WO2017081480A1 - Liquid formulation - Google Patents
Liquid formulation Download PDFInfo
- Publication number
- WO2017081480A1 WO2017081480A1 PCT/GB2016/053547 GB2016053547W WO2017081480A1 WO 2017081480 A1 WO2017081480 A1 WO 2017081480A1 GB 2016053547 W GB2016053547 W GB 2016053547W WO 2017081480 A1 WO2017081480 A1 WO 2017081480A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electronic cigarette
- stabiliser
- liquid formulation
- formulation according
- cigarette liquid
- Prior art date
Links
- 0 Cc(c(C)c1*)ccc1O Chemical compound Cc(c(C)c1*)ccc1O 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/10—Chemical features of tobacco products or tobacco substitutes
- A24B15/16—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes
- A24B15/167—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes in liquid or vaporisable form, e.g. liquid compositions for electronic cigarettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/045—Hydroxy compounds, e.g. alcohols; Salts thereof, e.g. alcoholates
- A61K31/05—Phenols
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/10—Chemical features of tobacco products or tobacco substitutes
- A24B15/16—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/28—Treatment of tobacco products or tobacco substitutes by chemical substances
- A24B15/30—Treatment of tobacco products or tobacco substitutes by chemical substances by organic substances
- A24B15/302—Treatment of tobacco products or tobacco substitutes by chemical substances by organic substances by natural substances obtained from animals or plants
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F40/00—Electrically operated smoking devices; Component parts thereof; Manufacture thereof; Maintenance or testing thereof; Charging means specially adapted therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/465—Nicotine; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/44—Oils, fats or waxes according to two or more groups of A61K47/02-A61K47/42; Natural or modified natural oils, fats or waxes, e.g. castor oil, polyethoxylated castor oil, montan wax, lignite, shellac, rosin, beeswax or lanolin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F40/00—Electrically operated smoking devices; Component parts thereof; Manufacture thereof; Maintenance or testing thereof; Charging means specially adapted therefor
- A24F40/10—Devices using liquid inhalable precursors
Definitions
- the present invention relates to liquid formulations and in particular to liquid formulations containing cannabidiol. It also relates to e-cigarette liquids (vaping liquids or vaping formulations) containing cannabidiol.
- E-cigarette liquids or formulations typically include nicotine in a liquid carrier comprising principally propylene glycol. However, it is proposed to replace some or all of the nicotine with cannabidiol.
- Cannabidiol (2-[(l , 6R)-6-isopropenyl-3-methylcyclohex-2-en-lyl]-5-pentylbenzene-l,3-diol) is one of at least 85 active cannabinoids found in cannabis.
- Cannabidiol (hereinafter referred to as "CBD") is considered to be a safer alternative to tetrahydrocannabinol (THC) as it produces less or no short term memory impairment in subjects. It is also considered not to generate the feelings of anxiety often associated with the use of THC.
- CBD The structure of CBD is as follows:
- the two hydroxyl groups on the benzene ring may be readily oxidised. It has been found that formulations of CBD in a liquid carrier comprising propylene glycol result in the discolouration of the liquid. Without wishing to be bound by theory, this is believed to be a result of the oxidation of at least one of the hydroxyl groups of the CBD molecule, likely by the propylene glycol component.
- an electronic cigarette liquid formulation comprising cannabidiol, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0-40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser is soluble or miscible with the liquid carrier.
- Electronic cigarette liquid formulations may also be referred to as an e-cigarette liquid or simply an e-liquid. They are also sometime referred to as vaping liquids or vaping formulations.
- the major component of the liquid carrier is propylene glycol, which comprises at least 60% by volume of the liquid carrier.
- the liquid carrier may also contain glycerol and/or one or more triglyceride compounds.
- triglyceride compounds tend to be derived from natural sources, they are typically provided in the form of a mixture of compounds.
- Further liquid components may be included in the liquid carrier.
- the head portion of the stabiliser is typically a stronger reducing agent than CBD and thus is preferentially oxidised. The head portion of the stabiliser therefore acts as an anti-oxidant for the CBD formulation.
- the tail portion of the stabiliser is required to ensure that the stabiliser is soluble or miscible in the formulation.
- the aliphatic group may be a branched or straight chain alkyl (alkane) group, a branched or straight chain alkene group or a branched or straight chain alkyne group. In embodiments in which the aliphatic chain is unsaturated, it may be mono unsaturated or polyunsaturated.
- the head portion of the stabiliser may be a hydroxyl substituted 5- or 6-membered carbocyclic or heterocyclic ring. In embodiments in which the ring is a heterocyclic ring, it may contain one or more heteroatoms each independently selected from oxygen, nitrogen and sulphur. Suitably, the heteroatom is an oxygen atom. Examples of suitable head portions include:
- Ascorbic acid derivatives such as:
- R 1 is a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety.
- Hydroquinone derivatives such as:
- R 2 and R 3 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion optionally coupled to the head portion via a linker moiety, provided that at least one of R 2 and R 3 comprises an aliphatic tail portion.
- Hydroxychromane derivatives such as:
- R 4 , R 5 , R 6 and R 7 are each independently selected from OH, H, and a C1-C3 alkyl group, wherein at least one of R 4 , R 5 , R 6 and R 7 is OH; and R 8 is a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety.
- Hydroxyanisole derivatives such as:
- R 9 and R 10 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety, provided that at least one of R 9 and R 10 comprises an aliphatic tail portion.
- Hydroxytoluene derivatives such as:
- R 11 and R 12 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety, provided that at least one of R 11 and R 12 comprises an aliphatic tail portion.
- Gallic acid derivatives such as:
- R 13 is a C3-C20 aliphatic tail portion.
- the linker group where present, may be selected from -0-, -0(0)C-, - C(O)-, -N(H)C(0)- and -N(H)-.
- the aliphatic tail portion is suitably a branched or straight chain alkyl group containing 3 to 20, suitably 6 to 20, 7 to 20, 10 to 20 or 12 to 18 carbon atoms.
- a suitable stabiliser comprises an ascorbic acid derivative as shown above, wherein Rl is a C6-C20 straight or branched aliphatic chain connected to the ascorbic acid head group via a -C(O)- linker group:
- R 14 is a C3-C20 aliphatic tail portion.
- R 14 may be a C3-C20 branched or straight chain alkyl group, such as for example a C6-C20 alkyl group, a C10-C20 alkyl group or a C12-C20 alkyl group.
- the stabiliser may be an ascorbate ester of a long chain fatty acid having from 6 to 20 carbon atoms, 7 to 20 carbon atoms, 8 to 18 carbon atoms or 10 to 16 carbon atoms.
- the long chain fatty acid may be unsaturated, monounsaturated or polyunsaturated.
- the stabiliser may be an ascorbyl ester of palmitic acid, which has a chain comprising 16 carbon atoms.
- the formulation is intended for human consumption, suitably in the form of an e-cigarette formulation, the stabiliser may already be approved for human consumption and/or as an approved food additive. For example, it may already have an "E" number.
- ascorbyl palmitate which is also known as E304 and is approved in the EU, the US, Australia and New Zealand.
- R 4 and R 6 are each independently H or CH 3 ;
- R 5 is OH;
- R 7 is CH 3 ; and
- R 8 is a Ci 6 branched chain alkyl group;
- R 13 is a propyl group
- R 2 is a tertiary butyl group and R 3 is H;
- R 11 and R 12 are each independently a tertiary butyl group.
- the CBD may be present in the formulation in an amount of lOmg/ml to 300mg/ml.
- the volume (ml) value refers to the volume of the liquid carrier.
- the lower limit on the amount of CBD in the formulation may be 15mg/ml, 20mg/ml, 25mg/ml, 30mg/ml, 35mg/ml. 40mg/ml, 45mg/ml or 50mg/ml.
- the upper limit on the amount of CBD present in the formulation may be 250mg/ml, 200mg/ml or 150mg/ml.
- the amount of the stabiliser present in the formulation may be 0.5% to 5% by weight of the CBD content.
- the stabiliser may be present in an amount of 0.5% to 2% by weight or 0.5 to 1.5% by weight of the CBD present in the formulation.
- the ratio (weight/ml) of CBD to stabiliser in the formulation may be 200:1 to 50:1.
- the stabiliser may be present in the formulation in an amount of 0.05mg/ml to 5mg/ml.
- the volume (ml) refers to the volume of the liquid carrier.
- the liquid carrier may include glycerol and/or one or more glyceride compounds, suitably one or more plant-derived glyceride compounds.
- Glycerol and/or glyceride compounds are often present in E-cigarette liquid formulations and facilitate the formation of the vapour for inhalation when heated within the electronic cigarette.
- the formulation may contain nicotine.
- the CBD is intended to replace at least some of the nicotine. Accordingly, the formulation may contain less nicotine than would otherwise be present in an E-cigarette formulation.
- Formulations according to the invention may be used to wean users away from nicotine.
- a number of different formulations may be available which contain a decreasing amount of nicotine.
- a first formulation which contains CBD and a first amount of nicotine
- a second formulation which contains CBD and a second amount of nicotine, wherein the second amount of nicotine is less than the first amount of nicotine.
- a third formulation may be provided, wherein the third formulation contains CBD and a third amount of nicotine, wherein the third amount of nicotine is less than the second amount of nicotine.
- Such formulations may be available separately or they may form part of a kit.
- a kit containing a first formulation which contains CBD and a first amount of nicotine and a second formulation which contains CBD and a second amount of nicotine, wherein the second amount of nicotine is less than the first amount of nicotine.
- a kit which further includes a third formulation which contains CBD and a third amount of nicotine, wherein and the third amount of nicotine is less than the second amount of nicotine.
- the formulations are as defined herein.
- the formulations discussed above are suitably formulations as defined in the first aspect of the invention, which also include nicotine.
- the first, second and optionally third formulations suitably comprise cannabidiol, nicotine, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0-40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser is soluble or miscible with the liquid carrier.
- the amount of nicotine in the first, second and third formulations decreases.
- the amount of nicotine present in the formulations of the invention may be 0 to 50mg/ml. Thus, no nicotine may be present in the formulation or, where present, it may be present in an amount of for example 5 to 40mg/ml, such as 10 to 30mg/ml.
- the formulation may further comprise a flavouring agent.
- a flavouring agent is common in the field of E-cigarette liquid formulations.
- the formulation may include a scent agent which releases a desired scent when the liquid is heated.
- the flavouring component and/or the scent component may be considered to form a part of the liquid carrier.
- the liquid carrier may further comprise 0-5% v/v of a liquid flavouring component and 0-5% v/v of a scent component.
- the liquid carrier comprises 0-2% v/v of a liquid flavouring component and 0-2% v/v of a scent component.
- the formulation is suitably substantially free from THC.
- substantially free it is meant that the formulation suitably includes less than 0.1 wt% THC, suitably less than 0.01 wt% or less than 0.001 wt% THC.
- substantially free means that THC is not detectable in the formulation by HPLC.
- a further aspect of the invention provides a cartridge containing an electronic cigarette liquid formulation as defined hereinabove.
- Such cartridges are suitably configured for location in an electronic cigarette.
- a yet further aspect of the invention provides an electronic cigarette including an electronic cigarette liquid formulation as defined herein.
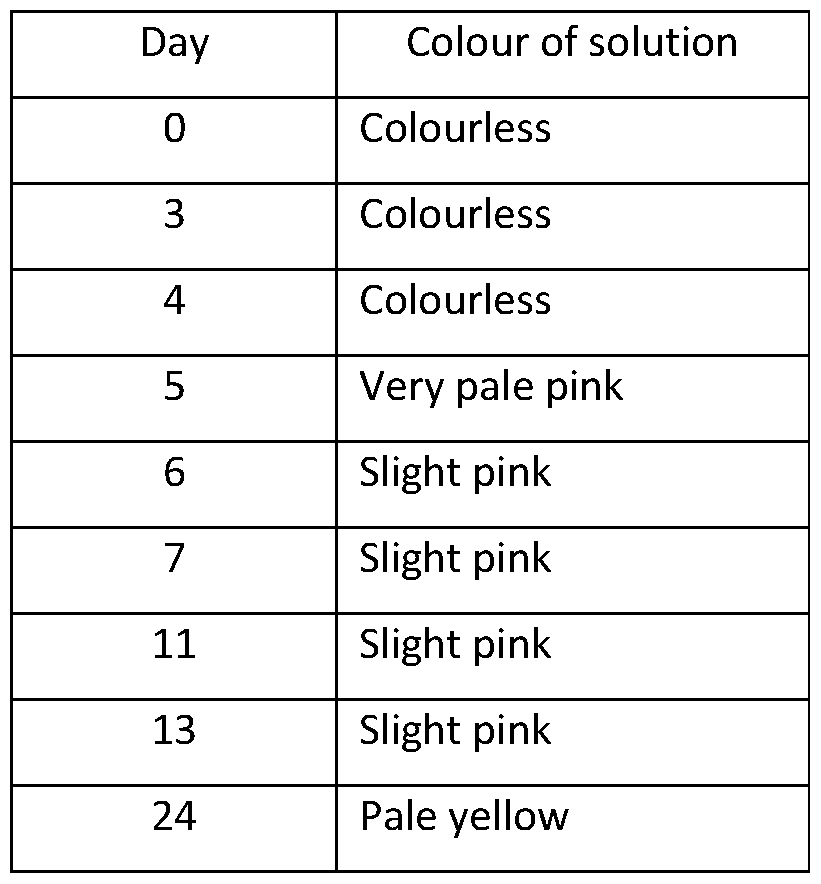
- Comparative Example 1 A 20% w/v solution of CBD in propylene glycol with no stabiliser was prepared by dissolving 2g of CBD in 8ml of propylene glycol. The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
- the colouration of the solution was due to the presence of an oxidised species of the CBD, wherein the CBD was being oxidised by the propylene glycol carrier.
- the oxidised species is thought to be a quinone derivative of CBD.
- Example 1 A stabilised CBD solution was prepared by adding 0.2% w/v butylated hydroxyanisole (BHA) to the solution of Comparative Example 1 (2g CBD and 20mg BHA in 8ml propylene glycol). The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
- the rate of oxidation of the CBD in solution is significantly slowed by the addition of the BHA.
- a stabilised CBD solution was prepared by adding 0.05% w/v 6-O-palmitoyl-L-ascorbic acid (6-0- AP) to the solution of Comparative Example 1 (2g CBD and 5mg 6-O-AP in 8ml propylene glycol). The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
- a stabilised CBD solution was prepared by adding 0.2% w/v 6-O-palmitoyl-L-ascorbic acid (6-O-AP) to the solution of Comparative Example 1 (2g CBD and 20mg 6-O-AP in 8ml propylene glycol). The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
- a stabilised CBD solution was prepared by adding 0.05% w/v 6-O-AP to the solution of
- a stabiliser as defined herein can reduce, slow down or prevent the oxidation of a solution of CBD in propylene glycol.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Agronomy & Crop Science (AREA)
- Botany (AREA)
- Toxicology (AREA)
- Medicinal Preparation (AREA)
Abstract
An electronic cigarette liquid formulation (e-liquid) comprising cannabidiol, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0-40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser is soluble or miscible with the liquid carrier.
Description
Liquid Formulation
The present invention relates to liquid formulations and in particular to liquid formulations containing cannabidiol. It also relates to e-cigarette liquids (vaping liquids or vaping formulations) containing cannabidiol.
Electronic cigarette (E-cigarette) liquids or formulations typically include nicotine in a liquid carrier comprising principally propylene glycol. However, it is proposed to replace some or all of the nicotine with cannabidiol.
Cannabidiol (2-[(l , 6R)-6-isopropenyl-3-methylcyclohex-2-en-lyl]-5-pentylbenzene-l,3-diol) is one of at least 85 active cannabinoids found in cannabis. Cannabidiol (hereinafter referred to as "CBD") is considered to be a safer alternative to tetrahydrocannabinol (THC) as it produces less or no short term memory impairment in subjects. It is also considered not to generate the feelings of anxiety often associated with the use of THC.
The structure of CBD is as follows:
It will be appreciated that the two hydroxyl groups on the benzene ring may be readily oxidised. It has been found that formulations of CBD in a liquid carrier comprising propylene glycol result in the discolouration of the liquid. Without wishing to be bound by theory, this is believed to be a result of the oxidation of at least one of the hydroxyl groups of the CBD molecule, likely by the propylene glycol component.
Furthermore, it is known that CBD is largely insoluble in water, but it is soluble in organic solvents, including propylene glycol.
According to a first aspect of the invention, there is provided an electronic cigarette liquid formulation (e-liquid) comprising cannabidiol, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0-40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser is soluble or miscible with the liquid carrier. Electronic cigarette liquid formulations may also be referred to as an e-cigarette liquid or simply an e-liquid. They are also sometime referred to as vaping liquids or vaping formulations.
It will be appreciated that the major component of the liquid carrier is propylene glycol, which comprises at least 60% by volume of the liquid carrier. Thus, reference to % v/v above refers to the percentage by volume of the liquid carrier. The liquid carrier may also contain glycerol and/or one or more triglyceride compounds. As triglyceride compounds tend to be derived from natural sources, they are typically provided in the form of a mixture of compounds. Further liquid components may be included in the liquid carrier. The head portion of the stabiliser is typically a stronger reducing agent than CBD and thus is preferentially oxidised. The head portion of the stabiliser therefore acts as an anti-oxidant for the CBD formulation.
The tail portion of the stabiliser is required to ensure that the stabiliser is soluble or miscible in the formulation. The aliphatic group may be a branched or straight chain alkyl (alkane) group, a branched or straight chain alkene group or a branched or straight chain alkyne group. In embodiments in which the aliphatic chain is unsaturated, it may be mono unsaturated or polyunsaturated. The head portion of the stabiliser may be a hydroxyl substituted 5- or 6-membered carbocyclic or heterocyclic ring. In embodiments in which the ring is a heterocyclic ring, it may contain one or more heteroatoms each independently selected from oxygen, nitrogen and sulphur. Suitably, the heteroatom is an oxygen atom.
Examples of suitable head portions include:
Ascorbic acid derivatives, such as:
Wherein R1 is a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety. Hydroquinone derivatives, such as:
Wherein R2 and R3 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion optionally coupled to the head portion via a linker moiety, provided that at least one of R2 and R3 comprises an aliphatic tail portion.
Hydroxychromane derivatives, such as:
Wherein R4, R5, R6 and R7 are each independently selected from OH, H, and a C1-C3 alkyl group, wherein at least one of R4, R5, R6 and R7 is OH; and R8 is a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety.
Hydroxyanisole derivatives, such as:
Wherein R9 and R10 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety, provided that at least one of R9 and R10 comprises an aliphatic tail portion. Hydroxytoluene derivatives, such as:
Wherein R11 and R12 are each independently selected from H, a C1-C6 alkyl group and a C3-C20 aliphatic tail portion, optionally coupled to the head portion via a linker moiety, provided that at least one of R11 and R12 comprises an aliphatic tail portion.
Gallic acid derivatives, such as:
Wherein R13 is a C3-C20 aliphatic tail portion.
In the above examples, the linker group, where present, may be selected from -0-, -0(0)C-, - C(O)-, -N(H)C(0)- and -N(H)-. The aliphatic tail portion is suitably a branched or straight chain alkyl group containing 3 to 20, suitably 6 to 20, 7 to 20, 10 to 20 or 12 to 18 carbon atoms.
A suitable stabiliser comprises an ascorbic acid derivative as shown above, wherein Rl is a C6-C20 straight or branched aliphatic chain connected to the ascorbic acid head group via a -C(O)- linker group:
Wherein 14 is a C3-C20 aliphatic tail portion. R14 may be a C3-C20 branched or straight chain alkyl group, such as for example a C6-C20 alkyl group, a C10-C20 alkyl group or a C12-C20 alkyl group. Thus, the stabiliser may be an ascorbate ester of a long chain fatty acid having from 6 to 20 carbon atoms, 7 to 20 carbon atoms, 8 to 18 carbon atoms or 10 to 16 carbon atoms. The long chain fatty acid may be unsaturated, monounsaturated or polyunsaturated. For example, the stabiliser may be an ascorbyl ester of palmitic acid, which has a chain comprising 16 carbon atoms. As the formulation is intended for human consumption, suitably in the form of an e-cigarette formulation, the stabiliser may already be approved for human consumption and/or as an approved food additive. For example, it may already have an "E" number.
One such example of an approved food additive is ascorbyl palmitate which is also known as E304 and is approved in the EU, the US, Australia and New Zealand.
Further examples include:
Tocopherols (E306) which have the following formula:
Wherein R4 and R6 are each independently H or CH3; R5 is OH; R7 is CH3; and R8 is a Ci6 branched chain alkyl group;
Propyl gallate (E310):
Wherein R13 is a propyl group;
Tertiary butylhydroquinone (E319):
Wherein R2 is a tertiary butyl group and R3 is H;
Wherein 9 is a tertiary butyl group and R10 is H; and Butylated hydroxytoluene (E321):
Wherein R11 and R12 are each independently a tertiary butyl group.
The CBD may be present in the formulation in an amount of lOmg/ml to 300mg/ml. The volume (ml) value refers to the volume of the liquid carrier. The lower limit on the amount of CBD in the formulation may be 15mg/ml, 20mg/ml, 25mg/ml, 30mg/ml, 35mg/ml. 40mg/ml, 45mg/ml or 50mg/ml. The upper limit on the amount of CBD present in the formulation may be 250mg/ml, 200mg/ml or 150mg/ml.
The amount of the stabiliser present in the formulation may be 0.5% to 5% by weight of the CBD content. For example, the stabiliser may be present in an amount of 0.5% to 2% by weight or 0.5 to 1.5% by weight of the CBD present in the formulation.
In other words, the ratio (weight/ml) of CBD to stabiliser in the formulation may be 200:1 to 50:1. In certain embodiments of the invention, the stabiliser may be present in the formulation in an
amount of 0.05mg/ml to 5mg/ml. Again, the volume (ml) refers to the volume of the liquid carrier.
As noted above, the liquid carrier may include glycerol and/or one or more glyceride compounds, suitably one or more plant-derived glyceride compounds. Glycerol and/or glyceride compounds are often present in E-cigarette liquid formulations and facilitate the formation of the vapour for inhalation when heated within the electronic cigarette.
The formulation may contain nicotine. In an embodiment of the invention, the CBD is intended to replace at least some of the nicotine. Accordingly, the formulation may contain less nicotine than would otherwise be present in an E-cigarette formulation.
Formulations according to the invention may be used to wean users away from nicotine.
Accordingly, a number of different formulations may be available which contain a decreasing amount of nicotine. Thus, there may be provided a first formulation which contains CBD and a first amount of nicotine, and a second formulation which contains CBD and a second amount of nicotine, wherein the second amount of nicotine is less than the first amount of nicotine.
Optionally, a third formulation may be provided, wherein the third formulation contains CBD and a third amount of nicotine, wherein the third amount of nicotine is less than the second amount of nicotine. Such formulations may be available separately or they may form part of a kit. Thus, an aspect of the invention provides a kit containing a first formulation which contains CBD and a first amount of nicotine and a second formulation which contains CBD and a second amount of nicotine, wherein the second amount of nicotine is less than the first amount of nicotine. In an embodiment of this aspect of the invention, there is provided a kit which further includes a third formulation which contains CBD and a third amount of nicotine, wherein and the third amount of nicotine is less than the second amount of nicotine. In this aspect of the invention, the formulations are as defined herein. The formulations discussed above are suitably formulations as defined in the first aspect of the invention, which also include nicotine. Accordingly, the first, second and optionally third formulations suitably comprise cannabidiol, nicotine, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0-40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser
is soluble or miscible with the liquid carrier. As noted above, the amount of nicotine in the first, second and third formulations decreases.
The amount of nicotine present in the formulations of the invention may be 0 to 50mg/ml. Thus, no nicotine may be present in the formulation or, where present, it may be present in an amount of for example 5 to 40mg/ml, such as 10 to 30mg/ml.
The formulation may further comprise a flavouring agent. Such flavouring agents are common in the field of E-cigarette liquid formulations. Additionally or alternatively, the formulation may include a scent agent which releases a desired scent when the liquid is heated. In embodiments in which the flavouring component and/or the scent component are liquids, the flavouring component and/or the scent component may be considered to form a part of the liquid carrier. Thus, the liquid carrier may further comprise 0-5% v/v of a liquid flavouring component and 0-5% v/v of a scent component. Suitably the liquid carrier comprises 0-2% v/v of a liquid flavouring component and 0-2% v/v of a scent component.
On the basis that there are numerous legal restrictions associated with tetrahydrocannabinol (THC), the formulation is suitably substantially free from THC. By the term "substantially free", it is meant that the formulation suitably includes less than 0.1 wt% THC, suitably less than 0.01 wt% or less than 0.001 wt% THC. Optionally, the term "substantially free" means that THC is not detectable in the formulation by HPLC.
A further aspect of the invention provides a cartridge containing an electronic cigarette liquid formulation as defined hereinabove. Such cartridges are suitably configured for location in an electronic cigarette.
A yet further aspect of the invention provides an electronic cigarette including an electronic cigarette liquid formulation as defined herein.
Examples:
Comparative Example 1
A 20% w/v solution of CBD in propylene glycol with no stabiliser was prepared by dissolving 2g of CBD in 8ml of propylene glycol. The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
It is believed that the colouration of the solution was due to the presence of an oxidised species of the CBD, wherein the CBD was being oxidised by the propylene glycol carrier. The oxidised species is thought to be a quinone derivative of CBD.
Comparative Example 2
A 5% w/v solution of CBD was prepared having the following formula:
2.5ml of the 20% w/v CBD solution of Comparative Example 1
3ml vegetable glycerine
4.1ml propylene glycol
0.4 ml menthol solution (200mg menthol in 1ml propylene glycol)
The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
3 Light pink
4 Pink
8 Pink
10 Pink
21 Pink
Example 1 A stabilised CBD solution was prepared by adding 0.2% w/v butylated hydroxyanisole (BHA) to the solution of Comparative Example 1 (2g CBD and 20mg BHA in 8ml propylene glycol). The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
As can be seen, the rate of oxidation of the CBD in solution is significantly slowed by the addition of the BHA.
Example 2
A stabilised CBD solution was prepared by adding 0.05% w/v 6-O-palmitoyl-L-ascorbic acid (6-0- AP) to the solution of Comparative Example 1 (2g CBD and 5mg 6-O-AP in 8ml propylene glycol).
The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
Example 3
A stabilised CBD solution was prepared by adding 0.2% w/v 6-O-palmitoyl-L-ascorbic acid (6-O-AP) to the solution of Comparative Example 1 (2g CBD and 20mg 6-O-AP in 8ml propylene glycol). The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
A stabilised CBD solution was prepared by adding 0.05% w/v 6-O-AP to the solution of
Comparative Example 2 to provide a solution having the following formulation:
2.5ml of the 20% w/v CBD solution of Example 3 (2g CBD and 20mg 6-O-AP in 8ml propylene glycol)
3ml vegetable glycerine
4.1ml propylene glycol
0.4 ml menthol solution (200mg menthol in 1ml propylene glycol)
The solution was placed in an open glass vial and monitored over a period of time. The colour of the solution was monitored during this time and the results are set out below:
It can be seen that the addition of a stabiliser as defined herein can reduce, slow down or prevent the oxidation of a solution of CBD in propylene glycol.
Claims
1. An electronic cigarette liquid formulation (e-liquid) comprising cannabidiol, a stabiliser and a liquid carrier; wherein the liquid carrier comprises 60-100% v/v propylene glycol, 0- 40% v/v glycerol, and 0-40% v/v of one or more triglyceride compounds; and the stabiliser comprises a head portion and a tail portion, wherein the head portion includes one or more reducing moieties and the tail portion comprises a C3-C20 straight or branched aliphatic chain, wherein the stabiliser is soluble or miscible with the liquid carrier.
2. An electronic cigarette liquid formulation according to Claim 1, wherein the head portion of the stabiliser includes a hydroxy substituted 5- or 6-membered carbocyclic or heterocyclic ring
3. An electronic cigarette liquid formulation according to Claim 2, wherein the head portion of the stabiliser is derived from ascorbic acid, hydroquinone, hydroxychromane, hydroxyanisole, hydroxytoluene or gallic acid.
4. An electronic cigarette liquid formulation according to any of Claims 1 to 3, wherein the aliphatic tail of the stabiliser is directly bonded to the head portion or is bonded via a linker group selected from -0-, -0(0)C-, -C(O)-, -N(H)C(0)- and -N(H)-.
5. An electronic cigarette liquid formulation according to Claim 1, wherein the stabiliser comprises a head group derived from ascorbic acid, a tail group which is a C6-C2o straight or branched aliphatic chain, and the tail group is connected to the head group via a carboxylate linker group.
6. An electronic cigarette liquid formulation according to Claim 5, wherein the stabiliser is an ascorbate ester of a C7-C20 long chain acid.
7. An electronic cigarette liquid formulation according to Claim 6, wherein the stabiliser is an ascorbate ester of palmitic acid.
8. An electronic cigarette liquid formulation according to any of Claims 1 to 7, wherein the cannabidiol is present in the formulation in an amount of lOmg/ml to 300mg/ml.
9. An electronic cigarette liquid formulation according to Claim 8, wherein the cannabidiol is present in an amount of 30mg/ml to 250mg/ml.
10. An electronic cigarette liquid formulation according to Claim 9, wherein the cannabidiol is present in an amount of 50mg/ml to 200mg/ml.
11. An electronic cigarette liquid formulation according to any of Claims 1 to 10, wherein the stabiliser is present in an amount of 0.05mg/ml to 5mg/ml.
12. An electronic cigarette liquid formulation according to any of Claims 1 to 11, wherein the ratio of cannabidiol to stabiliser in the mixture is 200:1 to 50:1.
13. An electronic cigarette liquid formulation according to any of Claims 1 to 12, wherein the or each triglyceride compound, where present, is derived from a plant source.
14. An electronic cigarette liquid formulation according to any of Claims 1 to 13, wherein the formulation further includes nicotine.
15. An electronic cigarette liquid formulation according to any of Claims 1 to 14, wherein the formulation further includes a flavouring agent and/or a scent agent.
16. An electronic cigarette liquid formulation according to any of Claims 1 to 15, wherein the formulation is substantially free from tetrahydrocannabinol (THC).
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16813111.8A EP3373974A1 (en) | 2015-11-12 | 2016-11-11 | Liquid formulation |
| CA3005301A CA3005301A1 (en) | 2015-11-12 | 2016-11-11 | Liquid formulation |
| US15/775,995 US20180325164A1 (en) | 2015-11-12 | 2016-11-11 | Liquid formulation |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1520014.0 | 2015-11-12 | ||
| GB1520014.0A GB2544468A (en) | 2015-11-12 | 2015-11-12 | Liquid formulation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017081480A1 true WO2017081480A1 (en) | 2017-05-18 |
Family
ID=55132718
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2016/053547 WO2017081480A1 (en) | 2015-11-12 | 2016-11-11 | Liquid formulation |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20180325164A1 (en) |
| EP (1) | EP3373974A1 (en) |
| CA (1) | CA3005301A1 (en) |
| GB (1) | GB2544468A (en) |
| WO (1) | WO2017081480A1 (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107343668A (en) * | 2017-08-31 | 2017-11-14 | 云南巴菰生物科技有限公司 | A kind of purposes of the electronics tobacco tar for lifting fragrance texture and preparation method thereof with it |
| CN108186566A (en) * | 2018-01-24 | 2018-06-22 | 云南汉木森生物科技有限责任公司 | A kind of nervous, relieving mental strain and helping sleep Alevaire and preparation method thereof of releiving |
| USD825102S1 (en) | 2016-07-28 | 2018-08-07 | Juul Labs, Inc. | Vaporizer device with cartridge |
| US10045568B2 (en) | 2013-12-23 | 2018-08-14 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10045567B2 (en) | 2013-12-23 | 2018-08-14 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10058130B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10076139B2 (en) | 2013-12-23 | 2018-09-18 | Juul Labs, Inc. | Vaporizer apparatus |
| US10104915B2 (en) | 2013-12-23 | 2018-10-23 | Juul Labs, Inc. | Securely attaching cartridges for vaporizer devices |
| US10111470B2 (en) | 2013-12-23 | 2018-10-30 | Juul Labs, Inc. | Vaporizer apparatus |
| USD836541S1 (en) | 2016-06-23 | 2018-12-25 | Pax Labs, Inc. | Charging device |
| USD842536S1 (en) | 2016-07-28 | 2019-03-05 | Juul Labs, Inc. | Vaporizer cartridge |
| US10244793B2 (en) | 2005-07-19 | 2019-04-02 | Juul Labs, Inc. | Devices for vaporization of a substance |
| US10279934B2 (en) | 2013-03-15 | 2019-05-07 | Juul Labs, Inc. | Fillable vaporizer cartridge and method of filling |
| USD849996S1 (en) | 2016-06-16 | 2019-05-28 | Pax Labs, Inc. | Vaporizer cartridge |
| USD851830S1 (en) | 2016-06-23 | 2019-06-18 | Pax Labs, Inc. | Combined vaporizer tamp and pick tool |
| US10405582B2 (en) | 2016-03-10 | 2019-09-10 | Pax Labs, Inc. | Vaporization device with lip sensing |
| WO2019211629A1 (en) * | 2018-05-03 | 2019-11-07 | Nicoventures Trading Limited | Vaporisable formulation |
| US10512282B2 (en) | 2014-12-05 | 2019-12-24 | Juul Labs, Inc. | Calibrated dose control |
| USD887632S1 (en) | 2017-09-14 | 2020-06-16 | Pax Labs, Inc. | Vaporizer cartridge |
| WO2020141178A1 (en) * | 2018-12-31 | 2020-07-09 | Philip Morris Products S.A. | Liquid nicotine formulation comprising partially water-soluble solvent |
| US10865001B2 (en) | 2016-02-11 | 2020-12-15 | Juul Labs, Inc. | Fillable vaporizer cartridge and method of filling |
| WO2023135408A1 (en) * | 2022-01-14 | 2023-07-20 | Nicoventures Trading Limited | Aerosolisable material |
| IT202200007478A1 (en) * | 2022-04-14 | 2023-10-14 | Daniele Tartaglia | EXTRACTION PROCEDURE TO OBTAIN A LIQUID FORMULATION BASED ON PROPYLENE GLYCOL AND LIPOSOLUBLE PHYTO CANNABINOIDS FOR ELECTRONIC CIGARETTES |
| GB2597170B (en) * | 2019-04-18 | 2023-12-13 | Kanabo Res Ltd | Diluents for compositions of cannabinoids and uses thereof |
| RU2816308C2 (en) * | 2018-12-31 | 2024-03-28 | Филип Моррис Продактс С.А. | Liquid formulation based on nicotine containing partially water-soluble solvent |
| US12114688B2 (en) | 2017-10-24 | 2024-10-15 | Rai Strategic Holdings, Inc. | Method for formulating aerosol precursor for aerosol delivery device |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4040999A1 (en) * | 2019-10-09 | 2022-08-17 | Nicoventures Trading Limited | Aerosolisable material |
| KR20230066372A (en) * | 2020-09-24 | 2023-05-15 | 니코벤처스 트레이딩 리미티드 | formulation |
| MX2023003451A (en) * | 2020-09-24 | 2023-06-22 | Nicoventures Trading Ltd | Packaged formulation. |
| GB202110560D0 (en) * | 2021-07-22 | 2021-09-08 | Nicoventures Trading Ltd | Aerosol generation |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2450753A (en) * | 2007-07-06 | 2009-01-07 | Gw Pharma Ltd | Composition comprising inverse agonist and neutral antagonist of the CB1 and / or CB2 receptor |
| US20090047234A1 (en) * | 2005-10-11 | 2009-02-19 | Elka Touitou | Compositions for nasal delivery |
| WO2010127033A1 (en) * | 2009-04-28 | 2010-11-04 | Alltranz Inc. | Formulations of cannabidiol and methods of using the same |
| US20110306660A1 (en) * | 2007-08-06 | 2011-12-15 | Goskonda Venkat R | Liquid cannabinoid formulations |
| DE102012105063A1 (en) * | 2012-06-12 | 2013-12-12 | Thc Pharm Gmbh | Stabilizing cannabinoids e.g. cannabidiol, comprises adding additives consisting of compounds that are metal ions complex, free radical scavengers, phenolic groups and/or a compound having basic properties to compounds/compositions |
| WO2015116463A1 (en) * | 2013-01-30 | 2015-08-06 | Larson Raymond Louis | Smokeless thc and administration method thereof |
| US20150231108A1 (en) * | 2014-02-19 | 2015-08-20 | Kind Consumer Limited | Cannabinoid Inhaler and Composition Therefor |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX2016015636A (en) * | 2014-05-29 | 2017-08-02 | Insys Pharma Inc | Stable cannabinoid formulations. |
-
2015
- 2015-11-12 GB GB1520014.0A patent/GB2544468A/en not_active Withdrawn
-
2016
- 2016-11-11 US US15/775,995 patent/US20180325164A1/en not_active Abandoned
- 2016-11-11 CA CA3005301A patent/CA3005301A1/en not_active Abandoned
- 2016-11-11 EP EP16813111.8A patent/EP3373974A1/en not_active Withdrawn
- 2016-11-11 WO PCT/GB2016/053547 patent/WO2017081480A1/en active Application Filing
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090047234A1 (en) * | 2005-10-11 | 2009-02-19 | Elka Touitou | Compositions for nasal delivery |
| GB2450753A (en) * | 2007-07-06 | 2009-01-07 | Gw Pharma Ltd | Composition comprising inverse agonist and neutral antagonist of the CB1 and / or CB2 receptor |
| US20110306660A1 (en) * | 2007-08-06 | 2011-12-15 | Goskonda Venkat R | Liquid cannabinoid formulations |
| WO2010127033A1 (en) * | 2009-04-28 | 2010-11-04 | Alltranz Inc. | Formulations of cannabidiol and methods of using the same |
| DE102012105063A1 (en) * | 2012-06-12 | 2013-12-12 | Thc Pharm Gmbh | Stabilizing cannabinoids e.g. cannabidiol, comprises adding additives consisting of compounds that are metal ions complex, free radical scavengers, phenolic groups and/or a compound having basic properties to compounds/compositions |
| WO2015116463A1 (en) * | 2013-01-30 | 2015-08-06 | Larson Raymond Louis | Smokeless thc and administration method thereof |
| US20150231108A1 (en) * | 2014-02-19 | 2015-08-20 | Kind Consumer Limited | Cannabinoid Inhaler and Composition Therefor |
Non-Patent Citations (3)
| Title |
|---|
| ANONYMOUS: "Delta Liquids CBD E Liquids - GREEN LABEL", 2017, XP002766909, Retrieved from the Internet <URL:https://www.legalherbalshop.com/delta-liquids-cbd-e-liquids-green-label.html> [retrieved on 20170220] * |
| CHRISTIAN GIROUD ET AL: "E-Cigarettes: A Review of New Trends in Cannabis Use", INTERNATIONAL JOURNAL OF ENVIRONMENTAL RESEARCH AND PUBLIC HEALTH, vol. 12, no. 8, 21 August 2015 (2015-08-21), pages 9988 - 10008, XP055343694, DOI: 10.3390/ijerph120809988 * |
| KRAMPUS: "Vaping CBD Juice and the Effects of CBD Oil", 18 September 2015 (2015-09-18), XP002766908, Retrieved from the Internet <URL:https://krampusbotanicals.com/vaping-cbd-juice-and-the-effects-of-cbd-oil/> [retrieved on 20170209] * |
Cited By (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10244793B2 (en) | 2005-07-19 | 2019-04-02 | Juul Labs, Inc. | Devices for vaporization of a substance |
| US10638792B2 (en) | 2013-03-15 | 2020-05-05 | Juul Labs, Inc. | Securely attaching cartridges for vaporizer devices |
| US10279934B2 (en) | 2013-03-15 | 2019-05-07 | Juul Labs, Inc. | Fillable vaporizer cartridge and method of filling |
| US10104915B2 (en) | 2013-12-23 | 2018-10-23 | Juul Labs, Inc. | Securely attaching cartridges for vaporizer devices |
| US11752283B2 (en) | 2013-12-23 | 2023-09-12 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10058129B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10058130B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10058124B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10070669B2 (en) | 2013-12-23 | 2018-09-11 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10912331B2 (en) | 2013-12-23 | 2021-02-09 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10701975B2 (en) | 2013-12-23 | 2020-07-07 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10111470B2 (en) | 2013-12-23 | 2018-10-30 | Juul Labs, Inc. | Vaporizer apparatus |
| US10117466B2 (en) | 2013-12-23 | 2018-11-06 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10117465B2 (en) | 2013-12-23 | 2018-11-06 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10159282B2 (en) | 2013-12-23 | 2018-12-25 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10667560B2 (en) | 2013-12-23 | 2020-06-02 | Juul Labs, Inc. | Vaporizer apparatus |
| US10201190B2 (en) | 2013-12-23 | 2019-02-12 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10045567B2 (en) | 2013-12-23 | 2018-08-14 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10045568B2 (en) | 2013-12-23 | 2018-08-14 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10264823B2 (en) | 2013-12-23 | 2019-04-23 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10076139B2 (en) | 2013-12-23 | 2018-09-18 | Juul Labs, Inc. | Vaporizer apparatus |
| US10512282B2 (en) | 2014-12-05 | 2019-12-24 | Juul Labs, Inc. | Calibrated dose control |
| US10865001B2 (en) | 2016-02-11 | 2020-12-15 | Juul Labs, Inc. | Fillable vaporizer cartridge and method of filling |
| US10405582B2 (en) | 2016-03-10 | 2019-09-10 | Pax Labs, Inc. | Vaporization device with lip sensing |
| USD929036S1 (en) | 2016-06-16 | 2021-08-24 | Pax Labs, Inc. | Vaporizer cartridge and device assembly |
| USD913583S1 (en) | 2016-06-16 | 2021-03-16 | Pax Labs, Inc. | Vaporizer device |
| USD849996S1 (en) | 2016-06-16 | 2019-05-28 | Pax Labs, Inc. | Vaporizer cartridge |
| USD836541S1 (en) | 2016-06-23 | 2018-12-25 | Pax Labs, Inc. | Charging device |
| USD851830S1 (en) | 2016-06-23 | 2019-06-18 | Pax Labs, Inc. | Combined vaporizer tamp and pick tool |
| USD842536S1 (en) | 2016-07-28 | 2019-03-05 | Juul Labs, Inc. | Vaporizer cartridge |
| USD825102S1 (en) | 2016-07-28 | 2018-08-07 | Juul Labs, Inc. | Vaporizer device with cartridge |
| CN107343668A (en) * | 2017-08-31 | 2017-11-14 | 云南巴菰生物科技有限公司 | A kind of purposes of the electronics tobacco tar for lifting fragrance texture and preparation method thereof with it |
| USD887632S1 (en) | 2017-09-14 | 2020-06-16 | Pax Labs, Inc. | Vaporizer cartridge |
| US12114688B2 (en) | 2017-10-24 | 2024-10-15 | Rai Strategic Holdings, Inc. | Method for formulating aerosol precursor for aerosol delivery device |
| CN108186566A (en) * | 2018-01-24 | 2018-06-22 | 云南汉木森生物科技有限责任公司 | A kind of nervous, relieving mental strain and helping sleep Alevaire and preparation method thereof of releiving |
| WO2019211629A1 (en) * | 2018-05-03 | 2019-11-07 | Nicoventures Trading Limited | Vaporisable formulation |
| CN113163845A (en) * | 2018-12-31 | 2021-07-23 | 菲利普莫里斯生产公司 | Liquid nicotine formulation comprising a partially water soluble solvent |
| RU2816308C2 (en) * | 2018-12-31 | 2024-03-28 | Филип Моррис Продактс С.А. | Liquid formulation based on nicotine containing partially water-soluble solvent |
| US11992038B2 (en) | 2018-12-31 | 2024-05-28 | Philip Morris Products S.A. | Liquid nicotine formulation comprising partially water-soluble solvent |
| WO2020141178A1 (en) * | 2018-12-31 | 2020-07-09 | Philip Morris Products S.A. | Liquid nicotine formulation comprising partially water-soluble solvent |
| GB2597170B (en) * | 2019-04-18 | 2023-12-13 | Kanabo Res Ltd | Diluents for compositions of cannabinoids and uses thereof |
| WO2023135408A1 (en) * | 2022-01-14 | 2023-07-20 | Nicoventures Trading Limited | Aerosolisable material |
| IT202200007478A1 (en) * | 2022-04-14 | 2023-10-14 | Daniele Tartaglia | EXTRACTION PROCEDURE TO OBTAIN A LIQUID FORMULATION BASED ON PROPYLENE GLYCOL AND LIPOSOLUBLE PHYTO CANNABINOIDS FOR ELECTRONIC CIGARETTES |
Also Published As
| Publication number | Publication date |
|---|---|
| GB201520014D0 (en) | 2015-12-30 |
| US20180325164A1 (en) | 2018-11-15 |
| GB2544468A (en) | 2017-05-24 |
| EP3373974A1 (en) | 2018-09-19 |
| CA3005301A1 (en) | 2017-05-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2017081480A1 (en) | Liquid formulation | |
| DE102012105063B4 (en) | Stabilization of cannabinoids and their pharmaceutical preparations | |
| RU2009116609A (en) | DELIVERY SYSTEM WITH CONTROLLED RELEASE FOR NASAL USE OF NEURO TRANSMITTERS | |
| ES2581212T3 (en) | Improved administration of tetahydrocannabinol | |
| TWI286941B (en) | Stabilized pharmaceutical and cosmetic composition of catechins or derivatives thereof | |
| US10561731B2 (en) | Method and compositions for solubilizing non-polar constituents | |
| PE20020261A1 (en) | SELF-EMULSING MEDICINE ADMINISTRATION SYSTEMS FOR EXTREMELY INSOLUBLE IN WATER LIPOPHILIC DRUGS | |
| JP2007238625A (en) | Stabilized ascorbic acid solution; use thereof; process for its preparation; and formulation comprising the same | |
| JP2005002123A5 (en) | ||
| RU2017111503A (en) | COMPOSITIONS (17-β) -3-OXOANDROST-4-EN-17-IL UNDECANOATE AND METHODS FOR PRODUCING AND USING THEM | |
| JP2003528142A (en) | Use of metal salts to stabilize taxane-based compositions | |
| WO2012071389A3 (en) | Stable cannabinoid compositions and methods for making and storing them | |
| JP4690672B2 (en) | Emulsion skin external preparation | |
| JP7186222B2 (en) | Stable cannabinoid composition | |
| SG185389A1 (en) | Non-aqueous taxane pro-emulsion formulations and methods of making and using the same | |
| BR112017008033B1 (en) | PHARMACEUTICAL COMPOSITION FOR ORAL ADMINISTRATION AND ITS PREPARATION PROCESS | |
| RU2011117230A (en) | STABILIZED COMPOSITION FOR THE TREATMENT OF PSORIASIS | |
| Moen et al. | Antioxidant efficacy of a new synergistic, multicomponent formulation for fish oil omega-3 concentrates | |
| JP5734331B2 (en) | Pharmaceutical composition comprising a bicyclic compound and method for stabilizing the bicyclic compound | |
| CA2695605C (en) | Pyrazolone derivative emulsion formulations | |
| CN108348498B (en) | Pharmaceutical composition for oral administration containing high concentration of taxane | |
| KR101755407B1 (en) | Pharmaceutical Composition for Preventing or Treating Psoriasis | |
| RU2020131982A (en) | PHARMACEUTICAL COMPOSITION WITH EXCELLENT STORAGE STABILITY | |
| JP2021024845A (en) | Eldecalcitol gelatin agent | |
| EP3456314A1 (en) | Topical formulation comprising 17-ketolic corticosteroid |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16813111 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3005301 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15775995 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016813111 Country of ref document: EP |