WO2021217041A1 - N-substituted alpha-amino and alpha-hydroxy carboxamide derivatives - Google Patents
N-substituted alpha-amino and alpha-hydroxy carboxamide derivatives Download PDFInfo
- Publication number
- WO2021217041A1 WO2021217041A1 PCT/US2021/028907 US2021028907W WO2021217041A1 WO 2021217041 A1 WO2021217041 A1 WO 2021217041A1 US 2021028907 W US2021028907 W US 2021028907W WO 2021217041 A1 WO2021217041 A1 WO 2021217041A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aminobut
- alkyl
- compound
- propanamide
- phenyl
- Prior art date
Links
- 0 CNC(*)N*I** Chemical compound CNC(*)N*I** 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/20—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by nitrogen atoms not being part of nitro or nitroso groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/12—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/16—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/18—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by doubly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/32—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of rings other than six-membered aromatic rings
- C07C271/34—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of rings other than six-membered aromatic rings with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/32—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of rings other than six-membered aromatic rings
- C07C271/36—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of rings other than six-membered aromatic rings with the nitrogen atom of at least one of the carbamate groups bound to a carbon atom of a ring other than a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/40—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of six-membered aromatic rings
- C07C271/42—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of six-membered aromatic rings with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/52—Esters of carbamic acids having oxygen atoms of carbamate groups bound to carbon atoms of six-membered aromatic rings with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by nitrogen atoms not being part of nitro or nitroso groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C275/00—Derivatives of urea, i.e. compounds containing any of the groups, the nitrogen atoms not being part of nitro or nitroso groups
- C07C275/04—Derivatives of urea, i.e. compounds containing any of the groups, the nitrogen atoms not being part of nitro or nitroso groups having nitrogen atoms of urea groups bound to acyclic carbon atoms
- C07C275/20—Derivatives of urea, i.e. compounds containing any of the groups, the nitrogen atoms not being part of nitro or nitroso groups having nitrogen atoms of urea groups bound to acyclic carbon atoms of an unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C317/00—Sulfones; Sulfoxides
- C07C317/44—Sulfones; Sulfoxides having sulfone or sulfoxide groups and carboxyl groups bound to the same carbon skeleton
- C07C317/46—Sulfones; Sulfoxides having sulfone or sulfoxide groups and carboxyl groups bound to the same carbon skeleton the carbon skeleton being further substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/54—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/56—Amides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/70—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings condensed with carbocyclic rings or ring systems
- C07D239/72—Quinazolines; Hydrogenated quinazolines
- C07D239/86—Quinazolines; Hydrogenated quinazolines with hetero atoms directly attached in position 4
- C07D239/88—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D275/00—Heterocyclic compounds containing 1,2-thiazole or hydrogenated 1,2-thiazole rings
- C07D275/04—Heterocyclic compounds containing 1,2-thiazole or hydrogenated 1,2-thiazole rings condensed with carbocyclic rings or ring systems
- C07D275/06—Heterocyclic compounds containing 1,2-thiazole or hydrogenated 1,2-thiazole rings condensed with carbocyclic rings or ring systems with hetero atoms directly attached to the ring sulfur atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/78—Benzo [b] furans; Hydrogenated benzo [b] furans
- C07D307/79—Benzo [b] furans; Hydrogenated benzo [b] furans with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the hetero ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/77—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D307/87—Benzo [c] furans; Hydrogenated benzo [c] furans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/24—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/52—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes
- C07D333/54—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the hetero ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/02—Systems containing only non-condensed rings with a three-membered ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/04—Systems containing only non-condensed rings with a four-membered ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/06—Systems containing only non-condensed rings with a five-membered ring
- C07C2601/08—Systems containing only non-condensed rings with a five-membered ring the ring being saturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2602/00—Systems containing two condensed rings
- C07C2602/02—Systems containing two condensed rings the rings having only two atoms in common
- C07C2602/04—One of the condensed rings being a six-membered aromatic ring
- C07C2602/06—One of the condensed rings being a six-membered aromatic ring the other ring being four-membered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2602/00—Systems containing two condensed rings
- C07C2602/02—Systems containing two condensed rings the rings having only two atoms in common
- C07C2602/04—One of the condensed rings being a six-membered aromatic ring
- C07C2602/08—One of the condensed rings being a six-membered aromatic ring the other ring being five-membered, e.g. indane
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2602/00—Systems containing two condensed rings
- C07C2602/36—Systems containing two condensed rings the rings having more than two atoms in common
- C07C2602/42—Systems containing two condensed rings the rings having more than two atoms in common the bicyclo ring system containing seven carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/56—Ring systems containing bridged rings
- C07C2603/58—Ring systems containing bridged rings containing three rings
- C07C2603/70—Ring systems containing bridged rings containing three rings containing only six-membered rings
- C07C2603/74—Adamantanes
Definitions
- This disclosure relates generally to N-substituted a-amino and ohydroxy carboxamides, pharmaceutical compositions comprising these compounds, and methods of using these compounds to treat various diseases and disorders.
- the inflammasome is a large multiprotein complex that plays an important role in both sterile tissue injury and infection.
- complex regulatory mechanisms have evolved to suppress inflammasome activation in the absence of injury or infection.
- the importance of such mechanisms is highlighted by activating mutations in the inflammasome pathway that lead to an array of autoinflammatory diseases which can be life threatening.
- Chronic and acute aberrant activation of the inflammasome pathway also contributes to the pathology of a wide range of arthritic, neurodegenerative, cardiovascular, dermatological, pulmonary and systemic inflammatory conditions.
- Signal 2 is provided by an array of pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) that are sensed by a family of cytosolic pattern recognition receptors termed nucleotide-binding oligomerization domain-like receptors (NLRs), as well as a few additional inflammasome forming sensors. Binding of the DAMP to its corresponding NLR leads to conformational changes in the NLR allowing self-oligomerization of the receptor. These NLR oligomers then recruits the adapter protein, apoptosis-associated speck-like protein containing a CARD (ASC), nucleating formation of a filament of repeating ASC subunits, mediated through the pyrin domain of ASC.
- PAMPs pathogen associated molecular patterns
- DAMPs damage associated molecular patterns
- NLRs nucleotide-binding oligomerization domain-like receptors
- ASC apoptosis-associated speck-
- the ASC filaments in turn creates a platform for recruitment of caspase-1 leading autocatalytic cleavage and activation, as well nucleating subsequent caspase-1 filament formation mediated through the caspase-1 CARD domain, which is thought to amplify caspase-1 activation.
- Active caspase-1 then cleaves pro-IL- 1b, pro-IL-18 and Gasdermin D into their active forms, releasing the inflammatory mediators from the cell and inducing Gasdermin-D mediated pyroptotic cell death.
- Inflammasome formation can be initiated by at least 11 different sensors including Pyrin, numerous NLRs including NLRP1, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4 and NLRC5, the PYHIN family members IFI-16 and AIM2, as well as RIG-I.
- Conventional approaches to inflammasome inhibition have targeted individual receptors, such as NLRP3, to inhibit a subset of inflammasomes.
- emerging evidence clearly demonstrates that activation of multiple different inflammasome forming receptors is a common pathological feature of many diseases including inflammatory bowel disease, arthritic diseases and neurodegenerative disorders among others.
- broader pharmaceutical approaches capable of simultaneously inhibiting multiple species of inflammasomes are needed to address pathological inflammasome-dependent inflammation.
- ASC filaments ASC filaments
- Assembly of the inflammasome is induced by a cytosolic sensor (e.g. NLRP3 or AIM2) which detect danger signals associated with infection or sterile injury. Upon sensing these signals, the sensor nucleates formation of long filaments composed of repeating units of the inflammasome adapter protein ASC. These filaments form a scaffold for the recruitment of Caspase-1 leading to autoproteolytic activation of the enzyme.
- a cytosolic sensor e.g. NLRP3 or AIM2
- the sensor nucleates formation of long filaments composed of repeating units of the inflammasome adapter protein ASC. These filaments form a scaffold for the recruitment of Caspase-1 leading to autoproteolytic activation of the enzyme.
- the inflammasome plays a protective role against infection and injury, aberrant activation of the complex in the absence of infection or injury contributes to a wide range of autoinflammatory conditions. Accordingly, the development of compositions and methods that disrupt assembly of
- One aspect of the disclosure provides compounds having the structural formula: and pharmaceutically acceptable salts thereof, and/or solvates or hydrates thereof, wherein:
- a 1 is C 1 -C 6 alkyl, -(C 0 -C 2 alkyl)-cycloalkyl, -(C 0 -C 2 alkyl)-heterocycloalkyl, -(C 0 -C 2 alkyl)-aryl, -(C 0 -C 2 alkyl)-heteroaryl, or -(C 0 -C 2 alkyl)-B 1 -C 1 , wherein B 1 and C 1 are each independently cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
- Q 1 is -N(R A1 )(R B1 ) or -OR A1 ;
- X 1 is -N(R A1 )-;
- W 1 is C(O) or S(O) n1 , wherein n1 is 1 or 2;
- Y 1 is a C 1 -C 8 alkyl, C 2 -C 8 alkenyl, C 2 -C 8 alkynyl, or -(C 0 -C 2 alkyl)-cycloalkyl;
- Z 1 is -(C 0 -C 2 alkyl )-O(R A ) or -(C 0 -C 2 alkyl)-N(R A1 )(R B1 ); wherein each R A1 is independently H, C 1 -C 6 alkyl, C 2 -C 6 alkenyl, or (C 0 -C 2 )-cyclopropyl; each R B1 is independently H, C 1 -C 6 alkyl, or C 2 -C 6 alkenyl; each alkyl, alkenyl, and alkynyl is unsubstituted, substituted with one or more halogens (e.g., fluorinated or perfluorinated), or substituted with one or two groups independently selected from methyl, ethyl, acyl, oxo and -C(O)N(R A1 )(R B1 ); each cycloalkyl has 3-10 ring carbons and is saturated or partially unsaturated
- compositions comprising a compound (e.g., a compound of formula (I)) as described herein.
- the disclosure provides methods for treating various diseases, such as inflammation-related diseases to a subject in need thereof.
- the methods include administering to the subject an effective amount of a compound as described herein (e.g., a compound of formula (I)).
- the disclosure provides intermediates useful to prepare the compounds of formula (I).
- the diseases that can be treated with the compounds or compositions as described herein include, but are not limited to Adult-Onset Still’s Disease (AOSD), Systemic Juvenile Idiopathic Arthritis (sJIA), Macrophage Activation Syndrome (MAS), Autoinflammation with Infantile Enterocolitic (AIFEC), Bullous Pemphigoid, Pemphigus Vulgaris, Idiopathic Pulmonary Fibrosis (IPF), Non-Alcoholic Steatohepatitis (NASH), Systemic Lupus Erythematosus (SLE), Multiple Sclerosis, Alzheimer’s Disease, Parkinson’s Disease, Traumatic Brain Injury (TBI), Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), Cryopyrin- Associated Periodic Syndromes (CAPS), Vitiligo, Multiple Self-Healing Palmoplantar Carcinoma (MSPC), Autoimmune Addison’s Disease, Familial Mediterranean
- the disclosure provides methods for treating an inflammatory condition, for example, an inflammatory condition that causes cell death, or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a coronavirus (e.g., SARS-CoV 2, SARS-CoV, or MERS), viral, bacterial, fungal, parasitic, or other type of infection in a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
- a coronavirus e.g., SARS-CoV 2, SARS-CoV, or MERS
- a compound as otherwise described herein e.g., a compound of formula (I)
- the disclosure provides methods for treating an inflammatory condition (e.g., cytokine storm syndrome, or cytokine release syndrome), for example, an inflammatory condition that causes cell death or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a cell therapy (e.g., CAR T cell therapy) administered to a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
- a cell therapy e.g., CAR T cell therapy
- FIG. 1 is a Western blot depicting autoproteolyic cleavage of Caspase-1 when treated with compositions according to example embodiments.
- the disclosure provides compounds, pharmaceutical compositions, methods and uses for treating a variety of diseases associated with inhibiting inflammasome formation.
- the compounds are targeted at inhibiting ASC assembly, blocking a structural motif of the inflammasome and preventing cytokine expression.
- ASC assembly blocking a structural motif of the inflammasome
- cytokine expression a structural motif of the inflammasome
- the compounds can be defined generically as with respect to formula (I) as appropriate, or in various subgenera formulae in which A 1 , Q 1 , W 1 , X 1 , Y 1 , and Z 1 are optionally independently selected from the groups (1a) etseq., (2a) etseq., (3a) etseq., (4a) etseq., (5a) et seq., and (6a) et seq. defined herein below (e.g., wherein the compound is of a structural formula as defined in any combination of the embodiments below):
- a 1 is independently selected from one of the following groups (1a) - (1n):
- cycloalkyl e.g., cyclopropyl
- (lm) C 2 -C 4 alkyl e.g., (propyl, such as isopropyl (prop-2-yl));
- Q 1 is independently selected from one of the following groups (2a) - (2I):
- R A1 is H, methyl, ethyl, or isopropyl
- W 1 is independently selected from one of the following groups (3a) - (3d):
- X 1 is independently selected from one of the following groups (4a) - (4b):
- Y 1 is independently selected from one of the following groups (5a) - (5f):
- C 2 -C 6 alkyl groups in (5b) include n-propyl, n-butyl, or n-pentyl
- alkenyl groups in (5c) include but-2-enyl, 2-methylbut-2-enyl, 2- fluorobut-2-enyl, and 2,3-difluorobut-2-enyl).
- C 2 -C 6 alkynyl groups in (5d) include but-2-ynyl.
- Z 1 is independently selected from one of the following groups (6a) - (6g):
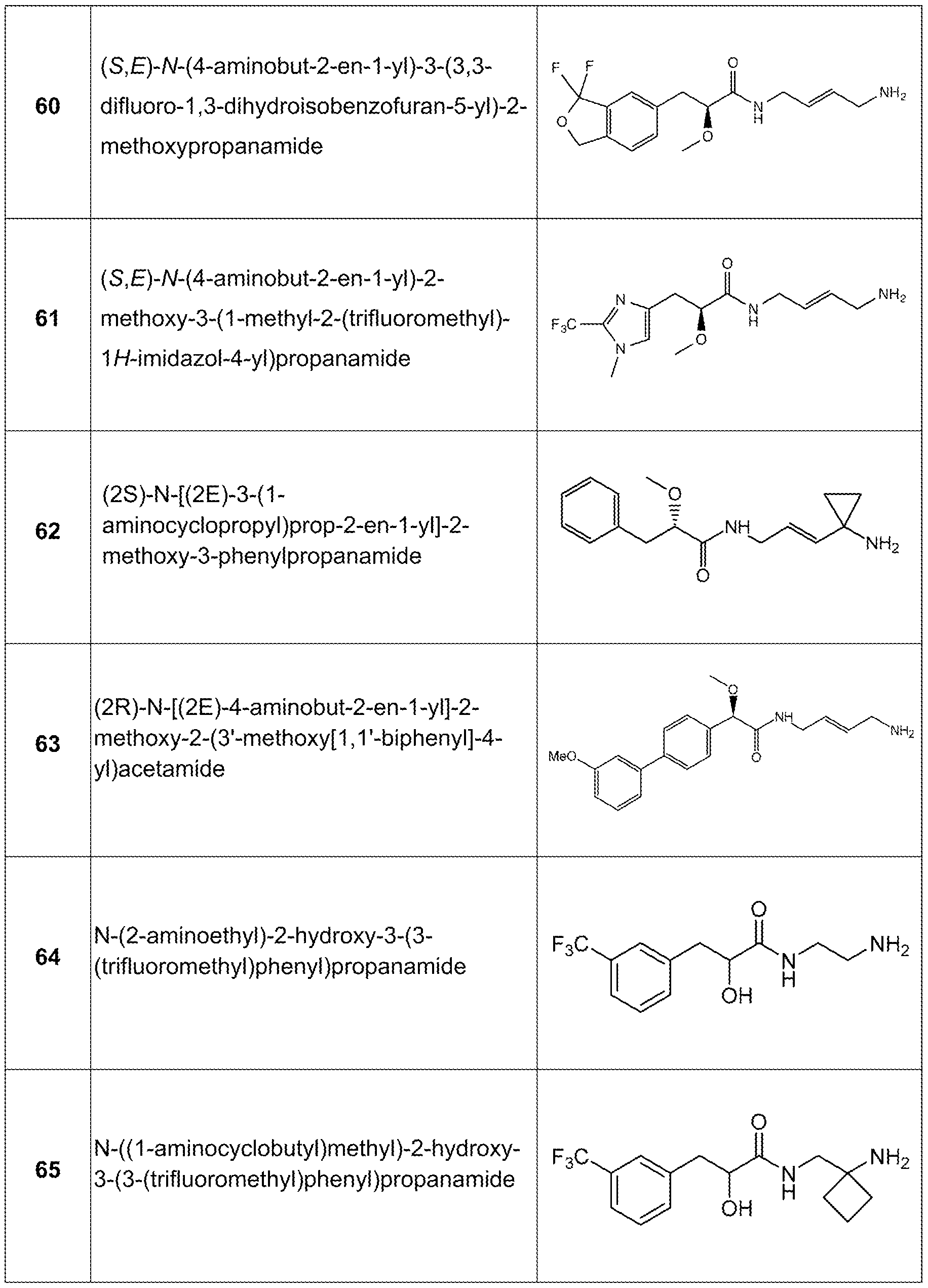
- the compound is not N-(2-aminoethyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, N-((1 - aminocyclobutyl)methyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, or N-(2- aminocyclohexyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide.
- each R C1 is independently selected from the group consisting of methyl, ethyl, -CF 3 , -NH 2 , -F, and -Cl.
- each R C1 is CH 3 , -CH 2 CH 3 , -CF 3 -F, or -Cl.
- each R A1 is independently H, methyl, butenyl, or -(C 0-1 )-cyclopropyl.
- each R A1 is H or methyl.
- each R B1 is H.
- the compound has the structural
- the compound has the structural formula
- n and m are independently 1 , 2, or 3 and s and t are independently 0, 1 , 2, or 3.
- the compound has the structural formula
- n and m are independently 1 , 2, or 3 and s and t are independently 0, 1 , 2, or 3.
- the compound has formula I-A1 , I-A2, 1-A3, 1-B1 , I-B2 or I-B3 wherein n, m, s and t are independently 1 or 2 and Q 1 is hydroxy or C 1 -C 6 alkoxy.
- the compound has formula I-A1 , I-A2, or I-A3, wherein n, m, s and t are independently 1 or 2 and Q 1 is hydroxy or C 1 -C 3 alkoxy, particularly methoxy or ethoxy.
- the compound has formula I-A1 , 1-A2, or I-A3, wherein n, m, s and t are independently 1 or 2, Q 1 is hydroxy, methoxy or ethoxy, and A 1 is phenyl optionally substituted with one or two of trifluoromethyl, halogen (particularly chloro or fluoro), hydroxy, amino, C 1 -C 2 alkyl, C 1 -C 2 alkoxy, or mono- or di-C 1 -C 2 alkyl amino.
- each optionally substituted alkyl, alkenyl, and alkynyl recited in any one of preceding embodiments is unsubstituted.
- each cycloalkyl recited in any one of the preceding embodiments is a 3-7 membered monocyclic cycloalkyl.
- each cycloalkyl recited in any one of the preceding embodiments is a cyclopropyl, a cyclobutyl, a cyclopentyl, a cyclopentenyl, a cyclohexyl or a cyclohexenyl.
- each heterocycloalkyl recited in any one of the preceding embodiments is a 4-7 membered monocyclic heterocycloalkyl having 1-2 heteroatoms selected from O, S and N.
- each heterocycloalkyl recited in any one of the preceding embodiments is a pyrrolidinyl, a tetrahydrofuranyl, a tetrahydrothienyl, a piperidinyl, a piperazinyl, a morpholinyl, a thiomorpholinyl, a tetrahydro-2H- pyranyl, or a tetrahydro-2H-thiopyranyl.
- each heterocycloalkyl recited in any one of the preceding embodiments is a pyrrolidine, a piperidine, a piperazine, a tetrahydrofuran, a (IH)dihydropyran, or a morpholine (e.g., each unsubstituted).
- each aryl is phenyl.
- each heteroaryl is a 5-6 membered monocyclic heteroaryl having 1-3 heteroatoms selected from O, S and N.
- each heteroaryl is a monocyclic heteroaryl is substituted with 0-3 R C1 , e.g., is unsubstituted, substituted with one R C1 or substituted with two R C1 .
- each heteroaryl is a furanyl, a thienyl, a pyrrolyl, a pyrazolyl, an imidazolyl, an oxazolyl or a thiazolyl.
- each R C1 is independently C 1 -C 4 alkyl (e.g., methyl, t-butyl, or -CF 3 ), -Cl, -F, -Br, -CN, -N(R A1 )(R B1 ), -OR A1 , -C(O)R A1 , or -C(O)NR B1 R A1 .
- each R C1 is independently methyl, -CF 3 , -F-, -CN, -OR A1 , or -C(O)R A1 .
- the carbon joining A 1 , Q 1 , and W 1 is a stereocenter, and has S stereochemistry, except in the case where W 1 contains sulfur, wherein the carbon has R stereochemistry.
- the stereochemistry of said carbon joining A 1 , Q 1 , and W 1 has stereochemistry selected so as to mimic the molecular geometry of an L-amino acid.
- the compound is in the form of a pharmaceutically acceptable salt of a compound as described herein.
- a pharmaceutically-acceptable salts may be provided, as described in additional detail below.
- a compound is in the form of a solvate (e.g., a hydrate) of a compound or salt as described herein.
- solvates and/or hydrates may be formed.
- phrase “optionally in the form of a pharmaceutically acceptable salt thereof, and/or a solvate or hydrate thereof includes compounds in the form of solvates and hydrates of base compounds or pharmaceutically acceptable salts as described above. But in certain embodiments as described above, the compound is not in the form of a solvate or hydrate.
- the disclosure also provides methods of treating various diseases, such as inflammatory conditions. These methods include administering to a subject in need of such treatment an effective amount of one or more compounds of the disclosure as described herein (e.g., compounds of formula (I)) or a pharmaceutical composition of the disclosure as described herein.
- the present disclosure provides for a method of treating an inflammatory condition in a subject thereof, wherein the method includes providing to the subject a compound as otherwise described herein.
- the inflammatory condition is an inflammatory bowel disease, an arthritic disease, or a neurodegenerative disorder.
- the inflammatory condition is an autoinflammatory syndrome.
- the inflammatory condition is an inflammasome-related condition.
- the diseases of the disclosure include, but are not limited to Adult- Onset Still’s Disease (AOSD), Systemic Juvenile Idiopathic Arthritis (sJIA), Macrophage Activation Syndrome (MAS), Autoinflammation with Infantile Enterocolitic (AIFEC), Bullous Pemphigoid, Pemphigus Vulgaris, Idiopathic Pulmonary Fibrosis (IPF), Non-Alcoholic Steatohepatitis (NASH), Systemic Lupus Erythematosus (SLE), Multiple Sclerosis, Alzheimer’s Disease, Parkinson’s Disease, Traumatic Brain Injury (TBI), Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), Cryopyrin-Associated Periodic Syndromes (CAPS), Vitiligo, Multiple Self-Healing Palmoplantar Carcinoma (MSPC), Autoimmune Addison’s Disease, Familial Mediterranean Fever (FMF), Autoimmune Thyroid
- the disclosure provides methods for treating an inflammatory condition, for example, an inflammatory condition that causes cell death, or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a coronavirus (e.g., SARS-CoV 2, SARS-CoV, or MERS), viral, bacterial, fungal, parasitic, or other type of infection in a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
- a coronavirus e.g., SARS-CoV 2, SARS-CoV, or MERS
- the inflammatory condition may cause at least one of cell death, release of pro-inflammatory cytokines, or other inflammatory mediators.
- the inflammatory condition suitable for treatment by compounds as otherwise described herein includes cytokine storm syndrome, cytokine release syndrome, sepsis, hemophagocytic lymphohistiocytosis, acute lung injury, acute respiratory distress syndrome, or macrophage activation syndrome. Further suitable inflammatory conditions will be apparent to one of skill in the art.
- the inflammatory condition may result from a cell therapy administered to a human subject.
- a CAR T cell therapy may comprise cytokine storm syndrome, or cytokine release syndrome.
- the inflammatory condition may be treated with a compound as otherwise described herein in co-therapy with at least one of an antiinfective, antimicrobial, antiviral (e.g., remdesivir), monoclonal antibody (e.g., tocilizumab), biologic, immunoglobulin, immunomodulator, anti-inflammatory drug, corticosteroid, small molecule, cell therapy, or other type of prophylactic or therapeutic agent.
- an antiinfective, antimicrobial, antiviral e.g., remdesivir
- monoclonal antibody e.g., tocilizumab
- biologic e.g., tocilizumab
- immunoglobulin e.g., tocilizumab
- immunomodulator e.g., anti-inflammatory drug, corticosteroid, small molecule, cell therapy, or other type of prophylactic or therapeutic agent.
- the disclosure provides methods for treating an inflammatory condition (e.g., cytokine storm syndrome, or cytokine release syndrome), for example, an inflammatory condition that causes cell death or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a cell therapy (e.g., CAR T cell therapy) administered to a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
- a cell therapy e.g., CAR T cell therapy
- a compound as otherwise described herein is administered in co-therapy with at least one of a monoclonal antibody (e.g., tocilizumab), a biologic, an immunoglobulin, an immunomodulatory, an anti-inflammatory drug, a corticosteroid, a small molecule, a cell therapy, or other type of prophylactic or therapaetuic agent.
- a monoclonal antibody e.g., tocilizumab
- a biologic e.g., an immunoglobulin, an immunomodulatory, an anti-inflammatory drug, a corticosteroid, a small molecule, a cell therapy, or other type of prophylactic or therapaetuic agent.
- the present disclosure provides for a method for suppressing ASC-filament formation in a subject, wherein the method includes administering a compound as otherwise described herein.
- the inhibition may be through interacting with the apoptosis-associated speck-like protein containing A CARD.
- a compound as described herein can usefully be provided in the form of a pharmaceutical composition.
- Such compositions include the compound according to any one of the preceding aspects or embodiments described herein, together with a pharmaceutically acceptable excipient, diluent, or carrier.
- the compounds may be formulated in the pharmaceutical composition per se, or in the form of a hydrate, solvate, or pharmaceutically acceptable salt, as previously described.
- such salts are more soluble in aqueous solutions than the corresponding free acids and bases, but salts having lower solubility than the corresponding free acids and bases may also be formed.
- the pharmaceutical composition can be, for example, in the form of a tablet, a capsule, or a parenteral formulation, but the person of ordinary skill in the art will appreciate that the compound can be provided in a wide variety of pharmaceutical compositions.
- the compounds of the disclosure can be administered, for example, orally, topically, parenterally, by inhalation or spray or rectally in dosage unit formulations containing one or more pharmaceutically acceptable carriers, diluents or excipients.
- parenteral as used herein includes percutaneous, subcutaneous, intravascular (e.g., intravenous), intramuscular, or intrathecal injection or infusion techniques and the like.
- a medicament including a compound of the disclosure can be provided in any appropriate of the formulations and dosage forms as described herein.
- compositions can be made using the presently disclosed compounds.
- a pharmaceutical composition includes a pharmaceutically acceptable carrier, diluent or excipient, and compound as described above with reference to any one of structural formulae.
- one or more compounds of the disclosure may be present in association with one or more pharmaceutically acceptable carriers, diluents or excipients, and, if desired, other active ingredients.
- the pharmaceutical compositions containing compounds of the disclosure may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
- compositions intended for oral use can be prepared according to any suitable method for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preservative agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients that are suitable for the manufacture of tablets.
- excipients can be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets can be uncoated or they can be coated by known techniques. In some cases such coatings can be prepared by suitable techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monostearate or glyceryl distearate can be employed.
- Formulations for oral use can also be presented as hard gelatin capsules, wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
- an inert solid diluent for example, calcium carbonate, calcium phosphate or kaolin
- water or an oil medium for example peanut oil, liquid paraffin or olive oil.
- Formulations for oral use can also be presented as lozenges.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients can be suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropylmethylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents such as a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example -olyethylene sorbit
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions can be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol.
- Sweetening agents and flavoring agents may be added to provide palatable oral preparations. These compositions may be preserved by the addition of an anti- oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives.
- a dispersing or wetting agent for example sweetening, flavoring and coloring agents, can also be present.
- compositions can also be in the form of oil-in-water emulsions.
- the oily phase can be a vegetable oil or a mineral oil or mixtures of these.
- Suitable emulsifying agents can be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally- occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate.
- the emulsions can also contain sweetening and flavoring agents.
- the pharmaceutically acceptable carrier, diluent, or excipient is not water.
- the water comprises less than 50% of the composition.
- compositions comprising less than 50% water have at least 1%, 2%, 3%, 4% or 5% water.
- the water content is present in the composition in a trace amount.
- the pharmaceutically acceptable carrier, diluent, or excipient is not alcohol.
- the alcohol comprises less than 50% of the composition.
- compositions comprising less than 50% alcohol have at least 1%, 2%, 3%, 4% or 5% alcohol.
- the alcohol content is present in the composition in a trace amount.
- Syrups and elixirs can be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol, glucose or sucrose. Such formulations can also contain a demulcent, a preservative, flavoring, and coloring agents.
- the pharmaceutical compositions can be in the form of a sterile injectable aqueous or oleaginous suspension.
- This suspension can be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents that have been mentioned above.
- the sterile injectable preparation can also be a sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol.
- the acceptable vehicles and solvents that can be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils can be employed as a solvent or suspending medium.
- any bland fixed oil can be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- compositions of the disclosure can also be administered in the form of suppositories, e.g., for rectal administration of the drug.
- suppositories e.g., for rectal administration of the drug.
- These compositions can be prepared by mixing the compound with a suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- suitable non-irritating excipient include cocoa butter and polyethylene glycols.
- Compounds of the disclosure can also be administered parenterally in a sterile medium.
- the drug depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle.
- adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
- compositions can be formulated in a unit dosage form of the active ingredient.
- unit dosage forms refers to physically discrete units suitable as unitary dosages for human subjects and other mammals, each unit containing a predetermined quantity of active material calculated to produce the desired therapeutic effect, in association with a suitable pharmaceutical excipient.
- the compound can be effective over a wide dosage range and is generally administered in a pharmaceutically effective amount. It will be understood, however, that the amount of the compound actually administered will usually be determined by a physician, according to the relevant circumstances, including the condition to be treated, the chosen route of administration, the actual compound administered, the age, weight, and response of the individual patient, the severity of the patient's symptoms, and the like.
- the principal active ingredient is mixed with a pharmaceutical excipient to form a solid preformulation composition containing a homogeneous mixture of a compound described herein.
- the active ingredient is typically dispersed evenly throughout the composition so that the composition can be readily subdivided into equally effective unit dosage forms such as tablets, pills and capsules.
- This solid preformulation is then subdivided into unit dosage forms of the type described above containing from, for example, 0.1 to about 500 mg of the active ingredient of a compound described herein.
- the tablets or pills can be coated or otherwise compounded to provide a dosage form affording the advantage of prolonged action.
- the tablet or pill can comprise an inner dosage and an outer dosage component, the latter being in the form of an envelope over the former.
- the two components can be separated by an enteric layer which serves to resist disintegration in the stomach and permit the inner component to pass intact into the duodenum or to be delayed in release.
- enteric layers or coatings such materials including a number of polymeric acids and mixtures of polymeric acids with such materials as shellac, cetyl alcohol, and cellulose acetate.
- compositions can be administered to a patient already suffering from a disease in an amount sufficient to cure or at least partially arrest the symptoms of the disease and its complications. Effective doses will depend on the disease condition being treated as well as by the judgment of the attending clinician depending upon factors such as the severity of the disease, the age, weight and general condition of the patient, and the like.
- compositions administered to a patient can be in the form of pharmaceutical compositions described above. These compositions can be sterilized by conventional sterilization techniques, or may be sterile filtered. Aqueous solutions can be packaged for use as is, or lyophilized, the lyophilized preparation being combined with a sterile aqueous carrier prior to administration.
- the pH of the compound preparations typically will be between 3 and 11 , more preferably from 5 to 9 and most preferably from 7 to 8. It will be understood that use of certain of the foregoing excipients, carriers, or stabilizers will result in the formation of pharmaceutical salts.
- the therapeutic dosage of the compounds can vary according to, for example, the particular use for which the treatment is made, the manner of administration of the compound, the health and condition of the patient, and the judgment of the prescribing physician.
- the proportion or concentration of a compound described herein in a pharmaceutical composition can vary depending upon a number of factors including dosage, chemical characteristics (e.g ., hydrophobicity), and the route of administration.
- the compounds described herein can be provided in an aqueous physiological buffer solution containing about 0.1 to about 10% w/v of the compound for parenteral administration. Some typical dose ranges are from about 1 pg/kg to about 1 g/kg of body weight per day.
- the dose range is from about 0.01 mg/kg to about 100 mg/kg of body weight per day.
- the dosage is likely to depend on such variables as the type and extent of progression of the disease or disorder, the overall health status of the particular patient, the relative biological efficacy of the compound selected, formulation of the excipient, and its route of administration. Effective doses can be extrapolated from dose-response curves derived from in vitro or animal model test systems.
- the compounds described herein can also be formulated in combination with or administered sequentially with one or more additional active ingredients which can include any pharmaceutical agent such as anti-viral agents, vaccines, antibodies, immune enhancers, immune suppressants, anti-inflammatory agents and the like.
- an “alkyl” moiety can refer to a monovalent radical (e.g., CH 3 -CH 2 -)
- a bivalent linking moiety can be “alkyl,” in which case those skilled in the art will understand the alkyl to be a divalent radical (e.g., -CH 2 -CH 2 -), which is equivalent to the term “alkylene.”
- alkyl in which case those skilled in the art will understand the alkyl to be a divalent radical (e.g., -CH 2 -CH 2 -), which is equivalent to the term “alkylene.”
- aryl refers to the corresponding divalent moiety, arylene.
- Nitrogens in the presently disclosed compounds can be hypervalent, e.g., an N-oxide or tetrasubstituted ammonium salt.
- a moiety may be defined, for example, as -B-(A) a , wherein a is 0 or 1. In such instances, when a is 0 the moiety is -B and when a is 1 the moiety is -B-A.
- alkyl includes a saturated hydrocarbon having a designed number of carbon atoms, such as 1 to 10 carbons (i.e., inclusive of 1 and 10), 1 to 8 carbons, 1 to 6 carbons, 1 to 3 carbons, or 1, 2, 3, 4, 5 or 6.
- Alkyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkylene group).
- the moiety “-(C 1 -C 6 alkyl)-0-” signifies connection of an oxygen through an alkylene bridge having from 1 to 6 carbons and C 1 -C 3 alkyl represents methyl, ethyl, and propyl moieties.
- alkyl examples include, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, iso-, sec- and tert-butyl, pentyl, and hexyl.
- alkoxy represents an alkyl group of indicated number of carbon atoms attached to the parent molecular moiety through an oxygen bridge.
- alkoxy include, but are not limited to, methoxy, ethoxy, propoxy, and isopropoxy.
- alkenyl as used herein, unsaturated hydrocarbon containing from 2 to 10 carbons (i.e., inclusive of 2 and 10), 2 to 8 carbons, 2 to 6 carbons, or 2, 3, 4, 5 or 6, unless otherwise specified, and containing at least one carbon-carbon double bond.
- Alkenyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkenylene group).
- the moiety “-(C 2 -C 6 alkenyl)-O-” signifies connection of an oxygen through an alkenylene bridge having from 2 to 6 carbons.
- alkenyl include, but are not limited to, ethenyl, 2-propenyl, 2- methyl-2-propenyl, 3-butenyl, 4-pentenyl, 5-hexenyl, 2-heptenyl, 2-methyl-1-heptenyl, 3- decenyl, and 3,7-dimethylocta-2,6-dienyl.
- alkynyl unsaturated hydrocarbon containing from 2 to 10 carbons (i.e., inclusive of 2 and 10), 2 to 8 carbons, 2 to 6 carbons, or 2, 3, 4, 5 or 6 unless otherwise specified, and containing at least one carbon-carbon triple bond.
- Alkynyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkynylene group).
- the moiety “-(C 2 -C 6 alkynyl)-O-” signifies connection of an oxygen through an alkynylene bridge having from 2 to 6 carbons.
- Representative examples of alkynyl include, but are not limited to, acetylenyl, 1-propynyl, 2- propynyl, 3-butynyl, 2-pentynyl, and 1-butynyl.
- aryl represents an aromatic ring system having a single ring (e.g., phenyl) which is optionally fused to other aromatic hydrocarbon rings or non-aromatic cycloalkyl or heterocycloalkyl rings.
- the aryl ring system is attached to the parent molecular moiety through any atom contained within the ring system.
- aryl groups include, but are not limited to, phenyl, naphthyl, indanyl, indenyl, dihydronaphthyl, fluorenyl, tetralinyl, 1, 2,3,4- tetrahydronaphthyl, 6,7,8,9-tetrahydro-5H-benzo[a]cycloheptenyl, 1 H-2,3-dihydrobenzofuranyl and tetrahydroisoquinolinyl.
- aryl is phenyl.
- aryl is phenyl or naphthyl.
- the aryl is (i) phenyl, (ii) naphthyl or (iii) a phenyl ring fused to either a 5 or 6 membered monocyclic cycloalkyl or cycloalkenyl, or a 5 or 6 membered monocyclic heterocycloalkyl.
- the aryl groups herein are unsubstituted or, when specified as “optionally substituted”, can unless stated otherwise be substituted in one or more substitutable positions with various groups as indicated.
- halogen or “halo” indicate fluorine, chlorine, bromine, and iodine. In certain embodiments of each and every embodiment as otherwise described herein, the term “halogen” or “halo” refers to fluorine or chlorine. In certain embodiments of each and every embodiment described herein, the term “halogen” or “halo” refers to fluorine.
- fluorinated indicates a group (such as alkyl as otherwise described herein) wherein at least one of the carbon-bound hydrogens is substituted by fluorine to form C-F groups. Examples of fluorinated alkyl groups are fluoromethyl, difluoromethyl and trifluoromethyl.
- Trifluoromethyl is a perfluorinated alkyl group.
- perfluorinated indicates a group (such as alkyl as otherwise described herein) wherein all carbon-bound hydrogens are substituted by fluorine to form C-F groups. .
- heteroaryl refers to an aromatic ring or ring system containing at least one aromatic heteroatom selected from boron, nitrogen, oxygen and sulfur in an aromatic ring. Most commonly, the heteroaryl groups will have 1 , 2, 3, or 4 heteroatoms.
- the heteroaryl may be fused to one or more non-aromatic rings, for example, cycloalkyl or heterocycloalkyl rings, wherein the cycloalkyl and heterocycloalkyl rings are described herein.
- the heteroaryl ring system is bonded to the parent molecular moiety through any atom contained within the ring system.
- heteroaryl groups include, but are not limited to, pyridyl, pyrimidinyl, quinolinyl, benzothienyl, indolyl, indolinyl, pyridazinyl, pyrazinyl, isoindolyl, isoquinolyl, quinazolinyl, quinoxalinyl, phthalazinyl, imidazolyl, isoxazolyl, pyrazolyl, oxazolyl, thiazolyl, indolizinyl, indazolyl, benzothiazolyl, benzimidazolyl, benzofuranyl, furanyl, thienyl, pyrrolyl, oxadiazolyl, thiadiazolyl, benzo[1 ,4]oxazinyl, triazolyl, tetrazolyl, isothiazolyl, naphthyridinyl, isochromanyl, chromoni
- each heteroaryl is selected from pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl, imidazolyl, isoxazolyl, pyrazolyl, oxazolyl, thiazolyl, furanyl, thienyl, pyrrolyl, oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, isothiazolyl, pyridinyl-N- oxide, pyrrolyl N- oxide, pyrimidinyl N- oxide, pyridazinyl N- oxide, pyrazinyl N- oxide, imidazolyl N- oxide, isoxazolyl N- oxide, oxazolyl N- oxide, thiazolyl N- oxide, pyrrolyl N- oxide, oxadiazolyl N- oxide, thiadiazolyl N- oxide, triazolyl N- oxide, and
- heteroaryl groups include pyridyl, pyrimidyl, quinolinyl, indolyl, pyrrolyl, furanyl, thienyl, imidazolyl, pyrazolyl, indazolyl, thiazolyl and benzothiazolyl.
- the heteroaryl groups herein are unsubstituted or, when specified as “optionally substituted”, can unless stated otherwise be substituted in one or more substitutable positions with various groups, as indicated.
- heterocycloalkyl refers to a non-aromatic ring or ring system containing at least one heteroatom that is preferably selected from boron, nitrogen, oxygen and sulfur, wherein said heteroatom is in a non-aromatic ring.
- the heterocycloalkyl may have 1 , 2, 3 or 4 heteroatoms.
- the heterocycloalkyl may be saturated or partially unsaturated (i.e., a heterocycloalkenyl).
- Heterocycloalkyl includes monocyclic groups of three to eight annular atoms as well as bicyclic and polycyclic ring systems, including bridged and fused systems, wherein each ring includes three to eight annular atoms.
- the heterocycloalkyl ring is optionally fused and/or bridged to other heterocycloalkyl and/or non-aromatic hydrocarbon rings.
- the heterocycloalkyl groups have from 3 to 7 members in a single ring.
- heterocycloalkyl groups have 5 or 6 members in a single ring.
- the heterocycloalkyl groups have 3, 4, 5, 6 or 7 members in a single ring.
- heterocycloalkyl groups include, but are not limited to, azabicyclo[2.2.2]octyl (in each case also “quinuclidinyl” or a quinuclidine derivative), azabicyclo[3.2.1]octyl, 2,5- diazabicyclo[2.2.1]heptyl, morpholinyl, thiomorpholinyl, thiomorpholinyl S-oxide, thiomorpholinyl S,S-dioxide, 2-oxazolidonyl, piperazinyl, homopiperazinyl, piperazinonyl, pyrrolidinyl, azepanyl, azetidinyl, pyrrolinyl, tetrahydropyranyl, piperidinyl, tetrahydrofuranyl, tetrahydrothienyl, 3,4- dihydroisoquinolin-2(1H)-yl, isoindolindion
- heterocycloalkyl groups include morpholinyl, 3,4-dihydroisoquinolin-2(1H)-yl, tetrahydropyranyl, piperidinyl, aza-bicyclo[2.2.2]octyl, g-butyrolactonyl (i.e., an oxo-substituted tetrahydrofuranyl), y-butryolactamyl (i.e., an oxo-substituted pyrrolidine), pyrrolidinyl, piperazinyl, azepanyl, azetidinyl, thiomorpholinyl, thiomorpholinyl S,S-dioxide, 2-oxazolidonyl, imidazolidonyl, isoindolindionyl, piperazinonyl.
- the heterocycloalkyl groups herein are unsubstituted or, when specified as “option
- cycloalkyl refers to a non-aromatic carbocyclic ring or ring system, which may be saturated or partially unsaturated (e.g., a cycloalkenyl).
- the cycloalkyl ring can be optionally fused and/or bridged with a bivalent bridge to other cycloalkyl rings.
- Certain examples of cycloalkyl groups present in the disclosed compounds have from 3 to 7 members in a single ring, such as having 5 or 6 members in a single ring. In some embodiments, the cycloalkyl groups have 3, 4, 5, 6 or 7 members in a single ring.
- cycloalkyl groups include, but are not limited to, cyclohexyl, cyclopentyl, cyclobutyl, cyclopropyl, tetrahydronaphthyl norbornanyl, norbornenyl, noborndienyl, and bicyclo[2.2.1]heptanyl.
- the cycloalkyl groups herein are unsubstituted or, when specified as “optionally substituted”, may be substituted in one or more substitutable positions with various groups, as indicated.
- a cycloalkyl may be bridged with a trivalent bridge. Examples of trivalently bridged cycloalkyl systems includes adamantanyl.
- ring system encompasses monocycles, as well as fused and/or bridged polycycles.
- substituted when used to modify a specified group or radical, means that one or more hydrogen atoms of the specified group or radical are each, independently of one another, replaced with the same or different substituent groups as defined below, unless specified otherwise.
- pharmaceutically acceptable salt refers to both pharmaceutically acceptable acid and base addition salts and solvates.
- Such pharmaceutically acceptable salts include salts of acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic, toluenesulfonic, methanesulfonic, nitric, benzoic, citric, tartaric, maleic, hydroiodic, alkanoic such as acetic, HOOC-(CH 2 ) n -COOH where n is 0-4, and the like.
- Non-toxic pharmaceutical base addition salts include salts of bases such as sodium, potassium, calcium, ammonium, and the like. Those skilled in the art will recognize a wide variety of non-toxic pharmaceutically acceptable addition salts.
- isotopes includes those atoms having the same atomic number but different mass numbers.
- certain atoms, such as hydrogen occur in different isotopic forms.
- hydrogen includes three isotopic forms, protium, deuterium and tritium.
- certain compounds can be enriched at a given position with a particular isotope of the atom at that position.
- compounds having a fluorine atom may be synthesized in a form enriched in the radioactive fluorine isotope 18 F.
- compounds may be enriched in the heavy isotopes of hydrogen: deuterium and tritium; and similarly can be enriched in a radioactive isotope of carbon, such as 13 C.
- isotopic variant compounds undergo different metabolic pathways and can be useful, for example, in studying the ubiquitination pathway and its role in disease.
- the compound has substantially the same isotopic character as naturally-occurring materials.
- the terms “individual,” “patient,” or “subject” are used interchangeably, refers to any animal, including mammals, preferably humans.
- terapéuticaally effective amount refers to the amount of active compound or pharmaceutical agent that elicits the biological or medicinal response that is being sought in a tissue, system, animal, individual or human by a researcher, veterinarian, medical doctor or other clinician.
- an effective amount can be an amount suitable for a
- prophylactic use for example, preventing or limiting development of a disease, condition or disorder in an individual who may be predisposed or otherwise at risk to the disease, condition or disorder but does not yet experience or display the pathology or symptomatology of the disease;
- inhibiting the disease for example, inhibiting a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder;
- ameliorating the referenced disease state for example, ameliorating a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e., reversing or improving the pathology and/or symptomatology) such as decreasing the severity of disease; or
- treatment means (i) ameliorating the referenced disease state, condition, or disorder (or a symptom thereof), such as, for example, ameliorating a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e., reversing or improving the pathology and/or symptomatology) such as decreasing the severity of disease or symptom thereof, or inhibiting the progression of disease; or (ii) eliciting the referenced biological effect (e.g inhibiting inflammasome formation or function, or inhibition of IL-1 ⁇ ).
- Compounds as described herein can be purified by any of the means known in the art, including chromatographic means, such as HPLC, preparative thin layer chromatography, flash column chromatography and ion exchange chromatography. Any suitable stationary phase can be used, including normal and reversed phases as well as ionic resins. Most typically the disclosed compounds are purified via silica gel and/or alumina chromatography. See, e.g., Introduction to Modern Liquid Chromatography, 2nd Edition, ed. L. R. Snyder and J. J. Kirkland, John Wiley and Sons, 1979; and Thin Layer Chromatography, ed E. Stahl, Springer-Verlag, New York, 1969.
- a “leaving group” as used herein refers to a moiety of a reactant (e.g., the alkylhalogenide of the disclosure) that is displaced from the first reactant in the chemical reaction.
- a reactant e.g., the alkylhalogenide of the disclosure
- suitable leaving groups include, but are not limited to, halogen (such as Cl or Br), acetoxy, and sulfonyloxy groups (such as methyl sulfonyloxy, trifluoromethylsulfonyloxy (“triflate”), p-toluenesulfonyloxy (“tosylate”)).
- Step 1 Synthesis of (S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2-methoxypropanamide
- Step 1 Synthesis of (S)-3-(3-chlorophenyl)-2-hydroxypropanoic acid
- a solution of (S)-2-amino-3-(3-chlorophenyl)propanoic acid (0.50 g, 2.513 mmol) in 1 (M) H 2 SO 4 (6 mL) was cooled to 0°C. To this mixture was then added a solution of sodium nitrite (1.04 g, 15.075 mmol) in water (3 mL) slowly over 15 min. Reaction mixture was stirred at 0°C for 2h, then warmed to 25°C and stirred for another 20h. The reaction mixture was diluted with EtOAc and organic layer was separated. Aqueous layer was re-extracted with EtOAc.
- Step 3 Terf-butyl (S,E)-(4-(3-(3-chlorophenyl)-2-methoxypropanamido)but-2-en-1- yl)carbamate
- Step 4 (S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2-methoxypropanamide
- reaction mixture stirred for 5 minutes and [(dimethylamino)( ⁇ 3H- [1,2,3]triazolo[4,5-b]pyridin-3-yloxy ⁇ )methylidene]dimethylazanium; hexafluoro-A 5 -phosphanuide (3.20 g, 8.42 mmol) was added.
- the reaction mixture stirred at room temperature for 4 h. Reaction was monitored by TLC and NMR. After completion of reaction, reaction mixture was diluted with water (50 mL) and extracted with ethyl acetate (2 x 20 mL).
- the human monocytic THP-1 cell line was purchased from ATCC (TIB-202).
- the human monocytic THP-1 ASC-GFP reporter cell line was purchased from Invivogen (thp- ascgfp).
- Cells were maintained in RPMI media supplemented 10% fetal bovine serum and 100 units of penicillin and streptomycin per mL at a cell density of 2-8 x 10 5 cells/mL
- PMA differentiation of THP-1 cells For ASC Spec assays, the human monocytic cell line THP-1 ASC-GFP cells were differentiated into a macrophage-like phenotype using Phorbol 12- myristate 13-acetate (PMA). THP-1 cells were suspended at a density of 1-2 x 10 6 cells/mL and supplemented with 100 ng/mL PMA. One hundred thousand cells were seeded into each well of a 96 well plate and incubated for 72 hours. Adherent cells were washed three times with PMA free THP-1 media, then cells were rested for 24 hours in PMA free media.
- PMA Phorbol 12- myristate 13-acetate
- NLRP3 Inflammasome and pyroptosis assay in THP-1 Cells THP-1 cells were primed with 300 ng/mL of ultra-pure LPS. One hour later, cells were treated as indicated in figure legend. Three hours post-LPS prime, the NLRP3 inflammasome was activated with 10 mM Nigericin. Two hours later, supernatants were collected and analyzed for IL-1 b by ELISA. Pyroptotic cell death was assessed by MTT assay as described below.
- MTT Cell Viability Assay MTT Cell Viability Assay: MTT reagent (0.6 mg/mL in THP-1 culture media) was prepared and 150 ⁇ L was added to each well. After 1-2 hours, cells were centrifuged at 500g for 5 minutes to pellet cells and crystals. Supernatants were removed, and formazan crystals were dissolved in 100 ⁇ L of isopropanol. Cell viability was assessed by measuring optical density at 560 nm, with a reference wavelength of 670 nm. Viability is expressed as percent signal relative to wells containing untreated cells.
- Cell Free Inflammasome Assay Cell free inflammasome activation and Caspase-1 cleavage was assessed as described previously. Briefly, lysates were prepared from THP-1 by hypotonic lysis to a final protein concentration > 7 mg/mL. Lysates were pretreated for 20 minutes with a final concentration of 1 mM of the indicated compound on ice. Inflammasome activation was induced by incubating samples for 60 minutes at 30°C. Inflammasome activity was assessed through monitoring Caspase-1 cleavage by Western blot.
- NLRP3 Inflammasome activation in PBMCs 2 x 10 5 freshly isolated PBMCs were primed with 300 ng/mL LPS for 1 hour, then treated with a dose titration of the indicated compounds for an additional 2 hours. The NLRP3 inflammasome was then activated by treating cells with 10 mM nigericin for 2 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
- AIM2 Inflammasome activation in PBMCs 2 x 10 5 freshly isolated PBMCs were transfected with poly dA:dT (100 ng/well) using Lipofectamine 2000 (1 ⁇ L/well) according to the manufacturer’s instructions in order to activate the AIM2 inflammasome. One hour later, cells were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
- NLRP1 Inflammasome activation in PBMCs 2 x 10 5 freshly isolated PBMCs were treated with 10 mM Talabostat, a constitutive repressor of the NLRP1 inflammasome. One hour later, cells were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
- NLRC4 Inflammasome activation PBMCs 2 x 10 5 freshly isolated PBMCs were transfected with bacterial flagellin protein (100 ng/well) using Lipofectamine 2000 (1 ⁇ L/well) according to the manufacturer’s instructions in order to activate the NLRC4 inflammasome. One hour later, cells were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
- Inflammasome spontaneously assemble at 30°C in THP-1 cell lysates with sufficient protein concentrations. This processing is dependent on ASC oligomerization.
- the indicated compounds, or vehicle control, were added to THP-1 lysates at a final concentration of 1 mM for 20 minutes prior to inflammasome activation. Samples were incubated for 60 minutes at 30°C to activate the inflammasome. Activation of Caspase-1 (Casp-1 ) through autoproteolytic cleavage was assessed by Western blot. Disappearance of full length Casp-1, along with increased cleaved Casp-1 in the untreated and vehicle control lanes indicates inflammasome activation. Compounds 1-5 were able to significantly inhibit the conversion of full-length Casp-1 into its active, cleaved form as shown in FIG. 1.
- Cytotoxicity was assessed by treating THP-1 cells with a dose titration of the indicated compound for 5 hours, after which cell viability was assessed by MTT assay.
- Inflammasome inhibition was assayed in THP-1 cells primed with LPS, then treated with a dose titration of the indicated compound for 2 hours prior to NLRP3 inflammasome activation with Nigericin.
- IL-1 b was assessed in cell supernatants by ELISA to monitor inflammasome activation.
- an MTT assay was performed on the cells assess Pyroptosis, a form of inflammasome dependent inflammatory cell death.
- ASC oligomerization was monitored using an ASC-GFP THP-1 reporter cell line. Upon inflammasome activation, ASC polymerizes and forms a single large ‘Spec’ per cell. By tagging ASC with GFP these ‘Specs’ are readily observable and the percentage of inflammasome containing cells can be assessed.
- THP-1 ASC-GFP cells were differentiated using PMA overnight, then rested in PMA-free media for one day. Cells were treated with a dose titration of the indicated drug 2 hour prior to NLRP3 inflammasome activation using nigericin. Forty-five minutes after nigericin treatment cells were fixed in 2% paraformaldehyde and imaged on a fluorescent microscope. The percentage of ASC-GFP Spec positive cells per field was quantified.
- Example 6 Inhibition of Multiple Inflammasomes in human PBMCs
- PBMCs peripheral blood mononuclear cells
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Disclosed are N-substituted α-amino and α-hydroxy carboxamides, pharmaceutical compositions comprising them, and methods of using them.
Description
N-Substituted Alpha-Amino and Alpha-Hydroxy Carboxamide Derivatives
BACKGROUND OF THE DISCLOSURE Cross-reference to related applications
[1] This application claims priority from U.S. Application No. 63/015024, filed April 24, 2020, and 63/166083, filed March 25, 2021, the disclosure of each of which is hereby incorporated by reference in its entirety.
Field of the Disclosure
[2] This disclosure relates generally to N-substituted a-amino and ohydroxy carboxamides, pharmaceutical compositions comprising these compounds, and methods of using these compounds to treat various diseases and disorders.
Technical Background
[3] The inflammasome is a large multiprotein complex that plays an important role in both sterile tissue injury and infection. However, due to the potently inflammatory nature of inflammasome- derived mediators, complex regulatory mechanisms have evolved to suppress inflammasome activation in the absence of injury or infection. The importance of such mechanisms is highlighted by activating mutations in the inflammasome pathway that lead to an array of autoinflammatory diseases which can be life threatening. Chronic and acute aberrant activation of the inflammasome pathway also contributes to the pathology of a wide range of arthritic, neurodegenerative, cardiovascular, dermatological, pulmonary and systemic inflammatory conditions.
[4] Assembly of the inflammasome complex is under the control of a ‘two-hit’ system whereby two independent signals are required for activation. Signal 1 stimulates transcription of inflammasome related genes, leading to upregulation of the individual components of the inflammasome, as well as the production of the pro-form of the inflammasome substrates interleukin-1 b (IL-1 b), interleukin-18 (IL-18) and Gasdermin-D within the cytosol. Signal 2 is provided by an array of pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) that are sensed by a family of cytosolic pattern recognition receptors termed nucleotide-binding oligomerization domain-like receptors (NLRs), as well as a few additional inflammasome forming sensors. Binding of the DAMP to its corresponding NLR leads to conformational changes in the NLR allowing self-oligomerization of the receptor. These NLR oligomers then recruits the adapter protein, apoptosis-associated
speck-like protein containing a CARD (ASC), nucleating formation of a filament of repeating ASC subunits, mediated through the pyrin domain of ASC. The ASC filaments in turn creates a platform for recruitment of caspase-1 leading autocatalytic cleavage and activation, as well nucleating subsequent caspase-1 filament formation mediated through the caspase-1 CARD domain, which is thought to amplify caspase-1 activation. Active caspase-1 then cleaves pro-IL- 1b, pro-IL-18 and Gasdermin D into their active forms, releasing the inflammatory mediators from the cell and inducing Gasdermin-D mediated pyroptotic cell death.
[5] Inflammasome formation can be initiated by at least 11 different sensors including Pyrin, numerous NLRs including NLRP1, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4 and NLRC5, the PYHIN family members IFI-16 and AIM2, as well as RIG-I. Conventional approaches to inflammasome inhibition have targeted individual receptors, such as NLRP3, to inhibit a subset of inflammasomes. However, emerging evidence clearly demonstrates that activation of multiple different inflammasome forming receptors is a common pathological feature of many diseases including inflammatory bowel disease, arthritic diseases and neurodegenerative disorders among others. Hence, broader pharmaceutical approaches capable of simultaneously inhibiting multiple species of inflammasomes are needed to address pathological inflammasome-dependent inflammation.
[6] One way to achieve broader inflammasome inhibition is to target the shared components of the inflammasome, such as ASC filaments. Assembly of the inflammasome is induced by a cytosolic sensor (e.g. NLRP3 or AIM2) which detect danger signals associated with infection or sterile injury. Upon sensing these signals, the sensor nucleates formation of long filaments composed of repeating units of the inflammasome adapter protein ASC. These filaments form a scaffold for the recruitment of Caspase-1 leading to autoproteolytic activation of the enzyme. While the inflammasome plays a protective role against infection and injury, aberrant activation of the complex in the absence of infection or injury contributes to a wide range of autoinflammatory conditions. Accordingly, the development of compositions and methods that disrupt assembly of the ASC filament in order to prevent inflammasome driven inflammation is an important challenge.
SUMMARY OF THE DISCLOSURE
[7] One aspect of the disclosure provides compounds having the structural formula:
and pharmaceutically acceptable salts thereof, and/or solvates or hydrates thereof, wherein:
A1 is C1-C6 alkyl, -(C0-C2 alkyl)-cycloalkyl, -(C0-C2 alkyl)-heterocycloalkyl, -(C0-C2 alkyl)-aryl, -(C0-C2 alkyl)-heteroaryl, or -(C0-C2 alkyl)-B1-C1, wherein B1 and C1 are each independently cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
Q1 is -N(RA1)(RB1) or -ORA1;
X1 is -N(RA1)-;
W1 is C(O) or S(O)n1 , wherein n1 is 1 or 2;
Y1 is a C1-C8 alkyl, C2-C8 alkenyl, C2-C8alkynyl, or -(C0-C2 alkyl)-cycloalkyl;
Z1 is -(C0-C2 alkyl )-O(RA) or -(C0-C2 alkyl)-N(RA1)(RB1); wherein each RA1 is independently H, C1-C6 alkyl, C2-C6 alkenyl, or (C0-C2)-cyclopropyl; each RB1 is independently H, C1-C6 alkyl, or C2-C6 alkenyl; each alkyl, alkenyl, and alkynyl is unsubstituted, substituted with one or more halogens (e.g., fluorinated or perfluorinated), or substituted with one or two groups independently selected from methyl, ethyl, acyl, oxo and -C(O)N(RA1)(RB1); each cycloalkyl has 3-10 ring carbons and is saturated or partially unsaturated, and optionally includes one or two fused and/or bridged cycloalkyl rings, each fused and/or bridged ring having 3-8 ring members, wherein the bridge is bivalent or trivalent, and is substituted with 0-5 RC1 or is perfluorinated; each heterocycloalkyl has 3-10 ring members and 1-4 heteroatoms where each heteroatom is independently boron, nitrogen, oxygen or sulfur and is saturated or partially unsaturated, and optionally includes one or two fused cycloalkyl or aryl rings, and/or one or two bridged cycloalkyl rings, each fused and/or bridged ring each having 3-8 ring members, wherein the bridge is bivalent or trivalent, and is substituted with 0-5 RC1 or is perfluorinated; each aryl is a phenyl or naphthyl, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and is substituted with 0-5 RC1, or is perfluorinated; each heteroaryl is a 5-6 membered monocyclic heteroaryl ring having 1-4 heteroatoms, where each heteroatom is independently boron, nitrogen, oxygen or sulfur, or is a
bicyclic heteroaryl having 1-5 heteroatoms where each heteroatom is independently boron, nitrogen, oxygen, or sulfur, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and wherein the heteroaryl is substituted with 0-5 RC1; wherein each RC1 is independently C1-C4 alkyl (e.g., methyl, t-butyl, or -CF3), -Cl, -F, -Br, -CN, -ORA1, -C(O)RA1, -C(O)NRB1RA1, -N(RA1)(RB1), SO3, or SO2(C1 alkyl).
[8] In another aspect, the disclosure provides pharmaceutical compositions comprising a compound (e.g., a compound of formula (I)) as described herein.
[9] In another aspect, the disclosure provides methods for treating various diseases, such as inflammation-related diseases to a subject in need thereof. The methods include administering to the subject an effective amount of a compound as described herein (e.g., a compound of formula (I)).
[10] In another aspect, the disclosure provides intermediates useful to prepare the compounds of formula (I).
[11] In certain embodiments, the diseases that can be treated with the compounds or compositions as described herein include, but are not limited to Adult-Onset Still’s Disease (AOSD), Systemic Juvenile Idiopathic Arthritis (sJIA), Macrophage Activation Syndrome (MAS), Autoinflammation with Infantile Enterocolitic (AIFEC), Bullous Pemphigoid, Pemphigus Vulgaris, Idiopathic Pulmonary Fibrosis (IPF), Non-Alcoholic Steatohepatitis (NASH), Systemic Lupus Erythematosus (SLE), Multiple Sclerosis, Alzheimer’s Disease, Parkinson’s Disease, Traumatic Brain Injury (TBI), Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), Cryopyrin- Associated Periodic Syndromes (CAPS), Vitiligo, Multiple Self-Healing Palmoplantar Carcinoma (MSPC), Autoimmune Addison’s Disease, Familial Mediterranean Fever (FMF), Autoimmune Thyroiditis, Stroke, Type 2 Diabetes (T2D), Osteoarthritis, Gout, Atherosclerosis, Hidradenitis Suppurativa, Psoriasis, and Pyrin Diseases.
[12] In another aspect, the disclosure provides methods for treating an inflammatory condition, for example, an inflammatory condition that causes cell death, or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a coronavirus (e.g., SARS-CoV 2, SARS-CoV, or MERS), viral, bacterial, fungal, parasitic, or other type of infection in a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
[13] In another aspect, the disclosure provides methods for treating an inflammatory condition (e.g., cytokine storm syndrome, or cytokine release syndrome), for example, an inflammatory condition that causes cell death or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a cell therapy (e.g., CAR T cell therapy) administered to a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
[14] Other aspects and embodiments of the disclosure are evident in view of the detailed description provided herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[15] FIG. 1 is a Western blot depicting autoproteolyic cleavage of Caspase-1 when treated with compositions according to example embodiments.
DETAILED DESCRIPTION
[16] The disclosure provides compounds, pharmaceutical compositions, methods and uses for treating a variety of diseases associated with inhibiting inflammasome formation. Specifically, the compounds are targeted at inhibiting ASC assembly, blocking a structural motif of the inflammasome and preventing cytokine expression. Without wishing to be bound by theory, it is hypothesized that effective binding, and therefore inhibition, can be accomplished by a roughly linear molecule with two distinct motifs: a bulky head group on one end, and a modified alkyl chain on the other. Modifications to these two groups have effectively tuned the observed activity, suggesting a highly tunable family that has demonstrated functionality.
[17] The compounds can be defined generically as with respect to formula (I) as appropriate, or in various subgenera formulae in which A1, Q1, W1, X1, Y1, and Z1 are optionally independently selected from the groups (1a) etseq., (2a) etseq., (3a) etseq., (4a) etseq., (5a) et seq., and (6a) et seq. defined herein below (e.g., wherein the compound is of a structural formula as defined in any combination of the embodiments below):
[18] In certain embodiments of the compounds as otherwise described herein, A1 is independently selected from one of the following groups (1a) - (1n):
(1a) -(C0-C2 alkyl)-cycloalkyl, -(C0-C2 alkyl)-heterocycloalkyl, -(C0-C2 alkyl)-aryl, or -(C0-C2 alkyl)-heteroaryl-CH2-N(RA)(RB) (e.g., -(C0-C2 alkyl)-heteroaryl-CH2-NH2);
(lb) t-butyl, -(C0-C2 alkyl)-O0-1 -cycloalkyl, -(C0-C2 alkyl)-O0-1-heterocycloalkyl, -(C0-C2 alkyl)-O0-1-aryl, or -(C0-C2 alkyl)-O0-1-heteroaryl;
(lc) -(C0-C1 alkyl)-cycloalkyl, -(C0-C1 alkyl)-heterocycloalkyl, -(C0-C1 alkyl)-aryl, or -(C0-C1 alkyl)-heteroaryl, wherein each cycle is either monocyclic or fused bicyclic;
(ld) -(C0-C2 alkyl)-aryl, or -(C0-C2 alkyl)-heteroaryl;
(le) -(CH2)-(C0-C1 alkyl)-aryl or -(CH2)-(C0-C1 alkyl)-heteroaryl;
(lf) phenyl or benzyl;
(lg) -(CH2)-phenyl (e.g., -(CH2)-phenyl, or -(CH2)-difluorophenyl);
(lh) -(CH2)-heteroaryl (e.g., thiophenyl);
(1 i) -(C0-C1 alkyl)-cycloalkyl or -(C0-C1 alkyl )-heterocycloalkyl;
(lj) cycloalkyl (e.g., cyclopropyl);
(lk) C1-C6 alkyl;
(ll) t-butyl;
(lm) C2-C4 alkyl, e.g., (propyl, such as isopropyl (prop-2-yl));
(ln) -(C0-C1 alkyl)-B1-C1, wherein B1 is cyclopropyl, cyclobutyl, or phenyl, and C1 is cyclopropyl or phenyl.
[19] Each of groups (1a)-(1 n) is unsubstituted or substituted as described in connection with formula (I) above.
[20] In certain embodiments of the compounds as otherwise described herein, Q1 is independently selected from one of the following groups (2a) - (2I):
(2a) -N(RA1)(RB1), wherein no more than one of RA1 and RB1 is oxo-substituted;
(2b) -N(RA1)(RB1), wherein at least one of RA1 and RB1 is H or unsubstituted C1-C4 alkyl;
(2c) -N(RA1)(RB1), wherein RB1 is H or methyl;
(2d) -NH2;
(2e) -NH(RA1), wherein RA1 is alkyl and unsubstituted, or substituted with an oxo group or hydroxy group;
(2f) -NH(C(O)CH3) or -ORA1 ;
(2g) -NH(C(O)CH3);
(2h) -ORA1;
(2i) -ORA1, wherein RA1 is H, methyl, ethyl, or isopropyl;
(2j) -OH, -OCH3, or -0-CH2CH3;
(2k) -OH;
(2I) -OMe.
[21] Each of groups ( 2a)-(2l ) is unsubstituted or substituted as described in connection with formula (I) above.
[22] In certain embodiments of the compounds as otherwise described herein, W1 is independently selected from one of the following groups (3a) - (3d):
(3a) C(O);
(3b) S(O) or S(O)2.
(3c) S(O);
(3d) S(O)2.
[23] In certain embodiments of the compounds as otherwise described herein, X1 is independently selected from one of the following groups (4a) - (4b):
(4a) -N(RA1)-, wherein RA1 is H or methyl;
(4b) -N(H)-.
[24] Group (4a) is unsubstituted or substituted as described in connection with formula (I) above.
[25] In certain embodiments of the compounds as otherwise described herein, Y1 is independently selected from one of the following groups (5a) - (5f):
(5a) C1-C8 alkyl, C2-C3 alkenyl, or C2-C3alkynyl;
(5b) C2-C6 alkyl;
(5c) C2-C6 alkenyl or C3-C6 alkenyl;
(5d) C2-C6 alkynyl;
(5e) -(C0-C1 alkyl)-cycloalkyl;
(5f) -(C2-C3 alkenyl)-cyclolakyl, wherein cycloalkyl is cyclopropyl or cyclobutyl.
[26] Each of groups (5a)-(5f) is unsubstituted or substituted as described in connection with formula (I) above.
[27] Specific examples of C2-C6 alkyl groups in (5b) include n-propyl, n-butyl, or n-pentyl
[28] Specific examples of alkenyl groups in (5c) include but-2-enyl, 2-methylbut-2-enyl, 2- fluorobut-2-enyl, and 2,3-difluorobut-2-enyl).
[29] Specific examples of C2-C6 alkynyl groups in (5d) include but-2-ynyl.
[30] Specific examples of -(C0-C1 alkyl)-cycloalkyl groups in (5e) include -CH2-cyclopropyl.
[31] In certain embodiments of the compounds as otherwise described herein, Z1 is independently selected from one of the following groups (6a) - (6g):
(6a) -(C0-C1 alkyl)-0(RA) or -(C0-C1 alkyl)-N(RA)(RB);
(6b) -(C0-C1 alkyl)-OH or -(C0-C1 alkyl)-NH2;
(6c) -CH2-N(RA)(Rb) (e.g., -CH2-NH2);
(6d) -0(RA) or -N(RA)(RB);
(6e) OH or NH2;
(6f) -0(RA) (e.g., -OH);
(6g) -NH(RA) (e.g., NH2 or NH(CH2CH3)).
[32] Each of groups (6a)-(6c), (6f) and (6g) is unsubstituted or substituted as described in connection with formula (I) above.
[33] In certain embodiments of the compounds as otherwise described herein, the compound is not N-(2-aminoethyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, N-((1 - aminocyclobutyl)methyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, or N-(2- aminocyclohexyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide.
[34] In certain embodiments as otherwise described herein, each RC1 is independently selected from the group consisting of methyl, ethyl, -CF3, -NH2, -F, and -Cl. For example, in certain embodiments, each RC1 is CH3, -CH2 CH3, -CF3 -F, or -Cl.
[35] In certain embodiments as otherwise described herein, each RA1 is independently H, methyl, butenyl, or -(C0-1)-cyclopropyl. For example, in certain embodiments, each RA1 is H or methyl.
[36] In certain embodiments as otherwise described herein, each RB1 is H.
[39] wherein in each of I-A1, I-A2, and I-A3, n and m are independently 1 , 2, or 3 and s and t are independently 0, 1 , 2, or 3.
[41] wherein in each of I-B1, I-B2, and I-B3, n and m are independently 1 , 2, or 3 and s and t are independently 0, 1 , 2, or 3.
[42] In certain embodiments, the compound has formula I-A1 , I-A2, 1-A3, 1-B1 , I-B2 or I-B3 wherein n, m, s and t are independently 1 or 2 and Q1 is hydroxy or C1-C6 alkoxy. In other embodiments, the compound has formula I-A1 , I-A2, or I-A3, wherein n, m, s and t are independently 1 or 2 and Q1 is hydroxy or C1-C3 alkoxy, particularly methoxy or ethoxy. In still other embodiments, the compound has formula I-A1 , 1-A2, or I-A3, wherein n, m, s and t are independently 1 or 2, Q1 is hydroxy, methoxy or ethoxy, and A1 is phenyl optionally substituted with one or two of trifluoromethyl, halogen (particularly chloro or fluoro), hydroxy, amino, C1-C2 alkyl, C1-C2 alkoxy, or mono- or di-C1-C2 alkyl amino.
[43] Various particular embodiments nos. 1-48 of compounds for use in the methods, compounds and uses of the disclosure include compounds of formula (I), each as defined in each of the following rows (or a pharmaceutically acceptable salt, or a solvate or hydrate thereof), wherein each entry is a group number as defined above:
[44] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above, each optionally substituted alkyl, alkenyl, and alkynyl recited in any one of preceding embodiments is unsubstituted.
[45] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and the embodiment described in the paragraph immediately above, each cycloalkyl recited in any one of the preceding embodiments is a 3-7 membered monocyclic cycloalkyl. For example, in certain particular embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and the embodiment described in the paragraph immediately above, each cycloalkyl recited in any one of the preceding embodiments is a cyclopropyl, a cyclobutyl, a cyclopentyl, a cyclopentenyl, a cyclohexyl or a cyclohexenyl.
[46] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the two paragraphs immediately above, each heterocycloalkyl recited in any one of the preceding embodiments is a 4-7 membered monocyclic heterocycloalkyl having 1-2 heteroatoms selected from O, S and N. For example, in certain particular embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the two paragraphs immediately above, each heterocycloalkyl recited in any one of the preceding embodiments is a pyrrolidinyl, a tetrahydrofuranyl, a tetrahydrothienyl, a piperidinyl, a piperazinyl, a morpholinyl, a thiomorpholinyl, a tetrahydro-2H- pyranyl, or a tetrahydro-2H-thiopyranyl. In certain particular embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the two paragraphs immediately above, each heterocycloalkyl recited in any one of the preceding embodiments is a pyrrolidine, a piperidine, a piperazine, a tetrahydrofuran, a (IH)dihydropyran, or a morpholine (e.g., each unsubstituted).
[47] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the three paragraphs immediately above, each aryl is phenyl.
[48] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the four paragraphs immediately above, each heteroaryl is a 5-6 membered monocyclic heteroaryl having 1-3 heteroatoms selected from O, S and N. For example, in certain particular embodiments, including any of the embodiments described with reference to formula (I) and
embodiments 1-48 above and any embodiment described in the four paragraphs immediately above, each heteroaryl is a monocyclic heteroaryl is substituted with 0-3 RC1, e.g., is unsubstituted, substituted with one RC1 or substituted with two RC1. In certain particular embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the four paragraphs immediately above, each heteroaryl is a furanyl, a thienyl, a pyrrolyl, a pyrazolyl, an imidazolyl, an oxazolyl or a thiazolyl.
[49] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the paragraphs immediately above, each RC1 is independently C1-C4 alkyl (e.g., methyl, t-butyl, or -CF3), -Cl, -F, -Br, -CN, -N(RA1)(RB1), -ORA1, -C(O)RA1, or -C(O)NRB1RA1. For example, in certain additional embodiments, each RC1 is independently methyl, -CF3, -F-, -CN, -ORA1, or -C(O)RA1.
[50] In certain additional embodiments, including any of the embodiments described with reference to formula (I) and embodiments 1-48 above and any embodiment described in the paragraphs immediately above, the carbon joining A1, Q1, and W1 is a stereocenter, and has S stereochemistry, except in the case where W1 contains sulfur, wherein the carbon has R stereochemistry. In additional embodiments, the stereochemistry of said carbon joining A1, Q1, and W1 has stereochemistry selected so as to mimic the molecular geometry of an L-amino acid.
[51] In certain embodiments of the compounds as otherwise described herein, the compound is in the form of a pharmaceutically acceptable salt of a compound as described herein. The person of ordinary skill in the art will appreciate that a variety of pharmaceutically-acceptable salts may be provided, as described in additional detail below. In certain embodiments of the compounds as otherwise described herein, a compound is in the form of a solvate (e.g., a hydrate) of a compound or salt as described herein. The person of ordinary skill in the art will appreciate that a variety of solvates and/or hydrates may be formed. The person of ordinary skill in the art will appreciate that the phrase “optionally in the form of a pharmaceutically acceptable salt thereof, and/or a solvate or hydrate thereof includes compounds in the form of solvates and hydrates of base compounds or pharmaceutically acceptable salts as described above. But in certain embodiments as described above, the compound is not in the form of a solvate or hydrate.
Therapeutic Applications
[52] The disclosure also provides methods of treating various diseases, such as inflammatory conditions. These methods include administering to a subject in need of such treatment an effective amount of one or more compounds of the disclosure as described herein (e.g., compounds of formula (I)) or a pharmaceutical composition of the disclosure as described herein.
[53] In certain embodiments, the present disclosure provides for a method of treating an inflammatory condition in a subject thereof, wherein the method includes providing to the subject a compound as otherwise described herein. In certain embodiments as otherwise described herein, the inflammatory condition is an inflammatory bowel disease, an arthritic disease, or a neurodegenerative disorder. In other embodiments, the inflammatory condition is an autoinflammatory syndrome. In still other embodiments, the inflammatory condition is an inflammasome-related condition. Some conditions and/or diseases may be combinations of the above embodiments, or otherwise fall into multiple categories simultaneously.
[54] In certain embodiments, the diseases of the disclosure include, but are not limited to Adult- Onset Still’s Disease (AOSD), Systemic Juvenile Idiopathic Arthritis (sJIA), Macrophage Activation Syndrome (MAS), Autoinflammation with Infantile Enterocolitic (AIFEC), Bullous Pemphigoid, Pemphigus Vulgaris, Idiopathic Pulmonary Fibrosis (IPF), Non-Alcoholic Steatohepatitis (NASH), Systemic Lupus Erythematosus (SLE), Multiple Sclerosis, Alzheimer’s Disease, Parkinson’s Disease, Traumatic Brain Injury (TBI), Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), Cryopyrin-Associated Periodic Syndromes (CAPS), Vitiligo, Multiple Self-Healing Palmoplantar Carcinoma (MSPC), Autoimmune Addison’s Disease, Familial Mediterranean Fever (FMF), Autoimmune Thyroiditis, Stroke, Type 2 Diabetes (T2D), Osteoarthritis, Gout, Atherosclerosis, Hidradenitis Suppurativa, Psoriasis, and Pyrin Diseases.
[55] In another aspect, the disclosure provides methods for treating an inflammatory condition, for example, an inflammatory condition that causes cell death, or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a coronavirus (e.g., SARS-CoV 2, SARS-CoV, or MERS), viral, bacterial, fungal, parasitic, or other type of infection in a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
[56] In certain embodiments as otherwise described herein, the inflammatory condition may cause at least one of cell death, release of pro-inflammatory cytokines, or other inflammatory mediators.
[57] In certain embodiments as otherwise described herein, the inflammatory condition suitable for treatment by compounds as otherwise described herein includes cytokine storm syndrome, cytokine release syndrome, sepsis, hemophagocytic lymphohistiocytosis, acute lung injury, acute respiratory distress syndrome, or macrophage activation syndrome. Further suitable inflammatory conditions will be apparent to one of skill in the art.
[58] In certain embodiments as otherwise described herein, the inflammatory condition may result from a cell therapy administered to a human subject. For example, a CAR T cell therapy. The inflammatory condition may comprise cytokine storm syndrome, or cytokine release syndrome.
[59] In certain embodiments as otherwise described herein, the inflammatory condition may be treated with a compound as otherwise described herein in co-therapy with at least one of an antiinfective, antimicrobial, antiviral (e.g., remdesivir), monoclonal antibody (e.g., tocilizumab), biologic, immunoglobulin, immunomodulator, anti-inflammatory drug, corticosteroid, small molecule, cell therapy, or other type of prophylactic or therapeutic agent. Such co-therapies may be selected to as to not interfere with the action of the compound of the present disclosure, while treating another aspect of the condition, or alleviating a symptom thereof.
[60] In another aspect, the disclosure provides methods for treating an inflammatory condition (e.g., cytokine storm syndrome, or cytokine release syndrome), for example, an inflammatory condition that causes cell death or release of pro-inflammatory cytokines or other inflammatory mediators, resulting from a cell therapy (e.g., CAR T cell therapy) administered to a human subject, comprising administering to a subject in need of such treatment a compound as otherwise described herein (e.g., a compound of formula (I)).
[61] In certain embodiments as otherwise described herein, a compound as otherwise described herein is administered in co-therapy with at least one of a monoclonal antibody (e.g., tocilizumab), a biologic, an immunoglobulin, an immunomodulatory, an anti-inflammatory drug, a corticosteroid, a small molecule, a cell therapy, or other type of prophylactic or therapaetuic agent.
[62] Without wishing to be bound by theory, it is presently believed that certain compounds of the present disclosure may aid in the treatment of inflammatory conditions by inhibiting
activation of the inflammasome, specifically through binding to the ASC domain, preventing interactions through the pyrin domain (PYD). Accordingly, in certain embodiments, the present disclosure provides for a method for suppressing ASC-filament formation in a subject, wherein the method includes administering a compound as otherwise described herein. In particular, in some embodiments, the inhibition may be through interacting with the apoptosis-associated speck-like protein containing A CARD.
Pharmaceutical Compositions and Dosage Forms
[63] A compound as described herein can usefully be provided in the form of a pharmaceutical composition. Such compositions include the compound according to any one of the preceding aspects or embodiments described herein, together with a pharmaceutically acceptable excipient, diluent, or carrier.
[64] The compounds may be formulated in the pharmaceutical composition per se, or in the form of a hydrate, solvate, or pharmaceutically acceptable salt, as previously described. Typically, such salts are more soluble in aqueous solutions than the corresponding free acids and bases, but salts having lower solubility than the corresponding free acids and bases may also be formed.
[65] The pharmaceutical composition can be, for example, in the form of a tablet, a capsule, or a parenteral formulation, but the person of ordinary skill in the art will appreciate that the compound can be provided in a wide variety of pharmaceutical compositions.
[66] The compounds of the disclosure can be administered, for example, orally, topically, parenterally, by inhalation or spray or rectally in dosage unit formulations containing one or more pharmaceutically acceptable carriers, diluents or excipients. The term parenteral as used herein includes percutaneous, subcutaneous, intravascular (e.g., intravenous), intramuscular, or intrathecal injection or infusion techniques and the like. A medicament including a compound of the disclosure can be provided in any appropriate of the formulations and dosage forms as described herein.
[67] Pharmaceutical compositions can be made using the presently disclosed compounds. For example, in one embodiment, a pharmaceutical composition includes a pharmaceutically acceptable carrier, diluent or excipient, and compound as described above with reference to any one of structural formulae.
[68] In the pharmaceutical compositions disclosed herein, one or more compounds of the disclosure may be present in association with one or more pharmaceutically acceptable carriers,
diluents or excipients, and, if desired, other active ingredients. The pharmaceutical compositions containing compounds of the disclosure may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
[69] Compositions intended for oral use can be prepared according to any suitable method for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preservative agents in order to provide pharmaceutically elegant and palatable preparations. Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients that are suitable for the manufacture of tablets. These excipients can be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc. The tablets can be uncoated or they can be coated by known techniques. In some cases such coatings can be prepared by suitable techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a time delay material such as glyceryl monostearate or glyceryl distearate can be employed.
[70] Formulations for oral use can also be presented as hard gelatin capsules, wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
[71] Formulations for oral use can also be presented as lozenges.
[72] Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions. Such excipients can be suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropylmethylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents such as a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example
-olyethylene sorbitan monooleate. The aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
[73] Oily suspensions can be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin. The oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents and flavoring agents may be added to provide palatable oral preparations. These compositions may be preserved by the addition of an anti- oxidant such as ascorbic acid.
[74] Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives. Suitable dispersing or wetting agents or suspending agents are exemplified by those already mentioned above. Additional excipients, for example sweetening, flavoring and coloring agents, can also be present.
[75] Pharmaceutical compositions can also be in the form of oil-in-water emulsions. The oily phase can be a vegetable oil or a mineral oil or mixtures of these. Suitable emulsifying agents can be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally- occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate. The emulsions can also contain sweetening and flavoring agents.
[76] In some embodiments, the pharmaceutically acceptable carrier, diluent, or excipient is not water. In other embodiments, the water comprises less than 50% of the composition. In some embodiments, compositions comprising less than 50% water have at least 1%, 2%, 3%, 4% or 5% water. In other embodiments, the water content is present in the composition in a trace amount.
[77] In some embodiments, the pharmaceutically acceptable carrier, diluent, or excipient is not alcohol. In other embodiments, the alcohol comprises less than 50% of the composition. In some embodiments, compositions comprising less than 50% alcohol have at least 1%, 2%, 3%, 4% or 5% alcohol. In other embodiments, the alcohol content is present in the composition in a trace amount.
[78] Syrups and elixirs can be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol, glucose or sucrose. Such formulations can also contain a demulcent, a preservative, flavoring, and coloring agents. The pharmaceutical compositions can be in the form of a sterile injectable aqueous or oleaginous suspension. This suspension can be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents that have been mentioned above. The sterile injectable preparation can also be a sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that can be employed are water, Ringer's solution and isotonic sodium chloride solution. In addition, sterile, fixed oils can be employed as a solvent or suspending medium. For this purpose any bland fixed oil can be employed including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables.
[79] Compounds of the disclosure can also be administered in the form of suppositories, e.g., for rectal administration of the drug. These compositions can be prepared by mixing the compound with a suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug. Such materials include cocoa butter and polyethylene glycols.
[80] Compounds of the disclosure can also be administered parenterally in a sterile medium. The drug, depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle. Advantageously, adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
[81] The compositions can be formulated in a unit dosage form of the active ingredient. The term “unit dosage forms” refers to physically discrete units suitable as unitary dosages for human subjects and other mammals, each unit containing a predetermined quantity of active material calculated to produce the desired therapeutic effect, in association with a suitable pharmaceutical excipient.
[82] The compound can be effective over a wide dosage range and is generally administered in a pharmaceutically effective amount. It will be understood, however, that the amount of the compound actually administered will usually be determined by a physician, according to the relevant circumstances, including the condition to be treated, the chosen route of administration, the actual compound administered, the age, weight, and response of the individual patient, the severity of the patient's symptoms, and the like.
[83] For preparing solid compositions such as tablets, the principal active ingredient is mixed with a pharmaceutical excipient to form a solid preformulation composition containing a homogeneous mixture of a compound described herein. When referring to these preformulation compositions as homogeneous, the active ingredient is typically dispersed evenly throughout the composition so that the composition can be readily subdivided into equally effective unit dosage forms such as tablets, pills and capsules. This solid preformulation is then subdivided into unit dosage forms of the type described above containing from, for example, 0.1 to about 500 mg of the active ingredient of a compound described herein.
[84] The tablets or pills can be coated or otherwise compounded to provide a dosage form affording the advantage of prolonged action. For example, the tablet or pill can comprise an inner dosage and an outer dosage component, the latter being in the form of an envelope over the former. The two components can be separated by an enteric layer which serves to resist disintegration in the stomach and permit the inner component to pass intact into the duodenum or to be delayed in release. A variety of materials can be used for such enteric layers or coatings, such materials including a number of polymeric acids and mixtures of polymeric acids with such materials as shellac, cetyl alcohol, and cellulose acetate.
[85] The amount of compound or composition administered to a patient will vary depending upon what is being administered, the purpose of the administration, such as prophylaxis or therapy, the state of the patient, the manner of administration, and the like. In therapeutic applications, compositions can be administered to a patient already suffering from a disease in an amount sufficient to cure or at least partially arrest the symptoms of the disease and its complications. Effective doses will depend on the disease condition being treated as well as by the judgment of the attending clinician depending upon factors such as the severity of the disease, the age, weight and general condition of the patient, and the like.
[86] The compositions administered to a patient can be in the form of pharmaceutical compositions described above. These compositions can be sterilized by conventional sterilization techniques, or may be sterile filtered. Aqueous solutions can be packaged for use as is, or lyophilized, the lyophilized preparation being combined with a sterile aqueous carrier prior to administration. The pH of the compound preparations typically will be between 3 and 11 , more preferably from 5 to 9 and most preferably from 7 to 8. It will be understood that use of certain of the foregoing excipients, carriers, or stabilizers will result in the formation of pharmaceutical salts.
[87] The therapeutic dosage of the compounds can vary according to, for example, the particular use for which the treatment is made, the manner of administration of the compound, the health and condition of the patient, and the judgment of the prescribing physician. The proportion or concentration of a compound described herein in a pharmaceutical composition can vary depending upon a number of factors including dosage, chemical characteristics ( e.g ., hydrophobicity), and the route of administration. For example, the compounds described herein can be provided in an aqueous physiological buffer solution containing about 0.1 to about 10% w/v of the compound for parenteral administration. Some typical dose ranges are from about 1 pg/kg to about 1 g/kg of body weight per day. In some embodiments, the dose range is from about 0.01 mg/kg to about 100 mg/kg of body weight per day. The dosage is likely to depend on such variables as the type and extent of progression of the disease or disorder, the overall health status of the particular patient, the relative biological efficacy of the compound selected, formulation of the excipient, and its route of administration. Effective doses can be extrapolated from dose-response curves derived from in vitro or animal model test systems.
[88] The compounds described herein can also be formulated in combination with or administered sequentially with one or more additional active ingredients which can include any pharmaceutical agent such as anti-viral agents, vaccines, antibodies, immune enhancers, immune suppressants, anti-inflammatory agents and the like.
[89] The person of ordinary skill in the art will formulate a compound as described into pharmaceutical formulations herein. For example, based on the physicochemical properties of the compound, one of ordinary skill in the art will recognize a pharmaceutically effective amount of the compound, and the desired route of administration.
Definitions
[90] Terms used herein may be preceded and/or followed by a single dash,
or a double dash, “=”, to indicate the bond order of the bond between the named substituent and its parent moiety; a single dash indicates a single bond and a double dash indicates a double bond or a pair of single bonds in the case of a spiro-substituent. In the absence of a single or double dash it is understood that a single bond is formed between the substituent and its parent moiety; further, substituents are intended to be read “left to right” with reference to the chemical structure referred to unless a dash indicates otherwise. For example, arylalkyl, arylalkyl-, and -alkylaryl indicate the same functionality.
[91] For simplicity, chemical moieties are defined and referred to throughout primarily as univalent chemical moieties (e.g., alkyl, aryl, etc.)· Nevertheless, such terms are also used to convey corresponding multivalent moieties under the appropriate structural circumstances clear to those skilled in the art. For example, while an “alkyl” moiety can refer to a monovalent radical (e.g., CH3-CH2-), in some circumstances a bivalent linking moiety can be “alkyl,” in which case those skilled in the art will understand the alkyl to be a divalent radical (e.g., -CH2-CH2-), which is equivalent to the term “alkylene.” Similarly, in circumstances in which a divalent moiety is required and is stated as being “aryl,” those skilled in the art will understand that the term “aryl” refers to the corresponding divalent moiety, arylene. All atoms are understood to have their normal number of valences for bond formation (i.e., 4 for carbon, 3 for N, 2 for O, and 2, 4, or 6 for S, depending on the oxidation state of the S). Nitrogens in the presently disclosed compounds can be hypervalent, e.g., an N-oxide or tetrasubstituted ammonium salt. On occasion a moiety may be defined, for example, as -B-(A)a, wherein a is 0 or 1. In such instances, when a is 0 the moiety is -B and when a is 1 the moiety is -B-A.
[92] As used herein, the term “alkyl” includes a saturated hydrocarbon having a designed number of carbon atoms, such as 1 to 10 carbons (i.e., inclusive of 1 and 10), 1 to 8 carbons, 1 to 6 carbons, 1 to 3 carbons, or 1, 2, 3, 4, 5 or 6. Alkyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkylene group). For example, the moiety “-(C1-C6 alkyl)-0-” signifies connection of an oxygen through an alkylene bridge having from 1 to 6 carbons and C1-C3 alkyl represents methyl, ethyl, and propyl moieties. Examples of “alkyl” include, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, iso-, sec- and tert-butyl, pentyl, and hexyl.
[93] The term “alkoxy” represents an alkyl group of indicated number of carbon atoms attached to the parent molecular moiety through an oxygen bridge. Examples of “alkoxy” include, but are not limited to, methoxy, ethoxy, propoxy, and isopropoxy.
[94] The term “alkenyl” as used herein, unsaturated hydrocarbon containing from 2 to 10 carbons (i.e., inclusive of 2 and 10), 2 to 8 carbons, 2 to 6 carbons, or 2, 3, 4, 5 or 6, unless otherwise specified, and containing at least one carbon-carbon double bond. Alkenyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkenylene group). For example, the moiety “-(C2-C6alkenyl)-O-” signifies connection of an oxygen through an alkenylene bridge having from 2 to 6 carbons. Representative examples of alkenyl include, but are not limited to, ethenyl, 2-propenyl, 2-
methyl-2-propenyl, 3-butenyl, 4-pentenyl, 5-hexenyl, 2-heptenyl, 2-methyl-1-heptenyl, 3- decenyl, and 3,7-dimethylocta-2,6-dienyl.
[95] The term “alkynyl” as used herein, unsaturated hydrocarbon containing from 2 to 10 carbons (i.e., inclusive of 2 and 10), 2 to 8 carbons, 2 to 6 carbons, or 2, 3, 4, 5 or 6 unless otherwise specified, and containing at least one carbon-carbon triple bond. Alkynyl group may be straight or branched and depending on context, may be a monovalent radical or a divalent radical (i.e., an alkynylene group). For example, the moiety “-(C2-C6alkynyl)-O-” signifies connection of an oxygen through an alkynylene bridge having from 2 to 6 carbons. Representative examples of alkynyl include, but are not limited to, acetylenyl, 1-propynyl, 2- propynyl, 3-butynyl, 2-pentynyl, and 1-butynyl.
[96] The term “aryl” represents an aromatic ring system having a single ring (e.g., phenyl) which is optionally fused to other aromatic hydrocarbon rings or non-aromatic cycloalkyl or heterocycloalkyl rings. The aryl ring system is attached to the parent molecular moiety through any atom contained within the ring system. Examples of aryl groups include, but are not limited to, phenyl, naphthyl, indanyl, indenyl, dihydronaphthyl, fluorenyl, tetralinyl, 1, 2,3,4- tetrahydronaphthyl, 6,7,8,9-tetrahydro-5H-benzo[a]cycloheptenyl, 1 H-2,3-dihydrobenzofuranyl and tetrahydroisoquinolinyl. In certain embodiments, aryl is phenyl. In certain embodiments, aryl is phenyl or naphthyl. In certain embodiments, the aryl is (i) phenyl, (ii) naphthyl or (iii) a phenyl ring fused to either a 5 or 6 membered monocyclic cycloalkyl or cycloalkenyl, or a 5 or 6 membered monocyclic heterocycloalkyl. The aryl groups herein are unsubstituted or, when specified as “optionally substituted”, can unless stated otherwise be substituted in one or more substitutable positions with various groups as indicated.
[97] The terms “halogen” or "halo" indicate fluorine, chlorine, bromine, and iodine. In certain embodiments of each and every embodiment as otherwise described herein, the term “halogen” or “halo” refers to fluorine or chlorine. In certain embodiments of each and every embodiment described herein, the term “halogen” or “halo” refers to fluorine. The term “fluorinated” indicates a group (such as alkyl as otherwise described herein) wherein at least one of the carbon-bound hydrogens is substituted by fluorine to form C-F groups. Examples of fluorinated alkyl groups are fluoromethyl, difluoromethyl and trifluoromethyl. Trifluoromethyl is a perfluorinated alkyl group. The term “perfluorinated” indicates a group (such as alkyl as otherwise described herein) wherein all carbon-bound hydrogens are substituted by fluorine to form C-F groups. .
[98] The term “heteroaryl” refers to an aromatic ring or ring system containing at least one aromatic heteroatom selected from boron, nitrogen, oxygen and sulfur in an aromatic ring. Most
commonly, the heteroaryl groups will have 1 , 2, 3, or 4 heteroatoms. The heteroaryl may be fused to one or more non-aromatic rings, for example, cycloalkyl or heterocycloalkyl rings, wherein the cycloalkyl and heterocycloalkyl rings are described herein. The heteroaryl ring system is bonded to the parent molecular moiety through any atom contained within the ring system. Examples of heteroaryl groups include, but are not limited to, pyridyl, pyrimidinyl, quinolinyl, benzothienyl, indolyl, indolinyl, pyridazinyl, pyrazinyl, isoindolyl, isoquinolyl, quinazolinyl, quinoxalinyl, phthalazinyl, imidazolyl, isoxazolyl, pyrazolyl, oxazolyl, thiazolyl, indolizinyl, indazolyl, benzothiazolyl, benzimidazolyl, benzofuranyl, furanyl, thienyl, pyrrolyl, oxadiazolyl, thiadiazolyl, benzo[1 ,4]oxazinyl, triazolyl, tetrazolyl, isothiazolyl, naphthyridinyl, isochromanyl, chromanyl, isoindolinyl, isobenzothienyl, benzoxazolyl, pyridopyridinyl, purinyl, benzodioxolyl, triazinyl, pteridinyl, benzothiazolyl, imidazopyridinyl, imidazothiazolyl, benzisoxazinyl, benzoxazinyl, benzopyranyl, benzothiopyranyl, chromonyl, chromanonyl, pyridinyl-N- oxide, isoindolinonyl, benzodioxanyl, benzoxazolinonyl, pyrrolyl N- oxide, pyrimidinyl N- oxide, pyridazinyl N- oxide, pyrazinyl N- oxide, quinolinyl N- oxide, indolyl N- oxide, indolinyl N- oxide, isoquinolyl N- oxide, quinazolinyl N- oxide, quinoxalinyl N- oxide, phthalazinyl N- oxide, imidazolyl N- oxide, isoxazolyl N- oxide, oxazolyl N- oxide, thiazolyl N- oxide, indolizinyl N- oxide, indazolyl N- oxide, benzothiazolyl N- oxide, benzimidazolyl N- oxide, pyrrolyl N- oxide, oxadiazolyl N- oxide, thiadiazolyl N- oxide, triazolyl N- oxide, tetrazolyl N- oxide, benzothiopyranyl S-oxide, benzothiopyranyl S,S-dioxide. In certain embodiments, each heteroaryl is selected from pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl, imidazolyl, isoxazolyl, pyrazolyl, oxazolyl, thiazolyl, furanyl, thienyl, pyrrolyl, oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, isothiazolyl, pyridinyl-N- oxide, pyrrolyl N- oxide, pyrimidinyl N- oxide, pyridazinyl N- oxide, pyrazinyl N- oxide, imidazolyl N- oxide, isoxazolyl N- oxide, oxazolyl N- oxide, thiazolyl N- oxide, pyrrolyl N- oxide, oxadiazolyl N- oxide, thiadiazolyl N- oxide, triazolyl N- oxide, and tetrazolyl N- oxide. In certain embodiments, heteroaryl groups include pyridyl, pyrimidyl, quinolinyl, indolyl, pyrrolyl, furanyl, thienyl, imidazolyl, pyrazolyl, indazolyl, thiazolyl and benzothiazolyl. The heteroaryl groups herein are unsubstituted or, when specified as “optionally substituted”, can unless stated otherwise be substituted in one or more substitutable positions with various groups, as indicated.
[99] The term “heterocycloalkyl” refers to a non-aromatic ring or ring system containing at least one heteroatom that is preferably selected from boron, nitrogen, oxygen and sulfur, wherein said heteroatom is in a non-aromatic ring. The heterocycloalkyl may have 1 , 2, 3 or 4 heteroatoms. The heterocycloalkyl may be saturated or partially unsaturated (i.e., a heterocycloalkenyl). Heterocycloalkyl includes monocyclic groups of three to eight annular atoms as well as bicyclic and polycyclic ring systems, including bridged and fused systems,
wherein each ring includes three to eight annular atoms. The heterocycloalkyl ring is optionally fused and/or bridged to other heterocycloalkyl and/or non-aromatic hydrocarbon rings. In certain embodiments, the heterocycloalkyl groups have from 3 to 7 members in a single ring. In other embodiments, heterocycloalkyl groups have 5 or 6 members in a single ring. In some embodiments, the heterocycloalkyl groups have 3, 4, 5, 6 or 7 members in a single ring. Examples of heterocycloalkyl groups include, but are not limited to, azabicyclo[2.2.2]octyl (in each case also “quinuclidinyl” or a quinuclidine derivative), azabicyclo[3.2.1]octyl, 2,5- diazabicyclo[2.2.1]heptyl, morpholinyl, thiomorpholinyl, thiomorpholinyl S-oxide, thiomorpholinyl S,S-dioxide, 2-oxazolidonyl, piperazinyl, homopiperazinyl, piperazinonyl, pyrrolidinyl, azepanyl, azetidinyl, pyrrolinyl, tetrahydropyranyl, piperidinyl, tetrahydrofuranyl, tetrahydrothienyl, 3,4- dihydroisoquinolin-2(1H)-yl, isoindolindionyl, homopiperidinyl, homomorpholinyl, homothiomorpholinyl, homothiomorpholinyl S,S-dioxide, oxazolidinonyl, dihydropyrazolyl, dihydropyrrolyl, dihydropyrazinyl, dihydropyridinyl, dihydropyrimidinyl, dihydrofuryl, dihydropyranyl, imidazolidonyl, tetrahydrothienyl S-oxide, tetrahydrothienyl S,S-dioxide and homothiomorpholinyl S-oxide. In certain embodiments, heterocycloalkyl groups include morpholinyl, 3,4-dihydroisoquinolin-2(1H)-yl, tetrahydropyranyl, piperidinyl, aza-bicyclo[2.2.2]octyl, g-butyrolactonyl (i.e., an oxo-substituted tetrahydrofuranyl), y-butryolactamyl (i.e., an oxo-substituted pyrrolidine), pyrrolidinyl, piperazinyl, azepanyl, azetidinyl, thiomorpholinyl, thiomorpholinyl S,S-dioxide, 2-oxazolidonyl, imidazolidonyl, isoindolindionyl, piperazinonyl. The heterocycloalkyl groups herein are unsubstituted or, when specified as “optionally substituted”, can unless stated otherwise be substituted in one or more substitutable positions with various groups, as indicated.
[100] The term “cycloalkyl” refers to a non-aromatic carbocyclic ring or ring system, which may be saturated or partially unsaturated (e.g., a cycloalkenyl). The cycloalkyl ring can be optionally fused and/or bridged with a bivalent bridge to other cycloalkyl rings. Certain examples of cycloalkyl groups present in the disclosed compounds have from 3 to 7 members in a single ring, such as having 5 or 6 members in a single ring. In some embodiments, the cycloalkyl groups have 3, 4, 5, 6 or 7 members in a single ring. Examples of cycloalkyl groups include, but are not limited to, cyclohexyl, cyclopentyl, cyclobutyl, cyclopropyl, tetrahydronaphthyl norbornanyl, norbornenyl, noborndienyl, and bicyclo[2.2.1]heptanyl. The cycloalkyl groups herein are unsubstituted or, when specified as “optionally substituted”, may be substituted in one or more substitutable positions with various groups, as indicated.
[101] Alternatively, a cycloalkyl may be bridged with a trivalent bridge. Examples of trivalently bridged cycloalkyl systems includes adamantanyl.
[102] The term “ring system” encompasses monocycles, as well as fused and/or bridged polycycles.
[103] The term “oxo” means a doubly bonded oxygen, sometimes designated as =0 or for example in describing a carbonyl “C(O)” may be used to show an oxo substituted carbon.
[104] The term “substituted,” when used to modify a specified group or radical, means that one or more hydrogen atoms of the specified group or radical are each, independently of one another, replaced with the same or different substituent groups as defined below, unless specified otherwise.
[105] As used herein, the phrase “pharmaceutically acceptable salt” refers to both pharmaceutically acceptable acid and base addition salts and solvates. Such pharmaceutically acceptable salts include salts of acids such as hydrochloric, phosphoric, hydrobromic, sulfuric, sulfinic, formic, toluenesulfonic, methanesulfonic, nitric, benzoic, citric, tartaric, maleic, hydroiodic, alkanoic such as acetic, HOOC-(CH2)n-COOH where n is 0-4, and the like. Non-toxic pharmaceutical base addition salts include salts of bases such as sodium, potassium, calcium, ammonium, and the like. Those skilled in the art will recognize a wide variety of non-toxic pharmaceutically acceptable addition salts.
[106] One of ordinary skill in the art of medicinal chemistry also will appreciate that the disclosed structures are intended to include isotopically enriched forms of the present compounds. As used herein “isotopes” includes those atoms having the same atomic number but different mass numbers. As is known to those of skill in the art, certain atoms, such as hydrogen occur in different isotopic forms. For example, hydrogen includes three isotopic forms, protium, deuterium and tritium. As will be apparent to those of skill in the art upon consideration of the present compounds, certain compounds can be enriched at a given position with a particular isotope of the atom at that position. For example, compounds having a fluorine atom, may be synthesized in a form enriched in the radioactive fluorine isotope 18F. Similarly, compounds may be enriched in the heavy isotopes of hydrogen: deuterium and tritium; and similarly can be enriched in a radioactive isotope of carbon, such as 13C. Such isotopic variant compounds undergo different metabolic pathways and can be useful, for example, in studying the ubiquitination pathway and its role in disease. Of course, in certain embodiments, the compound has substantially the same isotopic character as naturally-occurring materials.
[107] One of ordinary skill in the art of chemistry will also appreciate that the disclosed structures, unless otherwise indicated are intended to include all possible stereoisomers of the claimed molecule, including mixtures of certain or all stereoisomers. However, compounds drawn with certain stereochemistry at one or more stereocenters are intended to have the indicated stereochemistry. Compounds and stereocenters drawn with ambiguous stereochemistry are meant to convey any stereoisomer or mixture thereof, e.g., a racemic mixture of compounds or a purified subset of stereoisomers.
[108] As used herein, the terms “individual,” “patient,” or “subject” are used interchangeably, refers to any animal, including mammals, preferably humans.
[109] As used herein, the phrase “therapeutically effective amount” or “effective amount” refers to the amount of active compound or pharmaceutical agent that elicits the biological or medicinal response that is being sought in a tissue, system, animal, individual or human by a researcher, veterinarian, medical doctor or other clinician.
[110] In certain embodiments, an effective amount can be an amount suitable for
(i) inhibiting the progression the disease;
(ii) prophylactic use for example, preventing or limiting development of a disease, condition or disorder in an individual who may be predisposed or otherwise at risk to the disease, condition or disorder but does not yet experience or display the pathology or symptomatology of the disease;
(iii) inhibiting the disease; for example, inhibiting a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder;
(iv) ameliorating the referenced disease state, for example, ameliorating a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e., reversing or improving the pathology and/or symptomatology) such as decreasing the severity of disease; or
(v) eliciting the referenced biological effect.
[111] As used here, the terms “treatment” and “treating” mean (i) ameliorating the referenced disease state, condition, or disorder (or a symptom thereof), such as, for example, ameliorating a disease, condition or disorder in an individual who is experiencing or displaying the pathology or symptomatology of the disease, condition or disorder (i.e., reversing or improving the pathology and/or symptomatology) such as decreasing the severity of disease or symptom
thereof, or inhibiting the progression of disease; or (ii) eliciting the referenced biological effect ( e.g inhibiting inflammasome formation or function, or inhibition of IL-1 β).
Methods of Preparation
[112] Many general references providing commonly known chemical synthetic schemes and conditions useful for synthesizing the disclosed compounds are available (see, e.g., Smith and March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Fifth Edition, Wiley-lnterscience, 2001; or Vogel, A Textbook of Practical Organic Chemistry, Including Qualitative Organic Analysis, Fourth Edition, New York: Longman, 1978).
[113] Compounds as described herein can be purified by any of the means known in the art, including chromatographic means, such as HPLC, preparative thin layer chromatography, flash column chromatography and ion exchange chromatography. Any suitable stationary phase can be used, including normal and reversed phases as well as ionic resins. Most typically the disclosed compounds are purified via silica gel and/or alumina chromatography. See, e.g., Introduction to Modern Liquid Chromatography, 2nd Edition, ed. L. R. Snyder and J. J. Kirkland, John Wiley and Sons, 1979; and Thin Layer Chromatography, ed E. Stahl, Springer-Verlag, New York, 1969.
[114] During any of the processes for preparation of the subject compounds, it may be necessary and/or desirable to protect sensitive or reactive groups on any of the molecules concerned. This may be achieved by means of conventional protecting groups as described in standard works, such as J. F. W. McOmie, "Protective Groups in Organic Chemistry,” Plenum Press, London and New York 1973, in T. W. Greene and P. G. M. Wuts, "Protective Groups in Organic Synthesis,” Third edition, Wiley, New York 1999, in "The Peptides"; Volume 3 (editors: E. Gross and J. Meienhofer), Academic Press, London and New York 1981, in "Methoden der organischen Chemie,” Houben-Weyl, 4.sup.th edition, Vol. 15/1, Georg Thieme Verlag, Stuttgart 1974, in H.-D. Jakubke and H. Jescheit, "Aminosauren, Peptide, Proteine,” Verlag Chemie, Weinheim, Deerfield Beach, and Basel 1982, and/or in Jochen Lehmann, "Chemie der Kohlenhydrate: Monosaccharide and Derivate,” Georg Thieme Verlag, Stuttgart 1974. The protecting groups may be removed at a convenient subsequent stage using methods known from the art.
[115] A “leaving group” as used herein (e.g., suitable as LG) refers to a moiety of a reactant (e.g., the alkylhalogenide of the disclosure) that is displaced from the first reactant in the chemical reaction. A comprehensive list of suitable leaving groups can be found in J. March,
Advanced Organic Chemistry, John Wiley and Sons, N.Y. (2013). Examples of suitable leaving groups include, but are not limited to, halogen (such as Cl or Br), acetoxy, and sulfonyloxy groups (such as methyl sulfonyloxy, trifluoromethylsulfonyloxy (“triflate”), p-toluenesulfonyloxy (“tosylate”)).
[116] The compounds disclosed herein can be made using procedures familiar to the person of ordinary skill in the art and as described herein, for example in Schemes 1. One of skill in the art can adapt the reaction sequences of schemes and examples as provided herein to fit the desired target molecule. Of course, in certain situations one of skill in the art will use different reagents to affect one or more of the individual steps or to use protected versions of certain of the substituents. Additionally, one skilled in the art would recognize that compounds of the disclosure can be synthesized using different routes altogether. For example, the person of ordinary skill in the art may adapt the procedures described herein and/or other procedures familiar to the person of ordinary skill in the art to make the compounds described herein.
Example 1
Synthesis of (S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2-methoxypropanamide [117] Step 1 : Synthesis of (S)-3-(3-chlorophenyl)-2-hydroxypropanoic acid
[118] A solution of (S)-2-amino-3-(3-chlorophenyl)propanoic acid (0.50 g, 2.513 mmol) in 1 (M) H2SO4 (6 mL) was cooled to 0°C. To this mixture was then added a solution of sodium nitrite (1.04 g, 15.075 mmol) in water (3 mL) slowly over 15 min. Reaction mixture was stirred at 0°C for 2h, then warmed to 25°C and stirred for another 20h. The reaction mixture was diluted with EtOAc and organic layer was separated. Aqueous layer was re-extracted with EtOAc.
Combined organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to get crude (S)-3-(3- chlorophenyl)-2-hydroxypropanoic acid (380 mg) as colorless liquid which was used to next step without any further purification.
[119] [a]D25: -22.278 (c 0.52; DMSO)
[120] Step 2: (S)-3-(3-chlorophenyl)-2-methoxypropanoic acid
[121] To a stirred solution of (S)-3-(3-chlorophenyl)-2-hydroxypropanoic acid (2) (350 mg, 1.75 mmol) in THF (5 mL) was added NaH (60% in oil, 201 mg, 8.4 mmol) at 25°C, and the reaction mixture was stirred at that temperature for 10 min. To this mixture was then added iodomethane (0.218 mL, 3.5 mmol) 25°C, and the reaction mixture was stirred at that temperature for 4h. After completion of the reaction (as judged by TLC), reaction mixture was quenched with aqueous 1N HCI solution. Aqueous layer was extracted with EtOAc. Combined organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give S)-3-(3-chlorophenyl)-2-methoxypropanoic acid (170 mg, crude) as white solid which was used to next step without any further purification.
[122] LCMS: Column- XBRIDGE (4.6X50mm, 5μm), (mobile phase: from 90% [10 mM NH4OAc in water] and 10% [CH3CN] to 70% [10 mM NH4OAc in water] and 30% [CH3 CN] in 1.5 min, further to 10% [10 mM NH4OAc in water] and 90% [CH3 CN] in 3.0 min, held this mobile phase composition up to 4.0 min and finally back to initial condition in 5.0 min). Flow =1.2ml/min. Rt = 1.95 min. MS Calculated 214, MS found: 213.2 [M-H],
[123] Step 3: Terf-butyl (S,E)-(4-(3-(3-chlorophenyl)-2-methoxypropanamido)but-2-en-1- yl)carbamate
[124] To a stirred solution of (S)-3-(3-chlorophenyl)-2-methoxypropanoic acid (170 mg, 0.794 mmol) in DMF (2 mL) were added HATU (604 mg, 1.589 mmol) and DIPEA (0.415 mL, 2.383 mmol) at 25°C, and the reaction mixture was stirred at that temperature for 10 min. To this mixture was then added fert-butyl (E)- (4-aminobut-2-en-1-yl)carbamate (148 mg, 0.794 mmol) at 25°C, and the reaction mixture was stirred at 25°C for 16h. After completion of the reaction
(as judged by LC/MS), the mixture was diluted with EtOAc. Organic layer was sequentially washed with ice cooled water, aqueous solution of NaHC03 and brine, dried over anhydrous Na2SO4, filtered, and the volatiles were removed in vacuo. Resultant crude residue was purified by combi flash using ethyl acetate in hexane to give tert-butyl (S,E)-(4-(3-(3- chlorophenyl)-2- methoxypropanamido)but-2-en-1-yl)carbamate (70 mg, 7%, 3 steps) as off white solid.
[125] LCMS: Column- XBRIDGE (4.6X50mm, 5 μm- o)x,i (dmeobile phase: from 90% [10 mM NH4OAc in water] and 10% [CH3CN] to 70% [10 mM NH4OAc in water] and 30% [CH3 CN] in 1.5 min, further to 10% [10 mM NH4OAc in water] and 90% [CH3CN] in 3.0 min, held this mobile phase composition up to 4.0 min and finally back to initial condition in 5.0 min). Flow =1.2 ml/min. Rt = 3.33 min. MS Calculated 382, MS found: 383 [M+H],
[126] Step 4: (S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2-methoxypropanamide
[127] To a stirred solution of tert-butyl (S,E)-(4-(3-(3-chlorophenyl)-2-methoxypropanamido)but- 2-en-1- yl)carbamate (70 mg, 0.183 mmol) in DCM (5 mL) was added 4(N) HCI in dioxane (0.25 mL) at 0°C, and the reaction mixture was then stirred at 25°C for 3h. After completion of the reaction (as judged by TLC and LC/MS), volatiles were removed under reduced pressure.
Crude material thus obtained was purified by reverse phase prep-HPLC to give (S,E)- N-(4- aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2- methoxypropanamide (16 mg, 31%) as yellow sticky gum.
[128] LCMS: Column- BEH(50mm), (mobile phase: 98% [0.05% HCOOH in water] and 2% [CH3CN] held for 0.75 min, then to 90% [0.05% HCOOH in water] and 10% [CH3CN] in 1.0 min, further to 2% [0.05% HCOOH in water] and 98% [CH3CN] in 2.0 min, held this mobile phase composition up to 2.25 min and finally back to initial condition in 5.0 min). Flow =1.5ml/min. Purity is 95.83%, Rt = 1.40 min. MS Calculated 282, MS found: 283.18 [M+H],
[129] RP-HPLC condition: Preparative HPLC was done on Waters auto purification instrument. Column name: YMC Triart Actus C18 (250 x 20 mm, 5m) operating at ambient temperature and flow rate of 16 mL/min. Mobile phase: A = 20 mM Ammonium Bicarbonate in water, B=Acetonitrile; Gradient Profile: Mobile phase initial composition of 80% A and 20% B, then 80%A, and 20%B in 3 min, then to 50% A and 50% B in 20 min., then to 5% A and 95% B in 21 min., held this composition up to 23 min. for column washing, then returned to initial composition in 24 min. and held till 26 min.
[130] Step 1 . Synthesis of methyl (2S)-2-methoxy-2-phenylacetate
[131] To a stirred solution of (2S)-2-hydroxy-2-phenylacetic acid (700 mg, 4.60 mmol) in dichloromethane (20.0 mL) at room temperature was added iodomethane (2.61 g, 18.4 mmol) and silver oxide (2.15 g, 9.20 mmol). The reaction mixture was stirred at room temperature for 16 h. Reaction was monitored by TLC and LCMS. After completion of starting material in reaction, Reaction mixture was filtered and the filtrate was evaporated under reduced pressure to get crude compound methyl (2S)-2-methoxy-2-phenylacetate (450 mg, Crude) as a colorless viscous liquid. 1 HNMR (400 MHz, CdCI3): δ 7.25-7.43 (m, 5H), 4.77 (s, 1H), 3.72(m, 3H), 3.40 (s, 3H); LCMS did not ionize.
[132] Step 2. Synthesis of (2S)-2-methoxy-2-phenylacetic acid
[133] To a stirred solution of methyl (2S)-2-methoxy-2-phenylacetate (500 mg, 2.77 mmol) in tetrahydrofuran (10.0 mL) was added lithium hydroxide (332 mg, 13.9 mmol) and MeOH: H20 (1:1) (2 ml_:2 mL). The reaction mixture stirred at room temperature for 2 h. Reaction was monitored by TLC and LCMS. After completion of reaction, the solvent was evaporated under reduced pressure and the obtained crude was diluted with ice water and acidified with 5N HCL solution. It was then extracted with ethyl acetate (2 x 50 mL). The organic layer separated, dried over anhydrous Na2SO4 and evaporated under reduced pressure to get compound (2S)-2- methoxy-2-phenylacetic acid (395 mg, 84%) as a white solid. 1HNMR (400 MHz, DMSO-d6): δ 12.90 (s, 1H), 7.32-7.36 (m, 5H), 4.74 (s, 1H), 3.28 (s, 3H).
[134] Step 3. Synthesis of tert-butyl N-[(2E)-4-[(2S)-2-methoxy-2-phenylacetamido]but-2-en-1- yljcarbamate
[135] To a stirred solution of (2S)-2-methoxy-2-phenylacetic acid (700 mg, 4.21 mmol) in N,N dimethylformamide (15.0 mL) at room temperature was added ethylbis(propan-2-yl)amine (2.93 mL, 16.8 mmol) and tert-butyl N-[(2E)-4-aminobut-2-en-1-yl]carbamate hydrochloride (938 mg, 4.21 mmol). The reaction mixture stirred for 5 minutes and [(dimethylamino)({3H- [1,2,3]triazolo[4,5-b]pyridin-3-yloxy})methylidene]dimethylazanium; hexafluoro-A5-phosphanuide (3.20 g, 8.42 mmol) was added. The reaction mixture stirred at room temperature for 4 h. Reaction was monitored by TLC and NMR. After completion of reaction, reaction mixture was diluted with water (50 mL) and extracted with ethyl acetate (2 x 20 mL). The organic layer separated, dried over anhydrous Na2SO4 and evaporated under reduced pressure to get crude compound ferf-butyl N-[(2E)-4-[(2S)-2-methoxy-2-phenylacetamido]but-2-en-1-yl]carbamate (1.01 g, 72%) as a colorless viscous liquid. 1HNMR (400 MHz, DMSO-d6): δ 8.20 (t, J = 5.6 Hz 1H), 7.28-7.36 (m, 5H), 6.92-6.95 (m, 1H), 5.38-5.48 (m, 2H), 4.60 (s, 1H), 3.59-3.64 (m, 2H), 3.42-3.46 (m, 2H), 3.25 (s, 3H), 1.35 (s, 9H).
[136] 4. Synthesis of (2S)-N-[(2E)-4-aminobut-2-en-1-yl]-2-methoxy-2-phenylacetamide hydrochloride.
[137] To a stirred solution of ferf-butyl N-[(2E)-4-[(2S)-2-methoxy-2-phenylacetamido]but-2-en- 1-yl]carbamate (600 mg, 1.79 mmol) in 1,4-dioxane (2.00 mL) was added 4M HCL in Dioxane (2.00 mL) at room temperature. Reaction mixture stirred at room temperature for 16 h. Reaction was monitored by TLC and LCMS. After completion of reaction, reaction solvent was evaporated under reduced pressure to get crude compound. Crude was diluted with water (50
mL) and extracted with ethyl acetate (100 mL). The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude compound was purified by flash column chromatography using 70% ethyl acetate in hexane as eluents to afford pure compound (2S)-N-[(2E)-4-aminobut-2-en~1-yl]-2-methoxy-2-phenylacetamide (420 mg, 75%) as a colorless viscous liquid. 1 HNMR (400 MHz, DMSO-d6): δ 8.34 (t, J = 6 Hz, 1 H), 8.02 (br s, 3H), 7.27- 7.37 (m, 5H), 5.73-5.79 (m, 1H), 5.51-5.58 (m, 1H), 4.63 (s, 1H), 3.64-3.75 (m, 2H), 3.44-3.46 (m, 2H), 3.26 (s, 3H). LCMS (ES) m/z = 235.0 [M+H]+.
[138] The compounds shown below in Table 2 can be prepared essentially according to procedures known to those of skill in the art in view of Schemes 1 and 2.
Biological Methods
[139] Cell Culture: The human monocytic THP-1 cell line was purchased from ATCC (TIB-202). The human monocytic THP-1 ASC-GFP reporter cell line was purchased from Invivogen (thp- ascgfp). Cells were maintained in RPMI media supplemented 10% fetal bovine serum and 100 units of penicillin and streptomycin per mL at a cell density of 2-8 x 105 cells/mL
[140] Human peripheral blood mononuclear cells were isolated from whole blood using Lymphoprep™ density gradient according to the manufacturer’s instructions. PBMCs were used fresh.
[141] PMA differentiation of THP-1 cells: For ASC Spec assays, the human monocytic cell line THP-1 ASC-GFP cells were differentiated into a macrophage-like phenotype using Phorbol 12-
myristate 13-acetate (PMA). THP-1 cells were suspended at a density of 1-2 x 106 cells/mL and supplemented with 100 ng/mL PMA. One hundred thousand cells were seeded into each well of a 96 well plate and incubated for 72 hours. Adherent cells were washed three times with PMA free THP-1 media, then cells were rested for 24 hours in PMA free media.
[142] NLRP3 Inflammasome and pyroptosis assay in THP-1 Cells: THP-1 cells were primed with 300 ng/mL of ultra-pure LPS. One hour later, cells were treated as indicated in figure legend. Three hours post-LPS prime, the NLRP3 inflammasome was activated with 10 mM Nigericin. Two hours later, supernatants were collected and analyzed for IL-1 b by ELISA. Pyroptotic cell death was assessed by MTT assay as described below.
[143] Cytotoxicity Assay: THP-1 or PBMCs were treated with a dose titration of the indicated compound for 5 hours after which cell viability was assessed by MTT assay, as described below.
[144] MTT Cell Viability Assay: MTT reagent (0.6 mg/mL in THP-1 culture media) was prepared and 150 μL was added to each well. After 1-2 hours, cells were centrifuged at 500g for 5 minutes to pellet cells and crystals. Supernatants were removed, and formazan crystals were dissolved in 100 μL of isopropanol. Cell viability was assessed by measuring optical density at 560 nm, with a reference wavelength of 670 nm. Viability is expressed as percent signal relative to wells containing untreated cells.
[145] Cell Free Inflammasome Assay: Cell free inflammasome activation and Caspase-1 cleavage was assessed as described previously. Briefly, lysates were prepared from THP-1 by hypotonic lysis to a final protein concentration > 7 mg/mL. Lysates were pretreated for 20 minutes with a final concentration of 1 mM of the indicated compound on ice. Inflammasome activation was induced by incubating samples for 60 minutes at 30°C. Inflammasome activity was assessed through monitoring Caspase-1 cleavage by Western blot.
[146] NLRP3 Inflammasome activation in PBMCs: 2 x 105 freshly isolated PBMCs were primed with 300 ng/mL LPS for 1 hour, then treated with a dose titration of the indicated compounds for an additional 2 hours. The NLRP3 inflammasome was then activated by treating cells with 10 mM nigericin for 2 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
[147] AIM2 Inflammasome activation in PBMCs: 2 x 105 freshly isolated PBMCs were transfected with poly dA:dT (100 ng/well) using Lipofectamine 2000 (1 μL/well) according to the manufacturer’s instructions in order to activate the AIM2 inflammasome. One hour later, cells
were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
[148] NLRP1 Inflammasome activation in PBMCs: 2 x 105 freshly isolated PBMCs were treated with 10 mM Talabostat, a constitutive repressor of the NLRP1 inflammasome. One hour later, cells were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
[149] NLRC4 Inflammasome activation PBMCs: 2 x 105 freshly isolated PBMCs were transfected with bacterial flagellin protein (100 ng/well) using Lipofectamine 2000 (1 μL/well) according to the manufacturer’s instructions in order to activate the NLRC4 inflammasome. One hour later, cells were treated with a dose titration of the indicated compounds for an additional 17 hours. Inflammasome activity was assessed by IL-1 b ELISA according to manufacturer’s instructions.
Example 3: Inhibition of Caspase-1 Processing in Cell Free Inflammasome Assay
[150] Inflammasome spontaneously assemble at 30°C in THP-1 cell lysates with sufficient protein concentrations. This processing is dependent on ASC oligomerization. The indicated compounds, or vehicle control, were added to THP-1 lysates at a final concentration of 1 mM for 20 minutes prior to inflammasome activation. Samples were incubated for 60 minutes at 30°C to activate the inflammasome. Activation of Caspase-1 (Casp-1 ) through autoproteolytic cleavage was assessed by Western blot. Disappearance of full length Casp-1, along with increased cleaved Casp-1 in the untreated and vehicle control lanes indicates inflammasome activation. Compounds 1-5 were able to significantly inhibit the conversion of full-length Casp-1 into its active, cleaved form as shown in FIG. 1.
Example 4: Cellular Activity Assays
[151] The properties of each compound were assayed in a variety of cellular assays using THP- 1 human monocytic cells:
[152] Cytotoxicity was assessed by treating THP-1 cells with a dose titration of the indicated compound for 5 hours, after which cell viability was assessed by MTT assay.
[153] Inflammasome inhibition was assayed in THP-1 cells primed with LPS, then treated with a dose titration of the indicated compound for 2 hours prior to NLRP3 inflammasome activation with Nigericin. IL-1 b was assessed in cell supernatants by ELISA to monitor inflammasome
activation. Simultaneously, an MTT assay was performed on the cells assess Pyroptosis, a form of inflammasome dependent inflammatory cell death.
Example 5: Inhibition of ASC oligomerization
[154] ASC oligomerization was monitored using an ASC-GFP THP-1 reporter cell line. Upon inflammasome activation, ASC polymerizes and forms a single large ‘Spec’ per cell. By tagging ASC with GFP these ‘Specs’ are readily observable and the percentage of inflammasome containing cells can be assessed. THP-1 ASC-GFP cells were differentiated using PMA overnight, then rested in PMA-free media for one day. Cells were treated with a dose titration of the indicated drug 2 hour prior to NLRP3 inflammasome activation using nigericin. Forty-five
minutes after nigericin treatment cells were fixed in 2% paraformaldehyde and imaged on a fluorescent microscope. The percentage of ASC-GFP Spec positive cells per field was quantified.
Example 6: Inhibition of Multiple Inflammasomes in human PBMCs
[155] The ability of a compound of interest to inhibit multiple inflammasomes is assessed in fresh primary human peripheral blood mononuclear cells (PBMCs). A dose titration of compound is added at the same time as the inflammasome stimul as follows: a. NLRP3: LPS prime followed by 2-hour stimulation with nigericin. b. NLRP1 : Overnight treatment with Talabostat (DPP4 inhibitor) c. NLRC4: Overnight transfection with bacterial flagellin protein d. AIM2: Overnight transfection with double stranded DNA
[156] It is to be understood that the embodiments of the invention disclosed herein are illustrative of the principles of the present invention. Other modifications that may be employed are within the scope of the invention. Thus, by way of example, but not of limitation, alternative configurations of the present invention may be utilized in accordance with the teachings herein. Accordingly, the present invention is not limited to that precisely as shown and described.
Claims
1. A compound having the structural formula:
or a pharmaceutically acceptable salt thereof, or solvate or hydrate thereof, wherein:
A1 is C1-C6 alkyl, -(C0-C2 alkyl)-cycloalkyl, -(C0-C2 alkyl)-heterocycloalkyl, -(C0-C2 alkyl)~aryl, -(C0-C2 alkyl)-heteroaryl, or -(C0-C2 alkyl)-B1-C1, wherein B1 and C1 are each independently cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
Q1 is -N(RA1)(RB1) or -ORA1;
X1 is -N(RA1)-;
W1 is C(O) or S(O)n1, wherein n1 is 1 or 2;
Y1 is a C1C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, or -(C0-C2 alkyl)-cycloalkyl;
Z1 is -(C0-C2 alkyl)-0(RA1) or -(C0-C2 alkyl)-N(RA1)(RB1); wherein each RA1 is independently H, C1-C6 alkyl, C2-C6 alkenyl, or (C0-C2)-cyclopropyl; each RB1 is independently H, C1-C6 alkyl, or C2-C6 alkenyl; each alkyl, alkenyl, and alkynyl is unsubstituted, substituted with one or more halogens (e.g., fluorinated or perfluorinated), or substituted with one or two groups independently selected from methyl, ethyl, acyl, oxo and -C(O)N(RA1)(RB1); each cycloalkyl has 3-10 ring carbons and is saturated or partially unsaturated, and optionally includes one or two fused and/or bridged cycloalkyl rings, each fused and/or bridged ring having 3-8 ring members, wherein the bridge is bivalent or trivalent, and is substituted with 0-5 RC1 or is perfluorinated;
each heterocycloalkyl has 3-10 ring members and 1-4 heteroatoms where each heteroatom is independently boron, nitrogen, oxygen or sulfur and is saturated or partially unsaturated, and optionally includes one or two fused cycloalkyl or aryl rings, and/or one or two bridged cycloalkyl rings, each fused and/or bridged ring each having 3-8 ring members, wherein the bridge is bivalent or trivalent, and is substituted with 0-5 RC1 or is perfluorinated; each aryl is a phenyl or naphthyl, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and is substituted with 0-5 RC1, or is perfluorinated; each heteroaryl is a 5-6 membered monocyclic heteroaryl ring having 1-4 heteroatoms, where each heteroatom is independently boron, nitrogen, oxygen or sulfur, or is a bicyclic heteroaryl having 1-5 heteroatoms where each heteroatom is independently boron, nitrogen, oxygen, or sulfur, and optionally includes one or two fused cycloalkyl or heterocycloalkyl rings, each fused cycloalkyl or heterocycloalkyl ring having 4-8 ring members, and wherein the heteroaryl is substituted with 0-5 RC1; wherein each RC1 is independently C1-C4 alkyl (e.g., methyl, t-butyl, or -CF3), -Cl, -F, -Br, -CN, -ORA1, -C(O)RA1, -C(O)NRB1RA1, -SO3, or -SO2(Ci alkyl).
2. The compound of claim 1 , wherein W1 is -C(O)-.
3. The compound of claim 1 or 2, wherein Q1 is -NH-C(0)CH3 or -ORA1.
4. The compound of claim 1 or 2, wherein Q1 is -ORA1, wherein RA1 is H, C1-C4 alkyl, or C2-
C4 alkenyl.
5. The compound of claim 1 or 2, wherein Q1 is -OH, -OCH3, or -OCH2CH3.
6. The compound of any claims 1-5, wherein X1 is -N(H)-.
7. The compound of any of claims 1-6, wherein A1 is t-butyl, -(C0-C2 alkyl)-O0-1-cycloalkyl, -(C0-C2 alkyl)-0o-i-heterocycloalkyl, -(C0-C2 alkyl)-O0-1-aryl, or -(C0-C2 alkyl)-O0-1-heteroaryl.
8. The compound of any of claims 1-6, wherein A1 is t-butyl.
9. The compound of any of claims 1-6, wherein A1 is -(C0-C1 alkyl)-cycloalkyl, -(C0-C1 alkyl)-heterocycloalkyl, -(C0-C1 alkyl)-aryl, or -(C0-C1 alkyl)-heteroaryl, wherein each cycle is either monocyclic or fused bicyclic.
10. The compound of any of claims 1-6, wherein A1 is phenyl or benzyl.
11. The compound of any of claims 1-6, wherein A1 is -(C0-C1 alkyl)-B1-C1, wherein B1 is cyclopropyl, cyclobutyl, or phenyl, and C1 is cyclopropyl or phenyl.
12. The compound of any of claims 1-6, wherein Y1 is C1-C8 alkyl, C2-C8 alkenyl, or C2-C8 alkynyl.
13. The compound of any of claims 1-6, wherein Y1 is C3-C6 alkenyl.
14. The compound of any of claims 1-6, wherein Y1 is -(C2-C3 alkenyl)-cyclolakyl, wherein cycloalkyl is cyclopropyl or cyclobutyl.
15. The compound of any of claims 1-14, wherein Z1 is absent.
16. The compound of any of claims 1-14, wherein Z1 is -(C0-C1 alkyl)-OH or -(C0-C1 alkyl)-NH2.
17. The compound of any of claims 1-14, wherein Z1 is -OH or -Nhb.
18. The compound of anyof claims 1-17, wherein each RC1 is independently -CH3, -CH2CH3, -CF3, -F, or -Cl.
19. The compound of claim 1 , wherein the compound is: (S)-2-acetamido-N-(5-aminopentyl)-3-(thiophen-2-yl)propanamide; (S)-2-acetamido-N-(4-aminobut-2-en-1-yl)-3-phenylpropanamide; (S)-2-acetamido-N-(4-aminobutyl)-3-(3,4-difluorophenyl)propanamide; (S)-2-acetamido-N-(4-aminobutyl)-3-phenylpropanamide; (S)-2-acetamido-N-(3-aminopropyl)-3-phenylpropanamide; (S)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-phenyl propanamide; (S)-N-(4-aminobut-2-en-1-yl)-2-cyclopropyl-2-hydroxyacetamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-hydroxy-3-phenylpropanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-ethoxy-3-phenylpropanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-chlorophenyl)-2-methoxypropanamide; (R,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-phenylpropanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(3- (trifluoromethyl)phenyl)propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-ethoxy-3-(3-(trifluoromethyl)phenyl)propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-2-phenylacetamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-2-phenylacetamide;
(E)-N-(4-aminobut-2-en-1-yl)-2-(3-hydroxyphenyl)-2-methoxyacetamide;
(E)-N-(4-aminobut-2-en-1-yl)-2-(2,3-dihydrobenzofuran-6-yl)-2-methoxyacetamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-phenyl-2-propoxypropanamide;
(S,E)-2-(allyloxy)-N-(4-aminobut-2-en-1-yl)-3-phenylpropanamide; (S)-N-((E)-4-aminobut-2-en-1-yl)-2-(((E)-but-2-en-1-yl)oxy)-3-phenylpropanamide,
(S,E)-N-(4-aminobut-2-en-1-yl)-2-(cyclopropylmethoxy)-3-phenylpropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-cyclopropoxy-3-phenylpropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(4-oxo-3,4-dihydroquinazolin-7-yl) propanamide;
(S,E)-2-methoxy-N-(4-(methylamino)but-2-en-1-yl)-3-(3-(trifluoromethyl)phenyl) propanamide; (S)-N-((E)-4-aminobut-2-en-1-yl)-2-((1R,2S)-2-(3-chlorophenyl)cyclopropyl)-2- hyd roxyacetam ide ; (S)-N-((E)-4-aminobut-2-en-1-yl)-2-((1R,2S)-2-(3-chlorophenyl)cyclopropyl)-2- methoxyacetamide;
(S,E)-N-(4-amino-3-methylbut-2-en-1-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl) propanamide;
(S,E)-N-(4-amino-2-methylbut-2-en-1-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl) propanamide;
(S,Z)-N-(4-amino-3-fluorobut-2-en-1-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl) propanamide;
(S,Z)-N-(4-amino-2-fluorobut-2-en-1-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl) propanamide;
(S,E)-2-methoxy-N-(4-(methylamino)but-2-en-1-yl)-3-(3-(trifluoromethyl)phenyl) propanamide; (S)-N-(4-aminobut-2-yn-1-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl)pro panamide; (S)-N-((R)-5-aminopent-3-yn-2-yl)-2-methoxy-3-(3-(trifluoromethyl)phenyl) propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-hydroxy-4-phenylbutanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3,5-difluorophenyl)-2-methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-4-(3-chlorophenyl)-2-hydroxybutanamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-4-(3-chlorophenyl)-2-hydroxybutanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-4-(4-fluorophenyl)-2-hydroxybutanamide; (R,E)-N-(4-aminobut-2-en-1-yl)-4-(4-fluorophenyl)-2-hydroxybutanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-4-(4-fluorophenyl)-2-methoxybutanamide; (R,E)-N-(4-aminobut-2-en-1-yl)-4-(3-chlorophenyl)-2-methoxybutanamide; (S,E)-2-amino-N-(4-aminobut-2-en-1-yl)-3-phenyl propanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(3-methoxyphenyl)propanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-4-(3-methoxyphenyl)butanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide; (S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(6-(trifluoromethyl)pyridin-2-yl) propanamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-2-(2-fluorophenyl)-2-hydroxyacetamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-2-(2-fluorophenyl)-2-methoxyacetamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-2-hydroxy-2-(pyridin-3-yl)acetamide;
(R,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-2-(pyridin-3-yl)acetamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3,4-difluorophenyl)-2-methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-cyclopropylphenyl)-2-methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(2,3-dihydrobenzofuran-5-yl)-2-methoxy propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3-fluoro-5-(trifluoromethyl)phenyl)-2-methoxy propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(3-
(methylsulfonyl)phenyl)propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3,5-dimethylphenyl)-2-methoxypropanamide; (R,E)-N-(4-aminobut-2-en-1 -yl)-3, 3-d ifluoro-2-methoxy-3-phenyl propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(1,1-dioxido-3-oxo-2,3-dihydrobenzo[d]isothiazol-
6-yl)-2-methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-cyclohexyl-2-methoxypropanamide;
(S,E)-2-amino-N-(4-aminobut-2-en-1-yl)-3-phenyl propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(1,1-dioxidobenzo[b]thiophen-6-yl)-2- methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-2-methoxy-3-(3-methyl-1,1- dioxidobenzo[d]isothiazol-6-yl)propanamide;
(S,E)-N-(4-aminobut-2-en-1-yl)-3-(3,3-difluoro-1,3-dihydroisobenzofuran-5-yl)-2- methoxypropanamide;
(S,E)-N-(4-aminobut-2-en-1 -yl)-2-methoxy-3-(1 -methyl-2-(trifluoromethyl)-1 /-/- imidazol-4-yl)propanamide;
(2S)-N-[(2E)-3-(1-aminocyclopropyl)prop-2-en-1-yl]-2-methoxy-3- phenylpropanamide; or
(2R)-N-[(2E)-4-aminobut-2-en-1 -yl]-2-methoxy-2-(3'-methoxy[1 ,T-biphenyl]-4- yl)acetamide.
20. A method of treating an inflammatory condition in a subject in need thereof, comprising providing to the subject a compound according to any one of claims 1-19.
21. The method of claim 20, wherein the inflammatory condition is an inflammatory bowel disease, an arthritic disease, or a neurodegenerative disorder.
22. The method of claim 20, wherein the inflammatory condition is an autoinflammatory syndrome.
23. The method of claim 20, wherein the inflammatory condition is an inflammasome-related condition.
24. The method of claim 20, wherein the inflammatory condition is Adult-Onset Still’s Disease (AOSD), Systemic Juvenile Idiopathic Arthritis (sJIA), Macrophage Activation Syndrome (MAS), Autoinflammation with Infantile Enterocolitic (AIFEC), Bullous Pemphigoid, Pemphigus Vulgaris, Idiopathic Pulmonary Fibrosis (IPF), Non-Alcoholic Steatohepatitis (NASH), Systemic Lupus Erythematosus (SLE), Multiple Sclerosis, Alzheimer’s Disease, Parkinson’s Disease, Traumatic Brain Injury (TBI), Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), Cryopyrin-Associated Periodic Syndromes (CAPS), Vitiligo, Multiple Self-Healing Palmoplantar Carcinoma (MSPC), Autoimmune Addison’s Disease, Familial Mediterranean Fever (FMF), Autoimmune Thyroiditis, Stroke, Type 2 Diabetes (T2D), Osteoarthritis, Gout, Atherosclerosis, Hidradenitis Suppurativa, Psoriasis, and Pyrin Diseases.
25. A method for treating an inflammatory condition resulting from a coronavirus (e.g., SARS-CoV 2, SARS-CoV, or MERS), viral, bacterial, fungal, parasitic, or other type of infection in a human subject, comprising administering to a subject in need of such treatment a compound according to any one of claims 1-19.
26. A method according to claim 25, wherein the inflammatory condition causes cell death, or release of pro-inflammatory cytokines or other inflammatory mediators.
27. The method of claim 25 or 26, wherein the inflammatory condition is cytokine storm syndrome, cytokine release syndrome, sepsis, hemophagocytic lymphohistiocytosis, acute lung injury, acute respiratory distress syndrome, or macrophage activation syndrome.
28. The method of any one of claims 25-27, wherein the compound of any one of claim 1-19 is administered in co-therapy with at least one of an antiinfective, antimicrobial, antiviral (e.g., remdesivir), monoclonal antibody (e.g., tocilizumab), biologic, immunoglobulin, immunomodulator, anti-inflammatory drug, corticosteroid, small molecule, cell therapy, or other type of prophylactic or therapeutic agent.
29. A method for treating an inflammatory condition resulting from a cell therapy administered to a human subject, comprising administering to a subject in need of such treatment a compound according to any one of claims 1-19.
30. A method according to 29, wherein the inflammatory condition is cytokine storm syndrome or cytokine release syndrome.
31. A method according to claim 29 or claim 30, wherein the inflammatory condition causes cell death or release of pro-inflammatory cytokines or other inflammatory mediators.
32. The method of any one of claims 29-31 , wherein the compound according to any one of claims 1-19 is administered in co-therapy with at least one of a monoclonal antibody (e.g., tocilizumab), a biologic, an immunoglobulin, an immunomodulatory, an anti-inflammatory drug, a corticosteroid, a small molecule, a cell therapy, or other type of prophylactic or therapeutic agent.
33. A method of any one of claims 20-32, wherein the compound is N-(2-aminoethyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, N-((1- aminocyclobutyl)methyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, or N-(2- aminocyclohexyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, or a pharmaceutically acceptable salt thereof.
34. A pharmaceutical composition comprising a compound of any of claims 1-19 or N-(2- aminoethyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, N-((1-aminocyclobutyl) methyl)-2-hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide, or N-(2-aminocyclohexyl)-2- hydroxy-3-(3-(trifluoromethyl)phenyl)propanamide claims, or a pharmaceutically acceptable salt thereof.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202063015024P | 2020-04-24 | 2020-04-24 | |
| US63/015,024 | 2020-04-24 | ||
| US202163166083P | 2021-03-25 | 2021-03-25 | |
| US63/166,083 | 2021-03-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021217041A1 true WO2021217041A1 (en) | 2021-10-28 |
Family
ID=75905056
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2021/028906 WO2021217040A1 (en) | 2020-04-24 | 2021-04-23 | Carbamate and urea derivatives |
| PCT/US2021/028907 WO2021217041A1 (en) | 2020-04-24 | 2021-04-23 | N-substituted alpha-amino and alpha-hydroxy carboxamide derivatives |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2021/028906 WO2021217040A1 (en) | 2020-04-24 | 2021-04-23 | Carbamate and urea derivatives |
Country Status (1)
| Country | Link |
|---|---|
| WO (2) | WO2021217040A1 (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2458422A (en) * | 1947-11-22 | 1949-01-04 | Eastman Kodak Co | Acrylic diester-propionamides and polymers thereof |
| WO2004041827A2 (en) * | 2002-10-30 | 2004-05-21 | Boehringer Ingelheim Pharmaceuticals, Inc. | DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-α] IMIDAZOLE-3-SULFONYLAMINO]-PROPIONAMIDE AND THEIR USE AS INHIBITORS UPON THE INTERACTION OF CAMS AND LEUKO INTEGRINS |
| WO2006027252A1 (en) * | 2004-09-10 | 2006-03-16 | Ucb Pharma, Sa | Sigma receptor ligands |
| WO2008107137A2 (en) * | 2007-03-02 | 2008-09-12 | Givaudan Nederland Services B.V. | Compositions comprising a physiological coolant |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2877263A (en) * | 1956-12-26 | 1959-03-10 | George R Thomas | Nitration catalyst |
| DE3804490A1 (en) * | 1988-02-12 | 1989-08-24 | Heumann Pharma Gmbh & Co | SUBSTITUTED 6-PHENYLDIHYDRO-3 (2H) -PYRIDAZINONE, METHOD FOR THE PRODUCTION THEREOF AND MEDICINAL PRODUCTS CONTAINING THESE COMPOUNDS |
| KR100441458B1 (en) * | 1995-10-25 | 2004-09-18 | 미쓰비시 가가꾸 가부시키가이샤 | A process for producing an unsymmetrical chain carbonic ester |
| US6001856A (en) * | 1997-06-13 | 1999-12-14 | Pfizer Inc. | β-adrenergic agonists to reduce a wasting condition |
| EP1171136B1 (en) * | 1999-04-06 | 2005-06-22 | Genzyme Corporation | Immunomodulating compositions comprising urea derivatives for use in the treatment of inflammatory diseases |
| WO2010097479A2 (en) * | 2010-05-25 | 2010-09-02 | Symrise Gmbh & Co. Kg | Cyclohexyl carbamate compounds as active anti-cellulite ingredients |
| US10130714B2 (en) * | 2012-04-14 | 2018-11-20 | Academia Sinica | Enhanced anti-influenza agents conjugated with anti-inflammatory activity |
| WO2020010451A1 (en) * | 2018-07-10 | 2020-01-16 | Trillium Therapeutics Inc. | Heteroaromatic-fused imidazolyl amides, compositions and uses thereof as sting agonists |
-
2021
- 2021-04-23 WO PCT/US2021/028906 patent/WO2021217040A1/en active Application Filing
- 2021-04-23 WO PCT/US2021/028907 patent/WO2021217041A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2458422A (en) * | 1947-11-22 | 1949-01-04 | Eastman Kodak Co | Acrylic diester-propionamides and polymers thereof |
| WO2004041827A2 (en) * | 2002-10-30 | 2004-05-21 | Boehringer Ingelheim Pharmaceuticals, Inc. | DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-α] IMIDAZOLE-3-SULFONYLAMINO]-PROPIONAMIDE AND THEIR USE AS INHIBITORS UPON THE INTERACTION OF CAMS AND LEUKO INTEGRINS |
| WO2006027252A1 (en) * | 2004-09-10 | 2006-03-16 | Ucb Pharma, Sa | Sigma receptor ligands |
| WO2008107137A2 (en) * | 2007-03-02 | 2008-09-12 | Givaudan Nederland Services B.V. | Compositions comprising a physiological coolant |
Non-Patent Citations (12)
| Title |
|---|
| "Introduction to Modern Liquid Chromatography", 1979, JOHN WILEY AND SONS |
| "The Peptides", vol. 3, 1981, ACADEMIC PRESS |
| "Thin Layer Chromatography", 1969, SPRINGER-VERLAG |
| H.-D. JAKUBKEH. JESCHEIT: "Aminosauren, Peptide, Proteine", 1982, VERLAG CHEMIE |
| J. F. W. MCOMIE: "Protective Groups in Organic Chemistry", 1973, PLENUM PRESS |
| J. MARCH: "Advanced Organic Chemistry", 2013, JOHN WILEY AND SONS |
| JOCHEN LEHMANN: "Chemie der Kohlenhydrate: Monosaccharide and Derivate", vol. 15, 1974, GEORG THIEME VERLAG |
| LIMA RAFAELY N ET AL: "Biocatalytic aminolysis of ethyl (S)-mandelate by lipase fromCandida antarctica", CATALYSIS COMMUNICATIONS, ELSEVIER, AMSTERDAM, NL, vol. 100, 21 June 2017 (2017-06-21), pages 157 - 163, XP085145511, ISSN: 1566-7367, DOI: 10.1016/J.CATCOM.2017.06.020 * |
| SMITHMARCH: "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure", 2001, WILEY-INTERSCIENCE |
| T. W. GREENEP. G. M. WUTS: "Protective Groups in Organic Synthesis", 1999, WILEY |
| TOMASI S ET AL: "Solid phase organic synthesis of polyamine derivatives and initial biological evaluation of their antitumoral activity", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, ELSEVIER, AMSTERDAM, NL, vol. 8, no. 6, 17 March 1998 (1998-03-17), pages 635 - 640, XP004136936, ISSN: 0960-894X, DOI: 10.1016/S0960-894X(98)00086-9 * |
| VOGEL: "A Textbook of Practical Organic Chemistry, Including Qualitative Organic Analysis", 1978, LONGMAN |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2021217040A1 (en) | 2021-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA3133753A1 (en) | Novel small molecule inhibitors of tead transcription factors | |
| US11407740B2 (en) | Antibacterial compounds | |
| WO2003013527A1 (en) | Sulfonamide derivatives as gamma secretase inhibitors | |
| WO2013160418A1 (en) | Novel compounds | |
| CN111032034A (en) | Spiro compounds and methods of making and using the same | |
| EA015376B1 (en) | SALTS OF 6-HETEROARYL-(1,2,3,4,4a,10b)-HEXAHYDROPHENANTHRIDINE DERIVATIVES AND USE THEREOF | |
| TW201829381A (en) | Histone Deacetylase Inhibitors | |
| JP2022540434A (en) | cannabinoid derivatives | |
| EP4037676A1 (en) | Cannabinoid derivatives | |
| US20230286906A1 (en) | Cannabinoid derivatives | |
| EP3994129A1 (en) | Cannabinoid derivatives | |
| US20230024212A1 (en) | Cannabinoid derivatives | |
| EP4065568A1 (en) | Cannabinoid derivatives | |
| JP2023503601A (en) | Cannabigerol derivatives and their use as cannabinoid receptor modulators | |
| JP2011088826A (en) | Aromatic carboxylic acid compound | |
| EP3022201A1 (en) | Autotaxin inhibitors | |
| JP6770507B2 (en) | Quinoline derivative useful as a ubiquitination inhibitor | |
| EP3500558B1 (en) | Benzimidazole direct ampk activators | |
| WO2021217041A1 (en) | N-substituted alpha-amino and alpha-hydroxy carboxamide derivatives | |
| WO2017175185A1 (en) | Heteroaryl butanoic acid derivatives as lta4h inhibitors | |
| JP2024510651A (en) | Polymorphic forms of compounds and their preparation and use | |
| Xu et al. | Structural insights of sulfonamide-based NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization | |
| WO2024130173A1 (en) | Benzimidazole derivatives | |
| CN118541366A (en) | Dihydrooxazole derivative compound | |
| AU2020343737A1 (en) | MAGL inhibitor, preparation method therefor and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21725339 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21725339 Country of ref document: EP Kind code of ref document: A1 |