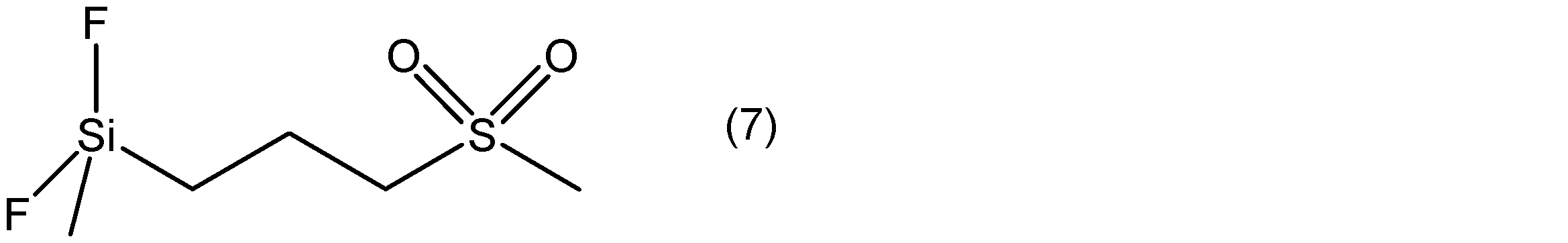WO2020116583A1 - Electrolyte and electrochemical device - Google Patents
Electrolyte and electrochemical device Download PDFInfo
- Publication number
- WO2020116583A1 WO2020116583A1 PCT/JP2019/047696 JP2019047696W WO2020116583A1 WO 2020116583 A1 WO2020116583 A1 WO 2020116583A1 JP 2019047696 W JP2019047696 W JP 2019047696W WO 2020116583 A1 WO2020116583 A1 WO 2020116583A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrolytic solution
- formula
- mass
- compound
- group
- Prior art date
Links
- 239000003792 electrolyte Substances 0.000 title claims abstract description 9
- -1 nitrile compound Chemical class 0.000 claims abstract description 57
- 150000001875 compounds Chemical class 0.000 claims abstract description 37
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 25
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical group [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims abstract description 21
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 19
- 229910052731 fluorine Inorganic materials 0.000 claims abstract description 14
- 125000000962 organic group Chemical group 0.000 claims abstract description 11
- 125000001153 fluoro group Chemical group F* 0.000 claims abstract description 10
- 229910052717 sulfur Inorganic materials 0.000 claims abstract description 10
- 125000004434 sulfur atom Chemical group 0.000 claims abstract description 10
- 229910052757 nitrogen Inorganic materials 0.000 claims abstract description 9
- 125000004433 nitrogen atom Chemical group N* 0.000 claims abstract description 9
- 125000002947 alkylene group Chemical group 0.000 claims abstract description 6
- 239000008151 electrolyte solution Substances 0.000 claims description 64
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 22
- 239000011255 nonaqueous electrolyte Substances 0.000 claims description 16
- IAHFWCOBPZCAEA-UHFFFAOYSA-N succinonitrile Chemical compound N#CCCC#N IAHFWCOBPZCAEA-UHFFFAOYSA-N 0.000 claims description 16
- 239000003575 carbonaceous material Substances 0.000 claims description 15
- 239000000463 material Substances 0.000 claims description 13
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 12
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 12
- 239000010703 silicon Substances 0.000 claims description 12
- 239000010439 graphite Substances 0.000 claims description 10
- 229910002804 graphite Inorganic materials 0.000 claims description 10
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 6
- 239000003990 capacitor Substances 0.000 claims description 6
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 3
- 239000000203 mixture Substances 0.000 description 41
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 31
- 229910001416 lithium ion Inorganic materials 0.000 description 31
- 230000000052 comparative effect Effects 0.000 description 25
- 229940126062 Compound A Drugs 0.000 description 20
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 20
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 16
- 238000012360 testing method Methods 0.000 description 13
- 230000014759 maintenance of location Effects 0.000 description 12
- 239000007773 negative electrode material Substances 0.000 description 12
- 229910052718 tin Inorganic materials 0.000 description 12
- 239000011135 tin Substances 0.000 description 12
- 229910052782 aluminium Inorganic materials 0.000 description 11
- 239000010408 film Substances 0.000 description 11
- 238000003860 storage Methods 0.000 description 11
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 10
- 238000000034 method Methods 0.000 description 10
- 239000011230 binding agent Substances 0.000 description 9
- 239000002612 dispersion medium Substances 0.000 description 9
- 239000011347 resin Substances 0.000 description 8
- 229920005989 resin Polymers 0.000 description 8
- 239000002562 thickening agent Substances 0.000 description 8
- 229910052799 carbon Inorganic materials 0.000 description 7
- SBLRHMKNNHXPHG-UHFFFAOYSA-N 4-fluoro-1,3-dioxolan-2-one Chemical compound FC1COC(=O)O1 SBLRHMKNNHXPHG-UHFFFAOYSA-N 0.000 description 6
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 6
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 6
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 6
- 125000004432 carbon atom Chemical group C* 0.000 description 6
- 229910052802 copper Inorganic materials 0.000 description 6
- 239000010949 copper Substances 0.000 description 6
- 229910052744 lithium Inorganic materials 0.000 description 6
- 239000007774 positive electrode material Substances 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- 229910052719 titanium Inorganic materials 0.000 description 6
- 239000010936 titanium Substances 0.000 description 6
- VAYTZRYEBVHVLE-UHFFFAOYSA-N 1,3-dioxol-2-one Chemical compound O=C1OC=CO1 VAYTZRYEBVHVLE-UHFFFAOYSA-N 0.000 description 5
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 238000007600 charging Methods 0.000 description 5
- 238000010277 constant-current charging Methods 0.000 description 5
- 150000005676 cyclic carbonates Chemical class 0.000 description 5
- 229910052759 nickel Inorganic materials 0.000 description 5
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 5
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 4
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 4
- 229910013870 LiPF 6 Inorganic materials 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- BTGRAWJCKBQKAO-UHFFFAOYSA-N adiponitrile Chemical compound N#CCCCCC#N BTGRAWJCKBQKAO-UHFFFAOYSA-N 0.000 description 4
- 239000003125 aqueous solvent Substances 0.000 description 4
- 239000006258 conductive agent Substances 0.000 description 4
- 238000010280 constant potential charging Methods 0.000 description 4
- 238000007599 discharging Methods 0.000 description 4
- 239000011737 fluorine Substances 0.000 description 4
- 239000005001 laminate film Substances 0.000 description 4
- FUJCRWPEOMXPAD-UHFFFAOYSA-N lithium oxide Chemical compound [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 4
- 229910001947 lithium oxide Inorganic materials 0.000 description 4
- 239000011572 manganese Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 239000011259 mixed solution Substances 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 239000002033 PVDF binder Substances 0.000 description 3
- 239000004743 Polypropylene Substances 0.000 description 3
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 3
- 239000006230 acetylene black Substances 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 229910003002 lithium salt Inorganic materials 0.000 description 3
- 159000000002 lithium salts Chemical class 0.000 description 3
- 239000002905 metal composite material Substances 0.000 description 3
- 150000004767 nitrides Chemical class 0.000 description 3
- 239000005020 polyethylene terephthalate Substances 0.000 description 3
- 229920000139 polyethylene terephthalate Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 229920001155 polypropylene Polymers 0.000 description 3
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 3
- 239000004810 polytetrafluoroethylene Substances 0.000 description 3
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 229910052814 silicon oxide Inorganic materials 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- OIFBSDVPJOWBCH-UHFFFAOYSA-N Diethyl carbonate Chemical compound CCOC(=O)OCC OIFBSDVPJOWBCH-UHFFFAOYSA-N 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- 229920000459 Nitrile rubber Polymers 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- 229910052581 Si3N4 Inorganic materials 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 239000002194 amorphous carbon material Substances 0.000 description 2
- 229910052787 antimony Inorganic materials 0.000 description 2
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011651 chromium Substances 0.000 description 2
- 229920001940 conductive polymer Polymers 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- JBTWLSYIZRCDFO-UHFFFAOYSA-N ethyl methyl carbonate Chemical compound CCOC(=O)OC JBTWLSYIZRCDFO-UHFFFAOYSA-N 0.000 description 2
- 229920001038 ethylene copolymer Polymers 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 229910052732 germanium Inorganic materials 0.000 description 2
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- LLEVMYXEJUDBTA-UHFFFAOYSA-N heptanedinitrile Chemical compound N#CCCCCCC#N LLEVMYXEJUDBTA-UHFFFAOYSA-N 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 229910001386 lithium phosphate Inorganic materials 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 229910021382 natural graphite Inorganic materials 0.000 description 2
- 125000002560 nitrile group Chemical group 0.000 description 2
- 239000004745 nonwoven fabric Substances 0.000 description 2
- BTNXBLUGMAMSSH-UHFFFAOYSA-N octanedinitrile Chemical compound N#CCCCCCCC#N BTNXBLUGMAMSSH-UHFFFAOYSA-N 0.000 description 2
- RXIMZKYZCDNHPG-UHFFFAOYSA-N pentane-1,3,5-tricarbonitrile Chemical compound N#CCCC(C#N)CCC#N RXIMZKYZCDNHPG-UHFFFAOYSA-N 0.000 description 2
- 229920002857 polybutadiene Polymers 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- MNAMONWYCZEPTE-UHFFFAOYSA-N propane-1,2,3-tricarbonitrile Chemical compound N#CCC(C#N)CC#N MNAMONWYCZEPTE-UHFFFAOYSA-N 0.000 description 2
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 2
- 229910010271 silicon carbide Inorganic materials 0.000 description 2
- LIVNPJMFVYWSIS-UHFFFAOYSA-N silicon monoxide Chemical compound [Si-]#[O+] LIVNPJMFVYWSIS-UHFFFAOYSA-N 0.000 description 2
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 229920006132 styrene block copolymer Polymers 0.000 description 2
- 229920003048 styrene butadiene rubber Polymers 0.000 description 2
- TWQULNDIKKJZPH-UHFFFAOYSA-K trilithium;phosphate Chemical compound [Li+].[Li+].[Li+].[O-]P([O-])([O-])=O TWQULNDIKKJZPH-UHFFFAOYSA-K 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- FSSPGSAQUIYDCN-UHFFFAOYSA-N 1,3-Propane sultone Chemical compound O=S1(=O)CCCO1 FSSPGSAQUIYDCN-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- VSKJLJHPAFKHBX-UHFFFAOYSA-N 2-methylbuta-1,3-diene;styrene Chemical compound CC(=C)C=C.C=CC1=CC=CC=C1.C=CC1=CC=CC=C1 VSKJLJHPAFKHBX-UHFFFAOYSA-N 0.000 description 1
- VUZHZBFVQSUQDP-UHFFFAOYSA-N 4,4,5,5-tetrafluoro-1,3-dioxolan-2-one Chemical compound FC1(F)OC(=O)OC1(F)F VUZHZBFVQSUQDP-UHFFFAOYSA-N 0.000 description 1
- CRJXZTRTJWAKMU-UHFFFAOYSA-N 4,4,5-trifluoro-1,3-dioxolan-2-one Chemical compound FC1OC(=O)OC1(F)F CRJXZTRTJWAKMU-UHFFFAOYSA-N 0.000 description 1
- ZTTYKFSKZIRTDP-UHFFFAOYSA-N 4,4-difluoro-1,3-dioxolan-2-one Chemical compound FC1(F)COC(=O)O1 ZTTYKFSKZIRTDP-UHFFFAOYSA-N 0.000 description 1
- DSMUTQTWFHVVGQ-UHFFFAOYSA-N 4,5-difluoro-1,3-dioxolan-2-one Chemical compound FC1OC(=O)OC1F DSMUTQTWFHVVGQ-UHFFFAOYSA-N 0.000 description 1
- KLLQVNFCMHPYGL-UHFFFAOYSA-N 5h-oxathiole 2,2-dioxide Chemical compound O=S1(=O)OCC=C1 KLLQVNFCMHPYGL-UHFFFAOYSA-N 0.000 description 1
- 229910000838 Al alloy Inorganic materials 0.000 description 1
- 238000007088 Archimedes method Methods 0.000 description 1
- 229910000925 Cd alloy Inorganic materials 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229910018871 CoO 2 Inorganic materials 0.000 description 1
- 229920000089 Cyclic olefin copolymer Polymers 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 229920000181 Ethylene propylene rubber Polymers 0.000 description 1
- 229910000733 Li alloy Inorganic materials 0.000 description 1
- 229910015044 LiB Inorganic materials 0.000 description 1
- 229910013063 LiBF 4 Inorganic materials 0.000 description 1
- 229910013684 LiClO 4 Inorganic materials 0.000 description 1
- 229910011281 LiCoPO 4 Inorganic materials 0.000 description 1
- 229910010707 LiFePO 4 Inorganic materials 0.000 description 1
- 229910013528 LiN(SO2 CF3)2 Inorganic materials 0.000 description 1
- 229910013398 LiN(SO2CF2CF3)2 Inorganic materials 0.000 description 1
- 229910002991 LiNi0.5Co0.2Mn0.3O2 Inorganic materials 0.000 description 1
- 229910011328 LiNi0.6Co0.2Mn0.2O2 Inorganic materials 0.000 description 1
- 229910002995 LiNi0.8Co0.15Al0.05O2 Inorganic materials 0.000 description 1
- 229910015872 LiNi0.8Co0.1Mn0.1O2 Inorganic materials 0.000 description 1
- 229910012573 LiSiO Inorganic materials 0.000 description 1
- 229910012404 LiSnO Inorganic materials 0.000 description 1
- 229910001228 Li[Ni1/3Co1/3Mn1/3]O2 (NCM 111) Inorganic materials 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- RFFFKMOABOFIDF-UHFFFAOYSA-N Pentanenitrile Chemical compound CCCCC#N RFFFKMOABOFIDF-UHFFFAOYSA-N 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 229910006404 SnO 2 Inorganic materials 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 239000002174 Styrene-butadiene Substances 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- YWJVFBOUPMWANA-UHFFFAOYSA-H [Li+].[V+5].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O Chemical compound [Li+].[V+5].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O YWJVFBOUPMWANA-UHFFFAOYSA-H 0.000 description 1
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 239000004760 aramid Substances 0.000 description 1
- 229920003235 aromatic polyamide Polymers 0.000 description 1
- 229910021383 artificial graphite Inorganic materials 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 1
- KVNRLNFWIYMESJ-UHFFFAOYSA-N butyronitrile Chemical compound CCCC#N KVNRLNFWIYMESJ-UHFFFAOYSA-N 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 229910021393 carbon nanotube Inorganic materials 0.000 description 1
- 239000002041 carbon nanotube Substances 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000003245 coal Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000000748 compression moulding Methods 0.000 description 1
- 239000011889 copper foil Substances 0.000 description 1
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 150000001993 dienes Chemical class 0.000 description 1
- 238000002050 diffraction method Methods 0.000 description 1
- NKDDWNXOKDWJAK-UHFFFAOYSA-N dimethoxymethane Chemical compound COCOC NKDDWNXOKDWJAK-UHFFFAOYSA-N 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000007606 doctor blade method Methods 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- HQQADJVZYDDRJT-UHFFFAOYSA-N ethene;prop-1-ene Chemical group C=C.CC=C HQQADJVZYDDRJT-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000010304 firing Methods 0.000 description 1
- 229920001973 fluoroelastomer Polymers 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 229910021389 graphene Inorganic materials 0.000 description 1
- 229910021480 group 4 element Inorganic materials 0.000 description 1
- SDAXRHHPNYTELL-UHFFFAOYSA-N heptanenitrile Chemical compound CCCCCCC#N SDAXRHHPNYTELL-UHFFFAOYSA-N 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 229920003049 isoprene rubber Polymers 0.000 description 1
- 239000003273 ketjen black Substances 0.000 description 1
- 229910052745 lead Inorganic materials 0.000 description 1
- 239000001989 lithium alloy Substances 0.000 description 1
- 229910000625 lithium cobalt oxide Inorganic materials 0.000 description 1
- GELKBWJHTRAYNV-UHFFFAOYSA-K lithium iron phosphate Chemical compound [Li+].[Fe+2].[O-]P([O-])([O-])=O GELKBWJHTRAYNV-UHFFFAOYSA-K 0.000 description 1
- SBWRUMICILYTAT-UHFFFAOYSA-K lithium;cobalt(2+);phosphate Chemical compound [Li+].[Co+2].[O-]P([O-])([O-])=O SBWRUMICILYTAT-UHFFFAOYSA-K 0.000 description 1
- ILXAVRFGLBYNEJ-UHFFFAOYSA-K lithium;manganese(2+);phosphate Chemical compound [Li+].[Mn+2].[O-]P([O-])([O-])=O ILXAVRFGLBYNEJ-UHFFFAOYSA-K 0.000 description 1
- BFZPBUKRYWOWDV-UHFFFAOYSA-N lithium;oxido(oxo)cobalt Chemical compound [Li+].[O-][Co]=O BFZPBUKRYWOWDV-UHFFFAOYSA-N 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000001254 oxidized starch Substances 0.000 description 1
- 235000013808 oxidized starch Nutrition 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 125000004817 pentamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000005011 phenolic resin Substances 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920002589 poly(vinylethylene) polymer Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 1
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 1
- 230000001603 reducing effect Effects 0.000 description 1
- 229910052706 scandium Inorganic materials 0.000 description 1
- 239000000565 sealant Substances 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000002639 sodium chloride Nutrition 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 229920002725 thermoplastic elastomer Polymers 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- QHGNHLZPVBIIPX-UHFFFAOYSA-N tin(II) oxide Inorganic materials [Sn]=O QHGNHLZPVBIIPX-UHFFFAOYSA-N 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/32—Carbon-based
- H01G11/42—Powders or particles, e.g. composition thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/54—Electrolytes
- H01G11/58—Liquid electrolytes
- H01G11/64—Liquid electrolytes characterised by additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H01M4/587—Carbonaceous material, e.g. graphite-intercalation compounds or CFx for inserting or intercalating light metals
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to an electrolytic solution and an electrochemical device.
- Patent Document 1 discloses an electrolyte solution for a non-aqueous electrolyte battery containing a specific siloxane compound in order to improve cycle characteristics and internal resistance characteristics.
- the present invention aims to provide an electrolytic solution capable of improving the performance of an electrochemical device.
- One aspect of the present invention is an electrolytic solution containing a compound represented by the following formula (1) and a nitrile compound having no silicon atom.
- R 1 to R 3 each independently represent an alkyl group or a fluorine atom
- R 4 represents an alkylene group
- R 5 represents an organic group containing a nitrogen atom or a sulfur atom.
- the capacity retention rate after the electrochemical device is stored at high temperature can be improved.
- the cycle characteristics of the electrochemical device can be improved.
- At least one of R 1 to R 3 may be a fluorine atom.
- the number of silicon atoms in one molecule of the compound represented by formula (1) may be one.
- R 5 may be an organic group containing a nitrogen atom.
- R 5 may be a group represented by the following formula (2). [In the formula (2), R 6 and R 7 each independently represent a hydrogen atom or an alkyl group, and * represents a bond. ]
- R 5 may be an organic group containing a sulfur atom.
- R 5 may be a group represented by the following formula (3), formula (4) or formula (5).
- R 8 represents an alkyl group, and * represents a bond.
- R 9 represents an alkyl group, and * represents a bond.
- R 10 represents an alkyl group, and * represents a bond.
- the nitrile compound may have two cyano groups.
- the nitrile compound may be succinonitrile.
- the total content of the compound represented by the formula (1) and the content of the nitrile compound may be 10% by mass or less based on the total amount of the electrolytic solution.
- Another aspect of the present invention is an electrochemical device including a positive electrode, a negative electrode, and the electrolytic solution.
- the negative electrode may contain a carbon material.
- the carbon material may contain graphite.
- the negative electrode may further contain a material containing at least one element selected from the group consisting of silicon and tin.
- the electrochemical device may be a non-aqueous electrolyte secondary battery or a capacitor.
- an electrolytic solution capable of improving the performance of an electrochemical device.
- FIG. 1 is a perspective view showing a non-aqueous electrolyte secondary battery as an electrochemical device according to an embodiment.
- FIG. 2 is an exploded perspective view showing an electrode group of the secondary battery shown in FIG. 1.
- FIG. 1 is a perspective view showing an electrochemical device according to one embodiment.
- the electrochemical device is a non-aqueous electrolyte secondary battery.
- the non-aqueous electrolyte secondary battery 1 includes an electrode group 2 including a positive electrode, a negative electrode, and a separator, and a bag-shaped battery exterior body 3 that houses the electrode group 2.
- a positive electrode current collecting tab 4 and a negative electrode current collecting tab 5 are provided on the positive electrode and the negative electrode, respectively.
- the positive electrode current collecting tab 4 and the negative electrode current collecting tab 5 project from the inside of the battery case 3 to the outside so that the positive electrode and the negative electrode can be electrically connected to the outside of the non-aqueous electrolyte secondary battery 1, respectively. ..
- the non-aqueous electrolyte secondary battery 1 may be a battery (coin type, cylindrical type, laminated type, etc.) having a shape other than the so-called “laminate type” as described above.
- the battery outer casing 3 may be a container formed of, for example, a laminated film.
- the laminated film may be, for example, a laminated film in which a resin film such as a polyethylene terephthalate (PET) film, a metal foil such as aluminum, copper and stainless steel, and a sealant layer such as polypropylene are laminated in this order.
- PET polyethylene terephthalate
- metal foil such as aluminum, copper and stainless steel
- a sealant layer such as polypropylene
- FIG. 2 is an exploded perspective view showing an embodiment of the electrode group 2 in the non-aqueous electrolyte secondary battery 1 shown in FIG.
- the electrode group 2 includes a positive electrode 6, a separator 7, and a negative electrode 8 in this order.
- the positive electrode 6 and the negative electrode 8 are arranged such that the surfaces on the positive electrode mixture layer 10 side and the negative electrode mixture layer 12 side face the separator 7, respectively.
- the positive electrode 6 includes a positive electrode current collector 9 and a positive electrode mixture layer 10 provided on the positive electrode current collector 9.
- the positive electrode current collector 9 is provided with the positive electrode current collector tab 4.
- the positive electrode current collector 9 is made of, for example, aluminum, titanium, stainless steel, nickel, baked carbon, conductive polymer, conductive glass, or the like.
- the positive electrode current collector 9 may be one in which the surface of aluminum, copper, or the like is treated with carbon, nickel, titanium, silver, or the like for the purpose of improving adhesiveness, conductivity, and oxidation resistance.
- the thickness of the positive electrode current collector 9 is, for example, 1 to 50 ⁇ m in terms of electrode strength and energy density.
- the positive electrode material mixture layer 10 contains a positive electrode active material, a conductive agent, and a binder.
- the thickness of the positive electrode mixture layer 10 is, for example, 20 to 200 ⁇ m.
- the positive electrode active material may be, for example, lithium oxide.
- the positive electrode active material may be, for example, a lithium phosphate.
- the lithium phosphate include lithium manganese phosphate (LiMnPO 4 ), lithium iron phosphate (LiFePO 4 ), lithium cobalt phosphate (LiCoPO 4 ), and lithium vanadium phosphate (Li 3 V 2 (PO 4 ). 3 ).
- the content of the positive electrode active material may be 80% by mass or more, or 85% by mass or more, and 99% by mass or less, based on the total amount of the positive electrode mixture layer.
- the conductive agent may be carbon black such as acetylene black or Ketjen black, or carbon material such as graphite, graphene or carbon nanotube.
- the content of the conductive agent may be, for example, 0.01% by mass or more, 0.1% by mass or more, or 1% by mass or more, and 50% by mass or less, 30% by mass, based on the total amount of the positive electrode mixture layer. Or less, or 15% by mass or less.
- binder examples include resins such as polyethylene, polypropylene, polyethylene terephthalate, polymethylmethacrylate, polyimide, aromatic polyamide, cellulose and nitrocellulose; SBR (styrene-butadiene rubber), NBR (acrylonitrile-butadiene rubber), fluororubber.
- resins such as polyethylene, polypropylene, polyethylene terephthalate, polymethylmethacrylate, polyimide, aromatic polyamide, cellulose and nitrocellulose
- SBR styrene-butadiene rubber
- NBR acrylonitrile-butadiene rubber
- fluororubber examples include fluororubber.
- a fluorine-containing resin a resin having a nitrile group-containing monomer as a monomer unit, a polymer composition having an alkali metal ion (for example, lithium ion) ion conductivity, and the like.
- the content of the binder may be, for example, 0.1% by mass or more, 1% by mass or more, or 1.5% by mass or more, based on the total amount of the positive electrode mixture layer, 30% by mass or less, 20% by mass. % Or less, or 10% by mass or less.
- the separator 7 is not particularly limited as long as it electrically insulates between the positive electrode 6 and the negative electrode 8 while allowing ions to pass therethrough and has resistance to the oxidizing property on the positive electrode 6 side and the reducing property on the negative electrode 8 side. Not done.
- Examples of the material (material) of the separator 7 include resins and inorganic materials.
- the separator 7 is preferably a porous sheet or a non-woven fabric formed of polyolefin such as polyethylene or polypropylene from the viewpoint of being stable with respect to the electrolytic solution and excellent in liquid retaining property.
- the inorganic substance examples include oxides such as alumina and silicon dioxide, nitrides such as aluminum nitride and silicon nitride, and sulfates such as barium sulfate and calcium sulfate.
- the separator 7 may be, for example, a thin film substrate such as a nonwoven fabric, a woven fabric, or a microporous film to which a fibrous or particulate inorganic substance is attached.
- the negative electrode 8 includes a negative electrode current collector 11 and a negative electrode mixture layer 12 provided on the negative electrode current collector 11.
- the negative electrode current collector 11 is provided with a negative electrode current collector tab 5.
- the negative electrode current collector 11 is made of copper, stainless steel, nickel, aluminum, titanium, baked carbon, conductive polymer, conductive glass, aluminum-cadmium alloy, or the like.
- the negative electrode current collector 11 may be one in which the surface of copper, aluminum, or the like is treated with carbon, nickel, titanium, silver, or the like for the purpose of improving adhesion, conductivity, and reduction resistance.
- the thickness of the negative electrode current collector 11 is, for example, 1 to 50 ⁇ m from the viewpoint of electrode strength and energy density.
- the negative electrode mixture layer 12 contains, for example, a negative electrode active material and a binder.
- the negative electrode active material is not particularly limited as long as it can absorb and release lithium ions.
- the negative electrode active material include carbon materials, metal composite oxides, oxides or nitrides of Group IV elements such as tin, germanium, and silicon, simple substances of lithium, lithium alloys such as lithium aluminum alloys, Sn, Si, and the like. And a metal capable of forming an alloy with lithium.
- the negative electrode active material is preferably at least one selected from the group consisting of carbon materials and metal composite oxides.
- the negative electrode active material may be one type of these alone or a mixture of two or more types.
- the shape of the negative electrode active material may be, for example, a particle shape.
- the carbon material amorphous carbon material, natural graphite, composite carbon material obtained by forming a film of amorphous carbon material on natural graphite, artificial graphite (resin raw material such as epoxy resin, phenol resin, or petroleum, coal, etc. Obtained by firing a pitch-based raw material obtained from the above).
- the metal composite oxide preferably contains one or both of titanium and lithium, and more preferably contains lithium.
- the negative electrode active materials carbon materials have high conductivity and are particularly excellent in low temperature characteristics and cycle stability.
- graphite is preferable from the viewpoint of high capacity.
- the carbon network plane layer (d002) in the X-ray wide-angle diffraction method is preferably less than 0.34 nm, and more preferably 0.3354 nm or more and 0.337 nm or less.
- a carbon material that satisfies such conditions may be referred to as pseudo-anisotropic carbon.
- the negative electrode active material may further contain a material containing at least one element selected from the group consisting of silicon and tin.
- the material containing at least one element selected from the group consisting of silicon and tin may be a simple substance of silicon or tin, or a compound containing at least one element selected from the group consisting of silicon and tin.
- the compound may be an alloy containing at least one element selected from the group consisting of silicon and tin. For example, in addition to silicon and tin, nickel, copper, iron, cobalt, manganese, zinc, indium, silver. , An alloy containing at least one selected from the group consisting of titanium, germanium, bismuth, antimony and chromium.
- the compound containing at least one element selected from the group consisting of silicon and tin may be an oxide, a nitride, or a carbide, and specifically, for example, silicon oxide such as SiO, SiO 2 , and LiSiO.
- silicon oxide such as SiO, SiO 2 , and LiSiO.
- a silicon nitride such as Si 3 N 4 or Si 2 N 2 O, a silicon carbide such as SiC, a tin oxide such as SnO, SnO 2 or LiSnO.
- the negative electrode 8 preferably contains a carbon material, more preferably graphite, and further preferably a carbon material, silicon and It contains a mixture with a material containing at least one element selected from the group consisting of tin, and particularly preferably contains a mixture of graphite and silicon oxide.
- the content of the carbon material (graphite) with respect to the material (silicon oxide) containing at least one element selected from the group consisting of silicon and tin in the mixture is 1% by mass or more based on the total amount of the mixture, or 3 It may be not less than 30% by mass and not more than 30% by mass.
- the content of the negative electrode active material may be 80% by mass or more, or 85% by mass or more, and 99% by mass or less, based on the total amount of the negative electrode mixture layer.
- the binder and the content thereof may be the same as the binder and the content thereof in the positive electrode mixture layer described above.
- the negative electrode mixture layer 12 may contain a thickener to adjust the viscosity.
- the thickener is not particularly limited, but may be carboxymethyl cellulose, methyl cellulose, hydroxymethyl cellulose, ethyl cellulose, polyvinyl alcohol, oxidized starch, phosphorylated starch, casein, salts thereof, or the like.
- the thickener may be one of these alone or a mixture of two or more thereof.
- the content thereof is not particularly limited.
- the content of the thickener may be 0.1% by mass or more, and preferably 0.2% by mass or more, based on the total amount of the negative electrode mixture layer, from the viewpoint of coatability of the negative electrode mixture layer. , And more preferably 0.5% by mass or more.
- the content of the thickener may be 5% by mass or less, preferably 3% by mass, based on the total amount of the negative electrode mixture layer, from the viewpoint of suppressing a decrease in battery capacity or an increase in resistance between the negative electrode active materials. % Or less, and more preferably 2% by mass or less.
- the electrolytic solution contains a compound represented by the following formula (1), a nitrile compound having no silicon atom, an electrolyte salt, and a nonaqueous solvent.
- R 1 to R 3 each independently represent an alkyl group or a fluorine atom
- R 4 represents an alkylene group
- R 5 represents an organic group containing a nitrogen atom or a sulfur atom.
- the alkyl group represented by R 1 to R 3 may have 1 or more carbon atoms and 3 or less carbon atoms.
- R 1 to R 3 may be a methyl group, an ethyl group, or a propyl group, and may be linear or branched.
- At least one of R 1 to R 3 is preferably a fluorine atom. May be any one of fluorine atom of R 1 ⁇ R 3, it may be any two of a fluorine atom of R 1 ⁇ R 3, all of R 1 ⁇ R 3 may be a fluorine atom.
- the carbon number of the alkylene group represented by R 4 may be 1 or more or 2 or more, and 5 or less or 4 or less.
- the alkylene group represented by R 4 may be a methylene group, an ethylene group, a propylene group, a butylene group, or a pentylene group, and may be linear or branched.
- the number of silicon atoms in one molecule of the compound represented by formula (1) is one. That is, in one embodiment, the organic group represented by R 5 does not include a silicon atom.
- R 5 is an organic group containing a nitrogen atom, and is preferably a group represented by the following formula (2) from the viewpoint of further improving the performance of the electrochemical device.
- R 6 and R 7 each independently represent a hydrogen atom or an alkyl group.
- the alkyl group represented by R 6 or R 7 may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
- R 5 is an organic group containing a sulfur atom and is preferably a compound represented by the following formula (3), formula (4) or formula from the viewpoint that the performance of the electrochemical device can be further improved. It may be a group represented by any of (5).
- R 8 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
- R 9 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
- R 10 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
- the content of the compound represented by the formula (1) is preferably 0.001% by mass or more and 0.005% by mass, based on the total amount of the electrolytic solution, from the viewpoint that the performance of the electrochemical device can be further improved. % Or more, 0.01 mass% or more, 0.05 mass% or more, or 0.1 mass% or more, preferably 8 mass% or less, 5 mass% or less, 3 mass% or less, 2 mass% or less, Alternatively, it is 1% by mass or less.
- a nitrile compound is a compound having at least one cyano group (nitrile group).
- the nitrile compound is a compound other than the compound represented by the above formula (1). In other words, the nitrile compound is a compound having no silicon atom.
- the nitrile compound may have 1 or 2 or more cyano groups, and may have 2 or 3 cyano groups.
- the nitrile compound having one cyano group may be, for example, butyronitrile, valeronitrile, n-heptanenitrile or the like.
- the nitrile compound having two cyano groups may be, for example, succinonitrile, glutarnitrile, adiponitrile, pimelonitrile, suberonitrile and the like.
- the nitrile compound having three cyano groups may be, for example, 1,2,3-propanetricarbonitrile, 1,3,5-pentanetricarbonitrile and the like.
- the nitrile compound has two or more cyano groups and excludes carbon atoms in the cyano group from the viewpoint that a stable film is formed on the positive electrode or the negative electrode and the expansion of the battery due to the decomposition of the electrolytic solution can be suppressed.
- Compounds having 2 or more carbon atoms are preferred.
- the nitrile compound is more preferably a compound having 2 or 3 cyano groups, and the number of carbon atoms excluding carbon atoms in the cyano group is 2 or more.
- the nitrile compound is more preferably succinonitrile, Glutarnitrile, adiponitrile, pimelonitrile, suberonitrile, 1,2,3-propanetricarbonitrile, or 1,3,5-pentanetricarbonitrile.
- the content of the nitrile compound is preferably 0.001 mass% or more, 0.005 mass% or more, 0.01 mass, based on the total amount of the electrolytic solution, from the viewpoint of further improving the performance of the electrochemical device. % Or more, 0.05% by mass or more, or 0.1% by mass or more, preferably 5% by mass or less, 3% by mass or less, 2% by mass or less, or 1% by mass or less.
- the total content of the compound represented by the formula (1) and the content of the nitrile compound is preferably 0.001 based on the total amount of the electrolytic solution, from the viewpoint that the performance of the electrochemical device can be further improved.
- % By mass 0.005% by mass or more, 0.01% by mass or more, 0.1% by mass or more, or 0.5% by mass or more, preferably 10% by mass or less, 7% by mass or less, 5% by mass % Or less, 3% by mass or less, or 2% by mass or less.
- the mass ratio of the content of the compound represented by the formula (1) to the content of the nitrile compound (the content of the compound represented by the formula (1)/the content of the nitrile compound) further improves the performance of the electrochemical device. From the viewpoint of being able to improve, it is preferably 0.01 or more, 0.05 or more, 0.1 or more, 0.2 or more, or 0.25 or more, and preferably 500 or less, 100 or less, It is 50 or less, 20 or less, 10 or less, 5 or less, 3 or less, 2 or less, or 1 or less.
- the electrolyte salt may be, for example, a lithium salt.
- the lithium salt is, for example, LiPF 6 , LiBF 4 , LiClO 4 , LiB(C 6 H 5 ) 4 , LiCH 3 SO 3 , CF 3 SO 2 OLi, LiN(SO 2 F) 2 (Li[FSI], lithium bis. Fluorosulfonylimide), LiN(SO 2 CF 3 ) 2 (Li[TFSI], lithium bistrifluoromethanesulfonylimide), and at least one selected from the group consisting of LiN(SO 2 CF 2 CF 3 ) 2 Good.
- the lithium salt preferably contains LiPF 6 from the viewpoint of further excellent solubility in a solvent, charge/discharge characteristics of a secondary battery, output characteristics, cycle characteristics, and the like.
- the concentration of the electrolyte salt is preferably 0.5 mol/L or more, more preferably 0.7 mol/L or more, and further preferably 0.8 mol/L or more, based on the total amount of the non-aqueous solvent. Further, it is preferably 1.5 mol/L or less, more preferably 1.3 mol/L or less, still more preferably 1.2 mol/L or less.
- the non-aqueous solvent is, for example, ethylene carbonate, propylene carbonate, dimethyl carbonate, diethyl carbonate, methyl ethyl carbonate, ⁇ -butyl lactone, 1,2-dimethoxyethane, dimethoxymethane, tetrahydrofuran, dioxolane, methylene chloride, methyl acetate, etc. You may.
- the non-aqueous solvent may be one of these alone or a mixture of two or more thereof, and preferably a mixture of two or more thereof.
- the electrolytic solution may further contain other materials other than the compound represented by the formula (1), the nitrile compound, the electrolyte salt and the non-aqueous solvent.
- Other materials include, for example, a cyclic carbonate such as a fluorine-containing cyclic carbonate, a cyclic carbonate having a carbon-carbon double bond, a nitrogen atom other than the compound represented by the formula (1), a sulfur atom, or a nitrogen atom and a sulfur atom. It may be a compound containing, a cyclic carboxylic acid ester, or the like.
- fluorine-containing cyclic carbonate examples include 4-fluoro-1,3-dioxolan-2-one (fluoroethylene carbonate; FEC), 1,2-difluoroethylene carbonate, 1,1-difluoroethylene carbonate, 1,1,2. It may be trifluoroethylene carbonate, 1,1,2,2-tetrafluoroethylene carbonate or the like, preferably 4-fluoro-1,3-dioxolan-2-one (fluoroethylene carbonate; FEC).
- the cyclic carbonate having a carbon-carbon double bond may be vinylene carbonate, for example.
- the compound containing a sulfur atom other than the compound represented by the formula (1) may be, for example, a cyclic sulfonate compound such as 1,3-propane sultone and 1-propene-1,3-sultone.
- the present inventors have improved the performance of an electrochemical device by applying the compound represented by the above formula (1) and a nitrile compound to an electrolytic solution. Revealed that can be made.
- the present inventors presume the action and effect of using the compound represented by the formula (1) and the nitrile compound in the electrolytic solution as follows. That is, the compound represented by the formula (1) and the nitrile compound act on the place where the effect is most likely to be exhibited in the lithium ion secondary battery, for example, to form a stable coating film of the positive electrode or the negative electrode, or the electrolytic solution.
- the performance of the electrochemical device such as the non-aqueous electrolyte secondary battery 1 is improved.
- the capacity retention rate after storing the electrochemical device at high temperature can be improved.
- this electrolytic solution it is possible to improve the capacity recovery rate after storing the electrochemical device at high temperature.
- this electrolytic solution it is possible to suppress an increase in volume of the electrochemical device after storage at high temperature.
- the use of this electrolytic solution can improve the cycle characteristics of the electrochemical device.
- the manufacturing method of the non-aqueous electrolyte secondary battery 1 includes a first step of obtaining the positive electrode 6, a second step of obtaining the negative electrode 8, and a third step of accommodating the electrode group 2 in the battery outer casing 3.
- the material used for the positive electrode mixture layer 10 is dispersed in a dispersion medium using a kneader, a disperser or the like to obtain a positive electrode mixture in a slurry state, and then this positive electrode mixture is treated by a doctor blade method,
- the positive electrode current collector 9 is coated with a dipping method, a spray method, or the like, and then the dispersion medium is volatilized to obtain the positive electrode 6.
- a compression molding step using a roll press may be provided, if necessary.
- the positive electrode mixture layer 10 may be formed as a positive electrode mixture layer having a multi-layer structure by performing the above-described steps from the application of the positive electrode mixture to the volatilization of the dispersion medium a plurality of times.
- the dispersion medium may be water, 1-methyl-2-pyrrolidone (hereinafter, also referred to as NMP), or the like.
- the second step may be the same as the above-mentioned first step, and the method of forming the negative electrode mixture layer 12 on the negative electrode current collector 11 may be the same method as the above-mentioned first step. ..
- the separator 7 is sandwiched between the produced positive electrode 6 and negative electrode 8 to form the electrode group 2.
- the electrode group 2 is housed in the battery case 3.
- the electrolytic solution is injected into the battery exterior body 3.
- the electrolytic solution can be prepared, for example, by first dissolving an electrolyte salt in a solvent and then dissolving other materials.
- the electrochemical device may be a capacitor.
- the capacitor may include an electrode group including a positive electrode, a negative electrode, and a separator, and a bag-shaped battery exterior body that houses the electrode group.
- the details of each component of the capacitor may be the same as those of the non-aqueous electrolyte secondary battery 1.
- Example 1 [Production of positive electrode] Lithium cobalt oxide (95% by mass) as a positive electrode active material, fibrous graphite (1% by mass) and acetylene black (AB) (1% by mass) as a conductive agent, and a binder (3% by mass). Were sequentially added and mixed. NMP as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry-like positive electrode mixture. A predetermined amount of this positive electrode mixture was applied uniformly and uniformly to an aluminum foil having a thickness of 20 ⁇ m as a positive electrode current collector. Then, the dispersion medium was volatilized and then pressed to consolidate it to a density of 3.6 g/cm 3 to obtain a positive electrode.
- NMP as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry-like positive electrode mixture. A predetermined amount of this positive electrode mixture was applied uniformly and uniformly to an aluminum
- Water as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry negative electrode mixture. A predetermined amount of this negative electrode mixture was uniformly and uniformly applied to a rolled copper foil having a thickness of 10 ⁇ m as a negative electrode current collector. After that, the dispersion medium was volatilized and then pressed to consolidate to a density of 1.6 g/cm 3 to obtain a negative electrode.
- the electrolytic solution a mixed solution of ethylene carbonate, dimethyl carbonate and ethylmethyl carbonate containing 1 mol/L of LiPF 6 , 1% by mass of vinylene carbonate (VC) based on the total amount of the mixed solution, 4-fluoro-1, Addition of 1% by mass of 3-dioxolan-2-one (fluoroethylene carbonate; FEC), 1% by mass of compound A represented by the following formula (6) and 1% by mass of succinonitrile (on the basis of the total amount of electrolytic solution). I used what I did.
- Example 2 A lithium ion secondary battery was produced in the same manner as in Example 1 except that the amount of the compound A added was 2% by mass based on the total amount of the electrolytic solution.
- Example 3 A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound B represented by the following formula (7) was added in place of Compound A in an amount of 0.5% by mass based on the total amount of the electrolytic solution. A battery was produced.
- Example 4 A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound C represented by the following formula (8) was added in place of Compound A in an amount of 0.1% by mass based on the total amount of the electrolytic solution. A battery was produced.
- Example 5 A lithium ion secondary battery was produced in the same manner as in Example 1 except that adiponitrile was used instead of succinonitrile.
- Example 2 A lithium ion secondary battery was produced in the same manner as in Example 1 except that the compound A was not used (1% by mass of succinonitrile was added).
- each of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 was taken out from the constant temperature bath and kept in an environment of 25° C. for 30 minutes, and then cut off at a current value of 0.1 C and a final voltage of 2.
- a constant current discharge of 5V was performed.
- the discharge capacity at this time was defined as Q2.
- constant current charging was performed at a current value of 0.1 C up to an upper limit voltage of 4.45 V, and then constant voltage charging was performed at 4.45 V.
- the charge termination condition was a current value of 0.01C.
- constant current discharge with a final voltage of 2.5 V was performed at a current value of 0.1 C.
- the discharge capacity at this time was set to Q3.
- the lithium ion secondary batteries to which the electrolytic solutions of Examples 1 to 5 containing the compound A, the compound B or the compound C, and the nitrile compound (succinonitrile or adiponitrile) were applied were the compound A
- the volume increase rate in the high temperature storage test is small, and the capacity retention rate is small. It was also found that the recovery rate is improved.
- the lithium-ion secondary battery described in Comparative Example 1 accelerated the deterioration and short-circuited during the test.
- the lithium ion secondary batteries to which the electrolytic solutions of Examples 1 to 5 were applied had a higher capacity retention rate in the cycle test than the lithium ion secondary batteries of Comparative Examples 1 and 2. It is considered that this is because the nitrile compound formed a stable film on the positive electrode, and the compound A, the compound B, or the compound C contributed to the stabilization of the electrolytic solution.
- Example 6 Preparation of positive electrode and negative electrode
- a positive electrode and a negative electrode were obtained in the same manner as in Example 1.
- the volume increase rate of Example 1 was 102.3%
- the volume increase rate of Comparative Example 1 was 107.8%
- the volume increase rate of Comparative Example 2 was 103.7%.
- the lithium ion secondary battery of Comparative Example 2 to which the electrolytic solution containing succinonitrile and not containing Compound A was applied was the lithium ion secondary battery of Comparative Example 1 to which the electrolytic solution containing neither Compound A nor succinonitrile was applied.
- the volume increase rate was reduced as compared with the battery. It is considered that this is because in the lithium ion secondary battery of Comparative Example 2, the gas derived from the electrolytic solution generated under a high temperature (80° C.) environment was reduced.
- the volume increase rate was further reduced as compared with the lithium ion secondary batteries of Comparative Examples 1 and 2.
- the capacity retention rate of Example 6 was 95.8%
- the capacity retention rate of Comparative Example 1 was 94.5%
- the capacity retention rate of Comparative Example 2 was 94.8%.
- the lithium ion secondary battery of Example 1 to which the electrolytic solution containing both the compound A and succinonitrile was applied was the lithium ion battery of Comparative Example 1 to which the electrolytic solution containing neither compound A nor succinonitrile was applied, and The capacity retention rate was better than that of the lithium ion secondary battery of Comparative Example 2 to which the electrolytic solution containing succinonitrile and containing no compound A was applied.
- the compound A contributed to the stabilization of the electrolytic solution. It is also considered that the side reaction at the positive electrode or the negative electrode was suppressed and the electrolytic solution was stabilized.
- the lithium ion secondary batteries of Examples 1 to 6 to which the electrolytic solution containing both the compound A, the compound B or the compound C, and the nitrile compound were applied were prepared by using the electrolytic solution containing neither the nitrile compound nor the compound A.
- excellent performance is exhibited.
- Non-aqueous electrolyte secondary battery electrochemical device
- 6 Positive electrode
- 7 Separator
- 8 Negative electrode
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Power Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Inorganic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Secondary Cells (AREA)
- Electric Double-Layer Capacitors Or The Like (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
The present invention provides an electrolyte containing: a compound indicated by formula (1); and a nitrile compound that does not have a silicon atom. [In formula (1), R1–R3 each independently indicate an alkyl group or a fluorine atom, R4 indicates an alkylene group, and R5 indicates an organic group including a nitrogen atom or a sulfur atom.]
Description
本発明は、電解液及び電気化学デバイスに関する。
The present invention relates to an electrolytic solution and an electrochemical device.
近年、携帯型電子機器、電気自動車等の普及により、リチウムイオン二次電池に代表される非水電解液二次電池、キャパシタ等の高性能な電気化学デバイスが必要とされている。電気化学デバイスの性能を向上させる手段としては、例えば、電解液に所定の添加剤を添加する方法が検討されている。特許文献1には、サイクル特性及び内部抵抗特性を改善するために、特定のシロキサン化合物を含有させた非水電解液電池用電解液が開示されている。
With the recent spread of portable electronic devices, electric vehicles, etc., high-performance electrochemical devices such as non-aqueous electrolyte secondary batteries represented by lithium-ion secondary batteries and capacitors are required. As a means for improving the performance of an electrochemical device, for example, a method of adding a predetermined additive to an electrolytic solution has been studied. Patent Document 1 discloses an electrolyte solution for a non-aqueous electrolyte battery containing a specific siloxane compound in order to improve cycle characteristics and internal resistance characteristics.
本発明は、電気化学デバイスの性能を向上させることが可能な電解液を提供することを目的とする。
The present invention aims to provide an electrolytic solution capable of improving the performance of an electrochemical device.
本発明の一側面は、下記式(1)で表される化合物と、ケイ素原子を有さないニトリル化合物と、を含有する電解液である。
[式(1)中、R1~R3は、それぞれ独立に、アルキル基又はフッ素原子を示し、R4はアルキレン基を示し、R5は、窒素原子又は硫黄原子を含む有機基を示す。]
One aspect of the present invention is an electrolytic solution containing a compound represented by the following formula (1) and a nitrile compound having no silicon atom.
[In the formula (1), R 1 to R 3 each independently represent an alkyl group or a fluorine atom, R 4 represents an alkylene group, and R 5 represents an organic group containing a nitrogen atom or a sulfur atom. ]
この電解液によれば、一側面において、電気化学デバイスの性能として、電気化学デバイスを高温下で保存した後の容量維持率を向上させることができる。また、この電解液によれば、他の一側面において、電気化学デバイスを高温下で保存した後の容量回復率を向上させることができる。また、この電解液によれば、他の一側面において、電気化学デバイスを高温下で保存した後の体積増加を抑制することができる。また、この電解液によれば、他の一側面において、電気化学デバイスのサイクル特性を向上させることができる。
With this electrolyte, in one aspect, as the performance of the electrochemical device, the capacity retention rate after the electrochemical device is stored at high temperature can be improved. Further, according to this electrolytic solution, in another aspect, it is possible to improve the capacity recovery rate after storing the electrochemical device at a high temperature. Further, according to this electrolytic solution, in another aspect, it is possible to suppress an increase in volume after the electrochemical device is stored at a high temperature. Further, according to this electrolytic solution, in another aspect, the cycle characteristics of the electrochemical device can be improved.
R1~R3の少なくとも1つは、フッ素原子であってよい。
At least one of R 1 to R 3 may be a fluorine atom.
式(1)で表される化合物1分子中のケイ素原子の数は、1個であってよい。
The number of silicon atoms in one molecule of the compound represented by formula (1) may be one.
R5は、窒素原子を含む有機基であってよい。R5は、下記式(2)で表される基であってよい。
[式(2)中、R6及びR7は、それぞれ独立に、水素原子又はアルキル基を示し、*は結合手を示す。]
R 5 may be an organic group containing a nitrogen atom. R 5 may be a group represented by the following formula (2).
[In the formula (2), R 6 and R 7 each independently represent a hydrogen atom or an alkyl group, and * represents a bond. ]
R5は、硫黄原子を含む有機基であってよい。R5は、下記式(3)、式(4)又は式(5)のいずれかで表される基であってよい。
[式(3)中、R8はアルキル基を示し、*は結合手を示す。]
[式(4)中、R9はアルキル基を示し、*は結合手を示す。]
[式(5)中、R10はアルキル基を示し、*は結合手を示す。]
R 5 may be an organic group containing a sulfur atom. R 5 may be a group represented by the following formula (3), formula (4) or formula (5).
[In the formula (3), R 8 represents an alkyl group, and * represents a bond. ]
[In the formula (4), R 9 represents an alkyl group, and * represents a bond. ]
[In the formula (5), R 10 represents an alkyl group, and * represents a bond. ]
ニトリル化合物は、シアノ基を2つ有していてよい。ニトリル化合物は、スクシノニトリルであってよい。
The nitrile compound may have two cyano groups. The nitrile compound may be succinonitrile.
式(1)で表される化合物の含有量及びニトリル化合物の含有量の合計は、電解液全量を基準として10質量%以下であってよい。
The total content of the compound represented by the formula (1) and the content of the nitrile compound may be 10% by mass or less based on the total amount of the electrolytic solution.
本発明の他の一側面は、正極と、負極と、上記電解液と、を備える電気化学デバイスである。
Another aspect of the present invention is an electrochemical device including a positive electrode, a negative electrode, and the electrolytic solution.
負極は、炭素材料を含有してよい。炭素材料は黒鉛を含有してよい。負極は、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料を更に含有してよい。
The negative electrode may contain a carbon material. The carbon material may contain graphite. The negative electrode may further contain a material containing at least one element selected from the group consisting of silicon and tin.
電気化学デバイスは、非水電解液二次電池又はキャパシタであってよい。
The electrochemical device may be a non-aqueous electrolyte secondary battery or a capacitor.
本発明によれば、電気化学デバイスの性能を向上させることが可能な電解液を提供することができる。
According to the present invention, it is possible to provide an electrolytic solution capable of improving the performance of an electrochemical device.
以下、図面を適宜参照しながら、本発明の実施形態について説明する。ただし、本発明は以下の実施形態に限定されるものではない。
Hereinafter, embodiments of the present invention will be described with reference to the drawings as appropriate. However, the present invention is not limited to the following embodiments.
図1は、一実施形態に係る電気化学デバイスを示す斜視図である。本実施形態において、電気化学デバイスは非水電解液二次電池である。図1に示すように、非水電解液二次電池1は、正極、負極及びセパレータから構成される電極群2と、電極群2を収容する袋状の電池外装体3とを備えている。正極及び負極には、それぞれ正極集電タブ4及び負極集電タブ5が設けられている。正極集電タブ4及び負極集電タブ5は、それぞれ正極及び負極が非水電解液二次電池1の外部と電気的に接続可能なように、電池外装体3の内部から外部へ突き出している。電池外装体3内には、電解液(図示せず)が充填されている。非水電解液二次電池1は、上述したようないわゆる「ラミネート型」以外の形状の電池(コイン型、円筒型、積層型等)であってもよい。
FIG. 1 is a perspective view showing an electrochemical device according to one embodiment. In this embodiment, the electrochemical device is a non-aqueous electrolyte secondary battery. As shown in FIG. 1, the non-aqueous electrolyte secondary battery 1 includes an electrode group 2 including a positive electrode, a negative electrode, and a separator, and a bag-shaped battery exterior body 3 that houses the electrode group 2. A positive electrode current collecting tab 4 and a negative electrode current collecting tab 5 are provided on the positive electrode and the negative electrode, respectively. The positive electrode current collecting tab 4 and the negative electrode current collecting tab 5 project from the inside of the battery case 3 to the outside so that the positive electrode and the negative electrode can be electrically connected to the outside of the non-aqueous electrolyte secondary battery 1, respectively. .. An electrolyte solution (not shown) is filled in the battery exterior body 3. The non-aqueous electrolyte secondary battery 1 may be a battery (coin type, cylindrical type, laminated type, etc.) having a shape other than the so-called “laminate type” as described above.
電池外装体3は、例えばラミネートフィルムで形成された容器であってよい。ラミネートフィルムは、例えば、ポリエチレンテレフタレート(PET)フィルム等の樹脂フィルムと、アルミニウム、銅、ステンレス鋼等の金属箔と、ポリプロピレン等のシーラント層とがこの順で積層された積層フィルムであってよい。
The battery outer casing 3 may be a container formed of, for example, a laminated film. The laminated film may be, for example, a laminated film in which a resin film such as a polyethylene terephthalate (PET) film, a metal foil such as aluminum, copper and stainless steel, and a sealant layer such as polypropylene are laminated in this order.
図2は、図1に示した非水電解液二次電池1における電極群2の一実施形態を示す分解斜視図である。図2に示すように、電極群2は、正極6と、セパレータ7と、負極8とをこの順に備えている。正極6及び負極8は、正極合剤層10側及び負極合剤層12側の面がそれぞれセパレータ7と対向するように配置されている。
FIG. 2 is an exploded perspective view showing an embodiment of the electrode group 2 in the non-aqueous electrolyte secondary battery 1 shown in FIG. As shown in FIG. 2, the electrode group 2 includes a positive electrode 6, a separator 7, and a negative electrode 8 in this order. The positive electrode 6 and the negative electrode 8 are arranged such that the surfaces on the positive electrode mixture layer 10 side and the negative electrode mixture layer 12 side face the separator 7, respectively.
正極6は、正極集電体9と、正極集電体9上に設けられた正極合剤層10とを備えている。正極集電体9には、正極集電タブ4が設けられている。
The positive electrode 6 includes a positive electrode current collector 9 and a positive electrode mixture layer 10 provided on the positive electrode current collector 9. The positive electrode current collector 9 is provided with the positive electrode current collector tab 4.
正極集電体9は、例えば、アルミニウム、チタン、ステンレス、ニッケル、焼成炭素、導電性高分子、導電性ガラス等で形成されている。正極集電体9は、接着性、導電性及び耐酸化性向上の目的で、アルミニウム、銅等の表面にカーボン、ニッケル、チタン、銀等で処理が施されたものであってもよい。正極集電体9の厚さは、電極強度及びエネルギー密度の点から、例えば1~50μmである。
The positive electrode current collector 9 is made of, for example, aluminum, titanium, stainless steel, nickel, baked carbon, conductive polymer, conductive glass, or the like. The positive electrode current collector 9 may be one in which the surface of aluminum, copper, or the like is treated with carbon, nickel, titanium, silver, or the like for the purpose of improving adhesiveness, conductivity, and oxidation resistance. The thickness of the positive electrode current collector 9 is, for example, 1 to 50 μm in terms of electrode strength and energy density.
正極合剤層10は、一実施形態において、正極活物質と、導電剤と、結着剤とを含有する。正極合剤層10の厚さは、例えば20~200μmである。
In one embodiment, the positive electrode material mixture layer 10 contains a positive electrode active material, a conductive agent, and a binder. The thickness of the positive electrode mixture layer 10 is, for example, 20 to 200 μm.
正極活物質は、例えばリチウム酸化物であってよい。リチウム酸化物としては、例えば、LixCoO2、LixNiO2、LixMnO2、LixCoyNi1-yO2、LixCoyM1-yOz、LixNi1-yMyOz、LixMn2O4及びLixMn2-yMyO4(各式中、Mは、Na、Mg、Sc、Y、Mn、Fe、Co、Cu、Zn、Al、Cr、Pb、Sb、V及びBからなる群より選ばれる少なくとも1種の元素を示す(ただし、Mは、各式中の他の元素と異なる元素である)。x=0~1.2、y=0~0.9、z=2.0~2.3である。)が挙げられる。LixNi1-yMyOzで表されるリチウム酸化物は、LixNi1-(y1+y2)Coy1Mny2Oz(ただし、x及びzは上述したものと同様であり、y1=0~0.9、y2=0~0.9であり、かつ、y1+y2=0~0.9である。)であってよく、例えばLiNi1/3Co1/3Mn1/3O2、LiNi0.5Co0.2Mn0.3O2、LiNi0.6Co0.2Mn0.2O2、LiNi0.8Co0.1Mn0.1O2であってよい。LixNi1-yMyOzで表されるリチウム酸化物は、LixNi1-(y3+y4)Coy3Aly4Oz(ただし、x及びzは上述したものと同様であり、y3=0~0.9、y4=0~0.9であり、かつ、y3+y4=0~0.9である。)であってよく、例えばLiNi0.8Co0.15Al0.05O2であってもよい。
The positive electrode active material may be, for example, lithium oxide. Examples of the lithium oxide include Li x CoO 2 , Li x NiO 2 , Li x MnO 2 , Li x Co y Ni 1-y O 2 , Li x Co y M 1-y O z , and Li x Ni 1-. y M y O z , Li x Mn 2 O 4 and Li x Mn 2 -y M y O 4 (In each formula, M is Na, Mg, Sc, Y, Mn, Fe, Co, Cu, Zn, Al. , At least one element selected from the group consisting of Cr, Pb, Sb, V and B (provided that M is an element different from the other elements in each formula), x=0 to 1.2. , Y=0 to 0.9 and z=2.0 to 2.3). The lithium oxide represented by Li x Ni 1-y M y O z is Li x Ni 1-(y1+y2) Co y1 Mn y2 O z (where x and z are the same as those described above, and y1= 0 to 0.9, y2=0 to 0.9, and y1+y2=0 to 0.9), for example, LiNi 1/3 Co 1/3 Mn 1/3 O 2 , LiNi 0.5 Co 0.2 Mn 0.3 O 2 , LiNi 0.6 Co 0.2 Mn 0.2 O 2, may be LiNi 0.8 Co 0.1 Mn 0.1 O 2 . The lithium oxide represented by Li x Ni 1-y M y O z is Li x Ni 1-(y3+y4) Co y3 Al y4 O z (where x and z are the same as those described above, and y3= 0 to 0.9, y4=0 to 0.9, and y3+y4=0 to 0.9), for example, LiNi 0.8 Co 0.15 Al 0.05 O 2 . It may be.
正極活物質は、例えばリチウムのリン酸塩であってもよい。リチウムのリン酸塩としては、例えば、リン酸マンガンリチウム(LiMnPO4)、リン酸鉄リチウム(LiFePO4)、リン酸コバルトリチウム(LiCoPO4)及びリン酸バナジウムリチウム(Li3V2(PO4)3)が挙げられる。
The positive electrode active material may be, for example, a lithium phosphate. Examples of the lithium phosphate include lithium manganese phosphate (LiMnPO 4 ), lithium iron phosphate (LiFePO 4 ), lithium cobalt phosphate (LiCoPO 4 ), and lithium vanadium phosphate (Li 3 V 2 (PO 4 ). 3 ).
正極活物質の含有量は、正極合剤層全量を基準として、80質量%以上、又は85質量%以上であってよく、99質量%以下であってよい。
The content of the positive electrode active material may be 80% by mass or more, or 85% by mass or more, and 99% by mass or less, based on the total amount of the positive electrode mixture layer.
導電剤は、アセチレンブラック、ケッチェンブラック等のカーボンブラック、黒鉛、グラフェン、カーボンナノチューブなどの炭素材料であってよい。導電剤の含有量は、正極合剤層全量を基準として、例えば、0.01質量%以上、0.1質量%以上、又は1質量%以上であってよく、50質量%以下、30質量%以下、又は15質量%以下であってよい。
The conductive agent may be carbon black such as acetylene black or Ketjen black, or carbon material such as graphite, graphene or carbon nanotube. The content of the conductive agent may be, for example, 0.01% by mass or more, 0.1% by mass or more, or 1% by mass or more, and 50% by mass or less, 30% by mass, based on the total amount of the positive electrode mixture layer. Or less, or 15% by mass or less.
結着剤は、例えば、ポリエチレン、ポリプロピレン、ポリエチレンテレフタレート、ポリメチルメタクリレート、ポリイミド、芳香族ポリアミド、セルロース、ニトロセルロース等の樹脂;SBR(スチレン-ブタジエンゴム)、NBR(アクリロニトリル-ブタジエンゴム)、フッ素ゴム、イソプレンゴム、ブタジエンゴム、エチレン-プロピレンゴム等のゴム;スチレン・ブタジエン・スチレンブロック共重合体又はその水素添加物、EPDM(エチレン・プロピレン・ジエン三元共重合体)、スチレン・エチレン・ブタジエン・エチレン共重合体、スチレン・イソプレン・スチレンブロック共重合体又はその水素添加物等の熱可塑性エラストマー;シンジオタクチック-1、2-ポリブタジエン、ポリ酢酸ビニル、エチレン・酢酸ビニル共重合体、プロピレン・α-オレフィン共重合体等の軟質樹脂;ポリフッ化ビニリデン(PVDF)、ポリテトラフルオロエチレン、フッ素化ポリフッ化ビニリデン、ポリテトラフルオロエチレン・エチレン共重合体、ポリテトラフルオロエチレン・フッ化ビニリデン共重合体等のフッ素含有樹脂;ニトリル基含有モノマーをモノマー単位として有する樹脂;アルカリ金属イオン(例えばリチウムイオン)のイオン伝導性を有する高分子組成物などが挙げられる。
Examples of the binder include resins such as polyethylene, polypropylene, polyethylene terephthalate, polymethylmethacrylate, polyimide, aromatic polyamide, cellulose and nitrocellulose; SBR (styrene-butadiene rubber), NBR (acrylonitrile-butadiene rubber), fluororubber. , Isoprene rubber, butadiene rubber, ethylene-propylene rubber and the like; styrene/butadiene/styrene block copolymers or hydrogenated products thereof, EPDM (ethylene/propylene/diene terpolymer), styrene/ethylene/butadiene/ Thermoplastic elastomers such as ethylene copolymers, styrene/isoprene/styrene block copolymers or hydrogenated products thereof; syndiotactic-1,2-polybutadiene, polyvinyl acetate, ethylene/vinyl acetate copolymers, propylene/α -Soft resin such as olefin copolymer; polyvinylidene fluoride (PVDF), polytetrafluoroethylene, fluorinated polyvinylidene fluoride, polytetrafluoroethylene/ethylene copolymer, polytetrafluoroethylene/vinylidene fluoride copolymer, etc. And a fluorine-containing resin, a resin having a nitrile group-containing monomer as a monomer unit, a polymer composition having an alkali metal ion (for example, lithium ion) ion conductivity, and the like.
結着剤の含有量は、正極合剤層全量を基準として、例えば、0.1質量%以上、1質量%以上、又は1.5質量%以上であってよく、30質量%以下、20質量%以下、又は10質量%以下であってよい。
The content of the binder may be, for example, 0.1% by mass or more, 1% by mass or more, or 1.5% by mass or more, based on the total amount of the positive electrode mixture layer, 30% by mass or less, 20% by mass. % Or less, or 10% by mass or less.
セパレータ7は、正極6及び負極8間を電子的には絶縁する一方でイオンを透過させ、かつ、正極6側における酸化性及び負極8側における還元性に対する耐性を備えるものであれば、特に制限されない。このようなセパレータ7の材料(材質)としては、樹脂、無機物等が挙げられる。
The separator 7 is not particularly limited as long as it electrically insulates between the positive electrode 6 and the negative electrode 8 while allowing ions to pass therethrough and has resistance to the oxidizing property on the positive electrode 6 side and the reducing property on the negative electrode 8 side. Not done. Examples of the material (material) of the separator 7 include resins and inorganic materials.
樹脂としては、オレフィン系ポリマー、フッ素系ポリマー、セルロース系ポリマー、ポリイミド、ナイロン等が挙げられる。セパレータ7は、電解液に対して安定で、保液性に優れる観点から、好ましくは、ポリエチレン、ポリプロピレン等のポリオレフィンで形成された多孔質シート又は不織布である。
Examples of resins include olefin polymers, fluorine polymers, cellulosic polymers, polyimides and nylons. The separator 7 is preferably a porous sheet or a non-woven fabric formed of polyolefin such as polyethylene or polypropylene from the viewpoint of being stable with respect to the electrolytic solution and excellent in liquid retaining property.
無機物としては、アルミナ、二酸化珪素等の酸化物、窒化アルミニウム、窒化珪素等の窒化物、硫酸バリウム、硫酸カルシウム等の硫酸塩が挙げられる。セパレータ7は、例えば、不織布、織布、微多孔性フィルム等の薄膜状基材に、繊維状又は粒子状の無機物を付着させたセパレータであってよい。
Examples of the inorganic substance include oxides such as alumina and silicon dioxide, nitrides such as aluminum nitride and silicon nitride, and sulfates such as barium sulfate and calcium sulfate. The separator 7 may be, for example, a thin film substrate such as a nonwoven fabric, a woven fabric, or a microporous film to which a fibrous or particulate inorganic substance is attached.
負極8は、負極集電体11と、負極集電体11上に設けられた負極合剤層12とを備えている。負極集電体11には、負極集電タブ5が設けられている。
The negative electrode 8 includes a negative electrode current collector 11 and a negative electrode mixture layer 12 provided on the negative electrode current collector 11. The negative electrode current collector 11 is provided with a negative electrode current collector tab 5.
負極集電体11は、銅、ステンレス、ニッケル、アルミニウム、チタン、焼成炭素、導電性高分子、導電性ガラス、アルミニウム-カドミウム合金等で形成されている。負極集電体11は、接着性、導電性、耐還元性向上の目的で、銅、アルミニウム等の表面にカーボン、ニッケル、チタン、銀等で処理が施されたものであってもよい。負極集電体11の厚さは、電極強度及びエネルギー密度の点から、例えば1~50μmである。
The negative electrode current collector 11 is made of copper, stainless steel, nickel, aluminum, titanium, baked carbon, conductive polymer, conductive glass, aluminum-cadmium alloy, or the like. The negative electrode current collector 11 may be one in which the surface of copper, aluminum, or the like is treated with carbon, nickel, titanium, silver, or the like for the purpose of improving adhesion, conductivity, and reduction resistance. The thickness of the negative electrode current collector 11 is, for example, 1 to 50 μm from the viewpoint of electrode strength and energy density.
負極合剤層12は、例えば、負極活物質と、結着剤とを含有する。
The negative electrode mixture layer 12 contains, for example, a negative electrode active material and a binder.
負極活物質は、リチウムイオンを吸蔵及び放出可能な物質であれば特に制限されない。負極活物質としては、例えば、炭素材料、金属複合酸化物、錫、ゲルマニウム、ケイ素等の第四族元素の酸化物又は窒化物、リチウムの単体、リチウムアルミニウム合金等のリチウム合金、Sn、Si等のリチウムと合金を形成可能な金属などが挙げられる。負極活物質は、安全性の観点からは、好ましくは炭素材料及び金属複合酸化物からなる群より選択される少なくとも1種である。負極活物質はこれらの1種単独又は2種以上の混合物であってよい。負極活物質の形状は、例えば、粒子状であってよい。
The negative electrode active material is not particularly limited as long as it can absorb and release lithium ions. Examples of the negative electrode active material include carbon materials, metal composite oxides, oxides or nitrides of Group IV elements such as tin, germanium, and silicon, simple substances of lithium, lithium alloys such as lithium aluminum alloys, Sn, Si, and the like. And a metal capable of forming an alloy with lithium. From the viewpoint of safety, the negative electrode active material is preferably at least one selected from the group consisting of carbon materials and metal composite oxides. The negative electrode active material may be one type of these alone or a mixture of two or more types. The shape of the negative electrode active material may be, for example, a particle shape.
炭素材料としては、非晶質炭素材料、天然黒鉛、天然黒鉛に非晶質炭素材料の被膜を形成した複合炭素材料、人造黒鉛(エポキシ樹脂、フェノール樹脂等の樹脂原料、又は、石油、石炭等から得られるピッチ系原料を焼成して得られるもの)などが挙げられる。金属複合酸化物は、高電流密度充放電特性の観点からは、好ましくはチタン及びリチウムのいずれか一方又は両方を含有し、より好ましくはリチウムを含有する。
As the carbon material, amorphous carbon material, natural graphite, composite carbon material obtained by forming a film of amorphous carbon material on natural graphite, artificial graphite (resin raw material such as epoxy resin, phenol resin, or petroleum, coal, etc. Obtained by firing a pitch-based raw material obtained from the above). From the viewpoint of high current density charge/discharge characteristics, the metal composite oxide preferably contains one or both of titanium and lithium, and more preferably contains lithium.
負極活物質の中でも炭素材料は、導電性が高く、低温特性及びサイクル安定性に特に優れている。炭素材料の中でも高容量化の観点からは、黒鉛が好ましい。黒鉛においては、好ましくはX線広角回折法における炭素網面層間(d002)が0.34nm未満であり、より好ましくは0.3354nm以上0.337nm以下である。このような条件を満たす炭素材料を、擬似異方性炭素と称する場合がある。
Among the negative electrode active materials, carbon materials have high conductivity and are particularly excellent in low temperature characteristics and cycle stability. Among the carbon materials, graphite is preferable from the viewpoint of high capacity. In graphite, the carbon network plane layer (d002) in the X-ray wide-angle diffraction method is preferably less than 0.34 nm, and more preferably 0.3354 nm or more and 0.337 nm or less. A carbon material that satisfies such conditions may be referred to as pseudo-anisotropic carbon.
負極活物質には、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料が更に含まれていてもよい。ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料は、ケイ素又はスズの単体、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む化合物であってよい。当該化合物は、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む合金であってよく、例えば、ケイ素及びスズの他に、ニッケル、銅、鉄、コバルト、マンガン、亜鉛、インジウム、銀、チタン、ゲルマニウム、ビスマス、アンチモン及びクロムからなる群より選ばれる少なくとも1種を含む合金である。ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む化合物は、酸化物、窒化物、又は炭化物であってもよく、具体的には、例えば、SiO、SiO2、LiSiO等のケイ素酸化物、Si3N4、Si2N2O等のケイ素窒化物、SiC等のケイ素炭化物、SnO、SnO2、LiSnO等のスズ酸化物などであってよい。
The negative electrode active material may further contain a material containing at least one element selected from the group consisting of silicon and tin. The material containing at least one element selected from the group consisting of silicon and tin may be a simple substance of silicon or tin, or a compound containing at least one element selected from the group consisting of silicon and tin. The compound may be an alloy containing at least one element selected from the group consisting of silicon and tin. For example, in addition to silicon and tin, nickel, copper, iron, cobalt, manganese, zinc, indium, silver. , An alloy containing at least one selected from the group consisting of titanium, germanium, bismuth, antimony and chromium. The compound containing at least one element selected from the group consisting of silicon and tin may be an oxide, a nitride, or a carbide, and specifically, for example, silicon oxide such as SiO, SiO 2 , and LiSiO. A silicon nitride such as Si 3 N 4 or Si 2 N 2 O, a silicon carbide such as SiC, a tin oxide such as SnO, SnO 2 or LiSnO.
負極8は、低温入力特性等の電気化学デバイスの性能を更に向上させる観点から、負極活物質として、好ましくは炭素材料を含み、より好ましくは黒鉛を含み、更に好ましくは、炭素材料と、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料との混合物を含み、特に好ましくは、黒鉛とケイ素酸化物との混合物を含む。当該混合物におけるケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料(ケイ素酸化物)に対する炭素材料(黒鉛)の含有量は、当該混合物全量を基準として、1質量%以上、又は3質量%以上であってよく、30質量%以下であってよい。
From the viewpoint of further improving the performance of the electrochemical device such as low temperature input characteristics, the negative electrode 8 preferably contains a carbon material, more preferably graphite, and further preferably a carbon material, silicon and It contains a mixture with a material containing at least one element selected from the group consisting of tin, and particularly preferably contains a mixture of graphite and silicon oxide. The content of the carbon material (graphite) with respect to the material (silicon oxide) containing at least one element selected from the group consisting of silicon and tin in the mixture is 1% by mass or more based on the total amount of the mixture, or 3 It may be not less than 30% by mass and not more than 30% by mass.
負極活物質の含有量は、負極合剤層全量を基準として、80質量%以上、又は85質量%以上であってよく、99質量%以下であってよい。
The content of the negative electrode active material may be 80% by mass or more, or 85% by mass or more, and 99% by mass or less, based on the total amount of the negative electrode mixture layer.
結着剤及びその含有量は、上述した正極合剤層における結着剤及びその含有量と同様であってよい。
The binder and the content thereof may be the same as the binder and the content thereof in the positive electrode mixture layer described above.
負極合剤層12は、粘度を調節するために増粘剤を含有してもよい。増粘剤は、特に制限されないが、カルボキシメチルセルロース、メチルセルロース、ヒドロキシメチルセルロース、エチルセルロース、ポリビニルアルコール、酸化スターチ、リン酸化スターチ、カゼイン、これらの塩等であってよい。増粘剤は、これらの1種単独又は2種以上の混合物であってよい。
The negative electrode mixture layer 12 may contain a thickener to adjust the viscosity. The thickener is not particularly limited, but may be carboxymethyl cellulose, methyl cellulose, hydroxymethyl cellulose, ethyl cellulose, polyvinyl alcohol, oxidized starch, phosphorylated starch, casein, salts thereof, or the like. The thickener may be one of these alone or a mixture of two or more thereof.
負極合剤層12が増粘剤を含む場合、その含有量は特に制限されない。増粘剤の含有量は、負極合剤層の塗布性の観点からは、負極合剤層全量を基準として、0.1質量%以上であってよく、好ましくは0.2質量%以上であり、より好ましくは0.5質量%以上である。増粘剤の含有量は、電池容量の低下又は負極活物質間の抵抗の上昇を抑制する観点からは、負極合剤層全量を基準として、5質量%以下であってよく、好ましくは3質量%以下であり、より好ましくは2質量%以下である。
When the negative electrode mixture layer 12 contains a thickener, the content thereof is not particularly limited. The content of the thickener may be 0.1% by mass or more, and preferably 0.2% by mass or more, based on the total amount of the negative electrode mixture layer, from the viewpoint of coatability of the negative electrode mixture layer. , And more preferably 0.5% by mass or more. The content of the thickener may be 5% by mass or less, preferably 3% by mass, based on the total amount of the negative electrode mixture layer, from the viewpoint of suppressing a decrease in battery capacity or an increase in resistance between the negative electrode active materials. % Or less, and more preferably 2% by mass or less.
電解液は、一実施形態において、下記式(1)で表される化合物と、ケイ素原子を有さないニトリル化合物と、電解質塩と、非水溶媒とを含有する。
式(1)中、R1~R3は、それぞれ独立に、アルキル基又はフッ素原子を示し、R4はアルキレン基を示し、R5は、窒素原子又は硫黄原子を含む有機基を示す。
In one embodiment, the electrolytic solution contains a compound represented by the following formula (1), a nitrile compound having no silicon atom, an electrolyte salt, and a nonaqueous solvent.
In formula (1), R 1 to R 3 each independently represent an alkyl group or a fluorine atom, R 4 represents an alkylene group, and R 5 represents an organic group containing a nitrogen atom or a sulfur atom.
R1~R3で表されるアルキル基の炭素数は、1以上であってよく、3以下であってよい。R1~R3は、メチル基、エチル基、又はプロピル基であってよく、直鎖状でも分岐状でもよい。R1~R3の少なくとも1つは、好ましくはフッ素原子である。R1~R3のいずれか1つがフッ素原子であってよく、R1~R3のいずれか2つがフッ素原子であってよく、R1~R3のすべてがフッ素原子であってよい。
The alkyl group represented by R 1 to R 3 may have 1 or more carbon atoms and 3 or less carbon atoms. R 1 to R 3 may be a methyl group, an ethyl group, or a propyl group, and may be linear or branched. At least one of R 1 to R 3 is preferably a fluorine atom. May be any one of fluorine atom of R 1 ~ R 3, it may be any two of a fluorine atom of R 1 ~ R 3, all of R 1 ~ R 3 may be a fluorine atom.
R4で表されるアルキレン基の炭素数は、1以上又は2以上であってよく、5以下又は4以下であってよい。R4で表されるアルキレン基は、メチレン基、エチレン基、プロピレン基、ブチレン基、又はペンチレン基であってよく、直鎖状でも分岐状でもよい。
The carbon number of the alkylene group represented by R 4 may be 1 or more or 2 or more, and 5 or less or 4 or less. The alkylene group represented by R 4 may be a methylene group, an ethylene group, a propylene group, a butylene group, or a pentylene group, and may be linear or branched.
一実施形態において、式(1)で表される化合物1分子中のケイ素原子の数は、1個である。すなわち、一実施形態において、R5で表される有機基は、ケイ素原子を含まない。
In one embodiment, the number of silicon atoms in one molecule of the compound represented by formula (1) is one. That is, in one embodiment, the organic group represented by R 5 does not include a silicon atom.
R5は、一実施形態において、窒素原子を含む有機基であり、電気化学デバイスの性能を更に向上させることができる観点から、好ましくは、下記式(2)で表される基である。
式(2)中、R6及びR7は、それぞれ独立に、水素原子又はアルキル基を示す。R6又はR7で表されるアルキル基は、上述したR1~R3で表されるアルキル基と同様であってよい。*は結合手を示す。
In one embodiment, R 5 is an organic group containing a nitrogen atom, and is preferably a group represented by the following formula (2) from the viewpoint of further improving the performance of the electrochemical device.
In formula (2), R 6 and R 7 each independently represent a hydrogen atom or an alkyl group. The alkyl group represented by R 6 or R 7 may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
R5は、他の一実施形態において、硫黄原子を含む有機基であり、電気化学デバイスの性能を更に向上させることができる観点から、好ましくは、下記式(3)、式(4)又は式(5)のいずれかで表される基であってよい。
式(3)中、R8はアルキル基を示す。アルキル基は、上述したR1~R3で表されるアルキル基と同様であってよい。*は結合手を示す。
式(4)中、R9はアルキル基を示す。アルキル基は、上述したR1~R3で表されるアルキル基と同様であってよい。*は結合手を示す。
式(5)中、R10はアルキル基を示す。アルキル基は、上述したR1~R3で表されるアルキル基と同様であってよい。*は結合手を示す。
In another embodiment, R 5 is an organic group containing a sulfur atom and is preferably a compound represented by the following formula (3), formula (4) or formula from the viewpoint that the performance of the electrochemical device can be further improved. It may be a group represented by any of (5).
In formula (3), R 8 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
In formula (4), R 9 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
In formula (5), R 10 represents an alkyl group. The alkyl group may be the same as the alkyl group represented by R 1 to R 3 described above. * Indicates a bond.
式(1)で表される化合物の含有量は、電気化学デバイスの性能を更に向上させることができる観点から、電解液全量を基準として、好ましくは、0.001質量%以上、0.005質量%以上、0.01質量%以上、0.05質量%以上、又は0.1質量%以上であり、好ましくは、8質量%以下、5質量%以下、3質量%以下、2質量%以下、又は1質量%以下である。
The content of the compound represented by the formula (1) is preferably 0.001% by mass or more and 0.005% by mass, based on the total amount of the electrolytic solution, from the viewpoint that the performance of the electrochemical device can be further improved. % Or more, 0.01 mass% or more, 0.05 mass% or more, or 0.1 mass% or more, preferably 8 mass% or less, 5 mass% or less, 3 mass% or less, 2 mass% or less, Alternatively, it is 1% by mass or less.
ニトリル化合物は、シアノ基(ニトリル基)を少なくとも1つ有する化合物である。なお、ニトリル化合物は、上記式(1)で表される化合物以外の化合物である。言い換えれば、ニトリル化合物は、ケイ素原子を有さない化合物である。ニトリル化合物は、シアノ基を1つ又は2つ以上有していてよく、シアノ基を2つ又は3つ有していてもよい。シアノ基を1つ有するニトリル化合物は、例えば、ブチロニトリル、バレロニトリル、n-ヘプタンニトリル等であってよい。シアノ基を2つ有するニトリル化合物は、例えば、スクシノニトリル、グルタルニトリル、アジポニトリル、ピメロニトリル、スベロニトリル等であってよい。シアノ基を3つ有するニトリル化合物は、例えば、1,2,3-プロパントリカルボニトリル、1,3,5-ペンタントリカルボニトリル等であってよい。ニトリル化合物としては、正極又は負極上にて安定な被膜が形成され、電解液の分解に起因する電池の膨張を抑制できる観点から、シアノ基を2つ以上有し、シアノ基における炭素原子を除く炭素原子の数が2以上の化合物が好ましい。ニトリル化合物は、より好ましくは、シアノ基を2つ又は3つ有し、シアノ基における炭素原子を除く炭素原子の数が2以上の化合物である、ニトリル化合物は、更に好ましくは、スクシノニトリル、グルタルニトリル、アジポニトリル、ピメロニトリル、スベロニトリル、1,2,3-プロパントリカルボニトリル、又は1,3,5-ペンタントリカルボニトリルである。
A nitrile compound is a compound having at least one cyano group (nitrile group). The nitrile compound is a compound other than the compound represented by the above formula (1). In other words, the nitrile compound is a compound having no silicon atom. The nitrile compound may have 1 or 2 or more cyano groups, and may have 2 or 3 cyano groups. The nitrile compound having one cyano group may be, for example, butyronitrile, valeronitrile, n-heptanenitrile or the like. The nitrile compound having two cyano groups may be, for example, succinonitrile, glutarnitrile, adiponitrile, pimelonitrile, suberonitrile and the like. The nitrile compound having three cyano groups may be, for example, 1,2,3-propanetricarbonitrile, 1,3,5-pentanetricarbonitrile and the like. The nitrile compound has two or more cyano groups and excludes carbon atoms in the cyano group from the viewpoint that a stable film is formed on the positive electrode or the negative electrode and the expansion of the battery due to the decomposition of the electrolytic solution can be suppressed. Compounds having 2 or more carbon atoms are preferred. The nitrile compound is more preferably a compound having 2 or 3 cyano groups, and the number of carbon atoms excluding carbon atoms in the cyano group is 2 or more. The nitrile compound is more preferably succinonitrile, Glutarnitrile, adiponitrile, pimelonitrile, suberonitrile, 1,2,3-propanetricarbonitrile, or 1,3,5-pentanetricarbonitrile.
ニトリル化合物の含有量は、電気化学デバイスの性能を更に向上させることができる観点から、電解液全量を基準として、好ましくは、0.001質量%以上、0.005質量%以上、0.01質量%以上、0.05質量%以上、又は0.1質量%以上であり、好ましくは、5質量%以下、3質量%以下、2質量%以下、又は1質量%以下である。
The content of the nitrile compound is preferably 0.001 mass% or more, 0.005 mass% or more, 0.01 mass, based on the total amount of the electrolytic solution, from the viewpoint of further improving the performance of the electrochemical device. % Or more, 0.05% by mass or more, or 0.1% by mass or more, preferably 5% by mass or less, 3% by mass or less, 2% by mass or less, or 1% by mass or less.
式(1)で表される化合物の含有量及びニトリル化合物の含有量の合計は、電気化学デバイスの性能を更に向上させることができる観点から、電解液全量を基準として、好ましくは、0.001質量%以上、0.005質量%以上、0.01質量%以上、0.1質量%以上、又は0.5質量%以上であり、好ましくは、10質量%以下、7質量%以下、5質量%以下、3質量%以下、又は2質量%以下である。
The total content of the compound represented by the formula (1) and the content of the nitrile compound is preferably 0.001 based on the total amount of the electrolytic solution, from the viewpoint that the performance of the electrochemical device can be further improved. % By mass, 0.005% by mass or more, 0.01% by mass or more, 0.1% by mass or more, or 0.5% by mass or more, preferably 10% by mass or less, 7% by mass or less, 5% by mass % Or less, 3% by mass or less, or 2% by mass or less.
ニトリル化合物の含有量に対する式(1)で表される化合物の含有量の質量比(式(1)で表される化合物の含有量/ニトリル化合物の含有量)は、電気化学デバイスの性能を更に向上させることができる観点から、好ましくは、0.01以上、0.05以上、0.1以上、0.2以上、又は0.25以上であり、また、好ましくは、500以下、100以下、50以下、20以下、10以下、5以下、3以下、2以下、又は1以下である。
The mass ratio of the content of the compound represented by the formula (1) to the content of the nitrile compound (the content of the compound represented by the formula (1)/the content of the nitrile compound) further improves the performance of the electrochemical device. From the viewpoint of being able to improve, it is preferably 0.01 or more, 0.05 or more, 0.1 or more, 0.2 or more, or 0.25 or more, and preferably 500 or less, 100 or less, It is 50 or less, 20 or less, 10 or less, 5 or less, 3 or less, 2 or less, or 1 or less.
電解質塩は、例えばリチウム塩であってよい。リチウム塩は、例えば、LiPF6、LiBF4、LiClO4、LiB(C6H5)4、LiCH3SO3、CF3SO2OLi、LiN(SO2F)2(Li[FSI]、リチウムビスフルオロスルホニルイミド)、LiN(SO2CF3)2(Li[TFSI]、リチウムビストリフルオロメタンスルホニルイミド)、及びLiN(SO2CF2CF3)2からなる群より選ばれる少なくとも1種であってよい。リチウム塩は、溶媒に対する溶解性、二次電池の充放電特性、出力特性、サイクル特性等に更に優れる観点から、好ましくはLiPF6を含む。
The electrolyte salt may be, for example, a lithium salt. The lithium salt is, for example, LiPF 6 , LiBF 4 , LiClO 4 , LiB(C 6 H 5 ) 4 , LiCH 3 SO 3 , CF 3 SO 2 OLi, LiN(SO 2 F) 2 (Li[FSI], lithium bis. Fluorosulfonylimide), LiN(SO 2 CF 3 ) 2 (Li[TFSI], lithium bistrifluoromethanesulfonylimide), and at least one selected from the group consisting of LiN(SO 2 CF 2 CF 3 ) 2 Good. The lithium salt preferably contains LiPF 6 from the viewpoint of further excellent solubility in a solvent, charge/discharge characteristics of a secondary battery, output characteristics, cycle characteristics, and the like.
電解質塩の濃度は、充放電特性に優れる観点から、非水溶媒全量を基準として、好ましくは0.5mol/L以上、より好ましくは0.7mol/L以上、更に好ましくは0.8mol/L以上であり、また、好ましくは1.5mol/L以下、より好ましくは1.3mol/L以下、更に好ましくは1.2mol/L以下である。
From the viewpoint of excellent charge/discharge characteristics, the concentration of the electrolyte salt is preferably 0.5 mol/L or more, more preferably 0.7 mol/L or more, and further preferably 0.8 mol/L or more, based on the total amount of the non-aqueous solvent. Further, it is preferably 1.5 mol/L or less, more preferably 1.3 mol/L or less, still more preferably 1.2 mol/L or less.
非水溶媒は、例えば、エチレンカーボネート、プロピレンカーボネート、ジメチルカーボネート、ジエチルカーボネート、メチルエチルカーボネート、γ-ブチルラクトン、1,2-ジメトキシエタン、ジメトキシメタン、テトラヒドロフラン、ジオキソラン、塩化メチレン、酢酸メチル等であってよい。非水溶媒は、これらの1種単独又は2種以上の混合物であってよく、好ましくは2種以上の混合物である。
The non-aqueous solvent is, for example, ethylene carbonate, propylene carbonate, dimethyl carbonate, diethyl carbonate, methyl ethyl carbonate, γ-butyl lactone, 1,2-dimethoxyethane, dimethoxymethane, tetrahydrofuran, dioxolane, methylene chloride, methyl acetate, etc. You may. The non-aqueous solvent may be one of these alone or a mixture of two or more thereof, and preferably a mixture of two or more thereof.
電解液は、式(1)で表される化合物、ニトリル化合物、電解質塩及び非水溶媒以外のその他の材料を更に含有してもよい。その他の材料は、例えば、フッ素含有環状カーボネート、炭素-炭素二重結合を有する環状カーボネート等の環状カーボネート、式(1)で表される化合物以外の窒素原子、硫黄原子、又は窒素原子及び硫黄原子を含有する化合物、環状カルボン酸エステル等であってよい。
The electrolytic solution may further contain other materials other than the compound represented by the formula (1), the nitrile compound, the electrolyte salt and the non-aqueous solvent. Other materials include, for example, a cyclic carbonate such as a fluorine-containing cyclic carbonate, a cyclic carbonate having a carbon-carbon double bond, a nitrogen atom other than the compound represented by the formula (1), a sulfur atom, or a nitrogen atom and a sulfur atom. It may be a compound containing, a cyclic carboxylic acid ester, or the like.
フッ素含有環状カーボネートは、例えば、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)、1,2-ジフルオロエチレンカーボネート、1,1-ジフルオロエチレンカーボネート、1,1,2-トリフルオロエチレンカーボネート、1,1,2,2-テトラフルオロエチレンカーボネート等であってよく、好ましくは、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)である。炭素-炭素二重結合を有する環状カーボネートは、例えばビニレンカーボネートであってよい。式(1)で表される化合物以外の硫黄原子を含有する化合物は、例えば、1,3-プロパンスルトン、1-プロペン-1,3-スルトン等の環状スルホン酸エステル化合物であってよい。
Examples of the fluorine-containing cyclic carbonate include 4-fluoro-1,3-dioxolan-2-one (fluoroethylene carbonate; FEC), 1,2-difluoroethylene carbonate, 1,1-difluoroethylene carbonate, 1,1,2. It may be trifluoroethylene carbonate, 1,1,2,2-tetrafluoroethylene carbonate or the like, preferably 4-fluoro-1,3-dioxolan-2-one (fluoroethylene carbonate; FEC). The cyclic carbonate having a carbon-carbon double bond may be vinylene carbonate, for example. The compound containing a sulfur atom other than the compound represented by the formula (1) may be, for example, a cyclic sulfonate compound such as 1,3-propane sultone and 1-propene-1,3-sultone.
本発明者らは、様々な構造及び官能基を有する化合物を検討した結果、上述した式(1)で表される化合物及びニトリル化合物を電解液に適用することによって、電気化学デバイスの性能を向上させることができることを明らかにした。本発明者らは、式(1)で表される化合物及びニトリル化合物を電解液に用いることによる作用効果を以下のように推察している。すなわち、式(1)で表される化合物及びニトリル化合物が、それぞれリチウムイオン二次電池内において最も効果を発現しやすい場所に作用して、例えば、正極又は負極の安定な被膜形成、又は電解液の安定化に寄与すると考えられる。その結果、非水電解液二次電池1のような電気化学デバイスの性能が向上する。例えば、この電解液を用いることにより、電気化学デバイスを高温下に保存した後の容量維持率を向上させることができる。他の一側面においては、この電解液を用いることにより、電気化学デバイスを高温下で保存した後の容量回復率を向上させることができる。他の一側面においては、この電解液を用いることにより、電気化学デバイスを高温下で保存した後の体積増加を抑制することができる。他の一側面においては、この電解液を用いることにより、電気化学デバイスのサイクル特性を向上させることができる。
As a result of examining compounds having various structures and functional groups, the present inventors have improved the performance of an electrochemical device by applying the compound represented by the above formula (1) and a nitrile compound to an electrolytic solution. Revealed that can be made. The present inventors presume the action and effect of using the compound represented by the formula (1) and the nitrile compound in the electrolytic solution as follows. That is, the compound represented by the formula (1) and the nitrile compound act on the place where the effect is most likely to be exhibited in the lithium ion secondary battery, for example, to form a stable coating film of the positive electrode or the negative electrode, or the electrolytic solution. Is considered to contribute to the stabilization of As a result, the performance of the electrochemical device such as the non-aqueous electrolyte secondary battery 1 is improved. For example, by using this electrolytic solution, the capacity retention rate after storing the electrochemical device at high temperature can be improved. In another aspect, by using this electrolytic solution, it is possible to improve the capacity recovery rate after storing the electrochemical device at high temperature. In another aspect, by using this electrolytic solution, it is possible to suppress an increase in volume of the electrochemical device after storage at high temperature. In another aspect, the use of this electrolytic solution can improve the cycle characteristics of the electrochemical device.
続いて、非水電解液二次電池1の製造方法を説明する。非水電解液二次電池1の製造方法は、正極6を得る第1の工程と、負極8を得る第2の工程と、電極群2を電池外装体3に収容する第3の工程と、電解液を電池外装体3に注液する第4の工程と、を備える。第1~第4の工程の順序は任意である。
Next, a method for manufacturing the non-aqueous electrolyte secondary battery 1 will be described. The manufacturing method of the non-aqueous electrolyte secondary battery 1 includes a first step of obtaining the positive electrode 6, a second step of obtaining the negative electrode 8, and a third step of accommodating the electrode group 2 in the battery outer casing 3. A fourth step of injecting the electrolytic solution into the battery exterior body 3. The order of the first to fourth steps is arbitrary.
第1の工程では、正極合剤層10に用いる材料を混練機、分散機等を用いて分散媒に分散させてスラリー状の正極合剤を得た後、この正極合剤をドクターブレード法、ディッピング法、スプレー法等により正極集電体9上に塗布し、その後分散媒を揮発させることにより正極6を得る。分散媒を揮発させた後、必要に応じて、ロールプレスによる圧縮成型工程が設けられてもよい。正極合剤層10は、上述した正極合剤の塗布から分散媒の揮発までの工程を複数回行うことにより、多層構造の正極合剤層として形成されてもよい。分散媒は、水、1-メチル-2-ピロリドン(以下、NMPともいう。)等であってよい。
In the first step, the material used for the positive electrode mixture layer 10 is dispersed in a dispersion medium using a kneader, a disperser or the like to obtain a positive electrode mixture in a slurry state, and then this positive electrode mixture is treated by a doctor blade method, The positive electrode current collector 9 is coated with a dipping method, a spray method, or the like, and then the dispersion medium is volatilized to obtain the positive electrode 6. After volatilizing the dispersion medium, a compression molding step using a roll press may be provided, if necessary. The positive electrode mixture layer 10 may be formed as a positive electrode mixture layer having a multi-layer structure by performing the above-described steps from the application of the positive electrode mixture to the volatilization of the dispersion medium a plurality of times. The dispersion medium may be water, 1-methyl-2-pyrrolidone (hereinafter, also referred to as NMP), or the like.
第2の工程は、上述した第1の工程と同様であってよく、負極集電体11に負極合剤層12を形成する方法は、上述した第1の工程と同様の方法であってよい。
The second step may be the same as the above-mentioned first step, and the method of forming the negative electrode mixture layer 12 on the negative electrode current collector 11 may be the same method as the above-mentioned first step. ..
第3の工程では、作製した正極6及び負極8の間にセパレータ7を挟み、電極群2を形成する。次いで、この電極群2を電池外装体3に収容する。
In the third step, the separator 7 is sandwiched between the produced positive electrode 6 and negative electrode 8 to form the electrode group 2. Next, the electrode group 2 is housed in the battery case 3.
第4の工程では、電解液を電池外装体3に注入する。電解液は、例えば、電解質塩をはじめに溶媒に溶解させてから、その他の材料を溶解させることにより調製することができる。
In the fourth step, the electrolytic solution is injected into the battery exterior body 3. The electrolytic solution can be prepared, for example, by first dissolving an electrolyte salt in a solvent and then dissolving other materials.
他の実施形態として、電気化学デバイスはキャパシタであってもよい。キャパシタは、上述した非水電解液二次電池1と同様に、正極、負極及びセパレータから構成される電極群と、電極群を収容する袋状の電池外装体とを備えていてよい。キャパシタにおける各構成要素の詳細は、非水電解液二次電池1と同様であってよい。
In another embodiment, the electrochemical device may be a capacitor. Like the above-described non-aqueous electrolyte secondary battery 1, the capacitor may include an electrode group including a positive electrode, a negative electrode, and a separator, and a bag-shaped battery exterior body that houses the electrode group. The details of each component of the capacitor may be the same as those of the non-aqueous electrolyte secondary battery 1.
以下、実施例により本発明を具体的に説明するが、本発明はこれらの実施例に限定されるものではない。
Hereinafter, the present invention will be specifically described with reference to examples, but the present invention is not limited to these examples.
(実施例1)
[正極の作製]
正極活物質としてのコバルト酸リチウム(95質量%)に、導電剤としての繊維状の黒鉛(1質量%)及びアセチレンブラック(AB)(1質量%)と、結着剤(3質量%)とを順次添加し、混合した。得られた混合物に対し、分散媒としてのNMPを添加し、混練することによりスラリー状の正極合剤を調製した。この正極合剤を正極集電体としての厚さ20μmのアルミニウム箔に均等かつ均質に所定量塗布した。その後、分散媒を揮発させてから、プレスすることにより密度3.6g/cm3まで圧密化して、正極を得た。 (Example 1)
[Production of positive electrode]
Lithium cobalt oxide (95% by mass) as a positive electrode active material, fibrous graphite (1% by mass) and acetylene black (AB) (1% by mass) as a conductive agent, and a binder (3% by mass). Were sequentially added and mixed. NMP as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry-like positive electrode mixture. A predetermined amount of this positive electrode mixture was applied uniformly and uniformly to an aluminum foil having a thickness of 20 μm as a positive electrode current collector. Then, the dispersion medium was volatilized and then pressed to consolidate it to a density of 3.6 g/cm 3 to obtain a positive electrode.
[正極の作製]
正極活物質としてのコバルト酸リチウム(95質量%)に、導電剤としての繊維状の黒鉛(1質量%)及びアセチレンブラック(AB)(1質量%)と、結着剤(3質量%)とを順次添加し、混合した。得られた混合物に対し、分散媒としてのNMPを添加し、混練することによりスラリー状の正極合剤を調製した。この正極合剤を正極集電体としての厚さ20μmのアルミニウム箔に均等かつ均質に所定量塗布した。その後、分散媒を揮発させてから、プレスすることにより密度3.6g/cm3まで圧密化して、正極を得た。 (Example 1)
[Production of positive electrode]
Lithium cobalt oxide (95% by mass) as a positive electrode active material, fibrous graphite (1% by mass) and acetylene black (AB) (1% by mass) as a conductive agent, and a binder (3% by mass). Were sequentially added and mixed. NMP as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry-like positive electrode mixture. A predetermined amount of this positive electrode mixture was applied uniformly and uniformly to an aluminum foil having a thickness of 20 μm as a positive electrode current collector. Then, the dispersion medium was volatilized and then pressed to consolidate it to a density of 3.6 g/cm 3 to obtain a positive electrode.
[負極の作製]
負極活物質としての黒鉛に、結着剤と、増粘剤としてのカルボキシメチルセルロースとを添加した。これらの質量比については、負極活物質:結着剤:増粘剤=98:1:1とした。得られた混合物に対し、分散媒としての水を添加し、混練することによりスラリー状の負極合剤を調製した。この負極合剤を負極集電体としての厚さ10μmの圧延銅箔に均等かつ均質に所定量塗布した。その後、分散媒を揮発させてから、プレスすることにより密度1.6g/cm3まで圧密化して、負極を得た。 [Fabrication of negative electrode]
A binder and carboxymethyl cellulose as a thickener were added to graphite as a negative electrode active material. Regarding the mass ratio of these materials, the negative electrode active material:binder:thickener=98:1:1. Water as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry negative electrode mixture. A predetermined amount of this negative electrode mixture was uniformly and uniformly applied to a rolled copper foil having a thickness of 10 μm as a negative electrode current collector. After that, the dispersion medium was volatilized and then pressed to consolidate to a density of 1.6 g/cm 3 to obtain a negative electrode.
負極活物質としての黒鉛に、結着剤と、増粘剤としてのカルボキシメチルセルロースとを添加した。これらの質量比については、負極活物質:結着剤:増粘剤=98:1:1とした。得られた混合物に対し、分散媒としての水を添加し、混練することによりスラリー状の負極合剤を調製した。この負極合剤を負極集電体としての厚さ10μmの圧延銅箔に均等かつ均質に所定量塗布した。その後、分散媒を揮発させてから、プレスすることにより密度1.6g/cm3まで圧密化して、負極を得た。 [Fabrication of negative electrode]
A binder and carboxymethyl cellulose as a thickener were added to graphite as a negative electrode active material. Regarding the mass ratio of these materials, the negative electrode active material:binder:thickener=98:1:1. Water as a dispersion medium was added to the obtained mixture, and the mixture was kneaded to prepare a slurry negative electrode mixture. A predetermined amount of this negative electrode mixture was uniformly and uniformly applied to a rolled copper foil having a thickness of 10 μm as a negative electrode current collector. After that, the dispersion medium was volatilized and then pressed to consolidate to a density of 1.6 g/cm 3 to obtain a negative electrode.
[リチウムイオン二次電池の作製]
13.5cm2の四角形に切断した正極電極を、セパレータであるポリエチレン製多孔質シート(商品名:ハイポア(登録商標)、旭化成株式会社製、厚さ30μm)で挟み、更に14.3cm2の四角形に切断した負極を重ね合わせて電極群を作製した。この電極群を、アルミニウム製のラミネートフィルム(商品名:アルミラミネートフィルム、大日本印刷株式会社製)で形成された容器(電池外装体)に収容した。次いで、容器の中に電解液を1mL添加し、容器を熱溶着させ、評価用のリチウムイオン二次電池を作製した。電解液としては、1mol/LのLiPF6を含むエチレンカーボネート、ジメチルカーボネート及びエチルメチルカーボネートの混合溶液に、混合溶液全量に対して、ビニレンカーボネート(VC)を1質量%、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)を1質量%と、下記式(6)で表される化合物Aを1質量%及びスクシノニトリルを1質量%(電解液全量基準)添加したものを使用した。
[Preparation of lithium-ion secondary battery]
The positive electrode cut into a 13.5 cm 2 square was sandwiched by a polyethylene porous sheet (trade name: Hypore (registered trademark), manufactured by Asahi Kasei Co., Ltd., thickness: 30 μm), which is a separator, and further a 14.3 cm 2 square. The negative electrodes cut into pieces were stacked to prepare an electrode group. This electrode group was housed in a container (battery outer casing) formed of an aluminum laminate film (trade name: aluminum laminate film, manufactured by Dai Nippon Printing Co., Ltd.). Next, 1 mL of the electrolytic solution was added to the container, and the container was heat-welded to produce a lithium ion secondary battery for evaluation. As the electrolytic solution, a mixed solution of ethylene carbonate, dimethyl carbonate and ethylmethyl carbonate containing 1 mol/L of LiPF 6 , 1% by mass of vinylene carbonate (VC) based on the total amount of the mixed solution, 4-fluoro-1, Addition of 1% by mass of 3-dioxolan-2-one (fluoroethylene carbonate; FEC), 1% by mass of compound A represented by the following formula (6) and 1% by mass of succinonitrile (on the basis of the total amount of electrolytic solution). I used what I did.
13.5cm2の四角形に切断した正極電極を、セパレータであるポリエチレン製多孔質シート(商品名:ハイポア(登録商標)、旭化成株式会社製、厚さ30μm)で挟み、更に14.3cm2の四角形に切断した負極を重ね合わせて電極群を作製した。この電極群を、アルミニウム製のラミネートフィルム(商品名:アルミラミネートフィルム、大日本印刷株式会社製)で形成された容器(電池外装体)に収容した。次いで、容器の中に電解液を1mL添加し、容器を熱溶着させ、評価用のリチウムイオン二次電池を作製した。電解液としては、1mol/LのLiPF6を含むエチレンカーボネート、ジメチルカーボネート及びエチルメチルカーボネートの混合溶液に、混合溶液全量に対して、ビニレンカーボネート(VC)を1質量%、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)を1質量%と、下記式(6)で表される化合物Aを1質量%及びスクシノニトリルを1質量%(電解液全量基準)添加したものを使用した。
The positive electrode cut into a 13.5 cm 2 square was sandwiched by a polyethylene porous sheet (trade name: Hypore (registered trademark), manufactured by Asahi Kasei Co., Ltd., thickness: 30 μm), which is a separator, and further a 14.3 cm 2 square. The negative electrodes cut into pieces were stacked to prepare an electrode group. This electrode group was housed in a container (battery outer casing) formed of an aluminum laminate film (trade name: aluminum laminate film, manufactured by Dai Nippon Printing Co., Ltd.). Next, 1 mL of the electrolytic solution was added to the container, and the container was heat-welded to produce a lithium ion secondary battery for evaluation. As the electrolytic solution, a mixed solution of ethylene carbonate, dimethyl carbonate and ethylmethyl carbonate containing 1 mol/L of LiPF 6 , 1% by mass of vinylene carbonate (VC) based on the total amount of the mixed solution, 4-fluoro-1, Addition of 1% by mass of 3-dioxolan-2-one (fluoroethylene carbonate; FEC), 1% by mass of compound A represented by the following formula (6) and 1% by mass of succinonitrile (on the basis of the total amount of electrolytic solution). I used what I did.
(実施例2)
実施例1において、化合物Aの添加量を、電解液全量基準で2質量%としたこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Example 2)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the amount of the compound A added was 2% by mass based on the total amount of the electrolytic solution.
実施例1において、化合物Aの添加量を、電解液全量基準で2質量%としたこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Example 2)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the amount of the compound A added was 2% by mass based on the total amount of the electrolytic solution.
(実施例3)
実施例1において、化合物Aの代わりに下記式(7)で表される化合物Bを、電解液全量基準で0.5質量%添加したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。
(Example 3)
A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound B represented by the following formula (7) was added in place of Compound A in an amount of 0.5% by mass based on the total amount of the electrolytic solution. A battery was produced.
実施例1において、化合物Aの代わりに下記式(7)で表される化合物Bを、電解液全量基準で0.5質量%添加したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。
A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound B represented by the following formula (7) was added in place of Compound A in an amount of 0.5% by mass based on the total amount of the electrolytic solution. A battery was produced.
(実施例4)
実施例1において、化合物Aの代わりに下記式(8)で表される化合物Cを、電解液全量基準で0.1質量%添加したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。
(Example 4)
A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound C represented by the following formula (8) was added in place of Compound A in an amount of 0.1% by mass based on the total amount of the electrolytic solution. A battery was produced.
実施例1において、化合物Aの代わりに下記式(8)で表される化合物Cを、電解液全量基準で0.1質量%添加したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。
A lithium ion secondary battery was prepared in the same manner as in Example 1 except that Compound C represented by the following formula (8) was added in place of Compound A in an amount of 0.1% by mass based on the total amount of the electrolytic solution. A battery was produced.
(実施例5)
実施例1において、スクシノニトリルの代わりにアジポニトリルを使用したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Example 5)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that adiponitrile was used instead of succinonitrile.
実施例1において、スクシノニトリルの代わりにアジポニトリルを使用したこと以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Example 5)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that adiponitrile was used instead of succinonitrile.
(比較例1)
実施例1において、化合物A及びスクシノニトリルを使用しなかった以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Comparative Example 1)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the compound A and succinonitrile were not used.
実施例1において、化合物A及びスクシノニトリルを使用しなかった以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Comparative Example 1)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the compound A and succinonitrile were not used.
(比較例2)
実施例1において、化合物Aを使用しなかった(スクシノニトリルは1質量%添加した)以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Comparative example 2)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the compound A was not used (1% by mass of succinonitrile was added).
実施例1において、化合物Aを使用しなかった(スクシノニトリルは1質量%添加した)以外は、実施例1と同様にしてリチウムイオン二次電池を作製した。 (Comparative example 2)
A lithium ion secondary battery was produced in the same manner as in Example 1 except that the compound A was not used (1% by mass of succinonitrile was added).
[初回充放電]
作製したリチウムイオン電池について、以下に示す方法で初回充放電を実施した。まず、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この充放電サイクルを3回繰り返した(電流値の単位として用いた「C」とは、「電流値(A)/電池容量(Ah)」を意味する。)。3サイクル目の放電容量をこの電池の容量Q1とした。 [First charge/discharge]
The lithium-ion battery thus prepared was subjected to initial charge/discharge by the method described below. First, in a 25° C. environment, constant current charging was performed at a current value of 0.1 C up to an upper limit voltage of 4.45 V, and then constant voltage charging was performed at 4.45 V. The charge termination condition was a current value of 0.01C. After that, constant current discharge with a final voltage of 2.5 V was performed at a current value of 0.1 C. This charging/discharging cycle was repeated 3 times (“C” used as a unit of current value means “current value (A)/battery capacity (Ah)”). The discharge capacity at the third cycle was defined as the capacity Q1 of this battery.
作製したリチウムイオン電池について、以下に示す方法で初回充放電を実施した。まず、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この充放電サイクルを3回繰り返した(電流値の単位として用いた「C」とは、「電流値(A)/電池容量(Ah)」を意味する。)。3サイクル目の放電容量をこの電池の容量Q1とした。 [First charge/discharge]
The lithium-ion battery thus prepared was subjected to initial charge/discharge by the method described below. First, in a 25° C. environment, constant current charging was performed at a current value of 0.1 C up to an upper limit voltage of 4.45 V, and then constant voltage charging was performed at 4.45 V. The charge termination condition was a current value of 0.01C. After that, constant current discharge with a final voltage of 2.5 V was performed at a current value of 0.1 C. This charging/discharging cycle was repeated 3 times (“C” used as a unit of current value means “current value (A)/battery capacity (Ah)”). The discharge capacity at the third cycle was defined as the capacity Q1 of this battery.
[高温保存試験(1)]
実施例1~5、比較例1~2の各二次電池を、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、それらの二次電池を60℃の恒温槽中で1週間保管した。 [High temperature storage test (1)]
Each of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 was subjected to constant current charging up to an upper limit voltage of 4.45 V at a current value of 0.1 C under an environment of 25° C., and then at 4.45 V. Voltage charging was performed. The charge termination condition was a current value of 0.01C. Then, the secondary batteries were stored for 1 week in a constant temperature bath at 60°C.
実施例1~5、比較例1~2の各二次電池を、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、それらの二次電池を60℃の恒温槽中で1週間保管した。 [High temperature storage test (1)]
Each of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 was subjected to constant current charging up to an upper limit voltage of 4.45 V at a current value of 0.1 C under an environment of 25° C., and then at 4.45 V. Voltage charging was performed. The charge termination condition was a current value of 0.01C. Then, the secondary batteries were stored for 1 week in a constant temperature bath at 60°C.
[体積増加率の測定]
実施例1~5、比較例1~2の各二次電池の体積をアルキメデス法に基づく比重計(電子比重計MDS-300、アルファミラージュ社製)により測定した。高温保存試験前の二次電池の体積(V1)、及び高温保存試験後25℃の環境下に30分間保持した二次電池の体積(V2)から、体積増加率を下記の式により算出した。これにより、二次電池の膨張の程度を評価した。結果を表1に示す。
体積増加率(%)=V2/V1×100 [Measurement of volume increase rate]
The volumes of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 were measured by a hydrometer based on the Archimedes method (electronic hydrometer MDS-300, manufactured by Alpha Mirage). The volume increase rate was calculated by the following formula from the volume (V1) of the secondary battery before the high temperature storage test and the volume (V2) of the secondary battery kept for 30 minutes in the environment of 25° C. after the high temperature storage test. From this, the degree of expansion of the secondary battery was evaluated. The results are shown in Table 1.
Volume increase rate (%)=V2/V1×100
実施例1~5、比較例1~2の各二次電池の体積をアルキメデス法に基づく比重計(電子比重計MDS-300、アルファミラージュ社製)により測定した。高温保存試験前の二次電池の体積(V1)、及び高温保存試験後25℃の環境下に30分間保持した二次電池の体積(V2)から、体積増加率を下記の式により算出した。これにより、二次電池の膨張の程度を評価した。結果を表1に示す。
体積増加率(%)=V2/V1×100 [Measurement of volume increase rate]
The volumes of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 were measured by a hydrometer based on the Archimedes method (electronic hydrometer MDS-300, manufactured by Alpha Mirage). The volume increase rate was calculated by the following formula from the volume (V1) of the secondary battery before the high temperature storage test and the volume (V2) of the secondary battery kept for 30 minutes in the environment of 25° C. after the high temperature storage test. From this, the degree of expansion of the secondary battery was evaluated. The results are shown in Table 1.
Volume increase rate (%)=V2/V1×100
[容量維持率及び回復率の測定]
高温保存試験後の実施例1~5、比較例1~2の各二次電池を恒温槽から取り出し、25℃の環境下に30分保持した後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この時の放電容量をQ2とした。次に、0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この時の放電容量をQ3とした。上述したQ1、Q2、Q3を用いて、容量維持率及び容量回復率を以下の式を用いて算出した。結果を表1に示す。
(容量維持率)(%)=Q2/Q1×100
(容量回復率)(%)=Q3/Q1×100 [Measurement of capacity maintenance rate and recovery rate]
After the high temperature storage test, each of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 was taken out from the constant temperature bath and kept in an environment of 25° C. for 30 minutes, and then cut off at a current value of 0.1 C and a final voltage of 2. A constant current discharge of 5V was performed. The discharge capacity at this time was defined as Q2. Next, constant current charging was performed at a current value of 0.1 C up to an upper limit voltage of 4.45 V, and then constant voltage charging was performed at 4.45 V. The charge termination condition was a current value of 0.01C. After that, constant current discharge with a final voltage of 2.5 V was performed at a current value of 0.1 C. The discharge capacity at this time was set to Q3. Using Q1, Q2, and Q3 described above, the capacity retention rate and capacity recovery rate were calculated using the following formulas. The results are shown in Table 1.
(Capacity maintenance rate) (%)=Q2/Q1×100
(Capacity recovery rate) (%)=Q3/Q1×100
高温保存試験後の実施例1~5、比較例1~2の各二次電池を恒温槽から取り出し、25℃の環境下に30分保持した後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この時の放電容量をQ2とした。次に、0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、0.1Cの電流値で終止電圧2.5Vの定電流放電を行った。この時の放電容量をQ3とした。上述したQ1、Q2、Q3を用いて、容量維持率及び容量回復率を以下の式を用いて算出した。結果を表1に示す。
(容量維持率)(%)=Q2/Q1×100
(容量回復率)(%)=Q3/Q1×100 [Measurement of capacity maintenance rate and recovery rate]
After the high temperature storage test, each of the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 was taken out from the constant temperature bath and kept in an environment of 25° C. for 30 minutes, and then cut off at a current value of 0.1 C and a final voltage of 2. A constant current discharge of 5V was performed. The discharge capacity at this time was defined as Q2. Next, constant current charging was performed at a current value of 0.1 C up to an upper limit voltage of 4.45 V, and then constant voltage charging was performed at 4.45 V. The charge termination condition was a current value of 0.01C. After that, constant current discharge with a final voltage of 2.5 V was performed at a current value of 0.1 C. The discharge capacity at this time was set to Q3. Using Q1, Q2, and Q3 described above, the capacity retention rate and capacity recovery rate were calculated using the following formulas. The results are shown in Table 1.
(Capacity maintenance rate) (%)=Q2/Q1×100
(Capacity recovery rate) (%)=Q3/Q1×100
[サイクル特性の評価]
初回充放電後に、充放電を繰り返すサイクル試験によって、各二次電池のサイクル特性を評価した。充電パターンとしては、45℃の環境下で、実施例1~5及び比較例1~2の二次電池を0.5Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.05Cとした。放電については、1Cで定電流放電を2.5Vまで行い、放電容量を求めた。この一連の充放電を100サイクル繰り返し、充放電の度に放電容量を測定した。1サイクル目の充放電後の放電容量に対する、100サイクル後の放電容量の相対値(放電容量維持率(%))を求めた。結果を表1に示す。 [Evaluation of cycle characteristics]
After the initial charge/discharge, the cycle characteristics of each secondary battery were evaluated by a cycle test in which charge/discharge was repeated. Regarding the charging pattern, in the environment of 45° C., the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 were subjected to constant current charging at a current value of 0.5 C up to an upper limit voltage of 4.45 V, and then 4 Constant voltage charging was performed at 0.45V. The charge termination condition was a current value of 0.05C. Regarding discharge, constant current discharge was performed up to 2.5 V at 1 C, and the discharge capacity was obtained. This series of charging/discharging was repeated 100 cycles, and the discharging capacity was measured every charging/discharging. The relative value (discharge capacity retention rate (%)) of the discharge capacity after 100 cycles with respect to the discharge capacity after charge and discharge in the first cycle was determined. The results are shown in Table 1.
初回充放電後に、充放電を繰り返すサイクル試験によって、各二次電池のサイクル特性を評価した。充電パターンとしては、45℃の環境下で、実施例1~5及び比較例1~2の二次電池を0.5Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.05Cとした。放電については、1Cで定電流放電を2.5Vまで行い、放電容量を求めた。この一連の充放電を100サイクル繰り返し、充放電の度に放電容量を測定した。1サイクル目の充放電後の放電容量に対する、100サイクル後の放電容量の相対値(放電容量維持率(%))を求めた。結果を表1に示す。 [Evaluation of cycle characteristics]
After the initial charge/discharge, the cycle characteristics of each secondary battery were evaluated by a cycle test in which charge/discharge was repeated. Regarding the charging pattern, in the environment of 45° C., the secondary batteries of Examples 1 to 5 and Comparative Examples 1 and 2 were subjected to constant current charging at a current value of 0.5 C up to an upper limit voltage of 4.45 V, and then 4 Constant voltage charging was performed at 0.45V. The charge termination condition was a current value of 0.05C. Regarding discharge, constant current discharge was performed up to 2.5 V at 1 C, and the discharge capacity was obtained. This series of charging/discharging was repeated 100 cycles, and the discharging capacity was measured every charging/discharging. The relative value (discharge capacity retention rate (%)) of the discharge capacity after 100 cycles with respect to the discharge capacity after charge and discharge in the first cycle was determined. The results are shown in Table 1.
表1に示すように、化合物A、化合物B又は化合物C、及びニトリル化合物(スクシノニトリル、又はアジポニトリル)を含有する実施例1~5の電解液を適用したリチウムイオン二次電池は、化合物A、化合物B又は化合物C及びニトリル化合物の少なくとも一方を含まない電解液を適用した比較例1~2のリチウムイオン二次電池と比較して、高温保存試験での体積増加率が小さく、容量維持率及び回復率が向上することが明らかになった。なお、比較例1に記載のリチウムイオン二次電池は劣化の進行が早く、試験途中で短絡した。また、実施例1~5の電解液を適用したリチウムイオン二次電池は、比較例1~2のリチウムイオン二次電池よりもサイクル試験での容量維持率が高いことが明らかになった。この理由としては、ニトリル化合物が正極にて安定な被膜を形成したことに加えて、化合物A、化合物B又は化合物Cが、電解液の安定化に寄与したためと考えられる。
As shown in Table 1, the lithium ion secondary batteries to which the electrolytic solutions of Examples 1 to 5 containing the compound A, the compound B or the compound C, and the nitrile compound (succinonitrile or adiponitrile) were applied were the compound A In comparison with the lithium ion secondary batteries of Comparative Examples 1 and 2 to which an electrolytic solution containing at least one of Compound B or Compound C and a nitrile compound is applied, the volume increase rate in the high temperature storage test is small, and the capacity retention rate is small. It was also found that the recovery rate is improved. The lithium-ion secondary battery described in Comparative Example 1 accelerated the deterioration and short-circuited during the test. Further, it was revealed that the lithium ion secondary batteries to which the electrolytic solutions of Examples 1 to 5 were applied had a higher capacity retention rate in the cycle test than the lithium ion secondary batteries of Comparative Examples 1 and 2. It is considered that this is because the nitrile compound formed a stable film on the positive electrode, and the compound A, the compound B, or the compound C contributed to the stabilization of the electrolytic solution.
(実施例6)
[正極、負極の作製]
実施例1と同様の方法により、正極及び負極を得た。 (Example 6)
[Preparation of positive electrode and negative electrode]
A positive electrode and a negative electrode were obtained in the same manner as in Example 1.
[正極、負極の作製]
実施例1と同様の方法により、正極及び負極を得た。 (Example 6)
[Preparation of positive electrode and negative electrode]
A positive electrode and a negative electrode were obtained in the same manner as in Example 1.
[リチウムイオン二次電池の作製]
13.5cm2の四角形に切断した正極電極を、セパレータであるポリエチレン製多孔質シート(商品名:ハイポア(登録商標)、旭化成株式会社製、厚さ30μm)で挟み、更に14.3cm2の四角形に切断した負極を重ね合わせて電極群を作製した。この電極群を、アルミニウム製のラミネートフィルム(商品名:アルミラミネートフィルム、大日本印刷株式会社製)で形成された容器(電池外装体)に収容した。次いで、容器の中に電解液を1mL添加し、容器を熱溶着させ、評価用のリチウムイオン二次電池を作製した。電解液としては、1mol/LのLiPF6を含むエチレンカーボネート、ジメチルカーボネート及びジエチルカーボネートの混合溶液に、混合溶液全量に対してビニレンカーボネート(VC)を1質量%、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)を0.5質量%と、上記式(6)で表される化合物Aを0.5質量%、及びスクシノニトリルを0.5質量%(電解液全量基準)添加したものを使用した。 [Preparation of lithium-ion secondary battery]
The positive electrode cut into a 13.5 cm 2 square was sandwiched by a polyethylene porous sheet (trade name: Hypore (registered trademark), manufactured by Asahi Kasei Co., Ltd., thickness: 30 μm), which is a separator, and further a 14.3 cm 2 square. The negative electrodes cut into pieces were stacked to prepare an electrode group. This electrode group was housed in a container (battery outer casing) formed of an aluminum laminate film (trade name: aluminum laminate film, manufactured by Dai Nippon Printing Co., Ltd.). Next, 1 mL of the electrolytic solution was added to the container, and the container was heat-welded to produce a lithium ion secondary battery for evaluation. As the electrolytic solution, a mixed solution of ethylene carbonate, dimethyl carbonate and diethyl carbonate containing 1 mol/L of LiPF 6 , 1 mass% of vinylene carbonate (VC) based on the total amount of the mixed solution, 4-fluoro-1,3- 0.5 mass% of dioxolan-2-one (fluoroethylene carbonate; FEC), 0.5 mass% of compound A represented by the above formula (6), and 0.5 mass% of succinonitrile (electrolysis The total amount of the liquid) was used.
13.5cm2の四角形に切断した正極電極を、セパレータであるポリエチレン製多孔質シート(商品名:ハイポア(登録商標)、旭化成株式会社製、厚さ30μm)で挟み、更に14.3cm2の四角形に切断した負極を重ね合わせて電極群を作製した。この電極群を、アルミニウム製のラミネートフィルム(商品名:アルミラミネートフィルム、大日本印刷株式会社製)で形成された容器(電池外装体)に収容した。次いで、容器の中に電解液を1mL添加し、容器を熱溶着させ、評価用のリチウムイオン二次電池を作製した。電解液としては、1mol/LのLiPF6を含むエチレンカーボネート、ジメチルカーボネート及びジエチルカーボネートの混合溶液に、混合溶液全量に対してビニレンカーボネート(VC)を1質量%、4-フルオロ-1,3-ジオキソラン-2-オン(フルオロエチレンカーボネート;FEC)を0.5質量%と、上記式(6)で表される化合物Aを0.5質量%、及びスクシノニトリルを0.5質量%(電解液全量基準)添加したものを使用した。 [Preparation of lithium-ion secondary battery]
The positive electrode cut into a 13.5 cm 2 square was sandwiched by a polyethylene porous sheet (trade name: Hypore (registered trademark), manufactured by Asahi Kasei Co., Ltd., thickness: 30 μm), which is a separator, and further a 14.3 cm 2 square. The negative electrodes cut into pieces were stacked to prepare an electrode group. This electrode group was housed in a container (battery outer casing) formed of an aluminum laminate film (trade name: aluminum laminate film, manufactured by Dai Nippon Printing Co., Ltd.). Next, 1 mL of the electrolytic solution was added to the container, and the container was heat-welded to produce a lithium ion secondary battery for evaluation. As the electrolytic solution, a mixed solution of ethylene carbonate, dimethyl carbonate and diethyl carbonate containing 1 mol/L of LiPF 6 , 1 mass% of vinylene carbonate (VC) based on the total amount of the mixed solution, 4-fluoro-1,3- 0.5 mass% of dioxolan-2-one (fluoroethylene carbonate; FEC), 0.5 mass% of compound A represented by the above formula (6), and 0.5 mass% of succinonitrile (electrolysis The total amount of the liquid) was used.
[高温保存試験(2)]
実施例1、及び上述した比較例1~2の各二次電池について、上述した初回充放電を行った後、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、それらの二次電池を80℃の恒温槽中で4時間保管した。 [High temperature storage test (2)]
For each of the secondary batteries of Example 1 and Comparative Examples 1 and 2 described above, after performing the initial charge and discharge described above, constant current charging was performed at a current value of 0.1 C under an environment of 25° C. to obtain an upper limit voltage of 4. It was performed up to 45V, and then constant voltage charging was performed at 4.45V. The charge termination condition was a current value of 0.01C. After that, these secondary batteries were stored for 4 hours in an 80° C. constant temperature bath.
実施例1、及び上述した比較例1~2の各二次電池について、上述した初回充放電を行った後、25℃の環境下において0.1Cの電流値で定電流充電を上限電圧4.45Vまで行い、続いて4.45Vで定電圧充電を行った。充電終止条件は、電流値0.01Cとした。その後、それらの二次電池を80℃の恒温槽中で4時間保管した。 [High temperature storage test (2)]
For each of the secondary batteries of Example 1 and Comparative Examples 1 and 2 described above, after performing the initial charge and discharge described above, constant current charging was performed at a current value of 0.1 C under an environment of 25° C. to obtain an upper limit voltage of 4. It was performed up to 45V, and then constant voltage charging was performed at 4.45V. The charge termination condition was a current value of 0.01C. After that, these secondary batteries were stored for 4 hours in an 80° C. constant temperature bath.
[体積増加率の測定]
上述した方法と同様の方法により、高温保存試験前の体積(V1)及び高温保存試験後25℃の環境下に30分間保持した二次電池の体積(V2)から、実施例6、及び比較例1~2の高温保存試験後の体積増加率を算出した。 [Measurement of volume increase rate]
By the same method as the above-mentioned method, from the volume before the high temperature storage test (V1) and the volume of the secondary battery (V2) kept for 30 minutes in the environment of 25° C. after the high temperature storage test, Example 6 and Comparative Example The volume increase rate after the high temperature storage test of 1-2 was calculated.
上述した方法と同様の方法により、高温保存試験前の体積(V1)及び高温保存試験後25℃の環境下に30分間保持した二次電池の体積(V2)から、実施例6、及び比較例1~2の高温保存試験後の体積増加率を算出した。 [Measurement of volume increase rate]
By the same method as the above-mentioned method, from the volume before the high temperature storage test (V1) and the volume of the secondary battery (V2) kept for 30 minutes in the environment of 25° C. after the high temperature storage test, Example 6 and Comparative Example The volume increase rate after the high temperature storage test of 1-2 was calculated.
その結果、実施例1の体積増加率は102.3%、比較例1の体積増加率は107.8%、比較例2の体積増加率は103.7%であった。スクシノニトリルを含み化合物Aを含まない電解液を適用した比較例2のリチウムイオン二次電池は、化合物A及びスクシノニトリルのどちらも含まない電解液を適用した比較例1のリチウムイオン二次電池と比較して、体積増加率が低減した。これは、比較例2のリチウムイオン二次電池では、高温(80℃)の環境下で発生した電解液由来のガスが低減したためと考えられる。一方、化合物A及びスクシノニトリルを両方含む電解液を適用した実施例6のリチウムイオン二次電池は、比較例1、2のリチウムイオン二次電池と比較して体積増加率が更に低減した。
As a result, the volume increase rate of Example 1 was 102.3%, the volume increase rate of Comparative Example 1 was 107.8%, and the volume increase rate of Comparative Example 2 was 103.7%. The lithium ion secondary battery of Comparative Example 2 to which the electrolytic solution containing succinonitrile and not containing Compound A was applied was the lithium ion secondary battery of Comparative Example 1 to which the electrolytic solution containing neither Compound A nor succinonitrile was applied. The volume increase rate was reduced as compared with the battery. It is considered that this is because in the lithium ion secondary battery of Comparative Example 2, the gas derived from the electrolytic solution generated under a high temperature (80° C.) environment was reduced. On the other hand, in the lithium ion secondary battery of Example 6 to which the electrolytic solution containing both the compound A and succinonitrile was applied, the volume increase rate was further reduced as compared with the lithium ion secondary batteries of Comparative Examples 1 and 2.
[容量維持率の測定]
80℃の恒温槽中で4時間保管した実施例6、比較例1~2の各二次電池を恒温槽から取り出し、25℃の環境下に30分間保持した後、上述した方法と同様の方法により、実施例6、及び比較例1~2の高温保存試験後の容量維持率を算出した。 [Measurement of capacity retention rate]
The secondary batteries of Example 6 and Comparative Examples 1 and 2 stored for 4 hours in a constant temperature bath of 80° C. were taken out from the constant temperature bath and kept in an environment of 25° C. for 30 minutes, and then the same method as the above-mentioned method. Thus, the capacity retention rate after the high temperature storage test of Example 6 and Comparative Examples 1 and 2 was calculated.
80℃の恒温槽中で4時間保管した実施例6、比較例1~2の各二次電池を恒温槽から取り出し、25℃の環境下に30分間保持した後、上述した方法と同様の方法により、実施例6、及び比較例1~2の高温保存試験後の容量維持率を算出した。 [Measurement of capacity retention rate]
The secondary batteries of Example 6 and Comparative Examples 1 and 2 stored for 4 hours in a constant temperature bath of 80° C. were taken out from the constant temperature bath and kept in an environment of 25° C. for 30 minutes, and then the same method as the above-mentioned method. Thus, the capacity retention rate after the high temperature storage test of Example 6 and Comparative Examples 1 and 2 was calculated.
その結果、実施例6の容量維持率は95.8%、比較例1の容量維持率は94.5%、比較例2の容量維持率は94.8%であった。化合物A及びスクシノニトリルを両方含む電解液を適用した実施例1のリチウムイオン二次電池は、化合物A及びスクシノニトリルのどちらも含まない電解液を適用した比較例1のリチウムイオン電池、及びスクシノニトリルを含み化合物Aを含まない電解液を適用した比較例2のリチウムイオン二次電池と比較して、容量維持率が良好であった。この理由としては、ニトリル化合物が正極にて安定な被膜を形成したことに加えて、化合物Aが電解液の安定化に寄与したためと考えられる。また、正極又は負極での副反応が抑制され、電解液が安定化されたためと考えられる。
As a result, the capacity retention rate of Example 6 was 95.8%, the capacity retention rate of Comparative Example 1 was 94.5%, and the capacity retention rate of Comparative Example 2 was 94.8%. The lithium ion secondary battery of Example 1 to which the electrolytic solution containing both the compound A and succinonitrile was applied was the lithium ion battery of Comparative Example 1 to which the electrolytic solution containing neither compound A nor succinonitrile was applied, and The capacity retention rate was better than that of the lithium ion secondary battery of Comparative Example 2 to which the electrolytic solution containing succinonitrile and containing no compound A was applied. The reason for this is considered that, in addition to the nitrile compound forming a stable film on the positive electrode, the compound A contributed to the stabilization of the electrolytic solution. It is also considered that the side reaction at the positive electrode or the negative electrode was suppressed and the electrolytic solution was stabilized.
以上のように、化合物A、化合物B又は化合物Cと、ニトリル化合物とを両方含む電解液を適用した実施例1~6のリチウムイオン二次電池は、ニトリル化合物及び化合物Aを含まない電解液を適用した比較例1のリチウムイオン二次電池、及びニトリル化合物を含み化合物Aを含まない電解液を適用した比較例2のリチウムイオン二次電池に比べて、優れた性能を示している。
As described above, the lithium ion secondary batteries of Examples 1 to 6 to which the electrolytic solution containing both the compound A, the compound B or the compound C, and the nitrile compound were applied were prepared by using the electrolytic solution containing neither the nitrile compound nor the compound A. Compared to the applied lithium ion secondary battery of Comparative Example 1 and the lithium ion secondary battery of Comparative Example 2 to which the electrolytic solution containing the nitrile compound and not containing the compound A was applied, excellent performance is exhibited.
1…非水電解液二次電池(電気化学デバイス)、6…正極、7…セパレータ、8…負極。
1... Non-aqueous electrolyte secondary battery (electrochemical device), 6... Positive electrode, 7... Separator, 8... Negative electrode.
Claims (15)
- 下記式(1)で表される化合物と、ケイ素原子を有さないニトリル化合物と、を含有する電解液。
- 前記R1~R3の少なくとも1つはフッ素原子である、請求項1に記載の電解液。 The electrolytic solution according to claim 1, wherein at least one of R 1 to R 3 is a fluorine atom.
- 前記式(1)で表される化合物1分子中のケイ素原子の数は1個である、請求項1又は2に記載の電解液。 The electrolytic solution according to claim 1 or 2, wherein the number of silicon atoms in one molecule of the compound represented by the formula (1) is one.
- 前記R5は窒素原子を含む有機基である、請求項1~3のいずれか一項に記載の電解液。 The electrolytic solution according to any one of claims 1 to 3, wherein R 5 is an organic group containing a nitrogen atom.
- 前記R5は下記式(2)で表される基である、請求項4に記載の電解液。
- 前記R5は硫黄原子を含む有機基である、請求項1~3のいずれか一項に記載の電解液。 The electrolytic solution according to any one of claims 1 to 3, wherein R 5 is an organic group containing a sulfur atom.
- 前記R5は、下記式(3)、式(4)又は式(5)のいずれかで表される基である、請求項6に記載の電解液。
- 前記ニトリル化合物はシアノ基を2つ有する、請求項1~7のいずれか一項に記載の電解液。 The electrolytic solution according to any one of claims 1 to 7, wherein the nitrile compound has two cyano groups.
- 前記ニトリル化合物はスクシノニトリルである、請求項1~8のいずれか一項に記載の電解液。 The electrolytic solution according to any one of claims 1 to 8, wherein the nitrile compound is succinonitrile.
- 前記式(1)で表される化合物の含有量及び前記ニトリル化合物の含有量の合計は、前記電解液全量を基準として10質量%以下である、請求項1~9のいずれか一項に記載の電解液。 10. The total of the content of the compound represented by the formula (1) and the content of the nitrile compound is 10% by mass or less based on the total amount of the electrolytic solution, according to any one of claims 1 to 9. Electrolyte.
- 正極と、負極と、請求項1~10のいずれか一項に記載の電解液と、を備える電気化学デバイス。 An electrochemical device comprising a positive electrode, a negative electrode, and the electrolytic solution according to any one of claims 1 to 10.
- 前記負極は炭素材料を含有する、請求項11に記載の電気化学デバイス。 The electrochemical device according to claim 11, wherein the negative electrode contains a carbon material.
- 前記炭素材料は黒鉛を含有する、請求項12に記載の電気化学デバイス。 The electrochemical device according to claim 12, wherein the carbon material contains graphite.
- 前記負極は、ケイ素及びスズからなる群より選ばれる少なくとも1種の元素を含む材料を更に含有する、請求項12又は13に記載の電気化学デバイス。 The electrochemical device according to claim 12 or 13, wherein the negative electrode further contains a material containing at least one element selected from the group consisting of silicon and tin.
- 前記電気化学デバイスは、非水電解液二次電池又はキャパシタである、請求項11~14のいずれか一項に記載の電気化学デバイス。 The electrochemical device according to any one of claims 11 to 14, wherein the electrochemical device is a non-aqueous electrolyte secondary battery or a capacitor.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020560024A JPWO2020116583A1 (en) | 2018-12-05 | 2019-12-05 | Electrolytes and electrochemical devices |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018228480 | 2018-12-05 | ||
| JP2018-228480 | 2018-12-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020116583A1 true WO2020116583A1 (en) | 2020-06-11 |
Family
ID=70973893
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/047696 WO2020116583A1 (en) | 2018-12-05 | 2019-12-05 | Electrolyte and electrochemical device |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JPWO2020116583A1 (en) |
| TW (1) | TWI835941B (en) |
| WO (1) | WO2020116583A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112271336A (en) * | 2020-11-25 | 2021-01-26 | 广州天赐高新材料股份有限公司 | Electrolyte and lithium secondary battery |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11354118A (en) * | 1998-06-08 | 1999-12-24 | Fuji Photo Film Co Ltd | Nonaqueous secondary battery |
| WO2013062056A1 (en) * | 2011-10-28 | 2013-05-02 | 旭化成株式会社 | Non-aqueous secondary battery |
| KR20130137941A (en) * | 2012-06-08 | 2013-12-18 | 주식회사 엘지화학 | Non-aqueous electrolyte solution for lithium secondary battery and lithium secondary battery comprising the same |
| JP2015213016A (en) * | 2014-05-02 | 2015-11-26 | ソニー株式会社 | Battery, battery pack, battery module, electronic device, electric motor vehicle, power storage device and electric power system |
| JP2017062955A (en) * | 2015-09-25 | 2017-03-30 | 日清紡ホールディングス株式会社 | Additive agent for electrolytic solution |
| JP2017538667A (en) * | 2014-10-03 | 2017-12-28 | シラトロニクス,インコーポレイテッド | Functionalized silane and electrolyte compositions and electrochemical devices containing them |
-
2019
- 2019-12-05 WO PCT/JP2019/047696 patent/WO2020116583A1/en active Application Filing
- 2019-12-05 TW TW108144546A patent/TWI835941B/en active
- 2019-12-05 JP JP2020560024A patent/JPWO2020116583A1/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11354118A (en) * | 1998-06-08 | 1999-12-24 | Fuji Photo Film Co Ltd | Nonaqueous secondary battery |
| WO2013062056A1 (en) * | 2011-10-28 | 2013-05-02 | 旭化成株式会社 | Non-aqueous secondary battery |
| KR20130137941A (en) * | 2012-06-08 | 2013-12-18 | 주식회사 엘지화학 | Non-aqueous electrolyte solution for lithium secondary battery and lithium secondary battery comprising the same |
| JP2015213016A (en) * | 2014-05-02 | 2015-11-26 | ソニー株式会社 | Battery, battery pack, battery module, electronic device, electric motor vehicle, power storage device and electric power system |
| JP2017538667A (en) * | 2014-10-03 | 2017-12-28 | シラトロニクス,インコーポレイテッド | Functionalized silane and electrolyte compositions and electrochemical devices containing them |
| JP2017062955A (en) * | 2015-09-25 | 2017-03-30 | 日清紡ホールディングス株式会社 | Additive agent for electrolytic solution |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112271336A (en) * | 2020-11-25 | 2021-01-26 | 广州天赐高新材料股份有限公司 | Electrolyte and lithium secondary battery |
| CN112271336B (en) * | 2020-11-25 | 2021-08-27 | 广州天赐高新材料股份有限公司 | Electrolyte and lithium secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2020116583A1 (en) | 2021-10-21 |
| TW202032846A (en) | 2020-09-01 |
| TWI835941B (en) | 2024-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2020116583A1 (en) | Electrolyte and electrochemical device | |
| JP7415947B2 (en) | Electrolytes and electrochemical devices | |
| WO2020116574A1 (en) | Electrolyte solution and electrochemical device | |
| JP7074132B2 (en) | Electrolytes and electrochemical devices | |
| US11444325B2 (en) | Electrolytic solution and electrochemical device | |
| WO2020116578A1 (en) | Electrolyte and electrochemical device | |
| JP2020004542A (en) | Negative electrode and electrochemical device | |
| WO2023190363A1 (en) | Electrochemical device, and electrolyte for electrochemical device | |
| WO2020027003A1 (en) | Electrolyte and electrochemical device | |
| WO2023190335A1 (en) | Electrochemical device, and electrolyte solution for electrochemical device | |
| WO2020027004A1 (en) | Electrolyte and electrochemical device | |
| CN110710047B (en) | Electrolyte and electrochemical device | |
| WO2018220799A1 (en) | Electrolytic solution and electrochemical device | |
| WO2022070312A1 (en) | Electrolyte solution and electrochemical device | |
| WO2018220795A1 (en) | Electrolytic solution and electrochemical device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19894069 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020560024 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19894069 Country of ref document: EP Kind code of ref document: A1 |