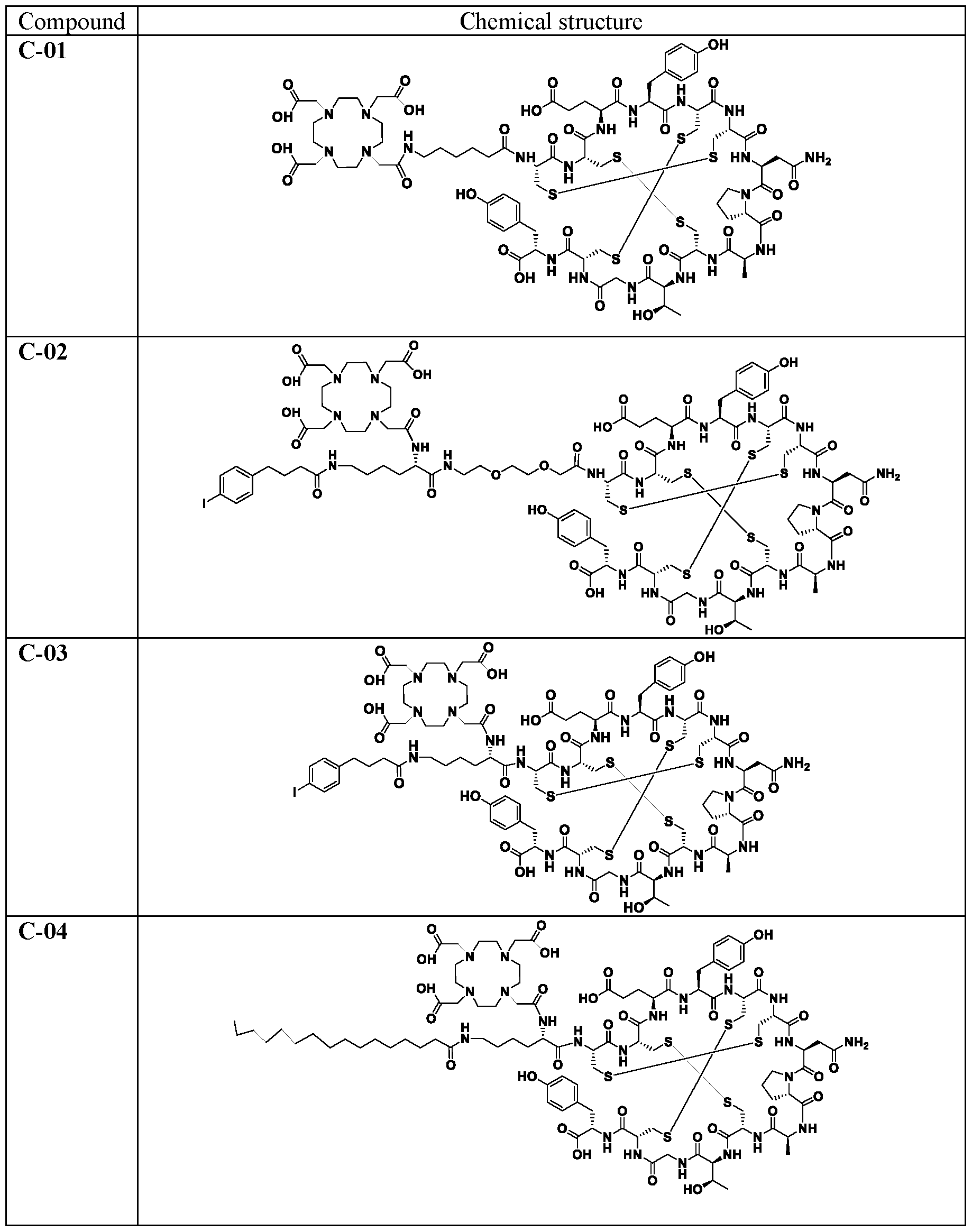
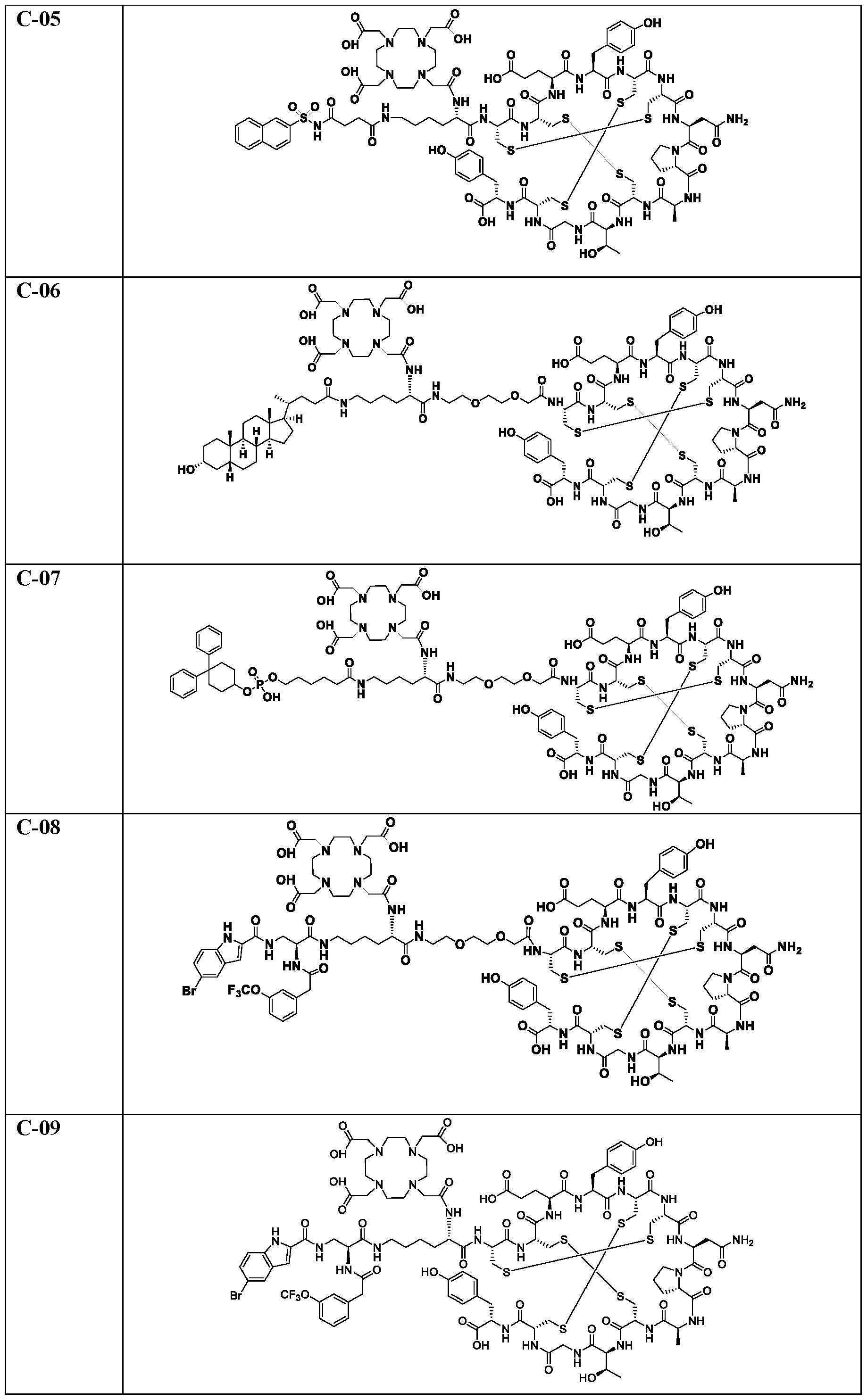
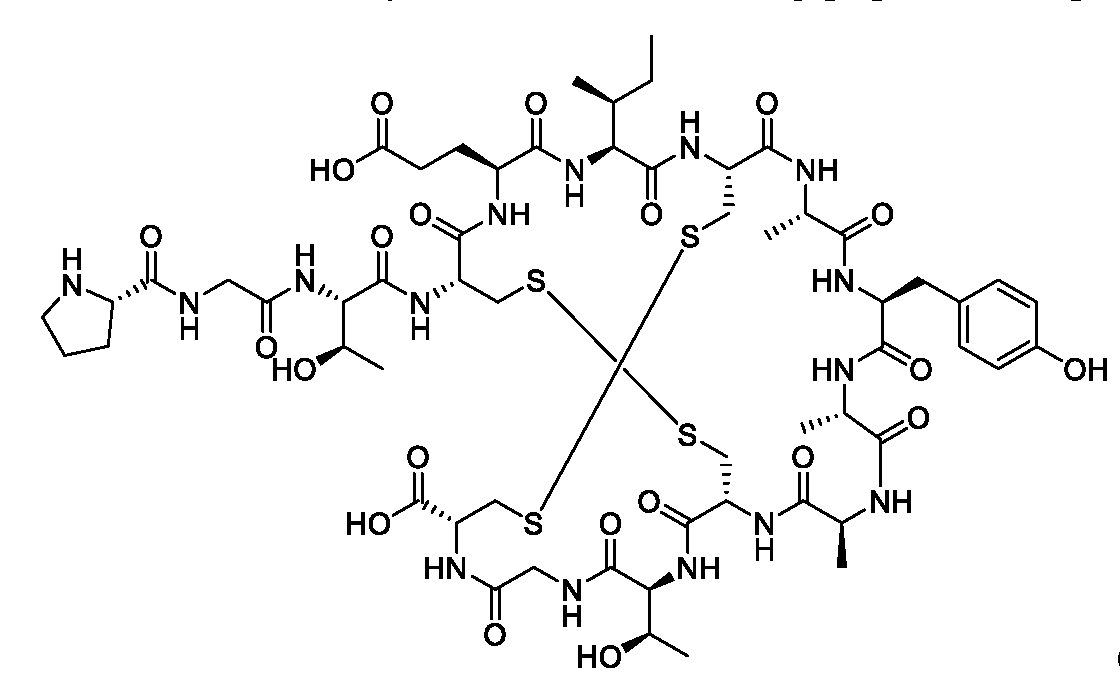
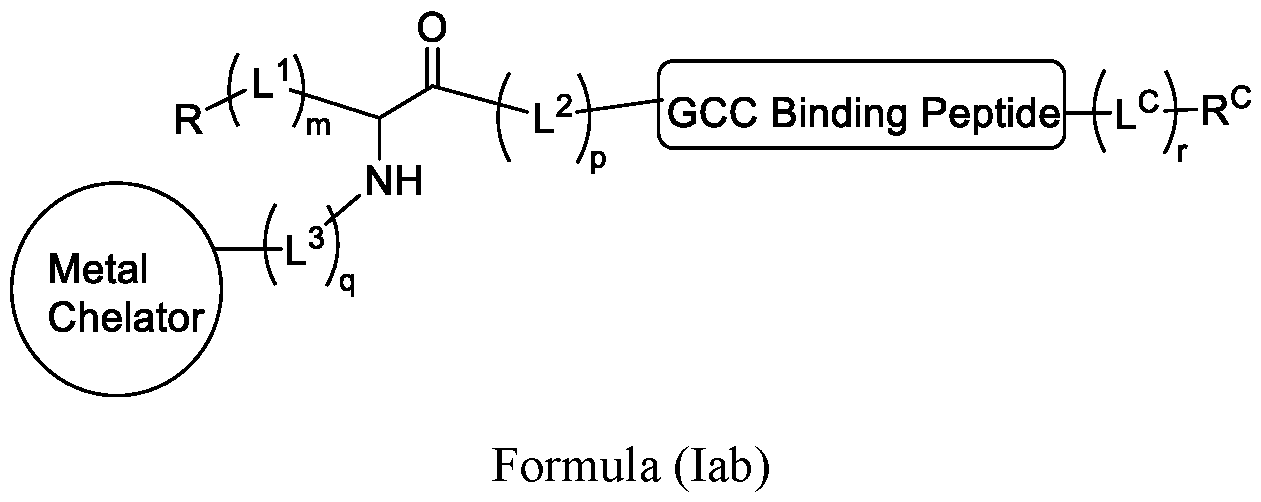
WO2022115799A1 - Radiopharmaceutical conjugates targeting guanylyl cyclase c, and compositions and uses thereof - Google Patents
Radiopharmaceutical conjugates targeting guanylyl cyclase c, and compositions and uses thereof Download PDFInfo
- Publication number
- WO2022115799A1 WO2022115799A1 PCT/US2021/061252 US2021061252W WO2022115799A1 WO 2022115799 A1 WO2022115799 A1 WO 2022115799A1 US 2021061252 W US2021061252 W US 2021061252W WO 2022115799 A1 WO2022115799 A1 WO 2022115799A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- substituted
- unsubstituted
- conjugate
- alkyl
- cycloalkyl
- Prior art date
Links
- 108010001687 Enterotoxin Receptors Proteins 0.000 title claims description 284
- 102000000820 Enterotoxin Receptors Human genes 0.000 title claims description 284
- 239000000203 mixture Substances 0.000 title abstract description 26
- 239000012217 radiopharmaceutical Substances 0.000 title abstract description 18
- 229940121896 radiopharmaceutical Drugs 0.000 title abstract description 18
- 230000002799 radiopharmaceutical effect Effects 0.000 title abstract description 18
- 230000008685 targeting Effects 0.000 title description 4
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 437
- 239000002738 chelating agent Substances 0.000 claims abstract description 179
- 229910052751 metal Inorganic materials 0.000 claims abstract description 178
- 239000002184 metal Substances 0.000 claims abstract description 178
- 238000000034 method Methods 0.000 claims abstract description 61
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 41
- 230000008030 elimination Effects 0.000 claims abstract description 38
- 238000003379 elimination reaction Methods 0.000 claims abstract description 38
- 201000011510 cancer Diseases 0.000 claims abstract description 8
- 125000001072 heteroaryl group Chemical group 0.000 claims description 242
- 125000003118 aryl group Chemical group 0.000 claims description 219
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 212
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 208
- 125000005647 linker group Chemical group 0.000 claims description 206
- -1 C1-C6aminoalkyl Chemical group 0.000 claims description 169
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 154
- 229940024606 amino acid Drugs 0.000 claims description 123
- 150000001413 amino acids Chemical class 0.000 claims description 101
- 125000004474 heteroalkylene group Chemical group 0.000 claims description 100
- 125000004429 atom Chemical group 0.000 claims description 96
- 125000002947 alkylene group Chemical group 0.000 claims description 90
- 229910052736 halogen Inorganic materials 0.000 claims description 88
- 125000004404 heteroalkyl group Chemical group 0.000 claims description 80
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 78
- 229910052739 hydrogen Inorganic materials 0.000 claims description 72
- 239000001257 hydrogen Substances 0.000 claims description 72
- 150000002367 halogens Chemical group 0.000 claims description 67
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 65
- 125000000217 alkyl group Chemical group 0.000 claims description 63
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 55
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 54
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 54
- 125000006716 (C1-C6) heteroalkyl group Chemical group 0.000 claims description 51
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 48
- 125000006577 C1-C6 hydroxyalkyl group Chemical group 0.000 claims description 48
- 125000000304 alkynyl group Chemical group 0.000 claims description 48
- 238000000338 in vitro Methods 0.000 claims description 44
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 43
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 43
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 42
- 125000000539 amino acid group Chemical group 0.000 claims description 41
- 125000004450 alkenylene group Chemical group 0.000 claims description 40
- 125000006730 (C2-C5) alkynyl group Chemical group 0.000 claims description 36
- 125000005843 halogen group Chemical group 0.000 claims description 35
- 125000004043 oxo group Chemical group O=* 0.000 claims description 32
- 150000003839 salts Chemical class 0.000 claims description 32
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 claims description 31
- 125000004419 alkynylene group Chemical group 0.000 claims description 27
- 125000001424 substituent group Chemical group 0.000 claims description 27
- 125000000739 C2-C30 alkenyl group Chemical group 0.000 claims description 25
- 125000000923 (C1-C30) alkyl group Chemical group 0.000 claims description 24
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 23
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 22
- WDLRUFUQRNWCPK-UHFFFAOYSA-N Tetraxetan Chemical group OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1 WDLRUFUQRNWCPK-UHFFFAOYSA-N 0.000 claims description 20
- 239000008194 pharmaceutical composition Substances 0.000 claims description 20
- 210000002966 serum Anatomy 0.000 claims description 19
- 239000012453 solvate Substances 0.000 claims description 17
- 150000001540 azides Chemical class 0.000 claims description 16
- 238000004128 high performance liquid chromatography Methods 0.000 claims description 16
- 150000001732 carboxylic acid derivatives Chemical class 0.000 claims description 14
- JUINSXZKUKVTMD-UHFFFAOYSA-N hydrogen azide Chemical group N=[N+]=[N-] JUINSXZKUKVTMD-UHFFFAOYSA-N 0.000 claims description 14
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 13
- 229930195729 fatty acid Natural products 0.000 claims description 13
- 239000000194 fatty acid Substances 0.000 claims description 13
- 229940125666 actinium-225 Drugs 0.000 claims description 12
- 239000003814 drug Substances 0.000 claims description 12
- 125000003107 substituted aryl group Chemical group 0.000 claims description 12
- QQINRWTZWGJFDB-YPZZEJLDSA-N actinium-225 Chemical group [225Ac] QQINRWTZWGJFDB-YPZZEJLDSA-N 0.000 claims description 11
- 150000004665 fatty acids Chemical class 0.000 claims description 11
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 11
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 11
- 229920001223 polyethylene glycol Polymers 0.000 claims description 11
- JHALWMSZGCVVEM-UHFFFAOYSA-N 2-[4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl]acetic acid Chemical compound OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CC1 JHALWMSZGCVVEM-UHFFFAOYSA-N 0.000 claims description 10
- 150000002191 fatty alcohols Chemical class 0.000 claims description 10
- OHSVLFRHMCKCQY-NJFSPNSNSA-N lutetium-177 Chemical group [177Lu] OHSVLFRHMCKCQY-NJFSPNSNSA-N 0.000 claims description 10
- 239000002202 Polyethylene glycol Substances 0.000 claims description 9
- 229930182558 Sterol Natural products 0.000 claims description 9
- BJAJDJDODCWPNS-UHFFFAOYSA-N dotp Chemical compound O=C1N2CCOC2=NC2=C1SC=C2 BJAJDJDODCWPNS-UHFFFAOYSA-N 0.000 claims description 9
- 150000003432 sterols Chemical class 0.000 claims description 9
- 235000003702 sterols Nutrition 0.000 claims description 9
- 125000005346 substituted cycloalkyl group Chemical group 0.000 claims description 9
- LDGWQMRUWMSZIU-LQDDAWAPSA-M 2,3-bis[(z)-octadec-9-enoxy]propyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCCOCC(C[N+](C)(C)C)OCCCCCCCC\C=C/CCCCCCCC LDGWQMRUWMSZIU-LQDDAWAPSA-M 0.000 claims description 8
- HHLZCENAOIROSL-UHFFFAOYSA-N 2-[4,7-bis(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetic acid Chemical compound OC(=O)CN1CCNCCN(CC(O)=O)CCN(CC(O)=O)CC1 HHLZCENAOIROSL-UHFFFAOYSA-N 0.000 claims description 8
- 125000000041 C6-C10 aryl group Chemical group 0.000 claims description 8
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 claims description 8
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 claims description 8
- 125000004103 aminoalkyl group Chemical group 0.000 claims description 8
- HCWPIIXVSYCSAN-OIOBTWANSA-N radium-223 Chemical compound [223Ra] HCWPIIXVSYCSAN-OIOBTWANSA-N 0.000 claims description 8
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 8
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 claims description 7
- 238000010494 dissociation reaction Methods 0.000 claims description 7
- 230000005593 dissociations Effects 0.000 claims description 7
- 125000001165 hydrophobic group Chemical group 0.000 claims description 7
- 239000002245 particle Substances 0.000 claims description 7
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 7
- 229940124530 sulfonamide Drugs 0.000 claims description 7
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 claims description 6
- FQIHLPGWBOBPSG-UHFFFAOYSA-N 2-[4,7,10-tris(2-amino-2-oxoethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetamide Chemical compound NC(=O)CN1CCN(CC(N)=O)CCN(CC(N)=O)CCN(CC(N)=O)CC1 FQIHLPGWBOBPSG-UHFFFAOYSA-N 0.000 claims description 6
- SYFGLWDDLZQFNI-UHFFFAOYSA-N 2-[4-(carboxymethyl)-1,4,8,11-tetrazabicyclo[6.6.2]hexadecan-11-yl]acetic acid Chemical compound C1CN(CC(O)=O)CCCN2CCN(CC(=O)O)CCCN1CC2 SYFGLWDDLZQFNI-UHFFFAOYSA-N 0.000 claims description 6
- UQQQAKFVWNQYTP-UHFFFAOYSA-N 3,6,10,13,16,19-hexazabicyclo[6.6.6]icosane-1,8-diamine Chemical compound C1NCCNCC2(N)CNCCNCC1(N)CNCCNC2 UQQQAKFVWNQYTP-UHFFFAOYSA-N 0.000 claims description 6
- 241000700159 Rattus Species 0.000 claims description 6
- RYXHOMYVWAEKHL-OUBTZVSYSA-N astatine-211 Chemical compound [211At] RYXHOMYVWAEKHL-OUBTZVSYSA-N 0.000 claims description 6
- 108700013553 diamsar chelate Proteins 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 6
- WHUUTDBJXJRKMK-VKHMYHEASA-L glutamate group Chemical group N[C@@H](CCC(=O)[O-])C(=O)[O-] WHUUTDBJXJRKMK-VKHMYHEASA-L 0.000 claims description 6
- 125000001624 naphthyl group Chemical group 0.000 claims description 6
- 229960005562 radium-223 Drugs 0.000 claims description 6
- 239000000018 receptor agonist Substances 0.000 claims description 6
- 229940044601 receptor agonist Drugs 0.000 claims description 6
- 150000003456 sulfonamides Chemical class 0.000 claims description 6
- ZSLUVFAKFWKJRC-FTXFMUIASA-N thorium-227 Chemical compound [227Th] ZSLUVFAKFWKJRC-FTXFMUIASA-N 0.000 claims description 6
- 229910019142 PO4 Inorganic materials 0.000 claims description 5
- SNHMUERNLJLMHN-UHFFFAOYSA-N iodobenzene Chemical compound IC1=CC=CC=C1 SNHMUERNLJLMHN-UHFFFAOYSA-N 0.000 claims description 5
- 239000010452 phosphate Substances 0.000 claims description 5
- AXDLCFOOGCNDST-VIFPVBQESA-N (2s)-3-(4-hydroxyphenyl)-2-(methylamino)propanoic acid Chemical compound CN[C@H](C(O)=O)CC1=CC=C(O)C=C1 AXDLCFOOGCNDST-VIFPVBQESA-N 0.000 claims description 4
- 206010009944 Colon cancer Diseases 0.000 claims description 4
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 4
- NHTGHBARYWONDQ-JTQLQIEISA-N L-α-methyl-Tyrosine Chemical compound OC(=O)[C@](N)(C)CC1=CC=C(O)C=C1 NHTGHBARYWONDQ-JTQLQIEISA-N 0.000 claims description 4
- OUYCCCASQSFEME-MRVPVSSYSA-N D-tyrosine Chemical compound OC(=O)[C@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-MRVPVSSYSA-N 0.000 claims description 3
- 229930195709 D-tyrosine Natural products 0.000 claims description 3
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 claims description 3
- PQFMNVGMJJMLAE-QMMMGPOBSA-N L-tyrosinamide Chemical compound NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 PQFMNVGMJJMLAE-QMMMGPOBSA-N 0.000 claims description 3
- 208000024558 digestive system cancer Diseases 0.000 claims description 3
- 201000010231 gastrointestinal system cancer Diseases 0.000 claims description 3
- 125000006303 iodophenyl group Chemical group 0.000 claims description 3
- 150000003668 tyrosines Chemical class 0.000 claims description 3
- LBDSXVIYZYSRII-IGMARMGPSA-N alpha-particle Chemical compound [4He+2] LBDSXVIYZYSRII-IGMARMGPSA-N 0.000 claims description 2
- 229940124597 therapeutic agent Drugs 0.000 claims description 2
- YYCZLGUOLIWZAW-GVETXGJZSA-N peptide 75 Chemical compound C([C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C1=CC=CC=C1 YYCZLGUOLIWZAW-GVETXGJZSA-N 0.000 claims 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 22
- 201000010099 disease Diseases 0.000 abstract description 14
- 239000000562 conjugate Substances 0.000 description 237
- 235000001014 amino acid Nutrition 0.000 description 117
- 150000001875 compounds Chemical class 0.000 description 67
- 150000002431 hydrogen Chemical class 0.000 description 48
- 125000004432 carbon atom Chemical group C* 0.000 description 40
- 102000004196 processed proteins & peptides Human genes 0.000 description 37
- 125000003342 alkenyl group Chemical group 0.000 description 31
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 28
- 239000000126 substance Substances 0.000 description 27
- 108010069514 Cyclic Peptides Proteins 0.000 description 26
- 102000001189 Cyclic Peptides Human genes 0.000 description 26
- 235000018102 proteins Nutrition 0.000 description 23
- 102000004169 proteins and genes Human genes 0.000 description 23
- 108090000623 proteins and genes Proteins 0.000 description 23
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 22
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 21
- 125000003545 alkoxy group Chemical group 0.000 description 18
- 229910052799 carbon Inorganic materials 0.000 description 18
- 125000001188 haloalkyl group Chemical group 0.000 description 18
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 16
- 210000001519 tissue Anatomy 0.000 description 16
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 14
- 102000004506 Blood Proteins Human genes 0.000 description 14
- 108010017384 Blood Proteins Proteins 0.000 description 14
- 125000004122 cyclic group Chemical group 0.000 description 14
- 239000003795 chemical substances by application Substances 0.000 description 13
- 229910052757 nitrogen Inorganic materials 0.000 description 13
- 150000002825 nitriles Chemical class 0.000 description 12
- 125000004433 nitrogen atom Chemical group N* 0.000 description 11
- 230000002285 radioactive effect Effects 0.000 description 11
- 238000011282 treatment Methods 0.000 description 11
- 101800004305 Guanylin Proteins 0.000 description 10
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 10
- 229940079593 drug Drugs 0.000 description 10
- 102000018009 guanylin Human genes 0.000 description 10
- 241000588724 Escherichia coli Species 0.000 description 9
- 125000002619 bicyclic group Chemical group 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 9
- KXGCNMMJRFDFNR-WDRJZQOASA-N linaclotide Chemical compound C([C@H](NC(=O)[C@@H]1CSSC[C@H]2C(=O)N[C@H]3CSSC[C@H](N)C(=O)N[C@H](C(N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N2)=O)CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(N)=O)NC3=O)C(=O)N[C@H](C(NCC(=O)N1)=O)[C@H](O)C)C(O)=O)C1=CC=C(O)C=C1 KXGCNMMJRFDFNR-WDRJZQOASA-N 0.000 description 9
- 108010024409 linaclotide Proteins 0.000 description 9
- 229960000812 linaclotide Drugs 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 150000003254 radicals Chemical class 0.000 description 9
- 239000002253 acid Substances 0.000 description 8
- 150000001721 carbon Chemical group 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 229910052760 oxygen Inorganic materials 0.000 description 8
- 238000007363 ring formation reaction Methods 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- SULKGYKWHKPPKO-RAJPIYRYSA-N (4s)-4-[[(2r)-2-[[(2s,3r)-2-[[(2s)-4-amino-4-oxo-2-[[(2s)-pyrrolidine-2-carbonyl]amino]butanoyl]amino]-3-hydroxybutanoyl]amino]-3-sulfanylpropanoyl]amino]-5-[[(2s,3s)-1-[[(2r)-1-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2r)-1-[[(2s,3r)-1-[[2-[[(1r)-1-carboxy Chemical compound N([C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CS)C(O)=O)[C@@H](C)O)C(=O)[C@@H]1CCCN1 SULKGYKWHKPPKO-RAJPIYRYSA-N 0.000 description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 7
- 102400000230 Uroguanylin Human genes 0.000 description 7
- 101800000255 Uroguanylin Proteins 0.000 description 7
- 150000001408 amides Chemical class 0.000 description 7
- 210000004899 c-terminal region Anatomy 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 125000000524 functional group Chemical group 0.000 description 7
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 7
- 229920001184 polypeptide Polymers 0.000 description 7
- 239000002904 solvent Substances 0.000 description 7
- 238000011191 terminal modification Methods 0.000 description 7
- SJMPVWVIVWEWJK-AXEIBBKLSA-N uroguanylin Chemical compound SC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(N)=O SJMPVWVIVWEWJK-AXEIBBKLSA-N 0.000 description 7
- 102100034605 Atrial natriuretic peptide receptor 3 Human genes 0.000 description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 6
- 101000924488 Homo sapiens Atrial natriuretic peptide receptor 3 Proteins 0.000 description 6
- 125000003282 alkyl amino group Chemical group 0.000 description 6
- 150000001412 amines Chemical class 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- XSCHRSMBECNVNS-UHFFFAOYSA-N benzopyrazine Natural products N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 125000002843 carboxylic acid group Chemical group 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 125000005842 heteroatom Chemical group 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 125000002950 monocyclic group Chemical group 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 125000003367 polycyclic group Chemical group 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 229910052717 sulfur Inorganic materials 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 229960004441 tyrosine Drugs 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 5
- 102000035195 Peptidases Human genes 0.000 description 5
- 108091005804 Peptidases Proteins 0.000 description 5
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 5
- 150000001345 alkine derivatives Chemical class 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 150000002148 esters Chemical class 0.000 description 5
- 150000002430 hydrocarbons Chemical group 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 230000000968 intestinal effect Effects 0.000 description 5
- 210000003734 kidney Anatomy 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- 238000007069 methylation reaction Methods 0.000 description 5
- 125000004430 oxygen atom Chemical group O* 0.000 description 5
- 230000002265 prevention Effects 0.000 description 5
- 102000005962 receptors Human genes 0.000 description 5
- 108020003175 receptors Proteins 0.000 description 5
- 239000011593 sulfur Substances 0.000 description 5
- 125000004434 sulfur atom Chemical group 0.000 description 5
- 150000003852 triazoles Chemical class 0.000 description 5
- NEMHIKRLROONTL-QMMMGPOBSA-N (2s)-2-azaniumyl-3-(4-azidophenyl)propanoate Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N=[N+]=[N-])C=C1 NEMHIKRLROONTL-QMMMGPOBSA-N 0.000 description 4
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 4
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 4
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 4
- 102000009027 Albumins Human genes 0.000 description 4
- 108010088751 Albumins Proteins 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- 230000021736 acetylation Effects 0.000 description 4
- 238000006640 acetylation reaction Methods 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 4
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 230000002068 genetic effect Effects 0.000 description 4
- 239000000543 intermediate Substances 0.000 description 4
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 4
- 230000002503 metabolic effect Effects 0.000 description 4
- 230000011987 methylation Effects 0.000 description 4
- 150000007524 organic acids Chemical class 0.000 description 4
- 235000021317 phosphate Nutrition 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 239000003053 toxin Substances 0.000 description 4
- 231100000765 toxin Toxicity 0.000 description 4
- 108700012359 toxins Proteins 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- JSXMFBNJRFXRCX-NSHDSACASA-N (2s)-2-amino-3-(4-prop-2-ynoxyphenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=C(OCC#C)C=C1 JSXMFBNJRFXRCX-NSHDSACASA-N 0.000 description 3
- FDSYTWVNUJTPMA-UHFFFAOYSA-N 2-[3,9-bis(carboxymethyl)-3,6,9,15-tetrazabicyclo[9.3.1]pentadeca-1(15),11,13-trien-6-yl]acetic acid Chemical compound C1N(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC2=CC=CC1=N2 FDSYTWVNUJTPMA-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- 150000008574 D-amino acids Chemical group 0.000 description 3
- 108010053187 Diphtheria Toxin Proteins 0.000 description 3
- 102000016607 Diphtheria Toxin Human genes 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- 108010078321 Guanylate Cyclase Proteins 0.000 description 3
- 102000014469 Guanylate cyclase Human genes 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 3
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 3
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 3
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- GEYBMYRBIABFTA-VIFPVBQESA-N O-methyl-L-tyrosine Chemical compound COC1=CC=C(C[C@H](N)C(O)=O)C=C1 GEYBMYRBIABFTA-VIFPVBQESA-N 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- VWQVUPCCIRVNHF-OUBTZVSYSA-N Yttrium-90 Chemical compound [90Y] VWQVUPCCIRVNHF-OUBTZVSYSA-N 0.000 description 3
- RCXMQNIDOFXYDO-UHFFFAOYSA-N [4,7,10-tris(phosphonomethyl)-1,4,7,10-tetrazacyclododec-1-yl]methylphosphonic acid Chemical compound OP(O)(=O)CN1CCN(CP(O)(O)=O)CCN(CP(O)(O)=O)CCN(CP(O)(O)=O)CC1 RCXMQNIDOFXYDO-UHFFFAOYSA-N 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 125000002015 acyclic group Chemical group 0.000 description 3
- 150000001576 beta-amino acids Chemical class 0.000 description 3
- XMIIGOLPHOKFCH-UHFFFAOYSA-N beta-phenylpropanoic acid Natural products OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 238000004422 calculation algorithm Methods 0.000 description 3
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 3
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000010949 copper Substances 0.000 description 3
- ZOOGRGPOEVQQDX-KHLHZJAASA-N cyclic guanosine monophosphate Chemical compound C([C@H]1O2)O[P@](O)(=O)O[C@@H]1[C@H](O)[C@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-KHLHZJAASA-N 0.000 description 3
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 3
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- 229960000975 daunorubicin Drugs 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 125000001153 fluoro group Chemical group F* 0.000 description 3
- 125000003709 fluoroalkyl group Chemical group 0.000 description 3
- 125000002541 furyl group Chemical group 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- 235000013922 glutamic acid Nutrition 0.000 description 3
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 3
- 229930195733 hydrocarbon Natural products 0.000 description 3
- 238000010348 incorporation Methods 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 3
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 3
- 125000000468 ketone group Chemical group 0.000 description 3
- WABPQHHGFIMREM-BKFZFHPZSA-N lead-212 Chemical group [212Pb] WABPQHHGFIMREM-BKFZFHPZSA-N 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 150000002678 macrocyclic compounds Chemical class 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 3
- 229960005190 phenylalanine Drugs 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 235000019419 proteases Nutrition 0.000 description 3
- 230000017854 proteolysis Effects 0.000 description 3
- 125000003373 pyrazinyl group Chemical group 0.000 description 3
- 125000004076 pyridyl group Chemical group 0.000 description 3
- 125000000714 pyrimidinyl group Chemical group 0.000 description 3
- HCWPIIXVSYCSAN-YPZZEJLDSA-N radium-224 Chemical compound [224Ra] HCWPIIXVSYCSAN-YPZZEJLDSA-N 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
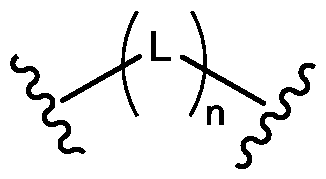
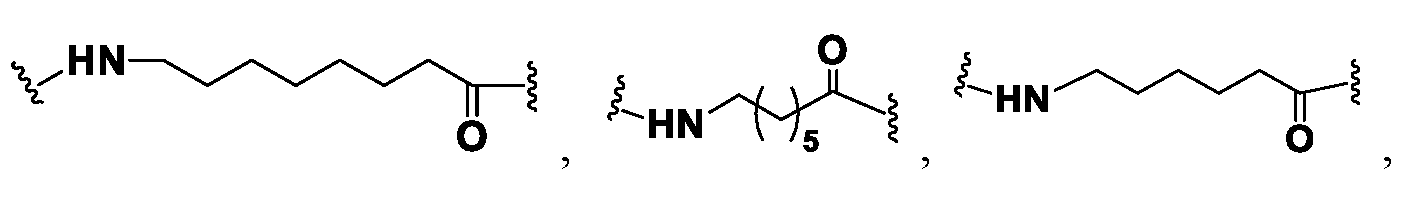
- 125000006850 spacer group Chemical group 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- GZCRRIHWUXGPOV-CBESVEIWSA-N terbium-149 Chemical compound [149Tb] GZCRRIHWUXGPOV-CBESVEIWSA-N 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 238000002411 thermogravimetry Methods 0.000 description 3
- 125000001113 thiadiazolyl group Chemical group 0.000 description 3
- 125000000335 thiazolyl group Chemical group 0.000 description 3
- 125000001544 thienyl group Chemical group 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- NSPHQWLKCGGCQR-DLJDZFDSSA-N (2s)-2-[[(1r,4s,7s,10s,13s,16r,21r,27s,34r,37s,40s)-10-(2-amino-2-oxoethyl)-34-[[(2s)-4-carboxy-2-[[(2s)-3-carboxy-2-[[(2s)-2,4-diamino-4-oxobutanoyl]amino]propanoyl]amino]butanoyl]amino]-37-(2-carboxyethyl)-27-[(1r)-1-hydroxyethyl]-4-methyl-40-(2-methylp Chemical compound N1C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)CSSC[C@@H]2NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CSSC[C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)CNC(=O)[C@H]([C@@H](C)O)NC2=O NSPHQWLKCGGCQR-DLJDZFDSSA-N 0.000 description 2
- MFRNYXJJRJQHNW-DEMKXPNLSA-N (2s)-2-[[(2r,3r)-3-methoxy-3-[(2s)-1-[(3r,4s,5s)-3-methoxy-5-methyl-4-[methyl-[(2s)-3-methyl-2-[[(2s)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MFRNYXJJRJQHNW-DEMKXPNLSA-N 0.000 description 2
- MSECZMWQBBVGEN-LURJTMIESA-N (2s)-2-azaniumyl-4-(1h-imidazol-5-yl)butanoate Chemical compound OC(=O)[C@@H](N)CCC1=CN=CN1 MSECZMWQBBVGEN-LURJTMIESA-N 0.000 description 2
- NOPNWHSMQOXAEI-PUCKCBAPSA-N (7s,9s)-7-[(2r,4s,5s,6s)-4-(2,3-dihydropyrrol-1-yl)-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione Chemical compound N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCC=C1 NOPNWHSMQOXAEI-PUCKCBAPSA-N 0.000 description 2
- 125000003161 (C1-C6) alkylene group Chemical group 0.000 description 2
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 2
- ITWBWJFEJCHKSN-UHFFFAOYSA-N 1,4,7-triazonane Chemical compound C1CNCCNCCN1 ITWBWJFEJCHKSN-UHFFFAOYSA-N 0.000 description 2
- FLBAYUMRQUHISI-UHFFFAOYSA-N 1,8-naphthyridine Chemical compound N1=CC=CC2=CC=CN=C21 FLBAYUMRQUHISI-UHFFFAOYSA-N 0.000 description 2
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 2
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- RAEOEMDZDMCHJA-UHFFFAOYSA-N 2-[2-[bis(carboxymethyl)amino]ethyl-[2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]ethyl]amino]acetic acid Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O RAEOEMDZDMCHJA-UHFFFAOYSA-N 0.000 description 2
- GRUVVLWKPGIYEG-UHFFFAOYSA-N 2-[2-[carboxymethyl-[(2-hydroxyphenyl)methyl]amino]ethyl-[(2-hydroxyphenyl)methyl]amino]acetic acid Chemical compound C=1C=CC=C(O)C=1CN(CC(=O)O)CCN(CC(O)=O)CC1=CC=CC=C1O GRUVVLWKPGIYEG-UHFFFAOYSA-N 0.000 description 2
- UDOPJKHABYSVIX-UHFFFAOYSA-N 2-[4,7,10-tris(carboxymethyl)-6-[(4-isothiocyanatophenyl)methyl]-1,4,7,10-tetrazacyclododec-1-yl]acetic acid Chemical compound C1N(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CCN(CC(O)=O)C1CC1=CC=C(N=C=S)C=C1 UDOPJKHABYSVIX-UHFFFAOYSA-N 0.000 description 2
- GTACSIONMHMRPD-UHFFFAOYSA-N 2-[4-[2-(benzenesulfonamido)ethylsulfanyl]-2,6-difluorophenoxy]acetamide Chemical compound C1=C(F)C(OCC(=O)N)=C(F)C=C1SCCNS(=O)(=O)C1=CC=CC=C1 GTACSIONMHMRPD-UHFFFAOYSA-N 0.000 description 2
- NEAQRZUHTPSBBM-UHFFFAOYSA-N 2-hydroxy-3,3-dimethyl-7-nitro-4h-isoquinolin-1-one Chemical class C1=C([N+]([O-])=O)C=C2C(=O)N(O)C(C)(C)CC2=C1 NEAQRZUHTPSBBM-UHFFFAOYSA-N 0.000 description 2
- ZRPLANDPDWYOMZ-UHFFFAOYSA-N 3-cyclopentylpropionic acid Chemical compound OC(=O)CCC1CCCC1 ZRPLANDPDWYOMZ-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 2
- GDRVFDDBLLKWRI-UHFFFAOYSA-N 4H-quinolizine Chemical compound C1=CC=CN2CC=CC=C21 GDRVFDDBLLKWRI-UHFFFAOYSA-N 0.000 description 2
- PKCFWCMMZOUICG-UHFFFAOYSA-N 6-[[16-[(6-carboxypyridin-2-yl)methyl]-1,4,10,13-tetraoxa-7,16-diazacyclooctadec-7-yl]methyl]-4-isothiocyanatopyridine-2-carboxylic acid Chemical compound OC(=O)C1=CC=CC(CN2CCOCCOCCN(CC3=NC(=CC(=C3)N=C=S)C(O)=O)CCOCCOCC2)=N1 PKCFWCMMZOUICG-UHFFFAOYSA-N 0.000 description 2
- FJHBVJOVLFPMQE-QFIPXVFZSA-N 7-Ethyl-10-Hydroxy-Camptothecin Chemical compound C1=C(O)C=C2C(CC)=C(CN3C(C4=C([C@@](C(=O)OC4)(O)CC)C=C33)=O)C3=NC2=C1 FJHBVJOVLFPMQE-QFIPXVFZSA-N 0.000 description 2
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 2
- 230000005730 ADP ribosylation Effects 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 101710130081 Aspergillopepsin-1 Proteins 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- 102100031007 Cytosolic non-specific dipeptidase Human genes 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical group OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 108010092160 Dactinomycin Proteins 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- GYHNNYVSQQEPJS-OIOBTWANSA-N Gallium-67 Chemical compound [67Ga] GYHNNYVSQQEPJS-OIOBTWANSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- IQUHNCOJRJBMSU-UHFFFAOYSA-N H3HP-DO3A Chemical compound CC(O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1 IQUHNCOJRJBMSU-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- 229930194542 Keto Natural products 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 229930126263 Maytansine Natural products 0.000 description 2
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 108010039491 Ricin Proteins 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 208000035896 Twin-reversed arterial perfusion sequence Diseases 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 241000607626 Vibrio cholerae Species 0.000 description 2
- 241000607447 Yersinia enterocolitica Species 0.000 description 2
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 2
- 230000006154 adenylylation Effects 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 125000005090 alkenylcarbonyl group Chemical group 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 2
- 125000005119 alkyl cycloalkyl group Chemical group 0.000 description 2
- 125000005213 alkyl heteroaryl group Chemical group 0.000 description 2
- 125000005087 alkynylcarbonyl group Chemical group 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 230000009435 amidation Effects 0.000 description 2
- 238000007112 amidation reaction Methods 0.000 description 2
- 125000003368 amide group Chemical group 0.000 description 2
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 150000005840 aryl radicals Chemical class 0.000 description 2
- 125000000732 arylene group Chemical group 0.000 description 2
- 108010044540 auristatin Proteins 0.000 description 2
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- RFRXIWQYSOIBDI-UHFFFAOYSA-N benzarone Chemical compound CCC=1OC2=CC=CC=C2C=1C(=O)C1=CC=C(O)C=C1 RFRXIWQYSOIBDI-UHFFFAOYSA-N 0.000 description 2
- 230000001851 biosynthetic effect Effects 0.000 description 2
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 2
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 2
- QARVLSVVCXYDNA-UHFFFAOYSA-N bromobenzene Chemical compound BrC1=CC=CC=C1 QARVLSVVCXYDNA-UHFFFAOYSA-N 0.000 description 2
- 229940127093 camptothecin Drugs 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- WCZVZNOTHYJIEI-UHFFFAOYSA-N cinnoline Chemical compound N1=NC=CC2=CC=CC=C21 WCZVZNOTHYJIEI-UHFFFAOYSA-N 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 238000004590 computer program Methods 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 125000006254 cycloalkyl carbonyl group Chemical group 0.000 description 2
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- XVOYSCVBGLVSOL-UHFFFAOYSA-N cysteic acid Chemical compound OC(=O)C(N)CS(O)(=O)=O XVOYSCVBGLVSOL-UHFFFAOYSA-N 0.000 description 2
- 125000004855 decalinyl group Chemical group C1(CCCC2CCCCC12)* 0.000 description 2
- 230000002939 deleterious effect Effects 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 229960003668 docetaxel Drugs 0.000 description 2
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 2
- 238000009509 drug development Methods 0.000 description 2
- 239000003792 electrolyte Substances 0.000 description 2
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 2
- 229960001842 estramustine Drugs 0.000 description 2
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 2
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 2
- 125000003838 furazanyl group Chemical group 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 125000003827 glycol group Chemical group 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 150000004677 hydrates Chemical class 0.000 description 2
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical group [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- APFVFJFRJDLVQX-AHCXROLUSA-N indium-111 Chemical compound [111In] APFVFJFRJDLVQX-AHCXROLUSA-N 0.000 description 2
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 2
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 2
- HOBCFUWDNJPFHB-UHFFFAOYSA-N indolizine Chemical compound C1=CC=CN2C=CC=C21 HOBCFUWDNJPFHB-UHFFFAOYSA-N 0.000 description 2
- 230000004968 inflammatory condition Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 210000004347 intestinal mucosa Anatomy 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 125000001786 isothiazolyl group Chemical group 0.000 description 2
- 125000000842 isoxazolyl group Chemical group 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 210000003712 lysosome Anatomy 0.000 description 2
- 230000001868 lysosomic effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 108010093470 monomethyl auristatin E Proteins 0.000 description 2
- 108010059074 monomethylauristatin F Proteins 0.000 description 2
- TXXHDPDFNKHHGW-UHFFFAOYSA-N muconic acid Chemical group OC(=O)C=CC=CC(O)=O TXXHDPDFNKHHGW-UHFFFAOYSA-N 0.000 description 2
- 230000007498 myristoylation Effects 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000002868 norbornyl group Chemical group C12(CCC(CC1)C2)* 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 125000001715 oxadiazolyl group Chemical group 0.000 description 2
- 125000002971 oxazolyl group Chemical group 0.000 description 2
- 230000026792 palmitoylation Effects 0.000 description 2
- 229950009215 phenylbutanoic acid Drugs 0.000 description 2
- 235000011007 phosphoric acid Nutrition 0.000 description 2
- 125000004437 phosphorous atom Chemical group 0.000 description 2
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 2
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical compound C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 2
- 108010018859 plecanatide Proteins 0.000 description 2
- 229950008515 plecanatide Drugs 0.000 description 2
- 238000000634 powder X-ray diffraction Methods 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- VQMWBBYLQSCNPO-RNFDNDRNSA-N promethium-149 Chemical compound [149Pm] VQMWBBYLQSCNPO-RNFDNDRNSA-N 0.000 description 2
- 230000006289 propionylation Effects 0.000 description 2
- 238000010515 propionylation reaction Methods 0.000 description 2
- CPNGPNLZQNNVQM-UHFFFAOYSA-N pteridine Chemical compound N1=CN=CC2=NC=CN=C21 CPNGPNLZQNNVQM-UHFFFAOYSA-N 0.000 description 2
- 125000003226 pyrazolyl group Chemical group 0.000 description 2
- 125000002098 pyridazinyl group Chemical group 0.000 description 2
- YUOCYTRGANSSRY-UHFFFAOYSA-N pyrrolo[2,3-i][1,2]benzodiazepine Chemical compound C1=CN=NC2=C3C=CN=C3C=CC2=C1 YUOCYTRGANSSRY-UHFFFAOYSA-N 0.000 description 2
- 125000000168 pyrrolyl group Chemical group 0.000 description 2
- 238000005956 quaternization reaction Methods 0.000 description 2
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 239000002464 receptor antagonist Substances 0.000 description 2
- 229940044551 receptor antagonist Drugs 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical group OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- KZUNJOHGWZRPMI-AKLPVKDBSA-N samarium-153 Chemical compound [153Sm] KZUNJOHGWZRPMI-AKLPVKDBSA-N 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 125000003003 spiro group Chemical group 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 125000000547 substituted alkyl group Chemical group 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 2
- 125000003831 tetrazolyl group Chemical group 0.000 description 2
- 150000003573 thiols Chemical class 0.000 description 2
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 2
- 229960000303 topotecan Drugs 0.000 description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 238000000844 transformation Methods 0.000 description 2
- 125000004306 triazinyl group Chemical group 0.000 description 2
- 125000001425 triazolyl group Chemical group 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical compound CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- PXOMSWXCVZBBIV-PQKSKRJKSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4S,6R)-4-amino-2-methyl-6-[[(1S,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,11-dioxo-2,4-dihydro-1H-tetracen-1-yl]oxy]oxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound C[C@H]1[C@@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)C(=O)O)O)O)O PXOMSWXCVZBBIV-PQKSKRJKSA-N 0.000 description 1
- FDKWRPBBCBCIGA-REOHCLBHSA-N (2r)-2-azaniumyl-3-$l^{1}-selanylpropanoate Chemical compound [Se]C[C@H](N)C(O)=O FDKWRPBBCBCIGA-REOHCLBHSA-N 0.000 description 1
- WOWDZACBATWTAU-FEFUEGSOSA-N (2s)-2-[[(2s)-2-(dimethylamino)-3-methylbutanoyl]amino]-n-[(3r,4s,5s)-1-[(2s)-2-[(1r,2r)-3-[[(1s,2r)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-n,3-dimethylbutanamide Chemical compound CC(C)[C@H](N(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)C1=CC=CC=C1 WOWDZACBATWTAU-FEFUEGSOSA-N 0.000 description 1
- YYTDJPUFAVPHQA-VKHMYHEASA-N (2s)-2-amino-3-(2,3,4,5,6-pentafluorophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=C(F)C(F)=C(F)C(F)=C1F YYTDJPUFAVPHQA-VKHMYHEASA-N 0.000 description 1
- NFIVJOSXJDORSP-QMMMGPOBSA-N (2s)-2-amino-3-(4-boronophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=C(B(O)O)C=C1 NFIVJOSXJDORSP-QMMMGPOBSA-N 0.000 description 1
- PEMUHKUIQHFMTH-QMMMGPOBSA-N (2s)-2-amino-3-(4-bromophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=C(Br)C=C1 PEMUHKUIQHFMTH-QMMMGPOBSA-N 0.000 description 1
- BJOQKIKXKGJLIJ-NSHDSACASA-N (2s)-2-amino-3-(4-prop-2-ynylphenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=C(CC#C)C=C1 BJOQKIKXKGJLIJ-NSHDSACASA-N 0.000 description 1
- HTFFMYRVHHNNBE-YFKPBYRVSA-N (2s)-2-amino-6-azidohexanoic acid Chemical compound OC(=O)[C@@H](N)CCCCN=[N+]=[N-] HTFFMYRVHHNNBE-YFKPBYRVSA-N 0.000 description 1
- ZXSBHXZKWRIEIA-JTQLQIEISA-N (2s)-3-(4-acetylphenyl)-2-azaniumylpropanoate Chemical compound CC(=O)C1=CC=C(C[C@H](N)C(O)=O)C=C1 ZXSBHXZKWRIEIA-JTQLQIEISA-N 0.000 description 1
- IWHCAJPPWOMXNW-ZESMOPTKSA-N (2s,3r)-2-[[(2s)-2-[[(2s)-4-amino-2-[[(2s,3r)-2-[[(2s,3r)-2-[[(2s,3r)-2-[[(2s)-2-[[(2r)-2-aminopropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxybutanoyl]amino]-4-oxobutanoyl]amino]-3-(4-hydroxyphenyl)pr Chemical compound C[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H]([C@H](O)C)C(O)=O)CC1=CC=C(O)C=C1 IWHCAJPPWOMXNW-ZESMOPTKSA-N 0.000 description 1
- APOKYMYZOKIMLM-LUMVZWMBSA-N (2s,3s,4s,5r,6s)-3,4,5-trihydroxy-6-[4-[[(2s,3s,4s,6r)-3-hydroxy-2-methyl-6-[[(1s,3s)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,11-dioxo-2,4-dihydro-1h-tetracen-1-yl]oxy]oxan-4-yl]carbamoyloxymethyl]-2-nitrophenoxy]oxane-2-carboxylic acid Chemical compound N([C@H]1C[C@@H](O[C@@H](C)[C@H]1O)O[C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)C(=O)OCC(C=C1[N+]([O-])=O)=CC=C1O[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O APOKYMYZOKIMLM-LUMVZWMBSA-N 0.000 description 1
- URCVASXWNJQAEH-HDWVWLDDSA-N (2s,3s,4s,5r,6s)-6-[4-[(5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-8-oxo-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[5,6-f][1,3]benzodioxol-9-yl]-2,6-dimethoxyphenoxy]-3,4,5-trihydrox Chemical compound COC1=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=CC(OC)=C1O[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O URCVASXWNJQAEH-HDWVWLDDSA-N 0.000 description 1
- DLKUYSQUHXBYPB-NSSHGSRYSA-N (2s,4r)-4-[[2-[(1r,3r)-1-acetyloxy-4-methyl-3-[3-methylbutanoyloxymethyl-[(2s,3s)-3-methyl-2-[[(2r)-1-methylpiperidine-2-carbonyl]amino]pentanoyl]amino]pentyl]-1,3-thiazole-4-carbonyl]amino]-2-methyl-5-(4-methylphenyl)pentanoic acid Chemical compound N([C@@H]([C@@H](C)CC)C(=O)N(COC(=O)CC(C)C)[C@H](C[C@@H](OC(C)=O)C=1SC=C(N=1)C(=O)N[C@H](C[C@H](C)C(O)=O)CC=1C=CC(C)=CC=1)C(C)C)C(=O)[C@H]1CCCCN1C DLKUYSQUHXBYPB-NSSHGSRYSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 description 1
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 description 1
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 1
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 description 1
- 125000006701 (C1-C7) alkyl group Chemical group 0.000 description 1
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 1
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 description 1
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 description 1
- 125000006729 (C2-C5) alkenyl group Chemical group 0.000 description 1
- 125000006645 (C3-C4) cycloalkyl group Chemical group 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 125000005988 1,1-dioxo-thiomorpholinyl group Chemical group 0.000 description 1
- AEBWATHAIVJLTA-UHFFFAOYSA-N 1,2,3,3a,4,5,6,6a-octahydropentalene Chemical compound C1CCC2CCCC21 AEBWATHAIVJLTA-UHFFFAOYSA-N 0.000 description 1
- KEIFWROAQVVDBN-UHFFFAOYSA-N 1,2-dihydronaphthalene Chemical compound C1=CC=C2C=CCCC2=C1 KEIFWROAQVVDBN-UHFFFAOYSA-N 0.000 description 1
- QBPPRVHXOZRESW-UHFFFAOYSA-N 1,4,7,10-tetraazacyclododecane Chemical compound C1CNCCNCCNCCN1 QBPPRVHXOZRESW-UHFFFAOYSA-N 0.000 description 1
- KIUIVKNVSSLOAG-UHFFFAOYSA-N 1,4,7,10-tetrazacyclotridecan-11-one Chemical compound O=C1CCNCCNCCNCCN1 KIUIVKNVSSLOAG-UHFFFAOYSA-N 0.000 description 1
- XJKSTNDFUHDPQJ-UHFFFAOYSA-N 1,4-diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=C(C=2C=CC=CC=2)C=C1 XJKSTNDFUHDPQJ-UHFFFAOYSA-N 0.000 description 1
- 102100025573 1-alkyl-2-acetylglycerophosphocholine esterase Human genes 0.000 description 1
- AMMPLVWPWSYRDR-UHFFFAOYSA-N 1-methylbicyclo[2.2.2]oct-2-ene-4-carboxylic acid Chemical compound C1CC2(C(O)=O)CCC1(C)C=C2 AMMPLVWPWSYRDR-UHFFFAOYSA-N 0.000 description 1
- ILHFCLNOTOJYHF-UHFFFAOYSA-N 1-n,3-n,5-n-tris(pyridin-2-ylmethyl)cyclohexane-1,3,5-triamine Chemical compound C=1C=CC=NC=1CNC(C1)CC(NCC=2N=CC=CC=2)CC1NCC1=CC=CC=N1 ILHFCLNOTOJYHF-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- 125000005987 1-oxo-thiomorpholinyl group Chemical group 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- HAWSQZCWOQZXHI-FQEVSTJZSA-N 10-Hydroxycamptothecin Chemical compound C1=C(O)C=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 HAWSQZCWOQZXHI-FQEVSTJZSA-N 0.000 description 1
- BFPYWIDHMRZLRN-UHFFFAOYSA-N 17alpha-ethynyl estradiol Natural products OC1=CC=C2C3CCC(C)(C(CC4)(O)C#C)C4C3CCC2=C1 BFPYWIDHMRZLRN-UHFFFAOYSA-N 0.000 description 1
- WUAPFZMCVAUBPE-NJFSPNSNSA-N 188Re Chemical compound [188Re] WUAPFZMCVAUBPE-NJFSPNSNSA-N 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- SKWCZPYWFRTSDD-UHFFFAOYSA-N 2,3-bis(azaniumyl)propanoate;chloride Chemical compound Cl.NCC(N)C(O)=O SKWCZPYWFRTSDD-UHFFFAOYSA-N 0.000 description 1
- HMBHAQMOBKLWRX-UHFFFAOYSA-N 2,3-dihydro-1,4-benzodioxine-3-carboxylic acid Chemical compound C1=CC=C2OC(C(=O)O)COC2=C1 HMBHAQMOBKLWRX-UHFFFAOYSA-N 0.000 description 1
- HCSBTDBGTNZOAB-UHFFFAOYSA-N 2,3-dinitrobenzoic acid Chemical compound OC(=O)C1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O HCSBTDBGTNZOAB-UHFFFAOYSA-N 0.000 description 1
- OMGHIGVFLOPEHJ-UHFFFAOYSA-N 2,5-dihydro-1h-pyrrol-1-ium-2-carboxylate Chemical compound OC(=O)C1NCC=C1 OMGHIGVFLOPEHJ-UHFFFAOYSA-N 0.000 description 1
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 1
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 1
- XBXHBDPVWILCAG-MUGJNUQGSA-N 2-[(2S,5S,8S,11S)-4,7,10-tris(carboxymethyl)-2,5,8,11-tetraethyl-1,4,7,10-tetrazacyclododec-1-yl]acetic acid Chemical group CC[C@H]1CN([C@H](CN([C@H](CN([C@H](CN1CC(=O)O)CC)CC(=O)O)CC)CC(=O)O)CC)CC(=O)O XBXHBDPVWILCAG-MUGJNUQGSA-N 0.000 description 1
- ZRLVKTFJWWRBOU-VGWMRTNUSA-N 2-[(2s,5s,8s,11s)-4,7,10-tris(carboxymethyl)-2,5,8,11-tetramethyl-1,4,7,10-tetrazacyclododec-1-yl]acetic acid Chemical group C[C@H]1CN(CC(O)=O)[C@@H](C)CN(CC(O)=O)[C@@H](C)CN(CC(O)=O)[C@@H](C)CN1CC(O)=O ZRLVKTFJWWRBOU-VGWMRTNUSA-N 0.000 description 1
- LZBLLVHUXJXXNS-UHFFFAOYSA-N 2-[4,7,10-tris(2-amino-2-oxoethyl)-6-[(4-isothiocyanatophenyl)methyl]-1,4,7,10-tetrazacyclododec-1-yl]acetamide Chemical compound C1N(CC(N)=O)CCN(CC(=O)N)CCN(CC(N)=O)CCN(CC(N)=O)C1CC1=CC=C(N=C=S)C=C1 LZBLLVHUXJXXNS-UHFFFAOYSA-N 0.000 description 1
- VOXBZHOHGGBLCQ-UHFFFAOYSA-N 2-amino-3,7-dihydropurine-6-thione;hydrate Chemical compound O.N1C(N)=NC(=S)C2=C1N=CN2.N1C(N)=NC(=S)C2=C1N=CN2 VOXBZHOHGGBLCQ-UHFFFAOYSA-N 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- STNZNCWQNMGRIM-UHFFFAOYSA-N 2-benzyl-1,4,7,10-tetrakis-(4-methylphenyl)sulfonyl-1,4,7,10-tetrazacyclododecane Chemical compound C1=CC(C)=CC=C1S(=O)(=O)N1CCN(S(=O)(=O)C=2C=CC(C)=CC=2)CC(CC=2C=CC=CC=2)N(S(=O)(=O)C=2C=CC(C)=CC=2)CCN(S(=O)(=O)C=2C=CC(C)=CC=2)CC1 STNZNCWQNMGRIM-UHFFFAOYSA-N 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- UPHOPMSGKZNELG-UHFFFAOYSA-N 2-hydroxynaphthalene-1-carboxylic acid Chemical group C1=CC=C2C(C(=O)O)=C(O)C=CC2=C1 UPHOPMSGKZNELG-UHFFFAOYSA-N 0.000 description 1
- 125000004918 2-methyl-2-pentyl group Chemical group CC(C)(CCC)* 0.000 description 1
- 125000004493 2-methylbut-1-yl group Chemical group CC(C*)CC 0.000 description 1
- 125000004638 2-oxopiperazinyl group Chemical group O=C1N(CCNC1)* 0.000 description 1
- 125000004637 2-oxopiperidinyl group Chemical group O=C1N(CCCC1)* 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- YIMDLWDNDGKDTJ-QLKYHASDSA-N 3'-deamino-3'-(3-cyanomorpholin-4-yl)doxorubicin Chemical compound N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCOCC1C#N YIMDLWDNDGKDTJ-QLKYHASDSA-N 0.000 description 1
- XLZYKTYMLBOINK-UHFFFAOYSA-N 3-(4-hydroxybenzoyl)benzoic acid Chemical compound OC(=O)C1=CC=CC(C(=O)C=2C=CC(O)=CC=2)=C1 XLZYKTYMLBOINK-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- RUXHWBMJNBBYNL-UHFFFAOYSA-N 3-hydroxy-1,2-dihydropyrrol-5-one Chemical compound OC1=CC(=O)NC1 RUXHWBMJNBBYNL-UHFFFAOYSA-N 0.000 description 1
- 125000004919 3-methyl-2-pentyl group Chemical group CC(C(C)*)CC 0.000 description 1
- JZRBSTONIYRNRI-VIFPVBQESA-N 3-methylphenylalanine Chemical compound CC1=CC=CC(C[C@H](N)C(O)=O)=C1 JZRBSTONIYRNRI-VIFPVBQESA-N 0.000 description 1
- IRZQDMYEJPNDEN-UHFFFAOYSA-N 3-phenyl-2-aminobutanoic acid Natural products OC(=O)C(N)C(C)C1=CC=CC=C1 IRZQDMYEJPNDEN-UHFFFAOYSA-N 0.000 description 1
- XMIIGOLPHOKFCH-UHFFFAOYSA-M 3-phenylpropionate Chemical compound [O-]C(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-M 0.000 description 1
- CMUHFUGDYMFHEI-QMMMGPOBSA-N 4-amino-L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N)C=C1 CMUHFUGDYMFHEI-QMMMGPOBSA-N 0.000 description 1
- SJZRECIVHVDYJC-UHFFFAOYSA-N 4-hydroxybutyric acid Chemical compound OCCCC(O)=O SJZRECIVHVDYJC-UHFFFAOYSA-N 0.000 description 1
- PZNQZSRPDOEBMS-QMMMGPOBSA-N 4-iodo-L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(I)C=C1 PZNQZSRPDOEBMS-QMMMGPOBSA-N 0.000 description 1
- 125000004920 4-methyl-2-pentyl group Chemical group CC(CC(C)*)C 0.000 description 1
- KJSJBKBZMGSIPT-UHFFFAOYSA-N 4-oxo-3-phenylmethoxypyran-2-carboxylic acid Chemical compound O1C=CC(=O)C(OCC=2C=CC=CC=2)=C1C(=O)O KJSJBKBZMGSIPT-UHFFFAOYSA-N 0.000 description 1
- 125000005986 4-piperidonyl group Chemical group 0.000 description 1
- CTQPPUPWHLGKOB-UHFFFAOYSA-N 4h-oxadiazol-5-one Chemical compound O=C1CN=NO1 CTQPPUPWHLGKOB-UHFFFAOYSA-N 0.000 description 1
- RXSFZYSNXZGVCU-UHFFFAOYSA-N 4h-oxadiazole-5-thione Chemical compound S=C1CN=NO1 RXSFZYSNXZGVCU-UHFFFAOYSA-N 0.000 description 1
- YQDGQEKUTLYWJU-UHFFFAOYSA-N 5,6,7,8-tetrahydroquinoline Chemical compound C1=CC=C2CCCCC2=N1 YQDGQEKUTLYWJU-UHFFFAOYSA-N 0.000 description 1
- LGZKGOGODCLQHG-CYBMUJFWSA-N 5-[(2r)-2-hydroxy-2-(3,4,5-trimethoxyphenyl)ethyl]-2-methoxyphenol Chemical compound C1=C(O)C(OC)=CC=C1C[C@@H](O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-CYBMUJFWSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- RKNCRHZZEDXQRF-UHFFFAOYSA-N 7,8-dihydroquinoline Chemical compound C1=CN=C2CC[CH][CH]C2=C1 RKNCRHZZEDXQRF-UHFFFAOYSA-N 0.000 description 1
- OOLRAQKFMNOZBV-UHFFFAOYSA-N 8-n-[(4-aminophenyl)methyl]-3,6,10,13,16,19-hexazabicyclo[6.6.6]icosane-1,8-diamine Chemical compound C1=CC(N)=CC=C1CNC1(CNCCNC2)CNCCNCC2(N)CNCCNC1 OOLRAQKFMNOZBV-UHFFFAOYSA-N 0.000 description 1
- 108010066676 Abrin Proteins 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- 108010027164 Amanitins Proteins 0.000 description 1
- 101100172211 Arabidopsis thaliana HAG3 gene Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- 125000006374 C2-C10 alkenyl group Chemical group 0.000 description 1
- 125000005865 C2-C10alkynyl group Chemical group 0.000 description 1
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 description 1
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 description 1
- HAWSQZCWOQZXHI-UHFFFAOYSA-N CPT-OH Natural products C1=C(O)C=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 HAWSQZCWOQZXHI-UHFFFAOYSA-N 0.000 description 1
- AUJXLBOHYWTPFV-BLWRDSOESA-N CS[C@H]1SC[C@H]2N(C)C(=O)[C@@H](C)NC(=O)[C@H](COC(=O)[C@@H](C(C)C)N(C)C(=O)[C@@H]1N(C)C(=O)[C@@H](C)NC(=O)[C@H](COC(=O)[C@@H](C(C)C)N(C)C2=O)NC(=O)c1cnc2ccccc2n1)NC(=O)c1cnc2ccccc2n1 Chemical compound CS[C@H]1SC[C@H]2N(C)C(=O)[C@@H](C)NC(=O)[C@H](COC(=O)[C@@H](C(C)C)N(C)C(=O)[C@@H]1N(C)C(=O)[C@@H](C)NC(=O)[C@H](COC(=O)[C@@H](C(C)C)N(C)C2=O)NC(=O)c1cnc2ccccc2n1)NC(=O)c1cnc2ccccc2n1 AUJXLBOHYWTPFV-BLWRDSOESA-N 0.000 description 1
- FVLVBPDQNARYJU-XAHDHGMMSA-N C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O Chemical compound C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O FVLVBPDQNARYJU-XAHDHGMMSA-N 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-NJFSPNSNSA-N Carbon-14 Chemical compound [14C] OKTJSMMVPCPJKN-NJFSPNSNSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 102000005367 Carboxypeptidases Human genes 0.000 description 1
- 108010006303 Carboxypeptidases Proteins 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102000005600 Cathepsins Human genes 0.000 description 1
- 108010084457 Cathepsins Proteins 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- FDKWRPBBCBCIGA-UWTATZPHSA-N D-Selenocysteine Natural products [Se]C[C@@H](N)C(O)=O FDKWRPBBCBCIGA-UWTATZPHSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Chemical group OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine group Chemical group N[C@H](CCCCN)C(=O)O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- 125000002849 D-tyrosine group Chemical group [H]N([H])[C@@]([H])(C(=O)[*])C([H])([H])C1=C([H])C([H])=C(O[H])C([H])=C1[H] 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 102000007260 Deoxyribonuclease I Human genes 0.000 description 1
- 108010008532 Deoxyribonuclease I Proteins 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 241000551547 Dione <red algae> Species 0.000 description 1
- 229940120146 EDTMP Drugs 0.000 description 1
- 108010009858 Echinomycin Proteins 0.000 description 1
- 241000305071 Enterobacterales Species 0.000 description 1
- 101710146739 Enterotoxin Proteins 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- BFPYWIDHMRZLRN-SLHNCBLASA-N Ethinyl estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 BFPYWIDHMRZLRN-SLHNCBLASA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- GYHNNYVSQQEPJS-YPZZEJLDSA-N Gallium-68 Chemical compound [68Ga] GYHNNYVSQQEPJS-YPZZEJLDSA-N 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 108700004714 Gelonium multiflorum GEL Proteins 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102100022662 Guanylyl cyclase C Human genes 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000899808 Homo sapiens Guanylyl cyclase C Proteins 0.000 description 1
- 238000006736 Huisgen cycloaddition reaction Methods 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- LCWXJXMHJVIJFK-UHFFFAOYSA-N Hydroxylysine Natural products NCC(O)CC(N)CC(O)=O LCWXJXMHJVIJFK-UHFFFAOYSA-N 0.000 description 1
- DOMWKUIIPQCAJU-LJHIYBGHSA-N Hydroxyprogesterone caproate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)CCCCC)[C@@]1(C)CC2 DOMWKUIIPQCAJU-LJHIYBGHSA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 238000004566 IR spectroscopy Methods 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 241000588747 Klebsiella pneumoniae Species 0.000 description 1
- WTDRDQBEARUVNC-LURJTMIESA-N L-DOPA Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-LURJTMIESA-N 0.000 description 1
- WTDRDQBEARUVNC-UHFFFAOYSA-N L-Dopa Natural products OC(=O)C(N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-UHFFFAOYSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 150000008575 L-amino acids Chemical class 0.000 description 1
- JTTHKOPSMAVJFE-VIFPVBQESA-N L-homophenylalanine Chemical compound OC(=O)[C@@H](N)CCC1=CC=CC=C1 JTTHKOPSMAVJFE-VIFPVBQESA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-N Metaphosphoric acid Chemical compound OP(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-N 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- HYFMSAFINFJTFH-UHFFFAOYSA-N Mitomycin-A Natural products O=C1C(OC)=C(C)C(=O)C2=C1C(COC(N)=O)C1(OC)N2CC2NC21 HYFMSAFINFJTFH-UHFFFAOYSA-N 0.000 description 1
- TXXHDPDFNKHHGW-CCAGOZQPSA-N Muconic acid Chemical group OC(=O)\C=C/C=C\C(O)=O TXXHDPDFNKHHGW-CCAGOZQPSA-N 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- CBQJSKKFNMDLON-JTQLQIEISA-N N-acetyl-L-phenylalanine Chemical compound CC(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 CBQJSKKFNMDLON-JTQLQIEISA-N 0.000 description 1
- 150000007945 N-acyl ureas Chemical class 0.000 description 1
- 238000007126 N-alkylation reaction Methods 0.000 description 1
- UBQYURCVBFRUQT-UHFFFAOYSA-N N-benzoyl-Ferrioxamine B Chemical compound CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN UBQYURCVBFRUQT-UHFFFAOYSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- ACHQFNGCBWWVRR-UHFFFAOYSA-N NOPO Chemical compound NOPO ACHQFNGCBWWVRR-UHFFFAOYSA-N 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- XIIDTTVOHTUJMS-UHFFFAOYSA-N O.[Na].OS(=O)(=O)CC(S)CS Chemical compound O.[Na].OS(=O)(=O)CC(S)CS XIIDTTVOHTUJMS-UHFFFAOYSA-N 0.000 description 1
- 102100027069 Odontogenic ameloblast-associated protein Human genes 0.000 description 1
- 101710091533 Odontogenic ameloblast-associated protein Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108010043958 Peptoids Proteins 0.000 description 1
- 244000025272 Persea americana Species 0.000 description 1
- 235000008673 Persea americana Nutrition 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 102000016611 Proteoglycans Human genes 0.000 description 1
- 108010067787 Proteoglycans Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 1
- 239000002262 Schiff base Substances 0.000 description 1
- 150000004753 Schiff bases Chemical class 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 229940100389 Sulfonylurea Drugs 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 238000006161 Suzuki-Miyaura coupling reaction Methods 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- GKLVYJBZJHMRIY-OUBTZVSYSA-N Technetium-99 Chemical compound [99Tc] GKLVYJBZJHMRIY-OUBTZVSYSA-N 0.000 description 1
- PDMMFKSKQVNJMI-BLQWBTBKSA-N Testosterone propionate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](OC(=O)CC)[C@@]1(C)CC2 PDMMFKSKQVNJMI-BLQWBTBKSA-N 0.000 description 1
- DPOPAJRDYZGTIR-UHFFFAOYSA-N Tetrazine Chemical compound C1=CN=NN=N1 DPOPAJRDYZGTIR-UHFFFAOYSA-N 0.000 description 1
- 229940123464 Thiazolidinedione Drugs 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 1
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 1
- 241000607253 Vibrio mimicus Species 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-L adipate(2-) Chemical compound [O-]C(=O)CCCCC([O-])=O WNLRTRBMVRJNCN-UHFFFAOYSA-L 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000004931 aggregating effect Effects 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 125000002355 alkine group Chemical group 0.000 description 1
- 125000005360 alkyl sulfoxide group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 230000005262 alpha decay Effects 0.000 description 1
- 229940061720 alpha hydroxy acid Drugs 0.000 description 1
- 150000001280 alpha hydroxy acids Chemical class 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- CIORWBWIBBPXCG-JZTFPUPKSA-N amanitin Chemical compound O=C1N[C@@H](CC(N)=O)C(=O)N2CC(O)C[C@H]2C(=O)N[C@@H](C(C)[C@@H](O)CO)C(=O)N[C@@H](C2)C(=O)NCC(=O)N[C@@H](C(C)CC)C(=O)NCC(=O)N[C@H]1CS(=O)C1=C2C2=CC=C(O)C=C2N1 CIORWBWIBBPXCG-JZTFPUPKSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- 239000004037 angiogenesis inhibitor Substances 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000005129 aryl carbonyl group Chemical group 0.000 description 1
- 125000005362 aryl sulfone group Chemical group 0.000 description 1
- 125000005361 aryl sulfoxide group Chemical group 0.000 description 1
- 125000005110 aryl thio group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- QQOBRRFOVWGIMD-OJAKKHQRSA-N azaribine Chemical compound CC(=O)O[C@@H]1[C@H](OC(C)=O)[C@@H](COC(=O)C)O[C@H]1N1C(=O)NC(=O)C=N1 QQOBRRFOVWGIMD-OJAKKHQRSA-N 0.000 description 1
- 229950010054 azaribine Drugs 0.000 description 1
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- GONOPSZTUGRENK-UHFFFAOYSA-N benzyl(trichloro)silane Chemical compound Cl[Si](Cl)(Cl)CC1=CC=CC=C1 GONOPSZTUGRENK-UHFFFAOYSA-N 0.000 description 1
- 230000005255 beta decay Effects 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229920006233 biaxially oriented polyamide Polymers 0.000 description 1
- 125000006580 bicyclic heterocycloalkyl group Chemical group 0.000 description 1
- JSMRMEYFZHIPJV-UHFFFAOYSA-N bicyclo[2.1.1]hexane Chemical compound C1C2CC1CC2 JSMRMEYFZHIPJV-UHFFFAOYSA-N 0.000 description 1
- GPRLTFBKWDERLU-UHFFFAOYSA-N bicyclo[2.2.2]octane Chemical compound C1CC2CCC1CC2 GPRLTFBKWDERLU-UHFFFAOYSA-N 0.000 description 1
- GNTFBMAGLFYMMZ-UHFFFAOYSA-N bicyclo[3.2.2]nonane Chemical compound C1CC2CCC1CCC2 GNTFBMAGLFYMMZ-UHFFFAOYSA-N 0.000 description 1
- WMRPOCDOMSNXCQ-UHFFFAOYSA-N bicyclo[3.3.2]decane Chemical compound C1CCC2CCCC1CC2 WMRPOCDOMSNXCQ-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 238000001815 biotherapy Methods 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- JCXGWMGPZLAOME-RNFDNDRNSA-N bismuth-213 Chemical compound [213Bi] JCXGWMGPZLAOME-RNFDNDRNSA-N 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 125000005621 boronate group Chemical class 0.000 description 1
- 125000005620 boronic acid group Chemical class 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 229960005539 bryostatin 1 Drugs 0.000 description 1
- MJQUEDHRCUIRLF-TVIXENOKSA-N bryostatin 1 Chemical compound C([C@@H]1CC(/[C@@H]([C@@](C(C)(C)/C=C/2)(O)O1)OC(=O)/C=C/C=C/CCC)=C\C(=O)OC)[C@H]([C@@H](C)O)OC(=O)C[C@H](O)C[C@@H](O1)C[C@H](OC(C)=O)C(C)(C)[C@]1(O)C[C@@H]1C\C(=C\C(=O)OC)C[C@H]\2O1 MJQUEDHRCUIRLF-TVIXENOKSA-N 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 230000011414 cGMP-mediated signaling Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 125000001314 canonical amino-acid group Chemical group 0.000 description 1
- 108010049145 capistruin Proteins 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 238000007623 carbamidomethylation reaction Methods 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229940047495 celebrex Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- PBAYDYUZOSNJGU-UHFFFAOYSA-N chelidonic acid Natural products OC(=O)C1=CC(=O)C=C(C(O)=O)O1 PBAYDYUZOSNJGU-UHFFFAOYSA-N 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 239000012829 chemotherapy agent Substances 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- KVSASDOGYIBWTA-UHFFFAOYSA-N chloro benzoate Chemical compound ClOC(=O)C1=CC=CC=C1 KVSASDOGYIBWTA-UHFFFAOYSA-N 0.000 description 1
- 125000004965 chloroalkyl group Chemical group 0.000 description 1
- 229940075419 choline hydroxide Drugs 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- NNBZCPXTIHJBJL-AOOOYVTPSA-N cis-decalin Chemical compound C1CCC[C@H]2CCCC[C@H]21 NNBZCPXTIHJBJL-AOOOYVTPSA-N 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960002436 cladribine Drugs 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- LGZKGOGODCLQHG-UHFFFAOYSA-N combretastatin Natural products C1=C(O)C(OC)=CC=C1CC(O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-UHFFFAOYSA-N 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 238000006352 cycloaddition reaction Methods 0.000 description 1
- 125000001162 cycloheptenyl group Chemical group C1(=CCCCCC1)* 0.000 description 1
- 125000000522 cyclooctenyl group Chemical group C1(=CCCCCCC1)* 0.000 description 1
- CIISBNCSMVCNIP-UHFFFAOYSA-N cyclopentane-1,2-dione Chemical class O=C1CCCC1=O CIISBNCSMVCNIP-UHFFFAOYSA-N 0.000 description 1
- LOGSONSNCYTHPS-UHFFFAOYSA-N cyclopentane-1,3-dione Chemical class O=C1CCC(=O)C1 LOGSONSNCYTHPS-UHFFFAOYSA-N 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 125000005507 decahydroisoquinolyl group Chemical group 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 1
- 229960000958 deferoxamine Drugs 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- YSMODUONRAFBET-UHFFFAOYSA-N delta-DL-hydroxylysine Natural products NCC(O)CCC(N)C(O)=O YSMODUONRAFBET-UHFFFAOYSA-N 0.000 description 1
- CFCUWKMKBJTWLW-UHFFFAOYSA-N deoliosyl-3C-alpha-L-digitoxosyl-MTM Natural products CC=1C(O)=C2C(O)=C3C(=O)C(OC4OC(C)C(O)C(OC5OC(C)C(O)C(OC6OC(C)C(O)C(C)(O)C6)C5)C4)C(C(OC)C(=O)C(O)C(C)O)CC3=CC2=CC=1OC(OC(C)C1O)CC1OC1CC(O)C(O)C(C)O1 CFCUWKMKBJTWLW-UHFFFAOYSA-N 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- 229950006137 dexfosfoserine Drugs 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- 229960000452 diethylstilbestrol Drugs 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-M dihydrogenphosphate Chemical compound OP(O)([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-M 0.000 description 1
- IZFHEQBZOYJLPK-UHFFFAOYSA-N dihydrolipoic acid Chemical compound OC(=O)CCCCC(S)CCS IZFHEQBZOYJLPK-UHFFFAOYSA-N 0.000 description 1
- 125000005594 diketone group Chemical group 0.000 description 1
- 125000005879 dioxolanyl group Chemical group 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 229930187817 disorazole Natural products 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- DUYCTCQXNHFCSJ-UHFFFAOYSA-N dtpmp Chemical compound OP(=O)(O)CN(CP(O)(O)=O)CCN(CP(O)(=O)O)CCN(CP(O)(O)=O)CP(O)(O)=O DUYCTCQXNHFCSJ-UHFFFAOYSA-N 0.000 description 1
- 229960005501 duocarmycin Drugs 0.000 description 1
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 description 1
- 229930184221 duocarmycin Natural products 0.000 description 1
- NFDRPXJGHKJRLJ-UHFFFAOYSA-N edtmp Chemical compound OP(O)(=O)CN(CP(O)(O)=O)CCN(CP(O)(O)=O)CP(O)(O)=O NFDRPXJGHKJRLJ-UHFFFAOYSA-N 0.000 description 1
- 210000001513 elbow Anatomy 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- XOPYFXBZMVTEJF-PDACKIITSA-N eleutherobin Chemical compound C(/[C@H]1[C@H](C(=CC[C@@H]1C(C)C)C)C[C@@H]([C@@]1(C)O[C@@]2(C=C1)OC)OC(=O)\C=C\C=1N=CN(C)C=1)=C2\CO[C@@H]1OC[C@@H](O)[C@@H](O)[C@@H]1OC(C)=O XOPYFXBZMVTEJF-PDACKIITSA-N 0.000 description 1
- XOPYFXBZMVTEJF-UHFFFAOYSA-N eleutherobin Natural products C1=CC2(OC)OC1(C)C(OC(=O)C=CC=1N=CN(C)C=1)CC(C(=CCC1C(C)C)C)C1C=C2COC1OCC(O)C(O)C1OC(C)=O XOPYFXBZMVTEJF-UHFFFAOYSA-N 0.000 description 1
- 238000003821 enantio-separation Methods 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000001842 enterocyte Anatomy 0.000 description 1
- 230000000688 enterotoxigenic effect Effects 0.000 description 1
- 239000000147 enterotoxin Substances 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- HESCAJZNRMSMJG-KKQRBIROSA-N epothilone A Chemical class C/C([C@@H]1C[C@@H]2O[C@@H]2CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 HESCAJZNRMSMJG-KKQRBIROSA-N 0.000 description 1
- YSMODUONRAFBET-UHNVWZDZSA-N erythro-5-hydroxy-L-lysine Chemical compound NC[C@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-UHNVWZDZSA-N 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 229960002568 ethinylestradiol Drugs 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- LIQODXNTTZAGID-OCBXBXKTSA-N etoposide phosphate Chemical compound COC1=C(OP(O)(O)=O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 LIQODXNTTZAGID-OCBXBXKTSA-N 0.000 description 1
- 229960000752 etoposide phosphate Drugs 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000004428 fluoroalkoxy group Chemical group 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 1
- 229960001751 fluoxymesterone Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 239000000174 gluconic acid Chemical group 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 229930182480 glucuronide Natural products 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- RQFCJASXJCIDSX-UUOKFMHZSA-N guanosine 5'-monophosphate Chemical class C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O RQFCJASXJCIDSX-UUOKFMHZSA-N 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005223 heteroarylcarbonyl group Chemical group 0.000 description 1
- KKLGDUSGQMHBPB-UHFFFAOYSA-N hex-2-ynedioic acid Chemical compound OC(=O)CCC#CC(O)=O KKLGDUSGQMHBPB-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000002017 high-resolution X-ray diffraction Methods 0.000 description 1
- 210000001624 hip Anatomy 0.000 description 1
- 150000002429 hydrazines Chemical class 0.000 description 1
- 150000007857 hydrazones Chemical class 0.000 description 1
- BNRNAKTVFSZAFA-UHFFFAOYSA-N hydrindane Chemical compound C1CCCC2CCCC21 BNRNAKTVFSZAFA-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- QJHBJHUKURJDLG-UHFFFAOYSA-N hydroxy-L-lysine Natural products NCCCCC(NO)C(O)=O QJHBJHUKURJDLG-UHFFFAOYSA-N 0.000 description 1
- 125000002349 hydroxyamino group Chemical group [H]ON([H])[*] 0.000 description 1
- 150000002443 hydroxylamines Chemical class 0.000 description 1
- 229950000801 hydroxyprogesterone caproate Drugs 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- HIFJCPQKFCZDDL-ACWOEMLNSA-N iloprost Chemical compound C1\C(=C/CCCC(O)=O)C[C@@H]2[C@@H](/C=C/[C@@H](O)C(C)CC#CC)[C@H](O)C[C@@H]21 HIFJCPQKFCZDDL-ACWOEMLNSA-N 0.000 description 1
- 150000002463 imidates Chemical class 0.000 description 1
- 125000002632 imidazolidinyl group Chemical group 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229940055742 indium-111 Drugs 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 125000002346 iodo group Chemical group I* 0.000 description 1
- 230000019948 ion homeostasis Effects 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000004628 isothiazolidinyl group Chemical group S1N(CCC1)* 0.000 description 1
- 150000002540 isothiocyanates Chemical class 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 125000003965 isoxazolidinyl group Chemical group 0.000 description 1
- 150000002576 ketones Chemical group 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical group [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- AGBQKNBQESQNJD-UHFFFAOYSA-M lipoate Chemical compound [O-]C(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-M 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 210000004937 luminal membrane Anatomy 0.000 description 1
- 108010077127 lymphoguanylin Proteins 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 125000005341 metaphosphate group Chemical group 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- FBOZXECLQNJBKD-UHFFFAOYSA-N methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-UHFFFAOYSA-N 0.000 description 1
- IZYBEMGNIUSSAX-UHFFFAOYSA-N methyl benzenecarboperoxoate Chemical compound COOC(=O)C1=CC=CC=C1 IZYBEMGNIUSSAX-UHFFFAOYSA-N 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- HYFMSAFINFJTFH-NGSRAFSJSA-N mitomycin A Chemical compound O=C1C(OC)=C(C)C(=O)C2=C1[C@@H](COC(N)=O)[C@]1(OC)N2C[C@@H]2N[C@@H]21 HYFMSAFINFJTFH-NGSRAFSJSA-N 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- 125000006578 monocyclic heterocycloalkyl group Chemical group 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- JFOHFDSMPQIOES-UHFFFAOYSA-N motexafin Chemical compound C1=NC2=CC(OCCOCCOCCOC)=C(OCCOCCOCCOC)C=C2N=CC(C(=C2CCCO)C)=NC2=CC(C(CC)=C2CC)=NC2=CC2=C(CCCO)C(C)=C1N2 JFOHFDSMPQIOES-UHFFFAOYSA-N 0.000 description 1
- 229950011637 motexafin Drugs 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- UXDAWVUDZLBBAM-UHFFFAOYSA-N n,n-diethylbenzeneacetamide Chemical compound CCN(CC)C(=O)CC1=CC=CC=C1 UXDAWVUDZLBBAM-UHFFFAOYSA-N 0.000 description 1
- SQDFHQJTAWCFIB-UHFFFAOYSA-N n-methylidenehydroxylamine Chemical compound ON=C SQDFHQJTAWCFIB-UHFFFAOYSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 201000002120 neuroendocrine carcinoma Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 125000006574 non-aromatic ring group Chemical group 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- UMRZSTCPUPJPOJ-KNVOCYPGSA-N norbornane Chemical compound C1C[C@H]2CC[C@@H]1C2 UMRZSTCPUPJPOJ-KNVOCYPGSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical group CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Chemical group CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 125000005060 octahydroindolyl group Chemical group N1(CCC2CCCCC12)* 0.000 description 1
- 125000005061 octahydroisoindolyl group Chemical group C1(NCC2CCCCC12)* 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 238000006053 organic reaction Methods 0.000 description 1
- 150000002905 orthoesters Chemical class 0.000 description 1
- 125000000160 oxazolidinyl group Chemical group 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- PSWJVKKJYCAPTI-UHFFFAOYSA-N oxido-oxo-phosphonophosphanylphosphanium Chemical compound OP(O)(=O)PP(=O)=O PSWJVKKJYCAPTI-UHFFFAOYSA-N 0.000 description 1
- 125000005476 oxopyrrolidinyl group Chemical group 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- KDLHZDBZIXYQEI-AKLPVKDBSA-N palladium-109 Chemical compound [109Pd] KDLHZDBZIXYQEI-AKLPVKDBSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Chemical group OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- TVIDEEHSOPHZBR-AWEZNQCLSA-N para-(benzoyl)-phenylalanine Chemical compound C1=CC(C[C@H](N)C(O)=O)=CC=C1C(=O)C1=CC=CC=C1 TVIDEEHSOPHZBR-AWEZNQCLSA-N 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- DCWXELXMIBXGTH-QMMMGPOBSA-N phosphonotyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(OP(O)(O)=O)C=C1 DCWXELXMIBXGTH-QMMMGPOBSA-N 0.000 description 1
- 150000003016 phosphoric acids Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229940075930 picrate Drugs 0.000 description 1
- OXNIZHLAWKMVMX-UHFFFAOYSA-M picrate anion Chemical compound [O-]C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O OXNIZHLAWKMVMX-UHFFFAOYSA-M 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- 229940012957 plasmin Drugs 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 229950008499 plitidepsin Drugs 0.000 description 1
- UUSZLLQJYRSZIS-LXNNNBEUSA-N plitidepsin Chemical compound CN([C@H](CC(C)C)C(=O)N[C@@H]1C(=O)N[C@@H]([C@H](CC(=O)O[C@H](C(=O)[C@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N(C)[C@@H](CC=2C=CC(OC)=CC=2)C(=O)O[C@@H]1C)C(C)C)O)[C@@H](C)CC)C(=O)[C@@H]1CCCN1C(=O)C(C)=O UUSZLLQJYRSZIS-LXNNNBEUSA-N 0.000 description 1
- 108010049948 plitidepsin Proteins 0.000 description 1
- 108700028325 pokeweed antiviral Proteins 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229960000688 pomalidomide Drugs 0.000 description 1
- UVSMNLNDYGZFPF-UHFFFAOYSA-N pomalidomide Chemical compound O=C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O UVSMNLNDYGZFPF-UHFFFAOYSA-N 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- WSHYKIAQCMIPTB-UHFFFAOYSA-M potassium;2-oxo-3-(3-oxo-1-phenylbutyl)chromen-4-olate Chemical compound [K+].[O-]C=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 WSHYKIAQCMIPTB-UHFFFAOYSA-M 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- KCXFHTAICRTXLI-UHFFFAOYSA-N propane-1-sulfonic acid Chemical compound CCCS(O)(=O)=O KCXFHTAICRTXLI-UHFFFAOYSA-N 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000003072 pyrazolidinyl group Chemical group 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- DGZUEIPKRRSMGK-UHFFFAOYSA-N quadricyclane Chemical compound C1C2C3C2C2C3C12 DGZUEIPKRRSMGK-UHFFFAOYSA-N 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- AUJXLBOHYWTPFV-UHFFFAOYSA-N quinomycin A Natural products CN1C(=O)C(C)NC(=O)C(NC(=O)C=2N=C3C=CC=CC3=NC=2)COC(=O)C(C(C)C)N(C)C(=O)C2N(C)C(=O)C(C)NC(=O)C(NC(=O)C=3N=C4C=CC=CC4=NC=3)COC(=O)C(C(C)C)N(C)C(=O)C1CSC2SC AUJXLBOHYWTPFV-UHFFFAOYSA-N 0.000 description 1
- 125000004621 quinuclidinyl group Chemical group N12C(CC(CC1)CC2)* 0.000 description 1
- 108010061338 ranpirnase Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- WUAPFZMCVAUBPE-IGMARMGPSA-N rhenium-186 Chemical compound [186Re] WUAPFZMCVAUBPE-IGMARMGPSA-N 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 125000006413 ring segment Chemical group 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 235000015598 salt intake Nutrition 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- CXMXRPHRNRROMY-UHFFFAOYSA-L sebacate(2-) Chemical compound [O-]C(=O)CCCCCCCCC([O-])=O CXMXRPHRNRROMY-UHFFFAOYSA-L 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- ZKZBPNGNEQAJSX-UHFFFAOYSA-N selenocysteine Natural products [SeH]CC(N)C(O)=O ZKZBPNGNEQAJSX-UHFFFAOYSA-N 0.000 description 1
- 229940055619 selenocysteine Drugs 0.000 description 1
- 235000016491 selenocysteine Nutrition 0.000 description 1
- 150000007659 semicarbazones Chemical class 0.000 description 1
- 229960003440 semustine Drugs 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000002832 shoulder Anatomy 0.000 description 1
- BQCADISMDOOEFD-AKLPVKDBSA-N silver-111 Chemical compound [111Ag] BQCADISMDOOEFD-AKLPVKDBSA-N 0.000 description 1
- 210000001286 simple columnar epithelial cell Anatomy 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000008117 stearic acid Chemical group 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- TYFQFVWCELRYAO-UHFFFAOYSA-L suberate(2-) Chemical compound [O-]C(=O)CCCCCCC([O-])=O TYFQFVWCELRYAO-UHFFFAOYSA-L 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- ACTRVOBWPAIOHC-XIXRPRMCSA-N succimer Chemical compound OC(=O)[C@@H](S)[C@@H](S)C(O)=O ACTRVOBWPAIOHC-XIXRPRMCSA-N 0.000 description 1
- 150000003455 sulfinic acids Chemical class 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 125000000542 sulfonic acid group Chemical group 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 238000003419 tautomerization reaction Methods 0.000 description 1
- 229940056501 technetium 99m Drugs 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001712 testosterone propionate Drugs 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- 229960003433 thalidomide Drugs 0.000 description 1
- 229940127044 therapeutic radiopharmaceutical Drugs 0.000 description 1
- 150000001467 thiazolidinediones Chemical class 0.000 description 1
- 125000001984 thiazolidinyl group Chemical group 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000005985 thienyl[1,3]dithianyl group Chemical group 0.000 description 1
- SRVJKTDHMYAMHA-WUXMJOGZSA-N thioacetazone Chemical compound CC(=O)NC1=CC=C(\C=N\NC(N)=S)C=C1 SRVJKTDHMYAMHA-WUXMJOGZSA-N 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 238000003354 tissue distribution assay Methods 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- NNBZCPXTIHJBJL-UHFFFAOYSA-N trans-decahydronaphthalene Natural products C1CCCC2CCCCC21 NNBZCPXTIHJBJL-UHFFFAOYSA-N 0.000 description 1
- NNBZCPXTIHJBJL-MGCOHNPYSA-N trans-decalin Chemical compound C1CCC[C@@H]2CCCC[C@H]21 NNBZCPXTIHJBJL-MGCOHNPYSA-N 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 125000005455 trithianyl group Chemical group 0.000 description 1
- 229960004799 tryptophan Drugs 0.000 description 1
- 229930184737 tubulysin Natural products 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 229940099039 velcade Drugs 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- QCWXUUIWCKQGHC-YPZZEJLDSA-N zirconium-89 Chemical group [89Zr] QCWXUUIWCKQGHC-YPZZEJLDSA-N 0.000 description 1
- JPZXHKDZASGCLU-LBPRGKRZSA-N β-(2-naphthyl)-alanine Chemical compound C1=CC=CC2=CC(C[C@H](N)C(O)=O)=CC=C21 JPZXHKDZASGCLU-LBPRGKRZSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/0402—Organic compounds carboxylic acid carriers, fatty acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/041—Heterocyclic compounds
- A61K51/044—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins
- A61K51/0446—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/0493—Steroids, e.g. cholesterol, testosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/0497—Organic compounds conjugates with a carrier being an organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/08—Peptides, e.g. proteins, carriers being peptides, polyamino acids, proteins
- A61K51/088—Peptides, e.g. proteins, carriers being peptides, polyamino acids, proteins conjugates with carriers being peptides, polyamino acids or proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/88—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86
- G01N2030/8809—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample
- G01N2030/8813—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample biological materials
- G01N2030/8831—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample biological materials involving peptides or proteins
Definitions
- the subject radiopharmaceutical conjugates are useful for the treatment of diseases, e.g., cancer.
- the present disclosure provides conjugates having improved elimination profile and metabolic, chemical and/or physical stabilities that are suitable for radiopharmaceutical applications while maintaining the protein binding affinity. It is surprisingly discovered that, for some conjugates, the target binding affinity, plasma stability and serum half-live can be adjusted by modifying the hydrophobicity and length of the linker connecting a guanylyl cyclase C (GCC) binding peptide with a metal chelator. Accordingly, in one aspect, disclosed herein are GCC binding conjugates with optimized linkers and configurations.
- GCC guanylyl cyclase C
- the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; a metal chelator configured to bind with a radionuclide, wherein the metal chelator is selected from FIGs.3-17; one or more linkers; and optionally a radionuclide, e.g., a radionuclide of Table 5A or Table 5B.
- GCC guanylyl cyclase C
- the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42, a metal chelator configured to bind with a radionuclide; a linker that covalently attaches the GCC binding peptide with the metal chelator; and an alpha particle-emitting radionuclide.
- GCC guanylyl cyclase C
- the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein a dissociation constant (Kd) between the linker and human serum albumin is at most 100 ⁇ M, as determined at room temperature in human serum condition.
- GCC guanylyl cyclase C
- the present disclosure provides a conjugate comprising (i) a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; (ii) a metal chelator configured to bind with a radionuclide; (iii) a linker that covalently attaches the GCC binding peptide with the metal chelator; and (iv) an alpha-particle emitting radionuclide bound to the metal chelator.
- GCC guanylyl cyclase C
- the present disclosure provides a conjugate comprising (i) a guanylyl cyclase C (GCC) binding peptide; (ii) a metal chelator configured to bind with a radionuclide; and (iii) a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein 5% to 99% of the conjugate binds to Human Serum Albumin (HSA) in vitro as determined by HSA-HPLC method.
- the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42.
- the GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 1. In some embodiments, the binding peptide further comprises 1, 2 or 3 intramolecular disulfide bonds. In some embodiments, the binding peptide further comprises one or more of alkylene, alkenylene, heteroalkylene, and heteroaryl. In some embodiments, the binding peptide is selected from: . In some embodiments, the binding peptide further comprises one intramolecular heteroalkylene, alkylene or alkenylene bond.
- the heteroalkylene, alkylene or alkenylene is optionally substituted with one or more R 10 , wherein each R 10 is independently halogen, amino, -OH, -SH, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 1 -C 6 hydroxyalkyl, C 1 -C 6 aminoalkyl, C 1 -C 6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R 10 on the same atoms are taken together to form a cycloalkyl or heterocycloalkyl; or two R 10 on different atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl.
- the binding peptide comprises an unnatural amino acid at the N-terminus, the C-terminus, or both. In some embodiments, the binding peptide comprises a modified tyrosine at the C-terminus. In some embodiments, the modified tyrosine is a D-tyrosine, N-methyl-L-tyrosine, ⁇ -methyl-L-tyrosine, or L-tyrosinamide.
- the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB- Cyclam, CB-TE2A, DOTA-4AMP, and NOTP.
- the metal chelator is DOTA.
- the linker is a bond.
- the linker connects to the metal chelator through L 3 and to the peptide through L 2 .
- the linker has a structure of Formula (II-2a).
- the linker has a structure of Formula (II-2b).
- the linker comprises a click chemistry residue.
- the linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and one or more amino acids.
- the linker comprises one or more of a substituted or unsubstituted C 6 -C 10 aryl, substituted or unsubstituted C 5 -C 9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, C 3 -C 20 alkylene, or amino acid residues.
- the linker comprises one or more lysine residues.
- the linker comprises one or more glutamate residues.
- the linker comprises phenyl iodide or carboxylic acid.
- the linker comprises 3 to 30 intervening atoms between the metal chelator and the binding peptide.
- the linker comprises 6 to 18 intervening atoms between the metal chelator and the binding peptide.
- a dissociation constant (Kd) between the linker and human serum albumin is at most 25 ⁇ M, as determined in vitro at room temperature in human serum condition.
- Kd dissociation constant
- about 40%-60% of the conjugate binds to HSA in vitro as determined by HSA- HPLC method.
- about 60%-80% of the conjugate binds to HSA in vitro as determined by HSA-HPLC method.
- the linker is attached to the binding peptide via the N terminus of the binding peptide.
- the conjugate comprises a second linker attached to the C terminus of the binding peptide.
- the conjugate comprises an alpha particle-emitting radionuclide bound to the metal chelator.
- the alpha particle-emitting radionuclide is actinium-225, astatine-211, radium-223, or thorium-227.
- the alpha particle-emitting radionuclide is actinium-225.
- the conjugate comprises two or more metal chelators.
- the conjugate has an elimination half-life in rats of about 1 to 120 hours. In some embodiments, the conjugate has an elimination half-life in rats of about 2 to 24 hours.
- a half-life of the conjugate in human serum condition is about 2 to 20 hours, 5 to 20 hours, 8 to 15 hours, or 10 to 14 hours at 37 °C.
- the linker –(L) n - comprises 3 to 20 intervening atoms between the metal chelator and the binding peptide. In some embodiments, –(L) n - is bound to the N-terminus of the GCC binding peptide and L C is bound to the C-terminus of the GCC binding peptide. In some embodiments, the linker –(L) n - comprises a hydrophobic group selected from a C 8 -C 30 fatty acid, C 8 -C 30 fatty alcohol, C 8 -C 30 alkyl, aryl, heteroaryl, one or more amino acid residues, or a combination thereof.
- –(L) n - comprises one or more lysine residues. In some embodiments, –(L) n - comprises one or more glutamate residues.
- the binding peptide comprises an unnatural amino acid, e.g., at the C-terminus. In some embodiments, the binding peptide comprises a modified tyrosine at the C- terminus. In some embodiments, the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide comprises an amino acid sequence selected from SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide further comprises 1 to 3 intramolecular bonds selected from heteroalkylene, alkylene and alkenylene bonds.
- the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, or NOTP.
- the metal chelator is DOTA.
- the conjugate comprises a radionuclide bound to the metal chelator.
- the radionuclide is an alpha particle-emitting radionuclide selected from actinium-225, astatine-211, radium-223, and thorium-227.
- the radionuclide is lutetium-177 or actinium-225.
- the present disclosure provides a conjugate selected from a structure of Table 1.
- the present disclosure provides a conjugate comprising: a structure selected from Table 1 and a radionuclide bound to a metal chelator of the structure.
- the present disclosure provides a conjugate, wherein the conjugate is a salt or solvate of a conjugate of described herein.
- the present disclosure provides a pharmaceutical composition comprising a herein-described conjugate and a pharmaceutically acceptable excipient or carrier.
- the present disclosure provides a method of treating a gastrointestinal tract cancer in a subject in need thereof, comprising administering to the subject a conjugate described herein, or a pharmaceutical composition described herein.
- the cancer is colorectal cancer.
- the method comprises administering a second therapeutic agent that is a GCC receptor agonist.
- the method comprises administering (i) a first conjugate comprising a radionuclide configured for companion diagnostic and (ii) a second conjugate comprising a radionuclide selected from an alpha or beta-particle emitter, wherein the first and the second conjugate have the same structure except for the radionuclide.
- the radionuclide of the first conjugate is selected from Lu- 177, Technetium-99m, In-111, Ga-68, Cu-64, and Zr-89.
- the present disclosure provides a method of treating a gastrointestinal tract cancer in a subject in need thereof, comprising administering to the subject a herein-described conjugate or a pharmaceutical composition comprising the conjugate.
- the cancer is colorectal cancer.
- the method comprises administering a second agent that binds to a GCC receptor.
- the method comprises administering a second agent that is a GCC receptor agonist.
- the method comprises administering a second agent that is a GCC receptor antagonist.
- FIG. 1A illustrates amino acid sequence for linaclotide
- FIG. 1B illustrates amino acid sequence for plecanatide
- FIG.1C illustrates amino acid sequence for F 19 - STh (1-19), an analogue of a wild-type Escherichia coli heat-stable peptide (STh) moiety with a Tyr to Phe alteration at position 19
- FIG.1D illustrates amino acid sequence for R 1,4 , F 19 -STh(1- 19), an analogue of a wild-type STh moiety with a Tyr to Phe alteration at position 19 and having arginine residues at positions 1 and 4
- FIG.1E illustrates amino acid sequence for F 9 -STh(6-19), an analogue of a wild-type STh moiety
- FIG.1F illustrates amino acid sequence for F 19 -STh (2- 19), an analogue of a wild-type STh moiety
- FIG.1F illustrates amino acid sequence for F 19 -STh (2- 19
- FIG.2A-2D illustrate conjugates comprising exemplary linker structures that contain one or more motifs.
- FIG.2A illustrates a conjugate comprising a metal chelator, a linker and a cyclized GCC binding peptide;
- FIG.2A illustrates a conjugate comprising a metal chelator, a linker and a cyclized GCC binding peptide;
- FIG.2B and FIG.2C illustrate conjugates comprising a metal chelator, a cyclized GCC binding peptide, and a linker that comprises two or more motifs
- FIG.2D illustrates a conjugate comprising a metal chelator, two cyclized GCC binding peptides, and a linker that comprises two or more motifs.
- FIG.3 illustrates the structures of representative metal chelators.
- FIG.4 illustrates the structures of representative metal chelators.
- FIG.5 illustrates the structures of representative metal chelators.
- FIG.6 illustrates the structures of representative metal chelators.
- FIG.7 illustrates the structures of representative metal chelators.
- FIG.8 illustrates the structures of representative metal chelators.
- FIG.9 illustrates the structures of representative metal chelators.
- FIG.10 illustrates the structures of representative metal chelators.
- FIG.11 illustrates the structures of representative metal chelators.
- FIG.12 illustrates the structures of representative metal chelators.
- FIG.13 illustrates the structures of representative metal chelators.
- FIG.14 illustrates the structures of representative metal chelators.
- FIG.15 illustrates the structures of representative metal chelators.
- FIG.16 illustrates the structures of representative metal chelators.
- FIG.17 illustrates the structures of representative metal chelators.
- FIG.18A illustrates plasma stability of linaclotide
- FIG.18B illustrates plasma stability of La-C-02.
- FIG.19 illustrates the structures of representative conjugates of the present disclosure.
- FIG.20 illustrates the structures of representative conjugates of the present disclosure.
- FIG.21 illustrates the structures of representative conjugates of the present disclosure.
- FIG.22 illustrates the structures of representative conjugates of the present disclosure.
- DETAILED DESCRIPTION [0052]
- the present disclosure relates to radiopharmaceutical conjugate compositions and their use. [0053]
- the following description and examples illustrate embodiments of the present disclosure in detail. It is to be understood that this present disclosure is not limited to the particular embodiments described herein and as such can vary.
- the term “about” or “approximately” can mean within an acceptable error range for the particular value as determined by one of ordinary skill in the art, which will depend in part on how the value is measured or determined, i.e., the limitations of the measurement system. For example, “about” can mean within 1 or more than 1 standard deviation, per the practice in the art. Alternatively, “about” can mean a range of up to 20%, up to 15%, up to 10%, up to 5%, or up to 1% of a given value. Alternatively, particularly with respect to biological systems or processes, the term can mean within an order of magnitude, within 5-fold, or within 2-fold, of a value.
- “Hydroxy” or “hydroxyl” refers to the -OH radical.
- “Hydroxyamino” refers to the -NH-OH radical.
- Acyl refers to a substituted or unsubstituted alkylcarbonyl, substituted or unsubstituted alkenylcarbonyl, substituted or unsubstituted alkynylcarbonyl, substituted or unsubstituted cycloalkylcarbonyl, substituted or unsubstituted heterocycloalkylcarbonyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted heteroarylcarbonyl, amide, or ester, wherein the carbonyl atom of the carbonyl group is the point of attachment.
- an alkylcarbonyl group, alkenylcarbonyl group, alkynylcarbonyl group, cycloalkylcarbonyl group, amide group, or ester group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- Alkyl refers to an optionally substituted straight-chain, or optionally substituted branched-chain saturated hydrocarbon monoradical.
- An alkyl group can have from one to about twenty carbon atoms, from one to about ten carbon atoms, or from one to six carbon atoms. Examples include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, 2-methyl-1-propyl, 2-methyl-2-propyl, 2-methyl-1-butyl, 3-methyl-1-butyl, 2-methyl-3-butyl, 2,2-dimethyl-1-propyl, 2-methyl-1-pentyl, 3-methyl-1-pentyl, 4-methyl-1-pentyl, 2-methyl-2-pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 2,2-dimethyl-1-butyl, 3,3-dimethyl-1-butyl, 2-ethyl-1-butyl, n-butyl, isobutyl, sec-butyl, t-butyl, n-pentyl, isopentyl, neopenty
- C 1 -C 6 alkyl means that the alkyl group consists of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkyl” where no numerical range is designated.
- the alkyl is a C 1 -C 10 alkyl, a C 1 -C 9 alkyl, a C 1 -C 8 alkyl, a C 1 -C 7 alkyl, a C 1 -C 6 alkyl, a C 1 -C 5 alkyl, a C 1 -C 4 alkyl, a C 1 -C 3 alkyl, a C 1 -C 2 alkyl, or a C 1 alkyl.
- an alkyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- the alkyl is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, -OMe, -NH 2 , -NO 2 , or -C ⁇ CH.
- the alkyl is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe.
- alkyl is optionally substituted with halogen.
- Alkylene refers to a straight or branched divalent hydrocarbon chain. Unless stated otherwise specifically in the specification, an alkylene group may be optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkylene is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- an alkylene is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe. In some embodiments, the alkylene is optionally substituted with halogen. In some embodiments, the alkylene is -CH 2 -, -CH 2 CH 2 -, -CH 2 CH 2 CH 2 -, or -CH 2 CH(CH 3 )CH 2 -. In some embodiments, the alkylene is -CH 2 -. In some embodiments, the alkylene is -CH 2 CH 2 -. In some embodiments, the alkylene is -CH 2 CH 2 CH 2 -.
- alkenyl refers to an optionally substituted straight-chain, or optionally substituted branched-chain hydrocarbon monoradical having one or more carbon-carbon double-bonds.
- C 2 -C 6 alkenyl means that the alkenyl group may consist of 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms, or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkenyl” where no numerical range is designated.
- the alkenyl is a C 2 -C 10 alkenyl, a C 2 -C 9 alkenyl, a C 2 -C 8 alkenyl, a C 2 -C 7 alkenyl, a C 2 -C 6 alkenyl, a C 2 -C 5 alkenyl, a C 2 -C 4 alkenyl, a C 2 -C 3 alkenyl, or a C 2 alkenyl.
- an alkenyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- an alkenyl is optionally substituted with oxo, halogen, -CN, - CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- an alkenyl is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe.
- the alkenyl is optionally substituted with halogen.
- alkenylene or “alkenylene chain” refers to an optionally substituted straight or branched divalent hydrocarbon chain in which at least one carbon-carbon double bond is present linking the rest of the molecule to a radical group.
- Alkynyl refers to an optionally substituted straight-chain or optionally substituted branched-chain hydrocarbon monoradical having one or more carbon-carbon triple-bonds.
- an alkynyl group has from two to about ten carbon atoms, more preferably from two to about six carbon atoms. Examples include, but are not limited to, ethynyl, 2-propynyl, 2-butynyl, 1,3-butadiynyl, and the like.
- C 2 -C 6 alkynyl means that the alkynyl group may consist of 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms, or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkynyl” where no numerical range is designated.
- the alkynyl is a C 2 -C 10 alkynyl, a C 2 -C 9 alkynyl, a C 2 -C 8 alkynyl, a C 2 -C 7 alkynyl, a C 2 -C 6 alkynyl, a C 2 -C 5 alkynyl, a C 2 -C 4 alkynyl, a C 2 -C 3 alkynyl, or a C 2 alkynyl.
- an alkynyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- an alkynyl is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- an alkynyl is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe.
- alkynyl is optionally substituted with halogen.
- alkynylene refers to an optionally substituted straight-chain or optionally substituted branched-chain divalent hydrocarbon having one or more carbon-carbon triple-bonds.
- Alkylamino refers to a radical of the formula -N(Ra) 2 where Ra is an alkyl radical as defined, or two R a , taken together with the nitrogen atom, can form a substituted or unsubstituted C 2 -C 7 heterocyloalkyl ring.
- an alkylamino group may be optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- an alkylamino is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, - OMe, -NH 2 , or -NO 2 .
- an alkylamino is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe.
- alkylamino is optionally substituted with halogen.
- Alkoxy refers to a radical of the formula -OR a where R a is an alkyl radical as defined. Unless stated otherwise specifically in the specification, an alkoxy group may be optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- an alkoxy is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 . In some embodiments, an alkoxy is optionally substituted with oxo, halogen, -CN, -CF 3 , -OH, or -OMe. In some embodiments, the alkoxy is optionally substituted with halogen. [0078] “Aminoalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more amines. In some embodiments, the alkyl is substituted with one amine.
- the alkyl is substituted with one, two, or three amines.
- Hydroxyalkyl include, for example, aminomethyl, aminoethyl, aminopropyl, aminobutyl, or aminopentyl. In some embodiments, the hydroxyalkyl is aminomethyl.
- “Hydroxyalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more hydroxyls. In some embodiments, the alkyl is substituted with one hydroxyl. In some embodiments, the alkyl is substituted with one, two, or three hydroxyls.
- Hydroxyalkyl include, for example, hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl, or hydroxypentyl. In some embodiments, the hydroxyalkyl is hydroxymethyl.
- aryl refers to a radical comprising at least one aromatic ring wherein each of the atoms forming the ring is a carbon atom.
- Aryl groups can be optionally substituted. Examples of aryl groups include, but are not limited to phenyl, and naphthyl. In some embodiments, the aryl is phenyl. Depending on the structure, an aryl group can be a monoradical or a diradical (i.e., an arylene group).
- an aryl group comprises a partially reduced cycloalkyl group defined herein (e.g., 1,2-dihydronaphthalene). In some embodiments, an aryl group comprises a fully reduced cycloalkyl group defined herein (e.g., 1,2,3,4-tetrahydronaphthalene). When aryl comprises a cycloalkyl group, the aryl is bonded to the rest of the molecule through an aromatic ring carbon atom.
- An aryl radical can be a monocyclic or polycyclic (e.g., bicyclic, tricyclic, or tetracyclic) ring system, which may include fused, spiro or bridged ring systems.
- an aryl may be optionally substituted, for example, with halogen, amino, alkylamino, aminoalkyl, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, heteroalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, -S(O) 2 NH-C 1 - C 6 alkyl, and the like.
- an aryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , -NO 2 , -S(O) 2 NH 2 , -S(O) 2 NHCH 3 , -S(O) 2 NHCH 2 CH 3 , - S(O) 2 NHCH(CH 3 ) 2 , -S(O) 2 N(CH 3 ) 2 , or -S(O) 2 NHC(CH 3 ) 3 .
- an aryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF 3 , -OH, or -OMe. In some embodiments, the aryl is optionally substituted with halogen.
- the aryl is substituted with alkyl, alkenyl, alkynyl, haloalkyl, or heteroalkyl, wherein each alkyl, alkenyl, alkynyl, haloalkyl, heteroalkyl is independently unsubstituted, or substituted with halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2.
- cycloalkyl refers to a monocyclic or polycyclic non-aromatic radical, wherein each of the atoms forming the ring (i.e. skeletal atoms) is a carbon atom.
- cycloalkyls are saturated or partially unsaturated. In some embodiments, cycloalkyls are spirocyclic or bridged compounds. In some embodiments, cycloalkyls are fused with an aromatic ring (in which case the cycloalkyl is bonded through a non-aromatic ring carbon atom). Cycloalkyl groups include groups having from 3 to 10 ring atoms. Representative cycloalkyls include, but are not limited to, cycloalkyls having from three to ten carbon atoms, from three to eight carbon atoms, from three to six carbon atoms, or from three to five carbon atoms.
- Monocyclic cycloalkyl radicals include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
- the monocyclic cycloalkyl is cyclopentyl.
- the monocyclic cycloalkyl is cyclopentenyl or cyclohexenyl.
- the monocyclic cycloalkyl is cyclopentenyl.
- Polycyclic radicals include, for example, adamantyl, 1,2-dihydronaphthalenyl, 1,4-dihydronaphthalenyl, tetrainyl, decalinyl, 3,4- dihydronaphthalenyl-1(2H)-one, spiro[2.2]pentyl, norbornyl and bicycle[1.1.1]pentyl. Unless otherwise stated specifically in the specification, a cycloalkyl group may be optionally substituted.
- Representative cycloalkyls include, but are not limited to, cycloalkyls having from three to fifteen carbon atoms (C 3 -C 15 cycloalkyl), from three to ten carbon atoms (C 3 -C 10 cycloalkyl), from three to eight carbon atoms (C 3 -C 8 cycloalkyl), from three to six carbon atoms (C 3 -C 6 cycloalkyl), from three to five carbon atoms (C 3 -C 5 cycloalkyl), or three to four carbon atoms (C 3 -C 4 cycloalkyl).
- the cycloalkyl is a 3- to 6-membered cycloalkyl.
- the cycloalkyl is a 5- to 6-membered cycloalkyl.
- Monocyclic cycloalkyls include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
- Polycyclic cycloalkyls or carbocycles include, for example, adamantyl, norbornyl, decalinyl, bicyclo[3.3.0]octane, bicyclo[4.3.0]nonane, cis-decalin, trans-decalin, bicyclo[2.1.1]hexane, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, bicyclo[3.2.2]nonane, and bicyclo[3.3.2]decane, and 7,7-dimethyl-bicyclo[2.2.1]heptanyl.
- Partially saturated cycloalkyls include, for example, cyclopentenyl, cyclohexenyl, cycloheptenyl, and cyclooctenyl. Unless stated otherwise specifically in the specification, a cycloalkyl is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- a cycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- a cycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, - CF 3 , -OH, or -OMe.
- the cycloalkyl is optionally substituted with halogen.
- Halo or “halogen” refers to bromo, chloro, fluoro, or iodo.
- Halogen is fluoro or chloro. In some embodiments, halogen is fluoro.
- Halogen is fluoro.
- Haloalkyl refers to an alkyl radical, as defined above, that is substituted by one or more halogens. In some embodiments, the alkyl is substituted with one, two, or three halogens. In some embodiments, the alkyl is substituted with one, two, three, four, five, or six halogens. Haloalkyl can include, for example, iodoalkyl, bromoalkyl, chloroalkyl, and fluoroalkyl.
- fluoroalkyl refers to an alkyl radical, as defined above, that is substituted by one or more fluoro radicals, as defined above, for example, trifluoromethyl, difluoromethyl, fluoromethyl, 2,2,2-trifluoroethyl, 1-fluoromethyl-2-fluoroethyl, and the like.
- the alkyl part of the fluoroalkyl radical is optionally substituted as defined above for an alkyl group.
- Heteroalkyl refers to an alkyl group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen (e.g., -NH-, -N(alkyl)-), sulfur, or combinations thereof.
- a heteroalkyl is attached to the rest of the molecule at a carbon atom of the heteroalkyl.
- a heteroalkyl is a C 1 -C 6 heteroalkyl wherein the heteroalkyl is comprised of 1 to 6 carbon atoms and one or more atoms other than carbon, e.g., oxygen, nitrogen (e.g.
- heteroalkyl is attached to the rest of the molecule at a carbon atom of the heteroalkyl.
- heteroalkyl are, for example, –CH 2 -O-CH 2 -, –CH 2 -N(alkyl)-CH 2 -, –CH 2 -N(aryl)-CH 2 -, -OCH 2 CH 2 O-, – OCH 2 CH 2 OCH 2 CH 2 O-, or –OCH 2 CH 2 OCH 2 CH 2 OCH 2 CH 2 O-.
- a heteroalkyl is optionally substituted for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- a heteroalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- a heteroalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, - CF 3 , -OH, or -OMe. In some embodiments, the heteroalkyl is optionally substituted with halogen.
- a “heteroalkylene” refers to divalent heteroalkyl group.
- the term “heterocycloalkyl” refers to a cycloalkyl group that includes at least one hetero ring atom, e.g., a heteroatom selected from nitrogen, oxygen, and sulfur. In some embodiment, heterocycloalkyl are saturated. In some embodiment, heterocycloalkyl are partially unsaturated.
- the heterocycloalkyl radical may be a monocyclic, or bicyclic ring system, which may include fused (when fused with an aryl or a heteroaryl ring, the heterocycloalkyl is bonded through a non-aromatic ring atom) or bridged ring systems.
- the nitrogen, carbon or sulfur atoms in the heterocyclyl radical may be optionally oxidized.
- the nitrogen atom may be optionally quaternized.
- the heterocycloalkyl radical is partially or fully saturated.
- heterocycloalkyl radicals include, but are not limited to, dioxolanyl, thienyl[1,3]dithianyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, decahydroisoquinolyl, imidazolinyl, imidazolidinyl, isothiazolidinyl, isoxazolidinyl, morpholinyl, octahydroindolyl, octahydroisoindolyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl, oxazolidinyl, piperidinyl, piperazinyl, 4-piperidonyl, pyrrolidinyl, pyrazolidinyl, quinuclidinyl, thiazolidinyl, tetrahydrofuryl, trithianyl, t
- heterocycloalkyl also includes all ring forms of carbohydrates, including but not limited to monosaccharides, disaccharides and oligosaccharides. Unless otherwise noted, heterocycloalkyls have from 2 to 12 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring and 1 or 2 N atoms. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring and 3 or 4 N atoms.
- heterocycloalkyls have from 2 to 12 carbons, 0-2 N atoms, 0-2 O atoms, 0-2 P atoms, and 0-1 S atoms in the ring. In some embodiments, heterocycloalkyls have from 2 to 12 carbons, 1-3 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. It is understood that when referring to the number of carbon atoms in a heterocycloalkyl, the number of carbon atoms in the heterocycloalkyl is not the same as the total number of atoms (including the heteroatoms) that make up the heterocycloalkyl (i.e.
- a heterocycloalkyl is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- a heterocycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- a heterocycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF 3 , -OH, or -OMe. In some embodiments, the heterocycloalkyl is optionally substituted with halogen.
- Heteroaryl refers to a ring system radical comprising carbon atom(s) and one or more ring heteroatoms selected from the group consisting of nitrogen, oxygen, phosphorous, and sulfur, and at least one aromatic ring. In some embodiments, heteroaryl is monocyclic, bicyclic or polycyclic.
- monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, furazanyl, indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine.
- monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, and furazanyl.
- bicyclic heteroaryls include indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine.
- heteroaryl is pyridinyl, pyrazinyl, pyrimidinyl, thiazolyl, thienyl, thiadiazolyl or furyl.
- a heteroaryl contains 0-6 N atoms in the ring.
- a heteroaryl contains 1-4 N atoms in the ring. In some embodiments, a heteroaryl contains 4-6 N atoms in the ring. In some embodiments, a heteroaryl contains 0-4 N atoms, 0-1 O atoms, 0-1 P atoms, and 0- 1 S atoms in the ring. In some embodiments, a heteroaryl contains 1-4 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. In some embodiments, heteroaryl is a C 1 -C 9 heteroaryl. In some embodiments, monocyclic heteroaryl is a C 1 -C 5 heteroaryl.
- monocyclic heteroaryl is a 5-membered or 6-membered heteroaryl.
- a bicyclic heteroaryl is a C 6 -C 9 heteroaryl.
- a heteroaryl group comprises a partially reduced cycloalkyl or heterocycloalkyl group defined herein (e.g., 7,8-dihydroquinoline).
- a heteroaryl group comprises a fully reduced cycloalkyl or heterocycloalkyl group defined herein (e.g., 5,6,7,8-tetrahydroquinoline).
- heteroaryl comprises a cycloalkyl or heterocycloalkyl group
- the heteroaryl is bonded to the rest of the molecule through a heteroaromatic ring carbon or hetero atom.
- a heteroaryl radical can be a monocyclic or polycyclic (e.g., bicyclic, tricyclic, or tetracyclic) ring system, which may include fused, spiro or bridged ring systems.
- a heteroaryl is optionally substituted, for example, with halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like.
- a heteroaryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF 3 , -OH, -OMe, -NH 2 , or -NO 2 .
- a heteroaryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF 3 , -OH, or -OMe. In some embodiments, the heteroaryl is optionally substituted with halogen.
- carboxylic acid or an isostere thereof refers to a carboxylic acid moiety, or a functional group or moiety that exhibits similar physical, biological and/or chemical properties as a carboxylic acid moiety.
- carboxylic acid isosteres include, but are not limited to, hydroxamic acids, hydroxamic esters, sulfinic acids, sulfonic acids, sulfonamides, acyl- sulfonamides, sulfonylureas, acylureas, tetrazole, thiazolidine diones, oxozolidine diones, oxadiazol-5(4H)-one, oxothiadiazole-2-oxide, oxadiazol-5(4H)-thione, isoxazole, tetramic acid, cyclopentane 1,3-diones, cyclopentane 1,2-diones, squaryl groups, phosphoric acids, phosphinic acids, and halogenated phenols.
- a carboxylic acid isostere can be: wherein each hydrogen bound to a carbon atom is optionally replaced with methyl, ethyl, -CN, - CF 3 , -OH, -OMe, -NH 2 , or -NO 2 , or a different halogen.
- moiety refers to a specific segment or functional group of a molecule. Chemical moieties are often recognized chemical entities embedded in or appended to a molecule.
- the disclosed methods can provide any amount of any level of treatment, prevention, amelioration, or inhibition of the disorder in a mammal.
- a disorder, including symptoms or conditions thereof may be reduced by, for example, about 100%, about 90%, about 80%, about 70%, about 60%, about 50%, about 40%, about 30%, about 20%, or about 10%.
- the treatment, prevention, amelioration, or inhibition provided by the methods disclosed herein can include treatment, prevention, amelioration, or inhibition of one or more conditions or symptoms of the disorder, e.g., cancer or an inflammatory disease.
- treating includes the concepts of “alleviating”, which refers to lessening the frequency of occurrence or recurrence, or the severity, of any symptoms or other ill effects related to a disorder and/or the associated side effects.
- the term “treating” also encompasses the concept of “managing” which refers to reducing the severity of a particular disease or disorder in a patient or delaying its recurrence, e.g., lengthening the period of remission in a patient who had suffered from the disease.
- the term “prevent” or “preventing” as related to a disease or disorder may refer to a compound that, in a statistical sample, reduces the occurrence of the disorder or condition in the treated sample relative to an untreated control sample, or delays the onset or reduces the severity of one or more symptoms of the disorder or condition relative to the untreated control sample.
- a therapeutically effective amount of the composition may vary depending on factors such as the individual's condition, age, sex, and weight, and the ability of the protein to elicit the desired response of the individual.
- a therapeutically effective amount can also be an amount that exceeds any toxic or deleterious effect of the composition that would have a beneficial effect on the treatment.
- the term “optional” or “optionally” means that the subsequently described event or circumstance may or may not occur, and that the description includes instances where said event or circumstance occurs and instances in which it does not.
- “optionally substituted alkyl” means either “alkyl” or “substituted alkyl” as defined above.
- an optionally substituted group may be un-substituted (e.g., -CH 2 CH 3 ), fully substituted (e.g., -CF 2 CF 3 ), mono- substituted (e.g., -CH 2 CH 2 F) or substituted at a level anywhere in-between fully substituted and mono-substituted (e.g., -CH 2 CHF 2 , -CH 2 CF 3 , -CF 2 CH 3 , -CFHCHF 2 , etc.).
- substituted means positional variables on the atoms of a core molecule that are substituted at a designated atom position, replacing one or more hydrogens on the designated atom, provided that the designated atom's normal valency is not exceeded, and that the substitution results in a stable compound. Combinations of substituents and/or variables are permissible only if such combinations result in stable compounds.
- any carbon as well as heteroatom with valences that appear to be unsatisfied as described or shown herein is assumed to have a sufficient number of hydrogen atom(s) to satisfy the valences described or shown.
- substituents having a double bond may be described, shown or listed herein within a substituent group, wherein the structure may only show a single bond as the point of attachment to the core structure.
- optional substituents are independently selected from D, halogen, -CN, -NH 2 , -OH, -NH(CH 3 ), -N(CH 3 ) 2 , -NH(cyclopropyl), -CH 3 , -CH 2 CH 3 , -CF 3 , -OCH 3 , and - OCF 3 .
- substituted groups are substituted with one or two of the preceding groups.
- a peptide described herein can comprise one or more unnatural amino acids.
- the term “peptide” also encompasses peptide mimetics.
- the term “amino acid” is used in its broadest meaning and it embraces not only natural amino acids but also derivatives thereof and artificial amino acids.
- the term “amino acid” encompasses unnatural amino acids.
- the term “unnatural amino acid” refers to an amino acid other than the 20 amino acids that occur naturally in protein.
- protein refers to a polypeptide (i.e., a string of at least 3 amino acids linked to one another by peptide bonds).
- Proteins can include moieties other than amino acids (e.g., may be glycoproteins, proteoglycans, etc.) and/or can be otherwise processed or modified.
- a protein can be a complete polypeptide as produced by and/or active in a cell (with or without a signal sequence).
- a protein is or comprises a characteristic portion such as a polypeptide as produced by and/or active in a cell.
- a protein can include more than one polypeptide chain. For example, polypeptide chains can be linked by one or more disulfide bonds or associated by other means.
- peptide mimetic refers to biologically active compounds that mimic the biological activity of a peptide or a protein but are no longer entirely peptidic in chemical nature, e.g.,, they can contain non-peptide bonds (that are, bonds other than amide bonds between amino acids).
- peptide mimetic is used in a broader sense to include molecules that are no longer completely peptidic in nature, such as pseudo-peptides, semi- peptides and peptoids.
- peptide mimetics described herein can provide a spatial arrangement of reactive chemical moieties that closely resemble the three-dimensional arrangement of active groups in the subject amino acid sequence or subject molecule on which the peptide mimetic is based. As a result of this similar active-site geometry, the peptide mimetic can have effects on biological systems that are similar to the biological activity of the subject entity. [0101] In some embodiments, the peptide mimetics are substantially similar in both three- dimensional shape and biological activity to the subject amino acid sequence or subject molecule on which the peptide mimetic is based.
- Examples of methods of structurally modifying a peptide to create a peptide mimetic include the inversion of backbone chiral centers leading to D-amino acid residue structures that may, particularly at the N-terminus, lead to enhanced stability for proteolytical degradation without adversely affecting activity.
- An example is described in the paper “Tritiated D-ala1-Peptide T Binding”, Smith C. S. et al., Drug Development Res., 15, pp. 371-379 (1988).
- a second method is altering cyclic structure for stability, such as N to C interchain imides and lactams (Ede et al. in Smith and Rivier (Eds.) “Peptides: Chemistry and Biology”, Escom, Leiden (1991), pp.
- GCC guanylyl cyclase C
- STAR GUC 2 C
- GUI2C ST receptor
- Human GCC refers to the protein shown in SEQ ID NO: 43 and naturally occurring allelic protein variants thereof (e.g., natural variants of SEQ ID NO: 43 as provided in UniProtKB Accession No. P25092).
- Natural variants of SEQ ID NO: 43 as provided in UniProtKB Accession No. P25092 include VAR_067724, VAR_068174, VAR_042221, VAR_042222, VAR_042223, VAR_049253, VAR_068174, VAR_042224, VAR_042225, VAR_067724, VAR_042226, VAR_042227, VAR_042228, etc. Other variants are known in the art.
- accession number Ensp0000261170 Ensembl Database, European Bioinformatics Institute and Wellcome Trust Sanger Institute, which has a leucine at residue 281; SEQ ID NO: 14 of published US patent application number 20060035852; or GenBank accession number AAB 19934.
- a naturally occur ring allelic variant has an amino acid sequence at least 95%, 97% or 99% identical to the GCC sequence of SEQ ID NO: 43.
- the transcript encodes a protein product of 1073 amino acids, and is described in GenBank accession no.: NM 004963.
- GCC protein is characterized as a transmembrane cell surface receptor protein, and is believed to play a critical role in the maintenance of intestinal fluid, electrolyte homeostasis and cell proliferation.
- Amino acid sequence for human GCC protein (UniProtKB Accession No. P25092; SEQ ID NO: 43):
- guanylyl cyclase C (GCC) binding peptide or “guanylate cyclase C (GCC) binding peptide” refers to peptides that bind to the class of guanylate cyclase C receptor proteins on any cell type.
- GCC binding peptide also includes all peptides that have amino acid sequences substantially equivalent to at least a portion of any one of SEQ ID NOs: 1-42 and derivatives thereof. This term also covers fragments and pro-peptides that bind to guanylate cyclase receptor.
- substantially equivalent refers to a peptide that has an amino acid sequence equivalent to that of the binding domain where certain residues may be deleted or replaced with other amino acids without impairing the peptide's ability to bind to a guanylate cyclase receptor.
- GCC binding peptide encompasses “GCC receptor agonists,” which refer to peptides and/or other compounds that bind to a guanylate cyclase C receptor and stimulate cGMP production.
- GCC binding peptide also encompasses “GCC receptor antagonist.”
- a range of 1 to 50 is understood to include any number, combination of numbers, or sub-range from the group consisting of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, or 50, as well as all intervening decimal values between the aforementioned integers such as, for example, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, and 1.9.
- sub-ranges “nested sub-ranges” that extend from either end point of the range are specifically contemplated.
- a nested sub-range of an exemplary range of 1 to 50 may comprise 1 to 10, 1 to 20, 1 to 30, and 1 to 40 in one direction, or 50 to 40, 50 to 30, 50 to 20, and 50 to 10 in the other direction.
- C 1 -C x (or C 1-x ) includes C 1 -C 2 , C 1 -C 3 ... C 1 -C x .
- a group designated as “C 1 -C 4 ” indicates that there are one to four carbon atoms in the moiety, i.e. groups containing 1 carbon atom, 2 carbon atoms, 3 carbon atoms or 4 carbon atoms.
- C 1 -C 4 alkyl indicates that there are one to four carbon atoms in the alkyl group, i.e., the alkyl group is selected from among methyl, ethyl, propyl, iso-propyl, n-butyl, iso- butyl, sec-butyl, and t-butyl.
- C 0 -C 2 alkylene includes a direct bond, - CH 2 -, and -CH 2 CH 2 - linkages.
- cyclized or “cyclization” as used herein means that two amino acids apart from each other by at least one amino acid bind directly or bind indirectly to each other in one peptide to form a cyclic structure in the molecule. In some cases, the two amino acids bind via a linker or the like.
- subject or “patient” encompasses mammals.
- mammals include, but are not limited to, any member of the Mammalian class: humans, non-human primates such as chimpanzees, and other apes and monkey species; farm animals such as cattle, horses, sheep, goats, swine; domestic animals such as rabbits, dogs, and cats; laboratory animals including rodents, such as rats, mice and guinea pigs, and the like.
- the mammal is a human.
- a therapeutically effective amount of a composition may vary depending on factors such as the individual's condition (e.g., age, sex, and weight), the radiopharmaceutical conjugate, and the method of administration (e.g., oral or parenteral).
- the individual's condition e.g., age, sex, and weight
- the radiopharmaceutical conjugate e.g., the method of administration
- the method of administration e.g., oral or parenteral.
- a conjugate that comprises a GCC binding peptide, a metal chelator that is configured to bind with a radionuclide, a linker that covalently attaches the GCC binding peptide with the metal chelator, and optionally a radionuclide.
- a conjugate that comprises a GCC binding peptide, a metal chelator that is configured to bind with a radionuclide, optionally a linker that covalently attaches the GCC binding peptide with the metal chelator, and optionally a radionuclide.
- a conjugate comprising: a GCC binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; a metal chelator configured to bind with a radionuclide; a linker that covalently attaches the GCC binding peptide with the metal chelator; and optionally an alpha particle- emitting radionuclide.
- a conjugate comprising: a GCC binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein a dissociation constant (Kd) between the linker and human serum albumin is at most 500 ⁇ M, as determined at room temperature in human serum condition.
- Kd dissociation constant
- the Kd is at most 100 ⁇ M.
- the Kd is at most 15 ⁇ M.
- the GCC binding peptide comprises 7 to 40 amino acid residues.
- the alpha particle-emitting radionuclide is 225 Ac bound to the metal chelator.
- the radiopharmaceutical conjugate comprises two or more GCC binding peptides. In some embodiments, the radiopharmaceutical conjugate comprises two GCC binding peptides. In some embodiments, the radiopharmaceutical conjugate comprises three GCC binding peptides. In some embodiments, the two or more GCC binding peptides are the same (e.g., both comprising SEQ ID NO: 1). In some embodiments, the two or more GCC binding peptides are different. [0116] In some embodiments, a conjugate described herein is designed to have a prescribed elimination profile.
- the elimination profile can be designed by adjusting the sequence and length of the peptide, the property of the linker, the type of radionuclide, etc.
- the conjugate has an elimination half-life of about 0.1 to 200 hours. In some embodiments, the conjugate has an elimination half-life of about 0.5 to 150 hours. In some embodiments, the conjugate has an elimination half-life of about 1 to 120 hours. In some embodiments, the conjugate has an elimination half-life of at least 0.1 hour, 0.5 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 7 hours, 10 hours, 11 hours, 12 hours, 13 hours, 14 hours, 15 hours, 16 hours, 17 hours, 18 hours, 19 hours, 20 hours, 21 hours, 22 hours, 23 hours, or 24 hours.
- the conjugate has an elimination half-life of at most 120 hour, 80 hours, 70 hours, 60 hours, 50 hours, 40 hours, 30 hours, 24 hours, 12 hours, 10 hours, 5 hours, or 1 hour. In some embodiments, the conjugate has an elimination half-life of about 0.5 to 48 hours. In some embodiments, the conjugate has an elimination half-life of about 1 to 36 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 24 hours. In some embodiments, the conjugate has an elimination half-life of about 3 to 9 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 12 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 8 hours.
- the conjugate has an elimination half-life of about 2 to 5 hours. In some embodiments, the conjugate has an elimination half-life of about 3 to 4 hours. In some embodiments, the blood elimination half-life is determined in rats. In some embodiments, the blood elimination half-life is determined in humans. [0117]
- a herein described conjugate can have an elimination half-life in a tumor and non-tumor tissue of the subject. The elimination half-life in a tumor can be the same as or different from (either longer or shorter than) the elimination half-life in a non-tumor issue. In some embodiments, the elimination half-life of the conjugate in a tumor is about 3 hours to 14 days, about 2 to 10 days, about 7 to 10 days, or about 4 to 7 days.
- the elimination half-life of the conjugate in a tumor is more than 14 days. In some embodiments, the elimination half-life of the conjugate in a non-tumor tissue is about 1 hour to 14 days, about 12 hours to 2 days, about 1 day to 3 days, about 2 to 10 days, about 7 to 10 days, or about 4 to 7 days. In some embodiments, the elimination half-life of the conjugate in a tumor is at least 1.1, 1.2, 1.3, 1.4, 1.5, 2.0, 2.5, 3.0, 4.0, or 5.0 fold of the elimination half-life of the conjugate in a non-tumor tissue of the subject.
- the “elimination half-life” can refer to the time it takes from the maximum concentration after administration to half maximum concentration.
- the elimination half-life is determined after intravenous administration.
- the elimination half-life is measured as biological half-life, which is the half-life of the cold pharmaceutical in the living system.
- the elimination half-life is measured as effective half-life, which is the half-life of a radiopharmaceutical in a living system taking into account the half-life of the radionuclide.
- a conjugate described herein can have a described time-integrated activity coefficient (i.e., ⁇ ) in a tumor or non-tumor tissues of a subject.
- ⁇ represents the cumulative number of nuclear transformations occurring in a source tissue over a dose-integration period per unit administered activity.
- the ⁇ value of a conjugate can be tuned by modifications of the peptide in the conjugate, e.g., modifying the amino acid sequences and length of the peptide.
- the ⁇ value can be determined using a method known in the art.
- the ⁇ value of the conjugate in a tumor is from about 6 hours to 14 days.
- the ⁇ value in a tumor is about 2 to 10 days.
- the ⁇ value in a tumor is about 4 to 7 days.
- the ⁇ value in a tumor is about 7 to 10 days.
- the ⁇ value in a tumor is from about 1 day, 2 days, 3 days, or 4 days to about 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ⁇ value in a tumor is about 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ⁇ value of the conjugate in a non-tumor tissue is from about 6 hours to 14 days. In some embodiments, the ⁇ value in a non-tumor tissue is about 2 to 10 days. In some embodiments, the ⁇ value in a non-tumor tissue is about 4 to 7 days.
- the ⁇ value in a non-tumor tissue is about 7 to 10 days. In some embodiments, the ⁇ value in a non-tumor tissue is from about 1 day, 2 days, 3 days, or 4 days to about 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ⁇ value in a non-tumor tissue is about 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days.
- the ⁇ value of the conjugate in a tumor can be the same as the ⁇ value of the conjugate in a non-tumor tissue of the subject.
- the ⁇ value of the conjugate in a tumor can be longer or shorter than the ⁇ value of the conjugate in a non-tumor tissue of the subject.
- the ⁇ value of the conjugate in a tumor is at least 1.1, 1.2, 1.3, 1.4, 1.5, 2.0, 2.5, 3.0, 4.0, or 5.0 fold of the ⁇ value of the conjugate in a non-tumor tissue of the subject.
- a conjugate described herein can have an ⁇ value in an organ of a subject.
- the conjugate has an ⁇ value in a kidney of the subject of at most 24 hours.
- the ⁇ value of the conjugate in a kidney of the subject is at most 18 hours, 15 hours, 12 hours, 10 hours, 8 hours, 6 hours, or 5 hours. In some embodiments, the ⁇ value of the conjugate in a kidney of the subject is about 30 minutes to about 24 hours. In some embodiments, the ⁇ value of the conjugate in a kidney of the subject is about 2 to 24 hours. In some embodiments, the ⁇ value of the conjugate in a kidney of the subject is more than 24 hours. In some embodiments, the ⁇ value of the conjugate in a liver of the subject is at most 24 hours.
- the ⁇ value of the conjugate in a liver of the subject is at most 18 hours, 15 hours, 12 hours, 10 hours, 8 hours, 6 hours, or 5 hours. In some embodiments, the ⁇ value of the conjugate in a liver of the subject is about 30 minutes to about 24 hours. In some embodiments, the ⁇ value of the conjugate in a liver of the subject is about 2 to 24 hours. In some embodiments, the ⁇ value of the conjugate in a liver of the subject is more than 24 hours. [0121] In some cases, the elimination profile of the conjugate can be adjusted by a reversible binding between the conjugate and a plasma protein such as albumin.
- a suitable affinity between the conjugate and the plasma protein can utilize the plasma protein as a reservoir for the conjugates, attaching and preserving the conjugates at high concentration and releasing the conjugates at a lower concentration, thereby improving elimination profile.
- a dissociation constant (Kd) between the conjugate and human serum albumin is at most 500 ⁇ M, as determined at room temperature in human serum condition. In some embodiments, the Kd is at most 100 ⁇ M. In some embodiments, the Kd is at most 15 ⁇ M. In some embodiments, the Kd is from about 0.1 nM to about 10 ⁇ M. In some embodiments, the Kd is from about 10 nM to about 10 ⁇ M.
- the Kd is from about 50 nM to about 1 ⁇ M. In some embodiments, the Kd is from about 100 nM to about 10 ⁇ M. In some embodiments, the Kd is from about 500 nM to about 5 ⁇ M.
- a conjugate described herein is bound is bound to plasma proteins. In some embodiments, a conjugate described herein is bound to albumin. In some embodiments, a conjugate described herein has a plasma protein binding percentage of between 40% to 100%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of 40% to 80%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of 40% to 60%.
- a conjugate described herein has a plasma protein binding percentage of 60% to 80%. In some embodiments, the conjugate described herein has a plasma protein binding percentage of about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, or about 100%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of between 40% to 45%, between 45% to 50%, between 50% to 55%, between 55% to 60%, between 60% to 65%, between 70% to 75%, between 75% to 80%, or between 80% to 85%. [0123] In one aspect, provided herein are conjugates having a prescribed serum stability.
- the human serum half-life of the conjugate is at least 0.1 hour, 0.5 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 11 hours, 12 hours, 13 hours, 14 hours, 15 hours, or 16 hours at 37 °C. In some embodiments, the human serum half- life of the conjugate is at most 48 hours, 36 hours, 24 hours, 23 hours, 22 hours, 21 hours, 20 hours, 19 hours, 18 hours, 17 hours, 16 hours, 15 hours, 14 hours, 13 hours, 12 hours, 11 hours, 10 hours, 9 hours, 8 hours, 7 hours, 6 hours, 5 hours, or 1 hour at 37 °C.
- the human serum half-life of the conjugate is about 2 to 20 hours, 5 to 20 hours, 8 to 15 hours, or 10 to 14 hours at 37 °C.
- a herein described conjugate is a conjugate of FIG. 19.
- a herein described conjugate is a conjugate of FIG.20.
- a herein described conjugate is a conjugate of FIG. 21.
- a herein described conjugate is a conjugate of FIG. 22.
- a herein described conjugate is a conjugate of Table 1 or Table 2.
- the conjugate further comprises a radionuclide such as Ac-225 or Lutetium-177.
- described herein is a conjugate that comprises a conjugate of FIGs 9-22 and a radionuclide of Table 5A or 5B. In some embodiments, described herein is a conjugate that comprises a conjugate of Table 1 and a radionuclide of Table 5A or 5B. In some embodiments, described herein is a conjugate that comprises a conjugate of Table 2 and a radionuclide of Table 5A or 5B. In some embodiments, a herein described conjugate is a conjugate of Table 6. In some embodiments, a herein described conjugate is a conjugate of Table 7. In some embodiments, a herein described conjugate is a conjugate of Table 8. In some embodiments, a herein described conjugate is a conjugate of Table 9. Table 1. Exemplary Conjugates
- a metal chelator such as DOTA can interact with a radionuclide (e.g., 177 Lu or 225 Ac) via one or more functional groups and/or atoms.
- a metal chelator can interact with a radionuclide via nitrogen and/or oxygen atoms.
- a metal chelator can interact with a radionuclide via carbonyl, carboxylic acid, amino, and/or amide groups of the metal chelator.
- the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as . In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as . In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as or .
- the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as .
- the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as .
- the radionuclide exists in a positive oxidation state e.g., 225 Ac 3+ , 177 Lu 3+ .
- the radionuclide exists in a salt form, e.g., as 225 Ac 3+ , 177 Lu 3+ .
- the radionuclide exists in a salt form, e.g., as 225 Ac 3+ , 177 Lu 3+ .
- the conjugate is in a salt form.
- one or more of the carboxylic acid groups of the conjugate may exist as carboxylate anions.
- one or more of the carboxylate anions of the conjugate may coordinate to the radionuclide.
- the dissociation of an acid can depend on the pH value of the environment and its pK value. Accordingly, in some embodiments, a conjugate described herein can exist in a completely ionized, partially ionized or non-ionized form. Table 2. Design of Exemplary Conjugates
- a conjugate described herein has a structure of Formula (I), (Ia), (Iaa) or (Iab), or a salt or solvate thereof, which is further described below.
- Peptide Ligands [0130] In one aspect, a conjugate described herein comprises a GCC binding peptide.
- the GCC binding peptide can be linear or cyclic (e.g., cyclized by disulfide bond or other bonds).
- the cyclization can occur between the N- and C-terminus, or it can occur between a terminal amino acid and a non-terminal amino acid. In some embodiments, the cyclization occurs between two non-terminal amino acids.
- the peptide is monocyclic. In some embodiments, the peptide is bicyclic or polycyclic.
- the peptide can comprise any suitable number of amino acid residues. In some embodiments, the peptide comprises from 5 to 50, 6 to 40, 7 to 30, 8 to 25, 12 to 25, or 9 to 20 amino acid residues. In some embodiments, the peptide comprises from 10 to 15 amino acid residues.
- the peptide comprises from 12 to 15 amino acid residues. In some embodiments, the peptide comprises from 13 to 16 amino acid residues. In some embodiments, the peptide comprises 9 amino acid residues. In some embodiments, the peptide comprises 10 amino acid residues. In some embodiments, the peptide comprises 11 amino acid residues. In some embodiments, the peptide comprises 12 amino acid residues. In some embodiments, the peptide comprises 13 amino acid residues. In some embodiments, the peptide comprises 14 amino acid residues. In some embodiments, the peptide comprises 15 amino acid residues. In some embodiments, the peptide comprises 16 amino acid residues. In some embodiments, the peptide comprises 17 amino acid residues.
- the peptide comprises 18 amino acid residues. In some embodiments, the peptide comprises 16 to 25 amino acid residues. In some embodiments, the peptide comprises 9 amino acid residues. In some embodiments, the peptide consists of 10 amino acid residues. In some embodiments, the peptide consists of 11 amino acid residues. In some embodiments, the peptide consists of 12 amino acid residues. In some embodiments, the peptide consists of 13 amino acid residues. In some embodiments, the peptide consists of 14 amino acid residues. In some embodiments, the peptide consists of 15 amino acid residues. In some embodiments, the peptide consists of 16 amino acid residues.
- the peptide consists of 17 amino acid residues.
- the GCC binding peptide is an endogenous GCC ligand, e.g., guanylin and uroguanylin.
- the GCC binding peptide is an enterotoxin, e.g., enterotoxigenic E. coli, K. pneumonia, V. cholera, and Y. enterocolitica.
- the GCC binding peptide is a synthetic peptide, e.g., linaclotide, plecanatide and dolcantide.
- Exemplary GCC binding peptides include, but are not limited to linaclotide, plecantide, dolcantide, E. coli ST, guanylin, Uroguanylin, limphoguanylin, E.coli STp, E.coli EAST1, V. cholerae STh, V. mimicus ST, Y. enterocolitica ST, and guanylin.
- GCC binding peptides are further provided in Lin et al., Toxins 2010, 2, 2028-2054 and Aka et al., Expert Rev Clin Pharmacol.2017 May ; 10(5): 549–557, each of which is hereby incorporated by reference in its entirety.
- the GCC binding peptide is linaclotide or a derivative thereof.
- a GCC binding peptide comprises an amino acid sequence that has at least 50% identity to any one of SEQ ID NOs: 1-42.
- a GCC binding peptide comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs: 1-42.
- a GCC binding peptide comprises an amino acid sequence that has at least 70% identity to any one of SEQ ID NOs: 1-42.
- a GCC binding peptide comprises an amino acid sequence that has at least 80% identity to any one of SEQ ID NOs: 1-42.
- a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 90% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 95% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, or a binding fragment thereof. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 70% identity to any one of SEQ ID NOs: 1-19.
- a GCC binding peptide comprises an amino acid sequence that has at least 80% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 90% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 95% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-19, or a binding fragment thereof.
- the GCC binding peptide comprises any one of sequences in FIGs. 1A-1J, or a binding fragment thereof. In some embodiments, the GCC binding peptide comprises any one of sequences in Table 2. Table 4. Amino acid sequences for exemplary GCC binding peptides
- the GCC binding peptide comprises SEQ ID NO: 1. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 2. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 3. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 4. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 5. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 6. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 7. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 8. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 9.
- the GCC binding peptide comprises SEQ ID NO: 10. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 11. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 12. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 13. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 14. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 15. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 16. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 17. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 18. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 19.
- the GCC binding peptide comprises SEQ ID NO: 20. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 21. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 22. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 23. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 24. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 25. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 26. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 27. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 28.
- the GCC binding peptide comprises SEQ ID NO: 29. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 30. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 31. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 32. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 33. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 34. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 35. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 36. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 37.
- the GCC binding peptide comprises SEQ ID NO: 38. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 39. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 40. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 41. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 42. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 1. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 2. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 3. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 4.
- the GCC binding peptide consists of SEQ ID NO: 5. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 6. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 7. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 8. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 9. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 10. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 11. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 12. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 13.
- the GCC binding peptide consists of SEQ ID NO: 14. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 15. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 16. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 17. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 18. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 19. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 20. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 21. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 22.
- the GCC binding peptide consists of SEQ ID NO: 23. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 24. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 25. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 26. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 27. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 28. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 29. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 30.
- the GCC binding peptide consists of SEQ ID NO: 31. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 32. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 33. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 34. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 35. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 36. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 37. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 38.
- the GCC binding peptide consists of SEQ ID NO: 39. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 40. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 41. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 42. In some embodiments, one or more amino acids in any one of the SEQ ID NOs: 1-42 are derivatized, modified, and/or replaced with an unnatural amino acid. [0134] Percent sequence identity can be calculated using computer programs or direct sequence comparison.
- Preferred computer program methods to determine identity between two sequences include, but are not limited to, the GCG program package, FASTA, BLASTP, and TBLASTN (see, e.g., D. W. Mount, 2001, Bioinformatics: Sequence and Genome Analysis, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.).
- the BLASTP and TBLASTN programs are publicly available from NCBI and other sources.
- the Smith Waterman algorithm can also be used to determine percent identity.
- Exemplary parameters for amino acid sequence comparison include the following: 1) algorithm from Needleman and Wunsch (J. Mol. Biol., 48:443-453 (1970)); 2) BLOSSUM62 comparison matrix from Hentikoff and Hentikoff (Proc. Nat. Acad. Sci.
- GCC binding peptide described herein is a GCC receptor agonist.
- Exemplary GCC receptor agonists are described in U.S. Pat.
- a peptide described herein can be a peptide mimetic.
- the peptide can comprise non-peptide bonds and it can comprise one or more unnatural amino acids.
- each of the amino acid in a peptide described herein can independently be in its D or L form. Both D and L forms are encompassed by the present disclosure.
- amino acid embraces derivatives of amino acids.
- the derivatives include, for example, amino acids obtained by modifying a natural amino acid constituting a protein produced by cellular DNA-encoded biological matter.
- non-natural amino acids include hydroxyproline and hydroxylysine, which are amino acids having a hydroxyl group introduced therein, and diaminopropionic acid, which is an amino acid having an amino group introduced therein.
- the derivatives also include derivatives having an amide, ester, or carboxyl group as the C-terminus and/or N-terminus thereof.
- derivatives of the peptide include those obtained by modification such as phosphorylation, methylation, acetylation, adenylylation, ADP-ribosylation, or glycosylation and fused protein obtained by fusion with another peptide or protein. These derivatives can be prepared by those skilled in the art in a known manner or a method based thereon.
- amino acids include natural protein L-amino acids, unnatural amino acids, and chemically synthesized compounds having properties known in the art as characteristics of an amino acid.
- unnatural amino acids include, but not limited to, ⁇ , ⁇ - disubstituted amino acids (such as ⁇ -methylalanine), N-alkyl- ⁇ -amino acids, D-amino acids, ⁇ - amino acids, and ⁇ -hydroxy acids, each having a backbone structure different from that of natural amino acids; amino acids (such as norleucine and homohistidine) having a side-chain structure different from that of natural amino acids; amino acids (such as “homo” amino acids, homophenylalanine, and homohistidine) having extra methylene in the side chain thereof; and amino acids (such as cysteic acid) obtained by substituting a carboxylic acid functional amino group in the side chain thereof by a sulfonic acid group.
- ⁇ , ⁇ - disubstituted amino acids such as ⁇ -methylalanine
- N-alkyl- ⁇ -amino acids such as ⁇ -methylalanine
- D-amino acids such as ⁇ - amino acids
- each amino acid residue of the GCC binding peptide can be independently derivatized.
- the N- and/or C-terminus of the residue of any of the sequences in Table 4 can be replaced with a derivatized and/or unnatural amino acid. Modifications of the amino acid residues can improve the properties of the peptide (e.g., stability) and the conjugate comprising the peptide.
- the GCC binding peptide comprises one or more D-amino acids.
- the GCC binding peptide comprises one or more ⁇ -amino acids.
- the GCC binding peptide comprises N-alkylation (such as N-methylation).
- the GCC binding peptide comprises one or more polyethylene glycol chains. In some embodiments, the GCC binding peptide is cyclized. In some embodiments, the GCC binding peptide comprises an amide to sulfonamide substitution. In some embodiments, the GCC binding peptide comprises terminal modification. [0140] A binding peptide (e.g., GCC binding peptide) described herein can be modified internally, and/or at N-terminus, C-terminus or both. In some embodiments, the GCC binding peptide comprises N-terminal methylation, N-terminal acetylation, N-terminal propionylation, N- terminal myristoylation, or N-terminal palmitoylation.
- the GCC binding peptide comprises C-terminal methylation, C-terminal acetylation, C-terminal propionylation, C- terminal myristoylation, or C-terminal palmitoylation.
- the GCC binding peptide comprises N-terminal modification such as amidation.
- the GCC binding peptide comprises C-terminal modification such as amidation.
- the GCC binding peptide comprises internal modification such as cyclization, carbamidomethylation, phosphorylation, or PEGylation.
- the modification can function to increase the bulkiness of the terminal amino acid thereby improving metabolic stability. In some embodiments, the modification increases half-life and/or proteolysis stability.
- Exemplary C- terminal modification on a tyrosine residue includes D- tyrosine, N-methyl-L-tyrosine, ⁇ -methyl-L-tyrosine, L-tyrosinamide, O-methyl-L-tyrosine, O-4- allyl-L-tyrosine, 4-propyl-L-tyrosine, O-propargyltyrosine, etc.
- the C- terminal modification on a tyrosine residue is D-tyrosine.
- the C-terminal modification on a tyrosine residue is N-methyl-L-tyrosine.
- a GCC binding peptide described herein can be modified by cyclization. In some embodiments, the cyclization occurs between the N- and C-terminus. In some embodiments, the cyclization occurs between a terminal amino acid and a non-terminal amino acid. In some embodiments, the cyclization occurs between two non-terminal amino acids. In some embodiments, a GCC binding peptide described herein comprises 1, 2 or 3 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises 2 or 3 intramolecular disulfide bonds.
- a GCC binding peptide comprises 2 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises 3 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises one intramolecular alkylene or alkenylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular alkylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular heteroalkylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular alkenylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular bond that is bridged by a triazole.
- a GCC binding peptide described herein comprises two intramolecular disulfide bonds and one intramolecular heteroalkene, alkylene or alkenylene bond.
- the intramolecular bond comprises a triazole bridge.
- a GCC binding peptide comprises intramolecular alkylene bridge.
- a GCC binding peptide comprises intramolecular heteroalkylene bridge.
- the alkylene bridge is C 2 -C 10 alkylene.
- the alkylene bridge is C 3 -C 8 alkylene.
- the alkylene bridge is C 3 -C 6 alkylene.
- the bridge is C 2 -C 10 heteroalkylene. In some embodiments, the bridge is C 3 -C 8 heteroalkylene. In some embodiments, the bridge is C 3 -C 6 heteroalkylene. In some embodiments, the alkylene bridge is C 3 alkylene. In some embodiments, the alkylene bridge is C 4 alkylene. In some embodiments, a GCC binding peptide comprises intramolecular heteroalkylene bridge. In some embodiments, the heteroalkylene bridge is C 2 -C 10 heteroalkylene. In some embodiments, the heteroalkylene bridge is C 2 -C 8 heteroalkylene. In some embodiments, the heteroalkylene bridge is C 2 -C 4 heteroalkylene.
- the heteroalkylene bridge is C 3 heteroalkylene. In some embodiments, the heteroalkylene bridge is C 4 heteroalkylene. In some embodiments, the heteroalkylene bridge comprises 1, 2, or 3 atoms each independently selected from O, N, P, S, and Se. In some embodiments, the heteroalkylene bridge comprises S. In some embodiments, the heteroalkylene bridge comprises Se.
- the heteroalkylene, alkylene or alkenylene is optionally substituted with one or more R 10 , wherein each R 10 is independently halogen, amino, -OH, -SH, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 1 -C 6 hydroxyalkyl, C 1 -C 6 aminoalkyl, C 1 -C 6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R 10 on the same atoms are taken together to form a cycloalkyl or heterocycloalkyl; or two R 10 on different atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl.
- a cyclized GCC binding peptide comprises a structure selected from:
- a cyclized GCC binding peptide comprises a structure of C-28. In some embodiments, a cyclized GCC binding peptide comprises a structure of C-29. In some embodiments, a cyclized GCC binding peptide comprises a structure of C-66. In some embodiments, a cyclized GCC binding peptide comprises a structure of C-69. In some embodiments, a cyclized GCC binding peptide comprises a structure of In some embodiments, a cyclized GCC binding peptide comprises a structure of C-85. In some embodiments, a cyclized GCC binding peptide comprises a structure of C-86.
- a cyclized GCC binding peptide comprises a structure of In some embodiments, a cyclized GCC binding peptide comprises a structure of [0146]
- a peptide having SEQ ID NO: 1 or a fragment thereof can contain one or more intramolecular bonds.
- a peptide having SEQ ID NO: 1-42 or a fragment thereof can contain one or more intramolecular bonds.
- a peptide of Tables 2 and 4 can contain one or more intramolecular bonds.
- the peptide can comprise one or more of Cys 1 - Cys 6 disulfide bond, Cys 2 -Cys 10 disulfide bond, and Cys 5 - Cys 13 disulfide bonds.
- the peptide comprises Cys 1 -Cys 6 alkylene, heteroalkylene, or alkenylene bond. In some embodiments, the peptide comprises Cys 2 - Cys 10 alkylene, heteroalkylene or alkenylene bond. In some embodiments, the peptide comprises Cys 5 - Cys 13 alkylene, heteroalkylene, or alkenylene bond. In some embodiments, the intramolecular bond comprises a triazole bridge. [0147] Peptide ligands disclosed herein can contain two or more cysteine residues. For example, a peptide sequence of Table 2 or 4 can contain two or more cysteine residues, e.g., 2, 3, 4, 5, or 6 cysteine residues.
- the first cysteine residue in the sequence forms a disulfide bond with the 4 th cysteine residue in the same sequence.
- the second cysteine residue in the sequence forms a disulfide bond with the 5 th cysteine residue in the same sequence.
- the third cysteine residue in the sequence forms a disulfide bond with the 6 th cysteine residue in the same sequence.
- the GCC binding peptide is more resistant to protease degradation than linaclotide.
- the human serum half-life of a GCC binding peptide described herein is at least 100%, 120%, 150%, 200%, or 300% compared to the human serum half-life of linaclotide at 37 °C.
- the human serum half-life of a GCC binding peptide described herein is at least 1.1, 1.2, 1.5, 2.0, 3.0, 5.0 or 10 fold compared to the human serum half-life of linaclotide at 37 °C.
- a peptide described herein has a molecular weight from 500 Da to 10,000 Da.
- a peptide described herein has a molecular weight from 500 Da to 5,000 Da. In some embodiments, a peptide described herein has a molecular weight from 500 Da to 3,000 Da. In some embodiments, the peptide has a molecular weight from 1,000 Da to 1,500 Da. In some embodiments, the peptide has a molecular weight from 1,500 Da to 2,000 Da. In some embodiments, the peptide has a molecular weight from 2,000 Da to 2,500 Da. In some embodiments, the peptide has a molecular weight from 2,500 Da to 3,000 Da. In some embodiments, the peptide has a molecular weight from 3,000 Da to 3.500 Da.
- the peptide has a molecular weight from 3.500 Da to 4,000 Da. In some embodiments, the peptide has a molecular weight from 4,000 Da to 4,500 Da. In some embodiments, the peptide has a molecular weight from 4,500 Da to 5,000 Da. In some embodiments, the peptide has a molecular weight from 5,000 Da to 5,500 Da. In some embodiments, the peptide has a molecular weight from 5,500 Da to 6,000 Da. In some embodiments, the peptide has a molecular weight from 6,000 Da to 6,500 Da. In some embodiments, the peptide has a molecular weight from 6,500 Da to 7,000 Da.
- the peptide has a molecular weight of at least 500 Da, 800 Da, 1,000 Da, 1,200 Da, 1,400 Da, 1,600 Da, 1,800 Da, 2,000 Da, 2,200 Da, 2,400 Da, 2,600 Da, 2,800 Da, 3,000 Da, 3,200 Da, 3,400 Da, 3,600 Da, 3,800 Da, 4,000 Da, 4,200 Da, 4,400 Da, 4,600 Da, 4,800 Da, 5,000 Da, 5,200 Da, 5,400 Da, 5,600 Da, 5,800 Da, 6,000 Da, 6,200 Da, 6,400 Da, 6,600 Da, 6,800 Da, or 7,000 Da.
- the peptide has a molecular weight of at most 600 Da, 800 Da, 1,000 Da, 1,200 Da, 1,400 Da, 1,600 Da, 1,800 Da, 2,000 Da, 2,200 Da, 2,400 Da, 2,600 Da, 2,800 Da, 3,000 Da, 3,200 Da, 3,400 Da, 3,600 Da, 3,800 Da, 4,000 Da, 4,200 Da, 4,400 Da, 4,600 Da, 4,800 Da, 5,000 Da, 5,200 Da, 5,400 Da, 5,600 Da, 5,800 Da, 6,000 Da, 6,200 Da, 6,400 Da, 6,600 Da, 6,800 Da, 7,000 Da, or 7,500 Da.
- a cyclic GCC binding peptide described herein has a net charge of -3 to +1.
- the cyclic peptide has a net charge of -3. In some embodiments, the cyclic peptide has a net charge of -2. In some embodiments, the cyclic peptide has a net charge of -1. In some embodiments, the cyclic peptide has a net charge of 0. In some embodiments, the cyclic peptide has a net charge of +1. The net charge can be determined by aggregating the charge of each of the amino acids in the peptide.
- a cyclic GCC binding peptide described herein has a net charge of at most -4. In some embodiments, the cyclic peptide has a net charge of -4. In some embodiments, a cyclic GCC binding peptide described herein has a net charge of at least +2. In some embodiments, the cyclic peptide has a net charge of +2.
- a GCC binding peptide comprises unnatural amino acid.
- a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, wherein the amino acid sequence of any one of SEQ ID NOs: 1-30 comprises unnatural amino acid.
- C-terminus of a GCC binding peptide comprises unnatural amino acid.
- a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, wherein C-terminus of the amino acid sequence of any one of SEQ ID NOs: 1-42 comprises unnatural amino acid.
- the peptides described herein can comprise one or more unnatural amino acids.
- unnatural amino acids include: p-acetyl-L-phenylalanine, p-iodo-L- phenylalanine, p-methoxyphenylalanine, O-methyl-L-tyrosine, p-propargyloxyphenylalanine, p- propargyl-phenylalanine, L-3-(2-naphthyl)alanine, 3-methyl-phenylalanine, O-4-allyl-L-tyrosine, 4-propyl-L-tyrosine, tri-O-acetyl-GlcNAcp-serine, L-Dopa, fluorinated phenylalanine, isopropyl- L-phenylalanine, p-azido-L-phenylalanine, p-acyl-L-phenylalanine, p-benzo
- the unnatural amino acid is an unnatural analogue of a tyrosine amino acid; an unnatural analogue of a glutamine amino acid; an unnatural analogue of a phenylalanine amino acid; an unnatural analogue of an alanine amino acid; an unnatural analogue of a serine amino acid; an unnatural analogue of a threonine amino acid; an alkyl, aryl, acyl, azido, cyano, halo, hydrazine, hydrazide, hydroxyl, alkenyl, alkynl, ether, thiol, sulfonyl, seleno, ester, thioacid, borate, boronate, phospho, phosphono, phosphine, heterocyclic, enone, imine, aldehyde, hydroxylamine, keto, or amino substituted amino acid; or a combination thereof.
- the unnatural amino acid is an amino acid with a photoactivatable cross-linker; a spin-labeled amino acid; a fluorescent amino acid; a metal binding amino acid; a metal-containing amino acid; a radioactive amino acid; a photocaged and/or photoisomerizable amino acid; a biotin or biotin-analogue containing amino acid; a keto containing amino acid; an amino acid comprising polyethylene glycol or polyether; a heavy atom substituted amino acid; a chemically cleavable or photocleavable amino acid; an amino acid with an elongated side chain; an amino acid containing a toxic group; a sugar substituted amino acid; a carbon-linked sugar-containing amino acid; a redox-active amino acid; an a-hydroxy containing acid; an amino thio acid; an ⁇ , ⁇ -disubstituted amino acid; a ⁇ -amino acid; a cyclic amino acid other than proline or hist
- the unnatural amino acids incorporated into the peptides include one or more of: 1) a ketone functional group (as found in para or meta acetyl-phenylalanine) that can be specifically reacted with hydrazines, hydroxylamines and their derivatives (Addition of the keto functional group to the genetic code of Escherichia coli. Wang L, Zhang Z, Brock A, Schultz P G. Proc Natl Acad Sci USA.2003 Jan.7; 100(1):56-61; Bioorg Med Chem Lett.2006 Oct.15; 16(20):5356-9. Genetic introduction of a diketone-containing amino acid into proteins.
- Zeng H, Xie J, Schultz P G 2) azides (as found in p-azido-phenylalanine) that can be reacted with alkynes via copper catalyzed “click chemistry” or strain promoted (3+2) cycloadditions to form the corresponding triazoles (Addition of p-azido-L-phenylalanine to the genetic code of Escherichia coli. Chin J W, Santoro S W, Martin A B, King D S, Wang L, Schultz P G. J Am Chem Soc.2002 Aug. 7; 124(31):9026-7; Adding amino acids with novel reactivity to the genetic code of Saccharomyces cerevisiae.
- a genetically encoded boronate-containing amino acid is a genetically encoded boronate-containing amino acid., Housead E, Bushey M L, Lee J W, Groff D, Liu W, Schultz P G), and 5) metal chelating amino acids, including those bearing bipyridyls, that can specifically co-ordinate a metal ion (Angew Chem Int Ed Engl.2007; 46(48):9239-42.
- a genetically encoded bidentate, metal-binding amino acid is Xie J, Liu W, Schultz P G).
- the peptide of the present disclosure embraces various derivatives thereof. Examples of the derivatives include derivatives having an amide, ester, or carboxyl group as the C-terminus and/or N-terminus thereof.
- the derivatives of the peptide include those obtained by modification such as phosphorylation, methylation, acetylation, adenylylation, ADP- ribosylation, or glycosylation and fused protein obtained by fusion with another peptide or protein. These derivatives can be prepared by those skilled in the art in a known manner or a method based thereon.
- the binding peptide is bicyclic or polycyclic.
- a conjugate described herein comprises a bicyclic peptide.
- Exemplary bicyclic peptides include the bicyclic targeting peptides of BT5528, BT1718, and BT8009.
- the binding peptide is a lasso peptide.
- Lasso peptides can be synthetic or naturally produced by bacteria, and they possess a distinctive threaded lariat fold that offers a 3D array of functionality for engaging biological targets.
- This lasso structure can enable beneficial properties such as affinity, stability and potent biological activities. Suitable lasso structure can be designed by algorithms.
- Exemplary lasso peptides are provided in Hegemann, J.D., et al., Lasso Peptides: An Intriguing Class of Bacterial Natural Products, Acc. Chem. Res., 2015, 48, 1909 ⁇ 1919; Tietz, J.I., et al., A new genome-mining tool redefines the lasso peptide biosynthetic landscape, Nature Chem Bio, 2017, 13, 470-478; DiCaprio, A.J., et al., Enzymatic Reconstitution and Biosynthetic Investigation of the Lasso Peptide Fusilassin, J. Am. Chem.
- a cyclic peptide described herein is configured to bind to a plasma protein with a prescribed affinity, for example, measured as Plasma Protein Albumin Binding (PPB) percentage.
- PPB Plasma Protein Albumin Binding
- PPB can be determined in vitro by HPLC (e.g., Example B4) or by other suitable means known in the art.
- 1% to 99% of the cyclic peptide binds to Human Serum Albumin (HSA) in vitro as determined by HPLC, according to the conditions described in Example B3.
- HSA Human Serum Albumin
- about 2% to about 99%, about 5% to about 99%, about 10% to about 99%, about 20% to about 99%, about 30% to about 99%, about 40% to about 99%, about 50% to about 99%, about 60% to about 99%, about 70% to about 99%, or about 80% to about 99% of the cyclic peptide binds to HSA in vitro as determined by HPLC.
- about 10% to about 95% of the cyclic peptide binds to HSA in vitro (i.e., PPB of about 10% to about 95%). In some embodiments, about 20% to about 90% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 20% to about 60% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 95% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 80% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 60% of the cyclic peptide binds to HSA in vitro.
- about 60% to about 99% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 95% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 80% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 70% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 50% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 50% to about 60% of the cyclic peptide binds to HSA in vitro.
- a conjugate described herein e.g., a conjugate of Table 1 or Table 2 is configured to bind to a plasma protein with a prescribed affinity, for example, measured as Plasma Protein Albumin Binding (PPB) percentage.
- PPB Plasma Protein Albumin Binding
- PPB can be determined in vitro by HPLC (e.g., Example B4) or by other suitable means known in the art.
- 1% to 99% of the conjugate binds to Human Serum Albumin (HSA) in vitro as determined by HPLC, according to the conditions described in Example B3.
- HSA Human Serum Albumin
- about 2% to about 99%, about 5% to about 99%, about 10% to about 99%, about 20% to about 99%, about 30% to about 99%, about 40% to about 99%, about 50% to about 99%, about 60% to about 99%, about 70% to about 99%, or about 80% to about 99% of the conjugate binds to HSA in vitro as determined by HPLC.
- about 10% to about 95% of the conjugate binds to HSA in vitro (i.e., PPB of about 10% to about 95%). In some embodiments, about 20% to about 90% of the conjugate binds to HSA in vitro. In some embodiments, about 20% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 95% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 99% of the conjugate binds to HSA in vitro.
- about 60% to about 95% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 70% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 50% of the conjugate binds to HSA in vitro. In some embodiments, about 50% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 70% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 80% to about 99% of the conjugate binds to HSA in vitro.
- GCC Guanylate cyclase C
- ST heat-stable toxin
- guanylin and uroguanylin act as regulators of fluid and electrolyte balance.
- these peptides are released into the intestinal lumen where they bind to guanylate cyclase C localized on the luminal membrane of enterocytes (simple columnar epithelial cells of the small intestines and colon).
- enterocytes simple columnar epithelial cells of the small intestines and colon.
- the binding of the guanylin peptides to guanylate cyclase C induces electrolyte and water excretion into the intestinal lumen via a complex intracellular signaling cascade that is initiated by an increase in cyclic guanosine monophosphate (cGMP).
- cGMP cyclic guanosine monophosphate
- the cGMP-mediated signaling that is initiated by the guanylin peptides can be important for the normal functioning of the gut. Any abnormality in this process could lead to gastrointestinal disorders.
- these peptides may also be involved in the continual renewal of gastrointestinal mucosa by maintaining the balance between proliferation and apoptosis.
- uroguanylin and guanylin peptides can promote apoptosis by controlling cellular ion flux.
- GCC as a tumor suppressor expressed by the intestinal epithelium, can be a target for the treatment of certain cancers. GCC is densely expressed throughout the intestine, and overexpressed by tumor tissue, which can enable GCC as a suitable target for diagnostic and therapeutic goals.
- Linkers [0163] A conjugate described herein can comprise one or more linkers. In some embodiments, the linker covalently attaches the GCC binding peptide with the metal chelator. In some embodiments, the GCC binding peptide attaches directly to the metal chelator without a linker. [0164] In some embodiments, the present disclosure describes linkers that function as a spacer.
- a linker can comprise a number of intervening atoms (on a linear chain, excluding pendant groups or substituents) between the metal chelator and the binding peptide thereby creating a distance between the metal chelator and the binding peptide.
- a linker comprises 10- 100 intervening atoms between the metal chelator and the binding peptide.
- a linker comprises 2-60 intervening atoms between the metal chelator and the binding peptide.
- a linker comprises 2 to 20, 2 to 50, 5 to 15, 5 to 25, 10 to 40, 30 to 60, or 10 to 20 intervening atoms between the metal chelator and the binding peptide.
- a linker comprises 3 to 30 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 5 to 25 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 6 to 18 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 10 to 20 intervening atoms between the metal chelator and the binding peptide.
- the intervening atoms can comprise 1 or more carbons, and optionally one or more heteroatoms such as O and N.
- the intervening atoms comprise 2 to 20, 2 to 50, 5 to 15, 5 to 25, 10 to 40, 30 to 60, or 10 to 20 carbons. In some embodiments, the intervening atoms comprise 0, 1, 2, 3, 4, 5, or 6 nitrogen. In some embodiments, the intervening atoms comprise 0, 1, 2, 3, 4, 5, 6, 7 or 8 oxygen. [0165] As an example, the intervening atoms between the metal chelator and the binding peptide of conjugate C-035 are illustrated in brackets below and there are 16 intervening atoms. . [0166] A linker can comprise one or more amino acid residues. In some embodiments, the linker comprises 1 to 3, 1 to 5, 1 to 10, 5 to 10, or 5 to 20 amino acid residues.
- the linker comprises 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 amino acid residues. In some embodiments, the linker comprises 1 to 5 amino acid residues.
- the linker can comprise one or more lysine (K) residues such as K, KK, or KKK sequences. In some embodiments, one or more amino acids of the linker are unnatural amino acids.
- K lysine
- a herein-described linker can attach to the N-terminus of the peptide, the C-terminus of the peptide, or a non-terminal amino acid of the peptide, or it can attach to the peptide through a combination of the above. In some embodiments, a linker is attached to the GCC binding peptide via its N-terminus.
- a linker is attached to the GCC binding peptide via its C-terminus. In some embodiments, a linker is attached to the GCC binding peptide via its N- terminus and via its C-terminus. In some embodiments, a linker is attached to the peptide via a non-terminal amino acid.
- a conjugate described herein can comprise 2, 3, 4, 5 or more linkers. In some embodiments, the conjugate comprises two linkers, one attached to the N-terminus of the peptide and the other attached to the C-terminus of the peptide. In some embodiments, the conjugate comprises two linkers, one attached to the N or C-terminus of the peptide and the other attached to a non-terminal amino acid of the peptide.
- the linker can be bonded to the peptide, the metal chelator, or both, for example, through a chemically reactive group.
- chemically reactive groups include, but are not limited to, a free amino, imino, hydroxyl, thiol or carboxyl group (e.g., to the N- or C-terminus, to the epsilon amino group of one or more lysine residues, the free carboxylic acid group of one or more glutamic acid or aspartic acid residues, or to the sulfhydryl group of one or more cysteinyl residues).
- the site to which the linker is bound to the peptide can be a natural or unnatural amino acid of the peptide and/or it can be introduced into the peptide, e.g., by DNA recombinant technology (e.g., by introducing a cysteine or protease cleavage site in the amino acid sequence) or by protein biochemistry (e.g., reduction, pH adjustment or proteolysis).
- Exemplary methods for attaching the linker includes carbodiimide reaction, reactions using bifunctional agents such as dialdehydes or imidoesters, Schiff base reaction, Suzuki-Miyaura cross-coupling reactions, Isothiocyanates as coupling agents, and click chemistry.
- the linker can have a prescribed length thereby linking the metal chelator (and optionally radionuclide) and the peptide while allowing an appropriate distance therebetween.
- the linker has 1 to 100 atoms, 1 to 60 atoms, 1 to 30 atoms, 1 to 15 atoms, 1 to 10 atoms, 1 to 5, or 2 to 20 atoms in length.
- the linker has 1 to 10 atoms in length.
- the linker can function as a “spacer” between the GCC binding peptide and the radionuclide.
- the linker is configured to provide a distance between the GCC binding peptide and the metal chelator of at least 3 atoms, 4 atoms, 5 atoms, 6 atoms, 7 atoms, 8 atoms, 9 atoms, 10 atoms, 15 atoms, 20 atoms, or 25 atoms in length.
- the linker comprises an alkylene or heteroalkylene bond moiety between the GCC binding peptide and the metal chelator.
- the alkylene or heteroalkylene comprises at least 3 atoms, 4 atoms, 5 atoms, 6 atoms, 7 atoms, 8 atoms, 9 atoms, 10 atoms or 20 atoms in length.
- the linker comprises one or more polyethylene glycol units between the GCC binding peptide and the metal chelator. In some embodiments, the linker comprises at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, or at least 10 – (CH 2 -CH 2 -O)- units between the GCC binding peptide and the metal chelator, wherein the units can be contiguous or noncontiguous.
- the linker can comprise flexible and/or rigid regions. Exemplary flexible linker regions include those comprising Gly and Ser residues (“GS” linker), glycine residues, alkylene chain, PEG chain, etc.
- Exemplary rigid linker regions include those comprising alpha helix-forming sequences (e.g., EAAAK (SEQ ID NO: 45)), proline-rich sequences, and regions rich in double and/or triple bonds.
- the linker can be cleavable, e.g., under physiological conditions, e.g., under intracellular conditions, such that cleavage of the linker releases the chelator and radionuclide in the intracellular environment.
- the linker can be, e.g., a peptidyl linker that is cleaved by an intracellular peptidase or protease enzyme, including, but not limited to, a lysosomal or endosomal protease.
- the peptidyl linker is at least two amino acids long or at least three amino acids long.
- Cleaving agents can include cathepsins B and D and plasmin.
- the linker is not cleavable.
- the linker is pH-sensitive, i.e., sensitive to hydrolysis at certain pH values.
- the pH-sensitive linker can be hydrolyzable under acidic conditions.
- a linker can be an acid-labile linker that is hydrolyzable in the lysosome (e.g., a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the like).
- Such linkers can be relatively stable under neutral pH conditions, such as those in the blood, but are unstable at below pH 5.5 or 5.0, the approximate pH of the lysosome.
- the hydrolyzable linker is a thioether linker.
- the linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl.
- the linker comprises substituted or unsubstituted C 1 -C 30 alkylene.
- the linker comprises polyethylene glycol such as (-CH 2 - CH 2 -O-) 1-10 .
- the linker comprises a structure selected from: , and structures derived from any one thereof.
- the linker comprises a click chemistry residue.
- the linker is attached to the peptide, to the metal chelator, or both via click chemistry, thereby forming a click chemistry residue.
- the peptide can comprise an azide group (at N- or C-terminus or at a non-terminal amino acid) that reacts with an alkyne moiety of the linker.
- the peptide can comprise an alkyne group (at N- or C-terminus or at a non-terminal amino acid) that reacts with an azide of the linker.
- the metal chelator and the linker can be attached similarly.
- the linker comprises an azide moiety, an alkyne moiety, or both. In some embodiments, the linker comprises a triazole. In some embodiments, the click chemistry residue is (DBCO-azide residue), , , , , , . In some embodiments, the click chemistry residue is a DIBO-azide residue, BARAC-azide residue, DBCO-azide residue, DIFO-azide residue, COMBO-azide residue, BCN-azide residue, or DIMAC-azide residue. In some embodiments, the linker comprises a residue of nitrone dipole cycloaddition.
- the linker comprises a residue of tetrazine ligation. In some embodiments, the linker comprises a residue of quadricyclane ligation. Exemplary groups of click chemistry residue are shown in Hein at al., “Click Chemistry, A Powerful Tool for Pharmaceutical Sciences,” Pharmaceutical Research volume 25, pages2216– 2230 (2008); Thirumurugan et al, “Click Chemistry for Drug Development and Diverse Chemical–Biology Applications,” Chem. Rev. 2013, 113, 7, 4905–4979; US20160107999A1; US10266502B2; and US20190204330A1, each of which is incorporated by reference in its entirety.
- a linker described herein comprises two or more motifs (see FIGs. 2A-2D).
- the motifs can be connected by any suitable covalent bond.
- one or more of the motifs are connected via click chemistry such that they can be clicked in/out of the linker.
- Each of the motifs in a linker can have independent functions.
- a linker can comprise a motif that functions to adjust plasma half-life and/or a motif that functions as a spacer between the peptide and metal chelator.
- each of the motifs independently comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl.
- the linker comprises substituted or unsubstituted C 1 -C 30 alkylene.
- the linker comprises polyethylene glycol such as (-CH 2 -CH 2 -O-) 1-10 .
- R is hydrogen, substituted or unsubstituted C 6 -C 10 aryl, substituted or unsubstituted C 5 -C 9 heteroaryl, or a sterol.
- at least one L 1 is unsubstituted C 3 -C 20 alkylene.
- the linker comprises a click chemistry residue. In some embodiments, the linker comprises one or more lysine residues.
- the linker comprises one or more of a substituted or unsubstituted C 6 -C 10 aryl, substituted or unsubstituted C 5 -C 9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, or C 3 -C 20 alkylene, or amino acid residues.
- the linker –(L) n - comprises 3 to 20 intervening atoms between the metal chelator and the binding peptide. In some embodiments, the linker –(L) n - comprises 3 to 50, 2 to 25, 2 to 75, 15 to 25, 10 to 30, or 15 to 40 intervening atoms between the metal chelator and the binding peptide. [0187] In some embodiments, –(L) n - is bound to the N-terminus of the GCC binding peptide and L C is bound to the C-terminus of the GCC binding peptide.
- –(L) n - is bound to the C-terminus of the GCC binding peptide and L C is bound to the N-terminus of the GCC binding peptide. In some embodiments, –(L) n - is bound to the N-terminus of the GCC binding peptide. In some embodiments, –(L) n - is bound to the C-terminus of the GCC binding peptide. In some embodiments, –(L) n - is bound to a non-terminus amino acid of the GCC binding peptide. In some embodiments, L C is bound to the N-terminus of the GCC binding peptide.
- L C is bound to the C-terminus of the GCC binding peptide. In some embodiments, L C is bound to a non-terminus amino acid of the GCC binding peptide.
- the linker –(L) n - comprises a hydrophobic group selected from a C 8 -C 30 fatty acid, C 8 -C 30 fatty alcohol, C 8 -C 30 alkyl, aryl, heteroaryl, one or more amino acid residues, or a combination thereof.
- the fatty acid contains 1, 2, 3 or more carboxylic acid groups.
- the one or more amino acids contain a hydrophobic group such as an alkyl chain or a phenyl or heteroaryl group.
- –(L) n - comprises one or more lysine residues. In some embodiments, –(L) n - comprises one or more glutamate residues. In some embodiments, –(L) n - comprises one or more tyrosine residues. In some embodiments, –(L) n - comprises one or more lysine residues.
- n is 0. In some embodiments, n is 1. In some embodiments, n is 2. In some embodiments, n is 3. In some embodiments, n is 4. In some embodiments, n is 5. In some embodiments, n is 6. In some embodiments, n is 7. In some embodiments, n is 8. In some embodiments, n is 9.
- n is 10. In some embodiments, n is 11. In some embodiments, n is 12. In some embodiments, n is 13. In some embodiments, n is 14. In some embodiments, n is 15. In some embodiments, n is 16. In some embodiments, n is 17. In some embodiments, n is 18. In some embodiments, n is 19. In some embodiments, n is 20. [0192] In some embodiments of Formula (I), (Ia), (Iaa) or (Iab), r is 0. In some embodiments, r is 1. In some embodiments of Formula (I), r is 2. In some embodiments of Formula (I), r is 3. In some embodiments of Formula (I), r is 4.
- At least one of L 1 , L 2 , and L 3 comprises a hydrophobic group selected from a C 8 -C 30 fatty acid, C 8 -C 30 fatty alcohol, C 4 -C 12 alkylene chain, heteroaryl, or aryl group, each of which is optionally substituted.
- m is 0 or 1-9. In some embodiments, m is 0. In some embodiments, m is 1. In some embodiments, m is 2. In some embodiments, m is 3. In some embodiments, m is 4. In some embodiments, m is 5. In some embodiments, m is 6. In some embodiments, m is 7. In some embodiments, m is 8. In some embodiments, m is 9. In some embodiments of Formula (Ia), m is 10. [0197] In some embodiments of Formula (Ia), (Iaa) or (Iab), p is 0 or 1-5. In some embodiments, p is 0. In some embodiments, p is 1.
- p is 2. In some embodiments, p is 3. In some embodiments, p is 4. In some embodiments, p is 5. In some embodiments of Formula (Ia), p is 6. [0198] In some embodiments of Formula (Ia), (Iaa) or (Iab), q is 0 or 1-5. In some embodiments, q is 0. In some embodiments, q is 1. In some embodiments, q is 2. In some embodiments, q is 3. In some embodiments, q is 4. In some embodiments, q is 5. In some embodiments of Formula (Ia), q is 6.
- R is a hydrophobic group.
- R is optionally substituted naphthyl. In some embodiments, R is optionally substituted monocyclic heteroaryl. In some embodiments, R is optionally substituted bicyclic heteroaryl. In some embodiments, R is optionally substituted monocyclic cycloalkyl. In some embodiments, R is optionally substituted bicyclic cycloalkyl. In some embodiments, R is optionally substituted monocyclic heterocycloalkyl. In some embodiments, R is optionally substituted bicyclic heterocycloalkyl.
- R is hydrogen, substituted or unsubstituted C 6 -C 10 aryl, substituted or unsubstituted C 5 -C 9 heteroaryl, or a sterol.
- R is H.
- R is a sterol or a derivative thereof.
- R is phenyl.
- R is phenyl substituted with one or more halogen.
- R is iodophenyl. In some embodiments, R is . In some embodiments, R is . [0203] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is C 6 -C 30 fatty acid, C 6 -C 30 fatty alcohol or C 6 -C 30 alkyl. In some embodiments, R is C 6 - C 12 fatty acid.
- R is C 12 -C 24 fatty acid. In some embodiments, R is C 6 -C 12 fatty alcohol. In some embodiments, R is C 12 -C 24 fatty alcohol. In some embodiments, R is C 6 -C 12 alkyl. In some embodiments, R is C 12 -C 24 alkyl. In some embodiments, R is C 6 -C 12 alkenyl. In some embodiments, R is C 12 -C 24 alkenyl.
- R C is a C 6 -C 30 fatty acid, C 6 -C 30 fatty alcohol or C 6 -C 30 alkyl.
- R C is C 6 -C 12 fatty acid.
- R C is C 12 -C 24 fatty acid.
- R C is C 6 -C 12 fatty alcohol.
- R C is C 12 -C 24 fatty alcohol.
- R C is C 6 -C 12 alkyl.
- R C is C 12 -C 24 alkyl.
- R C is C 6 -C 12 alkenyl.
- R C is C 12 -C 24 alkenyl.
- R C is C 12 -C 24 alkenyl.
- a conjugate of Formula (I) has a linker of Formula (II-1).
- L 2 is unsubstituted C 1 -C 12 alkylene.
- a linker of the present disclosure e.g., a linker of Formula (II- 1a)
- L 2 is C 1 -C 30 heteroalkylene that is optionally substituted with one or more substituents selected from -OH, -SH, oxo, amino, C 1 -C 6 alkyl, C 1 -C 6 hydroxyalkyl, C 1 -C 6 haloalkyl, and C 1 -C 6 aminoalkyl.
- a linker of the present disclosure e.g., a linker of Formula (II- 1a)
- a linker of Formula (II- 1a) has a structure of , , , [0216]
- a conjugate of Formula (Ia) has a linker of Formula (II-2).
- the linker of Formula (II-2a) or Formula (II-2b) connects to the metal chelator through L 3 and to the peptide through L 2 .
- the linker has a structure of Formula (II-2a).
- a conjugate of Formula (Ia) or (Iaa) has a linker of Formula (II-2aa).
- L 31 is -NH- and L 32 is substituted or unsubstituted C 1 -C 12 alkylene, or substituted or unsubstituted C 1 -C 30 heteroalkylene.
- L 31 is -NH- and L 32 is -(CH 2 ) 4 -.
- L 32 is absent, substituted or unsubstituted C 3 -C 15 cycloalkyl, substituted or unsubstituted C 1 -C 12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C 1 -C 20 alkylene, or substituted or unsubstituted C 1 -C 30 heteroalkylene.
- L 32 is substituted or unsubstituted C 1 -C 12 alkylene.
- L 32 is C 1 -C 30 heteroalkylene.
- L 12 is substituted or unsubstituted C 1 -C 12 alkylene. In some embodiments, L 12 is C 1 -C 30 heteroalkylene. In some embodiments, L 12 is C 10 -C 20 heteroalkylene. In some embodiments, L 12 is C 1 -C 12 alkenyl. In some embodiments, L 12 is absent.
- L 21 is absent, substituted or unsubstituted C 3 -C 15 cycloalkyl, substituted or unsubstituted C 1 -C 12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C 1 -C 20 alkylene, or substituted or unsubstituted C 1 -C 30 heteroalkylene.
- L 21 is substituted or unsubstituted C 1 -C 12 alkylene.
- L 21 is C 1 -C 30 heteroalkylene.
- q is 0. In some embodiments, q is 1. In some embodiments, q is 2. In some embodiments, q is 3. In some embodiments, q is 4. In some embodiments, q is 5.
- L 12 is substituted or unsubstituted C 1 -C 12 alkylene, or substituted or unsubstituted C 1 -C 30 heteroalkylene.
- L 12 is , -(CH 2 ) 2 -, -(CH 2 ) 3 -, -(CH 2 ) 4 -,
- L 22 and L 21 are both absent.
- -L 21 -L 22 - is .
- L 1 is substituted or unsubstituted C 2 -C 30 alkenylene. In some embodiments, L 1 is substituted or unsubstituted C 1 -C 30 heteroalkylene. In some embodiments, L 1 is substituted or unsubstituted C 5 -C 25 heteroalkylene. In some embodiments, L 1 is substituted or unsubstituted C 5 -C 12 heteroalkylene.
- L 2 is substituted or unsubstituted C 2 -C 30 alkenylene. In some embodiments, L 2 is substituted or unsubstituted C 1 -C 30 heteroalkylene. In some embodiments, L 2 is substituted or unsubstituted C 5 -C 25 heteroalkylene. In some embodiments, L 2 is substituted or unsubstituted C 5 -C 12 heteroalkylene.
- L 3 is substituted or unsubstituted C 2 -C 30 alkenylene. In some embodiments, L 3 is substituted or unsubstituted C 1 -C 30 heteroalkylene. In some embodiments, L 3 is substituted or unsubstituted C 5 -C 25 heteroalkylene. In some embodiments, L 3 is substituted or unsubstituted C 5 -C 12 heteroalkylene.
- a linker comprises a click chemistry residue.
- a linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and one or more amino acids.
- a linker comprises one or more of a substituted or unsubstituted C 6 -C 10 aryl, substituted or unsubstituted C 5 -C 9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, C 3 -C 20 alkylene, or amino acid residues.
- a linker comprises one or more lysine residues.
- a linker comprises one or more glutamate residues.
- the linker comprises phenyl iodide or carboxylic acid.
- a linker described herein can reversibly bind to human serum albumin.
- the linker binds to Sudlow Site I of the human serum albumin.
- the linker binds to Sudlow Site II of the human serum albumin.
- the linker binds to Sudlow Site I and site II of the human serum albumin.
- the linker comprises one or more negatively charged groups such as a carboxylic acid and aromatic carboxylic acid.
- the linker comprises a halo substituted aryl group.
- the linker comprises phenyl iodide.
- the linker comprises phenyl bromide.
- the linker comprises an unsubstituted or halo substituted 1,1'-biphenyl. In some embodiments, the linker comprises an unsubstituted or halo substituted 1,1':4',1''-terphenyl. In some embodiments, the linker comprises an unsubstituted or halo substituted naphthyl. In some embodiments, the linker comprises a carboxylic acid or an isostere thereof. In some embodiments, the linker comprises a carboxylic acid. The halo substituted aryl group and the carboxylic acid group can be located in the same linker or in two different linkers.
- the linker comprises a lysine residue.
- the lysine residue is D-lysine.
- a linker described herein comprises one or more structures selected from: , ,
- the linker comprises: . [0257] In some embodiments, the linker comprises: [0258] In some embodiments, a dissociation constant (Kd) between the linker and human serum albumin is at most 500 ⁇ M, as determined at room temperature in human serum condition. In some embodiments, the Kd is at most 100 ⁇ M. In some embodiments, the Kd is at most 15 ⁇ M. In some embodiments, the Kd is from about 0.1 nM to about 10 ⁇ M. In some embodiments, the Kd is from about 10 nM to about 10 ⁇ M.
- Kd dissociation constant
- conjugates that comprise a metal chelator that is configured to bind with a radionuclide.
- the metal chelator can refer to a moiety of the conjugate that is configured to bind with a radionuclide.
- a conjugate described herein comprises two or more independent metal chelators, e.g., 2, 3, 4, 5, or more metal chelators.
- a conjugate described herein comprises two metal chelators, which can be the same or different.
- a conjugate described herein comprises two or more metal chelators.
- the conjugate comprises two radionuclides bound to the metal chelators.
- the metal chelator can be attached to the linker or the peptide through any suitable group/atom of the chelator.
- the metal chelator is capable of binding a radioactive atom.
- the binding can be direct, e.g., the metal chelator can make hydrogen bonds or electrostatic interactions with the radioactive atom.
- the binding can also be indirect, e.g., the metal chelator binds to a molecule that comprises a radioactive atom.
- the metal chelator comprises, or is, a macrocycle.
- the metal chelator comprises, or is, 2,2′,2′′,2′′′-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA) or 1,4,7- triazacyclononane-1,4,7-triacetic acid (NOTA).
- the metal chelator comprises a macrocycle, e.g., a macrocycle comprising an O and/or a N, DOTA, NOTA, one or more amines, one or more ethers, one or more carboxylic acids, EDTA, DTPA, TETA, DO3A, PCTA, or desferrioxamine.
- the metal chelator comprises a plurality of amines. In some embodiments, the metal chelator includes 4 or more N, 4 or more carboxylic acid groups, or a combination thereof. In some embodiments, the metal chelator does not comprise S. In some embodiments, the metal chelator comprises a ring. In some embodiments, the ring comprises an O and/or an N. In some embodiments, the metal chelator is a ring that includes 3 or more N, 3 or more carboxylic acid groups, or a combination thereof. In some embodiments, the metal chelator is poly polydentate. [0262] In some embodiments, a metal chelator described herein comprises a cyclic chelating agent.
- Exemplary cyclic chelating agents include, but are not limited to, AAZTA, BAT, BAT- TM, Crown, Cyclen, DO2A, CB-DO2A, DO3A, H3HP-DO3A, Oxo-DO3A, p-NH 2 -Bn-Oxo- DO3A, DOTA, DOTA-3py, DOTA-PA, DOTA-GA, DOTA-4AMP, DOTA-2py, DOTA-1py, p- SCN-Bn-DOTA, CHX-A′′-EDTA, MeO-DOTA-NCS EDTA, DOTAMAP, DOTAGA, DOTAGA-anhydride, DOTMA, DOTASA, DOTAM, DOTP, CB-Cyclam, TE2A, CB-TE2A, CB-TE2P, DM-TE2A, MM-TE2A, NOTA, NOTP, HEHA, HEHA-NCS, p-SCN-Bn-HEHA, DT
- the metal chelator is DOTA, TRITA, TETA, DOTA-MA, DO3A-HP, DOTMA, DOTA-pNB, DOTP, DOTMP, DOTEP, DOTMPE, F-DOTPME, DOTPP, DOTBzP, DOTA-monoamide, p-NCS-DOTA, p-NCS-PADOTA, BAT, DO3TMP-Monoamide, p-NCS- TRITA, NOTA, and CHX-A′′-DTPA.
- a metal chelator described herein comprises an acyclic chelating agent.
- Exemplary acyclic chelating agents include, but are not limited to, DTA, CyEDTA, EDTMP, DTPMP, DTPA, CyDTPA, Cy2DTPA, DTPA-MA, DTPA- BA, and BOPA.
- a metal chelator described herein comprises DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, TACN, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, or NOTP.
- a metal chelator described herein comprises H 4 pypa, H 4 octox, H 4 octapa, p-NO 2 -Bn-neunpa, p-SCN–Bn– H 4 neunpa, TTHA, t Bu 4 pypa-C 7 -NHS, H 4 neunpa, H 2 macropa, HP-DO3A, BT-DO3A, DO3A- Nprop, DO3AP, DO2A2P, DOA3P, DOTP, DOTPMB, DOTAMAE, DOTAMAP, DO3AM Bu , DOTMA, TCE-DOTA, DEPA, PCTA, p-NO 2 -Bn-PCTA, p-NO 2 -Bn-DOTA, symPC 2 APA, symPCA2PA, asymPC 2 APA, asymPCA2PA, TRAP, AAZTA, DATA m , THP,
- the metal chelator is DO3A. In some embodiments, the metal chelator is PEPA. In some embodiments, the metal chelator is EDTA. In some embodiments, the metal chelator is CHX-A′′-DTPA. In some embodiments, the metal chelator is HEHA. In some embodiments, the metal chelator is DOTMP. In some embodiments, the metal chelator is t-Bu- calix[4]arene-tetracarboxylic acid. In some embodiments, the metal chelator is macropa. In some embodiments, the metal chelator is macropa-NCS. In some embodiments, the metal chelator is H4pypa.
- the metal chelator is H4octapa. In some embodiments, the metal chelator is H 4 CHXoctapa. In some embodiments, the metal chelator is DOTP. In some embodiments, the metal chelator is crown. [0264] In some embodiments, the metal chelator is DOTA. In some embodiments, the metal chelator is a chiral derivative of DOTA. Exemplary chiral DOTA chelators are described in Dai et al., Nature Communications (2018) 9:857.
- the metal chelator is 2,2',2'',2''-((2S,5S,8S,11S)-2,5,8,11-tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10- tetrayl)tetraacetic acid. In some embodiments, the metal chelator has a structure of .
- the metal chelator is 2,2',2'',2''- ((2S,5S,8S,11S)-2,5,8,11-tetraethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid.
- the metal chelator has a structure of .
- the metal chelator has a structure of wherein each R e is independently selected from hydrogen, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkylcycloalkyl, alkylheterocycloalkyl, alkylaryl, alkylheteroaryl, or an amino acid side chain.
- the metal chelator has a structure of wherein each R e is independently selected from hydrogen, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkylcycloalkyl, alkylheterocycloalkyl, alkylaryl, alkylheteroaryl, or an amino acid side chain.
- the conjugate comprises DOTA.
- the conjugate comprises a DOTA derivative such as p-SCN-Bn-DOTA and MeO-DOTA-NCS.
- the conjugate comprises two independent metal chelators, and at least one or both are DOTA.
- the structures of some exemplary metal chelators are illustrated in FIGs. 3-17 (without showing the attachment points).
- Exemplary metal chelators are further described in WO2012/174136; US20130183235A1; US20120219495A1; Ramogidaand et al., EJNMMI radiopharm. chem.4, 21 (2019); Thiele et al., Cancer Biotherapy and Radiopharmaceuticals 2018; Li et al., Bioconjugate Chem.2019, 30, 5, 1539–1553; and Baranyai et al., Eur. J. Inorg. Chem.
- Radionuclides [0267] In one aspect, described herein are conjugates that comprise a radionuclide. Generally, the type of radionuclide used in a therapeutic radiopharmaceutical can be tailored to the specific type of cancer, the type of targeting moiety (e.g., binding peptide), etc. Radionuclides that undergo ⁇ -decay produce particles composed of two neutrons and two protons, and radionuclides that undergo ⁇ -decay emit energetic electrons from their nuclei. Some radionuclides can also emit Auger. In some embodiments, the conjugate comprises an alpha particle-emitting radionuclide.
- Alpha radiation can cause direct, irreparable double-strand DNA breaks compared with gamma and beta radiation, which can cause single-stranded breaks via indirect DNA damage.
- the range of these particles in tissue and the half-life of the radionuclide can also be considered in designing the radiopharmaceutical conjugate.
- Tables 5A and 5B below illustrate some properties of exemplary radionuclides. Table 5A. Exemplary radionuclides
- a conjugate described herein comprises one or more independent radionuclides.
- the conjugate comprises two radionuclides.
- each of the one or more radionuclides is bound to a metal chelator of the conjugate.
- two radionuclides of a conjugate are bound to the same metal chelator.
- two radionuclides of a conjugate are bound to two independent metal chelators.
- each of the one or more radionuclides is an alpha particle- emitting radionuclide.
- a conjugate described herein comprises an alpha particle-emitting radionuclide.
- the alpha particle-emitting radionuclide is actinium-225 ( 225 Ac), astatine-211 ( 211 At), radium-223 ( 223 Ra), radium-224 ( 224 Ra), bismuth-213 ( 213 Bi), Terbium-149 ( 149 Tb), or thorium-227 ( 227 Th).
- the alpha particle-emitting radionuclide is 225 Ac.
- the alpha particle-emitting radionuclide is 213 Bi.
- the alpha particle-emitting radionuclide is 212 Bi.
- the alpha particle-emitting radionuclide is 212 Pb. In some embodiments, the alpha particle-emitting radionuclide is 224 Ra. In some embodiments, the alpha particle-emitting radionuclide is 223 Ra. In some embodiments, the alpha particle-emitting radionuclide is 227 Th. In some embodiments, the alpha particle-emitting radionuclide is 211 At. In some embodiments, the alpha particle-emitting radionuclide is 149 Tb. In some embodiments, the radionuclide is Zirconium-89 ( 89 Zr).
- a conjugate described herein comprises a radionuclide selected from 67 Cu, 64 Cu, 90 Y, 109 Pd, 111 Ag, 149 Pm, 153 Sm, 166 Ho, 99m Tc, 67 Ga, 68 Ga, 111 In, 90 Y, 177 Lu, 186 Re, 188 Re, 197 Au, 198 Au, 199 Au, 105 Rh, 165 Ho, 161 Tb, 149 Pm, 44 Sc, 47 Sc, 70 As, 71 As, 72 As, 73 As, 74 As, 76 As, 77 As, 212 Pb, 212 Bi, 213 Bi, 225 Ac, 117m Sn, 67 Ga, 201 Tl, 123 I, 131 I, 160 Gd, 148 Nd, 89 Sr, 89 Zr, and 211 At.
- a radionuclide selected from 67 Cu, 64 Cu, 90 Y, 109 Pd, 111 Ag, 149 Pm, 153 Sm, 166 Ho
- the radionuclide is 225 Ac. In some embodiments, the radionuclide is a decay daughter of 225 Ac such as 221 Fr, 217 At, 213 Bi, 213 Po, 209 Tl, 209 Pb, or 209 Bi. In some embodiments, the conjugate comprises two 225 Ac radionuclides. In some embodiments, the radionuclide is 177 Lu. In some embodiments, the conjugate comprises two 177 Lu radionuclides. [0270] In some embodiments, the conjugate comprises an alpha particle-emitting radionuclide bound to the metal chelator.
- the alpha particle-emitting radionuclide is actinium-225, astatine-211, thorium-227, or radium-223. In some embodiments, the alpha particle-emitting radionuclide is actinium-225. [0271] In some embodiments, the conjugate comprises a beta particle-emitting radionuclide bound to the metal chelator. In some embodiments, the beta particle emitting radionuclide is zircronium-89, yttrium-90, samarium-153, lutetium-177, or lead-212. [0272] In some embodiments, the conjugate comprises a gamma particle emitting radionuclide.
- the gamma particle emitting radionuclide is indium-111.
- conjugates described herein do not contain any radionuclide, i.e., a cold conjugate.
- a radionuclide can be replaced with a surrogate (e.g., 225 Ac replaced with lanthanum) for testing and experimental purposes.
- Conjugates Comprising non-radioactive Drugs [0274] In one aspect, described herein is a conjugate that comprises a GCC binding peptide, a non-radioactive drug, and optionally a linker.
- the conjugate further comprises a metal chelator and optionally a radionuclide bound to the metal chelator.
- the non- radioactive drug can be a toxin.
- the toxin is selected from pseudomonas exotoxin (PE), deBouganin, Bouganin, diphtheria toxin (DT) and ricin.
- the non-radioactive drug can be a chemotherapy agent. [0275]
- the non-radioactive drug can be a cytotoxic drug.
- cytotoxic drugs include aplidin, azaribine, anastrozole, azacytidine, bleomycin, bortezomib, bryostatin-1, busulfan, calicheamycin, camptothecin, 10-hydroxycamptothecin, carmustine, celebrex, chlorambucil, cisplatin, irinotecan (CPT-11), SN-38, carboplatin, cladribine, cyclophosphamide, cytarabine, dacarbazine, docetaxel, dactinomycin, daunomycin glucuronide, daunorubicin, dexamethasone, diethylstilbestrol, doxorubicin, 2-pyrrolinodoxorubicin (2P-DOX), cyano-morpholino doxorubicin, doxorubicin glucuronide, epirubicin glucuronide, ethinyl estradiol
- the non-radioactive drug is selected from duocarmycin and its analogues, dolastatins, combretastatin, calicheamicin, N-acetyl- ⁇ -calicheamycin (CMC), a calicheamycin derivative, maytansine and analogues thereof, DM-I, auristatin E, auristatin EB (AEB), auristatin EFP (AEFP), monomethyl auristatin E (MMAE), monomethyl auristatin F (MMAF), tubulysin, disorazole, the epothilones, Paclitaxel, docetaxel, Topotecan, echinomycin, estramustine, cemadotine, eleutherobin, methopterin, actinomycin, daunorubicin, the daunorubicin conjugates, mitomycin C, mitomycin A, vincristine, retinoic acid, camptothecin
- the compounds described herein exist as geometric isomers. In some embodiments, the compounds described herein possess one or more double bonds. The compounds presented herein include cis, trans, syn, anti,
- Z isomers as well as the corresponding mixtures thereof. In some situations, the compounds described herein possess one or more chiral centers and each center exists in the R configuration or S configuration. The compounds described herein include diastereomeric, enantiomeric, and epimeric forms as well as the corresponding mixtures thereof.
- mixtures of enantiomers and/or diastereoisomers, resulting from a single preparative step, combination, or interconversion are useful for the applications described herein.
- the compounds described herein are prepared as their individual stereoisomers by reacting a racemic mixture of the compound with an optically active resolving agent to form a pair of diastereoisomeric compounds, separating the diastereomers, and recovering the optically pure enantiomers.
- dissociable complexes are preferred.
- the diastereomers have distinct physical properties (e.g., melting points, boiling points, solubilities, reactivity, etc.) and are separated by taking advantage of these dissimilarities. In some embodiments, the diastereomers are separated by chiral chromatography, or preferably, by separation/resolution techniques based upon differences in solubility. In some embodiments, the optically pure enantiomer is then recovered, along with the resolving agent.
- Tautomers [0278] A "tautomer" refers to a molecule wherein a proton shift from one atom of a molecule to another atom of the same molecule is possible. The compounds presented herein, in certain embodiments, exist as tautomers.
- tautomeric equilibrium In circumstances where tautomerization is possible, a chemical equilibrium of the tautomers will exist. The exact ratio of the tautomers depends on several factors, including physical state, temperature, solvent, and pH. Some examples of tautomeric equilibrium include: [0279] In some instances, the compounds disclosed herein exist in tautomeric forms. The structures of said compounds are illustrated in the one tautomeric form for clarity. The alternative tautomeric forms are expressly included in this disclosure. Labeled compounds [0280] In some embodiments, the compounds described herein exist in their isotopically- labeled forms. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such isotopically-labeled compounds.
- the methods disclosed herein include methods of treating diseases by administering such isotopically-labeled compounds as pharmaceutical compositions.
- the compounds disclosed herein include isotopically-labeled compounds, which are identical to those recited herein, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature.
- isotopes that can be incorporated into compounds described herein, or a solvate, or stereoisomer thereof, include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, sulfur, fluorine, and chloride, such as 2 H, 3 H, 13 C, 14 C, l5 N, 18 O, 17 O, 31 P, 32 P, 35 S, 18 F, and 36 Cl, respectively.
- Compounds described herein, and the pharmaceutically acceptable salts, solvates, or stereoisomers thereof which contain the aforementioned isotopes and/or other isotopes of other atoms are within the scope of this disclosure.
- isotopically-labeled compounds for example those into which radioactive isotopes such as 3 H and 14 C are incorporated, are useful in drug and/or substrate tissue distribution assays. Tritiated, i.e., 3 H and carbon-14, i.e., 14 C, isotopes are notable for their ease of preparation and detectability. Further, substitution with heavy isotopes such as deuterium, i.e., 2 H, produces certain therapeutic advantages resulting from greater metabolic stability, for example increased in vivo half-life or reduced dosage requirements.
- the isotopically labeled compound or a pharmaceutically acceptable salt, solvate, or stereoisomer thereof is prepared by any suitable method.
- the compounds described herein are labeled by other means, including, but not limited to, the use of chromophores or fluorescent moieties, bioluminescent labels, or chemiluminescent labels.
- Pharmaceutically acceptable salts [0282] In some embodiments, the compounds described herein exist as their pharmaceutically acceptable salts. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such pharmaceutically acceptable salts. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such pharmaceutically acceptable salts as pharmaceutical compositions. As used herein, a “pharmaceutically acceptable salt” refers to any salt of a compound that is be useful for therapeutic purposes of a subject.
- the compounds described herein possess acidic or basic groups and therefore react with any of a number of inorganic or organic bases, and inorganic and organic acids, to form a pharmaceutically acceptable salt.
- these salts are prepared in situ during the final isolation and purification of the compounds disclosed herein, or by separately reacting a purified compound in its free form with a suitable acid or base, and isolating the salt thus formed.
- Examples of pharmaceutically acceptable salts include those salts prepared by reaction of the compounds described herein with a mineral acid, organic acid, or inorganic base, such salts including acetate, acrylate, adipate, alginate, aspartate, benzoate, benzenesulfonate, bisulfate, bisulfite, bromide, butyrate, butyn-1,4-dioate, camphorate, camphorsulfonate, caproate, caprylate, chlorobenzoate, chloride, citrate, cyclopentanepropionate, decanoate, digluconate, dihydrogenphosphate, dinitrobenzoate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptanoate, glycerophosphate, glycolate, hemisulfate, heptanoate, hexanoate, hexyne-1,6-dioate,
- the compounds described herein can be prepared as pharmaceutically acceptable salts formed by reacting the free base form of the compound with a pharmaceutically acceptable inorganic or organic acid, including, but not limited to, inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, metaphosphoric acid, and the like; and organic acids such as acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, p-toluenesulfonic acid, tartaric acid, trifluoroacetic acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, arylsulfonic acid, methanesulfonic acid, ethanesulfonic acid, 1,
- those compounds described herein which comprise a free acid group react with a suitable base, such as the hydroxide, carbonate, bicarbonate, or sulfate of a pharmaceutically acceptable metal cation, with ammonia, or with a pharmaceutically acceptable organic primary, secondary, tertiary, or quaternary amine.
- a suitable base such as the hydroxide, carbonate, bicarbonate, or sulfate of a pharmaceutically acceptable metal cation, with ammonia, or with a pharmaceutically acceptable organic primary, secondary, tertiary, or quaternary amine.
- Representative salts include the alkali or alkaline earth salts, like lithium, sodium, potassium, calcium, and magnesium, and aluminum salts, and the like.
- bases include sodium hydroxide, potassium hydroxide, choline hydroxide, sodium carbonate, N + (C 1-4 alkyl)4, and the like.
- Representative organic amines useful for the formation of base addition salts include ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine, piperazine, and the like. It should be understood that the compounds described herein also include the quaternization of any basic nitrogen-containing groups they contain. In some embodiments, water or oil-soluble or dispersible products are obtained by such quaternization. Solvates [0288] In some embodiments, the compounds described herein exist as solvates. This disclosure provides for methods of treating diseases by administering such solvates. This disclosure further provides for methods of treating diseases by administering such solvates as pharmaceutical compositions.
- Solvates contain either stoichiometric or non-stoichiometric amounts of a solvent, and, in some embodiments, are formed during the process of crystallization with pharmaceutically acceptable solvents such as water, ethanol, and the like. Hydrates are formed when the solvent is water, or alcoholates are formed when the solvent is alcohol. Solvates of the compounds described herein can be conveniently prepared or formed during the processes described herein. In addition, the compounds provided herein can exist in unsolvated as well as solvated forms. In general, the solvated forms are considered equivalent to the unsolvated forms for the purposes of the compounds and methods provided herein.
- one aspect of the present disclosure pertains to hydrates and solvates of compounds of the present disclosure and/or their pharmaceutical acceptable salts, as described herein, that can be isolated and characterized by methods known in the art, such as, thermogravimetric analysis (TGA), TGA-mass spectroscopy, TGA-Infrared spectroscopy, powder X-ray diffraction (PXRD), Karl Fisher titration, high resolution X-ray diffraction, and the like.
- TGA thermogravimetric analysis
- TGA-mass spectroscopy TGA-Infrared spectroscopy
- PXRD powder X-ray diffraction
- Karl Fisher titration high resolution X-ray diffraction
- “Commercially available chemicals” are obtained from standard commercial sources including Acros Organics (Pittsburgh, PA), Aldrich Chemical (Milwaukee, WI, including Sigma Chemical and Fluka), Apin Chemicals Ltd. (Milton Park, UK), Avocado Research (Lancashire, U.K.), BDH, Inc. (Toronto, Canada), Bionet (Cornwall, U.K.), Chem Service Inc. (West Chester, PA), Crescent Chemical Co. (Hauppauge, NY), Eastman Organic Chemicals, Eastman Kodak Company (Rochester, NY), Fisher Scientific Co. (Pittsburgh, PA), Fisons Chemicals (Leicestershire, UK), Frontier Scientific (Logan, UT), ICN Biomedicals, Inc.
- Suitable reference books and treatises that detail the synthesis of reactants useful in the preparation of compounds described herein, or provide references to articles that describe the preparation include for example, “Synthetic Organic Chemistry”, John Wiley & Sons, Inc., New York; S. R. Sandler et al., “Organic Functional Group Preparations,” 2nd Ed., Academic Press, New York, 1983; H. O. House, “Modern Synthetic Reactions”, 2nd Ed., W. A. Benjamin, Inc. Menlo Park, Calif.1972; T. L. Gilchrist, “Heterocyclic Chemistry”, 2nd Ed., John Wiley & Sons, New York, 1992; J.
- compositions [0293]
- the radiopharmaceutical conjugate described herein including e.g., pharmaceutically acceptable salt or solvate thereof, can be administered per se as a pure chemical or as a component of a pharmaceutically acceptable formulation.
- a conjugate described herein is combined with a pharmaceutically suitable or acceptable carrier selected on the basis of a chosen route of administration and standard pharmaceutical practice as described, for example, in Remington: The Science and Practice of Pharmacy (Gennaro, 21 st Ed. Mack Pub. Co., Easton, PA (2005)).
- a pharmaceutical composition comprising at least one conjugate described herein, or a stereoisomer, pharmaceutically acceptable salt, amide, ester, solvate, or N-oxide thereof, together with one or more pharmaceutically acceptable carriers.
- the carrier(s) or excipient(s)
- the disclosure provides a pharmaceutical composition comprising a herein described conjugate, or a pharmaceutically acceptable salt or solvate thereof, and a pharmaceutically acceptable excipient or carrier.
- the conjugate as described is substantially pure, in that it contains less than about 10%, less than about 5%, or less than about 1%, or less than about 0.1%, of other organic small molecules, such as unreacted intermediates or synthesis by-products that are created, for example, in one or more of the steps of a synthesis method.
- Pharmaceutical compositions can include pharmaceutically acceptable carriers, diluents or excipients.
- Exemplary pharmaceutically acceptable carriers include solvents (aqueous or non- aqueous), solutions, emulsions, dispersion media, coatings, isotonic and absorption promoting or delaying agents, compatible with pharmaceutical administration.
- compositions can be contained in a liquid; emulsion, suspension, syrup or elixir, or solid form; tablet (coated or uncoated), capsule (hard or soft), powder, granule, crystal, or microbead. Supplementary components (e.g., preservatives, antibacterial, antiviral and antifungal agents) can also be incorporated into the compositions.
- Supplementary components e.g., preservatives, antibacterial, antiviral and antifungal agents
- Pharmaceutical compositions can be formulated to be compatible with a particular local or systemic route of administration.
- pharmaceutical compositions include carriers, diluents, or excipients suitable for administration by particular routes.
- compositions of the current disclosure can be administered by any suitable means, including oral, topical (including buccal and sublingual), rectal, vaginal, transdermal, parenteral, subcutaneous, intraperitoneal, intrapulmonary, intradermal, intrathecal and epidural and intranasal, and, if desired for local treatment, intralesional administration.
- parenteral as used herein includes e.g., subcutaneous, intravenous, intramuscular, intrasternal, intraperitoneal, and infusion techniques.
- parenteral also includes injections, into the eye or ocular, intravitreal, intrabuccal, transdermal, intranasal, into the brain, including intracranial and intradural, into the joints, including ankles, knees, hips, shoulders, elbows, wrists, and the like, and in suppository form.
- the compounds and/or formulations are administered orally.
- the compounds and/or formulations are administered by systemic administration.
- the compounds and/or formulations are administered parenterally.
- the compounds and/or formulations are administered locally at a targeted site.
- conjugates, or pharmaceutically acceptable salts or solvates thereof, and pharmaceutical compositions described herein are administered via parenteral injection as liquid solution, which can include other chemical components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, preservatives, or excipients.
- Parenteral injections can be formulated for bolus injection or continuous infusion.
- the pharmaceutical compositions can be in a form suitable for parenteral injection as a sterile suspension, solution or emulsion in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing or dispersing agents.
- Pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water soluble form.
- Solutions or suspensions used for parenteral, intradermal, or subcutaneous application can include: a sterile diluent such as water for injection, saline solution, fixed oils, polyethylene glycols, glycerin, propylene glycol or other synthetic solvents; antibacterial agents such as benzyl alcohol or methyl parabens; antioxidants such as ascorbic acid, gentisic acid, or sodium bisulfite; surfactants such as polysorbate 80; chelating agents such as ethylenediaminetetraacetic acid; buffers such as acetates, citrates or phosphates and agents for the adjustment of tonicity such as sodium chloride or dextrose.
- a sterile diluent such as water for injection, saline solution, fixed oils, polyethylene glycols, glycerin, propylene glycol or other synthetic solvents
- antibacterial agents such as benzyl alcohol or methyl parabens
- antioxidants such as ascorbic acid,
- pH can be adjusted with acids or bases, such as hydrochloric acid or sodium hydroxide.
- the pharmaceutical composition comprises a reductant.
- the presence of a reductant can help minimize potential radiolysis.
- the reductant is ascorbic acid, gentisic acid, sodium thiosulfate, citric acid, tartaric acid, or a combination thereof.
- Pharmaceutical compositions comprising the conjugates or pharmaceutically acceptable salts or solvates thereof described herein can be prepared according to standard techniques and further comprise a pharmaceutically acceptable carrier. In some embodiments, normal saline can be employed as the pharmaceutically acceptable carrier.
- Suitable carriers include, e.g., water, buffered water, 0.9% isotonic saline, 0.4% saline, 0.3% glycine, and the like, including glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin, etc.
- These compositions can be sterilized by conventional sterilization techniques.
- the resulting aqueous solutions may be packaged for use or filtered under aseptic conditions and lyophilized.
- the lyophilized preparation is combined with a sterile aqueous solution prior to administration.
- compositions can contain pharmaceutically acceptable auxiliary substances as appropriate to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sodium lactate, sorbitan monolaurate, triethanolamine oleate, etc.
- Pharmaceutical compositions can be selected according to their physical characteristic, including, but not limited to fluid volumes, viscosities and other parameters in accordance with the particular mode of administration selected.
- the amount of conjugates administered can depend upon the particular targeting moiety used, the disease state being treated, the therapeutic agent being delivered, and the judgment of the clinician. [0299]
- concentration of the conjugates or pharmaceutically acceptable salts or solvates thereof described herein in the pharmaceutical formulations can vary.
- the conjugate is present in the pharmaceutical composition from about 0.05% to about 1% by weight, about 1% to about 2% by weight, about 2% to about 5% by weight, about 5% to about 10% by weight, about 10% to about 30% by weight, about 30% to about 50% by weight, about 50% to about 75% by weight, or about 75% to about 99% by weight.
- Pharmaceutical compositions are administered in a manner appropriate to the disease to be treated. An appropriate dose and a suitable duration and frequency of administration will be determined by such factors as the condition of the subject, the type and severity of the subject's disease, the particular form of the active ingredient, and the method of administration.
- an appropriate dose and treatment regimen provides the composition(s) in an amount sufficient to provide therapeutic and/or prophylactic benefit (e.g., an improved clinical outcome), or a lessening of symptom severity.
- Optimal doses are generally determined using experimental models and/or clinical trials. The optimal dose depends upon the body mass, weight, or blood volume of the subject.
- the amount of conjugates or pharmaceutically acceptable salts or solvates thereof and/or pharmaceutical compositions administered can be sufficient to deliver a therapeutically effective dose of the particular subject.
- conjugate dosages can be between about 0.1 pg and about 50 mg per kilogram of body weight, 1 ⁇ g and about 50 mg per kilogram of body weight, or between about 0.1 and about 10 mg/kg of body weight.
- Therapeutically effective dosages can also be determined at the discretion of a physician.
- the dose of the conjugate or a pharmaceutically acceptable salt or solvate thereof described herein for methods of treating a disease as described herein is about 0.001 mg/kg to about 1 mg/kg body weight of the subject per dose.
- the dose of conjugate or a pharmaceutically acceptable salt or solvate thereof described herein for the described methods is about 0.001 mg to about 1000 mg per dose for the subject being treated.
- a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of from about 0.01 mg to about 500 mg, from about 0.01 mg to about 100 mg, or from about 0.01mg to about 50 mg.
- a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of about 0.01 picomole to about 1 mole, about 0.1 picomole to about 0.1 mole, about 1 nanomole to about 0.1 mole, or about 0.01 micromole to about 0.1 millimole.
- a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of about 0.01 Gbq to about 1000 Gbq, about 0.5 Gbq to about 100 Gbq, or about 1 Gbq to about 50 Gbq.
- the dose is administered once a day, 1 to 3 times a week, 1 to 4 times a month, or 1 to 12 times a year.
- the pharmaceutical formulations can be packaged in unit dosage form for ease of administration and uniformity of dosage.
- a unit dosage form can refer to physically discrete units suited as unitary dosages for the subject to be treated; each unit containing a predetermined quantity of active compound calculated to produce the desired therapeutic effect in association with the pharmaceutical carrier or excipient.
- IV. Method of Treatment [0303]
- the disclosure provides methods of treating a disease or condition in a subject in need thereof.
- the methods comprise administering a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein, or a pharmaceutical composition comprising the same to the subject in need thereof.
- provided herein is a method of providing a therapeutic and/or prophylactic benefit to a subject in need thereof comprising administering a compound or pharmaceutical composition described herein.
- the methods comprise administering to a subject a therapeutically effective amount of a conjugate or a pharmaceutically acceptable salt or solvate thereof.
- the conjugate or pharmaceutically acceptable salt or solvate thereof is administered in a pharmaceutical composition.
- the subject has cancer.
- the cancer is a solid tumor or hematological cancer.
- the treatment is sufficient to reduce or inhibit the growth of the subject's tumor, reduce the number or size of metastatic lesions, reduce tumor load, reduce primary tumor load, reduce invasiveness, prolong survival time, or maintain or improve the quality of life.
- methods for killing a cell comprising contacting the cell with a conjugate or a pharmaceutically acceptable salt or solvate thereof.
- the cell expresses a guanylate cyclase C (GCC) receptor.
- GCC guanylate cyclase C
- the conjugate or pharmaceutically acceptable salt or solvate thereof binds to a structure on the cell, e.g., GCC.
- the conjugate or pharmaceutically acceptable salt or solvate thereof releases a number of alpha particles by natural radioactive decay. In some embodiments, the conjugate or pharmaceutically acceptable salt or solvate thereof releases a number of beta particles, gamma rays, and/or Auger electrons by natural radioactive decay.
- the conjugate described herein can kill a cell by radiation. In some embodiments, the conjugate kills the cell directly by radiation. In some embodiments, the radiation creates, in the cell, oxidized bases, abasic sites, single-stranded breaks, double- stranded breaks, DNA crosslink, chromosomal rearrangement, or a combination thereof. In some embodiments, the conjugate kills the cell by inducing double-stranded DNA breaks.
- the released alpha particles are sufficient to kill the cell. In some embodiments, the released alpha particles are sufficient to stop cell growth. In some embodiments, the conjugate kills the cell indirectly via the production of reactive oxygen species (ROS) such as free hydroxyl radicals. In some embodiments, the conjugate kills the cell indirectly by releasing tumor antigens from one or more different cells, which can have vaccine effect. In some embodiments, the conjugate kills the cell by abscopal effect. In some embodiments, the cell is a cancer cell. In some embodiments, the method comprises killing a cell with an alpha-particle emitting radionuclide. [0307] After contacting a cell, the described conjugate can be internalized by the cell.
- ROS reactive oxygen species
- the disclosed conjugate or a pharmaceutically acceptable salt or solvate thereof is configured to treat cancer by ablating tumor cells.
- the conjugate or a pharmaceutically acceptable salt or solvate thereof does not modulate the biology of the tumor cell and/or the surrounding stroma.
- the conjugate or a pharmaceutically acceptable salt or solvate thereof does not modulate immune cells.
- the ablating of tumor cells can lead to a downstream immunological cascade.
- Cancer includes tissue and organ carcinogenesis including metastases such as for example gastrointestinal cancer, (e.g., gastric cancer, esophageal cancer, pancreatic cancer colorectal cancer, intestinal cancer, anal cancer, liver cancer, gallbladder cancer, or colon cancer; lung cancer; thyroid cancer; skin cancer (e.g., melanoma); oral cancer; urinary tract cancer (e.g. bladder cancer or kidney cancer); blood cancer (e.g. myeloma or leukemia) or prostate cancer.
- gastrointestinal cancer e.g., gastric cancer, esophageal cancer, pancreatic cancer colorectal cancer, intestinal cancer, anal cancer, liver cancer, gallbladder cancer, or colon cancer
- lung cancer thyroid cancer
- skin cancer e.g., melanoma
- oral cancer e.g., urinary tract cancer (e.g. bladder cancer or kidney cancer); blood cancer (e.g. myeloma or leukemia) or prostate cancer.
- urinary tract cancer
- the present disclosure provides methods and compositions for treating gastrointestinal cancer in a subject in need thereof by administering an effective amount of a GCC binding peptide conjugate to the subject.
- gastrointestinal cancers that can be treated according to the methods of the present disclosure include gastric cancer, esophageal cancer, pancreatic cancer, lung cancer (small cell lung cancer and/or non small-cell lung cancer), colorectal cancer, intestinal cancer, anal cancer, liver cancer, gallbladder cancer, or colon cancer.
- the GCC-expressing cancer to be treated is a primary or metastatic cancer of gastrointestinal origin, such as colorectal cancer, stomach cancer, small intestine cancer, or esophageal cancer.
- the GCC-expressing cancer to be treated is primary or metastatic pancreatic cancer.
- the GCC-expressing cancer to be treated is primary or metastatic lung cancer, such as squamous cell carcinoma, adenosquamous carcinoma, or adenocarcinoma.
- the GCC-expressing cancer to be treated is a sarcoma, such as leiomyosarcoma or rhabdomyosarcoma.
- the GCC-expressing cancer to be treated is a primary or metastasized neuroectodermal tumor, such as aphaechromotcytoma or a paraganglioma.
- the GCC-expressing cancer is a primary or a metastasized bronchopulmonary or a gastrointestinal neuroendocrine tumor.
- the cancer is colorectal cancer.
- the method can be useful in treating a relevant disorder at any stage or subclassification. For example, method can be used to treat early or late stage colon cancer, or colon cancer of any of stages 0, I, IIA, IIB, IIIA, IIIB, IIIC, and IV.
- the compounds and compositions described herein can be used to image, and/or as part of a treatment for, any disease in which GCC is expressed, and in which a doctor wants to deliver a therapeutic to the GCC expressing tissue.
- a disease in which GCC is expressed, and in which a doctor wants to deliver a therapeutic to the GCC expressing tissue.
- An example of this type of disease is cancer (e.g., a cancer described above).
- GCC is expressed, for example, in colorectal cancer, gastric cancer, pancreatic cancer, esophageal cancer, cancer of the gastroesophageal junction, small intestine cancer, lung cancer, soft tissue sarcomas such as leiomyosarcomas and rhabdomyosarcomas, gastrointestinal and bronchopulmonary neuroendocrine tumors, and neuroectodermal tumors, and metastases derived from GCC- expressing primary tumors.
- An example of the last category is a liver metastasis derived from a primary colorectal tumor.
- Conjugates for imaging applications can comprise a radionuclide suitable for use as imaging isotopes such as the isotopes in Table 5B.
- SPECT single-photon emission computed tomography
- PET positron emission tomography
- the compounds can identify patients likely to respond to a GCC-targeted therapy, e.g., an anti-GCC antibody, e.g., an immunoconjugate comprising an anti-GCC antibody.
- the conjugate can be administered as a companion diagnostic for GCC-targeted therapy thereby informing whether a patient suffering from a proliferative disease such as cancer, or a gastrointestinal disorder such as inflammatory bowel syndrome, Crohn's Disease or constipation, or should be treated or not with a GCC-targeted therapy, based on the presence or absence, respectively, of GCC expression on the surface of or within the patient's cells or tissue.
- a patient having one more cells that express GCC on the cell surface or within the cell can be candidate for treatment with a GCC-targeted therapy.
- described herein is a method of treatments that comprises administering a first conjugate and a second conjugate.
- the first conjugate can be used as companion diagnostics and the second conjugate can be used for therapeutics.
- the first conjugate and the second conjugate have the same structure except for the radionuclide.
- the first conjugate comprises a gamma particle emitting radionuclide.
- the first conjugate comprises a radionuclide of Table 5B.
- the first conjugate comprises a radionuclide selected from Lu-177, In-111, Ga-68, Cu-64, and Zr- 89.
- the second conjugate comprises an alpha or beta-particle emitting radionuclide.
- the second conjugate comprises a radionuclide of Table 5A.
- the second conjugate comprises Ac-225.
- Combination Therapy [0315]
- a GCC binding peptide conjugate described herein can be administered alone or in combination with one or more additional therapeutic agents.
- the conjugate and a second therapeutic agent are administered in combination to prevent or treat inflammation, cancer and other disorders, particularly of the gastrointestinal tract.
- the combination therapy can include a composition comprising a conjugate described herein co-formulated with, and/or co-administered with, one or more additional therapeutic agents, e.g., one or more anti-cancer agents, e.g., cytotoxic or cytostatic agents, immune checkpoint inhibitors, hormone treatment, vaccines, and/or other immunotherapies.
- the conjugate is administered in combination with other therapeutic treatment modalities, including surgery, radiation, cryosurgery, and/or chemotherapy.
- combination therapies may advantageously utilize lower dosages of the administered therapeutic agents, thus avoiding possible toxicities or complications associated with the various monotherapies.
- two (or more) different treatments can be delivered to the subject during the course of the subject's affliction with the disorder, e.g., the two or more treatments are delivered after the subject has been diagnosed with the disorder and before the disorder has been cured or eliminated.
- the delivery of one treatment is still occurring when the delivery of the second begins, so that there is overlap.
- the delivery of one treatment ends before the delivery of the other treatment begins.
- the treatment is more effective because of combined administration.
- the second treatment is more effective, e.g., an equivalent effect is seen with less of the second treatment, or the second treatment reduces symptoms to a greater extent, than would be seen if the second treatment were administered in the absence of the first treatment, or the analogous situation is seen with the first treatment.
- delivery is such that the reduction in a symptom, or other parameter related to the disorder is greater than what would be observed with one treatment delivered in the absence of the other.
- the effect of the two treatments can be partially additive, wholly additive, or greater than additive.
- the delivery can be such that an effect of the first treatment delivered is still detectable when the second is delivered.
- the herein-described conjugate is used in combination with a chemotherapeutic agent, e.g., a DNA damaging chemotherapeutic agent.
- Non-limiting examples of DNA damaging chemotherapeutic agents include topoisomerase I inhibitors (e.g., irinotecan, topotecan, camptothecin and analogs or metabolites thereof, and doxorubicin); topoisomerase II inhibitors (e.g., etoposide, teniposide, and daunorubicin); alkylating agents (e.g., melphalan, chlorambucil, busulfan, thiotepa, ifosfamide, carmustine, lomustine, semustine, streptozocin, decarbazine, methotrexate, mitomycin C, and cyclophosphamide); DNA intercalators (e.g., cisplatin, oxaliplatin, and carboplatin); DNA intercalators and free radical generators such as bleomycin; and nucleoside mimetics (e.g., 5-fluorouracil, capecitibine
- the herein-described conjugate is used in combination with a radiation sensitizer, which makes tumor cells more sensitive to radiation therapy. In some embodiments, the herein-described conjugate is used in combination with a DNA damage repair inhibitor (or DNA damage response (DDR) inhibitor). [0319] In some embodiments, the method comprises administering a second agent that binds to a GCC receptor. In some embodiments, the method comprises administering a second agent that is a GCC receptor agonist. In some embodiments, the method comprises administering a second agent that is a GCC receptor antagonist. In some embodiments, a GCC binding peptide conjugate described herein is administered in combination with one or more of GCC binding peptides such as a GCC receptor agonist.
- the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-19.
- the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides consists of an amino acid sequence of any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. [0320] In some embodiments, a GCC binding peptide is co-administered in conjunction with the radiopharmaceutical.
- a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. [0321] In some embodiments, a GCC binding peptide conjugate described herein is administered in combination with one or more of GCC binding peptides.
- a GCC binding peptide conjugate described herein is administered with one or more of linaclotide, plecanatide and dolcantide. In some embodiments, the conjugate is administered in combination with linaclotide. [0322] In some embodiment, the GCC binding peptide conjugate is administered in combination with one or more additional therapeutic agents selected from the group consisting of phosphodiesterase inhibitors, cyclic nucleotides (such as cGMP and cAMP), a laxative (such as SENNA, METAMUCIL, MIRALAX, PEG, or calcium polycarbophil), a stool softener, an anti- tumor necrosis factor alpha therapy for IBD (such as REMICADE, ENBREL, or HUMAIRA), anti-inflammatory drugs (such as COX-2 inhibitors, sulfasalazine, 5-ASA derivatives and NSAIDS), anti-GCC antibody molecules, and GCC receptor agonists.
- additional therapeutic agents selected from the group consisting of phosphodieste
- the GCC binding peptide conjugate is administered in combination with an effective dose of an inhibitor of cGMP-specific phosphodiesterase (cGMP-PDE).
- cGMP-PDE inhibitors include, for example, suldinac sulfone, zaprinast, motapizone, vardenifil, and sildenafil.
- the herein-described conjugate can be administered simultaneously or sequentially with the second agent, and they can be administered via the same or different routes.
- the conjugate is administered in combination with a treatment that increases urinary excretion.
- the agent which increases urinary excretion is administered prior to, concurrently with and/or after administration with the radiolabeled peptide.
- the agent which ameliorates bladder toxicity associated with therapy is saline, e.g., intravenous saline, D5 half normal saline or D5 water.
- the subject is 4 to 100 years old.
- the subject is 5 to 10, 5 to 15, 5 to 18, 5 to 25, 5 to 35, 5 to 45, 5 to 55, 5 to 65, 5 to 75, 10 to 15, 10 to 18, 10 to 25, 10 to 35, 10 to 45, 10 to 55, 10 to 65, 10 to 75, 15 to 18, 15 to 25, 15 to 35, 15 to 45, 15 to 55, 15 to 65, 15 to 75, 18 to 25, 18 to 35, 18 to 45, 18 to 55, 18 to 65, 18 to 75, 25 to 35, 25 to 45, 25 to 55, 25 to 65, 25 to 75, 35 to 45, 35 to 55, 35 to 65, 35 to 75, 45 to 55, 45 to 65, 45 to 75, 55 to 65, 55 to 75, or 65 to 75 years old.
- the subject is at least 5, 10, 15, 18, 25, 35, 45, 55, or 65 years old. In some embodiments, the subject is at most 10, 15, 18, 25, 35, 45, 55, 65, or 75 years old.
- SPPS Solid phase peptide synthesis
- 2-CTC resin was purchased from Sunresin New Materials Co. (China). Fmoc protected amino acids were purchased from GL Biochem (China). HBTU and HATU were purchased from Highfine Biotech Co. (China). Piperidine was purchased from Damao Chemical Reagent Factory (China). The peptides and their derivatives were purified on a Gilson GX-281 preparative HPLC system using reverse-phase C18 columns (Gemini, 5 ⁇ m, 110 ⁇ + luna, 10 ⁇ m, 100 ⁇ ) at 30 °C. HPLC solvents consisted of H 2 O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B).
- HPLC high performance liquid chromatography
- Example A-1 Synthesis of 4-(naphthalene-2-sulfonamido)-4-oxobutanoic acid (2) [0331] To a solution of sulfonamide 1 (10.0 g, 48.3 mmol) in THF (20.0 mL) under N 2 atmosphere were added DMAP (7.07 g, 57.9 mmol), TEA (5.86 g, 57.9 mmol) and succinic anhydride (5.79 g, 57.9 mmol). The reaction mixture was stirred for 18 h at room temperature and then refluxed for 6 h.
- Example A2 Synthesis of C-01 and La-C-01.
- Example discloses SEQ ID NOS 46-48, respectively, in order of appearance.
- Fmoc-Ahx-OH (2.00 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF.
- the solution was mixed with the resin-bound peptide and then agitated for 30 min under nitrogen.
- the resin was then washed three times with DMF. Fmoc removal was carried out while the peptide was on resin using 20% piperidine in DMF followed by filtration and washing.
- Example A3 Synthesis of C-05 and La-C-05.
- Example discloses SEQ ID NOS 46, and 49-51, respectively, in order of appearance.
- Oxidative folding of DOTA-linker-peptide 17 To a solution of crude 17 (0.95 g) in water (330 mL) and MeCN (100 mL) was added GSSG (666 mg) and GSH (666 mg). The pH of the solution was adjusted to 8.0 using saturated NH 4 HCO 3 solution. The resulting mixture was stirred at room temperature for 12 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford compound ⁇ -05 (63.4 mg, 3.6% yield, 67.0% purity) as a white solid.
- La 3+ complexation To a solution of ⁇ -05 (45.1 mg, 67.0% purity, 13.0 ⁇ mol) in H 2 O (2.00 mL) and MeCN (1.00 mL) was added LaCl3 (3.82 mg, 15.6 ⁇ mol) and Na 2 CO 3 (1.37 mg, 13.0 ⁇ mol). The resulting mixture was stirred at 70 °C for 1 h. After filtration, the crude product was purified by preparative HPLC to afford La- ⁇ -05 (12.7 mg, 37.7% yield, 95.1% purity) as a white solid. [0353] Conjugates in Table 1 and their corresponding La 3+ complexed conjugates of Table 6 were synthesized similarly according to the methods described above.
- Example A4 Synthesis of conjugate of Table 1 [0355] Conjugates of Table 1 and corresponding conjugates containing chelated lutetium or lanthanum were synthesized according to the same methods described in Examples A1-A3. See Table 6 and Table 7 below. Table 6. Exemplary La 3+ -labelled conjugates
- HPLC solvents consisted of H 2 O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B).
- HPLC high performance liquid chromatography
- HPLC solvents consisted of H 2 O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B).
- a Waters Xbridge C-18 (3.5 ⁇ m, 3.1 ⁇ 30mm) column was used with a flow rate of 1.2 mL/min.
- the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing.
- 18-(tert-butoxy)-18-oxooctadecanoic acid (2.00 equiv), HBTU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF.
- the solution was mixed with the resin-bound peptide and then agitated for 30 min under nitrogen.
- the resin was then washed three times with DMF.
- Dde removal was carried out while the peptide was on resin using 3% hydrazine hydrate in DMF followed by filtration and washing.
- La 3+ complexation To a solution of La-C-25 (54 mg, 20.0 ⁇ mol) in H 2 O (2.50 mL) and MeCN (0.5 mL) was added LaCl3 (4.43 mg, 18.0 ⁇ mol) and Na 2 CO 3 (0.19 mg, 1.8 ⁇ mol). The resulting mixture was stirred at 70 °C for 1 h. After filtration, the crude product was purified by preparative HPLC to afford La-C-25 (10.9 mg, 95.6% purity) as a white solid. [0369]
- Example A6 Synthesis of conjugate of C-35 and Lu-C-35
- SPPS Solid phase peptide synthesis
- 2-CTC resin was purchased from Sunresin New Materials Co. (China). Fmoc protected amino acids were purchased from GL Biochem (China). HBTU and HATU were purchased from Highfine Biotech Co. (China). Piperidine was purchased from Damao Chemical Reagent Factory (China). The peptides and their derivatives were purified on a Gilson GX-281 preparative HPLC system using reverse-phase C18 columns (Gemini, 5 ⁇ m, 110 ⁇ + luna, 10 ⁇ m, 100 ⁇ ) at 30 °C. HPLC solvents consisted of H 2 O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B).
- HPLC high performance liquid chromatography
- HPLC solvents consisted of H 2 O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B).
- a Waters Xbridge C-18 (3.5 ⁇ m, 3.1 ⁇ 30mm) column was used with a flow rate of 1.2 mL/min.
- the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing.
- a solution of Fmoc-Tyr(tBu)-OH (0.69 g, 1.50 mmol), HBTU (542mg, 1.43mmol) and DIEA (0.35 mL, 2.00 mmol) in DMF (5 mL) was added to the resin and agitated with N 2 for 30 min at 20°C. The resin was then washed with DMF (15 mL * 3).
- the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing.
- Oxidative folding of DOTA-linker-peptide 3 To a solution of crude 3 (1.2 g) in water (400 mL) and MeCN (200 mL) was added GSSG (845 mg, 3eq) and GSH (845 mg, 6eq). The pH of the solution was adjusted to 8.0 using saturated NH4HCO3 solution. The resulting mixture was stirred at room temperature for 24 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford compound C-35 (2.1 mg, 95.06% purity and 67mg 70% purity) as a white solid.
- Example A8 Synthesis of 225-Actinium chelated conjugates
- Conjugates of Table 1 and Table 2 are synthesized according to the same methods described in Examples A1-A6.
- General procedure for 225 Ac-labeling [ 225 Ac]Ac(NO 3 ) 3 in 1 mM HCl (50 kBq) is added to a mixture of a DOTA-GCC construct (1 nmol) in NaOAc buffer (100 ⁇ L, 0.4 M, pH 5.5-6.5) in a 1.8 mL Eppendorf tube.
- conjugates of Table 9 can be synthesized according to this Example A8. Table 9. Exemplary 225-Actinium labelled conjugates
- Example B Biological Evaluation [0389]
- mice In vivo pharmacokinetic studies in female CD-1(ICR) mice [0394] The pharmacokinetics of linaclotide and the La 3+ -labelled peptide surrogates were determined using female CD-1 (ICR) mice purchased from Vital River Laboratory Animal Co., Ltd., Beijing, China. All animal studies were conducted in accordance with the highest standards of care as outlined in the NIH Guide for Care and Use of Laboratory. Following injection of the mice (10 mg/kg, 3 mice per test compound) with aliquots of the peptides in PBS (10 mM, pH 7.4) via the tail vein, blood samples were collected into pre-chilled tubes containing Heparin-Na (3 ⁇ L, 1000 I.U./mL) at 5, 30, 60, and 240 minutes.
- PBS 10 mM, pH 7.4
- Mobile phase A water/acetonitrile (95/5, v/v) with 0.1% formic acid and 2 mM ammonium formate
- Mobile phase B acetonitrile/water (95/5, v/v) with 0.1% formic acid and 2 mM ammonium formate.
- Mobile phase A water with 0.1% formic acid
- Mobile phase B acetonitrile with 0.1% formic acid.
- Column temperature 60 °C.
- each well was added T84 cell membrane protein 20 ⁇ g in 100 ⁇ L assay buffer (DMEM/20 mM HEPES, pH 7.4, 0.5% BSA), Reference-043-[ 125 I] (100 pM, 50 ⁇ L) and an unlabeled GCC construct (50 ⁇ L) at varying concentrations (0.3 pM – 5 uM).
- the plate was sealed and incubated at room temperature for 1 h with gentle agitation on a shaker set to 300 rpm.
- the incubation is stopped by filtration onto a Unifilter-96 GF/C filter plate (presoaked with 50 ⁇ L of 0.3% polyethyleneimine per well for at least 0.5 h at room temperature) using a Perkin Elmer Filtermate Harvester.
- the plate was washed with cold Dulbecco-PBS washing buffer (200 ⁇ L x 5 times) then dried at 50 ⁇ C for 1 h.
- the bottom of the filter plate was sealed using Perkin Elmer Unifilter-96 backing seal tape and the Perkin Elmer Microscint 20 cocktail (50 ⁇ L) was added to the wells.
- the top of the filter plate was sealed with Perkin Elmer TopSeal- A sealing film and the radioactivity was counted with a Perkin Elmer MicroBeta2 Reader.
- IC 50 values of compounds of the present disclosure are given in Table 11 below, where IC 5 00nM ⁇ A ⁇ 30nM; 30nM ⁇ B ⁇ 100nM; 100nM ⁇ C ⁇ 1000nM; and 1000nM ⁇ D ⁇ 6000nM. Table 11. IC 50 values of exemplary compounds of the present disclosure [0403] Example B4.
- HSA-HPLC Human Serum Albumin
- the logarithmic value of the gradient retention times from the experiment were plotted against the linearized values of the percent bound to plasma.
- Aqueous mobile phase (mobile phase A) was 50 mM ammonium acetate solution, pH 7.4 and the organic mobile phase (mobile phase B) was 2-propanol.
- the flow rate was set at 0.350mL/min and injection volume was 5uL, with samples prepared at 0.5mg/mL concentration in 50:50 mobile phase.
- the initial LC conditions were set at 0% B and ramped to 50% B over 8.5 minutes, then held at 50% B for 1.5 minutes before going back to initial conditions and re-equilibrating the column for 2.5 minutes. Chromatograms were recorded at 280 nm by a diode array UV absorption detector .
- the percent binding values of Table 12 are defined as the following: 90% bound to HSA ⁇ A ⁇ 100% bound to HSA; 70% bound to HSA ⁇ B ⁇ 90% bound to HSA; 20% bound to HSA ⁇ C ⁇ 70% bound to HSA; and 0% bound to HSA ⁇ D ⁇ 20% bound to HSA. Table 12. Percent HSA binding of compounds of the present disclosure
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
Abstract
Provided herein are radiopharmaceutical conjugate compositions and uses thereof. In one aspect, provided herein are conjugates that comprise a GCC binding peptide, a metal chelator configured to bind with a radionuclide, a linker that attaches the GCC binding peptide with the metal chelator, and optionally a radionuclide. Also provided herein are conjugates that have improved elimination profile and plasma stability that are suitable for radiopharmaceutical applications and methods of using the same in treating and/or diagnosing diseases such as cancer.
Description
RADIOPHARMACEUTICAL CONJUGATES TARGETING GUANYLYL CYCLASE C, AND COMPOSITIONS AND USES THEREOF CROSS-REFFERENCE [0001] This application claims the benefit of U.S. Provisional Application No.63/119,557, filed on November 30, 2020, which is incorporated herein by reference in its entirety. SEQUENCE LISTING [0002] The instant application contains a Sequence Listing which has been submitted electronically in ASCII format and is hereby incorporated by reference in its entirety. Said ASCII copy, created on November 24, 2021, is named 59541-708_601_SL.txt and is 27,279 bytes in size. BACKGROUND [0003] Cancers of the gastrointestinal tract such as colorectal cancer remain one of the leading cause of death in the United States. Despite process in early detection and treatment, a portion of the diagnosed patients still suffer late stage disease. Although many promising agents for metastatic disease have become clinically available (e.g., tyrosine kinase inhibitors, anti- angiogenesis agents, etc.), the five-year survival rate for this population remains low. Therefore, a need exists in the medicinal arts for compounds, formulations, and methods for treating these cancers. SUMMARY [0004] Provided herein are radiopharmaceutical conjugates and pharmaceutical compositions comprising said conjugates. In some embodiments, the subject radiopharmaceutical conjugates are useful for the treatment of diseases, e.g., cancer. In one aspect, the present disclosure provides conjugates having improved elimination profile and metabolic, chemical and/or physical stabilities that are suitable for radiopharmaceutical applications while maintaining the protein binding affinity. It is surprisingly discovered that, for some conjugates, the target binding affinity, plasma stability and serum half-live can be adjusted by modifying the hydrophobicity and length of the linker connecting a guanylyl cyclase C (GCC) binding peptide with a metal chelator. Accordingly, in one aspect, disclosed herein are GCC binding conjugates with optimized linkers and configurations. [0005] In one aspect, the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; a metal chelator configured to bind
with a radionuclide, wherein the metal chelator is selected from FIGs.3-17; one or more linkers; and optionally a radionuclide, e.g., a radionuclide of Table 5A or Table 5B. [0006] In one aspect, the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42, a metal chelator configured to bind with a radionuclide; a linker that covalently attaches the GCC binding peptide with the metal chelator; and an alpha particle-emitting radionuclide. [0007] In one aspect, the present disclosure provides a conjugate comprising a guanylyl cyclase C (GCC) binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein a dissociation constant (Kd) between the linker and human serum albumin is at most 100 µM, as determined at room temperature in human serum condition. [0008] In one aspect, the present disclosure provides a conjugate comprising (i) a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; (ii) a metal chelator configured to bind with a radionuclide; (iii) a linker that covalently attaches the GCC binding peptide with the metal chelator; and (iv) an alpha-particle emitting radionuclide bound to the metal chelator. In one aspect, the present disclosure provides a conjugate comprising (i) a guanylyl cyclase C (GCC) binding peptide; (ii) a metal chelator configured to bind with a radionuclide; and (iii) a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein 5% to 99% of the conjugate binds to Human Serum Albumin (HSA) in vitro as determined by HSA-HPLC method. In some embodiments, the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 1. In some embodiments, the binding peptide further comprises 1, 2 or 3 intramolecular disulfide bonds. In some embodiments, the binding peptide further comprises one or more of alkylene, alkenylene,
heteroalkylene, and heteroaryl. In some embodiments, the binding peptide is selected from:
.
In some embodiments, the binding peptide further comprises one intramolecular heteroalkylene, alkylene or alkenylene bond. In some embodiments, the heteroalkylene, alkylene or alkenylene is optionally substituted with one or more R10, wherein each R10 is independently halogen, amino, -OH, -SH, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R10 on the same atoms are taken together to form a cycloalkyl or heterocycloalkyl; or two R10 on different atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl. In some embodiments, the binding peptide comprises an unnatural amino acid at the N-terminus, the C-terminus, or both. In some embodiments, the binding peptide comprises a modified tyrosine at the C-terminus. In some embodiments, the modified tyrosine is a D-tyrosine, N-methyl-L-tyrosine, α-methyl-L-tyrosine, or L-tyrosinamide. In some embodiments, the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB- Cyclam, CB-TE2A, DOTA-4AMP, and NOTP. In some embodiments, the metal chelator is DOTA. In some embodiments, the linker is a bond. [0009] In some embodiments, the linker has a structure of Formula (II-1)
Formula (II-1) wherein each L is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)- , -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, - (C1-C30 alkylene)-O-, -O-( C1-C30 alkylene)-, -(C1-C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2-(C1-C30 alkylene)-; and each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; and n is 1 to 20.
[0010] In some embodiments, the linker comprises a structure of Formula (II-1a),
Formula (II-1a) wherein each of L1 and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; and L2 is absent, substituted or unsubstituted C1-C30 alkylene, or substituted or unsubstituted C1- C30 heteroalkylene. [0011] In some embodiments, the linker has a structure of Formula (II-2)
Formula (II-2), wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)- , substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2- C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, -(C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1- C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2- (C1-C30 alkylene)-; and R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted
or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RX is independently hydrogen, halo, -CN, -NO2, -OH, -SH, substituted or unsubstituted C1- C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2- C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; and q is 0, 1, 2, 3, 4, 5, and 6. [0012] In some embodiments, the linker has a structure of Formula (II-2a) or Formula (II-2b)
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9;
p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5. [0013] In some embodiments, the linker connects to the metal chelator through L3 and to the peptide through L2. In some embodiments, the linker has a structure of Formula (II-2a). [0014] In some embodiments, the linker of Formula (II-2a) has a structure of Formula (II-2aa),
Formula (II-2aa) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L31 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-;
L32 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5. [0015] In some embodiments, the linker has a structure of Formula (II-2b). In some embodiments, the linker of Formula (II-2b) has a structure of Formula (II-2ba),
Formula (II-2ba) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -
OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L3 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, - C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5. [0016] In some embodiments, the linker comprises a click chemistry residue. In some embodiments, the linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and one or more amino acids. In some embodiments, the linker comprises one or more of a substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, C3-C20 alkylene, or amino acid residues. In some embodiments, the linker comprises one or more lysine residues. In some embodiments, the linker comprises one or more glutamate residues. In some embodiments, the linker comprises phenyl iodide or carboxylic acid. In some embodiments, the linker comprises 3 to 30 intervening atoms between the metal chelator and the binding peptide. In some embodiments, the linker comprises 6 to 18 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a dissociation constant (Kd) between the linker and human serum albumin is at most 25 µM, as determined in vitro at room temperature in human serum condition. In some embodiments, about 40%-60% of the conjugate binds to HSA in vitro as determined by HSA- HPLC method. In some embodiments, about 60%-80% of the conjugate binds to HSA in vitro as
determined by HSA-HPLC method. In some embodiments, the linker is attached to the binding peptide via the N terminus of the binding peptide. In some embodiments, the conjugate comprises a second linker attached to the C terminus of the binding peptide. In some embodiments, the conjugate comprises an alpha particle-emitting radionuclide bound to the metal chelator. In some embodiments, the alpha particle-emitting radionuclide is actinium-225, astatine-211, radium-223, or thorium-227. n some embodiments, the alpha particle-emitting radionuclide is actinium-225. In some embodiments, the conjugate comprises two or more metal chelators. In some embodiments, the conjugate has an elimination half-life in rats of about 1 to 120 hours. In some embodiments, the conjugate has an elimination half-life in rats of about 2 to 24 hours. In some embodiments, a half-life of the conjugate in human serum condition is about 2 to 20 hours, 5 to 20 hours, 8 to 15 hours, or 10 to 14 hours at 37 °C. [0017] In one aspect, the present disclosure provides a conjugate comprising (i) a guanylyl cyclase C (GCC) binding peptide; (ii) a metal chelator configured to bind with a radionuclide; and (iii) a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein the conjugate has a structure of Formula (I)
Formula (I) wherein, the linker –(L)n- comprises 2 to 50 intervening atoms between the metal chelator and the binding peptide; each L is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, - C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1- C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30
alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C6 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); n is 0 or an integer from 1 to 20; and r is 0 or an integer from 1 to 5. [0018] In some embodiments, the linker –(L)n- comprises 3 to 20 intervening atoms between the metal chelator and the binding peptide. In some embodiments, –(L)n- is bound to the N-terminus of the GCC binding peptide and LC is bound to the C-terminus of the GCC binding peptide. In some embodiments, the linker –(L)n- comprises a hydrophobic group selected from a C8-C30 fatty acid, C8-C30 fatty alcohol, C8-C30 alkyl, aryl, heteroaryl, one or more amino acid residues, or a combination thereof. In some embodiments, –(L)n- comprises one or more lysine residues. In some embodiments, –(L)n- comprises one or more glutamate residues. In some embodiments, the conjugate has a structure of Formula (Ia),
Formula (Ia)
wherein, each L1, L2, and L3 is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, - C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R and RC are each independently hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RX is independently hydrogen, halo, substituted or unsubstituted C1-C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; q is 0, 1, 2, 3, 4, 5, or 6; and r is 0, 1, 2, 3, 4, or 5. [0019] In some embodiments, the conjugate has a structure of Formula (Iaa),
Formula (Iaa) wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl,
wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5. [0020] In some embodiments, the conjugate has a structure of Formula (Iab),
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -
C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5. [0021] In some embodiments, the binding peptide comprises an unnatural amino acid, e.g., at the C-terminus. In some embodiments, the binding peptide comprises a modified tyrosine at the C- terminus. In some embodiments, the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide comprises an amino acid sequence selected from SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide further comprises 1 to 3 intramolecular bonds selected from heteroalkylene, alkylene and alkenylene bonds. In some embodiments, the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, or NOTP. In some embodiments, the metal chelator is DOTA. In some embodiments, the conjugate comprises a radionuclide bound to the metal chelator. In some embodiments, the radionuclide is an alpha particle-emitting radionuclide selected from actinium-225, astatine-211, radium-223, and thorium-227. In some embodiments, the radionuclide is lutetium-177 or actinium-225. [0022] In one aspect, the present disclosure provides a conjugate selected from a structure of Table 1. [0023] In one aspect, the present disclosure provides a conjugate comprising: a structure selected from Table 1 and a radionuclide bound to a metal chelator of the structure. [0024] In one aspect, the present disclosure provides a conjugate, wherein the conjugate is a salt or solvate of a conjugate of described herein.
[0025] In one aspect, the present disclosure provides a pharmaceutical composition comprising a herein-described conjugate and a pharmaceutically acceptable excipient or carrier. [0026] In one aspect, the present disclosure provides a method of treating a gastrointestinal tract cancer in a subject in need thereof, comprising administering to the subject a conjugate described herein, or a pharmaceutical composition described herein. In some embodiments, the cancer is colorectal cancer. In some embodiments, the method comprises administering a second therapeutic agent that is a GCC receptor agonist. In some embodiments, the method comprises administering (i) a first conjugate comprising a radionuclide configured for companion diagnostic and (ii) a second conjugate comprising a radionuclide selected from an alpha or beta-particle emitter, wherein the first and the second conjugate have the same structure except for the radionuclide. In some embodiments, the radionuclide of the first conjugate is selected from Lu- 177, Technetium-99m, In-111, Ga-68, Cu-64, and Zr-89. [0027] In one aspect, the present disclosure provides a method of treating a gastrointestinal tract cancer in a subject in need thereof, comprising administering to the subject a herein-described conjugate or a pharmaceutical composition comprising the conjugate. In some embodiments, the cancer is colorectal cancer. In some embodiments, the method comprises administering a second agent that binds to a GCC receptor. In some embodiments, the method comprises administering a second agent that is a GCC receptor agonist. In some embodiments, the method comprises administering a second agent that is a GCC receptor antagonist. INCORPORATION BY REFERENCE [0028] All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference for the specific purposes identified herein. BRIEF DESCRIPTION OF THE DRAWINGS [0029] Various aspects of the disclosure are set forth with particularity in the appended claims. A better understanding of the features and advantages of the present disclosure will be obtained by reference to the following detailed description that sets forth illustrative embodiments, in which the principles of the disclosure are utilized, and the accompanying drawings of which: [0030] FIGs. 1A-1J illustrate amino acid sequences of exemplary GCC binding peptides, including disulfide bridges. FIG. 1A illustrates amino acid sequence for linaclotide; FIG. 1B illustrates amino acid sequence for plecanatide; FIG.1C illustrates amino acid sequence for F19- STh (1-19), an analogue of a wild-type Escherichia coli heat-stable peptide (STh) moiety with a Tyr to Phe alteration at position 19; FIG.1D illustrates amino acid sequence for R1,4, F19-STh(1- 19), an analogue of a wild-type STh moiety with a Tyr to Phe alteration at position 19 and having
arginine residues at positions 1 and 4; FIG.1E illustrates amino acid sequence for F9-STh(6-19), an analogue of a wild-type STh moiety; FIG.1F illustrates amino acid sequence for F19-STh (2- 19), an analogue of a wild-type STh moiety; FIG. 1G illustrates amino acid sequence for uroguanylin; FIG.1H illustrates amino acid sequence for guanylin; FIG.1I illustrates amino acid sequence for lymphoguanylin; and FIG.1J illustrates amino acid sequence for E. coli ST. [0031] FIGs.2A-2D illustrate conjugates comprising exemplary linker structures that contain one or more motifs. FIG.2A illustrates a conjugate comprising a metal chelator, a linker and a cyclized GCC binding peptide; FIG. 2B and FIG.2C illustrate conjugates comprising a metal chelator, a cyclized GCC binding peptide, and a linker that comprises two or more motifs; FIG.2D illustrates a conjugate comprising a metal chelator, two cyclized GCC binding peptides, and a linker that comprises two or more motifs. [0032] FIG.3 illustrates the structures of representative metal chelators. [0033] FIG.4 illustrates the structures of representative metal chelators. [0034] FIG.5 illustrates the structures of representative metal chelators. [0035] FIG.6 illustrates the structures of representative metal chelators. [0036] FIG.7 illustrates the structures of representative metal chelators. [0037] FIG.8 illustrates the structures of representative metal chelators. [0038] FIG.9 illustrates the structures of representative metal chelators. [0039] FIG.10 illustrates the structures of representative metal chelators. [0040] FIG.11 illustrates the structures of representative metal chelators. [0041] FIG.12 illustrates the structures of representative metal chelators. [0042] FIG.13 illustrates the structures of representative metal chelators. [0043] FIG.14 illustrates the structures of representative metal chelators. [0044] FIG.15 illustrates the structures of representative metal chelators. [0045] FIG.16 illustrates the structures of representative metal chelators. [0046] FIG.17 illustrates the structures of representative metal chelators. [0047] FIG.18A illustrates plasma stability of linaclotide; FIG.18B illustrates plasma stability of La-C-02. [0048] FIG.19 illustrates the structures of representative conjugates of the present disclosure. [0049] FIG.20 illustrates the structures of representative conjugates of the present disclosure. [0050] FIG.21 illustrates the structures of representative conjugates of the present disclosure. [0051] FIG.22 illustrates the structures of representative conjugates of the present disclosure.
DETAILED DESCRIPTION [0052] The present disclosure relates to radiopharmaceutical conjugate compositions and their use. [0053] The following description and examples illustrate embodiments of the present disclosure in detail. It is to be understood that this present disclosure is not limited to the particular embodiments described herein and as such can vary. Those of skill in the art will recognize that there are numerous variations and modifications of this present disclosure, which are encompassed within its scope. [0054] Although various features of the present disclosure may be described in the context of a single embodiment, the features may also be provided separately or in any suitable combination. Conversely, although the present disclosure may be described herein in the context of separate embodiments for clarity, the present disclosure may also be implemented in a single embodiment. [0055] The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. [0056] All terms are intended to be understood as they would be understood by a person skilled in the art. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which the disclosure pertains. [0057] The following definitions supplement those in the art and are directed to the current application and are not to be imputed to any related or unrelated case, e.g., to any commonly owned patent or application. Although any methods and materials similar or equivalent to those described herein can be used in the practice for testing of the present disclosure, the preferred materials and methods are described herein. Accordingly, the terminology used herein is for the purpose of describing particular embodiments only, and is not intended to be limiting. I. Definitions [0058] As used in the specification and appended claims, unless specified to the contrary, the following terms have the meaning indicated below. [0059] As used herein and in the appended claims, the singular forms “a,” “an,” and “the” include plural referents unless the context clearly dictates otherwise. Thus, for example, reference to “an agent” includes a plurality of such agents, and reference to “the cell” includes reference to one or more cells (or to a plurality of cells) and equivalents thereof known to those skilled in the art, and so forth. When ranges are used herein for physical properties, such as molecular weight, or chemical properties, such as chemical formulae, all combinations and subcombinations of ranges and specific embodiments therein are intended to be included.
[0060] The term “about” or “approximately” can mean within an acceptable error range for the particular value as determined by one of ordinary skill in the art, which will depend in part on how the value is measured or determined, i.e., the limitations of the measurement system. For example, “about” can mean within 1 or more than 1 standard deviation, per the practice in the art. Alternatively, “about” can mean a range of up to 20%, up to 15%, up to 10%, up to 5%, or up to 1% of a given value. Alternatively, particularly with respect to biological systems or processes, the term can mean within an order of magnitude, within 5-fold, or within 2-fold, of a value. [0061] The term “comprising” (and related terms such as “comprise” or “comprises” or “having” or “including”) is not intended to exclude that in other certain embodiments, for example, an embodiment of any composition of matter, composition, method, or process, or the like, described herein, “consist of” or “consist essentially of” the described features. [0062] "Amino" refers to the –NH2 radical. [0063] "Cyano" refers to the -CN radical. [0064] "Nitro" refers to the -NO2 radical. [0065] "Oxo" refers to the =O radical. [0066] "Imino" refers to the =N-H radical. [0067] "Oximo" refers to the =N-OH radical. [0068] “Hydroxy” or “hydroxyl” refers to the -OH radical. [0069] “Hydroxyamino” refers to the -NH-OH radical. [0070] “Acyl” refers to a substituted or unsubstituted alkylcarbonyl, substituted or unsubstituted alkenylcarbonyl, substituted or unsubstituted alkynylcarbonyl, substituted or unsubstituted cycloalkylcarbonyl, substituted or unsubstituted heterocycloalkylcarbonyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted heteroarylcarbonyl, amide, or ester, wherein the carbonyl atom of the carbonyl group is the point of attachment. Unless stated otherwise specifically in the specification, an alkylcarbonyl group, alkenylcarbonyl group, alkynylcarbonyl group, cycloalkylcarbonyl group, amide group, or ester group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. [0071] “Alkyl” refers to an optionally substituted straight-chain, or optionally substituted branched-chain saturated hydrocarbon monoradical. An alkyl group can have from one to about twenty carbon atoms, from one to about ten carbon atoms, or from one to six carbon atoms. Examples include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, 2-methyl-1-propyl, 2-methyl-2-propyl, 2-methyl-1-butyl, 3-methyl-1-butyl, 2-methyl-3-butyl, 2,2-dimethyl-1-propyl, 2-methyl-1-pentyl, 3-methyl-1-pentyl, 4-methyl-1-pentyl, 2-methyl-2-pentyl, 3-methyl-2-pentyl,
4-methyl-2-pentyl, 2,2-dimethyl-1-butyl, 3,3-dimethyl-1-butyl, 2-ethyl-1-butyl, n-butyl, isobutyl, sec-butyl, t-butyl, n-pentyl, isopentyl, neopentyl, tert-amyl, and hexyl, and longer alkyl groups, such as heptyl, octyl, and the like. Whenever it appears herein, a numerical range such as “C1-C6 alkyl” means that the alkyl group consists of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkyl” where no numerical range is designated. In some embodiments, the alkyl is a C1-C10 alkyl, a C1-C9 alkyl, a C1-C8 alkyl, a C1-C7 alkyl, a C1-C6 alkyl, a C1-C5 alkyl, a C1-C4 alkyl, a C1-C3 alkyl, a C1-C2 alkyl, or a C1 alkyl. Unless stated otherwise specifically in the specification, an alkyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, the alkyl is optionally substituted with oxo, halogen, -CN, -CF3, -OH, -OMe, -NH2, -NO2, or -C≡CH. In some embodiments, the alkyl is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkyl is optionally substituted with halogen. [0072] “Alkylene” refers to a straight or branched divalent hydrocarbon chain. Unless stated otherwise specifically in the specification, an alkylene group may be optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkylene is optionally substituted with oxo, halogen, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, an alkylene is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkylene is optionally substituted with halogen. In some embodiments, the alkylene is -CH2-, -CH2CH2-, -CH2CH2CH2-, or -CH2CH(CH3)CH2-. In some embodiments, the alkylene is -CH2-. In some embodiments, the alkylene is -CH2CH2-. In some embodiments, the alkylene is -CH2CH2CH2-. [0073] “Alkenyl” refers to an optionally substituted straight-chain, or optionally substituted branched-chain hydrocarbon monoradical having one or more carbon-carbon double-bonds. In some embodiments, an alkenyl group has from two to about ten carbon atoms, or two to about six carbon atoms. The group may be in either the cis or trans configuration about the double bond(s), and should be understood to include both isomers. Examples include, but are not limited to, ethenyl (-CH=CH2), 1-propenyl (-CH2CH=CH2), isopropenyl [-C(CH3)=CH2], butenyl, 1,3- butadienyl, and the like. Whenever it appears herein, a numerical range such as “C2-C6 alkenyl” means that the alkenyl group may consist of 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms, or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkenyl” where no numerical range is designated. In some embodiments, the alkenyl is a C2-C10 alkenyl, a C2-C9 alkenyl, a C2-C8 alkenyl, a C2-C7 alkenyl, a C2-C6 alkenyl, a C2-C5 alkenyl,
a C2-C4 alkenyl, a C2-C3 alkenyl, or a C2 alkenyl. Unless stated otherwise specifically in the specification, an alkenyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkenyl is optionally substituted with oxo, halogen, -CN, - CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, an alkenyl is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkenyl is optionally substituted with halogen. [0074] The term “alkenylene” or “alkenylene chain” refers to an optionally substituted straight or branched divalent hydrocarbon chain in which at least one carbon-carbon double bond is present linking the rest of the molecule to a radical group. In some embodiments, the alkenylene is –CH=CH-, -CH2CH=CH-, or –CH=CHCH2-. In some embodiments, the alkenylene is – CH=CH-. In some embodiments, the alkenylene is –CH2CH=CH-. In some embodiments, the alkenylene is –CH=CHCH2-. [0075] “Alkynyl” refers to an optionally substituted straight-chain or optionally substituted branched-chain hydrocarbon monoradical having one or more carbon-carbon triple-bonds. In some embodiments, an alkynyl group has from two to about ten carbon atoms, more preferably from two to about six carbon atoms. Examples include, but are not limited to, ethynyl, 2-propynyl, 2-butynyl, 1,3-butadiynyl, and the like. Whenever it appears herein, a numerical range such as “C2-C6 alkynyl” means that the alkynyl group may consist of 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms, or 6 carbon atoms, although the present definition also covers the occurrence of the term “alkynyl” where no numerical range is designated. In some embodiments, the alkynyl is a C2-C10 alkynyl, a C2-C9 alkynyl, a C2-C8 alkynyl, a C2-C7 alkynyl, a C2-C6 alkynyl, a C2-C5 alkynyl, a C2-C4 alkynyl, a C2-C3 alkynyl, or a C2 alkynyl. Unless stated otherwise specifically in the specification, an alkynyl group is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkynyl is optionally substituted with oxo, halogen, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, an alkynyl is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkynyl is optionally substituted with halogen. The term “alkynylene” refers to an optionally substituted straight-chain or optionally substituted branched-chain divalent hydrocarbon having one or more carbon-carbon triple-bonds. [0076] “Alkylamino” refers to a radical of the formula -N(Ra)2 where Ra is an alkyl radical as defined, or two Ra, taken together with the nitrogen atom, can form a substituted or unsubstituted C2-C7 heterocyloalkyl ring. Unless stated otherwise specifically in the specification, an alkylamino group may be optionally substituted, for example, with oxo, halogen, amino, nitrile,
nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkylamino is optionally substituted with oxo, halogen, -CN, -CF3, -OH, - OMe, -NH2, or -NO2. In some embodiments, an alkylamino is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkylamino is optionally substituted with halogen. [0077] “Alkoxy” refers to a radical of the formula -ORa where Ra is an alkyl radical as defined. Unless stated otherwise specifically in the specification, an alkoxy group may be optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, an alkoxy is optionally substituted with oxo, halogen, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, an alkoxy is optionally substituted with oxo, halogen, -CN, -CF3, -OH, or -OMe. In some embodiments, the alkoxy is optionally substituted with halogen. [0078] “Aminoalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more amines. In some embodiments, the alkyl is substituted with one amine. In some embodiments, the alkyl is substituted with one, two, or three amines. Hydroxyalkyl include, for example, aminomethyl, aminoethyl, aminopropyl, aminobutyl, or aminopentyl. In some embodiments, the hydroxyalkyl is aminomethyl. [0079] “Hydroxyalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more hydroxyls. In some embodiments, the alkyl is substituted with one hydroxyl. In some embodiments, the alkyl is substituted with one, two, or three hydroxyls. Hydroxyalkyl include, for example, hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl, or hydroxypentyl. In some embodiments, the hydroxyalkyl is hydroxymethyl. [0080] The term “aryl” refers to a radical comprising at least one aromatic ring wherein each of the atoms forming the ring is a carbon atom. Aryl groups can be optionally substituted. Examples of aryl groups include, but are not limited to phenyl, and naphthyl. In some embodiments, the aryl is phenyl. Depending on the structure, an aryl group can be a monoradical or a diradical (i.e., an arylene group). Unless stated otherwise specifically in the specification, the term “aryl” or the prefix “ar-”(such as in “aralkyl”) is meant to include aryl radicals that are optionally substituted. In some embodiments, an aryl group comprises a partially reduced cycloalkyl group defined herein (e.g., 1,2-dihydronaphthalene). In some embodiments, an aryl group comprises a fully reduced cycloalkyl group defined herein (e.g., 1,2,3,4-tetrahydronaphthalene). When aryl comprises a cycloalkyl group, the aryl is bonded to the rest of the molecule through an aromatic ring carbon atom. An aryl radical can be a monocyclic or polycyclic (e.g., bicyclic, tricyclic, or tetracyclic) ring system, which may include fused, spiro or bridged ring systems. Unless stated otherwise specifically in the specification, an aryl may be optionally substituted, for example, with
halogen, amino, alkylamino, aminoalkyl, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, heteroalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, -S(O)2NH-C1- C6alkyl, and the like. In some embodiments, an aryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, -NO2, -S(O)2NH2, -S(O)2NHCH3, -S(O)2NHCH2CH3, - S(O)2NHCH(CH3)2, -S(O)2N(CH3)2, or -S(O)2NHC(CH3)3. In some embodiments, an aryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF3, -OH, or -OMe. In some embodiments, the aryl is optionally substituted with halogen. In some embodiments, the aryl is substituted with alkyl, alkenyl, alkynyl, haloalkyl, or heteroalkyl, wherein each alkyl, alkenyl, alkynyl, haloalkyl, heteroalkyl is independently unsubstituted, or substituted with halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. [0081] The term “cycloalkyl” refers to a monocyclic or polycyclic non-aromatic radical, wherein each of the atoms forming the ring (i.e. skeletal atoms) is a carbon atom. In some embodiments, cycloalkyls are saturated or partially unsaturated. In some embodiments, cycloalkyls are spirocyclic or bridged compounds. In some embodiments, cycloalkyls are fused with an aromatic ring (in which case the cycloalkyl is bonded through a non-aromatic ring carbon atom). Cycloalkyl groups include groups having from 3 to 10 ring atoms. Representative cycloalkyls include, but are not limited to, cycloalkyls having from three to ten carbon atoms, from three to eight carbon atoms, from three to six carbon atoms, or from three to five carbon atoms. Monocyclic cycloalkyl radicals include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl. In some embodiments, the monocyclic cycloalkyl is cyclopentyl. In some embodiments, the monocyclic cycloalkyl is cyclopentenyl or cyclohexenyl. In some embodiments, the monocyclic cycloalkyl is cyclopentenyl. Polycyclic radicals include, for example, adamantyl, 1,2-dihydronaphthalenyl, 1,4-dihydronaphthalenyl, tetrainyl, decalinyl, 3,4- dihydronaphthalenyl-1(2H)-one, spiro[2.2]pentyl, norbornyl and bicycle[1.1.1]pentyl. Unless otherwise stated specifically in the specification, a cycloalkyl group may be optionally substituted. Representative cycloalkyls include, but are not limited to, cycloalkyls having from three to fifteen carbon atoms (C3-C15 cycloalkyl), from three to ten carbon atoms (C3-C10 cycloalkyl), from three to eight carbon atoms (C3-C8 cycloalkyl), from three to six carbon atoms (C3-C6 cycloalkyl), from three to five carbon atoms (C3-C5 cycloalkyl), or three to four carbon atoms (C3-C4 cycloalkyl). In some embodiments, the cycloalkyl is a 3- to 6-membered cycloalkyl. In some embodiments, the cycloalkyl is a 5- to 6-membered cycloalkyl. Monocyclic cycloalkyls include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl. Polycyclic cycloalkyls or carbocycles include, for example, adamantyl, norbornyl, decalinyl, bicyclo[3.3.0]octane, bicyclo[4.3.0]nonane, cis-decalin, trans-decalin, bicyclo[2.1.1]hexane, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, bicyclo[3.2.2]nonane, and bicyclo[3.3.2]decane, and
7,7-dimethyl-bicyclo[2.2.1]heptanyl. Partially saturated cycloalkyls include, for example, cyclopentenyl, cyclohexenyl, cycloheptenyl, and cyclooctenyl. Unless stated otherwise specifically in the specification, a cycloalkyl is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, a cycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, a cycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, - CF3, -OH, or -OMe. In some embodiments, the cycloalkyl is optionally substituted with halogen. [0082] “Halo” or “halogen” refers to bromo, chloro, fluoro, or iodo. In some embodiments, halogen is fluoro or chloro. In some embodiments, halogen is fluoro. [0083] “Haloalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more halogens. In some embodiments, the alkyl is substituted with one, two, or three halogens. In some embodiments, the alkyl is substituted with one, two, three, four, five, or six halogens. Haloalkyl can include, for example, iodoalkyl, bromoalkyl, chloroalkyl, and fluoroalkyl. For example, "fluoroalkyl" refers to an alkyl radical, as defined above, that is substituted by one or more fluoro radicals, as defined above, for example, trifluoromethyl, difluoromethyl, fluoromethyl, 2,2,2-trifluoroethyl, 1-fluoromethyl-2-fluoroethyl, and the like. In some embodiments, the alkyl part of the fluoroalkyl radical is optionally substituted as defined above for an alkyl group. [0084] “Heteroalkyl” refers to an alkyl group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen (e.g., -NH-, -N(alkyl)-), sulfur, or combinations thereof. A heteroalkyl is attached to the rest of the molecule at a carbon atom of the heteroalkyl. In one aspect, a heteroalkyl is a C1-C6 heteroalkyl wherein the heteroalkyl is comprised of 1 to 6 carbon atoms and one or more atoms other than carbon, e.g., oxygen, nitrogen (e.g. -NH-, -N(alkyl)-), sulfur, or combinations thereof wherein the heteroalkyl is attached to the rest of the molecule at a carbon atom of the heteroalkyl. Examples of such heteroalkyl are, for example, –CH2-O-CH2-, –CH2-N(alkyl)-CH2-, –CH2-N(aryl)-CH2-, -OCH2CH2O-, – OCH2CH2OCH2CH2O-, or –OCH2CH2OCH2CH2OCH2CH2O-. Unless stated otherwise specifically in the specification, a heteroalkyl is optionally substituted for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, a heteroalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, a heteroalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, - CF3, -OH, or -OMe. In some embodiments, the heteroalkyl is optionally substituted with halogen. As used herein, a “heteroalkylene” refers to divalent heteroalkyl group.
[0085] The term “heterocycloalkyl” refers to a cycloalkyl group that includes at least one hetero ring atom, e.g., a heteroatom selected from nitrogen, oxygen, and sulfur. In some embodiment, heterocycloalkyl are saturated. In some embodiment, heterocycloalkyl are partially unsaturated. Unless stated otherwise specifically in the specification, the heterocycloalkyl radical may be a monocyclic, or bicyclic ring system, which may include fused (when fused with an aryl or a heteroaryl ring, the heterocycloalkyl is bonded through a non-aromatic ring atom) or bridged ring systems. The nitrogen, carbon or sulfur atoms in the heterocyclyl radical may be optionally oxidized. The nitrogen atom may be optionally quaternized. The heterocycloalkyl radical is partially or fully saturated. Examples of heterocycloalkyl radicals include, but are not limited to, dioxolanyl, thienyl[1,3]dithianyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl, decahydroisoquinolyl, imidazolinyl, imidazolidinyl, isothiazolidinyl, isoxazolidinyl, morpholinyl, octahydroindolyl, octahydroisoindolyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl, oxazolidinyl, piperidinyl, piperazinyl, 4-piperidonyl, pyrrolidinyl, pyrazolidinyl, quinuclidinyl, thiazolidinyl, tetrahydrofuryl, trithianyl, tetrahydropyranyl, thiomorpholinyl, thiamorpholinyl, 1-oxo-thiomorpholinyl, 1,1-dioxo-thiomorpholinyl. The term heterocycloalkyl also includes all ring forms of carbohydrates, including but not limited to monosaccharides, disaccharides and oligosaccharides. Unless otherwise noted, heterocycloalkyls have from 2 to 12 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring and 1 or 2 N atoms. In some embodiments, heterocycloalkyls have from 2 to 10 carbons in the ring and 3 or 4 N atoms. In some embodiments, heterocycloalkyls have from 2 to 12 carbons, 0-2 N atoms, 0-2 O atoms, 0-2 P atoms, and 0-1 S atoms in the ring. In some embodiments, heterocycloalkyls have from 2 to 12 carbons, 1-3 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. It is understood that when referring to the number of carbon atoms in a heterocycloalkyl, the number of carbon atoms in the heterocycloalkyl is not the same as the total number of atoms (including the heteroatoms) that make up the heterocycloalkyl (i.e. skeletal atoms of the heterocycloalkyl ring). Unless stated otherwise specifically in the specification, a heterocycloalkyl is optionally substituted, for example, with oxo, halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, a heterocycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, a heterocycloalkyl is optionally substituted with oxo, halogen, methyl, ethyl, -CN, -CF3, -OH, or -OMe. In some embodiments, the heterocycloalkyl is optionally substituted with halogen. [0086] “Heteroaryl” refers to a ring system radical comprising carbon atom(s) and one or more ring heteroatoms selected from the group consisting of nitrogen, oxygen, phosphorous, and sulfur,
and at least one aromatic ring. In some embodiments, heteroaryl is monocyclic, bicyclic or polycyclic. Illustrative examples of monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, furazanyl, indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine. Illustrative examples of monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, and furazanyl. Illustrative examples of bicyclic heteroaryls include indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine. In some embodiments, heteroaryl is pyridinyl, pyrazinyl, pyrimidinyl, thiazolyl, thienyl, thiadiazolyl or furyl. In some embodiments, a heteroaryl contains 0-6 N atoms in the ring. In some embodiments, a heteroaryl contains 1-4 N atoms in the ring. In some embodiments, a heteroaryl contains 4-6 N atoms in the ring. In some embodiments, a heteroaryl contains 0-4 N atoms, 0-1 O atoms, 0-1 P atoms, and 0- 1 S atoms in the ring. In some embodiments, a heteroaryl contains 1-4 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. In some embodiments, heteroaryl is a C1-C9 heteroaryl. In some embodiments, monocyclic heteroaryl is a C1-C5 heteroaryl. In some embodiments, monocyclic heteroaryl is a 5-membered or 6-membered heteroaryl. In some embodiments, a bicyclic heteroaryl is a C6-C9 heteroaryl. In some embodiments, a heteroaryl group comprises a partially reduced cycloalkyl or heterocycloalkyl group defined herein (e.g., 7,8-dihydroquinoline). In some embodiments, a heteroaryl group comprises a fully reduced cycloalkyl or heterocycloalkyl group defined herein (e.g., 5,6,7,8-tetrahydroquinoline). When heteroaryl comprises a cycloalkyl or heterocycloalkyl group, the heteroaryl is bonded to the rest of the molecule through a heteroaromatic ring carbon or hetero atom. A heteroaryl radical can be a monocyclic or polycyclic (e.g., bicyclic, tricyclic, or tetracyclic) ring system, which may include fused, spiro or bridged ring systems. Unless stated otherwise specifically in the specification, a heteroaryl is optionally substituted, for example, with halogen, amino, nitrile, nitro, hydroxyl, alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, cycloalkyl, heterocycloalkyl, heteroaryl, and the like. In some embodiments, a heteroaryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF3, -OH, -OMe, -NH2, or -NO2. In some embodiments, a heteroaryl is optionally substituted with halogen, methyl, ethyl, -CN, -CF3, -OH, or -OMe. In some embodiments, the heteroaryl is optionally substituted with halogen.
[0087] As used herein, “carboxylic acid or an isostere thereof” refers to a carboxylic acid moiety, or a functional group or moiety that exhibits similar physical, biological and/or chemical properties as a carboxylic acid moiety. Examples of carboxylic acid isosteres include, but are not limited to, hydroxamic acids, hydroxamic esters, sulfinic acids, sulfonic acids, sulfonamides, acyl- sulfonamides, sulfonylureas, acylureas, tetrazole, thiazolidine diones, oxozolidine diones, oxadiazol-5(4H)-one, oxothiadiazole-2-oxide, oxadiazol-5(4H)-thione, isoxazole, tetramic acid, cyclopentane 1,3-diones, cyclopentane 1,2-diones, squaryl groups, phosphoric acids, phosphinic acids, and halogenated phenols. For example, a carboxylic acid isostere can be:
wherein each hydrogen bound to a carbon atom is optionally replaced with methyl, ethyl, -CN, - CF3, -OH, -OMe, -NH2, or -NO2, or a different halogen. [0088] The term “moiety” refers to a specific segment or functional group of a molecule. Chemical moieties are often recognized chemical entities embedded in or appended to a molecule. [0089] The terms “treat,” “prevent,” “ameliorate,” and “inhibit,” as well as words stemming therefrom, as used herein, do not necessarily imply 100% or complete treatment, prevention, amelioration, or inhibition. Rather, there are varying degrees of treatment, prevention, amelioration, and inhibition of which one of ordinary skill in the art recognizes as having a potential benefit or therapeutic effect. In this respect, the disclosed methods can provide any amount of any level of treatment, prevention, amelioration, or inhibition of the disorder in a
mammal. For example, a disorder, including symptoms or conditions thereof, may be reduced by, for example, about 100%, about 90%, about 80%, about 70%, about 60%, about 50%, about 40%, about 30%, about 20%, or about 10%. Furthermore, the treatment, prevention, amelioration, or inhibition provided by the methods disclosed herein can include treatment, prevention, amelioration, or inhibition of one or more conditions or symptoms of the disorder, e.g., cancer or an inflammatory disease. As used herein, “treating” includes the concepts of “alleviating”, which refers to lessening the frequency of occurrence or recurrence, or the severity, of any symptoms or other ill effects related to a disorder and/or the associated side effects. The term “treating” also encompasses the concept of “managing” which refers to reducing the severity of a particular disease or disorder in a patient or delaying its recurrence, e.g., lengthening the period of remission in a patient who had suffered from the disease. [0090] In certain embodiments, the term “prevent” or “preventing” as related to a disease or disorder may refer to a compound that, in a statistical sample, reduces the occurrence of the disorder or condition in the treated sample relative to an untreated control sample, or delays the onset or reduces the severity of one or more symptoms of the disorder or condition relative to the untreated control sample. [0091] The term "therapeutically effective amount" as used herein to refer to an amount effective at the dosage and duration necessary to achieve the desired therapeutic result. A therapeutically effective amount of the composition may vary depending on factors such as the individual's condition, age, sex, and weight, and the ability of the protein to elicit the desired response of the individual. A therapeutically effective amount can also be an amount that exceeds any toxic or deleterious effect of the composition that would have a beneficial effect on the treatment. [0092] The term “optional” or “optionally” means that the subsequently described event or circumstance may or may not occur, and that the description includes instances where said event or circumstance occurs and instances in which it does not. For example, “optionally substituted alkyl” means either “alkyl” or “substituted alkyl” as defined above. Further, an optionally substituted group may be un-substituted (e.g., -CH2CH3), fully substituted (e.g., -CF2CF3), mono- substituted (e.g., -CH2CH2F) or substituted at a level anywhere in-between fully substituted and mono-substituted (e.g., -CH2CHF2, -CH2CF3, -CF2CH3, -CFHCHF2, etc.). [0093] As used herein, the term "substituent" means positional variables on the atoms of a core molecule that are substituted at a designated atom position, replacing one or more hydrogens on the designated atom, provided that the designated atom's normal valency is not exceeded, and that the substitution results in a stable compound. Combinations of substituents and/or variables are permissible only if such combinations result in stable compounds. A person of ordinary skill in the art should note that any carbon as well as heteroatom with valences that appear to be
unsatisfied as described or shown herein is assumed to have a sufficient number of hydrogen atom(s) to satisfy the valences described or shown. In certain instances one or more substituents having a double bond (e.g., "oxo" or "=O") as the point of attachment may be described, shown or listed herein within a substituent group, wherein the structure may only show a single bond as the point of attachment to the core structure. A person of ordinary skill in the art would understand that, while only a single bond is shown, a double bond is intended for those substituents. [0094] The term “optionally substituted” or “substituted” means that the referenced group is optionally substituted with one or more additional group(s) individually and independently selected from D, halogen, -CN, -NH2, -NH(alkyl), -N(alkyl)2, -OH, -CO2H, -CO2alkyl, - C(=O)NH2, -C(=O)NH(alkyl), -C(=O)N(alkyl)2, -S(=O)2NH2, -S(=O)2NH(alkyl), - S(=O)2N(alkyl)2, alkyl, cycloalkyl, fluoroalkyl, heteroalkyl, alkoxy, fluoroalkoxy, heterocycloalkyl, aryl, heteroaryl, aryloxy, alkylthio, arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, and arylsulfone. In some other embodiments, optional substituents are independently selected from D, halogen, -CN, -NH2, -NH(CH3), -N(CH3)2, -OH, -CO2H, -CO2(C1-C4alkyl), - C(=O)NH2, -C(=O)NH(C1-C4alkyl), -C(=O)N(C1-C4alkyl)2, -S(=O)2NH2, -S(=O)2NH(C1- C4alkyl), -S(=O)2N(C1-C4alkyl)2, C1-C4alkyl, C3-C6cycloalkyl, C1-C4fluoroalkyl, C1- C4heteroalkyl, C1-C4alkoxy, C1-C4fluoroalkoxy, -SC1-C4alkyl, -S(=O)C1-C4alkyl, and -S(=O)2C1- C4alkyl. In some embodiments, optional substituents are independently selected from D, halogen, -CN, -NH2, -OH, -NH(CH3), -N(CH3)2, -NH(cyclopropyl), -CH3, -CH2CH3, -CF3, -OCH3, and - OCF3. In some embodiments, substituted groups are substituted with one or two of the preceding groups. In some embodiments, an optional substituent on an aliphatic carbon atom (acyclic or cyclic) includes oxo (=O). When indicating the number of substituents, the term “one or more” means from one substituent to the highest possible number of substitutions, i.e. replacement of one hydrogen up to replacement of all hydrogens by substituents. [0095] The term “unsubstituted” means that the specified group bears no substituents. [0096] Certain compounds described herein may exist in tautomeric forms, and all such tautomeric forms of the compounds being within the scope of the disclosure. Unless otherwise stated, structures depicted herein are also meant to include all stereochemical forms of the structure; i.e., the R and S configurations for each asymmetric center. Therefore, single stereochemical isomers as well as enantiomeric and diastereomeric mixtures of the present compounds are within the scope of the disclosure. [0097] The term “peptide” as used herein refers to a compound that includes two or more amino acids. A peptide described herein can comprise one or more unnatural amino acids. The term “peptide” also encompasses peptide mimetics. In the present disclosure, the term “amino acid” is used in its broadest meaning and it embraces not only natural amino acids but also derivatives
thereof and artificial amino acids. For example, the term “amino acid” encompasses unnatural amino acids. [0098] As used herein, the term “unnatural amino acid” refers to an amino acid other than the 20 amino acids that occur naturally in protein. [0099] The term “protein” as used herein refers to a polypeptide (i.e., a string of at least 3 amino acids linked to one another by peptide bonds). Proteins can include moieties other than amino acids (e.g., may be glycoproteins, proteoglycans, etc.) and/or can be otherwise processed or modified. A protein can be a complete polypeptide as produced by and/or active in a cell (with or without a signal sequence). In some embodiments, a protein is or comprises a characteristic portion such as a polypeptide as produced by and/or active in a cell. A protein can include more than one polypeptide chain. For example, polypeptide chains can be linked by one or more disulfide bonds or associated by other means. [0100] The term “peptide mimetic” or “mimetic” refers to biologically active compounds that mimic the biological activity of a peptide or a protein but are no longer entirely peptidic in chemical nature, e.g.,, they can contain non-peptide bonds (that are, bonds other than amide bonds between amino acids). As used herein, the term peptide mimetic is used in a broader sense to include molecules that are no longer completely peptidic in nature, such as pseudo-peptides, semi- peptides and peptoids. Whether completely or partially non-peptide, peptide mimetics described herein can provide a spatial arrangement of reactive chemical moieties that closely resemble the three-dimensional arrangement of active groups in the subject amino acid sequence or subject molecule on which the peptide mimetic is based. As a result of this similar active-site geometry, the peptide mimetic can have effects on biological systems that are similar to the biological activity of the subject entity. [0101] In some embodiments, the peptide mimetics are substantially similar in both three- dimensional shape and biological activity to the subject amino acid sequence or subject molecule on which the peptide mimetic is based. Examples of methods of structurally modifying a peptide to create a peptide mimetic include the inversion of backbone chiral centers leading to D-amino acid residue structures that may, particularly at the N-terminus, lead to enhanced stability for proteolytical degradation without adversely affecting activity. An example is described in the paper “Tritiated D-ala1-Peptide T Binding”, Smith C. S. et al., Drug Development Res., 15, pp. 371-379 (1988). A second method is altering cyclic structure for stability, such as N to C interchain imides and lactams (Ede et al. in Smith and Rivier (Eds.) “Peptides: Chemistry and Biology”, Escom, Leiden (1991), pp. 268-270). An example of this is provided in conformationally restricted thymopentin-like compounds, such as those disclosed in US4457489.
A third method is to substitute peptide bonds in the subject entity by pseudopeptide bonds that confer resistance to proteolysis. [0102] As used herein, “guanylyl cyclase C, ” also known as “GCC,” “STAR,” “GUC2C, “GUCY2C or “ST receptor” refers to mammalian GCC, preferably human GCC protein. Human GCC refers to the protein shown in SEQ ID NO: 43 and naturally occurring allelic protein variants thereof (e.g., natural variants of SEQ ID NO: 43 as provided in UniProtKB Accession No. P25092). Natural variants of SEQ ID NO: 43 as provided in UniProtKB Accession No. P25092 include VAR_067724, VAR_068174, VAR_042221, VAR_042222, VAR_042223, VAR_049253, VAR_068174, VAR_042224, VAR_042225, VAR_067724, VAR_042226, VAR_042227, VAR_042228, etc. Other variants are known in the art. See, e.g., accession number Ensp0000261170, Ensembl Database, European Bioinformatics Institute and Wellcome Trust Sanger Institute, which has a leucine at residue 281; SEQ ID NO: 14 of published US patent application number 20060035852; or GenBank accession number AAB 19934. Typically, a naturally occur ring allelic variant has an amino acid sequence at least 95%, 97% or 99% identical to the GCC sequence of SEQ ID NO: 43. The transcript encodes a protein product of 1073 amino acids, and is described in GenBank accession no.: NM 004963. GCC protein is characterized as a transmembrane cell surface receptor protein, and is believed to play a critical role in the maintenance of intestinal fluid, electrolyte homeostasis and cell proliferation. [0103] Amino acid sequence for human GCC protein (UniProtKB Accession No. P25092; SEQ ID NO: 43):
[0104] As used herein, the term “guanylyl cyclase C (GCC) binding peptide” or “guanylate cyclase C (GCC) binding peptide” refers to peptides that bind to the class of guanylate cyclase C receptor proteins on any cell type. The term “GCC binding peptide” also includes all peptides that have amino acid sequences substantially equivalent to at least a portion of any one of SEQ ID NOs: 1-42 and derivatives thereof. This term also covers fragments and pro-peptides that bind to guanylate cyclase receptor. The term “substantially equivalent” refers to a peptide that has an amino acid sequence equivalent to that of the binding domain where certain residues may be deleted or replaced with other amino acids without impairing the peptide's ability to bind to a guanylate cyclase receptor. [0105] As used herein, the term “GCC binding peptide” encompasses “GCC receptor agonists,” which refer to peptides and/or other compounds that bind to a guanylate cyclase C receptor and stimulate cGMP production. The term “GCC binding peptide” also encompasses “GCC receptor antagonist.” [0106] Ranges provided herein are understood to be shorthand for all of the values within the range. For example, a range of 1 to 50 is understood to include any number, combination of numbers, or sub-range from the group consisting of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, or 50, as well as all intervening decimal values between the aforementioned integers such as, for example, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, and 1.9. With respect to sub-ranges, “nested sub-ranges” that extend from either end point of the range are specifically contemplated. For example, a nested sub-range of an exemplary range of 1 to 50 may comprise 1 to 10, 1 to 20, 1 to 30, and 1 to 40 in one direction, or 50 to 40, 50 to 30, 50 to 20, and 50 to 10 in the other direction.
[0107] As used herein, C1-Cx (or C1-x) includes C1-C2, C1-C3... C1-Cx. By way of example only, a group designated as “C1-C4” indicates that there are one to four carbon atoms in the moiety, i.e. groups containing 1 carbon atom, 2 carbon atoms, 3 carbon atoms or 4 carbon atoms. Thus, by way of example only, “C1-C4 alkyl” indicates that there are one to four carbon atoms in the alkyl group, i.e., the alkyl group is selected from among methyl, ethyl, propyl, iso-propyl, n-butyl, iso- butyl, sec-butyl, and t-butyl. Also, by way of example, C0-C2 alkylene includes a direct bond, - CH2-, and -CH2CH2- linkages. [0108] The term “cyclized” or “cyclization” as used herein means that two amino acids apart from each other by at least one amino acid bind directly or bind indirectly to each other in one peptide to form a cyclic structure in the molecule. In some cases, the two amino acids bind via a linker or the like. [0109] The term “subject” or “patient” encompasses mammals. Examples of mammals include, but are not limited to, any member of the Mammalian class: humans, non-human primates such as chimpanzees, and other apes and monkey species; farm animals such as cattle, horses, sheep, goats, swine; domestic animals such as rabbits, dogs, and cats; laboratory animals including rodents, such as rats, mice and guinea pigs, and the like. In one aspect, the mammal is a human. [0110] The term "therapeutically effective amount" as used herein to refer to an amount effective at the dosage to achieve the desired therapeutic result. A therapeutically effective amount of a composition may vary depending on factors such as the individual's condition (e.g., age, sex, and weight), the radiopharmaceutical conjugate, and the method of administration (e.g., oral or parenteral). II. Radiopharmaceutical Conjugates [0111] Provided herein are radiopharmaceutical conjugates and pharmaceutical compositions comprising the conjugates. The conjugates and compositions are useful for treating guanylyl cyclase C (GCC) associated diseases or conditions. The conjugates and compositions can also be useful in disease diagnosis. [0112] In one aspect, described herein is a conjugate that comprises a GCC binding peptide, a metal chelator that is configured to bind with a radionuclide, a linker that covalently attaches the GCC binding peptide with the metal chelator, and optionally a radionuclide. In one aspect, described herein is a conjugate that comprises a GCC binding peptide, a metal chelator that is configured to bind with a radionuclide, optionally a linker that covalently attaches the GCC binding peptide with the metal chelator, and optionally a radionuclide. In some embodiments, described herein is a conjugate comprising: a GCC binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID
NOs: 1-42; a metal chelator configured to bind with a radionuclide; a linker that covalently attaches the GCC binding peptide with the metal chelator; and optionally an alpha particle- emitting radionuclide. [0113] In some embodiments, described herein is a conjugate comprising: a GCC binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein a dissociation constant (Kd) between the linker and human serum albumin is at most 500 µM, as determined at room temperature in human serum condition. In some embodiments, the Kd is at most 100 µM. In some embodiments, the Kd is at most 15 µM. [0114] In some embodiments, the GCC binding peptide comprises 7 to 40 amino acid residues. In some embodiments, the alpha particle-emitting radionuclide is 225Ac bound to the metal chelator. [0115] In some embodiments, the radiopharmaceutical conjugate comprises two or more GCC binding peptides. In some embodiments, the radiopharmaceutical conjugate comprises two GCC binding peptides. In some embodiments, the radiopharmaceutical conjugate comprises three GCC binding peptides. In some embodiments, the two or more GCC binding peptides are the same (e.g., both comprising SEQ ID NO: 1). In some embodiments, the two or more GCC binding peptides are different. [0116] In some embodiments, a conjugate described herein is designed to have a prescribed elimination profile. The elimination profile can be designed by adjusting the sequence and length of the peptide, the property of the linker, the type of radionuclide, etc. In some embodiments, the conjugate has an elimination half-life of about 0.1 to 200 hours. In some embodiments, the conjugate has an elimination half-life of about 0.5 to 150 hours. In some embodiments, the conjugate has an elimination half-life of about 1 to 120 hours. In some embodiments, the conjugate has an elimination half-life of at least 0.1 hour, 0.5 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 7 hours, 10 hours, 11 hours, 12 hours, 13 hours, 14 hours, 15 hours, 16 hours, 17 hours, 18 hours, 19 hours, 20 hours, 21 hours, 22 hours, 23 hours, or 24 hours. In some embodiments, the conjugate has an elimination half-life of at most 120 hour, 80 hours, 70 hours, 60 hours, 50 hours, 40 hours, 30 hours, 24 hours, 12 hours, 10 hours, 5 hours, or 1 hour. In some embodiments, the conjugate has an elimination half-life of about 0.5 to 48 hours. In some embodiments, the conjugate has an elimination half-life of about 1 to 36 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 24 hours. In some embodiments, the conjugate has an elimination half-life of about 3 to 9 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 12 hours. In some embodiments, the conjugate has an elimination half-life of about 2 to 8 hours. In some
embodiments, the conjugate has an elimination half-life of about 2 to 5 hours. In some embodiments, the conjugate has an elimination half-life of about 3 to 4 hours. In some embodiments, the blood elimination half-life is determined in rats. In some embodiments, the blood elimination half-life is determined in humans. [0117] A herein described conjugate can have an elimination half-life in a tumor and non-tumor tissue of the subject. The elimination half-life in a tumor can be the same as or different from (either longer or shorter than) the elimination half-life in a non-tumor issue. In some embodiments, the elimination half-life of the conjugate in a tumor is about 3 hours to 14 days, about 2 to 10 days, about 7 to 10 days, or about 4 to 7 days. In some embodiments, the elimination half-life of the conjugate in a tumor is more than 14 days. In some embodiments, the elimination half-life of the conjugate in a non-tumor tissue is about 1 hour to 14 days, about 12 hours to 2 days, about 1 day to 3 days, about 2 to 10 days, about 7 to 10 days, or about 4 to 7 days. In some embodiments, the elimination half-life of the conjugate in a tumor is at least 1.1, 1.2, 1.3, 1.4, 1.5, 2.0, 2.5, 3.0, 4.0, or 5.0 fold of the elimination half-life of the conjugate in a non-tumor tissue of the subject. [0118] As used herein, the “elimination half-life” can refer to the time it takes from the maximum concentration after administration to half maximum concentration. In some embodiments, the elimination half-life is determined after intravenous administration. In some embodiments, the elimination half-life is measured as biological half-life, which is the half-life of the cold pharmaceutical in the living system. In some embodiments, the elimination half-life is measured as effective half-life, which is the half-life of a radiopharmaceutical in a living system taking into account the half-life of the radionuclide. [0119] A conjugate described herein can have a described time-integrated activity coefficient (i.e., ã) in a tumor or non-tumor tissues of a subject. As used herein, ã represents the cumulative number of nuclear transformations occurring in a source tissue over a dose-integration period per unit administered activity. The ã value of a conjugate can be tuned by modifications of the peptide in the conjugate, e.g., modifying the amino acid sequences and length of the peptide. The ã value can be determined using a method known in the art. In some embodiments, the ã value of the conjugate in a tumor is from about 6 hours to 14 days. In some embodiments, the ã value in a tumor is about 2 to 10 days. In some embodiments, the ã value in a tumor is about 4 to 7 days. In some embodiments, the ã value in a tumor is about 7 to 10 days. In some embodiments, the ã value in a tumor is from about 1 day, 2 days, 3 days, or 4 days to about 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ã value in a tumor is about 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ã value of the conjugate in a non-tumor tissue is from
about 6 hours to 14 days. In some embodiments, the ã value in a non-tumor tissue is about 2 to 10 days. In some embodiments, the ã value in a non-tumor tissue is about 4 to 7 days. In some embodiments, the ã value in a non-tumor tissue is about 7 to 10 days. In some embodiments, the ã value in a non-tumor tissue is from about 1 day, 2 days, 3 days, or 4 days to about 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. In some embodiments, the ã value in a non-tumor tissue is about 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11 days, or 12 days. The ã value of the conjugate in a tumor can be the same as the ã value of the conjugate in a non-tumor tissue of the subject. The ã value of the conjugate in a tumor can be longer or shorter than the ã value of the conjugate in a non-tumor tissue of the subject. In some embodiments, the ã value of the conjugate in a tumor is at least 1.1, 1.2, 1.3, 1.4, 1.5, 2.0, 2.5, 3.0, 4.0, or 5.0 fold of the ã value of the conjugate in a non-tumor tissue of the subject. [0120] A conjugate described herein can have an ã value in an organ of a subject. In some embodiments, the conjugate has an ã value in a kidney of the subject of at most 24 hours. In some embodiments, the ã value of the conjugate in a kidney of the subject is at most 18 hours, 15 hours, 12 hours, 10 hours, 8 hours, 6 hours, or 5 hours. In some embodiments, the ã value of the conjugate in a kidney of the subject is about 30 minutes to about 24 hours. In some embodiments, the ã value of the conjugate in a kidney of the subject is about 2 to 24 hours. In some embodiments, the ã value of the conjugate in a kidney of the subject is more than 24 hours. In some embodiments, the ã value of the conjugate in a liver of the subject is at most 24 hours. In some embodiments, the ã value of the conjugate in a liver of the subject is at most 18 hours, 15 hours, 12 hours, 10 hours, 8 hours, 6 hours, or 5 hours. In some embodiments, the ã value of the conjugate in a liver of the subject is about 30 minutes to about 24 hours. In some embodiments, the ã value of the conjugate in a liver of the subject is about 2 to 24 hours. In some embodiments, the ã value of the conjugate in a liver of the subject is more than 24 hours. [0121] In some cases, the elimination profile of the conjugate can be adjusted by a reversible binding between the conjugate and a plasma protein such as albumin. A suitable affinity between the conjugate and the plasma protein can utilize the plasma protein as a reservoir for the conjugates, attaching and preserving the conjugates at high concentration and releasing the conjugates at a lower concentration, thereby improving elimination profile. In some embodiments, a dissociation constant (Kd) between the conjugate and human serum albumin is at most 500 µM, as determined at room temperature in human serum condition. In some embodiments, the Kd is at most 100 µM. In some embodiments, the Kd is at most 15 µM. In some embodiments, the Kd is from about 0.1 nM to about 10 µM. In some embodiments, the Kd is from about 10 nM to about 10 µM. In some embodiments, the Kd is from about 50 nM to about 1 µM. In some embodiments,
the Kd is from about 100 nM to about 10 µM. In some embodiments, the Kd is from about 500 nM to about 5 µM. [0122] In some embodiments, a conjugate described herein is bound is bound to plasma proteins. In some embodiments, a conjugate described herein is bound to albumin. In some embodiments, a conjugate described herein has a plasma protein binding percentage of between 40% to 100%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of 40% to 80%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of 40% to 60%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of 60% to 80%. In some embodiments, the conjugate described herein has a plasma protein binding percentage of about 40%, about 45%, about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%, about 95%, or about 100%. In some embodiments, a conjugate described herein has a plasma protein binding percentage of between 40% to 45%, between 45% to 50%, between 50% to 55%, between 55% to 60%, between 60% to 65%, between 70% to 75%, between 75% to 80%, or between 80% to 85%. [0123] In one aspect, provided herein are conjugates having a prescribed serum stability. In some embodiments, the human serum half-life of the conjugate is at least 0.1 hour, 0.5 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 11 hours, 12 hours, 13 hours, 14 hours, 15 hours, or 16 hours at 37 °C. In some embodiments, the human serum half- life of the conjugate is at most 48 hours, 36 hours, 24 hours, 23 hours, 22 hours, 21 hours, 20 hours, 19 hours, 18 hours, 17 hours, 16 hours, 15 hours, 14 hours, 13 hours, 12 hours, 11 hours, 10 hours, 9 hours, 8 hours, 7 hours, 6 hours, 5 hours, or 1 hour at 37 °C. In some embodiments, the human serum half-life of the conjugate is about 2 to 20 hours, 5 to 20 hours, 8 to 15 hours, or 10 to 14 hours at 37 °C. [0124] In some embodiments, a herein described conjugate is a conjugate of FIG. 19. In some embodiments, a herein described conjugate is a conjugate of FIG.20. In some embodiments, a herein described conjugate is a conjugate of FIG. 21. In some embodiments, a herein described conjugate is a conjugate of FIG. 22. In some embodiments, a herein described conjugate is a conjugate of Table 1 or Table 2. In some embodiments, the conjugate further comprises a radionuclide such as Ac-225 or Lutetium-177. In some embodiments, described herein is a conjugate that comprises a conjugate of FIGs 9-22 and a radionuclide of Table 5A or 5B. In some embodiments, described herein is a conjugate that comprises a conjugate of Table 1 and a radionuclide of Table 5A or 5B. In some embodiments, described herein is a conjugate that comprises a conjugate of Table 2 and a radionuclide of Table 5A or 5B. In some embodiments, a herein described conjugate is a conjugate of Table 6. In some embodiments, a herein described
conjugate is a conjugate of Table 7. In some embodiments, a herein described conjugate is a conjugate of Table 8. In some embodiments, a herein described conjugate is a conjugate of Table 9. Table 1. Exemplary Conjugates
[0125] It is understood that the structures of conjugates in Tables 6-7 are shown for illustration purposes. A person skilled in the art would appreciate that the bonding between the metal or radionuclide (La3+, 177Lu or 225Ac) and the metal chelator in the conjugates is for illustration purpose. [0126] A metal chelator such as DOTA can interact with a radionuclide (e.g., 177Lu or 225Ac) via one or more functional groups and/or atoms. For example, a metal chelator can interact with a
radionuclide via nitrogen and/or oxygen atoms. As another example, a metal chelator can interact with a radionuclide via carbonyl, carboxylic acid, amino, and/or amide groups of the metal chelator. In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
. In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
. In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
or
. In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
. In some embodiments, the interaction of a metal chelator and a radionuclide of the conjugates disclosed herein can be illustrated as
. In some embodiments, the radionuclide exists in a positive oxidation state e.g., 225Ac3+, 177Lu3+. In some embodiments, for example in certain aqueous conditions, the radionuclide exists in a salt form, e.g., as 225Ac3+, 177Lu3+. In some embodiments, for example in certain acidic aqueous conditions, the radionuclide exists in a salt form, e.g., as 225Ac3+, 177Lu3+. In some embodiments, the conjugate is in a salt form. In some embodiments, one or more of the carboxylic acid groups of the conjugate may exist as carboxylate anions. In some embodiments, one or more of the carboxylate anions of the conjugate may coordinate to the radionuclide. A person of ordinary skill would appreciate that the dissociation of an acid can depend on the pH value of the environment and its pK value. Accordingly, in some embodiments, a conjugate described herein can exist in a completely ionized, partially ionized or non-ionized form. Table 2. Design of Exemplary Conjugates
[0127] In Table 2, * denotes the side of a linker being connected to the N-terminus of the peptide sequence (also marked by *), while ** denotes the side of a linker being connected to the C- terminus of the peptide sequence (also marked by **).
[0128] Definitions for abbreviated compounds in Table 2 above are listed in Table 3. Table 3. Definitions of abbreviated compounds in Table 2.
[0129] In one aspect, a conjugate described herein has a structure of Formula (I), (Ia), (Iaa) or (Iab), or a salt or solvate thereof, which is further described below. Peptide Ligands [0130] In one aspect, a conjugate described herein comprises a GCC binding peptide. The GCC binding peptide can be linear or cyclic (e.g., cyclized by disulfide bond or other bonds). The cyclization can occur between the N- and C-terminus, or it can occur between a terminal amino acid and a non-terminal amino acid. In some embodiments, the cyclization occurs between two non-terminal amino acids. In some embodiments, the peptide is monocyclic. In some
embodiments, the peptide is bicyclic or polycyclic. The peptide can comprise any suitable number of amino acid residues. In some embodiments, the peptide comprises from 5 to 50, 6 to 40, 7 to 30, 8 to 25, 12 to 25, or 9 to 20 amino acid residues. In some embodiments, the peptide comprises from 10 to 15 amino acid residues. In some embodiments, the peptide comprises from 12 to 15 amino acid residues. In some embodiments, the peptide comprises from 13 to 16 amino acid residues. In some embodiments, the peptide comprises 9 amino acid residues. In some embodiments, the peptide comprises 10 amino acid residues. In some embodiments, the peptide comprises 11 amino acid residues. In some embodiments, the peptide comprises 12 amino acid residues. In some embodiments, the peptide comprises 13 amino acid residues. In some embodiments, the peptide comprises 14 amino acid residues. In some embodiments, the peptide comprises 15 amino acid residues. In some embodiments, the peptide comprises 16 amino acid residues. In some embodiments, the peptide comprises 17 amino acid residues. In some embodiments, the peptide comprises 18 amino acid residues. In some embodiments, the peptide comprises 16 to 25 amino acid residues. In some embodiments, the peptide comprises 9 amino acid residues. In some embodiments, the peptide consists of 10 amino acid residues. In some embodiments, the peptide consists of 11 amino acid residues. In some embodiments, the peptide consists of 12 amino acid residues. In some embodiments, the peptide consists of 13 amino acid residues. In some embodiments, the peptide consists of 14 amino acid residues. In some embodiments, the peptide consists of 15 amino acid residues. In some embodiments, the peptide consists of 16 amino acid residues. In some embodiments, the peptide consists of 17 amino acid residues. [0131] In some embodiments, the GCC binding peptide is an endogenous GCC ligand, e.g., guanylin and uroguanylin. In some embodiments, the GCC binding peptide is an enterotoxin, e.g., enterotoxigenic E. coli, K. pneumonia, V. cholera, and Y. enterocolitica. In some embodiments, the GCC binding peptide is a synthetic peptide, e.g., linaclotide, plecanatide and dolcantide. Exemplary GCC binding peptides include, but are not limited to linaclotide, plecantide, dolcantide, E. coli ST, guanylin, Uroguanylin, limphoguanylin, E.coli STp, E.coli EAST1, V. cholerae STh, V. mimicus ST, Y. enterocolitica ST, and guanylin. GCC binding peptides are further provided in Lin et al., Toxins 2010, 2, 2028-2054 and Aka et al., Expert Rev Clin Pharmacol.2017 May ; 10(5): 549–557, each of which is hereby incorporated by reference in its entirety. In some embodiments, the GCC binding peptide is linaclotide or a derivative thereof. [0132] In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 50% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that
has at least 70% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 80% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 90% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 95% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, or a binding fragment thereof. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 70% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 80% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 90% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 95% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-19, or a binding fragment thereof. In some embodiments, the GCC binding peptide comprises any one of sequences in FIGs. 1A-1J, or a binding fragment thereof. In some embodiments, the GCC binding peptide comprises any one of sequences in Table 2. Table 4. Amino acid sequences for exemplary GCC binding peptides
[0133] In some embodiments, the GCC binding peptide comprises SEQ ID NO: 1. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 2. In some embodiments, the
GCC binding peptide comprises SEQ ID NO: 3. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 4. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 5. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 6. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 7. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 8. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 9. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 10. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 11. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 12. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 13. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 14. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 15. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 16. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 17. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 18. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 19. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 20. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 21. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 22. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 23. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 24. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 25. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 26. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 27. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 28. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 29. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 30. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 31. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 32. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 33. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 34. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 35. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 36. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 37. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 38. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 39. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 40. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 41. In some embodiments, the GCC binding peptide comprises SEQ ID NO: 42. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 1. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 2. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 3. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 4. In some
embodiments, the GCC binding peptide consists of SEQ ID NO: 5. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 6. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 7. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 8. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 9. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 10. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 11. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 12. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 13. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 14. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 15. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 16. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 17. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 18. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 19. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 20. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 21. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 22. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 23. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 24. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 25. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 26. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 27. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 28. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 29. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 30. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 31. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 32. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 33. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 34. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 35. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 36. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 37. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 38. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 39. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 40. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 41. In some embodiments, the GCC binding peptide consists of SEQ ID NO: 42. In some embodiments, one or more amino acids in any one of the SEQ ID NOs: 1-42 are derivatized, modified, and/or replaced with an unnatural amino acid. [0134] Percent sequence identity can be calculated using computer programs or direct sequence comparison. Preferred computer program methods to determine identity between two sequences include, but are not limited to, the GCG program package, FASTA, BLASTP, and TBLASTN (see,
e.g., D. W. Mount, 2001, Bioinformatics: Sequence and Genome Analysis, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.). The BLASTP and TBLASTN programs are publicly available from NCBI and other sources. The Smith Waterman algorithm can also be used to determine percent identity. Exemplary parameters for amino acid sequence comparison include the following: 1) algorithm from Needleman and Wunsch (J. Mol. Biol., 48:443-453 (1970)); 2) BLOSSUM62 comparison matrix from Hentikoff and Hentikoff (Proc. Nat. Acad. Sci. USA., 89:10915-10919 (1992)) 3) gap penalty=12; and 4) gap length penalty=4. A program useful with these parameters can be publicly available as the “gap” program (Genetics Computer Group, Madison, Wis.). The aforementioned parameters are the default parameters for polypeptide comparisons (with no penalty for end gaps). Alternatively, polypeptide sequence identity can be calculated using the following equation: % identity—(the number of identical residues)/(alignment length in amino acid residues)*100. For this calculation, alignment length includes internal gaps but does not include terminal gaps. [0135] In some embodiments, the GCC binding peptide described herein is a GCC receptor agonist. Exemplary GCC receptor agonists are described in U.S. Pat. Nos.7,041,786, 7,799,897, and U.S. Patent Application Publication Nos. US2009/0048175, US 2010/0069306, US 2010/0120694, US 2010/0093635, and US 2010/0221329. [0136] A peptide described herein can be a peptide mimetic. For example, the peptide can comprise non-peptide bonds and it can comprise one or more unnatural amino acids. Unless stated otherwise, each of the amino acid in a peptide described herein (except the natural amino acid glycine) can independently be in its D or L form. Both D and L forms are encompassed by the present disclosure. [0137] In the present disclosure, the term amino acid embraces derivatives of amino acids. The derivatives include, for example, amino acids obtained by modifying a natural amino acid constituting a protein produced by cellular DNA-encoded biological matter. Examples of such non-natural amino acids include hydroxyproline and hydroxylysine, which are amino acids having a hydroxyl group introduced therein, and diaminopropionic acid, which is an amino acid having an amino group introduced therein. Examples of the derivatives also include derivatives having an amide, ester, or carboxyl group as the C-terminus and/or N-terminus thereof. Additional examples of the derivatives of the peptide include those obtained by modification such as phosphorylation, methylation, acetylation, adenylylation, ADP-ribosylation, or glycosylation and fused protein obtained by fusion with another peptide or protein. These derivatives can be prepared by those skilled in the art in a known manner or a method based thereon. [0138] Examples of the amino acids include natural protein L-amino acids, unnatural amino acids, and chemically synthesized compounds having properties known in the art as characteristics
of an amino acid. Examples of the unnatural amino acids include, but not limited to, α,α- disubstituted amino acids (such as α-methylalanine), N-alkyl-α-amino acids, D-amino acids, β- amino acids, and α-hydroxy acids, each having a backbone structure different from that of natural amino acids; amino acids (such as norleucine and homohistidine) having a side-chain structure different from that of natural amino acids; amino acids (such as “homo” amino acids, homophenylalanine, and homohistidine) having extra methylene in the side chain thereof; and amino acids (such as cysteic acid) obtained by substituting a carboxylic acid functional amino group in the side chain thereof by a sulfonic acid group. [0139] Accordingly, each amino acid residue of the GCC binding peptide can be independently derivatized. For example, the N- and/or C-terminus of the residue of any of the sequences in Table 4 can be replaced with a derivatized and/or unnatural amino acid. Modifications of the amino acid residues can improve the properties of the peptide (e.g., stability) and the conjugate comprising the peptide. In some embodiments, the GCC binding peptide comprises one or more D-amino acids. In some embodiments, the GCC binding peptide comprises one or more β-amino acids. In some embodiments, the GCC binding peptide comprises N-alkylation (such as N-methylation). In some embodiments, the GCC binding peptide comprises one or more polyethylene glycol chains. In some embodiments, the GCC binding peptide is cyclized. In some embodiments, the GCC binding peptide comprises an amide to sulfonamide substitution. In some embodiments, the GCC binding peptide comprises terminal modification. [0140] A binding peptide (e.g., GCC binding peptide) described herein can be modified internally, and/or at N-terminus, C-terminus or both. In some embodiments, the GCC binding peptide comprises N-terminal methylation, N-terminal acetylation, N-terminal propionylation, N- terminal myristoylation, or N-terminal palmitoylation. In some embodiments, the GCC binding peptide comprises C-terminal methylation, C-terminal acetylation, C-terminal propionylation, C- terminal myristoylation, or C-terminal palmitoylation. In some embodiments, the GCC binding peptide comprises N-terminal modification such as amidation. In some embodiments, the GCC binding peptide comprises C-terminal modification such as amidation. In some embodiments, the GCC binding peptide comprises internal modification such as cyclization, carbamidomethylation, phosphorylation, or PEGylation. In some embodiments, the modification can function to increase the bulkiness of the terminal amino acid thereby improving metabolic stability. In some embodiments, the modification increases half-life and/or proteolysis stability. Exemplary C- terminal modification on a tyrosine residue (e.g., peptide having SEQ ID NO: 1) includes D- tyrosine, N-methyl-L-tyrosine, α-methyl-L-tyrosine, L-tyrosinamide, O-methyl-L-tyrosine, O-4- allyl-L-tyrosine, 4-propyl-L-tyrosine, O-propargyltyrosine, etc. In some embodiments, the C- terminal modification on a tyrosine residue is D-tyrosine. In some embodiments, the C-terminal
modification on a tyrosine residue is N-methyl-L-tyrosine. In some embodiments, the C-terminal modification on a tyrosine residue is α-methyl-L-tyrosine. [0141] A GCC binding peptide described herein can be modified by cyclization. In some embodiments, the cyclization occurs between the N- and C-terminus. In some embodiments, the cyclization occurs between a terminal amino acid and a non-terminal amino acid. In some embodiments, the cyclization occurs between two non-terminal amino acids. In some embodiments, a GCC binding peptide described herein comprises 1, 2 or 3 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises 2 or 3 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises 2 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises 3 intramolecular disulfide bonds. In some embodiments, a GCC binding peptide comprises one intramolecular alkylene or alkenylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular alkylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular heteroalkylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular alkenylene bond. In some embodiments, a GCC binding peptide comprises an intramolecular bond that is bridged by a triazole. In some embodiments, a GCC binding peptide described herein comprises two intramolecular disulfide bonds and one intramolecular heteroalkene, alkylene or alkenylene bond. In some embodiments, the intramolecular bond comprises a triazole bridge. In some embodiments, a GCC binding peptide comprises intramolecular alkylene bridge. In some embodiments, a GCC binding peptide comprises intramolecular heteroalkylene bridge. In some embodiments, the alkylene bridge is C2-C10 alkylene. In some embodiments, the alkylene bridge is C3-C8 alkylene. In some embodiments, the alkylene bridge is C3-C6 alkylene. In some embodiments, the bridge is C2-C10 heteroalkylene. In some embodiments, the bridge is C3-C8 heteroalkylene. In some embodiments, the bridge is C3-C6 heteroalkylene. In some embodiments, the alkylene bridge is C3 alkylene. In some embodiments, the alkylene bridge is C4 alkylene. In some embodiments, a GCC binding peptide comprises intramolecular heteroalkylene bridge. In some embodiments, the heteroalkylene bridge is C2-C10 heteroalkylene. In some embodiments, the heteroalkylene bridge is C2-C8 heteroalkylene. In some embodiments, the heteroalkylene bridge is C2-C4 heteroalkylene. In some embodiments, the heteroalkylene bridge is C3 heteroalkylene. In some embodiments, the heteroalkylene bridge is C4 heteroalkylene. In some embodiments, the heteroalkylene bridge comprises 1, 2, or 3 atoms each independently selected from O, N, P, S, and Se. In some embodiments, the heteroalkylene bridge comprises S. In some embodiments, the heteroalkylene bridge comprises Se.
[0142] In some embodiments, the heteroalkylene, alkylene or alkenylene is optionally substituted with one or more R10, wherein each R10 is independently halogen, amino, -OH, -SH, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R10 on the same atoms are taken together to form a cycloalkyl or heterocycloalkyl; or two R10 on different atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl. [0143] In some embodiments, two R10 adjacent atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl. In some embodiments, two R10 adjacent atoms are taken together to form a heteroaryl such as triazole. In some embodiments, the intramolecular bridge is or
. [0144] In some embodiments, a cyclized GCC binding peptide comprises a structure selected from:
. [0145] In some embodiments, a cyclized GCC binding peptide comprises a structure of
C-28. In some embodiments, a cyclized GCC binding
peptide comprises a structure of
C-29. In some embodiments, a cyclized GCC binding peptide comprises a structure of
C-66. In some embodiments, a cyclized GCC binding peptide comprises a structure of
C-69. In some embodiments, a cyclized GCC binding peptide comprises a structure of
In some embodiments, a cyclized GCC binding peptide comprises a structure of
C-85. In some embodiments, a cyclized GCC binding peptide comprises a structure of
C-86. In some embodiments, a cyclized GCC binding peptide comprises a structure of
In some embodiments, a cyclized GCC binding peptide comprises a structure of
[0146] A peptide having SEQ ID NO: 1 or a fragment thereof can contain one or more intramolecular bonds. A peptide having SEQ ID NO: 1-42 or a fragment thereof can contain one or more intramolecular bonds. Similarly, a peptide of Tables 2 and 4 can contain one or more intramolecular bonds. For example, the peptide can comprise one or more of Cys1 - Cys6 disulfide bond, Cys2 -Cys10 disulfide bond, and Cys5 - Cys13 disulfide bonds. In some embodiments, the peptide comprises Cys1 -Cys6 alkylene, heteroalkylene, or alkenylene bond. In some embodiments, the peptide comprises Cys2 - Cys10 alkylene, heteroalkylene or alkenylene bond. In some embodiments, the peptide comprises Cys5 - Cys13 alkylene, heteroalkylene, or alkenylene bond. In some embodiments, the intramolecular bond comprises a triazole bridge. [0147] Peptide ligands disclosed herein can contain two or more cysteine residues. For example, a peptide sequence of Table 2 or 4 can contain two or more cysteine residues, e.g., 2, 3, 4, 5, or 6 cysteine residues. In some embodiments, the first cysteine residue in the sequence forms a disulfide bond with the 4th cysteine residue in the same sequence. In some embodiments, the second cysteine residue in the sequence forms a disulfide bond with the 5th cysteine residue in the same sequence. In some embodiments, the third cysteine residue in the sequence forms a disulfide bond with the 6th cysteine residue in the same sequence. [0148] In one aspect, provided herein are GCC binding peptides that have improved metabolic, chemical and/or physical stability against a variety of degradation mechanisms. In some embodiments, the GCC binding peptide is more resistant to carboxypeptidase hydrolysis than linaclotide. In some embodiments, the GCC binding peptide is more resistant to protease degradation than linaclotide. In some embodiments, the human serum half-life of a GCC binding peptide described herein is at least 100%, 120%, 150%, 200%, or 300% compared to the human serum half-life of linaclotide at 37 °C. In some embodiments, the human serum half-life of a GCC binding peptide described herein is at least 1.1, 1.2, 1.5, 2.0, 3.0, 5.0 or 10 fold compared to the human serum half-life of linaclotide at 37 °C. [0149] In some embodiments, a peptide described herein has a molecular weight from 500 Da to 10,000 Da. In some embodiments, a peptide described herein has a molecular weight from 500 Da to 5,000 Da. In some embodiments, a peptide described herein has a molecular weight from 500 Da to 3,000 Da. In some embodiments, the peptide has a molecular weight from 1,000 Da to 1,500 Da. In some embodiments, the peptide has a molecular weight from 1,500 Da to 2,000 Da. In some embodiments, the peptide has a molecular weight from 2,000 Da to 2,500 Da. In some embodiments, the peptide has a molecular weight from 2,500 Da to 3,000 Da. In some embodiments, the peptide has a molecular weight from 3,000 Da to 3.500 Da. In some embodiments, the peptide has a molecular weight from 3.500 Da to 4,000 Da. In some embodiments, the peptide has a molecular weight from 4,000 Da to 4,500 Da. In some
embodiments, the peptide has a molecular weight from 4,500 Da to 5,000 Da. In some embodiments, the peptide has a molecular weight from 5,000 Da to 5,500 Da. In some embodiments, the peptide has a molecular weight from 5,500 Da to 6,000 Da. In some embodiments, the peptide has a molecular weight from 6,000 Da to 6,500 Da. In some embodiments, the peptide has a molecular weight from 6,500 Da to 7,000 Da. In some embodiments, the peptide has a molecular weight of at least 500 Da, 800 Da, 1,000 Da, 1,200 Da, 1,400 Da, 1,600 Da, 1,800 Da, 2,000 Da, 2,200 Da, 2,400 Da, 2,600 Da, 2,800 Da, 3,000 Da, 3,200 Da, 3,400 Da, 3,600 Da, 3,800 Da, 4,000 Da, 4,200 Da, 4,400 Da, 4,600 Da, 4,800 Da, 5,000 Da, 5,200 Da, 5,400 Da, 5,600 Da, 5,800 Da, 6,000 Da, 6,200 Da, 6,400 Da, 6,600 Da, 6,800 Da, or 7,000 Da. In some embodiments, the peptide has a molecular weight of at most 600 Da, 800 Da, 1,000 Da, 1,200 Da, 1,400 Da, 1,600 Da, 1,800 Da, 2,000 Da, 2,200 Da, 2,400 Da, 2,600 Da, 2,800 Da, 3,000 Da, 3,200 Da, 3,400 Da, 3,600 Da, 3,800 Da, 4,000 Da, 4,200 Da, 4,400 Da, 4,600 Da, 4,800 Da, 5,000 Da, 5,200 Da, 5,400 Da, 5,600 Da, 5,800 Da, 6,000 Da, 6,200 Da, 6,400 Da, 6,600 Da, 6,800 Da, 7,000 Da, or 7,500 Da. [0150] In some embodiments, a cyclic GCC binding peptide described herein has a net charge of -3 to +1. In some embodiments, the cyclic peptide has a net charge of -3. In some embodiments, the cyclic peptide has a net charge of -2. In some embodiments, the cyclic peptide has a net charge of -1. In some embodiments, the cyclic peptide has a net charge of 0. In some embodiments, the cyclic peptide has a net charge of +1. The net charge can be determined by aggregating the charge of each of the amino acids in the peptide. For example, aspartic acid (D) and glutamic acid (E) each has a charge of -1, lysine (K), arginine (R) and histidine (H) each has a charge of +1, and the rest of the canonical amino acids each has a charge of 0. [0151] In some embodiments, a cyclic GCC binding peptide described herein has a net charge of at most -4. In some embodiments, the cyclic peptide has a net charge of -4. In some embodiments, a cyclic GCC binding peptide described herein has a net charge of at least +2. In some embodiments, the cyclic peptide has a net charge of +2. In some embodiments, the cyclic peptide has a net charge of +3. [0152] In some embodiments, a GCC binding peptide comprises unnatural amino acid. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, wherein the amino acid sequence of any one of SEQ ID NOs: 1-30 comprises unnatural amino acid. In some embodiments, C-terminus of a GCC binding peptide comprises unnatural amino acid. In some embodiments, a GCC binding peptide comprises an amino acid sequence of any one of SEQ ID NOs: 1-42, wherein C-terminus of the amino acid sequence of any one of SEQ ID NOs: 1-42 comprises unnatural amino acid.
[0153] The peptides described herein can comprise one or more unnatural amino acids. Non- limiting examples of unnatural amino acids include: p-acetyl-L-phenylalanine, p-iodo-L- phenylalanine, p-methoxyphenylalanine, O-methyl-L-tyrosine, p-propargyloxyphenylalanine, p- propargyl-phenylalanine, L-3-(2-naphthyl)alanine, 3-methyl-phenylalanine, O-4-allyl-L-tyrosine, 4-propyl-L-tyrosine, tri-O-acetyl-GlcNAcp-serine, L-Dopa, fluorinated phenylalanine, isopropyl- L-phenylalanine, p-azido-L-phenylalanine, p-acyl-L-phenylalanine, p-benzoyl-L-phenylalanine, Boronophenylalanine, O-propargyltyrosine, L-phosphoserine, phosphonoserine, phosphonotyrosine, p-bromophenylalanine, selenocysteine, p-amino-L- phenylalanine, isopropyl- L-phenylalanine, 3,4-dehydro-proline, and azido-lysine (AzK). In some embodiments, the unnatural amino acid is an unnatural analogue of a tyrosine amino acid; an unnatural analogue of a glutamine amino acid; an unnatural analogue of a phenylalanine amino acid; an unnatural analogue of an alanine amino acid; an unnatural analogue of a serine amino acid; an unnatural analogue of a threonine amino acid; an alkyl, aryl, acyl, azido, cyano, halo, hydrazine, hydrazide, hydroxyl, alkenyl, alkynl, ether, thiol, sulfonyl, seleno, ester, thioacid, borate, boronate, phospho, phosphono, phosphine, heterocyclic, enone, imine, aldehyde, hydroxylamine, keto, or amino substituted amino acid; or a combination thereof. In some embodiments, the unnatural amino acid is an amino acid with a photoactivatable cross-linker; a spin-labeled amino acid; a fluorescent amino acid; a metal binding amino acid; a metal-containing amino acid; a radioactive amino acid; a photocaged and/or photoisomerizable amino acid; a biotin or biotin-analogue containing amino acid; a keto containing amino acid; an amino acid comprising polyethylene glycol or polyether; a heavy atom substituted amino acid; a chemically cleavable or photocleavable amino acid; an amino acid with an elongated side chain; an amino acid containing a toxic group; a sugar substituted amino acid; a carbon-linked sugar-containing amino acid; a redox-active amino acid; an a-hydroxy containing acid; an amino thio acid; an α, α-disubstituted amino acid; a β-amino acid; a cyclic amino acid other than proline or histidine, or an aromatic amino acid other than phenylalanine, tyrosine or tryptophan. [0154] In some embodiments, the unnatural amino acids incorporated into the peptides include one or more of: 1) a ketone functional group (as found in para or meta acetyl-phenylalanine) that can be specifically reacted with hydrazines, hydroxylamines and their derivatives (Addition of the keto functional group to the genetic code of Escherichia coli. Wang L, Zhang Z, Brock A, Schultz P G. Proc Natl Acad Sci USA.2003 Jan.7; 100(1):56-61; Bioorg Med Chem Lett.2006 Oct.15; 16(20):5356-9. Genetic introduction of a diketone-containing amino acid into proteins. Zeng H, Xie J, Schultz P G), 2) azides (as found in p-azido-phenylalanine) that can be reacted with alkynes via copper catalyzed “click chemistry” or strain promoted (3+2) cycloadditions to form the corresponding triazoles (Addition of p-azido-L-phenylalanine to the genetic code of Escherichia
coli. Chin J W, Santoro S W, Martin A B, King D S, Wang L, Schultz P G. J Am Chem Soc.2002 Aug. 7; 124(31):9026-7; Adding amino acids with novel reactivity to the genetic code of Saccharomyces cerevisiae. Deiters A, Cropp T A, Mukherji M, Chin J W, Anderson J C, Schultz P G. J Am Chem Soc. 2003 Oct. 1; 125(39):11782-3), or azides that can be reacted with aryl phosphines, via a Staudinger ligation (Selective Staudinger modification of proteins containing p- azidophenylalanine. Tsao M L, Tian F, Schultz P G. Chembiochem.2005 December; 6(12):2147- 9), to form the corresponding amides, 3) alkynes that can be reacted with azides to form the corresponding triazole (In vivo incorporation of an alkyne into proteins in Escherichia coli. Deiters A, Schultz P G. Bioorg Med Chem Lett.2005 Mar.1; 15(5):1521-4), 4) boronic acids (boronates) than can be specifically reacted with compounds containing more than one appropriately spaced hydroxyl group or undergo palladium mediated coupling with halogenated compounds (Angew Chem Int Ed Engl.2008; 47(43):8220-3. A genetically encoded boronate-containing amino acid., Brustad E, Bushey M L, Lee J W, Groff D, Liu W, Schultz P G), and 5) metal chelating amino acids, including those bearing bipyridyls, that can specifically co-ordinate a metal ion (Angew Chem Int Ed Engl.2007; 46(48):9239-42. A genetically encoded bidentate, metal-binding amino acid. Xie J, Liu W, Schultz P G). [0155] The peptide of the present disclosure embraces various derivatives thereof. Examples of the derivatives include derivatives having an amide, ester, or carboxyl group as the C-terminus and/or N-terminus thereof. Additional examples of the derivatives of the peptide include those obtained by modification such as phosphorylation, methylation, acetylation, adenylylation, ADP- ribosylation, or glycosylation and fused protein obtained by fusion with another peptide or protein. These derivatives can be prepared by those skilled in the art in a known manner or a method based thereon. [0156] In some embodiments, the binding peptide is bicyclic or polycyclic. In some embodiments, a conjugate described herein comprises a bicyclic peptide. Exemplary bicyclic peptides include the bicyclic targeting peptides of BT5528, BT1718, and BT8009. Exemplary bicyclic peptides are described in US20180200378, US10441663, US8680022B2, US20180280525, and US20200215199, each of which is hereby incorporated by reference in its entirety. [0157] In some embodiments, the binding peptide is a lasso peptide. Lasso peptides can be synthetic or naturally produced by bacteria, and they possess a distinctive threaded lariat fold that offers a 3D array of functionality for engaging biological targets. This lasso structure can enable beneficial properties such as affinity, stability and potent biological activities. Suitable lasso structure can be designed by algorithms. Exemplary lasso peptides are provided in Hegemann, J.D., et al., Lasso Peptides: An Intriguing Class of Bacterial Natural Products, Acc. Chem. Res.,
2015, 48, 1909−1919; Tietz, J.I., et al., A new genome-mining tool redefines the lasso peptide biosynthetic landscape, Nature Chem Bio, 2017, 13, 470-478; DiCaprio, A.J., et al., Enzymatic Reconstitution and Biosynthetic Investigation of the Lasso Peptide Fusilassin, J. Am. Chem. Soc., 2019, 141, 290−297; Al Toma, R.S., et al., Site-Directed and Global Incorporation of Orthogonal and Isostructural Noncanonical Amino Acids into the Ribosomal Lasso Peptide Capistruin, ChemBioChem, 2015, 16, 503–509. [0158] In some embodiments, a cyclic peptide described herein is configured to bind to a plasma protein with a prescribed affinity, for example, measured as Plasma Protein Albumin Binding (PPB) percentage. The % bound can be determined by HSA-HPLC method (measurement of drug protein binding by immobilized human serum albumin-HPLC). PPB can be determined in vitro by HPLC (e.g., Example B4) or by other suitable means known in the art. In some embodiments, 1% to 99% of the cyclic peptide binds to Human Serum Albumin (HSA) in vitro as determined by HPLC, according to the conditions described in Example B3. In some embodiments, about 2% to about 99%, about 5% to about 99%, about 10% to about 99%, about 20% to about 99%, about 30% to about 99%, about 40% to about 99%, about 50% to about 99%, about 60% to about 99%, about 70% to about 99%, or about 80% to about 99% of the cyclic peptide binds to HSA in vitro as determined by HPLC. In some embodiments, about 10% to about 95% of the cyclic peptide binds to HSA in vitro (i.e., PPB of about 10% to about 95%). In some embodiments, about 20% to about 90% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 20% to about 60% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 95% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 80% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 60% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 99% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 95% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 80% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 60% to about 70% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 40% to about 50% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 50% to about 60% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 70% to about 80% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 80% to about 99% of the cyclic peptide binds to HSA in vitro. In some embodiments, about 80% to about 85% of the cyclic peptide binds to HSA in vitro. [0159] In some embodiments, a conjugate described herein (e.g., a conjugate of Table 1 or Table 2) is configured to bind to a plasma protein with a prescribed affinity, for example, measured as Plasma Protein Albumin Binding (PPB) percentage. The % bound can be determined by HSA-
HPLC method (measurement of drug protein binding by immobilized human serum albumin- HPLC). PPB can be determined in vitro by HPLC (e.g., Example B4) or by other suitable means known in the art. In some embodiments, 1% to 99% of the conjugate binds to Human Serum Albumin (HSA) in vitro as determined by HPLC, according to the conditions described in Example B3. In some embodiments, about 2% to about 99%, about 5% to about 99%, about 10% to about 99%, about 20% to about 99%, about 30% to about 99%, about 40% to about 99%, about 50% to about 99%, about 60% to about 99%, about 70% to about 99%, or about 80% to about 99% of the conjugate binds to HSA in vitro as determined by HPLC. In some embodiments, about 10% to about 95% of the conjugate binds to HSA in vitro (i.e., PPB of about 10% to about 95%). In some embodiments, about 20% to about 90% of the conjugate binds to HSA in vitro. In some embodiments, about 20% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 95% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 99% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 95% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 60% to about 70% of the conjugate binds to HSA in vitro. In some embodiments, about 40% to about 50% of the conjugate binds to HSA in vitro. In some embodiments, about 50% to about 60% of the conjugate binds to HSA in vitro. In some embodiments, about 70% to about 80% of the conjugate binds to HSA in vitro. In some embodiments, about 80% to about 99% of the conjugate binds to HSA in vitro. In some embodiments, about 80% to about 85% of the conjugate binds to HSA in vitro. Target [0160] Guanylate cyclase C (GCC) is a transmembrane form of guanylate cyclase that is expressed on various cells, including gastrointestinal epithelial. It was originally discovered as the intestinal receptor for the heat-stable toxin (ST) peptides secreted by enteric bacteria and which cause diarrhea. The ST peptides share a similar primary amino acid structure with two peptides isolated from intestinal mucosa and urine, guanylin and uroguanylin. [0161] In the intestines, guanylin and uroguanylin act as regulators of fluid and electrolyte balance. In response to high oral salt intake, these peptides are released into the intestinal lumen where they bind to guanylate cyclase C localized on the luminal membrane of enterocytes (simple columnar epithelial cells of the small intestines and colon). The binding of the guanylin peptides to guanylate cyclase C induces electrolyte and water excretion into the intestinal lumen via a
complex intracellular signaling cascade that is initiated by an increase in cyclic guanosine monophosphate (cGMP). The cGMP-mediated signaling that is initiated by the guanylin peptides can be important for the normal functioning of the gut. Any abnormality in this process could lead to gastrointestinal disorders. In addition to a role for uroguanylin and guanylin as modulators of intestinal fluid and ion secretion, these peptides may also be involved in the continual renewal of gastrointestinal mucosa by maintaining the balance between proliferation and apoptosis. For example, uroguanylin and guanylin peptides can promote apoptosis by controlling cellular ion flux. Given the prevalence of inflammatory conditions in Western societies a need exists to improve the treatment options for inflammatory conditions, particularly of the gastrointestinal tract. [0162] Further, GCC as a tumor suppressor expressed by the intestinal epithelium, can be a target for the treatment of certain cancers. GCC is densely expressed throughout the intestine, and overexpressed by tumor tissue, which can enable GCC as a suitable target for diagnostic and therapeutic goals. Linkers [0163] A conjugate described herein can comprise one or more linkers. In some embodiments, the linker covalently attaches the GCC binding peptide with the metal chelator. In some embodiments, the GCC binding peptide attaches directly to the metal chelator without a linker. [0164] In some embodiments, the present disclosure describes linkers that function as a spacer. A linker can comprise a number of intervening atoms (on a linear chain, excluding pendant groups or substituents) between the metal chelator and the binding peptide thereby creating a distance between the metal chelator and the binding peptide. In some embodiments, a linker comprises 10- 100 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 2-60 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 2 to 20, 2 to 50, 5 to 15, 5 to 25, 10 to 40, 30 to 60, or 10 to 20 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 3 to 30 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 5 to 25 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 6 to 18 intervening atoms between the metal chelator and the binding peptide. In some embodiments, a linker comprises 10 to 20 intervening atoms between the metal chelator and the binding peptide. The intervening atoms can comprise 1 or more carbons, and optionally one or more heteroatoms such as O and N. In some embodiments, the intervening atoms comprise 2 to 20, 2 to 50, 5 to 15, 5 to 25, 10 to 40, 30 to 60, or 10 to 20 carbons. In some embodiments, the intervening atoms
comprise 0, 1, 2, 3, 4, 5, or 6 nitrogen. In some embodiments, the intervening atoms comprise 0, 1, 2, 3, 4, 5, 6, 7 or 8 oxygen. [0165] As an example, the intervening atoms between the metal chelator and the binding peptide of conjugate C-035 are illustrated in brackets below and there are 16 intervening atoms.
. [0166] A linker can comprise one or more amino acid residues. In some embodiments, the linker comprises 1 to 3, 1 to 5, 1 to 10, 5 to 10, or 5 to 20 amino acid residues. In some embodiments, the linker comprises 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 amino acid residues. In some embodiments, the linker comprises 1 to 5 amino acid residues. For example, the linker can comprise one or more lysine (K) residues such as K, KK, or KKK sequences. In some embodiments, one or more amino acids of the linker are unnatural amino acids. [0167] A herein-described linker can attach to the N-terminus of the peptide, the C-terminus of the peptide, or a non-terminal amino acid of the peptide, or it can attach to the peptide through a combination of the above. In some embodiments, a linker is attached to the GCC binding peptide via its N-terminus. In some embodiments, a linker is attached to the GCC binding peptide via its C-terminus. In some embodiments, a linker is attached to the GCC binding peptide via its N- terminus and via its C-terminus. In some embodiments, a linker is attached to the peptide via a non-terminal amino acid. A conjugate described herein can comprise 2, 3, 4, 5 or more linkers. In some embodiments, the conjugate comprises two linkers, one attached to the N-terminus of the peptide and the other attached to the C-terminus of the peptide. In some embodiments, the conjugate comprises two linkers, one attached to the N or C-terminus of the peptide and the other attached to a non-terminal amino acid of the peptide. [0168] The linker can be bonded to the peptide, the metal chelator, or both, for example, through a chemically reactive group. Exemplary chemically reactive groups include, but are not limited to, a free amino, imino, hydroxyl, thiol or carboxyl group (e.g., to the N- or C-terminus, to the epsilon amino group of one or more lysine residues, the free carboxylic acid group of one or more glutamic acid or aspartic acid residues, or to the sulfhydryl group of one or more cysteinyl
residues). The site to which the linker is bound to the peptide can be a natural or unnatural amino acid of the peptide and/or it can be introduced into the peptide, e.g., by DNA recombinant technology (e.g., by introducing a cysteine or protease cleavage site in the amino acid sequence) or by protein biochemistry (e.g., reduction, pH adjustment or proteolysis). Exemplary methods for attaching the linker includes carbodiimide reaction, reactions using bifunctional agents such as dialdehydes or imidoesters, Schiff base reaction, Suzuki-Miyaura cross-coupling reactions, Isothiocyanates as coupling agents, and click chemistry. [0169] The linker can have a prescribed length thereby linking the metal chelator (and optionally radionuclide) and the peptide while allowing an appropriate distance therebetween. In some embodiments, the linker has 1 to 100 atoms, 1 to 60 atoms, 1 to 30 atoms, 1 to 15 atoms, 1 to 10 atoms, 1 to 5, or 2 to 20 atoms in length. In some embodiments, the linker has 1 to 10 atoms in length. [0170] The linker can function as a “spacer” between the GCC binding peptide and the radionuclide. In some embodiments, the linker is configured to provide a distance between the GCC binding peptide and the metal chelator of at least 3 atoms, 4 atoms, 5 atoms, 6 atoms, 7 atoms, 8 atoms, 9 atoms, 10 atoms, 15 atoms, 20 atoms, or 25 atoms in length. In some embodiments, the linker comprises an alkylene or heteroalkylene bond moiety between the GCC binding peptide and the metal chelator. In some embodiments, the alkylene or heteroalkylene comprises at least 3 atoms, 4 atoms, 5 atoms, 6 atoms, 7 atoms, 8 atoms, 9 atoms, 10 atoms or 20 atoms in length. In some embodiments, the linker comprises one or more polyethylene glycol units between the GCC binding peptide and the metal chelator. In some embodiments, the linker comprises at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, or at least 10 – (CH2-CH2-O)- units between the GCC binding peptide and the metal chelator, wherein the units can be contiguous or noncontiguous. [0171] The linker can comprise flexible and/or rigid regions. Exemplary flexible linker regions include those comprising Gly and Ser residues (“GS” linker), glycine residues, alkylene chain, PEG chain, etc. Exemplary rigid linker regions include those comprising alpha helix-forming sequences (e.g., EAAAK (SEQ ID NO: 45)), proline-rich sequences, and regions rich in double and/or triple bonds. [0172] The linker can be cleavable, e.g., under physiological conditions, e.g., under intracellular conditions, such that cleavage of the linker releases the chelator and radionuclide in the intracellular environment. The linker can be, e.g., a peptidyl linker that is cleaved by an intracellular peptidase or protease enzyme, including, but not limited to, a lysosomal or endosomal protease. In some embodiments, the peptidyl linker is at least two amino acids long or at least three amino acids long. Cleaving agents can include cathepsins B and D and plasmin. In other
embodiments, the linker is not cleavable. In some embodiments, the linker is pH-sensitive, i.e., sensitive to hydrolysis at certain pH values. For example, the pH-sensitive linker can be hydrolyzable under acidic conditions. For example, a linker can be an acid-labile linker that is hydrolyzable in the lysosome (e.g., a hydrazone, semicarbazone, thiosemicarbazone, cis-aconitic amide, orthoester, acetal, ketal, or the like). Such linkers can be relatively stable under neutral pH conditions, such as those in the blood, but are unstable at below pH 5.5 or 5.0, the approximate pH of the lysosome. In some embodiments, the hydrolyzable linker is a thioether linker. [0173] In some embodiments, the linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. In some embodiments, the linker comprises substituted or unsubstituted C1-C30 alkylene. In some embodiments, the linker comprises polyethylene glycol such as (-CH2- CH2-O-)1-10. In some embodiments, the linker comprises a structure selected from:
, and structures derived from any one thereof.
[0174] In some embodiments, the linker comprises a click chemistry residue. In some embodiments, the linker is attached to the peptide, to the metal chelator, or both via click chemistry, thereby forming a click chemistry residue. For example, the peptide can comprise an azide group (at N- or C-terminus or at a non-terminal amino acid) that reacts with an alkyne moiety of the linker. For another example, the peptide can comprise an alkyne group (at N- or C-terminus or at a non-terminal amino acid) that reacts with an azide of the linker. The metal chelator and the linker can be attached similarly. In some embodiments, the linker comprises an azide moiety, an alkyne moiety, or both. In some embodiments, the linker comprises a triazole. In some embodiments, the click chemistry residue is
(DBCO-azide residue),
, , , , ,
. In some embodiments, the click chemistry residue is a DIBO-azide residue, BARAC-azide residue, DBCO-azide residue, DIFO-azide residue, COMBO-azide residue, BCN-azide residue, or DIMAC-azide residue. In some embodiments, the linker comprises a residue of nitrone dipole cycloaddition. In some embodiments, the linker comprises a residue of tetrazine ligation. In some embodiments, the linker comprises a residue of quadricyclane ligation. Exemplary groups of click chemistry residue are shown in Hein at al., “Click Chemistry, A Powerful Tool for Pharmaceutical Sciences,” Pharmaceutical Research volume 25, pages2216– 2230 (2008); Thirumurugan et al, “Click Chemistry for Drug Development and Diverse Chemical–Biology Applications,” Chem. Rev. 2013, 113, 7, 4905–4979; US20160107999A1; US10266502B2; and US20190204330A1, each of which is incorporated by reference in its entirety. [0175] In some embodiments, a linker described herein comprises two or more motifs (see FIGs. 2A-2D). The motifs can be connected by any suitable covalent bond. In some embodiments, one or more of the motifs are connected via click chemistry such that they can be clicked in/out of the
linker. Each of the motifs in a linker can have independent functions. For example, a linker can comprise a motif that functions to adjust plasma half-life and/or a motif that functions as a spacer between the peptide and metal chelator. In some embodiments, each of the motifs independently comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. In some embodiments, the linker comprises substituted or unsubstituted C1-C30 alkylene. In some embodiments, the linker comprises polyethylene glycol such as (-CH2-CH2-O-)1-10. [0176] In some embodiments, the linker has a structure of
Formula (II-1), wherein each L is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)- , -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, - (C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1-C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2-(C1-C30 alkylene)-; and each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; and n is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. [0177] In some embodiments, the linker has a structure of
, wherein each L is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. [0178] In some embodiments, the linker has a structure of
Formula (II-2) wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)- , substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted C1-C15 arylene, -(C1-C30 alkylene)-O- , -O-(C1-C30 alkylene)-, -(C1-C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2-(C1-C30 alkylene)-; and R is hydrogen, azide, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted cycloalkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; X is N or CRL; and each of m, p, and q is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. [0179] In some embodiments, the linker has a structure of
, wherein each L1 and L2 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C20 alkylene, or -(CHRL-CHRL-O)1-10-;
RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted cycloalkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; m is 1 to 10; and p is 0 to 3. [0180] In some embodiments, L2 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C6 alkylene, or -(CH2-CH2-O)1-6-; L1 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, - C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C20 alkylene, or -(CH2-CH2-O)1-6-; RL is hydrogen or substituted or unsubstituted C1-C4 alkyl; R is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; m is 1 to 10; and p is 0 to 3. [0181] In some embodiments, the linker has a structure of
, wherein L2 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C6 alkylene, or -(CH2-CH2-O)1-6-;
L1 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, - C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C20 alkylene, or -(CH2-CH2-O)1-6-; RL is hydrogen or substituted or unsubstituted C1-C4 alkyl; R is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted C6-C10 aryl, or substituted or unsubstituted C5-C9 heteroaryl; m is 1 to 4; and p is 0 to 3. [0182] In some embodiments, R is hydrogen, substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, or a sterol. [0183] In some embodiments, at least one L1 is unsubstituted C3-C20 alkylene. In some embodiments, the linker comprises a click chemistry residue. In some embodiments, the linker comprises one or more lysine residues. [0184] In some embodiments, the linker comprises one or more of a substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, or C3-C20 alkylene, or amino acid residues. [0185] In one aspect, disclosed herein is a conjugate comprising a guanylyl cyclase C (GCC) binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein the conjugate has a structure of Formula (I)
Formula (I) wherein, the linker –(L)n- comprises 2 to 100 (e.g., 2 to 50 or 2 to 25) intervening atoms between the metal chelator and the binding peptide; each L is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, - C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1- C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted
C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C6 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); n is 0 or an integer from 1 to 20; and r is 0 or an integer from 1 to 5. [0186] In some embodiments, the linker –(L)n- comprises 3 to 20 intervening atoms between the metal chelator and the binding peptide. In some embodiments, the linker –(L)n- comprises 3 to 50, 2 to 25, 2 to 75, 15 to 25, 10 to 30, or 15 to 40 intervening atoms between the metal chelator and the binding peptide. [0187] In some embodiments, –(L)n- is bound to the N-terminus of the GCC binding peptide and LC is bound to the C-terminus of the GCC binding peptide. In some embodiments, –(L)n- is
bound to the C-terminus of the GCC binding peptide and LC is bound to the N-terminus of the GCC binding peptide. In some embodiments, –(L)n- is bound to the N-terminus of the GCC binding peptide. In some embodiments, –(L)n- is bound to the C-terminus of the GCC binding peptide. In some embodiments, –(L)n- is bound to a non-terminus amino acid of the GCC binding peptide. In some embodiments, LC is bound to the N-terminus of the GCC binding peptide. In some embodiments, LC is bound to the C-terminus of the GCC binding peptide. In some embodiments, LC is bound to a non-terminus amino acid of the GCC binding peptide. [0188] In some embodiments, the linker –(L)n- comprises a hydrophobic group selected from a C8-C30 fatty acid, C8-C30 fatty alcohol, C8-C30 alkyl, aryl, heteroaryl, one or more amino acid residues, or a combination thereof. In some embodiments, the fatty acid contains 1, 2, 3 or more carboxylic acid groups. In some embodiments, the one or more amino acids contain a hydrophobic group such as an alkyl chain or a phenyl or heteroaryl group. [0189] In some embodiments, each of -L- and - LC- is independently, optionally substituted with one or more R12, each R12 is independently halogen, -CN, -NO2, -OH, -ORa, -OC(=O)Ra, -OC(=O)ORb, - OC(=O)NRcRd, -SH, -SRa, -S(=O)Ra, -S(=O)2Ra, -S(=O)2NRcRd, -NRcRd, - NRbC(=O)NRcRd, -NRbC(=O)Ra, -NRbC(=O)ORb, -NRbS(=O)2Ra, -C(=O)Ra, -C(=O)ORb, - C(=O)NRcRd, -Si(Ra)3, -P(=O)(Rb)2, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl, wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or more R12a; or two R12 on the same atom are taken together to form an oxo; each R12a is independently halogen, -CN, -NO2, -OH, -ORa, -NRcRd, -C(=O)Ra, -C(=O)ORb, - C(=O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R12a on the same atom are taken together to form an oxo; each Ra is independently C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or two Ra are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf;
each Rb is independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or two Rb are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf; Rc and Rd are each independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or Rc and Rd are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf; and each Rf is independently halogen, -CN, -OH, -OCH3, -S(=O)CH3, -S(=O)2CH3, -S(=O)2NH2, - S(=O)2NHCH3, -S(=O)2N(CH3)2, -NH2, -NHCH3, -N(CH3)2, -C(=O)CH3, -C(=O)OH, - C(=O)OCH3, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, and C1-C6heteroalkyl, or two Rf on the same atom form an oxo. [0190] In some embodiments, –(L)n- comprises one or more lysine residues. In some embodiments, –(L)n- comprises one or more glutamate residues. In some embodiments, –(L)n- comprises one or more tyrosine residues. In some embodiments, –(L)n- comprises one or more lysine residues. In some embodiments, –(L)n- comprises one or more amino acid residues selected from glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). [0191] In some embodiments of Formula (I), n is 0. In some embodiments, n is 1. In some embodiments, n is 2. In some embodiments, n is 3. In some embodiments, n is 4. In some embodiments, n is 5. In some embodiments, n is 6. In some embodiments, n is 7. In some embodiments, n is 8. In some embodiments, n is 9. In some embodiments, n is 10. In some embodiments, n is 11. In some embodiments, n is 12. In some embodiments, n is 13. In some embodiments, n is 14. In some embodiments, n is 15. In some embodiments, n is 16. In some embodiments, n is 17. In some embodiments, n is 18. In some embodiments, n is 19. In some embodiments, n is 20.
[0192] In some embodiments of Formula (I), (Ia), (Iaa) or (Iab), r is 0. In some embodiments, r is 1. In some embodiments of Formula (I), r is 2. In some embodiments of Formula (I), r is 3. In some embodiments of Formula (I), r is 4. In some embodiments of Formula (I), r is 5. [0193] In some embodiments, the conjugate has a structure of Formula (Ia),
Formula (Ia) wherein, each L1, L2, and L3 is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, - C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R and RC are each independently hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RX is independently hydrogen, halo, substituted or unsubstituted C1-C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or
unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; q is 0, 1, 2, 3, 4, 5, or 6; and r is 0, 1, 2, 3, 4, or 5. [0194] In some embodiments, at least one of L1, L2, and L3 comprises a hydrophobic group selected from a C8-C30 fatty acid, C8-C30 fatty alcohol, C4-C12 alkylene chain, heteroaryl, or aryl group, each of which is optionally substituted. [0195] In some embodiments, the conjugate has a structure of Formula (Iaa),
Formula (Iaa) wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; r is 0, 1, 2, 3, 4, or 5; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5. [0196] In some embodiments of Formula (Ia), (Iaa) or (Iab), m is 0 or 1-9. In some embodiments, m is 0. In some embodiments, m is 1. In some embodiments, m is 2. In some embodiments, m is 3. In some embodiments, m is 4. In some embodiments, m is 5. In some embodiments, m is 6. In some embodiments, m is 7. In some embodiments, m is 8. In some embodiments, m is 9. In some embodiments of Formula (Ia), m is 10. [0197] In some embodiments of Formula (Ia), (Iaa) or (Iab), p is 0 or 1-5. In some embodiments, p is 0. In some embodiments, p is 1. In some embodiments, p is 2. In some embodiments, p is 3. In some embodiments, p is 4. In some embodiments, p is 5. In some embodiments of Formula (Ia), p is 6. [0198] In some embodiments of Formula (Ia), (Iaa) or (Iab), q is 0 or 1-5. In some embodiments, q is 0. In some embodiments, q is 1. In some embodiments, q is 2. In some embodiments, q is 3. In some embodiments, q is 4. In some embodiments, q is 5. In some embodiments of Formula (Ia), q is 6. [0199] In some embodiments, the conjugate has a structure of Formula (Iab),
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl,
wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; r is 0, 1, 2, 3, 4, or 5; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5. [0200] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is a hydrophobic group. [0201] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is phenyl, naphthyl, monocyclic heteroaryl, or bicyclic heteroaryl, each of which is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, - N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl). In some embodiments, R is optionally substituted naphthyl. In some embodiments, R is optionally substituted monocyclic heteroaryl. In some embodiments, R is optionally substituted bicyclic heteroaryl. In some embodiments, R is optionally substituted monocyclic cycloalkyl. In some embodiments, R is optionally substituted bicyclic cycloalkyl. In some embodiments, R is optionally substituted monocyclic heterocycloalkyl. In some embodiments, R is optionally substituted bicyclic heterocycloalkyl. In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II-2ba), R is hydrogen, substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, or a sterol. In some embodiments, R is H. In some embodiments, R is a sterol or a derivative thereof. In some embodiments, R is phenyl. In some embodiments, R is phenyl substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl. In some embodiments, is phenyl substituted with one or more halogen. In some embodiments, R is phenyl substituted with one or more substituents selected from halogen, -CN, -NO2, -OH, -NH2,
-C(=O)H, -C(=O)OH, -C(=O)NH2, C1-C6alkyl, C1-C6alkoxy, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, and C1-C6heteroalkyl. [0202] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is iodophenyl. In some embodiments, R is
. In some embodiments, R is
. [0203] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is C6-C30 fatty acid, C6-C30 fatty alcohol or C6-C30 alkyl. In some embodiments, R is C6- C12 fatty acid. In some embodiments, R is C12-C24 fatty acid. In some embodiments, R is C6-C12 fatty alcohol. In some embodiments, R is C12-C24 fatty alcohol. In some embodiments, R is C6-C12 alkyl. In some embodiments, R is C12-C24 alkyl. In some embodiments, R is C6-C12 alkenyl. In some embodiments, R is C12-C24 alkenyl. [0204] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), (II-2aa), or (II- 2ba), R is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl). [0205] In some embodiments of Formula (I), (Ia), (Iaa) or (Iab), RC is a C6-C30 fatty acid, C6-C30 fatty alcohol or C6-C30 alkyl. In some embodiments, RC is C6-C12 fatty acid. In some embodiments, RC is C12-C24 fatty acid. In some embodiments, RC is C6-C12 fatty alcohol. In some embodiments, RC is C12-C24 fatty alcohol. In some embodiments, RC is C6-C12 alkyl. In some embodiments, RC is C12-C24 alkyl. In some embodiments, RC is C6-C12 alkenyl. In some embodiments, RC is C12-C24 alkenyl. [0206] In some embodiments of Formula (I), (Ia), (Iaa) or (Iab),
is -OH. In some embodiments of Formula (I), (Ia), (Iaa) or (Iab),
is NH2. [0207] In some embodiments of Formula (I), (Ia), (Iaa) or (Iab), RC is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl,
C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl). [0208] In some embodiments, a conjugate of Formula (I) has a linker of Formula (II-1). [0209] In some embodiments, described herein is a linker that has a structure of Formula (II-1)
Formula (II-1) wherein each L is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)- , -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, - (C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1-C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2-(C1-C30 alkylene)-; and each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; and n is 1 to 20. [0210] In some embodiments, the linker comprises a structure of Formula (II-1a),
Formula (II-1a) wherein each of L1 and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-,
-C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; and L2 is absent, substituted or unsubstituted C1-C30 alkylene, or substituted or unsubstituted C1- C30 heteroalkylene. [0211] In some embodiments for Formula (II-1a), L1 is -NH-; L3 is -C(=O)-; L2 is unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene, wherein the heteroalkylene is optionally substituted with one or more substituents selected from -OH, -SH, oxo, amino, C1-C6 alkyl, C1-C6 hydroxyalkyl, C1-C6 haloalkyl, and C1-C6 aminoalkyl. [0212] In some embodiments for Formula (II-1a), L2 is unsubstituted C1-C12 alkylene. [0213] In some embodiments, a linker of the present disclosure (e.g., a linker of Formula (II- 1a)) has a structure of
, , [0214] In some embodiments for Formula (II-1a), L2 is C1-C30 heteroalkylene that is optionally substituted with one or more substituents selected from -OH, -SH, oxo, amino, C1-C6 alkyl, C1-C6 hydroxyalkyl, C1-C6 haloalkyl, and C1-C6 aminoalkyl. [0215] In some embodiments, a linker of the present disclosure (e.g., a linker of Formula (II- 1a)) has a structure of
, ,
,
[0216] In some embodiments, a conjugate of Formula (Ia) has a linker of Formula (II-2). [0217] In some embodiments, a linker of the present disclosure has a structure of Formula (II- 2)
Formula (II-2), wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)- , substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2- C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, -(C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1- C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2- (C1-C30 alkylene)-; and R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
RX is independently hydrogen, halo, -CN, -NO2, -OH, -SH, substituted or unsubstituted C1- C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2- C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; and q is 0, 1, 2, 3, 4, 5, and 6. [0218] In some embodiments, the linker has a structure of Formula (II-2a) or Formula (II-2b)
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5.
[0219] In some embodiments, the linker of Formula (II-2a) or Formula (II-2b) connects to the metal chelator through L3 and to the peptide through L2. [0220] In some embodiments, the linker has a structure of Formula (II-2a). In some embodiments, a conjugate of Formula (Ia) or (Iaa) has a linker of Formula (II-2aa). [0221] In some embodiments, the linker of Formula (II-2a) has a structure of Formula (II-2aa),
Formula (II-2aa) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L31 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-;
L32 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5. [0222] In some embodiments of a structure of Formula (II-2aa), L31 is -NH- and L32 is substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. [0223] In some embodiments, L31 is -NH- and L32 is -(CH2)4-. [0224] In some embodiments of a structure of Formula (II-2aa), L31 is absent, -O-, –NRL-, – N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O- , -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L31 is absent, -OP(=O)(OH)O-, -NHC(=O)-, -C(=O)NH-, or -S(=O)2NH-. In some embodiments, L31 is OP(=O)(ORL)O-. In some embodiments, L31 is OP(=O)(OH)O-. In some embodiments, L31 is -C(=O)NRL-. In some embodiments, L31 is -C(=O)NH-. In some embodiments, L31 is- S(=O)2NRL-. In some embodiments, L31 is- S(=O)2NH-. In some embodiments, L31 is absent. [0225] In some embodiments of a structure of Formula (II-2aa), L32 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L32 is substituted or unsubstituted C1-C12 alkylene. In some embodiments, L32 is C1-C30 heteroalkylene. In some embodiments, L32 is C10-C20 heteroalkylene. In some embodiments, L32 is C1-C12 alkenyl. In some embodiments, L32 is absent. [0226] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), wherein L11 is absent, -O-, -OP(=O)(ORL)O-, -S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-,
-NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L11 is absent, -OP(=O)(OH)O- , -NHC(=O)-, -C(=O)NH-, or -S(=O)2NH-. In some embodiments, L11 is OP(=O)(ORL)O-. In some embodiments, L11 is OP(=O)(OH)O-. In some embodiments, L11 is -C(=O)NRL-. In some embodiments, L11 is -C(=O)NH-. In some embodiments, L11 is- S(=O)2NRL-. In some embodiments, L11 is- S(=O)2NH-. In some embodiments, L11 is absent. [0227] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L12 is absent, -O-, - OP(=O)(ORL)O-, -S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L12 is substituted or unsubstituted C1-C12 alkylene. In some embodiments, L12 is C1-C30 heteroalkylene. In some embodiments, L12 is C10-C20 heteroalkylene. In some embodiments, L12 is C1-C12 alkenyl. In some embodiments, L12 is absent. [0228] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L21 is substituted or unsubstituted C1-C12 alkylene. In some embodiments, L21 is C1-C30 heteroalkylene. In some embodiments, L21 is C10-C20 heteroalkylene. In some embodiments, L21vis C1-C12 alkenyl. In some embodiments, L21 is absent. [0229] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L22 is -O-, –NRL-, – N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O- , -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L22 is absent, -OP(=O)(OH)O-, -NHC(=O)-, -C(=O)NH-, or -S(=O)2NH-. In some embodiments, L22 is OP(=O)(ORL)O-. In some embodiments, L22 is OP(=O)(OH)O-. In some embodiments, L22 is -C(=O)NRL-. In some embodiments, L22 is -C(=O)NH-. In some embodiments, L22 is- S(=O)2NRL-. In some embodiments, L22 is- S(=O)2NH-. In some embodiments, L22 is absent.
[0230] In some embodiments, L12 is
[0231] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L12 is -C(=O)- and L11 is absent. [0232] In some embodiments, the linker has a structure of Formula (II-2b). In some embodiments, a conjugate of Formula (Ia) or (Iab) has a linker of Formula (II-2ba). [0233] In some embodiments, a linker of Formula (II-2b) has a structure of Formula (II-2ba),
Formula (II-2ba) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L3 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, - C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -
OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5. [0234] I some embodiments of Formula (II-2ba), q is 0. In some embodiments, q is 1. In some embodiments, q is 2. In some embodiments, q is 3. In some embodiments, q is 4. In some embodiments, q is 5. [0235] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L11 is absent, -O-, - OP(=O)(ORL)O-, -S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L11 is absent, -OP(=O)(OH)O- , -NHC(=O)-, -C(=O)NH-, or -S(=O)2NH-. [0236] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L12 is substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. [0237] In some embodiments, L12 is
, -(CH2)2-, -(CH2)3-, -(CH2)4-,
. [0238] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L22 and L21 are both absent. [0239] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), L22 is -C(=O)- and L21 is substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene. [0240] In some embodiments of a structure of Formula (II-2aa) or (II-2ba), -L21-L22- is
. [0241] In some embodiments of a structure of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), or (II-2b), each of L1 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, or substituted or unsubstituted C1-C30 heteroalkylene, In some embodiments, L1 is -O-, –NRL-, - OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L1 is -O-, –NH-, -S(=O)-, -S(=O)2-, or -C(=O)-. In some embodiments, L1 is -C(=O)NH- or - NHC(=O)-. In some embodiments, L1 is substituted or unsubstituted C3-C15 cycloalkyl, or substituted or unsubstituted C1-C12 heterocycloalkyl. In some embodiments, L1 is substituted or unsubstituted aryl or substituted or unsubstituted heteroaryl. In some embodiments, L1 is
substituted or unsubstituted C1-C30 alkylene. In some embodiments, L1 is substituted or unsubstituted C2-C30 alkenylene. In some embodiments, L1 is substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L1 is substituted or unsubstituted C5-C25 heteroalkylene. In some embodiments, L1 is substituted or unsubstituted C5-C12 heteroalkylene. [0242] In some embodiments of a structure of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), or (II-2b), each of L2 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, or substituted or unsubstituted C1-C30 heteroalkylene, In some embodiments, L2 is -O-, –NRL-, - OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L2 is -O-, –NH-, -S(=O)-, -S(=O)2-, or -C(=O)-. In some embodiments, L2 is -C(=O)NH- or - NHC(=O)-. In some embodiments, L2 is substituted or unsubstituted C3-C15 cycloalkyl, or substituted or unsubstituted C1-C12 heterocycloalkyl. In some embodiments, L2 is substituted or unsubstituted aryl or substituted or unsubstituted heteroaryl. In some embodiments, L2 is substituted or unsubstituted C1-C30 alkylene. In some embodiments, L2 is substituted or unsubstituted C2-C30 alkenylene. In some embodiments, L2 is substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L2 is substituted or unsubstituted C5-C25 heteroalkylene. In some embodiments, L2 is substituted or unsubstituted C5-C12 heteroalkylene. [0243] In some embodiments of a structure of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a), (II-2b), or (II-2ba), each of L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, or substituted or unsubstituted C1-C30 heteroalkylene, In some embodiments, L3 is - O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, - OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -
NRLC(=S)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-. In some embodiments, L3 is -O-, –NH-, -S(=O)-, -S(=O)2-, or -C(=O)-. In some embodiments, L3 is -C(=O)NH- or -NHC(=O)-. In some embodiments, L3 is substituted or unsubstituted C3-C15 cycloalkyl, or substituted or unsubstituted C1-C12 heterocycloalkyl. In some embodiments, L3 is substituted or unsubstituted aryl or substituted or unsubstituted heteroaryl. In some embodiments, L3 is substituted or unsubstituted C1-C30 alkylene. In some embodiments, L3 is substituted or unsubstituted C2-C30 alkenylene. In some embodiments, L3 is substituted or unsubstituted C1-C30 heteroalkylene. In some embodiments, L3 is substituted or unsubstituted C5-C25 heteroalkylene. In some embodiments, L3 is substituted or unsubstituted C5-C12 heteroalkylene. [0244] In some embodiments of Formula (Ia), (Iaa), (Iab), (II-2), (II-2a) or (II-2b), each of L1, L2, L3, and LC is independently optionally substituted with one or more R12, each R12 is independently halogen, -CN, -NO2, -OH, -ORa, -OC(=O)Ra, -OC(=O)ORb, - OC(=O)NRcRd, -SH, -SRa, -S(=O)Ra, -S(=O)2Ra, -S(=O)2NRcRd, -NRcRd, - NRbC(=O)NRcRd, -NRbC(=O)Ra, -NRbC(=O)ORb, -NRbS(=O)2Ra, -C(=O)Ra, -C(=O)ORb, - C(=O)NRcRd, -Si(Ra)3, -P(=O)(Rb)2, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl, wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is optionally and independently substituted with one or more R12a; or two R12 on the same atom are taken together to form an oxo; each R12a is independently halogen, -CN, -NO2, -OH, -ORa, -NRcRd, -C(=O)Ra, -C(=O)ORb, - C(=O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R12a on the same atom are taken together to form an oxo; each Ra is independently C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or two Ra are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf; each Rb is independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl),
C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or two Rb are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf; Rc and Rd are each independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl), wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more Rf; or Rc and Rd are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more Rf; and each Rf is independently halogen, -CN, -OH, -OCH3, -S(=O)CH3, -S(=O)2CH3, -S(=O)2NH2, - S(=O)2NHCH3, -S(=O)2N(CH3)2, -NH2, -NHCH3, -N(CH3)2, -C(=O)CH3, -C(=O)OH, - C(=O)OCH3, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, and C1-C6heteroalkyl, or two Rf on the same atom form an oxo. [0245] In some embodiments of Formula (II-2aa) or (II-2ba), L11, L12, L21, L22, L31, and L32 are each independently, optionally substituted with one or more substituents selected from halogen, - CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl). [0246] In some embodiments, a linker comprises a click chemistry residue. [0247] In some embodiments, a linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and one or more amino acids. [0248] In some embodiments, a linker comprises one or more of a substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, C3-C20 alkylene, or amino acid residues.
[0249] In some embodiments, a linker comprises one or more lysine residues. [0250] In some embodiments, a linker comprises one or more glutamate residues. [0251] In some embodiments, the linker comprises phenyl iodide or carboxylic acid. [0252] A linker described herein can reversibly bind to human serum albumin. In some embodiments, the linker binds to Sudlow Site I of the human serum albumin. In some embodiments, the linker binds to Sudlow Site II of the human serum albumin. In some embodiments, the linker binds to Sudlow Site I and site II of the human serum albumin. In some embodiments, the linker comprises one or more negatively charged groups such as a carboxylic acid and aromatic carboxylic acid. [0253] In some embodiments, the linker comprises a halo substituted aryl group. In some embodiments, the linker comprises phenyl iodide. In some embodiments, the linker comprises phenyl bromide. In some embodiments, the linker comprises an unsubstituted or halo substituted 1,1'-biphenyl. In some embodiments, the linker comprises an unsubstituted or halo substituted 1,1':4',1''-terphenyl. In some embodiments, the linker comprises an unsubstituted or halo substituted naphthyl. In some embodiments, the linker comprises a carboxylic acid or an isostere thereof. In some embodiments, the linker comprises a carboxylic acid. The halo substituted aryl group and the carboxylic acid group can be located in the same linker or in two different linkers. It is revealed by the present disclosure that the presence of a halo substituted aryl group (e.g., phenyl iodide), the presence of a carboxylic acid or an isostere thereof, or both in the conjugate can improve elimination half-life of the conjugate. [0254] In some embodiments, the linker comprises a lysine residue. In some embodiments, the lysine residue is D-lysine. [0255] In some embodiments, a linker described herein comprises one or more structures selected from: ,
,
,
. [0256] In some embodiments, the linker comprises:
. [0257] In some embodiments, the linker comprises:
[0258] In some embodiments, a dissociation constant (Kd) between the linker and human serum albumin is at most 500 µM, as determined at room temperature in human serum condition. In some embodiments, the Kd is at most 100 µM. In some embodiments, the Kd is at most 15 µM. In some embodiments, the Kd is from about 0.1 nM to about 10 µM. In some embodiments, the Kd is from about 10 nM to about 10 µM. Metal Chelators [0259] In one aspect, described herein are conjugates that comprise a metal chelator that is configured to bind with a radionuclide. The metal chelator can refer to a moiety of the conjugate that is configured to bind with a radionuclide. In some embodiments, a conjugate described herein comprises two or more independent metal chelators, e.g., 2, 3, 4, 5, or more metal chelators. In some embodiments, a conjugate described herein comprises two metal chelators, which can be the same or different. In some embodiments, a conjugate described herein comprises two or more metal chelators. In some embodiments, the conjugate comprises two radionuclides bound to the metal chelators. The metal chelator can be attached to the linker or the peptide through any suitable group/atom of the chelator. [0260] In some embodiments, the metal chelator is capable of binding a radioactive atom. The binding can be direct, e.g., the metal chelator can make hydrogen bonds or electrostatic interactions with the radioactive atom. The binding can also be indirect, e.g., the metal chelator binds to a molecule that comprises a radioactive atom. In some embodiments, the metal chelator comprises, or is, a macrocycle. In some embodiments, the metal chelator comprises, or is, 2,2′,2′′,2′′′-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA) or 1,4,7- triazacyclononane-1,4,7-triacetic acid (NOTA). In some embodiments, the metal chelator comprises a macrocycle, e.g., a macrocycle comprising an O and/or a N, DOTA, NOTA, one or more amines, one or more ethers, one or more carboxylic acids, EDTA, DTPA, TETA, DO3A, PCTA, or desferrioxamine. [0261] In some embodiments, the metal chelator comprises a plurality of amines. In some embodiments, the metal chelator includes 4 or more N, 4 or more carboxylic acid groups, or a combination thereof. In some embodiments, the metal chelator does not comprise S. In some embodiments, the metal chelator comprises a ring. In some embodiments, the ring comprises an O and/or an N. In some embodiments, the metal chelator is a ring that includes 3 or more N, 3 or more carboxylic acid groups, or a combination thereof. In some embodiments, the metal chelator is poly polydentate. [0262] In some embodiments, a metal chelator described herein comprises a cyclic chelating agent. Exemplary cyclic chelating agents include, but are not limited to, AAZTA, BAT, BAT-
TM, Crown, Cyclen, DO2A, CB-DO2A, DO3A, H3HP-DO3A, Oxo-DO3A, p-NH2-Bn-Oxo- DO3A, DOTA, DOTA-3py, DOTA-PA, DOTA-GA, DOTA-4AMP, DOTA-2py, DOTA-1py, p- SCN-Bn-DOTA, CHX-A″-EDTA, MeO-DOTA-NCS EDTA, DOTAMAP, DOTAGA, DOTAGA-anhydride, DOTMA, DOTASA, DOTAM, DOTP, CB-Cyclam, TE2A, CB-TE2A, CB-TE2P, DM-TE2A, MM-TE2A, NOTA, NOTP, HEHA, HEHA-NCS, p-SCN-Bn-HEHA, DTPA, CHX-A″-DTPA, p-NH2-Bn-CHX-A″-DTPA, p-SCN-DTPA, p-SCN-Bz-Mx-DTPA, 1B4M-DTPA, p-SCN-Bn1B-DTPA, p-SCN-Bn-1B4M-DTPA, p-SCN-Bn-CHX-A″-DTPA, PEPA, p-SCN-Bn-PEPA, TETPA, DOTPA, DOTMP, DOTPM, t-Bu-calix[4]arene- tetracarboxylic acid, macropa, macropa-NCS, macropid, H3L1, H3L4, H2azapa, H5decapa, bispa2, H4pypa, H4octapa, H4CHXoctapa, p-SCN-Bn-H4octapa, p-SCN-Bn-H4octapa, TTHA, p-NO2-Bn- neunpa, H4octox, H2macropa, H2bispa2, H4phospa, H6phospa, p-SCN-Bn-H6phospa, TETA, p- NO2-Bn-TETA, TRAP, TPA, HBED, SHBED, HBED-CC, (HBED-CC)TFP, DMSA, DMPS, DHLA, lipoic acid, TGA, BAL, Bis-thioseminarabazones, p-SCN-NOTA, nNOTA, NODAGA, CB-TE1A1P, 3P-C-NETA-NCS, 3p-C-DEPA, 3P-C-DEPA-NCS, TCMC, PCTA, NODIA-Me, TACN, pycup1A1B, pycup2A, THP, DEDPA, H2DEDPA, p-SCN-Bn-H2DEDPA, p-SCN-Bn- TCMC, motexafin, NTA, NOC, 3p-C-NETA, p-NH2-Bn-TE3A, SarAr, DiAmSar, SarAr-NCS, AmBaSar, BaBaSar, TACN-TM, CP256, C-NE3TA, C-NE3TA-NCS, NODASA, NETA- monoamide, C-NETA, NOPO, BPCA, p-SCN-Bn-DFO, DFO-ChX-Mal, DFO, DFO-IAC, DFO- BAC, DiP-LICAM, EC, SBAD, BAPEN, TACHPYR, NEC-SP, Lpy, L1, L2, L3, and EuK-106. In some embodiments, the metal chelator is DOTA, TRITA, TETA, DOTA-MA, DO3A-HP, DOTMA, DOTA-pNB, DOTP, DOTMP, DOTEP, DOTMPE, F-DOTPME, DOTPP, DOTBzP, DOTA-monoamide, p-NCS-DOTA, p-NCS-PADOTA, BAT, DO3TMP-Monoamide, p-NCS- TRITA, NOTA, and CHX-A″-DTPA. In some embodiments, a metal chelator described herein comprises an acyclic chelating agent. Exemplary acyclic chelating agents include, but are not limited to, DTA, CyEDTA, EDTMP, DTPMP, DTPA, CyDTPA, Cy2DTPA, DTPA-MA, DTPA- BA, and BOPA. In some embodiments, a metal chelator described herein comprises DOTA, DOTP, DOTMA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, TACN, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, or NOTP. In some embodiments, a metal chelator described herein comprises H4pypa, H4octox, H4octapa, p-NO2-Bn-neunpa, p-SCN–Bn– H4neunpa, TTHA, tBu4pypa-C7-NHS, H4neunpa, H2macropa, HP-DO3A, BT-DO3A, DO3A- Nprop, DO3AP, DO2A2P, DOA3P, DOTP, DOTPMB, DOTAMAE, DOTAMAP, DO3AMBu, DOTMA, TCE-DOTA, DEPA, PCTA, p-NO2-Bn-PCTA, p-NO2-Bn-DOTA, symPC2APA, symPCA2PA, asymPC2APA, asymPCA2PA, TRAP, AAZTA, DATAm, THP, HEHA, or HBED. [0263] In some embodiments, the metal chelator is DO3A. In some embodiments, the metal chelator is PEPA. In some embodiments, the metal chelator is EDTA. In some embodiments, the
metal chelator is CHX-A″-DTPA. In some embodiments, the metal chelator is HEHA. In some embodiments, the metal chelator is DOTMP. In some embodiments, the metal chelator is t-Bu- calix[4]arene-tetracarboxylic acid. In some embodiments, the metal chelator is macropa. In some embodiments, the metal chelator is macropa-NCS. In some embodiments, the metal chelator is H4pypa. In some embodiments, the metal chelator is H4octapa. In some embodiments, the metal chelator is H4CHXoctapa. In some embodiments, the metal chelator is DOTP. In some embodiments, the metal chelator is crown. [0264] In some embodiments, the metal chelator is DOTA. In some embodiments, the metal chelator is a chiral derivative of DOTA. Exemplary chiral DOTA chelators are described in Dai et al., Nature Communications (2018) 9:857. In some embodiments, the metal chelator is 2,2',2'',2'''-((2S,5S,8S,11S)-2,5,8,11-tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10- tetrayl)tetraacetic acid. In some embodiments, the metal chelator has a structure of
. In some embodiments, the metal chelator is 2,2',2'',2'''- ((2S,5S,8S,11S)-2,5,8,11-tetraethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid. In some embodiments, the metal chelator has a structure of
. [0265] In some embodiments, the metal chelator has a structure of
wherein each Re is independently selected from hydrogen, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkylcycloalkyl, alkylheterocycloalkyl, alkylaryl, alkylheteroaryl, or an amino acid side chain. In
some embodiments, the metal chelator has a structure of
wherein each Re is independently selected from hydrogen, alkyl, haloalkyl, hydroxyalkyl, aminoalkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkylcycloalkyl, alkylheterocycloalkyl, alkylaryl, alkylheteroaryl, or an amino acid side chain. [0266] In some embodiments, the conjugate comprises DOTA. In some embodiments, the conjugate comprises a DOTA derivative such as p-SCN-Bn-DOTA and MeO-DOTA-NCS. In some embodiments, the conjugate comprises two independent metal chelators, and at least one or both are DOTA. The structures of some exemplary metal chelators are illustrated in FIGs. 3-17 (without showing the attachment points). Exemplary metal chelators are further described in WO2012/174136; US20130183235A1; US20120219495A1; Ramogidaand et al., EJNMMI radiopharm. chem.4, 21 (2019); Thiele et al., Cancer Biotherapy and Radiopharmaceuticals 2018; Li et al., Bioconjugate Chem.2019, 30, 5, 1539–1553; and Baranyai et al., Eur. J. Inorg. Chem. 36–56 (2020), each of which is incorporated by reference in its entirety. Radionuclides [0267] In one aspect, described herein are conjugates that comprise a radionuclide. Generally, the type of radionuclide used in a therapeutic radiopharmaceutical can be tailored to the specific type of cancer, the type of targeting moiety (e.g., binding peptide), etc. Radionuclides that undergo α-decay produce particles composed of two neutrons and two protons, and radionuclides that undergo β-decay emit energetic electrons from their nuclei. Some radionuclides can also emit Auger. In some embodiments, the conjugate comprises an alpha particle-emitting radionuclide. Alpha radiation can cause direct, irreparable double-strand DNA breaks compared with gamma and beta radiation, which can cause single-stranded breaks via indirect DNA damage. The range of these particles in tissue and the half-life of the radionuclide can also be considered in designing the radiopharmaceutical conjugate. Tables 5A and 5B below illustrate some properties of exemplary radionuclides. Table 5A. Exemplary radionuclides
Table 5B. Exemplary radionuclides
[0268] In some embodiments, a conjugate described herein comprises one or more independent radionuclides. In some embodiments, the conjugate comprises two radionuclides. In some embodiments, each of the one or more radionuclides is bound to a metal chelator of the conjugate. In some embodiments, two radionuclides of a conjugate are bound to the same metal chelator. In some embodiments, two radionuclides of a conjugate are bound to two independent metal chelators. In some embodiments, each of the one or more radionuclides is an alpha particle- emitting radionuclide. [0269] In some embodiments, a conjugate described herein comprises an alpha particle-emitting radionuclide. In some embodiments, the alpha particle-emitting radionuclide is actinium-225 (225Ac), astatine-211 (211At), radium-223 (223Ra), radium-224 (224Ra), bismuth-213 (213Bi), Terbium-149 (149Tb), or thorium-227 (227Th). In some embodiments, the alpha particle-emitting radionuclide is 225Ac. In some embodiments, the alpha particle-emitting radionuclide is 213Bi. In some embodiments, the alpha particle-emitting radionuclide is 212Bi. In some embodiments, the
alpha particle-emitting radionuclide is 212Pb. In some embodiments, the alpha particle-emitting radionuclide is 224Ra. In some embodiments, the alpha particle-emitting radionuclide is 223Ra. In some embodiments, the alpha particle-emitting radionuclide is 227Th. In some embodiments, the alpha particle-emitting radionuclide is 211At. In some embodiments, the alpha particle-emitting radionuclide is 149Tb. In some embodiments, the radionuclide is Zirconium-89 (89Zr). In some embodiments, a conjugate described herein comprises a radionuclide selected from 67Cu, 64Cu, 90Y, 109Pd, 111Ag, 149Pm, 153Sm, 166Ho, 99mTc, 67Ga, 68Ga, 111In, 90Y, 177Lu, 186Re, 188Re, 197Au, 198Au, 199Au, 105Rh, 165Ho, 161Tb, 149Pm, 44Sc, 47Sc, 70As, 71As, 72As, 73As, 74As, 76As, 77As, 212Pb, 212Bi, 213Bi, 225Ac, 117mSn, 67Ga, 201Tl, 123I, 131I, 160Gd, 148Nd, 89Sr, 89Zr, and 211At. In some embodiments, the radionuclide is 225Ac. In some embodiments, the radionuclide is a decay daughter of 225Ac such as 221Fr, 217At, 213Bi, 213Po, 209Tl, 209Pb, or 209Bi. In some embodiments, the conjugate comprises two 225Ac radionuclides. In some embodiments, the radionuclide is 177Lu. In some embodiments, the conjugate comprises two 177Lu radionuclides. [0270] In some embodiments, the conjugate comprises an alpha particle-emitting radionuclide bound to the metal chelator. In some embodiments, the alpha particle-emitting radionuclide is actinium-225, astatine-211, thorium-227, or radium-223. In some embodiments, the alpha particle-emitting radionuclide is actinium-225. [0271] In some embodiments, the conjugate comprises a beta particle-emitting radionuclide bound to the metal chelator. In some embodiments, the beta particle emitting radionuclide is zircronium-89, yttrium-90, samarium-153, lutetium-177, or lead-212. [0272] In some embodiments, the conjugate comprises a gamma particle emitting radionuclide. In some embodiments, the gamma particle emitting radionuclide is indium-111. [0273] In some embodiments, conjugates described herein do not contain any radionuclide, i.e., a cold conjugate. For example, in some cases, a radionuclide can be replaced with a surrogate (e.g., 225Ac replaced with lanthanum) for testing and experimental purposes. Conjugates Comprising non-radioactive Drugs [0274] In one aspect, described herein is a conjugate that comprises a GCC binding peptide, a non-radioactive drug, and optionally a linker. In some embodiments, the conjugate further comprises a metal chelator and optionally a radionuclide bound to the metal chelator. The non- radioactive drug can be a toxin. In some embodiments, the toxin is selected from pseudomonas exotoxin (PE), deBouganin, Bouganin, diphtheria toxin (DT) and ricin. The non-radioactive drug can be a chemotherapy agent. [0275] The non-radioactive drug can be a cytotoxic drug. Exemplary cytotoxic drugs include aplidin, azaribine, anastrozole, azacytidine, bleomycin, bortezomib, bryostatin-1, busulfan,
calicheamycin, camptothecin, 10-hydroxycamptothecin, carmustine, celebrex, chlorambucil, cisplatin, irinotecan (CPT-11), SN-38, carboplatin, cladribine, cyclophosphamide, cytarabine, dacarbazine, docetaxel, dactinomycin, daunomycin glucuronide, daunorubicin, dexamethasone, diethylstilbestrol, doxorubicin, 2-pyrrolinodoxorubicin (2P-DOX), cyano-morpholino doxorubicin, doxorubicin glucuronide, epirubicin glucuronide, ethinyl estradiol, estramustine, etoposide, etoposide glucuronide, etoposide phosphate, floxuridine (FUdR), 3′,5′-O-dioleoyl- FudR (FUdR-dO), fludarabine, flutamide, fluorouracil, fluoxymesterone, gemcitabine, hydroxyprogesterone caproate, hydroxyurea, idarubicin, ifosfamide, L-asparaginase, leucovorin, lomustine, mechlorethamine, medroprogesterone acetate, megestrol acetate, melphalan, mercaptopurine, 6-mercaptopurine, methotrexate, mitoxantrone, mithramycin, mitomycin, mitotane, phenyl butyrate, prednisone, procarbazine, paclitaxel, pentostatin, PSI-341, semustine streptozocin, tamoxifen, taxanes, taxol, testosterone propionate, thalidomide, thioguanine, thiotepa, teniposide, topotecan, uracil mustard, velcade, vinblastine, vinorelbine, vincristine, ricin, abrin, ribonuclease, onconase, rapLR1, DNase I, Staphylococcal enterotoxin-A, pokeweed antiviral protein, gelonin, diphtheria toxin, Pseudomonas exotoxin, Pseudomona endotoxin, or combinations of these. [0276] In some embodiments, the non-radioactive drug is selected from duocarmycin and its analogues, dolastatins, combretastatin, calicheamicin, N-acetyl-□-calicheamycin (CMC), a calicheamycin derivative, maytansine and analogues thereof, DM-I, auristatin E, auristatin EB (AEB), auristatin EFP (AEFP), monomethyl auristatin E (MMAE), monomethyl auristatin F (MMAF), tubulysin, disorazole, the epothilones, Paclitaxel, docetaxel, Topotecan, echinomycin, estramustine, cemadotine, eleutherobin, methopterin, actinomycin, daunorubicin, the daunorubicin conjugates, mitomycin C, mitomycin A, vincristine, retinoic acid, camptothecin, a camptothecin derivative, SN38, maytansine, a derivative of the maytansinoid type, DM1, DM4, TK1, amanitin, a pyrrolobenzodiazepine, a pyrrolobenzodiazepine dimer, methotrexate, ilomedine, aspirin, an IMIDs, lenalidomide, pomalidomide. Isomers/Stereoisomers [0277] In some embodiments, the compounds described herein exist as geometric isomers. In some embodiments, the compounds described herein possess one or more double bonds. The compounds presented herein include cis, trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the corresponding mixtures thereof. In some situations, the compounds described herein possess one or more chiral centers and each center exists in the R configuration or S configuration. The compounds described herein include diastereomeric, enantiomeric, and epimeric forms as well as the corresponding mixtures thereof. In additional embodiments of the
compounds and methods provided herein, mixtures of enantiomers and/or diastereoisomers, resulting from a single preparative step, combination, or interconversion are useful for the applications described herein. In some embodiments, the compounds described herein are prepared as their individual stereoisomers by reacting a racemic mixture of the compound with an optically active resolving agent to form a pair of diastereoisomeric compounds, separating the diastereomers, and recovering the optically pure enantiomers. In some embodiments, dissociable complexes are preferred. In some embodiments, the diastereomers have distinct physical properties (e.g., melting points, boiling points, solubilities, reactivity, etc.) and are separated by taking advantage of these dissimilarities. In some embodiments, the diastereomers are separated by chiral chromatography, or preferably, by separation/resolution techniques based upon differences in solubility. In some embodiments, the optically pure enantiomer is then recovered, along with the resolving agent. Tautomers [0278] A "tautomer" refers to a molecule wherein a proton shift from one atom of a molecule to another atom of the same molecule is possible. The compounds presented herein, in certain embodiments, exist as tautomers. In circumstances where tautomerization is possible, a chemical equilibrium of the tautomers will exist. The exact ratio of the tautomers depends on several factors, including physical state, temperature, solvent, and pH. Some examples of tautomeric equilibrium include:
[0279] In some instances, the compounds disclosed herein exist in tautomeric forms. The structures of said compounds are illustrated in the one tautomeric form for clarity. The alternative tautomeric forms are expressly included in this disclosure.
Labeled compounds [0280] In some embodiments, the compounds described herein exist in their isotopically- labeled forms. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such isotopically-labeled compounds. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such isotopically-labeled compounds as pharmaceutical compositions. Thus, in some embodiments, the compounds disclosed herein include isotopically-labeled compounds, which are identical to those recited herein, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature. Examples of isotopes that can be incorporated into compounds described herein, or a solvate, or stereoisomer thereof, include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, sulfur, fluorine, and chloride, such as 2H, 3H, 13C, 14C, l5N, 18O, 17O, 31P, 32P, 35S, 18F, and 36Cl, respectively. Compounds described herein, and the pharmaceutically acceptable salts, solvates, or stereoisomers thereof which contain the aforementioned isotopes and/or other isotopes of other atoms are within the scope of this disclosure. Certain isotopically-labeled compounds, for example those into which radioactive isotopes such as 3H and 14C are incorporated, are useful in drug and/or substrate tissue distribution assays. Tritiated, i.e., 3H and carbon-14, i.e., 14C, isotopes are notable for their ease of preparation and detectability. Further, substitution with heavy isotopes such as deuterium, i.e., 2H, produces certain therapeutic advantages resulting from greater metabolic stability, for example increased in vivo half-life or reduced dosage requirements. In some embodiments, the isotopically labeled compound or a pharmaceutically acceptable salt, solvate, or stereoisomer thereof is prepared by any suitable method. [0281] In some embodiments, the compounds described herein are labeled by other means, including, but not limited to, the use of chromophores or fluorescent moieties, bioluminescent labels, or chemiluminescent labels. Pharmaceutically acceptable salts [0282] In some embodiments, the compounds described herein exist as their pharmaceutically acceptable salts. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such pharmaceutically acceptable salts. In some embodiments, the methods disclosed herein include methods of treating diseases by administering such pharmaceutically acceptable salts as pharmaceutical compositions. As used herein, a “pharmaceutically acceptable salt” refers to any salt of a compound that is be useful for therapeutic purposes of a subject.
[0283] In some embodiments, the compounds described herein possess acidic or basic groups and therefore react with any of a number of inorganic or organic bases, and inorganic and organic acids, to form a pharmaceutically acceptable salt. In some embodiments, these salts are prepared in situ during the final isolation and purification of the compounds disclosed herein, or by separately reacting a purified compound in its free form with a suitable acid or base, and isolating the salt thus formed. [0284] Examples of pharmaceutically acceptable salts include those salts prepared by reaction of the compounds described herein with a mineral acid, organic acid, or inorganic base, such salts including acetate, acrylate, adipate, alginate, aspartate, benzoate, benzenesulfonate, bisulfate, bisulfite, bromide, butyrate, butyn-1,4-dioate, camphorate, camphorsulfonate, caproate, caprylate, chlorobenzoate, chloride, citrate, cyclopentanepropionate, decanoate, digluconate, dihydrogenphosphate, dinitrobenzoate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptanoate, glycerophosphate, glycolate, hemisulfate, heptanoate, hexanoate, hexyne-1,6-dioate, hydroxybenzoate, γ-hydroxybutyrate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, iodide, isobutyrate, lactate, maleate, malonate, methanesulfonate, mandelate, metaphosphate, methanesulfonate, methoxybenzoate, methylbenzoate, monohydrogenphosphate, 1-napthalenesulfonate, 2-napthalenesulfonate, nicotinate, nitrate, palmoate, pectinate, persulfate, 3-phenylpropionate, phosphate, picrate, pivalate, propionate, pyrosulfate, pyrophosphate, propiolate, phthalate, phenylacetate, phenylbutyrate, propanesulfonate, salicylate, succinate, sulfate, sulfite, succinate, suberate, sebacate, sulfonate, tartrate, thiocyanate, tosylate, undeconate, and xylenesulfonate. [0285] Further, the compounds described herein can be prepared as pharmaceutically acceptable salts formed by reacting the free base form of the compound with a pharmaceutically acceptable inorganic or organic acid, including, but not limited to, inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, metaphosphoric acid, and the like; and organic acids such as acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, p-toluenesulfonic acid, tartaric acid, trifluoroacetic acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, arylsulfonic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethanedisulfonic acid, 2- hydroxyethanesulfonic acid, benzenesulfonic acid, 2-naphthalenesulfonic acid, 4-methylbicyclo- [2.2.2]oct-2-ene-1-carboxylic acid, glucoheptonic acid, 4,4’-methylenebis-(3-hydroxy-2-ene-1- carboxylic acid), 3-phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid, gluconic acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, and muconic acid.
[0286] In some embodiments, those compounds described herein which comprise a free acid group react with a suitable base, such as the hydroxide, carbonate, bicarbonate, or sulfate of a pharmaceutically acceptable metal cation, with ammonia, or with a pharmaceutically acceptable organic primary, secondary, tertiary, or quaternary amine. Representative salts include the alkali or alkaline earth salts, like lithium, sodium, potassium, calcium, and magnesium, and aluminum salts, and the like. Illustrative examples of bases include sodium hydroxide, potassium hydroxide, choline hydroxide, sodium carbonate, N+(C1-4 alkyl)4, and the like. [0287] Representative organic amines useful for the formation of base addition salts include ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine, piperazine, and the like. It should be understood that the compounds described herein also include the quaternization of any basic nitrogen-containing groups they contain. In some embodiments, water or oil-soluble or dispersible products are obtained by such quaternization. Solvates [0288] In some embodiments, the compounds described herein exist as solvates. This disclosure provides for methods of treating diseases by administering such solvates. This disclosure further provides for methods of treating diseases by administering such solvates as pharmaceutical compositions. [0289] Solvates contain either stoichiometric or non-stoichiometric amounts of a solvent, and, in some embodiments, are formed during the process of crystallization with pharmaceutically acceptable solvents such as water, ethanol, and the like. Hydrates are formed when the solvent is water, or alcoholates are formed when the solvent is alcohol. Solvates of the compounds described herein can be conveniently prepared or formed during the processes described herein. In addition, the compounds provided herein can exist in unsolvated as well as solvated forms. In general, the solvated forms are considered equivalent to the unsolvated forms for the purposes of the compounds and methods provided herein. Accordingly, one aspect of the present disclosure pertains to hydrates and solvates of compounds of the present disclosure and/or their pharmaceutical acceptable salts, as described herein, that can be isolated and characterized by methods known in the art, such as, thermogravimetric analysis (TGA), TGA-mass spectroscopy, TGA-Infrared spectroscopy, powder X-ray diffraction (PXRD), Karl Fisher titration, high resolution X-ray diffraction, and the like. Preparation of the Compounds [0290] The compounds used in the reactions described herein are made according to organic synthesis techniques known to those skilled in this art, starting from commercially available
chemicals and/or from compounds described in the chemical literature. “Commercially available chemicals” are obtained from standard commercial sources including Acros Organics (Pittsburgh, PA), Aldrich Chemical (Milwaukee, WI, including Sigma Chemical and Fluka), Apin Chemicals Ltd. (Milton Park, UK), Avocado Research (Lancashire, U.K.), BDH, Inc. (Toronto, Canada), Bionet (Cornwall, U.K.), Chem Service Inc. (West Chester, PA), Crescent Chemical Co. (Hauppauge, NY), Eastman Organic Chemicals, Eastman Kodak Company (Rochester, NY), Fisher Scientific Co. (Pittsburgh, PA), Fisons Chemicals (Leicestershire, UK), Frontier Scientific (Logan, UT), ICN Biomedicals, Inc. (Costa Mesa, CA), Key Organics (Cornwall, U.K.), Lancaster Synthesis (Windham, NH), Maybridge Chemical Co. Ltd. (Cornwall, U.K.), Parish Chemical Co. (Orem, UT), Pfaltz & Bauer, Inc. (Waterbury, CN), Polyorganix (Houston, TX), Pierce Chemical Co. (Rockford, IL), Riedel de Haen AG (Hanover, Germany), Spectrum Quality Product, Inc. (New Brunswick, NJ), TCI America (Portland, OR), Trans World Chemicals, Inc. (Rockville, MD), and Wako Chemicals USA, Inc. (Richmond, VA). [0291] Suitable reference books and treatises that detail the synthesis of reactants useful in the preparation of compounds described herein, or provide references to articles that describe the preparation, include for example, “Synthetic Organic Chemistry”, John Wiley & Sons, Inc., New York; S. R. Sandler et al., “Organic Functional Group Preparations,” 2nd Ed., Academic Press, New York, 1983; H. O. House, “Modern Synthetic Reactions”, 2nd Ed., W. A. Benjamin, Inc. Menlo Park, Calif.1972; T. L. Gilchrist, “Heterocyclic Chemistry”, 2nd Ed., John Wiley & Sons, New York, 1992; J. March, “Advanced Organic Chemistry: Reactions, Mechanisms and Structure”, 4th Ed., Wiley-Interscience, New York, 1992. Additional suitable reference books and treatises that detail the synthesis of reactants useful in the preparation of compounds described herein, or provide references to articles that describe the preparation, include for example, Fuhrhop, J. and Penzlin G. “Organic Synthesis: Concepts, Methods, Starting Materials”, Second, Revised and Enlarged Edition (1994) John Wiley & Sons ISBN: 3-527-29074-5; Hoffman, R.V. “Organic Chemistry, An Intermediate Text” (1996) Oxford University Press, ISBN 0-19-509618-5; Larock, R. C. “Comprehensive Organic Transformations: A Guide to Functional Group Preparations” 2nd Edition (1999) Wiley-VCH, ISBN: 0-471-19031-4; March, J. “Advanced Organic Chemistry: Reactions, Mechanisms, and Structure” 4th Edition (1992) John Wiley & Sons, ISBN: 0-471-60180-2; Otera, J. (editor) “Modern Carbonyl Chemistry” (2000) Wiley- VCH, ISBN: 3-527-29871-1; Patai, S. “Patai's 1992 Guide to the Chemistry of Functional Groups” (1992) Interscience ISBN: 0-471-93022-9; Solomons, T. W. G. “Organic Chemistry” 7th Edition (2000) John Wiley & Sons, ISBN: 0-471-19095-0; Stowell, J.C., “Intermediate Organic Chemistry” 2nd Edition (1993) Wiley-Interscience, ISBN: 0-471-57456-2; “Industrial Organic Chemicals: Starting Materials and Intermediates: An Ullmann's Encyclopedia” (1999)
John Wiley & Sons, ISBN: 3-527-29645-X, in 8 volumes; “Organic Reactions” (1942-2000) John Wiley & Sons, in over 55 volumes; and “Chemistry of Functional Groups” John Wiley & Sons, in 73 volumes. [0292] Specific and analogous reactants are optionally identified through the indices of known chemicals prepared by the Chemical Abstract Service of the American Chemical Society, which are available in most public and university libraries, as well as on-line. Chemicals that are known but not commercially available in catalogs are optionally prepared by custom chemical synthesis houses, where many of the standard chemical supply houses (e.g., those listed above) provide custom synthesis services. A reference for the preparation and selection of pharmaceutical salts of the compounds described herein is P. H. Stahl & C. G. Wermuth “Handbook of Pharmaceutical Salts”, Verlag Helvetica Chimica Acta, Zurich, 2002. III. Pharmaceutical Compositions [0293] The radiopharmaceutical conjugate described herein, including e.g., pharmaceutically acceptable salt or solvate thereof, can be administered per se as a pure chemical or as a component of a pharmaceutically acceptable formulation. In some embodiments, a conjugate described herein is combined with a pharmaceutically suitable or acceptable carrier selected on the basis of a chosen route of administration and standard pharmaceutical practice as described, for example, in Remington: The Science and Practice of Pharmacy (Gennaro, 21st Ed. Mack Pub. Co., Easton, PA (2005)). Provided herein is a pharmaceutical composition comprising at least one conjugate described herein, or a stereoisomer, pharmaceutically acceptable salt, amide, ester, solvate, or N-oxide thereof, together with one or more pharmaceutically acceptable carriers. The carrier(s) (or excipient(s)) is acceptable or suitable if the carrier is compatible with the other ingredients of the composition and not deleterious to the recipient (i.e., the subject or patient) of the composition. [0294] In one aspect, the disclosure provides a pharmaceutical composition comprising a herein described conjugate, or a pharmaceutically acceptable salt or solvate thereof, and a pharmaceutically acceptable excipient or carrier. In certain embodiments, the conjugate as described is substantially pure, in that it contains less than about 10%, less than about 5%, or less than about 1%, or less than about 0.1%, of other organic small molecules, such as unreacted intermediates or synthesis by-products that are created, for example, in one or more of the steps of a synthesis method. [0295] Pharmaceutical compositions can include pharmaceutically acceptable carriers, diluents or excipients. Exemplary pharmaceutically acceptable carriers include solvents (aqueous or non- aqueous), solutions, emulsions, dispersion media, coatings, isotonic and absorption promoting or
delaying agents, compatible with pharmaceutical administration. Such formulations can be contained in a liquid; emulsion, suspension, syrup or elixir, or solid form; tablet (coated or uncoated), capsule (hard or soft), powder, granule, crystal, or microbead. Supplementary components (e.g., preservatives, antibacterial, antiviral and antifungal agents) can also be incorporated into the compositions. Pharmaceutical compositions can be formulated to be compatible with a particular local or systemic route of administration. Thus, pharmaceutical compositions include carriers, diluents, or excipients suitable for administration by particular routes. [0296] The compounds and pharmaceutical compositions of the current disclosure can be administered by any suitable means, including oral, topical (including buccal and sublingual), rectal, vaginal, transdermal, parenteral, subcutaneous, intraperitoneal, intrapulmonary, intradermal, intrathecal and epidural and intranasal, and, if desired for local treatment, intralesional administration. The term parenteral as used herein includes e.g., subcutaneous, intravenous, intramuscular, intrasternal, intraperitoneal, and infusion techniques. The term parenteral also includes injections, into the eye or ocular, intravitreal, intrabuccal, transdermal, intranasal, into the brain, including intracranial and intradural, into the joints, including ankles, knees, hips, shoulders, elbows, wrists, and the like, and in suppository form. In certain embodiments, the compounds and/or formulations are administered orally. In certain embodiments, the compounds and/or formulations are administered by systemic administration. In certain embodiments, the compounds and/or formulations are administered parenterally. In certain embodiments, the compounds and/or formulations are administered locally at a targeted site. [0297] In some embodiments, conjugates, or pharmaceutically acceptable salts or solvates thereof, and pharmaceutical compositions described herein are administered via parenteral injection as liquid solution, which can include other chemical components, such as carriers, stabilizers, diluents, dispersing agents, suspending agents, thickening agents, preservatives, or excipients. Parenteral injections can be formulated for bolus injection or continuous infusion. The pharmaceutical compositions can be in a form suitable for parenteral injection as a sterile suspension, solution or emulsion in oily or aqueous vehicles, and can contain formulatory agents such as suspending, stabilizing or dispersing agents. Pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water soluble form. Solutions or suspensions used for parenteral, intradermal, or subcutaneous application can include: a sterile diluent such as water for injection, saline solution, fixed oils, polyethylene glycols, glycerin, propylene glycol or other synthetic solvents; antibacterial agents such as benzyl alcohol or methyl parabens; antioxidants such as ascorbic acid, gentisic acid, or sodium bisulfite; surfactants such
as polysorbate 80; chelating agents such as ethylenediaminetetraacetic acid; buffers such as acetates, citrates or phosphates and agents for the adjustment of tonicity such as sodium chloride or dextrose. pH can be adjusted with acids or bases, such as hydrochloric acid or sodium hydroxide. In some embodiments, the pharmaceutical composition comprises a reductant. The presence of a reductant can help minimize potential radiolysis. In some embodiments, the reductant is ascorbic acid, gentisic acid, sodium thiosulfate, citric acid, tartaric acid, or a combination thereof. [0298] Pharmaceutical compositions comprising the conjugates or pharmaceutically acceptable salts or solvates thereof described herein can be prepared according to standard techniques and further comprise a pharmaceutically acceptable carrier. In some embodiments, normal saline can be employed as the pharmaceutically acceptable carrier. Other suitable carriers include, e.g., water, buffered water, 0.9% isotonic saline, 0.4% saline, 0.3% glycine, and the like, including glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin, etc. These compositions can be sterilized by conventional sterilization techniques. The resulting aqueous solutions may be packaged for use or filtered under aseptic conditions and lyophilized. In some embodiments, the lyophilized preparation is combined with a sterile aqueous solution prior to administration. The compositions can contain pharmaceutically acceptable auxiliary substances as appropriate to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sodium lactate, sorbitan monolaurate, triethanolamine oleate, etc. Pharmaceutical compositions can be selected according to their physical characteristic, including, but not limited to fluid volumes, viscosities and other parameters in accordance with the particular mode of administration selected. The amount of conjugates administered can depend upon the particular targeting moiety used, the disease state being treated, the therapeutic agent being delivered, and the judgment of the clinician. [0299] The concentration of the conjugates or pharmaceutically acceptable salts or solvates thereof described herein in the pharmaceutical formulations can vary. In some embodiments, the conjugate is present in the pharmaceutical composition from about 0.05% to about 1% by weight, about 1% to about 2% by weight, about 2% to about 5% by weight, about 5% to about 10% by weight, about 10% to about 30% by weight, about 30% to about 50% by weight, about 50% to about 75% by weight, or about 75% to about 99% by weight. [0300] Pharmaceutical compositions are administered in a manner appropriate to the disease to be treated. An appropriate dose and a suitable duration and frequency of administration will be determined by such factors as the condition of the subject, the type and severity of the subject's disease, the particular form of the active ingredient, and the method of administration. In some
embodiments, an appropriate dose and treatment regimen provides the composition(s) in an amount sufficient to provide therapeutic and/or prophylactic benefit (e.g., an improved clinical outcome), or a lessening of symptom severity. Optimal doses are generally determined using experimental models and/or clinical trials. The optimal dose depends upon the body mass, weight, or blood volume of the subject. [0301] The amount of conjugates or pharmaceutically acceptable salts or solvates thereof and/or pharmaceutical compositions administered can be sufficient to deliver a therapeutically effective dose of the particular subject. In some embodiments, conjugate dosages can be between about 0.1 pg and about 50 mg per kilogram of body weight, 1 µg and about 50 mg per kilogram of body weight, or between about 0.1 and about 10 mg/kg of body weight. Therapeutically effective dosages can also be determined at the discretion of a physician. By way of example only, the dose of the conjugate or a pharmaceutically acceptable salt or solvate thereof described herein for methods of treating a disease as described herein is about 0.001 mg/kg to about 1 mg/kg body weight of the subject per dose. In some embodiments, the dose of conjugate or a pharmaceutically acceptable salt or solvate thereof described herein for the described methods is about 0.001 mg to about 1000 mg per dose for the subject being treated. In some embodiments, a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of from about 0.01 mg to about 500 mg, from about 0.01 mg to about 100 mg, or from about 0.01mg to about 50 mg. In some embodiments, a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of about 0.01 picomole to about 1 mole, about 0.1 picomole to about 0.1 mole, about 1 nanomole to about 0.1 mole, or about 0.01 micromole to about 0.1 millimole. In some embodiments, a conjugate or a pharmaceutically acceptable salt or solvate thereof described herein is administered to a subject at a dosage of about 0.01 Gbq to about 1000 Gbq, about 0.5 Gbq to about 100 Gbq, or about 1 Gbq to about 50 Gbq. In some embodiments, the dose is administered once a day, 1 to 3 times a week, 1 to 4 times a month, or 1 to 12 times a year. [0302] The pharmaceutical formulations can be packaged in unit dosage form for ease of administration and uniformity of dosage. A unit dosage form can refer to physically discrete units suited as unitary dosages for the subject to be treated; each unit containing a predetermined quantity of active compound calculated to produce the desired therapeutic effect in association with the pharmaceutical carrier or excipient. IV. Method of Treatment. [0303] In one aspect, the disclosure provides methods of treating a disease or condition in a subject in need thereof. In some embodiments, the methods comprise administering a conjugate
or a pharmaceutically acceptable salt or solvate thereof described herein, or a pharmaceutical composition comprising the same to the subject in need thereof. In some embodiments, provided herein is a method of providing a therapeutic and/or prophylactic benefit to a subject in need thereof comprising administering a compound or pharmaceutical composition described herein. [0304] In some embodiments, the methods comprise administering to a subject a therapeutically effective amount of a conjugate or a pharmaceutically acceptable salt or solvate thereof. In some embodiments, the conjugate or pharmaceutically acceptable salt or solvate thereof is administered in a pharmaceutical composition. In some embodiments, the subject has cancer. In some embodiments, the cancer is a solid tumor or hematological cancer. [0305] In embodiments, the treatment is sufficient to reduce or inhibit the growth of the subject's tumor, reduce the number or size of metastatic lesions, reduce tumor load, reduce primary tumor load, reduce invasiveness, prolong survival time, or maintain or improve the quality of life. [0306] In some embodiments, provided herein are methods for killing a cell comprising contacting the cell with a conjugate or a pharmaceutically acceptable salt or solvate thereof. In some embodiments, the cell expresses a guanylate cyclase C (GCC) receptor. In some embodiments, the conjugate or pharmaceutically acceptable salt or solvate thereof binds to a structure on the cell, e.g., GCC. In some embodiments, the conjugate or pharmaceutically acceptable salt or solvate thereof releases a number of alpha particles by natural radioactive decay. In some embodiments, the conjugate or pharmaceutically acceptable salt or solvate thereof releases a number of beta particles, gamma rays, and/or Auger electrons by natural radioactive decay. The conjugate described herein can kill a cell by radiation. In some embodiments, the conjugate kills the cell directly by radiation. In some embodiments, the radiation creates, in the cell, oxidized bases, abasic sites, single-stranded breaks, double- stranded breaks, DNA crosslink, chromosomal rearrangement, or a combination thereof. In some embodiments, the conjugate kills the cell by inducing double-stranded DNA breaks. In some embodiments, the released alpha particles are sufficient to kill the cell. In some embodiments, the released alpha particles are sufficient to stop cell growth. In some embodiments, the conjugate kills the cell indirectly via the production of reactive oxygen species (ROS) such as free hydroxyl radicals. In some embodiments, the conjugate kills the cell indirectly by releasing tumor antigens from one or more different cells, which can have vaccine effect. In some embodiments, the conjugate kills the cell by abscopal effect. In some embodiments, the cell is a cancer cell. In some embodiments, the method comprises killing a cell with an alpha-particle emitting radionuclide. [0307] After contacting a cell, the described conjugate can be internalized by the cell. The internalization can be mediated by cell receptors, cell membrane endocytosis, etc. In some
embodiments, rapid internalization rate into cancer cells accompanied by a slow externalization rate can offer therapeutic benefit. [0308] In one aspect, the disclosed conjugate or a pharmaceutically acceptable salt or solvate thereof is configured to treat cancer by ablating tumor cells. In some embodiments, the conjugate or a pharmaceutically acceptable salt or solvate thereof does not modulate the biology of the tumor cell and/or the surrounding stroma. In some embodiments, the conjugate or a pharmaceutically acceptable salt or solvate thereof does not modulate immune cells. In some embodiments, the ablating of tumor cells can lead to a downstream immunological cascade. [0309] In one aspect, provided herein are methods and compositions for treating cancers. Cancer includes tissue and organ carcinogenesis including metastases such as for example gastrointestinal cancer, (e.g., gastric cancer, esophageal cancer, pancreatic cancer colorectal cancer, intestinal cancer, anal cancer, liver cancer, gallbladder cancer, or colon cancer; lung cancer; thyroid cancer; skin cancer (e.g., melanoma); oral cancer; urinary tract cancer (e.g. bladder cancer or kidney cancer); blood cancer (e.g. myeloma or leukemia) or prostate cancer. In some embodiments, the present disclosure provides methods and compositions for treating gastrointestinal cancer in a subject in need thereof by administering an effective amount of a GCC binding peptide conjugate to the subject. Non-limiting examples of gastrointestinal cancers that can be treated according to the methods of the present disclosure include gastric cancer, esophageal cancer, pancreatic cancer, lung cancer (small cell lung cancer and/or non small-cell lung cancer), colorectal cancer, intestinal cancer, anal cancer, liver cancer, gallbladder cancer, or colon cancer. [0310] In one aspect, provided herein are methods and compositions for treating a GCC- expressing cancer. In some embodiments, the GCC-expressing cancer to be treated is a primary or metastatic cancer of gastrointestinal origin, such as colorectal cancer, stomach cancer, small intestine cancer, or esophageal cancer. In some embodiments, the GCC-expressing cancer to be treated is primary or metastatic pancreatic cancer. In some embodiments, the GCC-expressing cancer to be treated is primary or metastatic lung cancer, such as squamous cell carcinoma, adenosquamous carcinoma, or adenocarcinoma. In some embodiments, the GCC-expressing cancer to be treated is a sarcoma, such as leiomyosarcoma or rhabdomyosarcoma. In some embodiments, the GCC-expressing cancer to be treated is a primary or metastasized neuroectodermal tumor, such as aphaechromotcytoma or a paraganglioma. In some embodiments, the GCC-expressing cancer is a primary or a metastasized bronchopulmonary or a gastrointestinal neuroendocrine tumor. In some embodiments, the cancer is colorectal cancer.
[0311] The method can be useful in treating a relevant disorder at any stage or subclassification. For example, method can be used to treat early or late stage colon cancer, or colon cancer of any of stages 0, I, IIA, IIB, IIIA, IIIB, IIIC, and IV. Disease to be Imaged [0312] In addition to the methods of treatment described above, the compounds and compositions described herein can be used to image, and/or as part of a treatment for, any disease in which GCC is expressed, and in which a doctor wants to deliver a therapeutic to the GCC expressing tissue. An example of this type of disease is cancer (e.g., a cancer described above). GCC is expressed, for example, in colorectal cancer, gastric cancer, pancreatic cancer, esophageal cancer, cancer of the gastroesophageal junction, small intestine cancer, lung cancer, soft tissue sarcomas such as leiomyosarcomas and rhabdomyosarcomas, gastrointestinal and bronchopulmonary neuroendocrine tumors, and neuroectodermal tumors, and metastases derived from GCC- expressing primary tumors. An example of the last category is a liver metastasis derived from a primary colorectal tumor. Conjugates for imaging applications, e.g., single-photon emission computed tomography (SPECT) and positron emission tomography (PET), can comprise a radionuclide suitable for use as imaging isotopes such as the isotopes in Table 5B. [0313] Further provided herein are methods and compositions that distinguish between GCC- expressing tumors and non-GCC-expressing tumors. In some embodiments, the compounds can identify patients likely to respond to a GCC-targeted therapy, e.g., an anti-GCC antibody, e.g., an immunoconjugate comprising an anti-GCC antibody. Accordingly, the conjugate can be administered as a companion diagnostic for GCC-targeted therapy thereby informing whether a patient suffering from a proliferative disease such as cancer, or a gastrointestinal disorder such as inflammatory bowel syndrome, Crohn's Disease or constipation, or should be treated or not with a GCC-targeted therapy, based on the presence or absence, respectively, of GCC expression on the surface of or within the patient's cells or tissue. A patient having one more cells that express GCC on the cell surface or within the cell can be candidate for treatment with a GCC-targeted therapy. [0314] In one aspect, described herein is a method of treatments that comprises administering a first conjugate and a second conjugate. The first conjugate can be used as companion diagnostics and the second conjugate can be used for therapeutics. In some embodiments, the first conjugate and the second conjugate have the same structure except for the radionuclide. In some embodiments, the first conjugate comprises a gamma particle emitting radionuclide. In some embodiments, the first conjugate comprises a radionuclide of Table 5B. In some embodiments, the first conjugate comprises a radionuclide selected from Lu-177, In-111, Ga-68, Cu-64, and Zr-
89. In some embodiments, the second conjugate comprises an alpha or beta-particle emitting radionuclide. In some embodiments, the second conjugate comprises a radionuclide of Table 5A. In some embodiments, the second conjugate comprises Ac-225. Combination Therapy [0315] In some embodiments, a GCC binding peptide conjugate described herein can be administered alone or in combination with one or more additional therapeutic agents. In some embodiments, the conjugate and a second therapeutic agent are administered in combination to prevent or treat inflammation, cancer and other disorders, particularly of the gastrointestinal tract. [0316] For example, the combination therapy can include a composition comprising a conjugate described herein co-formulated with, and/or co-administered with, one or more additional therapeutic agents, e.g., one or more anti-cancer agents, e.g., cytotoxic or cytostatic agents, immune checkpoint inhibitors, hormone treatment, vaccines, and/or other immunotherapies. In some embodiments, the conjugate is administered in combination with other therapeutic treatment modalities, including surgery, radiation, cryosurgery, and/or chemotherapy. Such combination therapies may advantageously utilize lower dosages of the administered therapeutic agents, thus avoiding possible toxicities or complications associated with the various monotherapies. [0317] When administered in combination, two (or more) different treatments can be delivered to the subject during the course of the subject's affliction with the disorder, e.g., the two or more treatments are delivered after the subject has been diagnosed with the disorder and before the disorder has been cured or eliminated. In some embodiments, the delivery of one treatment is still occurring when the delivery of the second begins, so that there is overlap. This is sometimes referred to herein as “simultaneous” or “concurrent delivery.” In some embodiments, the delivery of one treatment ends before the delivery of the other treatment begins. In some embodiments of either case, the treatment is more effective because of combined administration. For example, the second treatment is more effective, e.g., an equivalent effect is seen with less of the second treatment, or the second treatment reduces symptoms to a greater extent, than would be seen if the second treatment were administered in the absence of the first treatment, or the analogous situation is seen with the first treatment. In some embodiments, delivery is such that the reduction in a symptom, or other parameter related to the disorder is greater than what would be observed with one treatment delivered in the absence of the other. The effect of the two treatments can be partially additive, wholly additive, or greater than additive. The delivery can be such that an effect of the first treatment delivered is still detectable when the second is delivered. [0318] In some embodiments, the herein-described conjugate is used in combination with a chemotherapeutic agent, e.g., a DNA damaging chemotherapeutic agent. Non-limiting examples
of DNA damaging chemotherapeutic agents include topoisomerase I inhibitors (e.g., irinotecan, topotecan, camptothecin and analogs or metabolites thereof, and doxorubicin); topoisomerase II inhibitors (e.g., etoposide, teniposide, and daunorubicin); alkylating agents (e.g., melphalan, chlorambucil, busulfan, thiotepa, ifosfamide, carmustine, lomustine, semustine, streptozocin, decarbazine, methotrexate, mitomycin C, and cyclophosphamide); DNA intercalators (e.g., cisplatin, oxaliplatin, and carboplatin); DNA intercalators and free radical generators such as bleomycin; and nucleoside mimetics (e.g., 5-fluorouracil, capecitibine, gemcitabine, fludarabine, cytarabine, mercaptopurine, thioguanine, pentostatin, and hydroxyurea). In some embodiments, the herein-described conjugate is used in combination with a radiation sensitizer, which makes tumor cells more sensitive to radiation therapy. In some embodiments, the herein-described conjugate is used in combination with a DNA damage repair inhibitor (or DNA damage response (DDR) inhibitor). [0319] In some embodiments, the method comprises administering a second agent that binds to a GCC receptor. In some embodiments, the method comprises administering a second agent that is a GCC receptor agonist. In some embodiments, the method comprises administering a second agent that is a GCC receptor antagonist. In some embodiments, a GCC binding peptide conjugate described herein is administered in combination with one or more of GCC binding peptides such as a GCC receptor agonist. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-19. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides consists of an amino acid sequence of any one of SEQ ID NOs: 1-42. In some embodiments, the GCC binding peptide conjugate is administered in combination with one or more of GCC binding peptides, wherein the one or more of GCC binding peptides consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. [0320] In some embodiments, a GCC binding peptide is co-administered in conjunction with the radiopharmaceutical. In some embodiments, a GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-42. In some embodiments, a GCC binding peptide comprises an amino acid sequence
that has at least 85% identity to any one of SEQ ID NOs: 1-19. In some embodiments, a GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-19. [0321] In some embodiments, a GCC binding peptide conjugate described herein is administered in combination with one or more of GCC binding peptides. In some embodiments, a GCC binding peptide conjugate described herein is administered with one or more of linaclotide, plecanatide and dolcantide. In some embodiments, the conjugate is administered in combination with linaclotide. [0322] In some embodiment, the GCC binding peptide conjugate is administered in combination with one or more additional therapeutic agents selected from the group consisting of phosphodiesterase inhibitors, cyclic nucleotides (such as cGMP and cAMP), a laxative (such as SENNA, METAMUCIL, MIRALAX, PEG, or calcium polycarbophil), a stool softener, an anti- tumor necrosis factor alpha therapy for IBD (such as REMICADE, ENBREL, or HUMAIRA), anti-inflammatory drugs (such as COX-2 inhibitors, sulfasalazine, 5-ASA derivatives and NSAIDS), anti-GCC antibody molecules, and GCC receptor agonists. In certain embodiments, the GCC binding peptide conjugate is administered in combination with an effective dose of an inhibitor of cGMP-specific phosphodiesterase (cGMP-PDE). cGMP-PDE inhibitors include, for example, suldinac sulfone, zaprinast, motapizone, vardenifil, and sildenafil. When administered with a second therapeutic agent, the herein-described conjugate can be administered simultaneously or sequentially with the second agent, and they can be administered via the same or different routes. [0323] In some embodiments, the conjugate is administered in combination with a treatment that increases urinary excretion. In one embodiment, the agent which increases urinary excretion, is administered prior to, concurrently with and/or after administration with the radiolabeled peptide. In one embodiment, the agent which ameliorates bladder toxicity associated with therapy is saline, e.g., intravenous saline, D5 half normal saline or D5 water. [0324] In some embodiments, the subject is 4 to 100 years old. In some embodiments, the subject is 5 to 10, 5 to 15, 5 to 18, 5 to 25, 5 to 35, 5 to 45, 5 to 55, 5 to 65, 5 to 75, 10 to 15, 10 to 18, 10 to 25, 10 to 35, 10 to 45, 10 to 55, 10 to 65, 10 to 75, 15 to 18, 15 to 25, 15 to 35, 15 to 45, 15 to 55, 15 to 65, 15 to 75, 18 to 25, 18 to 35, 18 to 45, 18 to 55, 18 to 65, 18 to 75, 25 to 35, 25 to 45, 25 to 55, 25 to 65, 25 to 75, 35 to 45, 35 to 55, 35 to 65, 35 to 75, 45 to 55, 45 to 65, 45 to 75, 55 to 65, 55 to 75, or 65 to 75 years old. In some embodiments, the subject is at least 5, 10, 15, 18, 25, 35, 45, 55, or 65 years old. In some embodiments, the subject is at most 10, 15, 18, 25, 35, 45, 55, 65, or 75 years old.
[0325] Although the present disclosure and its advantages have been described in detail, it should be understood that various changes, substitutions and alterations can be made herein without departing from the spirit and scope of the disclosure as defined in the appended claims. [0326] The present disclosure is further illustrated in the following Examples which are given for illustration purposes only and are not intended to limit the disclosure in any way. EXAMPLES A: Synthesis of the Compounds [0327] Example A1: Experimental procedures [0328] Solid phase peptide synthesis (SPPS) was performed in a standard manual reaction vessel under nitrogen. 2-CTC resin was purchased from Sunresin New Materials Co. (China). Fmoc protected amino acids were purchased from GL Biochem (China). HBTU and HATU were purchased from Highfine Biotech Co. (China). Piperidine was purchased from Damao Chemical Reagent Factory (China). The peptides and their derivatives were purified on a Gilson GX-281 preparative HPLC system using reverse-phase C18 columns (Gemini, 5 µm, 110 Å + luna, 10 µm, 100 Å) at 30 °C. HPLC solvents consisted of H2O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B). [0329] High performance liquid chromatography (HPLC) analyses were performed on an Agilent 1260 series equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.2 network workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B). Conditions: a Phenomenex Gemini-NX C-18 (5 µm, 110 Å, 4.6 × 250 mm) column was used with a flow rate of 1.0 mL/min. [0330] LC-MS analyses were carried out on an Agilent 1200 series coupled to an Agilent MSD G6125C, equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.3 standalone workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B). LC-MS conditions are as follows:
* Method I uses acetonitrile containing 0.0375% trifluoroacetic acid for mobile phase B. Example A-1: Synthesis of 4-(naphthalene-2-sulfonamido)-4-oxobutanoic acid (2)
[0331] To a solution of sulfonamide 1 (10.0 g, 48.3 mmol) in THF (20.0 mL) under N2 atmosphere were added DMAP (7.07 g, 57.9 mmol), TEA (5.86 g, 57.9 mmol) and succinic anhydride (5.79 g, 57.9 mmol). The reaction mixture was stirred for 18 h at room temperature and then refluxed for 6 h. The solvent was concentrated in vacuo, and the resulting residue was partitioned between ethyl acetate and hydrochloric acid (1.0 N). The layers were separated, and the organic layer was washed with brine and saturated aqueous NaHCO3 solution. The basic aqueous layer was acidified with hydrochloric acid (1.0 N). The precipitate was collected through filtration and dried to give compound 2 (10.3 g, 27.6 mmol, 57.1%) as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 12.23 (s, 1H), 8.58 (s, 1 H), 8.22-8.15 (m, 1H), 8.09 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.74-7.72 (m, 1H), 7.70-7.69 (m, 2H), 2.46 (br d, J = 6.9 Hz, 2H), 2.38- 2.31 (m, 2H); MS(ESI): m/e 308.0 [M + H]+. Example A-2: Synthesis of 6-((((4,4-diphenylcyclohexyl)oxy)(hydroxy)phosphoryl)oxy)hexanoic acid (12)
[0332] To a solution of aldehyde 3 (5.00 g, 25.5 mmol) and alkene 4 (3.34 g, 47.7 mmol) in EtOH (20.0 mL) at 0 °C was slowly added a pre-cooled solution of KOH (715 mg, 12.7 mmol) in EtOH (10.0 mL) over 10 min. The reaction mixture was stirred at 0 °C for 3 h. After the solvent was removed in vacuo, the residue was partitioned between H2O (30.0 mL) and EtOAc (50.0 mL). The mixture was acidified to pH 3 with 2.0 M aqueous HCl. The layers were separated, and the organic fraction was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate=1/0 to 5/1, Rf = 0.60). to afford ketone 5 (4.00 g, 16.1 mmol, 63.2%) as a yellow solid.1H NMR (400 MHz, CDCl3): δ 7.31 - 7.38 (m, 5 H), 7.23 - 7.29 (m, 6 H), 6.23 (d, J = 10.2 Hz, 1 H), 2.65 - 2.78 (m, 2 H), 2.44 (dd, J = 7.2, 5.6 Hz, 2 H). [0333] An aqueous Raney-Ni (138 mg, 1.61 mmol) suspension, washed free of salt in advance, was added to ketone 5 (4.00 g, 16.1 mmol) in THF (50.0 mL). The resulting mixture was stirred vigorously at room temperature under hydrogen (50 psi) for 1 h. The mixture was filtered, and the filtrate was concentrated in vacuo to give alcohol 6 (4.00 g, 15.9 mmol, 98.4%) as a white solid. 1H NMR (400 MHz, CDCl3): δ 7.35 - 7.41 (m, 2 H), 7.19 - 7.33 (m, 6 H), 7.06 - 7.17 (m, 2 H), 4.44 (d, J = 4.4 Hz, 1 H), 3.52 - 3.64 (m, 1 H), 2.56 - 2.69 (m, 2 H), 1.97 (br t, J = 11.2 Hz, 2 H), 1.69 (br dd, J = 10.0, 3.8 Hz, 2 H), 1.24 - 1.41 (m, 2 H). [0334] The mixture of alcohol 7 (6.00 g, 37.5 mmol), 2-cyanoethyl N,N- diisopropylchlorophosphor-amidite 8 (9.31 g, 39.32 mmol) and DIEA (19.4 g, 150 mmol) in DCM (250 mL) was stirred at room temperature for 2 h. The mixture was washed with saturated aqueous NaHCO3 solution (50.0 mL × 2). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate=1/0 to 10/1, with 0.1 % TEA, Rf = 0.80) to afford Compound 9 (5.70 g, 15.8 mmol, 42.2%) as a colorless oil.1H NMR (400 MHz, CDCl3): δ 4.14 - 4.18 (m, 2 H), 3.84 - 3.63
(m, 2 H), 3.61 - 3.55 (m, 4 H), 2.65 - 2.62 (m, 2 H), 2.31 - 2.27 (m, 2 H), 1.64 - 1.62 (m, 4 H), 1.39 - 1.26 (m, 2 H), 1.24 - 1.22 (m, 3 H), 1.18 - 1.01 (m, 12 H). [0335] To a solution of Compound 9 (2.00 g, 5.55 mmol) and alcohol 6 (1.47 g, 5.83 mmol) in acetonitrile (30.0 mL) was added tetrazole (0.45 M, 13.0 mL). The resulting mixture was stirred under N2 for 3 h. t-Butyl hydroperoxide (5.64 g, 43.8 mmol, 70% purity) was added, and the reaction was stirred at 25 °C for an additional 1 h. The mixture was quenched with saturated Na2S2O6 solution (50.0 mL) and extracted with EtOAc (50.0 mL × 3). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate=1/0 to 1/1 with 0.1% TEA, Rf = 0.10) to afford Compound 10 (1.50 g, 2.93 mmol, 52.8%) as a colorless oil.1H NMR (400 MHz, CDCl3): δ 7.23 - 7.32 (m, 7 H), 7.26 (br d, J = 7.4 Hz, 1 H), 7.11 - 7.20 (m, 2 H), 4.52 - 4.62 (m, 1 H), 4.20 (dt, J = 8.0, 6.2 Hz, 2 H), 4.14 (br s, 1 H), 4.05 - 4.16 (m, 3 H), 2.74 (t, J = 6.2 Hz, 2 H), 2.52 - 2.59 (m, 2 H), 2.19 - 2.34 (m, 4 H), 1.90 - 2.00 (m, 2 H), 1.77 - 1.88 (m, 2 H), 1.59 - 1.76 (m, 5 H), 1.39 - 1.46 (m, 2 H), 1.22 - 1.26 (m, 2 H), 1.21 - 1.26 (m, 1 H). [0336] Compound 10 (1.50 g, 2.93 mmol) was dissolved in ammonia (50.0 mL, 2.00 M in MeOH). The mixture was stirred at 25 °C for 12 h under N2 and concentrated in vacuo to afford 11 (1.39 g, 2.93 mmol, 100%) as a colorless oil. The crude product was used directly in the next step.1H NMR (400 MHz, CDCl3): δ 7.21 - 7.33 (m, 8 H), 7.09 - 7.20 (m, 2 H), 4.25 (dt, J = 7.8, 3.8 Hz, 1 H), 4.04 - 4.15 (m, 2 H), 3.76 - 3.84 (m, 2 H), 2.52 - 2.60 (m, 2 H), 2.23 - 2.32 (m, 2 H), 2.14 (br t, J = 10.6 Hz, 2 H), 1.82 - 1.94 (m, 2 H), 1.68 (br d, J = 8.4 Hz, 2 H), 1.64 (br s, 4 H), 1.33 - 1.42 (m, 2 H), 1.27 - 1.32 (m, 1 H), 1.21 - 1.27 (m, 3 H). [0337] To a solution of Compound 11 (1.39 g, 2.93 mmol) in THF (20.0 mL) and H2O (2.00 mL) was added LiOH·H2O (246 mg, 5.86 mmol). The mixture was stirred at 25 °C for 2 h. The volatiles were removed in vacuo and water (20.0 mL) was added. The mixture was washed with EtOAc (20 mL × 3), acidified with HCl (1.0 N) until pH < 7 and extracted with EtOAc (20.0 mL × 3). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give acid 12 (1.20 g, 2.60 mmol, 88.8%, 96.8% purity) as a colorless oil.1H NMR (400 MHz, CDCl3): δ 7.23 - 7.29 (m, 8 H), 7.08 - 7.19 (m, 2 H), 4.47 (dt, J = 6.8, 3.6 Hz, 1 H), 4.00 (q, J = 6.6 Hz, 2 H), 2.49 - 2.60 (m, 2 H), 2.33 (t, J = 7.2 Hz, 2 H), 2.15 - 2.26 (m, 2 H), 1.79 - 1.95 (m, 4 H), 1.61 - 1.71 (m, 4 H), 1.38 - 1.47 (m, 2 H); MS (ESI): m/e 447.4 [M + H]+. Example A-3: Synthesis of peptidyl-resin 13. Example discloses SEQ ID NO: 46
[0338] To the swollen 2-CTC resin (0.50 mmol, 1.10 mmol/g, 1.00 equiv) were added Fmoc- Tyr(tBu)-OH (0.23 g, 0.50 mmol) and DIEA (0.35 mL, 2.00 mmol) in DCM (4.00 mL). The mixture was agitated for 2 h under nitrogen. MeOH (0.50 mL) was then added. The resulting mixture was agitated for 30 min. After the reaction solution was removed through filtration, the resin was washed three times with DMF (10 mL). The Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. [0339] Subsequent amino acids were coupled using Fmoc-protected amino acid (3.00 equiv), HBTU (2.85 equiv) and DIEA (6.00 equiv) in dry DMF, shaking for 30 min. Pre-activation of any amino acid was not performed prior to coupling. Between amino acid couplings, the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. Success of Fmoc removal steps and amino acid couplings were monitored qualitatively using a ninhydrin test. [0340] Example A2: Synthesis of C-01 and La-C-01. Example discloses SEQ ID NOS 46-48, respectively, in order of appearance.
[0341] Fmoc-Ahx-OH (2.00 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide and then agitated for 30 min
under nitrogen. The resin was then washed three times with DMF. Fmoc removal was carried out while the peptide was on resin using 20% piperidine in DMF followed by filtration and washing. [0342] 2-(4,7,10-Tris(2-(tert-butoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecan-1-yl)acetic acid (DOTA-(OtBu)3-OH, 1.50 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide-Ahx and then agitated for 30 min under nitrogen. The resin was then washed three times with DMF. [0343] After the resin was washed three times with MeOH and dried under vacuum, a cocktail of trifluoroacetic acid/H2O/triisopropylsilane/3-mercaptopropionic acid (90:2.5:2.5:5.0) was added. The resulting mixture was stirred for 2 h at room temperature. Cold isopropyl ether was added. The precipitated crude linear peptide-DOTA 14 was collected through filtration and dried under vacuum. [0344] Oxidative folding of DOTA-linker-peptide 14: To a solution of crude 14 (0.88 g) in water (330 mL) and MeCN (100 mL) were added GSSG (798 mg) and GSH (798 mg). The pH of the solution was adjusted to 8.0 using saturated NH4HCO3 solution. The resulting mixture was stirred at room temperature for 12 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford С-01 (91.2 mg, 7.3% yield, 80.8% purity) as a white solid. [0345] La3+ complexation: To a solution of С-01 (45.3 mg, 80.8% purity, 14.5 µmol) in H2O (2.50 mL) and MeCN (0.5 mL) was added LaCl3 (4.43 mg, 18.0 µmol) and Na2CO3 (0.19 mg, 1.8 µmol). The resulting mixture was stirred at 70 °C for 1 h. After filtration, the crude product was purified by preparative HPLC to afford La-С-01 (7.9 mg, 19.2% yield, 95.0% purity) as a white solid. [0346] Example A3: Synthesis of C-05 and La-C-05. Example discloses SEQ ID NOS 46, and 49-51, respectively, in order of appearance.
[0347] Fmoc-Lys(Dde)-OH (1.50 equiv), HATU (1.42 equiv) and DIEA (3.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide 13 and agitated for 30 min under nitrogen. The resin was then washed three times with DMF. Fmoc removal was carried out while the peptide was on resin using 20% piperidine in DMF followed by filtration and washing. [0348] 2-(4,7,10-Tris(2-(tert-butoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecan-1-yl)acetic acid (DOTA-(OtBu)3-OH, 1.50 equiv), HATU (1.42 equiv) and DIEA (3.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide-Lys(Dde) and agitated for 30 min under nitrogen. The resin was then washed three times with DMF and agitated in 3% hydrazine in DMF for 10 min. After the solvent was removed through filtration, the peptidyl-resin 15 was washed five times with DMF. [0349] 4-(Naphthalene-2-sulfonamido)-4-oxobutanoic acid 2 (2.00 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide 15 and then agitated for 30 min under nitrogen. The resin-bound peptide 16 was washed three times with DMF. [0350] After the resin was washed three times with MeOH and dried under vacuum, a cocktail of trifluoroacetic acid/H2O/triisopropylsilane/3-mercaptopropionic acid (90:2.5:2.5:5.0) was added. The resulting mixture was stirred for 2 h at room temperature. Cold isopropyl ether was used to precipitate the cleaved peptide-linker-DOTA. The crude linear peptide-DOTA 17 (0.95 g) was collected through filtration and dried under vacuum. [0351] Oxidative folding of DOTA-linker-peptide 17: To a solution of crude 17 (0.95 g) in water (330 mL) and MeCN (100 mL) was added GSSG (666 mg) and GSH (666 mg). The pH of the solution was adjusted to 8.0 using saturated NH4HCO3 solution. The resulting mixture was stirred at room temperature for 12 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford compound С-05 (63.4 mg, 3.6% yield, 67.0% purity) as a white solid. [0352] La3+ complexation: To a solution of С-05 (45.1 mg, 67.0% purity, 13.0 µmol) in H2O (2.00 mL) and MeCN (1.00 mL) was added LaCl3 (3.82 mg, 15.6 µmol) and Na2CO3 (1.37 mg, 13.0 µmol). The resulting mixture was stirred at 70 °C for 1 h. After filtration, the crude product was purified by preparative HPLC to afford La-С-05 (12.7 mg, 37.7% yield, 95.1% purity) as a white solid. [0353] Conjugates in Table 1 and their corresponding La3+ complexed conjugates of Table 6 were synthesized similarly according to the methods described above.
[0354] Example A4: Synthesis of conjugate of Table 1 [0355] Conjugates of Table 1 and corresponding conjugates containing chelated lutetium or lanthanum were synthesized according to the same methods described in Examples A1-A3. See Table 6 and Table 7 below. Table 6. Exemplary La3+-labelled conjugates
[0356] Conjugates in Table 1 and their corresponding Lu complexed conjugates of Table 7 were synthesized similarly according to the methods described above. Table 7. Exemplary [175]Lu labelled conjugates
[0357] Example A5: Synthesis of C-25 and La-C-25 [0358] Solid phase peptide synthesis (SPPS) was performed in a standard manual reaction vessel under nitrogen. 2-CTC resin was purchased from Sunresin New Materials Co. (China). Fmoc protected amino acids were purchased from GL Biochem (China). HBTU and HATU were purchased from Highfine Biotech Co. (China). Piperidine was purchased from Damao Chemical Reagent Factory (China). The peptides and their derivatives were purified on a Gilson GX-281 preparative HPLC system using reverse-phase C18 columns (Gemini, 5 µm, 110 Å + luna, 10 µm, 100 Å) at 30 °C. HPLC solvents consisted of H2O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B). [0359] High performance liquid chromatography (HPLC) analyses were performed on an Agilent 1260 series equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.2 network workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid
(mobile phase B). Conditions: a Phenomenex Gemini-NX C-18 (5 µm, 110 Å, 4.6 × 250 mm) column was used with a flow rate of 1.0 mL/min. [0360] LC-MS analyses were carried out on an Agilent 1200 series coupled to an Agilent MSD G6125C, equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.3 standalone workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B). Conditions: a Waters Xbridge C-18 (3.5 µm, 3.1× 30mm) column was used with a flow rate of 1.2 mL/min. Synthesis of peptidyl-resin 1
Scheme 1. Synthesis of peptidyl-resin 1
[0361] To the swollen 2-CTC resin (1.00 mmol, 1.10 mmol/g, 1.00 equiv) were added Dde- Lys(Fmoc)-OH (0.53 g, 1.00 mmol) and DIEA (0.70 mL, 4.00 mmol) in DCM (8.00 mL). The mixture was agitated for 2 h under nitrogen. MeOH (1.00 mL) was then added. The resulting mixture was agitated for 30 min. After the reaction solution was removed through filtration, the resin was washed three times with DMF (10 mL). The Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. [0362] 18-(tert-butoxy)-18-oxooctadecanoic acid (2.00 equiv), HBTU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide and then agitated for 30 min under nitrogen. The resin was then washed three times with DMF. Dde removal was carried out while the peptide was on resin using 3% hydrazine hydrate in DMF followed by filtration and washing. [0363] Subsequent amino acids were coupled using Fmoc-protected amino acid (3.00 equiv), HBTU (2.85 equiv) and DIEA (6.00 equiv) in dry DMF, shaking for 30 min. Pre-activation of any amino acid was not performed prior to coupling. Between amino acid couplings, the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. Success of Fmoc removal steps and amino acid couplings were monitored qualitatively using a ninhydrin test. Synthesis of C-025
Scheme 1. Synthesis of C-25 and La-C-25 [0364] Fmoc-AEEA-OH (2.00 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide and then agitated for 30 min under nitrogen. The resin was then washed three times with DMF. Fmoc removal was carried out while the peptide was on resin using 20% piperidine in DMF followed by filtration and washing. [0365] Fmoc-AEEA-OH (2.00 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide and then agitated for 30 min under nitrogen. The resin was then washed three times with DMF. Fmoc removal was carried out while the peptide was on resin using 20% piperidine in DMF followed by filtration and washing.
[0366] 2-(4,7,10-Tris(2-(tert-butoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecan-1-yl)acetic acid (DOTA-(OtBu)3-OH, 1.50 equiv), HATU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide-2 and then agitated for 30 min under nitrogen. The resin was then washed three times with DMF. [0367] After the resin was washed three times with MeOH and dried under vacuum, a cocktail of trifluoroacetic acid/H2O/triisopropylsilane/3-mercaptopropionic acid (90:2.5:2.5:5.0) was added. The resulting mixture was stirred for 2 h at room temperature. Cold isopropyl ether was added. The precipitated crude linear peptide-DOTA 3 was collected through filtration and dried under vacuum. [0368] Oxidative folding of DOTA-linker-peptide 3: To a solution of crude 3 (2.34 g) in water (700 mL) and MeCN (300 mL) were added GSSG (1.63 g) and GSH (1.63 g). The pH of the solution was adjusted to 8.0 using saturated NH4HCO3 solution. The resulting mixture was stirred at room temperature for 12 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford C-25 (8.0 mg, 95.59% purity; 13.6 mg, 94.78% purity and 54 mg, 70% purity) as a white solid. La3+ complexation: To a solution of La-C-25 (54 mg, 20.0 µmol) in H2O (2.50 mL) and MeCN (0.5 mL) was added LaCl3 (4.43 mg, 18.0 µmol) and Na2CO3 (0.19 mg, 1.8 µmol). The resulting mixture was stirred at 70 °C for 1 h. After filtration, the crude product was purified by preparative HPLC to afford La-C-25 (10.9 mg, 95.6% purity) as a white solid. [0369] Example A6: Synthesis of conjugate of C-35 and Lu-C-35 [0370] Solid phase peptide synthesis (SPPS) was performed in a standard manual reaction vessel under nitrogen. 2-CTC resin was purchased from Sunresin New Materials Co. (China). Fmoc protected amino acids were purchased from GL Biochem (China). HBTU and HATU were purchased from Highfine Biotech Co. (China). Piperidine was purchased from Damao Chemical Reagent Factory (China). The peptides and their derivatives were purified on a Gilson GX-281 preparative HPLC system using reverse-phase C18 columns (Gemini, 5 µm, 110 Å + luna, 10 µm, 100 Å) at 30 °C. HPLC solvents consisted of H2O containing 0.075% trifluoroacetic acid (mobile phase A) and acetonitrile (mobile phase B). [0371] High performance liquid chromatography (HPLC) analyses were performed on an Agilent 1260 series equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.2 network workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid
(mobile phase B). Conditions: a Phenomenex Gemini-NX C-18 (5 µm, 110 Å, 4.6 × 250 mm) column was used with a flow rate of 1.0 mL/min. [0372] LC-MS analyses were carried out on an Agilent 1200 series coupled to an Agilent MSD G6125C, equipped with a binary pump G7112A, micro vacuum degasser, standard autosampler ALS G7129A, thermostatted column compartment TCC G7116A, variable wavelength detector VWD G7114A, and data were analyzed by OpenLab CDS 2.3 standalone workstation software from Agilent Technologies. HPLC solvents consisted of H2O containing 0.1% trifluoroacetic acid (mobile phase A) and acetonitrile containing 0.075% trifluoroacetic acid (mobile phase B). Conditions: a Waters Xbridge C-18 (3.5 µm, 3.1× 30mm) column was used with a flow rate of 1.2 mL/min. Synthesis of peptidyl-resin 1
Scheme 2. Synthesis of peptidyl-resin 1 [0373] To the Rink amide-MBHA resin (0.50 mmol, 0.5 mmol/g, 1.00 equiv) were added in DMF (20mL). Then the mixture was agitated with N2 for 30 min. After the solution was removed through filtration, The Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. A solution of Fmoc-Tyr(tBu)-OH (0.69 g, 1.50 mmol), HBTU (542mg, 1.43mmol) and DIEA (0.35 mL, 2.00 mmol) in DMF (5 mL) was added to the resin and agitated with N2 for 30 min at 20°C. The resin was then washed with DMF (15 mL * 3). The Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. [0374] Subsequent amino acids were coupled using Fmoc-protected amino acid (3.00 equiv), HBTU (2.85 equiv) and DIEA (6.00 equiv) in dry DMF, shaking for 30 min. Pre-activation of any
amino acid was not performed prior to coupling. Between amino acid couplings, the Fmoc protecting group was removed via 30 min agitation with 20% piperidine in DMF followed by filtration and washing. Success of Fmoc removal steps and amino acid couplings were monitored qualitatively using a ninhydrin test. [0375] Synthesis of C-35
Scheme 3. Synthesis of C-35 and Lu-C-35
[0376] The resin was agitated in 3% hydrazine in DMF for 20 min. After the solvent was removed through filtration, the peptidyl-resin was washed five times with DMF. [0377] 2-(4,7,10-Tris(2-(tert-butoxy)-2-oxoethyl)-1,4,7,10-tetraazacyclododecan-1-yl)acetic acid (DOTA-(OtBu)3-OH, 1.50 equiv), HBTU (1.90 equiv) and DIEA (4.00 equiv) were dissolved in dry DMF. The solution was mixed with the resin-bound peptide 2 and then agitated for 30 min under nitrogen. The resin-bound peptide 2 was washed three times with DMF. [0378] After the resin was washed three times with MeOH and dried under vacuum, a cocktail of trifluoroacetic acid/H2O/triisopropylsilane/3-mercaptopropionic acid (90:2.5:2.5:5.0) was added. The resulting mixture was stirred for 2.5 h at room temperature. Cold isopropyl ether was used to precipitate the cleaved peptide-linker-DOTA. The crude linear peptide-DOTA 3 (1.2 g) was collected through filtration and dried under vacuum. [0379] Oxidative folding of DOTA-linker-peptide 3: To a solution of crude 3 (1.2 g) in water (400 mL) and MeCN (200 mL) was added GSSG (845 mg, 3eq) and GSH (845 mg, 6eq). The pH of the solution was adjusted to 8.0 using saturated NH4HCO3 solution. The resulting mixture was stirred at room temperature for 24 h. The pH of the solution was then adjusted to 5.0 using 1.0 N HCl. After lyophilization, the crude was purified by preparative HPLC to afford compound C-35 (2.1 mg, 95.06% purity and 67mg 70% purity) as a white solid. [0380] Lu3+ complexation: To a solution of C-35 (67 mg, 70% purity) in H2O (3.00 mL) and MeCN (1.00 mL) was added LuCl3 (36mg, 5eq) and then 1M Na2CO3 aqueous solution was adjusted to pH = 5~6. Then the mixture was heated to 40°C and stirred at 40 °C for 1h. The crude product was purified by preparative HPLC to afford Lu-C-35 (15.5 mg , 95.06% purity) as a white solid. [0381] Example A7: Synthesis of 177-Lutetium chelated conjugates [0382] Conjugates of Table 1 and Table 2 are synthesized according to the same methods described in Examples A1-A6. [0383] General procedure for 177Lu-labeling [177Lu]LuCl3 in HCl (50 MBq) is added to a mixture of a DOTA-GCC construct (1 nmol) in NaOAc buffer (5% EtOH, 0.25 M, pH 5.0-5.5, total volume 120 µL) in a 1.8 mL Eppendorf tube. The resulting mixture is heated at 80 °C in a thermal mixer at a shaking speed of 600 rpm for 15-30 min. If necessary, the mixture is purified using a C8 column. Radiochemical purity is determined by radio-RP-HPLC and iTLC. [0384] Accordingly, conjugates of Table 8 can be synthesized according to this Example A7. Table 8. Exemplary 177-Lu labelled conjugates
[0385] Example A8: Synthesis of 225-Actinium chelated conjugates [0386] Conjugates of Table 1 and Table 2 are synthesized according to the same methods described in Examples A1-A6. [0387] General procedure for 225Ac-labeling [225Ac]Ac(NO3)3 in 1 mM HCl (50 kBq) is added to a mixture of a DOTA-GCC construct (1 nmol) in NaOAc buffer (100 µL, 0.4 M, pH 5.5-6.5) in a 1.8 mL Eppendorf tube. The resulting mixture is heated at 80-100 °C in a thermal mixer at a shaking speed of 500 rpm for 15-30 min. Radiochemical purity is determined by iTLC. [0388] Accordingly, conjugates of Table 9 can be synthesized according to this Example A8. Table 9. Exemplary 225-Actinium labelled conjugates
B: Biological Evaluation [0389] Example B1. Plasma stability study [0390] The pooled frozen CD-1 (ICR) mouse plasma was thawed in a water bath at 37 ℃ prior to experiment. Plasma was centrifuged at 4000 rpm for 5 min and the clots were removed if any. The pH would be adjusted to 7.4 ± 0.1 if required. [0391] Plasma (98 µL) and test compounds (2 µL, 100 µM in DMSO) or reference compound propantheline bromide (2 µL, 100 µM in 40% v/v MeOH/H2O) were added to the individual wells of a 96-well microtiter plate in duplicate. The plate was incubated at 37 ℃. During the incubation, aliquots were withdrawn at 0, 10, 30, 60, 120 and 300 minutes.100 μL 4% H3PO4 and 800 μL of stop solution (200 ng/mL tolbutamide and 200 ng/mL labetalol in 100% acetonitrile) were added to precipitate proteins. After mixing thoroughly, the quenched aliquots were centrifuged at 4,000 rpm for 20 min. Aliquots (100 μL) of supernatant were transferred to a new plate. The samples were shaken at 800 rpm for 10 min before performing LC-MS/MS analysis. [0392] The analytes were detected by a multiple reaction monitoring method using a SCIEX Triple Quad 6500+ system equipped with an ACQUITY UPLC HSS T3 column (100 Å, 1.8 μm,
2.1 mm × 30 mm). Mobile phase A: water with 0.1% formic acid; Mobile phase B: acetonitrile with 0.1% formic acid. The % remaining of test compound after incubation in plasma was calculated using the following equation: % Remaining= PAR is the peak area ratio of analyte versus internal standard
(IS) [0393] Example B2. In vivo pharmacokinetic studies in female CD-1(ICR) mice [0394] The pharmacokinetics of linaclotide and the La3+-labelled peptide surrogates were determined using female CD-1 (ICR) mice purchased from Vital River Laboratory Animal Co., Ltd., Beijing, China. All animal studies were conducted in accordance with the highest standards of care as outlined in the NIH Guide for Care and Use of Laboratory. Following injection of the mice (10 mg/kg, 3 mice per test compound) with aliquots of the peptides in PBS (10 mM, pH 7.4) via the tail vein, blood samples were collected into pre-chilled tubes containing Heparin-Na (3 µL, 1000 I.U./mL) at 5, 30, 60, and 240 minutes. [0395] General sample processing procedure: An aliquot of 12 µL diluted blood sample (10× dilution factor for 30, 60, and 240 min blood samples; 20× dilution factor for 5 min blood samples), calibration standard, dilution quality control, single blank or double blank samples were added to the individual wells of a low binding 96-well plate. Each sample (except the double blank) was quenched with 120 µL IS in methanol respectively (double blank sample was quenched with 120 µL MeOH). The resulting mixtures were mixed for 10 min at 800 rpm and centrifuged at 3220 g (4000 rpm) for 15 min at 4 °C. Supernatant aliquots (50 µL) were transferred to a clean low binding 96-well plate and centrifuged at 3220 g (4000 rpm) for 5 min at 4 ℃, then the samples were directly injected for LC-MS/MS analysis. [0396] The analytes were detected by a multiple reaction monitoring method using a SCIEX Triple Quad 6500+ system equipped with an ACQUITY UPLC HSS T3 column (100 Å, 1.8 μm, 2.1 mm × 30 mm). Mobile phase A: water/acetonitrile (95/5, v/v) with 0.1% formic acid and 2 mM ammonium formate; Mobile phase B: acetonitrile/water (95/5, v/v) with 0.1% formic acid and 2 mM ammonium formate. Or Mobile phase A: water with 0.1% formic acid; Mobile phase B: acetonitrile with 0.1% formic acid. Column temperature: 60 °C. [0397] The plasma concentration-time data was subjected to IV-noncompartmental pharmacokinetics analysis by using Phoenix WinNonlin (version 6.3, Pharsight Corp., Mountain View, CA, USA). The linear/log trapezoidal rule was applied in obtaining the PK parameters.
[0398] Pharmacokinetics of linaclotide and compounds of the present disclosure are given in Table 10 below. Table 10. Pharmacokinetics of linaclotide and compounds of the present disclosure
[0399] Example B3. In vitro IC50 values for the GCC constructs determined by competitive radioligand displacement cell binding assay [0400] The IC50 values for the GCC constructs were determined by a competitive radioligand displacement cell binding assay, using compound Reference-043 radiolabeled with 125I, Reference-043-[125I]. In a 96 well plate, each well was added T84 cell membrane protein 20 µg in 100 µL assay buffer (DMEM/20 mM HEPES, pH 7.4, 0.5% BSA), Reference-043-[125I] (100 pM, 50 µL) and an unlabeled GCC construct (50 µL) at varying concentrations (0.3 pM – 5 uM). The plate was sealed and incubated at room temperature for 1 h with gentle agitation on a shaker set to 300 rpm. The incubation is stopped by filtration onto a Unifilter-96 GF/C filter plate (presoaked with 50 µL of 0.3% polyethyleneimine per well for at least 0.5 h at room temperature) using a Perkin Elmer Filtermate Harvester. The plate was washed with cold Dulbecco-PBS washing buffer (200 µL x 5 times) then dried at 50 ^C for 1 h. The bottom of the filter plate was sealed using Perkin Elmer Unifilter-96 backing seal tape and the Perkin Elmer Microscint 20 cocktail
(50 µL) was added to the wells. The top of the filter plate was sealed with Perkin Elmer TopSeal- A sealing film and the radioactivity was counted with a Perkin Elmer MicroBeta2 Reader. For statistical considerations, each GCC construct was tested in two separate competitive radioligand cell binding experiments performed in duplicate. IC50 calculations were performed using the four- parameter logistic model within GraphPad Prism 5.0 software. [0401] The structure of Reference-043 is the following:
. [0402] IC50 values of compounds of the present disclosure are given in Table 11 below, where IC500nM<A≤30nM; 30nM<B≤ 100nM; 100nM<C≤ 1000nM; and 1000nM<D≤ 6000nM. Table 11. IC50 values of exemplary compounds of the present disclosure
[0403] Example B4. In vitro determination of bound and unbound fraction of GCC binding constructs to Human Serum Albumin (HSA) [0404] HSA-HPLC method (measurement of drug protein binding by immobilized human serum albumin-HPLC). A 13-minute HPLC (Thermo Vanquish Horizon with Diode Array Detector) gradient method was used to determine the HSA (Human Serum Albumin) binding of novel compounds using a chemically bonded protein stationary phase (ChiralPAK HSA HPLC column, 50 x 4 mm). The HSA binding values were derived from the gradient retention times that were converted to the logarithm of the equilibrium constant using data from a calibration set of molecules. The % bound to plasma values for the calibrator compounds were converted to the linear free energy values using the following equation: LogK = log[%PPB/(101-%PPB)]. The logarithmic value of the gradient retention times from the experiment were plotted against the linearized values of the percent bound to plasma. The slope and the intercept were used to convert the retention times to linear free energy values (LogK), from which the estimated % protein binding was calculated using the following equation: %Binding = [(101·10LogK)/(1+10LogK)]. Aqueous mobile phase (mobile phase A) was 50 mM ammonium acetate solution, pH 7.4 and the organic mobile phase (mobile phase B) was 2-propanol. The flow rate was set at 0.350mL/min and injection volume was 5uL, with samples prepared at 0.5mg/mL concentration in 50:50 mobile phase. The initial LC conditions were set at 0% B and ramped to 50% B over 8.5 minutes, then held at 50% B for 1.5 minutes before going back to initial conditions and re-equilibrating the column for 2.5 minutes. Chromatograms were recorded at 280 nm by a diode array UV absorption detector . [0405] The percent binding values of Table 12 are defined as the following: 90% bound to HSA < A ≤100% bound to HSA; 70% bound to HSA < B ≤ 90% bound to HSA; 20% bound to HSA < C ≤ 70% bound to HSA; and 0% bound to HSA < D ≤ 20% bound to HSA. Table 12. Percent HSA binding of compounds of the present disclosure
Claims
CLAIMS We Claim: 1. A conjugate comprising a guanylyl cyclase C (GCC) binding peptide, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42; a metal chelator configured to bind with a radionuclide; a linker that covalently attaches the GCC binding peptide with the metal chelator; and an alpha-particle emitting radionuclide bound to the metal chelator.
2. A conjugate comprising a guanylyl cyclase C (GCC) binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein 5% to 99% of the conjugate binds to Human Serum Albumin (HSA) in vitro as determined by HSA-HPLC method.
3. The conjugate of claim 2, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42.
4. The conjugate of claim 1 or 3, wherein the GCC binding peptide consists of an amino acid sequence of any one of SEQ ID NOs: 1-19.
5. The conjugate of claim 1 or 3, wherein the GCC binding peptide comprises SEQ ID NO: 1.
6. The conjugate of any one of claims 1 to 5, wherein the binding peptide further comprises 1, 2 or 3 intramolecular disulfide bonds.
7. The conjugate of any one of claims 1 to 6, wherein the binding peptide further comprises one or more of alkylene, alkenylene, heteroalkylene, and heteroaryl.
9. The conjugate of any one of claims 1 to 6, wherein the binding peptide further comprises one intramolecular heteroalkylene, alkylene or alkenylene bond.
10. The conjugate of claim 9, wherein the heteroalkylene, alkylene or alkenylene is optionally substituted with one or more R10, wherein each R10 is independently halogen, amino, -OH, -SH, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two R10 on the same atoms are taken together to form a cycloalkyl or heterocycloalkyl;
or two R10 on different atoms are taken together to form a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl.
11. The conjugate of any one of claims 1 to 10, wherein the binding peptide comprises an unnatural amino acid at the N-terminus, the C-terminus, or both.
12. The conjugate of claim 11, wherein the binding peptide comprises a modified tyrosine at the C-terminus.
13. The conjugate of claim 12, wherein the modified tyrosine is a D-tyrosine, N-methyl-L- tyrosine, α-methyl-L-tyrosine, or L-tyrosinamide.
14. The conjugate of any one of claims 1 to 13, wherein the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAGA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, and NOTP.
15. The conjugate of any one of claims 1 to 14, wherein the metal chelator is DOTA.
16. The conjugate of any one of claims 1 to 15, wherein the linker is a bond.
17. The conjugate of any one of claims 1 to 15, wherein the linker has a structure of Formula (II- 1)
Formula (II-1) wherein each L is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, - S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)- , -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, - (C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1-C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2-(C1-C30 alkylene)-; and each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; and n is 1 to 20.
18. The conjugate of claim 17, wherein the linker comprises a structure of Formula (II-1a),
Formula (II-1a) wherein each of L1 and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; and L2 is absent, substituted or unsubstituted C1-C30 alkylene, or substituted or unsubstituted C1- C30 heteroalkylene.
19. The conjugate of claim 18, wherein L1 is -NH-; L3 is -C(=O)-; L2 is unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene, wherein the heteroalkylene is optionally substituted with one or more substituents selected from -OH, -SH, oxo, amino, C1-C6 alkyl, C1-C6 hydroxyalkyl, C1-C6 haloalkyl, and C1-C6 aminoalkyl.
22. The conjugate of claim 18 or 19, wherein L2 is C1-C30 heteroalkylene that is optionally substituted with one or more substituents selected from -OH, -SH, oxo, amino, C1-C6 alkyl, C1-C6 hydroxyalkyl, C1-C6 haloalkyl, and C1-C6 aminoalkyl.
24. The conjugate of any one of claims 1 to 15, wherein the linker has a structure of Formula (II- 2)
Formula (II-2), wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, - S(=O)-, -S(=O)2-, =CH-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, - NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, - CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)- , substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2- C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, -(C1-C30 alkylene)-O-, -O-(C1-C30 alkylene)-, -(C1- C30 alkylene)-NRL-, -NRL-(C1-C30 alkylene)-, -(C1-C30 alkylene)-N(RL)2-, or -N(RL)2- (C1-C30 alkylene)-; and R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl,
substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RX is independently hydrogen, halo, -CN, -NO2, -OH, -SH, substituted or unsubstituted C1- C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2- C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; and q is 0, 1, 2, 3, 4, 5, and 6.
25. The conjugate of claim 24, wherein the linker has a structure of Formula (II-2a) or Formula (II-2b)
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; p is 0, 1, 2, 3, 4, or 5; and
q is 0, 1, 2, 3, 4, or 5.
26. The conjugate of claim 25, wherein the linker connects to the metal chelator through L3 and to the peptide through L2.
27. The conjugate of claim 25 or 26, wherein the linker has a structure of Formula (II-2a).
28. The conjugate of claim 27, wherein the linker of Formula (II-2a) has a structure of Formula (II-2aa),
Formula (II-2aa) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L31 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-;
L32 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5.
29. The conjugate of claim 28, wherein L31 is -NH- and L32 is substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene.
30. The conjugate of claim 29, wherein L31 is -NH- and L32 is -(CH2)4-.
31. The conjugate of any one of claims 28 to 30, wherein L11 is absent, -O-, -OP(=O)(ORL)O-, - S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-.
32. The conjugate of any one of claims 28 to 30, wherein L11 is absent, -OP(=O)(OH)O-, - NHC(=O)-, -C(=O)NH-, or -S(=O)2NH-.
33. The conjugate of any one of claims 28 to 32, wherein L12 is absent, -O-, -OP(=O)(ORL)O-, - S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene.
35. The conjugate of any one of claims 28 to 33, wherein L12 is -C(=O)- and L11 is absent.
36. The conjugate of claim 25 or 26, wherein the linker has a structure of Formula (II-2b).
37. The conjugate of claim 36, wherein the linker of Formula (II-2b) has a structure of Formula (II-2ba),
Formula (II-2ba) wherein, L11 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-; L12 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L21 is absent, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1- C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; L22 is absent, -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, or -S(=O)2NRLC(=O)-;
L3 is -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, - C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, - OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C3- C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; q is 0, 1, 2, 3, 4, or 5.
38. The conjugate of claim 36 or 37, wherein q is 0.
39. The conjugate of claim 37 or 38, wherein L11 is absent, -O-, -OP(=O)(ORL)O-, -S-, -S(=O)2-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, or - S(=O)2NRLC(=O)-.
40. The conjugate of claim 37 or 38, wherein L11 is absent, -OP(=O)(OH)O-, -NHC(=O)-, - C(=O)NH-, or -S(=O)2NH-.
41. The conjugate of any one of claims 37 to 40, wherein L12 is substituted or unsubstituted C1- C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene.
43. The conjugate of any one of claims 28 to 42, wherein L22 and L21 are both absent.
44. The conjugate of any one of claims 28 to 42, wherein L22 is -C(=O)- and L21 is substituted or unsubstituted C1-C12 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene.
46. The conjugate of any one of claims 24 to 45, wherein R is hydrogen, substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, or a sterol.
47. The conjugate of claim 46, wherein R is phenyl, naphthyl, monocyclic heteroaryl, or bicyclic heteroaryl, each of which is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2- C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl).
48. The conjugate of claim 46 or 47, wherein R is a phenyl optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -OH, amino, -N(C1-C6alkyl)2, - NH(C1-C6alkyl), -C(=O)H, -C(=O)OH, -C(=O)NH2, C1-C6alkyl, C1-C6alkoxyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-
C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl.
50. The conjugate of any one of claims 24 to 45, wherein R is substituted or unsubstituted C1- C30 alkyl or substituted or unsubstituted C2-C30 alkenyl, each of which is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, - C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl).
51. The conjugate of claim 50, wherein R is C6-C30 fatty acid, C6-C30 fatty alcohol or C6-C30 alkyl.
52. The conjugate of any one of claims 1 to 15, 17, or 24, wherein the linker comprises a click chemistry residue.
53. The conjugate of any one of claims 1 to 52, wherein the linker comprises one or more of substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and one or more amino acids.
54. The conjugate of any one of claims 1 to 52, wherein the linker comprises one or more of a substituted or unsubstituted C6-C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, a sterol, sulfonamide, phosphate ester, polyethylene glycol, C3-C20 alkylene, or amino acid residues.
55. The conjugate of any one of claims 1 to 54, wherein the linker comprises one or more lysine residues.
56. The conjugate of any one of claims 1 to 55, wherein the linker comprises one or more glutamate residues.
57. The conjugate of any one of claims 1 to 56, wherein the linker comprises phenyl iodide or carboxylic acid.
58. The conjugate of any one of claims 1 to 15, wherein the linker comprises or has a structure selected from:
59. The conjugate of any one of claims 1 to 58, wherein the linker comprises 3 to 30 intervening atoms between the metal chelator and the binding peptide.
60. The conjugate of any one of claims 1 to 58, wherein the linker comprises 6 to 18 intervening atoms between the metal chelator and the binding peptide.
61. The conjugate of any one of claims 1 to 60, wherein a dissociation constant (Kd) between the linker and human serum albumin is at most 25 µM, as determined in vitro at room temperature in human serum condition.
62. The conjugate of any one of claims 1 to 61, wherein about 40%-60% of the conjugate binds to HSA in vitro as determined by HSA-HPLC method.
63. The conjugate of any one of claims 1 to 61, wherein about 60%-80% of the conjugate binds to HSA in vitro as determined by HSA-HPLC method .
64. The conjugate of any one of claims 1 to 63, wherein the linker is attached to the binding peptide via the N terminus of the binding peptide.
65. The conjugate of claim 64, wherein the conjugate comprises a second linker attached to the C terminus of the binding peptide.
66. The conjugate of any one of claims 1 to 65, wherein the conjugate comprises an alpha particle-emitting radionuclide bound to the metal chelator.
67. The conjugate of claim 66, wherein the alpha particle-emitting radionuclide is actinium-225, astatine-211, radium-223, or thorium-227.
68. The conjugate of claim 66, wherein the alpha particle-emitting radionuclide is actinium-225.
69. The conjugate of any one of claims 1 to 68, wherein the conjugate comprises two or more metal chelators.
70. The conjugate of any one of claims 1 to 69, wherein the conjugate has an elimination half- life in rats of about 1 to 120 hours.
71. The conjugate of any one of claims 1 to 69, wherein the conjugate has an elimination half- life in rats of about 2 to 24 hours.
72. The conjugate of any one of claims 1 to 71, wherein a half-life of the conjugate in human serum condition is about 2 to 20 hours, 5 to 20 hours, 8 to 15 hours, or 10 to 14 hours at 37 °C.
73. A conjugate comprising a guanylyl cyclase C (GCC) binding peptide; a metal chelator configured to bind with a radionuclide; and a linker that covalently attaches the GCC binding peptide with the metal chelator, wherein the conjugate has a structure of Formula (I)
Formula (I) wherein, the linker –(L)n- comprises 2 to 50 intervening atoms between the metal chelator and the binding peptide; each L is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, - C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1- C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted
C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; each RL is independently hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C6 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, substituted or unsubstituted C2-C7 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); n is 0 or an integer from 1 to 20; and r is 0 or an integer from 1 to 5.
74. The conjugate of claim 73, wherein the linker –(L)n- comprises 3 to 20 intervening atoms between the metal chelator and the binding peptide
75. The conjugate of claim 73 or 74, wherein –(L)n- is bound to the N-terminus of the GCC binding peptide and LC is bound to the C-terminus of the GCC binding peptide.
76. The conjugate of any one of claims 73 to 75, wherein the linker –(L)n- comprises a hydrophobic group selected from a C8-C30 fatty acid, C8-C30 fatty alcohol, C8-C30 alkyl, aryl, heteroaryl, one or more amino acid residues, or a combination thereof.
77. The conjugate of any one of claims 73 to 76, wherein –(L)n- comprises one or more lysine residues.
78. The conjugate of any one of claims 73 to 76, wherein –(L)n- comprises one or more glutamate residues.
79. The conjugate of any one of claims 73 to 75, wherein the conjugate has a structure of Formula (Ia),
Formula (Ia) wherein, each L1, L2, and L3 is independently -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, - C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, - NRLC(=O)O-, -NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, - S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R and RC are each independently hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
RX is independently hydrogen, halo, substituted or unsubstituted C1-C6 alkyl, substituted or unsubstituted C1-C6 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2-C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl, X is N or CRX; m is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; p is 0, 1, 2, 3, 4, 5, or 6; q is 0, 1, 2, 3, 4, 5, or 6; and r is 0, 1, 2, 3, 4, or 5.
80. The conjugate of claim 79, wherein at least one of L1, L2, and L3 comprises a hydrophobic group selected from a C8-C30 fatty acid, C8-C30 fatty alcohol, C4-C12 alkylene chain, heteroaryl, or aryl group, each of which is optionally substituted.
81. The conjugate of claim 79 or 80, wherein the conjugate has a structure of Formula (Iaa),
Formula (Iaa) wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted
or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; r is 0, 1, 2, 3, 4, or 5; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5.
82. The conjugate of 79 or 80, wherein the conjugate has a structure of Formula (Iab),
wherein each L1, L2, and L3 is independently -O-, –NRL-, –N(RL)2-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -CH=CH-, =CH-, -C≡C-, -C(=O)-, -C(=O)O-, -OC(=O)-, -OC(=O)O-, - C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, -NRLC(=O)NRL-, - NRLS(=O)2-, -S(=O)2NRL-, -C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or
unsubstituted C3-C15 cycloalkyl, substituted or unsubstituted C1-C12 heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted C1-C20 alkylene, or substituted or unsubstituted C1-C30 heteroalkylene; RL is hydrogen, substituted or unsubstituted C1-C4 alkyl, substituted or unsubstituted C1-C4 heteroalkyl, substituted or unsubstituted C2-C6 alkenyl, substituted or unsubstituted C2- C5 alkynyl, substituted or unsubstituted C3-C8 cycloalkyl, or substituted or unsubstituted C2-C7 heterocycloalkyl; R is hydrogen, azide, substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 heteroalkyl, substituted or unsubstituted C2-C30 alkenyl, substituted or unsubstituted C2-C30 alkynyl, substituted or unsubstituted C3-C30 cycloalkyl, substituted or unsubstituted C2-C30 heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; LC is absent, -O-, –NRL-, -OP(=O)(ORL)O-, -S-, -S(=O)-, -S(=O)2-, -C(=O)-, -C(=O)O-, - OC(=O)-, -OC(=O)O-, -C(=O)NRL-, -NRLC(=O)-, -OC(=O)NRL-, -NRLC(=O)O-, - NRLC(=O)NRL-, -NRLC(=S)NRL-, -CRL=N-, -N=CRL, -NRLS(=O)2-, -S(=O)2NRL-, - C(=O)NRLS(=O)2-, -S(=O)2NRLC(=O)-, substituted or unsubstituted C1-C30 alkylene, substituted or unsubstituted C2-C30 alkenylene, substituted or unsubstituted C2-C30 alkynylene, substituted or unsubstituted C1-C30 heteroalkylene, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or an amino acid; RC is hydrogen, halogen, -CN, -NO2, -SRa, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, - C(=O)NRaRa, azide, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 heteroalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); m is 0, 1, 2, 3, 4, 5, 6, 7, 8, or 9; r is 0, 1, 2, 3, 4, or 5; p is 0, 1, 2, 3, 4, or 5; and q is 0, 1, 2, 3, 4, or 5.
83. The conjugate of any one of claims 73 to 82, R is hydrogen, substituted or unsubstituted C6- C10 aryl, substituted or unsubstituted C5-C9 heteroaryl, or a sterol.
84. The conjugate of claim 83, wherein R is phenyl, naphthyl, monocyclic heteroaryl, or bicyclic heteroaryl, each of which is optionally substituted with one or more substituents selected from halogen, -CN, -NO2, -ORa, -N(Ra)2, -C(=O)Ra, -C(=O)ORa, -C(=O)NRaRa, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2- C6alkynyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, wherein each Ra is independently H, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl).
86. The conjugate of any one of claims 73 to 82, wherein R is C6-C30 fatty acid, C6-C30 fatty alcohol or C6-C30 alkyl.
87. The conjugate of any one of claims 73 to 86, wherein Rc is a C6-C30 fatty acid, C6-C30 fatty alcohol or C6-C30 alkyl.
89. The conjugate of any one of claims 73 to 88, wherein the binding peptide comprises an unnatural amino acid, e.g., at the C-terminus.
90. The conjugate any one of claims 73 to 89, wherein the binding peptide comprises a modified tyrosine at the C-terminus.
91. The conjugate of any one of claims 73 to 90, wherein the GCC binding peptide comprises an amino acid sequence that has at least 85% identity to any one of SEQ ID NOs: 1-42.
92. The conjugate of any one of claims 73 to 90, wherein the GCC binding peptide comprises an amino acid sequence selected from SEQ ID NOs: 1-42.
93. The conjugate of any one of claims 73 to 92, wherein the GCC binding peptide further comprises 1 to 3 intramolecular bonds selected from heteroalkylene, alkylene and alkenylene bonds.
94. The conjugate of any one of claims 73 to 93, wherein the metal chelator is selected from DOTA, DOTP, DOTMA, DOTAGA, DOTAM, DTPA, NTA, EDTA, DO3A, DO2A, NOC, NOTA, TETA, DiAmSar, CB-Cyclam, CB-TE2A, DOTA-4AMP, or NOTP.
95. The conjugate of claim 93, wherein the metal chelator is DOTA.
96. The conjugate of any one of claims 73 to 95, wherein the conjugate comprises a radionuclide bound to the metal chelator.
97. The conjugate of claim 96, wherein the radionuclide is an alpha particle-emitting radionuclide selected from actinium-225, astatine-211, radium-223, and thorium-227.
98. The conjugate of claim 96, wherein the radionuclide is lutetium-177 or actinium-225.
99. A conjugate having a structure selected from Table 1.
100. A conjugate comprising: a structure selected from Table 1 and a radionuclide bound to a metal chelator of the structure.
101. A conjugate, wherein the conjugate is a salt or solvate of a conjugate of any one of the preceding claims.
102. A pharmaceutical composition comprising a conjugate of any one of claims 1 to 101, and a pharmaceutically acceptable excipient or carrier.
103. A method of treating a gastrointestinal tract cancer in a subject in need thereof, comprising administering to the subject a conjugate of any one of claims 1 to 101, or a pharmaceutical composition of claim 102.
104. The method of claim 103, wherein the cancer is colorectal cancer.
105. The method of claim 103 or 104, wherein the method comprises administering a second therapeutic agent that is a GCC receptor agonist.
106. The method of claim 103 or 104, wherein the method comprises administering (i) a first conjugate comprising a radionuclide configured for companion diagnostic and (ii) a second conjugate comprising a radionuclide selected from an alpha or beta-particle emitter, wherein the first and the second conjugate have the same structure except for the radionuclide.
107. The method of claim 106, wherein the radionuclide of the first conjugate is selected from Lu-177, In-111, Ga-68, Cu-64, and Zr-89.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202063119557P | 2020-11-30 | 2020-11-30 | |
| US63/119,557 | 2020-11-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022115799A1 true WO2022115799A1 (en) | 2022-06-02 |
Family
ID=81754969
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2021/061252 WO2022115799A1 (en) | 2020-11-30 | 2021-11-30 | Radiopharmaceutical conjugates targeting guanylyl cyclase c, and compositions and uses thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2022115799A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11744873B2 (en) | 2021-01-20 | 2023-09-05 | Viking Therapeutics, Inc. | Compositions and methods for the treatment of metabolic and liver disorders |
| WO2023164777A1 (en) * | 2022-03-04 | 2023-09-07 | Fusion Pharmaceuticals Inc. | Gucy2c-targeted radiopharmaceuticals and use thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060258593A1 (en) * | 2003-01-28 | 2006-11-16 | Currie Mark G | Methods and compositions for the treatment of gastrointestinal disorders |
| US20140147380A1 (en) * | 2004-06-25 | 2014-05-29 | Thomas Jefferson University | Guanylylcyclase c ligands |
| WO2019177970A1 (en) * | 2018-03-12 | 2019-09-19 | Memorial Sloan Kettering Cancer Center | Bispecific binding agents and uses thereof |
| US20190321495A1 (en) * | 2016-06-24 | 2019-10-24 | University Of Iowa Research Foundation | Compositions and methods of treating melanoma |
| WO2019212357A1 (en) * | 2018-05-04 | 2019-11-07 | Tagworks Pharmaceuticals B.V. | Compounds comprising a linker for increasing transcyclooctene stability |
| US20200148791A1 (en) * | 2018-05-11 | 2020-05-14 | Ironwood Pharmaceuticals, Inc. | Method of Generating Anti-Linaclotide Antibodies and Uses Thereof |
-
2021
- 2021-11-30 WO PCT/US2021/061252 patent/WO2022115799A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060258593A1 (en) * | 2003-01-28 | 2006-11-16 | Currie Mark G | Methods and compositions for the treatment of gastrointestinal disorders |
| US20140147380A1 (en) * | 2004-06-25 | 2014-05-29 | Thomas Jefferson University | Guanylylcyclase c ligands |
| US20190321495A1 (en) * | 2016-06-24 | 2019-10-24 | University Of Iowa Research Foundation | Compositions and methods of treating melanoma |
| WO2019177970A1 (en) * | 2018-03-12 | 2019-09-19 | Memorial Sloan Kettering Cancer Center | Bispecific binding agents and uses thereof |
| WO2019212357A1 (en) * | 2018-05-04 | 2019-11-07 | Tagworks Pharmaceuticals B.V. | Compounds comprising a linker for increasing transcyclooctene stability |
| US20200148791A1 (en) * | 2018-05-11 | 2020-05-14 | Ironwood Pharmaceuticals, Inc. | Method of Generating Anti-Linaclotide Antibodies and Uses Thereof |
Non-Patent Citations (1)
| Title |
|---|
| VALKO KLARA, NUNHUCK SHENAZ, BEVAN CHRIS, ABRAHAM MICHAEL H., REYNOLDS DEREK P.: "Fast Gradient HPLC Method to Determine Compounds Binding to Human Serum Albumin. Relationships with Octanol/Water and Immobilized Artificial Membrane Lipophiliclty", JOURNAL OF PHARMACEUTICAL SCIENCES, vol. 92, no. 11, November 2003 (2003-11-01), pages 2236 - 2248, XP055942420, DOI: 10.1002/jps.10494 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11744873B2 (en) | 2021-01-20 | 2023-09-05 | Viking Therapeutics, Inc. | Compositions and methods for the treatment of metabolic and liver disorders |
| WO2023164777A1 (en) * | 2022-03-04 | 2023-09-07 | Fusion Pharmaceuticals Inc. | Gucy2c-targeted radiopharmaceuticals and use thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3702367B1 (en) | Novel pd-l1 binding polypeptides for imaging | |
| US20230158179A1 (en) | Radiopharmaceutical conjugate compositions and uses thereof | |
| JP7546574B2 (en) | Dual Ligand Drug Conjugates and Uses Thereof | |
| CN108699108B (en) | Radiolabeled pharmaceutical | |
| JP7414370B2 (en) | Complex consisting of a ligand, spacer, peptide linker and biomolecule | |
| US11564989B2 (en) | Camptothecine antibody-drug conjugates and methods of use thereof | |
| JP7063623B2 (en) | Therapeutic antibodies and their use | |
| WO2022115799A1 (en) | Radiopharmaceutical conjugates targeting guanylyl cyclase c, and compositions and uses thereof | |
| JP7425606B2 (en) | Quaternized Nicotinamide Adenine Dinucleotide Salvage Pathway Inhibitor Conjugate | |
| CN112912731A (en) | Fibroblast Activation Protein (FAP) targeted imaging and fibrosis therapy | |
| WO2016108587A1 (en) | Repebody derivative-drug conjugate, preparation method and use thereof | |
| CN115135628A (en) | Compounds for fast and efficient click release | |
| US20220096669A1 (en) | Silicon-fluoride acceptor substituted radiopharmaceuticals and precursors thereof | |
| JP2024506644A (en) | Bivalent Fibroblast Activation Protein Ligand for Targeted Delivery Applications | |
| CA3143924A1 (en) | Agents for cleaving labels from biomolecules in vivo | |
| WO2011094671A2 (en) | N-terminally conjugated polypeptides for targeted therapy and diagnosis | |
| JP2024503635A (en) | Compounds containing fibroblast activation protein ligands and uses thereof | |
| JP2020512348A (en) | Radiolabeled biomolecules and uses thereof | |
| JP2024503637A (en) | Compounds containing fibroblast activation protein ligands and uses thereof | |
| TW202320857A (en) | Linkers, drug linkers and conjugates thereof and methods of using the same | |
| JP2024532304A (en) | Methods of Using Antibody-Drug Conjugates | |
| KR20240040092A (en) | Fibroblast activation protein inhibitors and uses thereof | |
| TW201920128A (en) | Process for the preparation of TUBULYSINS and intermediates thereof | |
| US20220218852A1 (en) | Novel radiolabelled cxcr4-targeting compounds for diagnosis and therapy | |
| TW202304532A (en) | Methods of treating cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21899238 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21899238 Country of ref document: EP Kind code of ref document: A1 |