WO2020244969A1 - Pyridine derivatives and their use as fungicides - Google Patents
Pyridine derivatives and their use as fungicides Download PDFInfo
- Publication number
- WO2020244969A1 WO2020244969A1 PCT/EP2020/064576 EP2020064576W WO2020244969A1 WO 2020244969 A1 WO2020244969 A1 WO 2020244969A1 EP 2020064576 W EP2020064576 W EP 2020064576W WO 2020244969 A1 WO2020244969 A1 WO 2020244969A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- alkenyl
- alkynyl
- halogenalkyl
- cycloalkyl
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/72—Nitrogen atoms
- C07D213/75—Amino or imino radicals, acylated by carboxylic or carbonic acids, or by sulfur or nitrogen analogues thereof, e.g. carbamates
Definitions
- the present invention relates to the use of pyridine compounds and the N-oxides and the salts thereof as fungicides as well to new pyridine compounds.
- the invention also relates to the composition comprising at least one compound I, to the method for combating phytopathogenic fungi and to the ssed coated with at least one compound of the formula I.
- Another object of the present invention is to provide fungicides with improved toxicological properties or with improved environmental fate properties.
- the present invention relates to use of the compounds of formula I
- X is O or S
- R 1 is in each case independently selected from hydrogen, halogen, CN, CrC 6 -alkyl, C 1 -C 6 - halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, 0-CrC 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl, C 3 -C 6 -cycloalkyl, wherein the acyclic and cyclic moieties of R 1 are unsubstituted or substituted by one to six groups R 1a which independently of one another are selected from:
- halogen CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 - alkynyl, C 2 -C 6 -halogenalkynyl, 0-CrC 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl;
- R 2 is in each case independently selected from halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, 0-CrC 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl, C 3 -C 6 -cycloalkyl, wherein the acyclic and cyclic moieties of R 2 are unsubstituted or substituted by one to six groups R 2a which independently of one another are selected from:
- halogen CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 - alkynyl, C 2 -C 6 -halogenalkynyl, 0-CrC 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl;
- R 3 is in each case independently selected from halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, 0-CrC 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl, C 3 -C 6 -cycloalkyl, wherein the acyclic and cyclic moieties of R 3 are unsubstituted or substituted by one to six groups R 3a which independently of one another are selected from: halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -
- R 4 is in each case independently selected from hydrogen, halogen, CN, Ci-C 6 -alkyl, C 1 -C 6 - halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, 0-Ci-C 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl, C 3 -C 6 -cycloalkyl, wherein the acyclic and cyclic moieties of R 4 are unsubstituted or substituted by one to six groups R 4a which independently of one another are selected from:
- halogen CN, Ci-C 6 -alkyl, Ci-C 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 - alkynyl, C 2 -C 6 -halogenalkynyl, 0-Ci-C 6 -alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -C 6 -alkynyl;
- A is direct bond or C(R 7 R 8 );
- ⁇ are in each case independently selected from hydrogen, halogen, CN, Ci-C 6 -alkyl, C 1 -C 6 - halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, O-CrCe-alkyl, 0-C 2 -C 6 -alkenyl, 0-C 2 -Ce-alkynyl, Cs-Ce-cycloalkyl, O-Cs-Ce-cycloalkyl, CH 2 -C 3 -C 6 -cycloalkyl, C 3 -C 6 -cycloalkenyl, 0-C 3 -C 6 -cycloalkenyl, CH 2 -C 3 -C 6 -cycloalkenyl, wherein the acyclic moieties of R 5 , R 6
- R ' is as defined above;
- R 5 , R 6 , R 7 or R 8 can not all be H
- R 9 when A is C(R 7 R 8 ) R 9 can be also H;
- the N-oxides may be prepared from the inventive compounds according to conventional oxidation methods, e. g. by treating compounds I with an organic peracid such as
- metachloroperbenzoic acid cf. WO 03/64572 or J. Med. Chem. 38(11), 1892-903, 1995
- inorganic oxidizing agents such as hydrogen peroxide (cf. J. Heterocyc. Chem. 18(7), 1305-8, 1981) or oxone (cf. J. Am. Chem. Soc. 123(25), 5962-5973, 2001).
- the oxidation may lead to pure mono-N-oxides or to a mixture of different N-oxides, which can be separated by conventional methods such as chromatography.
- Agriculturally acceptable salts of the compounds of the formula I encompass especially the salts of those cations or the acid addition salts of those acids whose cations and anions, respectively, have no adverse effect on the fungicidal action of the compounds I.
- Suitable cations are thus in particular the ions of the alkali metals, preferably sodium and potassium, of the alkaline earth metals, preferably calcium, magnesium and barium, of the transition metals, preferably manganese, copper, zinc and iron, and also the ammonium ion which, if desired, may be substituted with one to four CrC4-alkyl substituents and/or one phenyl or benzyl substituent, preferably diisopropylammonium, tetramethylammonium, tetrabutylammonium,
- trimethylbenzylammonium furthermore phosphonium ions, sulfonium ions, preferably tri(Ci-C4- alkyl)sulfonium, and sulfoxonium ions, preferably tri(Ci-C4-alkyl)sulfoxonium.
- Anions of acceptable acid addition salts are primarily chloride, bromide, fluoride,
- Ci-C4-alkanoic acids preferably formate, acetate, propionate and butyrate. They can be formed by reacting a compound I with an acid of the corresponding anion, preferably of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid or nitric acid.
- Stereoisomers of the formula I can exist as one or more stereoisomers.
- the various stereoisomers include enantiomers, diastereomers, atropisomers arising from restricted rotation about a single bond of asymmetric groups and geometric isomers. They also form part of the subject matter of the present invention.
- one stereoisomer may be more active and/or may exhibit beneficial effects when enriched relative to the other stereoisomer(s) or when separated from the other stereoisomer(s). Additionally, the skilled artisan knows how to separate, enrich, and/or to selectively prepare said stereoisomers.
- the compounds of the invention may be present as a mixture of stereoisomers, e.g. a racemate, individual
- C n -C m indicates the number of carbon atoms possible in each case in the substituent or substituent moiety in question.
- halogen refers to fluorine, chlorine, bromine and iodine.
- CrC 6 -alkyl refers to a straight-chained or branched saturated hydrocarbon group having 1 to 6 carbon atoms, e.g. methyl, ethyl, propyl, 1-methylethyl, butyl, 1-methylpropyl, 2- methylpropyl, 1 , 1 -di methylethyl , pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl,
- C2-C4-alkyl refers to a straight-chained or branched alkyl group having 2 to 4 carbon atoms, such as ethyl, propyl (n-propyl), 1-methylethyl (iso-propoyl), butyl, 1-methylpropyl (sec.-butyl), 2-methylpropyl (iso-butyl), 1 , 1 -dimethylethyl (tert.-butyl).
- Ci-C 6 -halogenalkyl refers to an alkyl group having 1 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
- Ci-C2-halogenalkyl such as chloromethyl, bromomethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl, chlorodifluoromethyl, 1-chloroethyl, 1-bromoethyl, 1- fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl, 2-chloro- 2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl or pentafluoroethyl.
- CrC 6 -alkoxy refers to a straight-chain or branched alkyl group having 1 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkyl group.
- Examples are “C1-C4- alkoxy” groups, such as methoxy, ethoxy, n-propoxy, 1-methylethoxy, butoxy, 1-methyhprop- oxy, 2-methylpropoxy or 1 ,1-dimethylethoxy.
- CrC 6 -halogenalkoxy refers to a CrC 6 -alkoxy radical as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
- Examples are "CrC4-halogenalkoxy” groups, such as OCH2F, OCHF2, OCF 3 , OCH2CI, OCHCI2, OCCI 3 , chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 2-fluoroethoxy, 2-chlorothoxy, 2-bromoethoxy, 2-iodoethoxy, 2,2-difluoroethoxy, 2,2,2- trifluoroethoxy, 2-chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy, 2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy, OC2F5, 2-fluoropropoxy
- C2-C6-alkenyl refers to a straight-chain or branched unsaturated hydrocarbon radical having 2 to 6 carbon atoms and a double bond in any position.
- Examples are “C2-C4-alkenyl” groups, such as ethenyl, 1-propenyl, 2-propenyl (allyl), 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1 -methyl-1 -propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl, 2-methyl-2-propenyl.
- C2-C6-halogenalkenyl refers to an alkyl group having 2 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
- C2-C6-alkenyloxy refers to a straight-chain or branched alkenyl group having 2 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkenyl group. Examples are “C2-C4-alkenyloxy” groups.
- C2-C6-alkynyl refers to a straight-chain or branched unsaturated hydrocarbon radical having 2 to 6 carbon atoms and containing at least one triple bond.
- Examples are "C2-C4- alkynyl” groups, such as ethynyl, prop-1-ynyl, prop-2-ynyl (propargyl), but-1-ynyl, but-2-ynyl, but-3-ynyl , 1 -methyl-prop-2-ynyl .
- C2-C6-halogenalkynyl refers to an alkyl group having 2 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
- C2-C6-alkynyloxy refers to a straight-chain or branched alkynyl group having 2 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkynyl group. Examples are “C2-C4-alkynyloxy” groups.
- C3-C6-cycloalkyl refers to monocyclic saturated hydrocarbon radicals having 3 to 6 carbon ring members, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl. Accordingly, a saturated three-, four-, five-, six-, seven-, eight-, nine or ten-membered carbocyclyl or carbo- cycle is a "C3-Cio-cycloalkyl".
- C3-C6-cycloalkenyl refers to a monocyclic partially unsaturated 3-, 4- 5- or 6- membered carbocycle having 3 to 6 carbon ring members and at least one double bond, such as cyclopentenyl, cyclopentadienyl, cyclohexadienyl. Accordingly, a partially unsaturated three-, four-, five-, six-, seven-, eight-, nine or ten-membered carbocyclyl or carbocycle is a "C3-C10- cycloalkenyl".
- C3-C8-cycloalkyl-Ci-C4-alkyl refers to alkyl having 1 to 4 carbon atoms (as defined above), whereAccording to one hydrogen atom of the alkyl radical is replaced by a cycloalkyl radical having 3 to 8 carbon atoms (as defined above).
- heterocyclyl or heterocycle contains 1 , 2, 3 or 4 heteroatoms selected from N, O and S
- the ring member atoms of the heterocycle include besides carbon atoms 1 , 2, 3 or 4 heteroatoms independently selected from the group of O, N and S.
- a 3- or 4-membered saturated heterocycle which contains 1 or 2 heteroatoms from the group consisting of O, N and S as ring members such as oxirane, aziridine, thiirane, oxetane, azetidine, thiethane, [1 ,2]dioxetane, [1 ,2]dithietane, [1 ,2]diazetidine; and
- a 5- or 6-membered saturated or partially unsaturated heterocycle which contains 1 , 2 or 3 heteroatoms from the group consisting of O, N and S as ring members such as 2- tetrahydrofuranyl, 3-tetrahydrofuranyl, 2-tetrahydrothienyl, 3-tetrahydrothienyl, 2-pyrrolidinyl, 3- pyrrolidinyl, 3-isoxazolidinyl, 4-isoxazolidinyl, 5-isoxazolidinyl, 3-isothiazolidinyl,
- hexahydroazepinyl such as 2,3,4,5-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, 3, 4, 5, 6- tetrahydro[2H]azepin-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,4,7-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,6,7-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, hexahydroazepin-1-,-2-,-3- or-yl,
- substituted refers to substitued with 1 , 2, 3 or up to the maximum possible number of substituents.
- the term“5-or 6-membered heteroaryl” or“5-or 6-membered heteroaromatic” refers to aromatic ring systems incuding besides carbon atoms, 1 , 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O and S, for example, a 5-membered heteroaryl such as pyrrol-1 -yl, pyrrol-2-yl, pyrrol-3-yl, thien-2-yl, thien-3-yl, furan- 2-yl, furan-3-yl, pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl, imidazol-1-yl, imidazol-2-yl, imidazol-4-yl, imidazol-5-yl, oxazol-2-yl, oxazol-4-yl, oxazol-5-yl, isoxazol-3-yl, isox
- a 6-membered heteroaryl such as pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrimidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, pyrazin-2-yl and 1 ,3,5-triazin-2-yl and
- R 1 is H, halogen, Ci-C 6 -alkyl or Ci-C 6 -halogenalkyl, in particular H, F, Cl, CH 3 , C2H5, CF 3 more specifically H, CH 3 , F or Cl most preferred H, F or Cl, especially R 1 is hydrogen.
- R 1 Particularly preferred embodiments of R 1 according to the invention are in Table P1 below, wherein each line of lines P1-1 to P1-13 corresponds to one particular embodiment of the invention. Thereby, for every R 1 that is present in the inventive compounds, these specific embodiments and preferences apply independently of the meaning of any other R 1 that may be present in the ring:
- R 2 is selected from the group consisting of C 1 -C 6 - alkyl, CrC 6 -halogenalkyl, 0-CrC 6 -alkyl, C 3 -C 6 -cycloalkyl, in particular CH 3 , C 2 H 5 , CF 3 , CH 2 F, CHF 2 , OCH 3 , OC 2 H 5 , O-C 3 H 7 , O-C 4 H 9 , cyclopropyl, cyclobutyl, more specifically CH 3 , CH 2 F, CF 2 H, CF 3 , OCH3, OC2H5, most preferred OCH 3 , CH 3 .
- R 2 is halogen, in particular F, Cl, Br or I, more specifically F, Cl or Br, in particular F or Cl.
- R 2 is F.
- R 2 is Cl
- R 2 is Br
- R 2 is CrC6-alkyl, in particular CrC 4 -alkyl, such as CH 3 . or C 2 H 5 , in particular CH 3 or CH 2 CH 3 .
- R 2 is CrC6-halogenalkyl, in particular C 1 -C 4 - halogenalkyl, such as CF 3 , CCI 3 , FCH 2 , CICH 2 , F 2 CH, CI 2 CH, CF3CH2, CCI3CH2 or CF 2 CHF 2 .
- R 2 is C 2 -C 6 -alkynyl or C 2 -C 6 -halogenalkynyl, in particular C 2 -C 4 -alkynyl or C 2 -C 4 -halogenalkynyl, such as CECH, CFhCECH, CECCI,
- R 2 is 0-CrC 6 -alkyl, in particular C 1 -C 4 - alkyl, more specifically Ci-C 2 -alkoxy.
- R 2 is such as OCH 3 or OCH 2 CH 3 .
- R 2 is 0-CrC6-alkyl
- R 2 is 0-C2-C6-alkenyl in particular C 2 - C 4 -alkenyl, more specifically C 2 -C 3 -alkenyl.
- R 2 is 0-C2-C6-alkynyl, in particular C 2 - C6-alkynyl, in particular C 2 -C 4 -alkynyl, more specifically C 2 -C 3 -alkynyl.
- R 2 is such as O-CH 2 - CECH.
- R 2 is 0-CrC6-halogenalkyl, in particular OCF3, OCC , OFCH2, OCICH2, OF2CH, OCI2CH, OCF3CH2, OCCI3CH2 or OCF2CHF2, more specifically OCF 3 , OF 2 CH, OFCH 2.
- R 2 is C 3 -C 6 -cycloalkyl, in particular cyclopropyl or cyclobutyl.
- R 2 is C 3 -C 6 -halogencycloalkyl.
- R 2 is fully or partially halogenated cyclopropyl, such as 1-F-cyclopropyl, 1-CI- cyclopropyl, 2,2-F2-cyclopropyl, 2,2-Cl2-cyclopropyl .
- R 2 Particularly preferred embodiments of R 2 according to the invention are in Table P2 below, wherein each line of lines P2- 1 to P2-21 corresponds to one particular embodiment of the invention, wherein P2- 1 to P2-21 are also in any combination with one another a preferred embodiment of the present invention.
- the connection point to the carbon atom, to which R 2 is bound is marked with“#” in the drawings.
- R 3 is selected from the group consisting of C 1 -C 6 - alkyl, CrC 6 -halogenalkyl, 0-CrC 6 -alkyl, C 3 -C 6 -cycloalkyl, in particular CH 3 , C 2 H 5 , CF 3 , CH 2 F, CHF 2 , OCH 3 , OC 2 H 5 , O-C 3 H 7 , O-C 4 H 9 , cyclopropyl, cyclobutyl, more specifically CH 3 , CH 2 F, CF2H, CF3, cyclopropyl, cyclobutyl, most preferred CH3, CF2H, CF3.
- R 3 is halogen, in particular F, Cl, Br or I, more specifically F, Cl or Br, in particular F or Cl.
- R 3 is F.
- R 3 is Cl
- R 3 is Br.
- R 3 is CrC6-alkyl, in particular CrC 4 -alkyl, such as CH3 or C2H5, in particular CH3 or CH2CH3.
- R 3 is CrC6-halogenalkyl, in particular C1-C4- halogenalkyl, such as CF 3 , CCI 3 , FCH 2 , CICH 2 , F 2 CH, CI 2 CH, CF3CH2, CCI3CH2 or CF 2 CHF 2 .
- R 3 is C2-C6-alkynyl or C2-C6-halogenalkynyl, in particular C2-C4-alkynyl or C2-C4-halogenalkynyl, such as CECH, ChhCECH, CECCI,
- R 3 is 0-CrC 6 -alkyl, in particular C1-C4- alkyl, more specifically Ci-C 2 -alkoxy.
- R 3 is such as OCH 3 or OCH 2 CH 3 .
- R 3 is 0-C 2 -C 6 -alkenyl in particular C2- C4-alkenyl, more specifically C2-C3-alkenyl.
- R 3 is 0-C 2 -C 6 -alkynyl, in particular C2- C 6 -alkynyl, in particular C2-C4-alkynyl, more specifically C2-C3-alkynyl.
- R 3 is such as O-CH2- CECH.
- R 3 is 0-CrC 6 -halogenalkyl, in particular OCF3, OCC , OFCH2, OCICH2, OF2CH, OCI2CH, OCF3CH2, OCCI3CH2 or OCF2CHF2, more specifically OCF 3 , OF 2 CH, OFCH 2 .
- R 3 is C3-C6-cycloalkyl, in particular cyclopropyl, cyclobutyl.
- R 3 is C3-C6-halogencycloalkyl.
- R 3 is fully or partially halogenated cyclopropyl, such as 1-F-cyclopropyl, 1-CI- cyclopropyl, 2,2-F 2 -cyclopropyl, 2,2-Cl 2 -cyclopropyl .
- R 3 Particularly preferred embodiments of R 3 according to the invention are in Table P3 below, wherein each line of lines P3-1 to P3-21 corresponds to one particular embodiment of the invention, wherein P3-1 to P3-21 are also in any combination with one another a preferred embodiment of the present invention.
- the connection point to the carbon atom, to which R 3 is bound is marked with“#” in the drawings.
- R 4 is H, halogen, CrC 6 -alkyl or CrC 6 -halogenalkyl, in particular H, F, Cl, CH 3 , C2H5, CF 3 more specifically H, CH 3 , F or Cl most preferred H, F or Cl, especially R 4 is hydrogen.
- R 4 Particularly preferred embodiments of R 4 according to the invention are in Table P4 below, wherein each line of lines P4-1 to P4-10 corresponds to one particular embodiment of the invention. Thereby, for every R 4 that is present in the inventive compounds, these specific embodiments and preferences apply independently of the meaning of any other R 4 that may be present in the ring:
- ⁇ are in each case independently selected from hydrogen, halogen, CN, CrC 6 -alkyl, C 1 -C 6 - halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, C 2 -C 6 -alkynyl, C 2 -C 6 -halogenalkynyl, O-CrCe-alkyl, 0-C 2 -Ce-alkenyl, 0-C 2 -Ce-alkynyl, C 3 -Ce-cycloalkyl, 0-C 3 -Ce-cycloalkyl, CH 2 -C 3 -C 6 -cycloalkyl, C 3 -C 6 -cycloalkenyl, 0-C 3 -C 6 -cycloalkenyl, CH 2 -C 3 -C 6 -cycloalkenyl, wherein the acyclic moieties of R 5 , R 6
- R 5 , R 6 , R 7 , R 8 are in each case independently selected from H, halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C 2 -C 6 -alkenyl, C 2 -C 6 -halogenalkenyl, 0-CrC 6 -alkyl, C 3 -C 6 -cycloalkyl, 0-C 3 -C 6 -cycloalkyl, CH 2 -C 3 -C 6 -cycloalkyl, C 3 - C 6 -cycloalkenyl, 0-C 3 -C 6 -cycloalkenyl, CH 2 -C 3 -C 6 -cycloalkenyl.
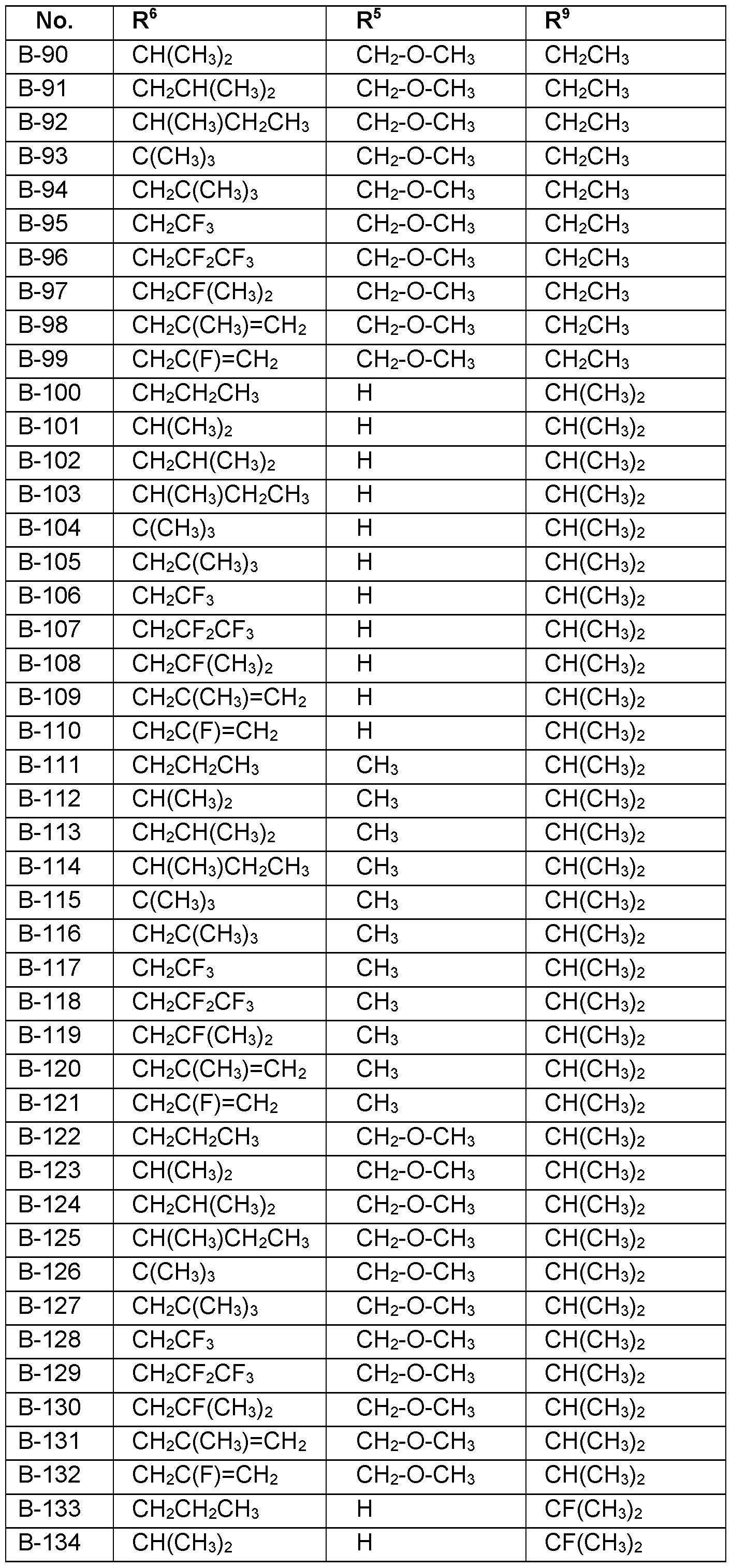
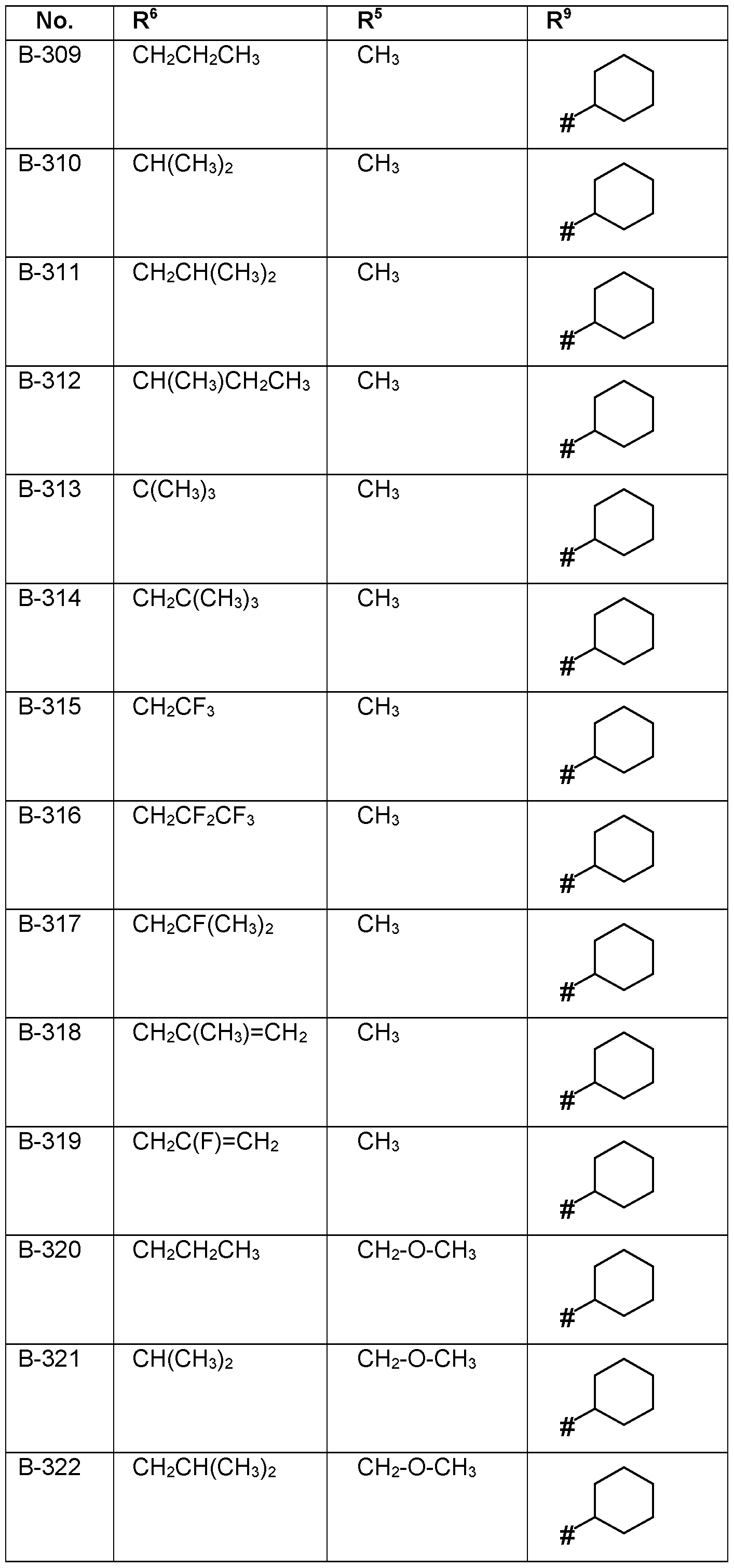
- R 5 is preferably H, CrC 6 -alkyl or CrC 6 -alkyl-0-Ci-C 6 -alkyl, more preferably H, CH 3, CH 2 OCH 3 .
- R 6 is preferably CrC 6 -alkyl, C 1 -C 4 - halogenalkyl, C 2 -C 4 -alkenyl, 0-CrC 6 -alkyl, C 2 -C 4 -halogenalkenyl, cyclopropyl, CFh-cyclopropyl.
- R 7 is preferably H or CrC 6 -alkyl, more preferably H and CH 3 .
- R 8 is preferably H or CrC 6 -alkyl, more preferably H and CH 3 .
- R 5 , R 6 , R 7 , R 8 are in each case independently H. According to still another embodiment of formula I, R 5 , R 6 , R 7 , R 8 are in each case independently halogen, preferably F or Cl, most preferably F.
- R 5 , R 6 , R 7 , R 8 are in each case independently CN.
- R 5 , R 6 , R 7 , R 8 are in each case
- CrC 6 -alkyl such as CH 3 , C 2 H 5 , n-propyl, i-propyl, n-butyl, i-butyl, tert-butyl, n- pentyl, i-pentyl or CH 2 -C(CH 3 ) 3 .
- R 5 , R 6 , R 7 , R 8 are in each case
- CrC 6 -halogenalkyl in particular CrC 4 -halogenalkyl, such as CF 3 , CC , FCH 2 , CICH2, F 2 CH, CI2CH, CF3CH2, CCI3CH2 or CF2CHF2.
- R 5 , R 6 , R 7 , R 8 are in each case
- R 5 , R 6 , R 7 , R 8 are in each case
- C 2 -C 6 -alkynyl or C 2 -C 6 -halogenalkynyl in particular C 2 -C 4 -alkynyl or C 2 -C 4 - halogenalkynyl, such as CECH, CFhCECH, CECCI, CFhCECCI, or CC CECCI.
- R 5 , R 6 , R 7 , R 8 are in each case independently 0-Ci-C 6 -alkanyl.
- R 5 , R 6 , R 7 , R 8 are in each case independently such as OCH 3 , OC 2 H 5 , O-n-propyl, O-iso-propyl, O-i-butyl, O-tert-butyl or 0-CH 2 -C(CH 3 ) 3 .
- R 5 , R 6 , R 7 , R 8 are in each case independently C 3 -C 6 -cycloalkyl, 0-C 3 -C 6 -cycloalkyl, CH 2 -C 3 -C 6 -cycloalkyl, C 3 -C 6 -cycloalkenyl, 0-C 3 -C 6 -cycloalkenyl, CH 2 -C 3 -C 6 -cycloalkenyl, wherein C 3 -C 6 -cycloalkyl is preferably selected from the group of cyclopropyl, 1-F-cyclopropyl, 1-CI-cyclopropyl, 2,2-F 2 -cyclopropyl, 2.2-CI 2 - cyclopropyl and cyclohexyl; and wherein C 3 -C 6 -cycloalkenyl is selected from the group of cyclohexen-1-yl, cyclohexen-2-yl
- R 5 , R 6 , R 7 , R 8 are in each case independently 0-C 2 -C 6 -alkenyl in particular C 2 -C 4 -alkenyl, more specifically C 2 -C 3 -alkenyl.
- R 5 , R 6 , R 7 , R 8 are in each case independently 0-C 2 -C 6 -alkenyl in particular C 2 -C 4 -alkenyl, more specifically C 2 -C 3 -alkenyl.
- R 5 , R 6 , R 7 , R 8 are in each case independently 0-C 2 -C 6 -alkynyl, in particular C 2 -C 6 -alkynyl, in particular C 2 -C 4 -alkynyl, more specifically C 2 -C 3 -alkynyl.
- R 5 , R 6 , R 7 , R 8 are in each case independently such as OCFhCECH.
- R 5 , R 6 , R 7 , R 8 are in Table P5 below, wherein each line of lines P5-1 to P5-30 corresponds to one particular embodiment of the invention, wherein P5-1 to P5-30 are also in any combination with one another a preferred embodiment of the present invention.
- the connection point to the carbon atom, to which R 5 is bound is marked with“#” in the drawings.
- R 9 is selected from the group consisting of CH 3 , CH2CH3, CH(CH 3 )2, CH(CH 3 )3, CF 3 , CF(CH 3 )2, CH 2 OCH(CH 3 )2, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, C 6 H 5 , 4-F-C 6 H 4 ; most preferred CH 3 and phenyl.
- R 9 is CrC 6 -alkyl, in particular CrC4-alkyl, such as CH 3 or C2H5, in particular CH 3 or CH2CH 3 .
- R 9 is CrC 6 -halogenalkyl, in particular C1 -C4- halogenalkyl, such as CF 3 , CC , FCH 2 , CICH 2 , F 2 CH, CI 2 CH, CF 3 CH 2 , CCI 3 CH 2 or CF 2 CHF 2 .
- R 9 is C 2 -C 6 -alkynyl or C 2 -C 6 -halogenalkynyl, in particular C 2 -C4-alkynyl or C 2 -C4-halogenalkynyl, such as CECH, CH 2 CECH, CECCI,
- R 9 is C 3 -C 6 -cycloalkyl, in particular cyclopropyl, cyclohexyl.
- R 9 is C 3 -C 6 -cycloalkenyl, in particular cyclopentenyl, or cyclohexenyl.
- R 9 is phenyl, wherein phenyl in each case is unsubstituted or substituted by identical or different groups R 9a which independently of one another are selected from halogen, CrC 2 -alkyl, CN.
- R 9 is a 5-membered heteroaryl such as pyrrol-1 -yl, pyrrol-2-yl, pyrrol-3-yl, thien-2-yl, thien-3-yl, furan-2-yl, furan-3-yl, pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl, imidazol-1-yl, imidazol-2-yl, imidazol-4-yl, imidazol-5-yl, oxazol-2-yl, oxazol4-yl, oxazol-5-yl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl, thiazol-2-yl, thiazol-4-yl, thiazol-5-yl, isothiazol-3-yl, isothiazol-4-yl, isothiazol-4
- 1.2.4-thiadiazol-5-yl preferred are pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl,
- R 9 is a 6-membered heteroaryl such as pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrimidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, pyrazin-2-yl and 1 ,3,5-triazin-2-yl and 1 ,2,4-triazin-3-yl, preferred are pyridin-2-yl, pyridin-3-yl, pyridin-4-yl.
- the 6-membered heteroaryl in each case is unsubstituted or substituted by identical or different groups R 9a which independently of one another are selected from halogen, Ci-C 2 -alkyl, CN.
- R 9 Particularly preferred embodiments of R 9 according to the invention are in Table P9 below, wherein each line of lines P9-1 to P9-104 corresponds to one particular embodiment of the invention, wherein P5-1 to P5-104 are also in any combination with one another a preferred embodiment of the present invention.
- the connection point to the carbon atom, to which R 9 is bound is marked with“#” in the drawings.
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O; A is C(R 7 R 8 );
- R 1 is H
- R 2 is selected from the group consisting of CrC 6 -alkyl, CrC 6 -halogenalkyl or 0-CrC 6 -alkyl
- R 3 is selected from the group consisting of CrC 6 -alkyl, CrC 6 -halogenalkyl or C3-C4-cycloalkyl
- R 4 is H
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O
- A is C(R 7 R 8 );
- R 1 is H
- R 2 is selected from the group consisting of CH 3 , C2H5, CF 3 , CH2F, CHF2, OCH 3 , OC2H5, O-C 3 H7, O-C4H 9 , cyclopropyl, cyclobutyl;
- R 3 is selected from the group consisting of CH 3 , C2H5, CF 3 , CH2F, CHF2, cyclopropyl, cyclobutyl;
- R 4 is H;
- R 5 , R 6 , R 7 , R 8 are independently selected from H, halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, C3-C6-cycloalkyl, 0-CrC 6 -alkyl, or two moieties:
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O
- A is C(R 7 R 8 );
- R 1 is H
- R 2 is selected from the group consisting of CH 3 , OCH 3 ;
- R 3 is selected from the group consisting of CH 3 , CF 3 , CF2H;
- R 4 is H
- R 5 is selected from the group consisting of H, F, Cl, CN, CH 3, CH2OCH 3 .
- R 6 is selected from the group consisting of CrC 6 -alkyl, CrC4-halogenalkyl, C2-C4-alkenyl, C2-C4- halogenalkenyl, cyclopropyl, CFh-cyclopropyl,
- R 7 is selected from the group consisting of H and CH 3 ;
- R 8 is selected from the group consisting of H and CH 3 .
- R 9 is selected from the group consisting of CH 3 , phenyl, pyridine-2-yl, pyridine-3-yl, pyridine-4-yl and cyclohexyl.
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O; A is a direct bond
- R 1 is H
- R 2 is selected from the group consisting of CH 3 , OCH 3 ;
- R 3 is selected from the group consisting of CH 3 , CF 3 , CF2H;
- R 4 is H
- R 5 is selected from the group consisting of H, F, Cl, CN, CH 3, CH2OCH 3 .
- R 6 is selected from the group consisting of CrC 6 -alkyl, CrC4-halogenalkyl, C2-C4-alkenyl, C2-C4- halogenalkenyl, cyclopropyl, CFh-cyclopropyl,
- R 7 is selected from the group consisting of H and CH 3 ;
- R 8 is selected from the group consisting of H and CH 3 .
- R 9 is selected from the group consisting of CH 3 , phenyl, pyridine-2-yl, pyridine-3-yl, pyridine-4-yl and cyclohexyl.
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O
- A is a direct bond
- R 1 is H
- R 2 is selected from the group consisting of CH 3 , C2H5, CF 3 , CH2F, CHF2, OCH 3 , OC2H5, O-C 3 H7, O-C4H 9 , cyclopropyl, cyclobutyl;
- R 3 is selected from the group consisting of CH 3 , C2H5, CF 3 , CH2F, CHF2, cyclopropyl, cyclobutyl;
- R 4 is H;
- R 5 , R 6 are independently selected from H, halogen, CN, CrC 6 -alkyl, CrC 6 -halogenalkyl, O-C1- C 6 -alkyl, or two moieties:
- the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
- X is O
- A is a direct bond
- R 1 is H
- R 2 is selected from the group consisting of CH 3 , OCH 3 ;
- R 3 is selected from the group consisting of CH 3 , CF 3 , CF2H;
- R 4 is H
- R 5 is selected from the group consisting of H, CH 3, CH2OCH 3 .
- R 6 is selected from the group consisting of CrC 6 -alkyl, CrC4-halogenalkyl, C2-C4-alkenyl,
- R 9 is selected from the group consisting of CH 3 and cyclopropyl.
- Preferred embodiments of the present invention are the following compounds I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6, I.A-6; compounds I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6.
- the substituents R 5 , R 6 , R 7 , R 8 and R 9 are independently as defined above or preferably defined herein:
- Table 1a Compounds of the formula I.A-1, I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1, I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CH2- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.1a.B-1 to I.A-1.1a.B-396, I.A-2.1a.B-1 to I.A-2.1a.B-396, I.A-3.1a.B-1 to I.A-3.1a.B-396, I.A-
- Table 2a Compounds of the formula I.A-1, I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1, I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CHF- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.2a.B-1 to I.A-1.2a.B-396, I.A-2.2a.B-1 to I.A-2.2a.B-396, I.A-3.2a.B-1 to I.A-3.2a.B-396, I.A-
- Table 3a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CH(CH3)- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
- Table 4a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CF(CH3)- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
- Table 5a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CF2- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.5a.B-1 to I.A-1.5a. B-396, I.A-2.5a.B-1 to I.A-2.5a.B-396, I.A-3.5a.B-1 to I.A-3.5a.B-396, I.A-
- Table 6a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -C(CH3)2- and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
- Table 7a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is direct bound and the meaning for the combination of R 5 , R 6 and R 9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-1.7a.B-1 to I.A-1.7a.B-396, I.A-2.7a.B-1 to I.A-2.7a.B-396, I.A-3.7a.B-1 to I.A-3.7a.B-396, I.A-4.7a.
- a robust method involves treatment of carboxylic acid of type II I (R 5 -R 9 and A are as defined for compounds of formula I) with an activating agent like phosgene, thionyl chloride or oxalyl chloride or an amide coupling reagent like dicyclohexylcarbodiimide in organic solvent like tetrahydrofuran (THF), dichloromethane (DCM) or dimethylformamide (DMF).
- an activating agent like phosgene, thionyl chloride or oxalyl chloride
- an amide coupling reagent like dicyclohexylcarbodiimide in organic solvent like tetrahydrofuran (THF), dichloromethane (DCM) or dimethylformamide (DMF).
- Compounds of formula (I . A) (wherein X is O and R 1 -R 9 and A are as defined for compounds of formula I) can also be prepared by the amide coupling reaction between 3- aminopyridines of type I I (R 1 -R 4 are as defined for compounds of formula I) and an ester of type IV (R 5 -R 9 and A are as defined for compounds of formula I and Y is Ci - C 6 alkyl or phenyl) in the presence of a Lewis acid such as trimethyl aluminium in an inert organic solvent like toluene under heating as shown in Scheme 2.
- a Lewis acid such as trimethyl aluminium in an inert organic solvent like toluene under heating as shown in Scheme 2.
- carboxylic acids of formula (III) (R 5 -R 9 and A are as defined for compounds of formula I) can be prepared from the corresponding esters such as compounds of formula (IV) (R 5 -R 9 and A are as defined for compounds of formula I and Y is Ci - C 6 alkyl or phenyl).
- esters such as compounds of formula (IV) (R 5 -R 9 and A are as defined for compounds of formula I and Y is Ci - C 6 alkyl or phenyl).
- alpha functionalization of these esters can be performed by deprotonation with a strong base like lithium
- carboxylic acids of formula (III) (R 5 -R 9 are as defined for compounds of formula I and A is a bond) can be prepared by various methods that involve functional group transformations, such as a triple bond can be obtained from an aldehyde using the Bestmann-Ohira reagent as described in J. Org. Chem., 1982, 47, 1837-1845).
- Other new methods for the preparation of carboxylic acids of formula (III) (R 5 -R 9 are as defined for compounds of formula I and A is a bond) are described in literatures (see:
- N-oxides may be prepared from the inventive compounds according to conventional oxidation methods as described in WO 03/64572 or J. Med. Chem. 1995,1892-903 or J.
- the compounds I and the compositions according to the invention are particularly important in the control of a multitude of phytopathogenic fungi on various cultivated plants, such as cereals, e. g. wheat, rye, barley, triticale, oats or rice; beet, e. g. sugar beet or fodder beet; fruits, such as pomes, stone fruits or soft fruits, e. g.
- compounds I and compositions thereof are used for controlling a multitude of fungi on field crops, such as potatoes sugar beets, tobacco, wheat, rye, barley, oats, rice, corn, cotton, soybeans, rape, legumes, sunflowers, coffee or sugar cane; fruits; vines; ornamentals; or vegetables, such as cucumbers, tomatoes, beans or squashes.
- field crops such as potatoes sugar beets, tobacco, wheat, rye, barley, oats, rice, corn, cotton, soybeans, rape, legumes, sunflowers, coffee or sugar cane; fruits; vines; ornamentals; or vegetables, such as cucumbers, tomatoes, beans or squashes.
- plant propagation material is to be understood to denote all the generative parts of the plant such as seeds and vegetative plant material such as cuttings and tubers (e. g.
- potatoes which can be used for the multiplication of the plant. This includes seeds, roots, fruits, tubers, bulbs, rhizomes, shoots, sprouts and other parts of plants, including seedlings and young plants, which are to be transplanted after germination or after emergence from soil.
- These young plants may also be protected before transplantation by a total or partial treatment by immersion or pouring.
- treatment of plant propagation materials with compounds I and compositions thereof, respectively is used for controlling a multitude of fungi on cereals, such as wheat, rye, barley and oats; rice, corn, cotton and soybeans.
- cultiva plants is to be understood as including plants which have been modified by mutagenesis or genetic engineering in order to provide a new trait to a plant or to modify an already present trait.
- Mutagenesis includes techniques of random mutagenesis using X-rays or mutagenic chemicals, but also techniques of targeted mutagenesis, to create mutations at a specific locus of a plant genome.
- Targeted mutagenesis techniques frequently use oligonucleotides or proteins like CRISPR/Cas, zinc-finger nucleases, TALENs or meganucleases to achieve the targeting effect.
- Genetic engineering usually uses recombinant DNA techniques to create modifications in a plant genome which under natural circumstances cannot readily be obtained by cross breeding, mutagenesis or natural recombination.
- one or more genes are integrated into the genome of a plant to add a trait or improve a trait.
- transgenic plants These integrated genes are also referred to as transgenes in the art, while plant comprising such transgenes are referred to as transgenic plants.
- the process of plant transformation usually produces several transformation events, wich differ in the genomic locus in which a transgene has been integrated. Plants comprising a specific transgene on a specific genomic locus are usually described as comprising a specific“event”, which is referred to by a specific event name. Traits which have been introduced in plants or have been modified include herbicide tolerance, insect resistance, increased yield and tolerance to abiotic conditions, like drought.
- Herbicide tolerance has been created by using mutagenesis as well as using genetic engineering. Plants which have been rendered tolerant to acetolactate synthase (ALS) inhibitor herbicides by mutagenesis and breeding comprise plant varieties commercially available under the name Clearfield®.
- ALS acetolactate synthase
- Herbicide tolerance has been created via the use of transgenes to glyphosate, glufosinate, 2,4-D, dicamba, oxynil herbicides, like bromoxynil and ioxynil, sulfonylurea herbicides, ALS inhibitors and 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors, like isoxaflutole and mesotrione.
- transgenes to glyphosate, glufosinate, 2,4-D, dicamba, oxynil herbicides, like bromoxynil and ioxynil, sulfonylurea herbicides, ALS inhibitors and 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors, like isoxaflutole and mesotrione.
- HPPD 4-hydroxyphenylpyruvate dioxygenase
- Transgenes wich have been used to provide herbicide tolerance traits comprise: for tolerance to glyphosate: cp4 epsps, epsps grg23ace5, mepsps, 2mepsps, gat4601 , gat4621 , goxv247; for tolerance to glufosinate: pat and bar, for tolerance to 2,4-D: aad-1 , aad-12; for tolerance to dicamba: dmo; for tolerance to oxynil herbicies: bxn; for tolerance to sulfonylurea herbicides: zm-hra, csr1-2, gm-hra, S4-HrA; for tolerance to ALS inhibitors: csr1-2; and for tolerance to HPPD inhibitors: hppdPF, W336, avhppd-03.
- Transgenic corn events comprising herbicide tolerance genes include, but are not limited to, DAS40278, MON801 , MON802, MON809, MON810, MON832, MON87411 , MON87419, MON87427, MON88017, MON89034, NK603, GA21 , MZHG0JG, HCEM485, VCO-01981-5, 676, 678, 680, 33121 , 4114, 59122, 98140, Bt10, Bt176, CBH-351 , DBT418, DLL25, MS3,
- Transgenic soybean events comprising herbicide tolerance genes include, but are not limited to, GTS 40-3-2, MON87705, MON87708, MON87712, MON87769, MON89788, A2704-12, A2704-21 , A5547-127, A5547-35, DP356043, DAS44406-6, DAS68416-4, DAS-81419-2, GU262, SYHT0H2, W62, W98, FG72 and CV127.
- Transgenic cotton events comprising herbicide tolerance genes include, but are not limited to, 19-51 a, 31707, 42317, 81910, 281-24-236, 3006-210-23, BXN10211 , BXN10215, BXN10222, BXN 10224, MON1445, MON1698, MON88701 , MON88913, GHB119, GHB614, LLCotton25, T303-3 and T304-40.
- Transgenic canola events comprising herbicide tolerance genes are for example, but not excluding others, MON88302, HCR-1 , HCN10, HCN28, HCN92, MS1 , MS8, PHY14, PHY23, PHY35, PHY36, RF1 , RF2 and RF3.
- Transgenes which have most frequently been used are toxin genes of Bacillus spp. and synthetic variants thereof, like cry1A, crylAb, cry1Ab-Ac, crylAc, cry1A.105, cry1 F, cry1 Fa2, cry2Ab2, cry2Ae, mcry3A, ecry3.1Ab, cry3Bb1 , cry34Ab1 , cry35Ab1 , cry9C, vip3A(a), vip3Aa20.
- genes of plant origin such as genes coding for protease inhibitors, like CpTI and pinll, have been transferred to other plants.
- a further approach uses transgenes such as dvsnf7 to produce double-stranded RNA in plants.
- Transgenic corn events comprising genes for insecticidal proteins or double stranded RNA include, but are not limited to, Bt10, Bt11 , Bt176, MON801 , MON802, MON809, MON810, MON863, MON87411 , MON88017, MON89034, 33121 , 4114, 5307, 59122, TC1507, TC6275, CBH-351 , MIR162, DBT418 and MZIR098.
- Transgenic soybean events comprising genes for insecticidal proteins include, but are not limited to, MON87701 , MON87751 and DAS-81419.
- Transgenic cotton events comprising genes for insecticidal proteins include, but are not limited to, SGK321 , MON531 , MON757, MON1076, MON15985, 31707, 31803, 31807, 31808, 42317, BN LA-601 , Eventl , COT67B, COT102, T303-3, T304-40, GFM Cry1A, GK12, MLS 9124, 281- 24-236, 3006-210-23, GHB119 and SGK321.
- transgene athb17 being present for example in corn event MON87403, or by using the transgene bbx32, being present for example in the soybean event MON87712.
- transgenes gm-fad2-1 , Pj.D6D, Nc.Fad3, fad2-1A and fatb1-A. Soybean events comprising at least one of these genes are: 260-05, MON87705 and MON87769.
- transgene cspB comprised by the corn event MON87460 and by using the transgene Hahb-4, comprised by soybean event IND-00410-5.
- Preferred combinations of traits are combinations of herbicide tolerance traits to different groups of herbicides, combinations of insect tolerance to different kind of insects, in particular tolerance to lepidopteran and coleopteran insects, combinations of herbicide tolerance with one or several types of insect resistance, combinations of herbicide tolerance with increased yield as well as combinations of herbicide tolerance and tolerance to abiotic conditions.
- CERA Cera-gmc.org/GMCropDatabase
- effects which are specific to a cultivated plant comprising a certain gene or event may result in effects which are specific to a cultivated plant comprising a certain gene or event. These effects might involve changes in growth behavior or changed resistance to biotic or abiotic stress factors. Such effects may in particular comprise enhanced yield, enhanced resistance or tolerance to insects, nematodes, fungal, bacterial, mycoplasma, viral or viroid pathogens as well as early vigour, early or delayed ripening, cold or heat tolerance as well as changed amino acid or fatty acid spectrum or content.
- the compounds I and compositions thereof, respectively, are particularly suitable for controlling the following plant diseases:
- Albugo spp. white rust on ornamentals, vegetables (e. g. A. Candida ) and sunflowers (e. g. A. tragopogonis ); Alternaria spp. (Alternaria leaf spot) on vegetables (e.g. A. dauci or A. porn), oilseed rape (A. brassicicola or brassicae), sugar beets (A. tenuis), fruits (e.g. A. grandis), rice, soybeans, potatoes and tomatoes (e. g. A. solani, A. grandis or A. alternata), tomatoes (e. g. A. solani or A. alternata) and wheat (e.g. A. triticina) ⁇ , Aphanomyces spp.
- Ascochyta spp. on cereals and vegetables e. g. A. tritici (anthracnose) on wheat and A. hordei on barley; Aureobasidium zeae (syn. Kapatiella zeae) on corn; Bipolaris and Drechslera spp. (teleomorph: Cochliobolus spp.), e. g. Southern leaf blight (D. maydis) or Northern leaf blight ( B . zeicola) on corn, e. g. spot blotch ( B . sorokiniana) on cereals and e. g.
- Botrytis cinerea teleomorph: Botryotinia fuckeliana grey mold) on fruits and berries (e. g. strawberries), vegetables (e. g. lettuce, carrots, celery and cabbages); B. squamosa
- Cochliobolus anamorph: Helminthosporium of Bipolaris
- spp. leaf spots
- cereals e. g. C. sativus, anamorph: B. sorokiniana
- rice e. g. C. miyabeanus, anamorph: H. oryzae
- Colletotrichum teleomorph: Glomerella
- spp. anthracnose on cotton (e. g. C. gossypii)
- corn e. g. C. graminicola: Anthracnose stalk rot
- soft fruits e. g.
- beans e. g. C. lindemuthianum
- soybeans e. g. C. truncatum or C. gloeosporioides
- vegetables e.g. C. lagenarium or C. capsici
- fruits e.g. C. acutatum
- coffee e.g. C. coffeanum or C. kahawae
- Corticium spp. e. g. C.
- sasakii sheath blight
- Corynespora cassiicola leaf spots
- Cycloconium spp. e. g. C. oleaginum on olive trees
- Helminthosporium, teleomorph Pyrenophora
- Phaeomoniella chlamydospora (formerly Phaeoacremonium chlamydosporum)
- Gibberella spp. on cereals e. g. G. zeae
- rice e. g. G. fujikuror. Bakanae disease
- Microdochium (syn. Fusarium) nivale (pink snow mold) on cereals (e. g. wheat or barley);
- Microsphaera diffusa (powdery mildew) on soybeans; Monilinia spp., e. g. M. laxa, M. fructicola and M. fructigena (syn. Monilia spp.: bloom and twig blight, brown rot) on stone fruits and other rosaceous plants; Mycosphaerella spp. on cereals, bananas, soft fruits and ground nuts, such as e. g. M. graminicola (anamorph: Zymoseptoria tritici formerly Septoria triticr. Septoria blotch) on wheat or M. fijiensis (syn. Pseudocercospora fijiensis ⁇ . black Sigatoka disease) and M.
- meibomiae (soybean rust) on soybeans; Phialophora spp. e. g. on vines (e. g. P. tracheiphila and P. tetraspora) and soybeans (e. g. P. gregata : stem rot); Phoma lingam (syn. Leptosphaeria biglobosa and L. maculans. root and stem rot) on oilseed rape and cabbage, P. betae (root rot, leaf spot and damping-off) on sugar beets and P. zeae-maydis (syn. Phyllostica zeae) on corn; Phomopsis spp.
- Plasmodiophora brassicae club root
- Plasmopara spp. e. g. P. viticola (grapevine downy mildew) on vines and P.
- Podosphaera spp. (powdery mildew) on rosaceous plants, hop, pome and soft fruits (e. g. P. leucotricha on apples) and curcurbits (P. xanthii ); Polymyxa spp., e. g. on cereals, such as barley and wheat (P. graminis) and sugar beets (P. betae) and thereby transmitted viral diseases; Pseudocercosporella herpotrichoides (syn. Oculimacula yallundae,
- O. acuformis eyespot, teleomorph: Tapesia yallundae) on cereals, e. g. wheat or barley;
- Pseudoperonospora downy mildew
- P. cubensis on cucurbits or P. humili on hop
- Pseudopezicula tracheiphila red fire disease or .rotbrenneR ' , anamorph
- Puccinia spp. rusts on various plants, e. g. P. triticina (brown or leaf rust), P. striiformis (stripe or yellow rust), P. hordei (dwarf rust), P. graminis (stem or black rust) or P. recondita (brown or leaf rust) on cereals, such as e. g. wheat, barley or rye, P. kuehnii (orange rust) on sugar cane and P. asparagi on asparagus; Pyrenopeziza spp., e.g. P.
- R. solani root and stem rot
- R. solani sheath blight
- R. cerealis Rhizoctonia spring blight
- Rhizopus stolonifer black mold, soft rot
- strawberries carrots, cabbage, vines and tomatoes
- Rhynchosporium secalis and R. commune scald
- Sarocladium oryzae and S. attenuatum (sheath rot) on rice
- Sclerotinia spp. stem rot or white mold
- vegetables S. minor and S. sclerotiorum
- field crops such as oilseed rape, sunflowers (e. g. S. sclerotiorum) and soybeans, S. rolfsii (syn.
- Athelia rolfsii on soybeans, peanut, vegetables, corn, cereals and ornamentals; Septoria spp. on various plants, e. g. S. glycines (brown spot) on soybeans, S. tritici (syn. Zymoseptoria tritici, Septoria blotch) on wheat and S. (syn. Stagonospora) nodorum (Stagonospora blotch) on cereals; Uncinula (syn. Erysiphe) necator ( powdery mildew, anamorph: Oidium tuckeri) on vines; Setosphaeria spp. (leaf blight) on corn (e. g.
- S. turcicum syn. Helminthosporium turcicum
- turf Sphacelotheca spp. (smut) on corn, (e. g. S. reiliana, syn. Ustilago reiliana. head smut), sorghum und sugar cane;
- Sphaerotheca fuliginea (syn. Podosphaera xanthir. powdery mildew) on cucurbits; Spongospora subterranea (powdery scab) on potatoes and thereby transmitted viral diseases; Stagonospora spp. on cereals, e. g. S. nodorum (Stagonospora blotch, teleomorph: Leptosphaeria [syn.
- phaseoli sugar beets (e. g. U. betae or U. beticola ) and on pulses (e.g. U. vignae, U. pisi, U. viciae-fabae and U. fabae) ⁇ Ustilago spp. (loose smut) on cereals (e. g. U. nuda and U.
- the compounds I, their mixtures with other active compounds as defined herein and compositions thereof, respectively, are particularly suitable for controlling the following plant diseases: Puccinia spp. (rusts) on various plants, for example, but not limited to P. triticina (brown or leaf rust), P. striiformis (stripe or yellow rust), P. hordei (dwarf rust), P. graminis (stem or black rust) or P. recondita (brown or leaf rust) on cereals, such as e. g. wheat, barley or rye, P. sorghi (common rust) on maize, P. polysora (southern rust) on maize; P.
- Puccinia spp. rusts
- rusts rusts
- P. triticina brown or leaf rust
- P. striiformis stripe or yellow rust
- P. hordei dwarf rust
- coronata e.g. on oats, P. sorghi und P. polysora on corn
- Puccinia spp. on other crops, e.g. P. heliathi on sunflower, P. arachidis on peanuts
- Uromyces spp. on pulses and other crops crops, e.g. Uromyces viciae-fabae, Uromyces vigniae, Uromyces pisi, U. ciceris-arietini, U. betae syn U. beticola
- Phakopsoraceae spp. on various plants, in particular Phakopsora pachyrhizi and P. meibomiae (soybean rust) on soybeans.
- Fungicide-resistant strains of the above-mentioned phytopathgenic fungi have been reported, with strains resistant to one or more fungicides from various fungicidal mode of action classes being observed including but not limited to beta-tubulin assembly inhibitors, sterol
- DMI demethylation-inhibitors
- Qol quinone-outside-inhibitors
- SDHI succinate dehydrogenase inhibitors
- the compounds I and compositions thereof, respectively, are also suitable for controlling harmful fungi in the protection of stored products or harvest and in the protection of materials.
- protection of materials is to be understood to denote the protection of technical and non-living materials, such as adhesives, glues, wood, paper and paperboard, textiles, leather, paint dispersions, plastics, cooling lubricants, fiber or fabrics, against the infestation and destruction by harmful microorganisms, such as fungi and bacteria.
- Ascomycetes such as Ophiostoma spp., Ceratocystis spp., Aureobasidium pullulans, Sclerophoma spp., Chaetomium spp., Humicola spp., Petriella spp., Trichurus spp.; Basidiomycetes such as Coniophora spp., Coriolus spp., Gloeophyllum spp., Lentinus spp., Pleurotus spp., Poria spp., Serpula spp.
- yeast fungi are worthy of note: Candida spp. and Saccharomyces cerevisae.
- the method of treatment according to the invention can also be used in the field of protecting stored products or harvest against attack of fungi and microorganisms.
- the term "stored products” is understood to denote natural substances of plant or animal origin and their processed forms, which have been taken from the natural life cycle and for which long-term protection is desired.
- Stored products of crop plant origin such as plants or parts thereof, for example stalks, leafs, tubers, seeds, fruits or grains, can be protected in the freshly harvested state or in processed form, such as pre-dried, moistened, comminuted, ground, pressed or roasted, which process is also known as post-harvest treatment.
- Also falling under the definition of stored products is timber, whether in the form of crude timber, such as construction timber, electricity pylons and barriers, or in the form of finished articles, such as furniture or objects made from wood.
- Stored products of animal origin are hides, leather, furs, hairs and the like. The combinations according the present invention can prevent
- stored products is understood to denote natural substances of plant origin and their processed forms, more preferably fruits and their processed forms, such as pomes, stone fruits, soft fruits and citrus fruits and their processed forms.
- the compounds I and compositions thereof, respectively, may be used for improving the health of a plant.
- the invention also relates to a method for improving plant health by treating a plant, its propagation material and/or the locus where the plant is growing or is to grow with an effective amount of compounds I and compositions thereof, respectively.
- plant health is to be understood to denote a condition of the plant and/or its products which is determined by several indicators alone or in combination with each other such as yield (e. g. increased biomass and/or increased content of valuable ingredients), plant vigor (e. g. improved plant growth and/or greener leaves (“greening effect”)), quality (e. g. improved content or composition of certain ingredients) and tolerance to abiotic and/or biotic stress.
- yield e. g. increased biomass and/or increased content of valuable ingredients
- plant vigor e. g. improved plant growth and/or greener leaves (“greening effect”)
- quality e. g. improved content or composition of certain ingredients
- tolerance to abiotic and/or biotic stress e. g. improved content or composition of certain ingredients
- the compounds of formula I can be present in different crystal modifications whose biological activity may differ. They are likewise subject matter of the present invention.
- the compounds I are employed as such or in form of compositions by treating the fungi or the plants, plant propagation materials, such as seeds, soil, surfaces, materials or rooms to be protected from fungal attack with a fungicidally effective amount of the active substances.
- the application can be carried out both before and after the infection of the plants, plant propagation materials, such as seeds, soil, surfaces, materials or rooms by the fungi.
- Plant propagation materials may be treated with compounds I as such or a composition comprising at least one compound I prophylactically either at or before planting or transplanting.
- the invention also relates to agrochemical compositions comprising an auxiliary and at least one compound I according to the invention.
- An agrochemical composition comprises a fungicidally effective amount of a compound I.
- effective amount denotes an amount of the composition or of the compounds I, which is sufficient for controlling harmful fungi on cultivated plants or in the protection of materials and which does not result in a substantial damage to the treated plants. Such an amount can vary in a broad range and is dependent on various factors, such as the fungal species to be controlled, the treated cultivated plant or material, the climatic conditions and the specific compound I used.
- compositions e. g. solutions, emulsions, suspensions, dusts, powders, pastes, granules, pressings, capsules, and mixtures thereof.
- composition types are suspensions (e. g. SC, OD, FS), emulsifiable concentrates (e. g. EC), emulsions (e. g. EW, EO, ES, ME), capsules (e. g. CS, ZC), pastes, pastilles, wettable powders or dusts (e. g. WP, SP, WS, DP, DS), pressings (e. g.
- compositions types are defined in the “Catalogue of pesticide formulation types and international coding system”, Technical
- compositions are prepared in a known manner, such as described by Mollet and
- Suitable auxiliaries are solvents, liquid carriers, solid carriers or fillers, surfactants,
- dispersants dispersants, emulsifiers, wetters, adjuvants, solubilizers, penetration enhancers, protective colloids, adhesion agents, thickeners, humectants, repellents, attractants, feeding stimulants, compatibilizers, bactericides, anti-freezing agents, anti-foaming agents, colorants, tackifiers and binders.
- Suitable solvents and liquid carriers are water and organic solvents, such as mineral oil fractions of medium to high boiling point, e. g. kerosene, diesel oil; oils of vegetable or animal origin; aliphatic, cyclic and aromatic hydrocarbons, e. g. toluene, paraffin,
- tetrahydronaphthalene alkylated naphthalenes
- alcohols e. g. ethanol, propanol, butanol, benzyl alcohol, cyclohexanol
- glycols DMSO; ketones, e. g. cyclohexanone; esters, e. g.
- lactates carbonates, fatty acid esters, gamma-butyrolactone; fatty acids; phosphonates;
- amines e. g. N-methyl pyrrolidone, fatty acid dimethyl amides; and mixtures thereof.
- Suitable solid carriers or fillers are mineral earths, e. g. silicates, silica gels, talc, kaolins, limestone, lime, chalk, clays, dolomite, diatomaceous earth, bentonite, calcium sulfate, magnesium sulfate, magnesium oxide; polysaccharides, e. g. cellulose, starch; fertilizers, e. g. ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas; products of vegetable origin, e. g. cereal meal, tree bark meal, wood meal, nutshell meal, and mixtures thereof.
- mineral earths e. g. silicates, silica gels, talc, kaolins, limestone, lime, chalk, clays, dolomite, diatomaceous earth, bentonite, calcium sulfate, magnesium sulfate, magnesium oxide
- polysaccharides e. g. cellulose, star
- Suitable surfactants are surface-active compounds, such as anionic, cationic, nonionic and amphoteric surfactants, block polymers, polyelectrolytes, and mixtures thereof. Such surfactants can be used as emulsifier, dispersant, solubilizer, wetter, penetration enhancer, protective colloid, or adjuvant. Examples of surfactants are listed in McCutcheon’s, Vol.1 : Emulsifiers & Detergents, McCutcheon’s Directories, Glen Rock, USA, 2008 (International Ed. or North American Ed.).
- Suitable anionic surfactants are alkali, alkaline earth or ammonium salts of sulfonates, sulfates, phosphates, carboxylates, and mixtures thereof.
- sulfonates are alkylaryl sulfonates, diphenyl sulfonates, alpha-olefin sulfonates, lignin sulfonates, sulfonates of fatty acids and oils, sulfonates of ethoxylated alkylphenols, sulfonates of alkoxylated arylphenols, sulfonates of condensed naphthalenes, sulfonates of dodecyl- and tridecylbenzenes, sulfonates of naphthalenes and alkyl naphthalenes, sulfosuccinates or sulfosuccinamates.
- Examples of sulfates are sulfates of fatty acids and oils, of ethoxylated alkylphenols, of alcohols, of ethoxylated alcohols, or of fatty acid esters.
- Examples of phosphates are phosphate esters.
- Examples of carboxylates are alkyl carboxylates, and carboxylated alcohol or alkylphenol ethoxylates.
- Suitable nonionic surfactants are alkoxylates, N-substituted fatty acid amides, amine oxides, esters, sugar-based surfactants, polymeric surfactants, and mixtures thereof.
- alkoxylates are compounds such as alcohols, alkylphenols, amines, amides, arylphenols, fatty acids or fatty acid esters which have been alkoxylated with 1 to 50 equivalents.
- Ethylene oxide and/or propylene oxide may be employed for the alkoxylation, preferably ethylene oxide.
- N-substituted fatty acid amides are fatty acid glucamides or fatty acid
- esters are fatty acid esters, glycerol esters or monoglycerides.
- sugar-based surfactants are sorbitans, ethoxylated sorbitans, sucrose and glucose esters or alkylpolyglucosides.
- polymeric surfactants are home- or copolymers of vinyl pyrrolidone, vinyl alcohols, or vinyl acetate.
- Suitable cationic surfactants are quaternary surfactants, for example quaternary ammonium compounds with one or two hydrophobic groups, or salts of long-chain primary amines.
- Suitable amphoteric surfactants are alkylbetains and imidazolines.
- Suitable block polymers are block polymers of the A-B or A-B-A type comprising blocks of polyethylene oxide and polypropylene oxide, or of the A-B-C type comprising alkanol, polyethylene oxide and polypropylene oxide.
- Suitable polyelectrolytes are polyacids or polybases. Examples of polyacids are alkali salts of polyacrylic acid or polyacid comb polymers. Examples of polybases are polyvinyl amines or polyethylene amines.
- Suitable adjuvants are compounds, which have a negligible or even no pesticidal activity themselves, and which improve the biological performance of the compound I on the target.
- examples are surfactants, mineral or vegetable oils, and other auxiliaries. Further examples are listed by Knowles, Adjuvants and additives, Agrow Reports DS256, T&F Informa UK, 2006, chapter 5.
- Suitable thickeners are polysaccharides (e. g. xanthan gum, carboxymethyl cellulose), inorganic clays (organically modified or unmodified), polycarboxylates, and silicates.
- Suitable bactericides are bronopol and isothiazolinone derivatives such as alkyliso- thiazolinones and benzisothiazolinones.
- Suitable anti-freezing agents are ethylene glycol, propylene glycol, urea and glycerin.
- Suitable anti-foaming agents are silicones, long chain alcohols, and salts of fatty acids.
- Suitable colorants are pigments of low water solubility and water- soluble dyes.
- examples are inorganic colorants (e. g. iron oxide, titan oxide, iron
- organic colorants e. g. alizarin-, azo- and phthalocyanine colorants.
- Suitable tackifiers or binders are polyvinyl pyrrolidones, polyvinyl acetates, polyvinyl alcohols, polyacrylates, biological or synthetic waxes, and cellulose ethers.
- composition types and their preparation are:
- a compound I and 5-15 wt% wetting agent e. g. alcohol alkoxylates
- a water-soluble solvent e. g. alcohols
- a compound I and 1-10 wt% dispersant e. g. polyvinyl pyrrolidone
- organic solvent e. g. cyclohexanone
- EC Emulsifiable concentrates
- emulsifiers e. g. calcium dodecylbenzenesulfonate and castor oil ethoxylate
- water-insoluble organic solvent e. g. aromatic hydrocarbon
- Emulsions (EW, EO, ES)
- emulsifiers e. g. calcium dodecylbenzenesulfonate and castor oil ethoxylate
- 20-40 wt% water-insoluble organic solvent e. g. aromatic hydrocarbon
- This mixture is introduced into water ad 100 wt% by means of an emulsifying machine and made into a homogeneous emulsion. Dilution with water gives an emulsion.
- a compound I In an agitated ball mill, 20-60 wt% of a compound I are comminuted with addition of 2-10 wt% dispersants and wetting agents (e. g. sodium lignosulfonate and alcohol ethoxylate), 0.1-2 wt% thickener (e. g. xanthan gum) and water ad 100 wt% to give a fine active substance suspension. Dilution with water gives a stable suspension of the active substance. For FS type composition up to 40 wt% binder (e. g. polyvinyl alcohol) is added.
- dispersants and wetting agents e. g. sodium lignosulfonate and alcohol ethoxylate
- 0.1-2 wt% thickener e. g. xanthan gum
- a compound I 50-80 wt% of a compound I are ground finely with addition of dispersants and wetting agents (e. g. sodium lignosulfonate and alcohol ethoxylate) ad 100 wt% and prepared as water- dispersible or water-soluble granules by means of technical appliances (e. g. extrusion, spray tower, fluidized bed). Dilution with water gives a stable dispersion or solution of the active substance.
- dispersants and wetting agents e. g. sodium lignosulfonate and alcohol ethoxylate
- dispersants e. g. sodium lignosulfonate
- 1-3 wt% wetting agents e. g. alcohol ethoxylate
- solid carrier e. g. silica gel
- a compound I In an agitated ball mill, 5-25 wt% of a compound I are comminuted with addition of 3-10 wt% dispersants (e. g. sodium lignosulfonate), 1-5 wt% thickener (e. g. carboxymethyl cellulose) and water ad 100 wt% to give a fine suspension of the active substance. Dilution with water gives a stable suspension of the active substance.
- dispersants e. g. sodium lignosulfonate
- 1-5 wt% thickener e. g. carboxymethyl cellulose
- wt% of a compound I are added to 5-30 wt% organic solvent blend (e. g. fatty acid dimethyl amide and cyclohexanone), 10-25 wt% surfactant blend (e. g. alcohol ethoxylate and arylphenol ethoxylate), and water ad 100 %. This mixture is stirred for 1 h to produce spontaneously a thermodynamically stable microemulsion.
- organic solvent blend e. g. fatty acid dimethyl amide and cyclohexanone
- surfactant blend e. g. alcohol ethoxylate and arylphenol ethoxylate
- An oil phase comprising 5-50 wt% of a compound I, 0-40 wt% water insoluble organic solvent (e. g. aromatic hydrocarbon), 2-15 wt% acrylic monomers (e. g. methylmethacrylate, methacrylic acid and a di- or triacrylate) are dispersed into an aqueous solution of a protective colloid (e. g. polyvinyl alcohol). Radical polymerization results in the formation of poly(meth)acrylate microcapsules.
- an oil phase comprising 5-50 wt% of a compound I according to the invention, 0-40 wt% water insoluble organic solvent (e. g. aromatic hydrocarbon), and an isocyanate monomer (e. g. diphenylmethene-4,4’-diisocyanatae) are dispersed into an aqueous solution of a protective colloid (e. g. polyvinyl alcohol).
- a polyamine e. g.
- hexamethylenediamine results in the formation of polyurea microcapsules.
- the monomers amount to 1-10 wt%.
- the wt% relate to the total CS composition.
- Dustable powders (DP, DS)
- 1-10 wt% of a compound I are ground finely and mixed intimately with solid carrier (e. g. finely divided kaolin) ad 100 wt%.
- solid carrier e. g. finely divided kaolin
- a compound I 0.5-30 wt% of a compound I is ground finely and associated with solid carrier (e. g. silicate) ad 100 wt%.
- solid carrier e. g. silicate
- Granulation is achieved by extrusion, spray-drying or fluidized bed.
- organic solvent e. g. aromatic hydrocarbon
- compositions types i) to xiii) may optionally comprise further auxiliaries, such as 0.1-1 wt% bactericides, 5-15 wt% anti-freezing agents, 0.1-1 wt% anti-foaming agents, and 0.1-1 wt% colorants.
- auxiliaries such as 0.1-1 wt% bactericides, 5-15 wt% anti-freezing agents, 0.1-1 wt% anti-foaming agents, and 0.1-1 wt% colorants.
- the agrochemical compositions generally comprise between 0.01 and 95%, preferably between 0.1 and 90%, more preferably between 1 and 70%, and in particular between 10 and 60%, by weight of active substance.
- the active substances are employed in a purity of from 90% to 100%, preferably from 95% to 100% (according to NMR spectrum).
- compositions in question give, after two-to-tenfold dilution, active substance concentrations of from 0.01 to 60% by weight, preferably from 0.1 to 40%, in the ready-to-use preparations.
- Methods for applying compound I and compositions thereof, respectively, onto plant propagation material, especially seeds include dressing, coating, pelleting, dusting, and soaking as well as in-furrow application methods.
- compound I or the compositions thereof, respectively are applied on to the plant propagation material by a method such that germination is not induced, e. g. by seed dressing, pelleting, coating and dusting.
- the amounts of active substances applied are, depending on the kind of effect desired, from 0.001 to 2 kg per ha, preferably from 0.005 to 2 kg per ha, more preferably from 0.05 to 0.9 kg per ha, and in particular from 0.1 to 0.75 kg per ha.
- amounts of active substance of from 0.1 to 1000 g, preferably from 1 to 1000 g, more preferably from 1 to 100 g and most preferably from 5 to 100 g, per 100 kilogram of plant propagation material (preferably seeds) are generally required.
- the amount of active substance applied depends on the kind of application area and on the desired effect. Amounts customarily applied in the protection of materials are 0.001 g to 2 kg, preferably 0.005 g to 1 kg, of active substance per cubic meter of treated material.
- oils, wetters, adjuvants, fertilizer, or micronutrients, and further pesticides may be added to the active substances or the compositions comprising them as premix or, if appropriate not until immediately prior to use (tank mix).
- pesticides e. g. herbicides, insecticides, fungicides, growth regulators, safeners, biopesticides
- These agents can be admixed with the compositions according to the invention in a weight ratio of 1 :100 to 100:1 , preferably 1 :10 to 10:1.
- a pesticide is generally a chemical or biological agent (such as pestidal active ingredient, compound, composition, virus, bacterium, antimicrobial or disinfectant) that through its effect deters, incapacitates, kills or otherwise discourages pests.
- Target pests can include insects, plant pathogens, weeds, mollusks, birds, mammals, fish, nematodes (roundworms), and microbes that destroy property, cause nuisance, spread disease or are vectors for disease.
- pesticide includes also plant growth regulators that alter the expected growth, flowering, or reproduction rate of plants; defoliants that cause leaves or other foliage to drop from a plant, usually to facilitate harvest; desiccants that promote drying of living tissues, such as unwanted plant tops; plant activators that activate plant physiology for defense of against certain pests; safeners that reduce unwanted herbicidal action of pesticides on crop plants; and plant growth promoters that affect plant physiology e.g. to increase plant growth, biomass, yield or any other quality parameter of the harvestable goods of a crop plant.
- composition according to the invention usually from a predosage device, a knapsack sprayer, a spray tank, a spray plane, or an irrigation system.
- a predosage device usually from a knapsack sprayer, a spray tank, a spray plane, or an irrigation system.
- agrochemical composition is made up with water, buffer, and/or further auxiliaries to the desired application concentration and the ready-to-use spray liquor or the agrochemical composition according to the invention is thus obtained.
- 20 to 2000 liters, preferably 50 to 400 liters, of the ready-to-use spray liquor are applied per hectare of agricultural useful area.
- composition according to the invention such as parts of a kit or parts of a binary or ternary mixture may be mixed by the user himself in a spray tank or any other kind of vessel used for applications (e. g. seed treater drums, seed pelleting machinery, knapsack sprayer) and further auxiliaries may be added, if appropriate.
- a spray tank or any other kind of vessel used for applications (e. g. seed treater drums, seed pelleting machinery, knapsack sprayer) and further auxiliaries may be added, if appropriate.
- one embodiment of the invention is a kit for preparing a usable pesticidal composition, the kit comprising a) a composition comprising component 1) as defined herein and at least one auxiliary; and b) a composition comprising component 2) as defined herein and at least one auxiliary; and optionally c) a composition comprising at least one auxiliary and optionally a further active component 3) as defined herein.
- pesticides II e. g. pesticidally-active substances and biopesticides
- the compounds I in conjunction with which the compounds I can be used, is intended to illustrate the possible combinations but does not limit them:
- coumoxystrobin (A.1.3), dimoxystrobin (A.1.4), enestroburin (A.1.5), fenaminstrobin (A.1.6), fenoxystrobin/flufenoxystrobin (A.1.7), fluoxastrobin (A.1.8), kresoxim-methyl (A.1.9), mandestrobin (A.1.10), metominostrobin (A.1.11), orysastrobin (A.1.12), picoxystrobin
- respiration inhibitors diflumetorim (A.4.1); nitrophenyl derivates: binapacryl (A.4.2), dinobuton (A.4.3), dinocap (A.4.4), fluazinam (A.4.5), meptyldinocap (A.4.6), ferimzone (A.4.7); organometal compounds: fentin salts, e. g. fentin-acetate (A.4.8), fentin chloride (A.4.9) or fentin hydroxide (A.4.10); ametoctradin (A.4.11); silthiofam (A.4.12);
- benalaxyl (C.1.1), benalaxyl-M (C.1.2), kiralaxyl (C.1.3), metalaxyl (C.1.4), metalaxyl-M (C.1.5), ofurace (C.1.6), oxadixyl (C.1.7);
- nucleic acid synthesis inhibitors hymexazole (C.2.1), octhilinone (C.2.2), oxolinic acid (C.2.3), bupirimate (C.2.4), 5-fluorocytosine (C.2.5), 5-fluoro-2-(p-tolylmethoxy)pyrimidin- 4-amine (C.2.6), 5-fluoro-2-(4-fluorophenylmethoxy)pyrimidin-4-amine (C.2.7), 5-fluoro- 2-(4-chlorophenylmethoxy)pyrimidin-4 amine (C.2.8);
- tubulin inhibitors benomyl (D.1.1), carbendazim (D.1.2), fuberidazole (D1.3), thiabendazole (D.1.4), thiophanate-methyl (D.1.5), pyridachlometyl (D.1.6), A/-ethyl-2-[(3-ethynyl-8-methyl- 6-quinolyl)oxy]butanamide (D.1.8), A/-ethyl-2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-2- methylsulfanyl-acetamide (D.1.9), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/ ⁇ /-(2- fluoroethyl)butanamide (D.1.10), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/ ⁇ /-(2-fluoroethyl)-2- methoxy-acetamide (D.1.11)
- diethofencarb (D.2.1), ethaboxam (D.2.2), pencycuron (D.2.3), fluopicolide (D.2.4), zoxamide (D.2.5), metrafenone (D.2.6), pyriofenone (D.2.7),
- cyprodinil E.1.1
- mepanipyrim E.1.2
- pyrimethanil E.1.3
- fluoroimid F.1.1
- iprodione F.1.2
- procymidone F.1.3
- vinclozolin F.1.4
- fludioxonil F.1.5
- quinoxyfen F.2.1
- edifenphos (G .1.1), iprobenfos (G.1.2), pyrazophos (G.1.3), isoprothiolane (G.1.4);
- dicloran G.2.1
- quintozene G.2.2
- tecnazene G.2.3
- tolclofos-methyl G.2.4
- biphenyl G.2.5
- chloroneb G.2.6
- etridiazole G.2.7
- zinc thiazole G.2.8
- dimethomorph G.3.1
- flumorph G.3.2
- mandipropamid G.3.3
- pyrimorph G.3.4
- benthiavalicarb G.3.5
- iprovalicarb G.3.6
- valifenalate G.3.7
- propamocarb (G.4.1);
- oxathiapiprolin G.5.1
- fluoxapiprolin G.5.3
- 4-[1-[2- [3-(difluoromethyl)-5-methyl-pyrazol-1-yl]acetyl]-4-piperidyl]-/ ⁇ /-tetralin-1-yl-pyridine-2- carboxamide G.5.4
- 4-[1-[2-[3,5-bis(difluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/ ⁇ /- tetralin-1-yl-pyridine-2-carboxamide G.5.5
- 4-[1-[2-[3-(difluoromethyl)-5-(tri- fluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/ ⁇ /-tetralin-1-yl-pyridine-2-carboxamide G.5.6
- organochlorine compounds anilazine (H.3.1), chlorothalonil (H.3.2), captafol (H.3.3), captan (H.3.4), folpet (H.3.5), dichlofluanid (H.3.6), dichlorophen (H.3.7), hexachlorobenzene (H.3.8), pentachlorphenole (H.3.9) and its salts, phthalide (H.3.10), tolylfluanid (H.3.11);
- guanidine H.4.1
- dodine H.4.2
- dodine free base H.4.3
- guazatine H.4.4
- guazatine-acetate H.4.5
- iminoctadine H.4.6
- iminoctadine-triacetate H.4.7
- iminoctadine-tris(albesilate) H.4.8
- dithianon H.4.9
- 2,6-dimethyl-1H,5H-[1 ,4]di- thiino[2,3-c:5,6-c']dipyrrole-1 ,3,5,7(2H,6H)-tetraone H.4.10);
- - melanin synthesis inhibitors pyroquilon (1.2.1), tricyclazole (1.2.2), carpropamid (1.2.3), dicyclomet (1.2.4), fenoxanil (1.2.5);
- abscisic acid M.1.1
- amidochlor ancymidol
- 6-benzylaminopurine brassinolide, butralin
- chlormequat chlormequat chloride, choline chloride, cyclanilide, daminozide, dikegulac, dimethipin, 2,6-dimethylpuridine, ethephon, flumetralin, flurprimidol, fluthiacet, forchlorfenuron, gibberellic acid, inabenfide, indole-3-acetic acid , maleic hydrazide, mefluidide, mepiquat, mepiquat chloride, naphthaleneacetic acid, A/-6-benzyladenine, paclobutrazol, prohexadione, prohexadione-calcium, prohydrojasmon, thidiazuron, triapenthenol, tributyl phosphorotrithioate, 2,3,5-
- Lipid biosynthesis inhibitors alloxydim, alloxydim-sodium, butroxydim, clethodim,
- clodinafop clodinafop-propargyl, cycloxydim, cyhalofop, cyhalofop-butyl, diclofop, diclofop- methyl, fenoxaprop, fenoxaprop-ethyl, fenoxaprop-P, fenoxaprop-P-ethyl, fluazifop, fluazifop- butyl, fluazifop-P, fluazifop-P-butyl, haloxyfop, haloxyfop-methyl, haloxyfop-P, haloxyfop-P- methyl, metamifop, pinoxaden, profoxydim, propaquizafop, quizalofop, quizalofop-ethyl, quizalofop-tefuryl, quizalofop-P, quizalofop-P-ethyl,
- N.2 ALS inhibitors amidosulfuron, azimsulfuron, bensulfuron, bensulfuron-methyl, chlorimuron, chlorimuron-ethyl, chlorsulfuron, cinosulfuron, cyclosulfamuron, ethametsulfuron, ethamet- sulfuron-methyl, ethoxysulfuron, flazasulfuron, flucetosulfuron, flupyrsulfuron, flupyrsulfuron- methyl-sodium, foramsulfuron, halosulfuron, halosulfuron-methyl, imazosulfuron, iodosulfu- ron, iodosulfuron-methyl-sodium, iofensulfuron, iofensulfuron-sodium, mesosulfuron, meta- zosulfuron, metsulfuron, metsulfuron-methyl, nic
- N.3 Photosynthesis inhibitors amicarbazone; chlorotriazine; ametryn, atrazine, chloridazone, cyanazine, desmetryn, dimethametryn.hexazinone, metribuzin, prometon, prometryn, pro- pazine, simazine, simetryn, terbumeton, terbuthylazin, terbutryn, trietazin; chlorobromuron, chlorotoluron, chloroxuron, dimefuron, diuron, fluometuron, isoproturon, isouron, linuron, metamitron, methabenzthiazuron, metobenzuron, metoxuron, monolinuron, neburon, sidu- ron, tebuthiuron, thiadiazuron, desmedipham, karbutilat, phenmedipham, phenmedipham- ethyl, bromofenoxim, brom
- N.4 protoporphyrinogen-IX oxidase inhibitors acifluorfen, acifluorfen-sodium, azafenidin, ben- carbazone, benzfendizone, bifenox, butafenacil, carfentrazone, carfentrazone-ethyl, chlor- methoxyfen, cinidon-ethyl, fluazolate, flufenpyr, flufenpyr-ethyl, flumiclorac, flumiclorac-pen- tyl, flumioxazin, fluoroglycofen, fluoroglycofen-ethyl, fluthiacet, fluthiacet-methyl, fomesafen, halosafen, lactofen, oxadiargyl, oxadiazon, oxyfluorfen, pentoxazone, profluazol, pyraclonil, pyraflufen, pyraflufen
- N.5 Bleacher herbicides beflubutamid, diflufenican, fluridone, flurochloridone, flurtamone,
- norflurazon picolinafen, 4-(3-trifluoromethyhphenoxy)-2-(4-trifluoromethylphenyl)pyrimidine (180608-33-7); benzobicyclon, benzofenap, bicyclopyrone, clomazone, fenquintrione, isoxaflutole, mesotrione, pyrasulfotole, pyrazolynate, pyrazoxyfen, sulcotrione, tefuryltrione, tembotrione, tolpyralate, topramezone; aclonifen, amitrole, flumeturon;
- N.6 EPSP synthase inhibitors glyphosate, glyphosate-isopropylammonium, glyposate- potassium, glyphosate-trimesium (sulfosate);
- Glutamine synthase inhibitors bilanaphos (bialaphos), bilanaphos-sodium, glufosinate, glufosinate-P, glufosinate-ammonium;
- Mitosis inhibitors benfluralin, butralin, dinitramine, ethalfluralin, fluchloralin, oryzalin, pen- dimethalin, prodiamine, trifluralin; amiprophos, amiprophos-methyl, butamiphos; chlorthal, chlorthal-dimethyl, dithiopyr, thiazopyr, propyzamide, tebutam; carbetamide, chlorpropham, flamprop, flamprop-isopropyl, flamprop-methyl, flamprop-M-isopropyl, flamprop-M-methyl, propham;
- N.10 VLCFA inhibitors acetochlor, alachlor, butachlor, dimethachlor, dimethenamid, dimethen- amid-P, metazachlor, metolachlor, metolachlor-S, pethoxamid, pretilachlor, propachlor, prop- isochlor, thenylchlor, flufenacet, mefenacet, diphenamid, naproanilide, napropamide, napro- pamide-M, fentrazamide, anilofos, cafenstrole, fenoxasulfone, ipfencarbazone, piperophos, pyroxasulfone, isoxazoline compounds of the formulae 11.1 , II.2, II.3, II.4, II.5, II.6, II.7, II.8 and II.9
- N.11 Cellulose biosynthesis inhibitors chlorthiamid, dichlobenil, flupoxam, indaziflam, isoxaben, triaziflam, 1-cyclohexyl-5-pentafluorphenyloxy-14-[1 ,2,4,6]thiatriazin-3-ylamine (175899-01- 1);
- N.12 Decoupler herbicides dinoseb, dinoterb, DNOC and its salts
- N.13 Auxinic herbicides 2,4-D and its salts and esters, clacyfos, 2,4-DB and its salts and
- esters aminocyclopyrachlor and its salts and esters, aminopyralid and its salts such as aminopyralid-dimethylammonium, aminopyralid-tris(2-hydroxypropyl)ammonium and its esters, benazolin, benazolin-ethyl, chloramben and its salts and esters, clomeprop, clopy- ralid and its salts and esters, dicamba and its salts and esters, dichlorprop and its salts and esters, dichlorprop-P and its salts and esters, fluroxypyr, fluroxypyr-butometyl, fluroxypyr- meptyl, halauxifen and its salts and esters (943832-60-8); MCPA and its salts and esters, MCPA-thioethyl, MCPB and its salts and esters, mecoprop and its salts and esters, meco- prop-P and its salts and esters, picloram and
- Acetylcholine esterase (AChE) inhibitors aldicarb (0.1.1), alanycarb (0.1.2), bendiocarb
- GABA-gated chloride channel antagonists endosulfan (0.2.1), chlordane (0.2.2), ethiprole (0.2.3), fipronil (0.2.4), flufiprole (0.2.5), pyrafluprole (0.2.6), pyriprole (0.2.7);
- Nicotinic acetylcholine receptor agonists acetamiprid (0.4.1), clothianidin (0.4.2), cycloxaprid (0.4.3), dinotefuran (0.4.4), imidacloprid (0.4.5), nitenpyram (0.4.6), thiacloprid (0.4.7), thiamethoxam (0.4.8), 4,5-dihydro-/ ⁇ /-nitro-1-(2-oxiranylmethyl)-1/-/-imidazol-2-amine (0.4.9), (2£)-1-[(6-chloropyridin-3-yl)methyl]-/ ⁇ /-nitro-2-pentylidene- hydrazinecarboximidamide (0.4.10), 1-[(6-chloropyridin-3-yl)methyl]-7-methyl-8-nitro-5- propoxy-1 ,2,3,5,6,7-hexahydroimidazo[1 ,2-a]pyridine (0.4.11), nicotine (0.4.11), nicotine (0.4.11
- Nicotinic acetylcholine receptor allosteric activators spinosad (0.5.1), spinetoram (0.5.2);
- Chloride channel activators abamectin (0.6.1), emamectin benzoate (0.6.2), ivermectin (0.6.3), lepimectin (0.6.4), milbemectin (0.6.5);
- 0.8 miscellaneous non-specific (multi-site) inhibitors methyl bromide (0.8.1) and other alkyl halides, chloropicrin (0.8.2), sulfuryl fluoride (0.8.3), borax (0.8.4), tartar emetic (0.8.5);
- 0.9 Chordotonal organ TRPV channel modulators pymetrozine (0.9.1), pyrifluquinazon (0.9.2), flonicamid (0.9.3);
- Mite growth inhibitors clofentezine (0.10.1), hexythiazox (0.10.2), diflovidazin (0.10.3), etoxazole (0.10.4);
- Israelensis (0.11.1), Bacillus sphaericus (0.11.2), Bacillus thuringiensis subsp. aizawai (0.11.3), Bacillus thuringiensis subsp. kurstaki (0.11.4), Bacillus thuringiensis subsp.
- Inhibitors of mitochondrial ATP synthase diafenthiuron (0.12.1), azocyclotin (0.12.2), cyhexatin (0.12.3), fenbutatin oxide (0.12.4), propargite (0.12.5), tetradifon (0.12.6);
- Nicotinic acetylcholine receptor (nAChR) channel blockers bensultap (0.14.1), cartap hydrochloride (0.14.2), thiocyclam (0.14.3), thiosultap sodium (0.14.4);
- Inhibitors of the chitin biosynthesis type 0 bistrifluron (0.15.1), chlorfluazuron (0.15.2), diflubenzuron (0.15.3), flucycloxuron (0.15.4), flufenoxuron (0.15.5), hexaflumuron
- Inhibitors of the chitin biosynthesis type 1 buprofezin (0.16.1);
- Moulting disruptors cyromazine (0.17.1); 0.18 Ecdyson receptor agonists: methoxyfenozide (0.18.1), tebufenozide (0.18.2), halofenozide (0.18.3), fufenozide (0.18.4), chromafenozide (0.18.5);
- Octopamin receptor agonists amitraz (0.19.1);
- Mitochondrial complex I electron transport inhibitors fenazaquin (0.21.1), fenpyroximate (0.21.2), pyrimidifen (0.21.3), pyridaben (0.21.4), tebufenpyrad (0.21.5), tolfenpyrad (0.21.6), rotenone (0.21.7);
- Inhibitors of the of acetyl CoA carboxylase spirodiclofen (0.23.1), spiromesifen (0.23.2), spirotetramat (0.23.3), spiropidion (0.23.4);
- Mitochondrial complex IV electron transport inhibitors aluminium phosphide (0.24.1), calcium phosphide (0.24.2), phosphine (0.24.3), zinc phosphide (0.24.4), cyanide (0.24.5);
- Mitochondrial complex II electron transport inhibitors cyenopyrafen (0.25.1), cyflumetofen (0.25.2);
- insecticidal active compounds of unknown or uncertain mode of action 0.28. insecticidal active compounds of unknown or uncertain mode of action: afidopyropen
- component 2 The active substances referred to as component 2, their preparation and their activity e. g. against harmful fungi is known (cf. : https://www.alanwood.net/pesticides/); these substances are commercially available.
- the compounds described by lUPAC nomenclature, their preparation and their pesticidal activity are also known (cf. Can. J. Plant Sci. 48(6), 587-94, 1968;
- WO 05/123690 WO 05/63721 ; WO 05/87772; WO 05/87773; WO 06/15866; WO 06/87325; WO 06/87343; WO 07/82098; WO 07/90624, WO 10/139271 , WO 11/028657, WO 12/168188, WO 07/006670, WO 11/77514; WO 13/047749, WO 10/069882, WO 13/047441 , WO 03/16303, WO 09/90181 , WO 13/007767, WO 13/010862, WO 13/127704, WO 13/024009, WO 13/24010, WO 13/047441 , WO 13/162072, WO 13/092224, WO 11/135833, CN 1907024, CN 1456054,
- the present invention furthermore relates to agrochemical compositions comprising a mixture of at least one compound I (component 1) and at least one further active substance useful for plant protection, e. g. selected from the groups A) to O) (component 2), in particular one further fungicide, e. g. one or more fungicide from the groups A) to K), as described above, and if desired one suitable solvent or solid carrier.
- agrochemical compositions comprising a mixture of at least one compound I (component 1) and at least one further active substance useful for plant protection, e. g. selected from the groups A) to O) (component 2), in particular one further fungicide, e. g. one or more fungicide from the groups A) to K), as described above, and if desired one suitable solvent or solid carrier.
- fungicide e. g. one or more fungicide from the groups A) to K
- combating harmful fungi with a mixture of compounds I and at least one fungicide from groups A) to K), as described above, is more efficient than combating those fungi with individual compounds I or individual fungicides from groups A) to K).
- the order of application is not essential for working of the present invention.
- the time between both applications may vary e. g. between 2 hours to 7 days. Also, a broader range is possible ranging from 0.25 hour to 30 days, preferably from 0.5 hour to 14 days, particularly from 1 hour to 7 days or from 1.5 hours to 5 days, even more preferred from 2 hours to 1 day.
- the weight ratio of the component 1) and the component 2) generally depends from the properties of the active components used, usually it is in the range of from 1 :10,000 to 10,000:1 , often it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 , even more preferably in the range of from 1 :4 to 4: 1 and in particular in the range of from 1 :2 to 2: 1.
- the weight ratio of the component 1) and the component 2) usually is in the range of from 1000:1 to 1 :1 , often in the range of from 100: 1 to 1 :1 , regularly in the range of from 50:1 to 1 :1 , preferably in the range of from 20:1 to 1 :1 , more preferably in the range of from 10:1 to 1 :1 , even more preferably in the range of from 4:1 to 1 :1 and in particular in the range of from 2:1 to 1 :1.
- the weight ratio of the component 1) and the component 2) usually is in the range of from 1 :1 to 1 :1000, often in the range of from 1 :1 to 1 :100, regularly in the range of from 1 :1 to 1 :50, preferably in the range of from 1 :1 to 1 :20, more preferably in the range of from 1 :1 to 1 :10, even more preferably in the range of from 1 :1 to 1 :4 and in particular in the range of from 1 :1 to 1 :2.
- the ternary mixtures i.e.
- compositions according to the invention comprising the component 1) and component 2) and a compound III (component 3), the weight ratio of component 1) and component 2) depends from the properties of the active substances used, usually it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 and in particular in the range of from 1 :4 to 4:1 , and the weight ratio of component 1) and component 3) usually it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 and in particular in the range of from 1 :4 to 4: 1.
- any further active components are, if desired, added in a ratio of from 20:1 to 1 :20 to the component 1).
- the present invention furthermore relates to mixtures comprising one compound of the formula I (component 1 , a group represented by the expression“(I)”) and one pesticide II (component 2), wherein pesticide II is an active ingredient selected from the groups A) to O) defined above.
- the mixtures of active substances can be prepared as compositions comprising besides the active ingredients at least one inert ingredient (auxiliary) by usual means, e. g. by the means given for the compositions of compounds I.
- the mixtures of active substances according to the present invention are suitable as fungicides, as are the compounds of formula I. They are distinguished by an outstanding effectiveness against a broad spectrum of phytopathogenic fungi, especially from the classes of the Ascomycetes, Basidiomycetes, Deuteromycetes and Peronosporomycetes (syn.
- reaction mixture was quenched with 200 ml aq. NH4CI-solution and extracted with methyl-t-butylether, the organic layer was dried over sodium sulfate and concentrated. The residue was purified by column- chromatography using petrolether/methyl-t-butylether-mixtures to give 12.5 g (73% of theory) 5- cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoate as yellow oil.
- the compound was dissolved in a mixture of acetone and/or dimethylsulfoxide and the wetting agent/emulsifier Wettol, which is based on ethoxylated alkylphenoles, in a ratio (volume) solvent-emulsifier of 99 to 1 to give a total volume of 5 ml. Subsequently, water was added to total volume of 100 ml.
- Wettol which is based on ethoxylated alkylphenoles
- This stock solution was then diluted with the described solvent-emulsifier-water mixture to the final concentration given in the table below.
- Example 1- Preventative fungicidal control of Botrytis cinerea on leaves of green pepper
- Young seedlings of green pepper were grown in pots to the 4 to 5 leaf stage. These plants were sprayed to run-off with previously described spray solution, containing the concentration of active ingredient or mixture mentioned in the table below. Seven days later the plants were inoculated with an aqueous DOB solution (or a DOB solution containing 10 percent glycerine), containing the spore suspension of Botrytis cinerea. Then the plants were immediately transferred to a humid chamber. After 5 days at 22 to 24 " C and a saturated relative humidity, the extent of fungal attack on the leaves was visually assessed as % diseased leaf area.
- aqueous DOB solution or a DOB solution containing 10 percent glycerine
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
The present invention relates to the use of compounds of formula I (formula I) wherein the variables are defined as given in the description and claims. The invention further relates to the compounds I and composition for compounds of formula I.
Description
PYRIDINE DERIVATIVES AND THEIR USE AS FUNGICIDES
The present invention relates to the use of pyridine compounds and the N-oxides and the salts thereof as fungicides as well to new pyridine compounds. The invention also relates to the composition comprising at least one compound I, to the method for combating phytopathogenic fungi and to the ssed coated with at least one compound of the formula I.
In many cases, in particular at low application rates, the fungicidal activity of known compounds is unsatisfactory. Based on this, it was an objective of the present invention to provide compounds having improved activity and/or a broader activity spectrum against
phytopathogenic fungi. Another object of the present invention is to provide fungicides with improved toxicological properties or with improved environmental fate properties. These and further objects are achieved by the use of pyridine carboxamides of formula (I), as defined below, and by their agriculturally suitable salts as well by the new pyridine carboxamides of formula (I).
Accordingly, the present invention relates to use of the compounds of formula I
X is O or S;
R1 is in each case independently selected from hydrogen, halogen, CN, CrC6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R1 are unsubstituted or substituted by one to six groups R1a which independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R1a are unsubstituted or substituted by one to six halogen or CN;
R2 is in each case independently selected from halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R2 are unsubstituted or substituted by one to six groups R2a which independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R2a are unsubstituted or substituted by one to six halogen or CN;
R3 is in each case independently selected from halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R3 are unsubstituted or substituted by one to six groups R3a which independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R3a are unsubstituted or substituted by one to six halogen or CN;
R4 is in each case independently selected from hydrogen, halogen, CN, Ci-C6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R4 are unsubstituted or substituted by one to six groups R4a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R4a are unsubstituted or substituted by one to six halogen or CN;
A is direct bond or C(R7R8);
R5, R6, R7, R8
are in each case independently selected from hydrogen, halogen, CN, Ci-C6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, O-CrCe-alkyl, 0-C2-C6-alkenyl, 0-C2-Ce-alkynyl, Cs-Ce-cycloalkyl, O-Cs-Ce-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl, wherein the acyclic moieties of R5, R6, R7, R8 are unsubstituted or substituted by one to six groups R5a, R6a, R7a, R8a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, Cs-Ce- cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, O-C3-C6- cycloalkenyl, CH2-C3-C6-cycloalkenyl, =N-OR';
R' is in each case independently selected from H, Ci-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C6-cycloalkyl, phenyl, -CH2-phenyl, a five- or six-membered heteroaryl, or -Chh- heteroaryl; wherein the heteroaryl contains 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S);
wherein the cyclic and acyclic moieties of R' are unsubstituted or substituted by one to six groups RR independently of one another are selected from: halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, 0-Ci-C6-alkyl, =N-OR';
R9 is in each case independently selected from Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6- alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, C3-C6-cycloalkyl, C3- C6-cycloalkenyl, saturated or partially unsaturated bicyclic carbocycle, a five- or six- membered heterocycle, aryl, heteroaryl; wherein the heterocycle and heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S);
wherein R' is as defined above;
wherein the cyclic and acyclic moieties of R9 are unsubstituted or substituted by one to six groups R9a independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, =N-OR'; and wherein the groups R9a are unsubstituted or substituted by one to six halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, 0-CrC6-alkyl,÷ wherein
wherein R5, R6, R7 or R8 can not all be H;
when A is C(R7R8) R9 can be also H;
and the N-oxides and the agriculturally acceptable salts thereof as fungicides.
The N-oxides may be prepared from the inventive compounds according to conventional oxidation methods, e. g. by treating compounds I with an organic peracid such as
metachloroperbenzoic acid (cf. WO 03/64572 or J. Med. Chem. 38(11), 1892-903, 1995); or with inorganic oxidizing agents such as hydrogen peroxide (cf. J. Heterocyc. Chem. 18(7), 1305-8, 1981) or oxone (cf. J. Am. Chem. Soc. 123(25), 5962-5973, 2001). The oxidation may lead to pure mono-N-oxides or to a mixture of different N-oxides, which can be separated by conventional methods such as chromatography.
Agriculturally acceptable salts of the compounds of the formula I encompass especially the salts of those cations or the acid addition salts of those acids whose cations and anions, respectively, have no adverse effect on the fungicidal action of the compounds I. Suitable cations are thus in particular the ions of the alkali metals, preferably sodium and potassium, of the alkaline earth metals, preferably calcium, magnesium and barium, of the transition metals, preferably manganese, copper, zinc and iron, and also the ammonium ion which, if desired, may be substituted with one to four CrC4-alkyl substituents and/or one phenyl or benzyl substituent, preferably diisopropylammonium, tetramethylammonium, tetrabutylammonium,
trimethylbenzylammonium, furthermore phosphonium ions, sulfonium ions, preferably tri(Ci-C4- alkyl)sulfonium, and sulfoxonium ions, preferably tri(Ci-C4-alkyl)sulfoxonium.
Anions of acceptable acid addition salts are primarily chloride, bromide, fluoride,
hydrogensulfate, sulfate, dihydrogenphosphate, hydrogenphosphate, phosphate, nitrate, bicarbonate, carbonate, hexafluorosilicate, hexafluorophosphate, benzoate, and the anions of Ci-C4-alkanoic acids, preferably formate, acetate, propionate and butyrate. They can be formed by reacting a compound I with an acid of the corresponding anion, preferably of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid or nitric acid.
Compounds of the formula I can exist as one or more stereoisomers. The various stereoisomers include enantiomers, diastereomers, atropisomers arising from restricted rotation about a single bond of asymmetric groups and geometric isomers. They also form part of the subject matter of the present invention. One skilled in the art will appreciate that one stereoisomer may be more active and/or may exhibit beneficial effects when enriched relative to the other stereoisomer(s) or when separated from the other stereoisomer(s). Additionally, the skilled artisan knows how to separate, enrich, and/or to selectively prepare said stereoisomers. The compounds of the invention may be present as a mixture of stereoisomers, e.g. a racemate, individual
stereoisomers, or as an optically active form.
Compounds of the formula I can be present in different crystal modifications whose biological activity may differ. They also form part of the subject matter of the present invention.
In respect of the variables, the embodiments of the intermediates obtained during preparation of compounds I correspond to the embodiments of the compounds of formula I. The term “compounds I” refers to compounds of the formula I.
In the following, the intermediate compounds are further described. A skilled person will readily understand that the preferences for the substituents, also in particular the ones given in the tables below for the respective substituents, given herein in connection with compounds I apply for the intermediates accordingly. Thereby, the substituents in each case have independently of each other or more preferably in combination the meanings as defined herein.
If the synthesis yields mixtures of isomers, a separation is generally not necessarily required since in some cases the individual isomers can be interconverted during work-up for use or during application (e. g. under the action of light, acids or bases). Such conversions may also take place after use, e. g. in the treatment of plants in the treated plant, or in the harmful fungus to be controlled.
In the definitions of the variables given above, collective terms are used which are generally representative for the substituents in question. The term "Cn-Cm" indicates the number of carbon atoms possible in each case in the substituent or substituent moiety in question.
The term "halogen" refers to fluorine, chlorine, bromine and iodine.
The term "CrC6-alkyl" refers to a straight-chained or branched saturated hydrocarbon group having 1 to 6 carbon atoms, e.g. methyl, ethyl, propyl, 1-methylethyl, butyl, 1-methylpropyl, 2- methylpropyl, 1 , 1 -di methylethyl , pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl,
2.2-dimethylpropyl, 1-ethylpropyl, 1 , 1 -dimethylpropyl, 1 ,2-dimethylpropyl, hexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 1 , 1 -dimethyl butyl, 1 ,2-dimethylbutyl,
1.3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl,
2-ethylbutyl, 1 , 1 ,2-trimethylpropyl, 1 ,2,2-trimethylpropyl, 1 -ethyl-1 -methylpropyl and 1 -ethyl-2- methylpropyl. Likewise, the term "C2-C4-alkyl" refers to a straight-chained or branched alkyl group having 2 to 4 carbon atoms, such as ethyl, propyl (n-propyl), 1-methylethyl (iso-propoyl), butyl, 1-methylpropyl (sec.-butyl), 2-methylpropyl (iso-butyl), 1 , 1 -dimethylethyl (tert.-butyl).
The term "Ci-C6-halogenalkyl" refers to an alkyl group having 1 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above. Examples are "Ci-C2-halogenalkyl" groups such as chloromethyl, bromomethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl, chlorodifluoromethyl, 1-chloroethyl, 1-bromoethyl, 1- fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl, 2-chloro- 2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl or pentafluoroethyl.
The term "CrC6-alkoxy" refers to a straight-chain or branched alkyl group having 1 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkyl group. Examples are "C1-C4- alkoxy" groups, such as methoxy, ethoxy, n-propoxy, 1-methylethoxy, butoxy, 1-methyhprop- oxy, 2-methylpropoxy or 1 ,1-dimethylethoxy.
The term "CrC6-halogenalkoxy" refers to a CrC6-alkoxy radical as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above. Examples are "CrC4-halogenalkoxy" groups, such as OCH2F, OCHF2, OCF3, OCH2CI, OCHCI2, OCCI3, chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 2-fluoroethoxy, 2-chlorothoxy, 2-bromoethoxy, 2-iodoethoxy, 2,2-difluoroethoxy, 2,2,2- trifluoroethoxy, 2-chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy, 2,2-dichloro-2-fluoroethoxy,
2,2,2-trichloroethoxy, OC2F5, 2-fluoropropoxy, 3-fluoropropoxy, 2,2-difluoropropoxy, 2,3-difluoro ,propoxy, 2 chloropropoxy, 3-chloropropoxy, 2,3-dichloropropoxy, 2-bromopropoxy, 3 bromopropoxy, 3,3,3-trifluoropropoxy, 3,3,3-trichloropropoxy, OCH2-C2F5, OCF2-C2F5, 1- fluoromethyl-2-fluoroethoxy, 1 -chloromethyl-2-chloroethoxy, 1 -bromomethyl-2-bromoethoxy, 4-fluorobutoxy, 4-chlorobutoxy, 4-bromobutoxy or nonafluorobutoxy.
The term "C2-C6-alkenyl" refers to a straight-chain or branched unsaturated hydrocarbon radical having 2 to 6 carbon atoms and a double bond in any position. Examples are "C2-C4-alkenyl" groups, such as ethenyl, 1-propenyl, 2-propenyl (allyl), 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1 -methyl-1 -propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl, 2-methyl-2-propenyl.
The term "C2-C6-halogenalkenyl" refers to an alkyl group having 2 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
The term "C2-C6-alkenyloxy" refers to a straight-chain or branched alkenyl group having 2 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkenyl group. Examples are "C2-C4-alkenyloxy" groups.
The term "C2-C6-alkynyl" refers to a straight-chain or branched unsaturated hydrocarbon radical having 2 to 6 carbon atoms and containing at least one triple bond. Examples are "C2-C4- alkynyl" groups, such as ethynyl, prop-1-ynyl, prop-2-ynyl (propargyl), but-1-ynyl, but-2-ynyl, but-3-ynyl , 1 -methyl-prop-2-ynyl .
The term "C2-C6-halogenalkynyl" refers to an alkyl group having 2 or 6 carbon atoms as defined above, wherein some or all of the hydrogen atoms in these groups may be replaced by halogen atoms as mentioned above.
The term "C2-C6-alkynyloxy" refers to a straight-chain or branched alkynyl group having 2 to 6 carbon atoms which is bonded via an oxygen, at any position in the alkynyl group. Examples are "C2-C4-alkynyloxy" groups.
The term "C3-C6-cycloalkyl" refers to monocyclic saturated hydrocarbon radicals having 3 to 6 carbon ring members, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl. Accordingly, a saturated three-, four-, five-, six-, seven-, eight-, nine or ten-membered carbocyclyl or carbo- cycle is a "C3-Cio-cycloalkyl".
The term "C3-C6-cycloalkenyl" refers to a monocyclic partially unsaturated 3-, 4- 5- or 6- membered carbocycle having 3 to 6 carbon ring members and at least one double bond, such as cyclopentenyl, cyclopentadienyl, cyclohexadienyl. Accordingly, a partially unsaturated three-, four-, five-, six-, seven-, eight-, nine or ten-membered carbocyclyl or carbocycle is a "C3-C10- cycloalkenyl".
The term "C3-C8-cycloalkyl-Ci-C4-alkyl" refers to alkyl having 1 to 4 carbon atoms (as defined above), whereAccording to one hydrogen atom of the alkyl radical is replaced by a cycloalkyl radical having 3 to 8 carbon atoms (as defined above).
The term“saturated or partially unsaturated three-, four-, five-, six-, seven-, eight-, nine or ten- membered heterocyclyl or heterocycle, wherein the heterocyclyl or heterocycle contains 1 , 2, 3 or 4 heteroatoms selected from N, O and S” is to be understood as meaning both saturated and partially unsaturated heterocycles, wherein the ring member atoms of the heterocycle include besides carbon atoms 1 , 2, 3 or 4 heteroatoms independently selected from the group of O, N
and S. For example:
a 3- or 4-membered saturated heterocycle which contains 1 or 2 heteroatoms from the group consisting of O, N and S as ring members such as oxirane, aziridine, thiirane, oxetane, azetidine, thiethane, [1 ,2]dioxetane, [1 ,2]dithietane, [1 ,2]diazetidine; and
a 5- or 6-membered saturated or partially unsaturated heterocycle which contains 1 , 2 or 3 heteroatoms from the group consisting of O, N and S as ring members such as 2- tetrahydrofuranyl, 3-tetrahydrofuranyl, 2-tetrahydrothienyl, 3-tetrahydrothienyl, 2-pyrrolidinyl, 3- pyrrolidinyl, 3-isoxazolidinyl, 4-isoxazolidinyl, 5-isoxazolidinyl, 3-isothiazolidinyl,
4-isothiazolidinyl, 5-isothiazolidinyl, 3-pyrazolidinyl, 4-pyrazolidinyl, 5-pyrazolidinyl, 2- oxazolidinyl, 4-oxazolidinyl, 5-oxazolidinyl, 2-thiazolidinyl, 4-thiazolidinyl, 5-thiazolidinyl,
2-imidazolidinyl, 4-imidazolidinyl, 1 ,2,4-oxadiazolidin-3-yl, 1 ,2,4-oxadiazolidin-5-yl, 1 ,2,4- thiadiazolidin-3-yl, 1 ,2,4-thiadiazolidin-5-yl, 1 ,2,4-triazolidin-3-yl, 1 ,3,4-oxadiazolidin-2-yl, 1 ,3,4- thiadiazolidin-2-yl, 1 ,3,4-triazolidin-2-yl, 2,3-dihydrofur-2-yl, 2,3-dihydrofur-3-yl, 2,4-dihydrofur-2- yl, 2,4-dihydrofur-3-yl, 2,3-dihydrothien-2-yl, 2,3-dihydrothien-3-yl, 2,4-dihydrothien-2-yl, 2,4- dihydrothien-3-yl, 2-pyrrolin-2-yl, 2-pyrrolin-3-yl, 3-pyrrolin-2-yl, 3-pyrrolin-3-yl, 2-isoxazolin-3-yl,
3-isoxazolin-3-yl, 4-isoxazolin-3-yl, 2-isoxazolin-4-yl, 3-isoxazolin-4-yl, 4-isoxazolin-4-yl, 2-isoxazolin-5-yl, 3-isoxazolin-5-yl, 4-isoxazolin-5-yl, 2-isothiazolin-3-yl, 3-isothiazolin-3-yl, 4- isothiazolin-3-yl, 2-isothiazolin-4-yl, 3-isothiazolin-4-yl, 4-isothiazolin-4-yl, 2-isothiazolin-5-yl, 3- isothiazolin-5-yl, 4-isothiazolin-5-yl, 2,3-dihydropyrazol-1-yl, 2,3-dihydropyrazol-2-yl, 2,3- dihydropyrazol-3-yl, 2,3-dihydropyrazol-4-yl, 2,3-dihydropyrazol-5-yl, 3,4-dihydropyrazol-1-yl, 3,4-dihydropyrazol-3-yl, 3,4-dihydropyrazol-4-yl, 3,4-dihydropyrazol-5-yl, 4,5-dihydropyrazol-1- yl, 4,5-dihydropyrazol-3-yl, 4,5-dihydropyrazol-4-yl, 4,5-dihydropyrazol-5-yl, 2,3-dihydrooxazol- 2-yl, 2,3-dihydrooxazol-3-yl, 2,3-dihydrooxazol-4-yl, 2,3-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2- yl, 3,4-dihydrooxazol-3-yl, 3,4-dihydrooxazol-4-yl, 3,4-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2- yl, 3,4-dihydrooxazol-3-yl, 3,4-dihydrooxazol-4-yl, 2-piperidinyl, 3-piperidinyl, 4-piperidinyl, 1 ,3- dioxan-5-yl, 2-tetrahydropyranyl, 4-tetrahydropyranyl, 2-tetrahydrothienyl, 3- hexahydropyridazinyl, 4-hexahydropyridazinyl, 2-hexahydropyrimidinyl, 4-hexahydropyrimidinyl,
5-hexahydropyrimidinyl, 2-piperazinyl, 1 ,3,5-hexahydrotriazin-2-yl and 1 ,2,4-hexahydrotriazin-3- yl and also the corresponding -ylidene radicals; and
a 7-membered saturated or partially unsaturated heterocycle such as tetra- and
hexahydroazepinyl, such as 2,3,4,5-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, 3, 4, 5, 6- tetrahydro[2H]azepin-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,4,7-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,6,7-tetrahydro[1 H]azepin-1-,-2-,-3-,-4-,-5-,-6- or-7-yl, hexahydroazepin-1-,-2-,-3- or-
4-yl, tetra- and hexahydrooxepinyl such as 2,3,4,5-tetrahydro[1 H]oxepin-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,4,7-tetrahydro[1 H]oxepin-2-,-3-,-4-,-5-,-6- or-7-yl, 2,3,6,7-tetrahydro[1 H]oxepin-2-, -3-, -4-, -5- ,-6- or-7-yl, hexahydroazepin-1-,-2-,-3- or-4-yl, tetra- and hexahydro-1 ,3-diazepinyl, tetra- and hexahydro-1 ,4-diazepinyl, tetra- and hexahydro-1 ,3-oxazepinyl, tetra- and hexahydro-1 ,4- oxazepinyl, tetra- and hexahydro-1 , 3-dioxepinyl, tetra- and hexahydro-1 , 4-dioxepinyl and the corresponding -ylidene radicals.
The term“substituted” refers to substitued with 1 , 2, 3 or up to the maximum possible number of substituents.
The term“5-or 6-membered heteroaryl” or“5-or 6-membered heteroaromatic” refers to aromatic ring systems incuding besides carbon atoms, 1 , 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O and S, for example,
a 5-membered heteroaryl such as pyrrol-1 -yl, pyrrol-2-yl, pyrrol-3-yl, thien-2-yl, thien-3-yl, furan- 2-yl, furan-3-yl, pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl, imidazol-1-yl, imidazol-2-yl, imidazol-4-yl, imidazol-5-yl, oxazol-2-yl, oxazol-4-yl, oxazol-5-yl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl, thiazol-2-yl, thiazol-4-yl, thiazol-5-yl, isothiazol-3-yl, isothiazol-4-yl, isothiazol-5-yl,
1.2.4-triazolyl-1-yl, 1 ,2,4-triazol-3-yl 1 ,2,4-triazol-5-yl, 1 ,2,4-oxadiazol-3-yl, 1 ,2,4-oxadiazol-5-yl and 1 ,2,4-thiadiazol-3-yl, 1 ,2,4-thiadiazol-5-yl; or
a 6-membered heteroaryl, such as pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrimidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, pyrazin-2-yl and 1 ,3,5-triazin-2-yl and
1.2.4-triazin-3-yl.
In the following, particular embodiments of the inventive compounds are described. Therein, specific meanings of the respective substituents are further detained, wherein the meanings are in each case on their own but also in any combination with one another, particular embodiments of the present invention.
Furthermore, in respect of the variables, generally, the embodiments of the compounds I also apply to the intermediates.
According to one embodiment of formula I, R1 is H, halogen, Ci-C6-alkyl or Ci-C6-halogenalkyl, in particular H, F, Cl, CH3, C2H5, CF3 more specifically H, CH3, F or Cl most preferred H, F or Cl, especially R1 is hydrogen.
Particularly preferred embodiments of R1 according to the invention are in Table P1 below, wherein each line of lines P1-1 to P1-13 corresponds to one particular embodiment of the invention. Thereby, for every R1 that is present in the inventive compounds, these specific embodiments and preferences apply independently of the meaning of any other R1 that may be present in the ring:
Table P1 :
P1-13 OCHF2
According to one embodiment of formula I, R2 is selected from the group consisting of C1-C6- alkyl, CrC6-halogenalkyl, 0-CrC6-alkyl, C3-C6-cycloalkyl, in particular CH3, C2H5, CF3, CH2F, CHF2, OCH3, OC2H5, O-C3H7, O-C4H9, cyclopropyl, cyclobutyl, more specifically CH3, CH2F, CF2H, CF3, OCH3, OC2H5, most preferred OCH3, CH3.
According to still another embodiment of formula I, R2 is halogen, in particular F, Cl, Br or I, more specifically F, Cl or Br, in particular F or Cl.
According to still another embodiment of formula I, R2 is F.
According to still another embodiment of formula I, R2 is Cl.
According to still another embodiment of formula I, R2 is Br.
According to still another embodiment of formula I, R2 is CrC6-alkyl, in particular CrC4-alkyl, such as CH3. or C2H5, in particular CH3 or CH2CH3.
According to still another embodiment of formula I, R2 is CrC6-halogenalkyl, in particular C1-C4- halogenalkyl, such as CF3, CCI3, FCH2, CICH2, F2CH, CI2CH, CF3CH2, CCI3CH2 or CF2CHF2.
According to still a further embodiment of formula I, R2 is C2-C6-alkenyl, in particular C2-C4-alk- enyl, such as CH=CH2, C(CH3)=CH2, CH2CH=CH2.
According to a further specific embodiment of formula I, R2 is C2-C6-halogenalkenyl, in particular C2-C4-halogenalkenyl, more specifically C2-C3-halogenalkenyl such as CH=CHF, CH=CHCI,
CH=CF2, CH=CCI2, CH2CH=CHF, CH2CH=CHCI, CH2CH=CF2, CH2CH=CCI2, CF2CH=CF2, CCI2CH=CCI2, CF2CF=CF2, CCI2CCI=CCI2.
According to still a further embodiment of formula I, R2 is C2-C6-alkynyl or C2-C6-halogenalkynyl, in particular C2-C4-alkynyl or C2-C4-halogenalkynyl, such as CECH, CFhCECH, CECCI,
CH2CECCI, or CC CECCI.
According to a further specific embodiment of formula I, R2 is 0-CrC6-alkyl, in particular C1-C4- alkyl, more specifically Ci-C2-alkoxy. R2 is such as OCH3 or OCH2CH3.
According to a further specific embodiment of formula I, R2 is 0-CrC6-alkyl,
According to a further specific embodiment of formula I, R2 is 0-C2-C6-alkenyl in particular C2- C4-alkenyl, more specifically C2-C3-alkenyl. R2 is such as OCH=CH2, OCH2CH=CH2.
According to a further specific embodiment of formula I, R2 is 0-C2-C6-alkynyl, in particular C2- C6-alkynyl, in particular C2-C4-alkynyl, more specifically C2-C3-alkynyl. R2 is such as O-CH2- CECH.
According to a further specific embodiment of formula I, R2 is 0-CrC6-halogenalkyl, in particular OCF3, OCC , OFCH2, OCICH2, OF2CH, OCI2CH, OCF3CH2, OCCI3CH2 or OCF2CHF2, more specifically OCF3, OF2CH, OFCH2.
According to still another embodiment of formula I, R2 is C3-C6-cycloalkyl, in particular cyclopropyl or cyclobutyl.
According to still another embodiment of formula I, R2 is C3-C6-halogencycloalkyl. In a special embodiment R2 is fully or partially halogenated cyclopropyl, such as 1-F-cyclopropyl, 1-CI- cyclopropyl, 2,2-F2-cyclopropyl, 2,2-Cl2-cyclopropyl .
Particularly preferred embodiments of R2 according to the invention are in Table P2 below, wherein each line of lines P2- 1 to P2-21 corresponds to one particular embodiment of the invention, wherein P2- 1 to P2-21 are also in any combination with one another a preferred
embodiment of the present invention. The connection point to the carbon atom, to which R2 is bound is marked with“#” in the drawings.
Table P2:
5
According to one embodiment of formula I, R3 is selected from the group consisting of C1-C6- alkyl, CrC6-halogenalkyl, 0-CrC6-alkyl, C3-C6-cycloalkyl, in particular CH3, C2H5, CF3, CH2F, CHF2, OCH3, OC2H5, O-C3H7, O-C4H9, cyclopropyl, cyclobutyl, more specifically CH3, CH2F, CF2H, CF3, cyclopropyl, cyclobutyl, most preferred CH3, CF2H, CF3.
According to still another embodiment of formula I, R3 is halogen, in particular F, Cl, Br or I, more specifically F, Cl or Br, in particular F or Cl.
According to still another embodiment of formula I, R3 is F.
According to still another embodiment of formula I, R3 is Cl.
According to still another embodiment of formula I, R3 is Br.
According to still another embodiment of formula I, R3 is CrC6-alkyl, in particular CrC4-alkyl, such as CH3 or C2H5, in particular CH3 or CH2CH3.
According to still another embodiment of formula I, R3 is CrC6-halogenalkyl, in particular C1-C4- halogenalkyl, such as CF3, CCI3, FCH2, CICH2, F2CH, CI2CH, CF3CH2, CCI3CH2 or CF2CHF2. According to still a further embodiment of formula I, R3 is C2-C6-alkenyl, in particular C2-C4-alk- enyl, such as CH=CH2, C(CH3)=CH2, CH2CH=CH2.
According to a further specific embodiment of formula I, R3 is C2-C6-halogenalkenyl, in particular C2-C4-halogenalkenyl, more specifically C2-C3-halogenalkenyl such as CH=CHF, CH=CHCI,
CH=CF2, CH=CCI2, CH2CH=CHF, CH2CH=CHCI, CH2CH=CF2, CH2CH=CCI2, CF2CH=CF2, CCI2CH=CCI2, CF2CF=CF2, CCI2CCI=CCI2.
According to still a further embodiment of formula I, R3 is C2-C6-alkynyl or C2-C6-halogenalkynyl, in particular C2-C4-alkynyl or C2-C4-halogenalkynyl, such as CECH, ChhCECH, CECCI,
CH2CECCI, or CC CECCI.
According to a further specific embodiment of formula I, R3 is 0-CrC6-alkyl, in particular C1-C4- alkyl, more specifically Ci-C2-alkoxy. R3 is such as OCH3 or OCH2CH3.
According to a further specific embodiment of formula I, R3 is 0-C2-C6-alkenyl in particular C2- C4-alkenyl, more specifically C2-C3-alkenyl. R3 is such as OCH=CH2, OCH2CH=CH2.
According to a further specific embodiment of formula I, R3 is 0-C2-C6-alkynyl, in particular C2- C6-alkynyl, in particular C2-C4-alkynyl, more specifically C2-C3-alkynyl. R3 is such as O-CH2- CECH.
According to a further specific embodiment of formula I, R3 is 0-CrC6-halogenalkyl, in particular OCF3, OCC , OFCH2, OCICH2, OF2CH, OCI2CH, OCF3CH2, OCCI3CH2 or OCF2CHF2, more specifically OCF3, OF2CH, OFCH2. According to still another embodiment of formula I, R3 is C3-C6-cycloalkyl, in particular cyclopropyl, cyclobutyl.
According to still another embodiment of formula I, R3 is C3-C6-halogencycloalkyl. In a special embodiment R3 is fully or partially halogenated cyclopropyl, such as 1-F-cyclopropyl, 1-CI- cyclopropyl, 2,2-F2-cyclopropyl, 2,2-Cl2-cyclopropyl .
Particularly preferred embodiments of R3 according to the invention are in Table P3 below, wherein each line of lines P3-1 to P3-21 corresponds to one particular embodiment of the invention, wherein P3-1 to P3-21 are also in any combination with one another a preferred embodiment of the present invention. The connection point to the carbon atom, to which R3 is bound is marked with“#” in the drawings.
Table P3:
According to one embodiment of formula I, R4 is H, halogen, CrC6-alkyl or CrC6-halogenalkyl, in particular H, F, Cl, CH3, C2H5, CF3 more specifically H, CH3, F or Cl most preferred H, F or Cl, especially R4 is hydrogen.
Particularly preferred embodiments of R4 according to the invention are in Table P4 below, wherein each line of lines P4-1 to P4-10 corresponds to one particular embodiment of the invention. Thereby, for every R4 that is present in the inventive compounds, these specific embodiments and preferences apply independently of the meaning of any other R4 that may be present in the ring:
Table P4:
are in each case independently selected from hydrogen, halogen, CN, CrC6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, O-CrCe-alkyl, 0-C2-Ce-alkenyl, 0-C2-Ce-alkynyl, C3-Ce-cycloalkyl, 0-C3-Ce-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl, wherein the acyclic moieties of R5, R6, R7, R8 are unsubstituted or substituted by one to six groups R5a, R6a, R7a, R8a which independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6- cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, O-C3-C6- cycloalkenyl, CH2-C3-C6-cycloalkenyl, =N-OR';
and wherein the groups R5a, R6a, R7a, R8a are unsubstituted or substituted by one to six halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, 0-CrC6-alkyl, =N-OR';
or two moieties: R5 and R6 or R7 and R8 form together with the C atoms to which they are bound a group C=N-OR'; wherein
R' is in each case independently selected from H, CrC6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C6-cycloalkyl, phenyl, -CFh-phenyl, a five- or six-membered heteroaryl, or CH2- heteroaryl; wherein the heteroaryl contains 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S);
wherein the acyclic moieties of R are unsubstituted or substituted by one to six groups RR independently of one another are selected from: halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, , 0-CrC6-alkyl, =N-OR'.
According to one embodiment of formula I, R5, R6, R7, R8 are in each case independently selected from H, halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6- halogenalkenyl, 0-CrC6-alkyl, C3-C6-cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3- C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl.
According to one further preferred embodiment of formula I, R5 is preferably H, CrC6-alkyl or CrC6-alkyl-0-Ci-C6-alkyl, more preferably H, CH3, CH2OCH3.
According to one further preferred embodiment of formula I, R5 is preferably H, CN, halogen, Ci- C6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, 0-CrC6-alkyl, C3-C6- cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl or more preferably H, CH3, C2H5, CN, F, Cl, CF3, CH2-CF3, OCH3, CH2- CH=CH2, cyclopropyl, CH2-cyclopropyl or more preferably H, CH3, C2H5, CN, F, Cl, OCH3.
According to one further preferred embodiment of formula I, R6 is preferably CrC6-alkyl, C1-C4- halogenalkyl, C2-C4-alkenyl, 0-CrC6-alkyl, C2-C4-halogenalkenyl, cyclopropyl, CFh-cyclopropyl. According to one further preferred embodiment of formula I, R7 is preferably H or CrC6-alkyl, more preferably H and CH3.
According to one further preferred embodiment of formula I, R8 is preferably H or CrC6-alkyl, more preferably H and CH3.
According to one embodiment of formula I, R5, R6, R7, R8 are in each case independently H. According to still another embodiment of formula I, R5, R6, R7, R8 are in each case
independently halogen, preferably F or Cl, most preferably F.
According to one embodiment of formula I, R5, R6, R7, R8 are in each case independently CN.
According to still another embodiment of formula I, R5, R6, R7, R8 are in each case
independently CrC6-alkyl, such as CH3, C2H5, n-propyl, i-propyl, n-butyl, i-butyl, tert-butyl, n- pentyl, i-pentyl or CH2-C(CH3)3. Most preferably is CH3, C2H5, n-propyl, i-butyl, tert-butyl or CH2- C(CH3)3.
According to still another embodiment of formula I, R5, R6, R7, R8 are in each case
independently CrC6-halogenalkyl, in particular CrC4-halogenalkyl, such as CF3, CC , FCH2, CICH2, F2CH, CI2CH, CF3CH2, CCI3CH2 or CF2CHF2.
According to still a further embodiment of formula I, R5, R6, R7, R8 are in each case
independently C2-C6-alkenyl, in particular C2-C4-alkenyl, such as C(CH3)=CH2, CH2CH=CH2,
CH2CH=C(CH3)2, CH2-CH2-CH=CH2.
According to a further specific embodiment of formula I, R5, R6, R7, R8 are in each case independently C2-C6-halogenalkenyl, in particular C2-C4-halogenalkenyl, more specifically C2- Cs-halogenalkenyl such as CH=CHF, CH=CHCI, CH=CF2, CH=CCI2, CH2CH=CHF,
CH2CH=CHCI, CH2CH=CF2, CH2CH=CCI2, CF2CH=CF2, CCI2CH=CCI2, CF2CF=CF2,
CCI2CCI=CCI2, CH(CI)-CH=CH2, CH2CH=CH(CI), CH2CH=CCI2, CH2CCI=CCI2.
According to still a further embodiment of formula I, R5, R6, R7, R8 are in each case
independently C2-C6-alkynyl or C2-C6-halogenalkynyl, in particular C2-C4-alkynyl or C2-C4- halogenalkynyl, such as CECH, CFhCECH, CECCI, CFhCECCI, or CC CECCI.
According to a further specific embodiment of formula I, R5, R6, R7, R8 are in each case independently 0-Ci-C6-alkanyl. R5, R6, R7, R8 are in each case independently such as OCH3, OC2H5, O-n-propyl, O-iso-propyl, O-i-butyl, O-tert-butyl or 0-CH2-C(CH3)3.
According to a further specific embodiment of formula I, R5, R6, R7, R8 are in each case independently C3-C6-cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl, wherein C3-C6-cycloalkyl is preferably selected from the group of cyclopropyl, 1-F-cyclopropyl, 1-CI-cyclopropyl, 2,2-F2-cyclopropyl, 2.2-CI2- cyclopropyl and cyclohexyl; and wherein C3-C6-cycloalkenyl is selected from the group of cyclohexen-1-yl, cyclohexen-2-yl, cyclohexen-3-yl, cyclohexen-4-yl.
According to a further specific embodiment of formula I, R5, R6, R7, R8 are in each case independently 0-C2-C6-alkenyl in particular C2-C4-alkenyl, more specifically C2-C3-alkenyl. R5,
R6, R7, R8 are in each case independently such as OCH=CH2, OCH2CH=CH2.
According to a further specific embodiment of formula I, R5, R6, R7, R8 are in each case independently 0-C2-C6-alkynyl, in particular C2-C6-alkynyl, in particular C2-C4-alkynyl, more specifically C2-C3-alkynyl. R5, R6, R7, R8 are in each case independently such as OCFhCECH.
According to a further specific embodiment of formula I, R5 and R6 or R7 and R8 form together with the C atoms to which they are bound a group C=N-OR' such as C=NOCH3, C=NO-CH2CH3 , C=NO-CH2CF3 , C=NO-CH2-CH=CH2 , C=NO-CH2-CCH, C=NO-CH2-C6H5. Particularly preferred embodiments of R5, R6, R7, R8 according to the invention are in Table P5 below, wherein each line of lines P5-1 to P5-30 corresponds to one particular embodiment of the invention, wherein P5-1 to P5-30 are also in any combination with one another a preferred
embodiment of the present invention. The connection point to the carbon atom, to which R5 is bound is marked with“#” in the drawings.
Table P5:
According to one embodiment of formula I, R9 is selected from CrC6-alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
According to one embodiment of formula I, when A is C(R7R8) R9 is selected from H, C1-C6- alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
According to one embodiment of formula I, R9 is selected from the group consisting of CH3, CH2CH3, CH(CH3)2, CH(CH3)3, CF3, CF(CH3)2, CH2OCH(CH3)2, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, C6H5, 4-F-C6H4; most preferred CH3 and phenyl.
According to still another embodiment of formula I, R9 is CrC6-alkyl, in particular CrC4-alkyl, such as CH3 or C2H5, in particular CH3 or CH2CH3.
According to still another embodiment of formula I, R9 is CrC6-halogenalkyl, in particular C1 -C4- halogenalkyl, such as CF3, CC , FCH2, CICH2, F2CH, CI2CH, CF3CH2, CCI3CH2 or CF2CHF2.
According to still a further embodiment of formula I, R9 is C2-C6-alkenyl, in particular C2-C4-alk- enyl, such as CH=CH2, C(CH3)=CH2, CH2CH=CH2.
According to a further specific embodiment of formula I, R9 is C2-C6-halogenalkenyl, in particular C2-C4-halogenalkenyl, more specifically C2-C3-halogenalkenyl such as CH=CHF, CH=CHCI,
CH=CF2, CH=CCI2, CH2CH=CHF, CH2CH=CHCI, CH2CH=CF2, CH2CH=CCI2, CF2CH=CF2, CCI2CH=CCI2, CF2CF=CF2, CCI2CCI=CCI2.
According to still a further embodiment of formula I, R9 is C2-C6-alkynyl or C2-C6-halogenalkynyl, in particular C2-C4-alkynyl or C2-C4-halogenalkynyl, such as CECH, CH2CECH, CECCI,
CH2CECCI, or CC CECCI.
According to still another embodiment of formula I, R9 is C3-C6-cycloalkyl, in particular cyclopropyl, cyclohexyl.
According to still another embodiment of formula I, R9 is C3-C6-cycloalkenyl, in particular cyclopentenyl, or cyclohexenyl.
According to still another embodiment of formula I, R9 is phenyl, wherein phenyl in each case is unsubstituted or substituted by identical or different groups R9a which independently of one another are selected from halogen, CrC2-alkyl, CN.
According to still another embodiment of formula I, R9 is a 5-membered heteroaryl such as pyrrol-1 -yl, pyrrol-2-yl, pyrrol-3-yl, thien-2-yl, thien-3-yl, furan-2-yl, furan-3-yl, pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl, imidazol-1-yl, imidazol-2-yl, imidazol-4-yl, imidazol-5-yl, oxazol-2-yl, oxazol4-yl, oxazol-5-yl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl, thiazol-2-yl, thiazol-4-yl, thiazol-5-yl, isothiazol-3-yl, isothiazol-4-yl, isothiazol-5-yl, 1 ,2,4-triazolyl-1 -yl, 1 ,2,4- triazol-3-yl 1 ,2,4-triazol-5-yl, 1 ,2,4-oxadiazol-3-yl, 1 ,2,4-oxadiazol-5-yl and 1 ,2,4-thiadiazol-3-yl,
1.2.4-thiadiazol-5-yl, preferred are pyrazol-1-yl, pyrazol-3-yl, pyrazol-4-yl, pyrazol-5-yl,
1.2.4-triazolyl-1 -yl, 1 ,2,4-triazol-3-yl 1 ,2,4-triazol-5-yl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl. The 5-membered heteroaryl in each case is unsubstituted or substituted by identical or different groups R9a which independently of one another are selected from halogen, CrC2-alkyl, CN.
According to still another embodiment of formula I, R9 is a 6-membered heteroaryl such as pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrimidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, pyrazin-2-yl and 1 ,3,5-triazin-2-yl and 1 ,2,4-triazin-3-yl, preferred are pyridin-2-yl, pyridin-3-yl, pyridin-4-yl. The 6-membered heteroaryl in each case is unsubstituted or substituted by identical or different groups R9a which independently of one another are selected from halogen, Ci-C2-alkyl, CN.
Particularly preferred embodiments of R9 according to the invention are in Table P9 below, wherein each line of lines P9-1 to P9-104 corresponds to one particular embodiment of the invention, wherein P5-1 to P5-104 are also in any combination with one another a preferred embodiment of the present invention. The connection point to the carbon atom, to which R9 is bound is marked with“#” in the drawings.
Table P9:
In one embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O; A is C(R7R8);
R1 is H;
R2 is selected from the group consisting of CrC6-alkyl, CrC6-halogenalkyl or 0-CrC6-alkyl;
R3 is selected from the group consisting of CrC6-alkyl, CrC6-halogenalkyl or C3-C4-cycloalkyl;
R4 is H;
R5, R6, R7, R8 are independently selected from H, halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, CrC6-alkenyl, CrC6-halogenalkenyl, 0-CrC6-alkyl, C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl or two moieties: R5 and R6 or R7 and R8 form together with the C atoms to which they are bound a group C=N-OR'; wherein R' is as defined above;
R9 is selected from the group consisting of CrC6-alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
In one further embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O;
A is C(R7R8);
R1 is H;
R2 is selected from the group consisting of CH3, C2H5, CF3, CH2F, CHF2, OCH3, OC2H5, O-C3H7, O-C4H9, cyclopropyl, cyclobutyl;
R3 is selected from the group consisting of CH3, C2H5, CF3, CH2F, CHF2, cyclopropyl, cyclobutyl; R4 is H;
R5, R6, R7, R8 are independently selected from H, halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, 0-CrC6-alkyl, or two moieties:
R5 and R6 or R7 and R8 form together with the C atoms to which they are bound a group C=N- OR'; wherein R' is as defined above;
R9 is selected from the group consisting of CrC6-alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
In one further embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O;
A is C(R7R8);
R1 is H;
R2 is selected from the group consisting of CH3, OCH3;
R3 is selected from the group consisting of CH3, CF3, CF2H;
R4 is H;
R5 is selected from the group consisting of H, F, Cl, CN, CH3, CH2OCH3.
R6 is selected from the group consisting of CrC6-alkyl, CrC4-halogenalkyl, C2-C4-alkenyl, C2-C4- halogenalkenyl, cyclopropyl, CFh-cyclopropyl,
R7 is selected from the group consisting of H and CH3;
R8 is selected from the group consisting of H and CH3.
R9 is selected from the group consisting of CH3, phenyl, pyridine-2-yl, pyridine-3-yl, pyridine-4-yl and cyclohexyl.
In one embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O;
A is a direct bond
R1 is H;
R2 is selected from the group consisting of CH3, OCH3;
R3 is selected from the group consisting of CH3, CF3, CF2H;
R4 is H;
R5 is selected from the group consisting of H, F, Cl, CN, CH3, CH2OCH3.
R6 is selected from the group consisting of CrC6-alkyl, CrC4-halogenalkyl, C2-C4-alkenyl, C2-C4- halogenalkenyl, cyclopropyl, CFh-cyclopropyl,
R7 is selected from the group consisting of H and CH3;
R8 is selected from the group consisting of H and CH3.
R9 is selected from the group consisting of CH3, phenyl, pyridine-2-yl, pyridine-3-yl, pyridine-4-yl and cyclohexyl.
In one further embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O;
A is a direct bond;
R1 is H;
R2 is selected from the group consisting of CH3, C2H5, CF3, CH2F, CHF2, OCH3, OC2H5, O-C3H7, O-C4H9, cyclopropyl, cyclobutyl;
R3 is selected from the group consisting of CH3, C2H5, CF3, CH2F, CHF2, cyclopropyl, cyclobutyl; R4 is H;
R5, R6 are independently selected from H, halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, O-C1- C6-alkyl, or two moieties:
R5 and R6 form together with the C atoms to which they are bound a group =N-OR'; wherein R' is as defined above;
R9 is selected from the group consisting of CrC6-alkyl, CrC6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
In one further embodiment, the invention relates to compounds of the formula I, or the N-oxides, or the agriculturally acceptable salts thereof, wherein
X is O;
A is a direct bond;
R1 is H;
R2 is selected from the group consisting of CH3, OCH3;
R3 is selected from the group consisting of CH3, CF3, CF2H;
R4 is H;
R5 is selected from the group consisting of H, CH3, CH2OCH3.
R6 is selected from the group consisting of CrC6-alkyl, CrC4-halogenalkyl, C2-C4-alkenyl,
R9 is selected from the group consisting of CH3 and cyclopropyl.
Preferred embodiments of the present invention are the following compounds I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6, I.A-6; compounds I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6. In these formulae, the substituents R5, R6, R7, R8 and R9 are independently as defined above or preferably defined herein:
In particular with a view to their use, according to one embodiment, preference is given to the compounds of the compounds I.A-1, I.A-2, I.A-3, I.A-4, I.A-5, I.A-6,; compounds I.B-1, I.B-2, I.B- 3, I.B-4, I.B-5, I.B-6 that are compiled in the Tables 1a to 6a. Each of the groups mentioned for a substituent in the tables is furthermore per se, independently of the combination in which it is mentioned, a particularly preferred aspect of the substituent in question.
Table 1a Compounds of the formula I.A-1, I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1, I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CH2- and the meaning for the combination of R5, R6and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.1a.B-1 to I.A-1.1a.B-396, I.A-2.1a.B-1 to I.A-2.1a.B-396, I.A-3.1a.B-1 to I.A-3.1a.B-396, I.A-
4.1a.B-1 to I.A-5.1a.B-396, I.A-5.1a.B-1 to I.A-3.1a.B-396, I.A-6.1a.B-1 to I.A-6.1a.B-396; B-
1.1a.B-1 to I.B-1.1a.B-396, I.B-2.1a.B-1 to I.B-2.1a.B-396, I.B-3.1a.B-1 to I.B-3.1a.B-396, I.B-
4.1a.B-1 to I.B-4.1a.B-396, I.B-5.1a.B-1 to I.B-5.1a.B-396, I.B-6.1a.B-1 to I.B-6.1a.B-396).
Table 2a Compounds of the formula I.A-1, I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1, I.B-2, I.B-3,
I.B-4, I.B-5, I.B-6 in which A is -CHF- and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.2a.B-1 to I.A-1.2a.B-396, I.A-2.2a.B-1 to I.A-2.2a.B-396, I.A-3.2a.B-1 to I.A-3.2a.B-396, I.A-
4.2a.B-1 to I.A-5.2a.B-396, I.A-5.2a.B-1 to I.A-3.2a.B-396, I.A-6.2a.B-1 to I.A-6.2a.B-396; B-
1.2a.B-1 to I.B-1.2a.B-396, I.B-2.2a.B-1 to I.B-2.2a.B-396, I.B-3.2a.B-1 to I.B-3.2a.B-396, I.B-
4.2a.B-1 to I.B-4.2a. B-396, I.B-5.2a.B-1 to I.B-5.2a.B-396, I.B-6.2a.B-1 to I.B-6.2a.B-396).
Table 3a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CH(CH3)- and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
1.3a.B-1 to I.A-1.3a. B-396, I.A-2.3a.B-1 to I.A-2.3a.B-396, I.A-3.3a.B-1 to I.A-3.3a.B-396, I.A-
4.3a.B-1 to I.A-5.3a. B-396, I.A-5.3a.B-1 to I.A-3.3a.B-396, I.A-6.3a.B-1 to I.A-6.3a.B-396; B-
1.3a.B-1 to I.B-1.3a. B-396, I.B-2.3a.B-1 to I.B-2.3a.B-396, I.B-3.3a.B-1 to I.B-3.3a.B-396, I.B-
4.3a.B-1 to I.B-4.3a. B-396, I.B-5.3a.B-1 to I.B-5.3a.B-396, I.B-6.3a.B-1 to I.B-6.3a.B-396).
Table 4a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CF(CH3)- and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
1.4a.B-1 to I.A-1.4a. B-396, I.A-2.4a.B-1 to I.A-2.4a.B-396, I.A-3.4a.B-1 to I.A-3.4a.B-396, I.A-
4.4a.B-1 to I.A-5.4a. B-396, I.A-5.4a.B-1 to I.A-3.4a.B-396, I.A-6.4a.B-1 to I.A-6.4a.B-396; B-
1.4a.B-1 to I.B-1.4a. B-396, I.B-2.4a.B-1 to I.B-2.4a.B-396, I.B-3.4a.B-1 to I.B-3.4a.B-396, I.B-
4.4a.B-1 to I.B-4.4a. B-396, I.B-5.4a.B-1 to I.B-5.4a.B-396, I.B-6.4a.B-1 to I.B-6.4a.B-396).
Table 5a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -CF2- and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A- 1.5a.B-1 to I.A-1.5a. B-396, I.A-2.5a.B-1 to I.A-2.5a.B-396, I.A-3.5a.B-1 to I.A-3.5a.B-396, I.A-
4.5a.B-1 to I.A-5.5a. B-396, I.A-5.5a.B-1 to I.A-3.5a.B-396, I.A-6.5a.B-1 to I.A-6.5a.B-396; B-
1.5a.B-1 to I.B-1.5a. B-396, I.B-2.5a.B-1 to I.B-2.5a.B-396, I.B-3.5a.B-1 to I.B-3.5a.B-396, I.B-
4.5a.B-1 to I.B-4.5a. B-396, I.B-5.5a.B-1 to I.B-5.5a.B-396, I.B-6.5a.B-1 to I.B-6.5a.B-396).
Table 6a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is -C(CH3)2- and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-
1.6a.B-1 to I.A-1.6a. B-396, I.A-2.6a.B-1 to I.A-2.6a.B-396, I.A-3.6a.B-1 to I.A-3.6a.B-396, I.A-
4.6a.B-1 to I.A-5.6a. B-396, I.A-5.6a.B-1 to I.A-3.6a.B-396, I.A-6.6a.B-1 to I.A-6.6a.B-396; B-
1.6a.B-1 to I.B-1.6a. B-396, I.B-2.6a.B-1 to I.B-2.6a.B-396, I.B-3.6a.B-1 to I.B-3.6a.B-396, I.B-
4.6a.B-1 to I.B-4.6a. B-396, I.B-5.6a.B-1 to I.B-5.6a.B-396, I.B-6.6a.B-1 to I.B-6.6a.B-396).
Table 7a Compounds of the formula I.A-1 , I.A-2, I.A-3, I.A-4, I.A-5, I.A-6; I.B-1 , I.B-2, I.B-3, I.B-4, I.B-5, I.B-6 in which A is direct bound and the meaning for the combination of R5, R6 and R9 for each individual compound corresponds in each case to one line of Table B (compounds I.A-1.7a.B-1 to I.A-1.7a.B-396, I.A-2.7a.B-1 to I.A-2.7a.B-396, I.A-3.7a.B-1 to I.A-3.7a.B-396, I.A-4.7a. B-1 to I.A-5.7a.B-396, I.A-5.7a.B-1 to I.A-3.7a.B-396, I.A-6.7a.B-1 to I.A-6.7a.B-396; B- 1.7a.B-1 to I. B-1.7a. B-396, I.B-2.7a.B-1 to I.B-2.7a.B-396, I.B-3.7a.B-1 to I.B-3.7a.B-396, I.B- 4.7a. B-1 to I.B-4.7a. B-396, I.B-5.7a.B-1 to I.B-5.7a.B-396, I.B-6.7a.B-1 to I.B-6.7a.B-396).
Table B
Compounds of the present invention can be made as shown in the following schemes, in which, unless otherwise stated, the definition of each variable is as defined above for a compound of formula I. The compounds of the formula I can be prepared according to methods or in analogy to methods that are described in the prior art. The synthesis takes advantage of starting materials that are commercially available or may be prepared according to conventional procedures starting from readily available compounds.
Compounds of the present invention can be made following the schemes 1 to 5 described below, in which, unless otherwise stated, the definition of each variable is as defined above for a compound of formula (I).
Among the various reported methods for preparing compounds of formula (I. A) (wherein X is O and R1-R9 and A are as defined for compounds of formula I) the most widely applied method is an amide coupling reaction of 3-aminopyridines of type I I with carboxylic acids of type I II . Among various reported methods for such amide coupling reactions, a robust method involves treatment of carboxylic acid of type II I (R5-R9 and A are as defined for compounds of formula I) with an activating agent like phosgene, thionyl chloride or oxalyl chloride or an amide coupling reagent like dicyclohexylcarbodiimide in organic solvent like tetrahydrofuran (THF), dichloromethane (DCM) or dimethylformamide (DMF). Subsequent addition of an amine of type I I (R1-R4 are as defined for compounds of formula I) in the presence of a base like triethylamine or dimethylaminopyridine gives the target compounds of formula I .A as described in Scheme 1 (see: Chem. Soc. Rev. 2009, 606-631 or
Tetrahedron 2005, 10827- 10852) .
Scheme 1
Compounds of formula (I . A) (wherein X is O and R1-R9 and A are as defined for compounds of formula I) can also be prepared by the amide coupling reaction between 3- aminopyridines of type I I (R1-R4 are as defined for compounds of formula I) and an ester of type IV (R5-R9 and A are as defined for compounds of formula I and Y is Ci - C6 alkyl or phenyl) in the presence of a Lewis acid such as trimethyl aluminium in an inert organic solvent like toluene under heating as shown in Scheme 2.
Scheme 2
Compounds of formula (I.A) (wherein X is O and R1-R9 and A are as defined for compounds of formula I) can alternatively be prepared by the coupling of 3-halopyridines of type V (R1-R4 are as defined for compounds of formula I and halogen are preferably chloro, bromo or iodo) with amide of type VI (R5-R9 and A are as defined for compounds of formula I) in the presence of a base such as cesium carbonate or sodium tertiary-butoxide in the presence of a transition metal catalyst such as a copper-based catalyst such as copper oxide, copper (I) acetylacetonate or copper (I) bromide-1 , 10-phenanthroline complex, a nickel catalyst such as Dichloro(1 ,3-bis(diphenylphosphino)propane)nickel or a palladium- based catalyst such as Chloro(2-dicyclohexylphosphino-2',4',6'-triisopropyl-1 ,1'-biphenyl)[2- (2'-amino-1 ,1'-biphenyl)]palladium(ll), X-Phos aminobiphenyl palladium chloride precatalyst or [1 ,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride in an aprotic solvent such as toluene or N,N-dimethylformamide at room temperature or while heating. This is shown in Scheme 3.
Scheme 3
Among the various reported methods for preparing compounds of formula VI (R5-R9 and A are as defined for compounds of formula I), the most widely applied method involves treatment of carboxylic acid III (R5-R9 and A are as defined for compounds of formula I) with an activating agent like phosgene, thionyl chloride or oxalyl chloride or an amide coupling reagent like dicyclohexylcarbodiimide in an inert organic solvent like tetrahydrofuran (THF), dichloromethane (DCM) or dimethylformamide (DMF). Subsequent addition of ammonia or an ammonium salt such as ammonium chloride or ammonium hydroxide in the presence or absence of a catalyst like dimethylaminopyridine gives the target compounds of formula VI as described in Scheme 4 (see: Chem. Soc. Rev. 2009, 606-631 or Tetrahedron 2005, 10827-10852).
Scheme 4
A skilled person will realize that carboxylic acids of formula (III) (R5-R9 and A are as defined for compounds of formula I) can be prepared from the corresponding esters such as compounds of formula (IV) (R5-R9 and A are as defined for compounds of formula I and Y is Ci - C6 alkyl or phenyl). A skilled person will also realize that the alpha functionalization of these esters can be performed by deprotonation with a strong base like lithium
diisopropylamine in an inert solvent like THF at temperatures between -78 °C and 20 °C followed by reaction with an electrophilic reagent like an alkyl iodide or a source of electrophilic fluorine, as described in March's Advanced Organic Chemistry, Smith and
March, 6^ edition, Wiley, 2007. This reaction can be repeated to prepare acids of formula (III) and the introduced alkyl, alkenyl and alkynyl groups can be further functionalized by
halogenation, cyclopropanation, oxidation or reduction, cross coupling (eg Sonogashira coupling) to prepare acid derivatives of formula (III) from commercially available esters.
A skilled person will realize that carboxylic acids of formula (III) (R5-R9 are as defined for compounds of formula I and A is a bond) can be prepared by various methods that involve functional group transformations, such as a triple bond can be obtained from an aldehyde using the Bestmann-Ohira reagent as described in J. Org. Chem., 1982, 47, 1837-1845). Other new methods for the preparation of carboxylic acids of formula (III) (R5-R9 are as defined for compounds of formula I and A is a bond) are described in literatures (see:
Chem. Commun. 2014, 13052-13055; Org. Lett. 2013, 1472-1475).
As shown in scheme 5, treatment of compounds of general formula (I. A) (wherein X is O and R1-R9 and A are as defined for compounds of formula I) with a deoxothionating agent like P4S10 or Lawesson’s reagent in an inert organic solvent like toluene at temperatures between 20 °C and 150 °C can afford compounds of general formula (I.B) (wherein X is S and R1-R9 and A are as defined for compounds of formula I).
Scheme 5
A skilled person will realize that using standard synthesis protocol compounds of formula (I. A) (wherein X is O and R1-R9 and A are as defined for compounds of formula I) can alternatively be obtained by transformation of another, closely related, compound of formula (I. A). Non-exhaustive examples include oxidation and reduction reactions, coupling reactions, nucleophilic or electrophilic aromatic substitution reactions, nucleophilic substitution reactions, nucleophilic addition reactions, cycloaddition reactions, halogenation reactions and hydrolysis reactions.
Certain intermediates described in the above schemes are novel and as such form a further aspect of the invention.
The compounds of the formula II and V are commercially available.
The N-oxides may be prepared from the inventive compounds according to conventional oxidation methods as described in WO 03/64572 or J. Med. Chem. 1995,1892-903 or J.
Heterocycl. Chem. 1981 ,1305-8 or. J. Am. Chem. Soc. 2001 , 5962-5973. The oxidation may lead to pure mono-N-oxides or to a mixture of different N-oxides, which can be separated by conventional methods such as chromatography.
The compounds I and the compositions according to the invention are particularly important in the control of a multitude of phytopathogenic fungi on various cultivated plants, such as cereals, e. g. wheat, rye, barley, triticale, oats or rice; beet, e. g. sugar beet or fodder beet; fruits, such as pomes, stone fruits or soft fruits, e. g. apples, pears, plums, peaches, almonds, cherries, strawberries, raspberries, blackberries or gooseberries; leguminous plants, such as lentils, peas, alfalfa or soybeans; oil plants, such as rape, mustard, olives, sunflowers, coconut, cocoa beans, castor oil plants, oil palms, ground nuts or soybeans; cucurbits, such as squashes, cucumber or melons; fiber plants, such as cotton, flax, hemp or jute; citrus fruit, such as
oranges, lemons, grapefruits or mandarins; vegetables, such as spinach, lettuce, asparagus, cabbages, carrots, onions, tomatoes, potatoes, cucurbits or paprika; lauraceous plants, such as avocados, cinnamon or camphor; energy and raw material plants, such as corn, soybean, rape, sugar cane or oil palm; corn; tobacco; nuts; coffee; tea; bananas; vines (table grapes and grape juice grape vines); hop; turf; sweet leaf (also called Stevia); natural rubber plants or ornamental and forestry plants, such as flowers, shrubs, broad-leaved trees or evergreens, e. g. conifers; and on the plant propagation material, such as seeds, and the crop material of these plants.
Preferably, compounds I and compositions thereof, respectively are used for controlling a multitude of fungi on field crops, such as potatoes sugar beets, tobacco, wheat, rye, barley, oats, rice, corn, cotton, soybeans, rape, legumes, sunflowers, coffee or sugar cane; fruits; vines; ornamentals; or vegetables, such as cucumbers, tomatoes, beans or squashes.
The term "plant propagation material" is to be understood to denote all the generative parts of the plant such as seeds and vegetative plant material such as cuttings and tubers (e. g.
potatoes), which can be used for the multiplication of the plant. This includes seeds, roots, fruits, tubers, bulbs, rhizomes, shoots, sprouts and other parts of plants, including seedlings and young plants, which are to be transplanted after germination or after emergence from soil.
These young plants may also be protected before transplantation by a total or partial treatment by immersion or pouring.
Preferably, treatment of plant propagation materials with compounds I and compositions thereof, respectively, is used for controlling a multitude of fungi on cereals, such as wheat, rye, barley and oats; rice, corn, cotton and soybeans.
The term "cultivated plants" is to be understood as including plants which have been modified by mutagenesis or genetic engineering in order to provide a new trait to a plant or to modify an already present trait.
Mutagenesis includes techniques of random mutagenesis using X-rays or mutagenic chemicals, but also techniques of targeted mutagenesis, to create mutations at a specific locus of a plant genome. Targeted mutagenesis techniques frequently use oligonucleotides or proteins like CRISPR/Cas, zinc-finger nucleases, TALENs or meganucleases to achieve the targeting effect. Genetic engineering usually uses recombinant DNA techniques to create modifications in a plant genome which under natural circumstances cannot readily be obtained by cross breeding, mutagenesis or natural recombination. Typically, one or more genes are integrated into the genome of a plant to add a trait or improve a trait. These integrated genes are also referred to as transgenes in the art, while plant comprising such transgenes are referred to as transgenic plants. The process of plant transformation usually produces several transformation events, wich differ in the genomic locus in which a transgene has been integrated. Plants comprising a specific transgene on a specific genomic locus are usually described as comprising a specific“event”, which is referred to by a specific event name. Traits which have been introduced in plants or have been modified include herbicide tolerance, insect resistance, increased yield and tolerance to abiotic conditions, like drought.
Herbicide tolerance has been created by using mutagenesis as well as using genetic engineering. Plants which have been rendered tolerant to acetolactate synthase (ALS) inhibitor herbicides by mutagenesis and breeding comprise plant varieties commercially available under the name Clearfield®.
Herbicide tolerance has been created via the use of transgenes to glyphosate, glufosinate, 2,4-D, dicamba, oxynil herbicides, like bromoxynil and ioxynil, sulfonylurea herbicides, ALS inhibitors and 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors, like isoxaflutole and mesotrione.
Transgenes wich have been used to provide herbicide tolerance traits comprise: for tolerance
to glyphosate: cp4 epsps, epsps grg23ace5, mepsps, 2mepsps, gat4601 , gat4621 , goxv247; for tolerance to glufosinate: pat and bar, for tolerance to 2,4-D: aad-1 , aad-12; for tolerance to dicamba: dmo; for tolerance to oxynil herbicies: bxn; for tolerance to sulfonylurea herbicides: zm-hra, csr1-2, gm-hra, S4-HrA; for tolerance to ALS inhibitors: csr1-2; and for tolerance to HPPD inhibitors: hppdPF, W336, avhppd-03.
Transgenic corn events comprising herbicide tolerance genes include, but are not limited to, DAS40278, MON801 , MON802, MON809, MON810, MON832, MON87411 , MON87419, MON87427, MON88017, MON89034, NK603, GA21 , MZHG0JG, HCEM485, VCO-01981-5, 676, 678, 680, 33121 , 4114, 59122, 98140, Bt10, Bt176, CBH-351 , DBT418, DLL25, MS3,
MS6, MZIR098, T25, TC1507 and TC6275.
Transgenic soybean events comprising herbicide tolerance genes include, but are not limited to, GTS 40-3-2, MON87705, MON87708, MON87712, MON87769, MON89788, A2704-12, A2704-21 , A5547-127, A5547-35, DP356043, DAS44406-6, DAS68416-4, DAS-81419-2, GU262, SYHT0H2, W62, W98, FG72 and CV127.
Transgenic cotton events comprising herbicide tolerance genes include, but are not limited to, 19-51 a, 31707, 42317, 81910, 281-24-236, 3006-210-23, BXN10211 , BXN10215, BXN10222, BXN 10224, MON1445, MON1698, MON88701 , MON88913, GHB119, GHB614, LLCotton25, T303-3 and T304-40.
Transgenic canola events comprising herbicide tolerance genes are for example, but not excluding others, MON88302, HCR-1 , HCN10, HCN28, HCN92, MS1 , MS8, PHY14, PHY23, PHY35, PHY36, RF1 , RF2 and RF3.
Insect resistance has mainly been created by transferring bacterial genes for insecticidal proteins to plants: Transgenes which have most frequently been used are toxin genes of Bacillus spp. and synthetic variants thereof, like cry1A, crylAb, cry1Ab-Ac, crylAc, cry1A.105, cry1 F, cry1 Fa2, cry2Ab2, cry2Ae, mcry3A, ecry3.1Ab, cry3Bb1 , cry34Ab1 , cry35Ab1 , cry9C, vip3A(a), vip3Aa20. However, also genes of plant origin, such as genes coding for protease inhibitors, like CpTI and pinll, have been transferred to other plants. A further approach uses transgenes such as dvsnf7 to produce double-stranded RNA in plants.
Transgenic corn events comprising genes for insecticidal proteins or double stranded RNA include, but are not limited to, Bt10, Bt11 , Bt176, MON801 , MON802, MON809, MON810, MON863, MON87411 , MON88017, MON89034, 33121 , 4114, 5307, 59122, TC1507, TC6275, CBH-351 , MIR162, DBT418 and MZIR098. Transgenic soybean events comprising genes for insecticidal proteins include, but are not limited to, MON87701 , MON87751 and DAS-81419. Transgenic cotton events comprising genes for insecticidal proteins include, but are not limited to, SGK321 , MON531 , MON757, MON1076, MON15985, 31707, 31803, 31807, 31808, 42317, BN LA-601 , Eventl , COT67B, COT102, T303-3, T304-40, GFM Cry1A, GK12, MLS 9124, 281- 24-236, 3006-210-23, GHB119 and SGK321.
Increased yield has been created by using the transgene athb17, being present for example in corn event MON87403, or by using the transgene bbx32, being present for example in the soybean event MON87712.
Cultivated plants comprising a modified oil content have been created by using the
transgenes: gm-fad2-1 , Pj.D6D, Nc.Fad3, fad2-1A and fatb1-A. Soybean events comprising at least one of these genes are: 260-05, MON87705 and MON87769.
Tolerance to abiotic conditions, such as drought, has been created by using the transgene cspB, comprised by the corn event MON87460 and by using the transgene Hahb-4, comprised by soybean event IND-00410-5.
Traits are frequently combined by combining genes in a transformation event or by combining different events during the breeding process resulting in a cultivated plant with stacked traits.
Preferred combinations of traits are combinations of herbicide tolerance traits to different groups of herbicides, combinations of insect tolerance to different kind of insects, in particular tolerance to lepidopteran and coleopteran insects, combinations of herbicide tolerance with one or several types of insect resistance, combinations of herbicide tolerance with increased yield as well as combinations of herbicide tolerance and tolerance to abiotic conditions.
Plants comprising singular or stacked traits as well as the genes and events providing these traits are well known in the art. For example, detailed information as to the mutagenized or integrated genes and the respective events are available from websites of the organizations “International Service for the Acquisition of Agri-biotech Applications (ISAAA)”
(https://www.isaaa.org/gmapprovaldatabase) and the“Center for Environmental Risk
Assessment (CERA)” (https://cera-gmc.org/GMCropDatabase). Further information on specific events and methods to detect them can be found for canola events MS1 , MS8, RF3, GT73, MON88302, KK179 in W001/031042, W001/041558, W001/041558, W002/036831 ,
W011/153186, W013/003558, for cotton events MON 1445, MON 15985, MON531
(MON 15985), LLCotton25, MON88913, COT102, 281-24-236, 3006-210-23, COT67B,
GHB614, T304-40, GHB119, MON88701 , 81910 in WO02/034946, W002/100163,
W002/100163, WO03/013224, WO04/072235, WO04/039986, W005/103266, W005/103266, WO06/128573, W007/017186, W008/122406, W008/151780, WO12/134808, W013/112527; for corn events GA21 , MON810, DLL25, TC1507, MON863, MIR604, LY038, MON88017, 3272, 59122, NK603, MIR162, MON89034, 98140, 32138, MON87460, 5307, 4114, MON87427, DAS40278, MON87411 , 33121 , MON87403, MON87419 in W098/044140, US02/102582, US03/126634, WO04/099447, WO04/011601 , W005/103301 , W005/061720, W005/059103, WO06/098952, WO06/039376, US2007/292854, W007/142840, W007/140256,
WO08/112019, WO09/103049, WO09/111263, W010/077816, WO11/084621 , W011/062904, W011/022469, W013/169923, W014/116854, WO15/053998, W015/142571 ; for potato events E12, F10, J3, J55, V11 , X17, Y9 in WO14/178910, W014/178913, W014/178941 ,
W014/179276, W016/183445, WO17/062831 , WO17/062825; for rice events LLRICE06, LLRICE601 , LLRICE62 in WO00/026345, WO00/026356, WO00/026345; and for soybean events H7-1 , MON89788, A2704-12, A5547-127, DP305423, DP356043, MON87701 ,
MON87769, CV127, MON87705, DAS68416-4, MON87708, MON87712, SYHT0H2,
DAS81419, DAS81419 x DAS44406-6, MON87751 in WO04/074492, W006/130436,
WO06/108674, WO06/108675, WO08/054747, W008/002872, WO09/064652, WO09/102873, W010/080829, W010/037016, W011/066384, W011/034704, WO12/051199, WO12/082548, W013/016527, WO13/016516, WO14/201235.
The use of compounds I and compositions according to the invention, respectively, on cultivated plants may result in effects which are specific to a cultivated plant comprising a certain gene or event. These effects might involve changes in growth behavior or changed resistance to biotic or abiotic stress factors. Such effects may in particular comprise enhanced yield, enhanced resistance or tolerance to insects, nematodes, fungal, bacterial, mycoplasma, viral or viroid pathogens as well as early vigour, early or delayed ripening, cold or heat tolerance as well as changed amino acid or fatty acid spectrum or content.
The compounds I and compositions thereof, respectively, are particularly suitable for controlling the following plant diseases:
Albugo spp. (white rust) on ornamentals, vegetables (e. g. A. Candida ) and sunflowers (e. g. A. tragopogonis ); Alternaria spp. (Alternaria leaf spot) on vegetables (e.g. A. dauci or A. porn), oilseed rape (A. brassicicola or brassicae), sugar beets (A. tenuis), fruits (e.g. A. grandis), rice, soybeans, potatoes and tomatoes (e. g. A. solani, A. grandis or A. alternata), tomatoes (e. g. A. solani or A. alternata) and wheat (e.g. A. triticina)·, Aphanomyces spp. on sugar beets and
vegetables; Ascochyta spp. on cereals and vegetables, e. g. A. tritici (anthracnose) on wheat and A. hordei on barley; Aureobasidium zeae (syn. Kapatiella zeae) on corn; Bipolaris and Drechslera spp. (teleomorph: Cochliobolus spp.), e. g. Southern leaf blight (D. maydis) or Northern leaf blight ( B . zeicola) on corn, e. g. spot blotch ( B . sorokiniana) on cereals and e. g.
B. oryzae on rice and turfs; Blumeria (formerly Erysiphe) graminis (powdery mildew) on cereals (e. g. on wheat or barley); Botrytis cinerea (teleomorph: Botryotinia fuckeliana grey mold) on fruits and berries (e. g. strawberries), vegetables (e. g. lettuce, carrots, celery and cabbages); B. squamosa or B. allii on onion family), oilseed rape, ornamentals (e.g. B eliptica), vines, forestry plants and wheat; Bremia lactucae (downy mildew) on lettuce; Ceratocystis (syn. Ophiostoma) spp. (rot or wilt) on broad-leaved trees and evergreens, e. g. C. ulmi (Dutch elm disease) on elms; Cercospora spp. (Cercospora leaf spots) on corn (e. g. Gray leaf spot: C. zeae-maydis ), rice, sugar beets (e. g. C. beticola), sugar cane, vegetables, coffee, soybeans (e. g. C. sojina or
C. kikuchii) and rice; Cladobotryum (syn. Dactylium) spp. (e.g. C. mycophilum
(formerly Dactylium dendroides, teleomorph: Nectria albertinii, Nectria rosella syn. Hypomyces rosellus) on mushrooms; Cladosporium spp. on tomatoes (e. g. C. fulvum leaf mold) and cereals, e. g. C. herbarum (black ear) on wheat; Claviceps purpurea (ergot) on cereals;
Cochliobolus (anamorph: Helminthosporium of Bipolaris) spp. (leaf spots) on corn (C.
carbonum ), cereals (e. g. C. sativus, anamorph: B. sorokiniana) and rice (e. g. C. miyabeanus, anamorph: H. oryzae)·, Colletotrichum (teleomorph: Glomerella) spp. (anthracnose) on cotton (e. g. C. gossypii), corn (e. g. C. graminicola: Anthracnose stalk rot), soft fruits, potatoes (e. g.
C. coccodes·. black dot), beans (e. g. C. lindemuthianum), soybeans (e. g. C. truncatum or C. gloeosporioides), vegetables (e.g. C. lagenarium or C. capsici), fruits (e.g. C. acutatum), coffee (e.g. C. coffeanum or C. kahawae) and C. gloeosporioides on various crops; Corticium spp., e. g. C. sasakii (sheath blight) on rice; Corynespora cassiicola (leaf spots) on soybeans, cotton and ornamentals; Cycloconium spp., e. g. C. oleaginum on olive trees; Cylindrocarpon spp.
(e. g. fruit tree canker or young vine decline, teleomorph: Nectria or Neonectria spp.) on fruit trees, vines (e. g. C. liriodendri, teleomorph: Neonectria liriodendrr. Black Foot Disease) and ornamentals; Dematophora (teleomorph: Rosellinia) necatrix (root and stem rot) on soybeans; Diaporthe spp., e. g. D. phaseolorum (damping off) on soybeans; Drechslera (syn.
Helminthosporium, teleomorph: Pyrenophora) spp. on corn, cereals, such as barley (e. g. D. teres, net blotch) and wheat (e. g. D. tritici-repentis·. tan spot), rice and turf; Esca (dieback, apoplexy) on vines, caused by Formitiporia (syn. Phellinus) punctata, F. mediterranea,
Phaeomoniella chlamydospora (formerly Phaeoacremonium chlamydosporum),
Phaeoacremonium aleophilum and/or Botryosphaeria obtusa\ Elsinoe spp. on pome fruits (E. pyri), soft fruits (E. veneta : anthracnose) and vines (E. ampelina\ anthracnose); Entyloma oryzae (leaf smut) on rice; Epicoccum spp. (black mold) on wheat; Erysiphe spp. (powdery mildew) on sugar beets (E. betae), vegetables (e. g. E. pisi), such as cucurbits (e. g. E.
cichoracearum), cabbages, oilseed rape (e. g. E. cruciferarum)·, Eutypa lata (Eutypa canker or dieback, anamorph: Cytosporina lata, syn. Libertella blepharis) on fruit trees, vines and ornamental woods; Exserohilum (syn. Helminthosporium) spp. on corn (e. g. E. turcicum)·, Fusarium (teleomorph: Gibberella) spp. (wilt, root or stem rot) on various plants, such as E. graminearum or E. culmorum (root rot, scab or head blight) on cereals (e. g. wheat or barley), E. oxysporum on tomatoes, E. solani (f. sp. glycines now syn. E. virguliforme ) and E. tucumaniae and E. brasiliense each causing sudden death syndrome on soybeans, and E. verticillioides on corn; Gaeumannomyces graminis (take-all) on cereals (e. g. wheat or barley) and corn;
Gibberella spp. on cereals (e. g. G. zeae) and rice (e. g. G. fujikuror. Bakanae disease);
Glomerella cingulata on vines, pome fruits and other plants and G. gossypii on cotton; Grain- staining complex on rice; Guignardia bidwellii (black rot) on vines; Gymnosporangium spp. on
rosaceous plants and junipers, e. g. G. sabinae (rust) on pears; Helminthosporium spp. (syn. Drechslera, teleomorph: Cochliobolus) on corn, cereals, potatoes and rice; Hemileia spp., e. g. H. vastatrix (coffee leaf rust) on coffee; Isariopsis clavispora (syn. Cladosporium vitis) on vines; Macrophomina phaseolina (syn. phaseoli) (root and stem rot) on soybeans and cotton;
Microdochium (syn. Fusarium) nivale (pink snow mold) on cereals (e. g. wheat or barley);
Microsphaera diffusa (powdery mildew) on soybeans; Monilinia spp., e. g. M. laxa, M. fructicola and M. fructigena (syn. Monilia spp.: bloom and twig blight, brown rot) on stone fruits and other rosaceous plants; Mycosphaerella spp. on cereals, bananas, soft fruits and ground nuts, such as e. g. M. graminicola (anamorph: Zymoseptoria tritici formerly Septoria triticr. Septoria blotch) on wheat or M. fijiensis (syn. Pseudocercospora fijiensis·. black Sigatoka disease) and M.
musicola on bananas, M. arachidicola (syn. M. arachidis or Cercospora arachidis), M. berkeleyi on peanuts, M. pisi on peas and M. brassiciola on brassicas; Peronospora spp. (downy mildew) on cabbage (e. g. P. brassicae ), oilseed rape (e. g. P. parasitica), onions (e. g. P. destructor), tobacco (P. tabacina) and soybeans (e. g. P. manshurica)·, Phakopsora pachyrhizi and P.
meibomiae (soybean rust) on soybeans; Phialophora spp. e. g. on vines (e. g. P. tracheiphila and P. tetraspora) and soybeans (e. g. P. gregata : stem rot); Phoma lingam (syn. Leptosphaeria biglobosa and L. maculans. root and stem rot) on oilseed rape and cabbage, P. betae (root rot, leaf spot and damping-off) on sugar beets and P. zeae-maydis (syn. Phyllostica zeae) on corn; Phomopsis spp. on sunflowers, vines (e. g. P. viticoia\ can and leaf spot) and soybeans (e. g. stem rot: P. phaseoli, teleomorph: Diaporthe phaseolorum)·, Physoderma maydis (brown spots) on corn; Phytophthora spp. (wilt, root, leaf, fruit and stem root) on various plants, such as paprika and cucurbits (e. g. P. capsici), soybeans (e. g. P. megasperma, syn. P. sojae), potatoes and tomatoes (e. g. P. infestans. late blight) and broad-leaved trees (e. g. P. ramorunr. sudden oak death); Plasmodiophora brassicae (club root) on cabbage, oilseed rape, radish and other plants; Plasmopara spp., e. g. P. viticola (grapevine downy mildew) on vines and P.
halstedii on sunflowers; Podosphaera spp. (powdery mildew) on rosaceous plants, hop, pome and soft fruits (e. g. P. leucotricha on apples) and curcurbits (P. xanthii ); Polymyxa spp., e. g. on cereals, such as barley and wheat (P. graminis) and sugar beets (P. betae) and thereby transmitted viral diseases; Pseudocercosporella herpotrichoides (syn. Oculimacula yallundae,
O. acuformis: eyespot, teleomorph: Tapesia yallundae) on cereals, e. g. wheat or barley;
Pseudoperonospora (downy mildew) on various plants, e. g. P. cubensis on cucurbits or P. humili on hop; Pseudopezicula tracheiphila (red fire disease or .rotbrenneR', anamorph:
Phialophora) on vines; Puccinia spp. (rusts) on various plants, e. g. P. triticina (brown or leaf rust), P. striiformis (stripe or yellow rust), P. hordei (dwarf rust), P. graminis (stem or black rust) or P. recondita (brown or leaf rust) on cereals, such as e. g. wheat, barley or rye, P. kuehnii (orange rust) on sugar cane and P. asparagi on asparagus; Pyrenopeziza spp., e.g. P.
brassicae on oilseed rape; Pyrenophora (anamorph: Drechslera) tritici-repentis (tan spot) on wheat or P. teres (net blotch) on barley; Pyricularia spp., e. g. P. oryzae (teleomorph:
Magnaporthe grisea\ rice blast) on rice and P. grisea on turf and cereals; Pythium spp.
(damping-off) on turf, rice, corn, wheat, cotton, oilseed rape, sunflowers, soybeans, sugar beets, vegetables and various other plants (e. g. P. ultimum or P. aphanidermatum) and P. oligandrum on mushrooms; Ramularia spp., e. g. R. collo-cygni (Ramularia leaf spots, Physiological leaf spots) on barley, R. areola (teleomorph: Mycosphaerella areola) on cotton and R. beticola on sugar beets; Rhizoctonia spp. on cotton, rice, potatoes, turf, corn, oilseed rape, potatoes, sugar beets, vegetables and various other plants, e. g. R. solani (root and stem rot) on soybeans, R. solani (sheath blight) on rice or R. cerealis (Rhizoctonia spring blight) on wheat or barley;
Rhizopus stolonifer (black mold, soft rot) on strawberries, carrots, cabbage, vines and tomatoes; Rhynchosporium secalis and R. commune (scald) on barley, rye and triticale; Sarocladium
oryzae and S. attenuatum (sheath rot) on rice; Sclerotinia spp. (stem rot or white mold) on vegetables (S. minor and S. sclerotiorum) and field crops, such as oilseed rape, sunflowers (e. g. S. sclerotiorum) and soybeans, S. rolfsii (syn. Athelia rolfsii) on soybeans, peanut, vegetables, corn, cereals and ornamentals; Septoria spp. on various plants, e. g. S. glycines (brown spot) on soybeans, S. tritici (syn. Zymoseptoria tritici, Septoria blotch) on wheat and S. (syn. Stagonospora) nodorum (Stagonospora blotch) on cereals; Uncinula (syn. Erysiphe) necator ( powdery mildew, anamorph: Oidium tuckeri) on vines; Setosphaeria spp. (leaf blight) on corn (e. g. S. turcicum, syn. Helminthosporium turcicum) and turf; Sphacelotheca spp. (smut) on corn, (e. g. S. reiliana, syn. Ustilago reiliana. head smut), sorghum und sugar cane;
Sphaerotheca fuliginea (syn. Podosphaera xanthir. powdery mildew) on cucurbits; Spongospora subterranea (powdery scab) on potatoes and thereby transmitted viral diseases; Stagonospora spp. on cereals, e. g. S. nodorum (Stagonospora blotch, teleomorph: Leptosphaeria [syn.
Phaeosphaeria ] nodorum, syn. Septoria nodorum ) on wheat; Synchytrium endobioticum on potatoes (potato wart disease); Taphrina spp., e. g. T. deformans (leaf curl disease) on peaches and T. pruni (plum pocket) on plums; Thielaviopsis spp. (black root rot) on tobacco, pome fruits, vegetables, soybeans and cotton, e. g. T. basicola (syn. Chalara elegans ); Tilletia spp.
(common bunt or stinking smut) on cereals, such as e. g. T. tritici (syn. T. caries, wheat bunt) and T. controversa (dwarf bunt) on wheat; Trichoderma harzianum on mushrooms ; Typhula incarnata (grey snow mold) on barley or wheat; Urocystis spp., e. g. U. occulta (stem smut) on rye; Uromyces spp. (rust) on vegetables, such as beans (e. g. U. appendiculatus, syn. U.
phaseoli), sugar beets (e. g. U. betae or U. beticola ) and on pulses (e.g. U. vignae, U. pisi, U. viciae-fabae and U. fabae)\ Ustilago spp. (loose smut) on cereals (e. g. U. nuda and U.
avaenae ), corn (e. g. U. maydis. corn smut) and sugar cane; Venturia spp. (scab) on apples (e. g. V. inaequalis ) and pears; and Verticillium spp. (wilt) on various plants, such as fruits and ornamentals, vines, soft fruits, vegetables and field crops, e. g. V. longisporum on oilseed rape, V. dahliae on strawberries, oilseed rape, potatoes and tomatoes, and V. fungicola on
mushrooms; Zymoseptoria tritici on cereals.
In a preferred embodiment the compounds I, their mixtures with other active compounds as defined herein and compositions thereof, respectively, are particularly suitable for controlling the following plant diseases: Puccinia spp. (rusts) on various plants, for example, but not limited to P. triticina (brown or leaf rust), P. striiformis (stripe or yellow rust), P. hordei (dwarf rust), P. graminis (stem or black rust) or P. recondita (brown or leaf rust) on cereals, such as e. g. wheat, barley or rye, P. sorghi (common rust) on maize, P. polysora (southern rust) on maize; P.
coronata e.g. on oats, P. sorghi und P. polysora on corn; Puccinia spp. on other crops, e.g. P. heliathi on sunflower, P. arachidis on peanuts; Uromyces spp. on pulses and other crops crops, e.g. Uromyces viciae-fabae, Uromyces vigniae, Uromyces pisi, U. ciceris-arietini, U. betae syn U. beticola; and Phakopsoraceae spp. on various plants, in particular Phakopsora pachyrhizi and P. meibomiae (soybean rust) on soybeans.
Additionally, to date, no cross-resistance has been observed between the compounds I and the current fungicidal solutions used to control phytopathogenic fungi including but not limited to Zymoseptoria tritici, Phakopsora pachyrhizi, Botrytis cinerea, Blumeria graminis, Pyrenophora tritici-repentis, Pyrenophora teres, Alternaria spp., Plasmopara viticola, preferably selected from Zymospeptoria tritici and Phakopsora pachyrhizi.
Fungicide-resistant strains of the above-mentioned phytopathgenic fungi have been reported, with strains resistant to one or more fungicides from various fungicidal mode of action classes being observed including but not limited to beta-tubulin assembly inhibitors, sterol
demethylation-inhibitors (DMI), quinone-outside-inhibitors (Qol) and succinate dehydrogenase inhibitors (SDHI). Thus, compounds I are useful to control phytopathogenic fungi comprising at
least one of the following mutations: E198A/G/K or F200Y in the beta-tubulin gene conferring resistance to beta-tubulin assembly inhibitors (Phytopathol (2008) 98: 397-404), I365N/S,
V368F, Q369H/P, N373S, T447S in the histidine kinase gene Os1 conferring resistance to MAP / histidin kinase inhibitors (dicarboximides; ibida); G143A, G137R or F129L in the mitochondrial cytochrome B Gene (Phytopathol (2003) 93: 891-900; Pest Manag Sci (2016) 72: 121 1-1215) resulting in resistance to Qol; V136A, Y137F (homologous to Y144F/H e.g. in
Parastagonospora nodorum or Y136F e.g. in Erysiphe necator), K147Q, A379G, 1381V, G461S or S509T in the Cyp51 gene resulting in resistance to DMI (Phytopathol (2016) 106: 1278- 1284); P225T/L/F, N225I/T, R265P, T268I/A, H272R/Y/L, H277Y or N230I in the succinate dehydrogenase iron-sulfur subunit gene SdhB, K49E, R64K, N75S, G79R, T79N/I, W80S, P80H/L, N86S/A, G91 R, H134R, S135R, H146R/LK, R151S/T/M, H152R, H153R, I161S, V166M, T168R and G171 D in the subunit C gene SdhC ; and I50F, D124E, M114V, H134R, D145G in the subunit D gene SdhD conferring resistance to SDHI ((2002) 58: 876-88; Pest Manag Sci (2014) 70: 378-388; Environ Microbiol (2014) 16: 2253-66; Pest Manag Sci (2018) 74: 672-681 ; https://www.frac.info/working-group/sdhi-fungicides), and I86F in the subunit C gene SdhC ( Journal of Plant Diseases and Protection 125, 21-26).
The compounds I and compositions thereof, respectively, are also suitable for controlling harmful fungi in the protection of stored products or harvest and in the protection of materials.
The term "protection of materials" is to be understood to denote the protection of technical and non-living materials, such as adhesives, glues, wood, paper and paperboard, textiles, leather, paint dispersions, plastics, cooling lubricants, fiber or fabrics, against the infestation and destruction by harmful microorganisms, such as fungi and bacteria. As to the protection of wood and other materials, the particular attention is paid to the following harmful fungi: Ascomycetes such as Ophiostoma spp., Ceratocystis spp., Aureobasidium pullulans, Sclerophoma spp., Chaetomium spp., Humicola spp., Petriella spp., Trichurus spp.; Basidiomycetes such as Coniophora spp., Coriolus spp., Gloeophyllum spp., Lentinus spp., Pleurotus spp., Poria spp., Serpula spp. and Tyromyces spp., Deuteromycetes such as Aspergillus spp., Cladosporium spp., Penicillium spp., Trichoderma spp., Alternaria spp., Paecilomyces spp. and Zygomycetes such as Mucor spp., and in addition in the protection of stored products and harvest the following yeast fungi are worthy of note: Candida spp. and Saccharomyces cerevisae.
The method of treatment according to the invention can also be used in the field of protecting stored products or harvest against attack of fungi and microorganisms. According to the present invention, the term "stored products" is understood to denote natural substances of plant or animal origin and their processed forms, which have been taken from the natural life cycle and for which long-term protection is desired. Stored products of crop plant origin, such as plants or parts thereof, for example stalks, leafs, tubers, seeds, fruits or grains, can be protected in the freshly harvested state or in processed form, such as pre-dried, moistened, comminuted, ground, pressed or roasted, which process is also known as post-harvest treatment. Also falling under the definition of stored products is timber, whether in the form of crude timber, such as construction timber, electricity pylons and barriers, or in the form of finished articles, such as furniture or objects made from wood. Stored products of animal origin are hides, leather, furs, hairs and the like. The combinations according the present invention can prevent
disadvantageous effects such as decay, discoloration or mold. Preferably "stored products" is understood to denote natural substances of plant origin and their processed forms, more preferably fruits and their processed forms, such as pomes, stone fruits, soft fruits and citrus fruits and their processed forms.
The compounds I and compositions thereof, respectively, may be used for improving the health of a plant. The invention also relates to a method for improving plant health by treating a
plant, its propagation material and/or the locus where the plant is growing or is to grow with an effective amount of compounds I and compositions thereof, respectively.
The term "plant health" is to be understood to denote a condition of the plant and/or its products which is determined by several indicators alone or in combination with each other such as yield (e. g. increased biomass and/or increased content of valuable ingredients), plant vigor (e. g. improved plant growth and/or greener leaves (“greening effect”)), quality (e. g. improved content or composition of certain ingredients) and tolerance to abiotic and/or biotic stress. The above identified indicators for the health condition of a plant may be interdependent or may result from each other.
The compounds of formula I can be present in different crystal modifications whose biological activity may differ. They are likewise subject matter of the present invention.
The compounds I are employed as such or in form of compositions by treating the fungi or the plants, plant propagation materials, such as seeds, soil, surfaces, materials or rooms to be protected from fungal attack with a fungicidally effective amount of the active substances. The application can be carried out both before and after the infection of the plants, plant propagation materials, such as seeds, soil, surfaces, materials or rooms by the fungi.
Plant propagation materials may be treated with compounds I as such or a composition comprising at least one compound I prophylactically either at or before planting or transplanting.
The invention also relates to agrochemical compositions comprising an auxiliary and at least one compound I according to the invention.
An agrochemical composition comprises a fungicidally effective amount of a compound I. The term "effective amount" denotes an amount of the composition or of the compounds I, which is sufficient for controlling harmful fungi on cultivated plants or in the protection of materials and which does not result in a substantial damage to the treated plants. Such an amount can vary in a broad range and is dependent on various factors, such as the fungal species to be controlled, the treated cultivated plant or material, the climatic conditions and the specific compound I used.
The compounds I, their N-oxides and salts can be converted into customary types of agrochemical compositions, e. g. solutions, emulsions, suspensions, dusts, powders, pastes, granules, pressings, capsules, and mixtures thereof. Examples for composition types are suspensions (e. g. SC, OD, FS), emulsifiable concentrates (e. g. EC), emulsions (e. g. EW, EO, ES, ME), capsules (e. g. CS, ZC), pastes, pastilles, wettable powders or dusts (e. g. WP, SP, WS, DP, DS), pressings (e. g. BR, TB, DT), granules (e. g. WG, SG, GR, FG, GG, MG), insecticidal articles (e. g. LN), as well as gel formulations for the treatment of plant propagation materials such as seeds (e. g. GF). These and further compositions types are defined in the “Catalogue of pesticide formulation types and international coding system”, Technical
Monograph No. 2, 6th Ed. May 2008, CropLife International.
The compositions are prepared in a known manner, such as described by Mollet and
Grubemann, Formulation technology, Wiley VCH, Weinheim, 2001 ; or Knowles, New
developments in crop protection product formulation, Agrow Reports DS243, T&F Informa, London, 2005.
Suitable auxiliaries are solvents, liquid carriers, solid carriers or fillers, surfactants,
dispersants, emulsifiers, wetters, adjuvants, solubilizers, penetration enhancers, protective colloids, adhesion agents, thickeners, humectants, repellents, attractants, feeding stimulants, compatibilizers, bactericides, anti-freezing agents, anti-foaming agents, colorants, tackifiers and binders.
Suitable solvents and liquid carriers are water and organic solvents, such as mineral oil fractions of medium to high boiling point, e. g. kerosene, diesel oil; oils of vegetable or animal
origin; aliphatic, cyclic and aromatic hydrocarbons, e. g. toluene, paraffin,
tetrahydronaphthalene, alkylated naphthalenes; alcohols, e. g. ethanol, propanol, butanol, benzyl alcohol, cyclohexanol; glycols; DMSO; ketones, e. g. cyclohexanone; esters, e. g.
lactates, carbonates, fatty acid esters, gamma-butyrolactone; fatty acids; phosphonates;
amines; amides, e. g. N-methyl pyrrolidone, fatty acid dimethyl amides; and mixtures thereof.
Suitable solid carriers or fillers are mineral earths, e. g. silicates, silica gels, talc, kaolins, limestone, lime, chalk, clays, dolomite, diatomaceous earth, bentonite, calcium sulfate, magnesium sulfate, magnesium oxide; polysaccharides, e. g. cellulose, starch; fertilizers, e. g. ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas; products of vegetable origin, e. g. cereal meal, tree bark meal, wood meal, nutshell meal, and mixtures thereof.
Suitable surfactants are surface-active compounds, such as anionic, cationic, nonionic and amphoteric surfactants, block polymers, polyelectrolytes, and mixtures thereof. Such surfactants can be used as emulsifier, dispersant, solubilizer, wetter, penetration enhancer, protective colloid, or adjuvant. Examples of surfactants are listed in McCutcheon’s, Vol.1 : Emulsifiers & Detergents, McCutcheon’s Directories, Glen Rock, USA, 2008 (International Ed. or North American Ed.).
Suitable anionic surfactants are alkali, alkaline earth or ammonium salts of sulfonates, sulfates, phosphates, carboxylates, and mixtures thereof. Examples of sulfonates are alkylaryl sulfonates, diphenyl sulfonates, alpha-olefin sulfonates, lignin sulfonates, sulfonates of fatty acids and oils, sulfonates of ethoxylated alkylphenols, sulfonates of alkoxylated arylphenols, sulfonates of condensed naphthalenes, sulfonates of dodecyl- and tridecylbenzenes, sulfonates of naphthalenes and alkyl naphthalenes, sulfosuccinates or sulfosuccinamates. Examples of sulfates are sulfates of fatty acids and oils, of ethoxylated alkylphenols, of alcohols, of ethoxylated alcohols, or of fatty acid esters. Examples of phosphates are phosphate esters. Examples of carboxylates are alkyl carboxylates, and carboxylated alcohol or alkylphenol ethoxylates.
Suitable nonionic surfactants are alkoxylates, N-substituted fatty acid amides, amine oxides, esters, sugar-based surfactants, polymeric surfactants, and mixtures thereof. Examples of alkoxylates are compounds such as alcohols, alkylphenols, amines, amides, arylphenols, fatty acids or fatty acid esters which have been alkoxylated with 1 to 50 equivalents. Ethylene oxide and/or propylene oxide may be employed for the alkoxylation, preferably ethylene oxide.
Examples of N-substituted fatty acid amides are fatty acid glucamides or fatty acid
alkanolamides. Examples of esters are fatty acid esters, glycerol esters or monoglycerides. Examples of sugar-based surfactants are sorbitans, ethoxylated sorbitans, sucrose and glucose esters or alkylpolyglucosides. Examples of polymeric surfactants are home- or copolymers of vinyl pyrrolidone, vinyl alcohols, or vinyl acetate.
Suitable cationic surfactants are quaternary surfactants, for example quaternary ammonium compounds with one or two hydrophobic groups, or salts of long-chain primary amines. Suitable amphoteric surfactants are alkylbetains and imidazolines. Suitable block polymers are block polymers of the A-B or A-B-A type comprising blocks of polyethylene oxide and polypropylene oxide, or of the A-B-C type comprising alkanol, polyethylene oxide and polypropylene oxide. Suitable polyelectrolytes are polyacids or polybases. Examples of polyacids are alkali salts of polyacrylic acid or polyacid comb polymers. Examples of polybases are polyvinyl amines or polyethylene amines.
Suitable adjuvants are compounds, which have a negligible or even no pesticidal activity themselves, and which improve the biological performance of the compound I on the target. Examples are surfactants, mineral or vegetable oils, and other auxiliaries. Further examples are listed by Knowles, Adjuvants and additives, Agrow Reports DS256, T&F Informa UK, 2006,
chapter 5.
Suitable thickeners are polysaccharides (e. g. xanthan gum, carboxymethyl cellulose), inorganic clays (organically modified or unmodified), polycarboxylates, and silicates.
Suitable bactericides are bronopol and isothiazolinone derivatives such as alkyliso- thiazolinones and benzisothiazolinones.
Suitable anti-freezing agents are ethylene glycol, propylene glycol, urea and glycerin.
Suitable anti-foaming agents are silicones, long chain alcohols, and salts of fatty acids.
Suitable colorants (e. g. in red, blue, or green) are pigments of low water solubility and water- soluble dyes. Examples are inorganic colorants (e. g. iron oxide, titan oxide, iron
hexacyanoferrate) and organic colorants (e. g. alizarin-, azo- and phthalocyanine colorants).
Suitable tackifiers or binders are polyvinyl pyrrolidones, polyvinyl acetates, polyvinyl alcohols, polyacrylates, biological or synthetic waxes, and cellulose ethers.
Examples for composition types and their preparation are:
i) Water-soluble concentrates (SL, LS)
10-60 wt% of a compound I and 5-15 wt% wetting agent (e. g. alcohol alkoxylates) are dissolved in water and/or in a water-soluble solvent (e. g. alcohols) ad 100 wt%. The active substance dissolves upon dilution with water.
ii) Dispersible concentrates (DC)
5-25 wt% of a compound I and 1-10 wt% dispersant (e. g. polyvinyl pyrrolidone) are dissolved in organic solvent (e. g. cyclohexanone) ad 100 wt%. Dilution with water gives a dispersion. iii) Emulsifiable concentrates (EC)
15-70 wt% of a compound I and 5-10 wt% emulsifiers (e. g. calcium dodecylbenzenesulfonate and castor oil ethoxylate) are dissolved in water-insoluble organic solvent (e. g. aromatic hydrocarbon) ad 100 wt%. Dilution with water gives an emulsion.
iv) Emulsions (EW, EO, ES)
5-40 wt% of a compound I and 1-10 wt% emulsifiers (e. g. calcium dodecylbenzenesulfonate and castor oil ethoxylate) are dissolved in 20-40 wt% water-insoluble organic solvent (e. g. aromatic hydrocarbon). This mixture is introduced into water ad 100 wt% by means of an emulsifying machine and made into a homogeneous emulsion. Dilution with water gives an emulsion.
v) Suspensions (SC, OD, FS)
In an agitated ball mill, 20-60 wt% of a compound I are comminuted with addition of 2-10 wt% dispersants and wetting agents (e. g. sodium lignosulfonate and alcohol ethoxylate), 0.1-2 wt% thickener (e. g. xanthan gum) and water ad 100 wt% to give a fine active substance suspension. Dilution with water gives a stable suspension of the active substance. For FS type composition up to 40 wt% binder (e. g. polyvinyl alcohol) is added.
vi) Water-dispersible granules and water-soluble granules (WG, SG)
50-80 wt% of a compound I are ground finely with addition of dispersants and wetting agents (e. g. sodium lignosulfonate and alcohol ethoxylate) ad 100 wt% and prepared as water- dispersible or water-soluble granules by means of technical appliances (e. g. extrusion, spray tower, fluidized bed). Dilution with water gives a stable dispersion or solution of the active substance.
vii) Water-dispersible powders and water-soluble powders (WP, SP, WS)
50-80 wt% of a compound I are ground in a rotor-stator mill with addition of 1-5 wt%
dispersants (e. g. sodium lignosulfonate), 1-3 wt% wetting agents (e. g. alcohol ethoxylate) and solid carrier (e. g. silica gel) ad 100 wt%. Dilution with water gives a stable dispersion or solution of the active substance.
viii) Gel (GW, GF)
In an agitated ball mill, 5-25 wt% of a compound I are comminuted with addition of 3-10 wt% dispersants (e. g. sodium lignosulfonate), 1-5 wt% thickener (e. g. carboxymethyl cellulose) and water ad 100 wt% to give a fine suspension of the active substance. Dilution with water gives a stable suspension of the active substance.
ix) Microemulsion (ME)
5-20 wt% of a compound I are added to 5-30 wt% organic solvent blend (e. g. fatty acid dimethyl amide and cyclohexanone), 10-25 wt% surfactant blend (e. g. alcohol ethoxylate and arylphenol ethoxylate), and water ad 100 %. This mixture is stirred for 1 h to produce spontaneously a thermodynamically stable microemulsion.
x) Microcapsules (CS)
An oil phase comprising 5-50 wt% of a compound I, 0-40 wt% water insoluble organic solvent (e. g. aromatic hydrocarbon), 2-15 wt% acrylic monomers (e. g. methylmethacrylate, methacrylic acid and a di- or triacrylate) are dispersed into an aqueous solution of a protective colloid (e. g. polyvinyl alcohol). Radical polymerization results in the formation of poly(meth)acrylate microcapsules. Alternatively, an oil phase comprising 5-50 wt% of a compound I according to the invention, 0-40 wt% water insoluble organic solvent (e. g. aromatic hydrocarbon), and an isocyanate monomer (e. g. diphenylmethene-4,4’-diisocyanatae) are dispersed into an aqueous solution of a protective colloid (e. g. polyvinyl alcohol). The addition of a polyamine (e. g.
hexamethylenediamine) results in the formation of polyurea microcapsules. The monomers amount to 1-10 wt%. The wt% relate to the total CS composition.
xi) Dustable powders (DP, DS)
1-10 wt% of a compound I are ground finely and mixed intimately with solid carrier (e. g. finely divided kaolin) ad 100 wt%.
xii) Granules (GR, FG)
0.5-30 wt% of a compound I is ground finely and associated with solid carrier (e. g. silicate) ad 100 wt%. Granulation is achieved by extrusion, spray-drying or fluidized bed.
xiii) Ultra-low volume liquids (UL)
1-50 wt% of a compound I are dissolved in organic solvent (e. g. aromatic hydrocarbon) ad 100 wt%.
The compositions types i) to xiii) may optionally comprise further auxiliaries, such as 0.1-1 wt% bactericides, 5-15 wt% anti-freezing agents, 0.1-1 wt% anti-foaming agents, and 0.1-1 wt% colorants.
The agrochemical compositions generally comprise between 0.01 and 95%, preferably between 0.1 and 90%, more preferably between 1 and 70%, and in particular between 10 and 60%, by weight of active substance. The active substances are employed in a purity of from 90% to 100%, preferably from 95% to 100% (according to NMR spectrum).
For the purposes of treatment of plant propagation materials, particularly seeds, solutions for seed treatment (LS), Suspoemulsions (SE), flowable concentrates (FS), powders for dry treatment (DS), water-dispersible powders for slurry treatment (WS), water-soluble powders (SS), emulsions (ES), emulsifiable concentrates (EC), and gels (GF) are usually employed. The compositions in question give, after two-to-tenfold dilution, active substance concentrations of from 0.01 to 60% by weight, preferably from 0.1 to 40%, in the ready-to-use preparations.
Application can be carried out before or during sowing. Methods for applying compound I and compositions thereof, respectively, onto plant propagation material, especially seeds, include dressing, coating, pelleting, dusting, and soaking as well as in-furrow application methods. Preferably, compound I or the compositions thereof, respectively, are applied on to the plant
propagation material by a method such that germination is not induced, e. g. by seed dressing, pelleting, coating and dusting.
When employed in plant protection, the amounts of active substances applied are, depending on the kind of effect desired, from 0.001 to 2 kg per ha, preferably from 0.005 to 2 kg per ha, more preferably from 0.05 to 0.9 kg per ha, and in particular from 0.1 to 0.75 kg per ha.
In treatment of plant propagation materials such as seeds, e. g. by dusting, coating or drenching seed, amounts of active substance of from 0.1 to 1000 g, preferably from 1 to 1000 g, more preferably from 1 to 100 g and most preferably from 5 to 100 g, per 100 kilogram of plant propagation material (preferably seeds) are generally required.
When used in the protection of materials or stored products, the amount of active substance applied depends on the kind of application area and on the desired effect. Amounts customarily applied in the protection of materials are 0.001 g to 2 kg, preferably 0.005 g to 1 kg, of active substance per cubic meter of treated material.
Various types of oils, wetters, adjuvants, fertilizer, or micronutrients, and further pesticides (e. g. herbicides, insecticides, fungicides, growth regulators, safeners, biopesticides) may be added to the active substances or the compositions comprising them as premix or, if appropriate not until immediately prior to use (tank mix). These agents can be admixed with the compositions according to the invention in a weight ratio of 1 :100 to 100:1 , preferably 1 :10 to 10:1.
A pesticide is generally a chemical or biological agent (such as pestidal active ingredient, compound, composition, virus, bacterium, antimicrobial or disinfectant) that through its effect deters, incapacitates, kills or otherwise discourages pests. Target pests can include insects, plant pathogens, weeds, mollusks, birds, mammals, fish, nematodes (roundworms), and microbes that destroy property, cause nuisance, spread disease or are vectors for disease. The term“pesticide” includes also plant growth regulators that alter the expected growth, flowering, or reproduction rate of plants; defoliants that cause leaves or other foliage to drop from a plant, usually to facilitate harvest; desiccants that promote drying of living tissues, such as unwanted plant tops; plant activators that activate plant physiology for defense of against certain pests; safeners that reduce unwanted herbicidal action of pesticides on crop plants; and plant growth promoters that affect plant physiology e.g. to increase plant growth, biomass, yield or any other quality parameter of the harvestable goods of a crop plant.
The user applies the composition according to the invention usually from a predosage device, a knapsack sprayer, a spray tank, a spray plane, or an irrigation system. Usually, the
agrochemical composition is made up with water, buffer, and/or further auxiliaries to the desired application concentration and the ready-to-use spray liquor or the agrochemical composition according to the invention is thus obtained. Usually, 20 to 2000 liters, preferably 50 to 400 liters, of the ready-to-use spray liquor are applied per hectare of agricultural useful area.
According to one embodiment, individual components of the composition according to the invention such as parts of a kit or parts of a binary or ternary mixture may be mixed by the user himself in a spray tank or any other kind of vessel used for applications (e. g. seed treater drums, seed pelleting machinery, knapsack sprayer) and further auxiliaries may be added, if appropriate.
Consequently, one embodiment of the invention is a kit for preparing a usable pesticidal composition, the kit comprising a) a composition comprising component 1) as defined herein and at least one auxiliary; and b) a composition comprising component 2) as defined herein and at least one auxiliary; and optionally c) a composition comprising at least one auxiliary and optionally a further active component 3) as defined herein.
Mixing the compounds I or the compositions comprising them in the use form as fungicides
with other fungicides results in many cases in an expansion of the fungicidal spectrum of activity being obtained or in a prevention of fungicide resistance development. Furthermore, in many cases, synergistic effects are obtained.
The following list of pesticides II (e. g. pesticidally-active substances and biopesticides), in conjunction with which the compounds I can be used, is intended to illustrate the possible combinations but does not limit them:
A) Respiration inhibitors
- Inhibitors of complex III at Q0 site: azoxystrobin (A.1.1), coumethoxystrobin (A.1.2),
coumoxystrobin (A.1.3), dimoxystrobin (A.1.4), enestroburin (A.1.5), fenaminstrobin (A.1.6), fenoxystrobin/flufenoxystrobin (A.1.7), fluoxastrobin (A.1.8), kresoxim-methyl (A.1.9), mandestrobin (A.1.10), metominostrobin (A.1.11), orysastrobin (A.1.12), picoxystrobin
(A.1.13), pyraclostrobin (A.1.14), pyrametostrobin (A.1.15), pyraoxystrobin (A.1.16), trifloxy- strobin (A.1.17), 2-(2-(3-(2,6-dichlorophenyl)-1-methyl-allylideneaminooxymethyl)-phenyl)- 2-methoxyimino-/\/-methyl-acetamide (A.1.18), pyribencarb (A.1.19), triclopyricarb/chloro- dincarb (A.1.20), famoxadone (A.1.21), fenamidone (A.1.21a), methyl-/\/-[2-[(1 ,4-dimethyl- 5-phenyl-pyrazol-3-yl)oxylmethyl]phenyl]-/\/-methoxy-carbamate (A.1.22), metyltetrapole (A.1.25), (Z,2£)-5-[1 -(2, 4-dichlorophenyl)pyrazol-3-yl]-oxy-2-methoxyimino-/\/, 3-dimethyl- pent-3-enamide (A.1.34), (Z,2£)-5-[1-(4-chlorophenyl)pyrazol-3-yl]oxy-2-methoxyimino-/V,3- dimethyl-pent-3-enamide (A.1.35), pyriminostrobin (A.1.36), bifujunzhi (A.1.37), 2-(ortho- ((2,5-dimethylphenyl-oxymethylen)phenyl)-3-methoxy-acrylic acid methylester (A.1.38);
- inhibitors of complex III at Q, site: cyazofamid (A.2.1), amisulbrom (A.2.2),
[(6S,7R,8R)-8-benzyl-3-[(3-hydroxy-4-methoxy-pyridine-2-carbonyl)amino]-6-methyl-4,9-di- oxo-1 ,5-dioxonan-7-yl] 2-methylpropanoate (A.2.3), fenpicoxamid (A.2.4), florylpicoxamid (A.2.5);
- inhibitors of complex II: benodanil (A.3.1), benzovindiflupyr (A.3.2), bixafen (A.3.3), boscalid (A.3.4), carboxin (A.3.5), fenfuram (A.3.6), fluopyram (A.3.7), flutolanil (A.3.8), fluxapyroxad (A.3.9), furametpyr (A.3.10), isofetamid (A.3.11), isopyrazam (A.3.12), mepronil (A.3.13), oxycarboxin (A.3.14), penflufen (A.3.15), penthiopyrad (A.3.16), pydiflumetofen (A.3.17), pyraziflumid (A.3.18), sedaxane (A.3.19), tecloftalam (A.3.20), thifluzamide (A.3.21), inpyrfluxam (A.3.22), pyrapropoyne (A.3.23), fluindapyr (A.3.28), N-[2-[2-chloro-4- (trifluoro-rnethyOphenoxyjphenylj-S-tdifluoromethyO-S-fluoro-l-methyl-pyrazole^- carboxamide (A.3.29), methyl (£)-2-[2-[(5-cyano-2-methyl-phenoxy)methyl]phenyl]-3- methoxy-prop-2-enoate (A.3.30), isoflucypram (A.3.31), 2-(difluoromethyl)-/\/-(1 , 1 ,3-trimethyl- indan-4-yl)pyridine-3-carboxamide (A.3.32), 2-(difluoromethyl)-/\/-[(3R)-1 ,1 ,3-trimethylindan- 4-yl]pyridine-3-carboxamide (A.3.33), 2-(difluoromethyl)-/\/-(3-ethyl-1 ,1-dimethyl-indan-4-yl)- pyridine-3-carboxamide (A.3.34), 2-(difluoromethyl)-/\/-[(3R)-3-ethyl-1 ,1-dimethyl-indan-4-yl]- pyridine-3-carboxamide (A.3.35), 2-(difluoromethyl)-/\/-(1 ,1-dimethyl-3-propyl-indan-4-yl)py- ridine-3-carboxamide (A.3.36), 2-(difluoromethyl)-/\/-[(3R)-1 ,1-dimethyl-3-propyl-indan-4-yl]- pyridine-3-carboxamide (A.3.37), 2-(difluoromethyl)-/\/-(3-isobutyl-1 ,1-dimethyl-indan-4-yl)- pyridine-3-carboxamide (A.3.38), 2-(difluoromethyl)-/\/-[(3R)-3-isobutyl-1 ,1-dimethyl-indan- 4-yl]pyridine-3-carboxamide (A.3.39);
- other respiration inhibitors: diflumetorim (A.4.1); nitrophenyl derivates: binapacryl (A.4.2), dinobuton (A.4.3), dinocap (A.4.4), fluazinam (A.4.5), meptyldinocap (A.4.6), ferimzone (A.4.7); organometal compounds: fentin salts, e. g. fentin-acetate (A.4.8), fentin chloride (A.4.9) or fentin hydroxide (A.4.10); ametoctradin (A.4.11); silthiofam (A.4.12);
B) Sterol biosynthesis inhibitors (SBI fungicides)
- C14 demethylase inhibitors: triazoles: azaconazole (B.1.1), bitertanol (B.1.2), bromu- conazole (B.1.3), cyproconazole (B.1.4), difenoconazole (B.1.5), diniconazole (B.1.6), diniconazole-M (B.1.7), epoxiconazole (B.1.8), fenbuconazole (B.1.9), fluquinconazole (B.1.10), flusilazole (B.1.11), flutriafol (B.1.12), hexaconazole (B.1.13), imibenconazole (B.1.14), ipconazole (B.1.15), metconazole (B.1.17), myclobutanil (B.1.18), oxpoconazole (B.1.19), paclobutrazole (B.1.20), penconazole (B.1.21), propiconazole (B.1.22), prothio- conazole (B.1.23), simeconazole (B.1.24), tebuconazole (B.1.25), tetraconazole (B.1.26), triadimefon (B.1.27), triadimenol (B.1.28), triticonazole (B.1.29), uniconazole (B.1.30), 2-(2,4-difluorophenyl)-1 ,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]- 2-pyridyl]propan-2-ol (B.1.31), 2-(2,4-difluorophenyl)-1 ,1-difluoro-3-(tetrazol-1-yl)-
1-[5-[4-(trifluoromethoxy)phenyl]-2-pyridyl]propan-2-ol (B.1.32), 4-[[6-[2-(2,4-difluorophenyl)- 1 ,1-difluoro-2-hydroxy-3-(5-sulfanyl-1 ,2,4-triazol-1-yl)propyl]-3-pyridyl]oxy]benzonitrile (B.1.33), ipfentrifluconazole (B.1.37), mefentrifluconazole (B.1.38), 2-(chloromethyl)-2- methyl-5-(p-tolylmethyl)-1-(1 ,2,4-triazol-1-ylmethyl)cyclopentanol (B.1.43); imidazoles:
i azalil (B.1.44), pefurazoate (B.1.45), prochloraz (B.1.46), triflumizol (B.1.47); pyrimidines, pyridines, piperazines: fenarimol (B.1.49), pyrifenox (B.1.50), triforine (B.1.51), [3-(4-chloro-
2-fluoro-phenyl)-5-(2,4-difluorophenyl)isoxazol-4-yl]-(3-pyridyl)methanol (B.1.52);
- Deltal 4-reductase inhibitors: aldimorph (B.2.1), dodemorph (B.2.2), dodemorph-acetate (B.2.3), fenpropimorph (B.2.4), tridemorph (B.2.5), fenpropidin (B.2.6), piperalin (B.2.7), spiroxamine (B.2.8);
- Inhibitors of 3-keto reductase: fenhexamid (B.3.1);
- Other Sterol biosynthesis inhibitors: chlorphenomizole (B.4.1);
C) Nucleic acid synthesis inhibitors
- phenylamides or acyl amino acid fungicides: benalaxyl (C.1.1), benalaxyl-M (C.1.2), kiralaxyl (C.1.3), metalaxyl (C.1.4), metalaxyl-M (C.1.5), ofurace (C.1.6), oxadixyl (C.1.7);
- other nucleic acid synthesis inhibitors: hymexazole (C.2.1), octhilinone (C.2.2), oxolinic acid (C.2.3), bupirimate (C.2.4), 5-fluorocytosine (C.2.5), 5-fluoro-2-(p-tolylmethoxy)pyrimidin- 4-amine (C.2.6), 5-fluoro-2-(4-fluorophenylmethoxy)pyrimidin-4-amine (C.2.7), 5-fluoro- 2-(4-chlorophenylmethoxy)pyrimidin-4 amine (C.2.8);
D) Inhibitors of cell division and cytoskeleton
- tubulin inhibitors: benomyl (D.1.1), carbendazim (D.1.2), fuberidazole (D1.3), thiabendazole (D.1.4), thiophanate-methyl (D.1.5), pyridachlometyl (D.1.6), A/-ethyl-2-[(3-ethynyl-8-methyl- 6-quinolyl)oxy]butanamide (D.1.8), A/-ethyl-2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-2- methylsulfanyl-acetamide (D.1.9), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/\/-(2- fluoroethyl)butanamide (D.1.10), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/\/-(2-fluoroethyl)-2- methoxy-acetamide (D.1.11), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/\/-propyl-butanamide (D.1.12), 2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-2-methoxy-/\/-propyl-acetamide (D.1.13), 2- [(3-ethynyl-8-methyl-6-quinolyl)oxy]-2-methylsulfanyl-/\/-propyl-acetamide (D.1.14),
2-[(3-ethynyl-8-methyl-6-quinolyl)oxy]-/\/-(2-fluoroethyl)-2-methylsulfanyl-acetamide (D.1.15), 4-(2-bromo-4-fluoro-phenyl)-/\/-(2-chloro-6-fluoro-phenyl)-2,5-dimethyl-pyrazol-3-amine (D.1.16);
- other cell division inhibitors: diethofencarb (D.2.1), ethaboxam (D.2.2), pencycuron (D.2.3), fluopicolide (D.2.4), zoxamide (D.2.5), metrafenone (D.2.6), pyriofenone (D.2.7),
phenamacril (D.2.8);
E) Inhibitors of amino acid and protein synthesis
- methionine synthesis inhibitors: cyprodinil (E.1.1), mepanipyrim (E.1.2), pyrimethanil (E.1.3);
- protein synthesis inhibitors: blasticidin-S (E.2.1), kasugamycin (E.2.2), kasugamycin hydro- chloride-hydrate (E.2.3), mildiomycin (E.2.4), streptomycin (E.2.5), oxytetracyclin (E.2.6);
F) Signal transduction inhibitors
- MAP / histidine kinase inhibitors: fluoroimid (F.1.1), iprodione (F.1.2), procymidone (F.1.3), vinclozolin (F.1.4), fludioxonil (F.1.5);
- G protein inhibitors: quinoxyfen (F.2.1);
G) Lipid and membrane synthesis inhibitors
- Phospholipid biosynthesis inhibitors: edifenphos (G .1.1), iprobenfos (G.1.2), pyrazophos (G.1.3), isoprothiolane (G.1.4);
- lipid peroxidation: dicloran (G.2.1), quintozene (G.2.2), tecnazene (G.2.3), tolclofos-methyl (G.2.4), biphenyl (G.2.5), chloroneb (G.2.6), etridiazole (G.2.7), zinc thiazole (G.2.8);
- phospholipid biosynthesis and cell wall deposition: dimethomorph (G.3.1), flumorph (G.3.2), mandipropamid (G.3.3), pyrimorph (G.3.4), benthiavalicarb (G.3.5), iprovalicarb (G.3.6), valifenalate (G.3.7);
- compounds affecting cell membrane permeability and fatty acides: propamocarb (G.4.1);
- inhibitors of oxysterol binding protein: oxathiapiprolin (G.5.1), fluoxapiprolin (G.5.3), 4-[1-[2- [3-(difluoromethyl)-5-methyl-pyrazol-1-yl]acetyl]-4-piperidyl]-/\/-tetralin-1-yl-pyridine-2- carboxamide (G.5.4), 4-[1-[2-[3,5-bis(difluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/\/- tetralin-1-yl-pyridine-2-carboxamide (G.5.5), 4-[1-[2-[3-(difluoromethyl)-5-(tri- fluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/\/-tetralin-1-yl-pyridine-2-carboxamide (G.5.6), 4-[1-[2-[5-cyclopropyl-3-(difluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/\/-tetralin-1-yl-pyri- dine-2-carboxamide (G.5.7), 4-[1-[2-[5-methyl-3-(trifluoromethyl)pyrazol-1-yl]acetyl]-4-piperi- dyl]-/\/-tetralin-1-yl-pyridine-2-carboxamide (G.5.8), 4-[1-[2-[5-(difluoromethyl)-3-(trifluoro- methyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/\/-tetralin-1-yl-pyridine-2-carboxamide (G.5.9),
4-[1-[2-[3,5-bis(trifluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]-/\/-tetralin-1-yl-pyridine-2-car- boxamide (G.5.10), (4-[1-[2-[5-cyclopropyl-3-(trifluoromethyl)pyrazol-1-yl]acetyl]-4-piperidyl]- A/-tetralin-1-yl-pyridine-2-carboxamide (G.5.11);
H) Inhibitors with Multi Site Action
- inorganic active substances: Bordeaux mixture (H.1.1), copper (H.1.2), copper acetate
(H.1.3), copper hydroxide (H.1.4), copper oxychloride (H.1.5), basic copper sulfate (H.1.6), sulfur (H.1.7);
- thio- and dithiocarbamates: ferbam (H.2.1), mancozeb (H.2.2), maneb (H.2.3), metam
(H.2.4), metiram (H.2.5), propineb (H.2.6), thiram (H.2.7), zineb (H.2.8), ziram (H.2.9);
- organochlorine compounds: anilazine (H.3.1), chlorothalonil (H.3.2), captafol (H.3.3), captan (H.3.4), folpet (H.3.5), dichlofluanid (H.3.6), dichlorophen (H.3.7), hexachlorobenzene (H.3.8), pentachlorphenole (H.3.9) and its salts, phthalide (H.3.10), tolylfluanid (H.3.11);
- guanidines and others: guanidine (H.4.1), dodine (H.4.2), dodine free base (H.4.3),
guazatine (H.4.4), guazatine-acetate (H.4.5), iminoctadine (H.4.6), iminoctadine-triacetate (H.4.7), iminoctadine-tris(albesilate) (H.4.8), dithianon (H.4.9), 2,6-dimethyl-1H,5H-[1 ,4]di- thiino[2,3-c:5,6-c']dipyrrole-1 ,3,5,7(2H,6H)-tetraone (H.4.10);
I) Cell wall synthesis inhibitors
- inhibitors of glucan synthesis: validamycin (1.1.1), polyoxin B (1.1.2);
- melanin synthesis inhibitors: pyroquilon (1.2.1), tricyclazole (1.2.2), carpropamid (1.2.3), dicyclomet (1.2.4), fenoxanil (1.2.5);
J) Plant defence inducers
- acibenzolar-S-methyl (J.1.1), probenazole (J.1.2), isotianil (J.1.3), tiadinil (J.1.4), prohexa- dione-calcium (J.1.5); phosphonates: fosetyl (J.1.6), fosetyl-aluminum (J.1.7), phosphorous acid and its salts (J.1.8), calcium phosphonate (J.1.11 ) , potassium phosphonate (J.1.12), potassium or sodium bicarbonate (J.1.9), 4-cyclopropyl-/\/-(2,4-dimethoxyphenyl)thiadiazole-
5-carboxamide (J.1.10);
K) Unknown mode of action
- bronopol (K.1.1), chinomethionat (K.1.2), cyflufenamid (K.1.3), cymoxanil (K.1.4), dazomet (K.1.5), debacarb (K.1.6), diclocymet (K.1.7), diclomezine (K.1.8), difenzoquat (K.1.9), di- fenzoquat-methylsulfate (K.1.10), diphenylamin (K.1.11), fenitropan (K.1.12), fenpyrazamine (K.1.13), flumetover (K.1.14), flusulfamide (K.1.15), flutianil (K.1.16), harpin (K.1.17), metha- sulfocarb (K.1.18), nitrapyrin (K.1.19), nitrothal-isopropyl (K.1.20), tolprocarb (K.1.21), oxin- copper (K.1.22), proquinazid (K.1.23), tebufloquin (K.1.24), tecloftala (K.1.25), triazoxide (K.1.26), L/ -(4-(4-chloro-3-trifluoromethyl-phenoxy)-2,5-dimethyl-phenyl)-/\/-ethyl-/\/-methyl formamidine (K.1.27), L/ -(4-(4-fluoro-3-trifluoromethyl-phenoxy)-2,5-dimethyl-phenyl)-/\/-eth- yl-/\/-methyl formamidine (K.1.28), A/-[4-[[3-[(4-chlorophenyl)methyl]-1 ,2,4-thiadiazol-5-yl]- oxy]-2,5-dimethyl-phenyl]-/\/-ethyl-/\/-methyl-formamidine (K.1.29), L/ -(5-bromo-6-indan-2- yloxy-2-methyl-3-pyridyl)-/\/-ethyl-/\/-methyl-formamidine (K.1.30), A/-[5-bromo-6-[1-(3,5-diflu- orophenyl)ethoxy]-2-methyl-3-pyridyl]-/\/-ethyl-/\/-methyl-formamidine (K.1.31), A/-[5-bromo- 6-(4-isopropylcyclohexoxy)-2-methyl-3-pyridyl]-/\/-ethyl-/\/-methyl-formamidine (K.1.32), A/-[5-bromo-2-methyl-6-(1-phenylethoxy)-3-pyridyl]-/\/-ethyl-/\/-methyl-formamidine (K.1.33), A/-(2-methyl-5-trifluoromethyl-4-(3-trimethylsilanyl-propoxy)-phenyl)-/\/-ethyl-/\/-methyl forma midine (K.1.34), L/ -(5-difluoromethyl-2-methyl-4-(3-trimethylsilanyl-propoxy)-phenyl)-/\/-ethyl- N- methyl formamidine (K.1.35), 2-(4-chloro-phenyl)-/\/-[4-(3,4-dimethoxy-phenyl)-isoxazol- 5-yl]-2-prop-2-ynyloxy-acetamide (K.1.36), 3-[5-(4-chloro-phenyl)-2,3-dimethyl-isoxazolidin- 3-yl]-pyridine (pyrisoxazole) (K.1.37), 3-[5-(4-methylphenyl)-2,3-dimethyl-isoxazolidin-3 yl]- pyridine (K.1.38), 5-chloro-1-(4,6-dimethoxy-pyrimidin-2-yl)-2-methyl-1/-/-benzoimidazole (K.1.39), ethyl (Z)-3-amino-2-cyano-3-phenyl-prop-2-enoate (K.1.40), picarbutrazox (K.1.41), pentyl A/-[6-[[(Z)-[(1-methyltetrazol-5-yl)-phenyl-methylene]amino]oxymethyl]-2-pyridyl]carba- mate (K.1.42), but-3-ynyl A/-[6-[[(Z)-[(1-methyltetrazol-5-yl)-phenyl-methylene]amino]oxy- methyl]-2-pyridyl]carbamate (K.1.43), ipflufenoquin (K.1.44), quinofumelin (K.1.47), benziothiazolinone (K.1.48), bromothalonil (K.1.49), 2-(6-benzyl-2-pyridyl)quinazoline (K.1.50), 2-[6-(3-fluoro-4-methoxy-phenyl)-5-methyl-2-pyridyl]quinazoline (K.1.51), dichlobentiazox (K.1.52), L/ -(2,5-dimethyl-4-phenoxy-phenyl)-/\/-ethyl-/\/-methyl-formamidine (K.1.53), pyrifenamine (K.1.54), fluopimomide (K.1.55), N'-[5-bromo-2-methyl-6-(1-methyl-2 propoxy-ethoxy)-3-pyridyl]-N-ethyl-N-methyl-formamidine (K.1.56);
M) Growth regulators
abscisic acid (M.1.1), amidochlor, ancymidol, 6-benzylaminopurine, brassinolide, butralin, chlormequat, chlormequat chloride, choline chloride, cyclanilide, daminozide, dikegulac, dimethipin, 2,6-dimethylpuridine, ethephon, flumetralin, flurprimidol, fluthiacet, forchlorfenuron, gibberellic acid, inabenfide, indole-3-acetic acid , maleic hydrazide, mefluidide, mepiquat, mepiquat chloride, naphthaleneacetic acid, A/-6-benzyladenine, paclobutrazol, prohexadione, prohexadione-calcium, prohydrojasmon, thidiazuron, triapenthenol, tributyl phosphorotrithioate, 2,3,5-tri-iodobenzoic acid , trinexapac-ethyl, uniconazole;
N) Herbicides from classes N.1 to N.15
N.1 Lipid biosynthesis inhibitors: alloxydim, alloxydim-sodium, butroxydim, clethodim,
clodinafop, clodinafop-propargyl, cycloxydim, cyhalofop, cyhalofop-butyl, diclofop, diclofop- methyl, fenoxaprop, fenoxaprop-ethyl, fenoxaprop-P, fenoxaprop-P-ethyl, fluazifop, fluazifop- butyl, fluazifop-P, fluazifop-P-butyl, haloxyfop, haloxyfop-methyl, haloxyfop-P, haloxyfop-P- methyl, metamifop, pinoxaden, profoxydim, propaquizafop, quizalofop, quizalofop-ethyl, quizalofop-tefuryl, quizalofop-P, quizalofop-P-ethyl, quizalofop-P-tefuryl, sethoxydim, tepraloxydim, tralkoxydim, 4-(4'-chloro-4-cyclo-,propyl-2'-fluoro[1 ,1'-biphenyl]-3-yl)-5-hy- droxy-2,2,6,6-tetramethyl-2/-/-pyran-3(6/-/)-one (1312337-72-6); 4-(2',4'-dichloro-4-cyclo- propyl[1 ,1'-biphenyl]-3-yl)-5-hydroxy-2,2,6,6-tetramethyl-2/-/-pyran-3(6/-/)-one (1312337-45-
3); 4-(4'-chloro-4-ethyl-2'-fluoro[1 ,1'-biphenyl]-3-yl)-5-hydroxy-2,2,6,6-tetramethyl-2/-/-pyran- 3(6H)-one (1033757-93-5); 4-(2',4'-dichloro-4-ethyl[1 ,T-biphenyl]-3-yl)-2,2,6,6-tetramethyl- 2/-/-pyran-3,5(4/-/,6/-/)-dione (1312340-84-3); 5-(acetyloxy)-4-(4'-chloro-4-cyclopropyl-2'- fluoro[1 ,1'-biphenyl]-3-yl)-3,6-dihydro-2,2,6,6-tetramethyl-2/-/-pyran-3-one (1312337-48-6); 5-(acetyloxy)-4-(2',4'-dichloro-4-cyclopropyl- [1 ,T-biphenyl]-3-yl)-3,6-dihydro-2,2,6,6-tetra- methyl-2/-/-pyran-3-one; 5-(acetyloxy)-4-(4'-chloro-4-ethyl-2'-fluoro[1 , 1 '-biphenyl]-3-yl)- 3,6-dihydro-2,2,6,6-tetramethyl-2/-/-pyran-3-one (1312340-82-1); 5-(acetyloxy)-4-(2',4'-di- chloro-4-ethyl[1 ,1'-biphenyl]-3-yl)-3,6-dihydro-2,2,6,6-tetramethyl-2/-/-pyran-3-one (1033760- 55-2); 4-(4'-chloro-4-cyclopropyl-2'-fluoro[1 ,1'-biphenyl]-3-yl)-5,6-dihydro-2,2,6,6-tetramethyl- 5-oxo-2/-/-pyran-3-yl carbonic acid methyl ester (1312337-51-1); 4-(2',4'-dichloro -4-cyclo- propyl- [1 ,T-biphenyl]-3-yl)-5,6-dihydro-2,2,6,6-tetramethyl-5-oxo-2/-/-pyran-3-yl carbonic acid methyl ester; 4-(4'-chloro-4-ethyl-2'-fluoro[1 ,1'-biphenyl]-3-yl)-5,6-dihydro-2,2,6,6-tetra- methyl-5-oxo-2/-/-pyran-3-yl carbonic acid methyl ester (1312340-83-2); 4-(2',4'-dichloro- 4-ethyh[1 , 1 '-biphenyl]-3-yl)-5,6-dihydro-2,2,6,6-tetramethyl-5-oxo-2/-/-pyran-3-yl carbonic acid methyl ester (1033760-58-5); benfuresate, butylate, cycloate, dalapon, dimepiperate, EPTC, esprocarb, ethofumesate, flupropanate, molinate, orbencarb, pebulate, prosulfocarb, TCA, thiobencarb, tiocarbazil, triallate, vernolate;
N.2 ALS inhibitors: amidosulfuron, azimsulfuron, bensulfuron, bensulfuron-methyl, chlorimuron, chlorimuron-ethyl, chlorsulfuron, cinosulfuron, cyclosulfamuron, ethametsulfuron, ethamet- sulfuron-methyl, ethoxysulfuron, flazasulfuron, flucetosulfuron, flupyrsulfuron, flupyrsulfuron- methyl-sodium, foramsulfuron, halosulfuron, halosulfuron-methyl, imazosulfuron, iodosulfu- ron, iodosulfuron-methyl-sodium, iofensulfuron, iofensulfuron-sodium, mesosulfuron, meta- zosulfuron, metsulfuron, metsulfuron-methyl, nicosulfuron, orthosulfamuron, oxasulfuron, primisulfuron, primisulfuron-methyl, propyrisulfuron, prosulfuron, pyrazosulfuron, pyrazo- sulfuron-ethyl, rimsulfuron, sulfometuron, sulfometuron-methyl, sulfosulfuron, thifensulfuron, thifensulfuron-methyl, triasulfuron, tribenuron, tribenuron-methyl, trifloxysulfuron, triflusul- furon, triflusulfuron-methyl, tritosulfuron, imazamethabenz, imazamethabenz-methyl, imaza- mox, imazapic, imazapyr, imazaquin, imazethapyr; cloransulam, cloransulam-methyl, diclo- sulam, flumetsulam, florasulam, metosulam, penoxsulam, pyrimisulfan, pyroxsulam; bispyri- bac, bispyribac-sodium, pyribenzoxim, pyriftalid, pyriminobac, pyriminobac-methyl, pyrithio- bac, pyrithiobac-sodium, 4-[[[2-[(4,6-dimethoxy-2-pyrimidinyl)oxy]phenyl]methyl]amino]- benzoic acid-1 -methyhethyl ester (420138-41-6), 4-[[[2-[(4,6-dimethoxy-2-pyrimidinyl)oxy]- phenylj-rnethyljaminoj-benzoic acid propyl ester (420138-40-5), A/-(4-bromophenyl)- 2-[(4,6-dimethoxy-2-pyrimidinyl)oxy]benzenemethanamine (420138-01-8); flucarbazone, flucarbazone-sodium, propoxycarbazone, propoxycarbazone-sodium, thiencarbazone, thiencarbazone-methyl; triafamone;
N.3 Photosynthesis inhibitors: amicarbazone; chlorotriazine; ametryn, atrazine, chloridazone, cyanazine, desmetryn, dimethametryn.hexazinone, metribuzin, prometon, prometryn, pro- pazine, simazine, simetryn, terbumeton, terbuthylazin, terbutryn, trietazin; chlorobromuron, chlorotoluron, chloroxuron, dimefuron, diuron, fluometuron, isoproturon, isouron, linuron, metamitron, methabenzthiazuron, metobenzuron, metoxuron, monolinuron, neburon, sidu- ron, tebuthiuron, thiadiazuron, desmedipham, karbutilat, phenmedipham, phenmedipham- ethyl, bromofenoxim, bromoxynil and its salts and esters, ioxynil and its salts and esters, bromacil, lenacil, terbacil, bentazon, bentazon-sodium, pyridate, pyridafol, pentanochlor, propanil; diquat, diquat-dibromide, paraquat, paraquat-dichloride, paraquat-dimetilsulfate, 1- (6-tert-butylpyrimidin-4-yl)-2-hydroxy-4-meth-Oxy-3-methyl-2H-pyrrol-5-one (1654744-66-7), 1-(5-tert-butylisoxazol-3-yl)-2-hydroxy-4 methoxy-3-methyl-2H-pyrrol-5-one (1637455-12-9), 1-(5-tert-butylisoxazol-3-yl)-4-chloro-2 hydroxy-3-methyl-2H-pyrrol-5-one (1637453-94-1), 1-
(5-tert-butyl-1-methyl-pyrazol-3-yl)-4 chloro-2-hydroxy-3-methyl-2H-pyrrol-5-one (1654057- 29-0), 1-(5-tert-butyl-1-methyl-pyrazol-3-yl)-3-chloro-2-hydroxy-4-methyl-2H-pyrrol-5-one (1654747-80-4), 4-hydroxy-1 methoxy-5-methyl-3-[4-(trifluoromethyl)-2-pyridyl]imidazolidin-
2-one; (2023785-78-4), 4 hydroxy-1 , 5-dimethyl-3-[4-(trifluoromethyl)-2-pyridyl]imidazolidin-2- one (2023785-79-5), 5 ethoxy-4-hydroxy-1-methyl-3-[4-(trifluoromethyl)-2- pyridyl]imidazolidin-2-one (1701416-69-4), 4-hydroxy-1-methyl-3-[4-(trifluoromethyl)-2- pyridyl]imidazolidin-2-one (1708087-22-2), 4 hydroxy-1 , 5-dimethyl-3-[1-methyl-5- (trifluoromethyl)pyrazol-3-yl]imidazolidin-2-one (2023785-80-8), 1-(5-tert-butylisoxazol-3-yl)-
4-ethoxy-5-hydroxy-3-methyl-imidazolidin-2-one (1844836-64-1);
N.4 protoporphyrinogen-IX oxidase inhibitors: acifluorfen, acifluorfen-sodium, azafenidin, ben- carbazone, benzfendizone, bifenox, butafenacil, carfentrazone, carfentrazone-ethyl, chlor- methoxyfen, cinidon-ethyl, fluazolate, flufenpyr, flufenpyr-ethyl, flumiclorac, flumiclorac-pen- tyl, flumioxazin, fluoroglycofen, fluoroglycofen-ethyl, fluthiacet, fluthiacet-methyl, fomesafen, halosafen, lactofen, oxadiargyl, oxadiazon, oxyfluorfen, pentoxazone, profluazol, pyraclonil, pyraflufen, pyraflufen-ethyl, saflufenacil, sulfentrazone, thidiazimin, tiafenacil, trifludimoxazin, ethyl [3-[2-chloro-4-fluoro-5-(1-methyl-6-trifluoromethyl-2,4-dioxo-1 ,2,3,4-tetrahydropyrimi- din-3-yl)phenoxy]-2-pyridyloxy]acetate (353292-31-6), A/-ethyl-3-(2,6-dichloro-4-trifluoro- methylphenoxy)-5-methyl-1/-/-pyrazole-1 -carboxamide (452098-92-9), /V-tetrahydrofurfuryl-
3-(2,6-dichloro-4-trifluoromethylphenoxy)-5-methyl-1/-/-pyrazole-1 -carboxamide (915396-43- 9), A/-ethyl-3-(2-chloro-6-fluoro-4-trifluoromethyhphenoxy)-5-methyl-1/-/-pyrazole-1 -carbox amide (452099-05-7), A/-tetrahydro-,furfuryl-3-(2-chloro-6-fluoro-4-trifluoro-,methylphenoxy)-
5-methyl-1/-/-pyrazole-1 -carboxamide (452100-03-7), 3-[7-fluoro-3-oxo-4-(prop-2-ynyl)-
3.4-di hydro-2/-/- be nzo[1 ,4]oxazin-6-yl]-1 ,5-dimethyl-6-thioxo-[1 ,3,5]triazinan-2,4-dione (451484-50-7), 2-(2,2,7-trifluoro-3-oxo-4-prop-2-ynyl-3,4-dihydro-2/-/-benzo[1 ,4]oxazin-6-yl)- 4,5,6,7-tetrahydro-isoindole-1 ,3-dione (1300118-96-0), l-methyl-e-trifluoro-rnethyl-
3-(2, 2, 7-tri-fluoro-3-oxo-4-prop-2-ynyl-3,4-dihydro-2/-/-benzo[1 ,4]oxazin-6-yl)-1 /-/-pyrimidine-
2.4-dione (1304113-05-0), methyl (£)-4-[2-chloro-5-[4-chloro-5-(difluoromethoxy)-1/-/-methyl- pyrazol-3-yl]-4-fluoro-phenoxy]-3-methoxy-but-2-enoate (948893-00-3), 3-[7-chloro-5-fluoro- 2-(trifluoromethyl)-1/-/-benzimidazol-4-yl]-1-methyl-6-(trifluoromethyl)-1 /-/-pyrimidine-2, 4-di- one (212754-02-4);
N.5 Bleacher herbicides: beflubutamid, diflufenican, fluridone, flurochloridone, flurtamone,
norflurazon, picolinafen, 4-(3-trifluoromethyhphenoxy)-2-(4-trifluoromethylphenyl)pyrimidine (180608-33-7); benzobicyclon, benzofenap, bicyclopyrone, clomazone, fenquintrione, isoxaflutole, mesotrione, pyrasulfotole, pyrazolynate, pyrazoxyfen, sulcotrione, tefuryltrione, tembotrione, tolpyralate, topramezone; aclonifen, amitrole, flumeturon;
N.6 EPSP synthase inhibitors: glyphosate, glyphosate-isopropylammonium, glyposate- potassium, glyphosate-trimesium (sulfosate);
N.7 Glutamine synthase inhibitors: bilanaphos (bialaphos), bilanaphos-sodium, glufosinate, glufosinate-P, glufosinate-ammonium;
N.8 DHP synthase inhibitors: asulam;
N.9 Mitosis inhibitors: benfluralin, butralin, dinitramine, ethalfluralin, fluchloralin, oryzalin, pen- dimethalin, prodiamine, trifluralin; amiprophos, amiprophos-methyl, butamiphos; chlorthal, chlorthal-dimethyl, dithiopyr, thiazopyr, propyzamide, tebutam; carbetamide, chlorpropham, flamprop, flamprop-isopropyl, flamprop-methyl, flamprop-M-isopropyl, flamprop-M-methyl, propham;
N.10 VLCFA inhibitors: acetochlor, alachlor, butachlor, dimethachlor, dimethenamid, dimethen- amid-P, metazachlor, metolachlor, metolachlor-S, pethoxamid, pretilachlor, propachlor, prop-
isochlor, thenylchlor, flufenacet, mefenacet, diphenamid, naproanilide, napropamide, napro- pamide-M, fentrazamide, anilofos, cafenstrole, fenoxasulfone, ipfencarbazone, piperophos, pyroxasulfone, isoxazoline compounds of the formulae 11.1 , II.2, II.3, II.4, II.5, II.6, II.7, II.8 and II.9
N.11 Cellulose biosynthesis inhibitors: chlorthiamid, dichlobenil, flupoxam, indaziflam, isoxaben, triaziflam, 1-cyclohexyl-5-pentafluorphenyloxy-14-[1 ,2,4,6]thiatriazin-3-ylamine (175899-01- 1);
N.12 Decoupler herbicides: dinoseb, dinoterb, DNOC and its salts;
N.13 Auxinic herbicides: 2,4-D and its salts and esters, clacyfos, 2,4-DB and its salts and
esters, aminocyclopyrachlor and its salts and esters, aminopyralid and its salts such as aminopyralid-dimethylammonium, aminopyralid-tris(2-hydroxypropyl)ammonium and its esters, benazolin, benazolin-ethyl, chloramben and its salts and esters, clomeprop, clopy- ralid and its salts and esters, dicamba and its salts and esters, dichlorprop and its salts and esters, dichlorprop-P and its salts and esters, fluroxypyr, fluroxypyr-butometyl, fluroxypyr- meptyl, halauxifen and its salts and esters (943832-60-8); MCPA and its salts and esters, MCPA-thioethyl, MCPB and its salts and esters, mecoprop and its salts and esters, meco- prop-P and its salts and esters, picloram and its salts and esters, quinclorac, quinmerac, TBA (2,3,6) and its salts and esters, triclopyr and its salts and esters, 4-amino-3-chloro- 6-(4-chloro-2-fluoro-3-methoxyphenyl)-5-fluoropyridine-2-carboxylic acid, florpyrauxifen- benzyl, florpyrauxifen;
N.14 Auxin transport inhibitors: diflufenzopyr, diflufenzopyr-sodium, naptalam, naptalam- sodium;
N.15 Other herbicides: bromobutide, chlorflurenol, chlorflurenol-methyl, cinmethylin, cumyluron, cyclopyrimorate (499223-49-3) and its salts and esters, dalapon, dazomet, difenzoquat, di- fenzoquat-metilsulfate, dimethipin, DSMA, dymron, endothal and its salts, etobenzanid, flu- renol, flurenol-butyl, flurprimidol, fosamine, fosamine-ammonium, indanofan, maleic hydra- zide, mefluidide, metam, methiozolin (403640-27-7), methyl azide, methyl bromide, methyl- dymron, methyl iodide, MSMA, oleic acid, oxaziclomefone, pelargonic acid, pyributicarb, quinoclamine, tridiphane;
O) Insecticides from classes 0.1 to 0.29
O.1 Acetylcholine esterase (AChE) inhibitors: aldicarb (0.1.1), alanycarb (0.1.2), bendiocarb
(0.1.3), benfuracarb (0.1.4), butocarboxim (0.1.5), butoxycarboxim (0.1.6), carbaryl (0.1.7), carbofuran (0.1.8), carbosulfan (0.1.9), ethiofencarb (0.1.10), fenobucarb (0.1.1 1), formetanate (0.1.12), furathiocarb (0.1.13), isoprocarb (0.1.14), methiocarb (0.1.15), methomyl (0.1.16), metolcarb (0.1.17), oxamyl (0.1.18), pirimicarb (0.1.19), propoxur (0.1.20), thiodicarb (0.1.21), thiofanox (0.1.22), trimethacarb (0.1.23), XMC (0.1.24), xylylcarb (0.1.25), triazamate (0.1.26), acephate (0.1.27), azamethiphos (0.1.28), azinphos-ethyl (0.1.29), azinphosmethyl (0.1.30), cadusafos (0.1.31), chlorethoxyfos (0.1.32), chlorfenvinphos (0.1.33), chlormephos (0.1.34), chlorpyrifos (0.1.35), chlorpyrifos- methyl (0.1.36), coumaphos (0.1.37), cyanophos (0.1.38), demeton-S-methyl (0.1.39), diazinon (0.1.40), dichlorvos/ DDVP (0.1.41), dicrotophos (0.1.42), dimethoate (0.1.43), dimethylvinphos (0.1.44), disulfoton (0.1.45), EPN (0.1.46), ethion (0.1.47), ethoprophos (0.1.48), famphur (0.1.49), fenamiphos (0.1.50), fenitrothion (0.1.51), fenthion (0.1.52), fosthiazate (0.1.53), heptenophos (0.1.54), imicyafos (0.1.55), isofenphos (0.1.56), isopropyl O-(methoxyaminothio-phosphoryl) salicylate (0.1.57), isoxathion (0.1.58), malathion (0.1.59), mecarbam (0.1.60), methamidophos (0.1.61), methidathion (0.1.62), mevinphos (0.1.63), monocrotophos (0.1.64), naled (0.1.65), omethoate (0.1.66), oxydemeton-methyl (0.1.67), parathion (0.1.68), parathion-methyl (0.1.69), phenthoate (0.1.70), phorate (0.1.71), phosalone (0.1.72), phosmet (0.1.73), phosphamidon (0.1.74), phoxim (0.1.75), pirimiphos- methyl (0.1.76), profenofos (0.1.77), propetamphos (0.1.78), prothiofos (0.1.79), pyraclofos (0.1.80), pyridaphenthion (0.1.81), quinalphos (0.1.82), sulfotep (0.1.83), tebupirimfos (0.1.84), temephos (0.1.85), terbufos (0.1.86),
tetrachlorvinphos (0.1.87), thiometon (0.1.88), triazophos (0.1.89), trichlorfon (0.1.90), vamidothion (0.1.91);
0.2 GABA-gated chloride channel antagonists: endosulfan (0.2.1), chlordane (0.2.2), ethiprole (0.2.3), fipronil (0.2.4), flufiprole (0.2.5), pyrafluprole (0.2.6), pyriprole (0.2.7);
0.3 Sodium channel modulators: acrinathrin (0.3.1), allethrin (0.3.2), d-cis-trans allethrin
(0.3.3), d-trans allethrin (0.3.4), bifenthrin (0.3.5), kappa-bifenthrin (0.3.6), bioallethrin (0.3.7), bioallethrin S-cylclopentenyl (0.3.8), bioresmethrin (0.3.9), cycloprothrin (0.3.10), cyfluthrin (0.3.1 1), beta-cyfluthrin (0.3.12), cyhalothrin (0.3.13), lambda-cyhalothrin
(0.3.14), gamma-cyhalothrin (0.3.15), cypermethrin (0.3.16), alpha-cypermethrin (0.3.17), beta-cypermethrin (0.3.18), theta-cypermethrin (0.3.19), zeta-cypermethrin (0.3.20), cyphenothrin (0.3.21), deltamethrin (0.3.22), empenthrin (0.3.23), esfenvalerate (0.3.24), etofenprox (0.3.25), fenpropathrin (0.3.26), fenvalerate (0.3.27), flucythrinate (0.3.28), flumethrin (0.3.29), tau-fluvalinate (0.3.30), halfenprox (0.3.31), heptafluthrin (0.3.32), imiprothrin (0.3.33), meperfluthrin (0.3.34), metofluthrin (0.3.35), momfluorothrin (0.3.36), epsilon-momfluorothrin (0.3.37), permethrin (0.3.38), phenothrin (0.3.39), prallethrin (0.3.40), profluthrin (0.3.41), pyrethrin (pyrethrum) (0.3.42), resmethrin (0.3.43), silafluofen
(0.3.44), tefluthrin (0.3.45), kappa-tefluthrin (0.3.46), tetramethylfluthrin (0.3.47), tetramethrin (0.3.48), tralomethrin (0.3.49), transfluthrin (0.3.50), DDT (0.3.51),
methoxychlor (0.3.52);
0.4 Nicotinic acetylcholine receptor agonists (nAChR): acetamiprid (0.4.1), clothianidin (0.4.2), cycloxaprid (0.4.3), dinotefuran (0.4.4), imidacloprid (0.4.5), nitenpyram (0.4.6), thiacloprid (0.4.7), thiamethoxam (0.4.8), 4,5-dihydro-/\/-nitro-1-(2-oxiranylmethyl)-1/-/-imidazol-2-amine (0.4.9), (2£)-1-[(6-chloropyridin-3-yl)methyl]-/\/-nitro-2-pentylidene- hydrazinecarboximidamide (0.4.10), 1-[(6-chloropyridin-3-yl)methyl]-7-methyl-8-nitro-5- propoxy-1 ,2,3,5,6,7-hexahydroimidazo[1 ,2-a]pyridine (0.4.11), nicotine (0.4.12), sulfoxaflor (0.4.13), flupyradifurone (0.4.14), triflumezopyrim (0.4.15), (3R)-3-(2-chlorothiazol-5-yl)-8- methyl-5-oxo-6-phenyl-2,3-dihydrothiazolo[3,2-a]pyrimidin-8-ium-7-olate (0.4.16), (3S)-3-(6- chloro-3-pyridyl)-8-methyl-5-oxo-6-phenyl-2,3-dihydrothiazolo[3,2-a]pyrimidin-8-ium-7-olate (0.4.17), (3S)-8-methyl-5-oxo-6-phenyl-3-pyrimidin-5-yl-2,3-dihydrothiazolo[3,2-a]pyrimidin- 8-ium-7-olate (0.4.18), (3R)-3-(2-chlorothiazol-5-yl)-8-methyl-5-oxo-6-[3- (trifluoromethyl)phenyl]-2,3-dihydrothiazolo[3,2-a]pyrimidin-8-ium-7-olate (0.4.19), (3R)-3-(2- chlorothiazol-5-yl)-6-(3,5-dichlorophenyl)-8-methyl-5-oxo-2,3-dihydrothiazolo[3,2-a]pyrimidin- 8-ium-7-olate (0.4.20), (3R)-3-(2-chlorothiazol-5-yl)-8-ethyl-5-oxo-6-phenyl-2,3-di- hydrothiazolo[3,2-a]pyrimidin-8-ium-7-olate (0.4.21);
0.5 Nicotinic acetylcholine receptor allosteric activators: spinosad (0.5.1), spinetoram (0.5.2);
0.6 Chloride channel activators: abamectin (0.6.1), emamectin benzoate (0.6.2), ivermectin (0.6.3), lepimectin (0.6.4), milbemectin (0.6.5);
0.7 Juvenile hormone mimics: hydroprene (0.7.1), kinoprene (0.7.2), methoprene (0.7.3), fenoxycarb (0.7.4), pyriproxyfen (0.7.5);
0.8 miscellaneous non-specific (multi-site) inhibitors: methyl bromide (0.8.1) and other alkyl halides, chloropicrin (0.8.2), sulfuryl fluoride (0.8.3), borax (0.8.4), tartar emetic (0.8.5);
0.9 Chordotonal organ TRPV channel modulators: pymetrozine (0.9.1), pyrifluquinazon (0.9.2), flonicamid (0.9.3);
0.10 Mite growth inhibitors: clofentezine (0.10.1), hexythiazox (0.10.2), diflovidazin (0.10.3), etoxazole (0.10.4);
0.11 Microbial disruptors of insect midgut membranes: Bacillus thuringiensis, Bacillus
sphaericus and the insecticdal proteins they produce: Bacillus thuringiensis subsp.
Israelensis (0.11.1), Bacillus sphaericus (0.11.2), Bacillus thuringiensis subsp. aizawai (0.11.3), Bacillus thuringiensis subsp. kurstaki (0.11.4), Bacillus thuringiensis subsp.
tenebrionis (0.11.5), the Bt crop proteins: CrylAb (0.11.6), CrylAc (0.11.7), Cryl Fa (0.11.8), Cry2Ab (0.11.9), mCry3A (0.11.10), Cry3Ab (0.11.11), Cry3Bb (0.11.12), Cry34/35Ab1 (0.11.13);
0.12 Inhibitors of mitochondrial ATP synthase: diafenthiuron (0.12.1), azocyclotin (0.12.2), cyhexatin (0.12.3), fenbutatin oxide (0.12.4), propargite (0.12.5), tetradifon (0.12.6);
0.13 Uncouplers of oxidative phosphorylation via disruption of the proton gradient: chlorfenapyr (0.13.1), DNOC (0.13.2), sulfluramid (0.13.3);
0.14 Nicotinic acetylcholine receptor (nAChR) channel blockers: bensultap (0.14.1), cartap hydrochloride (0.14.2), thiocyclam (0.14.3), thiosultap sodium (0.14.4);
0.15 Inhibitors of the chitin biosynthesis type 0: bistrifluron (0.15.1), chlorfluazuron (0.15.2), diflubenzuron (0.15.3), flucycloxuron (0.15.4), flufenoxuron (0.15.5), hexaflumuron
(0.15.6), lufenuron (0.15.7), novaluron (0.15.8), noviflumuron (0.15.9), teflubenzuron (0.15.10), triflumuron (0.15.11);
0.16 Inhibitors of the chitin biosynthesis type 1 : buprofezin (0.16.1);
0.17 Moulting disruptors: cyromazine (0.17.1);
0.18 Ecdyson receptor agonists: methoxyfenozide (0.18.1), tebufenozide (0.18.2), halofenozide (0.18.3), fufenozide (0.18.4), chromafenozide (0.18.5);
0.19 Octopamin receptor agonists: amitraz (0.19.1);
0.20 Mitochondrial complex III electron transport inhibitors: hydramethylnon (0.20.1),
acequinocyl (0.20.2), fluacrypyrim (0.20.3), bifenazate (0.20.4);
0.21 Mitochondrial complex I electron transport inhibitors: fenazaquin (0.21.1), fenpyroximate (0.21.2), pyrimidifen (0.21.3), pyridaben (0.21.4), tebufenpyrad (0.21.5), tolfenpyrad (0.21.6), rotenone (0.21.7);
0.22 Voltage-dependent sodium channel blockers: indoxacarb (0.22.1), metaflumizonev
(0.22.2), 2-[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidene]-/\/-[4- (difluoromethoxy)phenyl]-hydrazinecarboxamide (0.22.3), A/-(3-chloro-2-methylphenyl)-2-[(4- chlorophenyl)-[4-[methyl(methylsulfonyl)amino]phenyl]methylene]-hydrazinecarboxamide (0.22.4);
0.23 Inhibitors of the of acetyl CoA carboxylase: spirodiclofen (0.23.1), spiromesifen (0.23.2), spirotetramat (0.23.3), spiropidion (0.23.4);
0.24 Mitochondrial complex IV electron transport inhibitors: aluminium phosphide (0.24.1), calcium phosphide (0.24.2), phosphine (0.24.3), zinc phosphide (0.24.4), cyanide (0.24.5);
0.25 Mitochondrial complex II electron transport inhibitors: cyenopyrafen (0.25.1), cyflumetofen (0.25.2);
0.26 Ryanodine receptor-modulators: flubendiamide (0.26.1), chlorantraniliprole (0.26.2), cyantraniliprole (0.26.3), cyclaniliprole (0.26.4), tetraniliprole (0.26.5), (R)-3-chloro-/\/1-{2- methyl-4-[1 ,2,2,2 -tetrafluoro-l- trifluoromethyOethyl phenyl -A^-O-methyl^- methylsulfonylethyl)phthalamide (0.26.6), (S)-3-chloro-/\/1-{2-methyl-4-[1 ,2,2,2-tetrafluoro-1- (trifluoromethy ethylJphenylJ-A^-O-methyl^-methylsulfonylethyOphthalamide (0.26.7), methyl-2-[3,5-dibromo-2-({[3-bromo-1-(3-chloropyridin-2-yl)-1/-/-pyrazol-5-yl]carbonyl}- amino)benzoyl]-1 ,2-dimethylhydrazinecarboxylate (0.26.8), A/-[4,6-dichloro-2-[(diethyl- lambda-4-sulfanylidene)carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole- 3-carboxamide (0.26.9), A/-[4-chloro-2-[(diethyl-lambda-4-sulfanylidene)carbamoyl]-6- methyl-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole-3-carboxamide (0.26.10), A/-[4-chloro-2-[(di-2-propyl-lambda-4-sulfanylidene)carbamoyl]-6-methyl-phenyl]-2-(3-chloro- 2-pyridyl)-5-(trifluoromethyl)pyrazole-3-carboxamide (0.26.11), A/-[4,6-dichloro-2-[(di-2- propyl-lambda-4-sulfanylidene)carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5- (trifluoromethyl)pyrazole-3-carboxamide (0.26.12), A/-[4,6-dibromo-2-[(diethyl-lambda-4- sulfanylidene)carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole-3- carboxamide (0.26.13), A/-[2-(5-amino-1 ,3,4-thiadiazol-2-yl)-4-chloro-6-methylphenyl]-3- bromo-1-(3-chloro-2-pyridinyl)-1/-/-pyrazole-5-carboxamide (0.26.14), 3-chloro-1-(3-chloro-2- pyridinyl)-/\/-[2,4-dichloro-6-[[(1-cyano-1-methylethyl)amino]carbonyl]phenyl]-1/-/-pyrazole- 5-carboxamide (0.26.15), tetrachlorantraniliprole (0.26.16), A/-[4-chloro-2-[[(1 ,1- dimethylethyl)amino]carbonyl]-6-methylphenyl]-1-(3-chloro-2-pyridinyl)-3-(fluoromethoxy)- 1/-/-pyrazole-5-carboxamide (0.26.17), cyhalodiamide (0.26.18);
0.27: Chordotonal organ Modulators - undefined target site: flonicamid (0.27.1);
0.28. insecticidal active compounds of unknown or uncertain mode of action: afidopyropen
(0.28.1), afoxolaner (0.28.2), azadirachtin (0.28.3), amidoflumet (0.28.4), benzoximate (0.28.5), broflanilide (0.28.6), bromopropylate (0.28.7), chinomethionat (0.28.8), cryolite (0.28.9), dicloromezotiaz (0.28.10), dicofol (0.28.11), flufenerim (0.28.12), flometoquin (0.28.13), fluensulfone (0.28.14), fluhexafon (0.28.15), fluopyram (0.28.16), fluralaner (0.28.17), metoxadiazone (0.28.18), piperonyl butoxide (0.28.19), pyflubumide (0.28.20), pyridalyl (0.28.21), tioxazafen (0.28.22), 11-(4-chloro-2,6-dimethylphenyl)-12-hydroxy-1 ,4-
dioxa-9-azadispiro[4.2.4.2]-tetradec-11-en-10-one, 3-(4’-fluoro-2,4-dimethylbiphenyl-3-yl)-4- hydroxy-8-oxa-1-azaspiro[4.5]dec-3-en-2-one, 1-[2-fluoro-4-methyl-5-[(2,2,2- trifluoroethyl)sulfinyl]phenyl]-3-(trifluoromethyl)-1 HA ,2,4-triazole-5-amine (0.28.23), Bacillus firmus 1-1582 (0.28.24), flupyrimin (0.28.25), fluazaindolizine (0.28.26), 4-[5-(3,5-di- chlorophenyl)-5-(trifluoromethyl)-4/-/-isoxazol-3-yl]-2-methyl-/\/-(1-oxothietan-3-yl)benzamide (0.28.27), fluxametamide (0.28.28), 5-[3-[2,6-dichloro-4-(3,3- dichloroallyloxy)phenoxy]propoxy]-1/-/-pyrazole (0.28.1), 4-cyano-/\/-[2-cyano-5-[[2,6- dibromo-4-[1 ,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)propyl]phenyl]carbamoyl]phenyl]-2- methyl-benzamide (0.28.29), 4-cyano-3-[(4-cyano-2-methyl-benzoyl)amino]-/\/-[2,6-dichloro- 4-[1 ,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)propyl]phenyl]-2-fluoro-benzamide (0.28.30), A/-[5-[[2-chloro-6-cyano-4-[1 ,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)propyl]phenyl]carba- moyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide (0.28.31), A/-[5-[[2-bromo-6-chloro-
4-[2, 2, 2-trifluoro-1 -hydroxy-1 -(trifluoromethyl)ethyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cy- ano-2-methyl-benzamide (0.28.32), A/-[5-[[2-bromo-6-chloro-4-[1 ,2,2,3,3,3-hexafluoro-1- (trifluoromethyl)propyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide (0.28.33), 4-cyano-/\/-[2-cyano-5-[[2,6-dichloro-4-[1 ,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)- propyl]phenyl]carbamoyl]phenyl]-2-methyl-benzamide (0.28.34), 4-cyano-/\/-[2-cyano-
5-[[2,6-dichloro-4-[1 ,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]carbamoyl]phenyl]-2- methyl-benzamide (0.28.35), A/-[5-[[2-bromo-6-chloro-4-[1 ,2,2,2-tetrafluoro-1- (trifluoromethyl)ethyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide (0.28.36); 2-(1 ,3-dioxan-2-yl)-6-[2-(3-pyridinyl)-5-thiazolyl]-pyridine (0.28.37), 2-[6-[2-(5- fluoro-3-pyridinyl)-5-thiazolyl]-2-pyridinyl]-pyrimidine (0.28.38), 2-[6-[2-(3-pyridinyl)-5-thi- azolyl]-2-pyridinyl]-pyrimidine (0.28.39), A/-methylsulfonyl-6-[2-(3-pyridyl)thiazol-5-yl]pyri- dine-2-carboxamide (0.28.40), A/-methylsulfonyl-6-[2-(3-pyridyl)thiazol-5-yl]pyridine-2- carboxamide (0.28.41), 1-[(6-chloro-3-pyridinyl)methyl]-1 ,2,3,5,6,7-hexahydro-5-methoxy-7- methyl-8-nitro-imidazo[1 ,2-a]pyridine (0.28.42), 1-[(6-chloropyridin-3-yl)methyl]-7-methyl- 8-nitro-1 ,2,3,5,6,7-hexahydroimidazo[1 ,2-a]pyridin-5-ol (0.28.43), 1 -isopropyl-/V,5-dimethyl- A/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.44), 1-(1 ,2-dimethylpropyl)-/\/-ethyl-5-methyl- A/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.45), A/,5-dimethyl-/\/-pyridazin-4-yl-1 -(2,2,2- trifluoro-1-methyl-ethyl)pyrazole-4-carboxamide (0.28.46), 1-[1-(1-cyanocyclopropyl)ethyl]- /\/-ethyl-5-methyl-/\/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.47), A/-ethyl-1-(2-fluoro-1- methyl-propyl)-5-meth-yl-/\/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.48), 1 -(1 ,2- dimethylpropyl)-/\/,5-dimethyl-/\/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.49),
1-[1-(1-cyanocyclopropyl)ethyl]-/\/,5-dimethyl-/\/-pyridazin-4-yl-pyrazole-4-carboxamide (0.28.50), /\/-methyl-1-(2-fluoro-1-methyl-propyl]-5-methyl-/\/-pyridazin-4-yl-pyrazole-4- carboxamide (0.28.51), 1-(4,4-difluorocyclohexyl)-/\/-ethyl-5-methyl-/\/-pyridazin-4-yl-pyr- azole-4-carboxamide (0.28.52), 1-(4,4-difluorocyclohexyl)-/\/,5-dimethyl-/\/-pyridazin-4-yl- pyrazole-4-carboxamide (0.28.53), A/-(1-methylethyl)-2-(3-pyridinyl)-2/-/-indazole- 4-carboxamide (0.28.54), A/-cyclopropyl-2-(3-pyridinyl)-2/-/-indazole-4-carboxamide
(0.28.55), A/-cyclohexyl-2-(3-pyridinyl)-2/-/-indazole-4-carboxamide (0.28.56), 2-(3-pyridinyl)- A/-(2,2,2-trifluoroethyl)-2/-/-indazole-4-carboxamide (0.28.57), 2-(3-pyridinyl)-/V-[(tetrahydro-
2-furanyl)methyl]-2/-/-indazole-5-carboxamide (0.28.58), methyl 2-[[2-(3-pyridinyl)-2/-/- indazol-5-yl]carbonyl]hydrazinecarboxylate (0.28.59), A/-[(2,2-difluorocyclopropyl)methyl]-2- (3-pyridinyl)-2/-/-indazole-5-carboxamide (0.28.60), A/-(2,2-difluoropropyl)-2-(3-pyridinyl)-2/-/- indazole-5-carboxamide (0.28.61), 2-(3-pyridinyl )-A/-(2-pyrimidinylmethyl )-2/-/-indazole-5- carboxa ide (0.28.62), A/-[(5-methyl-2-pyrazinyl)methyl]-2-(3-pyridinyl)-2/-/-indazole-5-car- boxamide (0.28.63), tyclopyrazoflor (0.28.64), sarolaner (0.28.65), lotilaner (0.28.66),
A/-[4-chloro-3-[[(phenylmethyl)amino]carbonyl]phenyl]-1-methyl-3-(1 , 1 ,2,2, 2-penta- fluoroethyl)-4-(trifluoromethyl)-1H-pyrazole-5-carboxamide (0.28.67), M. UN.22a 2-(3- ethylsulfonyl-2-pyridyl)-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.68), 2-[3- ethylsulfonyl-5-(trifluoromethyl)-2-pyridyl]-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.69), iscocycloseram(O.28.70), A/-[4-chloro-3-(cyclopropylcarbamoyl)phenyl]-2-methyl- 5-(1 ,1 ,2,2,2-pentafluoroethyl)-4-(trifluoromethyl)pyrazole-3-carboxamide (0.28.72), N-[ 4- chloro-3-[(1 -cyanocyclopropyl)carbamoyl]phenyl]-2-methyl-5-(1 , 1 ,2,2,2-pentafluoroethyl)-4- (trifluoromethyl)pyrazole-3-carboxamide (0.28.73), acynonapyr (0.28.74), benzpyrimoxan (0.28.75), chloro-/\/-(1-cyanocyclopropyl)-5-[1-[2-methyl-5-(1 ,1 ,2,2,2-pentafluoroethyl)- 4-(trifluoromethyl)pyrazol-3-yl]pyrazol-4-yl]benzamide (0.28.76), oxazosulfyl (0.28.77), [(2S,3R,4R,5S,6S)-3,5-dimethoxy-6-methyl-4-propoxy-tetrahydropyran-2-yl]-/\/-[4-[1-[4-(tri- fluoromethoxy)phenyl]-1 ,2,4-triazol-3-yl]phenyl]carbamate (0.28.78),
[(2S,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyl-tetrahydropyran-2-yl] N-[4-[1-[4-(trifluoro- methoxy) phenyl]- 1 ,2,4-triazol-3-yl]phenyl]carbamate (0.28.79), [(2S,3R,4R,5S,6S)-3,5- dimethoxy-6-methyl-4-propoxy-tetrahydropyran-2-yl]-/\/-[4-[1 -[4-(1 , 1 ,2,2,2- pentafluoroethoxy)phenyl]-1 ,2,4-triazol-3-yl]phenyl]carbamate (0.28.80), [(2S,3R,4R,5S,6S)- 3,4,5-trimethoxy-6-methyl-tetrahydropyran-2-yl]-/\/-[4-[1-[4-(1 ,1 ,2,2,2-penta- fluoroethoxy)phenyl]-1 ,2,4-triazol-3-yl]phenyl]carbamate (0.28.81), (2Z)-3-(2- isopropylphenyl)-2-[(£)-[4-[1-[4-(1 ,1 ,2,2,2-pentafluoroethoxy)phenyl]-1 ,2,4-triazol-3- yl]phenyl]methylenehydrazono]thiazolidin-4-one (0.28.82), 2-(6-chloro-3-ethylsulfonyl- imidazo[1 ,2-a]pyridin-2-yl)-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.83), 2-(6- bromo-3-ethylsulfonyl-imidazo[1 ,2-a]pyridin-2-yl)-3-methyl-6-(trifluoromethyl)imidazo[4,5- b]pyridine (0.28.84), 2-(3-ethylsulfonyl-6-iodo-imidazo[1 ,2-a]pyridin-2-yl)-3-methyl-6- (trifluoromethyl)imidazo[4,5-b]pyridine (0.28.85), 2-[3-ethylsulfonyl-6- (trifluoromethyl)imidazo[1 ,2-a]pyridin-2-yl]-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.86), 2-(7-chloro-3-ethylsulfonyl-imidazo[1 ,2-a]pyridin-2-yl)-3-methyl-6- (trifluoromethyl)imidazo[4,5-b]pyridine (0.28.87), 2-(3-ethylsulfonyl-7-iodo-imidazo[1 ,2-a]py- ridin-2-yl)-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.88), 3-ethylsulfonyl-6- iodo-2-[3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridin-2-yl]imidazo[1 ,2-a]pyridine-8- carbonitrile (0.28.89), 2-[3-ethylsulfonyl-8-fluoro-6-(trifluoromethyl)imidazo[1 ,2-a]pyridin-2- yl]-3-methyl-6-(trifluoromethyl)imidazo[4,5-b]pyridine (0.28.90), 2-[3-ethylsulfonyl-7- (trifluoromethyl)imidazo[1 ,2-a]pyridin-2-yl]-3-methyl-6-(trifluoromethylsulfinyl)imidazo- [4,5-b]pyridine (0.28.91), 2-[3-ethylsulfonyl-7-(trifluoromethyl)imidazo[1 ,2-a]pyridin-2-yl]-3- methyl-6-(trifluoromethyl)imidazo[4,5-c]pyridine (0.28.92), 2-(6-bromo-3-ethylsulfonyl- imidazo[1 ,2-a]pyridin-2-yl)-6-(trifluoromethyl)pyrazolo[4,3-c]pyridine (0.28.93).
The active substances referred to as component 2, their preparation and their activity e. g. against harmful fungi is known (cf. : https://www.alanwood.net/pesticides/); these substances are commercially available. The compounds described by lUPAC nomenclature, their preparation and their pesticidal activity are also known (cf. Can. J. Plant Sci. 48(6), 587-94, 1968;
EP-A 141 317; EP-A 152 031 ; EP-A 226 917; EP-A 243 970; EP-A 256 503; EP-A 428 941 ; EP-A 532 022; EP-A 1 028 125; EP-A 1 035 122; EP-A 1 201 648; EP-A 1 122 244,
JP 2002316902; DE 19650197; DE 10021412; DE 102005009458; US 3,296,272;
US 3,325,503; WO 98/46608; WO 99/14187; WO 99/24413; WO 99/27783; WO 00/29404;
WO 00/46148; WO 00/65913; WO 01/54501 ; WO 01/56358; WO 02/22583; WO 02/40431 ;
WO 03/10149; WO 03/11853; WO 03/14103; WO 03/16286; WO 03/53145; WO 03/61388;
WO 03/66609; WO 03/74491 ; WO 04/49804; WO 04/83193; WO 05/120234; WO 05/123689;
WO 05/123690; WO 05/63721 ; WO 05/87772; WO 05/87773; WO 06/15866; WO 06/87325;
WO 06/87343; WO 07/82098; WO 07/90624, WO 10/139271 , WO 11/028657, WO 12/168188, WO 07/006670, WO 11/77514; WO 13/047749, WO 10/069882, WO 13/047441 , WO 03/16303, WO 09/90181 , WO 13/007767, WO 13/010862, WO 13/127704, WO 13/024009, WO 13/24010, WO 13/047441 , WO 13/162072, WO 13/092224, WO 11/135833, CN 1907024, CN 1456054,
CN 103387541 , CN 1309897, WO 12/84812, CN 1907024, WO 09094442, WO 14/60177,
WO 13/116251 , WO 08/013622, WO 15/65922, WO 94/01546, EP 2865265, WO 07/129454, WO 12/165511 , WO 11/081174, WO 13/47441 , JP2015089883, JP2015120675,
WO2015119246, WO2011135827, WO2012084812).
The present invention furthermore relates to agrochemical compositions comprising a mixture of at least one compound I (component 1) and at least one further active substance useful for plant protection, e. g. selected from the groups A) to O) (component 2), in particular one further fungicide, e. g. one or more fungicide from the groups A) to K), as described above, and if desired one suitable solvent or solid carrier. Those mixtures are of particular interest, since many of them at the same application rate show higher efficiencies against harmful fungi.
Furthermore, combating harmful fungi with a mixture of compounds I and at least one fungicide from groups A) to K), as described above, is more efficient than combating those fungi with individual compounds I or individual fungicides from groups A) to K).
By applying compounds I together with at least one active substance from groups A) to O) a synergistic effect can be obtained, i.e. more than simple addition of the individual effects is obtained (synergistic mixtures).
This can be obtained by applying the compounds I and at least one further active substance simultaneously, either jointly (e. g. as tank-mix) or seperately, or in succession, wherein the time interval between the individual applications is selected to ensure that the active substance applied first still occurs at the site of action in a sufficient amount at the time of application of the further active substance(s). The order of application is not essential for working of the present invention.
When applying compounds I and a pesticide II sequentially the time between both applications may vary e. g. between 2 hours to 7 days. Also, a broader range is possible ranging from 0.25 hour to 30 days, preferably from 0.5 hour to 14 days, particularly from 1 hour to 7 days or from 1.5 hours to 5 days, even more preferred from 2 hours to 1 day.
In the binary mixtures and compositions according to the invention the weight ratio of the component 1) and the component 2) generally depends from the properties of the active components used, usually it is in the range of from 1 :10,000 to 10,000:1 , often it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 , even more preferably in the range of from 1 :4 to 4: 1 and in particular in the range of from 1 :2 to 2: 1.
According to further embodiments of the binary mixtures and compositions, the weight ratio of the component 1) and the component 2) usually is in the range of from 1000:1 to 1 :1 , often in the range of from 100: 1 to 1 :1 , regularly in the range of from 50:1 to 1 :1 , preferably in the range of from 20:1 to 1 :1 , more preferably in the range of from 10:1 to 1 :1 , even more preferably in the range of from 4:1 to 1 :1 and in particular in the range of from 2:1 to 1 :1.
According to a further embodiments of the binary mixtures and compositions, the weight ratio of the component 1) and the component 2) usually is in the range of from 1 :1 to 1 :1000, often in the range of from 1 :1 to 1 :100, regularly in the range of from 1 :1 to 1 :50, preferably in the range of from 1 :1 to 1 :20, more preferably in the range of from 1 :1 to 1 :10, even more preferably in the range of from 1 :1 to 1 :4 and in particular in the range of from 1 :1 to 1 :2.
In the ternary mixtures, i.e. compositions according to the invention comprising the component 1) and component 2) and a compound III (component 3), the weight ratio of component 1) and component 2) depends from the properties of the active substances used, usually it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 and in particular in the range of from 1 :4 to 4:1 , and the weight ratio of component 1) and component 3) usually it is in the range of from 1 :100 to 100:1 , regularly in the range of from 1 :50 to 50:1 , preferably in the range of from 1 :20 to 20:1 , more preferably in the range of from 1 :10 to 10:1 and in particular in the range of from 1 :4 to 4: 1.
Any further active components are, if desired, added in a ratio of from 20:1 to 1 :20 to the component 1).
These ratios are also suitable for inventive mixtures applied by seed treatment.
Accordingly, the present invention furthermore relates to mixtures comprising one compound of the formula I (component 1 , a group represented by the expression“(I)”) and one pesticide II (component 2), wherein pesticide II is an active ingredient selected from the groups A) to O) defined above.
The mixtures of active substances can be prepared as compositions comprising besides the active ingredients at least one inert ingredient (auxiliary) by usual means, e. g. by the means given for the compositions of compounds I.
Concerning usual ingredients of such compositions reference is made to the explanations given for the compositions containing compounds I.
The mixtures of active substances according to the present invention are suitable as fungicides, as are the compounds of formula I. They are distinguished by an outstanding effectiveness against a broad spectrum of phytopathogenic fungi, especially from the classes of the Ascomycetes, Basidiomycetes, Deuteromycetes and Peronosporomycetes (syn.
Oomycetes). In addition, it is referred to the explanations regarding the fungicidal activity of the compounds and the compositions containing compounds I, respectively.
Example 1- Synthesis of 5-cyclopropyl-N-[6-(difluoromethyl)-5-methyl-3-pyridyl]-2-isobutyl-2- methyl-pent-4-ynamide
1.1. Ethyl 5-cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoate
44 ml 2m LDA-solution in tetrahydrofuran/hexanes (88 mmol) was added to a solution of 11 ,5 g (72,6 mmol) ethyl 2,4-dimethylpentanoate in 150 ml tetrahydrofuran at -78°C. Afterwards 14 g (80 mmol) HMPA (hexamethylphosphoric triamide) was added and the reaction mixture was stirred for 30 min at -78°C. Subsequently 15 g (94 mmol) 3-bromoprop-1-ynylcyclopropane was added and the reaction mixture was stirred for 3 h at -78°C. The reaction mixture was quenched with 200 ml aq. NH4CI-solution and extracted with methyl-t-butylether, the organic layer was dried over sodium sulfate and concentrated. The residue was purified by column- chromatography using petrolether/methyl-t-butylether-mixtures to give 12.5 g (73% of theory) 5- cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoate as yellow oil.
1H-NMR (CDCIs, d in ppm): 0.48 - 0.54 (m, 2 H) 0.61 - 0.65 (m, 2 H) 0.78 (dd, J=6.42, 1.41 Hz,
6 H) 1.08 - 1.14 (m, 1 H) 1.15 (s, 3 H) 1.19 (t, J=7.15 Hz, 3 H) 1.36 - 1.42 (m, 1 H) 1.51 - 1.60 (m, 2 H) 2.20 - 2.27 (m, 1 H) 2.32 - 2.38 (m, 1 H) 4.05 (qd, J=7.11 , 0.92 Hz, 2 H)
1.2. 5-Cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoic acid
A solution of 18 g (0,32 mol) KOH-solution in 40 ml water was added to a solution of 7,9 g (33,5 mmol) ethyl 5-cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoate in 80 ml methanol. The reaction mixture was stirred for 16 h at 80°C, concentrated i. vac. and the residue was diluted with 50 ml water. The aqueous layer was acidified to pH=2 with 2m HCI-solution and was extracted with ethyl acetate. The combined organic phases were dried over sodium sulfate and concentrated to give 6,2 g crude 5-cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoic acid as yellow oil.
1H-NMR (CDCIs, d in ppm): 0.50 - 0.55 (m, 2 H) 0.60 - 0.67 (m, 2 H) 0.82 (dd, J= 6.36, 1.10 Hz,
6 H) 1.09 - 1.14 (m, 1 H) 1.19 (s, 3 H) 1.41 - 1.46 (m, 1 H) 1.55 - 1.66 (m, 2 H) 2.24 - 2.31 (m, 1 H) 2.34 - 2.40 (m,1 H)
1.3. 5-Cyclopropyl-N-[6-(difluoromethyl)-5-methyl-3-pyridyl]-2-isobutyl-2-methyl-pent-4-ynamide
A mixture of 0,385 g (1 ,85 mmol) 5-cyclopropyl-2-isobutyl-2-methyl-pent-4-ynoic acid and 0,309 g (2,31 mmol) 1-chloro-/\/,/\/,2-trimethyl-1-propenylamine in 20 ml tetrahydrofuran was stirred for 2 h at 20°C. Subsequently 0,300 g (1 ,54 mmol) 6-(difluoromethyl)-5-methyl-pyridin-3-amine in 10 ml tetrahydrofuran and 0,622 g (6,16 mmol) triethylamine were added and the reaction mixture was stirred for 16 h at 20°C. Afterwards the reaction mixture was diluted with 50 ml water and extracted with 50 ml ethyl acetate, the organic layer was dried over sodium sulfate and concentrated. The residue was purified by column chromatography with petrolether/ethyl acetate mixtures to give 123 mg (23% of theory) 5-cyclopropyl-N-[6-(difluoromethyl)-5-methyl-3- pyridyl]-2-isobutyl-2-methyl-pent-4-ynamide as white solid.
1H-NMR (CDCIs, d in ppm): 0.47 - 0.52 (m, 2 H) 0.68 - 0.74 (m, 2 H) 0.91 (t, J=6.48 Hz, 6 H) 1.16 - 1.24 (m, 1 H) 1.37 (s, 3 H) 1.53 - 1.61 (m, 1 H) 1.70 - 1.84 (m, 2 H) 2.35 - 2.42 (m, 1 H) 2.49 - 2.60 (m, 4 H), 6.63 - 6.93 (m, 1 H) 8.04 (s, 1 H) 8.64 (d, J= 2.08 Hz, 1 H)
Example 2 - Synthesis of 2-tert-butyl-N-(5,6-dimethyl-3-pyridyl)-4-phenyl-but-3-ynamide
2.1. tert-Butyl 2-iodo-3,3-dimethyl-butanoate
76 ml 2m LDA-solution in tetrahydrofuran/hexanes (151 mmol) was added to a solution of 20 g (116 mmol) tert-butyl 3,3-dimethylbutanoate in 200 ml tetrahydrofuran at -78°C. After 0,5 h 44 g (174 mmol) Iodine dissolved in tetrahydrofuran was added and the reaction mixture was stirre for 2 h at -78°C. The reaction mixture was quenched with aqueous ammonium chloride solution and the aqueous phase was extracted with methyl-t-butyl ether. The organic phase was dried over sodium sulfate and concentrated and the residue was purified by colomn chromatography with petrol ether/methyl-t-butyl ether-mixtures to 17,8 g (51 % of theory) tert-Butyl 2-iodo-3,3- dimethyl-butanoate as yellow solid.
1H-NMR (CDCIs, d in ppm): 1.09 (s, 9 H) 1.37 - 1.40 (m, 1 H) 1.39 (s, 8 H) 4.08 (s, 1 H)
2.2. tert-butyl 2-tert-butyl-4-phenyl-but-3-ynoate
2,7 ml 2,5 m (6,71 mmol) n-butyl-lithium solution in hexanes was added to a solution of 0,685 g (6,71 mmol) ethynylbenzene in 20 ml diethylether at 0°C upon stirring. 1 ,2 g (6,71 mmol)
gallium trichloride was added and the reaction mixture was stirred 30 min at 0°C. Subsequently 1 g (3,36 mmol) tert-butyl 2-iodo-3,3-dimethyl-butanoate dissolved in diethylether and 1 ,3 ml (1 ,3 mmol) triethyl borane were added dropwise upon stirring at 20 °C. Then the reaction mixture was exposed to air and stirring was continued for further 2 h at 20°C. Afterwards the reaction mixture was quenched with 1 m HCI-solution and the aqueous layer was extracted with methyl-t-butyl ether. The combined organic layers were extracted with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography with petrol ether/methyl-t-butyl ether mixtures. The reaction was performed in 6 batches to give 0,85 g (15 % of theory) tert-butyl 2-tert-butyl-4-phenyl-but-3-ynoate as colourless oil.
1H-NMR (CDCIs, d in ppm): 1.17 (s, 9 H) 1.53 (s, 9 H) 3.24 - 3.26 (m, 1 H) 7.30 - 7.34 (m, 3 H) 7.46 (dd, J= 6.65, 3.01 Hz, 2 H)
2.3. 2-tert-butyl-4-phenyl-but-3-ynoic acid
A solution of (850 mg, 3.1 mmol) tert-butyl 2-tert-butyl-4-phenyl-but-3-ynoate in 30 ml
HCI\methyl-t-butyl ether was stirred for 16 h at 20° C. The reaction mixture was concentrated to give 2-tert-butyl-4-phenyl-but-3-ynoic acid (630 mg, crude) as yellow solid.
1H-NMR (CDCIs, d in ppm): 1.12 (s, 9 H) 7.21 - 7.25 (m, 3 H) 7.36 - 7.41 (m, 2 H)
2.4 2-tert-butyl-N-(5,6-dimethyl-3-pyridyl)-4-phenyl-but-3-ynamide
A mixture of 0,430 g (2 mmol) 2-tert-butyl-4-phenyl-but-3-ynoic acid and 0,401 g (3 mmol) 1- chloro-/\/,/\/,2-trimethyl-1-propenylamine in 20 ml tetrahydrofuran was stirred for 2 h at 20°C.
At 0°C this solution was added upon stirring to a mixture of 0,293 g (2,4 mmol) 5,6- dimethylpyridin-3-amine and 0,202 g (2 mmol) triethylamine in 10 ml tetrahydrofuran. After 3 h at 20 °C the reaction mixture was diluted with 50 ml water and the aqueous phase was extracted with ethyl acetate.. The combined organic layers were dried over sodium sulfate and concentrated. The residue was purified by column chromatography to give 0,34 g (53 % of theory) 2-tert-butyl-N-(5,6-dimethyl-3-pyridyl)-4-phenyl-but-3-ynamide as white solid.
1H-NMR (CDCIs, d in ppm): 1.23 (s, 9 H) 2.51 (s, 3 H) 2.69 (s, 3 H) 3.52 (s, 1 H) 7.29 - 7.39 (m, 3 H) 7.45 (dd, J= 6.69, 2.94 Hz, 2 H) 8.23 (s, 1 H) 9.13 (d, J= 2.00 Hz, 1 H).
Green House
The compound was dissolved in a mixture of acetone and/or dimethylsulfoxide and the wetting agent/emulsifier Wettol, which is based on ethoxylated alkylphenoles, in a ratio (volume) solvent-emulsifier of 99 to 1 to give a total volume of 5 ml. Subsequently, water was added to total volume of 100 ml.
This stock solution was then diluted with the described solvent-emulsifier-water mixture to the final concentration given in the table below.
Example 1- Preventative fungicidal control of Botrytis cinerea on leaves of green pepper
Young seedlings of green pepper were grown in pots to the 4 to 5 leaf stage. These plants were sprayed to run-off with previously described spray solution, containing the concentration of active ingredient or mixture mentioned in the table below. Seven days later the plants were inoculated with an aqueous DOB solution (or a DOB solution containing 10 percent glycerine), containing the spore suspension of Botrytis cinerea. Then the plants were immediately transferred to a humid chamber. After 5 days at 22 to 24"C and a saturated relative humidity, the extent of fungal attack on the leaves was visually assessed as % diseased leaf area.
In this test, the samples which had been treated with 250 ppm of the active substance from examples B-4 respectively, showed 18 % growth of the pathogen whereas the untreated plants were 90 % infected.
Claims
1. Use of the compounds of formula I
X is O or S;
R1 is in each case independently selected from hydrogen, halogen, CN, Ci-C6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R1 are unsubstituted or substituted by one to six groups R1a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R1a are unsubstituted or substituted by one to six halogen or CN;
R2 is in each case independently selected from halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R2 are unsubstituted or substituted by one to six groups R2a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R2a are unsubstituted or substituted by one to six halogen or CN;
R3 is in each case independently selected from halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R3 are unsubstituted or substituted by one to six groups R3a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R3a are unsubstituted or substituted by one to six halogen or CN;
R4 is in each case independently selected from hydrogen, halogen, CN, Ci-C6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the acyclic and cyclic moieties of R4 are unsubstituted or substituted by one to six groups R4a which independently of one another are selected from:
halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl;
and wherein the groups R4a are unsubstituted or substituted by one to six halogen or CN;
A is direct bond or C(R7R8);
R5, R6, R7, R8
are in each case independently selected from hydrogen, halogen, CN, Ci-C6-alkyl, C1-C6- halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, O-CrCe-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, Cs-Ce-cycloalkyl, O-Cs-Ce-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, 0-C3-C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl, wherein the acyclic moieties of R5, R6, R7, R8 are unsubstituted or substituted by one to six groups R5a, R6a, R7a, R8a which independently of one another are selected from:
halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-Ci-C6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, Cs-Ce- cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, O-C3-C6- cycloalkenyl, CH2-C3-C6-cycloalkenyl, =N-OR';
and wherein the groups R5a, R6a, R7a, R8a are unsubstituted or substituted by one to six halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, 0-Ci-C6-alkyl,=N-OR';
or two moieties: R5 and R6 or R7 and R8 form together with the C atoms to which they are bound a group C=N-OR'; wherein
R' is in each case independently selected from H, Ci-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C6-cycloalkyl, phenyl, -Chh-phenyl, a five- or six-membered heteroaryl, or -Chh- heteroaryl; wherein the heteroaryl contains 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S);
wherein the cyclic and acyclic moieties of R' are unsubstituted or substituted by one to six groups RR independently of one another are selected from: halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, 0-Ci-C6-alkyl, =N-OR';
R9 is in each case independently selected from Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6- alkenyl, C2-C6-halogenalkenyl, C2-C6-alkynyl, C2-C6-halogenalkynyl, C3-C6-cycloalkyl, C3- C6-cycloalkenyl, saturated or partially unsaturated bicyclic carbocycle, a five- or six- membered heterocycle, aryl, heteroaryl; wherein the heterocycle and heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S);
wherein R' is as defined above;
wherein the cyclic and acyclic moieties of R9 are unsubstituted or substituted by one to six groups R9a independently of one another are selected from: halogen, CN, CrC6-alkyl, CrC6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, C2-C6- alkynyl, C2-C6-halogenalkynyl, 0-CrC6-alkyl, 0-C2-C6-alkenyl, 0-C2-C6-alkynyl, =N-OR';
and wherein the groups R9a are unsubstituted or substituted by one to six halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, 0-Ci-C6-alkyl,÷ wherein
when A is C(R7R8) R9 can be also H;
wherein R5, R6, R7 or R8 can not all be H;
and the N-oxides and the agriculturally acceptable salts thereof as fungicides.
2. The use of claim 1 , wherein R1 is H.
3. The use of claims 1 or 2, wherein R2 is selected from Ci-C6-alkyl, Ci-C6-halogenalkyl, 0-Ci- C6-alkyl, C3-C4-cycloalkyl.
4. The use of any one of claims 1 to 3, wherein R3 is selected from Ci-C6-alkyl, C1-C6- halogenalkyl, 0-Ci-C6-alkyl, C3-C4-cycloalkyl.
5. The use of any one of claims 1 to 4, wherein R4 is H.
6. The use of any one of claims 1 to 5, wherein R5, R6, R7, R8 are independently selected from H, halogen, CN, Ci-C6-alkyl, Ci-C6-halogenalkyl, C2-C6-alkenyl, C2-C6-halogenalkenyl, 0-Ci- C6-alkyl, C3-C6-cycloalkyl, 0-C3-C6-cycloalkyl, CH2-C3-C6-cycloalkyl, C3-C6-cycloalkenyl, O-C3- C6-cycloalkenyl, CH2-C3-C6-cycloalkenyl.
7. The use of any one of claims 1 to 6, wherein R9 is selected from Ci-C6-alkyl, C1-C6- halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
8. The use of any one of claims 1 to 7, wherein A is C(R7R8). R9 is selected from H, Ci-C6-alkyl, Ci-C6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl; wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group
independently selected from C(=0) and C(=S).
9. The use of any one of claims 1 to 7, wherein when A is a single bond, R9 is selected from Ci- C6-alkyl, Ci-C6-halogenalkyl, C3-C6-cycloalkyl, a five- or six-membered aryl, heteroaryl;
wherein the heteroaryl contain 1 , 2 or 3 heteroatoms selected from N, O and S; and wherein in each case one or two CH2 groups of the carbo- or heterocycle may be replaced by a group independently selected from C(=0) and C(=S).
10. Compounds of the formula I as defined in claims 1 to 8,
wherein R1 , R2, R3, R4, R5, R6, R7, R8 and R9 are as defined above.
1 1. A composition, comprising one compound of formula I, as defined in any of the claims 1 to 9, an N-oxide or an agriculturally acceptable salt thereof.
12. A method for combating phytopathogenic fungi, comprising treating the fungi or the materials, plants, the soil or seeds to be protected against fungal attack with an effective amount of at least one compound of formula I, as defined in any of the claims 1 to 9 or with a composition, as defined in any of the claim 10.
13. Seed, coated with at least one compound of the formula I , as defined in any of the claims 1 to 9 or an agriculturally acceptable salt thereof or with a composition, as defined in any of the claim 10, in an amount of from 0.1 to 10 kg per 100 kg of seed.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP19178599 | 2019-06-06 | ||
| EP19178599.7 | 2019-06-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020244969A1 true WO2020244969A1 (en) | 2020-12-10 |
Family
ID=66776134
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2020/064576 WO2020244969A1 (en) | 2019-06-06 | 2020-05-26 | Pyridine derivatives and their use as fungicides |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2020244969A1 (en) |
Citations (153)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3296272A (en) | 1965-04-01 | 1967-01-03 | Dow Chemical Co | Sulfinyl- and sulfonylpyridines |
| US3325503A (en) | 1965-02-18 | 1967-06-13 | Diamond Alkali Co | Polychloro derivatives of mono- and dicyano pyridines and a method for their preparation |
| EP0141317A2 (en) | 1983-10-21 | 1985-05-15 | BASF Aktiengesellschaft | 7-Amino-azolo[1,5-a]pyrimidines and fungicides containing them |
| EP0152031A2 (en) | 1984-02-03 | 1985-08-21 | Shionogi & Co., Ltd. | Azolyl cycloalkanol derivatives and agricultural fungicides |
| EP0226917A1 (en) | 1985-12-20 | 1987-07-01 | BASF Aktiengesellschaft | Acrylic acid esters and fungicides containing these compounds |
| EP0243970A1 (en) | 1986-05-02 | 1987-11-04 | Stauffer Chemical Company | Fungicidal pyridyl imidates |
| EP0256503A2 (en) | 1986-08-12 | 1988-02-24 | Mitsubishi Kasei Corporation | Pyridinecarboxamide derivatives and their use as fungicide |
| EP0428941A1 (en) | 1989-11-10 | 1991-05-29 | Agro-Kanesho Co., Ltd. | Hexahydrotriazine compounds and insecticides |
| EP0532022A1 (en) | 1991-09-13 | 1993-03-17 | Ube Industries, Ltd. | Acrylate compound, preparation process thereof and fungicide using the same |
| WO1994001546A1 (en) | 1992-07-01 | 1994-01-20 | Cornell Research Foundation, Inc. | Elicitor of the hypersensitive response in plants |
| DE19650197A1 (en) | 1996-12-04 | 1998-06-10 | Bayer Ag | 3-thiocarbamoylpyrazole derivatives |
| WO1998044140A1 (en) | 1997-04-03 | 1998-10-08 | Dekalb Genetics Corporation | Glyphosate resistant maize lines |
| WO1998046608A1 (en) | 1997-04-14 | 1998-10-22 | American Cyanamid Company | Fungicidal trifluoromethylalkylamino-triazolopyrimidines |
| WO1999014187A1 (en) | 1997-09-18 | 1999-03-25 | Basf Aktiengesellschaft | Benzamidoxim derivatives, intermediate products and methods for preparing and using them as fungicides |
| WO1999024413A2 (en) | 1997-11-12 | 1999-05-20 | Bayer Aktiengesellschaft | Isothiazole carboxylic acid amides and the application thereof in order to protect plants |
| WO1999027783A1 (en) | 1997-12-04 | 1999-06-10 | Dow Agrosciences Llc | Fungicidal compositions and methods, and compounds and methods for the preparation thereof |
| WO2000026345A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N.V. | Glufosinate tolerant rice |
| WO2000026356A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N. V. | Glufosinate tolerant rice |
| WO2000029404A1 (en) | 1998-11-17 | 2000-05-25 | Kumiai Chemical Industry Co., Ltd. | Pyrimidinylbenzimidazole and triazinylbenzimidazole derivatives and agricultura/horticultural bactericides |
| WO2000046148A1 (en) | 1999-02-02 | 2000-08-10 | Sintokogio, Ltd. | Silica gel carrying titanium oxide photocatalyst in high concentration and method for preparation thereof |
| EP1028125A1 (en) | 1998-11-30 | 2000-08-16 | Isagro Ricerca S.r.l. | Dipeptide compounds having fungicidal activity and their agronomic use |
| EP1035122A1 (en) | 1999-03-11 | 2000-09-13 | Rohm And Haas Company | Heterocyclic subsituted isoxazolidines and their use as fungicides |
| WO2000065913A1 (en) | 1999-04-28 | 2000-11-09 | Takeda Chemical Industries, Ltd. | Sulfonamide derivatives |
| WO2001031042A2 (en) | 1999-10-29 | 2001-05-03 | Aventis Cropscience N.V. | Male-sterile brassica plants and methods for producing same |
| WO2001041558A1 (en) | 1999-12-08 | 2001-06-14 | Aventis Cropscience N.V. | Hybrid winter oilseed rape and methods for producing same |
| DE10021412A1 (en) | 1999-12-13 | 2001-06-21 | Bayer Ag | Fungicidal active ingredient combinations |
| WO2001054501A2 (en) | 2000-01-25 | 2001-08-02 | Syngenta Participations Ag | Herbicidal composition |
| EP1122244A1 (en) | 2000-02-04 | 2001-08-08 | Sumitomo Chemical Company, Limited | Uracil compounds and their use |
| WO2001056358A2 (en) | 2000-01-28 | 2001-08-09 | Rohm And Haas Company | Enhanced propertied pesticides |
| CN1309897A (en) | 2000-02-24 | 2001-08-29 | 沈阳化工研究院 | Unsaturated oximino ether bactericide |
| WO2002022583A2 (en) | 2000-09-18 | 2002-03-21 | E. I. Du Pont De Nemours And Company | Pyridinyl amides and imides for use as fungicides |
| WO2002034946A2 (en) | 2000-10-25 | 2002-05-02 | Monsanto Technology Llc | Cotton event pv-ghgt07(1445) and compositions and methods for detection thereof |
| EP1201648A1 (en) | 1999-08-05 | 2002-05-02 | Kumiai Chemical Industry Co., Ltd. | Carbamate derivatives and agricultural/horticultural bactericides |
| WO2002036831A2 (en) | 2000-10-30 | 2002-05-10 | Monsanto Technology Llc | Canola event pv-bngt04(rt73) and compositions and methods for detection thereof |
| WO2002040431A2 (en) | 2000-11-17 | 2002-05-23 | Dow Agrosciences Llc | Compounds having fungicidal activity and processes to make and use same |
| US20020102582A1 (en) | 2000-09-13 | 2002-08-01 | Levine Elaine B. | Corn event MON810 and compositions and methods for detection thereof |
| JP2002316902A (en) | 2001-04-20 | 2002-10-31 | Sumitomo Chem Co Ltd | Plant blight-preventing agent composition |
| WO2002100163A2 (en) | 2001-06-11 | 2002-12-19 | Monsanto Technology Llc | Cotton event moni5985 and compositions and methods for detection |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003011853A1 (en) | 2001-07-30 | 2003-02-13 | Dow Agrosciences Llc | 6-aryl-4-aminopicolinates and their use as herbicides |
| WO2003014103A1 (en) | 2001-08-03 | 2003-02-20 | Bayer Cropscience S.A. | Iodobenzopyran-4-one derivatives having fungicidal activity |
| WO2003013224A2 (en) | 2001-08-06 | 2003-02-20 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for producing and identifying same |
| WO2003016303A1 (en) | 2001-08-20 | 2003-02-27 | Dainippon Ink And Chemicals, Inc. | Tetrazoyl oxime derivative and agricultural chemical containing the same as active ingredient |
| WO2003016286A1 (en) | 2001-08-17 | 2003-02-27 | Sankyo Agro Company, Limited | 3-phenoxy-4-pyridazinol derivative and herbicide composition containing the same |
| WO2003053145A1 (en) | 2001-12-21 | 2003-07-03 | Nissan Chemical Industries, Ltd. | Bactericidal composition |
| US20030126634A1 (en) | 1990-08-09 | 2003-07-03 | Dekalb Genetics Corporation | Methods and compositions for the increase of yield in plants |
| WO2003061388A1 (en) | 2002-01-18 | 2003-07-31 | Sumitomo Chemical Takeda Agro Company, Limited | Fused heterocyclic sulfonylurea compound, herbicide containing the same, and method of controlling weed with the same |
| WO2003064572A1 (en) | 2002-01-31 | 2003-08-07 | Exxonmobil Research And Engineering Company | Lubricating oil compositions with improved friction properties |
| WO2003066609A1 (en) | 2002-02-04 | 2003-08-14 | Bayer Cropscience Aktiengesellschaft | Disubstituted thiazolyl carboxanilides and their use as microbicides |
| WO2003074491A1 (en) | 2002-03-05 | 2003-09-12 | Syngenta Participations Ag | O-cyclopropyl-carboxanilides and their use as fungicides |
| CN1456054A (en) | 2003-03-25 | 2003-11-19 | 浙江省化工研究院 | Methoxy methyl acrylate compounds as bactericidal agent |
| WO2004011601A2 (en) | 2002-07-29 | 2004-02-05 | Monsanto Technology, Llc | Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof |
| WO2004039986A1 (en) | 2002-10-29 | 2004-05-13 | Syngenta Participations Ag | Cot102 insecticidal cotton |
| WO2004049804A2 (en) | 2002-11-29 | 2004-06-17 | Syngenta Participations Ag | Fungicidal combinations for crop potection |
| WO2004072235A2 (en) | 2003-02-12 | 2004-08-26 | Monsanto Technology Llc | Cotton event mon 88913 and compositions and methods for detection thereof |
| WO2004074492A1 (en) | 2003-02-20 | 2004-09-02 | Kws Saat Ag | Glyphosate tolerant sugar beet |
| WO2004083193A1 (en) | 2003-03-17 | 2004-09-30 | Sumitomo Chemical Company, Limited | Amide compound and bactericide composition containing the same |
| WO2004099447A2 (en) | 2003-05-02 | 2004-11-18 | Dow Agrosciences Llc | Corn event tc1507 and methods for detection thereof |
| WO2005059103A2 (en) | 2003-12-15 | 2005-06-30 | Monsanto Technology Llc | Corn plant mon88017 and compositions and methods for detection thereof |
| WO2005061720A2 (en) | 2003-12-11 | 2005-07-07 | Monsanto Technology Llc | High lysine maize compositions and methods for detection thereof |
| WO2005063721A1 (en) | 2003-12-19 | 2005-07-14 | E.I. Dupont De Nemours And Company | Herbicidal pyrimidines |
| WO2005087773A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005087772A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005103266A1 (en) | 2004-03-26 | 2005-11-03 | Dow Agrosciences Llc | Cry1f and cry1ac transgenic cotton lines and event-specific identification thereof |
| WO2005103301A2 (en) | 2004-03-25 | 2005-11-03 | Syngenta Participations Ag | Corn event mir604 |
| WO2005120234A2 (en) | 2004-06-03 | 2005-12-22 | E.I. Dupont De Nemours And Company | Fungicidal mixtures of amidinylphenyl compounds |
| WO2005123690A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-difluoromethyl-pyrazol-4-carbonic acid-(ortho-phenyl)-anilides, and use thereof as a fungicide |
| WO2005123689A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-trifluoromethyl-pyrazole-4-carboxylic acid (ortho-phenyl)-anilides and to use thereof as fungicide |
| WO2006015866A1 (en) | 2004-08-12 | 2006-02-16 | Syngenta Participations Ag | Method for protecting useful plants or plant propagation material |
| WO2006039376A2 (en) | 2004-09-29 | 2006-04-13 | Pioneer Hi-Bred International, Inc. | Corn event das-59122-7 and methods for detection thereof |
| WO2006087343A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | Pyrazole carboxylic acid anilides, method for the production thereof and agents containing them for controlling pathogenic fungi |
| WO2006087325A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | 5-alkoxyalkyl-6-alkyl-7-amino-azolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said substances |
| DE102005009458A1 (en) | 2005-03-02 | 2006-09-07 | Bayer Cropscience Ag | pyrazolylcarboxanilides |
| WO2006098952A2 (en) | 2005-03-16 | 2006-09-21 | Syngenta Participations Ag | Corn event 3272 and methods of detection thereof |
| WO2006108675A2 (en) | 2005-04-11 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a5547-127 and methods and kits for identifying such event in biological samples |
| WO2006108674A2 (en) | 2005-04-08 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a2704-12 and methods and kits for identifying such event in biological samples |
| WO2006130436A2 (en) | 2005-05-27 | 2006-12-07 | Monsanto Technology Llc | Soybean event mon89788 and methods for detection thereof |
| WO2006128573A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce43- 67b, insecticidal transgenic cotton expressing cry1ab |
| WO2007006670A1 (en) | 2005-07-07 | 2007-01-18 | Basf Aktiengesellschaft | N-thio-anthranilamid compounds and their use as pesticides |
| CN1907024A (en) | 2005-08-03 | 2007-02-07 | 浙江化工科技集团有限公司 | Methoxyl group displacement methyl acrylate compound bactericidal agent |
| WO2007017186A1 (en) | 2005-08-08 | 2007-02-15 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for identifying same |
| WO2007082098A2 (en) | 2006-01-13 | 2007-07-19 | Dow Agrosciences Llc | 6-(poly-substituted aryl)-4-aminopicolinates and their use as herbicides |
| WO2007090624A2 (en) | 2006-02-09 | 2007-08-16 | Syngenta Participations Ag | A method of protecting a plant propagation material, a plant, and/or plant organs |
| WO2007129454A1 (en) | 2006-05-08 | 2007-11-15 | Kumiai Chemical Industry Co., Ltd. | 1,2-benzisothiazole derivative, and agricultural or horticultural plant disease-controlling agent |
| WO2007140256A1 (en) | 2006-05-26 | 2007-12-06 | Monsanto Technology, Llc | Corn plant and seed corresponding to transgenic event mon89034 and methods for detection and use thereof |
| WO2007142840A2 (en) | 2006-06-03 | 2007-12-13 | Syngenta Participations Ag | Corn event mir162 |
| US20070292854A1 (en) | 2000-06-22 | 2007-12-20 | Behr Carl F | Corn event PV-ZMGT32(nk603) and compositions and methods for detection thereof |
| WO2008002872A2 (en) | 2006-06-28 | 2008-01-03 | Pioneer Hi-Bred International, Inc. | Soybean event 3560.4.3.5 and compositions and methods for the identification and/or detection thereof |
| WO2008013622A2 (en) | 2006-07-27 | 2008-01-31 | E. I. Du Pont De Nemours And Company | Fungicidal azocyclic amides |
| WO2008054747A2 (en) | 2006-10-31 | 2008-05-08 | E. I. Du Pont De Nemours And Company | Soybean event dp-305423-1 and compositions and methods for the identification and/or detection thereof |
| WO2008112019A2 (en) | 2006-10-30 | 2008-09-18 | Pioneer Hi-Bred International, Inc. | Maize event dp-098140-6 and compositions and methods for the identification and/or detection thereof |
| WO2008122406A1 (en) | 2007-04-05 | 2008-10-16 | Bayer Bioscience N.V. | Insect resistant cotton plants and methods for identifying same |
| WO2008151780A1 (en) | 2007-06-11 | 2008-12-18 | Bayer Bioscience N.V. | Insect resistant cotton plants comprising elite event ee-gh6 and methods for identifying same |
| WO2009064652A1 (en) | 2007-11-15 | 2009-05-22 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87701 and methods for detection thereof |
| WO2009090181A2 (en) | 2008-01-15 | 2009-07-23 | Bayer Cropscience Sa | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| WO2009094442A2 (en) | 2008-01-22 | 2009-07-30 | Dow Agrosciences Llc | 5-fluoro pyrimidine derivatives |
| WO2009103049A2 (en) | 2008-02-14 | 2009-08-20 | Pioneer Hi-Bred International, Inc. | Plant genomic dna flanking spt event and methods for identifying spt event |
| WO2009102873A1 (en) | 2008-02-15 | 2009-08-20 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87769 and methods for detection thereof |
| WO2009111263A1 (en) | 2008-02-29 | 2009-09-11 | Monsanto Technology Llc | Corn plant event mon87460 and compositions and methods for detection thereof |
| WO2010037016A1 (en) | 2008-09-29 | 2010-04-01 | Monsanto Technology Llc | Soybean transgenic event mon87705 and methods for detection thereof |
| WO2010069882A1 (en) | 2008-12-17 | 2010-06-24 | Syngenta Participations Ag | Isoxazole derivatives for use as fungicides |
| WO2010077816A1 (en) | 2008-12-16 | 2010-07-08 | Syngenta Participations Ag | Corn event 5307 |
| WO2010080829A1 (en) | 2009-01-07 | 2010-07-15 | Basf Agrochemical Products B.V. | Soybean event 127 and methods related thereto |
| WO2010139271A1 (en) | 2009-06-05 | 2010-12-09 | 中国中化股份有限公司 | E-type phenyl acrylic ester compounds containing substituted anilino pyrimidine group and uses thereof |
| WO2011022469A2 (en) | 2009-08-19 | 2011-02-24 | Dow Agrosciences Llc | Aad-1 event das-40278-9, related transgenic corn lines, and event-specific identification thereof |
| WO2011028657A1 (en) | 2009-09-01 | 2011-03-10 | Dow Agrosciences Llc | Synergistic fungicidal compositions containing a 5-fluoropyrimidine derivative for fungal control in cereals |
| WO2011034704A1 (en) | 2009-09-17 | 2011-03-24 | Monsanto Technology Llc | Soybean transgenic event mon 87708 and methods of use thereof |
| WO2011062904A1 (en) | 2009-11-23 | 2011-05-26 | Monsanto Technology Llc | Transgenic maize event mon 87427 and the relative development scale |
| WO2011066384A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Aad-12 event 416, related transgenic soybean lines, and event-specific identification thereof |
| WO2011077514A1 (en) | 2009-12-22 | 2011-06-30 | 三井化学アグロ株式会社 | Plant disease control composition and method for controlling plant diseases by applying the composition |
| WO2011081174A1 (en) | 2010-01-04 | 2011-07-07 | 日本曹達株式会社 | Nitrogen-containing heterocyclic compound and agricultural/horticultural germicide |
| WO2011084621A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-004114-3 and methods for detection thereof |
| WO2011135827A1 (en) | 2010-04-27 | 2011-11-03 | Sumitomo Chemical Company, Limited | Pesticidal composition and its use |
| WO2011135833A1 (en) | 2010-04-28 | 2011-11-03 | Sumitomo Chemical Company, Limited | Plant disease control composition and its use |
| WO2011153186A1 (en) | 2010-06-04 | 2011-12-08 | Monsanto Technology Llc | Transgenic brassica event mon 88302 and methods of use thereof |
| WO2012051199A2 (en) | 2010-10-12 | 2012-04-19 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87712 and methods for detection thereof |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012084812A1 (en) | 2010-12-20 | 2012-06-28 | Isagro Ricerca S.R.L. | Aminoindanes amides having a high fungicidal activity and their phytosanitary compositions |
| WO2012134808A1 (en) | 2011-03-30 | 2012-10-04 | Monsanto Technology Llc | Cotton transgenic event mon 88701 and methods of use thereof |
| WO2012165511A1 (en) | 2011-05-31 | 2012-12-06 | クミアイ化学工業株式会社 | Method for controlling diseases in rice plant |
| WO2012168188A1 (en) | 2011-06-07 | 2012-12-13 | Bayer Intellectual Property Gmbh | Active compound combinations |
| WO2013003558A1 (en) | 2011-06-30 | 2013-01-03 | Monsanto Technology Llc | Alfalfa plant and seed corresponding to transgenic event kk 179-2 and methods for detection thereof |
| WO2013007767A1 (en) | 2011-07-13 | 2013-01-17 | Basf Se | Fungicidal substituted 2-[2-halogenalkyl-4-(phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013010862A1 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Fungicidal alkyl-substituted 2-[2-chloro-4-(4-chloro-phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013016516A1 (en) | 2011-07-26 | 2013-01-31 | Dow Agrosciences Llc | Insect resistant and herbicide tolerant breeding stack of soybean event pdab9582.814.19.1 and pdab4468.04.16.1 |
| WO2013024010A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013024009A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013047441A1 (en) | 2011-09-26 | 2013-04-04 | 日本曹達株式会社 | Agricultural and horticultural bactericide composition |
| WO2013047749A1 (en) | 2011-09-29 | 2013-04-04 | 三井化学アグロ株式会社 | Production method for 4, 4-difluoro-3,4-dihydroisoquinoline derivative |
| WO2013092224A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | Use of strobilurin type compounds for combating phytopathogenic fungi resistant to qo inhibitors |
| WO2013112527A1 (en) | 2012-01-23 | 2013-08-01 | Dow Agrosciences Llc | Herbicide tolerant cotton event pdab4468.19.10.3 |
| WO2013116251A2 (en) | 2012-02-01 | 2013-08-08 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazole mixtures |
| WO2013127704A1 (en) | 2012-02-27 | 2013-09-06 | Bayer Intellectual Property Gmbh | Active compound combinations containing a thiazoylisoxazoline and a fungicide |
| WO2013162072A1 (en) | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
| CN103387541A (en) | 2012-05-10 | 2013-11-13 | 中国中化股份有限公司 | Preparation method of substituted pyrazolylether compound |
| WO2013169923A2 (en) | 2012-05-08 | 2013-11-14 | Monsanto Technology Llc | Corn event mon 87411 |
| WO2014060177A1 (en) | 2012-10-16 | 2014-04-24 | Syngenta Participations Ag | Fungicidal compositions |
| WO2014116854A1 (en) | 2013-01-25 | 2014-07-31 | Pioneer Hi-Bred International, Inc. | Maize event dp-033121-3 and methods for detection thereof |
| WO2014178941A1 (en) | 2013-05-02 | 2014-11-06 | J.R. Simplot Company | Potato cultivar j3 |
| WO2014201235A2 (en) | 2013-06-14 | 2014-12-18 | Monsanto Technology Llc | Soybean transgenic event mon87751 and methods for detection and use thereof |
| WO2015053998A1 (en) | 2013-10-09 | 2015-04-16 | Monsanto Technology Llc | Transgenic corn event mon87403 and methods for detection thereof |
| EP2865265A1 (en) | 2014-02-13 | 2015-04-29 | Bayer CropScience AG | Active compound combinations comprising phenylamidine compounds and biological control agents |
| WO2015065922A1 (en) | 2013-10-28 | 2015-05-07 | Dexcom, Inc. | Devices used in connection with continuous analyte monitoring that provide the user with one or more notifications, and related methods |
| JP2015089883A (en) | 2013-11-06 | 2015-05-11 | アグロカネショウ株式会社 | 2-amino nicotinic acid benzyl ester derivative and antimicrobial agent comprising the same as active ingredient |
| JP2015120675A (en) | 2013-12-25 | 2015-07-02 | アグロカネショウ株式会社 | 6-substituted nicotinic acid ester derivative and antimicrobial agent containing the same as essential component |
| WO2015119246A1 (en) | 2014-02-07 | 2015-08-13 | 日産化学工業株式会社 | Sterilising or bactericidal composition and disease control method |
| WO2015142571A1 (en) | 2014-03-20 | 2015-09-24 | Monsanto Technology Llc | Transgenic maize event mon 87419 and methods of use thereof |
| WO2016183445A1 (en) | 2015-05-14 | 2016-11-17 | J.R. Simplot Company | Potato cultivar v11 |
| WO2017062831A1 (en) | 2015-10-08 | 2017-04-13 | J.R. Simplot Company | Potato cultivar x17 |
| WO2017062825A1 (en) | 2015-10-08 | 2017-04-13 | J.R. Simplot Company | Potato cultivar y9 |
| WO2018055135A1 (en) * | 2016-09-23 | 2018-03-29 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2019053027A1 (en) * | 2017-09-13 | 2019-03-21 | Syngenta Participations Ag | Microbiocidal quinoline (thio)carboxamide derivatives |
| WO2019053010A1 (en) * | 2017-09-13 | 2019-03-21 | Syngenta Participations Ag | Microbiocidal quinoline (thio)carboxamide derivatives |
-
2020
- 2020-05-26 WO PCT/EP2020/064576 patent/WO2020244969A1/en active Application Filing
Patent Citations (157)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3325503A (en) | 1965-02-18 | 1967-06-13 | Diamond Alkali Co | Polychloro derivatives of mono- and dicyano pyridines and a method for their preparation |
| US3296272A (en) | 1965-04-01 | 1967-01-03 | Dow Chemical Co | Sulfinyl- and sulfonylpyridines |
| EP0141317A2 (en) | 1983-10-21 | 1985-05-15 | BASF Aktiengesellschaft | 7-Amino-azolo[1,5-a]pyrimidines and fungicides containing them |
| EP0152031A2 (en) | 1984-02-03 | 1985-08-21 | Shionogi & Co., Ltd. | Azolyl cycloalkanol derivatives and agricultural fungicides |
| EP0226917A1 (en) | 1985-12-20 | 1987-07-01 | BASF Aktiengesellschaft | Acrylic acid esters and fungicides containing these compounds |
| EP0243970A1 (en) | 1986-05-02 | 1987-11-04 | Stauffer Chemical Company | Fungicidal pyridyl imidates |
| EP0256503A2 (en) | 1986-08-12 | 1988-02-24 | Mitsubishi Kasei Corporation | Pyridinecarboxamide derivatives and their use as fungicide |
| EP0428941A1 (en) | 1989-11-10 | 1991-05-29 | Agro-Kanesho Co., Ltd. | Hexahydrotriazine compounds and insecticides |
| US20030126634A1 (en) | 1990-08-09 | 2003-07-03 | Dekalb Genetics Corporation | Methods and compositions for the increase of yield in plants |
| EP0532022A1 (en) | 1991-09-13 | 1993-03-17 | Ube Industries, Ltd. | Acrylate compound, preparation process thereof and fungicide using the same |
| WO1994001546A1 (en) | 1992-07-01 | 1994-01-20 | Cornell Research Foundation, Inc. | Elicitor of the hypersensitive response in plants |
| DE19650197A1 (en) | 1996-12-04 | 1998-06-10 | Bayer Ag | 3-thiocarbamoylpyrazole derivatives |
| WO1998044140A1 (en) | 1997-04-03 | 1998-10-08 | Dekalb Genetics Corporation | Glyphosate resistant maize lines |
| WO1998046608A1 (en) | 1997-04-14 | 1998-10-22 | American Cyanamid Company | Fungicidal trifluoromethylalkylamino-triazolopyrimidines |
| WO1999014187A1 (en) | 1997-09-18 | 1999-03-25 | Basf Aktiengesellschaft | Benzamidoxim derivatives, intermediate products and methods for preparing and using them as fungicides |
| WO1999024413A2 (en) | 1997-11-12 | 1999-05-20 | Bayer Aktiengesellschaft | Isothiazole carboxylic acid amides and the application thereof in order to protect plants |
| WO1999027783A1 (en) | 1997-12-04 | 1999-06-10 | Dow Agrosciences Llc | Fungicidal compositions and methods, and compounds and methods for the preparation thereof |
| WO2000026345A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N.V. | Glufosinate tolerant rice |
| WO2000026356A1 (en) | 1998-11-03 | 2000-05-11 | Aventis Cropscience N. V. | Glufosinate tolerant rice |
| WO2000029404A1 (en) | 1998-11-17 | 2000-05-25 | Kumiai Chemical Industry Co., Ltd. | Pyrimidinylbenzimidazole and triazinylbenzimidazole derivatives and agricultura/horticultural bactericides |
| EP1028125A1 (en) | 1998-11-30 | 2000-08-16 | Isagro Ricerca S.r.l. | Dipeptide compounds having fungicidal activity and their agronomic use |
| WO2000046148A1 (en) | 1999-02-02 | 2000-08-10 | Sintokogio, Ltd. | Silica gel carrying titanium oxide photocatalyst in high concentration and method for preparation thereof |
| EP1035122A1 (en) | 1999-03-11 | 2000-09-13 | Rohm And Haas Company | Heterocyclic subsituted isoxazolidines and their use as fungicides |
| WO2000065913A1 (en) | 1999-04-28 | 2000-11-09 | Takeda Chemical Industries, Ltd. | Sulfonamide derivatives |
| EP1201648A1 (en) | 1999-08-05 | 2002-05-02 | Kumiai Chemical Industry Co., Ltd. | Carbamate derivatives and agricultural/horticultural bactericides |
| WO2001031042A2 (en) | 1999-10-29 | 2001-05-03 | Aventis Cropscience N.V. | Male-sterile brassica plants and methods for producing same |
| WO2001041558A1 (en) | 1999-12-08 | 2001-06-14 | Aventis Cropscience N.V. | Hybrid winter oilseed rape and methods for producing same |
| DE10021412A1 (en) | 1999-12-13 | 2001-06-21 | Bayer Ag | Fungicidal active ingredient combinations |
| WO2001054501A2 (en) | 2000-01-25 | 2001-08-02 | Syngenta Participations Ag | Herbicidal composition |
| WO2001056358A2 (en) | 2000-01-28 | 2001-08-09 | Rohm And Haas Company | Enhanced propertied pesticides |
| EP1122244A1 (en) | 2000-02-04 | 2001-08-08 | Sumitomo Chemical Company, Limited | Uracil compounds and their use |
| CN1309897A (en) | 2000-02-24 | 2001-08-29 | 沈阳化工研究院 | Unsaturated oximino ether bactericide |
| US20070292854A1 (en) | 2000-06-22 | 2007-12-20 | Behr Carl F | Corn event PV-ZMGT32(nk603) and compositions and methods for detection thereof |
| US20020102582A1 (en) | 2000-09-13 | 2002-08-01 | Levine Elaine B. | Corn event MON810 and compositions and methods for detection thereof |
| WO2002022583A2 (en) | 2000-09-18 | 2002-03-21 | E. I. Du Pont De Nemours And Company | Pyridinyl amides and imides for use as fungicides |
| WO2002034946A2 (en) | 2000-10-25 | 2002-05-02 | Monsanto Technology Llc | Cotton event pv-ghgt07(1445) and compositions and methods for detection thereof |
| WO2002036831A2 (en) | 2000-10-30 | 2002-05-10 | Monsanto Technology Llc | Canola event pv-bngt04(rt73) and compositions and methods for detection thereof |
| WO2002040431A2 (en) | 2000-11-17 | 2002-05-23 | Dow Agrosciences Llc | Compounds having fungicidal activity and processes to make and use same |
| JP2002316902A (en) | 2001-04-20 | 2002-10-31 | Sumitomo Chem Co Ltd | Plant blight-preventing agent composition |
| WO2002100163A2 (en) | 2001-06-11 | 2002-12-19 | Monsanto Technology Llc | Cotton event moni5985 and compositions and methods for detection |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003011853A1 (en) | 2001-07-30 | 2003-02-13 | Dow Agrosciences Llc | 6-aryl-4-aminopicolinates and their use as herbicides |
| WO2003014103A1 (en) | 2001-08-03 | 2003-02-20 | Bayer Cropscience S.A. | Iodobenzopyran-4-one derivatives having fungicidal activity |
| WO2003013224A2 (en) | 2001-08-06 | 2003-02-20 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for producing and identifying same |
| WO2003016286A1 (en) | 2001-08-17 | 2003-02-27 | Sankyo Agro Company, Limited | 3-phenoxy-4-pyridazinol derivative and herbicide composition containing the same |
| WO2003016303A1 (en) | 2001-08-20 | 2003-02-27 | Dainippon Ink And Chemicals, Inc. | Tetrazoyl oxime derivative and agricultural chemical containing the same as active ingredient |
| WO2003053145A1 (en) | 2001-12-21 | 2003-07-03 | Nissan Chemical Industries, Ltd. | Bactericidal composition |
| WO2003061388A1 (en) | 2002-01-18 | 2003-07-31 | Sumitomo Chemical Takeda Agro Company, Limited | Fused heterocyclic sulfonylurea compound, herbicide containing the same, and method of controlling weed with the same |
| WO2003064572A1 (en) | 2002-01-31 | 2003-08-07 | Exxonmobil Research And Engineering Company | Lubricating oil compositions with improved friction properties |
| WO2003066609A1 (en) | 2002-02-04 | 2003-08-14 | Bayer Cropscience Aktiengesellschaft | Disubstituted thiazolyl carboxanilides and their use as microbicides |
| WO2003074491A1 (en) | 2002-03-05 | 2003-09-12 | Syngenta Participations Ag | O-cyclopropyl-carboxanilides and their use as fungicides |
| WO2004011601A2 (en) | 2002-07-29 | 2004-02-05 | Monsanto Technology, Llc | Corn event pv-zmir13 (mon863) plants and compositions and methods for detection thereof |
| WO2004039986A1 (en) | 2002-10-29 | 2004-05-13 | Syngenta Participations Ag | Cot102 insecticidal cotton |
| WO2004049804A2 (en) | 2002-11-29 | 2004-06-17 | Syngenta Participations Ag | Fungicidal combinations for crop potection |
| WO2004072235A2 (en) | 2003-02-12 | 2004-08-26 | Monsanto Technology Llc | Cotton event mon 88913 and compositions and methods for detection thereof |
| WO2004074492A1 (en) | 2003-02-20 | 2004-09-02 | Kws Saat Ag | Glyphosate tolerant sugar beet |
| WO2004083193A1 (en) | 2003-03-17 | 2004-09-30 | Sumitomo Chemical Company, Limited | Amide compound and bactericide composition containing the same |
| CN1456054A (en) | 2003-03-25 | 2003-11-19 | 浙江省化工研究院 | Methoxy methyl acrylate compounds as bactericidal agent |
| WO2004099447A2 (en) | 2003-05-02 | 2004-11-18 | Dow Agrosciences Llc | Corn event tc1507 and methods for detection thereof |
| WO2005061720A2 (en) | 2003-12-11 | 2005-07-07 | Monsanto Technology Llc | High lysine maize compositions and methods for detection thereof |
| WO2005059103A2 (en) | 2003-12-15 | 2005-06-30 | Monsanto Technology Llc | Corn plant mon88017 and compositions and methods for detection thereof |
| WO2005063721A1 (en) | 2003-12-19 | 2005-07-14 | E.I. Dupont De Nemours And Company | Herbicidal pyrimidines |
| WO2005087773A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005087772A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005103301A2 (en) | 2004-03-25 | 2005-11-03 | Syngenta Participations Ag | Corn event mir604 |
| WO2005103266A1 (en) | 2004-03-26 | 2005-11-03 | Dow Agrosciences Llc | Cry1f and cry1ac transgenic cotton lines and event-specific identification thereof |
| WO2005120234A2 (en) | 2004-06-03 | 2005-12-22 | E.I. Dupont De Nemours And Company | Fungicidal mixtures of amidinylphenyl compounds |
| WO2005123690A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-difluoromethyl-pyrazol-4-carbonic acid-(ortho-phenyl)-anilides, and use thereof as a fungicide |
| WO2005123689A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-trifluoromethyl-pyrazole-4-carboxylic acid (ortho-phenyl)-anilides and to use thereof as fungicide |
| WO2006015866A1 (en) | 2004-08-12 | 2006-02-16 | Syngenta Participations Ag | Method for protecting useful plants or plant propagation material |
| WO2006039376A2 (en) | 2004-09-29 | 2006-04-13 | Pioneer Hi-Bred International, Inc. | Corn event das-59122-7 and methods for detection thereof |
| WO2006087343A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | Pyrazole carboxylic acid anilides, method for the production thereof and agents containing them for controlling pathogenic fungi |
| WO2006087325A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | 5-alkoxyalkyl-6-alkyl-7-amino-azolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said substances |
| DE102005009458A1 (en) | 2005-03-02 | 2006-09-07 | Bayer Cropscience Ag | pyrazolylcarboxanilides |
| WO2006098952A2 (en) | 2005-03-16 | 2006-09-21 | Syngenta Participations Ag | Corn event 3272 and methods of detection thereof |
| WO2006108674A2 (en) | 2005-04-08 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a2704-12 and methods and kits for identifying such event in biological samples |
| WO2006108675A2 (en) | 2005-04-11 | 2006-10-19 | Bayer Bioscience N.V. | Elite event a5547-127 and methods and kits for identifying such event in biological samples |
| WO2006130436A2 (en) | 2005-05-27 | 2006-12-07 | Monsanto Technology Llc | Soybean event mon89788 and methods for detection thereof |
| WO2006128573A2 (en) | 2005-06-02 | 2006-12-07 | Syngenta Participations Ag | Ce43- 67b, insecticidal transgenic cotton expressing cry1ab |
| WO2007006670A1 (en) | 2005-07-07 | 2007-01-18 | Basf Aktiengesellschaft | N-thio-anthranilamid compounds and their use as pesticides |
| CN1907024A (en) | 2005-08-03 | 2007-02-07 | 浙江化工科技集团有限公司 | Methoxyl group displacement methyl acrylate compound bactericidal agent |
| WO2007017186A1 (en) | 2005-08-08 | 2007-02-15 | Bayer Bioscience N.V. | Herbicide tolerant cotton plants and methods for identifying same |
| WO2007082098A2 (en) | 2006-01-13 | 2007-07-19 | Dow Agrosciences Llc | 6-(poly-substituted aryl)-4-aminopicolinates and their use as herbicides |
| WO2007090624A2 (en) | 2006-02-09 | 2007-08-16 | Syngenta Participations Ag | A method of protecting a plant propagation material, a plant, and/or plant organs |
| WO2007129454A1 (en) | 2006-05-08 | 2007-11-15 | Kumiai Chemical Industry Co., Ltd. | 1,2-benzisothiazole derivative, and agricultural or horticultural plant disease-controlling agent |
| WO2007140256A1 (en) | 2006-05-26 | 2007-12-06 | Monsanto Technology, Llc | Corn plant and seed corresponding to transgenic event mon89034 and methods for detection and use thereof |
| WO2007142840A2 (en) | 2006-06-03 | 2007-12-13 | Syngenta Participations Ag | Corn event mir162 |
| WO2008002872A2 (en) | 2006-06-28 | 2008-01-03 | Pioneer Hi-Bred International, Inc. | Soybean event 3560.4.3.5 and compositions and methods for the identification and/or detection thereof |
| WO2008013622A2 (en) | 2006-07-27 | 2008-01-31 | E. I. Du Pont De Nemours And Company | Fungicidal azocyclic amides |
| WO2008112019A2 (en) | 2006-10-30 | 2008-09-18 | Pioneer Hi-Bred International, Inc. | Maize event dp-098140-6 and compositions and methods for the identification and/or detection thereof |
| WO2008054747A2 (en) | 2006-10-31 | 2008-05-08 | E. I. Du Pont De Nemours And Company | Soybean event dp-305423-1 and compositions and methods for the identification and/or detection thereof |
| WO2008122406A1 (en) | 2007-04-05 | 2008-10-16 | Bayer Bioscience N.V. | Insect resistant cotton plants and methods for identifying same |
| WO2008151780A1 (en) | 2007-06-11 | 2008-12-18 | Bayer Bioscience N.V. | Insect resistant cotton plants comprising elite event ee-gh6 and methods for identifying same |
| WO2009064652A1 (en) | 2007-11-15 | 2009-05-22 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87701 and methods for detection thereof |
| WO2009090181A2 (en) | 2008-01-15 | 2009-07-23 | Bayer Cropscience Sa | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| WO2009094442A2 (en) | 2008-01-22 | 2009-07-30 | Dow Agrosciences Llc | 5-fluoro pyrimidine derivatives |
| WO2009103049A2 (en) | 2008-02-14 | 2009-08-20 | Pioneer Hi-Bred International, Inc. | Plant genomic dna flanking spt event and methods for identifying spt event |
| WO2009102873A1 (en) | 2008-02-15 | 2009-08-20 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87769 and methods for detection thereof |
| WO2009111263A1 (en) | 2008-02-29 | 2009-09-11 | Monsanto Technology Llc | Corn plant event mon87460 and compositions and methods for detection thereof |
| WO2010037016A1 (en) | 2008-09-29 | 2010-04-01 | Monsanto Technology Llc | Soybean transgenic event mon87705 and methods for detection thereof |
| WO2010077816A1 (en) | 2008-12-16 | 2010-07-08 | Syngenta Participations Ag | Corn event 5307 |
| WO2010069882A1 (en) | 2008-12-17 | 2010-06-24 | Syngenta Participations Ag | Isoxazole derivatives for use as fungicides |
| WO2010080829A1 (en) | 2009-01-07 | 2010-07-15 | Basf Agrochemical Products B.V. | Soybean event 127 and methods related thereto |
| WO2010139271A1 (en) | 2009-06-05 | 2010-12-09 | 中国中化股份有限公司 | E-type phenyl acrylic ester compounds containing substituted anilino pyrimidine group and uses thereof |
| WO2011022469A2 (en) | 2009-08-19 | 2011-02-24 | Dow Agrosciences Llc | Aad-1 event das-40278-9, related transgenic corn lines, and event-specific identification thereof |
| WO2011028657A1 (en) | 2009-09-01 | 2011-03-10 | Dow Agrosciences Llc | Synergistic fungicidal compositions containing a 5-fluoropyrimidine derivative for fungal control in cereals |
| WO2011034704A1 (en) | 2009-09-17 | 2011-03-24 | Monsanto Technology Llc | Soybean transgenic event mon 87708 and methods of use thereof |
| WO2011062904A1 (en) | 2009-11-23 | 2011-05-26 | Monsanto Technology Llc | Transgenic maize event mon 87427 and the relative development scale |
| WO2011066384A1 (en) | 2009-11-24 | 2011-06-03 | Dow Agrosciences Llc | Aad-12 event 416, related transgenic soybean lines, and event-specific identification thereof |
| WO2011084621A1 (en) | 2009-12-17 | 2011-07-14 | Pioneer Hi-Bred International, Inc. | Maize event dp-004114-3 and methods for detection thereof |
| WO2011077514A1 (en) | 2009-12-22 | 2011-06-30 | 三井化学アグロ株式会社 | Plant disease control composition and method for controlling plant diseases by applying the composition |
| WO2011081174A1 (en) | 2010-01-04 | 2011-07-07 | 日本曹達株式会社 | Nitrogen-containing heterocyclic compound and agricultural/horticultural germicide |
| WO2011135827A1 (en) | 2010-04-27 | 2011-11-03 | Sumitomo Chemical Company, Limited | Pesticidal composition and its use |
| WO2011135833A1 (en) | 2010-04-28 | 2011-11-03 | Sumitomo Chemical Company, Limited | Plant disease control composition and its use |
| WO2011153186A1 (en) | 2010-06-04 | 2011-12-08 | Monsanto Technology Llc | Transgenic brassica event mon 88302 and methods of use thereof |
| WO2012051199A2 (en) | 2010-10-12 | 2012-04-19 | Monsanto Technology Llc | Soybean plant and seed corresponding to transgenic event mon87712 and methods for detection thereof |
| WO2012082548A2 (en) | 2010-12-15 | 2012-06-21 | Syngenta Participations Ag | Soybean event syht0h2 and compositions and methods for detection thereof |
| WO2012084812A1 (en) | 2010-12-20 | 2012-06-28 | Isagro Ricerca S.R.L. | Aminoindanes amides having a high fungicidal activity and their phytosanitary compositions |
| WO2012134808A1 (en) | 2011-03-30 | 2012-10-04 | Monsanto Technology Llc | Cotton transgenic event mon 88701 and methods of use thereof |
| WO2012165511A1 (en) | 2011-05-31 | 2012-12-06 | クミアイ化学工業株式会社 | Method for controlling diseases in rice plant |
| WO2012168188A1 (en) | 2011-06-07 | 2012-12-13 | Bayer Intellectual Property Gmbh | Active compound combinations |
| WO2013003558A1 (en) | 2011-06-30 | 2013-01-03 | Monsanto Technology Llc | Alfalfa plant and seed corresponding to transgenic event kk 179-2 and methods for detection thereof |
| WO2013007767A1 (en) | 2011-07-13 | 2013-01-17 | Basf Se | Fungicidal substituted 2-[2-halogenalkyl-4-(phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013010862A1 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Fungicidal alkyl-substituted 2-[2-chloro-4-(4-chloro-phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013016516A1 (en) | 2011-07-26 | 2013-01-31 | Dow Agrosciences Llc | Insect resistant and herbicide tolerant breeding stack of soybean event pdab9582.814.19.1 and pdab4468.04.16.1 |
| WO2013016527A1 (en) | 2011-07-26 | 2013-01-31 | Dow Agrosciences Llc | Insect resistant and herbicide tolerant soybean event 9582.814.19.1 |
| WO2013024010A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013024009A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013047441A1 (en) | 2011-09-26 | 2013-04-04 | 日本曹達株式会社 | Agricultural and horticultural bactericide composition |
| WO2013047749A1 (en) | 2011-09-29 | 2013-04-04 | 三井化学アグロ株式会社 | Production method for 4, 4-difluoro-3,4-dihydroisoquinoline derivative |
| WO2013092224A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | Use of strobilurin type compounds for combating phytopathogenic fungi resistant to qo inhibitors |
| WO2013112527A1 (en) | 2012-01-23 | 2013-08-01 | Dow Agrosciences Llc | Herbicide tolerant cotton event pdab4468.19.10.3 |
| WO2013116251A2 (en) | 2012-02-01 | 2013-08-08 | E. I. Du Pont De Nemours And Company | Fungicidal pyrazole mixtures |
| WO2013127704A1 (en) | 2012-02-27 | 2013-09-06 | Bayer Intellectual Property Gmbh | Active compound combinations containing a thiazoylisoxazoline and a fungicide |
| WO2013162072A1 (en) | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
| WO2013169923A2 (en) | 2012-05-08 | 2013-11-14 | Monsanto Technology Llc | Corn event mon 87411 |
| CN103387541A (en) | 2012-05-10 | 2013-11-13 | 中国中化股份有限公司 | Preparation method of substituted pyrazolylether compound |
| WO2014060177A1 (en) | 2012-10-16 | 2014-04-24 | Syngenta Participations Ag | Fungicidal compositions |
| WO2014116854A1 (en) | 2013-01-25 | 2014-07-31 | Pioneer Hi-Bred International, Inc. | Maize event dp-033121-3 and methods for detection thereof |
| WO2014178941A1 (en) | 2013-05-02 | 2014-11-06 | J.R. Simplot Company | Potato cultivar j3 |
| WO2014179276A1 (en) | 2013-05-02 | 2014-11-06 | J.R. Simplot Company | Potato cultivar j55 |
| WO2014178910A1 (en) | 2013-05-02 | 2014-11-06 | J.R. Simplot Company | Potato cultivar e12 |
| WO2014178913A1 (en) | 2013-05-02 | 2014-11-06 | J.R. Simplot Company | Potato cultivar f10 |
| WO2014201235A2 (en) | 2013-06-14 | 2014-12-18 | Monsanto Technology Llc | Soybean transgenic event mon87751 and methods for detection and use thereof |
| WO2015053998A1 (en) | 2013-10-09 | 2015-04-16 | Monsanto Technology Llc | Transgenic corn event mon87403 and methods for detection thereof |
| WO2015065922A1 (en) | 2013-10-28 | 2015-05-07 | Dexcom, Inc. | Devices used in connection with continuous analyte monitoring that provide the user with one or more notifications, and related methods |
| JP2015089883A (en) | 2013-11-06 | 2015-05-11 | アグロカネショウ株式会社 | 2-amino nicotinic acid benzyl ester derivative and antimicrobial agent comprising the same as active ingredient |
| JP2015120675A (en) | 2013-12-25 | 2015-07-02 | アグロカネショウ株式会社 | 6-substituted nicotinic acid ester derivative and antimicrobial agent containing the same as essential component |
| WO2015119246A1 (en) | 2014-02-07 | 2015-08-13 | 日産化学工業株式会社 | Sterilising or bactericidal composition and disease control method |
| EP2865265A1 (en) | 2014-02-13 | 2015-04-29 | Bayer CropScience AG | Active compound combinations comprising phenylamidine compounds and biological control agents |
| WO2015142571A1 (en) | 2014-03-20 | 2015-09-24 | Monsanto Technology Llc | Transgenic maize event mon 87419 and methods of use thereof |
| WO2016183445A1 (en) | 2015-05-14 | 2016-11-17 | J.R. Simplot Company | Potato cultivar v11 |
| WO2017062831A1 (en) | 2015-10-08 | 2017-04-13 | J.R. Simplot Company | Potato cultivar x17 |
| WO2017062825A1 (en) | 2015-10-08 | 2017-04-13 | J.R. Simplot Company | Potato cultivar y9 |
| WO2018055135A1 (en) * | 2016-09-23 | 2018-03-29 | Syngenta Participations Ag | Microbiocidal oxadiazole derivatives |
| WO2019053027A1 (en) * | 2017-09-13 | 2019-03-21 | Syngenta Participations Ag | Microbiocidal quinoline (thio)carboxamide derivatives |
| WO2019053010A1 (en) * | 2017-09-13 | 2019-03-21 | Syngenta Participations Ag | Microbiocidal quinoline (thio)carboxamide derivatives |
Non-Patent Citations (23)
| Title |
|---|
| "McCutcheon's, Vol.1: Emulsifiers & Detergents", vol. 1, 2008, MCCUTCHEON'S DIRECTORIES |
| "Technical Monograph", May 2008, CROPLIFE INTERNATIONAL, article "Catalogue of pesticide formulation types and international coding system" |
| CAN. J. PLANT SCI., vol. 48, no. 6, 1968, pages 587 - 94 |
| CHEM. COMMUN., 2014, pages 13052 - 13055 |
| CHEM. SOC. REV., 2009, pages 606 - 631 |
| ENVIRON MICROBIOL, vol. 16, 2014, pages 2253 - 66 |
| J. AM. CHEM. SOC., vol. 123, no. 25, 2001, pages 5962 - 5973 |
| J. HETEROCYC. CHEM., vol. 18, no. 7, 1981, pages 1305 - 8 |
| J. HETEROCYCL. CHEM., 1981, pages 1305 - 8 |
| J. MED. CHEM., vol. 38, no. 11, 1995, pages 1892 - 903 |
| J. ORG. CHEM., vol. 47, 1982, pages 1837 - 1845 |
| JOURNAL OF PLANT DISEASES AND PROTECTION, vol. 125, pages 21 - 26 |
| KNOWLES: "Agrow Reports DS243", 2005, T&F INFORMA, article "New developments in crop protection product formulation" |
| MOLLETGRUBEMANN: "Formulation technology", 2001, WILEY VCH |
| ORG. LETT., 2013, pages 1472 - 1475 |
| PEST MANAG SCI, vol. 70, 2014, pages 378 - 388 |
| PEST MANAG SCI, vol. 72, no. 121, 2016, pages 1 - 1215 |
| PEST MANAG SCI, vol. 74, 2018, pages 672 - 681, Retrieved from the Internet <URL:https://www.frac.info/working-group/sdhi-fungicides> |
| PHYTOPATHOL, vol. 106, 2016, pages 1278 - 1284 |
| PHYTOPATHOL, vol. 93, 2003, pages 891 - 900 |
| PHYTOPATHOL, vol. 98, 2008, pages 397 - 404 |
| SMITHMARCH: "March's Advanced Organic Chemistry", 2007, WILEY |
| TETRAHEDRON, 2005, pages 10827 - 10852 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7160487B2 (en) | Substituted 5-(haloalkyl)-5-hydroxy-isoxazoles for combating plant pathogens | |
| EP3585773B1 (en) | Substituted oxadiazoles for combating phytopathogenic fungi | |
| JP2019502661A (en) | Substituted oxadiazoles for controlling plant pathogens | |
| WO2018202491A1 (en) | Substituted trifluoromethyloxadiazoles for combating phytopathogenic fungi | |
| EP3555056A1 (en) | Substituted oxadiazoles for combating phytopathogenic fungi | |
| EP3606912A1 (en) | Substituted oxadiazoles for combating phytopathogenic fungi | |
| WO2018073110A1 (en) | Quinoline compounds as fungicides | |
| US20200190043A1 (en) | 2-[[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]aryloxy](thio)acetamides for combating phytopathogenic fungi | |
| WO2018054711A1 (en) | Pyridine compounds for controlling phytopathogenic harmful fungi | |
| WO2018188962A1 (en) | Substituted oxadiazoles for combating phytopathogenic fungi | |
| WO2019154665A1 (en) | New pyridine carboxamides | |
| AU2020286573A1 (en) | Fungicidal n-(pyrid-3-yl)carboxamides | |
| WO2019038042A1 (en) | Substituted trifluoromethyloxadiazoles for combating phytopathogenic fungi | |
| EP3749660A1 (en) | New pyridine carboxamides | |
| WO2018054721A1 (en) | Pyridine compounds for controlling phytopathogenic harmful fungi | |
| WO2018065182A1 (en) | Reduced quinoline compounds as antifuni agents | |
| WO2018149754A1 (en) | Pyridine compounds | |
| WO2021063736A1 (en) | Bicyclic pyridine derivatives | |
| WO2020244970A1 (en) | New carbocyclic pyridine carboxamides | |
| US11147275B2 (en) | Substituted trifluoromethyloxadiazoles for combating phytopathogenic fungi | |
| AU2018278714B2 (en) | Pyridine and pyrazine compounds | |
| WO2019052932A1 (en) | Substituted trifluoromethyloxadiazoles for combating phytopathogenic fungi | |
| WO2019025250A1 (en) | Substituted trifluoromethyloxadiazoles for combating phytopathogenic fungi | |
| WO2020244969A1 (en) | Pyridine derivatives and their use as fungicides | |
| WO2021063735A1 (en) | New bicyclic pyridine derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20726875 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 20726875 Country of ref document: EP Kind code of ref document: A1 |