WO2016113741A1 - Process for preparing 1-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-1h-pyrazole - Google Patents
Process for preparing 1-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-1h-pyrazole Download PDFInfo
- Publication number
- WO2016113741A1 WO2016113741A1 PCT/IL2016/050047 IL2016050047W WO2016113741A1 WO 2016113741 A1 WO2016113741 A1 WO 2016113741A1 IL 2016050047 W IL2016050047 W IL 2016050047W WO 2016113741 A1 WO2016113741 A1 WO 2016113741A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- salt
- iii
- chlorophenyl
- Prior art date
Links
- 0 CCCC[C@@](CC1*CCC1)[C@](CC)C(C1)=C1C1C2=I[C@](*C)C#C[C@](C3*CC3)/C=C/[C@]12 Chemical compound CCCC[C@@](CC1*CCC1)[C@](CC)C(C1)=C1C1C2=I[C@](*C)C#C[C@](C3*CC3)/C=C/[C@]12 0.000 description 3
- CXOZQHPXKPDQGT-UHFFFAOYSA-N CC1C=CCC1 Chemical compound CC1C=CCC1 CXOZQHPXKPDQGT-UHFFFAOYSA-N 0.000 description 1
- QEWLOWAUHUOAEK-UHFFFAOYSA-O [OH2+]C(CC1)=NN1c(cc1)ccc1Cl Chemical compound [OH2+]C(CC1)=NN1c(cc1)ccc1Cl QEWLOWAUHUOAEK-UHFFFAOYSA-O 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D231/18—One oxygen or sulfur atom
- C07D231/20—One oxygen atom attached in position 3 or 5
- C07D231/22—One oxygen atom attached in position 3 or 5 with aryl radicals attached to ring nitrogen atoms
Definitions
- the present subject matter relates to a process for preparing l-(4-chlorophenyl)-
- the compound l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of the formula (I) is an important intermediate for preparing inter alia the fungicidal agents such as pyraclostrobin, as described in WO 96/01256.
- WO 96/01256 describes the preparation of 2-(3-pyrazolyloxy- methylene)nitrobenzene derivatives starting from o-nitrobenzyl bromides in general.
- U.S. Patent No. 6,133,451 describes a process for preparing 2-(3-pyrazolyloxymethylene)nitrobenzene derivatives by bromination of o-nitrotoluene to give o-nitrobenzyl bromide in the presence of a nonpolar, aprotic solvent and then reacting the o-nitrobenzyl bromide with a 3-hydroxypyrazole in the presence of a base.
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
- the salt of the compound of formula (III) may be an organic salt or an inorganic salt of the compound of formula (III).
- the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
- the dehydrogenation step may be carried out with an oxidizing agent, such as for example molecular oxygen or air in the presence of a base.
- the molar ratio of the compound of formula (IV) to the compound of formula (III) may be 1: 1 to 100: 1. In a further embodiment, the molar ratio of the compound of formula (IV) to the compound of formula (III) may be 1: 1 to 2: 1.
- the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
- the present subject matter provides a process for preparing the compound of formula (II) by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
- the solvent used in the dehydrogenation step may be the same solvent used in the preparation of the compound of formula (II).
- the compound of formula (II) is not isolated prior to the dehydrogenation step.
- the base used in the preparation of the compound of formula (II) may be the same base used in the dehydrogenation step.
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
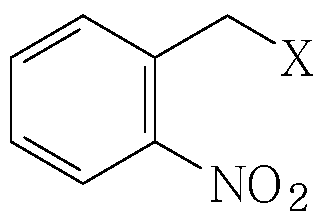
- X is one of bromide, chloride, iodide, tosylate, mesylate, triflate.
- the salt of l-(4-chlorophenyl)-3-hydroxy-lH- pyrazole of formula (III) is an organic salt or an inorganic salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole of formula (III).
- the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
- the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 100: 1.
- the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 2: 1.
- the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
- the compound of formula (III) and/or salt thereof is prepared by dehydrogenating l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
- the dehydrogenation step is carried out with an oxidizing agent in the presence of a base.
- the oxidizing agent comprises at least one of molecular oxygen and air.
- the compound of formula (II) is prepared by reacting 4-chlorophenylhydrazine h drochloride of formula (V)
- the solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (II), and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
- the base used in the dehydrogenation step is the same base used in the preparation of the compound of formula (II).
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
- the compound of formula (III) may be in the form of an organic salt or inorganic salt.
- the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
- the dehydrogenation step may be carried out with an oxidizing agent, such as for example molecular oxygen or air in the presence of a base.
- the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
- the process further includes the step of reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
- the compound of formula (II) may be prepared by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
- the solvent used in the preparation of the compound of formula (II) may be the same solvent used in the dehydrogenation step.
- the base used in the preparation of the compound of formula (II) may be the same base used in the dehydrogenation step.
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
- reaction is carried out in the solvent used in the dehydrogenation step, and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV) and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
- the herein described processes may be used in the production of pyraclostrobin.
- the present subject matter relates to the use of the compound of formula (I) as prepared according to processes disclosed herein in the preparation of pyraclostrobin.
- solvent system includes a mixture of at least two solvents.
- the present subject matter provides a process for preparing l-(4-chlorophenyl)- 3-[(2-nitrophenyl)methoxy]-lH-pyrazole formula (I)
- X is one of bromide, chloride, iodide, tosylate, mesylate, triflate
- reaction is carried out in the same solvent used in the dehydrogenation step.
- the process described herein is a one-pot process in which the resulting product of the dehydrogenation is directly used in the next step of producing 1- (4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole formula (I), without isolating l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole from the solvent and other components found in the mixture.
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or salt thereof
- the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I) wherein the process comprises: dehydrogenation of l-(4-chlorophenyl)-pyrazolidin-3- one of formula (II) to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof in the presence of a polar aprotic solvent and subsequent reaction of the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV); wherein the reaction is carried out in the solvent used in the dehydrogenation step and wherein (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
- the present process is advantageous in that it avoids the need for using expensive phase transfer catalysts.
- the process is highly efficient, providing a short reaction time.
- Another advantage of the present processes is that it provides a solvent which can be used in the dehydrogenation of the compound of formula (II) to obtain the compound of formula (III) and/or salt thereof as well as be used to dissolve both the compound of formula (III) and/or salt thereof and the compound of formula (IV) in the preparation of the compound of formula (I).
- the step of reacting l-(4-chlorophenyl)-3 -hydroxy- lH-pyrazole of formula (III) and/or salt thereof with the compound of formula (IV) to form l-(4- chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I) is conducted at temperatures lower when compared to those described in the prior art.
- the present one-pot process reduces the cost of production, simplifies work-up, and minimizes any effluent disposal problems. Further, the present process achieves high yields compared to the methods known in the prior art.
- the salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole is an organic or inorganic salt of l-(4-chlorophenyl)-3-hydroxy- lH-pyrazole.
- inorganic salts may include but are not limited to sodium salt, potassium salt, calcium salt, and magnesium salt.
- organic salts may include but are not limited to secondary amine salts, tertiary amine salts, quaternary amine salts, and aromatic carbocations such as pyridinium.
- the compound of formula (III) may be in the form of a salt due to the results of the dehydrogenation of the compound of formula (II).
- the compound of formula (III) may be converted into the form of a salt prior to or during its reaction with the compound of formula (IV).
- the polar aprotic solvent may include but is not limited to acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide, tetrahydrofuran, N- methyl pyrrolidinone and any combination thereof.
- the polar aprotic solvent is dimethyl sulfoxide.
- the polar aprotic solvent may include two or three or more polar aprotic solvents.
- the dehydrogenation may be conducted in the presence of a solvent system which may which comprises at least one polar aprotic solvent and may further include other suitable polar or non-polar solvents.
- suitable solvents may include but are not limited to (i) aliphatic hydrocarbons such as pentane, hexane, cyclohexane and petroleum ether; (ii) aromatic hydrocarbons such as toluene, o-xylene, m-xylene and p-xylene; (iii) halogenated hydrocarbons such as methylene chloride, chloroform and chlorobenzene; and (iv) alcohols such as methanol, ethanol, n-propanol, iso-propanol, n-butanol, and tert-butanol.
- the molar ratio between the polar aprotic solvent and the other solvents in the solvent is from about 1: 1 to about 6: 1. In another embodiment, the molar ratio is from about 1: 1 to about 5: 1. In yet another embodiment, the molar ratio is from about 1: 1 to about 4: 1. In a further embodiment, the molar ratio is from about 1: 1 to about 3: 1. In yet a further embodiment, the molar ratio is from about 1.5: 1 to about 3: 1.
- the dehydrogenation step may be carried out with an oxidizing agent in the presence of a base.
- oxidizing agents may include but are not limited to air and molecular oxygen. In a specific embodiment the oxidizing agent is molecular oxygen.
- bases may include but are not limited to alkali metal and alkaline earth metal alkoxides such as for example, sodium methoxide, sodium ethoxide, sodium butoxide, sodium isopropoxide, potassium ethoxide, potassium tert-butoxide and dimethoxy-magnesium and combinations thereof. In a specific embodiment the base is sodium methoxide.
- the dehydrogenation may be carried out in the presence of metal salts.
- the metal salts may include but are not limited to salts of iron in the oxidation state II or III (e.g. iron(II) chloride, iron(III) chloride, iron(II) sulfate and iron(III) sulfate), salts of copper in the oxidation state I or II (e.g. copper(I) chloride, copper(II) chloride, copper(I) sulfate and copper(II) sulfate) and also corresponding salts of main group or transition metals.
- iron salts may include but are not limited to salts of iron in the oxidation state II or III (e.g. iron(II) chloride, iron(III) chloride, iron(II) sulfate and iron(III) sulfate), salts of copper in the oxidation state I or II (e.g. copper(I) chloride, copper(II) chloride, copper(I
- the metal salts may be present in catalytic amounts. According to some embodiments, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.001 to about 1:0.1. In another embodiment, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.005 to about 1:0.05. In yet another embodiment, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.01 to about 1:0.05. In a specific embodiment, the molar ratio between the compound of formula (II) and the metal salts is about 1:0.125.
- the dehydrogenation step is conducted at a temperature from about 10°C to about 50°C, more preferably from about 10°C to about 35°C. In a specific example, the dehydrogenation step is conducted at a temperature of 15°C.
- the base e.g. sodium methoxide
- the sodium methoxide is added in portions to the reaction mixture of is 1- (4-chlorophenyl)-pyrazolidin-3-one of formula (II) over a period of about 30-40 minutes.
- the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 100: 1. In another embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 50: 1. In yet another embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 10: 1. In a further embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1 : 1 to about 2: 1. In a specific embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is about 1.7: 1.
- the step of reacting the compound of formula (III) and/or salt thereof with the compound of formula (IV) to form the compound of formula (I) is conducted at a temperature from about 25 C to about 50 C, more preferably from about 25 ° C to about 30 ° C.
- the present subject matter provides a process for preparing of l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
- the solvent used in the preparation of l-(4-chlorophenyl)-pyrazolidin-3-one may be the same solvent used in the abovementioned dehydrogenation step.
- the solvent may be a polar aprotic solvent may include but is not limited to acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide, tetrahydrofuran and any combination thereof.
- the same solvent may be used from the first step of preparing l-(4-chlorophenyl)-pyrazolidin-3-one to the second step of preparing l-(4- chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or salt thereof through to the third step of preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole without isolation of any of the intermediate products.
- the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1: 100. In another embodiment, the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1:50. In yet another embodiment, the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1: 10.
- the base used in the dehydrogenation step may be the same base used in the preparation of l-(4-chlorophenyl)-pyrazolidin-3-one .
- the bases may include but are not limited to alkali metal and alkaline earth metal alkoxides such as for example, sodium methoxide, sodium ethoxide, sodium butoxide, sodium isopropoxide, potassium ethoxide, potassium tert-butoxide and dimethoxy-magnesium and combinations thereof.
- alkyl acrylate used in the preparation of l-(4-chlorophenyl)- pyrazolidin-3-one may include but are not limited to methyl acrylate, ethyl acrylate, butyl acrylate, t-butyl acrylate, and any combination thereof.
- additional amounts of the base and/or the alkyl acrylate may be slowly added to the mixture until full conversion is achieved.
- the base and the alkyl acrylate may be added in alternate portions. For example, a portion of base may first be added to the mixture, after stirring a portion of the alkyl acrylate may be added to the mixture, after additional stirring an additional portion of base may be added etc. This may be continued until all of the necessary base and alkyl acrylate are added to the mixture.
- the compound of formula (I) is an important intermediate and may be used in the preparation of pyraclostrobin, as described in US 6,255,489, incorporated herein by reference in its entirety.
- reaction conditions in step (b) include but are not limited to oxidation, reduction, etherification, and esterification to obtain pyraclostrobin.
- the progress of the reaction can be monitored using any suitable method, which can include, for example, chromatographic methods such as, e.g., high performance liquid chromatography (HPLC), thin layer chromatography (TLC), and the like.
- chromatographic methods such as, e.g., high performance liquid chromatography (HPLC), thin layer chromatography (TLC), and the like.
- the compound of formula (I) can be isolated from the reaction mixture by any conventional techniques well-known in the art. Such isolation techniques can be selected, without limitation, from the group consisting of concentration, extraction, precipitation, cooling, filtration, crystallization, centrifugation, and a combination thereof, followed by drying.
- the compound of formula (I) can be optionally purified by any conventional techniques well-known in the art.
- purification techniques can be selected, without limitation, from the group consisting of precipitation, crystallization, slurrying, washing in a suitable solvent, filtration through a packed-bed column, dissolution in an appropriate solvent, re-precipitation by addition of a second solvent in which the compound is insoluble, and a combination thereof.
- l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II) was produced by adding 4.8 grams of methyl acrylate (55.9 mol) to the suspension and the suspension was then stirred for a few minutes. 3.02 grams (55.9 mol) of sodium methoxide was slowly added to the suspension at a rate of 0.1 mol every three minutes. This step was done in a N 2 atmosphere at 25°C.
- the crude product was recrystallized using MCB.
- the product was dried in a vacuum oven at 25 mbar at 70°C.
- l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II) was produced by adding 4.8 grams of methyl acrylate (55.9 mol) to the suspension and the suspension was then stirred for a few minutes. 3.02 grams (55.9 mol) of sodium methoxide was slowly added to the suspension at a rate of 0.1 mol every three minutes. This step was done in a N 2 atmosphere at 25°C.
- the crude product was recrystallized using MCB.
- the product was dried in a vacuum oven at 25 mbar at 70°C.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Abstract
A process for preparing 1-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-1H- pyrazole of formula (I) comprising: dehydrogenation of 1-(4-chlorophenyl)-pyrazolidin- 3-one of formula (II) to give 1-(4-chlorophenyl)-3-hydroxy-1H-pyrazole of formula (III) and/or a salt thereof in the presence of a solvent comprising at least one polar aprotic solvent and subsequent reaction of the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV) wherein the reaction is carried out in the solvent used in the dehydrogenation step and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
Description
PROCESS FOR PREPARING l-(4-CHLOROPHENYL)-3-[(2- NITROPHENYL)METHOXY]- 1H-PYRAZOLE
TECHNICAL FIELD
The present subject matter relates to a process for preparing l-(4-chlorophenyl)-
3-[(2-nitrophenyl)methoxy]-lH-pyrazole.
BACKGROUND
The compound l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of the formula (I) is an important intermediate for preparing inter alia the fungicidal agents such as pyraclostrobin, as described in WO 96/01256.
WO 96/01256 describes the preparation of 2-(3-pyrazolyloxy- methylene)nitrobenzene derivatives starting from o-nitrobenzyl bromides in general.
U.S. Patent No. 6,133,451 describes a process for preparing 2-(3-pyrazolyloxymethylene)nitrobenzene derivatives by bromination of o-nitrotoluene to give o-nitrobenzyl bromide in the presence of a nonpolar, aprotic solvent and then reacting the o-nitrobenzyl bromide with a 3-hydroxypyrazole in the presence of a base.
The o-nitrobenzyl bromide does not undergo any intermediate isolation prior to its reaction with 3-hydroxypyrazole.
It would be highly desirable to have an improved process for the production of compound of formula (I) which is suitable for industrial use, highly efficient, low-cost, environmentally friendly, and provides a high yield in a short reaction time, thereby overcoming the deficiencies of the prior art. The present subject matter provides such a process.
It is therefore a purpose of the present subject matter to provide a one-pot process for the preparation of l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH- pyrazole.
It is yet another purpose of the present subject matter to provide a process that overcomes the disadvantages of the known art.
SUMMARY
According to one aspect, the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
(I)
by the dehydrogenation of l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
OH
(II)
to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
(HI)
in the presence of a polar aprotic solvent and the subsequent reaction of the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV)
wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate; wherein the reaction is carried out in the reaction mixture resulting from the dehydrogenation step; and the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
According to an embodiment, the salt of the compound of formula (III) may be an organic salt or an inorganic salt of the compound of formula (III). The inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt. The dehydrogenation step may be carried out with an oxidizing agent, such as for example molecular oxygen or air in the presence of a base. According to an embodiment, the molar ratio of the compound of formula (IV) to the compound of formula (III) may be 1: 1 to 100: 1. In a further embodiment, the molar ratio of the compound of formula (IV) to the compound of formula (III) may be 1: 1 to 2: 1. The polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
According to another aspect, the present subject matter provides a process for preparing the compound of formula (II) by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate.
According to an embodiment, the solvent used in the dehydrogenation step may be the same solvent used in the preparation of the compound of formula (II). In this embodiment, the compound of formula (II) is not isolated prior to the dehydrogenation step. Further, the base used in the preparation of the compound of formula (II) may be the same base used in the dehydrogenation step.
According to a further aspect, the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
(I)
comprising:
reacting l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or thereof
(Ill)
(IV)
in the presence of a polar aprotic solvent; wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate.
According to an embodiment, the salt of l-(4-chlorophenyl)-3-hydroxy-lH- pyrazole of formula (III) is an organic salt or an inorganic salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole of formula (III). The inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt. According to an embodiment, the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 100: 1. In a further embodiment, the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 2: 1. The polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
According to an embodiment, the compound of formula (III) and/or salt thereof is prepared by dehydrogenating l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
(II)
in the presence of a polar aprotic solvent. The solvent used in the dehydrogenation step may be the same solvent used in the preparation of the compound of formula (I), and the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV). The dehydrogenation step is carried out with an oxidizing agent in the presence of a base. The oxidizing agent comprises at least one of molecular oxygen and air.
According to an embodiment, the compound of formula (II) is prepared by reacting 4-chlorophenylhydrazine h drochloride of formula (V)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate. The solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (II), and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step. The base used in the dehydrogenation step is the same base used in the preparation of the compound of formula (II).
According to a further aspect, the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
(III)
comprising:
dehydrogenation of l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
in the presence of a polar aprotic solvent.
According to an embodiment, the compound of formula (III) may be in the form of an organic salt or inorganic salt. The inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt. The dehydrogenation step may be carried out with an oxidizing agent, such as for example molecular oxygen or air in the presence of a base. The polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
According to an embodiment, the process further includes the step of reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV)
to give a compound of formula (I)
(I)
wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate; wherein the reaction is carried out in the solvent used in the dehydrogenation step and the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV). The compound of formula (II) may be prepared by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
(V)
with a base in the presence of a solvent and further reacting with an alkyl acrylate. The solvent used in the preparation of the compound of formula (II) may be the same
solvent used in the dehydrogenation step. The base used in the preparation of the compound of formula (II) may be the same base used in the dehydrogenation step.
According to an aspect, the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I)
(I)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate; (ii) dehydrogenating the formed l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
(II)
to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
(HI)
in the presence of a polar aprotic solvent and
(iii) subsequent reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate;
wherein the reaction is carried out in the solvent used in the dehydrogenation step, and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV) and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
In an embodiment, the herein described processes may be used in the production of pyraclostrobin.
In another embodiment, the present subject matter relates to the use of the compound of formula (I) as prepared according to processes disclosed herein in the preparation of pyraclostrobin.
DETAILED DESCRIPTION
Definitions
Prior to setting forth the present subject matter in detail, it may be helpful to provide definitions of certain terms to be used herein. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as is commonly understood by one of skill in the art to which this subject matter pertains.
The term "solvent system" as used herein includes a mixture of at least two solvents.
The term "a" or "an" as used herein includes the singular and the plural, unless specifically stated otherwise. Therefore, the terms "a," "an," or "at least one" can be used interchangeably in this application.
Throughout the application, descriptions of various embodiments use the term
"comprising"; however, it will be understood by one of skill in the art, that in some specific instances, an embodiment can alternatively be described using the language "consisting essentially of or "consisting of."
For purposes of better understanding the present teachings and in no way limiting the scope of the teachings, unless otherwise indicated, all numbers expressing
quantities, percentages, or proportions, and other numerical values used in the specification and claims, are to be understood as being modified in all instances by the term "about." Accordingly, unless indicated to the contrary, the numerical parameters set forth in the following specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained. At the very least, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques. In this regard, used of the term "about" herein specifically includes +10% from the indicated values in the range. In addition, the endpoints of all ranges directed to the same component or property herein are inclusive of the endpoints, are independently combinable, and include all intermediate points and ranges.
Process for Preparing l-(4-chlorophenyl)-3-r(2-nitrophenyl)methoxyl-lH-pyrazole
The present subject matter provides a process for preparing l-(4-chlorophenyl)- 3-[(2-nitrophenyl)methoxy]-lH-pyrazole formula (I)
(I)
wherein the process comprises the dehydrogenation of l-(4-chlorophenyl)-pyrazolidin- 3 -one of formula (II)
(II)
(III)
in the presence of a polar aprotic solvent and the subsequent reaction of the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV)
wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate;
wherein the reaction is carried out in the same solvent used in the dehydrogenation step. The process described herein is a one-pot process in which the resulting product of the dehydrogenation is directly used in the next step of producing 1- (4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole formula (I), without isolating l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole from the solvent and other components found in the mixture.
In another embodiment the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or salt thereof
(III)
wherein the process comprises: dehydrogenation of l-(4-chlorophenyl)-pyrazolidin-3- one of formula (II)
(Π)
in the presence of a polar aprotic solvent.
In another embodiment, the present subject matter provides a process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I) wherein the process comprises: dehydrogenation of l-(4-chlorophenyl)-pyrazolidin-3- one of formula (II) to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof in the presence of a polar aprotic solvent and subsequent reaction of the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV); wherein the reaction is carried out in the solvent used in the dehydrogenation step and wherein (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
The present process is advantageous in that it avoids the need for using expensive phase transfer catalysts. In addition, the process is highly efficient, providing a short reaction time.
Another advantage of the present processes is that it provides a solvent which can be used in the dehydrogenation of the compound of formula (II) to obtain the compound of formula (III) and/or salt thereof as well as be used to dissolve both the compound of formula (III) and/or salt thereof and the compound of formula (IV) in the preparation of the compound of formula (I).
Further, the step of reacting l-(4-chlorophenyl)-3 -hydroxy- lH-pyrazole of formula (III) and/or salt thereof with the compound of formula (IV) to form l-(4- chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole of formula (I) is conducted at temperatures lower when compared to those described in the prior art.
The present one-pot process reduces the cost of production, simplifies work-up, and minimizes any effluent disposal problems. Further, the present process achieves high yields compared to the methods known in the prior art.
In an embodiment of the present processes, the salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole is an organic or inorganic salt of l-(4-chlorophenyl)-3-hydroxy-
lH-pyrazole. Examples of inorganic salts may include but are not limited to sodium salt, potassium salt, calcium salt, and magnesium salt. Examples of organic salts may include but are not limited to secondary amine salts, tertiary amine salts, quaternary amine salts, and aromatic carbocations such as pyridinium.
According to an embodiment, the compound of formula (III) may be in the form of a salt due to the results of the dehydrogenation of the compound of formula (II). In another embodiment, the compound of formula (III) may be converted into the form of a salt prior to or during its reaction with the compound of formula (IV).
In one embodiment, the polar aprotic solvent may include but is not limited to acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide, tetrahydrofuran, N- methyl pyrrolidinone and any combination thereof.
In a specific embodiment the polar aprotic solvent is dimethyl sulfoxide.
In some embodiment, the polar aprotic solvent may include two or three or more polar aprotic solvents.
According to some embodiments, the dehydrogenation may be conducted in the presence of a solvent system which may which comprises at least one polar aprotic solvent and may further include other suitable polar or non-polar solvents. Examples of suitable solvents may include but are not limited to (i) aliphatic hydrocarbons such as pentane, hexane, cyclohexane and petroleum ether; (ii) aromatic hydrocarbons such as toluene, o-xylene, m-xylene and p-xylene; (iii) halogenated hydrocarbons such as methylene chloride, chloroform and chlorobenzene; and (iv) alcohols such as methanol, ethanol, n-propanol, iso-propanol, n-butanol, and tert-butanol.
In an embodiment of the present processes, the molar ratio between the polar aprotic solvent and the other solvents in the solvent is from about 1: 1 to about 6: 1. In another embodiment, the molar ratio is from about 1: 1 to about 5: 1. In yet another embodiment, the molar ratio is from about 1: 1 to about 4: 1. In a further embodiment, the molar ratio is from about 1: 1 to about 3: 1. In yet a further embodiment, the molar ratio is from about 1.5: 1 to about 3: 1.
In a further, optional, embodiment of the present processes, the dehydrogenation step may be carried out with an oxidizing agent in the presence of a base.
Examples of oxidizing agents may include but are not limited to air and molecular oxygen. In a specific embodiment the oxidizing agent is molecular oxygen.
Examples of bases may include but are not limited to alkali metal and alkaline earth metal alkoxides such as for example, sodium methoxide, sodium ethoxide, sodium butoxide, sodium isopropoxide, potassium ethoxide, potassium tert-butoxide and dimethoxy-magnesium and combinations thereof. In a specific embodiment the base is sodium methoxide.
In some embodiment, the dehydrogenation may be carried out in the presence of metal salts. The metal salts may include but are not limited to salts of iron in the oxidation state II or III (e.g. iron(II) chloride, iron(III) chloride, iron(II) sulfate and iron(III) sulfate), salts of copper in the oxidation state I or II (e.g. copper(I) chloride, copper(II) chloride, copper(I) sulfate and copper(II) sulfate) and also corresponding salts of main group or transition metals.
The metal salts may be present in catalytic amounts. According to some embodiments, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.001 to about 1:0.1. In another embodiment, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.005 to about 1:0.05. In yet another embodiment, the molar ratio between the compound of formula (II) and the metal salts may be from about 1:0.01 to about 1:0.05. In a specific embodiment, the molar ratio between the compound of formula (II) and the metal salts is about 1:0.125.
In one embodiment, the dehydrogenation step is conducted at a temperature from about 10°C to about 50°C, more preferably from about 10°C to about 35°C. In a specific example, the dehydrogenation step is conducted at a temperature of 15°C.
In another embodiment, during the dehydrogenation step, the base (e.g. sodium methoxide) may be added gradually to the reaction mixture over time. In a specific embodiment, the sodium methoxide is added in portions to the reaction mixture of is 1- (4-chlorophenyl)-pyrazolidin-3-one of formula (II) over a period of about 30-40 minutes.
In an embodiment of the present processes, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 100: 1. In another embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 50: 1. In yet another embodiment, the molar ratio between the
compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1: 1 to about 10: 1. In a further embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is from about 1 : 1 to about 2: 1. In a specific embodiment, the molar ratio between the compound of formula (IV) and the compound of formula (III) and/or salt thereof is about 1.7: 1.
In one embodiment, the step of reacting the compound of formula (III) and/or salt thereof with the compound of formula (IV) to form the compound of formula (I) is conducted at a temperature from about 25 C to about 50 C, more preferably from about 25°C to about 30°C.
Preparation of l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole
In another embodiment the present subject matter provides a process for preparing of l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
(II)
(V)
with a base in the presence of a solvent and further reacting with an alkyl acrylate. The solvent used in the preparation of l-(4-chlorophenyl)-pyrazolidin-3-one may be the same solvent used in the abovementioned dehydrogenation step. The solvent may be a polar aprotic solvent may include but is not limited to acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide, tetrahydrofuran and any combination thereof.
According to an embodiment, the same solvent may be used from the first step of preparing l-(4-chlorophenyl)-pyrazolidin-3-one to the second step of preparing l-(4- chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or salt thereof through to the third step of preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole
without isolation of any of the intermediate products.
In an embodiment of the present processes, the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1: 100. In another embodiment, the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1:50. In yet another embodiment, the weight ratio between 4- chlorophenylhydrazine hydrochloride of formula (V) and the solvent is from about 1 : 1 to about 1: 10.
Further, the base used in the dehydrogenation step may be the same base used in the preparation of l-(4-chlorophenyl)-pyrazolidin-3-one . The bases may include but are not limited to alkali metal and alkaline earth metal alkoxides such as for example, sodium methoxide, sodium ethoxide, sodium butoxide, sodium isopropoxide, potassium ethoxide, potassium tert-butoxide and dimethoxy-magnesium and combinations thereof.
Further, the alkyl acrylate used in the preparation of l-(4-chlorophenyl)- pyrazolidin-3-one may include but are not limited to methyl acrylate, ethyl acrylate, butyl acrylate, t-butyl acrylate, and any combination thereof.
In order to ensure full conversion of 4-chlorophenylhydrazine hydrochloride of formula (V) to l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II) additional amounts of the base and/or the alkyl acrylate may be slowly added to the mixture until full conversion is achieved. According to an embodiment, the base and the alkyl acrylate may be added in alternate portions. For example, a portion of base may first be added to the mixture, after stirring a portion of the alkyl acrylate may be added to the mixture, after additional stirring an additional portion of base may be added etc. This may be continued until all of the necessary base and alkyl acrylate are added to the mixture.
The compound of formula (I) is an important intermediate and may be used in the preparation of pyraclostrobin, as described in US 6,255,489, incorporated herein by reference in its entirety.
In a further aspect of the subject matter there is provided a process for preparation of pyraclostrobin comprising:
a) preparing compound of formula (I) as described herein;
b) providing reaction conditions for preparation of pyraclostrobin.
According to an embodiment the reaction conditions in step (b) include but are not limited to oxidation, reduction, etherification, and esterification to obtain pyraclostrobin.
The progress of the reaction can be monitored using any suitable method, which can include, for example, chromatographic methods such as, e.g., high performance liquid chromatography (HPLC), thin layer chromatography (TLC), and the like.
In yet another embodiment, the compound of formula (I) can be isolated from the reaction mixture by any conventional techniques well-known in the art. Such isolation techniques can be selected, without limitation, from the group consisting of concentration, extraction, precipitation, cooling, filtration, crystallization, centrifugation, and a combination thereof, followed by drying.
In yet another embodiment, the compound of formula (I) can be optionally purified by any conventional techniques well-known in the art. Such purification techniques can be selected, without limitation, from the group consisting of precipitation, crystallization, slurrying, washing in a suitable solvent, filtration through a packed-bed column, dissolution in an appropriate solvent, re-precipitation by addition of a second solvent in which the compound is insoluble, and a combination thereof.
The following examples illustrate the practice of the present subject matter in some of its embodiments, but should not be construed as limiting the scope of the present subject matter. Other embodiments will be apparent to one skilled in the art from consideration of the specification and examples. It is intended that the specification, including the examples, is considered exemplary only without limiting the scope and spirit of the present subject matter. EXAMPLE 1
Preparation of l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole
An exemplary experimental procedure is described as follows: 100 grams of dimethyl sulfoxide, 10 grams (55.9 mol) of 4-chlorophenylhydrazine hydrochloride of formula (V) was added to a 250 ml three-neck flask in a N2 atmosphere and at room temperature. Over a period of 30 minutes, 3.02 grams (55.9 mol) of sodium methoxide was added to the solution. The mixture was then mixed for 10 minutes, until the 4- chlorophenylhydrazine hydrochloride was neutralized to produce 4-
chlorophenylhydrazine. At this point the solution became darker in color and turned into a suspension.
l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II) was produced by adding 4.8 grams of methyl acrylate (55.9 mol) to the suspension and the suspension was then stirred for a few minutes. 3.02 grams (55.9 mol) of sodium methoxide was slowly added to the suspension at a rate of 0.1 mol every three minutes. This step was done in a N2 atmosphere at 25°C.
3.02 grams (55.9 mol) of sodium methoxide was slowly added to the l-(4- chlorophenyl)-pyrazolidin-3-one in a N2 atmosphere at 25-30°C. Oxygen was moderately bubbled through the solution for 2 hours using deep-pipe. The oxidation reaction was maintained at 40°C. The flask was kept in an ice-bath in order to prevent exotherm. Once the conversion of l-(4-chlorophenyl)-pyrazolidin-3-one to l-(4- chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) was complete, the oxygen bubbling was stopped.
Yield of l-(4-chlorophenyl)-3 -hydroxy- IH-pyrazole: 75%.
Preparation of l-(4-chlorophenyl)-3-r(2-nitrophenyl)methoxyl-lH-pyrazole
Using the same flask as used in the preparation of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole, a dropping funnel filled with 2-nitrobenzyl bromide of formula (IV) was connected. The ratio of 2-nitrobenzyl bromide to the l-(4-chlorophenyl)-3- hydroxy- IH-pyrazole present in the flask 1.7: 1 While being kept at a temperature of 25°C, the solution of 2-nitrobenzyl bromide was dropped into the flask at a rate of 0.5 ml/minute. After all of the 2-nitrobenzyl bromide was added to the flask, the reaction was mixed for 1 hour, until all of the l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole was consumed. A small amount of NaOH 15% was then added to the solution and the solution was then stirred for 30 minutes at 30°C. A small amount of H20 was added to the mixture in order to precipitate the crude product.
The crude product was recrystallized using MCB. The product was dried in a vacuum oven at 25 mbar at 70°C.
Yield of l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole: 70% with a purity of 99.1%.
EXAMPLE 2
Preparation of l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole
An exemplary experimental procedure is described as follows: 100 grams of dimethyl sulfoxide, 10 grams (55.9 mol) of 4-chlorophenylhydrazine hydrochloride of formula (V) was added to a 250 ml three-neck flask in a N2 atmosphere and at room temperature. Over a period of 30 minutes, 3.02 grams (55.9 mol) of sodium methoxide was added to the solution. The mixture was then mixed for 10 minutes, until the 4- chlorophenylhydrazine hydrochloride was neutralized to produce 4- chlorophenylhydrazine. At this point the solution became darker in color and turned into a suspension.
l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II) was produced by adding 4.8 grams of methyl acrylate (55.9 mol) to the suspension and the suspension was then stirred for a few minutes. 3.02 grams (55.9 mol) of sodium methoxide was slowly added to the suspension at a rate of 0.1 mol every three minutes. This step was done in a N2 atmosphere at 25°C.
CuCl22H20 (2.5 mol % relative to compound (II)) was added to the l-(4- chlorophenyl)-pyrazolidin-3-one at 25-30°C. Air was moderately bubbled through the solution for 5 hours using deep-pipe. The oxidation reaction was maintained at 60°C. The flask was kept in an ice-bath in order to prevent exotherm. Once the conversion of l-(4-chlorophenyl)-pyrazolidin-3-one to l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) was complete, the oxygen bubbling was stopped.
Yield of l-(4-chlorophenyl)-3 -hydroxy- lH-pyrazole: 83%.
Preparation of l-(4-chlorophenyl)-3-r(2-nitrophenyl)methoxyl-lH-pyrazole
Using the same flask as used in the preparation of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole, a dropping funnel filled with 2-nitrobenzyl bromide of formula (IV) was connected. The ratio of 2-nitrobenzyl bromide to the l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole present in the flask 1.7: 1. Sodium methoxide (1 mol equiv. versus compound (III) was added. While being kept at a temperature of 25°C, the solution of 2- nitrobenzyl bromide was dropped into the flask at a rate of 0.5 ml/minute. After all of the 2-nitrobenzyl bromide was added to the flask, the reaction was mixed for 1 hour, until all of the l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole was consumed. A small
amount of NaOH 15% was then added to the solution and the solution was then stirred for 30 minutes at 25°C. A small amount of H20 was added to the mixture in order to precipitate the crude product.
The crude product was recrystallized using MCB. The product was dried in a vacuum oven at 25 mbar at 70°C.
Yield of l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH-pyrazole: 70% with a purity of 99.1%.
As demonstrated in the above example, a high yield of l-(4-chlorophenyl)-3-[(2- nitrophenyl)methoxy]-lH-pyrazole may be produced using the one-pot process described hereinabove. The results show higher efficiency of the reaction.
While the present subject matter has been shown and described with reference to preferred embodiments thereof, it will be understood by those skilled in the art that many alternatives, modifications and variations may be made thereto without departing from the spirit and scope thereof. Accordingly, it is intended to embrace all such alternatives, modifications, and variations that fall within the spirit and broad scope of the appended claims.
All publications, patents and patent applications mentioned in this specification are herein incorporated in their entirety by reference into the specification, to the same extent as if each individual publication, patent or patent application was specifically and individually indicated to be incorporated herein by reference.
Claims
1. A process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH- pyrazole of formula (I)
(I)
(II)
to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
(in)
in the presence of a polar aprotic solvent and subsequent reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV) wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate; wherein the reaction is carried out in the solvent used in the dehydrogenation step, and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
2. The process of claim 1, wherein the salt of l-(4-chlorophenyl)-3-hydroxy-lH- pyrazole of formula (III) is an organic salt or inorganic salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole of formula (III).
3. The process of claim 2, wherein the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
4. The process of any one of claims 1-3, wherein the dehydrogenation step is carried out with an oxidizing agent in the presence of a base.
5. The process of claim 4, wherein the oxidizing agent comprises at least one of molecular oxygen and air.
6. The process of any one of claims 1-5, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 100: 1.
7. The process of any one of claims 1-5, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 2: 1.
8. The process of any one of claims 1-7, wherein the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
9. The process of any one of claims 1-8, wherein the compound of formula (II) is prepared by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate.
10. The process of claim 9, wherein the solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (II), and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
11. The process of claim 9 or 10, wherein the base used in the dehydrogenation step is the same base used in the preparation of the compound of formula (II).
12. A process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH- pyrazole of formula (I)
(I)
comprising: reacting l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
(IV) in the presence of a polar aprotic solvent; wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate.
13. The process of claim 12, wherein the salt of l-(4-chlorophenyl)-3-hydroxy-lH- pyrazole of formula (III) is an organic salt or an inorganic salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole of formula (III).
14. The process of claim 13, wherein the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
15. The process of any one of claims 12-14, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 100: 1.
16. The process of any one of claims 12-14, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 2: 1.
17. The process of any one of claims 12-16, wherein the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
18. The process of any one of claims 12-17, wherein the compound of formula (III) and/or salt thereof is prepared by dehydrogenating l-(4-chlorophenyl)-pyrazolidin-3- one of formula (II)
(II)
in the presence of a polar aprotic solvent.
19. The process of claim 18, wherein the solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (I), and the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
20. The process of claim 18 or 19, wherein the dehydrogenation step is carried out with an oxidizing agent in the presence of a base.
21. The process of claim 20, wherein the oxidizing agent comprises at least one of molecular oxygen and air.
22. The process of any one of claims 18-21, wherein the compound of formula (II) is prepared by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate.
23. The process of claim 22, wherein the solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (II), and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
24. The process of claim 22 or 23, wherein the base used in the dehydrogenation step is the same base used in the preparation of the compound of formula (II).
25. A process for preparing l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) and/or a salt thereof
H
(III) comprising: dehydrogenating l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
(II)
in the presence of a polar aprotic solvent.
26. The process of claim 25, wherein the compound of formula (III) is in the form of an organic salt or an inorganic salt.
27. The process of claim 26, wherein the salt is an inorganic salt comprising at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
28. The process of any one of claims 25-27, wherein the dehydrogenation step is carried out with an oxidizing agent in the presence of a base.
29. The process of claim 28, wherein the oxidizing agent comprises at least one of molecular oxygen and air.
30. The process of any one of claims 25-29, wherein the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
31. The process of any one of claims 25-30, further comprising the step of reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV) to give a compound of formula (I)
wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate; wherein the reaction is carried out in the solvent used in the dehydrogenation step and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV).
32. The process of any one of claims 25-31, wherein the compound of formula (II) is prepared by reacting 4-chlorophenylhydrazine hydrochloride of formula (V)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate.
33. The process of claim 32, wherein the solvent used in the dehydrogenation step is the same solvent used in the preparation of the compound of formula (II), and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
34. The process of claim 32 or 33, wherein the base used in the preparation of the compound of formula (II) is the same base used in the dehydrogenation step.
35. A process for preparing l-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-lH- pyrazole of formula (I)
(I)
(V)
with a base in the presence of a solvent, and further reacting with an alkyl acrylate; dehydrogenating the formed l-(4-chlorophenyl)-pyrazolidin-3-one of formula (II)
(Π)
to give l-(4-chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III) or a salt thereof
(Ill) in the presence of a polar aprotic solvent and subsequent reacting the resulting solution of formula (III) and/or salt thereof with the compound of formula (IV)
(IV) wherein X is one of bromide, chloride, iodide, tosylate, mesylate, triflate; wherein the reaction is carried out in the solvent used in the dehydrogenation step, and wherein the compound of formula (III) and/or salt thereof is not isolated prior to its reaction with the compound of formula (IV); and wherein the compound of formula (II) is not isolated prior to the dehydrogenation step.
36. The process of claim 35, wherein the base used in the dehydrogenation step is the same base used in the preparation of the compound of formula (II).
37. The process of claim 35 or 36, wherein the salt of l-(4-chlorophenyl)-3- hydroxy-lH-pyrazole of formula (III) is an organic or inorganic salt of l-(4- chlorophenyl)-3-hydroxy-lH-pyrazole of formula (III).
38. The process of claim 37, wherein the inorganic salt comprises at least one of sodium salt, potassium salt, calcium salt, and magnesium salt.
39. The process of any one of claims 35-38, wherein the dehydrogenation step is carried out with an oxidizing agent in the presence of a base.
40. The process of claim 39, wherein the oxidizing agent comprises at least one of molecular oxygen and air.
41. The process of any one of claims 35-40, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 100: 1.
42. The process of any one of claims 35-40, wherein the molar ratio of the compound of formula (IV) to the compound of formula (III) is 1: 1 to 2: 1.
43. The process of any one of claims 35-42, wherein the polar aprotic solvent comprises at least one of acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide and tetrahydrofuran.
44. Use of the compound of formula (I) as prepared according to any of the above claims for producing pyraclostrobin.
45. A process for preparation of pyraclostrobin comprising a) preparing compound of formula (I) according to any of claims 1-43 b) producing reaction conditions for the preparation of pyraclostrobin.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562103181P | 2015-01-14 | 2015-01-14 | |
| US62/103,181 | 2015-01-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016113741A1 true WO2016113741A1 (en) | 2016-07-21 |
Family
ID=55346154
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IL2016/050047 WO2016113741A1 (en) | 2015-01-14 | 2016-01-14 | Process for preparing 1-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-1h-pyrazole |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2016113741A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106928145A (en) * | 2017-04-17 | 2017-07-07 | 安徽广信农化股份有限公司 | A kind of synthesis system of pyraclostrobin Intermediate nitro benzene |
| CN106946785A (en) * | 2017-04-17 | 2017-07-14 | 安徽广信农化股份有限公司 | A kind of synthesis technique of pyraclostrobin intermediate pyrazole alcohol |
| CN107778246A (en) * | 2017-12-05 | 2018-03-09 | 利民化工股份有限公司 | A kind of process for purification of bactericide pyraclostrobin intermediate pyrazole alcohol |
| WO2018091338A1 (en) * | 2016-11-17 | 2018-05-24 | Basf Se | Process for the purification of 1-(4-chlorophenyl)pyrazol-3-ol |
| CN110105287A (en) * | 2019-05-23 | 2019-08-09 | 江苏禾本生化有限公司 | A kind of synthesis technology of pyraclostrobin |
| CN111018785A (en) * | 2019-12-26 | 2020-04-17 | 武汉科技大学 | Synthesis method and application of 1- (4-chlorphenyl) -3-pyrazole alcohol |
| CN111454208A (en) * | 2020-05-29 | 2020-07-28 | 安徽国星生物化学有限公司 | Production method of 2- [ (N-p-chlorophenyl) -3-pyrazolyloxymethyl ] nitrobenzene |
| CN113620879A (en) * | 2021-06-16 | 2021-11-09 | 浙江禾本科技股份有限公司 | Synthesis of 2[ (N-4-chlorphenyl) -1H-pyrazol-3-yloxymethyl ] nitrobenzene |
| CN115417818A (en) * | 2022-09-02 | 2022-12-02 | 江苏七洲绿色科技研究院有限公司 | Synthesis method of 1- (4-chlorphenyl) pyrazolidin-3-ketone |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996001256A1 (en) | 1994-07-06 | 1996-01-18 | Basf Aktiengesellschaft | Use of 2-[(dihydro)pyrazolyl-3'-oxymethylene]-anilides as pest-control agents and fungicides |
| US6133451A (en) | 1997-07-30 | 2000-10-17 | Basf Aktiengesellschaft | Method for producing 2-(3-pyrazolyl-oxymethylene) nitrobenzenes |
| US6255489B1 (en) | 1997-09-05 | 2001-07-03 | Basf Aktiengesellschaft | Method for producing (hetero)aromatic hydroxylamines |
| WO2013162072A1 (en) * | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
-
2016
- 2016-01-14 WO PCT/IL2016/050047 patent/WO2016113741A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996001256A1 (en) | 1994-07-06 | 1996-01-18 | Basf Aktiengesellschaft | Use of 2-[(dihydro)pyrazolyl-3'-oxymethylene]-anilides as pest-control agents and fungicides |
| US6133451A (en) | 1997-07-30 | 2000-10-17 | Basf Aktiengesellschaft | Method for producing 2-(3-pyrazolyl-oxymethylene) nitrobenzenes |
| US6255489B1 (en) | 1997-09-05 | 2001-07-03 | Basf Aktiengesellschaft | Method for producing (hetero)aromatic hydroxylamines |
| WO2013162072A1 (en) * | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10538493B2 (en) | 2016-11-17 | 2020-01-21 | Basf Se | Process for the purification of 1-(4-chlorophenyl)pyrazol-3-ol |
| CN109890794A (en) * | 2016-11-17 | 2019-06-14 | 巴斯夫欧洲公司 | The method for purifying 1- (4- chlorphenyl) pyrazoles -3- alcohol |
| CN109890794B (en) * | 2016-11-17 | 2023-04-11 | 巴斯夫欧洲公司 | Process for purifying 1- (4-chlorophenyl) pyrazol-3-ol |
| WO2018091338A1 (en) * | 2016-11-17 | 2018-05-24 | Basf Se | Process for the purification of 1-(4-chlorophenyl)pyrazol-3-ol |
| CN106928145A (en) * | 2017-04-17 | 2017-07-07 | 安徽广信农化股份有限公司 | A kind of synthesis system of pyraclostrobin Intermediate nitro benzene |
| CN106946785A (en) * | 2017-04-17 | 2017-07-14 | 安徽广信农化股份有限公司 | A kind of synthesis technique of pyraclostrobin intermediate pyrazole alcohol |
| CN107778246A (en) * | 2017-12-05 | 2018-03-09 | 利民化工股份有限公司 | A kind of process for purification of bactericide pyraclostrobin intermediate pyrazole alcohol |
| CN110105287A (en) * | 2019-05-23 | 2019-08-09 | 江苏禾本生化有限公司 | A kind of synthesis technology of pyraclostrobin |
| CN110105287B (en) * | 2019-05-23 | 2022-04-26 | 江苏禾本生化有限公司 | Synthesis process of pyraclostrobin |
| CN111018785A (en) * | 2019-12-26 | 2020-04-17 | 武汉科技大学 | Synthesis method and application of 1- (4-chlorphenyl) -3-pyrazole alcohol |
| CN111454208A (en) * | 2020-05-29 | 2020-07-28 | 安徽国星生物化学有限公司 | Production method of 2- [ (N-p-chlorophenyl) -3-pyrazolyloxymethyl ] nitrobenzene |
| CN113620879A (en) * | 2021-06-16 | 2021-11-09 | 浙江禾本科技股份有限公司 | Synthesis of 2[ (N-4-chlorphenyl) -1H-pyrazol-3-yloxymethyl ] nitrobenzene |
| CN113620879B (en) * | 2021-06-16 | 2023-05-05 | 浙江禾本科技股份有限公司 | Synthesis of 2[ (N-4-chlorophenyl) -1H-pyrazol-3-yloxymethyl ] nitrobenzene |
| CN115417818A (en) * | 2022-09-02 | 2022-12-02 | 江苏七洲绿色科技研究院有限公司 | Synthesis method of 1- (4-chlorphenyl) pyrazolidin-3-ketone |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2016113741A1 (en) | Process for preparing 1-(4-chlorophenyl)-3-[(2-nitrophenyl)methoxy]-1h-pyrazole | |
| IL175024A (en) | 2-dihaloacyl-3-amino-acrylic acid esters, a process for their preparation, their use for producing 3-dihalomethyl-1h-pyrazole-4-carboxylic acid esters and 3-dichloromethylpyrazole-4-carboxylic acid esters | |
| JP2018008985A (en) | Process for preparation of (5-fluoro-2-methyl-3-quinolin-2-ylmethyl-indol-1-1yl)-acetic acid esters | |
| CN108947884A (en) | A kind of Preparation Method And Their Intermediate of imrecoxib | |
| CN112441900B (en) | Preparation method of 4-felbinac | |
| CN112544621B (en) | Method for preparing 2- (4-chlorophenoxy) -propoxyamine | |
| CN110117256B (en) | Synthesis method of bixafen | |
| CN104402909A (en) | Synthetic method of cefoxitin acid | |
| KR101583851B1 (en) | Method for producing 3-methyl-2-thiophenecarboxylic acid | |
| EP3303274B1 (en) | Process for the synthesis of 9,9-bis(hydroxymethyl)fluorene | |
| CN109734656B (en) | Preparation method of nitrendipine | |
| EP2909224A2 (en) | An improved process for the preparation of fulvestrant | |
| CN112661668A (en) | N-substituted amide compound and preparation method thereof | |
| KR20160027536A (en) | Process for preparing an intermediate useful for the synthesis of silodosin | |
| CN112679477B (en) | Preparation method of celecoxib and intermediate thereof | |
| CN114349686B (en) | 1, 4-dihydropyridine chiral hybrid hydrogenation reagent, preparation method and application thereof | |
| CN104478799B (en) | The preparation method of 1,4-diallyl isoquinolin | |
| CN110734364A (en) | Synthesis method of 1- (4-chlorphenyl) -2-cyclopropyl-1-acetone | |
| CN104140430B (en) | A kind of racemization method of isomer | |
| EP2835371B1 (en) | Industrial method for manufacturing high-purity methiozoline | |
| AU2012324824B2 (en) | Processes for the preparation of 6-Chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide and of its precursors | |
| WO2007132990A1 (en) | Process for the preparation of chiral glycidylphthalimide in highly optical purity | |
| CN108503583B (en) | Alkylation method of nitrogen-hydrogen-containing compound and application thereof | |
| JP2009527511A (en) | Process for the preparation of 3,4-disubstituted phenylacetic acids and novel intermediates | |
| RU2702121C1 (en) | Method of producing 2-amino-nicotinic acid benzyl ester derivative |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16703860 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16703860 Country of ref document: EP Kind code of ref document: A1 |