WO2015156587A1 - Multi-component host material and organic electroluminescent device comprising the same - Google Patents
Multi-component host material and organic electroluminescent device comprising the same Download PDFInfo
- Publication number
- WO2015156587A1 WO2015156587A1 PCT/KR2015/003485 KR2015003485W WO2015156587A1 WO 2015156587 A1 WO2015156587 A1 WO 2015156587A1 KR 2015003485 W KR2015003485 W KR 2015003485W WO 2015156587 A1 WO2015156587 A1 WO 2015156587A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- substituted
- unsubstituted
- host
- alkyl
- Prior art date
Links
- 239000000463 material Substances 0.000 title description 45
- 150000001875 compounds Chemical class 0.000 claims abstract description 111
- 125000003118 aryl group Chemical group 0.000 claims abstract description 58
- 125000001072 heteroaryl group Chemical group 0.000 claims abstract description 58
- 239000002019 doping agent Substances 0.000 claims abstract description 33
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims abstract description 12
- 125000000609 carbazolyl group Chemical class C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 claims abstract description 6
- 125000000923 (C1-C30) alkyl group Chemical group 0.000 claims description 40
- 229910052739 hydrogen Inorganic materials 0.000 claims description 30
- 239000001257 hydrogen Substances 0.000 claims description 30
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 28
- 125000005104 aryl silyl group Chemical group 0.000 claims description 28
- 150000002431 hydrogen Chemical class 0.000 claims description 28
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 26
- 125000002950 monocyclic group Chemical group 0.000 claims description 26
- 229910052736 halogen Inorganic materials 0.000 claims description 25
- 125000004432 carbon atom Chemical group C* 0.000 claims description 24
- 150000002367 halogens Chemical class 0.000 claims description 23
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 22
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 22
- 229910052805 deuterium Inorganic materials 0.000 claims description 22
- 125000001424 substituent group Chemical group 0.000 claims description 22
- 229910052717 sulfur Inorganic materials 0.000 claims description 21
- 125000005842 heteroatom Chemical group 0.000 claims description 20
- 229910052757 nitrogen Inorganic materials 0.000 claims description 19
- 229910052760 oxygen Inorganic materials 0.000 claims description 19
- 125000006749 (C6-C60) aryl group Chemical group 0.000 claims description 18
- 125000002723 alicyclic group Chemical group 0.000 claims description 18
- 125000003367 polycyclic group Chemical group 0.000 claims description 18
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 14
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 14
- 239000001301 oxygen Substances 0.000 claims description 14
- 239000011593 sulfur Substances 0.000 claims description 14
- 125000000304 alkynyl group Chemical group 0.000 claims description 13
- -1 benzoimidazolyl Chemical group 0.000 claims description 13
- 125000000739 C2-C30 alkenyl group Chemical group 0.000 claims description 12
- 125000001769 aryl amino group Chemical group 0.000 claims description 12
- 125000006822 tri(C1-C30) alkylsilyl group Chemical group 0.000 claims description 11
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 10
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 9
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 9
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 9
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 9
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Chemical compound C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 claims description 8
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 8
- 229910052698 phosphorus Inorganic materials 0.000 claims description 8
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 7
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 claims description 6
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 claims description 6
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 claims description 6
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 claims description 5
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 claims description 5
- 125000005956 isoquinolyl group Chemical group 0.000 claims description 5
- 125000004076 pyridyl group Chemical group 0.000 claims description 5
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 5
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 claims description 5
- 125000005493 quinolyl group Chemical group 0.000 claims description 5
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 claims description 5
- 229910052710 silicon Inorganic materials 0.000 claims description 5
- 125000004306 triazinyl group Chemical group 0.000 claims description 5
- IANQTJSKSUMEQM-UHFFFAOYSA-N 1-benzofuran Chemical compound C1=CC=C2OC=CC2=C1 IANQTJSKSUMEQM-UHFFFAOYSA-N 0.000 claims description 4
- 125000005874 benzothiadiazolyl group Chemical group 0.000 claims description 4
- 229910052796 boron Inorganic materials 0.000 claims description 4
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 claims description 4
- 125000002883 imidazolyl group Chemical group 0.000 claims description 4
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 claims description 4
- 125000001041 indolyl group Chemical group 0.000 claims description 4
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 claims description 4
- 125000001624 naphthyl group Chemical group 0.000 claims description 4
- 125000004934 phenanthridinyl group Chemical group C1(=CC=CC2=NC=C3C=CC=CC3=C12)* 0.000 claims description 4
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 4
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 4
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 4
- 125000000168 pyrrolyl group Chemical group 0.000 claims description 4
- 125000005247 tetrazinyl group Chemical group N1=NN=NC(=C1)* 0.000 claims description 4
- 125000003831 tetrazolyl group Chemical group 0.000 claims description 4
- 125000001425 triazolyl group Chemical group 0.000 claims description 4
- 229910052720 vanadium Inorganic materials 0.000 claims description 4
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 claims description 3
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 claims description 3
- 125000000732 arylene group Chemical group 0.000 claims description 3
- 235000010290 biphenyl Nutrition 0.000 claims description 3
- 239000004305 biphenyl Substances 0.000 claims description 3
- 125000002676 chrysenyl group Chemical group C1(=CC=CC=2C3=CC=C4C=CC=CC4=C3C=CC12)* 0.000 claims description 3
- 125000003914 fluoranthenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC=C4C1=C23)* 0.000 claims description 3
- 125000003454 indenyl group Chemical group C1(C=CC2=CC=CC=C12)* 0.000 claims description 3
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 claims description 3
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 claims description 3
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 claims description 3
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 claims description 3
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 claims description 3
- 125000001725 pyrenyl group Chemical group 0.000 claims description 3
- 125000003960 triphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C3=CC=CC=C3C12)* 0.000 claims description 3
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims description 2
- 125000006761 (C6-C60) arylene group Chemical group 0.000 claims description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 2
- 229910052799 carbon Inorganic materials 0.000 claims description 2
- 125000005106 triarylsilyl group Chemical group 0.000 claims description 2
- RFRXIWQYSOIBDI-UHFFFAOYSA-N benzarone Chemical class CCC=1OC2=CC=CC=C2C=1C(=O)C1=CC=C(O)C=C1 RFRXIWQYSOIBDI-UHFFFAOYSA-N 0.000 claims 1
- 150000001555 benzenes Chemical class 0.000 claims 1
- 150000002469 indenes Chemical class 0.000 claims 1
- 150000002475 indoles Chemical class 0.000 claims 1
- 239000010410 layer Substances 0.000 description 121
- 230000005525 hole transport Effects 0.000 description 24
- 238000002347 injection Methods 0.000 description 21
- 239000007924 injection Substances 0.000 description 21
- 230000000052 comparative effect Effects 0.000 description 18
- 238000000151 deposition Methods 0.000 description 18
- 238000004519 manufacturing process Methods 0.000 description 17
- 0 c1ccc(C([N-]*2c3ccccc3)[N+]2c(cc2)ccc2-[n](c(cccc2)c2c2c3)c2cc(C2(c4ccccc4)c4ccccc4)c3-c3c2cccc3)cc1 Chemical compound c1ccc(C([N-]*2c3ccccc3)[N+]2c(cc2)ccc2-[n](c(cccc2)c2c2c3)c2cc(C2(c4ccccc4)c4ccccc4)c3-c3c2cccc3)cc1 0.000 description 13
- 230000000903 blocking effect Effects 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- VZSRBBMJRBPUNF-UHFFFAOYSA-N 2-(2,3-dihydro-1H-inden-2-ylamino)-N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]pyrimidine-5-carboxamide Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C(=O)NCCC(N1CC2=C(CC1)NN=N2)=O VZSRBBMJRBPUNF-UHFFFAOYSA-N 0.000 description 7
- 125000004429 atom Chemical group 0.000 description 7
- 239000012044 organic layer Substances 0.000 description 7
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 6
- TXCDCPKCNAJMEE-UHFFFAOYSA-N dibenzofuran Chemical group C1=CC=C2C3=CC=CC=C3OC2=C1 TXCDCPKCNAJMEE-UHFFFAOYSA-N 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 150000001716 carbazoles Chemical class 0.000 description 4
- 239000010949 copper Substances 0.000 description 4
- IYYZUPMFVPLQIF-UHFFFAOYSA-N dibenzothiophene Chemical compound C1=CC=C2C3=CC=CC=C3SC2=C1 IYYZUPMFVPLQIF-UHFFFAOYSA-N 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- SXAMGRAIZSSWIH-UHFFFAOYSA-N 2-[3-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]-1,2,4-oxadiazol-5-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C1=NOC(=N1)CC(=O)N1CC2=C(CC1)NN=N2 SXAMGRAIZSSWIH-UHFFFAOYSA-N 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 150000004770 chalcogenides Chemical class 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
- 229910052741 iridium Inorganic materials 0.000 description 3
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 3
- 229910001507 metal halide Inorganic materials 0.000 description 3
- 150000005309 metal halides Chemical class 0.000 description 3
- 229910044991 metal oxide Inorganic materials 0.000 description 3
- 150000004706 metal oxides Chemical class 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- WZFUQSJFWNHZHM-UHFFFAOYSA-N 2-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)CC(=O)N1CC2=C(CC1)NN=N2 WZFUQSJFWNHZHM-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- XOZYRGDWIYNWAR-UHFFFAOYSA-N CC(C)(c(cccc1)c1-c1c2c3c4)c1ccc2[n](C1N=C(c2ccccc2)N=C(c2ccccc2)N1C)c3ccc4-c1ccccc1 Chemical compound CC(C)(c(cccc1)c1-c1c2c3c4)c1ccc2[n](C1N=C(c2ccccc2)N=C(c2ccccc2)N1C)c3ccc4-c1ccccc1 XOZYRGDWIYNWAR-UHFFFAOYSA-N 0.000 description 2
- MOHRHAZGVCSGRE-UHFFFAOYSA-N CC(C)(c(cccc1)c1-c1c2c3c4cccc3)c1ccc2[n]4-c1nc(cccc2)c2c(-c(cc2)ccc2-c2ccccc2)n1 Chemical compound CC(C)(c(cccc1)c1-c1c2c3c4cccc3)c1ccc2[n]4-c1nc(cccc2)c2c(-c(cc2)ccc2-c2ccccc2)n1 MOHRHAZGVCSGRE-UHFFFAOYSA-N 0.000 description 2
- YSLKYXFJVNFAGD-UHFFFAOYSA-O CC(C)(c(cccc1)c1-c1c2c3c4cccc3)c1ccc2[n]4[NH+]1C(c2ccccc2)=NC1c1cccc2c1[o]c1c2cccc1 Chemical compound CC(C)(c(cccc1)c1-c1c2c3c4cccc3)c1ccc2[n]4[NH+]1C(c2ccccc2)=NC1c1cccc2c1[o]c1c2cccc1 YSLKYXFJVNFAGD-UHFFFAOYSA-O 0.000 description 2
- RSXONSQNWXWJSG-UHFFFAOYSA-O CC1(C)c2ccc3[n](C4[NH2+]c(cccc5)c5N=C4c4ccccc4)c4ccccc4c3c2-c2ccccc12 Chemical compound CC1(C)c2ccc3[n](C4[NH2+]c(cccc5)c5N=C4c4ccccc4)c4ccccc4c3c2-c2ccccc12 RSXONSQNWXWJSG-UHFFFAOYSA-O 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- AJCDPYKSSFAIFB-UHFFFAOYSA-N N#Cc1cc(C(N=C2c3ccccc3)=[N+]2c(cc2)ccc2-[n]2c3cc(-c4c5[s]c6ccccc6c5ccc4)ccc3c3c2cccc3)ccc1 Chemical compound N#Cc1cc(C(N=C2c3ccccc3)=[N+]2c(cc2)ccc2-[n]2c3cc(-c4c5[s]c6ccccc6c5ccc4)ccc3c3c2cccc3)ccc1 AJCDPYKSSFAIFB-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000006267 biphenyl group Chemical group 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000005281 excited state Effects 0.000 description 2
- RMBPEFMHABBEKP-UHFFFAOYSA-N fluorene Chemical compound C1=CC=C2C3=C[CH]C=CC3=CC2=C1 RMBPEFMHABBEKP-UHFFFAOYSA-N 0.000 description 2
- IMKMFBIYHXBKRX-UHFFFAOYSA-M lithium;quinoline-2-carboxylate Chemical compound [Li+].C1=CC=CC2=NC(C(=O)[O-])=CC=C21 IMKMFBIYHXBKRX-UHFFFAOYSA-M 0.000 description 2
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N o-biphenylenemethane Natural products C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 description 2
- 229910052762 osmium Inorganic materials 0.000 description 2
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229910052761 rare earth metal Inorganic materials 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 229910052814 silicon oxide Inorganic materials 0.000 description 2
- 239000002344 surface layer Substances 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 125000006736 (C6-C20) aryl group Chemical group 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000004972 1-butynyl group Chemical group [H]C([H])([H])C([H])([H])C#C* 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- 125000000530 1-propynyl group Chemical group [H]C([H])([H])C#C* 0.000 description 1
- OMDTUSYJJFBYMG-UHFFFAOYSA-N 2,4-bis(9,9-dimethylfluoren-2-yl)-6-naphthalen-2-yl-1,3,5-triazine Chemical compound C1=CC=C2C(C)(C)C3=CC(C=4N=C(N=C(N=4)C=4C=C5C=CC=CC5=CC=4)C4=CC=C5C6=CC=CC=C6C(C5=C4)(C)C)=CC=C3C2=C1 OMDTUSYJJFBYMG-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000000069 2-butynyl group Chemical group [H]C([H])([H])C#CC([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000000474 3-butynyl group Chemical group [H]C#CC([H])([H])C([H])([H])* 0.000 description 1
- GJWBRYKOJMOBHH-UHFFFAOYSA-N 9,9-dimethyl-n-[4-(9-phenylcarbazol-3-yl)phenyl]-n-(4-phenylphenyl)fluoren-2-amine Chemical compound C1=C2C(C)(C)C3=CC=CC=C3C2=CC=C1N(C=1C=CC(=CC=1)C=1C=C2C3=CC=CC=C3N(C=3C=CC=CC=3)C2=CC=1)C(C=C1)=CC=C1C1=CC=CC=C1 GJWBRYKOJMOBHH-UHFFFAOYSA-N 0.000 description 1
- KCOYLRXCNKJSSC-UHFFFAOYSA-N 9h-carbazole Chemical group C1=CC=C2C3=CC=CC=C3NC2=C1.C1=CC=C2C3=CC=CC=C3NC2=C1 KCOYLRXCNKJSSC-UHFFFAOYSA-N 0.000 description 1
- GNEXTYHIGKGOSL-UHFFFAOYSA-O C(C1)C=C(C2N=C(c(cc3)ccc3-[n]3c4ccccc4c4ccccc34)N=C(c3ccccc3)[NH2+]2)C=C1c(cc1)ccc1[Si](c1ccccc1)(c1ccccc1)c1ccccc1 Chemical compound C(C1)C=C(C2N=C(c(cc3)ccc3-[n]3c4ccccc4c4ccccc34)N=C(c3ccccc3)[NH2+]2)C=C1c(cc1)ccc1[Si](c1ccccc1)(c1ccccc1)c1ccccc1 GNEXTYHIGKGOSL-UHFFFAOYSA-O 0.000 description 1
- IXFLUGQXNJMZAG-UHFFFAOYSA-N C(C12)=CC(c3ccc4[s]c5ccccc5c4c3)=CC1c(cc(cc1)-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3ccccc3)c1N2c1ccccc1 Chemical compound C(C12)=CC(c3ccc4[s]c5ccccc5c4c3)=CC1c(cc(cc1)-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3ccccc3)c1N2c1ccccc1 IXFLUGQXNJMZAG-UHFFFAOYSA-N 0.000 description 1
- YRMRWJLTLIRKFW-UHFFFAOYSA-N C(CC(c(cc1c2ccccc22)ccc1[n]2-c1c(C(c2c-3cccc2)(c2ccccc2)c2ccccc2)c-3ccc1)=C1)c2c1c1ccccc1[n]2-c1cccc(-c2c3cccc2)c1C3(c1ccccc1)c1ccccc1 Chemical compound C(CC(c(cc1c2ccccc22)ccc1[n]2-c1c(C(c2c-3cccc2)(c2ccccc2)c2ccccc2)c-3ccc1)=C1)c2c1c1ccccc1[n]2-c1cccc(-c2c3cccc2)c1C3(c1ccccc1)c1ccccc1 YRMRWJLTLIRKFW-UHFFFAOYSA-N 0.000 description 1
- CHNYQCUZUQQSTQ-UHFFFAOYSA-N C(Cc1ccc23)C=Cc1c2c(c(cccc1)c1cc1)c1[n]3-c1cc(-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)ccc1 Chemical compound C(Cc1ccc23)C=Cc1c2c(c(cccc1)c1cc1)c1[n]3-c1cc(-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)ccc1 CHNYQCUZUQQSTQ-UHFFFAOYSA-N 0.000 description 1
- KLCKNBATNKCNKX-UHFFFAOYSA-N C(c(cccc1)c1-c1ccc2)c1c2-c(cc1c2c3ccc(-c(cc4)cc(c5c6cccc5)c4[n]6-c4ccccc4)c2)ccc1[n]3-c1ccccc1 Chemical compound C(c(cccc1)c1-c1ccc2)c1c2-c(cc1c2c3ccc(-c(cc4)cc(c5c6cccc5)c4[n]6-c4ccccc4)c2)ccc1[n]3-c1ccccc1 KLCKNBATNKCNKX-UHFFFAOYSA-N 0.000 description 1
- SUNYRHIPICDPSK-UHFFFAOYSA-N C1C=CC(c2nc(-c(cc3)ccc3-[n](c3c4c5ccccc5cc3)c3c4c4ccccc4cc3)nc(-c(cc3)cc4c3c(cccc3)c3[n]4-c3ccccc3)n2)=CC1 Chemical compound C1C=CC(c2nc(-c(cc3)ccc3-[n](c3c4c5ccccc5cc3)c3c4c4ccccc4cc3)nc(-c(cc3)cc4c3c(cccc3)c3[n]4-c3ccccc3)n2)=CC1 SUNYRHIPICDPSK-UHFFFAOYSA-N 0.000 description 1
- HNNNQHMBZJICEM-UHFFFAOYSA-N CC(C)(c1ccccc1-c1c2)c1ccc2-[n]1c(ccc(-c(cc2)cc(c3ccccc33)c2[n]3-c2cc(-c3ccccc3)ccc2)c2)c2c2c1cccc2 Chemical compound CC(C)(c1ccccc1-c1c2)c1ccc2-[n]1c(ccc(-c(cc2)cc(c3ccccc33)c2[n]3-c2cc(-c3ccccc3)ccc2)c2)c2c2c1cccc2 HNNNQHMBZJICEM-UHFFFAOYSA-N 0.000 description 1
- IXGSWYHRTCWTBK-UHFFFAOYSA-N CC(CC=C1)c2c1c(cc(cc1)-c(cc3)cc(c4ccccc44)c3[n]4-c3cccc-4c3C(C)(C)c3c-4cccc3)c1[n]2-c1c(C(C)(C)c2c-3cccc2)c-3ccc1 Chemical compound CC(CC=C1)c2c1c(cc(cc1)-c(cc3)cc(c4ccccc44)c3[n]4-c3cccc-4c3C(C)(C)c3c-4cccc3)c1[n]2-c1c(C(C)(C)c2c-3cccc2)c-3ccc1 IXGSWYHRTCWTBK-UHFFFAOYSA-N 0.000 description 1
- VLBNCULGMWXXAX-UHFFFAOYSA-N CC(CC=C1)c2c1c1cc(-c(cc3)cc4c3[s]c3ccccc43)ccc1[n]2-c(cc1)ccc1[N+]1=C(c2ccccc2)N=C1c1ccccc1 Chemical compound CC(CC=C1)c2c1c1cc(-c(cc3)cc4c3[s]c3ccccc43)ccc1[n]2-c(cc1)ccc1[N+]1=C(c2ccccc2)N=C1c1ccccc1 VLBNCULGMWXXAX-UHFFFAOYSA-N 0.000 description 1
- HSBHHCCVOZODRH-UHFFFAOYSA-N CC1(C)c(cc(c(c2c3cccc2)c2)[n]3-c3cc(C4=NC(c5ccccc5)N(C)C(c5cccc(S(c6ccccc6)c6ccccc6)c5)=N4)ccc3)c2-c2c1cccc2 Chemical compound CC1(C)c(cc(c(c2c3cccc2)c2)[n]3-c3cc(C4=NC(c5ccccc5)N(C)C(c5cccc(S(c6ccccc6)c6ccccc6)c5)=N4)ccc3)c2-c2c1cccc2 HSBHHCCVOZODRH-UHFFFAOYSA-N 0.000 description 1
- LAIUYYQRGYOFPB-UHFFFAOYSA-N CC1(C)c(cc(c(cccc2)c2[n]2-c3cc(C(N=C4c5ccccc5)=[N+]4c4ccccc4)ccc3)c2c2)c2-c2ccccc12 Chemical compound CC1(C)c(cc(c(cccc2)c2[n]2-c3cc(C(N=C4c5ccccc5)=[N+]4c4ccccc4)ccc3)c2c2)c2-c2ccccc12 LAIUYYQRGYOFPB-UHFFFAOYSA-N 0.000 description 1
- ONXSQBJULOFHHZ-UHFFFAOYSA-N CC1(C)c(ccc(-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccc(C(C)(C)c4ccccc4-4)c-4c3)c3)c3c3ccccc23)c2)c2-c2ccccc12 Chemical compound CC1(C)c(ccc(-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccc(C(C)(C)c4ccccc4-4)c-4c3)c3)c3c3ccccc23)c2)c2-c2ccccc12 ONXSQBJULOFHHZ-UHFFFAOYSA-N 0.000 description 1
- KEYHIVXRAYQDFI-UHFFFAOYSA-N CC1(C)c2cc(-[n](c3ccccc3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3ccc(C)cc3)ccc2-c2c1cccc2 Chemical compound CC1(C)c2cc(-[n](c3ccccc3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c3ccc(C)cc3)ccc2-c2c1cccc2 KEYHIVXRAYQDFI-UHFFFAOYSA-N 0.000 description 1
- MFSWLTSFIKMYSW-UHFFFAOYSA-N CC1(C)c2cc(-[n](c3ccccc3c3c4)c3ccc4-c(cc3c4ccccc44)ccc3[n]4-c(cc3)ccc3-c3cc(C#N)ccc3)ccc2-c2c1cccc2 Chemical compound CC1(C)c2cc(-[n](c3ccccc3c3c4)c3ccc4-c(cc3c4ccccc44)ccc3[n]4-c(cc3)ccc3-c3cc(C#N)ccc3)ccc2-c2c1cccc2 MFSWLTSFIKMYSW-UHFFFAOYSA-N 0.000 description 1
- GPCMCSOUROGNDV-UHFFFAOYSA-N CC1(C)c2cc(N(C3C=CC=CC3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c(cc3)ccc3C#N)ccc2-c2ccccc12 Chemical compound CC1(C)c2cc(N(C3C=CC=CC3c3c4)c3ccc4-c(cc3)cc(c4ccccc44)c3[n]4-c(cc3)ccc3-c(cc3)ccc3C#N)ccc2-c2ccccc12 GPCMCSOUROGNDV-UHFFFAOYSA-N 0.000 description 1
- RBRSRSQACNBFAQ-UHFFFAOYSA-O CC1(C=CC=CC1)C1=NC(c2ccccc2)=[NH+]C(c2cccc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc4c3[s]c3ccccc43)c2)N1 Chemical compound CC1(C=CC=CC1)C1=NC(c2ccccc2)=[NH+]C(c2cccc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc4c3[s]c3ccccc43)c2)N1 RBRSRSQACNBFAQ-UHFFFAOYSA-O 0.000 description 1
- APCOKPIQFVXVNN-UHFFFAOYSA-N CC1(c2c-3cccc2)c(cccc2c4cc(-c(cc5)cc(c6ccccc66)c5[n]6-c5cc(-c6ccccc6)ccc5)ccc44)c2[n]4-c2c1c-3ccc2 Chemical compound CC1(c2c-3cccc2)c(cccc2c4cc(-c(cc5)cc(c6ccccc66)c5[n]6-c5cc(-c6ccccc6)ccc5)ccc44)c2[n]4-c2c1c-3ccc2 APCOKPIQFVXVNN-UHFFFAOYSA-N 0.000 description 1
- CUYYESUSFARAPQ-UHFFFAOYSA-N CC12Sc3ccccc3C1=CC(c(cc1c3c4ccc5ccccc35)ccc1[n]4-c(cc1)ccc1[N+]1=C(c3ccccc3)N=C1c1ccccc1)=CC2 Chemical compound CC12Sc3ccccc3C1=CC(c(cc1c3c4ccc5ccccc35)ccc1[n]4-c(cc1)ccc1[N+]1=C(c3ccccc3)N=C1c1ccccc1)=CC2 CUYYESUSFARAPQ-UHFFFAOYSA-N 0.000 description 1
- KEZSHAYCFZIQRQ-UHFFFAOYSA-N CC1C(C(c2ccccc2-c2c3)(c2ccc3-[n]2c(ccc(-c(cc3)cc(c4ccccc44)c3[n]4-c3cc(-c4ccccc4)ccc3)c3)c3c3ccccc23)c2ccccc2)=CC=CC1 Chemical compound CC1C(C(c2ccccc2-c2c3)(c2ccc3-[n]2c(ccc(-c(cc3)cc(c4ccccc44)c3[n]4-c3cc(-c4ccccc4)ccc3)c3)c3c3ccccc23)c2ccccc2)=CC=CC1 KEZSHAYCFZIQRQ-UHFFFAOYSA-N 0.000 description 1
- SYZCPXVRYLOLGJ-UHFFFAOYSA-N CC1C=CC(C(N=C2c3ccccc3)=[N+]2c2ccccc2)=CC1c(cc1)ccc1N(C1(C)C2)c3ccccc3C1=Cc(c1c3cccc1)c2[n]3-c1ccccc1 Chemical compound CC1C=CC(C(N=C2c3ccccc3)=[N+]2c2ccccc2)=CC1c(cc1)ccc1N(C1(C)C2)c3ccccc3C1=Cc(c1c3cccc1)c2[n]3-c1ccccc1 SYZCPXVRYLOLGJ-UHFFFAOYSA-N 0.000 description 1
- JSRVMVHLHUFGOV-UHFFFAOYSA-N CC1CC=C2c(ccc(c3ccccc33)c4[n]3-c3cc(C(NC5c(cc6)ccc6C#N)=Nc6c5cccc6)ccc3)c4SC2CC1 Chemical compound CC1CC=C2c(ccc(c3ccccc33)c4[n]3-c3cc(C(NC5c(cc6)ccc6C#N)=Nc6c5cccc6)ccc3)c4SC2CC1 JSRVMVHLHUFGOV-UHFFFAOYSA-N 0.000 description 1
- SXPRQYHUTDRCPI-UHFFFAOYSA-O CCC(C1)=C(C2=CC=CCC2C)N=C(c2cccc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc4c3[o]c3ccccc43)c2)[NH+]=C1c1ccccc1 Chemical compound CCC(C1)=C(C2=CC=CCC2C)N=C(c2cccc(-[n](c(cccc3)c3c3c4)c3ccc4-c(cc3)cc4c3[o]c3ccccc43)c2)[NH+]=C1c1ccccc1 SXPRQYHUTDRCPI-UHFFFAOYSA-O 0.000 description 1
- CVQBVGKQZXQPMX-UHFFFAOYSA-N CN/C(/c1ccccc1)=N\C(c1ccccc1)=N Chemical compound CN/C(/c1ccccc1)=N\C(c1ccccc1)=N CVQBVGKQZXQPMX-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- HNLAOSYGUKPAKH-UHFFFAOYSA-N Cc1cccc(-[n](c(cccc2)c2c2c3)c2ccc3-c(cc2c3ccccc33)ccc2[n]3-c2cc(C)ccc2)c1 Chemical compound Cc1cccc(-[n](c(cccc2)c2c2c3)c2ccc3-c(cc2c3ccccc33)ccc2[n]3-c2cc(C)ccc2)c1 HNLAOSYGUKPAKH-UHFFFAOYSA-N 0.000 description 1
- JCICHJCASHANAC-UHFFFAOYSA-N Cc1cccc(-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 Chemical compound Cc1cccc(-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c3ccccc3)c3)c3c3ccccc23)c1 JCICHJCASHANAC-UHFFFAOYSA-N 0.000 description 1
- WOBKGYHYQKEAFY-UHFFFAOYSA-N Cc1cccc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c(cc3C4(c5ccccc5)c5ccccc5)ccc3-c3c4cccc3)c3)c3c3ccccc23)c1 Chemical compound Cc1cccc(-c(cc2)ccc2-[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c(cc3C4(c5ccccc5)c5ccccc5)ccc3-c3c4cccc3)c3)c3c3ccccc23)c1 WOBKGYHYQKEAFY-UHFFFAOYSA-N 0.000 description 1
- OQXSSFGKKMHLJS-UHFFFAOYSA-N Cc1ccccc1-c(c(Nc(cc1)ccc1-c(cc1)ccc1[N+]1=C(c2ccccc2)N=C1c1ccccc1)c1)cc(c2c3cccc2)c1[n]3-c1ccccc1 Chemical compound Cc1ccccc1-c(c(Nc(cc1)ccc1-c(cc1)ccc1[N+]1=C(c2ccccc2)N=C1c1ccccc1)c1)cc(c2c3cccc2)c1[n]3-c1ccccc1 OQXSSFGKKMHLJS-UHFFFAOYSA-N 0.000 description 1
- KOPBYBDAPCDYFK-UHFFFAOYSA-N Cs2O Inorganic materials [O-2].[Cs+].[Cs+] KOPBYBDAPCDYFK-UHFFFAOYSA-N 0.000 description 1
- 102100039856 Histone H1.1 Human genes 0.000 description 1
- 101001035402 Homo sapiens Histone H1.1 Proteins 0.000 description 1
- 239000002841 Lewis acid Substances 0.000 description 1
- FUJCRWPEOMXPAD-UHFFFAOYSA-N Li2O Inorganic materials [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- SZAUDYFHPVSKGY-UHFFFAOYSA-N N#Cc(cc1)cc(c2c3cccc2)c1[n]3-c1cc(C2=NC(c3ccccc3)=NC(c3ccccc3)N2)ccc1 Chemical compound N#Cc(cc1)cc(c2c3cccc2)c1[n]3-c1cc(C2=NC(c3ccccc3)=NC(c3ccccc3)N2)ccc1 SZAUDYFHPVSKGY-UHFFFAOYSA-N 0.000 description 1
- WZOLXCXEXKSLBW-UHFFFAOYSA-N N#Cc(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4cccc3)ccc2[n]4-c(cc2C3(c4ccccc4)c4ccccc4)ccc2-c2c3cccc2)c2)c2c2ccccc12 Chemical compound N#Cc(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4cccc3)ccc2[n]4-c(cc2C3(c4ccccc4)c4ccccc4)ccc2-c2c3cccc2)c2)c2c2ccccc12 WZOLXCXEXKSLBW-UHFFFAOYSA-N 0.000 description 1
- BJGDMDDUSCDYSP-UHFFFAOYSA-P N#Cc(cc1)ccc1-c1cc(C2[N+]([n]3c4c5[s]c6ccccc6c5ccc4c4c3cccc4)=C(c3ccccc3)[NH2+]2)ccc1 Chemical compound N#Cc(cc1)ccc1-c1cc(C2[N+]([n]3c4c5[s]c6ccccc6c5ccc4c4c3cccc4)=C(c3ccccc3)[NH2+]2)ccc1 BJGDMDDUSCDYSP-UHFFFAOYSA-P 0.000 description 1
- CETNUJMTBRNNCO-UHFFFAOYSA-O N#Cc1cccc(-c2cc(C(N3)[NH2+]C(c4ccccc4)NC3[n](c(cccc3)c3c3ccc45)c3c4OC3C5=CC=C3)ccc2)c1 Chemical compound N#Cc1cccc(-c2cc(C(N3)[NH2+]C(c4ccccc4)NC3[n](c(cccc3)c3c3ccc45)c3c4OC3C5=CC=C3)ccc2)c1 CETNUJMTBRNNCO-UHFFFAOYSA-O 0.000 description 1
- XHTYFBXVBDFAEP-UHFFFAOYSA-N N#Cc1cccc(C(C=C2)=CCC2[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c(cc3)cc(C4(c5ccccc5)c5ccccc5)c3-c3c4cccc3)c3)c3c3ccccc23)c1 Chemical compound N#Cc1cccc(C(C=C2)=CCC2[n]2c(ccc(-c(cc3c4ccccc44)ccc3[n]4-c(cc3)cc(C4(c5ccccc5)c5ccccc5)c3-c3c4cccc3)c3)c3c3ccccc23)c1 XHTYFBXVBDFAEP-UHFFFAOYSA-N 0.000 description 1
- ITHNTPYLPKFWPT-UHFFFAOYSA-N N#Cc1cccc(C2NC(c3cccc(-[n]4c5ccccc5c5c4cccc5)c3)=NC(c3ccccc3)[N-]2)c1 Chemical compound N#Cc1cccc(C2NC(c3cccc(-[n]4c5ccccc5c5c4cccc5)c3)=NC(c3ccccc3)[N-]2)c1 ITHNTPYLPKFWPT-UHFFFAOYSA-N 0.000 description 1
- CKHRKOFVUBRUBG-UHFFFAOYSA-O NC(c1ccccc1)[NH2+]C(c1cccc(C#N)c1)N=Cc(cc1)ccc1-[n]1c2c3[o]c4ccccc4c3ccc2c2c1cccc2 Chemical compound NC(c1ccccc1)[NH2+]C(c1cccc(C#N)c1)N=Cc(cc1)ccc1-[n]1c2c3[o]c4ccccc4c3ccc2c2c1cccc2 CKHRKOFVUBRUBG-UHFFFAOYSA-O 0.000 description 1
- 229910003564 SiAlON Inorganic materials 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- XSTXAVWGXDQKEL-UHFFFAOYSA-N Trichloroethylene Chemical group ClC=C(Cl)Cl XSTXAVWGXDQKEL-UHFFFAOYSA-N 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001339 alkali metal compounds Chemical class 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 150000004984 aromatic diamines Chemical class 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 125000005129 aryl carbonyl group Chemical group 0.000 description 1
- 125000005110 aryl thio group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000002047 benzodioxolyl group Chemical group O1OC(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- MHTPESFJWCJELK-UHFFFAOYSA-N c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c1ccccc1 Chemical compound c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2ccccc22)c1[n]2-c1ccccc1 MHTPESFJWCJELK-UHFFFAOYSA-N 0.000 description 1
- VKCAJZUHWAOGMT-UHFFFAOYSA-N c(cc1)ccc1-[n]1c(cc(c(c2c3cccc2)c2)[n]3-c3cc(C(N=C4c5cc6ccccc6cc5)=[N+]4c4ccccc4)ccc3)c2c2ccccc12 Chemical compound c(cc1)ccc1-[n]1c(cc(c(c2c3cccc2)c2)[n]3-c3cc(C(N=C4c5cc6ccccc6cc5)=[N+]4c4ccccc4)ccc3)c2c2ccccc12 VKCAJZUHWAOGMT-UHFFFAOYSA-N 0.000 description 1
- VULGVCBNDSXNAW-UHFFFAOYSA-N c(cc1)ccc1-[n]1c(ccc(-c(cc2c(cc3)c4cc3[Si](c3ccccc3)(c3ccccc3)c3ccccc3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 Chemical compound c(cc1)ccc1-[n]1c(ccc(-c(cc2c(cc3)c4cc3[Si](c3ccccc3)(c3ccccc3)c3ccccc3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 VULGVCBNDSXNAW-UHFFFAOYSA-N 0.000 description 1
- ZQUHWAMDAQNBLA-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2c3ccc(-c4ccc5[o]c(cccc6)c6c5c4)c2)c1[n]3-c1ccccc1 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2c3ccc(-c4ccc5[o]c(cccc6)c6c5c4)c2)c1[n]3-c1ccccc1 ZQUHWAMDAQNBLA-UHFFFAOYSA-N 0.000 description 1
- GLDCWBFVJVRKMS-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2cc(-c3c4[o]c5ccccc5c4ccc3)ccc22)c1[n]2-c1ccccc1 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n](c1ccccc1c1c2)c1ccc2-c(cc1)cc(c2cc(-c3c4[o]c5ccccc5c4ccc3)ccc22)c1[n]2-c1ccccc1 GLDCWBFVJVRKMS-UHFFFAOYSA-N 0.000 description 1
- DGIZBBUUPCJEGQ-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2)cc(c3cc(-c4c(c5ccccc5[o]5)c5ccc4)ccc33)c2[n]3-c2ccccc2)c2)c2c2c1cccc2 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2)cc(c3cc(-c4c(c5ccccc5[o]5)c5ccc4)ccc33)c2[n]3-c2ccccc2)c2)c2c2c1cccc2 DGIZBBUUPCJEGQ-UHFFFAOYSA-N 0.000 description 1
- WJITVZXLLFSBOU-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2)cc(c3cc(-c4c(c5ccccc5[s]5)c5ccc4)ccc33)c2[n]3-c2ccccc2)c2)c2c2c1cccc2 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2)cc(c3cc(-c4c(c5ccccc5[s]5)c5ccc4)ccc33)c2[n]3-c2ccccc2)c2)c2c2c1cccc2 WJITVZXLLFSBOU-UHFFFAOYSA-N 0.000 description 1
- SZAFXJGGOFYALN-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c(cc5)cc6c5c5ccccc5[o]6)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c(cc5)cc6c5c5ccccc5[o]6)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 SZAFXJGGOFYALN-UHFFFAOYSA-N 0.000 description 1
- KEOVSOBLTFDBII-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c(cc5)cc6c5c5ccccc5[s]6)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c(cc5)cc6c5c5ccccc5[s]6)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 KEOVSOBLTFDBII-UHFFFAOYSA-N 0.000 description 1
- SZMGYNYEUDVHAL-UHFFFAOYSA-N c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c5c6[s]c7ccccc7c6ccc5)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 Chemical compound c(cc1)ccc1-c(cc1)ccc1-[n]1c(ccc(-c(cc2c3c4ccc(-c5c6[s]c7ccccc7c6ccc5)c3)ccc2[n]4-c2ccccc2)c2)c2c2ccccc12 SZMGYNYEUDVHAL-UHFFFAOYSA-N 0.000 description 1
- RAJMZZYNYLWLHI-UHFFFAOYSA-N c(cc1)ccc1-c(cc12)c(cccc3)c3c1c(c(cccc1)c1c(-c(cc1)ccc1-c1cccc3c1ccc(-c(cc14)c(cccc5)c5c1c1c(cccc5)c5ccc1[n]4-c1cccc(-c4nc(-c5ccccc5)nc(-c5ccccc5)n4)c1)c3)c1)c1[n]2-c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c(cc12)c(cccc3)c3c1c(c(cccc1)c1c(-c(cc1)ccc1-c1cccc3c1ccc(-c(cc14)c(cccc5)c5c1c1c(cccc5)c5ccc1[n]4-c1cccc(-c4nc(-c5ccccc5)nc(-c5ccccc5)n4)c1)c3)c1)c1[n]2-c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccccc2)n1 RAJMZZYNYLWLHI-UHFFFAOYSA-N 0.000 description 1
- VMNKQDBWFNCWOB-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)cc(-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)c1 Chemical compound c(cc1)ccc1-c1cc(-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)cc(-c2nc(-c3ccccc3)nc(-c3ccccc3)n2)c1 VMNKQDBWFNCWOB-UHFFFAOYSA-N 0.000 description 1
- KXXSGPNWJJAKAP-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)cc(-c2ccc(c(cccc3)c3[o]3)c3c2)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)cc(-c2ccc(c(cccc3)c3[o]3)c3c2)n1 KXXSGPNWJJAKAP-UHFFFAOYSA-N 0.000 description 1
- JMXRCPIYMVELCU-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2c3[o]c4ccccc4c3ccc2)c1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2c3[o]c4ccccc4c3ccc2)c1 JMXRCPIYMVELCU-UHFFFAOYSA-N 0.000 description 1
- ZIIKJBXBDTWFFV-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2c3[s]c(cccc4)c4c3ccc2)c1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2c3[s]c(cccc4)c4c3ccc2)c1 ZIIKJBXBDTWFFV-UHFFFAOYSA-N 0.000 description 1
- GYJODXQGUQRZLI-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)cc(-c(cc2)cc3c2c(cccc2)c2[s]3)n1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)cc(-c(cc2)cc3c2c(cccc2)c2[s]3)n1 GYJODXQGUQRZLI-UHFFFAOYSA-N 0.000 description 1
- YMYYWEVQDREDMG-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c(cc2)cc3c2c2ccccc2[s]3)c1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c(cc2)cc3c2c2ccccc2[s]3)c1 YMYYWEVQDREDMG-UHFFFAOYSA-N 0.000 description 1
- BETRJRXTRMBONA-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc(c3ccccc3[o]3)c3c2)c1 Chemical compound c(cc1)ccc1-c1cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc(c3ccccc3[o]3)c3c2)c1 BETRJRXTRMBONA-UHFFFAOYSA-N 0.000 description 1
- KRAMKPMZYCWWAN-UHFFFAOYSA-N c(cc1)ccc1-c1cc(-c2c(cccc3-[n](c4c5c(cccc6)c6cc4)c4c5c5ccccc5cc4)c3ccc2)cc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1cc(-c2c(cccc3-[n](c4c5c(cccc6)c6cc4)c4c5c5ccccc5cc4)c3ccc2)cc(-c2ccccc2)n1 KRAMKPMZYCWWAN-UHFFFAOYSA-N 0.000 description 1
- KUYLQWZIDOVFDJ-UHFFFAOYSA-N c(cc1)ccc1-c1ccc(c2cc(-c(cc3)cc(c(c4c5)ccc5-c5ccccc5)c3[n]4-c3ccccc3)ccc2[n]2-c3ccccc3)c2c1 Chemical compound c(cc1)ccc1-c1ccc(c2cc(-c(cc3)cc(c(c4c5)ccc5-c5ccccc5)c3[n]4-c3ccccc3)ccc2[n]2-c3ccccc3)c2c1 KUYLQWZIDOVFDJ-UHFFFAOYSA-N 0.000 description 1
- FKFCRZIUBVZWDS-UHFFFAOYSA-N c(cc1)ccc1-c1cccc(C2NC(c3ccccc3)=NC(c(cc3)ccc3-[n]3c4ccccc4c4c3cccc4)N2)c1 Chemical compound c(cc1)ccc1-c1cccc(C2NC(c3ccccc3)=NC(c(cc3)ccc3-[n]3c4ccccc4c4c3cccc4)N2)c1 FKFCRZIUBVZWDS-UHFFFAOYSA-N 0.000 description 1
- YWCCNNUUKLXTJI-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(c2c3cccc2)ccc3-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2cccc3c2cccc3)n1 Chemical compound c(cc1)ccc1-c1nc(-c(c2c3cccc2)ccc3-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2cccc3c2cccc3)n1 YWCCNNUUKLXTJI-UHFFFAOYSA-N 0.000 description 1
- JLGGBHUOSTWAOP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(c2c3cccc2)ccc3-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(c2c3cccc2)ccc3-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2ccccc2)n1 JLGGBHUOSTWAOP-UHFFFAOYSA-N 0.000 description 1
- ZNIBHPQKGYDMRO-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c(cc2)cc3c2c(cccc2)c2[s]3)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c(cc2)cc3c2c(cccc2)c2[s]3)n1 ZNIBHPQKGYDMRO-UHFFFAOYSA-N 0.000 description 1
- CJLJFIVVSCIDCP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2ccc(c3ccccc3[o]3)c3c2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2ccc(c3ccccc3[o]3)c3c2)n1 CJLJFIVVSCIDCP-UHFFFAOYSA-N 0.000 description 1
- OFXHGYKDPQGSIP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)nc(-c2ccccc2)n1 OFXHGYKDPQGSIP-UHFFFAOYSA-N 0.000 description 1
- GOLSSBUFSKPRAK-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c(cc2c3c4cccc3)ccc2[n]4-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c(cc2c3c4cccc3)ccc2[n]4-c2ccccc2)n1 GOLSSBUFSKPRAK-UHFFFAOYSA-N 0.000 description 1
- VXFGRVXHRBYDRD-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2c3[o]c(cccc4)c4c3ccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2c3[o]c(cccc4)c4c3ccc2)n1 VXFGRVXHRBYDRD-UHFFFAOYSA-N 0.000 description 1
- KEZYYJSZBVDGQA-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2ccc3[o]c(cccc4)c4c3c2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2ccc3[o]c(cccc4)c4c3c2)n1 KEZYYJSZBVDGQA-UHFFFAOYSA-N 0.000 description 1
- WVGPMWONXXOZJD-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2cccc3c2cccc3)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c(cccc4)c4cc2)c2c3c3ccccc3cc2)nc(-c2cccc3c2cccc3)n1 WVGPMWONXXOZJD-UHFFFAOYSA-N 0.000 description 1
- LORPEPXAVHEAER-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)nc(-c2c3[s]c(cccc4)c4c3ccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)nc(-c2c3[s]c(cccc4)c4c3ccc2)n1 LORPEPXAVHEAER-UHFFFAOYSA-N 0.000 description 1
- SKUWLTAMTLLRRI-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc3[s]c(cccc4)c4c3c2)n1 Chemical compound c(cc1)ccc1-c1nc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc3[s]c(cccc4)c4c3c2)n1 SKUWLTAMTLLRRI-UHFFFAOYSA-N 0.000 description 1
- SZELSXHDKBMTLH-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2c3[o]c(cccc4)c4c3ccc2)cc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2c3[o]c(cccc4)c4c3ccc2)cc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)c1 SZELSXHDKBMTLH-UHFFFAOYSA-N 0.000 description 1
- PRXMULOSAVTAED-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2c3[s]c4ccccc4c3ccc2)cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2c3[s]c4ccccc4c3ccc2)cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)c1 PRXMULOSAVTAED-UHFFFAOYSA-N 0.000 description 1
- VLWJOYMRWYLDBP-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2cccc(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)nc(-c(cc2)cc3c2[o]c2ccccc32)n1 Chemical compound c(cc1)ccc1-c1nc(-c2cccc(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)nc(-c(cc2)cc3c2[o]c2ccccc32)n1 VLWJOYMRWYLDBP-UHFFFAOYSA-N 0.000 description 1
- QMBHCRZACCTWKW-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2cccc(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)nc(-c(cc2)cc3c2[s]c2c3cccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2cccc(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)nc(-c(cc2)cc3c2[s]c2c3cccc2)n1 QMBHCRZACCTWKW-UHFFFAOYSA-N 0.000 description 1
- ULHZKZZPOUJHEX-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2cccc(-[n]3c4ccc(cccc5)c5c4c4c3ccc3c4cccc3)c2)nc(-c(cc2)cc(c3ccccc33)c2[n]3-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2cccc(-[n]3c4ccc(cccc5)c5c4c4c3ccc3c4cccc3)c2)nc(-c(cc2)cc(c3ccccc33)c2[n]3-c2ccccc2)n1 ULHZKZZPOUJHEX-UHFFFAOYSA-N 0.000 description 1
- BSHLYDKZOPBTJI-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)cc(-c(cc2)c(cccc3)c3c2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)cc(-c(cc2)c(cccc3)c3c2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)c1 BSHLYDKZOPBTJI-UHFFFAOYSA-N 0.000 description 1
- WRUXHTZFUDIWDV-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)c1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)cc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)c1 WRUXHTZFUDIWDV-UHFFFAOYSA-N 0.000 description 1
- KPCONWBPCJIOLH-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n](c(cccc2)c2c2ccc3c4ccccc44)c2c3[n]4-c2ccccc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c(cc2)ccc2-[n](c(cccc2)c2c2ccc3c4ccccc44)c2c3[n]4-c2ccccc2)n1 KPCONWBPCJIOLH-UHFFFAOYSA-N 0.000 description 1
- JAOJIMCSVSIZNI-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2cc(cccc3)c3c(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2cc(cccc3)c3c(-[n](c3c4c(cccc5)c5cc3)c3c4c(cccc4)c4cc3)c2)n1 JAOJIMCSVSIZNI-UHFFFAOYSA-N 0.000 description 1
- VFEWOFQGOPHBKR-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2cccc3c2cccc3-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2cccc3c2cccc3-[n](c2c3c(cccc4)c4cc2)c2c3c(cccc3)c3cc2)n1 VFEWOFQGOPHBKR-UHFFFAOYSA-N 0.000 description 1
- JORQRKGCWTXLCM-UHFFFAOYSA-N c(cc1c2c3)ccc1[o]c2ccc3-c1nc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)nc(-c(cc2)cc3c2[s]c2c3cccc2)n1 Chemical compound c(cc1c2c3)ccc1[o]c2ccc3-c1nc(-c(cc2)ccc2-[n](c2c3c4ccccc4cc2)c2c3c3ccccc3cc2)nc(-c(cc2)cc3c2[s]c2c3cccc2)n1 JORQRKGCWTXLCM-UHFFFAOYSA-N 0.000 description 1
- LIOYFWWVHXJYDA-UHFFFAOYSA-N c(cc1c2c3)ccc1[s]c2ccc3-c1nc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc3[s]c(cccc4)c4c3c2)n1 Chemical compound c(cc1c2c3)ccc1[s]c2ccc3-c1nc(-c(cc2)ccc2-[n]2c3ccc(cccc4)c4c3c3c2ccc2c3cccc2)nc(-c2ccc3[s]c(cccc4)c4c3c2)n1 LIOYFWWVHXJYDA-UHFFFAOYSA-N 0.000 description 1
- MVANGRNPZMLKHW-UHFFFAOYSA-N c1ccc(C2(c(cc(c(c(cc3)c4cc3-c3ccccc3)c3)[n]4[N+]4=C(c5ccccc5)N=C4c4ccccc4)c3-c3c2cccc3)c2ccccc2)cc1 Chemical compound c1ccc(C2(c(cc(c(c(cc3)c4cc3-c3ccccc3)c3)[n]4[N+]4=C(c5ccccc5)N=C4c4ccccc4)c3-c3c2cccc3)c2ccccc2)cc1 MVANGRNPZMLKHW-UHFFFAOYSA-N 0.000 description 1
- FNAOWIIRVHABLD-UHFFFAOYSA-N c1ccc(C2N=C(c3ccccc3)N=C(c3cccc(-[n]4c5ccccc5c5c4cccc5)c3)N2)cc1 Chemical compound c1ccc(C2N=C(c3ccccc3)N=C(c3cccc(-[n]4c5ccccc5c5c4cccc5)c3)N2)cc1 FNAOWIIRVHABLD-UHFFFAOYSA-N 0.000 description 1
- IJNWXRXRYWZXEQ-UHFFFAOYSA-N c1ccc(C2NC(c3ccccc3)=NC(c3cccc(-[n]4c5cc(-c6cc(-c7c8[o]c(cccc9)c9c8ccc7)ccc6)ccc5c5c4cccc5)c3)N2)cc1 Chemical compound c1ccc(C2NC(c3ccccc3)=NC(c3cccc(-[n]4c5cc(-c6cc(-c7c8[o]c(cccc9)c9c8ccc7)ccc6)ccc5c5c4cccc5)c3)N2)cc1 IJNWXRXRYWZXEQ-UHFFFAOYSA-N 0.000 description 1
- JEHWTKPIZSRUMH-UHFFFAOYSA-O c1ccc(C2[NH+]=C(c3cccc(-[n]4c5cc(-c6c7[o]c8ccccc8c7ccc6)ccc5c(cc5)c4cc5-c4ccccc4)c3)N=C(c3ccccc3)N2)cc1 Chemical compound c1ccc(C2[NH+]=C(c3cccc(-[n]4c5cc(-c6c7[o]c8ccccc8c7ccc6)ccc5c(cc5)c4cc5-c4ccccc4)c3)N=C(c3ccccc3)N2)cc1 JEHWTKPIZSRUMH-UHFFFAOYSA-O 0.000 description 1
- IQHLFSJRTMRGFS-UHFFFAOYSA-O c1ccc(C2[NH2+]C(c3ccccc3)=NC(c3cccc(-[n]4c(ccc([Si](c5ccccc5)(c5ccccc5)c5ccccc5)c5)c5c5c4cccc5)c3)N2)cc1 Chemical compound c1ccc(C2[NH2+]C(c3ccccc3)=NC(c3cccc(-[n]4c(ccc([Si](c5ccccc5)(c5ccccc5)c5ccccc5)c5)c5c5c4cccc5)c3)N2)cc1 IQHLFSJRTMRGFS-UHFFFAOYSA-O 0.000 description 1
- IMKPFUJXAAMVSI-UHFFFAOYSA-O c1ccc(C2[NH2+]C(c3ccccc3)N=C(c3cccc(-[n](c(cccc4)c4c4c5)c4ccc5-c4ccccc4)c3)N2)cc1 Chemical compound c1ccc(C2[NH2+]C(c3ccccc3)N=C(c3cccc(-[n](c(cccc4)c4c4c5)c4ccc5-c4ccccc4)c3)N2)cc1 IMKPFUJXAAMVSI-UHFFFAOYSA-O 0.000 description 1
- KVQKYWXDJVPLHP-UHFFFAOYSA-N c1ccc(c(c2c3ccc4ccccc24)c(cc2)[n]3-c(cc3)ccc3-c3nc(-c4c(cccc5)c5ccc4)nc(-c4cccc5ccccc45)n3)c2c1 Chemical compound c1ccc(c(c2c3ccc4ccccc24)c(cc2)[n]3-c(cc3)ccc3-c3nc(-c4c(cccc5)c5ccc4)nc(-c4cccc5ccccc45)n3)c2c1 KVQKYWXDJVPLHP-UHFFFAOYSA-N 0.000 description 1
- WUKWITHWXAAZEY-UHFFFAOYSA-L calcium difluoride Chemical compound [F-].[F-].[Ca+2] WUKWITHWXAAZEY-UHFFFAOYSA-L 0.000 description 1
- 229910001634 calcium fluoride Inorganic materials 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 125000005105 dialkylarylsilyl group Chemical group 0.000 description 1
- IYYZUPMFVPLQIF-ALWQSETLSA-N dibenzothiophene Chemical group C1=CC=CC=2[34S]C3=C(C=21)C=CC=C3 IYYZUPMFVPLQIF-ALWQSETLSA-N 0.000 description 1
- 125000005509 dibenzothiophenyl group Chemical group 0.000 description 1
- AKUNKIJLSDQFLS-UHFFFAOYSA-M dicesium;hydroxide Chemical compound [OH-].[Cs+].[Cs+] AKUNKIJLSDQFLS-UHFFFAOYSA-M 0.000 description 1
- XUCJHNOBJLKZNU-UHFFFAOYSA-M dilithium;hydroxide Chemical compound [Li+].[Li+].[OH-] XUCJHNOBJLKZNU-UHFFFAOYSA-M 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- QPJFIVIVOOQUKD-UHFFFAOYSA-N dipyrazino[2,3-f:2,3-h]quinoxaline Chemical group C1=CN=C2C3=NC=CN=C3C3=NC=CN=C3C2=N1 QPJFIVIVOOQUKD-UHFFFAOYSA-N 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 125000003838 furazanyl group Chemical group 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 230000005283 ground state Effects 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 238000007733 ion plating Methods 0.000 description 1
- 125000001977 isobenzofuranyl group Chemical group C=1(OC=C2C=CC=CC12)* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 150000007517 lewis acids Chemical class 0.000 description 1
- PQXKHYXIUOZZFA-UHFFFAOYSA-M lithium fluoride Inorganic materials [Li+].[F-] PQXKHYXIUOZZFA-UHFFFAOYSA-M 0.000 description 1
- 229910001635 magnesium fluoride Inorganic materials 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- MESMXXUBQDBBSR-UHFFFAOYSA-N n,9-diphenyl-n-[4-[4-(n-(9-phenylcarbazol-3-yl)anilino)phenyl]phenyl]carbazol-3-amine Chemical compound C1=CC=CC=C1N(C=1C=C2C3=CC=CC=C3N(C=3C=CC=CC=3)C2=CC=1)C1=CC=C(C=2C=CC(=CC=2)N(C=2C=CC=CC=2)C=2C=C3C4=CC=CC=C4N(C=4C=CC=CC=4)C3=CC=2)C=C1 MESMXXUBQDBBSR-UHFFFAOYSA-N 0.000 description 1
- LCPYTQFVQRPZCV-UHFFFAOYSA-N n-[4-(4-carbazol-9-ylphenyl)phenyl]-4-phenyl-n-(4-phenylphenyl)aniline Chemical compound C1=CC=CC=C1C1=CC=C(N(C=2C=CC(=CC=2)C=2C=CC=CC=2)C=2C=CC(=CC=2)C=2C=CC(=CC=2)N2C3=CC=CC=C3C3=CC=CC=C32)C=C1 LCPYTQFVQRPZCV-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001715 oxadiazolyl group Chemical group 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 125000001644 phenoxazinyl group Chemical group C1(=CC=CC=2OC3=CC=CC=C3NC12)* 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000004528 spin coating Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 125000005017 substituted alkenyl group Chemical group 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- 125000004426 substituted alkynyl group Chemical group 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- RAOIDOHSFRTOEL-UHFFFAOYSA-N tetrahydrothiophene Chemical compound C1CCSC1 RAOIDOHSFRTOEL-UHFFFAOYSA-N 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 125000004665 trialkylsilyl group Chemical group 0.000 description 1
- UBOXGVDOUJQMTN-UHFFFAOYSA-N trichloroethylene Natural products ClCC(Cl)Cl UBOXGVDOUJQMTN-UHFFFAOYSA-N 0.000 description 1
- 238000001771 vacuum deposition Methods 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1033—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1037—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with sulfur
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1059—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1092—Heterocyclic compounds characterised by ligands containing sulfur as the only heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/10—Triplet emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/90—Multiple hosts in the emissive layer
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/342—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising iridium
Definitions
- the present invention relates to a multi-component host material and an organic electroluminescent device comprising the same.
- An electroluminescent (EL) device is a self-light-emitting device with the advantage of providing a wider viewing angle, a greater contrast ratio, and a faster response time.
- An organic EL device was first developed by Eastman Kodak, by using small aromatic diamine molecules and aluminum complexes as materials for forming a light-emitting layer (see Appl. Phys. Lett. 51, 913, 1987).
- the organic EL device changes electric energy into light by the injection of a charge into an organic light-emitting material and commonly comprises an anode, a cathode, and an organic layer formed between the two electrodes.
- the organic layer of the organic EL device may be composed of a hole injection layer (HIL), a hole transport layer (HTL), an electron blocking layer (EBL), a light-emitting layer (EML) (containing host and dopant materials), an electron buffer layer, a hole blocking layer (HBL), an electron transport layer (ETL), an electron injection layer (EIL), etc.; the materials used in the organic layer can be classified into a hole injection material, a hole transport material, an electron blocking material, a light-emitting material, an electron buffer material, a hole blocking material, an electron transport material, an electron injection material, etc., depending on functions.
- the organic EL device In the organic EL device, holes from an anode and electrons from a cathode are injected into a light-emitting layer by the injection of a charge, and an exciton having high energy is produced by the recombination of holes and electrons.
- the organic light-emitting compound moves into an excited state by the energy and emits light which changes from energy when the organic light-emitting compound returns to the ground state from the excited state.
- the most important factor determining luminescent efficiency in an organic EL device is the light-emitting material.
- the light-emitting material is required to have the following features: high quantum efficiency, high movement degree of an electron and a hole, formability of a uniform layer, and stability.
- the light-emitting material is classified into blue light-emitting materials, green light-emitting materials, and red light-emitting materials according to the light-emitting color, and further includes yellow light-emitting materials or orange light-emitting materials.
- the light-emitting material is classified into a host material and a dopant material in the functional aspect. Recently, an urgent task is the development of an organic EL device having high efficacy and long operating lifespan.
- a host material should have high purity and suitable molecular weight in order to be deposited under vacuum. Furthermore, a host material is required to have high glass transition temperature and pyrolysis temperature to guarantee thermal stability, high electrochemical stability to provide long lifespan, easy formability of an amorphous thin film, good adhesion with adjacent layers, and no movement between layers.
- a mixed system of a dopant/host material can be used as a light-emitting material to improve color purity, luminescent efficiency, and stability.
- the device having the most excellent EL properties comprises the light-emitting layer, wherein a dopant is doped onto a host. If the dopant/host material system is used, the selection of the host material is important since the host material greatly influences on efficiency and performance of a light-emitting device.
- WO 2013/168688 A1 Japanese Patent No. 3139321, Korean Patent No. 10-1170666, Korean Patent Application Laying-open No. 10-2012-0013173, and WO 2013/112557 A1 disclose organic EL devices comprising a dopant/host material system.
- the above literature use one host component having a carbazole-carbazole skeleton or exclude a host having a cabazole skeleton from second and third hosts.
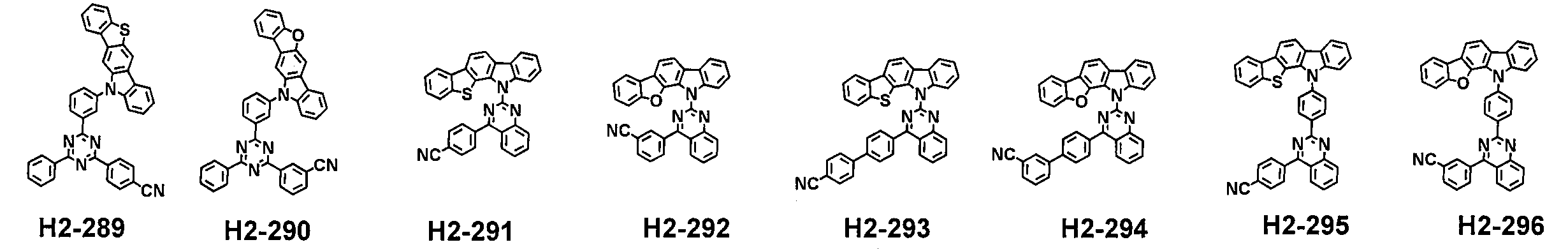
- an organic EL device using a multi-component host compounds having a specific bicarbazole derivative which contains an aryl group and a specific carbazole derivative which includes a nitrogen-containing heteroaryl group has high efficiency and long lifespan, compared with using one component host compound in a light-emitting layer.
- the object of the present invention is to provide an organic EL device having high efficiency and long lifespan.
- an organic electroluminescent device comprising at least one light-emitting layer between an anode and a cathode, wherein the light-emitting layer comprises a host and a phosphorescent dopant; the host consists of multi-component host compounds; at least a first host compound of the multi-component host compounds is represented by the following formula 1 which is a specific bicarbazole derivative containing an aryl group, and a second host compound is represented by the following formula 2 which is a specific carbazole derivative including a nitrogen-containing heteroaryl group:
- a 1 and A 2 each independently represent a substituted or unsubstituted (C6-C30)aryl group
- X 1 to X 16 each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsily
- Ma represents a substituted or unsubstituted nitrogen-containing 5- to 30-membered heteroaryl group
- La represents a single bond, or a substituted or unsubstituted (C6-C30)arylene group
- Xa to Xh each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsily
- the fused aromatic or heteroaromatic ring is selected from the group consisting of benzene, indole, indene, benzofuran and benzothiophene, which may be further substituted with a (C1-C10)alkyl group or a (C6-C15)aryl group; and
- the organic EL device having high efficiency and long lifespan is provided and the production of a display device or a lighting device is possible by using the organic EL device.
- the compound of formula 1 is represented by the following formula 3, 4, 5, or 6:
- a 1 , A 2 and X 1 to X 16 are as defined in formula 1.
- a 1 and A 2 each independently represent a substituted or unsubstituted (C6-C30)aryl group; preferably, a substituted or unsubstituted (C6-C18)aryl group; more preferably, a (C6-C18)aryl group which is unsubstituted or substituted with a (C1-C6)alkyl group, a (C6-C12)aryl group, or a tri(C6-C12)arylsilyl group ; and even more preferably, phenyl, biphenyl, terphenyl, naphthyl, fluorenyl, phenanthrenyl, anthracenyl, indenyl, triphenylenyl, pyrenyl, tetracenyl, perylenyl, chrysenyl, naphthacenyl, or fluoranthenyl.
- X 1 to X 16 each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsily
- the compound of formula 2 is represented by the following formula 7, 8, or 9:
- V and W each independently represent a single bond, NR 15 , CR 16 R 17 , S, or O, provided that both V and W neither represent a single bond nor represent NR 15 ;
- a 2 represents a substituted or unsubstituted (C6-C30)aryl group and may be bonded to Xn or Xo;
- L 3 and L 4 each independently represent a single bond, or a substituted or unsubstituted (C6-C60)arylene group;
- Xi represents hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a
- Xj to Xz each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, -NR 5 R 6 , or -SiR 7 R 8 R 9 ; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or
- Ma, La, Xa, Xb, and Xe to Xh are as defined in formula 2;
- R 5 to R 9 each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero
- R 16 and R 17 each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group; and
- R 15 represents hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group; preferably, a substituted or unsubstituted (C6-C30)aryl group; and more preferably, a substituted or unsubstituted phenyl group, an unsubstituted biphenyl group, an unsubstituted
- La represents a single bond, or a substituted or unsubstituted (C6-C30)arylene group; preferably, a single bond, or a substituted or unsubstituted (C6-C12)arylene group; and more preferably, a single bond, a (C6-C12)arylene group which is unsubstituted or substituted with a tri(C6-C10)arylsilyl group or a (C6-C12)aryl group.
- La represents a single bond, or is represented by one selected from the following formulas 10 to 19:
- Xi to Xp each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsily
- Ma represents a substituted or unsubstituted nitrogen-containing 5- to 11-membered heteroaryl group; preferably, a substituted or unsubstituted nitrogen-containing 6- to 10-membered heteroaryl group; and more preferably, a nitrogen-containing 6- to 10-membered heteroaryl group which is substituted with a substituents(s) selected from the group consisting of an unsubstituted (C6-C18)aryl group, a (C6-C12)aryl group substituted with a cyano group, a (C6-C12)aryl group substituted with a (C1-C6)alkyl group, a (C6-C12)aryl group substituted with a tri(C6-C12)arylsilyl group, and a 6- to 15-membered heteroaryl group.
- a substituents(s) selected from the group consisting of an unsubstituted (C6-C18)aryl group, a (C6-C12
- Ma represents a monocyclic-based heteroaryl group selected from the group consisting of pyrrolyl, imidazolyl, pyrazolyl, triazinyl, tetrazinyl, triazolyl, tetrazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, etc., or a fused ring-based heteroaryl group selected from the group consisting of benzoimidazolyl, isoindolyl, indolyl, indazolyl, benzothiadiazolyl, quinolyl, isoquinolyl, cinnolinyl, quinazolinyl, naphthyridinyl, quinoxalinyl, carbazolyl, phenanthridinyl, etc.; preferably, triazinyl, pyrimidinyl, pyridyl, quinolyl, isoquinolyl, quinazolin
- (C1-C30)alkyl(ene) is meant to be a linear or branched alkyl(ene) having 1 to 30 carbon atoms, in which the number of carbon atoms is preferably 1 to 20, more preferably 1 to 10, and includes methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, etc.
- (C2-C30)alkenyl is meant to be a linear or branched alkenyl having 2 to 30 carbon atoms, in which the number of carbon atoms is preferably 2 to 20, more preferably 2 to 10, and includes vinyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 2-methylbut-2-enyl, etc.
- (C2-C30)alkynyl is a linear or branched alkynyl having 2 to 30 carbon atoms, in which the number of carbon atoms is preferably 2 to 20, more preferably 2 to 10, and includes ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methylpent-2-ynyl, etc.
- (C3-C30)cycloalkyl is a mono- or polycyclic hydrocarbon having 3 to 30 carbon atoms, in which the number of carbon atoms is preferably 3 to 20, more preferably 3 to 7, and includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, etc.
- (C6-C30)aryl(ene) is a monocyclic or fused ring derived from an aromatic hydrocarbon having 6 to 30 carbon atoms, in which the number of carbon atoms is preferably 6 to 20, more preferably 6 to 15, and includes phenyl, biphenyl, terphenyl, naphthyl, fluorenyl, phenanthrenyl, anthracenyl, indenyl, triphenylenyl, pyrenyl, tetracenyl, perylenyl, chrysenyl, naphthacenyl, fluoranthenyl, etc.
- “Nitrogen-containing 5- to 30-membered heteroaryl(ene) group” is an aryl(ene) group having at least one heteroatom N and 5 to 30 ring backbone atoms. 5 to 20 ring backbone atoms and 1 to 4 heteroatom are preferable, and 5 to 15 ring backbone atoms are more preferable.
- substituted in the expression “substituted or unsubstituted” means that a hydrogen atom in a certain functional group is replaced with another atom or group, i.e., a substituent.
- Substituents of the substituted alkyl(ene) group, the substituted alkenyl group, the substituted alkynyl group, the substituted cycloalkyl group, the substituted aryl(ene) group, the substituted heteroaryl(ene) group, the substituted trialkylsilyl group, the substituted triarylsilyl group, the substituted dialkylarylsilyl group, the substituted mono- or di-arylamino group, or the substituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring are each independently at least one selected from the group consisting of deuterium; a halogen; a cyano group; a carboxyl group; a nitro group; a hydroxyl
- the substituents are each independently at least one selected from the group consisting of a (C1-C6)alkyl group; a 5- to 15-membered heteroaryl group; a (C6-C18)aryl group which is unsubstituted or substituted with a cyano group or a tri(C6-C12)arylsilyl group; a tri(C6-C12)arylsilyl group; and a (C1-C6)alkyl(C6-C12)aryl group.
- the compound of formula 1 as a first host compound may be selected from the group consisting of following compounds, but is not limited thereto:
- the compound of formula 2 as a second host compound may be selected from the group consisting of following compounds, but is not limited thereto:
- the organic EL device may comprise an anode, a cathode, and at least one organic layer between the two electrodes, wherein the organic layer comprises a light-emitting layer, the light-emitting layer comprises a host and a phosphorescent dopant; the host consists of multi-component host compounds; at least a first host compound of the multi-component host compounds is represented by formula 1 which is a specific bicarbazole derivative containing an aryl group, and a second host compound is represented by formula 2 which is a specific carbazole derivative including a nitrogen-containing heteroaryl group.
- the light-emitting layer means a layer emitting light and may be a single layer or multi-layers consisting of two or more layers.
- the doping concentration of dopant compounds to host compounds in the light-emitting layer is preferably less than 20 wt%.
- the dopants included in the organic EL device of the present invention are preferably one or more phosphorescent dopants.
- the phosphorescent dopant material applied to the organic electroluminescent device of the present invention is not specifically limited, but preferably may be selected from complex compounds of iridium (Ir), osmium (Os), copper (Cu), and platinum (Pt), more preferably ortho metallated complex compounds of iridium (Ir), osmium (Os), copper (Cu), and platinum (Pt), and even more preferably ortho metallated iridium complex compounds.
- the phosphorescent dopants may be selected from the group consisting of the compounds represented by the following formulae 101 to 103:
- L is selected from the following structures:
- R 100 represents hydrogen, or a substituted or unsubstituted (C1-C30)alkyl group
- R 101 to R 109 and R 111 to R 123 each independently represent hydrogen, deuterium, a halogen; a (C1-C30)alkyl group unsubstituted or substituted with halogen(s); a cyano group, a substituted or unsubstituted (C1-C30)alkoxy group, a substituted or unsubstituted (C6-C30)aryl group, or a substituted or unsubstituted (C3-C30)cycloalkyl group;
- R 120 to R 123 are linked to an adjacent substituent(s) to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring, for example, quinoline;
- R 124 to R 127 each independently represent hydrogen, deuterium, a halogen, a substitute
- the phosphorescent dopant material includes the following:
- the organic EL device of the present invention may further include at least one compound selected from the group consisting of arylamine-based compounds and styrylarylamine-based compounds in the organic layer.
- the organic layer may further comprise at least one metal selected from the group consisting of metals of Group 1, metals of Group 2, transition metals of the 4 th period, transition metals of the 5 th period, lanthanides, and organic metals of d-transition elements of the Periodic Table, or at least one complex compound comprising the metal.
- a surface layer selected from a chalcogenide layer, a metal halide layer and a metal oxide layer may be placed on an inner surface(s) of one or both electrode(s).
- a chalcogenide (including oxides) layer of silicon or aluminum is placed on an anode surface of a light-emitting medium layer, and a metal halide layer or metal oxide layer is placed on a cathode surface of an electroluminescent medium layer.
- the surface layer provides operating stability for the organic electroluminescent device.
- the chalcogenide includes SiO X (1 ⁇ X ⁇ 2), AlO X (1 ⁇ X ⁇ 1.5), SiON, SiAlON, etc.;
- the metal halide includes LiF, MgF 2 , CaF 2 , a rare earth metal fluoride, etc.; and the metal oxide includes Cs 2 O, Li 2 O, MgO, SrO, BaO, CaO, etc.
- a hole injection layer, a hole transport layer, an electron blocking layer, or their combinations can be used between an anode and a light-emitting layer.
- the hole injection layer may be multi-layers in order to lower a hole injection barrier (or hole injection voltage) from an anode to a hole transport layer or an electron blocking layer, wherein each of the multi-layers simultaneously uses two compounds.
- the hole transport layer or the electron blocking layer may also be multi-layers.
- An electron buffer layer, a hole blocking layer, an electron transport layer, an electron injection layer, or their combinations can be used between a light-emitting layer and a cathode.
- the electron buffer layer may be multi-layers in order to control the injection of an electron and improve interface properties between the light-emitting layer and the electron injection layer, wherein each of the multi-layers simultaneously uses two compounds.
- the hole blocking layer or the electron transport layer may also be multi-layers, wherein each of the multi-layers may use a multi-component of compounds.
- a mixed region of an electron transport compound and a reductive dopant, or a mixed region of a hole transport compound and an oxidative dopant may be placed on at least one surface of a pair of electrodes.
- the electron transport compound is reduced to an anion, and thus it becomes easier to inject and transport electrons from the mixed region to a light-emitting medium.
- the hole transport compound is oxidized to a cation, and thus it becomes easier to inject and transport holes from the mixed region to a light-emitting medium.
- the oxidative dopant includes various Lewis acids and acceptor compounds; and the reductive dopant includes alkali metals, alkali metal compounds, alkaline earth metals, rare-earth metals, and mixtures thereof.
- a reductive dopant layer may be employed as a charge-generating layer to prepare an organic electroluminescent device having two or more light-emitting layers and emitting white light.
- each layer constituting the organic electroluminescent device of the present invention dry film-forming methods, such as vacuum deposition, sputtering, plasma, ion plating methods, etc., or wet film-forming methods, such as spin coating, dip coating, flow coating methods, etc., can be used.
- dry film-forming methods such as vacuum deposition, sputtering, plasma, ion plating methods, etc.
- wet film-forming methods such as spin coating, dip coating, flow coating methods, etc.
- co-deposition or mixed-deposition may be used.
- a thin film is formed by dissolving or dispersing the material constituting each layer in suitable solvents, such as ethanol, chloroform, tetrahydrofuran, dioxane, etc.
- suitable solvents such as ethanol, chloroform, tetrahydrofuran, dioxane, etc.
- the solvents are not specifically limited as long as the material constituting each layer is soluble or dispersible in the solvents, which do not cause any problems in forming a layer.
- a display device or a light device can be produced by using the organic EL device of the present invention.
- Example 1-1 Production of an OLED device by co-deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device comprising the organic electroluminescent compound of the present invention was produced as follows: A transparent electrode indium tin oxide (ITO) thin film (10 ⁇ /sq) on a glass substrate for an OLED device (Samsung Corning, Republic of Korea) was subjected to an ultrasonic washing with trichloroethylene, acetone, ethanol, and distilled water, sequentially, and was then stored in isopropanol. Next, the ITO substrate was mounted on a substrate holder of a vacuum vapor depositing apparatus.
- ITO indium tin oxide
- N 4 ,N 4’ -diphenyl-N 4 ,N 4 ’-bis(9-phenyl-9H-carbazole-3-yl)-[1,1’-biphenyl]-4,4’-diamine as HI-1 was introduced into a cell of the vacuum vapor depositing apparatus, and the pressure in the chamber of the apparatus was then controlled to 10 -6 torr. Thereafter, an electric current was applied to the cell to evaporate the introduced material, thereby forming a hole injection layer 1 having a thickness of 80 nm on the ITO substrate.
- 1,4,5,8,9,12-hexaazatriphenylene hexacarbonitrile as HI-2 was then introduced into another cell of the vacuum vapor depositing apparatus, and an electric current was applied to the cell to evaporate the introduced material, thereby forming a hole injection layer 2 having a thickness of 5 nm on hole injection layer 1.
- N-([1,1’-biphenyl]-4-yl)-9,9-dimethyl-N-(4-(9-phenyl-9H-carbazole-3-yl)phenyl)-9H-fluorene-2-amine as HT-1 was introduced into one cell of the vacuum vapor depositing apparatus.
- compounds H1-1 and H2-2 as hosts were respectively introduced into two cells of the vacuum vapor depositing apparatus and compound D-96 as a dopant was introduced into another cell.
- the two host materials were evaporated at the same rates of 1:1, and the dopant was evaporated at a different rate and deposited in a doping amount of 3 wt%, based on the total weight of the host and dopant, to form a light-emitting layer having a thickness of 40 nm on the hole transport layer.
- the produced OLED device showed the driving voltage at a luminance of 1,000nit, luminescent efficiency, CIE color coordinate, and the lifespan taken to be reduced from 100% to 90% of the constant current at a luminance of 5,000nit as provided in Table 1 below.
- Comparative Example 1-1 Production of an OLED device by using only the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Example 1-1, except that only the second host compound was used as a host in a light-emitting layer.
- the luminescent properties of the OLED devices produced in Device Example 1-1 and Comparative Example 1-1 are provided in Table 1 below.
- Device Examples 2-1 to 2-13 Production of an OLED device by co -deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Example 1-1, except that hole injection layer 2 has a thickness of 3 nm, hole transport layer 1 has a thickness of 40 nm, hole transport layer 2 is not present, D-25 as a dopant was deposited in a doping amount on 15wt% in a light-emitting layer, the electron transport layer having a thickness of 35 nm was deposited via the evaporation rate of 4:6, the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 2-1 to 2-13 as provided in Table 2 below, and the lifespan taken to be reduced from 100% to 90% of the constant current at a luminance of 15,000nit as provided in Table 2 below.
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that hole injection layer 2 has a thickness of 3 nm, hole transport layer 1 has a thickness of 40 nm, hole transport layer 2 is not present, D-1 as a dopant was used in a light-emitting layer, the electron transport layer having a thickness of 35 nm was deposited via the evaporation rate of 4:6, the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 2-14 to 2-18 as provided in Table 2 below, and the lifespan taken to be reduced from 100% to 90% of the constant current at a luminance of 15,000nit as provided in Table 2 below.
- Device Examples 3-1 to 3-8 Production of an OLED device by co -deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that hole transport layer 1 has a thickness of 10 nm, hole transport layer 2 of HT-3 has a thickness of 30 nm, D-136 as a dopant was used in a light-emitting layer, and the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 3-1 to 3-8 as provided in Table 2 below.
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that hole transport layer 1 has a thickness of 10 nm, hole transport layer 2 of HT-3 has a thickness of 30 nm, D-164 as a dopant was used in a light-emitting layer, and the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Example 3-9 as provided in Table 2 below.
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that hole transport layer 1 has a thickness of 10 nm, hole transport layer 2 of HT-3 has a thickness of 30 nm, D-168 as a dopant was used in a light-emitting layer, and the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 3-10 to 3-12 as provided in Table 2 below.
- Example 3-13 Production of an OLED device by co-deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that hole transport layer 1 has a thickness of 10 nm, hole transport layer 2 of HT-3 has a thickness of 30 nm, D-180 as a dopant was used in a light-emitting layer, and the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Example 3-13 as provided in Table 2 below.
- Comparative Examples 2-1 to 2-3 Production of an OLED device by using only the first host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that the first host compound used as hosts in a light-emitting layer is based on Comparative Examples 2-1 to 2-3 as provided in Table 2 below.
- Comparative Examples 3-1 to 3-9 Production of an OLED device by using only the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 2-1 to 2-13, except that the second host compound used as hosts in a light-emitting layer is based on Comparative Examples 3-1 to 3-9 as provided in Table 2 below.
- Comparative Example 4-1 Production of an OLED device by using only the second host compound according to the present invention as a hos t
- An OLED device was produced in the same manner as in Device Examples 3-1 to 3-8, except that the second host compound used as hosts in a light-emitting layer is based on Comparative Example 4-1 as provided in Table 2 below.
- Device Examples 4-1 to 4-7 Production of an OLED device by co -deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Example 1-1, except that HT-4 was used as a hole transport layer 2, the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 4-1 to 4-7 as provided in Table 3 below, and the lifespan taken to be reduced from 100% to 95% of the constant current at a luminance of 5,000nit as provided in Table 3 below.
- Comparative Examples 5-1 and 5-2 Production of an OLED device by using only the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 4-1 to 4-7, except that the second host compound used as hosts in a light-emitting layer is based on Comparative Examples 5-1 and 5-2 as provided in Table 3 below.
- Device Examples 5-1 and 5-2 Production of an OLED device by co -deposition of the first host compound and the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 3-1 to 3-11, except that D-134 was used as a dopant in a light-emitting layer, the combinations of the first host compound and the second host compound used as hosts in a light-emitting layer are based on Device Examples 5-1 and 5-2 as provided in Table 4 below, and the lifespan taken to be reduced from 100% to 97% of the constant current at a luminance of 15,000nit as provided in Table 4 below.
- Comparative Examples 6-1 and 6-2 Production of an OLED device by using only the first host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 5-1 and 5-2, except that the first host compound used as hosts in a light-emitting layer is based on Comparative Examples 6-1 and 6-2 as provided in Table 4 below.
- Comparative Example 7-1 Production of an OLED device by using only the second host compound according to the present invention as a host
- An OLED device was produced in the same manner as in Device Examples 5-1 and 5-2, except that the second host compound used as hosts in a light-emitting layer is based on Comparative Example 7-1 as provided in Table 4 below.
- the organic electroluminescent device of the present invention provides longer lifespan compared with conventional devices by comprising a light-emitting layer containing a host and a phosphorescent dopant, wherein the host consists of multi-component host compounds, at least a first host compound of the multi-component host compounds has a specific bicarbazole derivative containing an aryl group, and a second host compound has a specific carbazole derivative including a nitrogen-containing heteroaryl group.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Organic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
Description
Claims (10)
- An organic electroluminescent device comprising at least one light-emitting layer between an anode and a cathode, wherein the light-emitting layer comprises a host and a phosphorescent dopant; the host consists of multi-component host compounds; at least a first host compound of the multi-component host compounds is represented by the following formula 1 which is a bicarbazole derivative containing an aryl group, and a second host compound is represented by the following formula 2 which is a carbazole derivative including a nitrogen-containing heteroaryl group:whereinA1 and A2 each independently represent a substituted or unsubstituted (C6-C30)aryl group;X1 to X16 each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a substituted or unsubstituted mono- or di-(C6-C30)arylamino group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur;Ma represents a substituted or unsubstituted nitrogen-containing 5- to 30-membered heteroaryl group;La represents a single bond, or a substituted or unsubstituted (C6-C30)arylene group;Xa to Xh each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a substituted or unsubstituted mono- or di-(C6-C30)arylamino group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur;the fused aromatic or heteroaromatic ring is selected from the group consisting of benzene, indole, indene, benzofuran and benzothiophene, which may be further substituted with a (C1-C10)alkyl group or a (C6-C15)aryl group; andthe heteroaryl group contains at least one hetero atom selected from B, N, O, S, P(=O), Si and P.
- The organic electroluminescent device according to claim 1, wherein the compound of formula 1 is represented by the following formula 3, 4, 5, or 6:whereinA1 and A2 each independently represent a substituted or unsubstituted (C6-C30)aryl group; andX1 to X16 each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a substituted or unsubstituted mono- or di-(C6-C30)arylamino group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur.
- The organic electroluminescent device according to claim 1, wherein the compound of formula 2 is represented by the following formula 7, 8, or 9:whereinV and W each independently represent a single bond, NR15, CR16R17, S, or O, provided that both V and W neither represent a single bond nor represent NR15;A2 represents a substituted or unsubstituted (C6-C30)aryl group and may be bonded to Xn or Xo;L3 and L4 each independently represent a single bond, or a substituted or unsubstituted (C6-C60)arylene group;Xi represents hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a substituted or unsubstituted mono- or di-(C6-C30)arylamino group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur;Xj to Xz each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, -NR5R6, or -SiR7R8R9; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur;Ma, La, Xa, Xb, and Xe to Xh are as defined in formula 2;R5 to R9 each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur;R16 and R17 each independently represent hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group; andR15 represents hydrogen, deuterium, a halogen, a cyano group, a carboxyl group, a nitro group, a hydroxyl group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C3-C30)cycloalkenyl group, a substituted or unsubstituted 3- to 7-membered heterocycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, or a substituted or unsubstituted 3- to 30-membered heteroaryl group.
- The organic electroluminescent device according to claim 1, wherein La in formula 2 represents a single bond, or is represented by one selected from the following formulas 10 to 19:whereinXi to Xp each independently represent hydrogen, deuterium, a halogen, a cyano group, a substituted or unsubstituted (C1-C30)alkyl group, a substituted or unsubstituted (C2-C30)alkenyl group, a substituted or unsubstituted (C2-C30)alkynyl group, a substituted or unsubstituted (C3-C30)cycloalkyl group, a substituted or unsubstituted (C6-C60)aryl group, a substituted or unsubstituted 3- to 30-membered heteroaryl group, a substituted or unsubstituted tri(C1-C30)alkylsilyl group, a substituted or unsubstituted tri(C6-C30)arylsilyl group, a substituted or unsubstituted di(C1-C30)alkyl(C6-C30)arylsilyl group, or a substituted or unsubstituted mono- or di-(C6-C30)arylamino group; or are linked between adjacent substituents to form a substituted or unsubstituted mono- or polycyclic, (C3-C30) alicyclic or aromatic ring whose carbon atom(s) ring may be replaced with at least one hetero atom selected from nitrogen, oxygen and sulfur.
- The organic electroluminescent device according to claim 1, wherein Ma in formula 2 is a monocyclic-based heteroaryl group selected from the group consisting of pyrrolyl, imidazolyl, pyrazolyl, triazinyl, tetrazinyl, triazolyl, tetrazolyl, pyridyl, pyrazinyl, pyrimidinyl and pyridazinyl, or a fused ring-based heteroaryl group selected from the group consisting of benzoimidazolyl, isoindolyl, indolyl, indazolyl, benzothiadiazolyl, quinolyl, isoquinolyl, cinnolinyl, quinazolinyl, naphthyridinyl, quinoxalinyl, carbazolyl and phenanthridinyl.
- The organic electroluminescent device according to claim 1, wherein A1 and A2 in formula 1 each independently represent phenyl, biphenyl, terphenyl, naphthyl, fluorenyl, phenanthrenyl, anthracenyl, indenyl, triphenylenyl, pyrenyl, tetracenyl, perylenyl, chrysenyl, naphthacenyl, or fluoranthenyl.
- The organic electroluminescent device according to claim 1, wherein Xa to Xh in formula 2 each independently represent hydrogen; a cyano group; a (C6-C15)aryl group which is unsubstituted or substituted with a tri(C6-C10)arylsilyl group, or a 10- to 20-membered heteroaryl group which is unsubstituted or substituted with a (C6-C12)aryl group; or are linked between adjacent substituents to form a substituted or unsubstituted benzene, a substituted or unsubstituted indole, a substituted or unsubstituted indene, a substituted or unsubstituted benzofuran, or a substituted or unsubstituted benzothiophene.
- The organic electroluminescent device according to claim 1, wherein the triarylsilyl as X1 to X16 in formula 1 is triphenylsilyl.
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016559431A JP6934299B2 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device containing it |
| EP24199330.2A EP4450590A3 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device comprising the same |
| US15/301,978 US20170117488A1 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device comprising the same |
| EP15777273.2A EP3129446A4 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device comprising the same |
| CN201580017400.6A CN106133113A (en) | 2014-04-08 | 2015-04-07 | Multicomponent material of main part and the Organnic electroluminescent device comprising it |
| US17/526,112 US20220077405A1 (en) | 2014-04-08 | 2021-11-15 | Multi-component host material and organic electroluminescent device comprising the same |
| US18/416,245 US20240206333A1 (en) | 2014-04-08 | 2024-01-18 | Multi-component host material and organic electroluminescent device comprising the same |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20140041844 | 2014-04-08 | ||
| KR10-2014-0041844 | 2014-04-08 | ||
| KR20140086754 | 2014-07-10 | ||
| KR10-2014-0086754 | 2014-07-10 | ||
| KR1020150042704A KR101754715B1 (en) | 2014-04-08 | 2015-03-26 | Multi-component host material and organic electroluminescence device comprising the same |
| KR10-2015-0042704 | 2015-03-26 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/301,978 A-371-Of-International US20170117488A1 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device comprising the same |
| US17/526,112 Continuation US20220077405A1 (en) | 2014-04-08 | 2021-11-15 | Multi-component host material and organic electroluminescent device comprising the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015156587A1 true WO2015156587A1 (en) | 2015-10-15 |
Family
ID=54288099
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2015/003485 WO2015156587A1 (en) | 2014-04-08 | 2015-04-07 | Multi-component host material and organic electroluminescent device comprising the same |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2015156587A1 (en) |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016013867A1 (en) * | 2014-07-22 | 2016-01-28 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device |
| WO2016129819A1 (en) * | 2015-02-12 | 2016-08-18 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| WO2016148390A1 (en) * | 2015-03-13 | 2016-09-22 | Rohm And Haas Electronic Materials Korea Ltd. | A plurality of host materials and organic electroluminescent device comprising the same |
| WO2016186321A1 (en) * | 2015-05-19 | 2016-11-24 | Rohm And Haas Electronic Materials Korea Ltd. | Phosphorous host material and organic electroluminescent device comprising the same |
| WO2016208873A1 (en) | 2015-06-26 | 2016-12-29 | Rohm And Haas Electronic Materials Korea Ltd. | Multi-component host material and organic electroluminescent device comprising the same |
| CN106432051A (en) * | 2016-09-20 | 2017-02-22 | 长春海谱润斯科技有限公司 | Carbazole derivatives as well as preparation method and application thereof |
| WO2017069428A1 (en) * | 2015-10-22 | 2017-04-27 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| CN107033128A (en) * | 2016-02-03 | 2017-08-11 | Sfc有限责任公司 | Organic luminescent compounds and the organic light emitting apparatus including it |
| CN107093677A (en) * | 2016-02-18 | 2017-08-25 | 三星显示有限公司 | Organic light emitting apparatus |
| JP2017175099A (en) * | 2016-03-25 | 2017-09-28 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | Compound for organic electroluminescent device and organic electroluminescent device using the same |
| CN107231801A (en) * | 2015-02-12 | 2017-10-03 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and the Organnic electroluminescent device comprising organic electroluminescent compounds |
| WO2018004096A1 (en) * | 2016-06-29 | 2018-01-04 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| WO2018004095A1 (en) * | 2016-06-29 | 2018-01-04 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| CN107771206A (en) * | 2015-06-26 | 2018-03-06 | 罗门哈斯电子材料韩国有限公司 | Multicomponent material of main part and the Organnic electroluminescent device including this material |
| WO2018050584A1 (en) | 2016-09-14 | 2018-03-22 | Merck Patent Gmbh | Compounds with spirobifluorene-structures |
| WO2018061446A1 (en) * | 2016-09-30 | 2018-04-05 | 新日鉄住金化学株式会社 | Organic electroluminescent element |
| US20180105740A1 (en) * | 2015-05-19 | 2018-04-19 | Rohm And Haas Electronic Materials Korea Ltd. | Phosphorous host material and organic electroluminescent device comprising the same |
| CN108137551A (en) * | 2015-10-22 | 2018-06-08 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and the Organnic electroluminescent device for including it |
| CN108475733A (en) * | 2015-12-28 | 2018-08-31 | 新日铁住金化学株式会社 | Organic electroluminescent element |
| CN108603109A (en) * | 2016-07-26 | 2018-09-28 | 株式会社Lg化学 | Organic luminescent device |
| WO2018198844A1 (en) * | 2017-04-27 | 2018-11-01 | 新日鉄住金化学株式会社 | Organic electroluminescent element |
| WO2019007866A1 (en) | 2017-07-05 | 2019-01-10 | Merck Patent Gmbh | Composition for organic electronic devices |
| CN109476988A (en) * | 2016-07-29 | 2019-03-15 | 三星Sdi株式会社 | Constituent, organic photoelectric device and display device for organic photoelectric device |
| CN109952357A (en) * | 2016-11-16 | 2019-06-28 | 三星Sdi株式会社 | Organic photodiode and display device |
| CN109983098A (en) * | 2016-11-24 | 2019-07-05 | 三星Sdi株式会社 | Organic photovoltaic component and display device |
| US10629821B2 (en) | 2015-04-08 | 2020-04-21 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescent elements using same, and organic electroluminescent element and electronic device each using same |
| EP3643761A1 (en) * | 2018-10-25 | 2020-04-29 | Idemitsu Kosan Co., Ltd. | Composition, organic electroluminescence device material, composition film, organic electroluminescence device, and electronic device |
| US10749119B2 (en) | 2015-03-13 | 2020-08-18 | Rohm And Haas Electronic Materials Korea Ltd | Plurality of host materials and organic electroluminescent device comprising the same |
| US10840458B2 (en) | 2016-05-25 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3605634A4 (en) * | 2017-03-23 | 2021-01-20 | NIPPON STEEL Chemical & Material Co., Ltd. | Organic electroluminescent element |
| US11084806B2 (en) | 2016-07-12 | 2021-08-10 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device, and display device |
| US11107997B2 (en) * | 2016-09-22 | 2021-08-31 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device comprising an electron buffer layer and an electron transport layer |
| WO2021180614A1 (en) | 2020-03-11 | 2021-09-16 | Merck Patent Gmbh | Organic electroluminescent apparatus |
| US11158817B2 (en) | 2017-01-05 | 2021-10-26 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| EP3767698A4 (en) * | 2018-03-16 | 2022-03-16 | NIPPON STEEL Chemical & Material Co., Ltd. | Organic electroluminescent element |
| US11696499B2 (en) | 2016-05-10 | 2023-07-04 | Samsung Display Co., Ltd. | Organic light-emitting device |
| US11856842B2 (en) | 2015-11-26 | 2023-12-26 | Samsung Display Co., Ltd. | Organic light-emitting device |
| US11910707B2 (en) | 2015-12-23 | 2024-02-20 | Samsung Display Co., Ltd. | Organic light-emitting device |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3139321B2 (en) | 1994-03-31 | 2001-02-26 | 東レ株式会社 | Light emitting element |
| WO2011155507A1 (en) * | 2010-06-08 | 2011-12-15 | 出光興産株式会社 | Organic electroluminescence element |
| KR20120013173A (en) | 2010-08-04 | 2012-02-14 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| KR101170666B1 (en) | 2009-03-03 | 2012-08-07 | 덕산하이메탈(주) | Bis-carbazole chemiclal and organic electroric element using the same, terminal thererof |
| WO2012176818A1 (en) | 2011-06-24 | 2012-12-27 | 出光興産株式会社 | Organic electroluminescent element |
| WO2013058343A1 (en) * | 2011-10-21 | 2013-04-25 | 出光興産株式会社 | Organic electroluminescence element and material for organic electroluminescence element |
| WO2013112557A1 (en) | 2012-01-26 | 2013-08-01 | Universal Display Corporation | Phosphorescent organic light emitting devices having a hole transporting cohost material in the emissive region |
| US20130234119A1 (en) * | 2011-12-05 | 2013-09-12 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescence device and organic electroluminescence device |
| WO2013147205A1 (en) * | 2012-03-29 | 2013-10-03 | 出光興産株式会社 | Organic electroluminescent element and material for organic electroluminescent elements |
| WO2013168688A1 (en) | 2012-05-10 | 2013-11-14 | コニカミノルタ株式会社 | Organic electroluminescence element, illumination device, and display device |
| WO2013187896A1 (en) * | 2012-06-14 | 2013-12-19 | Universal Display Corporation | Biscarbazole derivative host materials and green emitter for oled emissive region |
| US20140042469A1 (en) * | 2012-08-10 | 2014-02-13 | Semiconductor Energy Laboratory Co., Ltd. | Light-Emitting Element, Light-Emitting Device, Display Device, Electronic Device, and Lighting Device |
| WO2014038677A1 (en) * | 2012-09-07 | 2014-03-13 | 出光興産株式会社 | Novel aromatic heterocyclic derivative, organic electroluminescent element material, organic electroluminescent element material solution, and organic electroluminescent element |
| US20140070204A1 (en) | 2011-05-12 | 2014-03-13 | Toray Industries, Inc. | Light emitting device material and light emitting device |
| JP2014049539A (en) * | 2012-08-30 | 2014-03-17 | Idemitsu Kosan Co Ltd | Organic electroluminescent element |
| WO2014087913A1 (en) * | 2012-12-03 | 2014-06-12 | 出光興産株式会社 | Organic electroluminescent element |
| WO2014096961A2 (en) * | 2012-12-19 | 2014-06-26 | Marvell World Trade Ltd. | Systems and methods for adaptive scaling of digital images |
| EP2821459A2 (en) | 2013-07-01 | 2015-01-07 | Cheil Industries Inc. | Composition and organic optoelectric device and display device |
-
2015
- 2015-04-07 WO PCT/KR2015/003485 patent/WO2015156587A1/en active Application Filing
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3139321B2 (en) | 1994-03-31 | 2001-02-26 | 東レ株式会社 | Light emitting element |
| KR101170666B1 (en) | 2009-03-03 | 2012-08-07 | 덕산하이메탈(주) | Bis-carbazole chemiclal and organic electroric element using the same, terminal thererof |
| WO2011155507A1 (en) * | 2010-06-08 | 2011-12-15 | 出光興産株式会社 | Organic electroluminescence element |
| KR20120013173A (en) | 2010-08-04 | 2012-02-14 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| US20140070204A1 (en) | 2011-05-12 | 2014-03-13 | Toray Industries, Inc. | Light emitting device material and light emitting device |
| WO2012176818A1 (en) | 2011-06-24 | 2012-12-27 | 出光興産株式会社 | Organic electroluminescent element |
| US20140217378A1 (en) | 2011-06-24 | 2014-08-07 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element |
| WO2013058343A1 (en) * | 2011-10-21 | 2013-04-25 | 出光興産株式会社 | Organic electroluminescence element and material for organic electroluminescence element |
| US20130234119A1 (en) * | 2011-12-05 | 2013-09-12 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescence device and organic electroluminescence device |
| WO2013112557A1 (en) | 2012-01-26 | 2013-08-01 | Universal Display Corporation | Phosphorescent organic light emitting devices having a hole transporting cohost material in the emissive region |
| WO2013147205A1 (en) * | 2012-03-29 | 2013-10-03 | 出光興産株式会社 | Organic electroluminescent element and material for organic electroluminescent elements |
| WO2013168688A1 (en) | 2012-05-10 | 2013-11-14 | コニカミノルタ株式会社 | Organic electroluminescence element, illumination device, and display device |
| WO2013187896A1 (en) * | 2012-06-14 | 2013-12-19 | Universal Display Corporation | Biscarbazole derivative host materials and green emitter for oled emissive region |
| US20140042469A1 (en) * | 2012-08-10 | 2014-02-13 | Semiconductor Energy Laboratory Co., Ltd. | Light-Emitting Element, Light-Emitting Device, Display Device, Electronic Device, and Lighting Device |
| JP2014049539A (en) * | 2012-08-30 | 2014-03-17 | Idemitsu Kosan Co Ltd | Organic electroluminescent element |
| WO2014038677A1 (en) * | 2012-09-07 | 2014-03-13 | 出光興産株式会社 | Novel aromatic heterocyclic derivative, organic electroluminescent element material, organic electroluminescent element material solution, and organic electroluminescent element |
| WO2014087913A1 (en) * | 2012-12-03 | 2014-06-12 | 出光興産株式会社 | Organic electroluminescent element |
| WO2014096961A2 (en) * | 2012-12-19 | 2014-06-26 | Marvell World Trade Ltd. | Systems and methods for adaptive scaling of digital images |
| EP2821459A2 (en) | 2013-07-01 | 2015-01-07 | Cheil Industries Inc. | Composition and organic optoelectric device and display device |
Non-Patent Citations (2)
| Title |
|---|
| APPL. PHYS. LETT., vol. 51, 1987, pages 913 |
| See also references of EP3129446A4 |
Cited By (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016013867A1 (en) * | 2014-07-22 | 2016-01-28 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device |
| US11917907B2 (en) | 2014-07-22 | 2024-02-27 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device |
| CN107231801B (en) * | 2015-02-12 | 2021-03-23 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and organic electroluminescent devices comprising the same |
| WO2016129819A1 (en) * | 2015-02-12 | 2016-08-18 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| CN107231801A (en) * | 2015-02-12 | 2017-10-03 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and the Organnic electroluminescent device comprising organic electroluminescent compounds |
| WO2016148390A1 (en) * | 2015-03-13 | 2016-09-22 | Rohm And Haas Electronic Materials Korea Ltd. | A plurality of host materials and organic electroluminescent device comprising the same |
| US10749119B2 (en) | 2015-03-13 | 2020-08-18 | Rohm And Haas Electronic Materials Korea Ltd | Plurality of host materials and organic electroluminescent device comprising the same |
| US10629821B2 (en) | 2015-04-08 | 2020-04-21 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescent elements using same, and organic electroluminescent element and electronic device each using same |
| JP7127095B2 (en) | 2015-05-19 | 2022-08-29 | ローム・アンド・ハース・エレクトロニック・マテリアルズ・コリア・リミテッド | Phosphorus host material and organic electroluminescent device containing the same |
| JP2021022739A (en) * | 2015-05-19 | 2021-02-18 | ローム・アンド・ハース・エレクトロニック・マテリアルズ・コリア・リミテッド | Phosphorus host material and organic electroluminescent element including the same |
| US20180105740A1 (en) * | 2015-05-19 | 2018-04-19 | Rohm And Haas Electronic Materials Korea Ltd. | Phosphorous host material and organic electroluminescent device comprising the same |
| WO2016186321A1 (en) * | 2015-05-19 | 2016-11-24 | Rohm And Haas Electronic Materials Korea Ltd. | Phosphorous host material and organic electroluminescent device comprising the same |
| EP3298016A4 (en) * | 2015-05-19 | 2018-11-21 | Rohm And Haas Electronic Materials Korea Ltd. | Phosphorous host material and organic electroluminescent device comprising the same |
| WO2016208873A1 (en) | 2015-06-26 | 2016-12-29 | Rohm And Haas Electronic Materials Korea Ltd. | Multi-component host material and organic electroluminescent device comprising the same |
| CN107771206A (en) * | 2015-06-26 | 2018-03-06 | 罗门哈斯电子材料韩国有限公司 | Multicomponent material of main part and the Organnic electroluminescent device including this material |
| EP3636726A1 (en) * | 2015-06-26 | 2020-04-15 | Rohm And Haas Electronic Materials Korea Ltd. | Multi-component host material and organic electroluminescent device comprising the same |
| TWI699365B (en) * | 2015-06-26 | 2020-07-21 | 南韓商羅門哈斯電子材料韓國公司 | Multi-component host material and organic electroluminescent device comprising the same |
| CN113206210A (en) * | 2015-06-26 | 2021-08-03 | 罗门哈斯电子材料韩国有限公司 | Multi-component host material and organic electroluminescent device including the same |
| JP2018520513A (en) * | 2015-06-26 | 2018-07-26 | ローム・アンド・ハース・エレクトロニック・マテリアルズ・コリア・リミテッド | Multi-component host material and organic electroluminescent device including the same |
| CN108137551A (en) * | 2015-10-22 | 2018-06-08 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and the Organnic electroluminescent device for including it |
| CN114539224A (en) * | 2015-10-22 | 2022-05-27 | 罗门哈斯电子材料韩国有限公司 | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| WO2017069428A1 (en) * | 2015-10-22 | 2017-04-27 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| JP2019500316A (en) * | 2015-10-22 | 2019-01-10 | ローム・アンド・ハース・エレクトロニック・マテリアルズ・コリア・リミテッド | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| TWI719063B (en) * | 2015-10-22 | 2021-02-21 | 南韓商羅門哈斯電子材料韓國公司 | Organic electroluminescent compounds and organic electroluminescent device comprising the same |
| US11856842B2 (en) | 2015-11-26 | 2023-12-26 | Samsung Display Co., Ltd. | Organic light-emitting device |
| US11910707B2 (en) | 2015-12-23 | 2024-02-20 | Samsung Display Co., Ltd. | Organic light-emitting device |
| CN108475733A (en) * | 2015-12-28 | 2018-08-31 | 新日铁住金化学株式会社 | Organic electroluminescent element |
| CN107033128A (en) * | 2016-02-03 | 2017-08-11 | Sfc有限责任公司 | Organic luminescent compounds and the organic light emitting apparatus including it |
| US11476426B2 (en) | 2016-02-03 | 2022-10-18 | Sfc Co., Ltd. | Organic light emitting compounds and organic light emitting devices including the same |
| CN107093677A (en) * | 2016-02-18 | 2017-08-25 | 三星显示有限公司 | Organic light emitting apparatus |
| US11165024B2 (en) | 2016-02-18 | 2021-11-02 | Samsung Display Co., Ltd. | Organic light-emitting device |
| JP2017175099A (en) * | 2016-03-25 | 2017-09-28 | ▲いく▼▲雷▼光電科技股▲分▼有限公司 | Compound for organic electroluminescent device and organic electroluminescent device using the same |
| US11696499B2 (en) | 2016-05-10 | 2023-07-04 | Samsung Display Co., Ltd. | Organic light-emitting device |
| US10840458B2 (en) | 2016-05-25 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI649316B (en) * | 2016-06-29 | 2019-02-01 | 三星Sdi股份有限公司 | Compound for organic optoelectric device, composition for organic optoelectric device, and organic optoelectric device and display device |
| KR102027961B1 (en) | 2016-06-29 | 2019-10-02 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| CN109415624B (en) * | 2016-06-29 | 2022-08-26 | 三星Sdi株式会社 | Compound, composition, organic photoelectric device and display device |
| KR20180002351A (en) * | 2016-06-29 | 2018-01-08 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| CN109415625A (en) * | 2016-06-29 | 2019-03-01 | 三星Sdi株式会社 | Compound for organic photoelectric device, the constituent for organic photoelectric device and organic photoelectric device and display device |
| WO2018004095A1 (en) * | 2016-06-29 | 2018-01-04 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| WO2018004096A1 (en) * | 2016-06-29 | 2018-01-04 | 삼성에스디아이 주식회사 | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| CN109415624A (en) * | 2016-06-29 | 2019-03-01 | 三星Sdi株式会社 | Compound for organic photoelectric device, the composition for organic photoelectric device and organic photoelectric device and display device |
| US11678572B2 (en) | 2016-06-29 | 2023-06-13 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| US11706975B2 (en) | 2016-06-29 | 2023-07-18 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device and display apparatus |
| US11084806B2 (en) | 2016-07-12 | 2021-08-10 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device, organic optoelectronic device, and display device |
| US11751473B2 (en) | 2016-07-26 | 2023-09-05 | Lg Chem, Ltd. | Organic light emitting element |
| CN108603109B (en) * | 2016-07-26 | 2021-08-27 | 株式会社Lg化学 | Organic light emitting device |
| CN108603109A (en) * | 2016-07-26 | 2018-09-28 | 株式会社Lg化学 | Organic luminescent device |
| US11264574B2 (en) | 2016-07-29 | 2022-03-01 | Samsung Sdi Co., Ltd. | Composition for organic optoelectronic element, organic optoelectronic element, and display device |
| CN109476988A (en) * | 2016-07-29 | 2019-03-15 | 三星Sdi株式会社 | Constituent, organic photoelectric device and display device for organic photoelectric device |
| US11447464B2 (en) | 2016-09-14 | 2022-09-20 | Merck Patent Gmbh | Compounds with spirobifluorene-structures |
| WO2018050584A1 (en) | 2016-09-14 | 2018-03-22 | Merck Patent Gmbh | Compounds with spirobifluorene-structures |
| CN106432051B (en) * | 2016-09-20 | 2019-02-22 | 长春海谱润斯科技有限公司 | A kind of carbazole analog derivative and its preparation method and application |
| CN106432051A (en) * | 2016-09-20 | 2017-02-22 | 长春海谱润斯科技有限公司 | Carbazole derivatives as well as preparation method and application thereof |
| US11107997B2 (en) * | 2016-09-22 | 2021-08-31 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent device comprising an electron buffer layer and an electron transport layer |
| WO2018061446A1 (en) * | 2016-09-30 | 2018-04-05 | 新日鉄住金化学株式会社 | Organic electroluminescent element |
| TWI728164B (en) * | 2016-09-30 | 2021-05-21 | 日商日鐵化學材料股份有限公司 | Organic electroluminescent element and manufacturing method thereof |
| US11171295B2 (en) | 2016-09-30 | 2021-11-09 | Nippon Steel Chemical & Material Co., Ltd. | Organic electroluminescent element |
| CN109952357A (en) * | 2016-11-16 | 2019-06-28 | 三星Sdi株式会社 | Organic photodiode and display device |
| CN109952357B (en) * | 2016-11-16 | 2022-08-26 | 三星Sdi株式会社 | Organic photoelectric device and display device |
| CN109983098A (en) * | 2016-11-24 | 2019-07-05 | 三星Sdi株式会社 | Organic photovoltaic component and display device |
| US11158817B2 (en) | 2017-01-05 | 2021-10-26 | Samsung Sdi Co., Ltd. | Compound for organic optoelectronic device, composition for organic optoelectronic device and organic optoelectronic device and display device |
| EP3605634A4 (en) * | 2017-03-23 | 2021-01-20 | NIPPON STEEL Chemical & Material Co., Ltd. | Organic electroluminescent element |
| US11374178B2 (en) | 2017-03-23 | 2022-06-28 | Nippon Steel Chemical & Material Co., Ltd. | Organic electroluminescent element |
| CN110574180A (en) * | 2017-04-27 | 2019-12-13 | 日铁化学材料株式会社 | Organic electroluminescent element |
| CN110574180B (en) * | 2017-04-27 | 2022-11-15 | 日铁化学材料株式会社 | Organic electroluminescent device and method for manufacturing the same |
| JP7030794B2 (en) | 2017-04-27 | 2022-03-07 | 日鉄ケミカル&マテリアル株式会社 | Organic electroluminescent device |
| US11189802B2 (en) | 2017-04-27 | 2021-11-30 | Nippon Steel Chemical & Material Co., Ltd. | Organic electroluminescent element |
| JPWO2018198844A1 (en) * | 2017-04-27 | 2020-03-12 | 日鉄ケミカル&マテリアル株式会社 | Organic electroluminescent device |
| WO2018198844A1 (en) * | 2017-04-27 | 2018-11-01 | 新日鉄住金化学株式会社 | Organic electroluminescent element |
| US11591320B2 (en) | 2017-07-05 | 2023-02-28 | Merck Patent Gmbh | Composition for organic electronic devices |
| EP4186898A1 (en) | 2017-07-05 | 2023-05-31 | Merck Patent GmbH | Composition for organic electronic compounds |
| WO2019007866A1 (en) | 2017-07-05 | 2019-01-10 | Merck Patent Gmbh | Composition for organic electronic devices |
| EP3767698A4 (en) * | 2018-03-16 | 2022-03-16 | NIPPON STEEL Chemical & Material Co., Ltd. | Organic electroluminescent element |
| EP3643761A1 (en) * | 2018-10-25 | 2020-04-29 | Idemitsu Kosan Co., Ltd. | Composition, organic electroluminescence device material, composition film, organic electroluminescence device, and electronic device |
| WO2021180614A1 (en) | 2020-03-11 | 2021-09-16 | Merck Patent Gmbh | Organic electroluminescent apparatus |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3129446A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| WO2015156587A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| WO2016080791A1 (en) | A plurality of host materials and an organic electroluminescent device comprising the same | |
| EP3172780A1 (en) | Organic electroluminescent device | |
| EP3131879A1 (en) | Multi-component host material and an organic electroluminescence device comprising the same | |
| WO2016076629A1 (en) | A plurality of host materials and an organic electroluminescence device comprising the same | |
| WO2016013875A1 (en) | Organic electroluminescent device | |
| WO2015167259A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| WO2015178732A1 (en) | Multi-component host material and an organic electroluminescence device comprising the same | |
| EP3170206A1 (en) | Organic electroluminescent device | |
| EP3172779A1 (en) | Organic electroluminescent device | |
| EP3551623A1 (en) | Organic electroluminescent compound and organic electroluminescent device comprising the same | |
| EP3140367A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| EP3446345A1 (en) | A plurality of host materials and organic electroluminescent device comprising the same | |
| EP3494117A1 (en) | Organic electroluminescent compound and organic electroluminescent device comprising the same | |
| WO2015174738A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| WO2018021841A1 (en) | Organic electroluminescent compound and organic electroluminescent device comprising the same | |
| EP3183234A1 (en) | A plurality of host materials and an organic electroluminescence device comprising the same | |
| WO2016036171A1 (en) | A plurality of host materials and organic electroluminescent devices comprising the same | |
| EP3685453A1 (en) | A plurality of host materials and organic electroluminescent device comprising the same | |
| WO2016060516A1 (en) | A plurality of host materials and an organic electroluminescence device comprising the same | |
| EP3313958A1 (en) | Multi-component host material and organic electroluminescent device comprising the same | |
| EP3458457A1 (en) | Organic electroluminescent compound, organic electroluminescent material and organic electroluminescent device comprising the same | |
| WO2017183859A1 (en) | A plurality of host materials and organic electroluminescent device comprising the same | |
| EP3440155A1 (en) | Organic electroluminescent compound and organic electroluminescent device comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15777273 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2016559431 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15301978 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015777273 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015777273 Country of ref document: EP |