WO2019080724A1 - Nucleoside phosphate compound and preparation method therefor and use thereof - Google Patents
Nucleoside phosphate compound and preparation method therefor and use thereofInfo
- Publication number
- WO2019080724A1 WO2019080724A1 PCT/CN2018/109983 CN2018109983W WO2019080724A1 WO 2019080724 A1 WO2019080724 A1 WO 2019080724A1 CN 2018109983 W CN2018109983 W CN 2018109983W WO 2019080724 A1 WO2019080724 A1 WO 2019080724A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- hydrogen
- independently selected
- alkyl
- compound
- Prior art date
Links
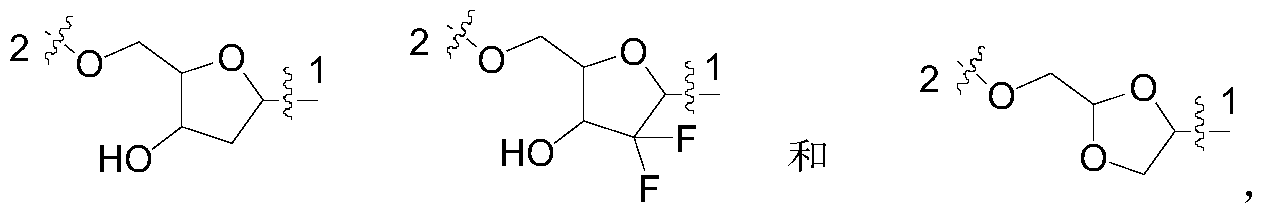
- 0 C*(*)C(C1(F)F)OC(CO)C1O Chemical compound C*(*)C(C1(F)F)OC(CO)C1O 0.000 description 4
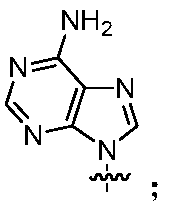
- WRXCXOUDSPTXNX-UHFFFAOYSA-N C[n]1c2ncnc(N)c2nc1 Chemical compound C[n]1c2ncnc(N)c2nc1 WRXCXOUDSPTXNX-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
- A61K31/7072—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid having two oxo groups directly attached to the pyrimidine ring, e.g. uridine, uridylic acid, thymidine, zidovudine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H1/00—Processes for the preparation of sugar derivatives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/06—Pyrimidine radicals
- C07H19/10—Pyrimidine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
Definitions
- the present invention relates to a nucleoside phosphate compound, a pharmaceutical composition and kit comprising the same, a process for the preparation thereof, and use thereof for preventing or treating cancer and/or a tumor and a related disease thereof.
- Nucleoside analogues are an important class of anti-tumor drugs.
- the main mechanism of action is to inhibit tumors by specifically interfering with DNA synthesis in tumor cells, affecting the transcription process of RNA or protein synthesis, or directly acting on these macromolecules.
- the cells divide and proliferate, thereby causing apoptosis.
- nucleoside antitumor drugs have very broad development prospects.
- the structural modification of known nucleoside antitumor drugs, improving the bioavailability of drugs and reducing adverse reactions have become the research hotspots of this class of antitumor drugs.
- nucleoside drugs such as the anticancer drug gemcitabine
- Traditional nucleoside drugs generate nucleoside triphosphates through phosphorylation and metabolism in the body.
- the latter is inserted into the DNA strand to inhibit DNA synthesis and prevent cell progression from G1 to S phase, resulting in tumor cells.
- the G1 phase arrests, thereby inhibiting the malignant proliferation of tumor cells (Oncology. 2002, 62(4), 354-362).
- these traditional nucleoside drugs often have resistance problems, mainly due to transporter variation and phosphorylation down-regulation.
- the former affects the absorption of the drug, resulting in poor bioavailability; while the latter affects the monophosphorylation of the nucleoside drug, which in turn causes the deficiency of the active component triphosphate.
- the phosphorylation process requires the participation of three different kinases. Nucleoside analogs and their monophosphate and diphosphate metabolites may not be good substrates for the corresponding kinases. Studies have shown that the first kinase is most selective for the substrate during phosphorylation. Therefore the first step of phosphorylation is usually the most difficult step. To overcome this difficulty, delivery of monophosphate to cells is a necessary means. However, nucleoside monophosphates are negatively charged, hard to pass through the cell membrane, and are easily degraded by phosphatase.
- the conversion of the nucleoside analog to the corresponding phosphate or phosphoramide prodrug can achieve the purpose of reducing the polarity of the drug, enhancing the biofilm penetration ability, and improving the bioavailability in the body.
- the efficiency of phosphorylation also determines the activity of nucleoside analogs as polymerase inhibitors or reverse transcriptase inhibitors. Specifically, the phosphorylation efficiency depends on the amount of nucleoside triphosphate produced, the rate of formation, and the time of existence. The more the amount of nucleoside triphosphate produced, the faster the rate of formation, and the longer the time of existence, the higher the inhibitory activity.
- nucleoside phosphate compound or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or precursor thereof
- the compound is particularly easily metabolized into active nucleoside triphosphate in liver tissue, and has an excellent preventive or therapeutic effect on liver cancer.
- the compound has the structure of the following formula (I):
- B is selected from:
- L is selected from C 1-6 alkylene, C 2-6 alkenylene, C 2-6 alkynylene, optionally substituted by one or more R b , said alkylene, alkenylene or sub
- An alkynyl group is optionally interrupted by one or more -O-, -NR- or -S-;
- X, Y and Z are each independently selected from CH 2 , O, S and NR at each occurrence;
- R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , -N 3 , C 1-6 alkyl, C 3- 6 cycloalkyl, C 2-6 alkenyl, halo C 2-6 alkenyl and C 2-6 alkynyl;
- q 0, 1, 2, 3, 4, 5, 6, 7, or 8, with the following conditions:
- q is not greater than the number of positions on the corresponding group that can be substituted
- each R b may be the same or different;
- n 0, 1, 2 or 3, provided that:
- n is not greater than the number of positions on the corresponding group that can be substituted
- each R a may be the same or different;
- Ar 1 and Ar 2 are each independently selected from C 6-14 aryl and 5-14 membered heteroaryl, the aryl and heteroaryl optionally being substituted by one or more R c ;
- R c is each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , C 1-6 alkyl, halo C 1-6 alkyl, C 1-6 alkoxy group, C 1-6 alkylthio group, C 3-6 cycloalkyl group, 3-10 membered heterocycloalkyl group and C 2-6 alkynyl group;
- R is each independently selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl;
- n 1, 2 or 3;
- p 1, 2 or 3;
- R 1 and R 2 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl.
- the alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
- R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 3 and R 4 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl.
- the alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
- R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 2 and R 3 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl and C 7-20 aralkyl, said alkyl, ring
- the alkyl, alkoxy, aryl and aralkyl groups are each optionally substituted by one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN and -NO 2 ;
- R 5 and the substituent R c on Ar 2 together with the carbon atom to which they are bonded form a C 4-6 cycloalkyl or 4-10 membered heterocycloalkyl fused to Ar 2 ;
- R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
- Ar 1 is not ⁇ -naphthyl or ⁇ -naphthyl
- Ar 2 is not 2-methylnaphthyl, 3-methylphenyl or 4-methylphenyl;
- R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
- Ar 2 is not 3-methylphenyl, 4-methylphenyl, 4-fluorophenyl, 2,6-dimethylphenyl or 2-methylnaphthyl;
- Ar 2 is not phenyl, 4-methylphenyl, 2-methoxyphenyl, 2-methoxy-4-methylphenyl, 2,4-dimethylphenyl, 2-methyl- 4-methoxyphenyl or 2-methyl-4-tert-butylphenyl;
- R 1 and R 2 are not the case where R 1 and R 2 are simultaneously hydrogen; or R 1 is hydrogen and R 2 is methyl; or R 1 is methyl and R 2 is hydrogen;
- Ar 2 is not a 2-methylnaphthyl group
- Ar 2 is not 2-methylphenyl
- Ar 2 is not 2-methylphenyl
- Ar 2 is not a 2-methylphenyl group.
- Another aspect of the invention provides a pharmaceutical composition
- a pharmaceutical composition comprising a prophylactically or therapeutically effective amount of a compound of the invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph thereof , solvate, hydrate, metabolite or prodrug, or mixtures thereof, and one or more pharmaceutically acceptable carriers.
- Another aspect of the present invention provides a method of preparing a pharmaceutical composition of the present invention, which comprises administering a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer thereof, Polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, are combined with one or more pharmaceutically acceptable carriers.
- kits comprising a compound of the invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof, hydrated thereof , metabolite or prodrug, or a mixture thereof, or a pharmaceutical composition of the invention.
- Another aspect of the invention provides a compound of the invention, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or the use of a mixture thereof or a pharmaceutical composition of the present invention for the preparation of a medicament for preventing or treating cancer and/or a tumor and a related disease thereof.
- Another aspect of the invention provides a compound of the invention, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or a mixture thereof or a pharmaceutical composition of the invention for use in the prevention or treatment of cancer and/or tumors and related diseases.
- Another aspect of the invention provides a method of preventing or treating cancer and/or a tumor and related diseases, the method comprising administering to an individual in need thereof an effective amount of a compound of the invention or a pharmaceutically acceptable salt or ester thereof , stereoisomers, tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
- Another aspect of the invention provides a method of preparing a compound of the invention, the method comprising the steps of:
- alkylene denotes a saturated divalent hydrocarbon group, preferably a saturated divalent hydrocarbon group having 1, 2, 3, 4, 5 or 6 carbon atoms, such as methylene, ethylene, Propylene or butylene.
- alkenylene denotes a divalent hydrocarbon radical containing one or more double bonds, preferably having 2, 3, 4, 5 or 6 carbon atoms, such as ethenylene, propenylene or Allylene.
- alkenylene groups the compounds may exist in pure E (ent ought) form, pure Z (zusammen) form, or any mixture thereof.
- alkynylene denotes a divalent hydrocarbon radical containing one or more triple bonds, preferably having 2, 3, 4, 5 or 6 carbon atoms, for example ethynylene or propynylene. .
- alkyl is defined as a straight or branched saturated aliphatic hydrocarbon group. In some embodiments, an alkyl group has from 1 to 12, such as from 1 to 6 carbon atoms.
- C1-6 alkyl refers to a straight or branched chain group having from 1 to 6 carbon atoms (eg, methyl, ethyl, n-propyl, isopropyl, N-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl or n-hexyl), which is optionally substituted by one or more (such as 1 to 3) suitable substituents such as halogen (at this time)
- haloalkyl such as CF 3 , C 2 F 5 , CHF 2 , CH 2 F, CH 2 CF 3 , CH 2 Cl or -CH 2 CH 2 CF 3 , and the like
- alkenyl is defined as an unsaturated straight or branched aliphatic hydrocarbon group containing at least one carbon to carbon double bond. In some embodiments, an alkenyl group has 2 to 10, such as 2 to 6 carbon atoms.
- C 2-6 alkenyl refers to a straight or branched alkenyl group containing from 2 to 6 carbon atoms, such as ethenyl, 1-propenyl, 2-propenyl, 1 a butenyl or 2-butenyl group which is optionally substituted by one or more (for example 1, 2, 3 or 4) suitable substituents.
- alkynyl is defined as an unsaturated straight or branched aliphatic hydrocarbon group containing at least one carbon-carbon triple bond.
- an alkynyl group has 2 to 10, such as 2 to 6 carbon atoms.
- C 2-6 alkynyl refers to a straight or branched alkynyl group containing from 2 to 6 carbon atoms, such as ethynyl, 1-propynyl, 2-propynyl. , 1-butynyl or 2-butynyl, optionally substituted by one or more (eg, 1, 2, 3 or 4) suitable substituents.
- cycloalkyl refers to a saturated or unsaturated, non-aromatic monocyclic or polycyclic (such as bicyclic) hydrocarbon ring (eg, a monocyclic ring such as cyclopropyl, cyclobutyl, cyclopentyl, Cyclohexyl, cycloheptyl, cyclooctyl, cyclodecyl, or bicyclic, including spiro, fused or bridged systems (such as bicyclo [1.1.1] pentyl, bicyclo [2.2.1] heptyl, bicyclo [ 3.2.1] octyl or bicyclo [5.2.0] decyl, decalinyl, etc.), which are optionally substituted by one or more (such as 1 to 3) suitable substituents.
- bicyclic hydrocarbon ring eg, a monocyclic ring such as cyclopropyl, cyclobutyl, cyclopentyl, Cyclohe
- C 3-6 cycloalkyl refers to a saturated or unsaturated, non-aromatic monocyclic or polycyclic ring having 3 to 6 ring-forming carbon atoms. (such as a bicyclic) hydrocarbon ring (such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl), which is optionally substituted by one or more (such as 1 to 3) suitable substituents, such as methyl substitution Cyclopropyl.
- aryl refers to an all-carbon monocyclic or fused-ring polycyclic aromatic group having a conjugated pi-electron system. In some embodiments, the aryl has 6 to 14, such as 6 to 10 carbon atoms.
- C6-14 aryl means an aromatic group containing from 6 to 14 carbon atoms, such as phenyl or naphthyl.
- Aryl is optionally substituted with one or more (such as 1 to 3) suitable substituent (e.g., halo, -OH, -CN, -NO 2, C 1-6 alkyl, etc.) substituted.
- aralkyl denotes an aryl-substituted alkyl group, wherein the aryl group and the alkyl group are as defined herein.
- the aryl group can have from 6 to 14 carbon atoms and the alkyl group can have from 1 to 6 carbon atoms.
- Exemplary aralkyl groups include, but are not limited to, benzyl, phenylethyl, phenylpropyl, phenylbutyl, and the like.
- heteroaryl refers to a monovalent monocyclic, bicyclic or tricyclic aromatic ring system having 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 rings.
- the heteroaryl is selected from the group consisting of thienyl, furyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, triazolyl, thia A oxazolyl group or the like, and a benzo derivative thereof; or a pyridyl group, a pyridazinyl group, a pyrimidinyl group, a pyrazinyl group, a triazinyl group or the like, and a benzo derivative thereof.
- halo or halogen group, as used herein, is defined to include F, Cl, Br or I.
- alkoxy refers to an alkyl group, as defined above, appended to the parent molecular moiety through an oxygen atom.
- Representative examples of C1-6 alkoxy include, but are not limited to, methoxy, ethoxy, propoxy, n-butoxy, isobutoxy, tert-butoxy, pentyloxy, hexyloxy, and the like. .
- alkylthio refers to an alkyl group, as defined above, appended to the parent molecular moiety through a sulfur atom.
- Representative examples of C1-6 alkylthio include, but are not limited to, methylthio, ethylthio, tert-butylthio, hexylthio, and the like.
- substituted means that one or more (eg, 1, 2, 3 or 4) hydrogens on the designated atom are replaced by the selection of the indicated group, provided that the specified atom is not exceeded.
- the normal valence of the current case and the substitution form a stable compound. Combinations of substituents and/or variables are permissible only if such combinations form stable compounds.
- substituent may be unsubstituted or (2) substituted. If the carbon of the substituent is described as being optionally substituted by one or more of the list of substituents, then one or more hydrogens on the carbon (to the extent of any hydrogen present) may be independently and/or together independently The optional substituents selected are substituted. If the nitrogen of the substituent is described as being optionally substituted by one or more of the list of substituents, then one or more hydrogens on the nitrogen (to the extent of any hydrogen present) may each be independently selected. Substitute substitution.
- each substituent is selected independently of the other.
- each substituent may be the same or different from another (other) substituent.
- one or more means 1 or more than 1, such as 2, 3, 4, 5 or 10 under reasonable conditions.
- a point of attachment of a substituent may come from any suitable position of the substituent.
- the invention also includes all pharmaceutically acceptable isotopically-labeled compounds which are identical to the compounds of the invention, except that one or more atoms are of the same atomic number but the atomic mass or mass number differs from the atomic mass prevailing in nature. Or atomic substitution of mass.
- isotopes in the compounds of the invention include, but are not limited to, hydrogen isotopes (e.g., 2 H, 3 H); carbon isotopes (e.g., 11 C, 13 C, and 14 C); chlorine isotopes (e.g., 36 Cl) Fluorine isotopes (eg 18 F); isotopes of iodine (eg 123 I and 125 I); isotopes of nitrogen (eg 13 N and 15 N); isotopes of oxygen (eg 15 O, 17 O and 18 O); phosphorus Isotope (eg 32 P); and sulfur isotope (eg 35 S).
- hydrogen isotopes e.g., 2 H, 3 H
- carbon isotopes e.g., 11 C, 13 C, and 14 C
- chlorine isotopes e.g., 36 Cl
- Fluorine isotopes eg 18 F
- Certain isotopically-labeled compounds of the invention are useful in drug and/or substrate tissue distribution studies (e.g., assays).
- the radioisotope ruthenium (i.e., 3 H) and carbon-14 (i.e., 14 C) are particularly useful for this purpose because of their ease of incorporation and ease of detection.
- Substitution with positron emitting isotopes eg, 11 C, 18 F, 15 O, and 13 N
- PET positron emission tomography
- Isotopically labeled compounds of the invention can be prepared by replacing the previously employed non-labeled reagents with suitable isotopically labeled reagents by methods analogous to those described in the accompanying routes and/or examples and preparations.
- the pharmaceutically acceptable solvates of the present invention include those in which the crystallization solvent can be substituted with an isotope, for example, D 2 O, acetone-d 6 or DMSO-d 6 .
- stereoisomer denotes an isomer formed by at least one asymmetric center.
- asymmetric center which can produce a racemic mixture, a single enantiomer, a mixture of diastereomers, and Separate diastereomers.
- Specific individual molecules can also exist as geometric isomers (cis/trans).
- the compounds of the invention may exist as mixtures (often referred to as tautomers) of two or more different forms in a rapidly balanced structure.
- tautomers include keto-enol tautomers, phenol-keto tautomers, nitroso-oxime tautomers, imine-enamine tautomers Wait. It is to be understood that the scope of the present application covers all such ratios in any ratio (eg, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99). %) isomer or a mixture thereof.
- Solid lines can be used in this article Solid wedge Virtual wedge
- the linkages in the compounds of the invention are depicted.
- the use of solid lines to delineate linkages to an asymmetric atom in a compound of the invention is meant to include all possible stereoisomers at the atom (e.g., specific enantiomers, racemic mixtures, and the like).
- a solid wedge or a virtual wedge is used to characterize the linkages to the asymmetric atoms in the compounds of the invention, indicating the stereoisomers shown.
- solid wedges and virtual wedges are used to define relative stereochemistry rather than absolute stereochemistry.
- the compounds of the invention may be stereoisomers (including cis and trans isomers, optical isomers (eg, R and S enantiomers), diastereomers, Geometric isomers, rotamers, conformers, atropisomers, and mixtures thereof exist.
- the compounds of the invention may exhibit more than one type of isomerism and consist of a mixture thereof (e.g., a racemic mixture and a diastereomeric pair).
- the invention encompasses all possible crystalline forms or polymorphs of the compounds of the invention, which may be a single polymorph or a mixture of more than one polymorph in any ratio.
- compositions of the invention may exist in free form for treatment or, where appropriate, in the form of their pharmaceutically acceptable derivatives.
- pharmaceutically acceptable derivatives include, but are not limited to, pharmaceutically acceptable salts, esters, solvates, metabolites or prodrugs, which can be directly administered to a patient in need thereof
- the compound of the invention or a metabolite or residue thereof is provided indirectly or indirectly.
- a compound of the invention it is also intended to encompass the various derivative forms described above for the compound.
- the pharmaceutically acceptable salts of the compounds of the present invention include the acid addition salts and base addition salts thereof.
- Suitable acid addition salts are formed from acids which form pharmaceutically acceptable salts. Examples include aspartate, bicarbonate/carbonate, hydrogen sulfate/sulfate, fumarate, glucoheptonate, gluconate, glucuronate, hexafluorophosphate, hydrobromine Acid salts/bromides, hydroiodides/iodides, maleates, methyl sulfates, nicotinates, nitrates, orotates, palmitates and other similar salts.
- Suitable base addition salts are formed from bases which form pharmaceutically acceptable salts. Examples include aluminum salts, arginine salts, choline salts, diethylamine salts, lysine salts, magnesium salts, meglumine salts, potassium salts, sodium salts, tromethamine salts, and other similar salts.
- esters means an ester derived from a compound of the formulae herein, which includes a physiologically hydrolyzable ester (which can be hydrolyzed under physiological conditions to release the free acid or alcohol form of the invention). Compound).
- the compounds of the invention may also be esters per se.
- the compound of the present invention may exist in the form of a solvate (preferably a hydrate) wherein the compound of the present invention contains a polar solvent as a structural element of the crystal lattice of the compound, particularly such as water, methanol or ethanol.
- a polar solvent as a structural element of the crystal lattice of the compound, particularly such as water, methanol or ethanol.
- the amount of polar solvent, particularly water, may be present in stoichiometric or non-stoichiometric ratios.
- metabolites of the compounds of the invention i.e., substances formed in vivo upon administration of a compound of the invention. Such products may be produced, for example, by oxidation, reduction, hydrolysis, amidation, deamidation, esterification, delipidization, enzymatic hydrolysis, and the like of the administered compound. Accordingly, the invention includes metabolites of the compounds of the invention, including compounds prepared by contacting a compound of the invention with a mammal for a time sufficient to produce a metabolic product thereof.
- the invention further includes within its scope prodrugs of the compounds of the invention which are certain derivatives of the compounds of the invention which may themselves have less or no pharmacological activity, when administered to or into the body It can be converted to a compound of the invention having the desired activity by, for example, hydrolytic cleavage.
- prodrugs will be functional group derivatives of the compounds which are readily converted in vivo to the desired therapeutically active compound. Additional information on the use of prodrugs can be found in "Pro-drugs as Novel Delivery Systems", Volume 14, ACS Symposium Series (T. Higuchi and V. Stella) and "Bioreversible Carriers in Drug Design," Pergamon Press, 1987 ( Edited by EBRoche, American Pharmaceutical Association).
- Prodrugs of the invention may, for example, be known by those skilled in the art as “pro-moiety” (e.g., “Design of Prodrugs", H. Bundgaard (Elsevier, 1985))" It is prepared in place of the appropriate functional groups present in the compounds of the invention.
- the invention also encompasses compounds of the invention containing a protecting group.
- a protecting group In any process for preparing a compound of the invention, it may be necessary and/or desirable to protect a sensitive group or reactive group on any of the molecules of interest, thereby forming a chemically protected form of the compound of the invention. This can be achieved by conventional protecting groups such as those described in Protective Groups in Organic Chemistry, ed. JFW McOmie, Plenum Press, 1973; and TW Greene & P. GM Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, 1991. Protecting groups, which are incorporated herein by reference. The protecting group can be removed at a suitable subsequent stage using methods known in the art.
- B is selected from:
- L is selected from C 1-6 alkylene, C 2-6 alkenylene, C 2-6 alkynylene, optionally substituted by one or more R b , said alkylene, alkenylene or sub
- An alkynyl group is optionally interrupted by one or more -O-, -NR- or -S-;
- X, Y and Z are each independently selected from CH 2 , O, S and NR at each occurrence;
- R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , -N 3 , C 1-6 alkyl, C 3- 6 cycloalkyl, C 2-6 alkenyl, halogenated C 2-6 alkenyl and C 2-6 alkynyl;
- q 0, 1, 2, 3, 4, 5, 6, 7, or 8, with the following conditions:
- q is not greater than the number of positions on the corresponding group that can be substituted
- each R b may be the same or different;
- n 0, 1, 2 or 3, provided that:
- n is not greater than the number of positions on the corresponding group that can be substituted
- each R a may be the same or different;
- Ar 1 and Ar 2 are each independently selected from C 6-14 aryl and 5-14 membered heteroaryl, the aryl and heteroaryl optionally being substituted by one or more R c ;
- R c is each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , C 1-6 alkyl, halo C 1-6 alkyl, C 1-6 alkoxy group, C 1-6 alkylthio group, C 3-6 cycloalkyl group, 3-10 membered heterocycloalkyl group and C 2-6 alkynyl group;
- R is each independently selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl;
- n 1, 2 or 3;
- p 1, 2 or 3;
- R 1 and R 2 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl.
- the alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
- R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 3 and R 4 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl.
- the alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
- R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 2 and R 3 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
- R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl and C 7-20 aralkyl, said alkyl, ring
- the alkyl, alkoxy, aryl and aralkyl groups are each optionally substituted by one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN and -NO 2 ;
- R 5 and the substituent R c on Ar 2 together with the carbon atom to which they are bonded form a C 4-6 cycloalkyl or 4-10 membered heterocycloalkyl fused to Ar 2 ;
- R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
- Ar 1 is not ⁇ -naphthyl or ⁇ -naphthyl
- Ar 2 is not 2-methylnaphthyl, 3-methylphenyl or 4-methylphenyl;
- R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
- Ar 2 is not 3-methylphenyl, 4-methylphenyl, 4-fluorophenyl, 2,6-dimethylphenyl or 2-methylnaphthyl;
- Ar 2 is not phenyl, 4-methylphenyl, 2-methoxyphenyl, 2-methoxy-4-methylphenyl, 2,4-dimethylphenyl, 2-methyl- 4-methoxyphenyl or 2-methyl-4-tert-butylphenyl;
- R 1 and R 2 are not the case where R 1 and R 2 are simultaneously hydrogen; or R 1 is hydrogen and R 2 is methyl; or R 1 is methyl and R 2 is hydrogen;
- Ar 2 is not a 2-methylnaphthyl group
- Ar 2 is not 2-methylphenyl
- Ar 2 is not 2-methylphenyl
- X is O or NR and R is selected from the group consisting of hydrogen, C1-6 alkyl and C3-6 cycloalkyl. In some preferred embodiments, X is O or NR and R is selected from the group consisting of hydrogen and C1-6 alkyl. In some more preferred embodiments, X is O, NH or NCH 3. In some further more preferred embodiments, X is NH.
- Y is O or S. In some preferred embodiments, Y is O.
- Z is O, S or CH 2 . In some preferred embodiments, Z is O.
- R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -N(R) 2 , -N 3 and C 1-6 alkyl. In some preferred embodiments, R a and R b are each independently selected from the group consisting of halogen, -OH, and -N(R) 2 at each occurrence. In some more preferred embodiments, R a and R b are each independently selected from the group consisting of F, -OH, and NH 2 at each occurrence.
- each occurrence of R c is independently selected from the group consisting of hydrogen, halogen, C 1-6 alkyl, and C 1-6 alkoxy.
- R c is each independently methyl, t-butyl, methoxy, fluoro or chloro at each occurrence.
- B is selected from:
- B is selected from the group consisting of:
- B is selected from the group consisting of
- L is selected from:
- One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
- L is selected from the group consisting of:
- One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
- L is selected from the group consisting of:
- One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
- Ar 1 and Ar 2 are each independently selected from the group consisting of phenyl, naphthyl and 5-6 membered heteroaryl (eg thienyl, pyridyl or pyrazolyl), said phenyl, naphthalene
- the base and the 5-6 membered heteroaryl are each independently optionally substituted with 1 or 2 substituents independently selected from the group consisting of hydrogen, C1-6 alkyl, halogen and C1-6 alkoxy.
- Ar 1 and Ar 2 are each independently selected from the group consisting of phenyl, naphthyl, thienyl, pyridyl and pyrazolyl, phenyl, naphthyl, thienyl, pyridyl and pyrazole
- the groups are each independently optionally substituted with 1 or 2 substituents independently selected from the group consisting of hydrogen, methyl, tert-butyl, methoxy, fluoro and chloro.
- Ar 1 and Ar 2 are each independently selected from phenyl, which is optionally substituted with 1 methyl.
- R 1 and R 2 are each independently selected from hydrogen and C 1-6 alkyl, optionally substituted by one or more selected from the group consisting of hydrogen, halogen, -OH, -CN Substituting with a substituent of -NO 2 ; or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group.
- R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group.
- R 1 and R 2 are each independently selected from the group consisting of hydrogen and methyl.
- R 3 and R 4 are each independently selected from hydrogen and C 1-6 alkyl, the alkyl optionally being selected from one or more selected from the group consisting of hydrogen, halogen, -OH, -CN, and Substituting a substituent of -NO 2 ; or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group.
- R 3 and R 4 are each independently selected from hydrogen and methyl; or R 3 and R 4 together with the carbon atom to which they are attached form a cyclopropyl group.
- both R 3 and R 4 are hydrogen.
- R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, each of which is optionally selected from one or more selected from the group consisting of hydrogen, Substituents for halogen, -OH, -CN, and -NO 2 are substituted. In some preferred embodiments, R 5 is hydrogen.
- m is 1 or 2.
- p is 1 or 2.
- the compound of the invention is a compound of formula (I-1), (I-2) or (I-3) or a pharmaceutically acceptable salt, ester, stereoisomer thereof, Tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof,
- X, Y, Z, R a , R b , q, n, Ar 1 , Ar 2 , m, p, R 1 , R 2 , R 3 , R 4 and R 5 are as defined above; preferably, X is NR, Y and Z are both O, and each occurrence of R a and R b is independently selected from the group consisting of halogen, -OH and -N(R) 2 , q is 0, 1, 2 or 3, and n is 1 or 2, Ar 1 and Ar 2 are each independently selected from a C 6-10 aryl group optionally substituted by 1 or 2 C 1-6 alkyl groups, m is 1 or 2, p is 1 or 2, and R 1 , R 2 , R 3 , R 4 , R 5 and R are each independently hydrogen or C 1-6 alkyl; and more preferably, X is NH, Y and Z are both O, and R a and R b are Each occurrence is independently selected from F, -OH and -
- the compound of the invention is a compound of formula (II) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof , hydrates, metabolites or prodrugs, or mixtures thereof,
- n, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted by 1 methyl group.
- the compound of the invention is a compound of formula (III) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof , hydrates, metabolites or prodrugs, or mixtures thereof,
- n, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m Is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted with 1 methyl group.
- the compound of the present invention is a compound of the formula (IV) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph or solvate thereof. , hydrates, metabolites or prodrugs, or mixtures thereof,
- n, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted by 1 methyl group.
- the present invention encompasses compounds obtained by any combination of the various embodiments.
- the compound of formula (I) of the invention is selected from the group consisting of
- Another object of the present invention is to provide a process for the preparation of a compound of the above formula (I) which comprises the steps of:
- step 1
- step 1 is carried out in the presence of an organic or inorganic base.
- the organic base includes, but is not limited to, sodium t-butoxide, triethylamine, DIPEA, pyridine or DMAP.
- the inorganic base includes, but is not limited to, NaH, NaOH, Na 2 CO 3 or K 2 CO 3 .
- step 1 is carried out at a temperature of from -80 °C to 0 °C, preferably from -70 °C to -20 °C.
- the molar ratio of the compound of formula a, the compound of formula b and the compound of formula c is 1: (0.5-2): (0.5-2), preferably 1: (0.8-2): ( 0.8-2), more preferably 1: (1-1.5): (1-1.5).
- the molar ratio of the compound of formula a, the compound of formula b, the compound of formula c to the organic or inorganic base is 1: (0.5-2): (0.5-2): (0.5-2
- step 2 is carried out in the presence of an organic or inorganic base.
- the organic base or inorganic base includes, but is not limited to, t-butyl magnesium chloride, n-butyl lithium, lithium diisopropylamide, sodium t-butoxide, triethylamine, DIPEA, pyridine, DMAP, NaH, NaOH, Na 2 CO 3 or K 2 CO 3 .
- step 2 is carried out at a temperature of from -80 °C to 25 °C, preferably from -30 °C to 10 °C.
- the molar ratio of the compound of formula d to the compound of formula e is 1: (0.5-2), preferably 1: (0.75-1.5).
- the molar ratio of the compound of formula d, the compound of formula e to the organic or inorganic base is 1: (0.5-2): (1-3), preferably 1: (0.75-1.5): (1-2).
- the above steps 1 and 2 can be carried out in an organic solvent.
- the organic solvent may be a reaction solvent commonly used in the art, such as, but not limited to, N,N-dimethylformamide, dimethyl sulfoxide, N-methylpyrrolidone, saturated hydrocarbons (such as cyclohexane, hexane). Etc.), halogenated hydrocarbons (such as dichloromethane, chloroform, 1,2-dichloroethane, etc.), ethers (such as tetrahydrofuran, diethyl ether, dioxane, 1,2-dimethoxyethane, etc.) , nitriles (such as acetonitrile, etc.) and their mixed solvents.
- a reaction solvent commonly used in the art, such as, but not limited to, N,N-dimethylformamide, dimethyl sulfoxide, N-methylpyrrolidone, saturated hydrocarbons (such as cyclohexane,
- Another object of the present invention is to provide a pharmaceutical composition
- a pharmaceutical composition comprising a prophylactically or therapeutically effective amount of a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph thereof , solvate, hydrate, metabolite or prodrug, or mixtures thereof, and one or more pharmaceutically acceptable carriers.
- “Pharmaceutically acceptable carrier” in the context of the present invention means a diluent, adjuvant, excipient or vehicle with which the therapeutic agent is administered, and which is suitable for contacting humans and/or within the scope of sound medical judgment. Tissues of other animals without excessive toxicity, irritation, allergic reactions, or other problems or complications corresponding to a reasonable benefit/risk ratio.
- Pharmaceutically acceptable carriers that can be used in the pharmaceutical compositions of the present invention include, but are not limited to, sterile liquids such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, minerals. Oil, sesame oil, etc. Water is an exemplary carrier when the pharmaceutical composition is administered intravenously. It is also possible to use physiological saline and an aqueous solution of glucose and glycerin as a liquid carrier, particularly for injection.
- Suitable pharmaceutical excipients include starch, glucose, lactose, sucrose, gelatin, maltose, chalk, silica gel, sodium stearate, glyceryl monostearate, talc, sodium chloride, skimmed milk powder, glycerin, propylene glycol, water, Ethanol and the like.
- the composition may also contain minor amounts of wetting agents, emulsifying agents or pH buffering agents as needed.
- Oral formulations may contain standard carriers such as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, cellulose, magnesium carbonate, and the like. Examples of suitable pharmaceutically acceptable carriers are as described in Remington's Pharmaceutical Sciences (1990).
- compositions of the invention may act systemically and/or locally.
- they may be administered in a suitable route, for example by injection (for example intravenous, intraarterial, subcutaneous, intraperitoneal, intramuscular, including instillation) or transdermal administration; or by oral, buccal, or oral administration.
- injection for example intravenous, intraarterial, subcutaneous, intraperitoneal, intramuscular, including instillation
- transdermal administration or by oral, buccal, or oral administration.
- the pharmaceutical compositions of the invention may be administered in a suitable dosage form.
- the dosage forms include, but are not limited to, tablets, capsules, troches, hard candy, powders, sprays, creams, ointments, suppositories, gels, pastes, lotions, ointments, aqueous suspensions. Injectable solutions, elixirs, syrups, and the like.
- the amount or amount of the compound of the present invention in the pharmaceutical composition may be from about 0.01 mg to about 1000 mg, suitably from 0.1 to 500 mg, preferably from 0.5 to 300 mg, more preferably from 1 to 150 mg, particularly preferably from 1 to 50 mg, for example, 1.5 mg, 2 mg, 4 mg, 10 mg, 25 mg, and the like.
- the pharmaceutical composition may further comprise one or more additional therapeutic agents, such as other therapeutic agents for preventing or treating cancer and/or tumors and related diseases, including but not limited to Cytotoxic anti-tumor drugs, drugs that affect endocrine balance, biological response modifiers, molecularly targeted anti-tumor drugs, and other ancillary treatments.
- additional therapeutic agents such as other therapeutic agents for preventing or treating cancer and/or tumors and related diseases, including but not limited to Cytotoxic anti-tumor drugs, drugs that affect endocrine balance, biological response modifiers, molecularly targeted anti-tumor drugs, and other ancillary treatments.
- Another object of the present invention is to provide a process for the preparation of a pharmaceutical composition of the present invention which comprises administering a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer thereof, Polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, are combined with one or more pharmaceutically acceptable carriers.
- Another object of the present invention is to provide a kit comprising a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof, Hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
- Another object of the present invention is to provide a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof Or a mixture thereof or a pharmaceutical composition of the invention for use in the manufacture of a medicament for the prevention or treatment of cancer and/or a tumor and a related disease thereof.
- Another object of the present invention is to provide a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof Or a mixture thereof or a pharmaceutical composition of the invention for use in the prevention or treatment of cancer and/or tumors and related diseases.
- Another object of the present invention is to provide a method for preventing or treating cancer and/or a tumor and a related disease thereof, which comprises administering to an individual in need thereof an effective amount of a compound of the present invention or a pharmaceutically acceptable salt or ester thereof , stereoisomers, tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
- a compound of the invention prevent or treat cancer and/or tumors and related diseases.
- the cancers and/or tumors described in the present invention include, but are not limited to, tumors and/or cancers occurring in the pancreas, lung (eg, non-small cell lung cancer), ovary, bladder, breast, stomach, colorectal, and liver, and related disease.
- lung eg, non-small cell lung cancer
- ovary e.g., bladder, breast, stomach, colorectal, and liver, and related disease.
- an effective amount refers to an amount of a compound that, to a certain extent, relieves one or more symptoms of the prophylactic or therapeutic condition after administration.
- the dosing regimen can be adjusted to provide the optimal desired response. For example, a single bolus may be administered, several divided doses may be administered over time, or the dose may be proportionally reduced or increased as indicated by the urgent need for treatment. It is noted that the dose value can vary with the type and severity of the condition to be alleviated and can include single or multiple doses. It is to be further understood that for any particular individual, the particular dosage regimen will be adjusted over time according to the individual needs and the professional judgment of the person administering the composition or the composition of the supervised composition.
- an effective dose will be from about 0.0001 to about 50 mg per kg body weight per day, for example from about 0.01 to about 10 mg/kg/day (single or divided doses). For a 70 kg person, this would add up to about 0.007 mg/day to about 3500 mg/day, such as from about 0.7 mg/day to about 700 mg/day.
- a dose level that is not higher than the lower limit of the aforementioned range may be sufficient, while in other cases, a larger dose may still be employed without causing any harmful side effects, provided that the larger The dose is divided into several smaller doses to be administered throughout the day.
- treating refers to reversing, alleviating, inhibiting the progression of a condition or condition to which such a term applies or the progression of one or more symptoms of such a condition or condition, or preventing such condition or condition or such One or more symptoms of a condition or condition.
- the term "individual” includes human or non-human animals.
- exemplary human individuals include a human individual (referred to as a patient) or a normal individual having a disease, such as the disease described herein.
- Non-human animals in the present invention include all vertebrates, such as non-mammals (eg, birds, amphibians, reptiles) and mammals, such as non-human primates, domestic animals, and/or domesticated animals (eg, sheep, dogs). , cats, cows, pigs, etc.).
- the structures of the compounds described in the following examples were confirmed by 1 H NMR or MS.
- the 1 H NMR measuring instrument was measured using a Bruker 400 MHz nuclear magnetic resonance apparatus, and the solvent was CD 3 OD, CDCl 3 or DMSO-d 6 , and the internal standard substance was TMS. The total ⁇ value was expressed by ppm.
- the mass spectrometer (MS) assay instrument used an Agilent (ESI) mass spectrometer, model Agilent 6120B.
- the mixture of diastereomers prepared in the examples can be separated by preparative high performance liquid chromatography to give the pure isomer.
- the preparative high performance liquid chromatography separation can be carried out according to methods known in the art. For example, under the following separation conditions: using octadecyl bonded silica as a filler, column temperature 30 ° C -50 ° C, flow rate 5.0-20.0 mL / min, detection wavelength 200-400 nm, using mobile phase A (for example, Water), mobile phase B (for example, methanol or acetonitrile), with a linear gradient elution.
- mobile phase A for example, Water
- mobile phase B for example, methanol or acetonitrile
- Step 1 Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
- Step 2 ((2R,3S,5R)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2- Synthesis of methyl-phenyl-((S)-1-(2-methylbenzyloxy)propan-2-yl)phosphoramidate (Compound 21)
- 5-Fluoro-2'-deoxyurea nucleoside (162 mg, 0.72 mmol) was added to a 100 mL 3-neck flask and dissolved in anhydrous tetrahydrofuran (10 mL). The temperature was lowered to -20 ° C, and a solution of t-butylmagnesium chloride in tetrahydrofuran (1.0 mL, 1.05 mmol, 1 M) was added dropwise, and the mixture was warmed to 0 ° C and stirred for 2 h.
- Step 1 Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
- Compound 21-2 was synthesized in the same manner as in the first step of the first embodiment.
- Step 2 ((2R,3R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl) Synthesis of phenyl-phenyl-((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 49)
- Step 1 Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
- Compound 21-2 was synthesized in the same manner as in the first step of the first embodiment.
- Step 2 ((2R,4R)-4-(4-Amino-2-oxopyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methyl-phenyl- Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 77)
- Compound 77 was synthesized in a similar manner to Step 2 of Example 1 except that instead of 5-fluoro-2'-deoxyurea nucleoside with tresapabine (426 mg, 2.0 mmol).
- nucleoside triphosphate 3P
- a metabolite of the compound of the present invention in human primary hepatocytes
- Human primary hepatocytes were purchased from Bioreclamation IVT, an in vitro technology company.
- the culture medium is diluted to a concentration of 6 ⁇ 10 5 cells/mL, and then the storage solution of the test compound of 25 mM concentration is diluted with the culture solution to a concentration of 50 ⁇ M working solution, wherein the organic solvent
- the final content is less than 1%.
- 250 ⁇ L of the hepatocyte solution and 250 ⁇ L of the test substance solution or the pure medium (NC group) containing the same solvent content were mixed and added to the 24-well plate so that the final concentration of the test compound was 25 ⁇ M. After incubating for 6 hours in a 37 ° C water bath, the sample was transferred to a test tube, and then the culture solution was removed by centrifugation.
- 3P generation rate (3P production amount * 150 ⁇ L) / (6 ⁇ 10 5 cells / mL * 250 ⁇ L * 6h).
- the compound of the present invention can be smoothly metabolized in hepatocytes to produce an active metabolite nucleoside triphosphate, and the metabolite nucleoside triphosphate is produced in a large amount and has a high production rate. Therefore, the compound of the present invention has an antitumor or anticancer effect, and particularly has an excellent anti-liver cancer effect.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Genetics & Genomics (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Disclosed are a nucleoside phosphate compound, a pharmaceutical composition comprising same, and a preparation method therefor and the use thereof. Such a compound can be successfully metabolized within liver cells, and can produce an active metabolite-nucleoside triphosphate, and hence can be used for treating or preventing related diseases, such as liver cancer.
Description
本发明涉及核苷磷酸类化合物、包含其的药物组合物和药盒、其制备方法及其在预防或治疗癌症和/或肿瘤及其相关疾病中的用途。The present invention relates to a nucleoside phosphate compound, a pharmaceutical composition and kit comprising the same, a process for the preparation thereof, and use thereof for preventing or treating cancer and/or a tumor and a related disease thereof.
发明背景Background of the invention
核苷类似物是一类重要的抗肿瘤药物,主要作用机制是通过特异性干扰肿瘤细胞中DNA的合成、影响RNA的转录过程或蛋白质的合成过程,或直接对这些大分子发生作用来抑制肿瘤细胞的分裂增殖,从而使之凋亡。随着对核苷类似物在药物动力学、体内代谢机制、转移机制和与诱导细胞凋亡等方面的研究的不断深入,人们发现核苷类抗肿瘤药物具有非常广阔的发展前景。近年来,对已知核苷类抗肿瘤药物进行结构修饰,提高药物的生物利用度,减少不良反应,已成为该类抗肿瘤新药的研究热点。Nucleoside analogues are an important class of anti-tumor drugs. The main mechanism of action is to inhibit tumors by specifically interfering with DNA synthesis in tumor cells, affecting the transcription process of RNA or protein synthesis, or directly acting on these macromolecules. The cells divide and proliferate, thereby causing apoptosis. With the deepening of research on nucleoside analogs in pharmacokinetics, metabolic mechanisms in vivo, metastasis mechanism and induction of apoptosis, it has been found that nucleoside antitumor drugs have very broad development prospects. In recent years, the structural modification of known nucleoside antitumor drugs, improving the bioavailability of drugs and reducing adverse reactions have become the research hotspots of this class of antitumor drugs.
传统核苷类药物(如抗癌药物吉西他滨)在体内通过磷酸化代谢过程生成三磷酸核苷,后者插入DNA链,抑制DNA的合成,阻止细胞由G1期向S期的进展,造成肿瘤细胞G1期周期阻滞,从而抑制肿瘤细胞的恶性增殖(Oncology.2002,62(4),354-362)。在长期使用中,这些传统核苷药物往往出现耐药性问题,主要是因为转运体变异和磷酸化下调。前者影响了药物的吸收,导致生物利用度差;而后者影响了核苷药物的单磷酸化,进而造成活性成分三磷酸生成的不足。Traditional nucleoside drugs (such as the anticancer drug gemcitabine) generate nucleoside triphosphates through phosphorylation and metabolism in the body. The latter is inserted into the DNA strand to inhibit DNA synthesis and prevent cell progression from G1 to S phase, resulting in tumor cells. The G1 phase arrests, thereby inhibiting the malignant proliferation of tumor cells (Oncology. 2002, 62(4), 354-362). In long-term use, these traditional nucleoside drugs often have resistance problems, mainly due to transporter variation and phosphorylation down-regulation. The former affects the absorption of the drug, resulting in poor bioavailability; while the latter affects the monophosphorylation of the nucleoside drug, which in turn causes the deficiency of the active component triphosphate.
磷酸化过程需要三种不同激酶的参与。而核苷类似物及其单磷酸和二磷酸代谢物可能并不是相应激酶的良好底物。研究表明在磷酸化过程中,第一种激酶对底物的选择性最强。因此第一步磷酸化通常是最困难的步骤。为了克服这一困难,将单磷酸输送到细胞内是必要的手段。但是,单磷酸核苷带负电荷,很难通过细胞膜,而且容易被磷酸酯酶降解。因此,将核苷类似物转化为相应的磷酸酯或者磷酰胺前药,即可达到减小药物极性、增强生物膜穿透能力、提高体内生物利用度的目的。此外,磷酸化的效率也决定了核苷类似物作为聚合酶抑制剂或者逆转录酶抑制剂的活性。具体而言,磷酸化效率取决于三磷酸核苷的生成量、生成速率和存在时间。三磷酸核苷的生成量越多、生成速率越快、存在时间越长,则抑制活性越高。The phosphorylation process requires the participation of three different kinases. Nucleoside analogs and their monophosphate and diphosphate metabolites may not be good substrates for the corresponding kinases. Studies have shown that the first kinase is most selective for the substrate during phosphorylation. Therefore the first step of phosphorylation is usually the most difficult step. To overcome this difficulty, delivery of monophosphate to cells is a necessary means. However, nucleoside monophosphates are negatively charged, hard to pass through the cell membrane, and are easily degraded by phosphatase. Therefore, the conversion of the nucleoside analog to the corresponding phosphate or phosphoramide prodrug can achieve the purpose of reducing the polarity of the drug, enhancing the biofilm penetration ability, and improving the bioavailability in the body. In addition, the efficiency of phosphorylation also determines the activity of nucleoside analogs as polymerase inhibitors or reverse transcriptase inhibitors. Specifically, the phosphorylation efficiency depends on the amount of nucleoside triphosphate produced, the rate of formation, and the time of existence. The more the amount of nucleoside triphosphate produced, the faster the rate of formation, and the longer the time of existence, the higher the inhibitory activity.
发明概述Summary of invention
本发明的一方面提供一种核苷磷酸类化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,所述化合物在体内代谢为具有活性的三磷酸核苷,具有良好的预防或治疗癌症和/或肿瘤及其相关病症的疾病的效果。所述化合物尤其可在肝组织中顺利代谢为具有活性的三磷酸核苷,对于肝癌具有优异的预防或治疗作用。所述化合物具有以下式(I)的结构:One aspect of the invention provides a nucleoside phosphate compound or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or precursor thereof A drug, or a mixture thereof, which is metabolized in vivo to an active nucleoside triphosphate, has a good effect of preventing or treating a disease of cancer and/or a tumor and a related condition thereof. The compound is particularly easily metabolized into active nucleoside triphosphate in liver tissue, and has an excellent preventive or therapeutic effect on liver cancer. The compound has the structure of the following formula (I):
其中,among them,
B选自:B is selected from:
L选自任选地被一个或多个R
b取代的C
1-6亚烷基、C
2-6亚烯基、C
2-6亚炔基,所述亚烷基、亚烯基或亚炔基任选地被一个或多个-O-、-NR-或-S-间断;
L is selected from C 1-6 alkylene, C 2-6 alkenylene, C 2-6 alkynylene, optionally substituted by one or more R b , said alkylene, alkenylene or sub An alkynyl group is optionally interrupted by one or more -O-, -NR- or -S-;
或者L选自下列基团:Or L is selected from the following groups:
其中
表示单键或双键,并且1位置处与B连接,2位置处与磷原子(P)连接;
among them Represents a single bond or a double bond, and is connected to B at the 1 position and to the phosphorus atom (P) at the 2 position;
X、Y和Z在每次出现时各自独立地选自CH
2、O、S和NR;
X, Y and Z are each independently selected from CH 2 , O, S and NR at each occurrence;
R
a和R
b在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO
2、-N(R)
2、-N
3、C
1-6烷基、C
3-6环烷基、C
2-6烯基,卤代C
2-6烯基和C
2-6炔基;
R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , -N 3 , C 1-6 alkyl, C 3- 6 cycloalkyl, C 2-6 alkenyl, halo C 2-6 alkenyl and C 2-6 alkynyl;
q为0、1、2、3、4、5、6、7或8,条件是:q is 0, 1, 2, 3, 4, 5, 6, 7, or 8, with the following conditions:
q不大于对应基团上可被取代的位置的数目;并且q is not greater than the number of positions on the corresponding group that can be substituted;
当q大于1时,每个R
b可以相同或不同;
When q is greater than 1, each R b may be the same or different;
n为0、1、2或3,条件是:n is 0, 1, 2 or 3, provided that:
n不大于对应基团上可被取代的位置的数目;并且n is not greater than the number of positions on the corresponding group that can be substituted;
当n大于1时,每个R
a可以相同或不同;
When n is greater than 1, each R a may be the same or different;
Ar
1和Ar
2各自独立地选自C
6-14芳基和5-14元杂芳基,所述芳基和杂芳基任选地被一个或多个R
c取代;
Ar 1 and Ar 2 are each independently selected from C 6-14 aryl and 5-14 membered heteroaryl, the aryl and heteroaryl optionally being substituted by one or more R c ;
R
c在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO
2、-N(R)
2、C
1-6烷基、卤代C
1-6烷基、C
1-6烷氧基、C
1-6烷硫基、C
3-6环烷基、3-10元杂环烷基和C
2-6炔基;
R c is each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , C 1-6 alkyl, halo C 1-6 alkyl, C 1-6 alkoxy group, C 1-6 alkylthio group, C 3-6 cycloalkyl group, 3-10 membered heterocycloalkyl group and C 2-6 alkynyl group;
R在每次出现时各自独立地选自氢、C
1-6烷基和C
3-6环烷基;
R is each independently selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl;
m为1、2或3;m is 1, 2 or 3;
p为1、2或3;p is 1, 2 or 3;
R
1和R
2各自独立地选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 1 and R 2 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
或者R
1和R
2连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;
Or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
R
3和R
4各自独立地选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 3 and R 4 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
或者R
3和R
4连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;
Or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
或者R
2和R
3连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;并且
Or R 2 and R 3 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
R
5选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl and C 7-20 aralkyl, said alkyl, ring The alkyl, alkoxy, aryl and aralkyl groups are each optionally substituted by one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN and -NO 2 ;
或者R
5与Ar
2上的取代基R
c连同其所连接的碳原子共同形成与Ar
2稠合的C
4-6环烷基或4-10元杂环烷基;
Or R 5 and the substituent R c on Ar 2 together with the carbon atom to which they are bonded form a C 4-6 cycloalkyl or 4-10 membered heterocycloalkyl fused to Ar 2 ;
条件是:requirement is:
1)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接; X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
2为2-甲基苯基;且Ar
1为苯基时,
1) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 2 is 2-methyl Phenyl; and when Ar 1 is phenyl,
R
1和R
2不为以下情况:R
1和R
2各自独立地选自氢和甲基;或者R
1和R
2连同其所连接的碳原子共同形成环丙基;
R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
2)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
2为2-甲基苯基时,
2) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 2 is 2-methylphenyl,
Ar
1不为α-萘基或β-萘基;
Ar 1 is not α-naphthyl or β-naphthyl;
3)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
3) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,
Ar
2不为2-甲基萘基、3-甲基苯基或4-甲基苯基;
Ar 2 is not 2-methylnaphthyl, 3-methylphenyl or 4-methylphenyl;
4)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
1为苯基;且Ar
2为2-甲基苯基时,
4) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is phenyl; When Ar 2 is 2-methylphenyl,
R
1和R
2不为以下情况:R
1和R
2各自独立地选自氢和甲基;或者R
1和R
2连同其所连接的碳原子共同形成环丙基;
R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
5)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
5) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,
Ar
2不为3-甲基苯基、4-甲基苯基、4-氟苯基、2,6-二甲基苯基或2-甲基萘基;
Ar 2 is not 3-methylphenyl, 4-methylphenyl, 4-fluorophenyl, 2,6-dimethylphenyl or 2-methylnaphthyl;
6)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
6) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a phenyl group,
Ar
2不为苯基、4-甲基苯基、2-甲氧基苯基、2-甲氧基-4-甲基苯基、2,4-二甲基苯基、2-甲基-4-甲氧基苯基或2-甲基-4-叔丁基苯基;
Ar 2 is not phenyl, 4-methylphenyl, 2-methoxyphenyl, 2-methoxy-4-methylphenyl, 2,4-dimethylphenyl, 2-methyl- 4-methoxyphenyl or 2-methyl-4-tert-butylphenyl;
7)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
1为α-萘基,且Ar
2为2-甲基苯基时,
7) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is α-naphthyl And when Ar 2 is 2-methylphenyl,
R
1和R
2不为以下情况:R
1和R
2同时为氢;或者R
1为氢且R
2为甲基;或者R
1为甲基且R
2为氢;
R 1 and R 2 are not the case where R 1 and R 2 are simultaneously hydrogen; or R 1 is hydrogen and R 2 is methyl; or R 1 is methyl and R 2 is hydrogen;
8)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;R
1和R
2不同并各自独立地选自氢和甲基;且Ar
1为β-萘基时,
8) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; R 1 and R 2 are different and Each of them is independently selected from hydrogen and methyl; and when Ar 1 is β-naphthyl,
Ar
2不为2-甲基萘基;
Ar 2 is not a 2-methylnaphthyl group;
9)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为4-氟苯基时,
9) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a 4-fluorophenyl group,
Ar
2不为2-甲基苯基;
Ar 2 is not 2-methylphenyl;
10)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NCH
3;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
10) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NCH 3 ; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; And when Ar 1 is a phenyl group,
Ar
2不为2-甲基苯基;
Ar 2 is not 2-methylphenyl;
11)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为β-萘基时,
11) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a β-naphthyl group,
Ar
2不为2-甲基苯基。
Ar 2 is not a 2-methylphenyl group.
本发明的另一方面提供一种药物组合物,其包含预防或治疗有效量的本发明的化合物或其药学上可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,以及一种或多种药学上可接受的载体。Another aspect of the invention provides a pharmaceutical composition comprising a prophylactically or therapeutically effective amount of a compound of the invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph thereof , solvate, hydrate, metabolite or prodrug, or mixtures thereof, and one or more pharmaceutically acceptable carriers.
本发明的另一方面提供一种制备本发明的药物组合物的方法,所述方法包括将本发明的化合物或其药学上可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物与一种或多种药学上可接受的载体组合。Another aspect of the present invention provides a method of preparing a pharmaceutical composition of the present invention, which comprises administering a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer thereof, Polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, are combined with one or more pharmaceutically acceptable carriers.
本发明的另一方面提供一种药盒,其包含本发明的化合物或其药学上可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者本发明的药物组合物。Another aspect of the invention provides a kit comprising a compound of the invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof, hydrated thereof , metabolite or prodrug, or a mixture thereof, or a pharmaceutical composition of the invention.
本发明的另一方面提供本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物或者本发明的药物组合物在制备用于预防或治疗癌症和/或肿瘤及其相关疾病的药物中的用途。Another aspect of the invention provides a compound of the invention, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or the use of a mixture thereof or a pharmaceutical composition of the present invention for the preparation of a medicament for preventing or treating cancer and/or a tumor and a related disease thereof.
本发明的另一方面提供本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物或者本发明的药物组合物,其用于预防或治疗癌症和/或肿瘤及其相关疾病。Another aspect of the invention provides a compound of the invention, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or a mixture thereof or a pharmaceutical composition of the invention for use in the prevention or treatment of cancer and/or tumors and related diseases.
本发明的另一方面提供预防或治疗癌症和/或肿瘤及其相关疾病的方法,所述方法包括向有此需要的个体给药有效量的本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者本发明的药物组合物。Another aspect of the invention provides a method of preventing or treating cancer and/or a tumor and related diseases, the method comprising administering to an individual in need thereof an effective amount of a compound of the invention or a pharmaceutically acceptable salt or ester thereof , stereoisomers, tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
本发明的另一方面提供制备本发明的化合物的方法,所述方法包括以下步骤:Another aspect of the invention provides a method of preparing a compound of the invention, the method comprising the steps of:
其中among them
B、L、X、Ar
1、Ar
2、R
1-R
5、m和p如上文所定义。
B, L, X, Ar 1 , Ar 2 , R 1 - R 5 , m and p are as defined above.
发明详细描述Detailed description of the invention
定义definition
除非在下文中另有定义,本文中所用的所有技术术语和科学术语的含义意图与本领域技术人员通常所理解的相同。提及本文中使用的技术意图指在本领域中通常所理解的技术,包括那些对本领域技术人员显而易见的技术的变化或等效技术的替换。虽然相信以下术语对于本领域技术人员很好理解,但仍然阐述以下定义以更好地解释本发明。Unless otherwise defined below, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art. References to techniques used herein are intended to refer to techniques that are generally understood in the art, including those that are obvious to those skilled in the art. While the following terms are believed to be well understood by those skilled in the art, the following definitions are set forth to better explain the invention.
如本文中所使用,术语“包括”、“包含”、“具有”、“含有”或“涉及”及其在本文中的其它变体形式为包含性的(inclusive)或开放式的,且不排除其它未列举的元素或方法步骤。The terms "including", "comprising", "having", "containing", or "comprising", as used herein, and other variants thereof, are inclusive or open, and not Exclude other unlisted elements or method steps.
如本文中所使用,术语“亚烷基”表示饱和二价烃基,优选表示具有1、2、3、4、5或6个碳原子的饱和二价烃基,例如亚甲基、亚乙基、亚丙基或亚丁基。The term "alkylene" as used herein denotes a saturated divalent hydrocarbon group, preferably a saturated divalent hydrocarbon group having 1, 2, 3, 4, 5 or 6 carbon atoms, such as methylene, ethylene, Propylene or butylene.
如本文中所使用,术语“亚烯基”表示包含一个或多个双键的二价烃基,其优选具有2、3、4、5或6个碳原子,例如亚乙烯基、亚丙烯基或亚烯丙基。当本发明的化合物含有亚烯基时,所述化合物可以纯E(异侧(entgegen))形式、纯Z(同侧(zusammen))形式或其任意混合物形式存在。The term "alkenylene" as used herein denotes a divalent hydrocarbon radical containing one or more double bonds, preferably having 2, 3, 4, 5 or 6 carbon atoms, such as ethenylene, propenylene or Allylene. When the compounds of the invention contain alkenylene groups, the compounds may exist in pure E (entgegen) form, pure Z (zusammen) form, or any mixture thereof.
如本文中所使用,术语“亚炔基”表示包含一个或多个三键的二价烃基,其优选具有2、3、4、5或6个碳原子,例如亚乙炔基或亚丙炔基。The term "alkynylene" as used herein denotes a divalent hydrocarbon radical containing one or more triple bonds, preferably having 2, 3, 4, 5 or 6 carbon atoms, for example ethynylene or propynylene. .
如本文中所使用,术语“烷基”定义为直链或支链的饱和脂肪族烃基。在一些实施方案中,烷基具有1至12个,例如1至6个碳原子。例如,如本文中所使用,术语“C
1-6烷基”指具有1至6个碳原子的直链或支链的基团(例如甲基、乙基、正丙基、异丙基、正丁基、异丁基、仲丁基、叔丁基、正戊基或正己基),其任选地被一个或多个(诸如1至3个)适合的取代基如卤素取代(此时该基团被称作“卤代烷基”,例如CF
3、C
2F
5、CHF
2、CH
2F、CH
2CF
3、CH
2Cl或-CH
2CH
2CF
3等)。
As used herein, the term "alkyl" is defined as a straight or branched saturated aliphatic hydrocarbon group. In some embodiments, an alkyl group has from 1 to 12, such as from 1 to 6 carbon atoms. For example, as used herein, the term " C1-6 alkyl" refers to a straight or branched chain group having from 1 to 6 carbon atoms (eg, methyl, ethyl, n-propyl, isopropyl, N-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl or n-hexyl), which is optionally substituted by one or more (such as 1 to 3) suitable substituents such as halogen (at this time) This group is referred to as "haloalkyl" such as CF 3 , C 2 F 5 , CHF 2 , CH 2 F, CH 2 CF 3 , CH 2 Cl or -CH 2 CH 2 CF 3 , and the like.
如本文中所使用,术语“烯基”定义为含有至少一个碳碳双键的不饱和直链或支链的脂肪族烃基。在一些实施方案中,烯基具有2至10个,例如2至6个碳原子。例如,如本文中所使用,术语“C
2-6烯基”指含有2至6个碳原子的直链或支链的烯基,例如乙烯基、1-丙烯基、2-丙烯基、1-丁烯基或2-丁烯基,其任选地被一个或多个(例如1个、2个、3个或4个)适合的取代基取代。
As used herein, the term "alkenyl" is defined as an unsaturated straight or branched aliphatic hydrocarbon group containing at least one carbon to carbon double bond. In some embodiments, an alkenyl group has 2 to 10, such as 2 to 6 carbon atoms. For example, as used herein, the term "C 2-6 alkenyl" refers to a straight or branched alkenyl group containing from 2 to 6 carbon atoms, such as ethenyl, 1-propenyl, 2-propenyl, 1 a butenyl or 2-butenyl group which is optionally substituted by one or more (for example 1, 2, 3 or 4) suitable substituents.
如本文中所使用,术语“炔基”定义为含有至少一个碳碳叁键的不饱和直链或支链的脂肪族烃基。在一些实施方案中,炔基具有2至10个,例如2至6个碳原子。例如,如本文中所使用,术语“C
2-6炔基”指含有2至6个碳原子的直链或支链的炔基,例如乙炔基、1-丙炔基、2-丙炔基、1-丁炔基或2-丁炔基,其任选地被一个或多个(例如1个、2个、3个或4个)适合的取代基取代。
As used herein, the term "alkynyl" is defined as an unsaturated straight or branched aliphatic hydrocarbon group containing at least one carbon-carbon triple bond. In some embodiments, an alkynyl group has 2 to 10, such as 2 to 6 carbon atoms. For example, as used herein, the term "C 2-6 alkynyl" refers to a straight or branched alkynyl group containing from 2 to 6 carbon atoms, such as ethynyl, 1-propynyl, 2-propynyl. , 1-butynyl or 2-butynyl, optionally substituted by one or more (eg, 1, 2, 3 or 4) suitable substituents.
如本文中所使用,术语“环烷基”指饱和或不饱和的非芳族单环或多环(诸如双环)烃环(例如单环,诸如环丙基、环丁基、环戊基、环己基、环庚基、环辛基、环壬基,或双环,包括螺环、稠合或桥连系统(诸如双环[1.1.1]戊基、双环[2.2.1]庚基、双环[3.2.1]辛基或双环[5.2.0]壬基、十氢化萘基等),其任选地被一个或多个(诸如1至3个)适合的取代基取代。所述环烷基具有3至15个,例如3至6个碳原 子。例如,术语“C
3-6环烷基”指具有3至6个成环碳原子的饱和或不饱和的非芳族单环或多环(诸如双环)烃环(例如环丙基、环丁基、环戊基或环己基),其任选地被一个或多个(诸如1至3个)适合的取代基取代,例如甲基取代的环丙基。
As used herein, the term "cycloalkyl" refers to a saturated or unsaturated, non-aromatic monocyclic or polycyclic (such as bicyclic) hydrocarbon ring (eg, a monocyclic ring such as cyclopropyl, cyclobutyl, cyclopentyl, Cyclohexyl, cycloheptyl, cyclooctyl, cyclodecyl, or bicyclic, including spiro, fused or bridged systems (such as bicyclo [1.1.1] pentyl, bicyclo [2.2.1] heptyl, bicyclo [ 3.2.1] octyl or bicyclo [5.2.0] decyl, decalinyl, etc.), which are optionally substituted by one or more (such as 1 to 3) suitable substituents. There are 3 to 15, for example 3 to 6 carbon atoms. For example, the term "C 3-6 cycloalkyl" refers to a saturated or unsaturated, non-aromatic monocyclic or polycyclic ring having 3 to 6 ring-forming carbon atoms. (such as a bicyclic) hydrocarbon ring (such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl), which is optionally substituted by one or more (such as 1 to 3) suitable substituents, such as methyl substitution Cyclopropyl.
如本文中所使用,术语“芳基”指具有共轭π电子系统的全碳单环或稠合环多环芳族基团。在一些实施方案中,芳基具有6至14个,例如6至10个碳原子。例如,如本文中所使用,术语“C
6-14芳基”意指含有6至14个碳原子的芳族基团,诸如苯基或萘基。芳基任选地被一个或多个(诸如1至3个)适合的取代基(例如卤素、-OH、-CN、-NO
2、C
1-6烷基等)取代。
As used herein, the term "aryl" refers to an all-carbon monocyclic or fused-ring polycyclic aromatic group having a conjugated pi-electron system. In some embodiments, the aryl has 6 to 14, such as 6 to 10 carbon atoms. For example, as used herein, the term " C6-14 aryl" means an aromatic group containing from 6 to 14 carbon atoms, such as phenyl or naphthyl. Aryl is optionally substituted with one or more (such as 1 to 3) suitable substituent (e.g., halo, -OH, -CN, -NO 2, C 1-6 alkyl, etc.) substituted.
如本文中所使用,术语“芳烷基”表示芳基取代的烷基,其中所述芳基和所述烷基如本文中所定义。通常,所述芳基可具有6-14个碳原子,并且所述烷基可具有1-6个碳原子。示例性芳烷基包括但不限于苄基、苯基乙基、苯基丙基、苯基丁基等。The term "aralkyl" as used herein denotes an aryl-substituted alkyl group, wherein the aryl group and the alkyl group are as defined herein. Typically, the aryl group can have from 6 to 14 carbon atoms and the alkyl group can have from 1 to 6 carbon atoms. Exemplary aralkyl groups include, but are not limited to, benzyl, phenylethyl, phenylpropyl, phenylbutyl, and the like.
如本文中所使用,术语“杂芳基”指一价单环、双环或三环芳族环系,其具有5、6、7、8、9、10、11、12、13或14个环原子,特别是1、2、3、4、5、6、7、8、9或10个碳原子,且其包含至少一个可以相同或不同的杂原子(例如氧、氮或硫),并且,另外在每一种情况下可为苯并稠合的。特别地,杂芳基选自噻吩基、呋喃基、吡咯基、噁唑基、噻唑基、咪唑基、吡唑基、异噁唑基、异噻唑基、噁二唑基、三唑基、噻二唑基等,以及它们的苯并衍生物;或吡啶基、哒嗪基、嘧啶基、吡嗪基、三嗪基等,以及它们的苯并衍生物。The term "heteroaryl" as used herein refers to a monovalent monocyclic, bicyclic or tricyclic aromatic ring system having 5, 6, 7, 8, 9, 10, 11, 12, 13 or 14 rings. An atom, particularly 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 carbon atoms, and which comprises at least one hetero atom which may be the same or different (for example, oxygen, nitrogen or sulfur), In addition, in each case, it may be benzo-fused. In particular, the heteroaryl is selected from the group consisting of thienyl, furyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, triazolyl, thia A oxazolyl group or the like, and a benzo derivative thereof; or a pyridyl group, a pyridazinyl group, a pyrimidinyl group, a pyrazinyl group, a triazinyl group or the like, and a benzo derivative thereof.
如本文中所使用,术语“卤代”或“卤素”基团定义为包括F、Cl、Br或I。The term "halo" or "halogen" group, as used herein, is defined to include F, Cl, Br or I.
如本文中所使用,术语“烷氧基”意指通过氧原子连接至母体分子部分的如上文所定义的烷基。C
1-6烷氧基的代表性实例包括但不限于甲氧基、乙氧基、丙氧基、正丁氧基、异丁氧基、叔丁氧基、戊氧基、己氧基等。
As used herein, the term "alkoxy" refers to an alkyl group, as defined above, appended to the parent molecular moiety through an oxygen atom. Representative examples of C1-6 alkoxy include, but are not limited to, methoxy, ethoxy, propoxy, n-butoxy, isobutoxy, tert-butoxy, pentyloxy, hexyloxy, and the like. .
如本文中所使用,术语“烷硫基”意指通过硫原子连接至母体分子部分的如上文所定义的烷基。C
1-6烷硫基的代表性实例包括但不限于甲硫基、乙硫基、叔丁硫基、己硫基等。
As used herein, the term "alkylthio" refers to an alkyl group, as defined above, appended to the parent molecular moiety through a sulfur atom. Representative examples of C1-6 alkylthio include, but are not limited to, methylthio, ethylthio, tert-butylthio, hexylthio, and the like.
如本文中所使用,术语“杂环烷基”指饱和的一价单环或双环基团,其在环中具有2、3、4、5、6、7、8或9个碳原子和一个或多个(例如1个、2个、3个或4个)选自C(=O)、O、S、S(=O)、S(=O)
2和NR(其中R表示氢原子、C
1-6烷基或C
3-6环烷基)的含杂原子的基团,例如但不限于环氧乙烷基、氮丙啶基、氮杂环丁基(azetidinyl)、氧杂环丁基(oxetanyl)、四氢呋喃基、吡咯烷基、吡咯烷酮基、咪唑烷基、吡唑烷基、四氢吡喃基、哌啶基、吗啉基、二噻烷基(dithianyl)、硫吗啉基、哌嗪基、三噻烷基(trithianyl)等。
The term "heterocycloalkyl" as used herein refers to a saturated monovalent monocyclic or bicyclic group having 2, 3, 4, 5, 6, 7, 8 or 9 carbon atoms and one in the ring. Or a plurality (for example, 1, 2, 3 or 4) selected from C(=O), O, S, S(=O), S(=O) 2 and NR (wherein R represents a hydrogen atom, a hetero atom-containing group of a C 1-6 alkyl group or a C 3-6 cycloalkyl group such as, but not limited to, an oxiranyl group, an aziridine group, an azetidinyl group, an oxoheterocyclic group Oxetanyl, tetrahydrofuranyl, pyrrolidinyl, pyrrolidinyl, imidazolidinyl, pyrazolidinyl, tetrahydropyranyl, piperidinyl, morpholinyl, dithianyl, thiomorpholine Base, piperazinyl, trithianyl and the like.
术语“取代”指所指定的原子上的一个或多个(例如1个、2个、3个或4个)氢被从所指出的基团的选择代替,条件是未超过所指定的原子在当前情况下的正常原子价并且所述取代形成稳定的化合物。取代基和/或变量的组合仅仅当这种组合形成稳定的化合物时才是允许的。The term "substituted" means that one or more (eg, 1, 2, 3 or 4) hydrogens on the designated atom are replaced by the selection of the indicated group, provided that the specified atom is not exceeded. The normal valence of the current case and the substitution form a stable compound. Combinations of substituents and/or variables are permissible only if such combinations form stable compounds.
如果取代基被描述为“任选地被取代”,则取代基可(1)未被取代或(2)被取代。如果取代基的碳被描述为任选地被取代基列表中的一个或多个取代,则碳上的一个或多个氢(至存在的任何氢的程度)可单独和/或一起被独立地选择的任选的取代基替代。如果取代基的氮被描述为任选地被取代基列表中的一个或多个取代,则氮上的一个或多个氢(至存在的任何氢的程度)可各自被独立地选择的任选的取代基替代。If a substituent is described as "optionally substituted," the substituent may be unsubstituted or (2) substituted. If the carbon of the substituent is described as being optionally substituted by one or more of the list of substituents, then one or more hydrogens on the carbon (to the extent of any hydrogen present) may be independently and/or together independently The optional substituents selected are substituted. If the nitrogen of the substituent is described as being optionally substituted by one or more of the list of substituents, then one or more hydrogens on the nitrogen (to the extent of any hydrogen present) may each be independently selected. Substitute substitution.
如果取代基被描述为“独立地选自”一组,则各取代基独立于另一者被选择。因此,各取代基可与另一(其他)取代基相同或不同。If a substituent is described as being "independently selected from" a group, each substituent is selected independently of the other. Thus, each substituent may be the same or different from another (other) substituent.
如本文中所使用,术语“一个或多个”意指在合理条件下的1个或超过1个,例如2个、3个、4个、5个或10个。As used herein, the term "one or more" means 1 or more than 1, such as 2, 3, 4, 5 or 10 under reasonable conditions.
除非指明,否则如本文中所使用,取代基的连接点可来自取代基的任意适宜位置。Unless otherwise indicated, a point of attachment of a substituent, as used herein, may come from any suitable position of the substituent.
当取代基的键显示为穿过环中连接两个原子的键时,则这样的取代基可键连至该可取代的环中的任一成环原子。When a bond of a substituent is shown to pass through a bond connecting two atoms in the ring, such a substituent may be bonded to any of the ring-forming atoms in the substitutable ring.
本发明还包括所有药学上可接受的同位素标记的化合物,其与本发明的化合物相同,除了一个或多个原子被具有相同原子序数但原子质量或质量数不同于在自然界中占优势的原子质量或质量数 的原子替代。本发明的化合物中的同位素的实例包括(但不限于)氢的同位素(例如
2H、
3H);碳的同位素(例如
11C、
13C及
14C);氯的同位素(例如
36Cl);氟的同位素(例如
18F);碘的同位素(例如
123I及
125I);氮的同位素(例如
13N及
15N);氧的同位素(例如
15O、
17O及
18O);磷的同位素(例如
32P);及硫的同位素(例如
35S)。某些同位素标记的本发明的化合物(例如掺入放射性同位素的那些)可用于药物和/或底物组织分布研究(例如分析)中。放射性同位素氚(即
3H)及碳-14(即
14C)因易于掺入且容易检测而特别可用于该目的。用正电子发射同位素(例如
11C、
18F、
15O及
13N)进行取代可在正电子发射断层显像术(PET)研究中用于检验底物受体占据情况。被同位素标记的本发明的化合物可通过与描述于随附路线和/或实施例及制备中的那些类似的方法通过使用适当的被同位素标记的试剂代替之前采用的非标记的试剂来制备。本发明的药学上可接受的溶剂合物包括其中结晶溶剂可被同位素取代的那些,例如,D
2O、丙酮-d
6或DMSO-d
6。
The invention also includes all pharmaceutically acceptable isotopically-labeled compounds which are identical to the compounds of the invention, except that one or more atoms are of the same atomic number but the atomic mass or mass number differs from the atomic mass prevailing in nature. Or atomic substitution of mass. Examples of isotopes in the compounds of the invention include, but are not limited to, hydrogen isotopes (e.g., 2 H, 3 H); carbon isotopes (e.g., 11 C, 13 C, and 14 C); chlorine isotopes (e.g., 36 Cl) Fluorine isotopes (eg 18 F); isotopes of iodine (eg 123 I and 125 I); isotopes of nitrogen (eg 13 N and 15 N); isotopes of oxygen (eg 15 O, 17 O and 18 O); phosphorus Isotope (eg 32 P); and sulfur isotope (eg 35 S). Certain isotopically-labeled compounds of the invention (e.g., those incorporating radioisotopes) are useful in drug and/or substrate tissue distribution studies (e.g., assays). The radioisotope ruthenium (i.e., 3 H) and carbon-14 (i.e., 14 C) are particularly useful for this purpose because of their ease of incorporation and ease of detection. Substitution with positron emitting isotopes (eg, 11 C, 18 F, 15 O, and 13 N) can be used to examine substrate receptor occupancy in positron emission tomography (PET) studies. Isotopically labeled compounds of the invention can be prepared by replacing the previously employed non-labeled reagents with suitable isotopically labeled reagents by methods analogous to those described in the accompanying routes and/or examples and preparations. The pharmaceutically acceptable solvates of the present invention include those in which the crystallization solvent can be substituted with an isotope, for example, D 2 O, acetone-d 6 or DMSO-d 6 .
如本文中所使用,术语“立体异构体”表示由于至少一个不对称中心形成的异构体。在具有一个或多个(例如1个、2个、3个或4个)不对称中心的化合物中,其可产生外消旋混合物、单一对映异构体、非对映异构体混合物和单独的非对映异构体。特定个别分子也可以几何异构体(顺式/反式)存在。类似地,本发明的化合物可以两种或更多种处于快速平衡的结构不同的形式的混合物(通常称作互变异构体)存在。互变异构体的代表性实例包括酮-烯醇互变异构体、苯酚-酮互变异构体、亚硝基-肟互变异构体、亚胺-烯胺互变异构体等。要理解,本申请的范围涵盖所有这样的以任意比例(例如60%、65%、70%、75%、80%、85%、90%、95%、96%、97%、98%、99%)的异构体或其混合物。The term "stereoisomer" as used herein denotes an isomer formed by at least one asymmetric center. In a compound having one or more (eg, 1, 2, 3, or 4) asymmetric centers, which can produce a racemic mixture, a single enantiomer, a mixture of diastereomers, and Separate diastereomers. Specific individual molecules can also exist as geometric isomers (cis/trans). Similarly, the compounds of the invention may exist as mixtures (often referred to as tautomers) of two or more different forms in a rapidly balanced structure. Representative examples of tautomers include keto-enol tautomers, phenol-keto tautomers, nitroso-oxime tautomers, imine-enamine tautomers Wait. It is to be understood that the scope of the present application covers all such ratios in any ratio (eg, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99). %) isomer or a mixture thereof.
本文中可使用实线
实楔形
或虚楔形
描绘本发明的化合物中的键。使用实线以描绘本发明化合物中键连至不对称原子的键,表明包括该原子处的所有可能的立体异构体(例如,特定的对映异构体、外消旋混合物等)。使用实楔或虚楔形以描绘本发明的化合物中键连至不对称原子的键,表明所示的立体异构体。当存在于外消旋混合物中时,使用实楔及虚楔形以定义相对立体化学,而非绝对立体化学。除非另外指明,否则本发明的化合物可以为立体异构体(其包括顺式及反式异构体、光学异构体(例如R及S对映异构体)、非对映异构体、几何异构体、旋转异构体、构象异构体、阻转异构体及其混合物)的形式存在。本发明的化合物可表现一种以上类型的异构现象,且由其混合物(例如外消旋混合物及非对映异构体对)组成。
Solid lines can be used in this article Solid wedge Virtual wedge The linkages in the compounds of the invention are depicted. The use of solid lines to delineate linkages to an asymmetric atom in a compound of the invention is meant to include all possible stereoisomers at the atom (e.g., specific enantiomers, racemic mixtures, and the like). A solid wedge or a virtual wedge is used to characterize the linkages to the asymmetric atoms in the compounds of the invention, indicating the stereoisomers shown. When present in a racemic mixture, solid wedges and virtual wedges are used to define relative stereochemistry rather than absolute stereochemistry. Unless otherwise indicated, the compounds of the invention may be stereoisomers (including cis and trans isomers, optical isomers (eg, R and S enantiomers), diastereomers, Geometric isomers, rotamers, conformers, atropisomers, and mixtures thereof exist. The compounds of the invention may exhibit more than one type of isomerism and consist of a mixture thereof (e.g., a racemic mixture and a diastereomeric pair).
本发明涵盖本发明的化合物的所有可能的结晶形式或多晶型物,其可为单一多晶型物或多于一种多晶型物的任意比例的混合物。The invention encompasses all possible crystalline forms or polymorphs of the compounds of the invention, which may be a single polymorph or a mixture of more than one polymorph in any ratio.
还应当理解,本发明的某些化合物可以游离形式存在用于治疗,或适当时,以其药学上可接受的衍生物形式存在。在本发明中,药学上可接受的衍生物包括但不限于:药学上可接受的盐、酯、溶剂合物、代谢物或前药,在将它们向需要其的患者给药后,能够直接或间接提供本发明的化合物或其代谢物或残余物。因此,当在本文中提及“本发明的化合物”时,也意在涵盖化合物的上述各种衍生物形式。It will also be understood that certain compounds of the invention may exist in free form for treatment or, where appropriate, in the form of their pharmaceutically acceptable derivatives. In the present invention, pharmaceutically acceptable derivatives include, but are not limited to, pharmaceutically acceptable salts, esters, solvates, metabolites or prodrugs, which can be directly administered to a patient in need thereof The compound of the invention or a metabolite or residue thereof is provided indirectly or indirectly. Thus, when reference is made herein to a "compound of the invention," it is also intended to encompass the various derivative forms described above for the compound.
本发明的化合物的药学上可接受的盐包括其酸加成盐及碱加成盐。The pharmaceutically acceptable salts of the compounds of the present invention include the acid addition salts and base addition salts thereof.
适合的酸加成盐由形成药学可接受盐的酸来形成。实例包括天冬氨酸盐、碳酸氢盐/碳酸盐、硫酸氢盐/硫酸盐、延胡索酸盐、葡庚糖酸盐、葡糖酸盐、葡糖醛酸盐、六氟磷酸盐、氢溴酸盐/溴化物、氢碘酸盐/碘化物、顺丁烯二酸盐、甲基硫酸盐、、烟酸盐、硝酸盐、乳清酸盐、棕榈酸盐及其它类似的盐。Suitable acid addition salts are formed from acids which form pharmaceutically acceptable salts. Examples include aspartate, bicarbonate/carbonate, hydrogen sulfate/sulfate, fumarate, glucoheptonate, gluconate, glucuronate, hexafluorophosphate, hydrobromine Acid salts/bromides, hydroiodides/iodides, maleates, methyl sulfates, nicotinates, nitrates, orotates, palmitates and other similar salts.
适合的碱加成盐由形成药学可接受盐的碱来形成。实例包括铝盐、精氨酸盐、胆碱盐、二乙胺盐、赖氨酸盐、镁盐、葡甲胺盐、钾盐、钠盐、氨丁三醇盐及其它类似的盐。Suitable base addition salts are formed from bases which form pharmaceutically acceptable salts. Examples include aluminum salts, arginine salts, choline salts, diethylamine salts, lysine salts, magnesium salts, meglumine salts, potassium salts, sodium salts, tromethamine salts, and other similar salts.
如本文中所使用,术语“酯”意指衍生自本申请中各个通式化合物的酯,其包括生理上可水解的酯(可在生理条件下水解以释放游离酸或醇形式的本发明的化合物)。本发明的化合物本身也可以是酯。As used herein, the term "ester" means an ester derived from a compound of the formulae herein, which includes a physiologically hydrolyzable ester (which can be hydrolyzed under physiological conditions to release the free acid or alcohol form of the invention). Compound). The compounds of the invention may also be esters per se.
本发明的化合物可以溶剂合物(优选水合物)的形式存在,其中本发明的化合物包含作为所述化合物晶格的结构要素的极性溶剂,特别是例如水、甲醇或乙醇。极性溶剂特别是水的量可以化学计量比或非化学计量比存在。The compound of the present invention may exist in the form of a solvate (preferably a hydrate) wherein the compound of the present invention contains a polar solvent as a structural element of the crystal lattice of the compound, particularly such as water, methanol or ethanol. The amount of polar solvent, particularly water, may be present in stoichiometric or non-stoichiometric ratios.
在本发明的范围内还包括本发明的化合物的代谢物,即在给药本发明的化合物时体内形成的物质。这样的产物可由例如被给药的化合物的氧化、还原、水解、酰胺化、脱酰胺化、酯化、脱脂化、酶解等产生。因此,本发明包括本发明的化合物的代谢物,包括通过使本发明的化合物与哺乳动物接触足以产生其代谢产物的时间的方法制得的化合物。Also included within the scope of the invention are metabolites of the compounds of the invention, i.e., substances formed in vivo upon administration of a compound of the invention. Such products may be produced, for example, by oxidation, reduction, hydrolysis, amidation, deamidation, esterification, delipidization, enzymatic hydrolysis, and the like of the administered compound. Accordingly, the invention includes metabolites of the compounds of the invention, including compounds prepared by contacting a compound of the invention with a mammal for a time sufficient to produce a metabolic product thereof.
本发明在其范围内进一步包括本发明的化合物的前药,其为自身可具有较小药理学活性或无药理学活性的本发明的化合物的某些衍生物当被给药至身体中或其上时可通过例如水解裂解转化成具有期望活性的本发明的化合物。通常这样的前药会是所述化合物的官能团衍生物,其易于在体内转化成期望的治疗活性化合物。关于前药的使用的其他信息可参见“Pro-drugs as Novel Delivery Systems”,第14卷,ACS Symposium Series(T.Higuchi及V.Stella)及“Bioreversible Carriers in Drug Design,”Pergamon Press,1987(E.B.Roche编辑,American Pharmaceutical Association)。本发明的前药可例如通过用本领域技术人员已知作为“前-部分(pro-moiety)(例如“Design of Prodrugs”,H.Bundgaard(Elsevier,1985)中所述)”的某些部分替代本发明的化合物中存在的适当官能团来制备。The invention further includes within its scope prodrugs of the compounds of the invention which are certain derivatives of the compounds of the invention which may themselves have less or no pharmacological activity, when administered to or into the body It can be converted to a compound of the invention having the desired activity by, for example, hydrolytic cleavage. Typically such prodrugs will be functional group derivatives of the compounds which are readily converted in vivo to the desired therapeutically active compound. Additional information on the use of prodrugs can be found in "Pro-drugs as Novel Delivery Systems", Volume 14, ACS Symposium Series (T. Higuchi and V. Stella) and "Bioreversible Carriers in Drug Design," Pergamon Press, 1987 ( Edited by EBRoche, American Pharmaceutical Association). Prodrugs of the invention may, for example, be known by those skilled in the art as "pro-moiety" (e.g., "Design of Prodrugs", H. Bundgaard (Elsevier, 1985))" It is prepared in place of the appropriate functional groups present in the compounds of the invention.
本发明还涵盖含有保护基的本发明的化合物。在制备本发明的化合物的任何过程中,保护在任何有关分子上的敏感基团或反应基团可能是必需的和/或期望的,由此形成本发明的化合物的化学保护的形式。这可以通过常规的保护基实现,例如,在Protective Groups in Organic Chemistry,ed.J.F.W.McOmie,Plenum Press,1973;和T.W.Greene&P.G.M.Wuts,Protective Groups in Organic Synthesis,John Wiley&Sons,1991中所述的那些保护基,这些参考文献通过援引加入本文。使用本领域已知的方法,在适当的后续阶段可以移除保护基。The invention also encompasses compounds of the invention containing a protecting group. In any process for preparing a compound of the invention, it may be necessary and/or desirable to protect a sensitive group or reactive group on any of the molecules of interest, thereby forming a chemically protected form of the compound of the invention. This can be achieved by conventional protecting groups such as those described in Protective Groups in Organic Chemistry, ed. JFW McOmie, Plenum Press, 1973; and TW Greene & P. GM Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, 1991. Protecting groups, which are incorporated herein by reference. The protecting group can be removed at a suitable subsequent stage using methods known in the art.
术语“约”是指在所述数值的±10%范围内,优选±5%范围内,更优选±2%范围内。The term "about" means within ±10% of the stated value, preferably within ±5%, more preferably within ±2%.
化合物Compound
本发明的一个目的在于提供式(I)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,It is an object of the present invention to provide a compound of the formula (I) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate or metabolite thereof Or prodrugs, or mixtures thereof,
其中,among them,
B选自:B is selected from:
L选自任选地被一个或多个R
b取代的C
1-6亚烷基、C
2-6亚烯基、C
2-6亚炔基,所述亚烷基、亚烯基或亚炔基任选地被一个或多个-O-、-NR-或-S-间断;
L is selected from C 1-6 alkylene, C 2-6 alkenylene, C 2-6 alkynylene, optionally substituted by one or more R b , said alkylene, alkenylene or sub An alkynyl group is optionally interrupted by one or more -O-, -NR- or -S-;
或者L选自下列基团:Or L is selected from the following groups:
其中
表示单键或双键,并且1位置处与B连接,2位置处与磷原子(P)连接;
among them Represents a single bond or a double bond, and is connected to B at the 1 position and to the phosphorus atom (P) at the 2 position;
X、Y和Z在每次出现时各自独立地选自CH
2、O、S和NR;
X, Y and Z are each independently selected from CH 2 , O, S and NR at each occurrence;
R
a和R
b在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO
2、-N(R)
2、-N
3、C
1-6烷基、 C
3-6环烷基、C
2-6烯基、卤代C
2-6烯基和C
2-6炔基;
R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , -N 3 , C 1-6 alkyl, C 3- 6 cycloalkyl, C 2-6 alkenyl, halogenated C 2-6 alkenyl and C 2-6 alkynyl;
q为0、1、2、3、4、5、6、7或8,条件是:q is 0, 1, 2, 3, 4, 5, 6, 7, or 8, with the following conditions:
q不大于对应基团上可被取代的位置的数目;并且q is not greater than the number of positions on the corresponding group that can be substituted;
当q大于1时,每个R
b可以相同或不同;
When q is greater than 1, each R b may be the same or different;
n为0、1、2或3,条件是:n is 0, 1, 2 or 3, provided that:
n不大于对应基团上可被取代的位置的数目;并且n is not greater than the number of positions on the corresponding group that can be substituted;
当n大于1时,每个R
a可以相同或不同;
When n is greater than 1, each R a may be the same or different;
Ar
1和Ar
2各自独立地选自C
6-14芳基和5-14元杂芳基,所述芳基和杂芳基任选地被一个或多个R
c取代;
Ar 1 and Ar 2 are each independently selected from C 6-14 aryl and 5-14 membered heteroaryl, the aryl and heteroaryl optionally being substituted by one or more R c ;
R
c在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO
2、-N(R)
2、C
1-6烷基、卤代C
1-6烷基、C
1-6烷氧基、C
1-6烷硫基、C
3-6环烷基、3-10元杂环烷基和C
2-6炔基;
R c is each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , C 1-6 alkyl, halo C 1-6 alkyl, C 1-6 alkoxy group, C 1-6 alkylthio group, C 3-6 cycloalkyl group, 3-10 membered heterocycloalkyl group and C 2-6 alkynyl group;
R在每次出现时各自独立地选自氢、C
1-6烷基和C
3-6环烷基;
R is each independently selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl;
m为1、2或3;m is 1, 2 or 3;
p为1、2或3;p is 1, 2 or 3;
R
1和R
2各自独立地选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 1 and R 2 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
或者R
1和R
2连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;
Or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
R
3和R
4各自独立地选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 3 and R 4 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl, and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN, and -NO 2 ;
或者R
3和R
4连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;
Or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
或者R
2和R
3连同其所连接的碳原子共同形成C
3-6环烷基或4-10元杂环烷基;并且
Or R 2 and R 3 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;
R
5选自氢、C
1-6烷基、C
3-6环烷基、C
1-6烷氧基、C
6-14芳基和C
7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;
R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl and C 7-20 aralkyl, said alkyl, ring The alkyl, alkoxy, aryl and aralkyl groups are each optionally substituted by one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN and -NO 2 ;
或者R
5与Ar
2上的取代基R
c连同其所连接的碳原子共同形成与Ar
2稠合的C
4-6环烷基或4-10元杂环烷基;
Or R 5 and the substituent R c on Ar 2 together with the carbon atom to which they are bonded form a C 4-6 cycloalkyl or 4-10 membered heterocycloalkyl fused to Ar 2 ;
条件是:requirement is:
1)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
2为2-甲基苯基;且Ar
1为苯基时,
1) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 2 is 2-methyl Phenyl; and when Ar 1 is phenyl,
R
1和R
2不为以下情况:R
1和R
2各自独立地选自氢和甲基;或者R
1和R
2连同其所连接的碳原子共同形成环丙基;
R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
2)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
2为2-甲基苯基时,
2) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 2 is 2-methylphenyl,
Ar
1不为α-萘基或β-萘基;
Ar 1 is not α-naphthyl or β-naphthyl;
3)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
3) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,
Ar
2不为2-甲基萘基、3-甲基苯基或4-甲基苯基;
Ar 2 is not 2-methylnaphthyl, 3-methylphenyl or 4-methylphenyl;
4)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
1为苯基;且Ar
2为2-甲基苯基时,
4) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is phenyl; When Ar 2 is 2-methylphenyl,
R
1和R
2不为以下情况:R
1和R
2各自独立地选自氢和甲基;或者R
1和R
2连同其所连接的碳原子共同形成环丙基;
R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;
5)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1和R
2不同并各自独立地选自氢和甲基;R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
5) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,
Ar
2不为3-甲基苯基、4-甲基苯基、4-氟苯基、2,6-二甲基苯基或2-甲基萘基;
Ar 2 is not 3-methylphenyl, 4-methylphenyl, 4-fluorophenyl, 2,6-dimethylphenyl or 2-methylnaphthyl;
6)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
6) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a phenyl group,
Ar
2不为苯基、4-甲基苯基、2-甲氧基苯基、2-甲氧基-4-甲基苯基、2,4-二甲基苯基、2-甲基-4-甲氧基苯基或2-甲基-4-叔丁基苯基;
Ar 2 is not phenyl, 4-methylphenyl, 2-methoxyphenyl, 2-methoxy-4-methylphenyl, 2,4-dimethylphenyl, 2-methyl- 4-methoxyphenyl or 2-methyl-4-tert-butylphenyl;
7)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;Ar
1为α-萘基,且Ar
2为2-甲基苯基时,
7) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is α-naphthyl And when Ar 2 is 2-methylphenyl,
R
1和R
2不为以下情况:R
1和R
2同时为氢;或者R
1为氢且R
2为甲基;或者R
1为甲基且R
2为氢;
R 1 and R 2 are not the case where R 1 and R 2 are simultaneously hydrogen; or R 1 is hydrogen and R 2 is methyl; or R 1 is methyl and R 2 is hydrogen;
8)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
3、R
4和R
5各自为氢;R
1和R
2不同并各自独立地选自氢和甲基;且Ar
1为β-萘基时,
8) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; R 1 and R 2 are different and Each of them is independently selected from hydrogen and methyl; and when Ar 1 is β-naphthyl,
Ar
2不为2-甲基萘基;
Ar 2 is not a 2-methylnaphthyl group;
9)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为4-氟苯基时,
9) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a 4-fluorophenyl group,
Ar
2不为2-甲基苯基;
Ar 2 is not 2-methylphenyl;
10)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NCH
3;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为苯基时,
10) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NCH 3 ; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; And when Ar 1 is a phenyl group,
Ar
2不为2-甲基苯基;
Ar 2 is not 2-methylphenyl;
11)当B为
L为
其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R
1、R
2、R
3、R
4和R
5各自为氢;且Ar
1为β-萘基时,Ar
2不为2-甲基苯基。
11) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a β-naphthyl group, Ar 2 is not a 2-methylphenyl group.
根据本发明的一些实施方案,X为O或NR,且R选自氢、C
1-6烷基和C
3-6环烷基。在一些优选的实施方案中,X为O或NR,且R选自氢和C
1-6烷基。在一些更优选的实施方案中,X为O、NH或NCH
3。在一些进一步更优选的实施方案中,X为NH。
According to some embodiments of the invention, X is O or NR and R is selected from the group consisting of hydrogen, C1-6 alkyl and C3-6 cycloalkyl. In some preferred embodiments, X is O or NR and R is selected from the group consisting of hydrogen and C1-6 alkyl. In some more preferred embodiments, X is O, NH or NCH 3. In some further more preferred embodiments, X is NH.
根据本发明的一些实施方案,Y为O或S。在一些优选的实施方案中,Y为O。According to some embodiments of the invention, Y is O or S. In some preferred embodiments, Y is O.
根据本发明的一些实施方案,Z为O、S或CH
2。在一些优选的实施方案中,Z为O。
According to some embodiments of the invention, Z is O, S or CH 2 . In some preferred embodiments, Z is O.
根据本发明的一些实施方案,R
a和R
b在每次出现时各自独立地选自氢、卤素、-OH、-N(R)
2、-N
3和C
1-6烷基。在一些优选的实施方案中,R
a和R
b在每次出现时各自独立地选自卤素、-OH和-N(R)
2。在一些更优选的实施方案中,R
a和R
b在每次出现时各自独立地选自F、-OH和NH
2。
According to some embodiments of the invention, R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -N(R) 2 , -N 3 and C 1-6 alkyl. In some preferred embodiments, R a and R b are each independently selected from the group consisting of halogen, -OH, and -N(R) 2 at each occurrence. In some more preferred embodiments, R a and R b are each independently selected from the group consisting of F, -OH, and NH 2 at each occurrence.
根据本发明的一些实施方案,R
c在每次出现时各自独立地选自氢、卤素、C
1-6烷基和C
1-6烷氧基。在一些优选的实施方案中,R
c在每次出现时各自独立地为甲基、叔丁基、甲氧基、氟或氯。
According to some embodiments of the invention, each occurrence of R c is independently selected from the group consisting of hydrogen, halogen, C 1-6 alkyl, and C 1-6 alkoxy. In some preferred embodiments, R c is each independently methyl, t-butyl, methoxy, fluoro or chloro at each occurrence.
根据本发明的一些实施方案,B选自:According to some embodiments of the invention, B is selected from:
在一些优选的实施方案中,B选自:In some preferred embodiments, B is selected from the group consisting of:
在特别优选的实施方案中,B选自:In a particularly preferred embodiment, B is selected from the group consisting of
根据本发明的一些实施方案,L选自:According to some embodiments of the invention, L is selected from:
其中1位置处与B连接,2位置处与磷原子(P)连接。One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
在一些优选的实施方案中,L选自:In some preferred embodiments, L is selected from the group consisting of:
其中1位置处与B连接,2位置处与磷原子(P)连接。One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
在特别优选的实施方案中,L选自:In a particularly preferred embodiment, L is selected from the group consisting of:
其中1位置处与B连接,2位置处与磷原子(P)连接。One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
根据本发明的一些实施方案,Ar
1和Ar
2各自独立地选自苯基、萘基和5-6元杂芳基(例如噻吩基、吡啶基或吡唑基),所述苯基、萘基和5-6元杂芳基各自独立地任选地被1或2个独立地选自氢、C
1-6烷基、卤素和C
1-6烷氧基的取代基取代。在一些优选的实施方案中,Ar
1和Ar
2各自独立地选自苯基、萘基、噻吩基、吡啶基和吡唑基,所述苯基、萘基、噻吩基、吡啶基和吡唑基各自独立地任选地被1或2个独立地选自氢、甲基、叔丁基、甲氧基、氟和氯的取代基取代。在一些更优选的实施方案中,Ar
1和Ar
2各自独立地选自苯基,所述苯基任选地被1个甲基取代。
According to some embodiments of the invention, Ar 1 and Ar 2 are each independently selected from the group consisting of phenyl, naphthyl and 5-6 membered heteroaryl (eg thienyl, pyridyl or pyrazolyl), said phenyl, naphthalene The base and the 5-6 membered heteroaryl are each independently optionally substituted with 1 or 2 substituents independently selected from the group consisting of hydrogen, C1-6 alkyl, halogen and C1-6 alkoxy. In some preferred embodiments, Ar 1 and Ar 2 are each independently selected from the group consisting of phenyl, naphthyl, thienyl, pyridyl and pyrazolyl, phenyl, naphthyl, thienyl, pyridyl and pyrazole The groups are each independently optionally substituted with 1 or 2 substituents independently selected from the group consisting of hydrogen, methyl, tert-butyl, methoxy, fluoro and chloro. In some more preferred embodiments, Ar 1 and Ar 2 are each independently selected from phenyl, which is optionally substituted with 1 methyl.
根据本发明的一些实施方案,R
1和R
2各自独立地选自氢和C
1-6烷基,所述烷基任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;或者R
1和R
2连同其所连接的碳原子共同形成C
3-6环烷基。在一些优选的实施方案中,R
1和R
2各自独立地选自氢和甲基;或者R
1和R
2连同其所连接的碳原子共同形成环丙基。在一些更优选的实施方案中,R
1和R
2各自独立地选自氢和甲基。
According to some embodiments of the invention, R 1 and R 2 are each independently selected from hydrogen and C 1-6 alkyl, optionally substituted by one or more selected from the group consisting of hydrogen, halogen, -OH, -CN Substituting with a substituent of -NO 2 ; or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group. In some preferred embodiments, R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group. In some more preferred embodiments, R 1 and R 2 are each independently selected from the group consisting of hydrogen and methyl.
根据本发明的一些实施方案,R
3和R
4各自独立地选自氢和C
1-6烷基,所述烷基选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代;或者R
3和R
4连同其所连接的碳原子共同形成C
3-6环烷基。在一些优选的实施方案中,R
3和R
4各自独立地选自氢和甲基;或者R
3和R
4连同其所连接的碳原子共同形成环丙基。在一些更优选的实施方案中,R
3和R
4均为氢。
According to some embodiments of the invention, R 3 and R 4 are each independently selected from hydrogen and C 1-6 alkyl, the alkyl optionally being selected from one or more selected from the group consisting of hydrogen, halogen, -OH, -CN, and Substituting a substituent of -NO 2 ; or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group. In some preferred embodiments, R 3 and R 4 are each independently selected from hydrogen and methyl; or R 3 and R 4 together with the carbon atom to which they are attached form a cyclopropyl group. In some more preferred embodiments, both R 3 and R 4 are hydrogen.
根据本发明的一些实施方案,R
5选自氢、C
1-6烷基、C
3-6环烷基,所述烷基、环烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO
2的取代基取代。在一些优选的实施方案中,R
5为 氢。
According to some embodiments of the invention, R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, each of which is optionally selected from one or more selected from the group consisting of hydrogen, Substituents for halogen, -OH, -CN, and -NO 2 are substituted. In some preferred embodiments, R 5 is hydrogen.
根据本发明的一些实施方案,m为1或2。According to some embodiments of the invention, m is 1 or 2.
根据本发明的一些实施方案,p为1或2。According to some embodiments of the invention, p is 1 or 2.
根据本发明的一些实施方案,本发明的化合物为式(I-1)、(I-2)或(I-3)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,According to some embodiments of the invention, the compound of the invention is a compound of formula (I-1), (I-2) or (I-3) or a pharmaceutically acceptable salt, ester, stereoisomer thereof, Tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof,
其中X、Y、Z、R
a、R
b、q、n、Ar
1、Ar
2、m、p、R
1、R
2、R
3、R
4和R
5如上所定义;优选地,X为NR,Y和Z均为O,R
a和R
b在每次出现时各自独立地选自卤素、-OH和-N(R)
2,q为0、1、2或3,n为1或2,Ar
1和Ar
2各自独立地选自任选地被1个或2个C
1-6烷基取代的C
6-10芳基,m为1或2,p为1或2,且R
1、R
2、R
3、R
4、R
5和R各自独立地为氢或C
1-6烷基;并且更优选地,X为NH,Y和Z均为O,R
a和R
b在每次出现时各自独立地选自F、-OH和-NH
2,q为1、2或3,n为1,Ar
1和Ar
2各自独立地选自任选地被1个或2个C
1-6烷基取代的C
6-10芳基,m为1,p为1,且R
1、R
2、R
3、R
4、R
5和R各自独立地为氢或甲基。
Wherein X, Y, Z, R a , R b , q, n, Ar 1 , Ar 2 , m, p, R 1 , R 2 , R 3 , R 4 and R 5 are as defined above; preferably, X is NR, Y and Z are both O, and each occurrence of R a and R b is independently selected from the group consisting of halogen, -OH and -N(R) 2 , q is 0, 1, 2 or 3, and n is 1 or 2, Ar 1 and Ar 2 are each independently selected from a C 6-10 aryl group optionally substituted by 1 or 2 C 1-6 alkyl groups, m is 1 or 2, p is 1 or 2, and R 1 , R 2 , R 3 , R 4 , R 5 and R are each independently hydrogen or C 1-6 alkyl; and more preferably, X is NH, Y and Z are both O, and R a and R b are Each occurrence is independently selected from F, -OH and -NH 2 , q is 1, 2 or 3, n is 1, and Ar 1 and Ar 2 are each independently selected from optionally 1 or 2 C. a 1-6 alkyl-substituted C 6-10 aryl group, m is 1, p is 1, and R 1 , R 2 , R 3 , R 4 , R 5 and R are each independently hydrogen or methyl.
根据本发明的一些实施方案,本发明的化合物为式(II)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,According to some embodiments of the invention, the compound of the invention is a compound of formula (II) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof , hydrates, metabolites or prodrugs, or mixtures thereof,
其中m、p、R
1、R
2、R
3、R
4、Ar
1、Ar
2和X如上所定义;优选地,m为1或2,且p为1或2;更优选地,m为1且p为1;并且优选Ar
1为苯基,且Ar
2为任选地被1个甲基取代的苯基。
Wherein m, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted by 1 methyl group.
根据本发明的一些实施方案,本发明的化合物为式(III)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,According to some embodiments of the invention, the compound of the invention is a compound of formula (III) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof , hydrates, metabolites or prodrugs, or mixtures thereof,
其中m、p、R
1、R
2、R
3、R
4、Ar
1、Ar
2和X如上所定义;,优选地,m为1或2,且p为1或2;更优选地,m为1且p为1;并且优选Ar
1为苯基,且Ar
2为任选地被1个甲基取代的苯基。
Wherein m, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m Is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted with 1 methyl group.
根据本发明的一些实施方案,本发明的化合物为式(IV)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,According to some embodiments of the present invention, the compound of the present invention is a compound of the formula (IV) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph or solvate thereof. , hydrates, metabolites or prodrugs, or mixtures thereof,
其中m、p、R
1、R
2、R
3、R
4、Ar
1、Ar
2和X如上所定义;优选地,m为1或2,且p为1或2;更优选地,m为1且p为1;并且优选Ar
1为苯基,且Ar
2为任选地被1个甲基取代的苯基。
Wherein m, p, R 1 , R 2 , R 3 , R 4 , Ar 1 , Ar 2 and X are as defined above; preferably, m is 1 or 2, and p is 1 or 2; more preferably, m is 1 and p is 1; and preferably Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted by 1 methyl group.
本发明涵盖对各个实施方案进行任意组合所得的化合物。The present invention encompasses compounds obtained by any combination of the various embodiments.
根据本发明的一些实施方案,本发明的式(I)的化合物选自:According to some embodiments of the invention, the compound of formula (I) of the invention is selected from the group consisting of
制备方法Preparation
本发明的另一目的在于提供上述式(I)的化合物的制备方法,其包括以下步骤:Another object of the present invention is to provide a process for the preparation of a compound of the above formula (I) which comprises the steps of:
其中among them
B、L、X、Ar
1、Ar
2、R
1-R
5、m和p如上文所定义。
B, L, X, Ar 1 , Ar 2 , R 1 - R 5 , m and p are as defined above.
步骤1:step 1:
根据本发明的一些实施方案,步骤1是在有机碱或无机碱的存在下进行的。所述有机碱包括但不限于叔丁醇钠、三乙胺、DIPEA、吡啶或DMAP。所述无机碱包括但不限于NaH、NaOH、Na
2CO
3或K
2CO
3。
According to some embodiments of the invention, step 1 is carried out in the presence of an organic or inorganic base. The organic base includes, but is not limited to, sodium t-butoxide, triethylamine, DIPEA, pyridine or DMAP. The inorganic base includes, but is not limited to, NaH, NaOH, Na 2 CO 3 or K 2 CO 3 .
根据本发明的一些实施方案,步骤1是在-80℃至0℃,优选-70℃至-20℃的温度下进行的。According to some embodiments of the invention, step 1 is carried out at a temperature of from -80 °C to 0 °C, preferably from -70 °C to -20 °C.
根据本发明的一些实施方案,式a的化合物、式b的化合物和式c的化合物的摩尔比为1:(0.5-2):(0.5-2),优选1:(0.8-2):(0.8-2),更优选1:(1-1.5):(1-1.5)。According to some embodiments of the invention, the molar ratio of the compound of formula a, the compound of formula b and the compound of formula c is 1: (0.5-2): (0.5-2), preferably 1: (0.8-2): ( 0.8-2), more preferably 1: (1-1.5): (1-1.5).
根据本发明的一些实施方案,式a的化合物、式b的化合物、式c的化合物与有机碱或无机碱的摩尔比为1:(0.5-2):(0.5-2):(0.5-2),优选1:(0.8-2):(0.8-2):(0.8-2),更优选1:(1-1.5):(1-1.5):(1-1.5)。According to some embodiments of the invention, the molar ratio of the compound of formula a, the compound of formula b, the compound of formula c to the organic or inorganic base is 1: (0.5-2): (0.5-2): (0.5-2 Preferably, 1: (0.8-2): (0.8-2): (0.8-2), more preferably 1: (1-1.5): (1-1.5): (1-1.5).
步骤2:Step 2:
根据本发明的一些实施方案,步骤2是在有机碱或无机碱的存在下进行的。所述有机碱或无机碱包括但不限于叔丁基氯化镁、正丁基锂、二异丙基氨基锂、叔丁醇钠、三乙胺、DIPEA、吡啶、DMAP、NaH、NaOH、Na
2CO
3或K
2CO
3。
According to some embodiments of the invention, step 2 is carried out in the presence of an organic or inorganic base. The organic base or inorganic base includes, but is not limited to, t-butyl magnesium chloride, n-butyl lithium, lithium diisopropylamide, sodium t-butoxide, triethylamine, DIPEA, pyridine, DMAP, NaH, NaOH, Na 2 CO 3 or K 2 CO 3 .
根据本发明的一些实施方案,步骤2是在-80℃至25℃,优选-30℃至10℃的温度下进行的。According to some embodiments of the invention, step 2 is carried out at a temperature of from -80 °C to 25 °C, preferably from -30 °C to 10 °C.
根据本发明的一些实施方案,式d的化合物与式e的化合物的摩尔比为1:(0.5-2),优选1:(0.75-1.5)。According to some embodiments of the invention, the molar ratio of the compound of formula d to the compound of formula e is 1: (0.5-2), preferably 1: (0.75-1.5).
根据本发明的一些实施方案,式d的化合物、式e的化合物与有机碱或无机碱的摩尔比为1:(0.5-2):(1-3),优选1:(0.75-1.5):(1-2)。According to some embodiments of the invention, the molar ratio of the compound of formula d, the compound of formula e to the organic or inorganic base is 1: (0.5-2): (1-3), preferably 1: (0.75-1.5): (1-2).
以上步骤1和2均可在有机溶剂中进行。所述有机溶剂可以是本领域常用的反应溶剂,例如但不限于N,N-二甲基甲酰胺、二甲基亚砜、N-甲基吡咯烷酮、饱和烃类(例如环己烷、己烷等)、卤代烃类(例如二氯甲烷、氯仿、1,2-二氯乙烷等)、醚类(例如四氢呋喃、乙醚、二噁烷、1,2-二甲氧基乙烷等)、腈类(例如乙腈等)和它们的混合溶剂等。The above steps 1 and 2 can be carried out in an organic solvent. The organic solvent may be a reaction solvent commonly used in the art, such as, but not limited to, N,N-dimethylformamide, dimethyl sulfoxide, N-methylpyrrolidone, saturated hydrocarbons (such as cyclohexane, hexane). Etc.), halogenated hydrocarbons (such as dichloromethane, chloroform, 1,2-dichloroethane, etc.), ethers (such as tetrahydrofuran, diethyl ether, dioxane, 1,2-dimethoxyethane, etc.) , nitriles (such as acetonitrile, etc.) and their mixed solvents.
药物组合物和药盒Pharmaceutical compositions and kits
本发明的另一目的在于提供一种药物组合物,其包含预防或治疗有效量的本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,以及一种或多种药学上可接受的载体。Another object of the present invention is to provide a pharmaceutical composition comprising a prophylactically or therapeutically effective amount of a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph thereof , solvate, hydrate, metabolite or prodrug, or mixtures thereof, and one or more pharmaceutically acceptable carriers.
本发明中“药学上可接受的载体”是指与治疗剂一同给药的稀释剂、辅剂、赋形剂或媒介物,并且其在合理的医学判断的范围内适于接触人类和/或其它动物的组织而没有过度的毒性、刺激、过敏反应或与合理的益处/风险比相应的其它问题或并发症。"Pharmaceutically acceptable carrier" in the context of the present invention means a diluent, adjuvant, excipient or vehicle with which the therapeutic agent is administered, and which is suitable for contacting humans and/or within the scope of sound medical judgment. Tissues of other animals without excessive toxicity, irritation, allergic reactions, or other problems or complications corresponding to a reasonable benefit/risk ratio.
在本发明的药物组合物中可使用的药学上可接受的载体包括但不限于无菌液体,例如水和油,包括那些石油、动物、植物或合成来源的油,例如花生油、大豆油、矿物油、芝麻油等。当所述药物组合物通过静脉内给药时,水是示例性载体。还可以使用生理盐水和葡萄糖及甘油水溶液作为液体载体,特别是用于注射液。适合的药物赋形剂包括淀粉、葡萄糖、乳糖、蔗糖、明胶、麦芽糖、白垩、硅胶、硬脂酸钠、单硬脂酸甘油酯、滑石、氯化钠、脱脂奶粉、甘油、丙二醇、水、乙醇等。所述组合物还可以视需要包含少量的湿润剂、乳化剂或pH缓冲剂。口服制剂可以包含标准载体,如药物级的甘露醇、乳糖、淀粉、硬脂酸镁、糖精钠、纤维素、碳酸镁等。适合的药学上可接受的载体的实例如在Remington’s Pharmaceutical Sciences(1990)中所述。Pharmaceutically acceptable carriers that can be used in the pharmaceutical compositions of the present invention include, but are not limited to, sterile liquids such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, minerals. Oil, sesame oil, etc. Water is an exemplary carrier when the pharmaceutical composition is administered intravenously. It is also possible to use physiological saline and an aqueous solution of glucose and glycerin as a liquid carrier, particularly for injection. Suitable pharmaceutical excipients include starch, glucose, lactose, sucrose, gelatin, maltose, chalk, silica gel, sodium stearate, glyceryl monostearate, talc, sodium chloride, skimmed milk powder, glycerin, propylene glycol, water, Ethanol and the like. The composition may also contain minor amounts of wetting agents, emulsifying agents or pH buffering agents as needed. Oral formulations may contain standard carriers such as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, cellulose, magnesium carbonate, and the like. Examples of suitable pharmaceutically acceptable carriers are as described in Remington's Pharmaceutical Sciences (1990).
本发明的药物组合物可以系统地作用和/或局部地作用。为此目的,它们可以适合的途径给药,例如通过注射(如静脉内、动脉内、皮下、腹膜内、肌内注射,包括滴注)或经皮给药;或者通过口服、含服、经鼻、透粘膜、局部、以眼用制剂的形式或通过吸入给药。The pharmaceutical compositions of the invention may act systemically and/or locally. For this purpose, they may be administered in a suitable route, for example by injection (for example intravenous, intraarterial, subcutaneous, intraperitoneal, intramuscular, including instillation) or transdermal administration; or by oral, buccal, or oral administration. Nasal, transmucosal, topical, in the form of ophthalmic preparations or by inhalation.
对于这些给药途径,可以适合的剂型给药本发明的药物组合物。所述剂型包括但不限于片剂、胶囊剂、锭剂、硬糖剂、散剂、喷雾剂、乳膏剂、软膏剂、栓剂、凝胶剂、糊剂、洗剂、软膏剂、水性混悬剂、可注射溶液剂、酏剂、糖浆剂等。For these routes of administration, the pharmaceutical compositions of the invention may be administered in a suitable dosage form. The dosage forms include, but are not limited to, tablets, capsules, troches, hard candy, powders, sprays, creams, ointments, suppositories, gels, pastes, lotions, ointments, aqueous suspensions. Injectable solutions, elixirs, syrups, and the like.
本发明的化合物在药物组合物中的含量或用量可以是约0.01mg至约1000mg,适合地是0.1-500mg,优选0.5-300mg,更优选1-150mg,特别优选1-50mg,例如1.5mg、2mg、4mg、10mg、25mg等。The amount or amount of the compound of the present invention in the pharmaceutical composition may be from about 0.01 mg to about 1000 mg, suitably from 0.1 to 500 mg, preferably from 0.5 to 300 mg, more preferably from 1 to 150 mg, particularly preferably from 1 to 50 mg, for example, 1.5 mg, 2 mg, 4 mg, 10 mg, 25 mg, and the like.
根据本发明的一些实施方案,所述药物组合物还可包含一种或多种其它治疗剂,例如用于预防或治疗癌症和/或肿瘤及其相关疾病的其它治疗剂,其包括但不限于细胞毒类抗肿瘤药物、影响内分泌平衡的药物、生物反应调节剂类药物、分子靶向抗肿瘤类药物、以及其他辅助治疗药物。According to some embodiments of the invention, the pharmaceutical composition may further comprise one or more additional therapeutic agents, such as other therapeutic agents for preventing or treating cancer and/or tumors and related diseases, including but not limited to Cytotoxic anti-tumor drugs, drugs that affect endocrine balance, biological response modifiers, molecularly targeted anti-tumor drugs, and other ancillary treatments.
本发明的另一目的在于提供一种制备本发明的药物组合物的方法,所述方法包括将本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物与一种或多种药学上可接受的载体组合。Another object of the present invention is to provide a process for the preparation of a pharmaceutical composition of the present invention which comprises administering a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer thereof, Polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, are combined with one or more pharmaceutically acceptable carriers.
本发明的另一目的在于提供一种药盒,其包含本发明的化合物或其药学上可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者本发明的药物组合物。Another object of the present invention is to provide a kit comprising a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate thereof, Hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
治疗方法和用途Treatment and use
本发明的另一目的在于提供本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物或者本发明的药物组合物在制备用于预防或治疗癌症和/或肿瘤及其相关疾病的药物中的用途。Another object of the present invention is to provide a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof Or a mixture thereof or a pharmaceutical composition of the invention for use in the manufacture of a medicament for the prevention or treatment of cancer and/or a tumor and a related disease thereof.
本发明的另一目的在于提供本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物或者本发明的药物组合物,其用于预防或治疗癌症和/或肿瘤及其相关疾病。Another object of the present invention is to provide a compound of the present invention or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof Or a mixture thereof or a pharmaceutical composition of the invention for use in the prevention or treatment of cancer and/or tumors and related diseases.
本发明的另一目的在于提供预防或治疗癌症和/或肿瘤及其相关疾病的方法,所述方法包括向需要其的个体给药有效量的本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者本发明的药物组合物。Another object of the present invention is to provide a method for preventing or treating cancer and/or a tumor and a related disease thereof, which comprises administering to an individual in need thereof an effective amount of a compound of the present invention or a pharmaceutically acceptable salt or ester thereof , stereoisomers, tautomers, polymorphs, solvates, hydrates, metabolites or prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention.
根据本发明的一些实施方案,可使用本发明的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者本发明的药物组合物预防或治疗癌症和/或肿瘤及其相关疾病。According to some embodiments of the invention, a compound of the invention, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite thereof or Prodrugs, or mixtures thereof, or pharmaceutical compositions of the invention prevent or treat cancer and/or tumors and related diseases.
本发明中所述的癌症和/或肿瘤包括但不限于胰腺、肺(如非小细胞肺癌)、卵巢、膀胱、乳腺癌、胃、结直肠和肝脏等部位发生的肿瘤和/或癌症以及相关疾病。The cancers and/or tumors described in the present invention include, but are not limited to, tumors and/or cancers occurring in the pancreas, lung (eg, non-small cell lung cancer), ovary, bladder, breast, stomach, colorectal, and liver, and related disease.
如本文中所使用的术语“有效量”指被给药后会在一定程度上缓解所预防或治疗病症的一或多种症状的化合物的量。The term "effective amount" as used herein refers to an amount of a compound that, to a certain extent, relieves one or more symptoms of the prophylactic or therapeutic condition after administration.
可调整给药方案以提供最佳所需响应。例如,可给药单次推注,可随时间给药数个分剂量,或可如治疗情况的急需所表明而按比例减少或增加剂量。要注意,剂量值可随要减轻的病况的类型及严重性而变化,且可包括单次或多次剂量。要进一步理解,对于任何特定个体,具体的给药方案应根据个体需要及给药组合物或监督组合物的给药的人员的专业判断来随时间调整。The dosing regimen can be adjusted to provide the optimal desired response. For example, a single bolus may be administered, several divided doses may be administered over time, or the dose may be proportionally reduced or increased as indicated by the urgent need for treatment. It is noted that the dose value can vary with the type and severity of the condition to be alleviated and can include single or multiple doses. It is to be further understood that for any particular individual, the particular dosage regimen will be adjusted over time according to the individual needs and the professional judgment of the person administering the composition or the composition of the supervised composition.
所给药的本发明的化合物的量会取决于所治疗的个体、病症或病况的严重性、给药的速率、化合物的处置及处方医师的判断。一般而言,有效剂量在每日每kg体重约0.0001至约50mg,例如约0.01至约10mg/kg/日(单次或分次给药)。对70kg的人而言,这会合计为约0.007mg/日至约3500mg/日,例如约0.7mg/日至约700mg/日。在一些情况下,不高于前述范围的下限的剂量水平可以是足够的,而在其它情况下,仍可在不引起任何有害副作用的情况下采用较大剂量,条件是首先将所述较大剂量分成数个较小剂量以在一整天中给药。The amount of the compound of the invention administered will depend on the severity of the individual, condition or condition being treated, the rate of administration, the handling of the compound, and the judgment of the prescribing physician. Generally, an effective dose will be from about 0.0001 to about 50 mg per kg body weight per day, for example from about 0.01 to about 10 mg/kg/day (single or divided doses). For a 70 kg person, this would add up to about 0.007 mg/day to about 3500 mg/day, such as from about 0.7 mg/day to about 700 mg/day. In some cases, a dose level that is not higher than the lower limit of the aforementioned range may be sufficient, while in other cases, a larger dose may still be employed without causing any harmful side effects, provided that the larger The dose is divided into several smaller doses to be administered throughout the day.
如本文中所使用,术语“治疗”是指逆转、减轻、抑制这样的术语所应用的病症或病况或者这样的病症或病况的一或多种症状的进展,或预防这样的病症或病况或者这样的病症或病况的一或多种症状。The term "treating" as used herein refers to reversing, alleviating, inhibiting the progression of a condition or condition to which such a term applies or the progression of one or more symptoms of such a condition or condition, or preventing such condition or condition or such One or more symptoms of a condition or condition.
如本文中所使用,术语“个体”包括人或非人动物。示例性人个体包括患有疾病(例如本文所述的疾病)的人个体(称为患者)或正常个体。本发明中“非人动物”包括所有脊椎动物,例如非哺乳动物(例 如鸟类、两栖动物、爬行动物)和哺乳动物,例如非人灵长类、家畜和/或驯化动物(例如绵羊、犬、猫、奶牛、猪等)。As used herein, the term "individual" includes human or non-human animals. Exemplary human individuals include a human individual (referred to as a patient) or a normal individual having a disease, such as the disease described herein. "Non-human animals" in the present invention include all vertebrates, such as non-mammals (eg, birds, amphibians, reptiles) and mammals, such as non-human primates, domestic animals, and/or domesticated animals (eg, sheep, dogs). , cats, cows, pigs, etc.).
为了使本发明的目的和技术方案更加清楚,以下结合具体实施例进一步阐述本发明。应理解,这些实施例仅用于说明本发明而不用于限制本发明的范围。并且,下列实施例中未提及的具体实验方法,均按照常规实验方法进行。In order to make the objects and technical solutions of the present invention clearer, the present invention will be further described below in conjunction with specific embodiments. It is to be understood that the examples are not intended to limit the scope of the invention. Further, specific experimental methods not mentioned in the following examples were carried out in accordance with a conventional experimental method.
本文中的缩写具有以下含义:The abbreviations in this article have the following meanings:
以下实施例中记载的化合物的结构通过
1H NMR或MS来确证。
1H NMR的测定仪器使用Bruker 400MHz核磁共振仪,测定溶剂为CD
3OD、CDCl
3或DMSO-d
6,内标物质为TMS,全部δ值用ppm值表示。质谱(MS)的测定仪器使用Agilent(ESI)质谱仪,型号为Agilent 6120B。
The structures of the compounds described in the following examples were confirmed by 1 H NMR or MS. The 1 H NMR measuring instrument was measured using a Bruker 400 MHz nuclear magnetic resonance apparatus, and the solvent was CD 3 OD, CDCl 3 or DMSO-d 6 , and the internal standard substance was TMS. The total δ value was expressed by ppm. The mass spectrometer (MS) assay instrument used an Agilent (ESI) mass spectrometer, model Agilent 6120B.
实施例中制备的非对映异构体混合物可以通过制备高效液相色谱进行分离,从而得到纯的异构体。所述制备高效液相色谱分离可以按照本领域已知的方法来进行。例如在以下分离条件 下进行:以十八烷基键合硅胶为填充剂,柱温30℃-50℃,流速5.0-20.0mL/min,检测波长200-400nm,使用流动相A(例如可以为水),流动相B(例如可以为甲醇或乙腈),进行线性梯度洗脱。The mixture of diastereomers prepared in the examples can be separated by preparative high performance liquid chromatography to give the pure isomer. The preparative high performance liquid chromatography separation can be carried out according to methods known in the art. For example, under the following separation conditions: using octadecyl bonded silica as a filler, column temperature 30 ° C -50 ° C, flow rate 5.0-20.0 mL / min, detection wavelength 200-400 nm, using mobile phase A (for example, Water), mobile phase B (for example, methanol or acetonitrile), with a linear gradient elution.
实施例一.((2R,3S,5R)-5-(5-氟-2,4-二氧代-3,4-二氢嘧啶-1(2H)-基)-3-羟基四氢呋喃-2-基)甲基-苯基-((S)-1-(2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物21)的制备Example 1. ((2R,3S,5R)-5-(5-Fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2 -Methyl-phenyl-((S)-1-(2-methylbenzyloxy)propan-2-yl)phosphoramidate (Compound 21) Preparation
步骤一:((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸五氟苯酯苯酯(化合物21-2)的合成Step 1: Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
将(S)-1-((2-甲基苄氧基)丙基)-2-胺(化合物21-1)(760mg,4.25mmol)加入至100mL三颈瓶中,加入无水二氯甲烷(10mL)。降温至-70℃,依次滴加三乙胺(450mg,4.46mmol)的无水二氯甲烷(3mL)溶液、二氯化磷酸苯酯(896mg,4.25mmol)的无水二氯甲烷(3mL)溶液,在-70℃下搅拌1h,然后升温至0℃并搅拌2h。降温至-70℃,缓慢滴加五氟苯酚(820mg,4.46mmol)和三乙胺(450mg,4.46mmol)的无水二氯甲烷(5mL)溶液。滴加完毕后,升温至室温,反应过夜,经后处理得到1.4g化合物21-2。ESI-MS(m/z):502[M+H]
+
(S)-1-((2-Methylbenzyloxy)propyl)-2-amine (Compound 21-1) (760 mg, 4.25 mmol) was added to a 100 mL 3-neck flask, and anhydrous dichloromethane was added. (10 mL). The temperature was lowered to -70 ° C, and a solution of triethylamine (450 mg, 4.46 mmol) in anhydrous dichloromethane (3 mL) and phenyl diphenyl phosphate (896 mg, 4.25 mmol) in anhydrous dichloromethane (3 mL) The solution was stirred at -70 ° C for 1 h, then warmed to 0 ° C and stirred for 2 h. The temperature was lowered to -70 ° C, and a solution of pentafluorophenol (820 mg, 4.46 mmol) and triethylamine (450 mg, 4.46 mmol) in anhydrous dichloromethane (5 mL) was slowly added dropwise. After completion of the dropwise addition, the mixture was warmed to room temperature and allowed to react overnight, and then worked up to give 1.4 g of Compound 21-2. ESI-MS (m/z): 502 [M+H] +
步骤二:((2R,3S,5R)-5-(5-氟-2,4-二氧代-3,4-二氢嘧啶-1(2H)-基)-3-羟基四氢呋喃-2-基)甲基-苯基-((S)-1-(2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物21)的合成Step 2: ((2R,3S,5R)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2- Synthesis of methyl-phenyl-((S)-1-(2-methylbenzyloxy)propan-2-yl)phosphoramidate (Compound 21)
将5-氟-2'-脱氧脲核苷(162mg,0.72mmol)加入至100mL三颈瓶中,用无水四氢呋喃(10mL)溶解。降温至-20℃,滴加叔丁基氯化镁的四氢呋喃溶液(1.0mL,1.05mmol,1M),升温至0℃,搅拌2h。然后缓慢滴加化合物21-2(300mg,0.60mmol)的无水四氢呋喃(3mL)溶液,升温至室温,反应过夜,经后处理得到粗品,经制备TLC(展开剂为DCM:MeOH=10:1)纯化得到23mg化合物21。5-Fluoro-2'-deoxyurea nucleoside (162 mg, 0.72 mmol) was added to a 100 mL 3-neck flask and dissolved in anhydrous tetrahydrofuran (10 mL). The temperature was lowered to -20 ° C, and a solution of t-butylmagnesium chloride in tetrahydrofuran (1.0 mL, 1.05 mmol, 1 M) was added dropwise, and the mixture was warmed to 0 ° C and stirred for 2 h. Then, a solution of the compound 21-2 (300 mg, 0.60 mmol) in anhydrous tetrahydrofuran (3 mL) was added dropwise, and the mixture was warmed to room temperature overnight, and then worked up to give a crude product (yield: DCM:MeOH = 10:1) Purification afforded 23 mg of compound 21.
其结构表征如下:Its structural representation is as follows:
1H NMR(400MHz,DMSO)δ11.85(s,1H),7.89(dd,J=9.1,6.9Hz,1H),7.33(t,J=7.9Hz,2H),7.27(d,J=7.0Hz,1H),7.22-7.11(m,6H),6.13(m,1H),5.48-5.37(m,2H),4.43(s,2H),4.21(m,1H),4.18-4.06(m,2H),3.96(m,1H),3.35(m,2H),3.22-3.16(m,1H),2.25(s,3H),2.12-1.97(m,2H),1.05(dd,J=6.2,2.4Hz,3H)。
1 H NMR (400MHz, DMSO) δ11.85 (s, 1H), 7.89 (dd, J = 9.1,6.9Hz, 1H), 7.33 (t, J = 7.9Hz, 2H), 7.27 (d, J = 7.0 Hz, 1H), 7.22-7.11 (m, 6H), 6.13 (m, 1H), 5.48-5.37 (m, 2H), 4.43 (s, 2H), 4.21 (m, 1H), 4.18-4.06 (m, 2H), 3.96 (m, 1H), 3.35 (m, 2H), 3.22-3.16 (m, 1H), 2.25 (s, 3H), 2.12-1.97 (m, 2H), 1.05 (dd, J = 6.2, 2.4Hz, 3H).
ESI-MS(m/z):564.2[M+H]
+。
ESI-MS (m/z): 564.2 [M+H] + .
实施例二 化合物21异构体A和化合物21异构体B的制备Example 2 Preparation of Compound 21 Isomer A and Compound 21 Isomer B
将化合物21(340mg)通过手性色谱法分离,分离条件如下:分离柱CHIRALPAK OZ-H 0.46cm I.D.×15cm,流动相:Hexane/EtOH=50/50(v/v),流速1.0ml/min,波长UV 254nm,温度35℃。Compound 21 (340 mg) was separated by chiral chromatography, and the separation conditions were as follows: separation column CHIRALPAK OZ-H 0.46 cm ID × 15 cm, mobile phase: Hexane/EtOH = 50/50 (v/v), flow rate 1.0 ml/min The wavelength is UV 254 nm and the temperature is 35 °C.
其中得到168mg的化合物21异构体A(Rt=3.965min,de%=98.9%),其结构表征如下:There were obtained 168 mg of Compound 21 isomer A (Rt = 3.965 min, de% = 98.9%), and its structure was characterized as follows:
1H NMR(400MHz,CDCl
3):δ9.58(s,1H),7.64(d,J=6.2Hz,1H),7.31-7.12(m,9H),6.20(t,J=5.7Hz,1H),4.44(d,J=2.3Hz,2H),4.47-4.35(m,2H),4.22-4.18(m,1H),4.00(s,1H),3.61-3.48(m,2H),3.34(d,J=4.1Hz,2H),2.29(s,3H),2.28-2.25(m,1H),1.86-1.80(m,2H),1.20(d,J=6.4Hz,3H)。
1 H NMR (400MHz, CDCl 3 ): δ9.58 (s, 1H), 7.64 (d, J = 6.2Hz, 1H), 7.31-7.12 (m, 9H), 6.20 (t, J = 5.7Hz, 1H ), 4.44 (d, J = 2.3 Hz, 2H), 4.47 - 4.35 (m, 2H), 4.22-4.18 (m, 1H), 4.00 (s, 1H), 3.61-3.48 (m, 2H), 3.34 ( d, J = 4.1 Hz, 2H), 2.29 (s, 3H), 2.28-2.25 (m, 1H), 1.86-1.80 (m, 2H), 1.20 (d, J = 6.4 Hz, 3H).
ESI-MS(m/z):564.2[M+H]
+。
ESI-MS (m/z): 564.2 [M+H] + .
其中得到214mg的化合物21异构体B(Rt=5.032min,de%=99.3%),其结构表征如下:There were obtained 214 mg of Compound 21 isomer B (Rt = 5.032 min, de% = 99.3%), and its structure was characterized as follows:
1H NMR(400MHz,CDCl
3):δ9.62(s,1H),7.65(d,J=6.2Hz,1H),7.31-7.12(m,9H),6.16(t,J=5.7Hz,1H),4.45(s,2H),4.36-4.28(m,3H),3.97(s,1H),3.62-3.48(m,2H),3.41-3.31(m,2H),2.29(s,3H),2.34-2.25(m,1H),2.06-1.87(m,2H),1.15(d,J=6.4Hz,3H)。
1 H NMR (400MHz, CDCl 3 ): δ9.62 (s, 1H), 7.65 (d, J = 6.2Hz, 1H), 7.31-7.12 (m, 9H), 6.16 (t, J = 5.7Hz, 1H ), 4.45 (s, 2H), 4.36-4.28 (m, 3H), 3.97 (s, 1H), 3.62-3.48 (m, 2H), 3.41-3.31 (m, 2H), 2.29 (s, 3H), 2.34-2.25 (m, 1H), 2.06-1.87 (m, 2H), 1.15 (d, J = 6.4 Hz, 3H).
ESI-MS(m/z):564.2[M+H]
+。
ESI-MS (m/z): 564.2 [M+H] + .
实施例三.((2R,3R,5R)-5-(4-氨基-2-氧代嘧啶-1(2H)-基)-4,4-二氟-3-羟基四氢呋喃-2-基)甲基-苯基-((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物49)的制备Example 3. ((2R,3R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl) Preparation of methyl-phenyl-((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 49)
步骤一:((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸五氟苯酯苯酯(化合物21-2)的合成Step 1: Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
采用与实施例一中步骤一相同的方法合成化合物21-2。Compound 21-2 was synthesized in the same manner as in the first step of the first embodiment.
步骤二:((2R,3R,5R)-5-(4-氨基-2-氧代嘧啶-1(2H)-基)-4,4-二氟-3-羟基四氢呋喃-2-基)甲基-苯基-((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物49)的合成Step 2: ((2R,3R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl) Synthesis of phenyl-phenyl-((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 49)
除了以吉西他滨(1.57g,6.0mmol))代替5-氟-2'-脱氧脲核苷外,以与实施例一的步骤二类似的方法合成600mg化合物49。In the same manner as in Step 2 of Example 1, except that gemcitabine (1.57 g, 6.0 mmol) was used instead of 5-fluoro-2'-deoxyuridine, 600 mg of Compound 49 was synthesized.
其结构表征如下:Its structural representation is as follows:
1H NMR(400MHz,CDCl
3):δ8.99-8.75(m,1H),7.41-7.28(m,1H),7.23-7.08(m,10H),6.11-5.98(m,2H),4.41-4.14(m,5H),3.96(s,1H),3.49-3.47(m,1H),3.31-3.19(m,2H),2.29-2.19(m,3H),1.15-1.08(m,3H)。
1 H NMR (400MHz, CDCl 3 ): δ8.99-8.75 (m, 1H), 7.41-7.28 (m, 1H), 7.23-7.08 (m, 10H), 6.11-5.98 (m, 2H), 4.41- 4.14 (m, 5H), 3.96 (s, 1H), 3.49-3.47 (m, 1H), 3.31-3.19 (m, 2H), 2.29-2.19 (m, 3H), 1.15-1.08 (m, 3H).
ESI-MS(m/z):581.2[M+H]
+。
ESI-MS (m/z): 581.2 [M+H] + .
实施例四.化合物49异构体A和化合物49异构体B的制备Example 4. Preparation of Compound 49 Isomer A and Compound 49 Isomer B
将化合物49(217mg)通过手性色谱法分离,分离条件如下:分离柱CHIRALPAK OZ-H 0.46cm I.D.×15cm,流动相:Hexane/EtOH=65/35(V/V),流速1.0ml/min,波长UV 254nm,温度35℃。Compound 49 (217 mg) was isolated by chiral chromatography. The separation conditions were as follows: separation column CHIRALPAK OZ-H 0.46 cm ID × 15 cm, mobile phase: Hexane/EtOH = 65/35 (V/V), flow rate 1.0 ml/min The wavelength is UV 254 nm and the temperature is 35 °C.
其中得到80mg的化合物49异构体A(Rt=6.352min,de%=99.6%),其结构表征如下:There were obtained 80 mg of Compound 49 isomer A (Rt = 6.352 min, de% = 99.6%), and its structure was characterized as follows:
1H NMR(400MHz,DMSO-d
6):δ8.02(s,1H),7.58(s,1H),7.50(d,J=7.7Hz,1H),7.33-7.14(m,9H),6.28-6.24(m,1H),5.89(d,J=7.6Hz,1H),4.68(t,J=11.9Hz,1H),4.45(s,2H),4.46-4.39(m,1H), 4.32-4.27(m,1H),4.24-4.16(m,1H),4.10-4.04(m,1H),3.58-3.48(m,1H),3.42-3.39(m,1H),3.35-3.32(m,1H),2.30(s,3H),1.18(d,J=6.6Hz,3H)。
1 H NMR (400MHz, DMSO- d 6): δ8.02 (s, 1H), 7.58 (s, 1H), 7.50 (d, J = 7.7Hz, 1H), 7.33-7.14 (m, 9H), 6.28 -6.24(m,1H), 5.89(d,J=7.6Hz,1H), 4.68(t,J=11.9Hz,1H),4.45(s,2H),4.46-4.39(m,1H), 4.32- 4.27(m,1H),4.24-4.16(m,1H),4.10-4.04(m,1H),3.58-3.48(m,1H),3.42-3.39(m,1H),3.35-3.32(m,1H) ), 2.30 (s, 3H), 1.18 (d, J = 6.6 Hz, 3H).
ESI-MS(m/z):581.2[M+H]
+。
ESI-MS (m/z): 581.2 [M+H] + .
其中得到160mg的化合物49异构体B(Rt=10.598min,de%=98.3%),其结构表征如下:Thereto was obtained 160 mg of Compound 49 isomer B (Rt = 10.598 min, de% = 98.3%), and its structure was characterized as follows:
1H NMR(400MHz,DMSO-d
6):δ7.88(s,1H),7.60(s,1H),7.50(d,J=7.7Hz,1H),7.33-7.14(m,9H),6.29(t,J=8.1Hz,1H),5.89(d,J=7.6Hz,1H),4.49(s,2H),4.44-4.39(m,1H),4.35-4.29(m,1H),4.21-4.14(m,1H),4.07-4.06(m,1H),3.60-3.48(m,1H),3.44-3.33(m,2H),2.30(s,3H),1.17(d,J=6.6Hz,3H)。
1 H NMR (400MHz, DMSO- d 6): δ7.88 (s, 1H), 7.60 (s, 1H), 7.50 (d, J = 7.7Hz, 1H), 7.33-7.14 (m, 9H), 6.29 (t, J = 8.1 Hz, 1H), 5.89 (d, J = 7.6 Hz, 1H), 4.49 (s, 2H), 4.44 - 4.39 (m, 1H), 4.35 - 4.29 (m, 1H), 4.21 4.14 (m, 1H), 4.07-4.06 (m, 1H), 3.60-3.48 (m, 1H), 3.44-3.33 (m, 2H), 2.30 (s, 3H), 1.17 (d, J = 6.6 Hz, 3H).
ESI-MS(m/z):581.2[M+H]
+。
ESI-MS (m/z): 581.2 [M+H] + .
实施例五.((2R,4R)-4-(4-氨基-2-氧代嘧啶-1(2H)-基)-1,3-二氧戊环-2-基)甲基-苯基-((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物77)的合成Example 5. ((2R,4R)-4-(4-Amino-2-oxopyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methyl-phenyl Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 77)
步骤一:((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸五氟苯酯苯酯(化合物21-2)的合成Step 1: Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoric acid pentafluorophenyl ester phenyl ester (Compound 21-2)
采用与实施例一中步骤一相同的方法合成化合物21-2。Compound 21-2 was synthesized in the same manner as in the first step of the first embodiment.
步骤二:((2R,4R)-4-(4-氨基-2-氧代嘧啶-1(2H)-基)-1,3-二氧戊环-2-基)甲基-苯基-((S)-1-((2-甲基苄氧基)丙)-2-基)氨基磷酸酯(化合物77)的合成Step 2: ((2R,4R)-4-(4-Amino-2-oxopyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methyl-phenyl- Synthesis of ((S)-1-((2-methylbenzyloxy)propyl)-2-yl)phosphoramidate (Compound 77)
除了以曲沙他滨(426mg,2.0mmol)代替5-氟-2'-脱氧脲核苷外,以实施例一的步骤二类似的方法合成2470mg化合物77。2470 mg of Compound 77 was synthesized in a similar manner to Step 2 of Example 1 except that instead of 5-fluoro-2'-deoxyurea nucleoside with tresapabine (426 mg, 2.0 mmol).
其结构表征如下:Its structural representation is as follows:
ESI-MS(m/z):531.2[M+H]
+。
ESI-MS (m/z): 531.2 [M+H] + .
参照实施例一至实施例五的方法,合成如下化合物:The following compounds were synthesized by the methods of Example 1 to Example 5:
生物试验Biological test
1.本发明化合物在人原代肝细胞中的代谢产物三磷酸核苷(3P)生成量的体外筛选评价1. In vitro screening and evaluation of the production of nucleoside triphosphate (3P), a metabolite of the compound of the present invention in human primary hepatocytes
1.1.测试系统1.1. Test System
人原代肝细胞购买自美国体外技术公司BioreclamationIVT。Human primary hepatocytes were purchased from Bioreclamation IVT, an in vitro technology company.
1.2.测试方法1.2. Test methods
将人原代肝细胞解冻后,用培养液稀释至6×10
5个细胞/mL的浓度,然后分别将25mM浓度的待测化合物的存储液用培养液稀释至50μM工作液浓度,其中有机溶剂的最终含量小于1%。将250μL的肝细胞溶液和250μL的测试物溶液或含相同溶剂含量的纯培养液(NC组)混合加入24-孔板,使得待测化合物的终浓度为25μM。在37℃水浴中孵育6小时后,将样品转移到试管,然后离心除去培养液,加入PBS磷酸缓冲液清洗细胞后,去除上清,加入180μL 70%甲醇,漩涡混匀,静置于-20℃过夜。在12000rpm及4℃下离心10min后,将150μL上清转移到上样管,经LC-MS/MS检测3P产物生成量。
After thawing human primary hepatocytes, the culture medium is diluted to a concentration of 6×10 5 cells/mL, and then the storage solution of the test compound of 25 mM concentration is diluted with the culture solution to a concentration of 50 μM working solution, wherein the organic solvent The final content is less than 1%. 250 μL of the hepatocyte solution and 250 μL of the test substance solution or the pure medium (NC group) containing the same solvent content were mixed and added to the 24-well plate so that the final concentration of the test compound was 25 μM. After incubating for 6 hours in a 37 ° C water bath, the sample was transferred to a test tube, and then the culture solution was removed by centrifugation. After washing the cells with PBS phosphate buffer, the supernatant was removed, 180 μL of 70% methanol was added, and the mixture was vortexed and placed at -20. °C overnight. After centrifugation at 12,000 rpm and 4 ° C for 10 min, 150 μL of the supernatant was transferred to a sample tube, and the amount of 3P product produced was measured by LC-MS/MS.
3P生成速率=(3P生成量*150μL)/(6×10
5个细胞/mL*250μL*6h)。
3P generation rate = (3P production amount * 150 μL) / (6 × 10 5 cells / mL * 250 μL * 6h).
1.3.实验结果1.3. Experimental results
待测化合物的3P生成量、生成速率见表1。The amount of 3P produced and the rate of formation of the test compound are shown in Table 1.
表1Table 1
由表1可见,本发明的化合物可以在肝细胞内顺利代谢,生成具有活性的代谢产物三磷酸核苷,并且代谢产物三磷酸核苷生成量大,生成速率快。因此,本发明的化合物具有抗肿瘤或抗癌作用,尤其具有优异的抗肝癌效果。As can be seen from Table 1, the compound of the present invention can be smoothly metabolized in hepatocytes to produce an active metabolite nucleoside triphosphate, and the metabolite nucleoside triphosphate is produced in a large amount and has a high production rate. Therefore, the compound of the present invention has an antitumor or anticancer effect, and particularly has an excellent anti-liver cancer effect.
Claims (17)
- 式(I)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,a compound of the formula (I) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, or a compound thereof mixture,其中,among them,B选自:B is selected from:L选自任选地被一个或多个R b取代的C 1-6亚烷基、C 2-6亚烯基、C 2-6亚炔基,所述亚烷基、亚烯基或亚炔基任选地被一个或多个-O-、-NR-或-S-间断; L is selected from C 1-6 alkylene, C 2-6 alkenylene, C 2-6 alkynylene, optionally substituted by one or more R b , said alkylene, alkenylene or sub An alkynyl group is optionally interrupted by one or more -O-, -NR- or -S-;或者L选自下列基团:Or L is selected from the following groups:其中 表示单键或双键,并且1位置处与B连接,2位置处与磷原子(P)连接; among them Represents a single bond or a double bond, and is connected to B at the 1 position and to the phosphorus atom (P) at the 2 position;X、Y和Z在每次出现时各自独立地选自CH 2、O、S和NR; X, Y and Z are each independently selected from CH 2 , O, S and NR at each occurrence;R a和R b在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO 2、-N(R) 2、-N 3、C 1-6烷基、C 3-6环烷基、C 2-6烯基、卤代C 2-6烯基和C 2-6炔基; R a and R b are each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , -N 3 , C 1-6 alkyl, C 3- 6 cycloalkyl, C 2-6 alkenyl, halogenated C 2-6 alkenyl and C 2-6 alkynyl;q为0、1、2、3、4、5、6、7或8,条件是:q is 0, 1, 2, 3, 4, 5, 6, 7, or 8, with the following conditions:q不大于对应基团上可被取代的位置的数目;并且q is not greater than the number of positions on the corresponding group that can be substituted;当q大于1时,每个R b可以相同或不同; When q is greater than 1, each R b may be the same or different;n为0、1、2或3,条件是:n is 0, 1, 2 or 3, provided that:n不大于对应基团上可被取代的位置的数目;并且n is not greater than the number of positions on the corresponding group that can be substituted;当n大于1时,每个R a可以相同或不同; When n is greater than 1, each R a may be the same or different;Ar 1和Ar 2各自独立地选自C 6-14芳基和5-14元杂芳基,所述芳基和杂芳基任选地被一个或多个(优选1、2、3、4或5个,特别优选1或2个)R c取代; Ar 1 and Ar 2 are each independently selected from a C 6-14 aryl group and a 5-14 membered heteroaryl group, which are optionally one or more (preferably 1, 2, 3, 4) Or 5, particularly preferably 1 or 2) R c substituted;R c在每次出现时各自独立地选自氢、卤素、-OH、-CN、-NO 2、-N(R) 2、C 1-6烷基、卤代C 1-6烷基、C 1-6烷氧基、C 1-6烷硫基、C 3-6环烷基、3-10元杂环烷基和C 2-6炔基; R c is each independently selected from the group consisting of hydrogen, halogen, -OH, -CN, -NO 2 , -N(R) 2 , C 1-6 alkyl, halo C 1-6 alkyl, C 1-6 alkoxy group, C 1-6 alkylthio group, C 3-6 cycloalkyl group, 3-10 membered heterocycloalkyl group and C 2-6 alkynyl group;R在每次出现时各自独立地选自氢、C 1-6烷基和C 3-6环烷基; R is each independently selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl;m为1、2或3,优选为1或2;m is 1, 2 or 3, preferably 1 or 2;p为1、2或3,优选为1或2;p is 1, 2 or 3, preferably 1 or 2;R 1和R 2各自独立地选自氢、C 1-6烷基、C 3-6环烷基、C 1-6烷氧基、C 6-14芳基和C 7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自卤素、-OH、-CN和-NO 2的取代基取代; R 1 and R 2 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of halogen, -OH, -CN and -NO 2 ;或者R 1和R 2连同其所连接的碳原子共同形成C 3-6环烷基或4-10元杂环烷基; Or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;R 3和R 4各自独立地选自氢、C 1-6烷基、C 3-6环烷基、C 1-6烷氧基、C 6-14芳基和C 7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自卤素、-OH、-CN和-NO 2的取代基取代; R 3 and R 4 are each independently selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl, and C 7-20 aralkyl. The alkyl, cycloalkyl, alkoxy, aryl and aralkyl groups are each optionally substituted with one or more substituents selected from the group consisting of halogen, -OH, -CN and -NO 2 ;或者R 3和R 4连同其所连接的碳原子共同形成C 3-6环烷基或4-10元杂环烷基; Or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;或者R 2和R 3连同其所连接的碳原子共同形成C 3-6环烷基或4-10元杂环烷基;并且 Or R 2 and R 3 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group or a 4-10 membered heterocycloalkyl group;R 5选自氢、C 1-6烷基、C 3-6环烷基、C 1-6烷氧基、C 6-14芳基和C 7-20芳烷基,所述烷基、环烷基、烷氧基、芳基和芳烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO 2的取代基取代; R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, C 1-6 alkoxy, C 6-14 aryl and C 7-20 aralkyl, said alkyl, ring The alkyl, alkoxy, aryl and aralkyl groups are each optionally substituted by one or more substituents selected from the group consisting of hydrogen, halogen, -OH, -CN and -NO 2 ;或者R 5与Ar 2上的取代基R c连同其所连接的碳原子共同形成与Ar 2稠合的C 4-6环烷基或4-10元杂环烷基; Or R 5 and the substituent R c on Ar 2 together with the carbon atom to which they are bonded form a C 4-6 cycloalkyl or 4-10 membered heterocycloalkyl fused to Ar 2 ;条件是:requirement is:1)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 3、R 4和R 5各自为氢;Ar 2为2-甲基苯基;且Ar 1为苯基时, 1) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 2 is 2-methyl Phenyl; and when Ar 1 is phenyl,R 1和R 2不为以下情况:R 1和R 2各自独立地选自氢和甲基;或者R 1和R 2连同其所连接的碳原子共同形成环丙基; R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;2)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1和R 2不同并各自独立地选自氢和甲基;R 3、R 4和R 5各自为氢;且Ar 2为2-甲基苯基时, 2) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 2 is 2-methylphenyl,Ar 1不为α-萘基或β-萘基; Ar 1 is not α-naphthyl or β-naphthyl;3)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1和R 2不同并各自独立地选自氢和甲基;R 3、R 4和R 5各自为氢;且Ar 1为苯基时, 3) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,Ar 2不为2-甲基萘基、3-甲基苯基或4-甲基苯基; Ar 2 is not 2-methylnaphthyl, 3-methylphenyl or 4-methylphenyl;4)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 3、R 4和R 5各自为氢;Ar 1为苯基;且Ar 2为2-甲基苯基时, 4) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is phenyl; When Ar 2 is 2-methylphenyl,R 1和R 2不为以下情况:R 1和R 2各自独立地选自氢和甲基;或者R 1和R 2连同其所连接的碳原子共同形成环丙基; R 1 and R 2 are not the case wherein R 1 and R 2 are each independently selected from hydrogen and methyl; or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group;5)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1和R 2不同并各自独立地选自氢和甲基;R 3、R 4和R 5各自为氢;且Ar 1为苯基时, 5) When B is L is Wherein at position B 1 is connected, at the second position to the phosphorus atom (P) is connected; X is NH; m is 1; P is 1; R & lt 1 and R 2 are different and are each independently selected from hydrogen and methyl; R 3 , R 4 and R 5 are each hydrogen; and when Ar 1 is a phenyl group,Ar 2不为3-甲基苯基、4-甲基苯基、4-氟苯基、2,6-二甲基苯基或2-甲基萘基; Ar 2 is not 3-methylphenyl, 4-methylphenyl, 4-fluorophenyl, 2,6-dimethylphenyl or 2-methylnaphthyl;6)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1、R 2、R 3、R 4和R 5各自为氢;且Ar 1为苯基时, 6) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a phenyl group,Ar 2不为苯基、4-甲基苯基、2-甲氧基苯基、2-甲氧基-4-甲基苯基、2,4-二甲基苯基、2-甲基-4-甲氧基苯基或2-甲基-4-叔丁基苯基; Ar 2 is not phenyl, 4-methylphenyl, 2-methoxyphenyl, 2-methoxy-4-methylphenyl, 2,4-dimethylphenyl, 2-methyl- 4-methoxyphenyl or 2-methyl-4-tert-butylphenyl;7)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 3、R 4和R 5各自为氢;Ar 1为α-萘基,且Ar 2为2-甲基苯基时, 7) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; Ar 1 is α-naphthyl And when Ar 2 is 2-methylphenyl,R 1和R 2不为以下情况:R 1和R 2同时为氢;或者R 1为氢且R 2为甲基;或者R 1为甲基且R 2为氢; R 1 and R 2 are not the case where R 1 and R 2 are simultaneously hydrogen; or R 1 is hydrogen and R 2 is methyl; or R 1 is methyl and R 2 is hydrogen;8)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 3、R 4和R 5各自为氢;R 1和R 2不同并各自独立地选自氢和甲基;且Ar 1为β-萘基时, 8) When B is L is Wherein the 1 position is linked to B, the 2 position is linked to the phosphorus atom (P); X is NH; m is 1; p is 1; R 3 , R 4 and R 5 are each hydrogen; R 1 and R 2 are different and Each of them is independently selected from hydrogen and methyl; and when Ar 1 is β-naphthyl,Ar 2不为2-甲基萘基; Ar 2 is not a 2-methylnaphthyl group;9)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1、R 2、R 3、R 4和R 5各自为氢;且Ar 1为4-氟苯基时, 9) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a 4-fluorophenyl group,Ar 2不为2-甲基苯基; Ar 2 is not 2-methylphenyl;10)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NCH 3;m为1;p为1;R 1、R 2、R 3、R 4和R 5各自为氢;且Ar 1为苯基时, 10) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NCH 3 ; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; And when Ar 1 is a phenyl group,Ar 2不为2-甲基苯基; Ar 2 is not 2-methylphenyl;11)当B为 L为 其中1位置处与B连接,2位置处与磷原子(P)连接;X为NH;m为1;p为1;R 1、R 2、R 3、R 4和R 5各自为氢;且Ar 1为β-萘基时, 11) When B is L is Wherein 1 is attached to B, 2 is attached to a phosphorus atom (P); X is NH; m is 1; p is 1; and R 1 , R 2 , R 3 , R 4 and R 5 are each hydrogen; When Ar 1 is a β-naphthyl group,Ar 2不为2-甲基苯基。 Ar 2 is not a 2-methylphenyl group.
- 权利要求1所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中Y为O或S,优选为O。The compound of claim 1 or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, or mixtures thereof Wherein Y is O or S, preferably O.
- 权利要求1或2所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中Z为O、S或CH 2,优选 为O。 The compound of claim 1 or 2, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, or a mixture wherein Z is O, S or CH 2 , preferably O.
- 权利要求1-3中任一项的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中B选自:A compound according to any one of claims 1 to 3, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or a mixture thereof, wherein B is selected from:优选地选自:Preferably selected from:更优选地选自:More preferably selected from:
- 权利要求1-4中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中L选自:A compound according to any one of claims 1 to 4, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Medicine, or a mixture thereof, wherein L is selected from:优选地选自:Preferably selected from:更优选地选自:More preferably selected from:其中1位置处与B连接,2位置处与磷原子(P)连接。One of the positions is connected to B, and the second position is connected to the phosphorus atom (P).
- 权利要求1-5中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中X为O或NR,且R选自氢、C 1-6烷基和C 3-6环烷基;优选地,X为O或NR,且R选自氢和C 1-6烷基;更优选地,X为O、NH或NCH 3;进一步更优选地,X为NH。 A compound of any one of claims 1 to 5, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Or a mixture thereof, wherein X is O or NR, and R is selected from the group consisting of hydrogen, C 1-6 alkyl and C 3-6 cycloalkyl; preferably, X is O or NR, and R is selected from hydrogen and C 1-6 alkyl; more preferably, X is O, NH or NCH 3 ; further more preferably, X is NH.
- 权利要求1-6中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中R 1和R 2各自独立地选自氢和C 1-6烷基,所述烷基任选地被一个或多个选自氢、卤素、-OH、-CN和-NO 2的取代基取代,或者R 1和R 2连同其所连接的碳原子共同形成C 3-6环烷基;优选地,R 1和R 2各自独立地选自氢和甲基,或者R 1和R 2连同其所连接的碳原子共同形成环丙基;更优选地,R 1和R 2各自独立地选自氢和甲基。 The compound of any one of claims 1 to 6, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Or a mixture thereof, wherein R 1 and R 2 are each independently selected from hydrogen and C 1-6 alkyl, the alkyl optionally being selected from one or more selected from the group consisting of hydrogen, halogen, -OH, -CN Substituting with a substituent of -NO 2 or R 1 and R 2 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group; preferably, R 1 and R 2 are each independently selected from hydrogen and methyl, Or R 1 and R 2 together with the carbon atom to which they are attached form a cyclopropyl group; more preferably, R 1 and R 2 are each independently selected from the group consisting of hydrogen and methyl.
- 权利要求1-7中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中R 3和R 4各自独立地选自氢和C 1-6烷基,所述烷基任选地被一个或多个选自氢、卤素、-OH、-CN和-NO 2的取代基取代,或者R 3和R 4连同其所连接的碳原子共同形成C 3-6环烷基;优选地,R 3和R 4各自独立地选自氢和甲基,或者R 3和R 4连同其所连接的碳原子共同形成环丙基;更优选地,R 3和R 4均为氢。 A compound according to any one of claims 1 to 7, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Or a mixture thereof, wherein R 3 and R 4 are each independently selected from hydrogen and C 1-6 alkyl, the alkyl optionally being selected from one or more selected from the group consisting of hydrogen, halogen, -OH, -CN Substituting with a substituent of -NO 2 or R 3 and R 4 together with the carbon atom to which they are attached form a C 3-6 cycloalkyl group; preferably, R 3 and R 4 are each independently selected from hydrogen and methyl, Or R 3 and R 4 together with the carbon atom to which they are attached form a cyclopropyl group; more preferably, both R 3 and R 4 are hydrogen.
- 权利要求1-8中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中R 5选自氢、C 1-6烷基、C 3-6环烷基,所述烷基、环烷基各自任选地被一个或多个选自氢、卤素、-OH、-CN和-NO 2的取代基取代;优选地,R 5为氢。 The compound of any one of claims 1-8, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Or a mixture thereof, wherein R 5 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 3-6 cycloalkyl, each of which is optionally selected from one or more selected from the group consisting of hydrogen, Substituted by a substituent of halogen, -OH, -CN and -NO 2 ; preferably, R 5 is hydrogen.
- 权利要求1-9中任一项所述的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中Ar 1和Ar 2各自独立地选自苯基、萘基和5-6元杂芳基,所述苯基、萘基和5-6元杂芳基各自独立地任选地被1或2个独立地选自氢、C 1-6烷基、卤素和C 1-6烷氧基的取代基取代;优选地,Ar 1和Ar 2各自独立地选自苯基、萘基、噻吩基、吡唑基和吡啶基,所述苯基、萘基、噻吩基、吡唑基和吡啶基各自独立地任选地被1或2个独立地选自氢、甲基、叔丁基、甲氧基、氟和氯的取代基取代基的取代基取代;更优选地,Ar 1和Ar 2各自独立地选自苯基,所述苯基任选地被1个甲基取代。 A compound according to any one of claims 1 to 9, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or pre-form thereof Or a mixture thereof, wherein Ar 1 and Ar 2 are each independently selected from the group consisting of phenyl, naphthyl and 5-6 membered heteroaryl, each independently of phenyl, naphthyl and 5-6 membered heteroaryl Optionally substituted with 1 or 2 substituents independently selected from the group consisting of hydrogen, C 1-6 alkyl, halogen and C 1-6 alkoxy; preferably, Ar 1 and Ar 2 are each independently selected from phenyl , naphthyl, thienyl, pyrazolyl and pyridyl, said phenyl, naphthyl, thienyl, pyrazolyl and pyridyl are each independently optionally 1 or 2 independently selected from hydrogen, methyl Substituted with a substituent of a substituent of a t-butyl group, a methoxy group, a fluorine group, and a chlorine group; more preferably, each of Ar 1 and Ar 2 is independently selected from a phenyl group, and the phenyl group is optionally 1 A Substituted.
- 式(I-1)、(I-2)或(I-3)所示的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,a compound represented by the formula (I-1), (I-2) or (I-3) or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph thereof, solvent combination thereof a substance, hydrate, metabolite or prodrug, or a mixture thereof,其中X、Y、Z、R a、R b、q、n、Ar 1、Ar 2、m、p、R 1、R 2、R 3、R 4和R 5如权利要求1-3和6-10中任一项所定义; Wherein X, Y, Z, R a , R b , q, n, Ar 1 , Ar 2 , m, p, R 1 , R 2 , R 3 , R 4 and R 5 are as defined in claims 1-3 and 6- Defined in any one of 10;优选地,X为NR,Y和Z均为O,R a和R b在每次出现时各自独立地选自卤素、-OH和-N(R) 2,q为0、1、2或3,n为1或2,Ar 1和Ar 2各自独立地选自任选地被1个或2个C 1-6烷基取代的C 6-10芳基,m为1或2,p为1或2,且R 1、R 2、R 3、R 4、R 5和R各自独立地为氢或C 1-6烷基;并且 Preferably, X is NR, Y and Z are both O, and R a and R b are each independently selected from halogen, -OH and -N(R) 2 at each occurrence, and q is 0, 1, 2 or 3 , n is 1 or 2, and Ar 1 and Ar 2 are each independently selected from a C 6-10 aryl group optionally substituted by 1 or 2 C 1-6 alkyl groups, m is 1 or 2, and p is 1 Or 2, and R 1 , R 2 , R 3 , R 4 , R 5 and R are each independently hydrogen or C 1-6 alkyl;更优选地,X为NH,Y和Z均为O,R a和R b在每次出现时各自独立地选自F、-OH和-NH 2,q为1、2或3,n为1,Ar 1和Ar 2各自独立地选自任选地被1个或2个C 1-6烷基取代的C 6-10芳基,m为1,p为1,且R 1、R 2、R 3、R 4、R 5和R各自独立地为氢或甲基。 More preferably, X is NH, Y and Z are both O, and R a and R b are each independently selected from F, -OH and -NH 2 at each occurrence, q is 1, 2 or 3, and n is 1 , Ar 1 and Ar 2 are each independently selected from C 6-10 aryl optionally substituted by 1 or 2 C 1-6 alkyl groups, m is 1, p is 1, and R 1 , R 2 , R 3 , R 4 , R 5 and R are each independently hydrogen or methyl.
- 权利要求11的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中所述化合物如式(II)、式(III)或式(IV)所示:The compound of claim 11 or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, or mixtures thereof, wherein The compound is as shown in formula (II), formula (III) or formula (IV):其中Ar 1为苯基,且Ar 2为任选地被1个甲基取代的苯基。 Wherein Ar 1 is a phenyl group, and Ar 2 is a phenyl group optionally substituted by one methyl group.
- 权利要求1-12中任一项的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,其中所述化合物选自:A compound according to any one of claims 1 to 12, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or a mixture thereof, wherein the compound is selected from the group consisting of:
- 药物组合物,其包含预防或治疗有效量的权利要求1-13中任一项的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,以及一种或多种药学上可接受的载体。A pharmaceutical composition comprising a prophylactically or therapeutically effective amount of a compound according to any one of claims 1 to 13, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvent thereof a compound, hydrate, metabolite or prodrug, or a mixture thereof, and one or more pharmaceutically acceptable carriers.
- 药盒,其包含权利要求1-13中任一项的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物,或者权利要求14的药物组合物。A kit comprising a compound according to any one of claims 1 to 13, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolism thereof Or prodrug, or a mixture thereof, or the pharmaceutical composition of claim 14.
- 权利要求1-13中任一项的化合物或其药学可接受的盐、酯、立体异构体、互变异构体、多晶型物、溶剂合物、水合物、代谢物或前药、或者它们的混合物或者权利要求14的药物组合物在制备用于预防或治疗癌症和/或肿瘤及其相关疾病的药物中的用途,其中所述药物优选为通过口服、静脉内、动脉内、皮下、腹膜内、肌内或经皮途径给药的药物,其中所述癌症和/或肿瘤及其相关疾病优选地选自胰腺癌、肺癌(如非小细胞肺癌)、卵巢癌、膀胱癌、乳腺癌、胃癌、结直肠癌和肝癌。A compound according to any one of claims 1 to 13, or a pharmaceutically acceptable salt, ester, stereoisomer, tautomer, polymorph, solvate, hydrate, metabolite or prodrug thereof, Or a mixture thereof or the use of the pharmaceutical composition according to claim 14 for the preparation of a medicament for preventing or treating cancer and/or a tumor and a related disease thereof, wherein the medicament is preferably administered orally, intravenously, intraarterially, subcutaneously Or a drug for administration by intraperitoneal, intramuscular or transdermal route, wherein the cancer and/or tumor and related diseases are preferably selected from the group consisting of pancreatic cancer, lung cancer (such as non-small cell lung cancer), ovarian cancer, bladder cancer, breast Cancer, stomach cancer, colorectal cancer and liver cancer.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201880054417.2A CN111051326A (en) | 2017-10-23 | 2018-10-12 | Nucleoside phosphate compound and preparation method and application thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201710991553.1 | 2017-10-23 | ||
| CN201710991553 | 2017-10-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019080724A1 true WO2019080724A1 (en) | 2019-05-02 |
Family
ID=66247180
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2018/109983 WO2019080724A1 (en) | 2017-10-23 | 2018-10-12 | Nucleoside phosphate compound and preparation method therefor and use thereof |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN111051326A (en) |
| WO (1) | WO2019080724A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112274516B (en) * | 2020-10-26 | 2022-08-05 | 中山大学附属第三医院 | Novel iodine-containing antiviral drug |
| CN114480568B (en) * | 2020-12-30 | 2024-04-26 | 安诺优达基因科技(北京)有限公司 | Detection method and kit for phosphorylation reaction |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010135520A1 (en) * | 2009-05-20 | 2010-11-25 | Chimerix, Inc. | Compounds, compositions and methods for treating viral infection |
| CN104761604A (en) * | 2014-01-02 | 2015-07-08 | 江苏豪森药业股份有限公司 | Uridine monophosphate analogue, and preparation method and applications thereof |
| CN105254695A (en) * | 2014-11-10 | 2016-01-20 | 南京曼杰生物科技有限公司 | Nucleoside phosphoramidate ramification and application thereof |
| CN105315319A (en) * | 2014-07-30 | 2016-02-10 | 南京圣和药业股份有限公司 | Hepatitis C virus inhibitor and application thereof |
| CN106554382A (en) * | 2015-09-29 | 2017-04-05 | 四川科伦药物研究院有限公司 | Application in nucleoside phosphoramidite class compound and preparation method thereof and medicine |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8716262B2 (en) * | 2008-12-23 | 2014-05-06 | Gilead Pharmasset Llc | Nucleoside phosphoramidates |
| KR20150130355A (en) * | 2013-03-15 | 2015-11-23 | 더 리전트 오브 더 유니버시티 오브 캘리포니아 | Acyclic nucleoside phosphonate diesters |
| WO2015158913A1 (en) * | 2014-04-17 | 2015-10-22 | Katholieke Universiteit Leuven | Novel antiviral and antitumoral compounds |
| WO2018113652A1 (en) * | 2016-12-23 | 2018-06-28 | 四川科伦博泰生物医药股份有限公司 | Nucleoside phosphate compound and preparation method and use thereof |
-
2018
- 2018-10-12 WO PCT/CN2018/109983 patent/WO2019080724A1/en active Application Filing
- 2018-10-12 CN CN201880054417.2A patent/CN111051326A/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010135520A1 (en) * | 2009-05-20 | 2010-11-25 | Chimerix, Inc. | Compounds, compositions and methods for treating viral infection |
| CN104761604A (en) * | 2014-01-02 | 2015-07-08 | 江苏豪森药业股份有限公司 | Uridine monophosphate analogue, and preparation method and applications thereof |
| CN105315319A (en) * | 2014-07-30 | 2016-02-10 | 南京圣和药业股份有限公司 | Hepatitis C virus inhibitor and application thereof |
| CN105254695A (en) * | 2014-11-10 | 2016-01-20 | 南京曼杰生物科技有限公司 | Nucleoside phosphoramidate ramification and application thereof |
| CN106554382A (en) * | 2015-09-29 | 2017-04-05 | 四川科伦药物研究院有限公司 | Application in nucleoside phosphoramidite class compound and preparation method thereof and medicine |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111051326A (en) | 2020-04-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111153901B (en) | Nitrogen-containing fused heterocyclic SHP2 inhibitor compound, preparation method and application | |
| CN109790169B (en) | Cyanopyrrolidine derivatives having activity as USP30 inhibitors | |
| CN111315747B (en) | Dihydropyrazolopyrimidine compound and preparation method and application thereof | |
| CN111094317B (en) | Phosphonic acid derivative with CD73 inhibitory activity, and preparation method and application thereof | |
| JP2020528062A (en) | Piperazine heteroaryl derivative, its production method, and its use in medicine | |
| KR101982951B1 (en) | New type of cytidine derivative and application thereof | |
| KR20190035870A (en) | As the FGFR inhibitor, the heterocyclic compound | |
| KR102658095B1 (en) | Diarylthiohydantoin compounds as androgen receptor antagonists | |
| CN109867675B (en) | Pyrrolopyrimidine derivative compound, pharmaceutical composition and application thereof | |
| IL215732A (en) | Compounds of reverse-turn mimetics, method for manufacturing the same and use thereof | |
| JP7384535B2 (en) | Quinazoline compounds and their preparation, use and pharmaceutical compositions | |
| WO2015101183A1 (en) | Uracil nucleotide analogs, preparation methods therefor and uses thereof | |
| JP6850361B2 (en) | Compounds that selectively inhibit kinases and their use | |
| WO2019080724A1 (en) | Nucleoside phosphate compound and preparation method therefor and use thereof | |
| EP3978498A1 (en) | Substituted fused bicyclic derivative, preparation method therefor, and application thereof in medicines | |
| EP3950693A1 (en) | Thienoheterocyclic derivative, preparation method therefor and medical use thereof | |
| TW201734028A (en) | Compounds for inhibiting cancer and virus | |
| EP3049400B1 (en) | New pi3k/akt/mtor inhibitors and pharmaceutical uses thereof | |
| CN113366008A (en) | CD73 inhibitor, preparation method and application thereof | |
| EP4403558A1 (en) | Azaindazole macrocyclic compound and use thereof | |
| KR20200065056A (en) | Tryptolide derivatives and methods for their preparation and application | |
| EP4219453A1 (en) | Pyrazole compound and preparation method therefor and use thereof | |
| EP1480984B9 (en) | Hexacyclic compounds | |
| CN114478532A (en) | Targeted Btk degradation compound and anti-tumor application thereof | |
| EP3816162A1 (en) | Diarylpyrazole compound, composition comprising same, and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18870611 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18870611 Country of ref document: EP Kind code of ref document: A1 |