WO2019167068A1 - Novel polymorphs of ribociclib succinate - Google Patents
Novel polymorphs of ribociclib succinate Download PDFInfo
- Publication number
- WO2019167068A1 WO2019167068A1 PCT/IN2019/050159 IN2019050159W WO2019167068A1 WO 2019167068 A1 WO2019167068 A1 WO 2019167068A1 IN 2019050159 W IN2019050159 W IN 2019050159W WO 2019167068 A1 WO2019167068 A1 WO 2019167068A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ribociclib succinate
- hours
- crystalline form
- ribociclib
- succinate
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- the present invention relates to novel polymorphs of ribociclib succinate and processes for preparation thereof.
- the present invention further provides a pharmaceutical composition comprising polymorphic forms of ribociclib succinate and one or more of pharmaceutically acceptable carriers, excipients or diluents used for the treatment of cancer.
- Formula I also chemically described as 7-cyclopentyl-N,N-dimethyl-2- ⁇ [5-(piperazin-l- yl)pyridin-2-yl]amino ⁇ -7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; is used as inhibitors of cyclin dependent kinases, and as modulators and/or inhibitors of glycogen synthase kinase-3 (GSK-3).
- WO 2010020675 Al discloses ribociclib and its use in treatments and therapies for protein kinase-associated disorders.
- Ribociclib is approved as ribociclib succinate salt of Formula II.
- WO 2012064805 discloses a new process for preparation of ribociclib and the novel succinic acid addition salt of ribociclib thereof.
- this application also describes the solid-state characteristics such as XRD, DSC, TGA and DVS, of anhydrous ribociclib succinate salt as well as the monohydrate thereof.
- Ribociclib succinate is stable at 80% RH but on exposure to 90%RH converts to hydrate form. Since for stability of drug substance and drug product, a physical form stability at 75% RH is desirable, ribociclib succinate of Formula (II) (non- hydrate) is suitable for development.
- the solubility of the non-hydrate form in water is about 40 mg/ml. In contrast, the solubility of the hydrate form is significantly lower and is less than 0.5 mg/ml.
- WO 2016091221 patent application publication discloses novel salts of ribociclib such as hemi succinate, adipate, maleate and glycolate.
- EP 3156406A discloses crystalline forms of ribociclib free base.
- WO2019/019959 Al patent application publication discloses crystalline forms X, III, V, characterised by XRD, DSC, TGA and crystalline forms II, S2 and S4 characterised by XRD.
- the pharmaceutical industry is often confronted with the phenomenon of multiple polymorphs of the same crystalline chemical entity.
- Polymorphism is often characterized as the ability of a drug substance to exist as two or more crystalline phases that have different arrangements and/or conformations of the molecules in the crystal lattices giving the crystals different physicochemical properties.
- Powder X-ray Diffraction is a powerful tool in identifying different crystal phases by their unique diffraction patterns.
- the object of the present invention is to provide novel crystalline forms of ribociclib succinate.
- Another object of the present invention is to provide process for the preparation of crystalline forms of ribociclib succinate.
- Yet another object of the present invention is to provide pharmaceutical composition comprising a therapeutically effective amount of crystalline forms of ribociclib succinate.
- Yet another object of the present invention is to provide a process which is simple, economical and suitable for industrial scale-up.
- the present invention provides novel polymorphic forms of ribociclib succinate.
- the present invention provides crystalline forms of ribociclib succinate, hereinafter referred to as Form- C2(hydrate Form), Form- C3 (anhydrous Form) and Form- C4(hydrate Form), Form- C5 (2-Pentanol solvate), Form- C6 (anhydrous Form), Form- C7 (anhydrous Form), Form- C8 (hydrate Form), Form- C9 (anhydrous Form) and Form-ClO (hydrate Form).
- the succinate salt of ribociclib may be in a pseudo polymorphic form. Accordingly, pseudo polymorphs are provided that include hydrates and/or solvates.
- the crystalline nature of forms according to the present invention is characterized by X-ray powder diffraction. Accordingly, the invention also provides methods for preparing these novel forms. In another aspect, the present invention relates to processes for preparing novel polymorphic forms of ribociclib succinate thereof.
- the invention also provides pharmaceutical compositions comprising a therapeutically effective amount of at least one of the above described forms of ribociclib succinate and at least one pharmaceutically acceptable excipient.
- the invention encompasses a process for preparing a pharmaceutical formulation comprising combining at least one of the above- described forms of ribociclib succinate with at least one pharmaceutically acceptable excipient.
- the invention also provides methods of treatment of diseases or symptoms wherein ribociclib succinate is useful.
- these new methods are for similar therapeutic indications to those described in the above identified patents and applications and are incorporated herein by reference.
- FIG. 1 represents a powder X-ray diffraction pattern of Form- C2 of ribociclib succinate.
- FIG. 2 represents a powder X-ray diffraction pattern of Form- C3 of ribociclib succinate.
- FIG. 3 represents a powder X-ray diffraction pattern of Form- C4 of ribociclib succinate.
- FIG. 4 represents a powder X-ray diffraction pattern of Form- C5 of ribociclib succinate.
- FIG. 5 represents a powder X-ray diffraction pattern of Form- C6 of ribociclib succinate.
- FIG. 6 represents a powder X-ray diffraction pattern of Form- C7 of ribociclib succinate.
- FIG. 7 represents a powder X-ray diffraction pattern of Form- C8 of ribociclib succinate
- FIG. 8 represents a powder X-ray diffraction pattern of Form- C9 of ribociclib succinate
- FIG. 9 represents a powder X-ray diffraction pattern of Form- C10 of ribociclib succinate
- FIG.10 illustrates Thermogravimetric analysis (TGA) of Form- C10 of ribociclib succinate
- FIG.11 illustrates Differential Scanning Calorimetry (DSC) of Form- C10 of ribociclib succinate
- the term "PXRD” refers to powder X-ray diffraction
- the term “IR” refers to infrared
- the term “NMR” refers to nuclear magnetic resonance
- the term “TGA” refers to thermogravimetric analysis
- the term “DSC” refers to differential scanning calorimetry
- the term “DVC” refers to dynamic vapour sorption isotherm.
- the term “substantially the same X-ray powder diffraction pattern” is understood to mean that those X-ray powder diffraction patterns having diffraction peaks with 2Q values within ⁇ 0.2° of the diffraction pattern referred to herein are within the scope of the referred to diffraction pattern.
- solvate refers to an association or complex of one or more solvent molecules and a compound of the invention. Such solvents for the invention may not interfere with the biological activity of the solute.
- the solvent used is a pharmaceutically acceptable solvent.
- solvents that form solvates include, but are not limited to, C1-C4 alcohol solvents such as isopropanol, ethanol, methanol, 2-pentanol, dimethyl sulfoxide (DMSO), 1, 4- dioxane, tetrahydrofuran(THF), ethyl acetate and acetone, other than water at levels of more than 1%.
- the solvate can be isolated either as an amorphous form or in a crystalline form, preferably in crystalline form.
- the solvate can be further isolated either in anhydrous form or hydrated form.
- hydrate refers to the complex where the solvent molecule is water.
- the skilled person will appreciate that the water molecules are absorbed, adsorbed or contained within a crystal lattice of the solid compounds, usually in defined stoichiometric ratio.
- the notation for a hydrated compound may be. nFhO, where n is the number of water molecules per formula unit of the compound. For example, in a hemihydrate, n is 0.5; in a monohydrate n is one; in a sesquihydrate, n is 1.5; in a dihydrate, n is 2; and so on.
- the novel polymorphs of the present invention may be isolated in pseudo polymorphic form as a solvate optionally in hydrated form, or as a non-hydrated solvate.
- the polymorphs of the present invention have been characterized by powder X-ray diffraction spectroscopy which produces a fingerprint of the crystalline form and is able to distinguish it from all other crystalline and amorphous forms of ribociclib succinate. Measurements of 20 values are accurate to within ⁇ 0.2 degrees. All the powder diffraction patterns were measured on a PANalytical X’Pert 3 X-ray powder diffractometer with a copper-K-a radiation source.
- the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form-C2”, which has good flow characteristics.
- ribociclib succinate hydrate referred in this specification includes various degrees of hydrates.
- hydrate is monohydrate.
- ribociclib succinate forms a crystalline monohydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3%(l .05 mole).
- the crystalline Form-C2 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
- the crystalline Form-C2 has an XRD pattern with characteristics peaks at 5.49, 7.72, 8.30, 10.57, 12.41, 13.01, 19.19, 20.04 and 21.04 ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 8.90, 11.03, 22.11 and 27.86 ⁇ 0.2 °20.
- the XRPD diffractogram may be as depicted in Figure 1.
- the crystalline Form-C2 has an XRPD pattern with those peaks at °2Q values ⁇ 0.2 °2Q as depicted in Table 1.
- Table 1 Table of values for the XRPD pattern depicted in Figure 1
- Form-C2 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form- C2 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C2 of ribociclib succinate comprising, suspending ribociclib base in a suitable first solvent; treating with succinic acid; isolating the precipitated solid and drying the solid.
- the ribociclib base used for the above process, as well as for the following processes may be in any polymorphic form or in a mixture of any polymorphic forms such as hydrated, solvated, non-solvated or mixture of hydrated, solvated or non-solvated forms thereof.
- Suitable first solvent/s include, but are not limited to THF and l,4-Dioxane, with l,4-Dioxane being preferred.
- ribociclib base is suspended in 1,4 dioxane at about room temperature.
- treating includes mixing, dissolving, slurrying or suspending the ribociclib base in the solvent.
- Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
- Suitable second solvent/s include, but are not limited to, polar solvents and non polar solvents.
- the addition of succinic acid as a solid is done at about room temperature.
- a suspension is obtained.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used, for a period of about 1 hour to about 10 hours, preferably 2 hours to about 8 hours, more preferably for about 4 hours.
- a precipitate is formed in the reaction mixture.
- removing the precipitate is done by filtration.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 50°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C2 of ribociclib succinate, obtained per the present invention is substantially free from other forms of ribociclib succinate.
- substantially freefrom other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C2 prepared by a process according to the invention described above.
- the process produces crystalline Form-C2 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C3”, which has good flow characteristics.
- the crystalline Form-C3 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
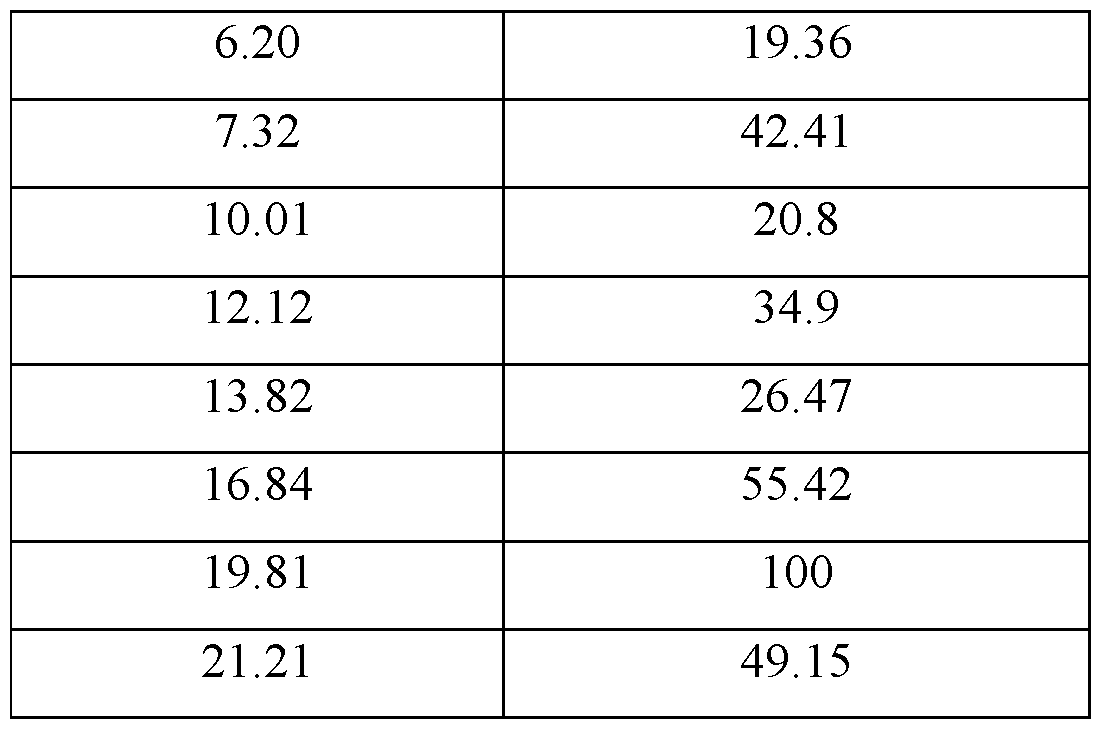
- the crystalline Form-C3 has an XRD pattern with characteristics peaks at 4.51, 6.20, 7.32, 12.12, 16.84 and 19.81. ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 10.01, 13.82 and 2l .2l ⁇ 0.2 °20.
- the XRPD diffractogram may be as depicted in Figure 2.
- the crystalline Form-C3 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 2.
- Table 2 Table of values for the XRPD pattern depicted in Figure 2
- Form-C3 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C3 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C3 of ribociclib succinate comprising, suspending ribociclib base in a suitable first solvent; treating with succinic acid; isolating the precipitated solid and drying the solid.
- Suitable first solvent/s include, but are not limited to esters such as ethyl acetate, methyl acetate, isopropyl acetate and butyl acetate with ethyl acetate being preferred.
- ribociclib base is suspended in ethyl acetate at about room temperature.
- treating includes mixing, dissolving, slurrying or suspending the ribociclib base in the solvent.
- Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
- Suitable second solvent/s include, but are not limited to, polar solvents and non polar solvents.
- the addition of succinic acid as a solid is done at about room temperature.
- a suspension is obtained.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 40°C to about 60°C , for a period of about 5 hours to about 20 hours, preferably 7 hours to about 15 hours, more preferably for about 10 hours.
- a precipitate is formed in the solution.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C3 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C3 prepared by a process according to the invention described above.
- the process produces crystalline Form-C3 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form-C4”, which has good flow characteristics.
- ribociclib succinate hydrate referred in this specification includes various degrees of hydrates.
- hydrate is monohydrate.
- ribociclib succinate forms a crystalline monohydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3% (1.05 moles).
- the crystalline Form-C4 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
- the crystalline Form-C4 has an XRD pattern with characteristics peaks at 4.57, 8.95, 10.68, 12.99 and 18.18 ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 16.12, 21.60 and 22.06 ⁇ 0.2 °20.
- the XRPD diffractogram may be as depicted in Figure 3.
- the crystalline Form-C4 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 3.
- Table 3 Table of values for the XRPD pattern depicted in Figure 3
- Form-C4 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C4 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C4 of ribociclib succinate comprising, dissolving ribociclib succinate in a suitable first solvent or solvents mixture; evaporating the solvent, treating with second solvent; isolating the precipitated solid and drying the solid.
- Suitable first solvent mixture includes polar solvent and non polar solvent.
- Polar solvents include but are not limited to C1-C4 alcohol such as methanol, ethanol, isopropanol, n-propanol, t-butanol, isobutanol and the like; ketones such as acetone, butanone, and methyl isobutyl ketone, methyl isobutyl ketone, methyl vinyl ketone; nitriles such as acetonitrile, propionitrile; polar aprotic solvents such as dimethyl formamide, dimethyl sulfoxide, tetrahydrofuran, l,4-dioxane, trioxane, N-methyl pyrrolidone and dimethyl acetamide and the like or mixture thereof.
- C1-C4 alcohol such as methanol, ethanol, isopropanol, n-propanol, t-butanol, isobutanol and
- Non polar solvent include but are not limited to esters such as ethyl acetate, isopropyl acetate, t-butyl acetate, and isobutyl acetate; chlorinated solvents such as chloroform, dichloromethane, ethylene dichloride; hydrocarbons such as toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof.
- esters such as ethyl acetate, isopropyl acetate, t-butyl acetate, and isobutyl acetate
- chlorinated solvents such as chloroform, dichloromethane, ethylene dichloride
- hydrocarbons such as toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof.
- ribociclib succinate is dissolved in a mixture of alcohol and chlorinated solvents, preferably methanol and DCM at about room temperature.
- a mixture of alcohol and chlorinated solvents preferably methanol and DCM at about room temperature.
- the ratio of methanol to DCM is about 1 : 1 to about 10: 1, more preferably, about 1 :9.
- Suitable second solvent/s include, non-polar solvents such as hydrocarbons for e.g toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof with hexane being preferred.
- non-polar solvents such as hydrocarbons for e.g toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof with hexane being preferred.
- the hexane is added at about room temperature.
- the first solvent mixture Prior to addition of second solvent, the first solvent mixture is removed from the reaction preferably by evaporation under reduced pressure at about 25°C to about 50°C.
- a suspension is obtained.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 50°C, for a period of about 1 day to about 7 days, preferably 2 hours to about 5 days, more preferably for about 4 days.
- a precipitate is formed in the solution.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 25°C to about 80°C, preferably at 30°C to about 40°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C4 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C4 prepared by a process according to the invention described above.
- the process produces crystalline Form-C4 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate 2-pentanol solvate which is herein and in the claims designated as“Form-C5”, which has good flow characteristics.
- the crystalline Form-C5 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
- the crystalline Form-C5 has an XRD pattern with characteristics peaks at 5.24, 6.57, 15.77 and 22.33 ⁇ 0.2°2Q.
- the XRPD diffractogram may comprise further peaks at 12.34, 13.03, 18.32, 19.71, 24.37 and 25.13 ⁇ 0.2 °20.
- the XRPD diffractogram may be as depicted in Figure 4.
- the crystalline Form-C5 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 4.
- Table 4 Table of values for the XRPD pattern depicted in Figure 4
- Form-C5 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form- C5 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C5 of ribociclib succinate comprising, dissolving ribociclib base in 2-pentanol, treating with succinic acid; isolating the precipitated solid and drying the solid.
- the ribociclib base used for the above process, as well as for the following processes, may be in any polymorphic form or in a mixture of any polymorphic forms such as hydrated, solvated, non-solvated or mixture of hydrated, solvated or non-solvated forms thereof.
- ribociclib base is suspended in 2-pentanol at about room temperature.
- suspension is heated at about 50°C to about 80°C, more preferably at about 60°C to about 70°C to get a clear solution.
- treating includes mixing, dissolving, slurrying or suspending the ribociclib base in 2-pentanol.
- Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
- the addition of succinic acid as a solid is done at about room temperature.
- the solution is cooled at about 40°C to about 50°C.
- a suspension is obtained.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 30°C , for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours.
- a precipitate is formed in the solution.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C5 of ribociclib succinate, obtained per the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C5 prepared by a process according to the invention described above.
- the process produces crystalline Form-C5 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C6”, which has good flow characteristics.
- the crystalline Form-C6 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
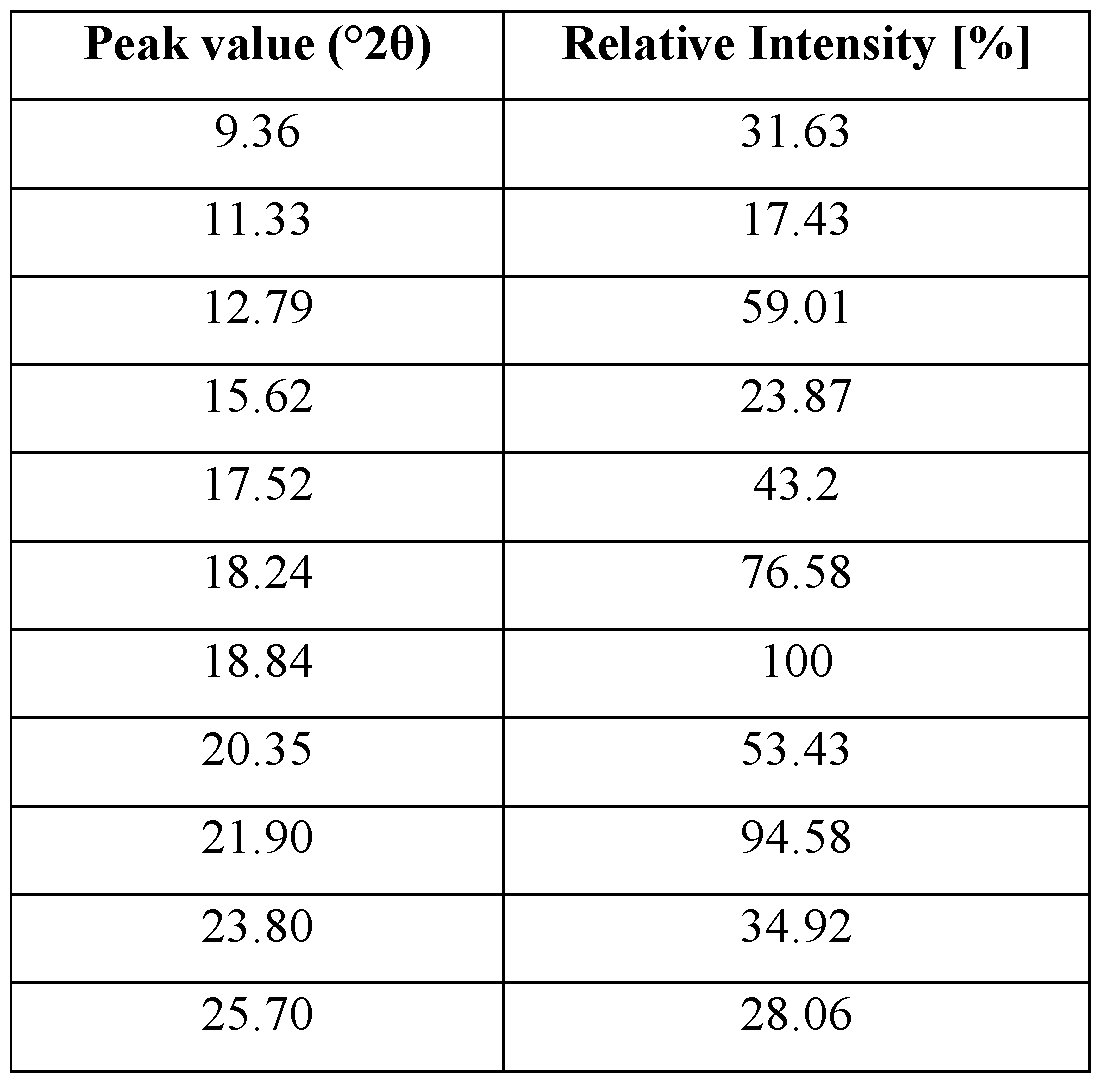
- the crystalline Form-C6 has an XRD pattern with characteristics peaks at 12.79,18.24, 18.84 and 21.90 ⁇ 0.2°2Q.
- the XRPD diffractogram may comprise further peaks at 9.36, 11.33, 15.62, 17.52, 20.35, 23.80 and 25.70 ⁇ 0.2 °20.
- the XRPD diffractogram may be as depicted in Figure 5.
- the crystalline Form-C6 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 5.
- Table 5 Table of values for the XRPD pattern depicted in Figure 5
- Form-C6 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C6 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C6 of ribociclib succinate comprising, suspending crystalline Form-C5 of ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
- Suitable solvent/s include, but are not limited to non-polar solvents such as heptane, hexane, toluene and xylene with heptane being preferred.
- crystalline Form-C5 of ribociclib succinate is suspended in heptane at about room temperature.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 90°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 3 hours to about 5 hours.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C6 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C6 prepared by a process according to the invention described above.
- the process produces crystalline Form-C6 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C7”, which has good flow characteristics.
- the crystalline Form-C7 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
- the crystalline Form-C7 has an XRD pattern with characteristics peaks at 10.66, 12.78, 18.25, 18.90, and 21.89 ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 4.56,9.36, 11.33, 13.80, 15.62, 16.2, 17.52, 20.35, 23.80 and 25.70 ⁇ 0.2°20.
- the XRPD diffractogram may be as depicted in Figure 6.
- the crystalline Form-C7 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 6.
- Table 6 Table of values for the XRPD pattern depicted in Figure 6
- Form-C7 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C7 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C7 of ribociclib succinate comprising, suspending crystalline Form-C7 of ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
- suitable solvent/s include, but are not limited to non-polar solvents such as heptane, hexane, toluene and xylene with heptane being preferred.
- crystalline Form-C5 of ribociclib succinate is suspended in heptane at about room temperature.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 90°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours to about 10 hours.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C7 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C7 prepared by a process according to the invention described above.
- the process produces crystalline Form-C7 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form- C8”, which has good flow characteristics.
- ribociclib succinate hydrate referred in this specification includes various degrees of hydrates.
- hydrate is monohydrate.
- ribociclib succinate forms a crystalline hydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3%(l .05 mole).
- the crystalline Form-C8 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
- the crystalline Form-C8 has an XRD pattern with characteristics peaks at 10.08, 12.29, 19.56 and 19.92 ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 12.64, 13.92, 14.83, 17.39, 22.00 and 23.56 ⁇ 0.2°20.
- the XRPD diffractogram may be as depicted in Figure 7.
- the crystalline Form-C8 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 7.
- Table 7 Table of values for the XRPD pattern depicted in Figure 7
- Form-C8 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C8 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C8 of ribociclib succinate comprising, suspending ribociclib base in a suitable solvent, treating with succinic acid; isolating the precipitated solid and drying the solid.
- Suitable solvent/s include, but are not limited to ester solvents such as ethyl acetate, methyl acetate, isopropyl acetate and n-propyl acetate with n-propyl acetate being preferred.
- treating includes mixing, dissolving, slurrying or suspending the ribociclib base in n-propyl acetate.
- ribociclib base is suspended in n-propyl acetate at about room temperature.
- Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
- solid succinic acid is added at room temperature.
- a suspension is obtained.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 30°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours.
- a precipitate is formed in the solution.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C8 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C8 prepared by a process according to the invention described above.
- the process produces crystalline Form-C8 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C9”, which has good flow characteristics.
- the crystalline Form-C9 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
- the crystalline Form-C9 has an XRD pattern with characteristics peaks at 8.79, 10.91, 12.93, 13.68, 19.9 and 24.78 ⁇ 0.2°2Q.
- the XRPD diffractogram may comprise further peaks at 7.80, 12.30, 14.95, 15.60, 21.05, 21.36, 21.95 and 22.94 ⁇ 0.2°20.
- the XRPD diffractogram may be as depicted in Figure 8.
- the crystalline Form-C9 has an XRPD pattern with those peaks at °20 values ⁇ 0.2 °20 as depicted in Table 8.
- Table 8 Table of values for the XRPD pattern depicted in Figure 8
- Form-C9 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-C9 of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-C9 of ribociclib succinate comprising, suspending ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
- Suitable solvent/s include, but are not limited to C1-C5 alcohol solvents such as methanol, ethanol, isopropanol, t-butanol and n-butanol with ethanol being preferred.
- ribociclib succinate is suspended in ethanol at about room temperature.
- the obtained suspension is preferably maintained while stirring.
- stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 80°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours to about 10 hours.
- removing the precipitate is done by filtration at room temperature.
- the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
- the crystalline Form-C9 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- ribociclib succinate substantially free from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-C9 prepared by a process according to the invention described above.
- the process produces crystalline Form-C9 in high yield and purity.
- the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form- C10”, which has good flow characteristics.
- ribociclib succinate hydrate referred in this specification includes various degrees of hydrates.
- the hydrate is dihydrate.
- ribociclib succinate forms a crystalline dihydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 :2 wherein water is present in the stoichiometric ratio ranging from 6.28% (2.0 mole) to 6.4% (2.05 mole).
- the crystalline Form-ClO is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
- the crystalline Form-ClO has an XRD pattern with characteristics peaks at 6.45, 10.13, 10.73, 20.18 and 20.65 ⁇ 0.2°20.
- the XRPD diffractogram may comprise further peaks at 7.45, 11.92, 14.50, 23.25 and 25.34 ⁇ 0.2°20.
- the XRPD diffractogram may be as depicted in Figure 9.
- the crystalline Form-ClO has an XRPD pattern with those peaks at °2Q values ⁇ 0.2 °2Q as depicted in Table 9.
- Table 9 Table of values for the XRPD pattern depicted in Figure 9
- crystalline Form-ClO of ribociclib succinate is characterized by having a thermogravimetric analysis profile as shown in Figure 10
- Crystalline Form-ClO of ribociclib succinate may also be characterized as having a DSC spectrum.
- the DSC plot for the sample shows one endotherm with an onset at 66.09°C, a peak maximum at 77.53°C; a second endotherm with an onset at l3 l . l l°C, a peak maximum at l42.88°C; and a third second endotherm with an onset at l80.07°C, a peak maximum at l83. l8°C.
- crystalline Form-ClO of ribociclib succinate is characterized by having a DSC spectrum as shown in Figure 11.
- Form-ClO may be further characterized by other methods including, but not limited to IR, solid state NMR, intrinsic dissolution and Raman spectroscopy.
- the crystalline Form-ClO of ribociclib succinate has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
- the invention encompasses a process for preparing the crystalline Form-ClO of ribociclib succinate comprising, exposing ribociclib succinate at about 90% RH in a humidifier.
- the solids are exposed at 40°C to about 60°C, preferably at 45°C to about 55°C, more preferably at 50°C; for a residence period of about 4 days to about 6 days, preferably 4 days to about 5 days, more preferably for about 5 days.
- a steep rise in moisture absorption is observed at 90% RH condition in each cycle and difference in sorption and desorption behavior reflects a formation of hydrate form is taking place at 90% RH condition.
- the crystalline Form-ClO of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate.
- the phrase "Substantially free” from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
- crystalline Form-ClO prepared by a process according to the invention described above.
- the process produces crystalline Form-ClO in high yield and purity.
- the process of the present invention may be used as a method for purifying any form of ribociclib succinate, as well as for the preparation of the new polymorphic forms and salts thereof.
- the present invention comprises 1) a pharmaceutical composition comprising any one, or combination of ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 described above and at least one pharmaceutically acceptable excipient; and 2) the use of any one, or combination, of the above-described ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 in the manufacture of a pharmaceutical composition, wherein the pharmaceutical composition can be useful for the treatment or prevention of cancer.
- the pharmaceutical composition of the present invention can be in solid or a non solid form. If the pharmaceutical composition is in a non-solid form, any one, or combination, of the ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 are retained as solid(s) in the non-solid pharmaceutical composition e.g., as a suspension, foam, ointment etc.
- the pharmaceutical composition can be prepared by a process comprising combining any one, or combination of the above-described ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10; with at least one pharmaceutically acceptable excipient.
- the pharmaceutical composition can be used to make appropriate dosage forms such as tablets, powders, capsules, suppositories, sachets, troches, and lozenges.
- any one, or combination, of the above-mentioned ribociclib succinate crystalline forms C2, C3,C4, C5, C6, C7, C8, C9 or C10 particularly in a pharmaceutical composition and dosage form can be used to treat or prevent cancer in a mammal such as a human, comprising administering a treatment effective amount of the one, or combination, of the ribociclib succinate crystalline forms C2, C3,C4, C5, C6, C7, C8, C9 or C10 in the mammal.
- the invention is further defined by reference to the following examples describing in detail the preparation of ribociclib succinate crystalline forms of the invention. It will be apparent to those skilled in the art that many modifications, both to materials and methods, may be practiced without departing from the scope of the invention.
- Ribociclib succinate (1.0 g) was dissolved in a mixture of methanol (2 ml) and DCM (18 ml) at 25-30°C. The solvent was removed under reduced pressure below 40°C. To the residue was added hexane (20 ml) and the mixture was stirred at 25- 30 °C for about 75-80 hr. The solids were isolated by filtration and dried at 25- 30°C under vacuum, resulted the title compound (0.85g) and identified by XRD as Form-C4, as presented in figure 3.
- Example 4 Preparation of ribociclib succinate Form-C5
- Ribociclib base (5.0g) was dissolved in 2-pentanol (150 ml) at 60-65°C.To the above solution was added succinic acid (1.45 g) at 40-45°C. The reaction mixture was stirred at 25-30°C for 4 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (6.30g) and identified by XRD as Form-C5, as presented in figure 4.
- Ribociclib succinate Form-C5 (2.5g) was suspended in n-Heptane (25ml). The reaction mixture was stirred at 75-80°C for 3 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (2.0g) and identified by XRD as Form-C6, as presented in figure 5.
- Ribociclib succinate Form-C5 (2.0g) was suspended in n-Heptane (20ml). The reaction mixture was stirred at 75-80°C for 5 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (l .70g) and identified by XRD as Form-C7, as presented in figure 6.
- Ribociclib base (l .Og) was suspended in n-propyl acetate (30ml) at 25-30°C.To the above suspension was added succinic acid (0.29g). The reaction mixture was stirred at 25-30°C for 4 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (T lOg) and identified by XRD as Form-C8, as presented in figure 7.
- Ribociclib succinate (l.Og) was exposed at 50°C, 90%RH in Humidification chamber for 4-5 days resulted the titled compound (1.0 g) and identified by XRD as Form-ClO, as presented in figure 9.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present invention discloses novel crystalline polymorphic forms of ribociclib succinate, methods of preparation, pharmaceutical compositions and methods of therapeutic treatment involving polymorphic forms thereof.
Description
NOVEL POLYMORPHS OF RIBOCICLIB SUCCINATE
Related Applications:
This application is Complete Cognate Application of the Provisional Patent Application No. 201821007800 filed on Ist March, 2018 and Provisional Patent Application No. 201821023064 filed on 20th June, 2018.
FIELD OF INVENTION:
The present invention relates to novel polymorphs of ribociclib succinate and processes for preparation thereof. The present invention further provides a pharmaceutical composition comprising polymorphic forms of ribociclib succinate and one or more of pharmaceutically acceptable carriers, excipients or diluents used for the treatment of cancer.
BACKGROUND OF INVENTION:
Ribociclib of Formula I
Formula I, also chemically described as 7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-l- yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; is used as inhibitors of cyclin dependent kinases, and as modulators and/or inhibitors of glycogen synthase kinase-3 (GSK-3).
WO 2010020675 Al discloses ribociclib and its use in treatments and therapies for protein kinase-associated disorders.
Ribociclib is approved as ribociclib succinate salt of Formula II.
Formula II
WO 2012064805 discloses a new process for preparation of ribociclib and the novel succinic acid addition salt of ribociclib thereof. In addition, this application also describes the solid-state characteristics such as XRD, DSC, TGA and DVS, of anhydrous ribociclib succinate salt as well as the monohydrate thereof. Ribociclib succinate is stable at 80% RH but on exposure to 90%RH converts to hydrate form. Since for stability of drug substance and drug product, a physical form stability at 75% RH is desirable, ribociclib succinate of Formula (II) (non- hydrate) is suitable for development.
The solubility of the non-hydrate form in water is about 40 mg/ml. In contrast, the solubility of the hydrate form is significantly lower and is less than 0.5 mg/ml.
WO 2016091221 patent application publication discloses novel salts of ribociclib such as hemi succinate, adipate, maleate and glycolate.
EP 3156406A discloses crystalline forms of ribociclib free base.
WO2019/019959 Al patent application publication discloses crystalline forms X, III, V, characterised by XRD, DSC, TGA and crystalline forms II, S2 and S4 characterised by XRD.
The pharmaceutical industry is often confronted with the phenomenon of multiple polymorphs of the same crystalline chemical entity. Polymorphism is often characterized as the ability of a drug substance to exist as two or more crystalline phases that have different arrangements and/or conformations of the molecules in the crystal lattices giving the crystals different physicochemical properties.
Multiple crystal forms with different solid state properties of a drug substance can exhibit differences in storage, stability, compressibility, density and dissolution rates (important in determining bioavailability) during processing. Powder X-ray Diffraction is a powerful tool in identifying different crystal phases by their unique diffraction patterns.
Regulatory agencies worldwide require a reasonable effort to identify the polymorphs of the drug substance and check for polymorph interconversions. Due to the often-unpredictable behaviour of polymorphs and their respective differences in physicochemical properties, consistency in manufacturing between batches of the same product must be demonstrated.
The ability to be able to manufacture the selected polymorphic form reliably is a key factor in determining the success of the drug product. Proper understanding of the polymorph landscape and nature of the polymorphs of a pharmaceutical will contribute to manufacturing consistency.
The discovery of new polymorphic forms of a pharmaceutically useful compound provides a new opportunity to improve the performance characteristics of a pharmaceutical product. It enlarges the repertoire of materials that a formulation scientist has available for designing, for example, a pharmaceutical dosage form of a drug with a targeted release profile or other desired characteristic.
There is a need in the art to provide a pharmaceutically active substance which not only is characterized by high pharmacological potency but also satisfies the above- mentioned physicochemical requirements as far as possible.
OBJECTS OF THE INVENTION:
The object of the present invention is to provide novel crystalline forms of ribociclib succinate.
Another object of the present invention is to provide process for the preparation of crystalline forms of ribociclib succinate.
Yet another object of the present invention is to provide pharmaceutical composition comprising a therapeutically effective amount of crystalline forms of ribociclib succinate.
Yet another object of the present invention is to provide a process which is simple, economical and suitable for industrial scale-up.
SUMMARY OF THE INVENTION:
The present invention provides novel polymorphic forms of ribociclib succinate.
In one embodiment, the present invention provides crystalline forms of ribociclib succinate, hereinafter referred to as Form- C2(hydrate Form), Form- C3 (anhydrous Form) and Form- C4(hydrate Form), Form- C5 (2-Pentanol solvate), Form- C6 (anhydrous Form), Form- C7 (anhydrous Form), Form- C8 (hydrate Form), Form- C9 (anhydrous Form) and Form-ClO (hydrate Form).
The succinate salt of ribociclib may be in a pseudo polymorphic form. Accordingly, pseudo polymorphs are provided that include hydrates and/or solvates.
The crystalline nature of forms according to the present invention is characterized by X-ray powder diffraction. Accordingly, the invention also provides methods for preparing these novel forms.
In another aspect, the present invention relates to processes for preparing novel polymorphic forms of ribociclib succinate thereof.
The invention also provides pharmaceutical compositions comprising a therapeutically effective amount of at least one of the above described forms of ribociclib succinate and at least one pharmaceutically acceptable excipient.
In yet another embodiment, the invention encompasses a process for preparing a pharmaceutical formulation comprising combining at least one of the above- described forms of ribociclib succinate with at least one pharmaceutically acceptable excipient.
The invention also provides methods of treatment of diseases or symptoms wherein ribociclib succinate is useful. In particular, these new methods are for similar therapeutic indications to those described in the above identified patents and applications and are incorporated herein by reference.
Further features of the present invention are defined in the dependent claims.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 represents a powder X-ray diffraction pattern of Form- C2 of ribociclib succinate.
FIG. 2 represents a powder X-ray diffraction pattern of Form- C3 of ribociclib succinate.
FIG. 3 represents a powder X-ray diffraction pattern of Form- C4 of ribociclib succinate.
FIG. 4 represents a powder X-ray diffraction pattern of Form- C5 of ribociclib succinate.
FIG. 5 represents a powder X-ray diffraction pattern of Form- C6 of ribociclib succinate.
FIG. 6 represents a powder X-ray diffraction pattern of Form- C7 of ribociclib succinate.
FIG. 7 represents a powder X-ray diffraction pattern of Form- C8 of ribociclib succinate
FIG. 8 represents a powder X-ray diffraction pattern of Form- C9 of ribociclib succinate
FIG. 9 represents a powder X-ray diffraction pattern of Form- C10 of ribociclib succinate
FIG.10 illustrates Thermogravimetric analysis (TGA) of Form- C10 of ribociclib succinate
FIG.11 illustrates Differential Scanning Calorimetry (DSC) of Form- C10 of ribociclib succinate
DETAILED DESCRIPTION OF THE INVENTION:
Unless defined otherwise, technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs, and are consistent with:
As used herein, the term "PXRD" refers to powder X-ray diffraction, the term "IR" refers to infrared, the term "NMR" refers to nuclear magnetic resonance, the term "TGA" refers to thermogravimetric analysis, the term "DSC" refers to differential scanning calorimetry and the term "DVC" refers to dynamic vapour sorption isotherm.
As used herein, the term "substantially the same X-ray powder diffraction pattern" is understood to mean that those X-ray powder diffraction patterns having diffraction peaks with 2Q values within ± 0.2° of the diffraction pattern referred to herein are within the scope of the referred to diffraction pattern.
As used herein, the term“solvate" refers to an association or complex of one or more solvent molecules and a compound of the invention. Such solvents for the invention may not interfere with the biological activity of the solute. Typically, the solvent used is a pharmaceutically acceptable solvent. Examples of solvents that form solvates include, but are not limited to, C1-C4 alcohol solvents such as isopropanol, ethanol, methanol, 2-pentanol, dimethyl sulfoxide (DMSO), 1, 4- dioxane, tetrahydrofuran(THF), ethyl acetate and acetone, other than water at levels of more than 1%.
The solvate can be isolated either as an amorphous form or in a crystalline form, preferably in crystalline form.
The solvate can be further isolated either in anhydrous form or hydrated form.
As used herein, the term "hydrate" refers to the complex where the solvent molecule is water. The skilled person will appreciate that the water molecules are absorbed, adsorbed or contained within a crystal lattice of the solid compounds, usually in defined stoichiometric ratio. The notation for a hydrated compound may be. nFhO, where n is the number of water molecules per formula unit of the compound. For example, in a hemihydrate, n is 0.5; in a monohydrate n is one; in a sesquihydrate, n is 1.5; in a dihydrate, n is 2; and so on.
The novel polymorphs of the present invention may be isolated in pseudo polymorphic form as a solvate optionally in hydrated form, or as a non-hydrated solvate.
As polymorphic forms are reliably characterized by peak positions in the X-ray diffractogram, the polymorphs of the present invention have been characterized by powder X-ray diffraction spectroscopy which produces a fingerprint of the crystalline form and is able to distinguish it from all other crystalline and amorphous forms of ribociclib succinate. Measurements of 20 values are accurate to within ± 0.2 degrees. All the powder diffraction patterns were measured on a PANalytical X’Pert3 X-ray powder diffractometer with a copper-K-a radiation source.
The invention will now be described in detail in connection with certain preferred and optional embodiments, so that various aspects thereof may be more fully understood and appreciated.
Thus, in one aspect, the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form-C2”, which has good flow characteristics.
In an embodiment, ribociclib succinate hydrate referred in this specification includes various degrees of hydrates. Preferably, hydrate is monohydrate.
Thus, ribociclib succinate forms a crystalline monohydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3%(l .05 mole).
In one embodiment, the crystalline Form-C2 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C2 has an XRD pattern with characteristics peaks at 5.49, 7.72, 8.30, 10.57, 12.41, 13.01, 19.19, 20.04 and 21.04 ± 0.2°20. The XRPD diffractogram may comprise further peaks at 8.90, 11.03, 22.11 and 27.86 ± 0.2 °20. The XRPD diffractogram may be as depicted in Figure 1.
In an embodiment, the crystalline Form-C2 has an XRPD pattern with those peaks at °2Q values ± 0.2 °2Q as depicted in Table 1.
Table 1: Table of values for the XRPD pattern depicted in Figure 1
Those skilled in the art would recognize that Form-C2 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form- C2 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C2 of ribociclib succinate comprising, suspending ribociclib base in a suitable first solvent; treating with succinic acid; isolating the precipitated solid and drying the solid.
The ribociclib base used for the above process, as well as for the following processes, may be in any polymorphic form or in a mixture of any polymorphic forms such as hydrated, solvated, non-solvated or mixture of hydrated, solvated or non-solvated forms thereof.
Suitable first solvent/s include, but are not limited to THF and l,4-Dioxane, with l,4-Dioxane being preferred.
In an embodiment, ribociclib base is suspended in 1,4 dioxane at about room temperature.
In one embodiment treating includes mixing, dissolving, slurrying or suspending the ribociclib base in the solvent.
Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
Suitable second solvent/s include, but are not limited to, polar solvents and non polar solvents. Preferably, the addition of succinic acid as a solid is done at about room temperature.
Typically, after the succinic acid addition, a suspension is obtained. The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used, for a period of about 1 hour to about 10 hours, preferably 2 hours to about 8 hours, more preferably for about 4 hours.
Typically, a precipitate is formed in the reaction mixture. Preferably, removing the precipitate is done by filtration. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 50°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C2 of ribociclib succinate, obtained per the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially freefrom other forms of ribociclib succinate” shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C2 prepared by a process according to the invention described above. The process produces crystalline Form-C2 in high yield and purity.
According to second aspect, the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C3”, which has good flow characteristics.
In one embodiment, the crystalline Form-C3 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C3 has an XRD pattern with characteristics peaks at 4.51, 6.20, 7.32, 12.12, 16.84 and 19.81. ± 0.2°20. The XRPD diffractogram may comprise further peaks at 10.01, 13.82 and 2l .2l± 0.2 °20. The XRPD diffractogram may be as depicted in Figure 2.
In an embodiment, the crystalline Form-C3 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 2.
Those skilled in the art would recognize that Form-C3 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C3 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C3 of ribociclib succinate comprising, suspending ribociclib base in a suitable first solvent; treating with succinic acid; isolating the precipitated solid and drying the solid.
Suitable first solvent/s include, but are not limited to esters such as ethyl acetate, methyl acetate, isopropyl acetate and butyl acetate with ethyl acetate being preferred.
In an embodiment, ribociclib base is suspended in ethyl acetate at about room temperature.
In one embodiment treating includes mixing, dissolving, slurrying or suspending the ribociclib base in the solvent.
Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid.
Suitable second solvent/s include, but are not limited to, polar solvents and non polar solvents. Preferably, the addition of succinic acid as a solid is done at about room temperature.
Typically, after the succinic acid addition, a suspension is obtained. The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 40°C to about 60°C , for a period of about 5 hours to about 20 hours, preferably 7 hours to about 15 hours, more preferably for about 10 hours.
Typically a precipitate is formed in the solution. Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C3 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C3 prepared by a process according to the invention described above. The process produces crystalline Form-C3 in high yield and purity.
According to third aspect, the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form-C4”, which has good flow characteristics.
In an embodiment, ribociclib succinate hydrate referred in this specification includes various degrees of hydrates. Preferably, hydrate is monohydrate.
Thus, ribociclib succinate forms a crystalline monohydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3% (1.05 moles).
In one embodiment, the crystalline Form-C4 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C4 has an XRD pattern with characteristics peaks at 4.57, 8.95, 10.68, 12.99 and 18.18± 0.2°20. The XRPD diffractogram may comprise further peaks at 16.12, 21.60 and 22.06± 0.2 °20. The XRPD diffractogram may be as depicted in Figure 3.
In an embodiment, the crystalline Form-C4 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 3.
Table 3: Table of values for the XRPD pattern depicted in Figure 3
Those skilled in the art would recognize that Form-C4 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C4 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C4 of ribociclib succinate comprising, dissolving ribociclib succinate in a suitable first solvent or solvents mixture; evaporating the solvent, treating with second solvent; isolating the precipitated solid and drying the solid.
Suitable first solvent mixture includes polar solvent and non polar solvent. Polar solvents include but are not limited to C1-C4 alcohol such as methanol, ethanol, isopropanol, n-propanol, t-butanol, isobutanol and the like; ketones such as acetone, butanone, and methyl isobutyl ketone, methyl isobutyl ketone, methyl vinyl ketone; nitriles such as acetonitrile, propionitrile; polar aprotic solvents such as dimethyl formamide, dimethyl sulfoxide, tetrahydrofuran, l,4-dioxane, trioxane, N-methyl pyrrolidone and dimethyl acetamide and the like or mixture thereof.
Non polar solvent include but are not limited to esters such as ethyl acetate, isopropyl acetate, t-butyl acetate, and isobutyl acetate; chlorinated solvents such as chloroform, dichloromethane, ethylene dichloride; hydrocarbons such as toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof.
In an embodiment ribociclib succinate is dissolved in a mixture of alcohol and chlorinated solvents, preferably methanol and DCM at about room temperature.
Preferably, the ratio of methanol to DCM is about 1 : 1 to about 10: 1, more preferably, about 1 :9.
Suitable second solvent/s include, non-polar solvents such as hydrocarbons for e.g toluene, xylene, heptane, hexane, cyclohexane and the like or mixture thereof with hexane being preferred. Preferably, the hexane is added at about room temperature.
Prior to addition of second solvent, the first solvent mixture is removed from the reaction preferably by evaporation under reduced pressure at about 25°C to about 50°C.
Typically, after the hexane addition, a suspension is obtained. The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 50°C, for a period of about 1 day to about 7 days, preferably 2 hours to about 5 days, more preferably for about 4 days.
Typically, a precipitate is formed in the solution. Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 25°C to about 80°C, preferably at 30°C to about 40°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C4 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C4 prepared by a process according to the invention described above. The process produces crystalline Form-C4 in high yield and purity.
According to the fourth aspect, the present invention provides the crystalline ribociclib succinate 2-pentanol solvate which is herein and in the claims designated as“Form-C5”, which has good flow characteristics.
In one embodiment, the crystalline Form-C5 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C5 has an XRD pattern with characteristics peaks at 5.24, 6.57, 15.77 and 22.33 ± 0.2°2Q. The XRPD diffractogram may comprise further peaks at 12.34, 13.03, 18.32, 19.71, 24.37 and 25.13 ± 0.2 °20. The XRPD diffractogram may be as depicted in Figure 4.
In an embodiment, the crystalline Form-C5 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 4.
Table 4: Table of values for the XRPD pattern depicted in Figure 4
Those skilled in the art would recognize that Form-C5 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form- C5 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C5 of ribociclib succinate comprising, dissolving ribociclib base in 2-pentanol, treating with succinic acid; isolating the precipitated solid and drying the solid.
The ribociclib base used for the above process, as well as for the following processes, may be in any polymorphic form or in a mixture of any polymorphic forms such as hydrated, solvated, non-solvated or mixture of hydrated, solvated or non-solvated forms thereof.
In an embodiment, ribociclib base is suspended in 2-pentanol at about room temperature. Preferably, suspension is heated at about 50°C to about 80°C, more preferably at about 60°C to about 70°C to get a clear solution.
In one embodiment treating includes mixing, dissolving, slurrying or suspending the ribociclib base in 2-pentanol.
Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid. Preferably, the addition of succinic acid as a solid is done at about room temperature.
Preferably, prior to addition of succinic acid, the solution is cooled at about 40°C to about 50°C.
Typically, after the succinic acid addition, a suspension is obtained. The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 30°C , for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours.
Typically, a precipitate is formed in the solution. Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C5 of ribociclib succinate, obtained per the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to the another aspect of the present invention, there is provided crystalline Form-C5 prepared by a process according to the invention described above. The process produces crystalline Form-C5 in high yield and purity.
According to the fifth aspect, the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C6”, which has good flow characteristics.
In one embodiment, the crystalline Form-C6 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C6 has an XRD pattern with characteristics peaks at 12.79,18.24, 18.84 and 21.90 ± 0.2°2Q. The XRPD diffractogram may comprise further peaks at 9.36, 11.33, 15.62, 17.52, 20.35, 23.80 and 25.70± 0.2 °20. The XRPD diffractogram may be as depicted in Figure 5.
In an embodiment, the crystalline Form-C6 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 5.
Table 5: Table of values for the XRPD pattern depicted in Figure 5
Those skilled in the art would recognize that Form-C6 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C6 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C6 of ribociclib succinate comprising, suspending crystalline Form-C5 of ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
Suitable solvent/s include, but are not limited to non-polar solvents such as heptane, hexane, toluene and xylene with heptane being preferred.
In an embodiment, crystalline Form-C5 of ribociclib succinate is suspended in heptane at about room temperature.
The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 90°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 3 hours to about 5 hours.
Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C6 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C6 prepared by a process according to the invention described above. The process produces crystalline Form-C6 in high yield and purity.
According to the sixth aspect, the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C7”, which has good flow characteristics.
In one embodiment, the crystalline Form-C7 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C7 has an XRD pattern with characteristics peaks at 10.66, 12.78, 18.25, 18.90, and 21.89 ± 0.2°20. The XRPD diffractogram may comprise further peaks at 4.56,9.36, 11.33, 13.80, 15.62, 16.2, 17.52, 20.35, 23.80 and 25.70± 0.2°20. The XRPD diffractogram may be as depicted in Figure 6. In an embodiment, the crystalline Form-C7 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 6.
Table 6: Table of values for the XRPD pattern depicted in Figure 6
Those skilled in the art would recognize that Form-C7 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C7 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C7 of ribociclib succinate comprising, suspending crystalline Form-C7 of ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid. Suitable solvent/s include, but are not limited to non-polar solvents such as heptane, hexane, toluene and xylene with heptane being preferred.
In an embodiment, crystalline Form-C5 of ribociclib succinate is suspended in heptane at about room temperature.
The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 90°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours to about 10 hours.
Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C7 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably
less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C7 prepared by a process according to the invention described above. The process produces crystalline Form-C7 in high yield and purity.
According to the seventh aspect, the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form- C8”, which has good flow characteristics.
In an embodiment, ribociclib succinate hydrate referred in this specification includes various degrees of hydrates. Preferably, hydrate is monohydrate.
Thus, ribociclib succinate forms a crystalline hydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 : 1 wherein water is present in the stoichiometric ratio ranging from 3.14% (1.0 mole) to 3.3%(l .05 mole).
In one embodiment, the crystalline Form-C8 is characterized by an X-ray powder diffraction pattern comprising the following 2Q values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C8 has an XRD pattern with characteristics peaks at 10.08, 12.29, 19.56 and 19.92 ± 0.2°20. The XRPD diffractogram may comprise further peaks at 12.64, 13.92, 14.83, 17.39, 22.00 and 23.56± 0.2°20. The XRPD diffractogram may be as depicted in Figure 7.
In an embodiment, the crystalline Form-C8 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 7.
Table 7: Table of values for the XRPD pattern depicted in Figure 7
Those skilled in the art would recognize that Form-C8 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C8 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C8 of ribociclib succinate comprising, suspending ribociclib base in a suitable solvent, treating with succinic acid; isolating the precipitated solid and drying the solid.
Suitable solvent/s include, but are not limited to ester solvents such as ethyl acetate, methyl acetate, isopropyl acetate and n-propyl acetate with n-propyl acetate being preferred.
In one embodiment treating includes mixing, dissolving, slurrying or suspending the ribociclib base in n-propyl acetate. Preferably, ribociclib base is suspended in n-propyl acetate at about room temperature.
Succinic acid can be added either as a solution in a suitable second solvent or it may be added as a solid. Preferably, solid succinic acid is added at room temperature.
Typically, after the succinic acid addition, a suspension is obtained. The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 25°C to about 30°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours.
Typically, a precipitate is formed in the solution. Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C8 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C8 prepared by a process according to the invention described above. The process produces crystalline Form-C8 in high yield and purity.
According to the eighth aspect, the present invention provides the crystalline ribociclib succinate which is herein and in the claims designated as“Form-C9”, which has good flow characteristics.
In one embodiment, the crystalline Form-C9 is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
In an embodiment, the crystalline Form-C9 has an XRD pattern with characteristics peaks at 8.79, 10.91, 12.93, 13.68, 19.9 and 24.78± 0.2°2Q. The XRPD diffractogram may comprise further peaks at 7.80, 12.30, 14.95, 15.60, 21.05, 21.36, 21.95 and 22.94 ± 0.2°20. The XRPD diffractogram may be as depicted in Figure 8.
In an embodiment, the crystalline Form-C9 has an XRPD pattern with those peaks at °20 values ± 0.2 °20 as depicted in Table 8.
Table 8: Table of values for the XRPD pattern depicted in Figure 8
Those skilled in the art would recognize that Form-C9 may be further characterized by other methods including, but not limited to IR, solid state NMR, DSC, TGA, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-C9 of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-C9 of ribociclib succinate comprising, suspending ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
Suitable solvent/s include, but are not limited to C1-C5 alcohol solvents such as methanol, ethanol, isopropanol, t-butanol and n-butanol with ethanol being preferred.
In an embodiment, ribociclib succinate is suspended in ethanol at about room temperature.
The obtained suspension is preferably maintained while stirring. Preferably, stirring is done at about 25°C to the boiling point of the solvent used. More preferably, stirring is done at about 50°C to about 80°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours to about 10 hours.
Preferably, removing the precipitate is done by filtration at room temperature. Preferably, the obtained solid is dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
The crystalline Form-C9 of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-C9 prepared by a process according to the invention described above. The process produces crystalline Form-C9 in high yield and purity.
According to the ninth aspect, the present invention provides the crystalline ribociclib succinate hydrate which is herein and in the claims designated as“Form- C10”, which has good flow characteristics.
In an embodiment, ribociclib succinate hydrate referred in this specification includes various degrees of hydrates. Preferably, the hydrate is dihydrate.
Thus, ribociclib succinate forms a crystalline dihydrate wherein the molar ratio of ribociclib succinate to water is approximately 1 :2 wherein water is present in the stoichiometric ratio ranging from 6.28% (2.0 mole) to 6.4% (2.05 mole).
In one embodiment, the crystalline Form-ClO is characterized by an X-ray powder diffraction pattern comprising the following 20 values measured using CuKa, radiation.
In an embodiment, the crystalline Form-ClO has an XRD pattern with characteristics peaks at 6.45, 10.13, 10.73, 20.18 and 20.65± 0.2°20. The XRPD diffractogram may comprise further peaks at 7.45, 11.92, 14.50, 23.25 and 25.34 ± 0.2°20. The XRPD diffractogram may be as depicted in Figure 9.
In an embodiment, the crystalline Form-ClO has an XRPD pattern with those peaks at °2Q values ± 0.2 °2Q as depicted in Table 9.
Table 9: Table of values for the XRPD pattern depicted in Figure 9
In another embodiment, crystalline Form-ClO of ribociclib succinate is characterized by having a thermogravimetric analysis profile as shown in Figure 10
Crystalline Form-ClO of ribociclib succinate may also be characterized as having a DSC spectrum. The DSC plot for the sample shows one endotherm with an onset at 66.09°C, a peak maximum at 77.53°C; a second endotherm with an onset at l3 l . l l°C, a peak maximum at l42.88°C; and a third second endotherm with an onset at l80.07°C, a peak maximum at l83. l8°C.
In an embodiment, crystalline Form-ClO of ribociclib succinate is characterized by having a DSC spectrum as shown in Figure 11.
Those skilled in the art would recognize that Form-ClO may be further characterized by other methods including, but not limited to IR, solid state NMR, intrinsic dissolution and Raman spectroscopy.
Preferably the crystalline Form-ClO of ribociclib succinate, has a crystalline purity of at least 80%, more preferably at least 90%, more preferably at least 95%, most preferably at least 99% by weight.
The invention encompasses a process for preparing the crystalline Form-ClO of ribociclib succinate comprising, exposing ribociclib succinate at about 90% RH in a humidifier.
Preferably, the solids are exposed at 40°C to about 60°C, preferably at 45°C to about 55°C, more preferably at 50°C; for a residence period of about 4 days to about 6 days, preferably 4 days to about 5 days, more preferably for about 5 days.
Preferably, a steep rise in moisture absorption is observed at 90% RH condition in each cycle and difference in sorption and desorption behavior reflects a formation of hydrate form is taking place at 90% RH condition.
The crystalline Form-ClO of ribociclib succinate, obtained according to the present invention is substantially free from other forms of ribociclib succinate. The phrase "Substantially free" from other forms of ribociclib succinate shall be understood to mean that the polymorphs of ribociclib succinate contain less than 10%, preferably less than 5%, of any other forms of ribociclib succinate and less than 1% of other impurities.
According to another aspect of the present invention, there is provided crystalline Form-ClO prepared by a process according to the invention described above. The process produces crystalline Form-ClO in high yield and purity.
The process of the present invention may be used as a method for purifying any form of ribociclib succinate, as well as for the preparation of the new polymorphic forms and salts thereof.
The present invention comprises 1) a pharmaceutical composition comprising any one, or combination of ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 described above and at least one pharmaceutically acceptable excipient; and 2) the use of any one, or combination, of the above-described ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 in the manufacture of a pharmaceutical composition, wherein the pharmaceutical composition can be useful for the treatment or prevention of cancer.
The pharmaceutical composition of the present invention can be in solid or a non solid form. If the pharmaceutical composition is in a non-solid form, any one, or combination, of the ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10 are retained as solid(s) in the non-solid pharmaceutical composition e.g., as a suspension, foam, ointment etc.
The pharmaceutical composition can be prepared by a process comprising combining any one, or combination of the above-described ribociclib succinate crystalline forms C2, C3, C4, C5, C6, C7, C8, C9 or C10; with at least one pharmaceutically acceptable excipient.
The pharmaceutical composition can be used to make appropriate dosage forms such as tablets, powders, capsules, suppositories, sachets, troches, and lozenges.
Any one, or combination, of the above-mentioned ribociclib succinate crystalline forms C2, C3,C4, C5, C6, C7, C8, C9 or C10 particularly in a pharmaceutical composition and dosage form, can be used to treat or prevent cancer in a mammal such as a human, comprising administering a treatment effective amount of the one, or combination, of the ribociclib succinate crystalline forms C2, C3,C4, C5, C6, C7, C8, C9 or C10 in the mammal.
The invention is further defined by reference to the following examples describing in detail the preparation of ribociclib succinate crystalline forms of the invention. It will be apparent to those skilled in the art that many modifications, both to materials and methods, may be practiced without departing from the scope of the invention.
EXAMPLES:
Example 1: Preparation of ribociclib succinate Form-C2
To a suspension of ribociclib base (1.0 g) in l,4-dioxane (30ml) was added succinic acid (0.28g ) at 25-30°C. The reaction mixture was stirred at 25-30°C for 4 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (T l5g) and identified by XRD as Form-C2, as presented in figure 1.
Example 2: Preparation of ribociclib succinate Form-C3
To a suspension of ribociclib base (1.0 g) in ethyl acetate (30ml) was added succinic acid (0.28g ) at 25-30°C. The reaction mixture was stirred at 50-55°C for 10 hr. The solids were isolated by filtration and dried at 40°C under vacuum, resulted the title compound (l . lOg) and identified by XRD as Form-C3, as presented in figure 2.
Example 3: Preparation of ribociclib succinate Form-C4
Ribociclib succinate (1.0 g) was dissolved in a mixture of methanol (2 ml) and DCM (18 ml) at 25-30°C. The solvent was removed under reduced pressure below 40°C. To the residue was added hexane (20 ml) and the mixture was stirred at 25- 30 °C for about 75-80 hr. The solids were isolated by filtration and dried at 25- 30°C under vacuum, resulted the title compound (0.85g) and identified by XRD as Form-C4, as presented in figure 3.
Example 4: Preparation of ribociclib succinate Form-C5
Ribociclib base (5.0g) was dissolved in 2-pentanol (150 ml) at 60-65°C.To the above solution was added succinic acid (1.45 g) at 40-45°C. The reaction mixture was stirred at 25-30°C for 4 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (6.30g) and identified by XRD as Form-C5, as presented in figure 4.
Example 5: Preparation of ribociclib succinate Form-C6
Ribociclib succinate Form-C5 (2.5g) was suspended in n-Heptane (25ml). The reaction mixture was stirred at 75-80°C for 3 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (2.0g) and identified by XRD as Form-C6, as presented in figure 5.
Example 6: Preparation of ribociclib succinate Form-C7
Ribociclib succinate Form-C5 (2.0g) was suspended in n-Heptane (20ml). The reaction mixture was stirred at 75-80°C for 5 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (l .70g) and identified by XRD as Form-C7, as presented in figure 6.
Example 7: Preparation of ribociclib succinate Form-C8
Ribociclib base (l .Og) was suspended in n-propyl acetate (30ml) at 25-30°C.To the above suspension was added succinic acid (0.29g). The reaction mixture was stirred at 25-30°C for 4 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (T lOg) and identified by XRD as Form-C8, as presented in figure 7.
Example 8: Preparation of ribociclib succinate Form-C9
Ribociclib succinate (2.0g) was suspended in Ethanol (40ml). The reaction mixture was stirred at 65-70°C for 6 hr. The solids were isolated by filtration and dried at 50°C under vacuum, resulted the title compound (1.65 g) and identified by XRD as Form-C9, as presented in figure 8.
Example 9: Preparation of ribociclib succinate Form-CIO
Ribociclib succinate (l.Og) was exposed at 50°C, 90%RH in Humidification chamber for 4-5 days resulted the titled compound (1.0 g) and identified by XRD as Form-ClO, as presented in figure 9.
Claims
1. Crystalline polymorphic forms of ribociclib succinate, selected from the group consisting of Form-C2, Form-C3, Form-C4, Form-C5, Form-C6, Form-C7, Form- C8, Form-C9 and Form-ClO.
2. The crystalline Form-ClO of ribociclib succinate as claimed in claim 1, wherein the Form-ClO of ribociclib succinate is characterized by having an XRPD diffractogram comprising peaks at 6.45, 10.13, 10.73, 20.18 and 20.65± 0.2°20, substantially as depicted in Figure 9.
3. The crystalline Form-ClO of ribociclib succinate as claimed in claim 2, wherein the Form-ClO of ribociclib succinate is characterized by having an XRPD diffractogram comprising further peaks at 7.45, 11.92, 14.50, 23.25 and 25.34 ± 0.2°20, substantially as depicted in Figure 9.
4. The crystalline Form-ClO of ribociclib succinate as claimed in claim 1, wherein the Form-ClO of ribociclib succinate is characterized by a TGA substantially as depicted in Figure 10.
5. The crystalline Form-ClO of ribociclib succinate as claimed in claim 1, wherein the Form-ClO of ribociclib succinate is characterized by a DSC, substantially as depicted in Figure 11.
6. The crystalline Form-C8 of ribociclib succinate as claimed in claim 1, wherein the Form-C8 of ribociclib succinate is characterized by having an XRPD diffractogram comprising peaks at 10.08, 12.29, 19.56 and 19.92 ± 0.2°20, substantially as depicted in Figure 7.
7. The crystalline Form-C8 of ribociclib succinate as claimed in claim 6, wherein the Form-C8 of ribociclib succinate is characterized by having an
XRPD diffractogram comprising further peaks at 12.64, 13.92, 14.83,17.39, 22.00 and 23.56 ± 0.2°20, substantially as depicted in Figure 7.
8. The crystalline Form-C6 of ribociclib succinate as claimed in claim 1, wherein the Form-C6 of ribociclib succinate is characterized by having an XRPD diffractogram comprising peaks at 12.79,18.24, 18.84 and 21.90 ± 0.2°20, substantially as depicted in Figure 5.
9. The crystalline Form-C6 of ribociclib succinate as claimed in claim 8, wherein the Form-C6 of ribociclib succinate is characterized by having an XRPD diffractogram comprising further peaks at 9.36, 11.33, 15.62, 17.52, 20.35, 23.80 and 25.70± 0.2°20, substantially as depicted in Figure 5.
10. A process for preparing crystalline Form- C10 of ribociclib succinate, as claimed in any one of the claims 2 to 5, wherein, the process comprises, exposing ribociclib succinate at about 90% RH in a humidifier.
11. The process according to the claim 10, wherein operation temperature in the said humidifier is about 40°C to about 60°C, preferably about 45°C to about 55°C, more preferably about 50°C.
12. The process as claimed in claim 10 or 11, wherein residence time in the said humidifier is typically anywhere between a period of about 4 days to about 6 days, preferably 4 days to about 5 days, more preferably for about 5 days.
13. Crystalline Form- C10 of ribociclib succinate prepared by a process according to any one of the claims 10 to 12.
14. A process for preparing crystalline Form- C8 of ribociclib succinate as claimed in claim 6 or 7, wherein, the process comprises, suspending ribociclib base in a suitable solvent, treating with succinic acid; isolating the precipitated solid and drying the solid.
15. The process according to the claim 14, wherein the suspension is stirred at about 25°C to the boiling point of the solvent used.
16. The process according to the claim 15, wherein the suitable solvent is selected from, but not limited to ester solvents such as ethyl acetate, methyl acetate, isopropyl acetate and n-propyl acetate.
17. The process according to the claim 16, wherein the solvent is n-propyl acetate.
18. The process according to any one of the claims 14 to 17, wherein the suspension is stirred at about 25°C to about 30°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 4 hours.
19. The process according to any one of the claims 14 tol8, wherein the precipitated solid is isolated by filtration and dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hour to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
20. Crystalline Form- C8 of ribociclib succinate prepared by a process according to any one of the claims 14 to 19.
21. A process for preparing crystalline Form- C6 of ribociclib succinate, as claimed in claim 8 or 9, wherein, the process comprises, suspending crystalline Form-C5 of ribociclib succinate in a suitable solvent; stirring, isolating the solid and drying the solid.
22. The process according to the claim 21, wherein the suspension is stirred at about 25°C to the boiling point of the solvent used.
23. The process according to the claim 22, wherein the solvent is selected from, but not limited to non-polar solvents such as heptane, hexane, toluene and xylene.
24. The process according to the claim 23, wherein solvent is heptane.
25. The process according to any one of the claims 21 to 24, wherein the suspension is stirred at about 50°C to about 90°C, for a period of about 1 hour to about 20 hours, preferably 2 hours to about 15 hours, more preferably for about 3 hours to about 5 hours.
26. The process according to any one of the claims 21 to 25, wherein the precipitated solid is isolated by filtration and dried under reduced pressure at 30°C to about 80°C, preferably at 40°C to about 60°C, for a period of about 2 hours to about 10 hours, preferably 4 hours to about 8 hours, more preferably for about 6 hours.
27. Crystalline Form- C6 of ribociclib succinate prepared by a process according to any one of the claims 21 to 26.
28. A pharmaceutical composition comprising: (a) a therapeutically effective amount of a crystalline Form-ClO of ribociclib succinate according to any of the claims 2 to 5 or 13 ; and (b) at least one pharmaceutically acceptable carrier, diluent, vehicle or excipient.
29. A pharmaceutical composition comprising: (a) a therapeutically effective amount of a crystalline Form-C8 of ribociclib succinate according to any of the claims 6 to 7 or 20; and (b) at least one pharmaceutically acceptable carrier, diluent, vehicle or excipient.
30. A pharmaceutical composition comprising: (a) a therapeutically effective amount of a crystalline Form-C6 of ribociclib succinate according to any of
the claims 8 to 9 or 27; and (b) at least one pharmaceutically acceptable carrier, diluent, vehicle or excipient.
31. A method for the prevention or treatment of cancer which method comprises administering therapeutically effective amounts to a patient in need thereof crystalline Form-ClO of ribociclib succinate according to any of the claims 2 to 5 or 13.
32. A method for the prevention or treatment of cancer which method comprises administering therapeutically effective amounts to a patient in need thereof crystalline Form-C8 of ribociclib succinate according to any of the claims 6 to 7 or 20.
33. A method for the prevention or treatment of cancer which method comprises administering therapeutically effective amounts to a patient in need thereof crystalline Form-C6 of ribociclib succinate according to any of the claims 8 to 9 or 27.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN201821007800 | 2018-03-01 | ||
| IN201821007800 | 2018-03-01 | ||
| IN201821023064 | 2018-06-20 | ||
| IN201821023064 | 2018-06-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019167068A1 true WO2019167068A1 (en) | 2019-09-06 |
Family
ID=65995809
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2019/050159 WO2019167068A1 (en) | 2018-03-01 | 2019-02-27 | Novel polymorphs of ribociclib succinate |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2019167068A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024115680A1 (en) | 2022-12-01 | 2024-06-06 | Krka, D.D., Novo Mesto | Ribociclib salts and formulations thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010020675A1 (en) | 2008-08-22 | 2010-02-25 | Novartis Ag | Pyrrolopyrimidine compounds as cdk inhibitors |
| WO2012064805A1 (en) | 2010-11-10 | 2012-05-18 | Novartis Ag | Salt(s) of 7-cyclopentyl-2 -(5-piperazin-1-yl-pyridin-2-ylamino)-7h-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof |
| WO2016091221A1 (en) | 2014-12-12 | 2016-06-16 | 苏州晶云药物科技有限公司 | Salt of pyrrolo[2,3-d]pyrimidine compound and novel polymorph of salt |
| EP3156406A1 (en) | 2015-10-14 | 2017-04-19 | ratiopharm GmbH | Crystalline forms of ribociclib free base |
| WO2018051280A1 (en) * | 2016-09-15 | 2018-03-22 | Dr. Reddy’S Laboratories Limited | Process for preparation of ribociclib, its acid addition salts |
| US20180282342A1 (en) * | 2014-12-12 | 2018-10-04 | Crystal Pharmatech Co., Ltd. | Salt of Pyrrolo[2,3-D]pyrimidine Compound and Novel Polymorph of Salt |
| WO2019019959A1 (en) | 2017-07-27 | 2019-01-31 | 苏州晶云药物科技股份有限公司 | Crystal form of monosuccinate of ribociclib and preparation method and use thereof |
-
2019
- 2019-02-27 WO PCT/IN2019/050159 patent/WO2019167068A1/en active Application Filing
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010020675A1 (en) | 2008-08-22 | 2010-02-25 | Novartis Ag | Pyrrolopyrimidine compounds as cdk inhibitors |
| WO2012064805A1 (en) | 2010-11-10 | 2012-05-18 | Novartis Ag | Salt(s) of 7-cyclopentyl-2 -(5-piperazin-1-yl-pyridin-2-ylamino)-7h-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof |
| WO2016091221A1 (en) | 2014-12-12 | 2016-06-16 | 苏州晶云药物科技有限公司 | Salt of pyrrolo[2,3-d]pyrimidine compound and novel polymorph of salt |
| EP3231805A1 (en) * | 2014-12-12 | 2017-10-18 | Crystal Pharmatech Co. Ltd. | Salt of pyrrolo[2,3-d]pyrimidine compound and novel polymorph of salt |
| US20180282342A1 (en) * | 2014-12-12 | 2018-10-04 | Crystal Pharmatech Co., Ltd. | Salt of Pyrrolo[2,3-D]pyrimidine Compound and Novel Polymorph of Salt |
| EP3156406A1 (en) | 2015-10-14 | 2017-04-19 | ratiopharm GmbH | Crystalline forms of ribociclib free base |
| WO2018051280A1 (en) * | 2016-09-15 | 2018-03-22 | Dr. Reddy’S Laboratories Limited | Process for preparation of ribociclib, its acid addition salts |
| WO2019019959A1 (en) | 2017-07-27 | 2019-01-31 | 苏州晶云药物科技股份有限公司 | Crystal form of monosuccinate of ribociclib and preparation method and use thereof |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024115680A1 (en) | 2022-12-01 | 2024-06-06 | Krka, D.D., Novo Mesto | Ribociclib salts and formulations thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2262793B1 (en) | Nilotinib hci crystalline forms | |
| JP7407740B2 (en) | Crystal forms of TLR7/TLR8 inhibitors | |
| CA2958139A1 (en) | Solid state forms of ibrutinib | |
| US20220144851A1 (en) | Novel polymorphs of integrase inhibitor | |
| WO2012061469A2 (en) | Crystalline forms of pralatrexate | |
| WO2015138933A1 (en) | Solid state forms of dolutegravir sodium | |
| WO2018109786A1 (en) | Novel polymoprphs and salts of polycyclic carbamoyl pyridone derivatives | |
| WO2019003249A1 (en) | Polymorphic forms of baricitinib | |
| AU2021277593A1 (en) | Solid forms of [(1 S)-1 -[(2S,4R,5R)-5-(5-amino-2-oxo-thiazolo[4,5-d]pyrimidin-3-yl)-4-hydroxy-te trahydrofuran-2-yl]propyl] acetate | |
| AU2015342444A1 (en) | Crystalline form of JAK kinase inhibitor bisulfate and a preparation method thereof | |
| US20220002302A1 (en) | Novel polymorphs of acalabrutinib, a bruton's tyrosine kinase inhibitor | |
| WO2019167068A1 (en) | Novel polymorphs of ribociclib succinate | |
| CA2724320A1 (en) | Solid states of aliskiren free base | |
| US20200407382A1 (en) | Polymorphic forms of (9-[(r)-2-[[(s)-[[(s)-1-(isopropoxycarbonyl)ethyl]amino]phenoxy phosphinyl]methoxy]propyl] adenine and pharmaceutically acceptable salts thereof | |
| WO2017114456A1 (en) | Salt of morpholine derivative and crystalline form thereof, as well as preparation method, pharmaceutical composition and use of the same | |
| US11339164B2 (en) | Crystalline form E1 of larotrectinib ethanesulfonate | |
| US20220009929A1 (en) | Polymorphic forms of ibrutinib | |
| JP2018518515A (en) | Polymorphs of phenylaminopyrimidine compounds or salts thereof | |
| JP2021533111A (en) | Crystal form of LTA4H inhibitor | |
| US8198470B2 (en) | Crystalline form II of tigecycline and processes for preparation thereof | |
| US20240239791A1 (en) | Processes for the synthesis of valbenazine | |
| US20240287090A1 (en) | Solid state forms of relugolix | |
| WO2022224269A1 (en) | Co-crystals, salts and solid forms of niraparib | |
| WO2022081502A1 (en) | Solid state forms of lorecivivint | |
| WO2008088900A2 (en) | Polymorphic forms of rimonabant base and processes for preparation thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19714809 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19714809 Country of ref document: EP Kind code of ref document: A1 |