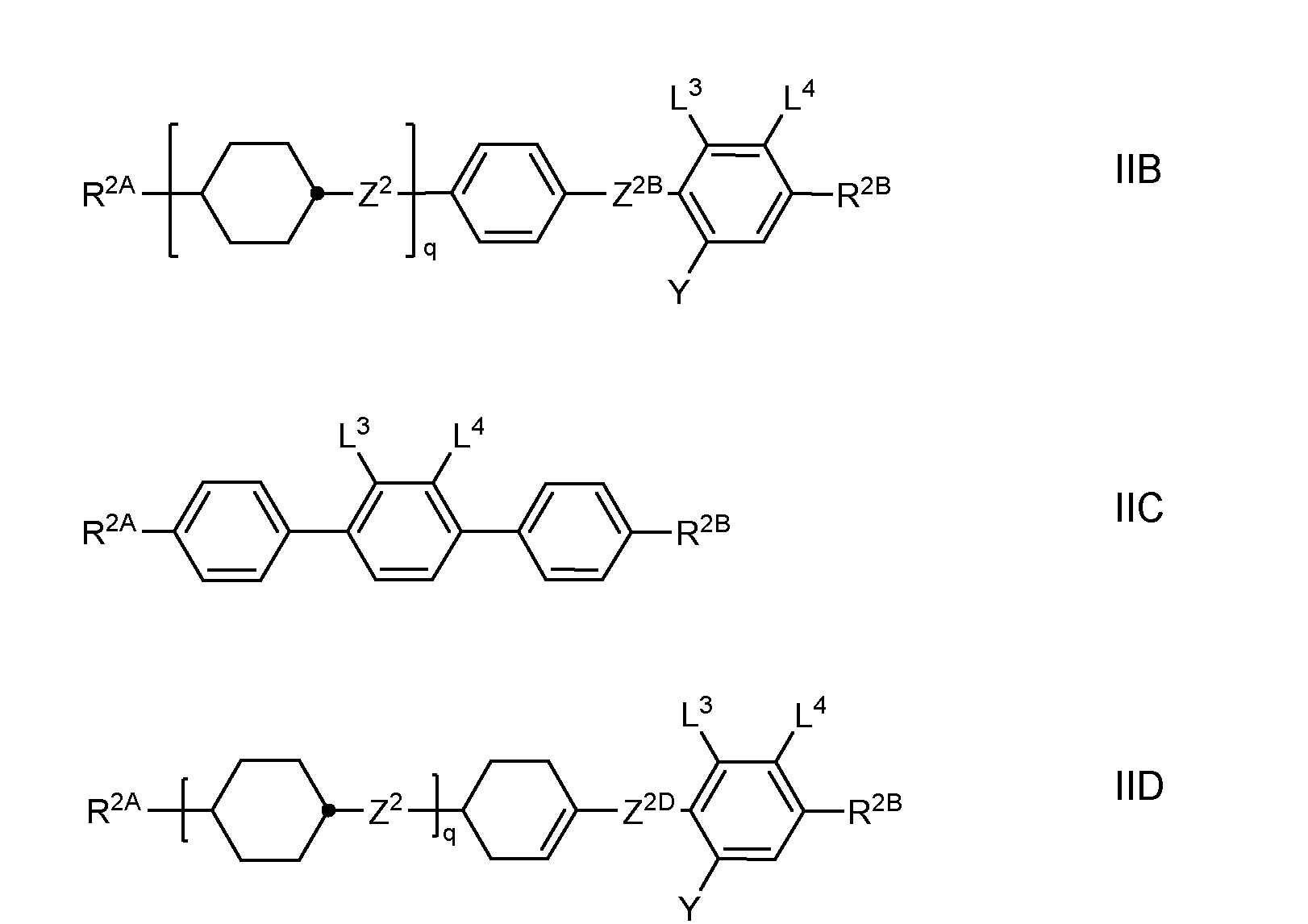
- the LC medium contains one or more compounds of formula II selected from the group consisting of compounds of the formulae IIA, IIB, IIC and IID
- R 2A and R 2B each, independently of one another, denote H, an alkyl or alkenyl radical having up to 15 C atoms which is unsubstituted, monosubstituted by CN or CF 3 or at least monosubstituted by halogen, where, in addition, one or more CH 2 groups in these radicals may be replaced by -O-, -S-, -C ⁇ C-, -CF 2 O-, -OCF 2 -, -OC-O- or -O-CO- in such a way that O atoms are not linked directly to one another, L 1 to L 4 each, independently of one another, denote F, Cl, CF 3 or CHF 2 , Y denotes H, F, Cl, CF 3 , CHF 2 or CH 3 , preferably H or CH 3 , particularly preferably H, Z 2 , Z 2B and Z 2D each, independently of one another, denote a single bond, -CH 2 CH 2 -,
- Preferred compounds of the formulae IIA, IIB, IIC and IID are those wherein R 2B denotes an alkyl or alkoxy radical having up to 15 C atoms, and very preferablydenotes (O)C v H 2v+1 wherein (O) is an oxygen atom or a single bond and v is 1, 2, 3, 4, 5 or 6.
- LC medium comprises one or more compounds of the formula IIA selected from the group consisting of formulae IIA-1 to IIA-76,
- index a denotes 1 or 2
- alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms
- alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms
- (O) denotes an oxygen atom or a single bond.
- Particularly preferred LC media according to the invention comprise one or more compounds selected from the group consisting of formulae IIA-2, IIA- 8, IIA-10, IIA-16, II-18, IIA-40, IIA-41, IIA-42 and IIA-43.
- the LC medium comprises one or more compounds of the formula IIB selected from the group consisting of formulae IIB-1 to IIB-26,
- LC media comprise one or more compounds selected from the group consisting of formulae IIB-2, IIB- 10 and IIB-16.
- the LC medium comprises one or more compounds of the formula IIC selected from the formula IIC-1, in which “alkyl” and “alkyl*” and (O) have the meanings given above.
- the LC medium comprises one or more compounds of the formula IID selected from the group consisting of formulae IID-1 to IID-10,
- Particularly preferred LC media according to the invention comprise one or more compounds of the formula IID-4.
- the proportion of compounds of the formulae IIA and/or IIB in the mixture as a whole is preferably at least 20 % by weight.
- the LC medium comprises one or more compounds of the formula III-1 and/or III-2 in which the occurring groups have the same meanings as given under formula III above and preferably R 11 and R 12 each, independently of one another, an alkyl, alkenyl or alkoxy radical having up to 15 C atoms, more preferably one or both of them denote an alkoxy radical and L 11 and L 12 each preferably denote F.
- the LC medium comprises one or more compounds of the formula III-1 selected from the group consisting of formulae III-1-1 to III-1-11, preferably of formula III-1-6, in which “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms, “alkoxy” and “alkoxy*” each, independently of one another, denote a straight-chain alkoxy radical having 1-6 C atoms, and L 11 and L 12 each, independently of one another, denote F or Cl, preferably both F.
- the LC medium comprises one or more compounds of the formula III-2 selected from the group consisting of formulae III-2-1 to III-2-10, preferably of formula III-2-6,
- alkyl and “alkyl*” each, independently of one another denote a straight-chain alkyl radical having 1-6 C atoms
- alkenyl and “alkenyl*” each, independently of one another denote a straight-chain alkenyl radical having 2-6 C atoms
- alkoxy and “alkoxy*” each, independently of one another denote a straight-chain alkoxy radical having 1-6 C atoms
- L 11 and L 12 each, independently of one another, denote F or Cl, preferably both F.
- the LC medium comprises one or more compounds selected from the group consisting of the following formulae
- the LC medium comprises one or more compounds of the formula IIIA-1 and/or IIIA-2 in which L 11 and L 12 have the same meanings as given under formula III, (O) denotes O or a single bond, R IIIA denotes alkyl or alkenyl having up to 7 C atoms or a group Cy- C m H 2m+1 -, m and n are, identically or differently, 0, 1, 2, 3, 4, 5 or 6, preferably 1, 2 or 3, very preferably 1, and Cy denotes a cycloaliphatic group having 3, 4 or 5 ring atoms, which is optionally substituted with alkyl or alkenyl each having up to 3 C atoms, or with halogen or CN, and preferably denotes cyclopropyl, cyclobutyl or cyclopentyl.
- the compounds of formula IIIA-1 and/or IIIA-2 are contained
- alkoxy denotes a straight-chain alkoxy radical having 1-6 C atoms, and preferably denotes n-propoxy, n-butyloxy, n-pentyloxy or n- hexyloxy.
- the compounds of formula III-3 are preferably selected from the group consisting of formulae III-3-1 to III-3-11:
- the LC medium comprises one or more compounds of the formulae III-4 to III-6, preferably of formula III-5,
- the LC medium comprises one or more compounds of the formula I selected from the group consisting of formulae III-7 to III-9, preferably of formula III-8, in which the parameters have the meanings given above, R 11 preferably denotes straight-chain alkyl and R 12 preferably denotes alkoxy each having 1 to 7 C atoms.
- the medium comprises one or more compounds of the formula IV, in which R 41 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkenyl radical having 2 to 7 C atoms, preferably an n-alkyl radical, particularly preferably having 2, 3, 4 or 5 C atoms, and R 42 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkoxy radical having 1 to 6 C atoms, both preferably having 2 to 5 C atoms, an unsub- stituted alkenyl radical having 2 to 7 C atoms, preferably having 2, 3 or 4 C atoms, more preferably a vinyl radical or a 1-propenyl radical and in particular a vinyl radical.
- R 41 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkenyl radical having 2 to 7 C atoms, preferably an
- the compounds of the formula IV are preferably selected from the group consisting of formulae IV-1 to IV-6, in which “alkyl” and “alkyl*“ independently of one another, denote alkyl having 1 to 7 C atoms, preferably having 2 to 5 C atoms, “alkenyl” denotes an alkenyl radical having 2 to 5 C atoms, prefer- ably having 2 to 4 C atoms, particularly preferably 2 C atoms, “alkenyl*“ denotes an alkenyl radical having 2 to 5 C atoms, prefer- ably having 2 to 4 C atoms, particularly preferably having 2 to 3 C atoms, and “alkoxy” denotes alkoxy having 1 to 5 C atoms, preferably having 2 to 4 C atoms.
- the LC medium comprises one or more compounds selected from the group consisting of formulae IV-1-1 to IV-1-9
- the LC medium according to the invention comprises one or more compounds of the formulae IV-2-1 and/or IV-2-2
- the LC medium according to the invention comprises a compound of formula IV-3, in particular selected from the group consisting of formulae IV-3-1 to IV-3-4
- the LC medium according to the invention comprises a compound of formula IV-4, in particular selected from the compounds of the formulae IV-4-1 and IV-4-2
- the LC medium according to the invention preferably comprises at least one compound of the formula IVa-1and/or formula IVa-2.
- the LC medium comprises one or more compounds of formula IVb-1 to IVb-3 in which “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1 to 6 C atoms, and “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2 to 6 C atoms.
- the proportion of the biphenyls of the formulae IV-1 to IV-3 in the mixture as a whole is preferably at least 3 % by weight, in particular ⁇ 5 % by weight.
- the compounds of the formula IVb-2 are particularly preferred.
- Particularly preferred biphenyls are in which “alkyl*” denotes an alkyl radical having 1 to 6 C atoms and preferably denotes n-propyl.
- the LC medium according to the invention particularly preferably comprises one or more compounds of the formulae IVb-1-1 and/or IVb-2-3.
- R 1 and R 2 have the meanings indicated for R 2A above.
- R 1 and R 2 preferably each, independently of one another, denote straight- chain alkyl or alkenyl.
- Preferred LC media comprise one or more compounds of the formulae V- 1, V-3, V-4, V-6, V-7, V-10, V-11, V-12, V-14, V-15, and/or V-16 LC media according to the invention very particularly preferably comprise the compounds of the formula V-10, V-12, V-16 and/or IV-1, in particular in amounts of 5 to 30 %.
- Preferred compounds of the formulae V-10 are indicated below:
- the LC medium according to the invention particularly preferably com- prises the tricyclic compounds of the formula V-10a and/or of the formula V-10b in combination with one or more bicyclic compounds of the formulae IV-1
- the total proportion of the compounds of the formulae V-10a and/or V- 10b in combination with one or more compounds selected from the bicyclohexyl compounds of the formula IV-1 is 5 to 40 %, very particularly preferably 15 to 35 %.
- Very particularly preferred LC media comprise compounds V-10a and CC- 2-3
- the compounds V-10a and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole.
- Further particularly preferred LC media comprise compounds V-10b and IV-1-1:
- the compounds V-10b and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole.
- Further particularly preferred LC media comprise the following three com- pounds:
- the compounds V-10a, V-10b and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole.
- Preferred LC media comprise at least one compound selected from the group consisting of the following formulae in which R 41 and R 42 , and R 51 and R 52 have the meanings indicated above.
- R 41 and R 51 denotes alkyl or alkenyl having 1 to 6 or 2 to 6 C atoms, respectively, and R 42 and R 52 denotes alkenyl having 2 to 6 C atoms.
- Preferred LC media comprise at least one compound of the formulae V-6a, V-6b, V-7a, V-7b, IV-4-1, IV-4-2, IV-3a and IV-3b: in which alkyl denotes an alkyl radical having 1 to 6 C atoms and alkenyl denotes an alkenyl radical having 2 to 6 C atoms.
- the compounds of the formulae V-6a, V-6b, V-7a, V-7b, IV-4-1, IV-4-2, IV- 3a and IV-3b are preferably present in the LC media according to the invention in amounts of 1 to 40 % by weight, preferably 5 to 35 % by weight and very particularly preferably 10 to 30 % by weight.
- the LC medium additionally comprises one or more compounds selected from the group consisting of formulae VI-1 to VI-9
- LC media comprising at least one compound of the formula V-9.
- the LC medium additionally comprises one or more compounds selected from the group consisting of the formulae VII-1 to VII-25,
- R denotes a straight-chain alkyl or alkoxy radical having 1 to 6 C atoms, (O) denotes -O- or a single bond, X denotes F, Cl, OCF 3 or OCHF 2 , L x denotes H or F, m is 0, 1, 2, 3, 4, 5 or 6 and n is 0, 1, 2, 3 or 4.
- R preferably denotes methyl, ethyl, propyl, butyl, pentyl, hexyl, methoxy, ethoxy, propoxy, butoxy, pentoxy.
- X preferably denotes F or OCH 3 , very preferably F.
- the LC medium according to the invention preferably comprises the ter- phenyls of the formulae VII-1 to VII-25 in amounts of 2 to 30 % by weight, in particular 5 to 20 % by weight.
- Particular preference is given to compounds of the formulae VII-1, VII-2, VII- 4, VII-20, VII-21, and VII-22 wherein X denotes F.
- R preferably denotes alkyl, furthermore alkoxy, each having 1 to 5 C atoms.
- R preferably denotes alkyl or alkenyl, in particular alkyl.
- R preferably denotes alkyl.
- X preferably denotes F.
- terphenyls of formula VII-1 to VII-25 are preferably employed in the LC media according to the invention if the ⁇ n value of the mixture is to be ⁇ 0.1.
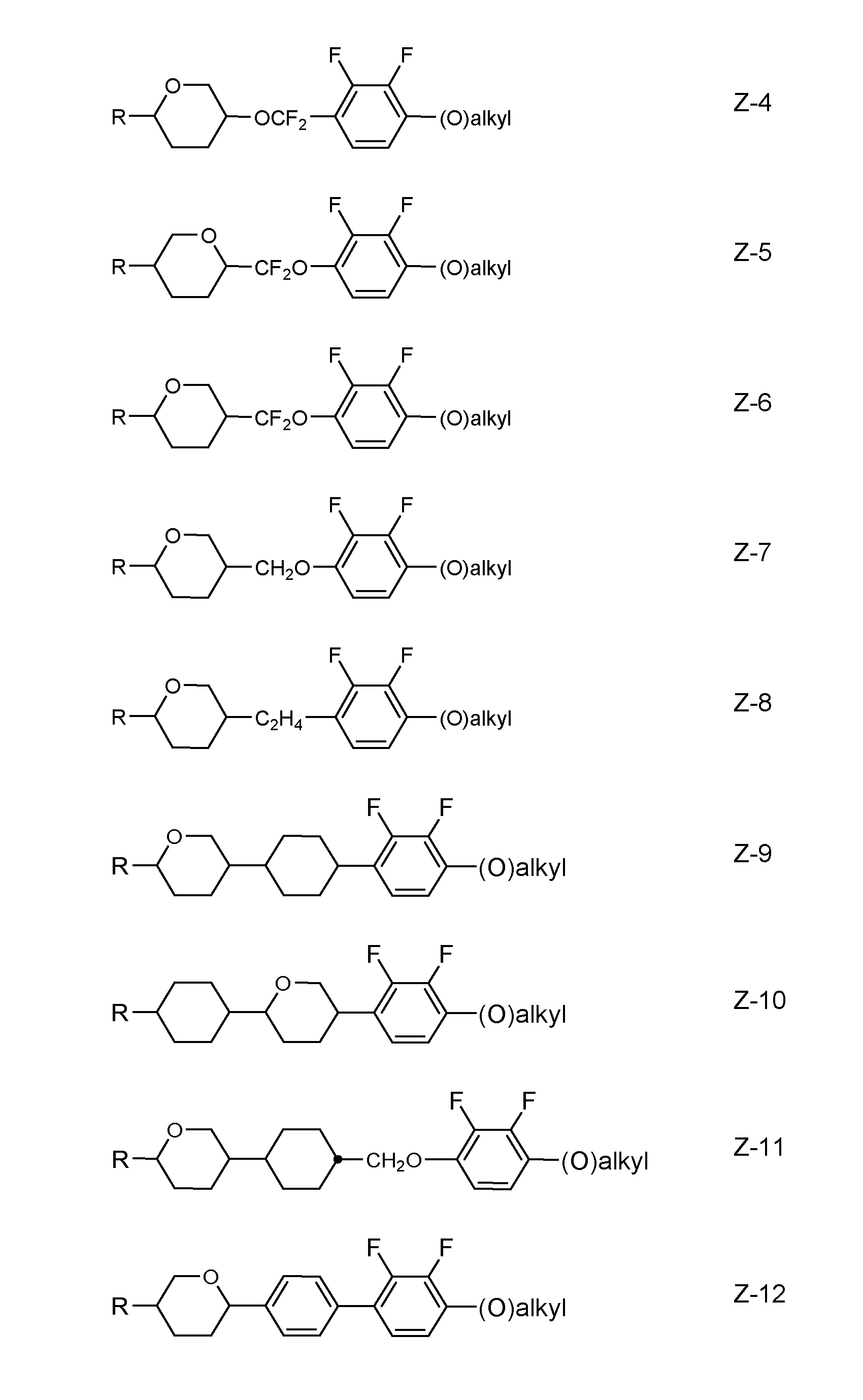
- Preferred LC media comprise 2 to 20 % by weight of one or more terphenyl compounds selected from the group of the compounds of formulae VII-1 to VII-25. Further preferred embodiments are listed below: a) LC medium comprising at least one compound selected from the group consisting of formulae Z-1 to Z-14, in which R, (O) and “alkyl” have the meanings indicated above for formula III.
- Preferred LC media comprise one or more compounds selected from the group of the difluorodibenzochroman compounds of the formula BC, chromans of the formula CR, and fluorinated phenanthrenes of the formulae PH-1 and PH-2, in which R B1 , R B2 , R CR1 , R CR2 , R 1 , R 2 each, independently of one another, have the meaning of R 2A .
- c is 0, 1 or 2.
- R 1 and R 2 preferably, independently of one another, denote alkyl or alkoxy having 1 to 6 C atoms.
- the LC media according to the invention preferably comprise the compounds of the formulae BC, CR, PH-1, PH-2 in amounts of 3 to 20 % by weight, in particular in amounts of 3 to 15 % by weight.
- Particularly preferred compounds of the formulae BC and CR are the compounds BC-1 to BC-7 and CR-1 to CR-5,
- alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1 to 6 C atoms
- alkenyl and alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2 to 6 C atoms.
- LC media comprising one, two or three compounds of the formula BC-2, BF-1 and/or BF-2.
- Preferred LC media comprise one or more indane compounds of the formula In, In in which R 11 , R 12 , R 13 each, independently of one another, denote a straight- chain alkyl, alkoxy, alkoxyalkyl or alkenyl radical having 1 to 6 C atoms, R 12 and R 13 additionally denote halogen, preferably F, i denotes 0, 1 or 2.
- Preferred compounds of the formula In are the compounds of the formulae In-1 to In-16 indicated below: Particular preference is given to the compounds of the formulae In-1, In-2, In-3 and In-4.
- Preferred LC media additionally comprise one or more compounds of the formulae L-1 to L-5, in which R and R 1 each, independently of one another, have the meanings indicated for R 2A in formula IIA above, and alkyl denotes an alkyl radical having 1 to 6 C atoms.
- the parameter s denotes 1 or 2.
- Preferred LC media additionally comprise one or more compounds of formula IIA-Y in which R 11 and R 12 have one of the meanings given for R 2A in formula IIA above, and L 1 and L 2 , identically or differently, denote F or Cl.
- Preferred compounds of the formula IIA-Y are selected from the group consisting of the following subformulae
- Alkoxy denotes a straight-chain alkoxy radical having 1-6 C atoms
- O denotes an oxygen atom or a single bond.
- Particularly preferred compounds of the formula IIA-Y are selected from the group consisting of following subformulae: in which Alkoxy and Alkoxy* have the meanings defined above and preferably denote methoxy, ethoxy, n- propyloxy, n-butyloxy or n- pentyloxy.
- LC medium which additionally comprises one or more quaterphenyl compounds selected from the following formula: wherein R Q is alkyl, alkoxy, oxaalkyl or alkoxyalkyl having 1 to 9 C atoms or alkenyl or alkenyloxy having 2 to 9 C atoms, all of which are optionally fluorinated, X Q is F, Cl, halogenated alkyl or alkoxy having 1 to 6 C atoms or halogenated alkenyl or alkenyloxy having 2 to 6 C atoms, L Q1 to L Q6 independently of each other are H or F, with at least one of L Q1 to L Q6 being F.
- R Q is alkyl, alkoxy, oxaalkyl or alkoxyalkyl having 1 to 9 C atoms or alkenyl or alkenyloxy having 2 to 9 C atoms, all of which are optionally fluorinated
- X Q is F, Cl, halogenated alkyl or al
- Preferred compounds of formula Q are those wherein R Q denotes straight-chain alkyl with 2 to 6 C-atoms, very preferably ethyl, n- propyl or n-butyl.
- Preferred compounds of formula Q are those wherein L Q3 and L Q4 are F.
- Further preferred compounds of formula Q are those wherein L Q3 , L Q4 and one or two of L Q1 and L Q2 are F.
- Preferred compounds of formula Q are those wherein X Q denotes F or OCF 3 , very preferably F.
- the compounds of formula Q are preferably selected from the following subformulae wherein R Q has one of the meanings of formula Q or one of its preferred meanings given above and below, and is preferably ethyl, n-propyl or n-butyl. Especially preferred are compounds of formula Q1, in particular those wherein R Q is n-propyl.
- the proportion of compounds of formula Q in the LC host mixture is from >0 to ⁇ 5% by weight, very preferably from 0.05 to 2% by weight, more preferably from 0.1 to 1% by weight, most preferably from 0.1 to 0.8% by weight.
- the LC medium contains 1 to 5, preferably 1 or 2 compounds of formula Q.
- quaterphenyl compounds of formula Q to the LC host mixture enables to reduce ODF mura, whilst maintaining high UV absorption, enabling quick and complete polymerization, enabling strong and quick tilt angle generation, and increasing the UV stability of the LC medium.
- compounds of formula Q which have positive dielectric anisotropy
- the LC media according to the invention preferably comprise - one or more compounds of formula I or its subformulae, preferably in a total concentration in the range of from 0.001% to 0.02%, more preferably from 0.002% to 0.015%, most preferably from 0.005% to 0.015%, and/or - one or more compounds of formula IIA, preferably in a total concentration in the range of from 5% to 30%, more preferably from 7% to 25%, particularly preferably from 10% to 20%; and/or - one or more compounds of formulae IIA and IIB, preferably in a total concentration in the range of from 30% to 45%; and/or - one or more compounds of formula IV, preferably in a total concentration in the range of from 35% to 70%, more preferably from 40 % to 65%, particularly preferably from 45% to 60%; and/or - one or more compounds of formula IV-3, preferably in a total concentration in the range of from 35% to 60%, more preferably from 40 % to 55%, particularly preferably from 45% to
- the medium comprises - one or more compounds CY-n-Om, in particular CY-3-O4, CY-5-O4 and/or CY-3-O2, preferably in a total concentration in the range of from 5% to 30%, preferably 10% to 20%; and/or - one or more compounds PY-n-Om, in particular PY-3-O2 and/or PY-1- O2, preferably in a total concentration in the range of from 5% to 30%, preferably 5% to 20%; and/or - CPY-n-Om, in particular CPY-2-O2, CPY-3-O2 and/or CPY-5-O2, pref- erably in concentrations > 5%, in particular 7% to 20%, based on the mixture as a whole, and/or - one or more compounds CCY-n-Om, preferably CCY-4-O2, CCY-3-O2, CCY-3-O3, CCY-3-O1 and/or CCY-5-O2, preferably in concentrations > 3%,
- the compound of the formula CC-3-V1 in a total concentration in the range of from 5 to 40%, more preferably from 15% to 35%, particularly preferably from 20% to 30%, and/or - one or more compounds of formula B-nO-Om and/or B(S)-nO-Om, in particular the compound B(S)-2O-O4 and/or B(S)-2O-O5, preferably in a concentration in the range of from 2 to 12 %.
- the invention furthermore relates to an electro-optical display having active-matrix addressing, characterised in that it contains, as dielectric, a liquid-crystalline medium according to claim 1 and wherein the display is a VA, SA-VA, IPS, U-IPS, FFS, UB-FFS, SA-FFS, PS-VA, PS-OCB, PS-IPS, PS-FFS, PS-UB-FFS, PS-posi-VA, PS-TN, polymer stabilised SA-VA or polymer stabilised SA-FFS display.
- active-matrix addressing characterised in that it contains, as dielectric, a liquid-crystalline medium according to claim 1 and wherein the display is a VA, SA-VA, IPS, U-IPS, FFS, UB-FFS, SA-FFS, PS-VA, PS-OCB, PS-IPS, PS-FFS, PS-UB-FFS, PS-posi-VA, PS-TN, polymer stabilised SA-VA or polymer stabilised SA-FFS display.
- the liquid-crystalline medium according to the invention preferably have a nematic phase from ⁇ -20°C to ⁇ 70°C, particularly preferably from ⁇ -30°C to ⁇ 80°C, very particularly preferably from ⁇ -40°C to ⁇ 90°C.
- the medium according to the invention preferably has a clearing temperature of 70°C or more, very preferably of 74°C or more.
- the LC medium has preferably a nematic LC phase.
- the expression "have a nematic phase” here means on the one hand that no smectic phase and no crystallisation are observed at low temperatures at the corresponding temperature and on the other hand that clearing still does not occur on heating from the nematic phase.
- the investigation at low temperatures is carried out in a flow viscometer at the corresponding temperature and checked by storage in test cells having a layer thickness corresponding to the electro-optical use for at least 100 hours. If the stor- age stability at a temperature of -20°C in a corresponding test cell is 1000 h or more, the medium is referred to as stable at this temperature. At temperatures of -30°C and -40°C, the corresponding times are 500 h and 250 h respectively. At high temperatures, the clearing point is measured by conventional methods in capillaries.
- the liquid-crystal mixture preferably has a nematic phase range of at least 60 K and a flow viscosity v 20 of at most 30 mm 2 ⁇ s -1 at 20°C.
- the mixture is nematic at a temperature of -20°C or less, preferably at - 30°C or less, very preferably at -40°C or less.
- the values of the birefringence ⁇ n in the liquid-crystal mixture are gener- ally between 0.07 and 0.16, preferably between 0.08 and 0.15, very preferably between 0.09 and 0.14.
- the medium has a birefringence in the range of from 0.090 to 0.110, preferably from 0.095 to 0.105, in particular from 0.100 to 0.105.
- the medium according to the invention has a birefringence of 0.120 or more, preferably in the range of from 0.125 to 0.145, more preferably from 0.130 to 0.140.
- the liquid-crystal mixture according to the invention has a dielectric anisotropy ⁇ ⁇ of -1.5 to -8.0, preferably of -2.0 to – 4.0, in particular -2.5 to -3.5,
- the rotational viscosity ⁇ 1 at 20°C is preferably ⁇ 120 mPa ⁇ s, in particular ⁇ 100 mPa ⁇ s.
- the rotational viscosity ⁇ 1 at 20°C is ⁇ 100mPa ⁇ s, in particular ⁇ 95 mPa ⁇ s.
- the liquid-crystal media according to the invention have relatively low val- ues for the threshold voltage (V 0 ).
- the term "threshold voltage” relates to the capa- citive threshold (V 0 ), also called the Freedericks threshold, unless explicitly indicated otherwise.
- the liquid-crystal media according to the invention have high values for the voltage holding ratio in liquid-crystal cells. In general, liquid-crystal media having a low addressing voltage or thresh- old voltage exhibit a lower voltage holding ratio than those having a higher addressing voltage or threshold voltage and vice versa.
- dielectrically positive compounds denotes compounds having a ⁇ ⁇ > 1.5
- dielectrically neutral com- pounds denotes those having -1.5 ⁇ ⁇ ⁇ ⁇ 1.5
- dielectrically negative compounds denotes those having ⁇ ⁇ ⁇ -1.5.
- the dielectric ani- sotropy of the compounds is determined here by dissolving 10 % of the compounds in a liquid-crystalline host and determining the capacitance of the resultant mixture in at least one test cell in each case having a layer thickness of 20 ⁇ m with homeotropic and with homogeneous surface alignment at 1 kHz.
- the measurement voltage is typically 0.5 V to 1.0 V, but is always lower than the capacitive threshold of the respective liquid- crystal mixture investigated. All temperature values indicated for the present invention are in °C.
- the LC media according to the invention are suitable for all VA-TFT (vertical alignment-thin film transistor) applications, such as, for example, VAN (vertically aligned nematic), MVA (multidomain VA), (S)-PVA (super patterned VA), ASV (advanced super view, or axially symmetric VA), or UV 2 A. They are furthermore suitable for IPS (in-plane switching) and FFS (fringe field switching) applications having negative ⁇ ⁇ .
- the nematic LC media in the displays according to the invention generally comprise two components NA and NB, which themselves consist of one or more individual compounds.
- Component NA has significantly negative dielectric anisotropy and gives the nematic phase a dielectric anisotropy of ⁇ -0.5.
- one or more compounds of the formula I it preferably comprises the compounds of the formulae IIA, IIB and/or IIC, furthermore one or more compounds of the formula IV-1.
- the proportion of component NA is preferably between 45 and 100 %, in particular between 60 and 85 %.
- component NA one (or more) individual compound(s) which has (have) a value of ⁇ ⁇ ⁇ -0.8 is (are) preferably selected.
- Component NB has pronounced nematogeneity and a flow viscosity of not greater than 30 mm 2 ⁇ s -1 , preferably not greater than 25 mm 2 ⁇ s -1 , at 20°C.
- Particularly preferred individual compounds in component NB are extremely low-viscosity nematic liquid crystals having a flow viscosity of not greater than 18 mm 2 ⁇ s -1 , preferably not greater than 12 mm 2 ⁇ s -1 , at 20°C.
- Component NB is monotropically or enantiotropically nematic, has no smectic phases and is able to prevent the occurrence of smectic phases down to very low temperatures in LC media. For example, if various materials of high nematogeneity are added to a smectic liquid-crystal mix- ture, the nematogeneity of these materials can be compared through the degree of suppression of smectic phases that is achieved.
- the mixture may optionally also comprise a component NC, comprising compounds having a dielectric anisotropy of ⁇ ⁇ ⁇ 1.5.
- the medium preferably comprises 4 to 15, in particular 5 to 12, and particularly preferably ⁇ 10, compounds of the formulae IIA, IIB and/or IIC and optionally one or more compounds of the formula IV-1.
- the other constituents are preferably selected from nematic or nemato- genic substances, in particular known substances, from the classes of the azoxybenzenes, benzylideneanilines, biphenyls, terphenyls, phenyl or cyclohexyl benzoates, phenyl or cyclohexyl cyclohexanecarboxylates, phenylcyclohexanes, cyclohexylbiphenyls, cyclohexylcyclohexanes, cyclo- hexylnaphthalenes, 1,4-biscyclohexylbiphenyls or cyclohexylpyrimidines, phenyl- or cyclohexyldioxanes, optionally halogenated stilbenes, benzyl phenyl ethers, tolanes and substituted cinnamic acid esters.
- R 20 and R 21 are different from one another, one of these radicals usually being an alkyl or alkoxy group.
- Other variants of the proposed substituents are also common. Many such substances or also mixtures thereof are commercially available. All these substances can be prepared by methods known from the literature. It goes without saying for the person skilled in the art that the VA, IPS or FFS mixture according to the invention may also comprise compounds in which, for example, H, N, O, Cl and F have been replaced by the corres- ponding isotopes.
- the combination of compounds of the preferred embodiments mentioned above with the polymerized compounds described above causes low threshold voltages, low rotational viscosities and very good low-tem- perature stabilities in the LC media according to the invention at the same time as constantly high clearing points and high HR values, and allows the rapid establishment of a particularly low tilt angle (i.e. a large tilt) in PSA displays.
- the LC media exhibit significantly shortened response times, in particular also the grey-shade response times, in PSA displays compared with the LC media from the prior art.
- the invention furthermore relates to an LC display comprising an LC medium as described above and below.
- the LC display is preferably a VA, IPS, FFS, UB-FFS or UV 2 A display.
- the structure of the displays according to the invention corresponds to the usual geometry for PSA displays, as described in the prior art cited at the outset. Geometries without protrusions are preferred, in particular those in which, in addition, the electrode on the colour filter side is unstructured and only the electrode on the TFT side has slots. Particularly suitable and preferred electrode structures for PS-VA displays are described, for example, in US 2006/0066793 A1.
- a preferred LC display of the present invention comprises: - a first substrate including a pixel electrode defining pixel areas, the pixel electrode being connected to a switching element disposed in each pixel area and optionally including a micro-slit pattern, and optionally a first alignment layer disposed on the pixel electrode, - a second substrate including a common electrode layer, which may be disposed on the entire portion of the second substrate facing the first substrate, and optionally a second alignment layer, - an LC layer disposed between the first and second substrates and including an LC medium as described above and below.
- the first and/or second alignment layer controls the alignment direction of the LC molecules of the LC layer.
- the alignment layer is selected such that it imparts to the LC molecules homeotropic (or vertical) alignment (i.e. perpendicular to the surface) or tilted alignment.
- Such an alignment layer may for example comprise a polyimide, which may also be rubbed, or may be prepared by a photoalignment method.
- UV 2 A displays the alignment layer is made by photopolymerization using linear polarized UV light and irradiation at an oblique angle.
- the LC layer with the LC medium can be deposited between the substrates of the display by methods that are conventionally used by display manufacturers, for example the so-called one-drop-filling (ODF) method.
- ODF one-drop-filling
- the LC display may comprise further elements, like a colour filter, a black matrix, a passivation layer, optical retardation layers, transistor elements for addressing the individual pixels, etc., all of which are well known to the person skilled in the art and can be employed without inventive skill.
- the electrode structure can be designed by the skilled person depending on the individual display type. For example for VA displays a multi-domain orientation of the LC molecules can be induced by providing electrodes having slits and/or bumps or protrusions in order to create two, four or more different tilt alignment directions.
- the the LC medium may also comprise, in addition to the compounds of formula I, one or more further stabilizers. Suitable types and amounts of stabilizers are known to the person skilled in the art and are described in the literature.
- the LC media contain one or more chiral dopants, preferably in a concentration from 0.01 to 1% by weight, very preferably from 0.05 to 1.0% by weight.
- the chiral dopants are preferably selected from the group consisting of compounds from Table B below, very preferably from the group consisting of R- or S-1011, R- or S-2011, R- or S-3011, R- or S-4011, and R- or S-5011.
- the LC media contain a racemate of one or more chiral dopants, which are preferably selected from the chiral dopants mentioned in the previous paragraph.
- a racemate of one or more chiral dopants which are preferably selected from the chiral dopants mentioned in the previous paragraph.
- it is possible to add to the LC media for example, 0 to 15% by weight of pleochroic dyes, furthermore nanoparticles, conductive salts, preferably ethyldimethyldodecylammonium 4-hexoxybenzoate, tetrabutyl- ammonium tetraphenylborate or complex salts of crown ethers (cf., for example, Haller et al., Mol. Cryst. Liq.
- Corresponding compounds of the formula ZK are described, for example, in DE-A- 2636 684 and DE-A-3321373.
- the LC media which can be used in accordance with the invention are prepared in a manner conventional per se, for example by mixing one or more of the above-mentioned compounds with one or more compounds of formula I as defined above, and optionally with further liquid-crystalline compounds and/or additives.
- the desired amount of the com- ponents used in lesser amount is dissolved in the components making up the principal constituent, advantageously at elevated temperature. It is also possible to mix solutions of the components in an organic solvent, for example in acetone, chloroform or methanol, and to remove the solvent again, for example by distillation, after thorough mixing.
- the invention furthermore relates to the process for the preparation of the LC media according to the invention.
- the LC media according to the invention may also comprise compounds in which, for example, H, N, O, Cl, F have been replaced by the corresponding isotopes like deuterium etc.
- the following examples explain the present invention without restricting it. However, they show the person skilled in the art preferred mixture con- cepts with compounds preferably to be employed and the respective con- centrations thereof and combinations thereof with one another. In addition, the examples illustrate which properties and property combinations are accessible. Preferred mixture components are shown in Table A below.
- m and n are independently of each other an integer from 1 to 12, preferably 1, 2, 3, 4, 5 or 6, k is 0, 1, 2, 3, 4, 5 or 6, and (O)C m H 2m+1 means C m H 2m+1 or OC m H 2m+1 .
- the LC media according to the invention comprise one or more compounds selected from the group consisting of compounds from Table A.
- Table B shows possible chiral dopants which can be added to the LC media according to the invention.
- the LC media preferably comprise 0 to 10% by weight, in particular 0.01 to 5% by weight, particularly preferably 0.1 to 3% by weight, of dopants.
- the LC media preferably comprise one or more dopants selected from the group consisting of compounds from Table B.
- Table C Table C shows possible stabilizers which can be added to the LC media according to the invention.
- n denotes an integer from 1 to 12, preferably 1, 2, 3, 4, 5, 6, 7 or 8, and terminal methyl groups are not shown.
- the LC media preferably comprise 0 to 10% by weight, in particular 1 ppm to 5% by weight, particularly preferably 1 ppm to 1% by weight, of stabilizers.
- the LC media preferably comprise one or more stabilizers selected from the group consisting of compounds from Table C. Examples The following examples explain the present invention without restricting it. However, they show the person skilled in the art preferred mixture con- cepts with compounds preferably to be employed and the respective con- centrations thereof and combinations thereof with one another. In addition, the examples illustrate which properties and property combinations are accessible.
- threshold voltage for the present invention relates to the capa- citive threshold (V 0 ), also known as the Freedericks threshold, unless explicitly indicated otherwise.
- the optical threshold may also, as generally usual, be quoted for 10% relative contrast (V 10 ).
- nematic LC host mixture N1 is formulated as follows Stabilised mixture S1.1 is prepared by adding 100ppm of the compound M1 according to formula I to the nematic LC host mixture N1. Stabilised mixture S1.2 is prepared by adding 200ppm of the compound M1 according to formula I to the nematic LC host mixture N1.
- the mixture C1 is prepared by adding 300ppm of the compound M1 to the nematic LC host mixture N1.
- Example 2 The nematic LC host mixture N2 is formulated as follows Stabilised mixture S2 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N2.
- Example 3 The nematic LC host mixture N3 is formulated as follows
- Stabilised mixture S3 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N3.
- Example 4 The nematic LC host mixture N4 is formulated as follows Stabilised mixture S4.1 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N4.
- Stabilised mixture S4.2 is prepared by adding 200ppm of the compound M1 to the nematic LC host mixture N4.
- Example 5 The nematic LC host mixture N5 is formulated as follows
- Stabilised mixture S5 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N5.
- Example 6 The nematic LC host mixture N6 is formulated as follows Stabilised mixture S6 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N6.
- Example 7 The nematic LC host mixture N7 is formulated as follows
- Stabilised mixture S7 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N7.
- Example 8 The nematic LC host mixture N8 is formulated as follows Stabilised mixture S8 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N8.
- Example 9 The nematic LC host mixture N9 is formulated as follows
- Stabilised mixture S9 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N9.
- Example 10 The nematic LC host mixture N10 is formulated as follows Stabilised mixture S10.1 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N10.
- Stabilised mixture S10.2 is prepared by adding 200ppm of the compound M1 to the nematic LC host mixture N10.
- Example 11 The nematic LC host mixture N11 is formulated as follows
- Stabilised mixture S11 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N11.
- VHR of stabilised mixtures S1.1 and S1.2 according to the present invention containing the compound M1 is measured compared to the pure host mixture N1.
- VHR Measurement The test cells used for VHR measurement consist of two plane-parallel glass outer plates at a separation of 3.2 ⁇ m, each of which has on the inside an electrode layer and a polyimide alignment layer on top, where the two polyimide layers effect homeotropic alignment of the liquid crystal molecules and photoalignment is applied to induce tilt directions at the two substrates crossed to one another. Six test cells are prepared for each LC mixture. After filling in the LC mixtures the cells are sealed by a sealent.
- VHR of host mixture N1 and mixtures S1.1 and S1.2 is measured at 60°C with application of a voltage of 1 V / 0.6 Hz before and after 120 hours of BL stress at 60°C.
- Light and thermal stress usually causes the generation of impurity and ion which decrease of VHR in LC mixtures, therefore the smaller the absolute decrease of VHR value after stress, the better the performance for display applications.
- Table 1 - VHR From Table 1 it can be seen that for mixtures S1.1 and S1.2 according to the present invention the initial VHR is already higher than the initial VHR of host mixture N1 without the compound M1.
- the tilt angle generation of stabilised mixture S1.1 according to the present invention is measured compared to the pure host mixture N1 and to comparison mixture C1 with a higher amount of compound M1.
- the test cells used for tilt angle measurement consist of two plane-parallel glass outer plates at a separation of 3.2 ⁇ m, each of which has on the inside an electrode layer and a polyimide alignment layer on top, where the two polyimide layers effect homeotropic alignment of the liquid crystal molecules and photoalignment is applied to induce tilt directions at the two substrates anti-parallel to one another.
- the LC mixtues are filled into the text cells which are then sealed by a sealent.
- the tilt angle generated in host mixture N1 and mixtures S1.1 and C1 is measured before and after exposure to BL and electric stress of 60 Vpp for 120h using the Mueller Matrix Polarimeter “AxoScan” from Axometrics.
- the tilt angle variation i.e. the change of the tilt angle ⁇ tilt in absolute values after stress is shown in Table 2 below.
- Table 2 – Tilt Variation From Table 2 it can be seen that mixture S1.1 according to the present invention with 100ppm of compound M1 shows a small tilt angle change which is similar to host mixture N1, which means that no significant tilt angle has been generated by the compound M1 at this concentration.
- comparison mixture C1 with 300ppm of compound M1 shows a stronger tilt angle change indicating that a tilt angle has been generated.
Landscapes
- Chemical & Material Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Liquid Crystal (AREA)
Abstract
The present invention relates to a liquid-crystal (LC) medium comprising a stabiliser, to its use for optical, electro-optical and electronic purposes, in particular in LC displays, especially in LC displays of the vertically aligned mode, to an LC display of the vertically aligned mode comprising the LC medium, and to a process of manufacturing the LC display.
Description
Liquid-Crystal Medium The present invention relates to a liquid-crystal (LC) medium comprising a stabiliser, to its use for optical, electro-optical and electronic purposes, in particular in LC displays, especially in LC displays of the vertically aligned mode, to an LC display of the vertically aligned mode comprising the LC medium, and to a process of manufacturing the LC display. The popularity of 8K and gaming monitors leads to an increased need for LC display (LCD) panels having higher refresh rates and thus for LC media having faster response times. Many of these LCD panels are using display modes wherein the LC molecules are aligned substantially perpendicular or slightly tilted relative to the electrode surface in the switched-off state. Thus, so-called VA (“vertically aligned”) displays are known which have a broad viewing angle and fast response times. The LC cell of a VA display contains a layer of an LC medium between two transparent electrodes, where the LC medium usually has a negative value of the dielectric anisotropy ( Δε). In the switched-off state, the molecules of the LC layer are aligned perpendicular to the electrode surfaces (homeotropically) or have a tilted homeotropic alignment. On application of a voltage to the two electrodes, a realignment of the LC molecules parallel to the electrode surfaces takes place. Furthermore, so-called FFS (“fringe-field switching”) displays have been reported (see, inter alia, S.H. Jung et al., Jpn. J. Appl. Phys., Volume 43, No.3, 2004, 1028), which contain two electrodes on the same substrate, one of which is structured in a comb-shaped manner and the other is unstructured. A strong, so-called "fringe field" is thereby generated, i.e. a strong electric field close to the edge of the electrodes, and, throughout the cell, an electric field which has both a strong vertical component and also a strong horizontal component. FFS displays have a low viewing-angle dependence of the contrast. FFS displays usually contain an LC medium with positive dielectric anisotropy, and an alignment layer, usually of polyimide, which provides planar alignment to the molecules of the LC medium.
FFS displays can be operated as active-matrix or passive-matrix displays. In the case of active-matrix displays, individual pixels are usually addressed by integrated, non-linear active elements, such as, for example, transistors (for example thin-film transistors ("TFTs")), while in the case of passive-matrix displays, individual pixels are usually addressed by the multiplex method, as known from the prior art. Also known are so-called IPS (“in-plane switching”) displays, which contain an LC layer between two substrates with planar orientation, where the two electrodes are arranged on only one of the two substrates and preferably have interdigitated, comb-shaped structures. On application of a voltage to the electrodes an electric field with a significant component parallel to the LC layer is generated between them. This causes realignment of the LC molecules in the layer plane. Furthermore, FFS displays have been disclosed (see S.H. Lee et al., Appl. Phys. Lett.73(20), 1998, 2882-2883 and S.H. Lee et al., Liquid Crystals 39(9), 2012, 1141-1148), which have similar electrode design and layer thickness as FFS displays, but comprise a layer of an LC medium with negative dielectric anisotropy instead of an LC medium with positive dielectric anisotropy. The LC medium with negative dielectric anisotropy shows a more favourable director orientation that has less tilt and more twist orientation compared to the LC medium with positive dielectric anisotropy, as a result of which these displays have a higher transmission. Furthermore VA displays have been disclosed which use an alignment layer that is prepared by photoalignment, also known as UV2A mode (see e.g. Q. Tang et al., SID Symposium Digest of Technical Papers 2018, 414-417). These displays utilize an alignment layer prepared from crosslinkable and photoorientable monomers or prepolymers, e.g. cinnamate chromophores which are irradiated obliquely with linearly polarized UV light. As a result a crosslinked alignment layer is formed which induces uniaxial alignment with a pretilt angle in the LC molecules close to its surface. By changing the irradiation direction a multidomain configuration with different pretilt directions can be obtained.
However, the use of LC media with negative dielectric anisotropy in VA or FFS displays has also several drawbacks. For example, they have a significantly lower reliability compared to LC media with positive dielectric anisotropy. The term "reliability" as used hereinafter means the quality of the performance of the display during time and with different stress loads, such as light load, temperature, humidity, or voltage which cause display defects such as image sticking (area and line image sticking), mura, yogore etc. and which are known to the skilled person in the field of LC displays. As a standard parameter for categorising the reliability usually the voltage holding ration (VHR) value is used, which is a measure for maintaining a constant electrical voltage in a test display. The higher the VHR value, the better the reliability of the medium. The reduced reliability of an LC medium with negative dielectric anisotropy in a VA or FFS display can be explained by an interaction of the LC molecules with the polyimide of the alignment layer, as a result of which ions are extracted from the polyimide alignment layer, and wherein LC molecules with negative dielectric anisotropy do more effectively extract such ions. This results in new requirements for LC media to be used in VA or FFS displays. In particular, the LC medium has to show a high reliability and a high VHR value after UV exposure. Further requirements are a high specific resistance, a large working-temperature range, short response times even at low temperatures, a low threshold voltage, a multiplicity of grey levels, high contrast and a broad viewing angle, and reduced image sticking. Thus, in displays known from prior art often the undesired effect of so- called "image sticking" or "image burn" is observed, wherein the image produced in the LC display by temporary addressing of individual pixels still remains visible even after the electric field in these pixels has been switched off, or after other pixels have been addressed.
This "image sticking" can occur on the one hand if LC media having a low VHR are used. The UV component of daylight or the backlight can cause undesired decomposition reactions of the LC molecules therein and thus initiate the production of ionic or free-radical impurities. These may accumulate, in particular, at the electrodes or the alignment layers, where they may reduce the effective applied voltage. Another problem observed in prior art is that LC media for use in displays, including but not limited to VA and FFS displays, do often exhibit high viscosities and, as a consequence, high switching times. In order to reduce the viscosity and switching time of the LC medium, it has been suggested in prior art to add LC compounds with an alkenyl group. However, it was observed that LC media containing alkenyl compounds often show a decrease of the reliability and stability, and a decrease of the VHR especially after exposure to UV radiation but also to visible light from the backlight of a display, that usually does not emit UV light. In order to reduce the decrease of the reliability and stability, the use of stabilisers was proposed, such as for example compounds of the HALS- (hindered amine light stabiliser) type. A typical example is Tinuvin 770, a compound of the formula
Nevertheless, these LC mixtures can still exhibit insufficient reliability during the operation of a display, e.g. upon irradiation with the typical CCFL-(Cold Cathode Fluorescent Lamp) backlight. A different class of compound used for the stabilisation of liquid crystals are antioxidants derived from phenol, such as for example the compound
as described in DE 19539141 A1. Such stabilisers can be used to stabilise LC mixtures against heat or the influence of oxygen but typically do not show advantages under light stress. Because of the complex modes of action of the different kinds of stabilisers and minute effects in a display, where the liquid crystal, a complex mixture of many different types of compounds itself, interacts with different kinds of species, including the polyimide, it is a challenging task also for the skilled person to choose the right stabiliser in order to identify the best material combination. Hence, there is still great demand for new types of stabilisers with different properties in order to broaden the range of applicable materials. It is therefore an object of the present invention to provide a process for providing improved LC media for use in VA-, IPS- or FFS displays, which do not exhibit the disadvantages described above or only do so to a small extent and have improved properties. A further object of the invention is to provide FFS displays with good transmission, high reliability, a VHR value especially after backlight exposure, a high specific resistance, a large working-temperature range, short response times even at low tempera- tures, a low threshold voltage, a multiplicity of grey levels, high contrast and a broad viewing angle, and reduced image sticking. It was found that one or more of these objects could be achieved by providing an LC medium as disclosed and claimed hereinafter. In particular, the inventors of the present invention have found that the above objects can be achieved by using an LC medium comprising a small amount of a stabiliser, which is a compound of formula I as described hereinafter, in a VA-, IPS or FFS display. It has also been found that when
using such stabilisers in an LC medium for use in an FFS display, surprisingly the reliability and the VHR value after backlight load are higher, compared to an LC medium without a stabiliser according to the present invention. Also, the use of an LC medium comprising a stabiliser as described hereinafter allows to exploit the known advantages of alkenyl-containing LC media, like reduced viscosity and faster switching time, and at the same time leads to improved reliability and high VHR value especially after backlight exposure. The invention thus relates to an LC medium comprising one or more compounds of formula I in a concentration of >0% and ≤0.02% by weight
wherein the individual radicals, independently of each other and on each occurrence identically or differently, have the following meanings P CW=CH-CO-O-, W H, F, Cl, CF3 or alkyl with 1 to 5 C atoms, preferably H or CH3, Sp1, Sp2, Sp3 a spacer group which is optionally substituted by P, or a single bond, L F, Cl, -CN, P-Sp- or straight chain, branched or cyclic alkyl having 1 to 25 C atoms, wherein one or more non-adjacent CH2-groups are optionally replaced by -O-, -S-, -CO-, -CO- O-, -O-CO-, -O-CO-O-, CR0=CR00-, -C ≡C-,
in such a manner that O- and/or S-atoms are not directly connected with each other, and wherein one or more H atoms are each optionally replaced by P-Sp-, F or Cl, r, s 0, 1, 2, 3 or 4, preferably 0, 1 or 2, and further comprising one or more compounds of formula II
wherein the individual radicals, independently of each other and on each occurrence identically or differently, have the following meanings R1, R2 straight chain, branched or cyclic alkyl having 1 to 25 C atoms, wherein one or more non-adjacent CH2-groups are optionally replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, CR0=CR00-, -C ≡C-,
in such a manner that O- and/or S-atoms are not directly connected with each other, and wherein one or more H atoms are each optionally replaced by F or Cl, preferably alkyl or alkoxy having 1 to 6 C atoms, A1, A2 a group selected from the following formulae
preferably from formulae A1, A2, A3, A4, A5, A6, A9 and A10, very preferably from formulae A1, A2, A3, A4, A5, A9 and A10, Z1, Z2 -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -CO-O-, -O-CO-, -C2F4-, -CF=CF-, -CH=CH-CH2O- or a single bond, preferably a single bond, L1, L2, L3, L4 F, Cl, OCF3, CF3, CH3, CH2F or CHF2, preferably F or Cl, very preferably F, Y H, F, Cl, CF3, CHF2 or CH3, preferably H or CH3, very preferably H, LC CH3 or OCH3, preferably CH3,
a1 1 or 2, a2 0 or 1. The invention further relates to the use of the LC medium as described above and below in LC displays, preferably in LC displays of the VA, IPS, FFS, UB-FFS or UV2A mode. The LC medium has negative dielectric anisotropy. The invention furthermore relates to a process for preparing an LC medium as described above and below, comprising the steps of mixing one or more compounds of formula I with one or more compounds of formula II and optionally with further LC compounds and/or additives. The invention furthermore relates to an LC display comprising an LC medium according to the invention as described above and below, preferably an LC display of the VA, IPS, FFS, UB-FFS or UV2A mode. The invention furthermore relates to a process for manufacturing an LC display as described above and below, comprising the steps of filling or otherwise providing an LC medium as described above and below between the substrates of the display. Surprisingly it was found that the compounds of formula I, although they carry potentially reactive groups P like acrylate or methacrylate, quite contrary to being harmful in terms of reliability of the LC, are able to stabilise LC mixtures under light stress. It was also found that, if the concentration of the compounds of formula I is kept low enough, an undesired generation of a pretilt angle in the LC medium, which is usually observed when using such compounds in VA mode displays and exposing them to UV irradiation, can be suppressed. It was also found that when using compounds of formula I in an LC medium for use in a VA or FFS mode display, surprisingly the reliability
and the VHR value after backlight load are higher, compared to an LC medium without a compound of formula I according to the present invention. Also, the use of an LC medium comprising a compound of formula I as described hereinafter allows to exploit the known advantages of alkenyl- containing LC media, like reduced viscosity and faster switching time, and at the same time leads to improved reliability and high VHR value especially after backlight exposure. Unless stated otherwise, the compounds of formula I are preferably selected from achiral compounds. As used herein, the terms "active layer" and "switchable layer" mean a layer in an electrooptical display, for example an LC display, that comprises one or more molecules having structural and optical anisotropy, like for example LC molecules, which change their orientation upon an external stimulus like an electric or magnetic field, resulting in a change of the transmission of the layer for polarized or unpolarized light. As used herein, the terms "tilt" and "tilt angle" will be understood to mean a tilted alignment of the LC molecules of an LC medium relative to the surfaces of the cell in an LC display (here preferably a PSA display), and will be understood to be inclusive of "pretilt" and "pretilt angle". The tilt angle here denotes the average angle (< 90°) between the longitudinal molecular axes of the LC molecules (LC director) and the surface of the plane-parallel outer plates which form the LC cell. A low absolute value for the tilt angle (i.e. a large deviation from the 90° angle) corresponds to a large tilt here. A suitable method for measurement of the tilt angle is given in the examples. Unless indicated otherwise, tilt angle values disclosed above and below relate to this measurement method. The term "mesogenic group" as used herein is known to the person skilled in the art and described in the literature, and means a group which, due to the anisotropy of its attracting and repelling interactions, essentially contributes to causing a liquid-crystal (LC) phase in low-molecular-weight
or polymeric substances. Compounds containing mesogenic groups (mesogenic compounds) do not necessarily have to have an LC phase themselves. It is also possible for mesogenic compounds to exhibit LC phase behaviour only after mixing with other compounds and/or after polymerization. Typical mesogenic groups are, for example, rigid rod- or disc-shaped units. An overview of the terms and definitions used in connection with mesogenic or LC compounds is given in Pure Appl. Chem. 2001, 73(5), 888 and C. Tschierske, G. Pelzl, S. Diele, Angew. Chem. 2004, 116, 6340-6368. The term "spacer group", hereinafter also referred to as "Sp", as used herein is known to the person skilled in the art and is described in the literature, see, for example, Pure Appl. Chem.2001, 73(5), 888 and C. Tschierske, G. Pelzl, S. Diele, Angew. Chem.2004, 116, 6340-6368. As used herein, the terms "spacer group" or "spacer" mean a flexible group, for example an alkylene group, which connects the mesogenic group and the polymerizable group(s) in a polymerizable mesogenic compound. Above and below, denotes a trans-1,4-cyclohexylene ring,
and
denotes a 1,4-phenylene ring. In a group
the single bond shown between the two ring atoms can be attached to any free position of the benzene ring. If in the formulae shown above and below a group R1-12, RQ, R or L denotes an alkyl radical and/or an alkoxy radical, this may be straight- chain or branched. It is preferably straight-chain, has 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes ethyl, propyl, butyl, pentyl, hexyl, heptyl, ethoxy, propoxy, butoxy, pentoxy, hexyloxy or heptyloxy, furthermore methyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetra- decyl, pentadecyl, methoxy, octyloxy, nonyloxy, decyloxy, undecyloxy, dodecyloxy, tridecyloxy or tetradecyloxy.
If in the formulae shown above and below a group R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L denotes an alkyl radical and/or an alkoxy radical, this may be straight-chain or branched. It is preferably straight-chain, has 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes ethyl, propyl, butyl, pentyl, hexyl, heptyl, ethoxy, propoxy, butoxy, pentoxy, hexyloxy or heptyloxy, furthermore methyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl, methoxy, octyloxy, nonyloxy, decyloxy, undecyloxy, dodecyloxy, tridecyloxy or tetradecyloxy. If in the formulae shown above and below a group R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L denotes an alkyl radical wherein one or more CH2 groups are replaced by S, this may be straight-chain or branched. It is preferably straight-chain, has 1, 2, 3, 4, 5, 6 or 7 C atoms and accordingly preferably denotes thiomethyl, thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl or thioheptyl. Oxaalkyl preferably denotes straight-chain 2-oxapropyl (= methoxymethyl), 2- (= ethoxymethyl) or 3-oxabutyl (= 2-methoxyethyl), 2-, 3- or 4-oxapentyl, 2-, 3-, 4- or 5-oxahexyl, 2-, 3-, 4-, 5- or 6-oxaheptyl, 2-, 3-, 4-, 5-, 6- or 7- oxaoctyl, 2-, 3-, 4-, 5-, 6-, 7- or 8-oxanonyl, 2-, 3-, 4-, 5-, 6-, 7-, 8- or 9-oxa- decyl. If in the formulae shown above and below a group R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L denotes an alkoxy or oxaalkyl group it may also contain one or more additional oxygen atoms, provided that oxygen atoms are not linked directly to one another. In another preferred embodiment, one or more of R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L are selected from the group consisting of
-S1-F, -O-S1-F, -O-S1-O-S2, wherein S1 is C1-12-alkylene or C2-12- alkenylene and S2 is H, C1-12-alkyl or C2-12-alkenyl, and very preferably are selected from the group consisting of
-OCH2OCH3, -O(CH2)2OCH3, -O(CH2)3OCH3, -O(CH2)4OCH3, -O(CH2)2F, - O(CH2)3F and -O(CH2)4F. If in the formulae shown above and below a group R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L denotes an alkyl radical in which one CH2 group has been replaced by -CH=CH-, this may be straight-chain or branched. It is preferably straight-chain and has 2 to 10 C atoms. Accordingly, it denotes, in particular, vinyl, prop-1- or -2-enyl, but-1-, -2- or -3-enyl, pent-1-, -2-, -3- or -4-enyl, hex-1-, -2-, -3-, -4- or -5- enyl, hept-1-, -2-, -3-, -4-, -5- or -6-enyl, oct-1-, -2-, -3-, -4-, -5-, -6- or -7- enyl, non-1-, -2-, -3-, -4-, -5-, -6-, -7- or -8-enyl, dec-1-, -2-, -3-, -4-, -5-, -6-, -7-, -8- or -9-enyl. If in the formulae shown above and below a group R1-13, R51, R52, RQ, R, R2A, R2B, RIIIA, R1N, R2N, RB1, RB2, RCR1, RCR2, R or L denotes an alkyl or alkenyl radical which is at least monosubstituted by halogen, this radical is preferably straight-chain, and halogen is preferably F or Cl. In the case of polysubstitution, halogen is preferably F. The resultant radicals also include perfluorinated radicals. In the case of monosubstitution, the fluorine or chlorine substituent may be in any desired position, but is preferably in the ω-position. Halogen is preferably F or Cl, very preferably F.
The group -CR0=CR00- is preferably -CH=CH-. -OC-, -CO-, -C(=O)- and -C(O)- denote a carbonyl group, i.e.
Preferred substituents L, are, for example, F, Cl, Br, I, -CN, -NO2, -NCO, - NCS, -OCN, -SCN, -C(=O)N(Rx)2, -C(=O)Y1, -C(=O)Rx, -N(Rx)2, straight- chain or branched alkyl, alkoxy, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or alkoxycarbonyloxy each having 1 to 25 C atoms, in which one or more H atoms may optionally be replaced by F or Cl, optionally substituted silyl having 1 to 20 Si atoms, or optionally substituted aryl having 6 to 25, preferably 6 to 15, C atoms, wherein Rx denotes H, F, Cl, CN, or straight chain, branched or cyclic alkyl having 1 to 25 C atoms, wherein one or more non-adjacent CH2-groups are optionally replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a manner that O- and/or S-atoms are not directly connected with each other, and wherein one or more H atoms are each optionally replaced by F, Cl, P- or P-Sp-, and Y1 denotes halogen. Particularly preferred substituents L are, for example, F, Cl, CN, NO2, CH3,C2H5, OCH3, OC2H5, COCH3, COC2H5, COOCH3, COOC2H5,CF3, OCF3, OCHF2, OC2F5, furthermore phenyl.
is preferably
in which L has one of the meanings indicated above. In the compounds of formula I and its subformulae the group P is preferably selected from the group consisting of acrylate, methacrylate, fluoroacrylate and chloroacrylate, more preferably from acrylate and methacrylate, most preferably P denotes methacrylate.
Very preferably all groups P in the compound of formula I and its subformulae have the same meaning, and very preferably denote acrylate or methacrylate, most preferably methacrylate. If one of the spacer groups Sp1-3 is different from a single bond, it is preferably of the formula Sp"-X", so that the respective radical P-Sp- conforms to the formula P-Sp"-X"-, wherein Sp" denotes linear or branched alkylene having 1 to 20, preferably 1 to 12, C atoms, which is optionally mono- or polysubstituted by F, Cl, Br, I or CN and in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -O-, -S-, -NH-, -N(R0)-, -Si(R0R00)-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, -S- CO-, -CO-S-, -N(R00)-CO-O-, -O-CO-N(R0)-, -N(R0)-CO-N(R00)- , -CH=CH- or -C ≡C- in such a way that O and/or S atoms are not linked directly to one another, X" denotes -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, -CO-N(R0)- , -N(R0)-CO-, -N(R0)-CO-N(R00)-, -OCH2-, -CH2O-, -SCH2-, -CH2S- , -CF2O-, -OCF2-, -CF2S-, -SCF2-, -CF2CH2-, -CH2CF2-, -CF2CF2- , -CH=N-, -N=CH-, -N=N-, -CH=CR0-, -CY2=CY3-, -C ≡C-, -CH=CH- CO-O-, -O-CO-CH=CH- or a single bond, R0 and R00 each, independently of one another, denote H or alkyl having 1 to 20 C atoms, and Y2 and Y3 each, independently of one another, denote H, F, Cl or CN. X" is preferably -O-, -S-, -CO-, -COO-, -OCO-, -O-COO-, -CO-NR0-, -NR0- CO-, -NR0-CO-NR00- or a single bond. Typical spacer groups Sp1-3 and -Sp"-X"- are, for example, -(CH2)p1-, - (CH2)p1-O-, -(CH2)p1-O-CO-, -(CH2)p1-CO-O-, -(CH2)p1-O-CO-O-, - (CH2CH2O)q1-CH2CH2-, -CH2CH2-S-CH2CH2-, -CH2CH2-NH-CH2CH2- or - (SiR0R00-O)p1-, in which p1 is an integer from 1 to 12, q1 is an integer from 1 to 3, and R0 and R00 have the meanings indicated above.
Particularly preferred groups Sp1-3 and -Sp"-X"- are -(CH2)p1-, -(CH2)p1-O-, - (CH2)p1-O-CO-, -(CH2)p1-CO-O-, -(CH2)p1-O-CO-O-, in which p1 and q1 have the meanings indicated above. Particularly preferred groups Sp" are, in each case straight-chain, ethylene, propylene, butylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, octadecylene, ethyleneoxyethylene, methyleneoxybutylene, ethylenethioethylene, ethylene-N-methyliminoethylene, 1-methylalkylene, ethenylene, propenylene and butenylene. Preferred compounds of formula I and its subformulae are those wherein at least one of the groups Sp1-3 is a single bond. Further preferred compounds or formula I and its subformulae are those wherein at least one of the groups Sp1-3 is different from a single bond. Very preferred are compounds of formula I and its subformulae, wherein Sp1-3 are selected from the group consisting of a single bond, -(CH2)p1-, - O-(CH2)p1-, -O-CO-(CH2)p1 and -CO-O-(CH2)p1, wherein p1 is 2, 3, 4, 5 or 6, and, if Sp is -O-(CH2)p1-, -O-CO-(CH2)p1 or -CO-O-(CH2)p1 the O-atom or CO-group, respectively, is linked to the benzene ring. Further preferred compounds of formula I and its subformulae as described above and below are selected from the following preferred embodiments, including any combination thereof: - all groups P have the same meaning, and very preferably denote acrylate or methacrylate, most preferably methacrylate, - all of Sp1-3 are a single bond - all of Sp1-3 are different from a single bond, - one of Sp1-3 is a single bond and the other two are different from a single bond, - one of Sp1-3 is different from a single bond and the other two each denote a single bond,
- Sp1 is a single bond and one or both of Sp2 and Sp3, preferably both of Sp2 and Sp3, are different from a single bond, - Sp1-3, when being different from a single bond, are selected from the group consisting of -(CH2)2-, -(CH2)3-, -(CH2)4-, -O-(CH2)2-, -O-(CH2)3-, - O-CO-(CH2)2 and -CO-O-(CH)2-, wherein the O atom or the CO group is attached to the benzene ring, - at least one of the groups Sp1-3 is different from a single bond, and is selected from the group consisting of -(CH2)2-, -(CH2)3-, -(CH2)4-, -O- (CH2)2-, -O-(CH2)3-, -O-CO-(CH2)2 and -CO-O-(CH)2-, wherein the O atom or the CO group is attached to the benzene ring, - Sp1-3, when being different from a single bond, denote alkylene with 2 to 6 C atoms, - r and s are independently of each other 0, 1 or 2, and the sum of r and s is 0, 1 or 2 - r=s=0 or r=0 and s=1 or r=1 and s=0 or r=s=1, - L denotes F, Cl, CN or OCH3, very preferably F or OCH3. Further preferred compounds of formula I are selected from the following subformulae:
wherein P and L have the meanings of formula I or one of the preferred meanings give above and below, and Sp’ has one of the meanings of Sp as given formula I, or one of the preferred meanings give above and below, which is different from a single bond. Sp’ in formulae I1 to I8 is preferably selected from the group consisting of - (CH2)2-, -(CH2)3-, -(CH2)4-, -O-(CH2)2-, -O-(CH2)3-, -O-CO-(CH2)2 and -CO- O-(CH)2-, wherein the O atom or the CO group is attached to the benzene ring. L in formulae I1 to I8 preferably denotes F, Cl, CN or OCH3, very preferably F or OCH3.
Very preferred compounds of formula I are selected from the following subformulae:
Further preferred are compounds of formula I1a to I6a wherein one, two or three of the methacrylate groups are replaced by acrylate groups. The concentration of the compounds of formula I and its subformulae in the LC medium is preferably from 0.001 to 0.02%, very preferably from 0.002 to 0.015%, most preferably from 0.005 to 0.015%. In another rpreferred embodiment the concentration of the compounds of formula I and its subformulae in the LC medium is from 10 to 250ppm, preferably from 20 to 200ppm, most preferably from 50 to 150 ppm.
The compounds of the formula I can be prepared analogously to processes known to the person skilled in the art and described in standard works of organic chemistry, such as, for example, in Houben-Weyl, Methoden der organischen Chemie [Methods of Organic Chemistry], Thieme-Verlag, Stuttgart. For example, acrylic or methacrylic esters can be prepared by esterification of the corresponding alcohols with acid derivatives like, for example, (meth)acryloyl chloride or (meth)acrylic anhydride in the presence of a base like pyridine or triethyl amine, and 4-(N,N-dimethylamino)pyridine (DMAP). Alternatively the esters can be prepared by esterification of the alcohols with (meth)acrylic acid in the presence of a dehydrating reagent, for example according to Steglich with dicyclohexylcarbodiimide (DCC), N-(3- dimethylaminopropyl)-N’-ethylcarbodiimide (EDC) or N-(3- dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride and DMAP. Besides the compounds of formula I described above, the LC media for use in the LC displays according to the invention comprise an LC mixture ("host mixture") comprising one or more, preferably two or more LC compounds, at least one of which is a compound of formula II. Particularly preferred embodiments of such an LC medium are shown below. Preferably the LC medium contains one or more compounds of formula II selected from the group consisting of compounds of the formulae IIA, IIB, IIC and IID
in which R2A and R2B each, independently of one another, denote H, an alkyl or alkenyl radical having up to 15 C atoms which is unsubstituted, monosubstituted by CN or CF3 or at least monosubstituted by halogen, where, in addition, one or more CH2 groups in these radicals may be replaced by -O-, -S-,
-C ≡C-, -CF2O-, -OCF2-, -OC-O- or -O-CO- in such a way that O atoms are not linked directly to one another, L1 to L4 each, independently of one another, denote F, Cl, CF3 or CHF2, Y denotes H, F, Cl, CF3, CHF2 or CH3, preferably H or CH3, particularly preferably H,
Z2, Z2B and Z2D each, independently of one another, denote a single bond, -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, - COO-, -OCO-, -C2F4-, -CF=CF-, -CH=CHCH2O-, p denotes 0, 1 or 2, and q on each occurrence, identically or differently, denotes 0 or 1. Preferred compounds of the formulae IIA, IIB, IIC and IID are those wherein R2B denotes an alkyl or alkoxy radical having up to 15 C atoms, and very preferablydenotes (O)CvH2v+1 wherein (O) is an oxygen atom or a single bond and v is 1, 2, 3, 4, 5 or 6. Further preferred compounds of the formulae IIA, IIB, IIC and IID are those wherein R2A or R2B denotes or contains cycloalkyl or cycloalkoxy radical, preferably selected from the group consisting of
wherein S1 is C1-5-alkylene or C2-5-alkenylene and S2 is H, C1-7-alkyl or C2- 7-alkenyl, and very preferably are selected from the group consisting of
In a preferred embodiment the LC medium comprises one or more compounds of the formula IIA selected from the group consisting of formulae IIA-1 to IIA-76,
in which the index a denotes 1 or 2, “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, “alkenyl” denotes a straight-chain alkenyl radical having 2-6 C atoms, and (O) denotes an oxygen atom or a single bond. “alkenyl” preferably denotes CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2- CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH- (CH2)2-. Particularly preferred LC media according to the invention comprise one or more compounds selected from the group consisting of formulae IIA-2, IIA- 8, IIA-10, IIA-16, II-18, IIA-40, IIA-41, IIA-42 and IIA-43. In another preferred embodiment the LC medium comprises one or more compounds of the formula IIB selected from the group consisting of formulae IIB-1 to IIB-26,
in which “alkyl”, “alkyl*”, “alkenyl” and (O) have the meanings given above. Particularly preferred LC media according to the invention comprise one or more compounds selected from the group consisting of formulae IIB-2, IIB- 10 and IIB-16. In another preferred embodiment the LC medium comprises one or more compounds of the formula IIC selected from the formula IIC-1,
in which “alkyl” and “alkyl*” and (O) have the meanings given above. In another preferred embodiment the LC medium comprises one or more compounds of the formula IID selected from the group consisting of formulae IID-1 to IID-10,
in which “alkyl”, “alkyl*”, “alkenyl” and (O) have the meanings given above. Particularly preferred LC media according to the invention comprise one or more compounds of the formula IID-4. The proportion of compounds of the formulae IIA and/or IIB in the mixture as a whole is preferably at least 20 % by weight. In another preferred embodiment the LC medium comprises one or more compounds of formula III
in which R11 and R12 each, independently of one another, denote H, an alkyl or alkoxy radical having 1 to 15 C atoms, where one or more
CH2 groups in these radicals may each be replaced, independently of one another, by
-O-, -S-, -C ≡C-, -CF2O-, -OCF2-, -CH=CH-, -OC-O- or -O-CO- in such a way that O atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by halogen, A3 on each occurrence, independently of one another, denotes a) 1,4-cyclohexenylene or 1,4-cyclohexylene radical, in which one or two non-adjacent CH2 groups may be replaced by -O- or -S-, b) a 1,4-phenylene radical, in which one or two CH groups may be replaced by N, or c) a radical selected from the group consisting of spiro[3.3]heptane-2,6-diyl, 1,4-bicyclo[2.2.2]octylene, naphthalene-2,6-diyl, decahydronaphthalene-2,6-diyl, 1,2,3,4-tetrahydronaphthalene-2,6-diyl, phenanthrene- 2,7-diyl and fluorene-2,7-diyl, wherein the radicals a), b) and c) may be mono- or polysubstituted by halogen atoms, n denotes 0, 1 or 2, preferably 0 or 1, Z1 on each occurrence independently of one another denotes - CO-O-, -O-CO-, -CF2O- , -OCF2-, -CH2O-, -OCH2-, -CH2-, - CH2CH2-, -(CH2)4-, -CH=CH-CH2O-, -C2F4-, -CH2CF2-, - CF2CH2 -, -CF=CF-, -CH=CF-, -CF=CH-, -CH=CH-, -C■C- or a single bond, and
L11 and L12 each, independently of one another, denote F, Cl, CF3 or CHF2, preferably H or F, most preferably F, and W denotes O or S. In a preferred embodiment of the present invention the LC medium comprises one or more compounds of the formula III-1 and/or III-2
in which the occurring groups have the same meanings as given under formula III above and preferably R11 and R12 each, independently of one another, an alkyl, alkenyl or alkoxy radical having up to 15 C atoms, more preferably one or both of them denote an alkoxy radical and L11 and L12 each preferably denote F. In another preferred embodiment the LC medium comprises one or more compounds of the formula III-1 selected from the group consisting of formulae III-1-1 to III-1-11, preferably of formula III-1-6,
in which “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms, “alkoxy” and “alkoxy*” each, independently of one another, denote a straight-chain alkoxy radical having 1-6 C atoms, and L11 and L12 each, independently of one another, denote F or Cl, preferably both F. In another preferred embodiment the LC medium comprises one or more compounds of the formula III-2 selected from the group consisting of formulae III-2-1 to III-2-10, preferably of formula III-2-6,
in which “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms, “alkoxy” and “alkoxy*” each, independently of one another, denote a straight-chain alkoxy radical having 1-6 C atoms, and L11 and L12 each, independently of one another, denote F or Cl, preferably both F. In a very preferred embodiment the LC medium comprises one or more compounds selected from the group consisting of the following formulae
In another preferred embodiment of the present invention the LC medium comprises one or more compounds of the formula IIIA-1 and/or IIIA-2
in which L11 and L12 have the same meanings as given under formula III, (O) denotes O or a single bond, RIIIA denotes alkyl or alkenyl having up to 7 C atoms or a group Cy- CmH2m+1-, m and n are, identically or differently, 0, 1, 2, 3, 4, 5 or 6, preferably 1, 2 or 3, very preferably 1, and Cy denotes a cycloaliphatic group having 3, 4 or 5 ring atoms, which is optionally substituted with alkyl or alkenyl each having up to 3 C atoms, or with halogen or CN, and preferably denotes cyclopropyl, cyclobutyl or cyclopentyl. The compounds of formula IIIA-1 and/or IIIA-2 are contained in the LC medium either alternatively or additionally to the compounds of formula III, preferably additionally. Very preferred compounds of the formulae IIIA-1 and IIIA-2 are the following:
in which “alkoxy” denotes a straight-chain alkoxy radical having 1-6 C atoms, and preferably denotes n-propoxy, n-butyloxy, n-pentyloxy or n- hexyloxy. In a preferred embodiment of the present invention, the LC medium comprises one or more compounds of formula III-3
in which
R11, R12 identically or differently, denote H, an alkyl or alkoxy radical having 1 to 15 C atoms, in which one or more CH2 groups in these radicals are optionally replaced, independently of one another, by -C ≡C-, -CF2O-, -OCF2-, -CH=CH-,
-O-, -S-, -CO-O- or -O-CO- in such a way that O atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by halogen. The compounds of formula III-3 are preferably selected from the group consisting of formulae III-3-1 to III-3-11:
in which R12 denotes alkyl having 1 to 7 C-atoms, preferably ethyl, n- propyl, n-butyl, n-pentyl or n-hexyl, or alternatively cyclopropylmethyl, cyclobutylmethyl or cyclopentylmethyl. In another preferred embodiment of the present invention, the LC medium comprises one or more compounds of the formulae III-4 to III-6, preferably of formula III-5,
in which the parameters have the meanings given above, R11 preferably denotes straight-chain alkyl and R12 preferably denotes alkoxy, each having 1 to 7 C atoms. In another preferred embodiment the LC medium comprises one or more compounds of the formula I selected from the group consisting of formulae III-7 to III-9, preferably of formula III-8,
in which the parameters have the meanings given above, R11 preferably denotes straight-chain alkyl and R12 preferably denotes alkoxy each having 1 to 7 C atoms. In a preferred embodiment, the medium comprises one or more compounds of the formula IV,
in which R41 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkenyl radical having 2 to 7 C atoms, preferably an n-alkyl radical, particularly preferably having 2, 3, 4 or 5 C atoms, and R42 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkoxy radical having 1 to 6 C atoms, both preferably having 2 to 5 C atoms, an unsub- stituted alkenyl radical having 2 to 7 C atoms, preferably having 2, 3 or 4 C atoms, more preferably a vinyl radical or a 1-propenyl radical and in particular a vinyl radical. The compounds of the formula IV are preferably selected from the group consisting of formulae IV-1 to IV-6,
in which “alkyl” and “alkyl*“ independently of one another, denote alkyl having 1 to 7 C atoms, preferably having 2 to 5 C atoms, “alkenyl” denotes an alkenyl radical having 2 to 5 C atoms, prefer- ably having 2 to 4 C atoms, particularly preferably 2 C atoms, “alkenyl*“ denotes an alkenyl radical having 2 to 5 C atoms, prefer- ably having 2 to 4 C atoms, particularly preferably having 2 to 3 C atoms, and “alkoxy” denotes alkoxy having 1 to 5 C atoms, preferably having 2 to 4 C atoms. Preferably, the LC medium comprises one or more compounds selected from the group consisting of formulae IV-1-1 to IV-1-9
wherein the propyl, butyl and pentyl groups are straight-chain groups. Very preferably, the LC medium according to the invention comprises one or more compounds of the formulae IV-2-1 and/or IV-2-2
Very preferably, the LC medium according to the invention comprises a compound of formula IV-3, in particular selected from the group consisting of formulae IV-3-1 to IV-3-4
Very preferably, the LC medium according to the invention comprises a compound of formula IV-4, in particular selected from the compounds of the formulae IV-4-1 and IV-4-2
The LC medium preferably additionally comprises one or more compounds of the formula IVa,
in which R41 and R42 each, independently of one another, denote a straight-chain alkyl, alkoxy, alkenyl, alkoxyalkyl or alkoxy radical having up to 12 C atoms, and
Z4 denotes a single bond, -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -COO-, -OCO-, -C2F4-, -C4H8- or -CF=CF-.
Preferred compounds of the formula IVa are indicated below:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1 to 6 C atoms. The LC medium according to the invention preferably comprises at least one compound of the formula IVa-1and/or formula IVa-2. The proportion of compounds of the formula IVa in the mixture as a whole is preferably at least 5 % by weight Preferably, the LC medium comprises one or more compounds of formula IVb-1 to IVb-3
in which “alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1 to 6 C atoms, and “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2 to 6 C atoms. The proportion of the biphenyls of the formulae IV-1 to IV-3 in the mixture as a whole is preferably at least 3 % by weight, in particular ≥ 5 % by weight. Of the compounds of the formulae IVb-1 to IVb-3, the compounds of the formula IVb-2 are particularly preferred. Particularly preferred biphenyls are
in which “alkyl*” denotes an alkyl radical having 1 to 6 C atoms and preferably denotes n-propyl.
The LC medium according to the invention particularly preferably comprises one or more compounds of the formulae IVb-1-1 and/or IVb-2-3. In a preferred embodiment, the LC medium comprises one or more compounds of formula V
in which R51 and R52 independently of one another, have one of the meanings given for R41 and R42 and preferably denote alkyl having 1 to 7 C atoms, preferably n-alkyl, particularly preferably n-alkyl having 1 to 5 C atoms, alkoxy having 1 to 7 C atoms, preferably n-alkoxy, particularly preferably n-alkoxy having 2 to 5 C atoms, alkoxyalkyl, alkenyl or alkenyloxy having 2 to 7 C atoms, preferably having 2 to 4 C atoms, preferably alkenyloxy,
, , identically or differently, denote
in which preferably denotes
Z51 , Z52 each, independently of one another, denote -CH2-CH2-, -CH2-O-,-CH=CH-, -C≡C-, -COO- or a single bond, preferably -CH2-CH2-, -CH2-O- or a single bond and particularly preferably a single bond, and n is 1 or 2. The compounds of formula V are preferably selected from the compounds of the formulae V-1 to V-16:
in which R1 and R2 have the meanings indicated for R2A above. R1 and R2 preferably each, independently of one another, denote straight- chain alkyl or alkenyl. Preferred LC media comprise one or more compounds of the formulae V- 1, V-3, V-4, V-6, V-7, V-10, V-11, V-12, V-14, V-15, and/or V-16 LC media according to the invention very particularly preferably comprise the compounds of the formula V-10, V-12, V-16 and/or IV-1, in particular in amounts of 5 to 30 %. Preferred compounds of the formulae V-10 are indicated below:
The LC medium according to the invention particularly preferably com- prises the tricyclic compounds of the formula V-10a and/or of the formula V-10b in combination with one or more bicyclic compounds of the formulae IV-1 The total proportion of the compounds of the formulae V-10a and/or V- 10b in combination with one or more compounds selected from the bicyclohexyl compounds of the formula IV-1 is 5 to 40 %, very particularly preferably 15 to 35 %. Very particularly preferred LC media comprise compounds V-10a and CC- 2-3
The compounds V-10a and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole. Further particularly preferred LC media comprise compounds V-10b and IV-1-1:
The compounds V-10b and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole. Further particularly preferred LC media comprise the following three com- pounds:
The compounds V-10a, V-10b and IV-1-1 are preferably present in these LC media in a concentration of 15 to 35 %, particularly preferably 15 to 25 % and especially preferably 18 to 22 %, based on the mixture as a whole. Preferred LC media comprise at least one compound selected from the group consisting of the following formulae
in which R41 and R42, and R51 and R52 have the meanings indicated above. Preferably in the compounds V-6, V-7 and IV-1, R41 and R51 denotes alkyl or alkenyl having 1 to 6 or 2 to 6 C atoms, respectively, and R42 and R52 denotes alkenyl having 2 to 6 C atoms. Preferred LC media comprise at least one compound of the formulae V-6a, V-6b, V-7a, V-7b, IV-4-1, IV-4-2, IV-3a and IV-3b:
in which alkyl denotes an alkyl radical having 1 to 6 C atoms and alkenyl denotes an alkenyl radical having 2 to 6 C atoms. The compounds of the formulae V-6a, V-6b, V-7a, V-7b, IV-4-1, IV-4-2, IV- 3a and IV-3b are preferably present in the LC media according to the invention in amounts of 1 to 40 % by weight, preferably 5 to 35 % by weight and very particularly preferably 10 to 30 % by weight. In a preferred embodiment of the present invention the LC medium additionally comprises one or more compounds selected from the group consisting of formulae VI-1 to VI-9
in which R7 each, independently of one another, have one of the meanings indicated for R2A in formula IIA, and w and x each, independently of one another, denote 1 to 6. Particular preference is given to LC media comprising at least one compound of the formula V-9. In a preferred embodiment of the present invention the LC medium additionally comprises one or more compounds selected from the group consisting of the formulae VII-1 to VII-25,
in which R denotes a straight-chain alkyl or alkoxy radical having 1 to 6 C atoms, (O) denotes -O- or a single bond, X denotes F, Cl, OCF3 or OCHF2, Lx denotes H or F, m is 0, 1, 2, 3, 4, 5 or 6 and n is 0, 1, 2, 3 or 4. R preferably denotes methyl, ethyl, propyl, butyl, pentyl, hexyl, methoxy, ethoxy, propoxy, butoxy, pentoxy. X preferably denotes F or OCH3, very preferably F.
The LC medium according to the invention preferably comprises the ter- phenyls of the formulae VII-1 to VII-25 in amounts of 2 to 30 % by weight, in particular 5 to 20 % by weight. Particular preference is given to compounds of the formulae VII-1, VII-2, VII- 4, VII-20, VII-21, and VII-22 wherein X denotes F. In these compounds, R preferably denotes alkyl, furthermore alkoxy, each having 1 to 5 C atoms. In the compounds of the formula VII-20, R preferably denotes alkyl or alkenyl, in particular alkyl. In the compounds of the formula VII-21, R preferably denotes alkyl. In the compounds of the formulae VII-22 to VII-25, X preferably denotes F. The terphenyls of formula VII-1 to VII-25 are preferably employed in the LC media according to the invention if the Δn value of the mixture is to be ≥ 0.1. Preferred LC media comprise 2 to 20 % by weight of one or more terphenyl compounds selected from the group of the compounds of formulae VII-1 to VII-25. Further preferred embodiments are listed below: a) LC medium comprising at least one compound selected from the group consisting of formulae Z-1 to Z-14,
in which R, (O) and “alkyl” have the meanings indicated above for formula III. b) Preferred LC media according to the invention comprise one or more substances which contain a tetrahydronaphthyl or naphthyl unit, such as, for example, the compounds of the formulae N-1 to N-5,
in which R1N and R2N each, independently of one another, have the meanings indicated for R2A, preferably denote straight-chain alkyl, straight-chain alkoxy or straight-chain alkenyl, and Z1 and Z2 each, independently of one another, denote -C2H4-, -CH=CH-, -(CH2)4-, -(CH2)3O-, -O(CH2)3-, -CH=CHCH2CH2-, -CH2CH2CH=CH-, -CH2O-, -OCH2-, - COO-, -OCO-, -C2F4-, -CF=CF-, -CF=CH-, -CH=CF-, - CF2O-, -OCF2-, -CH2- or a single bond. c) Preferred LC media comprise one or more compounds selected from the group of the difluorodibenzochroman compounds of the formula BC, chromans of the formula CR, and fluorinated phenanthrenes of the formulae PH-1 and PH-2,
in which RB1, RB2, RCR1, RCR2, R1, R2 each, independently of one another, have the meaning of R2A. c is 0, 1 or 2. R1 and R2 preferably, independently of one another, denote alkyl or alkoxy having 1 to 6 C atoms. The LC media according to the invention preferably comprise the compounds of the formulae BC, CR, PH-1, PH-2 in amounts of 3 to 20 % by weight, in particular in amounts of 3 to 15 % by weight. Particularly preferred compounds of the formulae BC and CR are the compounds BC-1 to BC-7 and CR-1 to CR-5,
in which
“alkyl” and “alkyl*” each, independently of one another, denote a straight-chain alkyl radical having 1 to 6 C atoms, and “alkenyl” and “alkenyl*” each, independently of one another, denote a straight-chain alkenyl radical having 2 to 6 C atoms. Very particular preference is given to LC media comprising one, two or three compounds of the formula BC-2, BF-1 and/or BF-2. d) Preferred LC media comprise one or more indane compounds of the formula In, In
in which R11, R12, R13 each, independently of one another, denote a straight- chain alkyl, alkoxy, alkoxyalkyl or alkenyl radical having 1 to 6 C atoms, R12 and R13 additionally denote halogen, preferably F,
i denotes 0, 1 or 2. Preferred compounds of the formula In are the compounds of the formulae In-1 to In-16 indicated below:
Particular preference is given to the compounds of the formulae In-1, In-2, In-3 and In-4. The compounds of the formula In and the sub-formulae In-1 to In-16 are preferably employed in the LC media according to the invention in concentrations ≥ 5 % by weight, in particular 5 to 30 % by weight and very particularly preferably 5 to 25 % by weight. e) Preferred LC media additionally comprise one or more compounds of the formulae L-1 to L-5,
in which R and R1 each, independently of one another, have the meanings indicated for R2A in formula IIA above, and alkyl denotes an alkyl radical having 1 to 6 C atoms. The parameter s denotes 1 or 2. The compounds of the formulae L-1 to L-5 are preferably employed in concentrations of 5 to 50 % by weight, in particular 5 to 40 % by weight and very particularly preferably 10 to 40 % by weight. f) Preferred LC media additionally comprise one or more compounds of formula IIA-Y
in which R11 and R12 have one of the meanings given for R2A in formula IIA above, and L1 and L2, identically or differently, denote F or Cl. Preferred compounds of the formula IIA-Y are selected from the group consisting of the following subformulae
in which, Alkyl and Alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, Alkoxy denotes a straight-chain alkoxy radical having 1-6 C atoms, Alkenyl and Alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms, and O denotes an oxygen atom or a single bond. Alkenyl and Alkenyl* preferably denote CH2=CH-,
CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-CH=CH-, CH3-(CH2)2- CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-(CH2)2-. Particularly preferred compounds of the formula IIA-Y are selected from the group consisting of following subformulae:
in which Alkoxy and Alkoxy* have the meanings defined above and preferably denote methoxy, ethoxy, n- propyloxy, n-butyloxy or n- pentyloxy. g) LC medium which additionally comprises one or more quaterphenyl compounds selected from the following formula:
wherein RQ is alkyl, alkoxy, oxaalkyl or alkoxyalkyl having 1 to 9 C atoms or alkenyl or alkenyloxy having 2 to 9 C atoms, all of which are optionally fluorinated, XQ is F, Cl, halogenated alkyl or alkoxy having 1 to 6 C atoms or halogenated alkenyl or alkenyloxy having 2 to 6 C atoms,
LQ1 to LQ6 independently of each other are H or F, with at least one of LQ1 to LQ6 being F. Preferred compounds of formula Q are those wherein RQ denotes straight-chain alkyl with 2 to 6 C-atoms, very preferably ethyl, n- propyl or n-butyl. Preferred compounds of formula Q are those wherein LQ3 and LQ4 are F. Further preferred compounds of formula Q are those wherein LQ3, LQ4 and one or two of LQ1 and LQ2 are F. Preferred compounds of formula Q are those wherein XQ denotes F or OCF3, very preferably F. The compounds of formula Q are preferably selected from the following subformulae
wherein RQ has one of the meanings of formula Q or one of its preferred meanings given above and below, and is preferably ethyl, n-propyl or n-butyl. Especially preferred are compounds of formula Q1, in particular those wherein RQ is n-propyl.
Preferably the proportion of compounds of formula Q in the LC host mixture is from >0 to ≤5% by weight, very preferably from 0.05 to 2% by weight, more preferably from 0.1 to 1% by weight, most preferably from 0.1 to 0.8% by weight. Preferably the LC medium contains 1 to 5, preferably 1 or 2 compounds of formula Q. The addition of quaterphenyl compounds of formula Q to the LC host mixture enables to reduce ODF mura, whilst maintaining high UV absorption, enabling quick and complete polymerization, enabling strong and quick tilt angle generation, and increasing the UV stability of the LC medium. Besides, the addition of compounds of formula Q, which have positive dielectric anisotropy, to the LC medium with negative dielectric anisotropy allows a better control of the values of the dielectric constants ε|| and ε┴, and in particular enables to achieve a high value of the dielectric constant ε|| while keeping the dielectric anisotropy Δ ε constant, thereby reducing the kick-back voltage and reducing image sticking. The LC media according to the invention preferably comprise - one or more compounds of formula I or its subformulae, preferably in a total concentration in the range of from 0.001% to 0.02%, more preferably from 0.002% to 0.015%, most preferably from 0.005% to 0.015%, and/or - one or more compounds of formula IIA, preferably in a total concentration in the range of from 5% to 30%, more preferably from 7% to 25%, particularly preferably from 10% to 20%; and/or - one or more compounds of formulae IIA and IIB, preferably in a total concentration in the range of from 30% to 45%;
and/or - one or more compounds of formula IV, preferably in a total concentration in the range of from 35% to 70%, more preferably from 40 % to 65%, particularly preferably from 45% to 60%; and/or - one or more compounds of formula IV-3, preferably in a total concentration in the range of from 35% to 60%, more preferably from 40 % to 55%, particularly preferably from 45% to 50%; and/or - one or more compounds of formula III-2, preferably of formula III-2-6, preferably in a total concentration in the range of from 2% to 25%, more preferably from 5% to 15%, particularly preferably from 5 to 12%. In particular, the medium comprises - one or more compounds CY-n-Om, in particular CY-3-O4, CY-5-O4 and/or CY-3-O2, preferably in a total concentration in the range of from 5% to 30%, preferably 10% to 20%; and/or - one or more compounds PY-n-Om, in particular PY-3-O2 and/or PY-1- O2, preferably in a total concentration in the range of from 5% to 30%, preferably 5% to 20%; and/or - CPY-n-Om, in particular CPY-2-O2, CPY-3-O2 and/or CPY-5-O2, pref- erably in concentrations > 5%, in particular 7% to 20%, based on the mixture as a whole, and/or - one or more compounds CCY-n-Om, preferably CCY-4-O2, CCY-3-O2, CCY-3-O3, CCY-3-O1 and/or CCY-5-O2, preferably in concentrations > 3%, in particular 5 to 15%, based on the mixture as a whole;
and/or - one or more compounds CPY-n-Om, preferably CPY-2-O2 and/or CPY- 3-O2, preferably in concentrations > 3%, in particular 5 to 15%, based on the mixture as a whole; and/or - CLY-n-Om, preferably CLY-2-O4, CLY-3-O2 and/or CLY-3-O3, preferably in concentrations > 5%, in particular 10 to 30%, very preferably 15 to 20%, based on the mixture as a whole; and/or - CPY-n-Om and CY-n-Om, preferably in concentrations of 10 to 80%, based on the mixture as a whole, and/or - CPY-n-Om and PY-n-Om, preferably CPY-2-O2 and/or CPY-3-O2 and PY-3-O2 or PY-1-O2, preferably in concentrations of 5 to 20%, more preferably 10 to 15% to based on the mixture as a whole, and/or - CC-3-V, preferably in concentrations of 5 to 50%, based on the mixture as a whole. and/or - the compound of the formula CC-3-V1, in a total concentration in the range of from 5 to 40%, more preferably from 15% to 35%, particularly preferably from 20% to 30%, and/or - one or more compounds of formula B-nO-Om and/or B(S)-nO-Om, in particular the compound B(S)-2O-O4 and/or B(S)-2O-O5, preferably in a concentration in the range of from 2 to 12 %. and/or
- 0.1% to 3% of the compound PPGU-3-F, and/or - the compound B(S)-(c5)1O-O2:
The invention furthermore relates to an electro-optical display having active-matrix addressing, characterised in that it contains, as dielectric, a liquid-crystalline medium according to claim 1 and wherein the display is a VA, SA-VA, IPS, U-IPS, FFS, UB-FFS, SA-FFS, PS-VA, PS-OCB, PS-IPS, PS-FFS, PS-UB-FFS, PS-posi-VA, PS-TN, polymer stabilised SA-VA or polymer stabilised SA-FFS display. It is advantageous for the liquid-crystalline medium according to the invention to preferably have a nematic phase from ≤ -20°C to ≥ 70°C, particularly preferably from ≤ -30°C to ≥ 80°C, very particularly preferably from ≤ -40°C to ≥ 90°C. The medium according to the invention preferably has a clearing temperature of 70°C or more, very preferably of 74°C or more. The LC medium has preferably a nematic LC phase. The expression "have a nematic phase" here means on the one hand that no smectic phase and no crystallisation are observed at low temperatures at the corresponding temperature and on the other hand that clearing still does not occur on heating from the nematic phase. The investigation at low temperatures is carried out in a flow viscometer at the corresponding temperature and checked by storage in test cells having a layer thickness corresponding to the electro-optical use for at least 100 hours. If the stor- age stability at a temperature of -20°C in a corresponding test cell is 1000 h or more, the medium is referred to as stable at this temperature. At temperatures of -30°C and -40°C, the corresponding times are 500 h and
250 h respectively. At high temperatures, the clearing point is measured by conventional methods in capillaries. The liquid-crystal mixture preferably has a nematic phase range of at least 60 K and a flow viscosity v20 of at most 30 mm2 · s-1 at 20°C. The mixture is nematic at a temperature of -20°C or less, preferably at - 30°C or less, very preferably at -40°C or less. The values of the birefringence Δn in the liquid-crystal mixture are gener- ally between 0.07 and 0.16, preferably between 0.08 and 0.15, very preferably between 0.09 and 0.14. In a preferred embodiment of the present invention, the medium has a birefringence in the range of from 0.090 to 0.110, preferably from 0.095 to 0.105, in particular from 0.100 to 0.105. In another preferred embodiment, the medium according to the invention has a birefringence of 0.120 or more, preferably in the range of from 0.125 to 0.145, more preferably from 0.130 to 0.140. The liquid-crystal mixture according to the invention has a dielectric anisotropy Δ ε of -1.5 to -8.0, preferably of -2.0 to – 4.0, in particular -2.5 to -3.5, The rotational viscosity γ1 at 20°C is preferably ≤ 120 mPa ·s, in particular ≤ 100 mPa ·s. In a preferred embodiment, the rotational viscosity γ1 at 20°C is ≤ 100mPa ·s, in particular ≤ 95 mPa ·s. The liquid-crystal media according to the invention have relatively low val- ues for the threshold voltage (V0). They are preferably in the range from 1.7 V to 3.0 V, particularly preferably ≤ 2.7 V and very particularly prefera- bly ≤ 2.5 V.
For the present invention, the term "threshold voltage" relates to the capa- citive threshold (V0), also called the Freedericks threshold, unless explicitly indicated otherwise. In addition, the liquid-crystal media according to the invention have high values for the voltage holding ratio in liquid-crystal cells. In general, liquid-crystal media having a low addressing voltage or thresh- old voltage exhibit a lower voltage holding ratio than those having a higher addressing voltage or threshold voltage and vice versa. For the present invention, the term "dielectrically positive compounds" denotes compounds having a Δ ε > 1.5, the term "dielectrically neutral com- pounds" denotes those having -1.5 ≤ Δ ε ≤ 1.5 and the term "dielectrically negative compounds” denotes those having Δ ε < -1.5. The dielectric ani- sotropy of the compounds is determined here by dissolving 10 % of the compounds in a liquid-crystalline host and determining the capacitance of the resultant mixture in at least one test cell in each case having a layer thickness of 20 µm with homeotropic and with homogeneous surface alignment at 1 kHz. The measurement voltage is typically 0.5 V to 1.0 V, but is always lower than the capacitive threshold of the respective liquid- crystal mixture investigated. All temperature values indicated for the present invention are in °C. The LC media according to the invention are suitable for all VA-TFT (vertical alignment-thin film transistor) applications, such as, for example, VAN (vertically aligned nematic), MVA (multidomain VA), (S)-PVA (super patterned VA), ASV (advanced super view, or axially symmetric VA), or UV2A. They are furthermore suitable for IPS (in-plane switching) and FFS (fringe field switching) applications having negative Δ ε. The nematic LC media in the displays according to the invention generally comprise two components NA and NB, which themselves consist of one or more individual compounds.
Component NA has significantly negative dielectric anisotropy and gives the nematic phase a dielectric anisotropy of ≤ -0.5. Besides one or more compounds of the formula I, it preferably comprises the compounds of the formulae IIA, IIB and/or IIC, furthermore one or more compounds of the formula IV-1. The proportion of component NA is preferably between 45 and 100 %, in particular between 60 and 85 %. For component NA, one (or more) individual compound(s) which has (have) a value of Δ ε ≤ -0.8 is (are) preferably selected. This value must be more negative, the smaller the proportion of NA in the mixture as a whole. Component NB has pronounced nematogeneity and a flow viscosity of not greater than 30 mm2 · s-1, preferably not greater than 25 mm2 · s-1, at 20°C. Particularly preferred individual compounds in component NB are extremely low-viscosity nematic liquid crystals having a flow viscosity of not greater than 18 mm2 · s-1, preferably not greater than 12 mm2 · s-1, at 20°C. Component NB is monotropically or enantiotropically nematic, has no smectic phases and is able to prevent the occurrence of smectic phases down to very low temperatures in LC media. For example, if various materials of high nematogeneity are added to a smectic liquid-crystal mix- ture, the nematogeneity of these materials can be compared through the degree of suppression of smectic phases that is achieved. The mixture may optionally also comprise a component NC, comprising compounds having a dielectric anisotropy of Δ ε ≥1.5. These so-called posi- tive compounds are generally present in a mixture of negative dielectric anisotropy in amounts of ≤ 20 % by weight, based on the mixture as a whole. Besides one or more compounds of the formula I or its subformulae, the medium preferably comprises 4 to 15, in particular 5 to 12, and particularly
preferably < 10, compounds of the formulae IIA, IIB and/or IIC and optionally one or more compounds of the formula IV-1. Besides compounds of the formula I or its subformulae and the compounds of the formulae IIA, IIB and/or IIC and optionally IV-1, other constituents may also be present, for example in an amount of up to 45 % of the mixture as a whole, but preferably up to 35 %, in particular up to 10 %. The other constituents are preferably selected from nematic or nemato- genic substances, in particular known substances, from the classes of the azoxybenzenes, benzylideneanilines, biphenyls, terphenyls, phenyl or cyclohexyl benzoates, phenyl or cyclohexyl cyclohexanecarboxylates, phenylcyclohexanes, cyclohexylbiphenyls, cyclohexylcyclohexanes, cyclo- hexylnaphthalenes, 1,4-biscyclohexylbiphenyls or cyclohexylpyrimidines, phenyl- or cyclohexyldioxanes, optionally halogenated stilbenes, benzyl phenyl ethers, tolanes and substituted cinnamic acid esters. The most important compounds which are suitable as constituents of liquid-crystal phases of this type can be characterised by the formula IV R20-L-G-E-R21 IV in which L and E each denote a carbo- or heterocyclic ring system from the group formed by 1,4-disubstituted benzene and cyclohexane rings, 4,4’-disubstituted biphenyl, phenylcyclohexane and cyclohexylcyclohexane systems, 2,5-disubstituted pyrimidine and 1,3-dioxane rings, 2,6-disubsti- tuted naphthalene, di- and tetrahydronaphthalene, quinazoline and tetra- hydroquinazoline, G denotes -CH=CH- -N(O)=N- -CH=CQ- -CH=N(O)- -C ≡C- -CH2-CH2- -CO-O- -CH2-O- -CO-S- -CH2-S- -CH=N- -COO-Phe-COO-
-CF2O- -CF=CF- -OCF2- -OCH2- -(CH2)4- -(CH2)3O- or a C-C single bond, Q denotes halogen, preferably chlorine, or -CN, and R20 and R21 each denote alkyl, alkenyl, alkoxy, alkoxyalkyl or alkoxycar- bonyloxy having up to 18, preferably up to 8, carbon atoms, or one of these radicals alternatively denotes CN, NC, NO2, NCS, CF3, SF5, OCF3, F, Cl or Br. In most of these compounds, R20 and R21 are different from one another, one of these radicals usually being an alkyl or alkoxy group. Other variants of the proposed substituents are also common. Many such substances or also mixtures thereof are commercially available. All these substances can be prepared by methods known from the literature. It goes without saying for the person skilled in the art that the VA, IPS or FFS mixture according to the invention may also comprise compounds in which, for example, H, N, O, Cl and F have been replaced by the corres- ponding isotopes. The combination of compounds of the preferred embodiments mentioned above with the polymerized compounds described above causes low threshold voltages, low rotational viscosities and very good low-tem- perature stabilities in the LC media according to the invention at the same time as constantly high clearing points and high HR values, and allows the rapid establishment of a particularly low tilt angle (i.e. a large tilt) in PSA displays. In particular, the LC media exhibit significantly shortened response times, in particular also the grey-shade response times, in PSA displays compared with the LC media from the prior art. The invention furthermore relates to an LC display comprising an LC medium as described above and below. The LC display is preferably a VA, IPS, FFS, UB-FFS or UV2A display.
The structure of the displays according to the invention corresponds to the usual geometry for PSA displays, as described in the prior art cited at the outset. Geometries without protrusions are preferred, in particular those in which, in addition, the electrode on the colour filter side is unstructured and only the electrode on the TFT side has slots. Particularly suitable and preferred electrode structures for PS-VA displays are described, for example, in US 2006/0066793 A1. A preferred LC display of the present invention comprises: - a first substrate including a pixel electrode defining pixel areas, the pixel electrode being connected to a switching element disposed in each pixel area and optionally including a micro-slit pattern, and optionally a first alignment layer disposed on the pixel electrode, - a second substrate including a common electrode layer, which may be disposed on the entire portion of the second substrate facing the first substrate, and optionally a second alignment layer, - an LC layer disposed between the first and second substrates and including an LC medium as described above and below. The first and/or second alignment layer controls the alignment direction of the LC molecules of the LC layer. For example, in VA displays the alignment layer is selected such that it imparts to the LC molecules homeotropic (or vertical) alignment (i.e. perpendicular to the surface) or tilted alignment. Such an alignment layer may for example comprise a polyimide, which may also be rubbed, or may be prepared by a photoalignment method. In UV2A displays the alignment layer is made by photopolymerization using linear polarized UV light and irradiation at an oblique angle. The LC layer with the LC medium can be deposited between the substrates of the display by methods that are conventionally used by display manufacturers, for example the so-called one-drop-filling (ODF) method.
The LC display may comprise further elements, like a colour filter, a black matrix, a passivation layer, optical retardation layers, transistor elements for addressing the individual pixels, etc., all of which are well known to the person skilled in the art and can be employed without inventive skill. The electrode structure can be designed by the skilled person depending on the individual display type. For example for VA displays a multi-domain orientation of the LC molecules can be induced by providing electrodes having slits and/or bumps or protrusions in order to create two, four or more different tilt alignment directions. The the LC medium may also comprise, in addition to the compounds of formula I, one or more further stabilizers. Suitable types and amounts of stabilizers are known to the person skilled in the art and are described in the literature. Particularly suitable are, for example, the commercially available stabilizers from the Irganox® series (Ciba AG), such as, for example, Irganox® 1076. If stabilizers are employed, their proportion is preferably 10-5000 ppm, particularly preferably 50-500 ppm. In a preferred embodiment the LC media contain one or more chiral dopants, preferably in a concentration from 0.01 to 1% by weight, very preferably from 0.05 to 1.0% by weight. The chiral dopants are preferably selected from the group consisting of compounds from Table B below, very preferably from the group consisting of R- or S-1011, R- or S-2011, R- or S-3011, R- or S-4011, and R- or S-5011. In another preferred embodiment the LC media contain a racemate of one or more chiral dopants, which are preferably selected from the chiral dopants mentioned in the previous paragraph. Furthermore, it is possible to add to the LC media, for example, 0 to 15% by weight of pleochroic dyes, furthermore nanoparticles, conductive salts, preferably ethyldimethyldodecylammonium 4-hexoxybenzoate, tetrabutyl- ammonium tetraphenylborate or complex salts of crown ethers (cf., for example, Haller et al., Mol. Cryst. Liq. Cryst.24, 249-258 (1973)), for improving the conductivity, or substances for modifying the dielectric
anisotropy, the viscosity and/or the alignment of the nematic phases. Sub- stances of this type are described, for example, in DE-A 2209127, 2240 864, 2321632, 2338281, 2450088, 2637430 and 2853728. The individual components of the above-listed preferred embodiments of the LC media according to the invention are either known or methods for the preparation thereof can readily be derived from the prior art by the person skilled in the relevant art, since they are based on standard methods described in the literature. Corresponding compounds of the formula CY are described, for example, in EP-A-0364538. Corresponding compounds of the formula ZK are described, for example, in DE-A- 2636 684 and DE-A-3321373. The LC media which can be used in accordance with the invention are prepared in a manner conventional per se, for example by mixing one or more of the above-mentioned compounds with one or more compounds of formula I as defined above, and optionally with further liquid-crystalline compounds and/or additives. In general, the desired amount of the com- ponents used in lesser amount is dissolved in the components making up the principal constituent, advantageously at elevated temperature. It is also possible to mix solutions of the components in an organic solvent, for example in acetone, chloroform or methanol, and to remove the solvent again, for example by distillation, after thorough mixing. The invention furthermore relates to the process for the preparation of the LC media according to the invention. It goes without saying to the person skilled in the art that the LC media according to the invention may also comprise compounds in which, for example, H, N, O, Cl, F have been replaced by the corresponding isotopes like deuterium etc. The following examples explain the present invention without restricting it. However, they show the person skilled in the art preferred mixture con- cepts with compounds preferably to be employed and the respective con- centrations thereof and combinations thereof with one another. In addition,
the examples illustrate which properties and property combinations are accessible. Preferred mixture components are shown in Table A below. Table A In Table A, m and n are independently of each other an integer from 1 to 12, preferably 1, 2, 3, 4, 5 or 6, k is 0, 1, 2, 3, 4, 5 or 6, and (O)CmH2m+1 means CmH2m+1 or OCmH2m+1.
In a preferred embodiment of the present invention, the LC media according to the invention comprise one or more compounds selected from the group consisting of compounds from Table A. Table B Table B shows possible chiral dopants which can be added to the LC media according to the invention.
The LC media preferably comprise 0 to 10% by weight, in particular 0.01 to 5% by weight, particularly preferably 0.1 to 3% by weight, of dopants. The LC media preferably comprise one or more dopants selected from the group consisting of compounds from Table B. Table C Table C shows possible stabilizers which can be added to the LC media according to the invention. Therein n denotes an integer from 1 to 12, preferably 1, 2, 3, 4, 5, 6, 7 or 8, and terminal methyl groups are not shown.
The LC media preferably comprise 0 to 10% by weight, in particular 1 ppm to 5% by weight, particularly preferably 1 ppm to 1% by weight, of stabilizers. The LC media preferably comprise one or more stabilizers selected from the group consisting of compounds from Table C. Examples The following examples explain the present invention without restricting it. However, they show the person skilled in the art preferred mixture con- cepts with compounds preferably to be employed and the respective con- centrations thereof and combinations thereof with one another. In addition, the examples illustrate which properties and property combinations are accessible. In addition, the following abbreviations and symbols are used: V0 threshold voltage, capacitive [V] at 20°C, ne extraordinary refractive index at 20°C and 589 nm, no ordinary refractive index at 20°C and 589 nm,
Δn optical anisotropy at 20°C and 589 nm, ε ┴ dielectric permittivity perpendicular to the director at 20°C and 1 kHz, ε|| dielectric permittivity parallel to the director at 20°C and 1 kHz, Δ ε dielectric anisotropy at 20°C and 1 kHz, cl.p., T(N,I) clearing point [°C], γ1 rotational viscosity at 20°C [mPa ·s], K1 elastic constant, "splay" deformation at 20°C [pN], K2 elastic constant, "twist" deformation at 20°C [pN], K3 elastic constant, "bend" deformation at 20°C [pN]. Unless explicitly noted otherwise, all concentrations in the present application are quoted in per cent by weight and relate to the corresponding mixture as a whole, comprising all solid or liquid-crystalline components, without solvents. Unless explicitly noted otherwise, all temperature values indicated in the present application, such as, for example, for the melting point T(C,N), the transition from the smectic (S) to the nematic (N) phase T(S,N) and the clearing point T(N,I), are quoted in degrees Celsius (°C). M.p. denotes melting point, cl.p. = clearing point. Furthermore, C = crystalline state, N = nematic phase, S = smectic phase and I = isotropic phase. The data between these symbols represent the transition temperatures. All physical properties are and have been determined in accordance with "Merck Liquid Crystals, Physical Properties of Liquid Crystals", Status Nov. 1997, Merck KGaA, Germany, and apply for a temperature of 20°C, and Δn is determined at 589 nm and Δ ε at 1 kHz, unless explicitly indicated otherwise in each case. The term "threshold voltage" for the present invention relates to the capa- citive threshold (V0), also known as the Freedericks threshold, unless explicitly indicated otherwise. In the examples, the optical threshold may also, as generally usual, be quoted for 10% relative contrast (V10).
Unless stated otherwise, methods of preparing test cells and measuring their electrooptical and other properties are carried out by the methods as described hereinafter or in analogy thereto. Unless stated otherwise, the term "tilt angle" means the angle between the LC director and the substrate, and "LC director" means in a layer of LC molecules with uniform orientation the preferred orientation direction of the optical main axis of the LC molecules, which corresponds, in case of calamitic, uniaxially positive birefringent LC molecules, to their molecular long axis. Example 1 The nematic LC host mixture N1 is formulated as follows
Stabilised mixture S1.1 is prepared by adding 100ppm of the compound M1 according to formula I to the nematic LC host mixture N1. Stabilised mixture S1.2 is prepared by adding 200ppm of the compound M1 according to formula I to the nematic LC host mixture N1.
Comparison Example 1 The mixture C1 is prepared by adding 300ppm of the compound M1 to the nematic LC host mixture N1. Example 2 The nematic LC host mixture N2 is formulated as follows
Stabilised mixture S2 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N2. Example 3 The nematic LC host mixture N3 is formulated as follows
Stabilised mixture S3 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N3. Example 4 The nematic LC host mixture N4 is formulated as follows
Stabilised mixture S4.1 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N4. Stabilised mixture S4.2 is prepared by adding 200ppm of the compound M1 to the nematic LC host mixture N4. Example 5 The nematic LC host mixture N5 is formulated as follows
Stabilised mixture S5 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N5. Example 6 The nematic LC host mixture N6 is formulated as follows
Stabilised mixture S6 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N6. Example 7 The nematic LC host mixture N7 is formulated as follows
Stabilised mixture S7 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N7. Example 8 The nematic LC host mixture N8 is formulated as follows
Stabilised mixture S8 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N8. Example 9 The nematic LC host mixture N9 is formulated as follows
Stabilised mixture S9 is prepared by adding 150ppm of the compound M1 to the nematic LC host mixture N9. Example 10 The nematic LC host mixture N10 is formulated as follows
Stabilised mixture S10.1 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N10. Stabilised mixture S10.2 is prepared by adding 200ppm of the compound M1 to the nematic LC host mixture N10. Example 11 The nematic LC host mixture N11 is formulated as follows
Stabilised mixture S11 is prepared by adding 100ppm of the compound M1 to the nematic LC host mixture N11. Use Examples The VHR of stabilised mixtures S1.1 and S1.2 according to the present invention containing the compound M1 is measured compared to the pure host mixture N1. VHR Measurement The test cells used for VHR measurement consist of two plane-parallel glass outer plates at a separation of 3.2 ^m, each of which has on the inside an electrode layer and a polyimide alignment layer on top, where the two polyimide layers effect homeotropic alignment of the liquid crystal molecules and photoalignment is applied to induce tilt directions at the two substrates crossed to one another. Six test cells are prepared for each LC mixture. After filling in the LC mixtures the cells are sealed by a sealent. Then the VHR of host mixture N1 and mixtures S1.1 and S1.2 is measured at 60°C with application of a voltage of 1 V / 0.6 Hz before and after 120 hours of BL stress at 60°C. Light and thermal stress usually causes the generation of impurity and ion which decrease of VHR in LC mixtures, therefore the smaller the absolute decrease of VHR value after stress, the better the performance for display applications.
The results are shown in Table 1. Table 1 - VHR
From Table 1 it can be seen that for mixtures S1.1 and S1.2 according to the present invention the initial VHR is already higher than the initial VHR of host mixture N1 without the compound M1. After 120h BL stress the VHR of host mixture N1 shows a strong decrease, whereas the VHR of mixtures S1.1 and S1.2 shows a much smaller decrease. Titl Angle Measurement The tilt angle generation of stabilised mixture S1.1 according to the present invention is measured compared to the pure host mixture N1 and to comparison mixture C1 with a higher amount of compound M1. The test cells used for tilt angle measurement consist of two plane-parallel glass outer plates at a separation of 3.2 ^m, each of which has on the inside an electrode layer and a polyimide alignment layer on top, where the two polyimide layers effect homeotropic alignment of the liquid crystal molecules and photoalignment is applied to induce tilt directions at the two substrates anti-parallel to one another. The LC mixtues are filled into the text cells which are then sealed by a sealent. The tilt angle generated in host mixture N1 and mixtures S1.1 and C1 is measured before and after exposure to BL and electric stress of 60 Vpp for 120h using the Mueller Matrix Polarimeter “AxoScan” from Axometrics.
The tilt angle variation, i.e. the change of the tilt angle Δtilt in absolute values after stress is shown in Table 2 below. Table 2 – Tilt Variation
From Table 2 it can be seen that mixture S1.1 according to the present invention with 100ppm of compound M1 shows a small tilt angle change which is similar to host mixture N1, which means that no significant tilt angle has been generated by the compound M1 at this concentration. Compared thereto, comparison mixture C1 with 300ppm of compound M1 shows a stronger tilt angle change indicating that a tilt angle has been generated. Overall the above results show that a compound of formula I, when added to a nematic host mixture in a low concentration, can increase the VHR without showing significant tilt angle generation.
Claims
Claims 1. An LC medium comprising one or more compounds of formula I in a concentration of >0% and ≤0.02% by weight
wherein the individual radicals, independently of each other and on each occurrence identically or differently, have the following meanings P CW=CH-CO-O-, W H, F, Cl, CF3 or alkyl with 1 to 5 C atoms, preferably H or CH3, Sp1, Sp2, Sp3 a spacer group or a single bond, L F, Cl, -CN, P-Sp- or straight chain, branched or cyclic alkyl having 1 to 25 C atoms, wherein one or more non-adjacent CH2-groups are optionally replaced by - O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, CR0=CR00-, -C ≡C-,
in such a manner that O- and/or S-atoms are not directly connected with each other, and wherein one or more H atoms are each optionally replaced by P- Sp-, F or Cl, r, s 0, 1, 2, 3 or 4,
and further comprising one or more compounds of formula II
wherein the individual radicals, independently of each other and on each occurrence identically or differently, have the following meanings R1, R2 straight chain, branched or cyclic alkyl having 1 to 25 C atoms, wherein one or more non-adjacent CH2-groups are optionally replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, CR0=CR00-, -C ≡C-,
in such a manner that O- and/or S-atoms are not directly connected with each other, and wherein one or more H atoms are each optionally replaced by F or Cl, A1, A2 a group selected from the following formulae
Z1, Z2 -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, - CO-O-, -O-CO-, -C2F4-, -CF=CF-, -CH=CH-CH2O- or a single bond, L1, L2, L3, L4 F, Cl, OCF3, CF3, CH3, CH2F or CHF2, Y H, F, Cl, CF3, CHF2 or CH3, LC CH3 or OCH3, a1 1 or 2, a2 0 or 1.
2. The LC medium according to Claim 1, characterized in that in the compounds of formula I P is acrylate or methacrylate.
3. The LC medium according to Claim 1 or 2, characterized in that in the compounds of formula I L denotes F, Cl, CN or OCH3.
4. The LC medium according to one or more of Claims 1 to 3, characterized in that in the compounds of formula I at least one group Sp is different from a single bond.
5. The LC medium according to one or more of Claims 1 to 4, characterized in that if Sp is different from a single bond, it is selected from the group consisting of -(CH2)2-, -(CH2)3-, -(CH2)4-, -O- (CH2)2-, -O-(CH2)3-, -O-CO-(CH2)2 and -CO-O-(CH)2-, wherein the O atom or the CO group is attached to the benzene ring.
6. The LC medium according to one or more of Claims 1 to 5, characterized in that the compounds of formula I are selected from the following subformulae:
7. The LC medium according to one or more of Claims 1 to 6, characterized in that the compounds of formula I are selected from the following subformulae:
8. The LC medium according to one or more of Claims 1 to 7, characterized in that it contains one or more compounds of formula II selected from the group consisting of compounds of the formulae IIA, IIB, IIC and IID
in which R2A and R2B each, independently of one another, denote H, an alkyl or alkenyl radical having up to 15 C atoms which is un- substituted, monosubstituted by CN or CF3 or at least monosubstituted by halogen, where, in addition, one or more CH2 groups in these radicals may be replaced by -O-, -S-,
-C ≡C-, -CF2O-, -OCF2-, -OC-O- or -O-CO- in such a way that O atoms are not linked directly to one another, L1 to L4 each, independently of one another, denote F, Cl, CF3 or CHF2, Y denotes H, F, Cl, CF3, CHF2 or CH3, preferably H or CH3, particularly preferably H, Z2, Z2B and Z2D each, independently of one another, denote a single bond, -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -COO-, -OCO-, -C2F4-, -CF=CF-, -CH=CHCH2O-, p denotes 0, 1 or 2, and q on each occurrence, identically or differently, denotes 0 or 1. 9. The LC medium according to any one of Claims 1 to 8, characterized in that it additionally comprises one or more compounds of formula III
in which R11 and R12 each, independently of one another, denote H, an alkyl or alkoxy radical having 1 to 15 C atoms, where one or more CH2 groups in these radicals may each be replaced, independently of one another, by
-O-, -S-, -C ≡C-, -CF2O-, -OCF2-, -CH=CH-, -OC-O- or -O-CO- in such a way that O atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by halogen, A3 on each occurrence, independently of one another, denotes a) 1,4-cyclohexenylene or 1,4-cyclohexylene radical, in which one or two non-adjacent CH2 groups may be replaced by -O- or -S-, b) a 1,4-phenylene radical, in which one or two CH groups may be replaced by N, or c) a radical selected from the group consisting of spiro[3.3]heptane-2,6-diyl, 1,4-bicyclo[2.2.2]octylene, naphthalene-2,6-diyl, decahydronaphthalene-2,6-diyl, 1,2,3,4-tetrahydronaphthalene-2,6-diyl, phenanthrene- 2,7-diyl and fluorene-2,7-diyl,
wherein the radicals a), b) and c) may be mono- or polysubstituted by halogen atoms, n denotes 0, 1 or 2, preferably 0 or 1, Z1 on each occurrence independently of one another denotes -CO-O-, -O-CO-, -CF2O- , -OCF2-, -CH2O-, -OCH2-, -CH2-, -CH2CH2-, -(CH2)4-, -CH=CH-CH2O-, -C2F4-, -CH2CF2-, - CF2CH2 -, -CF=CF-, -CH=CF-, -CF=CH-, -CH=CH-, -C■C- or a single bond, L11 and L12 each, independently of one another, denote F, Cl, CF3 or CHF2, preferably H or F, most preferably F, and W denotes O or S. 10. The LC medium according to any one of Claims 1 to 9, characterized in that it additionally comprises one or more compounds of formula IV
in which R41 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkenyl radical having 2 to 7 C atoms, preferably an n-alkyl radical, particularly preferably having 2, 3, 4 or 5 C atoms, and R42 denotes an unsubstituted alkyl radical having 1 to 7 C atoms or an unsubstituted alkoxy radical having 1 to 6 C atoms, both preferably having 2 to 5 C atoms, an unsubstituted alkenyl radical having 2 to 7 C atoms, preferably having 2, 3 or 4 C
atoms, more preferably a vinyl radical or a 1-propenyl radical and in particular a vinyl radical. 11. The LC medium according to any one of Claims 1 to 10, characterized in that it comprises one or more compounds of formula IV-3
in which “alkyl” denotes alkyl having 1 to 7 C atoms, preferably having 2 to 5 C atoms, and “alkenyl” denotes an alkenyl radical having 2 to 5 C atoms, prefer- ably having 2 to 4 C atoms, particularly preferably 2 C atoms. 12. The LC medium according to any one of Claims 1 to 11, characterized in that it additionally comprises one or more compounds of formula V
in which R51 and R52 independently of one another, have one of the meanings given for R41 and R42 and preferably denote alkyl having 1 to 7 C atoms, preferably n-alkyl, particularly preferably n-alkyl having 1 to 5 C atoms, alkoxy having 1 to 7 C atoms, preferably n-alkoxy, particularly preferably n- alkoxy having 2 to 5 C atoms, alkoxyalkyl, alkenyl or
alkenyloxy having 2 to 7 C atoms, preferably having 2 to 4 C atoms, preferably alkenyloxy,
identically or differently, denote in which
preferably denotes
Z51 , Z52 each, independently of one another, denote -CH2-CH2- , -CH2-O-,-CH=CH-, -C≡C-, -COO- or a single bond, pref- erably -CH2-CH2-, -CH2-O- or a single bond and particu- larly preferably a single bond, and n is 1 or 2. 13. The LC medium according to any one of Claims 1 to 12, characterized in that it additionally comprises one or more additives selected from the group consisting of stabilizers and chiral dopants. 14. A process of preparing an LC medium according to one or more of Claims 1 to 13, comprising the steps of mixing one or more one or more compounds of formula I as defined in one or more of Claims 1 to 7 with one or more compounds of formula II, III, IV and/or V as defined in one or more of Claims 1 or 12, and optionally with further liquid- crystalline compounds and/or additives.
15. An LC display comprising an LC medium as defined in one or more of Claims 1 to 13. 16. The LC display of Claim 15, which is a display of the VA, IPS, FFS or UV2A mode. 17. The LC display of Claim 15 or 16, characterized in that it comprises two substrates, at least one of which is transparent to light, an electrode provided on each substrate or two electrodes provided on only one of the substrates, and located between the substrates a layer of an LC medium according to one or more of Claims 1 to 13.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202280013417.4A CN116964177A (en) | 2021-03-02 | 2022-02-28 | Liquid-crystalline medium |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNPCT/CN2021/078689 | 2021-03-02 | ||
| CN2021078689 | 2021-03-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022184604A1 true WO2022184604A1 (en) | 2022-09-09 |
Family
ID=80952435
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2022/054906 WO2022184604A1 (en) | 2021-03-02 | 2022-02-28 | Liquid-crystal medium |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN116964177A (en) |
| TW (1) | TW202302822A (en) |
| WO (1) | WO2022184604A1 (en) |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2209127A1 (en) | 1972-02-26 | 1973-09-06 | Merck Patent Gmbh | MODIFIED NEMATIC PHASES |
| DE2338281A1 (en) | 1972-08-03 | 1974-02-21 | Ibm | PROCESS FOR THE CONTROLLED CHANGE OF THE ELECTRICAL PROPERTIES OF NEMATIC LIQUIDS AND DOPING AGENTS THEREFORE |
| DE2240864A1 (en) | 1972-08-19 | 1974-02-28 | Merck Patent Gmbh | NEMATIC ESTERS AND THEIR USE TO INFLUENCE THE ELECTROOPTICAL PROPERTIES OF NEMATIC PHASES |
| DE2321632A1 (en) | 1973-04-28 | 1974-11-21 | Merck Patent Gmbh | MODIFIED NEMATIC MIXTURES WITH POSITIVE DIELECTRIC ANISOTROPY |
| DE2450088A1 (en) | 1974-10-22 | 1976-04-29 | Merck Patent Gmbh | Liquid crystalline dielectrics for electronic components - contg biphenylyl carboxylic acid phenyl ester or benzoic acid biphenylyl ester components |
| DE2636684A1 (en) | 1976-08-14 | 1978-02-16 | Merck Patent Gmbh | CYCLOHEXAN DERIVATIVES |
| DE2637430A1 (en) | 1976-08-20 | 1978-02-23 | Merck Patent Gmbh | Heterocyclic diaza cpd. in liquid crystalline dielectric - for electrooptical registration devices, giving stable orientation parallel to electrode surfaces |
| DE2853728A1 (en) | 1978-12-13 | 1980-07-17 | Merck Patent Gmbh | LIQUID CRYSTALLINE CARBONIC ACID ESTER, METHOD FOR THE PRODUCTION THEREOF, ITS CONTAINING DIELECTRICS AND ELECTRO-OPTICAL DISPLAY ELEMENT |
| DE3321373A1 (en) | 1983-06-14 | 1984-12-20 | Merck Patent Gmbh, 6100 Darmstadt | BICYCLOHEXYLE |
| EP0364538A1 (en) | 1988-03-10 | 1990-04-25 | Merck Patent Gmbh | Difluorobenzol derivatives. |
| DE19539141A1 (en) | 1995-10-20 | 1997-04-24 | Merck Patent Gmbh | New 4-(cyclic-group)-2,6-di-tert.-butyl-phenol derivatives |
| US20060066793A1 (en) | 2004-09-24 | 2006-03-30 | Fujitsu Display Technologies Corporation | Liquid crystal display device |
| EP2980062A2 (en) * | 2014-07-30 | 2016-02-03 | Merck Patent GmbH | Polymerisable compounds and the use thereof in liquid-crystal displays |
-
2022
- 2022-02-28 CN CN202280013417.4A patent/CN116964177A/en active Pending
- 2022-02-28 WO PCT/EP2022/054906 patent/WO2022184604A1/en active Application Filing
- 2022-03-01 TW TW111107220A patent/TW202302822A/en unknown
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2209127A1 (en) | 1972-02-26 | 1973-09-06 | Merck Patent Gmbh | MODIFIED NEMATIC PHASES |
| DE2338281A1 (en) | 1972-08-03 | 1974-02-21 | Ibm | PROCESS FOR THE CONTROLLED CHANGE OF THE ELECTRICAL PROPERTIES OF NEMATIC LIQUIDS AND DOPING AGENTS THEREFORE |
| DE2240864A1 (en) | 1972-08-19 | 1974-02-28 | Merck Patent Gmbh | NEMATIC ESTERS AND THEIR USE TO INFLUENCE THE ELECTROOPTICAL PROPERTIES OF NEMATIC PHASES |
| DE2321632A1 (en) | 1973-04-28 | 1974-11-21 | Merck Patent Gmbh | MODIFIED NEMATIC MIXTURES WITH POSITIVE DIELECTRIC ANISOTROPY |
| DE2450088A1 (en) | 1974-10-22 | 1976-04-29 | Merck Patent Gmbh | Liquid crystalline dielectrics for electronic components - contg biphenylyl carboxylic acid phenyl ester or benzoic acid biphenylyl ester components |
| DE2636684A1 (en) | 1976-08-14 | 1978-02-16 | Merck Patent Gmbh | CYCLOHEXAN DERIVATIVES |
| DE2637430A1 (en) | 1976-08-20 | 1978-02-23 | Merck Patent Gmbh | Heterocyclic diaza cpd. in liquid crystalline dielectric - for electrooptical registration devices, giving stable orientation parallel to electrode surfaces |
| DE2853728A1 (en) | 1978-12-13 | 1980-07-17 | Merck Patent Gmbh | LIQUID CRYSTALLINE CARBONIC ACID ESTER, METHOD FOR THE PRODUCTION THEREOF, ITS CONTAINING DIELECTRICS AND ELECTRO-OPTICAL DISPLAY ELEMENT |
| DE3321373A1 (en) | 1983-06-14 | 1984-12-20 | Merck Patent Gmbh, 6100 Darmstadt | BICYCLOHEXYLE |
| EP0364538A1 (en) | 1988-03-10 | 1990-04-25 | Merck Patent Gmbh | Difluorobenzol derivatives. |
| DE19539141A1 (en) | 1995-10-20 | 1997-04-24 | Merck Patent Gmbh | New 4-(cyclic-group)-2,6-di-tert.-butyl-phenol derivatives |
| US20060066793A1 (en) | 2004-09-24 | 2006-03-30 | Fujitsu Display Technologies Corporation | Liquid crystal display device |
| EP2980062A2 (en) * | 2014-07-30 | 2016-02-03 | Merck Patent GmbH | Polymerisable compounds and the use thereof in liquid-crystal displays |
Non-Patent Citations (7)
| Title |
|---|
| C. TSCHIERSKEG. PELZLS. DIELE, ANGEW. CHEM., vol. 116, 2004, pages 6340 - 6368 |
| HALLER ET AL., MOL. CRYST. LIQ. CRYST., vol. 24, 1973, pages 249 - 258 |
| PURE APPL. CHEM., vol. 73, no. 5, 2001, pages 888 |
| Q. TANG ET AL., SID SYMPOSIUM DIGEST OF TECHNICAL PAPERS, 2018, pages 414 - 417 |
| S.H. JUNG ET AL., JPN. J. APPL. PHYS., vol. 43, no. 3, 2004, pages 1028 |
| S.H. LEE ET AL., APPL. PHYS. LETT., vol. 73, no. 20, 1998, pages 2882 - 2883 |
| S.H. LEE ET AL., LIQUID CRYSTALS, vol. 39, no. 9, 2012, pages 1141 - 1148 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW202302822A (en) | 2023-01-16 |
| CN116964177A (en) | 2023-10-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3248061B1 (en) | Light modulation element | |
| EP3248060B1 (en) | Light modulation element | |
| EP4176021A1 (en) | Liquid crystal medium | |
| EP4194529A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| EP4286493A1 (en) | Liquid-crystal medium | |
| EP4056667B1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| EP4219656A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| WO2022096483A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| WO2022184604A1 (en) | Liquid-crystal medium | |
| EP4086325B1 (en) | Dye-doped liquid-crystal medium comprising polymerizable compounds | |
| US11905448B2 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| EP4244303A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| EP4001378A1 (en) | Liquid-crystal medium | |
| EP4357438A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| EP4419622A1 (en) | Liquid-crystal medium comprising polymerizable compounds | |
| WO2023209049A1 (en) | Liquid-crystal medium | |
| WO2023203043A1 (en) | Liquid-crystal medium | |
| WO2023052283A1 (en) | Liquid-crystal medium | |
| WO2024223484A1 (en) | Liquid-crystal medium comprising polymerizable compound | |
| EP4400561A1 (en) | Liquid-crystal medium |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22712845 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202280013417.4 Country of ref document: CN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 22712845 Country of ref document: EP Kind code of ref document: A1 |